User login
Serotonin syndrome: Preventing, recognizing, and treating it
With a substantial increase in antidepressant use in the United States over the last 2 decades, serotonin syndrome has become an increasingly common and significant clinical concern. In 1999, 6.5% of adults age 18 and older were taking antidepressants; by 2010, the percentage had increased to 10.4%.1 Though the true incidence of serotonin syndrome is difficult to determine, the number of ingestions of selective serotonin reuptake inhibitors (SSRIs) associated with moderate to major effects reported to US poison control centers increased from 7,349 in 20022 to 8,585 in 2005.3
Though the clinical manifestations are often mild to moderate, patients with serotonin syndrome can deteriorate rapidly and require intensive care. Unlike neuroleptic malignant syndrome, serotonin syndrome should not be considered an extremely rare idiosyncratic reaction to medication, but rather a progression of serotonergic toxicity based on increasing concentration levels that can occur in any patient regardless of age.4
Because it has a nonspecific prodrome and protean manifestations, serotonin syndrome can easily be overlooked, misdiagnosed, or exacerbated if not carefully assessed. Diagnosis requires a low threshold for suspicion and a meticulous history and physical examination. In the syndrome’s mildest stage, symptoms are often misattributed to other causes, and in its most severe form, it can easily be mistaken for neuroleptic malignant syndrome.
WHAT IS SEROTONIN SYNDROME?
Serotonin syndrome classically presents as the triad of autonomic dysfunction, neuromuscular excitation, and altered mental status. These symptoms are a result of increased serotonin levels affecting the central and peripheral nervous systems. Serotonin affects a family of receptors that has seven members, of which 5-HT1A and 5-HT2A are most often responsible for serotonin syndrome.5
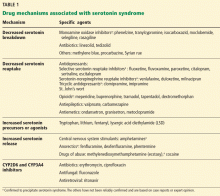
Conditions that can alter the regulation of serotonin include therapeutic doses, drug interactions, intentional or unintentional overdoses, and overlapping transitions between medications. As a result, drugs that have been associated with serotonin syndrome can be classified into the following five categories as shown below and in Table 1:
Drugs that decrease serotonin breakdown include monoamine oxidase inhibitors (MAOIs), linezolid,6 methylene blue, procarbazine, and Syrian rue.
Drugs that decrease serotonin reuptake include SSRIs, serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants, opioids (meperidine, buprenorphine, tramadol, tapentadol, dextromethorphan), antiepileptics (carbamazepine, valproate), and antiemetics (ondansetron, granisetron, metoclopramide), and the herbal preparation St. John’s wort.
Drugs that increase serotonin precursors or agonists include tryptophan, lithium, fentanyl, and lysergic acid diethylamide (LSD).
Drugs that increase serotonin release include fenfluramine, amphetamines, and methylenedioxymethamphetamine (ecstasy).
Drugs that prevent breakdown of the agents listed above are CYP2D6 and CYP3A4 inhibitors, eg, erythromycin,7 ciprofloxacin, fluconazole, ritonavir, and grapefruit juice.
However, the only drugs that have been reliably confirmed to precipitate serotonin syndrome are MAOIs, SSRIs, SNRIs, and serotonin releasers. Other listed drug interactions are based on case reports and have not been thoroughly evaluated.6–9
Currently, SSRIs are the most commonly prescribed antidepressant medications and, consequently, they are the ones most often implicated in serotonergic toxicity.1,10 An estimated 15% of SSRI overdoses lead to mild or moderate serotonin toxicity.11 Serotonergic agents used in conjunction can increase the risk for severe serotonin syndrome; an SSRI and an MAOI in combination poses the greatest risk.5
Ultimately, the incidence of serotonin syndrome is difficult to assess, but it is believed to be underreported because it is easy to misdiagnose and mild symptoms may be dismissed.
WHO IS AT RISK OF SEROTONIN SYNDROME?
Long-term antidepressant use has disproportionately increased in middle-aged and older adults and non-Hispanic whites.1,12,13 Intuitively, as the risk for depression increases dramatically in patients with chronic medical conditions, serotonin syndrome should be more prevalent among the elderly. In addition, patients with multiple comorbidities take more medications, increasing the risk of polypharmacy and adverse drug reactions.14
Although the epidemiology of serotonin syndrome has yet to be extensively studied, the combination of age and comorbidities may increase the risk for this condition.
HOW DOES IT PRESENT?
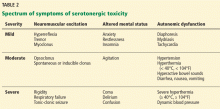
Serotonin syndrome characteristically presents as the triad of autonomic dysfunction, neuromuscular excitation, and altered mental status. However, these symptoms may not occur simultaneously: autonomic dysfunction is present in 40% of patients, neuromuscular excitation in 50%, and altered mental status in 40%.15 The symptoms can range from mild to life-threatening (Table 2).16
Autonomic dysfunction. Diaphoresis is present in 48.8% of cases, tachycardia in 44%, nausea and vomiting in 26.8%, and mydriasis in 19.5%. Other signs are hyperactive bowel sounds, diarrhea, and flushing.16
Neuromuscular excitation. Myoclonus is present in 48.8%, hyperreflexia in 41%, hyperthermia in 26.8%, and hypertonicity and rigidity in 19.5%. Other signs are spontaneous or inducible clonus, ocular clonus (continuous rhythmic oscillations of gaze), and tremor.
Altered mental status. Confusion is present in 41.2% and agitation in 36.5%. Other signs are anxiety, lethargy, and coma.
Symptoms of serotonin toxicity arise within an hour of a precipitating event (eg, ingestion) in approximately 28% of patients, and within 6 hours in 61%.16 Highly diagnostic features include hyperreflexia and induced or spontaneous clonus that are generally more pronounced in the lower limbs.11 Clonus can be elicited with ankle dorsiflexion.
In mild toxicity, patients may present with tremor or twitching and anxiety, as well as with hyperreflexia, tachycardia, diaphoresis, and mydriasis. Further investigation may uncover a recently initiated antidepressant or a cold-and-cough medication that contains dextromethorphan.15,17
In moderate toxicity, patients present in significant distress, with agitation and restlessness. Features may include hyperreflexia and clonus of the lower extremities, opsoclonus, hyperactive bowel sounds, diarrhea, nausea, vomiting, tachycardia, hypertension, diaphoresis, mydriasis, and hyperthermia (< 40°C, 104°F). The patient’s history may reveal use of ecstasy or combined treatment with serotonin-potentiating agents such as an antidepressant with a proserotonergic opioid, antiepileptic, or CYP2D6 or CYP3A4 inhibitor.15
Severe serotonin toxicity is a life-threatening condition that can lead to multiorgan failure within hours. It can be characterized by muscle rigidity, which can cause the body temperature to elevate rapidly to over 40°C. This hypertonicity can mask the classic and diagnostic signs of hyperreflexia and clonus. Patients may have unstable and dynamic vital signs with confusion or delirium and can experience tonic-clonic seizures.
If the muscle rigidity and resulting hyperthermia are not managed properly, patients can develop cellular damage and enzyme dysfunction leading to rhabdomyolysis, myoglobinuria, renal failure, metabolic acidosis, acute respiratory distress syndrome, and disseminated intravascular coagulation.16,18
Serotonin crisis is usually caused by the co-ingestion of multiple serotonergic agents, such as an antidepressant with an aforementioned opioid and antiemetic19; combining an SSRI and an MAOI poses the greatest risk. Alternatively, patients may have recently switched antidepressants without observing a safe washout period, leading to an overlap of serotonin levels.16
HOW DO WE DIAGNOSE SEROTONIN SYNDROME?
Serotonin syndrome is a clinical diagnosis and therefore requires a thorough review of medications and physical examination. Serum serotonin levels are an unreliable indicator of toxicity and do not correlate well with the clinical presentation.16
(based on information in reference 9).
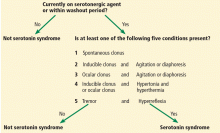
Currently, there are two clinical tools for diagnosing serotonin syndrome: the Hunter serotonin toxicity criteria (Figure 1) and the Sternbach criteria.
The Hunter criteria are based more heavily on physical findings. The patient must have taken a serotonergic agent and have one of the following:
- Spontaneous clonus
- Inducible clonus plus agitation or diaphoresis
- Ocular clonus plus agitation or diaphoresis
- Inducible clonus or ocular clonus, plus hypertonia and hyperthermia
- Tremor plus hyperreflexia.
The Sternbach criteria. The patient must be using a serotonergic agent, must have no other causes of symptoms, must not have recently used a neuroleptic agent, and must have three of the following:
- Mental status changes
- Agitation
- Hyperreflexia
- Myoclonus
- Diaphoresis
- Shivering
- Tremor
- Diarrhea
- Incoordination
- Fever
The Hunter criteria are recommended and are more specific (97% vs 96%) and more sensitive (84% vs 75%) than the Sternbach criteria when compared with the gold standard of diagnosis by a clinical toxicologist.1 The Hunter criteria are also less likely to yield false-positive results.11
Differential diagnosis
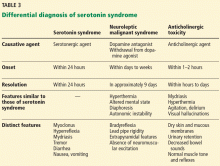
The differential diagnosis for serotonin syndrome includes neuroleptic malignant syndrome, anticholinergic poisoning (Table 3), metastatic carcinoma, central nervous system infection, gastroenteritis, and sepsis.
Neuroleptic malignant syndrome, the disorder most often misdiagnosed as serotonin syndrome, is an idiosyncratic reaction to a dopamine antagonist (eg, haloperidol, fluphenazine) that develops over days to weeks.20 In 70% of patients, agitated delirium with confusion appears first, followed by lead pipe rigidity and cogwheel tremor, then hyperthermia with body temperature greater than 40°C, and finally, profuse diaphoresis, tachycardia, hypertension, and tachypnea.21
Key elements that distinguish neuroleptic malignant syndrome are the timeline of the clinical course, bradyreflexia, and the absence of clonus. Prodromal symptoms of nausea, vomiting, and diarrhea are also rare in neuroleptic malignant syndrome. Neuroleptic malignant syndrome typically requires an average of 9 days to resolve.
Anticholinergic poisoning usually develops within 1 to 2 hours of oral ingestion. Symptoms include flushing, anhidrosis, anhidrotic hyperthermia, mydriasis, urinary retention, decreased bowel sounds, agitated delirium, and visual hallucinations. In contrast to serotonin syndrome, reflexes and muscle tone are normal with anticholinergic poisoning.
HOW CAN WE TREAT SEROTONIN SYNDROME?
The two mainstays of serotonin syndrome management are to discontinue the serotonergic agent and to give supportive care. Most patients improve within 24 hours of stopping the precipitating drug and starting therapy.16
For mild serotonin syndrome, treatment involves discontinuing the offending agent and supportive therapy with intravenous fluids, correction of vital signs, and symptomatic treatment with a benzodiazepine. Patients should be admitted and observed for 12 to 24 hours to prevent exacerbation.
For moderate serotonin syndrome, treatment also involves stopping the serotonergic agent and giving supportive care. Symptomatic treatment with a benzodiazepine and nonserotonergic antiemetics is recommended, and standard cooling measures should be implemented for hyperthermia. Patients should be admitted and observed for 12 to 24 hours to prevent exacerbation.
For severe serotonin toxicity, treatment should focus on management of airway, breathing, and circulation—ie, the “ABCs.” The two primary life-threatening concerns are hyperthermia (temperature > 40°C or 104°F) and rigidity, which can lead to hypoventilation.1,22 Controlling hyperthermia and rigidity can prevent other grave complications. Patients with severe serotonin toxicity should be sedated, paralyzed, and intubated.21 This will reverse ventilatory hypertonia and allow for mechanical ventilation. Paralysis will also prevent the exacerbation of hyperthermia, which is caused by muscle rigidity. Antipyretics have no role in the treatment of serotonin syndrome since the hyperthermia is not caused by a change in the hypothalamic temperature set point.21 Standard cooling measures should be used to manage hyperthermia.
Serotonin antagonists
Serotonin antagonists have had some success in case reports, but further studies are needed to confirm this.4,23,24
Cyproheptadine is a potent 5-HT2A antagonist; patients usually respond within 1 to 2 hours of administration. Signs and symptoms have resolved completely within times ranging from 20 minutes to 48 hours, depending on the severity of toxicity.
The recommended initial dose of cyproheptadine is 12 mg, followed by 2 mg every 2 hours if symptoms continue.16 Maintenance dosing with 8 mg every 6 hours should be prescribed once stabilization is achieved. The total daily dose for adults should not exceed 0.5 mg/kg/day. Cyproheptadine is available only in oral form but can be crushed and administered via a nasogastric tube.21
Chlorpromazine is a 5-HT1A and 5-HT2A antagonist and can be given intramuscularly. Despite case reports citing its effectiveness, the risk of hypotension, dystonic reactions, and neuroleptic malignant syndrome may make it a less desirable option.4,25
Cyproheptadine, chlorpromazine, and other serotonin receptor antagonists require further investigation beyond individual case reports to determine their effectiveness and reliability in treating serotonin syndrome.
Other agents
Benzodiazepines are considered a mainstay for symptomatic relief because of their anxiolytic and muscle relaxant effects.26 However, animal studies showed that treatment with benzodiazepines attenuated hyperthermia but had no effect on time to recovery or outcome.27
Neuromuscular blocking agents. The suggested neuromuscular blocking agent for severe toxicity is a nondepolarizing agent such as vecuronium. Succinylcholine should be avoided, as it can exacerbate rhabdomyolysis and hyperkalemia.21
Dantrolene has also been suggested for its muscle-relaxing effects and use in malignant hyperthermia. However, this treatment has not been successful in isolated case reports and has been ineffective in animal models.4,28
Physical restraints are ill-advised, since isometric muscle contractions can exacerbate hyperthermia and lactic acidosis in agitated patients.21 If physical restraints are necessary to deliver medications, they should be removed as soon as possible.
HOW CAN WE PREVENT SEROTONIN SYNDROME?
Prevention of serotonin syndrome begins with improving education and awareness in patients and healthcare providers. Patients should be primarily concerned with taking their medications carefully as prescribed and recognizing early signs and symptoms of serotonin toxicity.
As use of antidepressants among an aging population continues to increase, and as physicians in multiple disciplines prescribe them for evolving indications (eg, duloxetine to treat osteoarthritis, diabetic neuropathy, fibromyalgia, and chemotherapy-induced peripheral neuropathy), healthcare providers need to be prepared to see more cases of serotonin syndrome and its deleterious effects.29–31 Physicians should be vigilant in minimizing unnecessary use of serotonergic agents and reviewing drug regimens regularly to limit polypharmacy.
Electronic ordering systems should be designed to detect and alert the prescriber to possible interactions that can potentiate serotonin syndrome, and to not place the order until the prescriber overrides the alert. Combinations of SSRIs and MAOIs have the highest risk for inducing severe serotonin syndrome and should always be avoided.
If a patient is transitioning between serotonergic agents, physicians should observe a safe washout period to prevent overlap.16,32 Washout periods may differ among medications depending on their half-lives. For example, sertraline has a washout period of 2 weeks, while fluoxetine requires a washout period of 5 to 6 weeks.33 Consulting a pharmacist may be helpful when considering half-lives and washout periods.
We believe that educating both patients and physicians regarding prevention will help minimize the risk for serotonergic syndrome and will improve efficiency in assessment and management should toxicity develop.
- Mojtabai R, Olfson M. National trends in long-term use of antidepressant medications: results from the US National Health and Nutrition Examination Survey. J Clin Psychiatry 2014; 75:169–177.
- Watson WA, Litovitz TL, Rodgers GC Jr, et al. 2002 Annual Report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med. 2003;21:353–421.
- Lai MW, Klein-Schwartz W, Rodgers GC, et al. 2005 Annual Report of the American Association of Poison Control Centers’ national poisoning and exopsure database. Clin Toxicol (Phila) 2006; 44:803–932.
- Gillman PK. The serotonin syndrome and its treatment. J Psychopharmacol 1999; 13:100–109.
- Isbister GK, Buckley NA. The pathophysiology of serotonin toxicity in animals and humans: implications for diagnosis and treatment. Clin Neuropharmacol 2005; 28:205–214.
- Woytowish MR, Maynor LM. Clinical relevance of linezolid-associated serotonin toxicity. Ann Pharmacother 2013; 47:388–397.
- Lee DO, Lee CD. Serotonin syndrome in a child associated with erythromycin and sertraline. Pharmacotherapy 1999; 19:894–896.
- Gillman PK. Triptans, serotonin agonists, and serotonin syndrome (serotonin toxicity): a review. Headache 2010; 50:264–272.
- Isbister GK, Buckley NA, Whyte IM. Serotonin toxicity: a practical approach to diagnosis and treatment. Med J Aust 2007; 187:361–365.
- Mowry JB, Spyker DA, Cantilena LR Jr, McMillan N, Ford M. 2013 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol (Phila) 2014; 52:1032–1283.
- Dunkley EJ, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter serotonin toxicity criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM 2003; 96:635–642.
- Karkare SU, Bhattacharjee S, Kamble P, Aparasu R. Prevalence and predictors of antidepressant prescribing in nursing home residents in the United States. Am J Geriatr Pharmacother 2011; 9:109–119.
- Weissman J, Meyers BS, Ghosh S, Bruce ML. Demographic, clinical, and functional factors associated with antidepressant use in the home healthcare elderly. Am J Geriatr Psychiatry 2011; 19:1042–1045.
- Caughey GE, Roughead EE, Shakib S, Vitry AI, Gilbert AL. Co-morbidity and potential treatment conflicts in elderly heart failure patients: a retrospective, cross-sectional study of administrative claims data. Drugs Aging 2011; 28:575–581.
- Iqbal MM, Basil MJ, Kaplan J, Iqbal MT. Overview of serotonin syndrome. Ann Clin Psychiatry 2012; 24:310–318.
- Mason PJ, Morris VA, Balcezak TJ. Serotonin syndrome. Presentation of 2 cases and review of the literature. Medicine (Baltimore) 2000; 79:201–209.
- Prakash S, Patel V, Kakked S, Patel I, Yadav R. Mild serotonin syndrome: a report of 12 cases. Ann Indian Acad Neurol 2015; 18:226–230.
- Davies O, Batajoo-Shrestha B, Sosa-Popoteur J, Olibrice M. Full recovery after severe serotonin syndrome, severe rhabdomyolysis, multi-organ failure and disseminated intravascular coagulopathy from MDMA. Heart Lung 2014; 43:117–119.
- Pedavally S, Fugate JE, Rabinstein AA. Serotonin syndrome in the intensive care unit: clinical presentations and precipitating medications. Neurocrit Care 2014; 21:108–113.
- Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med 2005; 352:1112–1120.
- Velamoor VR, Norman RM, Caroff SN, Mann SC, Sullivan KA, Antelo RE. Progression of symptoms in neuroleptic malignant syndrome. J Nerv Ment Dis 1994; 182:168–173.
- Isbister GK, Hackett LP, Dawson AH, Whyte IM, Smith AJ. Moclobemide poisoning: toxicokinetics and occurrence of serotonin toxicity. Br J Clin Pharmacol 2003; 56:441–450.
- Graudins A, Stearman A, Chan B. Treatment of the serotonin syndrome with cyproheptadine. J Emerg Med 1998; 16:615–619.
- Lappin RI, Auchincloss EL. Treatment of the serotonin syndrome with cyproheptadine. N Engl J Med 1994; 331:1021–1022.
- Gillman PK. Successful treatment of serotonin syndrome with chlorpromazine. Med J Aust 1996; 165:345–346.
- Buckley NA, Dawson AH, Isbister GK. Serotonin syndrome. BMJ 2014; 348:g1626.
- Nisijima K, Shioda K, Yoshino T, Takano K, Kato S. Diazepam and chlormethiazole attenuate the development of hyperthermia in an animal model of the serotonin syndrome. Neurochem Int 2003; 43:155–164.
- Nisijima K, Yoshino T, Yui K, Katoh S. Potent serotonin (5-HT)(2A) receptor antagonists completely prevent the development of hyperthermia in an animal model of the 5-HT syndrome. Brain Res 2001; 890:23–31.
- Micca JL, Ruff D, Ahl J, Wohlreich MM. Safety and efficacy of duloxetine treatment in older and younger patients with osteoarthritis knee pain: a post hoc, subgroup analysis of two randomized, placebo-controlled trials. BMC Musculoskelet Disord 2013; 14:137.
- Smith EM, Pang H, Cirrincione C, et al; Alliance for Clinical Trials in Oncology. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA 2013; 309:1359–1367.
- Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev 2014; 1:CD007115.
- Caughey GE, Roughead EE, Shakib S, McDermott RA, Vitry AI, Gilbert AL. Comorbidity of chronic disease and potential treatment conflicts in older people dispensed antidepressants. Age Ageing 2010; 39:488–494.
- Gury C, Cousin F. Pharmacokinetics of SSRI antidepressants: half-life and clinical applicability. Encephale 1999; 25:470–476. French.
With a substantial increase in antidepressant use in the United States over the last 2 decades, serotonin syndrome has become an increasingly common and significant clinical concern. In 1999, 6.5% of adults age 18 and older were taking antidepressants; by 2010, the percentage had increased to 10.4%.1 Though the true incidence of serotonin syndrome is difficult to determine, the number of ingestions of selective serotonin reuptake inhibitors (SSRIs) associated with moderate to major effects reported to US poison control centers increased from 7,349 in 20022 to 8,585 in 2005.3
Though the clinical manifestations are often mild to moderate, patients with serotonin syndrome can deteriorate rapidly and require intensive care. Unlike neuroleptic malignant syndrome, serotonin syndrome should not be considered an extremely rare idiosyncratic reaction to medication, but rather a progression of serotonergic toxicity based on increasing concentration levels that can occur in any patient regardless of age.4
Because it has a nonspecific prodrome and protean manifestations, serotonin syndrome can easily be overlooked, misdiagnosed, or exacerbated if not carefully assessed. Diagnosis requires a low threshold for suspicion and a meticulous history and physical examination. In the syndrome’s mildest stage, symptoms are often misattributed to other causes, and in its most severe form, it can easily be mistaken for neuroleptic malignant syndrome.
WHAT IS SEROTONIN SYNDROME?
Serotonin syndrome classically presents as the triad of autonomic dysfunction, neuromuscular excitation, and altered mental status. These symptoms are a result of increased serotonin levels affecting the central and peripheral nervous systems. Serotonin affects a family of receptors that has seven members, of which 5-HT1A and 5-HT2A are most often responsible for serotonin syndrome.5
Conditions that can alter the regulation of serotonin include therapeutic doses, drug interactions, intentional or unintentional overdoses, and overlapping transitions between medications. As a result, drugs that have been associated with serotonin syndrome can be classified into the following five categories as shown below and in Table 1:
Drugs that decrease serotonin breakdown include monoamine oxidase inhibitors (MAOIs), linezolid,6 methylene blue, procarbazine, and Syrian rue.
Drugs that decrease serotonin reuptake include SSRIs, serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants, opioids (meperidine, buprenorphine, tramadol, tapentadol, dextromethorphan), antiepileptics (carbamazepine, valproate), and antiemetics (ondansetron, granisetron, metoclopramide), and the herbal preparation St. John’s wort.
Drugs that increase serotonin precursors or agonists include tryptophan, lithium, fentanyl, and lysergic acid diethylamide (LSD).
Drugs that increase serotonin release include fenfluramine, amphetamines, and methylenedioxymethamphetamine (ecstasy).
Drugs that prevent breakdown of the agents listed above are CYP2D6 and CYP3A4 inhibitors, eg, erythromycin,7 ciprofloxacin, fluconazole, ritonavir, and grapefruit juice.
However, the only drugs that have been reliably confirmed to precipitate serotonin syndrome are MAOIs, SSRIs, SNRIs, and serotonin releasers. Other listed drug interactions are based on case reports and have not been thoroughly evaluated.6–9
Currently, SSRIs are the most commonly prescribed antidepressant medications and, consequently, they are the ones most often implicated in serotonergic toxicity.1,10 An estimated 15% of SSRI overdoses lead to mild or moderate serotonin toxicity.11 Serotonergic agents used in conjunction can increase the risk for severe serotonin syndrome; an SSRI and an MAOI in combination poses the greatest risk.5
Ultimately, the incidence of serotonin syndrome is difficult to assess, but it is believed to be underreported because it is easy to misdiagnose and mild symptoms may be dismissed.
WHO IS AT RISK OF SEROTONIN SYNDROME?
Long-term antidepressant use has disproportionately increased in middle-aged and older adults and non-Hispanic whites.1,12,13 Intuitively, as the risk for depression increases dramatically in patients with chronic medical conditions, serotonin syndrome should be more prevalent among the elderly. In addition, patients with multiple comorbidities take more medications, increasing the risk of polypharmacy and adverse drug reactions.14
Although the epidemiology of serotonin syndrome has yet to be extensively studied, the combination of age and comorbidities may increase the risk for this condition.
HOW DOES IT PRESENT?
Serotonin syndrome characteristically presents as the triad of autonomic dysfunction, neuromuscular excitation, and altered mental status. However, these symptoms may not occur simultaneously: autonomic dysfunction is present in 40% of patients, neuromuscular excitation in 50%, and altered mental status in 40%.15 The symptoms can range from mild to life-threatening (Table 2).16
Autonomic dysfunction. Diaphoresis is present in 48.8% of cases, tachycardia in 44%, nausea and vomiting in 26.8%, and mydriasis in 19.5%. Other signs are hyperactive bowel sounds, diarrhea, and flushing.16
Neuromuscular excitation. Myoclonus is present in 48.8%, hyperreflexia in 41%, hyperthermia in 26.8%, and hypertonicity and rigidity in 19.5%. Other signs are spontaneous or inducible clonus, ocular clonus (continuous rhythmic oscillations of gaze), and tremor.
Altered mental status. Confusion is present in 41.2% and agitation in 36.5%. Other signs are anxiety, lethargy, and coma.
Symptoms of serotonin toxicity arise within an hour of a precipitating event (eg, ingestion) in approximately 28% of patients, and within 6 hours in 61%.16 Highly diagnostic features include hyperreflexia and induced or spontaneous clonus that are generally more pronounced in the lower limbs.11 Clonus can be elicited with ankle dorsiflexion.
In mild toxicity, patients may present with tremor or twitching and anxiety, as well as with hyperreflexia, tachycardia, diaphoresis, and mydriasis. Further investigation may uncover a recently initiated antidepressant or a cold-and-cough medication that contains dextromethorphan.15,17
In moderate toxicity, patients present in significant distress, with agitation and restlessness. Features may include hyperreflexia and clonus of the lower extremities, opsoclonus, hyperactive bowel sounds, diarrhea, nausea, vomiting, tachycardia, hypertension, diaphoresis, mydriasis, and hyperthermia (< 40°C, 104°F). The patient’s history may reveal use of ecstasy or combined treatment with serotonin-potentiating agents such as an antidepressant with a proserotonergic opioid, antiepileptic, or CYP2D6 or CYP3A4 inhibitor.15
Severe serotonin toxicity is a life-threatening condition that can lead to multiorgan failure within hours. It can be characterized by muscle rigidity, which can cause the body temperature to elevate rapidly to over 40°C. This hypertonicity can mask the classic and diagnostic signs of hyperreflexia and clonus. Patients may have unstable and dynamic vital signs with confusion or delirium and can experience tonic-clonic seizures.
If the muscle rigidity and resulting hyperthermia are not managed properly, patients can develop cellular damage and enzyme dysfunction leading to rhabdomyolysis, myoglobinuria, renal failure, metabolic acidosis, acute respiratory distress syndrome, and disseminated intravascular coagulation.16,18
Serotonin crisis is usually caused by the co-ingestion of multiple serotonergic agents, such as an antidepressant with an aforementioned opioid and antiemetic19; combining an SSRI and an MAOI poses the greatest risk. Alternatively, patients may have recently switched antidepressants without observing a safe washout period, leading to an overlap of serotonin levels.16
HOW DO WE DIAGNOSE SEROTONIN SYNDROME?
Serotonin syndrome is a clinical diagnosis and therefore requires a thorough review of medications and physical examination. Serum serotonin levels are an unreliable indicator of toxicity and do not correlate well with the clinical presentation.16
(based on information in reference 9).
Currently, there are two clinical tools for diagnosing serotonin syndrome: the Hunter serotonin toxicity criteria (Figure 1) and the Sternbach criteria.
The Hunter criteria are based more heavily on physical findings. The patient must have taken a serotonergic agent and have one of the following:
- Spontaneous clonus
- Inducible clonus plus agitation or diaphoresis
- Ocular clonus plus agitation or diaphoresis
- Inducible clonus or ocular clonus, plus hypertonia and hyperthermia
- Tremor plus hyperreflexia.
The Sternbach criteria. The patient must be using a serotonergic agent, must have no other causes of symptoms, must not have recently used a neuroleptic agent, and must have three of the following:
- Mental status changes
- Agitation
- Hyperreflexia
- Myoclonus
- Diaphoresis
- Shivering
- Tremor
- Diarrhea
- Incoordination
- Fever
The Hunter criteria are recommended and are more specific (97% vs 96%) and more sensitive (84% vs 75%) than the Sternbach criteria when compared with the gold standard of diagnosis by a clinical toxicologist.1 The Hunter criteria are also less likely to yield false-positive results.11
Differential diagnosis
The differential diagnosis for serotonin syndrome includes neuroleptic malignant syndrome, anticholinergic poisoning (Table 3), metastatic carcinoma, central nervous system infection, gastroenteritis, and sepsis.
Neuroleptic malignant syndrome, the disorder most often misdiagnosed as serotonin syndrome, is an idiosyncratic reaction to a dopamine antagonist (eg, haloperidol, fluphenazine) that develops over days to weeks.20 In 70% of patients, agitated delirium with confusion appears first, followed by lead pipe rigidity and cogwheel tremor, then hyperthermia with body temperature greater than 40°C, and finally, profuse diaphoresis, tachycardia, hypertension, and tachypnea.21
Key elements that distinguish neuroleptic malignant syndrome are the timeline of the clinical course, bradyreflexia, and the absence of clonus. Prodromal symptoms of nausea, vomiting, and diarrhea are also rare in neuroleptic malignant syndrome. Neuroleptic malignant syndrome typically requires an average of 9 days to resolve.
Anticholinergic poisoning usually develops within 1 to 2 hours of oral ingestion. Symptoms include flushing, anhidrosis, anhidrotic hyperthermia, mydriasis, urinary retention, decreased bowel sounds, agitated delirium, and visual hallucinations. In contrast to serotonin syndrome, reflexes and muscle tone are normal with anticholinergic poisoning.
HOW CAN WE TREAT SEROTONIN SYNDROME?
The two mainstays of serotonin syndrome management are to discontinue the serotonergic agent and to give supportive care. Most patients improve within 24 hours of stopping the precipitating drug and starting therapy.16
For mild serotonin syndrome, treatment involves discontinuing the offending agent and supportive therapy with intravenous fluids, correction of vital signs, and symptomatic treatment with a benzodiazepine. Patients should be admitted and observed for 12 to 24 hours to prevent exacerbation.
For moderate serotonin syndrome, treatment also involves stopping the serotonergic agent and giving supportive care. Symptomatic treatment with a benzodiazepine and nonserotonergic antiemetics is recommended, and standard cooling measures should be implemented for hyperthermia. Patients should be admitted and observed for 12 to 24 hours to prevent exacerbation.
For severe serotonin toxicity, treatment should focus on management of airway, breathing, and circulation—ie, the “ABCs.” The two primary life-threatening concerns are hyperthermia (temperature > 40°C or 104°F) and rigidity, which can lead to hypoventilation.1,22 Controlling hyperthermia and rigidity can prevent other grave complications. Patients with severe serotonin toxicity should be sedated, paralyzed, and intubated.21 This will reverse ventilatory hypertonia and allow for mechanical ventilation. Paralysis will also prevent the exacerbation of hyperthermia, which is caused by muscle rigidity. Antipyretics have no role in the treatment of serotonin syndrome since the hyperthermia is not caused by a change in the hypothalamic temperature set point.21 Standard cooling measures should be used to manage hyperthermia.
Serotonin antagonists
Serotonin antagonists have had some success in case reports, but further studies are needed to confirm this.4,23,24
Cyproheptadine is a potent 5-HT2A antagonist; patients usually respond within 1 to 2 hours of administration. Signs and symptoms have resolved completely within times ranging from 20 minutes to 48 hours, depending on the severity of toxicity.
The recommended initial dose of cyproheptadine is 12 mg, followed by 2 mg every 2 hours if symptoms continue.16 Maintenance dosing with 8 mg every 6 hours should be prescribed once stabilization is achieved. The total daily dose for adults should not exceed 0.5 mg/kg/day. Cyproheptadine is available only in oral form but can be crushed and administered via a nasogastric tube.21
Chlorpromazine is a 5-HT1A and 5-HT2A antagonist and can be given intramuscularly. Despite case reports citing its effectiveness, the risk of hypotension, dystonic reactions, and neuroleptic malignant syndrome may make it a less desirable option.4,25
Cyproheptadine, chlorpromazine, and other serotonin receptor antagonists require further investigation beyond individual case reports to determine their effectiveness and reliability in treating serotonin syndrome.
Other agents
Benzodiazepines are considered a mainstay for symptomatic relief because of their anxiolytic and muscle relaxant effects.26 However, animal studies showed that treatment with benzodiazepines attenuated hyperthermia but had no effect on time to recovery or outcome.27
Neuromuscular blocking agents. The suggested neuromuscular blocking agent for severe toxicity is a nondepolarizing agent such as vecuronium. Succinylcholine should be avoided, as it can exacerbate rhabdomyolysis and hyperkalemia.21
Dantrolene has also been suggested for its muscle-relaxing effects and use in malignant hyperthermia. However, this treatment has not been successful in isolated case reports and has been ineffective in animal models.4,28
Physical restraints are ill-advised, since isometric muscle contractions can exacerbate hyperthermia and lactic acidosis in agitated patients.21 If physical restraints are necessary to deliver medications, they should be removed as soon as possible.
HOW CAN WE PREVENT SEROTONIN SYNDROME?
Prevention of serotonin syndrome begins with improving education and awareness in patients and healthcare providers. Patients should be primarily concerned with taking their medications carefully as prescribed and recognizing early signs and symptoms of serotonin toxicity.
As use of antidepressants among an aging population continues to increase, and as physicians in multiple disciplines prescribe them for evolving indications (eg, duloxetine to treat osteoarthritis, diabetic neuropathy, fibromyalgia, and chemotherapy-induced peripheral neuropathy), healthcare providers need to be prepared to see more cases of serotonin syndrome and its deleterious effects.29–31 Physicians should be vigilant in minimizing unnecessary use of serotonergic agents and reviewing drug regimens regularly to limit polypharmacy.
Electronic ordering systems should be designed to detect and alert the prescriber to possible interactions that can potentiate serotonin syndrome, and to not place the order until the prescriber overrides the alert. Combinations of SSRIs and MAOIs have the highest risk for inducing severe serotonin syndrome and should always be avoided.
If a patient is transitioning between serotonergic agents, physicians should observe a safe washout period to prevent overlap.16,32 Washout periods may differ among medications depending on their half-lives. For example, sertraline has a washout period of 2 weeks, while fluoxetine requires a washout period of 5 to 6 weeks.33 Consulting a pharmacist may be helpful when considering half-lives and washout periods.
We believe that educating both patients and physicians regarding prevention will help minimize the risk for serotonergic syndrome and will improve efficiency in assessment and management should toxicity develop.
With a substantial increase in antidepressant use in the United States over the last 2 decades, serotonin syndrome has become an increasingly common and significant clinical concern. In 1999, 6.5% of adults age 18 and older were taking antidepressants; by 2010, the percentage had increased to 10.4%.1 Though the true incidence of serotonin syndrome is difficult to determine, the number of ingestions of selective serotonin reuptake inhibitors (SSRIs) associated with moderate to major effects reported to US poison control centers increased from 7,349 in 20022 to 8,585 in 2005.3
Though the clinical manifestations are often mild to moderate, patients with serotonin syndrome can deteriorate rapidly and require intensive care. Unlike neuroleptic malignant syndrome, serotonin syndrome should not be considered an extremely rare idiosyncratic reaction to medication, but rather a progression of serotonergic toxicity based on increasing concentration levels that can occur in any patient regardless of age.4
Because it has a nonspecific prodrome and protean manifestations, serotonin syndrome can easily be overlooked, misdiagnosed, or exacerbated if not carefully assessed. Diagnosis requires a low threshold for suspicion and a meticulous history and physical examination. In the syndrome’s mildest stage, symptoms are often misattributed to other causes, and in its most severe form, it can easily be mistaken for neuroleptic malignant syndrome.
WHAT IS SEROTONIN SYNDROME?
Serotonin syndrome classically presents as the triad of autonomic dysfunction, neuromuscular excitation, and altered mental status. These symptoms are a result of increased serotonin levels affecting the central and peripheral nervous systems. Serotonin affects a family of receptors that has seven members, of which 5-HT1A and 5-HT2A are most often responsible for serotonin syndrome.5
Conditions that can alter the regulation of serotonin include therapeutic doses, drug interactions, intentional or unintentional overdoses, and overlapping transitions between medications. As a result, drugs that have been associated with serotonin syndrome can be classified into the following five categories as shown below and in Table 1:
Drugs that decrease serotonin breakdown include monoamine oxidase inhibitors (MAOIs), linezolid,6 methylene blue, procarbazine, and Syrian rue.
Drugs that decrease serotonin reuptake include SSRIs, serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants, opioids (meperidine, buprenorphine, tramadol, tapentadol, dextromethorphan), antiepileptics (carbamazepine, valproate), and antiemetics (ondansetron, granisetron, metoclopramide), and the herbal preparation St. John’s wort.
Drugs that increase serotonin precursors or agonists include tryptophan, lithium, fentanyl, and lysergic acid diethylamide (LSD).
Drugs that increase serotonin release include fenfluramine, amphetamines, and methylenedioxymethamphetamine (ecstasy).
Drugs that prevent breakdown of the agents listed above are CYP2D6 and CYP3A4 inhibitors, eg, erythromycin,7 ciprofloxacin, fluconazole, ritonavir, and grapefruit juice.
However, the only drugs that have been reliably confirmed to precipitate serotonin syndrome are MAOIs, SSRIs, SNRIs, and serotonin releasers. Other listed drug interactions are based on case reports and have not been thoroughly evaluated.6–9
Currently, SSRIs are the most commonly prescribed antidepressant medications and, consequently, they are the ones most often implicated in serotonergic toxicity.1,10 An estimated 15% of SSRI overdoses lead to mild or moderate serotonin toxicity.11 Serotonergic agents used in conjunction can increase the risk for severe serotonin syndrome; an SSRI and an MAOI in combination poses the greatest risk.5
Ultimately, the incidence of serotonin syndrome is difficult to assess, but it is believed to be underreported because it is easy to misdiagnose and mild symptoms may be dismissed.
WHO IS AT RISK OF SEROTONIN SYNDROME?
Long-term antidepressant use has disproportionately increased in middle-aged and older adults and non-Hispanic whites.1,12,13 Intuitively, as the risk for depression increases dramatically in patients with chronic medical conditions, serotonin syndrome should be more prevalent among the elderly. In addition, patients with multiple comorbidities take more medications, increasing the risk of polypharmacy and adverse drug reactions.14
Although the epidemiology of serotonin syndrome has yet to be extensively studied, the combination of age and comorbidities may increase the risk for this condition.
HOW DOES IT PRESENT?
Serotonin syndrome characteristically presents as the triad of autonomic dysfunction, neuromuscular excitation, and altered mental status. However, these symptoms may not occur simultaneously: autonomic dysfunction is present in 40% of patients, neuromuscular excitation in 50%, and altered mental status in 40%.15 The symptoms can range from mild to life-threatening (Table 2).16
Autonomic dysfunction. Diaphoresis is present in 48.8% of cases, tachycardia in 44%, nausea and vomiting in 26.8%, and mydriasis in 19.5%. Other signs are hyperactive bowel sounds, diarrhea, and flushing.16
Neuromuscular excitation. Myoclonus is present in 48.8%, hyperreflexia in 41%, hyperthermia in 26.8%, and hypertonicity and rigidity in 19.5%. Other signs are spontaneous or inducible clonus, ocular clonus (continuous rhythmic oscillations of gaze), and tremor.
