User login
Menopausal hormone therapy
To the Editor: I much enjoyed the important article by Drs. Lipold, Batur, and Kagan on whether there is a time limit for systemic menopausal hormone therapy.1 The simple answer is no. The authors did a good job of reviewing the factors to consider in terms of contraindications and precautions when prescribing menopausal hormone therapy.
An important part of the discussion regarding stopping hormone therapy is the recent evidence from Finland that has shown increased risks of myocardial infarction and stroke, especially in women under age 60, when taken off hormone therapy.2 This fact is quite ironic, as many clinicians are trying to rush to get women off hormone therapy in order to protect the heart, when the evidence does not suggest this. Just as with other hormone-deficiency conditions, the status needs to be periodically reviewed, and doses may need to be adjusted. However, after age 60 or 65, women do not automatically start producing the sex hormone that they have been deficient in. While menopause is not a definite endocrinopathy, it is a potential endocrinopathy; and for some women, such as young women who are oophorectomized, it is an absolute endocrinopathy.
The International Menopause Society has published updated guidelines emphasizing that new data and reanalysis of older data show that for most women the benefits of menopausal hormone therapy are much greater than the risks, particularly when started within a few years of menopause.3
- Lipold LD, Batur P, Kagan R. Is there a time limit for systemic menopausal hormone therapy? Cleve Clin J Med 2016; 83:605–612.
- Mikkola TS, Tuomikoski P, Lyytinen H, et al. Increased cardiovascular mortality risk in women discontinuing postmenopausal hormone therapy. J Clin Endocrinol Metab 2015; 100:4588–4594.
- Baber RJ, Panay N, Fenton A; IMS Writing Group. 2016 IMS recommendations on women’s midlife health and menopause hormone therapy. Climacteric 2016; 19:109–150.
To the Editor: I much enjoyed the important article by Drs. Lipold, Batur, and Kagan on whether there is a time limit for systemic menopausal hormone therapy.1 The simple answer is no. The authors did a good job of reviewing the factors to consider in terms of contraindications and precautions when prescribing menopausal hormone therapy.
An important part of the discussion regarding stopping hormone therapy is the recent evidence from Finland that has shown increased risks of myocardial infarction and stroke, especially in women under age 60, when taken off hormone therapy.2 This fact is quite ironic, as many clinicians are trying to rush to get women off hormone therapy in order to protect the heart, when the evidence does not suggest this. Just as with other hormone-deficiency conditions, the status needs to be periodically reviewed, and doses may need to be adjusted. However, after age 60 or 65, women do not automatically start producing the sex hormone that they have been deficient in. While menopause is not a definite endocrinopathy, it is a potential endocrinopathy; and for some women, such as young women who are oophorectomized, it is an absolute endocrinopathy.
The International Menopause Society has published updated guidelines emphasizing that new data and reanalysis of older data show that for most women the benefits of menopausal hormone therapy are much greater than the risks, particularly when started within a few years of menopause.3
To the Editor: I much enjoyed the important article by Drs. Lipold, Batur, and Kagan on whether there is a time limit for systemic menopausal hormone therapy.1 The simple answer is no. The authors did a good job of reviewing the factors to consider in terms of contraindications and precautions when prescribing menopausal hormone therapy.
An important part of the discussion regarding stopping hormone therapy is the recent evidence from Finland that has shown increased risks of myocardial infarction and stroke, especially in women under age 60, when taken off hormone therapy.2 This fact is quite ironic, as many clinicians are trying to rush to get women off hormone therapy in order to protect the heart, when the evidence does not suggest this. Just as with other hormone-deficiency conditions, the status needs to be periodically reviewed, and doses may need to be adjusted. However, after age 60 or 65, women do not automatically start producing the sex hormone that they have been deficient in. While menopause is not a definite endocrinopathy, it is a potential endocrinopathy; and for some women, such as young women who are oophorectomized, it is an absolute endocrinopathy.
The International Menopause Society has published updated guidelines emphasizing that new data and reanalysis of older data show that for most women the benefits of menopausal hormone therapy are much greater than the risks, particularly when started within a few years of menopause.3
- Lipold LD, Batur P, Kagan R. Is there a time limit for systemic menopausal hormone therapy? Cleve Clin J Med 2016; 83:605–612.
- Mikkola TS, Tuomikoski P, Lyytinen H, et al. Increased cardiovascular mortality risk in women discontinuing postmenopausal hormone therapy. J Clin Endocrinol Metab 2015; 100:4588–4594.
- Baber RJ, Panay N, Fenton A; IMS Writing Group. 2016 IMS recommendations on women’s midlife health and menopause hormone therapy. Climacteric 2016; 19:109–150.
- Lipold LD, Batur P, Kagan R. Is there a time limit for systemic menopausal hormone therapy? Cleve Clin J Med 2016; 83:605–612.
- Mikkola TS, Tuomikoski P, Lyytinen H, et al. Increased cardiovascular mortality risk in women discontinuing postmenopausal hormone therapy. J Clin Endocrinol Metab 2015; 100:4588–4594.
- Baber RJ, Panay N, Fenton A; IMS Writing Group. 2016 IMS recommendations on women’s midlife health and menopause hormone therapy. Climacteric 2016; 19:109–150.
In reply: Menopausal hormone therapy
In Reply: We would like to thank Dr. Thacker for her interest in our article on the clinical considerations regarding optimal duration of hormone therapy.1 We agree that the simple answer to whether there is there a time limit for systemic menopausal hormone therapy is no, emphasizing an individualized approach to each patient. After appropriate counseling and shared decision-making, some women may elect a short duration of therapy while others prefer longer-term use.
As Dr. Thacker mentioned, Mikkola et al2 performed an observational study of more than 300,000 Finnish women who discontinued hormone therapy. Data on the number of deaths in this group were gathered from a national database and compared with the expected number of deaths in the background population; 30% of the listed causes of death were confirmed by autopsy. In women who had started hormone therapy before age 60, the risk of cardiac death was elevated within the first year after stopping it (standardized mortality ratio [SMR] 1.74; 95% confidence interval [CI] 1.37–2.19), as was the risk of stroke (SMR 2.59, 95% CI 2.08–3.19). This was not true in women who started hormone therapy at age 60 and older. These findings are consistent with our contemporary understanding that for many women younger than age 60 the benefits of hormone therapy outweigh the risks.
The study had several important limitations:
- A healthy-woman bias may have contributed to the reduction in cardiovascular risk.
- No dates for the myocardial infarctions or strokes were available, and the dates hormone therapy was discontined potentially had a 3-month error.
- No data were available on important confounding factors such as smoking, body mass index, blood pressure, lipid levels, and family history.
- Hormone therapy users were compared with an age-standardized background population, which also included hormone therapy users.
- Long-term follow-up data were also perplexing: although more women than expected died of stroke or coronary heart disease within the first year of stopping hormone therapy, after 1 year, significantly fewer women died of these conditions than expected, regardless of how long they had been on hormone therapy before stopping.
These observations highlight the need for long-term, randomized, prospective controlled studies that adequately assess all long-term outcomes (cardiovascular events, mortality, cancer, fracture) in women who initiate hormone therapy before age 60 and within 10 years of menopause, including long-term follow-up after discontinuation. Though future randomized controlled trials will be beneficial to help guide women to a more balanced understanding of long-term hormone therapy and the risks of discontinuation, the current evidence supports continuing hormone therapy in women who derive a net benefit.
- Lipold LD, Batur P, Kagan R. Is there a time limit for systemic menopausal hormone therapy? Cleve Clin J Med 2016; 83:605–612.
- Mikkola TS, Tuomikoski P, Lyytinen H, et al. Increased cardiovascular mortality risk in women discontinuing postmenopausal hormone therapy. J Clin Endocrinol Metab 2015; 100:4588–4594.
In Reply: We would like to thank Dr. Thacker for her interest in our article on the clinical considerations regarding optimal duration of hormone therapy.1 We agree that the simple answer to whether there is there a time limit for systemic menopausal hormone therapy is no, emphasizing an individualized approach to each patient. After appropriate counseling and shared decision-making, some women may elect a short duration of therapy while others prefer longer-term use.
As Dr. Thacker mentioned, Mikkola et al2 performed an observational study of more than 300,000 Finnish women who discontinued hormone therapy. Data on the number of deaths in this group were gathered from a national database and compared with the expected number of deaths in the background population; 30% of the listed causes of death were confirmed by autopsy. In women who had started hormone therapy before age 60, the risk of cardiac death was elevated within the first year after stopping it (standardized mortality ratio [SMR] 1.74; 95% confidence interval [CI] 1.37–2.19), as was the risk of stroke (SMR 2.59, 95% CI 2.08–3.19). This was not true in women who started hormone therapy at age 60 and older. These findings are consistent with our contemporary understanding that for many women younger than age 60 the benefits of hormone therapy outweigh the risks.
The study had several important limitations:
- A healthy-woman bias may have contributed to the reduction in cardiovascular risk.
- No dates for the myocardial infarctions or strokes were available, and the dates hormone therapy was discontined potentially had a 3-month error.
- No data were available on important confounding factors such as smoking, body mass index, blood pressure, lipid levels, and family history.
- Hormone therapy users were compared with an age-standardized background population, which also included hormone therapy users.
- Long-term follow-up data were also perplexing: although more women than expected died of stroke or coronary heart disease within the first year of stopping hormone therapy, after 1 year, significantly fewer women died of these conditions than expected, regardless of how long they had been on hormone therapy before stopping.
These observations highlight the need for long-term, randomized, prospective controlled studies that adequately assess all long-term outcomes (cardiovascular events, mortality, cancer, fracture) in women who initiate hormone therapy before age 60 and within 10 years of menopause, including long-term follow-up after discontinuation. Though future randomized controlled trials will be beneficial to help guide women to a more balanced understanding of long-term hormone therapy and the risks of discontinuation, the current evidence supports continuing hormone therapy in women who derive a net benefit.
In Reply: We would like to thank Dr. Thacker for her interest in our article on the clinical considerations regarding optimal duration of hormone therapy.1 We agree that the simple answer to whether there is there a time limit for systemic menopausal hormone therapy is no, emphasizing an individualized approach to each patient. After appropriate counseling and shared decision-making, some women may elect a short duration of therapy while others prefer longer-term use.
