User login
Preventing infection after cesarean delivery: Evidence-based guidance
Cesarean delivery is now the most commonly performed major operation in hospitals across the United States. Approximately 30% of the 4 million deliveries that occur each year are by cesarean. Endometritis and wound infection (superficial and deep surgical site infection) are the most common postoperative complications of cesarean delivery. These 2 infections usually can be treated in a straightforward manner with antibiotics or surgical drainage. In some cases, however, they can lead to serious sequelae, such as pelvic abscess, septic pelvic vein thrombophlebitis, and wound dehiscence/evisceration, thereby prolonging the patient’s hospitalization and significantly increasing medical expenses.
Accordingly, in the past 50 years many investigators have proposed various specific measures to reduce the risk of postcesarean infection. In this article, we critically evaluate 2 of these major interventions: methods of skin preparation and administration of prophylactic antibiotics. In part 2 of this series next month, we will review the evidence regarding preoperative bathing with an antiseptic, preoperative vaginal cleansing with an antiseptic solution, methods of placental extraction, closure of the deep subcutaneous layer of the abdomen, and closure of the skin.
CASE Cesarean delivery required for nonprogressing labor
A 26-year-old obese primigravid woman, body mass index (BMI) 37 kg m2, at 40 weeks’ gestation has been in labor for 20 hours. Her membranes have been ruptured for 16 hours. Her cervix is completely effaced and is 7 cm dilated. The fetal head is at −1 cm station. Her cervical examination findings have not changed in 4 hours despite adequate uterine contractility documented by intrauterine pressure catheter. You are now ready to proceed with cesarean delivery, and you want to do everything possible to prevent the patient from developing a postoperative infection.
What are the best practices for postcesarean infection prevention in this patient?
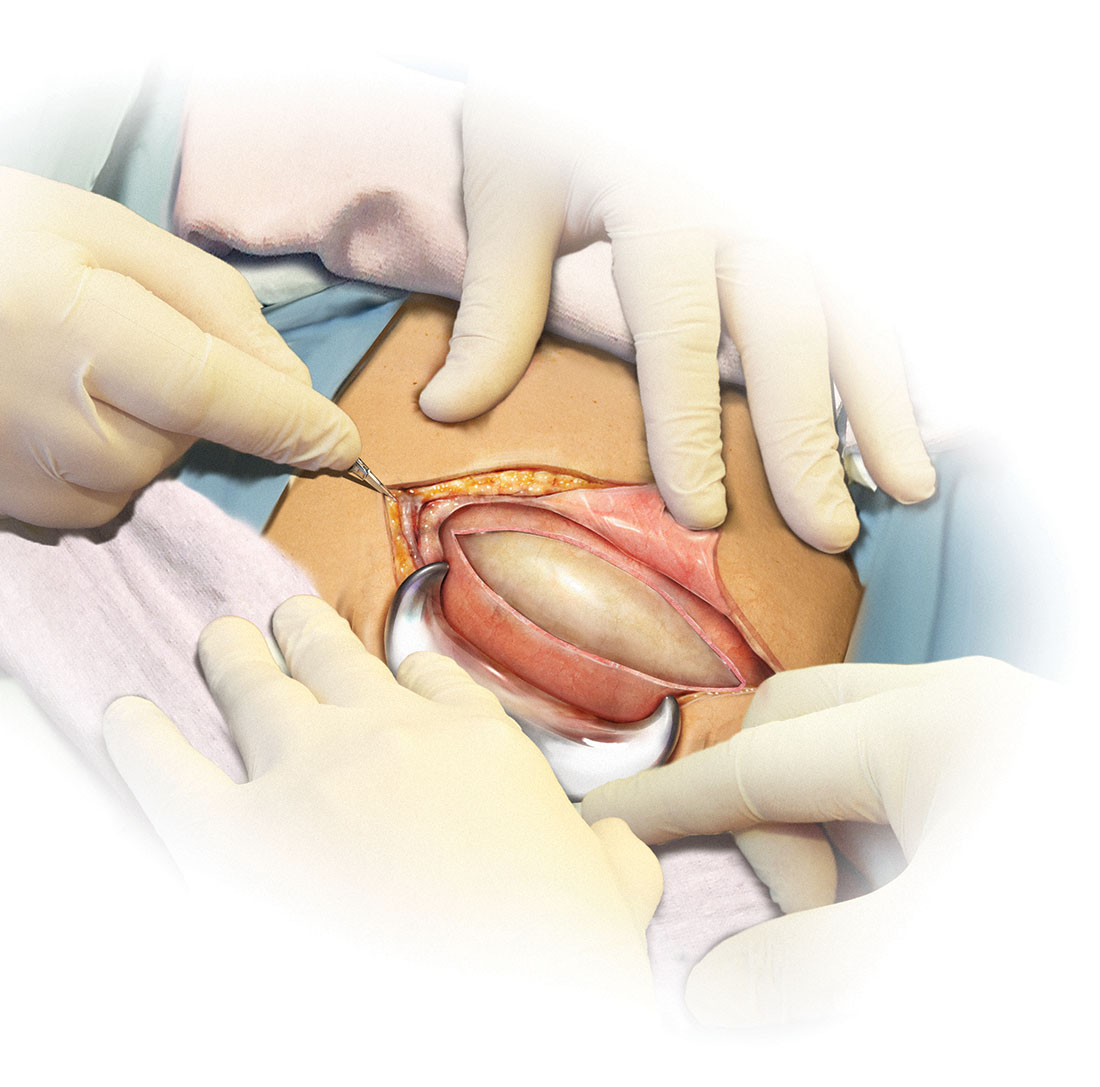
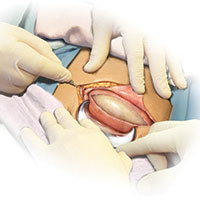
Skin preparation
Adequate preoperative skin preparation is an important first step in preventing post‑ cesarean infection.
How should you prepare the patient’s skin for surgery?
Two issues to address when preparing the abdominal wall for surgery are hair removal and skin cleansing. More than 40 years ago, Cruse and Foord definitively answered the question about hair removal.1 In a landmark cohort investigation of more than 23,000 patients having many different types of operative procedures, they demonstrated that shaving the hair on the evening before surgery resulted in a higher rate of wound infection than clipping the hair, removing the hair with a depilatory cream just before surgery, or not removing the hair at all.
Three recent investigations have thoughtfully addressed the issue of skin cleansing. Darouiche and colleagues conducted a prospective, randomized, multicenter trial comparing chlorhexidine-alcohol with povidone-iodine for skin preparation before surgery.2 Their investigation included 849 patients having many different types of surgical procedures, only a minority of which were in obstetric and gynecologic patients. They demonstrated fewer superficial wound infections in patients in the chlorhexidine-alcohol group (4.2% vs 8.6%, P = .008). Of even greater importance, patients in the chlorhexidine-alcohol group had fewer deep wound infections (1% vs 3%, P = .005).
Ngai and co-workers recently reported the results of a randomized controlled trial (RCT) in which women undergoing nonurgent cesarean delivery had their skin cleansed with povidone-iodine with alcohol, chlorhexidine with alcohol, or the sequential combination of both solutions.3 The overall rate of surgical site infection was just 4.3%. The 3 groups had comparable infection rates and, accordingly, the authors were unable to conclude that one type of skin preparation was superior to the other.
The most informative recent investigation was by Tuuli and colleagues, who evaluated 1,147 patients having cesarean delivery assigned to undergo skin preparation with either chlorhexidine-alcohol or iodine-alcohol.4 Unlike the study by Ngai and co-workers, in this study approximately 40% of the patients in each treatment arm had unscheduled, urgent cesarean deliveries.3,4 Overall, the rate of infection in the chlorhexidine-alcohol group was 4.0% compared with 7.3% in the iodine-alcohol group (relative risk [RR], 0.55; 95% confidence interval [CI], 0.34–0.90, P = .02).
What the evidence says
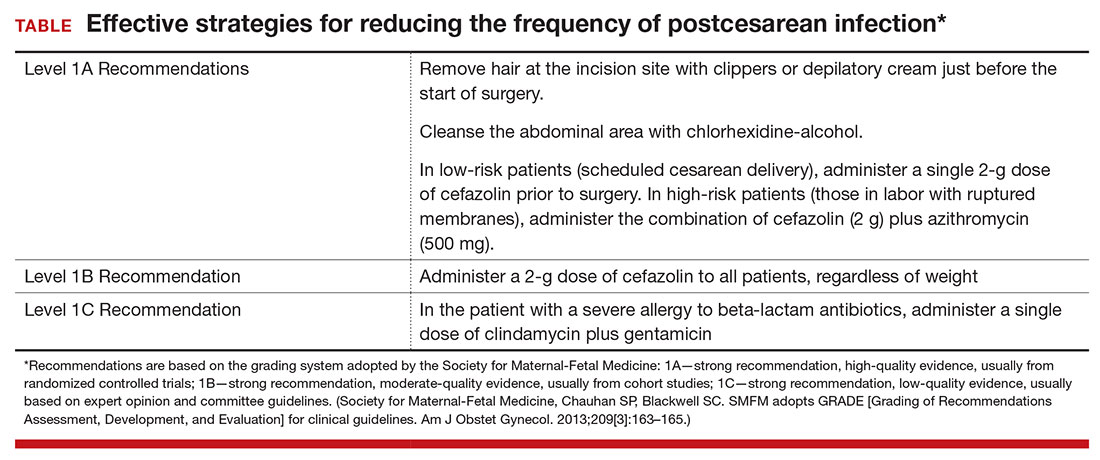
Based on the evidence cited above, we advise removing hair at the incision site with clippers or depilatory cream just before the start of surgery. The abdomen should then be cleansed with a chlorhexidine-alcohol solution (Level I Evidence, Level 1A Recommendation; TABLE).
Antibiotic prophylaxis
Questions to consider regarding antibiotic prophylaxis for cesarean delivery include appropriateness of treatment, antibiotic(s) selection, timing of administration, dose, and special circumstances.
Should you give the patient prophylactic antibiotics?
Prophylactic antibiotics are justified for surgical procedures whenever 3 major criteria are met5:
- the surgical site is inevitably contaminated with bacteria
- in the absence of prophylaxis, the frequency of infection at the operative site is unacceptably high
- operative site infections have the potential to lead to serious, potentially life-threatening sequelae.
Without a doubt, all 3 of these criteria are fulfilled when considering either urgent or nonurgent cesarean delivery. When cesarean delivery follows a long labor complicated by ruptured membranes, multiple internal vaginal examinations, and internal fetal monitoring, the operative site is inevitably contaminated with hundreds of thousands of pathogenic bacteria. Even when cesarean delivery is scheduled to occur before the onset of labor and ruptured membranes, a high concentration of vaginal organisms is introduced into the uterine and pelvic cavities coincident with making the hysterotomy incision.6
In the era before prophylactic antibiotics were used routinely, postoperative infection rates in some highly indigent patient populations approached 85%.5 Finally, as noted previously, postcesarean endometritis may progress to pelvic abscess formation, septic pelvic vein thrombophlebitis, and septic shock; wound infections may be complicated by dehiscence and evisceration.
When should you administer antibiotics: Before the surgical incision or after cord clamping?
More than 50 years ago, Burke conducted the classic sequence of basic science experiments that forms the foundation for use of prophylactic antibiotics.7 Using a guinea pig model, he showed that prophylactic antibiotics exert their most pronounced effect when they are administered before the surgical incision is made and before bacterial contamination occurs. Prophylaxis that is delayed more than 4 hours after the start of surgery will likely be ineffective.
Interestingly, however, when clinicians first began using prophylactic antibiotics for cesarean delivery, some investigators expressed concern about the possible exposure of the neonate to antibiotics just before delivery—specifically, whether this exposure would increase the frequency of evaluations for suspected sepsis or would promote resistance among organisms that would make neonatal sepsis more difficult to treat.
Gordon and colleagues published an important report in 1979 that showed that preoperative administration of ampicillin did not increase the frequency of immediate or delayed neonatal infections.8 However, delaying the administration of ampicillin until after the umbilical cord was clamped was just as effective in preventing post‑cesarean endometritis. Subsequently, Cunningham and co-workers showed that preoperative administration of prophylactic antibiotics significantly increased the frequency of sepsis workups in exposed neonates compared with infants with no preoperative antibiotic exposure (28% vs 15%; P<.025).9 Based on these 2 reports, obstetricians adopted a policy of delaying antibiotic administration until after the infant’s umbilical cord was clamped.
In 2007, Sullivan and colleagues challenged this long-standing practice.10 In a carefully designed prospective, randomized, double-blind trial, they showed that patients who received preoperative cefazolin had a significant reduction in the frequency of endometritis compared with women who received the same antibiotic after cord clamping (1% vs 5%; RR, 0.2; 95% CI, 0.2–0.94). The rate of wound infection was lower in the preoperative antibiotic group (3% vs 5%), but this difference did not reach statistical significance. The total infection-related morbidity was significantly reduced in women who received antibiotics preoperatively (4.0% vs 11.5%; RR, 0.4; 95% CI, 0.18–0.87). Additionally, there was no increase in the frequency of proven or suspected neonatal infection in the infants exposed to antibiotics before delivery.
Subsequent to the publication by Sullivan and colleagues, other reports have confirmed that administration of antibiotics prior to surgery is superior to administration after clamping of the umbilical cord.10–12 Thus, we have come full circle back to Burke’s principle established more than a half century ago.7
Which antibiotic(s) should you administer for prophylaxis, and how many doses?
In an earlier review, one of us (PD) examined the evidence regarding choice of antibiotics and number of doses, concluding that a single dose of a first-generation cephalosporin, such as cefazolin, was the preferred regimen.5 The single dose was comparable in effectiveness to 2- or 3-dose regimens and to single- or multiple-dose regimens of broader-spectrum agents. For more than 20 years now, the standard of care for antibiotic prophylaxis has been a single 1- to 2-g dose of cefazolin.
Several recent reports, however, have raised the question of whether the prophylactic effect could be enhanced if the spectrum of activity of the antibiotic regimen was broadened to include an agent effective against Ureaplasma species.
Tita and colleagues evaluated an indigent patient population with an inherently high rate of postoperative infection; they showed that adding azithromycin 500 mg to cefazolin significantly reduced the rate of postcesarean endometritis.13 In a follow-up report from the same institution, Tita and co-workers demonstrated that adding azithromycin also significantly reduced the frequency of wound infection.14 In both of these investigations, the antibiotics were administered after cord clamping.
In a subsequent report, Ward and Duff15 showed that the combination of azithromycin plus cefazolin administered preoperatively resulted in a very low rate of both endometritis and wound infection in a population similar to that studied by Tita et al.13,14
Very recently, Tita and associates published the results of the Cesarean Section Optimal Antibiotic Prophylaxis (C/SOAP) trial conducted at 14 US hospitals.16 This study included 2,013 women undergoing cesarean delivery during labor or after membrane rupture who were randomly assigned to receive intravenous azithromycin 500 mg (n = 1,019) or placebo (n = 994). All women also received standard antibiotic prophylaxis with cefazolin. The primary outcome (a composite of endometritis, wound infection, or other infection within 6 weeks) was significantly lower in the azithromycin group than in the placebo group (6.1% vs 12.0%, P<.001). In addition, there were significant differences between the treatment groups in the rates of endometritis (3.8% in the azithromycin group vs 6.1% in the placebo group, P = .02) as well as in the rates of wound infection (2.4% vs 6.6%, respectively, P<.001). Of additional note, there were no differences between the 2 groups in the composite neonatal outcome of death and serious neonatal complications (14.3% vs 13.6%, P = .63).The investigators concluded that extended-spectrum prophylaxis with adjunctive azithromycin safely reduces infection rates without raising the risk of neonatal adverse outcomes.
What the evidence says
We conclude that all patients, even those having a scheduled cesarean before the onset of labor or ruptured membranes, should receive prophylactic antibiotics in a single dose administered preoperatively rather than after cord clamping (Level I Evidence, Level 1A Recommendation; TABLE). In high-risk populations (eg, women in labor with ruptured membranes who are having an urgent cesarean), for whom the baseline risk of infection is high, administer the combination of cefazolin plus azithromycin in lieu of cefazolin alone (Level I Evidence, Level 1A Recommendation; TABLE).
If the patient has a history of an immediate hypersensitivity reaction to beta-lactam antibiotics, we recommend the combination of clindamycin (900 mg) plus gentamicin (1.5 mg/kg) as a single infusion prior to surgery. We base this recommendation on the need to provide reasonable coverage against a broad range of pathogens. Clindamycin covers gram-positive aerobes, such as staphylococci species and group B streptococci, and anaerobes; gentamicin covers aerobic gram-negative bacilli. A single agent, such as clindamycin or metronidazole, does not provide the broad-based coverage necessary for effective prophylaxis (Level III Evidence, Level 1C Recommendation; TABLE).
If the patient is overweight or obese, should you modify the antibiotic dose?
