User login
Chagas Disease: Creeping into Family Practice in the United States
CE/CME No: CR-1611
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand the prevalence and risks of Chagas disease in the United States.
• Explain the pathophysiology of Chagas disease, including the vector and transmission routes of the disease.
• Describe the clinical presentation of both the acute and chronic forms of the disease and learn when to suspect an infection.
• Outline a plan for diagnosis and treatment of Chagas disease.
• Educate women with Chagas disease about the risk of transmission for future offspring.
FACULTY
Jessica McDonald works in the Emergency Medicine Department at Dekalb Medical Center, Atlanta. Jill Mattingly is Academic Coordinator and Clinical Assistant Professor in the Physician Assistant Program at Mercer University, Atlanta.
The authors have no financial relationships to disclose.
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of November 2016.
Article begins on next page >>
Chagas disease, a parasitic infection, is increasingly being detected in the United States, most likely due to immigration from endemic countries in South and Central America. Approximately 300,000 persons in the US have chronic Chagas disease, and up to 30% of them will develop clinically evident cardiovascular and/or gastrointestinal disease. Here’s practical guidance to help you recognize the features of symptomatic Chagas disease and follow up with appropriate evaluation and management.
Chagas disease, also known as American trypanosomiasis, is caused by the protozoan parasite Trypanosoma cruzi.1 It is most commonly spread by triatomine bugs infected with T cruzi and is endemic in many parts of Mexico and Central and South America.2 Chagas disease was first described in 1909 by Brazilian physician Carlos Chagas.3 Since its discovery, it has often been considered a disease affecting only the poor living in endemic areas of Latin America. However, 6 million to 7 million people are infected with T cruzi worldwide, and estimates suggest that Mexico and the US rank third and seventh, respectively, in the number of persons with T cruzi infection in the Western Hemisphere.1,4
An estimated 300,000 persons in the US have Chagas disease; most of them are not aware that they are infected.5,6 The increasing presence of the disease in the US, which traditionally has been considered a nonendemic area, is due to immigration from endemic areas, with subsequent infections occurring through mechanisms that do not require contact with the triatomine vector (eg, congenital transmission).1 Between 1981 and 2005, more than 7 million people from T cruzi-endemic countries in Latin America moved to the US and became legal residents.3
Early detection and treatment of Chagas disease is important because up to 30% of patients with chronic infection will develop a heart disorder, which can range in severity from conduction system abnormalities to dilated cardiomyopathy.4 In some areas of southern Mexico, Chagas disease is the most common cause of dilated cardiomyopathy.1 Equally concerning is the fact that untreated mothers with Chagas disease can transmit T cruzi to their infants.1,3 An estimated 315 babies are born with congenital Chagas disease each year in the US, an incidence equivalent to that of phenylketonuria.7 It is estimated that congenital transmission is responsible for up to one-quarter of new infections worldwide.1 Unfortunately, obstetricians are not well informed about the risk factors for congenital Chagas disease, and very limited screening of at-risk women is performed. In a 2008 survey exploring health care providers’ knowledge of and understanding about Chagas disease, obstetricians and gynecologists had the greatest knowledge deficits about the disease, although considerable deficits were also seen among other specialties.1
KISSING BUG DISEASE: ETIOLOGY/PATHOPHYSIOLOGY
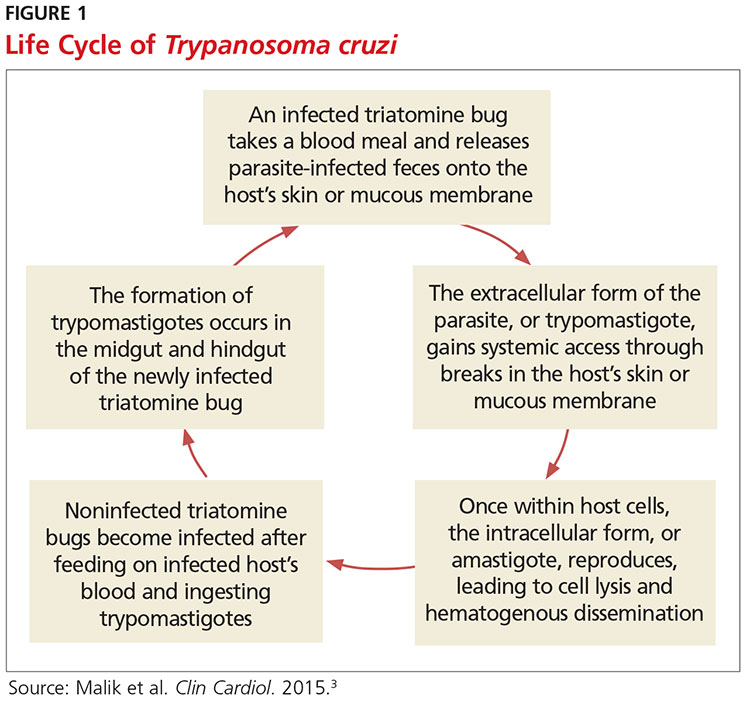
Exposure to the protozoan parasite T cruzi, the cause of Chagas disease, typically occurs following the bite of a triatomine bug. Also known as “kissing bugs” because they usually bite exposed areas of the skin such as the face, triatomine bugs feed on human blood, typically at night, and act as a vector for the parasite.8 The parasite lives in the feces and urine of the triatomine bugs and is excreted near the bite during or shortly after a blood meal. The bitten person will then unknowingly smear the infected feces into the bite wound, eyes, mouth, or any opening in the skin, which gives the parasites systemic access.4 Once in the host’s bloodstream, the parasite replicates in host cells, a process that ends in cell lysis and hematogenous spread. At this point, the parasites can be seen on peripheral blood smear. Noninfected triatomine insects become infected and continue the cycle when they feed on an infected human host (see Figure 1).3 Persons of lower socioeconomic status living in endemic areas in Latin America are at a higher risk for contracting Chagas disease because “kissing bugs” commonly live in wall or roof cracks of poorly built homes. Populations living in poverty are also at risk due to minimal access to health care and prenatal care.4 Transmission of T cruzi not involving triatomine vectors occurs congenitally or through blood transfusions, consumption of contaminated food, and organ donations.4
NATURAL HISTORY OF INFECTION AND PATIENT PRESENTATION
Acute phase
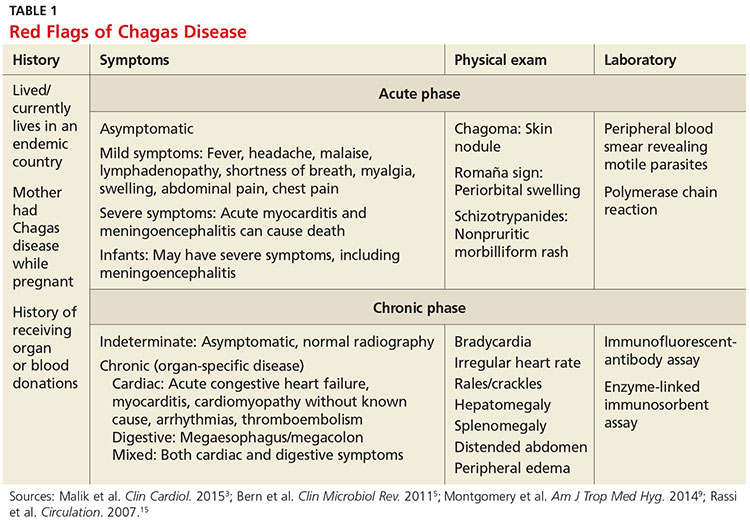
Infection with the T cruzi parasite is followed by an asymptomatic incubation period of one to two weeks, which is then followed by an acute phase that can last eight to 12 weeks.5 The acute phase is characterized by a large amount of parasites in the bloodstream (see Table 1). The patient is often asymptomatic but can have nonspecific symptoms such as fever, headache, lymphadenopathy, shortness of breath, myalgia, swelling, and abdominal or chest pain.4 Because symptoms during the acute phase are typically mild, many patients do not seek medical attention until they transition into the chronic phase.4 Infants are more likely to experience severe symptoms, including myocarditis or meningoencephalitis, and thus are more likely to present during the acute phase.9
If the patient acquired the infection through an organ transplant, the acute phase symptoms can be delayed, on average, up to 112 days.5 These patients will have more noticeable symptoms, including hepatosplenomegaly, myocarditis, and congestive heart failure. Due to the known risk for transmission through organ transplants, donors are often screened for Chagas disease. Unfortunately, this screening is selective and often inconsistent.5 Therefore, the presence of the previously mentioned symptoms in a person who recently received an organ transplant should raise suspicion of Chagas disease.5
Chronic phase
Patients not treated during the acute phase will pass into the chronic phase of Chagas disease.5 This may occur due to reactivation of T cruzi infection via immunosuppression.9 At this time, the previously asymptomatic patient will have typical signs and symptoms of chronic disease, along with nodules, panniculitis, and myocarditis.4,9,10 During the chronic phase, parasites are undetectable by microscopy, but the patient can still spread the disease to the vector as well as to others congenitally and through organ donation and blood transfusions.5,9
Patients with chronic T cruzi infection who remain without signs or symptoms of infection are considered to have the indeterminate form of chronic disease. Many patients will remain in the indeterminate form throughout their lives, but between 20% and 30% will progress to the determinate form of chronic disease over years to decades.3 The determinate form is characterized by clinically evident disease and is classically divided into cardiac Chagas disease and digestive Chagas disease.5 Symptoms of the chronic phase depend on the genotype of T cruzi that caused the infection. The AG genotype has a higher incidence of digestive disease.11
Cardiac Chagas disease is believed to occur due to parasite invasion and persistence in cardiac tissue, leading to immune-mediated myocardial injury.5 Chagas cardiomyopathy is characterized by chronic myocarditis affecting all cardiac chambers and disturbances in the electrical conduction system; patients also often develop apical aneurysms. Longstanding cardiac Chagas disease can lead to more serious complications, such as episodes of ventricular tachycardia, heart block, thromboembolic phenomena, severe bradycardia, dilated cardiomyopathy, and congestive heart failure. Patients may complain of presyncope, syncope, and episodes of palpitations. They are also at high risk for sudden cardiac death.5 Patients with cardiomyopathy or cardiac insufficiency secondary to Chagas disease have a worse prognosis than those with idiopathic cardiomyopathy or decompensated heart failure due to other etiologies.12
Less common than cardiac Chagas disease, digestive Chagas disease occurs mostly in Argentina, Bolivia, Chile, Paraguay, Uruguay, and parts of Peru and Brazil; it is rarely seen in northern South America, Central America, or Mexico.5 The parasite causes gastrointestinal symptoms by damaging intramural neurons, resulting in denervation of hollow viscera. Since it affects the esophagus and colon, patients may present with dysphagia, odynophagia, cough, reflux, weight loss, constipation, and abdominal pain.5
PHYSICAL EXAMINATION: A CRUCIAL STEP
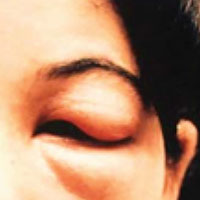
The physical examination of a patient with suspected Chagas disease can be crucial to the diagnosis. As noted, there are often few specific symptoms or physical exam findings during the acute phase. However, in some patients, swelling and inflammation may be evident at the site of inoculation, often on the face or extremities. This finding is called a chagoma. The Romaña sign, characterized by painless unilateral swelling of the upper and lower eyelid, can also be seen if the infection occurred through the conjunctiva.5 A nonpruritic morbilliform rash, called schizotrypanides, may be a presenting symptom in patients with acute disease.13 Children younger than 2 years of age are at increased risk for a severe acute infection, with signs and symptoms of pericardial effusion, myocarditis, and meningoencephalitis. Children can also develop generalized edema and lymphadenopathy. Those children who develop severe manifestations during acute infection have an increased risk for mortality.5
Chronic chagasic cardiomyopathy may present with signs of left-sided heart failure (pulmonary edema, dyspnea at rest or exertion), biventricular heart failure (hepatomegaly, peripheral edema, jugular venous distention), or thromboembolic events to the brain, lower extremities, and lungs.13 Chronic chagasic megaesophagus may lead to weight loss, esophageal dysmotility, pneumonitis due to aspiration of food trapped in the esophagus and stomach, salivary gland enlargement, and erosive esophagitis, which increases the risk for esophageal cancer. Chronic chagasic megacolon can present as an intestinal obstruction, volvulus, abdominal distention, or fecaloma.13
Clinicians should be alert to the possibility of congenital T cruzi infection in children born to women who emigrated from an endemic area or who visited an area with a high prevalence of Chagas disease. Most newborns with T cruzi infection are asymptomatic, but in some cases a thorough neonatal exam can lead to the diagnosis. Manifestations of symptomatic congenital infection include hepatosplenomegaly, low birth weight, premature birth, and low Apgar scores.5 Lab tests may reveal thrombocytopenia and anemia. Neonates with severe disease may also have respiratory distress, meningoencephalitis, and gastrointestinal problems.5
LABORATORY WORK-UP
Laboratory work-up for Chagas disease depends on the provider’s awareness of the disease and its symptoms. All patients should undergo routine blood work, including complete blood count (CBC) with differential, comprehensive metabolic panel (CMP), and liver function tests to rule out other etiologies that manifest with similar symptoms. If the patient presents during the acute phase, microscopy of blood smears with Giemsa stain should be done to visualize the parasites. In the patient who presents during the chronic phase with cardiac symptoms, measurement of B-type natriuretic peptide, troponin, C-reactive protein, and the erythrocyte sedimentation rate can be used to rule out other differential diagnoses. Electrocardiogram (ECG) may show a right bundle-branch block or left anterior fascicular block.5 Echocardiogram may show left ventricular wall motion abnormalities and/or cardiomyopathy with congestive heart failure.5,10 A work-up for digestive Chagas disease may include a barium swallow, kidney-ureter-bladder x-ray, or MRI/CT of the abdomen.14
DIAGNOSING ACUTE, CHRONIC, AND CONGENITAL CHAGAS
Accurate diagnosis of Chagas disease requires a thorough history and physical exam, as well as a high index of suspicion. Recent travel to an endemic area of Chagas disease in combination with the typical signs and symptoms—such as fever, headache, lymphadenopathy, shortness of breath, myalgia, swelling, and abdominal or chest pain—should prompt the provider to perform more specific tests.4 Inquiry about past medical history, blood transfusions, and surgeries is also imperative to make the correct diagnosis.5
The approach to diagnosis of Chagas disease depends on whether the patient presents during the acute or chronic phase. During the acute phase, the count of the trypomastigote, the mature extracellular form of the parasite T cruzi, is at its highest, making this the best time to obtain an accurate diagnosis if an infection is suspected.3 Microscopy of fresh preparations of anticoagulated blood or buffy coat may show motile parasites.10 Other options include visualization of parasites in a blood smear with Giemsa stain or hemoculture. Hemoculture is a sensitive test but takes several weeks to show growth of the parasites. Therefore, polymerase chain reaction (PCR) assay is the preferred diagnostic test due to its high sensitivity and quick turnaround time.5
Because no diagnostic gold standard exists for chronic disease, confidently diagnosing Chagas in the United States can be difficult.5 Past the acute phase (about three months after infection), microscopy and PCR cannot be used due to low parasitemia. If an infection with T cruzi is suspected but nine to 14 weeks have passed since exposure, serology is the method of choice for diagnosis. The enzyme-linked immunosorbent assay (ELISA) and immunofluorescent-antibody assay (IFA) are most often used to identify immunoglobulin (Ig) G antibodies to the parasite.
The difficulty of diagnosing Chagas disease in the chronic phase lies in the fact that neither ELISA or IFA alone is sensitive or specific enough to confirm the diagnosis.5 In order to make a serologic diagnosis of infection, positive results are needed from two serologic tests based on two different antigens or by using two different techniques (eg, ELISA or IFA). If the two tests are discordant, a third test must be done to determine the patient’s infection status. The radioimmunoprecipitation assay (RIPA) and trypomastigote excreted-secreted antigen immunoblot (TESA-blot) have been traditionally used as confirmatory tests, but even they do not have high sensitivity and specificity. A case of indeterminate Chagas disease is confirmed with positive serologic testing in a patient without symptoms and with normal ECG, chest x-ray, and imaging of the colon and esophagus.15
The preferred protocol for diagnosis of congenital Chagas disease first requires positive serologic testing confirming the infection in the mother (see Figure 2).16 Once that is determined, microscopic and PCR-based examinations of cord blood and peripheral blood specimens are carried out during the first one to two months of the infant’s life.10 PCR is the preferred test for early congenital Chagas disease, recipients of organ transplants, and after accidental exposure since results can determine if the patient is infected earlier than trypomastigotes (developmental stage of trypanosomes) can be seen on a peripheral blood smear.5
TREATMENT CONSIDERATIONS
If there is a suspicion of Chagas disease, the patient should be referred to an infectious disease specialist for diagnosis and treatment. Nifurtimox and benznidazole are the only drugs that have been shown to improve the course of Chagas disease.5 However, neither drug is approved by the FDA, and both can only be obtained from the CDC, which makes treatment a challenge.9 In addition, up to 30% of patients terminate treatment due to the many adverse effects of these drugs.17
The dosage regimen for nifurtimox is 8-10 mg/kg/d divided into three doses for 90 days.10 Anorexia, weight loss, nausea, vomiting, and abdominal pain occur in up to 70% of patients.5 Irritability, insomnia, disorientation, and tremors can also occur. Neurotoxicity leading to peripheral neuropathy is dose dependent and requires treatment termination.5
Benznidazole is better tolerated and is active against the trypomastigotes as well as the amastigotes or intracellular form of the parasite.10 The dosage regimen for benznidazole is 5-7 mg/kg/d divided into two doses for 60 days.10 Dermatologic reactions such as rash, photosensitivity, and exfoliative dermatitis are the most common adverse effects. Peripheral neuropathy and bone marrow suppression are dose dependent and require therapy cessation.5
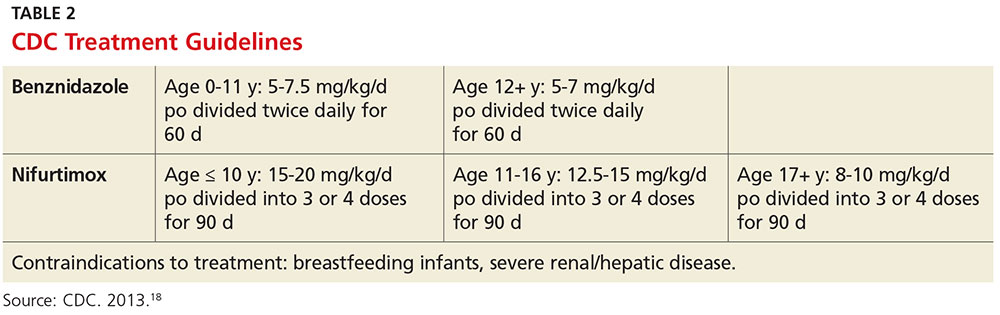
The CDC recommends treatment for all cases of acute disease (including congenital disease) regardless of age, and for chronic disease in patients up to age 50 who have not progressed to cardiomyopathy. In patients older than 50, treatment should be determined after weighing the potential risks and benefits (see Table 2).18
The success of treatment is determined in part by the phase of the disease. Cure rates in patients treated with either nifurtimox or benznidazole during the acute phase range from 65% to 80%.17 Chronic disease shows less of a response to traditional antiparasitic drug regimens, but higher rates of success are seen in younger patients.5 According to current estimates, successful treatment of chronic disease is limited to 15% to 30% patients.17 Treatment of congenital Chagas disease should begin as soon as the diagnosis is confirmed, and cure rates are greater than 90% if patients are treated within the first year of life.10 Treating congenital Chagas disease is important because the infection can be passed to future generations even if the disease never manifests with symptoms.19 However, if an expecting mother has known Chagas disease, antiparasitic medications are not recommended during the pregnancy because of a lack of fetal safety data for the two antiparasitic agents.20 Instead, it is recommended that women of childbearing age be treated before pregnancy, as rates of congenital infection are 25 times lower in women who are treated than in those who are not.21
PRE- AND POSTEXPOSURE PATIENT EDUCATION
Patient education mainly focuses on how to prevent Chagas disease and prognosis once diagnosed. During travel to endemic areas, the use of insecticides and residing in well-built households are the most important prevention measures. No vaccine is available, and primary chemoprophylaxis of persons visiting endemic areas is not recommended due to the low risk for infection and concerns about adverse effects.13
The survival rate of those who remain in the indeterminate phase is the same as that of the general population. However, findings that most strongly predict mortality include ventricular tachycardia, cardiomegaly, congestive heart failure (NYHA class III/IV), left ventricular systolic dysfunction, and male sex.10 Patients diagnosed with Chagas disease should be strongly encouraged not to donate blood or organs.10 Some organ and blood donation organizations selectively or universally screen donated specimens; however, this screening is not required by law.5 Family members of those diagnosed with the disease should also be tested, especially if the patient is a woman who has children or who plans to become pregnant.10
FOLLOW-UP
In patients confirmed to have Chagas disease but without symptoms and a normal ECG, further initial evaluation is not required.10 An annual history, physical exam, and ECG should be done. Those who have symptoms or ECG changes should have a complete cardiac work-up, including a 24-hour ambulatory ECG, exercise stress test, and echocardiogram to determine functional capacity. A barium swallow, barium enema, esophageal manometry, and endoscopy may be indicated in patients with gastrointestinal symptoms of Chagas disease but otherwise are not recommended. Patients taking antiparasitic drugs should have a CBC and CMP at the start of treatment and then bimonthly until the end of treatment to monitor for rare bone marrow suppression. Nifurtimox and benznidazole are also known to be mutagenic and increase the risk for lymphoma in animal studies, but this risk has not been documented in humans.10
CONCLUSION
Chagas disease is considered one of the neglected tropical diseases due to its high prevalence, chronic course, debilitating symptoms, and association with poverty.7 It is evident that incidence and prevalence of Chagas disease in the US are increasing due to recent immigration and mother-to-child transmission. Therefore, family practice clinicians must be able to recognize the red flags that suggest a T cruzi infection.5,9 Enhanced awareness of Chagas disease among health care providers will lead to better symptom control and cure rates for affected patients and may also prevent congenital infections. These efforts could serve to remove Chagas disease from the list of neglected tropical diseases.
1. Hotez PJ, Dumonteil E, Betancourt Cravioto M, et al. An unfolding tragedy of Chagas disease in North America. PLoS Negl Trop Dis. 2013; 7(10):e2300.
2. Verani JR, Seitz A, Gilman RH, et al. Geographic variation in the sensitivity of recombinant antigen-based rapid tests for chronic Trypanosoma cruzi infection. Am J Trop Med Hyg. 2009;80(3):410-415.
3. Malik LH, Singh GD, Amsterdam EA. The epidemiology, clinical manifestations, and management of Chagas heart disease. Clin Cardiol. 2015;38(9):565-569.
4. World Health Organization. Chagas disease (American trypanosomiasis). Fact sheet. Updated March 2016. www.who.int/mediacentre/factsheets/fs340/en/. Accessed October 20, 2016.
5. Bern C, Kjos S, Yabsley MJ, Montgomery SP. Trypanosoma cruzi and Chagas’ disease in the United States. Clin Microbiol Rev. 2011; 24(4):655-681.
6. Stimpert KK, Montgomery SP. Physician awareness of Chagas disease, USA. Emerg Infect Dis. 2010;16(5):871-872.
7. Hotez PJ. Neglected parasitic infections and poverty in the United States. PLoS Negl Trop Dis. 2014;8(9):e3012.
8. Goupil LS, McKerrow JH. Introduction: drug discovery and development for neglected diseases. Chem Rev. 2014;114(22):11131-11137.
9. Montgomery SP, Starr MC, Cantey P, et al. Neglected parasitic infections in the United States: Chagas disease. Am J Trop Med Hyg. 2014; 90(5):814-818.
10. Bern C, Montgomery SP, Herwaldt BL, et al. Evaluation and treatment of Chagas disease in the United States. JAMA. 2007;298(18):2171-2181.
11. de Oliveira AP, Bernardo CR, Camargo AV, et al. Genetic susceptibility to cardiac and digestive clinical forms of chronic Chagas disease: involvement of the CCR5 59029 A/G polymorphism. PLoS One. 2015; 10(11):e0141847.
12. Apt W, Arribada A, Zulantay I, et al. Trypanosoma cruzi burden, genotypes, and clinical evaluation of Chilean patients with chronic Chagas cardiopathy. Parasitol Res. 2015;114(8):3007-3018.
13. Kirchhoff LV. Chagas disease (American trypanosomiasis): Background, pathophysiology, epidemiology. Emedicine.medscape.com. 2015. http://emedicine.medscape.com/article/214581-overview. Accessed October 20, 2016.
14. Knipe H, St-Amant M. Chagas disease. Radiopaedia.org. 2015. http://radiopaedia.org/articles/chagas-disease. Accessed October 20, 2016.
15. Rassi A Jr, Rassi A, Rassi SG. Predictors of mortality in chronic Chagas disease: a systematic review of observational studies. Circulation. 2007;115(9):1101-1108.
16. Gomes YM, Lorena VM, Luquetti AO. Diagnosis of Chagas disease: what has been achieved? What remains to be done with regard to diagnosis and follow up studies? Mem Inst Oswaldo Cruz. 2009; 104(suppl 1):115-121.
17. Molina I, Gómez i Prat J, Salvador F, et al. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N Engl J Med. 2014;370:1899-1908.
18. CDC. Parasites – American trypanosomiasis (also known as Chagas Disease). Antiparasitic Treatment. Resources For Health Professionals. www.cdc.gov/parasites/chagas/health_professionals/tx.html. Accessed October 20, 2016.
19. Carlier Y, Truyens C. Congenital Chagas disease as an ecological model of interactions between Trypanosoma cruzi parasites, pregnant women, placenta and fetuses. Acta Trop. 2015;151:103-115.
20. Moscatelli G, Moroni S, García-Bournissen F, et al. Prevention of congenital Chagas through treatment of girls and women of childbearing age. Mem Inst Oswaldo Cruz. 2015;110(4):507-509.
21. Fabbro D, Danesi E, Olivera V, et al. Trypanocide treatment of women infected with Trypanosoma cruzi and its effect on preventing congenital Chagas. PLoS Negl Trop Dis. 2014;8(11):e3312.
CE/CME No: CR-1611
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand the prevalence and risks of Chagas disease in the United States.
• Explain the pathophysiology of Chagas disease, including the vector and transmission routes of the disease.
• Describe the clinical presentation of both the acute and chronic forms of the disease and learn when to suspect an infection.
• Outline a plan for diagnosis and treatment of Chagas disease.
• Educate women with Chagas disease about the risk of transmission for future offspring.
FACULTY
Jessica McDonald works in the Emergency Medicine Department at Dekalb Medical Center, Atlanta. Jill Mattingly is Academic Coordinator and Clinical Assistant Professor in the Physician Assistant Program at Mercer University, Atlanta.
The authors have no financial relationships to disclose.
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of November 2016.
Article begins on next page >>
Chagas disease, a parasitic infection, is increasingly being detected in the United States, most likely due to immigration from endemic countries in South and Central America. Approximately 300,000 persons in the US have chronic Chagas disease, and up to 30% of them will develop clinically evident cardiovascular and/or gastrointestinal disease. Here’s practical guidance to help you recognize the features of symptomatic Chagas disease and follow up with appropriate evaluation and management.
Chagas disease, also known as American trypanosomiasis, is caused by the protozoan parasite Trypanosoma cruzi.1 It is most commonly spread by triatomine bugs infected with T cruzi and is endemic in many parts of Mexico and Central and South America.2 Chagas disease was first described in 1909 by Brazilian physician Carlos Chagas.3 Since its discovery, it has often been considered a disease affecting only the poor living in endemic areas of Latin America. However, 6 million to 7 million people are infected with T cruzi worldwide, and estimates suggest that Mexico and the US rank third and seventh, respectively, in the number of persons with T cruzi infection in the Western Hemisphere.1,4
An estimated 300,000 persons in the US have Chagas disease; most of them are not aware that they are infected.5,6 The increasing presence of the disease in the US, which traditionally has been considered a nonendemic area, is due to immigration from endemic areas, with subsequent infections occurring through mechanisms that do not require contact with the triatomine vector (eg, congenital transmission).1 Between 1981 and 2005, more than 7 million people from T cruzi-endemic countries in Latin America moved to the US and became legal residents.3
Early detection and treatment of Chagas disease is important because up to 30% of patients with chronic infection will develop a heart disorder, which can range in severity from conduction system abnormalities to dilated cardiomyopathy.4 In some areas of southern Mexico, Chagas disease is the most common cause of dilated cardiomyopathy.1 Equally concerning is the fact that untreated mothers with Chagas disease can transmit T cruzi to their infants.1,3 An estimated 315 babies are born with congenital Chagas disease each year in the US, an incidence equivalent to that of phenylketonuria.7 It is estimated that congenital transmission is responsible for up to one-quarter of new infections worldwide.1 Unfortunately, obstetricians are not well informed about the risk factors for congenital Chagas disease, and very limited screening of at-risk women is performed. In a 2008 survey exploring health care providers’ knowledge of and understanding about Chagas disease, obstetricians and gynecologists had the greatest knowledge deficits about the disease, although considerable deficits were also seen among other specialties.1
KISSING BUG DISEASE: ETIOLOGY/PATHOPHYSIOLOGY
Exposure to the protozoan parasite T cruzi, the cause of Chagas disease, typically occurs following the bite of a triatomine bug. Also known as “kissing bugs” because they usually bite exposed areas of the skin such as the face, triatomine bugs feed on human blood, typically at night, and act as a vector for the parasite.8 The parasite lives in the feces and urine of the triatomine bugs and is excreted near the bite during or shortly after a blood meal. The bitten person will then unknowingly smear the infected feces into the bite wound, eyes, mouth, or any opening in the skin, which gives the parasites systemic access.4 Once in the host’s bloodstream, the parasite replicates in host cells, a process that ends in cell lysis and hematogenous spread. At this point, the parasites can be seen on peripheral blood smear. Noninfected triatomine insects become infected and continue the cycle when they feed on an infected human host (see Figure 1).3 Persons of lower socioeconomic status living in endemic areas in Latin America are at a higher risk for contracting Chagas disease because “kissing bugs” commonly live in wall or roof cracks of poorly built homes. Populations living in poverty are also at risk due to minimal access to health care and prenatal care.4 Transmission of T cruzi not involving triatomine vectors occurs congenitally or through blood transfusions, consumption of contaminated food, and organ donations.4

NATURAL HISTORY OF INFECTION AND PATIENT PRESENTATION
Acute phase
Infection with the T cruzi parasite is followed by an asymptomatic incubation period of one to two weeks, which is then followed by an acute phase that can last eight to 12 weeks.5 The acute phase is characterized by a large amount of parasites in the bloodstream (see Table 1). The patient is often asymptomatic but can have nonspecific symptoms such as fever, headache, lymphadenopathy, shortness of breath, myalgia, swelling, and abdominal or chest pain.4 Because symptoms during the acute phase are typically mild, many patients do not seek medical attention until they transition into the chronic phase.4 Infants are more likely to experience severe symptoms, including myocarditis or meningoencephalitis, and thus are more likely to present during the acute phase.9

If the patient acquired the infection through an organ transplant, the acute phase symptoms can be delayed, on average, up to 112 days.5 These patients will have more noticeable symptoms, including hepatosplenomegaly, myocarditis, and congestive heart failure. Due to the known risk for transmission through organ transplants, donors are often screened for Chagas disease. Unfortunately, this screening is selective and often inconsistent.5 Therefore, the presence of the previously mentioned symptoms in a person who recently received an organ transplant should raise suspicion of Chagas disease.5
Chronic phase
Patients not treated during the acute phase will pass into the chronic phase of Chagas disease.5 This may occur due to reactivation of T cruzi infection via immunosuppression.9 At this time, the previously asymptomatic patient will have typical signs and symptoms of chronic disease, along with nodules, panniculitis, and myocarditis.4,9,10 During the chronic phase, parasites are undetectable by microscopy, but the patient can still spread the disease to the vector as well as to others congenitally and through organ donation and blood transfusions.5,9
Patients with chronic T cruzi infection who remain without signs or symptoms of infection are considered to have the indeterminate form of chronic disease. Many patients will remain in the indeterminate form throughout their lives, but between 20% and 30% will progress to the determinate form of chronic disease over years to decades.3 The determinate form is characterized by clinically evident disease and is classically divided into cardiac Chagas disease and digestive Chagas disease.5 Symptoms of the chronic phase depend on the genotype of T cruzi that caused the infection. The AG genotype has a higher incidence of digestive disease.11
Cardiac Chagas disease is believed to occur due to parasite invasion and persistence in cardiac tissue, leading to immune-mediated myocardial injury.5 Chagas cardiomyopathy is characterized by chronic myocarditis affecting all cardiac chambers and disturbances in the electrical conduction system; patients also often develop apical aneurysms. Longstanding cardiac Chagas disease can lead to more serious complications, such as episodes of ventricular tachycardia, heart block, thromboembolic phenomena, severe bradycardia, dilated cardiomyopathy, and congestive heart failure. Patients may complain of presyncope, syncope, and episodes of palpitations. They are also at high risk for sudden cardiac death.5 Patients with cardiomyopathy or cardiac insufficiency secondary to Chagas disease have a worse prognosis than those with idiopathic cardiomyopathy or decompensated heart failure due to other etiologies.12
Less common than cardiac Chagas disease, digestive Chagas disease occurs mostly in Argentina, Bolivia, Chile, Paraguay, Uruguay, and parts of Peru and Brazil; it is rarely seen in northern South America, Central America, or Mexico.5 The parasite causes gastrointestinal symptoms by damaging intramural neurons, resulting in denervation of hollow viscera. Since it affects the esophagus and colon, patients may present with dysphagia, odynophagia, cough, reflux, weight loss, constipation, and abdominal pain.5
PHYSICAL EXAMINATION: A CRUCIAL STEP
The physical examination of a patient with suspected Chagas disease can be crucial to the diagnosis. As noted, there are often few specific symptoms or physical exam findings during the acute phase. However, in some patients, swelling and inflammation may be evident at the site of inoculation, often on the face or extremities. This finding is called a chagoma. The Romaña sign, characterized by painless unilateral swelling of the upper and lower eyelid, can also be seen if the infection occurred through the conjunctiva.5 A nonpruritic morbilliform rash, called schizotrypanides, may be a presenting symptom in patients with acute disease.13 Children younger than 2 years of age are at increased risk for a severe acute infection, with signs and symptoms of pericardial effusion, myocarditis, and meningoencephalitis. Children can also develop generalized edema and lymphadenopathy. Those children who develop severe manifestations during acute infection have an increased risk for mortality.5
Chronic chagasic cardiomyopathy may present with signs of left-sided heart failure (pulmonary edema, dyspnea at rest or exertion), biventricular heart failure (hepatomegaly, peripheral edema, jugular venous distention), or thromboembolic events to the brain, lower extremities, and lungs.13 Chronic chagasic megaesophagus may lead to weight loss, esophageal dysmotility, pneumonitis due to aspiration of food trapped in the esophagus and stomach, salivary gland enlargement, and erosive esophagitis, which increases the risk for esophageal cancer. Chronic chagasic megacolon can present as an intestinal obstruction, volvulus, abdominal distention, or fecaloma.13
Clinicians should be alert to the possibility of congenital T cruzi infection in children born to women who emigrated from an endemic area or who visited an area with a high prevalence of Chagas disease. Most newborns with T cruzi infection are asymptomatic, but in some cases a thorough neonatal exam can lead to the diagnosis. Manifestations of symptomatic congenital infection include hepatosplenomegaly, low birth weight, premature birth, and low Apgar scores.5 Lab tests may reveal thrombocytopenia and anemia. Neonates with severe disease may also have respiratory distress, meningoencephalitis, and gastrointestinal problems.5
LABORATORY WORK-UP
Laboratory work-up for Chagas disease depends on the provider’s awareness of the disease and its symptoms. All patients should undergo routine blood work, including complete blood count (CBC) with differential, comprehensive metabolic panel (CMP), and liver function tests to rule out other etiologies that manifest with similar symptoms. If the patient presents during the acute phase, microscopy of blood smears with Giemsa stain should be done to visualize the parasites. In the patient who presents during the chronic phase with cardiac symptoms, measurement of B-type natriuretic peptide, troponin, C-reactive protein, and the erythrocyte sedimentation rate can be used to rule out other differential diagnoses. Electrocardiogram (ECG) may show a right bundle-branch block or left anterior fascicular block.5 Echocardiogram may show left ventricular wall motion abnormalities and/or cardiomyopathy with congestive heart failure.5,10 A work-up for digestive Chagas disease may include a barium swallow, kidney-ureter-bladder x-ray, or MRI/CT of the abdomen.14
DIAGNOSING ACUTE, CHRONIC, AND CONGENITAL CHAGAS
Accurate diagnosis of Chagas disease requires a thorough history and physical exam, as well as a high index of suspicion. Recent travel to an endemic area of Chagas disease in combination with the typical signs and symptoms—such as fever, headache, lymphadenopathy, shortness of breath, myalgia, swelling, and abdominal or chest pain—should prompt the provider to perform more specific tests.4 Inquiry about past medical history, blood transfusions, and surgeries is also imperative to make the correct diagnosis.5
The approach to diagnosis of Chagas disease depends on whether the patient presents during the acute or chronic phase. During the acute phase, the count of the trypomastigote, the mature extracellular form of the parasite T cruzi, is at its highest, making this the best time to obtain an accurate diagnosis if an infection is suspected.3 Microscopy of fresh preparations of anticoagulated blood or buffy coat may show motile parasites.10 Other options include visualization of parasites in a blood smear with Giemsa stain or hemoculture. Hemoculture is a sensitive test but takes several weeks to show growth of the parasites. Therefore, polymerase chain reaction (PCR) assay is the preferred diagnostic test due to its high sensitivity and quick turnaround time.5
Because no diagnostic gold standard exists for chronic disease, confidently diagnosing Chagas in the United States can be difficult.5 Past the acute phase (about three months after infection), microscopy and PCR cannot be used due to low parasitemia. If an infection with T cruzi is suspected but nine to 14 weeks have passed since exposure, serology is the method of choice for diagnosis. The enzyme-linked immunosorbent assay (ELISA) and immunofluorescent-antibody assay (IFA) are most often used to identify immunoglobulin (Ig) G antibodies to the parasite.
The difficulty of diagnosing Chagas disease in the chronic phase lies in the fact that neither ELISA or IFA alone is sensitive or specific enough to confirm the diagnosis.5 In order to make a serologic diagnosis of infection, positive results are needed from two serologic tests based on two different antigens or by using two different techniques (eg, ELISA or IFA). If the two tests are discordant, a third test must be done to determine the patient’s infection status. The radioimmunoprecipitation assay (RIPA) and trypomastigote excreted-secreted antigen immunoblot (TESA-blot) have been traditionally used as confirmatory tests, but even they do not have high sensitivity and specificity. A case of indeterminate Chagas disease is confirmed with positive serologic testing in a patient without symptoms and with normal ECG, chest x-ray, and imaging of the colon and esophagus.15
The preferred protocol for diagnosis of congenital Chagas disease first requires positive serologic testing confirming the infection in the mother (see Figure 2).16 Once that is determined, microscopic and PCR-based examinations of cord blood and peripheral blood specimens are carried out during the first one to two months of the infant’s life.10 PCR is the preferred test for early congenital Chagas disease, recipients of organ transplants, and after accidental exposure since results can determine if the patient is infected earlier than trypomastigotes (developmental stage of trypanosomes) can be seen on a peripheral blood smear.5

TREATMENT CONSIDERATIONS
If there is a suspicion of Chagas disease, the patient should be referred to an infectious disease specialist for diagnosis and treatment. Nifurtimox and benznidazole are the only drugs that have been shown to improve the course of Chagas disease.5 However, neither drug is approved by the FDA, and both can only be obtained from the CDC, which makes treatment a challenge.9 In addition, up to 30% of patients terminate treatment due to the many adverse effects of these drugs.17
The dosage regimen for nifurtimox is 8-10 mg/kg/d divided into three doses for 90 days.10 Anorexia, weight loss, nausea, vomiting, and abdominal pain occur in up to 70% of patients.5 Irritability, insomnia, disorientation, and tremors can also occur. Neurotoxicity leading to peripheral neuropathy is dose dependent and requires treatment termination.5
Benznidazole is better tolerated and is active against the trypomastigotes as well as the amastigotes or intracellular form of the parasite.10 The dosage regimen for benznidazole is 5-7 mg/kg/d divided into two doses for 60 days.10 Dermatologic reactions such as rash, photosensitivity, and exfoliative dermatitis are the most common adverse effects. Peripheral neuropathy and bone marrow suppression are dose dependent and require therapy cessation.5
The CDC recommends treatment for all cases of acute disease (including congenital disease) regardless of age, and for chronic disease in patients up to age 50 who have not progressed to cardiomyopathy. In patients older than 50, treatment should be determined after weighing the potential risks and benefits (see Table 2).18

