User login
Poison ivy: How effective are available treatments?
ABSTRACT
Purpose To determine the characteristics and clinical course of Rhus dermatitis in patients who seek assistance from primary care clinicians, as well as treatment approaches used by patients and recommended by clinicians, and treatment approaches associated with better outcomes.
Methods This was a prospective cohort study with standardized baseline data collection on patients and their rashes, followed by examination of patient-completed diaries of signs, symptoms, and treatments.
Results Thirty-six clinicians identified 186 interested patients, of which 89 completed and returned diaries and consent forms. Of those 89 patients, 92% reported pruritus; 91%, erythema; 87%, papules; and 49%, vesicles or bullae at baseline. Their rashes involved the head/face/neck, 61%; trunk, 56%; legs, 54%; and arms, 22%.
From the date of clinical consultation, the mean (standard deviation [SD]; range) duration of any symptom or sign was 14.4 days (8.0; 1-43). Patients most often had tried a topical antipruritic, astringent, or low-potency corticosteroid before seeking care. Clinicians prescribed oral or parenteral corticosteroids 81% of the time, sometimes in combination with a high-potency topical c
Conclusions Patients who visit a primary care clinician for Rhus dermatitis can expect the rash to last another 2 weeks on average (total duration: one day to 6 weeks) regardless of what treatment is prescribed. Parenteral corticosteroids plus high-potency topical corticosteroids may reduce the duration of the itching.
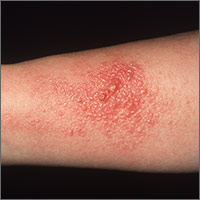
Rhus dermatitis (poison ivy, oak, and sumac) is a common cause of contact dermatitis throughout the United States. The condition is usually mild and often not brought to the attention of primary care clinicians. Some patients, however, do see a health care provider for treatment, most often because of pruritus. This form of contact dermatitis results from a type IV hypersensitivity reaction to urushiol, a colorless oil in the leaves, stem, root, and fruit of poison ivy, poison oak, and poison sumac. The reaction, which occurs 24 to 72 hours following contact with the skin, can be prevented by washing the skin promptly with a detergent soap after exposure. By the age of 8, most people are sensitized to urushiol.1
According to most standard texts and clinical reviews, untreated Rhus dermatitis usually resolves in one to 3 weeks. What is not known is whether particular patient or rash characteristics might affect prognosis and thereby influence treatment recommendations—eg, age, gender, race, location of the rash, prior episodes, chronic illnesses such as diabetes, or chronic use of medications such as nonsteroidal anti-inflammatory drugs and corticosteroids.
Impetus for our study. An informal survey of 10 clinician members of the Oklahoma Physicians Resource/Research Network (OKPRN), a statewide practice-based research network, suggested that primary care clinicians treat between one and 10 patients with poison ivy each week during the spring, summer, and fall (median 2.5). Their reported armamentarium included more than 15 different over-the-counter topical agents, several oral antihistamines, and a variety of topical, oral, and parenteral corticosteroids.
Surprisingly, there is very little published evidence on which to base treatment decisions. Using PubMed and the search terms, Rhus dermatitis, poison ivy, and poison oak, we found only 3 placebo-controlled clinical trials of Rhus dermatitis treatments in the English language literature after 1966. Based on these studies, Zanfel, a mixture of alcohol-soluble and anionic surfactant, may be somewhat effective, but pimecrolimus and jewelweed extract were no more effective than placebo.2-4 There is some evidence that topical corticosteroids are effective only before vesicles appear.5 In one uncontrolled study, intramuscular injection of betamethasone and dexamethasone yielded about a 30% reduction in symptoms within 48 hours.6 Assuming that systemic corticosteroids do produce benefit, however, the most effective dose and duration of treatment have not been determined.7,8
To address some of these gaps in our knowledge base, OKPRN members asked that we undertake a longitudinal cohort study of patients reporting to primary care practices.
METHODS
We conducted this study between May 2010 and October 2014. The project was approved by the University of Oklahoma Health Sciences Center Institutional Review Board. Clinician members of OKPRN were invited to participate in the study via listserv, fax, or letter. We instructed clinicians and office staff to ask patients with Rhus dermatitis if they might be interested in participating in a study, which would require that they keep a symptom diary and would earn them a $20 gift card. Interested patients were given a packet of information, and a member of the research team later called the patients with additional information, including an explanation of informed consent and instructions on completing and returning the diary and written consent form.
Clinicians recorded information about the patient and the rash on a customized template, releasing it to the team after written consent was obtained from the patient. Categories for characterizing the rash were head/face, arms/hands, trunk, and legs/feet. A subset of 5 participating clinicians, selected to include a variety of practice types and patient populations, were also asked to produce, from their billing software, the number of patients and encounters in which poison ivy was addressed in each month of 2013.
On the diary, patients were instructed to record the presence or absence of pruritus, erythema, raised lesions, and vesicles/bullae at the end of each day until the rash resolved, or for 6 weeks following onset of the rash, whichever came first. Patients were asked to mail their diaries to the principal investigator once they were free of symptoms for one week or after 6 weeks from the onset of symptoms, whichever came first.
We asked both patients and clinicians to report medications used before and after the primary care encounter. A member of the research team assigned these medications to one of 12 categories: topical antihistamines, topical soaps (eg, Zanfel or Tecnu), topical astringents, other topical antipruritics, topical aloe vera, topical bleach, low-potency topical corticosteroids, moderate-potency topical corticosteroids, high-potency topical corticosteroids, oral antihistamines, oral corticosteroids, and parenteral corticosteroids.
We used independent T-tests to evaluate associations between baseline variables, patient-initiated treatments, and clinician-initiated treatments and the time to complete resolution of individual signs and symptoms and complete resolution of all signs and symptoms following the clinical encounter. We created additional outcome variables for initial resolution followed by recurrence of itching, erythema, papules, and vesicles. The purpose of these variables was to determine if some treatments were initially effective but without lasting effect.
We used the chi square test to assess associations between clinician-initiated treatments and recurrence of signs or symptoms following initial resolution. To account for chance associations resulting from multiple analyses, we chose to set the level of statistical significance at P=.01. However, because of the lower-than-projected sample size, we chose to also report variables with P<.05 so that the reader could judge the likelihood that a larger sample might have disclosed other important associations.
We assumed that an average of 4 categories of treatment would be tried (eg, topical corticosteroids, systemic corticosteroids, topical antihistamines, and other topical agents), and that the mean number of days until resolution would be 21, with a standard deviation (SD) of 4 days. Setting power at 80% and alpha at .05, we calculated it would take 105 patients per group (N=420) to detect a difference of 2 days in time until resolution.
RESULTS
Over the 5-year study period, 36 clinicians identified 186 patients who expressed an interest in the study, and they transmitted the patient contact information to the research team. Patients were seen in a traditional primary care setting. All 186 patients were enrolled by phone. However, only 89 completed and returned their diaries and signed consent forms; of these, 60% were female, 92% were white, 4% were black, 4% were American Indians, 2% were Hispanic, and 7% had diabetes mellitus.
Five practices contributed data on numbers of poison ivy encounters per month and total encounters per month for the year 2013. They included an inner city academic practice in central Oklahoma and a rural community health center, a suburban private practice, and 2 private practices in a town of 30,000 in eastern Oklahoma. The largest average number of encounters occurred between April and August.
The distribution of enrolled-patient visits by month and season corresponded roughly to the proportions of all patient visits for poison ivy, with 1% occurring in the winter, 35% in the spring, 55% during the summer, and 9% in the fall. Virtually all study participants (92%) complained of pruritus and had erythema (91%) and papules (87%). Forty-nine percent had vesicles or bullae. The area of the body most often affected was the head/face/neck, 61%, followed by the trunk, 56%; legs, 54%; and arms, 22%.
From the date of initial clinical consultation, the mean/median (SD; range) duration of symptoms and signs were: pruritus, 10.9/9 days (7.1; 0-43); erythema, 13.7/13 days (7.7; 0-42); papules, 10.1/9.5 days (6.5; 0-37); and vesicles, 5.3/5 days (4.1; 0-15). The mean/median (SD; range) duration of any symptom or sign was 14.4/13.5 days (8; 1-43). Rashes with vesicles tended to last longer (16.1 vs 12.9 days), but this difference did not reach statistical significance.
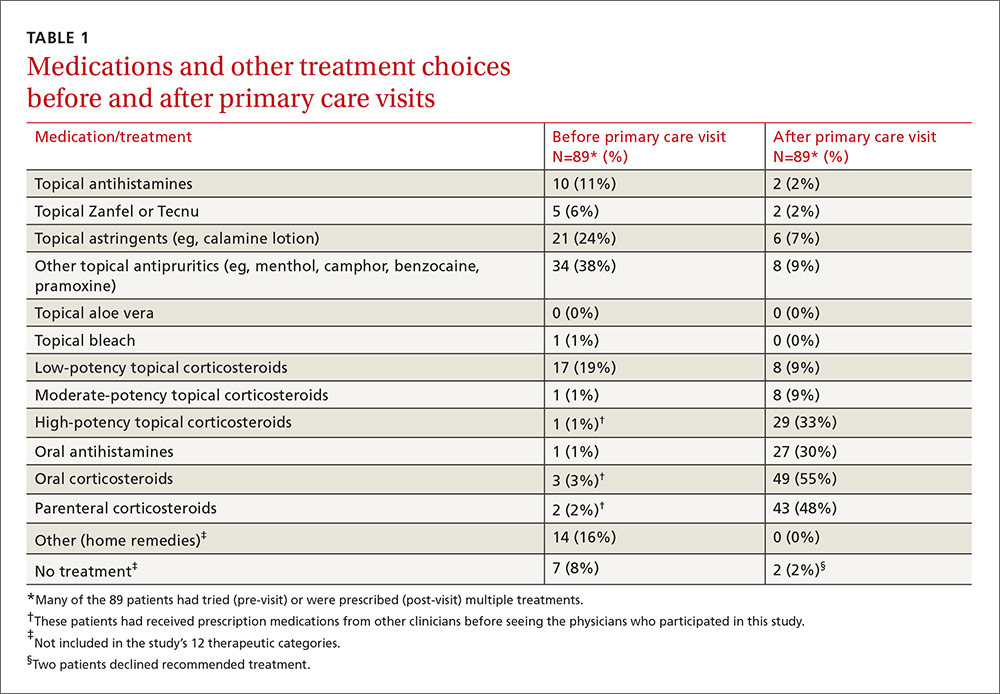
Treatments used by patients before and after their primary care visit are shown in TABLE 1. Seventy-three percent of patients had tried something from one treatment category before consulting a clinician, and 31% had tried something from more than one category. They were most likely to have used a topical antipruritic, astringent, or low-potency corticosteroid, or a combination of these. Clinicians always recommended some treatment and, in 76% of cases, treatments from more than one category. They most often prescribed oral or parenteral corticosteroids (81% of the time), sometimes in combination with a high-potency topical corticosteroid (25% of the time) or oral antihistamine (31%).
No statistically significant associations were found between the baseline non-treatment variables and duration of symptoms and signs. Patient-initiated treatments were also not associated with duration of symptoms and signs following the initial clinician visit.
Of the treatments prescribed by clinicians or independently chosen by patients following their initial office visit, only systemic corticosteroids plus high-potency topical corticosteroids were associated with a significantly shorter duration of itching (P=.005). No treatment was associated with reduced duration of erythema, papules, or vesicles. Use of topical soaps was associated with a longer duration of papules (P<.0001) and of total duration of signs or symptoms (P=.0004) compared with other treatments.
Location and characteristics of the rash were not associated with likelihood of recurrence following treatment. Post-visit use of a topical soap was associated with recurrence of itching (P=.001) and erythema (P=.01). Recurrence of erythema was also more frequent in patients prescribed topical astringents (beta coefficient=0.28; P=.008), and recurrence of papules was more common in patients treated with low-potency topical corticosteroids (P<.0001). These results and several others that almost reached statistical significance are shown in TABLE 2.
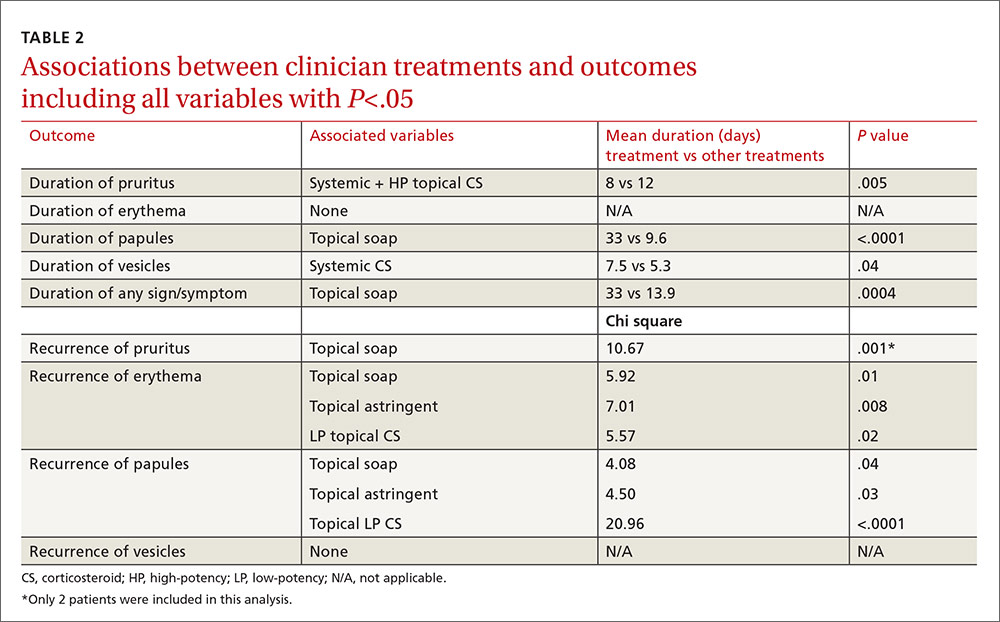
In the multivariable models, the only variable associated with duration of pruritus was the combination of systemic and high-potency topical corticosteroids (8 vs 12 days.) Use of only parenteral or only high-potency topical corticosteroids did not predict shorter duration of pruritus. Use of topical soaps was associated with longer duration of papules (33 vs 9.6 days) and longer duration of any symptoms (33 vs 13.9 days). It was also associated with a higher likelihood of recurrence of pruritus (chi square test [χ2], 10.67) and recurrence of erythema (χ2, 5.92) after initial resolution. Topical astringent use was predictive of recurrence of erythema (χ2, 7.01) and use of low-potency corticosteroids was associated with recurrence of papules (χ2, 20.96).
