User login
Study reveals ‘high-traffic’ routes of malaria importation
Results of an international study suggest France and the UK experience the highest number of malaria cases imported from other countries.
Researchers mapped the movement of malaria from endemic countries to 40 countries defined as being malaria-free.
The countries with the highest average number of imported infections per year over the past decade were France (2169), the UK (1898), the US (1511), Italy (637), and Germany (401).
Infection movement was strongly skewed to a small number of “high-traffic” routes, with malaria cases originating from West Africa accounting for 56% (13,947) of all cases detected in non-endemic countries.
These results were published in The Lancet Infectious Diseases.
“This is the first world-wide assessment of imported malaria cases in 20 years, and mapping this data is hugely valuable in helping us understand how we can mitigate against the effects of the global movements of the disease,” said study author Andrew Tatem, PhD, of the University of Southampton in the UK.
“Imported malaria can be expensive to treat, contribute to drug resistance, sometimes cause secondary local transmission, and threaten the long-term goal of eradication. This study forms part of wider efforts to understand patterns of human and malaria parasite movement to help guide elimination strategies.”
For this study, researchers analyzed a database of nationally reported statistics on imported malaria covering more than 50,000 individual cases over 10 years.
Although most incidents of malaria in non-endemic countries originated in West Africa, the study showed that 20% were from India (4988), 13% were from East Africa (3242), and 3% were from Papua New Guinea (748).
And although the routes from West Africa to France and the UK showed the strongest imported malaria link, there were other high-traffic routes. These included India to the US (149 cases on average per year), Papua New Guinea to Australia (97), Pakistan to the UK (69), and Haiti to the US (52).
By mapping this network of malaria movements across continents, the researchers showed that a number of factors, beyond geographic ones, may influence the strength of importation levels.
For example, the researchers believe that historical, economic, language, and cultural ties all play a part. They said population movements with former colonies had particular influence; such as Nigeria, Ghana, and Kenya with the UK, and Mali, Niger, and Chad with France.
The researchers hope to conduct further studies to examine which factors are the drivers behind the patterns of malaria spread between endemic and non-endemic countries. ![]()

Results of an international study suggest France and the UK experience the highest number of malaria cases imported from other countries.
Researchers mapped the movement of malaria from endemic countries to 40 countries defined as being malaria-free.
The countries with the highest average number of imported infections per year over the past decade were France (2169), the UK (1898), the US (1511), Italy (637), and Germany (401).
Infection movement was strongly skewed to a small number of “high-traffic” routes, with malaria cases originating from West Africa accounting for 56% (13,947) of all cases detected in non-endemic countries.
These results were published in The Lancet Infectious Diseases.
“This is the first world-wide assessment of imported malaria cases in 20 years, and mapping this data is hugely valuable in helping us understand how we can mitigate against the effects of the global movements of the disease,” said study author Andrew Tatem, PhD, of the University of Southampton in the UK.
“Imported malaria can be expensive to treat, contribute to drug resistance, sometimes cause secondary local transmission, and threaten the long-term goal of eradication. This study forms part of wider efforts to understand patterns of human and malaria parasite movement to help guide elimination strategies.”
For this study, researchers analyzed a database of nationally reported statistics on imported malaria covering more than 50,000 individual cases over 10 years.
Although most incidents of malaria in non-endemic countries originated in West Africa, the study showed that 20% were from India (4988), 13% were from East Africa (3242), and 3% were from Papua New Guinea (748).
And although the routes from West Africa to France and the UK showed the strongest imported malaria link, there were other high-traffic routes. These included India to the US (149 cases on average per year), Papua New Guinea to Australia (97), Pakistan to the UK (69), and Haiti to the US (52).
By mapping this network of malaria movements across continents, the researchers showed that a number of factors, beyond geographic ones, may influence the strength of importation levels.
For example, the researchers believe that historical, economic, language, and cultural ties all play a part. They said population movements with former colonies had particular influence; such as Nigeria, Ghana, and Kenya with the UK, and Mali, Niger, and Chad with France.
The researchers hope to conduct further studies to examine which factors are the drivers behind the patterns of malaria spread between endemic and non-endemic countries. ![]()

Results of an international study suggest France and the UK experience the highest number of malaria cases imported from other countries.
Researchers mapped the movement of malaria from endemic countries to 40 countries defined as being malaria-free.
The countries with the highest average number of imported infections per year over the past decade were France (2169), the UK (1898), the US (1511), Italy (637), and Germany (401).
Infection movement was strongly skewed to a small number of “high-traffic” routes, with malaria cases originating from West Africa accounting for 56% (13,947) of all cases detected in non-endemic countries.
These results were published in The Lancet Infectious Diseases.
“This is the first world-wide assessment of imported malaria cases in 20 years, and mapping this data is hugely valuable in helping us understand how we can mitigate against the effects of the global movements of the disease,” said study author Andrew Tatem, PhD, of the University of Southampton in the UK.
“Imported malaria can be expensive to treat, contribute to drug resistance, sometimes cause secondary local transmission, and threaten the long-term goal of eradication. This study forms part of wider efforts to understand patterns of human and malaria parasite movement to help guide elimination strategies.”
For this study, researchers analyzed a database of nationally reported statistics on imported malaria covering more than 50,000 individual cases over 10 years.
Although most incidents of malaria in non-endemic countries originated in West Africa, the study showed that 20% were from India (4988), 13% were from East Africa (3242), and 3% were from Papua New Guinea (748).
And although the routes from West Africa to France and the UK showed the strongest imported malaria link, there were other high-traffic routes. These included India to the US (149 cases on average per year), Papua New Guinea to Australia (97), Pakistan to the UK (69), and Haiti to the US (52).
By mapping this network of malaria movements across continents, the researchers showed that a number of factors, beyond geographic ones, may influence the strength of importation levels.
For example, the researchers believe that historical, economic, language, and cultural ties all play a part. They said population movements with former colonies had particular influence; such as Nigeria, Ghana, and Kenya with the UK, and Mali, Niger, and Chad with France.
The researchers hope to conduct further studies to examine which factors are the drivers behind the patterns of malaria spread between endemic and non-endemic countries. ![]()
Testing could ID cancer patients at high risk of VTE

Image by Andre E.X. Brown
Genetic testing could help identify breast cancer patients with a high risk of developing venous thromboembolism (VTE), according to a study published in Clinical Cancer Research.
The study showed that patients who received chemotherapy, had a higher genetic susceptibility for VTE according to a polygenic risk score (PRS), or had both of these risk factors were more likely to develop VTE than patients without these risk factors.
In addition, researchers found the impact of genetic susceptibility was most pronounced in older patients.
“The risk for [VTE] is increased in cancer patients, particularly in those receiving chemotherapy,” said study author Judith S. Brand, PhD, of Karolinska Institutet in Stockholm, Sweden.
“As one of the most common cancers, breast cancer accounts for a large number of cancer-associated VTE cases.”
Dr Brand and her colleagues sought to identify the individual and joint effects of chemotherapy and genetic susceptibility on VTE risk. They studied 4261 women in the Stockholm region diagnosed with primary invasive breast cancer between 2001 and 2008, and followed until 2012.
Risks were stratified based on chemotherapy status and genetic susceptibility, as determined by a PRS based on 9 established VTE loci, with the top 5% classified as having high genetic susceptibility.
The median follow-up was 7.6 years, and 276 patients experienced a VTE during that time.
The researchers found that receiving chemotherapy and having high genetic susceptibility independently increased the risk of VTE. The hazard ratio was 1.98 for patients receiving chemotherapy and 1.90 for patients in the highest 5% of the PRS.
The 1-year cumulative incidence of VTE was 9.5% among patients who both received chemotherapy and had high genetic susceptibility, compared with 1.3% in the patients who did not have either of these risk factors (P<0.001).
The researchers also found that patient age played a role in VTE risk. The team said the risk-increasing effect of the PRS was stronger in older patients (P interaction = 0.04).
In patients age 60 or older who underwent chemotherapy and had a high genetic susceptibility, the 1-year cumulative incidence of VTE was 25%.
“Breast cancer patients receiving chemotherapy are not routinely being examined for VTE prevention in today’s clinical practice,” Dr Brand said. “Our study demonstrates that information on genetic susceptibility can be used to identify patients at high risk of developing VTE.”
“Combined with other clinical risk factors and biomarkers, these findings will guide future studies evaluating routine VTE risk assessment in chemotherapy outpatients, and prophylaxis for those at highest risk. Because older patients demonstrated a stronger genetic effect and higher VTE incidence, this group requires special attention in future risk stratification efforts.”
Dr Brand added that a limitation of this study is the small number of older patients who had chemotherapy and a high genetic susceptibility. She said larger-scale studies would be necessary to provide more precise risk estimates. And further research is needed to assess the safety and potential benefit of thromboprophylaxis in high-risk cancer patients. ![]()

Image by Andre E.X. Brown
Genetic testing could help identify breast cancer patients with a high risk of developing venous thromboembolism (VTE), according to a study published in Clinical Cancer Research.
The study showed that patients who received chemotherapy, had a higher genetic susceptibility for VTE according to a polygenic risk score (PRS), or had both of these risk factors were more likely to develop VTE than patients without these risk factors.
In addition, researchers found the impact of genetic susceptibility was most pronounced in older patients.
“The risk for [VTE] is increased in cancer patients, particularly in those receiving chemotherapy,” said study author Judith S. Brand, PhD, of Karolinska Institutet in Stockholm, Sweden.
