User login
Leadless cardiac pacing: What primary care providers and non-EP cardiologists should know
WHY LEADLESS PACING?
The first clinical implantation of a cardiac pacemaker was performed surgically in 1958 by Drs. Elmvist and Senning via thoracotomy and direct attachment of electrodes to the myocardium. Transvenous pacing was introduced in 1962 by Drs. Lagergren, Parsonnet, and Welti.1,2 The general configuration of transvenous leads connected to a pulse generator situated in a surgical pocket has remained the standard of care ever since. Despite almost 60 years of technological innovation, contemporary permanent transvenous pacing continues to carry significant short- and long-term morbidity. Long-term composite complication rates are estimated at over 10%,3 further stratified as 12% in the 2 months post-implant (short-term) and 9% thereafter (long-term).4 Transvenous pacing complications are associated with an increase in both hospitalization days (hazard ratio 2.3) and unique hospitalizations (hazard ratio 4.4).5
Short-term complications
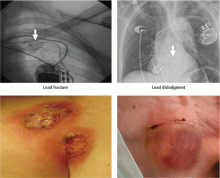
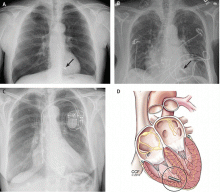
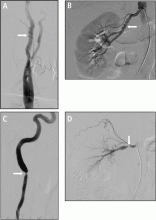
Short-term complications include lead dislodgment, pocket hematoma, pericardial effusion, and pneumothorax (Figure 1). Pocket hematomas are common with concurrent antiplatelet or anticoagulant administration, with incidence estimates varying from 5% to 33% depending on the definition.6 Morbidity associated with pocket hematoma include prolonged hospitalization, need for re-operation,7 and an almost eightfold increase in the rate of device infection over the long term compared with patients without pocket hematoma.8 New pericardial effusions after implant may affect up to 10% of patients; they are generally small, including 90% attributable to pericarditis or contained microperforation not requiring intervention. Overt lead perforation resulting in cardiac tamponade occurs in about 1% of transvenous pacemaker implants, of which 10% (0.1% overall) require open chest surgery, with the remainder treated with percutaneous drainage.9
Long-term complications
Long-term complications are predominantly lead and pocket-related but also include venous occlusive disease and tricuspid valve pathology.4 The development of primary lead failure due to insulation defects, conductor fracture, or dislodgment has been associated with major adverse events in 16% of patients, and an additional 6% if transvenous lead extraction is needed, which can rarely lead to hemorrhagic death by vascular tears involving the heart or superior vena cava.10 Fibrous tissue growth around the indwelling vascular leads can result in venous obstruction present in up to 14% of patients by 6 months after implant.11 This increases to 26% by the time of device replacement or upgrade, which is typically 5 to 10 years after the original implant, including 17% of patients with a complete venous occlusion.12 In addition, worsened tricuspid regurgitation due to lead impingement on the valve is seen in 7% to 40% of patients depending on definitions,13 with post-implant severe tricuspid regurgitation independently associated with increased mortality risk.14 The rate of device infection is 1% to 2% at 1 year,8,15 and 3% over the lifetime of the initial transvenous system; this increases to more than 10% after generator replacement.16
LEADLESS PACING TECHNOLOGY
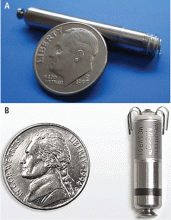
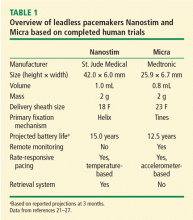
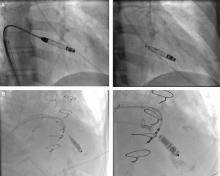
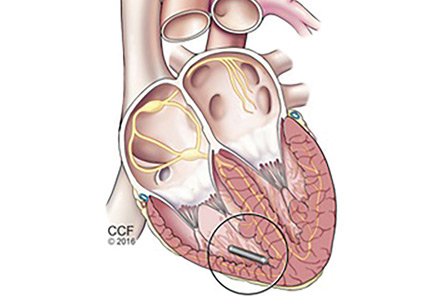
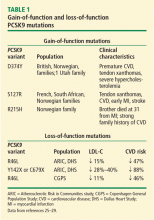
The principal goal of leadless pacing is to reduce short- and long-term pacemaker complications by eliminating the two most common sources of problems: the transvenous leads and the surgical pocket. Discussion of leadless pacing strategies began as early as 1970.17 Although several preclinical studies demonstrated efficacy with leadless prototypes,18–20 clinical implementation of fully leadless technology did not occur until recently. As shown in Figure 2, there are now two commercially available leadless pacing devices: Nanostim (St. Jude Medical Inc., St. Paul, MN) and Micra (Medtronic Inc., Dublin, Ireland). At the time of this writing, both have commercial approval in Europe. In the United States, Micra received commercial approval from the US Food and Drug Administration on April 6, 2016, with a similar decision expected on Nanostim. The current approved indications for leadless pacing are chronic atrial tachyarrhythmia with advanced atrioventricular (AV) block; advanced AV block with low level of physical activity or short expected lifespan; and infrequent pauses or unexplained syncope with abnormal findings at electrophysiologic study. Although differences exist between Nanostim and Micra, as shown in Table 1,21–27 there are fundamental similarities. Both are single-unit designs encapsulating the electrodes and pulse generator with rate-adaptive functionality. Both are delivered via an endovascular femoral venous approach without the need for incisional access, transvenous leads, or surgical pocket (Figures 3 and 4).21–27
Nanostim: Landmark trials
As the world’s first-in-man leadless pacemaker, Nanostim was evaluated in two prospective, non-randomized, multicenter, single-arm trials abbreviated LEADLESS22 and LEADLESS II.24 The first human feasibility study, LEADLESS, enrolled 33 patients with approved indications for ventricular-only pacing while excluding patients with expected pacemaker dependency. The most common indication was bradycardia in the presence of persistent atrial arrhythmias, thereby obviating the need for atrial pacing. The primary outcome was freedom from serious complications at 90 days. The secondary outcomes were implant success rate and device performance at 3 months. The results demonstrated 94% composite safety (31 of 33 patients) at 3 months. There was one cardiac perforation leading to tamponade and eventually death after prolonged hospitalization, and one inadvertent deployment into the left ventricle via patent foramen ovale that was successfully retrieved and redeployed without complication. The implant success rate was 97%, and the electrical parameters involving sensing, pacing thresholds, and impedance were as expected at 3 months. Results of 1-year follow-up were published for the LEADLESS cohort,25 revealing no additional complications from 3 to 12 months, no adverse changes in electrical performance parameters, and 100% effectiveness of rate-responsive programming.
The subsequent LEADLESS II trial enrolled 526 patients but did not exclude patients with expected pacemaker dependency, and its results were reported in a preplanned interim analysis when 300 patients had reached 6 months of follow-up (mean follow-up 6.9 ± 4.2 months).24 The primary efficacy end point involved electrical performance including capture thresholds and sensing. Initial deployment success was 96% with expected electrical parameters at implant that were stable at 6 months of follow-up. The rate of freedom from serious adverse events was 93%, with complications including device dislodgment (1.7%, mean 8 ± 6 days after implant), perforation (1.3%), performance deficiency requiring device retrieval and replacement (1.3%), and groin complications (1.3%). There were no device-related deaths, and all device dislodgments were successfully treated percutaneously.
There was no prospective control arm involving transvenous pacing in either the LEADLESS or LEADLESS II trial. Thus, in an effort to compare Nanostim (n = 718) vs transvenous pacing, complication rates were calculated for a propensity-matched registry cohort of 10,521 transvenous patients, and differences were reported.26 At 1 month, the composite complication rate was 5.8% for Nanostim (1.5% pericardial effusion, 1% dislodgment) and 12.7% for transvenous pacing (7.6% lead-related, 3.9% thoracic trauma, infection 1.9%) (P < .001). Between 1 month and 2 years, complication rates were only 0.6% for Nanostim vs 5.4% for transvenous pacing (P < .001). This lower complication rate at 2 years was driven almost entirely by a 2.6% infection rate and 2.4% lead-complication rate in the transvenous pacemaker group, nonexistent in the leadless group.
Micra: Landmark trials
Micra was evaluated in a prospective, nonrandomized, multicenter, single-arm trial, enrolling 725 patients with indications for ventricular-only pacing; approximately two-thirds of the cohort had bradycardia in the presence of persistent atrial arrhythmias, similar to the Nanostim cohort.27 The efficacy end point was stable capture threshold at 6 months. The safety end point was freedom from major complications resulting in new or prolonged hospitalization at 6 months. The implant success rate was 99%, and 98% of patients met the primary efficacy end point. The safety end point was met in 96% of patients. Complications included perforation or pericardial effusion (1.6%), groin complication (0.7%), elevated threshold (0.3%), venous thromboembolism (0.3%), and others (1.7%). No dislodgments were reported. There was no prospective, randomized control arm to compare Micra and transvenous pacing. A post hoc analysis was performed comparing major complication rates in this study with an unmatched 2,667-patient meta-analysis control cohort.27 The hazard ratio for the leadless pacing strategy was calculated at 0.49 (95% confidence interval 0.33 to 0.75, P = .001) with absolute risk reduction 3.4% at 6 months resulting in a number needed to treat of 29.4 patients. Further broken down, Micra patients compared with the control cohort had reduced rates of both subsequent hospitalizations (3.9% to 2.3%) and device revisions (3.5% to 0.4%).
ADVANTAGES OF LEADLESS PACING
As discussed above, the major observed benefit with both Nanostim and Micra compared with transvenous cohorts is the elimination of lead and pocket-related complications.25,27 Leadless pacing introduces a new 1% to 2% groin complication rate for both devices not present with transvenous pacing, and also a 1% device dislodgment rate in the case of Nanostim (all dislodgments were treated percutaneously). Data from both clinical trials suggest that the complication rates are largely compressed acutely. In contrast, there are considerable mid-term and long-term complications for transvenous systems.3–5 Indeed, the mid- to long-term window is where leadless pacing is expected to have the most favorable impact. As with any new disruptive technology, operator experience may be important, as evidenced by a near halving of the complication rate observed in the LEADLESS II trial after gaining the experience of 10 implants.25
Other benefits of leadless pacing include a generally quick procedure (average implant time was 30 minutes in LEADLESS and LEADLESS II)22,25 and full shoulder mobility afterwards, so that patients can resume driving once groin soreness has subsided, typically within a few days. (Current studies are investigating whether immediate shoulder mobility with leadless pacing is beneficial to older patients suffering from arthritis.) The lack of an incision allows patients to bathe and shower as soon as they desire, whereas after transvenous pacemaker implant, motion in the affected shoulder is usually restricted for several weeks to avoid lead dislodgment, and showering and bathing are restricted to avoid contamination of the incision with nonsterile tap water. (In some cases, a tightly adherent waterproof dressing can be used.) The leadless systems were designed for compatibility with magnetic resonance imaging (MRI), whereas not all transvenous pacemaker generators and leads are MRI compatible.
Leadless devices are not expected to span the tricuspid valve to create incident or worsening tricuspid regurgitation. In a recent small study of 22 patients undergoing Micra implant, there were no new cases of severe tricuspid regurgitation after the procedure, with only a 9% increase in mild and 5% increase in moderate tricuspid regurgitation,28 vs a rate of 40% of worsening tricuspid regurgitation and 10% of new severe tricuspid regurgitation with transvenous pacing.13,14
Transvenous pacemaker implant requires surgery for pulse generator exchange at a mean of 7 years, a procedure carrying significant risk of short- and long-term complications.10
END-OF-SERVICE QUESTIONS: ATTEMPT RETRIEVAL OR NOT?
Both leadless systems have favorable projected in-service battery life: a reported 15.0 years for Nanostim25 and mean 12.5 years for Micra.27 The inevitable question is what to do then. The Nanostim system was designed to be retrievable using a dedicated catheter system. Micra was not designed with an accompanying retrieval system. Pathologic examinations of leadless devices at autopsy or after explant have revealed a range of device endothelialization, from partial at 19 months to full at 4 months.29,30
As of this writing, no extraction complications have been observed with Nanostim explants up to 506 days after implant (n = 12, mean 197 days after implant).31 Needless to say, there is not yet enough experience worldwide with either system to know what the end-of-service will look like in 10 to 15 years. One strategy could involve first attempting percutaneous retrieval and replacement, if retrieval is not possible, abandoning the old device while implanting a new device alongside. Another strategy would be to forgo a retrieval attempt altogether. In the LEADLESS II study,24 the mean patient age was 75. In this cohort, forgoing elective retrieval for those who live to reach the end of pacemaker service between the age of 85 and 90 would seem reasonable assuming the next device provides similar longevity. For younger patients, careful consideration of long-term strategies is needed. It is not known what the replacement technology will look like in another decade with respect to device size or battery longevity. Preclinical studies using swine and human cadaver hearts have demonstrated the feasibility of multiple right-ventricular Micra implants without affecting cardiac function.32,33
OTHER LIMITATIONS AND CAUTIONARY NOTES
At present, leadless pacing is approved for single-chamber right-ventricular pacing. In the developed world, single right-ventricular pacing modes account for only 20% to 30% of new pacemaker implants, which total more than 1 million per year worldwide.34,35 As with any new technology, the up-front cost of leadless pacemaker implant is expected to be significantly higher than transvenous systems, which at this point remains poorly defined, as the field has not caught up in terms of charges, reimbursement, and billing codes. While those concerns fall outside the scope of this review, it is not known if the expected reductions in mid- and long-term complications will make up for an up-front cost difference. However, a cost-efficacy study reported that one complication of a transvenous pacemaker system was more expensive than the initial implant itself.36 The longest-term follow-up data currently available are with Nanostim, showing an absolute complication reduction of 11.7% at 2 years,24 a disparity only expected to widen with prolonged follow-up, particularly after transvenous generator exchange, when complication rates rapidly escalate.
FUTURE DIRECTIONS
The next horizon of leadless technology will be for right-atrial and dual-chamber pacing to treat the far more pervasive pacing indication of sinus node dysfunction with or without AV block. In the latter application, the two devices will communicate. Prototypes and early nonhuman evaluations are ongoing for both. Leadless pacing is also being investigated for use in tachycardia. Tjong et al37 reported on the safety and feasibility of an entirely leadless pacemaker plus an implantable cardioverter-defibrillator (ICD) system in two sheep and one human using both Nanostim and subcutaneous ICD. Currently, two important limitations of subcutaneous ICD are its inability to provide backup bradycardia and antitachycardia pacing (it provides only defibrillation). The EMBLEM PACE study will enroll 250 patients to receive a leadless pacemaker and Emblem subcutaneous ICD (Boston Scientific, Boston, MA), with patients subsequently receiving commanded antitachycardia pacing for ventricular arrhythmias and bradycardia pacing provided by the leadless device as indicated.
CONCLUSIONS
Leadless cardiac pacing is a safe and efficacious alternative to standard transvenous pacing systems. Although long-term data are limited, available short- and mid-term data show that the elimination of transvenous leads and the surgical pocket results in significant reductions in complication rates. Currently, leadless pacing is approved only for right-ventricular pacing, but investigation of right-atrial, dual-chamber, and fully leadless pacemaker-defibrillator hybrid systems is ongoing.
- Lagergren H. How it happened: my recollection of early pacing. Pacing Clin Electrophysiol 1978; 1:140–143.
- Parsonnet V. Permanent transvenous pacing in 1962. Pacing Clin Electrophysiol 1978; 1:265–268.
- Kirkfeldt RE, Johansen JB, Nohr EA, Jorgensen OD, Nielsen JC. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J 2014; 35:1186–1194.
- Udo EO, Zuithoff NP, van Hemel NM, et al. Incidence and predictors of short- and long-term complications in pacemaker therapy: the FOLLOWPACE study. Heart Rhythm 2012; 9:728–735.
- Palmisano P, Accogli M, Zaccaria M, et al. Rate, causes, and impact on patient outcome of implantable device complications requiring surgical revision: large population survey from two centres in Italy. Europace 2013; 15:531–540.
- De Sensi F, Miracapillo G, Cresti A, Severi S, Airaksinen KE. Pocket hematoma: a call for definition. Pacing Clin Electrophysiol Aug 2015; 38:909–913.
- Wiegand UK, LeJeune D, Boguschewski F, et al. Pocket hematoma after pacemaker or implantable cardioverter defibrillator surgery: influence of patient morbidity, operation strategy, and perioperative antiplatelet/anticoagulation therapy. Chest 2004; 126:1177–1186.
- Essebag V, Verma A, Healey JS, et al. Clinically significant pocket hematoma increases long-term risk of device infection: Bruise Control Infection Study. J Am Coll Cardiol 2016; 67:1300–1308.
- Ohlow MA, Lauer B, Brunelli M, Geller JC. Incidence and predictors of pericardial effusion after permanent heart rhythm device implantation: prospective evaluation of 968 consecutive patients. Circ J 2013; 77:975–981.
- Hauser RG, Hayes DL, Kallinen LM, et al. Clinical experience with pacemaker pulse generators and transvenous leads: an 8-year prospective multicenter study. Heart Rhythm 2007; 4:154–160.
- Korkeila P, Nyman K, Ylitalo A, et al. Venous obstruction after pacemaker implantation. Pacing Clin Electrophysiol 2007; 30:199–206.
- Haghjoo M, Nikoo MH, Fazelifar AF, Alizadeh A, Emkanjoo Z, Sadr-Ameli MA. Predictors of venous obstruction following pacemaker or implantable cardioverter-defibrillator implantation: a contrast venographic study on 100 patients admitted for generator change, lead revision, or device upgrade. Europace 2007; 9:328–332.
- Al-Mohaissen MA, Chan KL. Prevalence and mechanism of tricuspid regurgitation following implantation of endocardial leads for pacemaker or cardioverter-defibrillator. J Am Soc Echocardiogr 2012; 25:245–252.
- Al-Bawardy R, Krishnaswamy A, Rajeswaran J, et al. Tricuspid regurgitation and implantable devices. Pacing Clin Electrophysiol 2015; 38:259–266.
- Klug D, Balde M, Pavin D, et al. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation 2007; 116:1349–1355.
- Johansen JB, Jorgensen OD, Moller M, Arnsbo P, Mortensen PT, Nielsen JC. Infection after pacemaker implantation: infection rates and risk factors associated with infection in a population-based cohort study of 46,299 consecutive patients. Eur Heart J 2011; 32:991–998.
- Lown B, Kosowsky BD. Artificial cardiac pacemakers. I. N Engl J Med 1970; 283:907–916.
- Spickler JW, Rasor NS, Kezdi P, Misra SN, Robins KE, LeBoeuf C. Totally self-contained intracardiac pacemaker. J Electrocardiol 1970; 3:325–331.
- Sutton R. The first European journal on cardiac electrophysiology and pacing, the European Journal of Cardiac Pacing and Electrophysiology. Europace 2011; 13:1663–1664.
- Vardas PE, Politopoulous C, Manios E, Parthenakis F, Tsagarkis C. A miniature pacemaker introduced intravenously and implanted endocardially. Preliminary findings from an experimental study. Eur J Card Pacing Electrophysiol 1991; 1:27–30.
- Eggen MD, Grubac V, Bonner MD. Design and evaluation of a novel fixation mechanism for a transcatheter pacemaker. IEEE Trans Biomed Eng 2015; 62:2316–2323.
- Reddy VY, Knops RE, Sperzel J, et al. Permanent leadless cardiac pacing: results of the LEADLESS trial. Circulation 2014; 129:1466–1471.
- Ritter P, Duray GZ, Steinwender C, et al. Early performance of a miniaturized leadless cardiac pacemaker: the Micra Transcatheter Pacing Study. Eur Heart J 2015; 36:2510–2519.
- Reddy VY, Exner DV, Cantillon DJ, et al. Percutaneous implantation of an entirely intracardiac leadless pacemaker. N Engl J Med 2015; 373:1125–1135.
- Knops RE, Tjong FV, Neuzil P, et al. Chronic performance of a leadless cardiac pacemaker: 1-year follow-up of the LEADLESS trial. J Am Coll Cardiol 2015; 65:1497–1504.
- Reddy VY, Cantillon DJ, Ip J, et al. A comparative study of acute and mid-term complications of leadless versus transvenous pacemakers. Heart Rhythm 2016 July. [Epub ahead of print].
- Reynolds D, Duray GZ, Omar R, et al. A leadless intracardiac transcatheter pacing system. N Engl J Med 2016; 374:533–541.
- Garikipati NV, Karve A, Okabe T, et al. Tricuspid regurgitation after leadless pacemaker implantation. Abstract presented at Heart Rhythm Society Scientific Sessions, May 4–7, 2016, San Francisco, CA.
- Tjong FV, Stam OC, van der Wal AC, et al. Postmortem histopathological examination of a leadless pacemaker shows partial encapsulation after 19 months. Circ Arrhythm Electrophysiol 2015; 8:1293–1295.
- Borgquist R, Ljungstrom E, Koul B, Hoijer CJ. Leadless Medtronic Micra pacemaker almost completely endothelialized already after 4 months: first clinical experience from an explanted heart. Eur Heart J 2016; 37:2503.
- Reddy VY, Knops RE, Defaye P, et al. Worldwide clinical experience of the retrieval of leadless cardiac pacemakers. Abstract presented at Heart Rhythm Society Scientific Sessions, May 4–7, 2016, San Francisco, CA.
- Chen K, Zheng X, Dai Y, et al. Multiple leadless pacemakers implanted in the right ventricle of swine. Europace 2016 January 31. pii: euv418. [Epub ahead of print].
- Omdahl P, Eggen MD, Bonner MD, Iaizzo PA, Wika K. Right ventricular anatomy can accommodate multiple micra transcatheter pacemakers. Pacing Clin Electrophysiol 2016; 39:393–397.
- Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009—a World Society of Arrhythmia’s project. Pacing Clin Electrophysiol 2011; 34:1013–1027.
- Epstein AE, DiMarco JP, Ellenbogen KA, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Heart Rhythm Society. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2013; 127:e283–352.
- Tobin K, Stewart J, Westveer D, Frumin H. Acute complications of permanent pacemaker implantation: their financial implication and relation to volume and operator experience. Am J Cardiol 2000; 85:774–776, A9.
- Tjong FV, Brouwer TF, Smeding L, et al. Combined leadless pacemaker and subcutaneous implantable defibrillator therapy: feasibility, safety, and performance. Europace 2016 March 3. [Epub ahead of print].
WHY LEADLESS PACING?
The first clinical implantation of a cardiac pacemaker was performed surgically in 1958 by Drs. Elmvist and Senning via thoracotomy and direct attachment of electrodes to the myocardium. Transvenous pacing was introduced in 1962 by Drs. Lagergren, Parsonnet, and Welti.1,2 The general configuration of transvenous leads connected to a pulse generator situated in a surgical pocket has remained the standard of care ever since. Despite almost 60 years of technological innovation, contemporary permanent transvenous pacing continues to carry significant short- and long-term morbidity. Long-term composite complication rates are estimated at over 10%,3 further stratified as 12% in the 2 months post-implant (short-term) and 9% thereafter (long-term).4 Transvenous pacing complications are associated with an increase in both hospitalization days (hazard ratio 2.3) and unique hospitalizations (hazard ratio 4.4).5
Short-term complications
Short-term complications include lead dislodgment, pocket hematoma, pericardial effusion, and pneumothorax (Figure 1). Pocket hematomas are common with concurrent antiplatelet or anticoagulant administration, with incidence estimates varying from 5% to 33% depending on the definition.6 Morbidity associated with pocket hematoma include prolonged hospitalization, need for re-operation,7 and an almost eightfold increase in the rate of device infection over the long term compared with patients without pocket hematoma.8 New pericardial effusions after implant may affect up to 10% of patients; they are generally small, including 90% attributable to pericarditis or contained microperforation not requiring intervention. Overt lead perforation resulting in cardiac tamponade occurs in about 1% of transvenous pacemaker implants, of which 10% (0.1% overall) require open chest surgery, with the remainder treated with percutaneous drainage.9
Long-term complications
Long-term complications are predominantly lead and pocket-related but also include venous occlusive disease and tricuspid valve pathology.4 The development of primary lead failure due to insulation defects, conductor fracture, or dislodgment has been associated with major adverse events in 16% of patients, and an additional 6% if transvenous lead extraction is needed, which can rarely lead to hemorrhagic death by vascular tears involving the heart or superior vena cava.10 Fibrous tissue growth around the indwelling vascular leads can result in venous obstruction present in up to 14% of patients by 6 months after implant.11 This increases to 26% by the time of device replacement or upgrade, which is typically 5 to 10 years after the original implant, including 17% of patients with a complete venous occlusion.12 In addition, worsened tricuspid regurgitation due to lead impingement on the valve is seen in 7% to 40% of patients depending on definitions,13 with post-implant severe tricuspid regurgitation independently associated with increased mortality risk.14 The rate of device infection is 1% to 2% at 1 year,8,15 and 3% over the lifetime of the initial transvenous system; this increases to more than 10% after generator replacement.16
LEADLESS PACING TECHNOLOGY
The principal goal of leadless pacing is to reduce short- and long-term pacemaker complications by eliminating the two most common sources of problems: the transvenous leads and the surgical pocket. Discussion of leadless pacing strategies began as early as 1970.17 Although several preclinical studies demonstrated efficacy with leadless prototypes,18–20 clinical implementation of fully leadless technology did not occur until recently. As shown in Figure 2, there are now two commercially available leadless pacing devices: Nanostim (St. Jude Medical Inc., St. Paul, MN) and Micra (Medtronic Inc., Dublin, Ireland). At the time of this writing, both have commercial approval in Europe. In the United States, Micra received commercial approval from the US Food and Drug Administration on April 6, 2016, with a similar decision expected on Nanostim. The current approved indications for leadless pacing are chronic atrial tachyarrhythmia with advanced atrioventricular (AV) block; advanced AV block with low level of physical activity or short expected lifespan; and infrequent pauses or unexplained syncope with abnormal findings at electrophysiologic study. Although differences exist between Nanostim and Micra, as shown in Table 1,21–27 there are fundamental similarities. Both are single-unit designs encapsulating the electrodes and pulse generator with rate-adaptive functionality. Both are delivered via an endovascular femoral venous approach without the need for incisional access, transvenous leads, or surgical pocket (Figures 3 and 4).21–27
Nanostim: Landmark trials
As the world’s first-in-man leadless pacemaker, Nanostim was evaluated in two prospective, non-randomized, multicenter, single-arm trials abbreviated LEADLESS22 and LEADLESS II.24 The first human feasibility study, LEADLESS, enrolled 33 patients with approved indications for ventricular-only pacing while excluding patients with expected pacemaker dependency. The most common indication was bradycardia in the presence of persistent atrial arrhythmias, thereby obviating the need for atrial pacing. The primary outcome was freedom from serious complications at 90 days. The secondary outcomes were implant success rate and device performance at 3 months. The results demonstrated 94% composite safety (31 of 33 patients) at 3 months. There was one cardiac perforation leading to tamponade and eventually death after prolonged hospitalization, and one inadvertent deployment into the left ventricle via patent foramen ovale that was successfully retrieved and redeployed without complication. The implant success rate was 97%, and the electrical parameters involving sensing, pacing thresholds, and impedance were as expected at 3 months. Results of 1-year follow-up were published for the LEADLESS cohort,25 revealing no additional complications from 3 to 12 months, no adverse changes in electrical performance parameters, and 100% effectiveness of rate-responsive programming.
The subsequent LEADLESS II trial enrolled 526 patients but did not exclude patients with expected pacemaker dependency, and its results were reported in a preplanned interim analysis when 300 patients had reached 6 months of follow-up (mean follow-up 6.9 ± 4.2 months).24 The primary efficacy end point involved electrical performance including capture thresholds and sensing. Initial deployment success was 96% with expected electrical parameters at implant that were stable at 6 months of follow-up. The rate of freedom from serious adverse events was 93%, with complications including device dislodgment (1.7%, mean 8 ± 6 days after implant), perforation (1.3%), performance deficiency requiring device retrieval and replacement (1.3%), and groin complications (1.3%). There were no device-related deaths, and all device dislodgments were successfully treated percutaneously.
There was no prospective control arm involving transvenous pacing in either the LEADLESS or LEADLESS II trial. Thus, in an effort to compare Nanostim (n = 718) vs transvenous pacing, complication rates were calculated for a propensity-matched registry cohort of 10,521 transvenous patients, and differences were reported.26 At 1 month, the composite complication rate was 5.8% for Nanostim (1.5% pericardial effusion, 1% dislodgment) and 12.7% for transvenous pacing (7.6% lead-related, 3.9% thoracic trauma, infection 1.9%) (P < .001). Between 1 month and 2 years, complication rates were only 0.6% for Nanostim vs 5.4% for transvenous pacing (P < .001). This lower complication rate at 2 years was driven almost entirely by a 2.6% infection rate and 2.4% lead-complication rate in the transvenous pacemaker group, nonexistent in the leadless group.
Micra: Landmark trials
Micra was evaluated in a prospective, nonrandomized, multicenter, single-arm trial, enrolling 725 patients with indications for ventricular-only pacing; approximately two-thirds of the cohort had bradycardia in the presence of persistent atrial arrhythmias, similar to the Nanostim cohort.27 The efficacy end point was stable capture threshold at 6 months. The safety end point was freedom from major complications resulting in new or prolonged hospitalization at 6 months. The implant success rate was 99%, and 98% of patients met the primary efficacy end point. The safety end point was met in 96% of patients. Complications included perforation or pericardial effusion (1.6%), groin complication (0.7%), elevated threshold (0.3%), venous thromboembolism (0.3%), and others (1.7%). No dislodgments were reported. There was no prospective, randomized control arm to compare Micra and transvenous pacing. A post hoc analysis was performed comparing major complication rates in this study with an unmatched 2,667-patient meta-analysis control cohort.27 The hazard ratio for the leadless pacing strategy was calculated at 0.49 (95% confidence interval 0.33 to 0.75, P = .001) with absolute risk reduction 3.4% at 6 months resulting in a number needed to treat of 29.4 patients. Further broken down, Micra patients compared with the control cohort had reduced rates of both subsequent hospitalizations (3.9% to 2.3%) and device revisions (3.5% to 0.4%).
ADVANTAGES OF LEADLESS PACING
As discussed above, the major observed benefit with both Nanostim and Micra compared with transvenous cohorts is the elimination of lead and pocket-related complications.25,27 Leadless pacing introduces a new 1% to 2% groin complication rate for both devices not present with transvenous pacing, and also a 1% device dislodgment rate in the case of Nanostim (all dislodgments were treated percutaneously). Data from both clinical trials suggest that the complication rates are largely compressed acutely. In contrast, there are considerable mid-term and long-term complications for transvenous systems.3–5 Indeed, the mid- to long-term window is where leadless pacing is expected to have the most favorable impact. As with any new disruptive technology, operator experience may be important, as evidenced by a near halving of the complication rate observed in the LEADLESS II trial after gaining the experience of 10 implants.25
Other benefits of leadless pacing include a generally quick procedure (average implant time was 30 minutes in LEADLESS and LEADLESS II)22,25 and full shoulder mobility afterwards, so that patients can resume driving once groin soreness has subsided, typically within a few days. (Current studies are investigating whether immediate shoulder mobility with leadless pacing is beneficial to older patients suffering from arthritis.) The lack of an incision allows patients to bathe and shower as soon as they desire, whereas after transvenous pacemaker implant, motion in the affected shoulder is usually restricted for several weeks to avoid lead dislodgment, and showering and bathing are restricted to avoid contamination of the incision with nonsterile tap water. (In some cases, a tightly adherent waterproof dressing can be used.) The leadless systems were designed for compatibility with magnetic resonance imaging (MRI), whereas not all transvenous pacemaker generators and leads are MRI compatible.
Leadless devices are not expected to span the tricuspid valve to create incident or worsening tricuspid regurgitation. In a recent small study of 22 patients undergoing Micra implant, there were no new cases of severe tricuspid regurgitation after the procedure, with only a 9% increase in mild and 5% increase in moderate tricuspid regurgitation,28 vs a rate of 40% of worsening tricuspid regurgitation and 10% of new severe tricuspid regurgitation with transvenous pacing.13,14
Transvenous pacemaker implant requires surgery for pulse generator exchange at a mean of 7 years, a procedure carrying significant risk of short- and long-term complications.10
END-OF-SERVICE QUESTIONS: ATTEMPT RETRIEVAL OR NOT?
