User login
High protein intake moderately associated with improved breast cancer survival
Higher protein intake, particularly protein from animal sources, is associated with a modest but lower risk of breast cancer recurrence and death, regardless of insulin receptor status.
Using information gathered through biennial questionnaires from 6,348 women who were diagnosed with stage I to III breast cancer between 1976 and 2004, investigators found a significant inverse association between total protein intake and distant breast cancer recurrence (P = .02). This association was driven specifically by protein from animal sources (P = .003) rather than vegetable sources.
Pathology records were reviewed and histology samples were analyzed for insulin receptor and estrogen receptor expression. Associations between breast cancer recurrence and protein intake, amino acids, or protein-containing food groups did not differ by tumor receptor status or body mass index at time of cancer diagnosis. Given that the association between protein intake and recurrence was not confined to tumors expressing insulin receptors, “It is difficult to invoke the insulin pathway as a mechanism to explain these findings,” the investigators wrote.
Given the only “modest survival advantage” of higher protein intake among women with breast cancer, and given the “challenges involved in randomized trials of diet, this association is unlikely to ever be definitively tested in a randomized trial,” Dr. Holmes and her associates wrote.
“However, the modest survival advantage with higher protein intake has been found in several studies, and we feel it is important that patients with breast cancer and their clinicians know this. At the least, it may provide reassurance that consuming protein-containing foods is not likely to increase the risk of breast cancer recurrence,” the researchers concluded.
This study was sponsored by grants from the National Institutes of Health. Dr. Holmes and one other investigator reported receiving financial compensation from Bayer HealthCare Pharmaceuticals.
[email protected]
On Twitter @jessnicolecraig
Higher protein intake, particularly protein from animal sources, is associated with a modest but lower risk of breast cancer recurrence and death, regardless of insulin receptor status.
Using information gathered through biennial questionnaires from 6,348 women who were diagnosed with stage I to III breast cancer between 1976 and 2004, investigators found a significant inverse association between total protein intake and distant breast cancer recurrence (P = .02). This association was driven specifically by protein from animal sources (P = .003) rather than vegetable sources.
Pathology records were reviewed and histology samples were analyzed for insulin receptor and estrogen receptor expression. Associations between breast cancer recurrence and protein intake, amino acids, or protein-containing food groups did not differ by tumor receptor status or body mass index at time of cancer diagnosis. Given that the association between protein intake and recurrence was not confined to tumors expressing insulin receptors, “It is difficult to invoke the insulin pathway as a mechanism to explain these findings,” the investigators wrote.
Given the only “modest survival advantage” of higher protein intake among women with breast cancer, and given the “challenges involved in randomized trials of diet, this association is unlikely to ever be definitively tested in a randomized trial,” Dr. Holmes and her associates wrote.
“However, the modest survival advantage with higher protein intake has been found in several studies, and we feel it is important that patients with breast cancer and their clinicians know this. At the least, it may provide reassurance that consuming protein-containing foods is not likely to increase the risk of breast cancer recurrence,” the researchers concluded.
This study was sponsored by grants from the National Institutes of Health. Dr. Holmes and one other investigator reported receiving financial compensation from Bayer HealthCare Pharmaceuticals.
[email protected]
On Twitter @jessnicolecraig
Higher protein intake, particularly protein from animal sources, is associated with a modest but lower risk of breast cancer recurrence and death, regardless of insulin receptor status.
Using information gathered through biennial questionnaires from 6,348 women who were diagnosed with stage I to III breast cancer between 1976 and 2004, investigators found a significant inverse association between total protein intake and distant breast cancer recurrence (P = .02). This association was driven specifically by protein from animal sources (P = .003) rather than vegetable sources.
Pathology records were reviewed and histology samples were analyzed for insulin receptor and estrogen receptor expression. Associations between breast cancer recurrence and protein intake, amino acids, or protein-containing food groups did not differ by tumor receptor status or body mass index at time of cancer diagnosis. Given that the association between protein intake and recurrence was not confined to tumors expressing insulin receptors, “It is difficult to invoke the insulin pathway as a mechanism to explain these findings,” the investigators wrote.
Given the only “modest survival advantage” of higher protein intake among women with breast cancer, and given the “challenges involved in randomized trials of diet, this association is unlikely to ever be definitively tested in a randomized trial,” Dr. Holmes and her associates wrote.
“However, the modest survival advantage with higher protein intake has been found in several studies, and we feel it is important that patients with breast cancer and their clinicians know this. At the least, it may provide reassurance that consuming protein-containing foods is not likely to increase the risk of breast cancer recurrence,” the researchers concluded.
This study was sponsored by grants from the National Institutes of Health. Dr. Holmes and one other investigator reported receiving financial compensation from Bayer HealthCare Pharmaceuticals.
[email protected]
On Twitter @jessnicolecraig
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:
Major finding: The 5-year recurrence-free survival for women in the highest quintile of protein consumption was 94.0%, while those in the lowest quintile of protein consumption had 5-year recurrence-free survival of 92.1%.
Data source: Biennial questionnaires for 6,348 women diagnosed with any stage breast cancer between 1976 and 2004.
Disclosures: This study was sponsored by grants from the National Institutes of Health. Dr. Holmes and one coinvestigator reported receiving financial compensation from Bayer HealthCare Pharmaceuticals.
Study offers reassuring data on certolizumab use in pregnancy
VIENNA – Data from a large prospective registry of pregnancy outcomes in women on certolizumab are reassuring to date, Alexa B. Kimball, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“This unique dataset of pregnancies exposed to a single agent suggests that exposure is really not a problem, although we’ll continue to collect prospective data and anticipate doing so in women with psoriasis going forward,” said Dr. Kimball, professor of dermatology at Harvard Medical School, Boston.
Certolizumab is a tumor necrosis factor–alpha inhibitor currently approved in the United States for the treatment of Crohn’s disease, rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis. It is now in clinical trials for psoriasis, and Dr. Kimball said she expects that it will eventually receive an indication for that disease as well. In the interim, she switches her psoriasis patients who are pregnant or plan to become so to either off-label certolizumab or etanercept (Enbrel). She does the same for her psoriatic arthritis patients, although in that situation certolizumab is on-label therapy.
The reason she turns to etanercept or certolizumab in pregnancy or anticipated pregnancy is that these two biologics, unlike others, don’t cross the placenta in the third trimester.
“I’m concerned about the selective uptake of other monoclonal antibodies in the third trimester. We do see in babies born to moms exposed to these other drugs – like ustekinumab, infliximab, and adalimumab – that the baby’s drug blood levels at birth are higher than the mom’s, so you have potentially put them at some risk for infections unnecessarily and maybe have affected how their immune system develops. So if a woman comes to me who is pregnant and on a biologic agent I would either stop treatment in the second trimester or switch to etanercept or certolizumab,” the dermatologist said.
Of the 256 pregnancies prospectively followed in the UCB certolizumab registry, 80.9% resulted in live births, and 10.2% ended in spontaneous abortion or miscarriage. In addition, the induced abortion rate was 8.6%, and there was a single stillbirth.
Of note, the mean age at pregnancy was 31 years, and 29% of the women became pregnant at age 35 or older. In contrast, the mean age at first pregnancy in the general population is 26, and only about 10% are 35 or older. These data are consistent with Dr. Kimball’s own clinical experience, which is that women with moderate to severe psoriasis or psoriatic arthritis often have trouble conceiving, and if they eventually succeed it’s often at a more advanced age.
The rate of maternal complications in this series was unremarkable: preeclampsia in 3.5%, infection in 3.9%, disease flare in 5.1%, and gestational diabetes in 3.1%. The median gestational age at birth was 39 weeks. The rate of early preterm birth before 32 weeks was 3.5%, with 12.6% of babies arriving at 32-36 weeks. Considering these are older moms being treated for serious underlying systemic inflammatory diseases, these numbers look good, according to Dr. Kimball.
Most women were exposed to certolizumab during the first trimester at least, and many were on the drug throughout pregnancy.
She said about one-third of psoriasis patients experience improvement in their skin diseases during pregnancy.
“If they’re doing really well, I see no reason to keep them on systemic therapy during pregnancy. But I will say that I see a lot of very bad postpartum flares, and I’m quite cautious about that. For the women with psoriatic arthritis there may not be a choice; you may need to continue to treat them all the way through their pregnancy to keep from getting permanent joint destruction,” the dermatologist said.
Dr. Kimball reported receiving research grants from and serving as a consultant to UCB and numerous other pharmaceutical companies.
VIENNA – Data from a large prospective registry of pregnancy outcomes in women on certolizumab are reassuring to date, Alexa B. Kimball, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“This unique dataset of pregnancies exposed to a single agent suggests that exposure is really not a problem, although we’ll continue to collect prospective data and anticipate doing so in women with psoriasis going forward,” said Dr. Kimball, professor of dermatology at Harvard Medical School, Boston.
Certolizumab is a tumor necrosis factor–alpha inhibitor currently approved in the United States for the treatment of Crohn’s disease, rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis. It is now in clinical trials for psoriasis, and Dr. Kimball said she expects that it will eventually receive an indication for that disease as well. In the interim, she switches her psoriasis patients who are pregnant or plan to become so to either off-label certolizumab or etanercept (Enbrel). She does the same for her psoriatic arthritis patients, although in that situation certolizumab is on-label therapy.
The reason she turns to etanercept or certolizumab in pregnancy or anticipated pregnancy is that these two biologics, unlike others, don’t cross the placenta in the third trimester.
“I’m concerned about the selective uptake of other monoclonal antibodies in the third trimester. We do see in babies born to moms exposed to these other drugs – like ustekinumab, infliximab, and adalimumab – that the baby’s drug blood levels at birth are higher than the mom’s, so you have potentially put them at some risk for infections unnecessarily and maybe have affected how their immune system develops. So if a woman comes to me who is pregnant and on a biologic agent I would either stop treatment in the second trimester or switch to etanercept or certolizumab,” the dermatologist said.
Of the 256 pregnancies prospectively followed in the UCB certolizumab registry, 80.9% resulted in live births, and 10.2% ended in spontaneous abortion or miscarriage. In addition, the induced abortion rate was 8.6%, and there was a single stillbirth.
Of note, the mean age at pregnancy was 31 years, and 29% of the women became pregnant at age 35 or older. In contrast, the mean age at first pregnancy in the general population is 26, and only about 10% are 35 or older. These data are consistent with Dr. Kimball’s own clinical experience, which is that women with moderate to severe psoriasis or psoriatic arthritis often have trouble conceiving, and if they eventually succeed it’s often at a more advanced age.
