User login
Hormonal Contraceptives: Risk or Benefit in Migraine?
SAN DIEGO—Among women with migraine, low-dose combined estrogen–progestin contraceptives do not increase the risk of stroke, and continuous hormonal contraceptive use may reduce menstrual migraine, according to a lecture delivered at the 58th Annual Scientific Meeting of the American Headache Society.
High doses of combined oral contraceptives do increase stroke risk, and migraine with aura confers an independent increased risk of stroke, said Anne H. Calhoun, MD, Professor of Anesthesiology and Psychiatry at the University of North Carolina in Chapel Hill and partner and cofounder of the Carolina Headache Institute in Durham. Evidence does not suggest, however, that low-dose hormonal contraceptive use together with history of migraine confers additive risk, she said.
Over the last several decades, various combined oral contraceptive regimens (eg, triphasic, extended cycle, and continuous) with a range of estrogen doses have been developed. “They are all over the place, and we really need to know what we are dealing with,” Dr. Calhoun said. Risks of combined oral contraceptives, including myocardial infarction and stroke, are almost exclusively seen with smoking and high-dose pills, she said.
Dr. Calhoun prefers to prescribe pills that inhibit ovulation while using 20 μg or less of the synthetic estrogen ethinyl estradiol (EE) for women at increased risk of stroke. The highest dose pill in the United States contains 50 μg of estrogen. The lowest dose pill contains 10 μg.
Placebo Side Effects
In hormonal contraception, headache often develops during the placebo week. A typical contraceptive regimen includes 21 active pills and seven placebo pills. Sulak et al found that 70% of women taking birth control pills had headache during the placebo week, and peak incidence of headache was on the third day of the placebo week. “When you eliminated the placebo week, it improved mood scores, improved headache scores, there was less dysmenorrhea and pelvic pain,” Dr. Calhoun said.
When estrogen concentrations decrease upon taking placebo, 5HT concentration in the CNS declines, serotonin synthesis declines, monoamine oxidase and calcitonin gene-related peptide concentrations increase, and beta endorphins decline. “All pain is perceived as more intense,” she said. Furthermore, flow-mediated vasodilation parallels estrogen levels.
“You would think looking at all this, in a field where most of the patients are women, and the majority of them have menstrual migraine, and all of these things happen when estrogen falls, that headache specialists would be really interested in preventing estrogen from declining every month,” Dr. Calhoun said.
Reducing Migraine
In 2012, Dr. Calhoun and colleagues published a retrospective database review that identified 23 women in a subspecialty menstrual migraine clinic who had migraine with aura, a confirmed diagnosis of menstrual-related migraine, and who received treatment with extended-cycle dosing of a transvaginal ring contraceptive containing 0.120 mg of etonogestrel and 15 μg of EE for at least a month. The women were treated with the transvaginal ring for the purpose of migraine prevention, and not to prevent pregnancy. Participants were observed for an average of eight months. Women used the ultralow-dose ring contraceptive continuously. Instead of using the ring for three weeks followed by one week without the ring, women replaced the ring every three weeks. “With that, you stop ovulation with a level of estrogen that is lower than your own natural menstrual cycle. You do not have the high ovulatory peaks or even the peaks of the luteal phase,” she said.
At baseline, subjects experienced a median of 3.2 auras per month. With treatment, the median number of auras per month decreased to 0.2. Frequency of aura decreased from baseline for each participant, and menstrual migraine was eliminated in 91.3% of patients. Such use of hormonal contraceptives for the prevention of migraine is off label, Dr. Calhoun noted.
Risk of Stroke
How oral contraceptives affect risk of stroke has been studied for decades. In a 1975 study in JAMA, researchers looked at 140 cases of stroke in young women and compared them with hospital controls. There was a 4.6 times increased risk of stroke if a woman was taking high-dose oral contraceptives.
Hannaford et al in 1994 published a nested case–control analysis using data mainly from the 1970s and 1980s. They found that high-dose oral contraceptive use was associated with a sixfold increased risk of first stroke. Low-dose pills—including 30-μg and 35-μg pills, “which I do not think of as particularly low dose these days”—did not significantly increase risk, Dr. Calhoun said. However, the risk with these moderate-dose pills is usually around 1.2 to 2.5 times greater and is significant in some studies, she noted. Today’s “low-dose” pills (≤ 20 μg EE) are the best for decreasing risk.
In 1996, a study by Petitti et al looked at risk of stroke with “low-dose” (at that time meaning simply < 50 μg EE) hormonal contraceptives in the US. They identified 408 strokes in 3.6 million women-years and concluded that stroke is rare in this population. Low-dose pills did not increase the risk. At that time, 99.4% of prescriptions for hormonal contraception in the US contained less than 50 μg of estrogen.
ACOG Guideline
In 2006, however, the American College of Obstetrics and Gynecology (ACOG) recommended against using combination hormonal contraceptives in patients with migraine with aura. This guideline and some of the studies on which the recommendation was based are problematic, Dr. Calhoun said.
The recommendation stemmed in part from a concern that all women with migraine are at increased risk of stroke if they take combined hormonal contraceptives. That concern was based on a 1996 World Health Organization study that had no US sites. In the study, the majority of stroke cases were smokers and the average age was over 35. In the US, 35 tends to be the age at which physicians—in accordance with ACOG recommendations—no longer prescribe combined hormonal contraceptives for smokers, Dr. Calhoun said. In addition, the majority of the stroke cases were using high-dose pills. The authors concluded that migraine of greater than 12 years’ duration, migraine with aura, and frequent migraine with aura increased the risk of stroke. “But the authors concluded themselves, in no case did corrections for oral contraceptive use alter these observations. It was not a factor,” Dr. Calhoun said.
The ACOG guideline also was based on a Danish population-based case–control study by Lidegaard et al that found a threefold increased risk of ischemic stroke among migraineurs using oral contraceptives. In this study, however, they reported that only 6% of the control group had migraine, whereas 16% to 18% of women in Denmark have migraine—a rate similar to what was seen in the cases. That produced the threefold increased risk reported in the study, Dr. Calhoun said. A similar study in France did not find increased risk among migraineurs using oral contraceptives, but in that study, both controls and migraineurs had the normal frequency of migraine in the population.
Finally, the ACOG recommendation was based on a pooled analysis of two large US case–control studies that found a twofold increased risk of ischemic stroke among migraineurs using oral contraceptives. This finding by Schwartz et al was based on only four cases. The prevalence of migraine was virtually identical among ischemic stroke cases and controls who were using oral contraceptives (7.8% and 7.7%, respectively), but became significant after adjustment for other factors. But a key factor that was not taken into account was use of high-dose (≥ 50 μg EE) pills, Dr. Calhoun said. They were used by only 11 of the 1,564 ischemic and hemorrhagic stroke cases and controls, but accounted for four of the strokes.
Low Doses Appear Safe
Recent studies have had similar findings. A 15-year prospective population-based study by Lidegaard et al in 2012 analyzed 3,300 thrombotic strokes and 1,700 myocardial infarctions in more than 1.6 million women. The overall risk was low, and the absolute risk of thrombotic stroke and myocardial infarction was not increased with 20-μg pills. Pills with doses of 30 μg to 40 μg, however, as much as doubled the risk. “It is still better than the 4.5-times increased risk we had with the 50-μg pills, but we do not want to go there,” Dr. Calhoun said.
A 2013 study by Sidney et al compared a 20-μg oral birth control pill with a 30-μg oral birth control pill, a 20-μg patch birth control product, and a 15-μg ring birth control product. As expected, the 30-μg pill increased risk of thrombotic events, relative to the 20-μg pill, whereas the other products did not, Dr. Calhoun said.
“The argument against using combined hormonal contraceptives in migraine with aura is based on concerns that all women are at increased risk of stroke with oral contraceptives. That is false,” she said. “It is high-dose oral contraceptives that increase the risk.”
—Jake Remaly
Suggested Reading
ACOG Committee on Practice Bulletins-Gynecology. ACOG practice bulletin. No. 73: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2006;107(6):1453-1472.
Calhoun A, Ford S, Pruitt A. The impact of extended-cycle vaginal ring contraception on migraine aura: a retrospective case series. Headache. 2012;52(8):1246-1253.
Hannaford PC, Croft PR, Kay CR. Oral contraception and stroke. Evidence from the Royal College of General Practitioners’ Oral Contraception study. Stroke. 1994;25(5):935-942.
Ischaemic stroke and combined oral contraceptives: results of an international, multicentre, case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet. 1996;348(9026):498-505.
Lidegaard Ø, Kreiner S. Contraceptives and cerebral thrombosis: a five-year national case-control study. Contraception. 2002;65(3):197-205.
Lidegaard Ø, Løkkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366(24):2257-2266.
MacGregor EA. Contraception and headache. Headache. 2013;53(2):247-276.
Oral contraceptives and stroke in young women. Associated risk factors. JAMA. 1975;231(7):718-722.
Petitti DB, Sidney S, Bernstein A, et al. Stroke in users of low-dose oral contraceptives. N Engl J Med. 1996;335(1):8-15.
Schwartz SM, Petitti DB, Siscovick DS, et al. Stroke and use of low-dose oral contraceptives in young women: a pooled analysis of two US studies. Stroke. 1998;29(11):2277-2284.
Sidney S, Cheetham TC, Connell FA, et al. Recent combined hormonal contraceptives (CHCs) and the risk of thromboembolism and other cardiovascular events in new users. Contraception. 2013;87(1):93-100.
Sulak P, Willis S, Kuehl T, et al. Headaches and oral contraceptives: impact of eliminating the standard 7-day placebo interval. Headache. 2007;47(1):27-37.
Sulak PJ, Scow RD, Preece C, et al. Hormone withdrawal symptoms in oral contraceptive users. Obstet Gynecol. 2000;95(2):261-266.
SAN DIEGO—Among women with migraine, low-dose combined estrogen–progestin contraceptives do not increase the risk of stroke, and continuous hormonal contraceptive use may reduce menstrual migraine, according to a lecture delivered at the 58th Annual Scientific Meeting of the American Headache Society.
High doses of combined oral contraceptives do increase stroke risk, and migraine with aura confers an independent increased risk of stroke, said Anne H. Calhoun, MD, Professor of Anesthesiology and Psychiatry at the University of North Carolina in Chapel Hill and partner and cofounder of the Carolina Headache Institute in Durham. Evidence does not suggest, however, that low-dose hormonal contraceptive use together with history of migraine confers additive risk, she said.
Over the last several decades, various combined oral contraceptive regimens (eg, triphasic, extended cycle, and continuous) with a range of estrogen doses have been developed. “They are all over the place, and we really need to know what we are dealing with,” Dr. Calhoun said. Risks of combined oral contraceptives, including myocardial infarction and stroke, are almost exclusively seen with smoking and high-dose pills, she said.
Dr. Calhoun prefers to prescribe pills that inhibit ovulation while using 20 μg or less of the synthetic estrogen ethinyl estradiol (EE) for women at increased risk of stroke. The highest dose pill in the United States contains 50 μg of estrogen. The lowest dose pill contains 10 μg.
Placebo Side Effects
In hormonal contraception, headache often develops during the placebo week. A typical contraceptive regimen includes 21 active pills and seven placebo pills. Sulak et al found that 70% of women taking birth control pills had headache during the placebo week, and peak incidence of headache was on the third day of the placebo week. “When you eliminated the placebo week, it improved mood scores, improved headache scores, there was less dysmenorrhea and pelvic pain,” Dr. Calhoun said.
When estrogen concentrations decrease upon taking placebo, 5HT concentration in the CNS declines, serotonin synthesis declines, monoamine oxidase and calcitonin gene-related peptide concentrations increase, and beta endorphins decline. “All pain is perceived as more intense,” she said. Furthermore, flow-mediated vasodilation parallels estrogen levels.
“You would think looking at all this, in a field where most of the patients are women, and the majority of them have menstrual migraine, and all of these things happen when estrogen falls, that headache specialists would be really interested in preventing estrogen from declining every month,” Dr. Calhoun said.
Reducing Migraine
In 2012, Dr. Calhoun and colleagues published a retrospective database review that identified 23 women in a subspecialty menstrual migraine clinic who had migraine with aura, a confirmed diagnosis of menstrual-related migraine, and who received treatment with extended-cycle dosing of a transvaginal ring contraceptive containing 0.120 mg of etonogestrel and 15 μg of EE for at least a month. The women were treated with the transvaginal ring for the purpose of migraine prevention, and not to prevent pregnancy. Participants were observed for an average of eight months. Women used the ultralow-dose ring contraceptive continuously. Instead of using the ring for three weeks followed by one week without the ring, women replaced the ring every three weeks. “With that, you stop ovulation with a level of estrogen that is lower than your own natural menstrual cycle. You do not have the high ovulatory peaks or even the peaks of the luteal phase,” she said.
At baseline, subjects experienced a median of 3.2 auras per month. With treatment, the median number of auras per month decreased to 0.2. Frequency of aura decreased from baseline for each participant, and menstrual migraine was eliminated in 91.3% of patients. Such use of hormonal contraceptives for the prevention of migraine is off label, Dr. Calhoun noted.
Risk of Stroke
How oral contraceptives affect risk of stroke has been studied for decades. In a 1975 study in JAMA, researchers looked at 140 cases of stroke in young women and compared them with hospital controls. There was a 4.6 times increased risk of stroke if a woman was taking high-dose oral contraceptives.
Hannaford et al in 1994 published a nested case–control analysis using data mainly from the 1970s and 1980s. They found that high-dose oral contraceptive use was associated with a sixfold increased risk of first stroke. Low-dose pills—including 30-μg and 35-μg pills, “which I do not think of as particularly low dose these days”—did not significantly increase risk, Dr. Calhoun said. However, the risk with these moderate-dose pills is usually around 1.2 to 2.5 times greater and is significant in some studies, she noted. Today’s “low-dose” pills (≤ 20 μg EE) are the best for decreasing risk.