“As one of the most common cancers, breast cancer accounts for a large number of cancer-associated VTE cases.”
Dr Brand and her colleagues sought to identify the individual and joint effects of chemotherapy and genetic susceptibility on VTE risk. They studied 4261 women in the Stockholm region diagnosed with primary invasive breast cancer between 2001 and 2008, and followed until 2012.
Risks were stratified based on chemotherapy status and genetic susceptibility, as determined by a PRS based on 9 established VTE loci, with the top 5% classified as having high genetic susceptibility.
The median follow-up was 7.6 years, and 276 patients experienced a VTE during that time.
The researchers found that receiving chemotherapy and having high genetic susceptibility independently increased the risk of VTE. The hazard ratio was 1.98 for patients receiving chemotherapy and 1.90 for patients in the highest 5% of the PRS.
The 1-year cumulative incidence of VTE was 9.5% among patients who both received chemotherapy and had high genetic susceptibility, compared with 1.3% in the patients who did not have either of these risk factors (P<0.001).
The researchers also found that patient age played a role in VTE risk. The team said the risk-increasing effect of the PRS was stronger in older patients (P interaction = 0.04).
In patients age 60 or older who underwent chemotherapy and had a high genetic susceptibility, the 1-year cumulative incidence of VTE was 25%.
“Breast cancer patients receiving chemotherapy are not routinely being examined for VTE prevention in today’s clinical practice,” Dr Brand said. “Our study demonstrates that information on genetic susceptibility can be used to identify patients at high risk of developing VTE.”
“Combined with other clinical risk factors and biomarkers, these findings will guide future studies evaluating routine VTE risk assessment in chemotherapy outpatients, and prophylaxis for those at highest risk. Because older patients demonstrated a stronger genetic effect and higher VTE incidence, this group requires special attention in future risk stratification efforts.”
Dr Brand added that a limitation of this study is the small number of older patients who had chemotherapy and a high genetic susceptibility. She said larger-scale studies would be necessary to provide more precise risk estimates. And further research is needed to assess the safety and potential benefit of thromboprophylaxis in high-risk cancer patients. ![]()

Image by Andre E.X. Brown
Genetic testing could help identify breast cancer patients with a high risk of developing venous thromboembolism (VTE), according to a study published in Clinical Cancer Research.
The study showed that patients who received chemotherapy, had a higher genetic susceptibility for VTE according to a polygenic risk score (PRS), or had both of these risk factors were more likely to develop VTE than patients without these risk factors.
In addition, researchers found the impact of genetic susceptibility was most pronounced in older patients.
“The risk for [VTE] is increased in cancer patients, particularly in those receiving chemotherapy,” said study author Judith S. Brand, PhD, of Karolinska Institutet in Stockholm, Sweden.
“As one of the most common cancers, breast cancer accounts for a large number of cancer-associated VTE cases.”
Dr Brand and her colleagues sought to identify the individual and joint effects of chemotherapy and genetic susceptibility on VTE risk. They studied 4261 women in the Stockholm region diagnosed with primary invasive breast cancer between 2001 and 2008, and followed until 2012.
Risks were stratified based on chemotherapy status and genetic susceptibility, as determined by a PRS based on 9 established VTE loci, with the top 5% classified as having high genetic susceptibility.
The median follow-up was 7.6 years, and 276 patients experienced a VTE during that time.
The researchers found that receiving chemotherapy and having high genetic susceptibility independently increased the risk of VTE. The hazard ratio was 1.98 for patients receiving chemotherapy and 1.90 for patients in the highest 5% of the PRS.
The 1-year cumulative incidence of VTE was 9.5% among patients who both received chemotherapy and had high genetic susceptibility, compared with 1.3% in the patients who did not have either of these risk factors (P<0.001).
The researchers also found that patient age played a role in VTE risk. The team said the risk-increasing effect of the PRS was stronger in older patients (P interaction = 0.04).
In patients age 60 or older who underwent chemotherapy and had a high genetic susceptibility, the 1-year cumulative incidence of VTE was 25%.
“Breast cancer patients receiving chemotherapy are not routinely being examined for VTE prevention in today’s clinical practice,” Dr Brand said. “Our study demonstrates that information on genetic susceptibility can be used to identify patients at high risk of developing VTE.”
“Combined with other clinical risk factors and biomarkers, these findings will guide future studies evaluating routine VTE risk assessment in chemotherapy outpatients, and prophylaxis for those at highest risk. Because older patients demonstrated a stronger genetic effect and higher VTE incidence, this group requires special attention in future risk stratification efforts.”
Dr Brand added that a limitation of this study is the small number of older patients who had chemotherapy and a high genetic susceptibility. She said larger-scale studies would be necessary to provide more precise risk estimates. And further research is needed to assess the safety and potential benefit of thromboprophylaxis in high-risk cancer patients. ![]()
CTC analysis as good as BM biopsy in MM, team says

Photo by Juan D. Alfonso
Analysis of circulating tumor cells (CTCs) can provide at least as much genetic information about multiple myeloma (MM) as a bone marrow (BM) biopsy, according to a new study.
Researchers found evidence to suggest that analyzing CTCs isolated from the peripheral blood of MM patients could help physicians monitor disease progression over time, track the emergence of drug resistance, and tailor therapies to individual patients.
Jens Lohr, MD, PhD, of the Broad Institute of MIT and Harvard in Cambridge, Massachusetts, and colleagues conducted this research and detailed their findings in Science Translational Medicine.
The researchers said they devised a method that was “highly sensitive” in detecting and sequencing single CTCs, even from patients with low tumor burden.
Specifically, the team found they could isolate CTCs from, and detect mutations in, blood samples from an MM patient who had achieved a very good partial response to treatment and a patient with monoclonal gammopathy of undetermined significance.
Overall, the researchers found that CTCs could be used to identify mutations relevant for prognosis, and some of these mutations were more abundant in CTCs than in BM samples.
For example, the team detected somatic mutations in the KRAS, BRAF, IRF4, and TP53 genes in single CTCs from 3 MM patients. And the same mutations were present in single BM-derived MM cells.
In another 3 patients, the proportion of CTCs harboring TP53 R273C, BRAF G469A, and NRAS G13D mutations was higher than that observed in BM-derived cells. In 2 of the patients, the mutations weren’t detectable in BM cells because of insufficient sample material.
The researchers also found that CTCs could reveal patients with an overabundance of molecules expressed by MM cells—such as CD38 and SLAMF7—that can be targeted by therapies currently approved to treat MM—such as daratumumab and elotuzumab.
The team therefore believes that, with further development, CTC analysis could be a valuable tool for advancing precision medicine in MM. ![]()

Photo by Juan D. Alfonso
Analysis of circulating tumor cells (CTCs) can provide at least as much genetic information about multiple myeloma (MM) as a bone marrow (BM) biopsy, according to a new study.
Researchers found evidence to suggest that analyzing CTCs isolated from the peripheral blood of MM patients could help physicians monitor disease progression over time, track the emergence of drug resistance, and tailor therapies to individual patients.
Jens Lohr, MD, PhD, of the Broad Institute of MIT and Harvard in Cambridge, Massachusetts, and colleagues conducted this research and detailed their findings in Science Translational Medicine.
The researchers said they devised a method that was “highly sensitive” in detecting and sequencing single CTCs, even from patients with low tumor burden.
Specifically, the team found they could isolate CTCs from, and detect mutations in, blood samples from an MM patient who had achieved a very good partial response to treatment and a patient with monoclonal gammopathy of undetermined significance.
Overall, the researchers found that CTCs could be used to identify mutations relevant for prognosis, and some of these mutations were more abundant in CTCs than in BM samples.
For example, the team detected somatic mutations in the KRAS, BRAF, IRF4, and TP53 genes in single CTCs from 3 MM patients. And the same mutations were present in single BM-derived MM cells.
In another 3 patients, the proportion of CTCs harboring TP53 R273C, BRAF G469A, and NRAS G13D mutations was higher than that observed in BM-derived cells. In 2 of the patients, the mutations weren’t detectable in BM cells because of insufficient sample material.
The researchers also found that CTCs could reveal patients with an overabundance of molecules expressed by MM cells—such as CD38 and SLAMF7—that can be targeted by therapies currently approved to treat MM—such as daratumumab and elotuzumab.
The team therefore believes that, with further development, CTC analysis could be a valuable tool for advancing precision medicine in MM. ![]()

Photo by Juan D. Alfonso
Analysis of circulating tumor cells (CTCs) can provide at least as much genetic information about multiple myeloma (MM) as a bone marrow (BM) biopsy, according to a new study.
Researchers found evidence to suggest that analyzing CTCs isolated from the peripheral blood of MM patients could help physicians monitor disease progression over time, track the emergence of drug resistance, and tailor therapies to individual patients.
Jens Lohr, MD, PhD, of the Broad Institute of MIT and Harvard in Cambridge, Massachusetts, and colleagues conducted this research and detailed their findings in Science Translational Medicine.
The researchers said they devised a method that was “highly sensitive” in detecting and sequencing single CTCs, even from patients with low tumor burden.
Specifically, the team found they could isolate CTCs from, and detect mutations in, blood samples from an MM patient who had achieved a very good partial response to treatment and a patient with monoclonal gammopathy of undetermined significance.
