User login
Blood test can predict outcomes in DLBCL, team says

Photo by Juan D. Alfonso
A blood test can reveal genetic features linked to outcomes in patients with diffuse large B-cell lymphoma (DLBCL), according to research published in Science Translational Medicine.
Investigators used targeted sequencing to analyze circulating tumor DNA (ctDNA) in blood samples from DLBCL patients.
This allowed the team to identify the cell of origin, detect minimal residual disease (MRD), and predict progression-free survival (PFS) in these patients.
Florian Scherer, MD, of Stanford University in California, and his colleagues conducted this research.
They used cancer personalized profiling by deep sequencing (CAPP-Seq) to analyze tumor biopsies and cell-free DNA samples from 92 patients with DLBCL and 24 healthy controls.
The investigators found that CAPP-Seq could effectively detect somatic mutations in DLBCL plasma samples as well as tumor biopsies. They said their results suggest ctDNA is a “robust surrogate for direct assessment of primary tumor genotypes” in most DLBCL patients.
In addition, ctDNA profiling with CAPP-Seq revealed mutations associated with resistance to the BTK inhibitor ibrutinib.
The investigators also said their results suggest ctDNA profiling can be used to classify DLBCL subtypes. The overall concordance in cell of origin predictions between tumor tissue and plasma genotyping was 88%.
Another key finding of this study is that the amount of ctDNA at DLBCL diagnosis was predictive of PFS. The investigators said higher ctDNA levels at diagnosis were “continuously and independently” correlated with inferior PFS.
Dr Scherer and his colleagues also discovered that ctDNA profiling could detect MRD with greater accuracy than immunoglobulin sequencing and radiographic imaging. And patients with ctDNA in their plasma had significantly worse PFS than patients with undetectable ctDNA.
Finally, the investigators found evidence to suggest that ctDNA profiling could provide early detection of disease transformation. They identified “distinct patterns of clonal evolution” by which they could distinguish indolent follicular lymphomas from follicular lymphomas that transformed into DLBCL. ![]()

Photo by Juan D. Alfonso
A blood test can reveal genetic features linked to outcomes in patients with diffuse large B-cell lymphoma (DLBCL), according to research published in Science Translational Medicine.
Investigators used targeted sequencing to analyze circulating tumor DNA (ctDNA) in blood samples from DLBCL patients.
This allowed the team to identify the cell of origin, detect minimal residual disease (MRD), and predict progression-free survival (PFS) in these patients.
Florian Scherer, MD, of Stanford University in California, and his colleagues conducted this research.
They used cancer personalized profiling by deep sequencing (CAPP-Seq) to analyze tumor biopsies and cell-free DNA samples from 92 patients with DLBCL and 24 healthy controls.
The investigators found that CAPP-Seq could effectively detect somatic mutations in DLBCL plasma samples as well as tumor biopsies. They said their results suggest ctDNA is a “robust surrogate for direct assessment of primary tumor genotypes” in most DLBCL patients.
In addition, ctDNA profiling with CAPP-Seq revealed mutations associated with resistance to the BTK inhibitor ibrutinib.
The investigators also said their results suggest ctDNA profiling can be used to classify DLBCL subtypes. The overall concordance in cell of origin predictions between tumor tissue and plasma genotyping was 88%.
Another key finding of this study is that the amount of ctDNA at DLBCL diagnosis was predictive of PFS. The investigators said higher ctDNA levels at diagnosis were “continuously and independently” correlated with inferior PFS.
Dr Scherer and his colleagues also discovered that ctDNA profiling could detect MRD with greater accuracy than immunoglobulin sequencing and radiographic imaging. And patients with ctDNA in their plasma had significantly worse PFS than patients with undetectable ctDNA.
Finally, the investigators found evidence to suggest that ctDNA profiling could provide early detection of disease transformation. They identified “distinct patterns of clonal evolution” by which they could distinguish indolent follicular lymphomas from follicular lymphomas that transformed into DLBCL. ![]()

Photo by Juan D. Alfonso
A blood test can reveal genetic features linked to outcomes in patients with diffuse large B-cell lymphoma (DLBCL), according to research published in Science Translational Medicine.
Investigators used targeted sequencing to analyze circulating tumor DNA (ctDNA) in blood samples from DLBCL patients.
This allowed the team to identify the cell of origin, detect minimal residual disease (MRD), and predict progression-free survival (PFS) in these patients.
Florian Scherer, MD, of Stanford University in California, and his colleagues conducted this research.
They used cancer personalized profiling by deep sequencing (CAPP-Seq) to analyze tumor biopsies and cell-free DNA samples from 92 patients with DLBCL and 24 healthy controls.
The investigators found that CAPP-Seq could effectively detect somatic mutations in DLBCL plasma samples as well as tumor biopsies. They said their results suggest ctDNA is a “robust surrogate for direct assessment of primary tumor genotypes” in most DLBCL patients.
In addition, ctDNA profiling with CAPP-Seq revealed mutations associated with resistance to the BTK inhibitor ibrutinib.
The investigators also said their results suggest ctDNA profiling can be used to classify DLBCL subtypes. The overall concordance in cell of origin predictions between tumor tissue and plasma genotyping was 88%.
Another key finding of this study is that the amount of ctDNA at DLBCL diagnosis was predictive of PFS. The investigators said higher ctDNA levels at diagnosis were “continuously and independently” correlated with inferior PFS.
Dr Scherer and his colleagues also discovered that ctDNA profiling could detect MRD with greater accuracy than immunoglobulin sequencing and radiographic imaging. And patients with ctDNA in their plasma had significantly worse PFS than patients with undetectable ctDNA.
Finally, the investigators found evidence to suggest that ctDNA profiling could provide early detection of disease transformation. They identified “distinct patterns of clonal evolution” by which they could distinguish indolent follicular lymphomas from follicular lymphomas that transformed into DLBCL. ![]()
Ebola Treatment Is Promising—But Not Definitively Better
The experimental Ebola treatment ZMapp, which is composed of 3 different monoclonal antibodies, prevents progression of Ebola virus disease by targeting the main surface protein of the virus. According to findings from the clinical trial PREVAIL II, ZMapp is safe and well tolerated. But because the Ebola epidemic is “waning,” NIH says, the study enrolled too few people to determine definitively whether it is a better treatment than the best available standard of care.
Related: Novel Treatment for Ebola Virus
The study involved 72 men and women with confirmed infection. However, the researchers closed the study early because they could not enroll the target number of 200 participants due to the decline in cases. All patients received the optimized standard of care—IV fluids, electrolyte balance, maintaining oxygen and blood pressure levels—and half also received 3 IV infusions of ZMapp 3 days apart.
At 28 days, 13 of the 35 patients (37%) in the standard care group had died, compared with 8 of 36 (22%) in the ZMapp group. That difference, a 40% lower risk of death with ZMapp, still did not reach statistical significance.
Related: Ebola Virus Persists in Semen Long Term
The findings are “promising and provide valuable scientific data,” says Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases. Moreover, he adds, “Importantly, the study establishes that it is feasible to conduct a randomized, controlled trial during a major public health emergency in a scientifically and ethically sound manner.”
The experimental Ebola treatment ZMapp, which is composed of 3 different monoclonal antibodies, prevents progression of Ebola virus disease by targeting the main surface protein of the virus. According to findings from the clinical trial PREVAIL II, ZMapp is safe and well tolerated. But because the Ebola epidemic is “waning,” NIH says, the study enrolled too few people to determine definitively whether it is a better treatment than the best available standard of care.
Related: Novel Treatment for Ebola Virus
The study involved 72 men and women with confirmed infection. However, the researchers closed the study early because they could not enroll the target number of 200 participants due to the decline in cases. All patients received the optimized standard of care—IV fluids, electrolyte balance, maintaining oxygen and blood pressure levels—and half also received 3 IV infusions of ZMapp 3 days apart.
At 28 days, 13 of the 35 patients (37%) in the standard care group had died, compared with 8 of 36 (22%) in the ZMapp group. That difference, a 40% lower risk of death with ZMapp, still did not reach statistical significance.
Related: Ebola Virus Persists in Semen Long Term
The findings are “promising and provide valuable scientific data,” says Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases. Moreover, he adds, “Importantly, the study establishes that it is feasible to conduct a randomized, controlled trial during a major public health emergency in a scientifically and ethically sound manner.”
The experimental Ebola treatment ZMapp, which is composed of 3 different monoclonal antibodies, prevents progression of Ebola virus disease by targeting the main surface protein of the virus. According to findings from the clinical trial PREVAIL II, ZMapp is safe and well tolerated. But because the Ebola epidemic is “waning,” NIH says, the study enrolled too few people to determine definitively whether it is a better treatment than the best available standard of care.
Related: Novel Treatment for Ebola Virus
The study involved 72 men and women with confirmed infection. However, the researchers closed the study early because they could not enroll the target number of 200 participants due to the decline in cases. All patients received the optimized standard of care—IV fluids, electrolyte balance, maintaining oxygen and blood pressure levels—and half also received 3 IV infusions of ZMapp 3 days apart.
At 28 days, 13 of the 35 patients (37%) in the standard care group had died, compared with 8 of 36 (22%) in the ZMapp group. That difference, a 40% lower risk of death with ZMapp, still did not reach statistical significance.
Related: Ebola Virus Persists in Semen Long Term
The findings are “promising and provide valuable scientific data,” says Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases. Moreover, he adds, “Importantly, the study establishes that it is feasible to conduct a randomized, controlled trial during a major public health emergency in a scientifically and ethically sound manner.”
Rash for 20 years
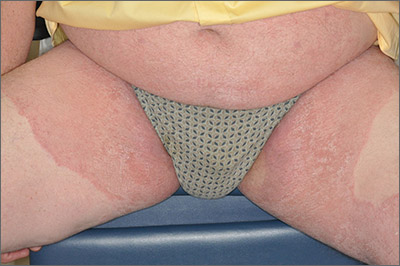
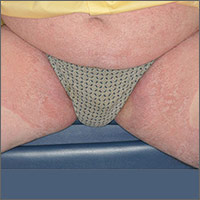
Based on the pattern of the rash and the patient’s history, the family physician (FP) considered tinea corporis and cruris, but the history of failing treatment seemed unusual. The FP also considered a diagnosis of pityriasis rubra pilaris because he observed a “skip” area on the left thigh.
The FP performed a potassium hydroxide (KOH) preparation using a fungal stain and found branching septate hyphae. (See video on how to perform a KOH preparation here.) He also wondered if the failed treatment was secondary to inadequate dosing or duration of the oral medicines previously used, given that the patient was 6 feet, 5 inches tall and weighed more than 250 pounds. The patient didn’t have liver disease and rarely drank alcohol. Baseline liver function tests (LFTs) were within normal limits.
The FP told the patient to use oral terbinafine for one month rather than the recommended 2 weeks. One month later, there was less erythema and scaling, but the rash had not completely resolved. At that time, the FP and patient decided together to do a punch biopsy to make sure the diagnosis was correct. The punch biopsy supported the diagnosis of tinea with a positive periodic acid–Schiff stain for fungal elements; no other pathology was noted.
Since the LFTs were still normal, the FP and patient discussed a second month of treatment. The FP also performed a fungal culture and requested that the lab test the fungus for identification and sensitivities. Two weeks later, the results showed Trichophyton rubrum that was sensitive to all oral antifungal medications tested, including terbinafine.
At this point, the FP became concerned about the patient’s immune system, so he ordered a complete blood count (CBC) and human immunodeficiency virus (HIV) test. The CBC came back normal and the HIV test was negative. At the end of the second month, the fungal infection was still present clinically and the KOH preparation was still positive.
The FP offered oral itraconazole 100 mg/d and the patient was happy to try another therapy. The LFTs remained normal and after one month of itraconazole, the tinea was still present. At this point, the patient decided that he could live with the condition and would use a topical antifungal when the rash was itchy.
This case demonstrates a situation in which the patient’s immune system is “blind” to the foreign fungus. This has been known to happen with human papillomavirus, when patients have warts that do not resolve even with the most aggressive therapies.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Smith M. Tinea cruris. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:795-798.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

Based on the pattern of the rash and the patient’s history, the family physician (FP) considered tinea corporis and cruris, but the history of failing treatment seemed unusual. The FP also considered a diagnosis of pityriasis rubra pilaris because he observed a “skip” area on the left thigh.
