User login
How EBV drives lymphomagenesis

Image by Ed Uthman
Results of research published in eLIFE appear to explain how Epstein-Barr virus (EBV) controls a pair of genes to drive lymphomagenesis.
Researchers set out to determine how EBV controls MYC, which is known to drive lymphoma development when activated, and BCL2L11, a gene that normally triggers apoptosis to prevent lymphoma but can be silenced by EBV.
The team discovered that EBV controls MYC and BCL2L11 by hijacking enhancer regions of DNA, which are situated far away from the genes.
These enhancers act as “control centers” and are able to contact and control genes from long distances by the looping out of the intervening stretches of DNA.
The researchers found that EBV activates MYC by increasing contacts between a specific set of enhancers and the gene.
The team said an Epstein-Barr nuclear antigen, EBNA2, activates multiple MYC enhancers and reconfigures the MYC locus to increase upstream enhancer-promoter interactions and decrease downstream interactions.
They noted that EBNA2 recruits the BRG1 ATPase of the SWI/SNF remodeller to MYC enhancers, and BRG1 is required for enhancer-promoter interactions in EBV-infected cells.
The researchers also discovered new enhancers that control BCL2L11. In this case, though, EBV stops these control centers from contacting the gene.
Specifically, the team found a hematopoietic enhancer hub that is inactivated by the Epstein-Barr nuclear antigens EBNA3A and EBNA3C through recruitment of the H3K27 methyltransferase EZH2.
Therefore, the researchers set out to determine if an EZH1/2 inhibitor, UNC1999, could reverse this effect. They found that UNC1999 did reverse enhancer inactivation, upregulated BCL2L11, and induced apoptosis in EBV-positive Burkitt lymphoma cells.
“This is a key step towards uncovering how this common virus, which affects thousands of people every year, causes blood cancer,” said study author Michelle West, PhD, of the University of Sussex in Brighton, UK.
“It is now important to carry out further studies to determine how the Epstein-Barr virus controls other genes that are associated with lymphoma. This will tell us more about how the virus drives lymphoma development and will help to identify new ways of targeting Epstein-Barr virus-infected cancer cells with specific drugs.” ![]()

Image by Ed Uthman
Results of research published in eLIFE appear to explain how Epstein-Barr virus (EBV) controls a pair of genes to drive lymphomagenesis.
Researchers set out to determine how EBV controls MYC, which is known to drive lymphoma development when activated, and BCL2L11, a gene that normally triggers apoptosis to prevent lymphoma but can be silenced by EBV.
The team discovered that EBV controls MYC and BCL2L11 by hijacking enhancer regions of DNA, which are situated far away from the genes.
These enhancers act as “control centers” and are able to contact and control genes from long distances by the looping out of the intervening stretches of DNA.
The researchers found that EBV activates MYC by increasing contacts between a specific set of enhancers and the gene.
The team said an Epstein-Barr nuclear antigen, EBNA2, activates multiple MYC enhancers and reconfigures the MYC locus to increase upstream enhancer-promoter interactions and decrease downstream interactions.
They noted that EBNA2 recruits the BRG1 ATPase of the SWI/SNF remodeller to MYC enhancers, and BRG1 is required for enhancer-promoter interactions in EBV-infected cells.
The researchers also discovered new enhancers that control BCL2L11. In this case, though, EBV stops these control centers from contacting the gene.
Specifically, the team found a hematopoietic enhancer hub that is inactivated by the Epstein-Barr nuclear antigens EBNA3A and EBNA3C through recruitment of the H3K27 methyltransferase EZH2.
Therefore, the researchers set out to determine if an EZH1/2 inhibitor, UNC1999, could reverse this effect. They found that UNC1999 did reverse enhancer inactivation, upregulated BCL2L11, and induced apoptosis in EBV-positive Burkitt lymphoma cells.
“This is a key step towards uncovering how this common virus, which affects thousands of people every year, causes blood cancer,” said study author Michelle West, PhD, of the University of Sussex in Brighton, UK.
“It is now important to carry out further studies to determine how the Epstein-Barr virus controls other genes that are associated with lymphoma. This will tell us more about how the virus drives lymphoma development and will help to identify new ways of targeting Epstein-Barr virus-infected cancer cells with specific drugs.” ![]()

Image by Ed Uthman
Results of research published in eLIFE appear to explain how Epstein-Barr virus (EBV) controls a pair of genes to drive lymphomagenesis.
Researchers set out to determine how EBV controls MYC, which is known to drive lymphoma development when activated, and BCL2L11, a gene that normally triggers apoptosis to prevent lymphoma but can be silenced by EBV.
The team discovered that EBV controls MYC and BCL2L11 by hijacking enhancer regions of DNA, which are situated far away from the genes.
These enhancers act as “control centers” and are able to contact and control genes from long distances by the looping out of the intervening stretches of DNA.
The researchers found that EBV activates MYC by increasing contacts between a specific set of enhancers and the gene.
The team said an Epstein-Barr nuclear antigen, EBNA2, activates multiple MYC enhancers and reconfigures the MYC locus to increase upstream enhancer-promoter interactions and decrease downstream interactions.
They noted that EBNA2 recruits the BRG1 ATPase of the SWI/SNF remodeller to MYC enhancers, and BRG1 is required for enhancer-promoter interactions in EBV-infected cells.
The researchers also discovered new enhancers that control BCL2L11. In this case, though, EBV stops these control centers from contacting the gene.
Specifically, the team found a hematopoietic enhancer hub that is inactivated by the Epstein-Barr nuclear antigens EBNA3A and EBNA3C through recruitment of the H3K27 methyltransferase EZH2.
Therefore, the researchers set out to determine if an EZH1/2 inhibitor, UNC1999, could reverse this effect. They found that UNC1999 did reverse enhancer inactivation, upregulated BCL2L11, and induced apoptosis in EBV-positive Burkitt lymphoma cells.
“This is a key step towards uncovering how this common virus, which affects thousands of people every year, causes blood cancer,” said study author Michelle West, PhD, of the University of Sussex in Brighton, UK.
“It is now important to carry out further studies to determine how the Epstein-Barr virus controls other genes that are associated with lymphoma. This will tell us more about how the virus drives lymphoma development and will help to identify new ways of targeting Epstein-Barr virus-infected cancer cells with specific drugs.” ![]()
November 2016 Digital Edition
Click here to access the November 2016 Digital Edition
Table of Contents
- Two Truths and One Hope Unite Us
- Taking a Team Approach to Treating Substance Use Disorders
- Who Overdoses at a VA Emergency Department?
- Implementation and Evaluation of an APRN-Led Opioid Monitoring Clinic
- The Overdose Education and Naloxone Distribution Program at a VA Hospital
- Kratom: A New Product in an Expanding Substance Abuse Market
- Posttraumatic Stress Disorder, Depression, and Other Comorbidities: Clinical and Systems Approaches to Diagnostic Uncertainties
- Abuse-Deterrent Opioids:What Practitioners Need to Know

Click here to access the November 2016 Digital Edition
Table of Contents
- Two Truths and One Hope Unite Us
- Taking a Team Approach to Treating Substance Use Disorders
- Who Overdoses at a VA Emergency Department?
- Implementation and Evaluation of an APRN-Led Opioid Monitoring Clinic
- The Overdose Education and Naloxone Distribution Program at a VA Hospital
- Kratom: A New Product in an Expanding Substance Abuse Market
- Posttraumatic Stress Disorder, Depression, and Other Comorbidities: Clinical and Systems Approaches to Diagnostic Uncertainties
- Abuse-Deterrent Opioids:What Practitioners Need to Know

Click here to access the November 2016 Digital Edition
Table of Contents
- Two Truths and One Hope Unite Us
- Taking a Team Approach to Treating Substance Use Disorders
- Who Overdoses at a VA Emergency Department?
- Implementation and Evaluation of an APRN-Led Opioid Monitoring Clinic
- The Overdose Education and Naloxone Distribution Program at a VA Hospital
- Kratom: A New Product in an Expanding Substance Abuse Market
- Posttraumatic Stress Disorder, Depression, and Other Comorbidities: Clinical and Systems Approaches to Diagnostic Uncertainties
- Abuse-Deterrent Opioids:What Practitioners Need to Know