Altered mental status. Confusion is present in 41.2% and agitation in 36.5%. Other signs are anxiety, lethargy, and coma.
Symptoms of serotonin toxicity arise within an hour of a precipitating event (eg, ingestion) in approximately 28% of patients, and within 6 hours in 61%.16 Highly diagnostic features include hyperreflexia and induced or spontaneous clonus that are generally more pronounced in the lower limbs.11 Clonus can be elicited with ankle dorsiflexion.
In mild toxicity, patients may present with tremor or twitching and anxiety, as well as with hyperreflexia, tachycardia, diaphoresis, and mydriasis. Further investigation may uncover a recently initiated antidepressant or a cold-and-cough medication that contains dextromethorphan.15,17
In moderate toxicity, patients present in significant distress, with agitation and restlessness. Features may include hyperreflexia and clonus of the lower extremities, opsoclonus, hyperactive bowel sounds, diarrhea, nausea, vomiting, tachycardia, hypertension, diaphoresis, mydriasis, and hyperthermia (< 40°C, 104°F). The patient’s history may reveal use of ecstasy or combined treatment with serotonin-potentiating agents such as an antidepressant with a proserotonergic opioid, antiepileptic, or CYP2D6 or CYP3A4 inhibitor.15
Severe serotonin toxicity is a life-threatening condition that can lead to multiorgan failure within hours. It can be characterized by muscle rigidity, which can cause the body temperature to elevate rapidly to over 40°C. This hypertonicity can mask the classic and diagnostic signs of hyperreflexia and clonus. Patients may have unstable and dynamic vital signs with confusion or delirium and can experience tonic-clonic seizures.
If the muscle rigidity and resulting hyperthermia are not managed properly, patients can develop cellular damage and enzyme dysfunction leading to rhabdomyolysis, myoglobinuria, renal failure, metabolic acidosis, acute respiratory distress syndrome, and disseminated intravascular coagulation.16,18
Serotonin crisis is usually caused by the co-ingestion of multiple serotonergic agents, such as an antidepressant with an aforementioned opioid and antiemetic19; combining an SSRI and an MAOI poses the greatest risk. Alternatively, patients may have recently switched antidepressants without observing a safe washout period, leading to an overlap of serotonin levels.16
HOW DO WE DIAGNOSE SEROTONIN SYNDROME?
Serotonin syndrome is a clinical diagnosis and therefore requires a thorough review of medications and physical examination. Serum serotonin levels are an unreliable indicator of toxicity and do not correlate well with the clinical presentation.16
(based on information in reference 9).
Currently, there are two clinical tools for diagnosing serotonin syndrome: the Hunter serotonin toxicity criteria (Figure 1) and the Sternbach criteria.
The Hunter criteria are based more heavily on physical findings. The patient must have taken a serotonergic agent and have one of the following:
- Spontaneous clonus
- Inducible clonus plus agitation or diaphoresis
- Ocular clonus plus agitation or diaphoresis
- Inducible clonus or ocular clonus, plus hypertonia and hyperthermia
- Tremor plus hyperreflexia.
The Sternbach criteria. The patient must be using a serotonergic agent, must have no other causes of symptoms, must not have recently used a neuroleptic agent, and must have three of the following:
- Mental status changes
- Agitation
- Hyperreflexia
- Myoclonus
- Diaphoresis
- Shivering
- Tremor
- Diarrhea
- Incoordination
- Fever
The Hunter criteria are recommended and are more specific (97% vs 96%) and more sensitive (84% vs 75%) than the Sternbach criteria when compared with the gold standard of diagnosis by a clinical toxicologist.1 The Hunter criteria are also less likely to yield false-positive results.11
Differential diagnosis
The differential diagnosis for serotonin syndrome includes neuroleptic malignant syndrome, anticholinergic poisoning (Table 3), metastatic carcinoma, central nervous system infection, gastroenteritis, and sepsis.
Neuroleptic malignant syndrome, the disorder most often misdiagnosed as serotonin syndrome, is an idiosyncratic reaction to a dopamine antagonist (eg, haloperidol, fluphenazine) that develops over days to weeks.20 In 70% of patients, agitated delirium with confusion appears first, followed by lead pipe rigidity and cogwheel tremor, then hyperthermia with body temperature greater than 40°C, and finally, profuse diaphoresis, tachycardia, hypertension, and tachypnea.21
Key elements that distinguish neuroleptic malignant syndrome are the timeline of the clinical course, bradyreflexia, and the absence of clonus. Prodromal symptoms of nausea, vomiting, and diarrhea are also rare in neuroleptic malignant syndrome. Neuroleptic malignant syndrome typically requires an average of 9 days to resolve.
Anticholinergic poisoning usually develops within 1 to 2 hours of oral ingestion. Symptoms include flushing, anhidrosis, anhidrotic hyperthermia, mydriasis, urinary retention, decreased bowel sounds, agitated delirium, and visual hallucinations. In contrast to serotonin syndrome, reflexes and muscle tone are normal with anticholinergic poisoning.
HOW CAN WE TREAT SEROTONIN SYNDROME?
The two mainstays of serotonin syndrome management are to discontinue the serotonergic agent and to give supportive care. Most patients improve within 24 hours of stopping the precipitating drug and starting therapy.16
For mild serotonin syndrome, treatment involves discontinuing the offending agent and supportive therapy with intravenous fluids, correction of vital signs, and symptomatic treatment with a benzodiazepine. Patients should be admitted and observed for 12 to 24 hours to prevent exacerbation.
For moderate serotonin syndrome, treatment also involves stopping the serotonergic agent and giving supportive care. Symptomatic treatment with a benzodiazepine and nonserotonergic antiemetics is recommended, and standard cooling measures should be implemented for hyperthermia. Patients should be admitted and observed for 12 to 24 hours to prevent exacerbation.
For severe serotonin toxicity, treatment should focus on management of airway, breathing, and circulation—ie, the “ABCs.” The two primary life-threatening concerns are hyperthermia (temperature > 40°C or 104°F) and rigidity, which can lead to hypoventilation.1,22 Controlling hyperthermia and rigidity can prevent other grave complications. Patients with severe serotonin toxicity should be sedated, paralyzed, and intubated.21 This will reverse ventilatory hypertonia and allow for mechanical ventilation. Paralysis will also prevent the exacerbation of hyperthermia, which is caused by muscle rigidity. Antipyretics have no role in the treatment of serotonin syndrome since the hyperthermia is not caused by a change in the hypothalamic temperature set point.21 Standard cooling measures should be used to manage hyperthermia.
Serotonin antagonists
Serotonin antagonists have had some success in case reports, but further studies are needed to confirm this.4,23,24
Cyproheptadine is a potent 5-HT2A antagonist; patients usually respond within 1 to 2 hours of administration. Signs and symptoms have resolved completely within times ranging from 20 minutes to 48 hours, depending on the severity of toxicity.
The recommended initial dose of cyproheptadine is 12 mg, followed by 2 mg every 2 hours if symptoms continue.16 Maintenance dosing with 8 mg every 6 hours should be prescribed once stabilization is achieved. The total daily dose for adults should not exceed 0.5 mg/kg/day. Cyproheptadine is available only in oral form but can be crushed and administered via a nasogastric tube.21
Chlorpromazine is a 5-HT1A and 5-HT2A antagonist and can be given intramuscularly. Despite case reports citing its effectiveness, the risk of hypotension, dystonic reactions, and neuroleptic malignant syndrome may make it a less desirable option.4,25
Cyproheptadine, chlorpromazine, and other serotonin receptor antagonists require further investigation beyond individual case reports to determine their effectiveness and reliability in treating serotonin syndrome.
Other agents
Benzodiazepines are considered a mainstay for symptomatic relief because of their anxiolytic and muscle relaxant effects.26 However, animal studies showed that treatment with benzodiazepines attenuated hyperthermia but had no effect on time to recovery or outcome.27
Neuromuscular blocking agents. The suggested neuromuscular blocking agent for severe toxicity is a nondepolarizing agent such as vecuronium. Succinylcholine should be avoided, as it can exacerbate rhabdomyolysis and hyperkalemia.21
Dantrolene has also been suggested for its muscle-relaxing effects and use in malignant hyperthermia. However, this treatment has not been successful in isolated case reports and has been ineffective in animal models.4,28
Physical restraints are ill-advised, since isometric muscle contractions can exacerbate hyperthermia and lactic acidosis in agitated patients.21 If physical restraints are necessary to deliver medications, they should be removed as soon as possible.
HOW CAN WE PREVENT SEROTONIN SYNDROME?
Prevention of serotonin syndrome begins with improving education and awareness in patients and healthcare providers. Patients should be primarily concerned with taking their medications carefully as prescribed and recognizing early signs and symptoms of serotonin toxicity.
As use of antidepressants among an aging population continues to increase, and as physicians in multiple disciplines prescribe them for evolving indications (eg, duloxetine to treat osteoarthritis, diabetic neuropathy, fibromyalgia, and chemotherapy-induced peripheral neuropathy), healthcare providers need to be prepared to see more cases of serotonin syndrome and its deleterious effects.29–31 Physicians should be vigilant in minimizing unnecessary use of serotonergic agents and reviewing drug regimens regularly to limit polypharmacy.
Electronic ordering systems should be designed to detect and alert the prescriber to possible interactions that can potentiate serotonin syndrome, and to not place the order until the prescriber overrides the alert. Combinations of SSRIs and MAOIs have the highest risk for inducing severe serotonin syndrome and should always be avoided.
If a patient is transitioning between serotonergic agents, physicians should observe a safe washout period to prevent overlap.16,32 Washout periods may differ among medications depending on their half-lives. For example, sertraline has a washout period of 2 weeks, while fluoxetine requires a washout period of 5 to 6 weeks.33 Consulting a pharmacist may be helpful when considering half-lives and washout periods.
We believe that educating both patients and physicians regarding prevention will help minimize the risk for serotonergic syndrome and will improve efficiency in assessment and management should toxicity develop.
- Mojtabai R, Olfson M. National trends in long-term use of antidepressant medications: results from the US National Health and Nutrition Examination Survey. J Clin Psychiatry 2014; 75:169–177.
- Watson WA, Litovitz TL, Rodgers GC Jr, et al. 2002 Annual Report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med. 2003;21:353–421.
- Lai MW, Klein-Schwartz W, Rodgers GC, et al. 2005 Annual Report of the American Association of Poison Control Centers’ national poisoning and exopsure database. Clin Toxicol (Phila) 2006; 44:803–932.
- Gillman PK. The serotonin syndrome and its treatment. J Psychopharmacol 1999; 13:100–109.
- Isbister GK, Buckley NA. The pathophysiology of serotonin toxicity in animals and humans: implications for diagnosis and treatment. Clin Neuropharmacol 2005; 28:205–214.
- Woytowish MR, Maynor LM. Clinical relevance of linezolid-associated serotonin toxicity. Ann Pharmacother 2013; 47:388–397.
- Lee DO, Lee CD. Serotonin syndrome in a child associated with erythromycin and sertraline. Pharmacotherapy 1999; 19:894–896.
- Gillman PK. Triptans, serotonin agonists, and serotonin syndrome (serotonin toxicity): a review. Headache 2010; 50:264–272.
- Isbister GK, Buckley NA, Whyte IM. Serotonin toxicity: a practical approach to diagnosis and treatment. Med J Aust 2007; 187:361–365.
- Mowry JB, Spyker DA, Cantilena LR Jr, McMillan N, Ford M. 2013 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol (Phila) 2014; 52:1032–1283.
- Dunkley EJ, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter serotonin toxicity criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM 2003; 96:635–642.
- Karkare SU, Bhattacharjee S, Kamble P, Aparasu R. Prevalence and predictors of antidepressant prescribing in nursing home residents in the United States. Am J Geriatr Pharmacother 2011; 9:109–119.
- Weissman J, Meyers BS, Ghosh S, Bruce ML. Demographic, clinical, and functional factors associated with antidepressant use in the home healthcare elderly. Am J Geriatr Psychiatry 2011; 19:1042–1045.
- Caughey GE, Roughead EE, Shakib S, Vitry AI, Gilbert AL. Co-morbidity and potential treatment conflicts in elderly heart failure patients: a retrospective, cross-sectional study of administrative claims data. Drugs Aging 2011; 28:575–581.
- Iqbal MM, Basil MJ, Kaplan J, Iqbal MT. Overview of serotonin syndrome. Ann Clin Psychiatry 2012; 24:310–318.
- Mason PJ, Morris VA, Balcezak TJ. Serotonin syndrome. Presentation of 2 cases and review of the literature. Medicine (Baltimore) 2000; 79:201–209.
- Prakash S, Patel V, Kakked S, Patel I, Yadav R. Mild serotonin syndrome: a report of 12 cases. Ann Indian Acad Neurol 2015; 18:226–230.
- Davies O, Batajoo-Shrestha B, Sosa-Popoteur J, Olibrice M. Full recovery after severe serotonin syndrome, severe rhabdomyolysis, multi-organ failure and disseminated intravascular coagulopathy from MDMA. Heart Lung 2014; 43:117–119.
- Pedavally S, Fugate JE, Rabinstein AA. Serotonin syndrome in the intensive care unit: clinical presentations and precipitating medications. Neurocrit Care 2014; 21:108–113.
- Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med 2005; 352:1112–1120.
- Velamoor VR, Norman RM, Caroff SN, Mann SC, Sullivan KA, Antelo RE. Progression of symptoms in neuroleptic malignant syndrome. J Nerv Ment Dis 1994; 182:168–173.
- Isbister GK, Hackett LP, Dawson AH, Whyte IM, Smith AJ. Moclobemide poisoning: toxicokinetics and occurrence of serotonin toxicity. Br J Clin Pharmacol 2003; 56:441–450.
- Graudins A, Stearman A, Chan B. Treatment of the serotonin syndrome with cyproheptadine. J Emerg Med 1998; 16:615–619.
- Lappin RI, Auchincloss EL. Treatment of the serotonin syndrome with cyproheptadine. N Engl J Med 1994; 331:1021–1022.
- Gillman PK. Successful treatment of serotonin syndrome with chlorpromazine. Med J Aust 1996; 165:345–346.
- Buckley NA, Dawson AH, Isbister GK. Serotonin syndrome. BMJ 2014; 348:g1626.
- Nisijima K, Shioda K, Yoshino T, Takano K, Kato S. Diazepam and chlormethiazole attenuate the development of hyperthermia in an animal model of the serotonin syndrome. Neurochem Int 2003; 43:155–164.
- Nisijima K, Yoshino T, Yui K, Katoh S. Potent serotonin (5-HT)(2A) receptor antagonists completely prevent the development of hyperthermia in an animal model of the 5-HT syndrome. Brain Res 2001; 890:23–31.
- Micca JL, Ruff D, Ahl J, Wohlreich MM. Safety and efficacy of duloxetine treatment in older and younger patients with osteoarthritis knee pain: a post hoc, subgroup analysis of two randomized, placebo-controlled trials. BMC Musculoskelet Disord 2013; 14:137.
- Smith EM, Pang H, Cirrincione C, et al; Alliance for Clinical Trials in Oncology. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA 2013; 309:1359–1367.
- Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev 2014; 1:CD007115.
- Caughey GE, Roughead EE, Shakib S, McDermott RA, Vitry AI, Gilbert AL. Comorbidity of chronic disease and potential treatment conflicts in older people dispensed antidepressants. Age Ageing 2010; 39:488–494.
- Gury C, Cousin F. Pharmacokinetics of SSRI antidepressants: half-life and clinical applicability. Encephale 1999; 25:470–476. French.
- Mojtabai R, Olfson M. National trends in long-term use of antidepressant medications: results from the US National Health and Nutrition Examination Survey. J Clin Psychiatry 2014; 75:169–177.
- Watson WA, Litovitz TL, Rodgers GC Jr, et al. 2002 Annual Report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med. 2003;21:353–421.
- Lai MW, Klein-Schwartz W, Rodgers GC, et al. 2005 Annual Report of the American Association of Poison Control Centers’ national poisoning and exopsure database. Clin Toxicol (Phila) 2006; 44:803–932.
- Gillman PK. The serotonin syndrome and its treatment. J Psychopharmacol 1999; 13:100–109.
- Isbister GK, Buckley NA. The pathophysiology of serotonin toxicity in animals and humans: implications for diagnosis and treatment. Clin Neuropharmacol 2005; 28:205–214.
- Woytowish MR, Maynor LM. Clinical relevance of linezolid-associated serotonin toxicity. Ann Pharmacother 2013; 47:388–397.
- Lee DO, Lee CD. Serotonin syndrome in a child associated with erythromycin and sertraline. Pharmacotherapy 1999; 19:894–896.
- Gillman PK. Triptans, serotonin agonists, and serotonin syndrome (serotonin toxicity): a review. Headache 2010; 50:264–272.
- Isbister GK, Buckley NA, Whyte IM. Serotonin toxicity: a practical approach to diagnosis and treatment. Med J Aust 2007; 187:361–365.
- Mowry JB, Spyker DA, Cantilena LR Jr, McMillan N, Ford M. 2013 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol (Phila) 2014; 52:1032–1283.
- Dunkley EJ, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter serotonin toxicity criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM 2003; 96:635–642.
- Karkare SU, Bhattacharjee S, Kamble P, Aparasu R. Prevalence and predictors of antidepressant prescribing in nursing home residents in the United States. Am J Geriatr Pharmacother 2011; 9:109–119.
- Weissman J, Meyers BS, Ghosh S, Bruce ML. Demographic, clinical, and functional factors associated with antidepressant use in the home healthcare elderly. Am J Geriatr Psychiatry 2011; 19:1042–1045.
- Caughey GE, Roughead EE, Shakib S, Vitry AI, Gilbert AL. Co-morbidity and potential treatment conflicts in elderly heart failure patients: a retrospective, cross-sectional study of administrative claims data. Drugs Aging 2011; 28:575–581.
- Iqbal MM, Basil MJ, Kaplan J, Iqbal MT. Overview of serotonin syndrome. Ann Clin Psychiatry 2012; 24:310–318.
- Mason PJ, Morris VA, Balcezak TJ. Serotonin syndrome. Presentation of 2 cases and review of the literature. Medicine (Baltimore) 2000; 79:201–209.
- Prakash S, Patel V, Kakked S, Patel I, Yadav R. Mild serotonin syndrome: a report of 12 cases. Ann Indian Acad Neurol 2015; 18:226–230.
- Davies O, Batajoo-Shrestha B, Sosa-Popoteur J, Olibrice M. Full recovery after severe serotonin syndrome, severe rhabdomyolysis, multi-organ failure and disseminated intravascular coagulopathy from MDMA. Heart Lung 2014; 43:117–119.
- Pedavally S, Fugate JE, Rabinstein AA. Serotonin syndrome in the intensive care unit: clinical presentations and precipitating medications. Neurocrit Care 2014; 21:108–113.
- Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med 2005; 352:1112–1120.
- Velamoor VR, Norman RM, Caroff SN, Mann SC, Sullivan KA, Antelo RE. Progression of symptoms in neuroleptic malignant syndrome. J Nerv Ment Dis 1994; 182:168–173.
- Isbister GK, Hackett LP, Dawson AH, Whyte IM, Smith AJ. Moclobemide poisoning: toxicokinetics and occurrence of serotonin toxicity. Br J Clin Pharmacol 2003; 56:441–450.
- Graudins A, Stearman A, Chan B. Treatment of the serotonin syndrome with cyproheptadine. J Emerg Med 1998; 16:615–619.
- Lappin RI, Auchincloss EL. Treatment of the serotonin syndrome with cyproheptadine. N Engl J Med 1994; 331:1021–1022.
- Gillman PK. Successful treatment of serotonin syndrome with chlorpromazine. Med J Aust 1996; 165:345–346.
- Buckley NA, Dawson AH, Isbister GK. Serotonin syndrome. BMJ 2014; 348:g1626.
- Nisijima K, Shioda K, Yoshino T, Takano K, Kato S. Diazepam and chlormethiazole attenuate the development of hyperthermia in an animal model of the serotonin syndrome. Neurochem Int 2003; 43:155–164.
- Nisijima K, Yoshino T, Yui K, Katoh S. Potent serotonin (5-HT)(2A) receptor antagonists completely prevent the development of hyperthermia in an animal model of the 5-HT syndrome. Brain Res 2001; 890:23–31.
- Micca JL, Ruff D, Ahl J, Wohlreich MM. Safety and efficacy of duloxetine treatment in older and younger patients with osteoarthritis knee pain: a post hoc, subgroup analysis of two randomized, placebo-controlled trials. BMC Musculoskelet Disord 2013; 14:137.
- Smith EM, Pang H, Cirrincione C, et al; Alliance for Clinical Trials in Oncology. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA 2013; 309:1359–1367.
- Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev 2014; 1:CD007115.
- Caughey GE, Roughead EE, Shakib S, McDermott RA, Vitry AI, Gilbert AL. Comorbidity of chronic disease and potential treatment conflicts in older people dispensed antidepressants. Age Ageing 2010; 39:488–494.
- Gury C, Cousin F. Pharmacokinetics of SSRI antidepressants: half-life and clinical applicability. Encephale 1999; 25:470–476. French.
KEY POINTS
- Serotonin syndrome is caused by elevated serotonin levels in the central and peripheral nervous systems.
- The classic presentation is the triad of autonomic dysfunction, neuromuscular excitation, and altered mental status. These symptoms vary based on the severity of serotonergic toxicity and often do not present concomitantly.
- Early recognition is critical to ensure appropriate resuscitative measures and to limit further use of drugs that can exacerbate symptoms.
Update on the management of intestinal failure
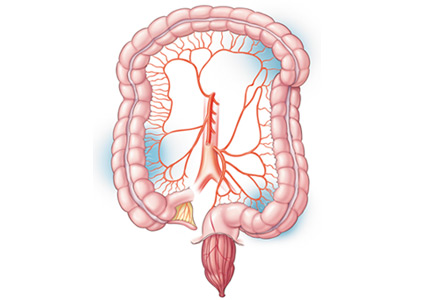
Intestinal failure, the inability of the gut to maintain nutritional homeostasis,1 is a complication of vascular thrombosis, inflammatory bowel disease, radiation enteritis, obstruction, and other conditions, and of removing segments of the small and large intestines in response to these diseases.1,2 Imbalances of fluids and electrolytes, dehydration, malabsorption, vitamin and mineral deficiencies, chronic diarrhea, and increased ostomy output contribute to a decline in the quality of life and in the survival rate in these patients.2,3
Referral to an intestinal rehabilitation program that combines gastroenterology, nutrition, pharmacy, nursing, and social work can improve nutritional status and quality of life.4 Whenever possible, the goal of rehabilitation is nutritional autonomy, helping the patient make the transition to an independent oral diet.4 In selected patients in whom rehabilitation is not effective, intestinal transplant may be an option.
In this article, we review the intestinal adaptations that follow surgical resection and provide an update on intestinal rehabilitation techniques such as dietary modification, drug therapy, and parenteral nutrition. We also review experience with intestinal transplant in patients with intestinal failure.
INTESTINAL FAILURE
Intestinal failure results from reduction in enterocyte cell mass, obstruction, dysmotility, surgical resection, congenital defects, or disease-associated loss of absorption with suboptimal nutritional autonomy.5 Patients often suffer from extensive nutrient, electrolyte, and fluid abnormalities proportional to the remnant length and part of the intestine removed.5
Epidemiologic studies have demonstrated that short-bowel syndrome is the most common cause of intestinal failure in adults and children.6,7 Short-bowel syndrome is defined as a small-bowel length less than 200 cm, most commonly from extensive resections for inflammatory bowel disease.6 In children, the syndrome is also defined by a residual small-bowel length of less than 25% expected for gestational age.7
Table 1 lists the frequencies of the underlying disorders leading to intestinal failure or short-bowel syndrome in one series.8
INTESTINAL ADAPTATION
The gastrointestinal tract is the only organ for nutrient, fluid, and electrolyte absorption.9 Every day, 8 to 9 L of fluids and secretions reach the small intestine, comprising about 2 to 3 L of oral fluids, 1 L of saliva, 2 L of gastric juices, 1 L of bile, and 2 L of pancreatic juices.9 Approximately 7 to 8 L are reabsorbed by the small intestine and 1 to 2 L by the colon.9
Although carbohydrates, lipids, and proteins are absorbed through the entire small intestine and colon, site-specific digestion and absorption of different nutrients occur in different parts of the gastrointestinal tract.10 Also, certain nutrients may need site-specific receptors or transporters for their absorption,10 for example:
- Iron in the duodenum and proximal jejunum1
- Lactose in the brush border membrane of the jejunum and proximal ileum, where most of the enzyme lactase is present
- Vitamin B12 and bile salts in the distal ileum.
Hence, resection of a specific part of the intestine may predict deficiencies the patient may encounter after surgery.
The diarrhea that occurs in short-bowel syndrome may be due partly to loss of neurohumoral mediators that govern gastrointestinal transit time, most importantly cholecystokinin, peptide YY, and glucagon-like peptide 1.11 After contact with lipid- or protein-rich nutrients, cholecystokinin is released from the proximal small intestine, which decreases the gastric emptying to maximize nutrient digestion.12 Additionally, release of peptide YY and glucagon-like peptide 1 from the ileal L cells decreases gastric and intestinal motility. These mediators prolong gastrointestinal transit, increase nutrient processing time, and enhance absorption.12
After massive intestinal resection, the remnant bowel undergoes physiologic and functional adaptation to maintain nutritional homeostasis.13 Enterocytes express membrane-bound transporters and undergo accelerated cell division to enhance the absorptive surface area.13 Intestinal hypertrophy, which includes an increase in villous diameter and crypt height, continues for 2 years or more after intestinal resection, leading to greater absorptive surface area.14 It is estimated that villous height may increase by as much as 80%, illustrating a dynamic process in response to intestinal stress.15
Luminal nutrients are essential to the stimulation of enterocyte cells through paracrine mechanisms as well as through the up-regulation of colonic peptide transporter PepT1.15 Furthermore, gut motility is initially decreased in order to increase the concentration of local luminal growth factors.16
Other factors that may affect intestinal adaptation are the length of the residual colon and small intestine, enteral growth, and enterotropic factors.16 And especially in patients with short-bowel syndrome, complications such as malabsorption secondary to pancreatic insufficiency or rapid transit, excessive gastric acid secretion, bile acid wasting due to terminal ileum resection, and bacterial overgrowth in the small intestine result in worsened nutritional status and poor quality of life.16
Key factors that affect the degree of nutritional deficiencies
The degree of nutritional deficiencies and fluid and electrolyte imbalances depends on the length and location of resection and whether the colon is still continuous with the small intestine.17 Normal small-bowel length in adults is highly variable and can be up to 600 cm. Malnutrition after surgical resection usually occurs when more than three-fourths of intestinal tissue is removed.17 However, because of intestinal adaptation, patients with 50% of remnant small bowel may be able to achieve nutritional autonomy.18 Furthermore, because absorption of nutrients occurs primarily in the first 150 cm of the small intestine, resections of this anatomic region have the highest probability of resulting in malnutrition.18
After extensive intestinal resection, absorption of water and electrolytes is better and intestinal transit time is longer if the colon is still continuous with the rest of the gastrointestinal system.19 Approximately 100 cm of remnant intestinal tissue without colonic continuity or 60 cm with colonic continuity is needed to ensure the possibility of nutritional autonomy and independence from parenteral nutrition.19 Severe malnutrition and fluid and electrolyte imbalances can be prevented by appropriate and timely multidisciplinary care and early referral for intestinal rehabilitation.
INTESTINAL REHABILITATION AND NUTRITIONAL AUTONOMY
The aim of intestinal rehabilitation is to improve quality of life by reversing malnutrition and promoting nutritional autonomy, ie, independence from parenteral nutrition (Table 2).20 The complex nature of intestinal failure necessitates collaboration of multiple specialists—gastroenterologists, surgeons, dietitians, nurses, psychiatrists or psychologists, pharmacists, and social workers.20
Although most patients with intestinal failure initially require parenteral nutrition to maintain nutritional homeostasis, progressive adaptation of the remnant intestine enables a transition to enteral nutrition.21 Stimulation of the remnant intestine by enteral feeding reduces the complications of parenteral nutrition and encourages intestinal adaptation.21
Outpatient participation in an intestinal rehabilitation program can facilitate weaning from parenteral nutrition. Patients are monitored and supported during dietary modification, pharmacologic interventions, and reconstructive surgeries.21 A study of 61 patients with short-bowel syndrome undergoing a 3-week program of intestinal rehabilitation (recombinant human growth hormone, glutamine, enteral nutrition, and parenteral nutrition) reported an overall survival rate of 95% with an 85% success rate in weaning from parenteral nutrition during a mean follow-up of 50 (± 24) months.22 Permanent dependence on parenteral nutrition despite rehabilitation was predicted by length of the small bowel less than 100 cm and by the absence of terminal ileum and colon.22
Permanent intestinal failure, defined by the inability to wean from parenteral nutrition and restore nutrition autonomy, may require early referral for evaluation for intestinal and multivisceral transplant. Early referral improves survival rates, possibly because of fewer complications from parenteral nutrition.4
DIETARY MODIFICATION
Dietary modification is the single most effective means of weaning patients safely from parenteral nutrition (Table 3).23,24 Small, frequent feedings help reduce symptoms associated with rapid intestinal transit and increase the activity of luminal growth factors.23 Likewise, limits on intake of simple sugars, stimulants such as caffeine or insoluble fiber, and hypo- or hypertonic fluids decrease intestinal losses and the risk of dehydration.23 Low sugar loads also aim to reduce the occurrence of d-lactic acidosis and bacterial overgrowth in the small intestine.23 Patients who cannot maintain positive fluid balance may require standardized oral rehydration (Table 4) to improve absorption by way of the sodium-glucose coupled transporters at the brush border membrane, or they may require intravenous fluid supplementation.25
Colonic continuity
Other dietary recommendations depend on colonic continuity. In 1994, Nordgaard et al26 compared the effects of high-carbohydrate and high-fat diets in eight patients with colonic continuity and six patients with jejunostomies. The authors noted that a high-carbohydrate diet (60% carbohydrate, 20% fat) reduced fecal loss of energy and increased energy absorption in patients with colonic continuity. However, patients with an end-jejunostomy experienced equal fecal losses of carbohydrates and fat proportional to the amount consumed. The authors concluded that the presence of colonic bacteria promoted carbohydrate salvage, ie, the fermentation of malabsorbed carbohydrates to easily absorbed short-chain fatty acids.26
The colon can salvage as much as 1,000 kcal/day in patients with less than 200 cm of small bowel, and the presence of at least 50% of colon in continuity has been shown to reduce parenteral nutrition requirements by half in patients with less than 100 cm of small bowel.27 As a result, a diet high in complex carbohydrates and soluble fiber supplements is recommended in cases of preserved colon to promote adaptation and nutritional autonomy.27
Another aim of a high-carbohydrate, low- fat diet is to prevent calcium oxalate-related nephrolithiasis and choleretic diarrhea.26
In summary, patients with short-bowel syndrome with or without colonic continuity need different dietary regimens to attain nutritional autonomy.
DRUG THERAPY
In addition to diet therapy, most patients with intestinal failure require pharmacologic therapy.28 High stool or stoma effluent is most commonly treated with an antidiarrheal to increase transit time; diphenoxylate-atropine, loperamide, codeine sulfate, paregoric, and opium tincture are commonly prescribed (Table 5).27 In severe high-output states, a somatostatin analogue (eg, octreotide) may be added.29
Postoperative increases in gastric secretion may be countered by histamine 2 receptor antagonists and proton pump inhibitors, but long-term use of these drugs may lead to nutritional deficiencies and bacterial overgrowth in the small intestine.29 Bile acid sequestrants (in cases of distal ileal resection) and pancreatic enzymes target fat malabsorption, resultant cases of choleretic diarrhea, deficiency of essential fatty acids, kidney stones, and deficiency of fat-soluble vitamins.29 Probiotics and antibiotics can also be given for prevention and treatment of small-intestinal bacterial overgrowth.29
When traditional dietary modification and medical therapy fail to achieve nutritional homeostasis, another option to consider is a glucagon-like peptide-2 analogue to enhance intestinal adaptation.30 Produced in the native distal ileum and colon, glucagon-like peptide 2 moderates the rate of gastric emptying and small-bowel transit and enhances epithelial cell proliferation, thereby promoting intestinal adaptation.30 Further, a randomized controlled trial of 83 patients reported efficacy of these agents in reducing parenteral nutrition requirements in patients with intestinal failure.31
Hence, in patients with intestinal failure who have increased stoma effluent, drug therapy may play an important role in maintaining fluid and nutritional homeostasis.
THE ROLE OF PARENTERAL NUTRITION IN INTESTINAL FAILURE
Despite the best efforts of an intestinal rehabilitation program, not all patients gain nutritional autonomy.32 Physiologic, psychological, social, and economic factors may contribute to dependence on parenteral nutrition.32 Currently, more than 40,000 US patients depend on it for survival.33
The need for short-term or long-term parenteral nutrition is determined by the patient’s medical needs.33 Patients requiring short-term parenteral nutrition (2–6 weeks) include those whose bowel function has not returned to normal postoperatively, and those who were severely malnourished preoperatively.34 Patients needing it long-term (from months to years to lifelong) are those with gastrointestinal dysmotility and short-bowel syndrome due to extensive bowel resections.33
Complications of parenteral nutrition
Catheter-related bloodstream infection is the most common complication and cause of hospitalization. Infection can be localized to the exit site or tunnel or can be systemic (eg, line sepsis).35Staphylococcus aureus and coagulase-negative staphylococci are most often implicated in catheter infection.35 When possible, catheter salvage is desirable, but the central venous catheter must be removed in cases of tunnel infection, port abscess, septic shock, paired blood cultures positive for fungi or highly virulent bacteria, endocarditis, septic thrombosis, and other conditions.35,36
Liver disease is a serious complication of long-term parenteral nutrition and may occur in up to 55% of patients on therapy for more than 2 years; it carries a mortality rate of 15%.37
Risk factors include younger age and use of excessive carbohydrate and fat compositions, mainly soybean-oil–based lipid emulsions.37 However, fish-oil–based lipid emulsions have recently shown promise in preventing and reversing parenteral nutrition-associated liver failure and cholestasis, especially in a pediatric population.38
Catheter thrombosis may occur in up to 30% of patients on long-term parenteral nutrition.39 However, this risk is reduced with appropriate positioning of the catheter tip in the mid or lower superior vena cava.37 Treatment of thrombosis of the central access includes either anticoagulation or thrombolysis.37
Hence, appropriate and timely follow-up of patients on parenteral nutrition is essential in reducing associated complications. Monitoring weight, fluid status, serum glucose, and patency of central access are critical to ensure that the patient maintains nutritional status effectively.40 To prevent complications, a specialized nutritional support team should monitor the patient’s parenteral nutrition both in the hospital and at home.
RECONSTRUCTIVE SURGERY
Patients with intestinal failure due to short- bowel syndrome should be considered for reconstructive surgery during different phases of the adaptation process. Options include reversed-segment procedures, stricturoplasty, bowel-lengthening procedures (eg, the Bianchi procedure), and serial transverse enteroplasty.41,42 If reconstructive surgery is ineffective, referral to an intestinal transplant program should be considered.
INTESTINAL AND MULTIVISCERAL TRANSPLANT
For patients who develop permanent intestinal failure and require lifelong parenteral nutrition, and for patients who experience significant complications of parenteral nutrition, such as infections and liver disease,43 intestinal transplant has emerged as a way to restore clinical nutritional autonomy.44 In one study, the 1-year survival rate after intestinal transplant was approximately 90%.44
There are currently three transplant procedures: isolated intestine transplant, combined liver-intestine transplant, and multivisceral transplant with or without a liver, depending on the presence of parenteral nutrition-associated liver disease.42,45 Close postoperative care is required to help the patient transition from parenteral to enteral nutrition.42 An intestinal rehabilitation team is equipped to provide this level of postoperative care.42
Intestinal and multivisceral transplant gained momentum in the early 1960s in preclinical and clinical studies.46,47 Since that time, the field has experienced remarkable advances due to standardization of surgical techniques, novel immunosuppressive therapies and induction protocols, and improved postoperative care.48 With the advent of tacrolimus in 1989, the rates of allograft rejection improved significantly, and the field of transplant emerged as a potentially lifesaving therapy for patients with permanent intestinal failure.48
Since 1990, more than 2,300 intestinal transplant procedures have been performed for various etiologies of intestinal failure, with short-bowel syndrome being the most common.49
The indications for intestinal transplant approved by the US Centers for Medicare and Medicaid services are detailed in Table 6.50 Despite ongoing challenges of graft rejection and maintenance immunosuppression, posttransplant quality-of-life questionnaires have indicated a significant improvement in functional status and a decrease in depressive symptoms.51 As such, it is evident that intestinal and multivisceral transplant offers substantial promise in restoring a patient’s quality of life and nutritional status.
- Parekh NR, Steiger E. Short bowel syndrome. Curr Treat Options Gastroenterol 2007; 10:10–23.
- Williamson RC. Intestinal adaptation (first of two parts). Structural, functional and cytokinetic changes. N Engl J Med 1978; 298:1393–1402.
- Vantini I, Benini L, Bonfante F, et al. Survival rate and prognostic factors in patients with intestinal failure. Dig Liver Dis 2004; 36:46–55.
- Abu-Elmagd KM, Bond GJ, Matarese L, et al. Gut rehabilitation and intestinal transplantation. Therapy 2005; 2:853–864.
- Nightingale JMD, Lennard-Jones JE. The short bowel syndrome: what’s new and old? Dig Dis 1993; 11:12–31.
- Parekh N, Seidner D, Steiger E. Managing short bowel syndrome: making the most of what the patient still has. Cleve Clin J Med 2005; 72:833–838.
- Wales PW. Surgical therapy for short bowel syndrome. Pediatr Surg Int 2004; 20:647–657.
- Parekh NR, Steiger E, Seidner DL. Determination of residual bowel length via surgical, radiological or historical data in patients with short bowel syndrome and intestinal failure (abstract). Gastroenterology 2006; 130:A605.
- Shatnawei A, Parekh NR, Rhoda KM, et al. Intestinal failure management at the Cleveland Clinic. Arch Surg 2010; 145:521–527.
- Kelly DG, Tappenden KA, Winkler MF. Short bowel syndrome: highlights of patient management, quality of life, and survival. JPEN J Parenter Enteral Nutr 2014; 38:427–437.
- Efsen E, Jeppesen PB. Modern treatment of adult short bowel syndrome patients. Minerva Gastroenterol Dietol 2011; 57:405–417.
- Wallis K, Walters JR, Gabe S. Short bowel syndrome: the role of GLP-2 on improving outcome. Curr Opin Clin Nutr Metab Care 2009; 12:526–532.
- Dowling RH, Booth DB. Functional compensation after small bowel resection in man. Lancet 1996; 2:146–147.
- Tappenden KA. Intestinal adaptation following resection. JPEN J Parenter Enteral Nutr 2014; 38(suppl 1):23S–31S.
- Friedman HI, Chandler JG, Peck CC, Nemeth TJ, Odum SK. Alterations in intestinal structure, fat absorption and body weight after intestinal bypass for morbid obesity. Surg Gynecol Obstet 1978; 146:757–767.
- O’Keefe SJ, Buchman AL, Fishbein TM, Jeejeebhoy KN, Jeppesen PB, Shaffer J. Short bowel syndrome and intestinal failure: consensus definitions and overview. Clin Gastroenterol Hepatol 2006; 4:6–10.
- Lennard-Jones JE. Review article: practical management of the short bowel. Aliment Pharmacol Ther 1994; 8:563–577.
- Goulet O, Colomb-Jung V, Joly F. Role of the colon in short bowel syndrome and intestinal transplantation. J Pediatr Gastroenterol Nutr 2009; 48(suppl 2):S66–S71.
- Jeppesen PB, Mortensen PB. Colonic digestion and absorption of energy from carbohydrates and medium-chain fat in small bowel failure. JPEN J Parenter Enteral Nutr 1999; 23(suppl 5):S101–S105.
