User login
Lipoic Acid Reduces Brain Atrophy in Secondary Progressive MS
LONDON—Daily oral intake of 1,200 mg of racemic lipoic acid significantly reduces the rate of whole brain atrophy among patients with secondary progressive multiple sclerosis (MS), according to data described at the 32nd Congress of the European Committee for Treatment and Research in MS (ECTRIMS). Overall, lipoic acid is safe and well tolerated.
“These results need further exploration in a larger sample size to clarify the effect size of lipoic acid, to determine the clinical benefits associated with reduction in brain atrophy, and to explore the mechanisms of action of lipoic acid in progressive MS,” said Rebecca Spain, MD, MSPH, neurologist at the VA Portland Health Care System and Oregon Health & Science University in Portland.
An Antioxidant With Many Roles
Lipoic acid is a small-molecule antioxidant with several biologic functions, such as participating in oxidative respiration in mitochondria, influencing endothelial cell function, and inhibiting inappropriate microglial activation. A synthetic version of the antioxidant is available commercially at low cost.
Several studies have indicated that lipoic acid reduces disability in mice with experimental autoimmune encephalomyelitis. Based on those results, Dr. Spain and colleagues conducted a two-year, double-blind trial in which patients with secondary progressive MS were randomized in equal groups to 1,200 mg/day of oral racemic lipoic acid or placebo. Participants underwent yearly MRI. The investigators’ primary outcome was the annualized percent change in brain volume, as measured by structural image evaluation, using normalization of atrophy (SIENA). They also sought to compare changes in disability and quality of life between the two groups and to assess the safety and tolerability of lipoic acid.
In their intention-to-treat analysis, the researchers used a linear mixed model approach to evaluate the effects of lipoic acid on annualized rates of change in outcomes. They corrected the models for age, sex, and disease duration.
In all, 54 patients were randomized, and three withdrew before receiving treatment. The intention-to-treat placebo cohort included 24 patients, and the lipoic-acid cohort had 27 patients. Five participants, all in the lipoic-acid cohort, dropped out of the study. One person had claustrophobia and could not tolerate the MRI. Another participant had nausea and vomiting that subsided on discontinuation of lipoic acid. The third dropout had a new diagnosis of prostate cancer. The fourth had new proteinuria, and the fifth had worsening renal function.
The study population was representative of people with secondary progressive MS, albeit with greater disability. Mean age was 60, and average disease duration was 30 years. The treatment arms were well matched at baseline.
Trend Toward Improved Walking
The annualized rate of brain atrophy was 0.65% for the placebo group, “which is comparable to [that of] other progressive MS natural history cohorts,” said Dr. Spain. Among patients receiving lipoic acid, the annualized rate of brain atrophy was 0.22%, which was significantly different from that of the placebo cohort. “This represents a 66% reduction in the rate of brain atrophy in the lipoic-acid cohort, compared with placebo,” said Dr. Spain.
No other outcomes were significantly different between groups at 96 weeks. Performance on the Timed 25-Foot Walk test, however, tended to improve by approximately 12% (ie, from 8 seconds to 7 seconds) among participants receiving lipoic acid.
Gastrointestinal upset was significantly more common among participants receiving lipoic acid, as would be expected because of previous studies. New-onset proteinuria and worsening renal function were observed in the lipoic-acid cohort, but a consulting nephrologist did not consider either adverse event to be related to treatment. Unexpectedly, the investigators observed half as many falls in the lipoic acid arm, compared with controls.
The US Department of Veterans Affairs and the NIH supported this research. Pure Encapsulations provided the lipoic acid and placebo.
—Erik Greb
Suggested Reading
Chaudhary P, Marracci G, Galipeau D, et al. Lipoic acid reduces inflammation in a mouse focal cortical experimental autoimmune encephalomyelitis model. J Neuroimmunol. 2015;289:68-74.
Plemel JR, Juzwik CA, Benson CA, et al. Over-the-counter anti-oxidant therapies for use in multiple sclerosis: A systematic review. Mult Scler. 2015;21(12):1485-1495.
Yadav V, Marracci G, Lovera J, et al. Lipoic acid in multiple sclerosis: a pilot study. Mult Scler. 2005;11(2):159-165.
LONDON—Daily oral intake of 1,200 mg of racemic lipoic acid significantly reduces the rate of whole brain atrophy among patients with secondary progressive multiple sclerosis (MS), according to data described at the 32nd Congress of the European Committee for Treatment and Research in MS (ECTRIMS). Overall, lipoic acid is safe and well tolerated.
“These results need further exploration in a larger sample size to clarify the effect size of lipoic acid, to determine the clinical benefits associated with reduction in brain atrophy, and to explore the mechanisms of action of lipoic acid in progressive MS,” said Rebecca Spain, MD, MSPH, neurologist at the VA Portland Health Care System and Oregon Health & Science University in Portland.
An Antioxidant With Many Roles
Lipoic acid is a small-molecule antioxidant with several biologic functions, such as participating in oxidative respiration in mitochondria, influencing endothelial cell function, and inhibiting inappropriate microglial activation. A synthetic version of the antioxidant is available commercially at low cost.
Several studies have indicated that lipoic acid reduces disability in mice with experimental autoimmune encephalomyelitis. Based on those results, Dr. Spain and colleagues conducted a two-year, double-blind trial in which patients with secondary progressive MS were randomized in equal groups to 1,200 mg/day of oral racemic lipoic acid or placebo. Participants underwent yearly MRI. The investigators’ primary outcome was the annualized percent change in brain volume, as measured by structural image evaluation, using normalization of atrophy (SIENA). They also sought to compare changes in disability and quality of life between the two groups and to assess the safety and tolerability of lipoic acid.
In their intention-to-treat analysis, the researchers used a linear mixed model approach to evaluate the effects of lipoic acid on annualized rates of change in outcomes. They corrected the models for age, sex, and disease duration.
In all, 54 patients were randomized, and three withdrew before receiving treatment. The intention-to-treat placebo cohort included 24 patients, and the lipoic-acid cohort had 27 patients. Five participants, all in the lipoic-acid cohort, dropped out of the study. One person had claustrophobia and could not tolerate the MRI. Another participant had nausea and vomiting that subsided on discontinuation of lipoic acid. The third dropout had a new diagnosis of prostate cancer. The fourth had new proteinuria, and the fifth had worsening renal function.
The study population was representative of people with secondary progressive MS, albeit with greater disability. Mean age was 60, and average disease duration was 30 years. The treatment arms were well matched at baseline.
Trend Toward Improved Walking
The annualized rate of brain atrophy was 0.65% for the placebo group, “which is comparable to [that of] other progressive MS natural history cohorts,” said Dr. Spain. Among patients receiving lipoic acid, the annualized rate of brain atrophy was 0.22%, which was significantly different from that of the placebo cohort. “This represents a 66% reduction in the rate of brain atrophy in the lipoic-acid cohort, compared with placebo,” said Dr. Spain.
No other outcomes were significantly different between groups at 96 weeks. Performance on the Timed 25-Foot Walk test, however, tended to improve by approximately 12% (ie, from 8 seconds to 7 seconds) among participants receiving lipoic acid.
Gastrointestinal upset was significantly more common among participants receiving lipoic acid, as would be expected because of previous studies. New-onset proteinuria and worsening renal function were observed in the lipoic-acid cohort, but a consulting nephrologist did not consider either adverse event to be related to treatment. Unexpectedly, the investigators observed half as many falls in the lipoic acid arm, compared with controls.
The US Department of Veterans Affairs and the NIH supported this research. Pure Encapsulations provided the lipoic acid and placebo.
—Erik Greb
Suggested Reading
Chaudhary P, Marracci G, Galipeau D, et al. Lipoic acid reduces inflammation in a mouse focal cortical experimental autoimmune encephalomyelitis model. J Neuroimmunol. 2015;289:68-74.
Plemel JR, Juzwik CA, Benson CA, et al. Over-the-counter anti-oxidant therapies for use in multiple sclerosis: A systematic review. Mult Scler. 2015;21(12):1485-1495.
Yadav V, Marracci G, Lovera J, et al. Lipoic acid in multiple sclerosis: a pilot study. Mult Scler. 2005;11(2):159-165.
LONDON—Daily oral intake of 1,200 mg of racemic lipoic acid significantly reduces the rate of whole brain atrophy among patients with secondary progressive multiple sclerosis (MS), according to data described at the 32nd Congress of the European Committee for Treatment and Research in MS (ECTRIMS). Overall, lipoic acid is safe and well tolerated.
“These results need further exploration in a larger sample size to clarify the effect size of lipoic acid, to determine the clinical benefits associated with reduction in brain atrophy, and to explore the mechanisms of action of lipoic acid in progressive MS,” said Rebecca Spain, MD, MSPH, neurologist at the VA Portland Health Care System and Oregon Health & Science University in Portland.
An Antioxidant With Many Roles
Lipoic acid is a small-molecule antioxidant with several biologic functions, such as participating in oxidative respiration in mitochondria, influencing endothelial cell function, and inhibiting inappropriate microglial activation. A synthetic version of the antioxidant is available commercially at low cost.
Several studies have indicated that lipoic acid reduces disability in mice with experimental autoimmune encephalomyelitis. Based on those results, Dr. Spain and colleagues conducted a two-year, double-blind trial in which patients with secondary progressive MS were randomized in equal groups to 1,200 mg/day of oral racemic lipoic acid or placebo. Participants underwent yearly MRI. The investigators’ primary outcome was the annualized percent change in brain volume, as measured by structural image evaluation, using normalization of atrophy (SIENA). They also sought to compare changes in disability and quality of life between the two groups and to assess the safety and tolerability of lipoic acid.
In their intention-to-treat analysis, the researchers used a linear mixed model approach to evaluate the effects of lipoic acid on annualized rates of change in outcomes. They corrected the models for age, sex, and disease duration.
In all, 54 patients were randomized, and three withdrew before receiving treatment. The intention-to-treat placebo cohort included 24 patients, and the lipoic-acid cohort had 27 patients. Five participants, all in the lipoic-acid cohort, dropped out of the study. One person had claustrophobia and could not tolerate the MRI. Another participant had nausea and vomiting that subsided on discontinuation of lipoic acid. The third dropout had a new diagnosis of prostate cancer. The fourth had new proteinuria, and the fifth had worsening renal function.
The study population was representative of people with secondary progressive MS, albeit with greater disability. Mean age was 60, and average disease duration was 30 years. The treatment arms were well matched at baseline.
Trend Toward Improved Walking
The annualized rate of brain atrophy was 0.65% for the placebo group, “which is comparable to [that of] other progressive MS natural history cohorts,” said Dr. Spain. Among patients receiving lipoic acid, the annualized rate of brain atrophy was 0.22%, which was significantly different from that of the placebo cohort. “This represents a 66% reduction in the rate of brain atrophy in the lipoic-acid cohort, compared with placebo,” said Dr. Spain.
No other outcomes were significantly different between groups at 96 weeks. Performance on the Timed 25-Foot Walk test, however, tended to improve by approximately 12% (ie, from 8 seconds to 7 seconds) among participants receiving lipoic acid.
Gastrointestinal upset was significantly more common among participants receiving lipoic acid, as would be expected because of previous studies. New-onset proteinuria and worsening renal function were observed in the lipoic-acid cohort, but a consulting nephrologist did not consider either adverse event to be related to treatment. Unexpectedly, the investigators observed half as many falls in the lipoic acid arm, compared with controls.
The US Department of Veterans Affairs and the NIH supported this research. Pure Encapsulations provided the lipoic acid and placebo.
—Erik Greb
Suggested Reading
Chaudhary P, Marracci G, Galipeau D, et al. Lipoic acid reduces inflammation in a mouse focal cortical experimental autoimmune encephalomyelitis model. J Neuroimmunol. 2015;289:68-74.
Plemel JR, Juzwik CA, Benson CA, et al. Over-the-counter anti-oxidant therapies for use in multiple sclerosis: A systematic review. Mult Scler. 2015;21(12):1485-1495.
Yadav V, Marracci G, Lovera J, et al. Lipoic acid in multiple sclerosis: a pilot study. Mult Scler. 2005;11(2):159-165.
New and Noteworthy Information—November 2016
Exercise may be associated with a small benefit for elderly people who have memory and thinking problems, according to a study published online ahead of print October 19 in Neurology. Researchers studied 70 adults randomized to six months of aerobic exercise training or usual care plus education on cognitive and everyday function. The aerobic exercise training group had significantly improved Alzheimer's Disease Assessment Scale-Cognitive subscale performance, compared with controls. This difference was not significant at six-month follow-up, however. There were no significant between-group differences at intervention completion and at the six-month follow-up in Executive Interview or Alzheimer's Disease Cooperative Study-Activities of Daily Living performance. Examination of secondary measures showed between-group differences at intervention completion favoring the exercise training program group in six-minute walk distance and in diastolic blood pressure.
The FDA has approved Carnexiv (carbamazepine) injection as a short-term replacement therapy for oral carbamazepine formulations in adults with certain seizure types when oral administration is temporarily not feasible. Carnexiv has received orphan drug designation for this indication and will be the first available IV formulation of carbamazepine. The drug is intended for people with partial seizures with complex symptomatology, generalized tonic-clonic seizures, mixed seizure patterns, or other partial or generalized seizures. Carnexiv is not indicated for the treatment of absence seizures. People taking Carnexiv should not discontinue the drug abruptly because of the risk of seizures, status epilepticus, and other withdrawal signs and symptoms. In addition, Carnexiv should not be used in patients with moderate or severe renal impairment. The drug is marketed by Lundbeck, which is headquartered in Deerfield, Illinois.
Chiropractic spinal manipulative therapy (CSMT) is no more effective than placebo for migraine, according to a study published online ahead of print October 2 in the European Journal of Neurology. Investigators randomized 104 migraineurs with at least one migraine attack per month to CSMT, sham chiropractic, or usual pharmacologic management for 17 months. Migraine days were significantly reduced within all three groups from baseline to post treatment. The effect continued in the CSMT and placebo groups at all follow-up time points, but the control group returned to baseline. The reduction in migraine days was not significantly different between the groups. Migraine duration and headache index were reduced significantly more in the CSMT group than in the control group toward the end of follow-up.
Video monitoring facilitates nocturnal surveillance of patients with epilepsy, but the costs are high, according to a study published online ahead of print September 30 in Epilepsia. For six months, researchers asked caregivers at an epilepsy unit to specify whether an acoustic detection system, bed motion sensor, or video monitoring alerted them to seizures and whether the alerts led to interventions. They identified 1,208 seizures in 37 people. Four people had no nocturnal seizures, and 33% of seizures were seen only on video. In 14% of seizures, including 10% of seizures seen only on video, an intervention was made. The extra costs of monitoring were 7,035 euro per seizure seen only on video and leading to an intervention. The results underscore the need for reliable seizure-detection devices, said the authors.
A higher level of physical activity may not reduce a woman's risk of multiple sclerosis (MS), according to a study published online ahead of print September 28 in Neurology. Researchers calculated total metabolic equivalent hours of physical activity per week for women participating in the Nurses' Health Study (NHS) and NHS II. There were 341 confirmed MS cases with first symptoms after baseline. Participants also reported early-life activity. The investigators analyzed the data with Cox proportional hazards models. Compared with women in the lowest baseline physical activity quartile, women in the highest quartile had a 27% reduced rate of MS. This trend was not present in six-year lagged analyses, however. In NHS II, total early life activity at ages 12 to 22 was not associated with MS.
Youth with primary hypertension have significantly worse performance on neurocognitive testing, compared with normotensive controls, according to a study published online ahead of print September 27 in the Journal of Pediatrics.Seventy-five children with newly diagnosed, untreated hypertension and 75 frequency-matched normotensive controls had baseline neurocognitive testing as part of a prospective multicenter study of cognition in primary hypertension. The participants completed general intelligence, attention, memory, executive function, and processing speed tests. Parents rated participants' executive function and sleep disordered breathing. The study groups were well matched. Hypertension was independently associated with worse memory, attention, and executive function, compared with normotension. Results indicated a significant interaction between disordered sleep and hypertension on ratings of executive function. Hypertension heightened the association between increased disordered sleep and worse executive function.
Headache disorders may be associated with an increased risk for the development of new-onset hypothyroidism, according to a study published online ahead of print September 27 in Headache. This longitudinal retrospective cohort study used data from 8,412 participants enrolled in the Fernald Medical Monitoring Program. Participants underwent physical examinations and thyroid function testing every three years during the 20-year program. The primary outcome measure was new-onset hypothyroidism, defined as the initiation of thyroid replacement therapy or thyroid-stimulating hormone test value greater than or equal to 10 without thyroid medication. Headache disorders were present in about 26% of the participants, and new-onset hypothyroidism developed in approximately 7% of participants. The hazard ratio for the development of new-onset hypothyroidism was 1.21 for people with headache disorders.
People with epilepsy can face various psychosocial adversities and extensively report feeling discriminated against, compared with the general population, according to a study published online ahead of print September 16 in Epilepsia. The Adult Psychiatric Morbidity Survey 2007 included comprehensive interviews with 7,403 people. Overall, people with epilepsy were sevenfold more likely to have reported experiencing discrimination due to health problems than the general population without epilepsy. People with epilepsy also had greater odds of experiencing domestic violence and sexual abuse than the general population, although these associations were also found in people with other chronic conditions. There was less evidence of an association between epilepsy and a history of physical abuse or having a greater burden of other stressful life events.
Short episodes of atrial tachycardia or fibrillation are not associated with increased risk of clinical events, compared with absence of these episodes, according to a study published October 18 in Circulation. The Registry of Atrial Tachycardia and Atrial Fibrillation Episodes enrolled 5,379 patients with pacemakers or implantable cardioverter defibrillators. There were 478 hospitalizations among 342 patients for clinical events. Study authors adjudicated 37,531 electrograms. Patients with clinical events were more likely than those without them to have long atrial tachycardia or fibrillation. Only short episodes of atrial tachycardia or fibrillation were documented in 9% of patients with pacemakers and in 16% of patients with implantable cardioverter defibrillators. Patients with clinical events were no more likely than those without them to have short atrial tachycardia or fibrillation.