The prevalence of obesity in the United States continues to increase. One-third of all US reproductive-aged women are obese, and 6% of women are extremely obese.17 Obesity increases the risk of postcesarean infection 3- to 5- fold.18 Because both pregnancy and obesity increase the total volume of a drug’s distribution, achieving adequate antibiotic tissue concentrations may be hindered by a dilutional effect. Furthermore, pharmacokinetic studies consistently have shown that the tissue concentration of an antibiotic—which, ideally, should be above the minimum inhibitory concentration (MIC) for common bacteria—determines the susceptibility of those tissues to infection, regardless of whether the serum concentration of the antibiotic is in the therapeutic range.19
These concerns have led to several recent investigations evaluating different doses of cefazolin for obese patients. Pevzner and colleagues conducted a prospective cohort study of 29 women having a scheduled cesarean delivery.20 The patients were divided into 3 groups: lean (BMI <30 kg m2), obese (BMI 30.0–39.9 kg m2), and extremely obese (BMI >40 kg m2). All women received a 2-g dose of cefazolin 30 to 60 minutes before surgery. Cefazolin concentrations in adipose tissue obtained at the time of skin incision were inversely proportional to maternal BMI (r, −0.67; P<.001). All specimens demonstrated a therapeutic concentration (>1 µg/g) of cefazolin for gram-positive cocci, but 20% of the obese women and 33% of the extremely obese women did not achieve the MIC (>4 µg/g) for gram-negative bacilli (P = .29 and P = .14, respectively). At the time of skin closure, 20% of obese women and 44% of extremely obese women did not have tissue concentrations that exceeded the MIC for gram-negative bacteria.
Swank and associates conducted a prospective cohort study that included 28 women.18 They demonstrated that, after a 2-g dose of cefazolin, only 20% of the obese women (BMI 30–40 kg m2) and 0% of the extremely obese women (BMI >40 kg m2) achieved an adipose tissue concentration that exceeded the MIC for gram-negative rods (8 µg/mL). However, 100% and 71.4%, respectively, achieved such a tissue concentration after a 3-g dose. When the women were stratified by actual weight, there was a statistically significant difference between those who weighed less than 120 kg and those who weighed more than 120 kg. Seventy-nine percent of the former had a tissue concentration of cefazolin greater than 8 µg/mL compared with 0% of the women who weighed more than 120 kg. Based on these observations, the authors recommended a 3-g dose of cefazolin for women who weigh more than 120 kg.
In a double-blind RCT with 26 obese women (BMI ≥30 kg m2), Young and colleagues demonstrated that, at the time of hysterotomy and fascial closure, significantly higher concentrations of cefazolin were found in the adipose tissue of obese women who received a 3-g dose of antibiotic compared with those who received a 2-g dose.21 However, all concentrations of cefazolin were consistently above the MIC of cefazolin for gram-positive cocci (1 µg/g) and gram-negative bacilli (4 µg/g). Further, Maggio and co-workers conducted a double-blind RCT comparing a 2-g dose of cefazolin versus a 3-g dose in 57 obese women (BMI ≥30 kg m2).22 They found no statistically significant difference in the percentage of women who had tissue concentrations of cefazolin greater than the MIC for gram-positive cocci (8 µg/g). All samples were above the MIC of cefazolin for gram-negative bacilli (2 µg/g). Based on these data, these investigators did not recommend increasing the dose of cefazolin from 2 g to 3 g in obese patients.21,22
The studies discussed above are difficult to compare for 3 reasons. First, each study used a different MIC of cefazolin for both gram-positive and gram-negative bacteria. Second, the authors sampled different maternal tissues or serum at varying times during the cesarean delivery. Third, the studies did not specifically investigate, or were not powered sufficiently to address, the more important clinical outcome of surgical site infection. In a recent historical cohort study, Ward and Duff were unable to show that increasing the dose of cefazolin to 2 g in all women with a BMI <30 kg m2 and to 3 g in all women with a BMI >30 kg m2 reduced the rate of endometritis and wound infection below the level already achieved with combined prophylaxis with cefazolin (1 g) plus azithromycin (500 mg).15
Sutton and colleagues recently assessed the pharmacokinetics of azithromycin when used as prophylaxis for cesarean delivery.23 They studied 30 women who had a scheduled cesarean delivery and who received a 500-mg intravenous dose of azithromycin that was initiated 15, 30, or 60 minutes before the surgical incision and then infused over 1 hour. They obtained maternal plasma samples multiple times during the first 8 hours after surgery. They also obtained samples of amniotic fluid, placenta, myometrium, adipose tissue, and umbilical cord blood intraoperatively. The median concentration of azithromycin in adipose tissue was 102 ng/g, which is below the MIC50 for Ureaplasma species (250 ng/mL). The median concentration in myometrial tissue was 402 ng/g. The concentration in maternal plasma consistently exceeded the MIC50 for Ureaplasma species.
What the evidence says
All women, regardless of weight,
CASE Resolved
For the 26-year-old obese laboring patient about to undergo cesarean delivery, reasonable steps for prevention of infection include removing the hair at the incision site with clippers or depilatory cream immediately prior to the start of surgery; cleansing the abdomen with a chlorhexidine-alcohol solution; and administering cefazolin (2 g) plus azithromycin (500 mg) preoperatively.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Cruse PJ, Foord R. A five‑year prospective study of 23,649 surgical wounds. Arch Surg. 1973;107(2):206–210.
- Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine‑alcohol versus povidone‑iodine for surgical‑site antisepsis. N Engl J Med. 2010;362(1):18–26.
- Ngai IM, Van Arsdale A, Govindappagari S, et al. Skin preparation for prevention of surgical site infection after cesarean delivery. Obstet Gynecol. 2015;126(6):1251–1257.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–655.
- Duff P. Prophylactic antibiotics for cesarean delivery: a simple cost‑effective strategy for prevention of postoperative morbidity. Am J Obstet Gynecol. 1987;157(4 pt 1):794–798.
- Dinsmoor MJ, Gilbert S, Landon MB, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development Maternal‑Fetal Medicine Units Network. Perioperative antibiotic prophylaxis for nonlaboring cesarean delivery. Obstet Gynecol. 2009;114(4):752–756.
- Burke JF. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery. 1961;50:161–168.
- Gordon HR, Phelps D, Blanchard K. Prophylactic cesarean section antibiotics: maternal and neonatal morbidity before or after cord clamping. Obstet Gynecol. 1979;53(2):151–156.
- Cunningham FG, Leveno KJ, DePalma RT, Roark M, Rosenfeld CR. Perioperative antimicrobials for cesarean delivery: before or after cord clamping? Obstet Gynecol. 1983;62(2):151–154.
- Sullivan SA, Smith T, Chang E, Hulsey T, Vandorsten JP, Soper D. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2007;196(5):455.e1–e5.
- Costantine MM, Rahman M, Ghulmiyah L, et al. Timing of perioperative antibiotics for cesarean delivery: a metaanalysis. Am J Obstet Gynecol. 2008;199(3):301.e1–e6.
- Owens SM, Brozanski BS, Meyn LA, Wiesenfeld HC. Antimicrobial prophylaxis for cesarean delivery before skin incision. Obstet Gynecol. 2009;114(3):573–579.
- Tita AT, Hauth JC, Grimes A, Owen J, Stamm AM, Andrews WW. Decreasing incidence of postcesarean endometritis with extended‑spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111(1):51–56.
- Tita AT, Owen J, Stamm AM, Grimes A, Hauth JC, Andrews WW. Impact of extended‑spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008;199(3):303.e1–e3.
- Ward E, Duff P. A comparison of 3 antibiotic regimens for prevention of postcesarean endometritis: an historical cohort study. Am J Obstet Gynecol. 2016;214(6):751.e1–e4.
- Tita AT, Szychowski JM, Boggess K, et al; C/SOAP Trial Consortium. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375(13):1231–1241.
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegel KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006:295(13):1549–1555.
- Swank ML, Wing DA, Nicolau DP, McNulty JA. Increased 3‑gram cefazolin dosing for cesarean delivery prophylaxis in obese women. Am J Obstet Gynecol. 2015;213(3):415.e1–e8.
- Liu P, Derendorf H. Antimicrobial tissue concentrations. Infect Dis Clin North Am. 2003:17(3):599–613.
- Pevzner L, Swank M, Krepel C, Wing DA, Chan K, Edmiston CE Jr. Effects of maternal obesity on tissue concentrations of prophylactic cefazolin during cesarean delivery. Obstet Gynecol. 2011;117(4):877–882.
- Young OM, Shaik IH, Twedt R, et al. Pharmacokinetics of cefazolin prophylaxis in obese gravidae at time of cesarean delivery. Am J Obstet Gynecol. 2015;213(4):541.e1–e7.
- Maggio L, Nicolau DP, DaCosta M, Rouse DJ, Hughes BL. Cefazolin prophylaxis in obese women undergoing cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;125(5):1205–1210.
- Sutton AL, Acosta EP, Larson KB, Kerstner‑Wood CD, Tita AT, Biggio JR. Perinatal pharmacokinetics of azithromycin for cesarean prophylaxis. Am J Obstet Gynecol. 2015;212(6):812. e1–e6.
Cesarean delivery is now the most commonly performed major operation in hospitals across the United States. Approximately 30% of the 4 million deliveries that occur each year are by cesarean. Endometritis and wound infection (superficial and deep surgical site infection) are the most common postoperative complications of cesarean delivery. These 2 infections usually can be treated in a straightforward manner with antibiotics or surgical drainage. In some cases, however, they can lead to serious sequelae, such as pelvic abscess, septic pelvic vein thrombophlebitis, and wound dehiscence/evisceration, thereby prolonging the patient’s hospitalization and significantly increasing medical expenses.
Accordingly, in the past 50 years many investigators have proposed various specific measures to reduce the risk of postcesarean infection. In this article, we critically evaluate 2 of these major interventions: methods of skin preparation and administration of prophylactic antibiotics. In part 2 of this series next month, we will review the evidence regarding preoperative bathing with an antiseptic, preoperative vaginal cleansing with an antiseptic solution, methods of placental extraction, closure of the deep subcutaneous layer of the abdomen, and closure of the skin.
CASE Cesarean delivery required for nonprogressing labor
A 26-year-old obese primigravid woman, body mass index (BMI) 37 kg m2, at 40 weeks’ gestation has been in labor for 20 hours. Her membranes have been ruptured for 16 hours. Her cervix is completely effaced and is 7 cm dilated. The fetal head is at −1 cm station. Her cervical examination findings have not changed in 4 hours despite adequate uterine contractility documented by intrauterine pressure catheter. You are now ready to proceed with cesarean delivery, and you want to do everything possible to prevent the patient from developing a postoperative infection.
What are the best practices for postcesarean infection prevention in this patient?

Skin preparation
Adequate preoperative skin preparation is an important first step in preventing post‑ cesarean infection.
How should you prepare the patient’s skin for surgery?
Two issues to address when preparing the abdominal wall for surgery are hair removal and skin cleansing. More than 40 years ago, Cruse and Foord definitively answered the question about hair removal.1 In a landmark cohort investigation of more than 23,000 patients having many different types of operative procedures, they demonstrated that shaving the hair on the evening before surgery resulted in a higher rate of wound infection than clipping the hair, removing the hair with a depilatory cream just before surgery, or not removing the hair at all.
Three recent investigations have thoughtfully addressed the issue of skin cleansing. Darouiche and colleagues conducted a prospective, randomized, multicenter trial comparing chlorhexidine-alcohol with povidone-iodine for skin preparation before surgery.2 Their investigation included 849 patients having many different types of surgical procedures, only a minority of which were in obstetric and gynecologic patients. They demonstrated fewer superficial wound infections in patients in the chlorhexidine-alcohol group (4.2% vs 8.6%, P = .008). Of even greater importance, patients in the chlorhexidine-alcohol group had fewer deep wound infections (1% vs 3%, P = .005).
Ngai and co-workers recently reported the results of a randomized controlled trial (RCT) in which women undergoing nonurgent cesarean delivery had their skin cleansed with povidone-iodine with alcohol, chlorhexidine with alcohol, or the sequential combination of both solutions.3 The overall rate of surgical site infection was just 4.3%. The 3 groups had comparable infection rates and, accordingly, the authors were unable to conclude that one type of skin preparation was superior to the other.
The most informative recent investigation was by Tuuli and colleagues, who evaluated 1,147 patients having cesarean delivery assigned to undergo skin preparation with either chlorhexidine-alcohol or iodine-alcohol.4 Unlike the study by Ngai and co-workers, in this study approximately 40% of the patients in each treatment arm had unscheduled, urgent cesarean deliveries.3,4 Overall, the rate of infection in the chlorhexidine-alcohol group was 4.0% compared with 7.3% in the iodine-alcohol group (relative risk [RR], 0.55; 95% confidence interval [CI], 0.34–0.90, P = .02).
What the evidence says
Based on the evidence cited above, we advise removing hair at the incision site with clippers or depilatory cream just before the start of surgery. The abdomen should then be cleansed with a chlorhexidine-alcohol solution (Level I Evidence, Level 1A Recommendation; TABLE).

Antibiotic prophylaxis
Questions to consider regarding antibiotic prophylaxis for cesarean delivery include appropriateness of treatment, antibiotic(s) selection, timing of administration, dose, and special circumstances.
Should you give the patient prophylactic antibiotics?
Prophylactic antibiotics are justified for surgical procedures whenever 3 major criteria are met5:
- the surgical site is inevitably contaminated with bacteria
- in the absence of prophylaxis, the frequency of infection at the operative site is unacceptably high
- operative site infections have the potential to lead to serious, potentially life-threatening sequelae.
Without a doubt, all 3 of these criteria are fulfilled when considering either urgent or nonurgent cesarean delivery. When cesarean delivery follows a long labor complicated by ruptured membranes, multiple internal vaginal examinations, and internal fetal monitoring, the operative site is inevitably contaminated with hundreds of thousands of pathogenic bacteria. Even when cesarean delivery is scheduled to occur before the onset of labor and ruptured membranes, a high concentration of vaginal organisms is introduced into the uterine and pelvic cavities coincident with making the hysterotomy incision.6
In the era before prophylactic antibiotics were used routinely, postoperative infection rates in some highly indigent patient populations approached 85%.5 Finally, as noted previously, postcesarean endometritis may progress to pelvic abscess formation, septic pelvic vein thrombophlebitis, and septic shock; wound infections may be complicated by dehiscence and evisceration.
When should you administer antibiotics: Before the surgical incision or after cord clamping?
More than 50 years ago, Burke conducted the classic sequence of basic science experiments that forms the foundation for use of prophylactic antibiotics.7 Using a guinea pig model, he showed that prophylactic antibiotics exert their most pronounced effect when they are administered before the surgical incision is made and before bacterial contamination occurs. Prophylaxis that is delayed more than 4 hours after the start of surgery will likely be ineffective.
Interestingly, however, when clinicians first began using prophylactic antibiotics for cesarean delivery, some investigators expressed concern about the possible exposure of the neonate to antibiotics just before delivery—specifically, whether this exposure would increase the frequency of evaluations for suspected sepsis or would promote resistance among organisms that would make neonatal sepsis more difficult to treat.
Gordon and colleagues published an important report in 1979 that showed that preoperative administration of ampicillin did not increase the frequency of immediate or delayed neonatal infections.8 However, delaying the administration of ampicillin until after the umbilical cord was clamped was just as effective in preventing post‑cesarean endometritis. Subsequently, Cunningham and co-workers showed that preoperative administration of prophylactic antibiotics significantly increased the frequency of sepsis workups in exposed neonates compared with infants with no preoperative antibiotic exposure (28% vs 15%; P<.025).9 Based on these 2 reports, obstetricians adopted a policy of delaying antibiotic administration until after the infant’s umbilical cord was clamped.
In 2007, Sullivan and colleagues challenged this long-standing practice.10 In a carefully designed prospective, randomized, double-blind trial, they showed that patients who received preoperative cefazolin had a significant reduction in the frequency of endometritis compared with women who received the same antibiotic after cord clamping (1% vs 5%; RR, 0.2; 95% CI, 0.2–0.94). The rate of wound infection was lower in the preoperative antibiotic group (3% vs 5%), but this difference did not reach statistical significance. The total infection-related morbidity was significantly reduced in women who received antibiotics preoperatively (4.0% vs 11.5%; RR, 0.4; 95% CI, 0.18–0.87). Additionally, there was no increase in the frequency of proven or suspected neonatal infection in the infants exposed to antibiotics before delivery.
Subsequent to the publication by Sullivan and colleagues, other reports have confirmed that administration of antibiotics prior to surgery is superior to administration after clamping of the umbilical cord.10–12 Thus, we have come full circle back to Burke’s principle established more than a half century ago.7
Which antibiotic(s) should you administer for prophylaxis, and how many doses?
In an earlier review, one of us (PD) examined the evidence regarding choice of antibiotics and number of doses, concluding that a single dose of a first-generation cephalosporin, such as cefazolin, was the preferred regimen.5 The single dose was comparable in effectiveness to 2- or 3-dose regimens and to single- or multiple-dose regimens of broader-spectrum agents. For more than 20 years now, the standard of care for antibiotic prophylaxis has been a single 1- to 2-g dose of cefazolin.
Several recent reports, however, have raised the question of whether the prophylactic effect could be enhanced if the spectrum of activity of the antibiotic regimen was broadened to include an agent effective against Ureaplasma species.
Tita and colleagues evaluated an indigent patient population with an inherently high rate of postoperative infection; they showed that adding azithromycin 500 mg to cefazolin significantly reduced the rate of postcesarean endometritis.13 In a follow-up report from the same institution, Tita and co-workers demonstrated that adding azithromycin also significantly reduced the frequency of wound infection.14 In both of these investigations, the antibiotics were administered after cord clamping.