The rate of maternal complications in this series was unremarkable: preeclampsia in 3.5%, infection in 3.9%, disease flare in 5.1%, and gestational diabetes in 3.1%. The median gestational age at birth was 39 weeks. The rate of early preterm birth before 32 weeks was 3.5%, with 12.6% of babies arriving at 32-36 weeks. Considering these are older moms being treated for serious underlying systemic inflammatory diseases, these numbers look good, according to Dr. Kimball.
Most women were exposed to certolizumab during the first trimester at least, and many were on the drug throughout pregnancy.
She said about one-third of psoriasis patients experience improvement in their skin diseases during pregnancy.
“If they’re doing really well, I see no reason to keep them on systemic therapy during pregnancy. But I will say that I see a lot of very bad postpartum flares, and I’m quite cautious about that. For the women with psoriatic arthritis there may not be a choice; you may need to continue to treat them all the way through their pregnancy to keep from getting permanent joint destruction,” the dermatologist said.
Dr. Kimball reported receiving research grants from and serving as a consultant to UCB and numerous other pharmaceutical companies.
VIENNA – Data from a large prospective registry of pregnancy outcomes in women on certolizumab are reassuring to date, Alexa B. Kimball, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“This unique dataset of pregnancies exposed to a single agent suggests that exposure is really not a problem, although we’ll continue to collect prospective data and anticipate doing so in women with psoriasis going forward,” said Dr. Kimball, professor of dermatology at Harvard Medical School, Boston.
Certolizumab is a tumor necrosis factor–alpha inhibitor currently approved in the United States for the treatment of Crohn’s disease, rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis. It is now in clinical trials for psoriasis, and Dr. Kimball said she expects that it will eventually receive an indication for that disease as well. In the interim, she switches her psoriasis patients who are pregnant or plan to become so to either off-label certolizumab or etanercept (Enbrel). She does the same for her psoriatic arthritis patients, although in that situation certolizumab is on-label therapy.
The reason she turns to etanercept or certolizumab in pregnancy or anticipated pregnancy is that these two biologics, unlike others, don’t cross the placenta in the third trimester.
“I’m concerned about the selective uptake of other monoclonal antibodies in the third trimester. We do see in babies born to moms exposed to these other drugs – like ustekinumab, infliximab, and adalimumab – that the baby’s drug blood levels at birth are higher than the mom’s, so you have potentially put them at some risk for infections unnecessarily and maybe have affected how their immune system develops. So if a woman comes to me who is pregnant and on a biologic agent I would either stop treatment in the second trimester or switch to etanercept or certolizumab,” the dermatologist said.
Of the 256 pregnancies prospectively followed in the UCB certolizumab registry, 80.9% resulted in live births, and 10.2% ended in spontaneous abortion or miscarriage. In addition, the induced abortion rate was 8.6%, and there was a single stillbirth.
Of note, the mean age at pregnancy was 31 years, and 29% of the women became pregnant at age 35 or older. In contrast, the mean age at first pregnancy in the general population is 26, and only about 10% are 35 or older. These data are consistent with Dr. Kimball’s own clinical experience, which is that women with moderate to severe psoriasis or psoriatic arthritis often have trouble conceiving, and if they eventually succeed it’s often at a more advanced age.
The rate of maternal complications in this series was unremarkable: preeclampsia in 3.5%, infection in 3.9%, disease flare in 5.1%, and gestational diabetes in 3.1%. The median gestational age at birth was 39 weeks. The rate of early preterm birth before 32 weeks was 3.5%, with 12.6% of babies arriving at 32-36 weeks. Considering these are older moms being treated for serious underlying systemic inflammatory diseases, these numbers look good, according to Dr. Kimball.
Most women were exposed to certolizumab during the first trimester at least, and many were on the drug throughout pregnancy.
She said about one-third of psoriasis patients experience improvement in their skin diseases during pregnancy.
“If they’re doing really well, I see no reason to keep them on systemic therapy during pregnancy. But I will say that I see a lot of very bad postpartum flares, and I’m quite cautious about that. For the women with psoriatic arthritis there may not be a choice; you may need to continue to treat them all the way through their pregnancy to keep from getting permanent joint destruction,” the dermatologist said.
Dr. Kimball reported receiving research grants from and serving as a consultant to UCB and numerous other pharmaceutical companies.
AT THE EADV CONGRESS
Key clinical point:
Major finding: The rate of major congenital malformations in a large prospective series of pregnancies in women on certolizumab was reassuringly low at 4.2%, with no pattern of malformations being seen.
Data source: This was a report on maternal and fetal outcomes of 256 prospectively followed pregnancies in women on certolizumab.
Disclosures: The presenter reported receiving research funds from and serving as a consultant to UCB, which markets certolizumab and maintains the pregnancy registry.
VIDEO: Topical antifungals win with patients
LAS VEGAS – New topical treatment options for onychomycosis represent significant improvements over older agents, and may approach the success seen with oral drugs, according to Dr. Theodore Rosen, professor of dermatology, Baylor College of Medicine, Houston.
Efinaconazole and tavaborole both permeate the nail and allow for spreading to the lateral nail folds and hyponychium, Dr. Rosen said in a video interview at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. Moreover, the topical treatments are popular with patients. Even if patients are not 100% clear, they are usually happy if their condition improves enough to wear sandals with confidence, Dr. Rosen added in the interview.
SDEF and this news organization are owned by the same parent company.
Dr. Rosen disclosed being a paid participant on the scientific advisory boards for Anacor and Valeant.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – New topical treatment options for onychomycosis represent significant improvements over older agents, and may approach the success seen with oral drugs, according to Dr. Theodore Rosen, professor of dermatology, Baylor College of Medicine, Houston.
Efinaconazole and tavaborole both permeate the nail and allow for spreading to the lateral nail folds and hyponychium, Dr. Rosen said in a video interview at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. Moreover, the topical treatments are popular with patients. Even if patients are not 100% clear, they are usually happy if their condition improves enough to wear sandals with confidence, Dr. Rosen added in the interview.
SDEF and this news organization are owned by the same parent company.
Dr. Rosen disclosed being a paid participant on the scientific advisory boards for Anacor and Valeant.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – New topical treatment options for onychomycosis represent significant improvements over older agents, and may approach the success seen with oral drugs, according to Dr. Theodore Rosen, professor of dermatology, Baylor College of Medicine, Houston.
Efinaconazole and tavaborole both permeate the nail and allow for spreading to the lateral nail folds and hyponychium, Dr. Rosen said in a video interview at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. Moreover, the topical treatments are popular with patients. Even if patients are not 100% clear, they are usually happy if their condition improves enough to wear sandals with confidence, Dr. Rosen added in the interview.
SDEF and this news organization are owned by the same parent company.
Dr. Rosen disclosed being a paid participant on the scientific advisory boards for Anacor and Valeant.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM SDEF LAS VEGAS SEMINAR
Tobacco-related cancer incidence, mortality drop
Tobacco-related cancer incidence and mortality rates dropped from 2004 to 2013, the Centers for Disease Control and Prevention reported in the Morbidity and Mortality Weekly Report published on Nov. 11, 2016.
Overall, tobacco-related invasive cancer incidence decreased from 206 cases per 100,000 during 2004-2008 to 193 cases per 100,000 during 2009-2013.
The announcement of this data marks a continuation of the downward trend that has been observed since the 1990s, lead author S. Jane Henley, MSPH, and her associates at the CDC wrote in the report (MMWR. 2016 Nov 11;65[44]:1212-8).
Despite this continued decline in tobacco-related cancer incidence and mortality rates, the tobacco-related cancer burden remains high, and disparities in the rates and decline of tobacco-related cancer persists.
“Tobacco use remains the leading preventable cause of disease and death in the United States,” reported Henley and her associates. For each year between 2009 and 2013, an estimated 660,000 Americans were diagnosed with tobacco-related cancer, and an estimated 343,000 people died from those cancers, according to the investigators’ analysis of data collected by the United States Cancer Statistics working group, which compiles data from multiple nationwide sources including the National Program of Cancer Registries and the National Cancer Institute’s Surveillance, Epidemiology, and End Results program.
Tobacco-related cancer incidence and deaths were higher among men than women and higher among blacks than any other ethnic group. However, the cancer incidence and mortality rates also declined the fastest among men and blacks, compared with women and other ethnic groups, respectively.
Cancer incidence and death were also highest and decreased the most slowly in counties with lower educational attainment or highest poverty. Conversely, cancer incidence and mortality was the lowest and decreased the most quickly in metropolitan areas with populations greater than 1 million people.
Given that an estimated 40% of cancers diagnosed in the country and 3 in 10 cancer deaths are attributable to cigarette smoking and the use of smokeless tobacco, it is imperative that the CDC implement programs to help the almost 6 million smokers quit, CDC director Tom Frieden, MD, said in an associated telebriefing. Most people who smoke want to quit, and the health care system should do all it can to help them, Dr. Frieden said. At the same time, he echoed a claim from Henley’s paper, which said many tobacco-related cancers could be prevented by reducing tobacco use through implementation of evidence-based tobacco prevention and control interventions, such as increasing tobacco product prices, enforcing smoke-free laws, and promoting anti-tobacco mass media campaigns. These programs should be tailored to local geographic areas and demographics given the continued inconsistent progress and persistent disparities in tobacco-related cancer incidence and mortality, Dr. Frieden added.
[email protected]
On Twitter @jessnicolecraig
Tobacco-related cancer incidence and mortality rates dropped from 2004 to 2013, the Centers for Disease Control and Prevention reported in the Morbidity and Mortality Weekly Report published on Nov. 11, 2016.
Overall, tobacco-related invasive cancer incidence decreased from 206 cases per 100,000 during 2004-2008 to 193 cases per 100,000 during 2009-2013.
The announcement of this data marks a continuation of the downward trend that has been observed since the 1990s, lead author S. Jane Henley, MSPH, and her associates at the CDC wrote in the report (MMWR. 2016 Nov 11;65[44]:1212-8).
Despite this continued decline in tobacco-related cancer incidence and mortality rates, the tobacco-related cancer burden remains high, and disparities in the rates and decline of tobacco-related cancer persists.