A brain signature identifies patients with fibromyalgia with 93% accuracy, according to a study published online ahead of print August 31 in Pain. Researchers examined 37 patients with fibromyalgia and 35 matched healthy controls. They analyzed participants' functional MRI responses to painful pressure and nonpainful multisensory stimulation. Investigators used machine-learning techniques to identify a brain-based fibromyalgia signature. When exposed to the same painful stimuli, patients with fibromyalgia had greater Neurologic Pain Signature responses. Furthermore, a new pain-related classifier revealed augmented responses in sensory integration and self-referential regions in fibromyalgia, and reduced responses in the lateral frontal cortex. Combined activity in the Neurologic Pain Signature, fibromyalgia pain, and multisensory patterns classified patients vs. controls with 92% sensitivity and 94% specificity in individuals who were not part of the study sample.
Children have measurable brain changes after a single season of youth football, even when they do not sustain a concussion, according to a study published online ahead of print October 24 in Radiology. Head impact data were recorded using the Head Impact Telemetry system and quantified as the combined-probability risk-weighted cumulative exposure. Twenty-five male participants were evaluated for seasonal fractional anisotropy changes in the inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, and superior longitudinal fasciculus. There were statistically significant linear relationships between risk-weighted cumulative exposure and decreased fractional anisotropy in the whole, core, and terminals of the left inferior fronto-occipital fasciculus. A trend toward statistical significance in the right superior longitudinal fasciculus was observed. Decrease in fractional anisotropy of the right superior longitudinal fasciculus terminal was significantly correlated with risk-weighted cumulative exposure.
Zika virus contributes to the development of Guillain-Barré syndrome, according to a study published online ahead of print October 5 in the New England Journal of Medicine. From November 2015 through March 2016, clusters of cases of Guillain-Barré syndrome were observed during an outbreak of Zika virus in Colombia. Researchers characterized the clinical features of 68 patients with Guillain-Barré syndrome during the outbreak and investigated their relationship with Zika virus infection. In all, 97% of patients had symptoms compatible with Zika virus infection before the onset of Guillain-Barré syndrome. Among the 42 patients who had samples tested for Zika virus infection, the results were positive in 40%. Most of the positive results were in urine samples, although three samples of CSF were also positive.
Among patients with amnestic mild cognitive impairment (aMCI), women have better verbal memory than men despite similar levels of brain hypometabolism, according to a study published online ahead of print October 5 in Neurology. In the Alzheimer's Disease Neuroimaging Initiative, 390 controls, 672 participants with aMCI, and 254 people with Alzheimer's disease dementia completed the Rey Auditory Verbal Learning Test and [18F]-fluorodeoxyglucose-PET. Female sex, higher temporal lobe glucose metabolic rates (TLGluMR), and the interaction of the two factors were associated with better verbal memory. The female advantage in verbal memory was greatest in people with moderate to high TLGluMR and minimal or absent among individuals with lower TLGluMR. Diagnosis-stratified analyses revealed that this interaction was driven by the aMCI group.
—Kimberly Williams
Exercise may be associated with a small benefit for elderly people who have memory and thinking problems, according to a study published online ahead of print October 19 in Neurology. Researchers studied 70 adults randomized to six months of aerobic exercise training or usual care plus education on cognitive and everyday function. The aerobic exercise training group had significantly improved Alzheimer's Disease Assessment Scale-Cognitive subscale performance, compared with controls. This difference was not significant at six-month follow-up, however. There were no significant between-group differences at intervention completion and at the six-month follow-up in Executive Interview or Alzheimer's Disease Cooperative Study-Activities of Daily Living performance. Examination of secondary measures showed between-group differences at intervention completion favoring the exercise training program group in six-minute walk distance and in diastolic blood pressure.
The FDA has approved Carnexiv (carbamazepine) injection as a short-term replacement therapy for oral carbamazepine formulations in adults with certain seizure types when oral administration is temporarily not feasible. Carnexiv has received orphan drug designation for this indication and will be the first available IV formulation of carbamazepine. The drug is intended for people with partial seizures with complex symptomatology, generalized tonic-clonic seizures, mixed seizure patterns, or other partial or generalized seizures. Carnexiv is not indicated for the treatment of absence seizures. People taking Carnexiv should not discontinue the drug abruptly because of the risk of seizures, status epilepticus, and other withdrawal signs and symptoms. In addition, Carnexiv should not be used in patients with moderate or severe renal impairment. The drug is marketed by Lundbeck, which is headquartered in Deerfield, Illinois.
Chiropractic spinal manipulative therapy (CSMT) is no more effective than placebo for migraine, according to a study published online ahead of print October 2 in the European Journal of Neurology. Investigators randomized 104 migraineurs with at least one migraine attack per month to CSMT, sham chiropractic, or usual pharmacologic management for 17 months. Migraine days were significantly reduced within all three groups from baseline to post treatment. The effect continued in the CSMT and placebo groups at all follow-up time points, but the control group returned to baseline. The reduction in migraine days was not significantly different between the groups. Migraine duration and headache index were reduced significantly more in the CSMT group than in the control group toward the end of follow-up.
Video monitoring facilitates nocturnal surveillance of patients with epilepsy, but the costs are high, according to a study published online ahead of print September 30 in Epilepsia. For six months, researchers asked caregivers at an epilepsy unit to specify whether an acoustic detection system, bed motion sensor, or video monitoring alerted them to seizures and whether the alerts led to interventions. They identified 1,208 seizures in 37 people. Four people had no nocturnal seizures, and 33% of seizures were seen only on video. In 14% of seizures, including 10% of seizures seen only on video, an intervention was made. The extra costs of monitoring were 7,035 euro per seizure seen only on video and leading to an intervention. The results underscore the need for reliable seizure-detection devices, said the authors.
A higher level of physical activity may not reduce a woman's risk of multiple sclerosis (MS), according to a study published online ahead of print September 28 in Neurology. Researchers calculated total metabolic equivalent hours of physical activity per week for women participating in the Nurses' Health Study (NHS) and NHS II. There were 341 confirmed MS cases with first symptoms after baseline. Participants also reported early-life activity. The investigators analyzed the data with Cox proportional hazards models. Compared with women in the lowest baseline physical activity quartile, women in the highest quartile had a 27% reduced rate of MS. This trend was not present in six-year lagged analyses, however. In NHS II, total early life activity at ages 12 to 22 was not associated with MS.
Youth with primary hypertension have significantly worse performance on neurocognitive testing, compared with normotensive controls, according to a study published online ahead of print September 27 in the Journal of Pediatrics.Seventy-five children with newly diagnosed, untreated hypertension and 75 frequency-matched normotensive controls had baseline neurocognitive testing as part of a prospective multicenter study of cognition in primary hypertension. The participants completed general intelligence, attention, memory, executive function, and processing speed tests. Parents rated participants' executive function and sleep disordered breathing. The study groups were well matched. Hypertension was independently associated with worse memory, attention, and executive function, compared with normotension. Results indicated a significant interaction between disordered sleep and hypertension on ratings of executive function. Hypertension heightened the association between increased disordered sleep and worse executive function.
Headache disorders may be associated with an increased risk for the development of new-onset hypothyroidism, according to a study published online ahead of print September 27 in Headache. This longitudinal retrospective cohort study used data from 8,412 participants enrolled in the Fernald Medical Monitoring Program. Participants underwent physical examinations and thyroid function testing every three years during the 20-year program. The primary outcome measure was new-onset hypothyroidism, defined as the initiation of thyroid replacement therapy or thyroid-stimulating hormone test value greater than or equal to 10 without thyroid medication. Headache disorders were present in about 26% of the participants, and new-onset hypothyroidism developed in approximately 7% of participants. The hazard ratio for the development of new-onset hypothyroidism was 1.21 for people with headache disorders.
People with epilepsy can face various psychosocial adversities and extensively report feeling discriminated against, compared with the general population, according to a study published online ahead of print September 16 in Epilepsia. The Adult Psychiatric Morbidity Survey 2007 included comprehensive interviews with 7,403 people. Overall, people with epilepsy were sevenfold more likely to have reported experiencing discrimination due to health problems than the general population without epilepsy. People with epilepsy also had greater odds of experiencing domestic violence and sexual abuse than the general population, although these associations were also found in people with other chronic conditions. There was less evidence of an association between epilepsy and a history of physical abuse or having a greater burden of other stressful life events.
Short episodes of atrial tachycardia or fibrillation are not associated with increased risk of clinical events, compared with absence of these episodes, according to a study published October 18 in Circulation. The Registry of Atrial Tachycardia and Atrial Fibrillation Episodes enrolled 5,379 patients with pacemakers or implantable cardioverter defibrillators. There were 478 hospitalizations among 342 patients for clinical events. Study authors adjudicated 37,531 electrograms. Patients with clinical events were more likely than those without them to have long atrial tachycardia or fibrillation. Only short episodes of atrial tachycardia or fibrillation were documented in 9% of patients with pacemakers and in 16% of patients with implantable cardioverter defibrillators. Patients with clinical events were no more likely than those without them to have short atrial tachycardia or fibrillation.
A brain signature identifies patients with fibromyalgia with 93% accuracy, according to a study published online ahead of print August 31 in Pain. Researchers examined 37 patients with fibromyalgia and 35 matched healthy controls. They analyzed participants' functional MRI responses to painful pressure and nonpainful multisensory stimulation. Investigators used machine-learning techniques to identify a brain-based fibromyalgia signature. When exposed to the same painful stimuli, patients with fibromyalgia had greater Neurologic Pain Signature responses. Furthermore, a new pain-related classifier revealed augmented responses in sensory integration and self-referential regions in fibromyalgia, and reduced responses in the lateral frontal cortex. Combined activity in the Neurologic Pain Signature, fibromyalgia pain, and multisensory patterns classified patients vs. controls with 92% sensitivity and 94% specificity in individuals who were not part of the study sample.
Children have measurable brain changes after a single season of youth football, even when they do not sustain a concussion, according to a study published online ahead of print October 24 in Radiology. Head impact data were recorded using the Head Impact Telemetry system and quantified as the combined-probability risk-weighted cumulative exposure. Twenty-five male participants were evaluated for seasonal fractional anisotropy changes in the inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, and superior longitudinal fasciculus. There were statistically significant linear relationships between risk-weighted cumulative exposure and decreased fractional anisotropy in the whole, core, and terminals of the left inferior fronto-occipital fasciculus. A trend toward statistical significance in the right superior longitudinal fasciculus was observed. Decrease in fractional anisotropy of the right superior longitudinal fasciculus terminal was significantly correlated with risk-weighted cumulative exposure.
Zika virus contributes to the development of Guillain-Barré syndrome, according to a study published online ahead of print October 5 in the New England Journal of Medicine. From November 2015 through March 2016, clusters of cases of Guillain-Barré syndrome were observed during an outbreak of Zika virus in Colombia. Researchers characterized the clinical features of 68 patients with Guillain-Barré syndrome during the outbreak and investigated their relationship with Zika virus infection. In all, 97% of patients had symptoms compatible with Zika virus infection before the onset of Guillain-Barré syndrome. Among the 42 patients who had samples tested for Zika virus infection, the results were positive in 40%. Most of the positive results were in urine samples, although three samples of CSF were also positive.
Among patients with amnestic mild cognitive impairment (aMCI), women have better verbal memory than men despite similar levels of brain hypometabolism, according to a study published online ahead of print October 5 in Neurology. In the Alzheimer's Disease Neuroimaging Initiative, 390 controls, 672 participants with aMCI, and 254 people with Alzheimer's disease dementia completed the Rey Auditory Verbal Learning Test and [18F]-fluorodeoxyglucose-PET. Female sex, higher temporal lobe glucose metabolic rates (TLGluMR), and the interaction of the two factors were associated with better verbal memory. The female advantage in verbal memory was greatest in people with moderate to high TLGluMR and minimal or absent among individuals with lower TLGluMR. Diagnosis-stratified analyses revealed that this interaction was driven by the aMCI group.
—Kimberly Williams
Exercise may be associated with a small benefit for elderly people who have memory and thinking problems, according to a study published online ahead of print October 19 in Neurology. Researchers studied 70 adults randomized to six months of aerobic exercise training or usual care plus education on cognitive and everyday function. The aerobic exercise training group had significantly improved Alzheimer's Disease Assessment Scale-Cognitive subscale performance, compared with controls. This difference was not significant at six-month follow-up, however. There were no significant between-group differences at intervention completion and at the six-month follow-up in Executive Interview or Alzheimer's Disease Cooperative Study-Activities of Daily Living performance. Examination of secondary measures showed between-group differences at intervention completion favoring the exercise training program group in six-minute walk distance and in diastolic blood pressure.
The FDA has approved Carnexiv (carbamazepine) injection as a short-term replacement therapy for oral carbamazepine formulations in adults with certain seizure types when oral administration is temporarily not feasible. Carnexiv has received orphan drug designation for this indication and will be the first available IV formulation of carbamazepine. The drug is intended for people with partial seizures with complex symptomatology, generalized tonic-clonic seizures, mixed seizure patterns, or other partial or generalized seizures. Carnexiv is not indicated for the treatment of absence seizures. People taking Carnexiv should not discontinue the drug abruptly because of the risk of seizures, status epilepticus, and other withdrawal signs and symptoms. In addition, Carnexiv should not be used in patients with moderate or severe renal impairment. The drug is marketed by Lundbeck, which is headquartered in Deerfield, Illinois.
Chiropractic spinal manipulative therapy (CSMT) is no more effective than placebo for migraine, according to a study published online ahead of print October 2 in the European Journal of Neurology. Investigators randomized 104 migraineurs with at least one migraine attack per month to CSMT, sham chiropractic, or usual pharmacologic management for 17 months. Migraine days were significantly reduced within all three groups from baseline to post treatment. The effect continued in the CSMT and placebo groups at all follow-up time points, but the control group returned to baseline. The reduction in migraine days was not significantly different between the groups. Migraine duration and headache index were reduced significantly more in the CSMT group than in the control group toward the end of follow-up.
Video monitoring facilitates nocturnal surveillance of patients with epilepsy, but the costs are high, according to a study published online ahead of print September 30 in Epilepsia. For six months, researchers asked caregivers at an epilepsy unit to specify whether an acoustic detection system, bed motion sensor, or video monitoring alerted them to seizures and whether the alerts led to interventions. They identified 1,208 seizures in 37 people. Four people had no nocturnal seizures, and 33% of seizures were seen only on video. In 14% of seizures, including 10% of seizures seen only on video, an intervention was made. The extra costs of monitoring were 7,035 euro per seizure seen only on video and leading to an intervention. The results underscore the need for reliable seizure-detection devices, said the authors.
A higher level of physical activity may not reduce a woman's risk of multiple sclerosis (MS), according to a study published online ahead of print September 28 in Neurology. Researchers calculated total metabolic equivalent hours of physical activity per week for women participating in the Nurses' Health Study (NHS) and NHS II. There were 341 confirmed MS cases with first symptoms after baseline. Participants also reported early-life activity. The investigators analyzed the data with Cox proportional hazards models. Compared with women in the lowest baseline physical activity quartile, women in the highest quartile had a 27% reduced rate of MS. This trend was not present in six-year lagged analyses, however. In NHS II, total early life activity at ages 12 to 22 was not associated with MS.
Youth with primary hypertension have significantly worse performance on neurocognitive testing, compared with normotensive controls, according to a study published online ahead of print September 27 in the Journal of Pediatrics.Seventy-five children with newly diagnosed, untreated hypertension and 75 frequency-matched normotensive controls had baseline neurocognitive testing as part of a prospective multicenter study of cognition in primary hypertension. The participants completed general intelligence, attention, memory, executive function, and processing speed tests. Parents rated participants' executive function and sleep disordered breathing. The study groups were well matched. Hypertension was independently associated with worse memory, attention, and executive function, compared with normotension. Results indicated a significant interaction between disordered sleep and hypertension on ratings of executive function. Hypertension heightened the association between increased disordered sleep and worse executive function.
Headache disorders may be associated with an increased risk for the development of new-onset hypothyroidism, according to a study published online ahead of print September 27 in Headache. This longitudinal retrospective cohort study used data from 8,412 participants enrolled in the Fernald Medical Monitoring Program. Participants underwent physical examinations and thyroid function testing every three years during the 20-year program. The primary outcome measure was new-onset hypothyroidism, defined as the initiation of thyroid replacement therapy or thyroid-stimulating hormone test value greater than or equal to 10 without thyroid medication. Headache disorders were present in about 26% of the participants, and new-onset hypothyroidism developed in approximately 7% of participants. The hazard ratio for the development of new-onset hypothyroidism was 1.21 for people with headache disorders.
People with epilepsy can face various psychosocial adversities and extensively report feeling discriminated against, compared with the general population, according to a study published online ahead of print September 16 in Epilepsia. The Adult Psychiatric Morbidity Survey 2007 included comprehensive interviews with 7,403 people. Overall, people with epilepsy were sevenfold more likely to have reported experiencing discrimination due to health problems than the general population without epilepsy. People with epilepsy also had greater odds of experiencing domestic violence and sexual abuse than the general population, although these associations were also found in people with other chronic conditions. There was less evidence of an association between epilepsy and a history of physical abuse or having a greater burden of other stressful life events.