The success of treatment is determined in part by the phase of the disease. Cure rates in patients treated with either nifurtimox or benznidazole during the acute phase range from 65% to 80%.17 Chronic disease shows less of a response to traditional antiparasitic drug regimens, but higher rates of success are seen in younger patients.5 According to current estimates, successful treatment of chronic disease is limited to 15% to 30% patients.17 Treatment of congenital Chagas disease should begin as soon as the diagnosis is confirmed, and cure rates are greater than 90% if patients are treated within the first year of life.10 Treating congenital Chagas disease is important because the infection can be passed to future generations even if the disease never manifests with symptoms.19 However, if an expecting mother has known Chagas disease, antiparasitic medications are not recommended during the pregnancy because of a lack of fetal safety data for the two antiparasitic agents.20 Instead, it is recommended that women of childbearing age be treated before pregnancy, as rates of congenital infection are 25 times lower in women who are treated than in those who are not.21
PRE- AND POSTEXPOSURE PATIENT EDUCATION
Patient education mainly focuses on how to prevent Chagas disease and prognosis once diagnosed. During travel to endemic areas, the use of insecticides and residing in well-built households are the most important prevention measures. No vaccine is available, and primary chemoprophylaxis of persons visiting endemic areas is not recommended due to the low risk for infection and concerns about adverse effects.13
The survival rate of those who remain in the indeterminate phase is the same as that of the general population. However, findings that most strongly predict mortality include ventricular tachycardia, cardiomegaly, congestive heart failure (NYHA class III/IV), left ventricular systolic dysfunction, and male sex.10 Patients diagnosed with Chagas disease should be strongly encouraged not to donate blood or organs.10 Some organ and blood donation organizations selectively or universally screen donated specimens; however, this screening is not required by law.5 Family members of those diagnosed with the disease should also be tested, especially if the patient is a woman who has children or who plans to become pregnant.10
FOLLOW-UP
In patients confirmed to have Chagas disease but without symptoms and a normal ECG, further initial evaluation is not required.10 An annual history, physical exam, and ECG should be done. Those who have symptoms or ECG changes should have a complete cardiac work-up, including a 24-hour ambulatory ECG, exercise stress test, and echocardiogram to determine functional capacity. A barium swallow, barium enema, esophageal manometry, and endoscopy may be indicated in patients with gastrointestinal symptoms of Chagas disease but otherwise are not recommended. Patients taking antiparasitic drugs should have a CBC and CMP at the start of treatment and then bimonthly until the end of treatment to monitor for rare bone marrow suppression. Nifurtimox and benznidazole are also known to be mutagenic and increase the risk for lymphoma in animal studies, but this risk has not been documented in humans.10
CONCLUSION
Chagas disease is considered one of the neglected tropical diseases due to its high prevalence, chronic course, debilitating symptoms, and association with poverty.7 It is evident that incidence and prevalence of Chagas disease in the US are increasing due to recent immigration and mother-to-child transmission. Therefore, family practice clinicians must be able to recognize the red flags that suggest a T cruzi infection.5,9 Enhanced awareness of Chagas disease among health care providers will lead to better symptom control and cure rates for affected patients and may also prevent congenital infections. These efforts could serve to remove Chagas disease from the list of neglected tropical diseases.
CE/CME No: CR-1611
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand the prevalence and risks of Chagas disease in the United States.
• Explain the pathophysiology of Chagas disease, including the vector and transmission routes of the disease.
• Describe the clinical presentation of both the acute and chronic forms of the disease and learn when to suspect an infection.
• Outline a plan for diagnosis and treatment of Chagas disease.
• Educate women with Chagas disease about the risk of transmission for future offspring.
FACULTY
Jessica McDonald works in the Emergency Medicine Department at Dekalb Medical Center, Atlanta. Jill Mattingly is Academic Coordinator and Clinical Assistant Professor in the Physician Assistant Program at Mercer University, Atlanta.
The authors have no financial relationships to disclose.
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of November 2016.
Article begins on next page >>
Chagas disease, a parasitic infection, is increasingly being detected in the United States, most likely due to immigration from endemic countries in South and Central America. Approximately 300,000 persons in the US have chronic Chagas disease, and up to 30% of them will develop clinically evident cardiovascular and/or gastrointestinal disease. Here’s practical guidance to help you recognize the features of symptomatic Chagas disease and follow up with appropriate evaluation and management.
Chagas disease, also known as American trypanosomiasis, is caused by the protozoan parasite Trypanosoma cruzi.1 It is most commonly spread by triatomine bugs infected with T cruzi and is endemic in many parts of Mexico and Central and South America.2 Chagas disease was first described in 1909 by Brazilian physician Carlos Chagas.3 Since its discovery, it has often been considered a disease affecting only the poor living in endemic areas of Latin America. However, 6 million to 7 million people are infected with T cruzi worldwide, and estimates suggest that Mexico and the US rank third and seventh, respectively, in the number of persons with T cruzi infection in the Western Hemisphere.1,4
An estimated 300,000 persons in the US have Chagas disease; most of them are not aware that they are infected.5,6 The increasing presence of the disease in the US, which traditionally has been considered a nonendemic area, is due to immigration from endemic areas, with subsequent infections occurring through mechanisms that do not require contact with the triatomine vector (eg, congenital transmission).1 Between 1981 and 2005, more than 7 million people from T cruzi-endemic countries in Latin America moved to the US and became legal residents.3
Early detection and treatment of Chagas disease is important because up to 30% of patients with chronic infection will develop a heart disorder, which can range in severity from conduction system abnormalities to dilated cardiomyopathy.4 In some areas of southern Mexico, Chagas disease is the most common cause of dilated cardiomyopathy.1 Equally concerning is the fact that untreated mothers with Chagas disease can transmit T cruzi to their infants.1,3 An estimated 315 babies are born with congenital Chagas disease each year in the US, an incidence equivalent to that of phenylketonuria.7 It is estimated that congenital transmission is responsible for up to one-quarter of new infections worldwide.1 Unfortunately, obstetricians are not well informed about the risk factors for congenital Chagas disease, and very limited screening of at-risk women is performed. In a 2008 survey exploring health care providers’ knowledge of and understanding about Chagas disease, obstetricians and gynecologists had the greatest knowledge deficits about the disease, although considerable deficits were also seen among other specialties.1
KISSING BUG DISEASE: ETIOLOGY/PATHOPHYSIOLOGY
Exposure to the protozoan parasite T cruzi, the cause of Chagas disease, typically occurs following the bite of a triatomine bug. Also known as “kissing bugs” because they usually bite exposed areas of the skin such as the face, triatomine bugs feed on human blood, typically at night, and act as a vector for the parasite.8 The parasite lives in the feces and urine of the triatomine bugs and is excreted near the bite during or shortly after a blood meal. The bitten person will then unknowingly smear the infected feces into the bite wound, eyes, mouth, or any opening in the skin, which gives the parasites systemic access.4 Once in the host’s bloodstream, the parasite replicates in host cells, a process that ends in cell lysis and hematogenous spread. At this point, the parasites can be seen on peripheral blood smear. Noninfected triatomine insects become infected and continue the cycle when they feed on an infected human host (see Figure 1).3 Persons of lower socioeconomic status living in endemic areas in Latin America are at a higher risk for contracting Chagas disease because “kissing bugs” commonly live in wall or roof cracks of poorly built homes. Populations living in poverty are also at risk due to minimal access to health care and prenatal care.4 Transmission of T cruzi not involving triatomine vectors occurs congenitally or through blood transfusions, consumption of contaminated food, and organ donations.4

NATURAL HISTORY OF INFECTION AND PATIENT PRESENTATION
Acute phase
Infection with the T cruzi parasite is followed by an asymptomatic incubation period of one to two weeks, which is then followed by an acute phase that can last eight to 12 weeks.5 The acute phase is characterized by a large amount of parasites in the bloodstream (see Table 1). The patient is often asymptomatic but can have nonspecific symptoms such as fever, headache, lymphadenopathy, shortness of breath, myalgia, swelling, and abdominal or chest pain.4 Because symptoms during the acute phase are typically mild, many patients do not seek medical attention until they transition into the chronic phase.4 Infants are more likely to experience severe symptoms, including myocarditis or meningoencephalitis, and thus are more likely to present during the acute phase.9

If the patient acquired the infection through an organ transplant, the acute phase symptoms can be delayed, on average, up to 112 days.5 These patients will have more noticeable symptoms, including hepatosplenomegaly, myocarditis, and congestive heart failure. Due to the known risk for transmission through organ transplants, donors are often screened for Chagas disease. Unfortunately, this screening is selective and often inconsistent.5 Therefore, the presence of the previously mentioned symptoms in a person who recently received an organ transplant should raise suspicion of Chagas disease.5
Chronic phase
Patients not treated during the acute phase will pass into the chronic phase of Chagas disease.5 This may occur due to reactivation of T cruzi infection via immunosuppression.9 At this time, the previously asymptomatic patient will have typical signs and symptoms of chronic disease, along with nodules, panniculitis, and myocarditis.4,9,10 During the chronic phase, parasites are undetectable by microscopy, but the patient can still spread the disease to the vector as well as to others congenitally and through organ donation and blood transfusions.5,9
Patients with chronic T cruzi infection who remain without signs or symptoms of infection are considered to have the indeterminate form of chronic disease. Many patients will remain in the indeterminate form throughout their lives, but between 20% and 30% will progress to the determinate form of chronic disease over years to decades.3 The determinate form is characterized by clinically evident disease and is classically divided into cardiac Chagas disease and digestive Chagas disease.5 Symptoms of the chronic phase depend on the genotype of T cruzi that caused the infection. The AG genotype has a higher incidence of digestive disease.11
Cardiac Chagas disease is believed to occur due to parasite invasion and persistence in cardiac tissue, leading to immune-mediated myocardial injury.5 Chagas cardiomyopathy is characterized by chronic myocarditis affecting all cardiac chambers and disturbances in the electrical conduction system; patients also often develop apical aneurysms. Longstanding cardiac Chagas disease can lead to more serious complications, such as episodes of ventricular tachycardia, heart block, thromboembolic phenomena, severe bradycardia, dilated cardiomyopathy, and congestive heart failure. Patients may complain of presyncope, syncope, and episodes of palpitations. They are also at high risk for sudden cardiac death.5 Patients with cardiomyopathy or cardiac insufficiency secondary to Chagas disease have a worse prognosis than those with idiopathic cardiomyopathy or decompensated heart failure due to other etiologies.12
Less common than cardiac Chagas disease, digestive Chagas disease occurs mostly in Argentina, Bolivia, Chile, Paraguay, Uruguay, and parts of Peru and Brazil; it is rarely seen in northern South America, Central America, or Mexico.5 The parasite causes gastrointestinal symptoms by damaging intramural neurons, resulting in denervation of hollow viscera. Since it affects the esophagus and colon, patients may present with dysphagia, odynophagia, cough, reflux, weight loss, constipation, and abdominal pain.5
PHYSICAL EXAMINATION: A CRUCIAL STEP
The physical examination of a patient with suspected Chagas disease can be crucial to the diagnosis. As noted, there are often few specific symptoms or physical exam findings during the acute phase. However, in some patients, swelling and inflammation may be evident at the site of inoculation, often on the face or extremities. This finding is called a chagoma. The Romaña sign, characterized by painless unilateral swelling of the upper and lower eyelid, can also be seen if the infection occurred through the conjunctiva.5 A nonpruritic morbilliform rash, called schizotrypanides, may be a presenting symptom in patients with acute disease.13 Children younger than 2 years of age are at increased risk for a severe acute infection, with signs and symptoms of pericardial effusion, myocarditis, and meningoencephalitis. Children can also develop generalized edema and lymphadenopathy. Those children who develop severe manifestations during acute infection have an increased risk for mortality.5
Chronic chagasic cardiomyopathy may present with signs of left-sided heart failure (pulmonary edema, dyspnea at rest or exertion), biventricular heart failure (hepatomegaly, peripheral edema, jugular venous distention), or thromboembolic events to the brain, lower extremities, and lungs.13 Chronic chagasic megaesophagus may lead to weight loss, esophageal dysmotility, pneumonitis due to aspiration of food trapped in the esophagus and stomach, salivary gland enlargement, and erosive esophagitis, which increases the risk for esophageal cancer. Chronic chagasic megacolon can present as an intestinal obstruction, volvulus, abdominal distention, or fecaloma.13
Clinicians should be alert to the possibility of congenital T cruzi infection in children born to women who emigrated from an endemic area or who visited an area with a high prevalence of Chagas disease. Most newborns with T cruzi infection are asymptomatic, but in some cases a thorough neonatal exam can lead to the diagnosis. Manifestations of symptomatic congenital infection include hepatosplenomegaly, low birth weight, premature birth, and low Apgar scores.5 Lab tests may reveal thrombocytopenia and anemia. Neonates with severe disease may also have respiratory distress, meningoencephalitis, and gastrointestinal problems.5
LABORATORY WORK-UP
Laboratory work-up for Chagas disease depends on the provider’s awareness of the disease and its symptoms. All patients should undergo routine blood work, including complete blood count (CBC) with differential, comprehensive metabolic panel (CMP), and liver function tests to rule out other etiologies that manifest with similar symptoms. If the patient presents during the acute phase, microscopy of blood smears with Giemsa stain should be done to visualize the parasites. In the patient who presents during the chronic phase with cardiac symptoms, measurement of B-type natriuretic peptide, troponin, C-reactive protein, and the erythrocyte sedimentation rate can be used to rule out other differential diagnoses. Electrocardiogram (ECG) may show a right bundle-branch block or left anterior fascicular block.5 Echocardiogram may show left ventricular wall motion abnormalities and/or cardiomyopathy with congestive heart failure.5,10 A work-up for digestive Chagas disease may include a barium swallow, kidney-ureter-bladder x-ray, or MRI/CT of the abdomen.14
DIAGNOSING ACUTE, CHRONIC, AND CONGENITAL CHAGAS
Accurate diagnosis of Chagas disease requires a thorough history and physical exam, as well as a high index of suspicion. Recent travel to an endemic area of Chagas disease in combination with the typical signs and symptoms—such as fever, headache, lymphadenopathy, shortness of breath, myalgia, swelling, and abdominal or chest pain—should prompt the provider to perform more specific tests.4 Inquiry about past medical history, blood transfusions, and surgeries is also imperative to make the correct diagnosis.5
The approach to diagnosis of Chagas disease depends on whether the patient presents during the acute or chronic phase. During the acute phase, the count of the trypomastigote, the mature extracellular form of the parasite T cruzi, is at its highest, making this the best time to obtain an accurate diagnosis if an infection is suspected.3 Microscopy of fresh preparations of anticoagulated blood or buffy coat may show motile parasites.10 Other options include visualization of parasites in a blood smear with Giemsa stain or hemoculture. Hemoculture is a sensitive test but takes several weeks to show growth of the parasites. Therefore, polymerase chain reaction (PCR) assay is the preferred diagnostic test due to its high sensitivity and quick turnaround time.5
Because no diagnostic gold standard exists for chronic disease, confidently diagnosing Chagas in the United States can be difficult.5 Past the acute phase (about three months after infection), microscopy and PCR cannot be used due to low parasitemia. If an infection with T cruzi is suspected but nine to 14 weeks have passed since exposure, serology is the method of choice for diagnosis. The enzyme-linked immunosorbent assay (ELISA) and immunofluorescent-antibody assay (IFA) are most often used to identify immunoglobulin (Ig) G antibodies to the parasite.
The difficulty of diagnosing Chagas disease in the chronic phase lies in the fact that neither ELISA or IFA alone is sensitive or specific enough to confirm the diagnosis.5 In order to make a serologic diagnosis of infection, positive results are needed from two serologic tests based on two different antigens or by using two different techniques (eg, ELISA or IFA). If the two tests are discordant, a third test must be done to determine the patient’s infection status. The radioimmunoprecipitation assay (RIPA) and trypomastigote excreted-secreted antigen immunoblot (TESA-blot) have been traditionally used as confirmatory tests, but even they do not have high sensitivity and specificity. A case of indeterminate Chagas disease is confirmed with positive serologic testing in a patient without symptoms and with normal ECG, chest x-ray, and imaging of the colon and esophagus.15
The preferred protocol for diagnosis of congenital Chagas disease first requires positive serologic testing confirming the infection in the mother (see Figure 2).16 Once that is determined, microscopic and PCR-based examinations of cord blood and peripheral blood specimens are carried out during the first one to two months of the infant’s life.10 PCR is the preferred test for early congenital Chagas disease, recipients of organ transplants, and after accidental exposure since results can determine if the patient is infected earlier than trypomastigotes (developmental stage of trypanosomes) can be seen on a peripheral blood smear.5

TREATMENT CONSIDERATIONS
If there is a suspicion of Chagas disease, the patient should be referred to an infectious disease specialist for diagnosis and treatment. Nifurtimox and benznidazole are the only drugs that have been shown to improve the course of Chagas disease.5 However, neither drug is approved by the FDA, and both can only be obtained from the CDC, which makes treatment a challenge.9 In addition, up to 30% of patients terminate treatment due to the many adverse effects of these drugs.17
The dosage regimen for nifurtimox is 8-10 mg/kg/d divided into three doses for 90 days.10 Anorexia, weight loss, nausea, vomiting, and abdominal pain occur in up to 70% of patients.5 Irritability, insomnia, disorientation, and tremors can also occur. Neurotoxicity leading to peripheral neuropathy is dose dependent and requires treatment termination.5
Benznidazole is better tolerated and is active against the trypomastigotes as well as the amastigotes or intracellular form of the parasite.10 The dosage regimen for benznidazole is 5-7 mg/kg/d divided into two doses for 60 days.10 Dermatologic reactions such as rash, photosensitivity, and exfoliative dermatitis are the most common adverse effects. Peripheral neuropathy and bone marrow suppression are dose dependent and require therapy cessation.5
The CDC recommends treatment for all cases of acute disease (including congenital disease) regardless of age, and for chronic disease in patients up to age 50 who have not progressed to cardiomyopathy. In patients older than 50, treatment should be determined after weighing the potential risks and benefits (see Table 2).18

The success of treatment is determined in part by the phase of the disease. Cure rates in patients treated with either nifurtimox or benznidazole during the acute phase range from 65% to 80%.17 Chronic disease shows less of a response to traditional antiparasitic drug regimens, but higher rates of success are seen in younger patients.5 According to current estimates, successful treatment of chronic disease is limited to 15% to 30% patients.17 Treatment of congenital Chagas disease should begin as soon as the diagnosis is confirmed, and cure rates are greater than 90% if patients are treated within the first year of life.10 Treating congenital Chagas disease is important because the infection can be passed to future generations even if the disease never manifests with symptoms.19 However, if an expecting mother has known Chagas disease, antiparasitic medications are not recommended during the pregnancy because of a lack of fetal safety data for the two antiparasitic agents.20 Instead, it is recommended that women of childbearing age be treated before pregnancy, as rates of congenital infection are 25 times lower in women who are treated than in those who are not.21
PRE- AND POSTEXPOSURE PATIENT EDUCATION
Patient education mainly focuses on how to prevent Chagas disease and prognosis once diagnosed. During travel to endemic areas, the use of insecticides and residing in well-built households are the most important prevention measures. No vaccine is available, and primary chemoprophylaxis of persons visiting endemic areas is not recommended due to the low risk for infection and concerns about adverse effects.13
The survival rate of those who remain in the indeterminate phase is the same as that of the general population. However, findings that most strongly predict mortality include ventricular tachycardia, cardiomegaly, congestive heart failure (NYHA class III/IV), left ventricular systolic dysfunction, and male sex.10 Patients diagnosed with Chagas disease should be strongly encouraged not to donate blood or organs.10 Some organ and blood donation organizations selectively or universally screen donated specimens; however, this screening is not required by law.5 Family members of those diagnosed with the disease should also be tested, especially if the patient is a woman who has children or who plans to become pregnant.10
FOLLOW-UP
In patients confirmed to have Chagas disease but without symptoms and a normal ECG, further initial evaluation is not required.10 An annual history, physical exam, and ECG should be done. Those who have symptoms or ECG changes should have a complete cardiac work-up, including a 24-hour ambulatory ECG, exercise stress test, and echocardiogram to determine functional capacity. A barium swallow, barium enema, esophageal manometry, and endoscopy may be indicated in patients with gastrointestinal symptoms of Chagas disease but otherwise are not recommended. Patients taking antiparasitic drugs should have a CBC and CMP at the start of treatment and then bimonthly until the end of treatment to monitor for rare bone marrow suppression. Nifurtimox and benznidazole are also known to be mutagenic and increase the risk for lymphoma in animal studies, but this risk has not been documented in humans.10
CONCLUSION
Chagas disease is considered one of the neglected tropical diseases due to its high prevalence, chronic course, debilitating symptoms, and association with poverty.7 It is evident that incidence and prevalence of Chagas disease in the US are increasing due to recent immigration and mother-to-child transmission. Therefore, family practice clinicians must be able to recognize the red flags that suggest a T cruzi infection.5,9 Enhanced awareness of Chagas disease among health care providers will lead to better symptom control and cure rates for affected patients and may also prevent congenital infections. These efforts could serve to remove Chagas disease from the list of neglected tropical diseases.
1. Hotez PJ, Dumonteil E, Betancourt Cravioto M, et al. An unfolding tragedy of Chagas disease in North America. PLoS Negl Trop Dis. 2013; 7(10):e2300.
2. Verani JR, Seitz A, Gilman RH, et al. Geographic variation in the sensitivity of recombinant antigen-based rapid tests for chronic Trypanosoma cruzi infection. Am J Trop Med Hyg. 2009;80(3):410-415.
3. Malik LH, Singh GD, Amsterdam EA. The epidemiology, clinical manifestations, and management of Chagas heart disease. Clin Cardiol. 2015;38(9):565-569.
4. World Health Organization. Chagas disease (American trypanosomiasis). Fact sheet. Updated March 2016. www.who.int/mediacentre/factsheets/fs340/en/. Accessed October 20, 2016.
5. Bern C, Kjos S, Yabsley MJ, Montgomery SP. Trypanosoma cruzi and Chagas’ disease in the United States. Clin Microbiol Rev. 2011; 24(4):655-681.
6. Stimpert KK, Montgomery SP. Physician awareness of Chagas disease, USA. Emerg Infect Dis. 2010;16(5):871-872.
7. Hotez PJ. Neglected parasitic infections and poverty in the United States. PLoS Negl Trop Dis. 2014;8(9):e3012.
8. Goupil LS, McKerrow JH. Introduction: drug discovery and development for neglected diseases. Chem Rev. 2014;114(22):11131-11137.
9. Montgomery SP, Starr MC, Cantey P, et al. Neglected parasitic infections in the United States: Chagas disease. Am J Trop Med Hyg. 2014; 90(5):814-818.
10. Bern C, Montgomery SP, Herwaldt BL, et al. Evaluation and treatment of Chagas disease in the United States. JAMA. 2007;298(18):2171-2181.
11. de Oliveira AP, Bernardo CR, Camargo AV, et al. Genetic susceptibility to cardiac and digestive clinical forms of chronic Chagas disease: involvement of the CCR5 59029 A/G polymorphism. PLoS One. 2015; 10(11):e0141847.
12. Apt W, Arribada A, Zulantay I, et al. Trypanosoma cruzi burden, genotypes, and clinical evaluation of Chilean patients with chronic Chagas cardiopathy. Parasitol Res. 2015;114(8):3007-3018.
13. Kirchhoff LV. Chagas disease (American trypanosomiasis): Background, pathophysiology, epidemiology. Emedicine.medscape.com. 2015. http://emedicine.medscape.com/article/214581-overview. Accessed October 20, 2016.
14. Knipe H, St-Amant M. Chagas disease. Radiopaedia.org. 2015. http://radiopaedia.org/articles/chagas-disease. Accessed October 20, 2016.
15. Rassi A Jr, Rassi A, Rassi SG. Predictors of mortality in chronic Chagas disease: a systematic review of observational studies. Circulation. 2007;115(9):1101-1108.
16. Gomes YM, Lorena VM, Luquetti AO. Diagnosis of Chagas disease: what has been achieved? What remains to be done with regard to diagnosis and follow up studies? Mem Inst Oswaldo Cruz. 2009; 104(suppl 1):115-121.
17. Molina I, Gómez i Prat J, Salvador F, et al. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N Engl J Med. 2014;370:1899-1908.
18. CDC. Parasites – American trypanosomiasis (also known as Chagas Disease). Antiparasitic Treatment. Resources For Health Professionals. www.cdc.gov/parasites/chagas/health_professionals/tx.html. Accessed October 20, 2016.
19. Carlier Y, Truyens C. Congenital Chagas disease as an ecological model of interactions between Trypanosoma cruzi parasites, pregnant women, placenta and fetuses. Acta Trop. 2015;151:103-115.
20. Moscatelli G, Moroni S, García-Bournissen F, et al. Prevention of congenital Chagas through treatment of girls and women of childbearing age. Mem Inst Oswaldo Cruz. 2015;110(4):507-509.
21. Fabbro D, Danesi E, Olivera V, et al. Trypanocide treatment of women infected with Trypanosoma cruzi and its effect on preventing congenital Chagas. PLoS Negl Trop Dis. 2014;8(11):e3312.
1. Hotez PJ, Dumonteil E, Betancourt Cravioto M, et al. An unfolding tragedy of Chagas disease in North America. PLoS Negl Trop Dis. 2013; 7(10):e2300.
2. Verani JR, Seitz A, Gilman RH, et al. Geographic variation in the sensitivity of recombinant antigen-based rapid tests for chronic Trypanosoma cruzi infection. Am J Trop Med Hyg. 2009;80(3):410-415.
3. Malik LH, Singh GD, Amsterdam EA. The epidemiology, clinical manifestations, and management of Chagas heart disease. Clin Cardiol. 2015;38(9):565-569.
4. World Health Organization. Chagas disease (American trypanosomiasis). Fact sheet. Updated March 2016. www.who.int/mediacentre/factsheets/fs340/en/. Accessed October 20, 2016.
5. Bern C, Kjos S, Yabsley MJ, Montgomery SP. Trypanosoma cruzi and Chagas’ disease in the United States. Clin Microbiol Rev. 2011; 24(4):655-681.
6. Stimpert KK, Montgomery SP. Physician awareness of Chagas disease, USA. Emerg Infect Dis. 2010;16(5):871-872.
7. Hotez PJ. Neglected parasitic infections and poverty in the United States. PLoS Negl Trop Dis. 2014;8(9):e3012.
8. Goupil LS, McKerrow JH. Introduction: drug discovery and development for neglected diseases. Chem Rev. 2014;114(22):11131-11137.
9. Montgomery SP, Starr MC, Cantey P, et al. Neglected parasitic infections in the United States: Chagas disease. Am J Trop Med Hyg. 2014; 90(5):814-818.
10. Bern C, Montgomery SP, Herwaldt BL, et al. Evaluation and treatment of Chagas disease in the United States. JAMA. 2007;298(18):2171-2181.
11. de Oliveira AP, Bernardo CR, Camargo AV, et al. Genetic susceptibility to cardiac and digestive clinical forms of chronic Chagas disease: involvement of the CCR5 59029 A/G polymorphism. PLoS One. 2015; 10(11):e0141847.
12. Apt W, Arribada A, Zulantay I, et al. Trypanosoma cruzi burden, genotypes, and clinical evaluation of Chilean patients with chronic Chagas cardiopathy. Parasitol Res. 2015;114(8):3007-3018.
13. Kirchhoff LV. Chagas disease (American trypanosomiasis): Background, pathophysiology, epidemiology. Emedicine.medscape.com. 2015. http://emedicine.medscape.com/article/214581-overview. Accessed October 20, 2016.
14. Knipe H, St-Amant M. Chagas disease. Radiopaedia.org. 2015. http://radiopaedia.org/articles/chagas-disease. Accessed October 20, 2016.
15. Rassi A Jr, Rassi A, Rassi SG. Predictors of mortality in chronic Chagas disease: a systematic review of observational studies. Circulation. 2007;115(9):1101-1108.
16. Gomes YM, Lorena VM, Luquetti AO. Diagnosis of Chagas disease: what has been achieved? What remains to be done with regard to diagnosis and follow up studies? Mem Inst Oswaldo Cruz. 2009; 104(suppl 1):115-121.
17. Molina I, Gómez i Prat J, Salvador F, et al. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N Engl J Med. 2014;370:1899-1908.
18. CDC. Parasites – American trypanosomiasis (also known as Chagas Disease). Antiparasitic Treatment. Resources For Health Professionals. www.cdc.gov/parasites/chagas/health_professionals/tx.html. Accessed October 20, 2016.
19. Carlier Y, Truyens C. Congenital Chagas disease as an ecological model of interactions between Trypanosoma cruzi parasites, pregnant women, placenta and fetuses. Acta Trop. 2015;151:103-115.
20. Moscatelli G, Moroni S, García-Bournissen F, et al. Prevention of congenital Chagas through treatment of girls and women of childbearing age. Mem Inst Oswaldo Cruz. 2015;110(4):507-509.
21. Fabbro D, Danesi E, Olivera V, et al. Trypanocide treatment of women infected with Trypanosoma cruzi and its effect on preventing congenital Chagas. PLoS Negl Trop Dis. 2014;8(11):e3312.
Which treatments are safe and effective for chronic sinusitis?
EVIDENCE-BASED ANSWER:
For adults with chronic rhinosinusitis (CRS), intranasal steroid (INS) therapy is more likely than placebo to improve symptoms (50% vs 32%; strength of recommendation [SOR]: A, systematic reviews).
Nasal saline irrigation (SI) alleviates symptoms better than no therapy (SOR: A, systematic reviews), but it’s probably not as effective as INS treatment (SOR: B, randomized controlled trial [RCT] with wide confidence interval).
Long-term (12 weeks) macrolide therapy doesn’t alter patient-oriented quality-of-life measures (SOR: A, systematic reviews).
Endoscopic sinus surgery improves CRS symptoms—nasal obstruction, discharge, and facial pain—over baseline (SOR: A, systematic reviews). Surgery and medical therapy appear about equivalent in terms of symptom improvement and quality-of-life measures (SOR: B, systematic reviews of low-quality RCTs).
EVIDENCE SUMMARY
The TABLE1-4 shows the major results of the meta-analyses for the various medical therapy trials.
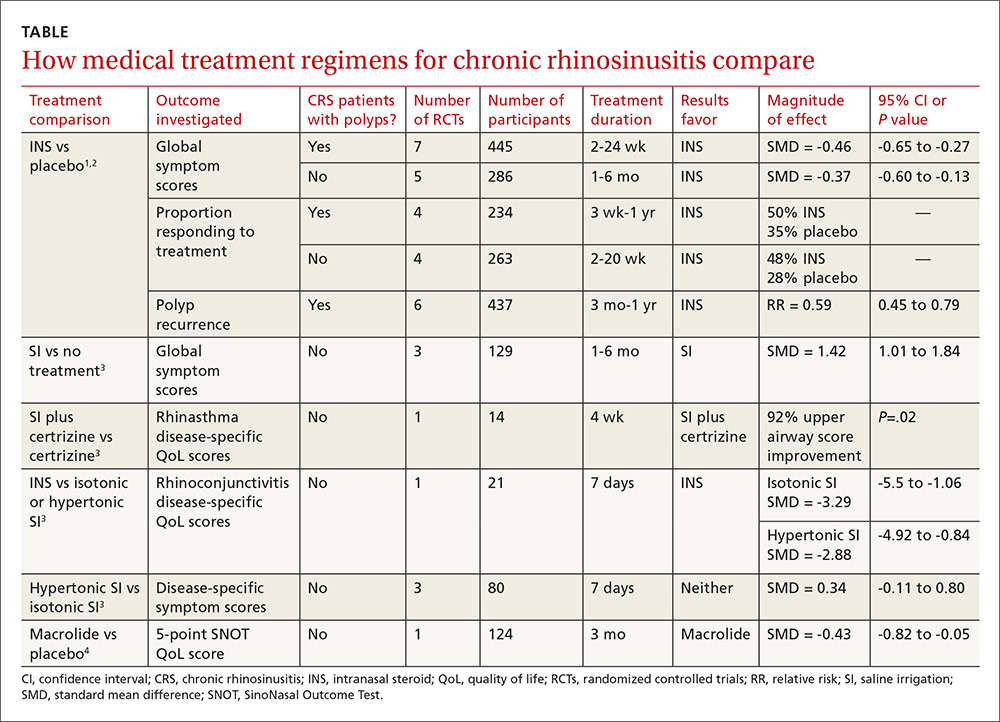
Two systematic reviews with meta-analyses evaluated treatment with INS for CRS with nasal polyps (40 RCTs; 3624 patients, mean age 48 years, 64% male) and without polyps (10 RCTs; 590 patients, mean age 39 years, 51% male).1,2 Trials reported sinonasal symptom outcomes differently and couldn’t be combined. In addition to reducing rate of polyp occurrence, for both CRS with and without polyps, key findings were:
- Global symptom scores were better for INS than placebo.
- Proportion of patients responding was greater for INS than with placebo.
There was no significant difference between adverse event rates with INS and placebo.
A systematic review and meta-analysis (8 RCTs, 389 patients) compared different SI regimens for CRS.3 The standardized mean difference was used to combine trials using various symptom outcomes. Key findings included the following:
- SI was better than no treatment.
- SI adjunctive therapy (with an antihistamine) improved disease-specific quality-of-life scores.
- SI was less effective than INS therapy for symptom improvement.
Hypertonic and isotonic saline yielded similar symptom scores. No adverse effects were reported.
One meta-analysis evaluated patient-reported outcomes with 12 weeks of macrolide therapy compared to placebo using the results of the SinoNasal Outcome Test (SNOT). The SNOT is a quality-of-life questionnaire that lists symptoms and the social-emotional consequences of CRS; a negative change in the SNOT score, on a 0 to 5 scale, indicates improvement. Overall the SNOT score improved 8% with macrolide therapy—statistically significant, but of uncertain clinical importance.4
Surgery improves nasal obstruction, pain, and postnasal discharge
A systematic review of 21 studies (prospective RCTs, prospective controlled clinical trials, cohort studies, case series, and retrospective record reviews) with a total of 2070 patients analyzed the effectiveness of endoscopic sinus surgery alone for improving CRS symptoms.5 Mean duration of post-operative follow-up was 14 months. Meta-analysis was performed separately for each symptom and the standard mean difference of the symptom severity score before and after surgery was reported as the effect size (ES) for the outcome measure (an ES of 0.2 is considered small; 0.6, moderate; 1.2, large; and 2, very large).
All symptoms improved compared to their preoperative severity scores. Nasal obstruction improved the most (ES=1.73; 95% CI, 1.45-2.02). Large symptom improvement was also observed for facial pain (ES=1.13; 95% CI, 0.96-1.31) and postnasal discharge (ES=1.19; 95% CI, 0.96-1.43).
Surgery and medical therapy may provide comparable symptom relief
A recent Cochrane review of 4 low-quality RCTs including 378 patients compared surgical with medical interventions for CRS with nasal polyps. Study heterogeneity and selective outcome reporting prevented meta-analysis.
The 3 comparison groups were endoscopic sinus surgery vs systemic steroids + INS; polypectomy vs systemic steroid + INS; and endoscopic surgery + INS vs antibiotic + “high-dose” INS. Overall, neither surgery nor medical therapy was superior in terms of patient-reported symptom scores or quality-of-life measures.6
1. Kalish L, Snidvongs K, Sivasubramaniam R, et al. Topical steroids for nasal polyps. Cochrane Database Syst Rev. 2012;(12):CD006549.
2. Snidvongs K, Kalish L, Sacks R, et al. Topical steroids for chronic rhinosinusitis without polyps. Cochrane Database Syst Rev. 2011;(8):CD009274.
3. Harvey R, Hannan SA, Badia L, et al. Nasal saline irrigation for the symptoms of chronic rhinosinusitis. Cochrane Database Syst Rev. 2007;(3):CD006394.
4. Pynnonen MA, Venkatraman G, Davis GE. Macrolide therapy for chronic rhinosinusitis: a meta-analysis. Otolaryngol Head Neck Surg. 2013;148:366-373.
5. Chester AC, Antisdel JL, Sindwani R. Symptom-specific outcomes of endoscopic sinus surgery: a systematic review. Otolaryngol Head Neck Surg. 2009;140:633-639.
6. Rimmer J, Fokkens W, Chong LY, et al. Surgical versus medical interventions for chronic rhinosinusitis with nasal polyps. Cochrane Database Syst Rev. 2014;(12):CD0069991.
EVIDENCE-BASED ANSWER:
For adults with chronic rhinosinusitis (CRS), intranasal steroid (INS) therapy is more likely than placebo to improve symptoms (50% vs 32%; strength of recommendation [SOR]: A, systematic reviews).
Nasal saline irrigation (SI) alleviates symptoms better than no therapy (SOR: A, systematic reviews), but it’s probably not as effective as INS treatment (SOR: B, randomized controlled trial [RCT] with wide confidence interval).
Long-term (12 weeks) macrolide therapy doesn’t alter patient-oriented quality-of-life measures (SOR: A, systematic reviews).
Endoscopic sinus surgery improves CRS symptoms—nasal obstruction, discharge, and facial pain—over baseline (SOR: A, systematic reviews). Surgery and medical therapy appear about equivalent in terms of symptom improvement and quality-of-life measures (SOR: B, systematic reviews of low-quality RCTs).
EVIDENCE SUMMARY
The TABLE1-4 shows the major results of the meta-analyses for the various medical therapy trials.

Two systematic reviews with meta-analyses evaluated treatment with INS for CRS with nasal polyps (40 RCTs; 3624 patients, mean age 48 years, 64% male) and without polyps (10 RCTs; 590 patients, mean age 39 years, 51% male).1,2 Trials reported sinonasal symptom outcomes differently and couldn’t be combined. In addition to reducing rate of polyp occurrence, for both CRS with and without polyps, key findings were:
- Global symptom scores were better for INS than placebo.
- Proportion of patients responding was greater for INS than with placebo.
There was no significant difference between adverse event rates with INS and placebo.
A systematic review and meta-analysis (8 RCTs, 389 patients) compared different SI regimens for CRS.3 The standardized mean difference was used to combine trials using various symptom outcomes. Key findings included the following:
- SI was better than no treatment.
- SI adjunctive therapy (with an antihistamine) improved disease-specific quality-of-life scores.
- SI was less effective than INS therapy for symptom improvement.
Hypertonic and isotonic saline yielded similar symptom scores. No adverse effects were reported.
One meta-analysis evaluated patient-reported outcomes with 12 weeks of macrolide therapy compared to placebo using the results of the SinoNasal Outcome Test (SNOT). The SNOT is a quality-of-life questionnaire that lists symptoms and the social-emotional consequences of CRS; a negative change in the SNOT score, on a 0 to 5 scale, indicates improvement. Overall the SNOT score improved 8% with macrolide therapy—statistically significant, but of uncertain clinical importance.4
Surgery improves nasal obstruction, pain, and postnasal discharge
A systematic review of 21 studies (prospective RCTs, prospective controlled clinical trials, cohort studies, case series, and retrospective record reviews) with a total of 2070 patients analyzed the effectiveness of endoscopic sinus surgery alone for improving CRS symptoms.5 Mean duration of post-operative follow-up was 14 months. Meta-analysis was performed separately for each symptom and the standard mean difference of the symptom severity score before and after surgery was reported as the effect size (ES) for the outcome measure (an ES of 0.2 is considered small; 0.6, moderate; 1.2, large; and 2, very large).
All symptoms improved compared to their preoperative severity scores. Nasal obstruction improved the most (ES=1.73; 95% CI, 1.45-2.02). Large symptom improvement was also observed for facial pain (ES=1.13; 95% CI, 0.96-1.31) and postnasal discharge (ES=1.19; 95% CI, 0.96-1.43).
Surgery and medical therapy may provide comparable symptom relief
A recent Cochrane review of 4 low-quality RCTs including 378 patients compared surgical with medical interventions for CRS with nasal polyps. Study heterogeneity and selective outcome reporting prevented meta-analysis.
The 3 comparison groups were endoscopic sinus surgery vs systemic steroids + INS; polypectomy vs systemic steroid + INS; and endoscopic surgery + INS vs antibiotic + “high-dose” INS. Overall, neither surgery nor medical therapy was superior in terms of patient-reported symptom scores or quality-of-life measures.6
EVIDENCE-BASED ANSWER:
For adults with chronic rhinosinusitis (CRS), intranasal steroid (INS) therapy is more likely than placebo to improve symptoms (50% vs 32%; strength of recommendation [SOR]: A, systematic reviews).
Nasal saline irrigation (SI) alleviates symptoms better than no therapy (SOR: A, systematic reviews), but it’s probably not as effective as INS treatment (SOR: B, randomized controlled trial [RCT] with wide confidence interval).
Long-term (12 weeks) macrolide therapy doesn’t alter patient-oriented quality-of-life measures (SOR: A, systematic reviews).
Endoscopic sinus surgery improves CRS symptoms—nasal obstruction, discharge, and facial pain—over baseline (SOR: A, systematic reviews). Surgery and medical therapy appear about equivalent in terms of symptom improvement and quality-of-life measures (SOR: B, systematic reviews of low-quality RCTs).
EVIDENCE SUMMARY
The TABLE1-4 shows the major results of the meta-analyses for the various medical therapy trials.

Two systematic reviews with meta-analyses evaluated treatment with INS for CRS with nasal polyps (40 RCTs; 3624 patients, mean age 48 years, 64% male) and without polyps (10 RCTs; 590 patients, mean age 39 years, 51% male).1,2 Trials reported sinonasal symptom outcomes differently and couldn’t be combined. In addition to reducing rate of polyp occurrence, for both CRS with and without polyps, key findings were:
- Global symptom scores were better for INS than placebo.
- Proportion of patients responding was greater for INS than with placebo.
There was no significant difference between adverse event rates with INS and placebo.
A systematic review and meta-analysis (8 RCTs, 389 patients) compared different SI regimens for CRS.3 The standardized mean difference was used to combine trials using various symptom outcomes. Key findings included the following:
- SI was better than no treatment.
- SI adjunctive therapy (with an antihistamine) improved disease-specific quality-of-life scores.
- SI was less effective than INS therapy for symptom improvement.
Hypertonic and isotonic saline yielded similar symptom scores. No adverse effects were reported.
One meta-analysis evaluated patient-reported outcomes with 12 weeks of macrolide therapy compared to placebo using the results of the SinoNasal Outcome Test (SNOT). The SNOT is a quality-of-life questionnaire that lists symptoms and the social-emotional consequences of CRS; a negative change in the SNOT score, on a 0 to 5 scale, indicates improvement. Overall the SNOT score improved 8% with macrolide therapy—statistically significant, but of uncertain clinical importance.4
Surgery improves nasal obstruction, pain, and postnasal discharge
A systematic review of 21 studies (prospective RCTs, prospective controlled clinical trials, cohort studies, case series, and retrospective record reviews) with a total of 2070 patients analyzed the effectiveness of endoscopic sinus surgery alone for improving CRS symptoms.5 Mean duration of post-operative follow-up was 14 months. Meta-analysis was performed separately for each symptom and the standard mean difference of the symptom severity score before and after surgery was reported as the effect size (ES) for the outcome measure (an ES of 0.2 is considered small; 0.6, moderate; 1.2, large; and 2, very large).
All symptoms improved compared to their preoperative severity scores. Nasal obstruction improved the most (ES=1.73; 95% CI, 1.45-2.02). Large symptom improvement was also observed for facial pain (ES=1.13; 95% CI, 0.96-1.31) and postnasal discharge (ES=1.19; 95% CI, 0.96-1.43).
Surgery and medical therapy may provide comparable symptom relief
A recent Cochrane review of 4 low-quality RCTs including 378 patients compared surgical with medical interventions for CRS with nasal polyps. Study heterogeneity and selective outcome reporting prevented meta-analysis.
The 3 comparison groups were endoscopic sinus surgery vs systemic steroids + INS; polypectomy vs systemic steroid + INS; and endoscopic surgery + INS vs antibiotic + “high-dose” INS. Overall, neither surgery nor medical therapy was superior in terms of patient-reported symptom scores or quality-of-life measures.6
1. Kalish L, Snidvongs K, Sivasubramaniam R, et al. Topical steroids for nasal polyps. Cochrane Database Syst Rev. 2012;(12):CD006549.
2. Snidvongs K, Kalish L, Sacks R, et al. Topical steroids for chronic rhinosinusitis without polyps. Cochrane Database Syst Rev. 2011;(8):CD009274.
3. Harvey R, Hannan SA, Badia L, et al. Nasal saline irrigation for the symptoms of chronic rhinosinusitis. Cochrane Database Syst Rev. 2007;(3):CD006394.
4. Pynnonen MA, Venkatraman G, Davis GE. Macrolide therapy for chronic rhinosinusitis: a meta-analysis. Otolaryngol Head Neck Surg. 2013;148:366-373.
5. Chester AC, Antisdel JL, Sindwani R. Symptom-specific outcomes of endoscopic sinus surgery: a systematic review. Otolaryngol Head Neck Surg. 2009;140:633-639.
6. Rimmer J, Fokkens W, Chong LY, et al. Surgical versus medical interventions for chronic rhinosinusitis with nasal polyps. Cochrane Database Syst Rev. 2014;(12):CD0069991.
1. Kalish L, Snidvongs K, Sivasubramaniam R, et al. Topical steroids for nasal polyps. Cochrane Database Syst Rev. 2012;(12):CD006549.
2. Snidvongs K, Kalish L, Sacks R, et al. Topical steroids for chronic rhinosinusitis without polyps. Cochrane Database Syst Rev. 2011;(8):CD009274.
3. Harvey R, Hannan SA, Badia L, et al. Nasal saline irrigation for the symptoms of chronic rhinosinusitis. Cochrane Database Syst Rev. 2007;(3):CD006394.
4. Pynnonen MA, Venkatraman G, Davis GE. Macrolide therapy for chronic rhinosinusitis: a meta-analysis. Otolaryngol Head Neck Surg. 2013;148:366-373.
5. Chester AC, Antisdel JL, Sindwani R. Symptom-specific outcomes of endoscopic sinus surgery: a systematic review. Otolaryngol Head Neck Surg. 2009;140:633-639.
6. Rimmer J, Fokkens W, Chong LY, et al. Surgical versus medical interventions for chronic rhinosinusitis with nasal polyps. Cochrane Database Syst Rev. 2014;(12):CD0069991.
Evidence-based answers from the Family Physicians Inquiries Network
How do clinical prediction rules compare with joint fluid analysis in diagnosing gout?
EVIDENCE-BASED ANSWER:
Clinical prediction rules effectively diagnose gout without joint fluid analysis. The American College of Rheumatology clinical prediction rules, the most accurate rules developed for research purposes, have a sensitivity of 92%, specificity of 89%, positive likelihood ratio of 8.36, and negative likelihood ratio of 0.09 (strength of recommendation [SOR]: A, prospective cohort studies).
The Netherlands criteria, developed for use in primary care, have a positive predictive value of more than 80%, a positive likelihood ratio of 3.48, and a negative likelihood ratio of 0.17 (SOR: A, prospective cohort study).
EVIDENCE SUMMARY
In 2015, the American College of Rheumatology (ACR) redefined the clinical criteria for diagnosis of gout based on a 3-step system1 that can be found at: http://goutclassificationcalculator.auckland.ac.nz. The ACR rule was derived from a cross-sectional study of 983 patients in 25 rheumatology centers in 16 countries who presented with a swollen joint.2 Of the 983 patients, 509 had gout; the prevalence was 52%. Data from 653 of these patients were used to develop the rule and then validated in the remaining 330 patients.
Compared with the gold standard of monosodium urate crystals in synovial fluid, the ACR rule has a sensitivity of 92% and a specificity of 89%. The rule, designed for the research setting, involves using synovial fluid analysis, ultrasound imaging, and radiography, which makes it less useful in a primary care setting.
The Netherlands rule for primary care
A prospective diagnostic study in 328 family medicine patients (74% male; mean age 57) with monoarthritis tested the ability of multiple clinical variables to diagnose gout using monosodium urate crystals in synovial fluid as the gold standard.3 The prevalence of gout in this population was 57%.
The best diagnostic rule (Netherlands rule) comprised the following predefined variables: male sex, previous patient-reported arthritis attack, onset within one day, joint redness, first metatarsophalangeal joint (MTP1) involvement, hypertension or cardiovascular disease (angina pectoris, myocardial infarction, heart failure, cerebrovascular accident, transient ischemic attack, or peripheral vascular disease), and serum uric acid level above 5.88 mg/dL. The rule gives one point for each item. A score >8 had a positive likelihood ratio for diagnosing gout of 3.48 (TABLE1) and a higher positive predictive value (PPV) than family physicians’ clinical impressions (83% vs 64%).
The prevalence of gout in patients with scores of <4, 4 to 8, and >8 were 2.8%, 27%, and 80%, respectively. For scores of 4 to 8, the probability of gout is indeterminate, and synovial fluid analysis is recommended.
The Netherlands rule, validated in a secondary care practice of 390 patients with monoarthritis, found that a score >8 had a PPV of 87% and a score <4 had a negative predictive value of 95%.4 The probability of gout based on this rule can be calculated at http://www.umcn.nl/goutcalc.
In the study used to develop the Netherlands rule, no patients with a high probability of gout had septic arthritis. The ability of the rule to differentiate between gout and septic arthritis was tested retrospectively in 33 patients with acute gout (podagra excluded) diagnosed by the presence of monosodium urate joint crystals and 27 patients with septic arthritis diagnosed by positive bacterial culture.5 Patients with gout had significantly higher scores than patients with septic arthritis (7.8 ± 1.59 vs 3.4 ± 2.3; P<.001).
American Rheumatology Association, New York, and Rome prediction rules
A study of 82 Veterans Administration patients compared the American Rheumatology Association (ARA), New York, and Rome prediction rules with regard to their ability to diagnose gout with synovial urate crystals.6 The ARA criteria for gout diagnosis require either tophi or monosodium urate crystals in synovial fluid, or 6 out of a list of 12 other criteria.7
The New York prediction rule requires that patients meet 2 or more of the following criteria: at least 2 attacks of painful joint swelling with complete resolution within 2 weeks, podagra, tophi, and rapid response to colchicine treatment, defined as a major reduction in the objective signs of inflammation within 48 hours.
The Rome prediction rule requires meeting 2 of 3 criteria: serum uric acid >7 mg/dL in men and >6 mg/dL in women, presence of tophi, and history of attacks of painful joint swelling with abrupt onset and resolution within 2 weeks.
The New York prediction rule had the highest positive likelihood ratio of 4.4 compared with the ARA (1.8) and Rome (4.3) rules.6 The utility of the New York and Rome rules, although they have fewer criteria than ARA, is limited by the fact that they include a previous episode of joint swelling and tophi. These criteria increase their specificity but make them less useful in diagnosing a first episode of gout, when tophi are unlikely to have developed.
Prediction rules are more sensitive in established gout
The new ACR prediction rule was compared with the ARA, Rome, and New York clinical prediction rules using urate crystals as the gold standard in early (less than 2 years) and established disease (longer than 2 years).8 All clinical prediction rules were more sensitive in established disease than early disease (95.3% vs 84.1%; P<.001) and more specific in early disease than established disease (79.9% vs 52.5%; P<.001).
1. Neogi T, Jansen TL, Dalbeth N, et al. 2015 Gout Classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2015;74:1789-1798.
2. Taylor WJ, Fransen J, Jansen TL, et al. Study for Updated Gout Classification Criteria (SUGAR): identification of features to classify gout. Arthritis Care Res (Hoboken). 2015;67:1304-1315.
3. Janssens HJ, Fransen J, van de Lisdonk EH, et al. A diagnostic rule for acute gouty arthritis in primary care without joint fluid analysis. Arch Intern Med. 2010;170:1120-1126.
4. Kienhorst LB, Janssens HJ, Fransen J, et al. The validation of a diagnostic rule for gout without joint fluid analysis: a prospective study. Rheumatology (Oxford). 2015;54:609-614.
5. Lee K, Choi ST, Kang EJ, et al. SAT0377 The performance of a novel scoring system in the differential diagnosis between acute gout and septic arthritis. Ann Rheum Dis. 2013;72:A711.
6. Malik A, Schumacher HR, Dinnella JE, et al. Clinical diagnostic criteria for gout: comparison with the gold standard of synovial fluid crystal analysis. J Clin Rheumatol. 2009;15:22.
7. Wallace SL, Robinson H, Masi AT, et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895-900.
8. Taylor WJ, Fransen J, Dalbeth N, et al. Performance of classification criteria for gout in early and established disease. Ann Rheum Dis. 2016;75:178-182.
EVIDENCE-BASED ANSWER:
Clinical prediction rules effectively diagnose gout without joint fluid analysis. The American College of Rheumatology clinical prediction rules, the most accurate rules developed for research purposes, have a sensitivity of 92%, specificity of 89%, positive likelihood ratio of 8.36, and negative likelihood ratio of 0.09 (strength of recommendation [SOR]: A, prospective cohort studies).
The Netherlands criteria, developed for use in primary care, have a positive predictive value of more than 80%, a positive likelihood ratio of 3.48, and a negative likelihood ratio of 0.17 (SOR: A, prospective cohort study).
EVIDENCE SUMMARY
In 2015, the American College of Rheumatology (ACR) redefined the clinical criteria for diagnosis of gout based on a 3-step system1 that can be found at: http://goutclassificationcalculator.auckland.ac.nz. The ACR rule was derived from a cross-sectional study of 983 patients in 25 rheumatology centers in 16 countries who presented with a swollen joint.2 Of the 983 patients, 509 had gout; the prevalence was 52%. Data from 653 of these patients were used to develop the rule and then validated in the remaining 330 patients.
Compared with the gold standard of monosodium urate crystals in synovial fluid, the ACR rule has a sensitivity of 92% and a specificity of 89%. The rule, designed for the research setting, involves using synovial fluid analysis, ultrasound imaging, and radiography, which makes it less useful in a primary care setting.
The Netherlands rule for primary care
A prospective diagnostic study in 328 family medicine patients (74% male; mean age 57) with monoarthritis tested the ability of multiple clinical variables to diagnose gout using monosodium urate crystals in synovial fluid as the gold standard.3 The prevalence of gout in this population was 57%.
The best diagnostic rule (Netherlands rule) comprised the following predefined variables: male sex, previous patient-reported arthritis attack, onset within one day, joint redness, first metatarsophalangeal joint (MTP1) involvement, hypertension or cardiovascular disease (angina pectoris, myocardial infarction, heart failure, cerebrovascular accident, transient ischemic attack, or peripheral vascular disease), and serum uric acid level above 5.88 mg/dL. The rule gives one point for each item. A score >8 had a positive likelihood ratio for diagnosing gout of 3.48 (TABLE1) and a higher positive predictive value (PPV) than family physicians’ clinical impressions (83% vs 64%).