In a subsequent report, Ward and Duff15 showed that the combination of azithromycin plus cefazolin administered preoperatively resulted in a very low rate of both endometritis and wound infection in a population similar to that studied by Tita et al.13,14
Very recently, Tita and associates published the results of the Cesarean Section Optimal Antibiotic Prophylaxis (C/SOAP) trial conducted at 14 US hospitals.16 This study included 2,013 women undergoing cesarean delivery during labor or after membrane rupture who were randomly assigned to receive intravenous azithromycin 500 mg (n = 1,019) or placebo (n = 994). All women also received standard antibiotic prophylaxis with cefazolin. The primary outcome (a composite of endometritis, wound infection, or other infection within 6 weeks) was significantly lower in the azithromycin group than in the placebo group (6.1% vs 12.0%, P<.001). In addition, there were significant differences between the treatment groups in the rates of endometritis (3.8% in the azithromycin group vs 6.1% in the placebo group, P = .02) as well as in the rates of wound infection (2.4% vs 6.6%, respectively, P<.001). Of additional note, there were no differences between the 2 groups in the composite neonatal outcome of death and serious neonatal complications (14.3% vs 13.6%, P = .63).The investigators concluded that extended-spectrum prophylaxis with adjunctive azithromycin safely reduces infection rates without raising the risk of neonatal adverse outcomes.
What the evidence says
We conclude that all patients, even those having a scheduled cesarean before the onset of labor or ruptured membranes, should receive prophylactic antibiotics in a single dose administered preoperatively rather than after cord clamping (Level I Evidence, Level 1A Recommendation; TABLE). In high-risk populations (eg, women in labor with ruptured membranes who are having an urgent cesarean), for whom the baseline risk of infection is high, administer the combination of cefazolin plus azithromycin in lieu of cefazolin alone (Level I Evidence, Level 1A Recommendation; TABLE).
If the patient has a history of an immediate hypersensitivity reaction to beta-lactam antibiotics, we recommend the combination of clindamycin (900 mg) plus gentamicin (1.5 mg/kg) as a single infusion prior to surgery. We base this recommendation on the need to provide reasonable coverage against a broad range of pathogens. Clindamycin covers gram-positive aerobes, such as staphylococci species and group B streptococci, and anaerobes; gentamicin covers aerobic gram-negative bacilli. A single agent, such as clindamycin or metronidazole, does not provide the broad-based coverage necessary for effective prophylaxis (Level III Evidence, Level 1C Recommendation; TABLE).
If the patient is overweight or obese, should you modify the antibiotic dose?
The prevalence of obesity in the United States continues to increase. One-third of all US reproductive-aged women are obese, and 6% of women are extremely obese.17 Obesity increases the risk of postcesarean infection 3- to 5- fold.18 Because both pregnancy and obesity increase the total volume of a drug’s distribution, achieving adequate antibiotic tissue concentrations may be hindered by a dilutional effect. Furthermore, pharmacokinetic studies consistently have shown that the tissue concentration of an antibiotic—which, ideally, should be above the minimum inhibitory concentration (MIC) for common bacteria—determines the susceptibility of those tissues to infection, regardless of whether the serum concentration of the antibiotic is in the therapeutic range.19
These concerns have led to several recent investigations evaluating different doses of cefazolin for obese patients. Pevzner and colleagues conducted a prospective cohort study of 29 women having a scheduled cesarean delivery.20 The patients were divided into 3 groups: lean (BMI <30 kg m2), obese (BMI 30.0–39.9 kg m2), and extremely obese (BMI >40 kg m2). All women received a 2-g dose of cefazolin 30 to 60 minutes before surgery. Cefazolin concentrations in adipose tissue obtained at the time of skin incision were inversely proportional to maternal BMI (r, −0.67; P<.001). All specimens demonstrated a therapeutic concentration (>1 µg/g) of cefazolin for gram-positive cocci, but 20% of the obese women and 33% of the extremely obese women did not achieve the MIC (>4 µg/g) for gram-negative bacilli (P = .29 and P = .14, respectively). At the time of skin closure, 20% of obese women and 44% of extremely obese women did not have tissue concentrations that exceeded the MIC for gram-negative bacteria.
Swank and associates conducted a prospective cohort study that included 28 women.18 They demonstrated that, after a 2-g dose of cefazolin, only 20% of the obese women (BMI 30–40 kg m2) and 0% of the extremely obese women (BMI >40 kg m2) achieved an adipose tissue concentration that exceeded the MIC for gram-negative rods (8 µg/mL). However, 100% and 71.4%, respectively, achieved such a tissue concentration after a 3-g dose. When the women were stratified by actual weight, there was a statistically significant difference between those who weighed less than 120 kg and those who weighed more than 120 kg. Seventy-nine percent of the former had a tissue concentration of cefazolin greater than 8 µg/mL compared with 0% of the women who weighed more than 120 kg. Based on these observations, the authors recommended a 3-g dose of cefazolin for women who weigh more than 120 kg.
In a double-blind RCT with 26 obese women (BMI ≥30 kg m2), Young and colleagues demonstrated that, at the time of hysterotomy and fascial closure, significantly higher concentrations of cefazolin were found in the adipose tissue of obese women who received a 3-g dose of antibiotic compared with those who received a 2-g dose.21 However, all concentrations of cefazolin were consistently above the MIC of cefazolin for gram-positive cocci (1 µg/g) and gram-negative bacilli (4 µg/g). Further, Maggio and co-workers conducted a double-blind RCT comparing a 2-g dose of cefazolin versus a 3-g dose in 57 obese women (BMI ≥30 kg m2).22 They found no statistically significant difference in the percentage of women who had tissue concentrations of cefazolin greater than the MIC for gram-positive cocci (8 µg/g). All samples were above the MIC of cefazolin for gram-negative bacilli (2 µg/g). Based on these data, these investigators did not recommend increasing the dose of cefazolin from 2 g to 3 g in obese patients.21,22
The studies discussed above are difficult to compare for 3 reasons. First, each study used a different MIC of cefazolin for both gram-positive and gram-negative bacteria. Second, the authors sampled different maternal tissues or serum at varying times during the cesarean delivery. Third, the studies did not specifically investigate, or were not powered sufficiently to address, the more important clinical outcome of surgical site infection. In a recent historical cohort study, Ward and Duff were unable to show that increasing the dose of cefazolin to 2 g in all women with a BMI <30 kg m2 and to 3 g in all women with a BMI >30 kg m2 reduced the rate of endometritis and wound infection below the level already achieved with combined prophylaxis with cefazolin (1 g) plus azithromycin (500 mg).15
Sutton and colleagues recently assessed the pharmacokinetics of azithromycin when used as prophylaxis for cesarean delivery.23 They studied 30 women who had a scheduled cesarean delivery and who received a 500-mg intravenous dose of azithromycin that was initiated 15, 30, or 60 minutes before the surgical incision and then infused over 1 hour. They obtained maternal plasma samples multiple times during the first 8 hours after surgery. They also obtained samples of amniotic fluid, placenta, myometrium, adipose tissue, and umbilical cord blood intraoperatively. The median concentration of azithromycin in adipose tissue was 102 ng/g, which is below the MIC50 for Ureaplasma species (250 ng/mL). The median concentration in myometrial tissue was 402 ng/g. The concentration in maternal plasma consistently exceeded the MIC50 for Ureaplasma species.
What the evidence says
All women, regardless of weight,
CASE Resolved
For the 26-year-old obese laboring patient about to undergo cesarean delivery, reasonable steps for prevention of infection include removing the hair at the incision site with clippers or depilatory cream immediately prior to the start of surgery; cleansing the abdomen with a chlorhexidine-alcohol solution; and administering cefazolin (2 g) plus azithromycin (500 mg) preoperatively.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Cesarean delivery is now the most commonly performed major operation in hospitals across the United States. Approximately 30% of the 4 million deliveries that occur each year are by cesarean. Endometritis and wound infection (superficial and deep surgical site infection) are the most common postoperative complications of cesarean delivery. These 2 infections usually can be treated in a straightforward manner with antibiotics or surgical drainage. In some cases, however, they can lead to serious sequelae, such as pelvic abscess, septic pelvic vein thrombophlebitis, and wound dehiscence/evisceration, thereby prolonging the patient’s hospitalization and significantly increasing medical expenses.
Accordingly, in the past 50 years many investigators have proposed various specific measures to reduce the risk of postcesarean infection. In this article, we critically evaluate 2 of these major interventions: methods of skin preparation and administration of prophylactic antibiotics. In part 2 of this series next month, we will review the evidence regarding preoperative bathing with an antiseptic, preoperative vaginal cleansing with an antiseptic solution, methods of placental extraction, closure of the deep subcutaneous layer of the abdomen, and closure of the skin.
CASE Cesarean delivery required for nonprogressing labor
A 26-year-old obese primigravid woman, body mass index (BMI) 37 kg m2, at 40 weeks’ gestation has been in labor for 20 hours. Her membranes have been ruptured for 16 hours. Her cervix is completely effaced and is 7 cm dilated. The fetal head is at −1 cm station. Her cervical examination findings have not changed in 4 hours despite adequate uterine contractility documented by intrauterine pressure catheter. You are now ready to proceed with cesarean delivery, and you want to do everything possible to prevent the patient from developing a postoperative infection.
What are the best practices for postcesarean infection prevention in this patient?

Skin preparation
Adequate preoperative skin preparation is an important first step in preventing post‑ cesarean infection.
How should you prepare the patient’s skin for surgery?
Two issues to address when preparing the abdominal wall for surgery are hair removal and skin cleansing. More than 40 years ago, Cruse and Foord definitively answered the question about hair removal.1 In a landmark cohort investigation of more than 23,000 patients having many different types of operative procedures, they demonstrated that shaving the hair on the evening before surgery resulted in a higher rate of wound infection than clipping the hair, removing the hair with a depilatory cream just before surgery, or not removing the hair at all.
Three recent investigations have thoughtfully addressed the issue of skin cleansing. Darouiche and colleagues conducted a prospective, randomized, multicenter trial comparing chlorhexidine-alcohol with povidone-iodine for skin preparation before surgery.2 Their investigation included 849 patients having many different types of surgical procedures, only a minority of which were in obstetric and gynecologic patients. They demonstrated fewer superficial wound infections in patients in the chlorhexidine-alcohol group (4.2% vs 8.6%, P = .008). Of even greater importance, patients in the chlorhexidine-alcohol group had fewer deep wound infections (1% vs 3%, P = .005).
Ngai and co-workers recently reported the results of a randomized controlled trial (RCT) in which women undergoing nonurgent cesarean delivery had their skin cleansed with povidone-iodine with alcohol, chlorhexidine with alcohol, or the sequential combination of both solutions.3 The overall rate of surgical site infection was just 4.3%. The 3 groups had comparable infection rates and, accordingly, the authors were unable to conclude that one type of skin preparation was superior to the other.
The most informative recent investigation was by Tuuli and colleagues, who evaluated 1,147 patients having cesarean delivery assigned to undergo skin preparation with either chlorhexidine-alcohol or iodine-alcohol.4 Unlike the study by Ngai and co-workers, in this study approximately 40% of the patients in each treatment arm had unscheduled, urgent cesarean deliveries.3,4 Overall, the rate of infection in the chlorhexidine-alcohol group was 4.0% compared with 7.3% in the iodine-alcohol group (relative risk [RR], 0.55; 95% confidence interval [CI], 0.34–0.90, P = .02).
What the evidence says
Based on the evidence cited above, we advise removing hair at the incision site with clippers or depilatory cream just before the start of surgery. The abdomen should then be cleansed with a chlorhexidine-alcohol solution (Level I Evidence, Level 1A Recommendation; TABLE).

Antibiotic prophylaxis
Questions to consider regarding antibiotic prophylaxis for cesarean delivery include appropriateness of treatment, antibiotic(s) selection, timing of administration, dose, and special circumstances.
Should you give the patient prophylactic antibiotics?
Prophylactic antibiotics are justified for surgical procedures whenever 3 major criteria are met5:
- the surgical site is inevitably contaminated with bacteria
- in the absence of prophylaxis, the frequency of infection at the operative site is unacceptably high
- operative site infections have the potential to lead to serious, potentially life-threatening sequelae.
Without a doubt, all 3 of these criteria are fulfilled when considering either urgent or nonurgent cesarean delivery. When cesarean delivery follows a long labor complicated by ruptured membranes, multiple internal vaginal examinations, and internal fetal monitoring, the operative site is inevitably contaminated with hundreds of thousands of pathogenic bacteria. Even when cesarean delivery is scheduled to occur before the onset of labor and ruptured membranes, a high concentration of vaginal organisms is introduced into the uterine and pelvic cavities coincident with making the hysterotomy incision.6
In the era before prophylactic antibiotics were used routinely, postoperative infection rates in some highly indigent patient populations approached 85%.5 Finally, as noted previously, postcesarean endometritis may progress to pelvic abscess formation, septic pelvic vein thrombophlebitis, and septic shock; wound infections may be complicated by dehiscence and evisceration.
When should you administer antibiotics: Before the surgical incision or after cord clamping?
More than 50 years ago, Burke conducted the classic sequence of basic science experiments that forms the foundation for use of prophylactic antibiotics.7 Using a guinea pig model, he showed that prophylactic antibiotics exert their most pronounced effect when they are administered before the surgical incision is made and before bacterial contamination occurs. Prophylaxis that is delayed more than 4 hours after the start of surgery will likely be ineffective.
Interestingly, however, when clinicians first began using prophylactic antibiotics for cesarean delivery, some investigators expressed concern about the possible exposure of the neonate to antibiotics just before delivery—specifically, whether this exposure would increase the frequency of evaluations for suspected sepsis or would promote resistance among organisms that would make neonatal sepsis more difficult to treat.
Gordon and colleagues published an important report in 1979 that showed that preoperative administration of ampicillin did not increase the frequency of immediate or delayed neonatal infections.8 However, delaying the administration of ampicillin until after the umbilical cord was clamped was just as effective in preventing post‑cesarean endometritis. Subsequently, Cunningham and co-workers showed that preoperative administration of prophylactic antibiotics significantly increased the frequency of sepsis workups in exposed neonates compared with infants with no preoperative antibiotic exposure (28% vs 15%; P<.025).9 Based on these 2 reports, obstetricians adopted a policy of delaying antibiotic administration until after the infant’s umbilical cord was clamped.
In 2007, Sullivan and colleagues challenged this long-standing practice.10 In a carefully designed prospective, randomized, double-blind trial, they showed that patients who received preoperative cefazolin had a significant reduction in the frequency of endometritis compared with women who received the same antibiotic after cord clamping (1% vs 5%; RR, 0.2; 95% CI, 0.2–0.94). The rate of wound infection was lower in the preoperative antibiotic group (3% vs 5%), but this difference did not reach statistical significance. The total infection-related morbidity was significantly reduced in women who received antibiotics preoperatively (4.0% vs 11.5%; RR, 0.4; 95% CI, 0.18–0.87). Additionally, there was no increase in the frequency of proven or suspected neonatal infection in the infants exposed to antibiotics before delivery.
Subsequent to the publication by Sullivan and colleagues, other reports have confirmed that administration of antibiotics prior to surgery is superior to administration after clamping of the umbilical cord.10–12 Thus, we have come full circle back to Burke’s principle established more than a half century ago.7
Which antibiotic(s) should you administer for prophylaxis, and how many doses?
In an earlier review, one of us (PD) examined the evidence regarding choice of antibiotics and number of doses, concluding that a single dose of a first-generation cephalosporin, such as cefazolin, was the preferred regimen.5 The single dose was comparable in effectiveness to 2- or 3-dose regimens and to single- or multiple-dose regimens of broader-spectrum agents. For more than 20 years now, the standard of care for antibiotic prophylaxis has been a single 1- to 2-g dose of cefazolin.
Several recent reports, however, have raised the question of whether the prophylactic effect could be enhanced if the spectrum of activity of the antibiotic regimen was broadened to include an agent effective against Ureaplasma species.
Tita and colleagues evaluated an indigent patient population with an inherently high rate of postoperative infection; they showed that adding azithromycin 500 mg to cefazolin significantly reduced the rate of postcesarean endometritis.13 In a follow-up report from the same institution, Tita and co-workers demonstrated that adding azithromycin also significantly reduced the frequency of wound infection.14 In both of these investigations, the antibiotics were administered after cord clamping.
In a subsequent report, Ward and Duff15 showed that the combination of azithromycin plus cefazolin administered preoperatively resulted in a very low rate of both endometritis and wound infection in a population similar to that studied by Tita et al.13,14
Very recently, Tita and associates published the results of the Cesarean Section Optimal Antibiotic Prophylaxis (C/SOAP) trial conducted at 14 US hospitals.16 This study included 2,013 women undergoing cesarean delivery during labor or after membrane rupture who were randomly assigned to receive intravenous azithromycin 500 mg (n = 1,019) or placebo (n = 994). All women also received standard antibiotic prophylaxis with cefazolin. The primary outcome (a composite of endometritis, wound infection, or other infection within 6 weeks) was significantly lower in the azithromycin group than in the placebo group (6.1% vs 12.0%, P<.001). In addition, there were significant differences between the treatment groups in the rates of endometritis (3.8% in the azithromycin group vs 6.1% in the placebo group, P = .02) as well as in the rates of wound infection (2.4% vs 6.6%, respectively, P<.001). Of additional note, there were no differences between the 2 groups in the composite neonatal outcome of death and serious neonatal complications (14.3% vs 13.6%, P = .63).The investigators concluded that extended-spectrum prophylaxis with adjunctive azithromycin safely reduces infection rates without raising the risk of neonatal adverse outcomes.