- Buchman AL. Etiology and initial management of short bowel syndrome. Gastroenterology 2006; 130(suppl 1):S5–S15.
- Donohoe CL, Reynolds JV. Short bowel syndrome. Surgeon 2010; 8:270–279.
- Gong JF, Zhu WM, Yu WK, Li N, Li JS. Role of enteral nutrition in adult short bowel syndrome undergoing intestinal rehabilitation: the long-term outcome. Asia Pac J Clin Nutr 2009; 18:155–163.
- Sundaram A, Koutkia P, Apovian CM. Nutritional management of short bowel syndrome in adults. J Clin Gastroenterol 2002; 34:207–220.
- Byrne TA, Wilmore DW, Iyer K, et al. Growth hormone, glutamine, and an optimal diet reduces parenteral nutrition in patients with short bowel syndrome: a prospective, randomized, placebo-controlled, double-blind clinical trial. Ann Surg 2005; 242:655–661.
- Matarese LE, Steiger E. Dietary and medical management of short bowel syndrome in adult patients. J Clin Gastroenterol 2006; 40(suppl 2):S85–S93.
- Nordgaard I, Hansen BS, Mortensen PB. Colon as a digestive organ in patients with short bowel. Lancet 1994; 343:373–376.
- Ukleja A, Scolapio JS, Buchman AL. Nutritional management of short bowel syndrome. Semin Gastrointest Dis 2002; 13:161–168.
- Jeejeebhoy KN. Short bowel syndrome: a nutritional and medical approach. CMAJ 2002; 166:1297–1302.
- Seetharam P, Rodrigues G. Short bowel syndrome: a review of management options. Saudi J Gastroenterol 2011; 17:229–235.
- Wallis K, Walters JR, Gabe S. Short bowel syndrome: the role of GLP-2 on improving outcome. Curr Opin Clin Nutr Metab Care 2009; 12:526–532.
- Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, O’Keefe SJ. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut 2011; 60:902–914.
- Pironi L, Joly F, Forbes A, et al; Home Artificial Nutrition & Chronic Intestinal Failure Working Group of the European Society for Clinical Nutrition and Metabolism (ESPEN). Long-term follow-up of patients on home parenteral nutrition in Europe: implications for intestinal transplantation. Gut 2011; 60:17–25.
- Ekema G, Milianti S, Boroni G. Total parenteral nutrition in patients with short bowel syndrome. Minerva Pediatr 2009; 61:283–291.
- Messing B, Crenn P, Beau P, Boutron-Ruault MC, Rambaud JC, Matuchansky C. Long-term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology 1999; 117:1043–1050.
- Opilla M. Epidemiology of bloodstream infection associated with parenteral nutrition. Am J Infect Control 2008; 36:S173.e5–e8.
- Ukleja A, Romano MM. Complications of parenteral nutrition. Gastroenterol Clin North Am 2007; 36:23–46.
- Buchman AI, Iyer K, Fryer J. Parenteral nutrition-associated liver disease and the role for isolated intestine and intestine/liver transplantation. Hepatology 2006; 43:9–19.
- Fürst P, Kuhn KS. Fish oil emulsions: what benefits can they bring? Clin Nutr 2000; 19:7–14.
- Verso M, Agnelli G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol 2003; 21:3665–3675.
- McMahon MM, Nystrom E, Braunschweig C, Miles J, Compher C; American Society for Parenteral and Enteral Nutrition (ASPEN) Board of Directors; American Society for Parenteral and Enteral Nutrition. American Society of Parenteral and Enteral Nutrition (ASPEN) Board of Directors. A.S.P.E.N. clinical guidelines: nutrition support of adult patients with hyperglycemia. JPEN J Parenter Enteral Nutr 2013; 37:23–36.
- Kim HB, Fauza D, Garza J, Oh JT, Nurko S, Jaksic T. Serial transverse enteroplasty (STEP): a novel bowel lengthening procedure. J Pediatr Surg 2003; 38:425–429.
- King B, Carlson G, Khalil BA, Morabito A. Intestinal bowel lengthening in children with short bowel syndrome: systematic review of the Bianchi and STEP procedures. World J Surg 2013; 37:694–704.
- Matarese LE, O’Keefe SJ, Kandil HM, Costa G, Abu-Elmagd KM. Short bowel syndrome: clinical guidelines for nutrition management. Nutr Clin Pract 2005; 20:493–502.
- Abu-Elmagd KM, Costa G, Bond GJ, et al. Five hundred intestinal and multivisceral transplantations at a single center: major advances with new challenges. Ann Surg 2009; 250:567–581.
- Abu-Elmagd K. The concept of gut rehabilitation and the future of visceral transplantation. Nat Rev Gastroenterol Hepatol 2015; 12:108–120.
- Lillehei RC, Goott B, Miller FA. The physiological response of the small bowel of the dog to ischemia including prolonged in vitro preservation of the bowel with successful replacement and survival. Ann Surg 1959; 150:543–559.
- Starzl TE, Kaupp HA. Mass homotransplantation of abdominal organs in dogs. Surg Forum 1960; 11:28–30.
- O’Keefe SJ, Matarese L. Small bowel transplantation. Curr Gastroenterol Rep 2006; 8:360–366.
- Horslen SP. Optimal management of the post-intestinal transplant patient. Gastroenterology 2006; 130(suppl 1):S163–S169.
- Buchman AL, Scolapio J, Fryer J. AGA technical review on short bowel syndrome and intestinal transplantation. Gastroenterology 2003; 124:1111–1134.
- DiMartini A, Rovera GM, Graham TO, et al. Quality of life after small intestinal transplantation and among home parenteral nutrition patients. JPEN J Parenter Enteral Nutr 1998; 22:357–362.
Intestinal failure, the inability of the gut to maintain nutritional homeostasis,1 is a complication of vascular thrombosis, inflammatory bowel disease, radiation enteritis, obstruction, and other conditions, and of removing segments of the small and large intestines in response to these diseases.1,2 Imbalances of fluids and electrolytes, dehydration, malabsorption, vitamin and mineral deficiencies, chronic diarrhea, and increased ostomy output contribute to a decline in the quality of life and in the survival rate in these patients.2,3
Referral to an intestinal rehabilitation program that combines gastroenterology, nutrition, pharmacy, nursing, and social work can improve nutritional status and quality of life.4 Whenever possible, the goal of rehabilitation is nutritional autonomy, helping the patient make the transition to an independent oral diet.4 In selected patients in whom rehabilitation is not effective, intestinal transplant may be an option.
In this article, we review the intestinal adaptations that follow surgical resection and provide an update on intestinal rehabilitation techniques such as dietary modification, drug therapy, and parenteral nutrition. We also review experience with intestinal transplant in patients with intestinal failure.
INTESTINAL FAILURE
Intestinal failure results from reduction in enterocyte cell mass, obstruction, dysmotility, surgical resection, congenital defects, or disease-associated loss of absorption with suboptimal nutritional autonomy.5 Patients often suffer from extensive nutrient, electrolyte, and fluid abnormalities proportional to the remnant length and part of the intestine removed.5
Epidemiologic studies have demonstrated that short-bowel syndrome is the most common cause of intestinal failure in adults and children.6,7 Short-bowel syndrome is defined as a small-bowel length less than 200 cm, most commonly from extensive resections for inflammatory bowel disease.6 In children, the syndrome is also defined by a residual small-bowel length of less than 25% expected for gestational age.7
Table 1 lists the frequencies of the underlying disorders leading to intestinal failure or short-bowel syndrome in one series.8
INTESTINAL ADAPTATION
The gastrointestinal tract is the only organ for nutrient, fluid, and electrolyte absorption.9 Every day, 8 to 9 L of fluids and secretions reach the small intestine, comprising about 2 to 3 L of oral fluids, 1 L of saliva, 2 L of gastric juices, 1 L of bile, and 2 L of pancreatic juices.9 Approximately 7 to 8 L are reabsorbed by the small intestine and 1 to 2 L by the colon.9
Although carbohydrates, lipids, and proteins are absorbed through the entire small intestine and colon, site-specific digestion and absorption of different nutrients occur in different parts of the gastrointestinal tract.10 Also, certain nutrients may need site-specific receptors or transporters for their absorption,10 for example:
- Iron in the duodenum and proximal jejunum1
- Lactose in the brush border membrane of the jejunum and proximal ileum, where most of the enzyme lactase is present
- Vitamin B12 and bile salts in the distal ileum.
Hence, resection of a specific part of the intestine may predict deficiencies the patient may encounter after surgery.
The diarrhea that occurs in short-bowel syndrome may be due partly to loss of neurohumoral mediators that govern gastrointestinal transit time, most importantly cholecystokinin, peptide YY, and glucagon-like peptide 1.11 After contact with lipid- or protein-rich nutrients, cholecystokinin is released from the proximal small intestine, which decreases the gastric emptying to maximize nutrient digestion.12 Additionally, release of peptide YY and glucagon-like peptide 1 from the ileal L cells decreases gastric and intestinal motility. These mediators prolong gastrointestinal transit, increase nutrient processing time, and enhance absorption.12
After massive intestinal resection, the remnant bowel undergoes physiologic and functional adaptation to maintain nutritional homeostasis.13 Enterocytes express membrane-bound transporters and undergo accelerated cell division to enhance the absorptive surface area.13 Intestinal hypertrophy, which includes an increase in villous diameter and crypt height, continues for 2 years or more after intestinal resection, leading to greater absorptive surface area.14 It is estimated that villous height may increase by as much as 80%, illustrating a dynamic process in response to intestinal stress.15
Luminal nutrients are essential to the stimulation of enterocyte cells through paracrine mechanisms as well as through the up-regulation of colonic peptide transporter PepT1.15 Furthermore, gut motility is initially decreased in order to increase the concentration of local luminal growth factors.16
Other factors that may affect intestinal adaptation are the length of the residual colon and small intestine, enteral growth, and enterotropic factors.16 And especially in patients with short-bowel syndrome, complications such as malabsorption secondary to pancreatic insufficiency or rapid transit, excessive gastric acid secretion, bile acid wasting due to terminal ileum resection, and bacterial overgrowth in the small intestine result in worsened nutritional status and poor quality of life.16
Key factors that affect the degree of nutritional deficiencies
The degree of nutritional deficiencies and fluid and electrolyte imbalances depends on the length and location of resection and whether the colon is still continuous with the small intestine.17 Normal small-bowel length in adults is highly variable and can be up to 600 cm. Malnutrition after surgical resection usually occurs when more than three-fourths of intestinal tissue is removed.17 However, because of intestinal adaptation, patients with 50% of remnant small bowel may be able to achieve nutritional autonomy.18 Furthermore, because absorption of nutrients occurs primarily in the first 150 cm of the small intestine, resections of this anatomic region have the highest probability of resulting in malnutrition.18
After extensive intestinal resection, absorption of water and electrolytes is better and intestinal transit time is longer if the colon is still continuous with the rest of the gastrointestinal system.19 Approximately 100 cm of remnant intestinal tissue without colonic continuity or 60 cm with colonic continuity is needed to ensure the possibility of nutritional autonomy and independence from parenteral nutrition.19 Severe malnutrition and fluid and electrolyte imbalances can be prevented by appropriate and timely multidisciplinary care and early referral for intestinal rehabilitation.
INTESTINAL REHABILITATION AND NUTRITIONAL AUTONOMY
The aim of intestinal rehabilitation is to improve quality of life by reversing malnutrition and promoting nutritional autonomy, ie, independence from parenteral nutrition (Table 2).20 The complex nature of intestinal failure necessitates collaboration of multiple specialists—gastroenterologists, surgeons, dietitians, nurses, psychiatrists or psychologists, pharmacists, and social workers.20
Although most patients with intestinal failure initially require parenteral nutrition to maintain nutritional homeostasis, progressive adaptation of the remnant intestine enables a transition to enteral nutrition.21 Stimulation of the remnant intestine by enteral feeding reduces the complications of parenteral nutrition and encourages intestinal adaptation.21
Outpatient participation in an intestinal rehabilitation program can facilitate weaning from parenteral nutrition. Patients are monitored and supported during dietary modification, pharmacologic interventions, and reconstructive surgeries.21 A study of 61 patients with short-bowel syndrome undergoing a 3-week program of intestinal rehabilitation (recombinant human growth hormone, glutamine, enteral nutrition, and parenteral nutrition) reported an overall survival rate of 95% with an 85% success rate in weaning from parenteral nutrition during a mean follow-up of 50 (± 24) months.22 Permanent dependence on parenteral nutrition despite rehabilitation was predicted by length of the small bowel less than 100 cm and by the absence of terminal ileum and colon.22
Permanent intestinal failure, defined by the inability to wean from parenteral nutrition and restore nutrition autonomy, may require early referral for evaluation for intestinal and multivisceral transplant. Early referral improves survival rates, possibly because of fewer complications from parenteral nutrition.4
DIETARY MODIFICATION
Dietary modification is the single most effective means of weaning patients safely from parenteral nutrition (Table 3).23,24 Small, frequent feedings help reduce symptoms associated with rapid intestinal transit and increase the activity of luminal growth factors.23 Likewise, limits on intake of simple sugars, stimulants such as caffeine or insoluble fiber, and hypo- or hypertonic fluids decrease intestinal losses and the risk of dehydration.23 Low sugar loads also aim to reduce the occurrence of d-lactic acidosis and bacterial overgrowth in the small intestine.23 Patients who cannot maintain positive fluid balance may require standardized oral rehydration (Table 4) to improve absorption by way of the sodium-glucose coupled transporters at the brush border membrane, or they may require intravenous fluid supplementation.25
Colonic continuity
Other dietary recommendations depend on colonic continuity. In 1994, Nordgaard et al26 compared the effects of high-carbohydrate and high-fat diets in eight patients with colonic continuity and six patients with jejunostomies. The authors noted that a high-carbohydrate diet (60% carbohydrate, 20% fat) reduced fecal loss of energy and increased energy absorption in patients with colonic continuity. However, patients with an end-jejunostomy experienced equal fecal losses of carbohydrates and fat proportional to the amount consumed. The authors concluded that the presence of colonic bacteria promoted carbohydrate salvage, ie, the fermentation of malabsorbed carbohydrates to easily absorbed short-chain fatty acids.26
The colon can salvage as much as 1,000 kcal/day in patients with less than 200 cm of small bowel, and the presence of at least 50% of colon in continuity has been shown to reduce parenteral nutrition requirements by half in patients with less than 100 cm of small bowel.27 As a result, a diet high in complex carbohydrates and soluble fiber supplements is recommended in cases of preserved colon to promote adaptation and nutritional autonomy.27
Another aim of a high-carbohydrate, low- fat diet is to prevent calcium oxalate-related nephrolithiasis and choleretic diarrhea.26
In summary, patients with short-bowel syndrome with or without colonic continuity need different dietary regimens to attain nutritional autonomy.
DRUG THERAPY
In addition to diet therapy, most patients with intestinal failure require pharmacologic therapy.28 High stool or stoma effluent is most commonly treated with an antidiarrheal to increase transit time; diphenoxylate-atropine, loperamide, codeine sulfate, paregoric, and opium tincture are commonly prescribed (Table 5).27 In severe high-output states, a somatostatin analogue (eg, octreotide) may be added.29
Postoperative increases in gastric secretion may be countered by histamine 2 receptor antagonists and proton pump inhibitors, but long-term use of these drugs may lead to nutritional deficiencies and bacterial overgrowth in the small intestine.29 Bile acid sequestrants (in cases of distal ileal resection) and pancreatic enzymes target fat malabsorption, resultant cases of choleretic diarrhea, deficiency of essential fatty acids, kidney stones, and deficiency of fat-soluble vitamins.29 Probiotics and antibiotics can also be given for prevention and treatment of small-intestinal bacterial overgrowth.29
When traditional dietary modification and medical therapy fail to achieve nutritional homeostasis, another option to consider is a glucagon-like peptide-2 analogue to enhance intestinal adaptation.30 Produced in the native distal ileum and colon, glucagon-like peptide 2 moderates the rate of gastric emptying and small-bowel transit and enhances epithelial cell proliferation, thereby promoting intestinal adaptation.30 Further, a randomized controlled trial of 83 patients reported efficacy of these agents in reducing parenteral nutrition requirements in patients with intestinal failure.31
Hence, in patients with intestinal failure who have increased stoma effluent, drug therapy may play an important role in maintaining fluid and nutritional homeostasis.
THE ROLE OF PARENTERAL NUTRITION IN INTESTINAL FAILURE
Despite the best efforts of an intestinal rehabilitation program, not all patients gain nutritional autonomy.32 Physiologic, psychological, social, and economic factors may contribute to dependence on parenteral nutrition.32 Currently, more than 40,000 US patients depend on it for survival.33
The need for short-term or long-term parenteral nutrition is determined by the patient’s medical needs.33 Patients requiring short-term parenteral nutrition (2–6 weeks) include those whose bowel function has not returned to normal postoperatively, and those who were severely malnourished preoperatively.34 Patients needing it long-term (from months to years to lifelong) are those with gastrointestinal dysmotility and short-bowel syndrome due to extensive bowel resections.33
Complications of parenteral nutrition
Catheter-related bloodstream infection is the most common complication and cause of hospitalization. Infection can be localized to the exit site or tunnel or can be systemic (eg, line sepsis).35Staphylococcus aureus and coagulase-negative staphylococci are most often implicated in catheter infection.35 When possible, catheter salvage is desirable, but the central venous catheter must be removed in cases of tunnel infection, port abscess, septic shock, paired blood cultures positive for fungi or highly virulent bacteria, endocarditis, septic thrombosis, and other conditions.35,36
Liver disease is a serious complication of long-term parenteral nutrition and may occur in up to 55% of patients on therapy for more than 2 years; it carries a mortality rate of 15%.37
Risk factors include younger age and use of excessive carbohydrate and fat compositions, mainly soybean-oil–based lipid emulsions.37 However, fish-oil–based lipid emulsions have recently shown promise in preventing and reversing parenteral nutrition-associated liver failure and cholestasis, especially in a pediatric population.38
Catheter thrombosis may occur in up to 30% of patients on long-term parenteral nutrition.39 However, this risk is reduced with appropriate positioning of the catheter tip in the mid or lower superior vena cava.37 Treatment of thrombosis of the central access includes either anticoagulation or thrombolysis.37
Hence, appropriate and timely follow-up of patients on parenteral nutrition is essential in reducing associated complications. Monitoring weight, fluid status, serum glucose, and patency of central access are critical to ensure that the patient maintains nutritional status effectively.40 To prevent complications, a specialized nutritional support team should monitor the patient’s parenteral nutrition both in the hospital and at home.
RECONSTRUCTIVE SURGERY
Patients with intestinal failure due to short- bowel syndrome should be considered for reconstructive surgery during different phases of the adaptation process. Options include reversed-segment procedures, stricturoplasty, bowel-lengthening procedures (eg, the Bianchi procedure), and serial transverse enteroplasty.41,42 If reconstructive surgery is ineffective, referral to an intestinal transplant program should be considered.
INTESTINAL AND MULTIVISCERAL TRANSPLANT
For patients who develop permanent intestinal failure and require lifelong parenteral nutrition, and for patients who experience significant complications of parenteral nutrition, such as infections and liver disease,43 intestinal transplant has emerged as a way to restore clinical nutritional autonomy.44 In one study, the 1-year survival rate after intestinal transplant was approximately 90%.44
There are currently three transplant procedures: isolated intestine transplant, combined liver-intestine transplant, and multivisceral transplant with or without a liver, depending on the presence of parenteral nutrition-associated liver disease.42,45 Close postoperative care is required to help the patient transition from parenteral to enteral nutrition.42 An intestinal rehabilitation team is equipped to provide this level of postoperative care.42
Intestinal and multivisceral transplant gained momentum in the early 1960s in preclinical and clinical studies.46,47 Since that time, the field has experienced remarkable advances due to standardization of surgical techniques, novel immunosuppressive therapies and induction protocols, and improved postoperative care.48 With the advent of tacrolimus in 1989, the rates of allograft rejection improved significantly, and the field of transplant emerged as a potentially lifesaving therapy for patients with permanent intestinal failure.48
Since 1990, more than 2,300 intestinal transplant procedures have been performed for various etiologies of intestinal failure, with short-bowel syndrome being the most common.49
The indications for intestinal transplant approved by the US Centers for Medicare and Medicaid services are detailed in Table 6.50 Despite ongoing challenges of graft rejection and maintenance immunosuppression, posttransplant quality-of-life questionnaires have indicated a significant improvement in functional status and a decrease in depressive symptoms.51 As such, it is evident that intestinal and multivisceral transplant offers substantial promise in restoring a patient’s quality of life and nutritional status.
Intestinal failure, the inability of the gut to maintain nutritional homeostasis,1 is a complication of vascular thrombosis, inflammatory bowel disease, radiation enteritis, obstruction, and other conditions, and of removing segments of the small and large intestines in response to these diseases.1,2 Imbalances of fluids and electrolytes, dehydration, malabsorption, vitamin and mineral deficiencies, chronic diarrhea, and increased ostomy output contribute to a decline in the quality of life and in the survival rate in these patients.2,3
Referral to an intestinal rehabilitation program that combines gastroenterology, nutrition, pharmacy, nursing, and social work can improve nutritional status and quality of life.4 Whenever possible, the goal of rehabilitation is nutritional autonomy, helping the patient make the transition to an independent oral diet.4 In selected patients in whom rehabilitation is not effective, intestinal transplant may be an option.
In this article, we review the intestinal adaptations that follow surgical resection and provide an update on intestinal rehabilitation techniques such as dietary modification, drug therapy, and parenteral nutrition. We also review experience with intestinal transplant in patients with intestinal failure.
INTESTINAL FAILURE
Intestinal failure results from reduction in enterocyte cell mass, obstruction, dysmotility, surgical resection, congenital defects, or disease-associated loss of absorption with suboptimal nutritional autonomy.5 Patients often suffer from extensive nutrient, electrolyte, and fluid abnormalities proportional to the remnant length and part of the intestine removed.5
Epidemiologic studies have demonstrated that short-bowel syndrome is the most common cause of intestinal failure in adults and children.6,7 Short-bowel syndrome is defined as a small-bowel length less than 200 cm, most commonly from extensive resections for inflammatory bowel disease.6 In children, the syndrome is also defined by a residual small-bowel length of less than 25% expected for gestational age.7
Table 1 lists the frequencies of the underlying disorders leading to intestinal failure or short-bowel syndrome in one series.8
INTESTINAL ADAPTATION
The gastrointestinal tract is the only organ for nutrient, fluid, and electrolyte absorption.9 Every day, 8 to 9 L of fluids and secretions reach the small intestine, comprising about 2 to 3 L of oral fluids, 1 L of saliva, 2 L of gastric juices, 1 L of bile, and 2 L of pancreatic juices.9 Approximately 7 to 8 L are reabsorbed by the small intestine and 1 to 2 L by the colon.9
Although carbohydrates, lipids, and proteins are absorbed through the entire small intestine and colon, site-specific digestion and absorption of different nutrients occur in different parts of the gastrointestinal tract.10 Also, certain nutrients may need site-specific receptors or transporters for their absorption,10 for example:
- Iron in the duodenum and proximal jejunum1
- Lactose in the brush border membrane of the jejunum and proximal ileum, where most of the enzyme lactase is present
- Vitamin B12 and bile salts in the distal ileum.
Hence, resection of a specific part of the intestine may predict deficiencies the patient may encounter after surgery.
The diarrhea that occurs in short-bowel syndrome may be due partly to loss of neurohumoral mediators that govern gastrointestinal transit time, most importantly cholecystokinin, peptide YY, and glucagon-like peptide 1.11 After contact with lipid- or protein-rich nutrients, cholecystokinin is released from the proximal small intestine, which decreases the gastric emptying to maximize nutrient digestion.12 Additionally, release of peptide YY and glucagon-like peptide 1 from the ileal L cells decreases gastric and intestinal motility. These mediators prolong gastrointestinal transit, increase nutrient processing time, and enhance absorption.12
After massive intestinal resection, the remnant bowel undergoes physiologic and functional adaptation to maintain nutritional homeostasis.13 Enterocytes express membrane-bound transporters and undergo accelerated cell division to enhance the absorptive surface area.13 Intestinal hypertrophy, which includes an increase in villous diameter and crypt height, continues for 2 years or more after intestinal resection, leading to greater absorptive surface area.14 It is estimated that villous height may increase by as much as 80%, illustrating a dynamic process in response to intestinal stress.15
Luminal nutrients are essential to the stimulation of enterocyte cells through paracrine mechanisms as well as through the up-regulation of colonic peptide transporter PepT1.15 Furthermore, gut motility is initially decreased in order to increase the concentration of local luminal growth factors.16
Other factors that may affect intestinal adaptation are the length of the residual colon and small intestine, enteral growth, and enterotropic factors.16 And especially in patients with short-bowel syndrome, complications such as malabsorption secondary to pancreatic insufficiency or rapid transit, excessive gastric acid secretion, bile acid wasting due to terminal ileum resection, and bacterial overgrowth in the small intestine result in worsened nutritional status and poor quality of life.16
Key factors that affect the degree of nutritional deficiencies
The degree of nutritional deficiencies and fluid and electrolyte imbalances depends on the length and location of resection and whether the colon is still continuous with the small intestine.17 Normal small-bowel length in adults is highly variable and can be up to 600 cm. Malnutrition after surgical resection usually occurs when more than three-fourths of intestinal tissue is removed.17 However, because of intestinal adaptation, patients with 50% of remnant small bowel may be able to achieve nutritional autonomy.18 Furthermore, because absorption of nutrients occurs primarily in the first 150 cm of the small intestine, resections of this anatomic region have the highest probability of resulting in malnutrition.18
After extensive intestinal resection, absorption of water and electrolytes is better and intestinal transit time is longer if the colon is still continuous with the rest of the gastrointestinal system.19 Approximately 100 cm of remnant intestinal tissue without colonic continuity or 60 cm with colonic continuity is needed to ensure the possibility of nutritional autonomy and independence from parenteral nutrition.19 Severe malnutrition and fluid and electrolyte imbalances can be prevented by appropriate and timely multidisciplinary care and early referral for intestinal rehabilitation.
INTESTINAL REHABILITATION AND NUTRITIONAL AUTONOMY
The aim of intestinal rehabilitation is to improve quality of life by reversing malnutrition and promoting nutritional autonomy, ie, independence from parenteral nutrition (Table 2).20 The complex nature of intestinal failure necessitates collaboration of multiple specialists—gastroenterologists, surgeons, dietitians, nurses, psychiatrists or psychologists, pharmacists, and social workers.20
Although most patients with intestinal failure initially require parenteral nutrition to maintain nutritional homeostasis, progressive adaptation of the remnant intestine enables a transition to enteral nutrition.21 Stimulation of the remnant intestine by enteral feeding reduces the complications of parenteral nutrition and encourages intestinal adaptation.21
Outpatient participation in an intestinal rehabilitation program can facilitate weaning from parenteral nutrition. Patients are monitored and supported during dietary modification, pharmacologic interventions, and reconstructive surgeries.21 A study of 61 patients with short-bowel syndrome undergoing a 3-week program of intestinal rehabilitation (recombinant human growth hormone, glutamine, enteral nutrition, and parenteral nutrition) reported an overall survival rate of 95% with an 85% success rate in weaning from parenteral nutrition during a mean follow-up of 50 (± 24) months.22 Permanent dependence on parenteral nutrition despite rehabilitation was predicted by length of the small bowel less than 100 cm and by the absence of terminal ileum and colon.22
Permanent intestinal failure, defined by the inability to wean from parenteral nutrition and restore nutrition autonomy, may require early referral for evaluation for intestinal and multivisceral transplant. Early referral improves survival rates, possibly because of fewer complications from parenteral nutrition.4
DIETARY MODIFICATION
Dietary modification is the single most effective means of weaning patients safely from parenteral nutrition (Table 3).23,24 Small, frequent feedings help reduce symptoms associated with rapid intestinal transit and increase the activity of luminal growth factors.23 Likewise, limits on intake of simple sugars, stimulants such as caffeine or insoluble fiber, and hypo- or hypertonic fluids decrease intestinal losses and the risk of dehydration.23 Low sugar loads also aim to reduce the occurrence of d-lactic acidosis and bacterial overgrowth in the small intestine.23 Patients who cannot maintain positive fluid balance may require standardized oral rehydration (Table 4) to improve absorption by way of the sodium-glucose coupled transporters at the brush border membrane, or they may require intravenous fluid supplementation.25
Colonic continuity
Other dietary recommendations depend on colonic continuity. In 1994, Nordgaard et al26 compared the effects of high-carbohydrate and high-fat diets in eight patients with colonic continuity and six patients with jejunostomies. The authors noted that a high-carbohydrate diet (60% carbohydrate, 20% fat) reduced fecal loss of energy and increased energy absorption in patients with colonic continuity. However, patients with an end-jejunostomy experienced equal fecal losses of carbohydrates and fat proportional to the amount consumed. The authors concluded that the presence of colonic bacteria promoted carbohydrate salvage, ie, the fermentation of malabsorbed carbohydrates to easily absorbed short-chain fatty acids.26
The colon can salvage as much as 1,000 kcal/day in patients with less than 200 cm of small bowel, and the presence of at least 50% of colon in continuity has been shown to reduce parenteral nutrition requirements by half in patients with less than 100 cm of small bowel.27 As a result, a diet high in complex carbohydrates and soluble fiber supplements is recommended in cases of preserved colon to promote adaptation and nutritional autonomy.27
Another aim of a high-carbohydrate, low- fat diet is to prevent calcium oxalate-related nephrolithiasis and choleretic diarrhea.26
In summary, patients with short-bowel syndrome with or without colonic continuity need different dietary regimens to attain nutritional autonomy.
DRUG THERAPY
In addition to diet therapy, most patients with intestinal failure require pharmacologic therapy.28 High stool or stoma effluent is most commonly treated with an antidiarrheal to increase transit time; diphenoxylate-atropine, loperamide, codeine sulfate, paregoric, and opium tincture are commonly prescribed (Table 5).27 In severe high-output states, a somatostatin analogue (eg, octreotide) may be added.29
Postoperative increases in gastric secretion may be countered by histamine 2 receptor antagonists and proton pump inhibitors, but long-term use of these drugs may lead to nutritional deficiencies and bacterial overgrowth in the small intestine.29 Bile acid sequestrants (in cases of distal ileal resection) and pancreatic enzymes target fat malabsorption, resultant cases of choleretic diarrhea, deficiency of essential fatty acids, kidney stones, and deficiency of fat-soluble vitamins.29 Probiotics and antibiotics can also be given for prevention and treatment of small-intestinal bacterial overgrowth.29
When traditional dietary modification and medical therapy fail to achieve nutritional homeostasis, another option to consider is a glucagon-like peptide-2 analogue to enhance intestinal adaptation.30 Produced in the native distal ileum and colon, glucagon-like peptide 2 moderates the rate of gastric emptying and small-bowel transit and enhances epithelial cell proliferation, thereby promoting intestinal adaptation.30 Further, a randomized controlled trial of 83 patients reported efficacy of these agents in reducing parenteral nutrition requirements in patients with intestinal failure.31
Hence, in patients with intestinal failure who have increased stoma effluent, drug therapy may play an important role in maintaining fluid and nutritional homeostasis.
THE ROLE OF PARENTERAL NUTRITION IN INTESTINAL FAILURE
Despite the best efforts of an intestinal rehabilitation program, not all patients gain nutritional autonomy.32 Physiologic, psychological, social, and economic factors may contribute to dependence on parenteral nutrition.32 Currently, more than 40,000 US patients depend on it for survival.33
The need for short-term or long-term parenteral nutrition is determined by the patient’s medical needs.33 Patients requiring short-term parenteral nutrition (2–6 weeks) include those whose bowel function has not returned to normal postoperatively, and those who were severely malnourished preoperatively.34 Patients needing it long-term (from months to years to lifelong) are those with gastrointestinal dysmotility and short-bowel syndrome due to extensive bowel resections.33
Complications of parenteral nutrition
Catheter-related bloodstream infection is the most common complication and cause of hospitalization. Infection can be localized to the exit site or tunnel or can be systemic (eg, line sepsis).35Staphylococcus aureus and coagulase-negative staphylococci are most often implicated in catheter infection.35 When possible, catheter salvage is desirable, but the central venous catheter must be removed in cases of tunnel infection, port abscess, septic shock, paired blood cultures positive for fungi or highly virulent bacteria, endocarditis, septic thrombosis, and other conditions.35,36
Liver disease is a serious complication of long-term parenteral nutrition and may occur in up to 55% of patients on therapy for more than 2 years; it carries a mortality rate of 15%.37
Risk factors include younger age and use of excessive carbohydrate and fat compositions, mainly soybean-oil–based lipid emulsions.37 However, fish-oil–based lipid emulsions have recently shown promise in preventing and reversing parenteral nutrition-associated liver failure and cholestasis, especially in a pediatric population.38
Catheter thrombosis may occur in up to 30% of patients on long-term parenteral nutrition.39 However, this risk is reduced with appropriate positioning of the catheter tip in the mid or lower superior vena cava.37 Treatment of thrombosis of the central access includes either anticoagulation or thrombolysis.37
Hence, appropriate and timely follow-up of patients on parenteral nutrition is essential in reducing associated complications. Monitoring weight, fluid status, serum glucose, and patency of central access are critical to ensure that the patient maintains nutritional status effectively.40 To prevent complications, a specialized nutritional support team should monitor the patient’s parenteral nutrition both in the hospital and at home.
RECONSTRUCTIVE SURGERY
Patients with intestinal failure due to short- bowel syndrome should be considered for reconstructive surgery during different phases of the adaptation process. Options include reversed-segment procedures, stricturoplasty, bowel-lengthening procedures (eg, the Bianchi procedure), and serial transverse enteroplasty.41,42 If reconstructive surgery is ineffective, referral to an intestinal transplant program should be considered.
INTESTINAL AND MULTIVISCERAL TRANSPLANT
For patients who develop permanent intestinal failure and require lifelong parenteral nutrition, and for patients who experience significant complications of parenteral nutrition, such as infections and liver disease,43 intestinal transplant has emerged as a way to restore clinical nutritional autonomy.44 In one study, the 1-year survival rate after intestinal transplant was approximately 90%.44
There are currently three transplant procedures: isolated intestine transplant, combined liver-intestine transplant, and multivisceral transplant with or without a liver, depending on the presence of parenteral nutrition-associated liver disease.42,45 Close postoperative care is required to help the patient transition from parenteral to enteral nutrition.42 An intestinal rehabilitation team is equipped to provide this level of postoperative care.42
Intestinal and multivisceral transplant gained momentum in the early 1960s in preclinical and clinical studies.46,47 Since that time, the field has experienced remarkable advances due to standardization of surgical techniques, novel immunosuppressive therapies and induction protocols, and improved postoperative care.48 With the advent of tacrolimus in 1989, the rates of allograft rejection improved significantly, and the field of transplant emerged as a potentially lifesaving therapy for patients with permanent intestinal failure.48
Since 1990, more than 2,300 intestinal transplant procedures have been performed for various etiologies of intestinal failure, with short-bowel syndrome being the most common.49
The indications for intestinal transplant approved by the US Centers for Medicare and Medicaid services are detailed in Table 6.50 Despite ongoing challenges of graft rejection and maintenance immunosuppression, posttransplant quality-of-life questionnaires have indicated a significant improvement in functional status and a decrease in depressive symptoms.51 As such, it is evident that intestinal and multivisceral transplant offers substantial promise in restoring a patient’s quality of life and nutritional status.
- Parekh NR, Steiger E. Short bowel syndrome. Curr Treat Options Gastroenterol 2007; 10:10–23.
- Williamson RC. Intestinal adaptation (first of two parts). Structural, functional and cytokinetic changes. N Engl J Med 1978; 298:1393–1402.
- Vantini I, Benini L, Bonfante F, et al. Survival rate and prognostic factors in patients with intestinal failure. Dig Liver Dis 2004; 36:46–55.
- Abu-Elmagd KM, Bond GJ, Matarese L, et al. Gut rehabilitation and intestinal transplantation. Therapy 2005; 2:853–864.
- Nightingale JMD, Lennard-Jones JE. The short bowel syndrome: what’s new and old? Dig Dis 1993; 11:12–31.
- Parekh N, Seidner D, Steiger E. Managing short bowel syndrome: making the most of what the patient still has. Cleve Clin J Med 2005; 72:833–838.
- Wales PW. Surgical therapy for short bowel syndrome. Pediatr Surg Int 2004; 20:647–657.
- Parekh NR, Steiger E, Seidner DL. Determination of residual bowel length via surgical, radiological or historical data in patients with short bowel syndrome and intestinal failure (abstract). Gastroenterology 2006; 130:A605.
- Shatnawei A, Parekh NR, Rhoda KM, et al. Intestinal failure management at the Cleveland Clinic. Arch Surg 2010; 145:521–527.
- Kelly DG, Tappenden KA, Winkler MF. Short bowel syndrome: highlights of patient management, quality of life, and survival. JPEN J Parenter Enteral Nutr 2014; 38:427–437.
- Efsen E, Jeppesen PB. Modern treatment of adult short bowel syndrome patients. Minerva Gastroenterol Dietol 2011; 57:405–417.
- Wallis K, Walters JR, Gabe S. Short bowel syndrome: the role of GLP-2 on improving outcome. Curr Opin Clin Nutr Metab Care 2009; 12:526–532.
- Dowling RH, Booth DB. Functional compensation after small bowel resection in man. Lancet 1996; 2:146–147.
- Tappenden KA. Intestinal adaptation following resection. JPEN J Parenter Enteral Nutr 2014; 38(suppl 1):23S–31S.
- Friedman HI, Chandler JG, Peck CC, Nemeth TJ, Odum SK. Alterations in intestinal structure, fat absorption and body weight after intestinal bypass for morbid obesity. Surg Gynecol Obstet 1978; 146:757–767.
- O’Keefe SJ, Buchman AL, Fishbein TM, Jeejeebhoy KN, Jeppesen PB, Shaffer J. Short bowel syndrome and intestinal failure: consensus definitions and overview. Clin Gastroenterol Hepatol 2006; 4:6–10.
- Lennard-Jones JE. Review article: practical management of the short bowel. Aliment Pharmacol Ther 1994; 8:563–577.
- Goulet O, Colomb-Jung V, Joly F. Role of the colon in short bowel syndrome and intestinal transplantation. J Pediatr Gastroenterol Nutr 2009; 48(suppl 2):S66–S71.
- Jeppesen PB, Mortensen PB. Colonic digestion and absorption of energy from carbohydrates and medium-chain fat in small bowel failure. JPEN J Parenter Enteral Nutr 1999; 23(suppl 5):S101–S105.
- Buchman AL. Etiology and initial management of short bowel syndrome. Gastroenterology 2006; 130(suppl 1):S5–S15.
- Donohoe CL, Reynolds JV. Short bowel syndrome. Surgeon 2010; 8:270–279.
- Gong JF, Zhu WM, Yu WK, Li N, Li JS. Role of enteral nutrition in adult short bowel syndrome undergoing intestinal rehabilitation: the long-term outcome. Asia Pac J Clin Nutr 2009; 18:155–163.
- Sundaram A, Koutkia P, Apovian CM. Nutritional management of short bowel syndrome in adults. J Clin Gastroenterol 2002; 34:207–220.
- Byrne TA, Wilmore DW, Iyer K, et al. Growth hormone, glutamine, and an optimal diet reduces parenteral nutrition in patients with short bowel syndrome: a prospective, randomized, placebo-controlled, double-blind clinical trial. Ann Surg 2005; 242:655–661.
- Matarese LE, Steiger E. Dietary and medical management of short bowel syndrome in adult patients. J Clin Gastroenterol 2006; 40(suppl 2):S85–S93.
- Nordgaard I, Hansen BS, Mortensen PB. Colon as a digestive organ in patients with short bowel. Lancet 1994; 343:373–376.
- Ukleja A, Scolapio JS, Buchman AL. Nutritional management of short bowel syndrome. Semin Gastrointest Dis 2002; 13:161–168.
