User login
CEA risk models fit for an app
COLUMBUS, OHIO – Carotid endarterectomy is an effective treatment for people with asymptomatic carotid artery disease when stroke rates are low and they survive long enough to benefit from the treatment. But determining who those patients are can be a challenge for vascular surgeons. A team of vascular specialists from around the country have developed risk prediction models to help surgeons better select asymptomatic patients for the procedure, Randall DeMartino, MD, said at the annual meeting of the Midwestern Vascular Surgical Society.
“These models will be used for mobile apps and web-based applications for point of care patient risk assessment,” said Dr. DeMartino of the Mayo Clinic in Rochester, Minn. He is the lead researcher for the study, which uses data from the Vascular Quality Initiative (VQI).
In developing the models, the researchers sampled asymptomatic patients in the VQI who had first-time elective CEA. There were 31,939 patients in the stroke analysis who had CEA from 2010-2015, and 24,086 patients in the mortality analysis who had procedures from 2010-2014. Dr. DeMartino and his colleagues evaluated all preoperative patient and surgeon characteristics, then used an algorithm to optimize the variables that were selected for the final logistic model.
The researchers also evaluated 30-day stroke rates and 1-year mortality at participating centers and found wide variability: an average of 0.9% for stroke, with a range of 0-8.3%; and 3.2% for mortality, with a range of 0-20%. “Actually, 22% of centers had a 1-year mortality rate that exceeded 5%,” Dr. DeMartino said.
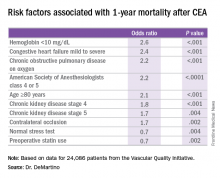
The model for 1-year mortality identified the following variables associated with the highest risk of death 1 year after CEA: age greater than or equal to 80 years; a preoperative hemoglobin less than 10 mg/dL; oxygen-dependent chronic obstructive pulmonary disease; mild to severe congestive heart failure; American Society of Anesthesiologists classification of IV or V; stage 4 or 5 chronic kidney disease; and a contralateral occlusion.
“Conversely, a normal stress test, when performed, and preoperative statin use were associated with reduced risk of death over a year,” Dr. DeMartino said.
“These data have been used to provide Center Opportunity for Improvement reports through VQI where centers can identify if they are selecting patients with risk factors for stroke or mortality more often compared to other centers,” Dr. DeMartino said. “This allows centers to see where opportunities for improvement exist.”
Also, physicians can see the proportion of patients they select with a predicted mortality risk over 5% at one year – “a group of patients who may gain little benefit from prophylactic CEA,” he said. “Physicians can compare their patient selection to those in their region or nationally.”
Dr. DeMartino had no relationships to disclose.
COLUMBUS, OHIO – Carotid endarterectomy is an effective treatment for people with asymptomatic carotid artery disease when stroke rates are low and they survive long enough to benefit from the treatment. But determining who those patients are can be a challenge for vascular surgeons. A team of vascular specialists from around the country have developed risk prediction models to help surgeons better select asymptomatic patients for the procedure, Randall DeMartino, MD, said at the annual meeting of the Midwestern Vascular Surgical Society.
“These models will be used for mobile apps and web-based applications for point of care patient risk assessment,” said Dr. DeMartino of the Mayo Clinic in Rochester, Minn. He is the lead researcher for the study, which uses data from the Vascular Quality Initiative (VQI).
In developing the models, the researchers sampled asymptomatic patients in the VQI who had first-time elective CEA. There were 31,939 patients in the stroke analysis who had CEA from 2010-2015, and 24,086 patients in the mortality analysis who had procedures from 2010-2014. Dr. DeMartino and his colleagues evaluated all preoperative patient and surgeon characteristics, then used an algorithm to optimize the variables that were selected for the final logistic model.
The researchers also evaluated 30-day stroke rates and 1-year mortality at participating centers and found wide variability: an average of 0.9% for stroke, with a range of 0-8.3%; and 3.2% for mortality, with a range of 0-20%. “Actually, 22% of centers had a 1-year mortality rate that exceeded 5%,” Dr. DeMartino said.
The model for 1-year mortality identified the following variables associated with the highest risk of death 1 year after CEA: age greater than or equal to 80 years; a preoperative hemoglobin less than 10 mg/dL; oxygen-dependent chronic obstructive pulmonary disease; mild to severe congestive heart failure; American Society of Anesthesiologists classification of IV or V; stage 4 or 5 chronic kidney disease; and a contralateral occlusion.
“Conversely, a normal stress test, when performed, and preoperative statin use were associated with reduced risk of death over a year,” Dr. DeMartino said.
“These data have been used to provide Center Opportunity for Improvement reports through VQI where centers can identify if they are selecting patients with risk factors for stroke or mortality more often compared to other centers,” Dr. DeMartino said. “This allows centers to see where opportunities for improvement exist.”
Also, physicians can see the proportion of patients they select with a predicted mortality risk over 5% at one year – “a group of patients who may gain little benefit from prophylactic CEA,” he said. “Physicians can compare their patient selection to those in their region or nationally.”
Dr. DeMartino had no relationships to disclose.
COLUMBUS, OHIO – Carotid endarterectomy is an effective treatment for people with asymptomatic carotid artery disease when stroke rates are low and they survive long enough to benefit from the treatment. But determining who those patients are can be a challenge for vascular surgeons. A team of vascular specialists from around the country have developed risk prediction models to help surgeons better select asymptomatic patients for the procedure, Randall DeMartino, MD, said at the annual meeting of the Midwestern Vascular Surgical Society.
“These models will be used for mobile apps and web-based applications for point of care patient risk assessment,” said Dr. DeMartino of the Mayo Clinic in Rochester, Minn. He is the lead researcher for the study, which uses data from the Vascular Quality Initiative (VQI).
In developing the models, the researchers sampled asymptomatic patients in the VQI who had first-time elective CEA. There were 31,939 patients in the stroke analysis who had CEA from 2010-2015, and 24,086 patients in the mortality analysis who had procedures from 2010-2014. Dr. DeMartino and his colleagues evaluated all preoperative patient and surgeon characteristics, then used an algorithm to optimize the variables that were selected for the final logistic model.
The researchers also evaluated 30-day stroke rates and 1-year mortality at participating centers and found wide variability: an average of 0.9% for stroke, with a range of 0-8.3%; and 3.2% for mortality, with a range of 0-20%. “Actually, 22% of centers had a 1-year mortality rate that exceeded 5%,” Dr. DeMartino said.
The model for 1-year mortality identified the following variables associated with the highest risk of death 1 year after CEA: age greater than or equal to 80 years; a preoperative hemoglobin less than 10 mg/dL; oxygen-dependent chronic obstructive pulmonary disease; mild to severe congestive heart failure; American Society of Anesthesiologists classification of IV or V; stage 4 or 5 chronic kidney disease; and a contralateral occlusion.
“Conversely, a normal stress test, when performed, and preoperative statin use were associated with reduced risk of death over a year,” Dr. DeMartino said.
“These data have been used to provide Center Opportunity for Improvement reports through VQI where centers can identify if they are selecting patients with risk factors for stroke or mortality more often compared to other centers,” Dr. DeMartino said. “This allows centers to see where opportunities for improvement exist.”
Also, physicians can see the proportion of patients they select with a predicted mortality risk over 5% at one year – “a group of patients who may gain little benefit from prophylactic CEA,” he said. “Physicians can compare their patient selection to those in their region or nationally.”
Dr. DeMartino had no relationships to disclose.
AT MIDWESTERN VASCULAR 2016
Key clinical point: Risk-prediction models may identify patients at greatest risk of stroke and 1-year death after carotid endarterectomy (CEA).
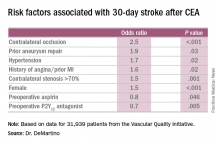
Major finding: Contralateral occlusion has odds ratios of 2.5 for 30-day stroke after CEA and 1.7 for death at 1 year.
Data source: Sampling of patients from the Vascular Quality Initiative who had first-time CEA: 31,939 in the stroke analysis and 24,086 in the mortality analysis.
Disclosures: Dr. DeMartino reported having no financial disclosures.
Infection, readmission linked after open lower-extremity procedures
COLUMBUS, OHIO – Infections account for more than one-third of readmissions after endovascular lower-extremity procedures, but an analysis of these procedures over a 6-year period has identified a handful of factors, including an extended hospital stay, that may help vascular surgeons identify patients at greatest risk and reduce infection-related readmissions.