Both leadless systems have favorable projected in-service battery life: a reported 15.0 years for Nanostim25 and mean 12.5 years for Micra.27 The inevitable question is what to do then. The Nanostim system was designed to be retrievable using a dedicated catheter system. Micra was not designed with an accompanying retrieval system. Pathologic examinations of leadless devices at autopsy or after explant have revealed a range of device endothelialization, from partial at 19 months to full at 4 months.29,30
As of this writing, no extraction complications have been observed with Nanostim explants up to 506 days after implant (n = 12, mean 197 days after implant).31 Needless to say, there is not yet enough experience worldwide with either system to know what the end-of-service will look like in 10 to 15 years. One strategy could involve first attempting percutaneous retrieval and replacement, if retrieval is not possible, abandoning the old device while implanting a new device alongside. Another strategy would be to forgo a retrieval attempt altogether. In the LEADLESS II study,24 the mean patient age was 75. In this cohort, forgoing elective retrieval for those who live to reach the end of pacemaker service between the age of 85 and 90 would seem reasonable assuming the next device provides similar longevity. For younger patients, careful consideration of long-term strategies is needed. It is not known what the replacement technology will look like in another decade with respect to device size or battery longevity. Preclinical studies using swine and human cadaver hearts have demonstrated the feasibility of multiple right-ventricular Micra implants without affecting cardiac function.32,33
OTHER LIMITATIONS AND CAUTIONARY NOTES
At present, leadless pacing is approved for single-chamber right-ventricular pacing. In the developed world, single right-ventricular pacing modes account for only 20% to 30% of new pacemaker implants, which total more than 1 million per year worldwide.34,35 As with any new technology, the up-front cost of leadless pacemaker implant is expected to be significantly higher than transvenous systems, which at this point remains poorly defined, as the field has not caught up in terms of charges, reimbursement, and billing codes. While those concerns fall outside the scope of this review, it is not known if the expected reductions in mid- and long-term complications will make up for an up-front cost difference. However, a cost-efficacy study reported that one complication of a transvenous pacemaker system was more expensive than the initial implant itself.36 The longest-term follow-up data currently available are with Nanostim, showing an absolute complication reduction of 11.7% at 2 years,24 a disparity only expected to widen with prolonged follow-up, particularly after transvenous generator exchange, when complication rates rapidly escalate.
FUTURE DIRECTIONS
The next horizon of leadless technology will be for right-atrial and dual-chamber pacing to treat the far more pervasive pacing indication of sinus node dysfunction with or without AV block. In the latter application, the two devices will communicate. Prototypes and early nonhuman evaluations are ongoing for both. Leadless pacing is also being investigated for use in tachycardia. Tjong et al37 reported on the safety and feasibility of an entirely leadless pacemaker plus an implantable cardioverter-defibrillator (ICD) system in two sheep and one human using both Nanostim and subcutaneous ICD. Currently, two important limitations of subcutaneous ICD are its inability to provide backup bradycardia and antitachycardia pacing (it provides only defibrillation). The EMBLEM PACE study will enroll 250 patients to receive a leadless pacemaker and Emblem subcutaneous ICD (Boston Scientific, Boston, MA), with patients subsequently receiving commanded antitachycardia pacing for ventricular arrhythmias and bradycardia pacing provided by the leadless device as indicated.
CONCLUSIONS
Leadless cardiac pacing is a safe and efficacious alternative to standard transvenous pacing systems. Although long-term data are limited, available short- and mid-term data show that the elimination of transvenous leads and the surgical pocket results in significant reductions in complication rates. Currently, leadless pacing is approved only for right-ventricular pacing, but investigation of right-atrial, dual-chamber, and fully leadless pacemaker-defibrillator hybrid systems is ongoing.
WHY LEADLESS PACING?
The first clinical implantation of a cardiac pacemaker was performed surgically in 1958 by Drs. Elmvist and Senning via thoracotomy and direct attachment of electrodes to the myocardium. Transvenous pacing was introduced in 1962 by Drs. Lagergren, Parsonnet, and Welti.1,2 The general configuration of transvenous leads connected to a pulse generator situated in a surgical pocket has remained the standard of care ever since. Despite almost 60 years of technological innovation, contemporary permanent transvenous pacing continues to carry significant short- and long-term morbidity. Long-term composite complication rates are estimated at over 10%,3 further stratified as 12% in the 2 months post-implant (short-term) and 9% thereafter (long-term).4 Transvenous pacing complications are associated with an increase in both hospitalization days (hazard ratio 2.3) and unique hospitalizations (hazard ratio 4.4).5
Short-term complications
Short-term complications include lead dislodgment, pocket hematoma, pericardial effusion, and pneumothorax (Figure 1). Pocket hematomas are common with concurrent antiplatelet or anticoagulant administration, with incidence estimates varying from 5% to 33% depending on the definition.6 Morbidity associated with pocket hematoma include prolonged hospitalization, need for re-operation,7 and an almost eightfold increase in the rate of device infection over the long term compared with patients without pocket hematoma.8 New pericardial effusions after implant may affect up to 10% of patients; they are generally small, including 90% attributable to pericarditis or contained microperforation not requiring intervention. Overt lead perforation resulting in cardiac tamponade occurs in about 1% of transvenous pacemaker implants, of which 10% (0.1% overall) require open chest surgery, with the remainder treated with percutaneous drainage.9
Long-term complications
Long-term complications are predominantly lead and pocket-related but also include venous occlusive disease and tricuspid valve pathology.4 The development of primary lead failure due to insulation defects, conductor fracture, or dislodgment has been associated with major adverse events in 16% of patients, and an additional 6% if transvenous lead extraction is needed, which can rarely lead to hemorrhagic death by vascular tears involving the heart or superior vena cava.10 Fibrous tissue growth around the indwelling vascular leads can result in venous obstruction present in up to 14% of patients by 6 months after implant.11 This increases to 26% by the time of device replacement or upgrade, which is typically 5 to 10 years after the original implant, including 17% of patients with a complete venous occlusion.12 In addition, worsened tricuspid regurgitation due to lead impingement on the valve is seen in 7% to 40% of patients depending on definitions,13 with post-implant severe tricuspid regurgitation independently associated with increased mortality risk.14 The rate of device infection is 1% to 2% at 1 year,8,15 and 3% over the lifetime of the initial transvenous system; this increases to more than 10% after generator replacement.16
LEADLESS PACING TECHNOLOGY
The principal goal of leadless pacing is to reduce short- and long-term pacemaker complications by eliminating the two most common sources of problems: the transvenous leads and the surgical pocket. Discussion of leadless pacing strategies began as early as 1970.17 Although several preclinical studies demonstrated efficacy with leadless prototypes,18–20 clinical implementation of fully leadless technology did not occur until recently. As shown in Figure 2, there are now two commercially available leadless pacing devices: Nanostim (St. Jude Medical Inc., St. Paul, MN) and Micra (Medtronic Inc., Dublin, Ireland). At the time of this writing, both have commercial approval in Europe. In the United States, Micra received commercial approval from the US Food and Drug Administration on April 6, 2016, with a similar decision expected on Nanostim. The current approved indications for leadless pacing are chronic atrial tachyarrhythmia with advanced atrioventricular (AV) block; advanced AV block with low level of physical activity or short expected lifespan; and infrequent pauses or unexplained syncope with abnormal findings at electrophysiologic study. Although differences exist between Nanostim and Micra, as shown in Table 1,21–27 there are fundamental similarities. Both are single-unit designs encapsulating the electrodes and pulse generator with rate-adaptive functionality. Both are delivered via an endovascular femoral venous approach without the need for incisional access, transvenous leads, or surgical pocket (Figures 3 and 4).21–27
Nanostim: Landmark trials
As the world’s first-in-man leadless pacemaker, Nanostim was evaluated in two prospective, non-randomized, multicenter, single-arm trials abbreviated LEADLESS22 and LEADLESS II.24 The first human feasibility study, LEADLESS, enrolled 33 patients with approved indications for ventricular-only pacing while excluding patients with expected pacemaker dependency. The most common indication was bradycardia in the presence of persistent atrial arrhythmias, thereby obviating the need for atrial pacing. The primary outcome was freedom from serious complications at 90 days. The secondary outcomes were implant success rate and device performance at 3 months. The results demonstrated 94% composite safety (31 of 33 patients) at 3 months. There was one cardiac perforation leading to tamponade and eventually death after prolonged hospitalization, and one inadvertent deployment into the left ventricle via patent foramen ovale that was successfully retrieved and redeployed without complication. The implant success rate was 97%, and the electrical parameters involving sensing, pacing thresholds, and impedance were as expected at 3 months. Results of 1-year follow-up were published for the LEADLESS cohort,25 revealing no additional complications from 3 to 12 months, no adverse changes in electrical performance parameters, and 100% effectiveness of rate-responsive programming.
The subsequent LEADLESS II trial enrolled 526 patients but did not exclude patients with expected pacemaker dependency, and its results were reported in a preplanned interim analysis when 300 patients had reached 6 months of follow-up (mean follow-up 6.9 ± 4.2 months).24 The primary efficacy end point involved electrical performance including capture thresholds and sensing. Initial deployment success was 96% with expected electrical parameters at implant that were stable at 6 months of follow-up. The rate of freedom from serious adverse events was 93%, with complications including device dislodgment (1.7%, mean 8 ± 6 days after implant), perforation (1.3%), performance deficiency requiring device retrieval and replacement (1.3%), and groin complications (1.3%). There were no device-related deaths, and all device dislodgments were successfully treated percutaneously.
There was no prospective control arm involving transvenous pacing in either the LEADLESS or LEADLESS II trial. Thus, in an effort to compare Nanostim (n = 718) vs transvenous pacing, complication rates were calculated for a propensity-matched registry cohort of 10,521 transvenous patients, and differences were reported.26 At 1 month, the composite complication rate was 5.8% for Nanostim (1.5% pericardial effusion, 1% dislodgment) and 12.7% for transvenous pacing (7.6% lead-related, 3.9% thoracic trauma, infection 1.9%) (P < .001). Between 1 month and 2 years, complication rates were only 0.6% for Nanostim vs 5.4% for transvenous pacing (P < .001). This lower complication rate at 2 years was driven almost entirely by a 2.6% infection rate and 2.4% lead-complication rate in the transvenous pacemaker group, nonexistent in the leadless group.
Micra: Landmark trials
Micra was evaluated in a prospective, nonrandomized, multicenter, single-arm trial, enrolling 725 patients with indications for ventricular-only pacing; approximately two-thirds of the cohort had bradycardia in the presence of persistent atrial arrhythmias, similar to the Nanostim cohort.27 The efficacy end point was stable capture threshold at 6 months. The safety end point was freedom from major complications resulting in new or prolonged hospitalization at 6 months. The implant success rate was 99%, and 98% of patients met the primary efficacy end point. The safety end point was met in 96% of patients. Complications included perforation or pericardial effusion (1.6%), groin complication (0.7%), elevated threshold (0.3%), venous thromboembolism (0.3%), and others (1.7%). No dislodgments were reported. There was no prospective, randomized control arm to compare Micra and transvenous pacing. A post hoc analysis was performed comparing major complication rates in this study with an unmatched 2,667-patient meta-analysis control cohort.27 The hazard ratio for the leadless pacing strategy was calculated at 0.49 (95% confidence interval 0.33 to 0.75, P = .001) with absolute risk reduction 3.4% at 6 months resulting in a number needed to treat of 29.4 patients. Further broken down, Micra patients compared with the control cohort had reduced rates of both subsequent hospitalizations (3.9% to 2.3%) and device revisions (3.5% to 0.4%).
ADVANTAGES OF LEADLESS PACING
As discussed above, the major observed benefit with both Nanostim and Micra compared with transvenous cohorts is the elimination of lead and pocket-related complications.25,27 Leadless pacing introduces a new 1% to 2% groin complication rate for both devices not present with transvenous pacing, and also a 1% device dislodgment rate in the case of Nanostim (all dislodgments were treated percutaneously). Data from both clinical trials suggest that the complication rates are largely compressed acutely. In contrast, there are considerable mid-term and long-term complications for transvenous systems.3–5 Indeed, the mid- to long-term window is where leadless pacing is expected to have the most favorable impact. As with any new disruptive technology, operator experience may be important, as evidenced by a near halving of the complication rate observed in the LEADLESS II trial after gaining the experience of 10 implants.25
Other benefits of leadless pacing include a generally quick procedure (average implant time was 30 minutes in LEADLESS and LEADLESS II)22,25 and full shoulder mobility afterwards, so that patients can resume driving once groin soreness has subsided, typically within a few days. (Current studies are investigating whether immediate shoulder mobility with leadless pacing is beneficial to older patients suffering from arthritis.) The lack of an incision allows patients to bathe and shower as soon as they desire, whereas after transvenous pacemaker implant, motion in the affected shoulder is usually restricted for several weeks to avoid lead dislodgment, and showering and bathing are restricted to avoid contamination of the incision with nonsterile tap water. (In some cases, a tightly adherent waterproof dressing can be used.) The leadless systems were designed for compatibility with magnetic resonance imaging (MRI), whereas not all transvenous pacemaker generators and leads are MRI compatible.
Leadless devices are not expected to span the tricuspid valve to create incident or worsening tricuspid regurgitation. In a recent small study of 22 patients undergoing Micra implant, there were no new cases of severe tricuspid regurgitation after the procedure, with only a 9% increase in mild and 5% increase in moderate tricuspid regurgitation,28 vs a rate of 40% of worsening tricuspid regurgitation and 10% of new severe tricuspid regurgitation with transvenous pacing.13,14
Transvenous pacemaker implant requires surgery for pulse generator exchange at a mean of 7 years, a procedure carrying significant risk of short- and long-term complications.10
END-OF-SERVICE QUESTIONS: ATTEMPT RETRIEVAL OR NOT?
Both leadless systems have favorable projected in-service battery life: a reported 15.0 years for Nanostim25 and mean 12.5 years for Micra.27 The inevitable question is what to do then. The Nanostim system was designed to be retrievable using a dedicated catheter system. Micra was not designed with an accompanying retrieval system. Pathologic examinations of leadless devices at autopsy or after explant have revealed a range of device endothelialization, from partial at 19 months to full at 4 months.29,30
As of this writing, no extraction complications have been observed with Nanostim explants up to 506 days after implant (n = 12, mean 197 days after implant).31 Needless to say, there is not yet enough experience worldwide with either system to know what the end-of-service will look like in 10 to 15 years. One strategy could involve first attempting percutaneous retrieval and replacement, if retrieval is not possible, abandoning the old device while implanting a new device alongside. Another strategy would be to forgo a retrieval attempt altogether. In the LEADLESS II study,24 the mean patient age was 75. In this cohort, forgoing elective retrieval for those who live to reach the end of pacemaker service between the age of 85 and 90 would seem reasonable assuming the next device provides similar longevity. For younger patients, careful consideration of long-term strategies is needed. It is not known what the replacement technology will look like in another decade with respect to device size or battery longevity. Preclinical studies using swine and human cadaver hearts have demonstrated the feasibility of multiple right-ventricular Micra implants without affecting cardiac function.32,33
OTHER LIMITATIONS AND CAUTIONARY NOTES
At present, leadless pacing is approved for single-chamber right-ventricular pacing. In the developed world, single right-ventricular pacing modes account for only 20% to 30% of new pacemaker implants, which total more than 1 million per year worldwide.34,35 As with any new technology, the up-front cost of leadless pacemaker implant is expected to be significantly higher than transvenous systems, which at this point remains poorly defined, as the field has not caught up in terms of charges, reimbursement, and billing codes. While those concerns fall outside the scope of this review, it is not known if the expected reductions in mid- and long-term complications will make up for an up-front cost difference. However, a cost-efficacy study reported that one complication of a transvenous pacemaker system was more expensive than the initial implant itself.36 The longest-term follow-up data currently available are with Nanostim, showing an absolute complication reduction of 11.7% at 2 years,24 a disparity only expected to widen with prolonged follow-up, particularly after transvenous generator exchange, when complication rates rapidly escalate.
FUTURE DIRECTIONS
The next horizon of leadless technology will be for right-atrial and dual-chamber pacing to treat the far more pervasive pacing indication of sinus node dysfunction with or without AV block. In the latter application, the two devices will communicate. Prototypes and early nonhuman evaluations are ongoing for both. Leadless pacing is also being investigated for use in tachycardia. Tjong et al37 reported on the safety and feasibility of an entirely leadless pacemaker plus an implantable cardioverter-defibrillator (ICD) system in two sheep and one human using both Nanostim and subcutaneous ICD. Currently, two important limitations of subcutaneous ICD are its inability to provide backup bradycardia and antitachycardia pacing (it provides only defibrillation). The EMBLEM PACE study will enroll 250 patients to receive a leadless pacemaker and Emblem subcutaneous ICD (Boston Scientific, Boston, MA), with patients subsequently receiving commanded antitachycardia pacing for ventricular arrhythmias and bradycardia pacing provided by the leadless device as indicated.
CONCLUSIONS
Leadless cardiac pacing is a safe and efficacious alternative to standard transvenous pacing systems. Although long-term data are limited, available short- and mid-term data show that the elimination of transvenous leads and the surgical pocket results in significant reductions in complication rates. Currently, leadless pacing is approved only for right-ventricular pacing, but investigation of right-atrial, dual-chamber, and fully leadless pacemaker-defibrillator hybrid systems is ongoing.
- Lagergren H. How it happened: my recollection of early pacing. Pacing Clin Electrophysiol 1978; 1:140–143.
- Parsonnet V. Permanent transvenous pacing in 1962. Pacing Clin Electrophysiol 1978; 1:265–268.
- Kirkfeldt RE, Johansen JB, Nohr EA, Jorgensen OD, Nielsen JC. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J 2014; 35:1186–1194.
- Udo EO, Zuithoff NP, van Hemel NM, et al. Incidence and predictors of short- and long-term complications in pacemaker therapy: the FOLLOWPACE study. Heart Rhythm 2012; 9:728–735.
- Palmisano P, Accogli M, Zaccaria M, et al. Rate, causes, and impact on patient outcome of implantable device complications requiring surgical revision: large population survey from two centres in Italy. Europace 2013; 15:531–540.
- De Sensi F, Miracapillo G, Cresti A, Severi S, Airaksinen KE. Pocket hematoma: a call for definition. Pacing Clin Electrophysiol Aug 2015; 38:909–913.
- Wiegand UK, LeJeune D, Boguschewski F, et al. Pocket hematoma after pacemaker or implantable cardioverter defibrillator surgery: influence of patient morbidity, operation strategy, and perioperative antiplatelet/anticoagulation therapy. Chest 2004; 126:1177–1186.
- Essebag V, Verma A, Healey JS, et al. Clinically significant pocket hematoma increases long-term risk of device infection: Bruise Control Infection Study. J Am Coll Cardiol 2016; 67:1300–1308.
- Ohlow MA, Lauer B, Brunelli M, Geller JC. Incidence and predictors of pericardial effusion after permanent heart rhythm device implantation: prospective evaluation of 968 consecutive patients. Circ J 2013; 77:975–981.
- Hauser RG, Hayes DL, Kallinen LM, et al. Clinical experience with pacemaker pulse generators and transvenous leads: an 8-year prospective multicenter study. Heart Rhythm 2007; 4:154–160.
- Korkeila P, Nyman K, Ylitalo A, et al. Venous obstruction after pacemaker implantation. Pacing Clin Electrophysiol 2007; 30:199–206.
- Haghjoo M, Nikoo MH, Fazelifar AF, Alizadeh A, Emkanjoo Z, Sadr-Ameli MA. Predictors of venous obstruction following pacemaker or implantable cardioverter-defibrillator implantation: a contrast venographic study on 100 patients admitted for generator change, lead revision, or device upgrade. Europace 2007; 9:328–332.
- Al-Mohaissen MA, Chan KL. Prevalence and mechanism of tricuspid regurgitation following implantation of endocardial leads for pacemaker or cardioverter-defibrillator. J Am Soc Echocardiogr 2012; 25:245–252.
- Al-Bawardy R, Krishnaswamy A, Rajeswaran J, et al. Tricuspid regurgitation and implantable devices. Pacing Clin Electrophysiol 2015; 38:259–266.
- Klug D, Balde M, Pavin D, et al. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation 2007; 116:1349–1355.
- Johansen JB, Jorgensen OD, Moller M, Arnsbo P, Mortensen PT, Nielsen JC. Infection after pacemaker implantation: infection rates and risk factors associated with infection in a population-based cohort study of 46,299 consecutive patients. Eur Heart J 2011; 32:991–998.
- Lown B, Kosowsky BD. Artificial cardiac pacemakers. I. N Engl J Med 1970; 283:907–916.
- Spickler JW, Rasor NS, Kezdi P, Misra SN, Robins KE, LeBoeuf C. Totally self-contained intracardiac pacemaker. J Electrocardiol 1970; 3:325–331.
- Sutton R. The first European journal on cardiac electrophysiology and pacing, the European Journal of Cardiac Pacing and Electrophysiology. Europace 2011; 13:1663–1664.
- Vardas PE, Politopoulous C, Manios E, Parthenakis F, Tsagarkis C. A miniature pacemaker introduced intravenously and implanted endocardially. Preliminary findings from an experimental study. Eur J Card Pacing Electrophysiol 1991; 1:27–30.
- Eggen MD, Grubac V, Bonner MD. Design and evaluation of a novel fixation mechanism for a transcatheter pacemaker. IEEE Trans Biomed Eng 2015; 62:2316–2323.
- Reddy VY, Knops RE, Sperzel J, et al. Permanent leadless cardiac pacing: results of the LEADLESS trial. Circulation 2014; 129:1466–1471.
- Ritter P, Duray GZ, Steinwender C, et al. Early performance of a miniaturized leadless cardiac pacemaker: the Micra Transcatheter Pacing Study. Eur Heart J 2015; 36:2510–2519.
- Reddy VY, Exner DV, Cantillon DJ, et al. Percutaneous implantation of an entirely intracardiac leadless pacemaker. N Engl J Med 2015; 373:1125–1135.
- Knops RE, Tjong FV, Neuzil P, et al. Chronic performance of a leadless cardiac pacemaker: 1-year follow-up of the LEADLESS trial. J Am Coll Cardiol 2015; 65:1497–1504.
- Reddy VY, Cantillon DJ, Ip J, et al. A comparative study of acute and mid-term complications of leadless versus transvenous pacemakers. Heart Rhythm 2016 July. [Epub ahead of print].
- Reynolds D, Duray GZ, Omar R, et al. A leadless intracardiac transcatheter pacing system. N Engl J Med 2016; 374:533–541.
- Garikipati NV, Karve A, Okabe T, et al. Tricuspid regurgitation after leadless pacemaker implantation. Abstract presented at Heart Rhythm Society Scientific Sessions, May 4–7, 2016, San Francisco, CA.
- Tjong FV, Stam OC, van der Wal AC, et al. Postmortem histopathological examination of a leadless pacemaker shows partial encapsulation after 19 months. Circ Arrhythm Electrophysiol 2015; 8:1293–1295.
- Borgquist R, Ljungstrom E, Koul B, Hoijer CJ. Leadless Medtronic Micra pacemaker almost completely endothelialized already after 4 months: first clinical experience from an explanted heart. Eur Heart J 2016; 37:2503.
- Reddy VY, Knops RE, Defaye P, et al. Worldwide clinical experience of the retrieval of leadless cardiac pacemakers. Abstract presented at Heart Rhythm Society Scientific Sessions, May 4–7, 2016, San Francisco, CA.
- Chen K, Zheng X, Dai Y, et al. Multiple leadless pacemakers implanted in the right ventricle of swine. Europace 2016 January 31. pii: euv418. [Epub ahead of print].
- Omdahl P, Eggen MD, Bonner MD, Iaizzo PA, Wika K. Right ventricular anatomy can accommodate multiple micra transcatheter pacemakers. Pacing Clin Electrophysiol 2016; 39:393–397.
- Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009—a World Society of Arrhythmia’s project. Pacing Clin Electrophysiol 2011; 34:1013–1027.
- Epstein AE, DiMarco JP, Ellenbogen KA, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Heart Rhythm Society. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2013; 127:e283–352.
- Tobin K, Stewart J, Westveer D, Frumin H. Acute complications of permanent pacemaker implantation: their financial implication and relation to volume and operator experience. Am J Cardiol 2000; 85:774–776, A9.
- Tjong FV, Brouwer TF, Smeding L, et al. Combined leadless pacemaker and subcutaneous implantable defibrillator therapy: feasibility, safety, and performance. Europace 2016 March 3. [Epub ahead of print].
- Lagergren H. How it happened: my recollection of early pacing. Pacing Clin Electrophysiol 1978; 1:140–143.
- Parsonnet V. Permanent transvenous pacing in 1962. Pacing Clin Electrophysiol 1978; 1:265–268.
- Kirkfeldt RE, Johansen JB, Nohr EA, Jorgensen OD, Nielsen JC. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J 2014; 35:1186–1194.
- Udo EO, Zuithoff NP, van Hemel NM, et al. Incidence and predictors of short- and long-term complications in pacemaker therapy: the FOLLOWPACE study. Heart Rhythm 2012; 9:728–735.
- Palmisano P, Accogli M, Zaccaria M, et al. Rate, causes, and impact on patient outcome of implantable device complications requiring surgical revision: large population survey from two centres in Italy. Europace 2013; 15:531–540.
- De Sensi F, Miracapillo G, Cresti A, Severi S, Airaksinen KE. Pocket hematoma: a call for definition. Pacing Clin Electrophysiol Aug 2015; 38:909–913.
- Wiegand UK, LeJeune D, Boguschewski F, et al. Pocket hematoma after pacemaker or implantable cardioverter defibrillator surgery: influence of patient morbidity, operation strategy, and perioperative antiplatelet/anticoagulation therapy. Chest 2004; 126:1177–1186.
- Essebag V, Verma A, Healey JS, et al. Clinically significant pocket hematoma increases long-term risk of device infection: Bruise Control Infection Study. J Am Coll Cardiol 2016; 67:1300–1308.
- Ohlow MA, Lauer B, Brunelli M, Geller JC. Incidence and predictors of pericardial effusion after permanent heart rhythm device implantation: prospective evaluation of 968 consecutive patients. Circ J 2013; 77:975–981.
- Hauser RG, Hayes DL, Kallinen LM, et al. Clinical experience with pacemaker pulse generators and transvenous leads: an 8-year prospective multicenter study. Heart Rhythm 2007; 4:154–160.
- Korkeila P, Nyman K, Ylitalo A, et al. Venous obstruction after pacemaker implantation. Pacing Clin Electrophysiol 2007; 30:199–206.
- Haghjoo M, Nikoo MH, Fazelifar AF, Alizadeh A, Emkanjoo Z, Sadr-Ameli MA. Predictors of venous obstruction following pacemaker or implantable cardioverter-defibrillator implantation: a contrast venographic study on 100 patients admitted for generator change, lead revision, or device upgrade. Europace 2007; 9:328–332.
- Al-Mohaissen MA, Chan KL. Prevalence and mechanism of tricuspid regurgitation following implantation of endocardial leads for pacemaker or cardioverter-defibrillator. J Am Soc Echocardiogr 2012; 25:245–252.
- Al-Bawardy R, Krishnaswamy A, Rajeswaran J, et al. Tricuspid regurgitation and implantable devices. Pacing Clin Electrophysiol 2015; 38:259–266.
- Klug D, Balde M, Pavin D, et al. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation 2007; 116:1349–1355.
- Johansen JB, Jorgensen OD, Moller M, Arnsbo P, Mortensen PT, Nielsen JC. Infection after pacemaker implantation: infection rates and risk factors associated with infection in a population-based cohort study of 46,299 consecutive patients. Eur Heart J 2011; 32:991–998.
- Lown B, Kosowsky BD. Artificial cardiac pacemakers. I. N Engl J Med 1970; 283:907–916.
- Spickler JW, Rasor NS, Kezdi P, Misra SN, Robins KE, LeBoeuf C. Totally self-contained intracardiac pacemaker. J Electrocardiol 1970; 3:325–331.
- Sutton R. The first European journal on cardiac electrophysiology and pacing, the European Journal of Cardiac Pacing and Electrophysiology. Europace 2011; 13:1663–1664.
- Vardas PE, Politopoulous C, Manios E, Parthenakis F, Tsagarkis C. A miniature pacemaker introduced intravenously and implanted endocardially. Preliminary findings from an experimental study. Eur J Card Pacing Electrophysiol 1991; 1:27–30.
- Eggen MD, Grubac V, Bonner MD. Design and evaluation of a novel fixation mechanism for a transcatheter pacemaker. IEEE Trans Biomed Eng 2015; 62:2316–2323.
- Reddy VY, Knops RE, Sperzel J, et al. Permanent leadless cardiac pacing: results of the LEADLESS trial. Circulation 2014; 129:1466–1471.
- Ritter P, Duray GZ, Steinwender C, et al. Early performance of a miniaturized leadless cardiac pacemaker: the Micra Transcatheter Pacing Study. Eur Heart J 2015; 36:2510–2519.
- Reddy VY, Exner DV, Cantillon DJ, et al. Percutaneous implantation of an entirely intracardiac leadless pacemaker. N Engl J Med 2015; 373:1125–1135.
- Knops RE, Tjong FV, Neuzil P, et al. Chronic performance of a leadless cardiac pacemaker: 1-year follow-up of the LEADLESS trial. J Am Coll Cardiol 2015; 65:1497–1504.
- Reddy VY, Cantillon DJ, Ip J, et al. A comparative study of acute and mid-term complications of leadless versus transvenous pacemakers. Heart Rhythm 2016 July. [Epub ahead of print].
- Reynolds D, Duray GZ, Omar R, et al. A leadless intracardiac transcatheter pacing system. N Engl J Med 2016; 374:533–541.
- Garikipati NV, Karve A, Okabe T, et al. Tricuspid regurgitation after leadless pacemaker implantation. Abstract presented at Heart Rhythm Society Scientific Sessions, May 4–7, 2016, San Francisco, CA.
- Tjong FV, Stam OC, van der Wal AC, et al. Postmortem histopathological examination of a leadless pacemaker shows partial encapsulation after 19 months. Circ Arrhythm Electrophysiol 2015; 8:1293–1295.
- Borgquist R, Ljungstrom E, Koul B, Hoijer CJ. Leadless Medtronic Micra pacemaker almost completely endothelialized already after 4 months: first clinical experience from an explanted heart. Eur Heart J 2016; 37:2503.
- Reddy VY, Knops RE, Defaye P, et al. Worldwide clinical experience of the retrieval of leadless cardiac pacemakers. Abstract presented at Heart Rhythm Society Scientific Sessions, May 4–7, 2016, San Francisco, CA.
- Chen K, Zheng X, Dai Y, et al. Multiple leadless pacemakers implanted in the right ventricle of swine. Europace 2016 January 31. pii: euv418. [Epub ahead of print].
- Omdahl P, Eggen MD, Bonner MD, Iaizzo PA, Wika K. Right ventricular anatomy can accommodate multiple micra transcatheter pacemakers. Pacing Clin Electrophysiol 2016; 39:393–397.
- Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009—a World Society of Arrhythmia’s project. Pacing Clin Electrophysiol 2011; 34:1013–1027.
- Epstein AE, DiMarco JP, Ellenbogen KA, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Heart Rhythm Society. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2013; 127:e283–352.
- Tobin K, Stewart J, Westveer D, Frumin H. Acute complications of permanent pacemaker implantation: their financial implication and relation to volume and operator experience. Am J Cardiol 2000; 85:774–776, A9.
- Tjong FV, Brouwer TF, Smeding L, et al. Combined leadless pacemaker and subcutaneous implantable defibrillator therapy: feasibility, safety, and performance. Europace 2016 March 3. [Epub ahead of print].
KEY POINTS
- Leadless cardiac pacing has emerged as a safe and effective alternative involving catheter-based delivery of a self-contained device directly into the right ventricle without incisional access, leads, or a surgical pocket. The procedure typically can be performed in 30 minutes or less, with fewer postprocedure restrictions.
- Leadless pacing is showing promising results, but it is currently limited to single-chamber pacing.
- Future directions include atrial and dual-chamber pacing and combining the procedure with a subcutaneous implantable cardioverter-defibrillator.
PCSK9 inhibition: A promise fulfilled?
Statin therapy has been shown to substantially reduce adverse events associated with low-density-lipoprotein cholesterol (LDL-C) and cardiovascular disease (CVD). Statins alone are often not adequate to achieve treatment goals, and residual CVD risk remains high. Combination therapies of statins with ezetimibe and resins to further lower LDL-C, fibrates and omega 3 fatty acids to lower triglycerides, and niacin to lower both and raise high-density-liproprotein cholesterol are available, but additional risk reduction has not been consistently demonstrated in clinical trials.
The link between atherogenic lipoproteins and CVD is strong, and the need to develop therapies in addition to statins to substantially and safely reduce LDL-C is a priority. The association of reduced proprotein convertase subtilisin/kexin type 9 (PCSK9) activity with reduced LDL-C and CVD events has led to the rapid development and approval of monoclonal antibody therapies to inhibit PCSK9.
