User login
Ryan White Program Announces New Funding Grants
“The Ryan White HIV/AIDS Program plays an instrumental role in the United States’ public health response to HIV,” said HHS Secretary Sylvia Burwell, announcing nearly $2.3 billion in grants to the program to ease access to HIV/AIDS care and medications.
The program provides primary medical care, drug assistance, education and training, and a number of other essential support services to more than half a million people—more than50% of those living with diagnosed HIV infection in the U..S. The services are crucial in “preserving health, extending life expectancy, and reducing HIV transmission,” said HRSA Acting Administrator Jim Macrae. “In 2014, more than 80% of Ryan White HIV/AIDS Program clients who received HIV medical care were retained in care, and more than 81% of program clients who received HIV medical care were virally suppressed.”
About $627.8 million was awarded to 24 metropolitan areas and 28 transitional grant areas with the highest number of people living with HIV and AIDS or those experiencing increases in HIV and AIDS cases and emerging care needs. Another approximate $1.3 billion was awarded to 59 states and territories for core medical and support services and for the AIDS Drug Assistance Program.
Sixteen states received Emerging Community grants based on the number of AIDS cases over the most recent 5-year period. Thirty-two states and territories were awarded $10.4 million in Part B Minority AIDS Initiative grants.
Local community-based organizations and other groups across the country also were awarded funding to provide family-centered comprehensive care for women and children; technical assistance, clinical training, and oral health services; and education and training for health care professionals. Grant money will support the demonstration and evaluation of innovative models of care delivery for hard-to-reach populations as well as efforts to reduce new HIV infections.
“The Ryan White HIV/AIDS Program plays an instrumental role in the United States’ public health response to HIV,” said HHS Secretary Sylvia Burwell, announcing nearly $2.3 billion in grants to the program to ease access to HIV/AIDS care and medications.
The program provides primary medical care, drug assistance, education and training, and a number of other essential support services to more than half a million people—more than50% of those living with diagnosed HIV infection in the U..S. The services are crucial in “preserving health, extending life expectancy, and reducing HIV transmission,” said HRSA Acting Administrator Jim Macrae. “In 2014, more than 80% of Ryan White HIV/AIDS Program clients who received HIV medical care were retained in care, and more than 81% of program clients who received HIV medical care were virally suppressed.”
About $627.8 million was awarded to 24 metropolitan areas and 28 transitional grant areas with the highest number of people living with HIV and AIDS or those experiencing increases in HIV and AIDS cases and emerging care needs. Another approximate $1.3 billion was awarded to 59 states and territories for core medical and support services and for the AIDS Drug Assistance Program.
Sixteen states received Emerging Community grants based on the number of AIDS cases over the most recent 5-year period. Thirty-two states and territories were awarded $10.4 million in Part B Minority AIDS Initiative grants.
Local community-based organizations and other groups across the country also were awarded funding to provide family-centered comprehensive care for women and children; technical assistance, clinical training, and oral health services; and education and training for health care professionals. Grant money will support the demonstration and evaluation of innovative models of care delivery for hard-to-reach populations as well as efforts to reduce new HIV infections.
“The Ryan White HIV/AIDS Program plays an instrumental role in the United States’ public health response to HIV,” said HHS Secretary Sylvia Burwell, announcing nearly $2.3 billion in grants to the program to ease access to HIV/AIDS care and medications.
The program provides primary medical care, drug assistance, education and training, and a number of other essential support services to more than half a million people—more than50% of those living with diagnosed HIV infection in the U..S. The services are crucial in “preserving health, extending life expectancy, and reducing HIV transmission,” said HRSA Acting Administrator Jim Macrae. “In 2014, more than 80% of Ryan White HIV/AIDS Program clients who received HIV medical care were retained in care, and more than 81% of program clients who received HIV medical care were virally suppressed.”
About $627.8 million was awarded to 24 metropolitan areas and 28 transitional grant areas with the highest number of people living with HIV and AIDS or those experiencing increases in HIV and AIDS cases and emerging care needs. Another approximate $1.3 billion was awarded to 59 states and territories for core medical and support services and for the AIDS Drug Assistance Program.
Sixteen states received Emerging Community grants based on the number of AIDS cases over the most recent 5-year period. Thirty-two states and territories were awarded $10.4 million in Part B Minority AIDS Initiative grants.
Local community-based organizations and other groups across the country also were awarded funding to provide family-centered comprehensive care for women and children; technical assistance, clinical training, and oral health services; and education and training for health care professionals. Grant money will support the demonstration and evaluation of innovative models of care delivery for hard-to-reach populations as well as efforts to reduce new HIV infections.
Food Security Can Help Reduce Cardiovascular Risk Factors
Food insecurity has been linked to hypertension, diabetes, elevated cholesterol, and obesity—all cardiovascular risk factors and dangerous for pregnant women and infants. Researchers from Massachusetts General Hospital theorized that enrolling pregnant women in a program to ensure their access to food banks and other resources could help reduce their risks.
The researchers conducted a retrospective analysis of 1,295 women who visited the obstetrics clinic at a community health center. Of those, 145 (11%) were referred to Food for Families, which connects patients to resources such as the Supplemental Nutrition Assistance Program (SNAP) and the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC).
Two-thirds of referred women enrolled in Food for Families. A majority rated their health as good, very good, or excellent. Most had never used a free meal program, soup kitchen, or food pantry, although 43% were eligible for SNAP, and 87% were enrolled in WIC.
The primary outcomes measured were trends in blood pressure (BP) and blood glucose during pregnancy. Blood pressure numbers trended “modestly better” for women in the intervention program. They had a better systolic BP (0.2015 mm Hg/wk lower) and diastolic BP (0.1049 mm Hg/wk lower) than those who were not referred. The researchers found no differences in blood glucose trend.
The findings suggest that programs to reduce food insecurity can improve cardiovascular health in pregnant women, the researchers say. If so, screening for food insecurity in obstetric care may be a useful tool—particularly if the next step is to get patients the food they need
Food insecurity has been linked to hypertension, diabetes, elevated cholesterol, and obesity—all cardiovascular risk factors and dangerous for pregnant women and infants. Researchers from Massachusetts General Hospital theorized that enrolling pregnant women in a program to ensure their access to food banks and other resources could help reduce their risks.
The researchers conducted a retrospective analysis of 1,295 women who visited the obstetrics clinic at a community health center. Of those, 145 (11%) were referred to Food for Families, which connects patients to resources such as the Supplemental Nutrition Assistance Program (SNAP) and the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC).
Two-thirds of referred women enrolled in Food for Families. A majority rated their health as good, very good, or excellent. Most had never used a free meal program, soup kitchen, or food pantry, although 43% were eligible for SNAP, and 87% were enrolled in WIC.
The primary outcomes measured were trends in blood pressure (BP) and blood glucose during pregnancy. Blood pressure numbers trended “modestly better” for women in the intervention program. They had a better systolic BP (0.2015 mm Hg/wk lower) and diastolic BP (0.1049 mm Hg/wk lower) than those who were not referred. The researchers found no differences in blood glucose trend.
The findings suggest that programs to reduce food insecurity can improve cardiovascular health in pregnant women, the researchers say. If so, screening for food insecurity in obstetric care may be a useful tool—particularly if the next step is to get patients the food they need
Food insecurity has been linked to hypertension, diabetes, elevated cholesterol, and obesity—all cardiovascular risk factors and dangerous for pregnant women and infants. Researchers from Massachusetts General Hospital theorized that enrolling pregnant women in a program to ensure their access to food banks and other resources could help reduce their risks.
The researchers conducted a retrospective analysis of 1,295 women who visited the obstetrics clinic at a community health center. Of those, 145 (11%) were referred to Food for Families, which connects patients to resources such as the Supplemental Nutrition Assistance Program (SNAP) and the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC).
Two-thirds of referred women enrolled in Food for Families. A majority rated their health as good, very good, or excellent. Most had never used a free meal program, soup kitchen, or food pantry, although 43% were eligible for SNAP, and 87% were enrolled in WIC.
The primary outcomes measured were trends in blood pressure (BP) and blood glucose during pregnancy. Blood pressure numbers trended “modestly better” for women in the intervention program. They had a better systolic BP (0.2015 mm Hg/wk lower) and diastolic BP (0.1049 mm Hg/wk lower) than those who were not referred. The researchers found no differences in blood glucose trend.
The findings suggest that programs to reduce food insecurity can improve cardiovascular health in pregnant women, the researchers say. If so, screening for food insecurity in obstetric care may be a useful tool—particularly if the next step is to get patients the food they need
Pain Management: How About Holistic?
I recently received the letter and instruction card for prescribing narcotic analgesics from US Surgeon General Vice Admiral Vivek H. Murthy, MD, MBA. While I agree in principle with the movement to improve pain management, I feel there is a lot being overlooked in this crusade and would like to suggest alternative evidence-based methods that don’t involve prescription narcotics.
I have extensive training in energy therapy, guided imagery, and ozonotherapy. Healing touch is one well-researched energy therapy that has been shown to reduce pain. I have performed and published research demonstrating its efficacy (see http://healingbeyondborders.org/index.php/research-integrative-health/research); patients’ functional abilities improve, and they are able to decrease or eliminate use of pain medication. Providers from many disciplines, including MDs, DOs, NPs, and DCs, practice healing touch. Training for healing touch is available worldwide. The certification process is similar to masters-level education, including both classroom and hands-on clinical practice experience. My practice uses healing touch for patients, and I teach classes using the international curriculum.
In addition to the research published on the efficacy of guided imagery (another method of pain relief therapy), I have personally witnessed and been part of several successful examples in my clinical practice. In the burn unit, Dr. Jean Achterberg Lawlis and I used guided imagery to relieve pain in patients with third- and fourth-degree burns over 70% or more of their body. We performed tanking and dressing changes without narcotic pain medications; patients were comfortable during treatment and slept peacefully after. In another instance, a 23-year-old man presented with major chest and spine injuries after a motorcycle accident. Morphine (100 mg IV) did nothing to relieve his pain. But guided imagery of racing his stock car around a racetrack eliminated any need for narcotics during dressing changes. I’ve also worked with women prenatally, teaching guided imagery for smooth, successful deliveries without pain medications or epidural.
Ozonotherapy has an extensive international evidence base, and many studies show that it relieves pain without the need for narcotics (see http://aaot.us/?page=Literature). I have seen many cases of chronic pain relieved by major autohemolytic therapy and prolozone injection therapies. Here, too, patients are able to decrease and eventually stop their narcotic medications. Some patients are able to avoid joint replacement surgery, achieving improved function and comfort without the adverse effects of steroids.
An effective way to release muscle tension and relieve pain from injury (eg, low back pain, plantar fasciitis, whiplash, carpal tunnel) is through massage therapy. Providers who refer patients to massage practitioners can avoid narcotic medication prescriptions by addressing the problem that is causing the pain. Chiropractic care is a standard care for low back pain; it can also resolve problems that cause migraines, trigeminal neuralgia, and Bell’s palsy without narcotics, steroids, or the sedating muscle relaxants and seizure medications. Yet several veterans in my community were denied chiropractic care until they had tried narcotics and physical therapy (which involved a four-hour roundtrip car ride, no less). Oh, and in the meantime, they were prescribed an additional narcotic!
By focusing only on narcotics, we miss out on other options to treat pain. If we overlook the full range of evidence, then the “evidence-based” mantra isn’t truthful, nor is it useful. To follow the pledge to “do no harm,” we must treat the causes of pain. Of the Surgeon General, I request: Please don’t just send us a teaching card on how to prescribe narcotics. Get providers involved in seeking continuing education credits in therapies that help us avoid prescribing them in the first place.

Susan Peck, PhD, GNP-BC, APNP, FAAO, APT, CHTP/I
Eau Claire, WI
I recently received the letter and instruction card for prescribing narcotic analgesics from US Surgeon General Vice Admiral Vivek H. Murthy, MD, MBA. While I agree in principle with the movement to improve pain management, I feel there is a lot being overlooked in this crusade and would like to suggest alternative evidence-based methods that don’t involve prescription narcotics.
I have extensive training in energy therapy, guided imagery, and ozonotherapy. Healing touch is one well-researched energy therapy that has been shown to reduce pain. I have performed and published research demonstrating its efficacy (see http://healingbeyondborders.org/index.php/research-integrative-health/research); patients’ functional abilities improve, and they are able to decrease or eliminate use of pain medication. Providers from many disciplines, including MDs, DOs, NPs, and DCs, practice healing touch. Training for healing touch is available worldwide. The certification process is similar to masters-level education, including both classroom and hands-on clinical practice experience. My practice uses healing touch for patients, and I teach classes using the international curriculum.
In addition to the research published on the efficacy of guided imagery (another method of pain relief therapy), I have personally witnessed and been part of several successful examples in my clinical practice. In the burn unit, Dr. Jean Achterberg Lawlis and I used guided imagery to relieve pain in patients with third- and fourth-degree burns over 70% or more of their body. We performed tanking and dressing changes without narcotic pain medications; patients were comfortable during treatment and slept peacefully after. In another instance, a 23-year-old man presented with major chest and spine injuries after a motorcycle accident. Morphine (100 mg IV) did nothing to relieve his pain. But guided imagery of racing his stock car around a racetrack eliminated any need for narcotics during dressing changes. I’ve also worked with women prenatally, teaching guided imagery for smooth, successful deliveries without pain medications or epidural.
Ozonotherapy has an extensive international evidence base, and many studies show that it relieves pain without the need for narcotics (see http://aaot.us/?page=Literature). I have seen many cases of chronic pain relieved by major autohemolytic therapy and prolozone injection therapies. Here, too, patients are able to decrease and eventually stop their narcotic medications. Some patients are able to avoid joint replacement surgery, achieving improved function and comfort without the adverse effects of steroids.
An effective way to release muscle tension and relieve pain from injury (eg, low back pain, plantar fasciitis, whiplash, carpal tunnel) is through massage therapy. Providers who refer patients to massage practitioners can avoid narcotic medication prescriptions by addressing the problem that is causing the pain. Chiropractic care is a standard care for low back pain; it can also resolve problems that cause migraines, trigeminal neuralgia, and Bell’s palsy without narcotics, steroids, or the sedating muscle relaxants and seizure medications. Yet several veterans in my community were denied chiropractic care until they had tried narcotics and physical therapy (which involved a four-hour roundtrip car ride, no less). Oh, and in the meantime, they were prescribed an additional narcotic!
By focusing only on narcotics, we miss out on other options to treat pain. If we overlook the full range of evidence, then the “evidence-based” mantra isn’t truthful, nor is it useful. To follow the pledge to “do no harm,” we must treat the causes of pain. Of the Surgeon General, I request: Please don’t just send us a teaching card on how to prescribe narcotics. Get providers involved in seeking continuing education credits in therapies that help us avoid prescribing them in the first place.

Susan Peck, PhD, GNP-BC, APNP, FAAO, APT, CHTP/I
Eau Claire, WI
I recently received the letter and instruction card for prescribing narcotic analgesics from US Surgeon General Vice Admiral Vivek H. Murthy, MD, MBA. While I agree in principle with the movement to improve pain management, I feel there is a lot being overlooked in this crusade and would like to suggest alternative evidence-based methods that don’t involve prescription narcotics.
I have extensive training in energy therapy, guided imagery, and ozonotherapy. Healing touch is one well-researched energy therapy that has been shown to reduce pain. I have performed and published research demonstrating its efficacy (see http://healingbeyondborders.org/index.php/research-integrative-health/research); patients’ functional abilities improve, and they are able to decrease or eliminate use of pain medication. Providers from many disciplines, including MDs, DOs, NPs, and DCs, practice healing touch. Training for healing touch is available worldwide. The certification process is similar to masters-level education, including both classroom and hands-on clinical practice experience. My practice uses healing touch for patients, and I teach classes using the international curriculum.
In addition to the research published on the efficacy of guided imagery (another method of pain relief therapy), I have personally witnessed and been part of several successful examples in my clinical practice. In the burn unit, Dr. Jean Achterberg Lawlis and I used guided imagery to relieve pain in patients with third- and fourth-degree burns over 70% or more of their body. We performed tanking and dressing changes without narcotic pain medications; patients were comfortable during treatment and slept peacefully after. In another instance, a 23-year-old man presented with major chest and spine injuries after a motorcycle accident. Morphine (100 mg IV) did nothing to relieve his pain. But guided imagery of racing his stock car around a racetrack eliminated any need for narcotics during dressing changes. I’ve also worked with women prenatally, teaching guided imagery for smooth, successful deliveries without pain medications or epidural.
Ozonotherapy has an extensive international evidence base, and many studies show that it relieves pain without the need for narcotics (see http://aaot.us/?page=Literature). I have seen many cases of chronic pain relieved by major autohemolytic therapy and prolozone injection therapies. Here, too, patients are able to decrease and eventually stop their narcotic medications. Some patients are able to avoid joint replacement surgery, achieving improved function and comfort without the adverse effects of steroids.
An effective way to release muscle tension and relieve pain from injury (eg, low back pain, plantar fasciitis, whiplash, carpal tunnel) is through massage therapy. Providers who refer patients to massage practitioners can avoid narcotic medication prescriptions by addressing the problem that is causing the pain. Chiropractic care is a standard care for low back pain; it can also resolve problems that cause migraines, trigeminal neuralgia, and Bell’s palsy without narcotics, steroids, or the sedating muscle relaxants and seizure medications. Yet several veterans in my community were denied chiropractic care until they had tried narcotics and physical therapy (which involved a four-hour roundtrip car ride, no less). Oh, and in the meantime, they were prescribed an additional narcotic!
By focusing only on narcotics, we miss out on other options to treat pain. If we overlook the full range of evidence, then the “evidence-based” mantra isn’t truthful, nor is it useful. To follow the pledge to “do no harm,” we must treat the causes of pain. Of the Surgeon General, I request: Please don’t just send us a teaching card on how to prescribe narcotics. Get providers involved in seeking continuing education credits in therapies that help us avoid prescribing them in the first place.