Overall, the researchers found that CTCs could be used to identify mutations relevant for prognosis, and some of these mutations were more abundant in CTCs than in BM samples.
For example, the team detected somatic mutations in the KRAS, BRAF, IRF4, and TP53 genes in single CTCs from 3 MM patients. And the same mutations were present in single BM-derived MM cells.
In another 3 patients, the proportion of CTCs harboring TP53 R273C, BRAF G469A, and NRAS G13D mutations was higher than that observed in BM-derived cells. In 2 of the patients, the mutations weren’t detectable in BM cells because of insufficient sample material.
The researchers also found that CTCs could reveal patients with an overabundance of molecules expressed by MM cells—such as CD38 and SLAMF7—that can be targeted by therapies currently approved to treat MM—such as daratumumab and elotuzumab.
The team therefore believes that, with further development, CTC analysis could be a valuable tool for advancing precision medicine in MM. ![]()
Making Fall Prevention “Routine”
Every second of every day in the U.S. an older adult falls, according to CDC data. Falls are the number one cause of injuries and deaths from injury among older Americans. The report cites 29 million falls in 2014 alone.
“Older adult falls are increasing and, sadly, often herald the end of independence,” said CDC Director Tom Frieden, MD, MPH. But he adds: “Health care providers can make fall prevention a routine part of care in their practice, and older adults can take steps to protect themselves.”
Related: Preventing Falls and Saving Costs
The CDC created Stopping Elderly Accidents, Deaths, and Injuries (STEADI), an initiative to make fall prevention routine in health care. The program provides information on how to screen for falls, online training for providers, videos on conducting functional assessments, and brochures for health care providers (HCPs), patients, and caregivers.
Among the suggestions for HCPs:
- Ask patients whether they have fallen in the past year, feel unsteady, or worry about falling
- Review medications and stop, switch, or reduce medicines that could increase the risk of falls
- Recommend vitamin D supplements
For older adults, the CDC recommends:
- Talk to a HCP about falls and fall prevention
- Tell a HCP if you have fallen—fewer than half of Americans who fall tell their doctor
- Have your eyes checked, and update eye prescriptions
- Participate in evidence-based programs like tai chi to improve balance and strengthen legs
- Get rid of fall hazards in your home
Every second of every day in the U.S. an older adult falls, according to CDC data. Falls are the number one cause of injuries and deaths from injury among older Americans. The report cites 29 million falls in 2014 alone.
“Older adult falls are increasing and, sadly, often herald the end of independence,” said CDC Director Tom Frieden, MD, MPH. But he adds: “Health care providers can make fall prevention a routine part of care in their practice, and older adults can take steps to protect themselves.”
Related: Preventing Falls and Saving Costs
The CDC created Stopping Elderly Accidents, Deaths, and Injuries (STEADI), an initiative to make fall prevention routine in health care. The program provides information on how to screen for falls, online training for providers, videos on conducting functional assessments, and brochures for health care providers (HCPs), patients, and caregivers.
Among the suggestions for HCPs:
- Ask patients whether they have fallen in the past year, feel unsteady, or worry about falling
- Review medications and stop, switch, or reduce medicines that could increase the risk of falls
- Recommend vitamin D supplements
For older adults, the CDC recommends:
- Talk to a HCP about falls and fall prevention
- Tell a HCP if you have fallen—fewer than half of Americans who fall tell their doctor
- Have your eyes checked, and update eye prescriptions
- Participate in evidence-based programs like tai chi to improve balance and strengthen legs
- Get rid of fall hazards in your home
Every second of every day in the U.S. an older adult falls, according to CDC data. Falls are the number one cause of injuries and deaths from injury among older Americans. The report cites 29 million falls in 2014 alone.
“Older adult falls are increasing and, sadly, often herald the end of independence,” said CDC Director Tom Frieden, MD, MPH. But he adds: “Health care providers can make fall prevention a routine part of care in their practice, and older adults can take steps to protect themselves.”
Related: Preventing Falls and Saving Costs
The CDC created Stopping Elderly Accidents, Deaths, and Injuries (STEADI), an initiative to make fall prevention routine in health care. The program provides information on how to screen for falls, online training for providers, videos on conducting functional assessments, and brochures for health care providers (HCPs), patients, and caregivers.
Among the suggestions for HCPs:
- Ask patients whether they have fallen in the past year, feel unsteady, or worry about falling
- Review medications and stop, switch, or reduce medicines that could increase the risk of falls
- Recommend vitamin D supplements
For older adults, the CDC recommends:
- Talk to a HCP about falls and fall prevention
- Tell a HCP if you have fallen—fewer than half of Americans who fall tell their doctor
- Have your eyes checked, and update eye prescriptions
- Participate in evidence-based programs like tai chi to improve balance and strengthen legs
- Get rid of fall hazards in your home
A Severe Sitchuation
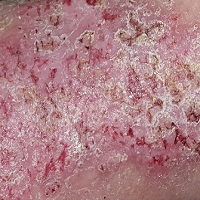
This 49-year-old man was first diagnosed with Darier disease at age 8. It has been contained, for the most part, by topical medications and acitretin. But the itching on his legs is especially severe, making it difficult for him to leave them alone.
EXAMINATION
Both legs have heavy scaling and lichenification from the lateral aspects of the knees down to the ankles. There is modest erythema but no suppurative signs. Other areas of scaling are seen on his chest and back, but these are less coarse and rough than the leg rash.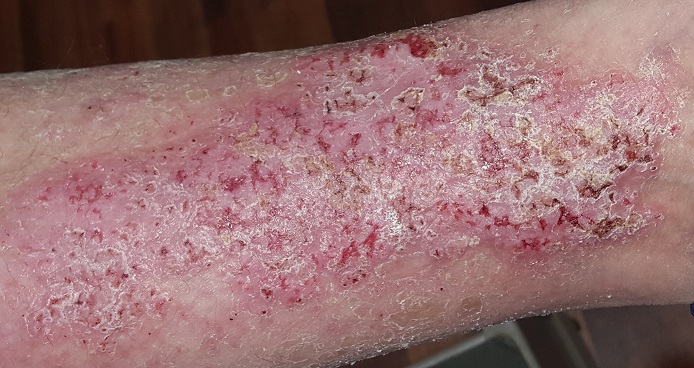
What is the diagnosis?
DISCUSSION
Lichen simplex chronicus (LSC) is the term given to this phenomenon. LSC is always secondary to a primary trigger, which can be dry skin, eczema, psoriasis, or even a bug bite. Excessive scratching induces more itching, and before the patient realizes it, he has jumped aboard the itch-scratch-itch train—a vicious cycle that can be hard to break.
The skin reacts to all this attention by thickening and becoming rough; on a microscopic level, the nerves become more sensitive. The urge to scratch becomes so irresistible that some patients fantasize about giving in.
The first goal of treating LSC is to stop the itching by helping the patient avoid contact with the site. High-potency topical steroids can be effective for this, especially if used under occlusion, which not only potentiates the steroid but also provides a barrier to protect the area from the patient’s fingernails (or hairbrush). Once the condition improves, steroids can be slowly withdrawn.
A biopsy, if it had been necessary, would have shown hypertrophic epidermis and acanthotic changes.
TAKE-HOME LEARNING POINTS
• Lichen simplex chronicus (LSC) is secondary to an inciting condition such as eczema, xerosis, psoriasis, or Darier disease.
• The manifestation of the itch-scratch-itch cycle leads to thickening and lichenification of the skin. The itch of LSC is so compelling that scratching is hard to resist.
• Despite treatment and patient education, recurrences are quite common.
This 49-year-old man was first diagnosed with Darier disease at age 8. It has been contained, for the most part, by topical medications and acitretin. But the itching on his legs is especially severe, making it difficult for him to leave them alone.
EXAMINATION
Both legs have heavy scaling and lichenification from the lateral aspects of the knees down to the ankles. There is modest erythema but no suppurative signs. Other areas of scaling are seen on his chest and back, but these are less coarse and rough than the leg rash.
What is the diagnosis?
DISCUSSION
Lichen simplex chronicus (LSC) is the term given to this phenomenon. LSC is always secondary to a primary trigger, which can be dry skin, eczema, psoriasis, or even a bug bite. Excessive scratching induces more itching, and before the patient realizes it, he has jumped aboard the itch-scratch-itch train—a vicious cycle that can be hard to break.
The skin reacts to all this attention by thickening and becoming rough; on a microscopic level, the nerves become more sensitive. The urge to scratch becomes so irresistible that some patients fantasize about giving in.
The first goal of treating LSC is to stop the itching by helping the patient avoid contact with the site. High-potency topical steroids can be effective for this, especially if used under occlusion, which not only potentiates the steroid but also provides a barrier to protect the area from the patient’s fingernails (or hairbrush). Once the condition improves, steroids can be slowly withdrawn.
A biopsy, if it had been necessary, would have shown hypertrophic epidermis and acanthotic changes.
TAKE-HOME LEARNING POINTS
• Lichen simplex chronicus (LSC) is secondary to an inciting condition such as eczema, xerosis, psoriasis, or Darier disease.
• The manifestation of the itch-scratch-itch cycle leads to thickening and lichenification of the skin. The itch of LSC is so compelling that scratching is hard to resist.
• Despite treatment and patient education, recurrences are quite common.
This 49-year-old man was first diagnosed with Darier disease at age 8. It has been contained, for the most part, by topical medications and acitretin. But the itching on his legs is especially severe, making it difficult for him to leave them alone.