In this review, we discuss trials of these therapies that have shown durable reductions in LDL-C of more than 50%, with acceptable tolerability. Now that PCSK9 inhibitors are approved by the US Food and Drug Administration (FDA), extended data are needed as to long-term tolerability, safety, and efficacy of these agents and, most importantly, demonstration of additional reduction in CVD events.
A CASE FOR ADDITIONAL THERAPIES
CVD is the leading cause of morbidity and death in the United States, responsible for one in four deaths. Hyperlipidemia and, specifically, elevated LDL-C have been found to be important drivers of atherosclerosis and, in turn, adverse cardiovascular (CV) events. Likewise, numerous observational and clinical trials have shown that reducing LDL-C, particularly with statins, decreases CVD events.1–4 More aggressive lowering with higher doses or more intensive statin therapy further reduces rates of adverse outcomes.3,4 In addition, the pleiotropic effects of statins imply that not all of their benefits are derived from LDL-C lowering alone.5 Consequently, it is now standard practice to use statins at the highest tolerable dose to reach target LDL-C levels and prevent CV events in high-risk patients with CVD or multiple coronary artery disease risk factors, regardless of the LDL-C levels.6,7
The American College of Cardiology (ACC) and the American Heart Association released cholesterol guidelines in 2013 that recommend a risk-based approach for statin therapy rather than targeting specific LDL-C levels.6 Although this evidence-based approach may better conform to clinical trials, the debate that lower LDL-C targets will further prevent CVD continues.
Indeed, it appears that lower is better, as demonstrated by the IMPROVE-IT trial.8 Although the control group receiving simvastatin monotherapy had low LDL-C levels (mean, 69.9 mg/dL; 1.8 mmol/L), the experimental group receiving simvastatin plus ezetimibe achieved even lower levels (mean, 53.2 mg/dL;1.4 mmol/L) after 1 year of therapy and had a significantly lower composite primary end point of CV death, major coronary event, or nonfatal stroke at 7 years (34.7% for simvastatin monotherapy vs 32.7% for combined therapy).9 Furthermore, the event-rate reduction with the addition of ezetimibe was the same as the average predicted by the Cholesterol Treatment Trialists’ meta-analysis: an LDL-C reduction of 1 mmol/L (38.6 mg/dL) yields a 23% risk reduction in major coronary events over 5 years.10 Although only a modest absolute reduction in outcomes, it supports the notion that further reduction of LDL-C levels by more potent therapies may offer greater benefit.
There is strong evidence that statin therapy reduces the risk of developing CVD in patients with or without a previous atherosclerotic event; however, residual CVD risk remains even for those on therapy. A contributing factor to this residual risk is that many statin-treated patients have insufficient response or intolerance and do not achieve adequate LDL-C reductions.
There are three clinically important patient populations who are inadequately managed with current therapies and remain at high risk of subsequent CV events; these are patients who would benefit from additional therapies.
1. Patients with familial hypercholesterolemia (FH). This is the most common genetic disorder in the world, yet it is frequently undiagnosed and untreated. Due to high baseline cholesterol levels, achieving LDL-C treatment goals is challenging.
- The prevalence may be closer to 1:200 to 1:250 rather than the often quoted 1:500.11
- Fewer than 12% of patients with heterzygous FH achieve the LDL-C goal of < 100 mg/dL with maximal statin treatment alone or with a second agent.12
2. Patients with hyperlipidemia not due to FH who are at elevated CV risk and undertreated. In US and European surveys, between 50% and 60% of patients receiving statins with or without other therapies failed to reach LDL-C reduction goals.13
- Variation in response to statin treatment between individuals may be considerable.
- Poor adherence to statin therapy is common.
3. Patients with side effects to statins, particularly muscle symptoms that prevent statin use or substantially limit the dose.
- Although the incidence of myopathy is low (< 0.1%) and rhabdomyolysis is even less common, observational studies suggest that 10% to 20% of patients may limit statin use due to muscle-associated complaints including muscle aching, cramps, or weakness.14
- Side effects may be dose-dependent, limiting the use of the high-intensity statin doses that are frequently necessary to achieve LDL-C goals.
Consequently, there is great interest in developing therapies beyond statins that may further reduce CV events. However, treatments other than ezetimibe for further management of hyperlipidemia and risk reduction have failed to demonstrate consistent benefit when added to statin therapy.15–19 The largest studies were with niacin and fibrates. Unfortunately, most trials demonstrated no overall outcomes benefit or only benefits in subgroup analyses, leaving the door open to other pharmacologic interventions.
Studies with the cholesterol ester transfer protein (CETP) inhibitor torcetrapib, in combination with statin therapy, actually demonstrated an overall increase in all-cause mortality in the treatment group.20 Two large outcome trials of the CETP inhibitors dalcetrapib and evacetrapib were stopped after interim analysis predicted no benefit. Although drugs such as lomitapide (a microsomal triglyceride transfer protein inhibitor) and mipomersen (an antisense oligonucleotide inhibitor of ApoB-100 synthesis) can lower LDL-C by reducing ApoB synthesis,21 they are approved only in the small population of individuals with homozygous FH and liver toxicity and side effects are a concern.
Accordingly, current cholesterol management guidelines continue to offer LDL-C as the main target of lipid-modifying therapy, with statins as the primary treatment choice. The desire to build on statin therapy to prevent further progression of atherosclerosis and clinical CVD has encouraged continued focus on strategies to lower LDL-C to even greater extents.
Fortunately for practitioners, for the first time since lovastatin was approved in 1987, there is a new therapy approved by the FDA that significantly lowers LDL-C and, potentially, improves CV outcomes—the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors. This review will focus on the PCSK9 inhibitors, a novel therapeutic class that reduces LDL-C through increased hepatic clearance. These drugs are rapidly emerging as an ideal adjunctive therapy to statins for patients at the highest risk and as a highly efficacious alternate therapy in patients intolerant of statins.
PCSK9 INHIBITORS: DISCOVERY, MECHANISM, AND THERAPEUTIC INTERVENTIONS
Two PCSK9 inhibitors have received FDA approval: alirocumab (Praluent) and evolocumab (Repatha). Among new molecular entities for clinical use, PCSK9 inhibitor therapies had one of the shortest durations from discovery to development and approval.
Mutations in the PCSK9 gene associated with autosomal dominant hypercholesterolemia were first identified in 2003 in a French family.22 The PCSK9 protein is now known to be a secreted enzymatic serine protease that is primarily synthesized in the liver and binds to the LDL receptor (LDL-R)/LDL-C complex on the surface of hepatocytes, marking the receptor for lysosomal degradation rather than recycling to the cell surface. Thus, it reduces the quantity of LDL-R that is available to remove LDL-C from circulation.23 As a result, higher levels of PCSK9 are associated with higher levels of plasma LDL-C.
The clinical importance of PCSK9 in regulating LDL-C is supported by observed mutations and polymorphisms. Gain-of-function mutations that increase the activity of PCSK9 have been shown to be associated with elevated LDL-C, premature CVD, and myocardial infarction (MI).24 Conversely, loss-of-function mutations (heterozygotes found in 1% to 3% of the population) result in decreased activity of PCSK9, lower LDL-C, and lower incidence of CVD (Table 1).25–29 These observations, combined with data showing that homozygote loss-of-function individuals with very low LDL-C were generally very healthy, sparked interest in developing inhibition of PCSK9 activity as a therapeutic strategy for hyperlipidemia.
Multiple pharmacologic developments are aimed at inhibiting PCSK9, with many compounds in clinical trials. The approaches include gene silencing with loss-of-function mutations, synthetic peptides, oral small molecules, and monoclonal antibodies. Gene silencing was first observed in 2007 when administration of antisense oligonucleotides targeted to selectively inhibit PCSK9 mRNA was found to up-regulate LDL-R, thereby decreasing serum levels of LDL-C.30
The first study to establish the role of synthetic peptides in PCSK9 inhibition was performed in 2008. In this study, the epidermal growth factor-like A synthetic peptide blocked the interaction between PCSK9 and LDL-R, thereby decreasing the degradation of LDL-R and preserving LDL uptake.31 Although studies are limited, synthetic peptides remain an area of great interest given their promising effects on lipid metabolism. Recently, a synthetic PCSK9-binding adnectin derived from the human fibronectin known as BMS-962476, had favorable results in a phase 1 clinical trial. An RNA interference molecule, subcutaneous ALN-PSC, inhibits PCSK9 gene expression by causing destruction of messenger RNA, thus inhibiting PCSK9 synthesis (Table 2).32
PCSK9 INHIBITORS: CLINICAL TRIALS
Subcutaneously administered monoclonal antibodies targeting PCSK9 currently are the only PCSK9 inhibitors FDA-approved for clinical use. The first study to demonstrate efficacy in enhancing uptake of serum LDL-C was performed in 2009.33 Multiple phase 1 and 2 studies soon followed, demonstrating acceptable safety and 50% to 70% reductions in LDL-C at upper-dose titrations.34 Additionally, there were significant reductions in total cholesterol, ApoB, triglycerides, and lipoprotein(a).
These early developments paved the way for larger phase 3 trials (Table 3).35–48 The PCSK9 inhibitors evolocumab and alirocumab have been shown in multiple phase 3 clinical trials to achieve a consistent dose-dependent 50% to 60% reduction in LDL-C across a broad range of CVD risk, pretreatment LDL-C levels, and background therapy: monotherapy (MENDEL-2, ODYSSEY COMBO I),35,44 added to statin therapy (LAPLACE-2, ODYSSEY CHOICE I),38,46 and in individuals with heterozygous FH (RUTHERFORD-2, ODYSSEY-FH).37,42 Trials with bococizumab are under way.
The GAUSS-2 clinical trial (Goal Achievement after Utilizing an Anti-PCSK9 Antibody in Statin Intolerant Subjects-2) demonstrated similar efficacy in reducing LDL-C in patients with clinically assessed statin intolerance due to muscle-related adverse symptoms.39 In GAUSS-3, patients were first identified as being statin intolerant secondary to muscle-associated symptoms based on a randomized, crossover trial of atorvastatin vs placebo.40 The 43% of participants who experienced intolerable muscle-related symptoms on the statin but not on placebo were then randomized to evolocumab vs ezetimibe. Results showed significant reduction in LDL-C in the evolocumab group (52.8%) compared with the ezetimibe group (16.7%). Additionally, among patients with muscle symptoms on statin therapy, PCSK9 therapy was discontinued for muscle symptoms in only 0.7% of evolocumab recipients and 6.8% of ezetimibe recipients.
Overall, the PCSK9 inhibitors are generally well tolerated with injection site reactions being the most common side effect. A meta-analysis published in 2015 of 25 trials including more than 12,000 patients treated with evolocumab and alirocumab reported no significant difference in adverse events or safety outcomes vs placebo or ezetimibe.49 Antidrug binding or neutralizing antibody production to these agents, thus far, has not been shown to be an issue. Additional analyses have not indicated an adverse effect on gonadal hormone levels or increased incidence of new-onset diabetes.
Two studies published in 2015 offer insight into longer term durability and safety as well as potential CVD outcome benefit (Table 4)50,51:
- OSLER-1 and 2: Open-Label Study of Long-Term Evaluation against LDL-Cholesterol (OSLER) trials—evolocumab trial;50
- ODYSSEY long term: Long-Term Safety and Tolerability of Alirocumab in High Cardiovascular Risk Patients with Hypercholesterolemia Not Adequately Controlled with Their Lipid Modifying Therapy—alirocumab trial.51
The OSLER trials reported durable LDL-C reductions of 61% and the ODYSSEY trial reported a LDL-C reduction of 62%.50,51 In both studies, the overall occurrence of adverse events was similar to placebo, but both reported a higher rate of neurocognitive effects in the active treatment groups (evolocumab 0.9% vs 0.3% for standard therapy; alirocumab 1.2% vs 0.5% for placebo). It must be noted that although the absolute rate of neurocognitive adverse events is low, it is unclear if these events were related to the drugs themselves or to extreme lowering of LDL-C. Nevertheless, the FDA has raised concerns about neurocognitive events. A sub-study of the ongoing FOURIER trial with evolocumab—EBBINGHAUS—is expected to address this concern.
In addition, analyses of CV events showed that the PCSK9 inhibitors effectively cut the CV rate in half in both studies (Figure 1).50,51 In the OSLER trials,50 evolocumab recipients had 53% reduction in major CV events (0.95% vs 2.18% in the standard therapy group; P = .003). In ODYSSEY,51 alirocumab recipients had a 48% reduction in major CV events (1.7% vs 3.3% for placebo; P = .02). Furthermore, a 2015 meta-analysis of 24 phase 2 and 3 trials reported a statistically significant 55% reduction in all-cause mortality and 50% reduction in CV mortality with PCSK9 inhibitors.52
For many reasons including short length of follow-up, study design, and small numbers of outcome events, the OSLER and ODYSSEY studies, although enticing, are exploratory and hypothesis-generating only and results need to be interpreted with caution. Nevertheless, they have set the stage for ongoing prospective randomized outcome trials that are studying the CV effects and tolerability of PCSK9 inhibitors over a longer time frame. These include the following trials.
- The Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) is an ongoing trial with the primary end point of CV death, MI, hospitalization for unstable angina, stroke, or coronary revascularization in high-risk patients receiving evolocumab or placebo.53
- The ODYSSEY trial is examining the effect of alirocumab vs placebo on the composite primary endpoint of coronary heart disease death, non-fatal MI, fatal and nonfatal ischemic stroke, and unstable angina requiring hospitalization in patients who have had an acute coronary syndrome event during the previous 4 to 52 weeks.54
- The Evaluation of Bococizumab in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects (SPIRE) trials are investigating the effect of bococizumab, a third PCSK9 “humanized” monoclonal antibody, vs placebo in reducing death, MI, stroke, or unstable angina in patients at high-risk of CVD who are receiving standard lipid-lowering therapy with LDL-C > 70 mg/dL (1.8 mmol/L) (SPIRE-1) or > 100 mg/dL (2.6 mmol/L) (SPIRE-2).55,56
Because these outcome trials are attempting to enroll more than 70,000 patients and are event driven, it is difficult to predict when they will be completed (Table 5).53–56 However, recent estimates indicate completion of at least one trial by the end of 2016 or early 2017, with interim analyses of others expected at that time. It is hoped that they will answer the all-important question of whether PCSK9 inhibitors are associated with further CV event reduction benefit.
CURRENT FDA INDICATIONS AND GUIDELINES
The two PCSK9 inhibitors approved by the FDA—alirocumab (subcutaneous 75 mg every 2 weeks up titrated to 150 mg) and evolocumab (subcutaneous 140 mg every 2 weeks or 420 mg every 4 weeks)—are both indicated for use with statins in patients with heterozygous FH or known atherosclerotic CVD who require further reduction in LDL-C levels despite lifestyle interventions and use of maximally tolerated statins. Evolocumab has also been approved for use in patients with homozygous FH.
Although PCSK9 inhibitors are not specifically approved for patients unable to tolerate statins, the results of GAUSS-3, which documented that statin intolerance is a real, definable entity and very responsive to PCSK9 inhibition, makes these drugs promising agents for patients intolerant of statins and, thus, unable to benefit from high-intensity stain therapy.
In April 2016, the ACC released a clinical consensus update to their 2013 cholesterol guidelines, which is their first recommendation specifically addressing the use of non-statin therapies, including the newer PCSK9 inhibitors.57 For high-risk patients with clinical atherosclerotic CVD or LDL-C > 190 and failure to achieve at least a 50% reduction in LDL-C on maximally tolerated statin, non-statins may be considered. Ezetimibe, given its safety and tolerability, should be the first additional medication added. Bile acid sequestrants may be used as a second-line therapy if ezetimibe is not tolerated and triglycerides are not elevated. If therapy goals are not met on maximally tolerated statin and ezetimibe, either approved PCSK9 inhibitor can be added or used to replace ezetimibe. The document also specifies that given the lack of long-term safety and efficacy data on the PCSK9 inhibitors, they are not recommended for use in primary prevention patients in the absence of FH.
CONCLUSION
Although statin therapy has been shown to substantially reduce LDL-C and CVD adverse events, there remains a high rate of inadequate goal achievement and residual CVD risk in patients receiving statins. Combination therapies with ezetimibe and resins to further lower LDL-C, fibrates and omega 3 fatty acids to lower triglycerides, and niacin to lower both and raise high-density-liproprotein cholesterol are available, even though additional CV risk reduction is minimal or elusive when these drugs are added to statin therapy.
The link between atherogenic lipoproteins and CVD is strong, and the need to develop therapies in addition to statins to substantially and safely reduce LDL-C remains a priority. The association of reduced PCSK9 activity with reduced LDL-C and CV events has led to rapid development and approval of monoclonal antibody therapies to inhibit PCSK9. In trials, these therapies have shown substantial and durable reductions in LDL-C of more than 50%, with acceptable tolerability. Now that PCSK9 inhibitors are approved by the FDA, extended data about long-term tolerability, safety, and efficacy and, most importantly, demonstration of additional reduction in CVD events are needed. It is hoped that the long-term ongoing trials will provide these data.
For the immediate future, statin therapy will continue to be the cornerstone of lipid and CVD risk management based on their low generic cost, proven CVD risk reduction, and clinicians’ comfort with their use. However, the reliable efficacy of PCSK9 inhibitors and the fact that statin therapy itself increases PCSK9 activity makes the addition of PCSK9 inhibitors to statins an attractive approach in high-risk patients failing to reach LDL-C treatment goals.
Although current indications are limited, there are patients at high CVD risk who would be appropriate candidates for these therapies. These include patients with the following:
- FH with lifetime burden of elevated LDL-C and associated low likelihood of achieving optimal LDL-C control on current available therapies
- Complete or partial statin intolerance with high-intensity statin dosing limited by side effects
- High CV risk who are not at LDL-C goal on current therapies.
Now that the first therapies are available, practitioners can expect newer approaches to tackle PCSK9-mediated LDL-C reduction. Bococizumab is lagging in phase 3 trials, but the SPIRE program is moving forward with special population studies expected to conclude in 2016 and simultaneous long-term outcomes trials. Other PCSK9 inhibitors being investigated include agents with more durable effect requiring less frequent injections, RNA-interference therapies, vaccinations, antisense therapies, and oral formulations.
The PCSK9 inhibitors hold promise as an adjunct to statin therapy. Their eventual clinical role will depend on a balance between substantial LDL-C reductions, long-term safety, tolerability, and reduction in CVD events vs the cost (estimated at $14,000 a year), access from payers, acceptance of injectable therapies, and magnitude of incremental benefit when added to current therapies. Nevertheless, initial clinical trial data are encouraging and these drugs may be an important addition to the therapeutic armamentarium against CVD.
- Scandinavian Simvastatin Survival Study Group. Randomized trial of cholesterol lowering in 4,444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- Sacks FM, Pfeiffer MA, Moye LA, et al; Cholesterol and Recurrent Events Trial Investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial Investigators. N Engl J Med 1996; 335:1001–1009.
- Schwartz GG, Olsson AG, Ezekowitz MD, et al; Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001; 285:1711–1718.
- Lipid Research Clinics Program. The Lipid Research Clinics Coronary Primary Prevention Trial results (reduction in incidence of coronary heart disease). JAMA 1984; 251:351–364.
- Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation 2004; 109(suppl 1):III39–III43.
- Stone N, Robinson J, Lichtenstein A, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(suppl 2):S1–S45.
- Jacobson TA, Ito MK, Maki KC, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 1—full report. J Clin Lipidol 2015; 9:129–169.
- Jarcho JA, Keaney JF Jr. Proof that lower is better–LDL cholesterol and IMPROVE-IT. N Engl J Med 2015; 372:2448–2450.
- Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015; 372:2387–2397.
- Baigent C, Keech A, Kearney PM, et al; Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- de Ferranti S, Rodday AM, Mendelson M, et al. Prevalence of familial hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES). Circulation 2016; 133:1067–1072.
- Perez de Isla L, Alonso R, Watts GF, et al; SAFEHEART investigators. Attainment of LDL-cholesterol treatment goals in patients with familial hypercholesterolemia: 5-year SAFEHEART registry follow-up. J Am Coll Cardiol 2016; 67:1278–1285.
- Unni SK, Quek RGW, Biskupiak J, et al. Assessment of statin therapy, LDL-C levels, and cardiovascular events among high-risk patients in the United States. J Clin Lipidol 2016; 10:63–71.
- Mammen AL, Amato AA. Statin myopathy: a review of recent progress. Curr Opin Rheum 2010; 22:644–650.
- AIM-HIGH Investigators; Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013; 34:1279–1291.
- ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010; 362:1563–1574.
- Keech A, Simes RJ, Barter P, et al; FIELD study investigators. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomized controlled trial. Lancet 2005; 366:1849–1861.
- Kaur N, Pandey A, Negi H, et al. Effect of HDL-raising drugs on cardiovascular outcomes: a systematic review and meta-regression. PLoS One 2014; 9:e94585.
- Barter PJ, Caulfield M, Eriksson M, et al; ILLUMINATE investigators. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007; 357:2109–2122.
- Rader D, Kastelein J. Lomitapide and mipomersen: two first-in-class drugs for reducing low-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. Circulation 2014; 129:1022–1032.
- Abifadel M, Varret M, Rabes JP, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 2003; 34:154–156.
- Verbeek R, Stoekenbroek RM, Hovingh GK. PCSK9 inhibitors: novel therapeutic agents for the treatment of hypercholesterolemia. Eur J of Pharm 2015; 763(Pt A):38–47.
- Steinberg D, Witztum JL. Inhibition of PCSK9: a powerful weapon for achieving ideal LDL cholesterol levels. Proc Natl Acad Sci USA 2009; 106:9546–9547.
- Abifadel M, Rabès J-P, Devillers M, et al. Mutations and polymorphisms in the proprotein convertase subtilisin kexin 9 (PCSK9) gene in cholesterol metabolism and disease. Hum Mutat 2009; 30:520–529.
- Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006; 354:1264–1272.
- Benn M, Nordestgaard BG, Grande P, Schnohr P, Tybærg-Hansen A. PCSK9 R46L, low-density lipoprotein cholesterol levels, and risk of ischemic heart disease. J Am Coll Cardiol 2010; 55:2833–2842.
- Mortensen MB, Afzal S, Nordestgaard BG, Falk E. The high-density lipoprotein-adjusted SCORE model worsens SCORE-based risk classification in a contemporary population of 30,824 Europeans: the Copenhagen General Population Study. Eur Heart J 2015; 36:2446–2453.
- Victor RG, Haley RW, Willett DL, et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol 2004; 93:1473–1480.
- Graham MJ, Lemonidis KM, Whipple CP, et al. Antisense inhibition of proprotein convertase subtilisin/kexin type 9 reduces serum LDL in hyperlipidemic mice. J Lipid Res 2007; 48:763–767.
- Shan L, Pang L, Zhang R, Murgolo NJ, Lan H, Hedrick JA. PCSK9 binds to multiple receptors and can be functionally inhibited by an EGF-A peptide. Biochem Biophys Res Comm 2008; 375:69–73.
- Stein EA, Raal F. Reduction of low-density lipoprotein cholesterol by monoclonal antibody inhibition of PCSK9. Annu Rev Med 2014; 65:417–431.
- Duff CJ, Scott MJ, Kirby IT, Hutchinson SE, Martin SL, Hooper NM. Antibody-mediated disruption of the interaction between PCSK9 and the low-density lipoprotein receptor. Biochem J 2009; 419:577–584.
- Stein EA, Mellis S, Yancopoulos GD, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Eng J Med 2012; 366:1108–1118.
- Koren MJ, Lundqvist P, Bolognese M, et al; MENDEL-2 Investigators. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol 2014; 63:2531–2540.
- Blom DJ, Hala T, Bolognese M, et al; DESCARTES investigators. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med 2014; 370:1809-1819.
- Raal FJ, Stein EA, Dufour R, et al; RUTHERFORD-2 Investigators. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet 2014; 385:331–340.
- Robinson JG, Nedergaard BS, Rogers WJ, et al; LAPLA C-2 Investigators. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA 2014; 311:1870–1882.
- Stroes E, Colquhoun D, Sullivan D, et al; GAUSS-2 Investigators. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol 2014; 63:2541–2548.
- Nissen SE, Stroes E, Dent-Acosta RE, et al; GAUSS-3 Investigators. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance, the GAUSS-3 randomized clinical trial. JAMA 2016; 315:1580–1590.
- Trial assessing long term use of PCSK9 inhibition in subjects with genetic LDL disorders (TAUSSIG). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT-1624142. Updated June 25, 2015. Accessed October 23, 2016.
- Kastelein JJ, Ginsberg HN, Langslet G, et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J 2015; 36:2996–3003.
- Efficacy and safety of alirocumab (SAR236553/REGN727) versus placebo on top of lipid-modifying therapy in patients with heterozygous familial hypercholesterolemia; the ODYSSEY HIGH FH trial. U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01617655. Updated September 27, 2016. Accessed October 23, 2016.
- Kereiakes DJ, Robinson JG, Cannon CP, et al. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: The ODYSSEY COMBO I study. Am Heart J 2015; 169:906–915.
- Efficacy and Safety of Alirocumab (SAR236553/REGN727) Versus Ezetimibe on Top of Statin in High Cardiovascular Risk Patients With Hypercholesterolemia (ODYSSEY COMBO II). U.S. National Institutes of Health website. Updated June 23, 2016. https://clinicaltrials.gov/ct2/show/NCT01644188. Accessed October 23, 2016.
- Roth EM, Moriarty P, Bergeron J, et al; ODYSSEY CHOICE I investigators. A phase III randomized trial evaluating alirocumab 300 mg every 4 weeks as monotherapy or add-on to statin: ODYSSEY CHOICE I. Atherosclerosis 2016, doi: 10.1016/j.atherosclerosis.2016.08.043.
- Phase III Study To Evaluate Alirocumab in Patients With Hypercholesterolemia Not Treated With a Statin (ODYSSEY CHOICE II). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT02023879. Updated November 2, 2015. Accessed October 23, 2016.
- Monthly and twice monthly subcutaneous dosing of PF-04950615 (RN316) in hypercholesterolemic subjects on a statin. U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/results?term=NCT01592240. Updated October 14, 2014. Accessed October 23, 2016.
- Zhang XL, Zhu QQ, Zhu L, et al. Safety and efficacy of anti-PCSK9 antibodies: a meta-analysis of 25 randomized, controlled trials. BMC Med 2015; 13:123.
- Sabatine MS, Giugliano RP, Wiviott SD, et al; OSLER Investigators. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372:1500–1509.
- Robinson JG, Farnier M, Krempf M, et al; ODYSSEY LONG TERM Investigators. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372:1489–1499.
- Navarese EP, Kolodziejczak M, Schulze V, et al. Effects of proprotein convertase subtilisin/kexin type 9 antibodies in adults with hypercholesterolemia: a systematic review and meta-analysis. Ann Intern Med 2015; 163:40–51.
- Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01764633. Updated July 26, 2016. Accessed October 23, 2016.
- ODYSSEY Outcomes: Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab. U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01663402. Updated October 23, 2016. Accessed September 13, 2016.
- The Evaluation of Bococizumab (PF-04950615;RN316) in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects (SPIRE-1). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01975376. Updated September 22, 2016. Accessed October 23, 2016.
- The Evaluation of Bococizumab (PF-04950615; RN316) in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects (SPIRE-2). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01975389. Updated July 26, 2016. Accessed October 23, 2016.
- Lloyd-Jones DM, Morris PB, Ballantyne CM, et al; Writing Committee. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology task force on clinical expert consensus documents. J Am Coll Cardiol 2016; 68:92–125.
Statin therapy has been shown to substantially reduce adverse events associated with low-density-lipoprotein cholesterol (LDL-C) and cardiovascular disease (CVD). Statins alone are often not adequate to achieve treatment goals, and residual CVD risk remains high. Combination therapies of statins with ezetimibe and resins to further lower LDL-C, fibrates and omega 3 fatty acids to lower triglycerides, and niacin to lower both and raise high-density-liproprotein cholesterol are available, but additional risk reduction has not been consistently demonstrated in clinical trials.
The link between atherogenic lipoproteins and CVD is strong, and the need to develop therapies in addition to statins to substantially and safely reduce LDL-C is a priority. The association of reduced proprotein convertase subtilisin/kexin type 9 (PCSK9) activity with reduced LDL-C and CVD events has led to the rapid development and approval of monoclonal antibody therapies to inhibit PCSK9.
In this review, we discuss trials of these therapies that have shown durable reductions in LDL-C of more than 50%, with acceptable tolerability. Now that PCSK9 inhibitors are approved by the US Food and Drug Administration (FDA), extended data are needed as to long-term tolerability, safety, and efficacy of these agents and, most importantly, demonstration of additional reduction in CVD events.
A CASE FOR ADDITIONAL THERAPIES
CVD is the leading cause of morbidity and death in the United States, responsible for one in four deaths. Hyperlipidemia and, specifically, elevated LDL-C have been found to be important drivers of atherosclerosis and, in turn, adverse cardiovascular (CV) events. Likewise, numerous observational and clinical trials have shown that reducing LDL-C, particularly with statins, decreases CVD events.1–4 More aggressive lowering with higher doses or more intensive statin therapy further reduces rates of adverse outcomes.3,4 In addition, the pleiotropic effects of statins imply that not all of their benefits are derived from LDL-C lowering alone.5 Consequently, it is now standard practice to use statins at the highest tolerable dose to reach target LDL-C levels and prevent CV events in high-risk patients with CVD or multiple coronary artery disease risk factors, regardless of the LDL-C levels.6,7
The American College of Cardiology (ACC) and the American Heart Association released cholesterol guidelines in 2013 that recommend a risk-based approach for statin therapy rather than targeting specific LDL-C levels.6 Although this evidence-based approach may better conform to clinical trials, the debate that lower LDL-C targets will further prevent CVD continues.
Indeed, it appears that lower is better, as demonstrated by the IMPROVE-IT trial.8 Although the control group receiving simvastatin monotherapy had low LDL-C levels (mean, 69.9 mg/dL; 1.8 mmol/L), the experimental group receiving simvastatin plus ezetimibe achieved even lower levels (mean, 53.2 mg/dL;1.4 mmol/L) after 1 year of therapy and had a significantly lower composite primary end point of CV death, major coronary event, or nonfatal stroke at 7 years (34.7% for simvastatin monotherapy vs 32.7% for combined therapy).9 Furthermore, the event-rate reduction with the addition of ezetimibe was the same as the average predicted by the Cholesterol Treatment Trialists’ meta-analysis: an LDL-C reduction of 1 mmol/L (38.6 mg/dL) yields a 23% risk reduction in major coronary events over 5 years.10 Although only a modest absolute reduction in outcomes, it supports the notion that further reduction of LDL-C levels by more potent therapies may offer greater benefit.
There is strong evidence that statin therapy reduces the risk of developing CVD in patients with or without a previous atherosclerotic event; however, residual CVD risk remains even for those on therapy. A contributing factor to this residual risk is that many statin-treated patients have insufficient response or intolerance and do not achieve adequate LDL-C reductions.
There are three clinically important patient populations who are inadequately managed with current therapies and remain at high risk of subsequent CV events; these are patients who would benefit from additional therapies.
1. Patients with familial hypercholesterolemia (FH). This is the most common genetic disorder in the world, yet it is frequently undiagnosed and untreated. Due to high baseline cholesterol levels, achieving LDL-C treatment goals is challenging.
- The prevalence may be closer to 1:200 to 1:250 rather than the often quoted 1:500.11
- Fewer than 12% of patients with heterzygous FH achieve the LDL-C goal of < 100 mg/dL with maximal statin treatment alone or with a second agent.12
2. Patients with hyperlipidemia not due to FH who are at elevated CV risk and undertreated. In US and European surveys, between 50% and 60% of patients receiving statins with or without other therapies failed to reach LDL-C reduction goals.13
- Variation in response to statin treatment between individuals may be considerable.
- Poor adherence to statin therapy is common.
3. Patients with side effects to statins, particularly muscle symptoms that prevent statin use or substantially limit the dose.
- Although the incidence of myopathy is low (< 0.1%) and rhabdomyolysis is even less common, observational studies suggest that 10% to 20% of patients may limit statin use due to muscle-associated complaints including muscle aching, cramps, or weakness.14
- Side effects may be dose-dependent, limiting the use of the high-intensity statin doses that are frequently necessary to achieve LDL-C goals.
Consequently, there is great interest in developing therapies beyond statins that may further reduce CV events. However, treatments other than ezetimibe for further management of hyperlipidemia and risk reduction have failed to demonstrate consistent benefit when added to statin therapy.15–19 The largest studies were with niacin and fibrates. Unfortunately, most trials demonstrated no overall outcomes benefit or only benefits in subgroup analyses, leaving the door open to other pharmacologic interventions.