In 1996, a study by Petitti et al looked at risk of stroke with “low-dose” (at that time meaning simply < 50 μg EE) hormonal contraceptives in the US. They identified 408 strokes in 3.6 million women-years and concluded that stroke is rare in this population. Low-dose pills did not increase the risk. At that time, 99.4% of prescriptions for hormonal contraception in the US contained less than 50 μg of estrogen.
ACOG Guideline
In 2006, however, the American College of Obstetrics and Gynecology (ACOG) recommended against using combination hormonal contraceptives in patients with migraine with aura. This guideline and some of the studies on which the recommendation was based are problematic, Dr. Calhoun said.
The recommendation stemmed in part from a concern that all women with migraine are at increased risk of stroke if they take combined hormonal contraceptives. That concern was based on a 1996 World Health Organization study that had no US sites. In the study, the majority of stroke cases were smokers and the average age was over 35. In the US, 35 tends to be the age at which physicians—in accordance with ACOG recommendations—no longer prescribe combined hormonal contraceptives for smokers, Dr. Calhoun said. In addition, the majority of the stroke cases were using high-dose pills. The authors concluded that migraine of greater than 12 years’ duration, migraine with aura, and frequent migraine with aura increased the risk of stroke. “But the authors concluded themselves, in no case did corrections for oral contraceptive use alter these observations. It was not a factor,” Dr. Calhoun said.
The ACOG guideline also was based on a Danish population-based case–control study by Lidegaard et al that found a threefold increased risk of ischemic stroke among migraineurs using oral contraceptives. In this study, however, they reported that only 6% of the control group had migraine, whereas 16% to 18% of women in Denmark have migraine—a rate similar to what was seen in the cases. That produced the threefold increased risk reported in the study, Dr. Calhoun said. A similar study in France did not find increased risk among migraineurs using oral contraceptives, but in that study, both controls and migraineurs had the normal frequency of migraine in the population.
Finally, the ACOG recommendation was based on a pooled analysis of two large US case–control studies that found a twofold increased risk of ischemic stroke among migraineurs using oral contraceptives. This finding by Schwartz et al was based on only four cases. The prevalence of migraine was virtually identical among ischemic stroke cases and controls who were using oral contraceptives (7.8% and 7.7%, respectively), but became significant after adjustment for other factors. But a key factor that was not taken into account was use of high-dose (≥ 50 μg EE) pills, Dr. Calhoun said. They were used by only 11 of the 1,564 ischemic and hemorrhagic stroke cases and controls, but accounted for four of the strokes.
Low Doses Appear Safe
Recent studies have had similar findings. A 15-year prospective population-based study by Lidegaard et al in 2012 analyzed 3,300 thrombotic strokes and 1,700 myocardial infarctions in more than 1.6 million women. The overall risk was low, and the absolute risk of thrombotic stroke and myocardial infarction was not increased with 20-μg pills. Pills with doses of 30 μg to 40 μg, however, as much as doubled the risk. “It is still better than the 4.5-times increased risk we had with the 50-μg pills, but we do not want to go there,” Dr. Calhoun said.
A 2013 study by Sidney et al compared a 20-μg oral birth control pill with a 30-μg oral birth control pill, a 20-μg patch birth control product, and a 15-μg ring birth control product. As expected, the 30-μg pill increased risk of thrombotic events, relative to the 20-μg pill, whereas the other products did not, Dr. Calhoun said.
“The argument against using combined hormonal contraceptives in migraine with aura is based on concerns that all women are at increased risk of stroke with oral contraceptives. That is false,” she said. “It is high-dose oral contraceptives that increase the risk.”
—Jake Remaly
Suggested Reading
ACOG Committee on Practice Bulletins-Gynecology. ACOG practice bulletin. No. 73: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2006;107(6):1453-1472.
Calhoun A, Ford S, Pruitt A. The impact of extended-cycle vaginal ring contraception on migraine aura: a retrospective case series. Headache. 2012;52(8):1246-1253.
Hannaford PC, Croft PR, Kay CR. Oral contraception and stroke. Evidence from the Royal College of General Practitioners’ Oral Contraception study. Stroke. 1994;25(5):935-942.
Ischaemic stroke and combined oral contraceptives: results of an international, multicentre, case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet. 1996;348(9026):498-505.
Lidegaard Ø, Kreiner S. Contraceptives and cerebral thrombosis: a five-year national case-control study. Contraception. 2002;65(3):197-205.
Lidegaard Ø, Løkkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366(24):2257-2266.
MacGregor EA. Contraception and headache. Headache. 2013;53(2):247-276.
Oral contraceptives and stroke in young women. Associated risk factors. JAMA. 1975;231(7):718-722.
Petitti DB, Sidney S, Bernstein A, et al. Stroke in users of low-dose oral contraceptives. N Engl J Med. 1996;335(1):8-15.
Schwartz SM, Petitti DB, Siscovick DS, et al. Stroke and use of low-dose oral contraceptives in young women: a pooled analysis of two US studies. Stroke. 1998;29(11):2277-2284.
Sidney S, Cheetham TC, Connell FA, et al. Recent combined hormonal contraceptives (CHCs) and the risk of thromboembolism and other cardiovascular events in new users. Contraception. 2013;87(1):93-100.
Sulak P, Willis S, Kuehl T, et al. Headaches and oral contraceptives: impact of eliminating the standard 7-day placebo interval. Headache. 2007;47(1):27-37.
Sulak PJ, Scow RD, Preece C, et al. Hormone withdrawal symptoms in oral contraceptive users. Obstet Gynecol. 2000;95(2):261-266.
SAN DIEGO—Among women with migraine, low-dose combined estrogen–progestin contraceptives do not increase the risk of stroke, and continuous hormonal contraceptive use may reduce menstrual migraine, according to a lecture delivered at the 58th Annual Scientific Meeting of the American Headache Society.
High doses of combined oral contraceptives do increase stroke risk, and migraine with aura confers an independent increased risk of stroke, said Anne H. Calhoun, MD, Professor of Anesthesiology and Psychiatry at the University of North Carolina in Chapel Hill and partner and cofounder of the Carolina Headache Institute in Durham. Evidence does not suggest, however, that low-dose hormonal contraceptive use together with history of migraine confers additive risk, she said.
Over the last several decades, various combined oral contraceptive regimens (eg, triphasic, extended cycle, and continuous) with a range of estrogen doses have been developed. “They are all over the place, and we really need to know what we are dealing with,” Dr. Calhoun said. Risks of combined oral contraceptives, including myocardial infarction and stroke, are almost exclusively seen with smoking and high-dose pills, she said.
Dr. Calhoun prefers to prescribe pills that inhibit ovulation while using 20 μg or less of the synthetic estrogen ethinyl estradiol (EE) for women at increased risk of stroke. The highest dose pill in the United States contains 50 μg of estrogen. The lowest dose pill contains 10 μg.
Placebo Side Effects
In hormonal contraception, headache often develops during the placebo week. A typical contraceptive regimen includes 21 active pills and seven placebo pills. Sulak et al found that 70% of women taking birth control pills had headache during the placebo week, and peak incidence of headache was on the third day of the placebo week. “When you eliminated the placebo week, it improved mood scores, improved headache scores, there was less dysmenorrhea and pelvic pain,” Dr. Calhoun said.
When estrogen concentrations decrease upon taking placebo, 5HT concentration in the CNS declines, serotonin synthesis declines, monoamine oxidase and calcitonin gene-related peptide concentrations increase, and beta endorphins decline. “All pain is perceived as more intense,” she said. Furthermore, flow-mediated vasodilation parallels estrogen levels.
“You would think looking at all this, in a field where most of the patients are women, and the majority of them have menstrual migraine, and all of these things happen when estrogen falls, that headache specialists would be really interested in preventing estrogen from declining every month,” Dr. Calhoun said.
Reducing Migraine
In 2012, Dr. Calhoun and colleagues published a retrospective database review that identified 23 women in a subspecialty menstrual migraine clinic who had migraine with aura, a confirmed diagnosis of menstrual-related migraine, and who received treatment with extended-cycle dosing of a transvaginal ring contraceptive containing 0.120 mg of etonogestrel and 15 μg of EE for at least a month. The women were treated with the transvaginal ring for the purpose of migraine prevention, and not to prevent pregnancy. Participants were observed for an average of eight months. Women used the ultralow-dose ring contraceptive continuously. Instead of using the ring for three weeks followed by one week without the ring, women replaced the ring every three weeks. “With that, you stop ovulation with a level of estrogen that is lower than your own natural menstrual cycle. You do not have the high ovulatory peaks or even the peaks of the luteal phase,” she said.
At baseline, subjects experienced a median of 3.2 auras per month. With treatment, the median number of auras per month decreased to 0.2. Frequency of aura decreased from baseline for each participant, and menstrual migraine was eliminated in 91.3% of patients. Such use of hormonal contraceptives for the prevention of migraine is off label, Dr. Calhoun noted.
Risk of Stroke
How oral contraceptives affect risk of stroke has been studied for decades. In a 1975 study in JAMA, researchers looked at 140 cases of stroke in young women and compared them with hospital controls. There was a 4.6 times increased risk of stroke if a woman was taking high-dose oral contraceptives.
Hannaford et al in 1994 published a nested case–control analysis using data mainly from the 1970s and 1980s. They found that high-dose oral contraceptive use was associated with a sixfold increased risk of first stroke. Low-dose pills—including 30-μg and 35-μg pills, “which I do not think of as particularly low dose these days”—did not significantly increase risk, Dr. Calhoun said. However, the risk with these moderate-dose pills is usually around 1.2 to 2.5 times greater and is significant in some studies, she noted. Today’s “low-dose” pills (≤ 20 μg EE) are the best for decreasing risk.
In 1996, a study by Petitti et al looked at risk of stroke with “low-dose” (at that time meaning simply < 50 μg EE) hormonal contraceptives in the US. They identified 408 strokes in 3.6 million women-years and concluded that stroke is rare in this population. Low-dose pills did not increase the risk. At that time, 99.4% of prescriptions for hormonal contraception in the US contained less than 50 μg of estrogen.
ACOG Guideline
In 2006, however, the American College of Obstetrics and Gynecology (ACOG) recommended against using combination hormonal contraceptives in patients with migraine with aura. This guideline and some of the studies on which the recommendation was based are problematic, Dr. Calhoun said.
The recommendation stemmed in part from a concern that all women with migraine are at increased risk of stroke if they take combined hormonal contraceptives. That concern was based on a 1996 World Health Organization study that had no US sites. In the study, the majority of stroke cases were smokers and the average age was over 35. In the US, 35 tends to be the age at which physicians—in accordance with ACOG recommendations—no longer prescribe combined hormonal contraceptives for smokers, Dr. Calhoun said. In addition, the majority of the stroke cases were using high-dose pills. The authors concluded that migraine of greater than 12 years’ duration, migraine with aura, and frequent migraine with aura increased the risk of stroke. “But the authors concluded themselves, in no case did corrections for oral contraceptive use alter these observations. It was not a factor,” Dr. Calhoun said.
The ACOG guideline also was based on a Danish population-based case–control study by Lidegaard et al that found a threefold increased risk of ischemic stroke among migraineurs using oral contraceptives. In this study, however, they reported that only 6% of the control group had migraine, whereas 16% to 18% of women in Denmark have migraine—a rate similar to what was seen in the cases. That produced the threefold increased risk reported in the study, Dr. Calhoun said. A similar study in France did not find increased risk among migraineurs using oral contraceptives, but in that study, both controls and migraineurs had the normal frequency of migraine in the population.
Finally, the ACOG recommendation was based on a pooled analysis of two large US case–control studies that found a twofold increased risk of ischemic stroke among migraineurs using oral contraceptives. This finding by Schwartz et al was based on only four cases. The prevalence of migraine was virtually identical among ischemic stroke cases and controls who were using oral contraceptives (7.8% and 7.7%, respectively), but became significant after adjustment for other factors. But a key factor that was not taken into account was use of high-dose (≥ 50 μg EE) pills, Dr. Calhoun said. They were used by only 11 of the 1,564 ischemic and hemorrhagic stroke cases and controls, but accounted for four of the strokes.
Low Doses Appear Safe
Recent studies have had similar findings. A 15-year prospective population-based study by Lidegaard et al in 2012 analyzed 3,300 thrombotic strokes and 1,700 myocardial infarctions in more than 1.6 million women. The overall risk was low, and the absolute risk of thrombotic stroke and myocardial infarction was not increased with 20-μg pills. Pills with doses of 30 μg to 40 μg, however, as much as doubled the risk. “It is still better than the 4.5-times increased risk we had with the 50-μg pills, but we do not want to go there,” Dr. Calhoun said.
A 2013 study by Sidney et al compared a 20-μg oral birth control pill with a 30-μg oral birth control pill, a 20-μg patch birth control product, and a 15-μg ring birth control product. As expected, the 30-μg pill increased risk of thrombotic events, relative to the 20-μg pill, whereas the other products did not, Dr. Calhoun said.
“The argument against using combined hormonal contraceptives in migraine with aura is based on concerns that all women are at increased risk of stroke with oral contraceptives. That is false,” she said. “It is high-dose oral contraceptives that increase the risk.”
—Jake Remaly
Suggested Reading
ACOG Committee on Practice Bulletins-Gynecology. ACOG practice bulletin. No. 73: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2006;107(6):1453-1472.
Calhoun A, Ford S, Pruitt A. The impact of extended-cycle vaginal ring contraception on migraine aura: a retrospective case series. Headache. 2012;52(8):1246-1253.
Hannaford PC, Croft PR, Kay CR. Oral contraception and stroke. Evidence from the Royal College of General Practitioners’ Oral Contraception study. Stroke. 1994;25(5):935-942.
Ischaemic stroke and combined oral contraceptives: results of an international, multicentre, case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet. 1996;348(9026):498-505.
Lidegaard Ø, Kreiner S. Contraceptives and cerebral thrombosis: a five-year national case-control study. Contraception. 2002;65(3):197-205.
Lidegaard Ø, Løkkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366(24):2257-2266.
MacGregor EA. Contraception and headache. Headache. 2013;53(2):247-276.
