User login
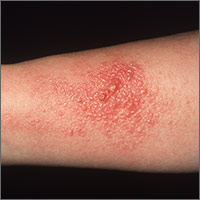
Poison ivy: How effective are available treatments?
ABSTRACT
Purpose To determine the characteristics and clinical course of Rhus dermatitis in patients who seek assistance from primary care clinicians, as well as treatment approaches used by patients and recommended by clinicians, and treatment approaches associated with better outcomes.
Methods This was a prospective cohort study with standardized baseline data collection on patients and their rashes, followed by examination of patient-completed diaries of signs, symptoms, and treatments.
Results Thirty-six clinicians identified 186 interested patients, of which 89 completed and returned diaries and consent forms. Of those 89 patients, 92% reported pruritus; 91%, erythema; 87%, papules; and 49%, vesicles or bullae at baseline. Their rashes involved the head/face/neck, 61%; trunk, 56%; legs, 54%; and arms, 22%.
From the date of clinical consultation, the mean (standard deviation [SD]; range) duration of any symptom or sign was 14.4 days (8.0; 1-43). Patients most often had tried a topical antipruritic, astringent, or low-potency corticosteroid before seeking care. Clinicians prescribed oral or parenteral corticosteroids 81% of the time, sometimes in combination with a high-potency topical c
Conclusions Patients who visit a primary care clinician for Rhus dermatitis can expect the rash to last another 2 weeks on average (total duration: one day to 6 weeks) regardless of what treatment is prescribed. Parenteral corticosteroids plus high-potency topical corticosteroids may reduce the duration of the itching.
Rhus dermatitis (poison ivy, oak, and sumac) is a common cause of contact dermatitis throughout the United States. The condition is usually mild and often not brought to the attention of primary care clinicians. Some patients, however, do see a health care provider for treatment, most often because of pruritus. This form of contact dermatitis results from a type IV hypersensitivity reaction to urushiol, a colorless oil in the leaves, stem, root, and fruit of poison ivy, poison oak, and poison sumac. The reaction, which occurs 24 to 72 hours following contact with the skin, can be prevented by washing the skin promptly with a detergent soap after exposure. By the age of 8, most people are sensitized to urushiol.1
According to most standard texts and clinical reviews, untreated Rhus dermatitis usually resolves in one to 3 weeks. What is not known is whether particular patient or rash characteristics might affect prognosis and thereby influence treatment recommendations—eg, age, gender, race, location of the rash, prior episodes, chronic illnesses such as diabetes, or chronic use of medications such as nonsteroidal anti-inflammatory drugs and corticosteroids.
Impetus for our study. An informal survey of 10 clinician members of the Oklahoma Physicians Resource/Research Network (OKPRN), a statewide practice-based research network, suggested that primary care clinicians treat between one and 10 patients with poison ivy each week during the spring, summer, and fall (median 2.5). Their reported armamentarium included more than 15 different over-the-counter topical agents, several oral antihistamines, and a variety of topical, oral, and parenteral corticosteroids.
Surprisingly, there is very little published evidence on which to base treatment decisions. Using PubMed and the search terms, Rhus dermatitis, poison ivy, and poison oak, we found only 3 placebo-controlled clinical trials of Rhus dermatitis treatments in the English language literature after 1966. Based on these studies, Zanfel, a mixture of alcohol-soluble and anionic surfactant, may be somewhat effective, but pimecrolimus and jewelweed extract were no more effective than placebo.2-4 There is some evidence that topical corticosteroids are effective only before vesicles appear.5 In one uncontrolled study, intramuscular injection of betamethasone and dexamethasone yielded about a 30% reduction in symptoms within 48 hours.6 Assuming that systemic corticosteroids do produce benefit, however, the most effective dose and duration of treatment have not been determined.7,8
To address some of these gaps in our knowledge base, OKPRN members asked that we undertake a longitudinal cohort study of patients reporting to primary care practices.
METHODS
We conducted this study between May 2010 and October 2014. The project was approved by the University of Oklahoma Health Sciences Center Institutional Review Board. Clinician members of OKPRN were invited to participate in the study via listserv, fax, or letter. We instructed clinicians and office staff to ask patients with Rhus dermatitis if they might be interested in participating in a study, which would require that they keep a symptom diary and would earn them a $20 gift card. Interested patients were given a packet of information, and a member of the research team later called the patients with additional information, including an explanation of informed consent and instructions on completing and returning the diary and written consent form.
Clinicians recorded information about the patient and the rash on a customized template, releasing it to the team after written consent was obtained from the patient. Categories for characterizing the rash were head/face, arms/hands, trunk, and legs/feet. A subset of 5 participating clinicians, selected to include a variety of practice types and patient populations, were also asked to produce, from their billing software, the number of patients and encounters in which poison ivy was addressed in each month of 2013.
On the diary, patients were instructed to record the presence or absence of pruritus, erythema, raised lesions, and vesicles/bullae at the end of each day until the rash resolved, or for 6 weeks following onset of the rash, whichever came first. Patients were asked to mail their diaries to the principal investigator once they were free of symptoms for one week or after 6 weeks from the onset of symptoms, whichever came first.
We asked both patients and clinicians to report medications used before and after the primary care encounter. A member of the research team assigned these medications to one of 12 categories: topical antihistamines, topical soaps (eg, Zanfel or Tecnu), topical astringents, other topical antipruritics, topical aloe vera, topical bleach, low-potency topical corticosteroids, moderate-potency topical corticosteroids, high-potency topical corticosteroids, oral antihistamines, oral corticosteroids, and parenteral corticosteroids.
We used independent T-tests to evaluate associations between baseline variables, patient-initiated treatments, and clinician-initiated treatments and the time to complete resolution of individual signs and symptoms and complete resolution of all signs and symptoms following the clinical encounter. We created additional outcome variables for initial resolution followed by recurrence of itching, erythema, papules, and vesicles. The purpose of these variables was to determine if some treatments were initially effective but without lasting effect.
We used the chi square test to assess associations between clinician-initiated treatments and recurrence of signs or symptoms following initial resolution. To account for chance associations resulting from multiple analyses, we chose to set the level of statistical significance at P=.01. However, because of the lower-than-projected sample size, we chose to also report variables with P<.05 so that the reader could judge the likelihood that a larger sample might have disclosed other important associations.
We assumed that an average of 4 categories of treatment would be tried (eg, topical corticosteroids, systemic corticosteroids, topical antihistamines, and other topical agents), and that the mean number of days until resolution would be 21, with a standard deviation (SD) of 4 days. Setting power at 80% and alpha at .05, we calculated it would take 105 patients per group (N=420) to detect a difference of 2 days in time until resolution.
RESULTS
Over the 5-year study period, 36 clinicians identified 186 patients who expressed an interest in the study, and they transmitted the patient contact information to the research team. Patients were seen in a traditional primary care setting. All 186 patients were enrolled by phone. However, only 89 completed and returned their diaries and signed consent forms; of these, 60% were female, 92% were white, 4% were black, 4% were American Indians, 2% were Hispanic, and 7% had diabetes mellitus.
Five practices contributed data on numbers of poison ivy encounters per month and total encounters per month for the year 2013. They included an inner city academic practice in central Oklahoma and a rural community health center, a suburban private practice, and 2 private practices in a town of 30,000 in eastern Oklahoma. The largest average number of encounters occurred between April and August.
The distribution of enrolled-patient visits by month and season corresponded roughly to the proportions of all patient visits for poison ivy, with 1% occurring in the winter, 35% in the spring, 55% during the summer, and 9% in the fall. Virtually all study participants (92%) complained of pruritus and had erythema (91%) and papules (87%). Forty-nine percent had vesicles or bullae. The area of the body most often affected was the head/face/neck, 61%, followed by the trunk, 56%; legs, 54%; and arms, 22%.
From the date of initial clinical consultation, the mean/median (SD; range) duration of symptoms and signs were: pruritus, 10.9/9 days (7.1; 0-43); erythema, 13.7/13 days (7.7; 0-42); papules, 10.1/9.5 days (6.5; 0-37); and vesicles, 5.3/5 days (4.1; 0-15). The mean/median (SD; range) duration of any symptom or sign was 14.4/13.5 days (8; 1-43). Rashes with vesicles tended to last longer (16.1 vs 12.9 days), but this difference did not reach statistical significance.
Treatments used by patients before and after their primary care visit are shown in TABLE 1. Seventy-three percent of patients had tried something from one treatment category before consulting a clinician, and 31% had tried something from more than one category. They were most likely to have used a topical antipruritic, astringent, or low-potency corticosteroid, or a combination of these. Clinicians always recommended some treatment and, in 76% of cases, treatments from more than one category. They most often prescribed oral or parenteral corticosteroids (81% of the time), sometimes in combination with a high-potency topical corticosteroid (25% of the time) or oral antihistamine (31%).
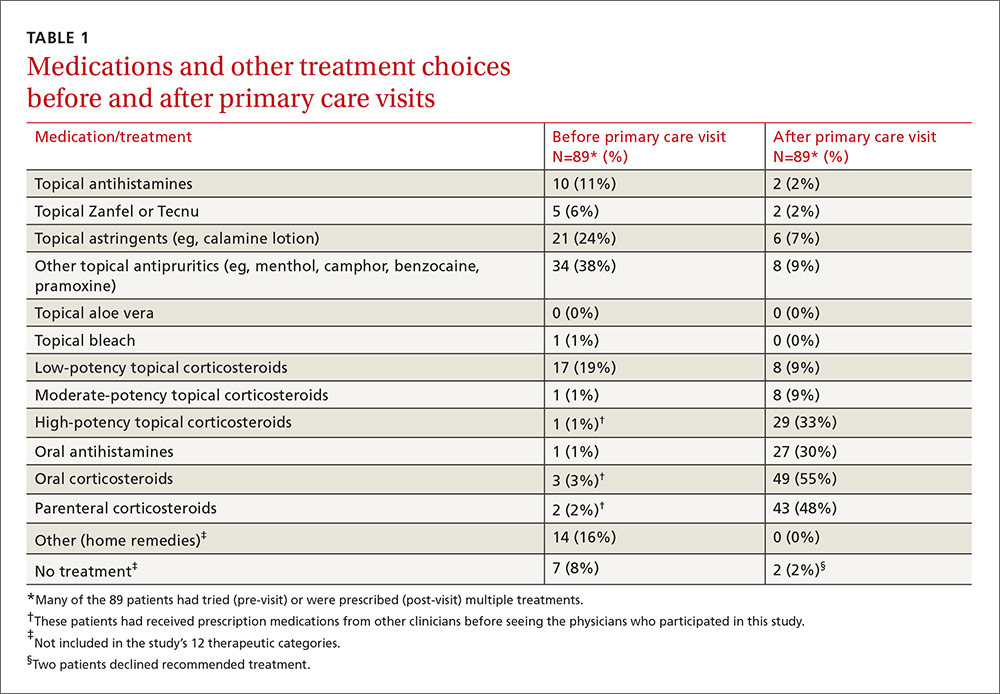
No statistically significant associations were found between the baseline non-treatment variables and duration of symptoms and signs. Patient-initiated treatments were also not associated with duration of symptoms and signs following the initial clinician visit.
Of the treatments prescribed by clinicians or independently chosen by patients following their initial office visit, only systemic corticosteroids plus high-potency topical corticosteroids were associated with a significantly shorter duration of itching (P=.005). No treatment was associated with reduced duration of erythema, papules, or vesicles. Use of topical soaps was associated with a longer duration of papules (P<.0001) and of total duration of signs or symptoms (P=.0004) compared with other treatments.
Location and characteristics of the rash were not associated with likelihood of recurrence following treatment. Post-visit use of a topical soap was associated with recurrence of itching (P=.001) and erythema (P=.01). Recurrence of erythema was also more frequent in patients prescribed topical astringents (beta coefficient=0.28; P=.008), and recurrence of papules was more common in patients treated with low-potency topical corticosteroids (P<.0001). These results and several others that almost reached statistical significance are shown in TABLE 2.
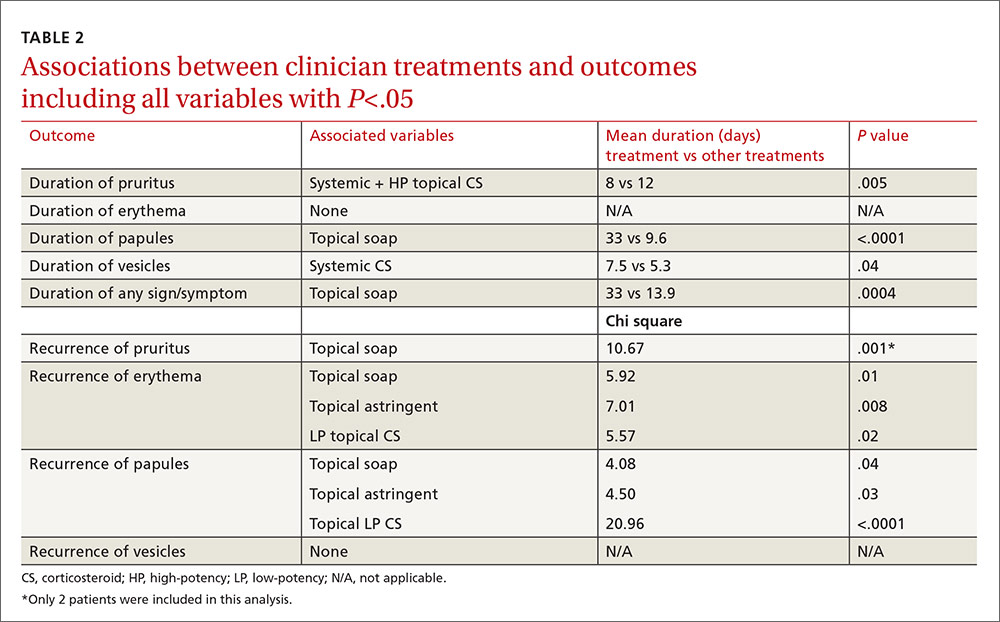
In the multivariable models, the only variable associated with duration of pruritus was the combination of systemic and high-potency topical corticosteroids (8 vs 12 days.) Use of only parenteral or only high-potency topical corticosteroids did not predict shorter duration of pruritus. Use of topical soaps was associated with longer duration of papules (33 vs 9.6 days) and longer duration of any symptoms (33 vs 13.9 days). It was also associated with a higher likelihood of recurrence of pruritus (chi square test [χ2], 10.67) and recurrence of erythema (χ2, 5.92) after initial resolution. Topical astringent use was predictive of recurrence of erythema (χ2, 7.01) and use of low-potency corticosteroids was associated with recurrence of papules (χ2, 20.96).
DISCUSSION
While network clinicians felt that studying poison ivy was of interest and importance, and we had preliminary survey information to suggest it was a common problem treated in primary care, our data suggest that clinical encounters for poison ivy are actually quite uncommon (less than 0.4% of all encounters) even during peak months. Our problems with recruitment were therefore unexpected, and we ended up with far fewer enrolled patients than we had projected, and needed, based on our power analysis. Also based on our preliminary survey, we anticipated considerably more variation in treatment approach than we found. Most clinicians recommended either an oral, parenteral, or high-potency topical corticosteroid, and some also recommended an oral antihistamine, usually diphenhydramine.
The literature and common sense suggest that most patients who seek medical treatment for poison ivy are primarily concerned about itching. Even with the smaller-than-anticipated number of participants in this study, we were able to show that the combination of a systemic (oral or parenteral) corticosteroid and a high-potency topical corticosteroid was associated with a statistically significant shorter duration of pruritus with no recurrence following treatment. We found no evidence that systemic corticosteroids alone, parenteral corticosteroids alone, or high-potency topical corticosteroids alone had any effect on duration of signs or symptoms, even at an alpha of .05. We also found no evidence that oral antihistamines were associated with a shorter duration of pruritus (P=.06); with a larger sample size, we might have found a difference.
Since only 2 patients used topical soaps following their initial clinician visit, the associations between use of these products and longer duration of signs and symptoms and with recurrence of signs and symptoms, although statistically significant, should be viewed with skepticism and with an eye toward possible confounders (eg, people who used these agents may have been more likely to notice and record minor symptoms). Furthermore, these agents have been effective only when used before or at the onset of the rash.
Study limitations. The study has a number of limitations. It had a high drop-out rate. Some patients might not have had poison ivy, but it is generally considered easy to diagnose with accuracy. We cannot be sure that all of the enrolled patients had Rhus dermatitis. Enrollment was based on the clinical impression of the patients’ primary care clinicians. The sample size reduced the power of the study to detect small differences in treatment effects and prevented more complex analyses (eg, combinations of medications, interactions).
The possibility of self-selection bias, weaknesses of the cohort design, and patient-reported outcome measures were additional limitations. The study was also carried out in a single southwestern state, which may not be representative of some other locations. However, it is one of only a few studies published on Rhus dermatitis and possibly the only one conducted in primary care settings.
CORRESPONDENCE
Cara Vaught, MPH, University of Oklahoma Health Sciences Center, Department of Family and Preventive Medicine, 900 NE 10th Street, Oklahoma City, OK 73104; [email protected].
ACKNOWLEDGEMENT
The authors thank the Oklahoma Physicians Resource/Research Network (OKPRN) and the OKPRN clinician members (as well as their staff and patients) for their contributions to this study. The authors also thank Bradley Long, Matthew Marr, and Kellie Hetherington for their involvement in the data collection for this study.
1. Epstein WL. Occupational poison ivy and oak dermatitis. Dermatol Clin. 1994;12:511-516.
2. Long D, Ballentine NH, Marks JG Jr. Treatment of poison ivy/oak allergic contact dermatitis with an extract of jewelweed. Am J Contact Dermat. 1997;8:150-153.
3. Amrol D, Keitel D, Hagaman D, et al. Topical pimecrolimus in the treatment of human allergic contact dermatitis. Ann Allergy Asthma Immunol. 2003;91:563-566.
4. Davila A, Laurora M, Fulton J, et al. A new topical agent, Zanfel, ameliorates urushiol-induced Toxicodendron allergic contact dermatitis [abstract 364]. Ann Emerg Med. 2003;42(suppl 4):S98.
5. Vernon HJ, Olsen EA. A controlled trial of clobetasol propionate ointment 0.05% in the treatment of experimentally induced Rhus dermatitis. J Am Acad Dermatol. 1990;23:829-832.
6. Dickey RF. Parenteral short-term corticosteroid therapy in moderate to severe dermatoses. A comparative multiclinic study. Cutis. 1976;17:179-193.
7. Goodall J. Oral corticosteroids for poison ivy dermatitis. CMAJ. 2002;166:300-301.
8. Moe JF. How much steroid for poison ivy? Postgrad Med. 1999;106:21,24.
ABSTRACT
Purpose To determine the characteristics and clinical course of Rhus dermatitis in patients who seek assistance from primary care clinicians, as well as treatment approaches used by patients and recommended by clinicians, and treatment approaches associated with better outcomes.
Methods This was a prospective cohort study with standardized baseline data collection on patients and their rashes, followed by examination of patient-completed diaries of signs, symptoms, and treatments.
Results Thirty-six clinicians identified 186 interested patients, of which 89 completed and returned diaries and consent forms. Of those 89 patients, 92% reported pruritus; 91%, erythema; 87%, papules; and 49%, vesicles or bullae at baseline. Their rashes involved the head/face/neck, 61%; trunk, 56%; legs, 54%; and arms, 22%.
From the date of clinical consultation, the mean (standard deviation [SD]; range) duration of any symptom or sign was 14.4 days (8.0; 1-43). Patients most often had tried a topical antipruritic, astringent, or low-potency corticosteroid before seeking care. Clinicians prescribed oral or parenteral corticosteroids 81% of the time, sometimes in combination with a high-potency topical c
Conclusions Patients who visit a primary care clinician for Rhus dermatitis can expect the rash to last another 2 weeks on average (total duration: one day to 6 weeks) regardless of what treatment is prescribed. Parenteral corticosteroids plus high-potency topical corticosteroids may reduce the duration of the itching.
Rhus dermatitis (poison ivy, oak, and sumac) is a common cause of contact dermatitis throughout the United States. The condition is usually mild and often not brought to the attention of primary care clinicians. Some patients, however, do see a health care provider for treatment, most often because of pruritus. This form of contact dermatitis results from a type IV hypersensitivity reaction to urushiol, a colorless oil in the leaves, stem, root, and fruit of poison ivy, poison oak, and poison sumac. The reaction, which occurs 24 to 72 hours following contact with the skin, can be prevented by washing the skin promptly with a detergent soap after exposure. By the age of 8, most people are sensitized to urushiol.1
According to most standard texts and clinical reviews, untreated Rhus dermatitis usually resolves in one to 3 weeks. What is not known is whether particular patient or rash characteristics might affect prognosis and thereby influence treatment recommendations—eg, age, gender, race, location of the rash, prior episodes, chronic illnesses such as diabetes, or chronic use of medications such as nonsteroidal anti-inflammatory drugs and corticosteroids.
Impetus for our study. An informal survey of 10 clinician members of the Oklahoma Physicians Resource/Research Network (OKPRN), a statewide practice-based research network, suggested that primary care clinicians treat between one and 10 patients with poison ivy each week during the spring, summer, and fall (median 2.5). Their reported armamentarium included more than 15 different over-the-counter topical agents, several oral antihistamines, and a variety of topical, oral, and parenteral corticosteroids.
Surprisingly, there is very little published evidence on which to base treatment decisions. Using PubMed and the search terms, Rhus dermatitis, poison ivy, and poison oak, we found only 3 placebo-controlled clinical trials of Rhus dermatitis treatments in the English language literature after 1966. Based on these studies, Zanfel, a mixture of alcohol-soluble and anionic surfactant, may be somewhat effective, but pimecrolimus and jewelweed extract were no more effective than placebo.2-4 There is some evidence that topical corticosteroids are effective only before vesicles appear.5 In one uncontrolled study, intramuscular injection of betamethasone and dexamethasone yielded about a 30% reduction in symptoms within 48 hours.6 Assuming that systemic corticosteroids do produce benefit, however, the most effective dose and duration of treatment have not been determined.7,8
To address some of these gaps in our knowledge base, OKPRN members asked that we undertake a longitudinal cohort study of patients reporting to primary care practices.
METHODS
We conducted this study between May 2010 and October 2014. The project was approved by the University of Oklahoma Health Sciences Center Institutional Review Board. Clinician members of OKPRN were invited to participate in the study via listserv, fax, or letter. We instructed clinicians and office staff to ask patients with Rhus dermatitis if they might be interested in participating in a study, which would require that they keep a symptom diary and would earn them a $20 gift card. Interested patients were given a packet of information, and a member of the research team later called the patients with additional information, including an explanation of informed consent and instructions on completing and returning the diary and written consent form.
Clinicians recorded information about the patient and the rash on a customized template, releasing it to the team after written consent was obtained from the patient. Categories for characterizing the rash were head/face, arms/hands, trunk, and legs/feet. A subset of 5 participating clinicians, selected to include a variety of practice types and patient populations, were also asked to produce, from their billing software, the number of patients and encounters in which poison ivy was addressed in each month of 2013.
On the diary, patients were instructed to record the presence or absence of pruritus, erythema, raised lesions, and vesicles/bullae at the end of each day until the rash resolved, or for 6 weeks following onset of the rash, whichever came first. Patients were asked to mail their diaries to the principal investigator once they were free of symptoms for one week or after 6 weeks from the onset of symptoms, whichever came first.
We asked both patients and clinicians to report medications used before and after the primary care encounter. A member of the research team assigned these medications to one of 12 categories: topical antihistamines, topical soaps (eg, Zanfel or Tecnu), topical astringents, other topical antipruritics, topical aloe vera, topical bleach, low-potency topical corticosteroids, moderate-potency topical corticosteroids, high-potency topical corticosteroids, oral antihistamines, oral corticosteroids, and parenteral corticosteroids.
We used independent T-tests to evaluate associations between baseline variables, patient-initiated treatments, and clinician-initiated treatments and the time to complete resolution of individual signs and symptoms and complete resolution of all signs and symptoms following the clinical encounter. We created additional outcome variables for initial resolution followed by recurrence of itching, erythema, papules, and vesicles. The purpose of these variables was to determine if some treatments were initially effective but without lasting effect.
We used the chi square test to assess associations between clinician-initiated treatments and recurrence of signs or symptoms following initial resolution. To account for chance associations resulting from multiple analyses, we chose to set the level of statistical significance at P=.01. However, because of the lower-than-projected sample size, we chose to also report variables with P<.05 so that the reader could judge the likelihood that a larger sample might have disclosed other important associations.
We assumed that an average of 4 categories of treatment would be tried (eg, topical corticosteroids, systemic corticosteroids, topical antihistamines, and other topical agents), and that the mean number of days until resolution would be 21, with a standard deviation (SD) of 4 days. Setting power at 80% and alpha at .05, we calculated it would take 105 patients per group (N=420) to detect a difference of 2 days in time until resolution.
RESULTS
Over the 5-year study period, 36 clinicians identified 186 patients who expressed an interest in the study, and they transmitted the patient contact information to the research team. Patients were seen in a traditional primary care setting. All 186 patients were enrolled by phone. However, only 89 completed and returned their diaries and signed consent forms; of these, 60% were female, 92% were white, 4% were black, 4% were American Indians, 2% were Hispanic, and 7% had diabetes mellitus.
Five practices contributed data on numbers of poison ivy encounters per month and total encounters per month for the year 2013. They included an inner city academic practice in central Oklahoma and a rural community health center, a suburban private practice, and 2 private practices in a town of 30,000 in eastern Oklahoma. The largest average number of encounters occurred between April and August.
The distribution of enrolled-patient visits by month and season corresponded roughly to the proportions of all patient visits for poison ivy, with 1% occurring in the winter, 35% in the spring, 55% during the summer, and 9% in the fall. Virtually all study participants (92%) complained of pruritus and had erythema (91%) and papules (87%). Forty-nine percent had vesicles or bullae. The area of the body most often affected was the head/face/neck, 61%, followed by the trunk, 56%; legs, 54%; and arms, 22%.
From the date of initial clinical consultation, the mean/median (SD; range) duration of symptoms and signs were: pruritus, 10.9/9 days (7.1; 0-43); erythema, 13.7/13 days (7.7; 0-42); papules, 10.1/9.5 days (6.5; 0-37); and vesicles, 5.3/5 days (4.1; 0-15). The mean/median (SD; range) duration of any symptom or sign was 14.4/13.5 days (8; 1-43). Rashes with vesicles tended to last longer (16.1 vs 12.9 days), but this difference did not reach statistical significance.
Treatments used by patients before and after their primary care visit are shown in TABLE 1. Seventy-three percent of patients had tried something from one treatment category before consulting a clinician, and 31% had tried something from more than one category. They were most likely to have used a topical antipruritic, astringent, or low-potency corticosteroid, or a combination of these. Clinicians always recommended some treatment and, in 76% of cases, treatments from more than one category. They most often prescribed oral or parenteral corticosteroids (81% of the time), sometimes in combination with a high-potency topical corticosteroid (25% of the time) or oral antihistamine (31%).

No statistically significant associations were found between the baseline non-treatment variables and duration of symptoms and signs. Patient-initiated treatments were also not associated with duration of symptoms and signs following the initial clinician visit.
Of the treatments prescribed by clinicians or independently chosen by patients following their initial office visit, only systemic corticosteroids plus high-potency topical corticosteroids were associated with a significantly shorter duration of itching (P=.005). No treatment was associated with reduced duration of erythema, papules, or vesicles. Use of topical soaps was associated with a longer duration of papules (P<.0001) and of total duration of signs or symptoms (P=.0004) compared with other treatments.
Location and characteristics of the rash were not associated with likelihood of recurrence following treatment. Post-visit use of a topical soap was associated with recurrence of itching (P=.001) and erythema (P=.01). Recurrence of erythema was also more frequent in patients prescribed topical astringents (beta coefficient=0.28; P=.008), and recurrence of papules was more common in patients treated with low-potency topical corticosteroids (P<.0001). These results and several others that almost reached statistical significance are shown in TABLE 2.