Short episodes of atrial tachycardia or fibrillation are not associated with increased risk of clinical events, compared with absence of these episodes, according to a study published October 18 in Circulation. The Registry of Atrial Tachycardia and Atrial Fibrillation Episodes enrolled 5,379 patients with pacemakers or implantable cardioverter defibrillators. There were 478 hospitalizations among 342 patients for clinical events. Study authors adjudicated 37,531 electrograms. Patients with clinical events were more likely than those without them to have long atrial tachycardia or fibrillation. Only short episodes of atrial tachycardia or fibrillation were documented in 9% of patients with pacemakers and in 16% of patients with implantable cardioverter defibrillators. Patients with clinical events were no more likely than those without them to have short atrial tachycardia or fibrillation.
A brain signature identifies patients with fibromyalgia with 93% accuracy, according to a study published online ahead of print August 31 in Pain. Researchers examined 37 patients with fibromyalgia and 35 matched healthy controls. They analyzed participants' functional MRI responses to painful pressure and nonpainful multisensory stimulation. Investigators used machine-learning techniques to identify a brain-based fibromyalgia signature. When exposed to the same painful stimuli, patients with fibromyalgia had greater Neurologic Pain Signature responses. Furthermore, a new pain-related classifier revealed augmented responses in sensory integration and self-referential regions in fibromyalgia, and reduced responses in the lateral frontal cortex. Combined activity in the Neurologic Pain Signature, fibromyalgia pain, and multisensory patterns classified patients vs. controls with 92% sensitivity and 94% specificity in individuals who were not part of the study sample.
Children have measurable brain changes after a single season of youth football, even when they do not sustain a concussion, according to a study published online ahead of print October 24 in Radiology. Head impact data were recorded using the Head Impact Telemetry system and quantified as the combined-probability risk-weighted cumulative exposure. Twenty-five male participants were evaluated for seasonal fractional anisotropy changes in the inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, and superior longitudinal fasciculus. There were statistically significant linear relationships between risk-weighted cumulative exposure and decreased fractional anisotropy in the whole, core, and terminals of the left inferior fronto-occipital fasciculus. A trend toward statistical significance in the right superior longitudinal fasciculus was observed. Decrease in fractional anisotropy of the right superior longitudinal fasciculus terminal was significantly correlated with risk-weighted cumulative exposure.
Zika virus contributes to the development of Guillain-Barré syndrome, according to a study published online ahead of print October 5 in the New England Journal of Medicine. From November 2015 through March 2016, clusters of cases of Guillain-Barré syndrome were observed during an outbreak of Zika virus in Colombia. Researchers characterized the clinical features of 68 patients with Guillain-Barré syndrome during the outbreak and investigated their relationship with Zika virus infection. In all, 97% of patients had symptoms compatible with Zika virus infection before the onset of Guillain-Barré syndrome. Among the 42 patients who had samples tested for Zika virus infection, the results were positive in 40%. Most of the positive results were in urine samples, although three samples of CSF were also positive.
Among patients with amnestic mild cognitive impairment (aMCI), women have better verbal memory than men despite similar levels of brain hypometabolism, according to a study published online ahead of print October 5 in Neurology. In the Alzheimer's Disease Neuroimaging Initiative, 390 controls, 672 participants with aMCI, and 254 people with Alzheimer's disease dementia completed the Rey Auditory Verbal Learning Test and [18F]-fluorodeoxyglucose-PET. Female sex, higher temporal lobe glucose metabolic rates (TLGluMR), and the interaction of the two factors were associated with better verbal memory. The female advantage in verbal memory was greatest in people with moderate to high TLGluMR and minimal or absent among individuals with lower TLGluMR. Diagnosis-stratified analyses revealed that this interaction was driven by the aMCI group.
—Kimberly Williams
Finding a Better Approach to Diagnosing Abnormal Uterine Bleeding

Click here to download the PDF.
Despite advances in diagnostic medicine, the evaluation of abnormal uterine bleeding (AUB) remains a challenge for physicians. In this supplement, learn about:
- Pathophysiology and differential diagnosis of AUB
- The limitations of blind endometrial biopsy
- The differences between diagnostic hysteroscopy options
Author
Steven R. Goldstein, MD
Professor of Obstetrics and Gynecology
New York University School of Medicine
Immediate Past President
American Institute of Ultrasound in Medicine
Director of Gynecologic Ultrasound and Co-Director of Bone Densitometry
New York University Medical Center
New York, New York
DISCLOSURES
Dr. Goldstein discloses that he is a paid consultant for CooperSurgical.

Click here to download the PDF.
Despite advances in diagnostic medicine, the evaluation of abnormal uterine bleeding (AUB) remains a challenge for physicians. In this supplement, learn about:
- Pathophysiology and differential diagnosis of AUB
- The limitations of blind endometrial biopsy
- The differences between diagnostic hysteroscopy options
Author
Steven R. Goldstein, MD
Professor of Obstetrics and Gynecology
New York University School of Medicine
Immediate Past President
American Institute of Ultrasound in Medicine
Director of Gynecologic Ultrasound and Co-Director of Bone Densitometry
New York University Medical Center
New York, New York
DISCLOSURES
Dr. Goldstein discloses that he is a paid consultant for CooperSurgical.

Click here to download the PDF.
Despite advances in diagnostic medicine, the evaluation of abnormal uterine bleeding (AUB) remains a challenge for physicians. In this supplement, learn about:
- Pathophysiology and differential diagnosis of AUB
- The limitations of blind endometrial biopsy
- The differences between diagnostic hysteroscopy options
Author
Steven R. Goldstein, MD
Professor of Obstetrics and Gynecology
New York University School of Medicine
Immediate Past President
American Institute of Ultrasound in Medicine
Director of Gynecologic Ultrasound and Co-Director of Bone Densitometry
New York University Medical Center
New York, New York
DISCLOSURES
Dr. Goldstein discloses that he is a paid consultant for CooperSurgical.
AATS Submission Opportunities
Don’t miss the opportunity to submit to one of these AATS scholarship programs.
Deadline: January 20, 2017
AATS Member for a Day
North American medical students, and general and up to third year integrated CT Surgery
(I-6) surgery residents can accompany an AATS Member during portions of the AATS Centennial as an AATS Member for a Day.
The meeting takes place April 29-May 3, 2017 in Boston, MA.
Those selected will receive free hotel accommodations for three to four night in an AATS Centennial hotel. They will also be given a $250 meal and $500 travel stipend at the end of the meeting.
Eligibility/More information
Summer Internship Opportunity for First/Second Year Medical Students
First and second year medical students can spend the summer being exposed to
cardiothoracic surgery thanks to the AATS Summer Intern Scholarship. For eight weeks
(June – September), students will work in the CT department of an AATS member.
Those chosen receive $2,500 for living expenses. They also will be able to attend the AATS Centennial gratis.
The meeting takes place April 29 – May 3, 2017
Boston, MA.
AATS Resident Poster Competition
International cardiothoracic surgery residents and/or congenital heart surgery fellows: Take advantage of this opportunity to represent your institution and present a scientific poster of your clinical/investigative research at The AATS Centennial.
The meeting will take place April 29 - May 3, 2017
Boston, MA.
Awardee institutions get a $500 stipend to offset meal/travel costs. Each winner receives free registration to the AATS Centennial and access to the Skills Course (April 30) and Postgraduate Course (May 1).
Non-MD CT Surgical Team Scientific Poster Competition
Non-MD cardiothoracic team professionals can submit a scientific poster for
the Perioperative/Team-Based Care Poster Competition.
Winning posters will be displayed at the AATS Centennial, April 29 – May 3, 2017
Boston, MA.
The competition winner will receive a $1,000 stipend to offset travel and accommodation costs.
Share:
Don’t miss the opportunity to submit to one of these AATS scholarship programs.
Deadline: January 20, 2017
AATS Member for a Day
North American medical students, and general and up to third year integrated CT Surgery
(I-6) surgery residents can accompany an AATS Member during portions of the AATS Centennial as an AATS Member for a Day.
The meeting takes place April 29-May 3, 2017 in Boston, MA.
Those selected will receive free hotel accommodations for three to four night in an AATS Centennial hotel. They will also be given a $250 meal and $500 travel stipend at the end of the meeting.
Eligibility/More information
Summer Internship Opportunity for First/Second Year Medical Students
First and second year medical students can spend the summer being exposed to
cardiothoracic surgery thanks to the AATS Summer Intern Scholarship. For eight weeks
(June – September), students will work in the CT department of an AATS member.
Those chosen receive $2,500 for living expenses. They also will be able to attend the AATS Centennial gratis.
The meeting takes place April 29 – May 3, 2017
Boston, MA.
AATS Resident Poster Competition
International cardiothoracic surgery residents and/or congenital heart surgery fellows: Take advantage of this opportunity to represent your institution and present a scientific poster of your clinical/investigative research at The AATS Centennial.
The meeting will take place April 29 - May 3, 2017
Boston, MA.
Awardee institutions get a $500 stipend to offset meal/travel costs. Each winner receives free registration to the AATS Centennial and access to the Skills Course (April 30) and Postgraduate Course (May 1).
Non-MD CT Surgical Team Scientific Poster Competition
Non-MD cardiothoracic team professionals can submit a scientific poster for
the Perioperative/Team-Based Care Poster Competition.
Winning posters will be displayed at the AATS Centennial, April 29 – May 3, 2017
Boston, MA.
The competition winner will receive a $1,000 stipend to offset travel and accommodation costs.
Share:
Don’t miss the opportunity to submit to one of these AATS scholarship programs.
Deadline: January 20, 2017
AATS Member for a Day
North American medical students, and general and up to third year integrated CT Surgery
(I-6) surgery residents can accompany an AATS Member during portions of the AATS Centennial as an AATS Member for a Day.
The meeting takes place April 29-May 3, 2017 in Boston, MA.
Those selected will receive free hotel accommodations for three to four night in an AATS Centennial hotel. They will also be given a $250 meal and $500 travel stipend at the end of the meeting.
Eligibility/More information
Summer Internship Opportunity for First/Second Year Medical Students
First and second year medical students can spend the summer being exposed to
cardiothoracic surgery thanks to the AATS Summer Intern Scholarship. For eight weeks
(June – September), students will work in the CT department of an AATS member.
Those chosen receive $2,500 for living expenses. They also will be able to attend the AATS Centennial gratis.
The meeting takes place April 29 – May 3, 2017
Boston, MA.
AATS Resident Poster Competition
International cardiothoracic surgery residents and/or congenital heart surgery fellows: Take advantage of this opportunity to represent your institution and present a scientific poster of your clinical/investigative research at The AATS Centennial.
The meeting will take place April 29 - May 3, 2017
Boston, MA.
Awardee institutions get a $500 stipend to offset meal/travel costs. Each winner receives free registration to the AATS Centennial and access to the Skills Course (April 30) and Postgraduate Course (May 1).
Non-MD CT Surgical Team Scientific Poster Competition
Non-MD cardiothoracic team professionals can submit a scientific poster for
the Perioperative/Team-Based Care Poster Competition.
Winning posters will be displayed at the AATS Centennial, April 29 – May 3, 2017
Boston, MA.
The competition winner will receive a $1,000 stipend to offset travel and accommodation costs.
Share:
AATS Mitral Conclave Call for Abstracts & Videos
AATS invites you to submit your abstracts and videos to the 2017 Mitral Conclave.
AATS Mitral Conclave
April 27-28, 2017
New York, NY
Submission Deadline:
Sunday, January 8, 2017 @ 11.59 pm EST
Share:
AATS invites you to submit your abstracts and videos to the 2017 Mitral Conclave.
AATS Mitral Conclave
April 27-28, 2017
New York, NY
Submission Deadline:
Sunday, January 8, 2017 @ 11.59 pm EST
Share:
AATS invites you to submit your abstracts and videos to the 2017 Mitral Conclave.
AATS Mitral Conclave
April 27-28, 2017
New York, NY
Submission Deadline:
Sunday, January 8, 2017 @ 11.59 pm EST
Share:
Gantenerumab Reduces Levels of Biomarkers Associated With Alzheimer’s Disease
TORONTO—The fully human monoclonal antibody gantenerumab may reduce levels of neurogranin, a postsynaptic degradation marker specific to Alzheimer’s disease, according to a post hoc analysis described at the Alzheimer’s Association International Conference. Gantenerumab, however, does not affect levels of YKL-40, also known as chitinase-3-like protein 1, which is thought to be an inflammation marker mainly derived from astrocytes.
Philip Scheltens, MD, PhD, Director of the Alzheimer Center at the VU University Medical Center in Amsterdam, and colleagues conducted a trial to determine whether gantenerumab would influence dementia severity. The researchers screened people for prodromal Alzheimer’s disease by administering the Free and Cued Selective Reminding Test and measuring their level of CSF amyloid beta-42. They randomized 797 patients to placebo or to 105-mg or 225-mg doses of gantenerumab administered every four weeks by subcutaneous injection. CSF was collected at week 52 for some patients and at week 104 for all patients. A subset of 114 patients underwent amyloid PET. The trial’s primary end point was the Clinical Dementia Rating Scale Sum of Boxes. The study was halted after a futility analysis found no difference between treatment groups.
During the study, the researchers measured levels of total tau, phospho tau, amyloid beta, neurogranin, and YKL-40. Post hoc analysis revealed that total tau tended to increase slightly in the placebo group, remain stable among patients receiving 105 mg of gantenerumab, and decline slightly among people receiving 225 mg of gantenerumab. Differences were significant at both time points.
At week 104, the investigators observed a significant decrease in phospho tau for both doses of gantenerumab. At the same time point, patients receiving the higher dose of gantenerumab had a decrease in neurogranin. “All three biomarkers showed a dose- and time-dependent decrease” following gantenerumab treatment, said Dr. Scheltens. Treatment did not affect levels of amyloid beta or YKL-40, however.
Among patients who underwent amyloid PET, the researchers observed a small and nonsignificant reduction in amyloid beta following treatment with gantenerumab. They noted a correlation between amyloid beta and phospho tau, but not between amyloid beta and total tau. “We do not exactly understand why that is the case,” said Dr. Scheltens.
“These biomarkers are … highly correlated among each other,” and this correlation is specific to Alzheimer’s disease, he added. Patients with Creutzfeldt–Jakob disease or stroke may have large increases in total tau, but not in phospho tau or neurogranin. Furthermore, neurogranin is not elevated in other neurodegenerative diseases such as frontotemporal dementia or dementia with Lewy bodies. “This is a characteristic pattern,” said Dr. Scheltens.
A higher dose of gantenerumab may reduce levels of amyloid beta, and further study of the therapy is needed, Dr. Scheltens concluded.
—Erik Greb
Suggested Reading
Bohrmann B, Baumann K, Benz J, et al. Gantenerumab: a novel human anti-Aβ antibody demonstrates sustained cerebral amyloid-β binding and elicits cell-mediated removal of human amyloid-β. J Alzheimers Dis. 2012;28(1):49-69.
Ostrowitzki S, Deptula D, Thurfjell L, et al. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch Neurol. 2012;69(2):198-207.
Panza F, Solfrizzi V, Imbimbo BP, et al. Efficacy and safety studies of gantenerumab in patients with Alzheimer’s disease. Expert Rev Neurother. 2014;14(9):973-986.
TORONTO—The fully human monoclonal antibody gantenerumab may reduce levels of neurogranin, a postsynaptic degradation marker specific to Alzheimer’s disease, according to a post hoc analysis described at the Alzheimer’s Association International Conference. Gantenerumab, however, does not affect levels of YKL-40, also known as chitinase-3-like protein 1, which is thought to be an inflammation marker mainly derived from astrocytes.
Philip Scheltens, MD, PhD, Director of the Alzheimer Center at the VU University Medical Center in Amsterdam, and colleagues conducted a trial to determine whether gantenerumab would influence dementia severity. The researchers screened people for prodromal Alzheimer’s disease by administering the Free and Cued Selective Reminding Test and measuring their level of CSF amyloid beta-42. They randomized 797 patients to placebo or to 105-mg or 225-mg doses of gantenerumab administered every four weeks by subcutaneous injection. CSF was collected at week 52 for some patients and at week 104 for all patients. A subset of 114 patients underwent amyloid PET. The trial’s primary end point was the Clinical Dementia Rating Scale Sum of Boxes. The study was halted after a futility analysis found no difference between treatment groups.
During the study, the researchers measured levels of total tau, phospho tau, amyloid beta, neurogranin, and YKL-40. Post hoc analysis revealed that total tau tended to increase slightly in the placebo group, remain stable among patients receiving 105 mg of gantenerumab, and decline slightly among people receiving 225 mg of gantenerumab. Differences were significant at both time points.
At week 104, the investigators observed a significant decrease in phospho tau for both doses of gantenerumab. At the same time point, patients receiving the higher dose of gantenerumab had a decrease in neurogranin. “All three biomarkers showed a dose- and time-dependent decrease” following gantenerumab treatment, said Dr. Scheltens. Treatment did not affect levels of amyloid beta or YKL-40, however.
Among patients who underwent amyloid PET, the researchers observed a small and nonsignificant reduction in amyloid beta following treatment with gantenerumab. They noted a correlation between amyloid beta and phospho tau, but not between amyloid beta and total tau. “We do not exactly understand why that is the case,” said Dr. Scheltens.
“These biomarkers are … highly correlated among each other,” and this correlation is specific to Alzheimer’s disease, he added. Patients with Creutzfeldt–Jakob disease or stroke may have large increases in total tau, but not in phospho tau or neurogranin. Furthermore, neurogranin is not elevated in other neurodegenerative diseases such as frontotemporal dementia or dementia with Lewy bodies. “This is a characteristic pattern,” said Dr. Scheltens.
A higher dose of gantenerumab may reduce levels of amyloid beta, and further study of the therapy is needed, Dr. Scheltens concluded.
—Erik Greb
Suggested Reading
Bohrmann B, Baumann K, Benz J, et al. Gantenerumab: a novel human anti-Aβ antibody demonstrates sustained cerebral amyloid-β binding and elicits cell-mediated removal of human amyloid-β. J Alzheimers Dis. 2012;28(1):49-69.