Studies with the cholesterol ester transfer protein (CETP) inhibitor torcetrapib, in combination with statin therapy, actually demonstrated an overall increase in all-cause mortality in the treatment group.20 Two large outcome trials of the CETP inhibitors dalcetrapib and evacetrapib were stopped after interim analysis predicted no benefit. Although drugs such as lomitapide (a microsomal triglyceride transfer protein inhibitor) and mipomersen (an antisense oligonucleotide inhibitor of ApoB-100 synthesis) can lower LDL-C by reducing ApoB synthesis,21 they are approved only in the small population of individuals with homozygous FH and liver toxicity and side effects are a concern.
Accordingly, current cholesterol management guidelines continue to offer LDL-C as the main target of lipid-modifying therapy, with statins as the primary treatment choice. The desire to build on statin therapy to prevent further progression of atherosclerosis and clinical CVD has encouraged continued focus on strategies to lower LDL-C to even greater extents.
Fortunately for practitioners, for the first time since lovastatin was approved in 1987, there is a new therapy approved by the FDA that significantly lowers LDL-C and, potentially, improves CV outcomes—the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors. This review will focus on the PCSK9 inhibitors, a novel therapeutic class that reduces LDL-C through increased hepatic clearance. These drugs are rapidly emerging as an ideal adjunctive therapy to statins for patients at the highest risk and as a highly efficacious alternate therapy in patients intolerant of statins.
PCSK9 INHIBITORS: DISCOVERY, MECHANISM, AND THERAPEUTIC INTERVENTIONS
Two PCSK9 inhibitors have received FDA approval: alirocumab (Praluent) and evolocumab (Repatha). Among new molecular entities for clinical use, PCSK9 inhibitor therapies had one of the shortest durations from discovery to development and approval.
Mutations in the PCSK9 gene associated with autosomal dominant hypercholesterolemia were first identified in 2003 in a French family.22 The PCSK9 protein is now known to be a secreted enzymatic serine protease that is primarily synthesized in the liver and binds to the LDL receptor (LDL-R)/LDL-C complex on the surface of hepatocytes, marking the receptor for lysosomal degradation rather than recycling to the cell surface. Thus, it reduces the quantity of LDL-R that is available to remove LDL-C from circulation.23 As a result, higher levels of PCSK9 are associated with higher levels of plasma LDL-C.
The clinical importance of PCSK9 in regulating LDL-C is supported by observed mutations and polymorphisms. Gain-of-function mutations that increase the activity of PCSK9 have been shown to be associated with elevated LDL-C, premature CVD, and myocardial infarction (MI).24 Conversely, loss-of-function mutations (heterozygotes found in 1% to 3% of the population) result in decreased activity of PCSK9, lower LDL-C, and lower incidence of CVD (Table 1).25–29 These observations, combined with data showing that homozygote loss-of-function individuals with very low LDL-C were generally very healthy, sparked interest in developing inhibition of PCSK9 activity as a therapeutic strategy for hyperlipidemia.
Multiple pharmacologic developments are aimed at inhibiting PCSK9, with many compounds in clinical trials. The approaches include gene silencing with loss-of-function mutations, synthetic peptides, oral small molecules, and monoclonal antibodies. Gene silencing was first observed in 2007 when administration of antisense oligonucleotides targeted to selectively inhibit PCSK9 mRNA was found to up-regulate LDL-R, thereby decreasing serum levels of LDL-C.30
The first study to establish the role of synthetic peptides in PCSK9 inhibition was performed in 2008. In this study, the epidermal growth factor-like A synthetic peptide blocked the interaction between PCSK9 and LDL-R, thereby decreasing the degradation of LDL-R and preserving LDL uptake.31 Although studies are limited, synthetic peptides remain an area of great interest given their promising effects on lipid metabolism. Recently, a synthetic PCSK9-binding adnectin derived from the human fibronectin known as BMS-962476, had favorable results in a phase 1 clinical trial. An RNA interference molecule, subcutaneous ALN-PSC, inhibits PCSK9 gene expression by causing destruction of messenger RNA, thus inhibiting PCSK9 synthesis (Table 2).32
PCSK9 INHIBITORS: CLINICAL TRIALS
Subcutaneously administered monoclonal antibodies targeting PCSK9 currently are the only PCSK9 inhibitors FDA-approved for clinical use. The first study to demonstrate efficacy in enhancing uptake of serum LDL-C was performed in 2009.33 Multiple phase 1 and 2 studies soon followed, demonstrating acceptable safety and 50% to 70% reductions in LDL-C at upper-dose titrations.34 Additionally, there were significant reductions in total cholesterol, ApoB, triglycerides, and lipoprotein(a).
These early developments paved the way for larger phase 3 trials (Table 3).35–48 The PCSK9 inhibitors evolocumab and alirocumab have been shown in multiple phase 3 clinical trials to achieve a consistent dose-dependent 50% to 60% reduction in LDL-C across a broad range of CVD risk, pretreatment LDL-C levels, and background therapy: monotherapy (MENDEL-2, ODYSSEY COMBO I),35,44 added to statin therapy (LAPLACE-2, ODYSSEY CHOICE I),38,46 and in individuals with heterozygous FH (RUTHERFORD-2, ODYSSEY-FH).37,42 Trials with bococizumab are under way.
The GAUSS-2 clinical trial (Goal Achievement after Utilizing an Anti-PCSK9 Antibody in Statin Intolerant Subjects-2) demonstrated similar efficacy in reducing LDL-C in patients with clinically assessed statin intolerance due to muscle-related adverse symptoms.39 In GAUSS-3, patients were first identified as being statin intolerant secondary to muscle-associated symptoms based on a randomized, crossover trial of atorvastatin vs placebo.40 The 43% of participants who experienced intolerable muscle-related symptoms on the statin but not on placebo were then randomized to evolocumab vs ezetimibe. Results showed significant reduction in LDL-C in the evolocumab group (52.8%) compared with the ezetimibe group (16.7%). Additionally, among patients with muscle symptoms on statin therapy, PCSK9 therapy was discontinued for muscle symptoms in only 0.7% of evolocumab recipients and 6.8% of ezetimibe recipients.
Overall, the PCSK9 inhibitors are generally well tolerated with injection site reactions being the most common side effect. A meta-analysis published in 2015 of 25 trials including more than 12,000 patients treated with evolocumab and alirocumab reported no significant difference in adverse events or safety outcomes vs placebo or ezetimibe.49 Antidrug binding or neutralizing antibody production to these agents, thus far, has not been shown to be an issue. Additional analyses have not indicated an adverse effect on gonadal hormone levels or increased incidence of new-onset diabetes.
Two studies published in 2015 offer insight into longer term durability and safety as well as potential CVD outcome benefit (Table 4)50,51:
- OSLER-1 and 2: Open-Label Study of Long-Term Evaluation against LDL-Cholesterol (OSLER) trials—evolocumab trial;50
- ODYSSEY long term: Long-Term Safety and Tolerability of Alirocumab in High Cardiovascular Risk Patients with Hypercholesterolemia Not Adequately Controlled with Their Lipid Modifying Therapy—alirocumab trial.51
The OSLER trials reported durable LDL-C reductions of 61% and the ODYSSEY trial reported a LDL-C reduction of 62%.50,51 In both studies, the overall occurrence of adverse events was similar to placebo, but both reported a higher rate of neurocognitive effects in the active treatment groups (evolocumab 0.9% vs 0.3% for standard therapy; alirocumab 1.2% vs 0.5% for placebo). It must be noted that although the absolute rate of neurocognitive adverse events is low, it is unclear if these events were related to the drugs themselves or to extreme lowering of LDL-C. Nevertheless, the FDA has raised concerns about neurocognitive events. A sub-study of the ongoing FOURIER trial with evolocumab—EBBINGHAUS—is expected to address this concern.
In addition, analyses of CV events showed that the PCSK9 inhibitors effectively cut the CV rate in half in both studies (Figure 1).50,51 In the OSLER trials,50 evolocumab recipients had 53% reduction in major CV events (0.95% vs 2.18% in the standard therapy group; P = .003). In ODYSSEY,51 alirocumab recipients had a 48% reduction in major CV events (1.7% vs 3.3% for placebo; P = .02). Furthermore, a 2015 meta-analysis of 24 phase 2 and 3 trials reported a statistically significant 55% reduction in all-cause mortality and 50% reduction in CV mortality with PCSK9 inhibitors.52
For many reasons including short length of follow-up, study design, and small numbers of outcome events, the OSLER and ODYSSEY studies, although enticing, are exploratory and hypothesis-generating only and results need to be interpreted with caution. Nevertheless, they have set the stage for ongoing prospective randomized outcome trials that are studying the CV effects and tolerability of PCSK9 inhibitors over a longer time frame. These include the following trials.
- The Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) is an ongoing trial with the primary end point of CV death, MI, hospitalization for unstable angina, stroke, or coronary revascularization in high-risk patients receiving evolocumab or placebo.53
- The ODYSSEY trial is examining the effect of alirocumab vs placebo on the composite primary endpoint of coronary heart disease death, non-fatal MI, fatal and nonfatal ischemic stroke, and unstable angina requiring hospitalization in patients who have had an acute coronary syndrome event during the previous 4 to 52 weeks.54
- The Evaluation of Bococizumab in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects (SPIRE) trials are investigating the effect of bococizumab, a third PCSK9 “humanized” monoclonal antibody, vs placebo in reducing death, MI, stroke, or unstable angina in patients at high-risk of CVD who are receiving standard lipid-lowering therapy with LDL-C > 70 mg/dL (1.8 mmol/L) (SPIRE-1) or > 100 mg/dL (2.6 mmol/L) (SPIRE-2).55,56
Because these outcome trials are attempting to enroll more than 70,000 patients and are event driven, it is difficult to predict when they will be completed (Table 5).53–56 However, recent estimates indicate completion of at least one trial by the end of 2016 or early 2017, with interim analyses of others expected at that time. It is hoped that they will answer the all-important question of whether PCSK9 inhibitors are associated with further CV event reduction benefit.
CURRENT FDA INDICATIONS AND GUIDELINES
The two PCSK9 inhibitors approved by the FDA—alirocumab (subcutaneous 75 mg every 2 weeks up titrated to 150 mg) and evolocumab (subcutaneous 140 mg every 2 weeks or 420 mg every 4 weeks)—are both indicated for use with statins in patients with heterozygous FH or known atherosclerotic CVD who require further reduction in LDL-C levels despite lifestyle interventions and use of maximally tolerated statins. Evolocumab has also been approved for use in patients with homozygous FH.
Although PCSK9 inhibitors are not specifically approved for patients unable to tolerate statins, the results of GAUSS-3, which documented that statin intolerance is a real, definable entity and very responsive to PCSK9 inhibition, makes these drugs promising agents for patients intolerant of statins and, thus, unable to benefit from high-intensity stain therapy.
In April 2016, the ACC released a clinical consensus update to their 2013 cholesterol guidelines, which is their first recommendation specifically addressing the use of non-statin therapies, including the newer PCSK9 inhibitors.57 For high-risk patients with clinical atherosclerotic CVD or LDL-C > 190 and failure to achieve at least a 50% reduction in LDL-C on maximally tolerated statin, non-statins may be considered. Ezetimibe, given its safety and tolerability, should be the first additional medication added. Bile acid sequestrants may be used as a second-line therapy if ezetimibe is not tolerated and triglycerides are not elevated. If therapy goals are not met on maximally tolerated statin and ezetimibe, either approved PCSK9 inhibitor can be added or used to replace ezetimibe. The document also specifies that given the lack of long-term safety and efficacy data on the PCSK9 inhibitors, they are not recommended for use in primary prevention patients in the absence of FH.
CONCLUSION
Although statin therapy has been shown to substantially reduce LDL-C and CVD adverse events, there remains a high rate of inadequate goal achievement and residual CVD risk in patients receiving statins. Combination therapies with ezetimibe and resins to further lower LDL-C, fibrates and omega 3 fatty acids to lower triglycerides, and niacin to lower both and raise high-density-liproprotein cholesterol are available, even though additional CV risk reduction is minimal or elusive when these drugs are added to statin therapy.
The link between atherogenic lipoproteins and CVD is strong, and the need to develop therapies in addition to statins to substantially and safely reduce LDL-C remains a priority. The association of reduced PCSK9 activity with reduced LDL-C and CV events has led to rapid development and approval of monoclonal antibody therapies to inhibit PCSK9. In trials, these therapies have shown substantial and durable reductions in LDL-C of more than 50%, with acceptable tolerability. Now that PCSK9 inhibitors are approved by the FDA, extended data about long-term tolerability, safety, and efficacy and, most importantly, demonstration of additional reduction in CVD events are needed. It is hoped that the long-term ongoing trials will provide these data.
For the immediate future, statin therapy will continue to be the cornerstone of lipid and CVD risk management based on their low generic cost, proven CVD risk reduction, and clinicians’ comfort with their use. However, the reliable efficacy of PCSK9 inhibitors and the fact that statin therapy itself increases PCSK9 activity makes the addition of PCSK9 inhibitors to statins an attractive approach in high-risk patients failing to reach LDL-C treatment goals.
Although current indications are limited, there are patients at high CVD risk who would be appropriate candidates for these therapies. These include patients with the following:
- FH with lifetime burden of elevated LDL-C and associated low likelihood of achieving optimal LDL-C control on current available therapies
- Complete or partial statin intolerance with high-intensity statin dosing limited by side effects
- High CV risk who are not at LDL-C goal on current therapies.
Now that the first therapies are available, practitioners can expect newer approaches to tackle PCSK9-mediated LDL-C reduction. Bococizumab is lagging in phase 3 trials, but the SPIRE program is moving forward with special population studies expected to conclude in 2016 and simultaneous long-term outcomes trials. Other PCSK9 inhibitors being investigated include agents with more durable effect requiring less frequent injections, RNA-interference therapies, vaccinations, antisense therapies, and oral formulations.
The PCSK9 inhibitors hold promise as an adjunct to statin therapy. Their eventual clinical role will depend on a balance between substantial LDL-C reductions, long-term safety, tolerability, and reduction in CVD events vs the cost (estimated at $14,000 a year), access from payers, acceptance of injectable therapies, and magnitude of incremental benefit when added to current therapies. Nevertheless, initial clinical trial data are encouraging and these drugs may be an important addition to the therapeutic armamentarium against CVD.
Statin therapy has been shown to substantially reduce adverse events associated with low-density-lipoprotein cholesterol (LDL-C) and cardiovascular disease (CVD). Statins alone are often not adequate to achieve treatment goals, and residual CVD risk remains high. Combination therapies of statins with ezetimibe and resins to further lower LDL-C, fibrates and omega 3 fatty acids to lower triglycerides, and niacin to lower both and raise high-density-liproprotein cholesterol are available, but additional risk reduction has not been consistently demonstrated in clinical trials.
The link between atherogenic lipoproteins and CVD is strong, and the need to develop therapies in addition to statins to substantially and safely reduce LDL-C is a priority. The association of reduced proprotein convertase subtilisin/kexin type 9 (PCSK9) activity with reduced LDL-C and CVD events has led to the rapid development and approval of monoclonal antibody therapies to inhibit PCSK9.
In this review, we discuss trials of these therapies that have shown durable reductions in LDL-C of more than 50%, with acceptable tolerability. Now that PCSK9 inhibitors are approved by the US Food and Drug Administration (FDA), extended data are needed as to long-term tolerability, safety, and efficacy of these agents and, most importantly, demonstration of additional reduction in CVD events.
A CASE FOR ADDITIONAL THERAPIES
CVD is the leading cause of morbidity and death in the United States, responsible for one in four deaths. Hyperlipidemia and, specifically, elevated LDL-C have been found to be important drivers of atherosclerosis and, in turn, adverse cardiovascular (CV) events. Likewise, numerous observational and clinical trials have shown that reducing LDL-C, particularly with statins, decreases CVD events.1–4 More aggressive lowering with higher doses or more intensive statin therapy further reduces rates of adverse outcomes.3,4 In addition, the pleiotropic effects of statins imply that not all of their benefits are derived from LDL-C lowering alone.5 Consequently, it is now standard practice to use statins at the highest tolerable dose to reach target LDL-C levels and prevent CV events in high-risk patients with CVD or multiple coronary artery disease risk factors, regardless of the LDL-C levels.6,7
The American College of Cardiology (ACC) and the American Heart Association released cholesterol guidelines in 2013 that recommend a risk-based approach for statin therapy rather than targeting specific LDL-C levels.6 Although this evidence-based approach may better conform to clinical trials, the debate that lower LDL-C targets will further prevent CVD continues.
Indeed, it appears that lower is better, as demonstrated by the IMPROVE-IT trial.8 Although the control group receiving simvastatin monotherapy had low LDL-C levels (mean, 69.9 mg/dL; 1.8 mmol/L), the experimental group receiving simvastatin plus ezetimibe achieved even lower levels (mean, 53.2 mg/dL;1.4 mmol/L) after 1 year of therapy and had a significantly lower composite primary end point of CV death, major coronary event, or nonfatal stroke at 7 years (34.7% for simvastatin monotherapy vs 32.7% for combined therapy).9 Furthermore, the event-rate reduction with the addition of ezetimibe was the same as the average predicted by the Cholesterol Treatment Trialists’ meta-analysis: an LDL-C reduction of 1 mmol/L (38.6 mg/dL) yields a 23% risk reduction in major coronary events over 5 years.10 Although only a modest absolute reduction in outcomes, it supports the notion that further reduction of LDL-C levels by more potent therapies may offer greater benefit.
There is strong evidence that statin therapy reduces the risk of developing CVD in patients with or without a previous atherosclerotic event; however, residual CVD risk remains even for those on therapy. A contributing factor to this residual risk is that many statin-treated patients have insufficient response or intolerance and do not achieve adequate LDL-C reductions.
There are three clinically important patient populations who are inadequately managed with current therapies and remain at high risk of subsequent CV events; these are patients who would benefit from additional therapies.
1. Patients with familial hypercholesterolemia (FH). This is the most common genetic disorder in the world, yet it is frequently undiagnosed and untreated. Due to high baseline cholesterol levels, achieving LDL-C treatment goals is challenging.
- The prevalence may be closer to 1:200 to 1:250 rather than the often quoted 1:500.11
- Fewer than 12% of patients with heterzygous FH achieve the LDL-C goal of < 100 mg/dL with maximal statin treatment alone or with a second agent.12
2. Patients with hyperlipidemia not due to FH who are at elevated CV risk and undertreated. In US and European surveys, between 50% and 60% of patients receiving statins with or without other therapies failed to reach LDL-C reduction goals.13
- Variation in response to statin treatment between individuals may be considerable.
- Poor adherence to statin therapy is common.
3. Patients with side effects to statins, particularly muscle symptoms that prevent statin use or substantially limit the dose.
- Although the incidence of myopathy is low (< 0.1%) and rhabdomyolysis is even less common, observational studies suggest that 10% to 20% of patients may limit statin use due to muscle-associated complaints including muscle aching, cramps, or weakness.14
- Side effects may be dose-dependent, limiting the use of the high-intensity statin doses that are frequently necessary to achieve LDL-C goals.
Consequently, there is great interest in developing therapies beyond statins that may further reduce CV events. However, treatments other than ezetimibe for further management of hyperlipidemia and risk reduction have failed to demonstrate consistent benefit when added to statin therapy.15–19 The largest studies were with niacin and fibrates. Unfortunately, most trials demonstrated no overall outcomes benefit or only benefits in subgroup analyses, leaving the door open to other pharmacologic interventions.
Studies with the cholesterol ester transfer protein (CETP) inhibitor torcetrapib, in combination with statin therapy, actually demonstrated an overall increase in all-cause mortality in the treatment group.20 Two large outcome trials of the CETP inhibitors dalcetrapib and evacetrapib were stopped after interim analysis predicted no benefit. Although drugs such as lomitapide (a microsomal triglyceride transfer protein inhibitor) and mipomersen (an antisense oligonucleotide inhibitor of ApoB-100 synthesis) can lower LDL-C by reducing ApoB synthesis,21 they are approved only in the small population of individuals with homozygous FH and liver toxicity and side effects are a concern.
Accordingly, current cholesterol management guidelines continue to offer LDL-C as the main target of lipid-modifying therapy, with statins as the primary treatment choice. The desire to build on statin therapy to prevent further progression of atherosclerosis and clinical CVD has encouraged continued focus on strategies to lower LDL-C to even greater extents.
Fortunately for practitioners, for the first time since lovastatin was approved in 1987, there is a new therapy approved by the FDA that significantly lowers LDL-C and, potentially, improves CV outcomes—the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors. This review will focus on the PCSK9 inhibitors, a novel therapeutic class that reduces LDL-C through increased hepatic clearance. These drugs are rapidly emerging as an ideal adjunctive therapy to statins for patients at the highest risk and as a highly efficacious alternate therapy in patients intolerant of statins.
PCSK9 INHIBITORS: DISCOVERY, MECHANISM, AND THERAPEUTIC INTERVENTIONS
Two PCSK9 inhibitors have received FDA approval: alirocumab (Praluent) and evolocumab (Repatha). Among new molecular entities for clinical use, PCSK9 inhibitor therapies had one of the shortest durations from discovery to development and approval.
Mutations in the PCSK9 gene associated with autosomal dominant hypercholesterolemia were first identified in 2003 in a French family.22 The PCSK9 protein is now known to be a secreted enzymatic serine protease that is primarily synthesized in the liver and binds to the LDL receptor (LDL-R)/LDL-C complex on the surface of hepatocytes, marking the receptor for lysosomal degradation rather than recycling to the cell surface. Thus, it reduces the quantity of LDL-R that is available to remove LDL-C from circulation.23 As a result, higher levels of PCSK9 are associated with higher levels of plasma LDL-C.
The clinical importance of PCSK9 in regulating LDL-C is supported by observed mutations and polymorphisms. Gain-of-function mutations that increase the activity of PCSK9 have been shown to be associated with elevated LDL-C, premature CVD, and myocardial infarction (MI).24 Conversely, loss-of-function mutations (heterozygotes found in 1% to 3% of the population) result in decreased activity of PCSK9, lower LDL-C, and lower incidence of CVD (Table 1).25–29 These observations, combined with data showing that homozygote loss-of-function individuals with very low LDL-C were generally very healthy, sparked interest in developing inhibition of PCSK9 activity as a therapeutic strategy for hyperlipidemia.
Multiple pharmacologic developments are aimed at inhibiting PCSK9, with many compounds in clinical trials. The approaches include gene silencing with loss-of-function mutations, synthetic peptides, oral small molecules, and monoclonal antibodies. Gene silencing was first observed in 2007 when administration of antisense oligonucleotides targeted to selectively inhibit PCSK9 mRNA was found to up-regulate LDL-R, thereby decreasing serum levels of LDL-C.30
The first study to establish the role of synthetic peptides in PCSK9 inhibition was performed in 2008. In this study, the epidermal growth factor-like A synthetic peptide blocked the interaction between PCSK9 and LDL-R, thereby decreasing the degradation of LDL-R and preserving LDL uptake.31 Although studies are limited, synthetic peptides remain an area of great interest given their promising effects on lipid metabolism. Recently, a synthetic PCSK9-binding adnectin derived from the human fibronectin known as BMS-962476, had favorable results in a phase 1 clinical trial. An RNA interference molecule, subcutaneous ALN-PSC, inhibits PCSK9 gene expression by causing destruction of messenger RNA, thus inhibiting PCSK9 synthesis (Table 2).32
PCSK9 INHIBITORS: CLINICAL TRIALS
Subcutaneously administered monoclonal antibodies targeting PCSK9 currently are the only PCSK9 inhibitors FDA-approved for clinical use. The first study to demonstrate efficacy in enhancing uptake of serum LDL-C was performed in 2009.33 Multiple phase 1 and 2 studies soon followed, demonstrating acceptable safety and 50% to 70% reductions in LDL-C at upper-dose titrations.34 Additionally, there were significant reductions in total cholesterol, ApoB, triglycerides, and lipoprotein(a).
These early developments paved the way for larger phase 3 trials (Table 3).35–48 The PCSK9 inhibitors evolocumab and alirocumab have been shown in multiple phase 3 clinical trials to achieve a consistent dose-dependent 50% to 60% reduction in LDL-C across a broad range of CVD risk, pretreatment LDL-C levels, and background therapy: monotherapy (MENDEL-2, ODYSSEY COMBO I),35,44 added to statin therapy (LAPLACE-2, ODYSSEY CHOICE I),38,46 and in individuals with heterozygous FH (RUTHERFORD-2, ODYSSEY-FH).37,42 Trials with bococizumab are under way.
The GAUSS-2 clinical trial (Goal Achievement after Utilizing an Anti-PCSK9 Antibody in Statin Intolerant Subjects-2) demonstrated similar efficacy in reducing LDL-C in patients with clinically assessed statin intolerance due to muscle-related adverse symptoms.39 In GAUSS-3, patients were first identified as being statin intolerant secondary to muscle-associated symptoms based on a randomized, crossover trial of atorvastatin vs placebo.40 The 43% of participants who experienced intolerable muscle-related symptoms on the statin but not on placebo were then randomized to evolocumab vs ezetimibe. Results showed significant reduction in LDL-C in the evolocumab group (52.8%) compared with the ezetimibe group (16.7%). Additionally, among patients with muscle symptoms on statin therapy, PCSK9 therapy was discontinued for muscle symptoms in only 0.7% of evolocumab recipients and 6.8% of ezetimibe recipients.
Overall, the PCSK9 inhibitors are generally well tolerated with injection site reactions being the most common side effect. A meta-analysis published in 2015 of 25 trials including more than 12,000 patients treated with evolocumab and alirocumab reported no significant difference in adverse events or safety outcomes vs placebo or ezetimibe.49 Antidrug binding or neutralizing antibody production to these agents, thus far, has not been shown to be an issue. Additional analyses have not indicated an adverse effect on gonadal hormone levels or increased incidence of new-onset diabetes.
Two studies published in 2015 offer insight into longer term durability and safety as well as potential CVD outcome benefit (Table 4)50,51:
- OSLER-1 and 2: Open-Label Study of Long-Term Evaluation against LDL-Cholesterol (OSLER) trials—evolocumab trial;50
- ODYSSEY long term: Long-Term Safety and Tolerability of Alirocumab in High Cardiovascular Risk Patients with Hypercholesterolemia Not Adequately Controlled with Their Lipid Modifying Therapy—alirocumab trial.51
The OSLER trials reported durable LDL-C reductions of 61% and the ODYSSEY trial reported a LDL-C reduction of 62%.50,51 In both studies, the overall occurrence of adverse events was similar to placebo, but both reported a higher rate of neurocognitive effects in the active treatment groups (evolocumab 0.9% vs 0.3% for standard therapy; alirocumab 1.2% vs 0.5% for placebo). It must be noted that although the absolute rate of neurocognitive adverse events is low, it is unclear if these events were related to the drugs themselves or to extreme lowering of LDL-C. Nevertheless, the FDA has raised concerns about neurocognitive events. A sub-study of the ongoing FOURIER trial with evolocumab—EBBINGHAUS—is expected to address this concern.
In addition, analyses of CV events showed that the PCSK9 inhibitors effectively cut the CV rate in half in both studies (Figure 1).50,51 In the OSLER trials,50 evolocumab recipients had 53% reduction in major CV events (0.95% vs 2.18% in the standard therapy group; P = .003). In ODYSSEY,51 alirocumab recipients had a 48% reduction in major CV events (1.7% vs 3.3% for placebo; P = .02). Furthermore, a 2015 meta-analysis of 24 phase 2 and 3 trials reported a statistically significant 55% reduction in all-cause mortality and 50% reduction in CV mortality with PCSK9 inhibitors.52
For many reasons including short length of follow-up, study design, and small numbers of outcome events, the OSLER and ODYSSEY studies, although enticing, are exploratory and hypothesis-generating only and results need to be interpreted with caution. Nevertheless, they have set the stage for ongoing prospective randomized outcome trials that are studying the CV effects and tolerability of PCSK9 inhibitors over a longer time frame. These include the following trials.
- The Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) is an ongoing trial with the primary end point of CV death, MI, hospitalization for unstable angina, stroke, or coronary revascularization in high-risk patients receiving evolocumab or placebo.53
- The ODYSSEY trial is examining the effect of alirocumab vs placebo on the composite primary endpoint of coronary heart disease death, non-fatal MI, fatal and nonfatal ischemic stroke, and unstable angina requiring hospitalization in patients who have had an acute coronary syndrome event during the previous 4 to 52 weeks.54
- The Evaluation of Bococizumab in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects (SPIRE) trials are investigating the effect of bococizumab, a third PCSK9 “humanized” monoclonal antibody, vs placebo in reducing death, MI, stroke, or unstable angina in patients at high-risk of CVD who are receiving standard lipid-lowering therapy with LDL-C > 70 mg/dL (1.8 mmol/L) (SPIRE-1) or > 100 mg/dL (2.6 mmol/L) (SPIRE-2).55,56
Because these outcome trials are attempting to enroll more than 70,000 patients and are event driven, it is difficult to predict when they will be completed (Table 5).53–56 However, recent estimates indicate completion of at least one trial by the end of 2016 or early 2017, with interim analyses of others expected at that time. It is hoped that they will answer the all-important question of whether PCSK9 inhibitors are associated with further CV event reduction benefit.
CURRENT FDA INDICATIONS AND GUIDELINES
The two PCSK9 inhibitors approved by the FDA—alirocumab (subcutaneous 75 mg every 2 weeks up titrated to 150 mg) and evolocumab (subcutaneous 140 mg every 2 weeks or 420 mg every 4 weeks)—are both indicated for use with statins in patients with heterozygous FH or known atherosclerotic CVD who require further reduction in LDL-C levels despite lifestyle interventions and use of maximally tolerated statins. Evolocumab has also been approved for use in patients with homozygous FH.
Although PCSK9 inhibitors are not specifically approved for patients unable to tolerate statins, the results of GAUSS-3, which documented that statin intolerance is a real, definable entity and very responsive to PCSK9 inhibition, makes these drugs promising agents for patients intolerant of statins and, thus, unable to benefit from high-intensity stain therapy.
In April 2016, the ACC released a clinical consensus update to their 2013 cholesterol guidelines, which is their first recommendation specifically addressing the use of non-statin therapies, including the newer PCSK9 inhibitors.57 For high-risk patients with clinical atherosclerotic CVD or LDL-C > 190 and failure to achieve at least a 50% reduction in LDL-C on maximally tolerated statin, non-statins may be considered. Ezetimibe, given its safety and tolerability, should be the first additional medication added. Bile acid sequestrants may be used as a second-line therapy if ezetimibe is not tolerated and triglycerides are not elevated. If therapy goals are not met on maximally tolerated statin and ezetimibe, either approved PCSK9 inhibitor can be added or used to replace ezetimibe. The document also specifies that given the lack of long-term safety and efficacy data on the PCSK9 inhibitors, they are not recommended for use in primary prevention patients in the absence of FH.
CONCLUSION
Although statin therapy has been shown to substantially reduce LDL-C and CVD adverse events, there remains a high rate of inadequate goal achievement and residual CVD risk in patients receiving statins. Combination therapies with ezetimibe and resins to further lower LDL-C, fibrates and omega 3 fatty acids to lower triglycerides, and niacin to lower both and raise high-density-liproprotein cholesterol are available, even though additional CV risk reduction is minimal or elusive when these drugs are added to statin therapy.
The link between atherogenic lipoproteins and CVD is strong, and the need to develop therapies in addition to statins to substantially and safely reduce LDL-C remains a priority. The association of reduced PCSK9 activity with reduced LDL-C and CV events has led to rapid development and approval of monoclonal antibody therapies to inhibit PCSK9. In trials, these therapies have shown substantial and durable reductions in LDL-C of more than 50%, with acceptable tolerability. Now that PCSK9 inhibitors are approved by the FDA, extended data about long-term tolerability, safety, and efficacy and, most importantly, demonstration of additional reduction in CVD events are needed. It is hoped that the long-term ongoing trials will provide these data.
For the immediate future, statin therapy will continue to be the cornerstone of lipid and CVD risk management based on their low generic cost, proven CVD risk reduction, and clinicians’ comfort with their use. However, the reliable efficacy of PCSK9 inhibitors and the fact that statin therapy itself increases PCSK9 activity makes the addition of PCSK9 inhibitors to statins an attractive approach in high-risk patients failing to reach LDL-C treatment goals.
Although current indications are limited, there are patients at high CVD risk who would be appropriate candidates for these therapies. These include patients with the following:
- FH with lifetime burden of elevated LDL-C and associated low likelihood of achieving optimal LDL-C control on current available therapies
- Complete or partial statin intolerance with high-intensity statin dosing limited by side effects
- High CV risk who are not at LDL-C goal on current therapies.