“Tobacco use remains the leading preventable cause of disease and death in the United States,” reported Henley and her associates. For each year between 2009 and 2013, an estimated 660,000 Americans were diagnosed with tobacco-related cancer, and an estimated 343,000 people died from those cancers, according to the investigators’ analysis of data collected by the United States Cancer Statistics working group, which compiles data from multiple nationwide sources including the National Program of Cancer Registries and the National Cancer Institute’s Surveillance, Epidemiology, and End Results program.
Tobacco-related cancer incidence and deaths were higher among men than women and higher among blacks than any other ethnic group. However, the cancer incidence and mortality rates also declined the fastest among men and blacks, compared with women and other ethnic groups, respectively.
Cancer incidence and death were also highest and decreased the most slowly in counties with lower educational attainment or highest poverty. Conversely, cancer incidence and mortality was the lowest and decreased the most quickly in metropolitan areas with populations greater than 1 million people.
Given that an estimated 40% of cancers diagnosed in the country and 3 in 10 cancer deaths are attributable to cigarette smoking and the use of smokeless tobacco, it is imperative that the CDC implement programs to help the almost 6 million smokers quit, CDC director Tom Frieden, MD, said in an associated telebriefing. Most people who smoke want to quit, and the health care system should do all it can to help them, Dr. Frieden said. At the same time, he echoed a claim from Henley’s paper, which said many tobacco-related cancers could be prevented by reducing tobacco use through implementation of evidence-based tobacco prevention and control interventions, such as increasing tobacco product prices, enforcing smoke-free laws, and promoting anti-tobacco mass media campaigns. These programs should be tailored to local geographic areas and demographics given the continued inconsistent progress and persistent disparities in tobacco-related cancer incidence and mortality, Dr. Frieden added.
[email protected]
On Twitter @jessnicolecraig
Tobacco-related cancer incidence and mortality rates dropped from 2004 to 2013, the Centers for Disease Control and Prevention reported in the Morbidity and Mortality Weekly Report published on Nov. 11, 2016.
Overall, tobacco-related invasive cancer incidence decreased from 206 cases per 100,000 during 2004-2008 to 193 cases per 100,000 during 2009-2013.
The announcement of this data marks a continuation of the downward trend that has been observed since the 1990s, lead author S. Jane Henley, MSPH, and her associates at the CDC wrote in the report (MMWR. 2016 Nov 11;65[44]:1212-8).
Despite this continued decline in tobacco-related cancer incidence and mortality rates, the tobacco-related cancer burden remains high, and disparities in the rates and decline of tobacco-related cancer persists.
“Tobacco use remains the leading preventable cause of disease and death in the United States,” reported Henley and her associates. For each year between 2009 and 2013, an estimated 660,000 Americans were diagnosed with tobacco-related cancer, and an estimated 343,000 people died from those cancers, according to the investigators’ analysis of data collected by the United States Cancer Statistics working group, which compiles data from multiple nationwide sources including the National Program of Cancer Registries and the National Cancer Institute’s Surveillance, Epidemiology, and End Results program.
Tobacco-related cancer incidence and deaths were higher among men than women and higher among blacks than any other ethnic group. However, the cancer incidence and mortality rates also declined the fastest among men and blacks, compared with women and other ethnic groups, respectively.
Cancer incidence and death were also highest and decreased the most slowly in counties with lower educational attainment or highest poverty. Conversely, cancer incidence and mortality was the lowest and decreased the most quickly in metropolitan areas with populations greater than 1 million people.
Given that an estimated 40% of cancers diagnosed in the country and 3 in 10 cancer deaths are attributable to cigarette smoking and the use of smokeless tobacco, it is imperative that the CDC implement programs to help the almost 6 million smokers quit, CDC director Tom Frieden, MD, said in an associated telebriefing. Most people who smoke want to quit, and the health care system should do all it can to help them, Dr. Frieden said. At the same time, he echoed a claim from Henley’s paper, which said many tobacco-related cancers could be prevented by reducing tobacco use through implementation of evidence-based tobacco prevention and control interventions, such as increasing tobacco product prices, enforcing smoke-free laws, and promoting anti-tobacco mass media campaigns. These programs should be tailored to local geographic areas and demographics given the continued inconsistent progress and persistent disparities in tobacco-related cancer incidence and mortality, Dr. Frieden added.
[email protected]
On Twitter @jessnicolecraig
FROM MMWR
Key clinical point:
Major finding: Tobacco-related cancer mortality dropped from 108 deaths per 100,000 during 2004-2008 to 100 per 100,000 during 2009-2013.
Data source: Retrospective analysis of United States Cancer Statistics data for 2004 to 2013.
Disclosures: This study was sponsored by the Centers for Disease Control and Prevention. The authors’ disclosures were not reported.
PCR will improve diagnostic testing for dermatophytes, expert says
LAS VEGAS – Until polymerase chain reaction (PCR) testing for diagnosing dermatophyte infections becomes available in the United States, the options remain the KOH test, periodic acid–Schiff (PAS) stain, and culture, Dr. Theodore Rosen said at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
PCR testing is both the most sensitive and most specific method to diagnose dermatophyte infections and eventually will become the gold standard for diagnosing such infections, noted Dr. Rosen, professor of dermatology, Baylor College of Medicine, Houston. A commercial PCR kit to diagnose dermatophytes, manufactured by a Danish company, is available outside of the United States but currently is not approved in this country.
He listed several studies that compared these methods, including a retrospective trial that analyzed the reliability of different tests in verifying a clinical diagnosis of onychomycosis in 108 patients (J Am Podiatr Med Assoc. 2015 Nov;105[6]:503-8). When toenail clippings from the study participants were tested, PAS produced the most consistent positive results (60%, compared with 43.5% for KOH and 39.8% for culture). Compared with the KOH test, PAS also had a higher sensitivity (0.79 vs. 0.64) for confirming fungal infection.
Dr. Rosen said that PCR testing should become available in the United States in the future. “PCR is not only good because we know that the fungus is there, but it can be very specific,” he said. A small percentage of onychomycosis cases are due to Candida, most often affecting the fingernails of people who have their hands in water frequently, such as bartenders and housekeepers.
“And then there’s another small percentage that are due to nondermatophyte molds, the kind of things that are everywhere in the environment,” said Dr. Rosen. Describing the appearance of these types of cases, he said, “sometimes they look different … they’re darker. The nails are a little more heavily affected.”
Dr. Rosen listed several PCR studies, including one that compared PCR with conventional diagnostic methods in 107 nail specimens of patients with clinically suspected onychomycosis. The study found that PCR use increased the diagnosis of specimens positive for dermatophytes by almost 40%. PCR was positive in 72% of the specimens, compared with 57% of the fungal cultures. In addition, PCR detected dermatophytes in 39 specimens that cultures missed (Australas J Dermatol. 2013 May;54[2]:105-8).
SDEF and this news organization are owned by the same parent company.
Dr. Rosen disclosed being a paid participant on the scientific advisory boards for Anacor and Valeant.
LAS VEGAS – Until polymerase chain reaction (PCR) testing for diagnosing dermatophyte infections becomes available in the United States, the options remain the KOH test, periodic acid–Schiff (PAS) stain, and culture, Dr. Theodore Rosen said at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
PCR testing is both the most sensitive and most specific method to diagnose dermatophyte infections and eventually will become the gold standard for diagnosing such infections, noted Dr. Rosen, professor of dermatology, Baylor College of Medicine, Houston. A commercial PCR kit to diagnose dermatophytes, manufactured by a Danish company, is available outside of the United States but currently is not approved in this country.
He listed several studies that compared these methods, including a retrospective trial that analyzed the reliability of different tests in verifying a clinical diagnosis of onychomycosis in 108 patients (J Am Podiatr Med Assoc. 2015 Nov;105[6]:503-8). When toenail clippings from the study participants were tested, PAS produced the most consistent positive results (60%, compared with 43.5% for KOH and 39.8% for culture). Compared with the KOH test, PAS also had a higher sensitivity (0.79 vs. 0.64) for confirming fungal infection.
Dr. Rosen said that PCR testing should become available in the United States in the future. “PCR is not only good because we know that the fungus is there, but it can be very specific,” he said. A small percentage of onychomycosis cases are due to Candida, most often affecting the fingernails of people who have their hands in water frequently, such as bartenders and housekeepers.
“And then there’s another small percentage that are due to nondermatophyte molds, the kind of things that are everywhere in the environment,” said Dr. Rosen. Describing the appearance of these types of cases, he said, “sometimes they look different … they’re darker. The nails are a little more heavily affected.”
Dr. Rosen listed several PCR studies, including one that compared PCR with conventional diagnostic methods in 107 nail specimens of patients with clinically suspected onychomycosis. The study found that PCR use increased the diagnosis of specimens positive for dermatophytes by almost 40%. PCR was positive in 72% of the specimens, compared with 57% of the fungal cultures. In addition, PCR detected dermatophytes in 39 specimens that cultures missed (Australas J Dermatol. 2013 May;54[2]:105-8).
SDEF and this news organization are owned by the same parent company.
Dr. Rosen disclosed being a paid participant on the scientific advisory boards for Anacor and Valeant.
LAS VEGAS – Until polymerase chain reaction (PCR) testing for diagnosing dermatophyte infections becomes available in the United States, the options remain the KOH test, periodic acid–Schiff (PAS) stain, and culture, Dr. Theodore Rosen said at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
PCR testing is both the most sensitive and most specific method to diagnose dermatophyte infections and eventually will become the gold standard for diagnosing such infections, noted Dr. Rosen, professor of dermatology, Baylor College of Medicine, Houston. A commercial PCR kit to diagnose dermatophytes, manufactured by a Danish company, is available outside of the United States but currently is not approved in this country.
He listed several studies that compared these methods, including a retrospective trial that analyzed the reliability of different tests in verifying a clinical diagnosis of onychomycosis in 108 patients (J Am Podiatr Med Assoc. 2015 Nov;105[6]:503-8). When toenail clippings from the study participants were tested, PAS produced the most consistent positive results (60%, compared with 43.5% for KOH and 39.8% for culture). Compared with the KOH test, PAS also had a higher sensitivity (0.79 vs. 0.64) for confirming fungal infection.
Dr. Rosen said that PCR testing should become available in the United States in the future. “PCR is not only good because we know that the fungus is there, but it can be very specific,” he said. A small percentage of onychomycosis cases are due to Candida, most often affecting the fingernails of people who have their hands in water frequently, such as bartenders and housekeepers.