Susan Peck, PhD, GNP-BC, APNP, FAAO, APT, CHTP/I
Eau Claire, WI
Statins in adults with CVD risk: The latest from the USPSTF
Do pedometers increase activity and improve health outcomes?
EVIDENCE SUMMARY
A systematic review and meta-analysis identified 26 studies evaluating activity and health outcomes with the use of pedometers.1 The studies included 8 RCTs and 18 observational studies with 2767 patients (mean body mass index [BMI]: 30 kg/m2; mean age: 49 years; 85% women). The studies ranged from 3 to 104 weeks. From the RCT data, patients using pedometers had an increase of 2491 steps per day (about one mile) more than control group patients (8 trials, n=305; 95% confidence interval [CI], 1098-3885 steps/day; P<.001).
Across all of the observational studies, pedometer users had a 26.9% increase from their baseline physical activity (P=.001). When data from all of the studies were combined, the researchers found a decrease from baseline BMI (18 studies, n=562; mean difference [MD]=0.38 kg/m2; 95% CI, 0.05-0.72; P=.03) and a decrease in systolic BP (12 studies, n=468; MD=3.8 mm Hg; 95% CI, 1.7-5.9 mm Hg; P<.001). No statistically significant change was noted in cholesterol or fasting glucose levels. Weaknesses of this review include the heterogeneity of the interventions, relatively small study sizes, and short study durations.
A systematic review and meta-analysis of 11 RCTs (N=1258) evaluated pedometer effects in overweight patients with type 2 diabetes.2 (One RCT was included in the above meta-analysis.) Studies ran from 6 to 48 weeks, and mean enrollment BMI (where reported) was 30 kg/m2 or more in at least one treatment arm. Compared to controls, patients using pedometers had greater reductions in weight (weighted mean difference [WMD]= -0.65 kg; 95% CI, -1.12 to -0.17 kg) and BMI (WMD= -0.15 kg/m2; 95% CI, -0.29 to -0.02 kg/m2). The effect persisted in the subset of studies in which the intervention and control groups both received dietary counseling (WMD weight= -0.86 kg; 95% CI, -1.45 to -0.27 kg; WMD BMI= -0.30 kg/m2; 95% CI, -0.50 to -0.10 kg/m2). Study quality was low to moderate, and 5 studies used per-protocol analysis instead of intention-to-treat analysis.
Pedometer use benefits patients with musculoskeletal diseases, too
A systematic review and meta-analysis examined the use of pedometers in patients with musculoskeletal diseases.3 It included 7 RCTs lasting 4 weeks to one year with 484 adults, 40 to 82 years of age, with musculoskeletal disorders (eg, back pain, knee pain, hip pain). (One RCT was also included in the diabetes meta-analysis.) Pedometer use resulted in a mean increase in physical activity of 1950 steps per day above baseline (range=818-2829 steps/day; P<.05). The authors noted that 4 of the 7 studies also demonstrated significant improvement in pain scores and physical function. BMI data were not tracked in this review.
Pedometers increase walking in older patients
A RCT compared the effects of pedometer-based activity prescriptions with standard time-based activity prescriptions in 330 patients ≥65 years of age with baseline low activity levels.4 All patients received an initial physician visit followed by 3 telephone counseling sessions encouraging increased activity. The pedometer group was counseled on increasing steps (without specific targets), while the standard activity prescription group received time-related activity goals.
At one year, “leisure walking” had increased more for the pedometer group than for the standard group (mean 50 minutes/week vs 28 minutes/week; P=.03), although both groups equally increased their amount of “total activity.”
1. Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296-2304.
2. Cai X, Qiu SH, Yin H, et al. Pedometer intervention and weight loss in overweight and obese adults with type 2 diabetes: a meta-analysis. Diabet Med. 2016;33:1035-1044.
3. Mansi S, Milosavljevic S, Baxter GD, et al. A systematic review of studies using pedometers as an intervention for musculoskeletal diseases. BMC Musculoskeletal Disorders. 2014;15:231.
4. Kolt GS, Schofield GM, Kerse N, et al. Healthy Steps trial: pedometer-based advice and physical activity for low-active older adults. Ann Fam Med. 2012;10:206-212.
EVIDENCE SUMMARY
A systematic review and meta-analysis identified 26 studies evaluating activity and health outcomes with the use of pedometers.1 The studies included 8 RCTs and 18 observational studies with 2767 patients (mean body mass index [BMI]: 30 kg/m2; mean age: 49 years; 85% women). The studies ranged from 3 to 104 weeks. From the RCT data, patients using pedometers had an increase of 2491 steps per day (about one mile) more than control group patients (8 trials, n=305; 95% confidence interval [CI], 1098-3885 steps/day; P<.001).
Across all of the observational studies, pedometer users had a 26.9% increase from their baseline physical activity (P=.001). When data from all of the studies were combined, the researchers found a decrease from baseline BMI (18 studies, n=562; mean difference [MD]=0.38 kg/m2; 95% CI, 0.05-0.72; P=.03) and a decrease in systolic BP (12 studies, n=468; MD=3.8 mm Hg; 95% CI, 1.7-5.9 mm Hg; P<.001). No statistically significant change was noted in cholesterol or fasting glucose levels. Weaknesses of this review include the heterogeneity of the interventions, relatively small study sizes, and short study durations.
A systematic review and meta-analysis of 11 RCTs (N=1258) evaluated pedometer effects in overweight patients with type 2 diabetes.2 (One RCT was included in the above meta-analysis.) Studies ran from 6 to 48 weeks, and mean enrollment BMI (where reported) was 30 kg/m2 or more in at least one treatment arm. Compared to controls, patients using pedometers had greater reductions in weight (weighted mean difference [WMD]= -0.65 kg; 95% CI, -1.12 to -0.17 kg) and BMI (WMD= -0.15 kg/m2; 95% CI, -0.29 to -0.02 kg/m2). The effect persisted in the subset of studies in which the intervention and control groups both received dietary counseling (WMD weight= -0.86 kg; 95% CI, -1.45 to -0.27 kg; WMD BMI= -0.30 kg/m2; 95% CI, -0.50 to -0.10 kg/m2). Study quality was low to moderate, and 5 studies used per-protocol analysis instead of intention-to-treat analysis.
Pedometer use benefits patients with musculoskeletal diseases, too
A systematic review and meta-analysis examined the use of pedometers in patients with musculoskeletal diseases.3 It included 7 RCTs lasting 4 weeks to one year with 484 adults, 40 to 82 years of age, with musculoskeletal disorders (eg, back pain, knee pain, hip pain). (One RCT was also included in the diabetes meta-analysis.) Pedometer use resulted in a mean increase in physical activity of 1950 steps per day above baseline (range=818-2829 steps/day; P<.05). The authors noted that 4 of the 7 studies also demonstrated significant improvement in pain scores and physical function. BMI data were not tracked in this review.
Pedometers increase walking in older patients
A RCT compared the effects of pedometer-based activity prescriptions with standard time-based activity prescriptions in 330 patients ≥65 years of age with baseline low activity levels.4 All patients received an initial physician visit followed by 3 telephone counseling sessions encouraging increased activity. The pedometer group was counseled on increasing steps (without specific targets), while the standard activity prescription group received time-related activity goals.
At one year, “leisure walking” had increased more for the pedometer group than for the standard group (mean 50 minutes/week vs 28 minutes/week; P=.03), although both groups equally increased their amount of “total activity.”
EVIDENCE SUMMARY
A systematic review and meta-analysis identified 26 studies evaluating activity and health outcomes with the use of pedometers.1 The studies included 8 RCTs and 18 observational studies with 2767 patients (mean body mass index [BMI]: 30 kg/m2; mean age: 49 years; 85% women). The studies ranged from 3 to 104 weeks. From the RCT data, patients using pedometers had an increase of 2491 steps per day (about one mile) more than control group patients (8 trials, n=305; 95% confidence interval [CI], 1098-3885 steps/day; P<.001).
Across all of the observational studies, pedometer users had a 26.9% increase from their baseline physical activity (P=.001). When data from all of the studies were combined, the researchers found a decrease from baseline BMI (18 studies, n=562; mean difference [MD]=0.38 kg/m2; 95% CI, 0.05-0.72; P=.03) and a decrease in systolic BP (12 studies, n=468; MD=3.8 mm Hg; 95% CI, 1.7-5.9 mm Hg; P<.001). No statistically significant change was noted in cholesterol or fasting glucose levels. Weaknesses of this review include the heterogeneity of the interventions, relatively small study sizes, and short study durations.
A systematic review and meta-analysis of 11 RCTs (N=1258) evaluated pedometer effects in overweight patients with type 2 diabetes.2 (One RCT was included in the above meta-analysis.) Studies ran from 6 to 48 weeks, and mean enrollment BMI (where reported) was 30 kg/m2 or more in at least one treatment arm. Compared to controls, patients using pedometers had greater reductions in weight (weighted mean difference [WMD]= -0.65 kg; 95% CI, -1.12 to -0.17 kg) and BMI (WMD= -0.15 kg/m2; 95% CI, -0.29 to -0.02 kg/m2). The effect persisted in the subset of studies in which the intervention and control groups both received dietary counseling (WMD weight= -0.86 kg; 95% CI, -1.45 to -0.27 kg; WMD BMI= -0.30 kg/m2; 95% CI, -0.50 to -0.10 kg/m2). Study quality was low to moderate, and 5 studies used per-protocol analysis instead of intention-to-treat analysis.
Pedometer use benefits patients with musculoskeletal diseases, too
A systematic review and meta-analysis examined the use of pedometers in patients with musculoskeletal diseases.3 It included 7 RCTs lasting 4 weeks to one year with 484 adults, 40 to 82 years of age, with musculoskeletal disorders (eg, back pain, knee pain, hip pain). (One RCT was also included in the diabetes meta-analysis.) Pedometer use resulted in a mean increase in physical activity of 1950 steps per day above baseline (range=818-2829 steps/day; P<.05). The authors noted that 4 of the 7 studies also demonstrated significant improvement in pain scores and physical function. BMI data were not tracked in this review.
Pedometers increase walking in older patients
A RCT compared the effects of pedometer-based activity prescriptions with standard time-based activity prescriptions in 330 patients ≥65 years of age with baseline low activity levels.4 All patients received an initial physician visit followed by 3 telephone counseling sessions encouraging increased activity. The pedometer group was counseled on increasing steps (without specific targets), while the standard activity prescription group received time-related activity goals.
At one year, “leisure walking” had increased more for the pedometer group than for the standard group (mean 50 minutes/week vs 28 minutes/week; P=.03), although both groups equally increased their amount of “total activity.”
1. Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296-2304.
2. Cai X, Qiu SH, Yin H, et al. Pedometer intervention and weight loss in overweight and obese adults with type 2 diabetes: a meta-analysis. Diabet Med. 2016;33:1035-1044.
3. Mansi S, Milosavljevic S, Baxter GD, et al. A systematic review of studies using pedometers as an intervention for musculoskeletal diseases. BMC Musculoskeletal Disorders. 2014;15:231.
4. Kolt GS, Schofield GM, Kerse N, et al. Healthy Steps trial: pedometer-based advice and physical activity for low-active older adults. Ann Fam Med. 2012;10:206-212.
1. Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296-2304.
2. Cai X, Qiu SH, Yin H, et al. Pedometer intervention and weight loss in overweight and obese adults with type 2 diabetes: a meta-analysis. Diabet Med. 2016;33:1035-1044.
3. Mansi S, Milosavljevic S, Baxter GD, et al. A systematic review of studies using pedometers as an intervention for musculoskeletal diseases. BMC Musculoskeletal Disorders. 2014;15:231.
4. Kolt GS, Schofield GM, Kerse N, et al. Healthy Steps trial: pedometer-based advice and physical activity for low-active older adults. Ann Fam Med. 2012;10:206-212.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
Yes. In overweight and obese patients, exercise interventions using a pedometer increase steps by about a mile per day over the same interventions without access to pedometer information (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs]) and are associated with a modest 4 mm Hg reduction in systolic blood pressure (BP) over baseline (SOR: B, meta-analysis of RCTs and cohort studies). In overweight patients with diabetes, pedometer use with nutritional counseling is associated with 0.86 kg greater weight loss than nutritional counseling alone (SOR: B, meta-analysis of lower quality RCTs).
Pedometers increase activity in patients with various musculoskeletal conditions and may help reduce pain (SOR: B, meta-analysis of RCTs with heterogeneous outcomes). In low-activity elderly patients, pedometers do not appear to increase total activity when added to an exercise program, but they do appear to increase walking (SOR: B, RCT).
There is no evidence concerning the impact of pedometers on cardiovascular outcomes.
Staring down the opioid epidemic
Nearly 80 people die every day in America from an opioid overdose.1 At the same time, sales of prescription painkillers have increased 4-fold since 1999.2 My own medical assistant was given an unsolicited prescription for 40 oxycodone after a wisdom tooth extraction.
Meanwhile, about 80% of the country’s 2 million opioid-dependent patients are not receiving the treatment they need.3,4 In Vermont, for example, more than 500 patients are on waiting lists to receive buprenorphine (the partial opioid agonist used to treat opioid addiction)—a wait that for many of them will last for more than a year and may cost them their life.5
Buprenorphine makes good sense. Fortunately, buprenorphine can reverse opioid cravings within minutes. Medication-assisted treatment with buprenorphine derivatives allows patients to lead normal, productive, and stable lives. Every dollar invested in treating opioid addiction saves society $7 in drug-related crime and criminal justice costs.6 In addition, 50% to 80% of opioid-dependent patients remain opioid-free for 12 months while taking buprenorphine.7
Steps we can take. As family physicians (FPs), we are frequently overwhelmed by regulatory concerns, overhead expenses, and providing meaningful use data to third-party payers. And we sometimes take the easy route of simply prescribing or refilling scheduled drugs. Instead, we should educate ourselves and our patients about alternative therapeutic interventions for pain control and addiction.
To that end, I encourage all FPs to take the 8-hour online course provided by the American Society of Addiction Medicine to obtain a US Drug Enforcement Administration waiver for prescribing buprenorphine (available at: http://www.asam.org/education/live-online-cme/buprenorphine-course). It costs less than $200 and successful completion of this CME program allows FPs to deliver office-based opioid dependency interventions as per the Drug Addiction Treatment Act of 2000.
Right now, monthly patient censuses indicate that there are about 3234 buprenorphine prescribers providing care for 245,016 opioid-dependent patients, and fewer than 20% of those prescribers are FPs.8 We need to change that. We have an opportunity to invest in the future of these high-risk patients. Let’s not let them down.
1. Democratic staff of the senate committee on finance. Dying waiting for treatment: the opioid use disorder treatment gap and the need for funding. October 10, 2016. Available at: https://www.finance.senate.gov/imo/media/doc/101116%20Opioid%20Treatment%20Gap%20Report%20Final.pdf. Accessed December 14, 2016.
2. Centers for Disease Control and Prevention. Vital signs: overdoses of prescription opioid pain relievers—United States, 1999-2008. MMWR Morb Mortal Wkly Rep. 2011;60:1487-1492.
3. Saloner B, Karthikeyan S. Changes in substance abuse treatment use among individuals with opioid use disorders in the United States. JAMA. 2015;314:1515-1517.
4. Substance Abuse and Mental Health Services Administration. Opioids. Available at: https://www.samhsa.gov/atod/opioids. Accessed December 14, 2016.
5. Vestal C. Waiting lists grow for medicine to fight opioid addiction. Stateline. February 11, 2016. Available at: http://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2016/02/11/waiting-lists-grow-for-medicine-to-fight-opioid-addiction. Accessed December 14, 2016.
6. National Institute on Drug Abuse. Principles of drug addiction treatment: a research-based guide (third edition). Is drug addiction treatment worth its cost? Available at: https://www.drugabuse.gov/publications/principles-drug-addiction-treatment-research-based-guide-third-edition/frequently-asked-questions/drug-addiction-treatment-worth-its-cost. Accessed December 14, 2016.
7. Kleber HD. Pharmacologic treatments for opioid dependence: detoxification and maintenance options. Dialogues Clin Neurosci. 2007; 9:455-470.
8. Stein BD, Sorbero MJ, Dick AW, et al. Physician capacity to treat opioid use disorder with buprenorphine-assisted treatment. JAMA. 2016;316:1211-1212.
Nearly 80 people die every day in America from an opioid overdose.1 At the same time, sales of prescription painkillers have increased 4-fold since 1999.2 My own medical assistant was given an unsolicited prescription for 40 oxycodone after a wisdom tooth extraction.
Meanwhile, about 80% of the country’s 2 million opioid-dependent patients are not receiving the treatment they need.3,4 In Vermont, for example, more than 500 patients are on waiting lists to receive buprenorphine (the partial opioid agonist used to treat opioid addiction)—a wait that for many of them will last for more than a year and may cost them their life.5
Buprenorphine makes good sense. Fortunately, buprenorphine can reverse opioid cravings within minutes. Medication-assisted treatment with buprenorphine derivatives allows patients to lead normal, productive, and stable lives. Every dollar invested in treating opioid addiction saves society $7 in drug-related crime and criminal justice costs.6 In addition, 50% to 80% of opioid-dependent patients remain opioid-free for 12 months while taking buprenorphine.7
Steps we can take. As family physicians (FPs), we are frequently overwhelmed by regulatory concerns, overhead expenses, and providing meaningful use data to third-party payers. And we sometimes take the easy route of simply prescribing or refilling scheduled drugs. Instead, we should educate ourselves and our patients about alternative therapeutic interventions for pain control and addiction.
To that end, I encourage all FPs to take the 8-hour online course provided by the American Society of Addiction Medicine to obtain a US Drug Enforcement Administration waiver for prescribing buprenorphine (available at: http://www.asam.org/education/live-online-cme/buprenorphine-course). It costs less than $200 and successful completion of this CME program allows FPs to deliver office-based opioid dependency interventions as per the Drug Addiction Treatment Act of 2000.