Adolescent depression climbs, but is not matched by treatment
Major depressive episodes among adolescents are on the rise but there hasn’t been a corresponding rise in treatment levels, suggesting many teens are left untreated.
The 12-month prevalence of major depressive episodes (MDE) in adolescents aged 12-17 years rose to 11.3% in 2014, from 8.7% in 2005, according to data from the National Surveys on Drug Use and Health. This corresponded to a 37% increase in odds over the time period studied (odds ratio, 1.37; 95% confidence interval, 1.27-1.48; P less than .001).
For young adults aged 18-25 years, the change was more modest, from 8.8% in 2005 to 9.6% in 2014 (OR, 1.13; 95%, CI, 1.05-1.22; P = .001), the researchers noted.
The trend of rising depression rates was limited to those in the 12-20 year age range and was more prominent among non-Hispanic whites and adolescent girls.
The researchers found no link between the increasing trend in depression and factors typically associated with adverse mental health outcomes, such as substance abuse, single parent homes, or income.
Of particular concern was the finding that the proportion of adolescents with depression who received treatment or counseling did not significantly change over the time period studied. While the use of specialty mental health providers increased in adolescents and young adults, most of the increases were limited to the years after 2011.
“In view of the growing prevalence of MDE in these age groups, stable treatment rates translate into a growing number of untreated depressed adolescents,” the researchers wrote. “These trends suggest that little progress has been made in narrowing the mental health treatment gap for adolescent depression. This lack of progress may reflect lingering reluctance on the part of providers to diagnose and treat depression in the wake of the FDA’s black box warning regarding the use of antidepressants.”
The researchers reported having no relevant financial disclosures.
Depression is a sizable and growing deadly threat to our U.S. adolescent population. The prioritization of youth depression treatment of our U.S. population health is imperative. In fact, the American Academy of Pediatrics recently updated its 2007 statement on recognizing suicide risks with a recommendation to routinely screen youth aged 11-21 for depression.
Sadly, even if this important update influences primary care providers to screen more youth, there will never be enough qualified mental health specialists to take care of the million or more adolescents per year, who, if screened and identified, will need treatment and monitoring for depression. The most recently updated Accreditation Council for Graduate Medical Education program requirements for graduate medical education in Pediatrics and Child and Adolescent Psychiatry are such that trainees in neither specialty are clearly required to gain specific skills to tackle the plague of youth depression at a population level.
Is it not time for educational requirements that reflect the urgent needs of our pediatric patients?
Anne Glowinski, MD, and Giuseppe D’Amelio are from Washington University in St. Louis. Dr. Glowinski serves on the Advisory Board of the Klingenstein Third Generation Foundation and the Accreditation Council for Graduate Medical Education Psychiatry Residency Review Committee. Mr. D’Amelio reported having no relevant financial disclosures. Their comments are adapted from an accompanying editorial (Pediatrics. 2016 Nov 14. doi: 10.1542/peds.2016-2869 ).
Depression is a sizable and growing deadly threat to our U.S. adolescent population. The prioritization of youth depression treatment of our U.S. population health is imperative. In fact, the American Academy of Pediatrics recently updated its 2007 statement on recognizing suicide risks with a recommendation to routinely screen youth aged 11-21 for depression.
Sadly, even if this important update influences primary care providers to screen more youth, there will never be enough qualified mental health specialists to take care of the million or more adolescents per year, who, if screened and identified, will need treatment and monitoring for depression. The most recently updated Accreditation Council for Graduate Medical Education program requirements for graduate medical education in Pediatrics and Child and Adolescent Psychiatry are such that trainees in neither specialty are clearly required to gain specific skills to tackle the plague of youth depression at a population level.
Is it not time for educational requirements that reflect the urgent needs of our pediatric patients?
Anne Glowinski, MD, and Giuseppe D’Amelio are from Washington University in St. Louis. Dr. Glowinski serves on the Advisory Board of the Klingenstein Third Generation Foundation and the Accreditation Council for Graduate Medical Education Psychiatry Residency Review Committee. Mr. D’Amelio reported having no relevant financial disclosures. Their comments are adapted from an accompanying editorial (Pediatrics. 2016 Nov 14. doi: 10.1542/peds.2016-2869 ).
Depression is a sizable and growing deadly threat to our U.S. adolescent population. The prioritization of youth depression treatment of our U.S. population health is imperative. In fact, the American Academy of Pediatrics recently updated its 2007 statement on recognizing suicide risks with a recommendation to routinely screen youth aged 11-21 for depression.
Sadly, even if this important update influences primary care providers to screen more youth, there will never be enough qualified mental health specialists to take care of the million or more adolescents per year, who, if screened and identified, will need treatment and monitoring for depression. The most recently updated Accreditation Council for Graduate Medical Education program requirements for graduate medical education in Pediatrics and Child and Adolescent Psychiatry are such that trainees in neither specialty are clearly required to gain specific skills to tackle the plague of youth depression at a population level.
Is it not time for educational requirements that reflect the urgent needs of our pediatric patients?
Anne Glowinski, MD, and Giuseppe D’Amelio are from Washington University in St. Louis. Dr. Glowinski serves on the Advisory Board of the Klingenstein Third Generation Foundation and the Accreditation Council for Graduate Medical Education Psychiatry Residency Review Committee. Mr. D’Amelio reported having no relevant financial disclosures. Their comments are adapted from an accompanying editorial (Pediatrics. 2016 Nov 14. doi: 10.1542/peds.2016-2869 ).
Major depressive episodes among adolescents are on the rise but there hasn’t been a corresponding rise in treatment levels, suggesting many teens are left untreated.
The 12-month prevalence of major depressive episodes (MDE) in adolescents aged 12-17 years rose to 11.3% in 2014, from 8.7% in 2005, according to data from the National Surveys on Drug Use and Health. This corresponded to a 37% increase in odds over the time period studied (odds ratio, 1.37; 95% confidence interval, 1.27-1.48; P less than .001).
For young adults aged 18-25 years, the change was more modest, from 8.8% in 2005 to 9.6% in 2014 (OR, 1.13; 95%, CI, 1.05-1.22; P = .001), the researchers noted.
The trend of rising depression rates was limited to those in the 12-20 year age range and was more prominent among non-Hispanic whites and adolescent girls.
The researchers found no link between the increasing trend in depression and factors typically associated with adverse mental health outcomes, such as substance abuse, single parent homes, or income.
Of particular concern was the finding that the proportion of adolescents with depression who received treatment or counseling did not significantly change over the time period studied. While the use of specialty mental health providers increased in adolescents and young adults, most of the increases were limited to the years after 2011.
“In view of the growing prevalence of MDE in these age groups, stable treatment rates translate into a growing number of untreated depressed adolescents,” the researchers wrote. “These trends suggest that little progress has been made in narrowing the mental health treatment gap for adolescent depression. This lack of progress may reflect lingering reluctance on the part of providers to diagnose and treat depression in the wake of the FDA’s black box warning regarding the use of antidepressants.”
The researchers reported having no relevant financial disclosures.
Major depressive episodes among adolescents are on the rise but there hasn’t been a corresponding rise in treatment levels, suggesting many teens are left untreated.
The 12-month prevalence of major depressive episodes (MDE) in adolescents aged 12-17 years rose to 11.3% in 2014, from 8.7% in 2005, according to data from the National Surveys on Drug Use and Health. This corresponded to a 37% increase in odds over the time period studied (odds ratio, 1.37; 95% confidence interval, 1.27-1.48; P less than .001).
For young adults aged 18-25 years, the change was more modest, from 8.8% in 2005 to 9.6% in 2014 (OR, 1.13; 95%, CI, 1.05-1.22; P = .001), the researchers noted.
The trend of rising depression rates was limited to those in the 12-20 year age range and was more prominent among non-Hispanic whites and adolescent girls.
The researchers found no link between the increasing trend in depression and factors typically associated with adverse mental health outcomes, such as substance abuse, single parent homes, or income.
Of particular concern was the finding that the proportion of adolescents with depression who received treatment or counseling did not significantly change over the time period studied. While the use of specialty mental health providers increased in adolescents and young adults, most of the increases were limited to the years after 2011.
“In view of the growing prevalence of MDE in these age groups, stable treatment rates translate into a growing number of untreated depressed adolescents,” the researchers wrote. “These trends suggest that little progress has been made in narrowing the mental health treatment gap for adolescent depression. This lack of progress may reflect lingering reluctance on the part of providers to diagnose and treat depression in the wake of the FDA’s black box warning regarding the use of antidepressants.”
The researchers reported having no relevant financial disclosures.
FROM PEDIATRICS
Key clinical finding: Adolescent depression is on the rise, but treatment rates have stayed the same indicating undertreatment in this population.
Main finding: The 12-month prevalence of major depressive episodes in adolescents (aged 12-17 years) was 11.3% in 2014, compared with 8.7% in 2005.
Source: Analysis of data from the National Surveys on Drug Use and Health from 2005 to 2014 involving 172,495 adolescents aged 12-17 years and 178,755 adults aged 18-25 years.
Disclosures: The researchers reported having no relevant financial disclosures.
Give a Hand
1. A 45-year-old construction worker accidentally fell 20 ft from a scaffolding. He is unable to flex or extend his right wrist due to pain. Examination of the wrist shows a moderate amount of soft-tissue swelling with moderate tenderness along the base of the first metacarpal.
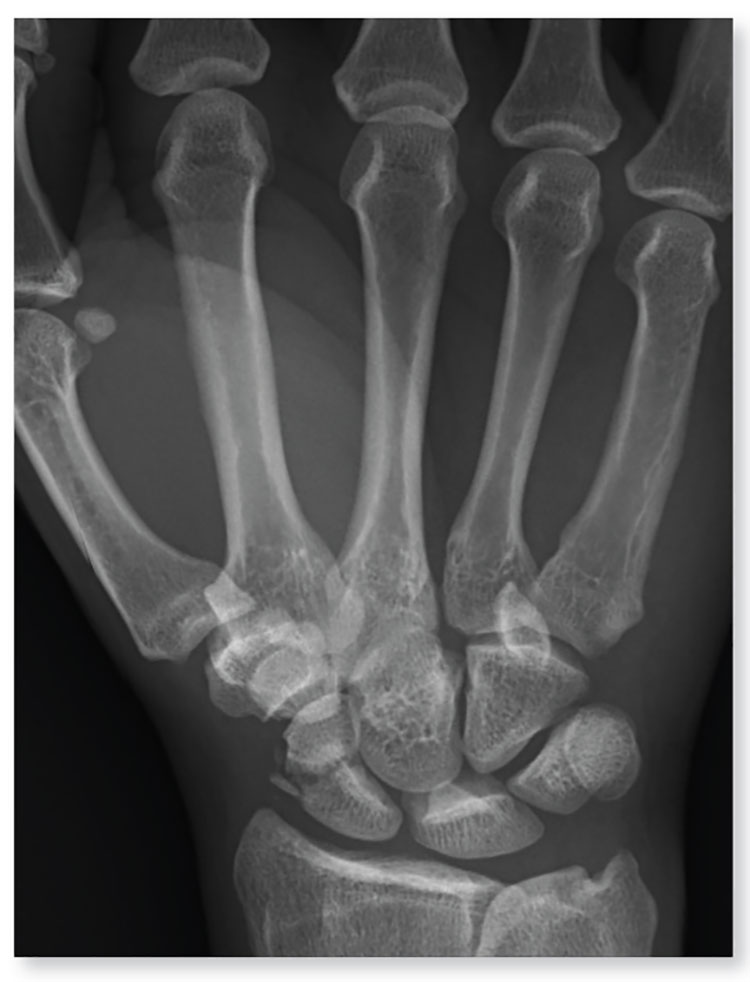
Diagnosis: The radiograph shows an acute, comminuted fracture of the scaphoid bone. The patient was placed in a thumb spica splint and sling. He was instructed to follow up in one to two days with the hand surgeon who was on call, with anticipation of subsequent open reduction and internal fixation.
For more information, see “Construction Worker Falls From Scaffolding.” Clinician Reviews. 2013;23(11):15.
2. A 90-year-old man “just passed out” in his yard, landing in an ant nest. He experiences bilateral wrist pain, presumably from multiple ant bites. Both wrists are tender; range of motion causes tenderness. Inspection demonstrates mild to moderate circumferential swelling with several raised, reddened bumps.
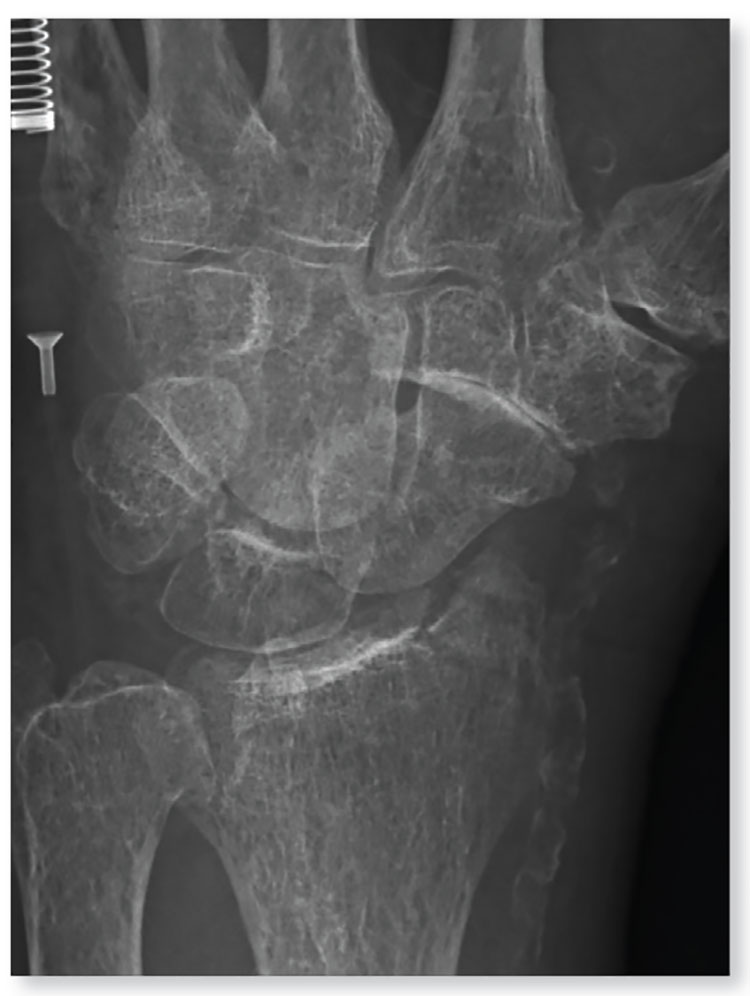
Diagnosis: The radiograph shows some osteopenia and significant vascular calcifications. Of note, there is a fracture of the styloid process of the radius, extending slightly to the joint space. The patient was placed in a splint and orthopedic referral was obtained.
Wrist Pain After a Fall. Clinician Reviews. 2012;22(9):22.
3. The middorsal aspect of a 48-year-old woman’s right hand was accidentally caught in a metal door as it was being shut. Examination shows mild to moderate soft tissue swelling and some early bruising. There is extreme tenderness over the fourth and fifth metacarpal bones. Although limited by swelling, she can flex her fingers somewhat.