As Dr. Thacker mentioned, Mikkola et al2 performed an observational study of more than 300,000 Finnish women who discontinued hormone therapy. Data on the number of deaths in this group were gathered from a national database and compared with the expected number of deaths in the background population; 30% of the listed causes of death were confirmed by autopsy. In women who had started hormone therapy before age 60, the risk of cardiac death was elevated within the first year after stopping it (standardized mortality ratio [SMR] 1.74; 95% confidence interval [CI] 1.37–2.19), as was the risk of stroke (SMR 2.59, 95% CI 2.08–3.19). This was not true in women who started hormone therapy at age 60 and older. These findings are consistent with our contemporary understanding that for many women younger than age 60 the benefits of hormone therapy outweigh the risks.
The study had several important limitations:
- A healthy-woman bias may have contributed to the reduction in cardiovascular risk.
- No dates for the myocardial infarctions or strokes were available, and the dates hormone therapy was discontined potentially had a 3-month error.
- No data were available on important confounding factors such as smoking, body mass index, blood pressure, lipid levels, and family history.
- Hormone therapy users were compared with an age-standardized background population, which also included hormone therapy users.
- Long-term follow-up data were also perplexing: although more women than expected died of stroke or coronary heart disease within the first year of stopping hormone therapy, after 1 year, significantly fewer women died of these conditions than expected, regardless of how long they had been on hormone therapy before stopping.
These observations highlight the need for long-term, randomized, prospective controlled studies that adequately assess all long-term outcomes (cardiovascular events, mortality, cancer, fracture) in women who initiate hormone therapy before age 60 and within 10 years of menopause, including long-term follow-up after discontinuation. Though future randomized controlled trials will be beneficial to help guide women to a more balanced understanding of long-term hormone therapy and the risks of discontinuation, the current evidence supports continuing hormone therapy in women who derive a net benefit.
- Lipold LD, Batur P, Kagan R. Is there a time limit for systemic menopausal hormone therapy? Cleve Clin J Med 2016; 83:605–612.
- Mikkola TS, Tuomikoski P, Lyytinen H, et al. Increased cardiovascular mortality risk in women discontinuing postmenopausal hormone therapy. J Clin Endocrinol Metab 2015; 100:4588–4594.
- Lipold LD, Batur P, Kagan R. Is there a time limit for systemic menopausal hormone therapy? Cleve Clin J Med 2016; 83:605–612.
- Mikkola TS, Tuomikoski P, Lyytinen H, et al. Increased cardiovascular mortality risk in women discontinuing postmenopausal hormone therapy. J Clin Endocrinol Metab 2015; 100:4588–4594.
ReACT: No benefit from routine coronary angiography after PCI
Routine follow-up coronary angiography after percutaneous coronary intervention leads to increased rates of coronary revascularization but without any significant benefits for outcomes, according to a study presented at the Transcatheter Cardiovascular Therapeutics annual meeting and published simultaneously on Nov. 1 in the Journal of the American College of Cardiology: Cardiovascular Interventions.
Hiroki Shiomi, MD, from Kyoto University, and his coauthors reported on ReACT, a prospective, open-label randomized controlled trial of routine follow-up coronary angiography in 700 patients who underwent successful percutaneous coronary intervention (PCI).
Among the 349 patients randomized to follow-up coronary angiography (FUCAG), 12.8% underwent any coronary revascularization within the first year after PCI, compared with 3.8% of the 351 patients randomized to standard clinical follow-up. The routine angiography group also had a higher incidence of target lesion revascularization in the first year after the index PCI (7.0% vs. 1.7%).
In both these cases, the cumulative 5-year incidence of coronary or target lesion revascularization was not significantly different between the routine angiography and control groups. However researchers saw no significant benefit from routine FUCAG in terms of the cumulative 5-year incidence of all-cause death, myocardial infarction, stroke, or emergency hospitalizations for acute coronary syndrome or heart failure, compared with clinical follow-up (22.4% vs. 24.7%; P = 0.70).
Nor were there any significant differences between the two groups in these individual components, or in the cumulative 5-year incidence of major bleeding (JACC Cardiovasc Interv. 2016 Nov 1.)
The authors commented that several previous studies have shown that routine FUCAG does not improve clinical outcomes, although it is still commonly performed in Japan after PCI.
“However, previous studies in the drug-eluting stents (DES) era were conducted in the context of pivotal randomized trials of DES and there have been no randomized clinical trials evaluating long-term clinical impact of routine FUCAG after PCI in the real world clinical practice including high-risk patients for cardiovascular events risk such as complex coronary artery disease and acute myocardial infarction (AMI) presentation,” the authors wrote.
Overall, 85.4% of patients in the routine angiography group and 12% of those in the clinical care group underwent coronary angiography in the first year, including for clinical reasons.
In the clinical follow-up group, coronary angiography was performed because of acute coronary syndrome (14%), recurrence of angina (60%), other clinical reasons (14%), or no clinical reason (12%). The control group also had more noninvasive physiological stress testing such as treadmill exercise test and stress nuclear study.
“Considering the invasive nature of coronary angiography and increased medical expenses, routine FUCAG after PCI would not be allowed as the usual clinical practice, unless patients have recurrent symptoms or objective evidence of ischemia,” the authors wrote.
“On the other hand, there was no excess of adverse clinical events with routine angiographic follow-up strategy except for the increased rate of 1-year repeat coronary revascularization.”
Given this, they suggested that scheduled angiographic follow-up might still be considered acceptable for early in vivo or significant coronary device trials.
While the authors said the trial ended up being underpowered because of a reduced final sample size and lower-than-anticipated event rate, it did warrant further larger-scale studies. In particular, they highlighted the question of what impact routine follow-up angiography might have in higher-risk patients, such as those with left main or multivessel coronary artery disease.
“Finally, because patient demographics, practice patterns including the indication of coronary revascularization, and clinical outcomes in Japan may be different from those outside Japan, generalizing the present study results to populations outside Japan should be done with caution.”
This study was supported by an educational grant from the Research Institute for Production Development (Kyoto). One author declared honoraria for education consulting from Boston Scientific Corporation.
Routine follow-up coronary angiography after percutaneous coronary intervention leads to increased rates of coronary revascularization but without any significant benefits for outcomes, according to a study presented at the Transcatheter Cardiovascular Therapeutics annual meeting and published simultaneously on Nov. 1 in the Journal of the American College of Cardiology: Cardiovascular Interventions.
Hiroki Shiomi, MD, from Kyoto University, and his coauthors reported on ReACT, a prospective, open-label randomized controlled trial of routine follow-up coronary angiography in 700 patients who underwent successful percutaneous coronary intervention (PCI).
Among the 349 patients randomized to follow-up coronary angiography (FUCAG), 12.8% underwent any coronary revascularization within the first year after PCI, compared with 3.8% of the 351 patients randomized to standard clinical follow-up. The routine angiography group also had a higher incidence of target lesion revascularization in the first year after the index PCI (7.0% vs. 1.7%).
In both these cases, the cumulative 5-year incidence of coronary or target lesion revascularization was not significantly different between the routine angiography and control groups. However researchers saw no significant benefit from routine FUCAG in terms of the cumulative 5-year incidence of all-cause death, myocardial infarction, stroke, or emergency hospitalizations for acute coronary syndrome or heart failure, compared with clinical follow-up (22.4% vs. 24.7%; P = 0.70).
Nor were there any significant differences between the two groups in these individual components, or in the cumulative 5-year incidence of major bleeding (JACC Cardiovasc Interv. 2016 Nov 1.)
The authors commented that several previous studies have shown that routine FUCAG does not improve clinical outcomes, although it is still commonly performed in Japan after PCI.
“However, previous studies in the drug-eluting stents (DES) era were conducted in the context of pivotal randomized trials of DES and there have been no randomized clinical trials evaluating long-term clinical impact of routine FUCAG after PCI in the real world clinical practice including high-risk patients for cardiovascular events risk such as complex coronary artery disease and acute myocardial infarction (AMI) presentation,” the authors wrote.
Overall, 85.4% of patients in the routine angiography group and 12% of those in the clinical care group underwent coronary angiography in the first year, including for clinical reasons.
In the clinical follow-up group, coronary angiography was performed because of acute coronary syndrome (14%), recurrence of angina (60%), other clinical reasons (14%), or no clinical reason (12%). The control group also had more noninvasive physiological stress testing such as treadmill exercise test and stress nuclear study.
“Considering the invasive nature of coronary angiography and increased medical expenses, routine FUCAG after PCI would not be allowed as the usual clinical practice, unless patients have recurrent symptoms or objective evidence of ischemia,” the authors wrote.
“On the other hand, there was no excess of adverse clinical events with routine angiographic follow-up strategy except for the increased rate of 1-year repeat coronary revascularization.”
Given this, they suggested that scheduled angiographic follow-up might still be considered acceptable for early in vivo or significant coronary device trials.
While the authors said the trial ended up being underpowered because of a reduced final sample size and lower-than-anticipated event rate, it did warrant further larger-scale studies. In particular, they highlighted the question of what impact routine follow-up angiography might have in higher-risk patients, such as those with left main or multivessel coronary artery disease.
“Finally, because patient demographics, practice patterns including the indication of coronary revascularization, and clinical outcomes in Japan may be different from those outside Japan, generalizing the present study results to populations outside Japan should be done with caution.”
This study was supported by an educational grant from the Research Institute for Production Development (Kyoto). One author declared honoraria for education consulting from Boston Scientific Corporation.
Routine follow-up coronary angiography after percutaneous coronary intervention leads to increased rates of coronary revascularization but without any significant benefits for outcomes, according to a study presented at the Transcatheter Cardiovascular Therapeutics annual meeting and published simultaneously on Nov. 1 in the Journal of the American College of Cardiology: Cardiovascular Interventions.
Hiroki Shiomi, MD, from Kyoto University, and his coauthors reported on ReACT, a prospective, open-label randomized controlled trial of routine follow-up coronary angiography in 700 patients who underwent successful percutaneous coronary intervention (PCI).
Among the 349 patients randomized to follow-up coronary angiography (FUCAG), 12.8% underwent any coronary revascularization within the first year after PCI, compared with 3.8% of the 351 patients randomized to standard clinical follow-up. The routine angiography group also had a higher incidence of target lesion revascularization in the first year after the index PCI (7.0% vs. 1.7%).
In both these cases, the cumulative 5-year incidence of coronary or target lesion revascularization was not significantly different between the routine angiography and control groups. However researchers saw no significant benefit from routine FUCAG in terms of the cumulative 5-year incidence of all-cause death, myocardial infarction, stroke, or emergency hospitalizations for acute coronary syndrome or heart failure, compared with clinical follow-up (22.4% vs. 24.7%; P = 0.70).