Ostrowitzki S, Deptula D, Thurfjell L, et al. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch Neurol. 2012;69(2):198-207.
Panza F, Solfrizzi V, Imbimbo BP, et al. Efficacy and safety studies of gantenerumab in patients with Alzheimer’s disease. Expert Rev Neurother. 2014;14(9):973-986.
TORONTO—The fully human monoclonal antibody gantenerumab may reduce levels of neurogranin, a postsynaptic degradation marker specific to Alzheimer’s disease, according to a post hoc analysis described at the Alzheimer’s Association International Conference. Gantenerumab, however, does not affect levels of YKL-40, also known as chitinase-3-like protein 1, which is thought to be an inflammation marker mainly derived from astrocytes.
Philip Scheltens, MD, PhD, Director of the Alzheimer Center at the VU University Medical Center in Amsterdam, and colleagues conducted a trial to determine whether gantenerumab would influence dementia severity. The researchers screened people for prodromal Alzheimer’s disease by administering the Free and Cued Selective Reminding Test and measuring their level of CSF amyloid beta-42. They randomized 797 patients to placebo or to 105-mg or 225-mg doses of gantenerumab administered every four weeks by subcutaneous injection. CSF was collected at week 52 for some patients and at week 104 for all patients. A subset of 114 patients underwent amyloid PET. The trial’s primary end point was the Clinical Dementia Rating Scale Sum of Boxes. The study was halted after a futility analysis found no difference between treatment groups.
During the study, the researchers measured levels of total tau, phospho tau, amyloid beta, neurogranin, and YKL-40. Post hoc analysis revealed that total tau tended to increase slightly in the placebo group, remain stable among patients receiving 105 mg of gantenerumab, and decline slightly among people receiving 225 mg of gantenerumab. Differences were significant at both time points.
At week 104, the investigators observed a significant decrease in phospho tau for both doses of gantenerumab. At the same time point, patients receiving the higher dose of gantenerumab had a decrease in neurogranin. “All three biomarkers showed a dose- and time-dependent decrease” following gantenerumab treatment, said Dr. Scheltens. Treatment did not affect levels of amyloid beta or YKL-40, however.
Among patients who underwent amyloid PET, the researchers observed a small and nonsignificant reduction in amyloid beta following treatment with gantenerumab. They noted a correlation between amyloid beta and phospho tau, but not between amyloid beta and total tau. “We do not exactly understand why that is the case,” said Dr. Scheltens.
“These biomarkers are … highly correlated among each other,” and this correlation is specific to Alzheimer’s disease, he added. Patients with Creutzfeldt–Jakob disease or stroke may have large increases in total tau, but not in phospho tau or neurogranin. Furthermore, neurogranin is not elevated in other neurodegenerative diseases such as frontotemporal dementia or dementia with Lewy bodies. “This is a characteristic pattern,” said Dr. Scheltens.
A higher dose of gantenerumab may reduce levels of amyloid beta, and further study of the therapy is needed, Dr. Scheltens concluded.
—Erik Greb
Suggested Reading
Bohrmann B, Baumann K, Benz J, et al. Gantenerumab: a novel human anti-Aβ antibody demonstrates sustained cerebral amyloid-β binding and elicits cell-mediated removal of human amyloid-β. J Alzheimers Dis. 2012;28(1):49-69.
Ostrowitzki S, Deptula D, Thurfjell L, et al. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch Neurol. 2012;69(2):198-207.
Panza F, Solfrizzi V, Imbimbo BP, et al. Efficacy and safety studies of gantenerumab in patients with Alzheimer’s disease. Expert Rev Neurother. 2014;14(9):973-986.
AAA screening showed no mortality reduction in new trial
In contrast to previous studies, screening for abdominal aortic aneurysms in older men does not appear to have a significant effect on overall mortality, according to a prospective, randomized study.
Mortality from ruptured AAA remains high in older men, which has prompted four previous large randomized trials to explore whether screening men aged 65 years and older might reduce mortality.
Writing in the October 31 online edition of JAMA Internal Medicine, the authors reported the long-term outcomes of an Australian population-based trial of screening for abdominal aortic aneurysms in 49,801 men aged 64-83 years, of whom 19 249 were invited to screening and 12,203 of those underwent screening (isrctn.org Identifier: ISRCTN16171472).
After a mean 12.8 years of follow-up, there was a non-significant 9% lower mortality in the invited screening group compared to the control group and a non-significant 8% lower mortality among men aged 65-74 years.
Overall, there were 90 deaths from ruptured AAA in the screening group and 98 in the control group (JAMA Internal Medicine 2016, October 31. DOI:10.1001/jamainternmed.2016.6633).
The prevalence of abdominal aortic aneurysms with a diameter at or above 30 mm was 6.6% in men aged 65-74, and 0.4% for those with a diameter of 55 mm or above.
While the rate of ruptured abdominal aortic aneurysms was significantly lower in the invited group compared to the control group (72 vs. 99, P = .04), the 30-day mortality after surgery for rupture was higher in the invited group compared to the control group (61.5% vs. 43.2%).
Screening had no meaningful impact on the risk of all-cause, cardiovascular, and other mortality, but men who had smoked had a higher risk of rupture and of death from a rupture than those who had never smoked, regardless of screening status.
The rate of total elective operations was significantly higher in the invited group compared to controls (536 vs. 414, P < .001), mainly in the first year after screening.
The authors calculated that to prevent one death from a ruptured abdominal aortic aneurysm in five years, 4784 men aged 64-83 years or 3290 men aged 65-74 years would need to be invited for screening.
While the strength of the study was that it was truly population-based – using the electoral roll – the authors said the lack of a benefit from screening was likely due to the relatively low rate of rupture and death from AAA, as well as a high rate of elective surgery for this condition, in the control group.
The non-significant 8% reduction in mortality observed in the study was significantly less than the 42% and 66% reductions seen in previous trials with a similar length of follow-up.
The authors suggested this may also have been related to a lower fraction of invited men participating in screening, but pointed out that the incidence of AAA in men is declining.
“The reason for the decrease in incidence and prevalence is multifactorial but is probably driven by differences in rates of smoking and cessation because the relative risk for AAA events is 3- to 6-fold higher in smokers compared with non-smokers,” they wrote.
The authors said selective screening of smokers or ex-smokers may be more effective, but pointed out that this approach would miss around one-quarter of aneurysms. However they suggested more targeted screening may yet achieve a benefit.
“The small overall benefit of population-wide screening does not mean that finding AAAs in suitable older men is not worthwhile because deaths from AAAs in men who actually attended for screening were halved by early detection and successful treatment.”
The study was supported by the National Health and Medical Research Council Project. The authors reported that they had no conflicts of interest.
These new data will not change the finding of robust reduction in AAA-related mortality from screening seen in all previous meta-analyses. However, the most recently updated meta-analyses now reveal the small reduction in all-cause mortality with screening to be statistically significant.
So although the findings of the Western Australian trial remain negative and raise some concerns about screening, their aggregation with other studies does not change the overall conclusions that screening substantially reduced AAA-related mortality and also resulted in a statistically significant reduction in all-cause mortality. Restricting screening to men who have smoked (the strongest risk factor for AAA) further lowers cost and increases efficiency.
Frank A. Lederle, MD, is from the Center for Chronic Disease Outcomes Research at the Veterans Affairs Medical Center. These comments are taken from an accompanying editorial (JAMA Internal Medicine 2016, October 31. DOI:10.1001/jamainternmed.2016.6663). No conflicts of interest were declared.
These new data will not change the finding of robust reduction in AAA-related mortality from screening seen in all previous meta-analyses. However, the most recently updated meta-analyses now reveal the small reduction in all-cause mortality with screening to be statistically significant.
So although the findings of the Western Australian trial remain negative and raise some concerns about screening, their aggregation with other studies does not change the overall conclusions that screening substantially reduced AAA-related mortality and also resulted in a statistically significant reduction in all-cause mortality. Restricting screening to men who have smoked (the strongest risk factor for AAA) further lowers cost and increases efficiency.
Frank A. Lederle, MD, is from the Center for Chronic Disease Outcomes Research at the Veterans Affairs Medical Center. These comments are taken from an accompanying editorial (JAMA Internal Medicine 2016, October 31. DOI:10.1001/jamainternmed.2016.6663). No conflicts of interest were declared.
These new data will not change the finding of robust reduction in AAA-related mortality from screening seen in all previous meta-analyses. However, the most recently updated meta-analyses now reveal the small reduction in all-cause mortality with screening to be statistically significant.
So although the findings of the Western Australian trial remain negative and raise some concerns about screening, their aggregation with other studies does not change the overall conclusions that screening substantially reduced AAA-related mortality and also resulted in a statistically significant reduction in all-cause mortality. Restricting screening to men who have smoked (the strongest risk factor for AAA) further lowers cost and increases efficiency.
Frank A. Lederle, MD, is from the Center for Chronic Disease Outcomes Research at the Veterans Affairs Medical Center. These comments are taken from an accompanying editorial (JAMA Internal Medicine 2016, October 31. DOI:10.1001/jamainternmed.2016.6663). No conflicts of interest were declared.
In contrast to previous studies, screening for abdominal aortic aneurysms in older men does not appear to have a significant effect on overall mortality, according to a prospective, randomized study.
Mortality from ruptured AAA remains high in older men, which has prompted four previous large randomized trials to explore whether screening men aged 65 years and older might reduce mortality.
Writing in the October 31 online edition of JAMA Internal Medicine, the authors reported the long-term outcomes of an Australian population-based trial of screening for abdominal aortic aneurysms in 49,801 men aged 64-83 years, of whom 19 249 were invited to screening and 12,203 of those underwent screening (isrctn.org Identifier: ISRCTN16171472).
After a mean 12.8 years of follow-up, there was a non-significant 9% lower mortality in the invited screening group compared to the control group and a non-significant 8% lower mortality among men aged 65-74 years.
Overall, there were 90 deaths from ruptured AAA in the screening group and 98 in the control group (JAMA Internal Medicine 2016, October 31. DOI:10.1001/jamainternmed.2016.6633).
The prevalence of abdominal aortic aneurysms with a diameter at or above 30 mm was 6.6% in men aged 65-74, and 0.4% for those with a diameter of 55 mm or above.
While the rate of ruptured abdominal aortic aneurysms was significantly lower in the invited group compared to the control group (72 vs. 99, P = .04), the 30-day mortality after surgery for rupture was higher in the invited group compared to the control group (61.5% vs. 43.2%).
Screening had no meaningful impact on the risk of all-cause, cardiovascular, and other mortality, but men who had smoked had a higher risk of rupture and of death from a rupture than those who had never smoked, regardless of screening status.
The rate of total elective operations was significantly higher in the invited group compared to controls (536 vs. 414, P < .001), mainly in the first year after screening.
The authors calculated that to prevent one death from a ruptured abdominal aortic aneurysm in five years, 4784 men aged 64-83 years or 3290 men aged 65-74 years would need to be invited for screening.
While the strength of the study was that it was truly population-based – using the electoral roll – the authors said the lack of a benefit from screening was likely due to the relatively low rate of rupture and death from AAA, as well as a high rate of elective surgery for this condition, in the control group.
The non-significant 8% reduction in mortality observed in the study was significantly less than the 42% and 66% reductions seen in previous trials with a similar length of follow-up.
The authors suggested this may also have been related to a lower fraction of invited men participating in screening, but pointed out that the incidence of AAA in men is declining.
“The reason for the decrease in incidence and prevalence is multifactorial but is probably driven by differences in rates of smoking and cessation because the relative risk for AAA events is 3- to 6-fold higher in smokers compared with non-smokers,” they wrote.
The authors said selective screening of smokers or ex-smokers may be more effective, but pointed out that this approach would miss around one-quarter of aneurysms. However they suggested more targeted screening may yet achieve a benefit.
“The small overall benefit of population-wide screening does not mean that finding AAAs in suitable older men is not worthwhile because deaths from AAAs in men who actually attended for screening were halved by early detection and successful treatment.”
The study was supported by the National Health and Medical Research Council Project. The authors reported that they had no conflicts of interest.
In contrast to previous studies, screening for abdominal aortic aneurysms in older men does not appear to have a significant effect on overall mortality, according to a prospective, randomized study.
Mortality from ruptured AAA remains high in older men, which has prompted four previous large randomized trials to explore whether screening men aged 65 years and older might reduce mortality.
Writing in the October 31 online edition of JAMA Internal Medicine, the authors reported the long-term outcomes of an Australian population-based trial of screening for abdominal aortic aneurysms in 49,801 men aged 64-83 years, of whom 19 249 were invited to screening and 12,203 of those underwent screening (isrctn.org Identifier: ISRCTN16171472).
After a mean 12.8 years of follow-up, there was a non-significant 9% lower mortality in the invited screening group compared to the control group and a non-significant 8% lower mortality among men aged 65-74 years.
Overall, there were 90 deaths from ruptured AAA in the screening group and 98 in the control group (JAMA Internal Medicine 2016, October 31. DOI:10.1001/jamainternmed.2016.6633).
The prevalence of abdominal aortic aneurysms with a diameter at or above 30 mm was 6.6% in men aged 65-74, and 0.4% for those with a diameter of 55 mm or above.
While the rate of ruptured abdominal aortic aneurysms was significantly lower in the invited group compared to the control group (72 vs. 99, P = .04), the 30-day mortality after surgery for rupture was higher in the invited group compared to the control group (61.5% vs. 43.2%).
Screening had no meaningful impact on the risk of all-cause, cardiovascular, and other mortality, but men who had smoked had a higher risk of rupture and of death from a rupture than those who had never smoked, regardless of screening status.
The rate of total elective operations was significantly higher in the invited group compared to controls (536 vs. 414, P < .001), mainly in the first year after screening.
The authors calculated that to prevent one death from a ruptured abdominal aortic aneurysm in five years, 4784 men aged 64-83 years or 3290 men aged 65-74 years would need to be invited for screening.
While the strength of the study was that it was truly population-based – using the electoral roll – the authors said the lack of a benefit from screening was likely due to the relatively low rate of rupture and death from AAA, as well as a high rate of elective surgery for this condition, in the control group.
The non-significant 8% reduction in mortality observed in the study was significantly less than the 42% and 66% reductions seen in previous trials with a similar length of follow-up.
The authors suggested this may also have been related to a lower fraction of invited men participating in screening, but pointed out that the incidence of AAA in men is declining.
“The reason for the decrease in incidence and prevalence is multifactorial but is probably driven by differences in rates of smoking and cessation because the relative risk for AAA events is 3- to 6-fold higher in smokers compared with non-smokers,” they wrote.
The authors said selective screening of smokers or ex-smokers may be more effective, but pointed out that this approach would miss around one-quarter of aneurysms. However they suggested more targeted screening may yet achieve a benefit.
“The small overall benefit of population-wide screening does not mean that finding AAAs in suitable older men is not worthwhile because deaths from AAAs in men who actually attended for screening were halved by early detection and successful treatment.”
The study was supported by the National Health and Medical Research Council Project. The authors reported that they had no conflicts of interest.
Key clinical point:
Major finding: Men invited to undergo screening for abdominal aortic aneurysms had a non-significant 9% lower mortality compared to a control group.
Data source: Prospective, population-based randomized controlled trial in 49,801 men aged 64-83 years.
Disclosures: The study was supported by the National Health and Medical Research Council Project. The authors reported that they had no conflicts of interest.
2016 Update on pelvic floor dysfunction
The genitourinary syndrome of menopause (GSM) is a constellation of symptoms and signs of a hypoestrogenic state resulting in some or all of the following: vaginal dryness, burning, irritation, dyspareunia, urinary urgency, dysuria, and recurrent urinary tract infections.1 In 2014, the International Society for the Study of Women’s Sexual Health and the North American Menopause Society endorsed “GSM” as a new term to replace the less comprehensive description, vulvovaginal atrophy (VVA).1
The prevalence of GSM is around 50%, but it may increase each year after menopause, reaching up to 84.2%.2,3 Only about half of women affected seek medical care, with the most commonly reported symptoms being vaginal dryness and dyspareunia.3,4
Nonhormonal vaginal moisturizers andlubricants remain first-line treatment. The benefits are temporary and short lived because these options do not change the physiologic makeup of the vaginal wall; these treatments therefore provide relief only if the GSM symptoms are limited or mild.5
In this Update on pelvic floor dysfunction, we review 2 randomized, placebo-controlled trials of hormonal options (vaginal estrogen and oral ospemifene) and examine the latest information regarding fractional CO2 vaginal laser treatment. Also included are evidence-based guidelines for vaginal estrogen use and recommendations and conclusions for use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. (The terms used in the studies described [ie, VVA versus GSM] have been maintained for accuracy of reporting.)
Low-dose estrogen vaginal cream ameliorates moderate to severe VVA with limited adverse events
Freedman M, Kaunitz AM, Reape KZ, Hait H, Shu H. Twice-weekly synthetic conjugated estrogens vaginal cream for the treatment of vaginal atrophy. Menopause. 2009;16(4):735-741.
In a multicenter, double-blind, randomized, placebo-controlled study, Freedman and colleagues evaluated the efficacy of a 1-g dose of synthetic conjugated estrogens A (SCE-A) cream versus placebo in postmenopausal women with moderate to severe VVA.
Details of the study
The investigators enrolled 305 participants aged 30 to 80 years (mean [SD] age, 60 [6.6] years) who were naturally or surgically postmenopausal. The enrollment criteria included ≤5% superficial cells on vaginal smear, vaginal pH >5.0, and at least 1 moderate or severe symptom of VVA (vaginal dryness, soreness, irritation/itching, pain with intercourse, or bleeding after intercourse).