In the multivariable models, the only variable associated with duration of pruritus was the combination of systemic and high-potency topical corticosteroids (8 vs 12 days.) Use of only parenteral or only high-potency topical corticosteroids did not predict shorter duration of pruritus. Use of topical soaps was associated with longer duration of papules (33 vs 9.6 days) and longer duration of any symptoms (33 vs 13.9 days). It was also associated with a higher likelihood of recurrence of pruritus (chi square test [χ2], 10.67) and recurrence of erythema (χ2, 5.92) after initial resolution. Topical astringent use was predictive of recurrence of erythema (χ2, 7.01) and use of low-potency corticosteroids was associated with recurrence of papules (χ2, 20.96).
DISCUSSION
While network clinicians felt that studying poison ivy was of interest and importance, and we had preliminary survey information to suggest it was a common problem treated in primary care, our data suggest that clinical encounters for poison ivy are actually quite uncommon (less than 0.4% of all encounters) even during peak months. Our problems with recruitment were therefore unexpected, and we ended up with far fewer enrolled patients than we had projected, and needed, based on our power analysis. Also based on our preliminary survey, we anticipated considerably more variation in treatment approach than we found. Most clinicians recommended either an oral, parenteral, or high-potency topical corticosteroid, and some also recommended an oral antihistamine, usually diphenhydramine.
The literature and common sense suggest that most patients who seek medical treatment for poison ivy are primarily concerned about itching. Even with the smaller-than-anticipated number of participants in this study, we were able to show that the combination of a systemic (oral or parenteral) corticosteroid and a high-potency topical corticosteroid was associated with a statistically significant shorter duration of pruritus with no recurrence following treatment. We found no evidence that systemic corticosteroids alone, parenteral corticosteroids alone, or high-potency topical corticosteroids alone had any effect on duration of signs or symptoms, even at an alpha of .05. We also found no evidence that oral antihistamines were associated with a shorter duration of pruritus (P=.06); with a larger sample size, we might have found a difference.
Since only 2 patients used topical soaps following their initial clinician visit, the associations between use of these products and longer duration of signs and symptoms and with recurrence of signs and symptoms, although statistically significant, should be viewed with skepticism and with an eye toward possible confounders (eg, people who used these agents may have been more likely to notice and record minor symptoms). Furthermore, these agents have been effective only when used before or at the onset of the rash.
Study limitations. The study has a number of limitations. It had a high drop-out rate. Some patients might not have had poison ivy, but it is generally considered easy to diagnose with accuracy. We cannot be sure that all of the enrolled patients had Rhus dermatitis. Enrollment was based on the clinical impression of the patients’ primary care clinicians. The sample size reduced the power of the study to detect small differences in treatment effects and prevented more complex analyses (eg, combinations of medications, interactions).
The possibility of self-selection bias, weaknesses of the cohort design, and patient-reported outcome measures were additional limitations. The study was also carried out in a single southwestern state, which may not be representative of some other locations. However, it is one of only a few studies published on Rhus dermatitis and possibly the only one conducted in primary care settings.
CORRESPONDENCE
Cara Vaught, MPH, University of Oklahoma Health Sciences Center, Department of Family and Preventive Medicine, 900 NE 10th Street, Oklahoma City, OK 73104; [email protected].
ACKNOWLEDGEMENT
The authors thank the Oklahoma Physicians Resource/Research Network (OKPRN) and the OKPRN clinician members (as well as their staff and patients) for their contributions to this study. The authors also thank Bradley Long, Matthew Marr, and Kellie Hetherington for their involvement in the data collection for this study.
ABSTRACT
Purpose To determine the characteristics and clinical course of Rhus dermatitis in patients who seek assistance from primary care clinicians, as well as treatment approaches used by patients and recommended by clinicians, and treatment approaches associated with better outcomes.
Methods This was a prospective cohort study with standardized baseline data collection on patients and their rashes, followed by examination of patient-completed diaries of signs, symptoms, and treatments.
Results Thirty-six clinicians identified 186 interested patients, of which 89 completed and returned diaries and consent forms. Of those 89 patients, 92% reported pruritus; 91%, erythema; 87%, papules; and 49%, vesicles or bullae at baseline. Their rashes involved the head/face/neck, 61%; trunk, 56%; legs, 54%; and arms, 22%.
From the date of clinical consultation, the mean (standard deviation [SD]; range) duration of any symptom or sign was 14.4 days (8.0; 1-43). Patients most often had tried a topical antipruritic, astringent, or low-potency corticosteroid before seeking care. Clinicians prescribed oral or parenteral corticosteroids 81% of the time, sometimes in combination with a high-potency topical c
Conclusions Patients who visit a primary care clinician for Rhus dermatitis can expect the rash to last another 2 weeks on average (total duration: one day to 6 weeks) regardless of what treatment is prescribed. Parenteral corticosteroids plus high-potency topical corticosteroids may reduce the duration of the itching.
Rhus dermatitis (poison ivy, oak, and sumac) is a common cause of contact dermatitis throughout the United States. The condition is usually mild and often not brought to the attention of primary care clinicians. Some patients, however, do see a health care provider for treatment, most often because of pruritus. This form of contact dermatitis results from a type IV hypersensitivity reaction to urushiol, a colorless oil in the leaves, stem, root, and fruit of poison ivy, poison oak, and poison sumac. The reaction, which occurs 24 to 72 hours following contact with the skin, can be prevented by washing the skin promptly with a detergent soap after exposure. By the age of 8, most people are sensitized to urushiol.1
According to most standard texts and clinical reviews, untreated Rhus dermatitis usually resolves in one to 3 weeks. What is not known is whether particular patient or rash characteristics might affect prognosis and thereby influence treatment recommendations—eg, age, gender, race, location of the rash, prior episodes, chronic illnesses such as diabetes, or chronic use of medications such as nonsteroidal anti-inflammatory drugs and corticosteroids.
Impetus for our study. An informal survey of 10 clinician members of the Oklahoma Physicians Resource/Research Network (OKPRN), a statewide practice-based research network, suggested that primary care clinicians treat between one and 10 patients with poison ivy each week during the spring, summer, and fall (median 2.5). Their reported armamentarium included more than 15 different over-the-counter topical agents, several oral antihistamines, and a variety of topical, oral, and parenteral corticosteroids.
Surprisingly, there is very little published evidence on which to base treatment decisions. Using PubMed and the search terms, Rhus dermatitis, poison ivy, and poison oak, we found only 3 placebo-controlled clinical trials of Rhus dermatitis treatments in the English language literature after 1966. Based on these studies, Zanfel, a mixture of alcohol-soluble and anionic surfactant, may be somewhat effective, but pimecrolimus and jewelweed extract were no more effective than placebo.2-4 There is some evidence that topical corticosteroids are effective only before vesicles appear.5 In one uncontrolled study, intramuscular injection of betamethasone and dexamethasone yielded about a 30% reduction in symptoms within 48 hours.6 Assuming that systemic corticosteroids do produce benefit, however, the most effective dose and duration of treatment have not been determined.7,8
To address some of these gaps in our knowledge base, OKPRN members asked that we undertake a longitudinal cohort study of patients reporting to primary care practices.
METHODS
We conducted this study between May 2010 and October 2014. The project was approved by the University of Oklahoma Health Sciences Center Institutional Review Board. Clinician members of OKPRN were invited to participate in the study via listserv, fax, or letter. We instructed clinicians and office staff to ask patients with Rhus dermatitis if they might be interested in participating in a study, which would require that they keep a symptom diary and would earn them a $20 gift card. Interested patients were given a packet of information, and a member of the research team later called the patients with additional information, including an explanation of informed consent and instructions on completing and returning the diary and written consent form.
Clinicians recorded information about the patient and the rash on a customized template, releasing it to the team after written consent was obtained from the patient. Categories for characterizing the rash were head/face, arms/hands, trunk, and legs/feet. A subset of 5 participating clinicians, selected to include a variety of practice types and patient populations, were also asked to produce, from their billing software, the number of patients and encounters in which poison ivy was addressed in each month of 2013.
On the diary, patients were instructed to record the presence or absence of pruritus, erythema, raised lesions, and vesicles/bullae at the end of each day until the rash resolved, or for 6 weeks following onset of the rash, whichever came first. Patients were asked to mail their diaries to the principal investigator once they were free of symptoms for one week or after 6 weeks from the onset of symptoms, whichever came first.
We asked both patients and clinicians to report medications used before and after the primary care encounter. A member of the research team assigned these medications to one of 12 categories: topical antihistamines, topical soaps (eg, Zanfel or Tecnu), topical astringents, other topical antipruritics, topical aloe vera, topical bleach, low-potency topical corticosteroids, moderate-potency topical corticosteroids, high-potency topical corticosteroids, oral antihistamines, oral corticosteroids, and parenteral corticosteroids.
We used independent T-tests to evaluate associations between baseline variables, patient-initiated treatments, and clinician-initiated treatments and the time to complete resolution of individual signs and symptoms and complete resolution of all signs and symptoms following the clinical encounter. We created additional outcome variables for initial resolution followed by recurrence of itching, erythema, papules, and vesicles. The purpose of these variables was to determine if some treatments were initially effective but without lasting effect.
We used the chi square test to assess associations between clinician-initiated treatments and recurrence of signs or symptoms following initial resolution. To account for chance associations resulting from multiple analyses, we chose to set the level of statistical significance at P=.01. However, because of the lower-than-projected sample size, we chose to also report variables with P<.05 so that the reader could judge the likelihood that a larger sample might have disclosed other important associations.
We assumed that an average of 4 categories of treatment would be tried (eg, topical corticosteroids, systemic corticosteroids, topical antihistamines, and other topical agents), and that the mean number of days until resolution would be 21, with a standard deviation (SD) of 4 days. Setting power at 80% and alpha at .05, we calculated it would take 105 patients per group (N=420) to detect a difference of 2 days in time until resolution.
RESULTS
Over the 5-year study period, 36 clinicians identified 186 patients who expressed an interest in the study, and they transmitted the patient contact information to the research team. Patients were seen in a traditional primary care setting. All 186 patients were enrolled by phone. However, only 89 completed and returned their diaries and signed consent forms; of these, 60% were female, 92% were white, 4% were black, 4% were American Indians, 2% were Hispanic, and 7% had diabetes mellitus.
Five practices contributed data on numbers of poison ivy encounters per month and total encounters per month for the year 2013. They included an inner city academic practice in central Oklahoma and a rural community health center, a suburban private practice, and 2 private practices in a town of 30,000 in eastern Oklahoma. The largest average number of encounters occurred between April and August.
The distribution of enrolled-patient visits by month and season corresponded roughly to the proportions of all patient visits for poison ivy, with 1% occurring in the winter, 35% in the spring, 55% during the summer, and 9% in the fall. Virtually all study participants (92%) complained of pruritus and had erythema (91%) and papules (87%). Forty-nine percent had vesicles or bullae. The area of the body most often affected was the head/face/neck, 61%, followed by the trunk, 56%; legs, 54%; and arms, 22%.
From the date of initial clinical consultation, the mean/median (SD; range) duration of symptoms and signs were: pruritus, 10.9/9 days (7.1; 0-43); erythema, 13.7/13 days (7.7; 0-42); papules, 10.1/9.5 days (6.5; 0-37); and vesicles, 5.3/5 days (4.1; 0-15). The mean/median (SD; range) duration of any symptom or sign was 14.4/13.5 days (8; 1-43). Rashes with vesicles tended to last longer (16.1 vs 12.9 days), but this difference did not reach statistical significance.
Treatments used by patients before and after their primary care visit are shown in TABLE 1. Seventy-three percent of patients had tried something from one treatment category before consulting a clinician, and 31% had tried something from more than one category. They were most likely to have used a topical antipruritic, astringent, or low-potency corticosteroid, or a combination of these. Clinicians always recommended some treatment and, in 76% of cases, treatments from more than one category. They most often prescribed oral or parenteral corticosteroids (81% of the time), sometimes in combination with a high-potency topical corticosteroid (25% of the time) or oral antihistamine (31%).

No statistically significant associations were found between the baseline non-treatment variables and duration of symptoms and signs. Patient-initiated treatments were also not associated with duration of symptoms and signs following the initial clinician visit.
Of the treatments prescribed by clinicians or independently chosen by patients following their initial office visit, only systemic corticosteroids plus high-potency topical corticosteroids were associated with a significantly shorter duration of itching (P=.005). No treatment was associated with reduced duration of erythema, papules, or vesicles. Use of topical soaps was associated with a longer duration of papules (P<.0001) and of total duration of signs or symptoms (P=.0004) compared with other treatments.
Location and characteristics of the rash were not associated with likelihood of recurrence following treatment. Post-visit use of a topical soap was associated with recurrence of itching (P=.001) and erythema (P=.01). Recurrence of erythema was also more frequent in patients prescribed topical astringents (beta coefficient=0.28; P=.008), and recurrence of papules was more common in patients treated with low-potency topical corticosteroids (P<.0001). These results and several others that almost reached statistical significance are shown in TABLE 2.

In the multivariable models, the only variable associated with duration of pruritus was the combination of systemic and high-potency topical corticosteroids (8 vs 12 days.) Use of only parenteral or only high-potency topical corticosteroids did not predict shorter duration of pruritus. Use of topical soaps was associated with longer duration of papules (33 vs 9.6 days) and longer duration of any symptoms (33 vs 13.9 days). It was also associated with a higher likelihood of recurrence of pruritus (chi square test [χ2], 10.67) and recurrence of erythema (χ2, 5.92) after initial resolution. Topical astringent use was predictive of recurrence of erythema (χ2, 7.01) and use of low-potency corticosteroids was associated with recurrence of papules (χ2, 20.96).
DISCUSSION
While network clinicians felt that studying poison ivy was of interest and importance, and we had preliminary survey information to suggest it was a common problem treated in primary care, our data suggest that clinical encounters for poison ivy are actually quite uncommon (less than 0.4% of all encounters) even during peak months. Our problems with recruitment were therefore unexpected, and we ended up with far fewer enrolled patients than we had projected, and needed, based on our power analysis. Also based on our preliminary survey, we anticipated considerably more variation in treatment approach than we found. Most clinicians recommended either an oral, parenteral, or high-potency topical corticosteroid, and some also recommended an oral antihistamine, usually diphenhydramine.
The literature and common sense suggest that most patients who seek medical treatment for poison ivy are primarily concerned about itching. Even with the smaller-than-anticipated number of participants in this study, we were able to show that the combination of a systemic (oral or parenteral) corticosteroid and a high-potency topical corticosteroid was associated with a statistically significant shorter duration of pruritus with no recurrence following treatment. We found no evidence that systemic corticosteroids alone, parenteral corticosteroids alone, or high-potency topical corticosteroids alone had any effect on duration of signs or symptoms, even at an alpha of .05. We also found no evidence that oral antihistamines were associated with a shorter duration of pruritus (P=.06); with a larger sample size, we might have found a difference.
Since only 2 patients used topical soaps following their initial clinician visit, the associations between use of these products and longer duration of signs and symptoms and with recurrence of signs and symptoms, although statistically significant, should be viewed with skepticism and with an eye toward possible confounders (eg, people who used these agents may have been more likely to notice and record minor symptoms). Furthermore, these agents have been effective only when used before or at the onset of the rash.
Study limitations. The study has a number of limitations. It had a high drop-out rate. Some patients might not have had poison ivy, but it is generally considered easy to diagnose with accuracy. We cannot be sure that all of the enrolled patients had Rhus dermatitis. Enrollment was based on the clinical impression of the patients’ primary care clinicians. The sample size reduced the power of the study to detect small differences in treatment effects and prevented more complex analyses (eg, combinations of medications, interactions).
The possibility of self-selection bias, weaknesses of the cohort design, and patient-reported outcome measures were additional limitations. The study was also carried out in a single southwestern state, which may not be representative of some other locations. However, it is one of only a few studies published on Rhus dermatitis and possibly the only one conducted in primary care settings.
CORRESPONDENCE
Cara Vaught, MPH, University of Oklahoma Health Sciences Center, Department of Family and Preventive Medicine, 900 NE 10th Street, Oklahoma City, OK 73104; [email protected].
ACKNOWLEDGEMENT
The authors thank the Oklahoma Physicians Resource/Research Network (OKPRN) and the OKPRN clinician members (as well as their staff and patients) for their contributions to this study. The authors also thank Bradley Long, Matthew Marr, and Kellie Hetherington for their involvement in the data collection for this study.
1. Epstein WL. Occupational poison ivy and oak dermatitis. Dermatol Clin. 1994;12:511-516.
2. Long D, Ballentine NH, Marks JG Jr. Treatment of poison ivy/oak allergic contact dermatitis with an extract of jewelweed. Am J Contact Dermat. 1997;8:150-153.
3. Amrol D, Keitel D, Hagaman D, et al. Topical pimecrolimus in the treatment of human allergic contact dermatitis. Ann Allergy Asthma Immunol. 2003;91:563-566.
4. Davila A, Laurora M, Fulton J, et al. A new topical agent, Zanfel, ameliorates urushiol-induced Toxicodendron allergic contact dermatitis [abstract 364]. Ann Emerg Med. 2003;42(suppl 4):S98.
5. Vernon HJ, Olsen EA. A controlled trial of clobetasol propionate ointment 0.05% in the treatment of experimentally induced Rhus dermatitis. J Am Acad Dermatol. 1990;23:829-832.
6. Dickey RF. Parenteral short-term corticosteroid therapy in moderate to severe dermatoses. A comparative multiclinic study. Cutis. 1976;17:179-193.
7. Goodall J. Oral corticosteroids for poison ivy dermatitis. CMAJ. 2002;166:300-301.
8. Moe JF. How much steroid for poison ivy? Postgrad Med. 1999;106:21,24.
1. Epstein WL. Occupational poison ivy and oak dermatitis. Dermatol Clin. 1994;12:511-516.
2. Long D, Ballentine NH, Marks JG Jr. Treatment of poison ivy/oak allergic contact dermatitis with an extract of jewelweed. Am J Contact Dermat. 1997;8:150-153.
3. Amrol D, Keitel D, Hagaman D, et al. Topical pimecrolimus in the treatment of human allergic contact dermatitis. Ann Allergy Asthma Immunol. 2003;91:563-566.
4. Davila A, Laurora M, Fulton J, et al. A new topical agent, Zanfel, ameliorates urushiol-induced Toxicodendron allergic contact dermatitis [abstract 364]. Ann Emerg Med. 2003;42(suppl 4):S98.
5. Vernon HJ, Olsen EA. A controlled trial of clobetasol propionate ointment 0.05% in the treatment of experimentally induced Rhus dermatitis. J Am Acad Dermatol. 1990;23:829-832.
6. Dickey RF. Parenteral short-term corticosteroid therapy in moderate to severe dermatoses. A comparative multiclinic study. Cutis. 1976;17:179-193.
7. Goodall J. Oral corticosteroids for poison ivy dermatitis. CMAJ. 2002;166:300-301.
8. Moe JF. How much steroid for poison ivy? Postgrad Med. 1999;106:21,24.
HIV update: Which single-tablet regimens, and when
› Offer all patients with human immunodeficiency virus (HIV) disease antiretroviral therapy (ART) regardless of disease state or CD4 cell lymphocyte count. A
› Consider one of 6 recommended ART regimens for ART-naive patients. A
› Offer one of 6 alternative antiretroviral regimens to patients unable to tolerate one of the recommended regimens for reasons of toxicity, a pre-existing medical condition, or baseline viral resistance. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › James G, age 43, recently had blood work performed for a life insurance policy, and his human immunodeficiency virus (HIV) test came back positive. At a follow-up office visit, Mr. G reports having anonymous male sexual partners when traveling to New York on business and rarely using condoms. His last HIV test was “about 4 years ago.” He is otherwise in good health, takes no regular medications, and is not married.
Having recently completed a primary care CME program on HIV disease, you order a CD4/T-cell count, an HIV RNA (viral load) test, and an HIV genotype drug resistance test on Mr. G, along with other baseline lab work, including a complete blood count, chemistry panel, and hepatitis panel. You schedule a follow-up visit with Mr. G in 2 weeks when all of the lab results will be available so that you can discuss his plan of care.
A diagnosis of HIV has moved from being a fatal disease to that of a chronic condition that can be effectively managed with combination antiretroviral therapy (ART) regimens over an almost normal lifespan. As a result, the role of the primary care practitioner in the ongoing care of patients with HIV has grown and will continue to do so, making knowledge of these drug combinations vital.
20 years have changed everything
Combination ART has existed since 1996 when the first protease inhibitors (PIs) were approved by the US Food and Drug Administration (FDA). Prior to this, treatment was limited to mono or dual therapy with nucleoside reverse transcriptase inhibitors (NRTIs). These agents provided some short-term clinical benefit, but didn’t significantly improve patient survival and ultimately failed due to viral resistance.1
Since the approval of zidovudine (AZT) in 1987, the FDA has approved more than 25 drugs in 6 different classes for the treatment of HIV disease.2 These include the NRTIs, non-nucleoside reverse transcriptase inhibitors (NNRTIs), PIs, a fusion inhibitor (FI), a CCR5 antagonist, and, more recently, integrase strand transfer inhibitors (INSTIs). In addition, 2 drugs, cobicistat and ritonavir, are used solely to improve or “boost” the pharmacokinetic profiles of several antiretroviral drugs.2
Most of these newer agents are more potent, have a higher genetic barrier to resistance, and a longer half-life than their predecessors. Moreover, many are less toxic and thus more tolerable than older drugs. With the progressive development and approval of single-tablet regimens (STRs) that contain 3 or 4 drugs, the majority of patients with HIV in the United States now take just one pill per day to treat their infection, facilitating far greater medication adherence.
Initiation of antiretroviral therapy
The US Department of Health and Human Services (DHHS) guidelines now recommend that all people infected with HIV, regardless of CD4 cell count, begin ART.2 The evidence for this recommendation comes largely from the START3 and TEMPRANO4 trials, which found that early initiation of ART significantly reduces morbidity and mortality associated with HIV. In addition, the HPTN 052 study concluded that early ART is associated with a 93% lower risk of viral transmission in serodiscordant heterosexual couples.5 The DHHS guidelines do note that when initiating ART, it is important to appropriately educate patients on the benefits of treatment and address strategies to optimize adherence.2 (For more on factors to consider when selecting an initial HIV regimen, see TABLE 1.2) On a case-by-case basis, ART may be deferred because of clinical and/or psychosocial factors, but it should never be withheld unless the risks clearly outweigh the benefits. Ideally, ART should be initiated as soon as possible after the initial diagnosis of HIV.
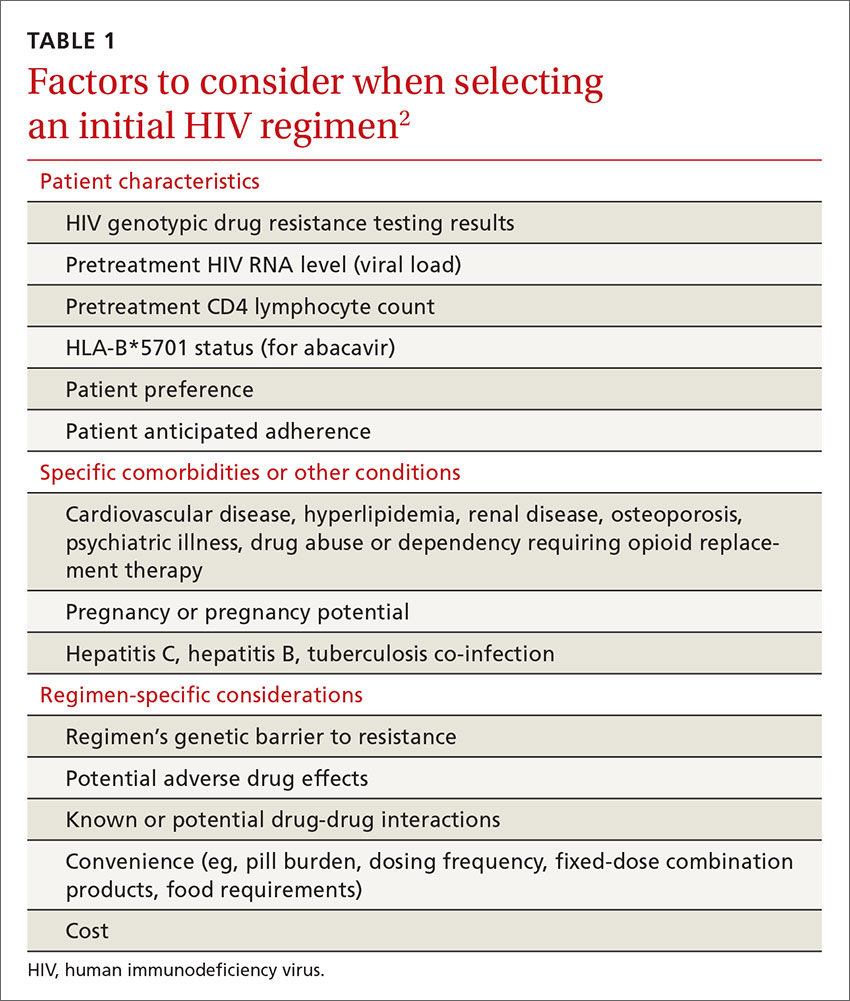
The DHHS guidelines divide treatment options into 3 categories:2
- Recommended regimens are backed by randomized controlled trials that show optimal and durable virologic efficacy, they have favorable tolerability and toxicity profiles, and they are easy to use.
- Alternative regimens have less or lower quality supporting data than recommended regimens. Although they are effective and may be optimal for certain individual patients, they have potential disadvantages and/or limitations in certain populations.
- Other regimens have limited supporting data, reduced virologic activity, a higher pill burden, more drug interactions, and greater toxicity.
Currently recommended first-line therapies
An antiretroviral regimen for a treatment-naive patient should consist of 2 NRTIs in combination with a third active antiretroviral drug from one of 3 drug classes. These include: an INSTI, a boosted PI, or, in some situations, an NNRTI. The DHHS guidelines panel currently recommends 6 different ART combinations as first-line treatment in treatment-naive patients (TABLE 2).2
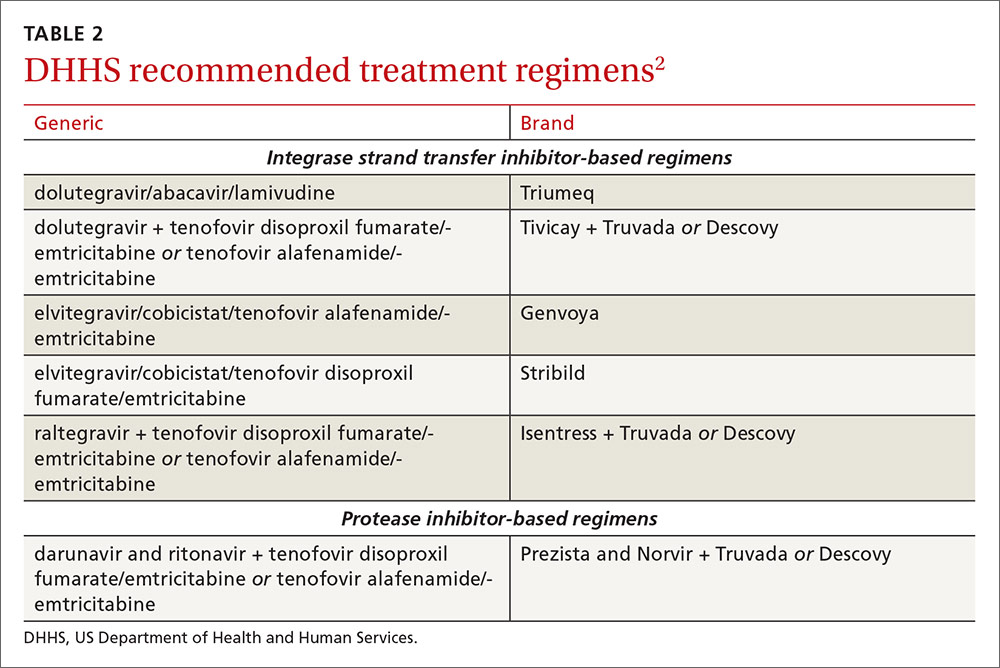
INSTI-based regimens
Dolutegravir/abacavir/lamivudine (Triumeq). Approved by the FDA as a single-tablet regimen in 2014, the combination of dolutegravir/abacavir/lamivudine has proven to be highly effective and well-tolerated in many clinical trials.6-9 However, before this regimen is started, patients must be screened for the HLA-B*5701 allele, which predicts hypersensitivity to abacavir.10 Assessing patients’ risk for cardiovascular disease is also advised because some data suggest that abacavir may increase the risk of cardiovascular events, although this remains controversial.2
Dolutegravir is generally well-tolerated with minimal adverse effects (≥2% incidence of headache and insomnia) and toxicity.11 Dolutegravir/abacavir/lamivudine should be taken 2 hours before or 6 hours after taking antacids or laxatives, sucralfate, and oral supplements with iron or calcium. However, it may be taken with calcium or iron supplements if it is also taken with food.11 Dolutegravir increases levels of metformin about 2-fold, so patients should not take more than 1000 mg/d of this oral hypoglycemic agent.11
Dolutegravir plus tenofovir disoproxil fumarate/emtricitabine (Tivicay plus Truvada). The combination of dolutegravir plus fixed-dose tenofovir disoproxil fumarate and emtricitabine is administered as 2 pills per day. Because tenofovir disoproxil fumarate can cause proximal renal tubular dysfunction, phosphate wasting, and decreased bone mineral density (BMD), avoid prescribing it for patients with underlying renal dysfunction (creatinine clearance [CrCl] <50 mL/min) and prescribe it cautiously for patients with hypertension or diabetes who are at increased risk of renal disease. Emtricitabine is generally safe and well tolerated, but the dose should be reduced in patients with renal insufficiency, which would preclude the use of this fixed-dose combination.12
Elvitegravir/cobicistat/tenofovir alafenamide/emtricitabine (Genvoya). The newer 4-drug combination of elvitegravir/cobicistat/tenofovir alafenamide/emtricitabine that was approved by the FDA in November 2015,13 contains the more recently approved form of tenofovir, which can be used in patients who have a CrCl as low as 30 mL/min. Compared to formulations containing tenofovir disoproxil fumarate, the newer tenofovir alafenamide formulation achieves higher intracellular levels in CD4 lymphocytes (but not in renal tubular cells). This allows for a lower dose of the drug and a smaller tablet size with co-formulation. It does not appear to cause kidney problems or loss of BMD as can be seen with tenofovir disoproxil fumarate.14 This newer single-tablet regimen may be best suited for older patients with HIV or those with comorbidities such as hypertension or diabetes.
Elvitegravir/cobicistat/tenofovir disoproxil fumarate/emtricitabine (Stribild). The FDA approved the combination of elvitegravir/cobicistat/tenofovir disoproxil fumarate/emtricitabine as a single-tablet regimen in 2012. The integrase inhibitor, elvitegravir, requires boosting with the CYP3A inhibitor, cobicistat, and should be taken with food.15 Two clinical trials demonstrated the superior efficacy of elvitegravir compared to a boosted PI and NNRTI-based regimen.16,17 Elvitegravir is generally well tolerated, but sometimes causes dyspepsia, nausea, or diarrhea.15 Similar to dolutegravir, it should not be taken concurrently with certain supplements—in this case, those containing aluminum, calcium, iron, magnesium, or zinc.15 Because it contains tenofovir disoproxil fumarate as an active agent, it should not be used in patients with a CrCl of <70 mL/min.15
Cobicistat inhibits tubular secretion of creatinine, so it may produce an elevation in serum creatinine without actually affecting glomerular function. Cobicistat may also cause drug-drug interactions with certain antiarrhythmics, sedative-hypnotics, and erectile dysfunction agents, and is contraindicated with some statins, anticonvulsants, and ergot derivatives.18
Raltegravir plus tenofovir disoproxil fumarate/emtricitabine (Isentress plus Truvada). The combination of the integrase inhibitor raltegravir plus fixed-dose tenofovir disoproxil fumarate and emtricitabine has been recommended by the DHHS as first-line therapy for approximately 5 years. The recommendation is based mainly on data from the STARTMRK trial, a phase III non-inferiority trial that followed more than 500 patients for 5 years and concluded that raltegravir/tenofovir/emtricitabine has superior efficacy with fewer drug-related adverse effects than efavirenz/tenofovir/emtricitabine.19 The overall pill burden with this regimen is 3 tablets per day. Although highly effective, the main drawbacks of raltegravir are that it must be dosed twice daily (which may be less preferable if adherence is a concern) and the genetic barrier to resistance is lower than that of the other 2 approved integrase inhibitors. There is a once-daily formulation of raltegravir that's expected to be available late in 2017.20
Adverse effects and toxicities (except the renal and bone effects due to tenofovir disoproxil fumarate mentioned earlier) and drug interactions with this regimen are infrequent. Raltegravir can be taken with or without food. Concurrent use of antacids that contain aluminum or magnesium may reduce absorption of raltegravir and so should be avoided.21
PI-based regimen
Darunavir (Prezista) and ritonavir (Norvir) plus tenofovir disoproxil fumarate/emtricitabine (Truvada). PIs were once the key component of all ART regimens; however, boosted darunavir is now the only PI-based regimen currently recommended as first-line therapy. It is taken as 3 tablets once daily. If the co-formulation with cobicistat is used, just 2 tablets daily are required. One advantage with darunavir with either of the boosting agents is that it does not appear to cause insulin resistance or dyslipidemia as occurs with older PIs, such as indinavir and lopinavir.2 The boosting agents do, however, increase the likelihood of drug-drug interactions. As with all PIs, darunavir has a very high genetic barrier to resistance, which is important in patients for whom adherence is a concern.
Adverse effects of the PIs may include nausea, vomiting, and diarrhea, all of which are typically mild and self-limiting.22 Co-formulation of darunavir with cobicistat, tenofovir alafenamide, and emtricitabine is in phase III studies. Projected to be available in late 2017, it will provide yet another daily STR option.23
The addition of fixed-dose tenofovir alafenamide/emtricitabine
In July 2016, the DHHS panel made some additions to their guidelines to reflect the FDA approval of 3 fixed-dose combination products that contain tenofovir alafenamide. Specifically, the combination of tenofovir alafenamide and emtricitabine is recommended for use with the integrase inhibitors—dolutegravir or raltegravir. It is also recommended in combination with ritonavir-boosted darunavir.
DHHS “alternative” and“other” regimens
The DHHS guidelines also include “alternative” (TABLE 32) and “other” regimens (available at: http://aidsinfo.nih.gov/guidelines) that may be used when first-line regimens may not. These second-line options are very effective, but have some possible clinical disadvantages or limitations. They are also less well supported by data from clinical trials. However, in certain situations, depending on an individual patient’s comorbidities, inability to tolerate one of the preferred regimens, or personal preferences, an alternative regimen may be the optimal choice.
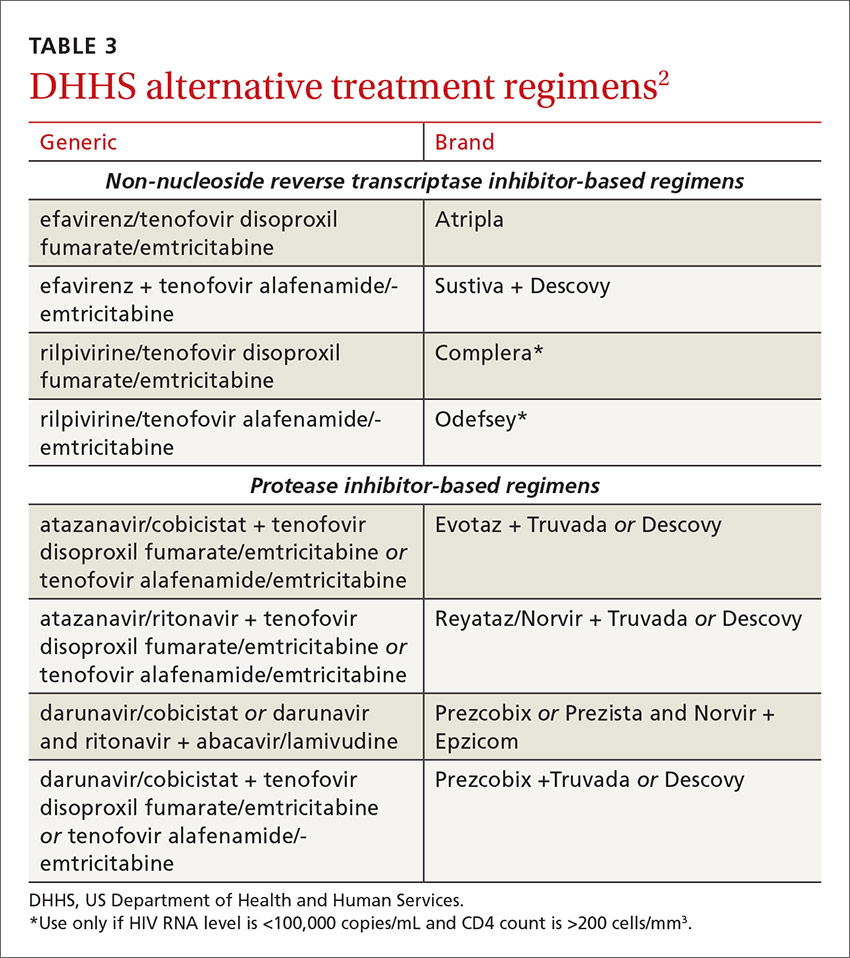
Under the category of alternative regimens, the panel has included tenofovir alafenamide and emtricitabine in combination with the NNRTI efavirenz or with ritonavir- or cobicistat-boosted atazanavir or darunavir.
The third group or “other” regimens have reduced virologic activity, increased toxicity, and even more limited data from clinical trials. Generally, medications from the DHHS “alternative” and “other” categories should be prescribed in consultation with an HIV specialist.
The future of ART
The currently available drugs are highly effective in fully suppressing HIV and allowing for immune recovery and clinical stability for most patients. Life expectancy for patients living with HIV is estimated to be approaching that of uninfected adults—provided they remain on ART.24 As a way to further simplify ART, current clinical trials are looking at 2-drug regimens including an integrase inhibitor with an NRTI, an INSTI, or an NNRTI, or a PI with one NRTI.25,26 This approach could further reduce pill burden and toxicity and substantially decrease the cost of long-term treatment.27 Also on the horizon are long-acting injectable antiretroviral drugs that will likely be available for clinical use in the next 2 to 3 years.28,29
CASE › At the 2-week follow-up visit, you discuss with Mr. G that his CD4+ count is 390 cells/mm3, his HIV RNA level is 32,450 copies/mL, and his HIV genotype test showed no antiviral drug resistance. Explaining that all patients with HIV should be treated with antiviral therapy regardless of CD4+ count, you recommend that Mr. G begin taking fixed-dose tenofovir disoproxil fumarate/emtricitabine/elvitegravir/cobicistat (Stribild), noting that it is one of the regimens recommended by the DHHS national treatment guidelines. You provide a patient handout that discusses dosing and adverse effects, including nausea and headache. The patient’s pharmacy was contacted and it was determined that Mr. G’s co-pay for the drug would be $50, which he found acceptable.
In addition, you discuss the importance of good adherence to this medication, and instruct Mr. G to contact the office via phone or patient portal for any concerns or questions that arise after starting the medication. Lastly, you advise him to return in 4 weeks for follow-up blood testing, including viral load monitoring, and additional care, if needed, and strongly recommend that he begin using condoms regularly.
CORRESPONDENCE
Jeffrey T. Kirchner, DO, FAAFP, AAHIVS, Medical Director, LGHP Comprehensive Care, 554 North Duke St., 3rd Floor, Lancaster, PA 1760; [email protected].
1. Concorde: MRC/ANRS randomised double-blind controlled trial of immediate and deferred zidovudine in symptom-free HIV infection. Concorde Coordinating Committee. Lancet. 1994;343:871-881.
2. Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available at: http://www.aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-treatment-guidelines/0. Accessed July 17, 2016.
3. The INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795-807.
4. The TEMPRANO ANRS 12136 Study Group. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373:808-822.
5. Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375:830-839.
6. Molina JM, Clotet B, van Lunzen J,et al. Once-daily dolutegravir versus darunavir plus ritonavir for treatment-naive adults with HIV-1 infection (FLAMINGO): 96 week results from a randomized, open-label, phase 3b study. Lancet HIV. 2015;2:e127-136.
7. Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369:1807-1818.
8. Van Lunzen J, Maggiolo F, Arribas JR, et al. Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naïve adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomized, phase 2b trial. Lancet Infect Dis. 2012;12:111-118.
9. Stellbrink HJ, Reynes J, Lazzarin A, et al. Dolutegravir in antiretroviral-naive adults with HIV-1: 96-week results from a randomized dose-ranging study. AIDS. 2013; 27:1771-1778.
10. Mallal S, Phillips E, Carosi G. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358:568-579.
11. AIDSinfo Drug Database. Dolutegravir. Available at: https://aidsinfo.nih.gov/drugs/509/dolutegravir/0/professional. Accessed July 17, 2016.
12. AIDSinfo Drug Database. Emtricitabine. Available at: https://aidsinfo.nih.gov/drugs/208/emtricitabine/0/patient. Accessed July 17, 2016.
13. AIDSinfo Drug Database. Elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide fumarate. Available at: https://aidsinfo.nih.gov/drugs/553/genvoya/0/professional. Accessed July 17, 2016.
14. Ray AS, Fordyce MW, Hitchcock, MJM. Tenofovir alafenamide: A novel prodrug of tenofovir for the treatment of human immunodeficiency virus. Antiviral Res. 2016;125:63-70.
15. AIDSinfo Drug Database. Elvitegravir. https://aidsinfo.nih.gov/drugs/421/elvitegravir/0/professional
16. Wohl DA, Cohen C, Gallant JE, et al. A randomized, double-blind comparison of single-tablet regimen elvitegravir/cobicistat/emtricitabine/tenofovir DF versus single-tablet regimen efavirenz/emtricitabine/tenofovir DF for initial treatment of HIV-1 infection: analysis of week 144 results. J Acquir Immune Defic Syndr. 2014;65:e118-120.
17. Clumeck N, Molina JM, Henry K, et al. A randomized, double-blind comparison of single-tablet regimen elvitegravir/cobicistat/emtricitabine/tenofovir DF vs ritonavir-boosted atazanavir plus emtricitabine/tenofovir for initial treatment of HIV-1 infection: analysis of week 144 results. J Acquir Immune Defic Syndr. 2014;65:e121-124.
18. AIDSinfo Drug Database. Cobicistat. Available at: https://aidsinfo.nih.gov/drugs/537/evotaz/0/patient/. Accessed July 17, 2016.
19. Rockstroh JK, DeJesus E, Lennox JL, et al. Durable efficacy and safety of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naïve HIV-1 infected patients: final 5-year results from STARTMRK. J Acquir Immune Defic Syndr. 2013;63:77-85.
20. Cahn P, Kaplan R, Sax P, et al. Raltegravir (RAL) 1200 mg once daily (QD) is non-inferior to RAL 400 mg twice daily (BID), in combination with tenofovir/emtricitabine, in treatment-naive HIV-1-infected subjects: week 48 results. Abstract FRAB0103LB presented at: 21st International AIDS Conference; July 18-22, 2016; Durban, South Africa.
21. Hicks C, Gulick RM. Raltegravir: the first HIV type 1 integrase inhibitor. Clin Infect Dis. 2009;48:931-939.
22. Prescriber’s Letter. HIV/AIDS Pharmacotherapy Review. Vol. 2015; Course no. 215. Available at: http://prescribersletter.therapeuticresearch.com/ce/cecourse.aspx?pc=15-215. Accessed October 6
23. AIDSinfo Drug Database. Tenofovir alafenamide. Available at: https://aidsinfo.nih.gov/drugs/514/tenofovir-alafenamide/0/patient. Accessed September 27, 2016.
24. Marcus JL, Chao C, Leyden W, et al. Narrowing the gap in life expectancy for HIV+ compared with HIV- individuals. Conference on Retroviruses and Opportunistic Infections. February 22-25, 2016, Boston. Abstract 54.
25. Gubavu C, Prazuck T, Niang M, et al. Dolutegravir-based monotherapy or dual therapy maintains a high proportion of viral suppression even in highly experienced HIV-1-infected patients. J Antimicrob Chemother. 2016;71:1046-1050.
26. Margolis DA, Brinson CC, Smith GHR. Cabotegravir plus rilpivirine, once a day, after induction with cabotegravir plus nucleoside reverse transcriptase inhibitors in antiretroviral naïve adults with HIV-1 infection (LATTE): a randomised, phase 2b, dose-ranging trial. Lancet Infect Dis. 2015;15:1145-1155.
27. Girouard MP, Sax PE, Parker RA, et al. The cost-effectiveness and budget impact of 2-drug dolutegravir-lamivudine regimens for the treatment of HIV infection in the United States. Clin Infect Dis. 2016; 62:784-791.
28. Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Cabotegravir + rilpivirine as long-acting maintenance therapy: LATTE-2 week 32 results. Abstract number 31 LB. Conference on Retroviruses and Opportunistic Infections. February 22-25, 2016; Boston, MA.
29. Murray MI, Markowitz M, Frank I, et al. Tolerability and acceptability of cabotegravir LA injection: results from ECLAIR study. Abstract number 471. Conference on Retroviruses and Opportunistic Infections. February 22-25, 2016; Boston, MA.
› Offer all patients with human immunodeficiency virus (HIV) disease antiretroviral therapy (ART) regardless of disease state or CD4 cell lymphocyte count. A
› Consider one of 6 recommended ART regimens for ART-naive patients. A
› Offer one of 6 alternative antiretroviral regimens to patients unable to tolerate one of the recommended regimens for reasons of toxicity, a pre-existing medical condition, or baseline viral resistance. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › James G, age 43, recently had blood work performed for a life insurance policy, and his human immunodeficiency virus (HIV) test came back positive. At a follow-up office visit, Mr. G reports having anonymous male sexual partners when traveling to New York on business and rarely using condoms. His last HIV test was “about 4 years ago.” He is otherwise in good health, takes no regular medications, and is not married.
Having recently completed a primary care CME program on HIV disease, you order a CD4/T-cell count, an HIV RNA (viral load) test, and an HIV genotype drug resistance test on Mr. G, along with other baseline lab work, including a complete blood count, chemistry panel, and hepatitis panel. You schedule a follow-up visit with Mr. G in 2 weeks when all of the lab results will be available so that you can discuss his plan of care.
A diagnosis of HIV has moved from being a fatal disease to that of a chronic condition that can be effectively managed with combination antiretroviral therapy (ART) regimens over an almost normal lifespan. As a result, the role of the primary care practitioner in the ongoing care of patients with HIV has grown and will continue to do so, making knowledge of these drug combinations vital.
20 years have changed everything
Combination ART has existed since 1996 when the first protease inhibitors (PIs) were approved by the US Food and Drug Administration (FDA). Prior to this, treatment was limited to mono or dual therapy with nucleoside reverse transcriptase inhibitors (NRTIs). These agents provided some short-term clinical benefit, but didn’t significantly improve patient survival and ultimately failed due to viral resistance.1
Since the approval of zidovudine (AZT) in 1987, the FDA has approved more than 25 drugs in 6 different classes for the treatment of HIV disease.2 These include the NRTIs, non-nucleoside reverse transcriptase inhibitors (NNRTIs), PIs, a fusion inhibitor (FI), a CCR5 antagonist, and, more recently, integrase strand transfer inhibitors (INSTIs). In addition, 2 drugs, cobicistat and ritonavir, are used solely to improve or “boost” the pharmacokinetic profiles of several antiretroviral drugs.2
Most of these newer agents are more potent, have a higher genetic barrier to resistance, and a longer half-life than their predecessors. Moreover, many are less toxic and thus more tolerable than older drugs. With the progressive development and approval of single-tablet regimens (STRs) that contain 3 or 4 drugs, the majority of patients with HIV in the United States now take just one pill per day to treat their infection, facilitating far greater medication adherence.
Initiation of antiretroviral therapy
The US Department of Health and Human Services (DHHS) guidelines now recommend that all people infected with HIV, regardless of CD4 cell count, begin ART.2 The evidence for this recommendation comes largely from the START3 and TEMPRANO4 trials, which found that early initiation of ART significantly reduces morbidity and mortality associated with HIV. In addition, the HPTN 052 study concluded that early ART is associated with a 93% lower risk of viral transmission in serodiscordant heterosexual couples.5 The DHHS guidelines do note that when initiating ART, it is important to appropriately educate patients on the benefits of treatment and address strategies to optimize adherence.2 (For more on factors to consider when selecting an initial HIV regimen, see TABLE 1.2) On a case-by-case basis, ART may be deferred because of clinical and/or psychosocial factors, but it should never be withheld unless the risks clearly outweigh the benefits. Ideally, ART should be initiated as soon as possible after the initial diagnosis of HIV.