The prevalence of gout in patients with scores of <4, 4 to 8, and >8 were 2.8%, 27%, and 80%, respectively. For scores of 4 to 8, the probability of gout is indeterminate, and synovial fluid analysis is recommended.
The Netherlands rule, validated in a secondary care practice of 390 patients with monoarthritis, found that a score >8 had a PPV of 87% and a score <4 had a negative predictive value of 95%.4 The probability of gout based on this rule can be calculated at http://www.umcn.nl/goutcalc.
In the study used to develop the Netherlands rule, no patients with a high probability of gout had septic arthritis. The ability of the rule to differentiate between gout and septic arthritis was tested retrospectively in 33 patients with acute gout (podagra excluded) diagnosed by the presence of monosodium urate joint crystals and 27 patients with septic arthritis diagnosed by positive bacterial culture.5 Patients with gout had significantly higher scores than patients with septic arthritis (7.8 ± 1.59 vs 3.4 ± 2.3; P<.001).
American Rheumatology Association, New York, and Rome prediction rules
A study of 82 Veterans Administration patients compared the American Rheumatology Association (ARA), New York, and Rome prediction rules with regard to their ability to diagnose gout with synovial urate crystals.6 The ARA criteria for gout diagnosis require either tophi or monosodium urate crystals in synovial fluid, or 6 out of a list of 12 other criteria.7
The New York prediction rule requires that patients meet 2 or more of the following criteria: at least 2 attacks of painful joint swelling with complete resolution within 2 weeks, podagra, tophi, and rapid response to colchicine treatment, defined as a major reduction in the objective signs of inflammation within 48 hours.
The Rome prediction rule requires meeting 2 of 3 criteria: serum uric acid >7 mg/dL in men and >6 mg/dL in women, presence of tophi, and history of attacks of painful joint swelling with abrupt onset and resolution within 2 weeks.
The New York prediction rule had the highest positive likelihood ratio of 4.4 compared with the ARA (1.8) and Rome (4.3) rules.6 The utility of the New York and Rome rules, although they have fewer criteria than ARA, is limited by the fact that they include a previous episode of joint swelling and tophi. These criteria increase their specificity but make them less useful in diagnosing a first episode of gout, when tophi are unlikely to have developed.
Prediction rules are more sensitive in established gout
The new ACR prediction rule was compared with the ARA, Rome, and New York clinical prediction rules using urate crystals as the gold standard in early (less than 2 years) and established disease (longer than 2 years).8 All clinical prediction rules were more sensitive in established disease than early disease (95.3% vs 84.1%; P<.001) and more specific in early disease than established disease (79.9% vs 52.5%; P<.001).
EVIDENCE-BASED ANSWER:
Clinical prediction rules effectively diagnose gout without joint fluid analysis. The American College of Rheumatology clinical prediction rules, the most accurate rules developed for research purposes, have a sensitivity of 92%, specificity of 89%, positive likelihood ratio of 8.36, and negative likelihood ratio of 0.09 (strength of recommendation [SOR]: A, prospective cohort studies).
The Netherlands criteria, developed for use in primary care, have a positive predictive value of more than 80%, a positive likelihood ratio of 3.48, and a negative likelihood ratio of 0.17 (SOR: A, prospective cohort study).
EVIDENCE SUMMARY
In 2015, the American College of Rheumatology (ACR) redefined the clinical criteria for diagnosis of gout based on a 3-step system1 that can be found at: http://goutclassificationcalculator.auckland.ac.nz. The ACR rule was derived from a cross-sectional study of 983 patients in 25 rheumatology centers in 16 countries who presented with a swollen joint.2 Of the 983 patients, 509 had gout; the prevalence was 52%. Data from 653 of these patients were used to develop the rule and then validated in the remaining 330 patients.
Compared with the gold standard of monosodium urate crystals in synovial fluid, the ACR rule has a sensitivity of 92% and a specificity of 89%. The rule, designed for the research setting, involves using synovial fluid analysis, ultrasound imaging, and radiography, which makes it less useful in a primary care setting.
The Netherlands rule for primary care
A prospective diagnostic study in 328 family medicine patients (74% male; mean age 57) with monoarthritis tested the ability of multiple clinical variables to diagnose gout using monosodium urate crystals in synovial fluid as the gold standard.3 The prevalence of gout in this population was 57%.
The best diagnostic rule (Netherlands rule) comprised the following predefined variables: male sex, previous patient-reported arthritis attack, onset within one day, joint redness, first metatarsophalangeal joint (MTP1) involvement, hypertension or cardiovascular disease (angina pectoris, myocardial infarction, heart failure, cerebrovascular accident, transient ischemic attack, or peripheral vascular disease), and serum uric acid level above 5.88 mg/dL. The rule gives one point for each item. A score >8 had a positive likelihood ratio for diagnosing gout of 3.48 (TABLE1) and a higher positive predictive value (PPV) than family physicians’ clinical impressions (83% vs 64%).

The prevalence of gout in patients with scores of <4, 4 to 8, and >8 were 2.8%, 27%, and 80%, respectively. For scores of 4 to 8, the probability of gout is indeterminate, and synovial fluid analysis is recommended.
The Netherlands rule, validated in a secondary care practice of 390 patients with monoarthritis, found that a score >8 had a PPV of 87% and a score <4 had a negative predictive value of 95%.4 The probability of gout based on this rule can be calculated at http://www.umcn.nl/goutcalc.
In the study used to develop the Netherlands rule, no patients with a high probability of gout had septic arthritis. The ability of the rule to differentiate between gout and septic arthritis was tested retrospectively in 33 patients with acute gout (podagra excluded) diagnosed by the presence of monosodium urate joint crystals and 27 patients with septic arthritis diagnosed by positive bacterial culture.5 Patients with gout had significantly higher scores than patients with septic arthritis (7.8 ± 1.59 vs 3.4 ± 2.3; P<.001).
American Rheumatology Association, New York, and Rome prediction rules
A study of 82 Veterans Administration patients compared the American Rheumatology Association (ARA), New York, and Rome prediction rules with regard to their ability to diagnose gout with synovial urate crystals.6 The ARA criteria for gout diagnosis require either tophi or monosodium urate crystals in synovial fluid, or 6 out of a list of 12 other criteria.7
The New York prediction rule requires that patients meet 2 or more of the following criteria: at least 2 attacks of painful joint swelling with complete resolution within 2 weeks, podagra, tophi, and rapid response to colchicine treatment, defined as a major reduction in the objective signs of inflammation within 48 hours.
The Rome prediction rule requires meeting 2 of 3 criteria: serum uric acid >7 mg/dL in men and >6 mg/dL in women, presence of tophi, and history of attacks of painful joint swelling with abrupt onset and resolution within 2 weeks.
The New York prediction rule had the highest positive likelihood ratio of 4.4 compared with the ARA (1.8) and Rome (4.3) rules.6 The utility of the New York and Rome rules, although they have fewer criteria than ARA, is limited by the fact that they include a previous episode of joint swelling and tophi. These criteria increase their specificity but make them less useful in diagnosing a first episode of gout, when tophi are unlikely to have developed.
Prediction rules are more sensitive in established gout
The new ACR prediction rule was compared with the ARA, Rome, and New York clinical prediction rules using urate crystals as the gold standard in early (less than 2 years) and established disease (longer than 2 years).8 All clinical prediction rules were more sensitive in established disease than early disease (95.3% vs 84.1%; P<.001) and more specific in early disease than established disease (79.9% vs 52.5%; P<.001).
1. Neogi T, Jansen TL, Dalbeth N, et al. 2015 Gout Classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2015;74:1789-1798.
2. Taylor WJ, Fransen J, Jansen TL, et al. Study for Updated Gout Classification Criteria (SUGAR): identification of features to classify gout. Arthritis Care Res (Hoboken). 2015;67:1304-1315.
3. Janssens HJ, Fransen J, van de Lisdonk EH, et al. A diagnostic rule for acute gouty arthritis in primary care without joint fluid analysis. Arch Intern Med. 2010;170:1120-1126.
4. Kienhorst LB, Janssens HJ, Fransen J, et al. The validation of a diagnostic rule for gout without joint fluid analysis: a prospective study. Rheumatology (Oxford). 2015;54:609-614.
5. Lee K, Choi ST, Kang EJ, et al. SAT0377 The performance of a novel scoring system in the differential diagnosis between acute gout and septic arthritis. Ann Rheum Dis. 2013;72:A711.
6. Malik A, Schumacher HR, Dinnella JE, et al. Clinical diagnostic criteria for gout: comparison with the gold standard of synovial fluid crystal analysis. J Clin Rheumatol. 2009;15:22.
7. Wallace SL, Robinson H, Masi AT, et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895-900.
8. Taylor WJ, Fransen J, Dalbeth N, et al. Performance of classification criteria for gout in early and established disease. Ann Rheum Dis. 2016;75:178-182.
1. Neogi T, Jansen TL, Dalbeth N, et al. 2015 Gout Classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2015;74:1789-1798.
2. Taylor WJ, Fransen J, Jansen TL, et al. Study for Updated Gout Classification Criteria (SUGAR): identification of features to classify gout. Arthritis Care Res (Hoboken). 2015;67:1304-1315.
3. Janssens HJ, Fransen J, van de Lisdonk EH, et al. A diagnostic rule for acute gouty arthritis in primary care without joint fluid analysis. Arch Intern Med. 2010;170:1120-1126.
4. Kienhorst LB, Janssens HJ, Fransen J, et al. The validation of a diagnostic rule for gout without joint fluid analysis: a prospective study. Rheumatology (Oxford). 2015;54:609-614.
5. Lee K, Choi ST, Kang EJ, et al. SAT0377 The performance of a novel scoring system in the differential diagnosis between acute gout and septic arthritis. Ann Rheum Dis. 2013;72:A711.
6. Malik A, Schumacher HR, Dinnella JE, et al. Clinical diagnostic criteria for gout: comparison with the gold standard of synovial fluid crystal analysis. J Clin Rheumatol. 2009;15:22.
7. Wallace SL, Robinson H, Masi AT, et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895-900.
8. Taylor WJ, Fransen J, Dalbeth N, et al. Performance of classification criteria for gout in early and established disease. Ann Rheum Dis. 2016;75:178-182.
Evidence-based answers from the Family Physicians Inquiries Network
Which patients with metabolic syndrome benefit from metformin?
EVIDENCE-BASED ANSWER:
Patients at highest risk for progression to diabetes benefit from metformin.
In patients with metabolic syndrome who are in the highest-risk quartile for progression to diabetes (predicted mean 3-year risk, 60%), metformin, 850 mg twice daily, reduces the absolute risk by about 20% over a 3-year period. Metformin doesn’t reduce the incidence in patients at lower risk of progression (strength of recommendation [SOR]: C, post-hoc analysis of a randomized controlled trial [RCT]).
Intensive lifestyle modification reduces absolute risk in all patients proportionate to risk quartile (from 5% reduction for the lowest quartile to 28% for the highest). Over a 10-year period, intensive lifestyle modification reduces the absolute risk of diabetes by 34% and metformin reduces the risk by 18% for all patients at increased risk (considered as a group)—that is, not separated by risk quartile (SOR: A, large prospective RCTs).
Lower doses or shorter courses of metformin reduce fasting plasma glucose (SOR: C, RCTs with laboratory outcomes) and may reduce the risk of developing diabetes by a smaller amount (SOR: C, flawed RCT).
EVIDENCE SUMMARY
A post-hoc analysis of a prospective RCT (the Diabetes Prevention Program) comprising 3081 patients with impaired glucose metabolism who received metformin, a lifestyle modification program, or no intervention (placebo) found that metformin reduced the risk of developing diabetes only for patients in the highest risk quartile over 2.8 years. Lifestyle modification reduced diabetes risk in all patients.1
Investigators stratified patients who met National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) criteria for metabolic syndrome into risk quartiles for progression to diabetes using a model they developed based on 7 parameters: fasting plasma glucose, hemoglobin A1c, history of high blood glucose, waist:hip ratio, waist circumference, triglycerides, and height (TABLE1). The model reasonably fit outcomes—the percentage of patients in each quartile who developed diabetes—with an area under the receiver operator characteristic curve of 0.73 (a measure of diagnostic accuracy where 1 is a perfect predictor and 0.5 is random).
Lifestyle modification reduced risk in all quartiles with progressively greater effect as risk increased (lowest risk quartile: ARR=4.9%, 3-year NNT=20.4; highest risk quartile: ARR=28.3%; 3-year NNT=3.5).
There were 2 key weaknesses of the risk model: It wasn’t validated in a separate population and the true incidence of diabetes among patients taking placebo was higher than predicted. The investigators compared their risk prediction model results with the Framingham Risk Score (FRS) for diabetes and found that they correlated well, although the FRS results were consistently about 6% (absolute) higher when corrected for duration. (The FRS calculator is available online at www.framinghamheartstudy.org/risk-functions/diabetes/.)
Lifestyle change reduces diabetes risk more than metformin
The original Diabetes Prevention Program found that intensive lifestyle intervention and metformin reduced the number of diabetes cases over 2.8 years among 3234 patients at risk for developing diabetes.2
Compared with no intervention, fewer patients developed diabetes with either metformin or lifestyle improvement, although lifestyle change had the larger effect (no intervention: 11 cases per 100 person-years; metformin: 7.8 cases; 95% confidence interval [CI], 6.8-8.8; ARR=3.2% per year vs no intervention; lifestyle improvements: 4.8 cases; 95% CI, 4.1-5.7; ARR=6.2% per year vs no intervention).
The effect of metformin and lifestyle change persists at 10 years
A 10-year follow-up study to the Diabetes Prevention Program found that, compared with no intervention, both metformin and lifestyle interventions continued to be associated with a lower incidence of diabetes (no intervention: 7.8 cases per 100 person-years; 95% CI, 4.8-6.5; metformin: 6.4 cases; 95% CI, 4.2-5.7; ARR=1.4% per year; lifestyle interventions: 5.3 cases; 95% CI, 5.1-6.8; ARR=2.5% per year).3
Researchers originally randomized 3234 patients with body mass index ≥24 kg/m2, fasting blood sugar 95 to 125 mg/dL, and 2-hour post 75-gm glucose value of 149 to 199 mg/dL to 3 groups: intensive lifestyle modification (weight loss goal of 7%, 150 minutes a week of exercise), metformin (850 mg twice daily), and no intervention. After the 2.8-year follow-up period, 2766 patients continued for another 5.7 years of follow-up. Investigators offered group lifestyle counseling to all patients and continued metformin at the same dose in the second group.
Earlier study shows an effect for metformin, but with a caveat
An earlier RCT found that metformin reduced the risk of developing diabetes in patients with metabolic syndrome.4 Investigators randomized 70 patients to metformin (250 mg 3 times daily) or placebo for a year. Fewer patients developed diabetes with metformin (3% vs 16.2%, P=.011; NNT=7.6) and more had a normal glucose tolerance test result (84.9% vs 51.4%, P=.011; NNT=3). However, by current American Diabetes Association criteria, half of the subjects had early diabetes at baseline.
Metformin lowers fasting blood sugar, but may not reverse metabolic syndrome
A post-hoc analysis of another RCT found that metformin reduced fasting plasma glucose (FPG) levels in patients with upper-body obesity and metabolic syndrome (by 1999 World Health Organization criteria but not NCEP ATP III criteria).5
Investigators randomized 457 patients to metformin 850 mg once daily or placebo and followed them for a year. FPG levels decreased with metformin but increased with placebo (reduction FPG 5.9 mg/dL vs increase FPG 12.3 mg/dL; P<.04). The investigators didn’t report whether any patients developed diabetes.
However, another RCT (155 patients) that compared metformin 850 mg twice daily with placebo in subjects with metabolic syndrome but without diabetes found greater normalization of FPG (5% vs 0%; P=.005), but no reversal of metabolic syndrome or change in Framingham 10-year risk score after 12 weeks.6
1. Sussman JB, Kent DM, Nelson JP, et al. Improving diabetes prevention with benefit based tailored treatment: risk based reanalysis of Diabetes Prevention Program. BMJ. 2015;350:h454.
2. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
3. Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Outcomes Study. Lancet. 2009:374:1677-1686.
4. Li CL, Pan CY, Lu JM, et al. Effect of metformin on patients with impaired glucose tolerance. Diabetes Med. 1999;16:477-481.
5. Fontbonne A, Diouf I, Baccara-Dinet M, et al. Effects of 1-year treatment with metformin on metabolic and cardiovascular risk factors in non-diabetic upper-body obese subjects with mild glucose anomalies: a post-hoc analysis of the BIGPRO1 trial. Diabetes Metab. 2009;35:385-391.
6. Nieuwdorp M, Stroes ESG, Kastelein JJP. Normalization of metabolic syndrome using fenofibrate, metformin or their combination. Diabetes Obesity Metab. 2007;9:869-878.
EVIDENCE-BASED ANSWER:
Patients at highest risk for progression to diabetes benefit from metformin.
In patients with metabolic syndrome who are in the highest-risk quartile for progression to diabetes (predicted mean 3-year risk, 60%), metformin, 850 mg twice daily, reduces the absolute risk by about 20% over a 3-year period. Metformin doesn’t reduce the incidence in patients at lower risk of progression (strength of recommendation [SOR]: C, post-hoc analysis of a randomized controlled trial [RCT]).
Intensive lifestyle modification reduces absolute risk in all patients proportionate to risk quartile (from 5% reduction for the lowest quartile to 28% for the highest). Over a 10-year period, intensive lifestyle modification reduces the absolute risk of diabetes by 34% and metformin reduces the risk by 18% for all patients at increased risk (considered as a group)—that is, not separated by risk quartile (SOR: A, large prospective RCTs).
Lower doses or shorter courses of metformin reduce fasting plasma glucose (SOR: C, RCTs with laboratory outcomes) and may reduce the risk of developing diabetes by a smaller amount (SOR: C, flawed RCT).
EVIDENCE SUMMARY
A post-hoc analysis of a prospective RCT (the Diabetes Prevention Program) comprising 3081 patients with impaired glucose metabolism who received metformin, a lifestyle modification program, or no intervention (placebo) found that metformin reduced the risk of developing diabetes only for patients in the highest risk quartile over 2.8 years. Lifestyle modification reduced diabetes risk in all patients.1
Investigators stratified patients who met National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) criteria for metabolic syndrome into risk quartiles for progression to diabetes using a model they developed based on 7 parameters: fasting plasma glucose, hemoglobin A1c, history of high blood glucose, waist:hip ratio, waist circumference, triglycerides, and height (TABLE1). The model reasonably fit outcomes—the percentage of patients in each quartile who developed diabetes—with an area under the receiver operator characteristic curve of 0.73 (a measure of diagnostic accuracy where 1 is a perfect predictor and 0.5 is random).

Lifestyle modification reduced risk in all quartiles with progressively greater effect as risk increased (lowest risk quartile: ARR=4.9%, 3-year NNT=20.4; highest risk quartile: ARR=28.3%; 3-year NNT=3.5).
There were 2 key weaknesses of the risk model: It wasn’t validated in a separate population and the true incidence of diabetes among patients taking placebo was higher than predicted. The investigators compared their risk prediction model results with the Framingham Risk Score (FRS) for diabetes and found that they correlated well, although the FRS results were consistently about 6% (absolute) higher when corrected for duration. (The FRS calculator is available online at www.framinghamheartstudy.org/risk-functions/diabetes/.)
Lifestyle change reduces diabetes risk more than metformin
The original Diabetes Prevention Program found that intensive lifestyle intervention and metformin reduced the number of diabetes cases over 2.8 years among 3234 patients at risk for developing diabetes.2
Compared with no intervention, fewer patients developed diabetes with either metformin or lifestyle improvement, although lifestyle change had the larger effect (no intervention: 11 cases per 100 person-years; metformin: 7.8 cases; 95% confidence interval [CI], 6.8-8.8; ARR=3.2% per year vs no intervention; lifestyle improvements: 4.8 cases; 95% CI, 4.1-5.7; ARR=6.2% per year vs no intervention).
The effect of metformin and lifestyle change persists at 10 years
A 10-year follow-up study to the Diabetes Prevention Program found that, compared with no intervention, both metformin and lifestyle interventions continued to be associated with a lower incidence of diabetes (no intervention: 7.8 cases per 100 person-years; 95% CI, 4.8-6.5; metformin: 6.4 cases; 95% CI, 4.2-5.7; ARR=1.4% per year; lifestyle interventions: 5.3 cases; 95% CI, 5.1-6.8; ARR=2.5% per year).3
Researchers originally randomized 3234 patients with body mass index ≥24 kg/m2, fasting blood sugar 95 to 125 mg/dL, and 2-hour post 75-gm glucose value of 149 to 199 mg/dL to 3 groups: intensive lifestyle modification (weight loss goal of 7%, 150 minutes a week of exercise), metformin (850 mg twice daily), and no intervention. After the 2.8-year follow-up period, 2766 patients continued for another 5.7 years of follow-up. Investigators offered group lifestyle counseling to all patients and continued metformin at the same dose in the second group.
Earlier study shows an effect for metformin, but with a caveat
An earlier RCT found that metformin reduced the risk of developing diabetes in patients with metabolic syndrome.4 Investigators randomized 70 patients to metformin (250 mg 3 times daily) or placebo for a year. Fewer patients developed diabetes with metformin (3% vs 16.2%, P=.011; NNT=7.6) and more had a normal glucose tolerance test result (84.9% vs 51.4%, P=.011; NNT=3). However, by current American Diabetes Association criteria, half of the subjects had early diabetes at baseline.
Metformin lowers fasting blood sugar, but may not reverse metabolic syndrome
A post-hoc analysis of another RCT found that metformin reduced fasting plasma glucose (FPG) levels in patients with upper-body obesity and metabolic syndrome (by 1999 World Health Organization criteria but not NCEP ATP III criteria).5
Investigators randomized 457 patients to metformin 850 mg once daily or placebo and followed them for a year. FPG levels decreased with metformin but increased with placebo (reduction FPG 5.9 mg/dL vs increase FPG 12.3 mg/dL; P<.04). The investigators didn’t report whether any patients developed diabetes.
However, another RCT (155 patients) that compared metformin 850 mg twice daily with placebo in subjects with metabolic syndrome but without diabetes found greater normalization of FPG (5% vs 0%; P=.005), but no reversal of metabolic syndrome or change in Framingham 10-year risk score after 12 weeks.6
EVIDENCE-BASED ANSWER:
Patients at highest risk for progression to diabetes benefit from metformin.
In patients with metabolic syndrome who are in the highest-risk quartile for progression to diabetes (predicted mean 3-year risk, 60%), metformin, 850 mg twice daily, reduces the absolute risk by about 20% over a 3-year period. Metformin doesn’t reduce the incidence in patients at lower risk of progression (strength of recommendation [SOR]: C, post-hoc analysis of a randomized controlled trial [RCT]).
Intensive lifestyle modification reduces absolute risk in all patients proportionate to risk quartile (from 5% reduction for the lowest quartile to 28% for the highest). Over a 10-year period, intensive lifestyle modification reduces the absolute risk of diabetes by 34% and metformin reduces the risk by 18% for all patients at increased risk (considered as a group)—that is, not separated by risk quartile (SOR: A, large prospective RCTs).
Lower doses or shorter courses of metformin reduce fasting plasma glucose (SOR: C, RCTs with laboratory outcomes) and may reduce the risk of developing diabetes by a smaller amount (SOR: C, flawed RCT).
EVIDENCE SUMMARY
A post-hoc analysis of a prospective RCT (the Diabetes Prevention Program) comprising 3081 patients with impaired glucose metabolism who received metformin, a lifestyle modification program, or no intervention (placebo) found that metformin reduced the risk of developing diabetes only for patients in the highest risk quartile over 2.8 years. Lifestyle modification reduced diabetes risk in all patients.1
Investigators stratified patients who met National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) criteria for metabolic syndrome into risk quartiles for progression to diabetes using a model they developed based on 7 parameters: fasting plasma glucose, hemoglobin A1c, history of high blood glucose, waist:hip ratio, waist circumference, triglycerides, and height (TABLE1). The model reasonably fit outcomes—the percentage of patients in each quartile who developed diabetes—with an area under the receiver operator characteristic curve of 0.73 (a measure of diagnostic accuracy where 1 is a perfect predictor and 0.5 is random).

Lifestyle modification reduced risk in all quartiles with progressively greater effect as risk increased (lowest risk quartile: ARR=4.9%, 3-year NNT=20.4; highest risk quartile: ARR=28.3%; 3-year NNT=3.5).
There were 2 key weaknesses of the risk model: It wasn’t validated in a separate population and the true incidence of diabetes among patients taking placebo was higher than predicted. The investigators compared their risk prediction model results with the Framingham Risk Score (FRS) for diabetes and found that they correlated well, although the FRS results were consistently about 6% (absolute) higher when corrected for duration. (The FRS calculator is available online at www.framinghamheartstudy.org/risk-functions/diabetes/.)
Lifestyle change reduces diabetes risk more than metformin
The original Diabetes Prevention Program found that intensive lifestyle intervention and metformin reduced the number of diabetes cases over 2.8 years among 3234 patients at risk for developing diabetes.2
Compared with no intervention, fewer patients developed diabetes with either metformin or lifestyle improvement, although lifestyle change had the larger effect (no intervention: 11 cases per 100 person-years; metformin: 7.8 cases; 95% confidence interval [CI], 6.8-8.8; ARR=3.2% per year vs no intervention; lifestyle improvements: 4.8 cases; 95% CI, 4.1-5.7; ARR=6.2% per year vs no intervention).
The effect of metformin and lifestyle change persists at 10 years
A 10-year follow-up study to the Diabetes Prevention Program found that, compared with no intervention, both metformin and lifestyle interventions continued to be associated with a lower incidence of diabetes (no intervention: 7.8 cases per 100 person-years; 95% CI, 4.8-6.5; metformin: 6.4 cases; 95% CI, 4.2-5.7; ARR=1.4% per year; lifestyle interventions: 5.3 cases; 95% CI, 5.1-6.8; ARR=2.5% per year).3
Researchers originally randomized 3234 patients with body mass index ≥24 kg/m2, fasting blood sugar 95 to 125 mg/dL, and 2-hour post 75-gm glucose value of 149 to 199 mg/dL to 3 groups: intensive lifestyle modification (weight loss goal of 7%, 150 minutes a week of exercise), metformin (850 mg twice daily), and no intervention. After the 2.8-year follow-up period, 2766 patients continued for another 5.7 years of follow-up. Investigators offered group lifestyle counseling to all patients and continued metformin at the same dose in the second group.
Earlier study shows an effect for metformin, but with a caveat
An earlier RCT found that metformin reduced the risk of developing diabetes in patients with metabolic syndrome.4 Investigators randomized 70 patients to metformin (250 mg 3 times daily) or placebo for a year. Fewer patients developed diabetes with metformin (3% vs 16.2%, P=.011; NNT=7.6) and more had a normal glucose tolerance test result (84.9% vs 51.4%, P=.011; NNT=3). However, by current American Diabetes Association criteria, half of the subjects had early diabetes at baseline.
Metformin lowers fasting blood sugar, but may not reverse metabolic syndrome
A post-hoc analysis of another RCT found that metformin reduced fasting plasma glucose (FPG) levels in patients with upper-body obesity and metabolic syndrome (by 1999 World Health Organization criteria but not NCEP ATP III criteria).5
Investigators randomized 457 patients to metformin 850 mg once daily or placebo and followed them for a year. FPG levels decreased with metformin but increased with placebo (reduction FPG 5.9 mg/dL vs increase FPG 12.3 mg/dL; P<.04). The investigators didn’t report whether any patients developed diabetes.
However, another RCT (155 patients) that compared metformin 850 mg twice daily with placebo in subjects with metabolic syndrome but without diabetes found greater normalization of FPG (5% vs 0%; P=.005), but no reversal of metabolic syndrome or change in Framingham 10-year risk score after 12 weeks.6
1. Sussman JB, Kent DM, Nelson JP, et al. Improving diabetes prevention with benefit based tailored treatment: risk based reanalysis of Diabetes Prevention Program. BMJ. 2015;350:h454.
2. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
3. Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Outcomes Study. Lancet. 2009:374:1677-1686.
4. Li CL, Pan CY, Lu JM, et al. Effect of metformin on patients with impaired glucose tolerance. Diabetes Med. 1999;16:477-481.
5. Fontbonne A, Diouf I, Baccara-Dinet M, et al. Effects of 1-year treatment with metformin on metabolic and cardiovascular risk factors in non-diabetic upper-body obese subjects with mild glucose anomalies: a post-hoc analysis of the BIGPRO1 trial. Diabetes Metab. 2009;35:385-391.
6. Nieuwdorp M, Stroes ESG, Kastelein JJP. Normalization of metabolic syndrome using fenofibrate, metformin or their combination. Diabetes Obesity Metab. 2007;9:869-878.
1. Sussman JB, Kent DM, Nelson JP, et al. Improving diabetes prevention with benefit based tailored treatment: risk based reanalysis of Diabetes Prevention Program. BMJ. 2015;350:h454.
2. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
3. Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Outcomes Study. Lancet. 2009:374:1677-1686.
4. Li CL, Pan CY, Lu JM, et al. Effect of metformin on patients with impaired glucose tolerance. Diabetes Med. 1999;16:477-481.
5. Fontbonne A, Diouf I, Baccara-Dinet M, et al. Effects of 1-year treatment with metformin on metabolic and cardiovascular risk factors in non-diabetic upper-body obese subjects with mild glucose anomalies: a post-hoc analysis of the BIGPRO1 trial. Diabetes Metab. 2009;35:385-391.
6. Nieuwdorp M, Stroes ESG, Kastelein JJP. Normalization of metabolic syndrome using fenofibrate, metformin or their combination. Diabetes Obesity Metab. 2007;9:869-878.
Evidence-based answers from the Family Physicians Inquiries Network
What can we do about the Zika virus in the United States?
Since Florida has seen several new cases of local mosquito-borne infection, controlling and preventing Zika infection has great urgency. Zika virus involves an arthropod-borne infection transmitted by Aedes aegypti and Aedes albopictus mosquitoes. Other modes of transmission include the maternal-fetal route, any sexual contact, blood transfusions, organ or tissue transplantation, and laboratory exposure.1
The first case of Zika infection in the United States and its territories occurred through international travel. According to the Centers for Disease Control and Prevention, as of October 12, 2016, there were 3807 travel-associated cases of Zika infection in the United States and 84 instances in its territories.2 As for local transmission, there were 128 people evidencing a Zika infection in the United States and 25,871 in US territories.2 Regions between Texas and Florida are at high risk because Aedes mosquitoes primarily inhabit the gulf coast.3 Many cases have occurred despite repellent use and eradication efforts, possibly due to resistance acquired by these mosquitoes.1
Control measures include using insect repellents, aerial spraying of insecticides, eliminating mosquito breeding sites, covering water tanks, and using mosquito nets or door and window screens. Infection during pregnancy is the greatest concern because of congenital anomalies (including microcephaly) that negatively affect brain development.4
Before a possible conception or any sexual contact, women exposed to Zika—with or without symptoms—must wait at least 8 weeks; men with or without symptoms should abstain for 6 months.4 Individuals should avoid traveling to areas with Zika infestation, wear long-sleeved clothing treated with permethrin, and minimize outside exposure, especially in evening hours.4
The World Health Organization is utilizing genetically modified mosquitoes to diminish Aedes populations; trials conducted in affected areas of Brazil revealed that the number of Aedes mosquitoes was reduced by 90%.5 This method of mosquito control is currently being studied in the United States.6 Vaccinations to prevent Zika infection are also under investigation.
Physicians should educate patients regarding the clinical manifestations and complications of Zika virus infection; people need to know that the Zika virus can be sexually transmitted. Doctors should also counsel patients to curtail travel to areas that have Zika infestations, or to at least wear protective clothing while in such areas to minimize mosquito bite risk. Educating travelers about appropriate postponement of sexual contact after any exposure to the Zika virus is also essential.4
Hema Madhuri Mekala, MD
Priyanga Jayakumar, MD
Rajashekar Reddy Yeruva, MD
Steven Lippmann, MD
Louisville, KY
1. Centers for Disease Control and Prevention. Zika virus: Transmission & risks. Available at: http://www.cdc.gov/zika/transmission/index.html. Accessed October 14, 2016.
2. Centers for Disease Control and Prevention. Zika virus: Case counts in the US. Available at: http://www.cdc.gov/zika/geo/united-states.html. Accessed October 14, 2016.
3. Castro L, Chen X, Dimitrov NB, et al. The University of Texas at Austin. Texas Arbovirus Risk. 2015. Available at: http://hdl.handle.net/2152/31934. Accessed October 14, 2016.
4. Centers for Disease Control and Prevention. Zika virus: Zika is in your area: What to do. Available at: http://www.cdc.gov/zika/intheus/what-to-do.html. Accessed October 14, 2016.
5. FL KEYS NEWS. Available at: http://www.flkeysnews.com/opinion/opn-columns-blogs/article83328707.html. Accessed October 14, 2016.
6. Ernst KC, Haenchen S, Dickinson K, et al. Awareness and support of release of genetically modified “sterile” mosquitoes, Key West, Florida, USA. Emerg Infect Dis. 2015;21:320-324.
Since Florida has seen several new cases of local mosquito-borne infection, controlling and preventing Zika infection has great urgency. Zika virus involves an arthropod-borne infection transmitted by Aedes aegypti and Aedes albopictus mosquitoes. Other modes of transmission include the maternal-fetal route, any sexual contact, blood transfusions, organ or tissue transplantation, and laboratory exposure.1
The first case of Zika infection in the United States and its territories occurred through international travel. According to the Centers for Disease Control and Prevention, as of October 12, 2016, there were 3807 travel-associated cases of Zika infection in the United States and 84 instances in its territories.2 As for local transmission, there were 128 people evidencing a Zika infection in the United States and 25,871 in US territories.2 Regions between Texas and Florida are at high risk because Aedes mosquitoes primarily inhabit the gulf coast.3 Many cases have occurred despite repellent use and eradication efforts, possibly due to resistance acquired by these mosquitoes.1
Control measures include using insect repellents, aerial spraying of insecticides, eliminating mosquito breeding sites, covering water tanks, and using mosquito nets or door and window screens. Infection during pregnancy is the greatest concern because of congenital anomalies (including microcephaly) that negatively affect brain development.4
Before a possible conception or any sexual contact, women exposed to Zika—with or without symptoms—must wait at least 8 weeks; men with or without symptoms should abstain for 6 months.4 Individuals should avoid traveling to areas with Zika infestation, wear long-sleeved clothing treated with permethrin, and minimize outside exposure, especially in evening hours.4
The World Health Organization is utilizing genetically modified mosquitoes to diminish Aedes populations; trials conducted in affected areas of Brazil revealed that the number of Aedes mosquitoes was reduced by 90%.5 This method of mosquito control is currently being studied in the United States.6 Vaccinations to prevent Zika infection are also under investigation.
Physicians should educate patients regarding the clinical manifestations and complications of Zika virus infection; people need to know that the Zika virus can be sexually transmitted. Doctors should also counsel patients to curtail travel to areas that have Zika infestations, or to at least wear protective clothing while in such areas to minimize mosquito bite risk. Educating travelers about appropriate postponement of sexual contact after any exposure to the Zika virus is also essential.4
Hema Madhuri Mekala, MD
Priyanga Jayakumar, MD
Rajashekar Reddy Yeruva, MD
Steven Lippmann, MD
Louisville, KY
Since Florida has seen several new cases of local mosquito-borne infection, controlling and preventing Zika infection has great urgency. Zika virus involves an arthropod-borne infection transmitted by Aedes aegypti and Aedes albopictus mosquitoes. Other modes of transmission include the maternal-fetal route, any sexual contact, blood transfusions, organ or tissue transplantation, and laboratory exposure.1
The first case of Zika infection in the United States and its territories occurred through international travel. According to the Centers for Disease Control and Prevention, as of October 12, 2016, there were 3807 travel-associated cases of Zika infection in the United States and 84 instances in its territories.2 As for local transmission, there were 128 people evidencing a Zika infection in the United States and 25,871 in US territories.2 Regions between Texas and Florida are at high risk because Aedes mosquitoes primarily inhabit the gulf coast.3 Many cases have occurred despite repellent use and eradication efforts, possibly due to resistance acquired by these mosquitoes.1
Control measures include using insect repellents, aerial spraying of insecticides, eliminating mosquito breeding sites, covering water tanks, and using mosquito nets or door and window screens. Infection during pregnancy is the greatest concern because of congenital anomalies (including microcephaly) that negatively affect brain development.4
Before a possible conception or any sexual contact, women exposed to Zika—with or without symptoms—must wait at least 8 weeks; men with or without symptoms should abstain for 6 months.4 Individuals should avoid traveling to areas with Zika infestation, wear long-sleeved clothing treated with permethrin, and minimize outside exposure, especially in evening hours.4
The World Health Organization is utilizing genetically modified mosquitoes to diminish Aedes populations; trials conducted in affected areas of Brazil revealed that the number of Aedes mosquitoes was reduced by 90%.5 This method of mosquito control is currently being studied in the United States.6 Vaccinations to prevent Zika infection are also under investigation.
Physicians should educate patients regarding the clinical manifestations and complications of Zika virus infection; people need to know that the Zika virus can be sexually transmitted. Doctors should also counsel patients to curtail travel to areas that have Zika infestations, or to at least wear protective clothing while in such areas to minimize mosquito bite risk. Educating travelers about appropriate postponement of sexual contact after any exposure to the Zika virus is also essential.4
Hema Madhuri Mekala, MD
Priyanga Jayakumar, MD
Rajashekar Reddy Yeruva, MD
Steven Lippmann, MD
Louisville, KY
1. Centers for Disease Control and Prevention. Zika virus: Transmission & risks. Available at: http://www.cdc.gov/zika/transmission/index.html. Accessed October 14, 2016.
2. Centers for Disease Control and Prevention. Zika virus: Case counts in the US. Available at: http://www.cdc.gov/zika/geo/united-states.html. Accessed October 14, 2016.
3. Castro L, Chen X, Dimitrov NB, et al. The University of Texas at Austin. Texas Arbovirus Risk. 2015. Available at: http://hdl.handle.net/2152/31934. Accessed October 14, 2016.
4. Centers for Disease Control and Prevention. Zika virus: Zika is in your area: What to do. Available at: http://www.cdc.gov/zika/intheus/what-to-do.html. Accessed October 14, 2016.
5. FL KEYS NEWS. Available at: http://www.flkeysnews.com/opinion/opn-columns-blogs/article83328707.html. Accessed October 14, 2016.
6. Ernst KC, Haenchen S, Dickinson K, et al. Awareness and support of release of genetically modified “sterile” mosquitoes, Key West, Florida, USA. Emerg Infect Dis. 2015;21:320-324.
1. Centers for Disease Control and Prevention. Zika virus: Transmission & risks. Available at: http://www.cdc.gov/zika/transmission/index.html. Accessed October 14, 2016.
2. Centers for Disease Control and Prevention. Zika virus: Case counts in the US. Available at: http://www.cdc.gov/zika/geo/united-states.html. Accessed October 14, 2016.
3. Castro L, Chen X, Dimitrov NB, et al. The University of Texas at Austin. Texas Arbovirus Risk. 2015. Available at: http://hdl.handle.net/2152/31934. Accessed October 14, 2016.
4. Centers for Disease Control and Prevention. Zika virus: Zika is in your area: What to do. Available at: http://www.cdc.gov/zika/intheus/what-to-do.html. Accessed October 14, 2016.
5. FL KEYS NEWS. Available at: http://www.flkeysnews.com/opinion/opn-columns-blogs/article83328707.html. Accessed October 14, 2016.
6. Ernst KC, Haenchen S, Dickinson K, et al. Awareness and support of release of genetically modified “sterile” mosquitoes, Key West, Florida, USA. Emerg Infect Dis. 2015;21:320-324.
Persistent fever investigation saves patient's life
THE CASE
A 47-year-old African American woman was admitted to the hospital with pulmonary edema revealed on a computed tomography (CT) scan. She had a history of systemic lupus erythematosus (SLE), hypertension, and end-stage renal disease (ESRD). The patient had been hospitalized one month earlier for lupus nephritis with a hypertensive emergency that led to a seizure. During this earlier hospitalization, she was given a diagnosis of posterior reversible encephalopathy syndrome.
Two weeks into her more recent hospitalization, the patient developed a fever that was accompanied by cough and fatigue. By the third week, there was no identified cause of the fever, and the patient met the criteria for fever of unknown origin (FUO).
Her medications included cyclophosphamide, prednisone, nebivolol, clonidine, phenytoin, and epoetin alfa. The patient was also receiving dialysis every other day. Chest x-ray findings suggested pneumonia, and the patient was treated with vancomycin and piperacillin/tazobactam. However, her fever persisted after completing the antibiotics. Central line sepsis was high in the differential, as the patient was on dialysis, but blood and catheter tip cultures were negative. Chest and abdominal CT scans showed no new disease process. Urine and sputum cultures were collected and were negative for infection. Drug-induced fever was then suspected, but was ruled out when the fever persisted after the removal of potential offending agents (phenytoin, nebivolol, and cyclophosphamide).
THE DIAGNOSIS
We then followed the American Academy of Family Physicians’ diagnostic protocol for FUO.1
Initial labs included a complete blood count (CBC), 2 blood cultures, a urine culture, erythrocyte sedimentation rate (ESR), a purified protein derivative skin test, chest and abdominal CT scans, and double-stranded DNA (dsDNA) levels (since this patient had known SLE). The patient’s hemoglobin level and mean corpuscular volume were consistent with normocytic anemia, which was attributed to the ESRD. The ESR was mildly elevated at 46 mm/hr, but dsDNA was not, ruling out a lupus flare. Thrombocytopenia (platelet count, 82 K/mcL) and lymphocytopenia (absolute lymphocyte count, 0.2 K/mcL) were assumed to be secondary to cyclophosphamide use.
Because the initial labs were non-diagnostic, we proceeded with a sputum stain and culture, human immunodeficiency virus testing, a hepatitis panel, and a peripheral blood smear.1 All were negative except for the peripheral blood smear, which showed hemophagocytic cells. This was the first finding that brought hemophagocytic lymphohistiocytosis (HLH) into the differential.
We then performed a bone marrow biopsy (FIGURE), which also revealed hemophagocytic cells, so we ordered HLH-specific labs (more on those in a bit). Liver enzymes were elevated to 3 times their normal value. Triglycerides (414 mg/dL), ferritin (>15,000 ng/mL), and interleukin-2 (IL-2) receptor levels (>20,000 pg/m) were also elevated.
The patient was tested for herpes simplex virus, Epstein-Barr virus (EBV), and cytomegalovirus (CMV), since these viruses are associated with HLH. She had 3.1 million copies/mL of CMV, leading to the diagnosis of secondary HLH. This diagnosis might not have been made if not for a persistent fever investigation.
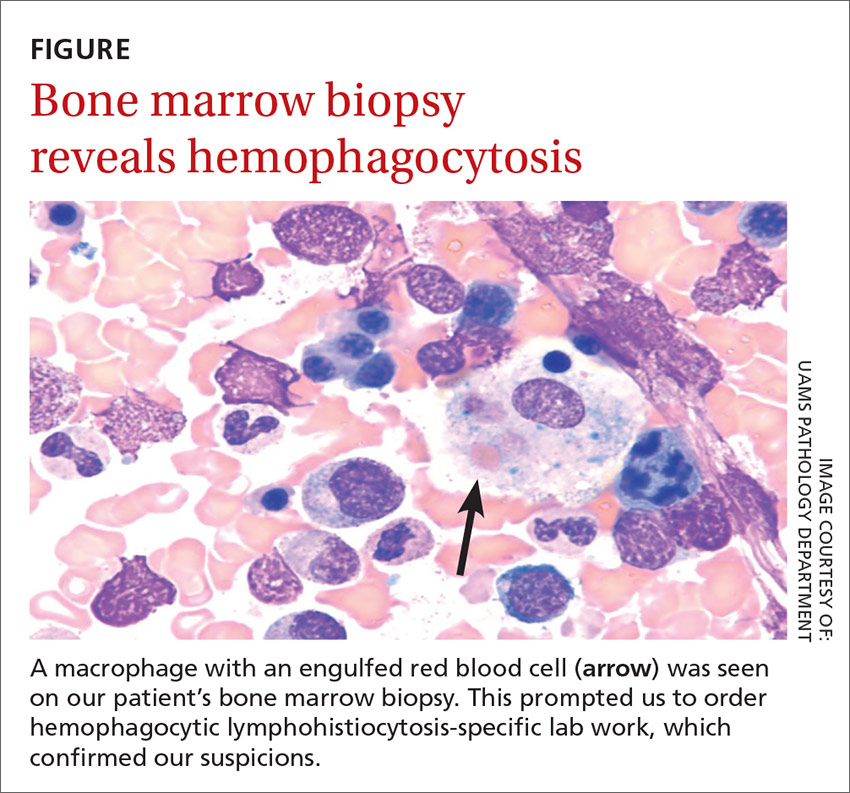
DISCUSSION
HLH is a life-threatening syndrome of excessive immune activation that results in tissue damage.2 There are primary and secondary forms, but they share the same mechanism of impaired regulation of cytotoxic granules and cytokines. Primary HLH results from a congenital gene mutation,3 while secondary HLH is triggered by an autoimmune or inflammatory disease or an infection.4 EBV is the most common viral etiology, followed closely by CMV.5
The diagnosis may be established genetically (based on mutations of the genes loci PRF1, UNC13D, or STX11) or by fulfillment of 5 out of 8 criteria: fever; splenomegaly; cytopenia; hypertriglyceridemia; hypofibrinogenemia; hemophagocytosis in the bone marrow, spleen, or lymph nodes; low or absent natural killer cell activity; and an elevated ferritin level (>500 ng/mL). Elevated soluble CD25 and IL-2 receptor markers are HLH-specific markers.3 This patient had fever, cytopenia, hypertriglyceridemia, hemophagocytosis, and elevated ferritin with elevated IL-2, meeting the criteria for secondary HLH.
First treat the underlying condition, then the HLH
Treatment for HLH includes treating the underlying condition (such as EBV or CMV) with antiretroviral medications, and using immunosuppressive agents such as chemotherapy drugs and steroids for the HLH.
Our patient was treated with valganciclovir 900 mg/d for 2 weeks for the CMV and an etoposide/prednisone taper for 3 months for HLH chemotherapy and suppression. Within one month, her CMV viral load decreased to <300 copies/mL and her fever resolved. Ferritin, triglycerides, and liver enzyme levels returned to normal within 3 months.
THE TAKEAWAY
FUO can be frustrating for both the physician and the patient. Not only is the differential large, but testing is extensive. It is important to get a thorough history and to consider medications as the cause. Testing should be patient-specific and systematic. Persistent investigation is critical to saving the patient’s life.
1. Roth AR, Basello GM. Approach to the adult patient with fever of unknown origin. Am Fam Physician. 2003;68:2223-2228.
2. Filipovich A, McClain K, Grom A. Histiocytic disorders: recentinsights into pathophysiology and practical guidelines. Biol Blood Marrow Transplant. 2010;16:S82-S89.
3. Larroche C. Hemophagocytic lymphohistiocytosis in adults: diagnosis and treatment. Joint Bone Spine. 2012;79:356-361.
4. Rouphael NG, Talati NJ, Vaughan C, et al. Infections associated with haemophagocytic syndrome. Lancet Infect Dis. 2007;7:814-822.
5. Janka GE, Lehmberg K. Hemophagocytic syndromes—an update. Blood Rev. 2014;28:135-142.
THE CASE
A 47-year-old African American woman was admitted to the hospital with pulmonary edema revealed on a computed tomography (CT) scan. She had a history of systemic lupus erythematosus (SLE), hypertension, and end-stage renal disease (ESRD). The patient had been hospitalized one month earlier for lupus nephritis with a hypertensive emergency that led to a seizure. During this earlier hospitalization, she was given a diagnosis of posterior reversible encephalopathy syndrome.
Two weeks into her more recent hospitalization, the patient developed a fever that was accompanied by cough and fatigue. By the third week, there was no identified cause of the fever, and the patient met the criteria for fever of unknown origin (FUO).
Her medications included cyclophosphamide, prednisone, nebivolol, clonidine, phenytoin, and epoetin alfa. The patient was also receiving dialysis every other day. Chest x-ray findings suggested pneumonia, and the patient was treated with vancomycin and piperacillin/tazobactam. However, her fever persisted after completing the antibiotics. Central line sepsis was high in the differential, as the patient was on dialysis, but blood and catheter tip cultures were negative. Chest and abdominal CT scans showed no new disease process. Urine and sputum cultures were collected and were negative for infection. Drug-induced fever was then suspected, but was ruled out when the fever persisted after the removal of potential offending agents (phenytoin, nebivolol, and cyclophosphamide).
THE DIAGNOSIS
We then followed the American Academy of Family Physicians’ diagnostic protocol for FUO.1
Initial labs included a complete blood count (CBC), 2 blood cultures, a urine culture, erythrocyte sedimentation rate (ESR), a purified protein derivative skin test, chest and abdominal CT scans, and double-stranded DNA (dsDNA) levels (since this patient had known SLE). The patient’s hemoglobin level and mean corpuscular volume were consistent with normocytic anemia, which was attributed to the ESRD. The ESR was mildly elevated at 46 mm/hr, but dsDNA was not, ruling out a lupus flare. Thrombocytopenia (platelet count, 82 K/mcL) and lymphocytopenia (absolute lymphocyte count, 0.2 K/mcL) were assumed to be secondary to cyclophosphamide use.
Because the initial labs were non-diagnostic, we proceeded with a sputum stain and culture, human immunodeficiency virus testing, a hepatitis panel, and a peripheral blood smear.1 All were negative except for the peripheral blood smear, which showed hemophagocytic cells. This was the first finding that brought hemophagocytic lymphohistiocytosis (HLH) into the differential.
We then performed a bone marrow biopsy (FIGURE), which also revealed hemophagocytic cells, so we ordered HLH-specific labs (more on those in a bit). Liver enzymes were elevated to 3 times their normal value. Triglycerides (414 mg/dL), ferritin (>15,000 ng/mL), and interleukin-2 (IL-2) receptor levels (>20,000 pg/m) were also elevated.
The patient was tested for herpes simplex virus, Epstein-Barr virus (EBV), and cytomegalovirus (CMV), since these viruses are associated with HLH. She had 3.1 million copies/mL of CMV, leading to the diagnosis of secondary HLH. This diagnosis might not have been made if not for a persistent fever investigation.