Participants were randomly assigned in a 1:1:1:1 ratio to twice-weekly therapy with 1 g (0.625 mg/g) SCE-A vaginal cream, 2 g SCE-A vaginal cream, 1 g placebo, or 2 g placebo. Study visits occurred on days 14, 21, 28, 56, and 84 (12-week end point). The 3 co-primary outcomes were cytology, vaginal pH, and most bothersome symptom (MBS). Primary outcomes and safety/adverse events (AEs) were recorded at each study visit, and transvaginal ultrasound and endometrial biopsy were performed for women with a uterus at the beginning and end of the study.
Mean change and percent change in the 3 primary outcomes were assessed between baseline and each study visit. MBS was scored on a scale of 0 to 3 (0 = none, 1 = mild, 2 = moderate, 3 = severe). The principal indicators of efficacy were the changes from baseline to the end of treatment (12 weeks) for each of the 3 end points. Since the 1-g and 2-g SCE-A dose groups showed a similar degree of efficacy on all 3 co-primary end points, approval from the US Food and Drug Administration (FDA) was sought only for the lower dose, in keeping with the use of the lowest effective dose; therefore, results from only the 1-g SCE-A dose group and matching placebo group were presented in the article. A sample size calculation determined that at least 111 participants in each group were needed to provide 90% power for statistical testing.
Estrogen reduced MBS severity, improved vaginal indices
The modified intent-to-treat (MITT) cohort was used for outcome analysis, and data from 275 participants were available at the 12-week end point. At baseline, 132 participants (48%) indicated vaginal dryness and 86 women (31.3%) indicated pain during intercourse as the MBS. In the SCE-A group at baseline, the vaginal maturation index (VMI) was 31.31 compared with 31.84 in the placebo group. At 12 weeks, the SCE-A group had a mean reduction of 1.71 in overall MBS severity compared with the placebo group’s mean reduction of 1.11 (P<.0001). The SCE-A group had a greater increase in the VMI (with a mean change of 31.46 vs 5.16 in the placebo group [P<.0001]) and a greater decrease in the vaginal pH (mean pH at the end of treatment for the SCE-A group was 4.84, a decrease of 1.48, and for the placebo group was 5.96, a decrease of 0.31 [P<.0001]).
Adverse events. The incidence of AEs was similar for the 1-g SCE-A group and the 1-g placebo group, with no AE occurring at a rate of higher than 5%. There were 15 (10%) treatment-related AEs in the estrogen group and 16 (10.3%) in the placebo group. The SCE-A group had 3 AEs (2%) leading to discontinuation, while the placebo group had 2 AEs (1.3%) leading to discontinuation. There were no clinically significant endometrial biopsy findings at the conclusion of the study.
Strengths and limitations. This study evaluated clinical and physiologic outcomes as well as uterine response to transvaginal estrogen. The use of MBS allows symptoms to be scored objectively compared with prior subjective symptom assessment, which varied widely. However, fewer indicated symptoms will permit limited conclusions.
For evidence-based recommended and suggested treatments for various genitourinary symptoms, we recommended as a resource the Society of Gynecologic Surgeons clinical practice guidelines on vaginal estrogen for the treatment of GSM (TABLE 1).5
In addition, for women with a history of estrogen-dependent breast cancer experiencing urogenital symptoms, the American College of Obstetricians and Gynecologists recommends nonhormonal agents as first-line therapy, with vaginal estrogen treatment reserved for woman whose symptoms are unresponsive to nonhormonal therapies (TABLE 2).6
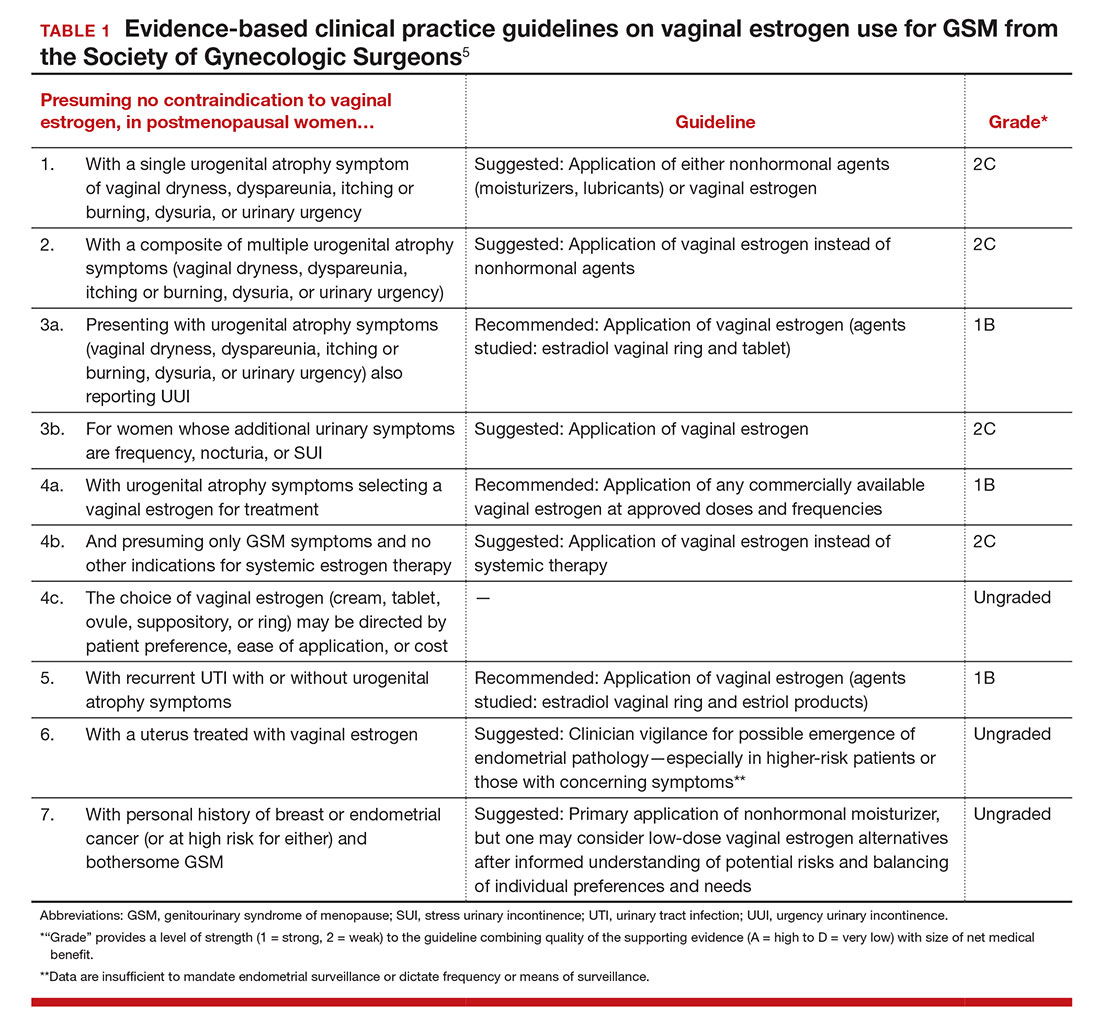

Ospemifene improves vaginal physiology and dyspareunia
Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17(3):480–486.
Bachmann and colleagues evaluated the efficacy and safety of ospemifene for the treatment of VVA. This is one of the efficacy studies on which FDA approval was based. Ospemifene is a selective estrogen receptor modulator (SERM) that acts as an estrogen agonist/antagonist.
Details of the study
The study included 826 postmenopausal women randomly assigned to 30 mg/day of ospemifene, 60 mg/day of ospemifene, or placebo for 12 weeks. Participants were aged 40 to 80 years and met the criteria for VVA (defined as ≤5% superficial cells on vaginal smear [maturation index], vaginal pH >5.0, and at least 1 moderate or severe symptom of VVA). All women were given a nonhormonal lubricant for use as needed.
There were 4 co-primary end points: percentage of superficial cells on the vaginal smear, percentage of parabasal cells on the vaginal smear, vaginal pH, and self-assessed MBS using a Likert scale (0, none; 1, mild; 2, moderate; 3, severe). The symptom score was calculated as the change from baseline to week 12 for each MBS. Safety was assessed by patient report; if a participant had an intact uterus and cervix, Pap test, endometrial thickness, and endometrial histologic analysis were performed at baseline and at 12 weeks. Baseline characteristics were similar among all treatment groups. A total of 46% of participants reported dyspareunia as their MBS, and 39% reported vaginal dryness.
Two dose levels of ospemifene effectively relieve symptoms
After 12 weeks of treatment, both the 30-mg and the 60-mg dose of ospemifene produced a statistically significant improvement in vaginal dryness and objective results of maturation index and vaginal pH compared with placebo. Vaginal dryness decreased in the ospemifene 30-mg group (1.22) and in the ospemifene 60-mg group (1.26) compared with placebo (0.84) (P = .04 for the 30-mg group and P = .021 for the 60-mg group). The percentage of superficial cells was increased in both treatment groups compared with placebo (7.8% for the 30-mg group, 10.8% for the 60-mg group, 2.2% for the placebo group; P<.001 for both). The percentage of parabasal cells decreased in both treatment groups compared with participants who received placebo (21.9% in the 30-mg group, 30.1% in the 60-mg group, and 3.98% in the placebo group; P<.001 for both). Both treatment groups had a decrease in vaginal pH versus the placebo group as well (0.67 decrease in the 30-mg group, 1.01 decrease in the 60-mg group, and 0.10 decrease in the placebo group; P<.001 for both). The 60-mg/day ospemifene dose improved dyspareunia compared with placebo and was more effective than the 30-mg dose for all end points.
Adverse effects. Hot flashes were reported in 9.6% of the 30-mg ospemifene group and in 8.3% of the 60-mg group, compared with 3.4% in the placebo group. The increased percentage of participants with hot flashes in the ospemifene groups did not lead to increased discontinuation with the study. Urinary tract infections, defined by symptoms only, were more common in the ospemifene groups (4.6% in the 30-mg group, 7.2% in the 60-mg group, and 2.2% in the placebo group). In each group, 5% of patients discontinued the study because of AEs. There were 5 serious AEs in the 30-mg ospemifene group, 4 serious AEs in the placebo group, and none in the 60-mg group. No venous thromboembolic events were reported.
Strengths and limitations. Vaginal physiology as well as common symptoms of GSM were assessed in this large study. However, AEs were self-reported. While ospemifene was found safe and well tolerated when the study was extended for an additional 52 weeks (in women without a uterus) and 40 weeks (in women with a uterus), longer follow-up is needed to determine endometrial safety.7,8
Some patients may prefer an oral agent over a vaginally applied medication. While ospemifene is not an estrogen, it is a SERM that may increase the risk of endometrial cancer and thromboembolic events as stated in the boxed warning of the ospemifene prescribing information.
Fractional CO2 laser for VVA shows efficacy, patient satisfaction
Sokol ER, Karram MM. An assessment of the safety and efficacy of a fractional CO2 laser system for the treatment of vulvovaginal atrophy. Menopause. 2016;23(10):1102–1107.
In this first US pilot study, postmenopausal women received 3 fractional CO2 laser treatments, 6 weeks apart. The investigators evaluated the safety and efficacy of the treatment for GSM.
Details of the study
Thirty women (mean age, 58.6 years) who were nonsmokers, postmenopausal, had less than stage 2 prolapse, no vaginal procedures for the past 6 months, and did not use vaginal creams, moisturizers, lubricants, or homeopathic preparations for the past 3 months were enrolled. Participants received 3 laser treatments with the SmartXide2, MonaLisa Touch (DEKA M.E.L.A. SRL, Florence, Italy) device at 6-week intervals followed by a 3-month follow-up.
The primary outcome was visual analog scale (VAS) change in 6 categories (vaginal pain, burning, itching, dryness, dyspareunia, and dysuria) assessed from baseline to after each treatment, including 3 months after the final treatment, using an 11-point scale with 0 the lowest (no symptoms) and 10 the highest (extreme bother). Secondary outcomes were Vaginal Health Index (VHI) score, maximal tolerable dilator size, Female Sexual Function Index (FSFI) questionnaire score, general quality of life, degree of difficulty performing the procedure, participant satisfaction, vaginal pH, adverse effects, and treatment discomfort assessed using the VAS.
Improved VVA symptoms and vaginal caliber
Twenty-seven women completed the study. There was a statistically significant change in all 6 symptom categories measured with the VAS. Improvement change (SD) on the VAS was 1.7 (3.2) for pain, 1.4 (2.9) for burning, 1.4 (1.9) for itching, 1.0 (2.4) for dysuria, comparing baseline scores to scores after 3 treatments (all with P<.05). A greater improvement was noted for dryness, 6.1 (2.7), and for dyspareunia, 5.4 (2.9) (both P<.001). There was also a statistically significant change in overall improvement on the VHI and the FSFI. The mean (SD) VHI score at baseline was 14.4 (2.9; range, 8 to 20) and the mean (SD) after 3 laser treatments was 21.4 (2.9; range, 16 to 25), with an overall mean (SD) improvement of 7.0 (3.1; P<.001).
Twenty-six participants completed a follow-up FSFI, with a mean (SD) baseline score of 11.3 (7.3; range, 2 to 25) and a follow-up mean (SD) score of 8.8 (7.3; range, −3.7 to 27.2) (P<.001). There was an increase in dilator size of 83% when comparing baseline to follow-up. At baseline, 24 participants (80%) could comfortably accept an XS or S dilator, and at follow-up 23 of 24 women (96%) could comfortably accept an M or L dilator.
Adverse effects. At their follow-up, 96% of participants were satisfied or extremely satisfied with treatment. Two women reported mild-to-moderate pain lasting 2 to 3 days, and 2 had minor bleeding; however, no women withdrew or discontinued treatment because of adverse events.
Study limitations. This study evaluated the majority of GSM symptoms as well as change in vaginal caliber after a nonhormonal therapy. The cohort was small and had no placebo group. In addition, with the limited observation period, it is difficult to determine the duration of effect and long-term safety of repeated treatments.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Portman DJ, Gass ML: Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Maturitas. 2014;79(3):349–354.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- Palma F, Volpe A, Villa P, Cagnacci A; Writing Groupt of AGATA Study. Vaginal atrophy of women in postmenopause. Results from a multicentric observational study: the AGATA study. Maturitas. 2016;83:40–44.
- Kingsberg SA, Sysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Rahn DD, Carberry C, Sanses TV, et al; Society of Gynecologic Surgeons Systematic Review Group. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
- Farrell R; American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127(3):e93–e96.
- Simon JA, Lin VH, Radovich C, Bachmann GA; Ospemiphene Study Group. One-year long-term safety extension study of ospemifene for the treatment of vulvar and vaginal atrophy in postmenopausal women with a uterus. Menopause. 2013;20(4):418–427.
- Simon J, Portman D, Mabey RG Jr; Ospemifene Study Group. Long-term safety of ospemifene (52-week extension) in the treatment of vulvar and vaginal atrophy in hysterectomized postmenopausal women. Maturitas. 2014;77(3):274–281.
The genitourinary syndrome of menopause (GSM) is a constellation of symptoms and signs of a hypoestrogenic state resulting in some or all of the following: vaginal dryness, burning, irritation, dyspareunia, urinary urgency, dysuria, and recurrent urinary tract infections.1 In 2014, the International Society for the Study of Women’s Sexual Health and the North American Menopause Society endorsed “GSM” as a new term to replace the less comprehensive description, vulvovaginal atrophy (VVA).1
The prevalence of GSM is around 50%, but it may increase each year after menopause, reaching up to 84.2%.2,3 Only about half of women affected seek medical care, with the most commonly reported symptoms being vaginal dryness and dyspareunia.3,4
Nonhormonal vaginal moisturizers andlubricants remain first-line treatment. The benefits are temporary and short lived because these options do not change the physiologic makeup of the vaginal wall; these treatments therefore provide relief only if the GSM symptoms are limited or mild.5
In this Update on pelvic floor dysfunction, we review 2 randomized, placebo-controlled trials of hormonal options (vaginal estrogen and oral ospemifene) and examine the latest information regarding fractional CO2 vaginal laser treatment. Also included are evidence-based guidelines for vaginal estrogen use and recommendations and conclusions for use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. (The terms used in the studies described [ie, VVA versus GSM] have been maintained for accuracy of reporting.)
Low-dose estrogen vaginal cream ameliorates moderate to severe VVA with limited adverse events
Freedman M, Kaunitz AM, Reape KZ, Hait H, Shu H. Twice-weekly synthetic conjugated estrogens vaginal cream for the treatment of vaginal atrophy. Menopause. 2009;16(4):735-741.
In a multicenter, double-blind, randomized, placebo-controlled study, Freedman and colleagues evaluated the efficacy of a 1-g dose of synthetic conjugated estrogens A (SCE-A) cream versus placebo in postmenopausal women with moderate to severe VVA.
Details of the study
The investigators enrolled 305 participants aged 30 to 80 years (mean [SD] age, 60 [6.6] years) who were naturally or surgically postmenopausal. The enrollment criteria included ≤5% superficial cells on vaginal smear, vaginal pH >5.0, and at least 1 moderate or severe symptom of VVA (vaginal dryness, soreness, irritation/itching, pain with intercourse, or bleeding after intercourse).
Participants were randomly assigned in a 1:1:1:1 ratio to twice-weekly therapy with 1 g (0.625 mg/g) SCE-A vaginal cream, 2 g SCE-A vaginal cream, 1 g placebo, or 2 g placebo. Study visits occurred on days 14, 21, 28, 56, and 84 (12-week end point). The 3 co-primary outcomes were cytology, vaginal pH, and most bothersome symptom (MBS). Primary outcomes and safety/adverse events (AEs) were recorded at each study visit, and transvaginal ultrasound and endometrial biopsy were performed for women with a uterus at the beginning and end of the study.