DISCUSSION
While network clinicians felt that studying poison ivy was of interest and importance, and we had preliminary survey information to suggest it was a common problem treated in primary care, our data suggest that clinical encounters for poison ivy are actually quite uncommon (less than 0.4% of all encounters) even during peak months. Our problems with recruitment were therefore unexpected, and we ended up with far fewer enrolled patients than we had projected, and needed, based on our power analysis. Also based on our preliminary survey, we anticipated considerably more variation in treatment approach than we found. Most clinicians recommended either an oral, parenteral, or high-potency topical corticosteroid, and some also recommended an oral antihistamine, usually diphenhydramine.
The literature and common sense suggest that most patients who seek medical treatment for poison ivy are primarily concerned about itching. Even with the smaller-than-anticipated number of participants in this study, we were able to show that the combination of a systemic (oral or parenteral) corticosteroid and a high-potency topical corticosteroid was associated with a statistically significant shorter duration of pruritus with no recurrence following treatment. We found no evidence that systemic corticosteroids alone, parenteral corticosteroids alone, or high-potency topical corticosteroids alone had any effect on duration of signs or symptoms, even at an alpha of .05. We also found no evidence that oral antihistamines were associated with a shorter duration of pruritus (P=.06); with a larger sample size, we might have found a difference.
Since only 2 patients used topical soaps following their initial clinician visit, the associations between use of these products and longer duration of signs and symptoms and with recurrence of signs and symptoms, although statistically significant, should be viewed with skepticism and with an eye toward possible confounders (eg, people who used these agents may have been more likely to notice and record minor symptoms). Furthermore, these agents have been effective only when used before or at the onset of the rash.
Study limitations. The study has a number of limitations. It had a high drop-out rate. Some patients might not have had poison ivy, but it is generally considered easy to diagnose with accuracy. We cannot be sure that all of the enrolled patients had Rhus dermatitis. Enrollment was based on the clinical impression of the patients’ primary care clinicians. The sample size reduced the power of the study to detect small differences in treatment effects and prevented more complex analyses (eg, combinations of medications, interactions).
The possibility of self-selection bias, weaknesses of the cohort design, and patient-reported outcome measures were additional limitations. The study was also carried out in a single southwestern state, which may not be representative of some other locations. However, it is one of only a few studies published on Rhus dermatitis and possibly the only one conducted in primary care settings.
CORRESPONDENCE
Cara Vaught, MPH, University of Oklahoma Health Sciences Center, Department of Family and Preventive Medicine, 900 NE 10th Street, Oklahoma City, OK 73104; [email protected].
ACKNOWLEDGEMENT
The authors thank the Oklahoma Physicians Resource/Research Network (OKPRN) and the OKPRN clinician members (as well as their staff and patients) for their contributions to this study. The authors also thank Bradley Long, Matthew Marr, and Kellie Hetherington for their involvement in the data collection for this study.
1. Epstein WL. Occupational poison ivy and oak dermatitis. Dermatol Clin. 1994;12:511-516.
2. Long D, Ballentine NH, Marks JG Jr. Treatment of poison ivy/oak allergic contact dermatitis with an extract of jewelweed. Am J Contact Dermat. 1997;8:150-153.
3. Amrol D, Keitel D, Hagaman D, et al. Topical pimecrolimus in the treatment of human allergic contact dermatitis. Ann Allergy Asthma Immunol. 2003;91:563-566.
4. Davila A, Laurora M, Fulton J, et al. A new topical agent, Zanfel, ameliorates urushiol-induced Toxicodendron allergic contact dermatitis [abstract 364]. Ann Emerg Med. 2003;42(suppl 4):S98.
5. Vernon HJ, Olsen EA. A controlled trial of clobetasol propionate ointment 0.05% in the treatment of experimentally induced Rhus dermatitis. J Am Acad Dermatol. 1990;23:829-832.
6. Dickey RF. Parenteral short-term corticosteroid therapy in moderate to severe dermatoses. A comparative multiclinic study. Cutis. 1976;17:179-193.
7. Goodall J. Oral corticosteroids for poison ivy dermatitis. CMAJ. 2002;166:300-301.
8. Moe JF. How much steroid for poison ivy? Postgrad Med. 1999;106:21,24.
ABSTRACT
Purpose To determine the characteristics and clinical course of Rhus dermatitis in patients who seek assistance from primary care clinicians, as well as treatment approaches used by patients and recommended by clinicians, and treatment approaches associated with better outcomes.
Methods This was a prospective cohort study with standardized baseline data collection on patients and their rashes, followed by examination of patient-completed diaries of signs, symptoms, and treatments.
Results Thirty-six clinicians identified 186 interested patients, of which 89 completed and returned diaries and consent forms. Of those 89 patients, 92% reported pruritus; 91%, erythema; 87%, papules; and 49%, vesicles or bullae at baseline. Their rashes involved the head/face/neck, 61%; trunk, 56%; legs, 54%; and arms, 22%.
From the date of clinical consultation, the mean (standard deviation [SD]; range) duration of any symptom or sign was 14.4 days (8.0; 1-43). Patients most often had tried a topical antipruritic, astringent, or low-potency corticosteroid before seeking care. Clinicians prescribed oral or parenteral corticosteroids 81% of the time, sometimes in combination with a high-potency topical c
Conclusions Patients who visit a primary care clinician for Rhus dermatitis can expect the rash to last another 2 weeks on average (total duration: one day to 6 weeks) regardless of what treatment is prescribed. Parenteral corticosteroids plus high-potency topical corticosteroids may reduce the duration of the itching.
Rhus dermatitis (poison ivy, oak, and sumac) is a common cause of contact dermatitis throughout the United States. The condition is usually mild and often not brought to the attention of primary care clinicians. Some patients, however, do see a health care provider for treatment, most often because of pruritus. This form of contact dermatitis results from a type IV hypersensitivity reaction to urushiol, a colorless oil in the leaves, stem, root, and fruit of poison ivy, poison oak, and poison sumac. The reaction, which occurs 24 to 72 hours following contact with the skin, can be prevented by washing the skin promptly with a detergent soap after exposure. By the age of 8, most people are sensitized to urushiol.1
According to most standard texts and clinical reviews, untreated Rhus dermatitis usually resolves in one to 3 weeks. What is not known is whether particular patient or rash characteristics might affect prognosis and thereby influence treatment recommendations—eg, age, gender, race, location of the rash, prior episodes, chronic illnesses such as diabetes, or chronic use of medications such as nonsteroidal anti-inflammatory drugs and corticosteroids.
Impetus for our study. An informal survey of 10 clinician members of the Oklahoma Physicians Resource/Research Network (OKPRN), a statewide practice-based research network, suggested that primary care clinicians treat between one and 10 patients with poison ivy each week during the spring, summer, and fall (median 2.5). Their reported armamentarium included more than 15 different over-the-counter topical agents, several oral antihistamines, and a variety of topical, oral, and parenteral corticosteroids.
Surprisingly, there is very little published evidence on which to base treatment decisions. Using PubMed and the search terms, Rhus dermatitis, poison ivy, and poison oak, we found only 3 placebo-controlled clinical trials of Rhus dermatitis treatments in the English language literature after 1966. Based on these studies, Zanfel, a mixture of alcohol-soluble and anionic surfactant, may be somewhat effective, but pimecrolimus and jewelweed extract were no more effective than placebo.2-4 There is some evidence that topical corticosteroids are effective only before vesicles appear.5 In one uncontrolled study, intramuscular injection of betamethasone and dexamethasone yielded about a 30% reduction in symptoms within 48 hours.6 Assuming that systemic corticosteroids do produce benefit, however, the most effective dose and duration of treatment have not been determined.7,8
To address some of these gaps in our knowledge base, OKPRN members asked that we undertake a longitudinal cohort study of patients reporting to primary care practices.
METHODS
We conducted this study between May 2010 and October 2014. The project was approved by the University of Oklahoma Health Sciences Center Institutional Review Board. Clinician members of OKPRN were invited to participate in the study via listserv, fax, or letter. We instructed clinicians and office staff to ask patients with Rhus dermatitis if they might be interested in participating in a study, which would require that they keep a symptom diary and would earn them a $20 gift card. Interested patients were given a packet of information, and a member of the research team later called the patients with additional information, including an explanation of informed consent and instructions on completing and returning the diary and written consent form.
Clinicians recorded information about the patient and the rash on a customized template, releasing it to the team after written consent was obtained from the patient. Categories for characterizing the rash were head/face, arms/hands, trunk, and legs/feet. A subset of 5 participating clinicians, selected to include a variety of practice types and patient populations, were also asked to produce, from their billing software, the number of patients and encounters in which poison ivy was addressed in each month of 2013.
On the diary, patients were instructed to record the presence or absence of pruritus, erythema, raised lesions, and vesicles/bullae at the end of each day until the rash resolved, or for 6 weeks following onset of the rash, whichever came first. Patients were asked to mail their diaries to the principal investigator once they were free of symptoms for one week or after 6 weeks from the onset of symptoms, whichever came first.
We asked both patients and clinicians to report medications used before and after the primary care encounter. A member of the research team assigned these medications to one of 12 categories: topical antihistamines, topical soaps (eg, Zanfel or Tecnu), topical astringents, other topical antipruritics, topical aloe vera, topical bleach, low-potency topical corticosteroids, moderate-potency topical corticosteroids, high-potency topical corticosteroids, oral antihistamines, oral corticosteroids, and parenteral corticosteroids.
We used independent T-tests to evaluate associations between baseline variables, patient-initiated treatments, and clinician-initiated treatments and the time to complete resolution of individual signs and symptoms and complete resolution of all signs and symptoms following the clinical encounter. We created additional outcome variables for initial resolution followed by recurrence of itching, erythema, papules, and vesicles. The purpose of these variables was to determine if some treatments were initially effective but without lasting effect.
We used the chi square test to assess associations between clinician-initiated treatments and recurrence of signs or symptoms following initial resolution. To account for chance associations resulting from multiple analyses, we chose to set the level of statistical significance at P=.01. However, because of the lower-than-projected sample size, we chose to also report variables with P<.05 so that the reader could judge the likelihood that a larger sample might have disclosed other important associations.
We assumed that an average of 4 categories of treatment would be tried (eg, topical corticosteroids, systemic corticosteroids, topical antihistamines, and other topical agents), and that the mean number of days until resolution would be 21, with a standard deviation (SD) of 4 days. Setting power at 80% and alpha at .05, we calculated it would take 105 patients per group (N=420) to detect a difference of 2 days in time until resolution.
RESULTS
Over the 5-year study period, 36 clinicians identified 186 patients who expressed an interest in the study, and they transmitted the patient contact information to the research team. Patients were seen in a traditional primary care setting. All 186 patients were enrolled by phone. However, only 89 completed and returned their diaries and signed consent forms; of these, 60% were female, 92% were white, 4% were black, 4% were American Indians, 2% were Hispanic, and 7% had diabetes mellitus.
Five practices contributed data on numbers of poison ivy encounters per month and total encounters per month for the year 2013. They included an inner city academic practice in central Oklahoma and a rural community health center, a suburban private practice, and 2 private practices in a town of 30,000 in eastern Oklahoma. The largest average number of encounters occurred between April and August.
The distribution of enrolled-patient visits by month and season corresponded roughly to the proportions of all patient visits for poison ivy, with 1% occurring in the winter, 35% in the spring, 55% during the summer, and 9% in the fall. Virtually all study participants (92%) complained of pruritus and had erythema (91%) and papules (87%). Forty-nine percent had vesicles or bullae. The area of the body most often affected was the head/face/neck, 61%, followed by the trunk, 56%; legs, 54%; and arms, 22%.
From the date of initial clinical consultation, the mean/median (SD; range) duration of symptoms and signs were: pruritus, 10.9/9 days (7.1; 0-43); erythema, 13.7/13 days (7.7; 0-42); papules, 10.1/9.5 days (6.5; 0-37); and vesicles, 5.3/5 days (4.1; 0-15). The mean/median (SD; range) duration of any symptom or sign was 14.4/13.5 days (8; 1-43). Rashes with vesicles tended to last longer (16.1 vs 12.9 days), but this difference did not reach statistical significance.