Now that the first therapies are available, practitioners can expect newer approaches to tackle PCSK9-mediated LDL-C reduction. Bococizumab is lagging in phase 3 trials, but the SPIRE program is moving forward with special population studies expected to conclude in 2016 and simultaneous long-term outcomes trials. Other PCSK9 inhibitors being investigated include agents with more durable effect requiring less frequent injections, RNA-interference therapies, vaccinations, antisense therapies, and oral formulations.
The PCSK9 inhibitors hold promise as an adjunct to statin therapy. Their eventual clinical role will depend on a balance between substantial LDL-C reductions, long-term safety, tolerability, and reduction in CVD events vs the cost (estimated at $14,000 a year), access from payers, acceptance of injectable therapies, and magnitude of incremental benefit when added to current therapies. Nevertheless, initial clinical trial data are encouraging and these drugs may be an important addition to the therapeutic armamentarium against CVD.
- Scandinavian Simvastatin Survival Study Group. Randomized trial of cholesterol lowering in 4,444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- Sacks FM, Pfeiffer MA, Moye LA, et al; Cholesterol and Recurrent Events Trial Investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial Investigators. N Engl J Med 1996; 335:1001–1009.
- Schwartz GG, Olsson AG, Ezekowitz MD, et al; Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001; 285:1711–1718.
- Lipid Research Clinics Program. The Lipid Research Clinics Coronary Primary Prevention Trial results (reduction in incidence of coronary heart disease). JAMA 1984; 251:351–364.
- Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation 2004; 109(suppl 1):III39–III43.
- Stone N, Robinson J, Lichtenstein A, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(suppl 2):S1–S45.
- Jacobson TA, Ito MK, Maki KC, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 1—full report. J Clin Lipidol 2015; 9:129–169.
- Jarcho JA, Keaney JF Jr. Proof that lower is better–LDL cholesterol and IMPROVE-IT. N Engl J Med 2015; 372:2448–2450.
- Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015; 372:2387–2397.
- Baigent C, Keech A, Kearney PM, et al; Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- de Ferranti S, Rodday AM, Mendelson M, et al. Prevalence of familial hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES). Circulation 2016; 133:1067–1072.
- Perez de Isla L, Alonso R, Watts GF, et al; SAFEHEART investigators. Attainment of LDL-cholesterol treatment goals in patients with familial hypercholesterolemia: 5-year SAFEHEART registry follow-up. J Am Coll Cardiol 2016; 67:1278–1285.
- Unni SK, Quek RGW, Biskupiak J, et al. Assessment of statin therapy, LDL-C levels, and cardiovascular events among high-risk patients in the United States. J Clin Lipidol 2016; 10:63–71.
- Mammen AL, Amato AA. Statin myopathy: a review of recent progress. Curr Opin Rheum 2010; 22:644–650.
- AIM-HIGH Investigators; Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013; 34:1279–1291.
- ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010; 362:1563–1574.
- Keech A, Simes RJ, Barter P, et al; FIELD study investigators. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomized controlled trial. Lancet 2005; 366:1849–1861.
- Kaur N, Pandey A, Negi H, et al. Effect of HDL-raising drugs on cardiovascular outcomes: a systematic review and meta-regression. PLoS One 2014; 9:e94585.
- Barter PJ, Caulfield M, Eriksson M, et al; ILLUMINATE investigators. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007; 357:2109–2122.
- Rader D, Kastelein J. Lomitapide and mipomersen: two first-in-class drugs for reducing low-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. Circulation 2014; 129:1022–1032.
- Abifadel M, Varret M, Rabes JP, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 2003; 34:154–156.
- Verbeek R, Stoekenbroek RM, Hovingh GK. PCSK9 inhibitors: novel therapeutic agents for the treatment of hypercholesterolemia. Eur J of Pharm 2015; 763(Pt A):38–47.
- Steinberg D, Witztum JL. Inhibition of PCSK9: a powerful weapon for achieving ideal LDL cholesterol levels. Proc Natl Acad Sci USA 2009; 106:9546–9547.
- Abifadel M, Rabès J-P, Devillers M, et al. Mutations and polymorphisms in the proprotein convertase subtilisin kexin 9 (PCSK9) gene in cholesterol metabolism and disease. Hum Mutat 2009; 30:520–529.
- Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006; 354:1264–1272.
- Benn M, Nordestgaard BG, Grande P, Schnohr P, Tybærg-Hansen A. PCSK9 R46L, low-density lipoprotein cholesterol levels, and risk of ischemic heart disease. J Am Coll Cardiol 2010; 55:2833–2842.
- Mortensen MB, Afzal S, Nordestgaard BG, Falk E. The high-density lipoprotein-adjusted SCORE model worsens SCORE-based risk classification in a contemporary population of 30,824 Europeans: the Copenhagen General Population Study. Eur Heart J 2015; 36:2446–2453.
- Victor RG, Haley RW, Willett DL, et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol 2004; 93:1473–1480.
- Graham MJ, Lemonidis KM, Whipple CP, et al. Antisense inhibition of proprotein convertase subtilisin/kexin type 9 reduces serum LDL in hyperlipidemic mice. J Lipid Res 2007; 48:763–767.
- Shan L, Pang L, Zhang R, Murgolo NJ, Lan H, Hedrick JA. PCSK9 binds to multiple receptors and can be functionally inhibited by an EGF-A peptide. Biochem Biophys Res Comm 2008; 375:69–73.
- Stein EA, Raal F. Reduction of low-density lipoprotein cholesterol by monoclonal antibody inhibition of PCSK9. Annu Rev Med 2014; 65:417–431.
- Duff CJ, Scott MJ, Kirby IT, Hutchinson SE, Martin SL, Hooper NM. Antibody-mediated disruption of the interaction between PCSK9 and the low-density lipoprotein receptor. Biochem J 2009; 419:577–584.
- Stein EA, Mellis S, Yancopoulos GD, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Eng J Med 2012; 366:1108–1118.
- Koren MJ, Lundqvist P, Bolognese M, et al; MENDEL-2 Investigators. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol 2014; 63:2531–2540.
- Blom DJ, Hala T, Bolognese M, et al; DESCARTES investigators. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med 2014; 370:1809-1819.
- Raal FJ, Stein EA, Dufour R, et al; RUTHERFORD-2 Investigators. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet 2014; 385:331–340.
- Robinson JG, Nedergaard BS, Rogers WJ, et al; LAPLA C-2 Investigators. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA 2014; 311:1870–1882.
- Stroes E, Colquhoun D, Sullivan D, et al; GAUSS-2 Investigators. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol 2014; 63:2541–2548.
- Nissen SE, Stroes E, Dent-Acosta RE, et al; GAUSS-3 Investigators. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance, the GAUSS-3 randomized clinical trial. JAMA 2016; 315:1580–1590.
- Trial assessing long term use of PCSK9 inhibition in subjects with genetic LDL disorders (TAUSSIG). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT-1624142. Updated June 25, 2015. Accessed October 23, 2016.
- Kastelein JJ, Ginsberg HN, Langslet G, et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J 2015; 36:2996–3003.
- Efficacy and safety of alirocumab (SAR236553/REGN727) versus placebo on top of lipid-modifying therapy in patients with heterozygous familial hypercholesterolemia; the ODYSSEY HIGH FH trial. U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01617655. Updated September 27, 2016. Accessed October 23, 2016.
- Kereiakes DJ, Robinson JG, Cannon CP, et al. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: The ODYSSEY COMBO I study. Am Heart J 2015; 169:906–915.
- Efficacy and Safety of Alirocumab (SAR236553/REGN727) Versus Ezetimibe on Top of Statin in High Cardiovascular Risk Patients With Hypercholesterolemia (ODYSSEY COMBO II). U.S. National Institutes of Health website. Updated June 23, 2016. https://clinicaltrials.gov/ct2/show/NCT01644188. Accessed October 23, 2016.
- Roth EM, Moriarty P, Bergeron J, et al; ODYSSEY CHOICE I investigators. A phase III randomized trial evaluating alirocumab 300 mg every 4 weeks as monotherapy or add-on to statin: ODYSSEY CHOICE I. Atherosclerosis 2016, doi: 10.1016/j.atherosclerosis.2016.08.043.
- Phase III Study To Evaluate Alirocumab in Patients With Hypercholesterolemia Not Treated With a Statin (ODYSSEY CHOICE II). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT02023879. Updated November 2, 2015. Accessed October 23, 2016.
- Monthly and twice monthly subcutaneous dosing of PF-04950615 (RN316) in hypercholesterolemic subjects on a statin. U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/results?term=NCT01592240. Updated October 14, 2014. Accessed October 23, 2016.
- Zhang XL, Zhu QQ, Zhu L, et al. Safety and efficacy of anti-PCSK9 antibodies: a meta-analysis of 25 randomized, controlled trials. BMC Med 2015; 13:123.
- Sabatine MS, Giugliano RP, Wiviott SD, et al; OSLER Investigators. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372:1500–1509.
- Robinson JG, Farnier M, Krempf M, et al; ODYSSEY LONG TERM Investigators. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372:1489–1499.
- Navarese EP, Kolodziejczak M, Schulze V, et al. Effects of proprotein convertase subtilisin/kexin type 9 antibodies in adults with hypercholesterolemia: a systematic review and meta-analysis. Ann Intern Med 2015; 163:40–51.
- Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01764633. Updated July 26, 2016. Accessed October 23, 2016.
- ODYSSEY Outcomes: Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab. U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01663402. Updated October 23, 2016. Accessed September 13, 2016.
- The Evaluation of Bococizumab (PF-04950615;RN316) in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects (SPIRE-1). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01975376. Updated September 22, 2016. Accessed October 23, 2016.
- The Evaluation of Bococizumab (PF-04950615; RN316) in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects (SPIRE-2). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01975389. Updated July 26, 2016. Accessed October 23, 2016.
- Lloyd-Jones DM, Morris PB, Ballantyne CM, et al; Writing Committee. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology task force on clinical expert consensus documents. J Am Coll Cardiol 2016; 68:92–125.
- Scandinavian Simvastatin Survival Study Group. Randomized trial of cholesterol lowering in 4,444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- Sacks FM, Pfeiffer MA, Moye LA, et al; Cholesterol and Recurrent Events Trial Investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial Investigators. N Engl J Med 1996; 335:1001–1009.
- Schwartz GG, Olsson AG, Ezekowitz MD, et al; Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001; 285:1711–1718.
- Lipid Research Clinics Program. The Lipid Research Clinics Coronary Primary Prevention Trial results (reduction in incidence of coronary heart disease). JAMA 1984; 251:351–364.
- Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation 2004; 109(suppl 1):III39–III43.
- Stone N, Robinson J, Lichtenstein A, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(suppl 2):S1–S45.
- Jacobson TA, Ito MK, Maki KC, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 1—full report. J Clin Lipidol 2015; 9:129–169.
- Jarcho JA, Keaney JF Jr. Proof that lower is better–LDL cholesterol and IMPROVE-IT. N Engl J Med 2015; 372:2448–2450.
- Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015; 372:2387–2397.
- Baigent C, Keech A, Kearney PM, et al; Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- de Ferranti S, Rodday AM, Mendelson M, et al. Prevalence of familial hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES). Circulation 2016; 133:1067–1072.
- Perez de Isla L, Alonso R, Watts GF, et al; SAFEHEART investigators. Attainment of LDL-cholesterol treatment goals in patients with familial hypercholesterolemia: 5-year SAFEHEART registry follow-up. J Am Coll Cardiol 2016; 67:1278–1285.
- Unni SK, Quek RGW, Biskupiak J, et al. Assessment of statin therapy, LDL-C levels, and cardiovascular events among high-risk patients in the United States. J Clin Lipidol 2016; 10:63–71.
- Mammen AL, Amato AA. Statin myopathy: a review of recent progress. Curr Opin Rheum 2010; 22:644–650.
- AIM-HIGH Investigators; Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013; 34:1279–1291.
- ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010; 362:1563–1574.
- Keech A, Simes RJ, Barter P, et al; FIELD study investigators. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomized controlled trial. Lancet 2005; 366:1849–1861.
- Kaur N, Pandey A, Negi H, et al. Effect of HDL-raising drugs on cardiovascular outcomes: a systematic review and meta-regression. PLoS One 2014; 9:e94585.
- Barter PJ, Caulfield M, Eriksson M, et al; ILLUMINATE investigators. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007; 357:2109–2122.
- Rader D, Kastelein J. Lomitapide and mipomersen: two first-in-class drugs for reducing low-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. Circulation 2014; 129:1022–1032.
- Abifadel M, Varret M, Rabes JP, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 2003; 34:154–156.
- Verbeek R, Stoekenbroek RM, Hovingh GK. PCSK9 inhibitors: novel therapeutic agents for the treatment of hypercholesterolemia. Eur J of Pharm 2015; 763(Pt A):38–47.
- Steinberg D, Witztum JL. Inhibition of PCSK9: a powerful weapon for achieving ideal LDL cholesterol levels. Proc Natl Acad Sci USA 2009; 106:9546–9547.
- Abifadel M, Rabès J-P, Devillers M, et al. Mutations and polymorphisms in the proprotein convertase subtilisin kexin 9 (PCSK9) gene in cholesterol metabolism and disease. Hum Mutat 2009; 30:520–529.
- Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006; 354:1264–1272.
- Benn M, Nordestgaard BG, Grande P, Schnohr P, Tybærg-Hansen A. PCSK9 R46L, low-density lipoprotein cholesterol levels, and risk of ischemic heart disease. J Am Coll Cardiol 2010; 55:2833–2842.
- Mortensen MB, Afzal S, Nordestgaard BG, Falk E. The high-density lipoprotein-adjusted SCORE model worsens SCORE-based risk classification in a contemporary population of 30,824 Europeans: the Copenhagen General Population Study. Eur Heart J 2015; 36:2446–2453.
- Victor RG, Haley RW, Willett DL, et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol 2004; 93:1473–1480.
- Graham MJ, Lemonidis KM, Whipple CP, et al. Antisense inhibition of proprotein convertase subtilisin/kexin type 9 reduces serum LDL in hyperlipidemic mice. J Lipid Res 2007; 48:763–767.
- Shan L, Pang L, Zhang R, Murgolo NJ, Lan H, Hedrick JA. PCSK9 binds to multiple receptors and can be functionally inhibited by an EGF-A peptide. Biochem Biophys Res Comm 2008; 375:69–73.
- Stein EA, Raal F. Reduction of low-density lipoprotein cholesterol by monoclonal antibody inhibition of PCSK9. Annu Rev Med 2014; 65:417–431.
- Duff CJ, Scott MJ, Kirby IT, Hutchinson SE, Martin SL, Hooper NM. Antibody-mediated disruption of the interaction between PCSK9 and the low-density lipoprotein receptor. Biochem J 2009; 419:577–584.
- Stein EA, Mellis S, Yancopoulos GD, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Eng J Med 2012; 366:1108–1118.
- Koren MJ, Lundqvist P, Bolognese M, et al; MENDEL-2 Investigators. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol 2014; 63:2531–2540.
- Blom DJ, Hala T, Bolognese M, et al; DESCARTES investigators. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med 2014; 370:1809-1819.
- Raal FJ, Stein EA, Dufour R, et al; RUTHERFORD-2 Investigators. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet 2014; 385:331–340.
- Robinson JG, Nedergaard BS, Rogers WJ, et al; LAPLA C-2 Investigators. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA 2014; 311:1870–1882.
- Stroes E, Colquhoun D, Sullivan D, et al; GAUSS-2 Investigators. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol 2014; 63:2541–2548.
- Nissen SE, Stroes E, Dent-Acosta RE, et al; GAUSS-3 Investigators. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance, the GAUSS-3 randomized clinical trial. JAMA 2016; 315:1580–1590.
- Trial assessing long term use of PCSK9 inhibition in subjects with genetic LDL disorders (TAUSSIG). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT-1624142. Updated June 25, 2015. Accessed October 23, 2016.
- Kastelein JJ, Ginsberg HN, Langslet G, et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J 2015; 36:2996–3003.
- Efficacy and safety of alirocumab (SAR236553/REGN727) versus placebo on top of lipid-modifying therapy in patients with heterozygous familial hypercholesterolemia; the ODYSSEY HIGH FH trial. U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01617655. Updated September 27, 2016. Accessed October 23, 2016.
- Kereiakes DJ, Robinson JG, Cannon CP, et al. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: The ODYSSEY COMBO I study. Am Heart J 2015; 169:906–915.
- Efficacy and Safety of Alirocumab (SAR236553/REGN727) Versus Ezetimibe on Top of Statin in High Cardiovascular Risk Patients With Hypercholesterolemia (ODYSSEY COMBO II). U.S. National Institutes of Health website. Updated June 23, 2016. https://clinicaltrials.gov/ct2/show/NCT01644188. Accessed October 23, 2016.
- Roth EM, Moriarty P, Bergeron J, et al; ODYSSEY CHOICE I investigators. A phase III randomized trial evaluating alirocumab 300 mg every 4 weeks as monotherapy or add-on to statin: ODYSSEY CHOICE I. Atherosclerosis 2016, doi: 10.1016/j.atherosclerosis.2016.08.043.
- Phase III Study To Evaluate Alirocumab in Patients With Hypercholesterolemia Not Treated With a Statin (ODYSSEY CHOICE II). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT02023879. Updated November 2, 2015. Accessed October 23, 2016.
- Monthly and twice monthly subcutaneous dosing of PF-04950615 (RN316) in hypercholesterolemic subjects on a statin. U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/results?term=NCT01592240. Updated October 14, 2014. Accessed October 23, 2016.
- Zhang XL, Zhu QQ, Zhu L, et al. Safety and efficacy of anti-PCSK9 antibodies: a meta-analysis of 25 randomized, controlled trials. BMC Med 2015; 13:123.
- Sabatine MS, Giugliano RP, Wiviott SD, et al; OSLER Investigators. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372:1500–1509.
- Robinson JG, Farnier M, Krempf M, et al; ODYSSEY LONG TERM Investigators. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372:1489–1499.
- Navarese EP, Kolodziejczak M, Schulze V, et al. Effects of proprotein convertase subtilisin/kexin type 9 antibodies in adults with hypercholesterolemia: a systematic review and meta-analysis. Ann Intern Med 2015; 163:40–51.
- Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01764633. Updated July 26, 2016. Accessed October 23, 2016.
- ODYSSEY Outcomes: Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab. U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01663402. Updated October 23, 2016. Accessed September 13, 2016.
- The Evaluation of Bococizumab (PF-04950615;RN316) in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects (SPIRE-1). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01975376. Updated September 22, 2016. Accessed October 23, 2016.
- The Evaluation of Bococizumab (PF-04950615; RN316) in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects (SPIRE-2). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01975389. Updated July 26, 2016. Accessed October 23, 2016.
- Lloyd-Jones DM, Morris PB, Ballantyne CM, et al; Writing Committee. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology task force on clinical expert consensus documents. J Am Coll Cardiol 2016; 68:92–125.
KEY POINTS
- Potential candidates for PCSK9 inhibitor therapy are patients with familial hypercholesterolemia with a lifetime burden of elevated low-density-lipoprotein cholesterol (LDL-C) and thus a low likelihood of optimal control on current therapies; patients with complete or partial statin intolerance, with high-intensity statin dosing limited by adverse effects; and patients at high CVD risk with LDL-C goals not achieved with current therapies.
- Subcutaneously administered monoclonal antibodies targeting PCSK9 are currently the only PCSK9 inhibitors with FDA approval.
- PCSK9 inhibitors under study include agents with more durable effect and that require less frequent injections, RNA-interference therapies, vaccinations, antisense therapies, and oral formulations.
Fibromuscular dysplasia: Advances in understanding and management
Fibromuscular dysplasia (FMD) is an uncommon vascular disease that leads to narrowing (with either a beaded appearance or, less commonly, focal stenosis), dissection, or aneurysm of medium-sized arteries. Awareness of FMD within the medical community has rapidly expanded during the past decade owing to heightened interest among clinicians, multicenter coordinated research initiatives, and patient advocacy efforts.
In addition, a better understanding of the clinical manifestations and natural history of the disease along with advances in diagnostic imaging have altered the clinical approach to management. There are many unanswered questions regarding FMD, but this review highlights recent insights and how this information has modified clinical care for those affected.
DISTINCT FROM ATHEROSCLEROSIS
FMD results from abnormal development of the arterial cell wall, most commonly the vessel media and less commonly the vessel intima (Figure 1).1,2 Distinct from atherosclerotic processes, FMD shares few typical cardiovascular risk factors aside from an association with tobacco smoking.3,4
The most common variant of FMD is the multifocal type, with the affected arteries resembling a string of beads due to alternating regions of stenosis and dilation.1,5 FMD can also cause a singular stenosis (focal type FMD) and has more recently been associated with findings of arterial tortuosity, aneurysm, and dissection.6,7
Though the disease typically affects the renal and extracranial carotid arteries, it has been noted in most medium-sized arteries throughout the body, most commonly the mesenteric, external iliac, and brachial arteries.1 The location of diseased segments determines symptoms, which commonly include hypertension, headache, and pulsatile tinnitus.8 The overwhelming majority of people affected (> 90%) are women.8
The diagnosis of FMD should be suspected in the case of young or middle-aged women presenting with migraine headaches, pulsatile tinnitus, or hypertension and for women with cervical bruits without typical risk factors for atherosclerotic disease. The diagnosis should also be suspected among patients who have suffered an arterial dissection or who are found to have a cerebral, carotid, or renal aneurysm.
THE US REGISTRY FOR FMD
Since it began enrolling patients in 2009, the US Registry for Fibromuscular Dysplasia has grown to include 13 active centers. It collects longitudinal data on the clinical characteristics, presentation, vascular bed involvement, vascular procedures, and clinical outcomes of patients with FMD.8,9Table 1 highlights key findings and lessons learned from registry publications, many of which have altered previous concepts of this disease.3,7,8,10–12
EPIDEMIOLOGY AND PATHOPHYSIOLOGY
Prevalence
Although FMD is considered a rare disease (and recognized as such by the National Organization of Rare Diseases), the exact prevalence is unknown. A review of 8 studies conducted from 1963 to 2011 found the prevalence of FMD ranged from 2.0% (3 of 150) to 6.6% (47 of 716) among healthy renal transplant donors for a mean prevalence of 3.3% (268 of 8,029) among all donors.13–21 Findings from the Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) trial, which studied the effectiveness of medical therapy alone vs medical therapy and stenting for treatment of significant renal artery stenosis and hypertension, found that 5.8% (58 of 997) of participants who underwent angiography had concomitant renal FMD.22 Importantly, patients with FMD were supposed to have been excluded from the trial, suggesting that FMD is often overlooked or underdiagnosed. A review published in 2010 reported the prevalence of cerebrovascular FMD to be 0.3% to 3.2% in patients undergoing cerebral angiography, but it noted significant heterogeneity in patient populations and definitions of FMD across published studies.23
Risk factors for FMD: Female sex and tobacco smoking
The mechanisms underlying the pathogenesis of FMD are still poorly understood, and its development is likely related to a combination of genetic and environmental factors. There seems to be a hormonal component to the pathogenesis of FMD, as most patients with this condition are women: approximately 91.5% of patients enrolled in the US Registry.10 Men with FMD, however, seem to have a more aggressive course with a rate of aneurysm or dissection two times higher than that in women with FMD.7
Studies have reported an increased risk of FMD in patients with a history of tobacco smoking.3,24 A US Registry report notes that FMD patients with a history of smoking had a statistically significant higher rate of aneurysm than those who had never smoked (24.8% vs 18.9%), and there was a trend toward increased prevalence of major vascular events in smokers, including subarachnoid hemorrhage, transient ischemic attack, stroke, mesenteric ischemia, renal infarction, and major coronary event.3 This study also found that patients with FMD who were smokers were more likely to have claudication symptoms (15.1% vs 7.4%) or to have undergone a vascular procedure (45.9% vs 36.7%).3 Further research is needed to fully understand the relationship between smoking and its interaction with other environmental, hormonal, and genetic factors.
FMD and connective tissue features
While studies have suggested a genetic component to the development of FMD, the specific genetic mechanisms are unknown.1 Studies have explored the potential relationship between FMD and genetic connective tissue disorders that can present with vascular manifestations, such as Loeys-Dietz, Marfan, and Ehlers-Danlos syndromes, and isolated case reports have noted concomitant FMD lesions in patients with these classical genetic disorders.25–31 In a series of patients with FMD from Cleveland Clinic who underwent genetic testing for selected connective tissue disorders, including Ehlers-Danlos syndrome and Loeys-Dietz syndrome, the overall yield of these tests was low.31 These studies suggest some overlap of FMD and other vascular connective tissue disorders, as well as the likelihood that the arterial manifestations of FMD may develop through multiple potential genetic pathways.
A series of 47 patients with FMD seen at the National Institutes of Health found a high incidence of connective tissue features on physical examination, with 95.7% of patients exhibiting at least four features of connective tissue disease, including marked hypermobility, scoliosis, craniofacial abnormalities, and pes planus (flat foot deformity).32 A study of a larger cohort of female patients seen at Cleveland Clinic did not find classical connective tissue features (such as pectus deformity, hypermobility, atrophic scaring, and club foot deformity) to a greater extent than what is reported in the general population, but it did find a significant prevalence of severe myopia (near sightedness), high-arched palate, dental crowding, and early-onset arthritis.33 Additional studies are needed to clarify the potential relationship between the spectrum of connective tissue disorders and FMD.
A BROADER SCOPE OF ARTERIAL MANIFESTATIONS
Since FMD was first described in the 1930s,34 most case reports have focused on its renal artery manifestations. In 1964, extrarenal involvement was first reported, which included carotid, iliac, and visceral arteries.35 The medical community has since recognized that the disease can affect medium-sized vessels throughout the body and, more recently, that it is a multifaceted disease with varying arterial manifestations outside of the typical string-of-beads appearance or focal FMD lesions.1 In addition to multifocal or focal narrowings, arterial manifestations of FMD include arterial tortuosity, aneurysm, and dissection.
Arterial tortuosity
Tortuosity or redundancy of the arteries, particularly the internal carotid arteries, has recently been reported in association with FMD.6 A study based on vascular ultrasonography findings identified this anatomic variant (described as having the appearance of an S-curvature of the internal carotid artery) in 31.9% (37 of 116) of FMD patients.6 This rate of tortuosity is higher than that in the general population, especially when compared with patients of similar age (under age 70). Arterial tortuosity is a common finding in FMD and may be seen in other arterial segments (Figure 2).
Aneurysm and dissection
Both arterial aneurysm and arterial dissection are recognized as manifestations of FMD. A US Registry report published in 2016 found a high prevalence of aneurysm and dissection in the FMD population.7 Of the 921 patients included in this analysis, 21.6% had an aneurysm, 25.7% had an arterial dissection, and 41.7% had either aneurysm or dissection. The most common locations for aneurysm were the extracranial carotid, renal, and intracranial arteries, whereas dissection commonly occurred in the extracranial carotid, vertebral, renal, and coronary arteries. The authors noted that these data may be an underestimation, because the entire cohort did not undergo comprehensive screening for asymptomatic aneurysm or dissection. Patients with aneurysm were more likely to have a history of smoking and subarachnoid hemorrhage, while those with dissection were younger and more likely to have headache, neck pain, and end-organ ischemia, including stroke, renal infarction, or myocardial infarction.
FMD of the coronary arteries
The association between FMD and spontaneous coronary artery dissection (SCAD) has recently been discovered (Figure 3). SCAD typically presents as troponin-positive acute coronary syndrome.36 FMD has been identified as a predisposing condition for SCAD in two case series from Vancouver General Hospital37 and Mayo Clinic.38 The case series from Mayo Clinic found that 45% of SCAD patients had FMD in the extracoronary vessels; the case series from Vancouver General Hospital found that 72% had FMD. A more recent study found that there seems to be other manifestations of FMD in the coronary arteries aside from SCAD.39 In this series, 32 patients with multifocal FMD (in the renal, external iliac, or cerebrovascular arteries) who underwent coronary angiography for suspected symptomatic coronary artery disease (either acute coronary syndrome or stable angina) were found to have coronary artery lesions different from those of atherosclerotic disease. In addition to coronary lesions of dissection (SCAD), the most common findings were marked coronary arterial tortuosity (the “S curve”), followed by areas of atypical-appearing irregular or smooth stenosis. More than half of patients in the series had segments of coronary artery ectasia (enlargement).
APPROACH TO MANAGEMENT
There is no cure for FMD, and thus management strategies focus on thorough evaluation and surveillance, lifestyle modification, and treatment of symptoms. Vascular procedures, such as angioplasty or treatment of aneurysms, are required for some patients. Because patients with FMD present with a diverse set of symptoms, consultation with a specialist who has experience with FMD and who works closely with an interdisciplinary team of experts is recommended.1 The interdisciplinary FMD care team may include a vascular medicine physician, cardiologist, nephrologist, neurologist, neurosurgeon, vascular surgeon, and vascular interventionalist (interventional cardiologist and radiologist).
Imaging and screening the vasculature in FMD patients
Because of the variability in location and manifestations of FMD and the high prevalence of aneurysm and dissection, all patients should undergo comprehensive one-time head-to-pelvis screening during the workup for FMD.1,7 Although the technical standard of diagnostic imaging is catheter angiography, noninvasive imaging—computed tomographic angiography (CTA), magnetic resonance angiography (MRA), duplex ultrasonography—is more commonly used to diagnose and monitor the disease.
A study from our group at Cleveland Clinic assessed the utility of a specialized CTA protocol of the chest, abdomen, and pelvis to image and diagnose manifestations of FMD outside of the cerebrovasculature.40 Incremental findings on imaging included areas of beading or focal narrowing in a new vascular territory and previously undiagnosed arterial aneurysm or dissection. These findings were seen in a variety of vascular beds, including the renal, iliac, and mesenteric arteries, although aortic abnormalities were rare. This study supports the diagnostic value of CTA for FMD to detect asymptomatic aneurysms and areas of arterial dissection, but it also suggests that routine vascular imaging of the thorax may not be necessary.40 In cases of SCAD, on-table renal and iliac angiography (performed after coronary angiography) can assist in diagnosis of FMD as an underlying cause.36 The cerebrovascular arteries (carotid, vertebral, and intracranial vessels) can be imaged later with noninvasive imaging (CTA, MRA).
As a general strategy, once patients with FMD undergo comprehensive imaging, a surveillance program is customized for the patient based on the anatomic location of the disease and the nature of the imaging findings. For example, renal and internal carotid artery FMD may be followed with annual duplex ultrasonography, whereas cerebral and renal or visceral aneurysms require periodic CTA or MRA.
Medical therapies
The medical regimen for patients with FMD varies based on disease location and symptoms, though there are no definitive treatment guidelines because of limited data. A study from the US Registry found that 72.9% of registrants were treated with antiplatelet medications,11 and this is a standard approach in our clinical practice for prevention of thromboembolic events. Antiplatelet drug therapy was more common in elderly patients, patients with a history of coronary artery disease or vascular intervention for FMD, and patients with isolated cerebrovascular FMD.11 Blood pressure management is also important in the medical therapy of patients with FMD who have hypertension. For patients with renal artery involvement, treatment with an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker has been suggested.1
Vascular intervention
The need for vascular intervention (eg, angioplasty or endovascular or surgical aneurysm treatment) is determined primarily by symptoms, with renal artery angioplasty for hypertension the most common FMD-related procedure. It is uncommon for vascular intervention to be performed for cerebrovascular FMD in the absence of recurrent transient ischemic attack or stroke despite antiplatelet therapy, arterial dissection that has failed medical management, or sizable aneurysm that requires treatment to prevent rupture.
When considering intervention for renal artery FMD, it is important to note that the appearance of multifocal FMD (beading) on angiography or noninvasive imaging does not reflect the hemodynamic severity of disease: translesional pressure gradients should be measured across the affected artery to determine if there is actually hemodynamic stenosis caused by an area of beading and to select patients for balloon angioplasty.1 Repeat pressure gradient assessment is done after angioplasty to confirm hemodynamic success.1 Surgical intervention for renal FMD is uncommon. It is generally reserved for complex cases in which endovascular techniques have failed.1
Asymptomatic patients with cerebral, visceral, or arterial aneurysm may require endovascular or surgical treatment. If surgery is indicated, the treatment approach (coiling, stenting, or open surgery) is determined by the size and location of the aneurysm, patient-related factors that may influence the risk of rupture (eg, uncontrolled hypertension, family history of ruptured aneurysm), the anatomic characteristics of the aneurysm, and the feasibility of endovascular vs open surgical repair. A US Registry study of 200 patients with an aneurysm reported that 31.5% underwent intervention to treat the aneurysm.7 Aneurysms requiring intervention were most commonly noted in the extracranial carotid, renal, and intracranial arteries.7
CONCLUSION
Awareness and understanding of FMD have substantially improved in recent years, and this has translated into better care for many patients with FMD. Important advancements have included the recognition of the variability of manifestations of this disease—ranging from an arterial string-of-beads appearance to aneurysm, dissection, and tortuosity—and establishing the need for comprehensive vascular imaging screening in FMD patients. Establishing the association of FMD with SCAD has led to better care for patients with SCAD and presents the opportunity to optimize management of these patients to prevent further vascular events. Research initiatives and heightened awareness have provided valuable insight into this disease, but further work is needed to determine the causal mechanisms of FMD and to continue to develop better management strategies.