Mean change and percent change in the 3 primary outcomes were assessed between baseline and each study visit. MBS was scored on a scale of 0 to 3 (0 = none, 1 = mild, 2 = moderate, 3 = severe). The principal indicators of efficacy were the changes from baseline to the end of treatment (12 weeks) for each of the 3 end points. Since the 1-g and 2-g SCE-A dose groups showed a similar degree of efficacy on all 3 co-primary end points, approval from the US Food and Drug Administration (FDA) was sought only for the lower dose, in keeping with the use of the lowest effective dose; therefore, results from only the 1-g SCE-A dose group and matching placebo group were presented in the article. A sample size calculation determined that at least 111 participants in each group were needed to provide 90% power for statistical testing.
Estrogen reduced MBS severity, improved vaginal indices
The modified intent-to-treat (MITT) cohort was used for outcome analysis, and data from 275 participants were available at the 12-week end point. At baseline, 132 participants (48%) indicated vaginal dryness and 86 women (31.3%) indicated pain during intercourse as the MBS. In the SCE-A group at baseline, the vaginal maturation index (VMI) was 31.31 compared with 31.84 in the placebo group. At 12 weeks, the SCE-A group had a mean reduction of 1.71 in overall MBS severity compared with the placebo group’s mean reduction of 1.11 (P<.0001). The SCE-A group had a greater increase in the VMI (with a mean change of 31.46 vs 5.16 in the placebo group [P<.0001]) and a greater decrease in the vaginal pH (mean pH at the end of treatment for the SCE-A group was 4.84, a decrease of 1.48, and for the placebo group was 5.96, a decrease of 0.31 [P<.0001]).
Adverse events. The incidence of AEs was similar for the 1-g SCE-A group and the 1-g placebo group, with no AE occurring at a rate of higher than 5%. There were 15 (10%) treatment-related AEs in the estrogen group and 16 (10.3%) in the placebo group. The SCE-A group had 3 AEs (2%) leading to discontinuation, while the placebo group had 2 AEs (1.3%) leading to discontinuation. There were no clinically significant endometrial biopsy findings at the conclusion of the study.
Strengths and limitations. This study evaluated clinical and physiologic outcomes as well as uterine response to transvaginal estrogen. The use of MBS allows symptoms to be scored objectively compared with prior subjective symptom assessment, which varied widely. However, fewer indicated symptoms will permit limited conclusions.
For evidence-based recommended and suggested treatments for various genitourinary symptoms, we recommended as a resource the Society of Gynecologic Surgeons clinical practice guidelines on vaginal estrogen for the treatment of GSM (TABLE 1).5
In addition, for women with a history of estrogen-dependent breast cancer experiencing urogenital symptoms, the American College of Obstetricians and Gynecologists recommends nonhormonal agents as first-line therapy, with vaginal estrogen treatment reserved for woman whose symptoms are unresponsive to nonhormonal therapies (TABLE 2).6


Ospemifene improves vaginal physiology and dyspareunia
Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17(3):480–486.
Bachmann and colleagues evaluated the efficacy and safety of ospemifene for the treatment of VVA. This is one of the efficacy studies on which FDA approval was based. Ospemifene is a selective estrogen receptor modulator (SERM) that acts as an estrogen agonist/antagonist.
Details of the study
The study included 826 postmenopausal women randomly assigned to 30 mg/day of ospemifene, 60 mg/day of ospemifene, or placebo for 12 weeks. Participants were aged 40 to 80 years and met the criteria for VVA (defined as ≤5% superficial cells on vaginal smear [maturation index], vaginal pH >5.0, and at least 1 moderate or severe symptom of VVA). All women were given a nonhormonal lubricant for use as needed.
There were 4 co-primary end points: percentage of superficial cells on the vaginal smear, percentage of parabasal cells on the vaginal smear, vaginal pH, and self-assessed MBS using a Likert scale (0, none; 1, mild; 2, moderate; 3, severe). The symptom score was calculated as the change from baseline to week 12 for each MBS. Safety was assessed by patient report; if a participant had an intact uterus and cervix, Pap test, endometrial thickness, and endometrial histologic analysis were performed at baseline and at 12 weeks. Baseline characteristics were similar among all treatment groups. A total of 46% of participants reported dyspareunia as their MBS, and 39% reported vaginal dryness.
Two dose levels of ospemifene effectively relieve symptoms
After 12 weeks of treatment, both the 30-mg and the 60-mg dose of ospemifene produced a statistically significant improvement in vaginal dryness and objective results of maturation index and vaginal pH compared with placebo. Vaginal dryness decreased in the ospemifene 30-mg group (1.22) and in the ospemifene 60-mg group (1.26) compared with placebo (0.84) (P = .04 for the 30-mg group and P = .021 for the 60-mg group). The percentage of superficial cells was increased in both treatment groups compared with placebo (7.8% for the 30-mg group, 10.8% for the 60-mg group, 2.2% for the placebo group; P<.001 for both). The percentage of parabasal cells decreased in both treatment groups compared with participants who received placebo (21.9% in the 30-mg group, 30.1% in the 60-mg group, and 3.98% in the placebo group; P<.001 for both). Both treatment groups had a decrease in vaginal pH versus the placebo group as well (0.67 decrease in the 30-mg group, 1.01 decrease in the 60-mg group, and 0.10 decrease in the placebo group; P<.001 for both). The 60-mg/day ospemifene dose improved dyspareunia compared with placebo and was more effective than the 30-mg dose for all end points.
Adverse effects. Hot flashes were reported in 9.6% of the 30-mg ospemifene group and in 8.3% of the 60-mg group, compared with 3.4% in the placebo group. The increased percentage of participants with hot flashes in the ospemifene groups did not lead to increased discontinuation with the study. Urinary tract infections, defined by symptoms only, were more common in the ospemifene groups (4.6% in the 30-mg group, 7.2% in the 60-mg group, and 2.2% in the placebo group). In each group, 5% of patients discontinued the study because of AEs. There were 5 serious AEs in the 30-mg ospemifene group, 4 serious AEs in the placebo group, and none in the 60-mg group. No venous thromboembolic events were reported.
Strengths and limitations. Vaginal physiology as well as common symptoms of GSM were assessed in this large study. However, AEs were self-reported. While ospemifene was found safe and well tolerated when the study was extended for an additional 52 weeks (in women without a uterus) and 40 weeks (in women with a uterus), longer follow-up is needed to determine endometrial safety.7,8
Some patients may prefer an oral agent over a vaginally applied medication. While ospemifene is not an estrogen, it is a SERM that may increase the risk of endometrial cancer and thromboembolic events as stated in the boxed warning of the ospemifene prescribing information.
Fractional CO2 laser for VVA shows efficacy, patient satisfaction
Sokol ER, Karram MM. An assessment of the safety and efficacy of a fractional CO2 laser system for the treatment of vulvovaginal atrophy. Menopause. 2016;23(10):1102–1107.
In this first US pilot study, postmenopausal women received 3 fractional CO2 laser treatments, 6 weeks apart. The investigators evaluated the safety and efficacy of the treatment for GSM.
Details of the study
Thirty women (mean age, 58.6 years) who were nonsmokers, postmenopausal, had less than stage 2 prolapse, no vaginal procedures for the past 6 months, and did not use vaginal creams, moisturizers, lubricants, or homeopathic preparations for the past 3 months were enrolled. Participants received 3 laser treatments with the SmartXide2, MonaLisa Touch (DEKA M.E.L.A. SRL, Florence, Italy) device at 6-week intervals followed by a 3-month follow-up.
The primary outcome was visual analog scale (VAS) change in 6 categories (vaginal pain, burning, itching, dryness, dyspareunia, and dysuria) assessed from baseline to after each treatment, including 3 months after the final treatment, using an 11-point scale with 0 the lowest (no symptoms) and 10 the highest (extreme bother). Secondary outcomes were Vaginal Health Index (VHI) score, maximal tolerable dilator size, Female Sexual Function Index (FSFI) questionnaire score, general quality of life, degree of difficulty performing the procedure, participant satisfaction, vaginal pH, adverse effects, and treatment discomfort assessed using the VAS.
Improved VVA symptoms and vaginal caliber
Twenty-seven women completed the study. There was a statistically significant change in all 6 symptom categories measured with the VAS. Improvement change (SD) on the VAS was 1.7 (3.2) for pain, 1.4 (2.9) for burning, 1.4 (1.9) for itching, 1.0 (2.4) for dysuria, comparing baseline scores to scores after 3 treatments (all with P<.05). A greater improvement was noted for dryness, 6.1 (2.7), and for dyspareunia, 5.4 (2.9) (both P<.001). There was also a statistically significant change in overall improvement on the VHI and the FSFI. The mean (SD) VHI score at baseline was 14.4 (2.9; range, 8 to 20) and the mean (SD) after 3 laser treatments was 21.4 (2.9; range, 16 to 25), with an overall mean (SD) improvement of 7.0 (3.1; P<.001).
Twenty-six participants completed a follow-up FSFI, with a mean (SD) baseline score of 11.3 (7.3; range, 2 to 25) and a follow-up mean (SD) score of 8.8 (7.3; range, −3.7 to 27.2) (P<.001). There was an increase in dilator size of 83% when comparing baseline to follow-up. At baseline, 24 participants (80%) could comfortably accept an XS or S dilator, and at follow-up 23 of 24 women (96%) could comfortably accept an M or L dilator.
Adverse effects. At their follow-up, 96% of participants were satisfied or extremely satisfied with treatment. Two women reported mild-to-moderate pain lasting 2 to 3 days, and 2 had minor bleeding; however, no women withdrew or discontinued treatment because of adverse events.
Study limitations. This study evaluated the majority of GSM symptoms as well as change in vaginal caliber after a nonhormonal therapy. The cohort was small and had no placebo group. In addition, with the limited observation period, it is difficult to determine the duration of effect and long-term safety of repeated treatments.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The genitourinary syndrome of menopause (GSM) is a constellation of symptoms and signs of a hypoestrogenic state resulting in some or all of the following: vaginal dryness, burning, irritation, dyspareunia, urinary urgency, dysuria, and recurrent urinary tract infections.1 In 2014, the International Society for the Study of Women’s Sexual Health and the North American Menopause Society endorsed “GSM” as a new term to replace the less comprehensive description, vulvovaginal atrophy (VVA).1
The prevalence of GSM is around 50%, but it may increase each year after menopause, reaching up to 84.2%.2,3 Only about half of women affected seek medical care, with the most commonly reported symptoms being vaginal dryness and dyspareunia.3,4
Nonhormonal vaginal moisturizers andlubricants remain first-line treatment. The benefits are temporary and short lived because these options do not change the physiologic makeup of the vaginal wall; these treatments therefore provide relief only if the GSM symptoms are limited or mild.5
In this Update on pelvic floor dysfunction, we review 2 randomized, placebo-controlled trials of hormonal options (vaginal estrogen and oral ospemifene) and examine the latest information regarding fractional CO2 vaginal laser treatment. Also included are evidence-based guidelines for vaginal estrogen use and recommendations and conclusions for use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. (The terms used in the studies described [ie, VVA versus GSM] have been maintained for accuracy of reporting.)
Low-dose estrogen vaginal cream ameliorates moderate to severe VVA with limited adverse events
Freedman M, Kaunitz AM, Reape KZ, Hait H, Shu H. Twice-weekly synthetic conjugated estrogens vaginal cream for the treatment of vaginal atrophy. Menopause. 2009;16(4):735-741.
In a multicenter, double-blind, randomized, placebo-controlled study, Freedman and colleagues evaluated the efficacy of a 1-g dose of synthetic conjugated estrogens A (SCE-A) cream versus placebo in postmenopausal women with moderate to severe VVA.
Details of the study
The investigators enrolled 305 participants aged 30 to 80 years (mean [SD] age, 60 [6.6] years) who were naturally or surgically postmenopausal. The enrollment criteria included ≤5% superficial cells on vaginal smear, vaginal pH >5.0, and at least 1 moderate or severe symptom of VVA (vaginal dryness, soreness, irritation/itching, pain with intercourse, or bleeding after intercourse).
Participants were randomly assigned in a 1:1:1:1 ratio to twice-weekly therapy with 1 g (0.625 mg/g) SCE-A vaginal cream, 2 g SCE-A vaginal cream, 1 g placebo, or 2 g placebo. Study visits occurred on days 14, 21, 28, 56, and 84 (12-week end point). The 3 co-primary outcomes were cytology, vaginal pH, and most bothersome symptom (MBS). Primary outcomes and safety/adverse events (AEs) were recorded at each study visit, and transvaginal ultrasound and endometrial biopsy were performed for women with a uterus at the beginning and end of the study.
Mean change and percent change in the 3 primary outcomes were assessed between baseline and each study visit. MBS was scored on a scale of 0 to 3 (0 = none, 1 = mild, 2 = moderate, 3 = severe). The principal indicators of efficacy were the changes from baseline to the end of treatment (12 weeks) for each of the 3 end points. Since the 1-g and 2-g SCE-A dose groups showed a similar degree of efficacy on all 3 co-primary end points, approval from the US Food and Drug Administration (FDA) was sought only for the lower dose, in keeping with the use of the lowest effective dose; therefore, results from only the 1-g SCE-A dose group and matching placebo group were presented in the article. A sample size calculation determined that at least 111 participants in each group were needed to provide 90% power for statistical testing.
Estrogen reduced MBS severity, improved vaginal indices
The modified intent-to-treat (MITT) cohort was used for outcome analysis, and data from 275 participants were available at the 12-week end point. At baseline, 132 participants (48%) indicated vaginal dryness and 86 women (31.3%) indicated pain during intercourse as the MBS. In the SCE-A group at baseline, the vaginal maturation index (VMI) was 31.31 compared with 31.84 in the placebo group. At 12 weeks, the SCE-A group had a mean reduction of 1.71 in overall MBS severity compared with the placebo group’s mean reduction of 1.11 (P<.0001). The SCE-A group had a greater increase in the VMI (with a mean change of 31.46 vs 5.16 in the placebo group [P<.0001]) and a greater decrease in the vaginal pH (mean pH at the end of treatment for the SCE-A group was 4.84, a decrease of 1.48, and for the placebo group was 5.96, a decrease of 0.31 [P<.0001]).
Adverse events. The incidence of AEs was similar for the 1-g SCE-A group and the 1-g placebo group, with no AE occurring at a rate of higher than 5%. There were 15 (10%) treatment-related AEs in the estrogen group and 16 (10.3%) in the placebo group. The SCE-A group had 3 AEs (2%) leading to discontinuation, while the placebo group had 2 AEs (1.3%) leading to discontinuation. There were no clinically significant endometrial biopsy findings at the conclusion of the study.
Strengths and limitations. This study evaluated clinical and physiologic outcomes as well as uterine response to transvaginal estrogen. The use of MBS allows symptoms to be scored objectively compared with prior subjective symptom assessment, which varied widely. However, fewer indicated symptoms will permit limited conclusions.
For evidence-based recommended and suggested treatments for various genitourinary symptoms, we recommended as a resource the Society of Gynecologic Surgeons clinical practice guidelines on vaginal estrogen for the treatment of GSM (TABLE 1).5
In addition, for women with a history of estrogen-dependent breast cancer experiencing urogenital symptoms, the American College of Obstetricians and Gynecologists recommends nonhormonal agents as first-line therapy, with vaginal estrogen treatment reserved for woman whose symptoms are unresponsive to nonhormonal therapies (TABLE 2).6


Ospemifene improves vaginal physiology and dyspareunia
Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17(3):480–486.
Bachmann and colleagues evaluated the efficacy and safety of ospemifene for the treatment of VVA. This is one of the efficacy studies on which FDA approval was based. Ospemifene is a selective estrogen receptor modulator (SERM) that acts as an estrogen agonist/antagonist.
Details of the study
The study included 826 postmenopausal women randomly assigned to 30 mg/day of ospemifene, 60 mg/day of ospemifene, or placebo for 12 weeks. Participants were aged 40 to 80 years and met the criteria for VVA (defined as ≤5% superficial cells on vaginal smear [maturation index], vaginal pH >5.0, and at least 1 moderate or severe symptom of VVA). All women were given a nonhormonal lubricant for use as needed.
There were 4 co-primary end points: percentage of superficial cells on the vaginal smear, percentage of parabasal cells on the vaginal smear, vaginal pH, and self-assessed MBS using a Likert scale (0, none; 1, mild; 2, moderate; 3, severe). The symptom score was calculated as the change from baseline to week 12 for each MBS. Safety was assessed by patient report; if a participant had an intact uterus and cervix, Pap test, endometrial thickness, and endometrial histologic analysis were performed at baseline and at 12 weeks. Baseline characteristics were similar among all treatment groups. A total of 46% of participants reported dyspareunia as their MBS, and 39% reported vaginal dryness.
Two dose levels of ospemifene effectively relieve symptoms
After 12 weeks of treatment, both the 30-mg and the 60-mg dose of ospemifene produced a statistically significant improvement in vaginal dryness and objective results of maturation index and vaginal pH compared with placebo. Vaginal dryness decreased in the ospemifene 30-mg group (1.22) and in the ospemifene 60-mg group (1.26) compared with placebo (0.84) (P = .04 for the 30-mg group and P = .021 for the 60-mg group). The percentage of superficial cells was increased in both treatment groups compared with placebo (7.8% for the 30-mg group, 10.8% for the 60-mg group, 2.2% for the placebo group; P<.001 for both). The percentage of parabasal cells decreased in both treatment groups compared with participants who received placebo (21.9% in the 30-mg group, 30.1% in the 60-mg group, and 3.98% in the placebo group; P<.001 for both). Both treatment groups had a decrease in vaginal pH versus the placebo group as well (0.67 decrease in the 30-mg group, 1.01 decrease in the 60-mg group, and 0.10 decrease in the placebo group; P<.001 for both). The 60-mg/day ospemifene dose improved dyspareunia compared with placebo and was more effective than the 30-mg dose for all end points.