Right now, monthly patient censuses indicate that there are about 3234 buprenorphine prescribers providing care for 245,016 opioid-dependent patients, and fewer than 20% of those prescribers are FPs.8 We need to change that. We have an opportunity to invest in the future of these high-risk patients. Let’s not let them down.
Nearly 80 people die every day in America from an opioid overdose.1 At the same time, sales of prescription painkillers have increased 4-fold since 1999.2 My own medical assistant was given an unsolicited prescription for 40 oxycodone after a wisdom tooth extraction.
Meanwhile, about 80% of the country’s 2 million opioid-dependent patients are not receiving the treatment they need.3,4 In Vermont, for example, more than 500 patients are on waiting lists to receive buprenorphine (the partial opioid agonist used to treat opioid addiction)—a wait that for many of them will last for more than a year and may cost them their life.5
Buprenorphine makes good sense. Fortunately, buprenorphine can reverse opioid cravings within minutes. Medication-assisted treatment with buprenorphine derivatives allows patients to lead normal, productive, and stable lives. Every dollar invested in treating opioid addiction saves society $7 in drug-related crime and criminal justice costs.6 In addition, 50% to 80% of opioid-dependent patients remain opioid-free for 12 months while taking buprenorphine.7
Steps we can take. As family physicians (FPs), we are frequently overwhelmed by regulatory concerns, overhead expenses, and providing meaningful use data to third-party payers. And we sometimes take the easy route of simply prescribing or refilling scheduled drugs. Instead, we should educate ourselves and our patients about alternative therapeutic interventions for pain control and addiction.
To that end, I encourage all FPs to take the 8-hour online course provided by the American Society of Addiction Medicine to obtain a US Drug Enforcement Administration waiver for prescribing buprenorphine (available at: http://www.asam.org/education/live-online-cme/buprenorphine-course). It costs less than $200 and successful completion of this CME program allows FPs to deliver office-based opioid dependency interventions as per the Drug Addiction Treatment Act of 2000.
Right now, monthly patient censuses indicate that there are about 3234 buprenorphine prescribers providing care for 245,016 opioid-dependent patients, and fewer than 20% of those prescribers are FPs.8 We need to change that. We have an opportunity to invest in the future of these high-risk patients. Let’s not let them down.
1. Democratic staff of the senate committee on finance. Dying waiting for treatment: the opioid use disorder treatment gap and the need for funding. October 10, 2016. Available at: https://www.finance.senate.gov/imo/media/doc/101116%20Opioid%20Treatment%20Gap%20Report%20Final.pdf. Accessed December 14, 2016.
2. Centers for Disease Control and Prevention. Vital signs: overdoses of prescription opioid pain relievers—United States, 1999-2008. MMWR Morb Mortal Wkly Rep. 2011;60:1487-1492.
3. Saloner B, Karthikeyan S. Changes in substance abuse treatment use among individuals with opioid use disorders in the United States. JAMA. 2015;314:1515-1517.
4. Substance Abuse and Mental Health Services Administration. Opioids. Available at: https://www.samhsa.gov/atod/opioids. Accessed December 14, 2016.
5. Vestal C. Waiting lists grow for medicine to fight opioid addiction. Stateline. February 11, 2016. Available at: http://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2016/02/11/waiting-lists-grow-for-medicine-to-fight-opioid-addiction. Accessed December 14, 2016.
6. National Institute on Drug Abuse. Principles of drug addiction treatment: a research-based guide (third edition). Is drug addiction treatment worth its cost? Available at: https://www.drugabuse.gov/publications/principles-drug-addiction-treatment-research-based-guide-third-edition/frequently-asked-questions/drug-addiction-treatment-worth-its-cost. Accessed December 14, 2016.
7. Kleber HD. Pharmacologic treatments for opioid dependence: detoxification and maintenance options. Dialogues Clin Neurosci. 2007; 9:455-470.
8. Stein BD, Sorbero MJ, Dick AW, et al. Physician capacity to treat opioid use disorder with buprenorphine-assisted treatment. JAMA. 2016;316:1211-1212.
1. Democratic staff of the senate committee on finance. Dying waiting for treatment: the opioid use disorder treatment gap and the need for funding. October 10, 2016. Available at: https://www.finance.senate.gov/imo/media/doc/101116%20Opioid%20Treatment%20Gap%20Report%20Final.pdf. Accessed December 14, 2016.
2. Centers for Disease Control and Prevention. Vital signs: overdoses of prescription opioid pain relievers—United States, 1999-2008. MMWR Morb Mortal Wkly Rep. 2011;60:1487-1492.
3. Saloner B, Karthikeyan S. Changes in substance abuse treatment use among individuals with opioid use disorders in the United States. JAMA. 2015;314:1515-1517.
4. Substance Abuse and Mental Health Services Administration. Opioids. Available at: https://www.samhsa.gov/atod/opioids. Accessed December 14, 2016.
5. Vestal C. Waiting lists grow for medicine to fight opioid addiction. Stateline. February 11, 2016. Available at: http://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2016/02/11/waiting-lists-grow-for-medicine-to-fight-opioid-addiction. Accessed December 14, 2016.
6. National Institute on Drug Abuse. Principles of drug addiction treatment: a research-based guide (third edition). Is drug addiction treatment worth its cost? Available at: https://www.drugabuse.gov/publications/principles-drug-addiction-treatment-research-based-guide-third-edition/frequently-asked-questions/drug-addiction-treatment-worth-its-cost. Accessed December 14, 2016.
7. Kleber HD. Pharmacologic treatments for opioid dependence: detoxification and maintenance options. Dialogues Clin Neurosci. 2007; 9:455-470.
8. Stein BD, Sorbero MJ, Dick AW, et al. Physician capacity to treat opioid use disorder with buprenorphine-assisted treatment. JAMA. 2016;316:1211-1212.
Need an add-on to metformin? Consider this
ILLUSTRATIVE CASE
A 58-year-old woman with type 2 diabetes mellitus (T2DM) and heart failure returns to your office for follow-up of her T2DM. She has been on the maximum dose of metformin alone for the past 6 months, but her HbA1c is now 7.8%. She is keen to avoid injections. What do you recommend next?
There is surprisingly little consensus about what to add to metformin for patients with T2DM who require a second agent to achieve their glycemic goal. Attainment of glycemic control earlier in the course of the disease may lead to reduced overall cardiovascular risk, so the choice of a second drug is an important one.2 While metformin is well established as initial pharmacotherapy because of its proven mortality benefit, wide availability, and low cost, no second-choice drug has amassed enough evidence of benefit to emerge as the add-on therapy of choice.
Furthermore, the professional societies and associations are of little assistance. Dual therapy recommendations from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes do not denote a specific preference, and while the American Association of Clinical Endocrinologists/American College of Endocrinology do suggest a hierarchy of choices, it is based upon expert consensus recommendation.3,4
Sulfonylureas can cause hypoglycemia and weight gain
Options for add-on therapy include sulfonylureas, thiazolidines, dipeptidyl peptidase-4 (DPP-4) inhibitors, sodium glucose cotransporter 2 (SGLT2) inhibitors, glucagon-like peptide 1 (GLP-1) agonists, and insulin. Providers have frequently prescribed a sulfonylurea after metformin because such agents are low in cost, have long-term safety data, and are effective at lowering HbA1c. Sulfonylureas work by directly stimulating insulin secretion by pancreatic beta cells in a glucose-independent manner. But as a 2010 meta-analysis revealed, they carry significant risks of hypoglycemia (relative risk [RR]=4.57; 95% confidence interval [CI], 2.11-11.45) and weight gain (2.06 kg; 95% CI, 1.15-2.96) compared to placebo.5
DPP-4 inhibitors, on the other hand, work by inducing insulin secretion in a glucose-dependent manner through an incretin mechanism. Combined with metformin, they provide glucose control similar to that achieved with the combination of a sulfonylurea and metformin.6 DPP-4 inhibitors were initially found to be associated with fewer cardiovascular events and less hypoglycemia than sulfonylureas, but were subsequently linked to an increased risk of hospitalization for heart failure.7
This latest large observational study provides more evidence on the effects of DPP-4s when added to metformin.1
STUDY SUMMARY
DPP-4s as effective as sulfonylureas with no increased risks
This population-based observational cohort study compared DPP-4 inhibitors and sulfonylureas when added to metformin for the treatment of T2DM.1 Outcomes were all-cause mortality, major adverse cardiovascular events (MACEs; defined as hospitalization for ischemic stroke or myocardial infarction [MI]), and hospitalizations for either heart failure or hypoglycemia. Using the National Health Insurance Research Database in Taiwan, the study included data on over 70,000 patients ages 20 years and older with a diagnosis of T2DM. Individuals adherent to metformin were considered to be enrolled into the cohort on the day they began using either a DPP-4 inhibitor or a sulfonylurea, in addition to metformin.
The researchers collected additional data on the enrolled individuals regarding socioeconomic factors, urbanization, robustness of the local health care system, Charlson Comorbidity Index, adapted Diabetes Complications Severity Index, and other comorbidities and medications that could affect the outcomes of interest. Using these data, enrollees were matched by propensity score into 10,089 pairs consisting of a DPP-4 inhibitor user and a sulfonylurea user.
After a mean follow-up period of 2.8 years, the authors of the study used Cox regression analysis to evaluate the relative hazards of the outcomes. Subgroup analysis performed by age, sex, Charlson Comorbidity Index, hypertension, chronic kidney disease, hospitalization for heart failure, MI, and cerebrovascular disease yielded results similar to those of the primary analysis for each outcome. Additionally, similar results were obtained when the data were analyzed without propensity-score matching.
The researchers found that users of DPP-4 inhibitors—when compared to users of sulfonylureas—had a lower risk of all-cause mortality (366 vs 488 deaths; hazard ratio [HR]=0.63; 95% CI, 0.55-0.72; number needed to treat [NNT]=117), MACE (209 vs 282 events; HR=0.68; 95% CI, 0.55-0.83; NNT=191), ischemic stroke (144 vs 203 strokes; HR 0.64; 95% CI, 0.51-0.81; NNT=246), and hypoglycemia (89 vs 170 events; HR=0.43; 95% CI, 0.33-0.56; NNT=201). Further, there were no significant differences in either the number of MIs that occurred (69 vs 88 MIs; HR=0.75; 95% CI, 0.52-1.07) or in the number of hospitalizations for heart failure (100 vs 100 events; HR=0.78; 95% CI, 0.57-1.06) between users of DPP-4 inhibitors and those of sulfonylureas.
WHAT’S NEW
Lower risks of death, CV events, and hypoglycemia
This study found that when added to metformin, DPP-4 inhibitors were associated with lower risks for all-cause mortality, cardiovascular events, and hypoglycemia when compared to sulfonylureas. Additionally, DPP-4 inhibitors did not increase the risk of hospitalization for heart failure. A recent multicenter observational study of nearly 1.5 million patients on the effects of incretin-based treatments, including both DPP-4 inhibitors and GLP-1 agonists, similarly found no increased risk of hospitalization for heart failure, with DPP-4 inhibitors compared to other combinations of oral T2DM agents.8
CAVEATS
Did unmeasured confounders play a role?
Unmeasured confounders potentially bias all observational population cohort results. In this study, in particular, there may have been unmeasured, but significant, patient factors that providers used to choose diabetes medications. Also, the study did not evaluate diabetes control, although previous studies have shown similar glucose control between sulfonylureas and DPP-4 inhibitors when they were added to metformin.6
Another caveat is that the results from this study group may not be fully generalizable to other populations due to physiologic differences. People of Asian ancestry are at risk of developing T2DM at a lower body mass index than people of European ancestry, which could affect the outcomes of interest.9
Furthermore, the study did not evaluate outcomes based on whether patients were taking first-, second-, or third-generation sulfonylureas. Some sulfonylureas, such as glyburide, carry a higher risk of hypoglycemia, which could bias the results if a large number of patients were taking them.10
Lastly, the study only provides guidance when choosing between a sulfonylurea and a DPP-4 inhibitor for second-line pharmacotherapy. The GRADE trial, due to be completed in 2023, is comparing sulfonylureas, DPP-4 inhibitors, GLP-1 agonists, and insulin as add-on medications to metformin, and may provide more data on which to base treatment decisions.11
CHALLENGES TO IMPLEMENTATION
DPP-4s have a higher price tag than sulfonylureas
Sulfonylureas and DPP-4 inhibitors are both available as generic medications, but the cost of DPP-4 inhibitors remains significantly higher.12 Higher copays and deductibles could affect patient preference. Furthermore, for patients without health insurance, sulfonylureas are available on the discounted drug lists of many major retailers, while DPP-4 inhibitors are not.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Ou SM, Shih CJ, Chao PW, et al. Effects of clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015;163:663-672.
2. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197-2206.
3. American Diabetes Association. Approaches to glycemic treatment. Sec 7. In Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl. 1):S52-S59. Diabetes Care. 2016; 39:e88-e89.
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes 4. Management Algorithm—2016 Executive Summary. Endocr Pract. 2016;22:84-113.
5. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
6. Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380:475-483.
7. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
8. Filion KB, Azoulay L, Platt RW, et al. A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med. 2016;374:1145-1154.
9. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, pathophysiology. JAMA. 2009;301:2129-2140.
10. Gangji AS, Cukierman T, Gerstein HC, et al. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389-394.
11. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36:2254-2261.
12. GoodRx. Gliptins. Available at: http://www.goodrx.com/gliptins. Accessed August 31, 2016.
ILLUSTRATIVE CASE
A 58-year-old woman with type 2 diabetes mellitus (T2DM) and heart failure returns to your office for follow-up of her T2DM. She has been on the maximum dose of metformin alone for the past 6 months, but her HbA1c is now 7.8%. She is keen to avoid injections. What do you recommend next?
There is surprisingly little consensus about what to add to metformin for patients with T2DM who require a second agent to achieve their glycemic goal. Attainment of glycemic control earlier in the course of the disease may lead to reduced overall cardiovascular risk, so the choice of a second drug is an important one.2 While metformin is well established as initial pharmacotherapy because of its proven mortality benefit, wide availability, and low cost, no second-choice drug has amassed enough evidence of benefit to emerge as the add-on therapy of choice.
Furthermore, the professional societies and associations are of little assistance. Dual therapy recommendations from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes do not denote a specific preference, and while the American Association of Clinical Endocrinologists/American College of Endocrinology do suggest a hierarchy of choices, it is based upon expert consensus recommendation.3,4
Sulfonylureas can cause hypoglycemia and weight gain
Options for add-on therapy include sulfonylureas, thiazolidines, dipeptidyl peptidase-4 (DPP-4) inhibitors, sodium glucose cotransporter 2 (SGLT2) inhibitors, glucagon-like peptide 1 (GLP-1) agonists, and insulin. Providers have frequently prescribed a sulfonylurea after metformin because such agents are low in cost, have long-term safety data, and are effective at lowering HbA1c. Sulfonylureas work by directly stimulating insulin secretion by pancreatic beta cells in a glucose-independent manner. But as a 2010 meta-analysis revealed, they carry significant risks of hypoglycemia (relative risk [RR]=4.57; 95% confidence interval [CI], 2.11-11.45) and weight gain (2.06 kg; 95% CI, 1.15-2.96) compared to placebo.5
DPP-4 inhibitors, on the other hand, work by inducing insulin secretion in a glucose-dependent manner through an incretin mechanism. Combined with metformin, they provide glucose control similar to that achieved with the combination of a sulfonylurea and metformin.6 DPP-4 inhibitors were initially found to be associated with fewer cardiovascular events and less hypoglycemia than sulfonylureas, but were subsequently linked to an increased risk of hospitalization for heart failure.7
This latest large observational study provides more evidence on the effects of DPP-4s when added to metformin.1
STUDY SUMMARY
DPP-4s as effective as sulfonylureas with no increased risks
This population-based observational cohort study compared DPP-4 inhibitors and sulfonylureas when added to metformin for the treatment of T2DM.1 Outcomes were all-cause mortality, major adverse cardiovascular events (MACEs; defined as hospitalization for ischemic stroke or myocardial infarction [MI]), and hospitalizations for either heart failure or hypoglycemia. Using the National Health Insurance Research Database in Taiwan, the study included data on over 70,000 patients ages 20 years and older with a diagnosis of T2DM. Individuals adherent to metformin were considered to be enrolled into the cohort on the day they began using either a DPP-4 inhibitor or a sulfonylurea, in addition to metformin.
The researchers collected additional data on the enrolled individuals regarding socioeconomic factors, urbanization, robustness of the local health care system, Charlson Comorbidity Index, adapted Diabetes Complications Severity Index, and other comorbidities and medications that could affect the outcomes of interest. Using these data, enrollees were matched by propensity score into 10,089 pairs consisting of a DPP-4 inhibitor user and a sulfonylurea user.
After a mean follow-up period of 2.8 years, the authors of the study used Cox regression analysis to evaluate the relative hazards of the outcomes. Subgroup analysis performed by age, sex, Charlson Comorbidity Index, hypertension, chronic kidney disease, hospitalization for heart failure, MI, and cerebrovascular disease yielded results similar to those of the primary analysis for each outcome. Additionally, similar results were obtained when the data were analyzed without propensity-score matching.
The researchers found that users of DPP-4 inhibitors—when compared to users of sulfonylureas—had a lower risk of all-cause mortality (366 vs 488 deaths; hazard ratio [HR]=0.63; 95% CI, 0.55-0.72; number needed to treat [NNT]=117), MACE (209 vs 282 events; HR=0.68; 95% CI, 0.55-0.83; NNT=191), ischemic stroke (144 vs 203 strokes; HR 0.64; 95% CI, 0.51-0.81; NNT=246), and hypoglycemia (89 vs 170 events; HR=0.43; 95% CI, 0.33-0.56; NNT=201). Further, there were no significant differences in either the number of MIs that occurred (69 vs 88 MIs; HR=0.75; 95% CI, 0.52-1.07) or in the number of hospitalizations for heart failure (100 vs 100 events; HR=0.78; 95% CI, 0.57-1.06) between users of DPP-4 inhibitors and those of sulfonylureas.