DISCUSSION
HLH is a life-threatening syndrome of excessive immune activation that results in tissue damage.2 There are primary and secondary forms, but they share the same mechanism of impaired regulation of cytotoxic granules and cytokines. Primary HLH results from a congenital gene mutation,3 while secondary HLH is triggered by an autoimmune or inflammatory disease or an infection.4 EBV is the most common viral etiology, followed closely by CMV.5
The diagnosis may be established genetically (based on mutations of the genes loci PRF1, UNC13D, or STX11) or by fulfillment of 5 out of 8 criteria: fever; splenomegaly; cytopenia; hypertriglyceridemia; hypofibrinogenemia; hemophagocytosis in the bone marrow, spleen, or lymph nodes; low or absent natural killer cell activity; and an elevated ferritin level (>500 ng/mL). Elevated soluble CD25 and IL-2 receptor markers are HLH-specific markers.3 This patient had fever, cytopenia, hypertriglyceridemia, hemophagocytosis, and elevated ferritin with elevated IL-2, meeting the criteria for secondary HLH.
First treat the underlying condition, then the HLH
Treatment for HLH includes treating the underlying condition (such as EBV or CMV) with antiretroviral medications, and using immunosuppressive agents such as chemotherapy drugs and steroids for the HLH.
Our patient was treated with valganciclovir 900 mg/d for 2 weeks for the CMV and an etoposide/prednisone taper for 3 months for HLH chemotherapy and suppression. Within one month, her CMV viral load decreased to <300 copies/mL and her fever resolved. Ferritin, triglycerides, and liver enzyme levels returned to normal within 3 months.
THE TAKEAWAY
FUO can be frustrating for both the physician and the patient. Not only is the differential large, but testing is extensive. It is important to get a thorough history and to consider medications as the cause. Testing should be patient-specific and systematic. Persistent investigation is critical to saving the patient’s life.
THE CASE
A 47-year-old African American woman was admitted to the hospital with pulmonary edema revealed on a computed tomography (CT) scan. She had a history of systemic lupus erythematosus (SLE), hypertension, and end-stage renal disease (ESRD). The patient had been hospitalized one month earlier for lupus nephritis with a hypertensive emergency that led to a seizure. During this earlier hospitalization, she was given a diagnosis of posterior reversible encephalopathy syndrome.
Two weeks into her more recent hospitalization, the patient developed a fever that was accompanied by cough and fatigue. By the third week, there was no identified cause of the fever, and the patient met the criteria for fever of unknown origin (FUO).
Her medications included cyclophosphamide, prednisone, nebivolol, clonidine, phenytoin, and epoetin alfa. The patient was also receiving dialysis every other day. Chest x-ray findings suggested pneumonia, and the patient was treated with vancomycin and piperacillin/tazobactam. However, her fever persisted after completing the antibiotics. Central line sepsis was high in the differential, as the patient was on dialysis, but blood and catheter tip cultures were negative. Chest and abdominal CT scans showed no new disease process. Urine and sputum cultures were collected and were negative for infection. Drug-induced fever was then suspected, but was ruled out when the fever persisted after the removal of potential offending agents (phenytoin, nebivolol, and cyclophosphamide).
THE DIAGNOSIS
We then followed the American Academy of Family Physicians’ diagnostic protocol for FUO.1
Initial labs included a complete blood count (CBC), 2 blood cultures, a urine culture, erythrocyte sedimentation rate (ESR), a purified protein derivative skin test, chest and abdominal CT scans, and double-stranded DNA (dsDNA) levels (since this patient had known SLE). The patient’s hemoglobin level and mean corpuscular volume were consistent with normocytic anemia, which was attributed to the ESRD. The ESR was mildly elevated at 46 mm/hr, but dsDNA was not, ruling out a lupus flare. Thrombocytopenia (platelet count, 82 K/mcL) and lymphocytopenia (absolute lymphocyte count, 0.2 K/mcL) were assumed to be secondary to cyclophosphamide use.
Because the initial labs were non-diagnostic, we proceeded with a sputum stain and culture, human immunodeficiency virus testing, a hepatitis panel, and a peripheral blood smear.1 All were negative except for the peripheral blood smear, which showed hemophagocytic cells. This was the first finding that brought hemophagocytic lymphohistiocytosis (HLH) into the differential.
We then performed a bone marrow biopsy (FIGURE), which also revealed hemophagocytic cells, so we ordered HLH-specific labs (more on those in a bit). Liver enzymes were elevated to 3 times their normal value. Triglycerides (414 mg/dL), ferritin (>15,000 ng/mL), and interleukin-2 (IL-2) receptor levels (>20,000 pg/m) were also elevated.
The patient was tested for herpes simplex virus, Epstein-Barr virus (EBV), and cytomegalovirus (CMV), since these viruses are associated with HLH. She had 3.1 million copies/mL of CMV, leading to the diagnosis of secondary HLH. This diagnosis might not have been made if not for a persistent fever investigation.

DISCUSSION
HLH is a life-threatening syndrome of excessive immune activation that results in tissue damage.2 There are primary and secondary forms, but they share the same mechanism of impaired regulation of cytotoxic granules and cytokines. Primary HLH results from a congenital gene mutation,3 while secondary HLH is triggered by an autoimmune or inflammatory disease or an infection.4 EBV is the most common viral etiology, followed closely by CMV.5
The diagnosis may be established genetically (based on mutations of the genes loci PRF1, UNC13D, or STX11) or by fulfillment of 5 out of 8 criteria: fever; splenomegaly; cytopenia; hypertriglyceridemia; hypofibrinogenemia; hemophagocytosis in the bone marrow, spleen, or lymph nodes; low or absent natural killer cell activity; and an elevated ferritin level (>500 ng/mL). Elevated soluble CD25 and IL-2 receptor markers are HLH-specific markers.3 This patient had fever, cytopenia, hypertriglyceridemia, hemophagocytosis, and elevated ferritin with elevated IL-2, meeting the criteria for secondary HLH.
First treat the underlying condition, then the HLH
Treatment for HLH includes treating the underlying condition (such as EBV or CMV) with antiretroviral medications, and using immunosuppressive agents such as chemotherapy drugs and steroids for the HLH.
Our patient was treated with valganciclovir 900 mg/d for 2 weeks for the CMV and an etoposide/prednisone taper for 3 months for HLH chemotherapy and suppression. Within one month, her CMV viral load decreased to <300 copies/mL and her fever resolved. Ferritin, triglycerides, and liver enzyme levels returned to normal within 3 months.
THE TAKEAWAY
FUO can be frustrating for both the physician and the patient. Not only is the differential large, but testing is extensive. It is important to get a thorough history and to consider medications as the cause. Testing should be patient-specific and systematic. Persistent investigation is critical to saving the patient’s life.
1. Roth AR, Basello GM. Approach to the adult patient with fever of unknown origin. Am Fam Physician. 2003;68:2223-2228.
2. Filipovich A, McClain K, Grom A. Histiocytic disorders: recentinsights into pathophysiology and practical guidelines. Biol Blood Marrow Transplant. 2010;16:S82-S89.
3. Larroche C. Hemophagocytic lymphohistiocytosis in adults: diagnosis and treatment. Joint Bone Spine. 2012;79:356-361.
4. Rouphael NG, Talati NJ, Vaughan C, et al. Infections associated with haemophagocytic syndrome. Lancet Infect Dis. 2007;7:814-822.
5. Janka GE, Lehmberg K. Hemophagocytic syndromes—an update. Blood Rev. 2014;28:135-142.
1. Roth AR, Basello GM. Approach to the adult patient with fever of unknown origin. Am Fam Physician. 2003;68:2223-2228.
2. Filipovich A, McClain K, Grom A. Histiocytic disorders: recentinsights into pathophysiology and practical guidelines. Biol Blood Marrow Transplant. 2010;16:S82-S89.
3. Larroche C. Hemophagocytic lymphohistiocytosis in adults: diagnosis and treatment. Joint Bone Spine. 2012;79:356-361.
4. Rouphael NG, Talati NJ, Vaughan C, et al. Infections associated with haemophagocytic syndrome. Lancet Infect Dis. 2007;7:814-822.
5. Janka GE, Lehmberg K. Hemophagocytic syndromes—an update. Blood Rev. 2014;28:135-142.
When can infants and children benefit from probiotics?
PRACTICE RECOMMENDATIONS
› Recommend a trial of Lactobacillus reuteri for breastfed infants with colic. A
› Consider Lactobacillus and Bifidobacterium species for the prevention of upper respiratory infections (URIs) and to shorten the course of URI illness. B
› Do not recommend probiotics for the prevention of respiratory or gastrointestinal allergies. A
› Consider probiotics for the reduction of abdominal pain in pediatric irritable bowel syndrome, as well as to reduce diarrhea associated with antibiotic use and acute gastroenteritis. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Ms. B, a 26-year-old woman, presents to your office with her 3-year-old son for a well-child examination. During the course of the conversation, she asks you if she should be giving her child probiotics to improve his general health. Many of her friends, who also have their children in day care, have told her that probiotics, “are nature’s way of fighting infection.” Her son currently takes no medications, and has no history of asthma or recent gastrointestinal disturbances. He was treated for 2 ear infections last winter, approximately 3 months apart. His physical exam is normal and, after today, his immunizations will be up to date. How should you respond?
The use of probiotics as over-the-counter treatments for a variety of conditions continues to grow, with retail sales of functional probiotic foods and supplements topping $35 billion worldwide in 2014.1 In children, claims of benefit for gastrointestinal (GI) disorders, colic, and allergy prevention, as well as prevention and treatment of upper respiratory infections (URIs) have existed for over 10 years.2-4 The human gut flora develops rapidly after birth and is known to be influenced by route of delivery (vaginal vs cesarean), type of feeding (breast vs formula), and other environmental factors.5 The use of probiotics to influence the types of bacteria in a child’s intestinal tract continues to be an area of active research. (For more on probiotic formulations, see TABLE 1.)
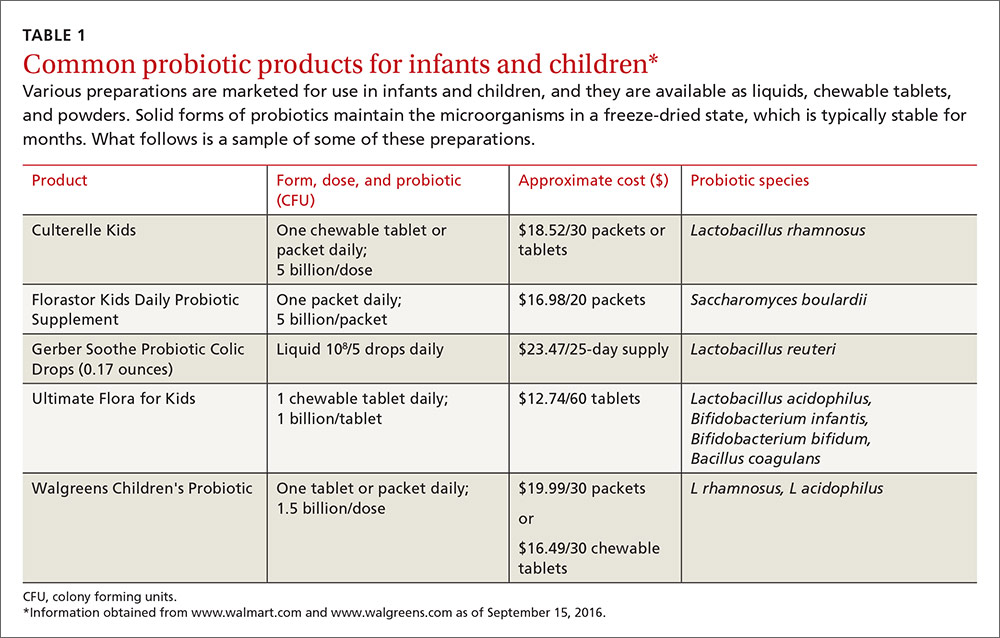
This article summarizes recent research on probiotic use in infants and children. New data support the use of probiotics for the treatment of colic and atopic eczema; however, the data on using probiotics in the management of URIs is less robust and mixed. And while probiotics improve irritable bowel syndrome (IBS) stomach pain, they do not help with related diarrhea or constipation. All of these data are summarized in TABLE 2.6-29
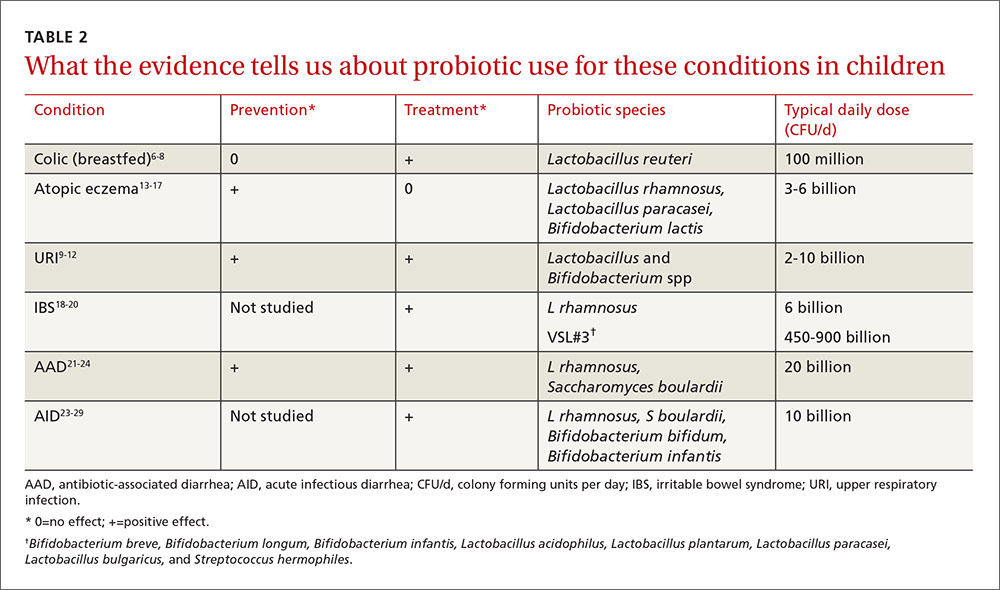
L reuteri improves symptoms in breastfed infants with colic
Infant colic is a relatively common condition known to negatively impact maternal mental health and the mother/child relationship.6 Numerous randomized controlled trials (RCTs) over the years have demonstrated mixed results with using probiotics to decrease crying times, with differences noted between infants who are solely breastfed and those who are not.7
In the most recent meta-analysis of 6 studies (n=427) that focused only on the probiotic Lactobacillus reuteri, breastfed infants with colic receiving a daily dose of 108 colony forming units (CFU) cried an average of 56 fewer minutes/day than those in the control group (95% confidence interval [CI], -64.4 to -47.3; P=.001) at day 21 of treatment.8 Although 2 studies in this meta-analysis included a small number of mixed-fed and formula-fed infants, the majority of trials do not show benefit for these infants. Trials assessing the use of L reuteri for prevention of colic have not shown positive results.7
Probiotics may help p
The mechanisms by which probiotics may prevent or shorten the course of URIs are not obvious. Current theories include boosting the immune function of the respiratory mucosa, acting as a competitive inhibitor for viruses, and secreting antiviral compounds.9 Multiple reviews published in the last 3 years, however, add to the evidence that the apparent benefit is real.
A 2013 meta-analysis assessed data from 4 RCTs (N=1805), which used Lactobacillus rhamnosus as the sole probiotic for prevention of URIs. In treated children, otitis media incidence was reduced by 24% (relative risk [RR] 0.76; 95% CI, 0.64-0.91) and risk of URI was reduced by 38% (RR 0.62; 95% CI, 0.50-0.78).10 The number needed to treat (NNT) was 4 for URI prevention, and the authors noted that adverse events were similar in the treatment and control groups.
A 2014 systematic review and meta-analysis of 20 RCTs examining duration of illness included 10 studies dedicated to pediatric subjects (age 12 months to 12 years).11 There were significantly fewer days of illness per person (standardized mean difference -0.31; 95% CI, -0.41 to -0.11) and each illness episode was shorter by three-quarters of a day (weighted mean difference -0.77; 95% CI, -1.5 to -0.04) in participants who received a probiotic vs those who received a placebo. Probiotics used in these studies belonged to the Lactobacillus and Bifidobacterium genera.
A 2015 systematic review of 14 RCTs assessing the benefits of probiotics, particularly Lactobacillus and Bifidobacterium strains, on URI occurrence and symptoms, showed mixed results.12 Seven of 12 studies found lowered rates of URI and otitis media incidence, 7 of 11 RCTs reported a significant reduction in severity scores for URI, and 4 of 8 RCTs reported significant reductions in school absenteeism between the probiotic and control groups. In a summary statement, the authors noted that “at least one beneficial effect of prophylactic probiotics was observed in the majority of RCTs,” and that “none of the studies reported any serious adverse events.”
Perinatal probiotics: No benefit for allergic conditions—except eczema
Allergic disease is on the rise and continues to plague children with reduced quality of life, potentially life-threatening reactions, and missed activities, including school. The gut microbiome likely influences a child’s allergic propensity through its effects on T-helper cells, transforming growth factor (TGF), and immunoglobulin A (IgA)—all known components of the allergic response. As the hygiene hypothesis suggests, the quantity and types of bacteria that inhabit the GI tract early in life play a significant role in determining a person’s later allergic responses.13
In a 2013 meta-analysis of 20 trials (N=4866), researchers looked specifically at probiotic use and the diagnosis of asthma and incident wheezing. Single and combination products of Lactobacillus and Bifidobacterium given prenatally and/or postnatally were included in the studies. The authors found no evidence to support a protective association between perinatal use of probiotics and diagnosed asthma (RR=0.99; 95% CI, 0.81-0.21) or childhood incident wheezing (RR=0.97; 95% CI, 0.87-1.09; 9 trials, 1949 infants).14
In a more recent meta-analysis (2015) conducted to inform the World Allergy Organization, 29 studies were evaluated to assess the impact of probiotics on allergic symptoms of the skin, respiratory system, and GI tract.15 No significant benefit was noted for any allergic condition except for eczema. Probiotics reduced the risk of eczema when given during the last trimester of pregnancy (RR=0.71; 95% CI, 0.60-0.84), when used by breastfeeding mothers (RR=0.57; 95% CI, 0.47-0.69), and when given to infants (RR=0.80; 95% CI, 0.68-0.94).
A 2014 systematic review and meta-analysis (N=2797) explored probiotic use specifically for the prevention of eczema.16 The pooled relative risk for all the studies was 0.74 (95% CI, 0.67-0.82). Evidence was strongest for probiotics containing the Lactobacillus species rhamnosus and paracasei, as well as for Bifidobacterium lactis. No benefit was noted with Lactobacillus acidophilus or other Bifidobacterium species. These newer reviews on eczema prevention contrast with an older Cochrane review published in 2008 (12 RCTs, N=781), which did not show significant benefit for the treatment of eczema.17
Probiotics improve IBS stomach pain, but not diarrhea or constipation
IBS is a functional disorder of the GI tract that affects up to 20% of children and teenagers and leads to a significant decrease in quality of life.18 Current theories of causation include bacterial overgrowth and neuronal hyperactivity, which may be amenable to change with supplemental probiotics.
A 2015 systematic review of non-pharmacological treatments for functional abdominal pain disorders identified 4 studies dedicated to IBS in children.19 A subgroup analysis of 3 RCTs (n=309) that looked at giving L rhamnosus to 5- to 17-year-olds with IBS showed improved abdominal pain (according to various pain scales) compared to the placebo group. Study participants received at least 3 x 109 CFU twice a day for 4 to 8 weeks. Relative risk for improvement was 1.7 (95% CI, 1.27-2.27) with an NNT of 4. None of these studies showed significant improvement in either frequency or severity of diarrhea or constipation.
A separate crossover RCT (N=59) compared placebo to VSL#3, a product containing 8 probiotics (Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, L acidophilus, Lactobacillus plantarum, L paracasei, Lactobacillus bulgaricus, and Streptococcus hermophiles), given in age-dependent doses for 6 weeks to children aged 4 to 18 years.20 The frequency and intensity of abdominal pain were measured on a 5-point Likert scale. The group treated with VSL#3 dropped 1.0 ± 0.2 points vs 0.5 ± 0.2 points in the control group (P<.05) and reported an improved quality of life.
These agents reduce antibiotic-associated diarrhea
Antibiotic-associated diarrhea (AAD) occurs in 5% to 30% of children who receive antibiotic therapy.21 It occurs most frequently with the use of cephalosporins, penicillin, fluoroquinolones, and clindamycin, and is likely caused by an alteration of the normal gut flora. Colitis caused by Clostridium difficile remains the most serious antibiotic-associated GI complication.
A systematic review of the specific probiotic Saccharomyces boulardii conducted in 2015 analyzed data from 6 RCTs (n=1653) to determine the effect of co-administration of this probiotic with antibiotics.22 The pooled relative risk for AAD in children receiving the probiotic was 0.43 (95% CI, 0.3-0.6) compared to antibiotics alone. The absolute risk of AAD dropped from 20.9% to 8.8%, translating to a NNT of 8. Two of the RCTs specifically looked at rates of C difficile infection (n=579). C difficile infection rates dropped by 75% (RR=.25; 95% CI, 0.08-0.73) in the treatment group. This dramatic treatment effect was not seen in studies involving adults.
A similar systematic review focusing on L rhamnosus conducted in 2015 pooled data from 5 RCTs (n=445) to see if the probiotic would decrease AAD in children if it was co-administered with antibiotics.23 The relative risk for AAD in this treatment group was 0.48 (95% CI, 0.26-0.89) with an absolute risk reduction of 13.4% (23% compared to 9.6%), translating to an NNT of 7.
A Cochrane review published in 2015 included 23 studies (N=3938) and found similar results with an RR for AAD of 0.46 for treated children (95% CI, 0.35-0.61).24 Doses of probiotics ranged from 5 to 40 billion CFU/day. Although many probiotic species were used in these studies, S boulardii and L rhamnosus were cited as having the strongest data to support use in this context.
Probiotics reduce the duration, frequency of acute infectious diarrhea
Diarrhea remains the second leading cause of death among children one to 59 months of age worldwide.25 Current World Health Organization recommendations include oral rehydration salts, continued feeding to avoid dehydration, and zinc to decrease the duration and severity of illness.26 Multiple studies in adults confirm that a variety of probiotics decrease both the duration and severity of diarrhea in acute gastroenteritis.27
The authors of a 2013 systematic review of probiotics for the treatment of community-acquired acute diarrhea in children less than 5 years of age analyzed data from 8 RCTs (N=1755).28 Various probiotics were used including Lactobacillus species, Streptococcus thermophilus, Bifidobacterium species, and Saccharomyces boulardii for between 4 and 10 days. Six of these studies (n=1164) measured diarrhea duration and found a 14% reduction (95% CI, 3.8%-24.2%) in days of illness for those children treated vs those receiving placebo. Five studies (n=925) measured the difference in stool frequency on Day 2 of illness and reported a reduction of 13.1% (95% CI, 0.8%-5.3%) in the number of stools in the treated group vs the placebo group.
This review augments a Cochrane meta-analysis of 63 studies (N=8014) published in 2010.27 Fifty-six of these studies included infants and children. Pooled analysis of the varied probiotic treatments showed a mean reduction in duration of diarrhea of just over a day (24.76 hours; 95% CI, 15.9-33.6 hours; n=4555, trials=35) and decreased stool frequency on Day 2 of treatment (mean difference 0.80; 95% CI, 0.45-1.14; n=2751, trials=20). The authors concluded that probiotics “have clear beneficial effects in shortening the duration and reducing stool frequency in acute infectious diarrhea.”
Pediatric society weighs in. In 2014, the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition issued guidelines regarding probiotic use for the treatment of acute gastroenteritis.29 In addition to rehydration therapy, these guidelines recommend the use of L rhamnosus and/or S boulardii as first-line treatments. Lower quality evidence is available for the use of L reuteri.
CASE › In response to Ms. B’s query about starting her young son on probiotics, you tell her that studies have shown that probiotics are safe for children when given in appropriate doses. They have been shown to help children recover from diarrheal illnesses and can help reduce the number of colds and ear infections when taken regularly. The reason you are giving them determines which strains you should use. You recommend giving her child a formulation of probiotic that contains Lactobacillus or Bifidobacterium with a dose range of 2 to 10 billion CFUs taken daily to reduce the risk of her child getting another ear infection.
CORRESPONDENCE
Paul Dassow, MD, MSPH, 1100 E. 3rd St, Chattanooga, TN 37403; [email protected].
1. Euromonitor International. Global and regional trends of the probiotics and omega fatty acids market. June 23, 2015. Available at: http://uschinahpa.org/wp-content/uploads/2015/07/EMI-US-China-HPA-Probiotic-and-Omega-2015-Final.pdf. Accessed September 9, 2016.
2. Du Toit G, Lack G. Can food allergy be prevented? The current evidence. Pediatr Clin North Am. 2011;58:481-509.
3. Gerritsen J, Smidt H, Rijkers GT, et al. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. 2011;6:209-240.
4. Versalovic J. The human microbiome and probiotics: implications for pediatrics. Ann Nutr Metab. 2013;63:42-52.
5. Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
6. Akman I, Kușçu K, Özdemir N, et al. Mothers’ postpartum psychological adjustment and infantile colic. Arch Dis Child. 2006;91:417-419.
7. Sung V, Collett S, de Gooyer T, et al. Probiotics to prevent or treat excessive infant crying systematic review and meta-analysis. JAMA Pediatr. 2013:167:1150-1157.
8. Harb T, Matsuyama M, David M, et al. Infant colic—what works: a systematic review of interventions for breastfed infants. J Pediatr Gastroenterol Nutr. 2016;62:668-686.
9. Hill C, Guarner F, Reid G, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506-514.
10. Liu S, Hu P, Du X, et al. Lactobacillus rhamnosus GG supplementation for preventing respiratory infections in children: a meta-analysis of randomized, placebo-controlled trials. Indian Pediatr. 2013;50:377-381.
11. King S, Glanville J, Sanders ME, et al. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112:41-54.
12. Ozen M, Kocabas Sandal G, Dinleyici EC. Probiotics for the prevention of pediatric upper respiratory tract infections: a systematic review. Expert Opin Biol Ther. 2015;15:9-20.
13. Azad MB, Konya T, Maughan H, et al. Infant gut microbiota and the hygiene hypothesis of allergic disease: impact of household pets and siblings on microbiota composition and diversity. Allergy Asthma Clin Immunol. 2013;9:15.
14. Azad MB, Coneys JG, Kozyrskyj AL, et al. Probiotic supplementation during pregnancy or infancy for the prevention of asthma and wheeze: systematic review and meta-analysis. Brit Med J. 2013;347:f6471.
15. Cuello-Garcia CA, Bro˙zek JL, Fiocchi A, et al. Probiotics for the prevention of allergy: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2015;136:952-961.
16. Mansfield JA, Bergin SW, Cooper JR, et al. Comparative probiotic strain efficacy in the prevention of eczema in infants and children: a systematic review and meta-analysis. Mil Med. 2014;179:580-592.
17. Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2008;(4):CD006135.
18. Chiou E, Nurko S. Management of functional abdominal pain and irritable bowel syndrome in children and adolescents. Expert Rev Gastroenterol Hepatol. 2010;4:293-304.
19. Rutten JMTM, Korterink JL, Venmans LMAJ, et al. Nonpharmacologic treatment of functional abdominal pain disorders: a systematic review. Pediatrics. 2015;135:522-535.
20. Guandalini S, Magazzù G, Chiaro A, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr. 2010;51:24-30.
21. Turck D, Bernet JP, Marx J, et al. Incidence and risk factors of oral antibiotic associated diarrhea in an outpatient pediatric population. J Pediatr Gastroenterol Nutr. 2003;37:22-26.
22. Szajewska H, Kołodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015;42:793-801.
23. Szajewska H, Kołodziej M. Systematic review with meta-analysis: Lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults. Aliment Pharmacol Ther. 2015;42:1149-1157.
24. Goldenberg JZ, Lytvyn L, Steurich J, et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2015;12:CD004827.
25. Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151-2161.
26. WHO/UNICEF Joint Statement: Clinical Management of Acute Diarrhea. August 2004. Available at: http://www.unicef.org/publications/files/ENAcute_Diarrhoea_reprint.pdf. Accessed September 9, 2016.
27. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhea. Cochrane Database Syst Rev. 2010;(11):CD003048.
28. Applegate JA, Fischer Walker CL, Ambikapathi R, et al. Systematic review of probiotics for the treatment of community-acquired acute diarrhea in children. BMC Public Health. 2013;13:S16.
29. Guarino A, Ashkenazi S, Gendrel D, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr. 2014;59:132-152.
PRACTICE RECOMMENDATIONS
› Recommend a trial of Lactobacillus reuteri for breastfed infants with colic. A
› Consider Lactobacillus and Bifidobacterium species for the prevention of upper respiratory infections (URIs) and to shorten the course of URI illness. B
› Do not recommend probiotics for the prevention of respiratory or gastrointestinal allergies. A
› Consider probiotics for the reduction of abdominal pain in pediatric irritable bowel syndrome, as well as to reduce diarrhea associated with antibiotic use and acute gastroenteritis. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Ms. B, a 26-year-old woman, presents to your office with her 3-year-old son for a well-child examination. During the course of the conversation, she asks you if she should be giving her child probiotics to improve his general health. Many of her friends, who also have their children in day care, have told her that probiotics, “are nature’s way of fighting infection.” Her son currently takes no medications, and has no history of asthma or recent gastrointestinal disturbances. He was treated for 2 ear infections last winter, approximately 3 months apart. His physical exam is normal and, after today, his immunizations will be up to date. How should you respond?
The use of probiotics as over-the-counter treatments for a variety of conditions continues to grow, with retail sales of functional probiotic foods and supplements topping $35 billion worldwide in 2014.1 In children, claims of benefit for gastrointestinal (GI) disorders, colic, and allergy prevention, as well as prevention and treatment of upper respiratory infections (URIs) have existed for over 10 years.2-4 The human gut flora develops rapidly after birth and is known to be influenced by route of delivery (vaginal vs cesarean), type of feeding (breast vs formula), and other environmental factors.5 The use of probiotics to influence the types of bacteria in a child’s intestinal tract continues to be an area of active research. (For more on probiotic formulations, see TABLE 1.)