Oral contraceptives and stroke in young women. Associated risk factors. JAMA. 1975;231(7):718-722.
Petitti DB, Sidney S, Bernstein A, et al. Stroke in users of low-dose oral contraceptives. N Engl J Med. 1996;335(1):8-15.
Schwartz SM, Petitti DB, Siscovick DS, et al. Stroke and use of low-dose oral contraceptives in young women: a pooled analysis of two US studies. Stroke. 1998;29(11):2277-2284.
Sidney S, Cheetham TC, Connell FA, et al. Recent combined hormonal contraceptives (CHCs) and the risk of thromboembolism and other cardiovascular events in new users. Contraception. 2013;87(1):93-100.
Sulak P, Willis S, Kuehl T, et al. Headaches and oral contraceptives: impact of eliminating the standard 7-day placebo interval. Headache. 2007;47(1):27-37.
Sulak PJ, Scow RD, Preece C, et al. Hormone withdrawal symptoms in oral contraceptive users. Obstet Gynecol. 2000;95(2):261-266.
Is regional anesthesia safer in CEA?
COLUMBUS, OHIO – General anesthesia during carotid endarterectomy carries almost twice the risk of complications and unplanned intubation as regional anesthesia, but the latter approach, which is not available in all hospitals, has its own issues, an analysis of procedures from a statewide database in Michigan found.
“This study is timely because of CMS [Center for Medicare & Medicaid Services] initiatives tying reimbursement to specific quality measures,” Ahmad S Hussain, MD, of Wayne State University in Detroit said in reporting the study results at the annual meeting of the Midwestern Vascular Surgery Society.
Regional anesthesia in CEA emerged in the 1990s, Dr. Hussain said, and allows for more reliable neurologic monitoring and more direct evaluation of the need for stenting during CEA than general anesthesia, which requires continuous monitoring of cerebral perfusion with carotid stump pressures, electroencephalogram, and transcranial doppler.
The researchers retrospectively analyzed 4,558 patients who had CEA at hospitals participating in the Michigan Surgical Quality Cooperative from 2012 to 2014 – 4,008 of whom had general anesthesia and 550 regional anesthesia.
“Advocates for carotid endarterectomy with regional anesthesia cite a reduction in hemodynamic instability and the ability for neurological monitoring, but many still prefer general anesthesia because the benefits of regional anesthesia have not been clearly demonstrated, allowing that regional anesthesia may not be available in all centers and allowing that a certain amount of patient movement during the procedure may not be uniformly tolerated,” Dr. Hussain said.
General anesthesia patients in the study had more than twice the rate of any morbidity at 30 days than those who had regional, 8.7% vs. 4.2%, and significantly higher rates of unplanned intervention, 2.1% vs. 0.6%. Dr. Hussain said. However, the study could not determine differences in 30-day mortality or other key outcomes, such as rates of pneumonia, sepsis, deep vein thrombosis, or pulmonary embolism, becauseof insufficient sample sizes, Dr. Hussain said
The study found less significant differences between general and regional anesthesia techniques, respectively, in rates of extended length of stay, 12.1% vs. 9.5%; readmissions, 9.2% vs. 6.1%; and reoperation, 4.5% vs. 3%.
The retrospective study used two models to analyze odds ratios: Model 1 adjusted for case mix; and model 2 adjusted for case mix as fixed effects and site as a random effect. While the retrospective nature of the study may be a limitation, the findings support the use of regional anesthesia for CEA when available, Dr. Hussain said.
Dr. Hussain had no relationships to disclose.
COLUMBUS, OHIO – General anesthesia during carotid endarterectomy carries almost twice the risk of complications and unplanned intubation as regional anesthesia, but the latter approach, which is not available in all hospitals, has its own issues, an analysis of procedures from a statewide database in Michigan found.
“This study is timely because of CMS [Center for Medicare & Medicaid Services] initiatives tying reimbursement to specific quality measures,” Ahmad S Hussain, MD, of Wayne State University in Detroit said in reporting the study results at the annual meeting of the Midwestern Vascular Surgery Society.
Regional anesthesia in CEA emerged in the 1990s, Dr. Hussain said, and allows for more reliable neurologic monitoring and more direct evaluation of the need for stenting during CEA than general anesthesia, which requires continuous monitoring of cerebral perfusion with carotid stump pressures, electroencephalogram, and transcranial doppler.
The researchers retrospectively analyzed 4,558 patients who had CEA at hospitals participating in the Michigan Surgical Quality Cooperative from 2012 to 2014 – 4,008 of whom had general anesthesia and 550 regional anesthesia.
“Advocates for carotid endarterectomy with regional anesthesia cite a reduction in hemodynamic instability and the ability for neurological monitoring, but many still prefer general anesthesia because the benefits of regional anesthesia have not been clearly demonstrated, allowing that regional anesthesia may not be available in all centers and allowing that a certain amount of patient movement during the procedure may not be uniformly tolerated,” Dr. Hussain said.
General anesthesia patients in the study had more than twice the rate of any morbidity at 30 days than those who had regional, 8.7% vs. 4.2%, and significantly higher rates of unplanned intervention, 2.1% vs. 0.6%. Dr. Hussain said. However, the study could not determine differences in 30-day mortality or other key outcomes, such as rates of pneumonia, sepsis, deep vein thrombosis, or pulmonary embolism, becauseof insufficient sample sizes, Dr. Hussain said
The study found less significant differences between general and regional anesthesia techniques, respectively, in rates of extended length of stay, 12.1% vs. 9.5%; readmissions, 9.2% vs. 6.1%; and reoperation, 4.5% vs. 3%.
The retrospective study used two models to analyze odds ratios: Model 1 adjusted for case mix; and model 2 adjusted for case mix as fixed effects and site as a random effect. While the retrospective nature of the study may be a limitation, the findings support the use of regional anesthesia for CEA when available, Dr. Hussain said.
Dr. Hussain had no relationships to disclose.
COLUMBUS, OHIO – General anesthesia during carotid endarterectomy carries almost twice the risk of complications and unplanned intubation as regional anesthesia, but the latter approach, which is not available in all hospitals, has its own issues, an analysis of procedures from a statewide database in Michigan found.
“This study is timely because of CMS [Center for Medicare & Medicaid Services] initiatives tying reimbursement to specific quality measures,” Ahmad S Hussain, MD, of Wayne State University in Detroit said in reporting the study results at the annual meeting of the Midwestern Vascular Surgery Society.
Regional anesthesia in CEA emerged in the 1990s, Dr. Hussain said, and allows for more reliable neurologic monitoring and more direct evaluation of the need for stenting during CEA than general anesthesia, which requires continuous monitoring of cerebral perfusion with carotid stump pressures, electroencephalogram, and transcranial doppler.
The researchers retrospectively analyzed 4,558 patients who had CEA at hospitals participating in the Michigan Surgical Quality Cooperative from 2012 to 2014 – 4,008 of whom had general anesthesia and 550 regional anesthesia.
“Advocates for carotid endarterectomy with regional anesthesia cite a reduction in hemodynamic instability and the ability for neurological monitoring, but many still prefer general anesthesia because the benefits of regional anesthesia have not been clearly demonstrated, allowing that regional anesthesia may not be available in all centers and allowing that a certain amount of patient movement during the procedure may not be uniformly tolerated,” Dr. Hussain said.
General anesthesia patients in the study had more than twice the rate of any morbidity at 30 days than those who had regional, 8.7% vs. 4.2%, and significantly higher rates of unplanned intervention, 2.1% vs. 0.6%. Dr. Hussain said. However, the study could not determine differences in 30-day mortality or other key outcomes, such as rates of pneumonia, sepsis, deep vein thrombosis, or pulmonary embolism, becauseof insufficient sample sizes, Dr. Hussain said
The study found less significant differences between general and regional anesthesia techniques, respectively, in rates of extended length of stay, 12.1% vs. 9.5%; readmissions, 9.2% vs. 6.1%; and reoperation, 4.5% vs. 3%.
The retrospective study used two models to analyze odds ratios: Model 1 adjusted for case mix; and model 2 adjusted for case mix as fixed effects and site as a random effect. While the retrospective nature of the study may be a limitation, the findings support the use of regional anesthesia for CEA when available, Dr. Hussain said.
Dr. Hussain had no relationships to disclose.
AT MIDWESTERN VASCULAR 2016
Key clinical point: General anesthesia for carotid endarterectomy carries a higher risk of complications and readmissions than regional anesthesia.
Major finding: Any morbidity after CEA with general anesthesia was 8.7% vs. 4.2% for regional anesthesia, and readmissions rates were 9.2% vs. 6.1%.
Data source: Retrospective analysis of 4,558 patients who had CEA between 2012 and 2014 at hospitals participating in the Michigan Surgical Quality Collaborative database.
Disclosures: Dr. Hussain reported having no financial disclosures.
Another Zika vaccine heads to Phase I trials
Scientists with the U.S. Department of Defense have launched a Phase I clinical trial to test an investigational Zika vaccine that relies on inactivated virus.
The candidate vaccine is known as the Zika Purified Inactivated Virus (ZPIV) vaccine and contains whole but inactivated Zika virus particles to stimulate an immune system response without replicating and causing illness.
“We urgently need a safe and effective vaccine to protect people from Zika virus infection as the virus continues to spread and cause serious public health consequences, particularly for pregnant women and their babies,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases (NIAID), said in a statement. “We are pleased to be part of the collaborative effort to advance this promising candidate vaccine into clinical trials.”
The NIAID helped support the preclinical development of the vaccine candidate and is part of a joint research agreement with the Department of Defense and other federal agencies to develop the vaccine for use in humans.
The trial at Walter Reed will enroll 75 individuals, ranging in age from 18-49 years, all of whom should have no history of a flavivirus infection. Twenty-five subjects will be given a pair of either intramuscular ZPIV injections, or a placebo (saline), with 28 days between injections.
The remaining 50 subjects will be divided into two groups of 25, with one group receiving two doses of a Japanese encephalitis virus vaccine and the other getting one dose of a yellow fever vaccine, before they both receive the two-dose ZPIV vaccine regimen.
A subgroup of 30 patients will then receive a third ZPIV dose 1 year later. Across all cohorts, the ZPIV dosage will be 5 micrograms.
In addition to the testing of the ZPIV vaccine, there are Phase I trials of a DNA-based Zika vaccine ongoing at the National Institutes of Health Clinical Center in Bethesda, Md., the Center for Vaccine Development at the University of Maryland in Baltimore, Md., and at Emory University in Atlanta, Ga. Those trials were launched in August 2016.
Scientists with the U.S. Department of Defense have launched a Phase I clinical trial to test an investigational Zika vaccine that relies on inactivated virus.
The candidate vaccine is known as the Zika Purified Inactivated Virus (ZPIV) vaccine and contains whole but inactivated Zika virus particles to stimulate an immune system response without replicating and causing illness.
“We urgently need a safe and effective vaccine to protect people from Zika virus infection as the virus continues to spread and cause serious public health consequences, particularly for pregnant women and their babies,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases (NIAID), said in a statement. “We are pleased to be part of the collaborative effort to advance this promising candidate vaccine into clinical trials.”
The NIAID helped support the preclinical development of the vaccine candidate and is part of a joint research agreement with the Department of Defense and other federal agencies to develop the vaccine for use in humans.
The trial at Walter Reed will enroll 75 individuals, ranging in age from 18-49 years, all of whom should have no history of a flavivirus infection. Twenty-five subjects will be given a pair of either intramuscular ZPIV injections, or a placebo (saline), with 28 days between injections.
The remaining 50 subjects will be divided into two groups of 25, with one group receiving two doses of a Japanese encephalitis virus vaccine and the other getting one dose of a yellow fever vaccine, before they both receive the two-dose ZPIV vaccine regimen.
A subgroup of 30 patients will then receive a third ZPIV dose 1 year later. Across all cohorts, the ZPIV dosage will be 5 micrograms.
In addition to the testing of the ZPIV vaccine, there are Phase I trials of a DNA-based Zika vaccine ongoing at the National Institutes of Health Clinical Center in Bethesda, Md., the Center for Vaccine Development at the University of Maryland in Baltimore, Md., and at Emory University in Atlanta, Ga. Those trials were launched in August 2016.
Scientists with the U.S. Department of Defense have launched a Phase I clinical trial to test an investigational Zika vaccine that relies on inactivated virus.
The candidate vaccine is known as the Zika Purified Inactivated Virus (ZPIV) vaccine and contains whole but inactivated Zika virus particles to stimulate an immune system response without replicating and causing illness.
“We urgently need a safe and effective vaccine to protect people from Zika virus infection as the virus continues to spread and cause serious public health consequences, particularly for pregnant women and their babies,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases (NIAID), said in a statement. “We are pleased to be part of the collaborative effort to advance this promising candidate vaccine into clinical trials.”
The NIAID helped support the preclinical development of the vaccine candidate and is part of a joint research agreement with the Department of Defense and other federal agencies to develop the vaccine for use in humans.
The trial at Walter Reed will enroll 75 individuals, ranging in age from 18-49 years, all of whom should have no history of a flavivirus infection. Twenty-five subjects will be given a pair of either intramuscular ZPIV injections, or a placebo (saline), with 28 days between injections.
The remaining 50 subjects will be divided into two groups of 25, with one group receiving two doses of a Japanese encephalitis virus vaccine and the other getting one dose of a yellow fever vaccine, before they both receive the two-dose ZPIV vaccine regimen.
A subgroup of 30 patients will then receive a third ZPIV dose 1 year later. Across all cohorts, the ZPIV dosage will be 5 micrograms.
In addition to the testing of the ZPIV vaccine, there are Phase I trials of a DNA-based Zika vaccine ongoing at the National Institutes of Health Clinical Center in Bethesda, Md., the Center for Vaccine Development at the University of Maryland in Baltimore, Md., and at Emory University in Atlanta, Ga. Those trials were launched in August 2016.
Low parental confidence in HPV vaccine stymies adolescent vaccination rates
More than a quarter of U.S. parents surveyed refused human papillomavirus (HPV) vaccination for their adolescents because of a lack of overall trust in adolescent vaccination programs and higher levels of perceived harm, a study found.