Treatments used by patients before and after their primary care visit are shown in TABLE 1. Seventy-three percent of patients had tried something from one treatment category before consulting a clinician, and 31% had tried something from more than one category. They were most likely to have used a topical antipruritic, astringent, or low-potency corticosteroid, or a combination of these. Clinicians always recommended some treatment and, in 76% of cases, treatments from more than one category. They most often prescribed oral or parenteral corticosteroids (81% of the time), sometimes in combination with a high-potency topical corticosteroid (25% of the time) or oral antihistamine (31%).

No statistically significant associations were found between the baseline non-treatment variables and duration of symptoms and signs. Patient-initiated treatments were also not associated with duration of symptoms and signs following the initial clinician visit.
Of the treatments prescribed by clinicians or independently chosen by patients following their initial office visit, only systemic corticosteroids plus high-potency topical corticosteroids were associated with a significantly shorter duration of itching (P=.005). No treatment was associated with reduced duration of erythema, papules, or vesicles. Use of topical soaps was associated with a longer duration of papules (P<.0001) and of total duration of signs or symptoms (P=.0004) compared with other treatments.
Location and characteristics of the rash were not associated with likelihood of recurrence following treatment. Post-visit use of a topical soap was associated with recurrence of itching (P=.001) and erythema (P=.01). Recurrence of erythema was also more frequent in patients prescribed topical astringents (beta coefficient=0.28; P=.008), and recurrence of papules was more common in patients treated with low-potency topical corticosteroids (P<.0001). These results and several others that almost reached statistical significance are shown in TABLE 2.

In the multivariable models, the only variable associated with duration of pruritus was the combination of systemic and high-potency topical corticosteroids (8 vs 12 days.) Use of only parenteral or only high-potency topical corticosteroids did not predict shorter duration of pruritus. Use of topical soaps was associated with longer duration of papules (33 vs 9.6 days) and longer duration of any symptoms (33 vs 13.9 days). It was also associated with a higher likelihood of recurrence of pruritus (chi square test [χ2], 10.67) and recurrence of erythema (χ2, 5.92) after initial resolution. Topical astringent use was predictive of recurrence of erythema (χ2, 7.01) and use of low-potency corticosteroids was associated with recurrence of papules (χ2, 20.96).
DISCUSSION
While network clinicians felt that studying poison ivy was of interest and importance, and we had preliminary survey information to suggest it was a common problem treated in primary care, our data suggest that clinical encounters for poison ivy are actually quite uncommon (less than 0.4% of all encounters) even during peak months. Our problems with recruitment were therefore unexpected, and we ended up with far fewer enrolled patients than we had projected, and needed, based on our power analysis. Also based on our preliminary survey, we anticipated considerably more variation in treatment approach than we found. Most clinicians recommended either an oral, parenteral, or high-potency topical corticosteroid, and some also recommended an oral antihistamine, usually diphenhydramine.
The literature and common sense suggest that most patients who seek medical treatment for poison ivy are primarily concerned about itching. Even with the smaller-than-anticipated number of participants in this study, we were able to show that the combination of a systemic (oral or parenteral) corticosteroid and a high-potency topical corticosteroid was associated with a statistically significant shorter duration of pruritus with no recurrence following treatment. We found no evidence that systemic corticosteroids alone, parenteral corticosteroids alone, or high-potency topical corticosteroids alone had any effect on duration of signs or symptoms, even at an alpha of .05. We also found no evidence that oral antihistamines were associated with a shorter duration of pruritus (P=.06); with a larger sample size, we might have found a difference.
Since only 2 patients used topical soaps following their initial clinician visit, the associations between use of these products and longer duration of signs and symptoms and with recurrence of signs and symptoms, although statistically significant, should be viewed with skepticism and with an eye toward possible confounders (eg, people who used these agents may have been more likely to notice and record minor symptoms). Furthermore, these agents have been effective only when used before or at the onset of the rash.
Study limitations. The study has a number of limitations. It had a high drop-out rate. Some patients might not have had poison ivy, but it is generally considered easy to diagnose with accuracy. We cannot be sure that all of the enrolled patients had Rhus dermatitis. Enrollment was based on the clinical impression of the patients’ primary care clinicians. The sample size reduced the power of the study to detect small differences in treatment effects and prevented more complex analyses (eg, combinations of medications, interactions).
The possibility of self-selection bias, weaknesses of the cohort design, and patient-reported outcome measures were additional limitations. The study was also carried out in a single southwestern state, which may not be representative of some other locations. However, it is one of only a few studies published on Rhus dermatitis and possibly the only one conducted in primary care settings.
CORRESPONDENCE
Cara Vaught, MPH, University of Oklahoma Health Sciences Center, Department of Family and Preventive Medicine, 900 NE 10th Street, Oklahoma City, OK 73104; [email protected].
ACKNOWLEDGEMENT
The authors thank the Oklahoma Physicians Resource/Research Network (OKPRN) and the OKPRN clinician members (as well as their staff and patients) for their contributions to this study. The authors also thank Bradley Long, Matthew Marr, and Kellie Hetherington for their involvement in the data collection for this study.
ABSTRACT
Purpose To determine the characteristics and clinical course of Rhus dermatitis in patients who seek assistance from primary care clinicians, as well as treatment approaches used by patients and recommended by clinicians, and treatment approaches associated with better outcomes.
Methods This was a prospective cohort study with standardized baseline data collection on patients and their rashes, followed by examination of patient-completed diaries of signs, symptoms, and treatments.
Results Thirty-six clinicians identified 186 interested patients, of which 89 completed and returned diaries and consent forms. Of those 89 patients, 92% reported pruritus; 91%, erythema; 87%, papules; and 49%, vesicles or bullae at baseline. Their rashes involved the head/face/neck, 61%; trunk, 56%; legs, 54%; and arms, 22%.
From the date of clinical consultation, the mean (standard deviation [SD]; range) duration of any symptom or sign was 14.4 days (8.0; 1-43). Patients most often had tried a topical antipruritic, astringent, or low-potency corticosteroid before seeking care. Clinicians prescribed oral or parenteral corticosteroids 81% of the time, sometimes in combination with a high-potency topical c
Conclusions Patients who visit a primary care clinician for Rhus dermatitis can expect the rash to last another 2 weeks on average (total duration: one day to 6 weeks) regardless of what treatment is prescribed. Parenteral corticosteroids plus high-potency topical corticosteroids may reduce the duration of the itching.
Rhus dermatitis (poison ivy, oak, and sumac) is a common cause of contact dermatitis throughout the United States. The condition is usually mild and often not brought to the attention of primary care clinicians. Some patients, however, do see a health care provider for treatment, most often because of pruritus. This form of contact dermatitis results from a type IV hypersensitivity reaction to urushiol, a colorless oil in the leaves, stem, root, and fruit of poison ivy, poison oak, and poison sumac. The reaction, which occurs 24 to 72 hours following contact with the skin, can be prevented by washing the skin promptly with a detergent soap after exposure. By the age of 8, most people are sensitized to urushiol.1
According to most standard texts and clinical reviews, untreated Rhus dermatitis usually resolves in one to 3 weeks. What is not known is whether particular patient or rash characteristics might affect prognosis and thereby influence treatment recommendations—eg, age, gender, race, location of the rash, prior episodes, chronic illnesses such as diabetes, or chronic use of medications such as nonsteroidal anti-inflammatory drugs and corticosteroids.
Impetus for our study. An informal survey of 10 clinician members of the Oklahoma Physicians Resource/Research Network (OKPRN), a statewide practice-based research network, suggested that primary care clinicians treat between one and 10 patients with poison ivy each week during the spring, summer, and fall (median 2.5). Their reported armamentarium included more than 15 different over-the-counter topical agents, several oral antihistamines, and a variety of topical, oral, and parenteral corticosteroids.
Surprisingly, there is very little published evidence on which to base treatment decisions. Using PubMed and the search terms, Rhus dermatitis, poison ivy, and poison oak, we found only 3 placebo-controlled clinical trials of Rhus dermatitis treatments in the English language literature after 1966. Based on these studies, Zanfel, a mixture of alcohol-soluble and anionic surfactant, may be somewhat effective, but pimecrolimus and jewelweed extract were no more effective than placebo.2-4 There is some evidence that topical corticosteroids are effective only before vesicles appear.5 In one uncontrolled study, intramuscular injection of betamethasone and dexamethasone yielded about a 30% reduction in symptoms within 48 hours.6 Assuming that systemic corticosteroids do produce benefit, however, the most effective dose and duration of treatment have not been determined.7,8
To address some of these gaps in our knowledge base, OKPRN members asked that we undertake a longitudinal cohort study of patients reporting to primary care practices.
METHODS
We conducted this study between May 2010 and October 2014. The project was approved by the University of Oklahoma Health Sciences Center Institutional Review Board. Clinician members of OKPRN were invited to participate in the study via listserv, fax, or letter. We instructed clinicians and office staff to ask patients with Rhus dermatitis if they might be interested in participating in a study, which would require that they keep a symptom diary and would earn them a $20 gift card. Interested patients were given a packet of information, and a member of the research team later called the patients with additional information, including an explanation of informed consent and instructions on completing and returning the diary and written consent form.
Clinicians recorded information about the patient and the rash on a customized template, releasing it to the team after written consent was obtained from the patient. Categories for characterizing the rash were head/face, arms/hands, trunk, and legs/feet. A subset of 5 participating clinicians, selected to include a variety of practice types and patient populations, were also asked to produce, from their billing software, the number of patients and encounters in which poison ivy was addressed in each month of 2013.
On the diary, patients were instructed to record the presence or absence of pruritus, erythema, raised lesions, and vesicles/bullae at the end of each day until the rash resolved, or for 6 weeks following onset of the rash, whichever came first. Patients were asked to mail their diaries to the principal investigator once they were free of symptoms for one week or after 6 weeks from the onset of symptoms, whichever came first.
We asked both patients and clinicians to report medications used before and after the primary care encounter. A member of the research team assigned these medications to one of 12 categories: topical antihistamines, topical soaps (eg, Zanfel or Tecnu), topical astringents, other topical antipruritics, topical aloe vera, topical bleach, low-potency topical corticosteroids, moderate-potency topical corticosteroids, high-potency topical corticosteroids, oral antihistamines, oral corticosteroids, and parenteral corticosteroids.
We used independent T-tests to evaluate associations between baseline variables, patient-initiated treatments, and clinician-initiated treatments and the time to complete resolution of individual signs and symptoms and complete resolution of all signs and symptoms following the clinical encounter. We created additional outcome variables for initial resolution followed by recurrence of itching, erythema, papules, and vesicles. The purpose of these variables was to determine if some treatments were initially effective but without lasting effect.
We used the chi square test to assess associations between clinician-initiated treatments and recurrence of signs or symptoms following initial resolution. To account for chance associations resulting from multiple analyses, we chose to set the level of statistical significance at P=.01. However, because of the lower-than-projected sample size, we chose to also report variables with P<.05 so that the reader could judge the likelihood that a larger sample might have disclosed other important associations.
We assumed that an average of 4 categories of treatment would be tried (eg, topical corticosteroids, systemic corticosteroids, topical antihistamines, and other topical agents), and that the mean number of days until resolution would be 21, with a standard deviation (SD) of 4 days. Setting power at 80% and alpha at .05, we calculated it would take 105 patients per group (N=420) to detect a difference of 2 days in time until resolution.
RESULTS
Over the 5-year study period, 36 clinicians identified 186 patients who expressed an interest in the study, and they transmitted the patient contact information to the research team. Patients were seen in a traditional primary care setting. All 186 patients were enrolled by phone. However, only 89 completed and returned their diaries and signed consent forms; of these, 60% were female, 92% were white, 4% were black, 4% were American Indians, 2% were Hispanic, and 7% had diabetes mellitus.
Five practices contributed data on numbers of poison ivy encounters per month and total encounters per month for the year 2013. They included an inner city academic practice in central Oklahoma and a rural community health center, a suburban private practice, and 2 private practices in a town of 30,000 in eastern Oklahoma. The largest average number of encounters occurred between April and August.
The distribution of enrolled-patient visits by month and season corresponded roughly to the proportions of all patient visits for poison ivy, with 1% occurring in the winter, 35% in the spring, 55% during the summer, and 9% in the fall. Virtually all study participants (92%) complained of pruritus and had erythema (91%) and papules (87%). Forty-nine percent had vesicles or bullae. The area of the body most often affected was the head/face/neck, 61%, followed by the trunk, 56%; legs, 54%; and arms, 22%.
From the date of initial clinical consultation, the mean/median (SD; range) duration of symptoms and signs were: pruritus, 10.9/9 days (7.1; 0-43); erythema, 13.7/13 days (7.7; 0-42); papules, 10.1/9.5 days (6.5; 0-37); and vesicles, 5.3/5 days (4.1; 0-15). The mean/median (SD; range) duration of any symptom or sign was 14.4/13.5 days (8; 1-43). Rashes with vesicles tended to last longer (16.1 vs 12.9 days), but this difference did not reach statistical significance.