This article summarizes recent research on probiotic use in infants and children. New data support the use of probiotics for the treatment of colic and atopic eczema; however, the data on using probiotics in the management of URIs is less robust and mixed. And while probiotics improve irritable bowel syndrome (IBS) stomach pain, they do not help with related diarrhea or constipation. All of these data are summarized in TABLE 2.6-29

L reuteri improves symptoms in breastfed infants with colic
Infant colic is a relatively common condition known to negatively impact maternal mental health and the mother/child relationship.6 Numerous randomized controlled trials (RCTs) over the years have demonstrated mixed results with using probiotics to decrease crying times, with differences noted between infants who are solely breastfed and those who are not.7
In the most recent meta-analysis of 6 studies (n=427) that focused only on the probiotic Lactobacillus reuteri, breastfed infants with colic receiving a daily dose of 108 colony forming units (CFU) cried an average of 56 fewer minutes/day than those in the control group (95% confidence interval [CI], -64.4 to -47.3; P=.001) at day 21 of treatment.8 Although 2 studies in this meta-analysis included a small number of mixed-fed and formula-fed infants, the majority of trials do not show benefit for these infants. Trials assessing the use of L reuteri for prevention of colic have not shown positive results.7
Probiotics may help p
The mechanisms by which probiotics may prevent or shorten the course of URIs are not obvious. Current theories include boosting the immune function of the respiratory mucosa, acting as a competitive inhibitor for viruses, and secreting antiviral compounds.9 Multiple reviews published in the last 3 years, however, add to the evidence that the apparent benefit is real.
A 2013 meta-analysis assessed data from 4 RCTs (N=1805), which used Lactobacillus rhamnosus as the sole probiotic for prevention of URIs. In treated children, otitis media incidence was reduced by 24% (relative risk [RR] 0.76; 95% CI, 0.64-0.91) and risk of URI was reduced by 38% (RR 0.62; 95% CI, 0.50-0.78).10 The number needed to treat (NNT) was 4 for URI prevention, and the authors noted that adverse events were similar in the treatment and control groups.
A 2014 systematic review and meta-analysis of 20 RCTs examining duration of illness included 10 studies dedicated to pediatric subjects (age 12 months to 12 years).11 There were significantly fewer days of illness per person (standardized mean difference -0.31; 95% CI, -0.41 to -0.11) and each illness episode was shorter by three-quarters of a day (weighted mean difference -0.77; 95% CI, -1.5 to -0.04) in participants who received a probiotic vs those who received a placebo. Probiotics used in these studies belonged to the Lactobacillus and Bifidobacterium genera.
A 2015 systematic review of 14 RCTs assessing the benefits of probiotics, particularly Lactobacillus and Bifidobacterium strains, on URI occurrence and symptoms, showed mixed results.12 Seven of 12 studies found lowered rates of URI and otitis media incidence, 7 of 11 RCTs reported a significant reduction in severity scores for URI, and 4 of 8 RCTs reported significant reductions in school absenteeism between the probiotic and control groups. In a summary statement, the authors noted that “at least one beneficial effect of prophylactic probiotics was observed in the majority of RCTs,” and that “none of the studies reported any serious adverse events.”
Perinatal probiotics: No benefit for allergic conditions—except eczema
Allergic disease is on the rise and continues to plague children with reduced quality of life, potentially life-threatening reactions, and missed activities, including school. The gut microbiome likely influences a child’s allergic propensity through its effects on T-helper cells, transforming growth factor (TGF), and immunoglobulin A (IgA)—all known components of the allergic response. As the hygiene hypothesis suggests, the quantity and types of bacteria that inhabit the GI tract early in life play a significant role in determining a person’s later allergic responses.13
In a 2013 meta-analysis of 20 trials (N=4866), researchers looked specifically at probiotic use and the diagnosis of asthma and incident wheezing. Single and combination products of Lactobacillus and Bifidobacterium given prenatally and/or postnatally were included in the studies. The authors found no evidence to support a protective association between perinatal use of probiotics and diagnosed asthma (RR=0.99; 95% CI, 0.81-0.21) or childhood incident wheezing (RR=0.97; 95% CI, 0.87-1.09; 9 trials, 1949 infants).14
In a more recent meta-analysis (2015) conducted to inform the World Allergy Organization, 29 studies were evaluated to assess the impact of probiotics on allergic symptoms of the skin, respiratory system, and GI tract.15 No significant benefit was noted for any allergic condition except for eczema. Probiotics reduced the risk of eczema when given during the last trimester of pregnancy (RR=0.71; 95% CI, 0.60-0.84), when used by breastfeeding mothers (RR=0.57; 95% CI, 0.47-0.69), and when given to infants (RR=0.80; 95% CI, 0.68-0.94).
A 2014 systematic review and meta-analysis (N=2797) explored probiotic use specifically for the prevention of eczema.16 The pooled relative risk for all the studies was 0.74 (95% CI, 0.67-0.82). Evidence was strongest for probiotics containing the Lactobacillus species rhamnosus and paracasei, as well as for Bifidobacterium lactis. No benefit was noted with Lactobacillus acidophilus or other Bifidobacterium species. These newer reviews on eczema prevention contrast with an older Cochrane review published in 2008 (12 RCTs, N=781), which did not show significant benefit for the treatment of eczema.17
Probiotics improve IBS stomach pain, but not diarrhea or constipation
IBS is a functional disorder of the GI tract that affects up to 20% of children and teenagers and leads to a significant decrease in quality of life.18 Current theories of causation include bacterial overgrowth and neuronal hyperactivity, which may be amenable to change with supplemental probiotics.
A 2015 systematic review of non-pharmacological treatments for functional abdominal pain disorders identified 4 studies dedicated to IBS in children.19 A subgroup analysis of 3 RCTs (n=309) that looked at giving L rhamnosus to 5- to 17-year-olds with IBS showed improved abdominal pain (according to various pain scales) compared to the placebo group. Study participants received at least 3 x 109 CFU twice a day for 4 to 8 weeks. Relative risk for improvement was 1.7 (95% CI, 1.27-2.27) with an NNT of 4. None of these studies showed significant improvement in either frequency or severity of diarrhea or constipation.
A separate crossover RCT (N=59) compared placebo to VSL#3, a product containing 8 probiotics (Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, L acidophilus, Lactobacillus plantarum, L paracasei, Lactobacillus bulgaricus, and Streptococcus hermophiles), given in age-dependent doses for 6 weeks to children aged 4 to 18 years.20 The frequency and intensity of abdominal pain were measured on a 5-point Likert scale. The group treated with VSL#3 dropped 1.0 ± 0.2 points vs 0.5 ± 0.2 points in the control group (P<.05) and reported an improved quality of life.
These agents reduce antibiotic-associated diarrhea
Antibiotic-associated diarrhea (AAD) occurs in 5% to 30% of children who receive antibiotic therapy.21 It occurs most frequently with the use of cephalosporins, penicillin, fluoroquinolones, and clindamycin, and is likely caused by an alteration of the normal gut flora. Colitis caused by Clostridium difficile remains the most serious antibiotic-associated GI complication.
A systematic review of the specific probiotic Saccharomyces boulardii conducted in 2015 analyzed data from 6 RCTs (n=1653) to determine the effect of co-administration of this probiotic with antibiotics.22 The pooled relative risk for AAD in children receiving the probiotic was 0.43 (95% CI, 0.3-0.6) compared to antibiotics alone. The absolute risk of AAD dropped from 20.9% to 8.8%, translating to a NNT of 8. Two of the RCTs specifically looked at rates of C difficile infection (n=579). C difficile infection rates dropped by 75% (RR=.25; 95% CI, 0.08-0.73) in the treatment group. This dramatic treatment effect was not seen in studies involving adults.
A similar systematic review focusing on L rhamnosus conducted in 2015 pooled data from 5 RCTs (n=445) to see if the probiotic would decrease AAD in children if it was co-administered with antibiotics.23 The relative risk for AAD in this treatment group was 0.48 (95% CI, 0.26-0.89) with an absolute risk reduction of 13.4% (23% compared to 9.6%), translating to an NNT of 7.
A Cochrane review published in 2015 included 23 studies (N=3938) and found similar results with an RR for AAD of 0.46 for treated children (95% CI, 0.35-0.61).24 Doses of probiotics ranged from 5 to 40 billion CFU/day. Although many probiotic species were used in these studies, S boulardii and L rhamnosus were cited as having the strongest data to support use in this context.
Probiotics reduce the duration, frequency of acute infectious diarrhea
Diarrhea remains the second leading cause of death among children one to 59 months of age worldwide.25 Current World Health Organization recommendations include oral rehydration salts, continued feeding to avoid dehydration, and zinc to decrease the duration and severity of illness.26 Multiple studies in adults confirm that a variety of probiotics decrease both the duration and severity of diarrhea in acute gastroenteritis.27
The authors of a 2013 systematic review of probiotics for the treatment of community-acquired acute diarrhea in children less than 5 years of age analyzed data from 8 RCTs (N=1755).28 Various probiotics were used including Lactobacillus species, Streptococcus thermophilus, Bifidobacterium species, and Saccharomyces boulardii for between 4 and 10 days. Six of these studies (n=1164) measured diarrhea duration and found a 14% reduction (95% CI, 3.8%-24.2%) in days of illness for those children treated vs those receiving placebo. Five studies (n=925) measured the difference in stool frequency on Day 2 of illness and reported a reduction of 13.1% (95% CI, 0.8%-5.3%) in the number of stools in the treated group vs the placebo group.
This review augments a Cochrane meta-analysis of 63 studies (N=8014) published in 2010.27 Fifty-six of these studies included infants and children. Pooled analysis of the varied probiotic treatments showed a mean reduction in duration of diarrhea of just over a day (24.76 hours; 95% CI, 15.9-33.6 hours; n=4555, trials=35) and decreased stool frequency on Day 2 of treatment (mean difference 0.80; 95% CI, 0.45-1.14; n=2751, trials=20). The authors concluded that probiotics “have clear beneficial effects in shortening the duration and reducing stool frequency in acute infectious diarrhea.”
Pediatric society weighs in. In 2014, the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition issued guidelines regarding probiotic use for the treatment of acute gastroenteritis.29 In addition to rehydration therapy, these guidelines recommend the use of L rhamnosus and/or S boulardii as first-line treatments. Lower quality evidence is available for the use of L reuteri.
CASE › In response to Ms. B’s query about starting her young son on probiotics, you tell her that studies have shown that probiotics are safe for children when given in appropriate doses. They have been shown to help children recover from diarrheal illnesses and can help reduce the number of colds and ear infections when taken regularly. The reason you are giving them determines which strains you should use. You recommend giving her child a formulation of probiotic that contains Lactobacillus or Bifidobacterium with a dose range of 2 to 10 billion CFUs taken daily to reduce the risk of her child getting another ear infection.
CORRESPONDENCE
Paul Dassow, MD, MSPH, 1100 E. 3rd St, Chattanooga, TN 37403; [email protected].
PRACTICE RECOMMENDATIONS
› Recommend a trial of Lactobacillus reuteri for breastfed infants with colic. A
› Consider Lactobacillus and Bifidobacterium species for the prevention of upper respiratory infections (URIs) and to shorten the course of URI illness. B
› Do not recommend probiotics for the prevention of respiratory or gastrointestinal allergies. A
› Consider probiotics for the reduction of abdominal pain in pediatric irritable bowel syndrome, as well as to reduce diarrhea associated with antibiotic use and acute gastroenteritis. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Ms. B, a 26-year-old woman, presents to your office with her 3-year-old son for a well-child examination. During the course of the conversation, she asks you if she should be giving her child probiotics to improve his general health. Many of her friends, who also have their children in day care, have told her that probiotics, “are nature’s way of fighting infection.” Her son currently takes no medications, and has no history of asthma or recent gastrointestinal disturbances. He was treated for 2 ear infections last winter, approximately 3 months apart. His physical exam is normal and, after today, his immunizations will be up to date. How should you respond?
The use of probiotics as over-the-counter treatments for a variety of conditions continues to grow, with retail sales of functional probiotic foods and supplements topping $35 billion worldwide in 2014.1 In children, claims of benefit for gastrointestinal (GI) disorders, colic, and allergy prevention, as well as prevention and treatment of upper respiratory infections (URIs) have existed for over 10 years.2-4 The human gut flora develops rapidly after birth and is known to be influenced by route of delivery (vaginal vs cesarean), type of feeding (breast vs formula), and other environmental factors.5 The use of probiotics to influence the types of bacteria in a child’s intestinal tract continues to be an area of active research. (For more on probiotic formulations, see TABLE 1.)

This article summarizes recent research on probiotic use in infants and children. New data support the use of probiotics for the treatment of colic and atopic eczema; however, the data on using probiotics in the management of URIs is less robust and mixed. And while probiotics improve irritable bowel syndrome (IBS) stomach pain, they do not help with related diarrhea or constipation. All of these data are summarized in TABLE 2.6-29