WHAT’S NEW
Lower risks of death, CV events, and hypoglycemia
This study found that when added to metformin, DPP-4 inhibitors were associated with lower risks for all-cause mortality, cardiovascular events, and hypoglycemia when compared to sulfonylureas. Additionally, DPP-4 inhibitors did not increase the risk of hospitalization for heart failure. A recent multicenter observational study of nearly 1.5 million patients on the effects of incretin-based treatments, including both DPP-4 inhibitors and GLP-1 agonists, similarly found no increased risk of hospitalization for heart failure, with DPP-4 inhibitors compared to other combinations of oral T2DM agents.8
CAVEATS
Did unmeasured confounders play a role?
Unmeasured confounders potentially bias all observational population cohort results. In this study, in particular, there may have been unmeasured, but significant, patient factors that providers used to choose diabetes medications. Also, the study did not evaluate diabetes control, although previous studies have shown similar glucose control between sulfonylureas and DPP-4 inhibitors when they were added to metformin.6
Another caveat is that the results from this study group may not be fully generalizable to other populations due to physiologic differences. People of Asian ancestry are at risk of developing T2DM at a lower body mass index than people of European ancestry, which could affect the outcomes of interest.9
Furthermore, the study did not evaluate outcomes based on whether patients were taking first-, second-, or third-generation sulfonylureas. Some sulfonylureas, such as glyburide, carry a higher risk of hypoglycemia, which could bias the results if a large number of patients were taking them.10
Lastly, the study only provides guidance when choosing between a sulfonylurea and a DPP-4 inhibitor for second-line pharmacotherapy. The GRADE trial, due to be completed in 2023, is comparing sulfonylureas, DPP-4 inhibitors, GLP-1 agonists, and insulin as add-on medications to metformin, and may provide more data on which to base treatment decisions.11
CHALLENGES TO IMPLEMENTATION
DPP-4s have a higher price tag than sulfonylureas
Sulfonylureas and DPP-4 inhibitors are both available as generic medications, but the cost of DPP-4 inhibitors remains significantly higher.12 Higher copays and deductibles could affect patient preference. Furthermore, for patients without health insurance, sulfonylureas are available on the discounted drug lists of many major retailers, while DPP-4 inhibitors are not.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 58-year-old woman with type 2 diabetes mellitus (T2DM) and heart failure returns to your office for follow-up of her T2DM. She has been on the maximum dose of metformin alone for the past 6 months, but her HbA1c is now 7.8%. She is keen to avoid injections. What do you recommend next?
There is surprisingly little consensus about what to add to metformin for patients with T2DM who require a second agent to achieve their glycemic goal. Attainment of glycemic control earlier in the course of the disease may lead to reduced overall cardiovascular risk, so the choice of a second drug is an important one.2 While metformin is well established as initial pharmacotherapy because of its proven mortality benefit, wide availability, and low cost, no second-choice drug has amassed enough evidence of benefit to emerge as the add-on therapy of choice.
Furthermore, the professional societies and associations are of little assistance. Dual therapy recommendations from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes do not denote a specific preference, and while the American Association of Clinical Endocrinologists/American College of Endocrinology do suggest a hierarchy of choices, it is based upon expert consensus recommendation.3,4
Sulfonylureas can cause hypoglycemia and weight gain
Options for add-on therapy include sulfonylureas, thiazolidines, dipeptidyl peptidase-4 (DPP-4) inhibitors, sodium glucose cotransporter 2 (SGLT2) inhibitors, glucagon-like peptide 1 (GLP-1) agonists, and insulin. Providers have frequently prescribed a sulfonylurea after metformin because such agents are low in cost, have long-term safety data, and are effective at lowering HbA1c. Sulfonylureas work by directly stimulating insulin secretion by pancreatic beta cells in a glucose-independent manner. But as a 2010 meta-analysis revealed, they carry significant risks of hypoglycemia (relative risk [RR]=4.57; 95% confidence interval [CI], 2.11-11.45) and weight gain (2.06 kg; 95% CI, 1.15-2.96) compared to placebo.5
DPP-4 inhibitors, on the other hand, work by inducing insulin secretion in a glucose-dependent manner through an incretin mechanism. Combined with metformin, they provide glucose control similar to that achieved with the combination of a sulfonylurea and metformin.6 DPP-4 inhibitors were initially found to be associated with fewer cardiovascular events and less hypoglycemia than sulfonylureas, but were subsequently linked to an increased risk of hospitalization for heart failure.7
This latest large observational study provides more evidence on the effects of DPP-4s when added to metformin.1
STUDY SUMMARY
DPP-4s as effective as sulfonylureas with no increased risks
This population-based observational cohort study compared DPP-4 inhibitors and sulfonylureas when added to metformin for the treatment of T2DM.1 Outcomes were all-cause mortality, major adverse cardiovascular events (MACEs; defined as hospitalization for ischemic stroke or myocardial infarction [MI]), and hospitalizations for either heart failure or hypoglycemia. Using the National Health Insurance Research Database in Taiwan, the study included data on over 70,000 patients ages 20 years and older with a diagnosis of T2DM. Individuals adherent to metformin were considered to be enrolled into the cohort on the day they began using either a DPP-4 inhibitor or a sulfonylurea, in addition to metformin.
The researchers collected additional data on the enrolled individuals regarding socioeconomic factors, urbanization, robustness of the local health care system, Charlson Comorbidity Index, adapted Diabetes Complications Severity Index, and other comorbidities and medications that could affect the outcomes of interest. Using these data, enrollees were matched by propensity score into 10,089 pairs consisting of a DPP-4 inhibitor user and a sulfonylurea user.
After a mean follow-up period of 2.8 years, the authors of the study used Cox regression analysis to evaluate the relative hazards of the outcomes. Subgroup analysis performed by age, sex, Charlson Comorbidity Index, hypertension, chronic kidney disease, hospitalization for heart failure, MI, and cerebrovascular disease yielded results similar to those of the primary analysis for each outcome. Additionally, similar results were obtained when the data were analyzed without propensity-score matching.
The researchers found that users of DPP-4 inhibitors—when compared to users of sulfonylureas—had a lower risk of all-cause mortality (366 vs 488 deaths; hazard ratio [HR]=0.63; 95% CI, 0.55-0.72; number needed to treat [NNT]=117), MACE (209 vs 282 events; HR=0.68; 95% CI, 0.55-0.83; NNT=191), ischemic stroke (144 vs 203 strokes; HR 0.64; 95% CI, 0.51-0.81; NNT=246), and hypoglycemia (89 vs 170 events; HR=0.43; 95% CI, 0.33-0.56; NNT=201). Further, there were no significant differences in either the number of MIs that occurred (69 vs 88 MIs; HR=0.75; 95% CI, 0.52-1.07) or in the number of hospitalizations for heart failure (100 vs 100 events; HR=0.78; 95% CI, 0.57-1.06) between users of DPP-4 inhibitors and those of sulfonylureas.
WHAT’S NEW
Lower risks of death, CV events, and hypoglycemia
This study found that when added to metformin, DPP-4 inhibitors were associated with lower risks for all-cause mortality, cardiovascular events, and hypoglycemia when compared to sulfonylureas. Additionally, DPP-4 inhibitors did not increase the risk of hospitalization for heart failure. A recent multicenter observational study of nearly 1.5 million patients on the effects of incretin-based treatments, including both DPP-4 inhibitors and GLP-1 agonists, similarly found no increased risk of hospitalization for heart failure, with DPP-4 inhibitors compared to other combinations of oral T2DM agents.8
CAVEATS
Did unmeasured confounders play a role?
Unmeasured confounders potentially bias all observational population cohort results. In this study, in particular, there may have been unmeasured, but significant, patient factors that providers used to choose diabetes medications. Also, the study did not evaluate diabetes control, although previous studies have shown similar glucose control between sulfonylureas and DPP-4 inhibitors when they were added to metformin.6
Another caveat is that the results from this study group may not be fully generalizable to other populations due to physiologic differences. People of Asian ancestry are at risk of developing T2DM at a lower body mass index than people of European ancestry, which could affect the outcomes of interest.9
Furthermore, the study did not evaluate outcomes based on whether patients were taking first-, second-, or third-generation sulfonylureas. Some sulfonylureas, such as glyburide, carry a higher risk of hypoglycemia, which could bias the results if a large number of patients were taking them.10
Lastly, the study only provides guidance when choosing between a sulfonylurea and a DPP-4 inhibitor for second-line pharmacotherapy. The GRADE trial, due to be completed in 2023, is comparing sulfonylureas, DPP-4 inhibitors, GLP-1 agonists, and insulin as add-on medications to metformin, and may provide more data on which to base treatment decisions.11
CHALLENGES TO IMPLEMENTATION
DPP-4s have a higher price tag than sulfonylureas
Sulfonylureas and DPP-4 inhibitors are both available as generic medications, but the cost of DPP-4 inhibitors remains significantly higher.12 Higher copays and deductibles could affect patient preference. Furthermore, for patients without health insurance, sulfonylureas are available on the discounted drug lists of many major retailers, while DPP-4 inhibitors are not.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Ou SM, Shih CJ, Chao PW, et al. Effects of clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015;163:663-672.
2. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197-2206.
3. American Diabetes Association. Approaches to glycemic treatment. Sec 7. In Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl. 1):S52-S59. Diabetes Care. 2016; 39:e88-e89.
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes 4. Management Algorithm—2016 Executive Summary. Endocr Pract. 2016;22:84-113.
5. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
6. Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380:475-483.
7. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
8. Filion KB, Azoulay L, Platt RW, et al. A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med. 2016;374:1145-1154.
9. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, pathophysiology. JAMA. 2009;301:2129-2140.
10. Gangji AS, Cukierman T, Gerstein HC, et al. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389-394.
11. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36:2254-2261.
12. GoodRx. Gliptins. Available at: http://www.goodrx.com/gliptins. Accessed August 31, 2016.
1. Ou SM, Shih CJ, Chao PW, et al. Effects of clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015;163:663-672.
2. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197-2206.
3. American Diabetes Association. Approaches to glycemic treatment. Sec 7. In Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl. 1):S52-S59. Diabetes Care. 2016; 39:e88-e89.
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes 4. Management Algorithm—2016 Executive Summary. Endocr Pract. 2016;22:84-113.
5. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
6. Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380:475-483.
7. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
8. Filion KB, Azoulay L, Platt RW, et al. A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med. 2016;374:1145-1154.
9. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, pathophysiology. JAMA. 2009;301:2129-2140.
10. Gangji AS, Cukierman T, Gerstein HC, et al. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389-394.
11. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36:2254-2261.
12. GoodRx. Gliptins. Available at: http://www.goodrx.com/gliptins. Accessed August 31, 2016.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Consider a dipeptidyl peptidase-4 inhibitor before a sulfonylurea for patients with type 2 diabetes mellitus who require therapy in addition to metformin.
Ou SM, Shih CJ, Chao PW, et al. Effects of clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015;163:663-672.1
STRENGTH OF RECOMMENDATION
B: Based on limited-quality, patient-oriented data from a high-quality, population-based cohort study.
Firm, non-tender mass in right breast • worsening, nonproductive cough • pleuritic pain • Dx?
THE CASE
A 44-year-old woman with a 15-year history of type 2 diabetes sought care for a firm, non-tender mass in the medial lower quadrant of her right breast. She hadn’t experienced any skin changes or axillary lymphadenopathy. The patient had immigrated to California from Afghanistan 22 years earlier, at which time she was briefly married to an Afghan man suffering from a chronic cough.
Mammography revealed a 3.5 x 4 x 4 cm lesion at the chest wall, which was highly suspicious for carcinoma (FIGURES 1A AND 1B). Sonography showed a heterogenous hypoechoic and isoechoic mass with posterior acoustic enhancement (FIGURE 1C). An excisional biopsy was performed.
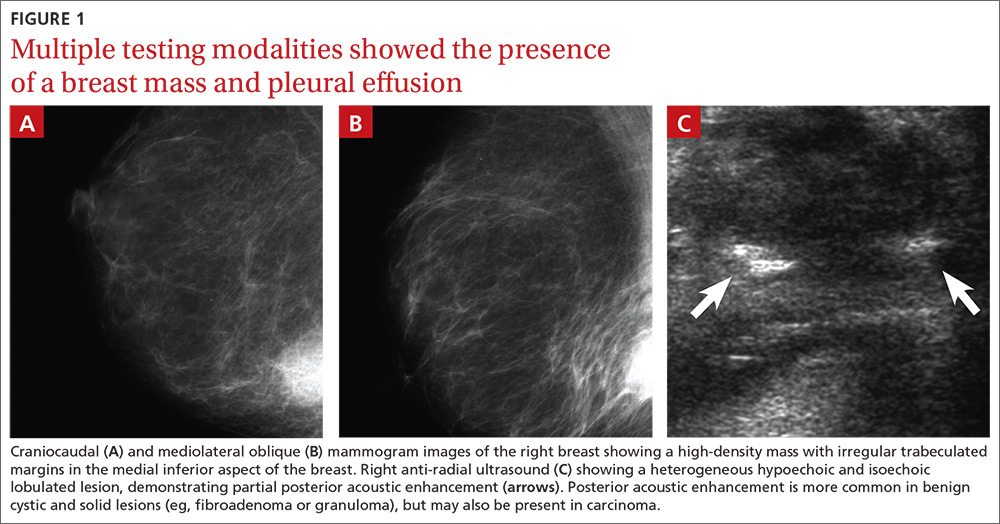
One week postoperatively, the patient presented to the emergency department for a worsening nonproductive cough that intensified when supine, and was associated with subscapular pleuritic pain. She denied fever or weight loss. Biopsy results were pending.
THE DIAGNOSIS
Chest x-rays revealed a large right pleural effusion that was presumed to be malignant (FIGURES 1D AND 1E). Thoracentesis yielded 1.5 liters of tea-colored exudate containing 2800 nucleated cells/mL—63% lymphocytes and 37% neutrophils—and a pleural fluid to serum protein ratio >0.5. Adenosine deaminase was <1 U/L. Fluid Gram stain, acid-fast bacillus (AFB) fluorescent antibody testing, AFB cultures, and cytology were negative. Computed tomography (CT) subsequently demonstrated recurrent effusion without hilar or mediastinal lymphadenopathy or pleural enhancement (FIGURE 1F).
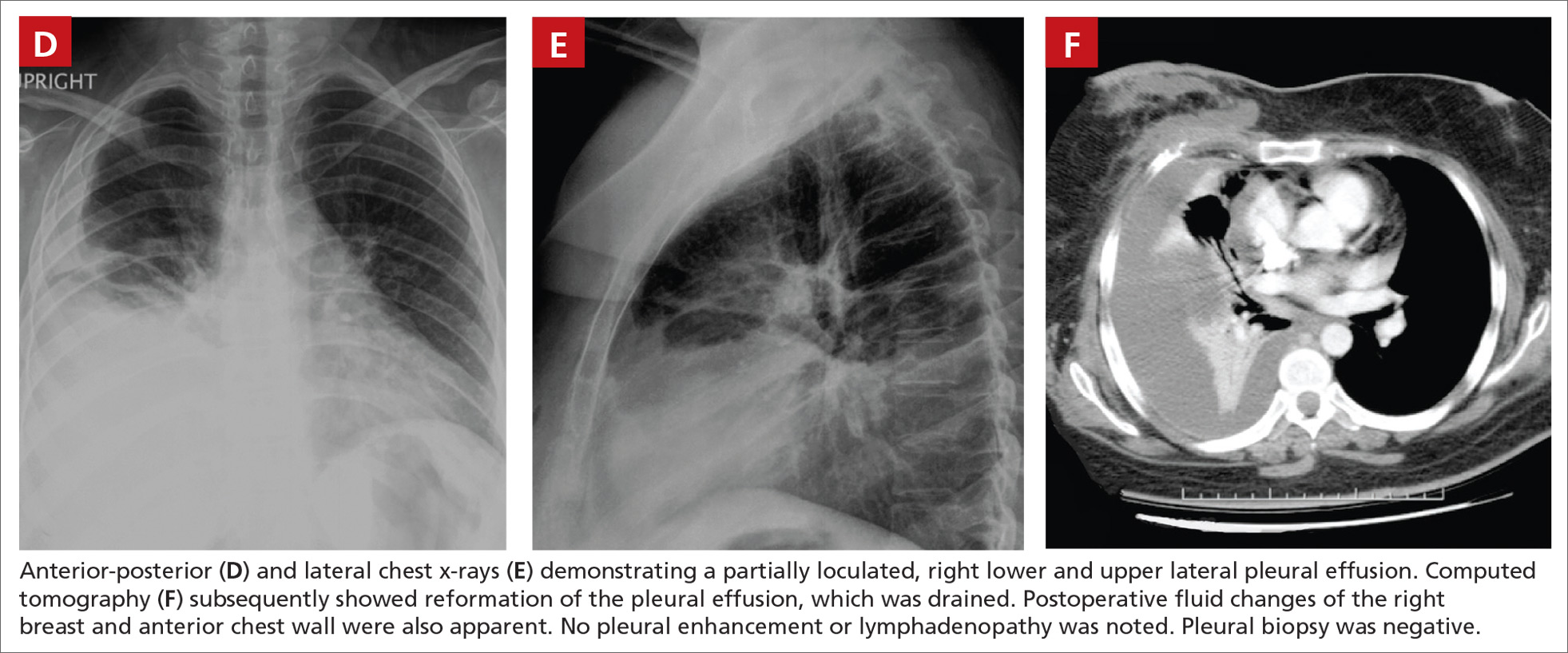
Histologically, the breast mass showed caseating granulomatous inflammation (FIGURES 1G AND 1H). An AFB stain was negative. Polymerase chain reaction (PCR) performed on DNA extracted from the formalin-fixed, paraffin-embedded biopsy material was positive for Mycobacterium tuberculosis.1 A CT-guided pleural biopsy showed only normal tissue. A follow-up tuberculin skin test (purified protein derivative [PPD]) yielded a 10-mm indurated reaction.