The FP performed a potassium hydroxide (KOH) preparation using a fungal stain and found branching septate hyphae. (See video on how to perform a KOH preparation here.) He also wondered if the failed treatment was secondary to inadequate dosing or duration of the oral medicines previously used, given that the patient was 6 feet, 5 inches tall and weighed more than 250 pounds. The patient didn’t have liver disease and rarely drank alcohol. Baseline liver function tests (LFTs) were within normal limits.
The FP told the patient to use oral terbinafine for one month rather than the recommended 2 weeks. One month later, there was less erythema and scaling, but the rash had not completely resolved. At that time, the FP and patient decided together to do a punch biopsy to make sure the diagnosis was correct. The punch biopsy supported the diagnosis of tinea with a positive periodic acid–Schiff stain for fungal elements; no other pathology was noted.
Since the LFTs were still normal, the FP and patient discussed a second month of treatment. The FP also performed a fungal culture and requested that the lab test the fungus for identification and sensitivities. Two weeks later, the results showed Trichophyton rubrum that was sensitive to all oral antifungal medications tested, including terbinafine.
At this point, the FP became concerned about the patient’s immune system, so he ordered a complete blood count (CBC) and human immunodeficiency virus (HIV) test. The CBC came back normal and the HIV test was negative. At the end of the second month, the fungal infection was still present clinically and the KOH preparation was still positive.
The FP offered oral itraconazole 100 mg/d and the patient was happy to try another therapy. The LFTs remained normal and after one month of itraconazole, the tinea was still present. At this point, the patient decided that he could live with the condition and would use a topical antifungal when the rash was itchy.
This case demonstrates a situation in which the patient’s immune system is “blind” to the foreign fungus. This has been known to happen with human papillomavirus, when patients have warts that do not resolve even with the most aggressive therapies.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Smith M. Tinea cruris. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:795-798.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

Based on the pattern of the rash and the patient’s history, the family physician (FP) considered tinea corporis and cruris, but the history of failing treatment seemed unusual. The FP also considered a diagnosis of pityriasis rubra pilaris because he observed a “skip” area on the left thigh.
The FP performed a potassium hydroxide (KOH) preparation using a fungal stain and found branching septate hyphae. (See video on how to perform a KOH preparation here.) He also wondered if the failed treatment was secondary to inadequate dosing or duration of the oral medicines previously used, given that the patient was 6 feet, 5 inches tall and weighed more than 250 pounds. The patient didn’t have liver disease and rarely drank alcohol. Baseline liver function tests (LFTs) were within normal limits.
The FP told the patient to use oral terbinafine for one month rather than the recommended 2 weeks. One month later, there was less erythema and scaling, but the rash had not completely resolved. At that time, the FP and patient decided together to do a punch biopsy to make sure the diagnosis was correct. The punch biopsy supported the diagnosis of tinea with a positive periodic acid–Schiff stain for fungal elements; no other pathology was noted.
Since the LFTs were still normal, the FP and patient discussed a second month of treatment. The FP also performed a fungal culture and requested that the lab test the fungus for identification and sensitivities. Two weeks later, the results showed Trichophyton rubrum that was sensitive to all oral antifungal medications tested, including terbinafine.
At this point, the FP became concerned about the patient’s immune system, so he ordered a complete blood count (CBC) and human immunodeficiency virus (HIV) test. The CBC came back normal and the HIV test was negative. At the end of the second month, the fungal infection was still present clinically and the KOH preparation was still positive.
The FP offered oral itraconazole 100 mg/d and the patient was happy to try another therapy. The LFTs remained normal and after one month of itraconazole, the tinea was still present. At this point, the patient decided that he could live with the condition and would use a topical antifungal when the rash was itchy.
This case demonstrates a situation in which the patient’s immune system is “blind” to the foreign fungus. This has been known to happen with human papillomavirus, when patients have warts that do not resolve even with the most aggressive therapies.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Smith M. Tinea cruris. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:795-798.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
As Girl Grows, Lesions Follow Suit
ANSWER
The correct diagnosis in this case is juvenile xanthogranuloma (JXG; choice “d”).
Anderson-Fabry disease (choice “a”) is a rare inherited disorder characterized by widespread red papules; these lesions, however, are much smaller and far more widespread than those of JXG.
Considered a possibility at initial presentation, molluscum contagiosum (choice “b”) was quickly ruled out upon further inspection. This patient’s condition lacked the typical features of molluscum: umbilicated, white, firm papules caused by a pox virus.
Eruptive xanthomata (choice “c”) is a collection of lipid-laden macrophages caused by hypertriglyceridemia. They present as papules and nodules under, rather than on, the skin.
DISCUSSION
Solitary JXG lesions are fairly common, developing on the trunk, face, or extremities as smooth, reddish brown to cream papules. Typically, they cause no problems—but when multiple lesions manifest at birth, the condition can affect the eye (especially the iris, as in this case).
JXG is considered a form of histiocytosis, specifically classified as a type II non-Langerhans cell-mediated lesion. It is believed to result from a disordered macrophage response to a nonspecific tissue injury, which leads to a distinct variety of granulomatous change. These lesions are part of a spectrum of related conditions that also includes Langerhans cell histiocytosis.
No perfect treatment exists for this patient’s multitudinous skin lesions, because her darker skin could easily be permanently changed by burning, freezing, laser, or other destructive modality. Fair or not, in many cases, insurance coverage (or lack thereof) ultimately dictates what treatment is used.
Once the biopsy confirmed the diagnosis and effectively ruled out the other items in the differential, she was referred to ophthalmology for ongoing care of her eyes. Beyond that, she’ll need an annual physical with labs, because JXG is known to affect internal organs as well.
ANSWER
The correct diagnosis in this case is juvenile xanthogranuloma (JXG; choice “d”).
Anderson-Fabry disease (choice “a”) is a rare inherited disorder characterized by widespread red papules; these lesions, however, are much smaller and far more widespread than those of JXG.
Considered a possibility at initial presentation, molluscum contagiosum (choice “b”) was quickly ruled out upon further inspection. This patient’s condition lacked the typical features of molluscum: umbilicated, white, firm papules caused by a pox virus.
Eruptive xanthomata (choice “c”) is a collection of lipid-laden macrophages caused by hypertriglyceridemia. They present as papules and nodules under, rather than on, the skin.
DISCUSSION
Solitary JXG lesions are fairly common, developing on the trunk, face, or extremities as smooth, reddish brown to cream papules. Typically, they cause no problems—but when multiple lesions manifest at birth, the condition can affect the eye (especially the iris, as in this case).
JXG is considered a form of histiocytosis, specifically classified as a type II non-Langerhans cell-mediated lesion. It is believed to result from a disordered macrophage response to a nonspecific tissue injury, which leads to a distinct variety of granulomatous change. These lesions are part of a spectrum of related conditions that also includes Langerhans cell histiocytosis.
No perfect treatment exists for this patient’s multitudinous skin lesions, because her darker skin could easily be permanently changed by burning, freezing, laser, or other destructive modality. Fair or not, in many cases, insurance coverage (or lack thereof) ultimately dictates what treatment is used.
Once the biopsy confirmed the diagnosis and effectively ruled out the other items in the differential, she was referred to ophthalmology for ongoing care of her eyes. Beyond that, she’ll need an annual physical with labs, because JXG is known to affect internal organs as well.
ANSWER
The correct diagnosis in this case is juvenile xanthogranuloma (JXG; choice “d”).
Anderson-Fabry disease (choice “a”) is a rare inherited disorder characterized by widespread red papules; these lesions, however, are much smaller and far more widespread than those of JXG.
Considered a possibility at initial presentation, molluscum contagiosum (choice “b”) was quickly ruled out upon further inspection. This patient’s condition lacked the typical features of molluscum: umbilicated, white, firm papules caused by a pox virus.
Eruptive xanthomata (choice “c”) is a collection of lipid-laden macrophages caused by hypertriglyceridemia. They present as papules and nodules under, rather than on, the skin.
DISCUSSION
Solitary JXG lesions are fairly common, developing on the trunk, face, or extremities as smooth, reddish brown to cream papules. Typically, they cause no problems—but when multiple lesions manifest at birth, the condition can affect the eye (especially the iris, as in this case).
JXG is considered a form of histiocytosis, specifically classified as a type II non-Langerhans cell-mediated lesion. It is believed to result from a disordered macrophage response to a nonspecific tissue injury, which leads to a distinct variety of granulomatous change. These lesions are part of a spectrum of related conditions that also includes Langerhans cell histiocytosis.
No perfect treatment exists for this patient’s multitudinous skin lesions, because her darker skin could easily be permanently changed by burning, freezing, laser, or other destructive modality. Fair or not, in many cases, insurance coverage (or lack thereof) ultimately dictates what treatment is used.
Once the biopsy confirmed the diagnosis and effectively ruled out the other items in the differential, she was referred to ophthalmology for ongoing care of her eyes. Beyond that, she’ll need an annual physical with labs, because JXG is known to affect internal organs as well.

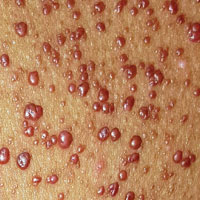
Since shortly after birth, a now 12-year-old African-American girl has had lesions on her trunk. She has never been given a diagnosis and has always been told she would “outgrow the problem.” Instead, the number and distribution of lesions continues to increase, and her pediatrician finally refers her to dermatology for evaluation.
About 150 to 200 nearly identical lesions scatter around the patient’s body, clustered mostly on the left upper back but also on the abdomen and bilateral upper thighs. The fleshy, reddish brown, mushroom-like papules range in size from 2 to 4 mm and exhibit no central umbilication. Two brown spots (each measuring 2 mm) are seen in the iris of the patient’s left eye.
There are no other apparent medical problems to report and no visual deficits. Aside from being unsightly, the lesions are asymptomatic. A shave biopsy of one of them is performed.
Broadly neutralizing antibody VRC01 fails to sustain HIV viral suppression
Passive immunization against HIV using the broadly neutralizing antibody VRC01 is associated with a delay in plasma viral rebound in individuals undergoing interruption of antiretroviral therapy, according to a new study, but the viral suppression is not sustained.
Two open-label trials investigated the impact of different dosing regiments of VRC01 in a total of 24 patients who were taking a break from antiretroviral therapy, according to a paper published Nov. 9 in the New England Journal of Medicine.
Katharine J. Bar, MD, of the Penn Center for AIDS Research at the University of Pennsylvania, Philadelphia, and her coauthors suggested broadly neutralizing antibodies such as VRC01 could target the persistent viral reservoir that leads to rapid viral rebound as soon as antiretroviral therapy is stopped (N Engl J Med. 2016 Nov 9. doi: 10.1056/NEJMoa1608243).
However, in these two studies, VRC01 did not achieve durable viral suppression. Overall, participants in both trials were significantly more likely than historical controls to maintain viral suppression 4 weeks after interrupting antiretroviral therapy (38% and 80% vs. 13%) but this difference was no longer significant by week 8.
In one trial – the A5340 – 12 of the 13 participants with data that could be evaluated showed viral rebound to more than 200 copies/mL by week 8, and in the second NIH trial, the median time to rebound of 40 copies/mL was 39 days.
All participants showed similar plasma levels of VRC01 that had been observed in previous trials – significantly above 50 mcg/mL for 8 weeks in the A5340 trial and above 100 mcg/mL in the NIH trial. Plasma VRC01 levels were consistently above 50 mcg/mL even at the time of viral rebound, except in one patient.
Researchers performed post hoc analyses of the sequence diversity at the time of viral rebound and compared these to samples from eight participants taken before initiation of antiretroviral therapy.
“Sequence-based and neutralization analyses suggest that VRC01 can restrict the clonality of rebounding virus in some participants, selecting for pre-existing resistance, and drive the emergence of VRC01-resistant virus,” the authors wrote.
However, they pointed out that the early years of antiretroviral drug development showed how quickly resistance could develop in a single-agent situation. Since that time, a multiagent approach directed at different targets has achieved much more potent and sustained viral suppression.
“Analogous to current regimens of highly successful combination ART that targets multiple HIV gene products, our data suggest that immunotherapy will probably require multiple bNAbs [broadly neutralizing antibodies] that target different sites on the HIV envelope glycoprotein,” the authors concluded.