“And then there’s another small percentage that are due to nondermatophyte molds, the kind of things that are everywhere in the environment,” said Dr. Rosen. Describing the appearance of these types of cases, he said, “sometimes they look different … they’re darker. The nails are a little more heavily affected.”
Dr. Rosen listed several PCR studies, including one that compared PCR with conventional diagnostic methods in 107 nail specimens of patients with clinically suspected onychomycosis. The study found that PCR use increased the diagnosis of specimens positive for dermatophytes by almost 40%. PCR was positive in 72% of the specimens, compared with 57% of the fungal cultures. In addition, PCR detected dermatophytes in 39 specimens that cultures missed (Australas J Dermatol. 2013 May;54[2]:105-8).
SDEF and this news organization are owned by the same parent company.
Dr. Rosen disclosed being a paid participant on the scientific advisory boards for Anacor and Valeant.
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
Misvalued codes lead to small cut in Medicare fee schedule
Instead of getting a 0.5% pay increase, doctors will lose 0.18% of their Medicare payments because the Centers for Medicare & Medicaid Services could not find enough misvalued services in the 2017 Medicare physician fee schedule.
A health reform law called the Protecting Access to Medicare Act of 2014 (H.R. 4302) was designed to hold down Medicare spending by requiring CMS to identify misvalued codes based on up to 16 criteria. Once identified, the value of those codes was to be lowered by CMS by an amount equal to 1% of the overall fee schedule so that physician pay could be increased by 1%. Codes up for consideration included codes that were the fastest-growing, codes for maturing technologies, codes for services that have undergone a change in service site, and codes with a significant difference in value based on service site.
A total of 19 potentially misvalued codes were found, including biopsy of finger or toe nail; injection of carpal tunnel; change of stomach feeding, accessed through skin; irrigation of vagina and/or application of drug to treat infection; wound closure utilizing tissue adhesive(s) only; and injection of anesthetic agent and/or steroid drug into nerve of foot. Lowering the value of those codes reduced the physician fee schedule by 0.32%, causing the 0.18% pay cut.
It is disappointing that a 0.5% fee schedule increase could not be accomplished, according to officials at the American Academy of Family Physicians.
“What I have to do in my office visit has greatly increased, whereas what it takes [specialists] to do their procedure has decreased. There needs to be an adjustment in the fee schedule,” AAFP President John Meigs, MD, said in an interview. This legislation “was supposed to spur Medicare and CMS to start making some adjustments and with these adjustments, give that tiny little pay increase to the fee schedule. That leads to the frustration of primary care physicians and family medicine physicians. We were promised a tiny pay increase and we are not going to get it.”
“CMS is paying lip service to increasing value of primary care, increasing the value of cognitive-based services, and they did come up with some new codes,” Dr. Meigs said. “They did some things with paying for diabetic patients that are things that they should have been paying for a long time ago. But the administrative hassle and regulatory burden of trying to do the documentation they require to actually get paid for some of these codes have a lot of physicians throwing their hands up saying, ‘it’s not worth my time to spend $5 to make a nickel.’ ”
Instead of getting a 0.5% pay increase, doctors will lose 0.18% of their Medicare payments because the Centers for Medicare & Medicaid Services could not find enough misvalued services in the 2017 Medicare physician fee schedule.
A health reform law called the Protecting Access to Medicare Act of 2014 (H.R. 4302) was designed to hold down Medicare spending by requiring CMS to identify misvalued codes based on up to 16 criteria. Once identified, the value of those codes was to be lowered by CMS by an amount equal to 1% of the overall fee schedule so that physician pay could be increased by 1%. Codes up for consideration included codes that were the fastest-growing, codes for maturing technologies, codes for services that have undergone a change in service site, and codes with a significant difference in value based on service site.
A total of 19 potentially misvalued codes were found, including biopsy of finger or toe nail; injection of carpal tunnel; change of stomach feeding, accessed through skin; irrigation of vagina and/or application of drug to treat infection; wound closure utilizing tissue adhesive(s) only; and injection of anesthetic agent and/or steroid drug into nerve of foot. Lowering the value of those codes reduced the physician fee schedule by 0.32%, causing the 0.18% pay cut.
It is disappointing that a 0.5% fee schedule increase could not be accomplished, according to officials at the American Academy of Family Physicians.
“What I have to do in my office visit has greatly increased, whereas what it takes [specialists] to do their procedure has decreased. There needs to be an adjustment in the fee schedule,” AAFP President John Meigs, MD, said in an interview. This legislation “was supposed to spur Medicare and CMS to start making some adjustments and with these adjustments, give that tiny little pay increase to the fee schedule. That leads to the frustration of primary care physicians and family medicine physicians. We were promised a tiny pay increase and we are not going to get it.”
“CMS is paying lip service to increasing value of primary care, increasing the value of cognitive-based services, and they did come up with some new codes,” Dr. Meigs said. “They did some things with paying for diabetic patients that are things that they should have been paying for a long time ago. But the administrative hassle and regulatory burden of trying to do the documentation they require to actually get paid for some of these codes have a lot of physicians throwing their hands up saying, ‘it’s not worth my time to spend $5 to make a nickel.’ ”
Instead of getting a 0.5% pay increase, doctors will lose 0.18% of their Medicare payments because the Centers for Medicare & Medicaid Services could not find enough misvalued services in the 2017 Medicare physician fee schedule.
A health reform law called the Protecting Access to Medicare Act of 2014 (H.R. 4302) was designed to hold down Medicare spending by requiring CMS to identify misvalued codes based on up to 16 criteria. Once identified, the value of those codes was to be lowered by CMS by an amount equal to 1% of the overall fee schedule so that physician pay could be increased by 1%. Codes up for consideration included codes that were the fastest-growing, codes for maturing technologies, codes for services that have undergone a change in service site, and codes with a significant difference in value based on service site.
A total of 19 potentially misvalued codes were found, including biopsy of finger or toe nail; injection of carpal tunnel; change of stomach feeding, accessed through skin; irrigation of vagina and/or application of drug to treat infection; wound closure utilizing tissue adhesive(s) only; and injection of anesthetic agent and/or steroid drug into nerve of foot. Lowering the value of those codes reduced the physician fee schedule by 0.32%, causing the 0.18% pay cut.
It is disappointing that a 0.5% fee schedule increase could not be accomplished, according to officials at the American Academy of Family Physicians.
“What I have to do in my office visit has greatly increased, whereas what it takes [specialists] to do their procedure has decreased. There needs to be an adjustment in the fee schedule,” AAFP President John Meigs, MD, said in an interview. This legislation “was supposed to spur Medicare and CMS to start making some adjustments and with these adjustments, give that tiny little pay increase to the fee schedule. That leads to the frustration of primary care physicians and family medicine physicians. We were promised a tiny pay increase and we are not going to get it.”
“CMS is paying lip service to increasing value of primary care, increasing the value of cognitive-based services, and they did come up with some new codes,” Dr. Meigs said. “They did some things with paying for diabetic patients that are things that they should have been paying for a long time ago. But the administrative hassle and regulatory burden of trying to do the documentation they require to actually get paid for some of these codes have a lot of physicians throwing their hands up saying, ‘it’s not worth my time to spend $5 to make a nickel.’ ”
Clindamycin, TMP-SMX both of benefit for small abscesses
NEW ORLEANS – Both clindamycin and trimethoprim-sulfamethoxazole (TMP-SMX) were superior to placebo when used after incision and drainage for the treatment of small, uncomplicated abscesses in children and adults in a prospective, randomized, placebo-controlled study.
Further, the cure rates were similar with both antibiotics, except in subjects with a clindamycin-resistant Staphylococcus aureus isolate, in whom the cure rate was lower, Robert S. Daum, MD, of the University of Chicago reported at an annual scientific meeting on infectious diseases.
Small, uncomplicated skin abscesses are common in ambulatory settings, but the optimal treatment strategy in the era of community-acquired methicillin-resistant S. aureus has been unclear. A prior study showed that clindamycin and TMP-SMX are both of benefit in the setting of large skin abscesses. The current findings further demonstrate that they also are of benefit when used in conjunction with incision and drainage for the treatment of small abscesses.
In 786 outpatient subjects, including 505 adults and 281 children who were randomized to receive 10 days of treatment with either clindamycin, TMP-SMX, or placebo following incision and drainage, mean cure rates at the 10-day posttherapy test of cure visit were 83% in the clindamycin group, 82% in the TMP-SMX group, and 69% in the placebo group, he said, noting that the differences were statistically significant for both treatments vs. placebo.
Study participants had a single skin abscess of 5 cm or less in diameter. Those with significant comorbidity, such as diabetes, were excluded.
S. aureus was isolated from 527 subjects (67%), and methicillin-resistant S. aureus was isolated from 388 (49%).
In clindamycin-treated subjects with an S. aureus lesion, 54% with a clindamycin-resistant isolate were cured, compared with 85% with a clindamycin-susceptible isolate.
Of note, subjects without S. aureus did not do better with antibiotics vs. placebo, Dr. Daum said.
“Staph aureus matters,” he said. People who did not grow Staph aureus did not do better with placebo than with antibiotic ... incision and drainage was basically all that was needed [in those patients],” he said.
Adverse events were more common in the clindamycin group (22% vs. 11% with TMP-SMX and 12.5% with placebo), but all events were mild and resolved without sequelae, and among those who were cured initially, fewer new skin infections were noted at a 1-month follow-up visit among clindamycin recipients, compared with those who received TMP-SMX or placebo, he noted at the combined annual meetings of the Infectious Diseases Society of America, the Society of Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
No cases of Clostridium difficile-associated diarrhea were reported among study subjects.
Dr. Daum reported having no disclosures.
NEW ORLEANS – Both clindamycin and trimethoprim-sulfamethoxazole (TMP-SMX) were superior to placebo when used after incision and drainage for the treatment of small, uncomplicated abscesses in children and adults in a prospective, randomized, placebo-controlled study.
Further, the cure rates were similar with both antibiotics, except in subjects with a clindamycin-resistant Staphylococcus aureus isolate, in whom the cure rate was lower, Robert S. Daum, MD, of the University of Chicago reported at an annual scientific meeting on infectious diseases.
Small, uncomplicated skin abscesses are common in ambulatory settings, but the optimal treatment strategy in the era of community-acquired methicillin-resistant S. aureus has been unclear. A prior study showed that clindamycin and TMP-SMX are both of benefit in the setting of large skin abscesses. The current findings further demonstrate that they also are of benefit when used in conjunction with incision and drainage for the treatment of small abscesses.