The DHHS guidelines divide treatment options into 3 categories:2
- Recommended regimens are backed by randomized controlled trials that show optimal and durable virologic efficacy, they have favorable tolerability and toxicity profiles, and they are easy to use.
- Alternative regimens have less or lower quality supporting data than recommended regimens. Although they are effective and may be optimal for certain individual patients, they have potential disadvantages and/or limitations in certain populations.
- Other regimens have limited supporting data, reduced virologic activity, a higher pill burden, more drug interactions, and greater toxicity.
Currently recommended first-line therapies
An antiretroviral regimen for a treatment-naive patient should consist of 2 NRTIs in combination with a third active antiretroviral drug from one of 3 drug classes. These include: an INSTI, a boosted PI, or, in some situations, an NNRTI. The DHHS guidelines panel currently recommends 6 different ART combinations as first-line treatment in treatment-naive patients (TABLE 2).2

INSTI-based regimens
Dolutegravir/abacavir/lamivudine (Triumeq). Approved by the FDA as a single-tablet regimen in 2014, the combination of dolutegravir/abacavir/lamivudine has proven to be highly effective and well-tolerated in many clinical trials.6-9 However, before this regimen is started, patients must be screened for the HLA-B*5701 allele, which predicts hypersensitivity to abacavir.10 Assessing patients’ risk for cardiovascular disease is also advised because some data suggest that abacavir may increase the risk of cardiovascular events, although this remains controversial.2
Dolutegravir is generally well-tolerated with minimal adverse effects (≥2% incidence of headache and insomnia) and toxicity.11 Dolutegravir/abacavir/lamivudine should be taken 2 hours before or 6 hours after taking antacids or laxatives, sucralfate, and oral supplements with iron or calcium. However, it may be taken with calcium or iron supplements if it is also taken with food.11 Dolutegravir increases levels of metformin about 2-fold, so patients should not take more than 1000 mg/d of this oral hypoglycemic agent.11
Dolutegravir plus tenofovir disoproxil fumarate/emtricitabine (Tivicay plus Truvada). The combination of dolutegravir plus fixed-dose tenofovir disoproxil fumarate and emtricitabine is administered as 2 pills per day. Because tenofovir disoproxil fumarate can cause proximal renal tubular dysfunction, phosphate wasting, and decreased bone mineral density (BMD), avoid prescribing it for patients with underlying renal dysfunction (creatinine clearance [CrCl] <50 mL/min) and prescribe it cautiously for patients with hypertension or diabetes who are at increased risk of renal disease. Emtricitabine is generally safe and well tolerated, but the dose should be reduced in patients with renal insufficiency, which would preclude the use of this fixed-dose combination.12
Elvitegravir/cobicistat/tenofovir alafenamide/emtricitabine (Genvoya). The newer 4-drug combination of elvitegravir/cobicistat/tenofovir alafenamide/emtricitabine that was approved by the FDA in November 2015,13 contains the more recently approved form of tenofovir, which can be used in patients who have a CrCl as low as 30 mL/min. Compared to formulations containing tenofovir disoproxil fumarate, the newer tenofovir alafenamide formulation achieves higher intracellular levels in CD4 lymphocytes (but not in renal tubular cells). This allows for a lower dose of the drug and a smaller tablet size with co-formulation. It does not appear to cause kidney problems or loss of BMD as can be seen with tenofovir disoproxil fumarate.14 This newer single-tablet regimen may be best suited for older patients with HIV or those with comorbidities such as hypertension or diabetes.
Elvitegravir/cobicistat/tenofovir disoproxil fumarate/emtricitabine (Stribild). The FDA approved the combination of elvitegravir/cobicistat/tenofovir disoproxil fumarate/emtricitabine as a single-tablet regimen in 2012. The integrase inhibitor, elvitegravir, requires boosting with the CYP3A inhibitor, cobicistat, and should be taken with food.15 Two clinical trials demonstrated the superior efficacy of elvitegravir compared to a boosted PI and NNRTI-based regimen.16,17 Elvitegravir is generally well tolerated, but sometimes causes dyspepsia, nausea, or diarrhea.15 Similar to dolutegravir, it should not be taken concurrently with certain supplements—in this case, those containing aluminum, calcium, iron, magnesium, or zinc.15 Because it contains tenofovir disoproxil fumarate as an active agent, it should not be used in patients with a CrCl of <70 mL/min.15
Cobicistat inhibits tubular secretion of creatinine, so it may produce an elevation in serum creatinine without actually affecting glomerular function. Cobicistat may also cause drug-drug interactions with certain antiarrhythmics, sedative-hypnotics, and erectile dysfunction agents, and is contraindicated with some statins, anticonvulsants, and ergot derivatives.18
Raltegravir plus tenofovir disoproxil fumarate/emtricitabine (Isentress plus Truvada). The combination of the integrase inhibitor raltegravir plus fixed-dose tenofovir disoproxil fumarate and emtricitabine has been recommended by the DHHS as first-line therapy for approximately 5 years. The recommendation is based mainly on data from the STARTMRK trial, a phase III non-inferiority trial that followed more than 500 patients for 5 years and concluded that raltegravir/tenofovir/emtricitabine has superior efficacy with fewer drug-related adverse effects than efavirenz/tenofovir/emtricitabine.19 The overall pill burden with this regimen is 3 tablets per day. Although highly effective, the main drawbacks of raltegravir are that it must be dosed twice daily (which may be less preferable if adherence is a concern) and the genetic barrier to resistance is lower than that of the other 2 approved integrase inhibitors. There is a once-daily formulation of raltegravir that's expected to be available late in 2017.20
Adverse effects and toxicities (except the renal and bone effects due to tenofovir disoproxil fumarate mentioned earlier) and drug interactions with this regimen are infrequent. Raltegravir can be taken with or without food. Concurrent use of antacids that contain aluminum or magnesium may reduce absorption of raltegravir and so should be avoided.21
PI-based regimen
Darunavir (Prezista) and ritonavir (Norvir) plus tenofovir disoproxil fumarate/emtricitabine (Truvada). PIs were once the key component of all ART regimens; however, boosted darunavir is now the only PI-based regimen currently recommended as first-line therapy. It is taken as 3 tablets once daily. If the co-formulation with cobicistat is used, just 2 tablets daily are required. One advantage with darunavir with either of the boosting agents is that it does not appear to cause insulin resistance or dyslipidemia as occurs with older PIs, such as indinavir and lopinavir.2 The boosting agents do, however, increase the likelihood of drug-drug interactions. As with all PIs, darunavir has a very high genetic barrier to resistance, which is important in patients for whom adherence is a concern.
Adverse effects of the PIs may include nausea, vomiting, and diarrhea, all of which are typically mild and self-limiting.22 Co-formulation of darunavir with cobicistat, tenofovir alafenamide, and emtricitabine is in phase III studies. Projected to be available in late 2017, it will provide yet another daily STR option.23
The addition of fixed-dose tenofovir alafenamide/emtricitabine
In July 2016, the DHHS panel made some additions to their guidelines to reflect the FDA approval of 3 fixed-dose combination products that contain tenofovir alafenamide. Specifically, the combination of tenofovir alafenamide and emtricitabine is recommended for use with the integrase inhibitors—dolutegravir or raltegravir. It is also recommended in combination with ritonavir-boosted darunavir.
DHHS “alternative” and“other” regimens
The DHHS guidelines also include “alternative” (TABLE 32) and “other” regimens (available at: http://aidsinfo.nih.gov/guidelines) that may be used when first-line regimens may not. These second-line options are very effective, but have some possible clinical disadvantages or limitations. They are also less well supported by data from clinical trials. However, in certain situations, depending on an individual patient’s comorbidities, inability to tolerate one of the preferred regimens, or personal preferences, an alternative regimen may be the optimal choice.

Under the category of alternative regimens, the panel has included tenofovir alafenamide and emtricitabine in combination with the NNRTI efavirenz or with ritonavir- or cobicistat-boosted atazanavir or darunavir.
The third group or “other” regimens have reduced virologic activity, increased toxicity, and even more limited data from clinical trials. Generally, medications from the DHHS “alternative” and “other” categories should be prescribed in consultation with an HIV specialist.
The future of ART
The currently available drugs are highly effective in fully suppressing HIV and allowing for immune recovery and clinical stability for most patients. Life expectancy for patients living with HIV is estimated to be approaching that of uninfected adults—provided they remain on ART.24 As a way to further simplify ART, current clinical trials are looking at 2-drug regimens including an integrase inhibitor with an NRTI, an INSTI, or an NNRTI, or a PI with one NRTI.25,26 This approach could further reduce pill burden and toxicity and substantially decrease the cost of long-term treatment.27 Also on the horizon are long-acting injectable antiretroviral drugs that will likely be available for clinical use in the next 2 to 3 years.28,29
CASE › At the 2-week follow-up visit, you discuss with Mr. G that his CD4+ count is 390 cells/mm3, his HIV RNA level is 32,450 copies/mL, and his HIV genotype test showed no antiviral drug resistance. Explaining that all patients with HIV should be treated with antiviral therapy regardless of CD4+ count, you recommend that Mr. G begin taking fixed-dose tenofovir disoproxil fumarate/emtricitabine/elvitegravir/cobicistat (Stribild), noting that it is one of the regimens recommended by the DHHS national treatment guidelines. You provide a patient handout that discusses dosing and adverse effects, including nausea and headache. The patient’s pharmacy was contacted and it was determined that Mr. G’s co-pay for the drug would be $50, which he found acceptable.
In addition, you discuss the importance of good adherence to this medication, and instruct Mr. G to contact the office via phone or patient portal for any concerns or questions that arise after starting the medication. Lastly, you advise him to return in 4 weeks for follow-up blood testing, including viral load monitoring, and additional care, if needed, and strongly recommend that he begin using condoms regularly.
CORRESPONDENCE
Jeffrey T. Kirchner, DO, FAAFP, AAHIVS, Medical Director, LGHP Comprehensive Care, 554 North Duke St., 3rd Floor, Lancaster, PA 1760; [email protected].
› Offer all patients with human immunodeficiency virus (HIV) disease antiretroviral therapy (ART) regardless of disease state or CD4 cell lymphocyte count. A
› Consider one of 6 recommended ART regimens for ART-naive patients. A
› Offer one of 6 alternative antiretroviral regimens to patients unable to tolerate one of the recommended regimens for reasons of toxicity, a pre-existing medical condition, or baseline viral resistance. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › James G, age 43, recently had blood work performed for a life insurance policy, and his human immunodeficiency virus (HIV) test came back positive. At a follow-up office visit, Mr. G reports having anonymous male sexual partners when traveling to New York on business and rarely using condoms. His last HIV test was “about 4 years ago.” He is otherwise in good health, takes no regular medications, and is not married.
Having recently completed a primary care CME program on HIV disease, you order a CD4/T-cell count, an HIV RNA (viral load) test, and an HIV genotype drug resistance test on Mr. G, along with other baseline lab work, including a complete blood count, chemistry panel, and hepatitis panel. You schedule a follow-up visit with Mr. G in 2 weeks when all of the lab results will be available so that you can discuss his plan of care.
A diagnosis of HIV has moved from being a fatal disease to that of a chronic condition that can be effectively managed with combination antiretroviral therapy (ART) regimens over an almost normal lifespan. As a result, the role of the primary care practitioner in the ongoing care of patients with HIV has grown and will continue to do so, making knowledge of these drug combinations vital.
20 years have changed everything
Combination ART has existed since 1996 when the first protease inhibitors (PIs) were approved by the US Food and Drug Administration (FDA). Prior to this, treatment was limited to mono or dual therapy with nucleoside reverse transcriptase inhibitors (NRTIs). These agents provided some short-term clinical benefit, but didn’t significantly improve patient survival and ultimately failed due to viral resistance.1
Since the approval of zidovudine (AZT) in 1987, the FDA has approved more than 25 drugs in 6 different classes for the treatment of HIV disease.2 These include the NRTIs, non-nucleoside reverse transcriptase inhibitors (NNRTIs), PIs, a fusion inhibitor (FI), a CCR5 antagonist, and, more recently, integrase strand transfer inhibitors (INSTIs). In addition, 2 drugs, cobicistat and ritonavir, are used solely to improve or “boost” the pharmacokinetic profiles of several antiretroviral drugs.2
Most of these newer agents are more potent, have a higher genetic barrier to resistance, and a longer half-life than their predecessors. Moreover, many are less toxic and thus more tolerable than older drugs. With the progressive development and approval of single-tablet regimens (STRs) that contain 3 or 4 drugs, the majority of patients with HIV in the United States now take just one pill per day to treat their infection, facilitating far greater medication adherence.
Initiation of antiretroviral therapy
The US Department of Health and Human Services (DHHS) guidelines now recommend that all people infected with HIV, regardless of CD4 cell count, begin ART.2 The evidence for this recommendation comes largely from the START3 and TEMPRANO4 trials, which found that early initiation of ART significantly reduces morbidity and mortality associated with HIV. In addition, the HPTN 052 study concluded that early ART is associated with a 93% lower risk of viral transmission in serodiscordant heterosexual couples.5 The DHHS guidelines do note that when initiating ART, it is important to appropriately educate patients on the benefits of treatment and address strategies to optimize adherence.2 (For more on factors to consider when selecting an initial HIV regimen, see TABLE 1.2) On a case-by-case basis, ART may be deferred because of clinical and/or psychosocial factors, but it should never be withheld unless the risks clearly outweigh the benefits. Ideally, ART should be initiated as soon as possible after the initial diagnosis of HIV.