The study was supported by the National Institute of Allergy and Infectious Diseases, the Penn Center for AIDS Research, the Penn Clinical Trials Unit, the University of Alabama at Birmingham Center for AIDS Research, the UAB Clinical Trials Unit, the AIDS Clinical Trials Group Statistical and Data Analysis Center, a Ruth L. Kirschstein National Research Service Award, and the National Institutes of Health.
Two authors declared personal fees from pharmaceutical industry outside the submitted work, and one author served as a contractor to the NIH through Columbus Technologies. No other conflicts of interest were declared.
Passive immunization against HIV using the broadly neutralizing antibody VRC01 is associated with a delay in plasma viral rebound in individuals undergoing interruption of antiretroviral therapy, according to a new study, but the viral suppression is not sustained.
Two open-label trials investigated the impact of different dosing regiments of VRC01 in a total of 24 patients who were taking a break from antiretroviral therapy, according to a paper published Nov. 9 in the New England Journal of Medicine.
Katharine J. Bar, MD, of the Penn Center for AIDS Research at the University of Pennsylvania, Philadelphia, and her coauthors suggested broadly neutralizing antibodies such as VRC01 could target the persistent viral reservoir that leads to rapid viral rebound as soon as antiretroviral therapy is stopped (N Engl J Med. 2016 Nov 9. doi: 10.1056/NEJMoa1608243).
However, in these two studies, VRC01 did not achieve durable viral suppression. Overall, participants in both trials were significantly more likely than historical controls to maintain viral suppression 4 weeks after interrupting antiretroviral therapy (38% and 80% vs. 13%) but this difference was no longer significant by week 8.
In one trial – the A5340 – 12 of the 13 participants with data that could be evaluated showed viral rebound to more than 200 copies/mL by week 8, and in the second NIH trial, the median time to rebound of 40 copies/mL was 39 days.
All participants showed similar plasma levels of VRC01 that had been observed in previous trials – significantly above 50 mcg/mL for 8 weeks in the A5340 trial and above 100 mcg/mL in the NIH trial. Plasma VRC01 levels were consistently above 50 mcg/mL even at the time of viral rebound, except in one patient.
Researchers performed post hoc analyses of the sequence diversity at the time of viral rebound and compared these to samples from eight participants taken before initiation of antiretroviral therapy.
“Sequence-based and neutralization analyses suggest that VRC01 can restrict the clonality of rebounding virus in some participants, selecting for pre-existing resistance, and drive the emergence of VRC01-resistant virus,” the authors wrote.
However, they pointed out that the early years of antiretroviral drug development showed how quickly resistance could develop in a single-agent situation. Since that time, a multiagent approach directed at different targets has achieved much more potent and sustained viral suppression.
“Analogous to current regimens of highly successful combination ART that targets multiple HIV gene products, our data suggest that immunotherapy will probably require multiple bNAbs [broadly neutralizing antibodies] that target different sites on the HIV envelope glycoprotein,” the authors concluded.
The study was supported by the National Institute of Allergy and Infectious Diseases, the Penn Center for AIDS Research, the Penn Clinical Trials Unit, the University of Alabama at Birmingham Center for AIDS Research, the UAB Clinical Trials Unit, the AIDS Clinical Trials Group Statistical and Data Analysis Center, a Ruth L. Kirschstein National Research Service Award, and the National Institutes of Health.
Two authors declared personal fees from pharmaceutical industry outside the submitted work, and one author served as a contractor to the NIH through Columbus Technologies. No other conflicts of interest were declared.
Passive immunization against HIV using the broadly neutralizing antibody VRC01 is associated with a delay in plasma viral rebound in individuals undergoing interruption of antiretroviral therapy, according to a new study, but the viral suppression is not sustained.
Two open-label trials investigated the impact of different dosing regiments of VRC01 in a total of 24 patients who were taking a break from antiretroviral therapy, according to a paper published Nov. 9 in the New England Journal of Medicine.
Katharine J. Bar, MD, of the Penn Center for AIDS Research at the University of Pennsylvania, Philadelphia, and her coauthors suggested broadly neutralizing antibodies such as VRC01 could target the persistent viral reservoir that leads to rapid viral rebound as soon as antiretroviral therapy is stopped (N Engl J Med. 2016 Nov 9. doi: 10.1056/NEJMoa1608243).
However, in these two studies, VRC01 did not achieve durable viral suppression. Overall, participants in both trials were significantly more likely than historical controls to maintain viral suppression 4 weeks after interrupting antiretroviral therapy (38% and 80% vs. 13%) but this difference was no longer significant by week 8.
In one trial – the A5340 – 12 of the 13 participants with data that could be evaluated showed viral rebound to more than 200 copies/mL by week 8, and in the second NIH trial, the median time to rebound of 40 copies/mL was 39 days.
All participants showed similar plasma levels of VRC01 that had been observed in previous trials – significantly above 50 mcg/mL for 8 weeks in the A5340 trial and above 100 mcg/mL in the NIH trial. Plasma VRC01 levels were consistently above 50 mcg/mL even at the time of viral rebound, except in one patient.
Researchers performed post hoc analyses of the sequence diversity at the time of viral rebound and compared these to samples from eight participants taken before initiation of antiretroviral therapy.
“Sequence-based and neutralization analyses suggest that VRC01 can restrict the clonality of rebounding virus in some participants, selecting for pre-existing resistance, and drive the emergence of VRC01-resistant virus,” the authors wrote.
However, they pointed out that the early years of antiretroviral drug development showed how quickly resistance could develop in a single-agent situation. Since that time, a multiagent approach directed at different targets has achieved much more potent and sustained viral suppression.
“Analogous to current regimens of highly successful combination ART that targets multiple HIV gene products, our data suggest that immunotherapy will probably require multiple bNAbs [broadly neutralizing antibodies] that target different sites on the HIV envelope glycoprotein,” the authors concluded.
The study was supported by the National Institute of Allergy and Infectious Diseases, the Penn Center for AIDS Research, the Penn Clinical Trials Unit, the University of Alabama at Birmingham Center for AIDS Research, the UAB Clinical Trials Unit, the AIDS Clinical Trials Group Statistical and Data Analysis Center, a Ruth L. Kirschstein National Research Service Award, and the National Institutes of Health.
Two authors declared personal fees from pharmaceutical industry outside the submitted work, and one author served as a contractor to the NIH through Columbus Technologies. No other conflicts of interest were declared.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Passive immunization against HIV using the broadly-neutralizing antibody VRC01 is associated with a brief delay in viral rebound in individuals undergoing interruption of antiretroviral therapy.
Major finding: Treatment with the broadly neutralizing antibody VRC01 was associated with a significant delay in viral rebound in individuals who have stopped antiretroviral therapy but this was not sustained beyond eight weeks.
Data source: Two prospective studies in 24 individuals with HIV infection undergoing a break from antiretroviral therapy.
Disclosures: The study was supported by the National Institute of Allergy and Infectious Diseases, the Penn Center for AIDS Research, the Penn Clinical Trials Unit, the University of Alabama at Birmingham Center for AIDS Research, the UAB Clinical Trials Unit, the AIDS Clinical Trials Group Statistical and Data Analysis Center, a Ruth L. Kirschstein National Research Service Award, and the National Institutes of Health. Two authors declared personal fees from pharmaceutical industry outside the submitted work, and one author served as a contractor to the NIH through Columbus Technologies. No other conflicts of interest were declared.
AHA Late-Breaking Clinical Trials preview
The emphasis on this year’s American Heart Association Scientific Sessions in New Orleans is bigness: “Big science, big technology, and big networking opportunities,” the AHA 16 website says.
And so the 19 abstracts out of thousands submitted that got the biggest score from program committee for AHA 2016, led by Frank Sellke, MD, were chosen for presentation at four Late-Breaking Clinical Trials session previewed the late-breaking science.
Big trials for big questions
The first late-breaker session, on Sunday, Nov. 13, at 3:45 p.m., CT, is titled will, as its title says present the long-awaited results of four trials with large enrollment and long-term outcomes.
EUCLID (A Study Comparing Cardiovascular Effects of Ticagrelor and Clopidogrel in Patients With Peripheral Artery Disease) randomized an estimated 16,000 patients with symptomatic PAD to long-term antiplatelet monotherapy with either ticagrelor or clopidogrel to see which one would be superior in preventing the composite of cardiovascular death, myocardial infarction and ischemic stroke up to 40 months. Secondarily, it looked at acute limb ischemia, need for revascularization, and disease progression. “This could have tremendous implications for patients treat for pad trying to prevent CV disease,” Dr. Sellke said.
PRECISION (Prospective Randomized Evaluation of Celecoxib Integrated Safety vs Ibuprofen or Naproxen) harks back to 2005, when the Food and Drug Administration, wrestling with the growing evidence that NSAIDs were linked with cardiovascular events, asked for a large, cardiovascular outcomes trial. PRECISION, sponsored by Pfizer but run by an academic-led steering committee led by Steven Nissen, MD, now chief of cardiovascular medicine at the Cleveland Clinic, randomized some 20,000 arthritis patients with or at risk for cardiovascular disease to long-term pain treatment with celecoxib, naproxen, or ibuprofen for a planned follow-up of 2 years. The primary endpoint is a composite of cardiovascular death, nonfatal MI, and nonfatal stroke. Dr. Sellke noted that the results will be important for many physicians and patients wanting to minimize the risks associated with NSAIDs.
HOPE 3 (Heart Outcomes Evaluation 3), presented in April this year at the American College of Cardiology meeting in Chicago, showed the combination of rosuvastatin plus candesartan and hydrochlorothiazide reduced cardiovascular events in intermediate-risk patients with hypertension, regardless of their baseline LDL cholesterol and inflammatory biomarker levels. The analysis to be presented at AHA will show whether the combination has any effect on cognitive function. As evidence builds of the cardiovascular benefit of aggressive treatment of hypertension, as in the SPRINT trial, the results could be tremendously important, Dr. Sellke said.
TRUE AHF (Efficacy and Safety of Ularitide for the Treatment of Acute Decompensated Heart Failure) randomized about 2,150 patients with acute decompensated heart failure to receive a 48-hour intravenous infusion of the natriuretic peptide ularitide or placebo. The primary outcome is a composite of 48-hour improved in-hospital worsening or unchanged clinical conditions, as well as long-term cardiovascular mortality with a median follow-up of 7 months. Because there are no effective treatments for acute systolic heart failure, the results of TRUE AHF could be of tremendous benefit, Dr. Sellke said.
Pioneering the Future of HeART Interventions
The trials with the greatest impact for practice to be presented at AHA 2015, according to the Dr. Sellke’s admitted bias as a cardiothoracic surgeon, will all be presented in this second of the late-breaker sessions, on Monday, Nov. 14, at 10:45 a.m., CT.
ART (Arterial Revascularization Trial) was a comparison of single vs. bilateral internal mammary artery grafting in more than 3,000 randomized patients undergoing coronary artery bypass surgery (CABG). The outcomes of mortality, stroke, MI, and repeat revascularization were published in 2010, showing no differences between groups. The 5-year results to be presented on Monday may resolve some of the controversy surrounding the two methods, as surgeons and cardiologists are strongly divided on the benefits and risks of single, compared with double, internal mammary artery grafting.
FUTURE (Functional Testing Underlying Coronary Revascularization) compared fractional flow reserve–guided management with conventional management in roughly 900 patients undergoing revascularization with multivessel coronary artery disease. The primary outcome is a composite of death, MI, coronary revascularization, and stroke. FFR has received a lot of attention recently, Dr. Sellke said, because it looks at the physiologic, rather than the anatomic, effects of lesion on catheterization. The results will show whether there’s clinical benefit to adding FFR to angiography that will offset the additional time it takes to perform before PCI or CABG.
PIONEER AF-PCI (An Open-label, Randomized, Controlled, Multicenter Study Exploring Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects With Atrial Fibrillation Who Undergo Percutaneous Coronary Intervention) addressed the conundrum of treating anticoagulated patients with atrial fibrillation who are undergoing PCI with adequate dual-antiplatelet therapy – and avoiding bleeding events. About 2,000 patients were randomized to varying combinations of rivaroxaban or warfarin plus aspirin, ticagrelor prasugrel, and/or clopidogrel for 1 year. The primary outcome is significant bleeding. Dr. Sellke said that because drug-eluting stents require at least a year of DAPT, the PIONEER AF-PCI results will add knowledge in an important and controversial area.