“Of a little over 7,000 patients that we evaluated with peripheral artery disease who underwent an elective lower-extremity procedure, we found an overall readmission rate of 10.9%; about 9.5% for those who underwent an open procedure and just over 12% for those who underwent an endovascular procedure,” Joseph C. Melvin, MD, of the University of Missouri Hospitals & Clinics in Columbia said at the annual meeting of the Midwestern Vascular Surgery Society.
While the readmission rate for open operations was lower, the infection rate at readmission was higher for open procedures: 45.5% (157 of 345 readmissions) vs. 31.1% (132 of 425 readmissions), Dr. Melvin said.
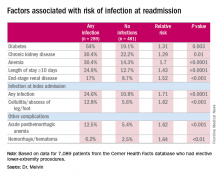
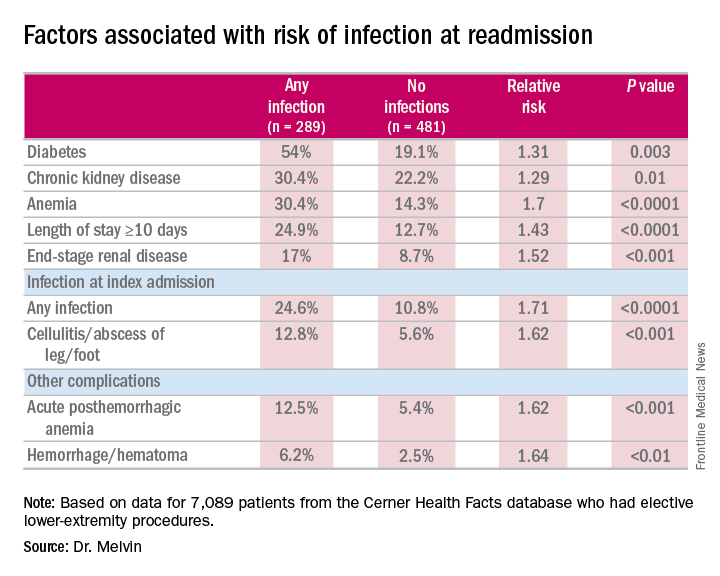
“The risk factors for diagnosis of infection at readmission we found to be significant were anemia, chronic kidney disease, and end-stage renal disease, any infection at the time of the index admission, specifically cellulitis or abscess of the lower extremity given the patient’s peripheral artery disease status, diabetes, and then complications including posthemorrhagic anemia,” Dr. Melvin said. Laboratory testing values at the time of index admissions confirmed the risk factors.
The investigators also used multivariable logistic regression models in the analysis and found that factors most predictive of an infection-related readmission were length of stay, having the procedure at a teaching facility, anemia, and infection at the index admission, Dr. Melvin said.
The surgical site was the most common source of the infection, and Staphylococcus “not surprisingly” accounted for 25% of pathogens, Dr. Melvin said. “But what we did find to be interesting was that just over 40% of patients were found to have a gram-negative bacteria isolated, which would come into play with our decision with regards to antibiotic treatment,” he said.
The data suggest that further evaluation of ways to decrease postoperative infections and use of broad-spectrum antibiotics during readmissions may improve outcomes after open lower-extremity procedures, Dr. Melvin said.
Dr. Melvin had no financial relationships to disclose.
COLUMBUS, OHIO – Infections account for more than one-third of readmissions after endovascular lower-extremity procedures, but an analysis of these procedures over a 6-year period has identified a handful of factors, including an extended hospital stay, that may help vascular surgeons identify patients at greatest risk and reduce infection-related readmissions.
“Of a little over 7,000 patients that we evaluated with peripheral artery disease who underwent an elective lower-extremity procedure, we found an overall readmission rate of 10.9%; about 9.5% for those who underwent an open procedure and just over 12% for those who underwent an endovascular procedure,” Joseph C. Melvin, MD, of the University of Missouri Hospitals & Clinics in Columbia said at the annual meeting of the Midwestern Vascular Surgery Society.
While the readmission rate for open operations was lower, the infection rate at readmission was higher for open procedures: 45.5% (157 of 345 readmissions) vs. 31.1% (132 of 425 readmissions), Dr. Melvin said.
“The risk factors for diagnosis of infection at readmission we found to be significant were anemia, chronic kidney disease, and end-stage renal disease, any infection at the time of the index admission, specifically cellulitis or abscess of the lower extremity given the patient’s peripheral artery disease status, diabetes, and then complications including posthemorrhagic anemia,” Dr. Melvin said. Laboratory testing values at the time of index admissions confirmed the risk factors.
The investigators also used multivariable logistic regression models in the analysis and found that factors most predictive of an infection-related readmission were length of stay, having the procedure at a teaching facility, anemia, and infection at the index admission, Dr. Melvin said.
The surgical site was the most common source of the infection, and Staphylococcus “not surprisingly” accounted for 25% of pathogens, Dr. Melvin said. “But what we did find to be interesting was that just over 40% of patients were found to have a gram-negative bacteria isolated, which would come into play with our decision with regards to antibiotic treatment,” he said.
The data suggest that further evaluation of ways to decrease postoperative infections and use of broad-spectrum antibiotics during readmissions may improve outcomes after open lower-extremity procedures, Dr. Melvin said.
Dr. Melvin had no financial relationships to disclose.
COLUMBUS, OHIO – Infections account for more than one-third of readmissions after endovascular lower-extremity procedures, but an analysis of these procedures over a 6-year period has identified a handful of factors, including an extended hospital stay, that may help vascular surgeons identify patients at greatest risk and reduce infection-related readmissions.
“Of a little over 7,000 patients that we evaluated with peripheral artery disease who underwent an elective lower-extremity procedure, we found an overall readmission rate of 10.9%; about 9.5% for those who underwent an open procedure and just over 12% for those who underwent an endovascular procedure,” Joseph C. Melvin, MD, of the University of Missouri Hospitals & Clinics in Columbia said at the annual meeting of the Midwestern Vascular Surgery Society.
While the readmission rate for open operations was lower, the infection rate at readmission was higher for open procedures: 45.5% (157 of 345 readmissions) vs. 31.1% (132 of 425 readmissions), Dr. Melvin said.
“The risk factors for diagnosis of infection at readmission we found to be significant were anemia, chronic kidney disease, and end-stage renal disease, any infection at the time of the index admission, specifically cellulitis or abscess of the lower extremity given the patient’s peripheral artery disease status, diabetes, and then complications including posthemorrhagic anemia,” Dr. Melvin said. Laboratory testing values at the time of index admissions confirmed the risk factors.
The investigators also used multivariable logistic regression models in the analysis and found that factors most predictive of an infection-related readmission were length of stay, having the procedure at a teaching facility, anemia, and infection at the index admission, Dr. Melvin said.
The surgical site was the most common source of the infection, and Staphylococcus “not surprisingly” accounted for 25% of pathogens, Dr. Melvin said. “But what we did find to be interesting was that just over 40% of patients were found to have a gram-negative bacteria isolated, which would come into play with our decision with regards to antibiotic treatment,” he said.
The data suggest that further evaluation of ways to decrease postoperative infections and use of broad-spectrum antibiotics during readmissions may improve outcomes after open lower-extremity procedures, Dr. Melvin said.
Dr. Melvin had no financial relationships to disclose.
AT MIDWESTERN VASCULAR 2016
Key clinical point: Extended hospital stay and other factors can help identify patients at greatest risk for readmission due to infection.
Major finding: More than one-third of readmissions from lower-extremity procedures are the result of infections.
Data source: 7,089 elective lower extremity procedures selected from the Cerner Health Facts database.
Disclosures: Dr. Melvin reported having no financial disclosures.
VIDEO: No effect of donor on FMT outcomes in C. difficile patients
NEW ORLEANS – Fecal microbiota transplantation, or FMT, is a highly effective treatment for Clostridium difficile infection (CDI) and other digestive and autoimmune disorders, but little is known about the role of donor characteristics with respect to outcomes in patients with recurrent CDI.
A study of nearly 1,999 patients with an 83.9% cure rate showed no significant difference between 28 donors in terms of clinical outcomes at 8 weeks, according to Majdi Osman, MD, of OpenBiome, a not-for-profit stool bank in the Boston area.