The DHHS guidelines divide treatment options into 3 categories:2
- Recommended regimens are backed by randomized controlled trials that show optimal and durable virologic efficacy, they have favorable tolerability and toxicity profiles, and they are easy to use.
- Alternative regimens have less or lower quality supporting data than recommended regimens. Although they are effective and may be optimal for certain individual patients, they have potential disadvantages and/or limitations in certain populations.
- Other regimens have limited supporting data, reduced virologic activity, a higher pill burden, more drug interactions, and greater toxicity.
Currently recommended first-line therapies
An antiretroviral regimen for a treatment-naive patient should consist of 2 NRTIs in combination with a third active antiretroviral drug from one of 3 drug classes. These include: an INSTI, a boosted PI, or, in some situations, an NNRTI. The DHHS guidelines panel currently recommends 6 different ART combinations as first-line treatment in treatment-naive patients (TABLE 2).2

INSTI-based regimens
Dolutegravir/abacavir/lamivudine (Triumeq). Approved by the FDA as a single-tablet regimen in 2014, the combination of dolutegravir/abacavir/lamivudine has proven to be highly effective and well-tolerated in many clinical trials.6-9 However, before this regimen is started, patients must be screened for the HLA-B*5701 allele, which predicts hypersensitivity to abacavir.10 Assessing patients’ risk for cardiovascular disease is also advised because some data suggest that abacavir may increase the risk of cardiovascular events, although this remains controversial.2
Dolutegravir is generally well-tolerated with minimal adverse effects (≥2% incidence of headache and insomnia) and toxicity.11 Dolutegravir/abacavir/lamivudine should be taken 2 hours before or 6 hours after taking antacids or laxatives, sucralfate, and oral supplements with iron or calcium. However, it may be taken with calcium or iron supplements if it is also taken with food.11 Dolutegravir increases levels of metformin about 2-fold, so patients should not take more than 1000 mg/d of this oral hypoglycemic agent.11
Dolutegravir plus tenofovir disoproxil fumarate/emtricitabine (Tivicay plus Truvada). The combination of dolutegravir plus fixed-dose tenofovir disoproxil fumarate and emtricitabine is administered as 2 pills per day. Because tenofovir disoproxil fumarate can cause proximal renal tubular dysfunction, phosphate wasting, and decreased bone mineral density (BMD), avoid prescribing it for patients with underlying renal dysfunction (creatinine clearance [CrCl] <50 mL/min) and prescribe it cautiously for patients with hypertension or diabetes who are at increased risk of renal disease. Emtricitabine is generally safe and well tolerated, but the dose should be reduced in patients with renal insufficiency, which would preclude the use of this fixed-dose combination.12
Elvitegravir/cobicistat/tenofovir alafenamide/emtricitabine (Genvoya). The newer 4-drug combination of elvitegravir/cobicistat/tenofovir alafenamide/emtricitabine that was approved by the FDA in November 2015,13 contains the more recently approved form of tenofovir, which can be used in patients who have a CrCl as low as 30 mL/min. Compared to formulations containing tenofovir disoproxil fumarate, the newer tenofovir alafenamide formulation achieves higher intracellular levels in CD4 lymphocytes (but not in renal tubular cells). This allows for a lower dose of the drug and a smaller tablet size with co-formulation. It does not appear to cause kidney problems or loss of BMD as can be seen with tenofovir disoproxil fumarate.14 This newer single-tablet regimen may be best suited for older patients with HIV or those with comorbidities such as hypertension or diabetes.
Elvitegravir/cobicistat/tenofovir disoproxil fumarate/emtricitabine (Stribild). The FDA approved the combination of elvitegravir/cobicistat/tenofovir disoproxil fumarate/emtricitabine as a single-tablet regimen in 2012. The integrase inhibitor, elvitegravir, requires boosting with the CYP3A inhibitor, cobicistat, and should be taken with food.15 Two clinical trials demonstrated the superior efficacy of elvitegravir compared to a boosted PI and NNRTI-based regimen.16,17 Elvitegravir is generally well tolerated, but sometimes causes dyspepsia, nausea, or diarrhea.15 Similar to dolutegravir, it should not be taken concurrently with certain supplements—in this case, those containing aluminum, calcium, iron, magnesium, or zinc.15 Because it contains tenofovir disoproxil fumarate as an active agent, it should not be used in patients with a CrCl of <70 mL/min.15
Cobicistat inhibits tubular secretion of creatinine, so it may produce an elevation in serum creatinine without actually affecting glomerular function. Cobicistat may also cause drug-drug interactions with certain antiarrhythmics, sedative-hypnotics, and erectile dysfunction agents, and is contraindicated with some statins, anticonvulsants, and ergot derivatives.18
Raltegravir plus tenofovir disoproxil fumarate/emtricitabine (Isentress plus Truvada). The combination of the integrase inhibitor raltegravir plus fixed-dose tenofovir disoproxil fumarate and emtricitabine has been recommended by the DHHS as first-line therapy for approximately 5 years. The recommendation is based mainly on data from the STARTMRK trial, a phase III non-inferiority trial that followed more than 500 patients for 5 years and concluded that raltegravir/tenofovir/emtricitabine has superior efficacy with fewer drug-related adverse effects than efavirenz/tenofovir/emtricitabine.19 The overall pill burden with this regimen is 3 tablets per day. Although highly effective, the main drawbacks of raltegravir are that it must be dosed twice daily (which may be less preferable if adherence is a concern) and the genetic barrier to resistance is lower than that of the other 2 approved integrase inhibitors. There is a once-daily formulation of raltegravir that's expected to be available late in 2017.20
Adverse effects and toxicities (except the renal and bone effects due to tenofovir disoproxil fumarate mentioned earlier) and drug interactions with this regimen are infrequent. Raltegravir can be taken with or without food. Concurrent use of antacids that contain aluminum or magnesium may reduce absorption of raltegravir and so should be avoided.21
PI-based regimen
Darunavir (Prezista) and ritonavir (Norvir) plus tenofovir disoproxil fumarate/emtricitabine (Truvada). PIs were once the key component of all ART regimens; however, boosted darunavir is now the only PI-based regimen currently recommended as first-line therapy. It is taken as 3 tablets once daily. If the co-formulation with cobicistat is used, just 2 tablets daily are required. One advantage with darunavir with either of the boosting agents is that it does not appear to cause insulin resistance or dyslipidemia as occurs with older PIs, such as indinavir and lopinavir.2 The boosting agents do, however, increase the likelihood of drug-drug interactions. As with all PIs, darunavir has a very high genetic barrier to resistance, which is important in patients for whom adherence is a concern.
Adverse effects of the PIs may include nausea, vomiting, and diarrhea, all of which are typically mild and self-limiting.22 Co-formulation of darunavir with cobicistat, tenofovir alafenamide, and emtricitabine is in phase III studies. Projected to be available in late 2017, it will provide yet another daily STR option.23
The addition of fixed-dose tenofovir alafenamide/emtricitabine
In July 2016, the DHHS panel made some additions to their guidelines to reflect the FDA approval of 3 fixed-dose combination products that contain tenofovir alafenamide. Specifically, the combination of tenofovir alafenamide and emtricitabine is recommended for use with the integrase inhibitors—dolutegravir or raltegravir. It is also recommended in combination with ritonavir-boosted darunavir.
DHHS “alternative” and“other” regimens
The DHHS guidelines also include “alternative” (TABLE 32) and “other” regimens (available at: http://aidsinfo.nih.gov/guidelines) that may be used when first-line regimens may not. These second-line options are very effective, but have some possible clinical disadvantages or limitations. They are also less well supported by data from clinical trials. However, in certain situations, depending on an individual patient’s comorbidities, inability to tolerate one of the preferred regimens, or personal preferences, an alternative regimen may be the optimal choice.

Under the category of alternative regimens, the panel has included tenofovir alafenamide and emtricitabine in combination with the NNRTI efavirenz or with ritonavir- or cobicistat-boosted atazanavir or darunavir.
The third group or “other” regimens have reduced virologic activity, increased toxicity, and even more limited data from clinical trials. Generally, medications from the DHHS “alternative” and “other” categories should be prescribed in consultation with an HIV specialist.
The future of ART
The currently available drugs are highly effective in fully suppressing HIV and allowing for immune recovery and clinical stability for most patients. Life expectancy for patients living with HIV is estimated to be approaching that of uninfected adults—provided they remain on ART.24 As a way to further simplify ART, current clinical trials are looking at 2-drug regimens including an integrase inhibitor with an NRTI, an INSTI, or an NNRTI, or a PI with one NRTI.25,26 This approach could further reduce pill burden and toxicity and substantially decrease the cost of long-term treatment.27 Also on the horizon are long-acting injectable antiretroviral drugs that will likely be available for clinical use in the next 2 to 3 years.28,29
CASE › At the 2-week follow-up visit, you discuss with Mr. G that his CD4+ count is 390 cells/mm3, his HIV RNA level is 32,450 copies/mL, and his HIV genotype test showed no antiviral drug resistance. Explaining that all patients with HIV should be treated with antiviral therapy regardless of CD4+ count, you recommend that Mr. G begin taking fixed-dose tenofovir disoproxil fumarate/emtricitabine/elvitegravir/cobicistat (Stribild), noting that it is one of the regimens recommended by the DHHS national treatment guidelines. You provide a patient handout that discusses dosing and adverse effects, including nausea and headache. The patient’s pharmacy was contacted and it was determined that Mr. G’s co-pay for the drug would be $50, which he found acceptable.
In addition, you discuss the importance of good adherence to this medication, and instruct Mr. G to contact the office via phone or patient portal for any concerns or questions that arise after starting the medication. Lastly, you advise him to return in 4 weeks for follow-up blood testing, including viral load monitoring, and additional care, if needed, and strongly recommend that he begin using condoms regularly.
CORRESPONDENCE
Jeffrey T. Kirchner, DO, FAAFP, AAHIVS, Medical Director, LGHP Comprehensive Care, 554 North Duke St., 3rd Floor, Lancaster, PA 1760; [email protected].
1. Concorde: MRC/ANRS randomised double-blind controlled trial of immediate and deferred zidovudine in symptom-free HIV infection. Concorde Coordinating Committee. Lancet. 1994;343:871-881.
2. Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available at: http://www.aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-treatment-guidelines/0. Accessed July 17, 2016.
3. The INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795-807.
4. The TEMPRANO ANRS 12136 Study Group. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373:808-822.
5. Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375:830-839.
6. Molina JM, Clotet B, van Lunzen J,et al. Once-daily dolutegravir versus darunavir plus ritonavir for treatment-naive adults with HIV-1 infection (FLAMINGO): 96 week results from a randomized, open-label, phase 3b study. Lancet HIV. 2015;2:e127-136.
7. Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369:1807-1818.
8. Van Lunzen J, Maggiolo F, Arribas JR, et al. Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naïve adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomized, phase 2b trial. Lancet Infect Dis. 2012;12:111-118.
9. Stellbrink HJ, Reynes J, Lazzarin A, et al. Dolutegravir in antiretroviral-naive adults with HIV-1: 96-week results from a randomized dose-ranging study. AIDS. 2013; 27:1771-1778.
10. Mallal S, Phillips E, Carosi G. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358:568-579.
11. AIDSinfo Drug Database. Dolutegravir. Available at: https://aidsinfo.nih.gov/drugs/509/dolutegravir/0/professional. Accessed July 17, 2016.
12. AIDSinfo Drug Database. Emtricitabine. Available at: https://aidsinfo.nih.gov/drugs/208/emtricitabine/0/patient. Accessed July 17, 2016.
13. AIDSinfo Drug Database. Elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide fumarate. Available at: https://aidsinfo.nih.gov/drugs/553/genvoya/0/professional. Accessed July 17, 2016.
14. Ray AS, Fordyce MW, Hitchcock, MJM. Tenofovir alafenamide: A novel prodrug of tenofovir for the treatment of human immunodeficiency virus. Antiviral Res. 2016;125:63-70.
15. AIDSinfo Drug Database. Elvitegravir. https://aidsinfo.nih.gov/drugs/421/elvitegravir/0/professional
16. Wohl DA, Cohen C, Gallant JE, et al. A randomized, double-blind comparison of single-tablet regimen elvitegravir/cobicistat/emtricitabine/tenofovir DF versus single-tablet regimen efavirenz/emtricitabine/tenofovir DF for initial treatment of HIV-1 infection: analysis of week 144 results. J Acquir Immune Defic Syndr. 2014;65:e118-120.
17. Clumeck N, Molina JM, Henry K, et al. A randomized, double-blind comparison of single-tablet regimen elvitegravir/cobicistat/emtricitabine/tenofovir DF vs ritonavir-boosted atazanavir plus emtricitabine/tenofovir for initial treatment of HIV-1 infection: analysis of week 144 results. J Acquir Immune Defic Syndr. 2014;65:e121-124.
18. AIDSinfo Drug Database. Cobicistat. Available at: https://aidsinfo.nih.gov/drugs/537/evotaz/0/patient/. Accessed July 17, 2016.
19. Rockstroh JK, DeJesus E, Lennox JL, et al. Durable efficacy and safety of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naïve HIV-1 infected patients: final 5-year results from STARTMRK. J Acquir Immune Defic Syndr. 2013;63:77-85.
20. Cahn P, Kaplan R, Sax P, et al. Raltegravir (RAL) 1200 mg once daily (QD) is non-inferior to RAL 400 mg twice daily (BID), in combination with tenofovir/emtricitabine, in treatment-naive HIV-1-infected subjects: week 48 results. Abstract FRAB0103LB presented at: 21st International AIDS Conference; July 18-22, 2016; Durban, South Africa.
21. Hicks C, Gulick RM. Raltegravir: the first HIV type 1 integrase inhibitor. Clin Infect Dis. 2009;48:931-939.
22. Prescriber’s Letter. HIV/AIDS Pharmacotherapy Review. Vol. 2015; Course no. 215. Available at: http://prescribersletter.therapeuticresearch.com/ce/cecourse.aspx?pc=15-215. Accessed October 6
23. AIDSinfo Drug Database. Tenofovir alafenamide. Available at: https://aidsinfo.nih.gov/drugs/514/tenofovir-alafenamide/0/patient. Accessed September 27, 2016.
24. Marcus JL, Chao C, Leyden W, et al. Narrowing the gap in life expectancy for HIV+ compared with HIV- individuals. Conference on Retroviruses and Opportunistic Infections. February 22-25, 2016, Boston. Abstract 54.
25. Gubavu C, Prazuck T, Niang M, et al. Dolutegravir-based monotherapy or dual therapy maintains a high proportion of viral suppression even in highly experienced HIV-1-infected patients. J Antimicrob Chemother. 2016;71:1046-1050.
26. Margolis DA, Brinson CC, Smith GHR. Cabotegravir plus rilpivirine, once a day, after induction with cabotegravir plus nucleoside reverse transcriptase inhibitors in antiretroviral naïve adults with HIV-1 infection (LATTE): a randomised, phase 2b, dose-ranging trial. Lancet Infect Dis. 2015;15:1145-1155.
27. Girouard MP, Sax PE, Parker RA, et al. The cost-effectiveness and budget impact of 2-drug dolutegravir-lamivudine regimens for the treatment of HIV infection in the United States. Clin Infect Dis. 2016; 62:784-791.
28. Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Cabotegravir + rilpivirine as long-acting maintenance therapy: LATTE-2 week 32 results. Abstract number 31 LB. Conference on Retroviruses and Opportunistic Infections. February 22-25, 2016; Boston, MA.
29. Murray MI, Markowitz M, Frank I, et al. Tolerability and acceptability of cabotegravir LA injection: results from ECLAIR study. Abstract number 471. Conference on Retroviruses and Opportunistic Infections. February 22-25, 2016; Boston, MA.
1. Concorde: MRC/ANRS randomised double-blind controlled trial of immediate and deferred zidovudine in symptom-free HIV infection. Concorde Coordinating Committee. Lancet. 1994;343:871-881.
2. Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available at: http://www.aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-treatment-guidelines/0. Accessed July 17, 2016.
3. The INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795-807.
4. The TEMPRANO ANRS 12136 Study Group. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373:808-822.
5. Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375:830-839.
6. Molina JM, Clotet B, van Lunzen J,et al. Once-daily dolutegravir versus darunavir plus ritonavir for treatment-naive adults with HIV-1 infection (FLAMINGO): 96 week results from a randomized, open-label, phase 3b study. Lancet HIV. 2015;2:e127-136.
7. Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369:1807-1818.
8. Van Lunzen J, Maggiolo F, Arribas JR, et al. Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naïve adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomized, phase 2b trial. Lancet Infect Dis. 2012;12:111-118.
9. Stellbrink HJ, Reynes J, Lazzarin A, et al. Dolutegravir in antiretroviral-naive adults with HIV-1: 96-week results from a randomized dose-ranging study. AIDS. 2013; 27:1771-1778.
10. Mallal S, Phillips E, Carosi G. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358:568-579.
11. AIDSinfo Drug Database. Dolutegravir. Available at: https://aidsinfo.nih.gov/drugs/509/dolutegravir/0/professional. Accessed July 17, 2016.
12. AIDSinfo Drug Database. Emtricitabine. Available at: https://aidsinfo.nih.gov/drugs/208/emtricitabine/0/patient. Accessed July 17, 2016.
13. AIDSinfo Drug Database. Elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide fumarate. Available at: https://aidsinfo.nih.gov/drugs/553/genvoya/0/professional. Accessed July 17, 2016.
14. Ray AS, Fordyce MW, Hitchcock, MJM. Tenofovir alafenamide: A novel prodrug of tenofovir for the treatment of human immunodeficiency virus. Antiviral Res. 2016;125:63-70.
15. AIDSinfo Drug Database. Elvitegravir. https://aidsinfo.nih.gov/drugs/421/elvitegravir/0/professional
16. Wohl DA, Cohen C, Gallant JE, et al. A randomized, double-blind comparison of single-tablet regimen elvitegravir/cobicistat/emtricitabine/tenofovir DF versus single-tablet regimen efavirenz/emtricitabine/tenofovir DF for initial treatment of HIV-1 infection: analysis of week 144 results. J Acquir Immune Defic Syndr. 2014;65:e118-120.
17. Clumeck N, Molina JM, Henry K, et al. A randomized, double-blind comparison of single-tablet regimen elvitegravir/cobicistat/emtricitabine/tenofovir DF vs ritonavir-boosted atazanavir plus emtricitabine/tenofovir for initial treatment of HIV-1 infection: analysis of week 144 results. J Acquir Immune Defic Syndr. 2014;65:e121-124.
18. AIDSinfo Drug Database. Cobicistat. Available at: https://aidsinfo.nih.gov/drugs/537/evotaz/0/patient/. Accessed July 17, 2016.
19. Rockstroh JK, DeJesus E, Lennox JL, et al. Durable efficacy and safety of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naïve HIV-1 infected patients: final 5-year results from STARTMRK. J Acquir Immune Defic Syndr. 2013;63:77-85.
20. Cahn P, Kaplan R, Sax P, et al. Raltegravir (RAL) 1200 mg once daily (QD) is non-inferior to RAL 400 mg twice daily (BID), in combination with tenofovir/emtricitabine, in treatment-naive HIV-1-infected subjects: week 48 results. Abstract FRAB0103LB presented at: 21st International AIDS Conference; July 18-22, 2016; Durban, South Africa.
21. Hicks C, Gulick RM. Raltegravir: the first HIV type 1 integrase inhibitor. Clin Infect Dis. 2009;48:931-939.
22. Prescriber’s Letter. HIV/AIDS Pharmacotherapy Review. Vol. 2015; Course no. 215. Available at: http://prescribersletter.therapeuticresearch.com/ce/cecourse.aspx?pc=15-215. Accessed October 6
23. AIDSinfo Drug Database. Tenofovir alafenamide. Available at: https://aidsinfo.nih.gov/drugs/514/tenofovir-alafenamide/0/patient. Accessed September 27, 2016.
24. Marcus JL, Chao C, Leyden W, et al. Narrowing the gap in life expectancy for HIV+ compared with HIV- individuals. Conference on Retroviruses and Opportunistic Infections. February 22-25, 2016, Boston. Abstract 54.
25. Gubavu C, Prazuck T, Niang M, et al. Dolutegravir-based monotherapy or dual therapy maintains a high proportion of viral suppression even in highly experienced HIV-1-infected patients. J Antimicrob Chemother. 2016;71:1046-1050.
26. Margolis DA, Brinson CC, Smith GHR. Cabotegravir plus rilpivirine, once a day, after induction with cabotegravir plus nucleoside reverse transcriptase inhibitors in antiretroviral naïve adults with HIV-1 infection (LATTE): a randomised, phase 2b, dose-ranging trial. Lancet Infect Dis. 2015;15:1145-1155.
27. Girouard MP, Sax PE, Parker RA, et al. The cost-effectiveness and budget impact of 2-drug dolutegravir-lamivudine regimens for the treatment of HIV infection in the United States. Clin Infect Dis. 2016; 62:784-791.
28. Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Cabotegravir + rilpivirine as long-acting maintenance therapy: LATTE-2 week 32 results. Abstract number 31 LB. Conference on Retroviruses and Opportunistic Infections. February 22-25, 2016; Boston, MA.
29. Murray MI, Markowitz M, Frank I, et al. Tolerability and acceptability of cabotegravir LA injection: results from ECLAIR study. Abstract number 471. Conference on Retroviruses and Opportunistic Infections. February 22-25, 2016; Boston, MA.
Is the Rx to blame for the patient’s weight gain?
One of my brothers has adult onset bipolar disorder. As luck would have it, he also has type 2 diabetes mellitus. He struggles constantly with blood sugar control since he needs to take 2 psychotropic medications, both of which cause weight gain.
His situation has prompted me to think about the responsibility we have as we care, and advocate, for our patients with major mental illness who require these effective medications. At a minimum, we must be knowledgeable about the adverse metabolic effects of these drugs, avoid prescribing them when possible, and advocate for dose reductions when feasible. Knowing, for example, that these drugs fall on a spectrum, with haloperidol causing the least weight gain and olanzapine causing the most, is important.1
An eye-opener. The article by Saunders in this issue provides advice on avoiding medications that commonly cause weight gain when prescribing for overweight or obese patients with diabetes, hypertension, and/or depression. I was unaware that some of the drugs on the list contribute to the problem. For example, I saw a new patient last week who has hypertension and is obese; she has been taking the beta-blocker metoprolol for the past 8 years. She has tried unsuccessfully to lose weight. She asked me if the metoprolol could be interfering with weight loss, and I mistakenly told her “No.” Thankfully, we decided to discontinue it anyway. I will admit to her my knowledge gap when I see her next month for follow-up. Errors are great teachers, especially when no harm is done.
The scope of the Saunders article is not meant to be comprehensive, since it focuses on medications for diabetes, hypertension, and depression. I think all of us are aware of the weight gain associated with other commonly prescribed drugs, such as systemic corticosteroids and long-acting progesterone for contraception. Thankfully, combination oral contraceptives do not appear to be associated with weight gain2—answering one of the more common questions I receive from patients about weight and medications.
The bottom line. Avoid prescribing medications that can cause weight gain in overweight and obese patients when possible, use the lowest effective dose when such agents are necessary, and warn patients of this adverse effect so that they can take precautions, such as walking an extra mile a day or giving up that high-calorie latte in the morning.
1. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951-962.
2. Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2011;CD003987.
One of my brothers has adult onset bipolar disorder. As luck would have it, he also has type 2 diabetes mellitus. He struggles constantly with blood sugar control since he needs to take 2 psychotropic medications, both of which cause weight gain.
His situation has prompted me to think about the responsibility we have as we care, and advocate, for our patients with major mental illness who require these effective medications. At a minimum, we must be knowledgeable about the adverse metabolic effects of these drugs, avoid prescribing them when possible, and advocate for dose reductions when feasible. Knowing, for example, that these drugs fall on a spectrum, with haloperidol causing the least weight gain and olanzapine causing the most, is important.1
An eye-opener. The article by Saunders in this issue provides advice on avoiding medications that commonly cause weight gain when prescribing for overweight or obese patients with diabetes, hypertension, and/or depression. I was unaware that some of the drugs on the list contribute to the problem. For example, I saw a new patient last week who has hypertension and is obese; she has been taking the beta-blocker metoprolol for the past 8 years. She has tried unsuccessfully to lose weight. She asked me if the metoprolol could be interfering with weight loss, and I mistakenly told her “No.” Thankfully, we decided to discontinue it anyway. I will admit to her my knowledge gap when I see her next month for follow-up. Errors are great teachers, especially when no harm is done.
The scope of the Saunders article is not meant to be comprehensive, since it focuses on medications for diabetes, hypertension, and depression. I think all of us are aware of the weight gain associated with other commonly prescribed drugs, such as systemic corticosteroids and long-acting progesterone for contraception. Thankfully, combination oral contraceptives do not appear to be associated with weight gain2—answering one of the more common questions I receive from patients about weight and medications.
The bottom line. Avoid prescribing medications that can cause weight gain in overweight and obese patients when possible, use the lowest effective dose when such agents are necessary, and warn patients of this adverse effect so that they can take precautions, such as walking an extra mile a day or giving up that high-calorie latte in the morning.
1. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951-962.
2. Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2011;CD003987.
One of my brothers has adult onset bipolar disorder. As luck would have it, he also has type 2 diabetes mellitus. He struggles constantly with blood sugar control since he needs to take 2 psychotropic medications, both of which cause weight gain.
His situation has prompted me to think about the responsibility we have as we care, and advocate, for our patients with major mental illness who require these effective medications. At a minimum, we must be knowledgeable about the adverse metabolic effects of these drugs, avoid prescribing them when possible, and advocate for dose reductions when feasible. Knowing, for example, that these drugs fall on a spectrum, with haloperidol causing the least weight gain and olanzapine causing the most, is important.1
An eye-opener. The article by Saunders in this issue provides advice on avoiding medications that commonly cause weight gain when prescribing for overweight or obese patients with diabetes, hypertension, and/or depression. I was unaware that some of the drugs on the list contribute to the problem. For example, I saw a new patient last week who has hypertension and is obese; she has been taking the beta-blocker metoprolol for the past 8 years. She has tried unsuccessfully to lose weight. She asked me if the metoprolol could be interfering with weight loss, and I mistakenly told her “No.” Thankfully, we decided to discontinue it anyway. I will admit to her my knowledge gap when I see her next month for follow-up. Errors are great teachers, especially when no harm is done.
The scope of the Saunders article is not meant to be comprehensive, since it focuses on medications for diabetes, hypertension, and depression. I think all of us are aware of the weight gain associated with other commonly prescribed drugs, such as systemic corticosteroids and long-acting progesterone for contraception. Thankfully, combination oral contraceptives do not appear to be associated with weight gain2—answering one of the more common questions I receive from patients about weight and medications.
The bottom line. Avoid prescribing medications that can cause weight gain in overweight and obese patients when possible, use the lowest effective dose when such agents are necessary, and warn patients of this adverse effect so that they can take precautions, such as walking an extra mile a day or giving up that high-calorie latte in the morning.
1. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951-962.
2. Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2011;CD003987.
Fibromyalgia management: A multimodal approach
VIDEO: Duodenal bulb sampling barely increased celiac yield in low-probability cohort
Separate sampling of the duodenal bulb increased detection of celiac disease by only 0.1% when endoscopy patients had a low pretest probability of celiac disease, according to research published in the November issue of Clinical Gastroenterology and Hepatology.
Duodenal bulb histology did reveal other abnormal findings, such as chronic peptic duodenitis, gastric heterotopia, and Brunner gland hyperplasia, wrote Samantha Stoven, MD, of Mayo Clinic, Rochester, Minn., and her associates. These findings did not seem to impede the identification of celiac disease, but their clinical implications were unclear, the researchers noted.
Most studies of the diagnostic yield of duodenal bulb specimens have been performed in patients with known celiac disease or positive serology. In past studies of these high-probability cohorts, duodenal bulb sampling increased the diagnostic yield of celiac disease anywhere from 1.8% to 18%, but whether and how that finding translates to low-probability cohorts is unclear, the researchers said. Therefore, they retrospectively analyzed data from 679 endoscopy patients who had both duodenal bulb and small bowel biopsies collected at three Mayo Clinic sites in 2011. These sites are “open access,” meaning that patients can be referred for endoscopy without the approval of a gastroenterologist.
The average age of the patients was 50 years, and 63% were female. They were most commonly referred for duodenal biopsy because of chronic dyspepsia (46% of patients), diarrhea (35%), or nausea (17%). Patients with either known celiac disease or positive serology were excluded from the study (Clin Gastroenterol Hepatol. 2016 Mar 7. doi: 10.1016/j.cgh.2016.02.026). A total of 265 patients (39%) had abnormal duodenal histology, which was most often diagnosed as chronic peptic duodenitis, the researchers said. Histologic abnormalities usually involved the duodenal bulb (36% of cases), not the distal duodenum (15%; P less than .0001). However, among the 16 patients (2%) found to have celiac disease, just one patient had disease only in the duodenal bulb. Thus, duodenal bulb sampling increased the diagnostic yield of celiac disease by only 0.1% when considering the overall cohort. The patient with celiac disease limited to the bulb was a 46-year-old female presenting with diarrhea and anemia who had normal serologies but a permissive human leukocyte antigen test. Her duodenal bulb had villous atrophy and more than 25 intraepithelial lymphocytes per 100 epithelial cells, while her distal duodenum was normal.
Among the 85% of patients who had normal distal duodenums, 28% had abnormal bulb histology, most often chronic peptic duodenitis, active chronic peptic duodenitis, or gastric heterotopia, the researchers said. Among the 59% of patients whose celiac serology before endoscopy was truly unknown, only two (0.5%) had histologic changes consistent with celiac disease, which in both cases were located in the distal duodenum.
SOURCE: American Gastroenterological Association
“Individual sampling of the duodenal bulb in patients with either negative or unknown celiac serologic status can be considered in practices where expert gastrointestinal pathologists are present and there is agreement that both samples can be submitted in the same bottle, or there is not a separate charge for the additional container. Further studies may be needed to assess the diagnostic yield of separate bulb biopsies for celiac detection in all comers.”
An American College of Gastroenterology Junior Faculty Development Award helped support the work. Senior author Joseph A. Murray, MD, disclosed ties to Alba Therapeutics, Alvine Pharmaceuticals, AMAG Pharmaceuticals, and several other corporate entities. The remaining authors had no disclosures.
Histologic diagnosis of celiac disease has traditionally relied upon endoscopic biopsies from the second and third portions of the duodenum. However, several recent studies indicate that duodenal bulb biopsies may show changes of celiac disease, despite normal histology in the more distal duodenum.
In their study, Dr. Stoven and her colleagues evaluated the diagnostic utility of endoscopic duodenal bulb biopsy in patients with a low probability for celiac disease. A new diagnosis of celiac disease was made in 16 of their 679 patients (2.4%). Only one patient showed villous atrophy of the duodenal bulb with normal histology of the more distal duodenum. Although a diagnosis of celiac disease was made, the case was atypical not only because distal duodenal biopsies were normal but also because multiple celiac serology tests were negative, raising the possibility of nonceliac villous atrophy. Thus, the added diagnostic yield of duodenal bulb biopsies in this low-risk population was extremely low (0.15% at most).
Ciaran P. Kelly, MD, AGAF, professor of medicine, Harvard Medical School, director Celiac Center, Beth Israel Deaconess Medical Center, Boston, has acted as a scientific adviser to companies including Celimmune, Cour Pharmaceuticals, ImmunogenX, and Takeda; he also acts as principal investigator on a research grant on celiac disease supported by Aptalis.
Histologic diagnosis of celiac disease has traditionally relied upon endoscopic biopsies from the second and third portions of the duodenum. However, several recent studies indicate that duodenal bulb biopsies may show changes of celiac disease, despite normal histology in the more distal duodenum.
In their study, Dr. Stoven and her colleagues evaluated the diagnostic utility of endoscopic duodenal bulb biopsy in patients with a low probability for celiac disease. A new diagnosis of celiac disease was made in 16 of their 679 patients (2.4%). Only one patient showed villous atrophy of the duodenal bulb with normal histology of the more distal duodenum. Although a diagnosis of celiac disease was made, the case was atypical not only because distal duodenal biopsies were normal but also because multiple celiac serology tests were negative, raising the possibility of nonceliac villous atrophy. Thus, the added diagnostic yield of duodenal bulb biopsies in this low-risk population was extremely low (0.15% at most).
Ciaran P. Kelly, MD, AGAF, professor of medicine, Harvard Medical School, director Celiac Center, Beth Israel Deaconess Medical Center, Boston, has acted as a scientific adviser to companies including Celimmune, Cour Pharmaceuticals, ImmunogenX, and Takeda; he also acts as principal investigator on a research grant on celiac disease supported by Aptalis.
Histologic diagnosis of celiac disease has traditionally relied upon endoscopic biopsies from the second and third portions of the duodenum. However, several recent studies indicate that duodenal bulb biopsies may show changes of celiac disease, despite normal histology in the more distal duodenum.
In their study, Dr. Stoven and her colleagues evaluated the diagnostic utility of endoscopic duodenal bulb biopsy in patients with a low probability for celiac disease. A new diagnosis of celiac disease was made in 16 of their 679 patients (2.4%). Only one patient showed villous atrophy of the duodenal bulb with normal histology of the more distal duodenum. Although a diagnosis of celiac disease was made, the case was atypical not only because distal duodenal biopsies were normal but also because multiple celiac serology tests were negative, raising the possibility of nonceliac villous atrophy. Thus, the added diagnostic yield of duodenal bulb biopsies in this low-risk population was extremely low (0.15% at most).
Ciaran P. Kelly, MD, AGAF, professor of medicine, Harvard Medical School, director Celiac Center, Beth Israel Deaconess Medical Center, Boston, has acted as a scientific adviser to companies including Celimmune, Cour Pharmaceuticals, ImmunogenX, and Takeda; he also acts as principal investigator on a research grant on celiac disease supported by Aptalis.
Separate sampling of the duodenal bulb increased detection of celiac disease by only 0.1% when endoscopy patients had a low pretest probability of celiac disease, according to research published in the November issue of Clinical Gastroenterology and Hepatology.
Duodenal bulb histology did reveal other abnormal findings, such as chronic peptic duodenitis, gastric heterotopia, and Brunner gland hyperplasia, wrote Samantha Stoven, MD, of Mayo Clinic, Rochester, Minn., and her associates. These findings did not seem to impede the identification of celiac disease, but their clinical implications were unclear, the researchers noted.
Most studies of the diagnostic yield of duodenal bulb specimens have been performed in patients with known celiac disease or positive serology. In past studies of these high-probability cohorts, duodenal bulb sampling increased the diagnostic yield of celiac disease anywhere from 1.8% to 18%, but whether and how that finding translates to low-probability cohorts is unclear, the researchers said. Therefore, they retrospectively analyzed data from 679 endoscopy patients who had both duodenal bulb and small bowel biopsies collected at three Mayo Clinic sites in 2011. These sites are “open access,” meaning that patients can be referred for endoscopy without the approval of a gastroenterologist.
The average age of the patients was 50 years, and 63% were female. They were most commonly referred for duodenal biopsy because of chronic dyspepsia (46% of patients), diarrhea (35%), or nausea (17%). Patients with either known celiac disease or positive serology were excluded from the study (Clin Gastroenterol Hepatol. 2016 Mar 7. doi: 10.1016/j.cgh.2016.02.026). A total of 265 patients (39%) had abnormal duodenal histology, which was most often diagnosed as chronic peptic duodenitis, the researchers said. Histologic abnormalities usually involved the duodenal bulb (36% of cases), not the distal duodenum (15%; P less than .0001). However, among the 16 patients (2%) found to have celiac disease, just one patient had disease only in the duodenal bulb. Thus, duodenal bulb sampling increased the diagnostic yield of celiac disease by only 0.1% when considering the overall cohort. The patient with celiac disease limited to the bulb was a 46-year-old female presenting with diarrhea and anemia who had normal serologies but a permissive human leukocyte antigen test. Her duodenal bulb had villous atrophy and more than 25 intraepithelial lymphocytes per 100 epithelial cells, while her distal duodenum was normal.
Among the 85% of patients who had normal distal duodenums, 28% had abnormal bulb histology, most often chronic peptic duodenitis, active chronic peptic duodenitis, or gastric heterotopia, the researchers said. Among the 59% of patients whose celiac serology before endoscopy was truly unknown, only two (0.5%) had histologic changes consistent with celiac disease, which in both cases were located in the distal duodenum.
SOURCE: American Gastroenterological Association
“Individual sampling of the duodenal bulb in patients with either negative or unknown celiac serologic status can be considered in practices where expert gastrointestinal pathologists are present and there is agreement that both samples can be submitted in the same bottle, or there is not a separate charge for the additional container. Further studies may be needed to assess the diagnostic yield of separate bulb biopsies for celiac detection in all comers.”
An American College of Gastroenterology Junior Faculty Development Award helped support the work. Senior author Joseph A. Murray, MD, disclosed ties to Alba Therapeutics, Alvine Pharmaceuticals, AMAG Pharmaceuticals, and several other corporate entities. The remaining authors had no disclosures.
Separate sampling of the duodenal bulb increased detection of celiac disease by only 0.1% when endoscopy patients had a low pretest probability of celiac disease, according to research published in the November issue of Clinical Gastroenterology and Hepatology.
Duodenal bulb histology did reveal other abnormal findings, such as chronic peptic duodenitis, gastric heterotopia, and Brunner gland hyperplasia, wrote Samantha Stoven, MD, of Mayo Clinic, Rochester, Minn., and her associates. These findings did not seem to impede the identification of celiac disease, but their clinical implications were unclear, the researchers noted.
Most studies of the diagnostic yield of duodenal bulb specimens have been performed in patients with known celiac disease or positive serology. In past studies of these high-probability cohorts, duodenal bulb sampling increased the diagnostic yield of celiac disease anywhere from 1.8% to 18%, but whether and how that finding translates to low-probability cohorts is unclear, the researchers said. Therefore, they retrospectively analyzed data from 679 endoscopy patients who had both duodenal bulb and small bowel biopsies collected at three Mayo Clinic sites in 2011. These sites are “open access,” meaning that patients can be referred for endoscopy without the approval of a gastroenterologist.
The average age of the patients was 50 years, and 63% were female. They were most commonly referred for duodenal biopsy because of chronic dyspepsia (46% of patients), diarrhea (35%), or nausea (17%). Patients with either known celiac disease or positive serology were excluded from the study (Clin Gastroenterol Hepatol. 2016 Mar 7. doi: 10.1016/j.cgh.2016.02.026). A total of 265 patients (39%) had abnormal duodenal histology, which was most often diagnosed as chronic peptic duodenitis, the researchers said. Histologic abnormalities usually involved the duodenal bulb (36% of cases), not the distal duodenum (15%; P less than .0001). However, among the 16 patients (2%) found to have celiac disease, just one patient had disease only in the duodenal bulb. Thus, duodenal bulb sampling increased the diagnostic yield of celiac disease by only 0.1% when considering the overall cohort. The patient with celiac disease limited to the bulb was a 46-year-old female presenting with diarrhea and anemia who had normal serologies but a permissive human leukocyte antigen test. Her duodenal bulb had villous atrophy and more than 25 intraepithelial lymphocytes per 100 epithelial cells, while her distal duodenum was normal.
Among the 85% of patients who had normal distal duodenums, 28% had abnormal bulb histology, most often chronic peptic duodenitis, active chronic peptic duodenitis, or gastric heterotopia, the researchers said. Among the 59% of patients whose celiac serology before endoscopy was truly unknown, only two (0.5%) had histologic changes consistent with celiac disease, which in both cases were located in the distal duodenum.
SOURCE: American Gastroenterological Association
“Individual sampling of the duodenal bulb in patients with either negative or unknown celiac serologic status can be considered in practices where expert gastrointestinal pathologists are present and there is agreement that both samples can be submitted in the same bottle, or there is not a separate charge for the additional container. Further studies may be needed to assess the diagnostic yield of separate bulb biopsies for celiac detection in all comers.”
An American College of Gastroenterology Junior Faculty Development Award helped support the work. Senior author Joseph A. Murray, MD, disclosed ties to Alba Therapeutics, Alvine Pharmaceuticals, AMAG Pharmaceuticals, and several other corporate entities. The remaining authors had no disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Separate sampling of the duodenal bulb increased detection of celiac disease by only 0.1% when endoscopy patients had a low pretest probability of celiac disease.
Major finding: One (0.1%) patient had celiac disease limited to the duodenal bulb.
Data source: A multicenter retrospective study of 679 patients without celiac disease or positive serology from whom duodenal bulb and small bowel biopsies were collected during endoscopy.
Disclosures: An American College of Gastroenterology Junior Faculty Development Award helped support the work. Senior author Joseph A. Murray, MD, disclosed ties to Alba Therapeutics, Alvine Pharmaceuticals, AMAG Pharmaceuticals, and several other corporate entities. The remaining authors had no disclosures.
Left ventricular thrombosis can still complicate acute myocardial infarction
A 62-year-old man with hypertension, type 2 diabetes mellitus, and hypercholesterolemia presented to the emergency department with substernal chest pain that started about 15 hours earlier while he was at rest watching television.
On examination, his pulse was 92 beats per minute and regular, his blood pressure was 160/88 mm Hg, and he had no evidence of jugular venous distention or pedal edema. Lung examination was positive for bibasilar crackles.
Electrocardiography revealed Q waves with ST elevation in leads I, aVL, V4, V5, and V6 with reciprocal ST depression in leads II, III, and aVF.
His troponin T level on presentation was markedly elevated.