Treatments used by patients before and after their primary care visit are shown in TABLE 1. Seventy-three percent of patients had tried something from one treatment category before consulting a clinician, and 31% had tried something from more than one category. They were most likely to have used a topical antipruritic, astringent, or low-potency corticosteroid, or a combination of these. Clinicians always recommended some treatment and, in 76% of cases, treatments from more than one category. They most often prescribed oral or parenteral corticosteroids (81% of the time), sometimes in combination with a high-potency topical corticosteroid (25% of the time) or oral antihistamine (31%).

No statistically significant associations were found between the baseline non-treatment variables and duration of symptoms and signs. Patient-initiated treatments were also not associated with duration of symptoms and signs following the initial clinician visit.
Of the treatments prescribed by clinicians or independently chosen by patients following their initial office visit, only systemic corticosteroids plus high-potency topical corticosteroids were associated with a significantly shorter duration of itching (P=.005). No treatment was associated with reduced duration of erythema, papules, or vesicles. Use of topical soaps was associated with a longer duration of papules (P<.0001) and of total duration of signs or symptoms (P=.0004) compared with other treatments.
Location and characteristics of the rash were not associated with likelihood of recurrence following treatment. Post-visit use of a topical soap was associated with recurrence of itching (P=.001) and erythema (P=.01). Recurrence of erythema was also more frequent in patients prescribed topical astringents (beta coefficient=0.28; P=.008), and recurrence of papules was more common in patients treated with low-potency topical corticosteroids (P<.0001). These results and several others that almost reached statistical significance are shown in TABLE 2.

In the multivariable models, the only variable associated with duration of pruritus was the combination of systemic and high-potency topical corticosteroids (8 vs 12 days.) Use of only parenteral or only high-potency topical corticosteroids did not predict shorter duration of pruritus. Use of topical soaps was associated with longer duration of papules (33 vs 9.6 days) and longer duration of any symptoms (33 vs 13.9 days). It was also associated with a higher likelihood of recurrence of pruritus (chi square test [χ2], 10.67) and recurrence of erythema (χ2, 5.92) after initial resolution. Topical astringent use was predictive of recurrence of erythema (χ2, 7.01) and use of low-potency corticosteroids was associated with recurrence of papules (χ2, 20.96).
DISCUSSION
While network clinicians felt that studying poison ivy was of interest and importance, and we had preliminary survey information to suggest it was a common problem treated in primary care, our data suggest that clinical encounters for poison ivy are actually quite uncommon (less than 0.4% of all encounters) even during peak months. Our problems with recruitment were therefore unexpected, and we ended up with far fewer enrolled patients than we had projected, and needed, based on our power analysis. Also based on our preliminary survey, we anticipated considerably more variation in treatment approach than we found. Most clinicians recommended either an oral, parenteral, or high-potency topical corticosteroid, and some also recommended an oral antihistamine, usually diphenhydramine.
The literature and common sense suggest that most patients who seek medical treatment for poison ivy are primarily concerned about itching. Even with the smaller-than-anticipated number of participants in this study, we were able to show that the combination of a systemic (oral or parenteral) corticosteroid and a high-potency topical corticosteroid was associated with a statistically significant shorter duration of pruritus with no recurrence following treatment. We found no evidence that systemic corticosteroids alone, parenteral corticosteroids alone, or high-potency topical corticosteroids alone had any effect on duration of signs or symptoms, even at an alpha of .05. We also found no evidence that oral antihistamines were associated with a shorter duration of pruritus (P=.06); with a larger sample size, we might have found a difference.
Since only 2 patients used topical soaps following their initial clinician visit, the associations between use of these products and longer duration of signs and symptoms and with recurrence of signs and symptoms, although statistically significant, should be viewed with skepticism and with an eye toward possible confounders (eg, people who used these agents may have been more likely to notice and record minor symptoms). Furthermore, these agents have been effective only when used before or at the onset of the rash.
Study limitations. The study has a number of limitations. It had a high drop-out rate. Some patients might not have had poison ivy, but it is generally considered easy to diagnose with accuracy. We cannot be sure that all of the enrolled patients had Rhus dermatitis. Enrollment was based on the clinical impression of the patients’ primary care clinicians. The sample size reduced the power of the study to detect small differences in treatment effects and prevented more complex analyses (eg, combinations of medications, interactions).
The possibility of self-selection bias, weaknesses of the cohort design, and patient-reported outcome measures were additional limitations. The study was also carried out in a single southwestern state, which may not be representative of some other locations. However, it is one of only a few studies published on Rhus dermatitis and possibly the only one conducted in primary care settings.
CORRESPONDENCE
Cara Vaught, MPH, University of Oklahoma Health Sciences Center, Department of Family and Preventive Medicine, 900 NE 10th Street, Oklahoma City, OK 73104; [email protected].
ACKNOWLEDGEMENT
The authors thank the Oklahoma Physicians Resource/Research Network (OKPRN) and the OKPRN clinician members (as well as their staff and patients) for their contributions to this study. The authors also thank Bradley Long, Matthew Marr, and Kellie Hetherington for their involvement in the data collection for this study.
1. Epstein WL. Occupational poison ivy and oak dermatitis. Dermatol Clin. 1994;12:511-516.
2. Long D, Ballentine NH, Marks JG Jr. Treatment of poison ivy/oak allergic contact dermatitis with an extract of jewelweed. Am J Contact Dermat. 1997;8:150-153.
3. Amrol D, Keitel D, Hagaman D, et al. Topical pimecrolimus in the treatment of human allergic contact dermatitis. Ann Allergy Asthma Immunol. 2003;91:563-566.
4. Davila A, Laurora M, Fulton J, et al. A new topical agent, Zanfel, ameliorates urushiol-induced Toxicodendron allergic contact dermatitis [abstract 364]. Ann Emerg Med. 2003;42(suppl 4):S98.
5. Vernon HJ, Olsen EA. A controlled trial of clobetasol propionate ointment 0.05% in the treatment of experimentally induced Rhus dermatitis. J Am Acad Dermatol. 1990;23:829-832.
6. Dickey RF. Parenteral short-term corticosteroid therapy in moderate to severe dermatoses. A comparative multiclinic study. Cutis. 1976;17:179-193.
7. Goodall J. Oral corticosteroids for poison ivy dermatitis. CMAJ. 2002;166:300-301.
8. Moe JF. How much steroid for poison ivy? Postgrad Med. 1999;106:21,24.
1. Epstein WL. Occupational poison ivy and oak dermatitis. Dermatol Clin. 1994;12:511-516.
2. Long D, Ballentine NH, Marks JG Jr. Treatment of poison ivy/oak allergic contact dermatitis with an extract of jewelweed. Am J Contact Dermat. 1997;8:150-153.
3. Amrol D, Keitel D, Hagaman D, et al. Topical pimecrolimus in the treatment of human allergic contact dermatitis. Ann Allergy Asthma Immunol. 2003;91:563-566.
4. Davila A, Laurora M, Fulton J, et al. A new topical agent, Zanfel, ameliorates urushiol-induced Toxicodendron allergic contact dermatitis [abstract 364]. Ann Emerg Med. 2003;42(suppl 4):S98.
5. Vernon HJ, Olsen EA. A controlled trial of clobetasol propionate ointment 0.05% in the treatment of experimentally induced Rhus dermatitis. J Am Acad Dermatol. 1990;23:829-832.
6. Dickey RF. Parenteral short-term corticosteroid therapy in moderate to severe dermatoses. A comparative multiclinic study. Cutis. 1976;17:179-193.
7. Goodall J. Oral corticosteroids for poison ivy dermatitis. CMAJ. 2002;166:300-301.
8. Moe JF. How much steroid for poison ivy? Postgrad Med. 1999;106:21,24.
Prevalence of night sweats in primary care patients
OBJECTIVE: To estimate the prevalence and factors associated with night sweats among adult primary care patients.
STUDY DESIGN: This was a cross-sectional study.
POPULATION: Adult patients in 2 primary care practice-based research networks (PBRNs) during 1 week in the summer and 1 week in the winter in the years 2000 and 2001.
OUTCOMES MEASURES: We measured the prevalence of pure night sweats and night and day sweats in all patients and subgroups defined by age and sex, clinical variables associated with night sweats, and the frequency, severity, and rate of reporting.
RESULTS: Of the 2267 patients who participated, 41% reported experiencing night sweats within the last month, including 23% with pure night sweats and an additional 18% with day and night sweats. The prevalence of night sweats in both men and women was highest in the group aged 41 years to 55 years. In multivariate analyses, factors associated with pure night sweats in women were hot flashes and panic attacks; in men, sleep problems. Variables associated with night and day sweats in women were increased weight, hot flashes, sleep disturbances, and use of antihistamines, selective serotonin reuptake inhibitors (SSRIs), and other (non-SSRI, non-tricyclic) antidepressants; in men, increased weight, hot flashes, and greater alcohol use. A majority of patients had not reported their night sweats to their physicians, even when frequent and severe.
CONCLUSIONS: Night sweats are common and under-reported. Pure night sweats and night and day sweats may have different causes. With regard to the etiologies of pure night sweats, panic attacks and sleep disorders need further investigation.
- Night sweats are a common experience for primary care patients, but they are frequently not reported to their physicians.
- There appear to be 2 somewhat distinct patterns of night sweats: pure night sweats and night and day sweats.
- A history of night sweats should prompt questions about menopause, panic attacks, sleep problems, and certain medications.
Night sweats have been attributed to tuberculosis, other acute and chronic febrile illnesses, menopause, pregnancy, hyperthyroidism, nocturnal hypoglycemia, other endocrine problems, neurologic diseases, sleep disorders (eg, sleep apnea and nightmares), malignancies, autoimmune diseases, coronary artery spasm, congestive heart failure, gastroesophageal reflux disease, psychiatric disorders, and certain medications. In 36 medical and surgical textbooks, night sweats were always discussed within sections covering specific diseases and never as a separate topic. References to the primary literature were never provided. We also searched Micromedix, a comprehensive source of information on medications, using “sweating” and “diaphoresis” as search terms.1 Table W1 contains a comprehensive list of proposed causes of night sweats identified in our searches and accompanying references.
Only 2 epidemiologic studies of night sweats were found in the English language literature. Lea and Aber2 interviewed 174 patients randomly selected from the inpatient units of a university hospital and found that 33% of nonobstetric patients and 60% of obstetric patients reported having had night sweats during the previous 3 months. Twenty-six percent of those with night sweats reported that their nighttime sweating was severe enough to require bathing and changing of bed linens. Reynolds,3 a gastroenterologist, queried 200 consecutive patients seen in his outpatient practice and found that 40% remembered experiencing night sweats at least once during the previous year. A total of 12% reported at least weekly night sweats. A review of the records of 750 patients at the Geriatric Continuity Clinic at the University of Oklahoma Family Medicine Center revealed that 10% reported having experienced night sweats during the previous month, when the question was asked as part of a standard review of systems questionnaire (J.W.M., unpublished data, 1999).
Our study was conducted in an effort to estimate the prevalence of night sweats in adult patients seen in primary care office settings, and to explore the associations of this symptom with demographic factors, physical characteristics, medical problems, and medications. We also sought to determine how distressing this symptom is to those who have it and to their sleep partners, whether patients are likely to report the symptom to their physicians, and what patients and their physicians think causes night sweats in individual cases.
Methods
Physician members of the Oklahoma Physicians Resource/Research Network (OKPRN) and the Texas Academy of Family Physicians Research Network (TAFP-Net) enrolled consecutive patients 18 years and older seen in their clinics during a 1-week period in the summer and a second 1-week period in the winter in the years 2000 and 2001. Patients who agreed to participate signed a consent form and then helped the nurse and physician complete a brief questionnaire on a preaddressed, stamped data collection card. For those who declined to participate, a card was generated containing the physician’s code number and the patient’s age and sex. Questions elicited demographic information; information about a selected set of medical conditions; medications, vitamins, herbs, and alcohol used regularly; and information about recent experiences with night sweats. Participating physicians were asked to check the questionnaires for accuracy and to record their opinions regarding the cause of the patients’ night sweats when they reported having had them. A laminated card with definitions of terms was provided to each physician.
“Night sweats” was defined as “sweating at night even when it isn’t excessively hot in your bedroom.” “Day sweats” was defined as “excessive sweating during the daytime.” “Pure night sweats” was defined as night sweats, but not day sweats, and “night and day sweats” as the combination of the 2. The time interval was specified as “during the last month.”
Completed questionnaires were mailed to the Oklahoma Center for Family Medicine Research for data entry and analysis. The data collection cards used by the Texas network included questions about race/ethnicity and panic attacks that were not included on the Oklahoma cards. Inadvertently, some of the Texas cards did not include the question about daytime sweating.
Statistix7 (Analytical Software, Tallahassee, Fla) was used for all statistical analyses. Medications were assigned to 1 of 47 categories according to their primary pharmacologic effects. Summary statistics were calculated for all participants and for the following subgroups: season (summer and winter), pattern of night sweats (excessive nighttime sweating only or night and day sweats), and age group. We anticipated that the majority of women with menopausal symptoms would be in the 41- to 55-year age group.
The two patterns of night sweats, “pure night sweats” and “night and day sweats,” were analyzed separately, and by sex and age. Logistic regression was used to identify the most significant predictors of night sweats while controlling for other variables. Variables were entered into the logistic models if they had a univariate association with the dependent variable at a P value of less than .05. They were then removed one at a time, in the order of largest to smallest P value, if they had a P value of greater than .01 after controlling for other variables. Conservative P values were chosen because of the large numbers of variables considered, in order to reduce the probability of type 1 errors. When appropriate, 95% confidence intervals were calculated.