Studies in inflammatory bowel diseases have suggested that donors do matter, but that does not appear to be the case when it comes to recurrent CDI, Dr. Osman said at an annual scientific meeting on infectious diseases.
“Broadly speaking, it seems like the efficacy rate is the same amongst all of our donors,” he said in a video interview at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Potential donors are subject to a rigorous screening process, and less than 3% are accepted, but given that donors were shown in previous studies to play a role in effectiveness in some other conditions, Dr. Osman said it was worth checking to see if outcomes in CDI could be further improved through donor selection.
In fact, it appears that “the donor doesn’t matter,” he said, noting that it may be that “we are selecting for a fairly narrow spectrum of the population, and actually the stool that we’re selecting is fairly similar in composition.”
Efforts are underway to look more closely at that possibility, and Dr. Osman said he hopes to see more standardized clinical trials and clinical follow-up. He also said he is excited about an FMT registry – a joint project of the American Gastroenterology Association and the Infectious Diseases Society of America – that will follow 4,000 patients for 10 years.
“We will be working closely with them to provide material and get some really good robust clinical data going forward,” he said.
Dr. Osman reported having no disclosures.
NEW ORLEANS – Fecal microbiota transplantation, or FMT, is a highly effective treatment for Clostridium difficile infection (CDI) and other digestive and autoimmune disorders, but little is known about the role of donor characteristics with respect to outcomes in patients with recurrent CDI.
A study of nearly 1,999 patients with an 83.9% cure rate showed no significant difference between 28 donors in terms of clinical outcomes at 8 weeks, according to Majdi Osman, MD, of OpenBiome, a not-for-profit stool bank in the Boston area.
Studies in inflammatory bowel diseases have suggested that donors do matter, but that does not appear to be the case when it comes to recurrent CDI, Dr. Osman said at an annual scientific meeting on infectious diseases.
“Broadly speaking, it seems like the efficacy rate is the same amongst all of our donors,” he said in a video interview at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Potential donors are subject to a rigorous screening process, and less than 3% are accepted, but given that donors were shown in previous studies to play a role in effectiveness in some other conditions, Dr. Osman said it was worth checking to see if outcomes in CDI could be further improved through donor selection.
In fact, it appears that “the donor doesn’t matter,” he said, noting that it may be that “we are selecting for a fairly narrow spectrum of the population, and actually the stool that we’re selecting is fairly similar in composition.”
Efforts are underway to look more closely at that possibility, and Dr. Osman said he hopes to see more standardized clinical trials and clinical follow-up. He also said he is excited about an FMT registry – a joint project of the American Gastroenterology Association and the Infectious Diseases Society of America – that will follow 4,000 patients for 10 years.
“We will be working closely with them to provide material and get some really good robust clinical data going forward,” he said.
Dr. Osman reported having no disclosures.
NEW ORLEANS – Fecal microbiota transplantation, or FMT, is a highly effective treatment for Clostridium difficile infection (CDI) and other digestive and autoimmune disorders, but little is known about the role of donor characteristics with respect to outcomes in patients with recurrent CDI.
A study of nearly 1,999 patients with an 83.9% cure rate showed no significant difference between 28 donors in terms of clinical outcomes at 8 weeks, according to Majdi Osman, MD, of OpenBiome, a not-for-profit stool bank in the Boston area.
Studies in inflammatory bowel diseases have suggested that donors do matter, but that does not appear to be the case when it comes to recurrent CDI, Dr. Osman said at an annual scientific meeting on infectious diseases.
“Broadly speaking, it seems like the efficacy rate is the same amongst all of our donors,” he said in a video interview at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Potential donors are subject to a rigorous screening process, and less than 3% are accepted, but given that donors were shown in previous studies to play a role in effectiveness in some other conditions, Dr. Osman said it was worth checking to see if outcomes in CDI could be further improved through donor selection.
In fact, it appears that “the donor doesn’t matter,” he said, noting that it may be that “we are selecting for a fairly narrow spectrum of the population, and actually the stool that we’re selecting is fairly similar in composition.”
Efforts are underway to look more closely at that possibility, and Dr. Osman said he hopes to see more standardized clinical trials and clinical follow-up. He also said he is excited about an FMT registry – a joint project of the American Gastroenterology Association and the Infectious Diseases Society of America – that will follow 4,000 patients for 10 years.
“We will be working closely with them to provide material and get some really good robust clinical data going forward,” he said.
Dr. Osman reported having no disclosures.
Shorter duration of untreated psychosis key to Navy program’s approach to schizophrenia care
WASHINGTON – Medical treatment of first-episode psychosis alone is a “cornerstone” intervention, but it’s not sufficient, according to a U.S. Navy psychiatrist who annually treats about 75 people with serious mental illness.
“We need coordinated, multimodal care for optimal treatment of psychosis,” said Michael C. Hann, MD, a Navy psychiatrist and a speaker during a panel on integrated care for schizophrenia at the American Psychiatric Association’s Institute on Psychiatric Services.
By moving away from the standard model in the past 3 years, and instead implementing a coordinated, recovery-oriented system of care as outlined in the National Institute of Mental Health’s RAISE study (Recovery After Initial Schizophrenia Episode), Dr. Hann said the Navy has seen impressive results: In six patients seen recently, the estimated duration of psychosis – the time between prodromal symptoms and first signs of a psychotic break – was as little as 6 weeks and no more than about 9 weeks.
“That is very, very short,” Dr. Hann said. “We’re very excited about that.”
The shorter the duration between first signs of psychosis and treatment, the greater chance a person has to sustain his capacity to function in his community, and enjoy higher a quality of life, according to the NIMH’s webpage about the RAISE trial.
Located at the Navy Medical Center San Diego, the Psychiatric Transition Program treats active-duty military personnel with first-episode psychosis, and also those with bipolar I disorder. Patients in the program are treated for up to 9 or 12 months, before being medically retired from service. Rates of psychosis seen in the military mirror those in the general population – about 1%. “That’s about 300 first breaks a year,” Dr. Hann, the program’s chief resident, said in an interview. “We capture about 20% of those, which is the upper limit of what we’re capable of [caring for],” he said in his presentation, noting that the program is growing as its reputation has spread across the service branches. Dr. Hann said part of the program’s success comes from the swift referrals by military commanders who are alert to signs and symptoms of psychosis.
Other strengths Dr. Hann listed are that all necessary services – including the emergency department, inpatient psychiatric services, and the outpatient clinic – are colocated. Access to inpatient psychiatric services means medication monitoring and modifications, such as being switched to a long-acting injectable antipsychotic, is easier to manage, particularly in high-risk patients. Peer support also is available through a group home model.
The program is staffed by psychiatrists, psychiatry residents, psychiatric technicians, social workers, and nurses who function as case managers. In an interview, Dr. Hann said the program typically has 30 patients in treatment at a time, with an annual average of 75 patients. Most of the patients are on the schizophrenia spectrum, although the program also accepts referrals for bipolar I.
“Currently, there is very little coordination between military and VA-based care systems,” Dr. Yoon said during the presentation. “After [these service personnel] are medically retired, they kind of go off into the wind, and it’s unclear what happens. Our preliminary data show it’s pretty bad.” This lack of coordinated transition puts affected veterans at greater risk of homelessness and suicide, Dr. Yoon said.
Because at present, there is no systematized way for medical personnel in the Department of Defense and the VA to communicate, simple measures that would help keep this patient population stable are not achieved, said Dr. Yoon. With its intended launch in January 2017, OPTICARE is intended to be the bridge between the two systems during the peritransition period, covering the 6 months prior to medical retirement to 1 year post discharge. “None of what we’re doing is rocket science, but none of it is currently being done,” he said.
Dr. Yoon, whose work focuses on how to stabilize faulty striatal dopamine signaling at the D2 receptor to minimize the duration of untreated psychosis, said using aripiprazole to maintain steady levels of D2 blocking is effective. In addition, Dr. Yoon said, he believes that emerging evidence for the stabilizing effects on D2 blocking that long-acting injectable antipsychotics provide mean they should be used more. However, this kind of evidence-based approach to care is frustrated by quirks between the two systems, such as the absence of a shared pharmacy formulary. This can lead to a person’s antipsychotic agent being switched or even noncompliance, and the possible end result can be relapse.
Dr. Yoon also emphasizes ways he expects OPTICARE can help use psychosocial support to minimize stress for patients, since stress disrupts a steady dopamine release in the brain.