- Jeejeebhoy KN. Short bowel syndrome: a nutritional and medical approach. CMAJ 2002; 166:1297–1302.
- Seetharam P, Rodrigues G. Short bowel syndrome: a review of management options. Saudi J Gastroenterol 2011; 17:229–235.
- Wallis K, Walters JR, Gabe S. Short bowel syndrome: the role of GLP-2 on improving outcome. Curr Opin Clin Nutr Metab Care 2009; 12:526–532.
- Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, O’Keefe SJ. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut 2011; 60:902–914.
- Pironi L, Joly F, Forbes A, et al; Home Artificial Nutrition & Chronic Intestinal Failure Working Group of the European Society for Clinical Nutrition and Metabolism (ESPEN). Long-term follow-up of patients on home parenteral nutrition in Europe: implications for intestinal transplantation. Gut 2011; 60:17–25.
- Ekema G, Milianti S, Boroni G. Total parenteral nutrition in patients with short bowel syndrome. Minerva Pediatr 2009; 61:283–291.
- Messing B, Crenn P, Beau P, Boutron-Ruault MC, Rambaud JC, Matuchansky C. Long-term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology 1999; 117:1043–1050.
- Opilla M. Epidemiology of bloodstream infection associated with parenteral nutrition. Am J Infect Control 2008; 36:S173.e5–e8.
- Ukleja A, Romano MM. Complications of parenteral nutrition. Gastroenterol Clin North Am 2007; 36:23–46.
- Buchman AI, Iyer K, Fryer J. Parenteral nutrition-associated liver disease and the role for isolated intestine and intestine/liver transplantation. Hepatology 2006; 43:9–19.
- Fürst P, Kuhn KS. Fish oil emulsions: what benefits can they bring? Clin Nutr 2000; 19:7–14.
- Verso M, Agnelli G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol 2003; 21:3665–3675.
- McMahon MM, Nystrom E, Braunschweig C, Miles J, Compher C; American Society for Parenteral and Enteral Nutrition (ASPEN) Board of Directors; American Society for Parenteral and Enteral Nutrition. American Society of Parenteral and Enteral Nutrition (ASPEN) Board of Directors. A.S.P.E.N. clinical guidelines: nutrition support of adult patients with hyperglycemia. JPEN J Parenter Enteral Nutr 2013; 37:23–36.
- Kim HB, Fauza D, Garza J, Oh JT, Nurko S, Jaksic T. Serial transverse enteroplasty (STEP): a novel bowel lengthening procedure. J Pediatr Surg 2003; 38:425–429.
- King B, Carlson G, Khalil BA, Morabito A. Intestinal bowel lengthening in children with short bowel syndrome: systematic review of the Bianchi and STEP procedures. World J Surg 2013; 37:694–704.
- Matarese LE, O’Keefe SJ, Kandil HM, Costa G, Abu-Elmagd KM. Short bowel syndrome: clinical guidelines for nutrition management. Nutr Clin Pract 2005; 20:493–502.
- Abu-Elmagd KM, Costa G, Bond GJ, et al. Five hundred intestinal and multivisceral transplantations at a single center: major advances with new challenges. Ann Surg 2009; 250:567–581.
- Abu-Elmagd K. The concept of gut rehabilitation and the future of visceral transplantation. Nat Rev Gastroenterol Hepatol 2015; 12:108–120.
- Lillehei RC, Goott B, Miller FA. The physiological response of the small bowel of the dog to ischemia including prolonged in vitro preservation of the bowel with successful replacement and survival. Ann Surg 1959; 150:543–559.
- Starzl TE, Kaupp HA. Mass homotransplantation of abdominal organs in dogs. Surg Forum 1960; 11:28–30.
- O’Keefe SJ, Matarese L. Small bowel transplantation. Curr Gastroenterol Rep 2006; 8:360–366.
- Horslen SP. Optimal management of the post-intestinal transplant patient. Gastroenterology 2006; 130(suppl 1):S163–S169.
- Buchman AL, Scolapio J, Fryer J. AGA technical review on short bowel syndrome and intestinal transplantation. Gastroenterology 2003; 124:1111–1134.
- DiMartini A, Rovera GM, Graham TO, et al. Quality of life after small intestinal transplantation and among home parenteral nutrition patients. JPEN J Parenter Enteral Nutr 1998; 22:357–362.
- Parekh NR, Steiger E. Short bowel syndrome. Curr Treat Options Gastroenterol 2007; 10:10–23.
- Williamson RC. Intestinal adaptation (first of two parts). Structural, functional and cytokinetic changes. N Engl J Med 1978; 298:1393–1402.
- Vantini I, Benini L, Bonfante F, et al. Survival rate and prognostic factors in patients with intestinal failure. Dig Liver Dis 2004; 36:46–55.
- Abu-Elmagd KM, Bond GJ, Matarese L, et al. Gut rehabilitation and intestinal transplantation. Therapy 2005; 2:853–864.
- Nightingale JMD, Lennard-Jones JE. The short bowel syndrome: what’s new and old? Dig Dis 1993; 11:12–31.
- Parekh N, Seidner D, Steiger E. Managing short bowel syndrome: making the most of what the patient still has. Cleve Clin J Med 2005; 72:833–838.
- Wales PW. Surgical therapy for short bowel syndrome. Pediatr Surg Int 2004; 20:647–657.
- Parekh NR, Steiger E, Seidner DL. Determination of residual bowel length via surgical, radiological or historical data in patients with short bowel syndrome and intestinal failure (abstract). Gastroenterology 2006; 130:A605.
- Shatnawei A, Parekh NR, Rhoda KM, et al. Intestinal failure management at the Cleveland Clinic. Arch Surg 2010; 145:521–527.
- Kelly DG, Tappenden KA, Winkler MF. Short bowel syndrome: highlights of patient management, quality of life, and survival. JPEN J Parenter Enteral Nutr 2014; 38:427–437.
- Efsen E, Jeppesen PB. Modern treatment of adult short bowel syndrome patients. Minerva Gastroenterol Dietol 2011; 57:405–417.
- Wallis K, Walters JR, Gabe S. Short bowel syndrome: the role of GLP-2 on improving outcome. Curr Opin Clin Nutr Metab Care 2009; 12:526–532.
- Dowling RH, Booth DB. Functional compensation after small bowel resection in man. Lancet 1996; 2:146–147.
- Tappenden KA. Intestinal adaptation following resection. JPEN J Parenter Enteral Nutr 2014; 38(suppl 1):23S–31S.
- Friedman HI, Chandler JG, Peck CC, Nemeth TJ, Odum SK. Alterations in intestinal structure, fat absorption and body weight after intestinal bypass for morbid obesity. Surg Gynecol Obstet 1978; 146:757–767.
- O’Keefe SJ, Buchman AL, Fishbein TM, Jeejeebhoy KN, Jeppesen PB, Shaffer J. Short bowel syndrome and intestinal failure: consensus definitions and overview. Clin Gastroenterol Hepatol 2006; 4:6–10.
- Lennard-Jones JE. Review article: practical management of the short bowel. Aliment Pharmacol Ther 1994; 8:563–577.
- Goulet O, Colomb-Jung V, Joly F. Role of the colon in short bowel syndrome and intestinal transplantation. J Pediatr Gastroenterol Nutr 2009; 48(suppl 2):S66–S71.
- Jeppesen PB, Mortensen PB. Colonic digestion and absorption of energy from carbohydrates and medium-chain fat in small bowel failure. JPEN J Parenter Enteral Nutr 1999; 23(suppl 5):S101–S105.
- Buchman AL. Etiology and initial management of short bowel syndrome. Gastroenterology 2006; 130(suppl 1):S5–S15.
- Donohoe CL, Reynolds JV. Short bowel syndrome. Surgeon 2010; 8:270–279.
- Gong JF, Zhu WM, Yu WK, Li N, Li JS. Role of enteral nutrition in adult short bowel syndrome undergoing intestinal rehabilitation: the long-term outcome. Asia Pac J Clin Nutr 2009; 18:155–163.
- Sundaram A, Koutkia P, Apovian CM. Nutritional management of short bowel syndrome in adults. J Clin Gastroenterol 2002; 34:207–220.
- Byrne TA, Wilmore DW, Iyer K, et al. Growth hormone, glutamine, and an optimal diet reduces parenteral nutrition in patients with short bowel syndrome: a prospective, randomized, placebo-controlled, double-blind clinical trial. Ann Surg 2005; 242:655–661.
- Matarese LE, Steiger E. Dietary and medical management of short bowel syndrome in adult patients. J Clin Gastroenterol 2006; 40(suppl 2):S85–S93.
- Nordgaard I, Hansen BS, Mortensen PB. Colon as a digestive organ in patients with short bowel. Lancet 1994; 343:373–376.
- Ukleja A, Scolapio JS, Buchman AL. Nutritional management of short bowel syndrome. Semin Gastrointest Dis 2002; 13:161–168.
- Jeejeebhoy KN. Short bowel syndrome: a nutritional and medical approach. CMAJ 2002; 166:1297–1302.
- Seetharam P, Rodrigues G. Short bowel syndrome: a review of management options. Saudi J Gastroenterol 2011; 17:229–235.
- Wallis K, Walters JR, Gabe S. Short bowel syndrome: the role of GLP-2 on improving outcome. Curr Opin Clin Nutr Metab Care 2009; 12:526–532.
- Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, O’Keefe SJ. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut 2011; 60:902–914.
- Pironi L, Joly F, Forbes A, et al; Home Artificial Nutrition & Chronic Intestinal Failure Working Group of the European Society for Clinical Nutrition and Metabolism (ESPEN). Long-term follow-up of patients on home parenteral nutrition in Europe: implications for intestinal transplantation. Gut 2011; 60:17–25.
- Ekema G, Milianti S, Boroni G. Total parenteral nutrition in patients with short bowel syndrome. Minerva Pediatr 2009; 61:283–291.
- Messing B, Crenn P, Beau P, Boutron-Ruault MC, Rambaud JC, Matuchansky C. Long-term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology 1999; 117:1043–1050.
- Opilla M. Epidemiology of bloodstream infection associated with parenteral nutrition. Am J Infect Control 2008; 36:S173.e5–e8.
- Ukleja A, Romano MM. Complications of parenteral nutrition. Gastroenterol Clin North Am 2007; 36:23–46.
- Buchman AI, Iyer K, Fryer J. Parenteral nutrition-associated liver disease and the role for isolated intestine and intestine/liver transplantation. Hepatology 2006; 43:9–19.
- Fürst P, Kuhn KS. Fish oil emulsions: what benefits can they bring? Clin Nutr 2000; 19:7–14.
- Verso M, Agnelli G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol 2003; 21:3665–3675.
- McMahon MM, Nystrom E, Braunschweig C, Miles J, Compher C; American Society for Parenteral and Enteral Nutrition (ASPEN) Board of Directors; American Society for Parenteral and Enteral Nutrition. American Society of Parenteral and Enteral Nutrition (ASPEN) Board of Directors. A.S.P.E.N. clinical guidelines: nutrition support of adult patients with hyperglycemia. JPEN J Parenter Enteral Nutr 2013; 37:23–36.
- Kim HB, Fauza D, Garza J, Oh JT, Nurko S, Jaksic T. Serial transverse enteroplasty (STEP): a novel bowel lengthening procedure. J Pediatr Surg 2003; 38:425–429.
- King B, Carlson G, Khalil BA, Morabito A. Intestinal bowel lengthening in children with short bowel syndrome: systematic review of the Bianchi and STEP procedures. World J Surg 2013; 37:694–704.
- Matarese LE, O’Keefe SJ, Kandil HM, Costa G, Abu-Elmagd KM. Short bowel syndrome: clinical guidelines for nutrition management. Nutr Clin Pract 2005; 20:493–502.
- Abu-Elmagd KM, Costa G, Bond GJ, et al. Five hundred intestinal and multivisceral transplantations at a single center: major advances with new challenges. Ann Surg 2009; 250:567–581.
- Abu-Elmagd K. The concept of gut rehabilitation and the future of visceral transplantation. Nat Rev Gastroenterol Hepatol 2015; 12:108–120.
- Lillehei RC, Goott B, Miller FA. The physiological response of the small bowel of the dog to ischemia including prolonged in vitro preservation of the bowel with successful replacement and survival. Ann Surg 1959; 150:543–559.
- Starzl TE, Kaupp HA. Mass homotransplantation of abdominal organs in dogs. Surg Forum 1960; 11:28–30.
- O’Keefe SJ, Matarese L. Small bowel transplantation. Curr Gastroenterol Rep 2006; 8:360–366.
- Horslen SP. Optimal management of the post-intestinal transplant patient. Gastroenterology 2006; 130(suppl 1):S163–S169.
- Buchman AL, Scolapio J, Fryer J. AGA technical review on short bowel syndrome and intestinal transplantation. Gastroenterology 2003; 124:1111–1134.
- DiMartini A, Rovera GM, Graham TO, et al. Quality of life after small intestinal transplantation and among home parenteral nutrition patients. JPEN J Parenter Enteral Nutr 1998; 22:357–362.
KEY POINTS
- Some patients with intestinal failure require lifelong parenteral nutrition, which increases the risk of complications such as infection and liver disease. For these patients, intestinal transplant has emerged as a therapeutic option toward the goal of restoring nutritional autonomy.
- The complexities of intestinal failure require collaboration of multiple specialists—gastroenterologists, surgeons, dietitians, nurses, psychiatrists or psychologists, pharmacists, and social workers. This multidisciplinary team is essential to intestinal rehabilitation.
- Dietary modification is the single most effective means of weaning patients safely from parenteral nutrition.
Influenza: Still more important than Zika virus
The mass media and the medical literature have been saturated in the last few years by concerns about a variety of emerging viral epidemics such as Ebola and Zika. We must always remember that influenza will continue to affect many more patients worldwide.
The Cleveland Clinic Journal of Medicine periodically publishes updates on influenza, a topic befitting the large proportion of internists and internal medicine subspecialists who regularly read the Journal. This series began in 1975 with an article by Steven R. Mostow, MD,1 which followed three pandemics that changed the world’s attitude about influenza.
A lot has changed since then, including another pandemic in 2009–2010. Here, I review recent information relevant to daily practice.
NO REASON FOR COMPLACENCY
The relatively mild 2015–2016 influenza season is no reason for complacency this season.
Influenza activity in 2015–2016 was milder than in most seasons in the last decade.2 Activity peaked in mid-March and resulted in fewer outpatient visits, hospitalizations, and deaths than in previous seasons. Influenza A (H1N1)pdm09 has remained the predominant circulating virus since 2009. Although the overall rate of influenza-related hospitalization was less than half that in previous years, the hospitalization rate of middle-aged adults was relatively high (16.8 per 100,000 population). Importantly, 92% of adults with influenza illness that required hospitalization had at least one underlying medical condition, alerting us as healthcare providers that there is plenty of room for improvement in preventing such hospitalizations.
We should remain vigilant. We should put forth our best efforts in vaccinating all individuals above the age of 6 months and in diagnosing influenza early in the course of the illness in order to prescribe antiviral therapy within 48 hours of onset of symptoms. These actions not only shorten the illness and prevent hospitalization and secondary bacterial infection, but also reduce contagion and thus reduce overall healthcare costs.
School closure as a measure to halt epidemics has been lately called into question,3 since there are not enough data to support doing this routinely. School closure in Western Kentucky during the 2013 influenza epidemic did not reduce transmission but caused additional economic and social difficulties for certain households.4
STUDIES REINFORCE EARLIER DATA THAT INFLUENZA VACCINE WORKS
In the several decades since influenza vaccine became available, hundreds of studies have demonstrated the value of the “flu shot.” A few recent papers that support these well-established data:
- In adults who sought medical care for acute respiratory illness, influenza vaccine was 58.4% effective in preventing laboratory-confirmed influenza illness in adults age 50 and older.5
- In the same age group, influenza vaccine was 56.8% effective in preventing laboratory-confirmed influenza hospitalizations.6
- Influenza vaccination in patients with heart failure reduced all-cause hospitalizations, particularly cardiovascular hospitalizations (30% reduction) and hospitalizations for respiratory infections (16% reduction).7 This effect lasted up to 4 months after influenza vaccination.
- Patients who were hospitalized with community-acquired, laboratory-confirmed influenza pneumonia were 43% less likely to have received the influenza vaccine than patients hospitalized with community-acquired pneumonia due to other pathogens.8
INFLUENZA VACCINE IS EVEN MORE VALUABLE DURING PREGNANCY
Influenza vaccination during pregnancy prevented one in five preterm deliveries in a developing country9 and reduced the risk of stillbirth by 50% in Australia.10
An interesting collateral benefit was demonstrated in a survey conducted in Minnesota, where children of mothers who self-reported prenatal influenza vaccination were more likely to complete their routine childhood vaccination series.11
ADDITIONAL BENEFITS OF INFLUENZA VACCINATION
A recently appreciated benefit is that influenza vaccine induces cross-reactive protective immune responses (“heterologous immunity”) to viral strains not included in the vaccine, even in immunosuppressed individuals such as kidney transplant recipients.12 Interestingly, patients were more likely to seroconvert for a cross-reactive “heterologous” antigen if they also seroconverted for the vaccine-specific “homologous” antigen.
In a study in mice, an influenza vaccine with an adjuvant protected mice not only from influenza virus challenge, but also from a Staphylococcus aureus superinfection challenge.13 This novel idea suggests that influenza vaccine protects not only against influenza virus infection, but also against a potentially fatal secondary bacterial infection. This has significant implications for curbing antibacterial use, with an expected reduction in antimicrobial resistance.
Another important benefit of influenza vaccination was recently demonstrated when ferrets were intranasally inoculated with the highly pathogenic influenza A(H5N1) strain and then received either influenza vaccine or prophylactic oseltamivir. Ferrets that received the vaccine were less likely to develop severe meningoencephalitis.14 Since influenza A(H5N1) is much more virulent than the current circulating influenza strains, and since it may be the cause of the next pandemic, preventing such a serious complication of influenza would be lifesaving.
SAFETY OF INFLUENZA VACCINATION
Hundreds of studies involving thousands of people have established the safety of influenza vaccination.
Issues related to Guillain-Barré syndrome have long been put to rest. A large retrospective study found no evidence of increased risk of Guillain-Barré syndrome following vaccination of any kind, including influenza vaccination.15
Local reactions after vaccination are transient and do not interfere with the ability to perform daily activities.
In this era of utilization review, it is reassuring to know that giving influenza vaccine to hospitalized surgical patients was not associated with an increased rate of postdischarge fever or other clinical concern for infection requiring emergency room visits or rehospitalization.16
WHY INFLUENZA VACCINE MAY NOT PREVENT ALL CASES OF INFLUENZA
Whether neutralizing antibodies to influenza virus hemagglutinin antigen should be the main immune correlate of protection for influenza vaccines remains in question. Although prepandemic avian influenza vaccines are poorly immunogenic in inducing neutralizing antibodies, they confer considerable protection. A recent study showed that antibody-dependent cell-mediated cytotoxicity to hemagglutinin antigen in an avian influenza vaccine was a better predictor of protective capacity than neutralizing antibodies.17
Patterns of immunity induced by the live-attenuated influenza vaccine and the inactivated influenza vaccine are different.18 In fact, no single cytokine or chemokine measurement predicts protection.
Even though adults age 50 and older mount statistically significant humoral and cell-mediated immune responses to the inactivated vaccine, two-thirds do not reach hemagglutination inhibition antibody titers of 40 or higher for influenza A(H1N1), and one-fifth do not reach hemagglutination inhibition antibody titers of 40 or higher for influenza A(H3N2).19 While age had some negative effect on vaccine responsiveness, prevaccination titers were much better at predicting postvaccination antibody levels.
ONGOING DEBATE OVER LIVE-ATTENUATED INFLUENZA VACCINE
Several studies had shown that the live-attenuated influenza vaccine, given intranasally, was not only more protective in vaccinated children, but also provided herd protection in unvaccinated contacts. However, a recently published study conducted in Canadian Hutterite children showed that the live-attenuated vaccine did not result in herd immunity when compared to the inactivated influenza vaccine.20
On June 22, 2016, the US Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommended against the use of the live-attenuated vaccine for the 2016–2017 season,21 based on data showing negligible protection conferred by the live-attenuated influenza vaccine in the three preceding influenza seasons.
This decision created significant debate among experts in the field. It is unclear why the live-attenuated influenza vaccine was much less protective in the last three seasons than in prior seasons. Recommending against its use in the United States will essentially eliminate any possibility of reassessing its efficacy in this country. Of note, the quadrivalent live-attenuated influenza vaccine had recently replaced the previous trivalent live-attenuated vaccine, which may have introduced some “competition” among the vaccine strains to infect enough cells to allow viral replication and subsequent immune response. Another potential explanation is that consistent annual vaccination may have resulted in a cumulative immunity that could hamper response to subsequent doses.
COMPOSITION OF THE 2016–2017 INFLUENZA VACCINE
The 2016–2017 quadrivalent inactivated influenza vaccine will contain22:
- A/California/7/2009 (H1N1)pdm09-like virus
- A/Hong Kong/4801/2014 (H3N2)-like virus
- B/Brisbane/60/2008-like virus (B/Victoria lineage)
- B/Phuket/3073/2013-like virus (B/Yamagata lineage).
This represents a change in the A (H3N2) component compared with the 2015–2016 vaccine.
Influenza vaccine manufacturers estimated they would produce 170 million doses for distribution in the United States for the upcoming influenza season. The previously mentioned recommendation against the use of the live-attenuated vaccine, which accounts for approximately 8% of the influenza vaccine supply, may affect vaccine uptake, particularly in children.
NEW ANTI-INFLUENZA AGENTS AND UPDATE ON EXISTING AGENTS
Neuraminidase inhibitors are the only class of antiviral drugs currently recommended for prevention and treatment of influenza. The three products currently available in the United States are oseltamivir, zanamivir, and peramivir. Oseltamivir is administered orally, and the first generic version was approved by the US Food and Drug Administration on August 3, 2016. Zanamivir is administered by oral inhalation. Both oseltamivir and zanamivir are approved for treatment and prevention of influenza. Peramivir is administered intravenously as a single dose and is approved only for the treatment of acute influenza, not prevention.
Unfortunately, the influenza vaccination rate during pregnancy in the United States remains only around 50%.23 Physicians’ recommendations are strongly associated with vaccine uptake, particularly when they emphasize protective effect on the newborn. Influenza during pregnancy carries higher mortality than in the general population, with collateral fetal loss.
Early initiation of antiviral therapy is particularly imperative during pregnancy. A recent study showed that starting antiviral therapy within 2 days of onset of illness in pregnant women hospitalized with severe influenza reduced length of stay by 5.6 days compared with those in whom therapy was started more than 2 days after illness onset.24
A single dose of laninamivir octanoate, a long-acting neuraminidase inhibitor currently approved in Japan for treating influenza, was recently shown to be effective as postexposure prophylaxis.25 This option may be convenient for people who prefer not to take a daily medication for several days, or in an outbreak in a healthcare facility.
- Mostow SR. Current perspectives of influenza. Cleve Clin J Med 1975; 42:63–70.
- Davlin SL, Blanton L, Kniss K, et al. Influenza activity — United States, 2015–16 season and composition of the 2016–17 influenza vaccine. MMWR Morb Mortal Wkly Rep 2016; 65:567–575.
- Sasaki A, Hoen AG, Al Ozonoff A, et al. Evidence-based tool for triggering school closures during influenza outbreaks, Japan. Emerg Infect Dis 2009; 15:1841–1843.
- Russell ES, Zheteyeva Y, Gao H, et al. Reactive school closure during increased influenza-like Illness (ILI) activity in Western Kentucky, 2013: a field evaluation of effect on ILI incidence and economic and social consequences for families. Open Forum Infect Dis (Summer 2016) 3 (3): first published online May 25, 2016. doi:10.1093/ofid/ofw113.
- Chen Q, Griffin MR, Nian H, et al. Influenza vaccine prevents medically attended influenza-associated acute respiratory illness in adults aged ≥ 50 years. J Infect Dis 2015, 211:1045–1050.
- Havers FP, Sokolow L, Shay DK, et al. Case-control study of vaccine effectiveness in preventing laboratory-confirmed influenza hospitalizations in older adults, United States, 2010–11. Clin Infect Dis 2016. [Epub ahead of print.].
- Influenza vaccination linked to fewer CV, respiratory hospitalizations in patients with HF. Helio Cardiology Today, May 25, 2016. www.healio.com/cardiology/hf-transplantation/news/online/%7B5292db4f-fc81-43f2-a28d-c124f2a6331b%7D/influenza-vaccination-linked-to-fewer-cv-respiratory-hospitalizations-in-patients-with-hf. Accessed October 6, 2016.
- Grijalva CG, Zhu Y, Williams DJ, et al. Association between hospitalization with community-acquired laboratory-confirmed influenza pneumonia and prior receipt of influenza vaccination. JAMA 2015, 314:1488–1497.
- Olsen SJ, Mirza SA, Vonglokham P, et al. The effect of influenza vaccination on birth outcomes in a cohort of pregnant women in Lao PDR, 2014–2015. Clin Infect Dis 2016; 63:487–494.
- Regan AK, Moore HC, de Klerk N, et al. Seasonal trivalent influenza vaccination during pregnancy and the incidence of stillbirth: population-based retrospective cohort study. Clin Infect Dis 2016; 62:1221–1227.
- Fuchs EL. Self-reported prenatal influenza vaccination and early childhood vaccine series completion. Prev Med 2016; 88:8–12.
- Kumar D, Ferreira VH, Campbell P, Hoschler K, Humar A. Heterologous immune responses to influenza vaccine in kidney transplant recipients. Am J Transplant 2016; accepted manuscript online: 12 Jul 2016; doi: 10.1111/ajt.13960. [Epub ahead of print.]
- Zurli V, Gallotta M, Taccone M, et al. Positive contribution of adjuvanted influenza vaccines to the resolution of bacterial superinfections. J Infect Dis 2016; 213:1876–1885.
- Siegers JY, van den Brand JM, Leijten LM, et al. Vaccination is more effective than prophylactic oseltamivir in preventing CNS invasion by H5N1 virus via the olfactory nerve. J Infect Dis 2016; 214:516–524.
- Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis 2013; 57:197–204.
- Tartof SY, Qian L, Rieg G, et al. Safety of seasonal Influenza vaccination in hospitalized surgical patients: a cohort study. Ann Intern Med 2016; 213:1876–1885.
- Zhong W, Liu F, Wilson JR, et al. Antibody-dependent cell-mediated cytotoxicity to hemagglutinin of influenza A viruses after influenza vaccination in humans. Open Forum Infect Dis 2016; 3: doi:10.1093/ofid/ofw102.
- Wright PF, Hoen AG, Ilyushina NA, et al. Correlates of immunity to influenza as determined by challenge of children with live, attenuated influenza vaccine. Open Forum Infect Dis 2016; 3: doi:10.1093/ofid/ofw108.
- Reber AJ, Kim JH, Biber R, et al. Preexisting immunity, more than aging, influences influenza vaccine responses. Open Forum Infect Dis 2015; 2 doi:10.1093/ofid/ofv052.
- Loeb M, Russell ML, Manning V, et al. Live attenuated versus inactivated influenza vaccine in Hutterite children: a cluster randomized blinded trial. Ann Intern Med published online August 16, 2016. doi:10.7326/M16-0513.
- CDC Newsroom. ACIP votes down use of LAIV for 2016-2017 flu season. June 22, 2016. www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Accessed October 6, 2016.
- Davlin SL, Blanton L, Kniss K, et al. Influenza activity—United States, 2015–16 season and composition of the 2016–17 influenza vaccine. MMWR Morb Mortal Wkly Rep 2016; 65:567–575.
- Goodman K, Mossad SB, Taksler GB, et al. Impact of video education on influenza vaccination in pregnancy. J Reprod Med 2015; 60:471–479.
- Oboho IK, Reed C, Gargiullo P, et al. Benefit of early initiation of influenza antiviral treatment to pregnant women hospitalized with laboratory-confirmed influenza. J Infect Dis 2016; 214:507–515.
- Kashiwagi S, Watanabe A, Ikematsu H, Uemori M, Awamura S, for the Laninamivir Prophylaxis Study Group. Long-acting neuraminidase inhibitor laninamivir octanoate as post-exposure prophylaxis for influenza. Clin Infect Dis 2016; 63:330–337.
The mass media and the medical literature have been saturated in the last few years by concerns about a variety of emerging viral epidemics such as Ebola and Zika. We must always remember that influenza will continue to affect many more patients worldwide.
The Cleveland Clinic Journal of Medicine periodically publishes updates on influenza, a topic befitting the large proportion of internists and internal medicine subspecialists who regularly read the Journal. This series began in 1975 with an article by Steven R. Mostow, MD,1 which followed three pandemics that changed the world’s attitude about influenza.
A lot has changed since then, including another pandemic in 2009–2010. Here, I review recent information relevant to daily practice.
NO REASON FOR COMPLACENCY
The relatively mild 2015–2016 influenza season is no reason for complacency this season.
Influenza activity in 2015–2016 was milder than in most seasons in the last decade.2 Activity peaked in mid-March and resulted in fewer outpatient visits, hospitalizations, and deaths than in previous seasons. Influenza A (H1N1)pdm09 has remained the predominant circulating virus since 2009. Although the overall rate of influenza-related hospitalization was less than half that in previous years, the hospitalization rate of middle-aged adults was relatively high (16.8 per 100,000 population). Importantly, 92% of adults with influenza illness that required hospitalization had at least one underlying medical condition, alerting us as healthcare providers that there is plenty of room for improvement in preventing such hospitalizations.
We should remain vigilant. We should put forth our best efforts in vaccinating all individuals above the age of 6 months and in diagnosing influenza early in the course of the illness in order to prescribe antiviral therapy within 48 hours of onset of symptoms. These actions not only shorten the illness and prevent hospitalization and secondary bacterial infection, but also reduce contagion and thus reduce overall healthcare costs.
School closure as a measure to halt epidemics has been lately called into question,3 since there are not enough data to support doing this routinely. School closure in Western Kentucky during the 2013 influenza epidemic did not reduce transmission but caused additional economic and social difficulties for certain households.4
STUDIES REINFORCE EARLIER DATA THAT INFLUENZA VACCINE WORKS
In the several decades since influenza vaccine became available, hundreds of studies have demonstrated the value of the “flu shot.” A few recent papers that support these well-established data:
- In adults who sought medical care for acute respiratory illness, influenza vaccine was 58.4% effective in preventing laboratory-confirmed influenza illness in adults age 50 and older.5
- In the same age group, influenza vaccine was 56.8% effective in preventing laboratory-confirmed influenza hospitalizations.6
- Influenza vaccination in patients with heart failure reduced all-cause hospitalizations, particularly cardiovascular hospitalizations (30% reduction) and hospitalizations for respiratory infections (16% reduction).7 This effect lasted up to 4 months after influenza vaccination.
- Patients who were hospitalized with community-acquired, laboratory-confirmed influenza pneumonia were 43% less likely to have received the influenza vaccine than patients hospitalized with community-acquired pneumonia due to other pathogens.8
INFLUENZA VACCINE IS EVEN MORE VALUABLE DURING PREGNANCY
Influenza vaccination during pregnancy prevented one in five preterm deliveries in a developing country9 and reduced the risk of stillbirth by 50% in Australia.10
An interesting collateral benefit was demonstrated in a survey conducted in Minnesota, where children of mothers who self-reported prenatal influenza vaccination were more likely to complete their routine childhood vaccination series.11
ADDITIONAL BENEFITS OF INFLUENZA VACCINATION
A recently appreciated benefit is that influenza vaccine induces cross-reactive protective immune responses (“heterologous immunity”) to viral strains not included in the vaccine, even in immunosuppressed individuals such as kidney transplant recipients.12 Interestingly, patients were more likely to seroconvert for a cross-reactive “heterologous” antigen if they also seroconverted for the vaccine-specific “homologous” antigen.
In a study in mice, an influenza vaccine with an adjuvant protected mice not only from influenza virus challenge, but also from a Staphylococcus aureus superinfection challenge.13 This novel idea suggests that influenza vaccine protects not only against influenza virus infection, but also against a potentially fatal secondary bacterial infection. This has significant implications for curbing antibacterial use, with an expected reduction in antimicrobial resistance.
Another important benefit of influenza vaccination was recently demonstrated when ferrets were intranasally inoculated with the highly pathogenic influenza A(H5N1) strain and then received either influenza vaccine or prophylactic oseltamivir. Ferrets that received the vaccine were less likely to develop severe meningoencephalitis.14 Since influenza A(H5N1) is much more virulent than the current circulating influenza strains, and since it may be the cause of the next pandemic, preventing such a serious complication of influenza would be lifesaving.
SAFETY OF INFLUENZA VACCINATION
Hundreds of studies involving thousands of people have established the safety of influenza vaccination.
Issues related to Guillain-Barré syndrome have long been put to rest. A large retrospective study found no evidence of increased risk of Guillain-Barré syndrome following vaccination of any kind, including influenza vaccination.15
Local reactions after vaccination are transient and do not interfere with the ability to perform daily activities.
In this era of utilization review, it is reassuring to know that giving influenza vaccine to hospitalized surgical patients was not associated with an increased rate of postdischarge fever or other clinical concern for infection requiring emergency room visits or rehospitalization.16
WHY INFLUENZA VACCINE MAY NOT PREVENT ALL CASES OF INFLUENZA
Whether neutralizing antibodies to influenza virus hemagglutinin antigen should be the main immune correlate of protection for influenza vaccines remains in question. Although prepandemic avian influenza vaccines are poorly immunogenic in inducing neutralizing antibodies, they confer considerable protection. A recent study showed that antibody-dependent cell-mediated cytotoxicity to hemagglutinin antigen in an avian influenza vaccine was a better predictor of protective capacity than neutralizing antibodies.17
Patterns of immunity induced by the live-attenuated influenza vaccine and the inactivated influenza vaccine are different.18 In fact, no single cytokine or chemokine measurement predicts protection.
Even though adults age 50 and older mount statistically significant humoral and cell-mediated immune responses to the inactivated vaccine, two-thirds do not reach hemagglutination inhibition antibody titers of 40 or higher for influenza A(H1N1), and one-fifth do not reach hemagglutination inhibition antibody titers of 40 or higher for influenza A(H3N2).19 While age had some negative effect on vaccine responsiveness, prevaccination titers were much better at predicting postvaccination antibody levels.
ONGOING DEBATE OVER LIVE-ATTENUATED INFLUENZA VACCINE
Several studies had shown that the live-attenuated influenza vaccine, given intranasally, was not only more protective in vaccinated children, but also provided herd protection in unvaccinated contacts. However, a recently published study conducted in Canadian Hutterite children showed that the live-attenuated vaccine did not result in herd immunity when compared to the inactivated influenza vaccine.20
On June 22, 2016, the US Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommended against the use of the live-attenuated vaccine for the 2016–2017 season,21 based on data showing negligible protection conferred by the live-attenuated influenza vaccine in the three preceding influenza seasons.
This decision created significant debate among experts in the field. It is unclear why the live-attenuated influenza vaccine was much less protective in the last three seasons than in prior seasons. Recommending against its use in the United States will essentially eliminate any possibility of reassessing its efficacy in this country. Of note, the quadrivalent live-attenuated influenza vaccine had recently replaced the previous trivalent live-attenuated vaccine, which may have introduced some “competition” among the vaccine strains to infect enough cells to allow viral replication and subsequent immune response. Another potential explanation is that consistent annual vaccination may have resulted in a cumulative immunity that could hamper response to subsequent doses.
COMPOSITION OF THE 2016–2017 INFLUENZA VACCINE
The 2016–2017 quadrivalent inactivated influenza vaccine will contain22:
- A/California/7/2009 (H1N1)pdm09-like virus
- A/Hong Kong/4801/2014 (H3N2)-like virus
- B/Brisbane/60/2008-like virus (B/Victoria lineage)
- B/Phuket/3073/2013-like virus (B/Yamagata lineage).
This represents a change in the A (H3N2) component compared with the 2015–2016 vaccine.
Influenza vaccine manufacturers estimated they would produce 170 million doses for distribution in the United States for the upcoming influenza season. The previously mentioned recommendation against the use of the live-attenuated vaccine, which accounts for approximately 8% of the influenza vaccine supply, may affect vaccine uptake, particularly in children.
NEW ANTI-INFLUENZA AGENTS AND UPDATE ON EXISTING AGENTS
Neuraminidase inhibitors are the only class of antiviral drugs currently recommended for prevention and treatment of influenza. The three products currently available in the United States are oseltamivir, zanamivir, and peramivir. Oseltamivir is administered orally, and the first generic version was approved by the US Food and Drug Administration on August 3, 2016. Zanamivir is administered by oral inhalation. Both oseltamivir and zanamivir are approved for treatment and prevention of influenza. Peramivir is administered intravenously as a single dose and is approved only for the treatment of acute influenza, not prevention.
Unfortunately, the influenza vaccination rate during pregnancy in the United States remains only around 50%.23 Physicians’ recommendations are strongly associated with vaccine uptake, particularly when they emphasize protective effect on the newborn. Influenza during pregnancy carries higher mortality than in the general population, with collateral fetal loss.
Early initiation of antiviral therapy is particularly imperative during pregnancy. A recent study showed that starting antiviral therapy within 2 days of onset of illness in pregnant women hospitalized with severe influenza reduced length of stay by 5.6 days compared with those in whom therapy was started more than 2 days after illness onset.24
A single dose of laninamivir octanoate, a long-acting neuraminidase inhibitor currently approved in Japan for treating influenza, was recently shown to be effective as postexposure prophylaxis.25 This option may be convenient for people who prefer not to take a daily medication for several days, or in an outbreak in a healthcare facility.
The mass media and the medical literature have been saturated in the last few years by concerns about a variety of emerging viral epidemics such as Ebola and Zika. We must always remember that influenza will continue to affect many more patients worldwide.
The Cleveland Clinic Journal of Medicine periodically publishes updates on influenza, a topic befitting the large proportion of internists and internal medicine subspecialists who regularly read the Journal. This series began in 1975 with an article by Steven R. Mostow, MD,1 which followed three pandemics that changed the world’s attitude about influenza.
A lot has changed since then, including another pandemic in 2009–2010. Here, I review recent information relevant to daily practice.
NO REASON FOR COMPLACENCY
The relatively mild 2015–2016 influenza season is no reason for complacency this season.
Influenza activity in 2015–2016 was milder than in most seasons in the last decade.2 Activity peaked in mid-March and resulted in fewer outpatient visits, hospitalizations, and deaths than in previous seasons. Influenza A (H1N1)pdm09 has remained the predominant circulating virus since 2009. Although the overall rate of influenza-related hospitalization was less than half that in previous years, the hospitalization rate of middle-aged adults was relatively high (16.8 per 100,000 population). Importantly, 92% of adults with influenza illness that required hospitalization had at least one underlying medical condition, alerting us as healthcare providers that there is plenty of room for improvement in preventing such hospitalizations.
We should remain vigilant. We should put forth our best efforts in vaccinating all individuals above the age of 6 months and in diagnosing influenza early in the course of the illness in order to prescribe antiviral therapy within 48 hours of onset of symptoms. These actions not only shorten the illness and prevent hospitalization and secondary bacterial infection, but also reduce contagion and thus reduce overall healthcare costs.
School closure as a measure to halt epidemics has been lately called into question,3 since there are not enough data to support doing this routinely. School closure in Western Kentucky during the 2013 influenza epidemic did not reduce transmission but caused additional economic and social difficulties for certain households.4
STUDIES REINFORCE EARLIER DATA THAT INFLUENZA VACCINE WORKS
In the several decades since influenza vaccine became available, hundreds of studies have demonstrated the value of the “flu shot.” A few recent papers that support these well-established data:
- In adults who sought medical care for acute respiratory illness, influenza vaccine was 58.4% effective in preventing laboratory-confirmed influenza illness in adults age 50 and older.5
- In the same age group, influenza vaccine was 56.8% effective in preventing laboratory-confirmed influenza hospitalizations.6
- Influenza vaccination in patients with heart failure reduced all-cause hospitalizations, particularly cardiovascular hospitalizations (30% reduction) and hospitalizations for respiratory infections (16% reduction).7 This effect lasted up to 4 months after influenza vaccination.