GERMANY is a report from the German Aortic Valve Registry (GARY) on the 1-year outcomes of patients with intermediate-risk severe aortic stenosis who underwent either transcatheter or surgical aortic replacement on the efficacy and outcomes of the two approaches. Dr. Sellke noted that these results will be important because the patients in this registry were not at high risk or ineligible for surgical aortic replacement.
Insights from New Therapeutic Trials for Lipids
Of the five trials presented in this session on Tuesday, Nov. 15, at 10:45 a.m., CT, only one is in an approved treatment for lowering lipids. That is GLAGOV (Global Assessment of Plaque Regression With a PCSK9 Antibody as Measured by Intravascular Ultrasound), is looking at whether LDL lowering with the PCSK9 inhibitor evolocumab reduces atheroma volume in almost 1,000 patients.
Guiding the Momentum to Effect HF Outcomes – Ironing Out the Wrinkles
Two of the six heart failure trials presented in this session on Wednesday, Nov. 16, at 10:45 a.m., CT, study cardiorespiratory effects of iron, thus the title, Dr. Sellke said.
REDUCE LAP HF (A Study to Evaluate the DC Devices, Inc. IASD System II to REDUCE Elevated Left Atrial Pressure in Patients With Heart Failure). The primary outcome is a composite of death, stroke, MI, or a systemic embolic event at 6 months. The trial evaluated a transcatheter interatrial shunt device to left atrial pressure in patients with heart failure with preserved ejection fraction (HFpEF). In this type of diastolic heart failure in which patients’ hearts cannot relax, there is really no treatment, Dr. Sellke said. So although this treatment seems “hokey,” a positive result could be important.
ATHENA HF (Aldosterone Targeted Neurohormonal Combined with Natriuresis Therapy in Heart Failure) tested the diuretic spironolactone in heart failure. The investigators randomized 360 patients to high-dose spironolactone or usual care to see whether they could provide greater reductions of n-terminal prohormone of brain natriuretic peptide (NT-proBNP) levels within 96 hours. There’s evidence that spironolactone can provide symptomatic relief for patients with heart failure, so these results could be important, Dr. Sellke said.
IRONOUT HF (Oral Iron Repletion Effects on Oxygen Up Take in Heart Failure) randomized heart failure patients with iron deficiency to oral iron supplementation or placebo and measured peak oxygen uptake at 16 weeks.
EFFECT-HF (Effect of Ferric Carboxymaltose on Exercise Capacity in Patients with Iron Deficiency and Chronic Heart Failure) also studied the effect of iron supplementation, intravenous in this case, on exercise capacity in heart failure patients at 24 weeks. Iron depletion is a hallmark of heart failure, Dr. Sellke pointed out, so iron repletion could be a simple way to improve functional capacity.
MOMENTUM 3 (Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HM3) evaluated the safety and effectiveness of the Thoratec HeartMate 3 left-ventricular assist device employing MagLev technology, which is said to facilitate the free flow of blood through the device. Roughly 1,000 patients with advanced, refractory heart failure were randomized to receive either the investigational HeartMate 3 or the HeartMate 2. The primary outcomes included short- and long-term survival and freedom from debilitating stroke. Trials such as this are very important, Dr. Sellke said, because the need for donor hearts far exceeds demand and better, cheaper LVADs that last longer could extend the lives of many thousands of patients every year.
MultiSENSE (Evaluation of Multisensor Data in Heart Failure Patients With Implanted Devices) collected information taken from sensors in an implanted cardiac synchronization therapy device in 1,000 patients to develop algorithms that would detect worsening heart failure. Multiple readmissions for heart failure are frequent and ineffective, and detecting the onset of worsening heart failure has the potential to bring those admissions way down, Dr. Sellke said.
The emphasis on this year’s American Heart Association Scientific Sessions in New Orleans is bigness: “Big science, big technology, and big networking opportunities,” the AHA 16 website says.
And so the 19 abstracts out of thousands submitted that got the biggest score from program committee for AHA 2016, led by Frank Sellke, MD, were chosen for presentation at four Late-Breaking Clinical Trials session previewed the late-breaking science.
Big trials for big questions
The first late-breaker session, on Sunday, Nov. 13, at 3:45 p.m., CT, is titled will, as its title says present the long-awaited results of four trials with large enrollment and long-term outcomes.
EUCLID (A Study Comparing Cardiovascular Effects of Ticagrelor and Clopidogrel in Patients With Peripheral Artery Disease) randomized an estimated 16,000 patients with symptomatic PAD to long-term antiplatelet monotherapy with either ticagrelor or clopidogrel to see which one would be superior in preventing the composite of cardiovascular death, myocardial infarction and ischemic stroke up to 40 months. Secondarily, it looked at acute limb ischemia, need for revascularization, and disease progression. “This could have tremendous implications for patients treat for pad trying to prevent CV disease,” Dr. Sellke said.
PRECISION (Prospective Randomized Evaluation of Celecoxib Integrated Safety vs Ibuprofen or Naproxen) harks back to 2005, when the Food and Drug Administration, wrestling with the growing evidence that NSAIDs were linked with cardiovascular events, asked for a large, cardiovascular outcomes trial. PRECISION, sponsored by Pfizer but run by an academic-led steering committee led by Steven Nissen, MD, now chief of cardiovascular medicine at the Cleveland Clinic, randomized some 20,000 arthritis patients with or at risk for cardiovascular disease to long-term pain treatment with celecoxib, naproxen, or ibuprofen for a planned follow-up of 2 years. The primary endpoint is a composite of cardiovascular death, nonfatal MI, and nonfatal stroke. Dr. Sellke noted that the results will be important for many physicians and patients wanting to minimize the risks associated with NSAIDs.
HOPE 3 (Heart Outcomes Evaluation 3), presented in April this year at the American College of Cardiology meeting in Chicago, showed the combination of rosuvastatin plus candesartan and hydrochlorothiazide reduced cardiovascular events in intermediate-risk patients with hypertension, regardless of their baseline LDL cholesterol and inflammatory biomarker levels. The analysis to be presented at AHA will show whether the combination has any effect on cognitive function. As evidence builds of the cardiovascular benefit of aggressive treatment of hypertension, as in the SPRINT trial, the results could be tremendously important, Dr. Sellke said.
TRUE AHF (Efficacy and Safety of Ularitide for the Treatment of Acute Decompensated Heart Failure) randomized about 2,150 patients with acute decompensated heart failure to receive a 48-hour intravenous infusion of the natriuretic peptide ularitide or placebo. The primary outcome is a composite of 48-hour improved in-hospital worsening or unchanged clinical conditions, as well as long-term cardiovascular mortality with a median follow-up of 7 months. Because there are no effective treatments for acute systolic heart failure, the results of TRUE AHF could be of tremendous benefit, Dr. Sellke said.
Pioneering the Future of HeART Interventions
The trials with the greatest impact for practice to be presented at AHA 2015, according to the Dr. Sellke’s admitted bias as a cardiothoracic surgeon, will all be presented in this second of the late-breaker sessions, on Monday, Nov. 14, at 10:45 a.m., CT.
ART (Arterial Revascularization Trial) was a comparison of single vs. bilateral internal mammary artery grafting in more than 3,000 randomized patients undergoing coronary artery bypass surgery (CABG). The outcomes of mortality, stroke, MI, and repeat revascularization were published in 2010, showing no differences between groups. The 5-year results to be presented on Monday may resolve some of the controversy surrounding the two methods, as surgeons and cardiologists are strongly divided on the benefits and risks of single, compared with double, internal mammary artery grafting.
FUTURE (Functional Testing Underlying Coronary Revascularization) compared fractional flow reserve–guided management with conventional management in roughly 900 patients undergoing revascularization with multivessel coronary artery disease. The primary outcome is a composite of death, MI, coronary revascularization, and stroke. FFR has received a lot of attention recently, Dr. Sellke said, because it looks at the physiologic, rather than the anatomic, effects of lesion on catheterization. The results will show whether there’s clinical benefit to adding FFR to angiography that will offset the additional time it takes to perform before PCI or CABG.
PIONEER AF-PCI (An Open-label, Randomized, Controlled, Multicenter Study Exploring Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects With Atrial Fibrillation Who Undergo Percutaneous Coronary Intervention) addressed the conundrum of treating anticoagulated patients with atrial fibrillation who are undergoing PCI with adequate dual-antiplatelet therapy – and avoiding bleeding events. About 2,000 patients were randomized to varying combinations of rivaroxaban or warfarin plus aspirin, ticagrelor prasugrel, and/or clopidogrel for 1 year. The primary outcome is significant bleeding. Dr. Sellke said that because drug-eluting stents require at least a year of DAPT, the PIONEER AF-PCI results will add knowledge in an important and controversial area.
GERMANY is a report from the German Aortic Valve Registry (GARY) on the 1-year outcomes of patients with intermediate-risk severe aortic stenosis who underwent either transcatheter or surgical aortic replacement on the efficacy and outcomes of the two approaches. Dr. Sellke noted that these results will be important because the patients in this registry were not at high risk or ineligible for surgical aortic replacement.
Insights from New Therapeutic Trials for Lipids
Of the five trials presented in this session on Tuesday, Nov. 15, at 10:45 a.m., CT, only one is in an approved treatment for lowering lipids. That is GLAGOV (Global Assessment of Plaque Regression With a PCSK9 Antibody as Measured by Intravascular Ultrasound), is looking at whether LDL lowering with the PCSK9 inhibitor evolocumab reduces atheroma volume in almost 1,000 patients.
Guiding the Momentum to Effect HF Outcomes – Ironing Out the Wrinkles
Two of the six heart failure trials presented in this session on Wednesday, Nov. 16, at 10:45 a.m., CT, study cardiorespiratory effects of iron, thus the title, Dr. Sellke said.
REDUCE LAP HF (A Study to Evaluate the DC Devices, Inc. IASD System II to REDUCE Elevated Left Atrial Pressure in Patients With Heart Failure). The primary outcome is a composite of death, stroke, MI, or a systemic embolic event at 6 months. The trial evaluated a transcatheter interatrial shunt device to left atrial pressure in patients with heart failure with preserved ejection fraction (HFpEF). In this type of diastolic heart failure in which patients’ hearts cannot relax, there is really no treatment, Dr. Sellke said. So although this treatment seems “hokey,” a positive result could be important.
ATHENA HF (Aldosterone Targeted Neurohormonal Combined with Natriuresis Therapy in Heart Failure) tested the diuretic spironolactone in heart failure. The investigators randomized 360 patients to high-dose spironolactone or usual care to see whether they could provide greater reductions of n-terminal prohormone of brain natriuretic peptide (NT-proBNP) levels within 96 hours. There’s evidence that spironolactone can provide symptomatic relief for patients with heart failure, so these results could be important, Dr. Sellke said.
IRONOUT HF (Oral Iron Repletion Effects on Oxygen Up Take in Heart Failure) randomized heart failure patients with iron deficiency to oral iron supplementation or placebo and measured peak oxygen uptake at 16 weeks.
EFFECT-HF (Effect of Ferric Carboxymaltose on Exercise Capacity in Patients with Iron Deficiency and Chronic Heart Failure) also studied the effect of iron supplementation, intravenous in this case, on exercise capacity in heart failure patients at 24 weeks. Iron depletion is a hallmark of heart failure, Dr. Sellke pointed out, so iron repletion could be a simple way to improve functional capacity.
MOMENTUM 3 (Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HM3) evaluated the safety and effectiveness of the Thoratec HeartMate 3 left-ventricular assist device employing MagLev technology, which is said to facilitate the free flow of blood through the device. Roughly 1,000 patients with advanced, refractory heart failure were randomized to receive either the investigational HeartMate 3 or the HeartMate 2. The primary outcomes included short- and long-term survival and freedom from debilitating stroke. Trials such as this are very important, Dr. Sellke said, because the need for donor hearts far exceeds demand and better, cheaper LVADs that last longer could extend the lives of many thousands of patients every year.
MultiSENSE (Evaluation of Multisensor Data in Heart Failure Patients With Implanted Devices) collected information taken from sensors in an implanted cardiac synchronization therapy device in 1,000 patients to develop algorithms that would detect worsening heart failure. Multiple readmissions for heart failure are frequent and ineffective, and detecting the onset of worsening heart failure has the potential to bring those admissions way down, Dr. Sellke said.