In 786 outpatient subjects, including 505 adults and 281 children who were randomized to receive 10 days of treatment with either clindamycin, TMP-SMX, or placebo following incision and drainage, mean cure rates at the 10-day posttherapy test of cure visit were 83% in the clindamycin group, 82% in the TMP-SMX group, and 69% in the placebo group, he said, noting that the differences were statistically significant for both treatments vs. placebo.
Study participants had a single skin abscess of 5 cm or less in diameter. Those with significant comorbidity, such as diabetes, were excluded.
S. aureus was isolated from 527 subjects (67%), and methicillin-resistant S. aureus was isolated from 388 (49%).
In clindamycin-treated subjects with an S. aureus lesion, 54% with a clindamycin-resistant isolate were cured, compared with 85% with a clindamycin-susceptible isolate.
Of note, subjects without S. aureus did not do better with antibiotics vs. placebo, Dr. Daum said.
“Staph aureus matters,” he said. People who did not grow Staph aureus did not do better with placebo than with antibiotic ... incision and drainage was basically all that was needed [in those patients],” he said.
Adverse events were more common in the clindamycin group (22% vs. 11% with TMP-SMX and 12.5% with placebo), but all events were mild and resolved without sequelae, and among those who were cured initially, fewer new skin infections were noted at a 1-month follow-up visit among clindamycin recipients, compared with those who received TMP-SMX or placebo, he noted at the combined annual meetings of the Infectious Diseases Society of America, the Society of Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
No cases of Clostridium difficile-associated diarrhea were reported among study subjects.
Dr. Daum reported having no disclosures.
NEW ORLEANS – Both clindamycin and trimethoprim-sulfamethoxazole (TMP-SMX) were superior to placebo when used after incision and drainage for the treatment of small, uncomplicated abscesses in children and adults in a prospective, randomized, placebo-controlled study.
Further, the cure rates were similar with both antibiotics, except in subjects with a clindamycin-resistant Staphylococcus aureus isolate, in whom the cure rate was lower, Robert S. Daum, MD, of the University of Chicago reported at an annual scientific meeting on infectious diseases.
Small, uncomplicated skin abscesses are common in ambulatory settings, but the optimal treatment strategy in the era of community-acquired methicillin-resistant S. aureus has been unclear. A prior study showed that clindamycin and TMP-SMX are both of benefit in the setting of large skin abscesses. The current findings further demonstrate that they also are of benefit when used in conjunction with incision and drainage for the treatment of small abscesses.
In 786 outpatient subjects, including 505 adults and 281 children who were randomized to receive 10 days of treatment with either clindamycin, TMP-SMX, or placebo following incision and drainage, mean cure rates at the 10-day posttherapy test of cure visit were 83% in the clindamycin group, 82% in the TMP-SMX group, and 69% in the placebo group, he said, noting that the differences were statistically significant for both treatments vs. placebo.
Study participants had a single skin abscess of 5 cm or less in diameter. Those with significant comorbidity, such as diabetes, were excluded.
S. aureus was isolated from 527 subjects (67%), and methicillin-resistant S. aureus was isolated from 388 (49%).
In clindamycin-treated subjects with an S. aureus lesion, 54% with a clindamycin-resistant isolate were cured, compared with 85% with a clindamycin-susceptible isolate.
Of note, subjects without S. aureus did not do better with antibiotics vs. placebo, Dr. Daum said.
“Staph aureus matters,” he said. People who did not grow Staph aureus did not do better with placebo than with antibiotic ... incision and drainage was basically all that was needed [in those patients],” he said.
Adverse events were more common in the clindamycin group (22% vs. 11% with TMP-SMX and 12.5% with placebo), but all events were mild and resolved without sequelae, and among those who were cured initially, fewer new skin infections were noted at a 1-month follow-up visit among clindamycin recipients, compared with those who received TMP-SMX or placebo, he noted at the combined annual meetings of the Infectious Diseases Society of America, the Society of Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
No cases of Clostridium difficile-associated diarrhea were reported among study subjects.
Dr. Daum reported having no disclosures.
AT IDWEEK 2016
Key clinical point:
Major finding: Mean cure rates were 83%, 82%, and 69% with clindamycin, TMP-SMX, and placebo, respectively.
Data source: A randomized, placebo-controlled, multicenter study of 786 subjects.
Disclosures: Dr. Daum reported having no disclosures.
Don’t Miss Out On. . .
The Challenging World of the Diagnosis and Treatment of Vascular Malformations: An Orphan Disease that Has Now Come of Age
(Sessions 92-98; Friday, 6:45 a.m. to 1:30 p.m.)
Location: Gramercy Suites East and West, 2nd Floor
Vascular malformations constitute one of the most challenging clinical entities encountered in Vascular Medicine today. They can occur in every anatomy in the body insinuating themselves throughout an organ or tissue. Being totally comprised of vascular structures, any surgical attempt at resection is fraught with potential hemorrhagic complications. Compounding their inherent difficulty in management , they are also very rare entities. The clinical presentations are extremely protean and can range from an asymptomatic birthmark, to fulminant, life-threatening CHF. Attributing any of these extremely varied symptoms that a patient may present with to a vascular malformation can be challenging to the most experienced clinician. Patients typically bounce from clinician to clinician experiencing disappointing outcomes, complications, and recurrence or worsening of their presenting symptoms. Due to their extreme rarity (>1% of the population), it is difficult for clinicians to gain any experience at all in their diagnosis and optimal management and make definitive statements.
The purpose of these Vascular Malformation Sessions is to offer the attendee the current state-of-the-art multi-disciplinary endovascular and surgical approaches to accurately diagnose and optimally treat all types of vascular malformations (AVMs, AVFs, venous malformations, lymphatic malformations, capillary-venous malformations, and mixed lesions) in all the various problematic anatomies in which they occur. Being that there are controversies regarding the various treatment strategies regarding vascular malformations, the attendees will be exposed to those multiple approaches and philosophies regarding the science, the multiple management strategies, the results, the long-term outcomes, and the complications inherent in the various palliative and curative treatments proffered by international experts.
Improving Outcomes in Hemodialysis Access
(Sessions 106-110; Saturday; 7:55 a.m. – 4:00 p.m.) Registration Begins At 6:00 a.m.
Location: Grand Ballroom West, 3rd Floor
The Hemodialysis Access sessions on Saturday (Sessions 106 – 110) will include presentations on planning, optimizing outcomes, political, economic and legal issues, new technologies and an update on clinical issues related to hemodialysis access. Experts will address specific topics including vein preservation and planning for access, use of ultrasound to facilitate cannulation, use of simulators, the role of drug eluting balloons and stents, management of complications including steal syndrome, infections and access hemorrhage, coding for access procedures, pharmacologic and mechanical approaches to improve fistula outcomes, treatment of fistula aneurysms, use of the HeRO graft and many more important topics. The keynote speaker will be Dr. Michael Brescia who will give his unique historical perspective on the development of the AV fistula. This session should be of interest to surgeons, nephrologists, interventionalists, nurses, dialysis technicians and others interested in the care of patients with end stage renal disease.
The Challenging World of the Diagnosis and Treatment of Vascular Malformations: An Orphan Disease that Has Now Come of Age
(Sessions 92-98; Friday, 6:45 a.m. to 1:30 p.m.)
Location: Gramercy Suites East and West, 2nd Floor
Vascular malformations constitute one of the most challenging clinical entities encountered in Vascular Medicine today. They can occur in every anatomy in the body insinuating themselves throughout an organ or tissue. Being totally comprised of vascular structures, any surgical attempt at resection is fraught with potential hemorrhagic complications. Compounding their inherent difficulty in management , they are also very rare entities. The clinical presentations are extremely protean and can range from an asymptomatic birthmark, to fulminant, life-threatening CHF. Attributing any of these extremely varied symptoms that a patient may present with to a vascular malformation can be challenging to the most experienced clinician. Patients typically bounce from clinician to clinician experiencing disappointing outcomes, complications, and recurrence or worsening of their presenting symptoms. Due to their extreme rarity (>1% of the population), it is difficult for clinicians to gain any experience at all in their diagnosis and optimal management and make definitive statements.
The purpose of these Vascular Malformation Sessions is to offer the attendee the current state-of-the-art multi-disciplinary endovascular and surgical approaches to accurately diagnose and optimally treat all types of vascular malformations (AVMs, AVFs, venous malformations, lymphatic malformations, capillary-venous malformations, and mixed lesions) in all the various problematic anatomies in which they occur. Being that there are controversies regarding the various treatment strategies regarding vascular malformations, the attendees will be exposed to those multiple approaches and philosophies regarding the science, the multiple management strategies, the results, the long-term outcomes, and the complications inherent in the various palliative and curative treatments proffered by international experts.
Improving Outcomes in Hemodialysis Access
(Sessions 106-110; Saturday; 7:55 a.m. – 4:00 p.m.) Registration Begins At 6:00 a.m.
Location: Grand Ballroom West, 3rd Floor
The Hemodialysis Access sessions on Saturday (Sessions 106 – 110) will include presentations on planning, optimizing outcomes, political, economic and legal issues, new technologies and an update on clinical issues related to hemodialysis access. Experts will address specific topics including vein preservation and planning for access, use of ultrasound to facilitate cannulation, use of simulators, the role of drug eluting balloons and stents, management of complications including steal syndrome, infections and access hemorrhage, coding for access procedures, pharmacologic and mechanical approaches to improve fistula outcomes, treatment of fistula aneurysms, use of the HeRO graft and many more important topics. The keynote speaker will be Dr. Michael Brescia who will give his unique historical perspective on the development of the AV fistula. This session should be of interest to surgeons, nephrologists, interventionalists, nurses, dialysis technicians and others interested in the care of patients with end stage renal disease.
The Challenging World of the Diagnosis and Treatment of Vascular Malformations: An Orphan Disease that Has Now Come of Age
(Sessions 92-98; Friday, 6:45 a.m. to 1:30 p.m.)
Location: Gramercy Suites East and West, 2nd Floor
Vascular malformations constitute one of the most challenging clinical entities encountered in Vascular Medicine today. They can occur in every anatomy in the body insinuating themselves throughout an organ or tissue. Being totally comprised of vascular structures, any surgical attempt at resection is fraught with potential hemorrhagic complications. Compounding their inherent difficulty in management , they are also very rare entities. The clinical presentations are extremely protean and can range from an asymptomatic birthmark, to fulminant, life-threatening CHF. Attributing any of these extremely varied symptoms that a patient may present with to a vascular malformation can be challenging to the most experienced clinician. Patients typically bounce from clinician to clinician experiencing disappointing outcomes, complications, and recurrence or worsening of their presenting symptoms. Due to their extreme rarity (>1% of the population), it is difficult for clinicians to gain any experience at all in their diagnosis and optimal management and make definitive statements.