- Olin JW, Gornik HL, Bacharach JM, et al. Fibromuscular dysplasia: state of the science and critical unanswered questions. A scientific statement from the American Heart Association. Circulation 2014; 129:1048–1078
- Poloskey SL, Olin JW, Mace P, Gornik HL. Fibromuscular dysplasia. Circulation 2012; 125:e636–e639.
- O’Connor S, Gornik HL, Froehlich JB, et al. Smoking and adverse outcomes in fibromuscular dysplasia: US registry report. J Am Coll Cardiol 2016; 67:1750–1751.
- Sang CN, Whelton PK, Hamper UM, et al. Etiologic factors in renovascular fibromuscular dysplasia. A case-control study. Hypertension 1989; 14:472–479.
- Persu A, Touzé E, Mousseaux E, Barral X, Joffre F, Plouin PF. Diagnosis and management of fibromuscular dysplasia: an expert consensus. Eur J Clin Invest 2012; 42:338–347.
- Sethi SS, Lau JF, Godbold J, Gustavson S, Olin JW. The S curve: a novel morphological finding in the internal carotid artery in patients with fibromuscular dysplasia. Vasc Med 2014; 19:356–362.
- Kadian-Dodov D, Gornik HL, Gu X, et al. Dissection and aneurysm in patients with fibromuscular dysplasia: findings for the U.S. registry for FMD. J Am Coll Cardiol 2016; 68:176–185.
- Olin JW, Froehlich J, Gu X, et al. The United States Registry for Fibromuscular Dysplasia: results in the first 447 patients. Circulation 2012; 125:3182–3190.
- Fibromuscular Dysplasia Society of America (FMDSA). The US Registry for FMD. www.fmdsa.org/research_network/fmd_registry. Accessed August 24, 2016.
- Kim ES, Olin JW, Froehlich JB, et al. Clinical manifestations of fibromuscular dysplasia vary by patient sex: a report of the United States Registry for Fibromuscular Dysplasia. J Am Coll Cardiol 2013; 62:2026–2028.
- Weinberg I, Gu X, Giri J, et al. Anti-platelet and anti-hypertension medication use in patients with fibromuscular dysplasia: results from the United States Registry for Fibromuscular Dysplasia. Vasc Med 2015; 20:447–453.
- Green R, Gu X, Kline-Rogers E, et al. Differences between the pediatric and adult presentation of fibromuscular dysplasia: results from the US Registry. Pediatr Nephrol 2016; 31:641–650.
- Shivapour DM, Erwin P, Kim ESH. Epidemiology of fibromuscular dysplasia: a review of the literature. Vasc Med 2016; 21:376–381.
- Spring DB, Satvatierra O Jr, Palubinskas AJ, Amend WJ Jr, Vincenti FG, Feduska NJ. Results and significance of angiography in potential kidney donors. Radiology 1979; 133:45–47.
- Cragg AH, Smith TP, Thompson BH, et al. Incidental fibromuscular dysplasia in potential renal donors: long-term clinical follow-up. Radiology 1989; 172:145–147.
- Neymark E, LaBerge JM, Hirose R, et al. Arteriographic detection of renovascular disease in potential renal donors: incidence and effect on donor surgery. Radiology 2000; 214:755–760.
- Andreoni KA, Weeks SM, Gerber DA, et al. Incidence of donor renal fibromuscular dysplasia: does it justify routine angiography? Transplantation 2002; 73:1112–1116.
- Blondin D, Lanzman R, Schellhammer F, et al. Fibromuscular dysplasia in living renal donors: still a challenge to computed tomographic angiography. Eur J Radiol 2010; 75:67–71.
- Lorenz EC, Vrtiska TJ, Lieske JC, et al. Prevalence of renal artery and kidney abnormalities by computed tomography among healthy adults. Clin J Am Soc Nephrol 2010; 5:431–438.
- McKenzie GA, Oderich GS, Kawashima A, Misra S. Renal artery fibromuscular dysplasia in 2,640 renal donor subjects: a CT angiography analysis. J Vasc Interv Radiol 2013; 24:1477–1480.
- Frick MP, Goldberg ME. Uro- and angiographic findings in a “normal” population: screening of 151 symptom-free potential transplant donors for renal disease. AJR Am J Roentgenol 1980; 134:503–505.
- Hendricks NJ, Matsumoto AH, Angle JF, et al. Is fibromuscular dysplasia underdiagnosed? A comparison of the prevalence of FMD seen in CORAL trial participants versus a single institution population of renal donor candidates. Vasc Med 2014; 19:363–367.
- Touzé E, Oppenheim C, Trystram D, et al. Fibromuscular dysplasia of cervical and intracranial arteries. Int J Stroke 2010; 5:296–305.
- Savard S, Azarine A, Jeunemaitre X, Azizi M, Plouin PF, Steichen O. Association of smoking with phenotype at diagnosis and vascular interventions in patients with renal artery fibromuscular dysplasia. Hypertension 2013; 61:1227–1232.
- Schievink WI, Limburg M. Angiographic abnormalities mimicking fibromuscular dysplasia in a patient with Ehlers-Danlos syndrome, type IV. Neurosurgery 1989; 25:482–483.
- Schievink WI, Bjornsson J, Piepgras DG. Coexistence of fibromuscular dysplasia and cystic medial necrosis in a patient with Marfan’s syndrome and bilateral carotid artery dissections. Stroke 1994; 25:2492–2496.
- Schievink WI, Bjournsson J, Parisi JE, Prakash UB. Arterial fibromuscular dysplasia associated with severe α1-antitrypsin deficiency. Mayo Clin Proc 1994; 69:1040–1043.
- Schievink WI, Puumala MR, Meyer FB, Raffel C, Katzmann JA, Parisi JE. Giant intracranial aneurysm and fibromuscular dysplasia in an adolescent with α1-antitrypsin deficiency. J Neurosurg 1996; 85:503–506.
- Schievink WI, Meyer FB, Parisi JE, Wijdicks EFM. Fibromuscular dysplasia of the internal carotid artery associated with alpha1-antitrypsin deficiency. Neurosurgery 1998; 43:229–233; discussion 233–234.
- Bofinger A, Hawley C, Fisher P, Daunt N, Stowasser M, Gordon R. Alpha-1-antitrypsin phenotypes in patients with renal arterial fibromuscular dysplasia. J Hum Hypertens 2000; 14:91–94.
- Poloskey SL, Kim ES, Sanghani R, et al. Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc Med 2012; 17:371–378.
- Ganesh SK, Morissette R, Xu Z, et al. Clinical and biochemical profiles suggest fibromuscular dysplasia is a systemic disease with altered TGF- expression and connective tissue features. FASEB J 2014; 28:3313–3324.
- O’Connor S, Kim ES, Brinza E, et al. Systemic connective tissue features in women with fibromuscular dysplasia. Vasc Med 2015; 20:454–462.
- Leadbetter WF, Burkland CE. Hypertension in unilateral renal disease. J Urol 1938; 39:611–626.
- Palubinskas AJ, Ripley HR. Fibromuscular hyperplasia in extrarenal arteries. Radiology 1964; 82:451–455.
- Saw J, Mancini GB, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016; 68:297–312.
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014; 7:645–655.
- Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015; 115:1672–1677.
- Saw J, Bezerra H, Gornik HL, Machan L, Mancini GB. Angiographic and intracoronary manifestations of coronary fibromuscular dysplasia. Circulation 2016; 133:1548–1559.
- Bolen MA, Brinza E, Renapurkar RD, Kim ES, Gornik HL. Screening CT angiography of the aorta, visceral branch vessels, and pelvic arteries in fibromuscular dysplasia. JACC Cardiovasc Imaging. 2016; doi: 10.1016/j.jcmg.2016.04.010. [Epub ahead of print].
Fibromuscular dysplasia (FMD) is an uncommon vascular disease that leads to narrowing (with either a beaded appearance or, less commonly, focal stenosis), dissection, or aneurysm of medium-sized arteries. Awareness of FMD within the medical community has rapidly expanded during the past decade owing to heightened interest among clinicians, multicenter coordinated research initiatives, and patient advocacy efforts.
In addition, a better understanding of the clinical manifestations and natural history of the disease along with advances in diagnostic imaging have altered the clinical approach to management. There are many unanswered questions regarding FMD, but this review highlights recent insights and how this information has modified clinical care for those affected.
DISTINCT FROM ATHEROSCLEROSIS
FMD results from abnormal development of the arterial cell wall, most commonly the vessel media and less commonly the vessel intima (Figure 1).1,2 Distinct from atherosclerotic processes, FMD shares few typical cardiovascular risk factors aside from an association with tobacco smoking.3,4
The most common variant of FMD is the multifocal type, with the affected arteries resembling a string of beads due to alternating regions of stenosis and dilation.1,5 FMD can also cause a singular stenosis (focal type FMD) and has more recently been associated with findings of arterial tortuosity, aneurysm, and dissection.6,7
Though the disease typically affects the renal and extracranial carotid arteries, it has been noted in most medium-sized arteries throughout the body, most commonly the mesenteric, external iliac, and brachial arteries.1 The location of diseased segments determines symptoms, which commonly include hypertension, headache, and pulsatile tinnitus.8 The overwhelming majority of people affected (> 90%) are women.8
The diagnosis of FMD should be suspected in the case of young or middle-aged women presenting with migraine headaches, pulsatile tinnitus, or hypertension and for women with cervical bruits without typical risk factors for atherosclerotic disease. The diagnosis should also be suspected among patients who have suffered an arterial dissection or who are found to have a cerebral, carotid, or renal aneurysm.
THE US REGISTRY FOR FMD
Since it began enrolling patients in 2009, the US Registry for Fibromuscular Dysplasia has grown to include 13 active centers. It collects longitudinal data on the clinical characteristics, presentation, vascular bed involvement, vascular procedures, and clinical outcomes of patients with FMD.8,9Table 1 highlights key findings and lessons learned from registry publications, many of which have altered previous concepts of this disease.3,7,8,10–12
EPIDEMIOLOGY AND PATHOPHYSIOLOGY
Prevalence
Although FMD is considered a rare disease (and recognized as such by the National Organization of Rare Diseases), the exact prevalence is unknown. A review of 8 studies conducted from 1963 to 2011 found the prevalence of FMD ranged from 2.0% (3 of 150) to 6.6% (47 of 716) among healthy renal transplant donors for a mean prevalence of 3.3% (268 of 8,029) among all donors.13–21 Findings from the Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) trial, which studied the effectiveness of medical therapy alone vs medical therapy and stenting for treatment of significant renal artery stenosis and hypertension, found that 5.8% (58 of 997) of participants who underwent angiography had concomitant renal FMD.22 Importantly, patients with FMD were supposed to have been excluded from the trial, suggesting that FMD is often overlooked or underdiagnosed. A review published in 2010 reported the prevalence of cerebrovascular FMD to be 0.3% to 3.2% in patients undergoing cerebral angiography, but it noted significant heterogeneity in patient populations and definitions of FMD across published studies.23
Risk factors for FMD: Female sex and tobacco smoking
The mechanisms underlying the pathogenesis of FMD are still poorly understood, and its development is likely related to a combination of genetic and environmental factors. There seems to be a hormonal component to the pathogenesis of FMD, as most patients with this condition are women: approximately 91.5% of patients enrolled in the US Registry.10 Men with FMD, however, seem to have a more aggressive course with a rate of aneurysm or dissection two times higher than that in women with FMD.7
Studies have reported an increased risk of FMD in patients with a history of tobacco smoking.3,24 A US Registry report notes that FMD patients with a history of smoking had a statistically significant higher rate of aneurysm than those who had never smoked (24.8% vs 18.9%), and there was a trend toward increased prevalence of major vascular events in smokers, including subarachnoid hemorrhage, transient ischemic attack, stroke, mesenteric ischemia, renal infarction, and major coronary event.3 This study also found that patients with FMD who were smokers were more likely to have claudication symptoms (15.1% vs 7.4%) or to have undergone a vascular procedure (45.9% vs 36.7%).3 Further research is needed to fully understand the relationship between smoking and its interaction with other environmental, hormonal, and genetic factors.
FMD and connective tissue features
While studies have suggested a genetic component to the development of FMD, the specific genetic mechanisms are unknown.1 Studies have explored the potential relationship between FMD and genetic connective tissue disorders that can present with vascular manifestations, such as Loeys-Dietz, Marfan, and Ehlers-Danlos syndromes, and isolated case reports have noted concomitant FMD lesions in patients with these classical genetic disorders.25–31 In a series of patients with FMD from Cleveland Clinic who underwent genetic testing for selected connective tissue disorders, including Ehlers-Danlos syndrome and Loeys-Dietz syndrome, the overall yield of these tests was low.31 These studies suggest some overlap of FMD and other vascular connective tissue disorders, as well as the likelihood that the arterial manifestations of FMD may develop through multiple potential genetic pathways.
A series of 47 patients with FMD seen at the National Institutes of Health found a high incidence of connective tissue features on physical examination, with 95.7% of patients exhibiting at least four features of connective tissue disease, including marked hypermobility, scoliosis, craniofacial abnormalities, and pes planus (flat foot deformity).32 A study of a larger cohort of female patients seen at Cleveland Clinic did not find classical connective tissue features (such as pectus deformity, hypermobility, atrophic scaring, and club foot deformity) to a greater extent than what is reported in the general population, but it did find a significant prevalence of severe myopia (near sightedness), high-arched palate, dental crowding, and early-onset arthritis.33 Additional studies are needed to clarify the potential relationship between the spectrum of connective tissue disorders and FMD.
A BROADER SCOPE OF ARTERIAL MANIFESTATIONS
Since FMD was first described in the 1930s,34 most case reports have focused on its renal artery manifestations. In 1964, extrarenal involvement was first reported, which included carotid, iliac, and visceral arteries.35 The medical community has since recognized that the disease can affect medium-sized vessels throughout the body and, more recently, that it is a multifaceted disease with varying arterial manifestations outside of the typical string-of-beads appearance or focal FMD lesions.1 In addition to multifocal or focal narrowings, arterial manifestations of FMD include arterial tortuosity, aneurysm, and dissection.
Arterial tortuosity
Tortuosity or redundancy of the arteries, particularly the internal carotid arteries, has recently been reported in association with FMD.6 A study based on vascular ultrasonography findings identified this anatomic variant (described as having the appearance of an S-curvature of the internal carotid artery) in 31.9% (37 of 116) of FMD patients.6 This rate of tortuosity is higher than that in the general population, especially when compared with patients of similar age (under age 70). Arterial tortuosity is a common finding in FMD and may be seen in other arterial segments (Figure 2).
Aneurysm and dissection
Both arterial aneurysm and arterial dissection are recognized as manifestations of FMD. A US Registry report published in 2016 found a high prevalence of aneurysm and dissection in the FMD population.7 Of the 921 patients included in this analysis, 21.6% had an aneurysm, 25.7% had an arterial dissection, and 41.7% had either aneurysm or dissection. The most common locations for aneurysm were the extracranial carotid, renal, and intracranial arteries, whereas dissection commonly occurred in the extracranial carotid, vertebral, renal, and coronary arteries. The authors noted that these data may be an underestimation, because the entire cohort did not undergo comprehensive screening for asymptomatic aneurysm or dissection. Patients with aneurysm were more likely to have a history of smoking and subarachnoid hemorrhage, while those with dissection were younger and more likely to have headache, neck pain, and end-organ ischemia, including stroke, renal infarction, or myocardial infarction.
FMD of the coronary arteries
The association between FMD and spontaneous coronary artery dissection (SCAD) has recently been discovered (Figure 3). SCAD typically presents as troponin-positive acute coronary syndrome.36 FMD has been identified as a predisposing condition for SCAD in two case series from Vancouver General Hospital37 and Mayo Clinic.38 The case series from Mayo Clinic found that 45% of SCAD patients had FMD in the extracoronary vessels; the case series from Vancouver General Hospital found that 72% had FMD. A more recent study found that there seems to be other manifestations of FMD in the coronary arteries aside from SCAD.39 In this series, 32 patients with multifocal FMD (in the renal, external iliac, or cerebrovascular arteries) who underwent coronary angiography for suspected symptomatic coronary artery disease (either acute coronary syndrome or stable angina) were found to have coronary artery lesions different from those of atherosclerotic disease. In addition to coronary lesions of dissection (SCAD), the most common findings were marked coronary arterial tortuosity (the “S curve”), followed by areas of atypical-appearing irregular or smooth stenosis. More than half of patients in the series had segments of coronary artery ectasia (enlargement).
APPROACH TO MANAGEMENT
There is no cure for FMD, and thus management strategies focus on thorough evaluation and surveillance, lifestyle modification, and treatment of symptoms. Vascular procedures, such as angioplasty or treatment of aneurysms, are required for some patients. Because patients with FMD present with a diverse set of symptoms, consultation with a specialist who has experience with FMD and who works closely with an interdisciplinary team of experts is recommended.1 The interdisciplinary FMD care team may include a vascular medicine physician, cardiologist, nephrologist, neurologist, neurosurgeon, vascular surgeon, and vascular interventionalist (interventional cardiologist and radiologist).
Imaging and screening the vasculature in FMD patients
Because of the variability in location and manifestations of FMD and the high prevalence of aneurysm and dissection, all patients should undergo comprehensive one-time head-to-pelvis screening during the workup for FMD.1,7 Although the technical standard of diagnostic imaging is catheter angiography, noninvasive imaging—computed tomographic angiography (CTA), magnetic resonance angiography (MRA), duplex ultrasonography—is more commonly used to diagnose and monitor the disease.
A study from our group at Cleveland Clinic assessed the utility of a specialized CTA protocol of the chest, abdomen, and pelvis to image and diagnose manifestations of FMD outside of the cerebrovasculature.40 Incremental findings on imaging included areas of beading or focal narrowing in a new vascular territory and previously undiagnosed arterial aneurysm or dissection. These findings were seen in a variety of vascular beds, including the renal, iliac, and mesenteric arteries, although aortic abnormalities were rare. This study supports the diagnostic value of CTA for FMD to detect asymptomatic aneurysms and areas of arterial dissection, but it also suggests that routine vascular imaging of the thorax may not be necessary.40 In cases of SCAD, on-table renal and iliac angiography (performed after coronary angiography) can assist in diagnosis of FMD as an underlying cause.36 The cerebrovascular arteries (carotid, vertebral, and intracranial vessels) can be imaged later with noninvasive imaging (CTA, MRA).
As a general strategy, once patients with FMD undergo comprehensive imaging, a surveillance program is customized for the patient based on the anatomic location of the disease and the nature of the imaging findings. For example, renal and internal carotid artery FMD may be followed with annual duplex ultrasonography, whereas cerebral and renal or visceral aneurysms require periodic CTA or MRA.
Medical therapies
The medical regimen for patients with FMD varies based on disease location and symptoms, though there are no definitive treatment guidelines because of limited data. A study from the US Registry found that 72.9% of registrants were treated with antiplatelet medications,11 and this is a standard approach in our clinical practice for prevention of thromboembolic events. Antiplatelet drug therapy was more common in elderly patients, patients with a history of coronary artery disease or vascular intervention for FMD, and patients with isolated cerebrovascular FMD.11 Blood pressure management is also important in the medical therapy of patients with FMD who have hypertension. For patients with renal artery involvement, treatment with an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker has been suggested.1
Vascular intervention
The need for vascular intervention (eg, angioplasty or endovascular or surgical aneurysm treatment) is determined primarily by symptoms, with renal artery angioplasty for hypertension the most common FMD-related procedure. It is uncommon for vascular intervention to be performed for cerebrovascular FMD in the absence of recurrent transient ischemic attack or stroke despite antiplatelet therapy, arterial dissection that has failed medical management, or sizable aneurysm that requires treatment to prevent rupture.
When considering intervention for renal artery FMD, it is important to note that the appearance of multifocal FMD (beading) on angiography or noninvasive imaging does not reflect the hemodynamic severity of disease: translesional pressure gradients should be measured across the affected artery to determine if there is actually hemodynamic stenosis caused by an area of beading and to select patients for balloon angioplasty.1 Repeat pressure gradient assessment is done after angioplasty to confirm hemodynamic success.1 Surgical intervention for renal FMD is uncommon. It is generally reserved for complex cases in which endovascular techniques have failed.1
Asymptomatic patients with cerebral, visceral, or arterial aneurysm may require endovascular or surgical treatment. If surgery is indicated, the treatment approach (coiling, stenting, or open surgery) is determined by the size and location of the aneurysm, patient-related factors that may influence the risk of rupture (eg, uncontrolled hypertension, family history of ruptured aneurysm), the anatomic characteristics of the aneurysm, and the feasibility of endovascular vs open surgical repair. A US Registry study of 200 patients with an aneurysm reported that 31.5% underwent intervention to treat the aneurysm.7 Aneurysms requiring intervention were most commonly noted in the extracranial carotid, renal, and intracranial arteries.7
CONCLUSION
Awareness and understanding of FMD have substantially improved in recent years, and this has translated into better care for many patients with FMD. Important advancements have included the recognition of the variability of manifestations of this disease—ranging from an arterial string-of-beads appearance to aneurysm, dissection, and tortuosity—and establishing the need for comprehensive vascular imaging screening in FMD patients. Establishing the association of FMD with SCAD has led to better care for patients with SCAD and presents the opportunity to optimize management of these patients to prevent further vascular events. Research initiatives and heightened awareness have provided valuable insight into this disease, but further work is needed to determine the causal mechanisms of FMD and to continue to develop better management strategies.
Fibromuscular dysplasia (FMD) is an uncommon vascular disease that leads to narrowing (with either a beaded appearance or, less commonly, focal stenosis), dissection, or aneurysm of medium-sized arteries. Awareness of FMD within the medical community has rapidly expanded during the past decade owing to heightened interest among clinicians, multicenter coordinated research initiatives, and patient advocacy efforts.
In addition, a better understanding of the clinical manifestations and natural history of the disease along with advances in diagnostic imaging have altered the clinical approach to management. There are many unanswered questions regarding FMD, but this review highlights recent insights and how this information has modified clinical care for those affected.
DISTINCT FROM ATHEROSCLEROSIS
FMD results from abnormal development of the arterial cell wall, most commonly the vessel media and less commonly the vessel intima (Figure 1).1,2 Distinct from atherosclerotic processes, FMD shares few typical cardiovascular risk factors aside from an association with tobacco smoking.3,4
The most common variant of FMD is the multifocal type, with the affected arteries resembling a string of beads due to alternating regions of stenosis and dilation.1,5 FMD can also cause a singular stenosis (focal type FMD) and has more recently been associated with findings of arterial tortuosity, aneurysm, and dissection.6,7
Though the disease typically affects the renal and extracranial carotid arteries, it has been noted in most medium-sized arteries throughout the body, most commonly the mesenteric, external iliac, and brachial arteries.1 The location of diseased segments determines symptoms, which commonly include hypertension, headache, and pulsatile tinnitus.8 The overwhelming majority of people affected (> 90%) are women.8
The diagnosis of FMD should be suspected in the case of young or middle-aged women presenting with migraine headaches, pulsatile tinnitus, or hypertension and for women with cervical bruits without typical risk factors for atherosclerotic disease. The diagnosis should also be suspected among patients who have suffered an arterial dissection or who are found to have a cerebral, carotid, or renal aneurysm.
THE US REGISTRY FOR FMD
Since it began enrolling patients in 2009, the US Registry for Fibromuscular Dysplasia has grown to include 13 active centers. It collects longitudinal data on the clinical characteristics, presentation, vascular bed involvement, vascular procedures, and clinical outcomes of patients with FMD.8,9Table 1 highlights key findings and lessons learned from registry publications, many of which have altered previous concepts of this disease.3,7,8,10–12
EPIDEMIOLOGY AND PATHOPHYSIOLOGY
Prevalence
Although FMD is considered a rare disease (and recognized as such by the National Organization of Rare Diseases), the exact prevalence is unknown. A review of 8 studies conducted from 1963 to 2011 found the prevalence of FMD ranged from 2.0% (3 of 150) to 6.6% (47 of 716) among healthy renal transplant donors for a mean prevalence of 3.3% (268 of 8,029) among all donors.13–21 Findings from the Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) trial, which studied the effectiveness of medical therapy alone vs medical therapy and stenting for treatment of significant renal artery stenosis and hypertension, found that 5.8% (58 of 997) of participants who underwent angiography had concomitant renal FMD.22 Importantly, patients with FMD were supposed to have been excluded from the trial, suggesting that FMD is often overlooked or underdiagnosed. A review published in 2010 reported the prevalence of cerebrovascular FMD to be 0.3% to 3.2% in patients undergoing cerebral angiography, but it noted significant heterogeneity in patient populations and definitions of FMD across published studies.23
Risk factors for FMD: Female sex and tobacco smoking
The mechanisms underlying the pathogenesis of FMD are still poorly understood, and its development is likely related to a combination of genetic and environmental factors. There seems to be a hormonal component to the pathogenesis of FMD, as most patients with this condition are women: approximately 91.5% of patients enrolled in the US Registry.10 Men with FMD, however, seem to have a more aggressive course with a rate of aneurysm or dissection two times higher than that in women with FMD.7
Studies have reported an increased risk of FMD in patients with a history of tobacco smoking.3,24 A US Registry report notes that FMD patients with a history of smoking had a statistically significant higher rate of aneurysm than those who had never smoked (24.8% vs 18.9%), and there was a trend toward increased prevalence of major vascular events in smokers, including subarachnoid hemorrhage, transient ischemic attack, stroke, mesenteric ischemia, renal infarction, and major coronary event.3 This study also found that patients with FMD who were smokers were more likely to have claudication symptoms (15.1% vs 7.4%) or to have undergone a vascular procedure (45.9% vs 36.7%).3 Further research is needed to fully understand the relationship between smoking and its interaction with other environmental, hormonal, and genetic factors.
FMD and connective tissue features
While studies have suggested a genetic component to the development of FMD, the specific genetic mechanisms are unknown.1 Studies have explored the potential relationship between FMD and genetic connective tissue disorders that can present with vascular manifestations, such as Loeys-Dietz, Marfan, and Ehlers-Danlos syndromes, and isolated case reports have noted concomitant FMD lesions in patients with these classical genetic disorders.25–31 In a series of patients with FMD from Cleveland Clinic who underwent genetic testing for selected connective tissue disorders, including Ehlers-Danlos syndrome and Loeys-Dietz syndrome, the overall yield of these tests was low.31 These studies suggest some overlap of FMD and other vascular connective tissue disorders, as well as the likelihood that the arterial manifestations of FMD may develop through multiple potential genetic pathways.
A series of 47 patients with FMD seen at the National Institutes of Health found a high incidence of connective tissue features on physical examination, with 95.7% of patients exhibiting at least four features of connective tissue disease, including marked hypermobility, scoliosis, craniofacial abnormalities, and pes planus (flat foot deformity).32 A study of a larger cohort of female patients seen at Cleveland Clinic did not find classical connective tissue features (such as pectus deformity, hypermobility, atrophic scaring, and club foot deformity) to a greater extent than what is reported in the general population, but it did find a significant prevalence of severe myopia (near sightedness), high-arched palate, dental crowding, and early-onset arthritis.33 Additional studies are needed to clarify the potential relationship between the spectrum of connective tissue disorders and FMD.
A BROADER SCOPE OF ARTERIAL MANIFESTATIONS
Since FMD was first described in the 1930s,34 most case reports have focused on its renal artery manifestations. In 1964, extrarenal involvement was first reported, which included carotid, iliac, and visceral arteries.35 The medical community has since recognized that the disease can affect medium-sized vessels throughout the body and, more recently, that it is a multifaceted disease with varying arterial manifestations outside of the typical string-of-beads appearance or focal FMD lesions.1 In addition to multifocal or focal narrowings, arterial manifestations of FMD include arterial tortuosity, aneurysm, and dissection.
Arterial tortuosity
Tortuosity or redundancy of the arteries, particularly the internal carotid arteries, has recently been reported in association with FMD.6 A study based on vascular ultrasonography findings identified this anatomic variant (described as having the appearance of an S-curvature of the internal carotid artery) in 31.9% (37 of 116) of FMD patients.6 This rate of tortuosity is higher than that in the general population, especially when compared with patients of similar age (under age 70). Arterial tortuosity is a common finding in FMD and may be seen in other arterial segments (Figure 2).
Aneurysm and dissection
Both arterial aneurysm and arterial dissection are recognized as manifestations of FMD. A US Registry report published in 2016 found a high prevalence of aneurysm and dissection in the FMD population.7 Of the 921 patients included in this analysis, 21.6% had an aneurysm, 25.7% had an arterial dissection, and 41.7% had either aneurysm or dissection. The most common locations for aneurysm were the extracranial carotid, renal, and intracranial arteries, whereas dissection commonly occurred in the extracranial carotid, vertebral, renal, and coronary arteries. The authors noted that these data may be an underestimation, because the entire cohort did not undergo comprehensive screening for asymptomatic aneurysm or dissection. Patients with aneurysm were more likely to have a history of smoking and subarachnoid hemorrhage, while those with dissection were younger and more likely to have headache, neck pain, and end-organ ischemia, including stroke, renal infarction, or myocardial infarction.
FMD of the coronary arteries
The association between FMD and spontaneous coronary artery dissection (SCAD) has recently been discovered (Figure 3). SCAD typically presents as troponin-positive acute coronary syndrome.36 FMD has been identified as a predisposing condition for SCAD in two case series from Vancouver General Hospital37 and Mayo Clinic.38 The case series from Mayo Clinic found that 45% of SCAD patients had FMD in the extracoronary vessels; the case series from Vancouver General Hospital found that 72% had FMD. A more recent study found that there seems to be other manifestations of FMD in the coronary arteries aside from SCAD.39 In this series, 32 patients with multifocal FMD (in the renal, external iliac, or cerebrovascular arteries) who underwent coronary angiography for suspected symptomatic coronary artery disease (either acute coronary syndrome or stable angina) were found to have coronary artery lesions different from those of atherosclerotic disease. In addition to coronary lesions of dissection (SCAD), the most common findings were marked coronary arterial tortuosity (the “S curve”), followed by areas of atypical-appearing irregular or smooth stenosis. More than half of patients in the series had segments of coronary artery ectasia (enlargement).
APPROACH TO MANAGEMENT
There is no cure for FMD, and thus management strategies focus on thorough evaluation and surveillance, lifestyle modification, and treatment of symptoms. Vascular procedures, such as angioplasty or treatment of aneurysms, are required for some patients. Because patients with FMD present with a diverse set of symptoms, consultation with a specialist who has experience with FMD and who works closely with an interdisciplinary team of experts is recommended.1 The interdisciplinary FMD care team may include a vascular medicine physician, cardiologist, nephrologist, neurologist, neurosurgeon, vascular surgeon, and vascular interventionalist (interventional cardiologist and radiologist).
Imaging and screening the vasculature in FMD patients
Because of the variability in location and manifestations of FMD and the high prevalence of aneurysm and dissection, all patients should undergo comprehensive one-time head-to-pelvis screening during the workup for FMD.1,7 Although the technical standard of diagnostic imaging is catheter angiography, noninvasive imaging—computed tomographic angiography (CTA), magnetic resonance angiography (MRA), duplex ultrasonography—is more commonly used to diagnose and monitor the disease.
A study from our group at Cleveland Clinic assessed the utility of a specialized CTA protocol of the chest, abdomen, and pelvis to image and diagnose manifestations of FMD outside of the cerebrovasculature.40 Incremental findings on imaging included areas of beading or focal narrowing in a new vascular territory and previously undiagnosed arterial aneurysm or dissection. These findings were seen in a variety of vascular beds, including the renal, iliac, and mesenteric arteries, although aortic abnormalities were rare. This study supports the diagnostic value of CTA for FMD to detect asymptomatic aneurysms and areas of arterial dissection, but it also suggests that routine vascular imaging of the thorax may not be necessary.40 In cases of SCAD, on-table renal and iliac angiography (performed after coronary angiography) can assist in diagnosis of FMD as an underlying cause.36 The cerebrovascular arteries (carotid, vertebral, and intracranial vessels) can be imaged later with noninvasive imaging (CTA, MRA).
As a general strategy, once patients with FMD undergo comprehensive imaging, a surveillance program is customized for the patient based on the anatomic location of the disease and the nature of the imaging findings. For example, renal and internal carotid artery FMD may be followed with annual duplex ultrasonography, whereas cerebral and renal or visceral aneurysms require periodic CTA or MRA.