Diagnosis: The radiograph shows a comminuted fracture of the proximal fifth phalanx. Soft tissue swelling is noted as well. The patient’s hand was splinted, and arrangements for outpatient orthopedic follow-up were made.
For more information, see “Hand Slammed in Door.” Clinician Reviews. 2013 May;23(5):20.
4. A trauma patient arrives in your facility after a motor vehicle collision. His right hand and wrist appear to be moderately swollen, and he has been placed in a splint.
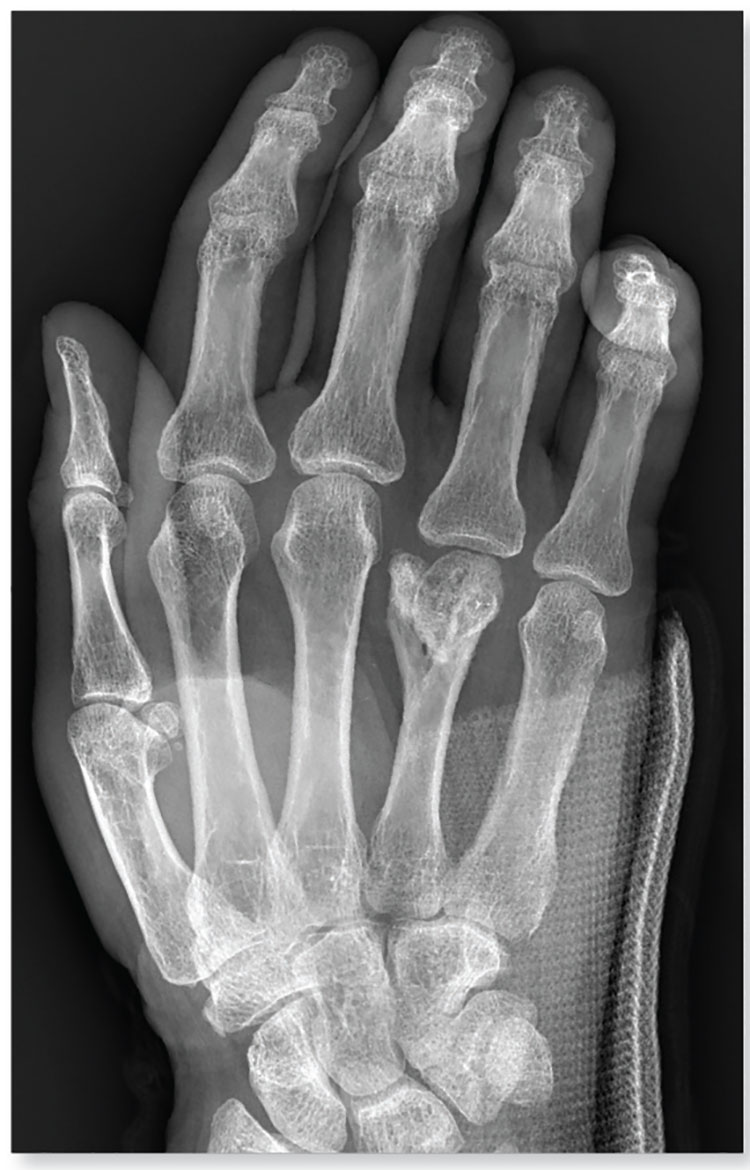
Diagnosis: The radiograph shows a slightly displaced fracture of the distal fourth metacarpal head. No other injuries are present.
The patient’s hand was left in the splint, and orthopedic evaluation was obtained.
For more information, see “Secondary Survey of Trauma Patient.” Clinician Reviews. 2015;25(12):10,35.
1. A 45-year-old construction worker accidentally fell 20 ft from a scaffolding. He is unable to flex or extend his right wrist due to pain. Examination of the wrist shows a moderate amount of soft-tissue swelling with moderate tenderness along the base of the first metacarpal.

Diagnosis: The radiograph shows an acute, comminuted fracture of the scaphoid bone. The patient was placed in a thumb spica splint and sling. He was instructed to follow up in one to two days with the hand surgeon who was on call, with anticipation of subsequent open reduction and internal fixation.
For more information, see “Construction Worker Falls From Scaffolding.” Clinician Reviews. 2013;23(11):15.
2. A 90-year-old man “just passed out” in his yard, landing in an ant nest. He experiences bilateral wrist pain, presumably from multiple ant bites. Both wrists are tender; range of motion causes tenderness. Inspection demonstrates mild to moderate circumferential swelling with several raised, reddened bumps.

Diagnosis: The radiograph shows some osteopenia and significant vascular calcifications. Of note, there is a fracture of the styloid process of the radius, extending slightly to the joint space. The patient was placed in a splint and orthopedic referral was obtained.
Wrist Pain After a Fall. Clinician Reviews. 2012;22(9):22.
3. The middorsal aspect of a 48-year-old woman’s right hand was accidentally caught in a metal door as it was being shut. Examination shows mild to moderate soft tissue swelling and some early bruising. There is extreme tenderness over the fourth and fifth metacarpal bones. Although limited by swelling, she can flex her fingers somewhat.

Diagnosis: The radiograph shows a comminuted fracture of the proximal fifth phalanx. Soft tissue swelling is noted as well. The patient’s hand was splinted, and arrangements for outpatient orthopedic follow-up were made.
For more information, see “Hand Slammed in Door.” Clinician Reviews. 2013 May;23(5):20.
4. A trauma patient arrives in your facility after a motor vehicle collision. His right hand and wrist appear to be moderately swollen, and he has been placed in a splint.

Diagnosis: The radiograph shows a slightly displaced fracture of the distal fourth metacarpal head. No other injuries are present.
The patient’s hand was left in the splint, and orthopedic evaluation was obtained.
For more information, see “Secondary Survey of Trauma Patient.” Clinician Reviews. 2015;25(12):10,35.
1. A 45-year-old construction worker accidentally fell 20 ft from a scaffolding. He is unable to flex or extend his right wrist due to pain. Examination of the wrist shows a moderate amount of soft-tissue swelling with moderate tenderness along the base of the first metacarpal.

Diagnosis: The radiograph shows an acute, comminuted fracture of the scaphoid bone. The patient was placed in a thumb spica splint and sling. He was instructed to follow up in one to two days with the hand surgeon who was on call, with anticipation of subsequent open reduction and internal fixation.
For more information, see “Construction Worker Falls From Scaffolding.” Clinician Reviews. 2013;23(11):15.
2. A 90-year-old man “just passed out” in his yard, landing in an ant nest. He experiences bilateral wrist pain, presumably from multiple ant bites. Both wrists are tender; range of motion causes tenderness. Inspection demonstrates mild to moderate circumferential swelling with several raised, reddened bumps.

