User login
January 2017: Click for Credit
Here are 5 articles in the January issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Gluten-free Adherence Triples While Celiac Disease Prevalence Remains Stable
To take the posttest, go to: http://bit.ly/2h2LFDu
Expires September 6, 2017
2. Fluoxetine Appears Safer for Bone Health in At-risk Older Patients
To take the posttest, go to: http://bit.ly/2he1FTD
Expires September 15, 2017
3. High Free T4 Levels Linked to Sudden Cardiac Death
To take the posttest, go to: http://bit.ly/2gMJqUz
Expires September 16, 2017
4. Morning Sickness Linked to Lower Risk for Pregnancy Loss
To take the posttest, go to: http://bit.ly/2uaWMkH
Expires September 26, 2017
5. Anxiety, Depression May Precede Parkinson's by 25 Years
To take the posttest, go to: http://bit.ly/2gMFQtr
Expires September 27, 2017
Here are 5 articles in the January issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Gluten-free Adherence Triples While Celiac Disease Prevalence Remains Stable
To take the posttest, go to: http://bit.ly/2h2LFDu
Expires September 6, 2017
2. Fluoxetine Appears Safer for Bone Health in At-risk Older Patients
To take the posttest, go to: http://bit.ly/2he1FTD
Expires September 15, 2017
3. High Free T4 Levels Linked to Sudden Cardiac Death
To take the posttest, go to: http://bit.ly/2gMJqUz
Expires September 16, 2017
4. Morning Sickness Linked to Lower Risk for Pregnancy Loss
To take the posttest, go to: http://bit.ly/2uaWMkH
Expires September 26, 2017
5. Anxiety, Depression May Precede Parkinson's by 25 Years
To take the posttest, go to: http://bit.ly/2gMFQtr
Expires September 27, 2017
Here are 5 articles in the January issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Gluten-free Adherence Triples While Celiac Disease Prevalence Remains Stable
To take the posttest, go to: http://bit.ly/2h2LFDu
Expires September 6, 2017
2. Fluoxetine Appears Safer for Bone Health in At-risk Older Patients
To take the posttest, go to: http://bit.ly/2he1FTD
Expires September 15, 2017
3. High Free T4 Levels Linked to Sudden Cardiac Death
To take the posttest, go to: http://bit.ly/2gMJqUz
Expires September 16, 2017
4. Morning Sickness Linked to Lower Risk for Pregnancy Loss
To take the posttest, go to: http://bit.ly/2uaWMkH
Expires September 26, 2017
5. Anxiety, Depression May Precede Parkinson's by 25 Years
To take the posttest, go to: http://bit.ly/2gMFQtr
Expires September 27, 2017
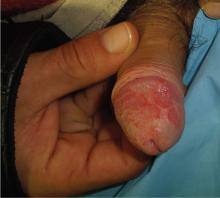
Did mother’s allergic reaction cause fetal injury?
Did mother’s allergic reaction cause fetal injury?
When a mother was admitted to the labor and delivery unit, she had strep throat; ampicillin was administered. She experienced anaphylactic symptoms that were attended to. The baby, delivered vaginally 3 hours later, was severely distressed and showed signs of asphyxia. He was found to have a permanent brain injury.
PARENTS’ CLAIM:
The ObGyn and hospital nurses failed to properly manage the mother’s anaphylactic reaction to ampicillin. Fetal heart-rate tracings indicated fetal distress. Standard of care required prompt intervention with epinephrine and/or emergency cesarean delivery. Brain injury occurred because these procedures were not performed.
DEFENDANTS’ DEFENSE:
The nurses denied fault and explained that they appropriately and immediately responded to mild anaphylactic symptoms in the mother. They could not administer epinephrine because the ObGyn did not order it.
The ObGyn denied violating the standard of care that included minimizing the mother’s allergic reaction. Because the mother didn’t have a rash, it was not necessary to order epinephrine. The baby sustained an unknown injury earlier in the pregnancy that was unrelated to labor.
VERDICT:
A Tennessee defense verdict was returned.
Resident blamed for shoulder dystocia
A mother presented to a federally funded health center in labor. A first-year resident managed labor and delivery under the supervision of the attending physician. Shoulder dystocia was encountered and the baby suffered a permanent brachial plexus injury.
PARENTS’ CLAIM:
Negligence occurred when the resident used excessive force by pulling on the infant’s neck during delivery. The resident, who had just received his medical license, was poorly supervised by the attending physician.
DEFENDANTS’ DEFENSE:
Suit was brought against the resident, the attending physician, the federal government, and the hospital’s residency program. The resident denied using excessive force. As soon as delivery became complex, the attending physician completed the delivery. The baby’s injuries were unpredictable and unavoidable.
VERDICT:
A $290,000 settlement with the federal government was reached before trial. A Pennsylvania defense verdict was returned for the other parties.
Related Article:
Tackle the challenging shoulder dystocia emergency by practicing delivery of the posterior arm
What caused brachial plexus injury?
An experienced midwife delivered a baby who sustained a brachial plexus injury resulting in flail arm syndrome.
PARENTS’ CLAIM:
The midwife mismanaged the delivery causing permanent injury. The child has gained little improvement with surgery and physical therapy.
DEFENDANTS’ DEFENSE:
The injury was caused by the natural forces of labor. The midwife used appropriate techniques during the birth.
VERDICT:
A Washington defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Did mother’s allergic reaction cause fetal injury?
When a mother was admitted to the labor and delivery unit, she had strep throat; ampicillin was administered. She experienced anaphylactic symptoms that were attended to. The baby, delivered vaginally 3 hours later, was severely distressed and showed signs of asphyxia. He was found to have a permanent brain injury.
PARENTS’ CLAIM:
The ObGyn and hospital nurses failed to properly manage the mother’s anaphylactic reaction to ampicillin. Fetal heart-rate tracings indicated fetal distress. Standard of care required prompt intervention with epinephrine and/or emergency cesarean delivery. Brain injury occurred because these procedures were not performed.
DEFENDANTS’ DEFENSE:
The nurses denied fault and explained that they appropriately and immediately responded to mild anaphylactic symptoms in the mother. They could not administer epinephrine because the ObGyn did not order it.
The ObGyn denied violating the standard of care that included minimizing the mother’s allergic reaction. Because the mother didn’t have a rash, it was not necessary to order epinephrine. The baby sustained an unknown injury earlier in the pregnancy that was unrelated to labor.
VERDICT:
A Tennessee defense verdict was returned.
Resident blamed for shoulder dystocia
A mother presented to a federally funded health center in labor. A first-year resident managed labor and delivery under the supervision of the attending physician. Shoulder dystocia was encountered and the baby suffered a permanent brachial plexus injury.
PARENTS’ CLAIM:
Negligence occurred when the resident used excessive force by pulling on the infant’s neck during delivery. The resident, who had just received his medical license, was poorly supervised by the attending physician.
DEFENDANTS’ DEFENSE:
Suit was brought against the resident, the attending physician, the federal government, and the hospital’s residency program. The resident denied using excessive force. As soon as delivery became complex, the attending physician completed the delivery. The baby’s injuries were unpredictable and unavoidable.
VERDICT:
A $290,000 settlement with the federal government was reached before trial. A Pennsylvania defense verdict was returned for the other parties.
Related Article:
Tackle the challenging shoulder dystocia emergency by practicing delivery of the posterior arm
What caused brachial plexus injury?
An experienced midwife delivered a baby who sustained a brachial plexus injury resulting in flail arm syndrome.
PARENTS’ CLAIM:
The midwife mismanaged the delivery causing permanent injury. The child has gained little improvement with surgery and physical therapy.
DEFENDANTS’ DEFENSE:
The injury was caused by the natural forces of labor. The midwife used appropriate techniques during the birth.
VERDICT:
A Washington defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Did mother’s allergic reaction cause fetal injury?
When a mother was admitted to the labor and delivery unit, she had strep throat; ampicillin was administered. She experienced anaphylactic symptoms that were attended to. The baby, delivered vaginally 3 hours later, was severely distressed and showed signs of asphyxia. He was found to have a permanent brain injury.
PARENTS’ CLAIM:
The ObGyn and hospital nurses failed to properly manage the mother’s anaphylactic reaction to ampicillin. Fetal heart-rate tracings indicated fetal distress. Standard of care required prompt intervention with epinephrine and/or emergency cesarean delivery. Brain injury occurred because these procedures were not performed.
DEFENDANTS’ DEFENSE:
The nurses denied fault and explained that they appropriately and immediately responded to mild anaphylactic symptoms in the mother. They could not administer epinephrine because the ObGyn did not order it.
The ObGyn denied violating the standard of care that included minimizing the mother’s allergic reaction. Because the mother didn’t have a rash, it was not necessary to order epinephrine. The baby sustained an unknown injury earlier in the pregnancy that was unrelated to labor.
VERDICT:
A Tennessee defense verdict was returned.
Resident blamed for shoulder dystocia
A mother presented to a federally funded health center in labor. A first-year resident managed labor and delivery under the supervision of the attending physician. Shoulder dystocia was encountered and the baby suffered a permanent brachial plexus injury.
PARENTS’ CLAIM:
Negligence occurred when the resident used excessive force by pulling on the infant’s neck during delivery. The resident, who had just received his medical license, was poorly supervised by the attending physician.
DEFENDANTS’ DEFENSE:
Suit was brought against the resident, the attending physician, the federal government, and the hospital’s residency program. The resident denied using excessive force. As soon as delivery became complex, the attending physician completed the delivery. The baby’s injuries were unpredictable and unavoidable.
VERDICT:
A $290,000 settlement with the federal government was reached before trial. A Pennsylvania defense verdict was returned for the other parties.
Related Article:
Tackle the challenging shoulder dystocia emergency by practicing delivery of the posterior arm
What caused brachial plexus injury?
An experienced midwife delivered a baby who sustained a brachial plexus injury resulting in flail arm syndrome.
PARENTS’ CLAIM:
The midwife mismanaged the delivery causing permanent injury. The child has gained little improvement with surgery and physical therapy.
DEFENDANTS’ DEFENSE:
The injury was caused by the natural forces of labor. The midwife used appropriate techniques during the birth.
VERDICT:
A Washington defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Removal of wrong ovary?
Removal of wrong ovary?
Several years earlier, a patient had undergone a hysterectomy but retained her ovaries and fallopian tubes. She reported recurrent pelvic pain, especially on the left side, to a gynecologic surgeon. Ultrasonography (US) results showed a small follicular cyst on the right ovary and a simple cyst on the left ovary. The patient consented to diagnostic laparoscopy with possible left salpingo-oophorectomy. During the procedure, the surgeon removed the right fallopian tube and ovary. After recovery, the patient continued to have left-sided pelvic pain. When she saw another surgeon a year later, US results showed that the left ovary and tube were still intact. The patient underwent left salpingo-oophorectomy.
PATIENT’S CLAIM:
The surgeon removed the wrong ovary and tube, a breach of the standard of care, and didn’t adequately explain his surgical actions.
DEFENDANTS’ DEFENSE:
Standard of care was maintained. During surgery, the surgeon encountered severe adhesions on the patient’s left side and was unable to visualize her left ovary. He decided that what had appeared to be an ovary on US most likely was a fluid collection, and that the patient’s left ovary must have been removed at hysterectomy. The surgeon concluded that the hemorrhagic cyst on the right ovary and adhesions were causing the patient’s pain, and removed them. The patient had given him permission to perform laparoscopic surgery, but he did not have her consent to convert to laparotomy, which would have been necessary to confirm the absence of her left ovary.
VERDICT:
An Alabama defense verdict was returned.
Related Article:
Medical errors: Meeting ethical obligations and reducing liability with proper communication
Was wrong hysterectomy procedure chosen?
After being treated by her ObGyn for postmenopausal bleeding with medication and dilation and curettage, a 50-year-old woman underwent total abdominal hysterectomy (TAH). At an office visit 3 weeks postsurgery, she reported uncontrollable urination. The patient was admitted to a hospital, where cystogram results showed a vesico-vaginal fistula (VVF). She was treated with catheter drainage and referred to a urologist. The patient underwent 2 unsuccessful repair operations. A third repair, performed 10 months after the TAH, was successful.
PATIENT’S CLAIM:
The ObGyn should have performed laparoscopic supracervical hysterectomy (LSH) instead of TAH because the patient’s cervix would have remained intact and VVF would not have developed. Medical bills totaled $194,000.
PHYSICIAN’S DEFENSE:
The standard of care did not require LSH. Had the ObGyn left the cervix intact, the patient could have continued bleeding with increased risk of cervical cancer. A bladder injury is a known complication of hysterectomy.
VERDICT:
A Mississippi defense verdict was returned.
Woman dies after uterine fibroid removal
A 39-year-old woman with a history of hypertension, diabetes, moderate obesity, and end-stage renal disease underwent myomectomy. A first-year resident assisted the attending anesthesiologist during the procedure. While the patient was under general anesthesia, her blood pressure (BP) dropped rapidly and remained at an abnormally low level for 45 minutes. Then the patient’s heart rate dropped to around 30 bpm and remained at that level for 15 minutes before her BP and heart rate were finally restored. The patient never regained consciousness and remained in an irreversible coma until she died 6 days later.
ESTATE’S CLAIM:
The anesthesiologist and resident negligently allowed the patient’s BP and heart rate to fall to dangerously low levels. Because the patient had hypertension, diabetes, and obesity, she required a higher BP to maintain adequate cerebral perfusion. The physicians precipitated the patient’s hypotension by giving her an excessive dose of morphine and bupivacaine via epidural catheter prior to induction of general anesthesia, and then failed to give her sufficient doses of vasopressors to increase her BP to safe levels. They failed to properly treat the condition in a timely manner, causing brain damage, and ultimately, death.
DEFENDANTS’ DEFENSE:
The case was settled during mediation.
VERDICT:
A $900,000 Massachusetts settlement was reached.
Related Article:
Total abdominal hysterectomy the Mayo Clinic way
Ureter injured during hysterectomy
A 47-year-old woman’s right ureter was damaged during laparoscopic hysterectomy. During surgery, the gynecologist called in a urologist to repair the injury. The patient reported postsurgical complications including renal function impairment. A computed tomography scan showed a right ureter obstruction. When surgery confirmed complete obstruction of the ureter, she had a temporary nephrostomy drain placed. After 4 weeks, the patient returned to the operating room to have the right ureter implanted into the bladder. The patient reported occasional painful urination with increased urinary frequency and decreased right kidney size.
PATIENT’S CLAIM:
The gynecologist lacerated the ureter because he did not adequately identify and protect the ureter; this error represented a departure from the standard of care. The urologist failed to properly repair the injury. The patient sought recovery of $990,000 for past and future pain and suffering.
DEFENDANTS’ CLAIM:
The suit against the urologist and hospital was dropped, but continued against the gynecologist. The gynecologist claimed that the patient’s injury was a thermal burn, and is a known complication of the procedure.
VERDICT:
A $500,000 New York verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Removal of wrong ovary?
Several years earlier, a patient had undergone a hysterectomy but retained her ovaries and fallopian tubes. She reported recurrent pelvic pain, especially on the left side, to a gynecologic surgeon. Ultrasonography (US) results showed a small follicular cyst on the right ovary and a simple cyst on the left ovary. The patient consented to diagnostic laparoscopy with possible left salpingo-oophorectomy. During the procedure, the surgeon removed the right fallopian tube and ovary. After recovery, the patient continued to have left-sided pelvic pain. When she saw another surgeon a year later, US results showed that the left ovary and tube were still intact. The patient underwent left salpingo-oophorectomy.
PATIENT’S CLAIM:
The surgeon removed the wrong ovary and tube, a breach of the standard of care, and didn’t adequately explain his surgical actions.
DEFENDANTS’ DEFENSE:
Standard of care was maintained. During surgery, the surgeon encountered severe adhesions on the patient’s left side and was unable to visualize her left ovary. He decided that what had appeared to be an ovary on US most likely was a fluid collection, and that the patient’s left ovary must have been removed at hysterectomy. The surgeon concluded that the hemorrhagic cyst on the right ovary and adhesions were causing the patient’s pain, and removed them. The patient had given him permission to perform laparoscopic surgery, but he did not have her consent to convert to laparotomy, which would have been necessary to confirm the absence of her left ovary.
VERDICT:
An Alabama defense verdict was returned.
Related Article:
Medical errors: Meeting ethical obligations and reducing liability with proper communication
Was wrong hysterectomy procedure chosen?
After being treated by her ObGyn for postmenopausal bleeding with medication and dilation and curettage, a 50-year-old woman underwent total abdominal hysterectomy (TAH). At an office visit 3 weeks postsurgery, she reported uncontrollable urination. The patient was admitted to a hospital, where cystogram results showed a vesico-vaginal fistula (VVF). She was treated with catheter drainage and referred to a urologist. The patient underwent 2 unsuccessful repair operations. A third repair, performed 10 months after the TAH, was successful.
PATIENT’S CLAIM:
The ObGyn should have performed laparoscopic supracervical hysterectomy (LSH) instead of TAH because the patient’s cervix would have remained intact and VVF would not have developed. Medical bills totaled $194,000.
PHYSICIAN’S DEFENSE:
The standard of care did not require LSH. Had the ObGyn left the cervix intact, the patient could have continued bleeding with increased risk of cervical cancer. A bladder injury is a known complication of hysterectomy.
VERDICT:
A Mississippi defense verdict was returned.
Woman dies after uterine fibroid removal
A 39-year-old woman with a history of hypertension, diabetes, moderate obesity, and end-stage renal disease underwent myomectomy. A first-year resident assisted the attending anesthesiologist during the procedure. While the patient was under general anesthesia, her blood pressure (BP) dropped rapidly and remained at an abnormally low level for 45 minutes. Then the patient’s heart rate dropped to around 30 bpm and remained at that level for 15 minutes before her BP and heart rate were finally restored. The patient never regained consciousness and remained in an irreversible coma until she died 6 days later.
ESTATE’S CLAIM:
The anesthesiologist and resident negligently allowed the patient’s BP and heart rate to fall to dangerously low levels. Because the patient had hypertension, diabetes, and obesity, she required a higher BP to maintain adequate cerebral perfusion. The physicians precipitated the patient’s hypotension by giving her an excessive dose of morphine and bupivacaine via epidural catheter prior to induction of general anesthesia, and then failed to give her sufficient doses of vasopressors to increase her BP to safe levels. They failed to properly treat the condition in a timely manner, causing brain damage, and ultimately, death.
DEFENDANTS’ DEFENSE:
The case was settled during mediation.
VERDICT:
A $900,000 Massachusetts settlement was reached.
Related Article:
Total abdominal hysterectomy the Mayo Clinic way
Ureter injured during hysterectomy
A 47-year-old woman’s right ureter was damaged during laparoscopic hysterectomy. During surgery, the gynecologist called in a urologist to repair the injury. The patient reported postsurgical complications including renal function impairment. A computed tomography scan showed a right ureter obstruction. When surgery confirmed complete obstruction of the ureter, she had a temporary nephrostomy drain placed. After 4 weeks, the patient returned to the operating room to have the right ureter implanted into the bladder. The patient reported occasional painful urination with increased urinary frequency and decreased right kidney size.
PATIENT’S CLAIM:
The gynecologist lacerated the ureter because he did not adequately identify and protect the ureter; this error represented a departure from the standard of care. The urologist failed to properly repair the injury. The patient sought recovery of $990,000 for past and future pain and suffering.
DEFENDANTS’ CLAIM:
The suit against the urologist and hospital was dropped, but continued against the gynecologist. The gynecologist claimed that the patient’s injury was a thermal burn, and is a known complication of the procedure.
VERDICT:
A $500,000 New York verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Removal of wrong ovary?
Several years earlier, a patient had undergone a hysterectomy but retained her ovaries and fallopian tubes. She reported recurrent pelvic pain, especially on the left side, to a gynecologic surgeon. Ultrasonography (US) results showed a small follicular cyst on the right ovary and a simple cyst on the left ovary. The patient consented to diagnostic laparoscopy with possible left salpingo-oophorectomy. During the procedure, the surgeon removed the right fallopian tube and ovary. After recovery, the patient continued to have left-sided pelvic pain. When she saw another surgeon a year later, US results showed that the left ovary and tube were still intact. The patient underwent left salpingo-oophorectomy.
PATIENT’S CLAIM:
The surgeon removed the wrong ovary and tube, a breach of the standard of care, and didn’t adequately explain his surgical actions.
DEFENDANTS’ DEFENSE:
Standard of care was maintained. During surgery, the surgeon encountered severe adhesions on the patient’s left side and was unable to visualize her left ovary. He decided that what had appeared to be an ovary on US most likely was a fluid collection, and that the patient’s left ovary must have been removed at hysterectomy. The surgeon concluded that the hemorrhagic cyst on the right ovary and adhesions were causing the patient’s pain, and removed them. The patient had given him permission to perform laparoscopic surgery, but he did not have her consent to convert to laparotomy, which would have been necessary to confirm the absence of her left ovary.
VERDICT:
An Alabama defense verdict was returned.
Related Article:
Medical errors: Meeting ethical obligations and reducing liability with proper communication
Was wrong hysterectomy procedure chosen?
After being treated by her ObGyn for postmenopausal bleeding with medication and dilation and curettage, a 50-year-old woman underwent total abdominal hysterectomy (TAH). At an office visit 3 weeks postsurgery, she reported uncontrollable urination. The patient was admitted to a hospital, where cystogram results showed a vesico-vaginal fistula (VVF). She was treated with catheter drainage and referred to a urologist. The patient underwent 2 unsuccessful repair operations. A third repair, performed 10 months after the TAH, was successful.
PATIENT’S CLAIM:
The ObGyn should have performed laparoscopic supracervical hysterectomy (LSH) instead of TAH because the patient’s cervix would have remained intact and VVF would not have developed. Medical bills totaled $194,000.
PHYSICIAN’S DEFENSE:
The standard of care did not require LSH. Had the ObGyn left the cervix intact, the patient could have continued bleeding with increased risk of cervical cancer. A bladder injury is a known complication of hysterectomy.
VERDICT:
A Mississippi defense verdict was returned.
Woman dies after uterine fibroid removal
A 39-year-old woman with a history of hypertension, diabetes, moderate obesity, and end-stage renal disease underwent myomectomy. A first-year resident assisted the attending anesthesiologist during the procedure. While the patient was under general anesthesia, her blood pressure (BP) dropped rapidly and remained at an abnormally low level for 45 minutes. Then the patient’s heart rate dropped to around 30 bpm and remained at that level for 15 minutes before her BP and heart rate were finally restored. The patient never regained consciousness and remained in an irreversible coma until she died 6 days later.
ESTATE’S CLAIM:
The anesthesiologist and resident negligently allowed the patient’s BP and heart rate to fall to dangerously low levels. Because the patient had hypertension, diabetes, and obesity, she required a higher BP to maintain adequate cerebral perfusion. The physicians precipitated the patient’s hypotension by giving her an excessive dose of morphine and bupivacaine via epidural catheter prior to induction of general anesthesia, and then failed to give her sufficient doses of vasopressors to increase her BP to safe levels. They failed to properly treat the condition in a timely manner, causing brain damage, and ultimately, death.
DEFENDANTS’ DEFENSE:
The case was settled during mediation.
VERDICT:
A $900,000 Massachusetts settlement was reached.
Related Article:
Total abdominal hysterectomy the Mayo Clinic way
Ureter injured during hysterectomy
A 47-year-old woman’s right ureter was damaged during laparoscopic hysterectomy. During surgery, the gynecologist called in a urologist to repair the injury. The patient reported postsurgical complications including renal function impairment. A computed tomography scan showed a right ureter obstruction. When surgery confirmed complete obstruction of the ureter, she had a temporary nephrostomy drain placed. After 4 weeks, the patient returned to the operating room to have the right ureter implanted into the bladder. The patient reported occasional painful urination with increased urinary frequency and decreased right kidney size.
PATIENT’S CLAIM:
The gynecologist lacerated the ureter because he did not adequately identify and protect the ureter; this error represented a departure from the standard of care. The urologist failed to properly repair the injury. The patient sought recovery of $990,000 for past and future pain and suffering.
DEFENDANTS’ CLAIM:
The suit against the urologist and hospital was dropped, but continued against the gynecologist. The gynecologist claimed that the patient’s injury was a thermal burn, and is a known complication of the procedure.
VERDICT:
A $500,000 New York verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Whether to anticoagulate: Toward a more reasoned approach
The article by Hagerty and Rich in this issue of the Cleveland Clinic Journal of Medicine1 covers an important topic—whether elderly patients with atrial fibrillation should receive anticoagulant therapy for it, or whether the risk of bleeding with this therapy outweighs the benefit of preventing stroke.
BETTER RISK PREDICTORS ARE NEEDED
Prediction tools are available for estimating the risk of stroke in patients with atrial fibrillation without anticoagulation2,3 and to estimate bleeding risk from anticoagulation4–7 (Table 1). Both tools have limitations, but as Hagerty and Rich point out, the stroke risk scales are likely better than the bleeding risk scales.
For example, Fang et al8 note that the risk of intracranial hemorrhage increases significantly after age 85. The bleeding risk scales use lower age cutoffs than this, perhaps increasing their sensitivity but decreasing their specificity.
Although HAS-BLED5,6 includes antiplatelet drugs such as nonsteroidal anti-inflammatory drugs and aspirin as risk factors for bleeding, ATRIA4 and HEMORR2HAGES7 do not.
Other drugs such as macrolides, quinolones, and high-dose corticosteroids raise the international normalized ratio (INR). These are typically used short-term, but can cause major fluctuations in the INR that may not be detected by monthly INR checks. Incorporating the short-term use of such drugs into bleeding risk scales would be difficult if not impossible a priori. Yet clinicians should be aware that these drugs can affect bleeding risk.
As Hagerty and Rich note,1 the bleeding risk scores were developed for warfarin, and their applicability to patients treated with novel oral anticoagulants is uncertain.
All three of the available bleeding risk scales consider prior bleeding as a risk factor, but the severity of the prior bleeding varies. Although it is understandable to include major bleeding as a risk factor since it carries an increased risk of death, minor bleeding can affect morbidity and quality of life. Only the ATRIA score4 considers both major and minor bleeding, while HEMORR2HAGES7 does not specify bleeding severity, and HAS-BLED5,6 considers only major bleeding. Clearly, there is a need to update these existing bleeding risk scores so that they can apply to novel oral anticoagulants and consider both major and minor bleeding.
As the authors note, for predicting the risk of stroke, the CHA2DS2-VASc score3 provides more precision than the CHADS2 score2 at the lower end of the benefit spectrum. Unfortunately, there is no similar screening tool to predict bleeding risk from anticoagulation with greater precision in the middle to lower part of the risk spectrum.
THE PATIENT’S PREFERENCES MATTER
The patient’s life expectancy and personal preferences are important independent factors that affect the decision of whether to anticoagulate or not. It is the responsibility of clinicians who care for older adults to make sure that these two important considerations are included in any anticoagulation decision-making for this group of patients.
- Hagerty T, Rich MW. Fall risk and anticoagulation for atrial fibrillation in the elderly: a delicate balance. Cleve Clin J Med 2017; 84:35–40.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest 2010; 137:263–272.
- Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) study. J Am Coll Cardiol 2011; 58:395–401.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol 2011; 57:173–180.
- Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006; 151:713–719.
- Fang MC, Chang Y, Hylek EM, et al. Advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for atrial fibrillation. Ann Intern Med 2004; 141:745–752.
The article by Hagerty and Rich in this issue of the Cleveland Clinic Journal of Medicine1 covers an important topic—whether elderly patients with atrial fibrillation should receive anticoagulant therapy for it, or whether the risk of bleeding with this therapy outweighs the benefit of preventing stroke.
BETTER RISK PREDICTORS ARE NEEDED
Prediction tools are available for estimating the risk of stroke in patients with atrial fibrillation without anticoagulation2,3 and to estimate bleeding risk from anticoagulation4–7 (Table 1). Both tools have limitations, but as Hagerty and Rich point out, the stroke risk scales are likely better than the bleeding risk scales.
For example, Fang et al8 note that the risk of intracranial hemorrhage increases significantly after age 85. The bleeding risk scales use lower age cutoffs than this, perhaps increasing their sensitivity but decreasing their specificity.
Although HAS-BLED5,6 includes antiplatelet drugs such as nonsteroidal anti-inflammatory drugs and aspirin as risk factors for bleeding, ATRIA4 and HEMORR2HAGES7 do not.
Other drugs such as macrolides, quinolones, and high-dose corticosteroids raise the international normalized ratio (INR). These are typically used short-term, but can cause major fluctuations in the INR that may not be detected by monthly INR checks. Incorporating the short-term use of such drugs into bleeding risk scales would be difficult if not impossible a priori. Yet clinicians should be aware that these drugs can affect bleeding risk.
As Hagerty and Rich note,1 the bleeding risk scores were developed for warfarin, and their applicability to patients treated with novel oral anticoagulants is uncertain.
All three of the available bleeding risk scales consider prior bleeding as a risk factor, but the severity of the prior bleeding varies. Although it is understandable to include major bleeding as a risk factor since it carries an increased risk of death, minor bleeding can affect morbidity and quality of life. Only the ATRIA score4 considers both major and minor bleeding, while HEMORR2HAGES7 does not specify bleeding severity, and HAS-BLED5,6 considers only major bleeding. Clearly, there is a need to update these existing bleeding risk scores so that they can apply to novel oral anticoagulants and consider both major and minor bleeding.
As the authors note, for predicting the risk of stroke, the CHA2DS2-VASc score3 provides more precision than the CHADS2 score2 at the lower end of the benefit spectrum. Unfortunately, there is no similar screening tool to predict bleeding risk from anticoagulation with greater precision in the middle to lower part of the risk spectrum.
THE PATIENT’S PREFERENCES MATTER
The patient’s life expectancy and personal preferences are important independent factors that affect the decision of whether to anticoagulate or not. It is the responsibility of clinicians who care for older adults to make sure that these two important considerations are included in any anticoagulation decision-making for this group of patients.
The article by Hagerty and Rich in this issue of the Cleveland Clinic Journal of Medicine1 covers an important topic—whether elderly patients with atrial fibrillation should receive anticoagulant therapy for it, or whether the risk of bleeding with this therapy outweighs the benefit of preventing stroke.
BETTER RISK PREDICTORS ARE NEEDED
Prediction tools are available for estimating the risk of stroke in patients with atrial fibrillation without anticoagulation2,3 and to estimate bleeding risk from anticoagulation4–7 (Table 1). Both tools have limitations, but as Hagerty and Rich point out, the stroke risk scales are likely better than the bleeding risk scales.
For example, Fang et al8 note that the risk of intracranial hemorrhage increases significantly after age 85. The bleeding risk scales use lower age cutoffs than this, perhaps increasing their sensitivity but decreasing their specificity.
Although HAS-BLED5,6 includes antiplatelet drugs such as nonsteroidal anti-inflammatory drugs and aspirin as risk factors for bleeding, ATRIA4 and HEMORR2HAGES7 do not.
Other drugs such as macrolides, quinolones, and high-dose corticosteroids raise the international normalized ratio (INR). These are typically used short-term, but can cause major fluctuations in the INR that may not be detected by monthly INR checks. Incorporating the short-term use of such drugs into bleeding risk scales would be difficult if not impossible a priori. Yet clinicians should be aware that these drugs can affect bleeding risk.
As Hagerty and Rich note,1 the bleeding risk scores were developed for warfarin, and their applicability to patients treated with novel oral anticoagulants is uncertain.
All three of the available bleeding risk scales consider prior bleeding as a risk factor, but the severity of the prior bleeding varies. Although it is understandable to include major bleeding as a risk factor since it carries an increased risk of death, minor bleeding can affect morbidity and quality of life. Only the ATRIA score4 considers both major and minor bleeding, while HEMORR2HAGES7 does not specify bleeding severity, and HAS-BLED5,6 considers only major bleeding. Clearly, there is a need to update these existing bleeding risk scores so that they can apply to novel oral anticoagulants and consider both major and minor bleeding.
As the authors note, for predicting the risk of stroke, the CHA2DS2-VASc score3 provides more precision than the CHADS2 score2 at the lower end of the benefit spectrum. Unfortunately, there is no similar screening tool to predict bleeding risk from anticoagulation with greater precision in the middle to lower part of the risk spectrum.
THE PATIENT’S PREFERENCES MATTER
The patient’s life expectancy and personal preferences are important independent factors that affect the decision of whether to anticoagulate or not. It is the responsibility of clinicians who care for older adults to make sure that these two important considerations are included in any anticoagulation decision-making for this group of patients.
- Hagerty T, Rich MW. Fall risk and anticoagulation for atrial fibrillation in the elderly: a delicate balance. Cleve Clin J Med 2017; 84:35–40.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest 2010; 137:263–272.
- Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) study. J Am Coll Cardiol 2011; 58:395–401.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol 2011; 57:173–180.
- Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006; 151:713–719.
- Fang MC, Chang Y, Hylek EM, et al. Advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for atrial fibrillation. Ann Intern Med 2004; 141:745–752.
- Hagerty T, Rich MW. Fall risk and anticoagulation for atrial fibrillation in the elderly: a delicate balance. Cleve Clin J Med 2017; 84:35–40.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest 2010; 137:263–272.
- Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) study. J Am Coll Cardiol 2011; 58:395–401.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol 2011; 57:173–180.
- Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006; 151:713–719.
- Fang MC, Chang Y, Hylek EM, et al. Advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for atrial fibrillation. Ann Intern Med 2004; 141:745–752.
Postexposure management of infectious diseases
People who have been exposed to an infectious disease should be evaluated promptly and systematically, whether they are healthcare professionals at work,1 patients, or contacts of patients. The primary goals are to prevent acquisition and transmission of the infection, allay the exposed person’s anxiety, and avoid unnecessary interventions and loss of work days.1,2 Some may need postexposure prophylaxis.
ESSENTIAL ELEMENTS OF POSTEXPOSURE MANAGEMENT
Because postexposure management can be challenging, an experienced clinician or expert consultant (eg, infectious disease specialist, infection control provider, or public health officer) should be involved. Institution-specific policies and procedures for postexposure prophylaxis and testing should be followed.1,2
Postexposure management should include the following elements:
- Immediate care of the wound or other site of exposure in cases of blood-borne exposures and tetanus- and rabies-prone injuries. This includes thoroughly washing with soap and water or cleansing with an antiseptic agent, flushing affected mucous membranes with water, and debridement of devitalized tissue.1–6
- Deciding whether postexposure prophylaxis is indicated and, if so, the type, dose, route, and duration.
- Initiating prophylaxis as soon as possible.
- Determining an appropriate baseline assessment and follow-up plan for the exposed individual.
- Counseling exposed women who are pregnant or breast-feeding about the risks and benefits of postexposure prophylaxis to mother, fetus, and infant.
- Identifying required infection control precautions, including work and school restriction, for exposed and source individuals.
- Counseling and psychological support for exposed individuals, who need to know about the risks of acquiring the infection and transmitting it to others, infection control precautions, benefits, and adverse effects of postexposure prophylaxis, the importance of adhering to the regimen, and the follow-up plan. They must understand that this treatment may not completely prevent the infection, and they should seek medical attention if they develop fever or any symptoms or signs of the infection of concern.1,2
IS POSTEXPOSURE PROPHYLAXIS INDICATED?
Postexposure management begins with an assessment to determine whether the exposure is likely to result in infection; whether the exposed individual is susceptible to the infection of concern or is at greater risk of complications from it than the general population; and whether postexposure prophylaxis is needed. This involves a complete focused history, physical examination, and laboratory testing of the potentially exposed individual and of the source, if possible.1,2
Postexposure prophylaxis should begin as soon as possible to maximize its effects while awaiting the results of further diagnostic tests. However, if the exposed individual seeks care after the recommended period, prophylactic therapy can still be effective for certain infections that have a long incubation period, such as tetanus and rabies.5,6 The choice of regimen should be guided by efficacy, safety, cost, toxicity, ease of adherence, drug interactions, and antimicrobial resistance.1,2
HOW GREAT IS THE RISK OF INFECTION?
Exposed individuals are not all at the same risk of acquiring a given infection. The risk depends on:
- Type and extent of exposure (see below)
- Characteristics of the infectious agent (eg, virulence, infectious dose)
- Status of the infectious source (eg, whether the disease is in its infectious period or is being treated); effective treatment can shorten the duration of microbial shedding and subsequently reduce risk of transmission of certain infections such as tuberculosis, meningococcal infection, invasive group A streptococcal infection, and pertussis7–10
- Immune status of the exposed individual (eg, prior infection or vaccination), since people who are immune to the infection of concern usually do not need postexposure prophylaxis2
- Adherence to infection prevention and control principles; postexposure prophylaxis may not be required if the potentially exposed individual was wearing appropriate personal protective equipment such as a surgical mask, gown, and gloves and was following standard precautions.1
WHO SHOULD BE RESTRICTED FROM WORK OR SCHOOL?
Most people without symptoms who were exposed to most types of infections do not need to stay home from work or school. However, susceptible people, particularly healthcare providers exposed to measles, mumps, rubella, and varicella, should be excluded from work while they are capable of transmitting these diseases, even if they have no symptoms.11,12 Moreover, people with symptoms with infections primarily transmitted via the airborne, droplet, or contact route should be restricted from work until no longer infectious.1,2,7,9–15
Most healthcare institutions have clear protocols for managing occupational exposures to infectious diseases, in particular for blood-borne pathogens such as human immunodeficiency virus (HIV). The protocol should include appropriate evaluation and laboratory testing of the source patient and exposed healthcare provider, as well as procedures for counseling the exposed provider, identifying and procuring an initial prophylactic regimen for timely administration, a mechanism for formal expert consultation (eg, with an in-house infectious diseases consultant), and a plan for outpatient follow-up.
The next section reviews postexposure management of common infections categorized by mode of transmission, including the risk of transmission, initial and follow-up evaluation, and considerations for postexposure prophylaxis.
BLOOD-BORNE INFECTIONS
Blood-borne pathogens can be transmitted by accidental needlesticks or cuts or by exposure of the eyes, mucous membranes, or nonintact skin to blood, tissue, or other potentially infectious body fluids—cerebrospinal, pericardial, pleural, peritoneal, synovial, and amniotic fluid, semen, and vaginal secretions. (Feces, nasal secretions, saliva, sputum, sweat, tears, urine, and vomitus are considered noninfectious for blood-borne pathogens unless they contain blood.16)
Healthcare professionals are commonly exposed to blood-borne pathogens as a result of needlestick injuries, and these exposures tend to be underreported.17
When someone has been exposed to blood or other infectious body fluids, the source individual and the exposed individual should be assessed for risk factors for hepatitis B virus, hepatitis C virus, HIV, and other blood-borne pathogens.3,4,16,18 If the disease status for these viruses is unknown, the source and exposed individual should be tested in accordance with institutional policies regarding consent to testing. Testing of needles or sharp instruments implicated in an exposure is not recommended.3,4,16,18
Determining the need for prophylaxis after exposure to an unknown source such as a disposed needle can be challenging. Assessment should be made on a case-by-case basis, depending on the known prevalence of the infection of concern in the local community. The risk of transmission in most source-unknown exposures is negligible.3,4,18 However, hepatitis B vaccine and hepatitis B immunoglobulin should be used liberally as postexposure prophylaxis for previously unvaccinated healthcare providers exposed to an unknown source.3,4,16,18
Hepatitis B
Hepatitis B virus (Table 1) is the most infectious of the common blood-borne viruses. The risk of transmission after percutaneous exposure to hepatitis B-infected blood ranges from 1% to 30% based on hepatitis Be antigen status and viral load (based on hepatitis B viral DNA).1,2,4,16
Hepatitis B vaccine or immunoglobulin, or both, are recommended for postexposure prophylaxis in pregnant women, based on evidence that perinatal transmission was reduced by 70% to 90% when these were given within 12 to 24 hours of exposure.4,16,19
Hepatitis C
The risk of infection after percutaneous exposure to hepatitis C virus-infected blood is estimated to be 1.8% per exposure.16 The risk is lower with exposure of a mucous membrane or nonintact skin to blood, fluids, or tissues from hepatitis C-infected patients.16,18
Since there is no effective postexposure prophylactic regimen, the goal of postexposure assessment of hepatitis C is early identification of infection (by monitoring the patient to see if he or she seroconverts) and, if infection is present, referral to an experienced clinician for further evaluation (Table 1). However, data supporting the utility of direct-acting anti-hepatitis C antiviral drugs as postexposure prophylaxis after occupational exposure to hepatitis C are lacking.
Human immunodeficiency virus
The estimated risk of HIV transmission from a known infected source after percutaneous exposure is 0.3%, and after mucosal exposures it is 0.09%.20
If postexposure prophylaxis is indicated, it should be a three-drug regimen (Table 1).3,18 The recommended antiretroviral therapies have been proven effective in clinical trials of HIV treatment, not for postexposure prophylaxis per se, but they are recommended because they are effective, safe, tolerable, and associated with high adherence rates.3,16,18,21 If the source individual is known to have HIV infection, information about his or her stage of infection, CD4+ T-cell count, results of viral load testing, current and previous antiretroviral therapy, and results of any genotypic viral resistance testing will guide the choice of postexposure prophylactic regimen.3,18
The clinician should give the exposed patient a starter pack of 5 to 7 days of medication, give the first dose then and there, and arrange follow-up with an experienced clinician within a few days of the exposure to determine whether a complete 30-day course is needed.3,16,18
SEXUALLY TRANSMITTED INFECTIONS
In the case of sexually transmitted infections, “exposure” means unprotected sexual contact with someone who has a sexually transmitted infection.22 People with sexually transmitted infections often have no symptoms but can still transmit the infection. Thus, people at risk should be identified and screened for all suspected sexually transmitted infections.23–25
Patients with sexually transmitted infections should be instructed to refer their sex partners for evaluation and treatment to prevent further transmission and reinfection. Assessment of exposed partners includes a medical history, physical examination, microbiologic testing for all potential sexually transmitted infections, and eligibility for hepatitis A virus, hepatitis B virus, and human papillomavirus vaccines.22 Ideally, exposed partners should be reassessed within 1 to 2 weeks to follow up testing results and to monitor for side effects of and adherence to postexposure prophylaxis, if applicable.
Public health departments should be notified of sexually transmitted infections such as gonorrhea, chlamydia, chancroid, and syphilis.22
Expedited partner therapy, in which index patients deliver the medication or a prescription for it directly to their partners, is an alternative for partner management where legally allowed by state and local health departments (see www.cdc.gov/std/ept/legal/).22
Recommended postexposure prophylactic regimens for sexually transmitted infections (Table 2) are based on their efficacy in the treatment of these infections.22,26–28 The regimen for HIV prophylaxis is the same as in Table 1.3,18,26
Chlamydia
Chlamydia is the most commonly reported communicable disease in the United States. The risk of transmission after sexual intercourse with a person who has an active infection is approximately 65% and increases with the number of exposures.22,29
Gonorrhea
Infection with Neisseria gonorrhoeae is the second most commonly reported communicable disease in the United States. The transmission rate of gonorrhea after sex with someone who has it ranges from 50% to 93%.22 When prescribing postexposure prophylaxis for gonorrhea, it is essential to consider the risk of antimicrobial resistance and local susceptibility data.22
Human immunodeficiency virus
Risk of HIV transmission through sexual contact varies depending on the nature of the exposure, ranging from 0.05% to 0.5%.30
Syphilis
The risk of transmission of syphilis in its early stages (primary and secondary) after sexual exposure is approximately 30%. Transmission requires open lesions such as chancres in primary syphilis and mucocutaneous lesions (mucous patches, condyloma lata) in secondary syphilis.22
After sexual assault
In cases of sexual assault, the risk of sexually transmitted infections may be increased due to trauma and bleeding. Testing for all sexually transmitted infections, including HIV, should be considered on a case-by-case basis.22
Survivors of sexual assault have been shown to be poorly compliant with follow-up visits, and thus provision of postexposure prophylaxis at the time of initial assessment is preferable to deferred treatment.22 The recommended regimen should cover chlamydia, gonorrhea, and trichomoniasis (a single dose of intramuscular ceftriaxone 250 mg, oral azithromycin 1 g, and either oral metronidazole 2 g or tinidazole 2 g), in addition to HIV if the victim presents within 72 hours of exposure (Table 2).22,26
Hepatitis B virus vaccine, not immunoglobulin, should be given if the hepatitis status of the assailant is unknown and the survivor has not been previously vaccinated. Both hepatitis B vaccine and immunoglobulin should be given to unvaccinated survivors if the assailant is known to be hepatitis B surface antigen-positive.22
Human papillomavirus vaccination is recommended for female survivors ages 9 to 26 and male survivors ages 9 to 21.