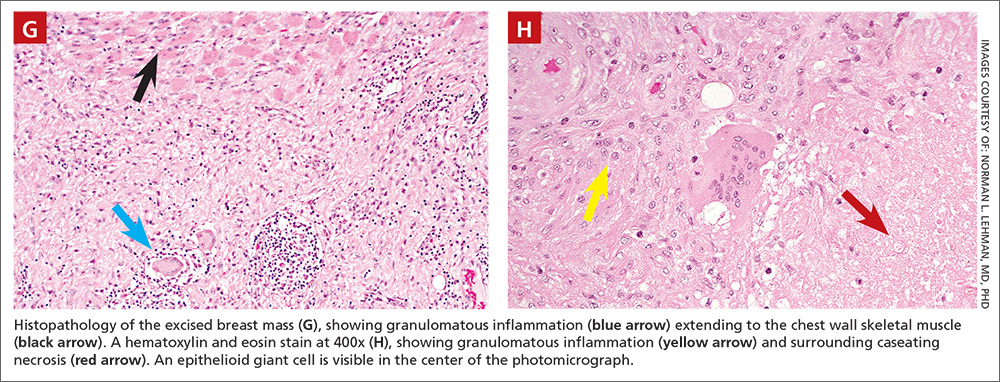
DISCUSSION
Granulomatous lesions, such as foreign body granuloma, idiopathic granulomatous mastitis (IGM), and sarcoidosis can mimic breast carcinoma.2,3 IGM is associated with elevated prolactin (eg, pregnancy or oral contraceptive use) and is usually subareolar.2 Infection, however, is also commonly subareolar. Sarcoidosis rarely exhibits unilateral pleural effusion and usually manifests with bilateral interstitial lung disease, hilar lymphadenopathy, and non-necrotizing granulomas.3,4
M tuberculosis and other granulomatous infections may also feign breast cancer.5-13 Breast TB, which is highly uncommon in the developed world, often demonstrates imaging similar to that which was seen in this case. Breast TB may appear nodular with ill-defined contours. Masses are sometimes attached to the chest wall and usually lack microcalcifications on mammography; they are also typically hypoechoic and heterogenous on ultrasound, often showing posterior enhancement.5,7,8 Like other breast infections, tuberculosis may show cutaneous sinus tract formation, which is seen in about one-third of patients.6,7 Alternatively, it may manifest as a diffuse mastitis with skin thickening and axillary lymphadenopathy.8
Primary breast TB without chest disease comprises up to 86% of mammary tuberculosis.6,7 Infection may occur via contamination of the skin or nipple.5-7 Lactation, pregnancy, and other causes of immunosuppression (especially human immunodeficiency virus) have been associated with an increased risk of breast infection.6-8 This patient was at risk for immunosuppression from longstanding diabetes.14
Many patients from TB-endemic areas have received the bacille Calmette-Guerin (BCG) vaccine and may exhibit equivocal or false-positive PPD results. Because interferon-gamma release assay TB blood tests (eg, QuantiFERON-TB Gold or T-SPOT.TB) are not affected by BCG, they are not associated with false-positive repeat testing results.15
Biopsy is necessary to rule out malignancy and diagnose breast TB
A pleural fluid to serum protein ratio >0.5 is consistent with infection, but also with sarcoidosis or malignancy.3,16 Elevated pleural fluid adenosine deaminase (>40 U/L) is sensitive, albeit nonspecific, for the presence of TB microorganisms. If a lymphocyte-dominant exudate is also present, however, its reliability greatly increases.16,17 Increased pleural fluid interferon-gamma is also sensitive and specific for TB pleurisy.18 Culture, along with drug sensitivity testing, should be performed on all unexplained pleural effusions.
A biopsy is often required to diagnose breast TB and should be performed on all suspicious lesions to exclude malignancy.5-7,9 AFB stains and cultures of aspirate fluids or tissue are often negative.7,9 PCR or other nucleic acid amplification tests of sputum, body fluids, or biopsy material may be positive in culture-negative cases and can rapidly confirm M tuberculosis infection.17,19 No testing modality offers 100% sensitivity or specificity; therefore, an additional confirmatory test is desirable.
Possible routes of transmission include activation of latent pulmonary tuberculosis and direct, lymphatic, or hematologic extension to the chest wall and breast.5-7 In this patient, we believe that activation of a latent breast granuloma may have resulted in a secondary or “sympathetic” pleural effusion, possibly triggered by surgical manipulation. This is compatible with her negative pleural adenosine deaminase result, negative culture, absence of pulmonary parenchymal disease, and negative pleural biopsy. Although we conducted a PubMed search, reviewing material as far back as 1966, we were unable to find a previous case of apparent sympathetic effusion associated with breast TB.
Our patient was treated with daily oral isoniazid, rifabutin, pyrazinamide, and ethambutol for 2 months, followed by isoniazid and rifabutin for 4 months. She has been disease-free for over 10 years.
THE TAKEAWAY
We describe a rare case of breast TB mimicking carcinoma that was associated with unilateral pleural effusion in a woman who had emigrated from Afghanistan. Patients at particular risk for breast TB include immigrants from endemic regions—especially parous females,6,7 those with a history of TB contacts, and those who are immunosuppressed.8 This case emphasizes the need for increased awareness of extrapulmonary TB by physicians in developed countries.
ACKNOWLEDGEMENTS
The authors thank Drs. Margie Scott, Harpreet Dhillon, Samir Vora, Todd Williams, Jeffrey Hawley, and Mr. Sergio Landeros. This report is dedicated to the memory of our friend and colleague in medicine, Dr. Jeanie Care Gillinta.
1. Bayer-Garner IB, Cox MD, Scott MA, et al. Mycobacteria other than Mycobacterium tuberculosis are not present in erythema induratum/nodular vasculitis: a case series and literature review of the clinical and histologic findings. J Cutan Pathol. 2005;32:220-226.
2. Verfaillie G, Breucq C, Sacre R, et al. Granulomatous lobular mastitis: a rare chronic inflammatory disease of the breast which can mimic breast carcinoma. Acta Chir Belg. 2006;106:222-224.
3. Fiorucci F, Conti V, Lucantoni G, et al. Sarcoidosis of the breast: a rare case report and a review. Eur Rev Med Pharmacol Sci. 2006;10:47-50.
4. Huggins JT, Doelken P, Sahn SA, et al. Pleural effusions in a series of 181 outpatients with sarcoidosis. Chest. 2006;129:1599-1604.
5. Zandrino F, Monetti F, Gandolfo N. Primary tuberculosis of the breast. A case report. Acta Radiol. 2000;41:61-63.
6. Khanna R, Prasanna GV, Gupta P, et al. Mammary tuberculosis: report on 52 cases. Postgrad Med J. 2002;78:422-424.
7. Harris SH, Khan MA, Khan R, et al. Mammary tuberculosis: analysis of thirty-eight patients. ANZ J Surg. 2006;76:234-237.
8. Meerkotter D, Spiegel K, Page-Shipp LS. Imaging of tuberculosis of the breast: 21 cases and a review of the literature. J Med Imaging Radiat Oncol. 2011;55:453-460.
9. Khodabakhshi B, Mehravar F. Breast tuberculosis in northeast Iran: review of 22 cases. BMC Womens Health. 2014;14:72.
10. Osborne BM. Granulomatous mastitis caused by histoplasma and mimicking inflammatory breast carcinoma. Hum Pathol. 1989;20:47-52.
11. Bocian JJ, Fahmy RN, Michas CA. A rare case of ‘coccidioidoma’ of the breast. Arch Pathol Lab Med. 1991;115:1064-1067.
12. Haddow LJ, Sahid F, Moosa MY. Cryptococcal breast abscess in an HIV-positive patient: arguments for reviewing the definition of immune reconstitution inflammatory syndrome. J Infect. 2008;57:82-84.
13. Lefkowitz M, Wear DJ. Cat-scratch disease masquerading as a solitary tumor of the breast. Arch Pathol Lab Med. 1989;113:473-475.
14. Ponce-De-Leon A, Garcia-Garcia Md Mde L, Garcia-Sancho MC, et al. Tuberculosis and diabetes in southern Mexico. Diabetes Care. 2004;27:1584-1590.
15. Mazurek GH, LoBue PA, Daley CL, et al. Comparison of a whole-blood interferon gamma assay with tuberculin skin testing for detecting latent Mycobacterium tuberculosis infection. JAMA. 2001;286:1740-1747.
16. Porcel JM, Light RW. Diagnostic approach to pleural effusion in adults. Am Fam Physician. 2006;73:1211-1220.
17. Burgess LJ, Maritz FJ, Le Roux I, et al. Combined use of pleural adenosine deaminase with lymphocyte/neutrophil ratio. Increased specificity for the diagnosis of tuberculous pleuritis. Chest. 1996;109:414-419.
18. Klimiuk J, Krenke R, Safianowska A, et al. Diagnostic performance of different pleural fluid biomarkers in tuberculous pleurisy. Adv Exp Med Biol. 2015;852:21-30.
19. Gopi A, Madhavan SM, Sharma SK, et al. Diagnosis and treatment of tuberculous pleural effusion in 2006. Chest. 2007;131:880-889.
THE CASE
A 44-year-old woman with a 15-year history of type 2 diabetes sought care for a firm, non-tender mass in the medial lower quadrant of her right breast. She hadn’t experienced any skin changes or axillary lymphadenopathy. The patient had immigrated to California from Afghanistan 22 years earlier, at which time she was briefly married to an Afghan man suffering from a chronic cough.
Mammography revealed a 3.5 x 4 x 4 cm lesion at the chest wall, which was highly suspicious for carcinoma (FIGURES 1A AND 1B). Sonography showed a heterogenous hypoechoic and isoechoic mass with posterior acoustic enhancement (FIGURE 1C). An excisional biopsy was performed.

One week postoperatively, the patient presented to the emergency department for a worsening nonproductive cough that intensified when supine, and was associated with subscapular pleuritic pain. She denied fever or weight loss. Biopsy results were pending.
THE DIAGNOSIS
Chest x-rays revealed a large right pleural effusion that was presumed to be malignant (FIGURES 1D AND 1E). Thoracentesis yielded 1.5 liters of tea-colored exudate containing 2800 nucleated cells/mL—63% lymphocytes and 37% neutrophils—and a pleural fluid to serum protein ratio >0.5. Adenosine deaminase was <1 U/L. Fluid Gram stain, acid-fast bacillus (AFB) fluorescent antibody testing, AFB cultures, and cytology were negative. Computed tomography (CT) subsequently demonstrated recurrent effusion without hilar or mediastinal lymphadenopathy or pleural enhancement (FIGURE 1F).

Histologically, the breast mass showed caseating granulomatous inflammation (FIGURES 1G AND 1H). An AFB stain was negative. Polymerase chain reaction (PCR) performed on DNA extracted from the formalin-fixed, paraffin-embedded biopsy material was positive for Mycobacterium tuberculosis.1 A CT-guided pleural biopsy showed only normal tissue. A follow-up tuberculin skin test (purified protein derivative [PPD]) yielded a 10-mm indurated reaction.

DISCUSSION
Granulomatous lesions, such as foreign body granuloma, idiopathic granulomatous mastitis (IGM), and sarcoidosis can mimic breast carcinoma.2,3 IGM is associated with elevated prolactin (eg, pregnancy or oral contraceptive use) and is usually subareolar.2 Infection, however, is also commonly subareolar. Sarcoidosis rarely exhibits unilateral pleural effusion and usually manifests with bilateral interstitial lung disease, hilar lymphadenopathy, and non-necrotizing granulomas.3,4
M tuberculosis and other granulomatous infections may also feign breast cancer.5-13 Breast TB, which is highly uncommon in the developed world, often demonstrates imaging similar to that which was seen in this case. Breast TB may appear nodular with ill-defined contours. Masses are sometimes attached to the chest wall and usually lack microcalcifications on mammography; they are also typically hypoechoic and heterogenous on ultrasound, often showing posterior enhancement.5,7,8 Like other breast infections, tuberculosis may show cutaneous sinus tract formation, which is seen in about one-third of patients.6,7 Alternatively, it may manifest as a diffuse mastitis with skin thickening and axillary lymphadenopathy.8
Primary breast TB without chest disease comprises up to 86% of mammary tuberculosis.6,7 Infection may occur via contamination of the skin or nipple.5-7 Lactation, pregnancy, and other causes of immunosuppression (especially human immunodeficiency virus) have been associated with an increased risk of breast infection.6-8 This patient was at risk for immunosuppression from longstanding diabetes.14
Many patients from TB-endemic areas have received the bacille Calmette-Guerin (BCG) vaccine and may exhibit equivocal or false-positive PPD results. Because interferon-gamma release assay TB blood tests (eg, QuantiFERON-TB Gold or T-SPOT.TB) are not affected by BCG, they are not associated with false-positive repeat testing results.15
Biopsy is necessary to rule out malignancy and diagnose breast TB
A pleural fluid to serum protein ratio >0.5 is consistent with infection, but also with sarcoidosis or malignancy.3,16 Elevated pleural fluid adenosine deaminase (>40 U/L) is sensitive, albeit nonspecific, for the presence of TB microorganisms. If a lymphocyte-dominant exudate is also present, however, its reliability greatly increases.16,17 Increased pleural fluid interferon-gamma is also sensitive and specific for TB pleurisy.18 Culture, along with drug sensitivity testing, should be performed on all unexplained pleural effusions.
A biopsy is often required to diagnose breast TB and should be performed on all suspicious lesions to exclude malignancy.5-7,9 AFB stains and cultures of aspirate fluids or tissue are often negative.7,9 PCR or other nucleic acid amplification tests of sputum, body fluids, or biopsy material may be positive in culture-negative cases and can rapidly confirm M tuberculosis infection.17,19 No testing modality offers 100% sensitivity or specificity; therefore, an additional confirmatory test is desirable.
Possible routes of transmission include activation of latent pulmonary tuberculosis and direct, lymphatic, or hematologic extension to the chest wall and breast.5-7 In this patient, we believe that activation of a latent breast granuloma may have resulted in a secondary or “sympathetic” pleural effusion, possibly triggered by surgical manipulation. This is compatible with her negative pleural adenosine deaminase result, negative culture, absence of pulmonary parenchymal disease, and negative pleural biopsy. Although we conducted a PubMed search, reviewing material as far back as 1966, we were unable to find a previous case of apparent sympathetic effusion associated with breast TB.
Our patient was treated with daily oral isoniazid, rifabutin, pyrazinamide, and ethambutol for 2 months, followed by isoniazid and rifabutin for 4 months. She has been disease-free for over 10 years.
THE TAKEAWAY
We describe a rare case of breast TB mimicking carcinoma that was associated with unilateral pleural effusion in a woman who had emigrated from Afghanistan. Patients at particular risk for breast TB include immigrants from endemic regions—especially parous females,6,7 those with a history of TB contacts, and those who are immunosuppressed.8 This case emphasizes the need for increased awareness of extrapulmonary TB by physicians in developed countries.
ACKNOWLEDGEMENTS
The authors thank Drs. Margie Scott, Harpreet Dhillon, Samir Vora, Todd Williams, Jeffrey Hawley, and Mr. Sergio Landeros. This report is dedicated to the memory of our friend and colleague in medicine, Dr. Jeanie Care Gillinta.
THE CASE
A 44-year-old woman with a 15-year history of type 2 diabetes sought care for a firm, non-tender mass in the medial lower quadrant of her right breast. She hadn’t experienced any skin changes or axillary lymphadenopathy. The patient had immigrated to California from Afghanistan 22 years earlier, at which time she was briefly married to an Afghan man suffering from a chronic cough.
Mammography revealed a 3.5 x 4 x 4 cm lesion at the chest wall, which was highly suspicious for carcinoma (FIGURES 1A AND 1B). Sonography showed a heterogenous hypoechoic and isoechoic mass with posterior acoustic enhancement (FIGURE 1C). An excisional biopsy was performed.

One week postoperatively, the patient presented to the emergency department for a worsening nonproductive cough that intensified when supine, and was associated with subscapular pleuritic pain. She denied fever or weight loss. Biopsy results were pending.
THE DIAGNOSIS
Chest x-rays revealed a large right pleural effusion that was presumed to be malignant (FIGURES 1D AND 1E). Thoracentesis yielded 1.5 liters of tea-colored exudate containing 2800 nucleated cells/mL—63% lymphocytes and 37% neutrophils—and a pleural fluid to serum protein ratio >0.5. Adenosine deaminase was <1 U/L. Fluid Gram stain, acid-fast bacillus (AFB) fluorescent antibody testing, AFB cultures, and cytology were negative. Computed tomography (CT) subsequently demonstrated recurrent effusion without hilar or mediastinal lymphadenopathy or pleural enhancement (FIGURE 1F).

Histologically, the breast mass showed caseating granulomatous inflammation (FIGURES 1G AND 1H). An AFB stain was negative. Polymerase chain reaction (PCR) performed on DNA extracted from the formalin-fixed, paraffin-embedded biopsy material was positive for Mycobacterium tuberculosis.1 A CT-guided pleural biopsy showed only normal tissue. A follow-up tuberculin skin test (purified protein derivative [PPD]) yielded a 10-mm indurated reaction.