Diagnosis: The radiograph shows some osteopenia and significant vascular calcifications. Of note, there is a fracture of the styloid process of the radius, extending slightly to the joint space. The patient was placed in a splint and orthopedic referral was obtained.
Wrist Pain After a Fall. Clinician Reviews. 2012;22(9):22.
3. The middorsal aspect of a 48-year-old woman’s right hand was accidentally caught in a metal door as it was being shut. Examination shows mild to moderate soft tissue swelling and some early bruising. There is extreme tenderness over the fourth and fifth metacarpal bones. Although limited by swelling, she can flex her fingers somewhat.

Diagnosis: The radiograph shows a comminuted fracture of the proximal fifth phalanx. Soft tissue swelling is noted as well. The patient’s hand was splinted, and arrangements for outpatient orthopedic follow-up were made.
For more information, see “Hand Slammed in Door.” Clinician Reviews. 2013 May;23(5):20.
4. A trauma patient arrives in your facility after a motor vehicle collision. His right hand and wrist appear to be moderately swollen, and he has been placed in a splint.

Diagnosis: The radiograph shows a slightly displaced fracture of the distal fourth metacarpal head. No other injuries are present.
The patient’s hand was left in the splint, and orthopedic evaluation was obtained.
For more information, see “Secondary Survey of Trauma Patient.” Clinician Reviews. 2015;25(12):10,35.
Pencils and paper ready!
No, this isn’t a test, this is an admonishment. For years, I have been using these letters to vent my frustration with the federal government and practice administrators who have foisted several generations of user-unfriendly electronic health records on us. Maybe it’s time to accept the ugly fact that, for the near future, clunky and time-gobbling EHRs are the reality, and we need to think of strategies to make the best of a bad situation.
It’s not only physicians who are complaining about EHRs. Listen to your friends and relatives at cookouts and in the line at the grocery story. You’ve heard what they are saying about us. “He always has his eyes on the computer screen. Never looks at me, and I’m not sure he’s listening.” “She asks me the same questions the nurse and that other woman already asked me. Hasn’t she already looked at my chart?” If you haven’t heard those complaints, make an appointment to see a doctor and experience the distortion of the doctor-patient interaction that the computer has created.
It might take some reordering of how you do things. Take a look at the patient’s chart before you go in to see the patient. Many of you may do this already. It’s the courteous thing to do. In the few cases you don’t think you can trust your memory on the trip between your office computer and the exam room, scribble a few notes on a scrap of paper.
Ask the patient to repeat his chief complaint; it may have a completely different ring to it than the one the nurse/receptionist entered in the computer. Apologize to the patient for asking the history again. Or even better, why not be the first and only person to take the history? Scribble a few more notes and a few more after the physical exam if necessary.
At the end of the visit, return to your office to order any lab work and prescriptions the visit required. Take a few minutes to look at the next patient’s medical record and then repeat, repeat. I have found that, in a general pediatric practice, when I was busy, I could batch three, rarely four, patients together before returning to my desk for a more lengthy sit down to finalize the charts, sometimes using my few scribbled notes to jog my memory.
I am confident that most of you are capable of the same mental gymnastics. You’ve passed the MCAT, graduated from medical school, passed the state board, and probably your specialty boards. You should be the master of retention. If a skilled wait person at a good restaurant can keep four patrons’ orders in his/her head, you should be able to retain the basic clinical information on a couple of patients with the help of a pencil and paper. The reward for your mental effort will be dramatically improved doctor-patient interaction. The patients will be impressed that you are looking at and listening to them, and not a computer screen. You will get more and better information from them, and this will make for more accurate diagnoses and better targeted therapies.
If you can’t imagine this working because your office system demands that a diagnosis and billing code be entered before that patient checks out, it may be time to demand a scribe.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.” Email him at [email protected] .
No, this isn’t a test, this is an admonishment. For years, I have been using these letters to vent my frustration with the federal government and practice administrators who have foisted several generations of user-unfriendly electronic health records on us. Maybe it’s time to accept the ugly fact that, for the near future, clunky and time-gobbling EHRs are the reality, and we need to think of strategies to make the best of a bad situation.
It’s not only physicians who are complaining about EHRs. Listen to your friends and relatives at cookouts and in the line at the grocery story. You’ve heard what they are saying about us. “He always has his eyes on the computer screen. Never looks at me, and I’m not sure he’s listening.” “She asks me the same questions the nurse and that other woman already asked me. Hasn’t she already looked at my chart?” If you haven’t heard those complaints, make an appointment to see a doctor and experience the distortion of the doctor-patient interaction that the computer has created.
It might take some reordering of how you do things. Take a look at the patient’s chart before you go in to see the patient. Many of you may do this already. It’s the courteous thing to do. In the few cases you don’t think you can trust your memory on the trip between your office computer and the exam room, scribble a few notes on a scrap of paper.
Ask the patient to repeat his chief complaint; it may have a completely different ring to it than the one the nurse/receptionist entered in the computer. Apologize to the patient for asking the history again. Or even better, why not be the first and only person to take the history? Scribble a few more notes and a few more after the physical exam if necessary.
At the end of the visit, return to your office to order any lab work and prescriptions the visit required. Take a few minutes to look at the next patient’s medical record and then repeat, repeat. I have found that, in a general pediatric practice, when I was busy, I could batch three, rarely four, patients together before returning to my desk for a more lengthy sit down to finalize the charts, sometimes using my few scribbled notes to jog my memory.
I am confident that most of you are capable of the same mental gymnastics. You’ve passed the MCAT, graduated from medical school, passed the state board, and probably your specialty boards. You should be the master of retention. If a skilled wait person at a good restaurant can keep four patrons’ orders in his/her head, you should be able to retain the basic clinical information on a couple of patients with the help of a pencil and paper. The reward for your mental effort will be dramatically improved doctor-patient interaction. The patients will be impressed that you are looking at and listening to them, and not a computer screen. You will get more and better information from them, and this will make for more accurate diagnoses and better targeted therapies.
If you can’t imagine this working because your office system demands that a diagnosis and billing code be entered before that patient checks out, it may be time to demand a scribe.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.” Email him at [email protected] .
No, this isn’t a test, this is an admonishment. For years, I have been using these letters to vent my frustration with the federal government and practice administrators who have foisted several generations of user-unfriendly electronic health records on us. Maybe it’s time to accept the ugly fact that, for the near future, clunky and time-gobbling EHRs are the reality, and we need to think of strategies to make the best of a bad situation.
It’s not only physicians who are complaining about EHRs. Listen to your friends and relatives at cookouts and in the line at the grocery story. You’ve heard what they are saying about us. “He always has his eyes on the computer screen. Never looks at me, and I’m not sure he’s listening.” “She asks me the same questions the nurse and that other woman already asked me. Hasn’t she already looked at my chart?” If you haven’t heard those complaints, make an appointment to see a doctor and experience the distortion of the doctor-patient interaction that the computer has created.
It might take some reordering of how you do things. Take a look at the patient’s chart before you go in to see the patient. Many of you may do this already. It’s the courteous thing to do. In the few cases you don’t think you can trust your memory on the trip between your office computer and the exam room, scribble a few notes on a scrap of paper.
Ask the patient to repeat his chief complaint; it may have a completely different ring to it than the one the nurse/receptionist entered in the computer. Apologize to the patient for asking the history again. Or even better, why not be the first and only person to take the history? Scribble a few more notes and a few more after the physical exam if necessary.
At the end of the visit, return to your office to order any lab work and prescriptions the visit required. Take a few minutes to look at the next patient’s medical record and then repeat, repeat. I have found that, in a general pediatric practice, when I was busy, I could batch three, rarely four, patients together before returning to my desk for a more lengthy sit down to finalize the charts, sometimes using my few scribbled notes to jog my memory.
I am confident that most of you are capable of the same mental gymnastics. You’ve passed the MCAT, graduated from medical school, passed the state board, and probably your specialty boards. You should be the master of retention. If a skilled wait person at a good restaurant can keep four patrons’ orders in his/her head, you should be able to retain the basic clinical information on a couple of patients with the help of a pencil and paper. The reward for your mental effort will be dramatically improved doctor-patient interaction. The patients will be impressed that you are looking at and listening to them, and not a computer screen. You will get more and better information from them, and this will make for more accurate diagnoses and better targeted therapies.
If you can’t imagine this working because your office system demands that a diagnosis and billing code be entered before that patient checks out, it may be time to demand a scribe.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.” Email him at [email protected] .
VIDEO: Allopurinol may not raise kidney disease risk in gout
WASHINGTON – Urate-lowering therapy (ULT) with allopurinol does not appear to increase the risk of chronic kidney disease in patients with gout who have normal or near-normal kidney function at diagnosis, according to a large retrospective study presented at the annual meeting of the American College of Rheumatology.
The study was based on electronic health records from The Health Improvement Network (THIN), a database that includes patients treated by general practitioners in the United Kingdom.