The emphasis on this year’s American Heart Association Scientific Sessions in New Orleans is bigness: “Big science, big technology, and big networking opportunities,” the AHA 16 website says.
And so the 19 abstracts out of thousands submitted that got the biggest score from program committee for AHA 2016, led by Frank Sellke, MD, were chosen for presentation at four Late-Breaking Clinical Trials session previewed the late-breaking science.
Big trials for big questions
The first late-breaker session, on Sunday, Nov. 13, at 3:45 p.m., CT, is titled will, as its title says present the long-awaited results of four trials with large enrollment and long-term outcomes.
EUCLID (A Study Comparing Cardiovascular Effects of Ticagrelor and Clopidogrel in Patients With Peripheral Artery Disease) randomized an estimated 16,000 patients with symptomatic PAD to long-term antiplatelet monotherapy with either ticagrelor or clopidogrel to see which one would be superior in preventing the composite of cardiovascular death, myocardial infarction and ischemic stroke up to 40 months. Secondarily, it looked at acute limb ischemia, need for revascularization, and disease progression. “This could have tremendous implications for patients treat for pad trying to prevent CV disease,” Dr. Sellke said.
PRECISION (Prospective Randomized Evaluation of Celecoxib Integrated Safety vs Ibuprofen or Naproxen) harks back to 2005, when the Food and Drug Administration, wrestling with the growing evidence that NSAIDs were linked with cardiovascular events, asked for a large, cardiovascular outcomes trial. PRECISION, sponsored by Pfizer but run by an academic-led steering committee led by Steven Nissen, MD, now chief of cardiovascular medicine at the Cleveland Clinic, randomized some 20,000 arthritis patients with or at risk for cardiovascular disease to long-term pain treatment with celecoxib, naproxen, or ibuprofen for a planned follow-up of 2 years. The primary endpoint is a composite of cardiovascular death, nonfatal MI, and nonfatal stroke. Dr. Sellke noted that the results will be important for many physicians and patients wanting to minimize the risks associated with NSAIDs.
HOPE 3 (Heart Outcomes Evaluation 3), presented in April this year at the American College of Cardiology meeting in Chicago, showed the combination of rosuvastatin plus candesartan and hydrochlorothiazide reduced cardiovascular events in intermediate-risk patients with hypertension, regardless of their baseline LDL cholesterol and inflammatory biomarker levels. The analysis to be presented at AHA will show whether the combination has any effect on cognitive function. As evidence builds of the cardiovascular benefit of aggressive treatment of hypertension, as in the SPRINT trial, the results could be tremendously important, Dr. Sellke said.
TRUE AHF (Efficacy and Safety of Ularitide for the Treatment of Acute Decompensated Heart Failure) randomized about 2,150 patients with acute decompensated heart failure to receive a 48-hour intravenous infusion of the natriuretic peptide ularitide or placebo. The primary outcome is a composite of 48-hour improved in-hospital worsening or unchanged clinical conditions, as well as long-term cardiovascular mortality with a median follow-up of 7 months. Because there are no effective treatments for acute systolic heart failure, the results of TRUE AHF could be of tremendous benefit, Dr. Sellke said.
Pioneering the Future of HeART Interventions
The trials with the greatest impact for practice to be presented at AHA 2015, according to the Dr. Sellke’s admitted bias as a cardiothoracic surgeon, will all be presented in this second of the late-breaker sessions, on Monday, Nov. 14, at 10:45 a.m., CT.
ART (Arterial Revascularization Trial) was a comparison of single vs. bilateral internal mammary artery grafting in more than 3,000 randomized patients undergoing coronary artery bypass surgery (CABG). The outcomes of mortality, stroke, MI, and repeat revascularization were published in 2010, showing no differences between groups. The 5-year results to be presented on Monday may resolve some of the controversy surrounding the two methods, as surgeons and cardiologists are strongly divided on the benefits and risks of single, compared with double, internal mammary artery grafting.
FUTURE (Functional Testing Underlying Coronary Revascularization) compared fractional flow reserve–guided management with conventional management in roughly 900 patients undergoing revascularization with multivessel coronary artery disease. The primary outcome is a composite of death, MI, coronary revascularization, and stroke. FFR has received a lot of attention recently, Dr. Sellke said, because it looks at the physiologic, rather than the anatomic, effects of lesion on catheterization. The results will show whether there’s clinical benefit to adding FFR to angiography that will offset the additional time it takes to perform before PCI or CABG.
PIONEER AF-PCI (An Open-label, Randomized, Controlled, Multicenter Study Exploring Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects With Atrial Fibrillation Who Undergo Percutaneous Coronary Intervention) addressed the conundrum of treating anticoagulated patients with atrial fibrillation who are undergoing PCI with adequate dual-antiplatelet therapy – and avoiding bleeding events. About 2,000 patients were randomized to varying combinations of rivaroxaban or warfarin plus aspirin, ticagrelor prasugrel, and/or clopidogrel for 1 year. The primary outcome is significant bleeding. Dr. Sellke said that because drug-eluting stents require at least a year of DAPT, the PIONEER AF-PCI results will add knowledge in an important and controversial area.
GERMANY is a report from the German Aortic Valve Registry (GARY) on the 1-year outcomes of patients with intermediate-risk severe aortic stenosis who underwent either transcatheter or surgical aortic replacement on the efficacy and outcomes of the two approaches. Dr. Sellke noted that these results will be important because the patients in this registry were not at high risk or ineligible for surgical aortic replacement.
Insights from New Therapeutic Trials for Lipids
Of the five trials presented in this session on Tuesday, Nov. 15, at 10:45 a.m., CT, only one is in an approved treatment for lowering lipids. That is GLAGOV (Global Assessment of Plaque Regression With a PCSK9 Antibody as Measured by Intravascular Ultrasound), is looking at whether LDL lowering with the PCSK9 inhibitor evolocumab reduces atheroma volume in almost 1,000 patients.
Guiding the Momentum to Effect HF Outcomes – Ironing Out the Wrinkles
Two of the six heart failure trials presented in this session on Wednesday, Nov. 16, at 10:45 a.m., CT, study cardiorespiratory effects of iron, thus the title, Dr. Sellke said.
REDUCE LAP HF (A Study to Evaluate the DC Devices, Inc. IASD System II to REDUCE Elevated Left Atrial Pressure in Patients With Heart Failure). The primary outcome is a composite of death, stroke, MI, or a systemic embolic event at 6 months. The trial evaluated a transcatheter interatrial shunt device to left atrial pressure in patients with heart failure with preserved ejection fraction (HFpEF). In this type of diastolic heart failure in which patients’ hearts cannot relax, there is really no treatment, Dr. Sellke said. So although this treatment seems “hokey,” a positive result could be important.
ATHENA HF (Aldosterone Targeted Neurohormonal Combined with Natriuresis Therapy in Heart Failure) tested the diuretic spironolactone in heart failure. The investigators randomized 360 patients to high-dose spironolactone or usual care to see whether they could provide greater reductions of n-terminal prohormone of brain natriuretic peptide (NT-proBNP) levels within 96 hours. There’s evidence that spironolactone can provide symptomatic relief for patients with heart failure, so these results could be important, Dr. Sellke said.
IRONOUT HF (Oral Iron Repletion Effects on Oxygen Up Take in Heart Failure) randomized heart failure patients with iron deficiency to oral iron supplementation or placebo and measured peak oxygen uptake at 16 weeks.
EFFECT-HF (Effect of Ferric Carboxymaltose on Exercise Capacity in Patients with Iron Deficiency and Chronic Heart Failure) also studied the effect of iron supplementation, intravenous in this case, on exercise capacity in heart failure patients at 24 weeks. Iron depletion is a hallmark of heart failure, Dr. Sellke pointed out, so iron repletion could be a simple way to improve functional capacity.
MOMENTUM 3 (Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HM3) evaluated the safety and effectiveness of the Thoratec HeartMate 3 left-ventricular assist device employing MagLev technology, which is said to facilitate the free flow of blood through the device. Roughly 1,000 patients with advanced, refractory heart failure were randomized to receive either the investigational HeartMate 3 or the HeartMate 2. The primary outcomes included short- and long-term survival and freedom from debilitating stroke. Trials such as this are very important, Dr. Sellke said, because the need for donor hearts far exceeds demand and better, cheaper LVADs that last longer could extend the lives of many thousands of patients every year.
MultiSENSE (Evaluation of Multisensor Data in Heart Failure Patients With Implanted Devices) collected information taken from sensors in an implanted cardiac synchronization therapy device in 1,000 patients to develop algorithms that would detect worsening heart failure. Multiple readmissions for heart failure are frequent and ineffective, and detecting the onset of worsening heart failure has the potential to bring those admissions way down, Dr. Sellke said.
Home oxygen upped survival in PAH with severely impaired DLCO
LOS ANGELES – Pulmonary arterial hypertension (PAH) patients with severely impaired diffusing capacity of the lung for carbon monoxide (DLCO) were much more likely to survive when they received home oxygen therapy, according to a disease registry analysis.
“We all know that supplemental oxygen is widely used with PAH,” said Harrison W. Farber, MD, director of the pulmonary hypertension center at Boston University. But there are practically no data showing that it is successful, and there are even fewer data for patients with PAH who have very low diffusion capacity, he added.
That knowledge gap prompted Dr. Farber and his colleagues to analyze data from the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL), the largest disease registry in the world of patients with PAH.
“Patients in that group – the severe DLCO group – who got oxygen had poorer prognostic features but improved overall survival relative to those who didn’t,” Dr. Farber explained during a presentation at the annual meeting of the American College of Chest Physicians. “Based on this, it makes us think that home oxygen, supplemental oxygen treatment, is associated with improved survival in patients, especially those with severe DLCO and PAH.”
The 3,046 patients analyzed by Dr. Farber and his colleagues had World Health Organization Group 1 PAH with right heart catheterization hemodynamic criteria: a mean pulmonary artery pressure greater than 25 mm Hg, a pulmonary capillary wedge pressure less than or equal to 15 mm Hg, and a pulmonary vascular resistance of at least 3 Wood units (WU). Patients were at least 18 years of age and grouped by oxygen use, which was defined as any use at any time from study enrollment to the end of follow-up, and by DLCO group.
A total of 57% of the patients (1,734) received oxygen, and the remaining 43% of the patients (1,312) did not receive oxygen. Among the patients who received oxygen, 71% (1,227) received the therapy continuously, and 24% (408) received oxygen at night only.
The 424 patients with a DLCO of less than 40% were considered to have a severe DLCO impairment. The other two groups comprised 505 patients with a moderate DLCO impairment (at least 40%, but less than 60%) and 844 patients with a mild to normal DLCO (at least 60%). The DLCOs of 1,273 patients analyzed were unknown.
Among those patients with severe DLCO impairment, the risk of death was significantly lower in those who received oxygen, compared with those who did not receive oxygen (hazard ratio, 0.56; P = .0033). Oxygen use was associated with significant improvements in overall survival in both the newly diagnosed (HR, 0.47; P = .029) and previously diagnosed (HR, 0.59; P = .026) severe DLCO cohorts, Dr. Farber said.
Patients receiving oxygen were more likely to be treated with PAH-specific medications, regardless of their DLCO group.
Among the analysis’s limitations was that the lengths of time patients had been undergoing oxygen treatment were unknown. That prevented adjustments for duration of oxygen treatment, according to Dr. Farber.
Dr. Farber disclosed serving on the steering committees or advisory boards for Actelion, Bayer, Bellerophon, Gilead, and United Therapeutics. He has received research support from Actelion, Gilead, and United Therapeutics, and has been a speaker for Actelion, Bayer, and Gilead.
LOS ANGELES – Pulmonary arterial hypertension (PAH) patients with severely impaired diffusing capacity of the lung for carbon monoxide (DLCO) were much more likely to survive when they received home oxygen therapy, according to a disease registry analysis.
“We all know that supplemental oxygen is widely used with PAH,” said Harrison W. Farber, MD, director of the pulmonary hypertension center at Boston University. But there are practically no data showing that it is successful, and there are even fewer data for patients with PAH who have very low diffusion capacity, he added.
That knowledge gap prompted Dr. Farber and his colleagues to analyze data from the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL), the largest disease registry in the world of patients with PAH.
“Patients in that group – the severe DLCO group – who got oxygen had poorer prognostic features but improved overall survival relative to those who didn’t,” Dr. Farber explained during a presentation at the annual meeting of the American College of Chest Physicians. “Based on this, it makes us think that home oxygen, supplemental oxygen treatment, is associated with improved survival in patients, especially those with severe DLCO and PAH.”