Nor were there any significant differences between the two groups in these individual components, or in the cumulative 5-year incidence of major bleeding (JACC Cardiovasc Interv. 2016 Nov 1.)
The authors commented that several previous studies have shown that routine FUCAG does not improve clinical outcomes, although it is still commonly performed in Japan after PCI.
“However, previous studies in the drug-eluting stents (DES) era were conducted in the context of pivotal randomized trials of DES and there have been no randomized clinical trials evaluating long-term clinical impact of routine FUCAG after PCI in the real world clinical practice including high-risk patients for cardiovascular events risk such as complex coronary artery disease and acute myocardial infarction (AMI) presentation,” the authors wrote.
Overall, 85.4% of patients in the routine angiography group and 12% of those in the clinical care group underwent coronary angiography in the first year, including for clinical reasons.
In the clinical follow-up group, coronary angiography was performed because of acute coronary syndrome (14%), recurrence of angina (60%), other clinical reasons (14%), or no clinical reason (12%). The control group also had more noninvasive physiological stress testing such as treadmill exercise test and stress nuclear study.
“Considering the invasive nature of coronary angiography and increased medical expenses, routine FUCAG after PCI would not be allowed as the usual clinical practice, unless patients have recurrent symptoms or objective evidence of ischemia,” the authors wrote.
“On the other hand, there was no excess of adverse clinical events with routine angiographic follow-up strategy except for the increased rate of 1-year repeat coronary revascularization.”
Given this, they suggested that scheduled angiographic follow-up might still be considered acceptable for early in vivo or significant coronary device trials.
While the authors said the trial ended up being underpowered because of a reduced final sample size and lower-than-anticipated event rate, it did warrant further larger-scale studies. In particular, they highlighted the question of what impact routine follow-up angiography might have in higher-risk patients, such as those with left main or multivessel coronary artery disease.
“Finally, because patient demographics, practice patterns including the indication of coronary revascularization, and clinical outcomes in Japan may be different from those outside Japan, generalizing the present study results to populations outside Japan should be done with caution.”
This study was supported by an educational grant from the Research Institute for Production Development (Kyoto). One author declared honoraria for education consulting from Boston Scientific Corporation.
Key clinical point: Routine follow-up coronary angiography after percutaneous coronary intervention increases rates of coronary revascularization but does not improve outcomes.
Major finding: Patients who underwent routine angiographic follow-up had a similar cumulative 5-year incidence of all-cause death, myocardial infarction, stroke, or emergency hospitalizations for acute coronary syndrome or heart failure as those who had standard clinical follow-up.
Data source: ReACT: a prospective, open-label randomized controlled trial in 700 patients after percutaneous coronary intervention.
Disclosures: This study was supported by an educational grant from the Research Institute for Production Development (Kyoto). One author declared honoraria for education consulting from Boston Scientific Corporation.
Interrupting oral anticoagulation in AF carries high thromboembolic cost
ROME – Temporary interruption of oral anticoagulation for stroke prevention in patients with atrial fibrillation occurs often and is associated with substantially increased risk of both cardioembolic events and all-cause mortality, according to a new prespecified secondary analysis of the ENGAGE-AF TIMI 48 trial.
The analysis showed that many of these treatment interruptions occur in response to nonserious adverse events such as minor bleeding, planned dental procedures, or simply because of patient wishes. The new ENGAGE-AF TIMI 48 findings should encourage physicians and patients to think twice before interrupting anticoagulant therapy for such reasons, which look pretty flimsy in light of the new evidence of the potentially serious consequences, Ilaria Cavallari, MD, said at the annual congress of the European Society of Cardiology.
The ENGAGE-AF TIMI 48 study was the pivotal phase III, double-blind, 21,105-patient clinical trial that led to Food and Drug Administration and European approval of the direct oral factor Xa inhibitor edoxaban (Savaysa) for stroke prevention in moderate- to high-risk patients with AF (N Engl J Med. 2013 Nov 28;369[22]:2093-104). The study showed that edoxaban at what later became the approved dose of 60 mg/day, or 30 mg/day in patients with impaired renal function, body weight of 60 kg or less, or on concomitant therapy with a platelet glycoprotein inhibitor, resulted in a 21% reduction in the risk of stroke or systemic embolism and a 20% reduction in major bleeding, compared with warfarin over 2.8 years of follow-up.
Dr. Cavallari presented a prespecified secondary retrospective analysis that focused on treatment interruptions: the reasons and the price paid in terms of thromboembolic events.
One or more treatment interruptions lasting for longer than 3 days occurred in 63% of patients during a median 2.8 years of follow-up. Since these were participants in a clinical trial with relatively close patient contact, it’s likely that the true interruption rate in real-world clinical practice is even higher, she said.
Interruptions were more significantly frequent in patients assigned to warfarin than among the groups assigned to edoxaban. The median duration of treatment interruptions was 9 days.
After excluding patients who were on any other anticoagulant during their interruption – low-molecular-weight heparin being the most common – investigators were left with 9,148 patients.
The endpoints of interest in this analysis were the major adverse events occurring during a time window lasting from 4 days after their last dose of oral anticoagulant until day 34 or when they resumed their study drug. The 30-day incidence of ischemic stroke or systemic embolism was 1.27%. The rate of a composite composed of cardiovascular death, MI, and ischemic stroke or systemic embolism was 4.99%. And the 30-day rate of an endpoint Dr. Cavallari termed primary net clinical outcome – a composite of stroke or systemic embolism, major bleeding, and all-cause mortality – was 7.16%.
These 30-day event rates among treatment interrupters are extremely high, compared with the 1-year rates in patients who didn’t interrupt oral anticoagulant therapy: 0.26% for ischemic stroke or systemic embolism; 0.36% for the composite of cardiovascular death, MI, and ischemic stroke; and 0.56% for the primary net clinical outcome, she continued.
The most common reason for treatment interruptions was adverse events, which accounted for 41% of the interruptions.
Drilling deeper into the types of adverse events that triggered treatment interruption, 1.5% of interrupters did so because of an on-treatment ischemic stroke or systemic embolism, 4.7% did so because of major bleeding, 8% had minor and clinically relevant nonmajor bleeding, another 30% interrupted treatment for other serious or nonserious adverse events.
Interrupting therapy because of an adverse event often had serious consequences, as reflected in an adjusted 3.94-fold increased risk of 30-day all-cause mortality, compared with patients who stopped for other reasons. Patients who stopped treatment because of a stroke, transient ischemic attack, or systemic embolism had a 30-day all-cause mortality of 29.3%. Those who interrupted treatment because of a major bleeding event had an 8.8% 30-day mortality. When minor or clinically relevant nonmajor bleeding was the impetus for a treatment interruption, the associated 30-day mortality was 3.4%.
Almost a third (29%) of treatment interruptions were the result of physician decisions in response to an upcoming invasive procedure, most often dental work.
The 30-day rates of ischemic stroke/systemic embolism and primary net clinical outcome didn’t differ significantly between patients who interrupted warfarin versus edoxaban at the approved dose. Nonetheless, this new secondary analysis from ENGAGE-AF TIMI 48 supports the parent study’s conclusion that edoxaban is preferable to warfarin in patients with AF, according to Dr. Cavallari.
“In light of the increased risk of ischemic events after interruption of oral anticoagulation, NOACs [new oral anticoagulants] represent an attractive alternative to vitamin K antagonists, given their faster onset of action, better adherence rates, safety, and tolerability profiles,” she concluded.
ENGAGE AF-TIMI 48 was funded by Daiichi Sankyo. Dr. Cavallari reported having no financial conflicts of interest regarding her presentation.
ROME – Temporary interruption of oral anticoagulation for stroke prevention in patients with atrial fibrillation occurs often and is associated with substantially increased risk of both cardioembolic events and all-cause mortality, according to a new prespecified secondary analysis of the ENGAGE-AF TIMI 48 trial.
The analysis showed that many of these treatment interruptions occur in response to nonserious adverse events such as minor bleeding, planned dental procedures, or simply because of patient wishes. The new ENGAGE-AF TIMI 48 findings should encourage physicians and patients to think twice before interrupting anticoagulant therapy for such reasons, which look pretty flimsy in light of the new evidence of the potentially serious consequences, Ilaria Cavallari, MD, said at the annual congress of the European Society of Cardiology.
The ENGAGE-AF TIMI 48 study was the pivotal phase III, double-blind, 21,105-patient clinical trial that led to Food and Drug Administration and European approval of the direct oral factor Xa inhibitor edoxaban (Savaysa) for stroke prevention in moderate- to high-risk patients with AF (N Engl J Med. 2013 Nov 28;369[22]:2093-104). The study showed that edoxaban at what later became the approved dose of 60 mg/day, or 30 mg/day in patients with impaired renal function, body weight of 60 kg or less, or on concomitant therapy with a platelet glycoprotein inhibitor, resulted in a 21% reduction in the risk of stroke or systemic embolism and a 20% reduction in major bleeding, compared with warfarin over 2.8 years of follow-up.
Dr. Cavallari presented a prespecified secondary retrospective analysis that focused on treatment interruptions: the reasons and the price paid in terms of thromboembolic events.
One or more treatment interruptions lasting for longer than 3 days occurred in 63% of patients during a median 2.8 years of follow-up. Since these were participants in a clinical trial with relatively close patient contact, it’s likely that the true interruption rate in real-world clinical practice is even higher, she said.
Interruptions were more significantly frequent in patients assigned to warfarin than among the groups assigned to edoxaban. The median duration of treatment interruptions was 9 days.
After excluding patients who were on any other anticoagulant during their interruption – low-molecular-weight heparin being the most common – investigators were left with 9,148 patients.
The endpoints of interest in this analysis were the major adverse events occurring during a time window lasting from 4 days after their last dose of oral anticoagulant until day 34 or when they resumed their study drug. The 30-day incidence of ischemic stroke or systemic embolism was 1.27%. The rate of a composite composed of cardiovascular death, MI, and ischemic stroke or systemic embolism was 4.99%. And the 30-day rate of an endpoint Dr. Cavallari termed primary net clinical outcome – a composite of stroke or systemic embolism, major bleeding, and all-cause mortality – was 7.16%.
These 30-day event rates among treatment interrupters are extremely high, compared with the 1-year rates in patients who didn’t interrupt oral anticoagulant therapy: 0.26% for ischemic stroke or systemic embolism; 0.36% for the composite of cardiovascular death, MI, and ischemic stroke; and 0.56% for the primary net clinical outcome, she continued.