“Although schizophrenia is incredibly complex and there is so much more we don’t know, enough coherent and consistent evidence is starting to emerge that I think can provide a unifying framework that should inform treatment decisions at these levels, Dr. Yoon said.
The opinions are the speakers’ own and do not represent those of the U.S. Navy.
[email protected]
On Twitter @whitneymcknight
WASHINGTON – Medical treatment of first-episode psychosis alone is a “cornerstone” intervention, but it’s not sufficient, according to a U.S. Navy psychiatrist who annually treats about 75 people with serious mental illness.
“We need coordinated, multimodal care for optimal treatment of psychosis,” said Michael C. Hann, MD, a Navy psychiatrist and a speaker during a panel on integrated care for schizophrenia at the American Psychiatric Association’s Institute on Psychiatric Services.
By moving away from the standard model in the past 3 years, and instead implementing a coordinated, recovery-oriented system of care as outlined in the National Institute of Mental Health’s RAISE study (Recovery After Initial Schizophrenia Episode), Dr. Hann said the Navy has seen impressive results: In six patients seen recently, the estimated duration of psychosis – the time between prodromal symptoms and first signs of a psychotic break – was as little as 6 weeks and no more than about 9 weeks.
“That is very, very short,” Dr. Hann said. “We’re very excited about that.”
The shorter the duration between first signs of psychosis and treatment, the greater chance a person has to sustain his capacity to function in his community, and enjoy higher a quality of life, according to the NIMH’s webpage about the RAISE trial.
Located at the Navy Medical Center San Diego, the Psychiatric Transition Program treats active-duty military personnel with first-episode psychosis, and also those with bipolar I disorder. Patients in the program are treated for up to 9 or 12 months, before being medically retired from service. Rates of psychosis seen in the military mirror those in the general population – about 1%. “That’s about 300 first breaks a year,” Dr. Hann, the program’s chief resident, said in an interview. “We capture about 20% of those, which is the upper limit of what we’re capable of [caring for],” he said in his presentation, noting that the program is growing as its reputation has spread across the service branches. Dr. Hann said part of the program’s success comes from the swift referrals by military commanders who are alert to signs and symptoms of psychosis.
Other strengths Dr. Hann listed are that all necessary services – including the emergency department, inpatient psychiatric services, and the outpatient clinic – are colocated. Access to inpatient psychiatric services means medication monitoring and modifications, such as being switched to a long-acting injectable antipsychotic, is easier to manage, particularly in high-risk patients. Peer support also is available through a group home model.
The program is staffed by psychiatrists, psychiatry residents, psychiatric technicians, social workers, and nurses who function as case managers. In an interview, Dr. Hann said the program typically has 30 patients in treatment at a time, with an annual average of 75 patients. Most of the patients are on the schizophrenia spectrum, although the program also accepts referrals for bipolar I.
“Currently, there is very little coordination between military and VA-based care systems,” Dr. Yoon said during the presentation. “After [these service personnel] are medically retired, they kind of go off into the wind, and it’s unclear what happens. Our preliminary data show it’s pretty bad.” This lack of coordinated transition puts affected veterans at greater risk of homelessness and suicide, Dr. Yoon said.
Because at present, there is no systematized way for medical personnel in the Department of Defense and the VA to communicate, simple measures that would help keep this patient population stable are not achieved, said Dr. Yoon. With its intended launch in January 2017, OPTICARE is intended to be the bridge between the two systems during the peritransition period, covering the 6 months prior to medical retirement to 1 year post discharge. “None of what we’re doing is rocket science, but none of it is currently being done,” he said.
Dr. Yoon, whose work focuses on how to stabilize faulty striatal dopamine signaling at the D2 receptor to minimize the duration of untreated psychosis, said using aripiprazole to maintain steady levels of D2 blocking is effective. In addition, Dr. Yoon said, he believes that emerging evidence for the stabilizing effects on D2 blocking that long-acting injectable antipsychotics provide mean they should be used more. However, this kind of evidence-based approach to care is frustrated by quirks between the two systems, such as the absence of a shared pharmacy formulary. This can lead to a person’s antipsychotic agent being switched or even noncompliance, and the possible end result can be relapse.
Dr. Yoon also emphasizes ways he expects OPTICARE can help use psychosocial support to minimize stress for patients, since stress disrupts a steady dopamine release in the brain.
“Although schizophrenia is incredibly complex and there is so much more we don’t know, enough coherent and consistent evidence is starting to emerge that I think can provide a unifying framework that should inform treatment decisions at these levels, Dr. Yoon said.
The opinions are the speakers’ own and do not represent those of the U.S. Navy.
[email protected]
On Twitter @whitneymcknight
WASHINGTON – Medical treatment of first-episode psychosis alone is a “cornerstone” intervention, but it’s not sufficient, according to a U.S. Navy psychiatrist who annually treats about 75 people with serious mental illness.
“We need coordinated, multimodal care for optimal treatment of psychosis,” said Michael C. Hann, MD, a Navy psychiatrist and a speaker during a panel on integrated care for schizophrenia at the American Psychiatric Association’s Institute on Psychiatric Services.
By moving away from the standard model in the past 3 years, and instead implementing a coordinated, recovery-oriented system of care as outlined in the National Institute of Mental Health’s RAISE study (Recovery After Initial Schizophrenia Episode), Dr. Hann said the Navy has seen impressive results: In six patients seen recently, the estimated duration of psychosis – the time between prodromal symptoms and first signs of a psychotic break – was as little as 6 weeks and no more than about 9 weeks.
“That is very, very short,” Dr. Hann said. “We’re very excited about that.”
The shorter the duration between first signs of psychosis and treatment, the greater chance a person has to sustain his capacity to function in his community, and enjoy higher a quality of life, according to the NIMH’s webpage about the RAISE trial.
Located at the Navy Medical Center San Diego, the Psychiatric Transition Program treats active-duty military personnel with first-episode psychosis, and also those with bipolar I disorder. Patients in the program are treated for up to 9 or 12 months, before being medically retired from service. Rates of psychosis seen in the military mirror those in the general population – about 1%. “That’s about 300 first breaks a year,” Dr. Hann, the program’s chief resident, said in an interview. “We capture about 20% of those, which is the upper limit of what we’re capable of [caring for],” he said in his presentation, noting that the program is growing as its reputation has spread across the service branches. Dr. Hann said part of the program’s success comes from the swift referrals by military commanders who are alert to signs and symptoms of psychosis.
Other strengths Dr. Hann listed are that all necessary services – including the emergency department, inpatient psychiatric services, and the outpatient clinic – are colocated. Access to inpatient psychiatric services means medication monitoring and modifications, such as being switched to a long-acting injectable antipsychotic, is easier to manage, particularly in high-risk patients. Peer support also is available through a group home model.
The program is staffed by psychiatrists, psychiatry residents, psychiatric technicians, social workers, and nurses who function as case managers. In an interview, Dr. Hann said the program typically has 30 patients in treatment at a time, with an annual average of 75 patients. Most of the patients are on the schizophrenia spectrum, although the program also accepts referrals for bipolar I.
“Currently, there is very little coordination between military and VA-based care systems,” Dr. Yoon said during the presentation. “After [these service personnel] are medically retired, they kind of go off into the wind, and it’s unclear what happens. Our preliminary data show it’s pretty bad.” This lack of coordinated transition puts affected veterans at greater risk of homelessness and suicide, Dr. Yoon said.
Because at present, there is no systematized way for medical personnel in the Department of Defense and the VA to communicate, simple measures that would help keep this patient population stable are not achieved, said Dr. Yoon. With its intended launch in January 2017, OPTICARE is intended to be the bridge between the two systems during the peritransition period, covering the 6 months prior to medical retirement to 1 year post discharge. “None of what we’re doing is rocket science, but none of it is currently being done,” he said.
Dr. Yoon, whose work focuses on how to stabilize faulty striatal dopamine signaling at the D2 receptor to minimize the duration of untreated psychosis, said using aripiprazole to maintain steady levels of D2 blocking is effective. In addition, Dr. Yoon said, he believes that emerging evidence for the stabilizing effects on D2 blocking that long-acting injectable antipsychotics provide mean they should be used more. However, this kind of evidence-based approach to care is frustrated by quirks between the two systems, such as the absence of a shared pharmacy formulary. This can lead to a person’s antipsychotic agent being switched or even noncompliance, and the possible end result can be relapse.