He underwent heart catheterization and was found to have 100% occlusion of the proximal left anterior descending artery. He underwent successful percutaneous coronary intervention with placement of a drug-eluting stent, and afterward had grade 3 flow on the Thrombolysis in Myocardial Infarction (TIMI) scale.
Echocardiography the next day revealed a mobile echo-dense mass in the left ventricular apex (Figure 1) and a left ventricular ejection fraction of 35%.
THE INCIDENCE OF LEFT VENTRICULAR THROMBOSIS IN ACUTE MI
1. What is the incidence of left ventricular thrombosis after acute myocardial infarction (MI), now that primary percutaneous coronary intervention is common?
- 0.1%
- 2%
- 20%
- 40%
Left ventricular thrombosis is a serious complication of acute MI that can cause systemic thromboembolism, including stroke.1 Before thrombolytic therapy was available, this complication occurred in 20% to 60% of patients with acute MI.2,3 But early reperfusion strategies, anticoagulation for the first 48 hours, and dual antiplatelet therapy have reduced the incidence of this complication significantly.
In the thrombolytic era, the incidence of left ventricular thrombosis was 5.1% in the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI) 3 study, which had 8,326 patients. A subset of patients who had an anterior MI had almost double the incidence (11.5%).3

The incidence has further declined with the advent of primary percutaneous coronary intervention, likely thanks to enhanced myocardial salvage, and now ranges from 2.5% to 15% (Table 1).4–11 The largest observational study, with 2,911 patients undergoing percutaneous coronary intervention, reported an incidence of 2.5% within 3 to 5 days of the MI.7 At our center, the incidence was found to be even lower, 1.8% in 1,700 patients presenting with ST-elevation MI undergoing primary percutaneous coronary intervention. Hence, of the answers to the question above, 2% would be closest.
Large infarct size with a low left ventricular ejection fraction (< 40%), anterior wall MI, hypertension, and delay in time from symptom onset to intervention were independent predictors of left ventricular thrombus formation in most studies.7,12 The risk is highest during the first 2 weeks after MI, and thrombosis almost never occurs more than 3 months after the index event.5,13–16
WHAT IS THE PATHOGENESIS OF LEFT VENTRICULAR THROMBOSIS?
A large transmural infarct results in loss of contractile function, which causes stagnation and pooling of blood adjacent to the infarcted ventricular segment. In addition, endocardial injury exposes tissue factor, which then initiates the coagulation cascade. To make matters worse, MI results in a hypercoagulable state through unclear mechanisms, which completes the Virchow triad for thrombus formation. Elevations of D-dimer, fibrinogen, anticardiolipin antibodies (IgM and IgG), and tissue factor have also been reported after acute MI.17
Thrombus formation begins with platelet aggregation at the site of endocardial damage, forming a platelet plug, followed by activation of clotting factors. These thrombi are referred to as “mural,” as they adhere to the chamber wall (endocardium). They are composed of fibrin and entrapped red and white blood cells (Figure 2).
The natural course of thrombus evolution is established but variable. A left ventricular thrombus may dislodge and embolize, resulting in stroke or other thromboembolic complications. Alternately, it can dissolve over time, aided by intrinsic fibrinolytic mechanisms. On other occasions, the thrombus may organize, a process characterized by ingrowth of smooth muscle cells, fibroblasts, and endothelium.
HOW IS LEFT VENTRICULAR THROMBOSIS DIAGNOSED?
2. What is the best imaging test for detecting a thrombus?
- Transesophageal echocardiography
- Transthoracic echocardiography
- Cardiac magnetic resonance imaging (MRI) without gadolinium contrast
- Cardiac MRI with gadolinium contrast
Evaluation of left ventricular function after acute MI carries a class I indication (ie, it should be performed).18
Echocardiography is commonly used, and it has a 60% sensitivity to detect a thrombus.19 In patients with poorer transthoracic echocardiographic windows, contrast can be used to better delineate the left ventricular cavity and show the thrombus. Transesophageal echocardiography is seldom useful, as the left ventricular apex is foreshortened and in the far field.
A left ventricular thrombus is confirmed if an echo-dense mass with well-demarcated margins distinct from the endocardium is seen throughout the cardiac cycle. It should be evident in at least two different views (apical and short-axis) and should be adjacent to a hypokinetic or akinetic left ventricular wall. False-positive findings can occur due to misidentified false tendons, papillary muscles, and trabeculae.
Cardiac MRI with late gadolinium enhancement is now the gold standard for diagnostic imaging, as it accurately characterizes the shape, size, and location of the thrombus (Figure 3). Gadolinium contrast increases the enhancement of the ventricular cavity, thus allowing easy detection of thrombus, which appears dark. Cardiac MRI with delayed enhancement has 88% to 91% sensitivity and 99% specificity to detect left ventricular thrombosis.20,21 However, compared with echocardiography, routine cardiac MRI is time-intensive, costly, and not routinely available. As a result, it should be performed only in patients with poor acoustic windows and a high clinical suspicion of left ventricular thrombosis.
Delayed-contrast cardiac computed tomography can be used to identify left ventricular thrombosis, using absence of contrast uptake. The need to use contrast is a disadvantage, but computed tomography can be an alternative in patients with contraindications to cardiac MRI.
WHAT COMPLICATIONS ARISE FROM LEFT VENTRICULAR THROMBOSIS?
The most feared complication of left ventricular thrombosis is thromboembolism. Cardioembolic stroke is generally severe, prone to early and long-term recurrence, and associated with a higher death rate than noncardioembolic ischemic stroke.22,23 Thrombi associated with thromboembolism are often acute and mobile rather than organized and immobile.24 They may embolize to the brain, spleen, kidneys, and bowel.25 In a meta-analysis of 11 studies, the pooled odds ratio for risk of embolization was 5.45 (95% confidence interval [CI] 3.02–9.83) with left ventricular thrombi vs without.26 Before systemic thrombolysis and antiplatelet therapy became available, stroke rates ranged from 1.5% to 10%.27–29
In a meta-analysis of 22 studies from 1978 to 2004, the incidence of ischemic stroke after MI during hospitalization was around 11.1 per 1,000 MIs.30 This study found that anterior MI was associated with a higher risk of stroke, but reported no difference in the incidence of stroke with percutaneous coronary intervention, systemic thrombolysis, or no reperfusion.
In a large prospective cohort study of 2,160 patients,31 259 (12%) had a stroke after MI. In multivariable analysis, age, diabetes, and previous stroke were predictors of stroke after MI. This study reported significantly fewer strokes in patients who underwent percutaneous coronary intervention than with other or no reperfusion therapies.31
ANTICOAGULATION TREATMENT
3. How would you treat a patient who has a drug-eluting stent in the left anterior descending artery and a new diagnosis of left ventricular thrombosis?
- Warfarin
- Aspirin and clopidogrel
- Aspirin, clopidogrel, and warfarin
- Aspirin and warfarin
The management of left ventricular thrombosis has been summarized in guidelines from the American College of Chest Physicians (ACCP) in 2012,32 and from the American College of Cardiology/American Heart Association in 2013,18 which recommend anticoagulation for at least 3 months, or indefinitely if bleeding risk is low, for all patients developing a left ventricular thrombus.
For patients with acute MI and left ventricular thrombosis, the ACCP guidelines recommend warfarin with a target international normalized ratio of 2.0 to 3.0 plus dual antiplatelet therapy (eg, aspirin plus clopidogrel) for 3 months, after which warfarin is discontinued but dual antiplatelet therapy is continued for up to 12 months.32
The European Society of Cardiology guidelines33 recommend 6 months of anticoagulation. However, if the patient is receiving dual antiplatelet therapy, they recommend repeated imaging of the left ventricle after 3 months of anticoagulation, which may allow for earlier discontinuation of anticoagulation if the thrombus has resolved and apical wall motion has recovered. Therefore, most experts recommend 3 months of anticoagulation when used in combination with dual antiplatelet therapy and repeating echocardiography at 3 months to safely discontinue anticoagulation. The best answer to the question posed here is aspirin, clopidogrel, and warfarin.
Decisions about antithrombotic therapy may also depend on stent type and the patient’s bleeding risk. With bare-metal stents, dual antiplatelet therapy along with anticoagulation should be used for 1 month, after which anticoagulation should be used with a single antiplatelet agent for another 2 months; after this, the anticoagulant can be discontinued and dual antiplatelet therapy can be resumed for a total of 12 months. Newer anticoagulants such as rivaroxaban, dabigatran, edoxaban, and apixaban may also have a role, but they have not yet been studied for this indication.
Surgical thrombectomy is rarely considered now, given the known efficacy of anticoagulants in dissolving the thrombus. It was done in the past for large, mobile, or protruding left ventricular thrombi, which have a higher potential for embolization.34 Currently, it can be done under very special circumstances, such as before placement of a left ventricular assist device or if the thrombus is large, to prevent embolism.35,36
BLEEDING COMPLICATIONS WITH TRIPLE ANTITHROMBOTIC THERAPY
After stent placement, almost all patients need to be on dual antiplatelet therapy for a specified duration depending on the type and generation of stent used. Such patients end up on “triple” antithrombotic therapy (two antiplatelet drugs plus an anticoagulant), which poses a high risk of bleeding.37 Consideration needs to be given to the risks of stroke, stent thrombosis, and major bleeding when selecting the antithrombotic regimen.38 Triple antithrombotic therapy has been associated with a risk of fatal and nonfatal bleeding of 4% to 16% when used for indications such as atrial fibrillation.39–41
Risks of triple antithrombotic therapy (aspirin 80–100 mg, clopidogrel 75 mg, and warfarin) were compared with those of clopidogrel plus warfarin in the What Is the Optimal Antiplatelet and Anticoagulant therapy in Patients With Oral Anticoagulation and Coronary Stenting Trial,37 which reported a significantly lower risk of major and minor bleeding with clopidogrel-plus-warfarin therapy than with triple antithrombotic therapy, 14.3% vs 31.7% (hazard ratio 0.40, 95% CI 0.28–0.58, P < .0001).
Additionally, the increased risk of major and minor bleeding associated with triple antithrombotic therapy has been confirmed in many observational studies; other studies found a trend toward lower risk with triple therapy, but this was not statistically significant (Table 2).38,40,42–55 A large multicenter European trial is being conducted to compare dual antiplatelet therapy vs triple antithrombotic therapy in patients with left ventricular thrombosis.
CASE FOLLOW-UP
Our patient was started on warfarin, clopidogrel 75 mg, and aspirin 75 mg at the time of discharge. He was continued on warfarin for 3 months, at which time a follow-up echocardiogram showed no thrombus in the left ventricle. Warfarin was discontinued, and he had no thromboembolic complications.
TAKE-HOME POINTS
Left ventricular thrombosis after an acute MI is very important to detect, as it can lead to serious complications through arterial embolism.
The incidence of left ventricular thrombosis has declined significantly with the use of percutaneous coronary intervention. However, it may still occur in a small number of patients with larger infarcts owing to delay in revascularization or proximal (left main or left anterior descending) occlusions with larger infarct size.
Echocardiography, which is routinely performed after acute MI to assess myocardial function, uncovers most left ventricular thrombi. In high-risk cases, MRI with late gadolinium enhancement can increase the diagnostic yield.
Anticoagulation with warfarin is recommended for at least 3 months. Post-MI patients undergoing stent implantation may need triple antithrombotic therapy, which, however, increases the bleeding risk significantly. Large randomized trials are needed to guide physicians in risk stratification of such patients.
- Lip GY, Piotrponikowski P, Andreotti F, et al; Heart Failure Association (EHFA) of the European Society of Cardiology (ESC) and the ESC Working Group on Thrombosis. Thromboembolism and antithrombotic therapy for heart failure in sinus rhythm: an executive summary of a joint consensus document from the ESC Heart Failure Association and the ESC Working Group on Thrombosis. Thromb Haemost 2012; 108:1009–1022.
- Turpie AG, Robinson JG, Doyle DJ, et al. Comparison of high-dose with low-dose subcutaneous heparin to prevent left ventricular mural thrombosis in patients with acute transmural anterior myocardial infarction. N Engl J Med 1989; 320:352–357.
- Chiarella F, Santoro E, Domenicucci S, Maggioni A, Vecchio C. Predischarge two-dimensional echocardiographic evaluation of left ventricular thrombosis after acute myocardial infarction in the GISSI-3 study. Am J Cardiol 1998; 81:822–827.
- Kalra A, Jang IK. Prevalence of early left ventricular thrombus after primary coronary intervention for acute myocardial infarction. J Thromb Thrombolysis 2000; 10:133–136.
- Nayak D, Aronow WS, Sukhija R, McClung JA, Monsen CE, Belkin RN. Comparison of frequency of left ventricular thrombi in patients with anterior wall versus non-anterior wall acute myocardial infarction treated with antithrombotic and antiplatelet therapy with or without coronary revascularization. Am J Cardiol 2004; 93:1529–1530.
- Rehan A, Kanwar M, Rosman H, et al. Incidence of post myocardial infarction left ventricular thrombus formation in the era of primary percutaneous intervention and glycoprotein IIb/IIIa inhibitors. A prospective observational study. Cardiovasc Ultrasound 2006;4:20.
- Zielinska M, Kaczmarek K, Tylkowski M. Predictors of left ventricular thrombus formation in acute myocardial infarction treated with successful primary angioplasty with stenting. Am J Med Sci 2008; 335:171–176.
- Osherov AB, Borovik-Raz M, Aronson D, et al. Incidence of early left ventricular thrombus after acute anterior wall myocardial infarction in the primary coronary intervention era. Am Heart J 2009; 157:1074–1080.
- Solheim S, Seljeflot I, Lunde K, et al. Frequency of left ventricular thrombus in patients with anterior wall acute myocardial infarction treated with percutaneous coronary intervention and dual antiplatelet therapy. Am J Cardiol 2010; 106:1197–1200.
- Shacham Y, Leshem-Rubinow E, Ben Assa E, et al. Comparison of C-reactive protein and fibrinogen levels in patients having anterior wall ST-segment elevation myocardial infarction with versus without left ventricular thrombus (from a primary percutaneous coronary intervention cohort). Am J Cardiol 2013; 112:57–60.
- Gianstefani S, Douiri A, Delithanasis I, et al. Incidence and predictors of early left ventricular thrombus after ST-elevation myocardial infarction in the contemporary era of primary percutaneous coronary intervention. Am J Cardiol 2014; 113:1111–1116.
- Shacham Y, Birati EY, Rogovski O, Cogan Y, Keren G, Roth A. Left ventricular thrombus formation and bleeding complications during continuous in-hospital anticoagulation for acute anterior myocardial infarction. Isr Med Assoc J 2012; 14:742–746.
- Asinger RW, Mikell FL, Elsperger J, Hodges M. Incidence of left-ventricular thrombosis after acute transmural myocardial infarction. Serial evaluation by two-dimensional echocardiography. N Engl J Med 1981; 305:297–302.
- Nihoyannopoulos P, Smith GC, Maseri A, Foale RA. The natural history of left ventricular thrombus in myocardial infarction: a rationale in support of masterly inactivity. J Am Coll Cardiol 1989; 14:903–911.
- Weinreich DJ, Burke JF, Pauletto FJ. Left ventricular mural thrombi complicating acute myocardial infarction. Long-term follow-up with serial echocardiography. Ann Intern Med 1984; 100:789–794.
- Greaves SC, Zhi G, Lee RT, et al. Incidence and natural history of left ventricular thrombus following anterior wall acute myocardial infarction. Am J Cardiol 1997; 80:442–448.
- Solheim S, Seljeflot I, Lunde K, et al. Prothrombotic markers in patients with acute myocardial infarction and left ventricular thrombus formation treated with pci and dual antiplatelet therapy. Thromb J 2013; 11:1.
- O’Gara PT, Kushner FG, Ascheim DD, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 127:e362–e425.
- Weinsaft JW, Kim HW, Crowley AL, et al. LV thrombus detection by routine echocardiography: insights into performance characteristics using delayed enhancement CMR. JACC Cardiovasc Imaging 2011; 4:702–712.
- Mollet NR, Dymarkowski S, Volders W, et al. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation 2002; 106:2873–2876.
- Srichai MB, Junor C, Rodriguez LL, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J 2006; 152:75–84.
- Eriksson SE, Olsson JE. Survival and recurrent strokes in patients with different subtypes of stroke: a fourteen-year follow-up study. Cerebrovasc Dis 2001; 12:171–180.
- Grau AJ, Weimar C, Buggle F, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German Stroke Data Bank. Stroke 2001; 32:2559–2566.
- Keren A, Goldberg S, Gottlieb S, et al. Natural history of left ventricular thrombi: their appearance and resolution in the posthospitalization period of acute myocardial infarction. J Am Coll Cardiol 1990; 15:790–800.
- Jordan RA, Miller RD, Edwards JE, Parker RL. Thrombo-embolism in acute and in healed myocardial infarction. I. Intracardiac mural thrombosis. Circulation 1952; 6:1–6.
- Vaitkus PT, Barnathan ES. Embolic potential, prevention and management of mural thrombus complicating anterior myocardial infarction: a meta-analysis. J Am Coll Cardiol 1993; 22:1004–1009.
- ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2:349–360.
- Cabin HS, Roberts WC. Left ventricular aneurysm, intraaneurysmal thrombus and systemic embolus in coronary heart disease. Chest 1980; 77:586–590.
- Keating EC, Gross SA, Schlamowitz RA, et al. Mural thrombi in myocardial infarctions. Prospective evaluation by two-dimensional echocardiography. Am J Med 1983; 74:989–995.
- Witt BJ, Ballman KV, Brown RD Jr, Meverden RA, Jacobsen SJ, Roger VL. The incidence of stroke after myocardial infarction: a meta-analysis. Am J Med 2006; 119:354.e1–354.e9.
- Witt BJ, Brown RD Jr, Jacobsen SJ, Weston SA, Yawn BP, Roger VL. A community-based study of stroke incidence after myocardial infarction. Ann Intern Med 2005; 143:785–792.
- Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e637S–e68S.
- Steg G, James SK, Atar D, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012; 33:2569–2619.
- Nili M, Deviri E, Jortner R, Strasberg B, Levy MJ. Surgical removal of a mobile, pedunculated left ventricular thrombus: report of 4 cases. Ann Thorac Surg 1988; 46:396–400.
- Kanemitsu S, Miyake Y, Okabe M. Surgical removal of a left ventricular thrombus associated with cardiac sarcoidosis. Interact Cardiovasc Thorac Surg 2008; 7:333–335.
- Engin C, Yagdi T, Balcioglu O, et al. Left ventricular assist device implantation in heart failure patients with a left ventricular thrombus. Transplant Proc 2013; 45:1017–1019.
- Dewilde WJ, Oirbans T, Verheugt FW, et al; WOEST study investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013; 381:1107–1115.
- Faxon DP, Eikelboom JW, Berger PB, et al. Antithrombotic therapy in patients with atrial fibrillation undergoing coronary stenting: a North American perspective: executive summary. Circ Cardiovasc Interv 2011; 4:522–534.
- Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010; 170:1433–1441.
- Karjalainen PP, Porela P, Ylitalo A, et al. Safety and efficacy of combined antiplatelet-warfarin therapy after coronary stenting. Eur Heart J 2007; 28:726–732.
- Doyle BJ, Rihal CS, Gastineau DA, Holmes DR Jr. Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J Am Coll Cardiol 2009; 53:2019–2027.
- Azoulay L, Dell’Aniello S, Simon T, Renoux C, Suissa S. The concurrent use of antithrombotic therapies and the risk of bleeding in patients with atrial fibrillation. Thromb Haemost 2013; 109:431–439.
- Deshmukh A, Hilleman DE, Del Core M, Nair CK. Antithrombotic regimens in patients with indication for long-term anticoagulation undergoing coronary interventions-systematic analysis, review of literature, and implications on management. Am J Ther 2013; 20:654–663.
- Fosbol EL, Wang TY, Li S, et al. Warfarin use among older atrial fibrillation patients with non-ST-segment elevation myocardial infarction managed with coronary stenting and dual antiplatelet therapy. Am Heart J 2013; 166:864–870.
- Gao F, Zhou YJ, Wang ZJ, et al. Meta-analysis of the combination of warfarin and dual antiplatelet therapy after coronary stenting in patients with indications for chronic oral anticoagulation. Int J Cardiol 2011; 148:96–101.
- Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010; 170:1433–1441.
- Hermosillo AJ, Spinler SA. Aspirin, clopidogrel, and warfarin: is the combination appropriate and effective or inappropriate and too dangerous? Ann Pharmacother 2008; 42:790–805.
- Holmes DR Jr, Kereiakes DJ, Kleiman NS, Moliterno DJ, Patti G, Grines CL. Combining antiplatelet and anticoagulant therapies. J Am Coll Cardiol 2009; 54:95–109.
- Khurram Z, Chou E, Minutello R, et al. Combination therapy with aspirin, clopidogrel and warfarin following coronary stenting is associated with a significant risk of bleeding. J Invasive Cardiol 2006; 18:162–164.
- Orford JL, Fasseas P, Melby S, et al. Safety and efficacy of aspirin, clopidogrel, and warfarin after coronary stent placement in patients with an indication for anticoagulation. Am Heart J 2004; 147:463–467.
- Porter A, Konstantino Y, Iakobishvili Z, Shachar L, Battler A, Hasdai D. Short-term triple therapy with aspirin, warfarin, and a thienopyridine among patients undergoing percutaneous coronary intervention. Catheter Cardiovasc Interv 2006; 68:56–61.
- DeEugenio D, Kolman L, DeCaro M, et al. Risk of major bleeding with concomitant dual antiplatelet therapy after percutaneous coronary intervention in patients receiving long-term warfarin therapy. Pharmacotherapy 2007; 27:691–696.
- Ruiz-Nodar JM, Marin F, Hurtado JA, et al. Anticoagulant and antiplatelet therapy use in 426 patients with atrial fibrillation undergoing percutaneous coronary intervention and stent implantation implications for bleeding risk and prognosis. J Am Coll Cardiol 2008; 51:818–825.
- Sarafoff N, Ndrepepa G, Mehilli J, et al. Aspirin and clopidogrel with or without phenprocoumon after drug eluting coronary stent placement in patients on chronic oral anticoagulation. J Intern Med 2008; 264:472–480.
- Rossini R, Musumeci GF, Lettieri CF, et al. Long-term outcomes in patients undergoing coronary stenting on dual oral antiplatelet treatment requiring oral anticoagulant therapy. Am J Cardiol 2008; 102:1618–1623.
A 62-year-old man with hypertension, type 2 diabetes mellitus, and hypercholesterolemia presented to the emergency department with substernal chest pain that started about 15 hours earlier while he was at rest watching television.
On examination, his pulse was 92 beats per minute and regular, his blood pressure was 160/88 mm Hg, and he had no evidence of jugular venous distention or pedal edema. Lung examination was positive for bibasilar crackles.
Electrocardiography revealed Q waves with ST elevation in leads I, aVL, V4, V5, and V6 with reciprocal ST depression in leads II, III, and aVF.
His troponin T level on presentation was markedly elevated.