“It is sad in my practice to see how many gout patients are not treated with ULT because patients fear the side effects of medication or just don’t want to be treated, especially when they are not in flare. Many general practitioners also don’t view gout as a serious condition requiring medication,” said lead author Ana Beatriz Vargas-Santos, PhD, a research fellow at Boston University and a rheumatologist at the State University of Rio de Janeiro in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The study enrolled 13,608 patients with newly diagnosed gout and normal kidney function who started ULT)with allopurinol and compared them with 13,608 gout patients in the THIN database who did not start ULT.
At a mean follow-up of 4 years, there was no increased risk of developing chronic kidney disease (CKD) stage 3 or higher in the allopurinol users: 1,401 of the allopurinol initiators versus 1,319 of nonusers developed CKD stage 3 or higher.
“Our study shows that there was no risk of harm to the kidney with allopurinol. This suggests that if a patient on gout presents with declining kidney function, it is better to look for other causes and keep the patient on allopurinol to lower serum urate. Accumulating evidence is in the same direction. Doctors have to be less fearful of prescribing allopurinol. Gout patients deserve better,” Dr. Vargas-Santos stated.
Dr. Vargas-Santos had no financial disclosures.
WASHINGTON – Urate-lowering therapy (ULT) with allopurinol does not appear to increase the risk of chronic kidney disease in patients with gout who have normal or near-normal kidney function at diagnosis, according to a large retrospective study presented at the annual meeting of the American College of Rheumatology.
The study was based on electronic health records from The Health Improvement Network (THIN), a database that includes patients treated by general practitioners in the United Kingdom.
“It is sad in my practice to see how many gout patients are not treated with ULT because patients fear the side effects of medication or just don’t want to be treated, especially when they are not in flare. Many general practitioners also don’t view gout as a serious condition requiring medication,” said lead author Ana Beatriz Vargas-Santos, PhD, a research fellow at Boston University and a rheumatologist at the State University of Rio de Janeiro in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The study enrolled 13,608 patients with newly diagnosed gout and normal kidney function who started ULT)with allopurinol and compared them with 13,608 gout patients in the THIN database who did not start ULT.
At a mean follow-up of 4 years, there was no increased risk of developing chronic kidney disease (CKD) stage 3 or higher in the allopurinol users: 1,401 of the allopurinol initiators versus 1,319 of nonusers developed CKD stage 3 or higher.
“Our study shows that there was no risk of harm to the kidney with allopurinol. This suggests that if a patient on gout presents with declining kidney function, it is better to look for other causes and keep the patient on allopurinol to lower serum urate. Accumulating evidence is in the same direction. Doctors have to be less fearful of prescribing allopurinol. Gout patients deserve better,” Dr. Vargas-Santos stated.
Dr. Vargas-Santos had no financial disclosures.
WASHINGTON – Urate-lowering therapy (ULT) with allopurinol does not appear to increase the risk of chronic kidney disease in patients with gout who have normal or near-normal kidney function at diagnosis, according to a large retrospective study presented at the annual meeting of the American College of Rheumatology.
The study was based on electronic health records from The Health Improvement Network (THIN), a database that includes patients treated by general practitioners in the United Kingdom.
“It is sad in my practice to see how many gout patients are not treated with ULT because patients fear the side effects of medication or just don’t want to be treated, especially when they are not in flare. Many general practitioners also don’t view gout as a serious condition requiring medication,” said lead author Ana Beatriz Vargas-Santos, PhD, a research fellow at Boston University and a rheumatologist at the State University of Rio de Janeiro in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The study enrolled 13,608 patients with newly diagnosed gout and normal kidney function who started ULT)with allopurinol and compared them with 13,608 gout patients in the THIN database who did not start ULT.
At a mean follow-up of 4 years, there was no increased risk of developing chronic kidney disease (CKD) stage 3 or higher in the allopurinol users: 1,401 of the allopurinol initiators versus 1,319 of nonusers developed CKD stage 3 or higher.
“Our study shows that there was no risk of harm to the kidney with allopurinol. This suggests that if a patient on gout presents with declining kidney function, it is better to look for other causes and keep the patient on allopurinol to lower serum urate. Accumulating evidence is in the same direction. Doctors have to be less fearful of prescribing allopurinol. Gout patients deserve better,” Dr. Vargas-Santos stated.
Dr. Vargas-Santos had no financial disclosures.
AT THE ACR ANNUAL MEETING
Inhaled laninamivir reduces risk of influenza in young children
The inhaled neuraminidase inhibitor laninamivir has been shown to significantly reduce the likelihood of developing influenza among children exposed to a family member with the infection, according to a study recently published in Pediatrics.
In a double-blind, placebo-controlled study, researchers randomized 343 children under 10 years old – who had an influenza-infected family member – to a single 20-mg dose of inhaled laninamivir octanoate or placebo.
Subgroup analyses suggested the treatment was more effective in children under 7 years old, with a relative risk reduction of 64%, compared with a non–statistically significant 28% reduction in those aged 7-10 years (Pediatrics. 2016 Nov 2. doi: 10.1542/peds.2016-0109).
The treatment was also effective among children where the index case was infected with influenza A (H3N2).
Dr. Takashi Nakano, from Kawasaki Hospital in Okayama, Japan, and coauthors reported a similar incidence of adverse events in the laninamivir and placebo groups, with no serious adverse events and no withdrawals due to adverse events. However, the authors noted that there were very few study participants considered at high risk, such as patients with chronic respiratory disease, and suggested further studies of the impact and efficacy of treatment in high-risk groups.
The researchers noted that, despite increasing rates of influenza vaccination and the availability of other neuraminidase inhibitors, such as oseltamivir and peramivir, pandemic outbreaks of influenza are still occurring. There has also been evidence of resistance to both oseltamivir and peramivir, for example, in the 2013/2014 outbreak of influenza A (H1N1) in Japan. “Given the limitations of vaccination, extensive variations in the option for antiinfluenza prophylaxis are desirable as an adjunct to influenza vaccine,” the researchers wrote.
Laninamivir has been studied in adults and children and shown to be effective at treating influenza infection, but its efficacy as prophylaxis in children under 10 years old had not previously been studied.
“Since a single 20-mg dose of laninamivir octanoate revealed prophylactic effect, the regimen in the current study is a highly user-friendly option,” the researchers wrote. “Although the numbers of infected individuals may differ by season, the number needed to treat based on the incidence of clinical influenza for the two groups in the current study was 11.”
The study was funded by Daiichi Sankyo. Two of the study authors reported being consultants for Daiichi Sankyo, as well as having financial relationships with other pharmaceutical companies. The other study authors are employees of Daiichi Sankyo.
Although vaccination remains the preferred approach for influenza prevention, additional options for influenza prophylaxis in children are important, given concerns for the emergence of resistance, the known antiviral adverse side effect profiles, possible limited supplies, and the potential for spotty patient compliance. This drug was well tolerated, without significant adverse events reported, and there were no neurologic symptoms or abnormal behavior, which have occurred with influenza illness and with other neuraminidase inhibitors in Japan.
Prompt initiation of influenza prophylaxis is necessary to ensure efficacy, which hinges on proper and prompt identification of index cases. Therefore, efforts to educate parents and families on the early signs and symptoms of influenza and the importance of seeking medical attention to confirm the diagnosis in the index case are crucial for timely initiation of prophylaxis in household contacts.
Flor M. Munoz, MD, is from the department of pediatrics at the Baylor College of Medicine and Texas Children’s Hospital in Houston, and Henry H. Bernstein, DO, is from the department of pediatrics, Hofstra Northwell School of Medicine, Hempstead, N.Y., and Cohen Children’s Medical Center of New York in New Hyde Park. These comments are adapted from an accompanying editorial (Pediatrics. 2016 Nov 2. doi: 10.1542/peds.2016-2371). The authors reported having no relevant financial disclosures.
Although vaccination remains the preferred approach for influenza prevention, additional options for influenza prophylaxis in children are important, given concerns for the emergence of resistance, the known antiviral adverse side effect profiles, possible limited supplies, and the potential for spotty patient compliance. This drug was well tolerated, without significant adverse events reported, and there were no neurologic symptoms or abnormal behavior, which have occurred with influenza illness and with other neuraminidase inhibitors in Japan.
Prompt initiation of influenza prophylaxis is necessary to ensure efficacy, which hinges on proper and prompt identification of index cases. Therefore, efforts to educate parents and families on the early signs and symptoms of influenza and the importance of seeking medical attention to confirm the diagnosis in the index case are crucial for timely initiation of prophylaxis in household contacts.
Flor M. Munoz, MD, is from the department of pediatrics at the Baylor College of Medicine and Texas Children’s Hospital in Houston, and Henry H. Bernstein, DO, is from the department of pediatrics, Hofstra Northwell School of Medicine, Hempstead, N.Y., and Cohen Children’s Medical Center of New York in New Hyde Park. These comments are adapted from an accompanying editorial (Pediatrics. 2016 Nov 2. doi: 10.1542/peds.2016-2371). The authors reported having no relevant financial disclosures.
Although vaccination remains the preferred approach for influenza prevention, additional options for influenza prophylaxis in children are important, given concerns for the emergence of resistance, the known antiviral adverse side effect profiles, possible limited supplies, and the potential for spotty patient compliance. This drug was well tolerated, without significant adverse events reported, and there were no neurologic symptoms or abnormal behavior, which have occurred with influenza illness and with other neuraminidase inhibitors in Japan.
Prompt initiation of influenza prophylaxis is necessary to ensure efficacy, which hinges on proper and prompt identification of index cases. Therefore, efforts to educate parents and families on the early signs and symptoms of influenza and the importance of seeking medical attention to confirm the diagnosis in the index case are crucial for timely initiation of prophylaxis in household contacts.
Flor M. Munoz, MD, is from the department of pediatrics at the Baylor College of Medicine and Texas Children’s Hospital in Houston, and Henry H. Bernstein, DO, is from the department of pediatrics, Hofstra Northwell School of Medicine, Hempstead, N.Y., and Cohen Children’s Medical Center of New York in New Hyde Park. These comments are adapted from an accompanying editorial (Pediatrics. 2016 Nov 2. doi: 10.1542/peds.2016-2371). The authors reported having no relevant financial disclosures.