- Patients who were hospitalized with community-acquired, laboratory-confirmed influenza pneumonia were 43% less likely to have received the influenza vaccine than patients hospitalized with community-acquired pneumonia due to other pathogens.8
INFLUENZA VACCINE IS EVEN MORE VALUABLE DURING PREGNANCY
Influenza vaccination during pregnancy prevented one in five preterm deliveries in a developing country9 and reduced the risk of stillbirth by 50% in Australia.10
An interesting collateral benefit was demonstrated in a survey conducted in Minnesota, where children of mothers who self-reported prenatal influenza vaccination were more likely to complete their routine childhood vaccination series.11
ADDITIONAL BENEFITS OF INFLUENZA VACCINATION
A recently appreciated benefit is that influenza vaccine induces cross-reactive protective immune responses (“heterologous immunity”) to viral strains not included in the vaccine, even in immunosuppressed individuals such as kidney transplant recipients.12 Interestingly, patients were more likely to seroconvert for a cross-reactive “heterologous” antigen if they also seroconverted for the vaccine-specific “homologous” antigen.
In a study in mice, an influenza vaccine with an adjuvant protected mice not only from influenza virus challenge, but also from a Staphylococcus aureus superinfection challenge.13 This novel idea suggests that influenza vaccine protects not only against influenza virus infection, but also against a potentially fatal secondary bacterial infection. This has significant implications for curbing antibacterial use, with an expected reduction in antimicrobial resistance.
Another important benefit of influenza vaccination was recently demonstrated when ferrets were intranasally inoculated with the highly pathogenic influenza A(H5N1) strain and then received either influenza vaccine or prophylactic oseltamivir. Ferrets that received the vaccine were less likely to develop severe meningoencephalitis.14 Since influenza A(H5N1) is much more virulent than the current circulating influenza strains, and since it may be the cause of the next pandemic, preventing such a serious complication of influenza would be lifesaving.
SAFETY OF INFLUENZA VACCINATION
Hundreds of studies involving thousands of people have established the safety of influenza vaccination.
Issues related to Guillain-Barré syndrome have long been put to rest. A large retrospective study found no evidence of increased risk of Guillain-Barré syndrome following vaccination of any kind, including influenza vaccination.15
Local reactions after vaccination are transient and do not interfere with the ability to perform daily activities.
In this era of utilization review, it is reassuring to know that giving influenza vaccine to hospitalized surgical patients was not associated with an increased rate of postdischarge fever or other clinical concern for infection requiring emergency room visits or rehospitalization.16
WHY INFLUENZA VACCINE MAY NOT PREVENT ALL CASES OF INFLUENZA
Whether neutralizing antibodies to influenza virus hemagglutinin antigen should be the main immune correlate of protection for influenza vaccines remains in question. Although prepandemic avian influenza vaccines are poorly immunogenic in inducing neutralizing antibodies, they confer considerable protection. A recent study showed that antibody-dependent cell-mediated cytotoxicity to hemagglutinin antigen in an avian influenza vaccine was a better predictor of protective capacity than neutralizing antibodies.17
Patterns of immunity induced by the live-attenuated influenza vaccine and the inactivated influenza vaccine are different.18 In fact, no single cytokine or chemokine measurement predicts protection.
Even though adults age 50 and older mount statistically significant humoral and cell-mediated immune responses to the inactivated vaccine, two-thirds do not reach hemagglutination inhibition antibody titers of 40 or higher for influenza A(H1N1), and one-fifth do not reach hemagglutination inhibition antibody titers of 40 or higher for influenza A(H3N2).19 While age had some negative effect on vaccine responsiveness, prevaccination titers were much better at predicting postvaccination antibody levels.
ONGOING DEBATE OVER LIVE-ATTENUATED INFLUENZA VACCINE
Several studies had shown that the live-attenuated influenza vaccine, given intranasally, was not only more protective in vaccinated children, but also provided herd protection in unvaccinated contacts. However, a recently published study conducted in Canadian Hutterite children showed that the live-attenuated vaccine did not result in herd immunity when compared to the inactivated influenza vaccine.20
On June 22, 2016, the US Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommended against the use of the live-attenuated vaccine for the 2016–2017 season,21 based on data showing negligible protection conferred by the live-attenuated influenza vaccine in the three preceding influenza seasons.
This decision created significant debate among experts in the field. It is unclear why the live-attenuated influenza vaccine was much less protective in the last three seasons than in prior seasons. Recommending against its use in the United States will essentially eliminate any possibility of reassessing its efficacy in this country. Of note, the quadrivalent live-attenuated influenza vaccine had recently replaced the previous trivalent live-attenuated vaccine, which may have introduced some “competition” among the vaccine strains to infect enough cells to allow viral replication and subsequent immune response. Another potential explanation is that consistent annual vaccination may have resulted in a cumulative immunity that could hamper response to subsequent doses.
COMPOSITION OF THE 2016–2017 INFLUENZA VACCINE
The 2016–2017 quadrivalent inactivated influenza vaccine will contain22:
- A/California/7/2009 (H1N1)pdm09-like virus
- A/Hong Kong/4801/2014 (H3N2)-like virus
- B/Brisbane/60/2008-like virus (B/Victoria lineage)
- B/Phuket/3073/2013-like virus (B/Yamagata lineage).
This represents a change in the A (H3N2) component compared with the 2015–2016 vaccine.
Influenza vaccine manufacturers estimated they would produce 170 million doses for distribution in the United States for the upcoming influenza season. The previously mentioned recommendation against the use of the live-attenuated vaccine, which accounts for approximately 8% of the influenza vaccine supply, may affect vaccine uptake, particularly in children.
NEW ANTI-INFLUENZA AGENTS AND UPDATE ON EXISTING AGENTS
Neuraminidase inhibitors are the only class of antiviral drugs currently recommended for prevention and treatment of influenza. The three products currently available in the United States are oseltamivir, zanamivir, and peramivir. Oseltamivir is administered orally, and the first generic version was approved by the US Food and Drug Administration on August 3, 2016. Zanamivir is administered by oral inhalation. Both oseltamivir and zanamivir are approved for treatment and prevention of influenza. Peramivir is administered intravenously as a single dose and is approved only for the treatment of acute influenza, not prevention.
Unfortunately, the influenza vaccination rate during pregnancy in the United States remains only around 50%.23 Physicians’ recommendations are strongly associated with vaccine uptake, particularly when they emphasize protective effect on the newborn. Influenza during pregnancy carries higher mortality than in the general population, with collateral fetal loss.
Early initiation of antiviral therapy is particularly imperative during pregnancy. A recent study showed that starting antiviral therapy within 2 days of onset of illness in pregnant women hospitalized with severe influenza reduced length of stay by 5.6 days compared with those in whom therapy was started more than 2 days after illness onset.24
A single dose of laninamivir octanoate, a long-acting neuraminidase inhibitor currently approved in Japan for treating influenza, was recently shown to be effective as postexposure prophylaxis.25 This option may be convenient for people who prefer not to take a daily medication for several days, or in an outbreak in a healthcare facility.
- Mostow SR. Current perspectives of influenza. Cleve Clin J Med 1975; 42:63–70.
- Davlin SL, Blanton L, Kniss K, et al. Influenza activity — United States, 2015–16 season and composition of the 2016–17 influenza vaccine. MMWR Morb Mortal Wkly Rep 2016; 65:567–575.
- Sasaki A, Hoen AG, Al Ozonoff A, et al. Evidence-based tool for triggering school closures during influenza outbreaks, Japan. Emerg Infect Dis 2009; 15:1841–1843.
- Russell ES, Zheteyeva Y, Gao H, et al. Reactive school closure during increased influenza-like Illness (ILI) activity in Western Kentucky, 2013: a field evaluation of effect on ILI incidence and economic and social consequences for families. Open Forum Infect Dis (Summer 2016) 3 (3): first published online May 25, 2016. doi:10.1093/ofid/ofw113.
- Chen Q, Griffin MR, Nian H, et al. Influenza vaccine prevents medically attended influenza-associated acute respiratory illness in adults aged ≥ 50 years. J Infect Dis 2015, 211:1045–1050.
- Havers FP, Sokolow L, Shay DK, et al. Case-control study of vaccine effectiveness in preventing laboratory-confirmed influenza hospitalizations in older adults, United States, 2010–11. Clin Infect Dis 2016. [Epub ahead of print.].
- Influenza vaccination linked to fewer CV, respiratory hospitalizations in patients with HF. Helio Cardiology Today, May 25, 2016. www.healio.com/cardiology/hf-transplantation/news/online/%7B5292db4f-fc81-43f2-a28d-c124f2a6331b%7D/influenza-vaccination-linked-to-fewer-cv-respiratory-hospitalizations-in-patients-with-hf. Accessed October 6, 2016.
- Grijalva CG, Zhu Y, Williams DJ, et al. Association between hospitalization with community-acquired laboratory-confirmed influenza pneumonia and prior receipt of influenza vaccination. JAMA 2015, 314:1488–1497.
- Olsen SJ, Mirza SA, Vonglokham P, et al. The effect of influenza vaccination on birth outcomes in a cohort of pregnant women in Lao PDR, 2014–2015. Clin Infect Dis 2016; 63:487–494.
- Regan AK, Moore HC, de Klerk N, et al. Seasonal trivalent influenza vaccination during pregnancy and the incidence of stillbirth: population-based retrospective cohort study. Clin Infect Dis 2016; 62:1221–1227.
- Fuchs EL. Self-reported prenatal influenza vaccination and early childhood vaccine series completion. Prev Med 2016; 88:8–12.
- Kumar D, Ferreira VH, Campbell P, Hoschler K, Humar A. Heterologous immune responses to influenza vaccine in kidney transplant recipients. Am J Transplant 2016; accepted manuscript online: 12 Jul 2016; doi: 10.1111/ajt.13960. [Epub ahead of print.]
- Zurli V, Gallotta M, Taccone M, et al. Positive contribution of adjuvanted influenza vaccines to the resolution of bacterial superinfections. J Infect Dis 2016; 213:1876–1885.
- Siegers JY, van den Brand JM, Leijten LM, et al. Vaccination is more effective than prophylactic oseltamivir in preventing CNS invasion by H5N1 virus via the olfactory nerve. J Infect Dis 2016; 214:516–524.
- Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis 2013; 57:197–204.
- Tartof SY, Qian L, Rieg G, et al. Safety of seasonal Influenza vaccination in hospitalized surgical patients: a cohort study. Ann Intern Med 2016; 213:1876–1885.
- Zhong W, Liu F, Wilson JR, et al. Antibody-dependent cell-mediated cytotoxicity to hemagglutinin of influenza A viruses after influenza vaccination in humans. Open Forum Infect Dis 2016; 3: doi:10.1093/ofid/ofw102.
- Wright PF, Hoen AG, Ilyushina NA, et al. Correlates of immunity to influenza as determined by challenge of children with live, attenuated influenza vaccine. Open Forum Infect Dis 2016; 3: doi:10.1093/ofid/ofw108.
- Reber AJ, Kim JH, Biber R, et al. Preexisting immunity, more than aging, influences influenza vaccine responses. Open Forum Infect Dis 2015; 2 doi:10.1093/ofid/ofv052.
- Loeb M, Russell ML, Manning V, et al. Live attenuated versus inactivated influenza vaccine in Hutterite children: a cluster randomized blinded trial. Ann Intern Med published online August 16, 2016. doi:10.7326/M16-0513.
- CDC Newsroom. ACIP votes down use of LAIV for 2016-2017 flu season. June 22, 2016. www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Accessed October 6, 2016.
- Davlin SL, Blanton L, Kniss K, et al. Influenza activity—United States, 2015–16 season and composition of the 2016–17 influenza vaccine. MMWR Morb Mortal Wkly Rep 2016; 65:567–575.
- Goodman K, Mossad SB, Taksler GB, et al. Impact of video education on influenza vaccination in pregnancy. J Reprod Med 2015; 60:471–479.
- Oboho IK, Reed C, Gargiullo P, et al. Benefit of early initiation of influenza antiviral treatment to pregnant women hospitalized with laboratory-confirmed influenza. J Infect Dis 2016; 214:507–515.
- Kashiwagi S, Watanabe A, Ikematsu H, Uemori M, Awamura S, for the Laninamivir Prophylaxis Study Group. Long-acting neuraminidase inhibitor laninamivir octanoate as post-exposure prophylaxis for influenza. Clin Infect Dis 2016; 63:330–337.
- Mostow SR. Current perspectives of influenza. Cleve Clin J Med 1975; 42:63–70.
- Davlin SL, Blanton L, Kniss K, et al. Influenza activity — United States, 2015–16 season and composition of the 2016–17 influenza vaccine. MMWR Morb Mortal Wkly Rep 2016; 65:567–575.
- Sasaki A, Hoen AG, Al Ozonoff A, et al. Evidence-based tool for triggering school closures during influenza outbreaks, Japan. Emerg Infect Dis 2009; 15:1841–1843.
- Russell ES, Zheteyeva Y, Gao H, et al. Reactive school closure during increased influenza-like Illness (ILI) activity in Western Kentucky, 2013: a field evaluation of effect on ILI incidence and economic and social consequences for families. Open Forum Infect Dis (Summer 2016) 3 (3): first published online May 25, 2016. doi:10.1093/ofid/ofw113.
- Chen Q, Griffin MR, Nian H, et al. Influenza vaccine prevents medically attended influenza-associated acute respiratory illness in adults aged ≥ 50 years. J Infect Dis 2015, 211:1045–1050.
- Havers FP, Sokolow L, Shay DK, et al. Case-control study of vaccine effectiveness in preventing laboratory-confirmed influenza hospitalizations in older adults, United States, 2010–11. Clin Infect Dis 2016. [Epub ahead of print.].
- Influenza vaccination linked to fewer CV, respiratory hospitalizations in patients with HF. Helio Cardiology Today, May 25, 2016. www.healio.com/cardiology/hf-transplantation/news/online/%7B5292db4f-fc81-43f2-a28d-c124f2a6331b%7D/influenza-vaccination-linked-to-fewer-cv-respiratory-hospitalizations-in-patients-with-hf. Accessed October 6, 2016.
- Grijalva CG, Zhu Y, Williams DJ, et al. Association between hospitalization with community-acquired laboratory-confirmed influenza pneumonia and prior receipt of influenza vaccination. JAMA 2015, 314:1488–1497.
- Olsen SJ, Mirza SA, Vonglokham P, et al. The effect of influenza vaccination on birth outcomes in a cohort of pregnant women in Lao PDR, 2014–2015. Clin Infect Dis 2016; 63:487–494.
- Regan AK, Moore HC, de Klerk N, et al. Seasonal trivalent influenza vaccination during pregnancy and the incidence of stillbirth: population-based retrospective cohort study. Clin Infect Dis 2016; 62:1221–1227.
- Fuchs EL. Self-reported prenatal influenza vaccination and early childhood vaccine series completion. Prev Med 2016; 88:8–12.
- Kumar D, Ferreira VH, Campbell P, Hoschler K, Humar A. Heterologous immune responses to influenza vaccine in kidney transplant recipients. Am J Transplant 2016; accepted manuscript online: 12 Jul 2016; doi: 10.1111/ajt.13960. [Epub ahead of print.]
- Zurli V, Gallotta M, Taccone M, et al. Positive contribution of adjuvanted influenza vaccines to the resolution of bacterial superinfections. J Infect Dis 2016; 213:1876–1885.
- Siegers JY, van den Brand JM, Leijten LM, et al. Vaccination is more effective than prophylactic oseltamivir in preventing CNS invasion by H5N1 virus via the olfactory nerve. J Infect Dis 2016; 214:516–524.
- Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis 2013; 57:197–204.
- Tartof SY, Qian L, Rieg G, et al. Safety of seasonal Influenza vaccination in hospitalized surgical patients: a cohort study. Ann Intern Med 2016; 213:1876–1885.
- Zhong W, Liu F, Wilson JR, et al. Antibody-dependent cell-mediated cytotoxicity to hemagglutinin of influenza A viruses after influenza vaccination in humans. Open Forum Infect Dis 2016; 3: doi:10.1093/ofid/ofw102.
- Wright PF, Hoen AG, Ilyushina NA, et al. Correlates of immunity to influenza as determined by challenge of children with live, attenuated influenza vaccine. Open Forum Infect Dis 2016; 3: doi:10.1093/ofid/ofw108.
- Reber AJ, Kim JH, Biber R, et al. Preexisting immunity, more than aging, influences influenza vaccine responses. Open Forum Infect Dis 2015; 2 doi:10.1093/ofid/ofv052.
- Loeb M, Russell ML, Manning V, et al. Live attenuated versus inactivated influenza vaccine in Hutterite children: a cluster randomized blinded trial. Ann Intern Med published online August 16, 2016. doi:10.7326/M16-0513.
- CDC Newsroom. ACIP votes down use of LAIV for 2016-2017 flu season. June 22, 2016. www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Accessed October 6, 2016.
- Davlin SL, Blanton L, Kniss K, et al. Influenza activity—United States, 2015–16 season and composition of the 2016–17 influenza vaccine. MMWR Morb Mortal Wkly Rep 2016; 65:567–575.
- Goodman K, Mossad SB, Taksler GB, et al. Impact of video education on influenza vaccination in pregnancy. J Reprod Med 2015; 60:471–479.
- Oboho IK, Reed C, Gargiullo P, et al. Benefit of early initiation of influenza antiviral treatment to pregnant women hospitalized with laboratory-confirmed influenza. J Infect Dis 2016; 214:507–515.
- Kashiwagi S, Watanabe A, Ikematsu H, Uemori M, Awamura S, for the Laninamivir Prophylaxis Study Group. Long-acting neuraminidase inhibitor laninamivir octanoate as post-exposure prophylaxis for influenza. Clin Infect Dis 2016; 63:330–337.
KEY POINTS
- Influenza vaccine remains the most effective way to prevent influenza. Healthcare providers should continue to vaccinate all people older than 6 months.
- For the 2016–2017 influenza season, only the inactivated influenza vaccine, not the live-attenuated vaccine, is recommended, regardless of age group or underlying disease.
- Early initiation of a neuraminidase inhibitor is advised for an influenza-like illness while awaiting a confirmatory diagnostic test.
VIDEO: Two PCI vs. CABG trials produce conflicting results
WASHINGTON – Results from two large, multicenter comparisons of coronary stenting and coronary bypass surgery for treating patients with unprotected left main coronary disease may have superficially shown sharp differences, but the bottom line will likely be greater empowerment of percutaneous coronary intervention as an option for selected patients with less complex coronary disease.
Prior to the results from the EXCEL and NOBLE trials, reported at the Transcatheter Cardiovascular Therapeutics annual meeting, “guidelines put PCI [percutaneous coronary intervention] into a class 1, 2 or 3 status for treating left main coronary disease depending on disease complexity, but in the United States, PCI for patients eligible for CABG [coronary artery bypass grafting] has not been frequently done. I think these results, in a very circumscribed subset of patients and using a state-of-the-art stent, will affect the guidelines,” predicted Gregg W. Stone, MD, lead investigator for EXCEL and professor of medicine at Columbia University in New York.
“What the guidelines have not addressed are the patients with low- or intermediate-complexity disease who have an acceptable risk for undergoing either PCI or CABG. I think the trial results answer this question,” said David Kandzari, MD, director of interventional cardiology and chief scientific officer at the Piedmont Heart Institute in Atlanta and an EXCEL investigator.
While the EXCEL and NOBLE results don’t provide a simple answer on the relative merits of PCI and CABG, many of their outcome differences seem explicable, several experts said at the meeting.
The Nordic-Baltic-British Left Main Revascularisation (NOBLE) trial randomized 1,201 patients who had unprotected left main coronary disease and were judged by a heart team to be reasonable candidates for both PCI or CABG at 36 centers in nine European countries during 2008-2015. The primary endpoint was death from any cause, nonprocedural MIs, stroke, or repeat revascularization.
The researchers followed patients for a median of just over 3 years, but they calculated the primary endpoint based on a Kaplan-Meier estimate for 5-year outcomes, which showed the primary endpoint in 29% of the PCI patients and in 19% of the CABG patients, a statistically significant benefit in favor of CABG, Evald H. Christiansen, MD, reported at the meeting, which was sponsored by the Cardiovascular Research Foundation. Concurrently with his report, the results were published online (Lancet. 2016 Oct. 31. doi: 10.1016/S0140-6736[16]32052-9).
This difference between PCI and CABG was largely driven by an excess of postprocedural MIs and repeat revascularizations among the PCI patients, said Dr. Christiansen, an interventional cardiologist at Aarhus University Hospital in Denmark. Another notable finding was that the superior outcomes with CABG primarily occurred among patients with a SYNTAX score – a measure of coronary disease complexity – of 22 or less, which identifies patients with low complexity disease. The outcomes of patients with SYNTAX scores of 23-32, which identifies intermediate complexity disease, and of patients with scores of 33 or higher, with very complex disease, were similar in the PCI and CABG arms, he reported. This finding was “very surprising,” Dr. Christiansen said, because it reversed the finding originally made in the SYNTAX trial that PCI performed best compared with CABG in patients with the lowest scores and least-complex coronary disease.
The superiority of CABG over PCI seen in the NOBLE results, especially in patients with lower SYNTAX scores, seemed at odds with the EXCEL results, reported at the meeting by Dr. Stone and simultaneously online (N Engl J Med. 2016 Oct. 31. doi: 10.1056/NEJMoa1610227). In EXCEL, which enrolled only patients with a SYNTAX score of 32 or less (low- or intermediate-complexity coronary disease), patients had a 3-year incidence of death, stroke or MI of 15% in both the PCI and CABG arms.
But the EXCEL and NOBLE trials had several important differences, and it seemed like cumulatively these differences account for their differing results.
“One of the biggest differences” was the exclusion of procedural MIs in the NOBLE tally of adverse events, noted Dr. Stone. These were diagnosed in EXCEL using the MI definition published in 2013 by a panel of the Society for Cardiovascular Angiography and Interventions (SCAI). NOBLE disregarded procedural MIs because many of its participating hospitals did not have the laboratory resources to make these diagnoses and because the trial’s design predated the SCAI definition by several years, Dr. Christiansen explained.
Other important differences included the shorter follow-up in EXCEL, the inclusion of revascularization as an endpoint component in NOBLE but not in EXCEL, and differences in the stents used. In EXCEL, all patients undergoing PCI received Xience everolimus-eluting stents. In NOBLE, the first 11% of the enrolled patients received first-generation, sirolimus-eluting Cypher stents; the next 89% of enrollees received a biolimus-eluting Biomatrix Flex stent. Dr. Christiansen acknowledged the confounding caused by having many patients in the NOBLE PCI arm who received outmoded Cypher stents, especially because their relatively longer follow-up made them overrepresented in the primary outcome results. Plus, the Biomatrix Flex stent was disparaged by Martin B. Leon, MD, an EXCEL investigator and professor of medicine at Columbia University, who called the device “not currently widely used for PCI and more of historic interest.”
Dr. Leon added that the EXCEL and NOBLE patients also had substantially different prevalence rates of diabetes and acute coronary syndrome.
“The huge difference [between EXCEL and NOBLE] is the endpoint,” declared Marc Ruel, MD, another EXCEL investigator and head of cardiac surgery at the Ottawa Heart Institute. “The EXCEL endpoint was driven by the high rate of periprocedural MIs in the CABG arm. Once you get past 30 days, the noninferiority is not met by PCI.”
Another big endpoint difference was leaving revascularizations out of the EXCEL composite. “Once you take revascularization out of the primary endpoint, the outcome [of EXCEL] was more or less preordained,” noted Craig R. Smith, MD, chairman of surgery at Columbia University and an EXCEL investigator. “It’s the slope of events [in the PCI arm] after 3 months that’s the story. I think the CABG and PCI slopes in EXCEL will continue to diverge with time” beyond the current 3-year follow-up, Dr. Smith said.
“I agree that after 30 days surgery was the more durable procedure,” said Dr. Stone. “There is a big upfront hit for patients to take with surgery compared with PCI. If patients get through that, then they have a more durable procedure. That’s the trade-off.”
Dr. Stone hinted that future reports of EXCEL data will highlight other hits that patients must endure upfront if they choose CABG over PCI. “The early difference was quite profound not only for the primary endpoint but also for renal failure, infections, arrhythmias, and blood transfusions,” he said. Choosing between PCI and CABG for patients with left main disease and a lower SYNTAX score “is a decision that should be made by the heart team and patients. Some patients will prefer surgery, and some will prefer PCI.”
The NOBLE trial received partial funding from Biosensors, the company that markets the Biomatrix Flex stent used in the trial. The EXCEL trial was sponsored by Abbott Vascular, the company that markets the Xience stent used in the trial. Dr. Stone, Dr. Kandzari, Dr. Christiansen, Dr. Ruel, and Dr. Smith had no disclosures. Dr. Leon has been a consultant to and received research support from Abbott Vascular and Boston Scientific and has also received research support from Edwards, Medtronic and St. Jude.
[email protected]
On Twitter @mitchelzoler
The results from EXCEL and NOBLE were not that different, but what was different was how the two trials were designed and how their endpoints were defined. The biggest difference between percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) seemed to be in the rate of late MIs, with a little higher rate following PCI, and more repeat revascularizations with PCI, but with similar mortality rates with both treatments. There was a lot of similarity in the results despite the differences in the trials.
The evidence in both studies gives more support to the concept that, for patients with simpler left main lesions, PCI is a competitive alternative to CABG. Until now, in U.S. practice patients with left main coronary disease have been preferentially referred for CABG. These results will open us up to giving selected patients a more balanced view of the two options. After we explain differences in recovery and late events patients can decide which treatment to receive.
Despite these new findings, PCI is still not for every patient. A substantial fraction of patients with left main coronary disease were excluded from these studies because they had more complex coronary anatomy, and for patients like that, CABG remains the clear standard of care.
David J. Cohen, MD, is director of cardiovascular research and an interventional cardiologist at Saint Luke’s Health System in Kansas City, Mo. He made these comments in a video interview. He had received research support from Abbott Vascular, and is an investigator in the EXCEL trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The results from EXCEL and NOBLE were not that different, but what was different was how the two trials were designed and how their endpoints were defined. The biggest difference between percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) seemed to be in the rate of late MIs, with a little higher rate following PCI, and more repeat revascularizations with PCI, but with similar mortality rates with both treatments. There was a lot of similarity in the results despite the differences in the trials.
The evidence in both studies gives more support to the concept that, for patients with simpler left main lesions, PCI is a competitive alternative to CABG. Until now, in U.S. practice patients with left main coronary disease have been preferentially referred for CABG. These results will open us up to giving selected patients a more balanced view of the two options. After we explain differences in recovery and late events patients can decide which treatment to receive.
Despite these new findings, PCI is still not for every patient. A substantial fraction of patients with left main coronary disease were excluded from these studies because they had more complex coronary anatomy, and for patients like that, CABG remains the clear standard of care.
David J. Cohen, MD, is director of cardiovascular research and an interventional cardiologist at Saint Luke’s Health System in Kansas City, Mo. He made these comments in a video interview. He had received research support from Abbott Vascular, and is an investigator in the EXCEL trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The results from EXCEL and NOBLE were not that different, but what was different was how the two trials were designed and how their endpoints were defined. The biggest difference between percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) seemed to be in the rate of late MIs, with a little higher rate following PCI, and more repeat revascularizations with PCI, but with similar mortality rates with both treatments. There was a lot of similarity in the results despite the differences in the trials.
The evidence in both studies gives more support to the concept that, for patients with simpler left main lesions, PCI is a competitive alternative to CABG. Until now, in U.S. practice patients with left main coronary disease have been preferentially referred for CABG. These results will open us up to giving selected patients a more balanced view of the two options. After we explain differences in recovery and late events patients can decide which treatment to receive.
Despite these new findings, PCI is still not for every patient. A substantial fraction of patients with left main coronary disease were excluded from these studies because they had more complex coronary anatomy, and for patients like that, CABG remains the clear standard of care.
David J. Cohen, MD, is director of cardiovascular research and an interventional cardiologist at Saint Luke’s Health System in Kansas City, Mo. He made these comments in a video interview. He had received research support from Abbott Vascular, and is an investigator in the EXCEL trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – Results from two large, multicenter comparisons of coronary stenting and coronary bypass surgery for treating patients with unprotected left main coronary disease may have superficially shown sharp differences, but the bottom line will likely be greater empowerment of percutaneous coronary intervention as an option for selected patients with less complex coronary disease.
Prior to the results from the EXCEL and NOBLE trials, reported at the Transcatheter Cardiovascular Therapeutics annual meeting, “guidelines put PCI [percutaneous coronary intervention] into a class 1, 2 or 3 status for treating left main coronary disease depending on disease complexity, but in the United States, PCI for patients eligible for CABG [coronary artery bypass grafting] has not been frequently done. I think these results, in a very circumscribed subset of patients and using a state-of-the-art stent, will affect the guidelines,” predicted Gregg W. Stone, MD, lead investigator for EXCEL and professor of medicine at Columbia University in New York.
“What the guidelines have not addressed are the patients with low- or intermediate-complexity disease who have an acceptable risk for undergoing either PCI or CABG. I think the trial results answer this question,” said David Kandzari, MD, director of interventional cardiology and chief scientific officer at the Piedmont Heart Institute in Atlanta and an EXCEL investigator.
While the EXCEL and NOBLE results don’t provide a simple answer on the relative merits of PCI and CABG, many of their outcome differences seem explicable, several experts said at the meeting.
The Nordic-Baltic-British Left Main Revascularisation (NOBLE) trial randomized 1,201 patients who had unprotected left main coronary disease and were judged by a heart team to be reasonable candidates for both PCI or CABG at 36 centers in nine European countries during 2008-2015. The primary endpoint was death from any cause, nonprocedural MIs, stroke, or repeat revascularization.
The researchers followed patients for a median of just over 3 years, but they calculated the primary endpoint based on a Kaplan-Meier estimate for 5-year outcomes, which showed the primary endpoint in 29% of the PCI patients and in 19% of the CABG patients, a statistically significant benefit in favor of CABG, Evald H. Christiansen, MD, reported at the meeting, which was sponsored by the Cardiovascular Research Foundation. Concurrently with his report, the results were published online (Lancet. 2016 Oct. 31. doi: 10.1016/S0140-6736[16]32052-9).
This difference between PCI and CABG was largely driven by an excess of postprocedural MIs and repeat revascularizations among the PCI patients, said Dr. Christiansen, an interventional cardiologist at Aarhus University Hospital in Denmark. Another notable finding was that the superior outcomes with CABG primarily occurred among patients with a SYNTAX score – a measure of coronary disease complexity – of 22 or less, which identifies patients with low complexity disease. The outcomes of patients with SYNTAX scores of 23-32, which identifies intermediate complexity disease, and of patients with scores of 33 or higher, with very complex disease, were similar in the PCI and CABG arms, he reported. This finding was “very surprising,” Dr. Christiansen said, because it reversed the finding originally made in the SYNTAX trial that PCI performed best compared with CABG in patients with the lowest scores and least-complex coronary disease.
The superiority of CABG over PCI seen in the NOBLE results, especially in patients with lower SYNTAX scores, seemed at odds with the EXCEL results, reported at the meeting by Dr. Stone and simultaneously online (N Engl J Med. 2016 Oct. 31. doi: 10.1056/NEJMoa1610227). In EXCEL, which enrolled only patients with a SYNTAX score of 32 or less (low- or intermediate-complexity coronary disease), patients had a 3-year incidence of death, stroke or MI of 15% in both the PCI and CABG arms.
But the EXCEL and NOBLE trials had several important differences, and it seemed like cumulatively these differences account for their differing results.
“One of the biggest differences” was the exclusion of procedural MIs in the NOBLE tally of adverse events, noted Dr. Stone. These were diagnosed in EXCEL using the MI definition published in 2013 by a panel of the Society for Cardiovascular Angiography and Interventions (SCAI). NOBLE disregarded procedural MIs because many of its participating hospitals did not have the laboratory resources to make these diagnoses and because the trial’s design predated the SCAI definition by several years, Dr. Christiansen explained.
Other important differences included the shorter follow-up in EXCEL, the inclusion of revascularization as an endpoint component in NOBLE but not in EXCEL, and differences in the stents used. In EXCEL, all patients undergoing PCI received Xience everolimus-eluting stents. In NOBLE, the first 11% of the enrolled patients received first-generation, sirolimus-eluting Cypher stents; the next 89% of enrollees received a biolimus-eluting Biomatrix Flex stent. Dr. Christiansen acknowledged the confounding caused by having many patients in the NOBLE PCI arm who received outmoded Cypher stents, especially because their relatively longer follow-up made them overrepresented in the primary outcome results. Plus, the Biomatrix Flex stent was disparaged by Martin B. Leon, MD, an EXCEL investigator and professor of medicine at Columbia University, who called the device “not currently widely used for PCI and more of historic interest.”
Dr. Leon added that the EXCEL and NOBLE patients also had substantially different prevalence rates of diabetes and acute coronary syndrome.
“The huge difference [between EXCEL and NOBLE] is the endpoint,” declared Marc Ruel, MD, another EXCEL investigator and head of cardiac surgery at the Ottawa Heart Institute. “The EXCEL endpoint was driven by the high rate of periprocedural MIs in the CABG arm. Once you get past 30 days, the noninferiority is not met by PCI.”
Another big endpoint difference was leaving revascularizations out of the EXCEL composite. “Once you take revascularization out of the primary endpoint, the outcome [of EXCEL] was more or less preordained,” noted Craig R. Smith, MD, chairman of surgery at Columbia University and an EXCEL investigator. “It’s the slope of events [in the PCI arm] after 3 months that’s the story. I think the CABG and PCI slopes in EXCEL will continue to diverge with time” beyond the current 3-year follow-up, Dr. Smith said.
“I agree that after 30 days surgery was the more durable procedure,” said Dr. Stone. “There is a big upfront hit for patients to take with surgery compared with PCI. If patients get through that, then they have a more durable procedure. That’s the trade-off.”
Dr. Stone hinted that future reports of EXCEL data will highlight other hits that patients must endure upfront if they choose CABG over PCI. “The early difference was quite profound not only for the primary endpoint but also for renal failure, infections, arrhythmias, and blood transfusions,” he said. Choosing between PCI and CABG for patients with left main disease and a lower SYNTAX score “is a decision that should be made by the heart team and patients. Some patients will prefer surgery, and some will prefer PCI.”
The NOBLE trial received partial funding from Biosensors, the company that markets the Biomatrix Flex stent used in the trial. The EXCEL trial was sponsored by Abbott Vascular, the company that markets the Xience stent used in the trial. Dr. Stone, Dr. Kandzari, Dr. Christiansen, Dr. Ruel, and Dr. Smith had no disclosures. Dr. Leon has been a consultant to and received research support from Abbott Vascular and Boston Scientific and has also received research support from Edwards, Medtronic and St. Jude.
[email protected]
On Twitter @mitchelzoler
WASHINGTON – Results from two large, multicenter comparisons of coronary stenting and coronary bypass surgery for treating patients with unprotected left main coronary disease may have superficially shown sharp differences, but the bottom line will likely be greater empowerment of percutaneous coronary intervention as an option for selected patients with less complex coronary disease.
Prior to the results from the EXCEL and NOBLE trials, reported at the Transcatheter Cardiovascular Therapeutics annual meeting, “guidelines put PCI [percutaneous coronary intervention] into a class 1, 2 or 3 status for treating left main coronary disease depending on disease complexity, but in the United States, PCI for patients eligible for CABG [coronary artery bypass grafting] has not been frequently done. I think these results, in a very circumscribed subset of patients and using a state-of-the-art stent, will affect the guidelines,” predicted Gregg W. Stone, MD, lead investigator for EXCEL and professor of medicine at Columbia University in New York.
“What the guidelines have not addressed are the patients with low- or intermediate-complexity disease who have an acceptable risk for undergoing either PCI or CABG. I think the trial results answer this question,” said David Kandzari, MD, director of interventional cardiology and chief scientific officer at the Piedmont Heart Institute in Atlanta and an EXCEL investigator.
While the EXCEL and NOBLE results don’t provide a simple answer on the relative merits of PCI and CABG, many of their outcome differences seem explicable, several experts said at the meeting.
The Nordic-Baltic-British Left Main Revascularisation (NOBLE) trial randomized 1,201 patients who had unprotected left main coronary disease and were judged by a heart team to be reasonable candidates for both PCI or CABG at 36 centers in nine European countries during 2008-2015. The primary endpoint was death from any cause, nonprocedural MIs, stroke, or repeat revascularization.
The researchers followed patients for a median of just over 3 years, but they calculated the primary endpoint based on a Kaplan-Meier estimate for 5-year outcomes, which showed the primary endpoint in 29% of the PCI patients and in 19% of the CABG patients, a statistically significant benefit in favor of CABG, Evald H. Christiansen, MD, reported at the meeting, which was sponsored by the Cardiovascular Research Foundation. Concurrently with his report, the results were published online (Lancet. 2016 Oct. 31. doi: 10.1016/S0140-6736[16]32052-9).
This difference between PCI and CABG was largely driven by an excess of postprocedural MIs and repeat revascularizations among the PCI patients, said Dr. Christiansen, an interventional cardiologist at Aarhus University Hospital in Denmark. Another notable finding was that the superior outcomes with CABG primarily occurred among patients with a SYNTAX score – a measure of coronary disease complexity – of 22 or less, which identifies patients with low complexity disease. The outcomes of patients with SYNTAX scores of 23-32, which identifies intermediate complexity disease, and of patients with scores of 33 or higher, with very complex disease, were similar in the PCI and CABG arms, he reported. This finding was “very surprising,” Dr. Christiansen said, because it reversed the finding originally made in the SYNTAX trial that PCI performed best compared with CABG in patients with the lowest scores and least-complex coronary disease.
The superiority of CABG over PCI seen in the NOBLE results, especially in patients with lower SYNTAX scores, seemed at odds with the EXCEL results, reported at the meeting by Dr. Stone and simultaneously online (N Engl J Med. 2016 Oct. 31. doi: 10.1056/NEJMoa1610227). In EXCEL, which enrolled only patients with a SYNTAX score of 32 or less (low- or intermediate-complexity coronary disease), patients had a 3-year incidence of death, stroke or MI of 15% in both the PCI and CABG arms.
But the EXCEL and NOBLE trials had several important differences, and it seemed like cumulatively these differences account for their differing results.
“One of the biggest differences” was the exclusion of procedural MIs in the NOBLE tally of adverse events, noted Dr. Stone. These were diagnosed in EXCEL using the MI definition published in 2013 by a panel of the Society for Cardiovascular Angiography and Interventions (SCAI). NOBLE disregarded procedural MIs because many of its participating hospitals did not have the laboratory resources to make these diagnoses and because the trial’s design predated the SCAI definition by several years, Dr. Christiansen explained.
Other important differences included the shorter follow-up in EXCEL, the inclusion of revascularization as an endpoint component in NOBLE but not in EXCEL, and differences in the stents used. In EXCEL, all patients undergoing PCI received Xience everolimus-eluting stents. In NOBLE, the first 11% of the enrolled patients received first-generation, sirolimus-eluting Cypher stents; the next 89% of enrollees received a biolimus-eluting Biomatrix Flex stent. Dr. Christiansen acknowledged the confounding caused by having many patients in the NOBLE PCI arm who received outmoded Cypher stents, especially because their relatively longer follow-up made them overrepresented in the primary outcome results. Plus, the Biomatrix Flex stent was disparaged by Martin B. Leon, MD, an EXCEL investigator and professor of medicine at Columbia University, who called the device “not currently widely used for PCI and more of historic interest.”
Dr. Leon added that the EXCEL and NOBLE patients also had substantially different prevalence rates of diabetes and acute coronary syndrome.
“The huge difference [between EXCEL and NOBLE] is the endpoint,” declared Marc Ruel, MD, another EXCEL investigator and head of cardiac surgery at the Ottawa Heart Institute. “The EXCEL endpoint was driven by the high rate of periprocedural MIs in the CABG arm. Once you get past 30 days, the noninferiority is not met by PCI.”