Emergency contraception should be given if there is a risk of pregnancy.22,26
In many jurisdictions, sexual assault centers provide trained examiners through Sexual Assault Nurse Examiners to perform evidence collection and to provide initial contact with the aftercare resources of the center.
Advice on medical management of sexual assault can be obtained by calling National PEPline (888–448–4911).
INFECTIONS TRANSMITTED BY THE AIRBORNE ROUTE
Airborne transmission of infections occurs by inhalation of droplet nuclei (diameter ≤ 5 μm) generated by coughing and sneezing. Certain procedures (eg, administration of nebulized medication, sputum induction, bronchoscopy) also generate droplets and aerosols, which can transmit organisms.1
Measles
Measles (Table 3) is highly contagious; up to 90% of susceptible individuals develop measles after exposure. The virus is transmitted by direct contact with infectious droplets and by the airborne route. It remains infectious in the air and on surfaces for up to 2 hours; therefore, any type of exposure, even transient, is an indication for postexposure prophylaxis in susceptible individuals.11
Both the measles, mumps, rubella (MMR) vaccine and immune globulin may prevent or modify disease severity in susceptible exposed individuals if given within 3 days of exposure (for the vaccine) or within 6 days of exposure (for immune globulin).31,32
Tuberculosis
Mycobacterium tuberculosis is transmitted from patients with pulmonary or laryngeal tuberculosis, particularly if patients cough and are sputum-positive for acid-fast bacilli. Patients with extrapulmonary tuberculosis or latent tuberculosis infection are not infectious.1,7
Postexposure management of tuberculosis occurs through contact investigation of a newly diagnosed index case of tuberculosis disease. Contacts are categorized as household contacts, close nonhousehold contacts (those having regular, extensive contact with the index case), casual contacts, and transient community contacts. The highest priority for contact investigations should be household contacts, close nonhousehold or casual contacts at high risk of progressing to tuberculosis disease (eg, those with HIV, those on dialysis, or transplant recipients), and unprotected healthcare providers exposed during aerosol-generating procedures.7,33
Postexposure management includes screening exposed individuals for tuberculosis symptoms and performing tuberculin skin testing or interferon-gamma release assay (blood testing) for those who had previously negative results (Table 3). Chest radiography is recommended for exposed immunocompromised individuals, due to high risk of tuberculosis disease and low sensitivity of skin or blood testing, and for those with a documented history of tuberculosis or previous positive skin or blood test.7,33,34
A positive tuberculin skin test for persons with recent contact with tuberculosis is defined as a wheal 5 mm or larger on baseline or follow-up screening. Prior bacillus Calmette-Guérin vaccination status should not be used in the interpretation of tuberculin skin testing in the setting of contact investigation.7,33
All exposed asymptomatic people with a positive result on testing should be treated for latent tuberculosis infection, since treatment reduces the risk of progression to tuberculosis disease by 60% to 90% .7,33,35–37
Varicella and disseminated herpes zoster
Varicella zoster virus is transmitted by direct contact with vesicular fluid of skin lesions and inhalation of aerosols from vesicular fluid or respiratory tract secretions. Varicella (chickenpox) is highly contagious, with a secondary attack rate in susceptible household contacts of 85%.12 Herpes zoster is less contagious than varicella.38
Postexposure prophylaxis against varicella is recommended for susceptible individuals who had household exposure, had face-to-face contact with an infectious patient while indoors, or shared the same hospital room with the patient.12
Postexposure prophylactic options for varicella and herpes zoster include varicella vaccine (not zoster vaccine) and varicella zoster immune globulin (Table 3).12,38–40
Varicella vaccine is approximately 90% effective if given within 3 days of exposure, and 70% effective if given within 5 days.12,39
Antiviral agents should be given if the exposed individual develops manifestations of varicella or herpes zoster.12,38
INFECTIONS TRANSMITTED BY THE DROPLET ROUTE
Droplet transmission occurs when respiratory droplets carrying infectious agents travel directly across short distances (3–6 feet) from the respiratory tract of the infected to mucosal surfaces of the susceptible exposed individual. Droplets are generated during coughing, sneezing, talking, and aerosol-generating procedures. Indirect contact with droplets can also transmit infection.1
Group A streptococcal infection
Although group A streptococcal infection (Table 4) may spread to close contacts of the index case and in closed populations (eg, military recruit camps, schools, institutions), secondary cases of invasive group A streptococcal infection rarely occur in family and institutional contacts.9,41,42
Postexposure prophylaxis for contacts of people with invasive group A streptococcal infection is debated, because it is unknown if antibiotic therapy will decrease the risk of acquiring the infection. It is generally agreed that it should not be routinely given to all contacts. The decision should be based on the clinician’s assessment of each individual’s risk and guidance from the local institution. If indicated, postexposure prophylaxis should be given to household and close contacts, particularly in high-risk groups (eg, Native Americans and those with risk factors such as old age, HIV infection, diabetes mellitus, heart disease, chickenpox, cancer, systemic corticosteroid therapy, other immunosuppressive medications, intravenous drug use, recent surgery or childbirth).9,41,42
Influenza
Influenza (Table 4) causes a significant burden in healthcare settings, given its prevalence and potential to cause outbreaks of severe respiratory illness in hospitalized patients and residents of long-term-care facilities.13,43
Neuraminidase inhibitors are effective as prophylaxis after unprotected exposure to influenza, particularly in outbreak situations. However, their use is not widely recommended, since overuse could lead to antiviral resistance. In selected cases, postexposure prophylaxis may be indicated for close contacts who are at high risk of complications of influenza (eg, age 65 or older, in third trimester of pregnancy or 2 weeks postpartum, morbid obesity, chronic comorbid conditions such as a cardiopulmonary and renal disorder, immunocompromising condition) or who are in close contact with persons at high risk of influenza-related complications.13,44,45
Meningococcal disease
N meningitidis is transmitted from individuals with meningococcal disease or from asymptomatic carriers.8
Postexposure prophylaxis is effective in eradicating N meningiditis and is recommended for all close contacts of patients with invasive meningococcal disease (Table 4).46 Close contacts include household contacts, childcare and preschool contacts, contacts exposed in dormitories or military training centers, those who had direct contact with the index case’s respiratory secretions (eg, intimate kissing, mouth-to-mouth resuscitation, unprotected contact during endotracheal intubation or endotracheal tube management), and passengers seated directly next to an index case on airplane flights of longer than 8 hours.
Postexposure prophylaxis is not indicated for those who had brief contact, those who had contact that did not involve exposure to oral or respiratory secretions, or for close contacts of patients with N meningitidis isolated in nonsterile sites only (eg, oropharynyx, trachea, conjunctiva).8,46
Pertussis
Pertussis is highly contagious, with a secondary attack rate of approximately 80% in susceptible individuals. Approximately one-third of susceptible household contacts develop pertussis after exposure.10
Postexposure prophylaxis for pertussis should be given to all household and close contacts (Table 4).10,47
Rubella
Transmission occurs through droplets or direct contact with nasopharyngeal secretions of an infectious case. Neither MMR vaccine nor immunoglobulin has been shown to prevent rubella in exposed contacts, and they are not recommended.11
INFECTIONS TRANSMITTED BY DIRECT CONTACT
Direct contact transmission includes infectious agents transmitted from an infected or colonized individual to another, whereas indirect contact transmission involves a contaminated intermediate object or person (eg, hands of healthcare providers, electronic thermometers, surgical instruments).1
There are no available postexposure prophylactic regimens for the organisms most commonly transmitted by this route (eg, methicillin-resistant Staphylococcus aureus, Clostridium difficile), but transmission can be prevented with adherence to standard precautions, including hand hygiene.1
Hepatitis A
Person-to-person transmission of hepatitis A virus occurs via the fecal-oral route. Common-source outbreaks and sporadic cases can occur from exposure to food or water contaminated with feces.1,15
Postexposure prophylaxis is indicated only for nonimmune close contacts (eg, household and sexual contacts) (Table 5). Without this treatment, secondary attack rates of 15% to 30% have been reported among households.15,48 Both hepatitis A vaccine and immune globulin are effective in preventing and ameliorating symptomatic hepatitis A infection. Advantages of vaccination include induction of longer-lasting immunity (at least 2 years), greater ease of administration, and lower cost than immune globulin.15,48
Scabies
Scabies is an infestation of the skin by the mite Sarcoptes scabiei var hominis. Person-to-person transmission typically occurs through direct, prolonged skin-to-skin contact with an infested person (eg, household and sexual contacts). However, crusted scabies can be transmitted after brief skin-to-skin contact or by exposure to bedding, clothing, or furniture used by the infested person.
All potentially infested persons should be treated concomitantly (Table 5).14,49
INFECTIONS TRANSMITTED BY MAMMAL BITES AND INJURIES
Bites and injury wounds account for approximately 1% of all visits to emergency departments.50 Human bites are associated with a risk of infection by blood-borne pathogens, herpes simplex infection, and bacterial infections (eg, skin and soft-tissue infections, bacteremia). Animal bites are associated with a risk of bacterial infections, rabies, tetanus, hepatitis B virus, and monkeypox.50
Rabies
Human rabies (Table 5) is almost always fatal. Essential factors in determining the need for postexposure prophylaxis include knowledge of the epidemiology of animal rabies in the area where the contact occurred and the species of animal involved, availability of the animal for observation or rabies testing, health status of the biting animal, and vaccination history of both the animal and exposed individual.6 Clinicians should seek assistance from public health officials for evaluating exposures and determining the need for postexposure prophylaxis in situations that are not routine.51
High-risk wild animals associated with rabies in North America include bats, raccoons, skunks, foxes, coyotes, bobcats, and woodchucks. Bats are the most common source of human rabies infections in the United States, and transmission can occur from minor, sometimes unnoticed, bites. The types of exposures that require postexposure prophylaxis include bites, abrasions, scratches, and contamination of mucous membranes or open wound with saliva or neural tissue of a suspected rabid animal.
Human-to-human transmission of rabies can rarely occur through exposure of mucous membrane or nonintact skin to an infectious material (saliva, tears, neural tissue), in addition to organ transplantation.6
Animal capture and testing is a strategy for excluding rabies risk and reducing the need for postexposure prophylaxis. A dog, cat, or ferret that bites a person should be confined and observed for 10 days without administering postexposure prophylaxis for rabies, unless the bite or exposure is on the face or neck, in which case this treatment should be given immediately.6 If the observed biting animal lives and remains healthy, postexposure prophylaxis is not recommended. However, if signs suggestive of rabies develop, postexposure prophylaxis should be given and the animal should be euthanized, with testing of brain tissue for rabies virus. Postexposure prophylaxis should be discontinued if rabies testing is negative.
The combination of rabies vaccine and human rabies immunoglobulin is nearly 100% effective in preventing rabies if administered in a timely and accurate fashion after exposure (Table 5).6
Tetanus
Tetanus transmission can occur through injuries ranging from small cuts to severe trauma and through contact with contaminated objects (eg, bites, nails, needles, splinters, neonates whose umbilical cord is cut with contaminated surgical instruments, and during circumcision or piercing with contaminated instruments).5
Tetanus is almost completely preventable with vaccination, and timely administration of postexposure prophylaxis (tetanus toxoid-containing vaccine, tetanus immune globulin) decreases disease severity (Table 5).2,5,52
- Siegel JD, Rhinehart E, Jackson M, Chiarello L; Health Care Infection Control Practices Advisory Committee. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control 2007; 35(suppl 2): S65–S164.
- Advisory Committee on Immunization Practices; Centers for Disease Control and Prevention. Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:1–45.
- Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol 2013; 34: 875–892.
- Schille S, Murphy TV, Sawyer M, et al; Centers for Disease Control and Prevention (CDC). CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR Recomm Rep 2013; 62:1–19.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:13–15.
- Manning SE, Rupprecht CE, Fishbein D, et al; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Human rabies prevention—United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2008; 57:1–28.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon R, Centers for Disease Control and Prevention (CDC). Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Morb Mortal Wkly Rep 2005; 54:1–141.
- Cohn AC, MacNeil JR, Clark TA, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2013; 62:1–28.
- Prevention of Invasive Group A Streptococcal Infections Workshop Participants. Prevention of invasive group A streptococcal disease among household contacts of case patients and among postpartum and postsurgical patients: recommendations from the Centers for Disease Control and Prevention. Clin Infect Dis 2002; 35:950–959.
- Tiwari T, Murphy TV, Moran J; National Immunization Program, Centers for Disease Control and Prevention (CDC). Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis: 2005 CDC Guidelines. MMWR Morb Mortal Wkly Rep 2005; 54:1–16.
- McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS; Centers for Disease Control and Prevention (CDC). Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2013; 62:1–34.
- Marin M, Guris D, Chaves SS, Schmid S, Seward JF; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56:1–40.
- Harper SA, Bradley JS, Englund JA, et al; Expert Panel of the Infectious Diseases Society of America. Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1003–1032.
- Centers for Disease Control and Prevention (CDC). Scabies. www.cdc.gov/parasites/scabies/. Accessed November 4, 2016.
- Advisory Committee on Immunization Practices (ACIP); Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2006; 55:1–23.
- US Public Health Service. Updated US Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep 2001; 50:1–52.
- Treakle AM, Schultz M, Giannakos GP, Joyce PC, Gordin FM. Evaluating a decade of exposures to blood and body fluids in an inner-city teaching hospital. Infect Control Hosp Epidemiol 2011; 32:903–907.
- New York State Department of Health AIDS Institute. Update: HIV prophylaxis following non-occupational exposure. www.hivguidelines.org/clinical-guidelines/post-exposure-prophylaxis/hiv-prophylaxis-following-non-occupational-exposure/. Accessed November 4, 2016.
- Beasley RP, Hwang LY, Lee GC, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet 1983; 2:1099–1102.
- Baggaley RF, Boily MC, White RG, Alary M. Risk of HIV-1 transmission for parenteral exposure and blood transfusion: a systematic review and meta-analysis. AIDS 2006; 20:805–812.
- McAllister J, Read P, McNulty A, Tong WW, Ingersoll A, Carr A. Raltegravir-emtricitabine-tenofovir as HIV nonoccupational post-exposure prophylaxis in men who have sex with men: safety, tolerability and adherence. HIV Med 2014; 15:13–22.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137.
- US Preventive Services Task Force (USPSTF). Final recommendation statement: chlamydia and gonorrhea: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/chlamydia-and-gonorrhea-screening. Accessed November 4, 2016.
- US Preventive Services Task Force (USPSTF). Human immunodeficiency virus (HIV) infection: screening. www.uspreventiveservicestaskforce.org/uspstf/uspshivi.htm. Accessed November 4, 2016.
- US Preventive Services Task Force (USPSTF). Screening for syphilis. www.uspreventiveservicestaskforce.org/uspstf/uspssyph.htm#update. Accessed November 4, 2016.
- Smith DK, Grohskopf LA, Black RJ, et al; US Department of Health and Human Services. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the US Department of Health and Human Services. MMWR Recomm Rep 2005; 54:1–20.
- Lin JS, Donegan SP, Heeren TC, et al. Transmission of Chlamydia trachomatis and Neisseria gonorrhoeae among men with urethritis and their female sex partners. J Infect Dis 1998; 178:1707–1712.
- Varghese B, Maher JE, Peterman TA, Branson BM, Steketee RW. Reducing the risk of sexual HIV transmission: quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use. Sex Transm Dis 2002; 29:38–43.
- Gülmezoglu AM, Azhar M. Interventions for trichomoniasis in pregnancy. Cochrane Database Syst Rev 2011; (5):CD000220.
- Forna F, Gülmezoglu AM. Interventions for treating trichomoniasis in women. Cochrane Database Syst Rev 2003; (2):CD000218.
- Rice P, Young Y, Cohen B, Ramsay M. MMR immunization after contact with measles virus. Lancet 2004; 363:569–570.
- Young MK, Nimmo GR, Cripps AW, Jones MA. Post-exposure passive immunization for preventing measles. Cochrane Database Syst Rev 2014; 4:CD010056.
- National Tuberculosis Controllers Association; Centers for Disease Control and Prevention (CDC). Guidelines for the investigation of contacts of persons with infectious tuberculosis. Recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Recomm Rep 2005; 54:1–47.
- Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Morb Mortal Wkly Rep 2010; 59:1–25.
- Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Morb Mortal Wkly Rep 2000; 49:1–51.
- Stagg HR, Zenner D, Harris RJ, Munoz L, Lipman MC, Abubakar I. Treatment of latent tuberculosis infection: a network meta-analysis. Ann Intern Med 2014; 161:419–428.
- Centers for Disease Control and Prevention (CDC). Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep 2011; 60:1650–1653.
- Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2008; 57:1–30.
- Macartney K, Heywood A, McIntyre P. Vaccines for post-exposure prophylaxis against varicella (chickenpox) in children and adults. Cochrane Database Syst Rev 2014; 6:CD001833.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of VariZIG—United States, 2013. MMWR Morb Mortal Wkly Rep 2013; 62: 574–576.
- Public Health Agency of Canada. Guidelines for the prevention and control of invasive group A streptococcal disease. Can Commun Dis Rep 2006; 32(suppl 2):1–26.
- Steer JA, Lamagni T, Healy B, et al. Guidelines for prevention and control of group A streptococcal infection in acute healthcare and maternity settings in the UK. J Infect 2012; 64:1–18.
- Grohskopf LA, Olsen SJ, Sokolow LZ, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2014–15 influenza season. MMWR Morb Mortal Wkly Rep 2014; 63: 691–697.
- Fiore AE, Fry A, Shay D, et al; Centers for Disease Control and Prevention (CDC). Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2011; 60:1–24.
- Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev 2014; 4:CD008965.
- Zalmanovici Trestioreanu A, Fraser A, Gafter-Gvili A, Paul M, Leibovici L. Antibiotics for preventing meningococcal infections. Cochrane Database Syst Rev 2013; 10:CD004785.
- Altunaiji S, Kukuruzovic R, Curtis N, Massie J. Antibiotics for whooping cough (pertussis). Cochrane Database Syst Rev 2007: CD004404.
- Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56:1080–1084.
- FitzGerald D, Grainger RJ, Reid A. Interventions for preventing the spread of infestation in close contacts of people with scabies. Cochrane Database Syst Rev 2014; 2:CD009943.
- Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:e10–e52.
- Rupprecht CE, Briggs D, Brown CM, et al; Centers for Disease Control and Prevention (CDC). Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies—recommendations of the Advisory Committee on Immunization Practice. MMWR Recomm Rep 2010; 59:1–9.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine in adults aged 65 years and older—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep 2012; 61:468–470.
People who have been exposed to an infectious disease should be evaluated promptly and systematically, whether they are healthcare professionals at work,1 patients, or contacts of patients. The primary goals are to prevent acquisition and transmission of the infection, allay the exposed person’s anxiety, and avoid unnecessary interventions and loss of work days.1,2 Some may need postexposure prophylaxis.
ESSENTIAL ELEMENTS OF POSTEXPOSURE MANAGEMENT
Because postexposure management can be challenging, an experienced clinician or expert consultant (eg, infectious disease specialist, infection control provider, or public health officer) should be involved. Institution-specific policies and procedures for postexposure prophylaxis and testing should be followed.1,2
Postexposure management should include the following elements:
- Immediate care of the wound or other site of exposure in cases of blood-borne exposures and tetanus- and rabies-prone injuries. This includes thoroughly washing with soap and water or cleansing with an antiseptic agent, flushing affected mucous membranes with water, and debridement of devitalized tissue.1–6
- Deciding whether postexposure prophylaxis is indicated and, if so, the type, dose, route, and duration.
- Initiating prophylaxis as soon as possible.
- Determining an appropriate baseline assessment and follow-up plan for the exposed individual.
- Counseling exposed women who are pregnant or breast-feeding about the risks and benefits of postexposure prophylaxis to mother, fetus, and infant.
- Identifying required infection control precautions, including work and school restriction, for exposed and source individuals.
- Counseling and psychological support for exposed individuals, who need to know about the risks of acquiring the infection and transmitting it to others, infection control precautions, benefits, and adverse effects of postexposure prophylaxis, the importance of adhering to the regimen, and the follow-up plan. They must understand that this treatment may not completely prevent the infection, and they should seek medical attention if they develop fever or any symptoms or signs of the infection of concern.1,2
IS POSTEXPOSURE PROPHYLAXIS INDICATED?
Postexposure management begins with an assessment to determine whether the exposure is likely to result in infection; whether the exposed individual is susceptible to the infection of concern or is at greater risk of complications from it than the general population; and whether postexposure prophylaxis is needed. This involves a complete focused history, physical examination, and laboratory testing of the potentially exposed individual and of the source, if possible.1,2
Postexposure prophylaxis should begin as soon as possible to maximize its effects while awaiting the results of further diagnostic tests. However, if the exposed individual seeks care after the recommended period, prophylactic therapy can still be effective for certain infections that have a long incubation period, such as tetanus and rabies.5,6 The choice of regimen should be guided by efficacy, safety, cost, toxicity, ease of adherence, drug interactions, and antimicrobial resistance.1,2
HOW GREAT IS THE RISK OF INFECTION?
Exposed individuals are not all at the same risk of acquiring a given infection. The risk depends on:
- Type and extent of exposure (see below)
- Characteristics of the infectious agent (eg, virulence, infectious dose)
- Status of the infectious source (eg, whether the disease is in its infectious period or is being treated); effective treatment can shorten the duration of microbial shedding and subsequently reduce risk of transmission of certain infections such as tuberculosis, meningococcal infection, invasive group A streptococcal infection, and pertussis7–10
- Immune status of the exposed individual (eg, prior infection or vaccination), since people who are immune to the infection of concern usually do not need postexposure prophylaxis2
- Adherence to infection prevention and control principles; postexposure prophylaxis may not be required if the potentially exposed individual was wearing appropriate personal protective equipment such as a surgical mask, gown, and gloves and was following standard precautions.1
WHO SHOULD BE RESTRICTED FROM WORK OR SCHOOL?
Most people without symptoms who were exposed to most types of infections do not need to stay home from work or school. However, susceptible people, particularly healthcare providers exposed to measles, mumps, rubella, and varicella, should be excluded from work while they are capable of transmitting these diseases, even if they have no symptoms.11,12 Moreover, people with symptoms with infections primarily transmitted via the airborne, droplet, or contact route should be restricted from work until no longer infectious.1,2,7,9–15
Most healthcare institutions have clear protocols for managing occupational exposures to infectious diseases, in particular for blood-borne pathogens such as human immunodeficiency virus (HIV). The protocol should include appropriate evaluation and laboratory testing of the source patient and exposed healthcare provider, as well as procedures for counseling the exposed provider, identifying and procuring an initial prophylactic regimen for timely administration, a mechanism for formal expert consultation (eg, with an in-house infectious diseases consultant), and a plan for outpatient follow-up.
The next section reviews postexposure management of common infections categorized by mode of transmission, including the risk of transmission, initial and follow-up evaluation, and considerations for postexposure prophylaxis.
BLOOD-BORNE INFECTIONS
Blood-borne pathogens can be transmitted by accidental needlesticks or cuts or by exposure of the eyes, mucous membranes, or nonintact skin to blood, tissue, or other potentially infectious body fluids—cerebrospinal, pericardial, pleural, peritoneal, synovial, and amniotic fluid, semen, and vaginal secretions. (Feces, nasal secretions, saliva, sputum, sweat, tears, urine, and vomitus are considered noninfectious for blood-borne pathogens unless they contain blood.16)
Healthcare professionals are commonly exposed to blood-borne pathogens as a result of needlestick injuries, and these exposures tend to be underreported.17
When someone has been exposed to blood or other infectious body fluids, the source individual and the exposed individual should be assessed for risk factors for hepatitis B virus, hepatitis C virus, HIV, and other blood-borne pathogens.3,4,16,18 If the disease status for these viruses is unknown, the source and exposed individual should be tested in accordance with institutional policies regarding consent to testing. Testing of needles or sharp instruments implicated in an exposure is not recommended.3,4,16,18
Determining the need for prophylaxis after exposure to an unknown source such as a disposed needle can be challenging. Assessment should be made on a case-by-case basis, depending on the known prevalence of the infection of concern in the local community. The risk of transmission in most source-unknown exposures is negligible.3,4,18 However, hepatitis B vaccine and hepatitis B immunoglobulin should be used liberally as postexposure prophylaxis for previously unvaccinated healthcare providers exposed to an unknown source.3,4,16,18
Hepatitis B
Hepatitis B virus (Table 1) is the most infectious of the common blood-borne viruses. The risk of transmission after percutaneous exposure to hepatitis B-infected blood ranges from 1% to 30% based on hepatitis Be antigen status and viral load (based on hepatitis B viral DNA).1,2,4,16
Hepatitis B vaccine or immunoglobulin, or both, are recommended for postexposure prophylaxis in pregnant women, based on evidence that perinatal transmission was reduced by 70% to 90% when these were given within 12 to 24 hours of exposure.4,16,19
Hepatitis C
The risk of infection after percutaneous exposure to hepatitis C virus-infected blood is estimated to be 1.8% per exposure.16 The risk is lower with exposure of a mucous membrane or nonintact skin to blood, fluids, or tissues from hepatitis C-infected patients.16,18
Since there is no effective postexposure prophylactic regimen, the goal of postexposure assessment of hepatitis C is early identification of infection (by monitoring the patient to see if he or she seroconverts) and, if infection is present, referral to an experienced clinician for further evaluation (Table 1). However, data supporting the utility of direct-acting anti-hepatitis C antiviral drugs as postexposure prophylaxis after occupational exposure to hepatitis C are lacking.
Human immunodeficiency virus
The estimated risk of HIV transmission from a known infected source after percutaneous exposure is 0.3%, and after mucosal exposures it is 0.09%.20
If postexposure prophylaxis is indicated, it should be a three-drug regimen (Table 1).3,18 The recommended antiretroviral therapies have been proven effective in clinical trials of HIV treatment, not for postexposure prophylaxis per se, but they are recommended because they are effective, safe, tolerable, and associated with high adherence rates.3,16,18,21 If the source individual is known to have HIV infection, information about his or her stage of infection, CD4+ T-cell count, results of viral load testing, current and previous antiretroviral therapy, and results of any genotypic viral resistance testing will guide the choice of postexposure prophylactic regimen.3,18
The clinician should give the exposed patient a starter pack of 5 to 7 days of medication, give the first dose then and there, and arrange follow-up with an experienced clinician within a few days of the exposure to determine whether a complete 30-day course is needed.3,16,18
SEXUALLY TRANSMITTED INFECTIONS
In the case of sexually transmitted infections, “exposure” means unprotected sexual contact with someone who has a sexually transmitted infection.22 People with sexually transmitted infections often have no symptoms but can still transmit the infection. Thus, people at risk should be identified and screened for all suspected sexually transmitted infections.23–25
Patients with sexually transmitted infections should be instructed to refer their sex partners for evaluation and treatment to prevent further transmission and reinfection. Assessment of exposed partners includes a medical history, physical examination, microbiologic testing for all potential sexually transmitted infections, and eligibility for hepatitis A virus, hepatitis B virus, and human papillomavirus vaccines.22 Ideally, exposed partners should be reassessed within 1 to 2 weeks to follow up testing results and to monitor for side effects of and adherence to postexposure prophylaxis, if applicable.
Public health departments should be notified of sexually transmitted infections such as gonorrhea, chlamydia, chancroid, and syphilis.22
Expedited partner therapy, in which index patients deliver the medication or a prescription for it directly to their partners, is an alternative for partner management where legally allowed by state and local health departments (see www.cdc.gov/std/ept/legal/).22
Recommended postexposure prophylactic regimens for sexually transmitted infections (Table 2) are based on their efficacy in the treatment of these infections.22,26–28 The regimen for HIV prophylaxis is the same as in Table 1.3,18,26
Chlamydia
Chlamydia is the most commonly reported communicable disease in the United States. The risk of transmission after sexual intercourse with a person who has an active infection is approximately 65% and increases with the number of exposures.22,29
Gonorrhea
Infection with Neisseria gonorrhoeae is the second most commonly reported communicable disease in the United States. The transmission rate of gonorrhea after sex with someone who has it ranges from 50% to 93%.22 When prescribing postexposure prophylaxis for gonorrhea, it is essential to consider the risk of antimicrobial resistance and local susceptibility data.22
Human immunodeficiency virus
Risk of HIV transmission through sexual contact varies depending on the nature of the exposure, ranging from 0.05% to 0.5%.30
Syphilis
The risk of transmission of syphilis in its early stages (primary and secondary) after sexual exposure is approximately 30%. Transmission requires open lesions such as chancres in primary syphilis and mucocutaneous lesions (mucous patches, condyloma lata) in secondary syphilis.22
After sexual assault
In cases of sexual assault, the risk of sexually transmitted infections may be increased due to trauma and bleeding. Testing for all sexually transmitted infections, including HIV, should be considered on a case-by-case basis.22
Survivors of sexual assault have been shown to be poorly compliant with follow-up visits, and thus provision of postexposure prophylaxis at the time of initial assessment is preferable to deferred treatment.22 The recommended regimen should cover chlamydia, gonorrhea, and trichomoniasis (a single dose of intramuscular ceftriaxone 250 mg, oral azithromycin 1 g, and either oral metronidazole 2 g or tinidazole 2 g), in addition to HIV if the victim presents within 72 hours of exposure (Table 2).22,26
Hepatitis B virus vaccine, not immunoglobulin, should be given if the hepatitis status of the assailant is unknown and the survivor has not been previously vaccinated. Both hepatitis B vaccine and immunoglobulin should be given to unvaccinated survivors if the assailant is known to be hepatitis B surface antigen-positive.22
Human papillomavirus vaccination is recommended for female survivors ages 9 to 26 and male survivors ages 9 to 21.
Emergency contraception should be given if there is a risk of pregnancy.22,26
In many jurisdictions, sexual assault centers provide trained examiners through Sexual Assault Nurse Examiners to perform evidence collection and to provide initial contact with the aftercare resources of the center.
Advice on medical management of sexual assault can be obtained by calling National PEPline (888–448–4911).
INFECTIONS TRANSMITTED BY THE AIRBORNE ROUTE
Airborne transmission of infections occurs by inhalation of droplet nuclei (diameter ≤ 5 μm) generated by coughing and sneezing. Certain procedures (eg, administration of nebulized medication, sputum induction, bronchoscopy) also generate droplets and aerosols, which can transmit organisms.1
Measles
Measles (Table 3) is highly contagious; up to 90% of susceptible individuals develop measles after exposure. The virus is transmitted by direct contact with infectious droplets and by the airborne route. It remains infectious in the air and on surfaces for up to 2 hours; therefore, any type of exposure, even transient, is an indication for postexposure prophylaxis in susceptible individuals.11
Both the measles, mumps, rubella (MMR) vaccine and immune globulin may prevent or modify disease severity in susceptible exposed individuals if given within 3 days of exposure (for the vaccine) or within 6 days of exposure (for immune globulin).31,32
Tuberculosis
Mycobacterium tuberculosis is transmitted from patients with pulmonary or laryngeal tuberculosis, particularly if patients cough and are sputum-positive for acid-fast bacilli. Patients with extrapulmonary tuberculosis or latent tuberculosis infection are not infectious.1,7
Postexposure management of tuberculosis occurs through contact investigation of a newly diagnosed index case of tuberculosis disease. Contacts are categorized as household contacts, close nonhousehold contacts (those having regular, extensive contact with the index case), casual contacts, and transient community contacts. The highest priority for contact investigations should be household contacts, close nonhousehold or casual contacts at high risk of progressing to tuberculosis disease (eg, those with HIV, those on dialysis, or transplant recipients), and unprotected healthcare providers exposed during aerosol-generating procedures.7,33
Postexposure management includes screening exposed individuals for tuberculosis symptoms and performing tuberculin skin testing or interferon-gamma release assay (blood testing) for those who had previously negative results (Table 3). Chest radiography is recommended for exposed immunocompromised individuals, due to high risk of tuberculosis disease and low sensitivity of skin or blood testing, and for those with a documented history of tuberculosis or previous positive skin or blood test.7,33,34
A positive tuberculin skin test for persons with recent contact with tuberculosis is defined as a wheal 5 mm or larger on baseline or follow-up screening. Prior bacillus Calmette-Guérin vaccination status should not be used in the interpretation of tuberculin skin testing in the setting of contact investigation.7,33
All exposed asymptomatic people with a positive result on testing should be treated for latent tuberculosis infection, since treatment reduces the risk of progression to tuberculosis disease by 60% to 90% .7,33,35–37
Varicella and disseminated herpes zoster
Varicella zoster virus is transmitted by direct contact with vesicular fluid of skin lesions and inhalation of aerosols from vesicular fluid or respiratory tract secretions. Varicella (chickenpox) is highly contagious, with a secondary attack rate in susceptible household contacts of 85%.12 Herpes zoster is less contagious than varicella.38
Postexposure prophylaxis against varicella is recommended for susceptible individuals who had household exposure, had face-to-face contact with an infectious patient while indoors, or shared the same hospital room with the patient.12
Postexposure prophylactic options for varicella and herpes zoster include varicella vaccine (not zoster vaccine) and varicella zoster immune globulin (Table 3).12,38–40
Varicella vaccine is approximately 90% effective if given within 3 days of exposure, and 70% effective if given within 5 days.12,39
Antiviral agents should be given if the exposed individual develops manifestations of varicella or herpes zoster.12,38
INFECTIONS TRANSMITTED BY THE DROPLET ROUTE
Droplet transmission occurs when respiratory droplets carrying infectious agents travel directly across short distances (3–6 feet) from the respiratory tract of the infected to mucosal surfaces of the susceptible exposed individual. Droplets are generated during coughing, sneezing, talking, and aerosol-generating procedures. Indirect contact with droplets can also transmit infection.1
Group A streptococcal infection
Although group A streptococcal infection (Table 4) may spread to close contacts of the index case and in closed populations (eg, military recruit camps, schools, institutions), secondary cases of invasive group A streptococcal infection rarely occur in family and institutional contacts.9,41,42
Postexposure prophylaxis for contacts of people with invasive group A streptococcal infection is debated, because it is unknown if antibiotic therapy will decrease the risk of acquiring the infection. It is generally agreed that it should not be routinely given to all contacts. The decision should be based on the clinician’s assessment of each individual’s risk and guidance from the local institution. If indicated, postexposure prophylaxis should be given to household and close contacts, particularly in high-risk groups (eg, Native Americans and those with risk factors such as old age, HIV infection, diabetes mellitus, heart disease, chickenpox, cancer, systemic corticosteroid therapy, other immunosuppressive medications, intravenous drug use, recent surgery or childbirth).9,41,42
Influenza
Influenza (Table 4) causes a significant burden in healthcare settings, given its prevalence and potential to cause outbreaks of severe respiratory illness in hospitalized patients and residents of long-term-care facilities.13,43
Neuraminidase inhibitors are effective as prophylaxis after unprotected exposure to influenza, particularly in outbreak situations. However, their use is not widely recommended, since overuse could lead to antiviral resistance. In selected cases, postexposure prophylaxis may be indicated for close contacts who are at high risk of complications of influenza (eg, age 65 or older, in third trimester of pregnancy or 2 weeks postpartum, morbid obesity, chronic comorbid conditions such as a cardiopulmonary and renal disorder, immunocompromising condition) or who are in close contact with persons at high risk of influenza-related complications.13,44,45
Meningococcal disease
N meningitidis is transmitted from individuals with meningococcal disease or from asymptomatic carriers.8
Postexposure prophylaxis is effective in eradicating N meningiditis and is recommended for all close contacts of patients with invasive meningococcal disease (Table 4).46 Close contacts include household contacts, childcare and preschool contacts, contacts exposed in dormitories or military training centers, those who had direct contact with the index case’s respiratory secretions (eg, intimate kissing, mouth-to-mouth resuscitation, unprotected contact during endotracheal intubation or endotracheal tube management), and passengers seated directly next to an index case on airplane flights of longer than 8 hours.
Postexposure prophylaxis is not indicated for those who had brief contact, those who had contact that did not involve exposure to oral or respiratory secretions, or for close contacts of patients with N meningitidis isolated in nonsterile sites only (eg, oropharynyx, trachea, conjunctiva).8,46
Pertussis
Pertussis is highly contagious, with a secondary attack rate of approximately 80% in susceptible individuals. Approximately one-third of susceptible household contacts develop pertussis after exposure.10
Postexposure prophylaxis for pertussis should be given to all household and close contacts (Table 4).10,47
Rubella
Transmission occurs through droplets or direct contact with nasopharyngeal secretions of an infectious case. Neither MMR vaccine nor immunoglobulin has been shown to prevent rubella in exposed contacts, and they are not recommended.11
INFECTIONS TRANSMITTED BY DIRECT CONTACT
Direct contact transmission includes infectious agents transmitted from an infected or colonized individual to another, whereas indirect contact transmission involves a contaminated intermediate object or person (eg, hands of healthcare providers, electronic thermometers, surgical instruments).1
There are no available postexposure prophylactic regimens for the organisms most commonly transmitted by this route (eg, methicillin-resistant Staphylococcus aureus, Clostridium difficile), but transmission can be prevented with adherence to standard precautions, including hand hygiene.1
Hepatitis A
Person-to-person transmission of hepatitis A virus occurs via the fecal-oral route. Common-source outbreaks and sporadic cases can occur from exposure to food or water contaminated with feces.1,15
Postexposure prophylaxis is indicated only for nonimmune close contacts (eg, household and sexual contacts) (Table 5). Without this treatment, secondary attack rates of 15% to 30% have been reported among households.15,48 Both hepatitis A vaccine and immune globulin are effective in preventing and ameliorating symptomatic hepatitis A infection. Advantages of vaccination include induction of longer-lasting immunity (at least 2 years), greater ease of administration, and lower cost than immune globulin.15,48
Scabies
Scabies is an infestation of the skin by the mite Sarcoptes scabiei var hominis. Person-to-person transmission typically occurs through direct, prolonged skin-to-skin contact with an infested person (eg, household and sexual contacts). However, crusted scabies can be transmitted after brief skin-to-skin contact or by exposure to bedding, clothing, or furniture used by the infested person.
All potentially infested persons should be treated concomitantly (Table 5).14,49
INFECTIONS TRANSMITTED BY MAMMAL BITES AND INJURIES
Bites and injury wounds account for approximately 1% of all visits to emergency departments.50 Human bites are associated with a risk of infection by blood-borne pathogens, herpes simplex infection, and bacterial infections (eg, skin and soft-tissue infections, bacteremia). Animal bites are associated with a risk of bacterial infections, rabies, tetanus, hepatitis B virus, and monkeypox.50
Rabies
Human rabies (Table 5) is almost always fatal. Essential factors in determining the need for postexposure prophylaxis include knowledge of the epidemiology of animal rabies in the area where the contact occurred and the species of animal involved, availability of the animal for observation or rabies testing, health status of the biting animal, and vaccination history of both the animal and exposed individual.6 Clinicians should seek assistance from public health officials for evaluating exposures and determining the need for postexposure prophylaxis in situations that are not routine.51
High-risk wild animals associated with rabies in North America include bats, raccoons, skunks, foxes, coyotes, bobcats, and woodchucks. Bats are the most common source of human rabies infections in the United States, and transmission can occur from minor, sometimes unnoticed, bites. The types of exposures that require postexposure prophylaxis include bites, abrasions, scratches, and contamination of mucous membranes or open wound with saliva or neural tissue of a suspected rabid animal.
Human-to-human transmission of rabies can rarely occur through exposure of mucous membrane or nonintact skin to an infectious material (saliva, tears, neural tissue), in addition to organ transplantation.6
Animal capture and testing is a strategy for excluding rabies risk and reducing the need for postexposure prophylaxis. A dog, cat, or ferret that bites a person should be confined and observed for 10 days without administering postexposure prophylaxis for rabies, unless the bite or exposure is on the face or neck, in which case this treatment should be given immediately.6 If the observed biting animal lives and remains healthy, postexposure prophylaxis is not recommended. However, if signs suggestive of rabies develop, postexposure prophylaxis should be given and the animal should be euthanized, with testing of brain tissue for rabies virus. Postexposure prophylaxis should be discontinued if rabies testing is negative.
The combination of rabies vaccine and human rabies immunoglobulin is nearly 100% effective in preventing rabies if administered in a timely and accurate fashion after exposure (Table 5).6
Tetanus
Tetanus transmission can occur through injuries ranging from small cuts to severe trauma and through contact with contaminated objects (eg, bites, nails, needles, splinters, neonates whose umbilical cord is cut with contaminated surgical instruments, and during circumcision or piercing with contaminated instruments).5
Tetanus is almost completely preventable with vaccination, and timely administration of postexposure prophylaxis (tetanus toxoid-containing vaccine, tetanus immune globulin) decreases disease severity (Table 5).2,5,52
People who have been exposed to an infectious disease should be evaluated promptly and systematically, whether they are healthcare professionals at work,1 patients, or contacts of patients. The primary goals are to prevent acquisition and transmission of the infection, allay the exposed person’s anxiety, and avoid unnecessary interventions and loss of work days.1,2 Some may need postexposure prophylaxis.
ESSENTIAL ELEMENTS OF POSTEXPOSURE MANAGEMENT
Because postexposure management can be challenging, an experienced clinician or expert consultant (eg, infectious disease specialist, infection control provider, or public health officer) should be involved. Institution-specific policies and procedures for postexposure prophylaxis and testing should be followed.1,2
Postexposure management should include the following elements:
- Immediate care of the wound or other site of exposure in cases of blood-borne exposures and tetanus- and rabies-prone injuries. This includes thoroughly washing with soap and water or cleansing with an antiseptic agent, flushing affected mucous membranes with water, and debridement of devitalized tissue.1–6
- Deciding whether postexposure prophylaxis is indicated and, if so, the type, dose, route, and duration.
- Initiating prophylaxis as soon as possible.
- Determining an appropriate baseline assessment and follow-up plan for the exposed individual.
- Counseling exposed women who are pregnant or breast-feeding about the risks and benefits of postexposure prophylaxis to mother, fetus, and infant.
- Identifying required infection control precautions, including work and school restriction, for exposed and source individuals.
- Counseling and psychological support for exposed individuals, who need to know about the risks of acquiring the infection and transmitting it to others, infection control precautions, benefits, and adverse effects of postexposure prophylaxis, the importance of adhering to the regimen, and the follow-up plan. They must understand that this treatment may not completely prevent the infection, and they should seek medical attention if they develop fever or any symptoms or signs of the infection of concern.1,2
IS POSTEXPOSURE PROPHYLAXIS INDICATED?