The 3,046 patients analyzed by Dr. Farber and his colleagues had World Health Organization Group 1 PAH with right heart catheterization hemodynamic criteria: a mean pulmonary artery pressure greater than 25 mm Hg, a pulmonary capillary wedge pressure less than or equal to 15 mm Hg, and a pulmonary vascular resistance of at least 3 Wood units (WU). Patients were at least 18 years of age and grouped by oxygen use, which was defined as any use at any time from study enrollment to the end of follow-up, and by DLCO group.
A total of 57% of the patients (1,734) received oxygen, and the remaining 43% of the patients (1,312) did not receive oxygen. Among the patients who received oxygen, 71% (1,227) received the therapy continuously, and 24% (408) received oxygen at night only.
The 424 patients with a DLCO of less than 40% were considered to have a severe DLCO impairment. The other two groups comprised 505 patients with a moderate DLCO impairment (at least 40%, but less than 60%) and 844 patients with a mild to normal DLCO (at least 60%). The DLCOs of 1,273 patients analyzed were unknown.
Among those patients with severe DLCO impairment, the risk of death was significantly lower in those who received oxygen, compared with those who did not receive oxygen (hazard ratio, 0.56; P = .0033). Oxygen use was associated with significant improvements in overall survival in both the newly diagnosed (HR, 0.47; P = .029) and previously diagnosed (HR, 0.59; P = .026) severe DLCO cohorts, Dr. Farber said.
Patients receiving oxygen were more likely to be treated with PAH-specific medications, regardless of their DLCO group.
Among the analysis’s limitations was that the lengths of time patients had been undergoing oxygen treatment were unknown. That prevented adjustments for duration of oxygen treatment, according to Dr. Farber.
Dr. Farber disclosed serving on the steering committees or advisory boards for Actelion, Bayer, Bellerophon, Gilead, and United Therapeutics. He has received research support from Actelion, Gilead, and United Therapeutics, and has been a speaker for Actelion, Bayer, and Gilead.
LOS ANGELES – Pulmonary arterial hypertension (PAH) patients with severely impaired diffusing capacity of the lung for carbon monoxide (DLCO) were much more likely to survive when they received home oxygen therapy, according to a disease registry analysis.
“We all know that supplemental oxygen is widely used with PAH,” said Harrison W. Farber, MD, director of the pulmonary hypertension center at Boston University. But there are practically no data showing that it is successful, and there are even fewer data for patients with PAH who have very low diffusion capacity, he added.
That knowledge gap prompted Dr. Farber and his colleagues to analyze data from the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL), the largest disease registry in the world of patients with PAH.
“Patients in that group – the severe DLCO group – who got oxygen had poorer prognostic features but improved overall survival relative to those who didn’t,” Dr. Farber explained during a presentation at the annual meeting of the American College of Chest Physicians. “Based on this, it makes us think that home oxygen, supplemental oxygen treatment, is associated with improved survival in patients, especially those with severe DLCO and PAH.”
The 3,046 patients analyzed by Dr. Farber and his colleagues had World Health Organization Group 1 PAH with right heart catheterization hemodynamic criteria: a mean pulmonary artery pressure greater than 25 mm Hg, a pulmonary capillary wedge pressure less than or equal to 15 mm Hg, and a pulmonary vascular resistance of at least 3 Wood units (WU). Patients were at least 18 years of age and grouped by oxygen use, which was defined as any use at any time from study enrollment to the end of follow-up, and by DLCO group.
A total of 57% of the patients (1,734) received oxygen, and the remaining 43% of the patients (1,312) did not receive oxygen. Among the patients who received oxygen, 71% (1,227) received the therapy continuously, and 24% (408) received oxygen at night only.
The 424 patients with a DLCO of less than 40% were considered to have a severe DLCO impairment. The other two groups comprised 505 patients with a moderate DLCO impairment (at least 40%, but less than 60%) and 844 patients with a mild to normal DLCO (at least 60%). The DLCOs of 1,273 patients analyzed were unknown.
Among those patients with severe DLCO impairment, the risk of death was significantly lower in those who received oxygen, compared with those who did not receive oxygen (hazard ratio, 0.56; P = .0033). Oxygen use was associated with significant improvements in overall survival in both the newly diagnosed (HR, 0.47; P = .029) and previously diagnosed (HR, 0.59; P = .026) severe DLCO cohorts, Dr. Farber said.
Patients receiving oxygen were more likely to be treated with PAH-specific medications, regardless of their DLCO group.
Among the analysis’s limitations was that the lengths of time patients had been undergoing oxygen treatment were unknown. That prevented adjustments for duration of oxygen treatment, according to Dr. Farber.
Dr. Farber disclosed serving on the steering committees or advisory boards for Actelion, Bayer, Bellerophon, Gilead, and United Therapeutics. He has received research support from Actelion, Gilead, and United Therapeutics, and has been a speaker for Actelion, Bayer, and Gilead.
Key clinical point:
Major finding: PAH patients with severe DLCO impairment who received oxygen had a significantly higher probability of survival than those who didn’t receive oxygen (HR, 0.56; P = .0033).
Data source: An analysis of 3,046 patients in the U.S. multicenter, observational REVEAL disease registry.
Disclosures: Dr. Farber disclosed serving on the steering committees or advisory boards for Actelion, Bayer, Bellerophon, Gilead, and United Therapeutics. He has received research support from Actelion, Gilead, and United Therapeutics, and has been a speaker for Actelion, Bayer, and Gilead.
Wide spectrum of feeding problems poses challenge for clinicians
SAN FRANCISCO – Clinicians are likely to encounter diverse feeding problems in daily practice that will challenge their diagnostic and treatment acumen, Dr. Irene Chatoor told attendees of the annual meeting of the American Academy of Pediatrics.
These problems run the gamut from the most prevalent but least serious picky eating, to the least prevalent but most serious feeding disorders, she noted. Correspondingly, management will range from simple reassurance of parents to more intensive behavioral and medical interventions.
Assessment
“When you assess a feeding problem, you have to look at both the mother and father, and the child,” she advised. The parents are evaluated for their feeding style, while the child is evaluated for three feeding problems: limited appetite, selective intake, and fear of feeding (Pediatrics. 2015;135[2]:344-53).
Clinicians must be alert for organic red flags, such as dysphagia, aspiration, and vomiting, and for behavioral red flags, such as food fixation, abrupt cessation of feeding after a trigger event, and anticipatory gagging. An overarching red flag is failure to thrive.
Sometimes, a child will have both an organic condition and a behavioral feeding disorder at the same time. “It’s very important that you don’t think one excludes the other,” she cautioned.
Diagnosis
“To delineate milder feeding difficulties from feeding disorders, there must be some form of impairment caused by the feeding problem,” Dr. Chatoor commented. “Why is this important? For two reasons. One is for the insurance companies, because they don’t pay unless there is a disorder. And then there is research: you cannot do research unless you clearly define what you are studying.”
Children are considered to have impairment if they have weight loss or growth faltering, considerable nutritional deficiency, or a marked interference with psychosocial functioning.
“When we diagnose feeding problems, it is best done with a multidisciplinary team,” Dr. Chatoor maintained. “I have learned many years ago that I’m not effective in helping parents deal with the feeding disorder if they are still in the back of their mind worried that the child has something organically wrong and that’s why the child does not want to eat.”
Accordingly, various team members perform a medical examination, a nutritional assessment, an oral motor and sensory evaluation, and a psychiatric or psychological assessment to identify the root cause or causes of the problem.
When it comes to behavioral etiologies, the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) now groups all feeding and eating disorders together in one section, reflecting the fact that disorders starting early in life can and often do track into adolescence and adulthood, according to Dr. Chatoor.
The manual also features a new diagnosis, avoidant/restrictive food intake disorder (ARFID). Key criteria include the presence of an eating or feeding disturbance that cannot be better explained by lack of available food, culturally sanctioned practices, or a concurrent medical condition or another mental disorder.
The child must have a persistent failure to meet appropriate nutritional and/or energy needs, associated with any of four findings: significant weight loss or failure to achieve expected weight gain or growth, significant nutritional deficiencies, dependence on enteral feeding or oral nutritional supplements, and marked interference with psychosocial functioning.
“You need to have at least one but often you have a combination of nutritional and emotional impairment in the same child,” she commented.
ARFID and its treatment
There are three subtypes of ARFID having different features, treatments, and prognosis, although they all share in common food refusal, according to Dr. Chatoor.
The first subtype – apparent lack of interest in eating or food – emerges by 3 years of age, most often during the transition to self-feeding between 9 and 18 months. Affected children refuse to eat an adequate amount of food for at least 1 month, rarely communicate hunger, lack interest in food, and prefer to play or talk. They typically present with growth deficiency.
“If you have a child who refuses to eat, it generates anxiety in the mother. The mother does things she would not normally do with the child who is eating well. She starts to distract, to cajole, to sometimes even force-feed the child,” Dr. Chatoor explained. “And the more she engages in these behaviors, the more resistant the child becomes. So they become trapped in this vicious cycle.”
Treatment is aimed at removing this conflict with three approaches: explaining to parents the infant’s special temperament (notably, high arousal and difficulty turning off excitement); addressing their background, including any eating issues of their own, and difficulty with setting limits; and providing specific feeding guidelines and a time-out procedure.
The guidelines stress regular feeding, withholding of all snacks, keeping the child at the table for 20 to 30 minutes, and not using any distractions or pressure. Also, importantly, the parents should have dinner with their child. “I always tell the parents what is good for the child is good for you. It’s good for your whole family,” she said. “If they have other children, these rules apply to everybody. Young children learn to eat by watching their parents eating.”
With early and consistent use of these interventions, about two-thirds of children outgrow this eating/feeding disturbance by mid-childhood, according to Dr. Chatoor.
Children with the second subtype of ARFID – avoidance based on the sensory characteristics of food – consistently refuse to eat certain foods having specific tastes, textures, temperatures, smells, and/or appearances. Onset occurs during the toddler years, when a new or different food is introduced.
“Not only do they refuse the same foods that were aversive, but they also generalize it. So these children are afraid to try other foods and they may end up limiting food groups – they don’t eat any vegetables and some of them don’t eat any meats,” she explained. “They are a challenge because this always causes a nutritional deficiency and they also have problems socially as they get older.”
Treatment varies by age, with gradual desensitization for infants and parent modeling of eating new foods for toddlers. A multifaceted approach is used for preschoolers, combining modeling, giving foods attractive shapes and names, having the child participate in food preparation, and using focused play therapy, such as feeding dolls who are “brave” and try new foods.
Clinicians can explain to affected school-aged children that they are “supertasters,” having more taste buds and therefore experiencing food more intensely, and that they can help their taste buds not react so strongly by starting to eat small amounts of new foods and gradually increasing over time. Parents can let the child make a list of 10 foods they would like to eat, and award them points for “courage” for every bite of a new food they try.
Prognosis of this type of ARFID varies, according to Dr. Chatoor. “Through gradual exposure, young children can expand the variety of foods they eat,” she elaborated. However, “some children become very rigid and brand sensitive in regard to the food they are willing to eat and begin to experience social problems during mid-childhood and adolescence. Some children grow up eating a limited diet, but finding ways to compensate nutritionally and socially.”
Children with the third subtype of ARFID – concern about aversive consequences of eating – have an acute onset of consistent food refusal at any age, from infancy onward, after experiencing a traumatic event or repeated traumatic insults to the oropharynx or gastrointestinal tract that trigger distress in the child. These children often have comorbidities such as gastroesophageal reflux, eosinophilic gastroenteritis, or anxiety disorder.
“Treatment involves gradual desensitization to feared objects: the highchair, bib, bottle, or spoon,” Dr. Chatoor explained. “It also involves training of the mother in behavioral techniques to feed the child in spite of the child’s fear and distress.”
Any underlying medical condition causing pain or distress should be treated. Additional measures may include, for example, use of a graduated approach, starting with liquids and progressing to purees, if the child fears solid foods, and prescribing anxiolytic medication in cases of severe anxiety.
Dr. Chatoor disclosed that she has lectured internationally at conferences on feeding disorders that were organized by Abbott Nutrition International and that the company provided a research grant for a study on feeding.