Results
Study population
A total of 2267 patients of 31 different physicians participated in this study, including 1888 patients of 24 Oklahoma physicians and 379 patients of 7 Texas physicians. Their mean (standard deviation) age was 50.7 (18.8) years, with a range of 18 to 97 years. Sixty-nine percent were women. A total of 99% of Oklahoma patients and 93% of Texas patients seen during the study weeks agreed to participate in the study. Among Texas participants, 53% were Hispanic whites, 33% were non-Hispanic whites, 13% were African Americans, and 1% were categorized as other. On the basis of prior OKPRN studies, we suspect that approximately 90% of Oklahoma patients were non-Hispanic whites, but exact proportions were not determined for this study.
Prevalence of night sweats
The prevalence of pure night sweats, night and day sweats, and any night sweats are shown in Table 1. While the prevalence of night and day sweats was lower for older patients, severity tended to be greater. Severity and frequency were positively correlated for all categories of night sweats and for all subgroups of patients (overall Spearman coefficient = 0.33; P < .001). Overall, the frequencies of night sweats among those who reported the condition were: almost never, 18%; 1 to 3 nights per month, 38%; 1 to 3 nights per week, 27%; and 4 to 7 nights per week, 16%. Ten percent of both women and men with night sweats said that their night sweats were bothersome to others.
TABLE 1
Percentage of patients with pure night sweats and night and day
| Patient group, by sex and age, in years | Pure night sweats % (95% CI) | Night and day sweats % (95% CI) | Any night sweats % (95% CI) |
|---|---|---|---|
| All patients | 23 (21-24) | 18 (16-20) | 41 (39-43) |
| Men | 22 (19-26) | 12 (9-14) | 34 (30-38) |
| 18-40 | 20 (14-26) | 14 (9-19) | 35 (28-42) |
| 41-55 | 25 (18-32) | 14 (9-19) | 40 (33-47) |
| 56-69 | 24 (16-32) | 12 (6-18) | 38 (30-46) |
| 70+ | 20 (13-27) | 6 (2-10) | 26 (19-33) |
| Women | 23 (21-25) | 21 (19-24) | 44 (42-47) |
| 18-40 | 22 (18-26) | 19 (15-23) | 42 (38-46) |
| 41-55 | 29 (24-34) | 32 (28-37) | 61 (56-66) |
| 56-69 | 22 (18-27) | 23 (18-28) | 43 (37-49) |
| 70+ | 19 (14-24) | 9 (5-13) | 29 (24-34) |
| CI denotes confidence interval. | |||
Frequency of reporting of night sweats
A minority of patients with night sweats (12%) had reported the symptom to their physicians. This was true even for those with severe night sweats (46%). Women younger than 70 years were more likely than men of the same age to have reported their night sweats to their physicians (15% vs. 6%; P < .001). The reverse was true for those 70 years and older (7% vs 13%; P =.08). Older patients with pure night sweats were more likely than younger patients to have reported them. After controlling for other variables, patients who were older (odds ratio [OR] = 1.03 per year of age; P < .001), those with night and day sweats (OR = 1.74; P =.0015), and those who reported that their night sweats bothered others (OR = 2.89; P =.001) were more likely to have reported the symptom to their physicians. Those who had reported their night sweats were also more likely to have hot flashes (OR = 2.98; P < .001) and to take estrogen (OR = 1.72; P =.003).
Factors associated with night sweats
The only variable associated with pure night sweats after controlling for all other variables was panic attacks. Variables associated with night and day sweats were younger age, greater body mass index, hot flashes, chronic infection, sleep disturbances, selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants, “other” (non–SSRI, non-tricyclic) antidepressants, and xanthines.
For women, the only variable clearly associated with pure night sweats in the multivariate model was hot flashes. Panic attacks nearly reached significance (P =.026) and improved the regression model substantially (deviance reduced from 1446 to 87). Variables associated with night and day sweats were weight, sleep problems, hot flashes, antihistamines, SSRIs, and other (non–SSRI, nontricyclic) antidepressants.
For men, the only variable associated with pure night sweats after controlling for other variables was sleep problems. After exclusion of sleep problems and sedatives from the model on the assumption that they might be the result rather than the cause of night sweats, significant predictors were hot flashes (OR = 2.70; 95% confidence interval [CI], 1.35-5.40; P =.005) and regular use of multivitamins (OR = 1.87; 95% CI, 1.17-2.99; P =.009). Variables associated with night and day sweats included greater weight, hot flashes, and greater alcohol use. The ORs and CIs are shown in Table 1.
Interestingly, 32 men (5%) reported hot flashes, and those who did were more likely to report night sweats of both types. Men with hot flashes were evenly distributed across age categories. Their night sweats were more frequent, but not more severe, and they were more likely to bother others than those without hot flashes. Men with hot flashes were more likely to have told their physicians about their night sweats. After controlling for other variables, men with hot flashes were much more likely to have panic attacks (OR = 28.28; P < .001).
Patients 70 years and older made up 19.5% of our sample (N=429). The only factor associated with pure night sweats in the multivariate model was sleep disturbances (OR = 2.04; = 95% CI, 1.21-3.42; P =.007). Exclusion of sleep disturbances left no associated variables. Variables associated with night and day sweats were hot flashes (OR = 15.14; = 95% CI, 6.43-35.68; P < .001) and corticosteroids (OR = 5.45; 95% CI 1.58-18.86; P =.007).
Suspected causes
In cases where patients reported night sweats, only 19% of the patients and 18% of their physicians recorded opinions regarding causation. The suspected causes listed by patients and physicians were similar. Both groups listed menopause most frequently (48% and 44%, respectively). Other etiologies proposed were stress (12% and 8%) and medications (9% and 10%). Physicians listed diabetes as a possible cause in 11% of cases while only 4% of patients listed it. Other suspected causes included obesity, pregnancy, gastroesophageal reflux disease, sleep discomforts, and ambient temperature.
TABLE 2
Associations between independent variables and night sweats in men and women after using logistic regression modeling to control for all other variables
| Patient group | Pure night sweats | Night and day sweats | ||
|---|---|---|---|---|
| Variable | OR (95% CI) | Variable | OR (95% CI) | |
| All | Panic attacks | 4.80 (1.69-13.63) | Age* | 0.99 per yr (0.98-0.99) |
| BMI | 1.03 per unit (1.02-1.05) | |||
| Hot flashes | 7.23 (5.45-9.58) | |||
| Chronic infections | 2.05 (1.22-3.42) | |||
| Sleep problems | 1.54 (1.16-2.04) | |||
| SSRIs | 1.82 (1.22-2.70) | |||
| TCAs | 2.43 (1.25-4.74) | |||
| Other antidepressants | 2.85 (1.66-4.89) | |||
| Xanthines | 5.48 (1.60-18.81) | |||
| Men | Sleep problems | 2.54 (1.7-3.8) | Weight | per lb (1.00-1.02) |
| Hot flashes | 9.41 (4.50-19.8) | |||
| Alcohol | 3.87 (1.60-9.20) | |||
| Women | Hot flashes | 3.35 (1.13-9.95) | Weight | 1.01 per lb (1.00-1.01) |
| Panic attacks | 4.47 (1.20-16.69) | Sleep problems | 1.74 (1.30-240) | |
| Hot flashes | 6.75 (5.00-9.20) | |||
| SSRIs | 2.01 (1.30-3.10) | |||
| Other antidepressants | 2.85 (1.70-5.90) | |||
| Antihistamines | 1.88 (1.20-2.90) | |||
| *Younger age was associated with a greater likelihood of night and day sweats. Otherwise, presence of or increasing amount of each variable was associated with a greater likelihood of night sweats. | ||||
| OR denotes odds ratio; CI, confidence interval; BMI, body mass index; SSRIs, selective serotonin reuptake inhibitors; TCAs, tricyclic antidepressants. | ||||
Discussion
As far as we know, this is the first systematic study of night sweats in a primary care population. It is exploratory in nature, and, because of its cross-sectional design, no firm conclusions can be drawn about causation.
Both pure night sweats and night and day sweats are extremely common, with a peak prevalence in men and women 41 to 55 years of age. In contrast to pure night sweats, night and day sweats are experienced infrequently by patients 70 years and older. The factors associated with pure night sweats are somewhat different than those associated with night and day sweats, suggesting different, though probably overlapping, sets of causes. The different associations seen for men and women, and for older and younger patients, are also noteworthy. Patients often fail to report night sweats to their primary care physician, even when frequent and severe, associated with sleep disturbances, or bothersome to others.
Because of the sampling method (ie, consecutive patients rather than a random sample of active patients), the prevalence estimates reflect the frequency at which physicians can expect to encounter patients with this symptom, rather than the prevalence of night sweats among active patients. Since patients with more symptoms probably see physicians more often, we assume we have overestimated the true prevalence of night sweats in the larger population. Participating physicians were also not selected randomly. It is impossible to know how this may have affected our results.
We were surprised that so few of our independent variables were associated with pure night sweats: only panic attacks (all patients), sleep disorders (men and older patients), and hot flashes (women). Factors not associated with pure night sweats included obesity; diabetes, insulin, or oral hypoglycemic agents; acute or chronic infections; gastroesophageal reflux disease; or thyroid medications. Pure night sweats were also not specifically associated with estrogen and progesterone, although they were associated with hot flashes. There was also no association of pure night sweats and alcohol consumption.
The fact that physicians and their patients could only speculate on a cause for night sweats in 1 out of 5 cases suggests a lack of familiarity with the multitude of suspected causes, a failure to detect certain common causes (eg, sleep disorders and panic attacks), or, most likely, that many common causes of night sweats have yet to be elucidated. If the last is correct, it may be an example of the bias in the primary and secondary clinical literature that occurs when clinical research is carried out primarily in the subspecialty clinics of academic medical centers.4-7 Our findings speak to the need for greater support for primary care practice-based research.8,9
In retrospect, the omission of the variable “panic attacks” from the Oklahoma cards was a mistake, since this variable was correlated with pure night sweats in women. It may have been more strongly associated with pure night sweats in men as well, if the number of respondents to this question had been larger. Also, some men complained of hot flashes, and when they did, they were more likely to have night sweats and panic attacks, suggesting that both hot flashes and night sweats in men should prompt physicians to ask additional questions about panic disorder. Although race was also omitted from the Oklahoma cards, this variable did not seem to be associated with differences in night sweats prevalence or association among those for whom this information was available.
The definition and description of night sweats used in this study were arbitrary and may have influenced the prevalence rates obtained. We attempted to exclude environmental temperature as a cause. Although the definitions provided clearly stated “within the last month,” the data collection cards did not specify a time interval. This may have resulted in some variation in interpretation.
The decisions that were made regarding logistic modeling strategies were conservative and may have excluded some important variables. However, with so many variables and no basis on which to judge a priori, we felt that a conservative approach was best. The decision to include in the models variables (eg, sleep problems and sedatives that might be considered consequences) rather than causes of night sweats, was also arbitrary and may have affected the results. An alternative explanation of the associations found between night sweats and sleep problems is that those who are unable to sleep for other reasons are more likely to notice excessive sweating than those who are asleep.
Future studies should more carefully examine factors found in this study to be associated with night sweats, such as panic attacks and sleep disorders, and other potential etiologic factors not considered, such as tobacco abuse, allergic diseases, migraines, congestive heart failure, and chronic lung disease. Given the high prevalence, future studies examining etiology should include appropriate control groups. Case-control and prospective studies should evaluate the natural history of both night sweats patterns and their association with quality and length of life. The potential value of night sweats as a clue to the early diagnosis of important under-recognized pathologies, such as sleep disorders and panic attacks, should be investigated. Finally, randomized trials of treatments to reduce the frequency, severity, and impact of night sweats should be undertaken once the potential causes have been better elucidated.
Acknowledgments
This research was made possible by a grant from the American Academy of Family Physicians Foundation. We would like to acknowledge the assistance of Lavonne Glover in preparing the manuscript and to the following practicing family physicians and their staff who made time in their busy schedules to collect the data: Nathan Boren, Jo Ann Carpenter, Stephen Cobb, Ed Farrow, Cary Fisher, Helen Franklin, Kurt Frantz, David Hadley, Terrill Hulson, Joe Jamison, Dee Legako, Migy Mathew, Tomas Owens, John Pittman, Mike Pontious, Paul Preslar, R. Scott Stewart, David Strickland, Clinton Strong, Terry Truong, Keith Underhill, Kyle Waugh, Dan Woiwode, Mike Woods, Rick Edwards, Bob C. Jones, Leah R. Mabry, Tom Mueller, Mike Ragsdale, Hugh Wilson, Frank D. Wright, and Samuel T. Coleridge.
1. “MICROMEDEX” Healthcare Series. Englewood, Colorado. Available online at http://www.micromedex.com/. Accessed in June 2001.
2. Lea MJ, Aber RC. Descriptive epidemiology of night sweats upon admission to a university hospital. South Med J 1985;78:1065-7.
3. Reynolds WA. Are night sweats a sign of esophageal reflux? [Letter] J Clin Gastroenterol 1989;11:590-1.
4. White KC, Williams FF, Greenburg BG. The ecology of medical care. N Engl J Med 1961;265:885-92.
5. Rosser WW, Green L. Update from the ambulatory sentinel practice network of North America. Can Fam Phys 1989;35:843-6.