He underwent heart catheterization and was found to have 100% occlusion of the proximal left anterior descending artery. He underwent successful percutaneous coronary intervention with placement of a drug-eluting stent, and afterward had grade 3 flow on the Thrombolysis in Myocardial Infarction (TIMI) scale.
Echocardiography the next day revealed a mobile echo-dense mass in the left ventricular apex (Figure 1) and a left ventricular ejection fraction of 35%.
THE INCIDENCE OF LEFT VENTRICULAR THROMBOSIS IN ACUTE MI
1. What is the incidence of left ventricular thrombosis after acute myocardial infarction (MI), now that primary percutaneous coronary intervention is common?
- 0.1%
- 2%
- 20%
- 40%
Left ventricular thrombosis is a serious complication of acute MI that can cause systemic thromboembolism, including stroke.1 Before thrombolytic therapy was available, this complication occurred in 20% to 60% of patients with acute MI.2,3 But early reperfusion strategies, anticoagulation for the first 48 hours, and dual antiplatelet therapy have reduced the incidence of this complication significantly.
In the thrombolytic era, the incidence of left ventricular thrombosis was 5.1% in the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI) 3 study, which had 8,326 patients. A subset of patients who had an anterior MI had almost double the incidence (11.5%).3

The incidence has further declined with the advent of primary percutaneous coronary intervention, likely thanks to enhanced myocardial salvage, and now ranges from 2.5% to 15% (Table 1).4–11 The largest observational study, with 2,911 patients undergoing percutaneous coronary intervention, reported an incidence of 2.5% within 3 to 5 days of the MI.7 At our center, the incidence was found to be even lower, 1.8% in 1,700 patients presenting with ST-elevation MI undergoing primary percutaneous coronary intervention. Hence, of the answers to the question above, 2% would be closest.
Large infarct size with a low left ventricular ejection fraction (< 40%), anterior wall MI, hypertension, and delay in time from symptom onset to intervention were independent predictors of left ventricular thrombus formation in most studies.7,12 The risk is highest during the first 2 weeks after MI, and thrombosis almost never occurs more than 3 months after the index event.5,13–16
WHAT IS THE PATHOGENESIS OF LEFT VENTRICULAR THROMBOSIS?
A large transmural infarct results in loss of contractile function, which causes stagnation and pooling of blood adjacent to the infarcted ventricular segment. In addition, endocardial injury exposes tissue factor, which then initiates the coagulation cascade. To make matters worse, MI results in a hypercoagulable state through unclear mechanisms, which completes the Virchow triad for thrombus formation. Elevations of D-dimer, fibrinogen, anticardiolipin antibodies (IgM and IgG), and tissue factor have also been reported after acute MI.17
Thrombus formation begins with platelet aggregation at the site of endocardial damage, forming a platelet plug, followed by activation of clotting factors. These thrombi are referred to as “mural,” as they adhere to the chamber wall (endocardium). They are composed of fibrin and entrapped red and white blood cells (Figure 2).
The natural course of thrombus evolution is established but variable. A left ventricular thrombus may dislodge and embolize, resulting in stroke or other thromboembolic complications. Alternately, it can dissolve over time, aided by intrinsic fibrinolytic mechanisms. On other occasions, the thrombus may organize, a process characterized by ingrowth of smooth muscle cells, fibroblasts, and endothelium.
HOW IS LEFT VENTRICULAR THROMBOSIS DIAGNOSED?
2. What is the best imaging test for detecting a thrombus?
- Transesophageal echocardiography
- Transthoracic echocardiography
- Cardiac magnetic resonance imaging (MRI) without gadolinium contrast
- Cardiac MRI with gadolinium contrast
Evaluation of left ventricular function after acute MI carries a class I indication (ie, it should be performed).18
Echocardiography is commonly used, and it has a 60% sensitivity to detect a thrombus.19 In patients with poorer transthoracic echocardiographic windows, contrast can be used to better delineate the left ventricular cavity and show the thrombus. Transesophageal echocardiography is seldom useful, as the left ventricular apex is foreshortened and in the far field.
A left ventricular thrombus is confirmed if an echo-dense mass with well-demarcated margins distinct from the endocardium is seen throughout the cardiac cycle. It should be evident in at least two different views (apical and short-axis) and should be adjacent to a hypokinetic or akinetic left ventricular wall. False-positive findings can occur due to misidentified false tendons, papillary muscles, and trabeculae.
Cardiac MRI with late gadolinium enhancement is now the gold standard for diagnostic imaging, as it accurately characterizes the shape, size, and location of the thrombus (Figure 3). Gadolinium contrast increases the enhancement of the ventricular cavity, thus allowing easy detection of thrombus, which appears dark. Cardiac MRI with delayed enhancement has 88% to 91% sensitivity and 99% specificity to detect left ventricular thrombosis.20,21 However, compared with echocardiography, routine cardiac MRI is time-intensive, costly, and not routinely available. As a result, it should be performed only in patients with poor acoustic windows and a high clinical suspicion of left ventricular thrombosis.
Delayed-contrast cardiac computed tomography can be used to identify left ventricular thrombosis, using absence of contrast uptake. The need to use contrast is a disadvantage, but computed tomography can be an alternative in patients with contraindications to cardiac MRI.
WHAT COMPLICATIONS ARISE FROM LEFT VENTRICULAR THROMBOSIS?
The most feared complication of left ventricular thrombosis is thromboembolism. Cardioembolic stroke is generally severe, prone to early and long-term recurrence, and associated with a higher death rate than noncardioembolic ischemic stroke.22,23 Thrombi associated with thromboembolism are often acute and mobile rather than organized and immobile.24 They may embolize to the brain, spleen, kidneys, and bowel.25 In a meta-analysis of 11 studies, the pooled odds ratio for risk of embolization was 5.45 (95% confidence interval [CI] 3.02–9.83) with left ventricular thrombi vs without.26 Before systemic thrombolysis and antiplatelet therapy became available, stroke rates ranged from 1.5% to 10%.27–29
In a meta-analysis of 22 studies from 1978 to 2004, the incidence of ischemic stroke after MI during hospitalization was around 11.1 per 1,000 MIs.30 This study found that anterior MI was associated with a higher risk of stroke, but reported no difference in the incidence of stroke with percutaneous coronary intervention, systemic thrombolysis, or no reperfusion.
In a large prospective cohort study of 2,160 patients,31 259 (12%) had a stroke after MI. In multivariable analysis, age, diabetes, and previous stroke were predictors of stroke after MI. This study reported significantly fewer strokes in patients who underwent percutaneous coronary intervention than with other or no reperfusion therapies.31
ANTICOAGULATION TREATMENT
3. How would you treat a patient who has a drug-eluting stent in the left anterior descending artery and a new diagnosis of left ventricular thrombosis?
- Warfarin
- Aspirin and clopidogrel
- Aspirin, clopidogrel, and warfarin
- Aspirin and warfarin
The management of left ventricular thrombosis has been summarized in guidelines from the American College of Chest Physicians (ACCP) in 2012,32 and from the American College of Cardiology/American Heart Association in 2013,18 which recommend anticoagulation for at least 3 months, or indefinitely if bleeding risk is low, for all patients developing a left ventricular thrombus.
For patients with acute MI and left ventricular thrombosis, the ACCP guidelines recommend warfarin with a target international normalized ratio of 2.0 to 3.0 plus dual antiplatelet therapy (eg, aspirin plus clopidogrel) for 3 months, after which warfarin is discontinued but dual antiplatelet therapy is continued for up to 12 months.32
The European Society of Cardiology guidelines33 recommend 6 months of anticoagulation. However, if the patient is receiving dual antiplatelet therapy, they recommend repeated imaging of the left ventricle after 3 months of anticoagulation, which may allow for earlier discontinuation of anticoagulation if the thrombus has resolved and apical wall motion has recovered. Therefore, most experts recommend 3 months of anticoagulation when used in combination with dual antiplatelet therapy and repeating echocardiography at 3 months to safely discontinue anticoagulation. The best answer to the question posed here is aspirin, clopidogrel, and warfarin.
Decisions about antithrombotic therapy may also depend on stent type and the patient’s bleeding risk. With bare-metal stents, dual antiplatelet therapy along with anticoagulation should be used for 1 month, after which anticoagulation should be used with a single antiplatelet agent for another 2 months; after this, the anticoagulant can be discontinued and dual antiplatelet therapy can be resumed for a total of 12 months. Newer anticoagulants such as rivaroxaban, dabigatran, edoxaban, and apixaban may also have a role, but they have not yet been studied for this indication.
Surgical thrombectomy is rarely considered now, given the known efficacy of anticoagulants in dissolving the thrombus. It was done in the past for large, mobile, or protruding left ventricular thrombi, which have a higher potential for embolization.34 Currently, it can be done under very special circumstances, such as before placement of a left ventricular assist device or if the thrombus is large, to prevent embolism.35,36
BLEEDING COMPLICATIONS WITH TRIPLE ANTITHROMBOTIC THERAPY
After stent placement, almost all patients need to be on dual antiplatelet therapy for a specified duration depending on the type and generation of stent used. Such patients end up on “triple” antithrombotic therapy (two antiplatelet drugs plus an anticoagulant), which poses a high risk of bleeding.37 Consideration needs to be given to the risks of stroke, stent thrombosis, and major bleeding when selecting the antithrombotic regimen.38 Triple antithrombotic therapy has been associated with a risk of fatal and nonfatal bleeding of 4% to 16% when used for indications such as atrial fibrillation.39–41
Risks of triple antithrombotic therapy (aspirin 80–100 mg, clopidogrel 75 mg, and warfarin) were compared with those of clopidogrel plus warfarin in the What Is the Optimal Antiplatelet and Anticoagulant therapy in Patients With Oral Anticoagulation and Coronary Stenting Trial,37 which reported a significantly lower risk of major and minor bleeding with clopidogrel-plus-warfarin therapy than with triple antithrombotic therapy, 14.3% vs 31.7% (hazard ratio 0.40, 95% CI 0.28–0.58, P < .0001).
Additionally, the increased risk of major and minor bleeding associated with triple antithrombotic therapy has been confirmed in many observational studies; other studies found a trend toward lower risk with triple therapy, but this was not statistically significant (Table 2).38,40,42–55 A large multicenter European trial is being conducted to compare dual antiplatelet therapy vs triple antithrombotic therapy in patients with left ventricular thrombosis.
CASE FOLLOW-UP
Our patient was started on warfarin, clopidogrel 75 mg, and aspirin 75 mg at the time of discharge. He was continued on warfarin for 3 months, at which time a follow-up echocardiogram showed no thrombus in the left ventricle. Warfarin was discontinued, and he had no thromboembolic complications.
TAKE-HOME POINTS
Left ventricular thrombosis after an acute MI is very important to detect, as it can lead to serious complications through arterial embolism.
The incidence of left ventricular thrombosis has declined significantly with the use of percutaneous coronary intervention. However, it may still occur in a small number of patients with larger infarcts owing to delay in revascularization or proximal (left main or left anterior descending) occlusions with larger infarct size.
Echocardiography, which is routinely performed after acute MI to assess myocardial function, uncovers most left ventricular thrombi. In high-risk cases, MRI with late gadolinium enhancement can increase the diagnostic yield.
Anticoagulation with warfarin is recommended for at least 3 months. Post-MI patients undergoing stent implantation may need triple antithrombotic therapy, which, however, increases the bleeding risk significantly. Large randomized trials are needed to guide physicians in risk stratification of such patients.
A 62-year-old man with hypertension, type 2 diabetes mellitus, and hypercholesterolemia presented to the emergency department with substernal chest pain that started about 15 hours earlier while he was at rest watching television.
On examination, his pulse was 92 beats per minute and regular, his blood pressure was 160/88 mm Hg, and he had no evidence of jugular venous distention or pedal edema. Lung examination was positive for bibasilar crackles.
Electrocardiography revealed Q waves with ST elevation in leads I, aVL, V4, V5, and V6 with reciprocal ST depression in leads II, III, and aVF.
His troponin T level on presentation was markedly elevated.

He underwent heart catheterization and was found to have 100% occlusion of the proximal left anterior descending artery. He underwent successful percutaneous coronary intervention with placement of a drug-eluting stent, and afterward had grade 3 flow on the Thrombolysis in Myocardial Infarction (TIMI) scale.
Echocardiography the next day revealed a mobile echo-dense mass in the left ventricular apex (Figure 1) and a left ventricular ejection fraction of 35%.
THE INCIDENCE OF LEFT VENTRICULAR THROMBOSIS IN ACUTE MI
1. What is the incidence of left ventricular thrombosis after acute myocardial infarction (MI), now that primary percutaneous coronary intervention is common?
- 0.1%
- 2%
- 20%
- 40%
Left ventricular thrombosis is a serious complication of acute MI that can cause systemic thromboembolism, including stroke.1 Before thrombolytic therapy was available, this complication occurred in 20% to 60% of patients with acute MI.2,3 But early reperfusion strategies, anticoagulation for the first 48 hours, and dual antiplatelet therapy have reduced the incidence of this complication significantly.
In the thrombolytic era, the incidence of left ventricular thrombosis was 5.1% in the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI) 3 study, which had 8,326 patients. A subset of patients who had an anterior MI had almost double the incidence (11.5%).3