What the evidence says
We conclude that all patients, even those having a scheduled cesarean before the onset of labor or ruptured membranes, should receive prophylactic antibiotics in a single dose administered preoperatively rather than after cord clamping (Level I Evidence, Level 1A Recommendation; TABLE). In high-risk populations (eg, women in labor with ruptured membranes who are having an urgent cesarean), for whom the baseline risk of infection is high, administer the combination of cefazolin plus azithromycin in lieu of cefazolin alone (Level I Evidence, Level 1A Recommendation; TABLE).
If the patient has a history of an immediate hypersensitivity reaction to beta-lactam antibiotics, we recommend the combination of clindamycin (900 mg) plus gentamicin (1.5 mg/kg) as a single infusion prior to surgery. We base this recommendation on the need to provide reasonable coverage against a broad range of pathogens. Clindamycin covers gram-positive aerobes, such as staphylococci species and group B streptococci, and anaerobes; gentamicin covers aerobic gram-negative bacilli. A single agent, such as clindamycin or metronidazole, does not provide the broad-based coverage necessary for effective prophylaxis (Level III Evidence, Level 1C Recommendation; TABLE).
If the patient is overweight or obese, should you modify the antibiotic dose?
The prevalence of obesity in the United States continues to increase. One-third of all US reproductive-aged women are obese, and 6% of women are extremely obese.17 Obesity increases the risk of postcesarean infection 3- to 5- fold.18 Because both pregnancy and obesity increase the total volume of a drug’s distribution, achieving adequate antibiotic tissue concentrations may be hindered by a dilutional effect. Furthermore, pharmacokinetic studies consistently have shown that the tissue concentration of an antibiotic—which, ideally, should be above the minimum inhibitory concentration (MIC) for common bacteria—determines the susceptibility of those tissues to infection, regardless of whether the serum concentration of the antibiotic is in the therapeutic range.19
These concerns have led to several recent investigations evaluating different doses of cefazolin for obese patients. Pevzner and colleagues conducted a prospective cohort study of 29 women having a scheduled cesarean delivery.20 The patients were divided into 3 groups: lean (BMI <30 kg m2), obese (BMI 30.0–39.9 kg m2), and extremely obese (BMI >40 kg m2). All women received a 2-g dose of cefazolin 30 to 60 minutes before surgery. Cefazolin concentrations in adipose tissue obtained at the time of skin incision were inversely proportional to maternal BMI (r, −0.67; P<.001). All specimens demonstrated a therapeutic concentration (>1 µg/g) of cefazolin for gram-positive cocci, but 20% of the obese women and 33% of the extremely obese women did not achieve the MIC (>4 µg/g) for gram-negative bacilli (P = .29 and P = .14, respectively). At the time of skin closure, 20% of obese women and 44% of extremely obese women did not have tissue concentrations that exceeded the MIC for gram-negative bacteria.
Swank and associates conducted a prospective cohort study that included 28 women.18 They demonstrated that, after a 2-g dose of cefazolin, only 20% of the obese women (BMI 30–40 kg m2) and 0% of the extremely obese women (BMI >40 kg m2) achieved an adipose tissue concentration that exceeded the MIC for gram-negative rods (8 µg/mL). However, 100% and 71.4%, respectively, achieved such a tissue concentration after a 3-g dose. When the women were stratified by actual weight, there was a statistically significant difference between those who weighed less than 120 kg and those who weighed more than 120 kg. Seventy-nine percent of the former had a tissue concentration of cefazolin greater than 8 µg/mL compared with 0% of the women who weighed more than 120 kg. Based on these observations, the authors recommended a 3-g dose of cefazolin for women who weigh more than 120 kg.
In a double-blind RCT with 26 obese women (BMI ≥30 kg m2), Young and colleagues demonstrated that, at the time of hysterotomy and fascial closure, significantly higher concentrations of cefazolin were found in the adipose tissue of obese women who received a 3-g dose of antibiotic compared with those who received a 2-g dose.21 However, all concentrations of cefazolin were consistently above the MIC of cefazolin for gram-positive cocci (1 µg/g) and gram-negative bacilli (4 µg/g). Further, Maggio and co-workers conducted a double-blind RCT comparing a 2-g dose of cefazolin versus a 3-g dose in 57 obese women (BMI ≥30 kg m2).22 They found no statistically significant difference in the percentage of women who had tissue concentrations of cefazolin greater than the MIC for gram-positive cocci (8 µg/g). All samples were above the MIC of cefazolin for gram-negative bacilli (2 µg/g). Based on these data, these investigators did not recommend increasing the dose of cefazolin from 2 g to 3 g in obese patients.21,22
The studies discussed above are difficult to compare for 3 reasons. First, each study used a different MIC of cefazolin for both gram-positive and gram-negative bacteria. Second, the authors sampled different maternal tissues or serum at varying times during the cesarean delivery. Third, the studies did not specifically investigate, or were not powered sufficiently to address, the more important clinical outcome of surgical site infection. In a recent historical cohort study, Ward and Duff were unable to show that increasing the dose of cefazolin to 2 g in all women with a BMI <30 kg m2 and to 3 g in all women with a BMI >30 kg m2 reduced the rate of endometritis and wound infection below the level already achieved with combined prophylaxis with cefazolin (1 g) plus azithromycin (500 mg).15
Sutton and colleagues recently assessed the pharmacokinetics of azithromycin when used as prophylaxis for cesarean delivery.23 They studied 30 women who had a scheduled cesarean delivery and who received a 500-mg intravenous dose of azithromycin that was initiated 15, 30, or 60 minutes before the surgical incision and then infused over 1 hour. They obtained maternal plasma samples multiple times during the first 8 hours after surgery. They also obtained samples of amniotic fluid, placenta, myometrium, adipose tissue, and umbilical cord blood intraoperatively. The median concentration of azithromycin in adipose tissue was 102 ng/g, which is below the MIC50 for Ureaplasma species (250 ng/mL). The median concentration in myometrial tissue was 402 ng/g. The concentration in maternal plasma consistently exceeded the MIC50 for Ureaplasma species.
What the evidence says
All women, regardless of weight,
CASE Resolved
For the 26-year-old obese laboring patient about to undergo cesarean delivery, reasonable steps for prevention of infection include removing the hair at the incision site with clippers or depilatory cream immediately prior to the start of surgery; cleansing the abdomen with a chlorhexidine-alcohol solution; and administering cefazolin (2 g) plus azithromycin (500 mg) preoperatively.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Cruse PJ, Foord R. A five‑year prospective study of 23,649 surgical wounds. Arch Surg. 1973;107(2):206–210.
- Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine‑alcohol versus povidone‑iodine for surgical‑site antisepsis. N Engl J Med. 2010;362(1):18–26.
- Ngai IM, Van Arsdale A, Govindappagari S, et al. Skin preparation for prevention of surgical site infection after cesarean delivery. Obstet Gynecol. 2015;126(6):1251–1257.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–655.
- Duff P. Prophylactic antibiotics for cesarean delivery: a simple cost‑effective strategy for prevention of postoperative morbidity. Am J Obstet Gynecol. 1987;157(4 pt 1):794–798.
- Dinsmoor MJ, Gilbert S, Landon MB, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development Maternal‑Fetal Medicine Units Network. Perioperative antibiotic prophylaxis for nonlaboring cesarean delivery. Obstet Gynecol. 2009;114(4):752–756.
- Burke JF. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery. 1961;50:161–168.
- Gordon HR, Phelps D, Blanchard K. Prophylactic cesarean section antibiotics: maternal and neonatal morbidity before or after cord clamping. Obstet Gynecol. 1979;53(2):151–156.
- Cunningham FG, Leveno KJ, DePalma RT, Roark M, Rosenfeld CR. Perioperative antimicrobials for cesarean delivery: before or after cord clamping? Obstet Gynecol. 1983;62(2):151–154.
- Sullivan SA, Smith T, Chang E, Hulsey T, Vandorsten JP, Soper D. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2007;196(5):455.e1–e5.
- Costantine MM, Rahman M, Ghulmiyah L, et al. Timing of perioperative antibiotics for cesarean delivery: a metaanalysis. Am J Obstet Gynecol. 2008;199(3):301.e1–e6.
- Owens SM, Brozanski BS, Meyn LA, Wiesenfeld HC. Antimicrobial prophylaxis for cesarean delivery before skin incision. Obstet Gynecol. 2009;114(3):573–579.
- Tita AT, Hauth JC, Grimes A, Owen J, Stamm AM, Andrews WW. Decreasing incidence of postcesarean endometritis with extended‑spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111(1):51–56.
- Tita AT, Owen J, Stamm AM, Grimes A, Hauth JC, Andrews WW. Impact of extended‑spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008;199(3):303.e1–e3.
- Ward E, Duff P. A comparison of 3 antibiotic regimens for prevention of postcesarean endometritis: an historical cohort study. Am J Obstet Gynecol. 2016;214(6):751.e1–e4.
- Tita AT, Szychowski JM, Boggess K, et al; C/SOAP Trial Consortium. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375(13):1231–1241.
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegel KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006:295(13):1549–1555.
- Swank ML, Wing DA, Nicolau DP, McNulty JA. Increased 3‑gram cefazolin dosing for cesarean delivery prophylaxis in obese women. Am J Obstet Gynecol. 2015;213(3):415.e1–e8.
- Liu P, Derendorf H. Antimicrobial tissue concentrations. Infect Dis Clin North Am. 2003:17(3):599–613.
- Pevzner L, Swank M, Krepel C, Wing DA, Chan K, Edmiston CE Jr. Effects of maternal obesity on tissue concentrations of prophylactic cefazolin during cesarean delivery. Obstet Gynecol. 2011;117(4):877–882.
- Young OM, Shaik IH, Twedt R, et al. Pharmacokinetics of cefazolin prophylaxis in obese gravidae at time of cesarean delivery. Am J Obstet Gynecol. 2015;213(4):541.e1–e7.
- Maggio L, Nicolau DP, DaCosta M, Rouse DJ, Hughes BL. Cefazolin prophylaxis in obese women undergoing cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;125(5):1205–1210.
- Sutton AL, Acosta EP, Larson KB, Kerstner‑Wood CD, Tita AT, Biggio JR. Perinatal pharmacokinetics of azithromycin for cesarean prophylaxis. Am J Obstet Gynecol. 2015;212(6):812. e1–e6.
- Cruse PJ, Foord R. A five‑year prospective study of 23,649 surgical wounds. Arch Surg. 1973;107(2):206–210.
- Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine‑alcohol versus povidone‑iodine for surgical‑site antisepsis. N Engl J Med. 2010;362(1):18–26.
- Ngai IM, Van Arsdale A, Govindappagari S, et al. Skin preparation for prevention of surgical site infection after cesarean delivery. Obstet Gynecol. 2015;126(6):1251–1257.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–655.
- Duff P. Prophylactic antibiotics for cesarean delivery: a simple cost‑effective strategy for prevention of postoperative morbidity. Am J Obstet Gynecol. 1987;157(4 pt 1):794–798.
- Dinsmoor MJ, Gilbert S, Landon MB, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development Maternal‑Fetal Medicine Units Network. Perioperative antibiotic prophylaxis for nonlaboring cesarean delivery. Obstet Gynecol. 2009;114(4):752–756.
- Burke JF. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery. 1961;50:161–168.
- Gordon HR, Phelps D, Blanchard K. Prophylactic cesarean section antibiotics: maternal and neonatal morbidity before or after cord clamping. Obstet Gynecol. 1979;53(2):151–156.
- Cunningham FG, Leveno KJ, DePalma RT, Roark M, Rosenfeld CR. Perioperative antimicrobials for cesarean delivery: before or after cord clamping? Obstet Gynecol. 1983;62(2):151–154.
- Sullivan SA, Smith T, Chang E, Hulsey T, Vandorsten JP, Soper D. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2007;196(5):455.e1–e5.
- Costantine MM, Rahman M, Ghulmiyah L, et al. Timing of perioperative antibiotics for cesarean delivery: a metaanalysis. Am J Obstet Gynecol. 2008;199(3):301.e1–e6.
- Owens SM, Brozanski BS, Meyn LA, Wiesenfeld HC. Antimicrobial prophylaxis for cesarean delivery before skin incision. Obstet Gynecol. 2009;114(3):573–579.
- Tita AT, Hauth JC, Grimes A, Owen J, Stamm AM, Andrews WW. Decreasing incidence of postcesarean endometritis with extended‑spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111(1):51–56.
- Tita AT, Owen J, Stamm AM, Grimes A, Hauth JC, Andrews WW. Impact of extended‑spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008;199(3):303.e1–e3.
- Ward E, Duff P. A comparison of 3 antibiotic regimens for prevention of postcesarean endometritis: an historical cohort study. Am J Obstet Gynecol. 2016;214(6):751.e1–e4.
- Tita AT, Szychowski JM, Boggess K, et al; C/SOAP Trial Consortium. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375(13):1231–1241.
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegel KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006:295(13):1549–1555.
- Swank ML, Wing DA, Nicolau DP, McNulty JA. Increased 3‑gram cefazolin dosing for cesarean delivery prophylaxis in obese women. Am J Obstet Gynecol. 2015;213(3):415.e1–e8.
- Liu P, Derendorf H. Antimicrobial tissue concentrations. Infect Dis Clin North Am. 2003:17(3):599–613.
- Pevzner L, Swank M, Krepel C, Wing DA, Chan K, Edmiston CE Jr. Effects of maternal obesity on tissue concentrations of prophylactic cefazolin during cesarean delivery. Obstet Gynecol. 2011;117(4):877–882.
- Young OM, Shaik IH, Twedt R, et al. Pharmacokinetics of cefazolin prophylaxis in obese gravidae at time of cesarean delivery. Am J Obstet Gynecol. 2015;213(4):541.e1–e7.
- Maggio L, Nicolau DP, DaCosta M, Rouse DJ, Hughes BL. Cefazolin prophylaxis in obese women undergoing cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;125(5):1205–1210.
- Sutton AL, Acosta EP, Larson KB, Kerstner‑Wood CD, Tita AT, Biggio JR. Perinatal pharmacokinetics of azithromycin for cesarean prophylaxis. Am J Obstet Gynecol. 2015;212(6):812. e1–e6.
In this Article
- Prepping the skin for surgery
- Selecting the antibiotic(s) for infection prevention
- Prophylaxis for the obese patient
Tips for sleep hygiene: A handout for patients
Are you in search of materials that can reinforce what you’ve told patients about how to get a good night’s sleep? Then download this handout, which includes 8 tips that cover the wake-promoting agents to avoid, the proper environment in which to go to sleep, and the dos and don’ts of before-bedtime activities. It also discusses when patients should seek professional help for a possible sleep disorder. This PDF from Neurology Reviews is available at: http://www.mdedge.com/neurologyreviews/article/115138/sleep-medicine/tips-sleep-hygiene/pdf.
Are you in search of materials that can reinforce what you’ve told patients about how to get a good night’s sleep? Then download this handout, which includes 8 tips that cover the wake-promoting agents to avoid, the proper environment in which to go to sleep, and the dos and don’ts of before-bedtime activities. It also discusses when patients should seek professional help for a possible sleep disorder. This PDF from Neurology Reviews is available at: http://www.mdedge.com/neurologyreviews/article/115138/sleep-medicine/tips-sleep-hygiene/pdf.
Are you in search of materials that can reinforce what you’ve told patients about how to get a good night’s sleep? Then download this handout, which includes 8 tips that cover the wake-promoting agents to avoid, the proper environment in which to go to sleep, and the dos and don’ts of before-bedtime activities. It also discusses when patients should seek professional help for a possible sleep disorder. This PDF from Neurology Reviews is available at: http://www.mdedge.com/neurologyreviews/article/115138/sleep-medicine/tips-sleep-hygiene/pdf.
Direct-acting antivirals: One of several keys to HCV eradication by 2030
Can the public health threat posed by the hepatitis C virus (HCV) be eliminated by 2030? Researchers in Italy say it can be done. Important elements of success will include the use of oral direct-acting antivirals and a global commitment to prevention. Earlier this year, the World Health Organization (WHO) announced plans to wipe out HCV worldwide by 2030 using the time between now and 2021 to reduce the number of annual new infections by 70%, and to slash the fatality rate by 60%. Find out what success in meeting the WHO challenge will hinge on by going to Family Practice News: http://www.mdedge.com/familypracticenews/article/114780/gastroenterology/direct-acting-antivirals-one-several-keys-hcv.
Can the public health threat posed by the hepatitis C virus (HCV) be eliminated by 2030? Researchers in Italy say it can be done. Important elements of success will include the use of oral direct-acting antivirals and a global commitment to prevention. Earlier this year, the World Health Organization (WHO) announced plans to wipe out HCV worldwide by 2030 using the time between now and 2021 to reduce the number of annual new infections by 70%, and to slash the fatality rate by 60%. Find out what success in meeting the WHO challenge will hinge on by going to Family Practice News: http://www.mdedge.com/familypracticenews/article/114780/gastroenterology/direct-acting-antivirals-one-several-keys-hcv.