Postexposure management begins with an assessment to determine whether the exposure is likely to result in infection; whether the exposed individual is susceptible to the infection of concern or is at greater risk of complications from it than the general population; and whether postexposure prophylaxis is needed. This involves a complete focused history, physical examination, and laboratory testing of the potentially exposed individual and of the source, if possible.1,2
Postexposure prophylaxis should begin as soon as possible to maximize its effects while awaiting the results of further diagnostic tests. However, if the exposed individual seeks care after the recommended period, prophylactic therapy can still be effective for certain infections that have a long incubation period, such as tetanus and rabies.5,6 The choice of regimen should be guided by efficacy, safety, cost, toxicity, ease of adherence, drug interactions, and antimicrobial resistance.1,2
HOW GREAT IS THE RISK OF INFECTION?
Exposed individuals are not all at the same risk of acquiring a given infection. The risk depends on:
- Type and extent of exposure (see below)
- Characteristics of the infectious agent (eg, virulence, infectious dose)
- Status of the infectious source (eg, whether the disease is in its infectious period or is being treated); effective treatment can shorten the duration of microbial shedding and subsequently reduce risk of transmission of certain infections such as tuberculosis, meningococcal infection, invasive group A streptococcal infection, and pertussis7–10
- Immune status of the exposed individual (eg, prior infection or vaccination), since people who are immune to the infection of concern usually do not need postexposure prophylaxis2
- Adherence to infection prevention and control principles; postexposure prophylaxis may not be required if the potentially exposed individual was wearing appropriate personal protective equipment such as a surgical mask, gown, and gloves and was following standard precautions.1
WHO SHOULD BE RESTRICTED FROM WORK OR SCHOOL?
Most people without symptoms who were exposed to most types of infections do not need to stay home from work or school. However, susceptible people, particularly healthcare providers exposed to measles, mumps, rubella, and varicella, should be excluded from work while they are capable of transmitting these diseases, even if they have no symptoms.11,12 Moreover, people with symptoms with infections primarily transmitted via the airborne, droplet, or contact route should be restricted from work until no longer infectious.1,2,7,9–15
Most healthcare institutions have clear protocols for managing occupational exposures to infectious diseases, in particular for blood-borne pathogens such as human immunodeficiency virus (HIV). The protocol should include appropriate evaluation and laboratory testing of the source patient and exposed healthcare provider, as well as procedures for counseling the exposed provider, identifying and procuring an initial prophylactic regimen for timely administration, a mechanism for formal expert consultation (eg, with an in-house infectious diseases consultant), and a plan for outpatient follow-up.
The next section reviews postexposure management of common infections categorized by mode of transmission, including the risk of transmission, initial and follow-up evaluation, and considerations for postexposure prophylaxis.
BLOOD-BORNE INFECTIONS
Blood-borne pathogens can be transmitted by accidental needlesticks or cuts or by exposure of the eyes, mucous membranes, or nonintact skin to blood, tissue, or other potentially infectious body fluids—cerebrospinal, pericardial, pleural, peritoneal, synovial, and amniotic fluid, semen, and vaginal secretions. (Feces, nasal secretions, saliva, sputum, sweat, tears, urine, and vomitus are considered noninfectious for blood-borne pathogens unless they contain blood.16)
Healthcare professionals are commonly exposed to blood-borne pathogens as a result of needlestick injuries, and these exposures tend to be underreported.17
When someone has been exposed to blood or other infectious body fluids, the source individual and the exposed individual should be assessed for risk factors for hepatitis B virus, hepatitis C virus, HIV, and other blood-borne pathogens.3,4,16,18 If the disease status for these viruses is unknown, the source and exposed individual should be tested in accordance with institutional policies regarding consent to testing. Testing of needles or sharp instruments implicated in an exposure is not recommended.3,4,16,18
Determining the need for prophylaxis after exposure to an unknown source such as a disposed needle can be challenging. Assessment should be made on a case-by-case basis, depending on the known prevalence of the infection of concern in the local community. The risk of transmission in most source-unknown exposures is negligible.3,4,18 However, hepatitis B vaccine and hepatitis B immunoglobulin should be used liberally as postexposure prophylaxis for previously unvaccinated healthcare providers exposed to an unknown source.3,4,16,18
Hepatitis B
Hepatitis B virus (Table 1) is the most infectious of the common blood-borne viruses. The risk of transmission after percutaneous exposure to hepatitis B-infected blood ranges from 1% to 30% based on hepatitis Be antigen status and viral load (based on hepatitis B viral DNA).1,2,4,16
Hepatitis B vaccine or immunoglobulin, or both, are recommended for postexposure prophylaxis in pregnant women, based on evidence that perinatal transmission was reduced by 70% to 90% when these were given within 12 to 24 hours of exposure.4,16,19
Hepatitis C
The risk of infection after percutaneous exposure to hepatitis C virus-infected blood is estimated to be 1.8% per exposure.16 The risk is lower with exposure of a mucous membrane or nonintact skin to blood, fluids, or tissues from hepatitis C-infected patients.16,18
Since there is no effective postexposure prophylactic regimen, the goal of postexposure assessment of hepatitis C is early identification of infection (by monitoring the patient to see if he or she seroconverts) and, if infection is present, referral to an experienced clinician for further evaluation (Table 1). However, data supporting the utility of direct-acting anti-hepatitis C antiviral drugs as postexposure prophylaxis after occupational exposure to hepatitis C are lacking.
Human immunodeficiency virus
The estimated risk of HIV transmission from a known infected source after percutaneous exposure is 0.3%, and after mucosal exposures it is 0.09%.20
If postexposure prophylaxis is indicated, it should be a three-drug regimen (Table 1).3,18 The recommended antiretroviral therapies have been proven effective in clinical trials of HIV treatment, not for postexposure prophylaxis per se, but they are recommended because they are effective, safe, tolerable, and associated with high adherence rates.3,16,18,21 If the source individual is known to have HIV infection, information about his or her stage of infection, CD4+ T-cell count, results of viral load testing, current and previous antiretroviral therapy, and results of any genotypic viral resistance testing will guide the choice of postexposure prophylactic regimen.3,18
The clinician should give the exposed patient a starter pack of 5 to 7 days of medication, give the first dose then and there, and arrange follow-up with an experienced clinician within a few days of the exposure to determine whether a complete 30-day course is needed.3,16,18
SEXUALLY TRANSMITTED INFECTIONS
In the case of sexually transmitted infections, “exposure” means unprotected sexual contact with someone who has a sexually transmitted infection.22 People with sexually transmitted infections often have no symptoms but can still transmit the infection. Thus, people at risk should be identified and screened for all suspected sexually transmitted infections.23–25
Patients with sexually transmitted infections should be instructed to refer their sex partners for evaluation and treatment to prevent further transmission and reinfection. Assessment of exposed partners includes a medical history, physical examination, microbiologic testing for all potential sexually transmitted infections, and eligibility for hepatitis A virus, hepatitis B virus, and human papillomavirus vaccines.22 Ideally, exposed partners should be reassessed within 1 to 2 weeks to follow up testing results and to monitor for side effects of and adherence to postexposure prophylaxis, if applicable.
Public health departments should be notified of sexually transmitted infections such as gonorrhea, chlamydia, chancroid, and syphilis.22
Expedited partner therapy, in which index patients deliver the medication or a prescription for it directly to their partners, is an alternative for partner management where legally allowed by state and local health departments (see www.cdc.gov/std/ept/legal/).22
Recommended postexposure prophylactic regimens for sexually transmitted infections (Table 2) are based on their efficacy in the treatment of these infections.22,26–28 The regimen for HIV prophylaxis is the same as in Table 1.3,18,26
Chlamydia
Chlamydia is the most commonly reported communicable disease in the United States. The risk of transmission after sexual intercourse with a person who has an active infection is approximately 65% and increases with the number of exposures.22,29
Gonorrhea
Infection with Neisseria gonorrhoeae is the second most commonly reported communicable disease in the United States. The transmission rate of gonorrhea after sex with someone who has it ranges from 50% to 93%.22 When prescribing postexposure prophylaxis for gonorrhea, it is essential to consider the risk of antimicrobial resistance and local susceptibility data.22
Human immunodeficiency virus
Risk of HIV transmission through sexual contact varies depending on the nature of the exposure, ranging from 0.05% to 0.5%.30
Syphilis
The risk of transmission of syphilis in its early stages (primary and secondary) after sexual exposure is approximately 30%. Transmission requires open lesions such as chancres in primary syphilis and mucocutaneous lesions (mucous patches, condyloma lata) in secondary syphilis.22
After sexual assault
In cases of sexual assault, the risk of sexually transmitted infections may be increased due to trauma and bleeding. Testing for all sexually transmitted infections, including HIV, should be considered on a case-by-case basis.22
Survivors of sexual assault have been shown to be poorly compliant with follow-up visits, and thus provision of postexposure prophylaxis at the time of initial assessment is preferable to deferred treatment.22 The recommended regimen should cover chlamydia, gonorrhea, and trichomoniasis (a single dose of intramuscular ceftriaxone 250 mg, oral azithromycin 1 g, and either oral metronidazole 2 g or tinidazole 2 g), in addition to HIV if the victim presents within 72 hours of exposure (Table 2).22,26
Hepatitis B virus vaccine, not immunoglobulin, should be given if the hepatitis status of the assailant is unknown and the survivor has not been previously vaccinated. Both hepatitis B vaccine and immunoglobulin should be given to unvaccinated survivors if the assailant is known to be hepatitis B surface antigen-positive.22
Human papillomavirus vaccination is recommended for female survivors ages 9 to 26 and male survivors ages 9 to 21.
Emergency contraception should be given if there is a risk of pregnancy.22,26
In many jurisdictions, sexual assault centers provide trained examiners through Sexual Assault Nurse Examiners to perform evidence collection and to provide initial contact with the aftercare resources of the center.
Advice on medical management of sexual assault can be obtained by calling National PEPline (888–448–4911).
INFECTIONS TRANSMITTED BY THE AIRBORNE ROUTE
Airborne transmission of infections occurs by inhalation of droplet nuclei (diameter ≤ 5 μm) generated by coughing and sneezing. Certain procedures (eg, administration of nebulized medication, sputum induction, bronchoscopy) also generate droplets and aerosols, which can transmit organisms.1
Measles
Measles (Table 3) is highly contagious; up to 90% of susceptible individuals develop measles after exposure. The virus is transmitted by direct contact with infectious droplets and by the airborne route. It remains infectious in the air and on surfaces for up to 2 hours; therefore, any type of exposure, even transient, is an indication for postexposure prophylaxis in susceptible individuals.11
Both the measles, mumps, rubella (MMR) vaccine and immune globulin may prevent or modify disease severity in susceptible exposed individuals if given within 3 days of exposure (for the vaccine) or within 6 days of exposure (for immune globulin).31,32
Tuberculosis
Mycobacterium tuberculosis is transmitted from patients with pulmonary or laryngeal tuberculosis, particularly if patients cough and are sputum-positive for acid-fast bacilli. Patients with extrapulmonary tuberculosis or latent tuberculosis infection are not infectious.1,7
Postexposure management of tuberculosis occurs through contact investigation of a newly diagnosed index case of tuberculosis disease. Contacts are categorized as household contacts, close nonhousehold contacts (those having regular, extensive contact with the index case), casual contacts, and transient community contacts. The highest priority for contact investigations should be household contacts, close nonhousehold or casual contacts at high risk of progressing to tuberculosis disease (eg, those with HIV, those on dialysis, or transplant recipients), and unprotected healthcare providers exposed during aerosol-generating procedures.7,33
Postexposure management includes screening exposed individuals for tuberculosis symptoms and performing tuberculin skin testing or interferon-gamma release assay (blood testing) for those who had previously negative results (Table 3). Chest radiography is recommended for exposed immunocompromised individuals, due to high risk of tuberculosis disease and low sensitivity of skin or blood testing, and for those with a documented history of tuberculosis or previous positive skin or blood test.7,33,34
A positive tuberculin skin test for persons with recent contact with tuberculosis is defined as a wheal 5 mm or larger on baseline or follow-up screening. Prior bacillus Calmette-Guérin vaccination status should not be used in the interpretation of tuberculin skin testing in the setting of contact investigation.7,33
All exposed asymptomatic people with a positive result on testing should be treated for latent tuberculosis infection, since treatment reduces the risk of progression to tuberculosis disease by 60% to 90% .7,33,35–37
Varicella and disseminated herpes zoster
Varicella zoster virus is transmitted by direct contact with vesicular fluid of skin lesions and inhalation of aerosols from vesicular fluid or respiratory tract secretions. Varicella (chickenpox) is highly contagious, with a secondary attack rate in susceptible household contacts of 85%.12 Herpes zoster is less contagious than varicella.38
Postexposure prophylaxis against varicella is recommended for susceptible individuals who had household exposure, had face-to-face contact with an infectious patient while indoors, or shared the same hospital room with the patient.12
Postexposure prophylactic options for varicella and herpes zoster include varicella vaccine (not zoster vaccine) and varicella zoster immune globulin (Table 3).12,38–40
Varicella vaccine is approximately 90% effective if given within 3 days of exposure, and 70% effective if given within 5 days.12,39
Antiviral agents should be given if the exposed individual develops manifestations of varicella or herpes zoster.12,38
INFECTIONS TRANSMITTED BY THE DROPLET ROUTE
Droplet transmission occurs when respiratory droplets carrying infectious agents travel directly across short distances (3–6 feet) from the respiratory tract of the infected to mucosal surfaces of the susceptible exposed individual. Droplets are generated during coughing, sneezing, talking, and aerosol-generating procedures. Indirect contact with droplets can also transmit infection.1
Group A streptococcal infection
Although group A streptococcal infection (Table 4) may spread to close contacts of the index case and in closed populations (eg, military recruit camps, schools, institutions), secondary cases of invasive group A streptococcal infection rarely occur in family and institutional contacts.9,41,42
Postexposure prophylaxis for contacts of people with invasive group A streptococcal infection is debated, because it is unknown if antibiotic therapy will decrease the risk of acquiring the infection. It is generally agreed that it should not be routinely given to all contacts. The decision should be based on the clinician’s assessment of each individual’s risk and guidance from the local institution. If indicated, postexposure prophylaxis should be given to household and close contacts, particularly in high-risk groups (eg, Native Americans and those with risk factors such as old age, HIV infection, diabetes mellitus, heart disease, chickenpox, cancer, systemic corticosteroid therapy, other immunosuppressive medications, intravenous drug use, recent surgery or childbirth).9,41,42
Influenza
Influenza (Table 4) causes a significant burden in healthcare settings, given its prevalence and potential to cause outbreaks of severe respiratory illness in hospitalized patients and residents of long-term-care facilities.13,43
Neuraminidase inhibitors are effective as prophylaxis after unprotected exposure to influenza, particularly in outbreak situations. However, their use is not widely recommended, since overuse could lead to antiviral resistance. In selected cases, postexposure prophylaxis may be indicated for close contacts who are at high risk of complications of influenza (eg, age 65 or older, in third trimester of pregnancy or 2 weeks postpartum, morbid obesity, chronic comorbid conditions such as a cardiopulmonary and renal disorder, immunocompromising condition) or who are in close contact with persons at high risk of influenza-related complications.13,44,45
Meningococcal disease
N meningitidis is transmitted from individuals with meningococcal disease or from asymptomatic carriers.8
Postexposure prophylaxis is effective in eradicating N meningiditis and is recommended for all close contacts of patients with invasive meningococcal disease (Table 4).46 Close contacts include household contacts, childcare and preschool contacts, contacts exposed in dormitories or military training centers, those who had direct contact with the index case’s respiratory secretions (eg, intimate kissing, mouth-to-mouth resuscitation, unprotected contact during endotracheal intubation or endotracheal tube management), and passengers seated directly next to an index case on airplane flights of longer than 8 hours.
Postexposure prophylaxis is not indicated for those who had brief contact, those who had contact that did not involve exposure to oral or respiratory secretions, or for close contacts of patients with N meningitidis isolated in nonsterile sites only (eg, oropharynyx, trachea, conjunctiva).8,46
Pertussis
Pertussis is highly contagious, with a secondary attack rate of approximately 80% in susceptible individuals. Approximately one-third of susceptible household contacts develop pertussis after exposure.10
Postexposure prophylaxis for pertussis should be given to all household and close contacts (Table 4).10,47
Rubella
Transmission occurs through droplets or direct contact with nasopharyngeal secretions of an infectious case. Neither MMR vaccine nor immunoglobulin has been shown to prevent rubella in exposed contacts, and they are not recommended.11
INFECTIONS TRANSMITTED BY DIRECT CONTACT
Direct contact transmission includes infectious agents transmitted from an infected or colonized individual to another, whereas indirect contact transmission involves a contaminated intermediate object or person (eg, hands of healthcare providers, electronic thermometers, surgical instruments).1
There are no available postexposure prophylactic regimens for the organisms most commonly transmitted by this route (eg, methicillin-resistant Staphylococcus aureus, Clostridium difficile), but transmission can be prevented with adherence to standard precautions, including hand hygiene.1
Hepatitis A
Person-to-person transmission of hepatitis A virus occurs via the fecal-oral route. Common-source outbreaks and sporadic cases can occur from exposure to food or water contaminated with feces.1,15
Postexposure prophylaxis is indicated only for nonimmune close contacts (eg, household and sexual contacts) (Table 5). Without this treatment, secondary attack rates of 15% to 30% have been reported among households.15,48 Both hepatitis A vaccine and immune globulin are effective in preventing and ameliorating symptomatic hepatitis A infection. Advantages of vaccination include induction of longer-lasting immunity (at least 2 years), greater ease of administration, and lower cost than immune globulin.15,48
Scabies
Scabies is an infestation of the skin by the mite Sarcoptes scabiei var hominis. Person-to-person transmission typically occurs through direct, prolonged skin-to-skin contact with an infested person (eg, household and sexual contacts). However, crusted scabies can be transmitted after brief skin-to-skin contact or by exposure to bedding, clothing, or furniture used by the infested person.
All potentially infested persons should be treated concomitantly (Table 5).14,49
INFECTIONS TRANSMITTED BY MAMMAL BITES AND INJURIES
Bites and injury wounds account for approximately 1% of all visits to emergency departments.50 Human bites are associated with a risk of infection by blood-borne pathogens, herpes simplex infection, and bacterial infections (eg, skin and soft-tissue infections, bacteremia). Animal bites are associated with a risk of bacterial infections, rabies, tetanus, hepatitis B virus, and monkeypox.50
Rabies
Human rabies (Table 5) is almost always fatal. Essential factors in determining the need for postexposure prophylaxis include knowledge of the epidemiology of animal rabies in the area where the contact occurred and the species of animal involved, availability of the animal for observation or rabies testing, health status of the biting animal, and vaccination history of both the animal and exposed individual.6 Clinicians should seek assistance from public health officials for evaluating exposures and determining the need for postexposure prophylaxis in situations that are not routine.51
High-risk wild animals associated with rabies in North America include bats, raccoons, skunks, foxes, coyotes, bobcats, and woodchucks. Bats are the most common source of human rabies infections in the United States, and transmission can occur from minor, sometimes unnoticed, bites. The types of exposures that require postexposure prophylaxis include bites, abrasions, scratches, and contamination of mucous membranes or open wound with saliva or neural tissue of a suspected rabid animal.
Human-to-human transmission of rabies can rarely occur through exposure of mucous membrane or nonintact skin to an infectious material (saliva, tears, neural tissue), in addition to organ transplantation.6
Animal capture and testing is a strategy for excluding rabies risk and reducing the need for postexposure prophylaxis. A dog, cat, or ferret that bites a person should be confined and observed for 10 days without administering postexposure prophylaxis for rabies, unless the bite or exposure is on the face or neck, in which case this treatment should be given immediately.6 If the observed biting animal lives and remains healthy, postexposure prophylaxis is not recommended. However, if signs suggestive of rabies develop, postexposure prophylaxis should be given and the animal should be euthanized, with testing of brain tissue for rabies virus. Postexposure prophylaxis should be discontinued if rabies testing is negative.
The combination of rabies vaccine and human rabies immunoglobulin is nearly 100% effective in preventing rabies if administered in a timely and accurate fashion after exposure (Table 5).6
Tetanus
Tetanus transmission can occur through injuries ranging from small cuts to severe trauma and through contact with contaminated objects (eg, bites, nails, needles, splinters, neonates whose umbilical cord is cut with contaminated surgical instruments, and during circumcision or piercing with contaminated instruments).5
Tetanus is almost completely preventable with vaccination, and timely administration of postexposure prophylaxis (tetanus toxoid-containing vaccine, tetanus immune globulin) decreases disease severity (Table 5).2,5,52
- Siegel JD, Rhinehart E, Jackson M, Chiarello L; Health Care Infection Control Practices Advisory Committee. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control 2007; 35(suppl 2): S65–S164.
- Advisory Committee on Immunization Practices; Centers for Disease Control and Prevention. Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:1–45.
- Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol 2013; 34: 875–892.
- Schille S, Murphy TV, Sawyer M, et al; Centers for Disease Control and Prevention (CDC). CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR Recomm Rep 2013; 62:1–19.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:13–15.
- Manning SE, Rupprecht CE, Fishbein D, et al; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Human rabies prevention—United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2008; 57:1–28.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon R, Centers for Disease Control and Prevention (CDC). Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Morb Mortal Wkly Rep 2005; 54:1–141.
- Cohn AC, MacNeil JR, Clark TA, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2013; 62:1–28.
- Prevention of Invasive Group A Streptococcal Infections Workshop Participants. Prevention of invasive group A streptococcal disease among household contacts of case patients and among postpartum and postsurgical patients: recommendations from the Centers for Disease Control and Prevention. Clin Infect Dis 2002; 35:950–959.
- Tiwari T, Murphy TV, Moran J; National Immunization Program, Centers for Disease Control and Prevention (CDC). Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis: 2005 CDC Guidelines. MMWR Morb Mortal Wkly Rep 2005; 54:1–16.
- McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS; Centers for Disease Control and Prevention (CDC). Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2013; 62:1–34.
- Marin M, Guris D, Chaves SS, Schmid S, Seward JF; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56:1–40.
- Harper SA, Bradley JS, Englund JA, et al; Expert Panel of the Infectious Diseases Society of America. Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1003–1032.
- Centers for Disease Control and Prevention (CDC). Scabies. www.cdc.gov/parasites/scabies/. Accessed November 4, 2016.
- Advisory Committee on Immunization Practices (ACIP); Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2006; 55:1–23.
- US Public Health Service. Updated US Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep 2001; 50:1–52.
- Treakle AM, Schultz M, Giannakos GP, Joyce PC, Gordin FM. Evaluating a decade of exposures to blood and body fluids in an inner-city teaching hospital. Infect Control Hosp Epidemiol 2011; 32:903–907.
- New York State Department of Health AIDS Institute. Update: HIV prophylaxis following non-occupational exposure. www.hivguidelines.org/clinical-guidelines/post-exposure-prophylaxis/hiv-prophylaxis-following-non-occupational-exposure/. Accessed November 4, 2016.
- Beasley RP, Hwang LY, Lee GC, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet 1983; 2:1099–1102.
- Baggaley RF, Boily MC, White RG, Alary M. Risk of HIV-1 transmission for parenteral exposure and blood transfusion: a systematic review and meta-analysis. AIDS 2006; 20:805–812.
- McAllister J, Read P, McNulty A, Tong WW, Ingersoll A, Carr A. Raltegravir-emtricitabine-tenofovir as HIV nonoccupational post-exposure prophylaxis in men who have sex with men: safety, tolerability and adherence. HIV Med 2014; 15:13–22.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137.
- US Preventive Services Task Force (USPSTF). Final recommendation statement: chlamydia and gonorrhea: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/chlamydia-and-gonorrhea-screening. Accessed November 4, 2016.
- US Preventive Services Task Force (USPSTF). Human immunodeficiency virus (HIV) infection: screening. www.uspreventiveservicestaskforce.org/uspstf/uspshivi.htm. Accessed November 4, 2016.
- US Preventive Services Task Force (USPSTF). Screening for syphilis. www.uspreventiveservicestaskforce.org/uspstf/uspssyph.htm#update. Accessed November 4, 2016.
- Smith DK, Grohskopf LA, Black RJ, et al; US Department of Health and Human Services. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the US Department of Health and Human Services. MMWR Recomm Rep 2005; 54:1–20.
- Lin JS, Donegan SP, Heeren TC, et al. Transmission of Chlamydia trachomatis and Neisseria gonorrhoeae among men with urethritis and their female sex partners. J Infect Dis 1998; 178:1707–1712.
- Varghese B, Maher JE, Peterman TA, Branson BM, Steketee RW. Reducing the risk of sexual HIV transmission: quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use. Sex Transm Dis 2002; 29:38–43.
- Gülmezoglu AM, Azhar M. Interventions for trichomoniasis in pregnancy. Cochrane Database Syst Rev 2011; (5):CD000220.
- Forna F, Gülmezoglu AM. Interventions for treating trichomoniasis in women. Cochrane Database Syst Rev 2003; (2):CD000218.
- Rice P, Young Y, Cohen B, Ramsay M. MMR immunization after contact with measles virus. Lancet 2004; 363:569–570.
- Young MK, Nimmo GR, Cripps AW, Jones MA. Post-exposure passive immunization for preventing measles. Cochrane Database Syst Rev 2014; 4:CD010056.
- National Tuberculosis Controllers Association; Centers for Disease Control and Prevention (CDC). Guidelines for the investigation of contacts of persons with infectious tuberculosis. Recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Recomm Rep 2005; 54:1–47.
- Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Morb Mortal Wkly Rep 2010; 59:1–25.
- Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Morb Mortal Wkly Rep 2000; 49:1–51.
- Stagg HR, Zenner D, Harris RJ, Munoz L, Lipman MC, Abubakar I. Treatment of latent tuberculosis infection: a network meta-analysis. Ann Intern Med 2014; 161:419–428.
- Centers for Disease Control and Prevention (CDC). Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep 2011; 60:1650–1653.
- Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2008; 57:1–30.
- Macartney K, Heywood A, McIntyre P. Vaccines for post-exposure prophylaxis against varicella (chickenpox) in children and adults. Cochrane Database Syst Rev 2014; 6:CD001833.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of VariZIG—United States, 2013. MMWR Morb Mortal Wkly Rep 2013; 62: 574–576.
- Public Health Agency of Canada. Guidelines for the prevention and control of invasive group A streptococcal disease. Can Commun Dis Rep 2006; 32(suppl 2):1–26.
- Steer JA, Lamagni T, Healy B, et al. Guidelines for prevention and control of group A streptococcal infection in acute healthcare and maternity settings in the UK. J Infect 2012; 64:1–18.
- Grohskopf LA, Olsen SJ, Sokolow LZ, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2014–15 influenza season. MMWR Morb Mortal Wkly Rep 2014; 63: 691–697.
- Fiore AE, Fry A, Shay D, et al; Centers for Disease Control and Prevention (CDC). Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2011; 60:1–24.
- Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev 2014; 4:CD008965.
- Zalmanovici Trestioreanu A, Fraser A, Gafter-Gvili A, Paul M, Leibovici L. Antibiotics for preventing meningococcal infections. Cochrane Database Syst Rev 2013; 10:CD004785.
- Altunaiji S, Kukuruzovic R, Curtis N, Massie J. Antibiotics for whooping cough (pertussis). Cochrane Database Syst Rev 2007: CD004404.
- Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56:1080–1084.
- FitzGerald D, Grainger RJ, Reid A. Interventions for preventing the spread of infestation in close contacts of people with scabies. Cochrane Database Syst Rev 2014; 2:CD009943.
- Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:e10–e52.
- Rupprecht CE, Briggs D, Brown CM, et al; Centers for Disease Control and Prevention (CDC). Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies—recommendations of the Advisory Committee on Immunization Practice. MMWR Recomm Rep 2010; 59:1–9.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine in adults aged 65 years and older—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep 2012; 61:468–470.
- Siegel JD, Rhinehart E, Jackson M, Chiarello L; Health Care Infection Control Practices Advisory Committee. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control 2007; 35(suppl 2): S65–S164.
- Advisory Committee on Immunization Practices; Centers for Disease Control and Prevention. Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:1–45.
- Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol 2013; 34: 875–892.
- Schille S, Murphy TV, Sawyer M, et al; Centers for Disease Control and Prevention (CDC). CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR Recomm Rep 2013; 62:1–19.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:13–15.
- Manning SE, Rupprecht CE, Fishbein D, et al; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Human rabies prevention—United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2008; 57:1–28.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon R, Centers for Disease Control and Prevention (CDC). Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Morb Mortal Wkly Rep 2005; 54:1–141.
- Cohn AC, MacNeil JR, Clark TA, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2013; 62:1–28.
- Prevention of Invasive Group A Streptococcal Infections Workshop Participants. Prevention of invasive group A streptococcal disease among household contacts of case patients and among postpartum and postsurgical patients: recommendations from the Centers for Disease Control and Prevention. Clin Infect Dis 2002; 35:950–959.
- Tiwari T, Murphy TV, Moran J; National Immunization Program, Centers for Disease Control and Prevention (CDC). Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis: 2005 CDC Guidelines. MMWR Morb Mortal Wkly Rep 2005; 54:1–16.
- McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS; Centers for Disease Control and Prevention (CDC). Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2013; 62:1–34.
- Marin M, Guris D, Chaves SS, Schmid S, Seward JF; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56:1–40.
- Harper SA, Bradley JS, Englund JA, et al; Expert Panel of the Infectious Diseases Society of America. Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1003–1032.
- Centers for Disease Control and Prevention (CDC). Scabies. www.cdc.gov/parasites/scabies/. Accessed November 4, 2016.
- Advisory Committee on Immunization Practices (ACIP); Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2006; 55:1–23.
- US Public Health Service. Updated US Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep 2001; 50:1–52.
- Treakle AM, Schultz M, Giannakos GP, Joyce PC, Gordin FM. Evaluating a decade of exposures to blood and body fluids in an inner-city teaching hospital. Infect Control Hosp Epidemiol 2011; 32:903–907.
- New York State Department of Health AIDS Institute. Update: HIV prophylaxis following non-occupational exposure. www.hivguidelines.org/clinical-guidelines/post-exposure-prophylaxis/hiv-prophylaxis-following-non-occupational-exposure/. Accessed November 4, 2016.
- Beasley RP, Hwang LY, Lee GC, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet 1983; 2:1099–1102.
- Baggaley RF, Boily MC, White RG, Alary M. Risk of HIV-1 transmission for parenteral exposure and blood transfusion: a systematic review and meta-analysis. AIDS 2006; 20:805–812.
- McAllister J, Read P, McNulty A, Tong WW, Ingersoll A, Carr A. Raltegravir-emtricitabine-tenofovir as HIV nonoccupational post-exposure prophylaxis in men who have sex with men: safety, tolerability and adherence. HIV Med 2014; 15:13–22.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137.
- US Preventive Services Task Force (USPSTF). Final recommendation statement: chlamydia and gonorrhea: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/chlamydia-and-gonorrhea-screening. Accessed November 4, 2016.
- US Preventive Services Task Force (USPSTF). Human immunodeficiency virus (HIV) infection: screening. www.uspreventiveservicestaskforce.org/uspstf/uspshivi.htm. Accessed November 4, 2016.
- US Preventive Services Task Force (USPSTF). Screening for syphilis. www.uspreventiveservicestaskforce.org/uspstf/uspssyph.htm#update. Accessed November 4, 2016.
- Smith DK, Grohskopf LA, Black RJ, et al; US Department of Health and Human Services. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the US Department of Health and Human Services. MMWR Recomm Rep 2005; 54:1–20.
- Lin JS, Donegan SP, Heeren TC, et al. Transmission of Chlamydia trachomatis and Neisseria gonorrhoeae among men with urethritis and their female sex partners. J Infect Dis 1998; 178:1707–1712.
- Varghese B, Maher JE, Peterman TA, Branson BM, Steketee RW. Reducing the risk of sexual HIV transmission: quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use. Sex Transm Dis 2002; 29:38–43.
- Gülmezoglu AM, Azhar M. Interventions for trichomoniasis in pregnancy. Cochrane Database Syst Rev 2011; (5):CD000220.
- Forna F, Gülmezoglu AM. Interventions for treating trichomoniasis in women. Cochrane Database Syst Rev 2003; (2):CD000218.
- Rice P, Young Y, Cohen B, Ramsay M. MMR immunization after contact with measles virus. Lancet 2004; 363:569–570.
- Young MK, Nimmo GR, Cripps AW, Jones MA. Post-exposure passive immunization for preventing measles. Cochrane Database Syst Rev 2014; 4:CD010056.
- National Tuberculosis Controllers Association; Centers for Disease Control and Prevention (CDC). Guidelines for the investigation of contacts of persons with infectious tuberculosis. Recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Recomm Rep 2005; 54:1–47.
- Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Morb Mortal Wkly Rep 2010; 59:1–25.
- Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Morb Mortal Wkly Rep 2000; 49:1–51.
- Stagg HR, Zenner D, Harris RJ, Munoz L, Lipman MC, Abubakar I. Treatment of latent tuberculosis infection: a network meta-analysis. Ann Intern Med 2014; 161:419–428.
- Centers for Disease Control and Prevention (CDC). Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep 2011; 60:1650–1653.
- Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2008; 57:1–30.
- Macartney K, Heywood A, McIntyre P. Vaccines for post-exposure prophylaxis against varicella (chickenpox) in children and adults. Cochrane Database Syst Rev 2014; 6:CD001833.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of VariZIG—United States, 2013. MMWR Morb Mortal Wkly Rep 2013; 62: 574–576.
- Public Health Agency of Canada. Guidelines for the prevention and control of invasive group A streptococcal disease. Can Commun Dis Rep 2006; 32(suppl 2):1–26.
- Steer JA, Lamagni T, Healy B, et al. Guidelines for prevention and control of group A streptococcal infection in acute healthcare and maternity settings in the UK. J Infect 2012; 64:1–18.
- Grohskopf LA, Olsen SJ, Sokolow LZ, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2014–15 influenza season. MMWR Morb Mortal Wkly Rep 2014; 63: 691–697.
- Fiore AE, Fry A, Shay D, et al; Centers for Disease Control and Prevention (CDC). Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2011; 60:1–24.
- Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev 2014; 4:CD008965.
- Zalmanovici Trestioreanu A, Fraser A, Gafter-Gvili A, Paul M, Leibovici L. Antibiotics for preventing meningococcal infections. Cochrane Database Syst Rev 2013; 10:CD004785.
- Altunaiji S, Kukuruzovic R, Curtis N, Massie J. Antibiotics for whooping cough (pertussis). Cochrane Database Syst Rev 2007: CD004404.
- Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56:1080–1084.
- FitzGerald D, Grainger RJ, Reid A. Interventions for preventing the spread of infestation in close contacts of people with scabies. Cochrane Database Syst Rev 2014; 2:CD009943.
- Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:e10–e52.
- Rupprecht CE, Briggs D, Brown CM, et al; Centers for Disease Control and Prevention (CDC). Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies—recommendations of the Advisory Committee on Immunization Practice. MMWR Recomm Rep 2010; 59:1–9.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine in adults aged 65 years and older—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep 2012; 61:468–470.
KEY POINTS
- Whether to give prophylactic therapy depends on the transmissibility of the infection, the susceptibility of the exposed individual, and the risk of infection-related complications.
- Postexposure prophylactic therapy should begin as soon as possible, while awaiting results of further diagnostic tests, to maximize the chances of preventing or ameliorating the infection.
- Keeping up-to-date with current institutional policies and national guidelines is essential. Sources include US Public Health Service guidelines and reports from the US Centers for Disease Control and Prevention, as well as consultation with an expert healthcare provider (eg, infectious diseases physician, infection control provider, public health officer).
Parsimonious blood use and lower transfusion triggers: What is the evidence?
For decades, physicians believed in the benefit of prompt transfusion of blood to keep the hemoglobin level at arbitrary, optimum levels, ie, close to normal values, especially in the critically ill, the elderly, and those with coronary syndromes, stroke, or renal failure.
However, the evidence supporting arbitrary hemoglobin values as an indication for transfusion was weak or nonexistent. Also, blood transfusion can have complications and adverse effects, and blood is costly and scarce. These considerations prompted research into when blood transfusion should be considered, and recommendations that it should be used more sparingly than in the past.
This review offers a perspective on the evidence supporting restrictive blood use. First, we focus on hemodilution studies that demonstrated that humans can tolerate anemia. Then, we look at studies that compared a restrictive transfusion strategy with a liberal one in patients with critical illness and active bleeding. We conclude with current recommendations for blood transfusion.
EVIDENCE FROM HEMODILUTION STUDIES
Hemoglobin is essential for tissue oxygenation, but the serum hemoglobin concentration is just one of several factors involved.1–5 In anemia, the body can adapt not only by increasing production of red blood cells, but also by:
- Increasing cardiac output
- Increasing synthesis of 2,3-diphosphoglycerate (2,3-DPG), with a consequent shift in the oxyhemoglobin dissociation curve to the right, allowing enhanced release of oxygen at the tissue level
- Moving more carbon dioxide into the blood (the Bohr effect), which decreases pH and also shifts the dissociation curve to the right.
Just 20 years ago, physicians were using arbitrary cutoffs such as hemoglobin 10 g/dL or hematocrit 30% as indications for blood transfusion, without reasonable evidence to support these values. Not until acute normovolemic hemodilution studies were performed were we able to progressively appraise how well patients could tolerate lower levels of hemoglobin without significant adverse outcomes.
Acute normovolemic hemodilution involves withdrawing blood and replacing it with crystalloid or colloid solution to maintain the volume.6
Initial studies were done in animals and focused on the safety of acute anemia regarding splanchnic perfusion. Subsequently, studies proved that healthy, elderly, and stable cardiac patients can tolerate acute anemia with normal cardiovascular response. The targets in these studies were modest at first, but researchers aimed progressively for more aggressive hemodilution with lower hemoglobin targets and demonstrated that the body can tolerate and adapt to more severe anemia.6–8
Studies in healthy patients
Weiskopf et al9 assessed the effect of severe anemia in 32 conscious healthy patients (11 presurgical patients and 21 volunteers not undergoing surgery) by performing acute normovolemic hemodilution with 5% human albumin, autologous plasma, or both, with a target hemoglobin level of 5 g/dL. The process was done gradually, obtaining aliquots of blood of 500 to 900 mL. Cardiac index increased, along with a mild increase in oxygen consumption with no increase in plasma lactate levels, suggesting that in conscious healthy patients, tissue oxygenation remains adequate even in severe anemia.
Leung et al10 addressed the electrocardiographic changes that occur with severe anemia (hemoglobin 5 g/dL) in 55 healthy volunteers. Three developed transient, reversible ST-segment depression, which was associated with a higher heart rate than in the volunteers with no electrocardiographic changes; however, the changes were reversible and asymptomatic, and thus were considered physiologic and benign.
Hemodilution in healthy elderly patients
Spahn et al11 performed 6 and 12 mL/kg isovolemic exchange of blood for 6% hydroxyethyl starch in 20 patients older than 65 years (mean age 76, range 65–88) without underlying coronary disease.
The patients’ mean hemoglobin level decreased from 11.6 g/dL to 8.8 g/dL. Their cardiac index and oxygen extraction values increased adequately, with stable oxygen consumption during hemodilution. There were no electrocardiographic signs of ischemia.
Hemodilution in coronary artery disease
Spahn et al12 performed hemodilution studies in 60 patients (ages 35–81) with coronary artery disease managed chronically with beta-blockers who were scheduled for coronary artery bypass graft surgery. Hemodilution was performed with 6- and 12-mL/kg isovolemic exchange of blood for 6% hydroxyethyl starch maintaining normovolemia and stable filling pressures. Hemoglobin levels decreased from 12.6 g/dL to 9.9 g/dL. The hemodilution process was done before the revascularization. The authors monitored hemodynamic variables, ST-segment deviation, and oxygen consumption before and after each hemodilution.
There was a compensatory increase in cardiac index and oxygen extraction with consequent stable oxygen consumption. These changes were independent of patient age or left ventricular function. In addition, there were no electrocardiographic signs of ischemia.
Licker et al13 studied the hemodynamic effect of preoperative hemodilution in 50 patients with coronary artery disease undergoing coronary artery bypass graft surgery, performing transesophageal echocardiography before and after hemodilution. The patients underwent isovolemic exchange with iso-oncotic starch to target a hematocrit of 28%.
Acute normovolemic hemodilution triggered an increase in cardiac stroke volume, which had a direct correlation with an increase in the central venous pressure and the left ventricular end-diastolic area. No signs of ischemia were seen in these patients on electrocardiography or echocardiography (eg, left ventricular wall-motion abnormalities).
Hemodilution in mitral regurgitation
Spahn et al14 performed acute isovolemic hemodilution with 6% hydroxyethyl starch in 20 patients with mitral regurgitation. The cardiac filling pressures were stable before and after hemodilution; the mean hemoglobin value decreased from 13 to 10.3 g/dL. The cardiac index and oxygen extraction increased proportionally, with stable oxygen consumption; these findings were the same regardless of whether the patient was in normal sinus rhythm or atrial fibrillation.
Effect of hemodilution on cognition
Weiskopf et al15 assessed the effect of anemia on executive and memory function by inducing progressive acute isovolemic anemia in 90 healthy volunteers (age 29 ± 5), reducing their hemoglobin values to 7, 6, and 5 g/dL and performing repetitive neuropsychological and memory testing before and after the hemodilution, as well as after autologous blood transfusion to return their hemoglobin level to 7 g/dL.
There were no changes in reaction time or error rate at a hemoglobin concentration of 7 g/dL compared with the performance at a baseline hemoglobin concentration of 14 g/dL. The volunteers got slower on a mathematics test at hemoglobin levels of 6 g/dL and 5 g/dL, but their error rate did not increase. Immediate and delayed memory were significantly impaired at hemoglobin of 5 g/dL but not at 6 g/dL. All tests normalized with blood transfusion once the hemoglobin level reached 7 g/dL.15
Weiskopf et al16 subsequently investigated whether giving supplemental oxygen to raise the arterial partial pressure of oxygen (Pao2) to 350 mm Hg or greater would overcome the neurocognitive effects of severe acute anemia. They followed a protocol similar to the one in the earlier study15 and induced anemia in 31 healthy volunteers, age 28 ± 4 years, with a mean baseline hemoglobin concentration of 12.7 g/dL.
When the volunteers reached a hemoglobin concentration of 5.7 ± 0.3 g/dL, they were significantly slower on the mathematics test, and their delayed memory was significantly impaired. Then, in a double-blind fashion, they were given either room air or oxygen. Oxygen increased the Pao2 to 406 mm Hg and normalized neurocognitive performance.
Hemodilution studies in surgical patients
A 2015 meta-analysis17 of 63 studies involving 3,819 surgical patients compared the risk of perioperative allogeneic blood transfusion as well as the overall volume of transfused blood in patients undergoing preoperative acute normovolemic hemodilution vs a control group. Though the overall data showed that the patients who underwent acute normovolemic hemodilution needed fewer transfusions and less blood (relative risk [RR] 0.74, 95% confidence interval [CI] 0.63–0.88, P = .0006), the authors noted significant heterogeneity and publication bias.
However, the hemodilution studies paved the way for justifying a more conservative and restrictive transfusion strategy, with a hemoglobin cutoff value of 7 g/dL, and in acute anemia, using oxygen to overcome acute neurocognitive effects while searching for and correcting the cause of the anemia.