L reuteri improves symptoms in breastfed infants with colic
Infant colic is a relatively common condition known to negatively impact maternal mental health and the mother/child relationship.6 Numerous randomized controlled trials (RCTs) over the years have demonstrated mixed results with using probiotics to decrease crying times, with differences noted between infants who are solely breastfed and those who are not.7
In the most recent meta-analysis of 6 studies (n=427) that focused only on the probiotic Lactobacillus reuteri, breastfed infants with colic receiving a daily dose of 108 colony forming units (CFU) cried an average of 56 fewer minutes/day than those in the control group (95% confidence interval [CI], -64.4 to -47.3; P=.001) at day 21 of treatment.8 Although 2 studies in this meta-analysis included a small number of mixed-fed and formula-fed infants, the majority of trials do not show benefit for these infants. Trials assessing the use of L reuteri for prevention of colic have not shown positive results.7
Probiotics may help p
The mechanisms by which probiotics may prevent or shorten the course of URIs are not obvious. Current theories include boosting the immune function of the respiratory mucosa, acting as a competitive inhibitor for viruses, and secreting antiviral compounds.9 Multiple reviews published in the last 3 years, however, add to the evidence that the apparent benefit is real.
A 2013 meta-analysis assessed data from 4 RCTs (N=1805), which used Lactobacillus rhamnosus as the sole probiotic for prevention of URIs. In treated children, otitis media incidence was reduced by 24% (relative risk [RR] 0.76; 95% CI, 0.64-0.91) and risk of URI was reduced by 38% (RR 0.62; 95% CI, 0.50-0.78).10 The number needed to treat (NNT) was 4 for URI prevention, and the authors noted that adverse events were similar in the treatment and control groups.
A 2014 systematic review and meta-analysis of 20 RCTs examining duration of illness included 10 studies dedicated to pediatric subjects (age 12 months to 12 years).11 There were significantly fewer days of illness per person (standardized mean difference -0.31; 95% CI, -0.41 to -0.11) and each illness episode was shorter by three-quarters of a day (weighted mean difference -0.77; 95% CI, -1.5 to -0.04) in participants who received a probiotic vs those who received a placebo. Probiotics used in these studies belonged to the Lactobacillus and Bifidobacterium genera.
A 2015 systematic review of 14 RCTs assessing the benefits of probiotics, particularly Lactobacillus and Bifidobacterium strains, on URI occurrence and symptoms, showed mixed results.12 Seven of 12 studies found lowered rates of URI and otitis media incidence, 7 of 11 RCTs reported a significant reduction in severity scores for URI, and 4 of 8 RCTs reported significant reductions in school absenteeism between the probiotic and control groups. In a summary statement, the authors noted that “at least one beneficial effect of prophylactic probiotics was observed in the majority of RCTs,” and that “none of the studies reported any serious adverse events.”
Perinatal probiotics: No benefit for allergic conditions—except eczema
Allergic disease is on the rise and continues to plague children with reduced quality of life, potentially life-threatening reactions, and missed activities, including school. The gut microbiome likely influences a child’s allergic propensity through its effects on T-helper cells, transforming growth factor (TGF), and immunoglobulin A (IgA)—all known components of the allergic response. As the hygiene hypothesis suggests, the quantity and types of bacteria that inhabit the GI tract early in life play a significant role in determining a person’s later allergic responses.13
In a 2013 meta-analysis of 20 trials (N=4866), researchers looked specifically at probiotic use and the diagnosis of asthma and incident wheezing. Single and combination products of Lactobacillus and Bifidobacterium given prenatally and/or postnatally were included in the studies. The authors found no evidence to support a protective association between perinatal use of probiotics and diagnosed asthma (RR=0.99; 95% CI, 0.81-0.21) or childhood incident wheezing (RR=0.97; 95% CI, 0.87-1.09; 9 trials, 1949 infants).14
In a more recent meta-analysis (2015) conducted to inform the World Allergy Organization, 29 studies were evaluated to assess the impact of probiotics on allergic symptoms of the skin, respiratory system, and GI tract.15 No significant benefit was noted for any allergic condition except for eczema. Probiotics reduced the risk of eczema when given during the last trimester of pregnancy (RR=0.71; 95% CI, 0.60-0.84), when used by breastfeeding mothers (RR=0.57; 95% CI, 0.47-0.69), and when given to infants (RR=0.80; 95% CI, 0.68-0.94).
A 2014 systematic review and meta-analysis (N=2797) explored probiotic use specifically for the prevention of eczema.16 The pooled relative risk for all the studies was 0.74 (95% CI, 0.67-0.82). Evidence was strongest for probiotics containing the Lactobacillus species rhamnosus and paracasei, as well as for Bifidobacterium lactis. No benefit was noted with Lactobacillus acidophilus or other Bifidobacterium species. These newer reviews on eczema prevention contrast with an older Cochrane review published in 2008 (12 RCTs, N=781), which did not show significant benefit for the treatment of eczema.17
Probiotics improve IBS stomach pain, but not diarrhea or constipation
IBS is a functional disorder of the GI tract that affects up to 20% of children and teenagers and leads to a significant decrease in quality of life.18 Current theories of causation include bacterial overgrowth and neuronal hyperactivity, which may be amenable to change with supplemental probiotics.
A 2015 systematic review of non-pharmacological treatments for functional abdominal pain disorders identified 4 studies dedicated to IBS in children.19 A subgroup analysis of 3 RCTs (n=309) that looked at giving L rhamnosus to 5- to 17-year-olds with IBS showed improved abdominal pain (according to various pain scales) compared to the placebo group. Study participants received at least 3 x 109 CFU twice a day for 4 to 8 weeks. Relative risk for improvement was 1.7 (95% CI, 1.27-2.27) with an NNT of 4. None of these studies showed significant improvement in either frequency or severity of diarrhea or constipation.
A separate crossover RCT (N=59) compared placebo to VSL#3, a product containing 8 probiotics (Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, L acidophilus, Lactobacillus plantarum, L paracasei, Lactobacillus bulgaricus, and Streptococcus hermophiles), given in age-dependent doses for 6 weeks to children aged 4 to 18 years.20 The frequency and intensity of abdominal pain were measured on a 5-point Likert scale. The group treated with VSL#3 dropped 1.0 ± 0.2 points vs 0.5 ± 0.2 points in the control group (P<.05) and reported an improved quality of life.
These agents reduce antibiotic-associated diarrhea
Antibiotic-associated diarrhea (AAD) occurs in 5% to 30% of children who receive antibiotic therapy.21 It occurs most frequently with the use of cephalosporins, penicillin, fluoroquinolones, and clindamycin, and is likely caused by an alteration of the normal gut flora. Colitis caused by Clostridium difficile remains the most serious antibiotic-associated GI complication.
A systematic review of the specific probiotic Saccharomyces boulardii conducted in 2015 analyzed data from 6 RCTs (n=1653) to determine the effect of co-administration of this probiotic with antibiotics.22 The pooled relative risk for AAD in children receiving the probiotic was 0.43 (95% CI, 0.3-0.6) compared to antibiotics alone. The absolute risk of AAD dropped from 20.9% to 8.8%, translating to a NNT of 8. Two of the RCTs specifically looked at rates of C difficile infection (n=579). C difficile infection rates dropped by 75% (RR=.25; 95% CI, 0.08-0.73) in the treatment group. This dramatic treatment effect was not seen in studies involving adults.
A similar systematic review focusing on L rhamnosus conducted in 2015 pooled data from 5 RCTs (n=445) to see if the probiotic would decrease AAD in children if it was co-administered with antibiotics.23 The relative risk for AAD in this treatment group was 0.48 (95% CI, 0.26-0.89) with an absolute risk reduction of 13.4% (23% compared to 9.6%), translating to an NNT of 7.
A Cochrane review published in 2015 included 23 studies (N=3938) and found similar results with an RR for AAD of 0.46 for treated children (95% CI, 0.35-0.61).24 Doses of probiotics ranged from 5 to 40 billion CFU/day. Although many probiotic species were used in these studies, S boulardii and L rhamnosus were cited as having the strongest data to support use in this context.
Probiotics reduce the duration, frequency of acute infectious diarrhea
Diarrhea remains the second leading cause of death among children one to 59 months of age worldwide.25 Current World Health Organization recommendations include oral rehydration salts, continued feeding to avoid dehydration, and zinc to decrease the duration and severity of illness.26 Multiple studies in adults confirm that a variety of probiotics decrease both the duration and severity of diarrhea in acute gastroenteritis.27
The authors of a 2013 systematic review of probiotics for the treatment of community-acquired acute diarrhea in children less than 5 years of age analyzed data from 8 RCTs (N=1755).28 Various probiotics were used including Lactobacillus species, Streptococcus thermophilus, Bifidobacterium species, and Saccharomyces boulardii for between 4 and 10 days. Six of these studies (n=1164) measured diarrhea duration and found a 14% reduction (95% CI, 3.8%-24.2%) in days of illness for those children treated vs those receiving placebo. Five studies (n=925) measured the difference in stool frequency on Day 2 of illness and reported a reduction of 13.1% (95% CI, 0.8%-5.3%) in the number of stools in the treated group vs the placebo group.
This review augments a Cochrane meta-analysis of 63 studies (N=8014) published in 2010.27 Fifty-six of these studies included infants and children. Pooled analysis of the varied probiotic treatments showed a mean reduction in duration of diarrhea of just over a day (24.76 hours; 95% CI, 15.9-33.6 hours; n=4555, trials=35) and decreased stool frequency on Day 2 of treatment (mean difference 0.80; 95% CI, 0.45-1.14; n=2751, trials=20). The authors concluded that probiotics “have clear beneficial effects in shortening the duration and reducing stool frequency in acute infectious diarrhea.”
Pediatric society weighs in. In 2014, the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition issued guidelines regarding probiotic use for the treatment of acute gastroenteritis.29 In addition to rehydration therapy, these guidelines recommend the use of L rhamnosus and/or S boulardii as first-line treatments. Lower quality evidence is available for the use of L reuteri.
CASE › In response to Ms. B’s query about starting her young son on probiotics, you tell her that studies have shown that probiotics are safe for children when given in appropriate doses. They have been shown to help children recover from diarrheal illnesses and can help reduce the number of colds and ear infections when taken regularly. The reason you are giving them determines which strains you should use. You recommend giving her child a formulation of probiotic that contains Lactobacillus or Bifidobacterium with a dose range of 2 to 10 billion CFUs taken daily to reduce the risk of her child getting another ear infection.
CORRESPONDENCE
Paul Dassow, MD, MSPH, 1100 E. 3rd St, Chattanooga, TN 37403; [email protected].
1. Euromonitor International. Global and regional trends of the probiotics and omega fatty acids market. June 23, 2015. Available at: http://uschinahpa.org/wp-content/uploads/2015/07/EMI-US-China-HPA-Probiotic-and-Omega-2015-Final.pdf. Accessed September 9, 2016.
2. Du Toit G, Lack G. Can food allergy be prevented? The current evidence. Pediatr Clin North Am. 2011;58:481-509.
3. Gerritsen J, Smidt H, Rijkers GT, et al. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. 2011;6:209-240.
4. Versalovic J. The human microbiome and probiotics: implications for pediatrics. Ann Nutr Metab. 2013;63:42-52.
5. Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
6. Akman I, Kușçu K, Özdemir N, et al. Mothers’ postpartum psychological adjustment and infantile colic. Arch Dis Child. 2006;91:417-419.
7. Sung V, Collett S, de Gooyer T, et al. Probiotics to prevent or treat excessive infant crying systematic review and meta-analysis. JAMA Pediatr. 2013:167:1150-1157.
8. Harb T, Matsuyama M, David M, et al. Infant colic—what works: a systematic review of interventions for breastfed infants. J Pediatr Gastroenterol Nutr. 2016;62:668-686.
9. Hill C, Guarner F, Reid G, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506-514.
10. Liu S, Hu P, Du X, et al. Lactobacillus rhamnosus GG supplementation for preventing respiratory infections in children: a meta-analysis of randomized, placebo-controlled trials. Indian Pediatr. 2013;50:377-381.
11. King S, Glanville J, Sanders ME, et al. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112:41-54.
12. Ozen M, Kocabas Sandal G, Dinleyici EC. Probiotics for the prevention of pediatric upper respiratory tract infections: a systematic review. Expert Opin Biol Ther. 2015;15:9-20.
13. Azad MB, Konya T, Maughan H, et al. Infant gut microbiota and the hygiene hypothesis of allergic disease: impact of household pets and siblings on microbiota composition and diversity. Allergy Asthma Clin Immunol. 2013;9:15.
14. Azad MB, Coneys JG, Kozyrskyj AL, et al. Probiotic supplementation during pregnancy or infancy for the prevention of asthma and wheeze: systematic review and meta-analysis. Brit Med J. 2013;347:f6471.
15. Cuello-Garcia CA, Bro˙zek JL, Fiocchi A, et al. Probiotics for the prevention of allergy: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2015;136:952-961.
16. Mansfield JA, Bergin SW, Cooper JR, et al. Comparative probiotic strain efficacy in the prevention of eczema in infants and children: a systematic review and meta-analysis. Mil Med. 2014;179:580-592.
17. Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2008;(4):CD006135.
18. Chiou E, Nurko S. Management of functional abdominal pain and irritable bowel syndrome in children and adolescents. Expert Rev Gastroenterol Hepatol. 2010;4:293-304.
19. Rutten JMTM, Korterink JL, Venmans LMAJ, et al. Nonpharmacologic treatment of functional abdominal pain disorders: a systematic review. Pediatrics. 2015;135:522-535.
20. Guandalini S, Magazzù G, Chiaro A, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr. 2010;51:24-30.
21. Turck D, Bernet JP, Marx J, et al. Incidence and risk factors of oral antibiotic associated diarrhea in an outpatient pediatric population. J Pediatr Gastroenterol Nutr. 2003;37:22-26.
22. Szajewska H, Kołodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015;42:793-801.
23. Szajewska H, Kołodziej M. Systematic review with meta-analysis: Lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults. Aliment Pharmacol Ther. 2015;42:1149-1157.
24. Goldenberg JZ, Lytvyn L, Steurich J, et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2015;12:CD004827.
25. Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151-2161.
26. WHO/UNICEF Joint Statement: Clinical Management of Acute Diarrhea. August 2004. Available at: http://www.unicef.org/publications/files/ENAcute_Diarrhoea_reprint.pdf. Accessed September 9, 2016.
27. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhea. Cochrane Database Syst Rev. 2010;(11):CD003048.
28. Applegate JA, Fischer Walker CL, Ambikapathi R, et al. Systematic review of probiotics for the treatment of community-acquired acute diarrhea in children. BMC Public Health. 2013;13:S16.
29. Guarino A, Ashkenazi S, Gendrel D, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr. 2014;59:132-152.
1. Euromonitor International. Global and regional trends of the probiotics and omega fatty acids market. June 23, 2015. Available at: http://uschinahpa.org/wp-content/uploads/2015/07/EMI-US-China-HPA-Probiotic-and-Omega-2015-Final.pdf. Accessed September 9, 2016.
2. Du Toit G, Lack G. Can food allergy be prevented? The current evidence. Pediatr Clin North Am. 2011;58:481-509.
3. Gerritsen J, Smidt H, Rijkers GT, et al. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. 2011;6:209-240.
4. Versalovic J. The human microbiome and probiotics: implications for pediatrics. Ann Nutr Metab. 2013;63:42-52.
5. Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
6. Akman I, Kușçu K, Özdemir N, et al. Mothers’ postpartum psychological adjustment and infantile colic. Arch Dis Child. 2006;91:417-419.
7. Sung V, Collett S, de Gooyer T, et al. Probiotics to prevent or treat excessive infant crying systematic review and meta-analysis. JAMA Pediatr. 2013:167:1150-1157.
8. Harb T, Matsuyama M, David M, et al. Infant colic—what works: a systematic review of interventions for breastfed infants. J Pediatr Gastroenterol Nutr. 2016;62:668-686.
9. Hill C, Guarner F, Reid G, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506-514.
10. Liu S, Hu P, Du X, et al. Lactobacillus rhamnosus GG supplementation for preventing respiratory infections in children: a meta-analysis of randomized, placebo-controlled trials. Indian Pediatr. 2013;50:377-381.
11. King S, Glanville J, Sanders ME, et al. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112:41-54.
12. Ozen M, Kocabas Sandal G, Dinleyici EC. Probiotics for the prevention of pediatric upper respiratory tract infections: a systematic review. Expert Opin Biol Ther. 2015;15:9-20.
13. Azad MB, Konya T, Maughan H, et al. Infant gut microbiota and the hygiene hypothesis of allergic disease: impact of household pets and siblings on microbiota composition and diversity. Allergy Asthma Clin Immunol. 2013;9:15.
14. Azad MB, Coneys JG, Kozyrskyj AL, et al. Probiotic supplementation during pregnancy or infancy for the prevention of asthma and wheeze: systematic review and meta-analysis. Brit Med J. 2013;347:f6471.
15. Cuello-Garcia CA, Bro˙zek JL, Fiocchi A, et al. Probiotics for the prevention of allergy: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2015;136:952-961.
16. Mansfield JA, Bergin SW, Cooper JR, et al. Comparative probiotic strain efficacy in the prevention of eczema in infants and children: a systematic review and meta-analysis. Mil Med. 2014;179:580-592.
17. Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2008;(4):CD006135.
18. Chiou E, Nurko S. Management of functional abdominal pain and irritable bowel syndrome in children and adolescents. Expert Rev Gastroenterol Hepatol. 2010;4:293-304.
19. Rutten JMTM, Korterink JL, Venmans LMAJ, et al. Nonpharmacologic treatment of functional abdominal pain disorders: a systematic review. Pediatrics. 2015;135:522-535.
20. Guandalini S, Magazzù G, Chiaro A, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr. 2010;51:24-30.
21. Turck D, Bernet JP, Marx J, et al. Incidence and risk factors of oral antibiotic associated diarrhea in an outpatient pediatric population. J Pediatr Gastroenterol Nutr. 2003;37:22-26.
22. Szajewska H, Kołodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015;42:793-801.
23. Szajewska H, Kołodziej M. Systematic review with meta-analysis: Lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults. Aliment Pharmacol Ther. 2015;42:1149-1157.
24. Goldenberg JZ, Lytvyn L, Steurich J, et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2015;12:CD004827.
25. Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151-2161.
26. WHO/UNICEF Joint Statement: Clinical Management of Acute Diarrhea. August 2004. Available at: http://www.unicef.org/publications/files/ENAcute_Diarrhoea_reprint.pdf. Accessed September 9, 2016.
27. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhea. Cochrane Database Syst Rev. 2010;(11):CD003048.
28. Applegate JA, Fischer Walker CL, Ambikapathi R, et al. Systematic review of probiotics for the treatment of community-acquired acute diarrhea in children. BMC Public Health. 2013;13:S16.
29. Guarino A, Ashkenazi S, Gendrel D, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr. 2014;59:132-152.
Deliver or wait with late preterm membrane rupture?
PRACTICE CHANGER
In the absence of clinical indications for delivery, consider expectant management in women with premature rupture of membranes in late preterm stages (34 weeks to 36 weeks, 6 days).
Strength of recommendation
B: Based on one well-designed randomized controlled trial.1
Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
ILLUSTRATIVE CASE
A 26-year-old G2P1001 at 35 weeks, 2 days of gestation presents with leakage of clear fluid for the last 2 hours. There is obvious pooling in the vaginal vault, and rupture of membranes is confirmed with appropriate testing. Her cervix is closed, she is not in labor, and tests of fetal well-being are reassuring. She had an uncomplicated vaginal delivery with her first child. How should you manage this situation?
Preterm premature rupture of membranes (PPROM)—when rupture of membranes occurs before 37 weeks’ gestation—affects about 3% of all pregnancies in the United States, and is a major contributor to perinatal morbidity and mortality.2,3 PPROM management remains controversial, especially during the late preterm stage (ie, 34 weeks to 36 weeks, 6 days). Non-reassuring fetal status, clinical chorioamnionitis, cord prolapse, and significant placental abruption are clear indications for delivery. In the absence of those factors, delivery vs expectant management is determined by gestational age. Between 23 and 34 weeks’ gestation, when the fetus is at or close to viability, expectant management is recommended, provided there are no signs of infection or maternal or fetal compromise.4 This is because of the significant morbidity and mortality associated with births before 34 weeks’ gestation.4
The American College of Obstetricians and Gynecologists (ACOG) currently recommends delivery for all women with rupture of membranes after 34 weeks’ gestation, while acknowledging that this recommendation is based on “limited and inconsistent scientific evidence.”5 The recommendation for delivery after 34 weeks is predicated on the belief that disability-free survival is high in late preterm infants. However, there is a growing body of evidence that shows negative short- and long-term effects for these children, including medical concerns, academic difficulties, and more frequent hospital admissions in early childhood.6,7
STUDY SUMMARY
Higher birth weights, fewer C-sections, and no increased sepsis with wait-and-see
The Preterm Pre-labour Rupture Of the Membranes close to Term (PPROMT) trial was a multicenter (65 institutions across 11 countries), randomized controlled trial (RCT) that included 1839 women with singleton pregnancies and confirmed rupture of membranes between 34 weeks and 36 weeks, 6 days’ gestation.1 Conducted from May 2004 to June 2013, participants were randomized to expectant management (915 women) vs immediate delivery by induction (924 women). Patients and care providers were not masked to treatment allocation, but those determining the primary outcome were masked to group allocation.
One woman in each group was lost to follow-up, and 2 additional women withdrew from the immediate birth group. Women already in active labor or with clinical indications for delivery (chorioamnionitis, abruption, cord prolapse, fetal distress) were excluded. The baseline characteristics of the 2 groups were similar.
Women in the induction group had delivery scheduled as soon as possible after randomization. Women in the expectant management group were allowed to go into spontaneous labor and were only induced if they reached term or the clinician identified other indications for immediate delivery.
The primary outcome was probable or confirmed neonatal sepsis. Secondary infant outcomes included a composite neonatal morbidity and mortality indicator (sepsis, mechanical ventilation ≥24 hours, still birth, or neonatal death), respiratory distress syndrome, any mechanical ventilation, birth weight, and duration of stay in a neonatal intensive care unit (NICU) or special care nursery. Secondary maternal outcomes included antepartum or intrapartum hemorrhage, intrapartum fever, mode of delivery, duration of hospital stay, and development of chorioamnionitis in the expectant management group.
The primary outcome of neonatal sepsis occurred in 2% of the neonates assigned to immediate delivery and 3% of neonates assigned to expectant management (relative risk [RR]=0.8; 95% confidence interval [CI], 0.5-1.3; P=.37). There was also no statistically significant difference in composite neonatal morbidity and mortality (RR=1.2; 95% CI, 0.9-1.6; P=.32). However, infants born in the immediate delivery group had significantly lower birth weights (2574.7 g vs 2673.2 g; absolute difference= -125 g; P<.0001), a higher incidence of respiratory distress (RR=1.6; 95% CI, 1.1-2.3; P=.008; number needed to treat [NNT]=32), and spent more time in the NICU/special care nursery (4 days vs 2 days; P<.0001).
Compared to immediate delivery, expectant management was associated with a higher likelihood of antepartum or intrapartum hemorrhage (RR=0.6; 95% CI, 0.4-0.9; P=.02; number needed to harm [NNH]=50) and intrapartum fever (RR=0.4; 95% CI, 0.2-0.9; P=.02; NNH=100). In the women assigned to immediate delivery, 26% had a cesarean section, compared to 19% in the expectant management group (RR=1.4; 95% CI, 1.2-1.7, P=.0001; NNT=14). A total of 56 women (6%) assigned to the expectant management group developed clinically significant chorioamnionitis requiring delivery. All other secondary maternal and neonatal outcomes were equivalent with no significant differences between the 2 groups.
WHAT'S NEW?
Largest study to show no increased sepsis with expectant management
Two prior RCTs (the PPROMEXIL trial8 and PPROMEXIL-29), involving a total of 736 women, evaluated expectant management vs induction in the late preterm stage of pregnancy. There was no increased risk of neonatal sepsis with expectant management in either study. However, those studies did not have sufficient power to show a statistically significant change in any of the outcomes.
The PPROMT study is the largest one to show that immediate birth increases the risk of respiratory distress and duration of NICU/special care stay for the baby and increases the risk of cesarean section for the mother. It also showed that the risk of neonatal sepsis was not higher in the expectant management group.
CAVEATS
Findings only apply to singleton pregnancies
Delivery of the infants in the expectant management group was not by specified protocol; each birth was managed according to the policies of the local center and clinician judgment. Because of this, there was variation in fetal and maternal monitoring. The vast majority of women in both groups (92% to 93%) received intrapartum antibiotics. Expectant management should include careful monitoring for infection and hemorrhage and may need to be changed to immediate delivery if one of these occurs.
The study participants all had singleton pregnancies; this recommendation cannot be extended to non-singleton pregnancies. However, a prior cesarean section was not an exclusion criterion for the study, and these recommendations would be valid for that group of women, too.
CHALLENGES TO IMPLEMENTATION
Going against the tide of ACOG
The most recent ACOG guidelines, updated October 2016, recommend induction of labor for women with ruptured membranes in the late preterm stages.5 This may present a challenge to widespread acceptance of expectant management for PPROM.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
2. Waters TP, Mercer B. Preterm PROM: prediction, prevention, principles. Clin Obstet Gynecol. 2011;54:307-312.
3. Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2010. Natl Vital Stat Rep. 2012;61:1-72.
4. Buchanan SL, Crowther CA, Levett KM, et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst Rev. 2010;3:CD004735.
5. Practice Bulletin Summary. Interim update. Premature rupture of membranes. Number 172, October 2016. Obstet Gynecol. 2016;128:934-936.
6. McGowan JE, Alderdice FA, Holmes VA, et al. Early childhood development of late-preterm infants: a systematic review. Pediatrics. 2011;127:1111-1124.
7. Teune MJ, Bakhuizen S, Gyamfi Bannerman C, et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205:374.
8. van der Ham DP, Vijgen SM, Nijhuis JG, et al; PPROMEXIL trial group. Induction of labor versus expectant management in women with preterm prelabor rupture of membranes between 34 and 37 weeks: a randomized controlled trial. PLoS Med. 2012;9:e1001208.
9. van der Ham DP, van der Heyden JL, Opmeer BC, et al. Management of late-preterm premature rupture of membranes: the PPROMEXIL-2 trial. Am J Obstet Gynecol. 2012;207:276.
PRACTICE CHANGER
In the absence of clinical indications for delivery, consider expectant management in women with premature rupture of membranes in late preterm stages (34 weeks to 36 weeks, 6 days).
Strength of recommendation
B: Based on one well-designed randomized controlled trial.1
Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
ILLUSTRATIVE CASE
A 26-year-old G2P1001 at 35 weeks, 2 days of gestation presents with leakage of clear fluid for the last 2 hours. There is obvious pooling in the vaginal vault, and rupture of membranes is confirmed with appropriate testing. Her cervix is closed, she is not in labor, and tests of fetal well-being are reassuring. She had an uncomplicated vaginal delivery with her first child. How should you manage this situation?
Preterm premature rupture of membranes (PPROM)—when rupture of membranes occurs before 37 weeks’ gestation—affects about 3% of all pregnancies in the United States, and is a major contributor to perinatal morbidity and mortality.2,3 PPROM management remains controversial, especially during the late preterm stage (ie, 34 weeks to 36 weeks, 6 days). Non-reassuring fetal status, clinical chorioamnionitis, cord prolapse, and significant placental abruption are clear indications for delivery. In the absence of those factors, delivery vs expectant management is determined by gestational age. Between 23 and 34 weeks’ gestation, when the fetus is at or close to viability, expectant management is recommended, provided there are no signs of infection or maternal or fetal compromise.4 This is because of the significant morbidity and mortality associated with births before 34 weeks’ gestation.4
The American College of Obstetricians and Gynecologists (ACOG) currently recommends delivery for all women with rupture of membranes after 34 weeks’ gestation, while acknowledging that this recommendation is based on “limited and inconsistent scientific evidence.”5 The recommendation for delivery after 34 weeks is predicated on the belief that disability-free survival is high in late preterm infants. However, there is a growing body of evidence that shows negative short- and long-term effects for these children, including medical concerns, academic difficulties, and more frequent hospital admissions in early childhood.6,7
STUDY SUMMARY
Higher birth weights, fewer C-sections, and no increased sepsis with wait-and-see
The Preterm Pre-labour Rupture Of the Membranes close to Term (PPROMT) trial was a multicenter (65 institutions across 11 countries), randomized controlled trial (RCT) that included 1839 women with singleton pregnancies and confirmed rupture of membranes between 34 weeks and 36 weeks, 6 days’ gestation.1 Conducted from May 2004 to June 2013, participants were randomized to expectant management (915 women) vs immediate delivery by induction (924 women). Patients and care providers were not masked to treatment allocation, but those determining the primary outcome were masked to group allocation.
One woman in each group was lost to follow-up, and 2 additional women withdrew from the immediate birth group. Women already in active labor or with clinical indications for delivery (chorioamnionitis, abruption, cord prolapse, fetal distress) were excluded. The baseline characteristics of the 2 groups were similar.
Women in the induction group had delivery scheduled as soon as possible after randomization. Women in the expectant management group were allowed to go into spontaneous labor and were only induced if they reached term or the clinician identified other indications for immediate delivery.
The primary outcome was probable or confirmed neonatal sepsis. Secondary infant outcomes included a composite neonatal morbidity and mortality indicator (sepsis, mechanical ventilation ≥24 hours, still birth, or neonatal death), respiratory distress syndrome, any mechanical ventilation, birth weight, and duration of stay in a neonatal intensive care unit (NICU) or special care nursery. Secondary maternal outcomes included antepartum or intrapartum hemorrhage, intrapartum fever, mode of delivery, duration of hospital stay, and development of chorioamnionitis in the expectant management group.
The primary outcome of neonatal sepsis occurred in 2% of the neonates assigned to immediate delivery and 3% of neonates assigned to expectant management (relative risk [RR]=0.8; 95% confidence interval [CI], 0.5-1.3; P=.37). There was also no statistically significant difference in composite neonatal morbidity and mortality (RR=1.2; 95% CI, 0.9-1.6; P=.32). However, infants born in the immediate delivery group had significantly lower birth weights (2574.7 g vs 2673.2 g; absolute difference= -125 g; P<.0001), a higher incidence of respiratory distress (RR=1.6; 95% CI, 1.1-2.3; P=.008; number needed to treat [NNT]=32), and spent more time in the NICU/special care nursery (4 days vs 2 days; P<.0001).
Compared to immediate delivery, expectant management was associated with a higher likelihood of antepartum or intrapartum hemorrhage (RR=0.6; 95% CI, 0.4-0.9; P=.02; number needed to harm [NNH]=50) and intrapartum fever (RR=0.4; 95% CI, 0.2-0.9; P=.02; NNH=100). In the women assigned to immediate delivery, 26% had a cesarean section, compared to 19% in the expectant management group (RR=1.4; 95% CI, 1.2-1.7, P=.0001; NNT=14). A total of 56 women (6%) assigned to the expectant management group developed clinically significant chorioamnionitis requiring delivery. All other secondary maternal and neonatal outcomes were equivalent with no significant differences between the 2 groups.
WHAT'S NEW?
Largest study to show no increased sepsis with expectant management
Two prior RCTs (the PPROMEXIL trial8 and PPROMEXIL-29), involving a total of 736 women, evaluated expectant management vs induction in the late preterm stage of pregnancy. There was no increased risk of neonatal sepsis with expectant management in either study. However, those studies did not have sufficient power to show a statistically significant change in any of the outcomes.
The PPROMT study is the largest one to show that immediate birth increases the risk of respiratory distress and duration of NICU/special care stay for the baby and increases the risk of cesarean section for the mother. It also showed that the risk of neonatal sepsis was not higher in the expectant management group.
CAVEATS
Findings only apply to singleton pregnancies
Delivery of the infants in the expectant management group was not by specified protocol; each birth was managed according to the policies of the local center and clinician judgment. Because of this, there was variation in fetal and maternal monitoring. The vast majority of women in both groups (92% to 93%) received intrapartum antibiotics. Expectant management should include careful monitoring for infection and hemorrhage and may need to be changed to immediate delivery if one of these occurs.
The study participants all had singleton pregnancies; this recommendation cannot be extended to non-singleton pregnancies. However, a prior cesarean section was not an exclusion criterion for the study, and these recommendations would be valid for that group of women, too.
CHALLENGES TO IMPLEMENTATION
Going against the tide of ACOG
The most recent ACOG guidelines, updated October 2016, recommend induction of labor for women with ruptured membranes in the late preterm stages.5 This may present a challenge to widespread acceptance of expectant management for PPROM.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
PRACTICE CHANGER
In the absence of clinical indications for delivery, consider expectant management in women with premature rupture of membranes in late preterm stages (34 weeks to 36 weeks, 6 days).
Strength of recommendation
B: Based on one well-designed randomized controlled trial.1
Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
ILLUSTRATIVE CASE
A 26-year-old G2P1001 at 35 weeks, 2 days of gestation presents with leakage of clear fluid for the last 2 hours. There is obvious pooling in the vaginal vault, and rupture of membranes is confirmed with appropriate testing. Her cervix is closed, she is not in labor, and tests of fetal well-being are reassuring. She had an uncomplicated vaginal delivery with her first child. How should you manage this situation?
Preterm premature rupture of membranes (PPROM)—when rupture of membranes occurs before 37 weeks’ gestation—affects about 3% of all pregnancies in the United States, and is a major contributor to perinatal morbidity and mortality.2,3 PPROM management remains controversial, especially during the late preterm stage (ie, 34 weeks to 36 weeks, 6 days). Non-reassuring fetal status, clinical chorioamnionitis, cord prolapse, and significant placental abruption are clear indications for delivery. In the absence of those factors, delivery vs expectant management is determined by gestational age. Between 23 and 34 weeks’ gestation, when the fetus is at or close to viability, expectant management is recommended, provided there are no signs of infection or maternal or fetal compromise.4 This is because of the significant morbidity and mortality associated with births before 34 weeks’ gestation.4
The American College of Obstetricians and Gynecologists (ACOG) currently recommends delivery for all women with rupture of membranes after 34 weeks’ gestation, while acknowledging that this recommendation is based on “limited and inconsistent scientific evidence.”5 The recommendation for delivery after 34 weeks is predicated on the belief that disability-free survival is high in late preterm infants. However, there is a growing body of evidence that shows negative short- and long-term effects for these children, including medical concerns, academic difficulties, and more frequent hospital admissions in early childhood.6,7
STUDY SUMMARY
Higher birth weights, fewer C-sections, and no increased sepsis with wait-and-see
The Preterm Pre-labour Rupture Of the Membranes close to Term (PPROMT) trial was a multicenter (65 institutions across 11 countries), randomized controlled trial (RCT) that included 1839 women with singleton pregnancies and confirmed rupture of membranes between 34 weeks and 36 weeks, 6 days’ gestation.1 Conducted from May 2004 to June 2013, participants were randomized to expectant management (915 women) vs immediate delivery by induction (924 women). Patients and care providers were not masked to treatment allocation, but those determining the primary outcome were masked to group allocation.
One woman in each group was lost to follow-up, and 2 additional women withdrew from the immediate birth group. Women already in active labor or with clinical indications for delivery (chorioamnionitis, abruption, cord prolapse, fetal distress) were excluded. The baseline characteristics of the 2 groups were similar.
Women in the induction group had delivery scheduled as soon as possible after randomization. Women in the expectant management group were allowed to go into spontaneous labor and were only induced if they reached term or the clinician identified other indications for immediate delivery.
The primary outcome was probable or confirmed neonatal sepsis. Secondary infant outcomes included a composite neonatal morbidity and mortality indicator (sepsis, mechanical ventilation ≥24 hours, still birth, or neonatal death), respiratory distress syndrome, any mechanical ventilation, birth weight, and duration of stay in a neonatal intensive care unit (NICU) or special care nursery. Secondary maternal outcomes included antepartum or intrapartum hemorrhage, intrapartum fever, mode of delivery, duration of hospital stay, and development of chorioamnionitis in the expectant management group.
The primary outcome of neonatal sepsis occurred in 2% of the neonates assigned to immediate delivery and 3% of neonates assigned to expectant management (relative risk [RR]=0.8; 95% confidence interval [CI], 0.5-1.3; P=.37). There was also no statistically significant difference in composite neonatal morbidity and mortality (RR=1.2; 95% CI, 0.9-1.6; P=.32). However, infants born in the immediate delivery group had significantly lower birth weights (2574.7 g vs 2673.2 g; absolute difference= -125 g; P<.0001), a higher incidence of respiratory distress (RR=1.6; 95% CI, 1.1-2.3; P=.008; number needed to treat [NNT]=32), and spent more time in the NICU/special care nursery (4 days vs 2 days; P<.0001).
Compared to immediate delivery, expectant management was associated with a higher likelihood of antepartum or intrapartum hemorrhage (RR=0.6; 95% CI, 0.4-0.9; P=.02; number needed to harm [NNH]=50) and intrapartum fever (RR=0.4; 95% CI, 0.2-0.9; P=.02; NNH=100). In the women assigned to immediate delivery, 26% had a cesarean section, compared to 19% in the expectant management group (RR=1.4; 95% CI, 1.2-1.7, P=.0001; NNT=14). A total of 56 women (6%) assigned to the expectant management group developed clinically significant chorioamnionitis requiring delivery. All other secondary maternal and neonatal outcomes were equivalent with no significant differences between the 2 groups.
WHAT'S NEW?
Largest study to show no increased sepsis with expectant management
Two prior RCTs (the PPROMEXIL trial8 and PPROMEXIL-29), involving a total of 736 women, evaluated expectant management vs induction in the late preterm stage of pregnancy. There was no increased risk of neonatal sepsis with expectant management in either study. However, those studies did not have sufficient power to show a statistically significant change in any of the outcomes.
The PPROMT study is the largest one to show that immediate birth increases the risk of respiratory distress and duration of NICU/special care stay for the baby and increases the risk of cesarean section for the mother. It also showed that the risk of neonatal sepsis was not higher in the expectant management group.
CAVEATS
Findings only apply to singleton pregnancies
Delivery of the infants in the expectant management group was not by specified protocol; each birth was managed according to the policies of the local center and clinician judgment. Because of this, there was variation in fetal and maternal monitoring. The vast majority of women in both groups (92% to 93%) received intrapartum antibiotics. Expectant management should include careful monitoring for infection and hemorrhage and may need to be changed to immediate delivery if one of these occurs.
The study participants all had singleton pregnancies; this recommendation cannot be extended to non-singleton pregnancies. However, a prior cesarean section was not an exclusion criterion for the study, and these recommendations would be valid for that group of women, too.
CHALLENGES TO IMPLEMENTATION
Going against the tide of ACOG
The most recent ACOG guidelines, updated October 2016, recommend induction of labor for women with ruptured membranes in the late preterm stages.5 This may present a challenge to widespread acceptance of expectant management for PPROM.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
2. Waters TP, Mercer B. Preterm PROM: prediction, prevention, principles. Clin Obstet Gynecol. 2011;54:307-312.
3. Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2010. Natl Vital Stat Rep. 2012;61:1-72.
4. Buchanan SL, Crowther CA, Levett KM, et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst Rev. 2010;3:CD004735.
5. Practice Bulletin Summary. Interim update. Premature rupture of membranes. Number 172, October 2016. Obstet Gynecol. 2016;128:934-936.
6. McGowan JE, Alderdice FA, Holmes VA, et al. Early childhood development of late-preterm infants: a systematic review. Pediatrics. 2011;127:1111-1124.
7. Teune MJ, Bakhuizen S, Gyamfi Bannerman C, et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205:374.
8. van der Ham DP, Vijgen SM, Nijhuis JG, et al; PPROMEXIL trial group. Induction of labor versus expectant management in women with preterm prelabor rupture of membranes between 34 and 37 weeks: a randomized controlled trial. PLoS Med. 2012;9:e1001208.
9. van der Ham DP, van der Heyden JL, Opmeer BC, et al. Management of late-preterm premature rupture of membranes: the PPROMEXIL-2 trial. Am J Obstet Gynecol. 2012;207:276.
1. Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
2. Waters TP, Mercer B. Preterm PROM: prediction, prevention, principles. Clin Obstet Gynecol. 2011;54:307-312.
3. Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2010. Natl Vital Stat Rep. 2012;61:1-72.
4. Buchanan SL, Crowther CA, Levett KM, et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst Rev. 2010;3:CD004735.
5. Practice Bulletin Summary. Interim update. Premature rupture of membranes. Number 172, October 2016. Obstet Gynecol. 2016;128:934-936.
6. McGowan JE, Alderdice FA, Holmes VA, et al. Early childhood development of late-preterm infants: a systematic review. Pediatrics. 2011;127:1111-1124.
7. Teune MJ, Bakhuizen S, Gyamfi Bannerman C, et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205:374.
8. van der Ham DP, Vijgen SM, Nijhuis JG, et al; PPROMEXIL trial group. Induction of labor versus expectant management in women with preterm prelabor rupture of membranes between 34 and 37 weeks: a randomized controlled trial. PLoS Med. 2012;9:e1001208.
9. van der Ham DP, van der Heyden JL, Opmeer BC, et al. Management of late-preterm premature rupture of membranes: the PPROMEXIL-2 trial. Am J Obstet Gynecol. 2012;207:276.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Recreational cannabinoid use: The hazards behind the “high”
PRACTICE RECOMMENDATIONS
› Screen all patients for use of addiction-prone substances. A
› Screen cannabis users with a validated secondary screen for problematic use. A
› Counsel patients that there is no evidence that use of recreational cannabis is safe; advise them that it can cause numerous physical, psychomotor, cognitive, and psychiatric effects. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Approximately 156 million Americans (49% of the population) have tried cannabis.1 About 5.7 million people ages 12 years and older use it daily or almost daily, a number that has nearly doubled since 2006.2 There are 6600 new users in the United States every day,2 and almost half of all high school students will have tried it by graduation.3
There is limited evidence that cannabis may have medical benefit in some circumstances.4 (See “Medical marijuana: A treatment worth trying?” J Fam Pract. 2016;65:178-185 or http://www.mdedge.com/jfponline/article/106836/medical-marijuana-treatment-worth-trying.) As a result, it is now legal for medical purposes in 25 states. Recreational use by adults is also legal in 4 states and the District of Columbia.5 The US Food and Drug Administration, however, has reaffirmed its stance that marijuana is a Schedule I drug on the basis of its “high potential for abuse” and the absence of “currently accepted medical uses.”6
The effects of legalizing the medical and recreational use of cannabis for individuals—and society as a whole—are uncertain. Debate is ongoing about the risks, benefits, and rights of individuals. Some argue it is safer than alcohol or that criminalization has been ineffective and even harmful. Others make the case for personal liberty and autonomy. Still, others are convinced legalization is a misdirected experiment that will result in diverse adverse outcomes. Regardless, it is important that primary care providers understand the ramifications of marijuana use. This evidence-based narrative highlights major negative consequences of non-medical cannabinoid use.
Potential adverse consequences of cannabis use
Although the potential adverse consequences are vast, the literature on this subject is limited for various reasons:
- Many studies are observational with a small sample size.
- Most studies examine smoked cannabis—not other routes of delivery.
- When smoked, the dose, frequency, duration, and smoking technique are variable.
- The quantity of Δ-9-tetrahydrocannabinol (THC), the primary psychoactive component in cannabis, is variable. (For more on the chemical properties of the marijuana plant, see “Cannabinoids: A diverse group of chemicals.”7)
- Most studies do not examine medical users, who are expected to use less cannabis or lower doses of THC.
- There are confounding effects of other drugs, notably tobacco, which is used by up to 90% of cannabis users.8
Lower quality of life. In general, regular non-medical cannabis use is associated with a lower quality of life and poorer socioeconomic outcomes (TABLE 1).9-12 Physical and mental health is ranked lower by heavy users as compared to extremely low users.9 Some who attempt butane extraction of THC from the plant have experienced explosions and severe burns.13
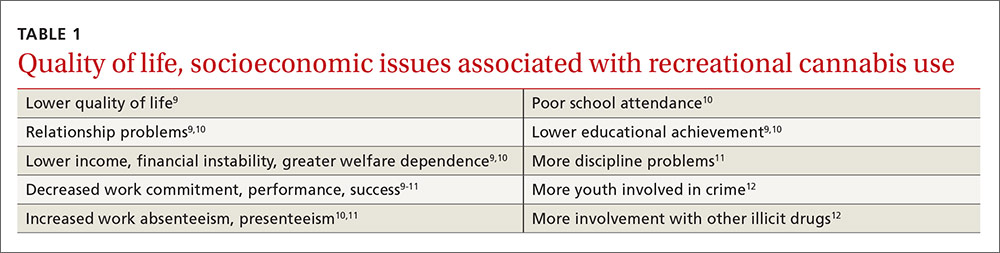
Studies regarding cannabis use and weight are conflicting. Appetite and weight may increase initially, and young adults who increase their use of the drug are more likely to find themselves on an increasing obesity trajectory.14 However, in an observational study of nearly 11,000 participants ages 20 to 59 years, cannabis users had a lower body mass index, better lipid parameters, and were less likely to have diabetes than non-using counterparts.15
Elevated rates of MI. Chronic effects may include oral health problems,16 gynecomastia, and changes in sexual function.17 Elevated rates of myocardial infarction, cardiomyopathy, limb arteritis, and stroke have been observed.18 Synthetic cannabinoids have been associated with heart attacks and acute renal injury in youth;19,20 however, plant-based marijuana does not affect the kidneys. In addition, high doses of plant-based marijuana can result in cannabinoid hyperemesis syndrome, characterized by cyclic vomiting and compulsive bathing that resolves with cessation of the drug.21
No major pulmonary effects. Interestingly, cannabis does not appear to have major negative pulmonary effects. Acutely, smoking marijuana causes bronchodilation.22 Chronic, low-level use over 20 years is associated with an increase in forced expiratory volume in one second (FEV1), but this upward trend diminishes and may reverse in high-level users.23 Although higher lung volumes are observed, cannabis does not appear to contribute to the development of chronic obstructive pulmonary disease, but can cause chronic bronchitis that resolves with smoking cessation.22 Chronic use has also been tied to airway infection. Lastly, fungal growth has been found on marijuana plants, which is concerning because of the potential to expose people to Aspergillus.22,24
Cannabis and cancer? The jury is out. Cannabis contains at least 33 carcinogens25 and may be contaminated with pesticides,26 but research about its relationship with cancer is incomplete. Although smoking results in histopathologic changes of the bronchial mucosa, evidence of lung cancer is mixed.22,25,27 Some studies have suggested associations with cancers of the brain, testis, prostate, and cervix,25,27 as well as certain rare cancers in children due to parental exposure.25,27
There are conflicting data about associations with head and neck squamous cell carcinoma,25,27,28 bladder cancer,25,29 and non-Hodgkin’s lymphoma.25,30 Some studies suggest marijuana offers protection against certain types of cancer. In fact, it appears that some cannabinoids found in marijuana, such as cannabidiol (CBD), may be antineoplastic.31 The potential oncogenic effects of edible and topical cannabinoid products have not been investigated.
Use linked to car accidents. More recent work indicates cannabis use is associated with injuries in motor vehicle,32 non-traffic,33 and workplace34 settings. In fact, a meta-analysis found a near-doubling of motor vehicle accidents with recent use.32 Risk is dose-dependent and heightened with alcohol.35-37 Psychomotor impairment persists for at least 6 hours after smoking cannabis,38 at least 10 hours after ingesting it,37 and may last up to 24 hours, as indicated by a study involving pilots using a flight simulator.39
In contrast to alcohol, there is a greater decrement in routine vs complex driving tasks in experimental studies.35,36 Behavioral strategies, like driving slowly, are employed to compensate for impairment, but the ability to do so is lost with alcohol co-ingestion.35 Importantly, individuals using marijuana may not recognize the presence or extent of the impairment they are experiencing,37,39 placing themselves and others in danger.
Data are insufficient to ascribe to marijuana an increase in overall mortality,40 and there have been no reported overdose deaths from respiratory depression. However, a few deaths and a greater number of hospitalizations, due mainly to central nervous system effects including agitation, depression, coma, delirium, and toxic psychosis, have been attributed to the use of synthetic cannabinoids.20
Cannabis use can pose a risk to the fetus. About 5% of pregnant women report recent marijuana use2 for recreational or medical reasons (eg, morning sickness), and there is concern about its effects on the developing fetus. Certain rare pediatric cancers22,25 and birth defects41 have been reported with cannabis use (TABLE 222,25,41,42). Neonatal withdrawal is minor, if present at all.42 Moderate evidence indicates prenatal and breastfeeding exposure can result in multiple developmental problems, as well as an increased likelihood of initiating tobacco and marijuana use as teens.41,42
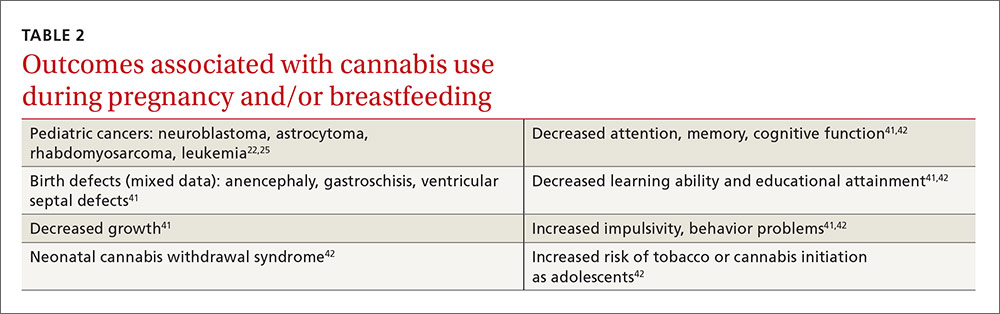
Cognitive effects of cannabis are a concern. The central nervous system is not fully myelinated until the age of 18, and complete maturation continues beyond that. Due to neuroplasticity, life experiences and exogenous agents may result in further changes. Cannabis produces changes in brain structure and function that are evident on neuroimaging.43 Although accidental pediatric intoxication is alarming, negative consequences are likely to be of short duration.
Regular use by youth, on the other hand, negatively affects cognition and delays brain maturation, especially for younger initiates.9,38,44 With abstinence, deficits tend to normalize, but they may last indefinitely among young people who continue to use marijuana.44
Dyscognition is less severe and is more likely to resolve with abstinence in adults,44 which may tip the scale for adults weighing whether to use cannabis for a medical purpose.45 Keep in mind that individuals may not be aware of their cognitive deficits,46 even though nearly all domains (from basic motor coordination to more complex executive function tasks, such as the ability to control emotions and behavior) are affected.44 A possible exception may be improvement in attention with acute use in daily, but not occasional, users.44 Highly focused attention, however, is not always beneficial if it delays redirection toward a new urgent stimulus.
Mood benefit? Research suggests otherwise. The psychiatric effects of cannabis are not fully understood. Users may claim mood benefit, but research suggests marijuana prompts the development or worsening of anxiety, depression, and suicidality.12,47 Violence, paranoia, and borderline personality features have also been associated with use.38,47 Amotivational syndrome, a disorder that includes apathy, callousness, and antisocial behavior, has been described, but the interplay between cannabis and motivation beyond recent use is unclear.48
Lifetime cannabis use is related to panic,49 yet correlational studies suggest both benefit and problems for individuals who use cannabis for posttraumatic stress disorder.50 It is now well established that marijuana use is an independent causal risk factor for the development of psychosis, particularly in vulnerable youth, and that it worsens schizophrenia in those who suffer from it.51 Human experimental studies suggest this may be because the effect of THC is counteracted by CBD.52 Synthetic cannabinoids are even more potent anxiogenic and psychogenic agents than plant-based marijuana.19,20
Cannabis Use Disorder
About 9% of those who try cannabis develop Cannabis Use Disorder, which is characterized by continued use of the substance despite significant distress or impairment.53 Cannabis Use Disorder is essentially an addiction. Primary risk factors include male gender, younger age at marijuana initiation, and personal or family history of other substance or psychiatric problems.53
Although cannabis use often precedes use of other addiction-prone substances, it remains unclear if it is a “gateway” to the use of other illicit drugs.54 Marijuana withdrawal is relatively minor and is comparable to that for tobacco.55 While there are no known effective pharmacotherapies for discontinuing cannabis use, addiction therapy—including cognitive behavioral therapy and trigger management—is effective.56
SIDEBAR
Cannabinoids: A diverse group of chemicalsCannabis, the genus name for 3 species of marijuana plant (sativa, indica, ruderalis), has come to mean any psychoactive part of the plant and is used interchangeably with “marijuana.” There are at least 85 different cannabinoids in the native plant.7
Cannabinoids are a diverse group of chemicals that have activity at cannabinoid receptors. Δ-9-tetrahydrocannabinol (THC), a partial agonist of the CB1 receptor, is the primary psychoactive component and is found in larger quantities in Cannabis sativa, which is preferred by non-medical users. Cannabidiol (CBD), a weak partial CB1 antagonist, exhibits few, if any, psychotropic properties and is more plentiful in Cannabis indica.
Synthetic cannabinioids are a heterogeneous group of manufactured drugs that are full CB1 agonists and that are more potent than THC, yet are often assumed to be safe by users. Typically, they are dissolved in solvents, sprayed onto inert plant materials, and marketed as herbal products like “K2” and “spice.”
So how should the evidence inform your care?
Screen all patients for use of cannabinoids and other addiction-prone substances.57 Follow any affirmative answers to your questions about cannabis use by asking about potential negative consequences of use. For example, ask patients:
- How often during the past 6 months did you find that you were unable to stop using cannabis once you started?
- How often during the past 6 months did you fail to do what was expected of you because of using cannabis? (For more questions, see the Cannabis Use Disorder Identification Test available at: http://www.otago.ac.nz/nationaladdictioncentre/pdfs/cudit-r.pdf.)
Other validated screening tools include the Severity of Dependence Scale, the Cannabis Abuse Screening Test, and the Problematic Use of Marijuana.58
Counsel patients about possible adverse effects and inform them there is no evidence that recreational marijuana or synthetic cannabinoids can be used safely over time. Consider medical use requests only if there is a favorable risk/benefit balance, other recognized treatment options have been exhausted, and you have a strong understanding of the use of cannabis in the medical condition being considered.4
Since brief interventions using motivational interviewing to reduce or eliminate recreational use have not been found to be effective,59 referral to an addiction specialist may be indicated. If a diagnosis of cannabis use disorder is established, then abstinence from addiction-prone substances including marijuana, programs like Marijuana Anonymous (Available at: https://www.marijuana-anonymous.org/), and individualized addiction therapy scaled to the severity of the condition can be effective.56 Because psychiatric conditions frequently co-occur and complicate addiction,53 they should be screened for and managed, as well.
Drug testing. Cannabis Use Disorder has significant relapse potential.60 Abstinence and treatment adherence should be ascertained through regular follow-up that includes a clinical interview, exam, and body fluid drug testing. Point-of-care urine analysis for substances of potential addiction has limited utility. Definitive testing of urine with gas chromotography/mass spectrometry (GC/MS) or liquid chromatography (LC/MS-MS) can eliminate THC false-positives and false-negatives that can occur with point-of-care urine immunoassays. In addition, GCMS and LC/MS-MS can identify synthetic cannabinoids; in-office immunoassays cannot.
If the patient relapses, subsequent medical care should be coordinated with an addiction specialist with the goal of helping the patient to abstain from cannabis.
CORRESPONDENCE
Steven Wright, MD, FAAFP, 5325 Ridge Trail, Littleton, CO 80123; [email protected].
1. Pew Research Center. 6 facts about marijuana. Available at: http://www.pewresearch.org/fact-tank/2015/04/14/6-facts-about-marijuana/. Accessed September 27, 2016.
2. Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. HHS Pub # (SMA) 14-4863. 2014. Available at: http://www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.pdf. Accessed September 27, 2015.
3. Johnston LD, O’Malley PM, Miech RA, et al. Monitoring the Future National Survey on Drug Use 1975-2015. Available at: http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2015.pdf. Accessed September 23, 2015.
4. Metts J, Wright S, Sundaram J, et al. Medical marijuana: a treatment worth trying? J Fam Pract. 2016;65:178-185.
5. Governing the states and localities. State marijuana laws map. Available at: http://www.governing.com/gov-data/state-marijuana-laws-map-medical-recreational.html. Accessed October 12, 2016.
6. US Drug Enforcement Administration. Drug scheduling. Available at: https://www.dea.gov/druginfo/ds.shtml. Accessed October 12, 2016.
7. El-Alfy AT, Ivey K, Robinson K, et al. Antidepressant-like effect of Δ9-tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L. Pharmacol Biochem Behav. 2010;95:434-442.
8. Peters EN, Budney AJ, Carroll KM. Clinical correlates of co-occurring cannabis and tobacco use: a systematic review. Addiction. 2012;107:1404-1417.
9. Gruber AJ, Pope HG, Hudson JI, et al. Attributes of long-term heavy cannabis users: a case-control study. Psychol Med. 2003;33:1415-1422.
10. Palamar JJ, Fenstermaker M, Kamboukos D, et al. Adverse psychosocial outcomes associated with drug use among US high school seniors: a comparison of alcohol and marijuana. Am J Drug Alcohol Abuse. 2014;40:438-446.
11. Zwerling C, Ryan J, Orav EJ. The efficacy of preemployment drug screening for marijuana and cocaine in predicting employment outcome. JAMA. 1990;264:2639-2643.
12. Fergusson DM, Horwood LJ, Swain-Campbell N. Cannabis use and psychosocial adjustment in adolescence and young adulthood. Addiction. 2002;97:1123-1135.
13. Bell C, Slim J, Flaten HK, et al. Butane hash oil burns associated with marijuana liberalization in Colorado. J Med Toxicol. 2015;11:422-425.
14. Huang DYC, Lanza HI, Anglin MD. Association between adolescent substance use and obesity in young adulthood: a group-based dual trajectory analysis. Addict Behav. 2013;38:2653-2660.
15. Rajavashisth TB, Shaheen M, Norris KC, et al. Decreased prevalence of diabetes in marijuana users: cross-sectional data from the National Health and Nutrition Examination Survey (NHANES) III. BMJ Open. 2012;2:e000494.
16. Cho CM, Hirsch R, Johnstone S. General and oral health implications of cannabis use. Aust Dent J. 2005;50:70-74.
17. Gorzalka BB, Hill MN, Chang SC. Male-female differences in the effects of cannabinoids on sexual behavior and gonadal hormone function. Horm Behav. 2010;58:91-99.
18. Desbois AC, Cacoub P. Cannabis-associated arterial disease. Ann Vasc Surg. 2013;27:996-1005.
19. Mills B, Yepes A, Nugent K. Synthetic cannabinoids. Am J Med Sci. 2015;350:59-62.
20. Tuv SS, Strand MC, Karinen R, et al. Effect and occurrence of synthetic cannabinoids. Tidsskr Nor Laegeforen. 2012;132:2285-2288.
21. Wallace EA, Andrews SE, Garmany CL, et al. Cannabinoid hyperemesis syndrome: literature review and proposed diagnosis and treatment algorithm. South Med J. 2011;104:659-964.
22. Gates P, Jaffe A, Copeland J. Cannabis smoking and respiratory health: considerations of the literature. Respirology. 2014;19:655-662.
23. Pletcher MJ, Vittinghoff E, Kalhan R, et al. Association between marijuana exposure and pulmonary function over 20 years: The Coronary Artery Risk Development in Young Adults (CARDIA) study. JAMA. 2012;307:173-181.
24. Verweij PE, Kerremans JJ, Vos A, et al. Fungal contamination of tobacco and marijuana. JAMA. 2000;284:2875.
25. Office of Environmental Health Hazard Assessment. Evidence on the carcinogenicity of marijuana smoke. August 2009. Available at: http://oehha.ca.gov/media/downloads/crnr/finalmjsmokehid.pdf. Accessed September 5, 2015.
26. Stone D. Cannabis, pesticides and conflicting laws: the dilemma for legalized States and implications for public health. Regul Toxicol Pharmacol. 2014;69:284-288.
27. Hashibe M, Straif K, Tashkin DP, et al. Epidemiologic review of marijuana and cancer risk. Alcohol. 2005;35:265-275.
28. Liang C, McClean MD, Marsit C, et al. A population-based case-control study of marijuana use and head and neck squamous cell carcinoma. Cancer Prev Res (Phila). 2009;2:759-768.
29. Thomas AA, Wallner LP, Quinn VP, et al. Association between cannabis use and the risk of bladder cancer: results from the California Men’s Health Study. Urology. 2015;85:388-392.
30. Holly EA, Lele C, Bracci PM, et al. Case-control study of non-Hodgkin’s lymphoma among women and heterosexual men in the San Francisco Bay area, California. Am J Epidemiol. 1999;150:375-389.
31. Massi P, Solinas M, Cinquina V, et al. Cannabidiol as potential anticancer drug. Br J Clin Pharmacol. 2013;75:303-312.
32. Ashbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ. 2012;344:e536.
33.Barrio G, Jimenez-Mejias E, Pulido J, et al. Association between cannabis use and non-traffic injuries. Accid Anal Prev. 2012;47:172-176.
34. MacDonald S, Hall W, Roman P, et al. Testing for cannabis in the work-place: a review of the evidence. Addiction. 2010;105:408-416.
35. Sewell RA, Poling J, Sofuoglu M. The effect of cannabis compared with alcohol on driving. Am J Addict. 2009;18:185-193.
36. Ramaekers JG, Berghaus G, van Laar M, et al. Dose related risk of motor vehicle crashes after cannabis use. Drug Alcohol Depend. 2004;73:109-119.
37. Menetrey A, Augsburger M, Favrat B, et al. Assessment of driving capability through the use of clinical and psychomotor tests in relation to blood cannabinoid levels following oral administration of 20 mg dronabinol or of a cannabis decoction made with 20 or 60 mg Δ9-THC. J Anal Toxicol. 2005;29:327-338.
38. Raemakers JG, Kaurert G, van Ruitenbeek P, et al. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology. 2006;31:2296-2303.
39. Leirer VO, Yesavage JA, Morrow DG. Marijuana carry-over effects on aircraft pilot performance. Aviat Space Environ Med. 1991;62:221-227.
40. Calabria B, Degenhardt L, Hall W, et al. Does cannabis use increase the risk of death? Systematic review of epidemiological evidence on adverse effects of cannabis use. Drug Alcohol Rev. 2010;29:318-330.
41. Colorado Department of Public Health and Environment. Monitoring health concerns related to marijuana in Colorado: 2014. Changes in marijuana use patterns, systematic literature review, and possible marijuana-related health effects. Available at: http://www2.cde.state.co.us/artemis/hemonos/he1282m332015internet/he1282m332015internet01.pdf. Accessed September 5, 2015.
42. Behnke M, Smith VC, Committee on Substance Abuse, Committee on Fetus and Newborn. Perinatal substance abuse: short- and long-term effects on the exposed fetus. Pediatrics. 2013;131:e1009-1024.
43. Batalla A, Bhattacharyya S, Yücel M, et al. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS One. 2013;8:e55821.
44. Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med. 2011;5:1-8.
45. Pavisian B, MacIntosh BJ, Szilagyi G, et al. Effects of cannabis on cognition in patients with multiple sclerosis: a psychometric and MRI study. Neurology. 2014;82:1879-1887.
46. Bartholomew J, Holroyd S, Heffernan TM. Does cannabis use affect prospective memory in young adults? J Psychopharmacol. 2010;24:241-246.
47. Copeland J, Rooke S, Swift W. Changes in cannabis use among young people: impact on mental health. Curr Opin Psychiatry. 2013;26:325-329.
48. Ari M, Sahpolat M, Kokacya H, et al. Amotivational syndrome: less known and diagnosed as a clinical. J Mood Disord. 2015;5:31-35.
49. Zvolensky MJ, Cougle JR, Johnson KA, et al. Marijuana use and panic psychopathology among a representative sample of adults. Exp Clin Psychopharmacol. 2010;18(2):129-134.
50. Yarnell S. The use of medicinal marijuana for posttraumatic stress disorder: a review of the current literature. Prim Care Companion CNS Disord. 2015;17(3).
51. Le Bec PY, Fatséas M, Denis C, et al. Cannabis and psychosis: search of a causal link through a critical and systematic review. Encephale. 2009;35:377-385.
52. Englund A, Morrison PD, Nottage J, et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol. 2013;27:19-27.
53. Lopez-Quintero C, Perez de los Cobos J, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 2011:115:120-130.
54. Degenhardt L, Dierker L, Chiu WT, et al. Evaluating the drug use “gateway” theory using cross-national data: consistency and associations of the order of initiation of drug use among participants in the WHO World Mental Health Surveys. Drug Alcohol Depend. 2010;108:84-97.
55. Vandrey RG, Budney AJ, Hughes JR, et al. A within subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depend. 2008;92:48-54.
56.Budney AJ, Roffman R, Stephens RS, et al. Marijuana dependence and its treatment. Addict Sci Clin Pract. 2007;4:4-16.
57. Turner SD, Spithoff S, Kahan M. Approach to cannabis use disorder in primary care: focus on youth and other high-risk users. Can Fam Phys. 2014;60:801-808.
58. Piontek D, Kraus L, Klempova D. Short scales to assess cannabis-related problems: a review of psychometric properties. Subst Abuse Treat Prev Policy. 2008;3:25.
59. Saitz R, Palfai TPA, Cheng DM, et al. Screening and brief intervention for drug use in primary care: the ASPIRE randomized clinical trial. JAMA. 2014;312:502-513.
60. McLellan AT, Lewis DC, O’Brien CP, et al. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689-1695.
PRACTICE RECOMMENDATIONS
› Screen all patients for use of addiction-prone substances. A
› Screen cannabis users with a validated secondary screen for problematic use. A
› Counsel patients that there is no evidence that use of recreational cannabis is safe; advise them that it can cause numerous physical, psychomotor, cognitive, and psychiatric effects. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Approximately 156 million Americans (49% of the population) have tried cannabis.1 About 5.7 million people ages 12 years and older use it daily or almost daily, a number that has nearly doubled since 2006.2 There are 6600 new users in the United States every day,2 and almost half of all high school students will have tried it by graduation.3
There is limited evidence that cannabis may have medical benefit in some circumstances.4 (See “Medical marijuana: A treatment worth trying?” J Fam Pract. 2016;65:178-185 or http://www.mdedge.com/jfponline/article/106836/medical-marijuana-treatment-worth-trying.) As a result, it is now legal for medical purposes in 25 states. Recreational use by adults is also legal in 4 states and the District of Columbia.5 The US Food and Drug Administration, however, has reaffirmed its stance that marijuana is a Schedule I drug on the basis of its “high potential for abuse” and the absence of “currently accepted medical uses.”6
The effects of legalizing the medical and recreational use of cannabis for individuals—and society as a whole—are uncertain. Debate is ongoing about the risks, benefits, and rights of individuals. Some argue it is safer than alcohol or that criminalization has been ineffective and even harmful. Others make the case for personal liberty and autonomy. Still, others are convinced legalization is a misdirected experiment that will result in diverse adverse outcomes. Regardless, it is important that primary care providers understand the ramifications of marijuana use. This evidence-based narrative highlights major negative consequences of non-medical cannabinoid use.
Potential adverse consequences of cannabis use
Although the potential adverse consequences are vast, the literature on this subject is limited for various reasons:
- Many studies are observational with a small sample size.
- Most studies examine smoked cannabis—not other routes of delivery.
- When smoked, the dose, frequency, duration, and smoking technique are variable.
- The quantity of Δ-9-tetrahydrocannabinol (THC), the primary psychoactive component in cannabis, is variable. (For more on the chemical properties of the marijuana plant, see “Cannabinoids: A diverse group of chemicals.”7)
- Most studies do not examine medical users, who are expected to use less cannabis or lower doses of THC.
- There are confounding effects of other drugs, notably tobacco, which is used by up to 90% of cannabis users.8
Lower quality of life. In general, regular non-medical cannabis use is associated with a lower quality of life and poorer socioeconomic outcomes (TABLE 1).9-12 Physical and mental health is ranked lower by heavy users as compared to extremely low users.9 Some who attempt butane extraction of THC from the plant have experienced explosions and severe burns.13

Studies regarding cannabis use and weight are conflicting. Appetite and weight may increase initially, and young adults who increase their use of the drug are more likely to find themselves on an increasing obesity trajectory.14 However, in an observational study of nearly 11,000 participants ages 20 to 59 years, cannabis users had a lower body mass index, better lipid parameters, and were less likely to have diabetes than non-using counterparts.15
Elevated rates of MI. Chronic effects may include oral health problems,16 gynecomastia, and changes in sexual function.17 Elevated rates of myocardial infarction, cardiomyopathy, limb arteritis, and stroke have been observed.18 Synthetic cannabinoids have been associated with heart attacks and acute renal injury in youth;19,20 however, plant-based marijuana does not affect the kidneys. In addition, high doses of plant-based marijuana can result in cannabinoid hyperemesis syndrome, characterized by cyclic vomiting and compulsive bathing that resolves with cessation of the drug.21
No major pulmonary effects. Interestingly, cannabis does not appear to have major negative pulmonary effects. Acutely, smoking marijuana causes bronchodilation.22 Chronic, low-level use over 20 years is associated with an increase in forced expiratory volume in one second (FEV1), but this upward trend diminishes and may reverse in high-level users.23 Although higher lung volumes are observed, cannabis does not appear to contribute to the development of chronic obstructive pulmonary disease, but can cause chronic bronchitis that resolves with smoking cessation.22 Chronic use has also been tied to airway infection. Lastly, fungal growth has been found on marijuana plants, which is concerning because of the potential to expose people to Aspergillus.22,24
Cannabis and cancer? The jury is out. Cannabis contains at least 33 carcinogens25 and may be contaminated with pesticides,26 but research about its relationship with cancer is incomplete. Although smoking results in histopathologic changes of the bronchial mucosa, evidence of lung cancer is mixed.22,25,27 Some studies have suggested associations with cancers of the brain, testis, prostate, and cervix,25,27 as well as certain rare cancers in children due to parental exposure.25,27
There are conflicting data about associations with head and neck squamous cell carcinoma,25,27,28 bladder cancer,25,29 and non-Hodgkin’s lymphoma.25,30 Some studies suggest marijuana offers protection against certain types of cancer. In fact, it appears that some cannabinoids found in marijuana, such as cannabidiol (CBD), may be antineoplastic.31 The potential oncogenic effects of edible and topical cannabinoid products have not been investigated.
Use linked to car accidents. More recent work indicates cannabis use is associated with injuries in motor vehicle,32 non-traffic,33 and workplace34 settings. In fact, a meta-analysis found a near-doubling of motor vehicle accidents with recent use.32 Risk is dose-dependent and heightened with alcohol.35-37 Psychomotor impairment persists for at least 6 hours after smoking cannabis,38 at least 10 hours after ingesting it,37 and may last up to 24 hours, as indicated by a study involving pilots using a flight simulator.39
In contrast to alcohol, there is a greater decrement in routine vs complex driving tasks in experimental studies.35,36 Behavioral strategies, like driving slowly, are employed to compensate for impairment, but the ability to do so is lost with alcohol co-ingestion.35 Importantly, individuals using marijuana may not recognize the presence or extent of the impairment they are experiencing,37,39 placing themselves and others in danger.
Data are insufficient to ascribe to marijuana an increase in overall mortality,40 and there have been no reported overdose deaths from respiratory depression. However, a few deaths and a greater number of hospitalizations, due mainly to central nervous system effects including agitation, depression, coma, delirium, and toxic psychosis, have been attributed to the use of synthetic cannabinoids.20
Cannabis use can pose a risk to the fetus. About 5% of pregnant women report recent marijuana use2 for recreational or medical reasons (eg, morning sickness), and there is concern about its effects on the developing fetus. Certain rare pediatric cancers22,25 and birth defects41 have been reported with cannabis use (TABLE 222,25,41,42). Neonatal withdrawal is minor, if present at all.42 Moderate evidence indicates prenatal and breastfeeding exposure can result in multiple developmental problems, as well as an increased likelihood of initiating tobacco and marijuana use as teens.41,42

Cognitive effects of cannabis are a concern. The central nervous system is not fully myelinated until the age of 18, and complete maturation continues beyond that. Due to neuroplasticity, life experiences and exogenous agents may result in further changes. Cannabis produces changes in brain structure and function that are evident on neuroimaging.43 Although accidental pediatric intoxication is alarming, negative consequences are likely to be of short duration.
Regular use by youth, on the other hand, negatively affects cognition and delays brain maturation, especially for younger initiates.9,38,44 With abstinence, deficits tend to normalize, but they may last indefinitely among young people who continue to use marijuana.44
Dyscognition is less severe and is more likely to resolve with abstinence in adults,44 which may tip the scale for adults weighing whether to use cannabis for a medical purpose.45 Keep in mind that individuals may not be aware of their cognitive deficits,46 even though nearly all domains (from basic motor coordination to more complex executive function tasks, such as the ability to control emotions and behavior) are affected.44 A possible exception may be improvement in attention with acute use in daily, but not occasional, users.44 Highly focused attention, however, is not always beneficial if it delays redirection toward a new urgent stimulus.
Mood benefit? Research suggests otherwise. The psychiatric effects of cannabis are not fully understood. Users may claim mood benefit, but research suggests marijuana prompts the development or worsening of anxiety, depression, and suicidality.12,47 Violence, paranoia, and borderline personality features have also been associated with use.38,47 Amotivational syndrome, a disorder that includes apathy, callousness, and antisocial behavior, has been described, but the interplay between cannabis and motivation beyond recent use is unclear.48
Lifetime cannabis use is related to panic,49 yet correlational studies suggest both benefit and problems for individuals who use cannabis for posttraumatic stress disorder.50 It is now well established that marijuana use is an independent causal risk factor for the development of psychosis, particularly in vulnerable youth, and that it worsens schizophrenia in those who suffer from it.51 Human experimental studies suggest this may be because the effect of THC is counteracted by CBD.52 Synthetic cannabinoids are even more potent anxiogenic and psychogenic agents than plant-based marijuana.19,20
Cannabis Use Disorder
About 9% of those who try cannabis develop Cannabis Use Disorder, which is characterized by continued use of the substance despite significant distress or impairment.53 Cannabis Use Disorder is essentially an addiction. Primary risk factors include male gender, younger age at marijuana initiation, and personal or family history of other substance or psychiatric problems.53
Although cannabis use often precedes use of other addiction-prone substances, it remains unclear if it is a “gateway” to the use of other illicit drugs.54 Marijuana withdrawal is relatively minor and is comparable to that for tobacco.55 While there are no known effective pharmacotherapies for discontinuing cannabis use, addiction therapy—including cognitive behavioral therapy and trigger management—is effective.56
SIDEBAR
Cannabinoids: A diverse group of chemicalsCannabis, the genus name for 3 species of marijuana plant (sativa, indica, ruderalis), has come to mean any psychoactive part of the plant and is used interchangeably with “marijuana.” There are at least 85 different cannabinoids in the native plant.7
Cannabinoids are a diverse group of chemicals that have activity at cannabinoid receptors. Δ-9-tetrahydrocannabinol (THC), a partial agonist of the CB1 receptor, is the primary psychoactive component and is found in larger quantities in Cannabis sativa, which is preferred by non-medical users. Cannabidiol (CBD), a weak partial CB1 antagonist, exhibits few, if any, psychotropic properties and is more plentiful in Cannabis indica.
Synthetic cannabinioids are a heterogeneous group of manufactured drugs that are full CB1 agonists and that are more potent than THC, yet are often assumed to be safe by users. Typically, they are dissolved in solvents, sprayed onto inert plant materials, and marketed as herbal products like “K2” and “spice.”
So how should the evidence inform your care?
Screen all patients for use of cannabinoids and other addiction-prone substances.57 Follow any affirmative answers to your questions about cannabis use by asking about potential negative consequences of use. For example, ask patients:
- How often during the past 6 months did you find that you were unable to stop using cannabis once you started?
- How often during the past 6 months did you fail to do what was expected of you because of using cannabis? (For more questions, see the Cannabis Use Disorder Identification Test available at: http://www.otago.ac.nz/nationaladdictioncentre/pdfs/cudit-r.pdf.)
Other validated screening tools include the Severity of Dependence Scale, the Cannabis Abuse Screening Test, and the Problematic Use of Marijuana.58
Counsel patients about possible adverse effects and inform them there is no evidence that recreational marijuana or synthetic cannabinoids can be used safely over time. Consider medical use requests only if there is a favorable risk/benefit balance, other recognized treatment options have been exhausted, and you have a strong understanding of the use of cannabis in the medical condition being considered.4
Since brief interventions using motivational interviewing to reduce or eliminate recreational use have not been found to be effective,59 referral to an addiction specialist may be indicated. If a diagnosis of cannabis use disorder is established, then abstinence from addiction-prone substances including marijuana, programs like Marijuana Anonymous (Available at: https://www.marijuana-anonymous.org/), and individualized addiction therapy scaled to the severity of the condition can be effective.56 Because psychiatric conditions frequently co-occur and complicate addiction,53 they should be screened for and managed, as well.
Drug testing. Cannabis Use Disorder has significant relapse potential.60 Abstinence and treatment adherence should be ascertained through regular follow-up that includes a clinical interview, exam, and body fluid drug testing. Point-of-care urine analysis for substances of potential addiction has limited utility. Definitive testing of urine with gas chromotography/mass spectrometry (GC/MS) or liquid chromatography (LC/MS-MS) can eliminate THC false-positives and false-negatives that can occur with point-of-care urine immunoassays. In addition, GCMS and LC/MS-MS can identify synthetic cannabinoids; in-office immunoassays cannot.
If the patient relapses, subsequent medical care should be coordinated with an addiction specialist with the goal of helping the patient to abstain from cannabis.
CORRESPONDENCE
Steven Wright, MD, FAAFP, 5325 Ridge Trail, Littleton, CO 80123; [email protected].
PRACTICE RECOMMENDATIONS
› Screen all patients for use of addiction-prone substances. A
› Screen cannabis users with a validated secondary screen for problematic use. A
› Counsel patients that there is no evidence that use of recreational cannabis is safe; advise them that it can cause numerous physical, psychomotor, cognitive, and psychiatric effects. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Approximately 156 million Americans (49% of the population) have tried cannabis.1 About 5.7 million people ages 12 years and older use it daily or almost daily, a number that has nearly doubled since 2006.2 There are 6600 new users in the United States every day,2 and almost half of all high school students will have tried it by graduation.3
There is limited evidence that cannabis may have medical benefit in some circumstances.4 (See “Medical marijuana: A treatment worth trying?” J Fam Pract. 2016;65:178-185 or http://www.mdedge.com/jfponline/article/106836/medical-marijuana-treatment-worth-trying.) As a result, it is now legal for medical purposes in 25 states. Recreational use by adults is also legal in 4 states and the District of Columbia.5 The US Food and Drug Administration, however, has reaffirmed its stance that marijuana is a Schedule I drug on the basis of its “high potential for abuse” and the absence of “currently accepted medical uses.”6
The effects of legalizing the medical and recreational use of cannabis for individuals—and society as a whole—are uncertain. Debate is ongoing about the risks, benefits, and rights of individuals. Some argue it is safer than alcohol or that criminalization has been ineffective and even harmful. Others make the case for personal liberty and autonomy. Still, others are convinced legalization is a misdirected experiment that will result in diverse adverse outcomes. Regardless, it is important that primary care providers understand the ramifications of marijuana use. This evidence-based narrative highlights major negative consequences of non-medical cannabinoid use.
Potential adverse consequences of cannabis use
Although the potential adverse consequences are vast, the literature on this subject is limited for various reasons:
- Many studies are observational with a small sample size.
- Most studies examine smoked cannabis—not other routes of delivery.
- When smoked, the dose, frequency, duration, and smoking technique are variable.
- The quantity of Δ-9-tetrahydrocannabinol (THC), the primary psychoactive component in cannabis, is variable. (For more on the chemical properties of the marijuana plant, see “Cannabinoids: A diverse group of chemicals.”7)
- Most studies do not examine medical users, who are expected to use less cannabis or lower doses of THC.
- There are confounding effects of other drugs, notably tobacco, which is used by up to 90% of cannabis users.8
Lower quality of life. In general, regular non-medical cannabis use is associated with a lower quality of life and poorer socioeconomic outcomes (TABLE 1).9-12 Physical and mental health is ranked lower by heavy users as compared to extremely low users.9 Some who attempt butane extraction of THC from the plant have experienced explosions and severe burns.13