SAN FRANCISCO – Clinicians are likely to encounter diverse feeding problems in daily practice that will challenge their diagnostic and treatment acumen, Dr. Irene Chatoor told attendees of the annual meeting of the American Academy of Pediatrics.
These problems run the gamut from the most prevalent but least serious picky eating, to the least prevalent but most serious feeding disorders, she noted. Correspondingly, management will range from simple reassurance of parents to more intensive behavioral and medical interventions.
Assessment
“When you assess a feeding problem, you have to look at both the mother and father, and the child,” she advised. The parents are evaluated for their feeding style, while the child is evaluated for three feeding problems: limited appetite, selective intake, and fear of feeding (Pediatrics. 2015;135[2]:344-53).
Clinicians must be alert for organic red flags, such as dysphagia, aspiration, and vomiting, and for behavioral red flags, such as food fixation, abrupt cessation of feeding after a trigger event, and anticipatory gagging. An overarching red flag is failure to thrive.
Sometimes, a child will have both an organic condition and a behavioral feeding disorder at the same time. “It’s very important that you don’t think one excludes the other,” she cautioned.
Diagnosis
“To delineate milder feeding difficulties from feeding disorders, there must be some form of impairment caused by the feeding problem,” Dr. Chatoor commented. “Why is this important? For two reasons. One is for the insurance companies, because they don’t pay unless there is a disorder. And then there is research: you cannot do research unless you clearly define what you are studying.”
Children are considered to have impairment if they have weight loss or growth faltering, considerable nutritional deficiency, or a marked interference with psychosocial functioning.
“When we diagnose feeding problems, it is best done with a multidisciplinary team,” Dr. Chatoor maintained. “I have learned many years ago that I’m not effective in helping parents deal with the feeding disorder if they are still in the back of their mind worried that the child has something organically wrong and that’s why the child does not want to eat.”
Accordingly, various team members perform a medical examination, a nutritional assessment, an oral motor and sensory evaluation, and a psychiatric or psychological assessment to identify the root cause or causes of the problem.
When it comes to behavioral etiologies, the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) now groups all feeding and eating disorders together in one section, reflecting the fact that disorders starting early in life can and often do track into adolescence and adulthood, according to Dr. Chatoor.
The manual also features a new diagnosis, avoidant/restrictive food intake disorder (ARFID). Key criteria include the presence of an eating or feeding disturbance that cannot be better explained by lack of available food, culturally sanctioned practices, or a concurrent medical condition or another mental disorder.
The child must have a persistent failure to meet appropriate nutritional and/or energy needs, associated with any of four findings: significant weight loss or failure to achieve expected weight gain or growth, significant nutritional deficiencies, dependence on enteral feeding or oral nutritional supplements, and marked interference with psychosocial functioning.
“You need to have at least one but often you have a combination of nutritional and emotional impairment in the same child,” she commented.
ARFID and its treatment
There are three subtypes of ARFID having different features, treatments, and prognosis, although they all share in common food refusal, according to Dr. Chatoor.
The first subtype – apparent lack of interest in eating or food – emerges by 3 years of age, most often during the transition to self-feeding between 9 and 18 months. Affected children refuse to eat an adequate amount of food for at least 1 month, rarely communicate hunger, lack interest in food, and prefer to play or talk. They typically present with growth deficiency.
“If you have a child who refuses to eat, it generates anxiety in the mother. The mother does things she would not normally do with the child who is eating well. She starts to distract, to cajole, to sometimes even force-feed the child,” Dr. Chatoor explained. “And the more she engages in these behaviors, the more resistant the child becomes. So they become trapped in this vicious cycle.”
Treatment is aimed at removing this conflict with three approaches: explaining to parents the infant’s special temperament (notably, high arousal and difficulty turning off excitement); addressing their background, including any eating issues of their own, and difficulty with setting limits; and providing specific feeding guidelines and a time-out procedure.
The guidelines stress regular feeding, withholding of all snacks, keeping the child at the table for 20 to 30 minutes, and not using any distractions or pressure. Also, importantly, the parents should have dinner with their child. “I always tell the parents what is good for the child is good for you. It’s good for your whole family,” she said. “If they have other children, these rules apply to everybody. Young children learn to eat by watching their parents eating.”
With early and consistent use of these interventions, about two-thirds of children outgrow this eating/feeding disturbance by mid-childhood, according to Dr. Chatoor.
Children with the second subtype of ARFID – avoidance based on the sensory characteristics of food – consistently refuse to eat certain foods having specific tastes, textures, temperatures, smells, and/or appearances. Onset occurs during the toddler years, when a new or different food is introduced.
“Not only do they refuse the same foods that were aversive, but they also generalize it. So these children are afraid to try other foods and they may end up limiting food groups – they don’t eat any vegetables and some of them don’t eat any meats,” she explained. “They are a challenge because this always causes a nutritional deficiency and they also have problems socially as they get older.”
Treatment varies by age, with gradual desensitization for infants and parent modeling of eating new foods for toddlers. A multifaceted approach is used for preschoolers, combining modeling, giving foods attractive shapes and names, having the child participate in food preparation, and using focused play therapy, such as feeding dolls who are “brave” and try new foods.
Clinicians can explain to affected school-aged children that they are “supertasters,” having more taste buds and therefore experiencing food more intensely, and that they can help their taste buds not react so strongly by starting to eat small amounts of new foods and gradually increasing over time. Parents can let the child make a list of 10 foods they would like to eat, and award them points for “courage” for every bite of a new food they try.
Prognosis of this type of ARFID varies, according to Dr. Chatoor. “Through gradual exposure, young children can expand the variety of foods they eat,” she elaborated. However, “some children become very rigid and brand sensitive in regard to the food they are willing to eat and begin to experience social problems during mid-childhood and adolescence. Some children grow up eating a limited diet, but finding ways to compensate nutritionally and socially.”
Children with the third subtype of ARFID – concern about aversive consequences of eating – have an acute onset of consistent food refusal at any age, from infancy onward, after experiencing a traumatic event or repeated traumatic insults to the oropharynx or gastrointestinal tract that trigger distress in the child. These children often have comorbidities such as gastroesophageal reflux, eosinophilic gastroenteritis, or anxiety disorder.
“Treatment involves gradual desensitization to feared objects: the highchair, bib, bottle, or spoon,” Dr. Chatoor explained. “It also involves training of the mother in behavioral techniques to feed the child in spite of the child’s fear and distress.”
Any underlying medical condition causing pain or distress should be treated. Additional measures may include, for example, use of a graduated approach, starting with liquids and progressing to purees, if the child fears solid foods, and prescribing anxiolytic medication in cases of severe anxiety.
Dr. Chatoor disclosed that she has lectured internationally at conferences on feeding disorders that were organized by Abbott Nutrition International and that the company provided a research grant for a study on feeding.
SAN FRANCISCO – Clinicians are likely to encounter diverse feeding problems in daily practice that will challenge their diagnostic and treatment acumen, Dr. Irene Chatoor told attendees of the annual meeting of the American Academy of Pediatrics.
These problems run the gamut from the most prevalent but least serious picky eating, to the least prevalent but most serious feeding disorders, she noted. Correspondingly, management will range from simple reassurance of parents to more intensive behavioral and medical interventions.
Assessment
“When you assess a feeding problem, you have to look at both the mother and father, and the child,” she advised. The parents are evaluated for their feeding style, while the child is evaluated for three feeding problems: limited appetite, selective intake, and fear of feeding (Pediatrics. 2015;135[2]:344-53).
Clinicians must be alert for organic red flags, such as dysphagia, aspiration, and vomiting, and for behavioral red flags, such as food fixation, abrupt cessation of feeding after a trigger event, and anticipatory gagging. An overarching red flag is failure to thrive.
Sometimes, a child will have both an organic condition and a behavioral feeding disorder at the same time. “It’s very important that you don’t think one excludes the other,” she cautioned.
Diagnosis
“To delineate milder feeding difficulties from feeding disorders, there must be some form of impairment caused by the feeding problem,” Dr. Chatoor commented. “Why is this important? For two reasons. One is for the insurance companies, because they don’t pay unless there is a disorder. And then there is research: you cannot do research unless you clearly define what you are studying.”
Children are considered to have impairment if they have weight loss or growth faltering, considerable nutritional deficiency, or a marked interference with psychosocial functioning.
“When we diagnose feeding problems, it is best done with a multidisciplinary team,” Dr. Chatoor maintained. “I have learned many years ago that I’m not effective in helping parents deal with the feeding disorder if they are still in the back of their mind worried that the child has something organically wrong and that’s why the child does not want to eat.”
Accordingly, various team members perform a medical examination, a nutritional assessment, an oral motor and sensory evaluation, and a psychiatric or psychological assessment to identify the root cause or causes of the problem.
When it comes to behavioral etiologies, the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) now groups all feeding and eating disorders together in one section, reflecting the fact that disorders starting early in life can and often do track into adolescence and adulthood, according to Dr. Chatoor.
The manual also features a new diagnosis, avoidant/restrictive food intake disorder (ARFID). Key criteria include the presence of an eating or feeding disturbance that cannot be better explained by lack of available food, culturally sanctioned practices, or a concurrent medical condition or another mental disorder.
The child must have a persistent failure to meet appropriate nutritional and/or energy needs, associated with any of four findings: significant weight loss or failure to achieve expected weight gain or growth, significant nutritional deficiencies, dependence on enteral feeding or oral nutritional supplements, and marked interference with psychosocial functioning.
“You need to have at least one but often you have a combination of nutritional and emotional impairment in the same child,” she commented.
ARFID and its treatment
There are three subtypes of ARFID having different features, treatments, and prognosis, although they all share in common food refusal, according to Dr. Chatoor.
The first subtype – apparent lack of interest in eating or food – emerges by 3 years of age, most often during the transition to self-feeding between 9 and 18 months. Affected children refuse to eat an adequate amount of food for at least 1 month, rarely communicate hunger, lack interest in food, and prefer to play or talk. They typically present with growth deficiency.
“If you have a child who refuses to eat, it generates anxiety in the mother. The mother does things she would not normally do with the child who is eating well. She starts to distract, to cajole, to sometimes even force-feed the child,” Dr. Chatoor explained. “And the more she engages in these behaviors, the more resistant the child becomes. So they become trapped in this vicious cycle.”
Treatment is aimed at removing this conflict with three approaches: explaining to parents the infant’s special temperament (notably, high arousal and difficulty turning off excitement); addressing their background, including any eating issues of their own, and difficulty with setting limits; and providing specific feeding guidelines and a time-out procedure.
The guidelines stress regular feeding, withholding of all snacks, keeping the child at the table for 20 to 30 minutes, and not using any distractions or pressure. Also, importantly, the parents should have dinner with their child. “I always tell the parents what is good for the child is good for you. It’s good for your whole family,” she said. “If they have other children, these rules apply to everybody. Young children learn to eat by watching their parents eating.”
With early and consistent use of these interventions, about two-thirds of children outgrow this eating/feeding disturbance by mid-childhood, according to Dr. Chatoor.
Children with the second subtype of ARFID – avoidance based on the sensory characteristics of food – consistently refuse to eat certain foods having specific tastes, textures, temperatures, smells, and/or appearances. Onset occurs during the toddler years, when a new or different food is introduced.
“Not only do they refuse the same foods that were aversive, but they also generalize it. So these children are afraid to try other foods and they may end up limiting food groups – they don’t eat any vegetables and some of them don’t eat any meats,” she explained. “They are a challenge because this always causes a nutritional deficiency and they also have problems socially as they get older.”
Treatment varies by age, with gradual desensitization for infants and parent modeling of eating new foods for toddlers. A multifaceted approach is used for preschoolers, combining modeling, giving foods attractive shapes and names, having the child participate in food preparation, and using focused play therapy, such as feeding dolls who are “brave” and try new foods.
Clinicians can explain to affected school-aged children that they are “supertasters,” having more taste buds and therefore experiencing food more intensely, and that they can help their taste buds not react so strongly by starting to eat small amounts of new foods and gradually increasing over time. Parents can let the child make a list of 10 foods they would like to eat, and award them points for “courage” for every bite of a new food they try.
Prognosis of this type of ARFID varies, according to Dr. Chatoor. “Through gradual exposure, young children can expand the variety of foods they eat,” she elaborated. However, “some children become very rigid and brand sensitive in regard to the food they are willing to eat and begin to experience social problems during mid-childhood and adolescence. Some children grow up eating a limited diet, but finding ways to compensate nutritionally and socially.”