Can the public health threat posed by the hepatitis C virus (HCV) be eliminated by 2030? Researchers in Italy say it can be done. Important elements of success will include the use of oral direct-acting antivirals and a global commitment to prevention. Earlier this year, the World Health Organization (WHO) announced plans to wipe out HCV worldwide by 2030 using the time between now and 2021 to reduce the number of annual new infections by 70%, and to slash the fatality rate by 60%. Find out what success in meeting the WHO challenge will hinge on by going to Family Practice News: http://www.mdedge.com/familypracticenews/article/114780/gastroenterology/direct-acting-antivirals-one-several-keys-hcv.
Palliative care boosts heart failure patient outcomes
Systematic introduction of palliative care interventions for patients with advanced heart failure improved patients’ quality of life and spurred their development of advanced-care preferences in a pair of independently performed, controlled pilot studies. But, despite demonstrating the ability of palliative-care interventions to help heart failure patients during their final months of life, the findings raised questions about the generalizability and reproducibility of palliative-care interventions that may depend upon the skills and experience of the individual specialists who deliver the care. To learn more about these 2 studies, go to Family Practice News: http://www.mdedge.com/familypracticenews/article/115737/cardiology/palliative-care-boosts-heart-failure-patient-outcomes.
Systematic introduction of palliative care interventions for patients with advanced heart failure improved patients’ quality of life and spurred their development of advanced-care preferences in a pair of independently performed, controlled pilot studies. But, despite demonstrating the ability of palliative-care interventions to help heart failure patients during their final months of life, the findings raised questions about the generalizability and reproducibility of palliative-care interventions that may depend upon the skills and experience of the individual specialists who deliver the care. To learn more about these 2 studies, go to Family Practice News: http://www.mdedge.com/familypracticenews/article/115737/cardiology/palliative-care-boosts-heart-failure-patient-outcomes.
Systematic introduction of palliative care interventions for patients with advanced heart failure improved patients’ quality of life and spurred their development of advanced-care preferences in a pair of independently performed, controlled pilot studies. But, despite demonstrating the ability of palliative-care interventions to help heart failure patients during their final months of life, the findings raised questions about the generalizability and reproducibility of palliative-care interventions that may depend upon the skills and experience of the individual specialists who deliver the care. To learn more about these 2 studies, go to Family Practice News: http://www.mdedge.com/familypracticenews/article/115737/cardiology/palliative-care-boosts-heart-failure-patient-outcomes.
Early menopause a risk factor for type 2 diabetes
Early age at menopause was associated with the incidence of type 2 diabetes, independent of obesity and a host of other potentially confounding factors, according to a prospect cohort study. “This association is independent of potential intermediate risk factors: obesity, insulin, glucose, inflammation, but also estradiol and other endogenous sex hormone levels,” said Taulant Muka, MD, PhD, a postdoctoral fellow at Erasmus Medical College, Rotterdam, the Netherlands. Among the 3210 participants in the study, 319 incident cases were identified over the median 10.9-year follow-up period, with a relative risk for incident diabetes of 2.29 for women undergoing menopause before age 40, and 1.49 for those experiencing menopause between the ages of 40 and 44. Read more at Family Practice News: http://www.mdedge.com/familypracticenews/article/115648/diabetes/early-menopause-risk-factor-type-2-diabetes.
Early age at menopause was associated with the incidence of type 2 diabetes, independent of obesity and a host of other potentially confounding factors, according to a prospect cohort study. “This association is independent of potential intermediate risk factors: obesity, insulin, glucose, inflammation, but also estradiol and other endogenous sex hormone levels,” said Taulant Muka, MD, PhD, a postdoctoral fellow at Erasmus Medical College, Rotterdam, the Netherlands. Among the 3210 participants in the study, 319 incident cases were identified over the median 10.9-year follow-up period, with a relative risk for incident diabetes of 2.29 for women undergoing menopause before age 40, and 1.49 for those experiencing menopause between the ages of 40 and 44. Read more at Family Practice News: http://www.mdedge.com/familypracticenews/article/115648/diabetes/early-menopause-risk-factor-type-2-diabetes.
Early age at menopause was associated with the incidence of type 2 diabetes, independent of obesity and a host of other potentially confounding factors, according to a prospect cohort study. “This association is independent of potential intermediate risk factors: obesity, insulin, glucose, inflammation, but also estradiol and other endogenous sex hormone levels,” said Taulant Muka, MD, PhD, a postdoctoral fellow at Erasmus Medical College, Rotterdam, the Netherlands. Among the 3210 participants in the study, 319 incident cases were identified over the median 10.9-year follow-up period, with a relative risk for incident diabetes of 2.29 for women undergoing menopause before age 40, and 1.49 for those experiencing menopause between the ages of 40 and 44. Read more at Family Practice News: http://www.mdedge.com/familypracticenews/article/115648/diabetes/early-menopause-risk-factor-type-2-diabetes.
Depression drops COPD medication adherence
Patients with chronic obstructive pulmonary disease (COPD) who also suffer from depression are less likely to take their COPD maintenance medications, according to a review of Medicare claims by researchers at the University of Maryland, Baltimore. Researchers found that patients with newly diagnosed depression were about 7% less likely to have good adherence to their medications. For more on this research, see the article in CHEST Physician, available at http://www.mdedge.com/chestphysician/article/115659/depression/depression-drops-copd-medication-adherence.
Patients with chronic obstructive pulmonary disease (COPD) who also suffer from depression are less likely to take their COPD maintenance medications, according to a review of Medicare claims by researchers at the University of Maryland, Baltimore. Researchers found that patients with newly diagnosed depression were about 7% less likely to have good adherence to their medications. For more on this research, see the article in CHEST Physician, available at http://www.mdedge.com/chestphysician/article/115659/depression/depression-drops-copd-medication-adherence.
Patients with chronic obstructive pulmonary disease (COPD) who also suffer from depression are less likely to take their COPD maintenance medications, according to a review of Medicare claims by researchers at the University of Maryland, Baltimore. Researchers found that patients with newly diagnosed depression were about 7% less likely to have good adherence to their medications. For more on this research, see the article in CHEST Physician, available at http://www.mdedge.com/chestphysician/article/115659/depression/depression-drops-copd-medication-adherence.
Rivaroxaban linked to more bleeding compared with dabigatran in elderly patients with nonvalvular AF
Rivaroxaban is associated with significantly more intra- and extracranial bleeding than dabigatran in patients ages 75 and older with nonvalvular atrial fibrillation (AF), according to a recent report published online in JAMA Internal Medicine. During the study period, rivaroxaban was used 2 to 3 times more often than dabigatran in AF patients in the United States. According to David J. Graham, MD, Center for Drug Evaluation and Research, FDA, that may be “partly because of prescriber misperceptions about bleeding risks with dabigatran, arising from FDA receipt of a large number of post-marketing case reports following its approval.” That’s ironic, according to Graham, since “we [now find] substantially higher bleeding risks with the use of rivaroxaban than dabigatran.” Further analysis on the data can be found in the article from Family Practice News: http://www.mdedge.com/familypracticenews/article/115021/acquired-cardiovascular-disease/rivaroxaban-linked-more-bleeding.
Rivaroxaban is associated with significantly more intra- and extracranial bleeding than dabigatran in patients ages 75 and older with nonvalvular atrial fibrillation (AF), according to a recent report published online in JAMA Internal Medicine. During the study period, rivaroxaban was used 2 to 3 times more often than dabigatran in AF patients in the United States. According to David J. Graham, MD, Center for Drug Evaluation and Research, FDA, that may be “partly because of prescriber misperceptions about bleeding risks with dabigatran, arising from FDA receipt of a large number of post-marketing case reports following its approval.” That’s ironic, according to Graham, since “we [now find] substantially higher bleeding risks with the use of rivaroxaban than dabigatran.” Further analysis on the data can be found in the article from Family Practice News: http://www.mdedge.com/familypracticenews/article/115021/acquired-cardiovascular-disease/rivaroxaban-linked-more-bleeding.
Rivaroxaban is associated with significantly more intra- and extracranial bleeding than dabigatran in patients ages 75 and older with nonvalvular atrial fibrillation (AF), according to a recent report published online in JAMA Internal Medicine. During the study period, rivaroxaban was used 2 to 3 times more often than dabigatran in AF patients in the United States. According to David J. Graham, MD, Center for Drug Evaluation and Research, FDA, that may be “partly because of prescriber misperceptions about bleeding risks with dabigatran, arising from FDA receipt of a large number of post-marketing case reports following its approval.” That’s ironic, according to Graham, since “we [now find] substantially higher bleeding risks with the use of rivaroxaban than dabigatran.” Further analysis on the data can be found in the article from Family Practice News: http://www.mdedge.com/familypracticenews/article/115021/acquired-cardiovascular-disease/rivaroxaban-linked-more-bleeding.
VIDEO: Bioresorbable Absorb unexpectedly humbled by metallic DES
WASHINGTON – The bioabsorbable vascular scaffold bubble suddenly burst with the first 3-year follow-up data from a randomized trial that unexpectedly showed that the Absorb device significantly underperformed compared with Xience, a widely-used, second-generation metallic drug-eluting stent.
“As a pioneer of BVS [bioresorbable vascular scaffold] I’m disappointed,” Patrick W. Serruys, MD, said at the Transcatheter Cardiovascular Therapeutics annual meeting. “The performance of the comparator stent was spectacular.”
Xience surpassed Absorb in several other secondary endpoints. For example, the in-device binary restenosis rate was 7.0% with Absorb and 0.7% with Xience; the in-segment binary restenosis rate was 8% with Absorb and 3% with Xience. Target-vessel MIs occurred in 7% of the Absorb patients and 1% of the Xience patients, while clinically indicated target-lesion revascularization occurred in 6% of the Absorb patients and 1% of the Xience patients.
Another notable finding was that definite or probable in-device thrombosis occurred in nine Absorb patents and in none of the Xience patients, a statistically significant difference. Six of the Absorb thrombotic events occurred more than 1 year after the device was placed, and in several instances these thromboses occurred more than 900 days after placement, when the BVS had largely resorbed.
“These thromboses are occurring at the late stages of BVS degradation,” Dr. Serruys noted. “The Absorb polymer is basically gone after 3 years, but it’s replaced by a proteoglycan, and some proteoglycans are quite thrombogenic,” a possible explanation for the “mysterious” very late thromboses, he said.
These “disappointing” results my be linked to inadequate lesion preparation, appropriate sizing of the BVS for the lesion, and inconsistent postdilatation of the BVS, three steps that became the guiding mantra for BVS use starting a couple of years ago, said Giulio G. Stefanini, MD, an interventional cardiologist at Humanitas Research Hospital in Milan and a discussant for the report at the meeting, which was sponsored by the Cardiovascular Research Foundation.
Dr. Stefanini said that even though the Absorb stent became available for routine use in Europe starting in 2012, the device gained little traction since then in his own practice and throughout Italy. Currently it’s used for fewer than 5% of coronary interventions in Italy, he estimated. That’s largely because “we have failed to identify a population that benefits.” Other issues include the extra time needed to place a BVS, and the need for longer treatment with dual antiplatelet therapy for patients who receive a BVS, compared with when they receive a modern metallic drug-eluting stent. The Absorb BVS received Food and Drug Administration approval for routine U.S. use in July 2016.
“It would be beautiful to have a fully bioresorbable stent. It’s a lovely concept, but we’re not there yet,” Dr. Stefanini observed.
ABSORB II was sponsored by Abbott Vascular, which markets the Absorb device. Dr. Serruys has received research support from Abbott Vascular and has been a consultant to several other device and drug companies. Dr. Stefanini has been a consultant to Boston Scientific, B.Braun, and Edwards.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
We were all disappointed by the ABSORB II 3-year results. It was really surprising that even the vasomotion endpoint was, if anything, a little better with Xience, which performed amazingly well in this trial. Both arms of the study did well out to 3 years, but the Xience patients did better.
The Absorb bioresorbable vascular scaffold (BVS) is an early-stage device, and based on these results I wouldn’t give up on the BVS concept. But we need to be very careful with Absorb and which patients we implant with it. We need to make sure we carefully use thorough lesion preparation, correct sizing, and postdilatation in every patient, and we need to carefully select the right patients.
The main issue with the Absorb BVS in this trial was scaffold thrombosis, so we need to use a BVS only in patients with the lowest thrombosis risk, which means younger patients without renal failure, calcified vessels, and a larger-diameter target coronary artery. Younger patients have the most to gain from receiving a BVS. Younger patients who need a coronary intervention often collect several stents over the balance of their life, and it’s in these patients where you’d prefer that the stents eventually disappear.
Paul S. Teirstein, MD , is chief of cardiology and director of interventional cardiology at the Scripps Clinic in La Jolla, Calif. He has received research support from and has been a consultant to Abbott Vascular, Boston Scientific, and Medtronic. He made these comments in an interview .
We were all disappointed by the ABSORB II 3-year results. It was really surprising that even the vasomotion endpoint was, if anything, a little better with Xience, which performed amazingly well in this trial. Both arms of the study did well out to 3 years, but the Xience patients did better.
The Absorb bioresorbable vascular scaffold (BVS) is an early-stage device, and based on these results I wouldn’t give up on the BVS concept. But we need to be very careful with Absorb and which patients we implant with it. We need to make sure we carefully use thorough lesion preparation, correct sizing, and postdilatation in every patient, and we need to carefully select the right patients.
The main issue with the Absorb BVS in this trial was scaffold thrombosis, so we need to use a BVS only in patients with the lowest thrombosis risk, which means younger patients without renal failure, calcified vessels, and a larger-diameter target coronary artery. Younger patients have the most to gain from receiving a BVS. Younger patients who need a coronary intervention often collect several stents over the balance of their life, and it’s in these patients where you’d prefer that the stents eventually disappear.
Paul S. Teirstein, MD , is chief of cardiology and director of interventional cardiology at the Scripps Clinic in La Jolla, Calif. He has received research support from and has been a consultant to Abbott Vascular, Boston Scientific, and Medtronic. He made these comments in an interview .
We were all disappointed by the ABSORB II 3-year results. It was really surprising that even the vasomotion endpoint was, if anything, a little better with Xience, which performed amazingly well in this trial. Both arms of the study did well out to 3 years, but the Xience patients did better.
The Absorb bioresorbable vascular scaffold (BVS) is an early-stage device, and based on these results I wouldn’t give up on the BVS concept. But we need to be very careful with Absorb and which patients we implant with it. We need to make sure we carefully use thorough lesion preparation, correct sizing, and postdilatation in every patient, and we need to carefully select the right patients.
The main issue with the Absorb BVS in this trial was scaffold thrombosis, so we need to use a BVS only in patients with the lowest thrombosis risk, which means younger patients without renal failure, calcified vessels, and a larger-diameter target coronary artery. Younger patients have the most to gain from receiving a BVS. Younger patients who need a coronary intervention often collect several stents over the balance of their life, and it’s in these patients where you’d prefer that the stents eventually disappear.
Paul S. Teirstein, MD , is chief of cardiology and director of interventional cardiology at the Scripps Clinic in La Jolla, Calif. He has received research support from and has been a consultant to Abbott Vascular, Boston Scientific, and Medtronic. He made these comments in an interview .
WASHINGTON – The bioabsorbable vascular scaffold bubble suddenly burst with the first 3-year follow-up data from a randomized trial that unexpectedly showed that the Absorb device significantly underperformed compared with Xience, a widely-used, second-generation metallic drug-eluting stent.
“As a pioneer of BVS [bioresorbable vascular scaffold] I’m disappointed,” Patrick W. Serruys, MD, said at the Transcatheter Cardiovascular Therapeutics annual meeting. “The performance of the comparator stent was spectacular.”
Xience surpassed Absorb in several other secondary endpoints. For example, the in-device binary restenosis rate was 7.0% with Absorb and 0.7% with Xience; the in-segment binary restenosis rate was 8% with Absorb and 3% with Xience. Target-vessel MIs occurred in 7% of the Absorb patients and 1% of the Xience patients, while clinically indicated target-lesion revascularization occurred in 6% of the Absorb patients and 1% of the Xience patients.
Another notable finding was that definite or probable in-device thrombosis occurred in nine Absorb patents and in none of the Xience patients, a statistically significant difference. Six of the Absorb thrombotic events occurred more than 1 year after the device was placed, and in several instances these thromboses occurred more than 900 days after placement, when the BVS had largely resorbed.
“These thromboses are occurring at the late stages of BVS degradation,” Dr. Serruys noted. “The Absorb polymer is basically gone after 3 years, but it’s replaced by a proteoglycan, and some proteoglycans are quite thrombogenic,” a possible explanation for the “mysterious” very late thromboses, he said.
These “disappointing” results my be linked to inadequate lesion preparation, appropriate sizing of the BVS for the lesion, and inconsistent postdilatation of the BVS, three steps that became the guiding mantra for BVS use starting a couple of years ago, said Giulio G. Stefanini, MD, an interventional cardiologist at Humanitas Research Hospital in Milan and a discussant for the report at the meeting, which was sponsored by the Cardiovascular Research Foundation.