The most common reason for treatment interruptions was adverse events, which accounted for 41% of the interruptions.
Drilling deeper into the types of adverse events that triggered treatment interruption, 1.5% of interrupters did so because of an on-treatment ischemic stroke or systemic embolism, 4.7% did so because of major bleeding, 8% had minor and clinically relevant nonmajor bleeding, another 30% interrupted treatment for other serious or nonserious adverse events.
Interrupting therapy because of an adverse event often had serious consequences, as reflected in an adjusted 3.94-fold increased risk of 30-day all-cause mortality, compared with patients who stopped for other reasons. Patients who stopped treatment because of a stroke, transient ischemic attack, or systemic embolism had a 30-day all-cause mortality of 29.3%. Those who interrupted treatment because of a major bleeding event had an 8.8% 30-day mortality. When minor or clinically relevant nonmajor bleeding was the impetus for a treatment interruption, the associated 30-day mortality was 3.4%.
Almost a third (29%) of treatment interruptions were the result of physician decisions in response to an upcoming invasive procedure, most often dental work.
The 30-day rates of ischemic stroke/systemic embolism and primary net clinical outcome didn’t differ significantly between patients who interrupted warfarin versus edoxaban at the approved dose. Nonetheless, this new secondary analysis from ENGAGE-AF TIMI 48 supports the parent study’s conclusion that edoxaban is preferable to warfarin in patients with AF, according to Dr. Cavallari.
“In light of the increased risk of ischemic events after interruption of oral anticoagulation, NOACs [new oral anticoagulants] represent an attractive alternative to vitamin K antagonists, given their faster onset of action, better adherence rates, safety, and tolerability profiles,” she concluded.
ENGAGE AF-TIMI 48 was funded by Daiichi Sankyo. Dr. Cavallari reported having no financial conflicts of interest regarding her presentation.
ROME – Temporary interruption of oral anticoagulation for stroke prevention in patients with atrial fibrillation occurs often and is associated with substantially increased risk of both cardioembolic events and all-cause mortality, according to a new prespecified secondary analysis of the ENGAGE-AF TIMI 48 trial.
The analysis showed that many of these treatment interruptions occur in response to nonserious adverse events such as minor bleeding, planned dental procedures, or simply because of patient wishes. The new ENGAGE-AF TIMI 48 findings should encourage physicians and patients to think twice before interrupting anticoagulant therapy for such reasons, which look pretty flimsy in light of the new evidence of the potentially serious consequences, Ilaria Cavallari, MD, said at the annual congress of the European Society of Cardiology.
The ENGAGE-AF TIMI 48 study was the pivotal phase III, double-blind, 21,105-patient clinical trial that led to Food and Drug Administration and European approval of the direct oral factor Xa inhibitor edoxaban (Savaysa) for stroke prevention in moderate- to high-risk patients with AF (N Engl J Med. 2013 Nov 28;369[22]:2093-104). The study showed that edoxaban at what later became the approved dose of 60 mg/day, or 30 mg/day in patients with impaired renal function, body weight of 60 kg or less, or on concomitant therapy with a platelet glycoprotein inhibitor, resulted in a 21% reduction in the risk of stroke or systemic embolism and a 20% reduction in major bleeding, compared with warfarin over 2.8 years of follow-up.
Dr. Cavallari presented a prespecified secondary retrospective analysis that focused on treatment interruptions: the reasons and the price paid in terms of thromboembolic events.
One or more treatment interruptions lasting for longer than 3 days occurred in 63% of patients during a median 2.8 years of follow-up. Since these were participants in a clinical trial with relatively close patient contact, it’s likely that the true interruption rate in real-world clinical practice is even higher, she said.
Interruptions were more significantly frequent in patients assigned to warfarin than among the groups assigned to edoxaban. The median duration of treatment interruptions was 9 days.
After excluding patients who were on any other anticoagulant during their interruption – low-molecular-weight heparin being the most common – investigators were left with 9,148 patients.
The endpoints of interest in this analysis were the major adverse events occurring during a time window lasting from 4 days after their last dose of oral anticoagulant until day 34 or when they resumed their study drug. The 30-day incidence of ischemic stroke or systemic embolism was 1.27%. The rate of a composite composed of cardiovascular death, MI, and ischemic stroke or systemic embolism was 4.99%. And the 30-day rate of an endpoint Dr. Cavallari termed primary net clinical outcome – a composite of stroke or systemic embolism, major bleeding, and all-cause mortality – was 7.16%.
These 30-day event rates among treatment interrupters are extremely high, compared with the 1-year rates in patients who didn’t interrupt oral anticoagulant therapy: 0.26% for ischemic stroke or systemic embolism; 0.36% for the composite of cardiovascular death, MI, and ischemic stroke; and 0.56% for the primary net clinical outcome, she continued.
The most common reason for treatment interruptions was adverse events, which accounted for 41% of the interruptions.
Drilling deeper into the types of adverse events that triggered treatment interruption, 1.5% of interrupters did so because of an on-treatment ischemic stroke or systemic embolism, 4.7% did so because of major bleeding, 8% had minor and clinically relevant nonmajor bleeding, another 30% interrupted treatment for other serious or nonserious adverse events.
Interrupting therapy because of an adverse event often had serious consequences, as reflected in an adjusted 3.94-fold increased risk of 30-day all-cause mortality, compared with patients who stopped for other reasons. Patients who stopped treatment because of a stroke, transient ischemic attack, or systemic embolism had a 30-day all-cause mortality of 29.3%. Those who interrupted treatment because of a major bleeding event had an 8.8% 30-day mortality. When minor or clinically relevant nonmajor bleeding was the impetus for a treatment interruption, the associated 30-day mortality was 3.4%.
Almost a third (29%) of treatment interruptions were the result of physician decisions in response to an upcoming invasive procedure, most often dental work.
The 30-day rates of ischemic stroke/systemic embolism and primary net clinical outcome didn’t differ significantly between patients who interrupted warfarin versus edoxaban at the approved dose. Nonetheless, this new secondary analysis from ENGAGE-AF TIMI 48 supports the parent study’s conclusion that edoxaban is preferable to warfarin in patients with AF, according to Dr. Cavallari.
“In light of the increased risk of ischemic events after interruption of oral anticoagulation, NOACs [new oral anticoagulants] represent an attractive alternative to vitamin K antagonists, given their faster onset of action, better adherence rates, safety, and tolerability profiles,” she concluded.
ENGAGE AF-TIMI 48 was funded by Daiichi Sankyo. Dr. Cavallari reported having no financial conflicts of interest regarding her presentation.
AT THE ESC CONGRESS 2016
Key clinical point:
Major finding: Atrial fibrillation patients who interrupted oral anticoagulation with edoxaban or warfarin for stroke prevention for longer than 3 days had a 127-fold increase in 30-day risk of the composite of stroke or systemic embolism, major bleeding, or all-cause mortality, compared with patients who didn’t interrupt treatment after 1 year.
Data source: A prespecified secondary analysis of 9,148 patients with atrial fibrillation who interrupted oral anticoagulation therapy with warfarin or edoxaban for longer than 3 days during a median follow-up of 2.8 years in the phase III, randomized, double-blind ENGAGE AF-TIMI 48 trial, and the consequences thereof.
Disclosures: The ENGAGE AF-TIMI 48 trial was funded by Daiichi Sankyo, which markets edoxaban. However, the presenter of this secondary analysis reported having no relevant financial interests.
Transcranial Direct Current Stimulation Enhances Cognitive Training in Parkinson’s Disease
PORTLAND, OR—Combining transcranial direct current stimulation (tDCS) and cognitive training resulted in an improvement in a greater number of cognitive outcomes than either intervention alone in a small, randomized, controlled trial of patients with Parkinson’s disease and mild cognitive impairment.
Researchers at Curtin University in Perth, Australia, conducted the trial comparing the effects of standard (ie, not individualized) cognitive training (SCT), tailored (ie, individualized) cognitive training (TCT), tDCS, and a combination of tDCS with either form of cognitive training on cognitive outcomes, activities of daily living, and quality of life. Previously, it was not known whether either form of cognitive training, tDCS, or a combination of the two would be most efficacious in improving cognition.
“Executive function, attention and working memory, memory, and language were the cognitive domains that improved for some groups, and we also found that activities of daily living and quality of life improved for the different groups as well,” PhD candidate Blake Lawrence said at the Fourth World Parkinson Congress.
Patients had cognitive deficits that did not interfere with functional independence and were responding to stable doses of antiparkinsonian medication. Forty-two eligible participants underwent neuropsychologic testing at baseline and were randomly and equally assigned to one of the following six groups: SCT, TCT, tDCS, SCT+tDCS, TCT+tDCS, or control.
Cognitive training consisted of three 45-minute sessions per week for four weeks using Smartbrain Pro software in participants’ homes. tDCS involved constant 1.5-mA stimulation for 20 minutes in one session per week for four weeks at the university, with the anode placed over area F3 to stimulate the left dorsal lateral prefrontal cortex. Neuropsychologic testing was conducted post intervention (ie, at five weeks). Follow-up evaluations were at 12 weeks.
The researchers administered tests to evaluate executive function (Stockings of Cambridge), attention and working memory (Stroop test), memory (paragraph recall), quality of life (Parkinson’s Disease Questionnaire), activities of daily living (Unified Parkinson’s Disease Rating Scale-II), and language (similarities test). In general, combining tDCS with either form of cognitive training resulted in significantly greater improvements in more outcomes than any of the modalities alone. SCT showed positive results when compared against the control group in memory improvement at follow-up (effect size, 1.30), as well as in quality of life and activities of daily living post intervention (effect sizes, 0.24 and 0.33, respectively). TCT showed benefits on quality of life at both time points (effect sizes, 0.26 and 0.12, respectively).
When combined with tDCS, SCT produced improvements in attention and working memory both post intervention and at 12-week follow-up (effect sizes, 0.60 and 0.24, respectively), and executive function at post intervention and follow-up (0.41 and 0.23, respectively). Improvement in activities of daily living and language were statistically significant only immediately post intervention.
Combining tDCS with TCT resulted in improvements post intervention and at follow-up on measures of memory (1.36 and 1.75, respectively) and executive function (0.19 and 0.92, respectively), as well as in language post intervention (1.06).