In an online survey of 1,484 U.S. parents, 28% of respondents reported they had refused the HPV vaccine on behalf of their children aged 11-17 years at least once. Another 8% responded they had elected to delay vaccination. The remaining two-thirds of respondents said they had neither refused nor delayed the vaccination, reported Melissa B. Gilkey, PhD, of Harvard Medical School, Boston, and her associates (Hum Vaccin Immunother. 2016. doi: 10.1080/21645515.2016.1247134).
Compared with parents who reported neither refusal nor delay, refusal was associated with lower confidence in adolescent vaccination (relative risk ratio = 0.66, 95% CI, 0.48-0.91), lower perceived HPV vaccine effectiveness (RRR = 0.68, 95% CI, 0.50-0.91), and higher perceived harms (RRR = 3.49, 95% CI, 2.65-4.60). Parents who reported delaying vaccination were more likely to endorse insufficient information as the reason (RRR = 1.76, 95% CI, 1.08-2.85). While 79% of parents who had delayed HPV vaccination said talking with a physician would help them with their decision, 61% of parents who refused the vaccination said it would. In addition, nearly half of parents who delayed vaccination said they did so out of a preference to wait until their children were older.
In adolescents whose parents had ever refused the vaccine, only 27% had received one HPV vaccine vs. 59% in those whose parents had elected to delay vaccination. Among adolescents whose parents responded they had neither refused nor delayed the vaccine, 56% had received one HPV vaccine.
Although the investigators did not find race, ethnicity, nor educational attainment were drivers of whether a parent chose to vaccinate, families with higher income levels tended to refuse the HPV vaccine more often than did other parents (RRR: 1.48, 95% confidence interval, 1.02-2.15).
Merck and the National Cancer Institute funded the study. Coauthor Noel T. Brewer, PhD, has received HPV vaccine-related grants from, or been on paid advisory boards for, Merck, GlaxoSmithKline, and Pfizer; he served on the National Vaccine Advisory Committee Working Group on HPV Vaccine and is chair of the National HPV Vaccination Roundtable.
[email protected]
On Twitter @whitneymcknight
More than a quarter of U.S. parents surveyed refused human papillomavirus (HPV) vaccination for their adolescents because of a lack of overall trust in adolescent vaccination programs and higher levels of perceived harm, a study found.
In an online survey of 1,484 U.S. parents, 28% of respondents reported they had refused the HPV vaccine on behalf of their children aged 11-17 years at least once. Another 8% responded they had elected to delay vaccination. The remaining two-thirds of respondents said they had neither refused nor delayed the vaccination, reported Melissa B. Gilkey, PhD, of Harvard Medical School, Boston, and her associates (Hum Vaccin Immunother. 2016. doi: 10.1080/21645515.2016.1247134).
Compared with parents who reported neither refusal nor delay, refusal was associated with lower confidence in adolescent vaccination (relative risk ratio = 0.66, 95% CI, 0.48-0.91), lower perceived HPV vaccine effectiveness (RRR = 0.68, 95% CI, 0.50-0.91), and higher perceived harms (RRR = 3.49, 95% CI, 2.65-4.60). Parents who reported delaying vaccination were more likely to endorse insufficient information as the reason (RRR = 1.76, 95% CI, 1.08-2.85). While 79% of parents who had delayed HPV vaccination said talking with a physician would help them with their decision, 61% of parents who refused the vaccination said it would. In addition, nearly half of parents who delayed vaccination said they did so out of a preference to wait until their children were older.
In adolescents whose parents had ever refused the vaccine, only 27% had received one HPV vaccine vs. 59% in those whose parents had elected to delay vaccination. Among adolescents whose parents responded they had neither refused nor delayed the vaccine, 56% had received one HPV vaccine.
Although the investigators did not find race, ethnicity, nor educational attainment were drivers of whether a parent chose to vaccinate, families with higher income levels tended to refuse the HPV vaccine more often than did other parents (RRR: 1.48, 95% confidence interval, 1.02-2.15).
Merck and the National Cancer Institute funded the study. Coauthor Noel T. Brewer, PhD, has received HPV vaccine-related grants from, or been on paid advisory boards for, Merck, GlaxoSmithKline, and Pfizer; he served on the National Vaccine Advisory Committee Working Group on HPV Vaccine and is chair of the National HPV Vaccination Roundtable.
[email protected]
On Twitter @whitneymcknight
More than a quarter of U.S. parents surveyed refused human papillomavirus (HPV) vaccination for their adolescents because of a lack of overall trust in adolescent vaccination programs and higher levels of perceived harm, a study found.
In an online survey of 1,484 U.S. parents, 28% of respondents reported they had refused the HPV vaccine on behalf of their children aged 11-17 years at least once. Another 8% responded they had elected to delay vaccination. The remaining two-thirds of respondents said they had neither refused nor delayed the vaccination, reported Melissa B. Gilkey, PhD, of Harvard Medical School, Boston, and her associates (Hum Vaccin Immunother. 2016. doi: 10.1080/21645515.2016.1247134).
Compared with parents who reported neither refusal nor delay, refusal was associated with lower confidence in adolescent vaccination (relative risk ratio = 0.66, 95% CI, 0.48-0.91), lower perceived HPV vaccine effectiveness (RRR = 0.68, 95% CI, 0.50-0.91), and higher perceived harms (RRR = 3.49, 95% CI, 2.65-4.60). Parents who reported delaying vaccination were more likely to endorse insufficient information as the reason (RRR = 1.76, 95% CI, 1.08-2.85). While 79% of parents who had delayed HPV vaccination said talking with a physician would help them with their decision, 61% of parents who refused the vaccination said it would. In addition, nearly half of parents who delayed vaccination said they did so out of a preference to wait until their children were older.
In adolescents whose parents had ever refused the vaccine, only 27% had received one HPV vaccine vs. 59% in those whose parents had elected to delay vaccination. Among adolescents whose parents responded they had neither refused nor delayed the vaccine, 56% had received one HPV vaccine.
Although the investigators did not find race, ethnicity, nor educational attainment were drivers of whether a parent chose to vaccinate, families with higher income levels tended to refuse the HPV vaccine more often than did other parents (RRR: 1.48, 95% confidence interval, 1.02-2.15).
Merck and the National Cancer Institute funded the study. Coauthor Noel T. Brewer, PhD, has received HPV vaccine-related grants from, or been on paid advisory boards for, Merck, GlaxoSmithKline, and Pfizer; he served on the National Vaccine Advisory Committee Working Group on HPV Vaccine and is chair of the National HPV Vaccination Roundtable.
[email protected]
On Twitter @whitneymcknight
Key clinical point:
Major finding: HPV vaccine refusal rate was 28% in parents of teens and preteens; the rate of vaccine delay was 8%.
Data source: Online survey conducted in 2014-2015 of 1,484 U.S. parents with children between ages of 11 and 17 years.
Disclosures: Merck and the National Cancer Institute funded the study. Coauthor Noel T. Brewer, PhD, has received HPV vaccine-related grants from, or been on paid advisory boards for, Merck, GlaxoSmithKline, and Pfizer; he served on the National Vaccine Advisory Committee Working Group on HPV Vaccine and is chair of the National HPV Vaccination Roundtable.
Malpractice Counsel: Missed Nodule
Case
A 48-year-old man presented to the ED with a 2-day history of cough and congestion. He described the cough as gradual in onset and, though initially nonproductive, it was now productive of green sputum. He denied fevers or chills, chest pain, nausea, vomiting, or diarrhea, and complained of only mild shortness of breath. His medical history was significant for hypertension, which was well managed with daily lisinopril-hydrochlorothiazide. He admitted to smoking one pack of cigarettes per day for the past 25 years, but denied alcohol or illicit drug use.
On physical examination, the patient’s vital signs were: blood pressure, 112/64 mm Hg; heart rate, 84 beats/min; respiratory rate, 20 breaths/min; and temperature, 98oF. Oxygen saturation was 97% on room air. The head, eyes, ears, nose, and throat examination was normal. Auscultation of the lungs revealed bilateral breath sounds with scattered, faint expiratory wheezing; the heart had a regular rate and rhythm, without murmurs, rubs, or gallops.
The emergency physician (EP) ordered posteroanterior and lateral chest X-rays (CXR), which he interpreted as normal. He also ordered an albuterol handheld nebulizer treatment for the patient. After the albuterol treatment, the patient felt he was breathing more easily. The frequency of his cough had also decreased following treatment and, on re-examination, he exhibited no wheezing and was given azithromycin 500 mg orally in the ED. The EP diagnosed the patient with acute bronchitis and discharged him home with an albuterol metered dose inhaler with a spacer, and a 4-day course of azithromycin. He also encouraged the patient to quit smoking.
The next day the radiologist’s official reading of the patient’s radiographs included the finding of a very small pulmonary nodule, which was seen only on the lateral X-ray. The radiologist recommended a repeat CXR or a computed tomography (CT) scan of the chest in 6 months.
Unfortunately, the EP never saw this information, and the patient was not contacted regarding the abnormal radiology finding and the need for follow-up. Approximately 20 months later, the patient was diagnosed with lung cancer with metastasis to the thoracic spine and liver. Despite chemotherapy and radiation treatment, he died from the cancer.
The patient’s family brought a malpractice suit against the EP, stating that the cancer could have been successfully treated prior to any metastasis if the patient had been informed of the abnormal radiology findings at his ED visit 20 months prior. The EP argued that he never saw the official radiology report, and therefore had no knowledge of the need for follow-up. At trial, a jury verdict was returned in favor of the defendant.
Discussion
Unfortunately, some version of this scenario occurs on a frequent basis. While imaging studies account for the majority of such cases, the same situation can occur with abnormal laboratory results, body-fluid cultures, or pathology reports in which an abnormality is identified (eg, positive blood culture, missed fracture) but, for a myriad of reasons, the critical information does not get related to the patient.
Because of the episodic nature of the practice of emergency medicine (EM), a process must be in place to ensure any “positive” test results or findings discovered after patient discharge are reviewed and compared to the ED diagnosis, and that any “misses” result in notifying the patient and/or his or her primary care physician and arranging follow-up. In cases such as the one presented here, a system issue existed—one that was not due to any fault or oversight of the EP. Ideally, EM leadership should work closely with leadership from radiology and laboratory services and hospital risk management to develop such a process—one that will be effective every day, including weekends and holidays.
Missed fractures on radiographs are a common cause of malpractice litigation against EPs. In one review by Kachalia et al1 examining malpractice claims involving EPs, missed fractures on radiographs accounted for 19% (the most common) of the 79 missed diagnoses identified in their study.In a similar study by Karcz et al,2 missed fractures ranked second in frequency and dollars lost in malpractice cases against EPs in Massachusetts.
While missed lesions on CXR do not occur with the same frequency as missed fractures, the results are much more devastating when the lesion turns out to be malignant. Three common areas where such lesions are missed on CXR include: the apex of the lung, obscured by overlying clavicle and ribs; the retrocardiac region (as in the patient in this case); and the lung bases obscured by the diaphragm.
Emergency physicians are neither trained nor expected to identify every single abnormality—especially subtle radiographic abnormalities. This is why there are radiology overreads, and a system or process must be in place to ensure patients are informed of any positive findings and to arrange proper follow-up.
1. Kachalia A, Gandhi TK, Puopolo AL, et al. Missed and delayed diagnoses in the emergency department: a study of closed malpractice claims from 4 liability insurers. Ann Emerg Med. 2007;49(2):196-205.
2. Karcz A, Korn R, Burke MC, et al. Malpractice claims against emergency physicians in Massachusetts: 1975-1993. Am J Emerg Med. 1996;14(4):341-345.
Case
A 48-year-old man presented to the ED with a 2-day history of cough and congestion. He described the cough as gradual in onset and, though initially nonproductive, it was now productive of green sputum. He denied fevers or chills, chest pain, nausea, vomiting, or diarrhea, and complained of only mild shortness of breath. His medical history was significant for hypertension, which was well managed with daily lisinopril-hydrochlorothiazide. He admitted to smoking one pack of cigarettes per day for the past 25 years, but denied alcohol or illicit drug use.
On physical examination, the patient’s vital signs were: blood pressure, 112/64 mm Hg; heart rate, 84 beats/min; respiratory rate, 20 breaths/min; and temperature, 98oF. Oxygen saturation was 97% on room air. The head, eyes, ears, nose, and throat examination was normal. Auscultation of the lungs revealed bilateral breath sounds with scattered, faint expiratory wheezing; the heart had a regular rate and rhythm, without murmurs, rubs, or gallops.
The emergency physician (EP) ordered posteroanterior and lateral chest X-rays (CXR), which he interpreted as normal. He also ordered an albuterol handheld nebulizer treatment for the patient. After the albuterol treatment, the patient felt he was breathing more easily. The frequency of his cough had also decreased following treatment and, on re-examination, he exhibited no wheezing and was given azithromycin 500 mg orally in the ED. The EP diagnosed the patient with acute bronchitis and discharged him home with an albuterol metered dose inhaler with a spacer, and a 4-day course of azithromycin. He also encouraged the patient to quit smoking.
The next day the radiologist’s official reading of the patient’s radiographs included the finding of a very small pulmonary nodule, which was seen only on the lateral X-ray. The radiologist recommended a repeat CXR or a computed tomography (CT) scan of the chest in 6 months.
Unfortunately, the EP never saw this information, and the patient was not contacted regarding the abnormal radiology finding and the need for follow-up. Approximately 20 months later, the patient was diagnosed with lung cancer with metastasis to the thoracic spine and liver. Despite chemotherapy and radiation treatment, he died from the cancer.
The patient’s family brought a malpractice suit against the EP, stating that the cancer could have been successfully treated prior to any metastasis if the patient had been informed of the abnormal radiology findings at his ED visit 20 months prior. The EP argued that he never saw the official radiology report, and therefore had no knowledge of the need for follow-up. At trial, a jury verdict was returned in favor of the defendant.