Dr. Yoon also emphasizes ways he expects OPTICARE can help use psychosocial support to minimize stress for patients, since stress disrupts a steady dopamine release in the brain.
“Although schizophrenia is incredibly complex and there is so much more we don’t know, enough coherent and consistent evidence is starting to emerge that I think can provide a unifying framework that should inform treatment decisions at these levels, Dr. Yoon said.
The opinions are the speakers’ own and do not represent those of the U.S. Navy.
[email protected]
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM INSTITUTE ON PSYCHIATRIC SERVICES
Indacaterol/glycopyrronium OK as preferred COPD treatment
AT CHEST 2016
LOS ANGELES – Indacaterol/glycopyrronium was superior to salmeterol/fluticasone at reducing the risk and rate of moderate to severe exacerbations in chronic obstructive pulmonary disease (COPD) patients with more than one or zero to one exacerbations in the previous year, results from an indirect comparison showed.
“Acute exacerbations of COPD are associated with accelerated decline in lung function and increased mortality,” Kenneth R. Chapman, MD, said at the annual meeting of the American College of Chest Physicians. “Current GOLD [Global Initiative for Chronic Obstructive Lung Disease] strategy recommends LABA/ICS [long-acting beta-agonist/inhaled corticosteroid] combination, and/or LAMA [long-acting muscarinic antagonist] as the first-line treatment, and LABA/LAMA as an alternative treatment for COPD patients at a high risk of exacerbations.”
In an effort to examine the reduction in moderate or severe exacerbations in COPD patients taking indacaterol/glycopyrronium (a combination of a LABA bronchodilator and a LAMA bronchodilator) or salmeterol/fluticasone (a LABA and inhaled glucocorticoid combination), researchers compared results from the FLAME and LANTERN trials. The FLAME study evaluated the rate and risk of exacerbations with indacaterol/glycopyrronium versus salmeterol/fluticasone in 3,362 moderate to very severe COPD patients with at least one exacerbation in the previous year (N Engl J Med. 2016;374[23]:2222-34).The LANTERN study compared the efficacy and safety of indacaterol/glycopyrronium versus salmeterol/fluticasone in 744 moderate to very severe COPD patients with 0-1 exacerbation in the previous year (Int J Chron Obstruct Pulmon Dis. 2015;10:1015-26).
Dr. Chapman, professor of medicine at the University of Toronto, reported that in the FLAME study, which was 52 weeks long, indacaterol/glycopyrronium significantly reduced the annualized rate of moderate or severe COPD exacerbations in patients who had one or more exacerbation in the previous year (a rate ratio of 0.83; P less than 0.001), which translated in to a clinically meaningful 17% reduction, compared with their counterparts taking salmeterol/fluticasone. In the LANTERN study, which was 26 weeks long, indacaterol/glycopyrronium also significantly reduced the annualized rate of patients who had 0-1 exacerbation in the previous year, compared with those taking salmeterol/fluticasone (RR, 0.69; P = .048).
In FLAME, indacaterol/glycopyrronium significantly delayed the time to first moderate or severe exacerbation, with a clinically meaningful 22% risk reduction, compared with salmeterol/fluticasone (hazard ratio, 0.78; P less than .001). Similar findings were observed in LANTERN; indacaterol/glycopyrronium significantly delayed the time to first moderate or severe exacerbation, with a clinically meaningful 35% risk reduction, compared with salmeterol/fluticasone (HR, 0.65; P less than .028).
“These results suggest that LABA/LAMA combinations such as indacaterol/glycopyrronium can be considered as a preferred treatment option in the management of COPD patients, irrespective of exacerbation history,” Dr. Chapman said.He went on to note that in FLAME, the incidence of pneumonia was 3.2% in the indacaterol/glycopyrronium group, compared with 4.8% in the salmeterol/fluticasone group P = .02). In LANTERN, the incidence of pneumonia was 0.8% in the indacaterol/glycopyrronium group, compared with 2.7% in the salmeterol/fluticasone group. Finally, in FLAME, the incidence of oral candidiasis was 1.2% in the indacaterol/glycopyrronium group, compared with 4.2% in the salmeterol/fluticasone group (P less than .001). In LANTERN, the respective values were 0% and 0.3%.
Dr. Chapman reported having numerous financial disclosures, including receiving consulting fees and research grants from Novartis.
[email protected]
AT CHEST 2016
LOS ANGELES – Indacaterol/glycopyrronium was superior to salmeterol/fluticasone at reducing the risk and rate of moderate to severe exacerbations in chronic obstructive pulmonary disease (COPD) patients with more than one or zero to one exacerbations in the previous year, results from an indirect comparison showed.
“Acute exacerbations of COPD are associated with accelerated decline in lung function and increased mortality,” Kenneth R. Chapman, MD, said at the annual meeting of the American College of Chest Physicians. “Current GOLD [Global Initiative for Chronic Obstructive Lung Disease] strategy recommends LABA/ICS [long-acting beta-agonist/inhaled corticosteroid] combination, and/or LAMA [long-acting muscarinic antagonist] as the first-line treatment, and LABA/LAMA as an alternative treatment for COPD patients at a high risk of exacerbations.”
In an effort to examine the reduction in moderate or severe exacerbations in COPD patients taking indacaterol/glycopyrronium (a combination of a LABA bronchodilator and a LAMA bronchodilator) or salmeterol/fluticasone (a LABA and inhaled glucocorticoid combination), researchers compared results from the FLAME and LANTERN trials. The FLAME study evaluated the rate and risk of exacerbations with indacaterol/glycopyrronium versus salmeterol/fluticasone in 3,362 moderate to very severe COPD patients with at least one exacerbation in the previous year (N Engl J Med. 2016;374[23]:2222-34).The LANTERN study compared the efficacy and safety of indacaterol/glycopyrronium versus salmeterol/fluticasone in 744 moderate to very severe COPD patients with 0-1 exacerbation in the previous year (Int J Chron Obstruct Pulmon Dis. 2015;10:1015-26).
Dr. Chapman, professor of medicine at the University of Toronto, reported that in the FLAME study, which was 52 weeks long, indacaterol/glycopyrronium significantly reduced the annualized rate of moderate or severe COPD exacerbations in patients who had one or more exacerbation in the previous year (a rate ratio of 0.83; P less than 0.001), which translated in to a clinically meaningful 17% reduction, compared with their counterparts taking salmeterol/fluticasone. In the LANTERN study, which was 26 weeks long, indacaterol/glycopyrronium also significantly reduced the annualized rate of patients who had 0-1 exacerbation in the previous year, compared with those taking salmeterol/fluticasone (RR, 0.69; P = .048).
In FLAME, indacaterol/glycopyrronium significantly delayed the time to first moderate or severe exacerbation, with a clinically meaningful 22% risk reduction, compared with salmeterol/fluticasone (hazard ratio, 0.78; P less than .001). Similar findings were observed in LANTERN; indacaterol/glycopyrronium significantly delayed the time to first moderate or severe exacerbation, with a clinically meaningful 35% risk reduction, compared with salmeterol/fluticasone (HR, 0.65; P less than .028).
“These results suggest that LABA/LAMA combinations such as indacaterol/glycopyrronium can be considered as a preferred treatment option in the management of COPD patients, irrespective of exacerbation history,” Dr. Chapman said.He went on to note that in FLAME, the incidence of pneumonia was 3.2% in the indacaterol/glycopyrronium group, compared with 4.8% in the salmeterol/fluticasone group P = .02). In LANTERN, the incidence of pneumonia was 0.8% in the indacaterol/glycopyrronium group, compared with 2.7% in the salmeterol/fluticasone group. Finally, in FLAME, the incidence of oral candidiasis was 1.2% in the indacaterol/glycopyrronium group, compared with 4.2% in the salmeterol/fluticasone group (P less than .001). In LANTERN, the respective values were 0% and 0.3%.
Dr. Chapman reported having numerous financial disclosures, including receiving consulting fees and research grants from Novartis.
[email protected]
AT CHEST 2016
LOS ANGELES – Indacaterol/glycopyrronium was superior to salmeterol/fluticasone at reducing the risk and rate of moderate to severe exacerbations in chronic obstructive pulmonary disease (COPD) patients with more than one or zero to one exacerbations in the previous year, results from an indirect comparison showed.