Studies regarding cannabis use and weight are conflicting. Appetite and weight may increase initially, and young adults who increase their use of the drug are more likely to find themselves on an increasing obesity trajectory.14 However, in an observational study of nearly 11,000 participants ages 20 to 59 years, cannabis users had a lower body mass index, better lipid parameters, and were less likely to have diabetes than non-using counterparts.15
Elevated rates of MI. Chronic effects may include oral health problems,16 gynecomastia, and changes in sexual function.17 Elevated rates of myocardial infarction, cardiomyopathy, limb arteritis, and stroke have been observed.18 Synthetic cannabinoids have been associated with heart attacks and acute renal injury in youth;19,20 however, plant-based marijuana does not affect the kidneys. In addition, high doses of plant-based marijuana can result in cannabinoid hyperemesis syndrome, characterized by cyclic vomiting and compulsive bathing that resolves with cessation of the drug.21
No major pulmonary effects. Interestingly, cannabis does not appear to have major negative pulmonary effects. Acutely, smoking marijuana causes bronchodilation.22 Chronic, low-level use over 20 years is associated with an increase in forced expiratory volume in one second (FEV1), but this upward trend diminishes and may reverse in high-level users.23 Although higher lung volumes are observed, cannabis does not appear to contribute to the development of chronic obstructive pulmonary disease, but can cause chronic bronchitis that resolves with smoking cessation.22 Chronic use has also been tied to airway infection. Lastly, fungal growth has been found on marijuana plants, which is concerning because of the potential to expose people to Aspergillus.22,24
Cannabis and cancer? The jury is out. Cannabis contains at least 33 carcinogens25 and may be contaminated with pesticides,26 but research about its relationship with cancer is incomplete. Although smoking results in histopathologic changes of the bronchial mucosa, evidence of lung cancer is mixed.22,25,27 Some studies have suggested associations with cancers of the brain, testis, prostate, and cervix,25,27 as well as certain rare cancers in children due to parental exposure.25,27
There are conflicting data about associations with head and neck squamous cell carcinoma,25,27,28 bladder cancer,25,29 and non-Hodgkin’s lymphoma.25,30 Some studies suggest marijuana offers protection against certain types of cancer. In fact, it appears that some cannabinoids found in marijuana, such as cannabidiol (CBD), may be antineoplastic.31 The potential oncogenic effects of edible and topical cannabinoid products have not been investigated.
Use linked to car accidents. More recent work indicates cannabis use is associated with injuries in motor vehicle,32 non-traffic,33 and workplace34 settings. In fact, a meta-analysis found a near-doubling of motor vehicle accidents with recent use.32 Risk is dose-dependent and heightened with alcohol.35-37 Psychomotor impairment persists for at least 6 hours after smoking cannabis,38 at least 10 hours after ingesting it,37 and may last up to 24 hours, as indicated by a study involving pilots using a flight simulator.39
In contrast to alcohol, there is a greater decrement in routine vs complex driving tasks in experimental studies.35,36 Behavioral strategies, like driving slowly, are employed to compensate for impairment, but the ability to do so is lost with alcohol co-ingestion.35 Importantly, individuals using marijuana may not recognize the presence or extent of the impairment they are experiencing,37,39 placing themselves and others in danger.
Data are insufficient to ascribe to marijuana an increase in overall mortality,40 and there have been no reported overdose deaths from respiratory depression. However, a few deaths and a greater number of hospitalizations, due mainly to central nervous system effects including agitation, depression, coma, delirium, and toxic psychosis, have been attributed to the use of synthetic cannabinoids.20
Cannabis use can pose a risk to the fetus. About 5% of pregnant women report recent marijuana use2 for recreational or medical reasons (eg, morning sickness), and there is concern about its effects on the developing fetus. Certain rare pediatric cancers22,25 and birth defects41 have been reported with cannabis use (TABLE 222,25,41,42). Neonatal withdrawal is minor, if present at all.42 Moderate evidence indicates prenatal and breastfeeding exposure can result in multiple developmental problems, as well as an increased likelihood of initiating tobacco and marijuana use as teens.41,42

Cognitive effects of cannabis are a concern. The central nervous system is not fully myelinated until the age of 18, and complete maturation continues beyond that. Due to neuroplasticity, life experiences and exogenous agents may result in further changes. Cannabis produces changes in brain structure and function that are evident on neuroimaging.43 Although accidental pediatric intoxication is alarming, negative consequences are likely to be of short duration.
Regular use by youth, on the other hand, negatively affects cognition and delays brain maturation, especially for younger initiates.9,38,44 With abstinence, deficits tend to normalize, but they may last indefinitely among young people who continue to use marijuana.44
Dyscognition is less severe and is more likely to resolve with abstinence in adults,44 which may tip the scale for adults weighing whether to use cannabis for a medical purpose.45 Keep in mind that individuals may not be aware of their cognitive deficits,46 even though nearly all domains (from basic motor coordination to more complex executive function tasks, such as the ability to control emotions and behavior) are affected.44 A possible exception may be improvement in attention with acute use in daily, but not occasional, users.44 Highly focused attention, however, is not always beneficial if it delays redirection toward a new urgent stimulus.
Mood benefit? Research suggests otherwise. The psychiatric effects of cannabis are not fully understood. Users may claim mood benefit, but research suggests marijuana prompts the development or worsening of anxiety, depression, and suicidality.12,47 Violence, paranoia, and borderline personality features have also been associated with use.38,47 Amotivational syndrome, a disorder that includes apathy, callousness, and antisocial behavior, has been described, but the interplay between cannabis and motivation beyond recent use is unclear.48
Lifetime cannabis use is related to panic,49 yet correlational studies suggest both benefit and problems for individuals who use cannabis for posttraumatic stress disorder.50 It is now well established that marijuana use is an independent causal risk factor for the development of psychosis, particularly in vulnerable youth, and that it worsens schizophrenia in those who suffer from it.51 Human experimental studies suggest this may be because the effect of THC is counteracted by CBD.52 Synthetic cannabinoids are even more potent anxiogenic and psychogenic agents than plant-based marijuana.19,20
Cannabis Use Disorder
About 9% of those who try cannabis develop Cannabis Use Disorder, which is characterized by continued use of the substance despite significant distress or impairment.53 Cannabis Use Disorder is essentially an addiction. Primary risk factors include male gender, younger age at marijuana initiation, and personal or family history of other substance or psychiatric problems.53
Although cannabis use often precedes use of other addiction-prone substances, it remains unclear if it is a “gateway” to the use of other illicit drugs.54 Marijuana withdrawal is relatively minor and is comparable to that for tobacco.55 While there are no known effective pharmacotherapies for discontinuing cannabis use, addiction therapy—including cognitive behavioral therapy and trigger management—is effective.56
SIDEBAR
Cannabinoids: A diverse group of chemicalsCannabis, the genus name for 3 species of marijuana plant (sativa, indica, ruderalis), has come to mean any psychoactive part of the plant and is used interchangeably with “marijuana.” There are at least 85 different cannabinoids in the native plant.7
Cannabinoids are a diverse group of chemicals that have activity at cannabinoid receptors. Δ-9-tetrahydrocannabinol (THC), a partial agonist of the CB1 receptor, is the primary psychoactive component and is found in larger quantities in Cannabis sativa, which is preferred by non-medical users. Cannabidiol (CBD), a weak partial CB1 antagonist, exhibits few, if any, psychotropic properties and is more plentiful in Cannabis indica.
Synthetic cannabinioids are a heterogeneous group of manufactured drugs that are full CB1 agonists and that are more potent than THC, yet are often assumed to be safe by users. Typically, they are dissolved in solvents, sprayed onto inert plant materials, and marketed as herbal products like “K2” and “spice.”
So how should the evidence inform your care?
Screen all patients for use of cannabinoids and other addiction-prone substances.57 Follow any affirmative answers to your questions about cannabis use by asking about potential negative consequences of use. For example, ask patients:
- How often during the past 6 months did you find that you were unable to stop using cannabis once you started?
- How often during the past 6 months did you fail to do what was expected of you because of using cannabis? (For more questions, see the Cannabis Use Disorder Identification Test available at: http://www.otago.ac.nz/nationaladdictioncentre/pdfs/cudit-r.pdf.)
Other validated screening tools include the Severity of Dependence Scale, the Cannabis Abuse Screening Test, and the Problematic Use of Marijuana.58
Counsel patients about possible adverse effects and inform them there is no evidence that recreational marijuana or synthetic cannabinoids can be used safely over time. Consider medical use requests only if there is a favorable risk/benefit balance, other recognized treatment options have been exhausted, and you have a strong understanding of the use of cannabis in the medical condition being considered.4
Since brief interventions using motivational interviewing to reduce or eliminate recreational use have not been found to be effective,59 referral to an addiction specialist may be indicated. If a diagnosis of cannabis use disorder is established, then abstinence from addiction-prone substances including marijuana, programs like Marijuana Anonymous (Available at: https://www.marijuana-anonymous.org/), and individualized addiction therapy scaled to the severity of the condition can be effective.56 Because psychiatric conditions frequently co-occur and complicate addiction,53 they should be screened for and managed, as well.
Drug testing. Cannabis Use Disorder has significant relapse potential.60 Abstinence and treatment adherence should be ascertained through regular follow-up that includes a clinical interview, exam, and body fluid drug testing. Point-of-care urine analysis for substances of potential addiction has limited utility. Definitive testing of urine with gas chromotography/mass spectrometry (GC/MS) or liquid chromatography (LC/MS-MS) can eliminate THC false-positives and false-negatives that can occur with point-of-care urine immunoassays. In addition, GCMS and LC/MS-MS can identify synthetic cannabinoids; in-office immunoassays cannot.
If the patient relapses, subsequent medical care should be coordinated with an addiction specialist with the goal of helping the patient to abstain from cannabis.
CORRESPONDENCE
Steven Wright, MD, FAAFP, 5325 Ridge Trail, Littleton, CO 80123; [email protected].
1. Pew Research Center. 6 facts about marijuana. Available at: http://www.pewresearch.org/fact-tank/2015/04/14/6-facts-about-marijuana/. Accessed September 27, 2016.
2. Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. HHS Pub # (SMA) 14-4863. 2014. Available at: http://www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.pdf. Accessed September 27, 2015.
3. Johnston LD, O’Malley PM, Miech RA, et al. Monitoring the Future National Survey on Drug Use 1975-2015. Available at: http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2015.pdf. Accessed September 23, 2015.
4. Metts J, Wright S, Sundaram J, et al. Medical marijuana: a treatment worth trying? J Fam Pract. 2016;65:178-185.
5. Governing the states and localities. State marijuana laws map. Available at: http://www.governing.com/gov-data/state-marijuana-laws-map-medical-recreational.html. Accessed October 12, 2016.
6. US Drug Enforcement Administration. Drug scheduling. Available at: https://www.dea.gov/druginfo/ds.shtml. Accessed October 12, 2016.
7. El-Alfy AT, Ivey K, Robinson K, et al. Antidepressant-like effect of Δ9-tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L. Pharmacol Biochem Behav. 2010;95:434-442.
8. Peters EN, Budney AJ, Carroll KM. Clinical correlates of co-occurring cannabis and tobacco use: a systematic review. Addiction. 2012;107:1404-1417.
9. Gruber AJ, Pope HG, Hudson JI, et al. Attributes of long-term heavy cannabis users: a case-control study. Psychol Med. 2003;33:1415-1422.
10. Palamar JJ, Fenstermaker M, Kamboukos D, et al. Adverse psychosocial outcomes associated with drug use among US high school seniors: a comparison of alcohol and marijuana. Am J Drug Alcohol Abuse. 2014;40:438-446.
11. Zwerling C, Ryan J, Orav EJ. The efficacy of preemployment drug screening for marijuana and cocaine in predicting employment outcome. JAMA. 1990;264:2639-2643.
12. Fergusson DM, Horwood LJ, Swain-Campbell N. Cannabis use and psychosocial adjustment in adolescence and young adulthood. Addiction. 2002;97:1123-1135.
13. Bell C, Slim J, Flaten HK, et al. Butane hash oil burns associated with marijuana liberalization in Colorado. J Med Toxicol. 2015;11:422-425.
14. Huang DYC, Lanza HI, Anglin MD. Association between adolescent substance use and obesity in young adulthood: a group-based dual trajectory analysis. Addict Behav. 2013;38:2653-2660.
15. Rajavashisth TB, Shaheen M, Norris KC, et al. Decreased prevalence of diabetes in marijuana users: cross-sectional data from the National Health and Nutrition Examination Survey (NHANES) III. BMJ Open. 2012;2:e000494.
16. Cho CM, Hirsch R, Johnstone S. General and oral health implications of cannabis use. Aust Dent J. 2005;50:70-74.
17. Gorzalka BB, Hill MN, Chang SC. Male-female differences in the effects of cannabinoids on sexual behavior and gonadal hormone function. Horm Behav. 2010;58:91-99.
18. Desbois AC, Cacoub P. Cannabis-associated arterial disease. Ann Vasc Surg. 2013;27:996-1005.
19. Mills B, Yepes A, Nugent K. Synthetic cannabinoids. Am J Med Sci. 2015;350:59-62.
20. Tuv SS, Strand MC, Karinen R, et al. Effect and occurrence of synthetic cannabinoids. Tidsskr Nor Laegeforen. 2012;132:2285-2288.
21. Wallace EA, Andrews SE, Garmany CL, et al. Cannabinoid hyperemesis syndrome: literature review and proposed diagnosis and treatment algorithm. South Med J. 2011;104:659-964.
22. Gates P, Jaffe A, Copeland J. Cannabis smoking and respiratory health: considerations of the literature. Respirology. 2014;19:655-662.
23. Pletcher MJ, Vittinghoff E, Kalhan R, et al. Association between marijuana exposure and pulmonary function over 20 years: The Coronary Artery Risk Development in Young Adults (CARDIA) study. JAMA. 2012;307:173-181.
24. Verweij PE, Kerremans JJ, Vos A, et al. Fungal contamination of tobacco and marijuana. JAMA. 2000;284:2875.
25. Office of Environmental Health Hazard Assessment. Evidence on the carcinogenicity of marijuana smoke. August 2009. Available at: http://oehha.ca.gov/media/downloads/crnr/finalmjsmokehid.pdf. Accessed September 5, 2015.
26. Stone D. Cannabis, pesticides and conflicting laws: the dilemma for legalized States and implications for public health. Regul Toxicol Pharmacol. 2014;69:284-288.
27. Hashibe M, Straif K, Tashkin DP, et al. Epidemiologic review of marijuana and cancer risk. Alcohol. 2005;35:265-275.
28. Liang C, McClean MD, Marsit C, et al. A population-based case-control study of marijuana use and head and neck squamous cell carcinoma. Cancer Prev Res (Phila). 2009;2:759-768.
29. Thomas AA, Wallner LP, Quinn VP, et al. Association between cannabis use and the risk of bladder cancer: results from the California Men’s Health Study. Urology. 2015;85:388-392.
30. Holly EA, Lele C, Bracci PM, et al. Case-control study of non-Hodgkin’s lymphoma among women and heterosexual men in the San Francisco Bay area, California. Am J Epidemiol. 1999;150:375-389.
31. Massi P, Solinas M, Cinquina V, et al. Cannabidiol as potential anticancer drug. Br J Clin Pharmacol. 2013;75:303-312.
32. Ashbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ. 2012;344:e536.
33.Barrio G, Jimenez-Mejias E, Pulido J, et al. Association between cannabis use and non-traffic injuries. Accid Anal Prev. 2012;47:172-176.
34. MacDonald S, Hall W, Roman P, et al. Testing for cannabis in the work-place: a review of the evidence. Addiction. 2010;105:408-416.
35. Sewell RA, Poling J, Sofuoglu M. The effect of cannabis compared with alcohol on driving. Am J Addict. 2009;18:185-193.
36. Ramaekers JG, Berghaus G, van Laar M, et al. Dose related risk of motor vehicle crashes after cannabis use. Drug Alcohol Depend. 2004;73:109-119.
37. Menetrey A, Augsburger M, Favrat B, et al. Assessment of driving capability through the use of clinical and psychomotor tests in relation to blood cannabinoid levels following oral administration of 20 mg dronabinol or of a cannabis decoction made with 20 or 60 mg Δ9-THC. J Anal Toxicol. 2005;29:327-338.
38. Raemakers JG, Kaurert G, van Ruitenbeek P, et al. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology. 2006;31:2296-2303.
39. Leirer VO, Yesavage JA, Morrow DG. Marijuana carry-over effects on aircraft pilot performance. Aviat Space Environ Med. 1991;62:221-227.
40. Calabria B, Degenhardt L, Hall W, et al. Does cannabis use increase the risk of death? Systematic review of epidemiological evidence on adverse effects of cannabis use. Drug Alcohol Rev. 2010;29:318-330.
41. Colorado Department of Public Health and Environment. Monitoring health concerns related to marijuana in Colorado: 2014. Changes in marijuana use patterns, systematic literature review, and possible marijuana-related health effects. Available at: http://www2.cde.state.co.us/artemis/hemonos/he1282m332015internet/he1282m332015internet01.pdf. Accessed September 5, 2015.
42. Behnke M, Smith VC, Committee on Substance Abuse, Committee on Fetus and Newborn. Perinatal substance abuse: short- and long-term effects on the exposed fetus. Pediatrics. 2013;131:e1009-1024.
43. Batalla A, Bhattacharyya S, Yücel M, et al. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS One. 2013;8:e55821.
44. Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med. 2011;5:1-8.
45. Pavisian B, MacIntosh BJ, Szilagyi G, et al. Effects of cannabis on cognition in patients with multiple sclerosis: a psychometric and MRI study. Neurology. 2014;82:1879-1887.
46. Bartholomew J, Holroyd S, Heffernan TM. Does cannabis use affect prospective memory in young adults? J Psychopharmacol. 2010;24:241-246.
47. Copeland J, Rooke S, Swift W. Changes in cannabis use among young people: impact on mental health. Curr Opin Psychiatry. 2013;26:325-329.
48. Ari M, Sahpolat M, Kokacya H, et al. Amotivational syndrome: less known and diagnosed as a clinical. J Mood Disord. 2015;5:31-35.
49. Zvolensky MJ, Cougle JR, Johnson KA, et al. Marijuana use and panic psychopathology among a representative sample of adults. Exp Clin Psychopharmacol. 2010;18(2):129-134.
50. Yarnell S. The use of medicinal marijuana for posttraumatic stress disorder: a review of the current literature. Prim Care Companion CNS Disord. 2015;17(3).
51. Le Bec PY, Fatséas M, Denis C, et al. Cannabis and psychosis: search of a causal link through a critical and systematic review. Encephale. 2009;35:377-385.
52. Englund A, Morrison PD, Nottage J, et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol. 2013;27:19-27.
53. Lopez-Quintero C, Perez de los Cobos J, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 2011:115:120-130.
54. Degenhardt L, Dierker L, Chiu WT, et al. Evaluating the drug use “gateway” theory using cross-national data: consistency and associations of the order of initiation of drug use among participants in the WHO World Mental Health Surveys. Drug Alcohol Depend. 2010;108:84-97.
55. Vandrey RG, Budney AJ, Hughes JR, et al. A within subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depend. 2008;92:48-54.
56.Budney AJ, Roffman R, Stephens RS, et al. Marijuana dependence and its treatment. Addict Sci Clin Pract. 2007;4:4-16.
57. Turner SD, Spithoff S, Kahan M. Approach to cannabis use disorder in primary care: focus on youth and other high-risk users. Can Fam Phys. 2014;60:801-808.
58. Piontek D, Kraus L, Klempova D. Short scales to assess cannabis-related problems: a review of psychometric properties. Subst Abuse Treat Prev Policy. 2008;3:25.
59. Saitz R, Palfai TPA, Cheng DM, et al. Screening and brief intervention for drug use in primary care: the ASPIRE randomized clinical trial. JAMA. 2014;312:502-513.
60. McLellan AT, Lewis DC, O’Brien CP, et al. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689-1695.
1. Pew Research Center. 6 facts about marijuana. Available at: http://www.pewresearch.org/fact-tank/2015/04/14/6-facts-about-marijuana/. Accessed September 27, 2016.
2. Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. HHS Pub # (SMA) 14-4863. 2014. Available at: http://www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.pdf. Accessed September 27, 2015.
3. Johnston LD, O’Malley PM, Miech RA, et al. Monitoring the Future National Survey on Drug Use 1975-2015. Available at: http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2015.pdf. Accessed September 23, 2015.
4. Metts J, Wright S, Sundaram J, et al. Medical marijuana: a treatment worth trying? J Fam Pract. 2016;65:178-185.
5. Governing the states and localities. State marijuana laws map. Available at: http://www.governing.com/gov-data/state-marijuana-laws-map-medical-recreational.html. Accessed October 12, 2016.
6. US Drug Enforcement Administration. Drug scheduling. Available at: https://www.dea.gov/druginfo/ds.shtml. Accessed October 12, 2016.
7. El-Alfy AT, Ivey K, Robinson K, et al. Antidepressant-like effect of Δ9-tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L. Pharmacol Biochem Behav. 2010;95:434-442.
8. Peters EN, Budney AJ, Carroll KM. Clinical correlates of co-occurring cannabis and tobacco use: a systematic review. Addiction. 2012;107:1404-1417.
9. Gruber AJ, Pope HG, Hudson JI, et al. Attributes of long-term heavy cannabis users: a case-control study. Psychol Med. 2003;33:1415-1422.
10. Palamar JJ, Fenstermaker M, Kamboukos D, et al. Adverse psychosocial outcomes associated with drug use among US high school seniors: a comparison of alcohol and marijuana. Am J Drug Alcohol Abuse. 2014;40:438-446.
11. Zwerling C, Ryan J, Orav EJ. The efficacy of preemployment drug screening for marijuana and cocaine in predicting employment outcome. JAMA. 1990;264:2639-2643.
12. Fergusson DM, Horwood LJ, Swain-Campbell N. Cannabis use and psychosocial adjustment in adolescence and young adulthood. Addiction. 2002;97:1123-1135.
13. Bell C, Slim J, Flaten HK, et al. Butane hash oil burns associated with marijuana liberalization in Colorado. J Med Toxicol. 2015;11:422-425.
14. Huang DYC, Lanza HI, Anglin MD. Association between adolescent substance use and obesity in young adulthood: a group-based dual trajectory analysis. Addict Behav. 2013;38:2653-2660.
15. Rajavashisth TB, Shaheen M, Norris KC, et al. Decreased prevalence of diabetes in marijuana users: cross-sectional data from the National Health and Nutrition Examination Survey (NHANES) III. BMJ Open. 2012;2:e000494.
16. Cho CM, Hirsch R, Johnstone S. General and oral health implications of cannabis use. Aust Dent J. 2005;50:70-74.
17. Gorzalka BB, Hill MN, Chang SC. Male-female differences in the effects of cannabinoids on sexual behavior and gonadal hormone function. Horm Behav. 2010;58:91-99.
18. Desbois AC, Cacoub P. Cannabis-associated arterial disease. Ann Vasc Surg. 2013;27:996-1005.
19. Mills B, Yepes A, Nugent K. Synthetic cannabinoids. Am J Med Sci. 2015;350:59-62.
20. Tuv SS, Strand MC, Karinen R, et al. Effect and occurrence of synthetic cannabinoids. Tidsskr Nor Laegeforen. 2012;132:2285-2288.
21. Wallace EA, Andrews SE, Garmany CL, et al. Cannabinoid hyperemesis syndrome: literature review and proposed diagnosis and treatment algorithm. South Med J. 2011;104:659-964.
22. Gates P, Jaffe A, Copeland J. Cannabis smoking and respiratory health: considerations of the literature. Respirology. 2014;19:655-662.
23. Pletcher MJ, Vittinghoff E, Kalhan R, et al. Association between marijuana exposure and pulmonary function over 20 years: The Coronary Artery Risk Development in Young Adults (CARDIA) study. JAMA. 2012;307:173-181.
24. Verweij PE, Kerremans JJ, Vos A, et al. Fungal contamination of tobacco and marijuana. JAMA. 2000;284:2875.
25. Office of Environmental Health Hazard Assessment. Evidence on the carcinogenicity of marijuana smoke. August 2009. Available at: http://oehha.ca.gov/media/downloads/crnr/finalmjsmokehid.pdf. Accessed September 5, 2015.
26. Stone D. Cannabis, pesticides and conflicting laws: the dilemma for legalized States and implications for public health. Regul Toxicol Pharmacol. 2014;69:284-288.
27. Hashibe M, Straif K, Tashkin DP, et al. Epidemiologic review of marijuana and cancer risk. Alcohol. 2005;35:265-275.
28. Liang C, McClean MD, Marsit C, et al. A population-based case-control study of marijuana use and head and neck squamous cell carcinoma. Cancer Prev Res (Phila). 2009;2:759-768.
29. Thomas AA, Wallner LP, Quinn VP, et al. Association between cannabis use and the risk of bladder cancer: results from the California Men’s Health Study. Urology. 2015;85:388-392.
30. Holly EA, Lele C, Bracci PM, et al. Case-control study of non-Hodgkin’s lymphoma among women and heterosexual men in the San Francisco Bay area, California. Am J Epidemiol. 1999;150:375-389.
31. Massi P, Solinas M, Cinquina V, et al. Cannabidiol as potential anticancer drug. Br J Clin Pharmacol. 2013;75:303-312.
32. Ashbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ. 2012;344:e536.
33.Barrio G, Jimenez-Mejias E, Pulido J, et al. Association between cannabis use and non-traffic injuries. Accid Anal Prev. 2012;47:172-176.
34. MacDonald S, Hall W, Roman P, et al. Testing for cannabis in the work-place: a review of the evidence. Addiction. 2010;105:408-416.
35. Sewell RA, Poling J, Sofuoglu M. The effect of cannabis compared with alcohol on driving. Am J Addict. 2009;18:185-193.
36. Ramaekers JG, Berghaus G, van Laar M, et al. Dose related risk of motor vehicle crashes after cannabis use. Drug Alcohol Depend. 2004;73:109-119.
37. Menetrey A, Augsburger M, Favrat B, et al. Assessment of driving capability through the use of clinical and psychomotor tests in relation to blood cannabinoid levels following oral administration of 20 mg dronabinol or of a cannabis decoction made with 20 or 60 mg Δ9-THC. J Anal Toxicol. 2005;29:327-338.
38. Raemakers JG, Kaurert G, van Ruitenbeek P, et al. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology. 2006;31:2296-2303.
39. Leirer VO, Yesavage JA, Morrow DG. Marijuana carry-over effects on aircraft pilot performance. Aviat Space Environ Med. 1991;62:221-227.
40. Calabria B, Degenhardt L, Hall W, et al. Does cannabis use increase the risk of death? Systematic review of epidemiological evidence on adverse effects of cannabis use. Drug Alcohol Rev. 2010;29:318-330.
41. Colorado Department of Public Health and Environment. Monitoring health concerns related to marijuana in Colorado: 2014. Changes in marijuana use patterns, systematic literature review, and possible marijuana-related health effects. Available at: http://www2.cde.state.co.us/artemis/hemonos/he1282m332015internet/he1282m332015internet01.pdf. Accessed September 5, 2015.
42. Behnke M, Smith VC, Committee on Substance Abuse, Committee on Fetus and Newborn. Perinatal substance abuse: short- and long-term effects on the exposed fetus. Pediatrics. 2013;131:e1009-1024.
43. Batalla A, Bhattacharyya S, Yücel M, et al. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS One. 2013;8:e55821.
44. Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med. 2011;5:1-8.
45. Pavisian B, MacIntosh BJ, Szilagyi G, et al. Effects of cannabis on cognition in patients with multiple sclerosis: a psychometric and MRI study. Neurology. 2014;82:1879-1887.
46. Bartholomew J, Holroyd S, Heffernan TM. Does cannabis use affect prospective memory in young adults? J Psychopharmacol. 2010;24:241-246.
47. Copeland J, Rooke S, Swift W. Changes in cannabis use among young people: impact on mental health. Curr Opin Psychiatry. 2013;26:325-329.
48. Ari M, Sahpolat M, Kokacya H, et al. Amotivational syndrome: less known and diagnosed as a clinical. J Mood Disord. 2015;5:31-35.
49. Zvolensky MJ, Cougle JR, Johnson KA, et al. Marijuana use and panic psychopathology among a representative sample of adults. Exp Clin Psychopharmacol. 2010;18(2):129-134.
50. Yarnell S. The use of medicinal marijuana for posttraumatic stress disorder: a review of the current literature. Prim Care Companion CNS Disord. 2015;17(3).
51. Le Bec PY, Fatséas M, Denis C, et al. Cannabis and psychosis: search of a causal link through a critical and systematic review. Encephale. 2009;35:377-385.
52. Englund A, Morrison PD, Nottage J, et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol. 2013;27:19-27.
53. Lopez-Quintero C, Perez de los Cobos J, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 2011:115:120-130.
54. Degenhardt L, Dierker L, Chiu WT, et al. Evaluating the drug use “gateway” theory using cross-national data: consistency and associations of the order of initiation of drug use among participants in the WHO World Mental Health Surveys. Drug Alcohol Depend. 2010;108:84-97.
55. Vandrey RG, Budney AJ, Hughes JR, et al. A within subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depend. 2008;92:48-54.
56.Budney AJ, Roffman R, Stephens RS, et al. Marijuana dependence and its treatment. Addict Sci Clin Pract. 2007;4:4-16.
57. Turner SD, Spithoff S, Kahan M. Approach to cannabis use disorder in primary care: focus on youth and other high-risk users. Can Fam Phys. 2014;60:801-808.
58. Piontek D, Kraus L, Klempova D. Short scales to assess cannabis-related problems: a review of psychometric properties. Subst Abuse Treat Prev Policy. 2008;3:25.
59. Saitz R, Palfai TPA, Cheng DM, et al. Screening and brief intervention for drug use in primary care: the ASPIRE randomized clinical trial. JAMA. 2014;312:502-513.
60. McLellan AT, Lewis DC, O’Brien CP, et al. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689-1695.
Drug-induced weight gain: Rethinking our choices
PRACTICE RECOMMENDATIONS
› Choose weight-loss-promoting medications, such as metformin, sodium-glucose co-transporter 2 inhibitors, and glucagon-like peptide-1 agonists, and weight-neutral medications, such as DPP-4 inhibitors, as first- and second-line agents for patients with type 2 diabetes who are overweight or obese. A
› Prescribe angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or calcium channel blockers as first- and second-line antihypertensive therapy for patients who are overweight or obese. A
› Select antidepressants that promote weight loss, such as bupropion, or weight-neutral agents, such as fluoxetine and sertraline, for patients who are overweight or obese and require treatment for depression. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Medications can have an unpredictable and variable effect on weight. Some drugs trigger weight gain in one patient while inducing weight loss in another. Others may lead to weight loss initially but cause weight gain when taken long term.1 Often, a drug’s effect on a patient’s weight depends on his or her medical history and lifestyle, including factors like insulin resistance, diet, and exercise level.
To make matters worse, clinical studies of drug-related effects on weight can be misleading. Because researchers often report a mean weight change—an average of those who had little or no change in weight when taking the drug and individuals who may have gained a significant amount of weight—a drug’s potential to cause weight gain may be underestimated. Few studies include an analysis of the range—eg, how many participants gained or lost various percentages of body weight. What’s more, pharmacology studies typically follow participants for a few months to a few years, whereas weight changes can be cumulative when a medication is taken for many years.
The nation’s continually growing obesity epidemic makes it crucial for physicians to consider the weight effects of medications being prescribed and to balance the benefits of treatment with the potential for weight gain. Until recently, the medical literature offered little guidance.
In 2015, the Endocrine Society published clinical practice guidelines for pharmacologic management of obesity, including data on medications that cause weight gain and suggesting alternatives that are weight-neutral or promote weight loss.2
In the pages that follow, we present case studies, tables, and a review of the latest evidence to highlight optimal drug treatment for patients who are overweight or obese, and are also being treated for diabetes, hypertension, and depression. You’ll find a brief discussion of weight management strategies related to other drugs and conditions in the sidebar.2-5
CASE 1 › 40-year-old man with diabetes and hyperlipidemia
Brian P, who has come in for an annual checkup, has a body mass index (BMI) of 30 kg/m2. He also has hyperlipidemia and type 2 diabetes, for which he has been taking metformin for several years. A year ago, his hemoglobin A1c (HbA1c) was 7.3%, so his physician added glyburide to his regimen.
In the year since, Mr. P has gained 12 lbs (5.4 kg) but achieved only a minimal reduction in HbA1c (to 6.8%). He expresses concern about the cardiovascular effects of the extra weight and says that diet and exercise have not helped him control his weight.
CASE 2 › Older woman with hypertension and hypothyroidism
Addie K, age 64, is obese (BMI, 37 kg/m2) and has hypertension and hypothyroidism, for which she takes metoprolol and levothyroxine. Ms. K says that she is careful about what she eats and exercises several times a week, but still has seen her weight increase steadily for the past several years.
CASE 3 › Young man with depression
Charlie D, a 21-year-old college student, is a new patient. He has depression and is obese (BMI, 34 kg/m2). The patient says he was diagnosed with depression by his former primary care physician, who prescribed paroxetine a year ago. He requests a refill of the paroxetine, which he reports has successfully boosted his mood. When asked about his weight, he admits that he has gained 8 lbs (3.6 kg) since he began taking the drug.
If these were your patients, what weight management steps would you take? Before we provide some recommendations, let’s review the evidence.
Antidiabetic agents and weight
While some antidiabetic agents are weight-neutral and others promote weight loss, several therapies are associated with weight gain6 (TABLE 13). Patients like Mr. P can gain as much as 10 kg in 3 to 6 months after beginning treatment with insulin, thiazolidinediones (TZDs), sulfonylureas, and other insulin secretagogues.2,7
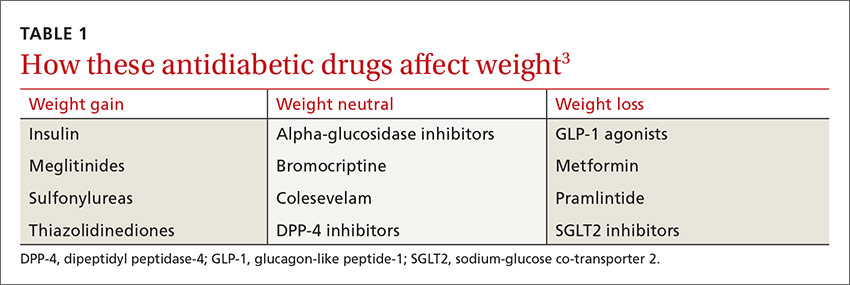
A recent systematic review and meta-analysis of 257 randomized controlled trials (RCTs) found weight gain to be associated with the use of pioglitazone (2.6 kg), glimepiride (2.1 kg), glyburide (2.6 kg), glipizide (2.2 kg), and sitagliptin (0.55 kg). A modest weight loss was associated with acarbose, exenatide, liraglutide, metformin, miglitol, and pramlintide.8
Sulfonylureas are generally associated with a 1.5 to 2.5 kg weight gain.9-11 In an analysis of 27 RCTs of noninsulin antidiabetic drugs in patients whose disease was not controlled by metformin alone, TZDs, sulfonylureas, and meglitinides were associated with a 1.77 to 2.08 kg weight gain.9 Furthermore, those taking sulfonylureas and meglitinides had higher rates of hypoglycemia compared with patients taking placebo (relative risk, 4.50-7.50). In fact, sulfonylureas have the highest risk of serious hypoglycemia of any noninsulin therapy.6
In contrast, metformin—the most commonly prescribed oral agent for type 2 diabetes—promotes mild weight loss by multiple mechanisms and has a good safety profile.12,13 Thus, some physicians use metformin off label for weight loss and diabetes prevention and have suggested that it be approved for these indications.13
Glycemic control and weight loss
Glucagon-like peptide-1 (GLP-1) agonists lead to weight loss by decreasing appetite and enhancing satiety, as well as improving glycemic control. Liraglutide received Food and Drug Administration (FDA) approval in 2014 as a treatment for chronic weight management at a higher dose (3 mg/d) than that used to treat diabetes (1.8 mg/d).14
Sodium-glucose co-transporter 2 (SGLT2) inhibitors are a relatively new class of antidiabetic medication that reduce glucose reabsorption by the kidneys, leading to increased urinary glucose excretion.15 The associated weight loss, in addition to a reduction in hyperglycemia, may be due to the subsequent calorie loss through glycosuria.
Both dipeptidyl peptidase-4 (DPP-4) inhibitors and alpha-glucosidase inhibitors (AGIs) appear to be weight-neutral or to induce minimal changes in weight.16 Although the systematic review mentioned earlier found a 0.55 kg weight gain associated with sitagliptin,8 most studies of DPP-4 inhibitors report weight neutrality.17-19 Pramlintide, the amylin analogue that has FDA approval for use in combination with existing insulin treatment, can prevent weight gain or lead to weight loss.20,21
The Endocrine Society Clinical Practice Guideline recommends concomitantly prescribing at least one weight loss-promoting medication (such as metformin, a GLP-1 agonist, or pramlintide) to patients with obesity and type 2 diabetes who require insulin to mitigate weight gain due to insulin.2
The 2016 Comprehensive Type 2 Diabetes Management Algorithm published by the American Association of Clinical Endocrinologists and American College of Endocrinology recommends that the initiation of diabetes therapies be based on the risks of weight gain and hypoglycemia, among other factors. The algorithm calls for metformin as first-line therapy, followed by a GLP-1 agonist as a second-line therapy, and an SGLT2 inhibitor as a third-line therapy.6
Finally, FDA-approved anti-obesity medications may be appropriate for patients with diabetes who are unable to lose weight with lifestyle interventions alone.22 Each medication is associated with improvements in glucose in addition to other metabolic parameters.
CASE 1 › A better choice for Mr. P
Because Mr. P has gained weight—and, indeed, developed obesity—since he started taking glyburide, it is clear that a sulfonylurea is not the best choice for this patient. An antidiabetic agent that is weight-neutral or that promotes weight loss, such as an SGLT2 inhibitor or a GLP-1 agonist, would be more suitable. The drug should be prescribed in conjunction with his metformin, which has a favorable weight profile and helps reduce HbA1c, as both SGLT2 inhibitors and GLP-1 agonists also do.
If Mr. P were to switch to an SGLT2 inhibitor, a combination pill containing metformin would be an effective way to limit the patient’s pill burden.
Treating hypertension without weight gain
Thiazide diuretics are often recommended as first-line agents for the treatment of hypertension, but their dose-related adverse effects, including dyslipidemia and insulin resistance, are undesirable for patients who are overweight or obese and at risk for metabolic syndrome and type 2 diabetes.23 Beta-adrenergic blockers have been shown to promote weight gain and prevent weight loss, especially in patients who have both hypertension and diabetes.24 In addition to having potential adverse metabolic effects on lipids and/or insulin sensitivity, beta-blockers can decrease metabolic rate by 10% and they may have other negative effects on energy metabolism, as well.25
In a meta-analysis of 8 RCTs that lasted ≥6 months, changes in body weight were higher in participants on beta-blockers, with a median difference of 1.2 kg (−0.4 to 3.5 kg) between those on beta-blockers and the control group.26 The evidence suggests that beta-blockers should not necessarily be first-line treatment for hypertension in patients who are overweight or obese and that obesity management in patients with hypertension may be harder if they are being treated with a beta-blocker.
When a different drug in the same class will do
There are exceptions, however. When beta-blockers are required—for patients with coronary artery disease, heart failure, or an arrhythmia, for example—a selective agent with a vasodilating component, such as carvedilol or nebivolol, is recommended.2 These drugs appear to have less potential for weight gain and to have minimal effect on lipid and glucose metabolism.26,27
In a study of 1106 patients with hypertension, those taking metoprolol had a statistically significant mean weight gain of 1.19 kg (P<.001) compared with patients taking carvedilol (mean weight gain, 0.17 kg; P=.36).24 While 4.5% of those in the metoprolol group gained ≥7% of their body weight, that was true of only 1.1% of those taking carvedilol. Thus, weight gain can sometimes be minimized by choosing a different medication within the same drug class.
ACE inhibitors, ARBs, and calcium channel blockers
Antihypertensive medications that are not associated with weight gain or insulin resistance include angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and calcium channel blockers (CCBs) (TABLE 2).3 Angiotensin contributes to obesity-related hypertension, as it is overexpressed in obesity, making ACE inhibitors and ARBs desirable options for the treatment of patients who are obese. And, because many patients who are obese also suffer from type 2 diabetes or prediabetes, they’re likely to benefit from the renal protection provided by ACE inhibitors and ARBs, as well.
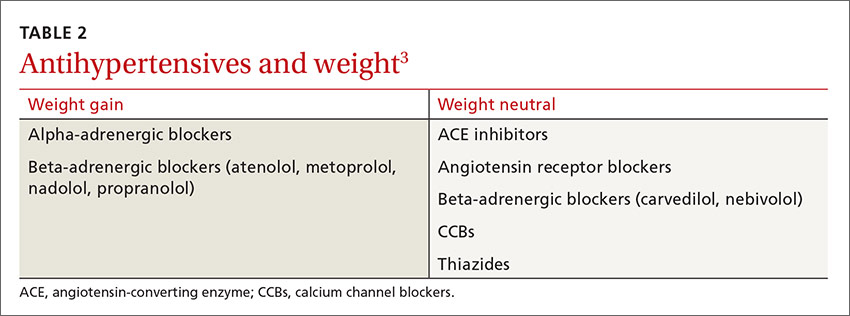
CASE 2 › Switching antihypertensives
Switching Ms. K from metoprolol, a beta-blocker, to an ACE inhibitor, ARB, or CCB may help prevent further weight gain, and possibly even lead to weight loss. Any drug in any of these 3 classes of medications would be a reasonable choice. However, if the patient had a condition that warranted use of a beta-blocker, a selective agent with a vasodilating component such as carvedilol or nebivolol might be helpful.
SIDEBAR
Weight management strategies for several other conditionsIn addition to medications for common conditions such as diabetes, hypertension, and depression, there are numerous other drugs that can cause unwanted weight gain. These include some antiseizure agents, antipsychotics, contraceptives, hormones, and migraine therapies, as well as corticosteroids. In view of both the nation’s obesity epidemic and the many drugs that are known to adversely affect weight maintenance, it is crucial to do a careful risk-benefit analysis and a search for alternatives whenever you prescribe a new medication for a patient who is overweight or obese or has metabolic risk factors.2-5
When weight-neutral substitutes exist, such medications should be considered, if appropriate, to prevent or lessen pharmacologic weight gain. For example, topiramate and zonisamide are preferable to other antiepileptics, such as valproic acid and gabapentin when it comes to weight management.2-4 It is essential to keep in mind, however, that medications in the same class are not always interchangeable.
For patients with inflammatory conditions such as rheumatoid arthritis, disease-modifying antirheumatic drugs (DMARDs) are preferable to corticosteroids whenever possible.2-4 For the many patients for whom steroids or other drugs known to cause weight gain are necessary, however, dietary and lifestyle counseling—advising patients to eat a healthful diet and maintain adequate activity levels, among other interventions—may help to mitigate the effects.
And when there are no alternative medications available, use the lowest possible dose for the shortest duration necessary.
Choosing an antidepressant when weight is an issue
For patients with psychiatric conditions, weight gain is often multifactorial. One key issue: Weight gain is a common adverse effect of many antidepressants (TABLE 3).3 Within classes of antidepressants, there is a range of weight gain potential, which can vary depending on the duration of therapy.2
In a meta-analysis of 116 studies, selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine and sertraline were associated with weight loss in short-term use (4-12 weeks) and weight neutrality when used for >4 months.1 Patients who had type 2 diabetes as well as depression had an average weight loss from fluoxetine of 5.1 kg (3.3–6.9 kg) at 24- to 26-week follow up.28
Among SSRI and tricyclic (TCA) antidepressants, paroxetine and amitriptyline, respectively, had the greatest risk for weight gain.1,29 No significant weight effect was observed for either citalopram or escitalopram. Keep in mind, however, that the effect of each antidepressant on weight may vary greatly from one patient to another.1 For example, while Mr. D gained 3.6 kg on paroxetine, some patients gain no weight at all.
In the systematic review and meta-analysis of 257 RCTs, weight gain was associated with the use of amitriptyline (1.8 kg) and mirtazapine (1.5 kg), while weight loss was associated with bupropion and fluoxetine (-1.3 kg for each).8
This antidepressant can decrease cravings
Bupropion, a norepinephrine and dopamine reuptake inhibitor, is the only antidepressant that has been consistently shown to cause weight loss.30,31 Clinical trials have found that it decreases body weight by suppressing appetite and reducing food cravings.30 Bupropion is approved for the treatment of depression and as a smoking cessation aide. And, in 2014, a bupropion-naltrexone combination received FDA approval for chronic weight management, sold under the brand name Contrave.32
As different classes of antidepressants are often prescribed for different types of depression, it is important to be aware that the few that are weight-neutral and weight-loss-promoting are not appropriate for all patients with depression. For example, bupropion is an activating agent and can exacerbate anxiety. Thus, a patient with concomitant depression and anxiety might be a better candidate for another antidepressant, which could lead to some weight gain but would better manage the individual’s symptoms. In such cases, the rule of thumb should be to prescribe the lowest dose required for clinical efficacy for the shortest duration necessary.
CASE 3 › Change antidepressants— and keep a close watch
Depending on the nature of Mr. D’s depression, bupropion, fluoxetine, or sertraline might be a reasonable alternative to paroxetine to prevent or reduce further drug-induced weight gain.
Frequent follow-up visits should be scheduled until the transition has been completed and his condition stabilized. If Mr. D’s new antidepressant is bupropion, monitoring him for signs of anxiety would be required.
CORRESPONDENCE
Katherine H. Saunders, MD, Comprehensive Weight Control Center, Weill Cornell Medicine, 1165 York Avenue, New York, NY 10065; [email protected].
1. Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71:1259-1272.
2. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2015;100:342-362.
3. Apovian CM, Aronne L, Powell AG. Clinical Management of Obesity. West Islip, NY: Professional Communications, Inc., 2015.
4. Aronne LJ. A Practical Guide to Drug-induced Weight Gain. Minneapolis, Minn: McGraw-Hill; 2002.
5. Leslie WS, Hankey CR, Lean ME. Weight gain as an adverse effect of some commonly prescribed drugs: a systematic review. QJM. 2007;100:395-404.
6. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm – 2016 executive summary. Endocr Pract. 2016;22:84-113.
7. Aronne LJ. Drug-induced weight gain: non-CNS medications. In: A Practical Guide to Drug-Induced Weight Gain. Minneapolis, Minn: McGraw-Hill: 2002:77-91.
8. Domecq JP, Prutsky G, Leppin A, et al. Clinical review: drugs commonly associated with weight change: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100:363-370.
9. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
10. Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427-2443.
11. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–481.
12. Malin SK, Kashyap SR. Effects of metformin on weight loss: potential mechanisms. Curr Opin Endocrinol Diabetes Obes. 2014;21:323-329.
13. Igel LI, Sinha A, Saunders KH, et al. Metformin: an old therapy that deserves a new indication for the treatment of obesity. Curr Atheroscler Rep. 2016;18:16.
14. US Food and Drug Administration. FDA approves weight-management drug Saxenda. December 23, 2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427913.htm. Accessed October 1, 2016.
15. Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8:495-502.
16. van de Laar FA, Lucassen PL, Akkermans RP, et al. Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care. 2005;28:154-163.
17. Hong ES, Khang AR, Yoon JW, et al. Comparison between sitagliptin as add-on therapy to insulin and insulin dose-increase therapy in uncontrolled Korean type 2 diabetes: CSI study. Diabetes Obes Metab. 2012;14:795-802.
18. Arnolds S, Dellweg S, Clair J, et al. Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with insulin glargine and metformin: a proof-of-concept study. Diabetes Care. 2010;33:1509-1515.
19. Scheen AJ. DPP-4 inhibitors in the management of type 2 diabetes: a critical review of head-to-head trials. Diabetes Metab. 2012;38:89-101.
20. Hollander PA, Levy P, Fineman MS, et al. Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care. 2003;26:784-790.
21. Aronne L, Fujioka K, Aroda V, et al. Progressive reduction in body weight after treatment with the amylin analog pramlintide in obese subjects: a phase 2, randomized, placebo-controlled, dose-escalation study. J Clin Endocrinol Metab. 2007;92:2977-2983.
22. Saunders KH, Kumar RB, Igel LI, et al. Pharmacologic approaches to weight management: recent gains and shortfalls in combating obesity. Curr Atheroscler Rep. 2016;18:36.
23. Landsberg L, Aronne LJ, Beilin LJ, et al. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment—a position paper of the Obesity Society and the American Society of Hypertension. Obesity (Silver Spring). 2013;21:8-24.
24. Messerli FH, Bell DS, Fonseca V, et al. Body weight changes with beta-blocker use: results from GEMINI. Am J Med. 2007;120:610-615.
25. Pischon T, Sharma AM. Use of beta-blockers in obesity hypertension: potential role of weight gain. Obes Rev. 2001;2:275-280.
26. Sharma AM, Pischon T, Hardt S, et al. Hypothesis: beta-adrenergic receptor blockers and weight gain: a systematic analysis. Hypertension. 2001;37:250-254.
27. Manrique C, Whaley-Connell A, Sowers JR. Nebivolol in obese and non-obese hypertensive patients. J Clin Hypertens (Greenwich). 2009;11:309-315.
28. Norris SL, Zhang X, Avenell A, et al. Pharmacotherapy for weight loss in adults with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;(1):CD004096.
29. Rosenzweig-Lipson S, Beyer CE, Hughes ZA, et al. Differentiating antidepressants of the future: efficacy and safety. Pharmacol Ther. 2007;113:134-153.
30. Gadde KM, Xiong GL. Bupropion for weight reduction. Expert Rev Neurother. 2007;7:17-24.
31. Arterburn D, Sofer T, Boudreau DM, et al. Long-term weight change after initiating second-generation antidepressants. J Clin Med. 2016;5:piiE48.
32. US Food and Drug Administration. FDA approves weight-management drug Contrave. September 10, 2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm413896.htm. Accessed October 1, 2016.
PRACTICE RECOMMENDATIONS
› Choose weight-loss-promoting medications, such as metformin, sodium-glucose co-transporter 2 inhibitors, and glucagon-like peptide-1 agonists, and weight-neutral medications, such as DPP-4 inhibitors, as first- and second-line agents for patients with type 2 diabetes who are overweight or obese. A
› Prescribe angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or calcium channel blockers as first- and second-line antihypertensive therapy for patients who are overweight or obese. A
› Select antidepressants that promote weight loss, such as bupropion, or weight-neutral agents, such as fluoxetine and sertraline, for patients who are overweight or obese and require treatment for depression. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Medications can have an unpredictable and variable effect on weight. Some drugs trigger weight gain in one patient while inducing weight loss in another. Others may lead to weight loss initially but cause weight gain when taken long term.1 Often, a drug’s effect on a patient’s weight depends on his or her medical history and lifestyle, including factors like insulin resistance, diet, and exercise level.
To make matters worse, clinical studies of drug-related effects on weight can be misleading. Because researchers often report a mean weight change—an average of those who had little or no change in weight when taking the drug and individuals who may have gained a significant amount of weight—a drug’s potential to cause weight gain may be underestimated. Few studies include an analysis of the range—eg, how many participants gained or lost various percentages of body weight. What’s more, pharmacology studies typically follow participants for a few months to a few years, whereas weight changes can be cumulative when a medication is taken for many years.
The nation’s continually growing obesity epidemic makes it crucial for physicians to consider the weight effects of medications being prescribed and to balance the benefits of treatment with the potential for weight gain. Until recently, the medical literature offered little guidance.
In 2015, the Endocrine Society published clinical practice guidelines for pharmacologic management of obesity, including data on medications that cause weight gain and suggesting alternatives that are weight-neutral or promote weight loss.2
In the pages that follow, we present case studies, tables, and a review of the latest evidence to highlight optimal drug treatment for patients who are overweight or obese, and are also being treated for diabetes, hypertension, and depression. You’ll find a brief discussion of weight management strategies related to other drugs and conditions in the sidebar.2-5