The inhaled neuraminidase inhibitor laninamivir has been shown to significantly reduce the likelihood of developing influenza among children exposed to a family member with the infection, according to a study recently published in Pediatrics.
In a double-blind, placebo-controlled study, researchers randomized 343 children under 10 years old – who had an influenza-infected family member – to a single 20-mg dose of inhaled laninamivir octanoate or placebo.
Subgroup analyses suggested the treatment was more effective in children under 7 years old, with a relative risk reduction of 64%, compared with a non–statistically significant 28% reduction in those aged 7-10 years (Pediatrics. 2016 Nov 2. doi: 10.1542/peds.2016-0109).
The treatment was also effective among children where the index case was infected with influenza A (H3N2).
Dr. Takashi Nakano, from Kawasaki Hospital in Okayama, Japan, and coauthors reported a similar incidence of adverse events in the laninamivir and placebo groups, with no serious adverse events and no withdrawals due to adverse events. However, the authors noted that there were very few study participants considered at high risk, such as patients with chronic respiratory disease, and suggested further studies of the impact and efficacy of treatment in high-risk groups.
The researchers noted that, despite increasing rates of influenza vaccination and the availability of other neuraminidase inhibitors, such as oseltamivir and peramivir, pandemic outbreaks of influenza are still occurring. There has also been evidence of resistance to both oseltamivir and peramivir, for example, in the 2013/2014 outbreak of influenza A (H1N1) in Japan. “Given the limitations of vaccination, extensive variations in the option for antiinfluenza prophylaxis are desirable as an adjunct to influenza vaccine,” the researchers wrote.
Laninamivir has been studied in adults and children and shown to be effective at treating influenza infection, but its efficacy as prophylaxis in children under 10 years old had not previously been studied.
“Since a single 20-mg dose of laninamivir octanoate revealed prophylactic effect, the regimen in the current study is a highly user-friendly option,” the researchers wrote. “Although the numbers of infected individuals may differ by season, the number needed to treat based on the incidence of clinical influenza for the two groups in the current study was 11.”
The study was funded by Daiichi Sankyo. Two of the study authors reported being consultants for Daiichi Sankyo, as well as having financial relationships with other pharmaceutical companies. The other study authors are employees of Daiichi Sankyo.
The inhaled neuraminidase inhibitor laninamivir has been shown to significantly reduce the likelihood of developing influenza among children exposed to a family member with the infection, according to a study recently published in Pediatrics.
In a double-blind, placebo-controlled study, researchers randomized 343 children under 10 years old – who had an influenza-infected family member – to a single 20-mg dose of inhaled laninamivir octanoate or placebo.
Subgroup analyses suggested the treatment was more effective in children under 7 years old, with a relative risk reduction of 64%, compared with a non–statistically significant 28% reduction in those aged 7-10 years (Pediatrics. 2016 Nov 2. doi: 10.1542/peds.2016-0109).
The treatment was also effective among children where the index case was infected with influenza A (H3N2).
Dr. Takashi Nakano, from Kawasaki Hospital in Okayama, Japan, and coauthors reported a similar incidence of adverse events in the laninamivir and placebo groups, with no serious adverse events and no withdrawals due to adverse events. However, the authors noted that there were very few study participants considered at high risk, such as patients with chronic respiratory disease, and suggested further studies of the impact and efficacy of treatment in high-risk groups.
The researchers noted that, despite increasing rates of influenza vaccination and the availability of other neuraminidase inhibitors, such as oseltamivir and peramivir, pandemic outbreaks of influenza are still occurring. There has also been evidence of resistance to both oseltamivir and peramivir, for example, in the 2013/2014 outbreak of influenza A (H1N1) in Japan. “Given the limitations of vaccination, extensive variations in the option for antiinfluenza prophylaxis are desirable as an adjunct to influenza vaccine,” the researchers wrote.
Laninamivir has been studied in adults and children and shown to be effective at treating influenza infection, but its efficacy as prophylaxis in children under 10 years old had not previously been studied.
“Since a single 20-mg dose of laninamivir octanoate revealed prophylactic effect, the regimen in the current study is a highly user-friendly option,” the researchers wrote. “Although the numbers of infected individuals may differ by season, the number needed to treat based on the incidence of clinical influenza for the two groups in the current study was 11.”
The study was funded by Daiichi Sankyo. Two of the study authors reported being consultants for Daiichi Sankyo, as well as having financial relationships with other pharmaceutical companies. The other study authors are employees of Daiichi Sankyo.
FROM PEDIATRICS
Key clinical point:
Major finding: Children treated with laninamivir showed a 45.8% reduction in the risk of influenza, compared with the placebo group.
Data source: Randomized, double-blind, placebo-controlled trial in 343 children under 10 years old.
Disclosures: The study was funded by Daiichi Sankyo. Two of the study authors reported being consultants for Daiichi Sankyo, as well as having financial relationships with other pharmaceutical companies. The other study authors are employees of Daiichi Sankyo.
Ticagrelor not superior to clopidogrel in peripheral artery disease
Ticagrelor was found to be “not superior” to clopidogrel at preventing cardiovascular events in the largest clinical trial to date involving patients with symptomatic peripheral artery disease (PAD), Manesh Patel, MD, reported at the American Heart Association scientific sessions.*
Ticagrelor also was no better than clopidogrel at preventing acute limb ischemia in this study of 13,885 patients.
Regarding safety issues, the two drugs had identical rates of major bleeding adverse events, but ticagrelor was discontinued significantly more often than clopidogrel was, because of its other well-known adverse effects, said Dr. Patel of Duke University, Durham, N.C.
The findings of the EUCLID (Examining the Use of Ticagrelor in PAD) study were presented at the meeting and simultaneously published online Nov. 13 in the New England Journal of Medicine (2016. doi: 10.1056/NEJMoa1611688).
In addition, the results of a substudy of the EUCLID trial involving the 7,875 participants who had previously undergone lower-limb revascularization were reported at the meeting and simultaneously published online in Circulation (2016 Nov 13; doi: 10.1161/CIRCULATIONAHA.116.025880).
Until now, there have been no large studies comparing antiplatelet therapies in patients with PAD. Clopidogrel is considered superior to aspirin in this patient population based on limited evidence, often extrapolated from studies of acute coronary syndromes or coronary artery disease. Ticagrelor also proved beneficial in these contexts, so researchers performed the EUCLID study, comparing the two medications head to head in patients with PAD.
The manufacturer-funded double-blind trial was conducted at 811 medical centers in eight countries. Patients aged 50 years or older (median age, 66 years) were randomly assigned to receive either oral ticagrelor, 90 mg twice daily (6,930 patients), or oral clopidogrel, 75 mg once daily (6,955 patients), and were followed for a median of 30 months.
The primary efficacy endpoint – the first occurrence of any event in the composite of cardiovascular death, myocardial infarction, or ischemic stroke – occurred in 10.8% of the ticagrelor group and 10.6% of the clopidogrel group, for a hazard ratio of 1.02.
When the components of this composite endpoint were considered individually, only the rate of ischemic stroke was significantly different between the two study groups, occurring in 1.9% of patients taking ticagrelor and 2.4% of those taking clopidogrel (HR, 0.78).
Other important secondary and composite efficacy endpoints, including acute limb ischemia and revascularization, were similar between the two study groups, Dr. Patel said.
The primary safety endpoint – the rate of major bleeding events – occurred in the same percentage of both study groups (1.6%), and individual rates of fatal bleeding, intracranial bleeding, and minor bleeding were similar.
“There were numerically fewer fatal bleeding events in the ticagrelor group than in the clopidogrel group (10 vs. 20), but there were significantly more bleeding events leading to drug discontinuation with ticagrelor than with clopidogrel (168 vs. 112),” he noted.
Ticagrelor was discontinued more often than clopidogrel during the study (30.1% of patients vs. 25.9%; HR, 1.21). Discontinuation was driven mainly by the occurrence of dyspnea (4.8% vs. 0.8%) and minor bleeding, both of which are well-described adverse effects of ticagrelor, Dr. Patel said.
“Our findings show the hazards of extrapolating evidence from patients with coronary artery disease to those with peripheral artery disease,” he added.
In a separate report in Circulation, the results were similar in the substudy of EUCLID participants who had already undergone lower-extremity revascularization procedures before enrollment, reported W. Schuyler Jones, MD, also of of Duke University.
The primary efficacy endpoint occurred in 11.4% of the ticagrelor group and 11.3% of the clopidogrel group, a nonsignificant difference (HR, 1.01). “Other key secondary and composite endpoints, including repeat revascularization, also were not different between the two study groups,” Dr. Jones said.
Regarding safety endpoints, the rates of major bleeding, fatal bleeding, intracranial bleeding, and minor bleeding all were similar between the two study groups.
“These findings suggest that patients with prior revascularization have a substantial residual rate of cardiovascular and acute limb events, despite high adherence to antiplatelet and statin medications, and require further study,” Dr. Jones noted.
*Correction 11/14/16: An earlier version of this article misstated the name of the investigator who presented the study at the meeting.
Ticagrelor was found to be “not superior” to clopidogrel at preventing cardiovascular events in the largest clinical trial to date involving patients with symptomatic peripheral artery disease (PAD), Manesh Patel, MD, reported at the American Heart Association scientific sessions.*
Ticagrelor also was no better than clopidogrel at preventing acute limb ischemia in this study of 13,885 patients.
Regarding safety issues, the two drugs had identical rates of major bleeding adverse events, but ticagrelor was discontinued significantly more often than clopidogrel was, because of its other well-known adverse effects, said Dr. Patel of Duke University, Durham, N.C.
The findings of the EUCLID (Examining the Use of Ticagrelor in PAD) study were presented at the meeting and simultaneously published online Nov. 13 in the New England Journal of Medicine (2016. doi: 10.1056/NEJMoa1611688).
In addition, the results of a substudy of the EUCLID trial involving the 7,875 participants who had previously undergone lower-limb revascularization were reported at the meeting and simultaneously published online in Circulation (2016 Nov 13; doi: 10.1161/CIRCULATIONAHA.116.025880).