Another big endpoint difference was leaving revascularizations out of the EXCEL composite. “Once you take revascularization out of the primary endpoint, the outcome [of EXCEL] was more or less preordained,” noted Craig R. Smith, MD, chairman of surgery at Columbia University and an EXCEL investigator. “It’s the slope of events [in the PCI arm] after 3 months that’s the story. I think the CABG and PCI slopes in EXCEL will continue to diverge with time” beyond the current 3-year follow-up, Dr. Smith said.
“I agree that after 30 days surgery was the more durable procedure,” said Dr. Stone. “There is a big upfront hit for patients to take with surgery compared with PCI. If patients get through that, then they have a more durable procedure. That’s the trade-off.”
Dr. Stone hinted that future reports of EXCEL data will highlight other hits that patients must endure upfront if they choose CABG over PCI. “The early difference was quite profound not only for the primary endpoint but also for renal failure, infections, arrhythmias, and blood transfusions,” he said. Choosing between PCI and CABG for patients with left main disease and a lower SYNTAX score “is a decision that should be made by the heart team and patients. Some patients will prefer surgery, and some will prefer PCI.”
The NOBLE trial received partial funding from Biosensors, the company that markets the Biomatrix Flex stent used in the trial. The EXCEL trial was sponsored by Abbott Vascular, the company that markets the Xience stent used in the trial. Dr. Stone, Dr. Kandzari, Dr. Christiansen, Dr. Ruel, and Dr. Smith had no disclosures. Dr. Leon has been a consultant to and received research support from Abbott Vascular and Boston Scientific and has also received research support from Edwards, Medtronic and St. Jude.
[email protected]
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM TCT 2016
Bilateral earlobe creases and coronary artery disease
A 70-year-old man with hypertension and hypercholesterolemia presented to the emergency department after the acute onset of substernal, pressure-like chest pain while climbing a flight of stairs. His physical examination was normal, but he was noted to have bilateral diagonal earlobe creases (the Frank sign) (Figure 1), considered by some to indicate risk of coronary artery disease.1–5
Electrocardiography showed atrial fibrillation with a ventricular rate of 149 beats per minute, ST-segment elevation in leads V1 and aVR, and ST-segment depression in leads V3 to V6, II, III, and aVF.
Urgent coronary arteriography showed severe coronary artery disease (Figure 2). Left ventriculography showed an ejection fraction of 50% with mild anterior wall hypokinesis. A drug-eluting stent was placed in the mid-left anterior descending artery. The patient tolerated the procedure well, and his chest pain resolved afterward.
A STILL-UNCLEAR ASSOCIATION
Sanders T. Frank, in 1973, first described a diagonal wrinkle-like line on the earlobe as a sign of coronary artery disease.1 Subsequently, autopsy studies suggested that deep bilateral earlobe creases could be an important sign of coronary atherosclerosis.2 Diagonal earlobe creases have been shown to be independently associated with increased prevalence, extent, and severity of coronary artery disease.3,4 They are also associated with major adverse cardiovascular events4 and ischemic stroke.5 The mechanism linking diagonal earlobe creases and atherosclerotic disease is not yet clear.
This patient’s presentation and evaluation remind us that bilateral earlobe creases may be useful to include in the clinical examination of patients with suspected coronary artery disease and may facilitate early recognition of disease in a patient at high risk.
- Frank ST. Aural sign of coronary-artery disease. N Engl J Med 1973; 289:327–328.
- Patel V, Champ C, Andrews PS, Gostelow BE, Gunasekara NP, Davidson AR. Diagonal earlobe creases and atheromatous disease: a postmortem study. J R Coll Phys Lond 1992; 26:274–277.
- Kaukola S, Manninen V, Valle M, Halonen PI. Ear-lobe crease and coronary atherosclerosis. Lancet 1979; 2:1377.
- Shmilovich H, Cheng VY, Rajani R, et al. Relation of diagonal ear lobe crease to the presence, extent, and severity of coronary artery disease determined by coronary computed tomography angiography. Am J Cardiol 2012; 109:1283–1287.
- Zapata-Wainberg G, Vivancos J. Images in clinical medicine: bilateral earlobe creases. N Engl J Med 2013; 368:e32.
A 70-year-old man with hypertension and hypercholesterolemia presented to the emergency department after the acute onset of substernal, pressure-like chest pain while climbing a flight of stairs. His physical examination was normal, but he was noted to have bilateral diagonal earlobe creases (the Frank sign) (Figure 1), considered by some to indicate risk of coronary artery disease.1–5
Electrocardiography showed atrial fibrillation with a ventricular rate of 149 beats per minute, ST-segment elevation in leads V1 and aVR, and ST-segment depression in leads V3 to V6, II, III, and aVF.
Urgent coronary arteriography showed severe coronary artery disease (Figure 2). Left ventriculography showed an ejection fraction of 50% with mild anterior wall hypokinesis. A drug-eluting stent was placed in the mid-left anterior descending artery. The patient tolerated the procedure well, and his chest pain resolved afterward.
A STILL-UNCLEAR ASSOCIATION
Sanders T. Frank, in 1973, first described a diagonal wrinkle-like line on the earlobe as a sign of coronary artery disease.1 Subsequently, autopsy studies suggested that deep bilateral earlobe creases could be an important sign of coronary atherosclerosis.2 Diagonal earlobe creases have been shown to be independently associated with increased prevalence, extent, and severity of coronary artery disease.3,4 They are also associated with major adverse cardiovascular events4 and ischemic stroke.5 The mechanism linking diagonal earlobe creases and atherosclerotic disease is not yet clear.
This patient’s presentation and evaluation remind us that bilateral earlobe creases may be useful to include in the clinical examination of patients with suspected coronary artery disease and may facilitate early recognition of disease in a patient at high risk.
A 70-year-old man with hypertension and hypercholesterolemia presented to the emergency department after the acute onset of substernal, pressure-like chest pain while climbing a flight of stairs. His physical examination was normal, but he was noted to have bilateral diagonal earlobe creases (the Frank sign) (Figure 1), considered by some to indicate risk of coronary artery disease.1–5
Electrocardiography showed atrial fibrillation with a ventricular rate of 149 beats per minute, ST-segment elevation in leads V1 and aVR, and ST-segment depression in leads V3 to V6, II, III, and aVF.
Urgent coronary arteriography showed severe coronary artery disease (Figure 2). Left ventriculography showed an ejection fraction of 50% with mild anterior wall hypokinesis. A drug-eluting stent was placed in the mid-left anterior descending artery. The patient tolerated the procedure well, and his chest pain resolved afterward.
A STILL-UNCLEAR ASSOCIATION
Sanders T. Frank, in 1973, first described a diagonal wrinkle-like line on the earlobe as a sign of coronary artery disease.1 Subsequently, autopsy studies suggested that deep bilateral earlobe creases could be an important sign of coronary atherosclerosis.2 Diagonal earlobe creases have been shown to be independently associated with increased prevalence, extent, and severity of coronary artery disease.3,4 They are also associated with major adverse cardiovascular events4 and ischemic stroke.5 The mechanism linking diagonal earlobe creases and atherosclerotic disease is not yet clear.
This patient’s presentation and evaluation remind us that bilateral earlobe creases may be useful to include in the clinical examination of patients with suspected coronary artery disease and may facilitate early recognition of disease in a patient at high risk.
- Frank ST. Aural sign of coronary-artery disease. N Engl J Med 1973; 289:327–328.
- Patel V, Champ C, Andrews PS, Gostelow BE, Gunasekara NP, Davidson AR. Diagonal earlobe creases and atheromatous disease: a postmortem study. J R Coll Phys Lond 1992; 26:274–277.
- Kaukola S, Manninen V, Valle M, Halonen PI. Ear-lobe crease and coronary atherosclerosis. Lancet 1979; 2:1377.
- Shmilovich H, Cheng VY, Rajani R, et al. Relation of diagonal ear lobe crease to the presence, extent, and severity of coronary artery disease determined by coronary computed tomography angiography. Am J Cardiol 2012; 109:1283–1287.
- Zapata-Wainberg G, Vivancos J. Images in clinical medicine: bilateral earlobe creases. N Engl J Med 2013; 368:e32.
- Frank ST. Aural sign of coronary-artery disease. N Engl J Med 1973; 289:327–328.
- Patel V, Champ C, Andrews PS, Gostelow BE, Gunasekara NP, Davidson AR. Diagonal earlobe creases and atheromatous disease: a postmortem study. J R Coll Phys Lond 1992; 26:274–277.
- Kaukola S, Manninen V, Valle M, Halonen PI. Ear-lobe crease and coronary atherosclerosis. Lancet 1979; 2:1377.
- Shmilovich H, Cheng VY, Rajani R, et al. Relation of diagonal ear lobe crease to the presence, extent, and severity of coronary artery disease determined by coronary computed tomography angiography. Am J Cardiol 2012; 109:1283–1287.
- Zapata-Wainberg G, Vivancos J. Images in clinical medicine: bilateral earlobe creases. N Engl J Med 2013; 368:e32.
Seeking medical care abroad: A challenge to empathy
On an otherwise pleasant evening during the first week of July 2016, a businessman who was a citizen of the United Arab Emirates visiting Cleveland for medical treatment was falsely accused of links to a terror organization. Officers stormed his hotel with assault rifles and handcuffed and arrested him—all this, apparently, because the man was dressed in traditional Emirati clothing.
This case highlights a level of complexity in providing medical care to foreigners far beyond language interpreting services and outside the borders of the institution where medical care is provided. In the current issue of the Journal, Cawcutt and Wilson1 review their experiences in the care of international patients and the unique challenges associated with it.
FROM THE TEMPLE OF AESCULAPIUS TO CLEVELAND CLINIC
In 2015, patients from more than 100 countries traveled to Cleveland seeking care at Cleveland Clinic. But medical travel was part of the practice of medicine long before major US hospitals became destinations for international patients, and it has been refined over the years.
Ancient cultures had a thriving tradition of patients traveling long distances for the best and most advanced medical treatment.2–4 In ancient Greece, people from all around the Mediterranean came to the city of Epidaurus to be cured in its famous temple of Aesculapius, built as a medical center.
Similarly, early Islamic cultures established a healthcare system that catered to foreigners. A noted example is the Mansuri hospital in Cairo, built in 1248 ce and considered the most advanced hospital of its time. Accommodating nearly 8,000 patients, the Mansuri hospital became a healthcare destination for foreigners regardless of race or religion.2–4
Europe also had a great tradition of providing medical care to foreign patients. Between the 15th and 17th centuries, belief in the healing power of mineral water led to the establishment of spas and the rise of spa towns, particularly in the south of France near mineral springs. The poor sanitary conditions of Europe at the time may have prompted the interest in the healing effect of mineral spas, but wealthy individuals from all over the world traveled to these destinations, creating local prosperity due to medical tourism.2–4
The city of Bath, in England, is a great example. In the 1720s, Bath was a popular destination for those traveling for healthcare. It became the first city in England to build a covered sewage system, ahead of London by several years. It also had paved roads, lights, hotels, and restaurants in much greater numbers than other cities in England, a likely result of prosperity associated with medical tourism.
ALL PATIENTS WANT TO BE TREATED WITH RESPECT AND KINDNESS
While medical knowledge and health delivery models have changed over the years, caring for foreign patients is perhaps as old as medicine itself. The central focus of restoring health is certainly not unique to international patients, but understanding their unique needs is important in order to achieve the best outcomes, something that Cawcutt and Wilson highlight well.1
A number of studies have addressed the question of what patients really want. Responses were surprisingly consistent: they want to be treated with respect and kindness.5,6 In other words, they want empathy, and this is true of all patients regardless of ethnicity or background. Empathy is a tremendous therapeutic force and can narrow what may look like an unbridgeable gap between patient and physician.7,8
EMPATHY REQUIRES EFFECTIVE COMMUNICATION
Empathy, though sometimes innate, requires effective communication and shared experiences. Neither of these two requirements is easily achievable in the care of foreign patients.
Communication is hampered by language barriers, although it can be enhanced significantly by language translating services and the work of certified medical interpreters. These often-invisible heroes should be recognized as essential members of the medical team. Their work requires cultural sensitivity and formal training to avoid miscommunication and medical errors. Codes of ethics for medical interpreters include confidentiality, accuracy in conveying the content and spirit of the message, freedom from personal biases, cultural training, and professional boundaries.9
TOWARD CULTURAL COMPETENCY
Lack of shared experiences between the foreign patient and care provider is an even greater obstacle to overcome in eliminating any empathy deficit. Shared experiences, whether cultural, religious, or social, help us to see the world through the eyes of the patient.
International patients may differ from us in background, ethnicity, religion, dress, expectations, and other areas. Cultural and religious backgrounds often dictate certain behaviors in the event of critical illness or death. Even in routine and less acute medical care, the background of a foreign patient may lead to logistical quandaries such as the need for same-sex caregivers or a private room.
A paradox currently exists in our efforts to meet patients’ need and desire for empathy. While culturally empathic care is necessary to achieve the best medical outcomes, this topic is not yet part of the curriculum for physicians or other healthcare providers in training. A culturally sensitive institution has many business advantages.10 Thorough and focused cultural training of medical staff is essential. Shared experiences can potentially be fashioned through a well-designed cultural competency training program to enhance empathy for foreign patients.
A SERVICE-ORIENTED APPROACH
Besides cultural competency and language training, a service-oriented approach to accommodate the needs of medical travelers and their family members is of paramount importance. Many of the complaints and burdens of medical visitors concern services that are not medical in nature, such as daily living necessities. Transportation, religious services, banking, extended-stay facilities, cell phone service, legal services, shopping, dining, and entertainment are among many other living needs for those receiving medical care abroad. These services are inconsistently provided throughout medical institutions in the United States, which provide care to thousands of international patients annually.
Unique challenges of providing medical care to international patients have direct effects on medical outcomes. A population-based cohort study of US-born and foreign-born adults with lung or colorectal cancer suggested disparities in quality and type of care.11 Foreign-born patients reported lower-quality care and were less likely to receive complex cancer treatments recommended by clinical guidelines. The authors proposed that quality of care and outcomes may be improved with greater emphasis on coordination of care and improving communication. Similar findings were reported in foreign-born patients with breast cancer.12
‘WHAT WOULD YOU THINK TO BE USED THUS?’
Four hundred years ago, in the play Sir Thomas More (a collaboration between several Elizabethan playwrights),13 the title character confronts a mob of anti-immigrant rioters, and in a speech believed to have been written by William Shakespeare (Act 2, Scene 4), asks them to imagine themselves banished to a foreign country and subjected to hostility such as they were meting out:
To be used thus?”
Empathy for foreigners seeking medical care is not merely an act of kindness; rather, it is a central piece of healing. Medical institutions interested in providing healthcare to this unique group of patients should take these principles into account and carefully examine their ability to deliver compassionate care collectively to local and foreign-born patients alike.
- Cawcutt KA, Wilson JW. The benefits and challenges of caring for international patients. Cleve Clin J Med 2016; 83:794–800.
- Health-Tourism.com. The history of medical tourism. Health-Tourism.com. www.health-tourism.com/medical-tourism/history/. Accessed September 21, 2016.
- Chen LH, Hochberg NS, Magill AJ. The pre-travel consultation. US Centers for Disease Control and Prevention. wwwnc.cdc.gov/travel/yellowbook/2016/the-pre-travel-consultation/the-pre-travel-consultation. Accessed September 21, 2016.
- Rogers K. Medical tourism. Encyclopedia Britannica. www.britannica.com/topic/medical-tourism. Accessed September 21, 2016.
- Detsky AS. What do patients really want from healthcare? JAMA 2011; 306:2500–2501.
- Shaywitz D. What do patients really want from healthcare? Forbes Dec 24, 2011. www.forbes.com/sites/davidshaywitz/2011/12/24/what-do-patients-really-want-from-health-care/print/. Accessed September 21, 2016.
- Lee TH. How to spread empathy in healthcare. Harvard Business Review July 17, 2014.
- Friedman R. Understanding empathy: can you feel my pain? New York Times April 24, 2007.
- National Council on Interpreting in Health Care. A national code of ethics for interpreters in healthcare. July 2004. www.ncihc.org/assets/documents/publications/NCIHC%20National%20Code%20of%20Ethics.pdf. Accessed September 21, 2016.
- Minguet L. Creating a culturally sensitive corporation. Harvard Business Review, September 2014.
- Nielsen SS, He Y, Ayanian JZ, Gomez SL, Khan KL, West DW, et al. Quality of cancer care among foreign-born patients with lung or colorectal cancer. Cancer 2010; 116:5497–5506.
- Kouri EM, He Y, Winer EP, Keating NL. Influence of birthplace on breast cancer diagnosis and treatment for Hispanic women. Breast Cancer Res Treat 2009; 121:743–751.
- Dyce A, editor. Sir Thomas More, a play. London: The Shakespeare Society, 1844. https://archive.org/details/sirthomasmorepla00mund. Accessed September 21, 2016.
On an otherwise pleasant evening during the first week of July 2016, a businessman who was a citizen of the United Arab Emirates visiting Cleveland for medical treatment was falsely accused of links to a terror organization. Officers stormed his hotel with assault rifles and handcuffed and arrested him—all this, apparently, because the man was dressed in traditional Emirati clothing.
This case highlights a level of complexity in providing medical care to foreigners far beyond language interpreting services and outside the borders of the institution where medical care is provided. In the current issue of the Journal, Cawcutt and Wilson1 review their experiences in the care of international patients and the unique challenges associated with it.
FROM THE TEMPLE OF AESCULAPIUS TO CLEVELAND CLINIC
In 2015, patients from more than 100 countries traveled to Cleveland seeking care at Cleveland Clinic. But medical travel was part of the practice of medicine long before major US hospitals became destinations for international patients, and it has been refined over the years.
Ancient cultures had a thriving tradition of patients traveling long distances for the best and most advanced medical treatment.2–4 In ancient Greece, people from all around the Mediterranean came to the city of Epidaurus to be cured in its famous temple of Aesculapius, built as a medical center.
Similarly, early Islamic cultures established a healthcare system that catered to foreigners. A noted example is the Mansuri hospital in Cairo, built in 1248 ce and considered the most advanced hospital of its time. Accommodating nearly 8,000 patients, the Mansuri hospital became a healthcare destination for foreigners regardless of race or religion.2–4
Europe also had a great tradition of providing medical care to foreign patients. Between the 15th and 17th centuries, belief in the healing power of mineral water led to the establishment of spas and the rise of spa towns, particularly in the south of France near mineral springs. The poor sanitary conditions of Europe at the time may have prompted the interest in the healing effect of mineral spas, but wealthy individuals from all over the world traveled to these destinations, creating local prosperity due to medical tourism.2–4
The city of Bath, in England, is a great example. In the 1720s, Bath was a popular destination for those traveling for healthcare. It became the first city in England to build a covered sewage system, ahead of London by several years. It also had paved roads, lights, hotels, and restaurants in much greater numbers than other cities in England, a likely result of prosperity associated with medical tourism.
ALL PATIENTS WANT TO BE TREATED WITH RESPECT AND KINDNESS
While medical knowledge and health delivery models have changed over the years, caring for foreign patients is perhaps as old as medicine itself. The central focus of restoring health is certainly not unique to international patients, but understanding their unique needs is important in order to achieve the best outcomes, something that Cawcutt and Wilson highlight well.1
A number of studies have addressed the question of what patients really want. Responses were surprisingly consistent: they want to be treated with respect and kindness.5,6 In other words, they want empathy, and this is true of all patients regardless of ethnicity or background. Empathy is a tremendous therapeutic force and can narrow what may look like an unbridgeable gap between patient and physician.7,8
EMPATHY REQUIRES EFFECTIVE COMMUNICATION
Empathy, though sometimes innate, requires effective communication and shared experiences. Neither of these two requirements is easily achievable in the care of foreign patients.
Communication is hampered by language barriers, although it can be enhanced significantly by language translating services and the work of certified medical interpreters. These often-invisible heroes should be recognized as essential members of the medical team. Their work requires cultural sensitivity and formal training to avoid miscommunication and medical errors. Codes of ethics for medical interpreters include confidentiality, accuracy in conveying the content and spirit of the message, freedom from personal biases, cultural training, and professional boundaries.9
TOWARD CULTURAL COMPETENCY
Lack of shared experiences between the foreign patient and care provider is an even greater obstacle to overcome in eliminating any empathy deficit. Shared experiences, whether cultural, religious, or social, help us to see the world through the eyes of the patient.
International patients may differ from us in background, ethnicity, religion, dress, expectations, and other areas. Cultural and religious backgrounds often dictate certain behaviors in the event of critical illness or death. Even in routine and less acute medical care, the background of a foreign patient may lead to logistical quandaries such as the need for same-sex caregivers or a private room.
A paradox currently exists in our efforts to meet patients’ need and desire for empathy. While culturally empathic care is necessary to achieve the best medical outcomes, this topic is not yet part of the curriculum for physicians or other healthcare providers in training. A culturally sensitive institution has many business advantages.10 Thorough and focused cultural training of medical staff is essential. Shared experiences can potentially be fashioned through a well-designed cultural competency training program to enhance empathy for foreign patients.
A SERVICE-ORIENTED APPROACH
Besides cultural competency and language training, a service-oriented approach to accommodate the needs of medical travelers and their family members is of paramount importance. Many of the complaints and burdens of medical visitors concern services that are not medical in nature, such as daily living necessities. Transportation, religious services, banking, extended-stay facilities, cell phone service, legal services, shopping, dining, and entertainment are among many other living needs for those receiving medical care abroad. These services are inconsistently provided throughout medical institutions in the United States, which provide care to thousands of international patients annually.
Unique challenges of providing medical care to international patients have direct effects on medical outcomes. A population-based cohort study of US-born and foreign-born adults with lung or colorectal cancer suggested disparities in quality and type of care.11 Foreign-born patients reported lower-quality care and were less likely to receive complex cancer treatments recommended by clinical guidelines. The authors proposed that quality of care and outcomes may be improved with greater emphasis on coordination of care and improving communication. Similar findings were reported in foreign-born patients with breast cancer.12
‘WHAT WOULD YOU THINK TO BE USED THUS?’
Four hundred years ago, in the play Sir Thomas More (a collaboration between several Elizabethan playwrights),13 the title character confronts a mob of anti-immigrant rioters, and in a speech believed to have been written by William Shakespeare (Act 2, Scene 4), asks them to imagine themselves banished to a foreign country and subjected to hostility such as they were meting out:
To be used thus?”
Empathy for foreigners seeking medical care is not merely an act of kindness; rather, it is a central piece of healing. Medical institutions interested in providing healthcare to this unique group of patients should take these principles into account and carefully examine their ability to deliver compassionate care collectively to local and foreign-born patients alike.
On an otherwise pleasant evening during the first week of July 2016, a businessman who was a citizen of the United Arab Emirates visiting Cleveland for medical treatment was falsely accused of links to a terror organization. Officers stormed his hotel with assault rifles and handcuffed and arrested him—all this, apparently, because the man was dressed in traditional Emirati clothing.
This case highlights a level of complexity in providing medical care to foreigners far beyond language interpreting services and outside the borders of the institution where medical care is provided. In the current issue of the Journal, Cawcutt and Wilson1 review their experiences in the care of international patients and the unique challenges associated with it.
FROM THE TEMPLE OF AESCULAPIUS TO CLEVELAND CLINIC
In 2015, patients from more than 100 countries traveled to Cleveland seeking care at Cleveland Clinic. But medical travel was part of the practice of medicine long before major US hospitals became destinations for international patients, and it has been refined over the years.
Ancient cultures had a thriving tradition of patients traveling long distances for the best and most advanced medical treatment.2–4 In ancient Greece, people from all around the Mediterranean came to the city of Epidaurus to be cured in its famous temple of Aesculapius, built as a medical center.
Similarly, early Islamic cultures established a healthcare system that catered to foreigners. A noted example is the Mansuri hospital in Cairo, built in 1248 ce and considered the most advanced hospital of its time. Accommodating nearly 8,000 patients, the Mansuri hospital became a healthcare destination for foreigners regardless of race or religion.2–4
Europe also had a great tradition of providing medical care to foreign patients. Between the 15th and 17th centuries, belief in the healing power of mineral water led to the establishment of spas and the rise of spa towns, particularly in the south of France near mineral springs. The poor sanitary conditions of Europe at the time may have prompted the interest in the healing effect of mineral spas, but wealthy individuals from all over the world traveled to these destinations, creating local prosperity due to medical tourism.2–4
The city of Bath, in England, is a great example. In the 1720s, Bath was a popular destination for those traveling for healthcare. It became the first city in England to build a covered sewage system, ahead of London by several years. It also had paved roads, lights, hotels, and restaurants in much greater numbers than other cities in England, a likely result of prosperity associated with medical tourism.
ALL PATIENTS WANT TO BE TREATED WITH RESPECT AND KINDNESS
While medical knowledge and health delivery models have changed over the years, caring for foreign patients is perhaps as old as medicine itself. The central focus of restoring health is certainly not unique to international patients, but understanding their unique needs is important in order to achieve the best outcomes, something that Cawcutt and Wilson highlight well.1
A number of studies have addressed the question of what patients really want. Responses were surprisingly consistent: they want to be treated with respect and kindness.5,6 In other words, they want empathy, and this is true of all patients regardless of ethnicity or background. Empathy is a tremendous therapeutic force and can narrow what may look like an unbridgeable gap between patient and physician.7,8
EMPATHY REQUIRES EFFECTIVE COMMUNICATION
Empathy, though sometimes innate, requires effective communication and shared experiences. Neither of these two requirements is easily achievable in the care of foreign patients.
Communication is hampered by language barriers, although it can be enhanced significantly by language translating services and the work of certified medical interpreters. These often-invisible heroes should be recognized as essential members of the medical team. Their work requires cultural sensitivity and formal training to avoid miscommunication and medical errors. Codes of ethics for medical interpreters include confidentiality, accuracy in conveying the content and spirit of the message, freedom from personal biases, cultural training, and professional boundaries.9
TOWARD CULTURAL COMPETENCY
Lack of shared experiences between the foreign patient and care provider is an even greater obstacle to overcome in eliminating any empathy deficit. Shared experiences, whether cultural, religious, or social, help us to see the world through the eyes of the patient.
International patients may differ from us in background, ethnicity, religion, dress, expectations, and other areas. Cultural and religious backgrounds often dictate certain behaviors in the event of critical illness or death. Even in routine and less acute medical care, the background of a foreign patient may lead to logistical quandaries such as the need for same-sex caregivers or a private room.
A paradox currently exists in our efforts to meet patients’ need and desire for empathy. While culturally empathic care is necessary to achieve the best medical outcomes, this topic is not yet part of the curriculum for physicians or other healthcare providers in training. A culturally sensitive institution has many business advantages.10 Thorough and focused cultural training of medical staff is essential. Shared experiences can potentially be fashioned through a well-designed cultural competency training program to enhance empathy for foreign patients.
A SERVICE-ORIENTED APPROACH
Besides cultural competency and language training, a service-oriented approach to accommodate the needs of medical travelers and their family members is of paramount importance. Many of the complaints and burdens of medical visitors concern services that are not medical in nature, such as daily living necessities. Transportation, religious services, banking, extended-stay facilities, cell phone service, legal services, shopping, dining, and entertainment are among many other living needs for those receiving medical care abroad. These services are inconsistently provided throughout medical institutions in the United States, which provide care to thousands of international patients annually.
Unique challenges of providing medical care to international patients have direct effects on medical outcomes. A population-based cohort study of US-born and foreign-born adults with lung or colorectal cancer suggested disparities in quality and type of care.11 Foreign-born patients reported lower-quality care and were less likely to receive complex cancer treatments recommended by clinical guidelines. The authors proposed that quality of care and outcomes may be improved with greater emphasis on coordination of care and improving communication. Similar findings were reported in foreign-born patients with breast cancer.12
‘WHAT WOULD YOU THINK TO BE USED THUS?’
Four hundred years ago, in the play Sir Thomas More (a collaboration between several Elizabethan playwrights),13 the title character confronts a mob of anti-immigrant rioters, and in a speech believed to have been written by William Shakespeare (Act 2, Scene 4), asks them to imagine themselves banished to a foreign country and subjected to hostility such as they were meting out:
To be used thus?”
Empathy for foreigners seeking medical care is not merely an act of kindness; rather, it is a central piece of healing. Medical institutions interested in providing healthcare to this unique group of patients should take these principles into account and carefully examine their ability to deliver compassionate care collectively to local and foreign-born patients alike.
- Cawcutt KA, Wilson JW. The benefits and challenges of caring for international patients. Cleve Clin J Med 2016; 83:794–800.
- Health-Tourism.com. The history of medical tourism. Health-Tourism.com. www.health-tourism.com/medical-tourism/history/. Accessed September 21, 2016.
- Chen LH, Hochberg NS, Magill AJ. The pre-travel consultation. US Centers for Disease Control and Prevention. wwwnc.cdc.gov/travel/yellowbook/2016/the-pre-travel-consultation/the-pre-travel-consultation. Accessed September 21, 2016.
- Rogers K. Medical tourism. Encyclopedia Britannica. www.britannica.com/topic/medical-tourism. Accessed September 21, 2016.
- Detsky AS. What do patients really want from healthcare? JAMA 2011; 306:2500–2501.
- Shaywitz D. What do patients really want from healthcare? Forbes Dec 24, 2011. www.forbes.com/sites/davidshaywitz/2011/12/24/what-do-patients-really-want-from-health-care/print/. Accessed September 21, 2016.
- Lee TH. How to spread empathy in healthcare. Harvard Business Review July 17, 2014.
- Friedman R. Understanding empathy: can you feel my pain? New York Times April 24, 2007.
- National Council on Interpreting in Health Care. A national code of ethics for interpreters in healthcare. July 2004. www.ncihc.org/assets/documents/publications/NCIHC%20National%20Code%20of%20Ethics.pdf. Accessed September 21, 2016.
- Minguet L. Creating a culturally sensitive corporation. Harvard Business Review, September 2014.
- Nielsen SS, He Y, Ayanian JZ, Gomez SL, Khan KL, West DW, et al. Quality of cancer care among foreign-born patients with lung or colorectal cancer. Cancer 2010; 116:5497–5506.
- Kouri EM, He Y, Winer EP, Keating NL. Influence of birthplace on breast cancer diagnosis and treatment for Hispanic women. Breast Cancer Res Treat 2009; 121:743–751.
- Dyce A, editor. Sir Thomas More, a play. London: The Shakespeare Society, 1844. https://archive.org/details/sirthomasmorepla00mund. Accessed September 21, 2016.
- Cawcutt KA, Wilson JW. The benefits and challenges of caring for international patients. Cleve Clin J Med 2016; 83:794–800.
- Health-Tourism.com. The history of medical tourism. Health-Tourism.com. www.health-tourism.com/medical-tourism/history/. Accessed September 21, 2016.
- Chen LH, Hochberg NS, Magill AJ. The pre-travel consultation. US Centers for Disease Control and Prevention. wwwnc.cdc.gov/travel/yellowbook/2016/the-pre-travel-consultation/the-pre-travel-consultation. Accessed September 21, 2016.
- Rogers K. Medical tourism. Encyclopedia Britannica. www.britannica.com/topic/medical-tourism. Accessed September 21, 2016.
- Detsky AS. What do patients really want from healthcare? JAMA 2011; 306:2500–2501.
- Shaywitz D. What do patients really want from healthcare? Forbes Dec 24, 2011. www.forbes.com/sites/davidshaywitz/2011/12/24/what-do-patients-really-want-from-health-care/print/. Accessed September 21, 2016.
- Lee TH. How to spread empathy in healthcare. Harvard Business Review July 17, 2014.
- Friedman R. Understanding empathy: can you feel my pain? New York Times April 24, 2007.
- National Council on Interpreting in Health Care. A national code of ethics for interpreters in healthcare. July 2004. www.ncihc.org/assets/documents/publications/NCIHC%20National%20Code%20of%20Ethics.pdf. Accessed September 21, 2016.
- Minguet L. Creating a culturally sensitive corporation. Harvard Business Review, September 2014.
- Nielsen SS, He Y, Ayanian JZ, Gomez SL, Khan KL, West DW, et al. Quality of cancer care among foreign-born patients with lung or colorectal cancer. Cancer 2010; 116:5497–5506.
- Kouri EM, He Y, Winer EP, Keating NL. Influence of birthplace on breast cancer diagnosis and treatment for Hispanic women. Breast Cancer Res Treat 2009; 121:743–751.
- Dyce A, editor. Sir Thomas More, a play. London: The Shakespeare Society, 1844. https://archive.org/details/sirthomasmorepla00mund. Accessed September 21, 2016.
The peacock and the doctor
Of the seven deadly sins, the worst is said to be pride, often represented in allegorical form as a peacock. In this month’s Journal, Kelly A. Cawcutt, MD and John W. Wilson, MD, and Nizar N. Zein, MD, note the rewards and challenges of caring for international patients. Pride, it seems to me, can get in the way of a successful relationship with these patients.
In the United States, we encounter a wide range of international patients, but there are two distinct categories: medical tourists, who come here by choice and often have significant financial means, and immigrants, who come here by choice or necessity and run the gamut of economic status.
The former group generally seeks care at major academic medical centers such as Cleveland Clinic and Mayo Clinic, which have built infrastructures to accommodate them, including paying special attention to the social aspects of the visit. For the medical center, there are immediate financial gains as well as potential long-term benefits, including international networking and philanthropy.
On the other hand, new immigrants, including refugees, generally seek care as needed where they have settled, mostly hoping that medical issues will not arise in the midst of the challenges of resettlement. They deserve and should expect to be able to establish a comfortable therapeutic relationship with a physician in a local medical practice, although one likely dissimilar from what they previously encountered.
For all of these patients, the focal point of interaction is us, the physician next to the examination table. Dr. Zein emphasizes the power of empathy and how our demeanor and choice of words are critical in building the therapeutic relationship. But pride can slip in, and the peacock subtly fans his tail.
While medical practice in the United States is technologically advanced in terms of tests and procedures, we are not the world leaders in outcomes or cost-effective care. We most certainly do not have a monopoly on delivering compassionate and empathic care or forging one-on-one doctor-patient relationships. We must be careful not to express a demeaning or dismissive attitude about the care our patients’ physicians provided in their home countries. That the laboratory and imaging reports are written in a different language, and perhaps reported in different units, should not imply any lower standard. We should also recognize that many of our physical examination skills have atrophied as we have come to over-rely on imaging studies. The apparent omission of an echocardiogram may in fact be an act of commission—a careful and confident physical examination may have resulted in a thoughtful decision to save the patient money. Careless words or a casually chauvinistic attitude can be disruptive to building a comfortable ongoing doctor-patient relationship.
At the same time, international patients come to see us with significant expectations (they may even have read our hospital’s marketing materials). But they may not be accustomed to their physician openly expressing a lack of certainty about a diagnosis. They may never have heard their at-home doctor say, “I don’t know.” The concept of patient involvement in the treatment plan may be totally foreign and discomforting to some, while others will expect that the entire family entourage (filling the exam room) will have an active role in decision-making.
Cultural awareness is critical as we sort these issues out so they do not stand in the way of successfully caring for the patient in front of us. We should avoid being too self-confident in our entrenched approach to healthcare delivery in the exam room (as well as in the redesign of our healthcare system). The peacock can be an attractive impediment.
Of the seven deadly sins, the worst is said to be pride, often represented in allegorical form as a peacock. In this month’s Journal, Kelly A. Cawcutt, MD and John W. Wilson, MD, and Nizar N. Zein, MD, note the rewards and challenges of caring for international patients. Pride, it seems to me, can get in the way of a successful relationship with these patients.
In the United States, we encounter a wide range of international patients, but there are two distinct categories: medical tourists, who come here by choice and often have significant financial means, and immigrants, who come here by choice or necessity and run the gamut of economic status.
The former group generally seeks care at major academic medical centers such as Cleveland Clinic and Mayo Clinic, which have built infrastructures to accommodate them, including paying special attention to the social aspects of the visit. For the medical center, there are immediate financial gains as well as potential long-term benefits, including international networking and philanthropy.
On the other hand, new immigrants, including refugees, generally seek care as needed where they have settled, mostly hoping that medical issues will not arise in the midst of the challenges of resettlement. They deserve and should expect to be able to establish a comfortable therapeutic relationship with a physician in a local medical practice, although one likely dissimilar from what they previously encountered.
For all of these patients, the focal point of interaction is us, the physician next to the examination table. Dr. Zein emphasizes the power of empathy and how our demeanor and choice of words are critical in building the therapeutic relationship. But pride can slip in, and the peacock subtly fans his tail.
While medical practice in the United States is technologically advanced in terms of tests and procedures, we are not the world leaders in outcomes or cost-effective care. We most certainly do not have a monopoly on delivering compassionate and empathic care or forging one-on-one doctor-patient relationships. We must be careful not to express a demeaning or dismissive attitude about the care our patients’ physicians provided in their home countries. That the laboratory and imaging reports are written in a different language, and perhaps reported in different units, should not imply any lower standard. We should also recognize that many of our physical examination skills have atrophied as we have come to over-rely on imaging studies. The apparent omission of an echocardiogram may in fact be an act of commission—a careful and confident physical examination may have resulted in a thoughtful decision to save the patient money. Careless words or a casually chauvinistic attitude can be disruptive to building a comfortable ongoing doctor-patient relationship.
At the same time, international patients come to see us with significant expectations (they may even have read our hospital’s marketing materials). But they may not be accustomed to their physician openly expressing a lack of certainty about a diagnosis. They may never have heard their at-home doctor say, “I don’t know.” The concept of patient involvement in the treatment plan may be totally foreign and discomforting to some, while others will expect that the entire family entourage (filling the exam room) will have an active role in decision-making.
Cultural awareness is critical as we sort these issues out so they do not stand in the way of successfully caring for the patient in front of us. We should avoid being too self-confident in our entrenched approach to healthcare delivery in the exam room (as well as in the redesign of our healthcare system). The peacock can be an attractive impediment.
Of the seven deadly sins, the worst is said to be pride, often represented in allegorical form as a peacock. In this month’s Journal, Kelly A. Cawcutt, MD and John W. Wilson, MD, and Nizar N. Zein, MD, note the rewards and challenges of caring for international patients. Pride, it seems to me, can get in the way of a successful relationship with these patients.
In the United States, we encounter a wide range of international patients, but there are two distinct categories: medical tourists, who come here by choice and often have significant financial means, and immigrants, who come here by choice or necessity and run the gamut of economic status.
The former group generally seeks care at major academic medical centers such as Cleveland Clinic and Mayo Clinic, which have built infrastructures to accommodate them, including paying special attention to the social aspects of the visit. For the medical center, there are immediate financial gains as well as potential long-term benefits, including international networking and philanthropy.
On the other hand, new immigrants, including refugees, generally seek care as needed where they have settled, mostly hoping that medical issues will not arise in the midst of the challenges of resettlement. They deserve and should expect to be able to establish a comfortable therapeutic relationship with a physician in a local medical practice, although one likely dissimilar from what they previously encountered.
For all of these patients, the focal point of interaction is us, the physician next to the examination table. Dr. Zein emphasizes the power of empathy and how our demeanor and choice of words are critical in building the therapeutic relationship. But pride can slip in, and the peacock subtly fans his tail.
While medical practice in the United States is technologically advanced in terms of tests and procedures, we are not the world leaders in outcomes or cost-effective care. We most certainly do not have a monopoly on delivering compassionate and empathic care or forging one-on-one doctor-patient relationships. We must be careful not to express a demeaning or dismissive attitude about the care our patients’ physicians provided in their home countries. That the laboratory and imaging reports are written in a different language, and perhaps reported in different units, should not imply any lower standard. We should also recognize that many of our physical examination skills have atrophied as we have come to over-rely on imaging studies. The apparent omission of an echocardiogram may in fact be an act of commission—a careful and confident physical examination may have resulted in a thoughtful decision to save the patient money. Careless words or a casually chauvinistic attitude can be disruptive to building a comfortable ongoing doctor-patient relationship.