STUDIES OF RESTRICTIVE VS LIBERAL TRANSFUSION STRATEGIES
Studies in critical care and high-risk patients
Hébert et al18 randomized 418 critical care patients to a restrictive transfusion approach (in which they were given red blood cells if their hemoglobin concentration dropped below 7.0 g/dL) and 420 patients to a liberal strategy (given red blood cells if their hemoglobin concentration dropped below 10.0 g/dL). Mortality rates (restrictive vs liberal strategy) were as follows:
- Overall at 30 days 18.7% vs 23.3%, P = .11
- In the subgroup with less-severe disease (Acute Physiology and Chronic Health Evaluation II [APACHE II] score < 20), 8.7% vs 16.1%, P = .03
- In the subgroup under age 55, 5.7% vs 13%, P = .02
- In the subgroup with clinically significant cardiac disease, 20.5% vs 22.9%, P = .69
- In the hospital, 22.2% vs 28.1%; P = .05.
This study demonstrated that parsimonious blood use did not worsen clinical outcomes in critical care patients.
Carson et al19 evaluated 2,016 patients age 50 and older who had a history of or risk factors for cardiovascular disease and a baseline hemoglobin level below 10 g/dL who underwent surgery for hip fracture. Patients were randomized to two transfusion strategies based on threshold hemoglobin level: restrictive (< 8 g/dL) or liberal (< 10 g/dL). The primary outcome was death or inability to walk without assistance at 60-day follow-up. The median number of units of blood used was 2 in the liberal group and 0 in the restrictive group.
There was no significant difference in the rates of the primary outcome (odds ratio [OR] 1.01, 95% CI 0.84–1.22), infection, venous thromboembolism, or reoperation. This study demonstrated that a liberal transfusion strategy offered no benefit over a restrictive one.
Rao et al20 analyzed the impact of blood transfusion in 24,112 patients with acute coronary syndromes enrolled in three large trials. Ten percent of the patients received at least 1 blood transfusion during their hospitalization, and they were older and had more complex comorbidity.
At 30 days, the group that had received blood had higher rates of death (adjusted hazard ratio [HR] 3.94, 95% CI 3.26–4.75) and the combined outcome of death or myocardial infarction (HR 2.92, 95% CI 2.55–3.35). Transfusion in patients whose nadir hematocrit was higher than 25% was associated with worse outcomes.
This study suggests being cautious about routinely transfusing blood in stable patients with ischemic heart disease solely on the basis of arbitrary hematocrit levels.
Carson et al,21 however, in a later trial, found a trend toward worse outcomes with a restrictive strategy than with a liberal one. Here, 110 patients with acute coronary syndrome or stable angina undergoing cardiac catheterization were randomized to a target hemoglobin level of either at least 8 mg/dL or at least 10 g/dL. The primary outcome (a composite of death, myocardial infarction, or unscheduled revascularization 30 days after randomization) occurred in 14 patients (25.5%) in the restrictive group and 6 patients (10.9%) in the liberal group (P = .054), and 7 (13.0%) vs 1 (1.8%) of the patients died (P = .032).
Murphy et al22 similarly found trends toward worse outcomes with a restrictive strategy in cardiac patients. The investigators randomized 2,007 elective cardiac surgery patients with a postoperative hemoglobin level lower than 9 g/dL to a hemoglobin transfusion threshold of either 7.5 or 9 g/dL. Outcomes (restrictive vs liberal strategies):
- Transfusion rates 53.4% vs 92.2%
- Rates of the primary outcome (a serious infection [sepsis or wound infection] or ischemic event [stroke, myocardial infarction, mesenteric ischemia, or acute kidney injury] within 3 months):
35.1% vs 33.0%, OR 1.11, 95% CI 0.91–1.34, P = .30) - Mortality rates 4.2% vs 2.6%, HR 1.64, 95% CI 1.00–2.67, P = .045
- Total costs did not differ significantly between the groups.
These studies21,22 suggest the need for more definitive trials in patients with active coronary disease and in cardiac surgery patients.
Holst et al23 randomized 998 intensive care patients in septic shock to hemoglobin thresholds for transfusion of 7 vs 9 g/dL. Mortality rates at 90 days (the primary outcome) were 43.0% vs 45.0%, RR 0.94, 95% CI 0.78–1.09, P = .44.
This study suggests that even in septic shock, a liberal transfusion strategy has no advantage over a parsimonious one.
Active bleeding, especially active gastrointestinal bleeding, poses a significant stress that may trigger empirical transfusion even without evidence of the real hemoglobin level.
Villanueva et al24 randomized 921 patients with severe acute upper-gastrointestinal bleeding to two groups, with hemoglobin transfusion triggers of 7 vs 9 g/dL. The findings were impressive:
- Freedom from transfusion 51% vs 14% (P < .001)
- Survival rates at 6 weeks 95% vs 91% (HR 0.55, 95% CI 0.33–0.92, P = .02)
- Rebleeding 10% vs 16% (P = .01).
Patients with peptic ulcer disease as well as those with cirrhosis stage Child-Pugh class A or B had higher survival rates with a restrictive transfusion strategy.
The RELIEVE trial25 compared the effect of a restrictive transfusion strategy in elderly patients on mechanical ventilation in 6 intensive care units in the United Kingdom. Transfusion triggers were hemoglobin 7 vs 9 g/dL, and the mortality rate at 180 days was 55% vs 37%, RR 0.68, 95% CI 0.44–1.05, P = .073.
Meta-analyses and observational studies
Rohde et al26 performed a systematic review and meta-analysis of 17 trials with 7,456 patients, which revealed that a restrictive strategy is associated with a lower risk of nosocomial infection, including pneumonia, wound infection, and sepsis.
The pooled risk of all serious infections was 10.6% in the restrictive group and 12.7% in the liberal group. Even after adjusting for the use of leukocyte reduction, the risk of infection was lower in the restrictive strategy group (RR 0.83, 95% CI 0.69–0.99). With a hemoglobin threshold of less than 7.0 g/dL, the risk of serious infection was 14% lower. Although this was not statistically significant overall (RR 0.86, 95% CI 0.72–1.02), the difference was statistically significant in the subgroup undergoing orthopedic surgery (RR 0.72, 95% CI 0.53–0.97) and the subgroup presenting with sepsis (RR 0.51, 95% CI 0.28–0.95).
Salpeter et al27 performed a meta-analysis and systematic review of three randomized trials (N = 2,364) comparing a restrictive hemoglobin transfusion trigger (hemoglobin < 7 g/dL) vs a more liberal trigger. The groups with restrictive transfusion triggers had lower rates of:
- In-hospital mortality (RR 0.74, 95% CI 0.60–0.92)
- Total mortality (RR 0.80, 95% CI 0.65–0.98)
- Rebleeding (RR 0.64, 95% CI 0.45–0.90)
- Acute coronary syndrome (RR 0.44, 95% CI 0.22–0.89)
- Pulmonary edema (RR 0.48, 95% CI 0.33–0.72)
- Bacterial infections (RR 0.86, 95% CI 0.73–1.00).
Wang et al28 performed a meta-analysis of 4 randomized controlled trials in patients with upper-gastrointestinal bleeding comparing restrictive (hemoglobin < 7 g/dL) vs liberal transfusion strategies. The primary outcomes were death and rebleeding. The restrictive strategy was associated with:
- A lower mortality rate (OR 0.52, 95% CI 0.31–0.87, P = .01)
- A lower rebleeding rate (OR 0.26, 95% CI 0.03–2.10, P = .21)
- Shorter hospitalizations (P = .009)
- Less blood transfused (P = .0005).
Vincent et al,29 in a prospective observational study of 3,534 patients in intensive care units in 146 facilities in Western Europe, found a correlation between transfusion and mortality. Transfusion was done most often in elderly patients and those with a longer stay in the intensive care unit. The 28-day mortality rate was 22.7% in patients who received a transfusion and 17.1% in those who did not (P = .02). The more units of blood the patients received, the more likely they were to die, and receiving more than 4 units was associated with worse outcomes (P = .01).
Dunne et al30 performed a study of 6,301 noncardiac surgical patients in the Veterans Affairs Maryland Healthcare System from the National Veterans Administration Surgical Quality Improvement Program from 1995 to 2000. Multiple logistic regression analysis revealed that the composite of low hematocrit before and after surgery and high transfusion rates (> 4 units per hospitalization) were associated with higher rates of death (P < .01) and postoperative pneumonia (P ≤ .05) and longer hospitalizations (P < .05). The risk of pneumonia increased proportionally with the decrease in hematocrit.
These findings support pharmacologic optimization of anemia with hematinic supplementation before surgery to decrease the risk of needing a transfusion, often with parenteral iron. The fact that the patient’s hemoglobin can be optimized preoperatively by nontransfusional means may decrease the likelihood of blood transfusion, as the hemoglobin will potentially remain above the transfusion threshold. For example, if a patient has a preoperative hemoglobin level of 10 g/dL, and it is optimized up to 12, then if postoperatively the hemoglobin level drops 3 g/dL instead of reaching the threshold of 7 g/dL, the nadir will be just 9 g/dL, far above that transfusion threshold.
Brunskill et al,31 in a Cochrane review of 6 trials with 2,722 patients undergoing surgery for hip fracture, found no difference in rates of mortality, functional recovery or postoperative morbidity with a restrictive transfusion strategy (hemoglobin target > 8 g/dL vs a liberal one (> 10 g/dL). However, the quality of evidence was rated as low. The authors concluded that there is no justification for liberal red blood cell transfusion thresholds (10 g/dL), and a more restrictive transfusion threshold is preferable.
Weinberg et al32 found that, in trauma patients, receiving more than 6 units of blood was associated with poor prognosis, and outcomes were worse when the blood was older than 2 weeks. However, the effect of blood age is not significant when using smaller transfusion volumes (1 to 2 units of red blood cells).
Studies in sickle cell disease
Sickle cell disease patients have high levels of hemoglobin S, which causes erythrocyte sickling and increases blood viscosity. Transfusion with normal erythrocytes increases the amount of hemoglobin A (the normal variant).33,34
In trials in surgical patients,35,36 conservative strategies for preoperative blood transfusion aiming at a hemoglobin level of 10 g/dL were as effective in preventing postoperative complications as decreasing the hemoglobin S levels to 30% by aggressive exchange transfusion.35
In nonsurgical patients, blood transfusion should be based on formal risk-benefit assessments. Therefore, the expert panel report on sickle cell management advises against blood transfusion in sickle cell patients with uncomplicated vaso-occlusive crises, priapism, asymptomatic anemia, or acute kidney injury in the absence of multisystem organ failure.34
Is hemoglobin the most relevant marker?
Most studies that compared restrictive and liberal transfusion strategies focused on using a lower hemoglobin threshold as the transfusion trigger, not on using fewer units of blood. Is the amount of blood transfused more important than the hemoglobin threshold? Perhaps a study focused both on a restrictive vs liberal strategy and also on the minimum amount of blood that each patient may benefit from would help to answer this question.
We should beware of routinely using the hemoglobin concentration as a threshold for transfusion and a surrogate marker of transfusion benefit because changes in hemoglobin concentration may not reflect changes in absolute red cell mass.37 Changes in plasma volume (an increase or decrease) affect the hematocrit concentration without necessarily affecting the total red cell mass. Unfortunately, red cell mass is very difficult to measure; hence, the hemoglobin and hematocrit values are used instead. Studies addressing changes in red cell mass may be needed, perhaps even to validate using the hemoglobin concentration as the sole indicator for transfusion.
Is fresh blood better than old blood?
Using blood that is more than 14 days old may be associated with poor outcomes, for several possible reasons. Red blood cells age rapidly in refrigeration, and usually just 75% may remain viable 24 hours after phlebotomy. Adenosine triphosphate and 2,3-DPG levels steadily decrease, with a consequent decrease in capacity for appropriate tissue oxygen delivery. In addition, loss of membrane phospholipids causes progressive rigidity of the red cell membrane with consequent formation of echynocytes after 14 to 21 days.38,39
The use of blood more than 14 days old in cardiac surgery patients has been associated with worse outcomes, including higher rates of death, prolonged intubation, acute renal failure, and sepsis.40 Similar poor outcomes have been seen in trauma patients.32
Lacroix et al,41 in a multicenter, randomized trial in critically ill adults, compared the outcomes of transfusion of fresh packed red cells (stored < 8 days) or old blood (stored for a mean of 22 days). The primary outcome was the mortality rate at 90 days: 37.0% in the fresh-blood group vs 35.3% in the old-blood group (HR 1.1, 95% CI 0.9–1.2, P = .38).
The authors concluded that using fresh blood compared with old blood was not associated with a lower 90-day mortality rate in critically ill adults.
RISKS ASSOCIATED WITH TRANSFUSION
Infections
The risk of infection from blood transfusion is small. Human immunodeficiency virus (HIV) is transmitted in 1 in 1.5 million transfused blood components, and hepatitis C virus in 1 in 1.1 million; these odds are similar to those of having a fatal airplane accident (1 in 1.7 million per flight). Hepatitis B virus infection is more common, the reported incidence being 1 in 357,000.42
Noninfectious complications
Transfusion-associated circulatory overload occurs in 4% to 6% of patients who receive a transfusion. Therefore, circulatory overload is a greater danger from transfusion than infection is.42
Febrile nonhemolytic transfusion reactions occur in 1.1% of patients with prestorage leukoreduction.
Transfusion-associated acute lung injury occurs in 0.8 per 10,000 blood components transfused.
Errors associated with blood transfusion include, in decreasing order of frequency, transfusion of the wrong blood component, handling and storage errors, inappropriate administration of anti-D immunoglobulin, and avoidable, delayed, or insufficient transfusions.43
Surgery and condition-specific complications of red blood cell transfusion
Cardiovascular surgery. Transfusion is associated with a higher risk of postoperative stroke, respiratory failure, acute respiratory distress syndrome, prolonged intubation time, reintubation, in-hospital death, sepsis, and longer postoperative length of stay.44
Malignancy. The use of blood in this setting has been found to be an independent predictor of recurrence, decreased survival, and increased risk of lymphoplasmacytic and marginal-zone lymphomas.44–47
Vascular, orthopedic, and other surgeries. Transfusion is associated with a higher risk of death, thromboembolic events, acute kidney injury, death, composite morbidity, reoperation, sepsis, and pulmonary complications.44
ST-segment elevation myocardial infarction, sepsis, and intensive care unit admissions. Transfusion is associated with an increased risk of rebleeding, death, and secondary infections.44
COST OF RED BLOOD CELL TRANSFUSION
Up to 85 million units of red blood cells are transfused per year worldwide, 15 million of them in the United States.42 At our hospital in 2013, 1 unit of leukocyte-reduced red blood cells cost $957.27, which included the costs of acquisition, processing, banking, patient testing, administration, and monitoring.
The Premier Healthcare Alliance48 analyzed data from 7.4 million discharges from 464 hospitals between April 2011 and March 2012. Blood use varied significantly among hospitals, and the hospitals in the lowest quartile of blood use had better patient outcomes. If all the hospitals used as little blood as those in the lowest quartile and had outcomes as good, blood product use would be reduced by 802,716 units, with savings of up to $165 million annually.
In addition to the economic cost of blood transfusion, the clinician must be aware of the cost in terms of comorbidities caused by unnecessary blood transfusion.49,50
RECOMMENDATIONS FROM THE AABB
In view of all the current compelling evidence, a restrictive approach to transfusion is the single best strategy to minimize adverse outcomes.51 Below, we outline the current recommendations from the AABB (formerly the American Association of Blood Banks),42 which are similar to the national clinical guideline on blood transfusion in the United Kingdom,52 and have recently been updated, confirming the initial recommendations.53
In critical care patients, transfusion should be considered if the hemoglobin concentration is 7 g/dL or less.
In postoperative patients and hospitalized patients with preexisting cardiovascular disease, transfusion should be considered if the hemoglobin concentration is 8 g/dL or less or if the patient has signs or symptoms of anemia such as chest pain, orthostatic hypotension, or tachycardia unresponsive to fluid resuscitation, or heart failure.
In hemodynamically stable patients with acute coronary syndrome, there is not enough evidence to allow a formal recommendation for or against a liberal or restrictive transfusion threshold.
Consider both the hemoglobin concentration and the symptoms when deciding whether to give a transfusion. This recommendation is shared by a National Institutes of Health consensus conference,54 which indicates that multiple factors related to the patient’s clinical status and oxygen delivery should be considered before deciding to transfuse red blood cells.
The Society of Hospital Medicine55 and the American Society of Hematology56 concur with a parsimonious approach to blood use in their Choosing Wisely campaigns. The American Society of Hematology recommends that if transfusion of red blood cells is necessary, the minimum number of units should be given that relieve the symptoms of anemia or achieve a safe hemoglobin range (7–8 g/dL in stable noncardiac inpatients).57
New electronic tools can monitor the ordering and use of blood products in real time and can identify the hemoglobin level used as the trigger for transfusion. They also provide data on blood use by physician, hospital, and department. These tools can reveal current practice at a glance and allow sharing of best practices among peers and institutions.52
CONSIDER TRANSFUSION FOR HEMOGLOBIN BELOW 7 G/DL
The routine use of blood has come under scrutiny, given its association with increased healthcare costs and morbidity. The accepted practice in stable medical patients is a restrictive threshold approach for blood transfusion, which is to consider (not necessarily give) a single unit of packed red blood cells for a hemoglobin less than 7 g/dL.
However, studies in acute coronary syndrome patients and postoperative cardiac surgery patients have not shown the restrictive threshold to be superior to a liberal threshold in terms of outcomes and costs. This variability suggests the need for further studies to determine the best course of action in different patient subpopulations (eg, surgical, oncologic, trauma, critical illness).
Also, a limitation of most of the clinical studies was that only the hemoglobin concentration was used as a marker of anemia, with no strict assessment of changes in red cell mass with transfusion.
Despite the variability in certain populations, the overall weight of current evidence favors a restrictive approach to blood transfusion (hemoglobin < 7 g/dL), although perhaps in patients who have active coronary disease or are undergoing cardiac surgery, a more lenient threshold (< 8 g/dL) for transfusion should be considered.
- Shander A, Gross I, Hill S, Javidroozi M, Sledge S; College of American Pathologists; American Society of Anesthesiologists; Society of Thoracic Surgeons and Society of Cardiovascular Anesthesiologists; Society of Critical Care Medicine; Italian Society of Transfusion Medicine and Immunohaematology; American Association of Blood Banks. A new perspective on best transfusion practices. Blood Transfus 2013; 11:193–202.
- Madjdpour C, Spahn DR. Allogeneic red blood cell transfusion: physiology of oxygen transport. Best Pract Res Clin Anaesthesiol 2007; 21:163–171.
- Tánczos K, Molnár Z. The oxygen supply-demand balance: a monitoring challenge. Best Pract Res Clin Anaesthesiol 2013; 27:201–207.
- Hebert PC, Van der Linden P, Biro G, Hu LQ. Physiologic aspects of anemia. Crit Care Clin 2004; 20:187–212.
- Spinelli E, Bartlett RH. Anemia and transfusion in critical care: physiology and management. J Intensive Care Med 2016; 31:295–306.
- Jamnicki M, Kocian R, Van Der Linden P, Zaugg M, Spahn DR. Acute normovolemic hemodilution: physiology, limitations, and clinical use. J Cardiothorac Vasc Anesth 2003; 17:747–754.
- Monk TG. Acute normovolemic hemodilution. Anesthesiol Clin North America 2005; 23:271–281.
- Shander A, Rijhwani TS. Acute normovolemic hemodilution. Transfusion 2004; 44(suppl 2):26S–34S.
- Weiskopf RB, Viele MK, Feiner J, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA 1998; 279:217–221.
- Leung JM, Weiskopf RB, Feiner J, et al. Electrocardiographic ST-segment changes during acute, severe isovolemic hemodilution in humans. Anesthesiology 2000; 93:1004–1010.
- Spahn DR, Zollinger A, Schlumpf RB, et al. Hemodilution tolerance in elderly patients without known cardiac disease. Anesth Analg 1996; 82:681–686.
- Spahn DR, Schmid ER, Seifert B, Pasch T. Hemodilution tolerance in patients with coronary artery disease who are receiving chronic beta-adrenergic blocker therapy. Anesth Analg 1996; 82:687–694.
- Licker M, Ellenberger C, Sierra J, Christenson J, Diaper J, Morel D. Cardiovascular response to acute normovolemic hemodilution in patients with coronary artery diseases: assessment with transesophageal echocardiography. Crit Care Med 2005; 33:591–597.
- Spahn DR, Seifert B, Pasch T, Schmid ER. Haemodilution tolerance in patients with mitral regurgitation. Anaesthesia 1998; 53:20–24.
- Weiskopf RB, Kramer JH, Viele M, et al. Acute severe isovolemic anemia impairs cognitive function and memory in humans. Anesthesiology 2000; 92:1646–1652.
- Weiskopf RB, Feiner J, Hopf HW, et al. Oxygen reverses deficits of cognitive function and memory and increased heart rate induced by acute severe isovolemic anemia. Anesthesiology 2002; 96:871–877.
- Zhou X, Zhang C, Wang Y, Yu L, Yan M. Preoperative acute normovolemic hemodilution for minimizing allogeneic blood transfusion: a meta-analysis. Anesth Analg 2015; 121:1443–1455.
- Hébert P, Wells G, Blajchman M, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med 1999: 340:409–417.
- Carson JL, Terrin ML, Noveck H, et al; FOCUS Investigators. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011; 365:2453–2462.
- Rao SV, Jollis JG, Harrington RA, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA 2004; 292:1555–1562.
- Carson JL, Brooks MM, Abbott JD, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J 2013; 165:964.e1–971.e1.
- Murphy GJ, Pike K, Rogers CA, et al; TITRe2 Investigators. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med 2015; 372:997–1008.
- Holst LB, Haase N, Wetterslev J, et al; TRISS Trial Group; Scandinavian Critical Care Trials Group. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med 2014; 371:1381–1391.
- Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013; 368:11–21.
- Walsh TS, Boyd JA, Watson D, et al; RELIEVE Investigators. Restrictive versus liberal transfusion strategies for older mechanically ventilated critically ill patients: a randomized pilot trial. Crit Care Med 2013; 41:2354–2363.
- Rohde JM, Dimcheff DE, Blumberg N, et al. Health care–associated infection after red blood cell transfusion. JAMA 2014; 311:1317–1326.
- Salpeter SR, Buckley JS, Chatterjee S. Impact of more restrictive blood transfusion strategies on clinical outcomes: a meta-analysis and systematic review. Am J Med 2014; 127:124.e3–131.e3.
- Wang J, Bao YX, Bai M, Zhang YG, Xu WD, Qi XS. Restrictive vs liberal transfusion for upper gastrointestinal bleeding: a meta-analysis of randomized controlled trials. World J Gastroenterol 2013; 19:6919–6927.
- Vincent JL, Baron JF, Reinhart K, et al; ABC (Anemia and Blood Transfusion in Critical Care) Investigators. Anemia and blood transfusion in critically ill patients. JAMA 2002; 288:1499–1507.
- Dunne JR, Malone D, Tracy JK, Gannon C, Napolitano LM. Perioperative anemia: an independent risk factor for infection, mortality, and resource utilization in surgery. J Surg Res 2002; 102:237–244.
- Brunskill SJ, Millette SL, Shokoohi A, et al. Red blood cell transfusion for people undergoing hip fracture surgery. Cochrane Database Syst Rev 2015; 4:CD009699.
- Weinberg JA, McGwin G Jr, Griffin RL, et al. Age of transfused blood: an independent predictor of mortality despite universal leukoreduction. J Trauma 2008; 65:279–284.
- Steinberg M. Management of sickle cell disease. N Engl J Med 1999; 340:1021–1030.
- Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease. JAMA 2014; 312:1033–1048.
- Vichinsky EP, Haberkern CM, Neumayr L, et al. A comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. The Preoperative Transfusion in Sickle Cell Disease Study Group. N Engl J Med 1995; 333:206–213.
- Howard J, Malfroy M, Llewelyn C, et al. The Transfusion Alternatives Preoperatively in Sickle Cell Disease (TAPS) study: a randomised, controlled, multicentre clinical trial. Lancet 2013; 381:930–938.
- Goodnough LT, Levy JH, Murphy MF. Concepts of blood transfusion in adults. Lancet 2013; 381:1845–1854.
- Holme S. Current issues related to the quality of stored RBCs. Transfus Apher Sci 2005; 33:55–61.
- Hovav T, Yedgar S, Manny N, Barshtein G. Alteration of red cell aggregability and shape during blood storage. Transfusion 1999; 39:277–281.
- Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med 2008; 358:1229–1239.
- Lacroix J, Hebert PC, Fergusson DA, et al. Age of transfused blood in critically ill adults. N Engl J Med 2015; 372:1410–1418.
- Carson JL, Grossman BJ, Kleinman S, et al; Clinical Transfusion Medicine Committee of the AABB. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2012; 157:49–58.
- Bolton-Maggs P, Watt A, Poles D, et al, on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group. The 2015 Annual SHOT Report. www.shotuk.org/wp-content/uploads/SHOT-2015-Annual-Report-Web-Edition-Final-bookmarked.pdf. Accessed November 30, 2016.
- Shander A, Javidroozi M, Ozawa S, Hare GMT. What is really dangerous: anaemia or transfusion? Br J Anaesth 2011; 107(suppl 1):i41–i59.
- Reeh M, Ghadban T, Dedow J, et al. Allogenic blood transfusion is associated with poor perioperative and long-term outcome in esophageal cancer. World J Surg 2016 Oct 11. [Epub ahead of print]
- Elmi M, Mahar A, Kagedan D, et al. The impact of blood transfusion on perioperative outcomes following gastric cancer resection: an analysis of the American College of Surgeons National Surgical Quality Improvement Program database. Can J Surg 2016; 59:322–329.
- Aquina CT, Blumberg N, Becerra AZ, et al. Association among blood transfusion, sepsis, and decreased long-term survival after colon cancer resection. Ann Surg 2016; Sep 14. [Epub ahead of print] PubMed PMID: 27631770.
- Premiere Analysis. Standardization of blood utilization practices could provide opportunity for improved outcomes, reduced costs. A Premiere Healthcare Alliance Analysis. 2012.
- Simeone F, Franchi F, Cevenini G, et al. A simple clinical model for planning transfusion quantities in heart surgery. BMC Med Inform Decis Mak 2011; 11:44.
- Spahn DR, Goodnough LT. Alternatives to blood transfusion. Lancet 2013; 381:1855–1865.
- Holst LB, Petersen MW, Haase N, Perner A, Wetterslev J. Restrictive versus liberal transfusion strategy for red blood cell transfusion: systematic review of randomised trials with meta-analysis and trial sequential analysis. BMJ 2015; 350:h1354.
- National Institute for Health and Care Excellence: Clinical Guidelines. London: National Institute for Health and Care Excellence (UK). www.ncbi.nlm.nih.gov/books/NBK11822/.
- Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA 2016 Oct 12. doi: 10.1001/jama.2016.9185. [Epub ahead of print]
- Consensus conference. Perioperative red blood cell transfusion. JAMA 1988; 260:2700–2703.
- Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med 2013; 8:486–492.
- Hicks LK, Bering H, Carson KR, et al. The ASH Choosing Wisely® campaign: five hematologic tests and treatments to question. Blood 2013; 122:3879–3883.
- Haemonetics IMPACT Online. The Blood Management Company. www.haemonetics.com/Products/Services/Consulting Services/IMPACT Online.aspx. Accessed November 30, 2016.
For decades, physicians believed in the benefit of prompt transfusion of blood to keep the hemoglobin level at arbitrary, optimum levels, ie, close to normal values, especially in the critically ill, the elderly, and those with coronary syndromes, stroke, or renal failure.
However, the evidence supporting arbitrary hemoglobin values as an indication for transfusion was weak or nonexistent. Also, blood transfusion can have complications and adverse effects, and blood is costly and scarce. These considerations prompted research into when blood transfusion should be considered, and recommendations that it should be used more sparingly than in the past.
This review offers a perspective on the evidence supporting restrictive blood use. First, we focus on hemodilution studies that demonstrated that humans can tolerate anemia. Then, we look at studies that compared a restrictive transfusion strategy with a liberal one in patients with critical illness and active bleeding. We conclude with current recommendations for blood transfusion.
EVIDENCE FROM HEMODILUTION STUDIES
Hemoglobin is essential for tissue oxygenation, but the serum hemoglobin concentration is just one of several factors involved.1–5 In anemia, the body can adapt not only by increasing production of red blood cells, but also by:
- Increasing cardiac output
- Increasing synthesis of 2,3-diphosphoglycerate (2,3-DPG), with a consequent shift in the oxyhemoglobin dissociation curve to the right, allowing enhanced release of oxygen at the tissue level
- Moving more carbon dioxide into the blood (the Bohr effect), which decreases pH and also shifts the dissociation curve to the right.
Just 20 years ago, physicians were using arbitrary cutoffs such as hemoglobin 10 g/dL or hematocrit 30% as indications for blood transfusion, without reasonable evidence to support these values. Not until acute normovolemic hemodilution studies were performed were we able to progressively appraise how well patients could tolerate lower levels of hemoglobin without significant adverse outcomes.
Acute normovolemic hemodilution involves withdrawing blood and replacing it with crystalloid or colloid solution to maintain the volume.6
Initial studies were done in animals and focused on the safety of acute anemia regarding splanchnic perfusion. Subsequently, studies proved that healthy, elderly, and stable cardiac patients can tolerate acute anemia with normal cardiovascular response. The targets in these studies were modest at first, but researchers aimed progressively for more aggressive hemodilution with lower hemoglobin targets and demonstrated that the body can tolerate and adapt to more severe anemia.6–8
Studies in healthy patients
Weiskopf et al9 assessed the effect of severe anemia in 32 conscious healthy patients (11 presurgical patients and 21 volunteers not undergoing surgery) by performing acute normovolemic hemodilution with 5% human albumin, autologous plasma, or both, with a target hemoglobin level of 5 g/dL. The process was done gradually, obtaining aliquots of blood of 500 to 900 mL. Cardiac index increased, along with a mild increase in oxygen consumption with no increase in plasma lactate levels, suggesting that in conscious healthy patients, tissue oxygenation remains adequate even in severe anemia.
Leung et al10 addressed the electrocardiographic changes that occur with severe anemia (hemoglobin 5 g/dL) in 55 healthy volunteers. Three developed transient, reversible ST-segment depression, which was associated with a higher heart rate than in the volunteers with no electrocardiographic changes; however, the changes were reversible and asymptomatic, and thus were considered physiologic and benign.
Hemodilution in healthy elderly patients
Spahn et al11 performed 6 and 12 mL/kg isovolemic exchange of blood for 6% hydroxyethyl starch in 20 patients older than 65 years (mean age 76, range 65–88) without underlying coronary disease.
The patients’ mean hemoglobin level decreased from 11.6 g/dL to 8.8 g/dL. Their cardiac index and oxygen extraction values increased adequately, with stable oxygen consumption during hemodilution. There were no electrocardiographic signs of ischemia.
Hemodilution in coronary artery disease
Spahn et al12 performed hemodilution studies in 60 patients (ages 35–81) with coronary artery disease managed chronically with beta-blockers who were scheduled for coronary artery bypass graft surgery. Hemodilution was performed with 6- and 12-mL/kg isovolemic exchange of blood for 6% hydroxyethyl starch maintaining normovolemia and stable filling pressures. Hemoglobin levels decreased from 12.6 g/dL to 9.9 g/dL. The hemodilution process was done before the revascularization. The authors monitored hemodynamic variables, ST-segment deviation, and oxygen consumption before and after each hemodilution.
There was a compensatory increase in cardiac index and oxygen extraction with consequent stable oxygen consumption. These changes were independent of patient age or left ventricular function. In addition, there were no electrocardiographic signs of ischemia.
Licker et al13 studied the hemodynamic effect of preoperative hemodilution in 50 patients with coronary artery disease undergoing coronary artery bypass graft surgery, performing transesophageal echocardiography before and after hemodilution. The patients underwent isovolemic exchange with iso-oncotic starch to target a hematocrit of 28%.
Acute normovolemic hemodilution triggered an increase in cardiac stroke volume, which had a direct correlation with an increase in the central venous pressure and the left ventricular end-diastolic area. No signs of ischemia were seen in these patients on electrocardiography or echocardiography (eg, left ventricular wall-motion abnormalities).
Hemodilution in mitral regurgitation
Spahn et al14 performed acute isovolemic hemodilution with 6% hydroxyethyl starch in 20 patients with mitral regurgitation. The cardiac filling pressures were stable before and after hemodilution; the mean hemoglobin value decreased from 13 to 10.3 g/dL. The cardiac index and oxygen extraction increased proportionally, with stable oxygen consumption; these findings were the same regardless of whether the patient was in normal sinus rhythm or atrial fibrillation.
Effect of hemodilution on cognition
Weiskopf et al15 assessed the effect of anemia on executive and memory function by inducing progressive acute isovolemic anemia in 90 healthy volunteers (age 29 ± 5), reducing their hemoglobin values to 7, 6, and 5 g/dL and performing repetitive neuropsychological and memory testing before and after the hemodilution, as well as after autologous blood transfusion to return their hemoglobin level to 7 g/dL.
There were no changes in reaction time or error rate at a hemoglobin concentration of 7 g/dL compared with the performance at a baseline hemoglobin concentration of 14 g/dL. The volunteers got slower on a mathematics test at hemoglobin levels of 6 g/dL and 5 g/dL, but their error rate did not increase. Immediate and delayed memory were significantly impaired at hemoglobin of 5 g/dL but not at 6 g/dL. All tests normalized with blood transfusion once the hemoglobin level reached 7 g/dL.15
Weiskopf et al16 subsequently investigated whether giving supplemental oxygen to raise the arterial partial pressure of oxygen (Pao2) to 350 mm Hg or greater would overcome the neurocognitive effects of severe acute anemia. They followed a protocol similar to the one in the earlier study15 and induced anemia in 31 healthy volunteers, age 28 ± 4 years, with a mean baseline hemoglobin concentration of 12.7 g/dL.
When the volunteers reached a hemoglobin concentration of 5.7 ± 0.3 g/dL, they were significantly slower on the mathematics test, and their delayed memory was significantly impaired. Then, in a double-blind fashion, they were given either room air or oxygen. Oxygen increased the Pao2 to 406 mm Hg and normalized neurocognitive performance.
Hemodilution studies in surgical patients
A 2015 meta-analysis17 of 63 studies involving 3,819 surgical patients compared the risk of perioperative allogeneic blood transfusion as well as the overall volume of transfused blood in patients undergoing preoperative acute normovolemic hemodilution vs a control group. Though the overall data showed that the patients who underwent acute normovolemic hemodilution needed fewer transfusions and less blood (relative risk [RR] 0.74, 95% confidence interval [CI] 0.63–0.88, P = .0006), the authors noted significant heterogeneity and publication bias.
However, the hemodilution studies paved the way for justifying a more conservative and restrictive transfusion strategy, with a hemoglobin cutoff value of 7 g/dL, and in acute anemia, using oxygen to overcome acute neurocognitive effects while searching for and correcting the cause of the anemia.
STUDIES OF RESTRICTIVE VS LIBERAL TRANSFUSION STRATEGIES
Studies in critical care and high-risk patients
Hébert et al18 randomized 418 critical care patients to a restrictive transfusion approach (in which they were given red blood cells if their hemoglobin concentration dropped below 7.0 g/dL) and 420 patients to a liberal strategy (given red blood cells if their hemoglobin concentration dropped below 10.0 g/dL). Mortality rates (restrictive vs liberal strategy) were as follows:
- Overall at 30 days 18.7% vs 23.3%, P = .11
- In the subgroup with less-severe disease (Acute Physiology and Chronic Health Evaluation II [APACHE II] score < 20), 8.7% vs 16.1%, P = .03
- In the subgroup under age 55, 5.7% vs 13%, P = .02
- In the subgroup with clinically significant cardiac disease, 20.5% vs 22.9%, P = .69
- In the hospital, 22.2% vs 28.1%; P = .05.
This study demonstrated that parsimonious blood use did not worsen clinical outcomes in critical care patients.
Carson et al19 evaluated 2,016 patients age 50 and older who had a history of or risk factors for cardiovascular disease and a baseline hemoglobin level below 10 g/dL who underwent surgery for hip fracture. Patients were randomized to two transfusion strategies based on threshold hemoglobin level: restrictive (< 8 g/dL) or liberal (< 10 g/dL). The primary outcome was death or inability to walk without assistance at 60-day follow-up. The median number of units of blood used was 2 in the liberal group and 0 in the restrictive group.
There was no significant difference in the rates of the primary outcome (odds ratio [OR] 1.01, 95% CI 0.84–1.22), infection, venous thromboembolism, or reoperation. This study demonstrated that a liberal transfusion strategy offered no benefit over a restrictive one.
Rao et al20 analyzed the impact of blood transfusion in 24,112 patients with acute coronary syndromes enrolled in three large trials. Ten percent of the patients received at least 1 blood transfusion during their hospitalization, and they were older and had more complex comorbidity.
At 30 days, the group that had received blood had higher rates of death (adjusted hazard ratio [HR] 3.94, 95% CI 3.26–4.75) and the combined outcome of death or myocardial infarction (HR 2.92, 95% CI 2.55–3.35). Transfusion in patients whose nadir hematocrit was higher than 25% was associated with worse outcomes.
This study suggests being cautious about routinely transfusing blood in stable patients with ischemic heart disease solely on the basis of arbitrary hematocrit levels.
Carson et al,21 however, in a later trial, found a trend toward worse outcomes with a restrictive strategy than with a liberal one. Here, 110 patients with acute coronary syndrome or stable angina undergoing cardiac catheterization were randomized to a target hemoglobin level of either at least 8 mg/dL or at least 10 g/dL. The primary outcome (a composite of death, myocardial infarction, or unscheduled revascularization 30 days after randomization) occurred in 14 patients (25.5%) in the restrictive group and 6 patients (10.9%) in the liberal group (P = .054), and 7 (13.0%) vs 1 (1.8%) of the patients died (P = .032).
Murphy et al22 similarly found trends toward worse outcomes with a restrictive strategy in cardiac patients. The investigators randomized 2,007 elective cardiac surgery patients with a postoperative hemoglobin level lower than 9 g/dL to a hemoglobin transfusion threshold of either 7.5 or 9 g/dL. Outcomes (restrictive vs liberal strategies):
- Transfusion rates 53.4% vs 92.2%
- Rates of the primary outcome (a serious infection [sepsis or wound infection] or ischemic event [stroke, myocardial infarction, mesenteric ischemia, or acute kidney injury] within 3 months):
35.1% vs 33.0%, OR 1.11, 95% CI 0.91–1.34, P = .30) - Mortality rates 4.2% vs 2.6%, HR 1.64, 95% CI 1.00–2.67, P = .045
- Total costs did not differ significantly between the groups.
These studies21,22 suggest the need for more definitive trials in patients with active coronary disease and in cardiac surgery patients.
Holst et al23 randomized 998 intensive care patients in septic shock to hemoglobin thresholds for transfusion of 7 vs 9 g/dL. Mortality rates at 90 days (the primary outcome) were 43.0% vs 45.0%, RR 0.94, 95% CI 0.78–1.09, P = .44.
This study suggests that even in septic shock, a liberal transfusion strategy has no advantage over a parsimonious one.
Active bleeding, especially active gastrointestinal bleeding, poses a significant stress that may trigger empirical transfusion even without evidence of the real hemoglobin level.
Villanueva et al24 randomized 921 patients with severe acute upper-gastrointestinal bleeding to two groups, with hemoglobin transfusion triggers of 7 vs 9 g/dL. The findings were impressive:
- Freedom from transfusion 51% vs 14% (P < .001)
- Survival rates at 6 weeks 95% vs 91% (HR 0.55, 95% CI 0.33–0.92, P = .02)
- Rebleeding 10% vs 16% (P = .01).
Patients with peptic ulcer disease as well as those with cirrhosis stage Child-Pugh class A or B had higher survival rates with a restrictive transfusion strategy.
The RELIEVE trial25 compared the effect of a restrictive transfusion strategy in elderly patients on mechanical ventilation in 6 intensive care units in the United Kingdom. Transfusion triggers were hemoglobin 7 vs 9 g/dL, and the mortality rate at 180 days was 55% vs 37%, RR 0.68, 95% CI 0.44–1.05, P = .073.
Meta-analyses and observational studies
Rohde et al26 performed a systematic review and meta-analysis of 17 trials with 7,456 patients, which revealed that a restrictive strategy is associated with a lower risk of nosocomial infection, including pneumonia, wound infection, and sepsis.
The pooled risk of all serious infections was 10.6% in the restrictive group and 12.7% in the liberal group. Even after adjusting for the use of leukocyte reduction, the risk of infection was lower in the restrictive strategy group (RR 0.83, 95% CI 0.69–0.99). With a hemoglobin threshold of less than 7.0 g/dL, the risk of serious infection was 14% lower. Although this was not statistically significant overall (RR 0.86, 95% CI 0.72–1.02), the difference was statistically significant in the subgroup undergoing orthopedic surgery (RR 0.72, 95% CI 0.53–0.97) and the subgroup presenting with sepsis (RR 0.51, 95% CI 0.28–0.95).
Salpeter et al27 performed a meta-analysis and systematic review of three randomized trials (N = 2,364) comparing a restrictive hemoglobin transfusion trigger (hemoglobin < 7 g/dL) vs a more liberal trigger. The groups with restrictive transfusion triggers had lower rates of:
- In-hospital mortality (RR 0.74, 95% CI 0.60–0.92)
- Total mortality (RR 0.80, 95% CI 0.65–0.98)
- Rebleeding (RR 0.64, 95% CI 0.45–0.90)
- Acute coronary syndrome (RR 0.44, 95% CI 0.22–0.89)
- Pulmonary edema (RR 0.48, 95% CI 0.33–0.72)
- Bacterial infections (RR 0.86, 95% CI 0.73–1.00).
Wang et al28 performed a meta-analysis of 4 randomized controlled trials in patients with upper-gastrointestinal bleeding comparing restrictive (hemoglobin < 7 g/dL) vs liberal transfusion strategies. The primary outcomes were death and rebleeding. The restrictive strategy was associated with:
- A lower mortality rate (OR 0.52, 95% CI 0.31–0.87, P = .01)
- A lower rebleeding rate (OR 0.26, 95% CI 0.03–2.10, P = .21)
- Shorter hospitalizations (P = .009)
- Less blood transfused (P = .0005).
Vincent et al,29 in a prospective observational study of 3,534 patients in intensive care units in 146 facilities in Western Europe, found a correlation between transfusion and mortality. Transfusion was done most often in elderly patients and those with a longer stay in the intensive care unit. The 28-day mortality rate was 22.7% in patients who received a transfusion and 17.1% in those who did not (P = .02). The more units of blood the patients received, the more likely they were to die, and receiving more than 4 units was associated with worse outcomes (P = .01).
Dunne et al30 performed a study of 6,301 noncardiac surgical patients in the Veterans Affairs Maryland Healthcare System from the National Veterans Administration Surgical Quality Improvement Program from 1995 to 2000. Multiple logistic regression analysis revealed that the composite of low hematocrit before and after surgery and high transfusion rates (> 4 units per hospitalization) were associated with higher rates of death (P < .01) and postoperative pneumonia (P ≤ .05) and longer hospitalizations (P < .05). The risk of pneumonia increased proportionally with the decrease in hematocrit.