Dr. Stefanini said that even though the Absorb stent became available for routine use in Europe starting in 2012, the device gained little traction since then in his own practice and throughout Italy. Currently it’s used for fewer than 5% of coronary interventions in Italy, he estimated. That’s largely because “we have failed to identify a population that benefits.” Other issues include the extra time needed to place a BVS, and the need for longer treatment with dual antiplatelet therapy for patients who receive a BVS, compared with when they receive a modern metallic drug-eluting stent. The Absorb BVS received Food and Drug Administration approval for routine U.S. use in July 2016.
“It would be beautiful to have a fully bioresorbable stent. It’s a lovely concept, but we’re not there yet,” Dr. Stefanini observed.
ABSORB II was sponsored by Abbott Vascular, which markets the Absorb device. Dr. Serruys has received research support from Abbott Vascular and has been a consultant to several other device and drug companies. Dr. Stefanini has been a consultant to Boston Scientific, B.Braun, and Edwards.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
WASHINGTON – The bioabsorbable vascular scaffold bubble suddenly burst with the first 3-year follow-up data from a randomized trial that unexpectedly showed that the Absorb device significantly underperformed compared with Xience, a widely-used, second-generation metallic drug-eluting stent.
“As a pioneer of BVS [bioresorbable vascular scaffold] I’m disappointed,” Patrick W. Serruys, MD, said at the Transcatheter Cardiovascular Therapeutics annual meeting. “The performance of the comparator stent was spectacular.”
Xience surpassed Absorb in several other secondary endpoints. For example, the in-device binary restenosis rate was 7.0% with Absorb and 0.7% with Xience; the in-segment binary restenosis rate was 8% with Absorb and 3% with Xience. Target-vessel MIs occurred in 7% of the Absorb patients and 1% of the Xience patients, while clinically indicated target-lesion revascularization occurred in 6% of the Absorb patients and 1% of the Xience patients.
Another notable finding was that definite or probable in-device thrombosis occurred in nine Absorb patents and in none of the Xience patients, a statistically significant difference. Six of the Absorb thrombotic events occurred more than 1 year after the device was placed, and in several instances these thromboses occurred more than 900 days after placement, when the BVS had largely resorbed.
“These thromboses are occurring at the late stages of BVS degradation,” Dr. Serruys noted. “The Absorb polymer is basically gone after 3 years, but it’s replaced by a proteoglycan, and some proteoglycans are quite thrombogenic,” a possible explanation for the “mysterious” very late thromboses, he said.
These “disappointing” results my be linked to inadequate lesion preparation, appropriate sizing of the BVS for the lesion, and inconsistent postdilatation of the BVS, three steps that became the guiding mantra for BVS use starting a couple of years ago, said Giulio G. Stefanini, MD, an interventional cardiologist at Humanitas Research Hospital in Milan and a discussant for the report at the meeting, which was sponsored by the Cardiovascular Research Foundation.
Dr. Stefanini said that even though the Absorb stent became available for routine use in Europe starting in 2012, the device gained little traction since then in his own practice and throughout Italy. Currently it’s used for fewer than 5% of coronary interventions in Italy, he estimated. That’s largely because “we have failed to identify a population that benefits.” Other issues include the extra time needed to place a BVS, and the need for longer treatment with dual antiplatelet therapy for patients who receive a BVS, compared with when they receive a modern metallic drug-eluting stent. The Absorb BVS received Food and Drug Administration approval for routine U.S. use in July 2016.
“It would be beautiful to have a fully bioresorbable stent. It’s a lovely concept, but we’re not there yet,” Dr. Stefanini observed.
ABSORB II was sponsored by Abbott Vascular, which markets the Absorb device. Dr. Serruys has received research support from Abbott Vascular and has been a consultant to several other device and drug companies. Dr. Stefanini has been a consultant to Boston Scientific, B.Braun, and Edwards.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
Key clinical point:
Major finding: In-stent or in-scaffold late luminal loss averaged 0.25 mm with Xience and 0.37 mm with Absorb, a statistically significant difference.
Data source: ABSORB II, a multicenter, randomized trial that enrolled 501 patients.
Disclosures: ABSORB II was sponsored by Abbott Vascular, which markets the Absorb device. Dr. Serruys has received research support from Abbott Vascular and has been a consultant to several other device and drug companies. Dr. Stefanini has been a consultant to Boston Scientific, B.Braun and Edwards.
Does one particular cesarean technique confer better maternal and neonatal outcomes?
EXPERT COMMENTARY
John M. Thorp Jr, MD, McAllister Distinguished Professor, Division Director, General Obstetrics and Gynecology, Vice Chair of Research, Department of Ob-Gyn, University of North Carolina Schools of Medicine and Public Health, Chapel Hill.
Five years ago one of our interns operating with the director of labor and delivery challenged him as to why we were not using evidenced-based surgical techniques for cesarean delivery. Bruised by the formidable (and at times misleading) club of “evidence-based medicine” that is held as sacrosanct by the modern obstetrician, the director responded to the charge by researching a systematic review on abdominal delivery that amalgamated studies of poor quality with precious few trials. He unilaterally decided that we needed an opening in the transparent portion of the drape overlying the incision site so that we might use “evidence” to prevent operative site infection. The end result: No change in the incidence of wound infections, and adhesive drapes that did not adhere well, thereby displacing the effluent of amniotic fluid and blood that are part of a cesarean delivery back into the first assistant’s socks, shoes, and clothing. It was as if the clock had been turned back to my early years as an attending when we had cloth drapes. So much for having an evidence-based protocol. I was thus elated at reading the results of the CORONIS trial.
Details of the study
The CORONIS trial, in which investigators randomly assigned almost 16,000 women from 7 countries (Argentina, Chile, Ghana, India, Kenya, Pakistan, and Sudan), used a sophisticated factorial design and followed up 13,153 (84%) of the women for 3 years. The investigators tested an array of technical questions about 5 intervention pairs used during abdominal delivery and reported the main outcomes of interest for each intervention, including:
- blunt versus sharp abdominal entry—no evidence of a difference in risk of abdominal hernias (adjusted risk ratio [RR], 0.66; 95% confidence interval [CI], 0.39–1.11)
- exteriorization of the uterus versus intra-abdominal repair—no evidence of a difference in risk of infertility (RR, 0.91; 95% CI, 0.71–1.18) or of ectopic pregnancy (RR, 0.50; CI, 0.15–1.66)
- single- versus double-layer closure of the uterus—no evidence of a difference in maternal death (RR, 0.78; 95% CI, 0.46–1.32) or a composite of pregnancy complications (RR, 1.20; 95% CI, 0.75–1.90)
- closure versus nonclosure of the peritoneum—no evidence of a difference in any outcomes relating to symptoms associated with pelvic adhesions, such as infertility (RR, 0.8; 95% CI, 0.61–1.06)
- chromic catgut versus polyglactin-910 sutures—no evidence of a difference in the main comparisons for adverse pregnancy outcomes in a subsequent pregnancy, such as uterine rupture (RR, 3.05; 95% CI, 0.32–29.29).
Strengths and limitations. The CORONIS trial included a large number of participants and had comprehensive follow-up, a rigorous data collection process, and the participation of many countries. The trial’s participating centers, however, were mostly large referral hospitals with high research interest; adverse outcomes might have been higher in other settings. As well, a lower incidence of subsequent pregnancy among participants limited the study’s power to detect differences in outcomes between the intervention pairs.
Conclusions. None of the alternative techniques produced any real benefits despite syntheses-suggested benefit reported in systematic reviews. Surgeon preference for cesarean delivery techniques likely will continue to guide clinical practice along with economic and institution factors.
A word to the wise: Evidence is not created equally, and pushing it into lumps does not increase its value.
--John M. Thorp Jr, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
John M. Thorp Jr, MD, McAllister Distinguished Professor, Division Director, General Obstetrics and Gynecology, Vice Chair of Research, Department of Ob-Gyn, University of North Carolina Schools of Medicine and Public Health, Chapel Hill.
Five years ago one of our interns operating with the director of labor and delivery challenged him as to why we were not using evidenced-based surgical techniques for cesarean delivery. Bruised by the formidable (and at times misleading) club of “evidence-based medicine” that is held as sacrosanct by the modern obstetrician, the director responded to the charge by researching a systematic review on abdominal delivery that amalgamated studies of poor quality with precious few trials. He unilaterally decided that we needed an opening in the transparent portion of the drape overlying the incision site so that we might use “evidence” to prevent operative site infection. The end result: No change in the incidence of wound infections, and adhesive drapes that did not adhere well, thereby displacing the effluent of amniotic fluid and blood that are part of a cesarean delivery back into the first assistant’s socks, shoes, and clothing. It was as if the clock had been turned back to my early years as an attending when we had cloth drapes. So much for having an evidence-based protocol. I was thus elated at reading the results of the CORONIS trial.
Details of the study
The CORONIS trial, in which investigators randomly assigned almost 16,000 women from 7 countries (Argentina, Chile, Ghana, India, Kenya, Pakistan, and Sudan), used a sophisticated factorial design and followed up 13,153 (84%) of the women for 3 years. The investigators tested an array of technical questions about 5 intervention pairs used during abdominal delivery and reported the main outcomes of interest for each intervention, including:
- blunt versus sharp abdominal entry—no evidence of a difference in risk of abdominal hernias (adjusted risk ratio [RR], 0.66; 95% confidence interval [CI], 0.39–1.11)
- exteriorization of the uterus versus intra-abdominal repair—no evidence of a difference in risk of infertility (RR, 0.91; 95% CI, 0.71–1.18) or of ectopic pregnancy (RR, 0.50; CI, 0.15–1.66)
- single- versus double-layer closure of the uterus—no evidence of a difference in maternal death (RR, 0.78; 95% CI, 0.46–1.32) or a composite of pregnancy complications (RR, 1.20; 95% CI, 0.75–1.90)
- closure versus nonclosure of the peritoneum—no evidence of a difference in any outcomes relating to symptoms associated with pelvic adhesions, such as infertility (RR, 0.8; 95% CI, 0.61–1.06)
- chromic catgut versus polyglactin-910 sutures—no evidence of a difference in the main comparisons for adverse pregnancy outcomes in a subsequent pregnancy, such as uterine rupture (RR, 3.05; 95% CI, 0.32–29.29).
Strengths and limitations. The CORONIS trial included a large number of participants and had comprehensive follow-up, a rigorous data collection process, and the participation of many countries. The trial’s participating centers, however, were mostly large referral hospitals with high research interest; adverse outcomes might have been higher in other settings. As well, a lower incidence of subsequent pregnancy among participants limited the study’s power to detect differences in outcomes between the intervention pairs.
Conclusions. None of the alternative techniques produced any real benefits despite syntheses-suggested benefit reported in systematic reviews. Surgeon preference for cesarean delivery techniques likely will continue to guide clinical practice along with economic and institution factors.
A word to the wise: Evidence is not created equally, and pushing it into lumps does not increase its value.
--John M. Thorp Jr, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
John M. Thorp Jr, MD, McAllister Distinguished Professor, Division Director, General Obstetrics and Gynecology, Vice Chair of Research, Department of Ob-Gyn, University of North Carolina Schools of Medicine and Public Health, Chapel Hill.
Five years ago one of our interns operating with the director of labor and delivery challenged him as to why we were not using evidenced-based surgical techniques for cesarean delivery. Bruised by the formidable (and at times misleading) club of “evidence-based medicine” that is held as sacrosanct by the modern obstetrician, the director responded to the charge by researching a systematic review on abdominal delivery that amalgamated studies of poor quality with precious few trials. He unilaterally decided that we needed an opening in the transparent portion of the drape overlying the incision site so that we might use “evidence” to prevent operative site infection. The end result: No change in the incidence of wound infections, and adhesive drapes that did not adhere well, thereby displacing the effluent of amniotic fluid and blood that are part of a cesarean delivery back into the first assistant’s socks, shoes, and clothing. It was as if the clock had been turned back to my early years as an attending when we had cloth drapes. So much for having an evidence-based protocol. I was thus elated at reading the results of the CORONIS trial.
Details of the study
The CORONIS trial, in which investigators randomly assigned almost 16,000 women from 7 countries (Argentina, Chile, Ghana, India, Kenya, Pakistan, and Sudan), used a sophisticated factorial design and followed up 13,153 (84%) of the women for 3 years. The investigators tested an array of technical questions about 5 intervention pairs used during abdominal delivery and reported the main outcomes of interest for each intervention, including:
- blunt versus sharp abdominal entry—no evidence of a difference in risk of abdominal hernias (adjusted risk ratio [RR], 0.66; 95% confidence interval [CI], 0.39–1.11)
- exteriorization of the uterus versus intra-abdominal repair—no evidence of a difference in risk of infertility (RR, 0.91; 95% CI, 0.71–1.18) or of ectopic pregnancy (RR, 0.50; CI, 0.15–1.66)
- single- versus double-layer closure of the uterus—no evidence of a difference in maternal death (RR, 0.78; 95% CI, 0.46–1.32) or a composite of pregnancy complications (RR, 1.20; 95% CI, 0.75–1.90)
- closure versus nonclosure of the peritoneum—no evidence of a difference in any outcomes relating to symptoms associated with pelvic adhesions, such as infertility (RR, 0.8; 95% CI, 0.61–1.06)
- chromic catgut versus polyglactin-910 sutures—no evidence of a difference in the main comparisons for adverse pregnancy outcomes in a subsequent pregnancy, such as uterine rupture (RR, 3.05; 95% CI, 0.32–29.29).
Strengths and limitations. The CORONIS trial included a large number of participants and had comprehensive follow-up, a rigorous data collection process, and the participation of many countries. The trial’s participating centers, however, were mostly large referral hospitals with high research interest; adverse outcomes might have been higher in other settings. As well, a lower incidence of subsequent pregnancy among participants limited the study’s power to detect differences in outcomes between the intervention pairs.
Conclusions. None of the alternative techniques produced any real benefits despite syntheses-suggested benefit reported in systematic reviews. Surgeon preference for cesarean delivery techniques likely will continue to guide clinical practice along with economic and institution factors.
A word to the wise: Evidence is not created equally, and pushing it into lumps does not increase its value.
--John M. Thorp Jr, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Your patients are talking: Isn’t it time you take responsibility for your online reputation?
In a web-focused world, it should not take much convincing that monitoring your online reputation is time well spent. For some of us, it may be hard to believe that online reviews have evolved beyond restaurants and plumbers, but today your patients are flocking to the Internet to read and leave reviews about you, your staff, and your services. What can you do to protect your online reputation?
We first addressed this topic in December 2014 (“Using the Internet in your practice. Part 4: Reputation management—how to gather kudos and combat negative online reviews”1). Have you implemented any of the tactics we offered then? We hope that you do take proactive steps to protect your online image.
What is a physician’s most precious asset?
You might answer this question with, “my patients” or “the training and education that I have obtained to practice my craft.” But the real answer is that your most precious asset is your reputation.
Physicians live and die by their reputations. We spend our entire medical careers polishing and protecting this status. The Internet dramatically has altered the way people gather information. It is sad but true that a single comment that only takes a few seconds and a single mouse-click to post can be seen by thousands and ruin that life-long effort.
How are physicians rated on the web?
Online physician reviews are positive 70% to 90% of the time.2 Most physicians have 5 or fewer reviews on any one site.3 Of the approximately 30 sites that monitor physicians and hospitals online, one of the most popular is AngiesList.com. This site requires registration and a fee; a member can review a physician every 6 months. On free websites such as Yelp.com and doctorsscorecard.com the reviewer can comment once. Other sites such as vitals.com or DrScore.com limit the reviews from 1 source, which prevents an angry patient from stuffing the ballot box.2
Pay attention
At a minimum, physicians should be monitoring their reputation by conducting periodic searches—“Googling” their name and practice name—to identify what information is already online. You may find that 3, 4, or even 10 reviews appear on various sites. If you are lucky, these reviews will be positive. Don’t be surprised, however, if 1 or 2 are not. Let’s face it: even the most accredited and experienced physician cannot possibly satisfy every patient who walks through the door.
Neil Baum, MD, and Ron Romano have offered tips on ways to manage online reputations in the past,1 and they urge Ob-Gyns to take an active role in this process in order to increase positive exposure to patients and maintain an active practice. Is active reputation management something that ObGyns are spending their valuable time on? To find out, OBG Management reached out to its Virtual Editorial Board. We found that many readers are paying attention to patient satisfaction. Some are soliciting online reviews and maintaining active upkeep on their online reputation. Here are a few responses we received from practicing ObGyns across the United States.
William E. McGrath Jr, MD, of Fernandina Beach, Florida, says that his office provides patients with a list of 5 popular review websites during their visits, and that approximately 1 in 10 will follow up with a review. Patient reviews are also prominently posted on his practice’s website. The large, private, single-specialty group to which his practice belongs requires patient satisfaction surveys for quality assurance review and insurance contract negotiations. “It is all about physician-patient communication,” he says.
Keith S. Merlin, MD, of Brockton, Massachusetts, says that he has checked online reviews to ensure their accuracy. His practice uses surveys, a suggestion box, and a mystery shopper to measure patient satisfaction, a worthwhile effort he says to understand where the practice is doing well and what needs to be done better.
Wesley Hambright, MD, of Jacksonville, North Carolina, reports that he has established a Google Alert to monitor for new content relating to his practice.