The purpose of these Vascular Malformation Sessions is to offer the attendee the current state-of-the-art multi-disciplinary endovascular and surgical approaches to accurately diagnose and optimally treat all types of vascular malformations (AVMs, AVFs, venous malformations, lymphatic malformations, capillary-venous malformations, and mixed lesions) in all the various problematic anatomies in which they occur. Being that there are controversies regarding the various treatment strategies regarding vascular malformations, the attendees will be exposed to those multiple approaches and philosophies regarding the science, the multiple management strategies, the results, the long-term outcomes, and the complications inherent in the various palliative and curative treatments proffered by international experts.
Improving Outcomes in Hemodialysis Access
(Sessions 106-110; Saturday; 7:55 a.m. – 4:00 p.m.) Registration Begins At 6:00 a.m.
Location: Grand Ballroom West, 3rd Floor
The Hemodialysis Access sessions on Saturday (Sessions 106 – 110) will include presentations on planning, optimizing outcomes, political, economic and legal issues, new technologies and an update on clinical issues related to hemodialysis access. Experts will address specific topics including vein preservation and planning for access, use of ultrasound to facilitate cannulation, use of simulators, the role of drug eluting balloons and stents, management of complications including steal syndrome, infections and access hemorrhage, coding for access procedures, pharmacologic and mechanical approaches to improve fistula outcomes, treatment of fistula aneurysms, use of the HeRO graft and many more important topics. The keynote speaker will be Dr. Michael Brescia who will give his unique historical perspective on the development of the AV fistula. This session should be of interest to surgeons, nephrologists, interventionalists, nurses, dialysis technicians and others interested in the care of patients with end stage renal disease.
Autoimmune Progesterone Dermatitis Presenting With Purpura
To the Editor:
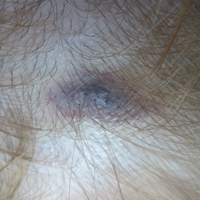
A 32-year-old woman presented with a recurrent painful eruption on the scalp of 1 year's duration. The lesion occurred on the left temporal region 1 week prior to menstruation and spontaneously resolved following menses; it recurred every month for 1 year. She had no notable medical history. She had taken oral contraceptive pills for 4 years and stopped 2 years prior to the development of the lesions. Dermatologic examination revealed a purple-colored, violaceous, centrally elevated, painful plaque that measured 2 cm in diameter in the left temporal region of the scalp (Figure, A). Laboratory test results were within reference range. The lesion spontaneously resolved with mild residual erythema at a follow-up visit after menstruation (Figure, B).
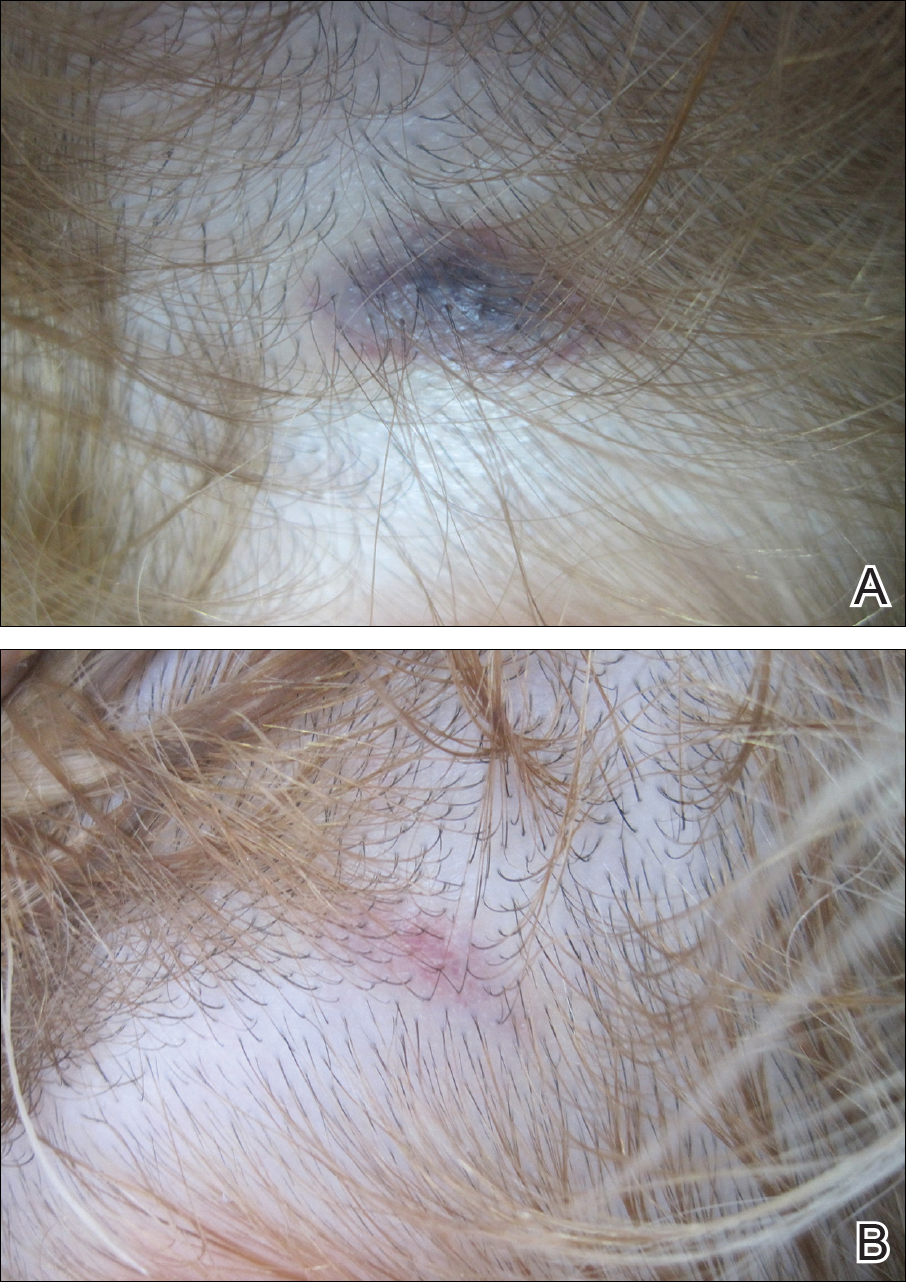
Because the eruption occurred and relapsed with the patient's menstrual cycle, we suspected progesterone hypersensitivity. An intradermal skin test was performed on the forearm with 0.05 mL of medroxyprogesterone acetate, and saline was used as a negative control. An indurated erythematous nodule occurred on the progesterone-treated side within 6 hours. Based on these findings and the patient's history, she was diagnosed with autoimmune progesterone dermatitis (APD). We recommended her to use gonadotropin-releasing hormone agonists as treatment, but the patient refused. At 6-month follow-up she had recurrent lesions but did not report any concerns.
Autoimmune progesterone dermatitis is a rare condition that is characterized by cyclical skin eruptions, typically occurring in the luteal phase of the menstrual cycle with spontaneous resolution after menses.1,2 It was first described by Geber3 in a patient with cyclical urticarial lesions. In 1964, Shelley et al4 characterized APD in a 27-year-old woman with a pruritic vesicular eruption with cyclical premenstrual exacerbations. Although it is believed there is no genetic predisposition to APD, a case series involving 3 sisters demonstrated that genetic susceptibility might play a role in the etiology.5 The etiology of APD is still unknown. It is thought to represent an autoimmune reaction to endogenous or exogenous progesterone.1 Our patient also had used oral contraceptives for 4 years and this exogenous progesterone might have played a role in the sensitization of the patient and the development of this autoimmune reaction.
The clinical features of APD usually begin 3 to 10 days prior to menstruation and end 1 to 2 days after menses. Autoimmune progesterone dermatitis can present in a variety of forms including eczema, erythema multiforme, erythema annulare centrifugum, fixed drug eruption, stomatitis, folliculitis, urticaria, and angioedema.6 A case of APD presenting with petechiae and purpura has been reported.7 There are no specific histologic findings for APD.8 Demonstration of progesterone sensitivity with a progesterone challenge test is the mainstay of diagnosis. Immediate urticaria may occur in some patients, with others experiencing a delayed reaction peaking at 24 to 96 hours.9 The main criteria of APD include the following: recurrent cyclic lesions related to the menstrual cycle; positive intradermal progesterone skin test; and prevention of lesions by inhibiting ovulation.1 Two of these criteria were positive in our patient, but we did not use any medications to prevent ovulation at the patient's request.
Current treatment modalities often attempt to inhibit the secretion of endogenous progesterone by suppressing ovulation. Oral contraceptives and conjugated estrogens have limited efficacy rates.8 Gonadotropin-releasing hormone agonists (ie, buserelin, triptorelin) have been used with success.1,6 Tamoxifen and danazol are other treatment options. For cases refractory to medical treatments, bilateral oophorectomy can be considered a definitive treatment.6
Autoimmune progesterone dermatitis may present in many different clinical forms. It should be considered in the differential diagnosis in patients with recurrent skin lesions related to menstrual cycle both in women of childbearing age and in men taking synthetic progesterone.
- Lee MK, Lee WY, Yong SJ, et al. A case of autoimmune progesterone dermatitis misdiagnosed as allergic contact dermatitis. Allergy Asthma Immunol Res. 2011;3:141-144.
- García-Ortega P, Scorza E. Progesterone autoimmune dermatitis with positive autologous serum skin test result. Obstet Gynecol. 2011;117:495-498.
- Geber J. Desensitization in the treatment of menstrual intoxication and other allergic symptoms. Br J Dermatol. 1930;51:265-268.
- Shelley WB, Preucel RW, Spoont SS. Autoimmune progesterone dermatitis: cure by oophorectomy. JAMA. 1964;190:35-38.
- Chawla SV, Quirk C, Sondheimer SJ, et al. Autoimmune progesterone dermatitis. Arch Dermatol. 2009;145:341-342.