“Acute exacerbations of COPD are associated with accelerated decline in lung function and increased mortality,” Kenneth R. Chapman, MD, said at the annual meeting of the American College of Chest Physicians. “Current GOLD [Global Initiative for Chronic Obstructive Lung Disease] strategy recommends LABA/ICS [long-acting beta-agonist/inhaled corticosteroid] combination, and/or LAMA [long-acting muscarinic antagonist] as the first-line treatment, and LABA/LAMA as an alternative treatment for COPD patients at a high risk of exacerbations.”
In an effort to examine the reduction in moderate or severe exacerbations in COPD patients taking indacaterol/glycopyrronium (a combination of a LABA bronchodilator and a LAMA bronchodilator) or salmeterol/fluticasone (a LABA and inhaled glucocorticoid combination), researchers compared results from the FLAME and LANTERN trials. The FLAME study evaluated the rate and risk of exacerbations with indacaterol/glycopyrronium versus salmeterol/fluticasone in 3,362 moderate to very severe COPD patients with at least one exacerbation in the previous year (N Engl J Med. 2016;374[23]:2222-34).The LANTERN study compared the efficacy and safety of indacaterol/glycopyrronium versus salmeterol/fluticasone in 744 moderate to very severe COPD patients with 0-1 exacerbation in the previous year (Int J Chron Obstruct Pulmon Dis. 2015;10:1015-26).
Dr. Chapman, professor of medicine at the University of Toronto, reported that in the FLAME study, which was 52 weeks long, indacaterol/glycopyrronium significantly reduced the annualized rate of moderate or severe COPD exacerbations in patients who had one or more exacerbation in the previous year (a rate ratio of 0.83; P less than 0.001), which translated in to a clinically meaningful 17% reduction, compared with their counterparts taking salmeterol/fluticasone. In the LANTERN study, which was 26 weeks long, indacaterol/glycopyrronium also significantly reduced the annualized rate of patients who had 0-1 exacerbation in the previous year, compared with those taking salmeterol/fluticasone (RR, 0.69; P = .048).
In FLAME, indacaterol/glycopyrronium significantly delayed the time to first moderate or severe exacerbation, with a clinically meaningful 22% risk reduction, compared with salmeterol/fluticasone (hazard ratio, 0.78; P less than .001). Similar findings were observed in LANTERN; indacaterol/glycopyrronium significantly delayed the time to first moderate or severe exacerbation, with a clinically meaningful 35% risk reduction, compared with salmeterol/fluticasone (HR, 0.65; P less than .028).
“These results suggest that LABA/LAMA combinations such as indacaterol/glycopyrronium can be considered as a preferred treatment option in the management of COPD patients, irrespective of exacerbation history,” Dr. Chapman said.He went on to note that in FLAME, the incidence of pneumonia was 3.2% in the indacaterol/glycopyrronium group, compared with 4.8% in the salmeterol/fluticasone group P = .02). In LANTERN, the incidence of pneumonia was 0.8% in the indacaterol/glycopyrronium group, compared with 2.7% in the salmeterol/fluticasone group. Finally, in FLAME, the incidence of oral candidiasis was 1.2% in the indacaterol/glycopyrronium group, compared with 4.2% in the salmeterol/fluticasone group (P less than .001). In LANTERN, the respective values were 0% and 0.3%.
Dr. Chapman reported having numerous financial disclosures, including receiving consulting fees and research grants from Novartis.
[email protected]
Key clinical point:
Major finding: Indacaterol/glycopyrronium significantly reduced the annualized rate of moderate or severe COPD exacerbations in patients who had one or more exacerbation in the previous year (a rate ratio of 0.83; P less than 0.001), which translated into a clinically meaningful 17% reduction, compared with their counterparts taking salmeterol/fluticasone.
Data source: An indirect comparison of 3,362 patients in the FLAME study and 744 patients in the LANTERN study.
Disclosures: Dr. Chapman reported having numerous financial disclosures, including receiving consulting fees and research grants from Novartis.
Meaningful use: CMS extends 90-day reporting period to 2016, 2017
Physicians who already participate in the EHR incentive program for meaningful use will have to demonstrate they are meaningful users for only 90 days in 2016 and 2017.
The Centers for Medicare & Medicaid Services finalized the 90-day reporting period in its annual update to the hospital outpatient prospective payment system, scheduled for publication in the Federal Register on Nov. 14.
The CMS was preparing to require a full year of reporting in 2017 to be eligible for bonuses under the EHR Incentive Program; but instead extended the 90-day reporting requirement to address concerns about technical functionalities and to help ease the transition to the Merit-based Incentive Payment System (MIPS) created by the 2015 MACRA law, according to an agency fact sheet.
The Outpatient Prospective Payment System (OPPS) final rule also eliminated clinical decision support and computerized order entry objectives and measures for eligible hospitals and critical access hospitals.
Instead of requiring that eligible professionals and hospitals new to the EHR Incentive Program in 2017 to meet Stage 3 requirements, the CMS has provided modified Stage 2 requirements.
Physicians who are new to the EHR Incentive Program in 2017 but are transitioning their practice to MIPS next year can apply for a significant hardship exemption to avoid any penalties to their 2018 Medicare payments.
Physicians who already participate in the EHR incentive program for meaningful use will have to demonstrate they are meaningful users for only 90 days in 2016 and 2017.
The Centers for Medicare & Medicaid Services finalized the 90-day reporting period in its annual update to the hospital outpatient prospective payment system, scheduled for publication in the Federal Register on Nov. 14.
The CMS was preparing to require a full year of reporting in 2017 to be eligible for bonuses under the EHR Incentive Program; but instead extended the 90-day reporting requirement to address concerns about technical functionalities and to help ease the transition to the Merit-based Incentive Payment System (MIPS) created by the 2015 MACRA law, according to an agency fact sheet.
The Outpatient Prospective Payment System (OPPS) final rule also eliminated clinical decision support and computerized order entry objectives and measures for eligible hospitals and critical access hospitals.
Instead of requiring that eligible professionals and hospitals new to the EHR Incentive Program in 2017 to meet Stage 3 requirements, the CMS has provided modified Stage 2 requirements.
Physicians who are new to the EHR Incentive Program in 2017 but are transitioning their practice to MIPS next year can apply for a significant hardship exemption to avoid any penalties to their 2018 Medicare payments.
Physicians who already participate in the EHR incentive program for meaningful use will have to demonstrate they are meaningful users for only 90 days in 2016 and 2017.
The Centers for Medicare & Medicaid Services finalized the 90-day reporting period in its annual update to the hospital outpatient prospective payment system, scheduled for publication in the Federal Register on Nov. 14.
The CMS was preparing to require a full year of reporting in 2017 to be eligible for bonuses under the EHR Incentive Program; but instead extended the 90-day reporting requirement to address concerns about technical functionalities and to help ease the transition to the Merit-based Incentive Payment System (MIPS) created by the 2015 MACRA law, according to an agency fact sheet.
The Outpatient Prospective Payment System (OPPS) final rule also eliminated clinical decision support and computerized order entry objectives and measures for eligible hospitals and critical access hospitals.
Instead of requiring that eligible professionals and hospitals new to the EHR Incentive Program in 2017 to meet Stage 3 requirements, the CMS has provided modified Stage 2 requirements.
Physicians who are new to the EHR Incentive Program in 2017 but are transitioning their practice to MIPS next year can apply for a significant hardship exemption to avoid any penalties to their 2018 Medicare payments.
November 2016 Quiz 2
Q2: Answer: B
This is likely a tapeworm infection with Diphyllobothrium latum, and most tapeworm infections are treated with praziquantel. D. latum infection can be acquired by ingesting certain forms of freshwater fish, and those who consume raw fish, including sushi, are at increased risk. Patients infected with D. latum may develop a megaloblastic anemia secondary to vitamin B12 deficiency given that D. latum competitively interferes with normal host absorption. All the other agents listed would not be used for treatment of tapeworm infection.
Reference
1. Scholz T., et al. Update on the human broad tapeworm (genus Diphyllobothrium), including clinical relevance. Clin Microbiol Rev. 2009;22:146-60.
Q2: Answer: B
This is likely a tapeworm infection with Diphyllobothrium latum, and most tapeworm infections are treated with praziquantel. D. latum infection can be acquired by ingesting certain forms of freshwater fish, and those who consume raw fish, including sushi, are at increased risk. Patients infected with D. latum may develop a megaloblastic anemia secondary to vitamin B12 deficiency given that D. latum competitively interferes with normal host absorption. All the other agents listed would not be used for treatment of tapeworm infection.