6. Smith FO. Practice-based research: opportunities for the clinician. So Med J 1991;84:479-82.
7. Green LA, Hames CG, Jr, Nutting PA. Potential of practice-based research networks: experiences from ASPN. J Fam Pract 1994;38:400-6.
8. Nutting PA, Beasley JW, Werner JJ. Practice-based research networks answer primary care questions. JAMA 1999;281:686-8.
9. Green LA, Dovey SM. Practice based primary care research networks. BMJ 2001;322:567-8.
OBJECTIVE: To estimate the prevalence and factors associated with night sweats among adult primary care patients.
STUDY DESIGN: This was a cross-sectional study.
POPULATION: Adult patients in 2 primary care practice-based research networks (PBRNs) during 1 week in the summer and 1 week in the winter in the years 2000 and 2001.
OUTCOMES MEASURES: We measured the prevalence of pure night sweats and night and day sweats in all patients and subgroups defined by age and sex, clinical variables associated with night sweats, and the frequency, severity, and rate of reporting.
RESULTS: Of the 2267 patients who participated, 41% reported experiencing night sweats within the last month, including 23% with pure night sweats and an additional 18% with day and night sweats. The prevalence of night sweats in both men and women was highest in the group aged 41 years to 55 years. In multivariate analyses, factors associated with pure night sweats in women were hot flashes and panic attacks; in men, sleep problems. Variables associated with night and day sweats in women were increased weight, hot flashes, sleep disturbances, and use of antihistamines, selective serotonin reuptake inhibitors (SSRIs), and other (non-SSRI, non-tricyclic) antidepressants; in men, increased weight, hot flashes, and greater alcohol use. A majority of patients had not reported their night sweats to their physicians, even when frequent and severe.
CONCLUSIONS: Night sweats are common and under-reported. Pure night sweats and night and day sweats may have different causes. With regard to the etiologies of pure night sweats, panic attacks and sleep disorders need further investigation.
- Night sweats are a common experience for primary care patients, but they are frequently not reported to their physicians.
- There appear to be 2 somewhat distinct patterns of night sweats: pure night sweats and night and day sweats.
- A history of night sweats should prompt questions about menopause, panic attacks, sleep problems, and certain medications.
Night sweats have been attributed to tuberculosis, other acute and chronic febrile illnesses, menopause, pregnancy, hyperthyroidism, nocturnal hypoglycemia, other endocrine problems, neurologic diseases, sleep disorders (eg, sleep apnea and nightmares), malignancies, autoimmune diseases, coronary artery spasm, congestive heart failure, gastroesophageal reflux disease, psychiatric disorders, and certain medications. In 36 medical and surgical textbooks, night sweats were always discussed within sections covering specific diseases and never as a separate topic. References to the primary literature were never provided. We also searched Micromedix, a comprehensive source of information on medications, using “sweating” and “diaphoresis” as search terms.1 Table W1 contains a comprehensive list of proposed causes of night sweats identified in our searches and accompanying references.
Only 2 epidemiologic studies of night sweats were found in the English language literature. Lea and Aber2 interviewed 174 patients randomly selected from the inpatient units of a university hospital and found that 33% of nonobstetric patients and 60% of obstetric patients reported having had night sweats during the previous 3 months. Twenty-six percent of those with night sweats reported that their nighttime sweating was severe enough to require bathing and changing of bed linens. Reynolds,3 a gastroenterologist, queried 200 consecutive patients seen in his outpatient practice and found that 40% remembered experiencing night sweats at least once during the previous year. A total of 12% reported at least weekly night sweats. A review of the records of 750 patients at the Geriatric Continuity Clinic at the University of Oklahoma Family Medicine Center revealed that 10% reported having experienced night sweats during the previous month, when the question was asked as part of a standard review of systems questionnaire (J.W.M., unpublished data, 1999).
Our study was conducted in an effort to estimate the prevalence of night sweats in adult patients seen in primary care office settings, and to explore the associations of this symptom with demographic factors, physical characteristics, medical problems, and medications. We also sought to determine how distressing this symptom is to those who have it and to their sleep partners, whether patients are likely to report the symptom to their physicians, and what patients and their physicians think causes night sweats in individual cases.
Methods
Physician members of the Oklahoma Physicians Resource/Research Network (OKPRN) and the Texas Academy of Family Physicians Research Network (TAFP-Net) enrolled consecutive patients 18 years and older seen in their clinics during a 1-week period in the summer and a second 1-week period in the winter in the years 2000 and 2001. Patients who agreed to participate signed a consent form and then helped the nurse and physician complete a brief questionnaire on a preaddressed, stamped data collection card. For those who declined to participate, a card was generated containing the physician’s code number and the patient’s age and sex. Questions elicited demographic information; information about a selected set of medical conditions; medications, vitamins, herbs, and alcohol used regularly; and information about recent experiences with night sweats. Participating physicians were asked to check the questionnaires for accuracy and to record their opinions regarding the cause of the patients’ night sweats when they reported having had them. A laminated card with definitions of terms was provided to each physician.
“Night sweats” was defined as “sweating at night even when it isn’t excessively hot in your bedroom.” “Day sweats” was defined as “excessive sweating during the daytime.” “Pure night sweats” was defined as night sweats, but not day sweats, and “night and day sweats” as the combination of the 2. The time interval was specified as “during the last month.”
Completed questionnaires were mailed to the Oklahoma Center for Family Medicine Research for data entry and analysis. The data collection cards used by the Texas network included questions about race/ethnicity and panic attacks that were not included on the Oklahoma cards. Inadvertently, some of the Texas cards did not include the question about daytime sweating.
Statistix7 (Analytical Software, Tallahassee, Fla) was used for all statistical analyses. Medications were assigned to 1 of 47 categories according to their primary pharmacologic effects. Summary statistics were calculated for all participants and for the following subgroups: season (summer and winter), pattern of night sweats (excessive nighttime sweating only or night and day sweats), and age group. We anticipated that the majority of women with menopausal symptoms would be in the 41- to 55-year age group.
The two patterns of night sweats, “pure night sweats” and “night and day sweats,” were analyzed separately, and by sex and age. Logistic regression was used to identify the most significant predictors of night sweats while controlling for other variables. Variables were entered into the logistic models if they had a univariate association with the dependent variable at a P value of less than .05. They were then removed one at a time, in the order of largest to smallest P value, if they had a P value of greater than .01 after controlling for other variables. Conservative P values were chosen because of the large numbers of variables considered, in order to reduce the probability of type 1 errors. When appropriate, 95% confidence intervals were calculated.
Results
Study population
A total of 2267 patients of 31 different physicians participated in this study, including 1888 patients of 24 Oklahoma physicians and 379 patients of 7 Texas physicians. Their mean (standard deviation) age was 50.7 (18.8) years, with a range of 18 to 97 years. Sixty-nine percent were women. A total of 99% of Oklahoma patients and 93% of Texas patients seen during the study weeks agreed to participate in the study. Among Texas participants, 53% were Hispanic whites, 33% were non-Hispanic whites, 13% were African Americans, and 1% were categorized as other. On the basis of prior OKPRN studies, we suspect that approximately 90% of Oklahoma patients were non-Hispanic whites, but exact proportions were not determined for this study.
Prevalence of night sweats
The prevalence of pure night sweats, night and day sweats, and any night sweats are shown in Table 1. While the prevalence of night and day sweats was lower for older patients, severity tended to be greater. Severity and frequency were positively correlated for all categories of night sweats and for all subgroups of patients (overall Spearman coefficient = 0.33; P < .001). Overall, the frequencies of night sweats among those who reported the condition were: almost never, 18%; 1 to 3 nights per month, 38%; 1 to 3 nights per week, 27%; and 4 to 7 nights per week, 16%. Ten percent of both women and men with night sweats said that their night sweats were bothersome to others.
TABLE 1
Percentage of patients with pure night sweats and night and day
| Patient group, by sex and age, in years | Pure night sweats % (95% CI) | Night and day sweats % (95% CI) | Any night sweats % (95% CI) |
|---|---|---|---|
| All patients | 23 (21-24) | 18 (16-20) | 41 (39-43) |
| Men | 22 (19-26) | 12 (9-14) | 34 (30-38) |
| 18-40 | 20 (14-26) | 14 (9-19) | 35 (28-42) |
| 41-55 | 25 (18-32) | 14 (9-19) | 40 (33-47) |
| 56-69 | 24 (16-32) | 12 (6-18) | 38 (30-46) |
| 70+ | 20 (13-27) | 6 (2-10) | 26 (19-33) |
| Women | 23 (21-25) | 21 (19-24) | 44 (42-47) |
| 18-40 | 22 (18-26) | 19 (15-23) | 42 (38-46) |
| 41-55 | 29 (24-34) | 32 (28-37) | 61 (56-66) |
| 56-69 | 22 (18-27) | 23 (18-28) | 43 (37-49) |
| 70+ | 19 (14-24) | 9 (5-13) | 29 (24-34) |
| CI denotes confidence interval. | |||
Frequency of reporting of night sweats
A minority of patients with night sweats (12%) had reported the symptom to their physicians. This was true even for those with severe night sweats (46%). Women younger than 70 years were more likely than men of the same age to have reported their night sweats to their physicians (15% vs. 6%; P < .001). The reverse was true for those 70 years and older (7% vs 13%; P =.08). Older patients with pure night sweats were more likely than younger patients to have reported them. After controlling for other variables, patients who were older (odds ratio [OR] = 1.03 per year of age; P < .001), those with night and day sweats (OR = 1.74; P =.0015), and those who reported that their night sweats bothered others (OR = 2.89; P =.001) were more likely to have reported the symptom to their physicians. Those who had reported their night sweats were also more likely to have hot flashes (OR = 2.98; P < .001) and to take estrogen (OR = 1.72; P =.003).
Factors associated with night sweats
The only variable associated with pure night sweats after controlling for all other variables was panic attacks. Variables associated with night and day sweats were younger age, greater body mass index, hot flashes, chronic infection, sleep disturbances, selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants, “other” (non–SSRI, non-tricyclic) antidepressants, and xanthines.
For women, the only variable clearly associated with pure night sweats in the multivariate model was hot flashes. Panic attacks nearly reached significance (P =.026) and improved the regression model substantially (deviance reduced from 1446 to 87). Variables associated with night and day sweats were weight, sleep problems, hot flashes, antihistamines, SSRIs, and other (non–SSRI, nontricyclic) antidepressants.
For men, the only variable associated with pure night sweats after controlling for other variables was sleep problems. After exclusion of sleep problems and sedatives from the model on the assumption that they might be the result rather than the cause of night sweats, significant predictors were hot flashes (OR = 2.70; 95% confidence interval [CI], 1.35-5.40; P =.005) and regular use of multivitamins (OR = 1.87; 95% CI, 1.17-2.99; P =.009). Variables associated with night and day sweats included greater weight, hot flashes, and greater alcohol use. The ORs and CIs are shown in Table 1.
Interestingly, 32 men (5%) reported hot flashes, and those who did were more likely to report night sweats of both types. Men with hot flashes were evenly distributed across age categories. Their night sweats were more frequent, but not more severe, and they were more likely to bother others than those without hot flashes. Men with hot flashes were more likely to have told their physicians about their night sweats. After controlling for other variables, men with hot flashes were much more likely to have panic attacks (OR = 28.28; P < .001).
Patients 70 years and older made up 19.5% of our sample (N=429). The only factor associated with pure night sweats in the multivariate model was sleep disturbances (OR = 2.04; = 95% CI, 1.21-3.42; P =.007). Exclusion of sleep disturbances left no associated variables. Variables associated with night and day sweats were hot flashes (OR = 15.14; = 95% CI, 6.43-35.68; P < .001) and corticosteroids (OR = 5.45; 95% CI 1.58-18.86; P =.007).
Suspected causes
In cases where patients reported night sweats, only 19% of the patients and 18% of their physicians recorded opinions regarding causation. The suspected causes listed by patients and physicians were similar. Both groups listed menopause most frequently (48% and 44%, respectively). Other etiologies proposed were stress (12% and 8%) and medications (9% and 10%). Physicians listed diabetes as a possible cause in 11% of cases while only 4% of patients listed it. Other suspected causes included obesity, pregnancy, gastroesophageal reflux disease, sleep discomforts, and ambient temperature.