DISCUSSION
Granulomatous lesions, such as foreign body granuloma, idiopathic granulomatous mastitis (IGM), and sarcoidosis can mimic breast carcinoma.2,3 IGM is associated with elevated prolactin (eg, pregnancy or oral contraceptive use) and is usually subareolar.2 Infection, however, is also commonly subareolar. Sarcoidosis rarely exhibits unilateral pleural effusion and usually manifests with bilateral interstitial lung disease, hilar lymphadenopathy, and non-necrotizing granulomas.3,4
M tuberculosis and other granulomatous infections may also feign breast cancer.5-13 Breast TB, which is highly uncommon in the developed world, often demonstrates imaging similar to that which was seen in this case. Breast TB may appear nodular with ill-defined contours. Masses are sometimes attached to the chest wall and usually lack microcalcifications on mammography; they are also typically hypoechoic and heterogenous on ultrasound, often showing posterior enhancement.5,7,8 Like other breast infections, tuberculosis may show cutaneous sinus tract formation, which is seen in about one-third of patients.6,7 Alternatively, it may manifest as a diffuse mastitis with skin thickening and axillary lymphadenopathy.8
Primary breast TB without chest disease comprises up to 86% of mammary tuberculosis.6,7 Infection may occur via contamination of the skin or nipple.5-7 Lactation, pregnancy, and other causes of immunosuppression (especially human immunodeficiency virus) have been associated with an increased risk of breast infection.6-8 This patient was at risk for immunosuppression from longstanding diabetes.14
Many patients from TB-endemic areas have received the bacille Calmette-Guerin (BCG) vaccine and may exhibit equivocal or false-positive PPD results. Because interferon-gamma release assay TB blood tests (eg, QuantiFERON-TB Gold or T-SPOT.TB) are not affected by BCG, they are not associated with false-positive repeat testing results.15
Biopsy is necessary to rule out malignancy and diagnose breast TB
A pleural fluid to serum protein ratio >0.5 is consistent with infection, but also with sarcoidosis or malignancy.3,16 Elevated pleural fluid adenosine deaminase (>40 U/L) is sensitive, albeit nonspecific, for the presence of TB microorganisms. If a lymphocyte-dominant exudate is also present, however, its reliability greatly increases.16,17 Increased pleural fluid interferon-gamma is also sensitive and specific for TB pleurisy.18 Culture, along with drug sensitivity testing, should be performed on all unexplained pleural effusions.
A biopsy is often required to diagnose breast TB and should be performed on all suspicious lesions to exclude malignancy.5-7,9 AFB stains and cultures of aspirate fluids or tissue are often negative.7,9 PCR or other nucleic acid amplification tests of sputum, body fluids, or biopsy material may be positive in culture-negative cases and can rapidly confirm M tuberculosis infection.17,19 No testing modality offers 100% sensitivity or specificity; therefore, an additional confirmatory test is desirable.
Possible routes of transmission include activation of latent pulmonary tuberculosis and direct, lymphatic, or hematologic extension to the chest wall and breast.5-7 In this patient, we believe that activation of a latent breast granuloma may have resulted in a secondary or “sympathetic” pleural effusion, possibly triggered by surgical manipulation. This is compatible with her negative pleural adenosine deaminase result, negative culture, absence of pulmonary parenchymal disease, and negative pleural biopsy. Although we conducted a PubMed search, reviewing material as far back as 1966, we were unable to find a previous case of apparent sympathetic effusion associated with breast TB.
Our patient was treated with daily oral isoniazid, rifabutin, pyrazinamide, and ethambutol for 2 months, followed by isoniazid and rifabutin for 4 months. She has been disease-free for over 10 years.
THE TAKEAWAY
We describe a rare case of breast TB mimicking carcinoma that was associated with unilateral pleural effusion in a woman who had emigrated from Afghanistan. Patients at particular risk for breast TB include immigrants from endemic regions—especially parous females,6,7 those with a history of TB contacts, and those who are immunosuppressed.8 This case emphasizes the need for increased awareness of extrapulmonary TB by physicians in developed countries.
ACKNOWLEDGEMENTS
The authors thank Drs. Margie Scott, Harpreet Dhillon, Samir Vora, Todd Williams, Jeffrey Hawley, and Mr. Sergio Landeros. This report is dedicated to the memory of our friend and colleague in medicine, Dr. Jeanie Care Gillinta.
1. Bayer-Garner IB, Cox MD, Scott MA, et al. Mycobacteria other than Mycobacterium tuberculosis are not present in erythema induratum/nodular vasculitis: a case series and literature review of the clinical and histologic findings. J Cutan Pathol. 2005;32:220-226.
2. Verfaillie G, Breucq C, Sacre R, et al. Granulomatous lobular mastitis: a rare chronic inflammatory disease of the breast which can mimic breast carcinoma. Acta Chir Belg. 2006;106:222-224.
3. Fiorucci F, Conti V, Lucantoni G, et al. Sarcoidosis of the breast: a rare case report and a review. Eur Rev Med Pharmacol Sci. 2006;10:47-50.
4. Huggins JT, Doelken P, Sahn SA, et al. Pleural effusions in a series of 181 outpatients with sarcoidosis. Chest. 2006;129:1599-1604.
5. Zandrino F, Monetti F, Gandolfo N. Primary tuberculosis of the breast. A case report. Acta Radiol. 2000;41:61-63.
6. Khanna R, Prasanna GV, Gupta P, et al. Mammary tuberculosis: report on 52 cases. Postgrad Med J. 2002;78:422-424.
7. Harris SH, Khan MA, Khan R, et al. Mammary tuberculosis: analysis of thirty-eight patients. ANZ J Surg. 2006;76:234-237.
8. Meerkotter D, Spiegel K, Page-Shipp LS. Imaging of tuberculosis of the breast: 21 cases and a review of the literature. J Med Imaging Radiat Oncol. 2011;55:453-460.
9. Khodabakhshi B, Mehravar F. Breast tuberculosis in northeast Iran: review of 22 cases. BMC Womens Health. 2014;14:72.
10. Osborne BM. Granulomatous mastitis caused by histoplasma and mimicking inflammatory breast carcinoma. Hum Pathol. 1989;20:47-52.
11. Bocian JJ, Fahmy RN, Michas CA. A rare case of ‘coccidioidoma’ of the breast. Arch Pathol Lab Med. 1991;115:1064-1067.
12. Haddow LJ, Sahid F, Moosa MY. Cryptococcal breast abscess in an HIV-positive patient: arguments for reviewing the definition of immune reconstitution inflammatory syndrome. J Infect. 2008;57:82-84.
13. Lefkowitz M, Wear DJ. Cat-scratch disease masquerading as a solitary tumor of the breast. Arch Pathol Lab Med. 1989;113:473-475.
14. Ponce-De-Leon A, Garcia-Garcia Md Mde L, Garcia-Sancho MC, et al. Tuberculosis and diabetes in southern Mexico. Diabetes Care. 2004;27:1584-1590.
15. Mazurek GH, LoBue PA, Daley CL, et al. Comparison of a whole-blood interferon gamma assay with tuberculin skin testing for detecting latent Mycobacterium tuberculosis infection. JAMA. 2001;286:1740-1747.
16. Porcel JM, Light RW. Diagnostic approach to pleural effusion in adults. Am Fam Physician. 2006;73:1211-1220.
17. Burgess LJ, Maritz FJ, Le Roux I, et al. Combined use of pleural adenosine deaminase with lymphocyte/neutrophil ratio. Increased specificity for the diagnosis of tuberculous pleuritis. Chest. 1996;109:414-419.
18. Klimiuk J, Krenke R, Safianowska A, et al. Diagnostic performance of different pleural fluid biomarkers in tuberculous pleurisy. Adv Exp Med Biol. 2015;852:21-30.
19. Gopi A, Madhavan SM, Sharma SK, et al. Diagnosis and treatment of tuberculous pleural effusion in 2006. Chest. 2007;131:880-889.
1. Bayer-Garner IB, Cox MD, Scott MA, et al. Mycobacteria other than Mycobacterium tuberculosis are not present in erythema induratum/nodular vasculitis: a case series and literature review of the clinical and histologic findings. J Cutan Pathol. 2005;32:220-226.
2. Verfaillie G, Breucq C, Sacre R, et al. Granulomatous lobular mastitis: a rare chronic inflammatory disease of the breast which can mimic breast carcinoma. Acta Chir Belg. 2006;106:222-224.
3. Fiorucci F, Conti V, Lucantoni G, et al. Sarcoidosis of the breast: a rare case report and a review. Eur Rev Med Pharmacol Sci. 2006;10:47-50.
4. Huggins JT, Doelken P, Sahn SA, et al. Pleural effusions in a series of 181 outpatients with sarcoidosis. Chest. 2006;129:1599-1604.
5. Zandrino F, Monetti F, Gandolfo N. Primary tuberculosis of the breast. A case report. Acta Radiol. 2000;41:61-63.
6. Khanna R, Prasanna GV, Gupta P, et al. Mammary tuberculosis: report on 52 cases. Postgrad Med J. 2002;78:422-424.
7. Harris SH, Khan MA, Khan R, et al. Mammary tuberculosis: analysis of thirty-eight patients. ANZ J Surg. 2006;76:234-237.
8. Meerkotter D, Spiegel K, Page-Shipp LS. Imaging of tuberculosis of the breast: 21 cases and a review of the literature. J Med Imaging Radiat Oncol. 2011;55:453-460.
9. Khodabakhshi B, Mehravar F. Breast tuberculosis in northeast Iran: review of 22 cases. BMC Womens Health. 2014;14:72.
10. Osborne BM. Granulomatous mastitis caused by histoplasma and mimicking inflammatory breast carcinoma. Hum Pathol. 1989;20:47-52.
11. Bocian JJ, Fahmy RN, Michas CA. A rare case of ‘coccidioidoma’ of the breast. Arch Pathol Lab Med. 1991;115:1064-1067.
12. Haddow LJ, Sahid F, Moosa MY. Cryptococcal breast abscess in an HIV-positive patient: arguments for reviewing the definition of immune reconstitution inflammatory syndrome. J Infect. 2008;57:82-84.
13. Lefkowitz M, Wear DJ. Cat-scratch disease masquerading as a solitary tumor of the breast. Arch Pathol Lab Med. 1989;113:473-475.
14. Ponce-De-Leon A, Garcia-Garcia Md Mde L, Garcia-Sancho MC, et al. Tuberculosis and diabetes in southern Mexico. Diabetes Care. 2004;27:1584-1590.
15. Mazurek GH, LoBue PA, Daley CL, et al. Comparison of a whole-blood interferon gamma assay with tuberculin skin testing for detecting latent Mycobacterium tuberculosis infection. JAMA. 2001;286:1740-1747.
16. Porcel JM, Light RW. Diagnostic approach to pleural effusion in adults. Am Fam Physician. 2006;73:1211-1220.
17. Burgess LJ, Maritz FJ, Le Roux I, et al. Combined use of pleural adenosine deaminase with lymphocyte/neutrophil ratio. Increased specificity for the diagnosis of tuberculous pleuritis. Chest. 1996;109:414-419.
18. Klimiuk J, Krenke R, Safianowska A, et al. Diagnostic performance of different pleural fluid biomarkers in tuberculous pleurisy. Adv Exp Med Biol. 2015;852:21-30.
19. Gopi A, Madhavan SM, Sharma SK, et al. Diagnosis and treatment of tuberculous pleural effusion in 2006. Chest. 2007;131:880-889.
Malodorous discharge, redness, and crusting of the feet
A 50-year-old man who worked in construction presented to a local urgent care facility complaining of 2 weeks of bilateral foot discomfort associated with local malodorous discharge, redness, and crusting. He had a past medical history of recurrent tinea pedis and had previously been treated with intravenous (IV) antibiotics for a severe episode of cellulitis. He denied recent fever, trauma, or swelling. Physical examination revealed extensive malodorous crusting of the interdigital webs of both feet, in addition to tenderness, erythema, and serous discharge. He was treated with topical clotrimazole and oral terbinafine for a presumed tinea pedis recurrence; cephalexin and triamcinolone were added a week later after minimal response. Two days later, he sought care at a local emergency department with progressive cellulitis, bullae formation, and extensive desquamation (FIGURES 1A AND 1B) and was hospitalized.
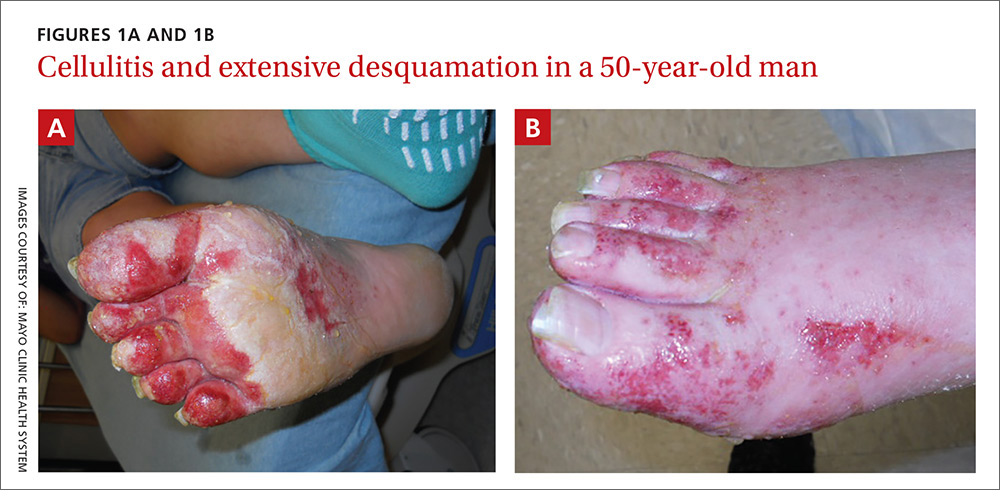
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Gram-negative foot intertrigo
Plain x-rays and computed tomography imaging did not reveal evidence of abscess formation, subcutaneous gas/air formation, or osteomyelitis. Laboratory testing was normal. The patient was empirically started on cefepime, clindamycin, and ketoconazole. Wound therapy, consisting of normal saline washes, use of xeroform gauze to cover the wound, and frequent absorbent dressing changes, was also initiated. Wound cultures were obtained, which later grew Pseudomonas aeruginosa and Enterococcus faecalis. (P aeruginosa was the predominant organism.) We made the diagnosis of gram-negative foot intertrigo.
First described in 1973, researchers have found that P aeruginosa, as well as E faecalis and Staphylococcus aureus, are commonly associated with tinea pedis1 and toe web intertrigo.2 The infection usually involves the lateral 3 toe webs, and can present with malodorous discharge, itchy maceration, edema, and erythema of the surrounding tissue. Non-purulent lower extremity cellulitis is typically caused by beta-hemolytic streptococci residing in the toe webs, and is usually treated empirically with cephalexin or similar antibiotics.3 The presentation of foot intertrigo varies along a spectrum that includes tinea pedis, superinfected maceration, and infectious eczematoid dermatitis, all of which can encourage bacterial superinfections.
In this patient, it is likely that sweating (and consequent skin maceration), tinea pedis, and perhaps even friction between the affected area and the patient’s shoes led to skin ulceration. This then became colonized and infected with bacteria, including gram-negative varieties, which sometimes harbor at the edges of ulcerations. Addressing each of these factors is important to heal the infection.
Differential diagnosis includes other bacterial skin infections
The differential diagnosis includes erysipelas, cellulitis, lipodermatosclerosis, venous eczema, and burns. Erysipelas affects the superficial dermis with well demarcated borders, while cellulitis involves the subcutaneous fat.4 Both are more likely to be caused by beta-hemolytic streptococci, but at first may be difficult to differentiate from similar appearing gram-negative skin/soft tissue infection without microbiologic data.
Patients with lipodermatosclerosis and venous eczema often have a history of chronic venous insufficiency. Lipodermatosclerosis often presents with a subcutaneous panniculitis and hyperpigmentation; venous eczema is often associated with scaling of the involved areas. Although burns can become secondarily superinfected with bacteria, they can be differentiated from a primary bacterial infection by the history or presentation.
Gram-negative infections (especially those caused by Pseudomonas species) should be suspected if a toe web infection does not respond to empiric antimicrobial therapy with first- or second-generation cephalosporins, as their spectrum of antimicrobial activity does not include P aeruginosa. If the infection is severe, it may impact ambulation.2 Predisposing factors for gram-negative toe web infections include obesity, diabetes, moist environments, tight interdigital spaces, and recurrent tinea pedis.4
Administer appropriate wound care and start antimicrobial therapy
Foot intertrigo provides an easy portal of entry for pathogenic organisms.5 Therefore, it is important to modify risk factors from the outset to help prevent superinfection (as occurred with this patient) and other complications. Aggressive treatment of tinea pedis and use of compression stockings to reduce lymphedema are vital for prevention of recurrent infections.3
Physicians should be aware of likely, as well as unlikely, causative pathogens; “typical” skin and soft tissue infections that do not resolve may be due to atypical or gram-negative organisms, and typical first-line antibiotics will do nothing to eradicate them. Antimicrobial susceptibilities and a bacterial culture will steer you to the appropriate antimicrobial therapy. Debridement of the edge of the ulceration is necessary to remove any lingering bacteria.6 And appropriate wound care is paramount and should include the use of techniques to keep the affected areas dry, such as the use of astringent powders along with avoidance of damp shoes and socks.
Our patient was switched from empiric therapy to culture-specific IV therapy with vancomycin 2 g every 12 hours, ciprofloxacin 400 mg every 12 hours, and fluconazole 400 mg daily. Two weeks later, he was discharged from the hospital on topical antifungals, oral fluconazole, and daily acetic acid soaks. He did, however, require further advanced topical treatments (miconazole 2% twice daily) due to recurrent flare-ups before complete resolution was achieved 6 weeks after presentation (FIGURES 2A AND 2B).