At the same time, international patients come to see us with significant expectations (they may even have read our hospital’s marketing materials). But they may not be accustomed to their physician openly expressing a lack of certainty about a diagnosis. They may never have heard their at-home doctor say, “I don’t know.” The concept of patient involvement in the treatment plan may be totally foreign and discomforting to some, while others will expect that the entire family entourage (filling the exam room) will have an active role in decision-making.
Cultural awareness is critical as we sort these issues out so they do not stand in the way of successfully caring for the patient in front of us. We should avoid being too self-confident in our entrenched approach to healthcare delivery in the exam room (as well as in the redesign of our healthcare system). The peacock can be an attractive impediment.
Plexiform neurofibroma
A 19-year-old woman presented with diffuse swelling on her neck and face that had been growing gradually over the past 12 years. There was no family history of similar swelling.
Examination revealed a diffuse, hyperpigmented, nodular swelling on the right side of her neck and extending upward to the lower part of the face and the scalp (Figure 1). The rest of the physical examination was normal. A diagnosis of plexiform neurofibroma was made based on the typical clinical presentation. The patient had come primarily because of cosmetic concerns but refused surgery. She was offered genetic counseling and was scheduled for regular follow-up.
Plexiform neurofibroma is mostly associated with autosomal dominant neurofibromatosis type 1, characterized by cutaneous findings such as café-au-lait spots, axillary freckling, skeletal dysplasias (usually of the tibia, fibula, ribs, and vertebrae), Lisch nodules in the eye (iris hamartomas), and neural tumors. Rarely, it may also be seen in germline p16 mutation-positive heritable melanoma and Cowden syndrome.
Diffuse plexiform neurofibroma of the face and neck rarely appears after the age of 1, and rarely develops on other parts of the body after adolescence. In contrast, deep nodular plexiform neurofibroma often originates from spinal nerve roots and usually becomes symptomatic in adulthood.
Plexiform neurofibroma has an 8% to 12% chance of changing into a malignant peripheral nerve-sheath tumor. Continuous pain in the tumor, rapid tumor growth, hardening of the tumor, or weakness or numbness in an arm or leg with a plexiform neurofibroma suggests malignant transformation.
The diagnosis is clinical, and the management involves a multidisciplinary team including a physician, geneticist, neurologist, surgeon, and ophthalmologist. Genetic counseling should be offered to patients for assessment of family risk, to look for other possible conditions such as Cowden syndrome, for marital and family planning, and whenever the diagnosis is unclear.
A 19-year-old woman presented with diffuse swelling on her neck and face that had been growing gradually over the past 12 years. There was no family history of similar swelling.
Examination revealed a diffuse, hyperpigmented, nodular swelling on the right side of her neck and extending upward to the lower part of the face and the scalp (Figure 1). The rest of the physical examination was normal. A diagnosis of plexiform neurofibroma was made based on the typical clinical presentation. The patient had come primarily because of cosmetic concerns but refused surgery. She was offered genetic counseling and was scheduled for regular follow-up.
Plexiform neurofibroma is mostly associated with autosomal dominant neurofibromatosis type 1, characterized by cutaneous findings such as café-au-lait spots, axillary freckling, skeletal dysplasias (usually of the tibia, fibula, ribs, and vertebrae), Lisch nodules in the eye (iris hamartomas), and neural tumors. Rarely, it may also be seen in germline p16 mutation-positive heritable melanoma and Cowden syndrome.
Diffuse plexiform neurofibroma of the face and neck rarely appears after the age of 1, and rarely develops on other parts of the body after adolescence. In contrast, deep nodular plexiform neurofibroma often originates from spinal nerve roots and usually becomes symptomatic in adulthood.
Plexiform neurofibroma has an 8% to 12% chance of changing into a malignant peripheral nerve-sheath tumor. Continuous pain in the tumor, rapid tumor growth, hardening of the tumor, or weakness or numbness in an arm or leg with a plexiform neurofibroma suggests malignant transformation.
The diagnosis is clinical, and the management involves a multidisciplinary team including a physician, geneticist, neurologist, surgeon, and ophthalmologist. Genetic counseling should be offered to patients for assessment of family risk, to look for other possible conditions such as Cowden syndrome, for marital and family planning, and whenever the diagnosis is unclear.
A 19-year-old woman presented with diffuse swelling on her neck and face that had been growing gradually over the past 12 years. There was no family history of similar swelling.
Examination revealed a diffuse, hyperpigmented, nodular swelling on the right side of her neck and extending upward to the lower part of the face and the scalp (Figure 1). The rest of the physical examination was normal. A diagnosis of plexiform neurofibroma was made based on the typical clinical presentation. The patient had come primarily because of cosmetic concerns but refused surgery. She was offered genetic counseling and was scheduled for regular follow-up.
Plexiform neurofibroma is mostly associated with autosomal dominant neurofibromatosis type 1, characterized by cutaneous findings such as café-au-lait spots, axillary freckling, skeletal dysplasias (usually of the tibia, fibula, ribs, and vertebrae), Lisch nodules in the eye (iris hamartomas), and neural tumors. Rarely, it may also be seen in germline p16 mutation-positive heritable melanoma and Cowden syndrome.
Diffuse plexiform neurofibroma of the face and neck rarely appears after the age of 1, and rarely develops on other parts of the body after adolescence. In contrast, deep nodular plexiform neurofibroma often originates from spinal nerve roots and usually becomes symptomatic in adulthood.
Plexiform neurofibroma has an 8% to 12% chance of changing into a malignant peripheral nerve-sheath tumor. Continuous pain in the tumor, rapid tumor growth, hardening of the tumor, or weakness or numbness in an arm or leg with a plexiform neurofibroma suggests malignant transformation.
The diagnosis is clinical, and the management involves a multidisciplinary team including a physician, geneticist, neurologist, surgeon, and ophthalmologist. Genetic counseling should be offered to patients for assessment of family risk, to look for other possible conditions such as Cowden syndrome, for marital and family planning, and whenever the diagnosis is unclear.
Older LGB veterans report less depression, PTSD than younger peers
Older sexual minority veterans are less likely to report depression and/or posttraumatic stress disorder than their younger peers, a cross-sectional study of 3,157 U.S. veterans showed.
“These findings have important public health implications,” wrote Joan K. Monin, PhD, of Yale University, New Haven, Conn., and her associates. “For instance, it may be beneficial to engage older [lesbian, gay, or bisexual] adults as a social support resource … to help improve and/or protect the mental health of younger LGB veterans.”
Dr. Monin and her associates analyzed data from the National Health and Resilience in Veterans Study (Depress Anxiety. 2013 May;30[5]:432-43), which recruited participants originally by telephone and later by mail in late 2011. Subjects were asked how they characterized their sexual status. Overall, 2,993 people (97.2%) identified as heterosexual, 39 (1.1%) as gay, 12 (0.4%) as lesbian, and 51 (1.4%) as bisexual, the researchers reported (Am J Geriatr Psychiatry 2016 Sep 23. doi: 10.1016/j.jagp.2016.09.006).
The participants’ mental health status was evaluated using several screens, including the Mini-International Neuropsychiatric Interview, a lifetime version of the PTSD Checklist, and the Trauma History Screen.
The researchers found a significant interaction between age and LGB status predicting lifetime diagnosis of depression and/or PTSD (P = .027; adjusted odds ratio, 1.03; 95% confidence interval, 1.01-1.06). Specifically, younger LGB veterans were more likely to screen positive for lifetime depression and/or PTSD than were their older counterparts.
“I was surprised to find that older LGB veterans were reporting less mental health problems than younger LGB veterans,” Dr. Monin said in an interview. “I was expecting to find that older and younger LGB veterans would have similar mental health disparity.”
She and her associates also found that younger LGB veterans have stronger support networks, a factor that further suggests that older age is tied to resilience in this population.
The study can be found here.
Older sexual minority veterans are less likely to report depression and/or posttraumatic stress disorder than their younger peers, a cross-sectional study of 3,157 U.S. veterans showed.
“These findings have important public health implications,” wrote Joan K. Monin, PhD, of Yale University, New Haven, Conn., and her associates. “For instance, it may be beneficial to engage older [lesbian, gay, or bisexual] adults as a social support resource … to help improve and/or protect the mental health of younger LGB veterans.”
Dr. Monin and her associates analyzed data from the National Health and Resilience in Veterans Study (Depress Anxiety. 2013 May;30[5]:432-43), which recruited participants originally by telephone and later by mail in late 2011. Subjects were asked how they characterized their sexual status. Overall, 2,993 people (97.2%) identified as heterosexual, 39 (1.1%) as gay, 12 (0.4%) as lesbian, and 51 (1.4%) as bisexual, the researchers reported (Am J Geriatr Psychiatry 2016 Sep 23. doi: 10.1016/j.jagp.2016.09.006).
The participants’ mental health status was evaluated using several screens, including the Mini-International Neuropsychiatric Interview, a lifetime version of the PTSD Checklist, and the Trauma History Screen.
The researchers found a significant interaction between age and LGB status predicting lifetime diagnosis of depression and/or PTSD (P = .027; adjusted odds ratio, 1.03; 95% confidence interval, 1.01-1.06). Specifically, younger LGB veterans were more likely to screen positive for lifetime depression and/or PTSD than were their older counterparts.
“I was surprised to find that older LGB veterans were reporting less mental health problems than younger LGB veterans,” Dr. Monin said in an interview. “I was expecting to find that older and younger LGB veterans would have similar mental health disparity.”
She and her associates also found that younger LGB veterans have stronger support networks, a factor that further suggests that older age is tied to resilience in this population.
The study can be found here.
Older sexual minority veterans are less likely to report depression and/or posttraumatic stress disorder than their younger peers, a cross-sectional study of 3,157 U.S. veterans showed.
“These findings have important public health implications,” wrote Joan K. Monin, PhD, of Yale University, New Haven, Conn., and her associates. “For instance, it may be beneficial to engage older [lesbian, gay, or bisexual] adults as a social support resource … to help improve and/or protect the mental health of younger LGB veterans.”
Dr. Monin and her associates analyzed data from the National Health and Resilience in Veterans Study (Depress Anxiety. 2013 May;30[5]:432-43), which recruited participants originally by telephone and later by mail in late 2011. Subjects were asked how they characterized their sexual status. Overall, 2,993 people (97.2%) identified as heterosexual, 39 (1.1%) as gay, 12 (0.4%) as lesbian, and 51 (1.4%) as bisexual, the researchers reported (Am J Geriatr Psychiatry 2016 Sep 23. doi: 10.1016/j.jagp.2016.09.006).
The participants’ mental health status was evaluated using several screens, including the Mini-International Neuropsychiatric Interview, a lifetime version of the PTSD Checklist, and the Trauma History Screen.
The researchers found a significant interaction between age and LGB status predicting lifetime diagnosis of depression and/or PTSD (P = .027; adjusted odds ratio, 1.03; 95% confidence interval, 1.01-1.06). Specifically, younger LGB veterans were more likely to screen positive for lifetime depression and/or PTSD than were their older counterparts.
“I was surprised to find that older LGB veterans were reporting less mental health problems than younger LGB veterans,” Dr. Monin said in an interview. “I was expecting to find that older and younger LGB veterans would have similar mental health disparity.”
She and her associates also found that younger LGB veterans have stronger support networks, a factor that further suggests that older age is tied to resilience in this population.
The study can be found here.
FROM THE AMERICAN JOURNAL OF GERIATRIC PSYCHIATRY
Breaking the pain contract: A better controlled-substance agreement for patients on chronic opioid therapy
Regulatory bodies and professional societies have encouraged or mandated written pain treatment agreements for over a decade as a way to establish informed consent, improve adherence, and mitigate risk. Unfortunately, the content of these agreements varies, their efficacy is uncertain, and some are stigmatizing or coercive and jeopardize trust. Additionally, many are written at reading levels beyond most patients’ understanding. However, we believe a well-written agreement is still an important tool in chronic pain management.
In this article, we explore common limitations of current pain treatment “contracts” and propose strategies to improve their usefulness and acceptance.
PAIN AND ITS TREATMENT HAVE COSTS
Chronic pain affects 100 million US adults and is estimated to cost $635 billion each year in treatment, lost wages, and reduced productivity.1
Opioid therapy for chronic noncancer pain is being called into question,2–5 and a 2016 guideline from the US Centers for Disease Control and Prevention has called for more limited and judicious use of opioids in primary care.6 Nevertheless, long-term opioid therapy is probably helpful in some circumstances and will likely continue to have a role in chronic pain management for the foreseeable future.7
Concerns about opioids include risks of overdose and death. Unintentional drug overdoses, typically with opioids, exceeded motor vehicle accidents in 2009 as the leading cause of accidental death in the United States8; by 2014, nearly one and a half times as many people were dying of a drug overdose than of a car accident.9 Even when used appropriately, opioids are associated with sedation, falls, motor vehicle accidents, addiction, and unintended overdose.10
The potential harm extends beyond the patient to the community at large. Diversion of prescription drugs for nonmedical use is common11 and, after marijuana and alcohol abuse, is the most common form of drug abuse in the United States.12 Misuse of prescription drugs costs health insurers an estimated $72.5 billion each year—a cost largely passed on to consumers through higher premiums.13 Most individuals who abuse prescription opioids get them from friends and family, sometimes by stealing them.14
THE SPECIAL ROLE OF THE PRIMARY CARE PHYSICIAN
Chronic pain is extremely prevalent in general internal medicine and primary care practice.15,16 It has tremendous associated medical, social, and economic costs.1
In light of the risks and complexity of opioid use and the increasing regulatory requirements for safe prescribing, some primary care physicians have stopped prescribing opioids altogether and refer patients elsewhere for pain management.
This does a disservice to patients. Primary care physicians cannot entirely avoid chronic pain management or absolutely refuse to prescribe opioids in all circumstances and still provide quality care. And although some primary care physicians may need more training in prescribing opioids, their comprehensive understanding of the patient’s other health issues enables them to address the psychosocial generators and consequences of the patient’s chronic pain more fully than a specialist can.
Furthermore, access to board-certified pain specialists is limited. There are only four such specialists for every 100,000 patients with chronic pain,17 and those who are available often restrict the types of insurance they accept, disproportionately excluding Medicaid patients.
We encourage primary care physicians to undertake continuing medical education and professional development as needed to prescribe opioids as safely and effectively as possible.
A CONTROLLED-SUBSTANCE AGREEMENT INSTEAD OF A ‘NARCOTIC CONTRACT’
To address the challenges of long-term opioid therapy, many state officials, medical licensing boards, professional societies, and other regulatory bodies recommend proactive monitoring and management of prescribing risks. Often promoted and sometimes mandated is the use of a written pain treatment agreement, sometimes called a “pain contract” or “narcotic contract,” in which the patient and the physician ostensibly agree to various conditions under which opioids will be prescribed or discontinued. Although well-intentioned, these documents can cause several problems.
Contracts were being advocated in treating opiate addiction as early as 1981.18 Since then, the term “narcotic contract” has become widely used, even as most professional guidelines have now moved away from using it. A Google search for the term on November 27, 2015, yielded 2,000 results, with numerous examples of the documents in clinical use.
But the phrase is misleading, and we believe physicians should avoid using it. Clinically, the word “narcotic” is imprecise and can refer to substances other than opioids. For example, the US Controlled Substances Act lists cocaine as a narcotic.19 The word also carries a stigma, as law enforcement agencies and drug abuse programs commonly use phrases such as “narcotic task force” or “narcotic treatment program.” On the other hand, the more accurate term “opioid” may be unfamiliar to patients. We recommend using the term “controlled substance” instead.
Similarly, the word “contract” can be perceived as coercive, can erode physician-patient trust, and implies that failure to agree to it will result in loss of access to pain medications.20–23
For these reasons, we encourage physicians to adopt the phrase “controlled-substance agreement” or something similar. This label accurately reflects the specificity of the treatment and connotes a partnership between patient and physician. Furthermore, it allows the physician to use the agreement when prescribing other controlled substances such as benzodiazepines and stimulants that also carry a risk of addiction, misuse, and adverse effects.
STIGMATIZING THE PATIENT
Although no studies have systematically assessed the style and tone of available treatment agreements, many of the agreements seem to stigmatize the patient, using language that is mistrustful, accusatory, and even confrontational and that implies that the patient will misuse or abuse the medications.21,24 For example, “Failure to comply with the terms of the contract will risk loss of medication or discharge from the medical practice” is inflammatory and coercive, but variations of this phrase appear in many of the results of the aforementioned Google search.
Such language defeats attempts to communicate openly and implies a deprecatory attitude towards patients. Stigmatization may result in undertreatment of pain, physician refusal to prescribe opioids, and patient refusal to submit to the terms of a one-sided agreement perceived as unfair. Therefore, poorly written opioid agreements impair the trust necessary for a therapeutic physician-patient relationship and can interfere with optimal pain management.20–23
Some physicians stigmatize inadvertently. Believing that they can identify which patients will misuse their prescriptions, they use controlled-substance agreements only in this subgroup. But in fact, physicians are notoriously poor at predicting which patients will misuse prescription opioids or suffer adverse effects.25 Therefore, it is important to be transparent and consistent with monitoring practices for all patients on chronic opioid therapy.26
Framing the controlled-substance agreement in terms of safety and using it universally can minimize miscommunication and unintentional stigmatization.
SHARED DECISION-MAKING AND CHRONIC OPIOID THERAPY
We recommend using controlled-substance agreements only in the context of personalized patient counseling and shared decision-making.
Shared decision-making promotes mutual respect between patients and physicians, is feasible to implement in primary care, and may improve health outcomes.27,28 A study found that physicians who received 2 hours of training in shared decision-making for chronic opioid therapy were more likely to complete treatment agreements and set mutually agreed-upon functional goals with patients, and they felt more confident, competent, and comfortable treating chronic pain.29 Additionally, after learning about the risks, some patients may choose to forgo opioid therapy.
To be consistent with shared decision-making, the controlled-substance agreement must:
- Engage the patient, emphasizing the shared, reciprocal obligations of physician and patient
- Address goals of treatment that are personalized and mutually agreed-upon and that incorporate the patient’s values and preferences
- Explain treatment options in a way that is understandable and informative for the patient.
Table 1 outlines other key elements in detail.27,30,31
Shared decision-making is especially useful when the balance between the risks and benefits of a treatment plan is uncertain. It is not a substitute for medical expertise, and a patient’s preferences do not override the physician’s clinical judgment. A physician should not offer or implement chronic opioid therapy if he or she believes it is not indicated or is contraindicated, or that the risks for that patient clearly outweigh the benefits.32
THE CONTROLLED-SUBSTANCE AGREEMENT: FOUR OBJECTIVES
Stigmatizing language in the controlled-substance agreement may result from physician ambivalence regarding its intent and objectives. For example, some may perceive the agreement as a way to facilitate communication, while others may use it in a possibly unethical manner to control patient behavior with the threat of cutting off access to pain medication.33
Controlled-substance agreements have four commonly identified objectives,34 explored further below:
- To improve adherence with the safe use of controlled substances while reducing aberrant behaviors
- To obtain informed consent
- To outline the prescribing policies of the practice
- To mitigate the prescriber’s legal risk.
Improving adherence
Many authors say that the primary goal of the controlled-substance agreement is to promote the use of the medication as prescribed, without variance, and from one physician only.35–38 This goal seems reasonable. However, many other classes of medications are also risky when used aberrantly, and we do not ask the patient to sign an agreement when we prescribe them. This double standard may reflect both the inherently higher risks associated with controlled substances and physician ambivalence regarding their use.
Regardless, the efficacy of controlledsubstance agreements in improving safe-use adherence and reducing aberrant medication-taking behaviors is uncertain. A 2010 systematic review based on observational and largely poor-quality studies concluded that using treatment agreements along with urine drug testing modestly reduced opioid misuse,39 while other reports have called their efficacy into question.40 We remain optimistic that well-written controlled-substance agreements can advance this objective, and that absence of evidence is not evidence of absence—ie, lack of efficacy. However, the data are not yet clear.
Interestingly, a 2014 survey found that most primary care physicians thought that controlled-substance agreements do not meaningfully reduce opioid misuse but do give a sense of protection against liability.41 Additionally, these documents are associated with a greater sense of physician satisfaction and mastery,42 and for some physicians these reasons may be enough to justify their use.
Somewhat alarmingly though, one study suggests that many patients do not even know that they signed a treatment agreement.43 Using a controlled-substance agreement without the full awareness and engagement of the patient cannot promote adherence and is likely counterproductive.
Obtaining informed consent
It is essential to discuss possible benefits and risks so that informed and shared decision-making can occur.
Controlled-substance agreements may advance this aim if carefully written, although medical practices often design them for use across a spectrum of patients with varying indications, contraindications, and risks, making these documents inherently inflexible. A one-size-fits-all document does not allow for meaningful personalization and is insufficient without patient-centered counseling.
We strongly recommend that treatment agreements complement but not replace personalized patient-centered counseling about individual risks and benefits. Well-written controlled-substance agreements may reduce the chance of overlooking key risks and launch further customized discussion. Additionally, they can be written in a manner that allows patients and physicians to agree on and document personalized goals (Table 2).
Furthermore, when crafted within a risk-benefit framework, a controlled-substance agreement can help to clarify an ethically important concept, ie, that the physician is judging the safety and appropriateness of the treatment, not the character of the patient.44 The prescriber can focus on evaluating the risks and benefits of treatment choices, not being a police officer or a judge of how “deserving” of opioid therapy the patient is.
Importantly, for patients to provide meaningful informed consent, the agreement must be understandable. A study of 162 opioid treatment agreements found that on average, they were written at a 14th grade level, which is beyond the reading comprehension of most patients.45 Another study evaluated patients’ ability to understand and follow instructions on labels for common prescriptions; even though 70% of the patients could read the labels, only 34.7% could demonstrate the instructions “take two tablets by mouth twice daily.”46
We recommend analyzing all controlled- substance agreements for readability by assessing their Flesch-Kincaid grade level or a similar literacy assessment, using readily available computer apps. The average education level of the patients cared for in each practice will vary based on the demographic served, and the controlled-substance agreement can be modified accordingly, but typically writing the document at the 6th- to 7th-grade reading level is suggested.
Outlining practice policies
Opioids are federally controlled substances with prescribing restrictions that vary based on the drug’s Drug Enforcement Agency schedule. State laws and regulations also govern opioid prescribing and are constantly evolving.47
Refilling opioid prescriptions should be a deliberate process during which the prescriber reviews the appropriateness of the medication and issues the prescription as safely as possible.
To promote practice consistency and to share expectations transparently with patients, we recommend spelling out in the agreement your policies on:
- Who can manage this patient’s opioid therapy
- How to handle refill requests after hours and on weekends
- When and how patients should request opioid refills
- Which pharmacies patients will use
- Whether the practice allows others to pick up refills for the patient.
This not only serves as a reference for patients, who keep a copy for their records, it also reduces the risk of inconsistent processes within the office, which will quickly lead to chaos and confusion among patients and physicians alike. Inconsistent prescription and refill practices can give the impression that a double standard exists and that some patients get more leeway than others, without apparent justification.
There is little evidence that this approach truly improves practice efficiency,34,48 but we believe that it may avert future confusion and conflict.
Mitigating the prescriber’s risk
Most licensing boards and clinical guidelines recommend controlled-substance agreements as part of opioid risk mitigation. These documents are now the standard of care, with many bodies recommending or mandating them, including the Federation of State Medical Boards,49 many states,50 Physicians for Responsible Opioid Prescribing,51 the American Academy of Pain Management,52 and the American Pain Society along with the American Academy of Pain Medicine.53
Historically, primary care physicians have used controlled-substance agreements inconsistently and primarily for patients believed to be at high risk of misuse.54 However, because physicians cannot accurately predict who will misuse or divert medications,25 controlled-substance agreements should be used universally, ie, for all patients prescribed controlled substances.
A controlled-substance agreement can serve as documentation. The patient can keep a copy for future reference, and a cosigned document is evidence that a discussion took place and may lower the risk of malpractice litigation.55 Further, if a state requires physicians to check their prescription monitoring database before prescribing opioids, the controlled-substance agreement can serve to both inform patients about this obligation and to obtain their consent when required.
At a minimum, we recommend that prescribers learn about the regulatory framework in their state and use controlled-substance agreements as legislatively mandated.
A CHECKLIST FOR THE PHYSICIAN AND PATIENT
To facilitate the development and use of ethically appropriate controlled-substance agreements with a focus on shared decision-making, we offer a sample tool in the form of a checklist (Table 2). It can be modified and implemented instead of a traditional controlled-substance agreement or can be used alongside other more comprehensive documents to facilitate discussion.
The model presents critical information for the patient and physician to discuss and acknowledge (initial) in writing. It is divided into three sections: shared responsibilities, patient responsibilities, and physician responsibilities. Each contains an approximately equal number of items; this is deliberate and visually conveys the notion of equivalent and shared responsibilities for patient and physician. The patient, physician, or both should initial each item to indicate their agreement.
The document is customizable for the specific treatment prescribed. It is written at a Flesch-Kincaid grade level of 6.8, consistent with current health literacy recommendations, and avoids medical jargon and complex compound sentences as much as possible.
We indicate key elements of shared decision-making27,30,31 in parentheses in Table 2 and cross-reference them with Table 1, which describes them more fully.
A BETTER TOOL
Both chronic pain and prescription drug abuse are highly prevalent and carry serious consequences. These overlapping epidemics put the prescriber in the difficult position of trying to prevent misuse, abuse, and diversion while simultaneously adequately treating pain.
Physicians and policy makers look to controlled-substance agreements as tools to help them balance the benefits and risks, but frequently at the expense of eroding trust between the patient and physician, stigmatizing the patient, using pejorative and coercive language, not adhering to health literacy guidelines, and failing to share decisions.
We believe a better tool is possible and suggest that controlled-substance agreements be universally applied, use deliberate and understandable language, be framed in terms of safety, and be implemented according to the principles of shared decision-making.
- Committee on Advancing Pain Research Care, Institute of Medicine. Relieving Pain In America: A Blueprint For Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011. 030921484X.
- Von Korff M, Kolodny A, Deyo RA, Chou R. Long-term opioid therapy reconsidered. Ann Intern Med 2011; 155:325–328.
- Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a national institutes of health pathways to prevention workshop. Ann Intern Med 2015; 162:276–286.
- Manchikanti L, Vallejo R, Manchikanti KN, Benyamin RM, Datta S, Christo PJ. Effectiveness of long-term opioid therapy for chronic non-cancer pain. Pain Physician 2011; 14:E133–E156.
- Trescot AM, Glaser SE, Hansen H, Benyamin R, Patel S, Manchikanti L. Effectiveness of opioids in the treatment of chronic non-cancer pain. Pain Physician 2008; 11(suppl):S181–S200.
- Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep 2016; 65(1):1–49.
- Brooks A, Kominek C, Pham TC, Fudin J. Exploring the use of chronic opioid therapy for chronic pain: when, how, and for whom? Med Clin North Am 2016; 100:81–102.
- Paulozzi L, Dellinger A, Degutis L. Lessons from the past. Injury Prev 2012; 18:70.
- Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths - United States, 2000-2014. MMWR Morb Mortal Wkly Rep 2016; 64(50-51):1378–1382.
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 2015; 156:569–576.
- Cicero TJ, Kurtz SP, Surratt HL, et al. Multiple determinants of specific modes of prescription opioid diversion. J Drug Issues 2011; 41:283–304.
- SAMHSA. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014: www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.htm. Accessed October 10, 2015.
- National Drug Intelligence Center, Drug Enforcement Administration. National Prescription Drug Threat Assessment. 2009.
- Jones CM, Paulozzi LJ, Mack KA. Sources of prescription opioid pain relievers by frequency of past-year nonmedical use: United States, 2008-2011. JAMA Intern Med 2014; 174:802–803.
- Clark JD. Chronic pain prevalence and analgesic prescribing in a general medical population. J Pain Symptom Manage 2002; 23:131–137.
- American Academy of Family Physicians. Pain management and opioid abuse: a public health concern. Position paper, executive summary. 2012; www.aafp.org/content/dam/AAFP/documents/patient_care/pain_management/opioid-abuse-position-paper.pdf. Accessed October 10, 2015.
- Breuer B, Pappagallo M, Tai JY, Portenoy RK. U.S. board-certified pain physician practices: uniformity and census data of their locations. J Pain 2007; 8:244–250.
- Rush AJ, Shaw BF. Psychotherapeutic treatment of opiate addiction. Am J Psychother 1981; 35:61–75.
- U.S. Department of Justice, Office of Diversion Control, Title 21 Code of Federal Regulations - Part 1300 - Definitions. 2015; www.deadiversion.usdoj.gov/21cfr/cfr/1300/1300_01.htm. Accessed October 10, 2016.
- McGee S, Silverman RD. Treatment agreements, informed consent, and the role of state medical boards in opioid prescribing. Pain Med 2015; 16:25–29.
- Buchman DZ, Ho A. What’s trust got to do with it? Revisiting opioid contracts. J Med Ethics 2014; 40:673–677.
- Deep K. Use of narcotics contracts. Virtual Mentor 2013; 15:416–420.
- Payne R, Anderson E, Arnold R, et al. A rose by any other name: pain contracts/agreements. Am J Bioethics 2010; 10:5–12.
- Goldberg DSDS. Job and the stigmatization of chronic pain. Perspect Biol Med 2010; 53:425–438.
- Bronstein K PS, Munitz L, Leider H. Can clinicians accurately predict which patients are misusing their medications? American Pain Society 30th Annual Scientific Meeting; May 18–21, 2011, 2011; Austin, TX.
- Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med 2005; 6:107–112.
- Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med 1997; 44:681–692.
- Murray E, Charles C, Gafni A. Shared decision-making in primary care: tailoring the Charles et al model to fit the context of general practice. Patient Educ Couns 2006; 62:205–211.
- Sullivan MD, Leigh J, Gaster B. Brief report: training internists in shared decision making about chronic opioid treatment for noncancer pain. J Gen Intern Med 2006; 21:360–362.
- Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med 1999; 49:651–661.
- Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns 2006; 60:301–312.
- Savage S. The patient-centered opioid treatment agreement. Am J Bioethics 2010; 10:18–19.
- Crowley-Matoka M. How to parse the protective, the punitive and the prejudicial in chronic opioid therapy? Pain 2013; 154:5–6.
- Arnold RM, Han PK, Seltzer D. Opioid contracts in chronic nonmalignant pain management: objectives and uncertainties. Am J Med 2006; 119:292–296.
- Kirkpatrick AF, Derasari M, Kovacs PL, Lamb BD, Miller R, Reading A. A protocol-contract for opioid use in patients with chronic pain not due to malignancy. J Clin Anesth 1998; 10:435–443.
- Fishman SM, Bandman TB, Edwards A, Borsook D. The opioid contract in the management of chronic pain. J Pain Symptom Manage 1999; 18:27–37.
- Hariharan J, Lamb GC, Neuner JM. Long-term opioid contract use for chronic pain management in primary care practice. A five year experience. J Gen Intern Med 2007; 22:485–490.
- Fishman SM, Wilsey B, Yang J, Reisfield GM, Bandman TB, Borsook D. Adherence monitoring and drug surveillance in chronic opioid therapy. J Pain Symptom Manage 2000; 20:293–307.
- Starrels JL, Becker WC, Alford DP, Kapoor A, Williams AR, Turner BJ. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med 2010; 152:712–720.
- King S. How useful are patient opioid agreements and urine drug testing? Psychiatric Times March 2, 2011; www.psychiatrictimes.com/how-useful-are-patient-opioid-agreements-and-urine-drug-testing. Accessed August 2, 2015.
- Starrels JL, Wu B, Peyser D, et al. It made my life a little easier: primary care providers’ beliefs and attitudes about using opioid treatment agreements. J Opioid Manag 2014; 10:95–102.
- Touchet BK, Yates WR, Coon KA. Opioid contract use is associated with physician training level and practice specialty. J Opioid Manage 2005; 1:195–200.
- Penko J, Mattson J, Miaskowski C, Kushel M. Do patients know they are on pain medication agreements? Results from a sample of high-risk patients on chronic opioid therapy. Pain Med 2012; 13:1174–1180.
- Nicolaidis C. Police officer, deal-maker, or health care provider? Moving to a patient-centered framework for chronic opioid management. Pain Med 2011; 12:890–897.
- Roskos SE, Keenum AJ, Newman LM, Wallace LS. Literacy demands and formatting characteristics of opioid contracts in chronic nonmalignant pain management. J Pain 2007; 8:753–758.
- Davis TC, Wolf MS, Bass PF 3rd, et al. Low literacy impairs comprehension of prescription drug warning labels. J Gen Intern Med 2006; 21:847–851.
- American Academy of Pain Medicine. State legislative updates. www.painmed.org/advocacy/state-updates/. Accessed August 5, 2016.
- Burchman SL, Pagel PS. Implementation of a formal treatment agreement for outpatient management of chronic nonmalignant pain with opioid analgesics. J Pain Symptom Manage 1995; 10:556–563.
- Federation of State Medical Boards. Model policy on the use of opioid analgesics in the treatment of chronic pain. 2013; www.fsmb.org/Media/Default/PDF/FSMB/Advocacy/pain_policy_july2013.pdf. Accessed August 2, 2016.
- University of Wisconsin-Madison. Pain & Policy Studies Group. Database of statutes, regulations, & other policies for pain management. www.painpolicy.wisc.edu/database-statutes-regulations-other-policies-pain-management. Accessed August 3, 2016.
- Cameron KA, Rintamaki LS, Kamanda-Kosseh M, Noskin GA, Baker DW, Makoul G. Using theoretical constructs to identify key issues for targeted message design: African American seniors’ perceptions about influenza and influenza vaccination. Health Commun 2009; 24:316–326.
- Kandula NR, Nsiah-Kumi PA, Makoul G, et al. The relationship between health literacy and knowledge improvement after a multimedia type 2 diabetes education program. Patient Educ Couns 2009; 75:321–327.
- Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009; 10:113–130.
- Adams NJ, Plane MB, Fleming MF, Mundt MP, Saunders LA, Stauffacher EA. Opioids and the treatment of chronic pain in a primary care sample. J Pain Symptom Manage 2001; 22:791–796.
- Richeimer SH. Opioids for pain: risk management. Semin Anesthesia Periop Med Pain 2005; 24:165–169.
Regulatory bodies and professional societies have encouraged or mandated written pain treatment agreements for over a decade as a way to establish informed consent, improve adherence, and mitigate risk. Unfortunately, the content of these agreements varies, their efficacy is uncertain, and some are stigmatizing or coercive and jeopardize trust. Additionally, many are written at reading levels beyond most patients’ understanding. However, we believe a well-written agreement is still an important tool in chronic pain management.
In this article, we explore common limitations of current pain treatment “contracts” and propose strategies to improve their usefulness and acceptance.
PAIN AND ITS TREATMENT HAVE COSTS
Chronic pain affects 100 million US adults and is estimated to cost $635 billion each year in treatment, lost wages, and reduced productivity.1
Opioid therapy for chronic noncancer pain is being called into question,2–5 and a 2016 guideline from the US Centers for Disease Control and Prevention has called for more limited and judicious use of opioids in primary care.6 Nevertheless, long-term opioid therapy is probably helpful in some circumstances and will likely continue to have a role in chronic pain management for the foreseeable future.7
Concerns about opioids include risks of overdose and death. Unintentional drug overdoses, typically with opioids, exceeded motor vehicle accidents in 2009 as the leading cause of accidental death in the United States8; by 2014, nearly one and a half times as many people were dying of a drug overdose than of a car accident.9 Even when used appropriately, opioids are associated with sedation, falls, motor vehicle accidents, addiction, and unintended overdose.10
The potential harm extends beyond the patient to the community at large. Diversion of prescription drugs for nonmedical use is common11 and, after marijuana and alcohol abuse, is the most common form of drug abuse in the United States.12 Misuse of prescription drugs costs health insurers an estimated $72.5 billion each year—a cost largely passed on to consumers through higher premiums.13 Most individuals who abuse prescription opioids get them from friends and family, sometimes by stealing them.14
THE SPECIAL ROLE OF THE PRIMARY CARE PHYSICIAN
Chronic pain is extremely prevalent in general internal medicine and primary care practice.15,16 It has tremendous associated medical, social, and economic costs.1
In light of the risks and complexity of opioid use and the increasing regulatory requirements for safe prescribing, some primary care physicians have stopped prescribing opioids altogether and refer patients elsewhere for pain management.
This does a disservice to patients. Primary care physicians cannot entirely avoid chronic pain management or absolutely refuse to prescribe opioids in all circumstances and still provide quality care. And although some primary care physicians may need more training in prescribing opioids, their comprehensive understanding of the patient’s other health issues enables them to address the psychosocial generators and consequences of the patient’s chronic pain more fully than a specialist can.
Furthermore, access to board-certified pain specialists is limited. There are only four such specialists for every 100,000 patients with chronic pain,17 and those who are available often restrict the types of insurance they accept, disproportionately excluding Medicaid patients.
We encourage primary care physicians to undertake continuing medical education and professional development as needed to prescribe opioids as safely and effectively as possible.
A CONTROLLED-SUBSTANCE AGREEMENT INSTEAD OF A ‘NARCOTIC CONTRACT’
To address the challenges of long-term opioid therapy, many state officials, medical licensing boards, professional societies, and other regulatory bodies recommend proactive monitoring and management of prescribing risks. Often promoted and sometimes mandated is the use of a written pain treatment agreement, sometimes called a “pain contract” or “narcotic contract,” in which the patient and the physician ostensibly agree to various conditions under which opioids will be prescribed or discontinued. Although well-intentioned, these documents can cause several problems.
Contracts were being advocated in treating opiate addiction as early as 1981.18 Since then, the term “narcotic contract” has become widely used, even as most professional guidelines have now moved away from using it. A Google search for the term on November 27, 2015, yielded 2,000 results, with numerous examples of the documents in clinical use.
But the phrase is misleading, and we believe physicians should avoid using it. Clinically, the word “narcotic” is imprecise and can refer to substances other than opioids. For example, the US Controlled Substances Act lists cocaine as a narcotic.19 The word also carries a stigma, as law enforcement agencies and drug abuse programs commonly use phrases such as “narcotic task force” or “narcotic treatment program.” On the other hand, the more accurate term “opioid” may be unfamiliar to patients. We recommend using the term “controlled substance” instead.
Similarly, the word “contract” can be perceived as coercive, can erode physician-patient trust, and implies that failure to agree to it will result in loss of access to pain medications.20–23
For these reasons, we encourage physicians to adopt the phrase “controlled-substance agreement” or something similar. This label accurately reflects the specificity of the treatment and connotes a partnership between patient and physician. Furthermore, it allows the physician to use the agreement when prescribing other controlled substances such as benzodiazepines and stimulants that also carry a risk of addiction, misuse, and adverse effects.
STIGMATIZING THE PATIENT
Although no studies have systematically assessed the style and tone of available treatment agreements, many of the agreements seem to stigmatize the patient, using language that is mistrustful, accusatory, and even confrontational and that implies that the patient will misuse or abuse the medications.21,24 For example, “Failure to comply with the terms of the contract will risk loss of medication or discharge from the medical practice” is inflammatory and coercive, but variations of this phrase appear in many of the results of the aforementioned Google search.
Such language defeats attempts to communicate openly and implies a deprecatory attitude towards patients. Stigmatization may result in undertreatment of pain, physician refusal to prescribe opioids, and patient refusal to submit to the terms of a one-sided agreement perceived as unfair. Therefore, poorly written opioid agreements impair the trust necessary for a therapeutic physician-patient relationship and can interfere with optimal pain management.20–23
Some physicians stigmatize inadvertently. Believing that they can identify which patients will misuse their prescriptions, they use controlled-substance agreements only in this subgroup. But in fact, physicians are notoriously poor at predicting which patients will misuse prescription opioids or suffer adverse effects.25 Therefore, it is important to be transparent and consistent with monitoring practices for all patients on chronic opioid therapy.26
Framing the controlled-substance agreement in terms of safety and using it universally can minimize miscommunication and unintentional stigmatization.
SHARED DECISION-MAKING AND CHRONIC OPIOID THERAPY
We recommend using controlled-substance agreements only in the context of personalized patient counseling and shared decision-making.