These findings support pharmacologic optimization of anemia with hematinic supplementation before surgery to decrease the risk of needing a transfusion, often with parenteral iron. The fact that the patient’s hemoglobin can be optimized preoperatively by nontransfusional means may decrease the likelihood of blood transfusion, as the hemoglobin will potentially remain above the transfusion threshold. For example, if a patient has a preoperative hemoglobin level of 10 g/dL, and it is optimized up to 12, then if postoperatively the hemoglobin level drops 3 g/dL instead of reaching the threshold of 7 g/dL, the nadir will be just 9 g/dL, far above that transfusion threshold.
Brunskill et al,31 in a Cochrane review of 6 trials with 2,722 patients undergoing surgery for hip fracture, found no difference in rates of mortality, functional recovery or postoperative morbidity with a restrictive transfusion strategy (hemoglobin target > 8 g/dL vs a liberal one (> 10 g/dL). However, the quality of evidence was rated as low. The authors concluded that there is no justification for liberal red blood cell transfusion thresholds (10 g/dL), and a more restrictive transfusion threshold is preferable.
Weinberg et al32 found that, in trauma patients, receiving more than 6 units of blood was associated with poor prognosis, and outcomes were worse when the blood was older than 2 weeks. However, the effect of blood age is not significant when using smaller transfusion volumes (1 to 2 units of red blood cells).
Studies in sickle cell disease
Sickle cell disease patients have high levels of hemoglobin S, which causes erythrocyte sickling and increases blood viscosity. Transfusion with normal erythrocytes increases the amount of hemoglobin A (the normal variant).33,34
In trials in surgical patients,35,36 conservative strategies for preoperative blood transfusion aiming at a hemoglobin level of 10 g/dL were as effective in preventing postoperative complications as decreasing the hemoglobin S levels to 30% by aggressive exchange transfusion.35
In nonsurgical patients, blood transfusion should be based on formal risk-benefit assessments. Therefore, the expert panel report on sickle cell management advises against blood transfusion in sickle cell patients with uncomplicated vaso-occlusive crises, priapism, asymptomatic anemia, or acute kidney injury in the absence of multisystem organ failure.34
Is hemoglobin the most relevant marker?
Most studies that compared restrictive and liberal transfusion strategies focused on using a lower hemoglobin threshold as the transfusion trigger, not on using fewer units of blood. Is the amount of blood transfused more important than the hemoglobin threshold? Perhaps a study focused both on a restrictive vs liberal strategy and also on the minimum amount of blood that each patient may benefit from would help to answer this question.
We should beware of routinely using the hemoglobin concentration as a threshold for transfusion and a surrogate marker of transfusion benefit because changes in hemoglobin concentration may not reflect changes in absolute red cell mass.37 Changes in plasma volume (an increase or decrease) affect the hematocrit concentration without necessarily affecting the total red cell mass. Unfortunately, red cell mass is very difficult to measure; hence, the hemoglobin and hematocrit values are used instead. Studies addressing changes in red cell mass may be needed, perhaps even to validate using the hemoglobin concentration as the sole indicator for transfusion.
Is fresh blood better than old blood?
Using blood that is more than 14 days old may be associated with poor outcomes, for several possible reasons. Red blood cells age rapidly in refrigeration, and usually just 75% may remain viable 24 hours after phlebotomy. Adenosine triphosphate and 2,3-DPG levels steadily decrease, with a consequent decrease in capacity for appropriate tissue oxygen delivery. In addition, loss of membrane phospholipids causes progressive rigidity of the red cell membrane with consequent formation of echynocytes after 14 to 21 days.38,39
The use of blood more than 14 days old in cardiac surgery patients has been associated with worse outcomes, including higher rates of death, prolonged intubation, acute renal failure, and sepsis.40 Similar poor outcomes have been seen in trauma patients.32
Lacroix et al,41 in a multicenter, randomized trial in critically ill adults, compared the outcomes of transfusion of fresh packed red cells (stored < 8 days) or old blood (stored for a mean of 22 days). The primary outcome was the mortality rate at 90 days: 37.0% in the fresh-blood group vs 35.3% in the old-blood group (HR 1.1, 95% CI 0.9–1.2, P = .38).
The authors concluded that using fresh blood compared with old blood was not associated with a lower 90-day mortality rate in critically ill adults.
RISKS ASSOCIATED WITH TRANSFUSION
Infections
The risk of infection from blood transfusion is small. Human immunodeficiency virus (HIV) is transmitted in 1 in 1.5 million transfused blood components, and hepatitis C virus in 1 in 1.1 million; these odds are similar to those of having a fatal airplane accident (1 in 1.7 million per flight). Hepatitis B virus infection is more common, the reported incidence being 1 in 357,000.42
Noninfectious complications
Transfusion-associated circulatory overload occurs in 4% to 6% of patients who receive a transfusion. Therefore, circulatory overload is a greater danger from transfusion than infection is.42
Febrile nonhemolytic transfusion reactions occur in 1.1% of patients with prestorage leukoreduction.
Transfusion-associated acute lung injury occurs in 0.8 per 10,000 blood components transfused.
Errors associated with blood transfusion include, in decreasing order of frequency, transfusion of the wrong blood component, handling and storage errors, inappropriate administration of anti-D immunoglobulin, and avoidable, delayed, or insufficient transfusions.43
Surgery and condition-specific complications of red blood cell transfusion
Cardiovascular surgery. Transfusion is associated with a higher risk of postoperative stroke, respiratory failure, acute respiratory distress syndrome, prolonged intubation time, reintubation, in-hospital death, sepsis, and longer postoperative length of stay.44
Malignancy. The use of blood in this setting has been found to be an independent predictor of recurrence, decreased survival, and increased risk of lymphoplasmacytic and marginal-zone lymphomas.44–47
Vascular, orthopedic, and other surgeries. Transfusion is associated with a higher risk of death, thromboembolic events, acute kidney injury, death, composite morbidity, reoperation, sepsis, and pulmonary complications.44
ST-segment elevation myocardial infarction, sepsis, and intensive care unit admissions. Transfusion is associated with an increased risk of rebleeding, death, and secondary infections.44
COST OF RED BLOOD CELL TRANSFUSION
Up to 85 million units of red blood cells are transfused per year worldwide, 15 million of them in the United States.42 At our hospital in 2013, 1 unit of leukocyte-reduced red blood cells cost $957.27, which included the costs of acquisition, processing, banking, patient testing, administration, and monitoring.
The Premier Healthcare Alliance48 analyzed data from 7.4 million discharges from 464 hospitals between April 2011 and March 2012. Blood use varied significantly among hospitals, and the hospitals in the lowest quartile of blood use had better patient outcomes. If all the hospitals used as little blood as those in the lowest quartile and had outcomes as good, blood product use would be reduced by 802,716 units, with savings of up to $165 million annually.
In addition to the economic cost of blood transfusion, the clinician must be aware of the cost in terms of comorbidities caused by unnecessary blood transfusion.49,50
RECOMMENDATIONS FROM THE AABB
In view of all the current compelling evidence, a restrictive approach to transfusion is the single best strategy to minimize adverse outcomes.51 Below, we outline the current recommendations from the AABB (formerly the American Association of Blood Banks),42 which are similar to the national clinical guideline on blood transfusion in the United Kingdom,52 and have recently been updated, confirming the initial recommendations.53
In critical care patients, transfusion should be considered if the hemoglobin concentration is 7 g/dL or less.
In postoperative patients and hospitalized patients with preexisting cardiovascular disease, transfusion should be considered if the hemoglobin concentration is 8 g/dL or less or if the patient has signs or symptoms of anemia such as chest pain, orthostatic hypotension, or tachycardia unresponsive to fluid resuscitation, or heart failure.
In hemodynamically stable patients with acute coronary syndrome, there is not enough evidence to allow a formal recommendation for or against a liberal or restrictive transfusion threshold.
Consider both the hemoglobin concentration and the symptoms when deciding whether to give a transfusion. This recommendation is shared by a National Institutes of Health consensus conference,54 which indicates that multiple factors related to the patient’s clinical status and oxygen delivery should be considered before deciding to transfuse red blood cells.
The Society of Hospital Medicine55 and the American Society of Hematology56 concur with a parsimonious approach to blood use in their Choosing Wisely campaigns. The American Society of Hematology recommends that if transfusion of red blood cells is necessary, the minimum number of units should be given that relieve the symptoms of anemia or achieve a safe hemoglobin range (7–8 g/dL in stable noncardiac inpatients).57
New electronic tools can monitor the ordering and use of blood products in real time and can identify the hemoglobin level used as the trigger for transfusion. They also provide data on blood use by physician, hospital, and department. These tools can reveal current practice at a glance and allow sharing of best practices among peers and institutions.52
CONSIDER TRANSFUSION FOR HEMOGLOBIN BELOW 7 G/DL
The routine use of blood has come under scrutiny, given its association with increased healthcare costs and morbidity. The accepted practice in stable medical patients is a restrictive threshold approach for blood transfusion, which is to consider (not necessarily give) a single unit of packed red blood cells for a hemoglobin less than 7 g/dL.
However, studies in acute coronary syndrome patients and postoperative cardiac surgery patients have not shown the restrictive threshold to be superior to a liberal threshold in terms of outcomes and costs. This variability suggests the need for further studies to determine the best course of action in different patient subpopulations (eg, surgical, oncologic, trauma, critical illness).
Also, a limitation of most of the clinical studies was that only the hemoglobin concentration was used as a marker of anemia, with no strict assessment of changes in red cell mass with transfusion.
Despite the variability in certain populations, the overall weight of current evidence favors a restrictive approach to blood transfusion (hemoglobin < 7 g/dL), although perhaps in patients who have active coronary disease or are undergoing cardiac surgery, a more lenient threshold (< 8 g/dL) for transfusion should be considered.
For decades, physicians believed in the benefit of prompt transfusion of blood to keep the hemoglobin level at arbitrary, optimum levels, ie, close to normal values, especially in the critically ill, the elderly, and those with coronary syndromes, stroke, or renal failure.
However, the evidence supporting arbitrary hemoglobin values as an indication for transfusion was weak or nonexistent. Also, blood transfusion can have complications and adverse effects, and blood is costly and scarce. These considerations prompted research into when blood transfusion should be considered, and recommendations that it should be used more sparingly than in the past.
This review offers a perspective on the evidence supporting restrictive blood use. First, we focus on hemodilution studies that demonstrated that humans can tolerate anemia. Then, we look at studies that compared a restrictive transfusion strategy with a liberal one in patients with critical illness and active bleeding. We conclude with current recommendations for blood transfusion.
EVIDENCE FROM HEMODILUTION STUDIES
Hemoglobin is essential for tissue oxygenation, but the serum hemoglobin concentration is just one of several factors involved.1–5 In anemia, the body can adapt not only by increasing production of red blood cells, but also by:
- Increasing cardiac output
- Increasing synthesis of 2,3-diphosphoglycerate (2,3-DPG), with a consequent shift in the oxyhemoglobin dissociation curve to the right, allowing enhanced release of oxygen at the tissue level
- Moving more carbon dioxide into the blood (the Bohr effect), which decreases pH and also shifts the dissociation curve to the right.
Just 20 years ago, physicians were using arbitrary cutoffs such as hemoglobin 10 g/dL or hematocrit 30% as indications for blood transfusion, without reasonable evidence to support these values. Not until acute normovolemic hemodilution studies were performed were we able to progressively appraise how well patients could tolerate lower levels of hemoglobin without significant adverse outcomes.
Acute normovolemic hemodilution involves withdrawing blood and replacing it with crystalloid or colloid solution to maintain the volume.6
Initial studies were done in animals and focused on the safety of acute anemia regarding splanchnic perfusion. Subsequently, studies proved that healthy, elderly, and stable cardiac patients can tolerate acute anemia with normal cardiovascular response. The targets in these studies were modest at first, but researchers aimed progressively for more aggressive hemodilution with lower hemoglobin targets and demonstrated that the body can tolerate and adapt to more severe anemia.6–8
Studies in healthy patients
Weiskopf et al9 assessed the effect of severe anemia in 32 conscious healthy patients (11 presurgical patients and 21 volunteers not undergoing surgery) by performing acute normovolemic hemodilution with 5% human albumin, autologous plasma, or both, with a target hemoglobin level of 5 g/dL. The process was done gradually, obtaining aliquots of blood of 500 to 900 mL. Cardiac index increased, along with a mild increase in oxygen consumption with no increase in plasma lactate levels, suggesting that in conscious healthy patients, tissue oxygenation remains adequate even in severe anemia.
Leung et al10 addressed the electrocardiographic changes that occur with severe anemia (hemoglobin 5 g/dL) in 55 healthy volunteers. Three developed transient, reversible ST-segment depression, which was associated with a higher heart rate than in the volunteers with no electrocardiographic changes; however, the changes were reversible and asymptomatic, and thus were considered physiologic and benign.
Hemodilution in healthy elderly patients
Spahn et al11 performed 6 and 12 mL/kg isovolemic exchange of blood for 6% hydroxyethyl starch in 20 patients older than 65 years (mean age 76, range 65–88) without underlying coronary disease.
The patients’ mean hemoglobin level decreased from 11.6 g/dL to 8.8 g/dL. Their cardiac index and oxygen extraction values increased adequately, with stable oxygen consumption during hemodilution. There were no electrocardiographic signs of ischemia.
Hemodilution in coronary artery disease
Spahn et al12 performed hemodilution studies in 60 patients (ages 35–81) with coronary artery disease managed chronically with beta-blockers who were scheduled for coronary artery bypass graft surgery. Hemodilution was performed with 6- and 12-mL/kg isovolemic exchange of blood for 6% hydroxyethyl starch maintaining normovolemia and stable filling pressures. Hemoglobin levels decreased from 12.6 g/dL to 9.9 g/dL. The hemodilution process was done before the revascularization. The authors monitored hemodynamic variables, ST-segment deviation, and oxygen consumption before and after each hemodilution.
There was a compensatory increase in cardiac index and oxygen extraction with consequent stable oxygen consumption. These changes were independent of patient age or left ventricular function. In addition, there were no electrocardiographic signs of ischemia.
Licker et al13 studied the hemodynamic effect of preoperative hemodilution in 50 patients with coronary artery disease undergoing coronary artery bypass graft surgery, performing transesophageal echocardiography before and after hemodilution. The patients underwent isovolemic exchange with iso-oncotic starch to target a hematocrit of 28%.
Acute normovolemic hemodilution triggered an increase in cardiac stroke volume, which had a direct correlation with an increase in the central venous pressure and the left ventricular end-diastolic area. No signs of ischemia were seen in these patients on electrocardiography or echocardiography (eg, left ventricular wall-motion abnormalities).
Hemodilution in mitral regurgitation
Spahn et al14 performed acute isovolemic hemodilution with 6% hydroxyethyl starch in 20 patients with mitral regurgitation. The cardiac filling pressures were stable before and after hemodilution; the mean hemoglobin value decreased from 13 to 10.3 g/dL. The cardiac index and oxygen extraction increased proportionally, with stable oxygen consumption; these findings were the same regardless of whether the patient was in normal sinus rhythm or atrial fibrillation.
Effect of hemodilution on cognition
Weiskopf et al15 assessed the effect of anemia on executive and memory function by inducing progressive acute isovolemic anemia in 90 healthy volunteers (age 29 ± 5), reducing their hemoglobin values to 7, 6, and 5 g/dL and performing repetitive neuropsychological and memory testing before and after the hemodilution, as well as after autologous blood transfusion to return their hemoglobin level to 7 g/dL.
There were no changes in reaction time or error rate at a hemoglobin concentration of 7 g/dL compared with the performance at a baseline hemoglobin concentration of 14 g/dL. The volunteers got slower on a mathematics test at hemoglobin levels of 6 g/dL and 5 g/dL, but their error rate did not increase. Immediate and delayed memory were significantly impaired at hemoglobin of 5 g/dL but not at 6 g/dL. All tests normalized with blood transfusion once the hemoglobin level reached 7 g/dL.15
Weiskopf et al16 subsequently investigated whether giving supplemental oxygen to raise the arterial partial pressure of oxygen (Pao2) to 350 mm Hg or greater would overcome the neurocognitive effects of severe acute anemia. They followed a protocol similar to the one in the earlier study15 and induced anemia in 31 healthy volunteers, age 28 ± 4 years, with a mean baseline hemoglobin concentration of 12.7 g/dL.
When the volunteers reached a hemoglobin concentration of 5.7 ± 0.3 g/dL, they were significantly slower on the mathematics test, and their delayed memory was significantly impaired. Then, in a double-blind fashion, they were given either room air or oxygen. Oxygen increased the Pao2 to 406 mm Hg and normalized neurocognitive performance.
Hemodilution studies in surgical patients
A 2015 meta-analysis17 of 63 studies involving 3,819 surgical patients compared the risk of perioperative allogeneic blood transfusion as well as the overall volume of transfused blood in patients undergoing preoperative acute normovolemic hemodilution vs a control group. Though the overall data showed that the patients who underwent acute normovolemic hemodilution needed fewer transfusions and less blood (relative risk [RR] 0.74, 95% confidence interval [CI] 0.63–0.88, P = .0006), the authors noted significant heterogeneity and publication bias.
However, the hemodilution studies paved the way for justifying a more conservative and restrictive transfusion strategy, with a hemoglobin cutoff value of 7 g/dL, and in acute anemia, using oxygen to overcome acute neurocognitive effects while searching for and correcting the cause of the anemia.
STUDIES OF RESTRICTIVE VS LIBERAL TRANSFUSION STRATEGIES
Studies in critical care and high-risk patients
Hébert et al18 randomized 418 critical care patients to a restrictive transfusion approach (in which they were given red blood cells if their hemoglobin concentration dropped below 7.0 g/dL) and 420 patients to a liberal strategy (given red blood cells if their hemoglobin concentration dropped below 10.0 g/dL). Mortality rates (restrictive vs liberal strategy) were as follows:
- Overall at 30 days 18.7% vs 23.3%, P = .11
- In the subgroup with less-severe disease (Acute Physiology and Chronic Health Evaluation II [APACHE II] score < 20), 8.7% vs 16.1%, P = .03
- In the subgroup under age 55, 5.7% vs 13%, P = .02
- In the subgroup with clinically significant cardiac disease, 20.5% vs 22.9%, P = .69
- In the hospital, 22.2% vs 28.1%; P = .05.
This study demonstrated that parsimonious blood use did not worsen clinical outcomes in critical care patients.
Carson et al19 evaluated 2,016 patients age 50 and older who had a history of or risk factors for cardiovascular disease and a baseline hemoglobin level below 10 g/dL who underwent surgery for hip fracture. Patients were randomized to two transfusion strategies based on threshold hemoglobin level: restrictive (< 8 g/dL) or liberal (< 10 g/dL). The primary outcome was death or inability to walk without assistance at 60-day follow-up. The median number of units of blood used was 2 in the liberal group and 0 in the restrictive group.
There was no significant difference in the rates of the primary outcome (odds ratio [OR] 1.01, 95% CI 0.84–1.22), infection, venous thromboembolism, or reoperation. This study demonstrated that a liberal transfusion strategy offered no benefit over a restrictive one.
Rao et al20 analyzed the impact of blood transfusion in 24,112 patients with acute coronary syndromes enrolled in three large trials. Ten percent of the patients received at least 1 blood transfusion during their hospitalization, and they were older and had more complex comorbidity.
At 30 days, the group that had received blood had higher rates of death (adjusted hazard ratio [HR] 3.94, 95% CI 3.26–4.75) and the combined outcome of death or myocardial infarction (HR 2.92, 95% CI 2.55–3.35). Transfusion in patients whose nadir hematocrit was higher than 25% was associated with worse outcomes.
This study suggests being cautious about routinely transfusing blood in stable patients with ischemic heart disease solely on the basis of arbitrary hematocrit levels.
Carson et al,21 however, in a later trial, found a trend toward worse outcomes with a restrictive strategy than with a liberal one. Here, 110 patients with acute coronary syndrome or stable angina undergoing cardiac catheterization were randomized to a target hemoglobin level of either at least 8 mg/dL or at least 10 g/dL. The primary outcome (a composite of death, myocardial infarction, or unscheduled revascularization 30 days after randomization) occurred in 14 patients (25.5%) in the restrictive group and 6 patients (10.9%) in the liberal group (P = .054), and 7 (13.0%) vs 1 (1.8%) of the patients died (P = .032).
Murphy et al22 similarly found trends toward worse outcomes with a restrictive strategy in cardiac patients. The investigators randomized 2,007 elective cardiac surgery patients with a postoperative hemoglobin level lower than 9 g/dL to a hemoglobin transfusion threshold of either 7.5 or 9 g/dL. Outcomes (restrictive vs liberal strategies):
- Transfusion rates 53.4% vs 92.2%
- Rates of the primary outcome (a serious infection [sepsis or wound infection] or ischemic event [stroke, myocardial infarction, mesenteric ischemia, or acute kidney injury] within 3 months):
35.1% vs 33.0%, OR 1.11, 95% CI 0.91–1.34, P = .30) - Mortality rates 4.2% vs 2.6%, HR 1.64, 95% CI 1.00–2.67, P = .045
- Total costs did not differ significantly between the groups.
These studies21,22 suggest the need for more definitive trials in patients with active coronary disease and in cardiac surgery patients.
Holst et al23 randomized 998 intensive care patients in septic shock to hemoglobin thresholds for transfusion of 7 vs 9 g/dL. Mortality rates at 90 days (the primary outcome) were 43.0% vs 45.0%, RR 0.94, 95% CI 0.78–1.09, P = .44.
This study suggests that even in septic shock, a liberal transfusion strategy has no advantage over a parsimonious one.
Active bleeding, especially active gastrointestinal bleeding, poses a significant stress that may trigger empirical transfusion even without evidence of the real hemoglobin level.
Villanueva et al24 randomized 921 patients with severe acute upper-gastrointestinal bleeding to two groups, with hemoglobin transfusion triggers of 7 vs 9 g/dL. The findings were impressive:
- Freedom from transfusion 51% vs 14% (P < .001)
- Survival rates at 6 weeks 95% vs 91% (HR 0.55, 95% CI 0.33–0.92, P = .02)
- Rebleeding 10% vs 16% (P = .01).
Patients with peptic ulcer disease as well as those with cirrhosis stage Child-Pugh class A or B had higher survival rates with a restrictive transfusion strategy.
The RELIEVE trial25 compared the effect of a restrictive transfusion strategy in elderly patients on mechanical ventilation in 6 intensive care units in the United Kingdom. Transfusion triggers were hemoglobin 7 vs 9 g/dL, and the mortality rate at 180 days was 55% vs 37%, RR 0.68, 95% CI 0.44–1.05, P = .073.
Meta-analyses and observational studies
Rohde et al26 performed a systematic review and meta-analysis of 17 trials with 7,456 patients, which revealed that a restrictive strategy is associated with a lower risk of nosocomial infection, including pneumonia, wound infection, and sepsis.
The pooled risk of all serious infections was 10.6% in the restrictive group and 12.7% in the liberal group. Even after adjusting for the use of leukocyte reduction, the risk of infection was lower in the restrictive strategy group (RR 0.83, 95% CI 0.69–0.99). With a hemoglobin threshold of less than 7.0 g/dL, the risk of serious infection was 14% lower. Although this was not statistically significant overall (RR 0.86, 95% CI 0.72–1.02), the difference was statistically significant in the subgroup undergoing orthopedic surgery (RR 0.72, 95% CI 0.53–0.97) and the subgroup presenting with sepsis (RR 0.51, 95% CI 0.28–0.95).
Salpeter et al27 performed a meta-analysis and systematic review of three randomized trials (N = 2,364) comparing a restrictive hemoglobin transfusion trigger (hemoglobin < 7 g/dL) vs a more liberal trigger. The groups with restrictive transfusion triggers had lower rates of:
- In-hospital mortality (RR 0.74, 95% CI 0.60–0.92)
- Total mortality (RR 0.80, 95% CI 0.65–0.98)
- Rebleeding (RR 0.64, 95% CI 0.45–0.90)
- Acute coronary syndrome (RR 0.44, 95% CI 0.22–0.89)
- Pulmonary edema (RR 0.48, 95% CI 0.33–0.72)
- Bacterial infections (RR 0.86, 95% CI 0.73–1.00).
Wang et al28 performed a meta-analysis of 4 randomized controlled trials in patients with upper-gastrointestinal bleeding comparing restrictive (hemoglobin < 7 g/dL) vs liberal transfusion strategies. The primary outcomes were death and rebleeding. The restrictive strategy was associated with:
- A lower mortality rate (OR 0.52, 95% CI 0.31–0.87, P = .01)
- A lower rebleeding rate (OR 0.26, 95% CI 0.03–2.10, P = .21)
- Shorter hospitalizations (P = .009)
- Less blood transfused (P = .0005).
Vincent et al,29 in a prospective observational study of 3,534 patients in intensive care units in 146 facilities in Western Europe, found a correlation between transfusion and mortality. Transfusion was done most often in elderly patients and those with a longer stay in the intensive care unit. The 28-day mortality rate was 22.7% in patients who received a transfusion and 17.1% in those who did not (P = .02). The more units of blood the patients received, the more likely they were to die, and receiving more than 4 units was associated with worse outcomes (P = .01).
Dunne et al30 performed a study of 6,301 noncardiac surgical patients in the Veterans Affairs Maryland Healthcare System from the National Veterans Administration Surgical Quality Improvement Program from 1995 to 2000. Multiple logistic regression analysis revealed that the composite of low hematocrit before and after surgery and high transfusion rates (> 4 units per hospitalization) were associated with higher rates of death (P < .01) and postoperative pneumonia (P ≤ .05) and longer hospitalizations (P < .05). The risk of pneumonia increased proportionally with the decrease in hematocrit.
These findings support pharmacologic optimization of anemia with hematinic supplementation before surgery to decrease the risk of needing a transfusion, often with parenteral iron. The fact that the patient’s hemoglobin can be optimized preoperatively by nontransfusional means may decrease the likelihood of blood transfusion, as the hemoglobin will potentially remain above the transfusion threshold. For example, if a patient has a preoperative hemoglobin level of 10 g/dL, and it is optimized up to 12, then if postoperatively the hemoglobin level drops 3 g/dL instead of reaching the threshold of 7 g/dL, the nadir will be just 9 g/dL, far above that transfusion threshold.
Brunskill et al,31 in a Cochrane review of 6 trials with 2,722 patients undergoing surgery for hip fracture, found no difference in rates of mortality, functional recovery or postoperative morbidity with a restrictive transfusion strategy (hemoglobin target > 8 g/dL vs a liberal one (> 10 g/dL). However, the quality of evidence was rated as low. The authors concluded that there is no justification for liberal red blood cell transfusion thresholds (10 g/dL), and a more restrictive transfusion threshold is preferable.
Weinberg et al32 found that, in trauma patients, receiving more than 6 units of blood was associated with poor prognosis, and outcomes were worse when the blood was older than 2 weeks. However, the effect of blood age is not significant when using smaller transfusion volumes (1 to 2 units of red blood cells).
Studies in sickle cell disease
Sickle cell disease patients have high levels of hemoglobin S, which causes erythrocyte sickling and increases blood viscosity. Transfusion with normal erythrocytes increases the amount of hemoglobin A (the normal variant).33,34
In trials in surgical patients,35,36 conservative strategies for preoperative blood transfusion aiming at a hemoglobin level of 10 g/dL were as effective in preventing postoperative complications as decreasing the hemoglobin S levels to 30% by aggressive exchange transfusion.35
In nonsurgical patients, blood transfusion should be based on formal risk-benefit assessments. Therefore, the expert panel report on sickle cell management advises against blood transfusion in sickle cell patients with uncomplicated vaso-occlusive crises, priapism, asymptomatic anemia, or acute kidney injury in the absence of multisystem organ failure.34
Is hemoglobin the most relevant marker?
Most studies that compared restrictive and liberal transfusion strategies focused on using a lower hemoglobin threshold as the transfusion trigger, not on using fewer units of blood. Is the amount of blood transfused more important than the hemoglobin threshold? Perhaps a study focused both on a restrictive vs liberal strategy and also on the minimum amount of blood that each patient may benefit from would help to answer this question.
We should beware of routinely using the hemoglobin concentration as a threshold for transfusion and a surrogate marker of transfusion benefit because changes in hemoglobin concentration may not reflect changes in absolute red cell mass.37 Changes in plasma volume (an increase or decrease) affect the hematocrit concentration without necessarily affecting the total red cell mass. Unfortunately, red cell mass is very difficult to measure; hence, the hemoglobin and hematocrit values are used instead. Studies addressing changes in red cell mass may be needed, perhaps even to validate using the hemoglobin concentration as the sole indicator for transfusion.
Is fresh blood better than old blood?
Using blood that is more than 14 days old may be associated with poor outcomes, for several possible reasons. Red blood cells age rapidly in refrigeration, and usually just 75% may remain viable 24 hours after phlebotomy. Adenosine triphosphate and 2,3-DPG levels steadily decrease, with a consequent decrease in capacity for appropriate tissue oxygen delivery. In addition, loss of membrane phospholipids causes progressive rigidity of the red cell membrane with consequent formation of echynocytes after 14 to 21 days.38,39
The use of blood more than 14 days old in cardiac surgery patients has been associated with worse outcomes, including higher rates of death, prolonged intubation, acute renal failure, and sepsis.40 Similar poor outcomes have been seen in trauma patients.32
Lacroix et al,41 in a multicenter, randomized trial in critically ill adults, compared the outcomes of transfusion of fresh packed red cells (stored < 8 days) or old blood (stored for a mean of 22 days). The primary outcome was the mortality rate at 90 days: 37.0% in the fresh-blood group vs 35.3% in the old-blood group (HR 1.1, 95% CI 0.9–1.2, P = .38).
The authors concluded that using fresh blood compared with old blood was not associated with a lower 90-day mortality rate in critically ill adults.
RISKS ASSOCIATED WITH TRANSFUSION
Infections
The risk of infection from blood transfusion is small. Human immunodeficiency virus (HIV) is transmitted in 1 in 1.5 million transfused blood components, and hepatitis C virus in 1 in 1.1 million; these odds are similar to those of having a fatal airplane accident (1 in 1.7 million per flight). Hepatitis B virus infection is more common, the reported incidence being 1 in 357,000.42
Noninfectious complications
Transfusion-associated circulatory overload occurs in 4% to 6% of patients who receive a transfusion. Therefore, circulatory overload is a greater danger from transfusion than infection is.42
Febrile nonhemolytic transfusion reactions occur in 1.1% of patients with prestorage leukoreduction.
Transfusion-associated acute lung injury occurs in 0.8 per 10,000 blood components transfused.
Errors associated with blood transfusion include, in decreasing order of frequency, transfusion of the wrong blood component, handling and storage errors, inappropriate administration of anti-D immunoglobulin, and avoidable, delayed, or insufficient transfusions.43
Surgery and condition-specific complications of red blood cell transfusion
Cardiovascular surgery. Transfusion is associated with a higher risk of postoperative stroke, respiratory failure, acute respiratory distress syndrome, prolonged intubation time, reintubation, in-hospital death, sepsis, and longer postoperative length of stay.44
Malignancy. The use of blood in this setting has been found to be an independent predictor of recurrence, decreased survival, and increased risk of lymphoplasmacytic and marginal-zone lymphomas.44–47
Vascular, orthopedic, and other surgeries. Transfusion is associated with a higher risk of death, thromboembolic events, acute kidney injury, death, composite morbidity, reoperation, sepsis, and pulmonary complications.44
ST-segment elevation myocardial infarction, sepsis, and intensive care unit admissions. Transfusion is associated with an increased risk of rebleeding, death, and secondary infections.44
COST OF RED BLOOD CELL TRANSFUSION
Up to 85 million units of red blood cells are transfused per year worldwide, 15 million of them in the United States.42 At our hospital in 2013, 1 unit of leukocyte-reduced red blood cells cost $957.27, which included the costs of acquisition, processing, banking, patient testing, administration, and monitoring.
The Premier Healthcare Alliance48 analyzed data from 7.4 million discharges from 464 hospitals between April 2011 and March 2012. Blood use varied significantly among hospitals, and the hospitals in the lowest quartile of blood use had better patient outcomes. If all the hospitals used as little blood as those in the lowest quartile and had outcomes as good, blood product use would be reduced by 802,716 units, with savings of up to $165 million annually.
In addition to the economic cost of blood transfusion, the clinician must be aware of the cost in terms of comorbidities caused by unnecessary blood transfusion.49,50
RECOMMENDATIONS FROM THE AABB
In view of all the current compelling evidence, a restrictive approach to transfusion is the single best strategy to minimize adverse outcomes.51 Below, we outline the current recommendations from the AABB (formerly the American Association of Blood Banks),42 which are similar to the national clinical guideline on blood transfusion in the United Kingdom,52 and have recently been updated, confirming the initial recommendations.53
In critical care patients, transfusion should be considered if the hemoglobin concentration is 7 g/dL or less.
In postoperative patients and hospitalized patients with preexisting cardiovascular disease, transfusion should be considered if the hemoglobin concentration is 8 g/dL or less or if the patient has signs or symptoms of anemia such as chest pain, orthostatic hypotension, or tachycardia unresponsive to fluid resuscitation, or heart failure.
In hemodynamically stable patients with acute coronary syndrome, there is not enough evidence to allow a formal recommendation for or against a liberal or restrictive transfusion threshold.
Consider both the hemoglobin concentration and the symptoms when deciding whether to give a transfusion. This recommendation is shared by a National Institutes of Health consensus conference,54 which indicates that multiple factors related to the patient’s clinical status and oxygen delivery should be considered before deciding to transfuse red blood cells.
The Society of Hospital Medicine55 and the American Society of Hematology56 concur with a parsimonious approach to blood use in their Choosing Wisely campaigns. The American Society of Hematology recommends that if transfusion of red blood cells is necessary, the minimum number of units should be given that relieve the symptoms of anemia or achieve a safe hemoglobin range (7–8 g/dL in stable noncardiac inpatients).57
New electronic tools can monitor the ordering and use of blood products in real time and can identify the hemoglobin level used as the trigger for transfusion. They also provide data on blood use by physician, hospital, and department. These tools can reveal current practice at a glance and allow sharing of best practices among peers and institutions.52
CONSIDER TRANSFUSION FOR HEMOGLOBIN BELOW 7 G/DL
The routine use of blood has come under scrutiny, given its association with increased healthcare costs and morbidity. The accepted practice in stable medical patients is a restrictive threshold approach for blood transfusion, which is to consider (not necessarily give) a single unit of packed red blood cells for a hemoglobin less than 7 g/dL.
However, studies in acute coronary syndrome patients and postoperative cardiac surgery patients have not shown the restrictive threshold to be superior to a liberal threshold in terms of outcomes and costs. This variability suggests the need for further studies to determine the best course of action in different patient subpopulations (eg, surgical, oncologic, trauma, critical illness).
Also, a limitation of most of the clinical studies was that only the hemoglobin concentration was used as a marker of anemia, with no strict assessment of changes in red cell mass with transfusion.
Despite the variability in certain populations, the overall weight of current evidence favors a restrictive approach to blood transfusion (hemoglobin < 7 g/dL), although perhaps in patients who have active coronary disease or are undergoing cardiac surgery, a more lenient threshold (< 8 g/dL) for transfusion should be considered.
- Shander A, Gross I, Hill S, Javidroozi M, Sledge S; College of American Pathologists; American Society of Anesthesiologists; Society of Thoracic Surgeons and Society of Cardiovascular Anesthesiologists; Society of Critical Care Medicine; Italian Society of Transfusion Medicine and Immunohaematology; American Association of Blood Banks. A new perspective on best transfusion practices. Blood Transfus 2013; 11:193–202.
- Madjdpour C, Spahn DR. Allogeneic red blood cell transfusion: physiology of oxygen transport. Best Pract Res Clin Anaesthesiol 2007; 21:163–171.
- Tánczos K, Molnár Z. The oxygen supply-demand balance: a monitoring challenge. Best Pract Res Clin Anaesthesiol 2013; 27:201–207.
- Hebert PC, Van der Linden P, Biro G, Hu LQ. Physiologic aspects of anemia. Crit Care Clin 2004; 20:187–212.
- Spinelli E, Bartlett RH. Anemia and transfusion in critical care: physiology and management. J Intensive Care Med 2016; 31:295–306.
- Jamnicki M, Kocian R, Van Der Linden P, Zaugg M, Spahn DR. Acute normovolemic hemodilution: physiology, limitations, and clinical use. J Cardiothorac Vasc Anesth 2003; 17:747–754.
- Monk TG. Acute normovolemic hemodilution. Anesthesiol Clin North America 2005; 23:271–281.
- Shander A, Rijhwani TS. Acute normovolemic hemodilution. Transfusion 2004; 44(suppl 2):26S–34S.
- Weiskopf RB, Viele MK, Feiner J, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA 1998; 279:217–221.
- Leung JM, Weiskopf RB, Feiner J, et al. Electrocardiographic ST-segment changes during acute, severe isovolemic hemodilution in humans. Anesthesiology 2000; 93:1004–1010.
- Spahn DR, Zollinger A, Schlumpf RB, et al. Hemodilution tolerance in elderly patients without known cardiac disease. Anesth Analg 1996; 82:681–686.
- Spahn DR, Schmid ER, Seifert B, Pasch T. Hemodilution tolerance in patients with coronary artery disease who are receiving chronic beta-adrenergic blocker therapy. Anesth Analg 1996; 82:687–694.
- Licker M, Ellenberger C, Sierra J, Christenson J, Diaper J, Morel D. Cardiovascular response to acute normovolemic hemodilution in patients with coronary artery diseases: assessment with transesophageal echocardiography. Crit Care Med 2005; 33:591–597.
- Spahn DR, Seifert B, Pasch T, Schmid ER. Haemodilution tolerance in patients with mitral regurgitation. Anaesthesia 1998; 53:20–24.
- Weiskopf RB, Kramer JH, Viele M, et al. Acute severe isovolemic anemia impairs cognitive function and memory in humans. Anesthesiology 2000; 92:1646–1652.
- Weiskopf RB, Feiner J, Hopf HW, et al. Oxygen reverses deficits of cognitive function and memory and increased heart rate induced by acute severe isovolemic anemia. Anesthesiology 2002; 96:871–877.
- Zhou X, Zhang C, Wang Y, Yu L, Yan M. Preoperative acute normovolemic hemodilution for minimizing allogeneic blood transfusion: a meta-analysis. Anesth Analg 2015; 121:1443–1455.
- Hébert P, Wells G, Blajchman M, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med 1999: 340:409–417.
- Carson JL, Terrin ML, Noveck H, et al; FOCUS Investigators. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011; 365:2453–2462.
- Rao SV, Jollis JG, Harrington RA, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA 2004; 292:1555–1562.
- Carson JL, Brooks MM, Abbott JD, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J 2013; 165:964.e1–971.e1.
- Murphy GJ, Pike K, Rogers CA, et al; TITRe2 Investigators. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med 2015; 372:997–1008.
- Holst LB, Haase N, Wetterslev J, et al; TRISS Trial Group; Scandinavian Critical Care Trials Group. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med 2014; 371:1381–1391.
- Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013; 368:11–21.
- Walsh TS, Boyd JA, Watson D, et al; RELIEVE Investigators. Restrictive versus liberal transfusion strategies for older mechanically ventilated critically ill patients: a randomized pilot trial. Crit Care Med 2013; 41:2354–2363.
- Rohde JM, Dimcheff DE, Blumberg N, et al. Health care–associated infection after red blood cell transfusion. JAMA 2014; 311:1317–1326.
- Salpeter SR, Buckley JS, Chatterjee S. Impact of more restrictive blood transfusion strategies on clinical outcomes: a meta-analysis and systematic review. Am J Med 2014; 127:124.e3–131.e3.
- Wang J, Bao YX, Bai M, Zhang YG, Xu WD, Qi XS. Restrictive vs liberal transfusion for upper gastrointestinal bleeding: a meta-analysis of randomized controlled trials. World J Gastroenterol 2013; 19:6919–6927.
- Vincent JL, Baron JF, Reinhart K, et al; ABC (Anemia and Blood Transfusion in Critical Care) Investigators. Anemia and blood transfusion in critically ill patients. JAMA 2002; 288:1499–1507.
- Dunne JR, Malone D, Tracy JK, Gannon C, Napolitano LM. Perioperative anemia: an independent risk factor for infection, mortality, and resource utilization in surgery. J Surg Res 2002; 102:237–244.
- Brunskill SJ, Millette SL, Shokoohi A, et al. Red blood cell transfusion for people undergoing hip fracture surgery. Cochrane Database Syst Rev 2015; 4:CD009699.
- Weinberg JA, McGwin G Jr, Griffin RL, et al. Age of transfused blood: an independent predictor of mortality despite universal leukoreduction. J Trauma 2008; 65:279–284.
- Steinberg M. Management of sickle cell disease. N Engl J Med 1999; 340:1021–1030.
- Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease. JAMA 2014; 312:1033–1048.
- Vichinsky EP, Haberkern CM, Neumayr L, et al. A comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. The Preoperative Transfusion in Sickle Cell Disease Study Group. N Engl J Med 1995; 333:206–213.
- Howard J, Malfroy M, Llewelyn C, et al. The Transfusion Alternatives Preoperatively in Sickle Cell Disease (TAPS) study: a randomised, controlled, multicentre clinical trial. Lancet 2013; 381:930–938.
- Goodnough LT, Levy JH, Murphy MF. Concepts of blood transfusion in adults. Lancet 2013; 381:1845–1854.
- Holme S. Current issues related to the quality of stored RBCs. Transfus Apher Sci 2005; 33:55–61.
- Hovav T, Yedgar S, Manny N, Barshtein G. Alteration of red cell aggregability and shape during blood storage. Transfusion 1999; 39:277–281.
- Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med 2008; 358:1229–1239.
- Lacroix J, Hebert PC, Fergusson DA, et al. Age of transfused blood in critically ill adults. N Engl J Med 2015; 372:1410–1418.
- Carson JL, Grossman BJ, Kleinman S, et al; Clinical Transfusion Medicine Committee of the AABB. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2012; 157:49–58.
- Bolton-Maggs P, Watt A, Poles D, et al, on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group. The 2015 Annual SHOT Report. www.shotuk.org/wp-content/uploads/SHOT-2015-Annual-Report-Web-Edition-Final-bookmarked.pdf. Accessed November 30, 2016.
- Shander A, Javidroozi M, Ozawa S, Hare GMT. What is really dangerous: anaemia or transfusion? Br J Anaesth 2011; 107(suppl 1):i41–i59.
- Reeh M, Ghadban T, Dedow J, et al. Allogenic blood transfusion is associated with poor perioperative and long-term outcome in esophageal cancer. World J Surg 2016 Oct 11. [Epub ahead of print]
- Elmi M, Mahar A, Kagedan D, et al. The impact of blood transfusion on perioperative outcomes following gastric cancer resection: an analysis of the American College of Surgeons National Surgical Quality Improvement Program database. Can J Surg 2016; 59:322–329.
- Aquina CT, Blumberg N, Becerra AZ, et al. Association among blood transfusion, sepsis, and decreased long-term survival after colon cancer resection. Ann Surg 2016; Sep 14. [Epub ahead of print] PubMed PMID: 27631770.
- Premiere Analysis. Standardization of blood utilization practices could provide opportunity for improved outcomes, reduced costs. A Premiere Healthcare Alliance Analysis. 2012.
- Simeone F, Franchi F, Cevenini G, et al. A simple clinical model for planning transfusion quantities in heart surgery. BMC Med Inform Decis Mak 2011; 11:44.