EXAMINATION
Both legs have heavy scaling and lichenification from the lateral aspects of the knees down to the ankles. There is modest erythema but no suppurative signs. Other areas of scaling are seen on his chest and back, but these are less coarse and rough than the leg rash.
What is the diagnosis?
DISCUSSION
Lichen simplex chronicus (LSC) is the term given to this phenomenon. LSC is always secondary to a primary trigger, which can be dry skin, eczema, psoriasis, or even a bug bite. Excessive scratching induces more itching, and before the patient realizes it, he has jumped aboard the itch-scratch-itch train—a vicious cycle that can be hard to break.
The skin reacts to all this attention by thickening and becoming rough; on a microscopic level, the nerves become more sensitive. The urge to scratch becomes so irresistible that some patients fantasize about giving in.
The first goal of treating LSC is to stop the itching by helping the patient avoid contact with the site. High-potency topical steroids can be effective for this, especially if used under occlusion, which not only potentiates the steroid but also provides a barrier to protect the area from the patient’s fingernails (or hairbrush). Once the condition improves, steroids can be slowly withdrawn.
A biopsy, if it had been necessary, would have shown hypertrophic epidermis and acanthotic changes.
TAKE-HOME LEARNING POINTS
• Lichen simplex chronicus (LSC) is secondary to an inciting condition such as eczema, xerosis, psoriasis, or Darier disease.
• The manifestation of the itch-scratch-itch cycle leads to thickening and lichenification of the skin. The itch of LSC is so compelling that scratching is hard to resist.
• Despite treatment and patient education, recurrences are quite common.
The Paradox of Pain Management
Pain was introduced as the “fifth vital sign” in the 1990s, ranking it as important a measure as blood pressure, heart and respiratory rate, and temperature.1 The American Pain Society promoted this notion to increase awareness of pain treatment among health care professionals. Emphasizing its importance, the Veterans Health Administration in 1999 launched the “Pain as the 5th Vital Sign” initiative, which mandated a pain intensity rating at all clinical encounters.2
Interestingly, the Joint Commission standards never stated that pain needed to be treated as a vital sign. But many organizations started to require documentation of routine pain screening for all patients. Health care providers were instructed to inquire about pain and to treat it as an essential element of health history.
These changes were quite controversial. The additional measure, while important, competed with other priority screening needs, including diabetes, cancer, and hypertension. There was—and continues to be—quite the debate on whether pain actually can be measured and what impact that information has on the quality of care.
I do not intend to enter that debate here. Instead, I want to discuss what continues to be a conundrum for me: the paradox of pain management.
For many patients, especially those in acute or emergency care settings, the presenting complaint is pain. I would submit that for many the expectation is for pain to be immediately and permanently relieved. But is this a realistic goal?
I recall a lecture on pain management I attended years ago; at that time, the approach involved early identification and prompt, aggressive treatment. When asked “How much medication and for how long?” the lecturer used diabetes as a treatment model, stating, “You would increase insulin until the blood glucose was controlled—don’t be afraid to increase pain medication until the pain is controlled.” In the early days of pain management, that was the accepted norm. The possibility that a “zero” on the pain scale was unattainable for some patients was not considered.
Yet seemingly overnight, once pain was decreed a vital sign, health care providers were mandated to measure it and faced with the responsibility to treat it. This resulted in a vague 0-1
Faced with growing concern for undertreated pain in the US, however, many of us strove to achieve a balance of sufficient yet appropriate treatment. We struggled to determine how to relieve the pain our patients experienced without creating other problems, such as undesirable side effects, misuse, or addiction. That predicament, paired with the ever-increasing direct-to-consumer advertisements about pain relief and the insistence by (some, not all) patients that nonnarcotic pain medication is ineffective, bred the crisis of opioid overuse and addiction we now face.
But just as I chose not to debate the impact of pain measurement on quality of care, I also choose not to debate the existence of the opioid crisis. What I want to emphasize is that all policy changes have consequences. I reach out to you, my colleagues, for innovative ideas to strike the delicate balance of appropriate use of narcotics. How do we address the needs of patients whose pain is more than just an inconvenience and for whom daily use of a narcotic allows them to function—while also avoiding the pitfalls that we are now regularly warned about?
I have no doubt that each of us knows at least one person—a patient, a family member, a neighbor—for whom pain is a daily occurrence. But we must put that in perspective; not all pain is a barrier to physical and emotional functioning. Data suggest that a “33% to 50% decrease in pain intensity is meaningful from a patient’s perspective and represents a reasonable standard of intervention efficacy.”3 For those who deal with chronic pain, even a slight improvement is progress.
So, while the American Medical Association and the American Pain Society bicker about whether pain is the “fifth vital sign,” we must find a better means to resolve the discord in our society.4 Banning all opioid use is not the answer, but neither is considering narcotics the default treatment for pain.
We must remind our patients, our policymakers, and ourselves that identifying and assessing pain is not equated with writing an opioid or narcotic prescription. Nor will removing those medications from our formulary mitigate the crisis. We need to communicate a clear, consistent message that pain is real, that some pain is a fact of life, and that we will help our patients.
However, it is incumbent upon us to adopt a systematic yet personalized plan of care that is effective, cost conscious, culturally and developmentally appropriate, and safe—and that plan may or may not include prescribing narcotics. We have much work ahead of us in order to minimize the potential for misuse of these medications without impeding patients’ access to necessary health care.
Please share your thoughts on this conundrum by writing to [email protected].
1. Veterans Health Administration. Pain as the 5th vital sign toolkit. www.va.gov/PAINMAN AGEMENT/docs/Pain_As_the_5th_Vital_Sign_Toolkit.pdf. Accessed October 5, 2016.
2. Mularski RA, White-Chu F, Overbay D, et al. Measuring pain as the 5th vital sign does not improve quality of pain management. J Gen Intern Med . 2006;21(6):607-612.
3. Gordon DB, Dahl JL, Miaskowski C, et al. American Pain Society recommendations for improving the quality of acute and cancer pain management. Arch Intern Med . 2005; 165(14):1574-1580.
4. Anson P. AMA drops pain as a vital sign . Pain News Network. June 16, 2016. www.painnewsnetwork.org/stories/2016/6/16/ama-drops-pain-as-vital-sign. Accessed October 5, 2016.
Pain was introduced as the “fifth vital sign” in the 1990s, ranking it as important a measure as blood pressure, heart and respiratory rate, and temperature.1 The American Pain Society promoted this notion to increase awareness of pain treatment among health care professionals. Emphasizing its importance, the Veterans Health Administration in 1999 launched the “Pain as the 5th Vital Sign” initiative, which mandated a pain intensity rating at all clinical encounters.2
Interestingly, the Joint Commission standards never stated that pain needed to be treated as a vital sign. But many organizations started to require documentation of routine pain screening for all patients. Health care providers were instructed to inquire about pain and to treat it as an essential element of health history.
These changes were quite controversial. The additional measure, while important, competed with other priority screening needs, including diabetes, cancer, and hypertension. There was—and continues to be—quite the debate on whether pain actually can be measured and what impact that information has on the quality of care.
I do not intend to enter that debate here. Instead, I want to discuss what continues to be a conundrum for me: the paradox of pain management.
For many patients, especially those in acute or emergency care settings, the presenting complaint is pain. I would submit that for many the expectation is for pain to be immediately and permanently relieved. But is this a realistic goal?
I recall a lecture on pain management I attended years ago; at that time, the approach involved early identification and prompt, aggressive treatment. When asked “How much medication and for how long?” the lecturer used diabetes as a treatment model, stating, “You would increase insulin until the blood glucose was controlled—don’t be afraid to increase pain medication until the pain is controlled.” In the early days of pain management, that was the accepted norm. The possibility that a “zero” on the pain scale was unattainable for some patients was not considered.
Yet seemingly overnight, once pain was decreed a vital sign, health care providers were mandated to measure it and faced with the responsibility to treat it. This resulted in a vague 0-1
Faced with growing concern for undertreated pain in the US, however, many of us strove to achieve a balance of sufficient yet appropriate treatment. We struggled to determine how to relieve the pain our patients experienced without creating other problems, such as undesirable side effects, misuse, or addiction. That predicament, paired with the ever-increasing direct-to-consumer advertisements about pain relief and the insistence by (some, not all) patients that nonnarcotic pain medication is ineffective, bred the crisis of opioid overuse and addiction we now face.
But just as I chose not to debate the impact of pain measurement on quality of care, I also choose not to debate the existence of the opioid crisis. What I want to emphasize is that all policy changes have consequences. I reach out to you, my colleagues, for innovative ideas to strike the delicate balance of appropriate use of narcotics. How do we address the needs of patients whose pain is more than just an inconvenience and for whom daily use of a narcotic allows them to function—while also avoiding the pitfalls that we are now regularly warned about?
I have no doubt that each of us knows at least one person—a patient, a family member, a neighbor—for whom pain is a daily occurrence. But we must put that in perspective; not all pain is a barrier to physical and emotional functioning. Data suggest that a “33% to 50% decrease in pain intensity is meaningful from a patient’s perspective and represents a reasonable standard of intervention efficacy.”3 For those who deal with chronic pain, even a slight improvement is progress.
So, while the American Medical Association and the American Pain Society bicker about whether pain is the “fifth vital sign,” we must find a better means to resolve the discord in our society.4 Banning all opioid use is not the answer, but neither is considering narcotics the default treatment for pain.