Discussion
Unfortunately, some version of this scenario occurs on a frequent basis. While imaging studies account for the majority of such cases, the same situation can occur with abnormal laboratory results, body-fluid cultures, or pathology reports in which an abnormality is identified (eg, positive blood culture, missed fracture) but, for a myriad of reasons, the critical information does not get related to the patient.
Because of the episodic nature of the practice of emergency medicine (EM), a process must be in place to ensure any “positive” test results or findings discovered after patient discharge are reviewed and compared to the ED diagnosis, and that any “misses” result in notifying the patient and/or his or her primary care physician and arranging follow-up. In cases such as the one presented here, a system issue existed—one that was not due to any fault or oversight of the EP. Ideally, EM leadership should work closely with leadership from radiology and laboratory services and hospital risk management to develop such a process—one that will be effective every day, including weekends and holidays.
Missed fractures on radiographs are a common cause of malpractice litigation against EPs. In one review by Kachalia et al1 examining malpractice claims involving EPs, missed fractures on radiographs accounted for 19% (the most common) of the 79 missed diagnoses identified in their study.In a similar study by Karcz et al,2 missed fractures ranked second in frequency and dollars lost in malpractice cases against EPs in Massachusetts.
While missed lesions on CXR do not occur with the same frequency as missed fractures, the results are much more devastating when the lesion turns out to be malignant. Three common areas where such lesions are missed on CXR include: the apex of the lung, obscured by overlying clavicle and ribs; the retrocardiac region (as in the patient in this case); and the lung bases obscured by the diaphragm.
Emergency physicians are neither trained nor expected to identify every single abnormality—especially subtle radiographic abnormalities. This is why there are radiology overreads, and a system or process must be in place to ensure patients are informed of any positive findings and to arrange proper follow-up.
Case
A 48-year-old man presented to the ED with a 2-day history of cough and congestion. He described the cough as gradual in onset and, though initially nonproductive, it was now productive of green sputum. He denied fevers or chills, chest pain, nausea, vomiting, or diarrhea, and complained of only mild shortness of breath. His medical history was significant for hypertension, which was well managed with daily lisinopril-hydrochlorothiazide. He admitted to smoking one pack of cigarettes per day for the past 25 years, but denied alcohol or illicit drug use.
On physical examination, the patient’s vital signs were: blood pressure, 112/64 mm Hg; heart rate, 84 beats/min; respiratory rate, 20 breaths/min; and temperature, 98oF. Oxygen saturation was 97% on room air. The head, eyes, ears, nose, and throat examination was normal. Auscultation of the lungs revealed bilateral breath sounds with scattered, faint expiratory wheezing; the heart had a regular rate and rhythm, without murmurs, rubs, or gallops.
The emergency physician (EP) ordered posteroanterior and lateral chest X-rays (CXR), which he interpreted as normal. He also ordered an albuterol handheld nebulizer treatment for the patient. After the albuterol treatment, the patient felt he was breathing more easily. The frequency of his cough had also decreased following treatment and, on re-examination, he exhibited no wheezing and was given azithromycin 500 mg orally in the ED. The EP diagnosed the patient with acute bronchitis and discharged him home with an albuterol metered dose inhaler with a spacer, and a 4-day course of azithromycin. He also encouraged the patient to quit smoking.
The next day the radiologist’s official reading of the patient’s radiographs included the finding of a very small pulmonary nodule, which was seen only on the lateral X-ray. The radiologist recommended a repeat CXR or a computed tomography (CT) scan of the chest in 6 months.
Unfortunately, the EP never saw this information, and the patient was not contacted regarding the abnormal radiology finding and the need for follow-up. Approximately 20 months later, the patient was diagnosed with lung cancer with metastasis to the thoracic spine and liver. Despite chemotherapy and radiation treatment, he died from the cancer.
The patient’s family brought a malpractice suit against the EP, stating that the cancer could have been successfully treated prior to any metastasis if the patient had been informed of the abnormal radiology findings at his ED visit 20 months prior. The EP argued that he never saw the official radiology report, and therefore had no knowledge of the need for follow-up. At trial, a jury verdict was returned in favor of the defendant.
Discussion
Unfortunately, some version of this scenario occurs on a frequent basis. While imaging studies account for the majority of such cases, the same situation can occur with abnormal laboratory results, body-fluid cultures, or pathology reports in which an abnormality is identified (eg, positive blood culture, missed fracture) but, for a myriad of reasons, the critical information does not get related to the patient.
Because of the episodic nature of the practice of emergency medicine (EM), a process must be in place to ensure any “positive” test results or findings discovered after patient discharge are reviewed and compared to the ED diagnosis, and that any “misses” result in notifying the patient and/or his or her primary care physician and arranging follow-up. In cases such as the one presented here, a system issue existed—one that was not due to any fault or oversight of the EP. Ideally, EM leadership should work closely with leadership from radiology and laboratory services and hospital risk management to develop such a process—one that will be effective every day, including weekends and holidays.
Missed fractures on radiographs are a common cause of malpractice litigation against EPs. In one review by Kachalia et al1 examining malpractice claims involving EPs, missed fractures on radiographs accounted for 19% (the most common) of the 79 missed diagnoses identified in their study.In a similar study by Karcz et al,2 missed fractures ranked second in frequency and dollars lost in malpractice cases against EPs in Massachusetts.
While missed lesions on CXR do not occur with the same frequency as missed fractures, the results are much more devastating when the lesion turns out to be malignant. Three common areas where such lesions are missed on CXR include: the apex of the lung, obscured by overlying clavicle and ribs; the retrocardiac region (as in the patient in this case); and the lung bases obscured by the diaphragm.
Emergency physicians are neither trained nor expected to identify every single abnormality—especially subtle radiographic abnormalities. This is why there are radiology overreads, and a system or process must be in place to ensure patients are informed of any positive findings and to arrange proper follow-up.
1. Kachalia A, Gandhi TK, Puopolo AL, et al. Missed and delayed diagnoses in the emergency department: a study of closed malpractice claims from 4 liability insurers. Ann Emerg Med. 2007;49(2):196-205.
2. Karcz A, Korn R, Burke MC, et al. Malpractice claims against emergency physicians in Massachusetts: 1975-1993. Am J Emerg Med. 1996;14(4):341-345.
1. Kachalia A, Gandhi TK, Puopolo AL, et al. Missed and delayed diagnoses in the emergency department: a study of closed malpractice claims from 4 liability insurers. Ann Emerg Med. 2007;49(2):196-205.
2. Karcz A, Korn R, Burke MC, et al. Malpractice claims against emergency physicians in Massachusetts: 1975-1993. Am J Emerg Med. 1996;14(4):341-345.
Emergency Ultrasound: Ultrasound-Guided Femoral Nerve Block
Case Scenario
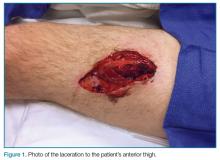
A young man presented to the ED for evaluation of a large laceration to the anterior thigh that resulted from an industrial accident (Figure 1).
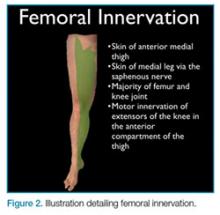
Femoral nerve blocks are useful in a variety of clinical scenarios, including fractures of the femur or hip1 and laceration repairs (Figure 2).
Identifying the Femoral Nerve on Ultrasound
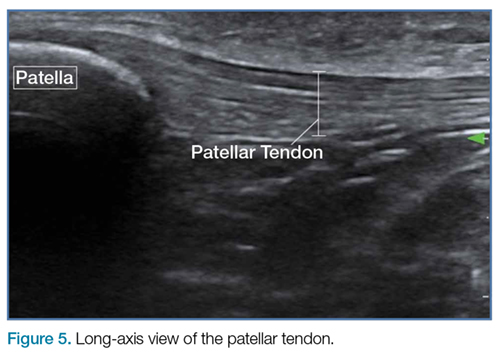
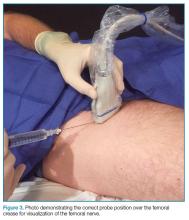
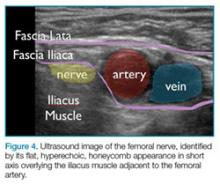
To perform this nerve block, one must recall the anatomy of femoral central-line placement. The femoral nerve lies lateral to the femoral artery and vein. The high-frequency probe should be placed over the femoral crease (Figure 3).
Performing the Block
An ultrasound-guided femoral nerve block can be performed using a 22-gauge blunt tip spinal needle, and an in-plane or out-of-plane technique can be employed. We prefer using an in-plane technique because the entire shaft of the needle can be visualized as it approaches the nerve. Anatomically, the femoral nerve lies in a separate fascial plane from the artery and vein, beneath the fascia iliaca (Figure 4). You can use this anatomic location of the femoral nerve to your advantage when performing the block. The needle can be advanced to a target slightly lateral to the nerve until it pops beneath the fascia iliaca. On the ultrasound, you can monitor the spread of anesthetic as it is injected. If the needle is in the right location, the hypoechoic fluid will spread medially toward the nerve, but will not track around the artery or vein. At least 15 cc to 20 cc of local anesthetic is typically required.2,3 If you prefer, the anesthetic can be diluted in normal saline, in a 1:1 ratio, to achieve adequate volume.
If you do not see the anesthetic spread during the injection, you should stop and check the needle placement, as it may be intravascular. Using a more lateral approach, targeting the injection at the fascial plane, rather than the nerve, helps to avoid direct intraneural injection or contact with the nerve—and it keeps the needle far away from the femoral vascular bundle.
Safety Considerations
As with any technique, prior to the procedure, aseptic measures should be taken, including the use of a sterile probe cover and sterile gloves. All patients undergoing ultrasound-guided nerve blocks proximal to the wrist or ankle should be placed on a cardiac monitor. In addition, intralipid emulsion should be readily available for administration in the unlikely event there is inadvertent intravascular injection of local anesthetic and cardiovascular collapse occurs.
Summary
With practice, ultrasound guidance can improve the procedural success of femoral nerve blocks and decrease the risk of nerve injury compared to blind nerve blocks
1. Dickman E, Pushkar I, Likourezos A, et al. Ultrasound-guided nerve blocks for intracapsular and extracapsular hip fractures. Am J Emerg Med. 2016;34(3):586-589.
2. Femoral nerve block. In: Hadzic A, Carrera A, Clark T, et al, eds. Hadzic’s Peripheral Nerve Blocks: An Anatomy for Ultrasound-Guided Regional Anesthesia. 2nd ed. New York, NY: The McGraw Hill Companies, Inc; 2012:267-279.
3. Ultrasound-guided femoral nerve block. In: Hadzic A, Carrera A, Clark T, et al, eds. Hadzic’s Peripheral Nerve Blocks: An Anatomy for Ultrasound-Guided Regional Anesthesia. 2nd ed. New York, NY: The McGraw Hill Companies, Inc; 2012:397-404.
Case Scenario
A young man presented to the ED for evaluation of a large laceration to the anterior thigh that resulted from an industrial accident (Figure 1).
Femoral nerve blocks are useful in a variety of clinical scenarios, including fractures of the femur or hip1 and laceration repairs (Figure 2).
Identifying the Femoral Nerve on Ultrasound
To perform this nerve block, one must recall the anatomy of femoral central-line placement. The femoral nerve lies lateral to the femoral artery and vein. The high-frequency probe should be placed over the femoral crease (Figure 3).
Performing the Block
An ultrasound-guided femoral nerve block can be performed using a 22-gauge blunt tip spinal needle, and an in-plane or out-of-plane technique can be employed. We prefer using an in-plane technique because the entire shaft of the needle can be visualized as it approaches the nerve. Anatomically, the femoral nerve lies in a separate fascial plane from the artery and vein, beneath the fascia iliaca (Figure 4). You can use this anatomic location of the femoral nerve to your advantage when performing the block. The needle can be advanced to a target slightly lateral to the nerve until it pops beneath the fascia iliaca. On the ultrasound, you can monitor the spread of anesthetic as it is injected. If the needle is in the right location, the hypoechoic fluid will spread medially toward the nerve, but will not track around the artery or vein. At least 15 cc to 20 cc of local anesthetic is typically required.2,3 If you prefer, the anesthetic can be diluted in normal saline, in a 1:1 ratio, to achieve adequate volume.
If you do not see the anesthetic spread during the injection, you should stop and check the needle placement, as it may be intravascular. Using a more lateral approach, targeting the injection at the fascial plane, rather than the nerve, helps to avoid direct intraneural injection or contact with the nerve—and it keeps the needle far away from the femoral vascular bundle.
Safety Considerations
As with any technique, prior to the procedure, aseptic measures should be taken, including the use of a sterile probe cover and sterile gloves. All patients undergoing ultrasound-guided nerve blocks proximal to the wrist or ankle should be placed on a cardiac monitor. In addition, intralipid emulsion should be readily available for administration in the unlikely event there is inadvertent intravascular injection of local anesthetic and cardiovascular collapse occurs.
Summary
With practice, ultrasound guidance can improve the procedural success of femoral nerve blocks and decrease the risk of nerve injury compared to blind nerve blocks
Case Scenario
A young man presented to the ED for evaluation of a large laceration to the anterior thigh that resulted from an industrial accident (Figure 1).
Femoral nerve blocks are useful in a variety of clinical scenarios, including fractures of the femur or hip1 and laceration repairs (Figure 2).
Identifying the Femoral Nerve on Ultrasound
To perform this nerve block, one must recall the anatomy of femoral central-line placement. The femoral nerve lies lateral to the femoral artery and vein. The high-frequency probe should be placed over the femoral crease (Figure 3).
Performing the Block
An ultrasound-guided femoral nerve block can be performed using a 22-gauge blunt tip spinal needle, and an in-plane or out-of-plane technique can be employed. We prefer using an in-plane technique because the entire shaft of the needle can be visualized as it approaches the nerve. Anatomically, the femoral nerve lies in a separate fascial plane from the artery and vein, beneath the fascia iliaca (Figure 4). You can use this anatomic location of the femoral nerve to your advantage when performing the block. The needle can be advanced to a target slightly lateral to the nerve until it pops beneath the fascia iliaca. On the ultrasound, you can monitor the spread of anesthetic as it is injected. If the needle is in the right location, the hypoechoic fluid will spread medially toward the nerve, but will not track around the artery or vein. At least 15 cc to 20 cc of local anesthetic is typically required.2,3 If you prefer, the anesthetic can be diluted in normal saline, in a 1:1 ratio, to achieve adequate volume.