- Spahn DR, Goodnough LT. Alternatives to blood transfusion. Lancet 2013; 381:1855–1865.
- Holst LB, Petersen MW, Haase N, Perner A, Wetterslev J. Restrictive versus liberal transfusion strategy for red blood cell transfusion: systematic review of randomised trials with meta-analysis and trial sequential analysis. BMJ 2015; 350:h1354.
- National Institute for Health and Care Excellence: Clinical Guidelines. London: National Institute for Health and Care Excellence (UK). www.ncbi.nlm.nih.gov/books/NBK11822/.
- Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA 2016 Oct 12. doi: 10.1001/jama.2016.9185. [Epub ahead of print]
- Consensus conference. Perioperative red blood cell transfusion. JAMA 1988; 260:2700–2703.
- Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med 2013; 8:486–492.
- Hicks LK, Bering H, Carson KR, et al. The ASH Choosing Wisely® campaign: five hematologic tests and treatments to question. Blood 2013; 122:3879–3883.
- Haemonetics IMPACT Online. The Blood Management Company. www.haemonetics.com/Products/Services/Consulting Services/IMPACT Online.aspx. Accessed November 30, 2016.
- Shander A, Gross I, Hill S, Javidroozi M, Sledge S; College of American Pathologists; American Society of Anesthesiologists; Society of Thoracic Surgeons and Society of Cardiovascular Anesthesiologists; Society of Critical Care Medicine; Italian Society of Transfusion Medicine and Immunohaematology; American Association of Blood Banks. A new perspective on best transfusion practices. Blood Transfus 2013; 11:193–202.
- Madjdpour C, Spahn DR. Allogeneic red blood cell transfusion: physiology of oxygen transport. Best Pract Res Clin Anaesthesiol 2007; 21:163–171.
- Tánczos K, Molnár Z. The oxygen supply-demand balance: a monitoring challenge. Best Pract Res Clin Anaesthesiol 2013; 27:201–207.
- Hebert PC, Van der Linden P, Biro G, Hu LQ. Physiologic aspects of anemia. Crit Care Clin 2004; 20:187–212.
- Spinelli E, Bartlett RH. Anemia and transfusion in critical care: physiology and management. J Intensive Care Med 2016; 31:295–306.
- Jamnicki M, Kocian R, Van Der Linden P, Zaugg M, Spahn DR. Acute normovolemic hemodilution: physiology, limitations, and clinical use. J Cardiothorac Vasc Anesth 2003; 17:747–754.
- Monk TG. Acute normovolemic hemodilution. Anesthesiol Clin North America 2005; 23:271–281.
- Shander A, Rijhwani TS. Acute normovolemic hemodilution. Transfusion 2004; 44(suppl 2):26S–34S.
- Weiskopf RB, Viele MK, Feiner J, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA 1998; 279:217–221.
- Leung JM, Weiskopf RB, Feiner J, et al. Electrocardiographic ST-segment changes during acute, severe isovolemic hemodilution in humans. Anesthesiology 2000; 93:1004–1010.
- Spahn DR, Zollinger A, Schlumpf RB, et al. Hemodilution tolerance in elderly patients without known cardiac disease. Anesth Analg 1996; 82:681–686.
- Spahn DR, Schmid ER, Seifert B, Pasch T. Hemodilution tolerance in patients with coronary artery disease who are receiving chronic beta-adrenergic blocker therapy. Anesth Analg 1996; 82:687–694.
- Licker M, Ellenberger C, Sierra J, Christenson J, Diaper J, Morel D. Cardiovascular response to acute normovolemic hemodilution in patients with coronary artery diseases: assessment with transesophageal echocardiography. Crit Care Med 2005; 33:591–597.
- Spahn DR, Seifert B, Pasch T, Schmid ER. Haemodilution tolerance in patients with mitral regurgitation. Anaesthesia 1998; 53:20–24.
- Weiskopf RB, Kramer JH, Viele M, et al. Acute severe isovolemic anemia impairs cognitive function and memory in humans. Anesthesiology 2000; 92:1646–1652.
- Weiskopf RB, Feiner J, Hopf HW, et al. Oxygen reverses deficits of cognitive function and memory and increased heart rate induced by acute severe isovolemic anemia. Anesthesiology 2002; 96:871–877.
- Zhou X, Zhang C, Wang Y, Yu L, Yan M. Preoperative acute normovolemic hemodilution for minimizing allogeneic blood transfusion: a meta-analysis. Anesth Analg 2015; 121:1443–1455.
- Hébert P, Wells G, Blajchman M, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med 1999: 340:409–417.
- Carson JL, Terrin ML, Noveck H, et al; FOCUS Investigators. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011; 365:2453–2462.
- Rao SV, Jollis JG, Harrington RA, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA 2004; 292:1555–1562.
- Carson JL, Brooks MM, Abbott JD, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J 2013; 165:964.e1–971.e1.
- Murphy GJ, Pike K, Rogers CA, et al; TITRe2 Investigators. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med 2015; 372:997–1008.
- Holst LB, Haase N, Wetterslev J, et al; TRISS Trial Group; Scandinavian Critical Care Trials Group. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med 2014; 371:1381–1391.
- Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013; 368:11–21.
- Walsh TS, Boyd JA, Watson D, et al; RELIEVE Investigators. Restrictive versus liberal transfusion strategies for older mechanically ventilated critically ill patients: a randomized pilot trial. Crit Care Med 2013; 41:2354–2363.
- Rohde JM, Dimcheff DE, Blumberg N, et al. Health care–associated infection after red blood cell transfusion. JAMA 2014; 311:1317–1326.
- Salpeter SR, Buckley JS, Chatterjee S. Impact of more restrictive blood transfusion strategies on clinical outcomes: a meta-analysis and systematic review. Am J Med 2014; 127:124.e3–131.e3.
- Wang J, Bao YX, Bai M, Zhang YG, Xu WD, Qi XS. Restrictive vs liberal transfusion for upper gastrointestinal bleeding: a meta-analysis of randomized controlled trials. World J Gastroenterol 2013; 19:6919–6927.
- Vincent JL, Baron JF, Reinhart K, et al; ABC (Anemia and Blood Transfusion in Critical Care) Investigators. Anemia and blood transfusion in critically ill patients. JAMA 2002; 288:1499–1507.
- Dunne JR, Malone D, Tracy JK, Gannon C, Napolitano LM. Perioperative anemia: an independent risk factor for infection, mortality, and resource utilization in surgery. J Surg Res 2002; 102:237–244.
- Brunskill SJ, Millette SL, Shokoohi A, et al. Red blood cell transfusion for people undergoing hip fracture surgery. Cochrane Database Syst Rev 2015; 4:CD009699.
- Weinberg JA, McGwin G Jr, Griffin RL, et al. Age of transfused blood: an independent predictor of mortality despite universal leukoreduction. J Trauma 2008; 65:279–284.
- Steinberg M. Management of sickle cell disease. N Engl J Med 1999; 340:1021–1030.
- Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease. JAMA 2014; 312:1033–1048.
- Vichinsky EP, Haberkern CM, Neumayr L, et al. A comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. The Preoperative Transfusion in Sickle Cell Disease Study Group. N Engl J Med 1995; 333:206–213.
- Howard J, Malfroy M, Llewelyn C, et al. The Transfusion Alternatives Preoperatively in Sickle Cell Disease (TAPS) study: a randomised, controlled, multicentre clinical trial. Lancet 2013; 381:930–938.
- Goodnough LT, Levy JH, Murphy MF. Concepts of blood transfusion in adults. Lancet 2013; 381:1845–1854.
- Holme S. Current issues related to the quality of stored RBCs. Transfus Apher Sci 2005; 33:55–61.
- Hovav T, Yedgar S, Manny N, Barshtein G. Alteration of red cell aggregability and shape during blood storage. Transfusion 1999; 39:277–281.
- Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med 2008; 358:1229–1239.
- Lacroix J, Hebert PC, Fergusson DA, et al. Age of transfused blood in critically ill adults. N Engl J Med 2015; 372:1410–1418.
- Carson JL, Grossman BJ, Kleinman S, et al; Clinical Transfusion Medicine Committee of the AABB. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2012; 157:49–58.
- Bolton-Maggs P, Watt A, Poles D, et al, on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group. The 2015 Annual SHOT Report. www.shotuk.org/wp-content/uploads/SHOT-2015-Annual-Report-Web-Edition-Final-bookmarked.pdf. Accessed November 30, 2016.
- Shander A, Javidroozi M, Ozawa S, Hare GMT. What is really dangerous: anaemia or transfusion? Br J Anaesth 2011; 107(suppl 1):i41–i59.
- Reeh M, Ghadban T, Dedow J, et al. Allogenic blood transfusion is associated with poor perioperative and long-term outcome in esophageal cancer. World J Surg 2016 Oct 11. [Epub ahead of print]
- Elmi M, Mahar A, Kagedan D, et al. The impact of blood transfusion on perioperative outcomes following gastric cancer resection: an analysis of the American College of Surgeons National Surgical Quality Improvement Program database. Can J Surg 2016; 59:322–329.
- Aquina CT, Blumberg N, Becerra AZ, et al. Association among blood transfusion, sepsis, and decreased long-term survival after colon cancer resection. Ann Surg 2016; Sep 14. [Epub ahead of print] PubMed PMID: 27631770.
- Premiere Analysis. Standardization of blood utilization practices could provide opportunity for improved outcomes, reduced costs. A Premiere Healthcare Alliance Analysis. 2012.
- Simeone F, Franchi F, Cevenini G, et al. A simple clinical model for planning transfusion quantities in heart surgery. BMC Med Inform Decis Mak 2011; 11:44.
- Spahn DR, Goodnough LT. Alternatives to blood transfusion. Lancet 2013; 381:1855–1865.
- Holst LB, Petersen MW, Haase N, Perner A, Wetterslev J. Restrictive versus liberal transfusion strategy for red blood cell transfusion: systematic review of randomised trials with meta-analysis and trial sequential analysis. BMJ 2015; 350:h1354.
- National Institute for Health and Care Excellence: Clinical Guidelines. London: National Institute for Health and Care Excellence (UK). www.ncbi.nlm.nih.gov/books/NBK11822/.
- Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA 2016 Oct 12. doi: 10.1001/jama.2016.9185. [Epub ahead of print]
- Consensus conference. Perioperative red blood cell transfusion. JAMA 1988; 260:2700–2703.
- Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med 2013; 8:486–492.
- Hicks LK, Bering H, Carson KR, et al. The ASH Choosing Wisely® campaign: five hematologic tests and treatments to question. Blood 2013; 122:3879–3883.
- Haemonetics IMPACT Online. The Blood Management Company. www.haemonetics.com/Products/Services/Consulting Services/IMPACT Online.aspx. Accessed November 30, 2016.
KEY POINTS
- In critical care patients, transfusion should be considered when the hemoglobin concentration reaches 7 g/dL or less.
- In postoperative patients and hospitalized patients with preexisting cardiovascular disease, transfusion should be considered at a hemoglobin concentration of 8 g/dL or less or for symptoms such as chest pain, orthostatic hypotension, or tachycardia unresponsive to fluid resuscitation, or heart failure.
- Consider both the hemoglobin concentration and the symptoms when deciding whether to give a patient a transfusion.
A patient with altered mental status and an acid-base disturbance
A 78-year-old black woman with a history of osteoarthrosis and chronic diffuse joint pain presents with altered mental status and tachypnea, which began 3 hours earlier. She lives alone, and her family suspects she abuses both alcohol and her pain medications. She has not been eating well and has lost approximately 10 pounds over the past 3 months. Her analgesic regimen includes acetaminophen and acetaminophen-oxycodone.
In the emergency department her temperature is 98.6°F (37.0°C), pulse 100 beats per minute and regular, respiratory rate 22 per minute, and blood pressure 136/98 mm Hg. She is obtunded but has no focal neurologic defects or meningismus. She has no signs of heart failure (jugular venous distention, cardiomegaly, or gallops), and examination of the lungs and abdomen is unremarkable.
Suspecting that the patient may have taken too much oxycodone, the physician gives her naloxone, but her mental status does not improve. Results of chest radiography and cranial computed tomography are unremarkable. The physician’s initial impression is that the patient has “metabolic encephalopathy of unknown etiology.”
The patient’s laboratory values are shown in Table 1.
WHICH ACID-BASE DISORDER DOES SHE HAVE?
1. Which acid-base disorder does this patient have?
- Metabolic acidosis and respiratory alkalosis
- Metabolic acidosis and respiratory acidosis
- Metabolic acidosis with an elevated anion gap
- A triple disturbance: metabolic acidosis, respiratory acidosis, and metabolic alkalosis
A 5-step approach
Acid-base disorders can be diagnosed and characterized using a systematic approach known as the “Rules of 5” (Table 2)1:
1. Determine the arterial pH status.
2. Determine whether the primary process is respiratory, metabolic, or both.
3. Calculate the anion gap.
4. Check the degree of compensation (respiratory or metabolic).
5. If the patient has metabolic acidosis with an elevated anion gap, check whether the bicarbonate level has decreased as much as the anion gap has increased (ie, whether there is a delta gap).
Let us apply this approach to the patient described above.
1. What is her pH status?
An arterial pH less than 7.40 is acidemic, whereas a pH higher than 7.44 is alkalemic. (Acidemia and alkalemia refer to the abnormal laboratory value, while acidosis and alkalosis refer to the process causing the abnormal value—a subtle distinction, but worth keeping in mind.)
Caveat. A patient may have a significant acid-base disorder even if the pH is normal. Therefore, even if the pH is normal, one should verify that the partial pressure of carbon dioxide (Pco2), bicarbonate level, and anion gap are normal. If they are not, the patient may have a mixed acid-base disorder such as respiratory acidosis superimposed on metabolic alkalosis.
Our patient’s pH is 7.25, which is in the acidemic range.
2. Is her acidosis respiratory, metabolic, or both?
Respiratory acidosis and alkalosis affect the Pco2. The Pco2 is high in respiratory acidosis (due to failure to get rid of excess carbon dioxide), whereas it is low in respiratory alkalosis (due to loss of too much carbon dioxide through hyperventilation).
Metabolic acidosis and alkalosis, on the other hand, affect the serum bicarbonate level. In metabolic acidosis the bicarbonate level is low, whereas in metabolic alkalosis the bicarbonate level is high.
Moreover, in mixed respiratory and metabolic acidosis, the bicarbonate level can be low and the Pco2 can be high. In mixed metabolic and respiratory alkalosis, the bicarbonate level can be high and the Pco2 can be low (Table 2).
Our patient’s serum bicarbonate level is low at 16.0 mmol/L, indicating that the process is metabolic. Her Pco2 is also low (28 mm Hg), which reflects an appropriate response to compensate for the acidosis.
3. What is her anion gap?
Always calculate the anion gap, ie, the serum sodium concentration minus the serum chloride and serum bicarbonate concentrations. If the patient’s serum albumin level is low, for every 1 gram it is below normal, an additional 2.5 mmol/L should be added to the calculated anion gap. We consider an anion gap of 10 mmol/L or less as normal.
Caveats. The blood sample used to calculate the anion gap should be drawn close in time to the arterial blood gas sample.
Although the anion gap is an effective tool in assessing acid-base disorders, further investigation is warranted if clinical judgment suggests that an anion gap calculation is inconsistent with the patient’s circumstances.2
Our patient’s anion gap is elevated (21 mmol/L). Her serum albumin level is in the normal range, so her anion gap does not need to be adjusted.
4. Is the degree of compensation appropriate for the primary acid-base disturbance?
The kidneys compensate for the lungs, and vice versa. That is, in respiratory acidosis or alkalosis, the kidneys adjust the bicarbonate levels, and in metabolic acidosis, the lungs adjust the Pco2 (although in metabolic alkalosis, it is hard for patients to breathe less, especially if they are already hypoxic).
In metabolic acidosis, people compensate by breathing harder to get rid of more carbon dioxide. For every 1-mmol/L decrease in the bicarbonate level, the Pco2 should decrease by 1.3 mm Hg.
Compensation does not return pH to normal; rather, it mitigates the impact of an acid or alkali excess or deficit. If the pH is normalized with an underlying acid-base disturbance, there may be mixed acid-base processes rather than compensation.
Our patient’s bicarbonate level is 16 mmol/L, which is 9 mmol/L lower than normal (for acid-base calculations, we use 25 mmol/L as the nominal normal level). If she is compensating appropriately, her Pco2 should decline from 40 mm Hg (the nominal normal level) by about 11.7 mm Hg (9 × 1.3), to approximately 28.3 mm Hg. Her Pco2 is, indeed, 28 mm Hg, indicating that she is compensating adequately for her metabolic acidosis.
If we use Winter’s formula instead (Pco2 = [1.5 × the bicarbonate level] + 8 ± 2),3 the lowest calculated Pco2 would be 30 mm Hg, which is within 2 mm Hg of the Rules of 5 calculation. Other formulas for calculating compensation are available.3
This information rules out the first two answers to question 1, ie, metabolic acidosis with respiratory alkalosis or acidosis.
5. Is there a delta gap?
Although we know the patient has metabolic acidosis with an elevated anion gap, we have not ruled out the possibility that she may have a triple disturbance. For this reason we need to check her delta gap.
In metabolic acidosis with an elevated anion gap, as the bicarbonate level decreases, the anion gap should increase by the same amount. If the bicarbonate level decreases more than the anion gap increases, the additional decline is the result of a second process—an additional normal-anion-gap acidosis. If the bicarbonate level does not decrease as much as the anion gap increases, there is an additional metabolic alkalosis.
Our patient’s bicarbonate level decreased 9 mmol/L (from the nominal normal level of 25 to 16), and therefore her anion gap should have increased approximately the same amount—and it did. (A normal anion gap for problem-solving is 10, and this patient’s anion gap has increased to 21. A difference of ± 2 is insignificant.) This conclusion verifies that a triple acid-base disturbance is not present, so the last answer is incorrect.
So, the correct answer to the question posed above is metabolic acidosis with an elevated anion gap (that is, metabolic acidosis with appropriate respiratory compensation).
‘MUD PILES’: FINDING THE CAUSE OF ANION GAP METABOLIC ACIDOSIS
The possible causes of metabolic acidosis with an elevated anion gap (as in our patient) can be summarized in the mnemonic MUD PILES (methanol, uremia, diabetes, paraldehyde, isoniazid, lactate, ethylene glycol, and salicylates), which has been used for many years. Parts of it are no longer useful, but rather than discard it, we propose to update it (Table 3).
Methanol and ethylene glycol
We will address toxic ingestion of methanol and ethylene glycol (the “M” and “E” of MUD PILES) at the same time.
In cases of suspected ingestion of toxic substances such as these, it is useful to examine the osmol gap, ie, the difference between the calculated and the measured serum osmolality. Serum osmolality (in mOsm/kg) is calculated as the sodium concentration in mmol/L times 2, plus the glucose concentration in mg/dL divided by 18, plus the blood urea nitrogen concentration in mg/dL divided by 2.8 (Table 4). If the measured osmolality is higher than this calculated value, the difference may be due to solutes in the blood that should not be there such as ethylene glycol, diethylene glycol, methanol, and their many metabolic products.
In our patient, ingestion of both methanol and ethylene glycol should be considered, since she lives alone and has been suspected of alcohol and opioid abuse. Her calculated osmol gap is 278 mOsm/kg. Her measured osmolality is 318 mOsm/kg (Table 1). The osmol gap is 40 mOsm/kg (normal is ≤ 10).4,5 Therefore, her osmol gap is elevated.
Identifying the specific substance the patient ingested that caused metabolic acidosis with anion gap may be difficult. Poisonings with these agents do not always increase the osmol gap.6 A high index of suspicion is essential. It is helpful to have the family search for any sources of ethylene glycol and methanol at home and initiate treatment early if an ingestion is suspected, using fomepizole (an alcohol dehydrogenase inhibitor) or parenteral ethanol and hemodialysis.7 Liquid chromatography identifies these two toxins, but results are not available emergently.
Diethylene glycol ingestion should also be considered.8 Since it is diagnosed and treated like ethylene glycol intoxication, it can be placed with the “E” of (di)ethylene glycol in the mnemonic.
Uremia
Renal failure can lead to metabolic acidosis.9 Our patient has no history of kidney disease, but her blood urea nitrogen and creatinine concentrations are above normal, and her estimated glomerular filtration rate by the Modification of Diet in Renal Disease formula is 48 mL/min/1.73 m2—low, but not uremic.
Rhabdomyolysis (suspected by elevated creatine kinase values) should be considered in any patient with mental status changes, suspected toxic ingestion, and metabolic acidosis (see the “I” in MUD PILES below). Compartment syndromes with muscle necrosis may present in a subtle fashion. Therefore, renal failure from rhabdomyolysis may complicate this patient’s course later, and should be kept in mind.
Diabetes
The patient has no history of diabetes and has a normal blood glucose level. Blood testing did not reveal ketones. She is not taking metformin (alleged to cause lactic acidosis) or a sodium-glucose cotransporter 2 inhibitor (which have been associated with ketoacidosis).10
There is another, less common cause of ketoacidosis: alcohol.11 Although alcoholism is common, alcoholic ketoacidosis is uncommon, even in heavy drinkers. Ethyl alcohol causing metabolic acidosis is similar to metabolic acidosis with (di)ethylene glycol and methanol, and if suspected it should be treated empirically (first with thiamine, then dextrose and saline, and correcting other electrolyte disturbances such as hypokalemia and hypomagnesemia) before specific identification is made. Ketones (predominantly beta-hydroxybutyrate) may persist up to 2 weeks after alcohol ingestion has stopped.11 Ketosis in the setting of alcoholic ketoacidosis is frequently accompanied by other markers of alcohol target organ injury: elevated bilirubin, aspartate aminotransferase, alanine aminotransferase, and gamma-glutamyl transferase levels. The term “ketohepatitis” has been suggested as an alternative to alcoholic ketosis.11
This patient did not have an elevated blood ethanol level, and her liver markers were otherwise normal.
THE NEW MUD PILES
2. Which of the following is (are) true? Regarding the remaining letters of the MUD PILES mnemonic:
- The “P” (paraldehyde) has been replaced by pyroglutamic acid (5-oxoproline) and propylene glycol.
- There are two isomers of lactate (dextro and levo), and consequently two clinical varieties of lactic acidosis.
- Isoniazid is no longer associated with metabolic acidosis with elevated anion gap.
- Salicylates can paradoxically be associated both with elevated and low anion gaps.
Isoniazid is still associated with metabolic acidosis with elevated anion gap, and so the third answer choice is false; the rest are true.
Paraldehyde, isoniazid, lactate
The “P,” “I,” and “L” (d-lactate) of the revamped MUD PILES acronym are less common than the others. They should be considered when the more typical causes of metabolic acidosis are not present, as in this patient.
UPDATING THE ‘P’ IN MUD PILES
Paraldehyde is rarely prescribed anymore. A PubMed search on December 21, 2015 applying the terms paraldehyde and metabolic acidosis yielded 17 results. Those specific to anion gap metabolic acidosis were from 1957 to 1986 (n = 9).12–20
Therefore, we can eliminate paraldehyde from the MUD PILES mnemonic and replace it with pyroglutamic acid and propylene glycol.
5-Oxoproline or pyroglutamic acid, a metabolite of acetaminophen
Acetaminophen depletes glutathione stores in acute overdoses, in patients with inborn errors of metabolism, and after chronic ingestion of excessive, frequent doses. Depletion of glutathione increases metabolic products, including pyroglutamic acid, which dissociates into hydrogen ions (leading to metabolic acidosis and an anion gap), and 5-oxoproline, (which can be detected in the urine).21,22
Risk factors for metabolic acidosis with acetaminophen ingestion include malnutrition, chronic alcoholism, liver disease, and female sex. In fact, most cases have been reported in females, and altered mental status has been common.
Metabolic acidosis with pyroglutamic acid can occur without elevated acetaminophen levels. Serum and urine levels of pyroglutamic acid may assist with diagnosis. Since identification of urine pyroglutamic acid usually requires outside laboratory assistance, a clinical diagnosis is often made initially and corroborated later by laboratory results. When the anion gap metabolic acidosis is multifactorial, as it was suspected to be in a case reported by Tan et al,23 the osmol gap may be elevated as a consequence of additional toxic ingestions, as it was in the reported patient.
No controlled studies of treatment have been done. n-Acetylcysteine may be of benefit. Occasional patients have been dialyzed for removal of excess pyroglutamic acid.
Propylene glycol, a component of parenteral lorazepam
Lorazepam is a hydrophobic drug, so when it is given parenterally, it must be mixed with a suitable solvent. A typical formulation adds propylene glycol. In patients receiving high doses of lorazepam as relaxation therapy for acute respiratory distress syndrome in the intensive care unit, or as treatment of alcohol withdrawal, the propylene glycol component can precipitate anion gap metabolic acidosis.24,25
Although nearly one-half of the administered propylene glycol is excreted by the kidneys, the remaining substrate is metabolized by alcohol dehydrogenase into d,l-lactaldehyde, then converted into d- or l-lactate. l-Lactate can be metabolized, but d-lactate cannot and leads to anion gap metabolic acidosis. This is another toxic metabolic acidosis associated with an elevated osmol gap. An increasing osmol gap in the intensive care unit can serve as a surrogate marker of excessive propylene glycol administration.23
Isoniazid
Although it is uncommon, there are reports of isoniazid-induced anion gap metabolic acidosis,26 either due to overdoses, or less commonly, with normal dosing. Isoniazid should therefore remain in the mnemonic MUD PILES and may be suspected when metabolic acidosis is accompanied by seizures unresponsive to usual therapy. The seizures respond to pyridoxine.
The “I” should also be augmented by newer causes of metabolic acidosis associated with “ingestions.” Ecstasy, or 3,4-methylenedioxymethamphetamine, can cause metabolic acidosis and seizures. Ecstasy has been associated with rhabdomyolysis and uremia, also leading to anion gap metabolic acidosis.27 A newer class of abused substances, synthetic cathinones (“bath salts”), are associated with metabolic acidosis, compartment syndrome, and renal failure.28
Lactic acidosis
Lactic acidosis and metabolic acidosis can result from hypoperfusion (type A) or other causes (type B). Not all lactic acidosis is contingent on l-lactate, which humans can metabolize. Metabolic acidosis may be a consequence of d-lactate (mammals have no d-lactate dehydrogenase). d-Lactic acidosis as a result of short bowel syndrome has been known for more than a generation.29 However, d-lactic acidosis occurs in another new setting. The new “P” in MUD PILES, propylene glycol, can generate substantial amounts of d-lactate.29
d-lactic metabolic acidosis is always accompanied by neurologic manifestations (slurred speech, confusion, somnolence, ataxia, abusive behavior, and others).30 With short bowel syndrome, the neurologic manifestations occur after eating and clear later.30
Although our patient’s anion gap is more than 20 mmol/L, her blood level of lactate is not elevated, and she had no history to suggest short-bowel syndrome.
Salicylates
Salicylate overdose can cause a mixed acid-base disorder: metabolic acidosis with elevated anion gap and respiratory alkalosis.
Although our patient does not have respiratory alkalosis, an aspirin overdose must be considered. A salicylate level was ordered; it was negative.
Despite the typical association of salicylates with an elevated anion gap, they may also cause a negative anion gap.31 Chloride-sensing ion-specific electrodes contain a membrane permeable to chloride. Salicylates can increase the chloride permeability of these membranes, generating pseudohyperchloremia, and consequently, a negative anion gap.
WHAT ELSE MUST BE CONSIDERED?
3. In view of her anion gap metabolic acidosis, elevated osmol gap, and absence of diabetes, renal failure, or lactate excess, what are the remaining diagnoses to consider in this patient? (Choose all that are potential sources of metabolic acidosis and an increased anion gap.)
- Methanol, ethylene, or diethylene glycol
- Excessive, chronic acetaminophen ingestion
- Salicylate toxicity
- Alcoholic ketoacidosis
All of the above can potentially contribute to metabolic acidosis.
A search of the patient’s home did not reveal a source of methanol or either ethylene or diethylene glycol. Similarly, no aspirin was found, and the patient’s salicylate levels were not elevated. The patient’s laboratory work did not reveal increased ketones.
Since none of the common causes of metabolic acidosis were discovered, and since the patient had been taking acetaminophen, the diagnosis of excessive chronic acetaminophen ingestion was suspected pending laboratory verification. Identification of 5-oxoproline in the urine may take a week or more since the sample is usually sent to special laboratories. Acetaminophen levels in this patient were significantly elevated, as were urinary oxyproline levels, which returned later.
The patient was diagnosed with pyroglutamic acid metabolic acidosis. She was treated supportively and with n-acetylcysteine intravenously, although there have been no controlled studies of the efficacy of this drug. Seventy-two hours after admission, she had improved. Her acid-base status returned to normal.
GOLD MARK: ANOTHER WAY TO REMEMBER
Another mnemonic device for remembering the causes of metabolic acidosis with elevated anion gap is “GOLD MARK”: glycols (ethylene and propylene), oxoproline (instead of pyroglutamic acid from acetaminophen), l-lactate, d-lactate, methanol, aspirin, renal failure, and ketoacidosis).32
ACID-BASE DISORDERS IN DIFFERENT DISEASES
Diverse diseases cause distinctive acid-base abnormalities. Matching the appropriate acid-base abnormality with its associated disease may lead to more timely diagnosis and treatment:
Type 2 diabetes mellitus, for example, can lead to lactic acidosis, ketoacidosis, or type 4 renal tubular acidosis.33
Heart failure, although not typically framed in the context of acid-base physiology, can lead to elevated lactate, which is associated with a worse prognosis.34
Acquired immunodeficiency syndrome. Abacavir can cause normal anion gap metabolic acidosis.35,36
Cancer37,38 can be associated with proximal tubular renal tubular acidosis and lactic acidosis.
An expanding array of toxic ingestions
Metabolic acidosis may be the most prominent and potentially lethal clinical acid-base disturbance. When metabolic acidosis occurs in certain disease states—lactic acidosis with hypoperfusion or methanol ingestion with metabolic acidosis, for example—there is increased morbidity and mortality.
As reflected in the revisions to MUD PILES and in the newer GOLD MARK acronym, the osmol gap has become more valuable in differential diagnosis of metabolic acidosis with an elevated anion gap consequent to an expanding array of toxic ingestions (methanol, propylene glycol, pyroglutamic acid-oxoproline, ethylene glycol, and diethylene glycol), which may accompany pyroglutamic acid-oxoproline.
- Whittier WL, Rutecki GW. Primer on clinical acid-base problem solving. Dis Mon 2004; 50:122–162.
- Kraut JA, Madias NE. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol 2007; 2:162–174.
- Adrogué HJ, Madias NE. Secondary responses to altered acid-base status: the rules of engagement. J Am Soc Nephrol 2010; 21:920–923.
- Krasowski MD, Wilcoxon RM, Miron J. A retrospective analysis of glycol and toxic alcohol ingestion: utility of anion and osmolal gaps. BMC Clin Pathol 2012;12:1.
- Latus J, Kimmel M, Alscher MD, Braun N. Ethylene glycol poisoning: a rare but life-threatening cause of metabolic acidosis—a single-centre experience. Clin Kidney J 2012; 5:120–123.
- Kraut JA. Diagnosis of toxic alcohols: limitations of present methods. Clin Toxicol (Phila) 2015; 53:589–595.
- Ghannoum M, Hoffman RS, Mowry JB, Lavergne V. Trends in toxic alcohol exposures in the United States from 2000 to 2013: a focus on the use of antidotes and extracorporeal treatments. Semin Dial 2014; 27:395–401.
- Schep LJ, Slaughter RJ, Temple WA, Beasley DM. Diethylene glycol poisoning. Clin Toxicol (Phila) 2009; 47:525–535.
- Kraut JA, Madias NE. Metabolic acidosis of CKD: an update. Am J Kidney Dis 2016; 67:307–317.
- Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 2015; 100:2849–2852.
- Yokoyama A, Yokoyama T, Mizukami T, et al. Alcoholic ketosis: prevalence, determinants, and ketohepatitis in Japanese alcoholic men. Alcohol Alcohol 2014; 49:618–625.
- Hayward JN, Boshell BR. Paraldehyde intoxication with metabolic acidosis; report of two cases, experimental data and a critical review of the literature. Am J Med 1957; 23:965–976.
- Elkinton JR, Huth EJ, Clark JK, Barker ES, Seligson D. Renal tubular acidosis with organic aciduria during paraldehyde ingestion; six year study of an unusual case. Am J Med 1957; 23:977–986.
- Waterhouse C, Stern EA. Metabolic acidosis occurring during administration of paraldehyde. Am J Med 1957; 23:987–989.
- Beier LS, Pitts WH, Gonick HC. Metabolic acidosis occurring during paraldehyde intoxication. Ann Intern Med 1963; 58:155–158.
- Hiemcke T. Metabolic acidosis due to paraldehyde. Ned Tijdschr Geneeskd 1964; 108:2165–2167. Dutch.
- Gailitis RJ. Paraldehyde acidosis syndrome. IMJ III Med J 1966; 129:258–262.
- Gutman RA, Burnell JM. Paraldehyde acidosis. Am J Med 1967; 42:435–440.
- Hadden JW, Metzner RJ. Pseudoketosis and hyperacetaldehydemia in paraldehyde acidosis. Am J Med 1969; 47:642–647.
- Linter CM, Linter SP. Severe lactic acidosis following paraldehyde administration. Br J Psychiatry 1986; 149:650–651.
- Zand L, Muriithi A, Nelsen E, et al. Severe anion gap metabolic acidosis from acetaminophen use secondary to 5-oxoproline (pyroglutamic acid) accumulation. Am J Med Sci 2012; 344:501–504.
- Abkur TM, Mohammed W, Ali M, Casserly L. Acetaminophen-induced anion gap metabolic acidosis secondary to 5-oxoproline: a case report. J Med Case Rep 2014; 8:409.
- Tan EM, Kalimullah E, Sohail MR, Ramar K. Diagnostic challenge in a patient with severe anion gap metabolic acidosis. Case Rep Crit Care 2015; 2015:272914.
- Jorens PG, Demey HE, Schepens PJ, et al. Unusual d-lactic acid acidosis from propylene glycol metabolism in overdose. J Toxicol Clin Toxicol 2004; 42:163–169.
- Barnes BJ, Gerst C, Smith JR, Terrell AR, Mullins ME. Osmol gap as a surrogate marker for serum propylene glycol concentrations in patients receiving lorazepam for sedation. Pharmacotherapy 2006; 26:23–33.
- Gokhale YA, Vaidya MS, Mehta AD, Rathod NN. Isoniazid toxicity presenting as status epilepticus and severe metabolic acidosis. J Assoc Physicians India 2009; 57:70–71.
- Ben-Abraham R, Szold O, Rudick V, Weinbroum AA. ‘Ecstasy’ intoxication: life-threatening manifestations and resuscitative measures in the intensive care setting. Eur J Emerg Med 2003; 10:309–313.
- German CL, Fleckenstein AE, Hanson GR. Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life Sci 2014; 97:2–8.
- Jorens PG, Demey HE, Schepens PJ, et al. Unusual d-lactic acidosis from propylene glycol metabolism in overdose. J Toxicol Clin Toxicol 2004; 42:163–169.
- Kang KP, Le S, Kang SK. d-Lactic acidosis in humans: review and update. Electrolyte Blood Press 2006; 4:53–56.
- Emmett M. Approach to the patient with a negative anion gap. Am J Kidney Dis 2016; 67:143–150.
- Mehta AN, Emmett JB, Emmett M. GOLD MARK: an anion gap mnemonic for the 21st Century. Lancet 2008; 372:892.
- Palmer BF, Clegg DJ. Electrolyte and acid-base disturbances in patients with diabetes mellitus. N Engl J Med 2015; 373:548–559.
- Park JJ, Choi DJ, Yoon CH, et al; KorHF Registry. The prognostic value of arterial blood gas analysis in high-risk acute heart failure patients: an analysis of the Korean Heart Failure (KorHF) registry. Eur J Heart Fail 2015; 17:601–611.
- Musso CG, Belloso WH, Glassock RJ. Water, electrolytes, and acid-base alterations in human immunodeficiency virus infected patients. World J Nephrol 2016; 5:33–42.
- Camara-Lemarroy CR, Flores-Cantu H, Calderon-Hernandez HJ, Diaz-Torres MA, Villareal-Velazquez HJ. Drug-induced haemolysis, renal failure, thrombocytopenia and lactic acidosis in patients with HIV and cryptococcal meningitis: a diagnostic challenge. Int J STD AIDS 2015; 26:1052–1054.
- Miltiadous G, Christidis D, Kalogirou M, Elisaf M. Causes and mechanisms of acid-base and electrolyte abnormalities in cancer. Eur J Intern Med 2008; 19:1–7.
- Vlachostergios PJ, Oikonomou KG, Gibilaro E, Apergis G. Elevated lactic acid is a negative prognostic factor in metastatic lung cancer. Cancer Biomark 2015; 15:725–734.
A 78-year-old black woman with a history of osteoarthrosis and chronic diffuse joint pain presents with altered mental status and tachypnea, which began 3 hours earlier. She lives alone, and her family suspects she abuses both alcohol and her pain medications. She has not been eating well and has lost approximately 10 pounds over the past 3 months. Her analgesic regimen includes acetaminophen and acetaminophen-oxycodone.
In the emergency department her temperature is 98.6°F (37.0°C), pulse 100 beats per minute and regular, respiratory rate 22 per minute, and blood pressure 136/98 mm Hg. She is obtunded but has no focal neurologic defects or meningismus. She has no signs of heart failure (jugular venous distention, cardiomegaly, or gallops), and examination of the lungs and abdomen is unremarkable.
Suspecting that the patient may have taken too much oxycodone, the physician gives her naloxone, but her mental status does not improve. Results of chest radiography and cranial computed tomography are unremarkable. The physician’s initial impression is that the patient has “metabolic encephalopathy of unknown etiology.”
The patient’s laboratory values are shown in Table 1.
WHICH ACID-BASE DISORDER DOES SHE HAVE?
1. Which acid-base disorder does this patient have?
- Metabolic acidosis and respiratory alkalosis
- Metabolic acidosis and respiratory acidosis
- Metabolic acidosis with an elevated anion gap
- A triple disturbance: metabolic acidosis, respiratory acidosis, and metabolic alkalosis
A 5-step approach
Acid-base disorders can be diagnosed and characterized using a systematic approach known as the “Rules of 5” (Table 2)1:
1. Determine the arterial pH status.
2. Determine whether the primary process is respiratory, metabolic, or both.
3. Calculate the anion gap.
4. Check the degree of compensation (respiratory or metabolic).
5. If the patient has metabolic acidosis with an elevated anion gap, check whether the bicarbonate level has decreased as much as the anion gap has increased (ie, whether there is a delta gap).
Let us apply this approach to the patient described above.
1. What is her pH status?
An arterial pH less than 7.40 is acidemic, whereas a pH higher than 7.44 is alkalemic. (Acidemia and alkalemia refer to the abnormal laboratory value, while acidosis and alkalosis refer to the process causing the abnormal value—a subtle distinction, but worth keeping in mind.)
Caveat. A patient may have a significant acid-base disorder even if the pH is normal. Therefore, even if the pH is normal, one should verify that the partial pressure of carbon dioxide (Pco2), bicarbonate level, and anion gap are normal. If they are not, the patient may have a mixed acid-base disorder such as respiratory acidosis superimposed on metabolic alkalosis.
Our patient’s pH is 7.25, which is in the acidemic range.
2. Is her acidosis respiratory, metabolic, or both?
Respiratory acidosis and alkalosis affect the Pco2. The Pco2 is high in respiratory acidosis (due to failure to get rid of excess carbon dioxide), whereas it is low in respiratory alkalosis (due to loss of too much carbon dioxide through hyperventilation).
Metabolic acidosis and alkalosis, on the other hand, affect the serum bicarbonate level. In metabolic acidosis the bicarbonate level is low, whereas in metabolic alkalosis the bicarbonate level is high.
Moreover, in mixed respiratory and metabolic acidosis, the bicarbonate level can be low and the Pco2 can be high. In mixed metabolic and respiratory alkalosis, the bicarbonate level can be high and the Pco2 can be low (Table 2).
Our patient’s serum bicarbonate level is low at 16.0 mmol/L, indicating that the process is metabolic. Her Pco2 is also low (28 mm Hg), which reflects an appropriate response to compensate for the acidosis.
3. What is her anion gap?
Always calculate the anion gap, ie, the serum sodium concentration minus the serum chloride and serum bicarbonate concentrations. If the patient’s serum albumin level is low, for every 1 gram it is below normal, an additional 2.5 mmol/L should be added to the calculated anion gap. We consider an anion gap of 10 mmol/L or less as normal.
Caveats. The blood sample used to calculate the anion gap should be drawn close in time to the arterial blood gas sample.
Although the anion gap is an effective tool in assessing acid-base disorders, further investigation is warranted if clinical judgment suggests that an anion gap calculation is inconsistent with the patient’s circumstances.2
Our patient’s anion gap is elevated (21 mmol/L). Her serum albumin level is in the normal range, so her anion gap does not need to be adjusted.
4. Is the degree of compensation appropriate for the primary acid-base disturbance?
The kidneys compensate for the lungs, and vice versa. That is, in respiratory acidosis or alkalosis, the kidneys adjust the bicarbonate levels, and in metabolic acidosis, the lungs adjust the Pco2 (although in metabolic alkalosis, it is hard for patients to breathe less, especially if they are already hypoxic).
In metabolic acidosis, people compensate by breathing harder to get rid of more carbon dioxide. For every 1-mmol/L decrease in the bicarbonate level, the Pco2 should decrease by 1.3 mm Hg.
Compensation does not return pH to normal; rather, it mitigates the impact of an acid or alkali excess or deficit. If the pH is normalized with an underlying acid-base disturbance, there may be mixed acid-base processes rather than compensation.
Our patient’s bicarbonate level is 16 mmol/L, which is 9 mmol/L lower than normal (for acid-base calculations, we use 25 mmol/L as the nominal normal level). If she is compensating appropriately, her Pco2 should decline from 40 mm Hg (the nominal normal level) by about 11.7 mm Hg (9 × 1.3), to approximately 28.3 mm Hg. Her Pco2 is, indeed, 28 mm Hg, indicating that she is compensating adequately for her metabolic acidosis.
If we use Winter’s formula instead (Pco2 = [1.5 × the bicarbonate level] + 8 ± 2),3 the lowest calculated Pco2 would be 30 mm Hg, which is within 2 mm Hg of the Rules of 5 calculation. Other formulas for calculating compensation are available.3
This information rules out the first two answers to question 1, ie, metabolic acidosis with respiratory alkalosis or acidosis.
5. Is there a delta gap?
Although we know the patient has metabolic acidosis with an elevated anion gap, we have not ruled out the possibility that she may have a triple disturbance. For this reason we need to check her delta gap.
In metabolic acidosis with an elevated anion gap, as the bicarbonate level decreases, the anion gap should increase by the same amount. If the bicarbonate level decreases more than the anion gap increases, the additional decline is the result of a second process—an additional normal-anion-gap acidosis. If the bicarbonate level does not decrease as much as the anion gap increases, there is an additional metabolic alkalosis.
Our patient’s bicarbonate level decreased 9 mmol/L (from the nominal normal level of 25 to 16), and therefore her anion gap should have increased approximately the same amount—and it did. (A normal anion gap for problem-solving is 10, and this patient’s anion gap has increased to 21. A difference of ± 2 is insignificant.) This conclusion verifies that a triple acid-base disturbance is not present, so the last answer is incorrect.