We must remind our patients, our policymakers, and ourselves that identifying and assessing pain is not equated with writing an opioid or narcotic prescription. Nor will removing those medications from our formulary mitigate the crisis. We need to communicate a clear, consistent message that pain is real, that some pain is a fact of life, and that we will help our patients.
However, it is incumbent upon us to adopt a systematic yet personalized plan of care that is effective, cost conscious, culturally and developmentally appropriate, and safe—and that plan may or may not include prescribing narcotics. We have much work ahead of us in order to minimize the potential for misuse of these medications without impeding patients’ access to necessary health care.
Please share your thoughts on this conundrum by writing to [email protected].
Pain was introduced as the “fifth vital sign” in the 1990s, ranking it as important a measure as blood pressure, heart and respiratory rate, and temperature.1 The American Pain Society promoted this notion to increase awareness of pain treatment among health care professionals. Emphasizing its importance, the Veterans Health Administration in 1999 launched the “Pain as the 5th Vital Sign” initiative, which mandated a pain intensity rating at all clinical encounters.2
Interestingly, the Joint Commission standards never stated that pain needed to be treated as a vital sign. But many organizations started to require documentation of routine pain screening for all patients. Health care providers were instructed to inquire about pain and to treat it as an essential element of health history.
These changes were quite controversial. The additional measure, while important, competed with other priority screening needs, including diabetes, cancer, and hypertension. There was—and continues to be—quite the debate on whether pain actually can be measured and what impact that information has on the quality of care.
I do not intend to enter that debate here. Instead, I want to discuss what continues to be a conundrum for me: the paradox of pain management.
For many patients, especially those in acute or emergency care settings, the presenting complaint is pain. I would submit that for many the expectation is for pain to be immediately and permanently relieved. But is this a realistic goal?
I recall a lecture on pain management I attended years ago; at that time, the approach involved early identification and prompt, aggressive treatment. When asked “How much medication and for how long?” the lecturer used diabetes as a treatment model, stating, “You would increase insulin until the blood glucose was controlled—don’t be afraid to increase pain medication until the pain is controlled.” In the early days of pain management, that was the accepted norm. The possibility that a “zero” on the pain scale was unattainable for some patients was not considered.
Yet seemingly overnight, once pain was decreed a vital sign, health care providers were mandated to measure it and faced with the responsibility to treat it. This resulted in a vague 0-1
Faced with growing concern for undertreated pain in the US, however, many of us strove to achieve a balance of sufficient yet appropriate treatment. We struggled to determine how to relieve the pain our patients experienced without creating other problems, such as undesirable side effects, misuse, or addiction. That predicament, paired with the ever-increasing direct-to-consumer advertisements about pain relief and the insistence by (some, not all) patients that nonnarcotic pain medication is ineffective, bred the crisis of opioid overuse and addiction we now face.
But just as I chose not to debate the impact of pain measurement on quality of care, I also choose not to debate the existence of the opioid crisis. What I want to emphasize is that all policy changes have consequences. I reach out to you, my colleagues, for innovative ideas to strike the delicate balance of appropriate use of narcotics. How do we address the needs of patients whose pain is more than just an inconvenience and for whom daily use of a narcotic allows them to function—while also avoiding the pitfalls that we are now regularly warned about?
I have no doubt that each of us knows at least one person—a patient, a family member, a neighbor—for whom pain is a daily occurrence. But we must put that in perspective; not all pain is a barrier to physical and emotional functioning. Data suggest that a “33% to 50% decrease in pain intensity is meaningful from a patient’s perspective and represents a reasonable standard of intervention efficacy.”3 For those who deal with chronic pain, even a slight improvement is progress.
So, while the American Medical Association and the American Pain Society bicker about whether pain is the “fifth vital sign,” we must find a better means to resolve the discord in our society.4 Banning all opioid use is not the answer, but neither is considering narcotics the default treatment for pain.
We must remind our patients, our policymakers, and ourselves that identifying and assessing pain is not equated with writing an opioid or narcotic prescription. Nor will removing those medications from our formulary mitigate the crisis. We need to communicate a clear, consistent message that pain is real, that some pain is a fact of life, and that we will help our patients.
However, it is incumbent upon us to adopt a systematic yet personalized plan of care that is effective, cost conscious, culturally and developmentally appropriate, and safe—and that plan may or may not include prescribing narcotics. We have much work ahead of us in order to minimize the potential for misuse of these medications without impeding patients’ access to necessary health care.
Please share your thoughts on this conundrum by writing to [email protected].
1. Veterans Health Administration. Pain as the 5th vital sign toolkit. www.va.gov/PAINMAN AGEMENT/docs/Pain_As_the_5th_Vital_Sign_Toolkit.pdf. Accessed October 5, 2016.
2. Mularski RA, White-Chu F, Overbay D, et al. Measuring pain as the 5th vital sign does not improve quality of pain management. J Gen Intern Med . 2006;21(6):607-612.
3. Gordon DB, Dahl JL, Miaskowski C, et al. American Pain Society recommendations for improving the quality of acute and cancer pain management. Arch Intern Med . 2005; 165(14):1574-1580.
4. Anson P. AMA drops pain as a vital sign . Pain News Network. June 16, 2016. www.painnewsnetwork.org/stories/2016/6/16/ama-drops-pain-as-vital-sign. Accessed October 5, 2016.
1. Veterans Health Administration. Pain as the 5th vital sign toolkit. www.va.gov/PAINMAN AGEMENT/docs/Pain_As_the_5th_Vital_Sign_Toolkit.pdf. Accessed October 5, 2016.
2. Mularski RA, White-Chu F, Overbay D, et al. Measuring pain as the 5th vital sign does not improve quality of pain management. J Gen Intern Med . 2006;21(6):607-612.
3. Gordon DB, Dahl JL, Miaskowski C, et al. American Pain Society recommendations for improving the quality of acute and cancer pain management. Arch Intern Med . 2005; 165(14):1574-1580.
4. Anson P. AMA drops pain as a vital sign . Pain News Network. June 16, 2016. www.painnewsnetwork.org/stories/2016/6/16/ama-drops-pain-as-vital-sign. Accessed October 5, 2016.
7 tips for successful value-based care contracts
During a recent webinar presented by the American Bar Association, legal experts provided guidance on how doctors can successfully draft pay-for-performance contracts and prevent disputes with payers.
1. Clearly define terms
Contract terms and definitions should be clearly outlined and understood by both parties before value-based agreements are signed, said Melissa J. Hulke, a director in the Berkeley Research Group, LLC health analytics practice.
“Contract terms become central to resolving differences, so it’s important the contract is well defined, especially when moving away from fee for service,” she said. “These are more complex arrangements.”
Another area that needs thorough definition surrounds referral volume. Contracts can include the phrases, “predominately refer” or “primarily refer,” without defining the meaning of “predominately” or “primarily,” Ms. Hulke said.
“If that’s not defined in the contract, it’s hard to establish a benchmark to not only project the provider’s performance under the arrangement and how much revenue they can expect to receive, but it’s hard to know if you’re meeting that benchmark, as well,” she said. “I strongly encourage that if [a term states] “primarily, predominately, [or] mostly refer,” that you have a specific percentage benchmark included. That will help to avoid any disagreement later on.”
2. Review payment calculations
Ensure that payment formulas are examined and agreed upon.
“I think that should be baked into the contracts of today because they are so complex and there is so much money at stake,” she said. “Walk through that compensation exhibit, walk through the examples of what’s supposed to happen.
If not in agreement with calculations, talk to the payer’s financial personnel about how the numbers were reached and try to resolve any differences.
3. Monitor results
Actively monitor projected-to-actual financial performance under the contract. If performance is not being monitored and results are not being tested, it’s impossible to tell whether the contract is succeeding or failing, Ms. Hulke said.
It’s a good idea to monitor projected-to-actual financial performance monthly or at least quarterly, she advised.
“Tracking budget to actual performance and investigating successes and failures are important if the contracts are material to the provider’s business,” Ms. Hulke said.
4. Institute time frames for government methodologies
Be specific about when contracts are linked to Medicare reimbursement methodologies.
If commercial payers are tying payment rates to government programs, which often occurs, contracts should include whether the reimbursement is based on Medicare rates and methodologies as of a particular date and time, according to Ms. Hulke. For instance, the contract could specify that rates are tied to Medicare methodologies at the time the contract was executed. Alternatively, contracts could allow the physician payment rate to fluctuate depending on changes the government makes to Medicare rates and methodologies during the span of the contract.
“If this is not defined, when Medicare makes a change, the parties may have differing opinions on the appropriate rate of reimbursement that’s being paid,” she said.
5. Structure around state and federal laws
Conduct a regulatory analysis before inking any value based payment arrangement/transaction, and structure and document around potential legal constraints, Ms. Hanna said.
Know the laws in your state, she advised. Some states have requirements for provider incentive programs, while others may have rules for provider organizations or intermediaries that assume certain financial risk. When working with federal health care payers, review the Stark Law and Anti-Kickback regulations and ensure that if triggered by the arrangement, the venture falls within an exception of the statutes. Other legal considerations when drafting contracts include:
• HIPAA and state privacy laws.
• Antitrust laws.
• Telemedicine and telehealth laws.
• Medicare Advantage benefit guidelines for programs designed for specific Medicare Advantage populations.
• Scope of practice laws applicable to mid-level medical providers.