Patrick Pevoto, MD, MBA, of Austin, Texas, informs us that he has just started to think about ways to manage his online reputation. He has created a website and is writing a monthly blog, which he posts on his site. He acknowledges the importance of assessing patient satisfaction in his practice but is not applying large-scale measurement techniques yet. To keep his patients happy, he handles concerns that arise on a personal, case-by-case basis.
John Armstrong, MD, MS, of Napa, California, also reports that management of his online reputation is in the beginning stages. He uses focus groups and feels that listening to his patients when they do comment on their experience is important to his overall practice. Listening helps to “identify areas to improve and reaffirms when we are doing well,” he says. To keep his patients happy, he strives to “give extraordinary care and simply be nice to people.” When issues arise, making it right and being polite are important elements, he asserts.
Delos J. Clow, DO, MS, of Chillicothe, Missouri, does measure patient satisfaction, and feels this is very important to his practice in order to identify and correct any negative trends. He does not actively monitor his practice reputation online.
Robert del Rosario, MD, of Camp Hill, Pennsylvania, similarly does not actively manage an online reputation, but does focus on patient satisfaction. To enhance satisfaction, he tries to de-emphasize the electronic medical record to “make visits more personal and less interrogative.” Additionally, his practice objectively gauges aspects of care that might be able to be improved upon.
Reference
- Romano R, Baum NH. Using the Internet in your practice. Part 4: Reputation management—how to gather kudos and combat negative online reviews. OBG Manag. 2014;26(12):23,24,26,28.
Tell your own story
As physicians, you may not have control over what others say about you, but you can take ownership of your online presence by establishing a website, blog, and social media platforms, ensuring your story is being properly communicated. Without an online presence, you are entirely at the mercy of directory and review sites.
Optimize your website
A site that successfully uses search engine optimization (SEO) will have the upper hand when patients hunt for a physician in your area because the information will be posted at the top of the search page, well above the reviews and listings left by patients and other third-party sources. This is a critical step for your online brand because it will be difficult for other sites to mask your credibility. This should motivate you to develop an online presence, regularly update information, and participate in Internet dialog with other sites.
Generate quality, natural reviews
If your site is in good standing in search results, the next step is to implement a patient reviews strategy to start acquiring positive online reviews. A third-party provider can work with you to launch a local search engine optimization strategy and a natural reviews management program tailored for your practice’s needs. For the most part, however, we do not recommend using an online reputation management company. It is far better and more economical to ask satisfied patients to provide reviews.
At first, you may be tempted to actively petition or solicit reviews through survey software, but this method is manipulative and can lead to reputation problems for your practice. Google actively tracks where reviews originate and uses advanced algorithms to determine the review’s integrity. A petitioned review is classified as less valid, and therefore Google will assume it was not written under the same pretense as a natural, unsolicited review.
Quality customer service and outstanding patient care are often what achieve the organic reviews you are striving for. To encourage a steady flow, administer a process that encourages your most satisfied, loyal patients to review your practice.
Keep the process simple. Capture positive compliments at the point of service. Before a patient leaves your office, hand her a card (FIGURE) with easy steps for posting an online review, or offer her a tablet that links directly to your website review section. If your patient is not computer savvy, ask her to complete a 4- to 5-question survey and give her a clipboard and a pen. Then have a staff member post it on your website.

In Dr. Baum’s practice, there is a poster in every exam room and in the reception area where patients can scan the quick response (QR) code and immediately submit a testimonial. Using this system, the practice is able to collect 3 to 5 positive reviews every day.
A patient pleased with your staff’s service will happily take 5 minutes to submit a review. Acquire 5 to 10 reviews monthly and within a year’s time you will have generated enough positive reviews to negate any damaging comments that inevitably will emerge from time to time.
CASE Patient criticizes physician in a review forum
A physician with a robust Internet presence will have his or her name and the practice appear at the top of search engine results pages (as is the case with Dr. Neil Baum when “urologist” plus “New Orleans” is typed into the Google search engine window). By far most of Dr. Baum’s reviews are positive. In one instance, however, a patient on a physician review website referred to Dr. Baum as “technologically advanced but more motivated to increase his income by performing too many diagnostic tests.”
If you find a negative comment in an online directory or review website, what should you do?
The Office for Civil Rights (OCR) within the US Department of Health and Human Services (HHS) is responsible for handling Health Insurance Portability and Accountability Act of 1996 (HIPAA)1 complaints. Deven McGraw, OCR’s deputy director of health information privacy, states that “just because patients have rated their health provider publicly doesn’t give their health provider permission to rate them in return.”2,3 In fact, some health care providers who responded to poor online reviews ran into trouble with privacy rules established by HIPAA.2,3
Mr. McGraw notes that, when responding to online reviews, health professionals should speak generally about the way they treat patients while complying with HIPAA regulations. He suggests, “If the complaint is about poor patient care … say, ‘I provide all of my patients with good patient care’ and ‘I’ve been reviewed in other contexts and have good reviews.’”2,3
According to Yelp’s senior director of litigation, Aaron Schur, most patient complaints center on practice-based concerns such as wait times, office staff, and billing, not about the medical service delivered. Although most physicians do not respond, says Mr. Schur, those who do, tend to ask patients to discuss the matter in private or to apologize.2,3
What are the consequences of a HIPAA violation?
OCR Director Jocelyn Samuels says that the office’s primary role is to help health providers follow HIPAA regulations.2,5 The OCR can resolve HIPPA violations privately and informally, impose fines of up to $50,000 per violation, or it can file criminal charges against violators.2,4
The majority of the office’s investigation and enforcement of HIPAA has been against large medical data breaches.2,5 Small privacy breaches by large health care providers (eg, CVS, Walmart, Lab Corp, Quest Diagnostics, and others) generally do not result in legal consequences; the providers are privately warned. According to ProPublica, even repeated HIPAA violations tend not to be fined.2,4
Small-scale infractions can be more damaging on a personal level to both patients and physicians. However, the OCR does not typically become involved in privacy breaches that include only a few individuals. Health care providers are rarely punished for small HIPPA breaches; instead, the OCR typically settles for pledges to fix any problems and issues reminders of HIPPA requirements.2,5
Although the OCR is often the only place patients can go to seek vindication, HIPAA does not support the right to sue for violation of personal privacy. People who seek a legal remedy must find another means, which is easier in some states than in others.2,5
Health care providers have tried myriad ways to attempt to combat negative reviews. Some have sued patients, attracting a flood of attention but achieving little legal success. Others have asked patients to remove their complaints.2,3
Best practices
Create and circulate a policy. Medical privacy breaches involving sensitive health details can occur when office or hospital staff share patient information due to personal hostility or lack of understanding of HIPPA policy.2,5 Have a practice policy for responding to online reviews by patients, and make sure the staff members who have access to the practice’s online accounts understand your policy and the possible repercussions of not following it. Teach and continue to remind your staff about HIPPA regulations and hold them to a high ethical level of privacy.
Solicit reviews on an ongoing basis. Jeffrey Segal, a review site critic, says that all reviews are valuable. Physicians should respond carefully to negative comments and encourage satisfied patients to post positive reviews. “’For doctors who get bent out of shape to get rid of negative reviews, it’s a denominator problem,’ he said. ‘If they only have three reviews and two are negative, the denominator is the problem. … If you can figure out a way to cultivate reviews from hundreds of patients rather than a few patients, the problem is solved.’”2,3
CASE Resolved
Dr. Baum never responded directly to the negative patient review, and others he has received. He balances the rare negative response with numerous and plentiful positive responses by making it a practice to encourage reviews from all of his patients.
References
- HIPAA for Professionals. US Department of Health & Human Services. http://www.hhs.gov/hipaa/for-professionals/index.html. Accessed October 11, 2016.
- Hall SD. Providers responding to Yelp reviews must be mindful of HIPAA. FierceHealthcare. http://www.fiercehealthcare.com/it/providers-responding-to-yelp-reviews-must-be-mindful-hipaa. Published May 31, 2016. Accessed October 7, 2016.
- Ornstein C. Stung by Yelp reviews, health providers spill patient secrets. ProPublica. https://www.propublica.org/article/stung-by-yelp-reviews-health -providers-spill-patient-secrets. Published May 27, 2016. Accessed October 11, 2016.
- Ornstein C, Waldman A. Few consequences for health privacy law’s repeat offenders. ProPublica. https://www.propublica.org/article/few-consequences-for-health-privacy-law-repeat-offenders. Published December 29, 2015. Accessed October 11, 2016.
- Ornstein C. Small-scale violations of medical privacy often cause the most harm. ProPublica. https://www.propublica.org/article/small-scale-violations-of-medical-privacy-often-cause-the-most-harm. Published December 10, 2015. Accessed October 11, 2016.
The bottom line
Patients are seeking and leaving reviews about you and your practice online and you need to actively manage your online reputation. Do not let one disgruntled patient ruin your reputation. Our advice: Do not wait for a negative review to begin your reputation management. Take an active role and generate positive reviews to drown out negative remarks made by an occasional patient. This is an inexpensive process that does work.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Romano R, Baum NH. Using the Internet in your practice. Part 4: Reputation management-how to gather kudos and combat negative online reviews. OBG Manag. 2014;26(12):23,24,26,28.
- Lagu T, Hannon NS, Rothberg MB, Lindenauer PK. Patients' evaluations of health care providers in the era of social networking: an analysis of physician rating websites. J Gen Intern Med. 2010;25(9):942-946.
- Gunter J. For better or maybe, worse, patients are judging your care online. OBG Manag. 2011;23(3):47-51.
In a web-focused world, it should not take much convincing that monitoring your online reputation is time well spent. For some of us, it may be hard to believe that online reviews have evolved beyond restaurants and plumbers, but today your patients are flocking to the Internet to read and leave reviews about you, your staff, and your services. What can you do to protect your online reputation?
We first addressed this topic in December 2014 (“Using the Internet in your practice. Part 4: Reputation management—how to gather kudos and combat negative online reviews”1). Have you implemented any of the tactics we offered then? We hope that you do take proactive steps to protect your online image.
What is a physician’s most precious asset?
You might answer this question with, “my patients” or “the training and education that I have obtained to practice my craft.” But the real answer is that your most precious asset is your reputation.
Physicians live and die by their reputations. We spend our entire medical careers polishing and protecting this status. The Internet dramatically has altered the way people gather information. It is sad but true that a single comment that only takes a few seconds and a single mouse-click to post can be seen by thousands and ruin that life-long effort.
How are physicians rated on the web?
Online physician reviews are positive 70% to 90% of the time.2 Most physicians have 5 or fewer reviews on any one site.3 Of the approximately 30 sites that monitor physicians and hospitals online, one of the most popular is AngiesList.com. This site requires registration and a fee; a member can review a physician every 6 months. On free websites such as Yelp.com and doctorsscorecard.com the reviewer can comment once. Other sites such as vitals.com or DrScore.com limit the reviews from 1 source, which prevents an angry patient from stuffing the ballot box.2
Pay attention
At a minimum, physicians should be monitoring their reputation by conducting periodic searches—“Googling” their name and practice name—to identify what information is already online. You may find that 3, 4, or even 10 reviews appear on various sites. If you are lucky, these reviews will be positive. Don’t be surprised, however, if 1 or 2 are not. Let’s face it: even the most accredited and experienced physician cannot possibly satisfy every patient who walks through the door.
Neil Baum, MD, and Ron Romano have offered tips on ways to manage online reputations in the past,1 and they urge Ob-Gyns to take an active role in this process in order to increase positive exposure to patients and maintain an active practice. Is active reputation management something that ObGyns are spending their valuable time on? To find out, OBG Management reached out to its Virtual Editorial Board. We found that many readers are paying attention to patient satisfaction. Some are soliciting online reviews and maintaining active upkeep on their online reputation. Here are a few responses we received from practicing ObGyns across the United States.
William E. McGrath Jr, MD, of Fernandina Beach, Florida, says that his office provides patients with a list of 5 popular review websites during their visits, and that approximately 1 in 10 will follow up with a review. Patient reviews are also prominently posted on his practice’s website. The large, private, single-specialty group to which his practice belongs requires patient satisfaction surveys for quality assurance review and insurance contract negotiations. “It is all about physician-patient communication,” he says.
Keith S. Merlin, MD, of Brockton, Massachusetts, says that he has checked online reviews to ensure their accuracy. His practice uses surveys, a suggestion box, and a mystery shopper to measure patient satisfaction, a worthwhile effort he says to understand where the practice is doing well and what needs to be done better.
Wesley Hambright, MD, of Jacksonville, North Carolina, reports that he has established a Google Alert to monitor for new content relating to his practice.
Patrick Pevoto, MD, MBA, of Austin, Texas, informs us that he has just started to think about ways to manage his online reputation. He has created a website and is writing a monthly blog, which he posts on his site. He acknowledges the importance of assessing patient satisfaction in his practice but is not applying large-scale measurement techniques yet. To keep his patients happy, he handles concerns that arise on a personal, case-by-case basis.
John Armstrong, MD, MS, of Napa, California, also reports that management of his online reputation is in the beginning stages. He uses focus groups and feels that listening to his patients when they do comment on their experience is important to his overall practice. Listening helps to “identify areas to improve and reaffirms when we are doing well,” he says. To keep his patients happy, he strives to “give extraordinary care and simply be nice to people.” When issues arise, making it right and being polite are important elements, he asserts.
Delos J. Clow, DO, MS, of Chillicothe, Missouri, does measure patient satisfaction, and feels this is very important to his practice in order to identify and correct any negative trends. He does not actively monitor his practice reputation online.
Robert del Rosario, MD, of Camp Hill, Pennsylvania, similarly does not actively manage an online reputation, but does focus on patient satisfaction. To enhance satisfaction, he tries to de-emphasize the electronic medical record to “make visits more personal and less interrogative.” Additionally, his practice objectively gauges aspects of care that might be able to be improved upon.
Reference
- Romano R, Baum NH. Using the Internet in your practice. Part 4: Reputation management—how to gather kudos and combat negative online reviews. OBG Manag. 2014;26(12):23,24,26,28.
Tell your own story
As physicians, you may not have control over what others say about you, but you can take ownership of your online presence by establishing a website, blog, and social media platforms, ensuring your story is being properly communicated. Without an online presence, you are entirely at the mercy of directory and review sites.
Optimize your website
A site that successfully uses search engine optimization (SEO) will have the upper hand when patients hunt for a physician in your area because the information will be posted at the top of the search page, well above the reviews and listings left by patients and other third-party sources. This is a critical step for your online brand because it will be difficult for other sites to mask your credibility. This should motivate you to develop an online presence, regularly update information, and participate in Internet dialog with other sites.
Generate quality, natural reviews
If your site is in good standing in search results, the next step is to implement a patient reviews strategy to start acquiring positive online reviews. A third-party provider can work with you to launch a local search engine optimization strategy and a natural reviews management program tailored for your practice’s needs. For the most part, however, we do not recommend using an online reputation management company. It is far better and more economical to ask satisfied patients to provide reviews.
At first, you may be tempted to actively petition or solicit reviews through survey software, but this method is manipulative and can lead to reputation problems for your practice. Google actively tracks where reviews originate and uses advanced algorithms to determine the review’s integrity. A petitioned review is classified as less valid, and therefore Google will assume it was not written under the same pretense as a natural, unsolicited review.
Quality customer service and outstanding patient care are often what achieve the organic reviews you are striving for. To encourage a steady flow, administer a process that encourages your most satisfied, loyal patients to review your practice.
Keep the process simple. Capture positive compliments at the point of service. Before a patient leaves your office, hand her a card (FIGURE) with easy steps for posting an online review, or offer her a tablet that links directly to your website review section. If your patient is not computer savvy, ask her to complete a 4- to 5-question survey and give her a clipboard and a pen. Then have a staff member post it on your website.

In Dr. Baum’s practice, there is a poster in every exam room and in the reception area where patients can scan the quick response (QR) code and immediately submit a testimonial. Using this system, the practice is able to collect 3 to 5 positive reviews every day.
A patient pleased with your staff’s service will happily take 5 minutes to submit a review. Acquire 5 to 10 reviews monthly and within a year’s time you will have generated enough positive reviews to negate any damaging comments that inevitably will emerge from time to time.
CASE Patient criticizes physician in a review forum
A physician with a robust Internet presence will have his or her name and the practice appear at the top of search engine results pages (as is the case with Dr. Neil Baum when “urologist” plus “New Orleans” is typed into the Google search engine window). By far most of Dr. Baum’s reviews are positive. In one instance, however, a patient on a physician review website referred to Dr. Baum as “technologically advanced but more motivated to increase his income by performing too many diagnostic tests.”
If you find a negative comment in an online directory or review website, what should you do?
The Office for Civil Rights (OCR) within the US Department of Health and Human Services (HHS) is responsible for handling Health Insurance Portability and Accountability Act of 1996 (HIPAA)1 complaints. Deven McGraw, OCR’s deputy director of health information privacy, states that “just because patients have rated their health provider publicly doesn’t give their health provider permission to rate them in return.”2,3 In fact, some health care providers who responded to poor online reviews ran into trouble with privacy rules established by HIPAA.2,3
Mr. McGraw notes that, when responding to online reviews, health professionals should speak generally about the way they treat patients while complying with HIPAA regulations. He suggests, “If the complaint is about poor patient care … say, ‘I provide all of my patients with good patient care’ and ‘I’ve been reviewed in other contexts and have good reviews.’”2,3
According to Yelp’s senior director of litigation, Aaron Schur, most patient complaints center on practice-based concerns such as wait times, office staff, and billing, not about the medical service delivered. Although most physicians do not respond, says Mr. Schur, those who do, tend to ask patients to discuss the matter in private or to apologize.2,3
What are the consequences of a HIPAA violation?