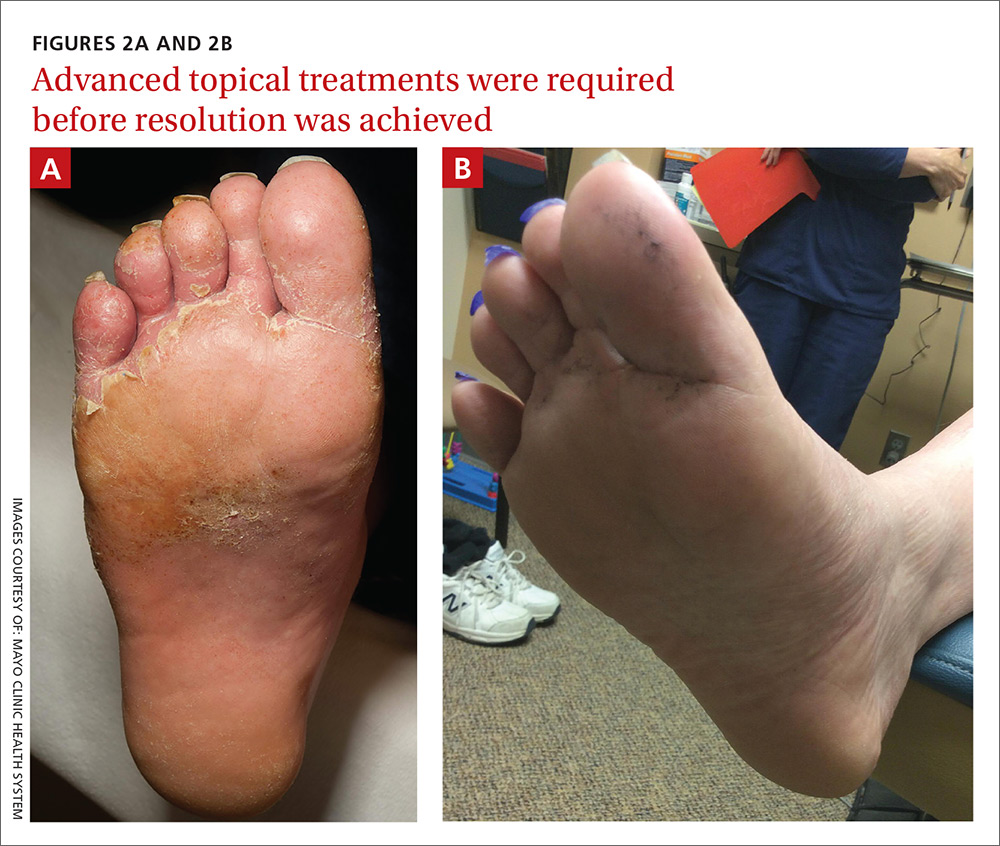
CORRESPONDENCE
Alberto Marcelin, MD, Department of Family Medicine, Mayo Clinic Health System, 1000 1st Dr NW, Austin, MN 55912; [email protected].
1. Westmoreland TA, Ross EV, Yeager JK. Pseudomonas toe web infections. Cutis. 1992;49:185-186.
2. Lin JY, Shih YL, Ho HC. Foot bacterial intertrigo mimicking interdigital tinea pedis. Chang Gung Med J. 2011;34:44-49.
3. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:e10-e52.
4. Hirschmann JV, Raugi GJ. Lower limb cellulitis and its mimics: part II. Conditions that simulate lower limb cellulitis. J Am Acad Dermatol. 2012;67:177, e1-e9; quiz 185-186.
5. Semel JD, Goldin H. Association of athlete’s foot with cellulitis of the lower extremities: diagnostic value of bacterial cultures of ipsilateral interdigital space samples. Clin Infect Dis. 1996;23:1162-1164.
6. Fangman W, Burton C. Hyperkeratotic rim of gram-negative toe web infections. Arch Dermatol. 2005;141:658.
A 50-year-old man who worked in construction presented to a local urgent care facility complaining of 2 weeks of bilateral foot discomfort associated with local malodorous discharge, redness, and crusting. He had a past medical history of recurrent tinea pedis and had previously been treated with intravenous (IV) antibiotics for a severe episode of cellulitis. He denied recent fever, trauma, or swelling. Physical examination revealed extensive malodorous crusting of the interdigital webs of both feet, in addition to tenderness, erythema, and serous discharge. He was treated with topical clotrimazole and oral terbinafine for a presumed tinea pedis recurrence; cephalexin and triamcinolone were added a week later after minimal response. Two days later, he sought care at a local emergency department with progressive cellulitis, bullae formation, and extensive desquamation (FIGURES 1A AND 1B) and was hospitalized.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Gram-negative foot intertrigo
Plain x-rays and computed tomography imaging did not reveal evidence of abscess formation, subcutaneous gas/air formation, or osteomyelitis. Laboratory testing was normal. The patient was empirically started on cefepime, clindamycin, and ketoconazole. Wound therapy, consisting of normal saline washes, use of xeroform gauze to cover the wound, and frequent absorbent dressing changes, was also initiated. Wound cultures were obtained, which later grew Pseudomonas aeruginosa and Enterococcus faecalis. (P aeruginosa was the predominant organism.) We made the diagnosis of gram-negative foot intertrigo.
First described in 1973, researchers have found that P aeruginosa, as well as E faecalis and Staphylococcus aureus, are commonly associated with tinea pedis1 and toe web intertrigo.2 The infection usually involves the lateral 3 toe webs, and can present with malodorous discharge, itchy maceration, edema, and erythema of the surrounding tissue. Non-purulent lower extremity cellulitis is typically caused by beta-hemolytic streptococci residing in the toe webs, and is usually treated empirically with cephalexin or similar antibiotics.3 The presentation of foot intertrigo varies along a spectrum that includes tinea pedis, superinfected maceration, and infectious eczematoid dermatitis, all of which can encourage bacterial superinfections.
In this patient, it is likely that sweating (and consequent skin maceration), tinea pedis, and perhaps even friction between the affected area and the patient’s shoes led to skin ulceration. This then became colonized and infected with bacteria, including gram-negative varieties, which sometimes harbor at the edges of ulcerations. Addressing each of these factors is important to heal the infection.
Differential diagnosis includes other bacterial skin infections
The differential diagnosis includes erysipelas, cellulitis, lipodermatosclerosis, venous eczema, and burns. Erysipelas affects the superficial dermis with well demarcated borders, while cellulitis involves the subcutaneous fat.4 Both are more likely to be caused by beta-hemolytic streptococci, but at first may be difficult to differentiate from similar appearing gram-negative skin/soft tissue infection without microbiologic data.
Patients with lipodermatosclerosis and venous eczema often have a history of chronic venous insufficiency. Lipodermatosclerosis often presents with a subcutaneous panniculitis and hyperpigmentation; venous eczema is often associated with scaling of the involved areas. Although burns can become secondarily superinfected with bacteria, they can be differentiated from a primary bacterial infection by the history or presentation.
Gram-negative infections (especially those caused by Pseudomonas species) should be suspected if a toe web infection does not respond to empiric antimicrobial therapy with first- or second-generation cephalosporins, as their spectrum of antimicrobial activity does not include P aeruginosa. If the infection is severe, it may impact ambulation.2 Predisposing factors for gram-negative toe web infections include obesity, diabetes, moist environments, tight interdigital spaces, and recurrent tinea pedis.4
Administer appropriate wound care and start antimicrobial therapy
Foot intertrigo provides an easy portal of entry for pathogenic organisms.5 Therefore, it is important to modify risk factors from the outset to help prevent superinfection (as occurred with this patient) and other complications. Aggressive treatment of tinea pedis and use of compression stockings to reduce lymphedema are vital for prevention of recurrent infections.3
Physicians should be aware of likely, as well as unlikely, causative pathogens; “typical” skin and soft tissue infections that do not resolve may be due to atypical or gram-negative organisms, and typical first-line antibiotics will do nothing to eradicate them. Antimicrobial susceptibilities and a bacterial culture will steer you to the appropriate antimicrobial therapy. Debridement of the edge of the ulceration is necessary to remove any lingering bacteria.6 And appropriate wound care is paramount and should include the use of techniques to keep the affected areas dry, such as the use of astringent powders along with avoidance of damp shoes and socks.
Our patient was switched from empiric therapy to culture-specific IV therapy with vancomycin 2 g every 12 hours, ciprofloxacin 400 mg every 12 hours, and fluconazole 400 mg daily. Two weeks later, he was discharged from the hospital on topical antifungals, oral fluconazole, and daily acetic acid soaks. He did, however, require further advanced topical treatments (miconazole 2% twice daily) due to recurrent flare-ups before complete resolution was achieved 6 weeks after presentation (FIGURES 2A AND 2B).

CORRESPONDENCE
Alberto Marcelin, MD, Department of Family Medicine, Mayo Clinic Health System, 1000 1st Dr NW, Austin, MN 55912; [email protected].
A 50-year-old man who worked in construction presented to a local urgent care facility complaining of 2 weeks of bilateral foot discomfort associated with local malodorous discharge, redness, and crusting. He had a past medical history of recurrent tinea pedis and had previously been treated with intravenous (IV) antibiotics for a severe episode of cellulitis. He denied recent fever, trauma, or swelling. Physical examination revealed extensive malodorous crusting of the interdigital webs of both feet, in addition to tenderness, erythema, and serous discharge. He was treated with topical clotrimazole and oral terbinafine for a presumed tinea pedis recurrence; cephalexin and triamcinolone were added a week later after minimal response. Two days later, he sought care at a local emergency department with progressive cellulitis, bullae formation, and extensive desquamation (FIGURES 1A AND 1B) and was hospitalized.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Gram-negative foot intertrigo
Plain x-rays and computed tomography imaging did not reveal evidence of abscess formation, subcutaneous gas/air formation, or osteomyelitis. Laboratory testing was normal. The patient was empirically started on cefepime, clindamycin, and ketoconazole. Wound therapy, consisting of normal saline washes, use of xeroform gauze to cover the wound, and frequent absorbent dressing changes, was also initiated. Wound cultures were obtained, which later grew Pseudomonas aeruginosa and Enterococcus faecalis. (P aeruginosa was the predominant organism.) We made the diagnosis of gram-negative foot intertrigo.
First described in 1973, researchers have found that P aeruginosa, as well as E faecalis and Staphylococcus aureus, are commonly associated with tinea pedis1 and toe web intertrigo.2 The infection usually involves the lateral 3 toe webs, and can present with malodorous discharge, itchy maceration, edema, and erythema of the surrounding tissue. Non-purulent lower extremity cellulitis is typically caused by beta-hemolytic streptococci residing in the toe webs, and is usually treated empirically with cephalexin or similar antibiotics.3 The presentation of foot intertrigo varies along a spectrum that includes tinea pedis, superinfected maceration, and infectious eczematoid dermatitis, all of which can encourage bacterial superinfections.
In this patient, it is likely that sweating (and consequent skin maceration), tinea pedis, and perhaps even friction between the affected area and the patient’s shoes led to skin ulceration. This then became colonized and infected with bacteria, including gram-negative varieties, which sometimes harbor at the edges of ulcerations. Addressing each of these factors is important to heal the infection.
Differential diagnosis includes other bacterial skin infections
The differential diagnosis includes erysipelas, cellulitis, lipodermatosclerosis, venous eczema, and burns. Erysipelas affects the superficial dermis with well demarcated borders, while cellulitis involves the subcutaneous fat.4 Both are more likely to be caused by beta-hemolytic streptococci, but at first may be difficult to differentiate from similar appearing gram-negative skin/soft tissue infection without microbiologic data.
Patients with lipodermatosclerosis and venous eczema often have a history of chronic venous insufficiency. Lipodermatosclerosis often presents with a subcutaneous panniculitis and hyperpigmentation; venous eczema is often associated with scaling of the involved areas. Although burns can become secondarily superinfected with bacteria, they can be differentiated from a primary bacterial infection by the history or presentation.
Gram-negative infections (especially those caused by Pseudomonas species) should be suspected if a toe web infection does not respond to empiric antimicrobial therapy with first- or second-generation cephalosporins, as their spectrum of antimicrobial activity does not include P aeruginosa. If the infection is severe, it may impact ambulation.2 Predisposing factors for gram-negative toe web infections include obesity, diabetes, moist environments, tight interdigital spaces, and recurrent tinea pedis.4
Administer appropriate wound care and start antimicrobial therapy
Foot intertrigo provides an easy portal of entry for pathogenic organisms.5 Therefore, it is important to modify risk factors from the outset to help prevent superinfection (as occurred with this patient) and other complications. Aggressive treatment of tinea pedis and use of compression stockings to reduce lymphedema are vital for prevention of recurrent infections.3
Physicians should be aware of likely, as well as unlikely, causative pathogens; “typical” skin and soft tissue infections that do not resolve may be due to atypical or gram-negative organisms, and typical first-line antibiotics will do nothing to eradicate them. Antimicrobial susceptibilities and a bacterial culture will steer you to the appropriate antimicrobial therapy. Debridement of the edge of the ulceration is necessary to remove any lingering bacteria.6 And appropriate wound care is paramount and should include the use of techniques to keep the affected areas dry, such as the use of astringent powders along with avoidance of damp shoes and socks.
Our patient was switched from empiric therapy to culture-specific IV therapy with vancomycin 2 g every 12 hours, ciprofloxacin 400 mg every 12 hours, and fluconazole 400 mg daily. Two weeks later, he was discharged from the hospital on topical antifungals, oral fluconazole, and daily acetic acid soaks. He did, however, require further advanced topical treatments (miconazole 2% twice daily) due to recurrent flare-ups before complete resolution was achieved 6 weeks after presentation (FIGURES 2A AND 2B).

CORRESPONDENCE
Alberto Marcelin, MD, Department of Family Medicine, Mayo Clinic Health System, 1000 1st Dr NW, Austin, MN 55912; [email protected].
1. Westmoreland TA, Ross EV, Yeager JK. Pseudomonas toe web infections. Cutis. 1992;49:185-186.
2. Lin JY, Shih YL, Ho HC. Foot bacterial intertrigo mimicking interdigital tinea pedis. Chang Gung Med J. 2011;34:44-49.
3. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:e10-e52.
4. Hirschmann JV, Raugi GJ. Lower limb cellulitis and its mimics: part II. Conditions that simulate lower limb cellulitis. J Am Acad Dermatol. 2012;67:177, e1-e9; quiz 185-186.
5. Semel JD, Goldin H. Association of athlete’s foot with cellulitis of the lower extremities: diagnostic value of bacterial cultures of ipsilateral interdigital space samples. Clin Infect Dis. 1996;23:1162-1164.
6. Fangman W, Burton C. Hyperkeratotic rim of gram-negative toe web infections. Arch Dermatol. 2005;141:658.
1. Westmoreland TA, Ross EV, Yeager JK. Pseudomonas toe web infections. Cutis. 1992;49:185-186.
2. Lin JY, Shih YL, Ho HC. Foot bacterial intertrigo mimicking interdigital tinea pedis. Chang Gung Med J. 2011;34:44-49.
3. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:e10-e52.
4. Hirschmann JV, Raugi GJ. Lower limb cellulitis and its mimics: part II. Conditions that simulate lower limb cellulitis. J Am Acad Dermatol. 2012;67:177, e1-e9; quiz 185-186.
5. Semel JD, Goldin H. Association of athlete’s foot with cellulitis of the lower extremities: diagnostic value of bacterial cultures of ipsilateral interdigital space samples. Clin Infect Dis. 1996;23:1162-1164.
6. Fangman W, Burton C. Hyperkeratotic rim of gram-negative toe web infections. Arch Dermatol. 2005;141:658.
Rash on eyebrows and periumbilical region
An 8-year-old girl was brought to her family physician’s office (RU) because of a persistent rash on her lateral eyebrows and periumbilical region. The family indicated that she’d had the rash for more than 6 months. They also mentioned that the child had received a new pair of eyeglasses 8 months earlier. The child was otherwise in good health. The physical examination revealed erythematous scaling plaques near both lateral eyebrows and around the belly button (FIGURES 1 AND 2).
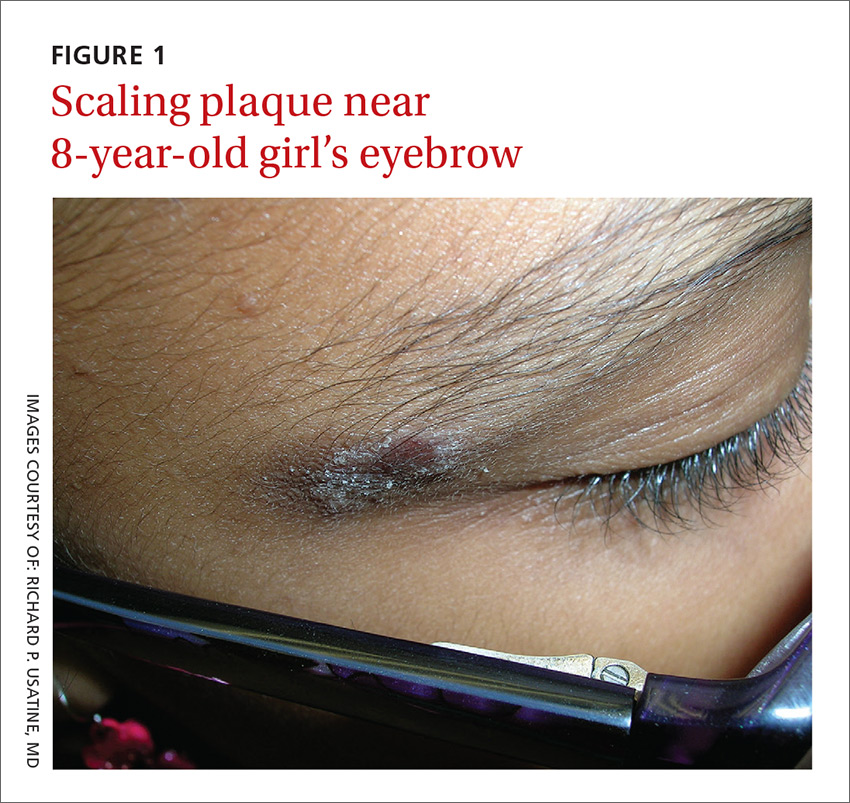
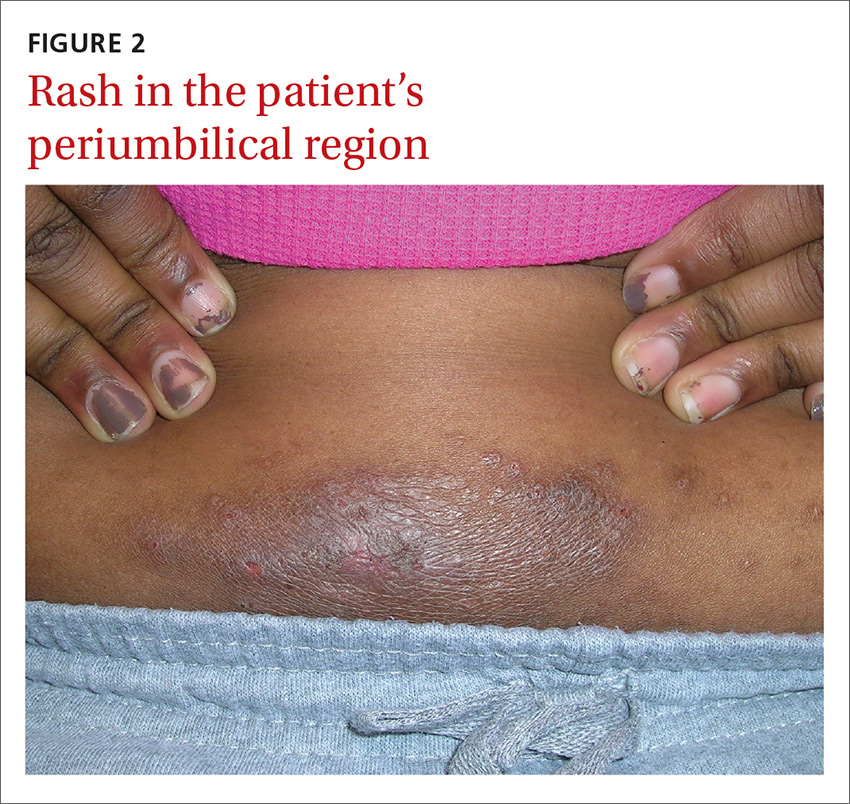
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Allergic contact dermatitis
We recognized that this was a case of allergic contact dermatitis (ACD), based on the clinical presentation. The distribution of the erythema, scale, and postinflammatory hyperpigmentation was highly suggestive of an ACD to nickel. In this case, the nickel in the patient’s eyeglasses and the snaps found on her pants were the culprits.