So, the correct answer to the question posed above is metabolic acidosis with an elevated anion gap (that is, metabolic acidosis with appropriate respiratory compensation).
‘MUD PILES’: FINDING THE CAUSE OF ANION GAP METABOLIC ACIDOSIS
The possible causes of metabolic acidosis with an elevated anion gap (as in our patient) can be summarized in the mnemonic MUD PILES (methanol, uremia, diabetes, paraldehyde, isoniazid, lactate, ethylene glycol, and salicylates), which has been used for many years. Parts of it are no longer useful, but rather than discard it, we propose to update it (Table 3).
Methanol and ethylene glycol
We will address toxic ingestion of methanol and ethylene glycol (the “M” and “E” of MUD PILES) at the same time.
In cases of suspected ingestion of toxic substances such as these, it is useful to examine the osmol gap, ie, the difference between the calculated and the measured serum osmolality. Serum osmolality (in mOsm/kg) is calculated as the sodium concentration in mmol/L times 2, plus the glucose concentration in mg/dL divided by 18, plus the blood urea nitrogen concentration in mg/dL divided by 2.8 (Table 4). If the measured osmolality is higher than this calculated value, the difference may be due to solutes in the blood that should not be there such as ethylene glycol, diethylene glycol, methanol, and their many metabolic products.
In our patient, ingestion of both methanol and ethylene glycol should be considered, since she lives alone and has been suspected of alcohol and opioid abuse. Her calculated osmol gap is 278 mOsm/kg. Her measured osmolality is 318 mOsm/kg (Table 1). The osmol gap is 40 mOsm/kg (normal is ≤ 10).4,5 Therefore, her osmol gap is elevated.
Identifying the specific substance the patient ingested that caused metabolic acidosis with anion gap may be difficult. Poisonings with these agents do not always increase the osmol gap.6 A high index of suspicion is essential. It is helpful to have the family search for any sources of ethylene glycol and methanol at home and initiate treatment early if an ingestion is suspected, using fomepizole (an alcohol dehydrogenase inhibitor) or parenteral ethanol and hemodialysis.7 Liquid chromatography identifies these two toxins, but results are not available emergently.
Diethylene glycol ingestion should also be considered.8 Since it is diagnosed and treated like ethylene glycol intoxication, it can be placed with the “E” of (di)ethylene glycol in the mnemonic.
Uremia
Renal failure can lead to metabolic acidosis.9 Our patient has no history of kidney disease, but her blood urea nitrogen and creatinine concentrations are above normal, and her estimated glomerular filtration rate by the Modification of Diet in Renal Disease formula is 48 mL/min/1.73 m2—low, but not uremic.
Rhabdomyolysis (suspected by elevated creatine kinase values) should be considered in any patient with mental status changes, suspected toxic ingestion, and metabolic acidosis (see the “I” in MUD PILES below). Compartment syndromes with muscle necrosis may present in a subtle fashion. Therefore, renal failure from rhabdomyolysis may complicate this patient’s course later, and should be kept in mind.
Diabetes
The patient has no history of diabetes and has a normal blood glucose level. Blood testing did not reveal ketones. She is not taking metformin (alleged to cause lactic acidosis) or a sodium-glucose cotransporter 2 inhibitor (which have been associated with ketoacidosis).10
There is another, less common cause of ketoacidosis: alcohol.11 Although alcoholism is common, alcoholic ketoacidosis is uncommon, even in heavy drinkers. Ethyl alcohol causing metabolic acidosis is similar to metabolic acidosis with (di)ethylene glycol and methanol, and if suspected it should be treated empirically (first with thiamine, then dextrose and saline, and correcting other electrolyte disturbances such as hypokalemia and hypomagnesemia) before specific identification is made. Ketones (predominantly beta-hydroxybutyrate) may persist up to 2 weeks after alcohol ingestion has stopped.11 Ketosis in the setting of alcoholic ketoacidosis is frequently accompanied by other markers of alcohol target organ injury: elevated bilirubin, aspartate aminotransferase, alanine aminotransferase, and gamma-glutamyl transferase levels. The term “ketohepatitis” has been suggested as an alternative to alcoholic ketosis.11
This patient did not have an elevated blood ethanol level, and her liver markers were otherwise normal.
THE NEW MUD PILES
2. Which of the following is (are) true? Regarding the remaining letters of the MUD PILES mnemonic:
- The “P” (paraldehyde) has been replaced by pyroglutamic acid (5-oxoproline) and propylene glycol.
- There are two isomers of lactate (dextro and levo), and consequently two clinical varieties of lactic acidosis.
- Isoniazid is no longer associated with metabolic acidosis with elevated anion gap.
- Salicylates can paradoxically be associated both with elevated and low anion gaps.
Isoniazid is still associated with metabolic acidosis with elevated anion gap, and so the third answer choice is false; the rest are true.
Paraldehyde, isoniazid, lactate
The “P,” “I,” and “L” (d-lactate) of the revamped MUD PILES acronym are less common than the others. They should be considered when the more typical causes of metabolic acidosis are not present, as in this patient.
UPDATING THE ‘P’ IN MUD PILES
Paraldehyde is rarely prescribed anymore. A PubMed search on December 21, 2015 applying the terms paraldehyde and metabolic acidosis yielded 17 results. Those specific to anion gap metabolic acidosis were from 1957 to 1986 (n = 9).12–20
Therefore, we can eliminate paraldehyde from the MUD PILES mnemonic and replace it with pyroglutamic acid and propylene glycol.
5-Oxoproline or pyroglutamic acid, a metabolite of acetaminophen
Acetaminophen depletes glutathione stores in acute overdoses, in patients with inborn errors of metabolism, and after chronic ingestion of excessive, frequent doses. Depletion of glutathione increases metabolic products, including pyroglutamic acid, which dissociates into hydrogen ions (leading to metabolic acidosis and an anion gap), and 5-oxoproline, (which can be detected in the urine).21,22
Risk factors for metabolic acidosis with acetaminophen ingestion include malnutrition, chronic alcoholism, liver disease, and female sex. In fact, most cases have been reported in females, and altered mental status has been common.
Metabolic acidosis with pyroglutamic acid can occur without elevated acetaminophen levels. Serum and urine levels of pyroglutamic acid may assist with diagnosis. Since identification of urine pyroglutamic acid usually requires outside laboratory assistance, a clinical diagnosis is often made initially and corroborated later by laboratory results. When the anion gap metabolic acidosis is multifactorial, as it was suspected to be in a case reported by Tan et al,23 the osmol gap may be elevated as a consequence of additional toxic ingestions, as it was in the reported patient.
No controlled studies of treatment have been done. n-Acetylcysteine may be of benefit. Occasional patients have been dialyzed for removal of excess pyroglutamic acid.
Propylene glycol, a component of parenteral lorazepam
Lorazepam is a hydrophobic drug, so when it is given parenterally, it must be mixed with a suitable solvent. A typical formulation adds propylene glycol. In patients receiving high doses of lorazepam as relaxation therapy for acute respiratory distress syndrome in the intensive care unit, or as treatment of alcohol withdrawal, the propylene glycol component can precipitate anion gap metabolic acidosis.24,25
Although nearly one-half of the administered propylene glycol is excreted by the kidneys, the remaining substrate is metabolized by alcohol dehydrogenase into d,l-lactaldehyde, then converted into d- or l-lactate. l-Lactate can be metabolized, but d-lactate cannot and leads to anion gap metabolic acidosis. This is another toxic metabolic acidosis associated with an elevated osmol gap. An increasing osmol gap in the intensive care unit can serve as a surrogate marker of excessive propylene glycol administration.23
Isoniazid
Although it is uncommon, there are reports of isoniazid-induced anion gap metabolic acidosis,26 either due to overdoses, or less commonly, with normal dosing. Isoniazid should therefore remain in the mnemonic MUD PILES and may be suspected when metabolic acidosis is accompanied by seizures unresponsive to usual therapy. The seizures respond to pyridoxine.
The “I” should also be augmented by newer causes of metabolic acidosis associated with “ingestions.” Ecstasy, or 3,4-methylenedioxymethamphetamine, can cause metabolic acidosis and seizures. Ecstasy has been associated with rhabdomyolysis and uremia, also leading to anion gap metabolic acidosis.27 A newer class of abused substances, synthetic cathinones (“bath salts”), are associated with metabolic acidosis, compartment syndrome, and renal failure.28
Lactic acidosis
Lactic acidosis and metabolic acidosis can result from hypoperfusion (type A) or other causes (type B). Not all lactic acidosis is contingent on l-lactate, which humans can metabolize. Metabolic acidosis may be a consequence of d-lactate (mammals have no d-lactate dehydrogenase). d-Lactic acidosis as a result of short bowel syndrome has been known for more than a generation.29 However, d-lactic acidosis occurs in another new setting. The new “P” in MUD PILES, propylene glycol, can generate substantial amounts of d-lactate.29
d-lactic metabolic acidosis is always accompanied by neurologic manifestations (slurred speech, confusion, somnolence, ataxia, abusive behavior, and others).30 With short bowel syndrome, the neurologic manifestations occur after eating and clear later.30
Although our patient’s anion gap is more than 20 mmol/L, her blood level of lactate is not elevated, and she had no history to suggest short-bowel syndrome.
Salicylates
Salicylate overdose can cause a mixed acid-base disorder: metabolic acidosis with elevated anion gap and respiratory alkalosis.
Although our patient does not have respiratory alkalosis, an aspirin overdose must be considered. A salicylate level was ordered; it was negative.
Despite the typical association of salicylates with an elevated anion gap, they may also cause a negative anion gap.31 Chloride-sensing ion-specific electrodes contain a membrane permeable to chloride. Salicylates can increase the chloride permeability of these membranes, generating pseudohyperchloremia, and consequently, a negative anion gap.
WHAT ELSE MUST BE CONSIDERED?
3. In view of her anion gap metabolic acidosis, elevated osmol gap, and absence of diabetes, renal failure, or lactate excess, what are the remaining diagnoses to consider in this patient? (Choose all that are potential sources of metabolic acidosis and an increased anion gap.)
- Methanol, ethylene, or diethylene glycol
- Excessive, chronic acetaminophen ingestion
- Salicylate toxicity
- Alcoholic ketoacidosis
All of the above can potentially contribute to metabolic acidosis.
A search of the patient’s home did not reveal a source of methanol or either ethylene or diethylene glycol. Similarly, no aspirin was found, and the patient’s salicylate levels were not elevated. The patient’s laboratory work did not reveal increased ketones.
Since none of the common causes of metabolic acidosis were discovered, and since the patient had been taking acetaminophen, the diagnosis of excessive chronic acetaminophen ingestion was suspected pending laboratory verification. Identification of 5-oxoproline in the urine may take a week or more since the sample is usually sent to special laboratories. Acetaminophen levels in this patient were significantly elevated, as were urinary oxyproline levels, which returned later.
The patient was diagnosed with pyroglutamic acid metabolic acidosis. She was treated supportively and with n-acetylcysteine intravenously, although there have been no controlled studies of the efficacy of this drug. Seventy-two hours after admission, she had improved. Her acid-base status returned to normal.
GOLD MARK: ANOTHER WAY TO REMEMBER
Another mnemonic device for remembering the causes of metabolic acidosis with elevated anion gap is “GOLD MARK”: glycols (ethylene and propylene), oxoproline (instead of pyroglutamic acid from acetaminophen), l-lactate, d-lactate, methanol, aspirin, renal failure, and ketoacidosis).32
ACID-BASE DISORDERS IN DIFFERENT DISEASES
Diverse diseases cause distinctive acid-base abnormalities. Matching the appropriate acid-base abnormality with its associated disease may lead to more timely diagnosis and treatment:
Type 2 diabetes mellitus, for example, can lead to lactic acidosis, ketoacidosis, or type 4 renal tubular acidosis.33
Heart failure, although not typically framed in the context of acid-base physiology, can lead to elevated lactate, which is associated with a worse prognosis.34
Acquired immunodeficiency syndrome. Abacavir can cause normal anion gap metabolic acidosis.35,36
Cancer37,38 can be associated with proximal tubular renal tubular acidosis and lactic acidosis.
An expanding array of toxic ingestions
Metabolic acidosis may be the most prominent and potentially lethal clinical acid-base disturbance. When metabolic acidosis occurs in certain disease states—lactic acidosis with hypoperfusion or methanol ingestion with metabolic acidosis, for example—there is increased morbidity and mortality.
As reflected in the revisions to MUD PILES and in the newer GOLD MARK acronym, the osmol gap has become more valuable in differential diagnosis of metabolic acidosis with an elevated anion gap consequent to an expanding array of toxic ingestions (methanol, propylene glycol, pyroglutamic acid-oxoproline, ethylene glycol, and diethylene glycol), which may accompany pyroglutamic acid-oxoproline.
A 78-year-old black woman with a history of osteoarthrosis and chronic diffuse joint pain presents with altered mental status and tachypnea, which began 3 hours earlier. She lives alone, and her family suspects she abuses both alcohol and her pain medications. She has not been eating well and has lost approximately 10 pounds over the past 3 months. Her analgesic regimen includes acetaminophen and acetaminophen-oxycodone.
In the emergency department her temperature is 98.6°F (37.0°C), pulse 100 beats per minute and regular, respiratory rate 22 per minute, and blood pressure 136/98 mm Hg. She is obtunded but has no focal neurologic defects or meningismus. She has no signs of heart failure (jugular venous distention, cardiomegaly, or gallops), and examination of the lungs and abdomen is unremarkable.
Suspecting that the patient may have taken too much oxycodone, the physician gives her naloxone, but her mental status does not improve. Results of chest radiography and cranial computed tomography are unremarkable. The physician’s initial impression is that the patient has “metabolic encephalopathy of unknown etiology.”
The patient’s laboratory values are shown in Table 1.
WHICH ACID-BASE DISORDER DOES SHE HAVE?
1. Which acid-base disorder does this patient have?
- Metabolic acidosis and respiratory alkalosis
- Metabolic acidosis and respiratory acidosis
- Metabolic acidosis with an elevated anion gap
- A triple disturbance: metabolic acidosis, respiratory acidosis, and metabolic alkalosis
A 5-step approach
Acid-base disorders can be diagnosed and characterized using a systematic approach known as the “Rules of 5” (Table 2)1:
1. Determine the arterial pH status.
2. Determine whether the primary process is respiratory, metabolic, or both.
3. Calculate the anion gap.
4. Check the degree of compensation (respiratory or metabolic).
5. If the patient has metabolic acidosis with an elevated anion gap, check whether the bicarbonate level has decreased as much as the anion gap has increased (ie, whether there is a delta gap).
Let us apply this approach to the patient described above.
1. What is her pH status?
An arterial pH less than 7.40 is acidemic, whereas a pH higher than 7.44 is alkalemic. (Acidemia and alkalemia refer to the abnormal laboratory value, while acidosis and alkalosis refer to the process causing the abnormal value—a subtle distinction, but worth keeping in mind.)
Caveat. A patient may have a significant acid-base disorder even if the pH is normal. Therefore, even if the pH is normal, one should verify that the partial pressure of carbon dioxide (Pco2), bicarbonate level, and anion gap are normal. If they are not, the patient may have a mixed acid-base disorder such as respiratory acidosis superimposed on metabolic alkalosis.
Our patient’s pH is 7.25, which is in the acidemic range.
2. Is her acidosis respiratory, metabolic, or both?
Respiratory acidosis and alkalosis affect the Pco2. The Pco2 is high in respiratory acidosis (due to failure to get rid of excess carbon dioxide), whereas it is low in respiratory alkalosis (due to loss of too much carbon dioxide through hyperventilation).
Metabolic acidosis and alkalosis, on the other hand, affect the serum bicarbonate level. In metabolic acidosis the bicarbonate level is low, whereas in metabolic alkalosis the bicarbonate level is high.
Moreover, in mixed respiratory and metabolic acidosis, the bicarbonate level can be low and the Pco2 can be high. In mixed metabolic and respiratory alkalosis, the bicarbonate level can be high and the Pco2 can be low (Table 2).
Our patient’s serum bicarbonate level is low at 16.0 mmol/L, indicating that the process is metabolic. Her Pco2 is also low (28 mm Hg), which reflects an appropriate response to compensate for the acidosis.
3. What is her anion gap?
Always calculate the anion gap, ie, the serum sodium concentration minus the serum chloride and serum bicarbonate concentrations. If the patient’s serum albumin level is low, for every 1 gram it is below normal, an additional 2.5 mmol/L should be added to the calculated anion gap. We consider an anion gap of 10 mmol/L or less as normal.
Caveats. The blood sample used to calculate the anion gap should be drawn close in time to the arterial blood gas sample.
Although the anion gap is an effective tool in assessing acid-base disorders, further investigation is warranted if clinical judgment suggests that an anion gap calculation is inconsistent with the patient’s circumstances.2
Our patient’s anion gap is elevated (21 mmol/L). Her serum albumin level is in the normal range, so her anion gap does not need to be adjusted.
4. Is the degree of compensation appropriate for the primary acid-base disturbance?
The kidneys compensate for the lungs, and vice versa. That is, in respiratory acidosis or alkalosis, the kidneys adjust the bicarbonate levels, and in metabolic acidosis, the lungs adjust the Pco2 (although in metabolic alkalosis, it is hard for patients to breathe less, especially if they are already hypoxic).
In metabolic acidosis, people compensate by breathing harder to get rid of more carbon dioxide. For every 1-mmol/L decrease in the bicarbonate level, the Pco2 should decrease by 1.3 mm Hg.
Compensation does not return pH to normal; rather, it mitigates the impact of an acid or alkali excess or deficit. If the pH is normalized with an underlying acid-base disturbance, there may be mixed acid-base processes rather than compensation.
Our patient’s bicarbonate level is 16 mmol/L, which is 9 mmol/L lower than normal (for acid-base calculations, we use 25 mmol/L as the nominal normal level). If she is compensating appropriately, her Pco2 should decline from 40 mm Hg (the nominal normal level) by about 11.7 mm Hg (9 × 1.3), to approximately 28.3 mm Hg. Her Pco2 is, indeed, 28 mm Hg, indicating that she is compensating adequately for her metabolic acidosis.
If we use Winter’s formula instead (Pco2 = [1.5 × the bicarbonate level] + 8 ± 2),3 the lowest calculated Pco2 would be 30 mm Hg, which is within 2 mm Hg of the Rules of 5 calculation. Other formulas for calculating compensation are available.3
This information rules out the first two answers to question 1, ie, metabolic acidosis with respiratory alkalosis or acidosis.
5. Is there a delta gap?
Although we know the patient has metabolic acidosis with an elevated anion gap, we have not ruled out the possibility that she may have a triple disturbance. For this reason we need to check her delta gap.
In metabolic acidosis with an elevated anion gap, as the bicarbonate level decreases, the anion gap should increase by the same amount. If the bicarbonate level decreases more than the anion gap increases, the additional decline is the result of a second process—an additional normal-anion-gap acidosis. If the bicarbonate level does not decrease as much as the anion gap increases, there is an additional metabolic alkalosis.
Our patient’s bicarbonate level decreased 9 mmol/L (from the nominal normal level of 25 to 16), and therefore her anion gap should have increased approximately the same amount—and it did. (A normal anion gap for problem-solving is 10, and this patient’s anion gap has increased to 21. A difference of ± 2 is insignificant.) This conclusion verifies that a triple acid-base disturbance is not present, so the last answer is incorrect.
So, the correct answer to the question posed above is metabolic acidosis with an elevated anion gap (that is, metabolic acidosis with appropriate respiratory compensation).
‘MUD PILES’: FINDING THE CAUSE OF ANION GAP METABOLIC ACIDOSIS
The possible causes of metabolic acidosis with an elevated anion gap (as in our patient) can be summarized in the mnemonic MUD PILES (methanol, uremia, diabetes, paraldehyde, isoniazid, lactate, ethylene glycol, and salicylates), which has been used for many years. Parts of it are no longer useful, but rather than discard it, we propose to update it (Table 3).
Methanol and ethylene glycol
We will address toxic ingestion of methanol and ethylene glycol (the “M” and “E” of MUD PILES) at the same time.
In cases of suspected ingestion of toxic substances such as these, it is useful to examine the osmol gap, ie, the difference between the calculated and the measured serum osmolality. Serum osmolality (in mOsm/kg) is calculated as the sodium concentration in mmol/L times 2, plus the glucose concentration in mg/dL divided by 18, plus the blood urea nitrogen concentration in mg/dL divided by 2.8 (Table 4). If the measured osmolality is higher than this calculated value, the difference may be due to solutes in the blood that should not be there such as ethylene glycol, diethylene glycol, methanol, and their many metabolic products.
In our patient, ingestion of both methanol and ethylene glycol should be considered, since she lives alone and has been suspected of alcohol and opioid abuse. Her calculated osmol gap is 278 mOsm/kg. Her measured osmolality is 318 mOsm/kg (Table 1). The osmol gap is 40 mOsm/kg (normal is ≤ 10).4,5 Therefore, her osmol gap is elevated.
Identifying the specific substance the patient ingested that caused metabolic acidosis with anion gap may be difficult. Poisonings with these agents do not always increase the osmol gap.6 A high index of suspicion is essential. It is helpful to have the family search for any sources of ethylene glycol and methanol at home and initiate treatment early if an ingestion is suspected, using fomepizole (an alcohol dehydrogenase inhibitor) or parenteral ethanol and hemodialysis.7 Liquid chromatography identifies these two toxins, but results are not available emergently.
Diethylene glycol ingestion should also be considered.8 Since it is diagnosed and treated like ethylene glycol intoxication, it can be placed with the “E” of (di)ethylene glycol in the mnemonic.
Uremia
Renal failure can lead to metabolic acidosis.9 Our patient has no history of kidney disease, but her blood urea nitrogen and creatinine concentrations are above normal, and her estimated glomerular filtration rate by the Modification of Diet in Renal Disease formula is 48 mL/min/1.73 m2—low, but not uremic.
Rhabdomyolysis (suspected by elevated creatine kinase values) should be considered in any patient with mental status changes, suspected toxic ingestion, and metabolic acidosis (see the “I” in MUD PILES below). Compartment syndromes with muscle necrosis may present in a subtle fashion. Therefore, renal failure from rhabdomyolysis may complicate this patient’s course later, and should be kept in mind.
Diabetes
The patient has no history of diabetes and has a normal blood glucose level. Blood testing did not reveal ketones. She is not taking metformin (alleged to cause lactic acidosis) or a sodium-glucose cotransporter 2 inhibitor (which have been associated with ketoacidosis).10
There is another, less common cause of ketoacidosis: alcohol.11 Although alcoholism is common, alcoholic ketoacidosis is uncommon, even in heavy drinkers. Ethyl alcohol causing metabolic acidosis is similar to metabolic acidosis with (di)ethylene glycol and methanol, and if suspected it should be treated empirically (first with thiamine, then dextrose and saline, and correcting other electrolyte disturbances such as hypokalemia and hypomagnesemia) before specific identification is made. Ketones (predominantly beta-hydroxybutyrate) may persist up to 2 weeks after alcohol ingestion has stopped.11 Ketosis in the setting of alcoholic ketoacidosis is frequently accompanied by other markers of alcohol target organ injury: elevated bilirubin, aspartate aminotransferase, alanine aminotransferase, and gamma-glutamyl transferase levels. The term “ketohepatitis” has been suggested as an alternative to alcoholic ketosis.11
This patient did not have an elevated blood ethanol level, and her liver markers were otherwise normal.
THE NEW MUD PILES
2. Which of the following is (are) true? Regarding the remaining letters of the MUD PILES mnemonic:
- The “P” (paraldehyde) has been replaced by pyroglutamic acid (5-oxoproline) and propylene glycol.
- There are two isomers of lactate (dextro and levo), and consequently two clinical varieties of lactic acidosis.
- Isoniazid is no longer associated with metabolic acidosis with elevated anion gap.
- Salicylates can paradoxically be associated both with elevated and low anion gaps.
Isoniazid is still associated with metabolic acidosis with elevated anion gap, and so the third answer choice is false; the rest are true.
Paraldehyde, isoniazid, lactate
The “P,” “I,” and “L” (d-lactate) of the revamped MUD PILES acronym are less common than the others. They should be considered when the more typical causes of metabolic acidosis are not present, as in this patient.
UPDATING THE ‘P’ IN MUD PILES
Paraldehyde is rarely prescribed anymore. A PubMed search on December 21, 2015 applying the terms paraldehyde and metabolic acidosis yielded 17 results. Those specific to anion gap metabolic acidosis were from 1957 to 1986 (n = 9).12–20
Therefore, we can eliminate paraldehyde from the MUD PILES mnemonic and replace it with pyroglutamic acid and propylene glycol.
5-Oxoproline or pyroglutamic acid, a metabolite of acetaminophen
Acetaminophen depletes glutathione stores in acute overdoses, in patients with inborn errors of metabolism, and after chronic ingestion of excessive, frequent doses. Depletion of glutathione increases metabolic products, including pyroglutamic acid, which dissociates into hydrogen ions (leading to metabolic acidosis and an anion gap), and 5-oxoproline, (which can be detected in the urine).21,22
Risk factors for metabolic acidosis with acetaminophen ingestion include malnutrition, chronic alcoholism, liver disease, and female sex. In fact, most cases have been reported in females, and altered mental status has been common.
Metabolic acidosis with pyroglutamic acid can occur without elevated acetaminophen levels. Serum and urine levels of pyroglutamic acid may assist with diagnosis. Since identification of urine pyroglutamic acid usually requires outside laboratory assistance, a clinical diagnosis is often made initially and corroborated later by laboratory results. When the anion gap metabolic acidosis is multifactorial, as it was suspected to be in a case reported by Tan et al,23 the osmol gap may be elevated as a consequence of additional toxic ingestions, as it was in the reported patient.
No controlled studies of treatment have been done. n-Acetylcysteine may be of benefit. Occasional patients have been dialyzed for removal of excess pyroglutamic acid.
Propylene glycol, a component of parenteral lorazepam
Lorazepam is a hydrophobic drug, so when it is given parenterally, it must be mixed with a suitable solvent. A typical formulation adds propylene glycol. In patients receiving high doses of lorazepam as relaxation therapy for acute respiratory distress syndrome in the intensive care unit, or as treatment of alcohol withdrawal, the propylene glycol component can precipitate anion gap metabolic acidosis.24,25
Although nearly one-half of the administered propylene glycol is excreted by the kidneys, the remaining substrate is metabolized by alcohol dehydrogenase into d,l-lactaldehyde, then converted into d- or l-lactate. l-Lactate can be metabolized, but d-lactate cannot and leads to anion gap metabolic acidosis. This is another toxic metabolic acidosis associated with an elevated osmol gap. An increasing osmol gap in the intensive care unit can serve as a surrogate marker of excessive propylene glycol administration.23
Isoniazid
Although it is uncommon, there are reports of isoniazid-induced anion gap metabolic acidosis,26 either due to overdoses, or less commonly, with normal dosing. Isoniazid should therefore remain in the mnemonic MUD PILES and may be suspected when metabolic acidosis is accompanied by seizures unresponsive to usual therapy. The seizures respond to pyridoxine.
The “I” should also be augmented by newer causes of metabolic acidosis associated with “ingestions.” Ecstasy, or 3,4-methylenedioxymethamphetamine, can cause metabolic acidosis and seizures. Ecstasy has been associated with rhabdomyolysis and uremia, also leading to anion gap metabolic acidosis.27 A newer class of abused substances, synthetic cathinones (“bath salts”), are associated with metabolic acidosis, compartment syndrome, and renal failure.28
Lactic acidosis
Lactic acidosis and metabolic acidosis can result from hypoperfusion (type A) or other causes (type B). Not all lactic acidosis is contingent on l-lactate, which humans can metabolize. Metabolic acidosis may be a consequence of d-lactate (mammals have no d-lactate dehydrogenase). d-Lactic acidosis as a result of short bowel syndrome has been known for more than a generation.29 However, d-lactic acidosis occurs in another new setting. The new “P” in MUD PILES, propylene glycol, can generate substantial amounts of d-lactate.29
d-lactic metabolic acidosis is always accompanied by neurologic manifestations (slurred speech, confusion, somnolence, ataxia, abusive behavior, and others).30 With short bowel syndrome, the neurologic manifestations occur after eating and clear later.30
Although our patient’s anion gap is more than 20 mmol/L, her blood level of lactate is not elevated, and she had no history to suggest short-bowel syndrome.
Salicylates
Salicylate overdose can cause a mixed acid-base disorder: metabolic acidosis with elevated anion gap and respiratory alkalosis.
Although our patient does not have respiratory alkalosis, an aspirin overdose must be considered. A salicylate level was ordered; it was negative.
Despite the typical association of salicylates with an elevated anion gap, they may also cause a negative anion gap.31 Chloride-sensing ion-specific electrodes contain a membrane permeable to chloride. Salicylates can increase the chloride permeability of these membranes, generating pseudohyperchloremia, and consequently, a negative anion gap.
WHAT ELSE MUST BE CONSIDERED?
3. In view of her anion gap metabolic acidosis, elevated osmol gap, and absence of diabetes, renal failure, or lactate excess, what are the remaining diagnoses to consider in this patient? (Choose all that are potential sources of metabolic acidosis and an increased anion gap.)
- Methanol, ethylene, or diethylene glycol
- Excessive, chronic acetaminophen ingestion
- Salicylate toxicity
- Alcoholic ketoacidosis
All of the above can potentially contribute to metabolic acidosis.
A search of the patient’s home did not reveal a source of methanol or either ethylene or diethylene glycol. Similarly, no aspirin was found, and the patient’s salicylate levels were not elevated. The patient’s laboratory work did not reveal increased ketones.
Since none of the common causes of metabolic acidosis were discovered, and since the patient had been taking acetaminophen, the diagnosis of excessive chronic acetaminophen ingestion was suspected pending laboratory verification. Identification of 5-oxoproline in the urine may take a week or more since the sample is usually sent to special laboratories. Acetaminophen levels in this patient were significantly elevated, as were urinary oxyproline levels, which returned later.
The patient was diagnosed with pyroglutamic acid metabolic acidosis. She was treated supportively and with n-acetylcysteine intravenously, although there have been no controlled studies of the efficacy of this drug. Seventy-two hours after admission, she had improved. Her acid-base status returned to normal.
GOLD MARK: ANOTHER WAY TO REMEMBER
Another mnemonic device for remembering the causes of metabolic acidosis with elevated anion gap is “GOLD MARK”: glycols (ethylene and propylene), oxoproline (instead of pyroglutamic acid from acetaminophen), l-lactate, d-lactate, methanol, aspirin, renal failure, and ketoacidosis).32
ACID-BASE DISORDERS IN DIFFERENT DISEASES
Diverse diseases cause distinctive acid-base abnormalities. Matching the appropriate acid-base abnormality with its associated disease may lead to more timely diagnosis and treatment:
Type 2 diabetes mellitus, for example, can lead to lactic acidosis, ketoacidosis, or type 4 renal tubular acidosis.33
Heart failure, although not typically framed in the context of acid-base physiology, can lead to elevated lactate, which is associated with a worse prognosis.34
Acquired immunodeficiency syndrome. Abacavir can cause normal anion gap metabolic acidosis.35,36
Cancer37,38 can be associated with proximal tubular renal tubular acidosis and lactic acidosis.
An expanding array of toxic ingestions
Metabolic acidosis may be the most prominent and potentially lethal clinical acid-base disturbance. When metabolic acidosis occurs in certain disease states—lactic acidosis with hypoperfusion or methanol ingestion with metabolic acidosis, for example—there is increased morbidity and mortality.
As reflected in the revisions to MUD PILES and in the newer GOLD MARK acronym, the osmol gap has become more valuable in differential diagnosis of metabolic acidosis with an elevated anion gap consequent to an expanding array of toxic ingestions (methanol, propylene glycol, pyroglutamic acid-oxoproline, ethylene glycol, and diethylene glycol), which may accompany pyroglutamic acid-oxoproline.
- Whittier WL, Rutecki GW. Primer on clinical acid-base problem solving. Dis Mon 2004; 50:122–162.
- Kraut JA, Madias NE. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol 2007; 2:162–174.
- Adrogué HJ, Madias NE. Secondary responses to altered acid-base status: the rules of engagement. J Am Soc Nephrol 2010; 21:920–923.
- Krasowski MD, Wilcoxon RM, Miron J. A retrospective analysis of glycol and toxic alcohol ingestion: utility of anion and osmolal gaps. BMC Clin Pathol 2012;12:1.
- Latus J, Kimmel M, Alscher MD, Braun N. Ethylene glycol poisoning: a rare but life-threatening cause of metabolic acidosis—a single-centre experience. Clin Kidney J 2012; 5:120–123.
- Kraut JA. Diagnosis of toxic alcohols: limitations of present methods. Clin Toxicol (Phila) 2015; 53:589–595.
- Ghannoum M, Hoffman RS, Mowry JB, Lavergne V. Trends in toxic alcohol exposures in the United States from 2000 to 2013: a focus on the use of antidotes and extracorporeal treatments. Semin Dial 2014; 27:395–401.
- Schep LJ, Slaughter RJ, Temple WA, Beasley DM. Diethylene glycol poisoning. Clin Toxicol (Phila) 2009; 47:525–535.
- Kraut JA, Madias NE. Metabolic acidosis of CKD: an update. Am J Kidney Dis 2016; 67:307–317.
- Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 2015; 100:2849–2852.
- Yokoyama A, Yokoyama T, Mizukami T, et al. Alcoholic ketosis: prevalence, determinants, and ketohepatitis in Japanese alcoholic men. Alcohol Alcohol 2014; 49:618–625.
- Hayward JN, Boshell BR. Paraldehyde intoxication with metabolic acidosis; report of two cases, experimental data and a critical review of the literature. Am J Med 1957; 23:965–976.
- Elkinton JR, Huth EJ, Clark JK, Barker ES, Seligson D. Renal tubular acidosis with organic aciduria during paraldehyde ingestion; six year study of an unusual case. Am J Med 1957; 23:977–986.
- Waterhouse C, Stern EA. Metabolic acidosis occurring during administration of paraldehyde. Am J Med 1957; 23:987–989.
- Beier LS, Pitts WH, Gonick HC. Metabolic acidosis occurring during paraldehyde intoxication. Ann Intern Med 1963; 58:155–158.
- Hiemcke T. Metabolic acidosis due to paraldehyde. Ned Tijdschr Geneeskd 1964; 108:2165–2167. Dutch.
- Gailitis RJ. Paraldehyde acidosis syndrome. IMJ III Med J 1966; 129:258–262.
- Gutman RA, Burnell JM. Paraldehyde acidosis. Am J Med 1967; 42:435–440.
- Hadden JW, Metzner RJ. Pseudoketosis and hyperacetaldehydemia in paraldehyde acidosis. Am J Med 1969; 47:642–647.
- Linter CM, Linter SP. Severe lactic acidosis following paraldehyde administration. Br J Psychiatry 1986; 149:650–651.
- Zand L, Muriithi A, Nelsen E, et al. Severe anion gap metabolic acidosis from acetaminophen use secondary to 5-oxoproline (pyroglutamic acid) accumulation. Am J Med Sci 2012; 344:501–504.
- Abkur TM, Mohammed W, Ali M, Casserly L. Acetaminophen-induced anion gap metabolic acidosis secondary to 5-oxoproline: a case report. J Med Case Rep 2014; 8:409.
- Tan EM, Kalimullah E, Sohail MR, Ramar K. Diagnostic challenge in a patient with severe anion gap metabolic acidosis. Case Rep Crit Care 2015; 2015:272914.
- Jorens PG, Demey HE, Schepens PJ, et al. Unusual d-lactic acid acidosis from propylene glycol metabolism in overdose. J Toxicol Clin Toxicol 2004; 42:163–169.
- Barnes BJ, Gerst C, Smith JR, Terrell AR, Mullins ME. Osmol gap as a surrogate marker for serum propylene glycol concentrations in patients receiving lorazepam for sedation. Pharmacotherapy 2006; 26:23–33.
- Gokhale YA, Vaidya MS, Mehta AD, Rathod NN. Isoniazid toxicity presenting as status epilepticus and severe metabolic acidosis. J Assoc Physicians India 2009; 57:70–71.
- Ben-Abraham R, Szold O, Rudick V, Weinbroum AA. ‘Ecstasy’ intoxication: life-threatening manifestations and resuscitative measures in the intensive care setting. Eur J Emerg Med 2003; 10:309–313.
- German CL, Fleckenstein AE, Hanson GR. Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life Sci 2014; 97:2–8.
- Jorens PG, Demey HE, Schepens PJ, et al. Unusual d-lactic acidosis from propylene glycol metabolism in overdose. J Toxicol Clin Toxicol 2004; 42:163–169.
- Kang KP, Le S, Kang SK. d-Lactic acidosis in humans: review and update. Electrolyte Blood Press 2006; 4:53–56.
- Emmett M. Approach to the patient with a negative anion gap. Am J Kidney Dis 2016; 67:143–150.
- Mehta AN, Emmett JB, Emmett M. GOLD MARK: an anion gap mnemonic for the 21st Century. Lancet 2008; 372:892.
- Palmer BF, Clegg DJ. Electrolyte and acid-base disturbances in patients with diabetes mellitus. N Engl J Med 2015; 373:548–559.
- Park JJ, Choi DJ, Yoon CH, et al; KorHF Registry. The prognostic value of arterial blood gas analysis in high-risk acute heart failure patients: an analysis of the Korean Heart Failure (KorHF) registry. Eur J Heart Fail 2015; 17:601–611.
- Musso CG, Belloso WH, Glassock RJ. Water, electrolytes, and acid-base alterations in human immunodeficiency virus infected patients. World J Nephrol 2016; 5:33–42.
- Camara-Lemarroy CR, Flores-Cantu H, Calderon-Hernandez HJ, Diaz-Torres MA, Villareal-Velazquez HJ. Drug-induced haemolysis, renal failure, thrombocytopenia and lactic acidosis in patients with HIV and cryptococcal meningitis: a diagnostic challenge. Int J STD AIDS 2015; 26:1052–1054.
- Miltiadous G, Christidis D, Kalogirou M, Elisaf M. Causes and mechanisms of acid-base and electrolyte abnormalities in cancer. Eur J Intern Med 2008; 19:1–7.
- Vlachostergios PJ, Oikonomou KG, Gibilaro E, Apergis G. Elevated lactic acid is a negative prognostic factor in metastatic lung cancer. Cancer Biomark 2015; 15:725–734.
- Whittier WL, Rutecki GW. Primer on clinical acid-base problem solving. Dis Mon 2004; 50:122–162.
- Kraut JA, Madias NE. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol 2007; 2:162–174.
- Adrogué HJ, Madias NE. Secondary responses to altered acid-base status: the rules of engagement. J Am Soc Nephrol 2010; 21:920–923.
- Krasowski MD, Wilcoxon RM, Miron J. A retrospective analysis of glycol and toxic alcohol ingestion: utility of anion and osmolal gaps. BMC Clin Pathol 2012;12:1.
- Latus J, Kimmel M, Alscher MD, Braun N. Ethylene glycol poisoning: a rare but life-threatening cause of metabolic acidosis—a single-centre experience. Clin Kidney J 2012; 5:120–123.
- Kraut JA. Diagnosis of toxic alcohols: limitations of present methods. Clin Toxicol (Phila) 2015; 53:589–595.
- Ghannoum M, Hoffman RS, Mowry JB, Lavergne V. Trends in toxic alcohol exposures in the United States from 2000 to 2013: a focus on the use of antidotes and extracorporeal treatments. Semin Dial 2014; 27:395–401.
- Schep LJ, Slaughter RJ, Temple WA, Beasley DM. Diethylene glycol poisoning. Clin Toxicol (Phila) 2009; 47:525–535.
- Kraut JA, Madias NE. Metabolic acidosis of CKD: an update. Am J Kidney Dis 2016; 67:307–317.
- Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 2015; 100:2849–2852.
- Yokoyama A, Yokoyama T, Mizukami T, et al. Alcoholic ketosis: prevalence, determinants, and ketohepatitis in Japanese alcoholic men. Alcohol Alcohol 2014; 49:618–625.
- Hayward JN, Boshell BR. Paraldehyde intoxication with metabolic acidosis; report of two cases, experimental data and a critical review of the literature. Am J Med 1957; 23:965–976.
- Elkinton JR, Huth EJ, Clark JK, Barker ES, Seligson D. Renal tubular acidosis with organic aciduria during paraldehyde ingestion; six year study of an unusual case. Am J Med 1957; 23:977–986.
- Waterhouse C, Stern EA. Metabolic acidosis occurring during administration of paraldehyde. Am J Med 1957; 23:987–989.
- Beier LS, Pitts WH, Gonick HC. Metabolic acidosis occurring during paraldehyde intoxication. Ann Intern Med 1963; 58:155–158.
- Hiemcke T. Metabolic acidosis due to paraldehyde. Ned Tijdschr Geneeskd 1964; 108:2165–2167. Dutch.
- Gailitis RJ. Paraldehyde acidosis syndrome. IMJ III Med J 1966; 129:258–262.
- Gutman RA, Burnell JM. Paraldehyde acidosis. Am J Med 1967; 42:435–440.
- Hadden JW, Metzner RJ. Pseudoketosis and hyperacetaldehydemia in paraldehyde acidosis. Am J Med 1969; 47:642–647.
- Linter CM, Linter SP. Severe lactic acidosis following paraldehyde administration. Br J Psychiatry 1986; 149:650–651.
- Zand L, Muriithi A, Nelsen E, et al. Severe anion gap metabolic acidosis from acetaminophen use secondary to 5-oxoproline (pyroglutamic acid) accumulation. Am J Med Sci 2012; 344:501–504.
- Abkur TM, Mohammed W, Ali M, Casserly L. Acetaminophen-induced anion gap metabolic acidosis secondary to 5-oxoproline: a case report. J Med Case Rep 2014; 8:409.
- Tan EM, Kalimullah E, Sohail MR, Ramar K. Diagnostic challenge in a patient with severe anion gap metabolic acidosis. Case Rep Crit Care 2015; 2015:272914.
- Jorens PG, Demey HE, Schepens PJ, et al. Unusual d-lactic acid acidosis from propylene glycol metabolism in overdose. J Toxicol Clin Toxicol 2004; 42:163–169.
- Barnes BJ, Gerst C, Smith JR, Terrell AR, Mullins ME. Osmol gap as a surrogate marker for serum propylene glycol concentrations in patients receiving lorazepam for sedation. Pharmacotherapy 2006; 26:23–33.
- Gokhale YA, Vaidya MS, Mehta AD, Rathod NN. Isoniazid toxicity presenting as status epilepticus and severe metabolic acidosis. J Assoc Physicians India 2009; 57:70–71.
- Ben-Abraham R, Szold O, Rudick V, Weinbroum AA. ‘Ecstasy’ intoxication: life-threatening manifestations and resuscitative measures in the intensive care setting. Eur J Emerg Med 2003; 10:309–313.
- German CL, Fleckenstein AE, Hanson GR. Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life Sci 2014; 97:2–8.
- Jorens PG, Demey HE, Schepens PJ, et al. Unusual d-lactic acidosis from propylene glycol metabolism in overdose. J Toxicol Clin Toxicol 2004; 42:163–169.
- Kang KP, Le S, Kang SK. d-Lactic acidosis in humans: review and update. Electrolyte Blood Press 2006; 4:53–56.
- Emmett M. Approach to the patient with a negative anion gap. Am J Kidney Dis 2016; 67:143–150.