Children with the third subtype of ARFID – concern about aversive consequences of eating – have an acute onset of consistent food refusal at any age, from infancy onward, after experiencing a traumatic event or repeated traumatic insults to the oropharynx or gastrointestinal tract that trigger distress in the child. These children often have comorbidities such as gastroesophageal reflux, eosinophilic gastroenteritis, or anxiety disorder.
“Treatment involves gradual desensitization to feared objects: the highchair, bib, bottle, or spoon,” Dr. Chatoor explained. “It also involves training of the mother in behavioral techniques to feed the child in spite of the child’s fear and distress.”
Any underlying medical condition causing pain or distress should be treated. Additional measures may include, for example, use of a graduated approach, starting with liquids and progressing to purees, if the child fears solid foods, and prescribing anxiolytic medication in cases of severe anxiety.
Dr. Chatoor disclosed that she has lectured internationally at conferences on feeding disorders that were organized by Abbott Nutrition International and that the company provided a research grant for a study on feeding.
EXPERT ANALYSIS FROM AAP 16
The Robots Are Coming to Vascular Surgery
Emerging technologies in robotics systems will be the subject of a key session at the 2016 Veithsymposium, entitled “Vascular Robotics and Guidance Systems” and co-chaired by Dr. Kenneth Ouriel, founder of Syntactx, and Dr. Anton N. Sidawy of George Washington University.
According to Dr. Sidawy, the major focus of the session will be the Magellan System, which is a steerable catheter system controlled by a remote physician console. The system has two major components: a robotic arm and the aforementioned remote console. This design is meant to keep the operator away from the table, thereby decreasing exposure to the radiation source.
The promise of such technology is becoming increasingly acknowledged in the field of vascular surgery. The Magellan can be used in a number of endovascular procedures, including – but not necessarily limited to – embolization, endovascular aneurysm repair (EVAR), angioplasty, stenting, crossing total occlusions, and venous procedures. Such applications will be highlighted in-depth during this session, with presentations by some of the most prominent names in the field of vascular robotic surgery.
The session will conclude with a panel discussion involving all the speakers and which will cover the current state and future of robotics in vascular surgery.
Thursday, November 17, 2016
Session 53: VASCULAR ROBOTICS AND GUIDANCE SYSTEMS
Moderators: Kenneth Ouriel, MD, MBA / Anton N. Sidawy, MD, MPH
3:52 PM - 4:52 PM
Emerging technologies in robotics systems will be the subject of a key session at the 2016 Veithsymposium, entitled “Vascular Robotics and Guidance Systems” and co-chaired by Dr. Kenneth Ouriel, founder of Syntactx, and Dr. Anton N. Sidawy of George Washington University.
According to Dr. Sidawy, the major focus of the session will be the Magellan System, which is a steerable catheter system controlled by a remote physician console. The system has two major components: a robotic arm and the aforementioned remote console. This design is meant to keep the operator away from the table, thereby decreasing exposure to the radiation source.
The promise of such technology is becoming increasingly acknowledged in the field of vascular surgery. The Magellan can be used in a number of endovascular procedures, including – but not necessarily limited to – embolization, endovascular aneurysm repair (EVAR), angioplasty, stenting, crossing total occlusions, and venous procedures. Such applications will be highlighted in-depth during this session, with presentations by some of the most prominent names in the field of vascular robotic surgery.
The session will conclude with a panel discussion involving all the speakers and which will cover the current state and future of robotics in vascular surgery.
Thursday, November 17, 2016
Session 53: VASCULAR ROBOTICS AND GUIDANCE SYSTEMS
Moderators: Kenneth Ouriel, MD, MBA / Anton N. Sidawy, MD, MPH
3:52 PM - 4:52 PM
Emerging technologies in robotics systems will be the subject of a key session at the 2016 Veithsymposium, entitled “Vascular Robotics and Guidance Systems” and co-chaired by Dr. Kenneth Ouriel, founder of Syntactx, and Dr. Anton N. Sidawy of George Washington University.
According to Dr. Sidawy, the major focus of the session will be the Magellan System, which is a steerable catheter system controlled by a remote physician console. The system has two major components: a robotic arm and the aforementioned remote console. This design is meant to keep the operator away from the table, thereby decreasing exposure to the radiation source.
The promise of such technology is becoming increasingly acknowledged in the field of vascular surgery. The Magellan can be used in a number of endovascular procedures, including – but not necessarily limited to – embolization, endovascular aneurysm repair (EVAR), angioplasty, stenting, crossing total occlusions, and venous procedures. Such applications will be highlighted in-depth during this session, with presentations by some of the most prominent names in the field of vascular robotic surgery.
The session will conclude with a panel discussion involving all the speakers and which will cover the current state and future of robotics in vascular surgery.
Thursday, November 17, 2016
Session 53: VASCULAR ROBOTICS AND GUIDANCE SYSTEMS
Moderators: Kenneth Ouriel, MD, MBA / Anton N. Sidawy, MD, MPH
3:52 PM - 4:52 PM
Addiction potential
By nature, my wife and I are risk-averse people. Our investment strategy is just a few baby steps short of hiding our money under the mattress. We have never tried marijuana, though to some extent this is because we were out of college and already married when its popularity surged across this country’s campuses. We do drink alcohol, which was so ubiquitous when we were teenagers that it seemed innocuous.
Given my personality, you can understand why I have trouble understanding why anyone would ever try heroin or any opiate. I realize that many of the young addicts don’t follow the news closely enough to realize the risks. But the epidemic of addiction is so entrenched here in rural Maine that they must have known someone who has died of an overdose.
A recent op-ed piece in the New York Times describes a program that attempts to do just that (“The 4 Traits That Put Kids at Risk for Addiction,” by Maia Szalavitz, Sept. 29, 2016). The program is called Preventure and is the result of some work by Patricia Conrod, a psychiatry professor at the University of Montreal. It has been tried in Britain, Canada, Australia, and the Netherlands with some success in reducing binge drinking. In other studies, it reduced symptoms of depression, panic attacks, and impulsive behavior.
The program begins with testing middle school students and focuses on the traits of hopelessness, sensation-seeking, impulsiveness, and anxiety sensitivity. Hopeless is not a surprising choice given that many of the areas of this country with highest rates of opiate addiction are economically depressed. And it is easy to see why impulsivity and sensation-seeking are related to addiction potential. Anxiety-sensitivity is a less intuitive choice.
With the results of this testing in hand, the program administrators wait several months before approaching the outliers. The next step offers two 90-minute workshops to the entire school with the stated goal of showing how the students can channel their personalities toward success. Although the workshops are advertised as being open to everyone but limited in number of attendees, only the students identified as being at the highest risk are actually selected. It is hoped that this deception will avoid having the participants feel that they have been labeled. However, if a student asks about the selection process, he is given an honest answer. The workshops are targeted to the students’ specific emotional and behavioral vulnerabilities, and teaches cognitive behavioral techniques on how they can be managed.
While I think the deceptive selection process is a clever to wrinkle to avoid labeling, I wonder how long the ruse will survive should the program become more universally adopted. With increased popularity and publicity, every parent and most of the children in a school will realize why the test is being administered and what being selected for the workshop means. There is the threat that being identified as at risk for addiction will become a self-fulfilling prophecy and a trigger for depression.
Preventure sounds like a program worth watching. If larger series and long-term outcomes continue to be favorable, it will remain to be seen whether labeling is a hazard worth worrying about.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.” Email him at [email protected].
By nature, my wife and I are risk-averse people. Our investment strategy is just a few baby steps short of hiding our money under the mattress. We have never tried marijuana, though to some extent this is because we were out of college and already married when its popularity surged across this country’s campuses. We do drink alcohol, which was so ubiquitous when we were teenagers that it seemed innocuous.
Given my personality, you can understand why I have trouble understanding why anyone would ever try heroin or any opiate. I realize that many of the young addicts don’t follow the news closely enough to realize the risks. But the epidemic of addiction is so entrenched here in rural Maine that they must have known someone who has died of an overdose.
A recent op-ed piece in the New York Times describes a program that attempts to do just that (“The 4 Traits That Put Kids at Risk for Addiction,” by Maia Szalavitz, Sept. 29, 2016). The program is called Preventure and is the result of some work by Patricia Conrod, a psychiatry professor at the University of Montreal. It has been tried in Britain, Canada, Australia, and the Netherlands with some success in reducing binge drinking. In other studies, it reduced symptoms of depression, panic attacks, and impulsive behavior.
The program begins with testing middle school students and focuses on the traits of hopelessness, sensation-seeking, impulsiveness, and anxiety sensitivity. Hopeless is not a surprising choice given that many of the areas of this country with highest rates of opiate addiction are economically depressed. And it is easy to see why impulsivity and sensation-seeking are related to addiction potential. Anxiety-sensitivity is a less intuitive choice.
With the results of this testing in hand, the program administrators wait several months before approaching the outliers. The next step offers two 90-minute workshops to the entire school with the stated goal of showing how the students can channel their personalities toward success. Although the workshops are advertised as being open to everyone but limited in number of attendees, only the students identified as being at the highest risk are actually selected. It is hoped that this deception will avoid having the participants feel that they have been labeled. However, if a student asks about the selection process, he is given an honest answer. The workshops are targeted to the students’ specific emotional and behavioral vulnerabilities, and teaches cognitive behavioral techniques on how they can be managed.
While I think the deceptive selection process is a clever to wrinkle to avoid labeling, I wonder how long the ruse will survive should the program become more universally adopted. With increased popularity and publicity, every parent and most of the children in a school will realize why the test is being administered and what being selected for the workshop means. There is the threat that being identified as at risk for addiction will become a self-fulfilling prophecy and a trigger for depression.
Preventure sounds like a program worth watching. If larger series and long-term outcomes continue to be favorable, it will remain to be seen whether labeling is a hazard worth worrying about.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.” Email him at [email protected].
By nature, my wife and I are risk-averse people. Our investment strategy is just a few baby steps short of hiding our money under the mattress. We have never tried marijuana, though to some extent this is because we were out of college and already married when its popularity surged across this country’s campuses. We do drink alcohol, which was so ubiquitous when we were teenagers that it seemed innocuous.
Given my personality, you can understand why I have trouble understanding why anyone would ever try heroin or any opiate. I realize that many of the young addicts don’t follow the news closely enough to realize the risks. But the epidemic of addiction is so entrenched here in rural Maine that they must have known someone who has died of an overdose.
A recent op-ed piece in the New York Times describes a program that attempts to do just that (“The 4 Traits That Put Kids at Risk for Addiction,” by Maia Szalavitz, Sept. 29, 2016). The program is called Preventure and is the result of some work by Patricia Conrod, a psychiatry professor at the University of Montreal. It has been tried in Britain, Canada, Australia, and the Netherlands with some success in reducing binge drinking. In other studies, it reduced symptoms of depression, panic attacks, and impulsive behavior.
The program begins with testing middle school students and focuses on the traits of hopelessness, sensation-seeking, impulsiveness, and anxiety sensitivity. Hopeless is not a surprising choice given that many of the areas of this country with highest rates of opiate addiction are economically depressed. And it is easy to see why impulsivity and sensation-seeking are related to addiction potential. Anxiety-sensitivity is a less intuitive choice.
With the results of this testing in hand, the program administrators wait several months before approaching the outliers. The next step offers two 90-minute workshops to the entire school with the stated goal of showing how the students can channel their personalities toward success. Although the workshops are advertised as being open to everyone but limited in number of attendees, only the students identified as being at the highest risk are actually selected. It is hoped that this deception will avoid having the participants feel that they have been labeled. However, if a student asks about the selection process, he is given an honest answer. The workshops are targeted to the students’ specific emotional and behavioral vulnerabilities, and teaches cognitive behavioral techniques on how they can be managed.
While I think the deceptive selection process is a clever to wrinkle to avoid labeling, I wonder how long the ruse will survive should the program become more universally adopted. With increased popularity and publicity, every parent and most of the children in a school will realize why the test is being administered and what being selected for the workshop means. There is the threat that being identified as at risk for addiction will become a self-fulfilling prophecy and a trigger for depression.
Preventure sounds like a program worth watching. If larger series and long-term outcomes continue to be favorable, it will remain to be seen whether labeling is a hazard worth worrying about.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.” Email him at [email protected].