Adverse effects. Hot flashes were reported in 9.6% of the 30-mg ospemifene group and in 8.3% of the 60-mg group, compared with 3.4% in the placebo group. The increased percentage of participants with hot flashes in the ospemifene groups did not lead to increased discontinuation with the study. Urinary tract infections, defined by symptoms only, were more common in the ospemifene groups (4.6% in the 30-mg group, 7.2% in the 60-mg group, and 2.2% in the placebo group). In each group, 5% of patients discontinued the study because of AEs. There were 5 serious AEs in the 30-mg ospemifene group, 4 serious AEs in the placebo group, and none in the 60-mg group. No venous thromboembolic events were reported.
Strengths and limitations. Vaginal physiology as well as common symptoms of GSM were assessed in this large study. However, AEs were self-reported. While ospemifene was found safe and well tolerated when the study was extended for an additional 52 weeks (in women without a uterus) and 40 weeks (in women with a uterus), longer follow-up is needed to determine endometrial safety.7,8
Some patients may prefer an oral agent over a vaginally applied medication. While ospemifene is not an estrogen, it is a SERM that may increase the risk of endometrial cancer and thromboembolic events as stated in the boxed warning of the ospemifene prescribing information.
Fractional CO2 laser for VVA shows efficacy, patient satisfaction
Sokol ER, Karram MM. An assessment of the safety and efficacy of a fractional CO2 laser system for the treatment of vulvovaginal atrophy. Menopause. 2016;23(10):1102–1107.
In this first US pilot study, postmenopausal women received 3 fractional CO2 laser treatments, 6 weeks apart. The investigators evaluated the safety and efficacy of the treatment for GSM.
Details of the study
Thirty women (mean age, 58.6 years) who were nonsmokers, postmenopausal, had less than stage 2 prolapse, no vaginal procedures for the past 6 months, and did not use vaginal creams, moisturizers, lubricants, or homeopathic preparations for the past 3 months were enrolled. Participants received 3 laser treatments with the SmartXide2, MonaLisa Touch (DEKA M.E.L.A. SRL, Florence, Italy) device at 6-week intervals followed by a 3-month follow-up.
The primary outcome was visual analog scale (VAS) change in 6 categories (vaginal pain, burning, itching, dryness, dyspareunia, and dysuria) assessed from baseline to after each treatment, including 3 months after the final treatment, using an 11-point scale with 0 the lowest (no symptoms) and 10 the highest (extreme bother). Secondary outcomes were Vaginal Health Index (VHI) score, maximal tolerable dilator size, Female Sexual Function Index (FSFI) questionnaire score, general quality of life, degree of difficulty performing the procedure, participant satisfaction, vaginal pH, adverse effects, and treatment discomfort assessed using the VAS.
Improved VVA symptoms and vaginal caliber
Twenty-seven women completed the study. There was a statistically significant change in all 6 symptom categories measured with the VAS. Improvement change (SD) on the VAS was 1.7 (3.2) for pain, 1.4 (2.9) for burning, 1.4 (1.9) for itching, 1.0 (2.4) for dysuria, comparing baseline scores to scores after 3 treatments (all with P<.05). A greater improvement was noted for dryness, 6.1 (2.7), and for dyspareunia, 5.4 (2.9) (both P<.001). There was also a statistically significant change in overall improvement on the VHI and the FSFI. The mean (SD) VHI score at baseline was 14.4 (2.9; range, 8 to 20) and the mean (SD) after 3 laser treatments was 21.4 (2.9; range, 16 to 25), with an overall mean (SD) improvement of 7.0 (3.1; P<.001).
Twenty-six participants completed a follow-up FSFI, with a mean (SD) baseline score of 11.3 (7.3; range, 2 to 25) and a follow-up mean (SD) score of 8.8 (7.3; range, −3.7 to 27.2) (P<.001). There was an increase in dilator size of 83% when comparing baseline to follow-up. At baseline, 24 participants (80%) could comfortably accept an XS or S dilator, and at follow-up 23 of 24 women (96%) could comfortably accept an M or L dilator.
Adverse effects. At their follow-up, 96% of participants were satisfied or extremely satisfied with treatment. Two women reported mild-to-moderate pain lasting 2 to 3 days, and 2 had minor bleeding; however, no women withdrew or discontinued treatment because of adverse events.
Study limitations. This study evaluated the majority of GSM symptoms as well as change in vaginal caliber after a nonhormonal therapy. The cohort was small and had no placebo group. In addition, with the limited observation period, it is difficult to determine the duration of effect and long-term safety of repeated treatments.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Portman DJ, Gass ML: Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Maturitas. 2014;79(3):349–354.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- Palma F, Volpe A, Villa P, Cagnacci A; Writing Groupt of AGATA Study. Vaginal atrophy of women in postmenopause. Results from a multicentric observational study: the AGATA study. Maturitas. 2016;83:40–44.
- Kingsberg SA, Sysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Rahn DD, Carberry C, Sanses TV, et al; Society of Gynecologic Surgeons Systematic Review Group. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
- Farrell R; American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127(3):e93–e96.
- Simon JA, Lin VH, Radovich C, Bachmann GA; Ospemiphene Study Group. One-year long-term safety extension study of ospemifene for the treatment of vulvar and vaginal atrophy in postmenopausal women with a uterus. Menopause. 2013;20(4):418–427.
- Simon J, Portman D, Mabey RG Jr; Ospemifene Study Group. Long-term safety of ospemifene (52-week extension) in the treatment of vulvar and vaginal atrophy in hysterectomized postmenopausal women. Maturitas. 2014;77(3):274–281.
- Portman DJ, Gass ML: Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Maturitas. 2014;79(3):349–354.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- Palma F, Volpe A, Villa P, Cagnacci A; Writing Groupt of AGATA Study. Vaginal atrophy of women in postmenopause. Results from a multicentric observational study: the AGATA study. Maturitas. 2016;83:40–44.
- Kingsberg SA, Sysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Rahn DD, Carberry C, Sanses TV, et al; Society of Gynecologic Surgeons Systematic Review Group. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
- Farrell R; American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127(3):e93–e96.
- Simon JA, Lin VH, Radovich C, Bachmann GA; Ospemiphene Study Group. One-year long-term safety extension study of ospemifene for the treatment of vulvar and vaginal atrophy in postmenopausal women with a uterus. Menopause. 2013;20(4):418–427.
- Simon J, Portman D, Mabey RG Jr; Ospemifene Study Group. Long-term safety of ospemifene (52-week extension) in the treatment of vulvar and vaginal atrophy in hysterectomized postmenopausal women. Maturitas. 2014;77(3):274–281.
In this Article
- Low-dose estrogen vaginal cream
- Ospemifene therapy
- Fractional CO2 laser treatment
Can a Diabetes Drug Treat Parkinson’s Disease?
PORTLAND, OR—Researchers have completed a double-blinded, placebo-controlled trial examining the potential benefits of a new formulation of exenatide in patients with Parkinson’s disease, according to an overview provided at the Fourth World Parkinson Congress. Results will be published in early 2017. If the findings are similar to those of the early proof-of-concept trial, then “this will be the first-ever demonstration of long-term disease modification and slowing of neurodegeneration in patients with Parkinson’s disease,” said Richard Wyse, MD, Director of Research at the Cure Parkinson’s Trust in London.
Exenatide is a treatment for type 2 diabetes that the Cure Parkinson’s Trust selected for study. As part of the charity’s international PD Linked Clinical Trials initiative, investigators conducted an open-label trial that suggested that 12 months of treatment with exenatide improved motor and nonmotor function in patients with mid-stage Parkinson’s disease. A follow-up study examined the same participants at 12 months after the end of the original study (ie, one year after the active group stopped receiving exenatide). Motor function and cognition were better among patients previously exposed to exenatide, compared with controls. These findings prompted the initiation of the recently completed study.
The Linked Clinical Trials Initiative
The goal of the Linked Clinical Trials initiative is to identify approved treatments for various conditions that may slow, stop, or reverse Parkinson’s disease. The initiative began in 2012 after research indicated that, among people with hypertension, patients who took the calcium channel blocker isradipine were approximately 30% less likely to develop Parkinson’s disease than those who did not. The Cure Parkinson’s Trust brought together international experts in calcium channels and funded research into the brains of healthy controls and patients with Parkinson’s disease. This research helped inform the design of a phase II trial that identified the maximum tolerable dose of isradipine. A phase III trial of isradipine’s efficacy in Parkinson’s disease is ongoing.
To date, the Cure Parkinson’s Trust has evaluated approximately 100 biologic targets relevant to Parkinson’s disease and has funded laboratory screens of more than 100,000 drugs for activity on some of these targets. Researchers have so far compiled detailed dossiers on more than 100 drugs that appear to have strong biochemical evidence for a disease-modifying effect in Parkinson’s disease. These drugs include parkin agonists, parkin interacting substrate (PARIS) inhibitors, mitochondrial and GLP-1 modulators, and various anti-inflammatory and alpha synuclein therapeutic approaches.
An international committee established by the charity regularly assesses these drug dossiers. During annual meetings, committee members prioritize the medicines for proof-of-concept trials. Their decisions are based on the therapies’ safety, efficacy, preclinical data, mechanism of action, and bioavailability. The process ensures that the selected drugs “have strong credentials for their disease-modifying potential in patients with Parkinson’s disease, and that they are also proven to be safe,” said Dr. Wyse.
Since 2012, between four and 10 therapies per year have been prioritized to enter clinical trials. Exenatide is one of these drugs. Six additional clinical trials are under way, and four more trials are in the advanced planning stages. Four of the trials are expected to conclude in 2017, and other trials in this program will launch soon.
Examining Drugs and Combination Therapies
Exenatide is not the only diabetes treatment that the initiative has considered. Among the other drugs in this program are liraglutide and lixisenatide, two injectable medicines for diabetes in the same drug class as exenatide. The liraglutide trial is already under way. In a study scheduled to begin soon, researchers will examine whether lixisenatide has benefits for patients in the early stage of Parkinson’s disease. Participants will begin treatment soon after their initial diagnosis, said Dr. Wyse.
Researchers in 23 UK centers are analyzing whether simvastatin’s anti-inflammatory properties could provide neuroprotection in Parkinson’s disease. The drug is approved to treat high cholesterol. Recruitment for a European trial of deferiprone, an iron chelator, is under way. Investigators are conducting a proof-of-concept trial in Boston, London, Tübingen, Los Angeles, and San Francisco to determine whether EPI-589, which was designed to treat pediatric mitochondrial diseases, may benefit patients with Parkinson’s disease.
Clinical trials for other drugs are in the planning stages. These treatments include N-acetyl cysteine, a therapy for cystic fibrosis and chronic obstructive pulmonary disease; nilotinib, a leukemia treatment; ursodeoxycholic acid, which treats liver disease; and the diabetes drugs alogliptin and MSDC-0160, all of which have strong biochemical rationales for disease-modifying potential in Parkinson’s disease, said Dr. Wyse.
Furthermore, the Cure Parkinson’s Trust plans to investigate the efficacy of various drug combinations. “The effective combinations of disease-modifying therapies are likely to involve two dissimilar biological approaches that work well individually, but complement each other,” said Dr. Wyse. For example, an alpha synuclein antiaggregation therapy might be more effective if it is administered with a mitochondrial therapy that increases energy availability.
“The need for effective disease-modifying therapies for Parkinson’s disease is urgent,” said Dr. Wyse. The Linked Clinical Trials initiative has prompted the initiation of clinical trials for various drugs that otherwise may not have been investigated as disease-modifying therapies for Parkinson’s disease, and many more trials are planned for the future. “It is the funding, not the science, that is slowing down the delivery of these fundamental new therapies for Parkinson’s disease,” Dr. Wyse concluded.
—Erik Greb
Suggested Reading
Aviles-Olmos I, Dickson J, Kefalopoulou Z, et al. Exenatide and the treatment of patients with Parkinson’s disease. J Clin Invest. 2013;123(6):2730-2736.
Aviles-Olmos I, Dickson J, Kefalopoulou Z, et al. Motor and cognitive advantages persist 12 months after exenatide exposure in Parkinson’s disease. J Parkinsons Dis. 2014;4(3):337-344.
Brundin P, Barker RA, Conn PJ, et al. Linked clinical trials--the development of new clinical learning studies in Parkinson’s disease using screening of multiple prospective new treatments. J Parkinsons Dis. 2013;3(3):231-239.
Parkinson Study Group. Phase II safety, tolerability, and dose selection study of isradipine as a potential disease-modifying intervention in early Parkinson’s disease (STEADY-PD). Mov Disord. 2013;28(13):1823-1831.
Roy A, Pahan K. Prospects of statins in Parkinson disease. Neuroscientist. 2011;17(3):244-255.
PORTLAND, OR—Researchers have completed a double-blinded, placebo-controlled trial examining the potential benefits of a new formulation of exenatide in patients with Parkinson’s disease, according to an overview provided at the Fourth World Parkinson Congress. Results will be published in early 2017. If the findings are similar to those of the early proof-of-concept trial, then “this will be the first-ever demonstration of long-term disease modification and slowing of neurodegeneration in patients with Parkinson’s disease,” said Richard Wyse, MD, Director of Research at the Cure Parkinson’s Trust in London.
Exenatide is a treatment for type 2 diabetes that the Cure Parkinson’s Trust selected for study. As part of the charity’s international PD Linked Clinical Trials initiative, investigators conducted an open-label trial that suggested that 12 months of treatment with exenatide improved motor and nonmotor function in patients with mid-stage Parkinson’s disease. A follow-up study examined the same participants at 12 months after the end of the original study (ie, one year after the active group stopped receiving exenatide). Motor function and cognition were better among patients previously exposed to exenatide, compared with controls. These findings prompted the initiation of the recently completed study.
The Linked Clinical Trials Initiative
The goal of the Linked Clinical Trials initiative is to identify approved treatments for various conditions that may slow, stop, or reverse Parkinson’s disease. The initiative began in 2012 after research indicated that, among people with hypertension, patients who took the calcium channel blocker isradipine were approximately 30% less likely to develop Parkinson’s disease than those who did not. The Cure Parkinson’s Trust brought together international experts in calcium channels and funded research into the brains of healthy controls and patients with Parkinson’s disease. This research helped inform the design of a phase II trial that identified the maximum tolerable dose of isradipine. A phase III trial of isradipine’s efficacy in Parkinson’s disease is ongoing.
To date, the Cure Parkinson’s Trust has evaluated approximately 100 biologic targets relevant to Parkinson’s disease and has funded laboratory screens of more than 100,000 drugs for activity on some of these targets. Researchers have so far compiled detailed dossiers on more than 100 drugs that appear to have strong biochemical evidence for a disease-modifying effect in Parkinson’s disease. These drugs include parkin agonists, parkin interacting substrate (PARIS) inhibitors, mitochondrial and GLP-1 modulators, and various anti-inflammatory and alpha synuclein therapeutic approaches.
An international committee established by the charity regularly assesses these drug dossiers. During annual meetings, committee members prioritize the medicines for proof-of-concept trials. Their decisions are based on the therapies’ safety, efficacy, preclinical data, mechanism of action, and bioavailability. The process ensures that the selected drugs “have strong credentials for their disease-modifying potential in patients with Parkinson’s disease, and that they are also proven to be safe,” said Dr. Wyse.
Since 2012, between four and 10 therapies per year have been prioritized to enter clinical trials. Exenatide is one of these drugs. Six additional clinical trials are under way, and four more trials are in the advanced planning stages. Four of the trials are expected to conclude in 2017, and other trials in this program will launch soon.
Examining Drugs and Combination Therapies
Exenatide is not the only diabetes treatment that the initiative has considered. Among the other drugs in this program are liraglutide and lixisenatide, two injectable medicines for diabetes in the same drug class as exenatide. The liraglutide trial is already under way. In a study scheduled to begin soon, researchers will examine whether lixisenatide has benefits for patients in the early stage of Parkinson’s disease. Participants will begin treatment soon after their initial diagnosis, said Dr. Wyse.
Researchers in 23 UK centers are analyzing whether simvastatin’s anti-inflammatory properties could provide neuroprotection in Parkinson’s disease. The drug is approved to treat high cholesterol. Recruitment for a European trial of deferiprone, an iron chelator, is under way. Investigators are conducting a proof-of-concept trial in Boston, London, Tübingen, Los Angeles, and San Francisco to determine whether EPI-589, which was designed to treat pediatric mitochondrial diseases, may benefit patients with Parkinson’s disease.
Clinical trials for other drugs are in the planning stages. These treatments include N-acetyl cysteine, a therapy for cystic fibrosis and chronic obstructive pulmonary disease; nilotinib, a leukemia treatment; ursodeoxycholic acid, which treats liver disease; and the diabetes drugs alogliptin and MSDC-0160, all of which have strong biochemical rationales for disease-modifying potential in Parkinson’s disease, said Dr. Wyse.
Furthermore, the Cure Parkinson’s Trust plans to investigate the efficacy of various drug combinations. “The effective combinations of disease-modifying therapies are likely to involve two dissimilar biological approaches that work well individually, but complement each other,” said Dr. Wyse. For example, an alpha synuclein antiaggregation therapy might be more effective if it is administered with a mitochondrial therapy that increases energy availability.
“The need for effective disease-modifying therapies for Parkinson’s disease is urgent,” said Dr. Wyse. The Linked Clinical Trials initiative has prompted the initiation of clinical trials for various drugs that otherwise may not have been investigated as disease-modifying therapies for Parkinson’s disease, and many more trials are planned for the future. “It is the funding, not the science, that is slowing down the delivery of these fundamental new therapies for Parkinson’s disease,” Dr. Wyse concluded.
—Erik Greb
Suggested Reading
Aviles-Olmos I, Dickson J, Kefalopoulou Z, et al. Exenatide and the treatment of patients with Parkinson’s disease. J Clin Invest. 2013;123(6):2730-2736.
Aviles-Olmos I, Dickson J, Kefalopoulou Z, et al. Motor and cognitive advantages persist 12 months after exenatide exposure in Parkinson’s disease. J Parkinsons Dis. 2014;4(3):337-344.