“The groups that completed both cognitive training and brain stimulation improved to a greater extent and in more outcomes than the groups that just completed the brain training or the stimulation individually,” Mr. Lawrence said. “The majority of the effects were shown immediately after the intervention, but some of the promising results ... actually maintained improvement at the 12-week follow-up, so that was after about eight weeks, when they did not complete any intervention whatsoever.”
The improvements are probably clinically meaningful to patients, since they themselves reported the outcomes on quality of life and activities of daily living scales, he said.
—Daniel M. Keller
PORTLAND, OR—Combining transcranial direct current stimulation (tDCS) and cognitive training resulted in an improvement in a greater number of cognitive outcomes than either intervention alone in a small, randomized, controlled trial of patients with Parkinson’s disease and mild cognitive impairment.
Researchers at Curtin University in Perth, Australia, conducted the trial comparing the effects of standard (ie, not individualized) cognitive training (SCT), tailored (ie, individualized) cognitive training (TCT), tDCS, and a combination of tDCS with either form of cognitive training on cognitive outcomes, activities of daily living, and quality of life. Previously, it was not known whether either form of cognitive training, tDCS, or a combination of the two would be most efficacious in improving cognition.
“Executive function, attention and working memory, memory, and language were the cognitive domains that improved for some groups, and we also found that activities of daily living and quality of life improved for the different groups as well,” PhD candidate Blake Lawrence said at the Fourth World Parkinson Congress.
Patients had cognitive deficits that did not interfere with functional independence and were responding to stable doses of antiparkinsonian medication. Forty-two eligible participants underwent neuropsychologic testing at baseline and were randomly and equally assigned to one of the following six groups: SCT, TCT, tDCS, SCT+tDCS, TCT+tDCS, or control.
Cognitive training consisted of three 45-minute sessions per week for four weeks using Smartbrain Pro software in participants’ homes. tDCS involved constant 1.5-mA stimulation for 20 minutes in one session per week for four weeks at the university, with the anode placed over area F3 to stimulate the left dorsal lateral prefrontal cortex. Neuropsychologic testing was conducted post intervention (ie, at five weeks). Follow-up evaluations were at 12 weeks.
The researchers administered tests to evaluate executive function (Stockings of Cambridge), attention and working memory (Stroop test), memory (paragraph recall), quality of life (Parkinson’s Disease Questionnaire), activities of daily living (Unified Parkinson’s Disease Rating Scale-II), and language (similarities test). In general, combining tDCS with either form of cognitive training resulted in significantly greater improvements in more outcomes than any of the modalities alone. SCT showed positive results when compared against the control group in memory improvement at follow-up (effect size, 1.30), as well as in quality of life and activities of daily living post intervention (effect sizes, 0.24 and 0.33, respectively). TCT showed benefits on quality of life at both time points (effect sizes, 0.26 and 0.12, respectively).
When combined with tDCS, SCT produced improvements in attention and working memory both post intervention and at 12-week follow-up (effect sizes, 0.60 and 0.24, respectively), and executive function at post intervention and follow-up (0.41 and 0.23, respectively). Improvement in activities of daily living and language were statistically significant only immediately post intervention.
Combining tDCS with TCT resulted in improvements post intervention and at follow-up on measures of memory (1.36 and 1.75, respectively) and executive function (0.19 and 0.92, respectively), as well as in language post intervention (1.06).
“The groups that completed both cognitive training and brain stimulation improved to a greater extent and in more outcomes than the groups that just completed the brain training or the stimulation individually,” Mr. Lawrence said. “The majority of the effects were shown immediately after the intervention, but some of the promising results ... actually maintained improvement at the 12-week follow-up, so that was after about eight weeks, when they did not complete any intervention whatsoever.”
The improvements are probably clinically meaningful to patients, since they themselves reported the outcomes on quality of life and activities of daily living scales, he said.
—Daniel M. Keller
PORTLAND, OR—Combining transcranial direct current stimulation (tDCS) and cognitive training resulted in an improvement in a greater number of cognitive outcomes than either intervention alone in a small, randomized, controlled trial of patients with Parkinson’s disease and mild cognitive impairment.
Researchers at Curtin University in Perth, Australia, conducted the trial comparing the effects of standard (ie, not individualized) cognitive training (SCT), tailored (ie, individualized) cognitive training (TCT), tDCS, and a combination of tDCS with either form of cognitive training on cognitive outcomes, activities of daily living, and quality of life. Previously, it was not known whether either form of cognitive training, tDCS, or a combination of the two would be most efficacious in improving cognition.
“Executive function, attention and working memory, memory, and language were the cognitive domains that improved for some groups, and we also found that activities of daily living and quality of life improved for the different groups as well,” PhD candidate Blake Lawrence said at the Fourth World Parkinson Congress.
Patients had cognitive deficits that did not interfere with functional independence and were responding to stable doses of antiparkinsonian medication. Forty-two eligible participants underwent neuropsychologic testing at baseline and were randomly and equally assigned to one of the following six groups: SCT, TCT, tDCS, SCT+tDCS, TCT+tDCS, or control.
Cognitive training consisted of three 45-minute sessions per week for four weeks using Smartbrain Pro software in participants’ homes. tDCS involved constant 1.5-mA stimulation for 20 minutes in one session per week for four weeks at the university, with the anode placed over area F3 to stimulate the left dorsal lateral prefrontal cortex. Neuropsychologic testing was conducted post intervention (ie, at five weeks). Follow-up evaluations were at 12 weeks.
The researchers administered tests to evaluate executive function (Stockings of Cambridge), attention and working memory (Stroop test), memory (paragraph recall), quality of life (Parkinson’s Disease Questionnaire), activities of daily living (Unified Parkinson’s Disease Rating Scale-II), and language (similarities test). In general, combining tDCS with either form of cognitive training resulted in significantly greater improvements in more outcomes than any of the modalities alone. SCT showed positive results when compared against the control group in memory improvement at follow-up (effect size, 1.30), as well as in quality of life and activities of daily living post intervention (effect sizes, 0.24 and 0.33, respectively). TCT showed benefits on quality of life at both time points (effect sizes, 0.26 and 0.12, respectively).
When combined with tDCS, SCT produced improvements in attention and working memory both post intervention and at 12-week follow-up (effect sizes, 0.60 and 0.24, respectively), and executive function at post intervention and follow-up (0.41 and 0.23, respectively). Improvement in activities of daily living and language were statistically significant only immediately post intervention.
Combining tDCS with TCT resulted in improvements post intervention and at follow-up on measures of memory (1.36 and 1.75, respectively) and executive function (0.19 and 0.92, respectively), as well as in language post intervention (1.06).
“The groups that completed both cognitive training and brain stimulation improved to a greater extent and in more outcomes than the groups that just completed the brain training or the stimulation individually,” Mr. Lawrence said. “The majority of the effects were shown immediately after the intervention, but some of the promising results ... actually maintained improvement at the 12-week follow-up, so that was after about eight weeks, when they did not complete any intervention whatsoever.”
The improvements are probably clinically meaningful to patients, since they themselves reported the outcomes on quality of life and activities of daily living scales, he said.
—Daniel M. Keller
ASCO: Patients with advanced cancer should receive palliative care within 8 weeks of diagnosis
Patients with advanced cancer should receive dedicated palliative care services early in the disease course, concurrently with active treatment, according to the American Society of Clinical Oncology’s new guidelines on the integration of palliative care into standard oncology care.
Ideally, patients should be referred to interdisciplinary palliative care teams within 8 weeks of cancer diagnosis, and palliative care should be available in both the inpatient and outpatient setting, recommended ASCO.
The guidelines, which updated and expanded the 2012 ASCO provisional clinical opinion, were developed by a multidisciplinary expert panel that systematically reviewed phase III randomized controlled trials, secondary analyses of those trials, and meta-analyses that were published between March 2010 and January 2016.
According to the panel, essential components of palliative care include:
• Rapport and relationship building with patient and family caregivers.
• Symptom, distress, and functional status management.
• Exploration of understanding and education about illness and prognosis.
• Clarification of treatment goals.
• Assessment and support of coping needs.
• Assistance with medical decision making.
• Provision of referrals to other care providers as indicated.
The panel makes the case that not only does palliative care improve care for patients and families, it also likely reduces the total cost of care, often substantially. However, “race, poverty and low socioeconomic and/or immigration status are determinants of barriers to palliative care,” wrote the expert panel, which was cochaired by Betty Ferrell, PhD, of the City of Hope Medical Center, Duarte, Calif., and Thomas Smith, MD, of the Sidney Kimmel Comprehensive Cancer Center in Baltimore.
Read the full guidelines here.
[email protected]
On Twitter @jessnicolecraig
Patients with advanced cancer should receive dedicated palliative care services early in the disease course, concurrently with active treatment, according to the American Society of Clinical Oncology’s new guidelines on the integration of palliative care into standard oncology care.
Ideally, patients should be referred to interdisciplinary palliative care teams within 8 weeks of cancer diagnosis, and palliative care should be available in both the inpatient and outpatient setting, recommended ASCO.
The guidelines, which updated and expanded the 2012 ASCO provisional clinical opinion, were developed by a multidisciplinary expert panel that systematically reviewed phase III randomized controlled trials, secondary analyses of those trials, and meta-analyses that were published between March 2010 and January 2016.
According to the panel, essential components of palliative care include:
• Rapport and relationship building with patient and family caregivers.
• Symptom, distress, and functional status management.
• Exploration of understanding and education about illness and prognosis.
• Clarification of treatment goals.
• Assessment and support of coping needs.
• Assistance with medical decision making.
• Provision of referrals to other care providers as indicated.
The panel makes the case that not only does palliative care improve care for patients and families, it also likely reduces the total cost of care, often substantially. However, “race, poverty and low socioeconomic and/or immigration status are determinants of barriers to palliative care,” wrote the expert panel, which was cochaired by Betty Ferrell, PhD, of the City of Hope Medical Center, Duarte, Calif., and Thomas Smith, MD, of the Sidney Kimmel Comprehensive Cancer Center in Baltimore.
Read the full guidelines here.
[email protected]
On Twitter @jessnicolecraig
Patients with advanced cancer should receive dedicated palliative care services early in the disease course, concurrently with active treatment, according to the American Society of Clinical Oncology’s new guidelines on the integration of palliative care into standard oncology care.
Ideally, patients should be referred to interdisciplinary palliative care teams within 8 weeks of cancer diagnosis, and palliative care should be available in both the inpatient and outpatient setting, recommended ASCO.