If you do not see the anesthetic spread during the injection, you should stop and check the needle placement, as it may be intravascular. Using a more lateral approach, targeting the injection at the fascial plane, rather than the nerve, helps to avoid direct intraneural injection or contact with the nerve—and it keeps the needle far away from the femoral vascular bundle.
Safety Considerations
As with any technique, prior to the procedure, aseptic measures should be taken, including the use of a sterile probe cover and sterile gloves. All patients undergoing ultrasound-guided nerve blocks proximal to the wrist or ankle should be placed on a cardiac monitor. In addition, intralipid emulsion should be readily available for administration in the unlikely event there is inadvertent intravascular injection of local anesthetic and cardiovascular collapse occurs.
Summary
With practice, ultrasound guidance can improve the procedural success of femoral nerve blocks and decrease the risk of nerve injury compared to blind nerve blocks
1. Dickman E, Pushkar I, Likourezos A, et al. Ultrasound-guided nerve blocks for intracapsular and extracapsular hip fractures. Am J Emerg Med. 2016;34(3):586-589.
2. Femoral nerve block. In: Hadzic A, Carrera A, Clark T, et al, eds. Hadzic’s Peripheral Nerve Blocks: An Anatomy for Ultrasound-Guided Regional Anesthesia. 2nd ed. New York, NY: The McGraw Hill Companies, Inc; 2012:267-279.
3. Ultrasound-guided femoral nerve block. In: Hadzic A, Carrera A, Clark T, et al, eds. Hadzic’s Peripheral Nerve Blocks: An Anatomy for Ultrasound-Guided Regional Anesthesia. 2nd ed. New York, NY: The McGraw Hill Companies, Inc; 2012:397-404.
1. Dickman E, Pushkar I, Likourezos A, et al. Ultrasound-guided nerve blocks for intracapsular and extracapsular hip fractures. Am J Emerg Med. 2016;34(3):586-589.
2. Femoral nerve block. In: Hadzic A, Carrera A, Clark T, et al, eds. Hadzic’s Peripheral Nerve Blocks: An Anatomy for Ultrasound-Guided Regional Anesthesia. 2nd ed. New York, NY: The McGraw Hill Companies, Inc; 2012:267-279.
3. Ultrasound-guided femoral nerve block. In: Hadzic A, Carrera A, Clark T, et al, eds. Hadzic’s Peripheral Nerve Blocks: An Anatomy for Ultrasound-Guided Regional Anesthesia. 2nd ed. New York, NY: The McGraw Hill Companies, Inc; 2012:397-404.
Carotid stenting tied to cardiovascular events in real-world study
ROME – Carotid stenting was associated with a roughly 30% higher risk of cardiovascular events than that of carotid endarterectomy during 12 years of follow-up in a large, real-world, population-based cohort study, Mohamad A. Hussain, MD, reported at the annual congress of the European Society of Cardiology.
“Our data raise concerns about the external validity of randomized controlled trials of carotid endarterectomy versus stenting and question the potential interchangeability of carotid endarterectomy and stenting as stated in clinical practice guidelines,” said Dr. Hussain of the University of Toronto.
Major practice guidelines cite randomized trial evidence in suggesting that CEA and stenting can be used interchangeably in treating low- or average-risk patients with significant carotid artery disease. Dr. Hussain and his coinvestigators, suspicious that the generalizability of the randomized trial findings may be limited because of operator and institutional selection bias, decided to conduct a retrospective cohort study of all patients over age 40 years who underwent CEA or carotid stenting in the province of Ontario from April 2002 through March 2013.
Using validated chart abstraction software, they identified 12,529 patients who had CEA and 1,935 with carotid stenting. The two groups were similar in terms of most baseline characteristics. Notably, however, stent recipients were significantly more likely to have symptomatic carotid disease and also had more comorbid conditions as reflected in a higher Charlson Comorbidity Index score.
The primary outcome in the study was the 12-year rate of a composite comprising ischemic stroke, transient ischemic attack (TIA), MI, or death. The rate was 35.4% in the CEA group and 44.5% in the stent group. After adjustment for the baseline differences, the stent group still had a statistically significant 28% greater risk of the primary outcome.
“We found the difference remained significant in all of our subgroup analyses, regardless of age, sex, year of procedure, symptomatic or asymptomatic carotid artery disease, CAD [coronary artery disease] or no CAD, diabetes (type 1 or 2) or no diabetes. Outcomes with endarterectomy were always significantly better,” said Dr. Hussain.
“I think our study shows that in clinical practice we’re not quite seeing the outcomes reported in the clinical trials,” he added.
As for the individual components of the composite endpoint, the 12-year rate of ischemic stroke or TIA was 9% in the CEA group and 14% with stenting, for an adjusted 40% increased risk in the stent group. The 12-year all-cause mortality rate was 26% in the CEA group and 34% with stenting, for an adjusted 28% increased risk. The incidence of MI was 8% in both groups.
The investigators next conducted a confirmatory propensity-matched analysis in which 1,927 of the stented patients were closely matched to 3,844 surgical patients, eliminating baseline differences in the prevalence of symptomatic carotid artery disease and other disparities. In this matched cohort, the primary outcome occurred in 37.4% of the CEA group and 44.3% of stent patients, for an adjusted 32% increase in risk in the stented group.
The differences in outcome were driven by sharply higher periprocedural risk in the stented group. After the periprocedural period, the outcome curves remained parallel in the two treatment groups.
In that first 30 days post procedure, the primary composite outcome occurred in 5.4% of the CEA group and 10% of stented patients, for an adjusted 40% increase in relative risk in percutaneously treated patients. The 30-day rate of ischemic stroke or TIA was 3.4% in the surgical group compared with 6.4% in stented patients. Thirty-day mortality was 0.9% with CEA versus 3.3% with stenting.
Asked by the award panel for his thoughts on the disparity between the results of his real-world study and the major randomized trials of CEA versus stenting, Dr. Hussain replied, “It may be because the trials had high-volume operators at high-volume centers who are really experts in carotid stenting, while in the real world many physicians may not be selecting the right people for carotid stenting.”
Differences in sample size may also figure in the disparity, he continued. He noted that in the recent 10-year report from the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST), the composite endpoint of stroke, MI, or death occurred in 9.9% of the CEA group compared with 11.8% of the stenting group (N Engl J Med. 2016 Mar 17;374[11]:1021-31), but this difference in favor of CEA didn’t achieve statistical significance because of the wide confidence intervals resulting from a smaller sample size than in the Ontario study.
Looking to the future, Dr. Hussain said he thinks the ongoing CREST-2 trial is “very important.” It is randomizing patients with asymptomatic high-grade carotid stenosis to uniform intensive medical management either alone or in combination with CEA or stenting with embolic protection.
“That study might end up showing us that medical therapy is as good as or even better than stenting or CEA, especially in asymptomatic patients,” he said.
Dr. Hussain reported having no financial conflicts regarding his academically funded study.
ROME – Carotid stenting was associated with a roughly 30% higher risk of cardiovascular events than that of carotid endarterectomy during 12 years of follow-up in a large, real-world, population-based cohort study, Mohamad A. Hussain, MD, reported at the annual congress of the European Society of Cardiology.
“Our data raise concerns about the external validity of randomized controlled trials of carotid endarterectomy versus stenting and question the potential interchangeability of carotid endarterectomy and stenting as stated in clinical practice guidelines,” said Dr. Hussain of the University of Toronto.
Major practice guidelines cite randomized trial evidence in suggesting that CEA and stenting can be used interchangeably in treating low- or average-risk patients with significant carotid artery disease. Dr. Hussain and his coinvestigators, suspicious that the generalizability of the randomized trial findings may be limited because of operator and institutional selection bias, decided to conduct a retrospective cohort study of all patients over age 40 years who underwent CEA or carotid stenting in the province of Ontario from April 2002 through March 2013.
Using validated chart abstraction software, they identified 12,529 patients who had CEA and 1,935 with carotid stenting. The two groups were similar in terms of most baseline characteristics. Notably, however, stent recipients were significantly more likely to have symptomatic carotid disease and also had more comorbid conditions as reflected in a higher Charlson Comorbidity Index score.
The primary outcome in the study was the 12-year rate of a composite comprising ischemic stroke, transient ischemic attack (TIA), MI, or death. The rate was 35.4% in the CEA group and 44.5% in the stent group. After adjustment for the baseline differences, the stent group still had a statistically significant 28% greater risk of the primary outcome.
“We found the difference remained significant in all of our subgroup analyses, regardless of age, sex, year of procedure, symptomatic or asymptomatic carotid artery disease, CAD [coronary artery disease] or no CAD, diabetes (type 1 or 2) or no diabetes. Outcomes with endarterectomy were always significantly better,” said Dr. Hussain.
“I think our study shows that in clinical practice we’re not quite seeing the outcomes reported in the clinical trials,” he added.
As for the individual components of the composite endpoint, the 12-year rate of ischemic stroke or TIA was 9% in the CEA group and 14% with stenting, for an adjusted 40% increased risk in the stent group. The 12-year all-cause mortality rate was 26% in the CEA group and 34% with stenting, for an adjusted 28% increased risk. The incidence of MI was 8% in both groups.
The investigators next conducted a confirmatory propensity-matched analysis in which 1,927 of the stented patients were closely matched to 3,844 surgical patients, eliminating baseline differences in the prevalence of symptomatic carotid artery disease and other disparities. In this matched cohort, the primary outcome occurred in 37.4% of the CEA group and 44.3% of stent patients, for an adjusted 32% increase in risk in the stented group.
The differences in outcome were driven by sharply higher periprocedural risk in the stented group. After the periprocedural period, the outcome curves remained parallel in the two treatment groups.
In that first 30 days post procedure, the primary composite outcome occurred in 5.4% of the CEA group and 10% of stented patients, for an adjusted 40% increase in relative risk in percutaneously treated patients. The 30-day rate of ischemic stroke or TIA was 3.4% in the surgical group compared with 6.4% in stented patients. Thirty-day mortality was 0.9% with CEA versus 3.3% with stenting.
Asked by the award panel for his thoughts on the disparity between the results of his real-world study and the major randomized trials of CEA versus stenting, Dr. Hussain replied, “It may be because the trials had high-volume operators at high-volume centers who are really experts in carotid stenting, while in the real world many physicians may not be selecting the right people for carotid stenting.”
Differences in sample size may also figure in the disparity, he continued. He noted that in the recent 10-year report from the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST), the composite endpoint of stroke, MI, or death occurred in 9.9% of the CEA group compared with 11.8% of the stenting group (N Engl J Med. 2016 Mar 17;374[11]:1021-31), but this difference in favor of CEA didn’t achieve statistical significance because of the wide confidence intervals resulting from a smaller sample size than in the Ontario study.
Looking to the future, Dr. Hussain said he thinks the ongoing CREST-2 trial is “very important.” It is randomizing patients with asymptomatic high-grade carotid stenosis to uniform intensive medical management either alone or in combination with CEA or stenting with embolic protection.
“That study might end up showing us that medical therapy is as good as or even better than stenting or CEA, especially in asymptomatic patients,” he said.
Dr. Hussain reported having no financial conflicts regarding his academically funded study.
ROME – Carotid stenting was associated with a roughly 30% higher risk of cardiovascular events than that of carotid endarterectomy during 12 years of follow-up in a large, real-world, population-based cohort study, Mohamad A. Hussain, MD, reported at the annual congress of the European Society of Cardiology.
“Our data raise concerns about the external validity of randomized controlled trials of carotid endarterectomy versus stenting and question the potential interchangeability of carotid endarterectomy and stenting as stated in clinical practice guidelines,” said Dr. Hussain of the University of Toronto.
Major practice guidelines cite randomized trial evidence in suggesting that CEA and stenting can be used interchangeably in treating low- or average-risk patients with significant carotid artery disease. Dr. Hussain and his coinvestigators, suspicious that the generalizability of the randomized trial findings may be limited because of operator and institutional selection bias, decided to conduct a retrospective cohort study of all patients over age 40 years who underwent CEA or carotid stenting in the province of Ontario from April 2002 through March 2013.
Using validated chart abstraction software, they identified 12,529 patients who had CEA and 1,935 with carotid stenting. The two groups were similar in terms of most baseline characteristics. Notably, however, stent recipients were significantly more likely to have symptomatic carotid disease and also had more comorbid conditions as reflected in a higher Charlson Comorbidity Index score.
The primary outcome in the study was the 12-year rate of a composite comprising ischemic stroke, transient ischemic attack (TIA), MI, or death. The rate was 35.4% in the CEA group and 44.5% in the stent group. After adjustment for the baseline differences, the stent group still had a statistically significant 28% greater risk of the primary outcome.
“We found the difference remained significant in all of our subgroup analyses, regardless of age, sex, year of procedure, symptomatic or asymptomatic carotid artery disease, CAD [coronary artery disease] or no CAD, diabetes (type 1 or 2) or no diabetes. Outcomes with endarterectomy were always significantly better,” said Dr. Hussain.
“I think our study shows that in clinical practice we’re not quite seeing the outcomes reported in the clinical trials,” he added.
As for the individual components of the composite endpoint, the 12-year rate of ischemic stroke or TIA was 9% in the CEA group and 14% with stenting, for an adjusted 40% increased risk in the stent group. The 12-year all-cause mortality rate was 26% in the CEA group and 34% with stenting, for an adjusted 28% increased risk. The incidence of MI was 8% in both groups.
The investigators next conducted a confirmatory propensity-matched analysis in which 1,927 of the stented patients were closely matched to 3,844 surgical patients, eliminating baseline differences in the prevalence of symptomatic carotid artery disease and other disparities. In this matched cohort, the primary outcome occurred in 37.4% of the CEA group and 44.3% of stent patients, for an adjusted 32% increase in risk in the stented group.