Reference
1. Scholz T., et al. Update on the human broad tapeworm (genus Diphyllobothrium), including clinical relevance. Clin Microbiol Rev. 2009;22:146-60.
Q2: Answer: B
This is likely a tapeworm infection with Diphyllobothrium latum, and most tapeworm infections are treated with praziquantel. D. latum infection can be acquired by ingesting certain forms of freshwater fish, and those who consume raw fish, including sushi, are at increased risk. Patients infected with D. latum may develop a megaloblastic anemia secondary to vitamin B12 deficiency given that D. latum competitively interferes with normal host absorption. All the other agents listed would not be used for treatment of tapeworm infection.
Reference
1. Scholz T., et al. Update on the human broad tapeworm (genus Diphyllobothrium), including clinical relevance. Clin Microbiol Rev. 2009;22:146-60.
A 40-year-old man presents to the clinic with 2 months of diarrhea and significant fatigue. He has no significant past medical history and works as a chef in a local sushi bar. He has nonbloody watery stools with nocturnal symptoms. The diarrhea is associated with abdominal cramps. His physical examination is unrevealing. His hemoglobin is 9.8 g/dL, with a mean corpuscular volume of 110 fL. Peripheral eosinophilia is noted. A stool sample is sent to the lab and is pending.
Hand Hygiene Improves Patient Safety
Clinical Question: Does improving hand hygiene compliance from a high level (>80%) to a very high level (>95%) reduce healthcare-associated infections?
Background: Hand hygiene compliance remains an elusive infection prevention parameter to master. Studies show a correlation in reduction of healthcare-associated infections with improved hand hygiene compliance from a low to medium level, but little data exist on very high rates of hand hygiene compliance.
Study Design: Prospective observational.
Setting: University of North Carolina Hospitals.
Synopsis: Researchers recruited all hospital staff to be hand hygiene monitors, thereby using the Hawthorne effect to drive hand hygiene compliance rates. Over a 17-month period, >4,000 unique observers made >140,000 observations. Data showed a significant increase in hand hygiene compliance rates of about 10% (P<0.001) and a significant decrease in overall healthcare-associated infection rates of about 6% (P=0.0066). A reduction in healthcare-associated Clostridium difficile infection of 14% was observed in association with the improved hand hygiene compliance. No association with multidrug-resistant organisms was found.
Bottom Line: There is continued correlation between improved hand hygiene compliance and reduced healthcare-associated infection rates even at very high levels (>95%) of hand hygiene compliance.
Citation: Sickbert-Bennett EE, DiBiase LM, Willis TM, Wolak ES, Weber DJ, Rutala WA. Reduction of healthcare-associated infections by exceeding high compliance with hand hygiene practices. Emerg Infect Dis. 2016;22(9):1628-1630.
Short Take
Avoid Fluoroquinolones in Acute Sinusitis, Acute Exacerbations of Bronchitis, and Uncomplicated Urinary Tract Infections If Other Treatment Options Exist
Because fluoroquinolones have been associated with potentially permanent side effects involving tendons, muscles, joints, and nerves, the FDA recently updated the boxed warning to state that the risk of use likely outweighs the benefit for uncomplicated infections.
Citation: Fluoroquinolone Antibacterial Drugs for Systemic Use: Drug Safety Communication - Warnings Updated Due to Disabling Side Effects. FDA website. Accessed September 9, 2016.
Clinical Question: Does improving hand hygiene compliance from a high level (>80%) to a very high level (>95%) reduce healthcare-associated infections?
Background: Hand hygiene compliance remains an elusive infection prevention parameter to master. Studies show a correlation in reduction of healthcare-associated infections with improved hand hygiene compliance from a low to medium level, but little data exist on very high rates of hand hygiene compliance.
Study Design: Prospective observational.
Setting: University of North Carolina Hospitals.
Synopsis: Researchers recruited all hospital staff to be hand hygiene monitors, thereby using the Hawthorne effect to drive hand hygiene compliance rates. Over a 17-month period, >4,000 unique observers made >140,000 observations. Data showed a significant increase in hand hygiene compliance rates of about 10% (P<0.001) and a significant decrease in overall healthcare-associated infection rates of about 6% (P=0.0066). A reduction in healthcare-associated Clostridium difficile infection of 14% was observed in association with the improved hand hygiene compliance. No association with multidrug-resistant organisms was found.
Bottom Line: There is continued correlation between improved hand hygiene compliance and reduced healthcare-associated infection rates even at very high levels (>95%) of hand hygiene compliance.
Citation: Sickbert-Bennett EE, DiBiase LM, Willis TM, Wolak ES, Weber DJ, Rutala WA. Reduction of healthcare-associated infections by exceeding high compliance with hand hygiene practices. Emerg Infect Dis. 2016;22(9):1628-1630.
Short Take
Avoid Fluoroquinolones in Acute Sinusitis, Acute Exacerbations of Bronchitis, and Uncomplicated Urinary Tract Infections If Other Treatment Options Exist
Because fluoroquinolones have been associated with potentially permanent side effects involving tendons, muscles, joints, and nerves, the FDA recently updated the boxed warning to state that the risk of use likely outweighs the benefit for uncomplicated infections.
Citation: Fluoroquinolone Antibacterial Drugs for Systemic Use: Drug Safety Communication - Warnings Updated Due to Disabling Side Effects. FDA website. Accessed September 9, 2016.
Clinical Question: Does improving hand hygiene compliance from a high level (>80%) to a very high level (>95%) reduce healthcare-associated infections?
Background: Hand hygiene compliance remains an elusive infection prevention parameter to master. Studies show a correlation in reduction of healthcare-associated infections with improved hand hygiene compliance from a low to medium level, but little data exist on very high rates of hand hygiene compliance.
Study Design: Prospective observational.
Setting: University of North Carolina Hospitals.
Synopsis: Researchers recruited all hospital staff to be hand hygiene monitors, thereby using the Hawthorne effect to drive hand hygiene compliance rates. Over a 17-month period, >4,000 unique observers made >140,000 observations. Data showed a significant increase in hand hygiene compliance rates of about 10% (P<0.001) and a significant decrease in overall healthcare-associated infection rates of about 6% (P=0.0066). A reduction in healthcare-associated Clostridium difficile infection of 14% was observed in association with the improved hand hygiene compliance. No association with multidrug-resistant organisms was found.
Bottom Line: There is continued correlation between improved hand hygiene compliance and reduced healthcare-associated infection rates even at very high levels (>95%) of hand hygiene compliance.
Citation: Sickbert-Bennett EE, DiBiase LM, Willis TM, Wolak ES, Weber DJ, Rutala WA. Reduction of healthcare-associated infections by exceeding high compliance with hand hygiene practices. Emerg Infect Dis. 2016;22(9):1628-1630.
Short Take
Avoid Fluoroquinolones in Acute Sinusitis, Acute Exacerbations of Bronchitis, and Uncomplicated Urinary Tract Infections If Other Treatment Options Exist
Because fluoroquinolones have been associated with potentially permanent side effects involving tendons, muscles, joints, and nerves, the FDA recently updated the boxed warning to state that the risk of use likely outweighs the benefit for uncomplicated infections.
Citation: Fluoroquinolone Antibacterial Drugs for Systemic Use: Drug Safety Communication - Warnings Updated Due to Disabling Side Effects. FDA website. Accessed September 9, 2016.
Traditional Hand Hygiene Audits Can Lead to Inaccurate Conclusions about Physician Performance
Clinical Question: Does direct observation underestimate physician compliance with hand hygiene (HH) compared to other professional groups due to the Hawthorne effect?
Background: Although it is well-known that HH is imperative to infection control, physician compliance remains suboptimal and is often reported to be below that of nurses. The Hawthorne effect may be contributing to this perceived difference because nurses, who work on the same unit consistently, may more readily recognize hospital auditors.
Study Design: Observational.
Setting: 800-bed acute-care academic hospital in Canada.
Synopsis: Two students were trained to covertly observe physician and nursing HH compliance on inpatient units. For two months, students rotated units every week to minimize risk of discovery. Their findings were compared with data gathered by hospital auditors over the same time period.