The incidence has further declined with the advent of primary percutaneous coronary intervention, likely thanks to enhanced myocardial salvage, and now ranges from 2.5% to 15% (Table 1).4–11 The largest observational study, with 2,911 patients undergoing percutaneous coronary intervention, reported an incidence of 2.5% within 3 to 5 days of the MI.7 At our center, the incidence was found to be even lower, 1.8% in 1,700 patients presenting with ST-elevation MI undergoing primary percutaneous coronary intervention. Hence, of the answers to the question above, 2% would be closest.
Large infarct size with a low left ventricular ejection fraction (< 40%), anterior wall MI, hypertension, and delay in time from symptom onset to intervention were independent predictors of left ventricular thrombus formation in most studies.7,12 The risk is highest during the first 2 weeks after MI, and thrombosis almost never occurs more than 3 months after the index event.5,13–16
WHAT IS THE PATHOGENESIS OF LEFT VENTRICULAR THROMBOSIS?
A large transmural infarct results in loss of contractile function, which causes stagnation and pooling of blood adjacent to the infarcted ventricular segment. In addition, endocardial injury exposes tissue factor, which then initiates the coagulation cascade. To make matters worse, MI results in a hypercoagulable state through unclear mechanisms, which completes the Virchow triad for thrombus formation. Elevations of D-dimer, fibrinogen, anticardiolipin antibodies (IgM and IgG), and tissue factor have also been reported after acute MI.17
Thrombus formation begins with platelet aggregation at the site of endocardial damage, forming a platelet plug, followed by activation of clotting factors. These thrombi are referred to as “mural,” as they adhere to the chamber wall (endocardium). They are composed of fibrin and entrapped red and white blood cells (Figure 2).
The natural course of thrombus evolution is established but variable. A left ventricular thrombus may dislodge and embolize, resulting in stroke or other thromboembolic complications. Alternately, it can dissolve over time, aided by intrinsic fibrinolytic mechanisms. On other occasions, the thrombus may organize, a process characterized by ingrowth of smooth muscle cells, fibroblasts, and endothelium.
HOW IS LEFT VENTRICULAR THROMBOSIS DIAGNOSED?
2. What is the best imaging test for detecting a thrombus?
- Transesophageal echocardiography
- Transthoracic echocardiography
- Cardiac magnetic resonance imaging (MRI) without gadolinium contrast
- Cardiac MRI with gadolinium contrast
Evaluation of left ventricular function after acute MI carries a class I indication (ie, it should be performed).18
Echocardiography is commonly used, and it has a 60% sensitivity to detect a thrombus.19 In patients with poorer transthoracic echocardiographic windows, contrast can be used to better delineate the left ventricular cavity and show the thrombus. Transesophageal echocardiography is seldom useful, as the left ventricular apex is foreshortened and in the far field.
A left ventricular thrombus is confirmed if an echo-dense mass with well-demarcated margins distinct from the endocardium is seen throughout the cardiac cycle. It should be evident in at least two different views (apical and short-axis) and should be adjacent to a hypokinetic or akinetic left ventricular wall. False-positive findings can occur due to misidentified false tendons, papillary muscles, and trabeculae.
Cardiac MRI with late gadolinium enhancement is now the gold standard for diagnostic imaging, as it accurately characterizes the shape, size, and location of the thrombus (Figure 3). Gadolinium contrast increases the enhancement of the ventricular cavity, thus allowing easy detection of thrombus, which appears dark. Cardiac MRI with delayed enhancement has 88% to 91% sensitivity and 99% specificity to detect left ventricular thrombosis.20,21 However, compared with echocardiography, routine cardiac MRI is time-intensive, costly, and not routinely available. As a result, it should be performed only in patients with poor acoustic windows and a high clinical suspicion of left ventricular thrombosis.
Delayed-contrast cardiac computed tomography can be used to identify left ventricular thrombosis, using absence of contrast uptake. The need to use contrast is a disadvantage, but computed tomography can be an alternative in patients with contraindications to cardiac MRI.
WHAT COMPLICATIONS ARISE FROM LEFT VENTRICULAR THROMBOSIS?
The most feared complication of left ventricular thrombosis is thromboembolism. Cardioembolic stroke is generally severe, prone to early and long-term recurrence, and associated with a higher death rate than noncardioembolic ischemic stroke.22,23 Thrombi associated with thromboembolism are often acute and mobile rather than organized and immobile.24 They may embolize to the brain, spleen, kidneys, and bowel.25 In a meta-analysis of 11 studies, the pooled odds ratio for risk of embolization was 5.45 (95% confidence interval [CI] 3.02–9.83) with left ventricular thrombi vs without.26 Before systemic thrombolysis and antiplatelet therapy became available, stroke rates ranged from 1.5% to 10%.27–29
In a meta-analysis of 22 studies from 1978 to 2004, the incidence of ischemic stroke after MI during hospitalization was around 11.1 per 1,000 MIs.30 This study found that anterior MI was associated with a higher risk of stroke, but reported no difference in the incidence of stroke with percutaneous coronary intervention, systemic thrombolysis, or no reperfusion.
In a large prospective cohort study of 2,160 patients,31 259 (12%) had a stroke after MI. In multivariable analysis, age, diabetes, and previous stroke were predictors of stroke after MI. This study reported significantly fewer strokes in patients who underwent percutaneous coronary intervention than with other or no reperfusion therapies.31
ANTICOAGULATION TREATMENT
3. How would you treat a patient who has a drug-eluting stent in the left anterior descending artery and a new diagnosis of left ventricular thrombosis?
- Warfarin
- Aspirin and clopidogrel
- Aspirin, clopidogrel, and warfarin
- Aspirin and warfarin
The management of left ventricular thrombosis has been summarized in guidelines from the American College of Chest Physicians (ACCP) in 2012,32 and from the American College of Cardiology/American Heart Association in 2013,18 which recommend anticoagulation for at least 3 months, or indefinitely if bleeding risk is low, for all patients developing a left ventricular thrombus.
For patients with acute MI and left ventricular thrombosis, the ACCP guidelines recommend warfarin with a target international normalized ratio of 2.0 to 3.0 plus dual antiplatelet therapy (eg, aspirin plus clopidogrel) for 3 months, after which warfarin is discontinued but dual antiplatelet therapy is continued for up to 12 months.32
The European Society of Cardiology guidelines33 recommend 6 months of anticoagulation. However, if the patient is receiving dual antiplatelet therapy, they recommend repeated imaging of the left ventricle after 3 months of anticoagulation, which may allow for earlier discontinuation of anticoagulation if the thrombus has resolved and apical wall motion has recovered. Therefore, most experts recommend 3 months of anticoagulation when used in combination with dual antiplatelet therapy and repeating echocardiography at 3 months to safely discontinue anticoagulation. The best answer to the question posed here is aspirin, clopidogrel, and warfarin.
Decisions about antithrombotic therapy may also depend on stent type and the patient’s bleeding risk. With bare-metal stents, dual antiplatelet therapy along with anticoagulation should be used for 1 month, after which anticoagulation should be used with a single antiplatelet agent for another 2 months; after this, the anticoagulant can be discontinued and dual antiplatelet therapy can be resumed for a total of 12 months. Newer anticoagulants such as rivaroxaban, dabigatran, edoxaban, and apixaban may also have a role, but they have not yet been studied for this indication.
Surgical thrombectomy is rarely considered now, given the known efficacy of anticoagulants in dissolving the thrombus. It was done in the past for large, mobile, or protruding left ventricular thrombi, which have a higher potential for embolization.34 Currently, it can be done under very special circumstances, such as before placement of a left ventricular assist device or if the thrombus is large, to prevent embolism.35,36
BLEEDING COMPLICATIONS WITH TRIPLE ANTITHROMBOTIC THERAPY
After stent placement, almost all patients need to be on dual antiplatelet therapy for a specified duration depending on the type and generation of stent used. Such patients end up on “triple” antithrombotic therapy (two antiplatelet drugs plus an anticoagulant), which poses a high risk of bleeding.37 Consideration needs to be given to the risks of stroke, stent thrombosis, and major bleeding when selecting the antithrombotic regimen.38 Triple antithrombotic therapy has been associated with a risk of fatal and nonfatal bleeding of 4% to 16% when used for indications such as atrial fibrillation.39–41
Risks of triple antithrombotic therapy (aspirin 80–100 mg, clopidogrel 75 mg, and warfarin) were compared with those of clopidogrel plus warfarin in the What Is the Optimal Antiplatelet and Anticoagulant therapy in Patients With Oral Anticoagulation and Coronary Stenting Trial,37 which reported a significantly lower risk of major and minor bleeding with clopidogrel-plus-warfarin therapy than with triple antithrombotic therapy, 14.3% vs 31.7% (hazard ratio 0.40, 95% CI 0.28–0.58, P < .0001).
Additionally, the increased risk of major and minor bleeding associated with triple antithrombotic therapy has been confirmed in many observational studies; other studies found a trend toward lower risk with triple therapy, but this was not statistically significant (Table 2).38,40,42–55 A large multicenter European trial is being conducted to compare dual antiplatelet therapy vs triple antithrombotic therapy in patients with left ventricular thrombosis.
CASE FOLLOW-UP
Our patient was started on warfarin, clopidogrel 75 mg, and aspirin 75 mg at the time of discharge. He was continued on warfarin for 3 months, at which time a follow-up echocardiogram showed no thrombus in the left ventricle. Warfarin was discontinued, and he had no thromboembolic complications.
TAKE-HOME POINTS
Left ventricular thrombosis after an acute MI is very important to detect, as it can lead to serious complications through arterial embolism.
The incidence of left ventricular thrombosis has declined significantly with the use of percutaneous coronary intervention. However, it may still occur in a small number of patients with larger infarcts owing to delay in revascularization or proximal (left main or left anterior descending) occlusions with larger infarct size.
Echocardiography, which is routinely performed after acute MI to assess myocardial function, uncovers most left ventricular thrombi. In high-risk cases, MRI with late gadolinium enhancement can increase the diagnostic yield.
Anticoagulation with warfarin is recommended for at least 3 months. Post-MI patients undergoing stent implantation may need triple antithrombotic therapy, which, however, increases the bleeding risk significantly. Large randomized trials are needed to guide physicians in risk stratification of such patients.
- Lip GY, Piotrponikowski P, Andreotti F, et al; Heart Failure Association (EHFA) of the European Society of Cardiology (ESC) and the ESC Working Group on Thrombosis. Thromboembolism and antithrombotic therapy for heart failure in sinus rhythm: an executive summary of a joint consensus document from the ESC Heart Failure Association and the ESC Working Group on Thrombosis. Thromb Haemost 2012; 108:1009–1022.
- Turpie AG, Robinson JG, Doyle DJ, et al. Comparison of high-dose with low-dose subcutaneous heparin to prevent left ventricular mural thrombosis in patients with acute transmural anterior myocardial infarction. N Engl J Med 1989; 320:352–357.
- Chiarella F, Santoro E, Domenicucci S, Maggioni A, Vecchio C. Predischarge two-dimensional echocardiographic evaluation of left ventricular thrombosis after acute myocardial infarction in the GISSI-3 study. Am J Cardiol 1998; 81:822–827.
- Kalra A, Jang IK. Prevalence of early left ventricular thrombus after primary coronary intervention for acute myocardial infarction. J Thromb Thrombolysis 2000; 10:133–136.
- Nayak D, Aronow WS, Sukhija R, McClung JA, Monsen CE, Belkin RN. Comparison of frequency of left ventricular thrombi in patients with anterior wall versus non-anterior wall acute myocardial infarction treated with antithrombotic and antiplatelet therapy with or without coronary revascularization. Am J Cardiol 2004; 93:1529–1530.
- Rehan A, Kanwar M, Rosman H, et al. Incidence of post myocardial infarction left ventricular thrombus formation in the era of primary percutaneous intervention and glycoprotein IIb/IIIa inhibitors. A prospective observational study. Cardiovasc Ultrasound 2006;4:20.
- Zielinska M, Kaczmarek K, Tylkowski M. Predictors of left ventricular thrombus formation in acute myocardial infarction treated with successful primary angioplasty with stenting. Am J Med Sci 2008; 335:171–176.
- Osherov AB, Borovik-Raz M, Aronson D, et al. Incidence of early left ventricular thrombus after acute anterior wall myocardial infarction in the primary coronary intervention era. Am Heart J 2009; 157:1074–1080.
- Solheim S, Seljeflot I, Lunde K, et al. Frequency of left ventricular thrombus in patients with anterior wall acute myocardial infarction treated with percutaneous coronary intervention and dual antiplatelet therapy. Am J Cardiol 2010; 106:1197–1200.
- Shacham Y, Leshem-Rubinow E, Ben Assa E, et al. Comparison of C-reactive protein and fibrinogen levels in patients having anterior wall ST-segment elevation myocardial infarction with versus without left ventricular thrombus (from a primary percutaneous coronary intervention cohort). Am J Cardiol 2013; 112:57–60.
- Gianstefani S, Douiri A, Delithanasis I, et al. Incidence and predictors of early left ventricular thrombus after ST-elevation myocardial infarction in the contemporary era of primary percutaneous coronary intervention. Am J Cardiol 2014; 113:1111–1116.
- Shacham Y, Birati EY, Rogovski O, Cogan Y, Keren G, Roth A. Left ventricular thrombus formation and bleeding complications during continuous in-hospital anticoagulation for acute anterior myocardial infarction. Isr Med Assoc J 2012; 14:742–746.
- Asinger RW, Mikell FL, Elsperger J, Hodges M. Incidence of left-ventricular thrombosis after acute transmural myocardial infarction. Serial evaluation by two-dimensional echocardiography. N Engl J Med 1981; 305:297–302.
- Nihoyannopoulos P, Smith GC, Maseri A, Foale RA. The natural history of left ventricular thrombus in myocardial infarction: a rationale in support of masterly inactivity. J Am Coll Cardiol 1989; 14:903–911.
- Weinreich DJ, Burke JF, Pauletto FJ. Left ventricular mural thrombi complicating acute myocardial infarction. Long-term follow-up with serial echocardiography. Ann Intern Med 1984; 100:789–794.
- Greaves SC, Zhi G, Lee RT, et al. Incidence and natural history of left ventricular thrombus following anterior wall acute myocardial infarction. Am J Cardiol 1997; 80:442–448.
- Solheim S, Seljeflot I, Lunde K, et al. Prothrombotic markers in patients with acute myocardial infarction and left ventricular thrombus formation treated with pci and dual antiplatelet therapy. Thromb J 2013; 11:1.
- O’Gara PT, Kushner FG, Ascheim DD, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 127:e362–e425.
- Weinsaft JW, Kim HW, Crowley AL, et al. LV thrombus detection by routine echocardiography: insights into performance characteristics using delayed enhancement CMR. JACC Cardiovasc Imaging 2011; 4:702–712.
- Mollet NR, Dymarkowski S, Volders W, et al. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation 2002; 106:2873–2876.
- Srichai MB, Junor C, Rodriguez LL, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J 2006; 152:75–84.
- Eriksson SE, Olsson JE. Survival and recurrent strokes in patients with different subtypes of stroke: a fourteen-year follow-up study. Cerebrovasc Dis 2001; 12:171–180.
- Grau AJ, Weimar C, Buggle F, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German Stroke Data Bank. Stroke 2001; 32:2559–2566.
- Keren A, Goldberg S, Gottlieb S, et al. Natural history of left ventricular thrombi: their appearance and resolution in the posthospitalization period of acute myocardial infarction. J Am Coll Cardiol 1990; 15:790–800.
- Jordan RA, Miller RD, Edwards JE, Parker RL. Thrombo-embolism in acute and in healed myocardial infarction. I. Intracardiac mural thrombosis. Circulation 1952; 6:1–6.
- Vaitkus PT, Barnathan ES. Embolic potential, prevention and management of mural thrombus complicating anterior myocardial infarction: a meta-analysis. J Am Coll Cardiol 1993; 22:1004–1009.
- ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2:349–360.
- Cabin HS, Roberts WC. Left ventricular aneurysm, intraaneurysmal thrombus and systemic embolus in coronary heart disease. Chest 1980; 77:586–590.
- Keating EC, Gross SA, Schlamowitz RA, et al. Mural thrombi in myocardial infarctions. Prospective evaluation by two-dimensional echocardiography. Am J Med 1983; 74:989–995.
- Witt BJ, Ballman KV, Brown RD Jr, Meverden RA, Jacobsen SJ, Roger VL. The incidence of stroke after myocardial infarction: a meta-analysis. Am J Med 2006; 119:354.e1–354.e9.
- Witt BJ, Brown RD Jr, Jacobsen SJ, Weston SA, Yawn BP, Roger VL. A community-based study of stroke incidence after myocardial infarction. Ann Intern Med 2005; 143:785–792.
- Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e637S–e68S.
- Steg G, James SK, Atar D, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012; 33:2569–2619.
- Nili M, Deviri E, Jortner R, Strasberg B, Levy MJ. Surgical removal of a mobile, pedunculated left ventricular thrombus: report of 4 cases. Ann Thorac Surg 1988; 46:396–400.
- Kanemitsu S, Miyake Y, Okabe M. Surgical removal of a left ventricular thrombus associated with cardiac sarcoidosis. Interact Cardiovasc Thorac Surg 2008; 7:333–335.
- Engin C, Yagdi T, Balcioglu O, et al. Left ventricular assist device implantation in heart failure patients with a left ventricular thrombus. Transplant Proc 2013; 45:1017–1019.
- Dewilde WJ, Oirbans T, Verheugt FW, et al; WOEST study investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013; 381:1107–1115.
- Faxon DP, Eikelboom JW, Berger PB, et al. Antithrombotic therapy in patients with atrial fibrillation undergoing coronary stenting: a North American perspective: executive summary. Circ Cardiovasc Interv 2011; 4:522–534.
- Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010; 170:1433–1441.
- Karjalainen PP, Porela P, Ylitalo A, et al. Safety and efficacy of combined antiplatelet-warfarin therapy after coronary stenting. Eur Heart J 2007; 28:726–732.
- Doyle BJ, Rihal CS, Gastineau DA, Holmes DR Jr. Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J Am Coll Cardiol 2009; 53:2019–2027.
- Azoulay L, Dell’Aniello S, Simon T, Renoux C, Suissa S. The concurrent use of antithrombotic therapies and the risk of bleeding in patients with atrial fibrillation. Thromb Haemost 2013; 109:431–439.
- Deshmukh A, Hilleman DE, Del Core M, Nair CK. Antithrombotic regimens in patients with indication for long-term anticoagulation undergoing coronary interventions-systematic analysis, review of literature, and implications on management. Am J Ther 2013; 20:654–663.
- Fosbol EL, Wang TY, Li S, et al. Warfarin use among older atrial fibrillation patients with non-ST-segment elevation myocardial infarction managed with coronary stenting and dual antiplatelet therapy. Am Heart J 2013; 166:864–870.
- Gao F, Zhou YJ, Wang ZJ, et al. Meta-analysis of the combination of warfarin and dual antiplatelet therapy after coronary stenting in patients with indications for chronic oral anticoagulation. Int J Cardiol 2011; 148:96–101.
- Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010; 170:1433–1441.
- Hermosillo AJ, Spinler SA. Aspirin, clopidogrel, and warfarin: is the combination appropriate and effective or inappropriate and too dangerous? Ann Pharmacother 2008; 42:790–805.
- Holmes DR Jr, Kereiakes DJ, Kleiman NS, Moliterno DJ, Patti G, Grines CL. Combining antiplatelet and anticoagulant therapies. J Am Coll Cardiol 2009; 54:95–109.
- Khurram Z, Chou E, Minutello R, et al. Combination therapy with aspirin, clopidogrel and warfarin following coronary stenting is associated with a significant risk of bleeding. J Invasive Cardiol 2006; 18:162–164.
- Orford JL, Fasseas P, Melby S, et al. Safety and efficacy of aspirin, clopidogrel, and warfarin after coronary stent placement in patients with an indication for anticoagulation. Am Heart J 2004; 147:463–467.
- Porter A, Konstantino Y, Iakobishvili Z, Shachar L, Battler A, Hasdai D. Short-term triple therapy with aspirin, warfarin, and a thienopyridine among patients undergoing percutaneous coronary intervention. Catheter Cardiovasc Interv 2006; 68:56–61.
- DeEugenio D, Kolman L, DeCaro M, et al. Risk of major bleeding with concomitant dual antiplatelet therapy after percutaneous coronary intervention in patients receiving long-term warfarin therapy. Pharmacotherapy 2007; 27:691–696.
- Ruiz-Nodar JM, Marin F, Hurtado JA, et al. Anticoagulant and antiplatelet therapy use in 426 patients with atrial fibrillation undergoing percutaneous coronary intervention and stent implantation implications for bleeding risk and prognosis. J Am Coll Cardiol 2008; 51:818–825.
- Sarafoff N, Ndrepepa G, Mehilli J, et al. Aspirin and clopidogrel with or without phenprocoumon after drug eluting coronary stent placement in patients on chronic oral anticoagulation. J Intern Med 2008; 264:472–480.
- Rossini R, Musumeci GF, Lettieri CF, et al. Long-term outcomes in patients undergoing coronary stenting on dual oral antiplatelet treatment requiring oral anticoagulant therapy. Am J Cardiol 2008; 102:1618–1623.
- Lip GY, Piotrponikowski P, Andreotti F, et al; Heart Failure Association (EHFA) of the European Society of Cardiology (ESC) and the ESC Working Group on Thrombosis. Thromboembolism and antithrombotic therapy for heart failure in sinus rhythm: an executive summary of a joint consensus document from the ESC Heart Failure Association and the ESC Working Group on Thrombosis. Thromb Haemost 2012; 108:1009–1022.
- Turpie AG, Robinson JG, Doyle DJ, et al. Comparison of high-dose with low-dose subcutaneous heparin to prevent left ventricular mural thrombosis in patients with acute transmural anterior myocardial infarction. N Engl J Med 1989; 320:352–357.
- Chiarella F, Santoro E, Domenicucci S, Maggioni A, Vecchio C. Predischarge two-dimensional echocardiographic evaluation of left ventricular thrombosis after acute myocardial infarction in the GISSI-3 study. Am J Cardiol 1998; 81:822–827.
- Kalra A, Jang IK. Prevalence of early left ventricular thrombus after primary coronary intervention for acute myocardial infarction. J Thromb Thrombolysis 2000; 10:133–136.
- Nayak D, Aronow WS, Sukhija R, McClung JA, Monsen CE, Belkin RN. Comparison of frequency of left ventricular thrombi in patients with anterior wall versus non-anterior wall acute myocardial infarction treated with antithrombotic and antiplatelet therapy with or without coronary revascularization. Am J Cardiol 2004; 93:1529–1530.
- Rehan A, Kanwar M, Rosman H, et al. Incidence of post myocardial infarction left ventricular thrombus formation in the era of primary percutaneous intervention and glycoprotein IIb/IIIa inhibitors. A prospective observational study. Cardiovasc Ultrasound 2006;4:20.
- Zielinska M, Kaczmarek K, Tylkowski M. Predictors of left ventricular thrombus formation in acute myocardial infarction treated with successful primary angioplasty with stenting. Am J Med Sci 2008; 335:171–176.
- Osherov AB, Borovik-Raz M, Aronson D, et al. Incidence of early left ventricular thrombus after acute anterior wall myocardial infarction in the primary coronary intervention era. Am Heart J 2009; 157:1074–1080.
- Solheim S, Seljeflot I, Lunde K, et al. Frequency of left ventricular thrombus in patients with anterior wall acute myocardial infarction treated with percutaneous coronary intervention and dual antiplatelet therapy. Am J Cardiol 2010; 106:1197–1200.
- Shacham Y, Leshem-Rubinow E, Ben Assa E, et al. Comparison of C-reactive protein and fibrinogen levels in patients having anterior wall ST-segment elevation myocardial infarction with versus without left ventricular thrombus (from a primary percutaneous coronary intervention cohort). Am J Cardiol 2013; 112:57–60.
- Gianstefani S, Douiri A, Delithanasis I, et al. Incidence and predictors of early left ventricular thrombus after ST-elevation myocardial infarction in the contemporary era of primary percutaneous coronary intervention. Am J Cardiol 2014; 113:1111–1116.
- Shacham Y, Birati EY, Rogovski O, Cogan Y, Keren G, Roth A. Left ventricular thrombus formation and bleeding complications during continuous in-hospital anticoagulation for acute anterior myocardial infarction. Isr Med Assoc J 2012; 14:742–746.
- Asinger RW, Mikell FL, Elsperger J, Hodges M. Incidence of left-ventricular thrombosis after acute transmural myocardial infarction. Serial evaluation by two-dimensional echocardiography. N Engl J Med 1981; 305:297–302.
- Nihoyannopoulos P, Smith GC, Maseri A, Foale RA. The natural history of left ventricular thrombus in myocardial infarction: a rationale in support of masterly inactivity. J Am Coll Cardiol 1989; 14:903–911.
- Weinreich DJ, Burke JF, Pauletto FJ. Left ventricular mural thrombi complicating acute myocardial infarction. Long-term follow-up with serial echocardiography. Ann Intern Med 1984; 100:789–794.
- Greaves SC, Zhi G, Lee RT, et al. Incidence and natural history of left ventricular thrombus following anterior wall acute myocardial infarction. Am J Cardiol 1997; 80:442–448.
- Solheim S, Seljeflot I, Lunde K, et al. Prothrombotic markers in patients with acute myocardial infarction and left ventricular thrombus formation treated with pci and dual antiplatelet therapy. Thromb J 2013; 11:1.
- O’Gara PT, Kushner FG, Ascheim DD, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 127:e362–e425.
- Weinsaft JW, Kim HW, Crowley AL, et al. LV thrombus detection by routine echocardiography: insights into performance characteristics using delayed enhancement CMR. JACC Cardiovasc Imaging 2011; 4:702–712.
- Mollet NR, Dymarkowski S, Volders W, et al. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation 2002; 106:2873–2876.
- Srichai MB, Junor C, Rodriguez LL, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J 2006; 152:75–84.
- Eriksson SE, Olsson JE. Survival and recurrent strokes in patients with different subtypes of stroke: a fourteen-year follow-up study. Cerebrovasc Dis 2001; 12:171–180.
- Grau AJ, Weimar C, Buggle F, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German Stroke Data Bank. Stroke 2001; 32:2559–2566.
- Keren A, Goldberg S, Gottlieb S, et al. Natural history of left ventricular thrombi: their appearance and resolution in the posthospitalization period of acute myocardial infarction. J Am Coll Cardiol 1990; 15:790–800.
- Jordan RA, Miller RD, Edwards JE, Parker RL. Thrombo-embolism in acute and in healed myocardial infarction. I. Intracardiac mural thrombosis. Circulation 1952; 6:1–6.
- Vaitkus PT, Barnathan ES. Embolic potential, prevention and management of mural thrombus complicating anterior myocardial infarction: a meta-analysis. J Am Coll Cardiol 1993; 22:1004–1009.
- ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2:349–360.
- Cabin HS, Roberts WC. Left ventricular aneurysm, intraaneurysmal thrombus and systemic embolus in coronary heart disease. Chest 1980; 77:586–590.
- Keating EC, Gross SA, Schlamowitz RA, et al. Mural thrombi in myocardial infarctions. Prospective evaluation by two-dimensional echocardiography. Am J Med 1983; 74:989–995.
- Witt BJ, Ballman KV, Brown RD Jr, Meverden RA, Jacobsen SJ, Roger VL. The incidence of stroke after myocardial infarction: a meta-analysis. Am J Med 2006; 119:354.e1–354.e9.
- Witt BJ, Brown RD Jr, Jacobsen SJ, Weston SA, Yawn BP, Roger VL. A community-based study of stroke incidence after myocardial infarction. Ann Intern Med 2005; 143:785–792.
- Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e637S–e68S.
- Steg G, James SK, Atar D, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012; 33:2569–2619.
- Nili M, Deviri E, Jortner R, Strasberg B, Levy MJ. Surgical removal of a mobile, pedunculated left ventricular thrombus: report of 4 cases. Ann Thorac Surg 1988; 46:396–400.
- Kanemitsu S, Miyake Y, Okabe M. Surgical removal of a left ventricular thrombus associated with cardiac sarcoidosis. Interact Cardiovasc Thorac Surg 2008; 7:333–335.
- Engin C, Yagdi T, Balcioglu O, et al. Left ventricular assist device implantation in heart failure patients with a left ventricular thrombus. Transplant Proc 2013; 45:1017–1019.
- Dewilde WJ, Oirbans T, Verheugt FW, et al; WOEST study investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013; 381:1107–1115.
- Faxon DP, Eikelboom JW, Berger PB, et al. Antithrombotic therapy in patients with atrial fibrillation undergoing coronary stenting: a North American perspective: executive summary. Circ Cardiovasc Interv 2011; 4:522–534.
- Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010; 170:1433–1441.
- Karjalainen PP, Porela P, Ylitalo A, et al. Safety and efficacy of combined antiplatelet-warfarin therapy after coronary stenting. Eur Heart J 2007; 28:726–732.
- Doyle BJ, Rihal CS, Gastineau DA, Holmes DR Jr. Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J Am Coll Cardiol 2009; 53:2019–2027.
- Azoulay L, Dell’Aniello S, Simon T, Renoux C, Suissa S. The concurrent use of antithrombotic therapies and the risk of bleeding in patients with atrial fibrillation. Thromb Haemost 2013; 109:431–439.
- Deshmukh A, Hilleman DE, Del Core M, Nair CK. Antithrombotic regimens in patients with indication for long-term anticoagulation undergoing coronary interventions-systematic analysis, review of literature, and implications on management. Am J Ther 2013; 20:654–663.
- Fosbol EL, Wang TY, Li S, et al. Warfarin use among older atrial fibrillation patients with non-ST-segment elevation myocardial infarction managed with coronary stenting and dual antiplatelet therapy. Am Heart J 2013; 166:864–870.
- Gao F, Zhou YJ, Wang ZJ, et al. Meta-analysis of the combination of warfarin and dual antiplatelet therapy after coronary stenting in patients with indications for chronic oral anticoagulation. Int J Cardiol 2011; 148:96–101.
- Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010; 170:1433–1441.
- Hermosillo AJ, Spinler SA. Aspirin, clopidogrel, and warfarin: is the combination appropriate and effective or inappropriate and too dangerous? Ann Pharmacother 2008; 42:790–805.
- Holmes DR Jr, Kereiakes DJ, Kleiman NS, Moliterno DJ, Patti G, Grines CL. Combining antiplatelet and anticoagulant therapies. J Am Coll Cardiol 2009; 54:95–109.
- Khurram Z, Chou E, Minutello R, et al. Combination therapy with aspirin, clopidogrel and warfarin following coronary stenting is associated with a significant risk of bleeding. J Invasive Cardiol 2006; 18:162–164.
- Orford JL, Fasseas P, Melby S, et al. Safety and efficacy of aspirin, clopidogrel, and warfarin after coronary stent placement in patients with an indication for anticoagulation. Am Heart J 2004; 147:463–467.
- Porter A, Konstantino Y, Iakobishvili Z, Shachar L, Battler A, Hasdai D. Short-term triple therapy with aspirin, warfarin, and a thienopyridine among patients undergoing percutaneous coronary intervention. Catheter Cardiovasc Interv 2006; 68:56–61.
- DeEugenio D, Kolman L, DeCaro M, et al. Risk of major bleeding with concomitant dual antiplatelet therapy after percutaneous coronary intervention in patients receiving long-term warfarin therapy. Pharmacotherapy 2007; 27:691–696.
- Ruiz-Nodar JM, Marin F, Hurtado JA, et al. Anticoagulant and antiplatelet therapy use in 426 patients with atrial fibrillation undergoing percutaneous coronary intervention and stent implantation implications for bleeding risk and prognosis. J Am Coll Cardiol 2008; 51:818–825.
- Sarafoff N, Ndrepepa G, Mehilli J, et al. Aspirin and clopidogrel with or without phenprocoumon after drug eluting coronary stent placement in patients on chronic oral anticoagulation. J Intern Med 2008; 264:472–480.
- Rossini R, Musumeci GF, Lettieri CF, et al. Long-term outcomes in patients undergoing coronary stenting on dual oral antiplatelet treatment requiring oral anticoagulant therapy. Am J Cardiol 2008; 102:1618–1623.
AGA Clinical Practice Update: Refer early Barrett’s dysplasia to a specialist
Low-grade esophageal dysplasia must be confirmed by a GI pathologist with a special interest in Barrett’s esophagus, one who deals with the problem on a daily basis and whom peers recognize as an expert in the field, according to a new expert review from the American Gastroenterological Association (Gastroenterology. doi: http://dx.doi.org/10.1053/j.gastro.2016.09.040).
In the absence of reliable biomarkers, it’s the best guarantee that low-grade dysplasia (LGD) is truly present. Overdiagnosis of early dysplasia is common, and it leads to mistreatment and uncertainty about study results, the AGA said.
In the absence of reliable biomarkers, AGA turned to expertise to combat mistreatment. Although it’s clear that high-grade disease and esophageal adenocarcinoma need intervention, LGD sometimes seems to regress on its own, but it’s unclear if it’s due to natural history or diagnosis in patients who don’t really have it.
For now, expertise is the best solution. “Unfortunately, there is no database that clearly identifies experts in the field of Barrett’s esophagus,” but most practitioners can identify people to “refer these patients to within their state or region.” Meanwhile, “we are working tirelessly” to establish a referral and outcome database. “We owe it to” patients to let them know “how good the” people we are referring them to are, Dr. Wani said.
Given the diagnosis uncertainty, AGA side-stepped the biggest controversy in LGD: whether patients should be treated or watched. Among “patients with confirmed Barrett’s esophagus with LGD by expert GI pathology review that persists on a [second] endoscopy despite intensification of acid-suppressive therapy” at 8-12 weeks with proton pump inhibitors twice a day, “risks and benefits of management options of endoscopic eradication therapy (specifically adverse events associated with endoscopic resection and ablation) and ongoing surveillance should be discussed and documented,” the group said.
When patients opt for treatment, endoscopic eradication should proceed “with the goal of achieving complete eradication of intestinal metaplasia ... radiofrequency ablation should be used,” AGA said.
Meanwhile, “patients with LGD undergoing surveillance rather than endoscopic eradication therapy should undergo surveillance every 6 months times two, then annually unless there is reversion to nondysplastic Barrett’s esophagus. Biopsies should be obtained in 4-quadrants every 1-2 cm and of any visible lesions.”
AGA funded the work. Dr. Wani is a Medtronic and Boston Scientific consultant.
Low-grade esophageal dysplasia must be confirmed by a GI pathologist with a special interest in Barrett’s esophagus, one who deals with the problem on a daily basis and whom peers recognize as an expert in the field, according to a new expert review from the American Gastroenterological Association (Gastroenterology. doi: http://dx.doi.org/10.1053/j.gastro.2016.09.040).
In the absence of reliable biomarkers, it’s the best guarantee that low-grade dysplasia (LGD) is truly present. Overdiagnosis of early dysplasia is common, and it leads to mistreatment and uncertainty about study results, the AGA said.
In the absence of reliable biomarkers, AGA turned to expertise to combat mistreatment. Although it’s clear that high-grade disease and esophageal adenocarcinoma need intervention, LGD sometimes seems to regress on its own, but it’s unclear if it’s due to natural history or diagnosis in patients who don’t really have it.
For now, expertise is the best solution. “Unfortunately, there is no database that clearly identifies experts in the field of Barrett’s esophagus,” but most practitioners can identify people to “refer these patients to within their state or region.” Meanwhile, “we are working tirelessly” to establish a referral and outcome database. “We owe it to” patients to let them know “how good the” people we are referring them to are, Dr. Wani said.
Given the diagnosis uncertainty, AGA side-stepped the biggest controversy in LGD: whether patients should be treated or watched. Among “patients with confirmed Barrett’s esophagus with LGD by expert GI pathology review that persists on a [second] endoscopy despite intensification of acid-suppressive therapy” at 8-12 weeks with proton pump inhibitors twice a day, “risks and benefits of management options of endoscopic eradication therapy (specifically adverse events associated with endoscopic resection and ablation) and ongoing surveillance should be discussed and documented,” the group said.
When patients opt for treatment, endoscopic eradication should proceed “with the goal of achieving complete eradication of intestinal metaplasia ... radiofrequency ablation should be used,” AGA said.
Meanwhile, “patients with LGD undergoing surveillance rather than endoscopic eradication therapy should undergo surveillance every 6 months times two, then annually unless there is reversion to nondysplastic Barrett’s esophagus. Biopsies should be obtained in 4-quadrants every 1-2 cm and of any visible lesions.”
AGA funded the work. Dr. Wani is a Medtronic and Boston Scientific consultant.
Low-grade esophageal dysplasia must be confirmed by a GI pathologist with a special interest in Barrett’s esophagus, one who deals with the problem on a daily basis and whom peers recognize as an expert in the field, according to a new expert review from the American Gastroenterological Association (Gastroenterology. doi: http://dx.doi.org/10.1053/j.gastro.2016.09.040).
In the absence of reliable biomarkers, it’s the best guarantee that low-grade dysplasia (LGD) is truly present. Overdiagnosis of early dysplasia is common, and it leads to mistreatment and uncertainty about study results, the AGA said.
In the absence of reliable biomarkers, AGA turned to expertise to combat mistreatment. Although it’s clear that high-grade disease and esophageal adenocarcinoma need intervention, LGD sometimes seems to regress on its own, but it’s unclear if it’s due to natural history or diagnosis in patients who don’t really have it.
For now, expertise is the best solution. “Unfortunately, there is no database that clearly identifies experts in the field of Barrett’s esophagus,” but most practitioners can identify people to “refer these patients to within their state or region.” Meanwhile, “we are working tirelessly” to establish a referral and outcome database. “We owe it to” patients to let them know “how good the” people we are referring them to are, Dr. Wani said.
Given the diagnosis uncertainty, AGA side-stepped the biggest controversy in LGD: whether patients should be treated or watched. Among “patients with confirmed Barrett’s esophagus with LGD by expert GI pathology review that persists on a [second] endoscopy despite intensification of acid-suppressive therapy” at 8-12 weeks with proton pump inhibitors twice a day, “risks and benefits of management options of endoscopic eradication therapy (specifically adverse events associated with endoscopic resection and ablation) and ongoing surveillance should be discussed and documented,” the group said.
When patients opt for treatment, endoscopic eradication should proceed “with the goal of achieving complete eradication of intestinal metaplasia ... radiofrequency ablation should be used,” AGA said.
Meanwhile, “patients with LGD undergoing surveillance rather than endoscopic eradication therapy should undergo surveillance every 6 months times two, then annually unless there is reversion to nondysplastic Barrett’s esophagus. Biopsies should be obtained in 4-quadrants every 1-2 cm and of any visible lesions.”
AGA funded the work. Dr. Wani is a Medtronic and Boston Scientific consultant.
ACP on gout: Treat to symptoms, not to urate targets
New gout treatment guidelines support the use of urate-lowering therapy, but find no place for treating patients to achieve any specific serum urate target – a dichotomy that has some rheumatologists scratching their heads.
Created by the American College of Physicians, the gout diagnosis and treatment guideline doesn’t recommend monitoring physiologic response to urate-lowering therapy or treating to specific serum urate target (Ann Int Med. 2016 Nov 1. doi: 10.7326/M16-0570). Instead, it says patients should be treated according to their symptomatic response – a recommendation that flies in the face of accepted clinical practice.
“When we step back and ask the question, ‘Has a randomized, controlled trial looked at this approach?’ The answer is simply no. And we are not willing to take that leap of faith without data.”
Reliance on lower-grade clinical evidence is simply no longer a strong-enough basis for a clinical practice recommendation, Dr. McLean said in an interview. In 2011, the Institute of Medicine raised the bar for guidelines evidence in its report, “Finding What Works in Health Care: Standards for Systematic Reviews.”
That report explicitly states that a clinical guideline cannot be driven by low-grade evidence, including meta-analysis and expert opinion, Dr. McLean said. The Agency for Healthcare Research and Quality National Guideline Clearinghouse incorporated this into its 2013 revision of guidelines acceptance policy: A review must be based on the highest level of evidence – randomized, controlled studies – and not be driven by expert opinion or review articles. Additionally, the literature reviews upon which guidelines are based must be published in a peer-reviewed journal. Guidelines that don’t meet these criteria will no longer be accepted into the clearinghouse.
“The 2012 ACR guidelines didn’t meet that criteria,” he said. “The authors clearly point out in their methodology section where the evidence is weak and admit that 80% of it is low grade. How can you make a guideline that is 80% based on weak evidence? The ACP doesn’t allow us to do that.”