Brundin P, Barker RA, Conn PJ, et al. Linked clinical trials--the development of new clinical learning studies in Parkinson’s disease using screening of multiple prospective new treatments. J Parkinsons Dis. 2013;3(3):231-239.
Parkinson Study Group. Phase II safety, tolerability, and dose selection study of isradipine as a potential disease-modifying intervention in early Parkinson’s disease (STEADY-PD). Mov Disord. 2013;28(13):1823-1831.
Roy A, Pahan K. Prospects of statins in Parkinson disease. Neuroscientist. 2011;17(3):244-255.
PORTLAND, OR—Researchers have completed a double-blinded, placebo-controlled trial examining the potential benefits of a new formulation of exenatide in patients with Parkinson’s disease, according to an overview provided at the Fourth World Parkinson Congress. Results will be published in early 2017. If the findings are similar to those of the early proof-of-concept trial, then “this will be the first-ever demonstration of long-term disease modification and slowing of neurodegeneration in patients with Parkinson’s disease,” said Richard Wyse, MD, Director of Research at the Cure Parkinson’s Trust in London.
Exenatide is a treatment for type 2 diabetes that the Cure Parkinson’s Trust selected for study. As part of the charity’s international PD Linked Clinical Trials initiative, investigators conducted an open-label trial that suggested that 12 months of treatment with exenatide improved motor and nonmotor function in patients with mid-stage Parkinson’s disease. A follow-up study examined the same participants at 12 months after the end of the original study (ie, one year after the active group stopped receiving exenatide). Motor function and cognition were better among patients previously exposed to exenatide, compared with controls. These findings prompted the initiation of the recently completed study.
The Linked Clinical Trials Initiative
The goal of the Linked Clinical Trials initiative is to identify approved treatments for various conditions that may slow, stop, or reverse Parkinson’s disease. The initiative began in 2012 after research indicated that, among people with hypertension, patients who took the calcium channel blocker isradipine were approximately 30% less likely to develop Parkinson’s disease than those who did not. The Cure Parkinson’s Trust brought together international experts in calcium channels and funded research into the brains of healthy controls and patients with Parkinson’s disease. This research helped inform the design of a phase II trial that identified the maximum tolerable dose of isradipine. A phase III trial of isradipine’s efficacy in Parkinson’s disease is ongoing.
To date, the Cure Parkinson’s Trust has evaluated approximately 100 biologic targets relevant to Parkinson’s disease and has funded laboratory screens of more than 100,000 drugs for activity on some of these targets. Researchers have so far compiled detailed dossiers on more than 100 drugs that appear to have strong biochemical evidence for a disease-modifying effect in Parkinson’s disease. These drugs include parkin agonists, parkin interacting substrate (PARIS) inhibitors, mitochondrial and GLP-1 modulators, and various anti-inflammatory and alpha synuclein therapeutic approaches.
An international committee established by the charity regularly assesses these drug dossiers. During annual meetings, committee members prioritize the medicines for proof-of-concept trials. Their decisions are based on the therapies’ safety, efficacy, preclinical data, mechanism of action, and bioavailability. The process ensures that the selected drugs “have strong credentials for their disease-modifying potential in patients with Parkinson’s disease, and that they are also proven to be safe,” said Dr. Wyse.
Since 2012, between four and 10 therapies per year have been prioritized to enter clinical trials. Exenatide is one of these drugs. Six additional clinical trials are under way, and four more trials are in the advanced planning stages. Four of the trials are expected to conclude in 2017, and other trials in this program will launch soon.
Examining Drugs and Combination Therapies
Exenatide is not the only diabetes treatment that the initiative has considered. Among the other drugs in this program are liraglutide and lixisenatide, two injectable medicines for diabetes in the same drug class as exenatide. The liraglutide trial is already under way. In a study scheduled to begin soon, researchers will examine whether lixisenatide has benefits for patients in the early stage of Parkinson’s disease. Participants will begin treatment soon after their initial diagnosis, said Dr. Wyse.
Researchers in 23 UK centers are analyzing whether simvastatin’s anti-inflammatory properties could provide neuroprotection in Parkinson’s disease. The drug is approved to treat high cholesterol. Recruitment for a European trial of deferiprone, an iron chelator, is under way. Investigators are conducting a proof-of-concept trial in Boston, London, Tübingen, Los Angeles, and San Francisco to determine whether EPI-589, which was designed to treat pediatric mitochondrial diseases, may benefit patients with Parkinson’s disease.
Clinical trials for other drugs are in the planning stages. These treatments include N-acetyl cysteine, a therapy for cystic fibrosis and chronic obstructive pulmonary disease; nilotinib, a leukemia treatment; ursodeoxycholic acid, which treats liver disease; and the diabetes drugs alogliptin and MSDC-0160, all of which have strong biochemical rationales for disease-modifying potential in Parkinson’s disease, said Dr. Wyse.
Furthermore, the Cure Parkinson’s Trust plans to investigate the efficacy of various drug combinations. “The effective combinations of disease-modifying therapies are likely to involve two dissimilar biological approaches that work well individually, but complement each other,” said Dr. Wyse. For example, an alpha synuclein antiaggregation therapy might be more effective if it is administered with a mitochondrial therapy that increases energy availability.
“The need for effective disease-modifying therapies for Parkinson’s disease is urgent,” said Dr. Wyse. The Linked Clinical Trials initiative has prompted the initiation of clinical trials for various drugs that otherwise may not have been investigated as disease-modifying therapies for Parkinson’s disease, and many more trials are planned for the future. “It is the funding, not the science, that is slowing down the delivery of these fundamental new therapies for Parkinson’s disease,” Dr. Wyse concluded.
—Erik Greb
Suggested Reading
Aviles-Olmos I, Dickson J, Kefalopoulou Z, et al. Exenatide and the treatment of patients with Parkinson’s disease. J Clin Invest. 2013;123(6):2730-2736.
Aviles-Olmos I, Dickson J, Kefalopoulou Z, et al. Motor and cognitive advantages persist 12 months after exenatide exposure in Parkinson’s disease. J Parkinsons Dis. 2014;4(3):337-344.
Brundin P, Barker RA, Conn PJ, et al. Linked clinical trials--the development of new clinical learning studies in Parkinson’s disease using screening of multiple prospective new treatments. J Parkinsons Dis. 2013;3(3):231-239.
Parkinson Study Group. Phase II safety, tolerability, and dose selection study of isradipine as a potential disease-modifying intervention in early Parkinson’s disease (STEADY-PD). Mov Disord. 2013;28(13):1823-1831.
Roy A, Pahan K. Prospects of statins in Parkinson disease. Neuroscientist. 2011;17(3):244-255.
Letters to the Editor: Extended use IUDs

“IN WHICH CLINICAL SITUATIONS CAN THE USE OF THE 52-MG LEVONORGESTREL-RELEASING IUD (MIRENA) AND THE TCU380A COPPER-IUD (PARAGARD) BE EXTENDED?”
ROBERT L. BARBIERI, MD (SEPTEMBER 2016)
Extended-use IUDs and infection risk
For some time now I have been leaving hormonal intrauterine devices (IUDs) in place for 6 to 7 years, until menses returns. In my practice, long-term use of copper-IUDs has been associated with the presence of actinomycosis in the endometrial cavity, although usually without sepsis.
George Haber, MD
Montreal, Canada
Suppressing menses, pain with an IUD
I have a number of patients using the 52-mg levonorgestrel-releasing (LNG) IUD (Mirena) for noncontraceptive reasons, especially for reduction or elimination of menstrual flow and/or pain. Many have permanent sterilization in place (tubal sterilization, partner vasectomy) and I tell them we can leave the IUD in as long as they are satisfied with the results, since we are not concerned with pregnancy. Several have continued IUD use well past the 5-year mark.
Alan Smith, MD
Savannah, Georgia
LNG-IUD effective for multiple uses
In our practice, we have used the LNG-IUD Mirena off label for over a decade successfully for men-strual suppression in perimenopausal and postmenopausal women effectively for up to 8 years. We often place this device in the uterus after an endometrial ablation. We also offer it extended use as an alternative for menopausal hormone therapy when a progestin is indicated due to the presence of a uterus. Progestin delivery by this IUD is maximized in the endometrium and minimized in the breast and other systemic sites.
John Lenihan Jr, MD
Tacoma, Washington
Dr. Barbieri responds
I thank Dr. Haber for his observations. He notes that users of IUDs may have Actinomyces organisms identified on cervical cytology. These women should be informed of the finding and examined for evidence of active pelvic infection. If the women are asympto-matic and have a normal physical exam, the IUD does not need to be removed and antibiotic treatment is not recommended. If the woman has evidence of pelvic infection, the IUD should be removed and sent for anaerobic culture.
I appreciate that Drs. Smith and Lenihan shared their clinical pearls with readers. Dr. Smith notes that when an LNG-IUD is used to control bleeding in women who are sterilized, there are few concerns about the duration of its contraceptive efficacy, and adequate control of bleeding is a clinically useful end point demonstrating the IUD’s continued efficacy. If bleeding begins to increase after 5 years, the clinician might choose to remove the old device and replace it with a new one. Dr. Lenihan reports his use of the 52-mg LNG-IUD as the progestin in a regimen of menopausal hormone therapy. Of note, there are multiple reports from Finland that use of an LNG-IUD in premenopausal and menopausal women may be associated with an increased risk of breast cancer.1,2 Conflicting reports from Finland and Germany did not detect an increased risk of breast cancer in women who used an LNG-IUD.3,4 Clinicians should be aware that when Mirena is used past its approved 5-year time limit, it is an off-label use of the device.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Soini T, Hurskainen R, Grenman S, et al. Levonorgestrel-releasing intrauterine system and the risk of breast cancer: a nationwide cohort study. Acta Oncol. 2016;55(2):188–192.
- Soini T, Hurskainen R, Grenman S, Maenpaa J, Paavonen J, Pukkala E. Cancer risk in women using the levonorgestrel-releasing intrauterine system in Finland. Obstet Gynecol. 2014;124(2 pt 1):292–299.
- Dinger J, Bardenheuer K, Minhn TD. Levonorgestrel-releasing and copper intrauterine devices and the risk of breast cancer. Contraception. 2011;83(3):211–217.
- Backman T, Rauramo I, Jaakkola K, et al. Use of the levonorgestrel-releasing intrauterine system and breast cancer. Obstet Gynecol. 2005;106(4):813–817.

“IN WHICH CLINICAL SITUATIONS CAN THE USE OF THE 52-MG LEVONORGESTREL-RELEASING IUD (MIRENA) AND THE TCU380A COPPER-IUD (PARAGARD) BE EXTENDED?”
ROBERT L. BARBIERI, MD (SEPTEMBER 2016)
Extended-use IUDs and infection risk
For some time now I have been leaving hormonal intrauterine devices (IUDs) in place for 6 to 7 years, until menses returns. In my practice, long-term use of copper-IUDs has been associated with the presence of actinomycosis in the endometrial cavity, although usually without sepsis.
George Haber, MD
Montreal, Canada
Suppressing menses, pain with an IUD
I have a number of patients using the 52-mg levonorgestrel-releasing (LNG) IUD (Mirena) for noncontraceptive reasons, especially for reduction or elimination of menstrual flow and/or pain. Many have permanent sterilization in place (tubal sterilization, partner vasectomy) and I tell them we can leave the IUD in as long as they are satisfied with the results, since we are not concerned with pregnancy. Several have continued IUD use well past the 5-year mark.
Alan Smith, MD
Savannah, Georgia
LNG-IUD effective for multiple uses
In our practice, we have used the LNG-IUD Mirena off label for over a decade successfully for men-strual suppression in perimenopausal and postmenopausal women effectively for up to 8 years. We often place this device in the uterus after an endometrial ablation. We also offer it extended use as an alternative for menopausal hormone therapy when a progestin is indicated due to the presence of a uterus. Progestin delivery by this IUD is maximized in the endometrium and minimized in the breast and other systemic sites.
John Lenihan Jr, MD
Tacoma, Washington
Dr. Barbieri responds
I thank Dr. Haber for his observations. He notes that users of IUDs may have Actinomyces organisms identified on cervical cytology. These women should be informed of the finding and examined for evidence of active pelvic infection. If the women are asympto-matic and have a normal physical exam, the IUD does not need to be removed and antibiotic treatment is not recommended. If the woman has evidence of pelvic infection, the IUD should be removed and sent for anaerobic culture.
I appreciate that Drs. Smith and Lenihan shared their clinical pearls with readers. Dr. Smith notes that when an LNG-IUD is used to control bleeding in women who are sterilized, there are few concerns about the duration of its contraceptive efficacy, and adequate control of bleeding is a clinically useful end point demonstrating the IUD’s continued efficacy. If bleeding begins to increase after 5 years, the clinician might choose to remove the old device and replace it with a new one. Dr. Lenihan reports his use of the 52-mg LNG-IUD as the progestin in a regimen of menopausal hormone therapy. Of note, there are multiple reports from Finland that use of an LNG-IUD in premenopausal and menopausal women may be associated with an increased risk of breast cancer.1,2 Conflicting reports from Finland and Germany did not detect an increased risk of breast cancer in women who used an LNG-IUD.3,4 Clinicians should be aware that when Mirena is used past its approved 5-year time limit, it is an off-label use of the device.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.

“IN WHICH CLINICAL SITUATIONS CAN THE USE OF THE 52-MG LEVONORGESTREL-RELEASING IUD (MIRENA) AND THE TCU380A COPPER-IUD (PARAGARD) BE EXTENDED?”
ROBERT L. BARBIERI, MD (SEPTEMBER 2016)
Extended-use IUDs and infection risk
For some time now I have been leaving hormonal intrauterine devices (IUDs) in place for 6 to 7 years, until menses returns. In my practice, long-term use of copper-IUDs has been associated with the presence of actinomycosis in the endometrial cavity, although usually without sepsis.
George Haber, MD
Montreal, Canada
Suppressing menses, pain with an IUD
I have a number of patients using the 52-mg levonorgestrel-releasing (LNG) IUD (Mirena) for noncontraceptive reasons, especially for reduction or elimination of menstrual flow and/or pain. Many have permanent sterilization in place (tubal sterilization, partner vasectomy) and I tell them we can leave the IUD in as long as they are satisfied with the results, since we are not concerned with pregnancy. Several have continued IUD use well past the 5-year mark.
Alan Smith, MD
Savannah, Georgia
LNG-IUD effective for multiple uses
In our practice, we have used the LNG-IUD Mirena off label for over a decade successfully for men-strual suppression in perimenopausal and postmenopausal women effectively for up to 8 years. We often place this device in the uterus after an endometrial ablation. We also offer it extended use as an alternative for menopausal hormone therapy when a progestin is indicated due to the presence of a uterus. Progestin delivery by this IUD is maximized in the endometrium and minimized in the breast and other systemic sites.
John Lenihan Jr, MD
Tacoma, Washington
Dr. Barbieri responds
I thank Dr. Haber for his observations. He notes that users of IUDs may have Actinomyces organisms identified on cervical cytology. These women should be informed of the finding and examined for evidence of active pelvic infection. If the women are asympto-matic and have a normal physical exam, the IUD does not need to be removed and antibiotic treatment is not recommended. If the woman has evidence of pelvic infection, the IUD should be removed and sent for anaerobic culture.
I appreciate that Drs. Smith and Lenihan shared their clinical pearls with readers. Dr. Smith notes that when an LNG-IUD is used to control bleeding in women who are sterilized, there are few concerns about the duration of its contraceptive efficacy, and adequate control of bleeding is a clinically useful end point demonstrating the IUD’s continued efficacy. If bleeding begins to increase after 5 years, the clinician might choose to remove the old device and replace it with a new one. Dr. Lenihan reports his use of the 52-mg LNG-IUD as the progestin in a regimen of menopausal hormone therapy. Of note, there are multiple reports from Finland that use of an LNG-IUD in premenopausal and menopausal women may be associated with an increased risk of breast cancer.1,2 Conflicting reports from Finland and Germany did not detect an increased risk of breast cancer in women who used an LNG-IUD.3,4 Clinicians should be aware that when Mirena is used past its approved 5-year time limit, it is an off-label use of the device.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Soini T, Hurskainen R, Grenman S, et al. Levonorgestrel-releasing intrauterine system and the risk of breast cancer: a nationwide cohort study. Acta Oncol. 2016;55(2):188–192.
- Soini T, Hurskainen R, Grenman S, Maenpaa J, Paavonen J, Pukkala E. Cancer risk in women using the levonorgestrel-releasing intrauterine system in Finland. Obstet Gynecol. 2014;124(2 pt 1):292–299.
- Dinger J, Bardenheuer K, Minhn TD. Levonorgestrel-releasing and copper intrauterine devices and the risk of breast cancer. Contraception. 2011;83(3):211–217.
- Backman T, Rauramo I, Jaakkola K, et al. Use of the levonorgestrel-releasing intrauterine system and breast cancer. Obstet Gynecol. 2005;106(4):813–817.
- Soini T, Hurskainen R, Grenman S, et al. Levonorgestrel-releasing intrauterine system and the risk of breast cancer: a nationwide cohort study. Acta Oncol. 2016;55(2):188–192.
- Soini T, Hurskainen R, Grenman S, Maenpaa J, Paavonen J, Pukkala E. Cancer risk in women using the levonorgestrel-releasing intrauterine system in Finland. Obstet Gynecol. 2014;124(2 pt 1):292–299.
- Dinger J, Bardenheuer K, Minhn TD. Levonorgestrel-releasing and copper intrauterine devices and the risk of breast cancer. Contraception. 2011;83(3):211–217.
- Backman T, Rauramo I, Jaakkola K, et al. Use of the levonorgestrel-releasing intrauterine system and breast cancer. Obstet Gynecol. 2005;106(4):813–817.