Covertly observed HH compliance was 50% (799/1,597 opportunities) compared with 83.7% (2,769/3,309) reported by hospital auditors (P<0.0002). The difference in physician compliance was 19% (73.2% compliance with overt observation versus 54.2% with covert observation). The difference was much higher for nurses at 40.7% (85.8% compliance with overt observation versus 45.1% with covert observation). Attending physician behaviors heavily influenced team behaviors—79.5% of trainees were compliant if their attending was compliant compared with 18.9% if attending was not (P<0.0002).
Bottom Line: Traditional HH audit findings that physicians are less compliant than nurses may be at least partially due to the Hawthorne effect. Nonetheless, all healthcare providers have substantial room for improvement, and attending physicians are powerful role models to effect this change.
Citation: Kovacs-Litman A, Wong K, Shojania KJ, Callery S, Vearncombe M, Leis J. Do physicians clean their hands? Insights from a covert observational study [published online ahead of print July 5, 2016]. J Hosp Med.
Clinical Question: Does direct observation underestimate physician compliance with hand hygiene (HH) compared to other professional groups due to the Hawthorne effect?
Background: Although it is well-known that HH is imperative to infection control, physician compliance remains suboptimal and is often reported to be below that of nurses. The Hawthorne effect may be contributing to this perceived difference because nurses, who work on the same unit consistently, may more readily recognize hospital auditors.
Study Design: Observational.
Setting: 800-bed acute-care academic hospital in Canada.
Synopsis: Two students were trained to covertly observe physician and nursing HH compliance on inpatient units. For two months, students rotated units every week to minimize risk of discovery. Their findings were compared with data gathered by hospital auditors over the same time period.
Covertly observed HH compliance was 50% (799/1,597 opportunities) compared with 83.7% (2,769/3,309) reported by hospital auditors (P<0.0002). The difference in physician compliance was 19% (73.2% compliance with overt observation versus 54.2% with covert observation). The difference was much higher for nurses at 40.7% (85.8% compliance with overt observation versus 45.1% with covert observation). Attending physician behaviors heavily influenced team behaviors—79.5% of trainees were compliant if their attending was compliant compared with 18.9% if attending was not (P<0.0002).
Bottom Line: Traditional HH audit findings that physicians are less compliant than nurses may be at least partially due to the Hawthorne effect. Nonetheless, all healthcare providers have substantial room for improvement, and attending physicians are powerful role models to effect this change.
Citation: Kovacs-Litman A, Wong K, Shojania KJ, Callery S, Vearncombe M, Leis J. Do physicians clean their hands? Insights from a covert observational study [published online ahead of print July 5, 2016]. J Hosp Med.
Clinical Question: Does direct observation underestimate physician compliance with hand hygiene (HH) compared to other professional groups due to the Hawthorne effect?
Background: Although it is well-known that HH is imperative to infection control, physician compliance remains suboptimal and is often reported to be below that of nurses. The Hawthorne effect may be contributing to this perceived difference because nurses, who work on the same unit consistently, may more readily recognize hospital auditors.
Study Design: Observational.
Setting: 800-bed acute-care academic hospital in Canada.
Synopsis: Two students were trained to covertly observe physician and nursing HH compliance on inpatient units. For two months, students rotated units every week to minimize risk of discovery. Their findings were compared with data gathered by hospital auditors over the same time period.
Covertly observed HH compliance was 50% (799/1,597 opportunities) compared with 83.7% (2,769/3,309) reported by hospital auditors (P<0.0002). The difference in physician compliance was 19% (73.2% compliance with overt observation versus 54.2% with covert observation). The difference was much higher for nurses at 40.7% (85.8% compliance with overt observation versus 45.1% with covert observation). Attending physician behaviors heavily influenced team behaviors—79.5% of trainees were compliant if their attending was compliant compared with 18.9% if attending was not (P<0.0002).
Bottom Line: Traditional HH audit findings that physicians are less compliant than nurses may be at least partially due to the Hawthorne effect. Nonetheless, all healthcare providers have substantial room for improvement, and attending physicians are powerful role models to effect this change.
Citation: Kovacs-Litman A, Wong K, Shojania KJ, Callery S, Vearncombe M, Leis J. Do physicians clean their hands? Insights from a covert observational study [published online ahead of print July 5, 2016]. J Hosp Med.
Vector may make gene therapy safer

Photo courtesy of Washington
State University Spokane
Using modified foamy retroviral vectors to deliver gene therapy may reduce the risk of genotoxicity, according to research published in Scientific Reports.
These vectors have demonstrated promise in vitro, and researchers believe they could be used to treat X-linked severe combined immunodeficiency, thalassemia, and other diseases.
“We’ve started to translate this in collaboration with other scientists and medical doctors into the clinic,” said study author Grant Trobridge, PhD, of Washington State University Spokane.
Dr Trobridge and his colleagues said they decided to pursue foamy retroviral vectors as a delivery system for gene therapy because these vectors are less likely than gammaretroviral vectors or lentiviral vectors to activate nearby genes, including proto-oncogenes.
Still, the researchers altered the foamy retroviral vectors to change how they interact with target stem cells in an attempt to ensure the vectors would insert themselves into safer parts of the genome.
The team said they were able to retarget the foamy retroviral vectors away from genes and into satellite regions enriched for trimethylated histone H3 at lysine 9 by modifying the foamy virus Gag and Pol proteins.
These retargeted foamy retroviral vectors integrated near genes significantly less often than unmodified foamy retroviral vectors (P<0.001).
The researchers also noted that their retargeted foamy retroviral vectors can be produced at clinically relevant titers, and engineered target cells are not needed. Any target cell can be used by using alternate foamy helper plasmids during vector production. ![]()

Photo courtesy of Washington
State University Spokane
Using modified foamy retroviral vectors to deliver gene therapy may reduce the risk of genotoxicity, according to research published in Scientific Reports.
These vectors have demonstrated promise in vitro, and researchers believe they could be used to treat X-linked severe combined immunodeficiency, thalassemia, and other diseases.
“We’ve started to translate this in collaboration with other scientists and medical doctors into the clinic,” said study author Grant Trobridge, PhD, of Washington State University Spokane.
Dr Trobridge and his colleagues said they decided to pursue foamy retroviral vectors as a delivery system for gene therapy because these vectors are less likely than gammaretroviral vectors or lentiviral vectors to activate nearby genes, including proto-oncogenes.
Still, the researchers altered the foamy retroviral vectors to change how they interact with target stem cells in an attempt to ensure the vectors would insert themselves into safer parts of the genome.
The team said they were able to retarget the foamy retroviral vectors away from genes and into satellite regions enriched for trimethylated histone H3 at lysine 9 by modifying the foamy virus Gag and Pol proteins.
These retargeted foamy retroviral vectors integrated near genes significantly less often than unmodified foamy retroviral vectors (P<0.001).
The researchers also noted that their retargeted foamy retroviral vectors can be produced at clinically relevant titers, and engineered target cells are not needed. Any target cell can be used by using alternate foamy helper plasmids during vector production. ![]()

Photo courtesy of Washington
State University Spokane
Using modified foamy retroviral vectors to deliver gene therapy may reduce the risk of genotoxicity, according to research published in Scientific Reports.
These vectors have demonstrated promise in vitro, and researchers believe they could be used to treat X-linked severe combined immunodeficiency, thalassemia, and other diseases.
“We’ve started to translate this in collaboration with other scientists and medical doctors into the clinic,” said study author Grant Trobridge, PhD, of Washington State University Spokane.
Dr Trobridge and his colleagues said they decided to pursue foamy retroviral vectors as a delivery system for gene therapy because these vectors are less likely than gammaretroviral vectors or lentiviral vectors to activate nearby genes, including proto-oncogenes.
Still, the researchers altered the foamy retroviral vectors to change how they interact with target stem cells in an attempt to ensure the vectors would insert themselves into safer parts of the genome.
The team said they were able to retarget the foamy retroviral vectors away from genes and into satellite regions enriched for trimethylated histone H3 at lysine 9 by modifying the foamy virus Gag and Pol proteins.
These retargeted foamy retroviral vectors integrated near genes significantly less often than unmodified foamy retroviral vectors (P<0.001).
The researchers also noted that their retargeted foamy retroviral vectors can be produced at clinically relevant titers, and engineered target cells are not needed. Any target cell can be used by using alternate foamy helper plasmids during vector production. ![]()