6. Design a dispute-resolution strategy
Include a thorough dispute-resolution strategy within the contract. The strategies help facilitate an orderly process for resolving disputes cost effectively, Ms. Hanna said. She suggested that policies start with an informal dispute-resolution process that sends unresolved matters to senior executives at each organization.
“This allows business leaders – and not the lawyers – to find a business solution to a thorny and potentially costly problem before each party gets entrenched in its own position and own sense of having been wronged,” she said in an interview. “The business solution may or may not rely on contract language but may be tailored to keep the relationship moving forward. However, if the informal process proves unsuccessful, then the process gets punted to the legal system – whether in a court proceeding or arbitration.”
7. Have an exit strategy
Include an exit strategy that ensures an end to the arrangement goes as smoothly and fairly as possible. The strategy will depend on the nature and structure of the payer-provider alignment and the value-based payment arrangement, Ms. Hanna said in an interview.
For example, in a true, corporate joint venture, the exit strategy may include buyout rights of the newly formed entity. In this case, it’s important to consider and negotiate the price of the buyout and what circumstances that will trigger the buyout. Termination rights should also be included in an exit strategy, Ms. Hanna said. One option is to allow termination “without case,” by either party.
“Clients often do not like this approach because the intention of the parties going in is to build a strong, long-term relationship where provider and payer benefit,” she said. “A ‘without cause’ termination rights gives the parties a safety valve if circumstances change or the relationship is just not successful.”
An alternative is to allow the relationship time to mature and grow before either party can exercise a without cause termination. A related approach is to develop triggering events that would give one or both parties the right to terminate the agreement, without the other party “having committed a bad act,” Ms. Hanna said in the interview.
“For instance, if the relationship does not meet certain financial or other benchmarks within an agreed-upon time frame, this may be a sufficient reason to terminate the relationship, abandon, or renegotiate the value-based purchasing payment formula or, perhaps, end an exclusive or other preferential relationship,” she said.
[email protected]
On Twitter @legal_med
During a recent webinar presented by the American Bar Association, legal experts provided guidance on how doctors can successfully draft pay-for-performance contracts and prevent disputes with payers.
1. Clearly define terms
Contract terms and definitions should be clearly outlined and understood by both parties before value-based agreements are signed, said Melissa J. Hulke, a director in the Berkeley Research Group, LLC health analytics practice.
“Contract terms become central to resolving differences, so it’s important the contract is well defined, especially when moving away from fee for service,” she said. “These are more complex arrangements.”
Another area that needs thorough definition surrounds referral volume. Contracts can include the phrases, “predominately refer” or “primarily refer,” without defining the meaning of “predominately” or “primarily,” Ms. Hulke said.
“If that’s not defined in the contract, it’s hard to establish a benchmark to not only project the provider’s performance under the arrangement and how much revenue they can expect to receive, but it’s hard to know if you’re meeting that benchmark, as well,” she said. “I strongly encourage that if [a term states] “primarily, predominately, [or] mostly refer,” that you have a specific percentage benchmark included. That will help to avoid any disagreement later on.”
2. Review payment calculations
Ensure that payment formulas are examined and agreed upon.
“I think that should be baked into the contracts of today because they are so complex and there is so much money at stake,” she said. “Walk through that compensation exhibit, walk through the examples of what’s supposed to happen.
If not in agreement with calculations, talk to the payer’s financial personnel about how the numbers were reached and try to resolve any differences.
3. Monitor results
Actively monitor projected-to-actual financial performance under the contract. If performance is not being monitored and results are not being tested, it’s impossible to tell whether the contract is succeeding or failing, Ms. Hulke said.
It’s a good idea to monitor projected-to-actual financial performance monthly or at least quarterly, she advised.
“Tracking budget to actual performance and investigating successes and failures are important if the contracts are material to the provider’s business,” Ms. Hulke said.
4. Institute time frames for government methodologies
Be specific about when contracts are linked to Medicare reimbursement methodologies.
If commercial payers are tying payment rates to government programs, which often occurs, contracts should include whether the reimbursement is based on Medicare rates and methodologies as of a particular date and time, according to Ms. Hulke. For instance, the contract could specify that rates are tied to Medicare methodologies at the time the contract was executed. Alternatively, contracts could allow the physician payment rate to fluctuate depending on changes the government makes to Medicare rates and methodologies during the span of the contract.
“If this is not defined, when Medicare makes a change, the parties may have differing opinions on the appropriate rate of reimbursement that’s being paid,” she said.
5. Structure around state and federal laws
Conduct a regulatory analysis before inking any value based payment arrangement/transaction, and structure and document around potential legal constraints, Ms. Hanna said.
Know the laws in your state, she advised. Some states have requirements for provider incentive programs, while others may have rules for provider organizations or intermediaries that assume certain financial risk. When working with federal health care payers, review the Stark Law and Anti-Kickback regulations and ensure that if triggered by the arrangement, the venture falls within an exception of the statutes. Other legal considerations when drafting contracts include:
• HIPAA and state privacy laws.
• Antitrust laws.
• Telemedicine and telehealth laws.
• Medicare Advantage benefit guidelines for programs designed for specific Medicare Advantage populations.
• Scope of practice laws applicable to mid-level medical providers.
6. Design a dispute-resolution strategy
Include a thorough dispute-resolution strategy within the contract. The strategies help facilitate an orderly process for resolving disputes cost effectively, Ms. Hanna said. She suggested that policies start with an informal dispute-resolution process that sends unresolved matters to senior executives at each organization.
“This allows business leaders – and not the lawyers – to find a business solution to a thorny and potentially costly problem before each party gets entrenched in its own position and own sense of having been wronged,” she said in an interview. “The business solution may or may not rely on contract language but may be tailored to keep the relationship moving forward. However, if the informal process proves unsuccessful, then the process gets punted to the legal system – whether in a court proceeding or arbitration.”
7. Have an exit strategy
Include an exit strategy that ensures an end to the arrangement goes as smoothly and fairly as possible. The strategy will depend on the nature and structure of the payer-provider alignment and the value-based payment arrangement, Ms. Hanna said in an interview.
For example, in a true, corporate joint venture, the exit strategy may include buyout rights of the newly formed entity. In this case, it’s important to consider and negotiate the price of the buyout and what circumstances that will trigger the buyout. Termination rights should also be included in an exit strategy, Ms. Hanna said. One option is to allow termination “without case,” by either party.
“Clients often do not like this approach because the intention of the parties going in is to build a strong, long-term relationship where provider and payer benefit,” she said. “A ‘without cause’ termination rights gives the parties a safety valve if circumstances change or the relationship is just not successful.”
An alternative is to allow the relationship time to mature and grow before either party can exercise a without cause termination. A related approach is to develop triggering events that would give one or both parties the right to terminate the agreement, without the other party “having committed a bad act,” Ms. Hanna said in the interview.
“For instance, if the relationship does not meet certain financial or other benchmarks within an agreed-upon time frame, this may be a sufficient reason to terminate the relationship, abandon, or renegotiate the value-based purchasing payment formula or, perhaps, end an exclusive or other preferential relationship,” she said.
[email protected]
On Twitter @legal_med
During a recent webinar presented by the American Bar Association, legal experts provided guidance on how doctors can successfully draft pay-for-performance contracts and prevent disputes with payers.
1. Clearly define terms
Contract terms and definitions should be clearly outlined and understood by both parties before value-based agreements are signed, said Melissa J. Hulke, a director in the Berkeley Research Group, LLC health analytics practice.
“Contract terms become central to resolving differences, so it’s important the contract is well defined, especially when moving away from fee for service,” she said. “These are more complex arrangements.”
Another area that needs thorough definition surrounds referral volume. Contracts can include the phrases, “predominately refer” or “primarily refer,” without defining the meaning of “predominately” or “primarily,” Ms. Hulke said.
“If that’s not defined in the contract, it’s hard to establish a benchmark to not only project the provider’s performance under the arrangement and how much revenue they can expect to receive, but it’s hard to know if you’re meeting that benchmark, as well,” she said. “I strongly encourage that if [a term states] “primarily, predominately, [or] mostly refer,” that you have a specific percentage benchmark included. That will help to avoid any disagreement later on.”
2. Review payment calculations
Ensure that payment formulas are examined and agreed upon.
“I think that should be baked into the contracts of today because they are so complex and there is so much money at stake,” she said. “Walk through that compensation exhibit, walk through the examples of what’s supposed to happen.
If not in agreement with calculations, talk to the payer’s financial personnel about how the numbers were reached and try to resolve any differences.
3. Monitor results
Actively monitor projected-to-actual financial performance under the contract. If performance is not being monitored and results are not being tested, it’s impossible to tell whether the contract is succeeding or failing, Ms. Hulke said.
It’s a good idea to monitor projected-to-actual financial performance monthly or at least quarterly, she advised.
“Tracking budget to actual performance and investigating successes and failures are important if the contracts are material to the provider’s business,” Ms. Hulke said.
4. Institute time frames for government methodologies
Be specific about when contracts are linked to Medicare reimbursement methodologies.
If commercial payers are tying payment rates to government programs, which often occurs, contracts should include whether the reimbursement is based on Medicare rates and methodologies as of a particular date and time, according to Ms. Hulke. For instance, the contract could specify that rates are tied to Medicare methodologies at the time the contract was executed. Alternatively, contracts could allow the physician payment rate to fluctuate depending on changes the government makes to Medicare rates and methodologies during the span of the contract.