- Medeiros S, Rodrigues-Alves R, Costa M, et al. Autoimmune progesterone dermatitis: treatment with oophorectomy. Clin Exp Dermatol. 2010;35:e12-e13.
- Wintzen M, Goor-van Egmond MB, Noz KC. Autoimmune progesterone dermatitis presenting with purpura and petechiae. Clin Exp Dermatol. 2004;29:316.
- Baptist AP, Baldwin JL. Autoimmune progesterone dermatitis in a patient with endometriosis: case report and review of the literature. Clin Mol Allergy. 2004;2:10.
- Le K, Wood G. A case of autoimmune progesterone dermatitis diagnosed by progesterone pessary. Australas J Dermatol. 2011;52:139-141.
To the Editor:
A 32-year-old woman presented with a recurrent painful eruption on the scalp of 1 year's duration. The lesion occurred on the left temporal region 1 week prior to menstruation and spontaneously resolved following menses; it recurred every month for 1 year. She had no notable medical history. She had taken oral contraceptive pills for 4 years and stopped 2 years prior to the development of the lesions. Dermatologic examination revealed a purple-colored, violaceous, centrally elevated, painful plaque that measured 2 cm in diameter in the left temporal region of the scalp (Figure, A). Laboratory test results were within reference range. The lesion spontaneously resolved with mild residual erythema at a follow-up visit after menstruation (Figure, B).

Because the eruption occurred and relapsed with the patient's menstrual cycle, we suspected progesterone hypersensitivity. An intradermal skin test was performed on the forearm with 0.05 mL of medroxyprogesterone acetate, and saline was used as a negative control. An indurated erythematous nodule occurred on the progesterone-treated side within 6 hours. Based on these findings and the patient's history, she was diagnosed with autoimmune progesterone dermatitis (APD). We recommended her to use gonadotropin-releasing hormone agonists as treatment, but the patient refused. At 6-month follow-up she had recurrent lesions but did not report any concerns.
Autoimmune progesterone dermatitis is a rare condition that is characterized by cyclical skin eruptions, typically occurring in the luteal phase of the menstrual cycle with spontaneous resolution after menses.1,2 It was first described by Geber3 in a patient with cyclical urticarial lesions. In 1964, Shelley et al4 characterized APD in a 27-year-old woman with a pruritic vesicular eruption with cyclical premenstrual exacerbations. Although it is believed there is no genetic predisposition to APD, a case series involving 3 sisters demonstrated that genetic susceptibility might play a role in the etiology.5 The etiology of APD is still unknown. It is thought to represent an autoimmune reaction to endogenous or exogenous progesterone.1 Our patient also had used oral contraceptives for 4 years and this exogenous progesterone might have played a role in the sensitization of the patient and the development of this autoimmune reaction.
The clinical features of APD usually begin 3 to 10 days prior to menstruation and end 1 to 2 days after menses. Autoimmune progesterone dermatitis can present in a variety of forms including eczema, erythema multiforme, erythema annulare centrifugum, fixed drug eruption, stomatitis, folliculitis, urticaria, and angioedema.6 A case of APD presenting with petechiae and purpura has been reported.7 There are no specific histologic findings for APD.8 Demonstration of progesterone sensitivity with a progesterone challenge test is the mainstay of diagnosis. Immediate urticaria may occur in some patients, with others experiencing a delayed reaction peaking at 24 to 96 hours.9 The main criteria of APD include the following: recurrent cyclic lesions related to the menstrual cycle; positive intradermal progesterone skin test; and prevention of lesions by inhibiting ovulation.1 Two of these criteria were positive in our patient, but we did not use any medications to prevent ovulation at the patient's request.
Current treatment modalities often attempt to inhibit the secretion of endogenous progesterone by suppressing ovulation. Oral contraceptives and conjugated estrogens have limited efficacy rates.8 Gonadotropin-releasing hormone agonists (ie, buserelin, triptorelin) have been used with success.1,6 Tamoxifen and danazol are other treatment options. For cases refractory to medical treatments, bilateral oophorectomy can be considered a definitive treatment.6
Autoimmune progesterone dermatitis may present in many different clinical forms. It should be considered in the differential diagnosis in patients with recurrent skin lesions related to menstrual cycle both in women of childbearing age and in men taking synthetic progesterone.
To the Editor:
A 32-year-old woman presented with a recurrent painful eruption on the scalp of 1 year's duration. The lesion occurred on the left temporal region 1 week prior to menstruation and spontaneously resolved following menses; it recurred every month for 1 year. She had no notable medical history. She had taken oral contraceptive pills for 4 years and stopped 2 years prior to the development of the lesions. Dermatologic examination revealed a purple-colored, violaceous, centrally elevated, painful plaque that measured 2 cm in diameter in the left temporal region of the scalp (Figure, A). Laboratory test results were within reference range. The lesion spontaneously resolved with mild residual erythema at a follow-up visit after menstruation (Figure, B).

Because the eruption occurred and relapsed with the patient's menstrual cycle, we suspected progesterone hypersensitivity. An intradermal skin test was performed on the forearm with 0.05 mL of medroxyprogesterone acetate, and saline was used as a negative control. An indurated erythematous nodule occurred on the progesterone-treated side within 6 hours. Based on these findings and the patient's history, she was diagnosed with autoimmune progesterone dermatitis (APD). We recommended her to use gonadotropin-releasing hormone agonists as treatment, but the patient refused. At 6-month follow-up she had recurrent lesions but did not report any concerns.
Autoimmune progesterone dermatitis is a rare condition that is characterized by cyclical skin eruptions, typically occurring in the luteal phase of the menstrual cycle with spontaneous resolution after menses.1,2 It was first described by Geber3 in a patient with cyclical urticarial lesions. In 1964, Shelley et al4 characterized APD in a 27-year-old woman with a pruritic vesicular eruption with cyclical premenstrual exacerbations. Although it is believed there is no genetic predisposition to APD, a case series involving 3 sisters demonstrated that genetic susceptibility might play a role in the etiology.5 The etiology of APD is still unknown. It is thought to represent an autoimmune reaction to endogenous or exogenous progesterone.1 Our patient also had used oral contraceptives for 4 years and this exogenous progesterone might have played a role in the sensitization of the patient and the development of this autoimmune reaction.
The clinical features of APD usually begin 3 to 10 days prior to menstruation and end 1 to 2 days after menses. Autoimmune progesterone dermatitis can present in a variety of forms including eczema, erythema multiforme, erythema annulare centrifugum, fixed drug eruption, stomatitis, folliculitis, urticaria, and angioedema.6 A case of APD presenting with petechiae and purpura has been reported.7 There are no specific histologic findings for APD.8 Demonstration of progesterone sensitivity with a progesterone challenge test is the mainstay of diagnosis. Immediate urticaria may occur in some patients, with others experiencing a delayed reaction peaking at 24 to 96 hours.9 The main criteria of APD include the following: recurrent cyclic lesions related to the menstrual cycle; positive intradermal progesterone skin test; and prevention of lesions by inhibiting ovulation.1 Two of these criteria were positive in our patient, but we did not use any medications to prevent ovulation at the patient's request.
Current treatment modalities often attempt to inhibit the secretion of endogenous progesterone by suppressing ovulation. Oral contraceptives and conjugated estrogens have limited efficacy rates.8 Gonadotropin-releasing hormone agonists (ie, buserelin, triptorelin) have been used with success.1,6 Tamoxifen and danazol are other treatment options. For cases refractory to medical treatments, bilateral oophorectomy can be considered a definitive treatment.6
Autoimmune progesterone dermatitis may present in many different clinical forms. It should be considered in the differential diagnosis in patients with recurrent skin lesions related to menstrual cycle both in women of childbearing age and in men taking synthetic progesterone.
- Lee MK, Lee WY, Yong SJ, et al. A case of autoimmune progesterone dermatitis misdiagnosed as allergic contact dermatitis. Allergy Asthma Immunol Res. 2011;3:141-144.
- García-Ortega P, Scorza E. Progesterone autoimmune dermatitis with positive autologous serum skin test result. Obstet Gynecol. 2011;117:495-498.
- Geber J. Desensitization in the treatment of menstrual intoxication and other allergic symptoms. Br J Dermatol. 1930;51:265-268.
- Shelley WB, Preucel RW, Spoont SS. Autoimmune progesterone dermatitis: cure by oophorectomy. JAMA. 1964;190:35-38.
- Chawla SV, Quirk C, Sondheimer SJ, et al. Autoimmune progesterone dermatitis. Arch Dermatol. 2009;145:341-342.
- Medeiros S, Rodrigues-Alves R, Costa M, et al. Autoimmune progesterone dermatitis: treatment with oophorectomy. Clin Exp Dermatol. 2010;35:e12-e13.
- Wintzen M, Goor-van Egmond MB, Noz KC. Autoimmune progesterone dermatitis presenting with purpura and petechiae. Clin Exp Dermatol. 2004;29:316.
- Baptist AP, Baldwin JL. Autoimmune progesterone dermatitis in a patient with endometriosis: case report and review of the literature. Clin Mol Allergy. 2004;2:10.
- Le K, Wood G. A case of autoimmune progesterone dermatitis diagnosed by progesterone pessary. Australas J Dermatol. 2011;52:139-141.
- Lee MK, Lee WY, Yong SJ, et al. A case of autoimmune progesterone dermatitis misdiagnosed as allergic contact dermatitis. Allergy Asthma Immunol Res. 2011;3:141-144.
- García-Ortega P, Scorza E. Progesterone autoimmune dermatitis with positive autologous serum skin test result. Obstet Gynecol. 2011;117:495-498.
- Geber J. Desensitization in the treatment of menstrual intoxication and other allergic symptoms. Br J Dermatol. 1930;51:265-268.
- Shelley WB, Preucel RW, Spoont SS. Autoimmune progesterone dermatitis: cure by oophorectomy. JAMA. 1964;190:35-38.
- Chawla SV, Quirk C, Sondheimer SJ, et al. Autoimmune progesterone dermatitis. Arch Dermatol. 2009;145:341-342.
- Medeiros S, Rodrigues-Alves R, Costa M, et al. Autoimmune progesterone dermatitis: treatment with oophorectomy. Clin Exp Dermatol. 2010;35:e12-e13.
- Wintzen M, Goor-van Egmond MB, Noz KC. Autoimmune progesterone dermatitis presenting with purpura and petechiae. Clin Exp Dermatol. 2004;29:316.