The argument about whether or not to treat to a specific urate target is not simply philosophical, said Dr. McLean, who authored an accompanying editorial (Ann Int Med. 2016 Nov 1. doi: 10.7326/M16-2401). In it, he argued that treating to a prespecified target would certainly help some patients but would probably hurt others.
“Treating to a target necessarily means increasing doses of medication in patients who may be asymptomatic,” he wrote. “Examples exist from other studies using intermediary biomarkers (such as elevated blood pressure or blood glucose level or low hemoglobin level), in which treating to a target resulted in more adverse effects than benefits. Thus, despite the strong biologic appeal of such a strategy and its advocacy by major specialty society guidelines, we judged the strength of evidence for monitoring to be low.”
“This paradigm also has not been formally tested in a randomized clinical trial, and there’s no scientific evidence to support that strategy and a lot of evidence to show its harm,” she said in an interview. “We have a large clinical experience about the ineffectiveness of that strategy. Patients eventually develop tophi, joint damage, and functional limitations when their physicians only treat their gout flares using anti-inflammatory therapy without addressing their underlying cause of gout – high uric acid. They are often dismayed and upset when they realize their physician had let their gout get to that point by just treating their gout flares.”
In other important ways, the two documents are complementary. Both put NSAIDs, corticosteroids, and colchicine at the heart of treating acute gout attacks.
According to the ACP guideline, these treatment strategies are all supported by high-level evidence, which was drawn from a review of 28 studies.
• Corticosteroids, NSAIDs, and colchicine are effective treatments to reduce pain in patients with acute gout.
• Lower doses of colchicine (1.2 mg, followed by 0.6 mg 1 hour later) are as effective as higher doses (1.2 mg, followed by 0.6 mg/hour for 6 hours) at reducing pain and are associated with fewer gastrointestinal adverse effects.
• Do not initiate long-term uric acid–lowering therapy in most patients after a first gout attack or in patients with infrequent attacks.
• Although evidence supports the benefits of using uric acid–lowering therapy for shorter durations to reduce gout flares, the benefits of long-term use in patients with a single or infrequent gout attacks have not been studied.
• Clinicians should discuss benefits, harms, costs, and individual preferences with patients before initiating uric acid–lowering therapy, including concomitant prophylaxis, in patients with recurrent gout attacks. (Grade: strong recommendation, moderate-quality evidence).
• Febuxostat (40 mg/day) and allopurinol (300 mg/day) are equally effective at decreasing serum urate levels, and prophylactic therapy with low-dose colchicine or low-dose NSAIDs reduces the risk for acute gout attacks in patients initiating urate-lowering therapy.
Allopurinol dosage, however, is one key point upon which the ACP and ACR guidelines are sharply divided. The ACR guideline calls for starting at low doses – 100 mg/day, and even lower in patients with kidney disease – with very slow upward titration, only if necessary, to 300 mg/day or more. Allopurinol is not without risk and should be used with caution, Dr. Neogi wrote in her commentary.
“Continuing a management strategy of starting allopurinol at an inappropriately high dose will perpetuate the problem of unnecessarily increasing flare risk, because this risk in the early treatment phase is directly proportional to the potency of the urate-lowering therapy used.”
However, allopurinol doses of 300 mg/day or less leave more than half of all patients undertreated, she said. This dilemma points up the difficulty with symptomatic treatment. Without checking urate levels, clinicians may be shooting blind when trying to dose appropriately.
She refuted any suggestion that the ACR guidelines were based on a lesser degree of evidence than the ACP document.
“The ACR uses a rigorous evidence-based method to develop treatment guidelines. It is inaccurate to say that ACR relies on lower-class evidence or expert opinion ‘to a large extent.’ In the absence of randomized controlled data, other peer-reviewed, available published data are used to formulate guidance, supported by the known biology.”
Current practices outlined in the ACR document are based on a thorough understanding of the biology of gout and how it progresses. Hard data on uric acid targets would greatly impact current therapeutic thinking. But in the meantime, there are patients to be treated, Dr. Neogi said.
“Of course, it would be ideal if we had randomized trial data to definitively provide insights, but we don’t have that right now. So does that mean that patients should suffer unnecessarily because we can’t use the remaining existing body of scientific knowledge to guide rational treatment decisions? What if funding agencies never fund such a study – should gout patients remain poorly managed? Should we abandon all scientific knowledge that isn’t randomized trial data and just not treat at all while waiting to see if a randomized trial will ever be funded and conducted?”
Dr. McLean sees the flip side of that coin.
“I think the effect of our guideline will be to help push the need for more explicit evidence in treat to target being the right way to go. But right now there is not enough evidence now to endorse that approach.”
Dr. McLean reports personal fees from Takeda Pharmaceuticals speakers’ bureau before 2015. Dr. Neogi had no financial disclosures.
New gout treatment guidelines support the use of urate-lowering therapy, but find no place for treating patients to achieve any specific serum urate target – a dichotomy that has some rheumatologists scratching their heads.
Created by the American College of Physicians, the gout diagnosis and treatment guideline doesn’t recommend monitoring physiologic response to urate-lowering therapy or treating to specific serum urate target (Ann Int Med. 2016 Nov 1. doi: 10.7326/M16-0570). Instead, it says patients should be treated according to their symptomatic response – a recommendation that flies in the face of accepted clinical practice.
“When we step back and ask the question, ‘Has a randomized, controlled trial looked at this approach?’ The answer is simply no. And we are not willing to take that leap of faith without data.”
Reliance on lower-grade clinical evidence is simply no longer a strong-enough basis for a clinical practice recommendation, Dr. McLean said in an interview. In 2011, the Institute of Medicine raised the bar for guidelines evidence in its report, “Finding What Works in Health Care: Standards for Systematic Reviews.”
That report explicitly states that a clinical guideline cannot be driven by low-grade evidence, including meta-analysis and expert opinion, Dr. McLean said. The Agency for Healthcare Research and Quality National Guideline Clearinghouse incorporated this into its 2013 revision of guidelines acceptance policy: A review must be based on the highest level of evidence – randomized, controlled studies – and not be driven by expert opinion or review articles. Additionally, the literature reviews upon which guidelines are based must be published in a peer-reviewed journal. Guidelines that don’t meet these criteria will no longer be accepted into the clearinghouse.
“The 2012 ACR guidelines didn’t meet that criteria,” he said. “The authors clearly point out in their methodology section where the evidence is weak and admit that 80% of it is low grade. How can you make a guideline that is 80% based on weak evidence? The ACP doesn’t allow us to do that.”
The argument about whether or not to treat to a specific urate target is not simply philosophical, said Dr. McLean, who authored an accompanying editorial (Ann Int Med. 2016 Nov 1. doi: 10.7326/M16-2401). In it, he argued that treating to a prespecified target would certainly help some patients but would probably hurt others.
“Treating to a target necessarily means increasing doses of medication in patients who may be asymptomatic,” he wrote. “Examples exist from other studies using intermediary biomarkers (such as elevated blood pressure or blood glucose level or low hemoglobin level), in which treating to a target resulted in more adverse effects than benefits. Thus, despite the strong biologic appeal of such a strategy and its advocacy by major specialty society guidelines, we judged the strength of evidence for monitoring to be low.”
“This paradigm also has not been formally tested in a randomized clinical trial, and there’s no scientific evidence to support that strategy and a lot of evidence to show its harm,” she said in an interview. “We have a large clinical experience about the ineffectiveness of that strategy. Patients eventually develop tophi, joint damage, and functional limitations when their physicians only treat their gout flares using anti-inflammatory therapy without addressing their underlying cause of gout – high uric acid. They are often dismayed and upset when they realize their physician had let their gout get to that point by just treating their gout flares.”
In other important ways, the two documents are complementary. Both put NSAIDs, corticosteroids, and colchicine at the heart of treating acute gout attacks.
According to the ACP guideline, these treatment strategies are all supported by high-level evidence, which was drawn from a review of 28 studies.
• Corticosteroids, NSAIDs, and colchicine are effective treatments to reduce pain in patients with acute gout.
• Lower doses of colchicine (1.2 mg, followed by 0.6 mg 1 hour later) are as effective as higher doses (1.2 mg, followed by 0.6 mg/hour for 6 hours) at reducing pain and are associated with fewer gastrointestinal adverse effects.
• Do not initiate long-term uric acid–lowering therapy in most patients after a first gout attack or in patients with infrequent attacks.
• Although evidence supports the benefits of using uric acid–lowering therapy for shorter durations to reduce gout flares, the benefits of long-term use in patients with a single or infrequent gout attacks have not been studied.
• Clinicians should discuss benefits, harms, costs, and individual preferences with patients before initiating uric acid–lowering therapy, including concomitant prophylaxis, in patients with recurrent gout attacks. (Grade: strong recommendation, moderate-quality evidence).
• Febuxostat (40 mg/day) and allopurinol (300 mg/day) are equally effective at decreasing serum urate levels, and prophylactic therapy with low-dose colchicine or low-dose NSAIDs reduces the risk for acute gout attacks in patients initiating urate-lowering therapy.
Allopurinol dosage, however, is one key point upon which the ACP and ACR guidelines are sharply divided. The ACR guideline calls for starting at low doses – 100 mg/day, and even lower in patients with kidney disease – with very slow upward titration, only if necessary, to 300 mg/day or more. Allopurinol is not without risk and should be used with caution, Dr. Neogi wrote in her commentary.
“Continuing a management strategy of starting allopurinol at an inappropriately high dose will perpetuate the problem of unnecessarily increasing flare risk, because this risk in the early treatment phase is directly proportional to the potency of the urate-lowering therapy used.”
However, allopurinol doses of 300 mg/day or less leave more than half of all patients undertreated, she said. This dilemma points up the difficulty with symptomatic treatment. Without checking urate levels, clinicians may be shooting blind when trying to dose appropriately.
She refuted any suggestion that the ACR guidelines were based on a lesser degree of evidence than the ACP document.
“The ACR uses a rigorous evidence-based method to develop treatment guidelines. It is inaccurate to say that ACR relies on lower-class evidence or expert opinion ‘to a large extent.’ In the absence of randomized controlled data, other peer-reviewed, available published data are used to formulate guidance, supported by the known biology.”
Current practices outlined in the ACR document are based on a thorough understanding of the biology of gout and how it progresses. Hard data on uric acid targets would greatly impact current therapeutic thinking. But in the meantime, there are patients to be treated, Dr. Neogi said.
“Of course, it would be ideal if we had randomized trial data to definitively provide insights, but we don’t have that right now. So does that mean that patients should suffer unnecessarily because we can’t use the remaining existing body of scientific knowledge to guide rational treatment decisions? What if funding agencies never fund such a study – should gout patients remain poorly managed? Should we abandon all scientific knowledge that isn’t randomized trial data and just not treat at all while waiting to see if a randomized trial will ever be funded and conducted?”
Dr. McLean sees the flip side of that coin.
“I think the effect of our guideline will be to help push the need for more explicit evidence in treat to target being the right way to go. But right now there is not enough evidence now to endorse that approach.”
Dr. McLean reports personal fees from Takeda Pharmaceuticals speakers’ bureau before 2015. Dr. Neogi had no financial disclosures.
New gout treatment guidelines support the use of urate-lowering therapy, but find no place for treating patients to achieve any specific serum urate target – a dichotomy that has some rheumatologists scratching their heads.
Created by the American College of Physicians, the gout diagnosis and treatment guideline doesn’t recommend monitoring physiologic response to urate-lowering therapy or treating to specific serum urate target (Ann Int Med. 2016 Nov 1. doi: 10.7326/M16-0570). Instead, it says patients should be treated according to their symptomatic response – a recommendation that flies in the face of accepted clinical practice.
“When we step back and ask the question, ‘Has a randomized, controlled trial looked at this approach?’ The answer is simply no. And we are not willing to take that leap of faith without data.”
Reliance on lower-grade clinical evidence is simply no longer a strong-enough basis for a clinical practice recommendation, Dr. McLean said in an interview. In 2011, the Institute of Medicine raised the bar for guidelines evidence in its report, “Finding What Works in Health Care: Standards for Systematic Reviews.”
That report explicitly states that a clinical guideline cannot be driven by low-grade evidence, including meta-analysis and expert opinion, Dr. McLean said. The Agency for Healthcare Research and Quality National Guideline Clearinghouse incorporated this into its 2013 revision of guidelines acceptance policy: A review must be based on the highest level of evidence – randomized, controlled studies – and not be driven by expert opinion or review articles. Additionally, the literature reviews upon which guidelines are based must be published in a peer-reviewed journal. Guidelines that don’t meet these criteria will no longer be accepted into the clearinghouse.
“The 2012 ACR guidelines didn’t meet that criteria,” he said. “The authors clearly point out in their methodology section where the evidence is weak and admit that 80% of it is low grade. How can you make a guideline that is 80% based on weak evidence? The ACP doesn’t allow us to do that.”
The argument about whether or not to treat to a specific urate target is not simply philosophical, said Dr. McLean, who authored an accompanying editorial (Ann Int Med. 2016 Nov 1. doi: 10.7326/M16-2401). In it, he argued that treating to a prespecified target would certainly help some patients but would probably hurt others.
“Treating to a target necessarily means increasing doses of medication in patients who may be asymptomatic,” he wrote. “Examples exist from other studies using intermediary biomarkers (such as elevated blood pressure or blood glucose level or low hemoglobin level), in which treating to a target resulted in more adverse effects than benefits. Thus, despite the strong biologic appeal of such a strategy and its advocacy by major specialty society guidelines, we judged the strength of evidence for monitoring to be low.”
“This paradigm also has not been formally tested in a randomized clinical trial, and there’s no scientific evidence to support that strategy and a lot of evidence to show its harm,” she said in an interview. “We have a large clinical experience about the ineffectiveness of that strategy. Patients eventually develop tophi, joint damage, and functional limitations when their physicians only treat their gout flares using anti-inflammatory therapy without addressing their underlying cause of gout – high uric acid. They are often dismayed and upset when they realize their physician had let their gout get to that point by just treating their gout flares.”
In other important ways, the two documents are complementary. Both put NSAIDs, corticosteroids, and colchicine at the heart of treating acute gout attacks.
According to the ACP guideline, these treatment strategies are all supported by high-level evidence, which was drawn from a review of 28 studies.
• Corticosteroids, NSAIDs, and colchicine are effective treatments to reduce pain in patients with acute gout.
• Lower doses of colchicine (1.2 mg, followed by 0.6 mg 1 hour later) are as effective as higher doses (1.2 mg, followed by 0.6 mg/hour for 6 hours) at reducing pain and are associated with fewer gastrointestinal adverse effects.
• Do not initiate long-term uric acid–lowering therapy in most patients after a first gout attack or in patients with infrequent attacks.
• Although evidence supports the benefits of using uric acid–lowering therapy for shorter durations to reduce gout flares, the benefits of long-term use in patients with a single or infrequent gout attacks have not been studied.
• Clinicians should discuss benefits, harms, costs, and individual preferences with patients before initiating uric acid–lowering therapy, including concomitant prophylaxis, in patients with recurrent gout attacks. (Grade: strong recommendation, moderate-quality evidence).
• Febuxostat (40 mg/day) and allopurinol (300 mg/day) are equally effective at decreasing serum urate levels, and prophylactic therapy with low-dose colchicine or low-dose NSAIDs reduces the risk for acute gout attacks in patients initiating urate-lowering therapy.
Allopurinol dosage, however, is one key point upon which the ACP and ACR guidelines are sharply divided. The ACR guideline calls for starting at low doses – 100 mg/day, and even lower in patients with kidney disease – with very slow upward titration, only if necessary, to 300 mg/day or more. Allopurinol is not without risk and should be used with caution, Dr. Neogi wrote in her commentary.
“Continuing a management strategy of starting allopurinol at an inappropriately high dose will perpetuate the problem of unnecessarily increasing flare risk, because this risk in the early treatment phase is directly proportional to the potency of the urate-lowering therapy used.”
However, allopurinol doses of 300 mg/day or less leave more than half of all patients undertreated, she said. This dilemma points up the difficulty with symptomatic treatment. Without checking urate levels, clinicians may be shooting blind when trying to dose appropriately.
She refuted any suggestion that the ACR guidelines were based on a lesser degree of evidence than the ACP document.
“The ACR uses a rigorous evidence-based method to develop treatment guidelines. It is inaccurate to say that ACR relies on lower-class evidence or expert opinion ‘to a large extent.’ In the absence of randomized controlled data, other peer-reviewed, available published data are used to formulate guidance, supported by the known biology.”
Current practices outlined in the ACR document are based on a thorough understanding of the biology of gout and how it progresses. Hard data on uric acid targets would greatly impact current therapeutic thinking. But in the meantime, there are patients to be treated, Dr. Neogi said.
“Of course, it would be ideal if we had randomized trial data to definitively provide insights, but we don’t have that right now. So does that mean that patients should suffer unnecessarily because we can’t use the remaining existing body of scientific knowledge to guide rational treatment decisions? What if funding agencies never fund such a study – should gout patients remain poorly managed? Should we abandon all scientific knowledge that isn’t randomized trial data and just not treat at all while waiting to see if a randomized trial will ever be funded and conducted?”
Dr. McLean sees the flip side of that coin.
“I think the effect of our guideline will be to help push the need for more explicit evidence in treat to target being the right way to go. But right now there is not enough evidence now to endorse that approach.”
Dr. McLean reports personal fees from Takeda Pharmaceuticals speakers’ bureau before 2015. Dr. Neogi had no financial disclosures.
Selected liver-transplant patients thrive off immunosuppression
MONTREAL – Three-fifths of pediatric liver-transplant recipients who were doing well enough to attempt weaning from their immunosuppression regimen succeeded in getting off immunosuppression and staying off for more than a year. In the process, they also significantly improved their health-related quality of life.
“Health-related quality of life domains associated with social interactions, worry, and medications improved” in pediatric liver recipients who had undergone immunosuppression withdrawal, Saeed Mohammad, MD, said at the World Congress of Pediatric Gastroenterology, Hepatology and Nutrition.
Patients who succeeded in staying off immunosuppressant drugs for at least 2 years after they first began ratcheting down their regimen showed better quality of life scores compared with their scores at baseline, and also compared with the scores of other pediatric liver transplant patients who unsuccessfully tried coming off immunosuppression.
Not every pediatric liver transplant patient should attempt withdrawing immunosuppression, cautioned Dr. Mohammad, a pediatric gastroenterologist at Northwestern University in Chicago. “To be successful withdrawal of immunosuppression needs to be in selected patients; not every patient is a good candidate.”
The Immunosuppression Withdrawal for Stable Pediatric Liver Transplant Recipients (iWITH) study ran at 11 U.S. center and one center in Toronto during October 2012 through June 2014. Pediatric liver transplant recipients were eligible to start a 9-10 month graduated withdrawal from their immunosuppression regimen if they met several criteria of stability including no rejection episode over at least the prior 12 months, normal laboratory-test results, no autoimmune disease and no problems detected in a liver biopsy. The prospective study enrolled 88 patients who averaged 10 years old. Patients underwent comprehensive examinations and laboratory testing at baseline and again several times during the subsequent 2 years including assessment of several quality of life measures.
During follow-up, 35 of the 88 patients (40%) developed symptoms of rejection and had to go back on immunosuppression. Most of these patients developed their rejection symptoms early during immunosuppression weaning, but a few patients failed later including one patient who failed 22 months after starting immunosuppression withdrawal, Dr. Mohammad said. Researchers from the iWITH study first reported these results at the American Transplant Congress in June 2016.
The quality of life findings reported by Dr. Mohammad came from assessments at baseline, after 12 months, and after 24 months, and included 30 of the patients who resumed immunosuppression and 48 patients who remained off immunosuppression for 2 years. All of these 78 patients had relatively robust quality of life profiles at baseline. Their scores for both physical and social subscales as well as for total score were significantly superior to the average scores for a large number of primarily U.S. pediatric liver transplant patients in the SPLIT database. Dr. Mohammad called the patients who attempted immunosuppression discontinuation as the “creme de la creme” of pediatric liver transplant patients in terms of their clinical status.
Analysis of scores after 2 years compared with baseline showed statistically significant improvements among patients who stayed off immunosuppression for the domains of social function, treatment attitudes and compliance, communication, and worry. A comparison of changes in quality of life scores from baseline to 2 years showed that patients who stayed off immunosuppression had improvements in several of their scores while patients who went back onto immunosuppression had on average a small deterioration of their scores.
Dr. Mohammad had no disclosures.
[email protected]
On Twitter @mitchelzoler
MONTREAL – Three-fifths of pediatric liver-transplant recipients who were doing well enough to attempt weaning from their immunosuppression regimen succeeded in getting off immunosuppression and staying off for more than a year. In the process, they also significantly improved their health-related quality of life.
“Health-related quality of life domains associated with social interactions, worry, and medications improved” in pediatric liver recipients who had undergone immunosuppression withdrawal, Saeed Mohammad, MD, said at the World Congress of Pediatric Gastroenterology, Hepatology and Nutrition.
Patients who succeeded in staying off immunosuppressant drugs for at least 2 years after they first began ratcheting down their regimen showed better quality of life scores compared with their scores at baseline, and also compared with the scores of other pediatric liver transplant patients who unsuccessfully tried coming off immunosuppression.
Not every pediatric liver transplant patient should attempt withdrawing immunosuppression, cautioned Dr. Mohammad, a pediatric gastroenterologist at Northwestern University in Chicago. “To be successful withdrawal of immunosuppression needs to be in selected patients; not every patient is a good candidate.”
The Immunosuppression Withdrawal for Stable Pediatric Liver Transplant Recipients (iWITH) study ran at 11 U.S. center and one center in Toronto during October 2012 through June 2014. Pediatric liver transplant recipients were eligible to start a 9-10 month graduated withdrawal from their immunosuppression regimen if they met several criteria of stability including no rejection episode over at least the prior 12 months, normal laboratory-test results, no autoimmune disease and no problems detected in a liver biopsy. The prospective study enrolled 88 patients who averaged 10 years old. Patients underwent comprehensive examinations and laboratory testing at baseline and again several times during the subsequent 2 years including assessment of several quality of life measures.
During follow-up, 35 of the 88 patients (40%) developed symptoms of rejection and had to go back on immunosuppression. Most of these patients developed their rejection symptoms early during immunosuppression weaning, but a few patients failed later including one patient who failed 22 months after starting immunosuppression withdrawal, Dr. Mohammad said. Researchers from the iWITH study first reported these results at the American Transplant Congress in June 2016.
The quality of life findings reported by Dr. Mohammad came from assessments at baseline, after 12 months, and after 24 months, and included 30 of the patients who resumed immunosuppression and 48 patients who remained off immunosuppression for 2 years. All of these 78 patients had relatively robust quality of life profiles at baseline. Their scores for both physical and social subscales as well as for total score were significantly superior to the average scores for a large number of primarily U.S. pediatric liver transplant patients in the SPLIT database. Dr. Mohammad called the patients who attempted immunosuppression discontinuation as the “creme de la creme” of pediatric liver transplant patients in terms of their clinical status.
Analysis of scores after 2 years compared with baseline showed statistically significant improvements among patients who stayed off immunosuppression for the domains of social function, treatment attitudes and compliance, communication, and worry. A comparison of changes in quality of life scores from baseline to 2 years showed that patients who stayed off immunosuppression had improvements in several of their scores while patients who went back onto immunosuppression had on average a small deterioration of their scores.
Dr. Mohammad had no disclosures.
[email protected]
On Twitter @mitchelzoler
MONTREAL – Three-fifths of pediatric liver-transplant recipients who were doing well enough to attempt weaning from their immunosuppression regimen succeeded in getting off immunosuppression and staying off for more than a year. In the process, they also significantly improved their health-related quality of life.
“Health-related quality of life domains associated with social interactions, worry, and medications improved” in pediatric liver recipients who had undergone immunosuppression withdrawal, Saeed Mohammad, MD, said at the World Congress of Pediatric Gastroenterology, Hepatology and Nutrition.
Patients who succeeded in staying off immunosuppressant drugs for at least 2 years after they first began ratcheting down their regimen showed better quality of life scores compared with their scores at baseline, and also compared with the scores of other pediatric liver transplant patients who unsuccessfully tried coming off immunosuppression.
Not every pediatric liver transplant patient should attempt withdrawing immunosuppression, cautioned Dr. Mohammad, a pediatric gastroenterologist at Northwestern University in Chicago. “To be successful withdrawal of immunosuppression needs to be in selected patients; not every patient is a good candidate.”
The Immunosuppression Withdrawal for Stable Pediatric Liver Transplant Recipients (iWITH) study ran at 11 U.S. center and one center in Toronto during October 2012 through June 2014. Pediatric liver transplant recipients were eligible to start a 9-10 month graduated withdrawal from their immunosuppression regimen if they met several criteria of stability including no rejection episode over at least the prior 12 months, normal laboratory-test results, no autoimmune disease and no problems detected in a liver biopsy. The prospective study enrolled 88 patients who averaged 10 years old. Patients underwent comprehensive examinations and laboratory testing at baseline and again several times during the subsequent 2 years including assessment of several quality of life measures.
During follow-up, 35 of the 88 patients (40%) developed symptoms of rejection and had to go back on immunosuppression. Most of these patients developed their rejection symptoms early during immunosuppression weaning, but a few patients failed later including one patient who failed 22 months after starting immunosuppression withdrawal, Dr. Mohammad said. Researchers from the iWITH study first reported these results at the American Transplant Congress in June 2016.
The quality of life findings reported by Dr. Mohammad came from assessments at baseline, after 12 months, and after 24 months, and included 30 of the patients who resumed immunosuppression and 48 patients who remained off immunosuppression for 2 years. All of these 78 patients had relatively robust quality of life profiles at baseline. Their scores for both physical and social subscales as well as for total score were significantly superior to the average scores for a large number of primarily U.S. pediatric liver transplant patients in the SPLIT database. Dr. Mohammad called the patients who attempted immunosuppression discontinuation as the “creme de la creme” of pediatric liver transplant patients in terms of their clinical status.
Analysis of scores after 2 years compared with baseline showed statistically significant improvements among patients who stayed off immunosuppression for the domains of social function, treatment attitudes and compliance, communication, and worry. A comparison of changes in quality of life scores from baseline to 2 years showed that patients who stayed off immunosuppression had improvements in several of their scores while patients who went back onto immunosuppression had on average a small deterioration of their scores.
Dr. Mohammad had no disclosures.
[email protected]
On Twitter @mitchelzoler
AT WCPGHAN 2016
Key clinical point: Selected pediatric liver-transplant patients who successfully weaned off immunosuppression responded with significantly improved quality of life scores.
Major finding: Patient and parent treatment satisfaction improved by 6-7 points when patients stopped immunosuppression and fell by 2-3 points when they did not.
Data source: iWISH, a multicenter study with 88 enrolled patients.
Disclosures: Dr. Mohammad had no disclosures.
Resorbable scaffold appears safe, effective in diabetes patients
An everolimus-eluting resorbable scaffold appeared to be safe and effective for percutaneous coronary intervention (PCI) in patients with diabetes and noncomplex coronary lesions, according to a study presented at the Transcatheter Cardiovascular Therapeutics annual meeting and published simultaneously in the Journal of the American College of Cardiology: Cardiovascular Interventions.
Patients with diabetes constitute an important and increasingly prevalent subgroup of PCI patients, who are at high risk of adverse clinical and angiographic outcomes such as MI, stent thrombosis, restenosis, and death. This is thought to be due to diabetic patients’ greater level of vascular inflammation and tendency toward a prothrombotic state and more complex angiographic features, said Dean J. Kereiakes, MD, of the Christ Hospital Heart and Vascular Center, Lindner Research Center, Cincinnati.
The substudy participants all received at least one resorbable scaffold in at least one target lesion. A total of 27.3% were insulin dependent and nearly 60% had HbA1c levels exceeding 7.0%. Notably, 18% of all the treated lesions in this analysis were less than 2.25 mm in diameter as assessed by quantitative coronary angiography, and approximately 60% had moderately to severely complex morphology.
The primary endpoint – the rate of target-lesion failure at 1-year follow-up – was 8.3%, which was well below the prespecified performance goal of 12.7%. This rate ranged from 4.4% to 10.9% across the different trials. A sensitivity analysis confirmed that the 1-year rate of target-lesion failure was significantly lower than the prespecified performance goal.
The rates of target-lesion failure, target-vessel MI, ischemia-driven target-lesion revascularization, and scaffold thrombosis were significantly higher in diabetic patients who required insulin than in those who did not. Older patient age, insulin dependency, and small target-vessel diameter all were independent predictors of target-lesion failure at 1 year.
The overall 1-year rate of scaffold thrombosis in this study was 2.3%, which is not surprising given the study population’s risk factors. For diabetic patients with appropriately sized vessels of greater than 2.25 mm diameter, the scaffold thrombosis rate was lower (1.3%).
In addition to being underpowered to assess rare adverse events, this study was limited in that it reported outcomes at 1 year, before resorption of the device was complete. It also reflects the first-time clinical experience with a resorbable scaffold for most of the participating investigators, “and one would expect that as with all new medical procedures, results will improve over time with increased operator experience,” the coauthors wrote.
Dr. Kereiakes reported being a consultant to Abbott Vascular, and his associates also reported ties to the company and to other industry sources.
An everolimus-eluting resorbable scaffold appeared to be safe and effective for percutaneous coronary intervention (PCI) in patients with diabetes and noncomplex coronary lesions, according to a study presented at the Transcatheter Cardiovascular Therapeutics annual meeting and published simultaneously in the Journal of the American College of Cardiology: Cardiovascular Interventions.
Patients with diabetes constitute an important and increasingly prevalent subgroup of PCI patients, who are at high risk of adverse clinical and angiographic outcomes such as MI, stent thrombosis, restenosis, and death. This is thought to be due to diabetic patients’ greater level of vascular inflammation and tendency toward a prothrombotic state and more complex angiographic features, said Dean J. Kereiakes, MD, of the Christ Hospital Heart and Vascular Center, Lindner Research Center, Cincinnati.
The substudy participants all received at least one resorbable scaffold in at least one target lesion. A total of 27.3% were insulin dependent and nearly 60% had HbA1c levels exceeding 7.0%. Notably, 18% of all the treated lesions in this analysis were less than 2.25 mm in diameter as assessed by quantitative coronary angiography, and approximately 60% had moderately to severely complex morphology.
The primary endpoint – the rate of target-lesion failure at 1-year follow-up – was 8.3%, which was well below the prespecified performance goal of 12.7%. This rate ranged from 4.4% to 10.9% across the different trials. A sensitivity analysis confirmed that the 1-year rate of target-lesion failure was significantly lower than the prespecified performance goal.
The rates of target-lesion failure, target-vessel MI, ischemia-driven target-lesion revascularization, and scaffold thrombosis were significantly higher in diabetic patients who required insulin than in those who did not. Older patient age, insulin dependency, and small target-vessel diameter all were independent predictors of target-lesion failure at 1 year.
The overall 1-year rate of scaffold thrombosis in this study was 2.3%, which is not surprising given the study population’s risk factors. For diabetic patients with appropriately sized vessels of greater than 2.25 mm diameter, the scaffold thrombosis rate was lower (1.3%).
In addition to being underpowered to assess rare adverse events, this study was limited in that it reported outcomes at 1 year, before resorption of the device was complete. It also reflects the first-time clinical experience with a resorbable scaffold for most of the participating investigators, “and one would expect that as with all new medical procedures, results will improve over time with increased operator experience,” the coauthors wrote.
Dr. Kereiakes reported being a consultant to Abbott Vascular, and his associates also reported ties to the company and to other industry sources.
An everolimus-eluting resorbable scaffold appeared to be safe and effective for percutaneous coronary intervention (PCI) in patients with diabetes and noncomplex coronary lesions, according to a study presented at the Transcatheter Cardiovascular Therapeutics annual meeting and published simultaneously in the Journal of the American College of Cardiology: Cardiovascular Interventions.
Patients with diabetes constitute an important and increasingly prevalent subgroup of PCI patients, who are at high risk of adverse clinical and angiographic outcomes such as MI, stent thrombosis, restenosis, and death. This is thought to be due to diabetic patients’ greater level of vascular inflammation and tendency toward a prothrombotic state and more complex angiographic features, said Dean J. Kereiakes, MD, of the Christ Hospital Heart and Vascular Center, Lindner Research Center, Cincinnati.
The substudy participants all received at least one resorbable scaffold in at least one target lesion. A total of 27.3% were insulin dependent and nearly 60% had HbA1c levels exceeding 7.0%. Notably, 18% of all the treated lesions in this analysis were less than 2.25 mm in diameter as assessed by quantitative coronary angiography, and approximately 60% had moderately to severely complex morphology.
The primary endpoint – the rate of target-lesion failure at 1-year follow-up – was 8.3%, which was well below the prespecified performance goal of 12.7%. This rate ranged from 4.4% to 10.9% across the different trials. A sensitivity analysis confirmed that the 1-year rate of target-lesion failure was significantly lower than the prespecified performance goal.
The rates of target-lesion failure, target-vessel MI, ischemia-driven target-lesion revascularization, and scaffold thrombosis were significantly higher in diabetic patients who required insulin than in those who did not. Older patient age, insulin dependency, and small target-vessel diameter all were independent predictors of target-lesion failure at 1 year.
The overall 1-year rate of scaffold thrombosis in this study was 2.3%, which is not surprising given the study population’s risk factors. For diabetic patients with appropriately sized vessels of greater than 2.25 mm diameter, the scaffold thrombosis rate was lower (1.3%).
In addition to being underpowered to assess rare adverse events, this study was limited in that it reported outcomes at 1 year, before resorption of the device was complete. It also reflects the first-time clinical experience with a resorbable scaffold for most of the participating investigators, “and one would expect that as with all new medical procedures, results will improve over time with increased operator experience,” the coauthors wrote.
Dr. Kereiakes reported being a consultant to Abbott Vascular, and his associates also reported ties to the company and to other industry sources.
Key clinical point:
Major finding: The primary endpoint – the rate of target-lesion failure at 1 year follow-up – was 8.3%, which was well below the prespecified performance goal of 12.7%.
Data source: A prespecified formal substudy of 754 patients with diabetes who participated in three clinical trials and one device registry, assessing 1-year outcomes after PCI.
Disclosures: This pooled analysis, plus all the contributing trials and the device registry, were funded by Abbott Vascular, maker of the resorbable scaffold. Dr. Kereiakes reported being a consultant to Abbott Vascular, and his associates also reported ties to the company and to other industry sources.