Until now, there have been no large studies comparing antiplatelet therapies in patients with PAD. Clopidogrel is considered superior to aspirin in this patient population based on limited evidence, often extrapolated from studies of acute coronary syndromes or coronary artery disease. Ticagrelor also proved beneficial in these contexts, so researchers performed the EUCLID study, comparing the two medications head to head in patients with PAD.
The manufacturer-funded double-blind trial was conducted at 811 medical centers in eight countries. Patients aged 50 years or older (median age, 66 years) were randomly assigned to receive either oral ticagrelor, 90 mg twice daily (6,930 patients), or oral clopidogrel, 75 mg once daily (6,955 patients), and were followed for a median of 30 months.
The primary efficacy endpoint – the first occurrence of any event in the composite of cardiovascular death, myocardial infarction, or ischemic stroke – occurred in 10.8% of the ticagrelor group and 10.6% of the clopidogrel group, for a hazard ratio of 1.02.
When the components of this composite endpoint were considered individually, only the rate of ischemic stroke was significantly different between the two study groups, occurring in 1.9% of patients taking ticagrelor and 2.4% of those taking clopidogrel (HR, 0.78).
Other important secondary and composite efficacy endpoints, including acute limb ischemia and revascularization, were similar between the two study groups, Dr. Patel said.
The primary safety endpoint – the rate of major bleeding events – occurred in the same percentage of both study groups (1.6%), and individual rates of fatal bleeding, intracranial bleeding, and minor bleeding were similar.
“There were numerically fewer fatal bleeding events in the ticagrelor group than in the clopidogrel group (10 vs. 20), but there were significantly more bleeding events leading to drug discontinuation with ticagrelor than with clopidogrel (168 vs. 112),” he noted.
Ticagrelor was discontinued more often than clopidogrel during the study (30.1% of patients vs. 25.9%; HR, 1.21). Discontinuation was driven mainly by the occurrence of dyspnea (4.8% vs. 0.8%) and minor bleeding, both of which are well-described adverse effects of ticagrelor, Dr. Patel said.
“Our findings show the hazards of extrapolating evidence from patients with coronary artery disease to those with peripheral artery disease,” he added.
In a separate report in Circulation, the results were similar in the substudy of EUCLID participants who had already undergone lower-extremity revascularization procedures before enrollment, reported W. Schuyler Jones, MD, also of of Duke University.
The primary efficacy endpoint occurred in 11.4% of the ticagrelor group and 11.3% of the clopidogrel group, a nonsignificant difference (HR, 1.01). “Other key secondary and composite endpoints, including repeat revascularization, also were not different between the two study groups,” Dr. Jones said.
Regarding safety endpoints, the rates of major bleeding, fatal bleeding, intracranial bleeding, and minor bleeding all were similar between the two study groups.
“These findings suggest that patients with prior revascularization have a substantial residual rate of cardiovascular and acute limb events, despite high adherence to antiplatelet and statin medications, and require further study,” Dr. Jones noted.
*Correction 11/14/16: An earlier version of this article misstated the name of the investigator who presented the study at the meeting.
Ticagrelor was found to be “not superior” to clopidogrel at preventing cardiovascular events in the largest clinical trial to date involving patients with symptomatic peripheral artery disease (PAD), Manesh Patel, MD, reported at the American Heart Association scientific sessions.*
Ticagrelor also was no better than clopidogrel at preventing acute limb ischemia in this study of 13,885 patients.
Regarding safety issues, the two drugs had identical rates of major bleeding adverse events, but ticagrelor was discontinued significantly more often than clopidogrel was, because of its other well-known adverse effects, said Dr. Patel of Duke University, Durham, N.C.
The findings of the EUCLID (Examining the Use of Ticagrelor in PAD) study were presented at the meeting and simultaneously published online Nov. 13 in the New England Journal of Medicine (2016. doi: 10.1056/NEJMoa1611688).
In addition, the results of a substudy of the EUCLID trial involving the 7,875 participants who had previously undergone lower-limb revascularization were reported at the meeting and simultaneously published online in Circulation (2016 Nov 13; doi: 10.1161/CIRCULATIONAHA.116.025880).
Until now, there have been no large studies comparing antiplatelet therapies in patients with PAD. Clopidogrel is considered superior to aspirin in this patient population based on limited evidence, often extrapolated from studies of acute coronary syndromes or coronary artery disease. Ticagrelor also proved beneficial in these contexts, so researchers performed the EUCLID study, comparing the two medications head to head in patients with PAD.
The manufacturer-funded double-blind trial was conducted at 811 medical centers in eight countries. Patients aged 50 years or older (median age, 66 years) were randomly assigned to receive either oral ticagrelor, 90 mg twice daily (6,930 patients), or oral clopidogrel, 75 mg once daily (6,955 patients), and were followed for a median of 30 months.
The primary efficacy endpoint – the first occurrence of any event in the composite of cardiovascular death, myocardial infarction, or ischemic stroke – occurred in 10.8% of the ticagrelor group and 10.6% of the clopidogrel group, for a hazard ratio of 1.02.
When the components of this composite endpoint were considered individually, only the rate of ischemic stroke was significantly different between the two study groups, occurring in 1.9% of patients taking ticagrelor and 2.4% of those taking clopidogrel (HR, 0.78).
Other important secondary and composite efficacy endpoints, including acute limb ischemia and revascularization, were similar between the two study groups, Dr. Patel said.
The primary safety endpoint – the rate of major bleeding events – occurred in the same percentage of both study groups (1.6%), and individual rates of fatal bleeding, intracranial bleeding, and minor bleeding were similar.
“There were numerically fewer fatal bleeding events in the ticagrelor group than in the clopidogrel group (10 vs. 20), but there were significantly more bleeding events leading to drug discontinuation with ticagrelor than with clopidogrel (168 vs. 112),” he noted.
Ticagrelor was discontinued more often than clopidogrel during the study (30.1% of patients vs. 25.9%; HR, 1.21). Discontinuation was driven mainly by the occurrence of dyspnea (4.8% vs. 0.8%) and minor bleeding, both of which are well-described adverse effects of ticagrelor, Dr. Patel said.
“Our findings show the hazards of extrapolating evidence from patients with coronary artery disease to those with peripheral artery disease,” he added.
In a separate report in Circulation, the results were similar in the substudy of EUCLID participants who had already undergone lower-extremity revascularization procedures before enrollment, reported W. Schuyler Jones, MD, also of of Duke University.
The primary efficacy endpoint occurred in 11.4% of the ticagrelor group and 11.3% of the clopidogrel group, a nonsignificant difference (HR, 1.01). “Other key secondary and composite endpoints, including repeat revascularization, also were not different between the two study groups,” Dr. Jones said.
Regarding safety endpoints, the rates of major bleeding, fatal bleeding, intracranial bleeding, and minor bleeding all were similar between the two study groups.
“These findings suggest that patients with prior revascularization have a substantial residual rate of cardiovascular and acute limb events, despite high adherence to antiplatelet and statin medications, and require further study,” Dr. Jones noted.
*Correction 11/14/16: An earlier version of this article misstated the name of the investigator who presented the study at the meeting.
FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: The primary efficacy end point – the first occurrence of any event in the composite of cardiovascular death, myocardial infarction, or ischemic stroke – occurred in 10.8% of the ticagrelor group and 10.6% of the clopidogrel group (HR, 1.02).
Data source: An international double-blind, randomized trial involving 13,885 patients followed for a median of 30 months.
Disclosures: The study was funded by AstraZeneca, maker of ticagrelor. Dr. Patel reported receiving funding from AstraZeneca and several pharmaceutical companies, and his associates reported ties to numerous industry sources.
Rheumatology malpractice litigation investigated for first time
WASHINGTON – Failure to diagnose and a failure to treat in a timely manner were the two most commonly alleged malpractice claims in an analysis of rheumatic disease–related cases presented at the annual meeting of the American College of Rheumatology.
The study of 20 rheumatic disease cases during a 31-year period is thought by the investigators to be the first analysis of malpractice litigation in rheumatology.
Rheumatologists accounted for one-third of all named defendants in the 20 cases, which constituted a convenience sample. They excluded 10 cases because of duplication or lack of relevance to rheumatology. They searched the online legal database WestLaw using the terms “rheumatology” and “medical malpractice” to find all state and federal jury verdicts and settlements related to rheumatology during 1985-2015. The 20 cases occurred in 10 states. The plaintiffs had a mean age of about 52 years, and 45% were female.
Plaintiffs in the medical malpractice suits won only four of the cases, but those had a mean payout of about $2.78 million, which Mr. Prabhu described as lower than what’s been reported in other specialties that he and his colleagues are investigating, such as oncology and neurosurgery. The jury found in favor of the defendant in 12 of the cases, and in 3 cases the parties reached a settlement in which most payouts were not disclosed. The verdict was undisclosed in one case.
Mr. Prabhu said that the investigators discovered an additional 34 cases in their search of the database that they plan on characterizing in another study.
The researchers reported having no relevant financial disclosures.
WASHINGTON – Failure to diagnose and a failure to treat in a timely manner were the two most commonly alleged malpractice claims in an analysis of rheumatic disease–related cases presented at the annual meeting of the American College of Rheumatology.
The study of 20 rheumatic disease cases during a 31-year period is thought by the investigators to be the first analysis of malpractice litigation in rheumatology.
Rheumatologists accounted for one-third of all named defendants in the 20 cases, which constituted a convenience sample. They excluded 10 cases because of duplication or lack of relevance to rheumatology. They searched the online legal database WestLaw using the terms “rheumatology” and “medical malpractice” to find all state and federal jury verdicts and settlements related to rheumatology during 1985-2015. The 20 cases occurred in 10 states. The plaintiffs had a mean age of about 52 years, and 45% were female.
Plaintiffs in the medical malpractice suits won only four of the cases, but those had a mean payout of about $2.78 million, which Mr. Prabhu described as lower than what’s been reported in other specialties that he and his colleagues are investigating, such as oncology and neurosurgery. The jury found in favor of the defendant in 12 of the cases, and in 3 cases the parties reached a settlement in which most payouts were not disclosed. The verdict was undisclosed in one case.
Mr. Prabhu said that the investigators discovered an additional 34 cases in their search of the database that they plan on characterizing in another study.
The researchers reported having no relevant financial disclosures.