Medical therapies
The medical regimen for patients with FMD varies based on disease location and symptoms, though there are no definitive treatment guidelines because of limited data. A study from the US Registry found that 72.9% of registrants were treated with antiplatelet medications,11 and this is a standard approach in our clinical practice for prevention of thromboembolic events. Antiplatelet drug therapy was more common in elderly patients, patients with a history of coronary artery disease or vascular intervention for FMD, and patients with isolated cerebrovascular FMD.11 Blood pressure management is also important in the medical therapy of patients with FMD who have hypertension. For patients with renal artery involvement, treatment with an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker has been suggested.1
Vascular intervention
The need for vascular intervention (eg, angioplasty or endovascular or surgical aneurysm treatment) is determined primarily by symptoms, with renal artery angioplasty for hypertension the most common FMD-related procedure. It is uncommon for vascular intervention to be performed for cerebrovascular FMD in the absence of recurrent transient ischemic attack or stroke despite antiplatelet therapy, arterial dissection that has failed medical management, or sizable aneurysm that requires treatment to prevent rupture.
When considering intervention for renal artery FMD, it is important to note that the appearance of multifocal FMD (beading) on angiography or noninvasive imaging does not reflect the hemodynamic severity of disease: translesional pressure gradients should be measured across the affected artery to determine if there is actually hemodynamic stenosis caused by an area of beading and to select patients for balloon angioplasty.1 Repeat pressure gradient assessment is done after angioplasty to confirm hemodynamic success.1 Surgical intervention for renal FMD is uncommon. It is generally reserved for complex cases in which endovascular techniques have failed.1
Asymptomatic patients with cerebral, visceral, or arterial aneurysm may require endovascular or surgical treatment. If surgery is indicated, the treatment approach (coiling, stenting, or open surgery) is determined by the size and location of the aneurysm, patient-related factors that may influence the risk of rupture (eg, uncontrolled hypertension, family history of ruptured aneurysm), the anatomic characteristics of the aneurysm, and the feasibility of endovascular vs open surgical repair. A US Registry study of 200 patients with an aneurysm reported that 31.5% underwent intervention to treat the aneurysm.7 Aneurysms requiring intervention were most commonly noted in the extracranial carotid, renal, and intracranial arteries.7
CONCLUSION
Awareness and understanding of FMD have substantially improved in recent years, and this has translated into better care for many patients with FMD. Important advancements have included the recognition of the variability of manifestations of this disease—ranging from an arterial string-of-beads appearance to aneurysm, dissection, and tortuosity—and establishing the need for comprehensive vascular imaging screening in FMD patients. Establishing the association of FMD with SCAD has led to better care for patients with SCAD and presents the opportunity to optimize management of these patients to prevent further vascular events. Research initiatives and heightened awareness have provided valuable insight into this disease, but further work is needed to determine the causal mechanisms of FMD and to continue to develop better management strategies.
- Olin JW, Gornik HL, Bacharach JM, et al. Fibromuscular dysplasia: state of the science and critical unanswered questions. A scientific statement from the American Heart Association. Circulation 2014; 129:1048–1078
- Poloskey SL, Olin JW, Mace P, Gornik HL. Fibromuscular dysplasia. Circulation 2012; 125:e636–e639.
- O’Connor S, Gornik HL, Froehlich JB, et al. Smoking and adverse outcomes in fibromuscular dysplasia: US registry report. J Am Coll Cardiol 2016; 67:1750–1751.
- Sang CN, Whelton PK, Hamper UM, et al. Etiologic factors in renovascular fibromuscular dysplasia. A case-control study. Hypertension 1989; 14:472–479.
- Persu A, Touzé E, Mousseaux E, Barral X, Joffre F, Plouin PF. Diagnosis and management of fibromuscular dysplasia: an expert consensus. Eur J Clin Invest 2012; 42:338–347.
- Sethi SS, Lau JF, Godbold J, Gustavson S, Olin JW. The S curve: a novel morphological finding in the internal carotid artery in patients with fibromuscular dysplasia. Vasc Med 2014; 19:356–362.
- Kadian-Dodov D, Gornik HL, Gu X, et al. Dissection and aneurysm in patients with fibromuscular dysplasia: findings for the U.S. registry for FMD. J Am Coll Cardiol 2016; 68:176–185.
- Olin JW, Froehlich J, Gu X, et al. The United States Registry for Fibromuscular Dysplasia: results in the first 447 patients. Circulation 2012; 125:3182–3190.
- Fibromuscular Dysplasia Society of America (FMDSA). The US Registry for FMD. www.fmdsa.org/research_network/fmd_registry. Accessed August 24, 2016.
- Kim ES, Olin JW, Froehlich JB, et al. Clinical manifestations of fibromuscular dysplasia vary by patient sex: a report of the United States Registry for Fibromuscular Dysplasia. J Am Coll Cardiol 2013; 62:2026–2028.
- Weinberg I, Gu X, Giri J, et al. Anti-platelet and anti-hypertension medication use in patients with fibromuscular dysplasia: results from the United States Registry for Fibromuscular Dysplasia. Vasc Med 2015; 20:447–453.
- Green R, Gu X, Kline-Rogers E, et al. Differences between the pediatric and adult presentation of fibromuscular dysplasia: results from the US Registry. Pediatr Nephrol 2016; 31:641–650.
- Shivapour DM, Erwin P, Kim ESH. Epidemiology of fibromuscular dysplasia: a review of the literature. Vasc Med 2016; 21:376–381.
- Spring DB, Satvatierra O Jr, Palubinskas AJ, Amend WJ Jr, Vincenti FG, Feduska NJ. Results and significance of angiography in potential kidney donors. Radiology 1979; 133:45–47.
- Cragg AH, Smith TP, Thompson BH, et al. Incidental fibromuscular dysplasia in potential renal donors: long-term clinical follow-up. Radiology 1989; 172:145–147.
- Neymark E, LaBerge JM, Hirose R, et al. Arteriographic detection of renovascular disease in potential renal donors: incidence and effect on donor surgery. Radiology 2000; 214:755–760.
- Andreoni KA, Weeks SM, Gerber DA, et al. Incidence of donor renal fibromuscular dysplasia: does it justify routine angiography? Transplantation 2002; 73:1112–1116.
- Blondin D, Lanzman R, Schellhammer F, et al. Fibromuscular dysplasia in living renal donors: still a challenge to computed tomographic angiography. Eur J Radiol 2010; 75:67–71.
- Lorenz EC, Vrtiska TJ, Lieske JC, et al. Prevalence of renal artery and kidney abnormalities by computed tomography among healthy adults. Clin J Am Soc Nephrol 2010; 5:431–438.
- McKenzie GA, Oderich GS, Kawashima A, Misra S. Renal artery fibromuscular dysplasia in 2,640 renal donor subjects: a CT angiography analysis. J Vasc Interv Radiol 2013; 24:1477–1480.
- Frick MP, Goldberg ME. Uro- and angiographic findings in a “normal” population: screening of 151 symptom-free potential transplant donors for renal disease. AJR Am J Roentgenol 1980; 134:503–505.
- Hendricks NJ, Matsumoto AH, Angle JF, et al. Is fibromuscular dysplasia underdiagnosed? A comparison of the prevalence of FMD seen in CORAL trial participants versus a single institution population of renal donor candidates. Vasc Med 2014; 19:363–367.
- Touzé E, Oppenheim C, Trystram D, et al. Fibromuscular dysplasia of cervical and intracranial arteries. Int J Stroke 2010; 5:296–305.
- Savard S, Azarine A, Jeunemaitre X, Azizi M, Plouin PF, Steichen O. Association of smoking with phenotype at diagnosis and vascular interventions in patients with renal artery fibromuscular dysplasia. Hypertension 2013; 61:1227–1232.
- Schievink WI, Limburg M. Angiographic abnormalities mimicking fibromuscular dysplasia in a patient with Ehlers-Danlos syndrome, type IV. Neurosurgery 1989; 25:482–483.
- Schievink WI, Bjornsson J, Piepgras DG. Coexistence of fibromuscular dysplasia and cystic medial necrosis in a patient with Marfan’s syndrome and bilateral carotid artery dissections. Stroke 1994; 25:2492–2496.
- Schievink WI, Bjournsson J, Parisi JE, Prakash UB. Arterial fibromuscular dysplasia associated with severe α1-antitrypsin deficiency. Mayo Clin Proc 1994; 69:1040–1043.
- Schievink WI, Puumala MR, Meyer FB, Raffel C, Katzmann JA, Parisi JE. Giant intracranial aneurysm and fibromuscular dysplasia in an adolescent with α1-antitrypsin deficiency. J Neurosurg 1996; 85:503–506.
- Schievink WI, Meyer FB, Parisi JE, Wijdicks EFM. Fibromuscular dysplasia of the internal carotid artery associated with alpha1-antitrypsin deficiency. Neurosurgery 1998; 43:229–233; discussion 233–234.
- Bofinger A, Hawley C, Fisher P, Daunt N, Stowasser M, Gordon R. Alpha-1-antitrypsin phenotypes in patients with renal arterial fibromuscular dysplasia. J Hum Hypertens 2000; 14:91–94.
- Poloskey SL, Kim ES, Sanghani R, et al. Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc Med 2012; 17:371–378.
- Ganesh SK, Morissette R, Xu Z, et al. Clinical and biochemical profiles suggest fibromuscular dysplasia is a systemic disease with altered TGF- expression and connective tissue features. FASEB J 2014; 28:3313–3324.
- O’Connor S, Kim ES, Brinza E, et al. Systemic connective tissue features in women with fibromuscular dysplasia. Vasc Med 2015; 20:454–462.
- Leadbetter WF, Burkland CE. Hypertension in unilateral renal disease. J Urol 1938; 39:611–626.
- Palubinskas AJ, Ripley HR. Fibromuscular hyperplasia in extrarenal arteries. Radiology 1964; 82:451–455.
- Saw J, Mancini GB, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016; 68:297–312.
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014; 7:645–655.
- Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015; 115:1672–1677.
- Saw J, Bezerra H, Gornik HL, Machan L, Mancini GB. Angiographic and intracoronary manifestations of coronary fibromuscular dysplasia. Circulation 2016; 133:1548–1559.
- Bolen MA, Brinza E, Renapurkar RD, Kim ES, Gornik HL. Screening CT angiography of the aorta, visceral branch vessels, and pelvic arteries in fibromuscular dysplasia. JACC Cardiovasc Imaging. 2016; doi: 10.1016/j.jcmg.2016.04.010. [Epub ahead of print].
- Olin JW, Gornik HL, Bacharach JM, et al. Fibromuscular dysplasia: state of the science and critical unanswered questions. A scientific statement from the American Heart Association. Circulation 2014; 129:1048–1078
- Poloskey SL, Olin JW, Mace P, Gornik HL. Fibromuscular dysplasia. Circulation 2012; 125:e636–e639.
- O’Connor S, Gornik HL, Froehlich JB, et al. Smoking and adverse outcomes in fibromuscular dysplasia: US registry report. J Am Coll Cardiol 2016; 67:1750–1751.
- Sang CN, Whelton PK, Hamper UM, et al. Etiologic factors in renovascular fibromuscular dysplasia. A case-control study. Hypertension 1989; 14:472–479.
- Persu A, Touzé E, Mousseaux E, Barral X, Joffre F, Plouin PF. Diagnosis and management of fibromuscular dysplasia: an expert consensus. Eur J Clin Invest 2012; 42:338–347.
- Sethi SS, Lau JF, Godbold J, Gustavson S, Olin JW. The S curve: a novel morphological finding in the internal carotid artery in patients with fibromuscular dysplasia. Vasc Med 2014; 19:356–362.
- Kadian-Dodov D, Gornik HL, Gu X, et al. Dissection and aneurysm in patients with fibromuscular dysplasia: findings for the U.S. registry for FMD. J Am Coll Cardiol 2016; 68:176–185.
- Olin JW, Froehlich J, Gu X, et al. The United States Registry for Fibromuscular Dysplasia: results in the first 447 patients. Circulation 2012; 125:3182–3190.
- Fibromuscular Dysplasia Society of America (FMDSA). The US Registry for FMD. www.fmdsa.org/research_network/fmd_registry. Accessed August 24, 2016.
- Kim ES, Olin JW, Froehlich JB, et al. Clinical manifestations of fibromuscular dysplasia vary by patient sex: a report of the United States Registry for Fibromuscular Dysplasia. J Am Coll Cardiol 2013; 62:2026–2028.
- Weinberg I, Gu X, Giri J, et al. Anti-platelet and anti-hypertension medication use in patients with fibromuscular dysplasia: results from the United States Registry for Fibromuscular Dysplasia. Vasc Med 2015; 20:447–453.
- Green R, Gu X, Kline-Rogers E, et al. Differences between the pediatric and adult presentation of fibromuscular dysplasia: results from the US Registry. Pediatr Nephrol 2016; 31:641–650.
- Shivapour DM, Erwin P, Kim ESH. Epidemiology of fibromuscular dysplasia: a review of the literature. Vasc Med 2016; 21:376–381.
- Spring DB, Satvatierra O Jr, Palubinskas AJ, Amend WJ Jr, Vincenti FG, Feduska NJ. Results and significance of angiography in potential kidney donors. Radiology 1979; 133:45–47.
- Cragg AH, Smith TP, Thompson BH, et al. Incidental fibromuscular dysplasia in potential renal donors: long-term clinical follow-up. Radiology 1989; 172:145–147.
- Neymark E, LaBerge JM, Hirose R, et al. Arteriographic detection of renovascular disease in potential renal donors: incidence and effect on donor surgery. Radiology 2000; 214:755–760.
- Andreoni KA, Weeks SM, Gerber DA, et al. Incidence of donor renal fibromuscular dysplasia: does it justify routine angiography? Transplantation 2002; 73:1112–1116.
- Blondin D, Lanzman R, Schellhammer F, et al. Fibromuscular dysplasia in living renal donors: still a challenge to computed tomographic angiography. Eur J Radiol 2010; 75:67–71.
- Lorenz EC, Vrtiska TJ, Lieske JC, et al. Prevalence of renal artery and kidney abnormalities by computed tomography among healthy adults. Clin J Am Soc Nephrol 2010; 5:431–438.
- McKenzie GA, Oderich GS, Kawashima A, Misra S. Renal artery fibromuscular dysplasia in 2,640 renal donor subjects: a CT angiography analysis. J Vasc Interv Radiol 2013; 24:1477–1480.
- Frick MP, Goldberg ME. Uro- and angiographic findings in a “normal” population: screening of 151 symptom-free potential transplant donors for renal disease. AJR Am J Roentgenol 1980; 134:503–505.
- Hendricks NJ, Matsumoto AH, Angle JF, et al. Is fibromuscular dysplasia underdiagnosed? A comparison of the prevalence of FMD seen in CORAL trial participants versus a single institution population of renal donor candidates. Vasc Med 2014; 19:363–367.
- Touzé E, Oppenheim C, Trystram D, et al. Fibromuscular dysplasia of cervical and intracranial arteries. Int J Stroke 2010; 5:296–305.
- Savard S, Azarine A, Jeunemaitre X, Azizi M, Plouin PF, Steichen O. Association of smoking with phenotype at diagnosis and vascular interventions in patients with renal artery fibromuscular dysplasia. Hypertension 2013; 61:1227–1232.
- Schievink WI, Limburg M. Angiographic abnormalities mimicking fibromuscular dysplasia in a patient with Ehlers-Danlos syndrome, type IV. Neurosurgery 1989; 25:482–483.
- Schievink WI, Bjornsson J, Piepgras DG. Coexistence of fibromuscular dysplasia and cystic medial necrosis in a patient with Marfan’s syndrome and bilateral carotid artery dissections. Stroke 1994; 25:2492–2496.
- Schievink WI, Bjournsson J, Parisi JE, Prakash UB. Arterial fibromuscular dysplasia associated with severe α1-antitrypsin deficiency. Mayo Clin Proc 1994; 69:1040–1043.
- Schievink WI, Puumala MR, Meyer FB, Raffel C, Katzmann JA, Parisi JE. Giant intracranial aneurysm and fibromuscular dysplasia in an adolescent with α1-antitrypsin deficiency. J Neurosurg 1996; 85:503–506.
- Schievink WI, Meyer FB, Parisi JE, Wijdicks EFM. Fibromuscular dysplasia of the internal carotid artery associated with alpha1-antitrypsin deficiency. Neurosurgery 1998; 43:229–233; discussion 233–234.
- Bofinger A, Hawley C, Fisher P, Daunt N, Stowasser M, Gordon R. Alpha-1-antitrypsin phenotypes in patients with renal arterial fibromuscular dysplasia. J Hum Hypertens 2000; 14:91–94.
- Poloskey SL, Kim ES, Sanghani R, et al. Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc Med 2012; 17:371–378.
- Ganesh SK, Morissette R, Xu Z, et al. Clinical and biochemical profiles suggest fibromuscular dysplasia is a systemic disease with altered TGF- expression and connective tissue features. FASEB J 2014; 28:3313–3324.
- O’Connor S, Kim ES, Brinza E, et al. Systemic connective tissue features in women with fibromuscular dysplasia. Vasc Med 2015; 20:454–462.
- Leadbetter WF, Burkland CE. Hypertension in unilateral renal disease. J Urol 1938; 39:611–626.
- Palubinskas AJ, Ripley HR. Fibromuscular hyperplasia in extrarenal arteries. Radiology 1964; 82:451–455.
- Saw J, Mancini GB, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016; 68:297–312.
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014; 7:645–655.
- Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015; 115:1672–1677.
- Saw J, Bezerra H, Gornik HL, Machan L, Mancini GB. Angiographic and intracoronary manifestations of coronary fibromuscular dysplasia. Circulation 2016; 133:1548–1559.
- Bolen MA, Brinza E, Renapurkar RD, Kim ES, Gornik HL. Screening CT angiography of the aorta, visceral branch vessels, and pelvic arteries in fibromuscular dysplasia. JACC Cardiovasc Imaging. 2016; doi: 10.1016/j.jcmg.2016.04.010. [Epub ahead of print].
KEY POINTS
- There is no cure for FMD. Management focuses on thorough evaluation and surveillance, lifestyle modification, and treatment of symptoms. Vascular procedures, such as angioplasty or treatment of aneurysms, are required for some patients.
- The overwhelming majority (> 90%) of patients with FMD are women. But men seem to have a more aggressive course, with a rate of aneurysm or dissection two times higher than that in women.
- The disease can affect medium-sized vessels throughout the body. In addition to the typical “string-of-beads” appearance or focal lesions, manifestations include arterial tortuosity, aneurysm, and dissection.
Dr. Hospitalist: Visa Problems Must Be Addressed
Dear Dr. Hospitalist:
I completed my residency approximately a year ago and was hired by a large academic hospital medicine program with an H-1B visa. After six to eight months in what I thought was the “application process” for permanent residency, I discovered that the people responsible for filing the necessary paperwork had not done anything. During this delayed application period, it is too risky to travel internationally. While I’m still gracious for the opportunity to train and work in the U.S., I am depressed and angry because I haven’t seen my family for almost two years. Should I escalate and complain about my situation beyond the director of my division or just sit back and wait it out?
Dr. Angry and in Limbo
Dr. Hospitalist responds:
There were 2,576 H-1B petitions approved for physicians and surgeons in FY 2012.1 Even though the Society of Hospital Medicine does not currently track the number of international medical graduates (IMGs) in the U.S. practicing hospital medicine, most authorities believe it’s between 20 and 25 percent of the current workforce. 2 Undoubtedly, many of these docs are already U.S. citizens, but most work in the U.S. on employer-sponsored programs (H1-B), with a few taking the J-1 visa tract. Both programs are often used as a catalyst to permanent residency, but the J-1 requires the individual to work in an underserved area for three years before being eligible.
Because the H-1B visa individual can only maintain this status for three years at a time for a maximum of six years, I’m assuming you used three years of the program to complete your residency and will either need to obtain permanent residency (a green card) very soon or will have to leave the country for at least a year before you can apply again. The law does grant extensions beyond this six-year period but only when certain parts of the green card process have been pending for one year prior to the requested H1-B start date.
Assuming you have no culpability for the delay in processing the application (e.g., you turned in necessary paperwork on time, responded timely to correspondence from processors), you have every right to be angry. The application process is tedious and very complex, with very rigid time constraints. Many organizations have used physicians with H-1B visas to fill the gaps in their recruiting without the necessary infrastructure to support the needs of this group. While I recognize it would be difficult for small groups or hospitals to afford knowledgeable and skilled support staff, the days of having part-time administrative support to manage this task are long gone. There are web-based organizations that are skilled and affordable for the smaller groups, and larger groups should invest in administrative staff to support their physicians with visas. After all, in this era of “we’re all part of the team,” it’s difficult to feel valued when your ability to borrow money or travel internationally is limited or even worse: You could be deported.
As an ex-military guy, following the chain of command is in my blood. However, if after given reasonable opportunity to assist and rectify your issues, the division director is unresponsive or unable to assist, I would escalate to the department chair and beyond if necessary.
Good luck! TH
References
- U.S. Department of Homeland Security, U.S. Citizenship and Immigration Services. Characteristics of H1B Specialty Occupation Workers: Fiscal Year 21012 Annual Report to Congress. U.S. Citizenship and Immigration Services website. Accessed October 17, 2016
- Educational Commission for Foreign Medical Graduates [10-06-2012];Annual Report.
Accessed October 17, 2016
Dear Dr. Hospitalist:
I completed my residency approximately a year ago and was hired by a large academic hospital medicine program with an H-1B visa. After six to eight months in what I thought was the “application process” for permanent residency, I discovered that the people responsible for filing the necessary paperwork had not done anything. During this delayed application period, it is too risky to travel internationally. While I’m still gracious for the opportunity to train and work in the U.S., I am depressed and angry because I haven’t seen my family for almost two years. Should I escalate and complain about my situation beyond the director of my division or just sit back and wait it out?
Dr. Angry and in Limbo
Dr. Hospitalist responds:
There were 2,576 H-1B petitions approved for physicians and surgeons in FY 2012.1 Even though the Society of Hospital Medicine does not currently track the number of international medical graduates (IMGs) in the U.S. practicing hospital medicine, most authorities believe it’s between 20 and 25 percent of the current workforce. 2 Undoubtedly, many of these docs are already U.S. citizens, but most work in the U.S. on employer-sponsored programs (H1-B), with a few taking the J-1 visa tract. Both programs are often used as a catalyst to permanent residency, but the J-1 requires the individual to work in an underserved area for three years before being eligible.
Because the H-1B visa individual can only maintain this status for three years at a time for a maximum of six years, I’m assuming you used three years of the program to complete your residency and will either need to obtain permanent residency (a green card) very soon or will have to leave the country for at least a year before you can apply again. The law does grant extensions beyond this six-year period but only when certain parts of the green card process have been pending for one year prior to the requested H1-B start date.
Assuming you have no culpability for the delay in processing the application (e.g., you turned in necessary paperwork on time, responded timely to correspondence from processors), you have every right to be angry. The application process is tedious and very complex, with very rigid time constraints. Many organizations have used physicians with H-1B visas to fill the gaps in their recruiting without the necessary infrastructure to support the needs of this group. While I recognize it would be difficult for small groups or hospitals to afford knowledgeable and skilled support staff, the days of having part-time administrative support to manage this task are long gone. There are web-based organizations that are skilled and affordable for the smaller groups, and larger groups should invest in administrative staff to support their physicians with visas. After all, in this era of “we’re all part of the team,” it’s difficult to feel valued when your ability to borrow money or travel internationally is limited or even worse: You could be deported.
As an ex-military guy, following the chain of command is in my blood. However, if after given reasonable opportunity to assist and rectify your issues, the division director is unresponsive or unable to assist, I would escalate to the department chair and beyond if necessary.
Good luck! TH
References
- U.S. Department of Homeland Security, U.S. Citizenship and Immigration Services. Characteristics of H1B Specialty Occupation Workers: Fiscal Year 21012 Annual Report to Congress. U.S. Citizenship and Immigration Services website. Accessed October 17, 2016
- Educational Commission for Foreign Medical Graduates [10-06-2012];Annual Report.
Accessed October 17, 2016
Dear Dr. Hospitalist:
I completed my residency approximately a year ago and was hired by a large academic hospital medicine program with an H-1B visa. After six to eight months in what I thought was the “application process” for permanent residency, I discovered that the people responsible for filing the necessary paperwork had not done anything. During this delayed application period, it is too risky to travel internationally. While I’m still gracious for the opportunity to train and work in the U.S., I am depressed and angry because I haven’t seen my family for almost two years. Should I escalate and complain about my situation beyond the director of my division or just sit back and wait it out?
Dr. Angry and in Limbo
Dr. Hospitalist responds:
There were 2,576 H-1B petitions approved for physicians and surgeons in FY 2012.1 Even though the Society of Hospital Medicine does not currently track the number of international medical graduates (IMGs) in the U.S. practicing hospital medicine, most authorities believe it’s between 20 and 25 percent of the current workforce. 2 Undoubtedly, many of these docs are already U.S. citizens, but most work in the U.S. on employer-sponsored programs (H1-B), with a few taking the J-1 visa tract. Both programs are often used as a catalyst to permanent residency, but the J-1 requires the individual to work in an underserved area for three years before being eligible.
Because the H-1B visa individual can only maintain this status for three years at a time for a maximum of six years, I’m assuming you used three years of the program to complete your residency and will either need to obtain permanent residency (a green card) very soon or will have to leave the country for at least a year before you can apply again. The law does grant extensions beyond this six-year period but only when certain parts of the green card process have been pending for one year prior to the requested H1-B start date.
Assuming you have no culpability for the delay in processing the application (e.g., you turned in necessary paperwork on time, responded timely to correspondence from processors), you have every right to be angry. The application process is tedious and very complex, with very rigid time constraints. Many organizations have used physicians with H-1B visas to fill the gaps in their recruiting without the necessary infrastructure to support the needs of this group. While I recognize it would be difficult for small groups or hospitals to afford knowledgeable and skilled support staff, the days of having part-time administrative support to manage this task are long gone. There are web-based organizations that are skilled and affordable for the smaller groups, and larger groups should invest in administrative staff to support their physicians with visas. After all, in this era of “we’re all part of the team,” it’s difficult to feel valued when your ability to borrow money or travel internationally is limited or even worse: You could be deported.
As an ex-military guy, following the chain of command is in my blood. However, if after given reasonable opportunity to assist and rectify your issues, the division director is unresponsive or unable to assist, I would escalate to the department chair and beyond if necessary.
Good luck! TH
References
- U.S. Department of Homeland Security, U.S. Citizenship and Immigration Services. Characteristics of H1B Specialty Occupation Workers: Fiscal Year 21012 Annual Report to Congress. U.S. Citizenship and Immigration Services website. Accessed October 17, 2016
- Educational Commission for Foreign Medical Graduates [10-06-2012];Annual Report.
Accessed October 17, 2016
Drug can fight adenovirus in HSCT recipients
Image by Yale Rosen
NEW ORLEANS—Interim results of a phase 3 trial suggest brincidofovir can treat adenovirus (AdV) infection in recipients of allogeneic hematopoietic stem cell transplant (HSCT).
Both pediatric and adult patients experienced a decline in AdV viral load after brincidofovir treatment, but pediatric patients were more likely to respond.
Overall survival rates were better for patients who had a rapid response and were therefore better among pediatric patients than adults.
Investigators said the adverse events (AEs) in this study were consistent with the known safety profile of brincidofovir.
Michael Grimley, MD, of Cincinnati Children’s Hospital in Ohio, and his colleagues presented these results at IDWeek 2016 (abstract 2339). The research was supported by Chimerix, the company developing brincidofovir.
This trial, known as AdVise, was designed to evaluate brincidofovir for the treatment of AdV infection in pediatric and adult patients divided into 3 cohorts:
- Cohort A consists of allogeneic HSCT recipients with asymptomatic or limited AdV infection
- Cohort B consists of allogeneic HSCT recipients with disseminated AdV disease
- Cohort C consists of autologous HSCT recipients, solid organ transplant recipients, and other immunocompromised patients.
All patients were assigned to 12 weeks of oral brincidofovir, administered twice weekly. An additional 12 weeks of treatment was allowed in patients with ongoing or recurrent infection. After completing treatment, all patients were followed until week 36.
Interim analysis
The investigators examined outcomes at 24 weeks after the first brincidofovir dose (12 weeks after prescribed dosing duration) in 158 patients, including:
- Cohort A—23 adults and 43 pediatric patients
- Cohort B—35 adults and 57 pediatric patients.
The investigators noted that many of the patients did not complete the study. The team said this is a reflection of the significant mortality risk of AdV because most of these patients died before they could finish.
Sixty-five percent of adults and 33% of children in Cohort A did not complete the study. The same was true for 71% of adults and 49% of children in Cohort B.
Mortality
The study’s primary efficacy endpoint is all-cause mortality at day 60 after the first brincidofovir dose in allogeneic HSCT recipients with disseminated AdV disease (Cohort B). All-cause mortality at day 60 in this cohort was 19% in pediatric patients and 43% in adults.
In Cohorts A and B, all-cause mortality at 24 weeks was lower in children than adults.
At 24 weeks, pediatric all-cause mortality was 33% in Cohort A and 42% in Cohort B. Adult all-cause mortality was 48% in Cohort A and 71% in Cohort B.
AdV-related mortality at 24 weeks in pediatric patients was 9% in Cohort A and 14% in Cohort B. AdV-related mortality in adults was 4% in Cohort A and 46% in Cohort B.
Declines in viremia
In Cohort A, 61% of patients achieved undetectable viremia at the end of treatment—43% of adults and 70% of children.
In Cohort B, 49% of patients achieved undetectable viremia at the end of treatment—29% of adults and 63% of children.
The median time to undetectable AdV viremia was 43 days (range, 8 to non-estimable) for adults in Cohort A, 14 days (range, 5 to 23) for children in Cohort A, non-estimable (range, 29 days to non-estimable) for adults in Cohort B, and 22 days (range, 15 to 36) for children in Cohort B.
Link between response and survival
The investigators conducted post-hoc analyses to assess the correlation between rapid virologic response to brincidofovir and time to subsequent mortality.
The team compared patients who responded to treatment—defined as achieving a ≥ 2-log10 copies/mL decline, undetectable AdV viremia at week 4, or undetectable AdV viremia at week 6—with non-responders.
Fifty percent of adults and 84% of children who were still alive at week 4 had achieved a ≥ 2 log decline or undetectable AdV viremia at that time.
This type of response was associated with improved survival at week 24. In adults, the mortality rate was 46% in responders and 85% in non-responders (P=0.03). In pediatric patients, the mortality rate was 25% in responders and 71% in non-responders (P=0.01).
In patients who were alive at week 6, 42% of adults and 68% of children achieved undetectable AdV viremia by that time.
This response was associated with improved survival at week 24. In adults, the mortality rate was 30% in responders and 86% in non-responders (P=0.001). In pediatric patients, the mortality rate was 18% in responders and 54% in non-responders (P=0.01).
Safety
All adults had treatment-emergent AEs, as did all pediatric patients in Cohort B and 95% of pediatric patients in Cohort A.
The most common treatment-emergent AEs were gastrointestinal (GI) events, which occurred in 70% of adults and 81% of children in Cohort A, as well as 83% of adults and 74% of children in Cohort B.
Acute graft-versus-host disease (GVHD) was also common, occurring in 22% of adults and 37% of children in Cohort A and 43% of adults and 40% of children in Cohort B. Some patients did have acute GVHD at baseline, however—22%, 26%, 34%, and 19%, respectively.
The percentage of patients with AEs leading to treatment discontinuation was 26% for adults and 28% for children in Cohort A and 31% for adults and 14% for children in Cohort B.
Overall, 20% of pediatric patients and 29% of adults discontinued brincidofovir due to AEs. GI events were cited as the most common reason—5% and 14%, respectively.
The investigators said there were no events reported that were suggestive of drug-related nephrotoxicity or myelosuppression. ![]()

Image by Yale Rosen
NEW ORLEANS—Interim results of a phase 3 trial suggest brincidofovir can treat adenovirus (AdV) infection in recipients of allogeneic hematopoietic stem cell transplant (HSCT).
Both pediatric and adult patients experienced a decline in AdV viral load after brincidofovir treatment, but pediatric patients were more likely to respond.
Overall survival rates were better for patients who had a rapid response and were therefore better among pediatric patients than adults.
Investigators said the adverse events (AEs) in this study were consistent with the known safety profile of brincidofovir.
Michael Grimley, MD, of Cincinnati Children’s Hospital in Ohio, and his colleagues presented these results at IDWeek 2016 (abstract 2339). The research was supported by Chimerix, the company developing brincidofovir.