Shared decision-making promotes mutual respect between patients and physicians, is feasible to implement in primary care, and may improve health outcomes.27,28 A study found that physicians who received 2 hours of training in shared decision-making for chronic opioid therapy were more likely to complete treatment agreements and set mutually agreed-upon functional goals with patients, and they felt more confident, competent, and comfortable treating chronic pain.29 Additionally, after learning about the risks, some patients may choose to forgo opioid therapy.
To be consistent with shared decision-making, the controlled-substance agreement must:
- Engage the patient, emphasizing the shared, reciprocal obligations of physician and patient
- Address goals of treatment that are personalized and mutually agreed-upon and that incorporate the patient’s values and preferences
- Explain treatment options in a way that is understandable and informative for the patient.
Table 1 outlines other key elements in detail.27,30,31
Shared decision-making is especially useful when the balance between the risks and benefits of a treatment plan is uncertain. It is not a substitute for medical expertise, and a patient’s preferences do not override the physician’s clinical judgment. A physician should not offer or implement chronic opioid therapy if he or she believes it is not indicated or is contraindicated, or that the risks for that patient clearly outweigh the benefits.32
THE CONTROLLED-SUBSTANCE AGREEMENT: FOUR OBJECTIVES
Stigmatizing language in the controlled-substance agreement may result from physician ambivalence regarding its intent and objectives. For example, some may perceive the agreement as a way to facilitate communication, while others may use it in a possibly unethical manner to control patient behavior with the threat of cutting off access to pain medication.33
Controlled-substance agreements have four commonly identified objectives,34 explored further below:
- To improve adherence with the safe use of controlled substances while reducing aberrant behaviors
- To obtain informed consent
- To outline the prescribing policies of the practice
- To mitigate the prescriber’s legal risk.
Improving adherence
Many authors say that the primary goal of the controlled-substance agreement is to promote the use of the medication as prescribed, without variance, and from one physician only.35–38 This goal seems reasonable. However, many other classes of medications are also risky when used aberrantly, and we do not ask the patient to sign an agreement when we prescribe them. This double standard may reflect both the inherently higher risks associated with controlled substances and physician ambivalence regarding their use.
Regardless, the efficacy of controlledsubstance agreements in improving safe-use adherence and reducing aberrant medication-taking behaviors is uncertain. A 2010 systematic review based on observational and largely poor-quality studies concluded that using treatment agreements along with urine drug testing modestly reduced opioid misuse,39 while other reports have called their efficacy into question.40 We remain optimistic that well-written controlled-substance agreements can advance this objective, and that absence of evidence is not evidence of absence—ie, lack of efficacy. However, the data are not yet clear.
Interestingly, a 2014 survey found that most primary care physicians thought that controlled-substance agreements do not meaningfully reduce opioid misuse but do give a sense of protection against liability.41 Additionally, these documents are associated with a greater sense of physician satisfaction and mastery,42 and for some physicians these reasons may be enough to justify their use.
Somewhat alarmingly though, one study suggests that many patients do not even know that they signed a treatment agreement.43 Using a controlled-substance agreement without the full awareness and engagement of the patient cannot promote adherence and is likely counterproductive.
Obtaining informed consent
It is essential to discuss possible benefits and risks so that informed and shared decision-making can occur.
Controlled-substance agreements may advance this aim if carefully written, although medical practices often design them for use across a spectrum of patients with varying indications, contraindications, and risks, making these documents inherently inflexible. A one-size-fits-all document does not allow for meaningful personalization and is insufficient without patient-centered counseling.
We strongly recommend that treatment agreements complement but not replace personalized patient-centered counseling about individual risks and benefits. Well-written controlled-substance agreements may reduce the chance of overlooking key risks and launch further customized discussion. Additionally, they can be written in a manner that allows patients and physicians to agree on and document personalized goals (Table 2).
Furthermore, when crafted within a risk-benefit framework, a controlled-substance agreement can help to clarify an ethically important concept, ie, that the physician is judging the safety and appropriateness of the treatment, not the character of the patient.44 The prescriber can focus on evaluating the risks and benefits of treatment choices, not being a police officer or a judge of how “deserving” of opioid therapy the patient is.
Importantly, for patients to provide meaningful informed consent, the agreement must be understandable. A study of 162 opioid treatment agreements found that on average, they were written at a 14th grade level, which is beyond the reading comprehension of most patients.45 Another study evaluated patients’ ability to understand and follow instructions on labels for common prescriptions; even though 70% of the patients could read the labels, only 34.7% could demonstrate the instructions “take two tablets by mouth twice daily.”46
We recommend analyzing all controlled- substance agreements for readability by assessing their Flesch-Kincaid grade level or a similar literacy assessment, using readily available computer apps. The average education level of the patients cared for in each practice will vary based on the demographic served, and the controlled-substance agreement can be modified accordingly, but typically writing the document at the 6th- to 7th-grade reading level is suggested.
Outlining practice policies
Opioids are federally controlled substances with prescribing restrictions that vary based on the drug’s Drug Enforcement Agency schedule. State laws and regulations also govern opioid prescribing and are constantly evolving.47
Refilling opioid prescriptions should be a deliberate process during which the prescriber reviews the appropriateness of the medication and issues the prescription as safely as possible.
To promote practice consistency and to share expectations transparently with patients, we recommend spelling out in the agreement your policies on:
- Who can manage this patient’s opioid therapy
- How to handle refill requests after hours and on weekends
- When and how patients should request opioid refills
- Which pharmacies patients will use
- Whether the practice allows others to pick up refills for the patient.
This not only serves as a reference for patients, who keep a copy for their records, it also reduces the risk of inconsistent processes within the office, which will quickly lead to chaos and confusion among patients and physicians alike. Inconsistent prescription and refill practices can give the impression that a double standard exists and that some patients get more leeway than others, without apparent justification.
There is little evidence that this approach truly improves practice efficiency,34,48 but we believe that it may avert future confusion and conflict.
Mitigating the prescriber’s risk
Most licensing boards and clinical guidelines recommend controlled-substance agreements as part of opioid risk mitigation. These documents are now the standard of care, with many bodies recommending or mandating them, including the Federation of State Medical Boards,49 many states,50 Physicians for Responsible Opioid Prescribing,51 the American Academy of Pain Management,52 and the American Pain Society along with the American Academy of Pain Medicine.53
Historically, primary care physicians have used controlled-substance agreements inconsistently and primarily for patients believed to be at high risk of misuse.54 However, because physicians cannot accurately predict who will misuse or divert medications,25 controlled-substance agreements should be used universally, ie, for all patients prescribed controlled substances.
A controlled-substance agreement can serve as documentation. The patient can keep a copy for future reference, and a cosigned document is evidence that a discussion took place and may lower the risk of malpractice litigation.55 Further, if a state requires physicians to check their prescription monitoring database before prescribing opioids, the controlled-substance agreement can serve to both inform patients about this obligation and to obtain their consent when required.
At a minimum, we recommend that prescribers learn about the regulatory framework in their state and use controlled-substance agreements as legislatively mandated.
A CHECKLIST FOR THE PHYSICIAN AND PATIENT
To facilitate the development and use of ethically appropriate controlled-substance agreements with a focus on shared decision-making, we offer a sample tool in the form of a checklist (Table 2). It can be modified and implemented instead of a traditional controlled-substance agreement or can be used alongside other more comprehensive documents to facilitate discussion.
The model presents critical information for the patient and physician to discuss and acknowledge (initial) in writing. It is divided into three sections: shared responsibilities, patient responsibilities, and physician responsibilities. Each contains an approximately equal number of items; this is deliberate and visually conveys the notion of equivalent and shared responsibilities for patient and physician. The patient, physician, or both should initial each item to indicate their agreement.
The document is customizable for the specific treatment prescribed. It is written at a Flesch-Kincaid grade level of 6.8, consistent with current health literacy recommendations, and avoids medical jargon and complex compound sentences as much as possible.
We indicate key elements of shared decision-making27,30,31 in parentheses in Table 2 and cross-reference them with Table 1, which describes them more fully.
A BETTER TOOL
Both chronic pain and prescription drug abuse are highly prevalent and carry serious consequences. These overlapping epidemics put the prescriber in the difficult position of trying to prevent misuse, abuse, and diversion while simultaneously adequately treating pain.
Physicians and policy makers look to controlled-substance agreements as tools to help them balance the benefits and risks, but frequently at the expense of eroding trust between the patient and physician, stigmatizing the patient, using pejorative and coercive language, not adhering to health literacy guidelines, and failing to share decisions.
We believe a better tool is possible and suggest that controlled-substance agreements be universally applied, use deliberate and understandable language, be framed in terms of safety, and be implemented according to the principles of shared decision-making.
Regulatory bodies and professional societies have encouraged or mandated written pain treatment agreements for over a decade as a way to establish informed consent, improve adherence, and mitigate risk. Unfortunately, the content of these agreements varies, their efficacy is uncertain, and some are stigmatizing or coercive and jeopardize trust. Additionally, many are written at reading levels beyond most patients’ understanding. However, we believe a well-written agreement is still an important tool in chronic pain management.
In this article, we explore common limitations of current pain treatment “contracts” and propose strategies to improve their usefulness and acceptance.
PAIN AND ITS TREATMENT HAVE COSTS
Chronic pain affects 100 million US adults and is estimated to cost $635 billion each year in treatment, lost wages, and reduced productivity.1
Opioid therapy for chronic noncancer pain is being called into question,2–5 and a 2016 guideline from the US Centers for Disease Control and Prevention has called for more limited and judicious use of opioids in primary care.6 Nevertheless, long-term opioid therapy is probably helpful in some circumstances and will likely continue to have a role in chronic pain management for the foreseeable future.7
Concerns about opioids include risks of overdose and death. Unintentional drug overdoses, typically with opioids, exceeded motor vehicle accidents in 2009 as the leading cause of accidental death in the United States8; by 2014, nearly one and a half times as many people were dying of a drug overdose than of a car accident.9 Even when used appropriately, opioids are associated with sedation, falls, motor vehicle accidents, addiction, and unintended overdose.10
The potential harm extends beyond the patient to the community at large. Diversion of prescription drugs for nonmedical use is common11 and, after marijuana and alcohol abuse, is the most common form of drug abuse in the United States.12 Misuse of prescription drugs costs health insurers an estimated $72.5 billion each year—a cost largely passed on to consumers through higher premiums.13 Most individuals who abuse prescription opioids get them from friends and family, sometimes by stealing them.14
THE SPECIAL ROLE OF THE PRIMARY CARE PHYSICIAN
Chronic pain is extremely prevalent in general internal medicine and primary care practice.15,16 It has tremendous associated medical, social, and economic costs.1
In light of the risks and complexity of opioid use and the increasing regulatory requirements for safe prescribing, some primary care physicians have stopped prescribing opioids altogether and refer patients elsewhere for pain management.
This does a disservice to patients. Primary care physicians cannot entirely avoid chronic pain management or absolutely refuse to prescribe opioids in all circumstances and still provide quality care. And although some primary care physicians may need more training in prescribing opioids, their comprehensive understanding of the patient’s other health issues enables them to address the psychosocial generators and consequences of the patient’s chronic pain more fully than a specialist can.
Furthermore, access to board-certified pain specialists is limited. There are only four such specialists for every 100,000 patients with chronic pain,17 and those who are available often restrict the types of insurance they accept, disproportionately excluding Medicaid patients.
We encourage primary care physicians to undertake continuing medical education and professional development as needed to prescribe opioids as safely and effectively as possible.
A CONTROLLED-SUBSTANCE AGREEMENT INSTEAD OF A ‘NARCOTIC CONTRACT’
To address the challenges of long-term opioid therapy, many state officials, medical licensing boards, professional societies, and other regulatory bodies recommend proactive monitoring and management of prescribing risks. Often promoted and sometimes mandated is the use of a written pain treatment agreement, sometimes called a “pain contract” or “narcotic contract,” in which the patient and the physician ostensibly agree to various conditions under which opioids will be prescribed or discontinued. Although well-intentioned, these documents can cause several problems.
Contracts were being advocated in treating opiate addiction as early as 1981.18 Since then, the term “narcotic contract” has become widely used, even as most professional guidelines have now moved away from using it. A Google search for the term on November 27, 2015, yielded 2,000 results, with numerous examples of the documents in clinical use.
But the phrase is misleading, and we believe physicians should avoid using it. Clinically, the word “narcotic” is imprecise and can refer to substances other than opioids. For example, the US Controlled Substances Act lists cocaine as a narcotic.19 The word also carries a stigma, as law enforcement agencies and drug abuse programs commonly use phrases such as “narcotic task force” or “narcotic treatment program.” On the other hand, the more accurate term “opioid” may be unfamiliar to patients. We recommend using the term “controlled substance” instead.
Similarly, the word “contract” can be perceived as coercive, can erode physician-patient trust, and implies that failure to agree to it will result in loss of access to pain medications.20–23
For these reasons, we encourage physicians to adopt the phrase “controlled-substance agreement” or something similar. This label accurately reflects the specificity of the treatment and connotes a partnership between patient and physician. Furthermore, it allows the physician to use the agreement when prescribing other controlled substances such as benzodiazepines and stimulants that also carry a risk of addiction, misuse, and adverse effects.
STIGMATIZING THE PATIENT
Although no studies have systematically assessed the style and tone of available treatment agreements, many of the agreements seem to stigmatize the patient, using language that is mistrustful, accusatory, and even confrontational and that implies that the patient will misuse or abuse the medications.21,24 For example, “Failure to comply with the terms of the contract will risk loss of medication or discharge from the medical practice” is inflammatory and coercive, but variations of this phrase appear in many of the results of the aforementioned Google search.
Such language defeats attempts to communicate openly and implies a deprecatory attitude towards patients. Stigmatization may result in undertreatment of pain, physician refusal to prescribe opioids, and patient refusal to submit to the terms of a one-sided agreement perceived as unfair. Therefore, poorly written opioid agreements impair the trust necessary for a therapeutic physician-patient relationship and can interfere with optimal pain management.20–23
Some physicians stigmatize inadvertently. Believing that they can identify which patients will misuse their prescriptions, they use controlled-substance agreements only in this subgroup. But in fact, physicians are notoriously poor at predicting which patients will misuse prescription opioids or suffer adverse effects.25 Therefore, it is important to be transparent and consistent with monitoring practices for all patients on chronic opioid therapy.26
Framing the controlled-substance agreement in terms of safety and using it universally can minimize miscommunication and unintentional stigmatization.
SHARED DECISION-MAKING AND CHRONIC OPIOID THERAPY
We recommend using controlled-substance agreements only in the context of personalized patient counseling and shared decision-making.
Shared decision-making promotes mutual respect between patients and physicians, is feasible to implement in primary care, and may improve health outcomes.27,28 A study found that physicians who received 2 hours of training in shared decision-making for chronic opioid therapy were more likely to complete treatment agreements and set mutually agreed-upon functional goals with patients, and they felt more confident, competent, and comfortable treating chronic pain.29 Additionally, after learning about the risks, some patients may choose to forgo opioid therapy.
To be consistent with shared decision-making, the controlled-substance agreement must:
- Engage the patient, emphasizing the shared, reciprocal obligations of physician and patient
- Address goals of treatment that are personalized and mutually agreed-upon and that incorporate the patient’s values and preferences
- Explain treatment options in a way that is understandable and informative for the patient.
Table 1 outlines other key elements in detail.27,30,31
Shared decision-making is especially useful when the balance between the risks and benefits of a treatment plan is uncertain. It is not a substitute for medical expertise, and a patient’s preferences do not override the physician’s clinical judgment. A physician should not offer or implement chronic opioid therapy if he or she believes it is not indicated or is contraindicated, or that the risks for that patient clearly outweigh the benefits.32
THE CONTROLLED-SUBSTANCE AGREEMENT: FOUR OBJECTIVES
Stigmatizing language in the controlled-substance agreement may result from physician ambivalence regarding its intent and objectives. For example, some may perceive the agreement as a way to facilitate communication, while others may use it in a possibly unethical manner to control patient behavior with the threat of cutting off access to pain medication.33
Controlled-substance agreements have four commonly identified objectives,34 explored further below:
- To improve adherence with the safe use of controlled substances while reducing aberrant behaviors
- To obtain informed consent
- To outline the prescribing policies of the practice
- To mitigate the prescriber’s legal risk.
Improving adherence
Many authors say that the primary goal of the controlled-substance agreement is to promote the use of the medication as prescribed, without variance, and from one physician only.35–38 This goal seems reasonable. However, many other classes of medications are also risky when used aberrantly, and we do not ask the patient to sign an agreement when we prescribe them. This double standard may reflect both the inherently higher risks associated with controlled substances and physician ambivalence regarding their use.
Regardless, the efficacy of controlledsubstance agreements in improving safe-use adherence and reducing aberrant medication-taking behaviors is uncertain. A 2010 systematic review based on observational and largely poor-quality studies concluded that using treatment agreements along with urine drug testing modestly reduced opioid misuse,39 while other reports have called their efficacy into question.40 We remain optimistic that well-written controlled-substance agreements can advance this objective, and that absence of evidence is not evidence of absence—ie, lack of efficacy. However, the data are not yet clear.
Interestingly, a 2014 survey found that most primary care physicians thought that controlled-substance agreements do not meaningfully reduce opioid misuse but do give a sense of protection against liability.41 Additionally, these documents are associated with a greater sense of physician satisfaction and mastery,42 and for some physicians these reasons may be enough to justify their use.
Somewhat alarmingly though, one study suggests that many patients do not even know that they signed a treatment agreement.43 Using a controlled-substance agreement without the full awareness and engagement of the patient cannot promote adherence and is likely counterproductive.
Obtaining informed consent
It is essential to discuss possible benefits and risks so that informed and shared decision-making can occur.
Controlled-substance agreements may advance this aim if carefully written, although medical practices often design them for use across a spectrum of patients with varying indications, contraindications, and risks, making these documents inherently inflexible. A one-size-fits-all document does not allow for meaningful personalization and is insufficient without patient-centered counseling.
We strongly recommend that treatment agreements complement but not replace personalized patient-centered counseling about individual risks and benefits. Well-written controlled-substance agreements may reduce the chance of overlooking key risks and launch further customized discussion. Additionally, they can be written in a manner that allows patients and physicians to agree on and document personalized goals (Table 2).
Furthermore, when crafted within a risk-benefit framework, a controlled-substance agreement can help to clarify an ethically important concept, ie, that the physician is judging the safety and appropriateness of the treatment, not the character of the patient.44 The prescriber can focus on evaluating the risks and benefits of treatment choices, not being a police officer or a judge of how “deserving” of opioid therapy the patient is.
Importantly, for patients to provide meaningful informed consent, the agreement must be understandable. A study of 162 opioid treatment agreements found that on average, they were written at a 14th grade level, which is beyond the reading comprehension of most patients.45 Another study evaluated patients’ ability to understand and follow instructions on labels for common prescriptions; even though 70% of the patients could read the labels, only 34.7% could demonstrate the instructions “take two tablets by mouth twice daily.”46
We recommend analyzing all controlled- substance agreements for readability by assessing their Flesch-Kincaid grade level or a similar literacy assessment, using readily available computer apps. The average education level of the patients cared for in each practice will vary based on the demographic served, and the controlled-substance agreement can be modified accordingly, but typically writing the document at the 6th- to 7th-grade reading level is suggested.
Outlining practice policies
Opioids are federally controlled substances with prescribing restrictions that vary based on the drug’s Drug Enforcement Agency schedule. State laws and regulations also govern opioid prescribing and are constantly evolving.47
Refilling opioid prescriptions should be a deliberate process during which the prescriber reviews the appropriateness of the medication and issues the prescription as safely as possible.
To promote practice consistency and to share expectations transparently with patients, we recommend spelling out in the agreement your policies on:
- Who can manage this patient’s opioid therapy
- How to handle refill requests after hours and on weekends
- When and how patients should request opioid refills
- Which pharmacies patients will use
- Whether the practice allows others to pick up refills for the patient.
This not only serves as a reference for patients, who keep a copy for their records, it also reduces the risk of inconsistent processes within the office, which will quickly lead to chaos and confusion among patients and physicians alike. Inconsistent prescription and refill practices can give the impression that a double standard exists and that some patients get more leeway than others, without apparent justification.
There is little evidence that this approach truly improves practice efficiency,34,48 but we believe that it may avert future confusion and conflict.
Mitigating the prescriber’s risk
Most licensing boards and clinical guidelines recommend controlled-substance agreements as part of opioid risk mitigation. These documents are now the standard of care, with many bodies recommending or mandating them, including the Federation of State Medical Boards,49 many states,50 Physicians for Responsible Opioid Prescribing,51 the American Academy of Pain Management,52 and the American Pain Society along with the American Academy of Pain Medicine.53
Historically, primary care physicians have used controlled-substance agreements inconsistently and primarily for patients believed to be at high risk of misuse.54 However, because physicians cannot accurately predict who will misuse or divert medications,25 controlled-substance agreements should be used universally, ie, for all patients prescribed controlled substances.
A controlled-substance agreement can serve as documentation. The patient can keep a copy for future reference, and a cosigned document is evidence that a discussion took place and may lower the risk of malpractice litigation.55 Further, if a state requires physicians to check their prescription monitoring database before prescribing opioids, the controlled-substance agreement can serve to both inform patients about this obligation and to obtain their consent when required.
At a minimum, we recommend that prescribers learn about the regulatory framework in their state and use controlled-substance agreements as legislatively mandated.
A CHECKLIST FOR THE PHYSICIAN AND PATIENT
To facilitate the development and use of ethically appropriate controlled-substance agreements with a focus on shared decision-making, we offer a sample tool in the form of a checklist (Table 2). It can be modified and implemented instead of a traditional controlled-substance agreement or can be used alongside other more comprehensive documents to facilitate discussion.
The model presents critical information for the patient and physician to discuss and acknowledge (initial) in writing. It is divided into three sections: shared responsibilities, patient responsibilities, and physician responsibilities. Each contains an approximately equal number of items; this is deliberate and visually conveys the notion of equivalent and shared responsibilities for patient and physician. The patient, physician, or both should initial each item to indicate their agreement.
The document is customizable for the specific treatment prescribed. It is written at a Flesch-Kincaid grade level of 6.8, consistent with current health literacy recommendations, and avoids medical jargon and complex compound sentences as much as possible.
We indicate key elements of shared decision-making27,30,31 in parentheses in Table 2 and cross-reference them with Table 1, which describes them more fully.
A BETTER TOOL
Both chronic pain and prescription drug abuse are highly prevalent and carry serious consequences. These overlapping epidemics put the prescriber in the difficult position of trying to prevent misuse, abuse, and diversion while simultaneously adequately treating pain.
Physicians and policy makers look to controlled-substance agreements as tools to help them balance the benefits and risks, but frequently at the expense of eroding trust between the patient and physician, stigmatizing the patient, using pejorative and coercive language, not adhering to health literacy guidelines, and failing to share decisions.
We believe a better tool is possible and suggest that controlled-substance agreements be universally applied, use deliberate and understandable language, be framed in terms of safety, and be implemented according to the principles of shared decision-making.
- Committee on Advancing Pain Research Care, Institute of Medicine. Relieving Pain In America: A Blueprint For Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011. 030921484X.
- Von Korff M, Kolodny A, Deyo RA, Chou R. Long-term opioid therapy reconsidered. Ann Intern Med 2011; 155:325–328.
- Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a national institutes of health pathways to prevention workshop. Ann Intern Med 2015; 162:276–286.
- Manchikanti L, Vallejo R, Manchikanti KN, Benyamin RM, Datta S, Christo PJ. Effectiveness of long-term opioid therapy for chronic non-cancer pain. Pain Physician 2011; 14:E133–E156.
- Trescot AM, Glaser SE, Hansen H, Benyamin R, Patel S, Manchikanti L. Effectiveness of opioids in the treatment of chronic non-cancer pain. Pain Physician 2008; 11(suppl):S181–S200.
- Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep 2016; 65(1):1–49.
- Brooks A, Kominek C, Pham TC, Fudin J. Exploring the use of chronic opioid therapy for chronic pain: when, how, and for whom? Med Clin North Am 2016; 100:81–102.
- Paulozzi L, Dellinger A, Degutis L. Lessons from the past. Injury Prev 2012; 18:70.
- Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths - United States, 2000-2014. MMWR Morb Mortal Wkly Rep 2016; 64(50-51):1378–1382.
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 2015; 156:569–576.
- Cicero TJ, Kurtz SP, Surratt HL, et al. Multiple determinants of specific modes of prescription opioid diversion. J Drug Issues 2011; 41:283–304.
- SAMHSA. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014: www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.htm. Accessed October 10, 2015.
- National Drug Intelligence Center, Drug Enforcement Administration. National Prescription Drug Threat Assessment. 2009.
- Jones CM, Paulozzi LJ, Mack KA. Sources of prescription opioid pain relievers by frequency of past-year nonmedical use: United States, 2008-2011. JAMA Intern Med 2014; 174:802–803.
- Clark JD. Chronic pain prevalence and analgesic prescribing in a general medical population. J Pain Symptom Manage 2002; 23:131–137.
- American Academy of Family Physicians. Pain management and opioid abuse: a public health concern. Position paper, executive summary. 2012; www.aafp.org/content/dam/AAFP/documents/patient_care/pain_management/opioid-abuse-position-paper.pdf. Accessed October 10, 2015.
- Breuer B, Pappagallo M, Tai JY, Portenoy RK. U.S. board-certified pain physician practices: uniformity and census data of their locations. J Pain 2007; 8:244–250.
- Rush AJ, Shaw BF. Psychotherapeutic treatment of opiate addiction. Am J Psychother 1981; 35:61–75.
- U.S. Department of Justice, Office of Diversion Control, Title 21 Code of Federal Regulations - Part 1300 - Definitions. 2015; www.deadiversion.usdoj.gov/21cfr/cfr/1300/1300_01.htm. Accessed October 10, 2016.
- McGee S, Silverman RD. Treatment agreements, informed consent, and the role of state medical boards in opioid prescribing. Pain Med 2015; 16:25–29.
- Buchman DZ, Ho A. What’s trust got to do with it? Revisiting opioid contracts. J Med Ethics 2014; 40:673–677.
- Deep K. Use of narcotics contracts. Virtual Mentor 2013; 15:416–420.
- Payne R, Anderson E, Arnold R, et al. A rose by any other name: pain contracts/agreements. Am J Bioethics 2010; 10:5–12.
- Goldberg DSDS. Job and the stigmatization of chronic pain. Perspect Biol Med 2010; 53:425–438.
- Bronstein K PS, Munitz L, Leider H. Can clinicians accurately predict which patients are misusing their medications? American Pain Society 30th Annual Scientific Meeting; May 18–21, 2011, 2011; Austin, TX.
- Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med 2005; 6:107–112.
- Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med 1997; 44:681–692.
- Murray E, Charles C, Gafni A. Shared decision-making in primary care: tailoring the Charles et al model to fit the context of general practice. Patient Educ Couns 2006; 62:205–211.
- Sullivan MD, Leigh J, Gaster B. Brief report: training internists in shared decision making about chronic opioid treatment for noncancer pain. J Gen Intern Med 2006; 21:360–362.
- Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med 1999; 49:651–661.
- Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns 2006; 60:301–312.
- Savage S. The patient-centered opioid treatment agreement. Am J Bioethics 2010; 10:18–19.
- Crowley-Matoka M. How to parse the protective, the punitive and the prejudicial in chronic opioid therapy? Pain 2013; 154:5–6.
- Arnold RM, Han PK, Seltzer D. Opioid contracts in chronic nonmalignant pain management: objectives and uncertainties. Am J Med 2006; 119:292–296.
- Kirkpatrick AF, Derasari M, Kovacs PL, Lamb BD, Miller R, Reading A. A protocol-contract for opioid use in patients with chronic pain not due to malignancy. J Clin Anesth 1998; 10:435–443.
- Fishman SM, Bandman TB, Edwards A, Borsook D. The opioid contract in the management of chronic pain. J Pain Symptom Manage 1999; 18:27–37.
- Hariharan J, Lamb GC, Neuner JM. Long-term opioid contract use for chronic pain management in primary care practice. A five year experience. J Gen Intern Med 2007; 22:485–490.
- Fishman SM, Wilsey B, Yang J, Reisfield GM, Bandman TB, Borsook D. Adherence monitoring and drug surveillance in chronic opioid therapy. J Pain Symptom Manage 2000; 20:293–307.
- Starrels JL, Becker WC, Alford DP, Kapoor A, Williams AR, Turner BJ. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med 2010; 152:712–720.
- King S. How useful are patient opioid agreements and urine drug testing? Psychiatric Times March 2, 2011; www.psychiatrictimes.com/how-useful-are-patient-opioid-agreements-and-urine-drug-testing. Accessed August 2, 2015.
- Starrels JL, Wu B, Peyser D, et al. It made my life a little easier: primary care providers’ beliefs and attitudes about using opioid treatment agreements. J Opioid Manag 2014; 10:95–102.
- Touchet BK, Yates WR, Coon KA. Opioid contract use is associated with physician training level and practice specialty. J Opioid Manage 2005; 1:195–200.
- Penko J, Mattson J, Miaskowski C, Kushel M. Do patients know they are on pain medication agreements? Results from a sample of high-risk patients on chronic opioid therapy. Pain Med 2012; 13:1174–1180.
- Nicolaidis C. Police officer, deal-maker, or health care provider? Moving to a patient-centered framework for chronic opioid management. Pain Med 2011; 12:890–897.
- Roskos SE, Keenum AJ, Newman LM, Wallace LS. Literacy demands and formatting characteristics of opioid contracts in chronic nonmalignant pain management. J Pain 2007; 8:753–758.
- Davis TC, Wolf MS, Bass PF 3rd, et al. Low literacy impairs comprehension of prescription drug warning labels. J Gen Intern Med 2006; 21:847–851.
- American Academy of Pain Medicine. State legislative updates. www.painmed.org/advocacy/state-updates/. Accessed August 5, 2016.
- Burchman SL, Pagel PS. Implementation of a formal treatment agreement for outpatient management of chronic nonmalignant pain with opioid analgesics. J Pain Symptom Manage 1995; 10:556–563.
- Federation of State Medical Boards. Model policy on the use of opioid analgesics in the treatment of chronic pain. 2013; www.fsmb.org/Media/Default/PDF/FSMB/Advocacy/pain_policy_july2013.pdf. Accessed August 2, 2016.
- University of Wisconsin-Madison. Pain & Policy Studies Group. Database of statutes, regulations, & other policies for pain management. www.painpolicy.wisc.edu/database-statutes-regulations-other-policies-pain-management. Accessed August 3, 2016.
- Cameron KA, Rintamaki LS, Kamanda-Kosseh M, Noskin GA, Baker DW, Makoul G. Using theoretical constructs to identify key issues for targeted message design: African American seniors’ perceptions about influenza and influenza vaccination. Health Commun 2009; 24:316–326.
- Kandula NR, Nsiah-Kumi PA, Makoul G, et al. The relationship between health literacy and knowledge improvement after a multimedia type 2 diabetes education program. Patient Educ Couns 2009; 75:321–327.
- Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009; 10:113–130.
- Adams NJ, Plane MB, Fleming MF, Mundt MP, Saunders LA, Stauffacher EA. Opioids and the treatment of chronic pain in a primary care sample. J Pain Symptom Manage 2001; 22:791–796.
- Richeimer SH. Opioids for pain: risk management. Semin Anesthesia Periop Med Pain 2005; 24:165–169.
- Committee on Advancing Pain Research Care, Institute of Medicine. Relieving Pain In America: A Blueprint For Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011. 030921484X.
- Von Korff M, Kolodny A, Deyo RA, Chou R. Long-term opioid therapy reconsidered. Ann Intern Med 2011; 155:325–328.
- Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a national institutes of health pathways to prevention workshop. Ann Intern Med 2015; 162:276–286.
- Manchikanti L, Vallejo R, Manchikanti KN, Benyamin RM, Datta S, Christo PJ. Effectiveness of long-term opioid therapy for chronic non-cancer pain. Pain Physician 2011; 14:E133–E156.
- Trescot AM, Glaser SE, Hansen H, Benyamin R, Patel S, Manchikanti L. Effectiveness of opioids in the treatment of chronic non-cancer pain. Pain Physician 2008; 11(suppl):S181–S200.
- Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep 2016; 65(1):1–49.
- Brooks A, Kominek C, Pham TC, Fudin J. Exploring the use of chronic opioid therapy for chronic pain: when, how, and for whom? Med Clin North Am 2016; 100:81–102.
- Paulozzi L, Dellinger A, Degutis L. Lessons from the past. Injury Prev 2012; 18:70.
- Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths - United States, 2000-2014. MMWR Morb Mortal Wkly Rep 2016; 64(50-51):1378–1382.
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 2015; 156:569–576.
- Cicero TJ, Kurtz SP, Surratt HL, et al. Multiple determinants of specific modes of prescription opioid diversion. J Drug Issues 2011; 41:283–304.
- SAMHSA. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014: www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.htm. Accessed October 10, 2015.
- National Drug Intelligence Center, Drug Enforcement Administration. National Prescription Drug Threat Assessment. 2009.
- Jones CM, Paulozzi LJ, Mack KA. Sources of prescription opioid pain relievers by frequency of past-year nonmedical use: United States, 2008-2011. JAMA Intern Med 2014; 174:802–803.
- Clark JD. Chronic pain prevalence and analgesic prescribing in a general medical population. J Pain Symptom Manage 2002; 23:131–137.
- American Academy of Family Physicians. Pain management and opioid abuse: a public health concern. Position paper, executive summary. 2012; www.aafp.org/content/dam/AAFP/documents/patient_care/pain_management/opioid-abuse-position-paper.pdf. Accessed October 10, 2015.
- Breuer B, Pappagallo M, Tai JY, Portenoy RK. U.S. board-certified pain physician practices: uniformity and census data of their locations. J Pain 2007; 8:244–250.
- Rush AJ, Shaw BF. Psychotherapeutic treatment of opiate addiction. Am J Psychother 1981; 35:61–75.
- U.S. Department of Justice, Office of Diversion Control, Title 21 Code of Federal Regulations - Part 1300 - Definitions. 2015; www.deadiversion.usdoj.gov/21cfr/cfr/1300/1300_01.htm. Accessed October 10, 2016.
- McGee S, Silverman RD. Treatment agreements, informed consent, and the role of state medical boards in opioid prescribing. Pain Med 2015; 16:25–29.
- Buchman DZ, Ho A. What’s trust got to do with it? Revisiting opioid contracts. J Med Ethics 2014; 40:673–677.
- Deep K. Use of narcotics contracts. Virtual Mentor 2013; 15:416–420.
- Payne R, Anderson E, Arnold R, et al. A rose by any other name: pain contracts/agreements. Am J Bioethics 2010; 10:5–12.
- Goldberg DSDS. Job and the stigmatization of chronic pain. Perspect Biol Med 2010; 53:425–438.
- Bronstein K PS, Munitz L, Leider H. Can clinicians accurately predict which patients are misusing their medications? American Pain Society 30th Annual Scientific Meeting; May 18–21, 2011, 2011; Austin, TX.
- Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med 2005; 6:107–112.
- Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med 1997; 44:681–692.
- Murray E, Charles C, Gafni A. Shared decision-making in primary care: tailoring the Charles et al model to fit the context of general practice. Patient Educ Couns 2006; 62:205–211.
- Sullivan MD, Leigh J, Gaster B. Brief report: training internists in shared decision making about chronic opioid treatment for noncancer pain. J Gen Intern Med 2006; 21:360–362.
- Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med 1999; 49:651–661.
- Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns 2006; 60:301–312.
- Savage S. The patient-centered opioid treatment agreement. Am J Bioethics 2010; 10:18–19.
- Crowley-Matoka M. How to parse the protective, the punitive and the prejudicial in chronic opioid therapy? Pain 2013; 154:5–6.
- Arnold RM, Han PK, Seltzer D. Opioid contracts in chronic nonmalignant pain management: objectives and uncertainties. Am J Med 2006; 119:292–296.
- Kirkpatrick AF, Derasari M, Kovacs PL, Lamb BD, Miller R, Reading A. A protocol-contract for opioid use in patients with chronic pain not due to malignancy. J Clin Anesth 1998; 10:435–443.
- Fishman SM, Bandman TB, Edwards A, Borsook D. The opioid contract in the management of chronic pain. J Pain Symptom Manage 1999; 18:27–37.
- Hariharan J, Lamb GC, Neuner JM. Long-term opioid contract use for chronic pain management in primary care practice. A five year experience. J Gen Intern Med 2007; 22:485–490.
- Fishman SM, Wilsey B, Yang J, Reisfield GM, Bandman TB, Borsook D. Adherence monitoring and drug surveillance in chronic opioid therapy. J Pain Symptom Manage 2000; 20:293–307.
- Starrels JL, Becker WC, Alford DP, Kapoor A, Williams AR, Turner BJ. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med 2010; 152:712–720.
- King S. How useful are patient opioid agreements and urine drug testing? Psychiatric Times March 2, 2011; www.psychiatrictimes.com/how-useful-are-patient-opioid-agreements-and-urine-drug-testing. Accessed August 2, 2015.
- Starrels JL, Wu B, Peyser D, et al. It made my life a little easier: primary care providers’ beliefs and attitudes about using opioid treatment agreements. J Opioid Manag 2014; 10:95–102.
- Touchet BK, Yates WR, Coon KA. Opioid contract use is associated with physician training level and practice specialty. J Opioid Manage 2005; 1:195–200.
- Penko J, Mattson J, Miaskowski C, Kushel M. Do patients know they are on pain medication agreements? Results from a sample of high-risk patients on chronic opioid therapy. Pain Med 2012; 13:1174–1180.
- Nicolaidis C. Police officer, deal-maker, or health care provider? Moving to a patient-centered framework for chronic opioid management. Pain Med 2011; 12:890–897.
- Roskos SE, Keenum AJ, Newman LM, Wallace LS. Literacy demands and formatting characteristics of opioid contracts in chronic nonmalignant pain management. J Pain 2007; 8:753–758.
- Davis TC, Wolf MS, Bass PF 3rd, et al. Low literacy impairs comprehension of prescription drug warning labels. J Gen Intern Med 2006; 21:847–851.
- American Academy of Pain Medicine. State legislative updates. www.painmed.org/advocacy/state-updates/. Accessed August 5, 2016.
- Burchman SL, Pagel PS. Implementation of a formal treatment agreement for outpatient management of chronic nonmalignant pain with opioid analgesics. J Pain Symptom Manage 1995; 10:556–563.
- Federation of State Medical Boards. Model policy on the use of opioid analgesics in the treatment of chronic pain. 2013; www.fsmb.org/Media/Default/PDF/FSMB/Advocacy/pain_policy_july2013.pdf. Accessed August 2, 2016.
- University of Wisconsin-Madison. Pain & Policy Studies Group. Database of statutes, regulations, & other policies for pain management. www.painpolicy.wisc.edu/database-statutes-regulations-other-policies-pain-management. Accessed August 3, 2016.
- Cameron KA, Rintamaki LS, Kamanda-Kosseh M, Noskin GA, Baker DW, Makoul G. Using theoretical constructs to identify key issues for targeted message design: African American seniors’ perceptions about influenza and influenza vaccination. Health Commun 2009; 24:316–326.
- Kandula NR, Nsiah-Kumi PA, Makoul G, et al. The relationship between health literacy and knowledge improvement after a multimedia type 2 diabetes education program. Patient Educ Couns 2009; 75:321–327.
- Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009; 10:113–130.
- Adams NJ, Plane MB, Fleming MF, Mundt MP, Saunders LA, Stauffacher EA. Opioids and the treatment of chronic pain in a primary care sample. J Pain Symptom Manage 2001; 22:791–796.
- Richeimer SH. Opioids for pain: risk management. Semin Anesthesia Periop Med Pain 2005; 24:165–169.
KEY POINTS
- Both chronic pain and opioid therapy impose costs and risks. Though controversial, long-term opioid therapy will probably have a role for the foreseeable future.
- The term “controlled-substance agreement” is preferable to “pain contract” or “narcotic contract.”
- Controlled-substance agreements should be used only in the context of personalized patient counseling and shared decision-making.
- Objectives of controlled-substance agreements are to improve adherence, obtain informed consent, outline the prescribing policies of the practice, and mitigate risk.