WASHINGTON – Failure to diagnose and a failure to treat in a timely manner were the two most commonly alleged malpractice claims in an analysis of rheumatic disease–related cases presented at the annual meeting of the American College of Rheumatology.
The study of 20 rheumatic disease cases during a 31-year period is thought by the investigators to be the first analysis of malpractice litigation in rheumatology.
Rheumatologists accounted for one-third of all named defendants in the 20 cases, which constituted a convenience sample. They excluded 10 cases because of duplication or lack of relevance to rheumatology. They searched the online legal database WestLaw using the terms “rheumatology” and “medical malpractice” to find all state and federal jury verdicts and settlements related to rheumatology during 1985-2015. The 20 cases occurred in 10 states. The plaintiffs had a mean age of about 52 years, and 45% were female.
Plaintiffs in the medical malpractice suits won only four of the cases, but those had a mean payout of about $2.78 million, which Mr. Prabhu described as lower than what’s been reported in other specialties that he and his colleagues are investigating, such as oncology and neurosurgery. The jury found in favor of the defendant in 12 of the cases, and in 3 cases the parties reached a settlement in which most payouts were not disclosed. The verdict was undisclosed in one case.
Mr. Prabhu said that the investigators discovered an additional 34 cases in their search of the database that they plan on characterizing in another study.
The researchers reported having no relevant financial disclosures.
Key clinical point:
Major finding: Plaintiffs in the malpractice suits won only 20% of the cases, but those had a mean payout of about $2.78 million.
Data source: A retrospective study of 20 rheumatic disease malpractice cases occurring in 1985-2015.
Disclosures: The researchers reported having no relevant financial disclosures.
Study found no link between direct-acting antivirals, hepatocellular carcinoma
BOSTON – Direct-acting antiviral therapy does not appear to increase the risk of de novo hepatocellular carcinoma in cirrhotic patients with hepatitis C virus (HCV) infection, according to findings from a prospective cohort study of more than 2,000 patients.
But curing HCV also did not protect patients from hepatocellular carcinoma (HCC), at least during the first 6-12 months after starting treatment, Antonietta Romano, MD, said at the annual meeting of the American Association for the Study of Liver Diseases. Clinicians should continue monitoring HCV patients with advanced liver disease, even after they have achieved sustained viral response, she said.
Recent studies had raised concerns about whether direct-acting antivirals (DAA) increase the risk of HCC, said Dr. Romano of the University of Padova (Italy). To clarify the issue, she and her coinvestigators followed 2,279 HCV patients who received approved DAA regimens. Over a median follow-up time of 305 days, 41 patients developed de novo hepatocellular carcinoma, for an incidence of 1.64 cases per 100 patient-years, Dr. Romano said.
“These results indicate that in cirrhotic patients, the incidence of HCC during the first 6-9 months after initiation of direct-acting antiviral therapy is not different from what we expect in untreated patients, according to historical controls,” she added.
Fully 86% of patients had cirrhosis, of which 91% were Child-Pugh A and 9% were Child-Pugh B, indicating more advanced liver disease, Dr. Romano said. Noncirrhotic patients had an HCC rate of 0.23 cases per 100 patient years – less than one-eighth that for cirrhotic patients (1.93 cases per 100 patient-years). Child-Pugh B patients developed 2.9 cases of HCC per 100 patient years, versus 1.6 for Child-Pugh A patients.
These rates resemble those in historical controls, suggesting that stage of liver disease is the crucial predictor of HCC risk – not DAA therapy, Dr. Romano said. Multivariable analyses supported that conclusion, linking HCC to elevated liver enzymes and to thrombocytopenia but not to DAA regimen, gender, age, or HCV genotype, she reported.
Most HCC cases were diagnosed at least 12 weeks after starting therapy, according to Dr. Romano. Half of patients had a single nodular tumor with a typical vascular pattern, but the other half had a more aggressive multifocal infiltrative pattern. The reason for the high proportion of atypical aggressive pattern was unclear, Dr. Romano said. However, DAA therapy causes HCV to abruptly stop replicating in the liver, leading to marked molecular and immunologic changes that might initially cause microscopic tumors to grow faster than before. So while DAA therapy does not increase the overall risk of HCC, it clearly is not protective, she said.
Nearly 70% of patients in this study were male. About 60% had HCV-1 infection, 22% had HCV-2, 19% had HCV-3, and 8% had HCV-4, Dr. Romano said. In all, 28 of 41 patients who developed HCC had achieved sustained virologic response at 12 weeks, while the other 13 had relapsed.
The investigators received no outside funding for this study. Dr. Romano reported ties to Gilead, Abbvie, and Bristol Myers Squibb.
BOSTON – Direct-acting antiviral therapy does not appear to increase the risk of de novo hepatocellular carcinoma in cirrhotic patients with hepatitis C virus (HCV) infection, according to findings from a prospective cohort study of more than 2,000 patients.
But curing HCV also did not protect patients from hepatocellular carcinoma (HCC), at least during the first 6-12 months after starting treatment, Antonietta Romano, MD, said at the annual meeting of the American Association for the Study of Liver Diseases. Clinicians should continue monitoring HCV patients with advanced liver disease, even after they have achieved sustained viral response, she said.
Recent studies had raised concerns about whether direct-acting antivirals (DAA) increase the risk of HCC, said Dr. Romano of the University of Padova (Italy). To clarify the issue, she and her coinvestigators followed 2,279 HCV patients who received approved DAA regimens. Over a median follow-up time of 305 days, 41 patients developed de novo hepatocellular carcinoma, for an incidence of 1.64 cases per 100 patient-years, Dr. Romano said.
“These results indicate that in cirrhotic patients, the incidence of HCC during the first 6-9 months after initiation of direct-acting antiviral therapy is not different from what we expect in untreated patients, according to historical controls,” she added.
Fully 86% of patients had cirrhosis, of which 91% were Child-Pugh A and 9% were Child-Pugh B, indicating more advanced liver disease, Dr. Romano said. Noncirrhotic patients had an HCC rate of 0.23 cases per 100 patient years – less than one-eighth that for cirrhotic patients (1.93 cases per 100 patient-years). Child-Pugh B patients developed 2.9 cases of HCC per 100 patient years, versus 1.6 for Child-Pugh A patients.
These rates resemble those in historical controls, suggesting that stage of liver disease is the crucial predictor of HCC risk – not DAA therapy, Dr. Romano said. Multivariable analyses supported that conclusion, linking HCC to elevated liver enzymes and to thrombocytopenia but not to DAA regimen, gender, age, or HCV genotype, she reported.
Most HCC cases were diagnosed at least 12 weeks after starting therapy, according to Dr. Romano. Half of patients had a single nodular tumor with a typical vascular pattern, but the other half had a more aggressive multifocal infiltrative pattern. The reason for the high proportion of atypical aggressive pattern was unclear, Dr. Romano said. However, DAA therapy causes HCV to abruptly stop replicating in the liver, leading to marked molecular and immunologic changes that might initially cause microscopic tumors to grow faster than before. So while DAA therapy does not increase the overall risk of HCC, it clearly is not protective, she said.
Nearly 70% of patients in this study were male. About 60% had HCV-1 infection, 22% had HCV-2, 19% had HCV-3, and 8% had HCV-4, Dr. Romano said. In all, 28 of 41 patients who developed HCC had achieved sustained virologic response at 12 weeks, while the other 13 had relapsed.
The investigators received no outside funding for this study. Dr. Romano reported ties to Gilead, Abbvie, and Bristol Myers Squibb.
BOSTON – Direct-acting antiviral therapy does not appear to increase the risk of de novo hepatocellular carcinoma in cirrhotic patients with hepatitis C virus (HCV) infection, according to findings from a prospective cohort study of more than 2,000 patients.
But curing HCV also did not protect patients from hepatocellular carcinoma (HCC), at least during the first 6-12 months after starting treatment, Antonietta Romano, MD, said at the annual meeting of the American Association for the Study of Liver Diseases. Clinicians should continue monitoring HCV patients with advanced liver disease, even after they have achieved sustained viral response, she said.
Recent studies had raised concerns about whether direct-acting antivirals (DAA) increase the risk of HCC, said Dr. Romano of the University of Padova (Italy). To clarify the issue, she and her coinvestigators followed 2,279 HCV patients who received approved DAA regimens. Over a median follow-up time of 305 days, 41 patients developed de novo hepatocellular carcinoma, for an incidence of 1.64 cases per 100 patient-years, Dr. Romano said.
“These results indicate that in cirrhotic patients, the incidence of HCC during the first 6-9 months after initiation of direct-acting antiviral therapy is not different from what we expect in untreated patients, according to historical controls,” she added.
Fully 86% of patients had cirrhosis, of which 91% were Child-Pugh A and 9% were Child-Pugh B, indicating more advanced liver disease, Dr. Romano said. Noncirrhotic patients had an HCC rate of 0.23 cases per 100 patient years – less than one-eighth that for cirrhotic patients (1.93 cases per 100 patient-years). Child-Pugh B patients developed 2.9 cases of HCC per 100 patient years, versus 1.6 for Child-Pugh A patients.
These rates resemble those in historical controls, suggesting that stage of liver disease is the crucial predictor of HCC risk – not DAA therapy, Dr. Romano said. Multivariable analyses supported that conclusion, linking HCC to elevated liver enzymes and to thrombocytopenia but not to DAA regimen, gender, age, or HCV genotype, she reported.
Most HCC cases were diagnosed at least 12 weeks after starting therapy, according to Dr. Romano. Half of patients had a single nodular tumor with a typical vascular pattern, but the other half had a more aggressive multifocal infiltrative pattern. The reason for the high proportion of atypical aggressive pattern was unclear, Dr. Romano said. However, DAA therapy causes HCV to abruptly stop replicating in the liver, leading to marked molecular and immunologic changes that might initially cause microscopic tumors to grow faster than before. So while DAA therapy does not increase the overall risk of HCC, it clearly is not protective, she said.
Nearly 70% of patients in this study were male. About 60% had HCV-1 infection, 22% had HCV-2, 19% had HCV-3, and 8% had HCV-4, Dr. Romano said. In all, 28 of 41 patients who developed HCC had achieved sustained virologic response at 12 weeks, while the other 13 had relapsed.
The investigators received no outside funding for this study. Dr. Romano reported ties to Gilead, Abbvie, and Bristol Myers Squibb.
AT THE LIVER MEETING
Key clinical point:
Major finding: The overall incidence of de novo hepatocellular carcinoma was 1.64 cases per 100 patient-years during and after treatment, with a median follow-up period of about 305 days.
Data source: A prospective cohort study of 2,279 patients (86% with cirrhosis) who received direct-acting antivirals for HCV.
Disclosures: The investigators received no outside funding for the study. Dr. Romano reported ties to Gilead, Abbvie, and Bristol Myers Squibb.