This trial, known as AdVise, was designed to evaluate brincidofovir for the treatment of AdV infection in pediatric and adult patients divided into 3 cohorts:
- Cohort A consists of allogeneic HSCT recipients with asymptomatic or limited AdV infection
- Cohort B consists of allogeneic HSCT recipients with disseminated AdV disease
- Cohort C consists of autologous HSCT recipients, solid organ transplant recipients, and other immunocompromised patients.
All patients were assigned to 12 weeks of oral brincidofovir, administered twice weekly. An additional 12 weeks of treatment was allowed in patients with ongoing or recurrent infection. After completing treatment, all patients were followed until week 36.
Interim analysis
The investigators examined outcomes at 24 weeks after the first brincidofovir dose (12 weeks after prescribed dosing duration) in 158 patients, including:
- Cohort A—23 adults and 43 pediatric patients
- Cohort B—35 adults and 57 pediatric patients.
The investigators noted that many of the patients did not complete the study. The team said this is a reflection of the significant mortality risk of AdV because most of these patients died before they could finish.
Sixty-five percent of adults and 33% of children in Cohort A did not complete the study. The same was true for 71% of adults and 49% of children in Cohort B.
Mortality
The study’s primary efficacy endpoint is all-cause mortality at day 60 after the first brincidofovir dose in allogeneic HSCT recipients with disseminated AdV disease (Cohort B). All-cause mortality at day 60 in this cohort was 19% in pediatric patients and 43% in adults.
In Cohorts A and B, all-cause mortality at 24 weeks was lower in children than adults.
At 24 weeks, pediatric all-cause mortality was 33% in Cohort A and 42% in Cohort B. Adult all-cause mortality was 48% in Cohort A and 71% in Cohort B.
AdV-related mortality at 24 weeks in pediatric patients was 9% in Cohort A and 14% in Cohort B. AdV-related mortality in adults was 4% in Cohort A and 46% in Cohort B.
Declines in viremia
In Cohort A, 61% of patients achieved undetectable viremia at the end of treatment—43% of adults and 70% of children.
In Cohort B, 49% of patients achieved undetectable viremia at the end of treatment—29% of adults and 63% of children.
The median time to undetectable AdV viremia was 43 days (range, 8 to non-estimable) for adults in Cohort A, 14 days (range, 5 to 23) for children in Cohort A, non-estimable (range, 29 days to non-estimable) for adults in Cohort B, and 22 days (range, 15 to 36) for children in Cohort B.
Link between response and survival
The investigators conducted post-hoc analyses to assess the correlation between rapid virologic response to brincidofovir and time to subsequent mortality.
The team compared patients who responded to treatment—defined as achieving a ≥ 2-log10 copies/mL decline, undetectable AdV viremia at week 4, or undetectable AdV viremia at week 6—with non-responders.
Fifty percent of adults and 84% of children who were still alive at week 4 had achieved a ≥ 2 log decline or undetectable AdV viremia at that time.
This type of response was associated with improved survival at week 24. In adults, the mortality rate was 46% in responders and 85% in non-responders (P=0.03). In pediatric patients, the mortality rate was 25% in responders and 71% in non-responders (P=0.01).
In patients who were alive at week 6, 42% of adults and 68% of children achieved undetectable AdV viremia by that time.
This response was associated with improved survival at week 24. In adults, the mortality rate was 30% in responders and 86% in non-responders (P=0.001). In pediatric patients, the mortality rate was 18% in responders and 54% in non-responders (P=0.01).
Safety
All adults had treatment-emergent AEs, as did all pediatric patients in Cohort B and 95% of pediatric patients in Cohort A.
The most common treatment-emergent AEs were gastrointestinal (GI) events, which occurred in 70% of adults and 81% of children in Cohort A, as well as 83% of adults and 74% of children in Cohort B.
Acute graft-versus-host disease (GVHD) was also common, occurring in 22% of adults and 37% of children in Cohort A and 43% of adults and 40% of children in Cohort B. Some patients did have acute GVHD at baseline, however—22%, 26%, 34%, and 19%, respectively.
The percentage of patients with AEs leading to treatment discontinuation was 26% for adults and 28% for children in Cohort A and 31% for adults and 14% for children in Cohort B.
Overall, 20% of pediatric patients and 29% of adults discontinued brincidofovir due to AEs. GI events were cited as the most common reason—5% and 14%, respectively.
The investigators said there were no events reported that were suggestive of drug-related nephrotoxicity or myelosuppression. ![]()

Image by Yale Rosen
NEW ORLEANS—Interim results of a phase 3 trial suggest brincidofovir can treat adenovirus (AdV) infection in recipients of allogeneic hematopoietic stem cell transplant (HSCT).
Both pediatric and adult patients experienced a decline in AdV viral load after brincidofovir treatment, but pediatric patients were more likely to respond.
Overall survival rates were better for patients who had a rapid response and were therefore better among pediatric patients than adults.
Investigators said the adverse events (AEs) in this study were consistent with the known safety profile of brincidofovir.
Michael Grimley, MD, of Cincinnati Children’s Hospital in Ohio, and his colleagues presented these results at IDWeek 2016 (abstract 2339). The research was supported by Chimerix, the company developing brincidofovir.
This trial, known as AdVise, was designed to evaluate brincidofovir for the treatment of AdV infection in pediatric and adult patients divided into 3 cohorts:
- Cohort A consists of allogeneic HSCT recipients with asymptomatic or limited AdV infection
- Cohort B consists of allogeneic HSCT recipients with disseminated AdV disease
- Cohort C consists of autologous HSCT recipients, solid organ transplant recipients, and other immunocompromised patients.
All patients were assigned to 12 weeks of oral brincidofovir, administered twice weekly. An additional 12 weeks of treatment was allowed in patients with ongoing or recurrent infection. After completing treatment, all patients were followed until week 36.
Interim analysis
The investigators examined outcomes at 24 weeks after the first brincidofovir dose (12 weeks after prescribed dosing duration) in 158 patients, including:
- Cohort A—23 adults and 43 pediatric patients
- Cohort B—35 adults and 57 pediatric patients.
The investigators noted that many of the patients did not complete the study. The team said this is a reflection of the significant mortality risk of AdV because most of these patients died before they could finish.
Sixty-five percent of adults and 33% of children in Cohort A did not complete the study. The same was true for 71% of adults and 49% of children in Cohort B.
Mortality
The study’s primary efficacy endpoint is all-cause mortality at day 60 after the first brincidofovir dose in allogeneic HSCT recipients with disseminated AdV disease (Cohort B). All-cause mortality at day 60 in this cohort was 19% in pediatric patients and 43% in adults.
In Cohorts A and B, all-cause mortality at 24 weeks was lower in children than adults.
At 24 weeks, pediatric all-cause mortality was 33% in Cohort A and 42% in Cohort B. Adult all-cause mortality was 48% in Cohort A and 71% in Cohort B.
AdV-related mortality at 24 weeks in pediatric patients was 9% in Cohort A and 14% in Cohort B. AdV-related mortality in adults was 4% in Cohort A and 46% in Cohort B.
Declines in viremia
In Cohort A, 61% of patients achieved undetectable viremia at the end of treatment—43% of adults and 70% of children.
In Cohort B, 49% of patients achieved undetectable viremia at the end of treatment—29% of adults and 63% of children.
The median time to undetectable AdV viremia was 43 days (range, 8 to non-estimable) for adults in Cohort A, 14 days (range, 5 to 23) for children in Cohort A, non-estimable (range, 29 days to non-estimable) for adults in Cohort B, and 22 days (range, 15 to 36) for children in Cohort B.
Link between response and survival
The investigators conducted post-hoc analyses to assess the correlation between rapid virologic response to brincidofovir and time to subsequent mortality.
The team compared patients who responded to treatment—defined as achieving a ≥ 2-log10 copies/mL decline, undetectable AdV viremia at week 4, or undetectable AdV viremia at week 6—with non-responders.
Fifty percent of adults and 84% of children who were still alive at week 4 had achieved a ≥ 2 log decline or undetectable AdV viremia at that time.
This type of response was associated with improved survival at week 24. In adults, the mortality rate was 46% in responders and 85% in non-responders (P=0.03). In pediatric patients, the mortality rate was 25% in responders and 71% in non-responders (P=0.01).
In patients who were alive at week 6, 42% of adults and 68% of children achieved undetectable AdV viremia by that time.
This response was associated with improved survival at week 24. In adults, the mortality rate was 30% in responders and 86% in non-responders (P=0.001). In pediatric patients, the mortality rate was 18% in responders and 54% in non-responders (P=0.01).
Safety
All adults had treatment-emergent AEs, as did all pediatric patients in Cohort B and 95% of pediatric patients in Cohort A.
The most common treatment-emergent AEs were gastrointestinal (GI) events, which occurred in 70% of adults and 81% of children in Cohort A, as well as 83% of adults and 74% of children in Cohort B.
Acute graft-versus-host disease (GVHD) was also common, occurring in 22% of adults and 37% of children in Cohort A and 43% of adults and 40% of children in Cohort B. Some patients did have acute GVHD at baseline, however—22%, 26%, 34%, and 19%, respectively.
The percentage of patients with AEs leading to treatment discontinuation was 26% for adults and 28% for children in Cohort A and 31% for adults and 14% for children in Cohort B.
Overall, 20% of pediatric patients and 29% of adults discontinued brincidofovir due to AEs. GI events were cited as the most common reason—5% and 14%, respectively.
The investigators said there were no events reported that were suggestive of drug-related nephrotoxicity or myelosuppression. ![]()
Two-drug combination targets LSCs in CML

Image by Difu Wu
Targeting a pair of transcription factors might improve the treatment of chronic myeloid leukemia (CML), according to researchers.
The team found that p53 and c-MYC have “defining roles” in the survival of leukemia stem cells (LSCs) in CML.
And by targeting these transcription factors with a pair of investigational drugs, the researchers were able to kill LSCs.
The team described this work in Nature.
“This collaborative study combined proteomics, transcriptomics, and systems biology to identify a novel, precision medicine-based approach for eradicating leukemic stem cells,” said study author Tony Whetton, PhD, of the University of Manchester in the UK.
Dr Whetton and his colleagues first discovered that p53 and c-MYC are “central hubs” in a CML network of deregulated proteins. The team also found that CML cells express increased c-MYC and decreased p53 levels.
So the researchers theorized that simultaneously activating p53 and inhibiting c-MYC could be a method for treating CML.
To that end, the team tested 2 drugs—RITA (or NSC652287), which binds p53 and blocks its degradation, and CPI-203, a BET inhibitor that hinders transcription by disrupting chromatin-dependent signal transduction.
The researchers found that CPI-203 successfully downregulated c-MYC but also reduced p53, while RITA increased p53.
Treating CML CD34+ cells with RITA or CPI-203 for 72 hours reduced cell viability and induced significant apoptosis, the team said. Combining the drugs enhanced these effects.
The researchers also found evidence to suggest that c-MYC inhibition induces differentiation of CML CD34+ cells. The team said that labelling with the cell-division tracker carboxyfluorescein succinimidyl ester (CFSE) and CD34 antibody showed that, as CML cells divided in the presence of CPI-203, there was a clear and rapid loss of CD34 expression that was not seen in the presence of RITA.
The researchers did not observe any differences in the effects of RITA and CPI-203 when they were tested in CML CD34+ cells pretreated with imatinib.
Furthermore, RITA and CPI-203, either alone or in combination, had no significant effects on normal CD34+ cells when tested at lower concentrations. However, when CPI-203 was used alone at higher concentrations (2 or 5 μ M) or with RITA at the highest concentrations tested (RITA at 25 nM, CPI-203 at 5 μ M), apoptosis did occur.
In CML cells, the researchers observed “significant apoptosis” with all concentrations of CPI-203 and RITA tested.
The team also exposed CML LSCs, defined as either CFSEmax or CD34+CD38− cells, to CPI-203 and RITA as well as a pair of tyrosine kinase inhibitors.
The CFSEmax population persisted despite 5 days of treatment with dasatinib or nilotinib, but the cells were “significantly reduced” after 5 days of treatment with CPI-203 alone and in combination with RITA.
Similarly, 72 hours of treatment with RITA with CPI-203 eliminated residual CD34+CD38− cells.
The researchers also assessed LSC engraftment after treatment with RITA and/or CPI-203, as well as dasatinib. They exposed CML CD34+ cells to the drugs for 48 hours before transplanting the cells into sublethally irradiated NSG mice.
The team said dasatinib had no significant effect on NSG-repopulating CML LSCs. However, RITA, CPI-203, and the drugs in combination reduced engraftment, as indicated by decreased CD45+, CD34+, CD33+, CD11b+, CD19+ and CD14+ cells. ![]()

Image by Difu Wu
Targeting a pair of transcription factors might improve the treatment of chronic myeloid leukemia (CML), according to researchers.
The team found that p53 and c-MYC have “defining roles” in the survival of leukemia stem cells (LSCs) in CML.
And by targeting these transcription factors with a pair of investigational drugs, the researchers were able to kill LSCs.
The team described this work in Nature.
“This collaborative study combined proteomics, transcriptomics, and systems biology to identify a novel, precision medicine-based approach for eradicating leukemic stem cells,” said study author Tony Whetton, PhD, of the University of Manchester in the UK.
Dr Whetton and his colleagues first discovered that p53 and c-MYC are “central hubs” in a CML network of deregulated proteins. The team also found that CML cells express increased c-MYC and decreased p53 levels.
So the researchers theorized that simultaneously activating p53 and inhibiting c-MYC could be a method for treating CML.
To that end, the team tested 2 drugs—RITA (or NSC652287), which binds p53 and blocks its degradation, and CPI-203, a BET inhibitor that hinders transcription by disrupting chromatin-dependent signal transduction.
The researchers found that CPI-203 successfully downregulated c-MYC but also reduced p53, while RITA increased p53.
Treating CML CD34+ cells with RITA or CPI-203 for 72 hours reduced cell viability and induced significant apoptosis, the team said. Combining the drugs enhanced these effects.
The researchers also found evidence to suggest that c-MYC inhibition induces differentiation of CML CD34+ cells. The team said that labelling with the cell-division tracker carboxyfluorescein succinimidyl ester (CFSE) and CD34 antibody showed that, as CML cells divided in the presence of CPI-203, there was a clear and rapid loss of CD34 expression that was not seen in the presence of RITA.
The researchers did not observe any differences in the effects of RITA and CPI-203 when they were tested in CML CD34+ cells pretreated with imatinib.
Furthermore, RITA and CPI-203, either alone or in combination, had no significant effects on normal CD34+ cells when tested at lower concentrations. However, when CPI-203 was used alone at higher concentrations (2 or 5 μ M) or with RITA at the highest concentrations tested (RITA at 25 nM, CPI-203 at 5 μ M), apoptosis did occur.
In CML cells, the researchers observed “significant apoptosis” with all concentrations of CPI-203 and RITA tested.
The team also exposed CML LSCs, defined as either CFSEmax or CD34+CD38− cells, to CPI-203 and RITA as well as a pair of tyrosine kinase inhibitors.
The CFSEmax population persisted despite 5 days of treatment with dasatinib or nilotinib, but the cells were “significantly reduced” after 5 days of treatment with CPI-203 alone and in combination with RITA.
Similarly, 72 hours of treatment with RITA with CPI-203 eliminated residual CD34+CD38− cells.
The researchers also assessed LSC engraftment after treatment with RITA and/or CPI-203, as well as dasatinib. They exposed CML CD34+ cells to the drugs for 48 hours before transplanting the cells into sublethally irradiated NSG mice.
The team said dasatinib had no significant effect on NSG-repopulating CML LSCs. However, RITA, CPI-203, and the drugs in combination reduced engraftment, as indicated by decreased CD45+, CD34+, CD33+, CD11b+, CD19+ and CD14+ cells. ![]()

Image by Difu Wu
Targeting a pair of transcription factors might improve the treatment of chronic myeloid leukemia (CML), according to researchers.
The team found that p53 and c-MYC have “defining roles” in the survival of leukemia stem cells (LSCs) in CML.
And by targeting these transcription factors with a pair of investigational drugs, the researchers were able to kill LSCs.
The team described this work in Nature.
“This collaborative study combined proteomics, transcriptomics, and systems biology to identify a novel, precision medicine-based approach for eradicating leukemic stem cells,” said study author Tony Whetton, PhD, of the University of Manchester in the UK.
Dr Whetton and his colleagues first discovered that p53 and c-MYC are “central hubs” in a CML network of deregulated proteins. The team also found that CML cells express increased c-MYC and decreased p53 levels.
So the researchers theorized that simultaneously activating p53 and inhibiting c-MYC could be a method for treating CML.
To that end, the team tested 2 drugs—RITA (or NSC652287), which binds p53 and blocks its degradation, and CPI-203, a BET inhibitor that hinders transcription by disrupting chromatin-dependent signal transduction.
The researchers found that CPI-203 successfully downregulated c-MYC but also reduced p53, while RITA increased p53.
Treating CML CD34+ cells with RITA or CPI-203 for 72 hours reduced cell viability and induced significant apoptosis, the team said. Combining the drugs enhanced these effects.
The researchers also found evidence to suggest that c-MYC inhibition induces differentiation of CML CD34+ cells. The team said that labelling with the cell-division tracker carboxyfluorescein succinimidyl ester (CFSE) and CD34 antibody showed that, as CML cells divided in the presence of CPI-203, there was a clear and rapid loss of CD34 expression that was not seen in the presence of RITA.
The researchers did not observe any differences in the effects of RITA and CPI-203 when they were tested in CML CD34+ cells pretreated with imatinib.
Furthermore, RITA and CPI-203, either alone or in combination, had no significant effects on normal CD34+ cells when tested at lower concentrations. However, when CPI-203 was used alone at higher concentrations (2 or 5 μ M) or with RITA at the highest concentrations tested (RITA at 25 nM, CPI-203 at 5 μ M), apoptosis did occur.
In CML cells, the researchers observed “significant apoptosis” with all concentrations of CPI-203 and RITA tested.
The team also exposed CML LSCs, defined as either CFSEmax or CD34+CD38− cells, to CPI-203 and RITA as well as a pair of tyrosine kinase inhibitors.
The CFSEmax population persisted despite 5 days of treatment with dasatinib or nilotinib, but the cells were “significantly reduced” after 5 days of treatment with CPI-203 alone and in combination with RITA.
Similarly, 72 hours of treatment with RITA with CPI-203 eliminated residual CD34+CD38− cells.
The researchers also assessed LSC engraftment after treatment with RITA and/or CPI-203, as well as dasatinib. They exposed CML CD34+ cells to the drugs for 48 hours before transplanting the cells into sublethally irradiated NSG mice.
The team said dasatinib had no significant effect on NSG-repopulating CML LSCs. However, RITA, CPI-203, and the drugs in combination reduced engraftment, as indicated by decreased CD45+, CD34+, CD33+, CD11b+, CD19+ and CD14+ cells. ![]()
Tranexamic acid safely reduces need for transfusion, study suggests

Photo by Piotr Bodzek
Results of a large study suggest that tranexamic acid can reduce the need for blood transfusion without increasing the risk of thrombotic complications or death in patients undergoing coronary artery surgery.
Patients who received tranexamic acid had a lower risk of excessive bleeding, required fewer units of blood products, and had a lower risk of emergency reoperation after surgery than patients who received placebo.
In addition, patients who received tranexamic acid had no higher risk of death or thrombotic complications than those who received placebo.
Paul S. Myles, MBBS, MD, of Alfred Hospital in Melbourne, Australia, and his colleagues conducted this study and reported the results in NEJM. The study was also presented at the ANESTHESIOLOGY® 2016 annual meeting.
The study included 4631 patients who underwent surgery and had available outcomes data, 2311 who were assigned to receive tranexamic acid and 2320 who were assigned to receive placebo.
The study’s primary outcome was a composite of death and thrombotic complications (nonfatal myocardial infarction, stroke, pulmonary embolism, renal failure, or bowel infarction) within 30 days after surgery.
There was no significant difference in the primary outcome between the 2 treatment groups. Thrombotic complications/death occurred in 16.7% of patients in the tranexamic acid group and 18.1% in the placebo group (relative risk=0.92; P=0.22).
Patients who received placebo required significantly more units of blood products than patients who received tranexamic acid—7994 and 4331 units, respectively (P<0.001).
And significantly fewer patients in the tranexamic acid group than the placebo group had major hemorrhage or cardiac tamponade leading to emergency reoperations—1.4% and 2.8%, respectively (P=0.001).
However, patients in the tranexamic group had a significantly higher incidence of seizures—0.7% and 0.1%, respectively (P=0.002).
Dr Myles said that although this study was conducted in patients undergoing coronary artery surgery, the results are relevant for patients having many other types of surgery where bleeding and the need for blood transfusion may occur. ![]()

Photo by Piotr Bodzek
Results of a large study suggest that tranexamic acid can reduce the need for blood transfusion without increasing the risk of thrombotic complications or death in patients undergoing coronary artery surgery.
Patients who received tranexamic acid had a lower risk of excessive bleeding, required fewer units of blood products, and had a lower risk of emergency reoperation after surgery than patients who received placebo.
In addition, patients who received tranexamic acid had no higher risk of death or thrombotic complications than those who received placebo.
Paul S. Myles, MBBS, MD, of Alfred Hospital in Melbourne, Australia, and his colleagues conducted this study and reported the results in NEJM. The study was also presented at the ANESTHESIOLOGY® 2016 annual meeting.
The study included 4631 patients who underwent surgery and had available outcomes data, 2311 who were assigned to receive tranexamic acid and 2320 who were assigned to receive placebo.
The study’s primary outcome was a composite of death and thrombotic complications (nonfatal myocardial infarction, stroke, pulmonary embolism, renal failure, or bowel infarction) within 30 days after surgery.
There was no significant difference in the primary outcome between the 2 treatment groups. Thrombotic complications/death occurred in 16.7% of patients in the tranexamic acid group and 18.1% in the placebo group (relative risk=0.92; P=0.22).
Patients who received placebo required significantly more units of blood products than patients who received tranexamic acid—7994 and 4331 units, respectively (P<0.001).
And significantly fewer patients in the tranexamic acid group than the placebo group had major hemorrhage or cardiac tamponade leading to emergency reoperations—1.4% and 2.8%, respectively (P=0.001).
However, patients in the tranexamic group had a significantly higher incidence of seizures—0.7% and 0.1%, respectively (P=0.002).
Dr Myles said that although this study was conducted in patients undergoing coronary artery surgery, the results are relevant for patients having many other types of surgery where bleeding and the need for blood transfusion may occur. ![]()

Photo by Piotr Bodzek
Results of a large study suggest that tranexamic acid can reduce the need for blood transfusion without increasing the risk of thrombotic complications or death in patients undergoing coronary artery surgery.
Patients who received tranexamic acid had a lower risk of excessive bleeding, required fewer units of blood products, and had a lower risk of emergency reoperation after surgery than patients who received placebo.
In addition, patients who received tranexamic acid had no higher risk of death or thrombotic complications than those who received placebo.
Paul S. Myles, MBBS, MD, of Alfred Hospital in Melbourne, Australia, and his colleagues conducted this study and reported the results in NEJM. The study was also presented at the ANESTHESIOLOGY® 2016 annual meeting.
The study included 4631 patients who underwent surgery and had available outcomes data, 2311 who were assigned to receive tranexamic acid and 2320 who were assigned to receive placebo.
The study’s primary outcome was a composite of death and thrombotic complications (nonfatal myocardial infarction, stroke, pulmonary embolism, renal failure, or bowel infarction) within 30 days after surgery.
There was no significant difference in the primary outcome between the 2 treatment groups. Thrombotic complications/death occurred in 16.7% of patients in the tranexamic acid group and 18.1% in the placebo group (relative risk=0.92; P=0.22).
Patients who received placebo required significantly more units of blood products than patients who received tranexamic acid—7994 and 4331 units, respectively (P<0.001).
And significantly fewer patients in the tranexamic acid group than the placebo group had major hemorrhage or cardiac tamponade leading to emergency reoperations—1.4% and 2.8%, respectively (P=0.001).
However, patients in the tranexamic group had a significantly higher incidence of seizures—0.7% and 0.1%, respectively (P=0.002).
Dr Myles said that although this study was conducted in patients undergoing coronary artery surgery, the results are relevant for patients having many other types of surgery where bleeding and the need for blood transfusion may occur. ![]()
Tobacco plants used to manufacture malaria drug

Tobacco plants can be engineered to manufacture artemisinin at therapeutic levels, according research published in Molecular Plant.
The researchers noted that the majority of people who live in malaria-endemic areas cannot afford to buy artemisinin.
The drug’s high cost is due to the extraction process and the fact that it’s difficult to grow Artemisia annua, the original source of the drug, in climates where malaria is common.
Advances in synthetic biology have made it possible to produce artemisinin in yeast, but the manufacturing process is difficult to scale up.
Earlier studies showed that artemisinin can be grown in tobacco—a plant that’s relatively easy to genetically manipulate and that grows well in areas where malaria is endemic. But yields of artemisinin from those plants were low.
Now, Shashi Kumar, PhD, of the International Centre for Genetic
Engineering and Biotechnology in New Delhi, India, and his colleagues say they have overcome this problem.
In the Molecular Plant paper, Dr Kumar and his colleagues reported using a dual-transformation approach to boost the production of artemisinin in the tobacco plants.
The team first generated plants that contained transgenic chloroplasts, and the same plants were then manipulated again to insert genes into the nuclear genome as well.
Extract from the plants was shown to stop the growth of Plasmodium falciparum in vitro. Whole cells from the plant were also fed to mice infected with Plasmodium berghei, which greatly reduced levels of the parasite in the blood.
In fact, the researchers found the whole plant material was more effective in attacking the parasite than pure artemisinin, likely because encapsulation inside the plant cells protected the compound from degradation by digestive enzymes.
The researchers acknowledged that convincing people to eat tobacco plants is likely to be a hard sell. For that reason, they are now aiming to genetically engineer lettuce plants to produce artemisinin at therapeutic levels.
They said the lettuce containing the drug could be freeze dried, ground into a powder, and put into capsules for cost-effective delivery.
“Plant and animal science are increasingly coming together,” Dr Kumar said. “In the near future, you will see more drugs produced inside plants will be commercialized to reduce the drug cost.” ![]()

Tobacco plants can be engineered to manufacture artemisinin at therapeutic levels, according research published in Molecular Plant.
The researchers noted that the majority of people who live in malaria-endemic areas cannot afford to buy artemisinin.
The drug’s high cost is due to the extraction process and the fact that it’s difficult to grow Artemisia annua, the original source of the drug, in climates where malaria is common.
Advances in synthetic biology have made it possible to produce artemisinin in yeast, but the manufacturing process is difficult to scale up.
Earlier studies showed that artemisinin can be grown in tobacco—a plant that’s relatively easy to genetically manipulate and that grows well in areas where malaria is endemic. But yields of artemisinin from those plants were low.
Now, Shashi Kumar, PhD, of the International Centre for Genetic
Engineering and Biotechnology in New Delhi, India, and his colleagues say they have overcome this problem.
In the Molecular Plant paper, Dr Kumar and his colleagues reported using a dual-transformation approach to boost the production of artemisinin in the tobacco plants.
The team first generated plants that contained transgenic chloroplasts, and the same plants were then manipulated again to insert genes into the nuclear genome as well.
Extract from the plants was shown to stop the growth of Plasmodium falciparum in vitro. Whole cells from the plant were also fed to mice infected with Plasmodium berghei, which greatly reduced levels of the parasite in the blood.
In fact, the researchers found the whole plant material was more effective in attacking the parasite than pure artemisinin, likely because encapsulation inside the plant cells protected the compound from degradation by digestive enzymes.
The researchers acknowledged that convincing people to eat tobacco plants is likely to be a hard sell. For that reason, they are now aiming to genetically engineer lettuce plants to produce artemisinin at therapeutic levels.
They said the lettuce containing the drug could be freeze dried, ground into a powder, and put into capsules for cost-effective delivery.
“Plant and animal science are increasingly coming together,” Dr Kumar said. “In the near future, you will see more drugs produced inside plants will be commercialized to reduce the drug cost.” ![]()

Tobacco plants can be engineered to manufacture artemisinin at therapeutic levels, according research published in Molecular Plant.
The researchers noted that the majority of people who live in malaria-endemic areas cannot afford to buy artemisinin.
The drug’s high cost is due to the extraction process and the fact that it’s difficult to grow Artemisia annua, the original source of the drug, in climates where malaria is common.
Advances in synthetic biology have made it possible to produce artemisinin in yeast, but the manufacturing process is difficult to scale up.
Earlier studies showed that artemisinin can be grown in tobacco—a plant that’s relatively easy to genetically manipulate and that grows well in areas where malaria is endemic. But yields of artemisinin from those plants were low.
Now, Shashi Kumar, PhD, of the International Centre for Genetic
Engineering and Biotechnology in New Delhi, India, and his colleagues say they have overcome this problem.
In the Molecular Plant paper, Dr Kumar and his colleagues reported using a dual-transformation approach to boost the production of artemisinin in the tobacco plants.
The team first generated plants that contained transgenic chloroplasts, and the same plants were then manipulated again to insert genes into the nuclear genome as well.
Extract from the plants was shown to stop the growth of Plasmodium falciparum in vitro. Whole cells from the plant were also fed to mice infected with Plasmodium berghei, which greatly reduced levels of the parasite in the blood.
In fact, the researchers found the whole plant material was more effective in attacking the parasite than pure artemisinin, likely because encapsulation inside the plant cells protected the compound from degradation by digestive enzymes.
The researchers acknowledged that convincing people to eat tobacco plants is likely to be a hard sell. For that reason, they are now aiming to genetically engineer lettuce plants to produce artemisinin at therapeutic levels.
They said the lettuce containing the drug could be freeze dried, ground into a powder, and put into capsules for cost-effective delivery.
“Plant and animal science are increasingly coming together,” Dr Kumar said. “In the near future, you will see more drugs produced inside plants will be commercialized to reduce the drug cost.” ![]()
Emergency Test for Absorbed Radiation
In a large-scale emergency involving radiation, health care providers need to know how much radiation a survivor has absorbed to be able to determine treatment. Devices are available that detect radiation externally, for example, on skin, but no biodosimetry tests are approved to measure radiation absorbed into the body.
To help save more people in such an emergency, the HHS is sponsoring development of 2 biodosimetry tests to determine radiation absorption. The Biomedical Advanced Research and Development Authority will provide more than $22.4 million over 2 years to DxTerity Diagnostics in Los Angeles and more than $21.3 million over 4 years to MRIGloba in Kansas City, Missouri.
Related: FDA Approves Rescue Drug for Chemotherapy Overdose
Both tests, which are being designed for use in clinical health care labs, analyze blood samples to measure how genes respond to different amounts of radiation. The tests are expected to generate results in about 8 hours and to be used up to 7 days after exposure. The manufacturers estimate a potential to process 400,000 or more tests a week.
In a large-scale emergency involving radiation, health care providers need to know how much radiation a survivor has absorbed to be able to determine treatment. Devices are available that detect radiation externally, for example, on skin, but no biodosimetry tests are approved to measure radiation absorbed into the body.
To help save more people in such an emergency, the HHS is sponsoring development of 2 biodosimetry tests to determine radiation absorption. The Biomedical Advanced Research and Development Authority will provide more than $22.4 million over 2 years to DxTerity Diagnostics in Los Angeles and more than $21.3 million over 4 years to MRIGloba in Kansas City, Missouri.
Related: FDA Approves Rescue Drug for Chemotherapy Overdose
Both tests, which are being designed for use in clinical health care labs, analyze blood samples to measure how genes respond to different amounts of radiation. The tests are expected to generate results in about 8 hours and to be used up to 7 days after exposure. The manufacturers estimate a potential to process 400,000 or more tests a week.
In a large-scale emergency involving radiation, health care providers need to know how much radiation a survivor has absorbed to be able to determine treatment. Devices are available that detect radiation externally, for example, on skin, but no biodosimetry tests are approved to measure radiation absorbed into the body.
To help save more people in such an emergency, the HHS is sponsoring development of 2 biodosimetry tests to determine radiation absorption. The Biomedical Advanced Research and Development Authority will provide more than $22.4 million over 2 years to DxTerity Diagnostics in Los Angeles and more than $21.3 million over 4 years to MRIGloba in Kansas City, Missouri.
Related: FDA Approves Rescue Drug for Chemotherapy Overdose
Both tests, which are being designed for use in clinical health care labs, analyze blood samples to measure how genes respond to different amounts of radiation. The tests are expected to generate results in about 8 hours and to be used up to 7 days after exposure. The manufacturers estimate a potential to process 400,000 or more tests a week.
Tips for Living With Trigeminal Neuralgia
Click here to download the PDF.
Click here to download the PDF.
Click here to download the PDF.