The guidelines, which updated and expanded the 2012 ASCO provisional clinical opinion, were developed by a multidisciplinary expert panel that systematically reviewed phase III randomized controlled trials, secondary analyses of those trials, and meta-analyses that were published between March 2010 and January 2016.
According to the panel, essential components of palliative care include:
• Rapport and relationship building with patient and family caregivers.
• Symptom, distress, and functional status management.
• Exploration of understanding and education about illness and prognosis.
• Clarification of treatment goals.
• Assessment and support of coping needs.
• Assistance with medical decision making.
• Provision of referrals to other care providers as indicated.
The panel makes the case that not only does palliative care improve care for patients and families, it also likely reduces the total cost of care, often substantially. However, “race, poverty and low socioeconomic and/or immigration status are determinants of barriers to palliative care,” wrote the expert panel, which was cochaired by Betty Ferrell, PhD, of the City of Hope Medical Center, Duarte, Calif., and Thomas Smith, MD, of the Sidney Kimmel Comprehensive Cancer Center in Baltimore.
Read the full guidelines here.
[email protected]
On Twitter @jessnicolecraig
FROM THE JOURNAL OF CLINICAL ONCOLOGY
The march of technology
Each year the American Academy of Pediatrics National Conference and Exhibition fills a huge convention hall with the latest products that can improve health and generate practice revenue.
Some products are solutions to the minor annoyances of everyday practice. For instance, there are ear curettes equipped with their own LED light and a magnifying lens. There are countless creams to treat rashes. There are new automated devices for testing hearing, vision, and attention. And at the far extreme, there are products with the potential to revolutionize clinical care or to bankrupt it. The latest technology in that category is whole exome sequencing.
A couple weeks earlier I had listened to a national meeting of pediatric ethicists discuss this technology. Some proponents discussed the possibility of doing whole exome sequencing (WES) for every newborn. Alas, many ethicists can’t do math. Even if the cost goes below $1,000 per test, at 4 million babies per year in the United States, that is $4 billion per year. That sounds like a small sum, compared with the current federal deficit, but the original budget for the entire, 10-year-long Human Genome Project (HGP) was $4.5 billion. There were complaints in that era that diverting such an enormous amount of money into the HGP would cut the funding of a lot of other very good research at the National Institutes of Health. Conversely, Medicare spends $4.5 billion on hepatitis C treatment.
Viewed differently, the yearly per capita payment to general pediatricians, excluding vaccine costs, is around $1,000. Perhaps I’m biased, but I think I provide much more value than a genetic sequence.
Precision medicine has a lot of potential. So far, it is mostly potential. One colleague related that, in the past year, he has done WES on three patients, at about $4,000 charge for each, and gotten positive results in two cases. He figures soon he will be ordering it on every child with symptoms of autism, developmental delay, or failure to thrive. Is that a wise idea? That, it seems, is the area in which there is the least illuminating research.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis.
Each year the American Academy of Pediatrics National Conference and Exhibition fills a huge convention hall with the latest products that can improve health and generate practice revenue.
Some products are solutions to the minor annoyances of everyday practice. For instance, there are ear curettes equipped with their own LED light and a magnifying lens. There are countless creams to treat rashes. There are new automated devices for testing hearing, vision, and attention. And at the far extreme, there are products with the potential to revolutionize clinical care or to bankrupt it. The latest technology in that category is whole exome sequencing.
A couple weeks earlier I had listened to a national meeting of pediatric ethicists discuss this technology. Some proponents discussed the possibility of doing whole exome sequencing (WES) for every newborn. Alas, many ethicists can’t do math. Even if the cost goes below $1,000 per test, at 4 million babies per year in the United States, that is $4 billion per year. That sounds like a small sum, compared with the current federal deficit, but the original budget for the entire, 10-year-long Human Genome Project (HGP) was $4.5 billion. There were complaints in that era that diverting such an enormous amount of money into the HGP would cut the funding of a lot of other very good research at the National Institutes of Health. Conversely, Medicare spends $4.5 billion on hepatitis C treatment.
Viewed differently, the yearly per capita payment to general pediatricians, excluding vaccine costs, is around $1,000. Perhaps I’m biased, but I think I provide much more value than a genetic sequence.
Precision medicine has a lot of potential. So far, it is mostly potential. One colleague related that, in the past year, he has done WES on three patients, at about $4,000 charge for each, and gotten positive results in two cases. He figures soon he will be ordering it on every child with symptoms of autism, developmental delay, or failure to thrive. Is that a wise idea? That, it seems, is the area in which there is the least illuminating research.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis.
Each year the American Academy of Pediatrics National Conference and Exhibition fills a huge convention hall with the latest products that can improve health and generate practice revenue.
Some products are solutions to the minor annoyances of everyday practice. For instance, there are ear curettes equipped with their own LED light and a magnifying lens. There are countless creams to treat rashes. There are new automated devices for testing hearing, vision, and attention. And at the far extreme, there are products with the potential to revolutionize clinical care or to bankrupt it. The latest technology in that category is whole exome sequencing.
A couple weeks earlier I had listened to a national meeting of pediatric ethicists discuss this technology. Some proponents discussed the possibility of doing whole exome sequencing (WES) for every newborn. Alas, many ethicists can’t do math. Even if the cost goes below $1,000 per test, at 4 million babies per year in the United States, that is $4 billion per year. That sounds like a small sum, compared with the current federal deficit, but the original budget for the entire, 10-year-long Human Genome Project (HGP) was $4.5 billion. There were complaints in that era that diverting such an enormous amount of money into the HGP would cut the funding of a lot of other very good research at the National Institutes of Health. Conversely, Medicare spends $4.5 billion on hepatitis C treatment.
Viewed differently, the yearly per capita payment to general pediatricians, excluding vaccine costs, is around $1,000. Perhaps I’m biased, but I think I provide much more value than a genetic sequence.
Precision medicine has a lot of potential. So far, it is mostly potential. One colleague related that, in the past year, he has done WES on three patients, at about $4,000 charge for each, and gotten positive results in two cases. He figures soon he will be ordering it on every child with symptoms of autism, developmental delay, or failure to thrive. Is that a wise idea? That, it seems, is the area in which there is the least illuminating research.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis.
Clinical Challenges - November 2016
What’s your diagnosis?
Answer to “What’s your diagnosis?” on page X: Collagenous gastritis and collagenous sprue
Recent studies have also shown the importance of obtaining at least 1 biopsy from the duodenal bulb to avoid missing the diagnosis of celiac disease. In 126 patients with newly established celiac disease and 85 patients with a previous diagnosis on a gluten-free diet presenting for reevaluation, villous atrophy was limited to the duodenal bulb in 9% and 14% of cases, respectively.3
References
1. Brain, O., Rajaguru, C., Warren, B. et al. Collagenous gastritis: reports and systematic review. Eur J Gastroenterol Hepatol. 2009;21:1419-24.
2. Gopal, P., McKenna, B.J. The collagenous gastroenteritides: similarities and differences. Arch Pathol Lab Med. 2010;134:1485-9.
3. Evans, K.E., Aziz, I., Cross, S.S. et al. A prospective study of duodenal bulb biopsy in newly diagnosed and established adult celiac disease. Am J Gastroenterol. 2011;106:1837-742.
Answer to “What’s your diagnosis?” on page X: Collagenous gastritis and collagenous sprue
Recent studies have also shown the importance of obtaining at least 1 biopsy from the duodenal bulb to avoid missing the diagnosis of celiac disease. In 126 patients with newly established celiac disease and 85 patients with a previous diagnosis on a gluten-free diet presenting for reevaluation, villous atrophy was limited to the duodenal bulb in 9% and 14% of cases, respectively.3
References
1. Brain, O., Rajaguru, C., Warren, B. et al. Collagenous gastritis: reports and systematic review. Eur J Gastroenterol Hepatol. 2009;21:1419-24.
2. Gopal, P., McKenna, B.J. The collagenous gastroenteritides: similarities and differences. Arch Pathol Lab Med. 2010;134:1485-9.
3. Evans, K.E., Aziz, I., Cross, S.S. et al. A prospective study of duodenal bulb biopsy in newly diagnosed and established adult celiac disease. Am J Gastroenterol. 2011;106:1837-742.
Answer to “What’s your diagnosis?” on page X: Collagenous gastritis and collagenous sprue
Recent studies have also shown the importance of obtaining at least 1 biopsy from the duodenal bulb to avoid missing the diagnosis of celiac disease. In 126 patients with newly established celiac disease and 85 patients with a previous diagnosis on a gluten-free diet presenting for reevaluation, villous atrophy was limited to the duodenal bulb in 9% and 14% of cases, respectively.3
References
1. Brain, O., Rajaguru, C., Warren, B. et al. Collagenous gastritis: reports and systematic review. Eur J Gastroenterol Hepatol. 2009;21:1419-24.
2. Gopal, P., McKenna, B.J. The collagenous gastroenteritides: similarities and differences. Arch Pathol Lab Med. 2010;134:1485-9.
3. Evans, K.E., Aziz, I., Cross, S.S. et al. A prospective study of duodenal bulb biopsy in newly diagnosed and established adult celiac disease. Am J Gastroenterol. 2011;106:1837-742.
What’s your diagnosis?
What’s your diagnosis?
What’s your diagnosis?
By Benjamin Kloesel, MD, Vishal S. Chandan, MD, and Glenn L. Alexander, MD. Published previously in Gastroenterology (2012;143:1439, 1692).
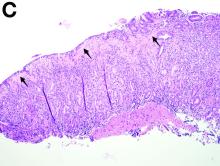
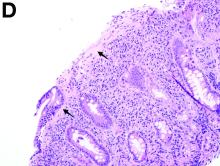
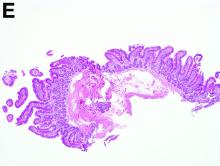
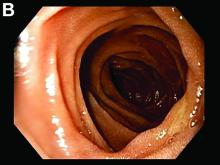
A 30-year-old woman with a past medical history of hypothyroidism presents for evaluation of epigastric discomfort, nausea without emesis, abdominal bloating, and watery, nonbloody diarrhea for 5 months. This was associated with a 15-pound weight loss. Complete blood count, liver function tests, thyroid-stimulating hormone, immunoglobulin (Ig) levels, and IgG/IgA tissue transglutaminase (tTG) were within normal limits. Stool studies for bacterial pathogens, Giardia, Clostridium difficile toxin, and ova/parasites were negative.