“If this is not defined, when Medicare makes a change, the parties may have differing opinions on the appropriate rate of reimbursement that’s being paid,” she said.
5. Structure around state and federal laws
Conduct a regulatory analysis before inking any value based payment arrangement/transaction, and structure and document around potential legal constraints, Ms. Hanna said.
Know the laws in your state, she advised. Some states have requirements for provider incentive programs, while others may have rules for provider organizations or intermediaries that assume certain financial risk. When working with federal health care payers, review the Stark Law and Anti-Kickback regulations and ensure that if triggered by the arrangement, the venture falls within an exception of the statutes. Other legal considerations when drafting contracts include:
• HIPAA and state privacy laws.
• Antitrust laws.
• Telemedicine and telehealth laws.
• Medicare Advantage benefit guidelines for programs designed for specific Medicare Advantage populations.
• Scope of practice laws applicable to mid-level medical providers.
6. Design a dispute-resolution strategy
Include a thorough dispute-resolution strategy within the contract. The strategies help facilitate an orderly process for resolving disputes cost effectively, Ms. Hanna said. She suggested that policies start with an informal dispute-resolution process that sends unresolved matters to senior executives at each organization.
“This allows business leaders – and not the lawyers – to find a business solution to a thorny and potentially costly problem before each party gets entrenched in its own position and own sense of having been wronged,” she said in an interview. “The business solution may or may not rely on contract language but may be tailored to keep the relationship moving forward. However, if the informal process proves unsuccessful, then the process gets punted to the legal system – whether in a court proceeding or arbitration.”
7. Have an exit strategy
Include an exit strategy that ensures an end to the arrangement goes as smoothly and fairly as possible. The strategy will depend on the nature and structure of the payer-provider alignment and the value-based payment arrangement, Ms. Hanna said in an interview.
For example, in a true, corporate joint venture, the exit strategy may include buyout rights of the newly formed entity. In this case, it’s important to consider and negotiate the price of the buyout and what circumstances that will trigger the buyout. Termination rights should also be included in an exit strategy, Ms. Hanna said. One option is to allow termination “without case,” by either party.
“Clients often do not like this approach because the intention of the parties going in is to build a strong, long-term relationship where provider and payer benefit,” she said. “A ‘without cause’ termination rights gives the parties a safety valve if circumstances change or the relationship is just not successful.”
An alternative is to allow the relationship time to mature and grow before either party can exercise a without cause termination. A related approach is to develop triggering events that would give one or both parties the right to terminate the agreement, without the other party “having committed a bad act,” Ms. Hanna said in the interview.
“For instance, if the relationship does not meet certain financial or other benchmarks within an agreed-upon time frame, this may be a sufficient reason to terminate the relationship, abandon, or renegotiate the value-based purchasing payment formula or, perhaps, end an exclusive or other preferential relationship,” she said.
[email protected]
On Twitter @legal_med
‘Committed’ is culmination of journey examining involuntary care
After 3 years of work, Anne Hanson and I are delighted that our book, “Committed: The Battle over Involuntary Psychiatric Care” was officially released Nov. 1! Our publisher, Johns Hopkins University Press, asked me to write a post for its blog to start the launch and with permission, Clinical Psychiatry News has let me begin the story of our research here as well.
So how did I find myself sitting in courtrooms and riding alongside a police officer? Let me tell you a little about the process of writing this book, because it was a quite the adventure for me. The title implies that this is another book by psychiatrists for psychiatrists, but for me, the days I spent working on this manuscript were days off from psychiatry. Those mornings I woke up a psychiatrist and felt like I walked into a phone booth (maybe it was just my shower) and emerged as a journalist.
To read more, please visit the Johns Hopkins University blog here.
After 3 years of work, Anne Hanson and I are delighted that our book, “Committed: The Battle over Involuntary Psychiatric Care” was officially released Nov. 1! Our publisher, Johns Hopkins University Press, asked me to write a post for its blog to start the launch and with permission, Clinical Psychiatry News has let me begin the story of our research here as well.
So how did I find myself sitting in courtrooms and riding alongside a police officer? Let me tell you a little about the process of writing this book, because it was a quite the adventure for me. The title implies that this is another book by psychiatrists for psychiatrists, but for me, the days I spent working on this manuscript were days off from psychiatry. Those mornings I woke up a psychiatrist and felt like I walked into a phone booth (maybe it was just my shower) and emerged as a journalist.
To read more, please visit the Johns Hopkins University blog here.
After 3 years of work, Anne Hanson and I are delighted that our book, “Committed: The Battle over Involuntary Psychiatric Care” was officially released Nov. 1! Our publisher, Johns Hopkins University Press, asked me to write a post for its blog to start the launch and with permission, Clinical Psychiatry News has let me begin the story of our research here as well.
So how did I find myself sitting in courtrooms and riding alongside a police officer? Let me tell you a little about the process of writing this book, because it was a quite the adventure for me. The title implies that this is another book by psychiatrists for psychiatrists, but for me, the days I spent working on this manuscript were days off from psychiatry. Those mornings I woke up a psychiatrist and felt like I walked into a phone booth (maybe it was just my shower) and emerged as a journalist.
To read more, please visit the Johns Hopkins University blog here.
Two factors associated with vocal cord dysfunction in study
LOS ANGELES – Female sex and the absence of wheezing were the only factors significantly associated with vocal cord dysfunction in patients with high pretest probability of disease, a retrospective analysis showed.
The findings differ from those of the Pittsburgh Vocal Cord Index, which identified symptoms of throat tightness, dysphonia, absence of wheezing, and the presence of odors as key features predictive of vocal cord dysfunction (VCD). “This proves the point that VCD is an elusive diagnosis,” lead study author Phalgoon Shah, MD, said, in an interview, at the annual meeting of the American College of Chest Physicians.
The researchers found that a history of depression or anxiety, throat tightness, dysphonia, odor symptom trigger, lack of response to bronchodilator or truncation, and flattening of the inspiratory volume curve did not predict VCD.
The patients were active duty military personnel and veterans who were referred to the pulmonary function lab at Tripler Army Medical Center, Honolulu, for suspected VCD between 2010 and 2014. The researchers identified patients by laryngoscopy procedure code and collected numerous variables, including demographic information, past medical history, pulmonary function test data, and clinical variables such as ED visits for dyspnea.
Dr. Shah of the division of pulmonary and critical care at Tripler said that direct laryngoscopic visualization is used to diagnose VCD in military personnel because the diagnosis is grounds for dismissal, but in other practice settings, the technique is not readily available, even though it is considered the gold standard. “You might still have a very high clinical suspicion of VCD in spite of a negative laryngoscopy, and you send these patients to speech therapy and they get better,” he said. “By logic, you can assume that these patients had VCD, you just didn’t catch it right in the lab. For the first time, we are saying that exercise laryngoscopy is not the gold standard.”
He emphasized that more research is required to develop a validated scoring system to predict a diagnosis of VCD and to distinguish it from asthma. He reported having no financial disclosures.
LOS ANGELES – Female sex and the absence of wheezing were the only factors significantly associated with vocal cord dysfunction in patients with high pretest probability of disease, a retrospective analysis showed.
The findings differ from those of the Pittsburgh Vocal Cord Index, which identified symptoms of throat tightness, dysphonia, absence of wheezing, and the presence of odors as key features predictive of vocal cord dysfunction (VCD). “This proves the point that VCD is an elusive diagnosis,” lead study author Phalgoon Shah, MD, said, in an interview, at the annual meeting of the American College of Chest Physicians.
The researchers found that a history of depression or anxiety, throat tightness, dysphonia, odor symptom trigger, lack of response to bronchodilator or truncation, and flattening of the inspiratory volume curve did not predict VCD.
The patients were active duty military personnel and veterans who were referred to the pulmonary function lab at Tripler Army Medical Center, Honolulu, for suspected VCD between 2010 and 2014. The researchers identified patients by laryngoscopy procedure code and collected numerous variables, including demographic information, past medical history, pulmonary function test data, and clinical variables such as ED visits for dyspnea.
Dr. Shah of the division of pulmonary and critical care at Tripler said that direct laryngoscopic visualization is used to diagnose VCD in military personnel because the diagnosis is grounds for dismissal, but in other practice settings, the technique is not readily available, even though it is considered the gold standard. “You might still have a very high clinical suspicion of VCD in spite of a negative laryngoscopy, and you send these patients to speech therapy and they get better,” he said. “By logic, you can assume that these patients had VCD, you just didn’t catch it right in the lab. For the first time, we are saying that exercise laryngoscopy is not the gold standard.”
He emphasized that more research is required to develop a validated scoring system to predict a diagnosis of VCD and to distinguish it from asthma. He reported having no financial disclosures.
LOS ANGELES – Female sex and the absence of wheezing were the only factors significantly associated with vocal cord dysfunction in patients with high pretest probability of disease, a retrospective analysis showed.
The findings differ from those of the Pittsburgh Vocal Cord Index, which identified symptoms of throat tightness, dysphonia, absence of wheezing, and the presence of odors as key features predictive of vocal cord dysfunction (VCD). “This proves the point that VCD is an elusive diagnosis,” lead study author Phalgoon Shah, MD, said, in an interview, at the annual meeting of the American College of Chest Physicians.