The differences in outcome were driven by sharply higher periprocedural risk in the stented group. After the periprocedural period, the outcome curves remained parallel in the two treatment groups.
In that first 30 days post procedure, the primary composite outcome occurred in 5.4% of the CEA group and 10% of stented patients, for an adjusted 40% increase in relative risk in percutaneously treated patients. The 30-day rate of ischemic stroke or TIA was 3.4% in the surgical group compared with 6.4% in stented patients. Thirty-day mortality was 0.9% with CEA versus 3.3% with stenting.
Asked by the award panel for his thoughts on the disparity between the results of his real-world study and the major randomized trials of CEA versus stenting, Dr. Hussain replied, “It may be because the trials had high-volume operators at high-volume centers who are really experts in carotid stenting, while in the real world many physicians may not be selecting the right people for carotid stenting.”
Differences in sample size may also figure in the disparity, he continued. He noted that in the recent 10-year report from the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST), the composite endpoint of stroke, MI, or death occurred in 9.9% of the CEA group compared with 11.8% of the stenting group (N Engl J Med. 2016 Mar 17;374[11]:1021-31), but this difference in favor of CEA didn’t achieve statistical significance because of the wide confidence intervals resulting from a smaller sample size than in the Ontario study.
Looking to the future, Dr. Hussain said he thinks the ongoing CREST-2 trial is “very important.” It is randomizing patients with asymptomatic high-grade carotid stenosis to uniform intensive medical management either alone or in combination with CEA or stenting with embolic protection.
“That study might end up showing us that medical therapy is as good as or even better than stenting or CEA, especially in asymptomatic patients,” he said.
Dr. Hussain reported having no financial conflicts regarding his academically funded study.
AT THE ESC CONGRESS 2016
Key clinical point:
Major finding: The composite rate of ischemic stroke, TIA, MI, or death over 12 years of follow-up occurred in 35.4% of patients with carotid artery disease treated by carotid endarterectomy and 44.5% of those who underwent stenting.
Data source: A retrospective population-based cohort study comprising all 14,464 patients who underwent carotid endarterectomy or stenting for carotid artery disease in Ontario during 2002-2013.
Disclosures: The presenter reported having no financial conflicts of interest regarding his academically funded study.
Delayed bleeding possible with EBUS-TBNA on antiplatelets
LOS ANGELES – There might be a slight increase in delayed bleeding when patients have endobronchial ultrasound with transbronchial needle aspiration within 5 days of taking oral antiplatelets, according to a review of 404 patients at Riverside Methodist Hospital in Columbus, Ohio.
This study is unusual in that it looked at the 48 hour mark. Previous studies have tended to focus on immediate bleeding events that require the procedure to be stopped; only some of that research has found an increased bleeding risk with antiplatelet therapy.
In the study at Riverside Methodist, none of the 20 patients on dual antiplatelet therapy – clopidogrel (Plavix) plus aspirin – bled during the procedure, but one (5%) had a hemoglobin drop of more than 2 g within 48 hours and another was readmitted to the hospital within 48 hours for procedure-related hemoptysis. Overall, the delayed bleeding event rate for patients using the dual antiplatelet therapy was 10%. Additionally, one of the 13 patients (7.7%) on clopidogrel alone experienced a greater than 2 g drop in hemoglobin.
Among the 270 patients not exposed to antiplatelets, the overall bleeding event rate was 2.6%, and the event rate for delayed bleeding was 1.1%. Four patients (1.5%) bled during the procedure, two (0.7%) had hemoglobin drops greater than 2 g within 48 hours, and one (0.4%) was readmitted for hemoptysis.
There were no bleeding events in the 101 patients who only took aspirin.
“There was a trend toward delayed bleeding events in patients” on clopidogrel or dual antiplatelets. “It’s worth considering a thoughtful pause in decision making. Maybe with the bleeding events we’re seeing, it would be worthwhile, if possible, to defer” endobronchial ultrasound with transbronchial needle aspiration “until after the antiplatelet therapy,” said Kevin Swiatek, DO, a medicine resident at Riverside.
Patients were excluded from the study if they had histories of bleeding or clotting disorders; low platelet counts; or if they were on anticoagulation. Subjects on antiplatelets were about 10 years older, on average, than those who were not (about 68 versus 59 years old), and more likely to have had a heart attack or stroke, and to be hypertensive.
There was no industry funding for the work, and the investigators had no disclosures.
LOS ANGELES – There might be a slight increase in delayed bleeding when patients have endobronchial ultrasound with transbronchial needle aspiration within 5 days of taking oral antiplatelets, according to a review of 404 patients at Riverside Methodist Hospital in Columbus, Ohio.
This study is unusual in that it looked at the 48 hour mark. Previous studies have tended to focus on immediate bleeding events that require the procedure to be stopped; only some of that research has found an increased bleeding risk with antiplatelet therapy.
In the study at Riverside Methodist, none of the 20 patients on dual antiplatelet therapy – clopidogrel (Plavix) plus aspirin – bled during the procedure, but one (5%) had a hemoglobin drop of more than 2 g within 48 hours and another was readmitted to the hospital within 48 hours for procedure-related hemoptysis. Overall, the delayed bleeding event rate for patients using the dual antiplatelet therapy was 10%. Additionally, one of the 13 patients (7.7%) on clopidogrel alone experienced a greater than 2 g drop in hemoglobin.
Among the 270 patients not exposed to antiplatelets, the overall bleeding event rate was 2.6%, and the event rate for delayed bleeding was 1.1%. Four patients (1.5%) bled during the procedure, two (0.7%) had hemoglobin drops greater than 2 g within 48 hours, and one (0.4%) was readmitted for hemoptysis.
There were no bleeding events in the 101 patients who only took aspirin.
“There was a trend toward delayed bleeding events in patients” on clopidogrel or dual antiplatelets. “It’s worth considering a thoughtful pause in decision making. Maybe with the bleeding events we’re seeing, it would be worthwhile, if possible, to defer” endobronchial ultrasound with transbronchial needle aspiration “until after the antiplatelet therapy,” said Kevin Swiatek, DO, a medicine resident at Riverside.
Patients were excluded from the study if they had histories of bleeding or clotting disorders; low platelet counts; or if they were on anticoagulation. Subjects on antiplatelets were about 10 years older, on average, than those who were not (about 68 versus 59 years old), and more likely to have had a heart attack or stroke, and to be hypertensive.
There was no industry funding for the work, and the investigators had no disclosures.
LOS ANGELES – There might be a slight increase in delayed bleeding when patients have endobronchial ultrasound with transbronchial needle aspiration within 5 days of taking oral antiplatelets, according to a review of 404 patients at Riverside Methodist Hospital in Columbus, Ohio.
This study is unusual in that it looked at the 48 hour mark. Previous studies have tended to focus on immediate bleeding events that require the procedure to be stopped; only some of that research has found an increased bleeding risk with antiplatelet therapy.
In the study at Riverside Methodist, none of the 20 patients on dual antiplatelet therapy – clopidogrel (Plavix) plus aspirin – bled during the procedure, but one (5%) had a hemoglobin drop of more than 2 g within 48 hours and another was readmitted to the hospital within 48 hours for procedure-related hemoptysis. Overall, the delayed bleeding event rate for patients using the dual antiplatelet therapy was 10%. Additionally, one of the 13 patients (7.7%) on clopidogrel alone experienced a greater than 2 g drop in hemoglobin.
Among the 270 patients not exposed to antiplatelets, the overall bleeding event rate was 2.6%, and the event rate for delayed bleeding was 1.1%. Four patients (1.5%) bled during the procedure, two (0.7%) had hemoglobin drops greater than 2 g within 48 hours, and one (0.4%) was readmitted for hemoptysis.
There were no bleeding events in the 101 patients who only took aspirin.
“There was a trend toward delayed bleeding events in patients” on clopidogrel or dual antiplatelets. “It’s worth considering a thoughtful pause in decision making. Maybe with the bleeding events we’re seeing, it would be worthwhile, if possible, to defer” endobronchial ultrasound with transbronchial needle aspiration “until after the antiplatelet therapy,” said Kevin Swiatek, DO, a medicine resident at Riverside.
Patients were excluded from the study if they had histories of bleeding or clotting disorders; low platelet counts; or if they were on anticoagulation. Subjects on antiplatelets were about 10 years older, on average, than those who were not (about 68 versus 59 years old), and more likely to have had a heart attack or stroke, and to be hypertensive.
There was no industry funding for the work, and the investigators had no disclosures.
AT CHEST 2016
Key clinical point:
Major finding: Ten percent of patients on dual antiplatelet therapy bled within 48 hours, versus 1.1% of those not on antiplatelet therapy.
Data source: Single-center review of 404 patients.
Disclosures: There was no industry funding for the work, and the investigators had no disclosures.
Expanding the portfolio of the contemporary psychiatrist: The physician as arbiter of morality, normalcy, and social justice
A few miles from New York City’s “Million Dollar Blocks” in Brownsville—single city blocks where the state spends more than a million dollars per year to incarcerate people who once lived there—is the one of the busiest psychiatric emergency rooms in the country. Through the doors come people with “behavioral disturbances” who may be a risk to themself or others and who might have a psychiatric disorder that causes functional or cognitive impairment. Sometimes, a clear-headed individual will rattle off an impressive list of diagnoses (schizophrenia, bipolar disorder, posttraumatic stress disorder, depression, anxiety), chronic, not in remission, on medication, which makes one wonder if a dartboard would provide better diagnostic accuracy. Without the construct validity that our colleagues in other specialties have to support our diagnoses, what is first and foremost a medical specialty has been expanded to a catch-all for what ails the human condition.
Being comfortable with uncertainty
Hyperbole aside, speculative pathologizing is risky business. Our conjectures could be influenced by personal bias and also are subject to the surrounding social climate. By watering down the field, our sins run the gamut of incorrectly diagnosing and stigmatizing a vulnerable patient to failing to protect the public from a mentally ill individual. Psychiatry, as it turns out, is not for the faint of heart.
I often am surprised at the number of medical students—it seems more than in past years—who are curious about psychiatry. I tell them one must be comfortable with some degree of uncertainty. Neurologists, for example, are far more certain of their uncertain, mysterious diseases. Likewise, internists resolutely say “idiopathic” without batting an eyelash. Psychiatrists, however, on the whole, are inclined to be an introspective and self-critical bunch. Extrapolated to the field at large, it creates a divisiveness not seen in other medical specialties. Psychiatry has its factions that are fortunately, are not mutually exclusive. Sometimes the twain can and do meet.
With flexible explanatory models come flexible roles in health care. We take on the shape of administrators, directors of clinical service, and policy advocates. Psychiatry, by assuming the mantle of phenomenology, naturally intersects with the areas of human rights, philosophy, and law. Without regard for our considerably influential positions, it is tempting to overstep our bounds as a medical provider. We are, woefully, powerful.
Refocusing community psychiatry
The well-intentioned public psychiatry movement, borne out of deinstitutionalization, endeavored to treat patients in a least restrictive, community-based setting with a focus on rehabilitation and integration. In practice, however, community psychiatry takes on a less rosy view. More often used as a Band-Aid for crime and problems associated with increasing socioeconomic divide, community psychiatry has been rebranded as mental health services for the indigent. Subverting the rights of this doubly vulnerable population is astonishingly easy when paired with a risk-averse litigious environment. “Danger to self or others” becomes our mantra; we unwittingly become ill-trained policemen.
I suggest we narrow the scope of psychiatry and we resist opining on social ills and conflating them with nebulous mental ills. Violent behavior, although troubling, is not a psychiatric symptom and unintentionally correlating it with psychiatric diagnoses does our patients a disservice. When we override an individual’s civil liberties with involuntary hospitalization or involuntary treatment, perhaps it is helpful to consider if we would do the same if the patient had more resources. It is a heavy burden to pretend that we can fix public policy with medications, therapy, and hospitalization. Not only should our actions safeguard the rights of our patients, but also the integrity of our field. In doing so, we might enjoy a less controversial place in health care.
A few miles from New York City’s “Million Dollar Blocks” in Brownsville—single city blocks where the state spends more than a million dollars per year to incarcerate people who once lived there—is the one of the busiest psychiatric emergency rooms in the country. Through the doors come people with “behavioral disturbances” who may be a risk to themself or others and who might have a psychiatric disorder that causes functional or cognitive impairment. Sometimes, a clear-headed individual will rattle off an impressive list of diagnoses (schizophrenia, bipolar disorder, posttraumatic stress disorder, depression, anxiety), chronic, not in remission, on medication, which makes one wonder if a dartboard would provide better diagnostic accuracy. Without the construct validity that our colleagues in other specialties have to support our diagnoses, what is first and foremost a medical specialty has been expanded to a catch-all for what ails the human condition.
Being comfortable with uncertainty
Hyperbole aside, speculative pathologizing is risky business. Our conjectures could be influenced by personal bias and also are subject to the surrounding social climate. By watering down the field, our sins run the gamut of incorrectly diagnosing and stigmatizing a vulnerable patient to failing to protect the public from a mentally ill individual. Psychiatry, as it turns out, is not for the faint of heart.
I often am surprised at the number of medical students—it seems more than in past years—who are curious about psychiatry. I tell them one must be comfortable with some degree of uncertainty. Neurologists, for example, are far more certain of their uncertain, mysterious diseases. Likewise, internists resolutely say “idiopathic” without batting an eyelash. Psychiatrists, however, on the whole, are inclined to be an introspective and self-critical bunch. Extrapolated to the field at large, it creates a divisiveness not seen in other medical specialties. Psychiatry has its factions that are fortunately, are not mutually exclusive. Sometimes the twain can and do meet.
With flexible explanatory models come flexible roles in health care. We take on the shape of administrators, directors of clinical service, and policy advocates. Psychiatry, by assuming the mantle of phenomenology, naturally intersects with the areas of human rights, philosophy, and law. Without regard for our considerably influential positions, it is tempting to overstep our bounds as a medical provider. We are, woefully, powerful.