- Mehta AN, Emmett JB, Emmett M. GOLD MARK: an anion gap mnemonic for the 21st Century. Lancet 2008; 372:892.
- Palmer BF, Clegg DJ. Electrolyte and acid-base disturbances in patients with diabetes mellitus. N Engl J Med 2015; 373:548–559.
- Park JJ, Choi DJ, Yoon CH, et al; KorHF Registry. The prognostic value of arterial blood gas analysis in high-risk acute heart failure patients: an analysis of the Korean Heart Failure (KorHF) registry. Eur J Heart Fail 2015; 17:601–611.
- Musso CG, Belloso WH, Glassock RJ. Water, electrolytes, and acid-base alterations in human immunodeficiency virus infected patients. World J Nephrol 2016; 5:33–42.
- Camara-Lemarroy CR, Flores-Cantu H, Calderon-Hernandez HJ, Diaz-Torres MA, Villareal-Velazquez HJ. Drug-induced haemolysis, renal failure, thrombocytopenia and lactic acidosis in patients with HIV and cryptococcal meningitis: a diagnostic challenge. Int J STD AIDS 2015; 26:1052–1054.
- Miltiadous G, Christidis D, Kalogirou M, Elisaf M. Causes and mechanisms of acid-base and electrolyte abnormalities in cancer. Eur J Intern Med 2008; 19:1–7.
- Vlachostergios PJ, Oikonomou KG, Gibilaro E, Apergis G. Elevated lactic acid is a negative prognostic factor in metastatic lung cancer. Cancer Biomark 2015; 15:725–734.
What is the role of roflumilast in chronic obstructive pulmonary disease?
Roflumilast has been shown to reduce rates of acute exacerbation in patients with severe chronic obstructive pulmonary disease (COPD), ie, forced expiratory volume in 1 second (FEV1) less than 50% with symptoms of chronic bronchitis and a history of exacerbations.
Roflumilast is a selective phosphodiesterase 4 (PDE4) inhibitor that acts on airway smooth muscle cells and various inflammatory cells. By blocking PDE4, roflumilast raises cyclic adenosine monophosphate levels within these cells, curtailing the inflammatory response.1,2
Roflumilast is not a bronchodilator, although modest improvements in FEV1 have been documented in clinical trials when it was used as maintenance therapy.
TRIALS OF ROFLUMILAST
Several trials have investigated the efficacy of roflumilast in COPD (Table 1).
The RECORD trial
The RECORD trial1 in 2005 was the first large randomized controlled trial of roflumilast in moderate to severe COPD. At a dose of 500 µg orally daily, there was a modest but statistically significant improvement in the postbronchodilator FEV1. There was also improvement in the St. George Respiratory Questionnaire score in the treatment arm, but this was not statistically significant. The study also found a reduction in acute exacerbations of COPD with roflumilast, which was a secondary end point.1
The results of this study spurred interest in roflumilast as well as criticism of the design of the study. First, COPD patients on inhaled maintenance therapy such as an inhaled corticosteroid and long-acting beta-agonist combination or a long-acting muscarinic antagonist had their medications held during the study. Second, the average FEV1 was 54% of predicted, indicative of a study population with less severe disease.1
The RATIO trial
Taking into account the results of the RECORD trial, the RATIO trial3 in 2007 recruited patients with more severe COPD—ie, Global Initiative for Chronic Obstructive Lung Disease (GOLD) class III and IV—and included the rate of acute exacerbations as a primary end point. Maintenance therapy with inhaled corticosteroids was continued in patients already taking them. However, long-acting beta-agonists and long-acting muscarinic antagonist therapies were held.3
Again, roflumilast improved postbronchodilator FEV1 compared with placebo. A reduction in acute exacerbations was seen but was not statistically significant except in subgroup analysis, where a statistically significant reduction in acute exacerbations was noted for patients with very severe (GOLD class IV) COPD.3
Post hoc analysis from the RATIO trial suggested that patients with chronic bronchitis and patients with a history of frequent exacerbations were more likely to respond to roflumilast.2
The EOS and HELIOS trials
In 2009, the results of the EOS and HELIOS trials of roflumilast in patients with severe COPD were published.4 These trials allowed continuation of long-acting beta-agonists and muscarinic antagonists. The prebronchodilator FEV1 improved modestly when roflumilast was added to a long-acting bronchodilator. These studies ran for only 24 weeks, and the rate of acute exacerbations was not a primary end point, although the results did show a trend toward reduction of exacerbations.4
The AURA and HERMES trials
Also in 2009 was the publication of the results of two 52-week placebo-controlled trials (AURA and HERMES) of roflumilast in patients with severe COPD with chronic bronchitis and a history of frequent exacerbations.5 Maintenance therapy with long-acting beta-agonists was continued, whereas inhaled corticosteroids and long-acting muscarinic antagonists were held. Statistically significant improvements in prebronchodilator FEV1 and reduction in the rate of exacerbations were observed in the roflumilast group (17% reduction, 95% confidence interval 8–25, P < .0003).5
The REACT trial
The REACT trial6 randomized 1,945 patients with severe COPD already on maximal recommended combination inhaled corticosteroid and long-acting beta-agonist therapy to receive either roflumilast or placebo. The patients’ ratio of FEV1 to forced vital capacity was less than 70%, their postbronchodilator FEV1 was less than 50%, and they had chronic bronchitis and a history of at least two acute exacerbations during the past year. They had also been on combination therapy for the previous year. Patients who were on long-acting muscarinic-antagonist therapy (70% of the cohort) were included, and continued with their medication.
Patients were followed for 52 weeks. There was a significant reduction in the rate of exacerbations in the roflumilast group vs placebo (0.823 vs 0.959; risk ratio 0.858; 95% confidence interval 0.740–0.995; P = .0424).6 As in previous trials, the roflumilast group showed an improvement in postbronchodilator FEV1. The study also showed a reduction in hospital admissions in the treatment group.6
ADVERSE EFFECTS OF ROFLUMILAST
Roflumilast is known to have adverse effects significant enough to reduce compliance, the most common being diarrhea, weight loss, and nausea.2,6,7 In the REACT trial,6 11% of patients in the roflumilast group vs 5% in the placebo group dropped out of the study because of adverse drug effects. Diarrhea was reported in 10% and weight loss in 9% of patients taking roflumilast. Weight loss has been shown to be reversible upon stopping roflumilast.2 There has been no evidence of increased risk of death or serious adverse events in studies of roflumilast in patients with COPD.2 However, the benefit-to-harm ratio suggests that roflumilast provides a net benefit only in patients at high risk of severe exacerbations.7
- Rabe KF, Bateman ED, O’Donnell DE, Witte S, Bredenbroker D, Bethke TD. Roflumilast—an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomized controlled trial. Lancet 2005; 366:63–71.
- Field SK. Roflumilast, a novel phosphodiesterase 4 inhibitor, for COPD patients with a history of exacerbations. Clin Med Insights Circ Respir Pulm Med 2011; 5:57–70.
- Calverley PM, Sanchez-Toril F, McIvor A, Teichmann P, Bredenbroeker D, Fabbri LM. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 176:154–161.
- Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al; M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomized clinical trials. Lancet 2009; 374:695–703.
- Calverley PM, Rabe KF, Goehring U-M, Kristiansen S, Fabbri LM, Martinez FJ. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomized clinical trials. Lancet 2009; 374:684–95.
- Martinez FJ, Calverley PM, Goehring UM, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet 2015; 385:857–866.
- Yu T, Fain K, Boyd CM, et al. Benefits and harms of roflumilast in moderate to severe COPD. Thorax 2014; 69:616–622.
Roflumilast has been shown to reduce rates of acute exacerbation in patients with severe chronic obstructive pulmonary disease (COPD), ie, forced expiratory volume in 1 second (FEV1) less than 50% with symptoms of chronic bronchitis and a history of exacerbations.
Roflumilast is a selective phosphodiesterase 4 (PDE4) inhibitor that acts on airway smooth muscle cells and various inflammatory cells. By blocking PDE4, roflumilast raises cyclic adenosine monophosphate levels within these cells, curtailing the inflammatory response.1,2
Roflumilast is not a bronchodilator, although modest improvements in FEV1 have been documented in clinical trials when it was used as maintenance therapy.
TRIALS OF ROFLUMILAST
Several trials have investigated the efficacy of roflumilast in COPD (Table 1).
The RECORD trial
The RECORD trial1 in 2005 was the first large randomized controlled trial of roflumilast in moderate to severe COPD. At a dose of 500 µg orally daily, there was a modest but statistically significant improvement in the postbronchodilator FEV1. There was also improvement in the St. George Respiratory Questionnaire score in the treatment arm, but this was not statistically significant. The study also found a reduction in acute exacerbations of COPD with roflumilast, which was a secondary end point.1
The results of this study spurred interest in roflumilast as well as criticism of the design of the study. First, COPD patients on inhaled maintenance therapy such as an inhaled corticosteroid and long-acting beta-agonist combination or a long-acting muscarinic antagonist had their medications held during the study. Second, the average FEV1 was 54% of predicted, indicative of a study population with less severe disease.1
The RATIO trial
Taking into account the results of the RECORD trial, the RATIO trial3 in 2007 recruited patients with more severe COPD—ie, Global Initiative for Chronic Obstructive Lung Disease (GOLD) class III and IV—and included the rate of acute exacerbations as a primary end point. Maintenance therapy with inhaled corticosteroids was continued in patients already taking them. However, long-acting beta-agonists and long-acting muscarinic antagonist therapies were held.3
Again, roflumilast improved postbronchodilator FEV1 compared with placebo. A reduction in acute exacerbations was seen but was not statistically significant except in subgroup analysis, where a statistically significant reduction in acute exacerbations was noted for patients with very severe (GOLD class IV) COPD.3
Post hoc analysis from the RATIO trial suggested that patients with chronic bronchitis and patients with a history of frequent exacerbations were more likely to respond to roflumilast.2
The EOS and HELIOS trials
In 2009, the results of the EOS and HELIOS trials of roflumilast in patients with severe COPD were published.4 These trials allowed continuation of long-acting beta-agonists and muscarinic antagonists. The prebronchodilator FEV1 improved modestly when roflumilast was added to a long-acting bronchodilator. These studies ran for only 24 weeks, and the rate of acute exacerbations was not a primary end point, although the results did show a trend toward reduction of exacerbations.4
The AURA and HERMES trials
Also in 2009 was the publication of the results of two 52-week placebo-controlled trials (AURA and HERMES) of roflumilast in patients with severe COPD with chronic bronchitis and a history of frequent exacerbations.5 Maintenance therapy with long-acting beta-agonists was continued, whereas inhaled corticosteroids and long-acting muscarinic antagonists were held. Statistically significant improvements in prebronchodilator FEV1 and reduction in the rate of exacerbations were observed in the roflumilast group (17% reduction, 95% confidence interval 8–25, P < .0003).5
The REACT trial
The REACT trial6 randomized 1,945 patients with severe COPD already on maximal recommended combination inhaled corticosteroid and long-acting beta-agonist therapy to receive either roflumilast or placebo. The patients’ ratio of FEV1 to forced vital capacity was less than 70%, their postbronchodilator FEV1 was less than 50%, and they had chronic bronchitis and a history of at least two acute exacerbations during the past year. They had also been on combination therapy for the previous year. Patients who were on long-acting muscarinic-antagonist therapy (70% of the cohort) were included, and continued with their medication.
Patients were followed for 52 weeks. There was a significant reduction in the rate of exacerbations in the roflumilast group vs placebo (0.823 vs 0.959; risk ratio 0.858; 95% confidence interval 0.740–0.995; P = .0424).6 As in previous trials, the roflumilast group showed an improvement in postbronchodilator FEV1. The study also showed a reduction in hospital admissions in the treatment group.6
ADVERSE EFFECTS OF ROFLUMILAST
Roflumilast is known to have adverse effects significant enough to reduce compliance, the most common being diarrhea, weight loss, and nausea.2,6,7 In the REACT trial,6 11% of patients in the roflumilast group vs 5% in the placebo group dropped out of the study because of adverse drug effects. Diarrhea was reported in 10% and weight loss in 9% of patients taking roflumilast. Weight loss has been shown to be reversible upon stopping roflumilast.2 There has been no evidence of increased risk of death or serious adverse events in studies of roflumilast in patients with COPD.2 However, the benefit-to-harm ratio suggests that roflumilast provides a net benefit only in patients at high risk of severe exacerbations.7
Roflumilast has been shown to reduce rates of acute exacerbation in patients with severe chronic obstructive pulmonary disease (COPD), ie, forced expiratory volume in 1 second (FEV1) less than 50% with symptoms of chronic bronchitis and a history of exacerbations.
Roflumilast is a selective phosphodiesterase 4 (PDE4) inhibitor that acts on airway smooth muscle cells and various inflammatory cells. By blocking PDE4, roflumilast raises cyclic adenosine monophosphate levels within these cells, curtailing the inflammatory response.1,2
Roflumilast is not a bronchodilator, although modest improvements in FEV1 have been documented in clinical trials when it was used as maintenance therapy.
TRIALS OF ROFLUMILAST
Several trials have investigated the efficacy of roflumilast in COPD (Table 1).
The RECORD trial
The RECORD trial1 in 2005 was the first large randomized controlled trial of roflumilast in moderate to severe COPD. At a dose of 500 µg orally daily, there was a modest but statistically significant improvement in the postbronchodilator FEV1. There was also improvement in the St. George Respiratory Questionnaire score in the treatment arm, but this was not statistically significant. The study also found a reduction in acute exacerbations of COPD with roflumilast, which was a secondary end point.1
The results of this study spurred interest in roflumilast as well as criticism of the design of the study. First, COPD patients on inhaled maintenance therapy such as an inhaled corticosteroid and long-acting beta-agonist combination or a long-acting muscarinic antagonist had their medications held during the study. Second, the average FEV1 was 54% of predicted, indicative of a study population with less severe disease.1
The RATIO trial
Taking into account the results of the RECORD trial, the RATIO trial3 in 2007 recruited patients with more severe COPD—ie, Global Initiative for Chronic Obstructive Lung Disease (GOLD) class III and IV—and included the rate of acute exacerbations as a primary end point. Maintenance therapy with inhaled corticosteroids was continued in patients already taking them. However, long-acting beta-agonists and long-acting muscarinic antagonist therapies were held.3
Again, roflumilast improved postbronchodilator FEV1 compared with placebo. A reduction in acute exacerbations was seen but was not statistically significant except in subgroup analysis, where a statistically significant reduction in acute exacerbations was noted for patients with very severe (GOLD class IV) COPD.3
Post hoc analysis from the RATIO trial suggested that patients with chronic bronchitis and patients with a history of frequent exacerbations were more likely to respond to roflumilast.2
The EOS and HELIOS trials
In 2009, the results of the EOS and HELIOS trials of roflumilast in patients with severe COPD were published.4 These trials allowed continuation of long-acting beta-agonists and muscarinic antagonists. The prebronchodilator FEV1 improved modestly when roflumilast was added to a long-acting bronchodilator. These studies ran for only 24 weeks, and the rate of acute exacerbations was not a primary end point, although the results did show a trend toward reduction of exacerbations.4
The AURA and HERMES trials
Also in 2009 was the publication of the results of two 52-week placebo-controlled trials (AURA and HERMES) of roflumilast in patients with severe COPD with chronic bronchitis and a history of frequent exacerbations.5 Maintenance therapy with long-acting beta-agonists was continued, whereas inhaled corticosteroids and long-acting muscarinic antagonists were held. Statistically significant improvements in prebronchodilator FEV1 and reduction in the rate of exacerbations were observed in the roflumilast group (17% reduction, 95% confidence interval 8–25, P < .0003).5
The REACT trial
The REACT trial6 randomized 1,945 patients with severe COPD already on maximal recommended combination inhaled corticosteroid and long-acting beta-agonist therapy to receive either roflumilast or placebo. The patients’ ratio of FEV1 to forced vital capacity was less than 70%, their postbronchodilator FEV1 was less than 50%, and they had chronic bronchitis and a history of at least two acute exacerbations during the past year. They had also been on combination therapy for the previous year. Patients who were on long-acting muscarinic-antagonist therapy (70% of the cohort) were included, and continued with their medication.
Patients were followed for 52 weeks. There was a significant reduction in the rate of exacerbations in the roflumilast group vs placebo (0.823 vs 0.959; risk ratio 0.858; 95% confidence interval 0.740–0.995; P = .0424).6 As in previous trials, the roflumilast group showed an improvement in postbronchodilator FEV1. The study also showed a reduction in hospital admissions in the treatment group.6
ADVERSE EFFECTS OF ROFLUMILAST
Roflumilast is known to have adverse effects significant enough to reduce compliance, the most common being diarrhea, weight loss, and nausea.2,6,7 In the REACT trial,6 11% of patients in the roflumilast group vs 5% in the placebo group dropped out of the study because of adverse drug effects. Diarrhea was reported in 10% and weight loss in 9% of patients taking roflumilast. Weight loss has been shown to be reversible upon stopping roflumilast.2 There has been no evidence of increased risk of death or serious adverse events in studies of roflumilast in patients with COPD.2 However, the benefit-to-harm ratio suggests that roflumilast provides a net benefit only in patients at high risk of severe exacerbations.7
- Rabe KF, Bateman ED, O’Donnell DE, Witte S, Bredenbroker D, Bethke TD. Roflumilast—an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomized controlled trial. Lancet 2005; 366:63–71.
- Field SK. Roflumilast, a novel phosphodiesterase 4 inhibitor, for COPD patients with a history of exacerbations. Clin Med Insights Circ Respir Pulm Med 2011; 5:57–70.
- Calverley PM, Sanchez-Toril F, McIvor A, Teichmann P, Bredenbroeker D, Fabbri LM. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 176:154–161.
- Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al; M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomized clinical trials. Lancet 2009; 374:695–703.
- Calverley PM, Rabe KF, Goehring U-M, Kristiansen S, Fabbri LM, Martinez FJ. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomized clinical trials. Lancet 2009; 374:684–95.
- Martinez FJ, Calverley PM, Goehring UM, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet 2015; 385:857–866.
- Yu T, Fain K, Boyd CM, et al. Benefits and harms of roflumilast in moderate to severe COPD. Thorax 2014; 69:616–622.
- Rabe KF, Bateman ED, O’Donnell DE, Witte S, Bredenbroker D, Bethke TD. Roflumilast—an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomized controlled trial. Lancet 2005; 366:63–71.
- Field SK. Roflumilast, a novel phosphodiesterase 4 inhibitor, for COPD patients with a history of exacerbations. Clin Med Insights Circ Respir Pulm Med 2011; 5:57–70.
- Calverley PM, Sanchez-Toril F, McIvor A, Teichmann P, Bredenbroeker D, Fabbri LM. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 176:154–161.
- Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al; M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomized clinical trials. Lancet 2009; 374:695–703.
- Calverley PM, Rabe KF, Goehring U-M, Kristiansen S, Fabbri LM, Martinez FJ. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomized clinical trials. Lancet 2009; 374:684–95.
- Martinez FJ, Calverley PM, Goehring UM, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet 2015; 385:857–866.
- Yu T, Fain K, Boyd CM, et al. Benefits and harms of roflumilast in moderate to severe COPD. Thorax 2014; 69:616–622.
Erythematous patches with keratotic annular borders on the glans penis
A 31-year-old man presented to the emergency department with meatal inflammation, dysuria, and mucopurulent penile discharge, diagnosed as urethritis and treated empirically with levofloxacin. He was referred to the genitourinary medicine clinic for a full screening for sexually transmitted disease. The results were negative.
Two months later, he returned with pain and redness in his left eye and inflammatory lumbar pain. The glans penis had small pustules that ruptured, leaving painless superficial erosions that coalesced to form a serpiginous pattern (Figure 1). Radiography and magnetic resonance imaging revealed features of grade 3 bilateral sacroiliitis (Figure 2): subchondral sclerosis of both sacral and iliac articular margins (predominantly on the iliac side), erosions, reduced articular space, widening of the joint space, and incipient ankylosis. A diagnosis of reactive arthritis was made based on the presence of urethritis, ocular symptoms, circinate balanitis, and radiologic evidence of sacroiliitis. In addition, the chronic inflammatory back pain and bilateral sacroiliitis indicated developing ankylosing spondylitis according to the modified New York criteria.
Our patient’s circinate balanitis resolved with hydrocortisone cream, and treatment with a nonsteroidal anti-inflammatory drug brought improvement of the joint symptoms.
THE MANY FEATURES OF REACTIVE ARTHRITIS
The American Rheumatology Association diagnostic criteria for reactive arthritis include asymmetric arthritis that lasts at least 1 month and at least one of the following symptoms: urethritis, inflammatory eye disease, mouth ulcers, circinate balanitis, and radiographic evidence of sarcoiliitis, periostitis, or heel spurs.1,2
Keratoderma blennorrhagicum is another extra-articular manifestation. These symptoms typically start within 1 to 6 weeks after urogenital infection with Chlamydia trachomatis or gastrointestinal infection with Salmonella, Shigella, Yersinia, or Campylobacter species.1–3
There is great variation in the severity, number, and timing of clinical features in reactive arthritis. The diagnosis can be difficult because only about one-third of patients show the complete classic triad (conjunctivitis, urethritis, arthritis). HLA-B27 positivity is associated with more frequent skin lesions and axial involvement.4,5
- Wu IB, Schwartz RA. Reiter’s syndrome: the classic triad and more. J Am Acad Dermatol 2008; 59:113–121.
- Koga T, Miyashita T, Watanabe T, et al. Reactive arthritis which occurred one year after acute chlamydial urethritis. Intern Med 2008; 47:663–666.
- Carter JD. Treating reactive arthritis: insights for the clinician. Ther Adv Musculoskelet Dis 2010; 2:45–54.
- Willkens RF, Arnett FC, Bitter T, et al. Reiter’s syndrome: evaluation of preliminary criteria for definite disease. Arthritis Rheum 1981; 24:844–849.
- Bakkour W, Chularojanamontri L, Motta L, Chalmers RJ. Successful use of dapsone for the management of circinate balanitis. Clin Exp Dermatol 2014; 39:333–335.
A 31-year-old man presented to the emergency department with meatal inflammation, dysuria, and mucopurulent penile discharge, diagnosed as urethritis and treated empirically with levofloxacin. He was referred to the genitourinary medicine clinic for a full screening for sexually transmitted disease. The results were negative.
Two months later, he returned with pain and redness in his left eye and inflammatory lumbar pain. The glans penis had small pustules that ruptured, leaving painless superficial erosions that coalesced to form a serpiginous pattern (Figure 1). Radiography and magnetic resonance imaging revealed features of grade 3 bilateral sacroiliitis (Figure 2): subchondral sclerosis of both sacral and iliac articular margins (predominantly on the iliac side), erosions, reduced articular space, widening of the joint space, and incipient ankylosis. A diagnosis of reactive arthritis was made based on the presence of urethritis, ocular symptoms, circinate balanitis, and radiologic evidence of sacroiliitis. In addition, the chronic inflammatory back pain and bilateral sacroiliitis indicated developing ankylosing spondylitis according to the modified New York criteria.
Our patient’s circinate balanitis resolved with hydrocortisone cream, and treatment with a nonsteroidal anti-inflammatory drug brought improvement of the joint symptoms.
THE MANY FEATURES OF REACTIVE ARTHRITIS
The American Rheumatology Association diagnostic criteria for reactive arthritis include asymmetric arthritis that lasts at least 1 month and at least one of the following symptoms: urethritis, inflammatory eye disease, mouth ulcers, circinate balanitis, and radiographic evidence of sarcoiliitis, periostitis, or heel spurs.1,2
Keratoderma blennorrhagicum is another extra-articular manifestation. These symptoms typically start within 1 to 6 weeks after urogenital infection with Chlamydia trachomatis or gastrointestinal infection with Salmonella, Shigella, Yersinia, or Campylobacter species.1–3
There is great variation in the severity, number, and timing of clinical features in reactive arthritis. The diagnosis can be difficult because only about one-third of patients show the complete classic triad (conjunctivitis, urethritis, arthritis). HLA-B27 positivity is associated with more frequent skin lesions and axial involvement.4,5
A 31-year-old man presented to the emergency department with meatal inflammation, dysuria, and mucopurulent penile discharge, diagnosed as urethritis and treated empirically with levofloxacin. He was referred to the genitourinary medicine clinic for a full screening for sexually transmitted disease. The results were negative.
Two months later, he returned with pain and redness in his left eye and inflammatory lumbar pain. The glans penis had small pustules that ruptured, leaving painless superficial erosions that coalesced to form a serpiginous pattern (Figure 1). Radiography and magnetic resonance imaging revealed features of grade 3 bilateral sacroiliitis (Figure 2): subchondral sclerosis of both sacral and iliac articular margins (predominantly on the iliac side), erosions, reduced articular space, widening of the joint space, and incipient ankylosis. A diagnosis of reactive arthritis was made based on the presence of urethritis, ocular symptoms, circinate balanitis, and radiologic evidence of sacroiliitis. In addition, the chronic inflammatory back pain and bilateral sacroiliitis indicated developing ankylosing spondylitis according to the modified New York criteria.
Our patient’s circinate balanitis resolved with hydrocortisone cream, and treatment with a nonsteroidal anti-inflammatory drug brought improvement of the joint symptoms.
THE MANY FEATURES OF REACTIVE ARTHRITIS
The American Rheumatology Association diagnostic criteria for reactive arthritis include asymmetric arthritis that lasts at least 1 month and at least one of the following symptoms: urethritis, inflammatory eye disease, mouth ulcers, circinate balanitis, and radiographic evidence of sarcoiliitis, periostitis, or heel spurs.1,2
Keratoderma blennorrhagicum is another extra-articular manifestation. These symptoms typically start within 1 to 6 weeks after urogenital infection with Chlamydia trachomatis or gastrointestinal infection with Salmonella, Shigella, Yersinia, or Campylobacter species.1–3
There is great variation in the severity, number, and timing of clinical features in reactive arthritis. The diagnosis can be difficult because only about one-third of patients show the complete classic triad (conjunctivitis, urethritis, arthritis). HLA-B27 positivity is associated with more frequent skin lesions and axial involvement.4,5
- Wu IB, Schwartz RA. Reiter’s syndrome: the classic triad and more. J Am Acad Dermatol 2008; 59:113–121.
- Koga T, Miyashita T, Watanabe T, et al. Reactive arthritis which occurred one year after acute chlamydial urethritis. Intern Med 2008; 47:663–666.
- Carter JD. Treating reactive arthritis: insights for the clinician. Ther Adv Musculoskelet Dis 2010; 2:45–54.
- Willkens RF, Arnett FC, Bitter T, et al. Reiter’s syndrome: evaluation of preliminary criteria for definite disease. Arthritis Rheum 1981; 24:844–849.
- Bakkour W, Chularojanamontri L, Motta L, Chalmers RJ. Successful use of dapsone for the management of circinate balanitis. Clin Exp Dermatol 2014; 39:333–335.
- Wu IB, Schwartz RA. Reiter’s syndrome: the classic triad and more. J Am Acad Dermatol 2008; 59:113–121.
- Koga T, Miyashita T, Watanabe T, et al. Reactive arthritis which occurred one year after acute chlamydial urethritis. Intern Med 2008; 47:663–666.
- Carter JD. Treating reactive arthritis: insights for the clinician. Ther Adv Musculoskelet Dis 2010; 2:45–54.
- Willkens RF, Arnett FC, Bitter T, et al. Reiter’s syndrome: evaluation of preliminary criteria for definite disease. Arthritis Rheum 1981; 24:844–849.
- Bakkour W, Chularojanamontri L, Motta L, Chalmers RJ. Successful use of dapsone for the management of circinate balanitis. Clin Exp Dermatol 2014; 39:333–335.
Lymphoplasmacytic lymphoma presenting as retinal hemorrhage
A 51-year-old woman with no significant medical history presented to her primary care physician because of blurred vision, increased fatigue, palpitations, and intermittent episodes of epistaxis. Vital signs were within normal limits except for a heart rate of 110 beats per minute. Physical examination revealed pale conjunctivae and bilateral white-centered retinal hemorrhages and microaneurysms
(Figures 1–4).
The results of laboratory studies:
- Hemoglobin 2.4 g/dL (reference range 12–16)
- Platelet count 78 × 109/L (150–400)
- White blood cell count 4.0 × 109/L (3.7–11.0)
- Atypical lymphocytes 18% (0.0–3.0%)
- Reticulocyte index 0.3 (0.5–2.5%)
- Gamma gap 7.0 g/dL (< 4)
- Immunoglobulin A (IgA) 5,560 mg/dL (61–356).
Bone marrow biopsy study showed complete effacement of the hematopoietic elements of normal marrow, and the diagnosis of B cell lymphoplasmacytic lymphoma was made. Serum electrophoresis showed an elevated kappa-to-lambda ratio of 4.6500 (reference range 0.2600–1.6500). B cells expressed monotypic kappa surface immunoglobulin light chains CD19, CD20, and CD22. They did not express CD5. No testing was done for the MYD88 point mutation.
ROTH SPOTS
White-centered retinal hemorrhages, or Roth spots, are the result of rupture of retinal vessels with subsequent accumulation of platelets and fibrin surrounded by blood.1 Although Roth spots are mistakenly believed to be caused only by infective endocarditis, they are seen in a variety of conditions, including leukemia, anemia, thrombocytopenia, and hypoxia. Each of these conditions has a different mechanism for vessel rupture, which can include fragility of the smooth muscle vessel wall from hypoxemia and increased hydrostatic pressure in hyperviscosity syndrome.
Hypergammaglobulinemia is the most common cause of hyperviscosity syndrome and is usually associated with Waldenström macroglobulinemia, a type of lymphoplasmacytic lymphoma associated with the largest immunoglobulin, IgM. However, our patient presented with a variant of Waldenström macroglobulinemia with high levels of IgA, a small molecule that in high quantities can also cause hyperviscosity.
Immediate treatment is aimed at decreasing blood viscosity with plasmapheresis and controlling the underlying disease with chemotherapy.2 There have been cases of cancer-related Roth spots, in which the lesions disappeared after chemotherapy.3
DIFFERENTIAL DIAGNOSIS OF ROTH SPOTS
Roth spots share morphologic features with other retinal abnormalities such as “cotton wool” spots, Purtscher retinopathy, and cytomegalovirus retinitis. A thorough history and careful ophthalmologic examination can help differentiate these from Roth spots.
Cotton wool spots are a nonspecific sign of vascular insufficiency and represent an ischemic, inflammatory, or infectious condition or an embolic or neoplastic process. Most often, they represent retinopathy from poorly controlled diabetes mellitus or hypertension. On funduscopic examination, cotton wool spots are small, irregularly shaped, yellow-white plaques that may appear raised. They are often found over the posterior pole of the fundus. In contrast, Roth spots appear white or pale on a background of hemorrhage.
Purtscher retinopathy. Polygonal retinal whitening with sharp demarcation against the normal retina is pathognomonic of Purtscher retinopathy.4 Purtscher lesions may represent a traumatic or nontraumatic condition, including closed head injury, barotrauma, pancreatic disorder, connective tissue disease, fat embolism after long bone fracture, and even microangiopathic hemolytic anemia. Patients usually describe diminished visual acuity in the context of injury or other systemic illness.
Cytomegalovirus retinitis is an important consideration in the immunocompromised patient presenting with retinal hemorrhage and is the most common cause of blindness in acquired immunodeficiency syndrome (AIDS).5 It is a slowly progressive disease, initially asymptomatic and involving one eye, but in some untreated patients with AIDS it may progress to the contralateral eye. When it progresses, patients usually present with floaters or vision loss. The retina can have cotton wool spots with a more diffuse pattern of hemorrhage on the periphery and white sheathing along blood vessels, eloquently described as “frosted branch angiitis.”
Our patient’s lesions appeared more punctate, in contrast to the often fulminant hemorrhagic necrosis or perivascular white lesions surrounding retinal vessels characteristic of cytomegalovirus retinitis.
THE NEED FOR ACTION
New-onset blurred vision should prompt a comprehensive history and physical examination, as it may be secondary to a life-threatening systemic disease. A thorough funduscopic examination may provide vital information and guide management and expeditious referrals for patients with time-sensitive conditions such as cancer.
Our patient decided to undergo treatment outside our hospital and so was lost to follow-up.
- Ling R, James B. White-centred retinal haemorrhages (Roth spots). Postgrad Med J 1998; 74:581–582.
- Mehta J, Singhal S. Hyperviscosity syndrome in plasma cell dyscrasias. Semin Thromb Hemost 2003; 29:467–471.
- Docherty SM, Reza M, Turner G, Bowles K. Resolution of Roth spots in chronic myeloid leukaemia after treatment with imatinib. Br J Haematol 2015; 170:744.
- Miguel AI, Henriques F, Azevedo LF, Loureiro AJ, Maberley DA. Systematic review of Purtscher’s and Purtscher-like retinopathies. Eye (Lond) 2013; 27:1–13.
- Kestelyn PG, Cunningham ET Jr. HIV/AIDS and blindness. Bull World Health Organ 2001; 79:208–213.
A 51-year-old woman with no significant medical history presented to her primary care physician because of blurred vision, increased fatigue, palpitations, and intermittent episodes of epistaxis. Vital signs were within normal limits except for a heart rate of 110 beats per minute. Physical examination revealed pale conjunctivae and bilateral white-centered retinal hemorrhages and microaneurysms
(Figures 1–4).
The results of laboratory studies:
- Hemoglobin 2.4 g/dL (reference range 12–16)
- Platelet count 78 × 109/L (150–400)
- White blood cell count 4.0 × 109/L (3.7–11.0)
- Atypical lymphocytes 18% (0.0–3.0%)
- Reticulocyte index 0.3 (0.5–2.5%)
- Gamma gap 7.0 g/dL (< 4)
- Immunoglobulin A (IgA) 5,560 mg/dL (61–356).
Bone marrow biopsy study showed complete effacement of the hematopoietic elements of normal marrow, and the diagnosis of B cell lymphoplasmacytic lymphoma was made. Serum electrophoresis showed an elevated kappa-to-lambda ratio of 4.6500 (reference range 0.2600–1.6500). B cells expressed monotypic kappa surface immunoglobulin light chains CD19, CD20, and CD22. They did not express CD5. No testing was done for the MYD88 point mutation.
ROTH SPOTS
White-centered retinal hemorrhages, or Roth spots, are the result of rupture of retinal vessels with subsequent accumulation of platelets and fibrin surrounded by blood.1 Although Roth spots are mistakenly believed to be caused only by infective endocarditis, they are seen in a variety of conditions, including leukemia, anemia, thrombocytopenia, and hypoxia. Each of these conditions has a different mechanism for vessel rupture, which can include fragility of the smooth muscle vessel wall from hypoxemia and increased hydrostatic pressure in hyperviscosity syndrome.
Hypergammaglobulinemia is the most common cause of hyperviscosity syndrome and is usually associated with Waldenström macroglobulinemia, a type of lymphoplasmacytic lymphoma associated with the largest immunoglobulin, IgM. However, our patient presented with a variant of Waldenström macroglobulinemia with high levels of IgA, a small molecule that in high quantities can also cause hyperviscosity.
Immediate treatment is aimed at decreasing blood viscosity with plasmapheresis and controlling the underlying disease with chemotherapy.2 There have been cases of cancer-related Roth spots, in which the lesions disappeared after chemotherapy.3
DIFFERENTIAL DIAGNOSIS OF ROTH SPOTS
Roth spots share morphologic features with other retinal abnormalities such as “cotton wool” spots, Purtscher retinopathy, and cytomegalovirus retinitis. A thorough history and careful ophthalmologic examination can help differentiate these from Roth spots.
Cotton wool spots are a nonspecific sign of vascular insufficiency and represent an ischemic, inflammatory, or infectious condition or an embolic or neoplastic process. Most often, they represent retinopathy from poorly controlled diabetes mellitus or hypertension. On funduscopic examination, cotton wool spots are small, irregularly shaped, yellow-white plaques that may appear raised. They are often found over the posterior pole of the fundus. In contrast, Roth spots appear white or pale on a background of hemorrhage.
Purtscher retinopathy. Polygonal retinal whitening with sharp demarcation against the normal retina is pathognomonic of Purtscher retinopathy.4 Purtscher lesions may represent a traumatic or nontraumatic condition, including closed head injury, barotrauma, pancreatic disorder, connective tissue disease, fat embolism after long bone fracture, and even microangiopathic hemolytic anemia. Patients usually describe diminished visual acuity in the context of injury or other systemic illness.
Cytomegalovirus retinitis is an important consideration in the immunocompromised patient presenting with retinal hemorrhage and is the most common cause of blindness in acquired immunodeficiency syndrome (AIDS).5 It is a slowly progressive disease, initially asymptomatic and involving one eye, but in some untreated patients with AIDS it may progress to the contralateral eye. When it progresses, patients usually present with floaters or vision loss. The retina can have cotton wool spots with a more diffuse pattern of hemorrhage on the periphery and white sheathing along blood vessels, eloquently described as “frosted branch angiitis.”
Our patient’s lesions appeared more punctate, in contrast to the often fulminant hemorrhagic necrosis or perivascular white lesions surrounding retinal vessels characteristic of cytomegalovirus retinitis.
THE NEED FOR ACTION
New-onset blurred vision should prompt a comprehensive history and physical examination, as it may be secondary to a life-threatening systemic disease. A thorough funduscopic examination may provide vital information and guide management and expeditious referrals for patients with time-sensitive conditions such as cancer.
Our patient decided to undergo treatment outside our hospital and so was lost to follow-up.
A 51-year-old woman with no significant medical history presented to her primary care physician because of blurred vision, increased fatigue, palpitations, and intermittent episodes of epistaxis. Vital signs were within normal limits except for a heart rate of 110 beats per minute. Physical examination revealed pale conjunctivae and bilateral white-centered retinal hemorrhages and microaneurysms
(Figures 1–4).
The results of laboratory studies:
- Hemoglobin 2.4 g/dL (reference range 12–16)
- Platelet count 78 × 109/L (150–400)
- White blood cell count 4.0 × 109/L (3.7–11.0)
- Atypical lymphocytes 18% (0.0–3.0%)
- Reticulocyte index 0.3 (0.5–2.5%)
- Gamma gap 7.0 g/dL (< 4)
- Immunoglobulin A (IgA) 5,560 mg/dL (61–356).
Bone marrow biopsy study showed complete effacement of the hematopoietic elements of normal marrow, and the diagnosis of B cell lymphoplasmacytic lymphoma was made. Serum electrophoresis showed an elevated kappa-to-lambda ratio of 4.6500 (reference range 0.2600–1.6500). B cells expressed monotypic kappa surface immunoglobulin light chains CD19, CD20, and CD22. They did not express CD5. No testing was done for the MYD88 point mutation.
ROTH SPOTS
White-centered retinal hemorrhages, or Roth spots, are the result of rupture of retinal vessels with subsequent accumulation of platelets and fibrin surrounded by blood.1 Although Roth spots are mistakenly believed to be caused only by infective endocarditis, they are seen in a variety of conditions, including leukemia, anemia, thrombocytopenia, and hypoxia. Each of these conditions has a different mechanism for vessel rupture, which can include fragility of the smooth muscle vessel wall from hypoxemia and increased hydrostatic pressure in hyperviscosity syndrome.
Hypergammaglobulinemia is the most common cause of hyperviscosity syndrome and is usually associated with Waldenström macroglobulinemia, a type of lymphoplasmacytic lymphoma associated with the largest immunoglobulin, IgM. However, our patient presented with a variant of Waldenström macroglobulinemia with high levels of IgA, a small molecule that in high quantities can also cause hyperviscosity.
Immediate treatment is aimed at decreasing blood viscosity with plasmapheresis and controlling the underlying disease with chemotherapy.2 There have been cases of cancer-related Roth spots, in which the lesions disappeared after chemotherapy.3
DIFFERENTIAL DIAGNOSIS OF ROTH SPOTS
Roth spots share morphologic features with other retinal abnormalities such as “cotton wool” spots, Purtscher retinopathy, and cytomegalovirus retinitis. A thorough history and careful ophthalmologic examination can help differentiate these from Roth spots.
Cotton wool spots are a nonspecific sign of vascular insufficiency and represent an ischemic, inflammatory, or infectious condition or an embolic or neoplastic process. Most often, they represent retinopathy from poorly controlled diabetes mellitus or hypertension. On funduscopic examination, cotton wool spots are small, irregularly shaped, yellow-white plaques that may appear raised. They are often found over the posterior pole of the fundus. In contrast, Roth spots appear white or pale on a background of hemorrhage.
Purtscher retinopathy. Polygonal retinal whitening with sharp demarcation against the normal retina is pathognomonic of Purtscher retinopathy.4 Purtscher lesions may represent a traumatic or nontraumatic condition, including closed head injury, barotrauma, pancreatic disorder, connective tissue disease, fat embolism after long bone fracture, and even microangiopathic hemolytic anemia. Patients usually describe diminished visual acuity in the context of injury or other systemic illness.
Cytomegalovirus retinitis is an important consideration in the immunocompromised patient presenting with retinal hemorrhage and is the most common cause of blindness in acquired immunodeficiency syndrome (AIDS).5 It is a slowly progressive disease, initially asymptomatic and involving one eye, but in some untreated patients with AIDS it may progress to the contralateral eye. When it progresses, patients usually present with floaters or vision loss. The retina can have cotton wool spots with a more diffuse pattern of hemorrhage on the periphery and white sheathing along blood vessels, eloquently described as “frosted branch angiitis.”
Our patient’s lesions appeared more punctate, in contrast to the often fulminant hemorrhagic necrosis or perivascular white lesions surrounding retinal vessels characteristic of cytomegalovirus retinitis.
THE NEED FOR ACTION
New-onset blurred vision should prompt a comprehensive history and physical examination, as it may be secondary to a life-threatening systemic disease. A thorough funduscopic examination may provide vital information and guide management and expeditious referrals for patients with time-sensitive conditions such as cancer.
Our patient decided to undergo treatment outside our hospital and so was lost to follow-up.
- Ling R, James B. White-centred retinal haemorrhages (Roth spots). Postgrad Med J 1998; 74:581–582.
- Mehta J, Singhal S. Hyperviscosity syndrome in plasma cell dyscrasias. Semin Thromb Hemost 2003; 29:467–471.
- Docherty SM, Reza M, Turner G, Bowles K. Resolution of Roth spots in chronic myeloid leukaemia after treatment with imatinib. Br J Haematol 2015; 170:744.
- Miguel AI, Henriques F, Azevedo LF, Loureiro AJ, Maberley DA. Systematic review of Purtscher’s and Purtscher-like retinopathies. Eye (Lond) 2013; 27:1–13.
- Kestelyn PG, Cunningham ET Jr. HIV/AIDS and blindness. Bull World Health Organ 2001; 79:208–213.
- Ling R, James B. White-centred retinal haemorrhages (Roth spots). Postgrad Med J 1998; 74:581–582.
- Mehta J, Singhal S. Hyperviscosity syndrome in plasma cell dyscrasias. Semin Thromb Hemost 2003; 29:467–471.
- Docherty SM, Reza M, Turner G, Bowles K. Resolution of Roth spots in chronic myeloid leukaemia after treatment with imatinib. Br J Haematol 2015; 170:744.
- Miguel AI, Henriques F, Azevedo LF, Loureiro AJ, Maberley DA. Systematic review of Purtscher’s and Purtscher-like retinopathies. Eye (Lond) 2013; 27:1–13.
- Kestelyn PG, Cunningham ET Jr. HIV/AIDS and blindness. Bull World Health Organ 2001; 79:208–213.