The researchers found that a history of depression or anxiety, throat tightness, dysphonia, odor symptom trigger, lack of response to bronchodilator or truncation, and flattening of the inspiratory volume curve did not predict VCD.
The patients were active duty military personnel and veterans who were referred to the pulmonary function lab at Tripler Army Medical Center, Honolulu, for suspected VCD between 2010 and 2014. The researchers identified patients by laryngoscopy procedure code and collected numerous variables, including demographic information, past medical history, pulmonary function test data, and clinical variables such as ED visits for dyspnea.
Dr. Shah of the division of pulmonary and critical care at Tripler said that direct laryngoscopic visualization is used to diagnose VCD in military personnel because the diagnosis is grounds for dismissal, but in other practice settings, the technique is not readily available, even though it is considered the gold standard. “You might still have a very high clinical suspicion of VCD in spite of a negative laryngoscopy, and you send these patients to speech therapy and they get better,” he said. “By logic, you can assume that these patients had VCD, you just didn’t catch it right in the lab. For the first time, we are saying that exercise laryngoscopy is not the gold standard.”
He emphasized that more research is required to develop a validated scoring system to predict a diagnosis of VCD and to distinguish it from asthma. He reported having no financial disclosures.
AT CHEST 2016
Key clinical point:
Major finding: The only variables significantly associated with VCD were female sex (P = .006) and the absence of wheezing (P = .037).
Data source: A retrospective analysis of 244 patients referred to a pulmonary function lab for suspected VCD.
Disclosures: Dr. Shah reported having no financial disclosures.
Prenatal triple ART arrests HIV transmission
A triple-drug antiretroviral therapy given to HIV-infected pregnant women significantly reduced transmission of the disease to their newborns, but with greater risk of adverse outcomes for mothers and infants, a study showed.
The findings were based on data from three treatment regimens in approximately 3,500 women and infant sets. The three treatments were zidovudine plus intrapartum single-dose nevirapine with 6-14 days of tenofovir and emtricitabine post partum (zidovudine alone); zidovudine, lamivudine, and lopinavir–ritonavir (zidovudine-based antiretroviral therapy [ART]); or tenofovir, emtricitabine, and lopinavir–ritonavir (tenofovir-based ART). All infants received nevirapine once daily, and infants of mothers coinfected with hepatitis B also received hepatitis B vaccination.
The Promoting Maternal and Infant Survival Everywhere (PROMISE) trial included patients at 14 sites in seven countries (India, Malawi, South Africa, Tanzania, Uganda, Zambia, and Zimbabwe). The current study presented findings from women with a CD4 count of at least 350 cells per cubic millimeter who were randomized at 14 weeks’ gestation to one of the three treatment regimens.
Maternal adverse events (grade 2 or higher) were significantly more common in the zidovudine-based ART group than in the zidovudine-only group (21.1% vs. 17.3%), as was the rate of grade 2 or higher abnormal blood chemical values (5.8% vs. 1.3%).
In addition, rates of abnormal blood chemical values grade 2 or higher were significantly more common in women treated with tenofovir-based ART than with zidovudine alone (2.9% vs. 0.8%).
Low birth weight (less than 2,500 g) was significantly more likely for infants of mothers in the zidovudine-based ART group, compared with the zidovudine-only group (23.0% vs. 12.0%) and in the tenofovir-based ART group, compared with the zidovudine-only group (16.9% vs. 8.9%). Preterm delivery and early infant death rates were significantly more likely in the tenofovir-based ART group than in the zidovudine-based ART group. Overall, the rate of HIV-free survival was highest among infants whose mothers received zidovudine-based ART, the investigators reported.
The findings were limited by several factors, and the safest and most effective regimens have yet to be determined, the researchers said. “Our findings emphasize the need for continued research to assess ART in pregnancy to ensure safer pregnancies for HIV-infected women and healthier outcomes for their uninfected infants,” they wrote.
A study coauthor reported receiving grant support from Gilead Sciences and ViiV Healthcare, and consulting fees from Janssen, paid directly to her institution. None of the other researchers, disclosed any financial conflicts.
A triple-drug antiretroviral therapy given to HIV-infected pregnant women significantly reduced transmission of the disease to their newborns, but with greater risk of adverse outcomes for mothers and infants, a study showed.
The findings were based on data from three treatment regimens in approximately 3,500 women and infant sets. The three treatments were zidovudine plus intrapartum single-dose nevirapine with 6-14 days of tenofovir and emtricitabine post partum (zidovudine alone); zidovudine, lamivudine, and lopinavir–ritonavir (zidovudine-based antiretroviral therapy [ART]); or tenofovir, emtricitabine, and lopinavir–ritonavir (tenofovir-based ART). All infants received nevirapine once daily, and infants of mothers coinfected with hepatitis B also received hepatitis B vaccination.
The Promoting Maternal and Infant Survival Everywhere (PROMISE) trial included patients at 14 sites in seven countries (India, Malawi, South Africa, Tanzania, Uganda, Zambia, and Zimbabwe). The current study presented findings from women with a CD4 count of at least 350 cells per cubic millimeter who were randomized at 14 weeks’ gestation to one of the three treatment regimens.
Maternal adverse events (grade 2 or higher) were significantly more common in the zidovudine-based ART group than in the zidovudine-only group (21.1% vs. 17.3%), as was the rate of grade 2 or higher abnormal blood chemical values (5.8% vs. 1.3%).
In addition, rates of abnormal blood chemical values grade 2 or higher were significantly more common in women treated with tenofovir-based ART than with zidovudine alone (2.9% vs. 0.8%).
Low birth weight (less than 2,500 g) was significantly more likely for infants of mothers in the zidovudine-based ART group, compared with the zidovudine-only group (23.0% vs. 12.0%) and in the tenofovir-based ART group, compared with the zidovudine-only group (16.9% vs. 8.9%). Preterm delivery and early infant death rates were significantly more likely in the tenofovir-based ART group than in the zidovudine-based ART group. Overall, the rate of HIV-free survival was highest among infants whose mothers received zidovudine-based ART, the investigators reported.
The findings were limited by several factors, and the safest and most effective regimens have yet to be determined, the researchers said. “Our findings emphasize the need for continued research to assess ART in pregnancy to ensure safer pregnancies for HIV-infected women and healthier outcomes for their uninfected infants,” they wrote.
A study coauthor reported receiving grant support from Gilead Sciences and ViiV Healthcare, and consulting fees from Janssen, paid directly to her institution. None of the other researchers, disclosed any financial conflicts.
A triple-drug antiretroviral therapy given to HIV-infected pregnant women significantly reduced transmission of the disease to their newborns, but with greater risk of adverse outcomes for mothers and infants, a study showed.
The findings were based on data from three treatment regimens in approximately 3,500 women and infant sets. The three treatments were zidovudine plus intrapartum single-dose nevirapine with 6-14 days of tenofovir and emtricitabine post partum (zidovudine alone); zidovudine, lamivudine, and lopinavir–ritonavir (zidovudine-based antiretroviral therapy [ART]); or tenofovir, emtricitabine, and lopinavir–ritonavir (tenofovir-based ART). All infants received nevirapine once daily, and infants of mothers coinfected with hepatitis B also received hepatitis B vaccination.
The Promoting Maternal and Infant Survival Everywhere (PROMISE) trial included patients at 14 sites in seven countries (India, Malawi, South Africa, Tanzania, Uganda, Zambia, and Zimbabwe). The current study presented findings from women with a CD4 count of at least 350 cells per cubic millimeter who were randomized at 14 weeks’ gestation to one of the three treatment regimens.
Maternal adverse events (grade 2 or higher) were significantly more common in the zidovudine-based ART group than in the zidovudine-only group (21.1% vs. 17.3%), as was the rate of grade 2 or higher abnormal blood chemical values (5.8% vs. 1.3%).
In addition, rates of abnormal blood chemical values grade 2 or higher were significantly more common in women treated with tenofovir-based ART than with zidovudine alone (2.9% vs. 0.8%).
Low birth weight (less than 2,500 g) was significantly more likely for infants of mothers in the zidovudine-based ART group, compared with the zidovudine-only group (23.0% vs. 12.0%) and in the tenofovir-based ART group, compared with the zidovudine-only group (16.9% vs. 8.9%). Preterm delivery and early infant death rates were significantly more likely in the tenofovir-based ART group than in the zidovudine-based ART group. Overall, the rate of HIV-free survival was highest among infants whose mothers received zidovudine-based ART, the investigators reported.
The findings were limited by several factors, and the safest and most effective regimens have yet to be determined, the researchers said. “Our findings emphasize the need for continued research to assess ART in pregnancy to ensure safer pregnancies for HIV-infected women and healthier outcomes for their uninfected infants,” they wrote.
A study coauthor reported receiving grant support from Gilead Sciences and ViiV Healthcare, and consulting fees from Janssen, paid directly to her institution. None of the other researchers, disclosed any financial conflicts.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Prenatal ART significantly lowered rates of early HIV transmission from HIV-infected pregnant women to their newborns, compared with zidovudine alone.
Major finding: The transmission rate for HIV was significantly lower in patients who underwent ART, compared with zidovudine alone (0.5% vs. 1.8%).
Data source: A randomized trial including 3,529 HIV-positive pregnant women at at least 14 weeks’ gestation.
Disclosures: A study coauthor reported receiving grant support from Gilead Sciences and ViiV Healthcare, and consulting fees from Janssen, paid directly to her institution. None of the other researchers disclosed any financial conflicts.