Refocusing community psychiatry
The well-intentioned public psychiatry movement, borne out of deinstitutionalization, endeavored to treat patients in a least restrictive, community-based setting with a focus on rehabilitation and integration. In practice, however, community psychiatry takes on a less rosy view. More often used as a Band-Aid for crime and problems associated with increasing socioeconomic divide, community psychiatry has been rebranded as mental health services for the indigent. Subverting the rights of this doubly vulnerable population is astonishingly easy when paired with a risk-averse litigious environment. “Danger to self or others” becomes our mantra; we unwittingly become ill-trained policemen.
I suggest we narrow the scope of psychiatry and we resist opining on social ills and conflating them with nebulous mental ills. Violent behavior, although troubling, is not a psychiatric symptom and unintentionally correlating it with psychiatric diagnoses does our patients a disservice. When we override an individual’s civil liberties with involuntary hospitalization or involuntary treatment, perhaps it is helpful to consider if we would do the same if the patient had more resources. It is a heavy burden to pretend that we can fix public policy with medications, therapy, and hospitalization. Not only should our actions safeguard the rights of our patients, but also the integrity of our field. In doing so, we might enjoy a less controversial place in health care.
A few miles from New York City’s “Million Dollar Blocks” in Brownsville—single city blocks where the state spends more than a million dollars per year to incarcerate people who once lived there—is the one of the busiest psychiatric emergency rooms in the country. Through the doors come people with “behavioral disturbances” who may be a risk to themself or others and who might have a psychiatric disorder that causes functional or cognitive impairment. Sometimes, a clear-headed individual will rattle off an impressive list of diagnoses (schizophrenia, bipolar disorder, posttraumatic stress disorder, depression, anxiety), chronic, not in remission, on medication, which makes one wonder if a dartboard would provide better diagnostic accuracy. Without the construct validity that our colleagues in other specialties have to support our diagnoses, what is first and foremost a medical specialty has been expanded to a catch-all for what ails the human condition.
Being comfortable with uncertainty
Hyperbole aside, speculative pathologizing is risky business. Our conjectures could be influenced by personal bias and also are subject to the surrounding social climate. By watering down the field, our sins run the gamut of incorrectly diagnosing and stigmatizing a vulnerable patient to failing to protect the public from a mentally ill individual. Psychiatry, as it turns out, is not for the faint of heart.
I often am surprised at the number of medical students—it seems more than in past years—who are curious about psychiatry. I tell them one must be comfortable with some degree of uncertainty. Neurologists, for example, are far more certain of their uncertain, mysterious diseases. Likewise, internists resolutely say “idiopathic” without batting an eyelash. Psychiatrists, however, on the whole, are inclined to be an introspective and self-critical bunch. Extrapolated to the field at large, it creates a divisiveness not seen in other medical specialties. Psychiatry has its factions that are fortunately, are not mutually exclusive. Sometimes the twain can and do meet.
With flexible explanatory models come flexible roles in health care. We take on the shape of administrators, directors of clinical service, and policy advocates. Psychiatry, by assuming the mantle of phenomenology, naturally intersects with the areas of human rights, philosophy, and law. Without regard for our considerably influential positions, it is tempting to overstep our bounds as a medical provider. We are, woefully, powerful.
Refocusing community psychiatry
The well-intentioned public psychiatry movement, borne out of deinstitutionalization, endeavored to treat patients in a least restrictive, community-based setting with a focus on rehabilitation and integration. In practice, however, community psychiatry takes on a less rosy view. More often used as a Band-Aid for crime and problems associated with increasing socioeconomic divide, community psychiatry has been rebranded as mental health services for the indigent. Subverting the rights of this doubly vulnerable population is astonishingly easy when paired with a risk-averse litigious environment. “Danger to self or others” becomes our mantra; we unwittingly become ill-trained policemen.
I suggest we narrow the scope of psychiatry and we resist opining on social ills and conflating them with nebulous mental ills. Violent behavior, although troubling, is not a psychiatric symptom and unintentionally correlating it with psychiatric diagnoses does our patients a disservice. When we override an individual’s civil liberties with involuntary hospitalization or involuntary treatment, perhaps it is helpful to consider if we would do the same if the patient had more resources. It is a heavy burden to pretend that we can fix public policy with medications, therapy, and hospitalization. Not only should our actions safeguard the rights of our patients, but also the integrity of our field. In doing so, we might enjoy a less controversial place in health care.
Remission, low lupus activity predict lower risk of organ damage
Fulfilling definitions for prolonged lupus remission and reaching a low disease activity state are both associated with a lower risk of damage accrual in lupus patients, new research confirms.
“Identifying predictors of organ damage holds great importance for improving outcomes in SLE [systemic lupus erythematosus]” in light of the strength of organ damage in predicting mortality in people with SLE, wrote the investigators of the new study, led by Michael W. P. Tsang-A-Sjoe of the Amsterdam Rheumatology and Immunology Center, VU University Medical Center, the Netherlands.
The investigators prospectively assessed 183 patients from the Amsterdam SLE cohort for flares and once a year for retrospectively determined remission as defined in a 2015 study (Ann Rheum Dis. 2015;74:2117-22) and in Lupus Low Disease Activity State (LLDAS) criteria set by the Asia-Pacific Lupus Collaboration (Ann Rheum Dis. 2016;75:1615-21). The research team developed a prediction model for damage accrual during the 5-year follow-up through the use of backward logistic regression analyses that took these definitions into account (Rheumatology [Oxford]. 2016 Nov 1. doi: 10.1093/rheumatology/kew377).
The occurrence of at least one flare and the average daily prednisone dose during follow-up were significant predictors of damage accrual (Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index increase of 5 or more during follow-up) in the cohort of patients with or without prolonged remission and with or without LLDAS in half or more of the observations. For example, patients had a higher risk of damage accrual with both a mean daily oral prednisone dose greater than 7.5 mg (odds ratio, 3.6; 95% confidence interval, 1.8-7.2) and greater than 5 mg (OR, 2.8; 95% CI, 1.5-5.3), compared with those taking lower doses.
The researchers also identified a history of nephrological manifestations at baseline as a significant predictor of damage accrual, despite not being part of the criteria for remission or LLDAS.
Overall, 38 (32.5%) of 117 patients met the prolonged remission criteria, whereas LLDAS in half or more of the available observations occurred in 118 (64.5%) of 183 patients.
Prolonged remission during 5 years of follow-up and LLDAS in 50% or more of observations were associated with a reduced risk of damage accrual (OR, 0.20; 95% CI, 0.07-0.53; P = .001; and OR, 0.52; 95% CI, 0.28-0.99, P = .046, respectively).
The effects of both remission and LLDAS, according to the definitions used in the study, don’t settle the matter of which might be used in a treat-to-target strategy. Neither the remission criteria nor the LLDAS definition showed clear superiority over the other, although patients in prolonged remission seemed to accrue less damage, compared with patients classified as being in LLDAS. However, the study did not prove this point, and the investigators noted that “future studies with sufficient power are needed in order to directly compare both sets of criteria for superiority.”
The results might also point to the possibility of lengthening the interval between disease activity assessments. The current study assessed LLDAS and remission at 1 year, compared with other studies that have generally assessed patients at quarterly intervals.
“This finding is interesting because it suggests that in clinical practice, yearly – rather than quarterly – assessments of LLDAS and remission might be sufficient to identify patients at risk of damage accrual, although future studies are needed to confirm this finding,” they wrote.
The study had no specific funding source, and the authors declared having no conflicts of interest.
Fulfilling definitions for prolonged lupus remission and reaching a low disease activity state are both associated with a lower risk of damage accrual in lupus patients, new research confirms.
“Identifying predictors of organ damage holds great importance for improving outcomes in SLE [systemic lupus erythematosus]” in light of the strength of organ damage in predicting mortality in people with SLE, wrote the investigators of the new study, led by Michael W. P. Tsang-A-Sjoe of the Amsterdam Rheumatology and Immunology Center, VU University Medical Center, the Netherlands.
The investigators prospectively assessed 183 patients from the Amsterdam SLE cohort for flares and once a year for retrospectively determined remission as defined in a 2015 study (Ann Rheum Dis. 2015;74:2117-22) and in Lupus Low Disease Activity State (LLDAS) criteria set by the Asia-Pacific Lupus Collaboration (Ann Rheum Dis. 2016;75:1615-21). The research team developed a prediction model for damage accrual during the 5-year follow-up through the use of backward logistic regression analyses that took these definitions into account (Rheumatology [Oxford]. 2016 Nov 1. doi: 10.1093/rheumatology/kew377).
The occurrence of at least one flare and the average daily prednisone dose during follow-up were significant predictors of damage accrual (Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index increase of 5 or more during follow-up) in the cohort of patients with or without prolonged remission and with or without LLDAS in half or more of the observations. For example, patients had a higher risk of damage accrual with both a mean daily oral prednisone dose greater than 7.5 mg (odds ratio, 3.6; 95% confidence interval, 1.8-7.2) and greater than 5 mg (OR, 2.8; 95% CI, 1.5-5.3), compared with those taking lower doses.
The researchers also identified a history of nephrological manifestations at baseline as a significant predictor of damage accrual, despite not being part of the criteria for remission or LLDAS.
Overall, 38 (32.5%) of 117 patients met the prolonged remission criteria, whereas LLDAS in half or more of the available observations occurred in 118 (64.5%) of 183 patients.
Prolonged remission during 5 years of follow-up and LLDAS in 50% or more of observations were associated with a reduced risk of damage accrual (OR, 0.20; 95% CI, 0.07-0.53; P = .001; and OR, 0.52; 95% CI, 0.28-0.99, P = .046, respectively).
The effects of both remission and LLDAS, according to the definitions used in the study, don’t settle the matter of which might be used in a treat-to-target strategy. Neither the remission criteria nor the LLDAS definition showed clear superiority over the other, although patients in prolonged remission seemed to accrue less damage, compared with patients classified as being in LLDAS. However, the study did not prove this point, and the investigators noted that “future studies with sufficient power are needed in order to directly compare both sets of criteria for superiority.”
The results might also point to the possibility of lengthening the interval between disease activity assessments. The current study assessed LLDAS and remission at 1 year, compared with other studies that have generally assessed patients at quarterly intervals.
“This finding is interesting because it suggests that in clinical practice, yearly – rather than quarterly – assessments of LLDAS and remission might be sufficient to identify patients at risk of damage accrual, although future studies are needed to confirm this finding,” they wrote.
The study had no specific funding source, and the authors declared having no conflicts of interest.
Fulfilling definitions for prolonged lupus remission and reaching a low disease activity state are both associated with a lower risk of damage accrual in lupus patients, new research confirms.
“Identifying predictors of organ damage holds great importance for improving outcomes in SLE [systemic lupus erythematosus]” in light of the strength of organ damage in predicting mortality in people with SLE, wrote the investigators of the new study, led by Michael W. P. Tsang-A-Sjoe of the Amsterdam Rheumatology and Immunology Center, VU University Medical Center, the Netherlands.
The investigators prospectively assessed 183 patients from the Amsterdam SLE cohort for flares and once a year for retrospectively determined remission as defined in a 2015 study (Ann Rheum Dis. 2015;74:2117-22) and in Lupus Low Disease Activity State (LLDAS) criteria set by the Asia-Pacific Lupus Collaboration (Ann Rheum Dis. 2016;75:1615-21). The research team developed a prediction model for damage accrual during the 5-year follow-up through the use of backward logistic regression analyses that took these definitions into account (Rheumatology [Oxford]. 2016 Nov 1. doi: 10.1093/rheumatology/kew377).
The occurrence of at least one flare and the average daily prednisone dose during follow-up were significant predictors of damage accrual (Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index increase of 5 or more during follow-up) in the cohort of patients with or without prolonged remission and with or without LLDAS in half or more of the observations. For example, patients had a higher risk of damage accrual with both a mean daily oral prednisone dose greater than 7.5 mg (odds ratio, 3.6; 95% confidence interval, 1.8-7.2) and greater than 5 mg (OR, 2.8; 95% CI, 1.5-5.3), compared with those taking lower doses.
The researchers also identified a history of nephrological manifestations at baseline as a significant predictor of damage accrual, despite not being part of the criteria for remission or LLDAS.
Overall, 38 (32.5%) of 117 patients met the prolonged remission criteria, whereas LLDAS in half or more of the available observations occurred in 118 (64.5%) of 183 patients.
Prolonged remission during 5 years of follow-up and LLDAS in 50% or more of observations were associated with a reduced risk of damage accrual (OR, 0.20; 95% CI, 0.07-0.53; P = .001; and OR, 0.52; 95% CI, 0.28-0.99, P = .046, respectively).
The effects of both remission and LLDAS, according to the definitions used in the study, don’t settle the matter of which might be used in a treat-to-target strategy. Neither the remission criteria nor the LLDAS definition showed clear superiority over the other, although patients in prolonged remission seemed to accrue less damage, compared with patients classified as being in LLDAS. However, the study did not prove this point, and the investigators noted that “future studies with sufficient power are needed in order to directly compare both sets of criteria for superiority.”
The results might also point to the possibility of lengthening the interval between disease activity assessments. The current study assessed LLDAS and remission at 1 year, compared with other studies that have generally assessed patients at quarterly intervals.
“This finding is interesting because it suggests that in clinical practice, yearly – rather than quarterly – assessments of LLDAS and remission might be sufficient to identify patients at risk of damage accrual, although future studies are needed to confirm this finding,” they wrote.
The study had no specific funding source, and the authors declared having no conflicts of interest.
FROM RHEUMATOLOGY
Key clinical finding:
Major finding: At least one flare, average daily prednisone dose during follow-up, and nephrological manifestations at baseline were the most significant predictors of damage accrual in lupus patients.
Data source: Retrospective, longitudinal study of 183 patients from the Amsterdam SLE cohort with a median follow-up of 5 years.
Disclosures: The study had no specific funding source, and the authors declared having no conflicts of interest.