TABLE 2
Associations between independent variables and night sweats in men and women after using logistic regression modeling to control for all other variables
| Patient group | Pure night sweats | Night and day sweats | ||
|---|---|---|---|---|
| Variable | OR (95% CI) | Variable | OR (95% CI) | |
| All | Panic attacks | 4.80 (1.69-13.63) | Age* | 0.99 per yr (0.98-0.99) |
| BMI | 1.03 per unit (1.02-1.05) | |||
| Hot flashes | 7.23 (5.45-9.58) | |||
| Chronic infections | 2.05 (1.22-3.42) | |||
| Sleep problems | 1.54 (1.16-2.04) | |||
| SSRIs | 1.82 (1.22-2.70) | |||
| TCAs | 2.43 (1.25-4.74) | |||
| Other antidepressants | 2.85 (1.66-4.89) | |||
| Xanthines | 5.48 (1.60-18.81) | |||
| Men | Sleep problems | 2.54 (1.7-3.8) | Weight | per lb (1.00-1.02) |
| Hot flashes | 9.41 (4.50-19.8) | |||
| Alcohol | 3.87 (1.60-9.20) | |||
| Women | Hot flashes | 3.35 (1.13-9.95) | Weight | 1.01 per lb (1.00-1.01) |
| Panic attacks | 4.47 (1.20-16.69) | Sleep problems | 1.74 (1.30-240) | |
| Hot flashes | 6.75 (5.00-9.20) | |||
| SSRIs | 2.01 (1.30-3.10) | |||
| Other antidepressants | 2.85 (1.70-5.90) | |||
| Antihistamines | 1.88 (1.20-2.90) | |||
| *Younger age was associated with a greater likelihood of night and day sweats. Otherwise, presence of or increasing amount of each variable was associated with a greater likelihood of night sweats. | ||||
| OR denotes odds ratio; CI, confidence interval; BMI, body mass index; SSRIs, selective serotonin reuptake inhibitors; TCAs, tricyclic antidepressants. | ||||
Discussion
As far as we know, this is the first systematic study of night sweats in a primary care population. It is exploratory in nature, and, because of its cross-sectional design, no firm conclusions can be drawn about causation.
Both pure night sweats and night and day sweats are extremely common, with a peak prevalence in men and women 41 to 55 years of age. In contrast to pure night sweats, night and day sweats are experienced infrequently by patients 70 years and older. The factors associated with pure night sweats are somewhat different than those associated with night and day sweats, suggesting different, though probably overlapping, sets of causes. The different associations seen for men and women, and for older and younger patients, are also noteworthy. Patients often fail to report night sweats to their primary care physician, even when frequent and severe, associated with sleep disturbances, or bothersome to others.
Because of the sampling method (ie, consecutive patients rather than a random sample of active patients), the prevalence estimates reflect the frequency at which physicians can expect to encounter patients with this symptom, rather than the prevalence of night sweats among active patients. Since patients with more symptoms probably see physicians more often, we assume we have overestimated the true prevalence of night sweats in the larger population. Participating physicians were also not selected randomly. It is impossible to know how this may have affected our results.
We were surprised that so few of our independent variables were associated with pure night sweats: only panic attacks (all patients), sleep disorders (men and older patients), and hot flashes (women). Factors not associated with pure night sweats included obesity; diabetes, insulin, or oral hypoglycemic agents; acute or chronic infections; gastroesophageal reflux disease; or thyroid medications. Pure night sweats were also not specifically associated with estrogen and progesterone, although they were associated with hot flashes. There was also no association of pure night sweats and alcohol consumption.
The fact that physicians and their patients could only speculate on a cause for night sweats in 1 out of 5 cases suggests a lack of familiarity with the multitude of suspected causes, a failure to detect certain common causes (eg, sleep disorders and panic attacks), or, most likely, that many common causes of night sweats have yet to be elucidated. If the last is correct, it may be an example of the bias in the primary and secondary clinical literature that occurs when clinical research is carried out primarily in the subspecialty clinics of academic medical centers.4-7 Our findings speak to the need for greater support for primary care practice-based research.8,9
In retrospect, the omission of the variable “panic attacks” from the Oklahoma cards was a mistake, since this variable was correlated with pure night sweats in women. It may have been more strongly associated with pure night sweats in men as well, if the number of respondents to this question had been larger. Also, some men complained of hot flashes, and when they did, they were more likely to have night sweats and panic attacks, suggesting that both hot flashes and night sweats in men should prompt physicians to ask additional questions about panic disorder. Although race was also omitted from the Oklahoma cards, this variable did not seem to be associated with differences in night sweats prevalence or association among those for whom this information was available.
The definition and description of night sweats used in this study were arbitrary and may have influenced the prevalence rates obtained. We attempted to exclude environmental temperature as a cause. Although the definitions provided clearly stated “within the last month,” the data collection cards did not specify a time interval. This may have resulted in some variation in interpretation.
The decisions that were made regarding logistic modeling strategies were conservative and may have excluded some important variables. However, with so many variables and no basis on which to judge a priori, we felt that a conservative approach was best. The decision to include in the models variables (eg, sleep problems and sedatives that might be considered consequences) rather than causes of night sweats, was also arbitrary and may have affected the results. An alternative explanation of the associations found between night sweats and sleep problems is that those who are unable to sleep for other reasons are more likely to notice excessive sweating than those who are asleep.
Future studies should more carefully examine factors found in this study to be associated with night sweats, such as panic attacks and sleep disorders, and other potential etiologic factors not considered, such as tobacco abuse, allergic diseases, migraines, congestive heart failure, and chronic lung disease. Given the high prevalence, future studies examining etiology should include appropriate control groups. Case-control and prospective studies should evaluate the natural history of both night sweats patterns and their association with quality and length of life. The potential value of night sweats as a clue to the early diagnosis of important under-recognized pathologies, such as sleep disorders and panic attacks, should be investigated. Finally, randomized trials of treatments to reduce the frequency, severity, and impact of night sweats should be undertaken once the potential causes have been better elucidated.
Acknowledgments
This research was made possible by a grant from the American Academy of Family Physicians Foundation. We would like to acknowledge the assistance of Lavonne Glover in preparing the manuscript and to the following practicing family physicians and their staff who made time in their busy schedules to collect the data: Nathan Boren, Jo Ann Carpenter, Stephen Cobb, Ed Farrow, Cary Fisher, Helen Franklin, Kurt Frantz, David Hadley, Terrill Hulson, Joe Jamison, Dee Legako, Migy Mathew, Tomas Owens, John Pittman, Mike Pontious, Paul Preslar, R. Scott Stewart, David Strickland, Clinton Strong, Terry Truong, Keith Underhill, Kyle Waugh, Dan Woiwode, Mike Woods, Rick Edwards, Bob C. Jones, Leah R. Mabry, Tom Mueller, Mike Ragsdale, Hugh Wilson, Frank D. Wright, and Samuel T. Coleridge.
OBJECTIVE: To estimate the prevalence and factors associated with night sweats among adult primary care patients.
STUDY DESIGN: This was a cross-sectional study.
POPULATION: Adult patients in 2 primary care practice-based research networks (PBRNs) during 1 week in the summer and 1 week in the winter in the years 2000 and 2001.
OUTCOMES MEASURES: We measured the prevalence of pure night sweats and night and day sweats in all patients and subgroups defined by age and sex, clinical variables associated with night sweats, and the frequency, severity, and rate of reporting.
RESULTS: Of the 2267 patients who participated, 41% reported experiencing night sweats within the last month, including 23% with pure night sweats and an additional 18% with day and night sweats. The prevalence of night sweats in both men and women was highest in the group aged 41 years to 55 years. In multivariate analyses, factors associated with pure night sweats in women were hot flashes and panic attacks; in men, sleep problems. Variables associated with night and day sweats in women were increased weight, hot flashes, sleep disturbances, and use of antihistamines, selective serotonin reuptake inhibitors (SSRIs), and other (non-SSRI, non-tricyclic) antidepressants; in men, increased weight, hot flashes, and greater alcohol use. A majority of patients had not reported their night sweats to their physicians, even when frequent and severe.
CONCLUSIONS: Night sweats are common and under-reported. Pure night sweats and night and day sweats may have different causes. With regard to the etiologies of pure night sweats, panic attacks and sleep disorders need further investigation.
- Night sweats are a common experience for primary care patients, but they are frequently not reported to their physicians.
- There appear to be 2 somewhat distinct patterns of night sweats: pure night sweats and night and day sweats.
- A history of night sweats should prompt questions about menopause, panic attacks, sleep problems, and certain medications.
Night sweats have been attributed to tuberculosis, other acute and chronic febrile illnesses, menopause, pregnancy, hyperthyroidism, nocturnal hypoglycemia, other endocrine problems, neurologic diseases, sleep disorders (eg, sleep apnea and nightmares), malignancies, autoimmune diseases, coronary artery spasm, congestive heart failure, gastroesophageal reflux disease, psychiatric disorders, and certain medications. In 36 medical and surgical textbooks, night sweats were always discussed within sections covering specific diseases and never as a separate topic. References to the primary literature were never provided. We also searched Micromedix, a comprehensive source of information on medications, using “sweating” and “diaphoresis” as search terms.1 Table W1 contains a comprehensive list of proposed causes of night sweats identified in our searches and accompanying references.
Only 2 epidemiologic studies of night sweats were found in the English language literature. Lea and Aber2 interviewed 174 patients randomly selected from the inpatient units of a university hospital and found that 33% of nonobstetric patients and 60% of obstetric patients reported having had night sweats during the previous 3 months. Twenty-six percent of those with night sweats reported that their nighttime sweating was severe enough to require bathing and changing of bed linens. Reynolds,3 a gastroenterologist, queried 200 consecutive patients seen in his outpatient practice and found that 40% remembered experiencing night sweats at least once during the previous year. A total of 12% reported at least weekly night sweats. A review of the records of 750 patients at the Geriatric Continuity Clinic at the University of Oklahoma Family Medicine Center revealed that 10% reported having experienced night sweats during the previous month, when the question was asked as part of a standard review of systems questionnaire (J.W.M., unpublished data, 1999).
Our study was conducted in an effort to estimate the prevalence of night sweats in adult patients seen in primary care office settings, and to explore the associations of this symptom with demographic factors, physical characteristics, medical problems, and medications. We also sought to determine how distressing this symptom is to those who have it and to their sleep partners, whether patients are likely to report the symptom to their physicians, and what patients and their physicians think causes night sweats in individual cases.
Methods
Physician members of the Oklahoma Physicians Resource/Research Network (OKPRN) and the Texas Academy of Family Physicians Research Network (TAFP-Net) enrolled consecutive patients 18 years and older seen in their clinics during a 1-week period in the summer and a second 1-week period in the winter in the years 2000 and 2001. Patients who agreed to participate signed a consent form and then helped the nurse and physician complete a brief questionnaire on a preaddressed, stamped data collection card. For those who declined to participate, a card was generated containing the physician’s code number and the patient’s age and sex. Questions elicited demographic information; information about a selected set of medical conditions; medications, vitamins, herbs, and alcohol used regularly; and information about recent experiences with night sweats. Participating physicians were asked to check the questionnaires for accuracy and to record their opinions regarding the cause of the patients’ night sweats when they reported having had them. A laminated card with definitions of terms was provided to each physician.
“Night sweats” was defined as “sweating at night even when it isn’t excessively hot in your bedroom.” “Day sweats” was defined as “excessive sweating during the daytime.” “Pure night sweats” was defined as night sweats, but not day sweats, and “night and day sweats” as the combination of the 2. The time interval was specified as “during the last month.”
Completed questionnaires were mailed to the Oklahoma Center for Family Medicine Research for data entry and analysis. The data collection cards used by the Texas network included questions about race/ethnicity and panic attacks that were not included on the Oklahoma cards. Inadvertently, some of the Texas cards did not include the question about daytime sweating.
Statistix7 (Analytical Software, Tallahassee, Fla) was used for all statistical analyses. Medications were assigned to 1 of 47 categories according to their primary pharmacologic effects. Summary statistics were calculated for all participants and for the following subgroups: season (summer and winter), pattern of night sweats (excessive nighttime sweating only or night and day sweats), and age group. We anticipated that the majority of women with menopausal symptoms would be in the 41- to 55-year age group.
The two patterns of night sweats, “pure night sweats” and “night and day sweats,” were analyzed separately, and by sex and age. Logistic regression was used to identify the most significant predictors of night sweats while controlling for other variables. Variables were entered into the logistic models if they had a univariate association with the dependent variable at a P value of less than .05. They were then removed one at a time, in the order of largest to smallest P value, if they had a P value of greater than .01 after controlling for other variables. Conservative P values were chosen because of the large numbers of variables considered, in order to reduce the probability of type 1 errors. When appropriate, 95% confidence intervals were calculated.
Results
Study population
A total of 2267 patients of 31 different physicians participated in this study, including 1888 patients of 24 Oklahoma physicians and 379 patients of 7 Texas physicians. Their mean (standard deviation) age was 50.7 (18.8) years, with a range of 18 to 97 years. Sixty-nine percent were women. A total of 99% of Oklahoma patients and 93% of Texas patients seen during the study weeks agreed to participate in the study. Among Texas participants, 53% were Hispanic whites, 33% were non-Hispanic whites, 13% were African Americans, and 1% were categorized as other. On the basis of prior OKPRN studies, we suspect that approximately 90% of Oklahoma patients were non-Hispanic whites, but exact proportions were not determined for this study.
Prevalence of night sweats
The prevalence of pure night sweats, night and day sweats, and any night sweats are shown in Table 1. While the prevalence of night and day sweats was lower for older patients, severity tended to be greater. Severity and frequency were positively correlated for all categories of night sweats and for all subgroups of patients (overall Spearman coefficient = 0.33; P < .001). Overall, the frequencies of night sweats among those who reported the condition were: almost never, 18%; 1 to 3 nights per month, 38%; 1 to 3 nights per week, 27%; and 4 to 7 nights per week, 16%. Ten percent of both women and men with night sweats said that their night sweats were bothersome to others.