The lichenification of the plaque near the umbilicus suggested that the dermatitis was not acute and that the patient had likely been scratching the area due to itching. The plaque near the patient’s eye was actually in the shape of the metal on the inside of her glasses.
Most prevalent contact allergens. Patch testing data indicate that the 5 most prevalent contact allergens out of more than 3700 that are known are: nickel (14.3% of patients tested), fragrance mix (14%), the topical antibiotic neomycin (11.6%), balsam of Peru (used in some perfumes, toiletries, and pharmaceutical items) (10.4%), and the mercury-based vaccine preservative thimerosal (10.4%).1
ACD is a delayed-type hypersensitivity reaction in which a foreign substance comes into contact with the skin and is linked to skin proteins forming an antigen complex that leads to sensitization. When the epidermis is re-exposed to the antigen, the sensitized T cells initiate an inflammatory cascade, leading to the skin changes seen in ACD.2
Silverberg et al reported that in 30 children with a personal history of umbilical or wrist dermatitis or a family history of nickel ACD, 100% demonstrated a positive reaction to nickel sulfate.3 Nickel continues to be used (and unregulated) in a wide range of products, including costume jewelry, piercing posts, belt buckles, eyeglasses, and personal electronics (eg, tablets, cell phones, and laptop computers).
Making the diagnosis. Contact dermatitis can sometimes be diagnosed clinically with a good history and physical exam. However, there are many cases in which patch testing is needed to find the offending allergens or confirm the suspicion regarding a specific allergen. The only convenient and ready-to-use patch test in the United States is the T.R.U.E. test.
The differential includes other superficial skin infections
ACD characteristically presents with eczematoid plaques that are primarily in the area(s) of cutaneous contact with an allergen. The condition typically appears within a few days of exposure.
The differential diagnosis for ACD includes cutaneous candidiasis, impetigo, plaque psoriasis, and seborrheic dermatitis.
Cutaneous candidiasis is a superficial infection of the skin with a candida species. It can present as beefy red erythematous plaques on the buttocks, lower abdomen, thighs, or in intertriginous areas or oral commissures. A hallmark sign is pinpoint pustulovesicular satellite lesions.
Impetigo is a superficial bacterial skin infection that presents with edema, erythema, tenderness on palpation, and possible purulent drainage. It appears as honey-colored crusts with erythema and occurs most often on the face—especially around a child’s nose and mouth—but can occur anywhere on the head and body.
Plaque psoriasis presents as erythematous silver-scaled plaques on extensor surfaces, including the elbows and knees. Inverse psoriasis may present as erythema and maceration in intertriginous areas.
Seborrheic dermatitis appears as well-circumscribed greasy scale overlying erythematous skin. It is commonly found on the scalp, eyebrows, nasolabial folds, chest, face, and in the ear canals. It is thought to be an inflammatory reaction to Malassezia furfur.
Cool compresses, topical steroids can relieve symptoms
Patients with ACD should avoid the allergen that is causing the reaction. In cases of nickel ACD, the patient may cover the metal tab of their jeans with an iron-on patch or a few coats of clear nail polish. A better option is to buy jeans and pants that do not have nickel in the metal tab. (Levi’s has removed nickel from their pants.) Cool compresses can soothe the symptoms of acute cases of ACD.4 Calamine and colloidal oatmeal baths may help to dry and soothe acute, oozing lesions. Localized acute ACD lesions respond best to mid-potency (eg, 0.1% triamcinolone) to high-potency (eg, 0.05% clobetasol) topical steroids.4
On areas of thinner skin (eg, flexural surfaces, eyelids, face, anogenital region), lower-potency steroids such as desonide ointment can minimize the risk of skin atrophy.3,4 Be aware that some patients are actually allergic to topical steroids. This unfortunate situation can be diagnosed with patch testing.
We recommended that our patient get different glasses that were nickel-free. Fortunately, there are many frames for glasses that have no nickel in them. We also gave her advice on how to avoid the nickel that still exists in some pants. We gave her desonide 0.05% cream to apply to the affected area for symptomatic relief.
CORRESPONDENCE
Richard P. Usatine, MD, Skin clinic, University of Texas Health Science Center at San Antonio, 903 W. Martin, San Antonio, TX 78207; [email protected].
1. Krob HA, Fleischer AB Jr, D’Agostino R Jr, et al. Prevalence and relevance of contact dermatitis allergens: a meta-analysis of 15 years of published T.R.U.E. test data. J Am Acad Dermatol. 2004;51:349-353.
2. Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
3. Silverberg NB, Licht J, Friedler S, et al. Nickel contact hypersensitivity in children. Pediatr Dermatol. 2002;19:110-113.
4. Beltrani VS, Bernstein IL, Cohen DE, et al. Contact dermatitis: a practice parameter. Ann Allergy Asthma Immunol. 2006;97:S1-S38.
An 8-year-old girl was brought to her family physician’s office (RU) because of a persistent rash on her lateral eyebrows and periumbilical region. The family indicated that she’d had the rash for more than 6 months. They also mentioned that the child had received a new pair of eyeglasses 8 months earlier. The child was otherwise in good health. The physical examination revealed erythematous scaling plaques near both lateral eyebrows and around the belly button (FIGURES 1 AND 2).


WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Allergic contact dermatitis
We recognized that this was a case of allergic contact dermatitis (ACD), based on the clinical presentation. The distribution of the erythema, scale, and postinflammatory hyperpigmentation was highly suggestive of an ACD to nickel. In this case, the nickel in the patient’s eyeglasses and the snaps found on her pants were the culprits.
The lichenification of the plaque near the umbilicus suggested that the dermatitis was not acute and that the patient had likely been scratching the area due to itching. The plaque near the patient’s eye was actually in the shape of the metal on the inside of her glasses.
Most prevalent contact allergens. Patch testing data indicate that the 5 most prevalent contact allergens out of more than 3700 that are known are: nickel (14.3% of patients tested), fragrance mix (14%), the topical antibiotic neomycin (11.6%), balsam of Peru (used in some perfumes, toiletries, and pharmaceutical items) (10.4%), and the mercury-based vaccine preservative thimerosal (10.4%).1
ACD is a delayed-type hypersensitivity reaction in which a foreign substance comes into contact with the skin and is linked to skin proteins forming an antigen complex that leads to sensitization. When the epidermis is re-exposed to the antigen, the sensitized T cells initiate an inflammatory cascade, leading to the skin changes seen in ACD.2
Silverberg et al reported that in 30 children with a personal history of umbilical or wrist dermatitis or a family history of nickel ACD, 100% demonstrated a positive reaction to nickel sulfate.3 Nickel continues to be used (and unregulated) in a wide range of products, including costume jewelry, piercing posts, belt buckles, eyeglasses, and personal electronics (eg, tablets, cell phones, and laptop computers).
Making the diagnosis. Contact dermatitis can sometimes be diagnosed clinically with a good history and physical exam. However, there are many cases in which patch testing is needed to find the offending allergens or confirm the suspicion regarding a specific allergen. The only convenient and ready-to-use patch test in the United States is the T.R.U.E. test.
The differential includes other superficial skin infections
ACD characteristically presents with eczematoid plaques that are primarily in the area(s) of cutaneous contact with an allergen. The condition typically appears within a few days of exposure.
The differential diagnosis for ACD includes cutaneous candidiasis, impetigo, plaque psoriasis, and seborrheic dermatitis.
Cutaneous candidiasis is a superficial infection of the skin with a candida species. It can present as beefy red erythematous plaques on the buttocks, lower abdomen, thighs, or in intertriginous areas or oral commissures. A hallmark sign is pinpoint pustulovesicular satellite lesions.
Impetigo is a superficial bacterial skin infection that presents with edema, erythema, tenderness on palpation, and possible purulent drainage. It appears as honey-colored crusts with erythema and occurs most often on the face—especially around a child’s nose and mouth—but can occur anywhere on the head and body.
Plaque psoriasis presents as erythematous silver-scaled plaques on extensor surfaces, including the elbows and knees. Inverse psoriasis may present as erythema and maceration in intertriginous areas.
Seborrheic dermatitis appears as well-circumscribed greasy scale overlying erythematous skin. It is commonly found on the scalp, eyebrows, nasolabial folds, chest, face, and in the ear canals. It is thought to be an inflammatory reaction to Malassezia furfur.
Cool compresses, topical steroids can relieve symptoms
Patients with ACD should avoid the allergen that is causing the reaction. In cases of nickel ACD, the patient may cover the metal tab of their jeans with an iron-on patch or a few coats of clear nail polish. A better option is to buy jeans and pants that do not have nickel in the metal tab. (Levi’s has removed nickel from their pants.) Cool compresses can soothe the symptoms of acute cases of ACD.4 Calamine and colloidal oatmeal baths may help to dry and soothe acute, oozing lesions. Localized acute ACD lesions respond best to mid-potency (eg, 0.1% triamcinolone) to high-potency (eg, 0.05% clobetasol) topical steroids.4
On areas of thinner skin (eg, flexural surfaces, eyelids, face, anogenital region), lower-potency steroids such as desonide ointment can minimize the risk of skin atrophy.3,4 Be aware that some patients are actually allergic to topical steroids. This unfortunate situation can be diagnosed with patch testing.
We recommended that our patient get different glasses that were nickel-free. Fortunately, there are many frames for glasses that have no nickel in them. We also gave her advice on how to avoid the nickel that still exists in some pants. We gave her desonide 0.05% cream to apply to the affected area for symptomatic relief.
CORRESPONDENCE
Richard P. Usatine, MD, Skin clinic, University of Texas Health Science Center at San Antonio, 903 W. Martin, San Antonio, TX 78207; [email protected].
An 8-year-old girl was brought to her family physician’s office (RU) because of a persistent rash on her lateral eyebrows and periumbilical region. The family indicated that she’d had the rash for more than 6 months. They also mentioned that the child had received a new pair of eyeglasses 8 months earlier. The child was otherwise in good health. The physical examination revealed erythematous scaling plaques near both lateral eyebrows and around the belly button (FIGURES 1 AND 2).


WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Allergic contact dermatitis
We recognized that this was a case of allergic contact dermatitis (ACD), based on the clinical presentation. The distribution of the erythema, scale, and postinflammatory hyperpigmentation was highly suggestive of an ACD to nickel. In this case, the nickel in the patient’s eyeglasses and the snaps found on her pants were the culprits.
The lichenification of the plaque near the umbilicus suggested that the dermatitis was not acute and that the patient had likely been scratching the area due to itching. The plaque near the patient’s eye was actually in the shape of the metal on the inside of her glasses.
Most prevalent contact allergens. Patch testing data indicate that the 5 most prevalent contact allergens out of more than 3700 that are known are: nickel (14.3% of patients tested), fragrance mix (14%), the topical antibiotic neomycin (11.6%), balsam of Peru (used in some perfumes, toiletries, and pharmaceutical items) (10.4%), and the mercury-based vaccine preservative thimerosal (10.4%).1
ACD is a delayed-type hypersensitivity reaction in which a foreign substance comes into contact with the skin and is linked to skin proteins forming an antigen complex that leads to sensitization. When the epidermis is re-exposed to the antigen, the sensitized T cells initiate an inflammatory cascade, leading to the skin changes seen in ACD.2
Silverberg et al reported that in 30 children with a personal history of umbilical or wrist dermatitis or a family history of nickel ACD, 100% demonstrated a positive reaction to nickel sulfate.3 Nickel continues to be used (and unregulated) in a wide range of products, including costume jewelry, piercing posts, belt buckles, eyeglasses, and personal electronics (eg, tablets, cell phones, and laptop computers).
Making the diagnosis. Contact dermatitis can sometimes be diagnosed clinically with a good history and physical exam. However, there are many cases in which patch testing is needed to find the offending allergens or confirm the suspicion regarding a specific allergen. The only convenient and ready-to-use patch test in the United States is the T.R.U.E. test.
The differential includes other superficial skin infections
ACD characteristically presents with eczematoid plaques that are primarily in the area(s) of cutaneous contact with an allergen. The condition typically appears within a few days of exposure.
The differential diagnosis for ACD includes cutaneous candidiasis, impetigo, plaque psoriasis, and seborrheic dermatitis.
Cutaneous candidiasis is a superficial infection of the skin with a candida species. It can present as beefy red erythematous plaques on the buttocks, lower abdomen, thighs, or in intertriginous areas or oral commissures. A hallmark sign is pinpoint pustulovesicular satellite lesions.
Impetigo is a superficial bacterial skin infection that presents with edema, erythema, tenderness on palpation, and possible purulent drainage. It appears as honey-colored crusts with erythema and occurs most often on the face—especially around a child’s nose and mouth—but can occur anywhere on the head and body.
Plaque psoriasis presents as erythematous silver-scaled plaques on extensor surfaces, including the elbows and knees. Inverse psoriasis may present as erythema and maceration in intertriginous areas.
Seborrheic dermatitis appears as well-circumscribed greasy scale overlying erythematous skin. It is commonly found on the scalp, eyebrows, nasolabial folds, chest, face, and in the ear canals. It is thought to be an inflammatory reaction to Malassezia furfur.
Cool compresses, topical steroids can relieve symptoms
Patients with ACD should avoid the allergen that is causing the reaction. In cases of nickel ACD, the patient may cover the metal tab of their jeans with an iron-on patch or a few coats of clear nail polish. A better option is to buy jeans and pants that do not have nickel in the metal tab. (Levi’s has removed nickel from their pants.) Cool compresses can soothe the symptoms of acute cases of ACD.4 Calamine and colloidal oatmeal baths may help to dry and soothe acute, oozing lesions. Localized acute ACD lesions respond best to mid-potency (eg, 0.1% triamcinolone) to high-potency (eg, 0.05% clobetasol) topical steroids.4
On areas of thinner skin (eg, flexural surfaces, eyelids, face, anogenital region), lower-potency steroids such as desonide ointment can minimize the risk of skin atrophy.3,4 Be aware that some patients are actually allergic to topical steroids. This unfortunate situation can be diagnosed with patch testing.
We recommended that our patient get different glasses that were nickel-free. Fortunately, there are many frames for glasses that have no nickel in them. We also gave her advice on how to avoid the nickel that still exists in some pants. We gave her desonide 0.05% cream to apply to the affected area for symptomatic relief.
CORRESPONDENCE
Richard P. Usatine, MD, Skin clinic, University of Texas Health Science Center at San Antonio, 903 W. Martin, San Antonio, TX 78207; [email protected].
1. Krob HA, Fleischer AB Jr, D’Agostino R Jr, et al. Prevalence and relevance of contact dermatitis allergens: a meta-analysis of 15 years of published T.R.U.E. test data. J Am Acad Dermatol. 2004;51:349-353.
2. Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
3. Silverberg NB, Licht J, Friedler S, et al. Nickel contact hypersensitivity in children. Pediatr Dermatol. 2002;19:110-113.
4. Beltrani VS, Bernstein IL, Cohen DE, et al. Contact dermatitis: a practice parameter. Ann Allergy Asthma Immunol. 2006;97:S1-S38.
1. Krob HA, Fleischer AB Jr, D’Agostino R Jr, et al. Prevalence and relevance of contact dermatitis allergens: a meta-analysis of 15 years of published T.R.U.E. test data. J Am Acad Dermatol. 2004;51:349-353.
2. Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
3. Silverberg NB, Licht J, Friedler S, et al. Nickel contact hypersensitivity in children. Pediatr Dermatol. 2002;19:110-113.
4. Beltrani VS, Bernstein IL, Cohen DE, et al. Contact dermatitis: a practice parameter. Ann Allergy Asthma Immunol. 2006;97:S1-S38.