- Baptist AP, Baldwin JL. Autoimmune progesterone dermatitis in a patient with endometriosis: case report and review of the literature. Clin Mol Allergy. 2004;2:10.
- Le K, Wood G. A case of autoimmune progesterone dermatitis diagnosed by progesterone pessary. Australas J Dermatol. 2011;52:139-141.
Practice Points
- Autoimmune progesterone dermatitis is characterized by cyclical skin eruptions, typically occurring in the second half of the menstrual cycle.
- Autoimmune progesterone dermatitis is thought to be an autoimmune reaction to endogenous or exogenous progesterone.
- This condition should be considered in female patients with recurrent skin lesions related to their menstrual cycle.
Combine qSOFA and SIRS for best sepsis score
LOS ANGELES – Instead of replacing the Systemic Inflammatory Response Syndrome (SIRS) score with the new quick Sequential Organ Failure Assessment (qSOFA) score to identify severe sepsis patients, it might be best to use both, according to two studies presented at the American College of Chest Physicians annual meeting.
The gold standard 3rd International Consensus Definitions for Sepsis and Septic Shock Task Force recently introduced qSOFA to replace SIRS, in part because SIRS is too sensitive. With criteria that include a temperature above 38° C; a heart rate above 90 bpm, and a respiratory rate above 20 breaths per minute, it’s possible to score positive on SIRS by walking up a flight of stairs, audience members at the study presentations noted.
The first study at the meeting session – a prospective cohort of 152 patients scored by both systems within 8 hours of ICU admission at the New York–Presbyterian Hospital – found that qSOFA was slightly better at predicting in-hospital mortality and ICU-free days, but no better than SIRS at predicting ventilator- or organ failure–free days.
However, of the 36% of patients (55) who met only one of the three qSOFA criteria - a respiratory rate of 22 breaths per minute, altered mental status, or a systolic blood pressure of 100 mg Hg or less - 6% (3) died in the hospital. Of those patients, two-thirds (2) were SIRS positive, meaning that they met two or more SIRS criteria.
“Having a borderline qSOFA of 1 point, which is considered negative, with the addition of having SIRS criteria, should raise concerns that patients need further evaluation. SIRS criteria should not be [entirely] discarded” in favor of qSOFA, said lead investigator Eli Finkelsztein, MD, of the New York–Presbyterian Hospital in New York City
The second study – a review of 6,811 severe sepsis/septic shock patients scored by both systems within 3 hours of emergency department admission at the University of Kansas Hospital emergency department in Kansas City – found that the two scores performed largely the same when it came to predicting ICU admission and 30-day mortality, but that people who met two or more criteria in both systems were of special concern.
Twenty-five percent of patients (1,713) scored 2 or more on both SIRS and qSOFA. These patients were more likely to be admitted to the ICU and be readmitted to the hospital after a month, compared with those patients who were positive in only one scoring system or negative in both. Additional factors associated with these patients were that they had the longest ICU and hospital lengths of stay. Two hundred (12%) of these patients scoring 2 or more on both SIRS and qSOFA died within 30 days.
“SIRS criteria continue to be more sensitive at identifying severe sepsis, but they are equally as accurate [as qSOFA criteria] at predicting adverse patient outcomes,” said lead investigator and Kansas University medical student Amanda Deis.
SIRS and qSOFA take only a few seconds to assess at the bedside. Using both builds “a clinical picture,” she said.
There was no industry funding for the work, and the investigators had no relevant financial disclosures.
Everybody got fed up with SIRS because it’s overly sensitive, but now we’ve swung in the other direction. It’s absolutely true that qSOFA is more specific, but one of the presenters had a 6% rate of qSOFA missing sick patients.
We want to be somewhere in the middle in terms of not missing too many of these cases. I thought 6% was reasonable, but others may not.
Zaza Cohen, MD, is the director of critical care at Mountainside Hospital in Montclair, N.J. He moderated - but was not involved with - the two studies.
Everybody got fed up with SIRS because it’s overly sensitive, but now we’ve swung in the other direction. It’s absolutely true that qSOFA is more specific, but one of the presenters had a 6% rate of qSOFA missing sick patients.
We want to be somewhere in the middle in terms of not missing too many of these cases. I thought 6% was reasonable, but others may not.
Zaza Cohen, MD, is the director of critical care at Mountainside Hospital in Montclair, N.J. He moderated - but was not involved with - the two studies.
Everybody got fed up with SIRS because it’s overly sensitive, but now we’ve swung in the other direction. It’s absolutely true that qSOFA is more specific, but one of the presenters had a 6% rate of qSOFA missing sick patients.
We want to be somewhere in the middle in terms of not missing too many of these cases. I thought 6% was reasonable, but others may not.
Zaza Cohen, MD, is the director of critical care at Mountainside Hospital in Montclair, N.J. He moderated - but was not involved with - the two studies.
LOS ANGELES – Instead of replacing the Systemic Inflammatory Response Syndrome (SIRS) score with the new quick Sequential Organ Failure Assessment (qSOFA) score to identify severe sepsis patients, it might be best to use both, according to two studies presented at the American College of Chest Physicians annual meeting.
The gold standard 3rd International Consensus Definitions for Sepsis and Septic Shock Task Force recently introduced qSOFA to replace SIRS, in part because SIRS is too sensitive. With criteria that include a temperature above 38° C; a heart rate above 90 bpm, and a respiratory rate above 20 breaths per minute, it’s possible to score positive on SIRS by walking up a flight of stairs, audience members at the study presentations noted.
The first study at the meeting session – a prospective cohort of 152 patients scored by both systems within 8 hours of ICU admission at the New York–Presbyterian Hospital – found that qSOFA was slightly better at predicting in-hospital mortality and ICU-free days, but no better than SIRS at predicting ventilator- or organ failure–free days.
However, of the 36% of patients (55) who met only one of the three qSOFA criteria - a respiratory rate of 22 breaths per minute, altered mental status, or a systolic blood pressure of 100 mg Hg or less - 6% (3) died in the hospital. Of those patients, two-thirds (2) were SIRS positive, meaning that they met two or more SIRS criteria.
“Having a borderline qSOFA of 1 point, which is considered negative, with the addition of having SIRS criteria, should raise concerns that patients need further evaluation. SIRS criteria should not be [entirely] discarded” in favor of qSOFA, said lead investigator Eli Finkelsztein, MD, of the New York–Presbyterian Hospital in New York City
The second study – a review of 6,811 severe sepsis/septic shock patients scored by both systems within 3 hours of emergency department admission at the University of Kansas Hospital emergency department in Kansas City – found that the two scores performed largely the same when it came to predicting ICU admission and 30-day mortality, but that people who met two or more criteria in both systems were of special concern.
Twenty-five percent of patients (1,713) scored 2 or more on both SIRS and qSOFA. These patients were more likely to be admitted to the ICU and be readmitted to the hospital after a month, compared with those patients who were positive in only one scoring system or negative in both. Additional factors associated with these patients were that they had the longest ICU and hospital lengths of stay. Two hundred (12%) of these patients scoring 2 or more on both SIRS and qSOFA died within 30 days.
“SIRS criteria continue to be more sensitive at identifying severe sepsis, but they are equally as accurate [as qSOFA criteria] at predicting adverse patient outcomes,” said lead investigator and Kansas University medical student Amanda Deis.
SIRS and qSOFA take only a few seconds to assess at the bedside. Using both builds “a clinical picture,” she said.
There was no industry funding for the work, and the investigators had no relevant financial disclosures.
LOS ANGELES – Instead of replacing the Systemic Inflammatory Response Syndrome (SIRS) score with the new quick Sequential Organ Failure Assessment (qSOFA) score to identify severe sepsis patients, it might be best to use both, according to two studies presented at the American College of Chest Physicians annual meeting.
The gold standard 3rd International Consensus Definitions for Sepsis and Septic Shock Task Force recently introduced qSOFA to replace SIRS, in part because SIRS is too sensitive. With criteria that include a temperature above 38° C; a heart rate above 90 bpm, and a respiratory rate above 20 breaths per minute, it’s possible to score positive on SIRS by walking up a flight of stairs, audience members at the study presentations noted.
The first study at the meeting session – a prospective cohort of 152 patients scored by both systems within 8 hours of ICU admission at the New York–Presbyterian Hospital – found that qSOFA was slightly better at predicting in-hospital mortality and ICU-free days, but no better than SIRS at predicting ventilator- or organ failure–free days.
However, of the 36% of patients (55) who met only one of the three qSOFA criteria - a respiratory rate of 22 breaths per minute, altered mental status, or a systolic blood pressure of 100 mg Hg or less - 6% (3) died in the hospital. Of those patients, two-thirds (2) were SIRS positive, meaning that they met two or more SIRS criteria.
“Having a borderline qSOFA of 1 point, which is considered negative, with the addition of having SIRS criteria, should raise concerns that patients need further evaluation. SIRS criteria should not be [entirely] discarded” in favor of qSOFA, said lead investigator Eli Finkelsztein, MD, of the New York–Presbyterian Hospital in New York City
The second study – a review of 6,811 severe sepsis/septic shock patients scored by both systems within 3 hours of emergency department admission at the University of Kansas Hospital emergency department in Kansas City – found that the two scores performed largely the same when it came to predicting ICU admission and 30-day mortality, but that people who met two or more criteria in both systems were of special concern.
Twenty-five percent of patients (1,713) scored 2 or more on both SIRS and qSOFA. These patients were more likely to be admitted to the ICU and be readmitted to the hospital after a month, compared with those patients who were positive in only one scoring system or negative in both. Additional factors associated with these patients were that they had the longest ICU and hospital lengths of stay. Two hundred (12%) of these patients scoring 2 or more on both SIRS and qSOFA died within 30 days.
“SIRS criteria continue to be more sensitive at identifying severe sepsis, but they are equally as accurate [as qSOFA criteria] at predicting adverse patient outcomes,” said lead investigator and Kansas University medical student Amanda Deis.
SIRS and qSOFA take only a few seconds to assess at the bedside. Using both builds “a clinical picture,” she said.
There was no industry funding for the work, and the investigators had no relevant financial disclosures.
AT CHEST 2016
Key clinical point:
Major finding: Of the 36% of patients who met only one of the three qSOFA criteria, 6% died in the hospital. Of those patients, two-thirds were SIRS positive, meaning that they met two or more SIRS criteria.
Data source: Two studies of almost 7,000 septic patients.
Disclosures: There was no industry funding for the work, and the investigators had no relevant financial disclosures.