TABLE 1
Percentage of patients with pure night sweats and night and day
| Patient group, by sex and age, in years | Pure night sweats % (95% CI) | Night and day sweats % (95% CI) | Any night sweats % (95% CI) |
|---|---|---|---|
| All patients | 23 (21-24) | 18 (16-20) | 41 (39-43) |
| Men | 22 (19-26) | 12 (9-14) | 34 (30-38) |
| 18-40 | 20 (14-26) | 14 (9-19) | 35 (28-42) |
| 41-55 | 25 (18-32) | 14 (9-19) | 40 (33-47) |
| 56-69 | 24 (16-32) | 12 (6-18) | 38 (30-46) |
| 70+ | 20 (13-27) | 6 (2-10) | 26 (19-33) |
| Women | 23 (21-25) | 21 (19-24) | 44 (42-47) |
| 18-40 | 22 (18-26) | 19 (15-23) | 42 (38-46) |
| 41-55 | 29 (24-34) | 32 (28-37) | 61 (56-66) |
| 56-69 | 22 (18-27) | 23 (18-28) | 43 (37-49) |
| 70+ | 19 (14-24) | 9 (5-13) | 29 (24-34) |
| CI denotes confidence interval. | |||
Frequency of reporting of night sweats
A minority of patients with night sweats (12%) had reported the symptom to their physicians. This was true even for those with severe night sweats (46%). Women younger than 70 years were more likely than men of the same age to have reported their night sweats to their physicians (15% vs. 6%; P < .001). The reverse was true for those 70 years and older (7% vs 13%; P =.08). Older patients with pure night sweats were more likely than younger patients to have reported them. After controlling for other variables, patients who were older (odds ratio [OR] = 1.03 per year of age; P < .001), those with night and day sweats (OR = 1.74; P =.0015), and those who reported that their night sweats bothered others (OR = 2.89; P =.001) were more likely to have reported the symptom to their physicians. Those who had reported their night sweats were also more likely to have hot flashes (OR = 2.98; P < .001) and to take estrogen (OR = 1.72; P =.003).
Factors associated with night sweats
The only variable associated with pure night sweats after controlling for all other variables was panic attacks. Variables associated with night and day sweats were younger age, greater body mass index, hot flashes, chronic infection, sleep disturbances, selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants, “other” (non–SSRI, non-tricyclic) antidepressants, and xanthines.
For women, the only variable clearly associated with pure night sweats in the multivariate model was hot flashes. Panic attacks nearly reached significance (P =.026) and improved the regression model substantially (deviance reduced from 1446 to 87). Variables associated with night and day sweats were weight, sleep problems, hot flashes, antihistamines, SSRIs, and other (non–SSRI, nontricyclic) antidepressants.
For men, the only variable associated with pure night sweats after controlling for other variables was sleep problems. After exclusion of sleep problems and sedatives from the model on the assumption that they might be the result rather than the cause of night sweats, significant predictors were hot flashes (OR = 2.70; 95% confidence interval [CI], 1.35-5.40; P =.005) and regular use of multivitamins (OR = 1.87; 95% CI, 1.17-2.99; P =.009). Variables associated with night and day sweats included greater weight, hot flashes, and greater alcohol use. The ORs and CIs are shown in Table 1.
Interestingly, 32 men (5%) reported hot flashes, and those who did were more likely to report night sweats of both types. Men with hot flashes were evenly distributed across age categories. Their night sweats were more frequent, but not more severe, and they were more likely to bother others than those without hot flashes. Men with hot flashes were more likely to have told their physicians about their night sweats. After controlling for other variables, men with hot flashes were much more likely to have panic attacks (OR = 28.28; P < .001).
Patients 70 years and older made up 19.5% of our sample (N=429). The only factor associated with pure night sweats in the multivariate model was sleep disturbances (OR = 2.04; = 95% CI, 1.21-3.42; P =.007). Exclusion of sleep disturbances left no associated variables. Variables associated with night and day sweats were hot flashes (OR = 15.14; = 95% CI, 6.43-35.68; P < .001) and corticosteroids (OR = 5.45; 95% CI 1.58-18.86; P =.007).
Suspected causes
In cases where patients reported night sweats, only 19% of the patients and 18% of their physicians recorded opinions regarding causation. The suspected causes listed by patients and physicians were similar. Both groups listed menopause most frequently (48% and 44%, respectively). Other etiologies proposed were stress (12% and 8%) and medications (9% and 10%). Physicians listed diabetes as a possible cause in 11% of cases while only 4% of patients listed it. Other suspected causes included obesity, pregnancy, gastroesophageal reflux disease, sleep discomforts, and ambient temperature.
TABLE 2
Associations between independent variables and night sweats in men and women after using logistic regression modeling to control for all other variables
| Patient group | Pure night sweats | Night and day sweats | ||
|---|---|---|---|---|
| Variable | OR (95% CI) | Variable | OR (95% CI) | |
| All | Panic attacks | 4.80 (1.69-13.63) | Age* | 0.99 per yr (0.98-0.99) |
| BMI | 1.03 per unit (1.02-1.05) | |||
| Hot flashes | 7.23 (5.45-9.58) | |||
| Chronic infections | 2.05 (1.22-3.42) | |||
| Sleep problems | 1.54 (1.16-2.04) | |||
| SSRIs | 1.82 (1.22-2.70) | |||
| TCAs | 2.43 (1.25-4.74) | |||
| Other antidepressants | 2.85 (1.66-4.89) | |||
| Xanthines | 5.48 (1.60-18.81) | |||
| Men | Sleep problems | 2.54 (1.7-3.8) | Weight | per lb (1.00-1.02) |
| Hot flashes | 9.41 (4.50-19.8) | |||
| Alcohol | 3.87 (1.60-9.20) | |||
| Women | Hot flashes | 3.35 (1.13-9.95) | Weight | 1.01 per lb (1.00-1.01) |
| Panic attacks | 4.47 (1.20-16.69) | Sleep problems | 1.74 (1.30-240) | |
| Hot flashes | 6.75 (5.00-9.20) | |||
| SSRIs | 2.01 (1.30-3.10) | |||
| Other antidepressants | 2.85 (1.70-5.90) | |||
| Antihistamines | 1.88 (1.20-2.90) | |||
| *Younger age was associated with a greater likelihood of night and day sweats. Otherwise, presence of or increasing amount of each variable was associated with a greater likelihood of night sweats. | ||||
| OR denotes odds ratio; CI, confidence interval; BMI, body mass index; SSRIs, selective serotonin reuptake inhibitors; TCAs, tricyclic antidepressants. | ||||
Discussion
As far as we know, this is the first systematic study of night sweats in a primary care population. It is exploratory in nature, and, because of its cross-sectional design, no firm conclusions can be drawn about causation.
Both pure night sweats and night and day sweats are extremely common, with a peak prevalence in men and women 41 to 55 years of age. In contrast to pure night sweats, night and day sweats are experienced infrequently by patients 70 years and older. The factors associated with pure night sweats are somewhat different than those associated with night and day sweats, suggesting different, though probably overlapping, sets of causes. The different associations seen for men and women, and for older and younger patients, are also noteworthy. Patients often fail to report night sweats to their primary care physician, even when frequent and severe, associated with sleep disturbances, or bothersome to others.
Because of the sampling method (ie, consecutive patients rather than a random sample of active patients), the prevalence estimates reflect the frequency at which physicians can expect to encounter patients with this symptom, rather than the prevalence of night sweats among active patients. Since patients with more symptoms probably see physicians more often, we assume we have overestimated the true prevalence of night sweats in the larger population. Participating physicians were also not selected randomly. It is impossible to know how this may have affected our results.
We were surprised that so few of our independent variables were associated with pure night sweats: only panic attacks (all patients), sleep disorders (men and older patients), and hot flashes (women). Factors not associated with pure night sweats included obesity; diabetes, insulin, or oral hypoglycemic agents; acute or chronic infections; gastroesophageal reflux disease; or thyroid medications. Pure night sweats were also not specifically associated with estrogen and progesterone, although they were associated with hot flashes. There was also no association of pure night sweats and alcohol consumption.
The fact that physicians and their patients could only speculate on a cause for night sweats in 1 out of 5 cases suggests a lack of familiarity with the multitude of suspected causes, a failure to detect certain common causes (eg, sleep disorders and panic attacks), or, most likely, that many common causes of night sweats have yet to be elucidated. If the last is correct, it may be an example of the bias in the primary and secondary clinical literature that occurs when clinical research is carried out primarily in the subspecialty clinics of academic medical centers.4-7 Our findings speak to the need for greater support for primary care practice-based research.8,9
In retrospect, the omission of the variable “panic attacks” from the Oklahoma cards was a mistake, since this variable was correlated with pure night sweats in women. It may have been more strongly associated with pure night sweats in men as well, if the number of respondents to this question had been larger. Also, some men complained of hot flashes, and when they did, they were more likely to have night sweats and panic attacks, suggesting that both hot flashes and night sweats in men should prompt physicians to ask additional questions about panic disorder. Although race was also omitted from the Oklahoma cards, this variable did not seem to be associated with differences in night sweats prevalence or association among those for whom this information was available.
The definition and description of night sweats used in this study were arbitrary and may have influenced the prevalence rates obtained. We attempted to exclude environmental temperature as a cause. Although the definitions provided clearly stated “within the last month,” the data collection cards did not specify a time interval. This may have resulted in some variation in interpretation.
The decisions that were made regarding logistic modeling strategies were conservative and may have excluded some important variables. However, with so many variables and no basis on which to judge a priori, we felt that a conservative approach was best. The decision to include in the models variables (eg, sleep problems and sedatives that might be considered consequences) rather than causes of night sweats, was also arbitrary and may have affected the results. An alternative explanation of the associations found between night sweats and sleep problems is that those who are unable to sleep for other reasons are more likely to notice excessive sweating than those who are asleep.
Future studies should more carefully examine factors found in this study to be associated with night sweats, such as panic attacks and sleep disorders, and other potential etiologic factors not considered, such as tobacco abuse, allergic diseases, migraines, congestive heart failure, and chronic lung disease. Given the high prevalence, future studies examining etiology should include appropriate control groups. Case-control and prospective studies should evaluate the natural history of both night sweats patterns and their association with quality and length of life. The potential value of night sweats as a clue to the early diagnosis of important under-recognized pathologies, such as sleep disorders and panic attacks, should be investigated. Finally, randomized trials of treatments to reduce the frequency, severity, and impact of night sweats should be undertaken once the potential causes have been better elucidated.
Acknowledgments
This research was made possible by a grant from the American Academy of Family Physicians Foundation. We would like to acknowledge the assistance of Lavonne Glover in preparing the manuscript and to the following practicing family physicians and their staff who made time in their busy schedules to collect the data: Nathan Boren, Jo Ann Carpenter, Stephen Cobb, Ed Farrow, Cary Fisher, Helen Franklin, Kurt Frantz, David Hadley, Terrill Hulson, Joe Jamison, Dee Legako, Migy Mathew, Tomas Owens, John Pittman, Mike Pontious, Paul Preslar, R. Scott Stewart, David Strickland, Clinton Strong, Terry Truong, Keith Underhill, Kyle Waugh, Dan Woiwode, Mike Woods, Rick Edwards, Bob C. Jones, Leah R. Mabry, Tom Mueller, Mike Ragsdale, Hugh Wilson, Frank D. Wright, and Samuel T. Coleridge.
1. “MICROMEDEX” Healthcare Series. Englewood, Colorado. Available online at http://www.micromedex.com/. Accessed in June 2001.
2. Lea MJ, Aber RC. Descriptive epidemiology of night sweats upon admission to a university hospital. South Med J 1985;78:1065-7.
3. Reynolds WA. Are night sweats a sign of esophageal reflux? [Letter] J Clin Gastroenterol 1989;11:590-1.
4. White KC, Williams FF, Greenburg BG. The ecology of medical care. N Engl J Med 1961;265:885-92.
5. Rosser WW, Green L. Update from the ambulatory sentinel practice network of North America. Can Fam Phys 1989;35:843-6.
6. Smith FO. Practice-based research: opportunities for the clinician. So Med J 1991;84:479-82.
7. Green LA, Hames CG, Jr, Nutting PA. Potential of practice-based research networks: experiences from ASPN. J Fam Pract 1994;38:400-6.
8. Nutting PA, Beasley JW, Werner JJ. Practice-based research networks answer primary care questions. JAMA 1999;281:686-8.
9. Green LA, Dovey SM. Practice based primary care research networks. BMJ 2001;322:567-8.
1. “MICROMEDEX” Healthcare Series. Englewood, Colorado. Available online at http://www.micromedex.com/. Accessed in June 2001.
2. Lea MJ, Aber RC. Descriptive epidemiology of night sweats upon admission to a university hospital. South Med J 1985;78:1065-7.
3. Reynolds WA. Are night sweats a sign of esophageal reflux? [Letter] J Clin Gastroenterol 1989;11:590-1.
4. White KC, Williams FF, Greenburg BG. The ecology of medical care. N Engl J Med 1961;265:885-92.
5. Rosser WW, Green L. Update from the ambulatory sentinel practice network of North America. Can Fam Phys 1989;35:843-6.
6. Smith FO. Practice-based research: opportunities for the clinician. So Med J 1991;84:479-82.
7. Green LA, Hames CG, Jr, Nutting PA. Potential of practice-based research networks: experiences from ASPN. J Fam Pract 1994;38:400-6.
8. Nutting PA, Beasley JW, Werner JJ. Practice-based research networks answer primary care questions. JAMA 1999;281:686-8.
9. Green LA, Dovey SM. Practice based primary care research networks. BMJ 2001;322:567-8.