Results puzzling for embolic protection during TAVR
The largest randomized clinical trial to assess the safety and efficacy of cerebral embolic protection systems during transcatheter aortic valve replacement yielded puzzling and somewhat contradictory results, according to a report presented at the Transcatheter Cardiovascular Therapeutics annual meeting and published simultaneously in the Journal of the American College of Cardiology.
Virtually every device in this industry-sponsored study involving 363 elderly patients (mean age, 83.4 years) with severe aortic stenosis trapped particulate debris as intended, the mean volume of new lesions in the protected areas of the brain was reduced by 42%, and the number and volume of new lesions correlated with neurocognitive outcomes at 30 days.
However, the reduction in lesion volume did not achieve statistical significance, and the improvement in neurocognitive function also did not reach statistical significance.
In addition, “the sample size was clearly too low to assess clinical outcomes, and in retrospect, was also too low to evaluate follow-up MRI findings or neurocognitive outcomes.” Nevertheless, the trial “provides reassuring evidence of device safety,” said Samir R. Kapadia, MD, of the Cleveland Clinic (J Am Coll Cardiol. 2016 Nov 1. doi: 10.1016/j.jacc.2016.10.023).
In this prospective study, the investigators assessed patients at 17 medical centers in the United States and 2 in Germany. In addition to being elderly, the study patients were at high risk because of frequent comorbidities, including atrial fibrillation (31.7%) and prior stroke (5.8%).
The remaining 123 patients underwent TAVR but not MRI in a safety arm of the trial.
The protection devices were placed “without safety concerns” in most patients. The rate of major adverse events with the device was 7.3%, markedly less than the 18.3% prespecified performance goal for this outcome. Total procedure time was lengthened by only 13 minutes when the device was used, and total fluoroscopy time was increased by only 3 minutes. These findings demonstrate the overall safety of using the device, Dr. Kapadia said.
Debris including thrombus with tissue elements, artery wall particles, calcifications, valve tissue, and foreign materials was retrieved from the filters in 99% of patients.
The mean volume of new cerebral lesions in areas of the brain protected by the device was reduced by 42%, compared with that in patients who underwent TAVR without the protection device. However, this reduction was not statistically significant, so the primary efficacy endpoint of the study was not met.
Similarly, neurocognitive testing at 30 days showed that the volume of new lesions correlated with poorer outcomes. However, the difference in neurocognitive function between the intervention group and the control group did not reach statistical significance.
Several limitations likely contributed to this lack of statistical significance, Dr. Kapadia said.
First, the 5-day “window” for MRI assessment was too long. Both the number and the volume of new lesions rapidly changed over time, which led to marked variance in MRI findings depending on when the images were taken.
In addition, only one TAVR device was available at the time the trial was designed, so the study wasn’t stratified by type of valve device. But several new devices became available during the study, and the study investigators were permitted to use any of them. Both pre- and postimplantation techniques differ among these TAVR devices, but these differences could not be accounted for, given the study design.
Also, certain risk factors for stroke, especially certain findings on baseline MRI, were not understood when the trial was designed, and those factors also were not accounted for, Dr. Kapadia said.
Claret Medical funded the study. Dr. Kapadia reported having no relevant financial disclosures; his associates reported numerous ties to industry sources. The meeting was sponsored by the Cardiovascular Research Foundation.
From a logical standpoint, a device that collects cerebral embolic material in 99% of cases should prevent ischemic brain injury, yet the findings from this randomized trial don’t appear to support the routine use of such devices. But it would be inappropriate and unfair to close the book on cerebral protection after this chapter.
The authors acknowledge that an MRI “window” of 5 days creates too much heterogeneity in the data, that multiple TAVR devices requiring different implantation techniques further muddy the picture, and that in retrospect the sample size was inadequate and the study was underpowered. In addition, rigorous neurocognitive assessment can be challenging in elderly, recovering patients, and results can depend on the time of day and the patient’s alertness.
Despite the negative findings regarding both primary and secondary endpoints, the data do show the overall safety of embolic protection devices. We are dealing with a potential benefit that cannot be ignored as TAVR shifts to younger and lower-risk patients.
Azeem Latib, MD, is in the interventional cardiology unit at San Raffaele Scientific Institute in Milan. Matteo Pagnesi, MD, is in the interventional cardiology unit at EMO-GVM Centro Cuore Columbus in Milan. San Raffaele Scientific Institute has been involved in clinical studies of embolic protection devices made by Claret Medical, Innovative Cardiovascular Solutions, and Keystone Heart. Dr. Latib and Dr. Pagnesi reported having no other relevant financial disclosures. They made these remarks in an editorial accompanying Dr. Kapadia’s report (J Am Coll Cardiol. 2016 Nov 1. doi: 10.1016/j.jacc.2016.10.036).
From a logical standpoint, a device that collects cerebral embolic material in 99% of cases should prevent ischemic brain injury, yet the findings from this randomized trial don’t appear to support the routine use of such devices. But it would be inappropriate and unfair to close the book on cerebral protection after this chapter.
The authors acknowledge that an MRI “window” of 5 days creates too much heterogeneity in the data, that multiple TAVR devices requiring different implantation techniques further muddy the picture, and that in retrospect the sample size was inadequate and the study was underpowered. In addition, rigorous neurocognitive assessment can be challenging in elderly, recovering patients, and results can depend on the time of day and the patient’s alertness.
Despite the negative findings regarding both primary and secondary endpoints, the data do show the overall safety of embolic protection devices. We are dealing with a potential benefit that cannot be ignored as TAVR shifts to younger and lower-risk patients.
Azeem Latib, MD, is in the interventional cardiology unit at San Raffaele Scientific Institute in Milan. Matteo Pagnesi, MD, is in the interventional cardiology unit at EMO-GVM Centro Cuore Columbus in Milan. San Raffaele Scientific Institute has been involved in clinical studies of embolic protection devices made by Claret Medical, Innovative Cardiovascular Solutions, and Keystone Heart. Dr. Latib and Dr. Pagnesi reported having no other relevant financial disclosures. They made these remarks in an editorial accompanying Dr. Kapadia’s report (J Am Coll Cardiol. 2016 Nov 1. doi: 10.1016/j.jacc.2016.10.036).
From a logical standpoint, a device that collects cerebral embolic material in 99% of cases should prevent ischemic brain injury, yet the findings from this randomized trial don’t appear to support the routine use of such devices. But it would be inappropriate and unfair to close the book on cerebral protection after this chapter.
The authors acknowledge that an MRI “window” of 5 days creates too much heterogeneity in the data, that multiple TAVR devices requiring different implantation techniques further muddy the picture, and that in retrospect the sample size was inadequate and the study was underpowered. In addition, rigorous neurocognitive assessment can be challenging in elderly, recovering patients, and results can depend on the time of day and the patient’s alertness.
Despite the negative findings regarding both primary and secondary endpoints, the data do show the overall safety of embolic protection devices. We are dealing with a potential benefit that cannot be ignored as TAVR shifts to younger and lower-risk patients.
Azeem Latib, MD, is in the interventional cardiology unit at San Raffaele Scientific Institute in Milan. Matteo Pagnesi, MD, is in the interventional cardiology unit at EMO-GVM Centro Cuore Columbus in Milan. San Raffaele Scientific Institute has been involved in clinical studies of embolic protection devices made by Claret Medical, Innovative Cardiovascular Solutions, and Keystone Heart. Dr. Latib and Dr. Pagnesi reported having no other relevant financial disclosures. They made these remarks in an editorial accompanying Dr. Kapadia’s report (J Am Coll Cardiol. 2016 Nov 1. doi: 10.1016/j.jacc.2016.10.036).
The largest randomized clinical trial to assess the safety and efficacy of cerebral embolic protection systems during transcatheter aortic valve replacement yielded puzzling and somewhat contradictory results, according to a report presented at the Transcatheter Cardiovascular Therapeutics annual meeting and published simultaneously in the Journal of the American College of Cardiology.
Virtually every device in this industry-sponsored study involving 363 elderly patients (mean age, 83.4 years) with severe aortic stenosis trapped particulate debris as intended, the mean volume of new lesions in the protected areas of the brain was reduced by 42%, and the number and volume of new lesions correlated with neurocognitive outcomes at 30 days.
However, the reduction in lesion volume did not achieve statistical significance, and the improvement in neurocognitive function also did not reach statistical significance.
In addition, “the sample size was clearly too low to assess clinical outcomes, and in retrospect, was also too low to evaluate follow-up MRI findings or neurocognitive outcomes.” Nevertheless, the trial “provides reassuring evidence of device safety,” said Samir R. Kapadia, MD, of the Cleveland Clinic (J Am Coll Cardiol. 2016 Nov 1. doi: 10.1016/j.jacc.2016.10.023).
In this prospective study, the investigators assessed patients at 17 medical centers in the United States and 2 in Germany. In addition to being elderly, the study patients were at high risk because of frequent comorbidities, including atrial fibrillation (31.7%) and prior stroke (5.8%).
The remaining 123 patients underwent TAVR but not MRI in a safety arm of the trial.
The protection devices were placed “without safety concerns” in most patients. The rate of major adverse events with the device was 7.3%, markedly less than the 18.3% prespecified performance goal for this outcome. Total procedure time was lengthened by only 13 minutes when the device was used, and total fluoroscopy time was increased by only 3 minutes. These findings demonstrate the overall safety of using the device, Dr. Kapadia said.
Debris including thrombus with tissue elements, artery wall particles, calcifications, valve tissue, and foreign materials was retrieved from the filters in 99% of patients.
The mean volume of new cerebral lesions in areas of the brain protected by the device was reduced by 42%, compared with that in patients who underwent TAVR without the protection device. However, this reduction was not statistically significant, so the primary efficacy endpoint of the study was not met.
Similarly, neurocognitive testing at 30 days showed that the volume of new lesions correlated with poorer outcomes. However, the difference in neurocognitive function between the intervention group and the control group did not reach statistical significance.
Several limitations likely contributed to this lack of statistical significance, Dr. Kapadia said.
First, the 5-day “window” for MRI assessment was too long. Both the number and the volume of new lesions rapidly changed over time, which led to marked variance in MRI findings depending on when the images were taken.
In addition, only one TAVR device was available at the time the trial was designed, so the study wasn’t stratified by type of valve device. But several new devices became available during the study, and the study investigators were permitted to use any of them. Both pre- and postimplantation techniques differ among these TAVR devices, but these differences could not be accounted for, given the study design.
Also, certain risk factors for stroke, especially certain findings on baseline MRI, were not understood when the trial was designed, and those factors also were not accounted for, Dr. Kapadia said.