OCR Director Jocelyn Samuels says that the office’s primary role is to help health providers follow HIPAA regulations.2,5 The OCR can resolve HIPPA violations privately and informally, impose fines of up to $50,000 per violation, or it can file criminal charges against violators.2,4
The majority of the office’s investigation and enforcement of HIPAA has been against large medical data breaches.2,5 Small privacy breaches by large health care providers (eg, CVS, Walmart, Lab Corp, Quest Diagnostics, and others) generally do not result in legal consequences; the providers are privately warned. According to ProPublica, even repeated HIPAA violations tend not to be fined.2,4
Small-scale infractions can be more damaging on a personal level to both patients and physicians. However, the OCR does not typically become involved in privacy breaches that include only a few individuals. Health care providers are rarely punished for small HIPPA breaches; instead, the OCR typically settles for pledges to fix any problems and issues reminders of HIPPA requirements.2,5
Although the OCR is often the only place patients can go to seek vindication, HIPAA does not support the right to sue for violation of personal privacy. People who seek a legal remedy must find another means, which is easier in some states than in others.2,5
Health care providers have tried myriad ways to attempt to combat negative reviews. Some have sued patients, attracting a flood of attention but achieving little legal success. Others have asked patients to remove their complaints.2,3
Best practices
Create and circulate a policy. Medical privacy breaches involving sensitive health details can occur when office or hospital staff share patient information due to personal hostility or lack of understanding of HIPPA policy.2,5 Have a practice policy for responding to online reviews by patients, and make sure the staff members who have access to the practice’s online accounts understand your policy and the possible repercussions of not following it. Teach and continue to remind your staff about HIPPA regulations and hold them to a high ethical level of privacy.
Solicit reviews on an ongoing basis. Jeffrey Segal, a review site critic, says that all reviews are valuable. Physicians should respond carefully to negative comments and encourage satisfied patients to post positive reviews. “’For doctors who get bent out of shape to get rid of negative reviews, it’s a denominator problem,’ he said. ‘If they only have three reviews and two are negative, the denominator is the problem. … If you can figure out a way to cultivate reviews from hundreds of patients rather than a few patients, the problem is solved.’”2,3
CASE Resolved
Dr. Baum never responded directly to the negative patient review, and others he has received. He balances the rare negative response with numerous and plentiful positive responses by making it a practice to encourage reviews from all of his patients.
References
- HIPAA for Professionals. US Department of Health & Human Services. http://www.hhs.gov/hipaa/for-professionals/index.html. Accessed October 11, 2016.
- Hall SD. Providers responding to Yelp reviews must be mindful of HIPAA. FierceHealthcare. http://www.fiercehealthcare.com/it/providers-responding-to-yelp-reviews-must-be-mindful-hipaa. Published May 31, 2016. Accessed October 7, 2016.
- Ornstein C. Stung by Yelp reviews, health providers spill patient secrets. ProPublica. https://www.propublica.org/article/stung-by-yelp-reviews-health -providers-spill-patient-secrets. Published May 27, 2016. Accessed October 11, 2016.
- Ornstein C, Waldman A. Few consequences for health privacy law’s repeat offenders. ProPublica. https://www.propublica.org/article/few-consequences-for-health-privacy-law-repeat-offenders. Published December 29, 2015. Accessed October 11, 2016.
- Ornstein C. Small-scale violations of medical privacy often cause the most harm. ProPublica. https://www.propublica.org/article/small-scale-violations-of-medical-privacy-often-cause-the-most-harm. Published December 10, 2015. Accessed October 11, 2016.
The bottom line
Patients are seeking and leaving reviews about you and your practice online and you need to actively manage your online reputation. Do not let one disgruntled patient ruin your reputation. Our advice: Do not wait for a negative review to begin your reputation management. Take an active role and generate positive reviews to drown out negative remarks made by an occasional patient. This is an inexpensive process that does work.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In a web-focused world, it should not take much convincing that monitoring your online reputation is time well spent. For some of us, it may be hard to believe that online reviews have evolved beyond restaurants and plumbers, but today your patients are flocking to the Internet to read and leave reviews about you, your staff, and your services. What can you do to protect your online reputation?
We first addressed this topic in December 2014 (“Using the Internet in your practice. Part 4: Reputation management—how to gather kudos and combat negative online reviews”1). Have you implemented any of the tactics we offered then? We hope that you do take proactive steps to protect your online image.
What is a physician’s most precious asset?
You might answer this question with, “my patients” or “the training and education that I have obtained to practice my craft.” But the real answer is that your most precious asset is your reputation.
Physicians live and die by their reputations. We spend our entire medical careers polishing and protecting this status. The Internet dramatically has altered the way people gather information. It is sad but true that a single comment that only takes a few seconds and a single mouse-click to post can be seen by thousands and ruin that life-long effort.
How are physicians rated on the web?
Online physician reviews are positive 70% to 90% of the time.2 Most physicians have 5 or fewer reviews on any one site.3 Of the approximately 30 sites that monitor physicians and hospitals online, one of the most popular is AngiesList.com. This site requires registration and a fee; a member can review a physician every 6 months. On free websites such as Yelp.com and doctorsscorecard.com the reviewer can comment once. Other sites such as vitals.com or DrScore.com limit the reviews from 1 source, which prevents an angry patient from stuffing the ballot box.2
Pay attention
At a minimum, physicians should be monitoring their reputation by conducting periodic searches—“Googling” their name and practice name—to identify what information is already online. You may find that 3, 4, or even 10 reviews appear on various sites. If you are lucky, these reviews will be positive. Don’t be surprised, however, if 1 or 2 are not. Let’s face it: even the most accredited and experienced physician cannot possibly satisfy every patient who walks through the door.
Neil Baum, MD, and Ron Romano have offered tips on ways to manage online reputations in the past,1 and they urge Ob-Gyns to take an active role in this process in order to increase positive exposure to patients and maintain an active practice. Is active reputation management something that ObGyns are spending their valuable time on? To find out, OBG Management reached out to its Virtual Editorial Board. We found that many readers are paying attention to patient satisfaction. Some are soliciting online reviews and maintaining active upkeep on their online reputation. Here are a few responses we received from practicing ObGyns across the United States.
William E. McGrath Jr, MD, of Fernandina Beach, Florida, says that his office provides patients with a list of 5 popular review websites during their visits, and that approximately 1 in 10 will follow up with a review. Patient reviews are also prominently posted on his practice’s website. The large, private, single-specialty group to which his practice belongs requires patient satisfaction surveys for quality assurance review and insurance contract negotiations. “It is all about physician-patient communication,” he says.
Keith S. Merlin, MD, of Brockton, Massachusetts, says that he has checked online reviews to ensure their accuracy. His practice uses surveys, a suggestion box, and a mystery shopper to measure patient satisfaction, a worthwhile effort he says to understand where the practice is doing well and what needs to be done better.
Wesley Hambright, MD, of Jacksonville, North Carolina, reports that he has established a Google Alert to monitor for new content relating to his practice.
Patrick Pevoto, MD, MBA, of Austin, Texas, informs us that he has just started to think about ways to manage his online reputation. He has created a website and is writing a monthly blog, which he posts on his site. He acknowledges the importance of assessing patient satisfaction in his practice but is not applying large-scale measurement techniques yet. To keep his patients happy, he handles concerns that arise on a personal, case-by-case basis.
John Armstrong, MD, MS, of Napa, California, also reports that management of his online reputation is in the beginning stages. He uses focus groups and feels that listening to his patients when they do comment on their experience is important to his overall practice. Listening helps to “identify areas to improve and reaffirms when we are doing well,” he says. To keep his patients happy, he strives to “give extraordinary care and simply be nice to people.” When issues arise, making it right and being polite are important elements, he asserts.
Delos J. Clow, DO, MS, of Chillicothe, Missouri, does measure patient satisfaction, and feels this is very important to his practice in order to identify and correct any negative trends. He does not actively monitor his practice reputation online.
Robert del Rosario, MD, of Camp Hill, Pennsylvania, similarly does not actively manage an online reputation, but does focus on patient satisfaction. To enhance satisfaction, he tries to de-emphasize the electronic medical record to “make visits more personal and less interrogative.” Additionally, his practice objectively gauges aspects of care that might be able to be improved upon.
Reference
- Romano R, Baum NH. Using the Internet in your practice. Part 4: Reputation management—how to gather kudos and combat negative online reviews. OBG Manag. 2014;26(12):23,24,26,28.
Tell your own story
As physicians, you may not have control over what others say about you, but you can take ownership of your online presence by establishing a website, blog, and social media platforms, ensuring your story is being properly communicated. Without an online presence, you are entirely at the mercy of directory and review sites.
Optimize your website
A site that successfully uses search engine optimization (SEO) will have the upper hand when patients hunt for a physician in your area because the information will be posted at the top of the search page, well above the reviews and listings left by patients and other third-party sources. This is a critical step for your online brand because it will be difficult for other sites to mask your credibility. This should motivate you to develop an online presence, regularly update information, and participate in Internet dialog with other sites.
Generate quality, natural reviews
If your site is in good standing in search results, the next step is to implement a patient reviews strategy to start acquiring positive online reviews. A third-party provider can work with you to launch a local search engine optimization strategy and a natural reviews management program tailored for your practice’s needs. For the most part, however, we do not recommend using an online reputation management company. It is far better and more economical to ask satisfied patients to provide reviews.
At first, you may be tempted to actively petition or solicit reviews through survey software, but this method is manipulative and can lead to reputation problems for your practice. Google actively tracks where reviews originate and uses advanced algorithms to determine the review’s integrity. A petitioned review is classified as less valid, and therefore Google will assume it was not written under the same pretense as a natural, unsolicited review.
Quality customer service and outstanding patient care are often what achieve the organic reviews you are striving for. To encourage a steady flow, administer a process that encourages your most satisfied, loyal patients to review your practice.
Keep the process simple. Capture positive compliments at the point of service. Before a patient leaves your office, hand her a card (FIGURE) with easy steps for posting an online review, or offer her a tablet that links directly to your website review section. If your patient is not computer savvy, ask her to complete a 4- to 5-question survey and give her a clipboard and a pen. Then have a staff member post it on your website.

In Dr. Baum’s practice, there is a poster in every exam room and in the reception area where patients can scan the quick response (QR) code and immediately submit a testimonial. Using this system, the practice is able to collect 3 to 5 positive reviews every day.
A patient pleased with your staff’s service will happily take 5 minutes to submit a review. Acquire 5 to 10 reviews monthly and within a year’s time you will have generated enough positive reviews to negate any damaging comments that inevitably will emerge from time to time.
CASE Patient criticizes physician in a review forum
A physician with a robust Internet presence will have his or her name and the practice appear at the top of search engine results pages (as is the case with Dr. Neil Baum when “urologist” plus “New Orleans” is typed into the Google search engine window). By far most of Dr. Baum’s reviews are positive. In one instance, however, a patient on a physician review website referred to Dr. Baum as “technologically advanced but more motivated to increase his income by performing too many diagnostic tests.”
If you find a negative comment in an online directory or review website, what should you do?
The Office for Civil Rights (OCR) within the US Department of Health and Human Services (HHS) is responsible for handling Health Insurance Portability and Accountability Act of 1996 (HIPAA)1 complaints. Deven McGraw, OCR’s deputy director of health information privacy, states that “just because patients have rated their health provider publicly doesn’t give their health provider permission to rate them in return.”2,3 In fact, some health care providers who responded to poor online reviews ran into trouble with privacy rules established by HIPAA.2,3
Mr. McGraw notes that, when responding to online reviews, health professionals should speak generally about the way they treat patients while complying with HIPAA regulations. He suggests, “If the complaint is about poor patient care … say, ‘I provide all of my patients with good patient care’ and ‘I’ve been reviewed in other contexts and have good reviews.’”2,3
According to Yelp’s senior director of litigation, Aaron Schur, most patient complaints center on practice-based concerns such as wait times, office staff, and billing, not about the medical service delivered. Although most physicians do not respond, says Mr. Schur, those who do, tend to ask patients to discuss the matter in private or to apologize.2,3
What are the consequences of a HIPAA violation?
OCR Director Jocelyn Samuels says that the office’s primary role is to help health providers follow HIPAA regulations.2,5 The OCR can resolve HIPPA violations privately and informally, impose fines of up to $50,000 per violation, or it can file criminal charges against violators.2,4
The majority of the office’s investigation and enforcement of HIPAA has been against large medical data breaches.2,5 Small privacy breaches by large health care providers (eg, CVS, Walmart, Lab Corp, Quest Diagnostics, and others) generally do not result in legal consequences; the providers are privately warned. According to ProPublica, even repeated HIPAA violations tend not to be fined.2,4
Small-scale infractions can be more damaging on a personal level to both patients and physicians. However, the OCR does not typically become involved in privacy breaches that include only a few individuals. Health care providers are rarely punished for small HIPPA breaches; instead, the OCR typically settles for pledges to fix any problems and issues reminders of HIPPA requirements.2,5
Although the OCR is often the only place patients can go to seek vindication, HIPAA does not support the right to sue for violation of personal privacy. People who seek a legal remedy must find another means, which is easier in some states than in others.2,5
Health care providers have tried myriad ways to attempt to combat negative reviews. Some have sued patients, attracting a flood of attention but achieving little legal success. Others have asked patients to remove their complaints.2,3
Best practices
Create and circulate a policy. Medical privacy breaches involving sensitive health details can occur when office or hospital staff share patient information due to personal hostility or lack of understanding of HIPPA policy.2,5 Have a practice policy for responding to online reviews by patients, and make sure the staff members who have access to the practice’s online accounts understand your policy and the possible repercussions of not following it. Teach and continue to remind your staff about HIPPA regulations and hold them to a high ethical level of privacy.
Solicit reviews on an ongoing basis. Jeffrey Segal, a review site critic, says that all reviews are valuable. Physicians should respond carefully to negative comments and encourage satisfied patients to post positive reviews. “’For doctors who get bent out of shape to get rid of negative reviews, it’s a denominator problem,’ he said. ‘If they only have three reviews and two are negative, the denominator is the problem. … If you can figure out a way to cultivate reviews from hundreds of patients rather than a few patients, the problem is solved.’”2,3
CASE Resolved
Dr. Baum never responded directly to the negative patient review, and others he has received. He balances the rare negative response with numerous and plentiful positive responses by making it a practice to encourage reviews from all of his patients.
References
- HIPAA for Professionals. US Department of Health & Human Services. http://www.hhs.gov/hipaa/for-professionals/index.html. Accessed October 11, 2016.
- Hall SD. Providers responding to Yelp reviews must be mindful of HIPAA. FierceHealthcare. http://www.fiercehealthcare.com/it/providers-responding-to-yelp-reviews-must-be-mindful-hipaa. Published May 31, 2016. Accessed October 7, 2016.
- Ornstein C. Stung by Yelp reviews, health providers spill patient secrets. ProPublica. https://www.propublica.org/article/stung-by-yelp-reviews-health -providers-spill-patient-secrets. Published May 27, 2016. Accessed October 11, 2016.
- Ornstein C, Waldman A. Few consequences for health privacy law’s repeat offenders. ProPublica. https://www.propublica.org/article/few-consequences-for-health-privacy-law-repeat-offenders. Published December 29, 2015. Accessed October 11, 2016.
- Ornstein C. Small-scale violations of medical privacy often cause the most harm. ProPublica. https://www.propublica.org/article/small-scale-violations-of-medical-privacy-often-cause-the-most-harm. Published December 10, 2015. Accessed October 11, 2016.
The bottom line
Patients are seeking and leaving reviews about you and your practice online and you need to actively manage your online reputation. Do not let one disgruntled patient ruin your reputation. Our advice: Do not wait for a negative review to begin your reputation management. Take an active role and generate positive reviews to drown out negative remarks made by an occasional patient. This is an inexpensive process that does work.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Romano R, Baum NH. Using the Internet in your practice. Part 4: Reputation management-how to gather kudos and combat negative online reviews. OBG Manag. 2014;26(12):23,24,26,28.
- Lagu T, Hannon NS, Rothberg MB, Lindenauer PK. Patients' evaluations of health care providers in the era of social networking: an analysis of physician rating websites. J Gen Intern Med. 2010;25(9):942-946.
- Gunter J. For better or maybe, worse, patients are judging your care online. OBG Manag. 2011;23(3):47-51.
- Romano R, Baum NH. Using the Internet in your practice. Part 4: Reputation management-how to gather kudos and combat negative online reviews. OBG Manag. 2014;26(12):23,24,26,28.
- Lagu T, Hannon NS, Rothberg MB, Lindenauer PK. Patients' evaluations of health care providers in the era of social networking: an analysis of physician rating websites. J Gen Intern Med. 2010;25(9):942-946.
- Gunter J. For better or maybe, worse, patients are judging your care online. OBG Manag. 2011;23(3):47-51.
In this Article
- How your peers manage online reputation
- Should you respond to a negative review?
- Generate quality, natural reviews