CASE 1 › 40-year-old man with diabetes and hyperlipidemia
Brian P, who has come in for an annual checkup, has a body mass index (BMI) of 30 kg/m2. He also has hyperlipidemia and type 2 diabetes, for which he has been taking metformin for several years. A year ago, his hemoglobin A1c (HbA1c) was 7.3%, so his physician added glyburide to his regimen.
In the year since, Mr. P has gained 12 lbs (5.4 kg) but achieved only a minimal reduction in HbA1c (to 6.8%). He expresses concern about the cardiovascular effects of the extra weight and says that diet and exercise have not helped him control his weight.
CASE 2 › Older woman with hypertension and hypothyroidism
Addie K, age 64, is obese (BMI, 37 kg/m2) and has hypertension and hypothyroidism, for which she takes metoprolol and levothyroxine. Ms. K says that she is careful about what she eats and exercises several times a week, but still has seen her weight increase steadily for the past several years.
CASE 3 › Young man with depression
Charlie D, a 21-year-old college student, is a new patient. He has depression and is obese (BMI, 34 kg/m2). The patient says he was diagnosed with depression by his former primary care physician, who prescribed paroxetine a year ago. He requests a refill of the paroxetine, which he reports has successfully boosted his mood. When asked about his weight, he admits that he has gained 8 lbs (3.6 kg) since he began taking the drug.
If these were your patients, what weight management steps would you take? Before we provide some recommendations, let’s review the evidence.
Antidiabetic agents and weight
While some antidiabetic agents are weight-neutral and others promote weight loss, several therapies are associated with weight gain6 (TABLE 13). Patients like Mr. P can gain as much as 10 kg in 3 to 6 months after beginning treatment with insulin, thiazolidinediones (TZDs), sulfonylureas, and other insulin secretagogues.2,7

A recent systematic review and meta-analysis of 257 randomized controlled trials (RCTs) found weight gain to be associated with the use of pioglitazone (2.6 kg), glimepiride (2.1 kg), glyburide (2.6 kg), glipizide (2.2 kg), and sitagliptin (0.55 kg). A modest weight loss was associated with acarbose, exenatide, liraglutide, metformin, miglitol, and pramlintide.8
Sulfonylureas are generally associated with a 1.5 to 2.5 kg weight gain.9-11 In an analysis of 27 RCTs of noninsulin antidiabetic drugs in patients whose disease was not controlled by metformin alone, TZDs, sulfonylureas, and meglitinides were associated with a 1.77 to 2.08 kg weight gain.9 Furthermore, those taking sulfonylureas and meglitinides had higher rates of hypoglycemia compared with patients taking placebo (relative risk, 4.50-7.50). In fact, sulfonylureas have the highest risk of serious hypoglycemia of any noninsulin therapy.6
In contrast, metformin—the most commonly prescribed oral agent for type 2 diabetes—promotes mild weight loss by multiple mechanisms and has a good safety profile.12,13 Thus, some physicians use metformin off label for weight loss and diabetes prevention and have suggested that it be approved for these indications.13
Glycemic control and weight loss
Glucagon-like peptide-1 (GLP-1) agonists lead to weight loss by decreasing appetite and enhancing satiety, as well as improving glycemic control. Liraglutide received Food and Drug Administration (FDA) approval in 2014 as a treatment for chronic weight management at a higher dose (3 mg/d) than that used to treat diabetes (1.8 mg/d).14
Sodium-glucose co-transporter 2 (SGLT2) inhibitors are a relatively new class of antidiabetic medication that reduce glucose reabsorption by the kidneys, leading to increased urinary glucose excretion.15 The associated weight loss, in addition to a reduction in hyperglycemia, may be due to the subsequent calorie loss through glycosuria.
Both dipeptidyl peptidase-4 (DPP-4) inhibitors and alpha-glucosidase inhibitors (AGIs) appear to be weight-neutral or to induce minimal changes in weight.16 Although the systematic review mentioned earlier found a 0.55 kg weight gain associated with sitagliptin,8 most studies of DPP-4 inhibitors report weight neutrality.17-19 Pramlintide, the amylin analogue that has FDA approval for use in combination with existing insulin treatment, can prevent weight gain or lead to weight loss.20,21
The Endocrine Society Clinical Practice Guideline recommends concomitantly prescribing at least one weight loss-promoting medication (such as metformin, a GLP-1 agonist, or pramlintide) to patients with obesity and type 2 diabetes who require insulin to mitigate weight gain due to insulin.2
The 2016 Comprehensive Type 2 Diabetes Management Algorithm published by the American Association of Clinical Endocrinologists and American College of Endocrinology recommends that the initiation of diabetes therapies be based on the risks of weight gain and hypoglycemia, among other factors. The algorithm calls for metformin as first-line therapy, followed by a GLP-1 agonist as a second-line therapy, and an SGLT2 inhibitor as a third-line therapy.6
Finally, FDA-approved anti-obesity medications may be appropriate for patients with diabetes who are unable to lose weight with lifestyle interventions alone.22 Each medication is associated with improvements in glucose in addition to other metabolic parameters.
CASE 1 › A better choice for Mr. P
Because Mr. P has gained weight—and, indeed, developed obesity—since he started taking glyburide, it is clear that a sulfonylurea is not the best choice for this patient. An antidiabetic agent that is weight-neutral or that promotes weight loss, such as an SGLT2 inhibitor or a GLP-1 agonist, would be more suitable. The drug should be prescribed in conjunction with his metformin, which has a favorable weight profile and helps reduce HbA1c, as both SGLT2 inhibitors and GLP-1 agonists also do.
If Mr. P were to switch to an SGLT2 inhibitor, a combination pill containing metformin would be an effective way to limit the patient’s pill burden.
Treating hypertension without weight gain
Thiazide diuretics are often recommended as first-line agents for the treatment of hypertension, but their dose-related adverse effects, including dyslipidemia and insulin resistance, are undesirable for patients who are overweight or obese and at risk for metabolic syndrome and type 2 diabetes.23 Beta-adrenergic blockers have been shown to promote weight gain and prevent weight loss, especially in patients who have both hypertension and diabetes.24 In addition to having potential adverse metabolic effects on lipids and/or insulin sensitivity, beta-blockers can decrease metabolic rate by 10% and they may have other negative effects on energy metabolism, as well.25
In a meta-analysis of 8 RCTs that lasted ≥6 months, changes in body weight were higher in participants on beta-blockers, with a median difference of 1.2 kg (−0.4 to 3.5 kg) between those on beta-blockers and the control group.26 The evidence suggests that beta-blockers should not necessarily be first-line treatment for hypertension in patients who are overweight or obese and that obesity management in patients with hypertension may be harder if they are being treated with a beta-blocker.
When a different drug in the same class will do
There are exceptions, however. When beta-blockers are required—for patients with coronary artery disease, heart failure, or an arrhythmia, for example—a selective agent with a vasodilating component, such as carvedilol or nebivolol, is recommended.2 These drugs appear to have less potential for weight gain and to have minimal effect on lipid and glucose metabolism.26,27
In a study of 1106 patients with hypertension, those taking metoprolol had a statistically significant mean weight gain of 1.19 kg (P<.001) compared with patients taking carvedilol (mean weight gain, 0.17 kg; P=.36).24 While 4.5% of those in the metoprolol group gained ≥7% of their body weight, that was true of only 1.1% of those taking carvedilol. Thus, weight gain can sometimes be minimized by choosing a different medication within the same drug class.
ACE inhibitors, ARBs, and calcium channel blockers
Antihypertensive medications that are not associated with weight gain or insulin resistance include angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and calcium channel blockers (CCBs) (TABLE 2).3 Angiotensin contributes to obesity-related hypertension, as it is overexpressed in obesity, making ACE inhibitors and ARBs desirable options for the treatment of patients who are obese. And, because many patients who are obese also suffer from type 2 diabetes or prediabetes, they’re likely to benefit from the renal protection provided by ACE inhibitors and ARBs, as well.

CASE 2 › Switching antihypertensives
Switching Ms. K from metoprolol, a beta-blocker, to an ACE inhibitor, ARB, or CCB may help prevent further weight gain, and possibly even lead to weight loss. Any drug in any of these 3 classes of medications would be a reasonable choice. However, if the patient had a condition that warranted use of a beta-blocker, a selective agent with a vasodilating component such as carvedilol or nebivolol might be helpful.
SIDEBAR
Weight management strategies for several other conditionsIn addition to medications for common conditions such as diabetes, hypertension, and depression, there are numerous other drugs that can cause unwanted weight gain. These include some antiseizure agents, antipsychotics, contraceptives, hormones, and migraine therapies, as well as corticosteroids. In view of both the nation’s obesity epidemic and the many drugs that are known to adversely affect weight maintenance, it is crucial to do a careful risk-benefit analysis and a search for alternatives whenever you prescribe a new medication for a patient who is overweight or obese or has metabolic risk factors.2-5
When weight-neutral substitutes exist, such medications should be considered, if appropriate, to prevent or lessen pharmacologic weight gain. For example, topiramate and zonisamide are preferable to other antiepileptics, such as valproic acid and gabapentin when it comes to weight management.2-4 It is essential to keep in mind, however, that medications in the same class are not always interchangeable.
For patients with inflammatory conditions such as rheumatoid arthritis, disease-modifying antirheumatic drugs (DMARDs) are preferable to corticosteroids whenever possible.2-4 For the many patients for whom steroids or other drugs known to cause weight gain are necessary, however, dietary and lifestyle counseling—advising patients to eat a healthful diet and maintain adequate activity levels, among other interventions—may help to mitigate the effects.
And when there are no alternative medications available, use the lowest possible dose for the shortest duration necessary.
Choosing an antidepressant when weight is an issue
For patients with psychiatric conditions, weight gain is often multifactorial. One key issue: Weight gain is a common adverse effect of many antidepressants (TABLE 3).3 Within classes of antidepressants, there is a range of weight gain potential, which can vary depending on the duration of therapy.2

In a meta-analysis of 116 studies, selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine and sertraline were associated with weight loss in short-term use (4-12 weeks) and weight neutrality when used for >4 months.1 Patients who had type 2 diabetes as well as depression had an average weight loss from fluoxetine of 5.1 kg (3.3–6.9 kg) at 24- to 26-week follow up.28
Among SSRI and tricyclic (TCA) antidepressants, paroxetine and amitriptyline, respectively, had the greatest risk for weight gain.1,29 No significant weight effect was observed for either citalopram or escitalopram. Keep in mind, however, that the effect of each antidepressant on weight may vary greatly from one patient to another.1 For example, while Mr. D gained 3.6 kg on paroxetine, some patients gain no weight at all.
In the systematic review and meta-analysis of 257 RCTs, weight gain was associated with the use of amitriptyline (1.8 kg) and mirtazapine (1.5 kg), while weight loss was associated with bupropion and fluoxetine (-1.3 kg for each).8
This antidepressant can decrease cravings
Bupropion, a norepinephrine and dopamine reuptake inhibitor, is the only antidepressant that has been consistently shown to cause weight loss.30,31 Clinical trials have found that it decreases body weight by suppressing appetite and reducing food cravings.30 Bupropion is approved for the treatment of depression and as a smoking cessation aide. And, in 2014, a bupropion-naltrexone combination received FDA approval for chronic weight management, sold under the brand name Contrave.32
As different classes of antidepressants are often prescribed for different types of depression, it is important to be aware that the few that are weight-neutral and weight-loss-promoting are not appropriate for all patients with depression. For example, bupropion is an activating agent and can exacerbate anxiety. Thus, a patient with concomitant depression and anxiety might be a better candidate for another antidepressant, which could lead to some weight gain but would better manage the individual’s symptoms. In such cases, the rule of thumb should be to prescribe the lowest dose required for clinical efficacy for the shortest duration necessary.
CASE 3 › Change antidepressants— and keep a close watch
Depending on the nature of Mr. D’s depression, bupropion, fluoxetine, or sertraline might be a reasonable alternative to paroxetine to prevent or reduce further drug-induced weight gain.
Frequent follow-up visits should be scheduled until the transition has been completed and his condition stabilized. If Mr. D’s new antidepressant is bupropion, monitoring him for signs of anxiety would be required.
CORRESPONDENCE
Katherine H. Saunders, MD, Comprehensive Weight Control Center, Weill Cornell Medicine, 1165 York Avenue, New York, NY 10065; [email protected].
PRACTICE RECOMMENDATIONS
› Choose weight-loss-promoting medications, such as metformin, sodium-glucose co-transporter 2 inhibitors, and glucagon-like peptide-1 agonists, and weight-neutral medications, such as DPP-4 inhibitors, as first- and second-line agents for patients with type 2 diabetes who are overweight or obese. A
› Prescribe angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or calcium channel blockers as first- and second-line antihypertensive therapy for patients who are overweight or obese. A
› Select antidepressants that promote weight loss, such as bupropion, or weight-neutral agents, such as fluoxetine and sertraline, for patients who are overweight or obese and require treatment for depression. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Medications can have an unpredictable and variable effect on weight. Some drugs trigger weight gain in one patient while inducing weight loss in another. Others may lead to weight loss initially but cause weight gain when taken long term.1 Often, a drug’s effect on a patient’s weight depends on his or her medical history and lifestyle, including factors like insulin resistance, diet, and exercise level.
To make matters worse, clinical studies of drug-related effects on weight can be misleading. Because researchers often report a mean weight change—an average of those who had little or no change in weight when taking the drug and individuals who may have gained a significant amount of weight—a drug’s potential to cause weight gain may be underestimated. Few studies include an analysis of the range—eg, how many participants gained or lost various percentages of body weight. What’s more, pharmacology studies typically follow participants for a few months to a few years, whereas weight changes can be cumulative when a medication is taken for many years.
The nation’s continually growing obesity epidemic makes it crucial for physicians to consider the weight effects of medications being prescribed and to balance the benefits of treatment with the potential for weight gain. Until recently, the medical literature offered little guidance.
In 2015, the Endocrine Society published clinical practice guidelines for pharmacologic management of obesity, including data on medications that cause weight gain and suggesting alternatives that are weight-neutral or promote weight loss.2
In the pages that follow, we present case studies, tables, and a review of the latest evidence to highlight optimal drug treatment for patients who are overweight or obese, and are also being treated for diabetes, hypertension, and depression. You’ll find a brief discussion of weight management strategies related to other drugs and conditions in the sidebar.2-5

CASE 1 › 40-year-old man with diabetes and hyperlipidemia
Brian P, who has come in for an annual checkup, has a body mass index (BMI) of 30 kg/m2. He also has hyperlipidemia and type 2 diabetes, for which he has been taking metformin for several years. A year ago, his hemoglobin A1c (HbA1c) was 7.3%, so his physician added glyburide to his regimen.
In the year since, Mr. P has gained 12 lbs (5.4 kg) but achieved only a minimal reduction in HbA1c (to 6.8%). He expresses concern about the cardiovascular effects of the extra weight and says that diet and exercise have not helped him control his weight.
CASE 2 › Older woman with hypertension and hypothyroidism
Addie K, age 64, is obese (BMI, 37 kg/m2) and has hypertension and hypothyroidism, for which she takes metoprolol and levothyroxine. Ms. K says that she is careful about what she eats and exercises several times a week, but still has seen her weight increase steadily for the past several years.
CASE 3 › Young man with depression
Charlie D, a 21-year-old college student, is a new patient. He has depression and is obese (BMI, 34 kg/m2). The patient says he was diagnosed with depression by his former primary care physician, who prescribed paroxetine a year ago. He requests a refill of the paroxetine, which he reports has successfully boosted his mood. When asked about his weight, he admits that he has gained 8 lbs (3.6 kg) since he began taking the drug.
If these were your patients, what weight management steps would you take? Before we provide some recommendations, let’s review the evidence.
Antidiabetic agents and weight
While some antidiabetic agents are weight-neutral and others promote weight loss, several therapies are associated with weight gain6 (TABLE 13). Patients like Mr. P can gain as much as 10 kg in 3 to 6 months after beginning treatment with insulin, thiazolidinediones (TZDs), sulfonylureas, and other insulin secretagogues.2,7

A recent systematic review and meta-analysis of 257 randomized controlled trials (RCTs) found weight gain to be associated with the use of pioglitazone (2.6 kg), glimepiride (2.1 kg), glyburide (2.6 kg), glipizide (2.2 kg), and sitagliptin (0.55 kg). A modest weight loss was associated with acarbose, exenatide, liraglutide, metformin, miglitol, and pramlintide.8
Sulfonylureas are generally associated with a 1.5 to 2.5 kg weight gain.9-11 In an analysis of 27 RCTs of noninsulin antidiabetic drugs in patients whose disease was not controlled by metformin alone, TZDs, sulfonylureas, and meglitinides were associated with a 1.77 to 2.08 kg weight gain.9 Furthermore, those taking sulfonylureas and meglitinides had higher rates of hypoglycemia compared with patients taking placebo (relative risk, 4.50-7.50). In fact, sulfonylureas have the highest risk of serious hypoglycemia of any noninsulin therapy.6
In contrast, metformin—the most commonly prescribed oral agent for type 2 diabetes—promotes mild weight loss by multiple mechanisms and has a good safety profile.12,13 Thus, some physicians use metformin off label for weight loss and diabetes prevention and have suggested that it be approved for these indications.13
Glycemic control and weight loss
Glucagon-like peptide-1 (GLP-1) agonists lead to weight loss by decreasing appetite and enhancing satiety, as well as improving glycemic control. Liraglutide received Food and Drug Administration (FDA) approval in 2014 as a treatment for chronic weight management at a higher dose (3 mg/d) than that used to treat diabetes (1.8 mg/d).14
Sodium-glucose co-transporter 2 (SGLT2) inhibitors are a relatively new class of antidiabetic medication that reduce glucose reabsorption by the kidneys, leading to increased urinary glucose excretion.15 The associated weight loss, in addition to a reduction in hyperglycemia, may be due to the subsequent calorie loss through glycosuria.
Both dipeptidyl peptidase-4 (DPP-4) inhibitors and alpha-glucosidase inhibitors (AGIs) appear to be weight-neutral or to induce minimal changes in weight.16 Although the systematic review mentioned earlier found a 0.55 kg weight gain associated with sitagliptin,8 most studies of DPP-4 inhibitors report weight neutrality.17-19 Pramlintide, the amylin analogue that has FDA approval for use in combination with existing insulin treatment, can prevent weight gain or lead to weight loss.20,21
The Endocrine Society Clinical Practice Guideline recommends concomitantly prescribing at least one weight loss-promoting medication (such as metformin, a GLP-1 agonist, or pramlintide) to patients with obesity and type 2 diabetes who require insulin to mitigate weight gain due to insulin.2
The 2016 Comprehensive Type 2 Diabetes Management Algorithm published by the American Association of Clinical Endocrinologists and American College of Endocrinology recommends that the initiation of diabetes therapies be based on the risks of weight gain and hypoglycemia, among other factors. The algorithm calls for metformin as first-line therapy, followed by a GLP-1 agonist as a second-line therapy, and an SGLT2 inhibitor as a third-line therapy.6
Finally, FDA-approved anti-obesity medications may be appropriate for patients with diabetes who are unable to lose weight with lifestyle interventions alone.22 Each medication is associated with improvements in glucose in addition to other metabolic parameters.
CASE 1 › A better choice for Mr. P
Because Mr. P has gained weight—and, indeed, developed obesity—since he started taking glyburide, it is clear that a sulfonylurea is not the best choice for this patient. An antidiabetic agent that is weight-neutral or that promotes weight loss, such as an SGLT2 inhibitor or a GLP-1 agonist, would be more suitable. The drug should be prescribed in conjunction with his metformin, which has a favorable weight profile and helps reduce HbA1c, as both SGLT2 inhibitors and GLP-1 agonists also do.
If Mr. P were to switch to an SGLT2 inhibitor, a combination pill containing metformin would be an effective way to limit the patient’s pill burden.
Treating hypertension without weight gain
Thiazide diuretics are often recommended as first-line agents for the treatment of hypertension, but their dose-related adverse effects, including dyslipidemia and insulin resistance, are undesirable for patients who are overweight or obese and at risk for metabolic syndrome and type 2 diabetes.23 Beta-adrenergic blockers have been shown to promote weight gain and prevent weight loss, especially in patients who have both hypertension and diabetes.24 In addition to having potential adverse metabolic effects on lipids and/or insulin sensitivity, beta-blockers can decrease metabolic rate by 10% and they may have other negative effects on energy metabolism, as well.25
In a meta-analysis of 8 RCTs that lasted ≥6 months, changes in body weight were higher in participants on beta-blockers, with a median difference of 1.2 kg (−0.4 to 3.5 kg) between those on beta-blockers and the control group.26 The evidence suggests that beta-blockers should not necessarily be first-line treatment for hypertension in patients who are overweight or obese and that obesity management in patients with hypertension may be harder if they are being treated with a beta-blocker.
When a different drug in the same class will do
There are exceptions, however. When beta-blockers are required—for patients with coronary artery disease, heart failure, or an arrhythmia, for example—a selective agent with a vasodilating component, such as carvedilol or nebivolol, is recommended.2 These drugs appear to have less potential for weight gain and to have minimal effect on lipid and glucose metabolism.26,27
In a study of 1106 patients with hypertension, those taking metoprolol had a statistically significant mean weight gain of 1.19 kg (P<.001) compared with patients taking carvedilol (mean weight gain, 0.17 kg; P=.36).24 While 4.5% of those in the metoprolol group gained ≥7% of their body weight, that was true of only 1.1% of those taking carvedilol. Thus, weight gain can sometimes be minimized by choosing a different medication within the same drug class.
ACE inhibitors, ARBs, and calcium channel blockers
Antihypertensive medications that are not associated with weight gain or insulin resistance include angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and calcium channel blockers (CCBs) (TABLE 2).3 Angiotensin contributes to obesity-related hypertension, as it is overexpressed in obesity, making ACE inhibitors and ARBs desirable options for the treatment of patients who are obese. And, because many patients who are obese also suffer from type 2 diabetes or prediabetes, they’re likely to benefit from the renal protection provided by ACE inhibitors and ARBs, as well.

CASE 2 › Switching antihypertensives
Switching Ms. K from metoprolol, a beta-blocker, to an ACE inhibitor, ARB, or CCB may help prevent further weight gain, and possibly even lead to weight loss. Any drug in any of these 3 classes of medications would be a reasonable choice. However, if the patient had a condition that warranted use of a beta-blocker, a selective agent with a vasodilating component such as carvedilol or nebivolol might be helpful.
SIDEBAR
Weight management strategies for several other conditionsIn addition to medications for common conditions such as diabetes, hypertension, and depression, there are numerous other drugs that can cause unwanted weight gain. These include some antiseizure agents, antipsychotics, contraceptives, hormones, and migraine therapies, as well as corticosteroids. In view of both the nation’s obesity epidemic and the many drugs that are known to adversely affect weight maintenance, it is crucial to do a careful risk-benefit analysis and a search for alternatives whenever you prescribe a new medication for a patient who is overweight or obese or has metabolic risk factors.2-5
When weight-neutral substitutes exist, such medications should be considered, if appropriate, to prevent or lessen pharmacologic weight gain. For example, topiramate and zonisamide are preferable to other antiepileptics, such as valproic acid and gabapentin when it comes to weight management.2-4 It is essential to keep in mind, however, that medications in the same class are not always interchangeable.
For patients with inflammatory conditions such as rheumatoid arthritis, disease-modifying antirheumatic drugs (DMARDs) are preferable to corticosteroids whenever possible.2-4 For the many patients for whom steroids or other drugs known to cause weight gain are necessary, however, dietary and lifestyle counseling—advising patients to eat a healthful diet and maintain adequate activity levels, among other interventions—may help to mitigate the effects.
And when there are no alternative medications available, use the lowest possible dose for the shortest duration necessary.
Choosing an antidepressant when weight is an issue
For patients with psychiatric conditions, weight gain is often multifactorial. One key issue: Weight gain is a common adverse effect of many antidepressants (TABLE 3).3 Within classes of antidepressants, there is a range of weight gain potential, which can vary depending on the duration of therapy.2

In a meta-analysis of 116 studies, selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine and sertraline were associated with weight loss in short-term use (4-12 weeks) and weight neutrality when used for >4 months.1 Patients who had type 2 diabetes as well as depression had an average weight loss from fluoxetine of 5.1 kg (3.3–6.9 kg) at 24- to 26-week follow up.28
Among SSRI and tricyclic (TCA) antidepressants, paroxetine and amitriptyline, respectively, had the greatest risk for weight gain.1,29 No significant weight effect was observed for either citalopram or escitalopram. Keep in mind, however, that the effect of each antidepressant on weight may vary greatly from one patient to another.1 For example, while Mr. D gained 3.6 kg on paroxetine, some patients gain no weight at all.
In the systematic review and meta-analysis of 257 RCTs, weight gain was associated with the use of amitriptyline (1.8 kg) and mirtazapine (1.5 kg), while weight loss was associated with bupropion and fluoxetine (-1.3 kg for each).8
This antidepressant can decrease cravings
Bupropion, a norepinephrine and dopamine reuptake inhibitor, is the only antidepressant that has been consistently shown to cause weight loss.30,31 Clinical trials have found that it decreases body weight by suppressing appetite and reducing food cravings.30 Bupropion is approved for the treatment of depression and as a smoking cessation aide. And, in 2014, a bupropion-naltrexone combination received FDA approval for chronic weight management, sold under the brand name Contrave.32
As different classes of antidepressants are often prescribed for different types of depression, it is important to be aware that the few that are weight-neutral and weight-loss-promoting are not appropriate for all patients with depression. For example, bupropion is an activating agent and can exacerbate anxiety. Thus, a patient with concomitant depression and anxiety might be a better candidate for another antidepressant, which could lead to some weight gain but would better manage the individual’s symptoms. In such cases, the rule of thumb should be to prescribe the lowest dose required for clinical efficacy for the shortest duration necessary.
CASE 3 › Change antidepressants— and keep a close watch
Depending on the nature of Mr. D’s depression, bupropion, fluoxetine, or sertraline might be a reasonable alternative to paroxetine to prevent or reduce further drug-induced weight gain.
Frequent follow-up visits should be scheduled until the transition has been completed and his condition stabilized. If Mr. D’s new antidepressant is bupropion, monitoring him for signs of anxiety would be required.
CORRESPONDENCE
Katherine H. Saunders, MD, Comprehensive Weight Control Center, Weill Cornell Medicine, 1165 York Avenue, New York, NY 10065; [email protected].
1. Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71:1259-1272.
2. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2015;100:342-362.
3. Apovian CM, Aronne L, Powell AG. Clinical Management of Obesity. West Islip, NY: Professional Communications, Inc., 2015.
4. Aronne LJ. A Practical Guide to Drug-induced Weight Gain. Minneapolis, Minn: McGraw-Hill; 2002.
5. Leslie WS, Hankey CR, Lean ME. Weight gain as an adverse effect of some commonly prescribed drugs: a systematic review. QJM. 2007;100:395-404.
6. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm – 2016 executive summary. Endocr Pract. 2016;22:84-113.
7. Aronne LJ. Drug-induced weight gain: non-CNS medications. In: A Practical Guide to Drug-Induced Weight Gain. Minneapolis, Minn: McGraw-Hill: 2002:77-91.
8. Domecq JP, Prutsky G, Leppin A, et al. Clinical review: drugs commonly associated with weight change: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100:363-370.
9. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
10. Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427-2443.
11. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–481.
12. Malin SK, Kashyap SR. Effects of metformin on weight loss: potential mechanisms. Curr Opin Endocrinol Diabetes Obes. 2014;21:323-329.
13. Igel LI, Sinha A, Saunders KH, et al. Metformin: an old therapy that deserves a new indication for the treatment of obesity. Curr Atheroscler Rep. 2016;18:16.
14. US Food and Drug Administration. FDA approves weight-management drug Saxenda. December 23, 2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427913.htm. Accessed October 1, 2016.
15. Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8:495-502.
16. van de Laar FA, Lucassen PL, Akkermans RP, et al. Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care. 2005;28:154-163.
17. Hong ES, Khang AR, Yoon JW, et al. Comparison between sitagliptin as add-on therapy to insulin and insulin dose-increase therapy in uncontrolled Korean type 2 diabetes: CSI study. Diabetes Obes Metab. 2012;14:795-802.
18. Arnolds S, Dellweg S, Clair J, et al. Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with insulin glargine and metformin: a proof-of-concept study. Diabetes Care. 2010;33:1509-1515.
19. Scheen AJ. DPP-4 inhibitors in the management of type 2 diabetes: a critical review of head-to-head trials. Diabetes Metab. 2012;38:89-101.
20. Hollander PA, Levy P, Fineman MS, et al. Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care. 2003;26:784-790.
21. Aronne L, Fujioka K, Aroda V, et al. Progressive reduction in body weight after treatment with the amylin analog pramlintide in obese subjects: a phase 2, randomized, placebo-controlled, dose-escalation study. J Clin Endocrinol Metab. 2007;92:2977-2983.
22. Saunders KH, Kumar RB, Igel LI, et al. Pharmacologic approaches to weight management: recent gains and shortfalls in combating obesity. Curr Atheroscler Rep. 2016;18:36.
23. Landsberg L, Aronne LJ, Beilin LJ, et al. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment—a position paper of the Obesity Society and the American Society of Hypertension. Obesity (Silver Spring). 2013;21:8-24.
24. Messerli FH, Bell DS, Fonseca V, et al. Body weight changes with beta-blocker use: results from GEMINI. Am J Med. 2007;120:610-615.
25. Pischon T, Sharma AM. Use of beta-blockers in obesity hypertension: potential role of weight gain. Obes Rev. 2001;2:275-280.
26. Sharma AM, Pischon T, Hardt S, et al. Hypothesis: beta-adrenergic receptor blockers and weight gain: a systematic analysis. Hypertension. 2001;37:250-254.
27. Manrique C, Whaley-Connell A, Sowers JR. Nebivolol in obese and non-obese hypertensive patients. J Clin Hypertens (Greenwich). 2009;11:309-315.
28. Norris SL, Zhang X, Avenell A, et al. Pharmacotherapy for weight loss in adults with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;(1):CD004096.
29. Rosenzweig-Lipson S, Beyer CE, Hughes ZA, et al. Differentiating antidepressants of the future: efficacy and safety. Pharmacol Ther. 2007;113:134-153.
30. Gadde KM, Xiong GL. Bupropion for weight reduction. Expert Rev Neurother. 2007;7:17-24.
31. Arterburn D, Sofer T, Boudreau DM, et al. Long-term weight change after initiating second-generation antidepressants. J Clin Med. 2016;5:piiE48.
32. US Food and Drug Administration. FDA approves weight-management drug Contrave. September 10, 2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm413896.htm. Accessed October 1, 2016.
1. Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71:1259-1272.
2. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2015;100:342-362.
3. Apovian CM, Aronne L, Powell AG. Clinical Management of Obesity. West Islip, NY: Professional Communications, Inc., 2015.
4. Aronne LJ. A Practical Guide to Drug-induced Weight Gain. Minneapolis, Minn: McGraw-Hill; 2002.
5. Leslie WS, Hankey CR, Lean ME. Weight gain as an adverse effect of some commonly prescribed drugs: a systematic review. QJM. 2007;100:395-404.
6. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm – 2016 executive summary. Endocr Pract. 2016;22:84-113.
7. Aronne LJ. Drug-induced weight gain: non-CNS medications. In: A Practical Guide to Drug-Induced Weight Gain. Minneapolis, Minn: McGraw-Hill: 2002:77-91.
8. Domecq JP, Prutsky G, Leppin A, et al. Clinical review: drugs commonly associated with weight change: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100:363-370.
9. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
10. Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427-2443.
11. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–481.
12. Malin SK, Kashyap SR. Effects of metformin on weight loss: potential mechanisms. Curr Opin Endocrinol Diabetes Obes. 2014;21:323-329.
13. Igel LI, Sinha A, Saunders KH, et al. Metformin: an old therapy that deserves a new indication for the treatment of obesity. Curr Atheroscler Rep. 2016;18:16.
14. US Food and Drug Administration. FDA approves weight-management drug Saxenda. December 23, 2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427913.htm. Accessed October 1, 2016.
15. Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8:495-502.
16. van de Laar FA, Lucassen PL, Akkermans RP, et al. Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care. 2005;28:154-163.
17. Hong ES, Khang AR, Yoon JW, et al. Comparison between sitagliptin as add-on therapy to insulin and insulin dose-increase therapy in uncontrolled Korean type 2 diabetes: CSI study. Diabetes Obes Metab. 2012;14:795-802.
18. Arnolds S, Dellweg S, Clair J, et al. Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with insulin glargine and metformin: a proof-of-concept study. Diabetes Care. 2010;33:1509-1515.
19. Scheen AJ. DPP-4 inhibitors in the management of type 2 diabetes: a critical review of head-to-head trials. Diabetes Metab. 2012;38:89-101.
20. Hollander PA, Levy P, Fineman MS, et al. Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care. 2003;26:784-790.
21. Aronne L, Fujioka K, Aroda V, et al. Progressive reduction in body weight after treatment with the amylin analog pramlintide in obese subjects: a phase 2, randomized, placebo-controlled, dose-escalation study. J Clin Endocrinol Metab. 2007;92:2977-2983.
22. Saunders KH, Kumar RB, Igel LI, et al. Pharmacologic approaches to weight management: recent gains and shortfalls in combating obesity. Curr Atheroscler Rep. 2016;18:36.
23. Landsberg L, Aronne LJ, Beilin LJ, et al. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment—a position paper of the Obesity Society and the American Society of Hypertension. Obesity (Silver Spring). 2013;21:8-24.
24. Messerli FH, Bell DS, Fonseca V, et al. Body weight changes with beta-blocker use: results from GEMINI. Am J Med. 2007;120:610-615.
25. Pischon T, Sharma AM. Use of beta-blockers in obesity hypertension: potential role of weight gain. Obes Rev. 2001;2:275-280.
26. Sharma AM, Pischon T, Hardt S, et al. Hypothesis: beta-adrenergic receptor blockers and weight gain: a systematic analysis. Hypertension. 2001;37:250-254.
27. Manrique C, Whaley-Connell A, Sowers JR. Nebivolol in obese and non-obese hypertensive patients. J Clin Hypertens (Greenwich). 2009;11:309-315.
28. Norris SL, Zhang X, Avenell A, et al. Pharmacotherapy for weight loss in adults with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;(1):CD004096.
29. Rosenzweig-Lipson S, Beyer CE, Hughes ZA, et al. Differentiating antidepressants of the future: efficacy and safety. Pharmacol Ther. 2007;113:134-153.
30. Gadde KM, Xiong GL. Bupropion for weight reduction. Expert Rev Neurother. 2007;7:17-24.
31. Arterburn D, Sofer T, Boudreau DM, et al. Long-term weight change after initiating second-generation antidepressants. J Clin Med. 2016;5:piiE48.
32. US Food and Drug Administration. FDA approves weight-management drug Contrave. September 10, 2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm413896.htm. Accessed October 1, 2016.