Claret Medical funded the study. Dr. Kapadia reported having no relevant financial disclosures; his associates reported numerous ties to industry sources. The meeting was sponsored by the Cardiovascular Research Foundation.
The largest randomized clinical trial to assess the safety and efficacy of cerebral embolic protection systems during transcatheter aortic valve replacement yielded puzzling and somewhat contradictory results, according to a report presented at the Transcatheter Cardiovascular Therapeutics annual meeting and published simultaneously in the Journal of the American College of Cardiology.
Virtually every device in this industry-sponsored study involving 363 elderly patients (mean age, 83.4 years) with severe aortic stenosis trapped particulate debris as intended, the mean volume of new lesions in the protected areas of the brain was reduced by 42%, and the number and volume of new lesions correlated with neurocognitive outcomes at 30 days.
However, the reduction in lesion volume did not achieve statistical significance, and the improvement in neurocognitive function also did not reach statistical significance.
In addition, “the sample size was clearly too low to assess clinical outcomes, and in retrospect, was also too low to evaluate follow-up MRI findings or neurocognitive outcomes.” Nevertheless, the trial “provides reassuring evidence of device safety,” said Samir R. Kapadia, MD, of the Cleveland Clinic (J Am Coll Cardiol. 2016 Nov 1. doi: 10.1016/j.jacc.2016.10.023).
In this prospective study, the investigators assessed patients at 17 medical centers in the United States and 2 in Germany. In addition to being elderly, the study patients were at high risk because of frequent comorbidities, including atrial fibrillation (31.7%) and prior stroke (5.8%).
The remaining 123 patients underwent TAVR but not MRI in a safety arm of the trial.
The protection devices were placed “without safety concerns” in most patients. The rate of major adverse events with the device was 7.3%, markedly less than the 18.3% prespecified performance goal for this outcome. Total procedure time was lengthened by only 13 minutes when the device was used, and total fluoroscopy time was increased by only 3 minutes. These findings demonstrate the overall safety of using the device, Dr. Kapadia said.
Debris including thrombus with tissue elements, artery wall particles, calcifications, valve tissue, and foreign materials was retrieved from the filters in 99% of patients.
The mean volume of new cerebral lesions in areas of the brain protected by the device was reduced by 42%, compared with that in patients who underwent TAVR without the protection device. However, this reduction was not statistically significant, so the primary efficacy endpoint of the study was not met.
Similarly, neurocognitive testing at 30 days showed that the volume of new lesions correlated with poorer outcomes. However, the difference in neurocognitive function between the intervention group and the control group did not reach statistical significance.
Several limitations likely contributed to this lack of statistical significance, Dr. Kapadia said.
First, the 5-day “window” for MRI assessment was too long. Both the number and the volume of new lesions rapidly changed over time, which led to marked variance in MRI findings depending on when the images were taken.
In addition, only one TAVR device was available at the time the trial was designed, so the study wasn’t stratified by type of valve device. But several new devices became available during the study, and the study investigators were permitted to use any of them. Both pre- and postimplantation techniques differ among these TAVR devices, but these differences could not be accounted for, given the study design.
Also, certain risk factors for stroke, especially certain findings on baseline MRI, were not understood when the trial was designed, and those factors also were not accounted for, Dr. Kapadia said.
Claret Medical funded the study. Dr. Kapadia reported having no relevant financial disclosures; his associates reported numerous ties to industry sources. The meeting was sponsored by the Cardiovascular Research Foundation.
Key clinical point: The largest randomized clinical trial to assess the safety and efficacy of cerebral embolic protection systems during TAVR yielded puzzling and contradictory results.
Major finding: Debris including thrombus with tissue elements, artery wall particles, calcifications, valve tissue, and foreign materials was retrieved from the cerebral protection filters in 99% of patients.
Data source: A prospective, international, randomized trial involving 363 elderly patients undergoing TAVR for severe aortic stenosis.
Disclosures: Claret Medical funded the study. Dr. Kapadia reported having no relevant financial disclosures; his associates reported numerous ties to industry sources.
Does Transdermal Nicotine Benefit Patients With Parkinson’s Disease?
PORTLAND, OR—High doses of transdermal nicotine failed to improve off motor symptoms in patients with Parkinson’s disease, according to trial results presented at the Fourth World Parkinson Congress. Nicotine may have provided benefit on secondary outcome measures, researchers said.
Gabriel Villafane, MD, a neurologist at Henri Mondor University Hospital in Créteil, France, and colleagues conducted the Nicopark2 Study, a single-blind, controlled, randomized trial to evaluate the effect of high doses of transdermal nicotine (approximately 90 mg per day) on motor symptoms in Parkinson’s disease. Cigarette smoking is associated with a dose-dependent reduction in risk of Parkinson’s disease. Nicotine’s effect on motor symptoms in Parkinson’s disease is controversial. Seven of eight open-label studies suggested that nicotine improves motor symptoms, but four placebo-controlled studies were negative.
The investigators enrolled 40 patients with Parkinson’s disease in the study. Eligible patients were nonsmokers age 35 to 70 with a Hoehn and Yahr off stage of four or less and a Hoehn and Yahr on stage of three or less. Patients had received levodopa treatment for at least three years. Exclusion criteria included neurosurgery, psychiatric disease, and symptomatic orthostatic hypotension.
The primary outcome was mean difference between groups in change of Unified Parkinson’s Disease Rating Scale off motor score from baseline to week 39 on blinded video rating.
Twenty patients were randomized to receive transdermal nicotine, and 20 patients were randomized to a control group. The change in motor score between groups was not statistically significant. Change in quality of life assessed by the Parkinson’s Disease Questionnaire was not statistically significant. At 39 weeks, a reduction in levodopa doses, a reduction in dyskinesias, and an improvement in activities of daily living were observed in the nicotine group, the researchers said.Adverse events occurred in more patients in the nicotine group than in the control group. They included worsening of parkinsonism (30% vs 5%), cutaneous reactions (35% vs 5%), gastrointestinal complaints (65% vs 15%), hypotension (25% vs 5%), insomnia (25% vs 5%), and nervousness and anxiety (20% vs 0%). Ten patients in the nicotine group had a serious adverse event, compared with three patients in the control group.
—Jake Remaly
PORTLAND, OR—High doses of transdermal nicotine failed to improve off motor symptoms in patients with Parkinson’s disease, according to trial results presented at the Fourth World Parkinson Congress. Nicotine may have provided benefit on secondary outcome measures, researchers said.
Gabriel Villafane, MD, a neurologist at Henri Mondor University Hospital in Créteil, France, and colleagues conducted the Nicopark2 Study, a single-blind, controlled, randomized trial to evaluate the effect of high doses of transdermal nicotine (approximately 90 mg per day) on motor symptoms in Parkinson’s disease. Cigarette smoking is associated with a dose-dependent reduction in risk of Parkinson’s disease. Nicotine’s effect on motor symptoms in Parkinson’s disease is controversial. Seven of eight open-label studies suggested that nicotine improves motor symptoms, but four placebo-controlled studies were negative.
The investigators enrolled 40 patients with Parkinson’s disease in the study. Eligible patients were nonsmokers age 35 to 70 with a Hoehn and Yahr off stage of four or less and a Hoehn and Yahr on stage of three or less. Patients had received levodopa treatment for at least three years. Exclusion criteria included neurosurgery, psychiatric disease, and symptomatic orthostatic hypotension.
The primary outcome was mean difference between groups in change of Unified Parkinson’s Disease Rating Scale off motor score from baseline to week 39 on blinded video rating.
Twenty patients were randomized to receive transdermal nicotine, and 20 patients were randomized to a control group. The change in motor score between groups was not statistically significant. Change in quality of life assessed by the Parkinson’s Disease Questionnaire was not statistically significant. At 39 weeks, a reduction in levodopa doses, a reduction in dyskinesias, and an improvement in activities of daily living were observed in the nicotine group, the researchers said.Adverse events occurred in more patients in the nicotine group than in the control group. They included worsening of parkinsonism (30% vs 5%), cutaneous reactions (35% vs 5%), gastrointestinal complaints (65% vs 15%), hypotension (25% vs 5%), insomnia (25% vs 5%), and nervousness and anxiety (20% vs 0%). Ten patients in the nicotine group had a serious adverse event, compared with three patients in the control group.
—Jake Remaly
PORTLAND, OR—High doses of transdermal nicotine failed to improve off motor symptoms in patients with Parkinson’s disease, according to trial results presented at the Fourth World Parkinson Congress. Nicotine may have provided benefit on secondary outcome measures, researchers said.
Gabriel Villafane, MD, a neurologist at Henri Mondor University Hospital in Créteil, France, and colleagues conducted the Nicopark2 Study, a single-blind, controlled, randomized trial to evaluate the effect of high doses of transdermal nicotine (approximately 90 mg per day) on motor symptoms in Parkinson’s disease. Cigarette smoking is associated with a dose-dependent reduction in risk of Parkinson’s disease. Nicotine’s effect on motor symptoms in Parkinson’s disease is controversial. Seven of eight open-label studies suggested that nicotine improves motor symptoms, but four placebo-controlled studies were negative.
The investigators enrolled 40 patients with Parkinson’s disease in the study. Eligible patients were nonsmokers age 35 to 70 with a Hoehn and Yahr off stage of four or less and a Hoehn and Yahr on stage of three or less. Patients had received levodopa treatment for at least three years. Exclusion criteria included neurosurgery, psychiatric disease, and symptomatic orthostatic hypotension.
The primary outcome was mean difference between groups in change of Unified Parkinson’s Disease Rating Scale off motor score from baseline to week 39 on blinded video rating.
Twenty patients were randomized to receive transdermal nicotine, and 20 patients were randomized to a control group. The change in motor score between groups was not statistically significant. Change in quality of life assessed by the Parkinson’s Disease Questionnaire was not statistically significant. At 39 weeks, a reduction in levodopa doses, a reduction in dyskinesias, and an improvement in activities of daily living were observed in the nicotine group, the researchers said.Adverse events occurred in more patients in the nicotine group than in the control group. They included worsening of parkinsonism (30% vs 5%), cutaneous reactions (35% vs 5%), gastrointestinal complaints (65% vs 15%), hypotension (25% vs 5%), insomnia (25% vs 5%), and nervousness and anxiety (20% vs 0%). Ten patients in the nicotine group had a serious adverse event, compared with three patients in the control group.
—Jake Remaly