User login
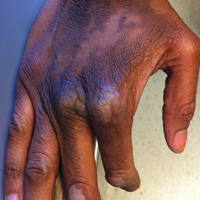
Clinicians Should Retain the Ability to Choose a Pathologist
As employers search for ways to reduce the cost of providing health care to their employees, there is a growing trend toward narrowed provider networks and exclusive laboratory contracts. In the case of clinical pathology, some of these choices make sense from the employer’s perspective. A complete blood cell count or comprehensive metabolic panel is done on a machine and the result is much the same regardless of the laboratory. So why not have all laboratory tests performed by the lowest bidder?
Laboratories vary in quality and anatomic pathology services are different from blood tests. Each slide must be interpreted by a physician and skill in the interpretation of skin specimens varies widely. Dermatopathology was one of the first subspecialties to be recognized within pathology, as it requires a high level of expertise. Clinicopathological correlation often is key to the accurate interpretation of a specimen. The stakes are high, and a delay in diagnosis of melanoma remains one of the most serious errors in medicine and one of the most common causes for litigation in dermatology.
The accurate interpretation of skin biopsy specimens becomes especially difficult when inadequate or misleading clinical information accompanies the specimen. A study of 589 biopsies submitted by primary care physicians and reported by general pathologists demonstrated a 6.5% error rate. False-negative errors were the most common, but false-positives also were observed.1 A study of pigmented lesions referred to the University of California, San Francisco, demonstrated a discordance rate of 14.3%.2 The degree of discordance would be expected to vary based on the range of diagnoses included in each study.
Board-certified dermatopathologists have varying areas of expertise and there is notable subjectivity in the interpretation of biopsy specimens. In the case of problematic pigmented lesions such as atypical Spitz nevi, there can be low interobserver agreement even among the experts in categorizing lesions as malignant versus nonmalignant (κ=0.30).3 The low concordance among expert dermatopathologists demonstrates that light microscopic features alone often are inadequate for diagnosis. Advanced studies, including immunohistochemical stains, can help to clarify the diagnosis. In the case of atypical Spitz tumors, the contribution of special stains to the final diagnosis is statistically similar to that of hematoxylin and eosin sections and age, suggesting that nothing alone is sufficiently reliable to establish a definitive diagnosis in every case.4 Although helpful, these studies are costly, and savings obtained by sending cases to the lowest bidder can evaporate quickly. Costs are higher when factoring in molecular studies, which can run upwards of $3000 per slide; the cost of litigation related to incorrect diagnoses; or the human costs of an incorrect diagnosis.
As a group, dermatopathologists are highly skilled in the interpretation of skin specimens, but challenging lesions are common in the routine practice of dermatopathology. A study of 1249 pigmented melanocytic lesions demonstrated substantial agreement among expert dermatopathologists for less problematic lesions, though agreement was greater for patients 40 years and older (κ=0.67) than for younger patients (κ=0.49). Agreement was lower for patients with atypical mole syndrome (κ=0.31).5 These discrepancies occur despite the fact that there is good interobserver reproducibility for grading of individual histological features such as asymmetry, circumscription, irregular confluent nests, single melanocytes predominating, absence of maturation, suprabasal melanocytes, symmetrical melanin, deep melanin, cytological atypia, mitoses, dermal lymphocytic infiltrate, and necrosis.6 These results indicate that accurate diagnoses cannot be reliably established simply by grading a list of histological features. Accurate diagnosis requires complex pattern recognition and integration of findings. Conflicting criteria often are present and an accurate interpretation requires considerable judgment as to which features are significant and which are not.
Separation of sebaceous adenoma, sebaceoma, and well-differentiated sebaceous carcinoma is another challenging area, and interobserver consensus can be as low as 11%,7 which suggests notable subjectivity in the criteria for diagnosis of nonmelanocytic tumors and emphasizes the importance of communication between the dermatopathologist and clinician when determining how to manage an ambiguous lesion. The interpretation of inflammatory skin diseases, alopecia, and lymphoid proliferations also can be problematic, and expert consultation often is required.
All dermatologists receive substantial training in dermatopathology, which puts them in an excellent position to interpret ambiguous findings in the context of the clinical presentation. Sometimes the dermatologist who has seen the clinical presentation can be in the best position to make the diagnosis. All clinicians must be wary of bias and an objective set of eyes often can be helpful. Communication is crucial to ensure appropriate care for each patient, and policies that restrict the choice of pathologist can be damaging.
- Trotter MJ, Bruecks AK. Interpretation of skin biopsies by general pathologists: diagnostic discrepancy rate measured by blinded review. Arch Pathol Lab Med. 2003;127:1489-1492.
- Shoo BA, Sagebiel RW, Kashani-Sabet M. Discordance in the histopathologic diagnosis of melanoma at a melanoma referral center [published online March 19, 2010]. J Am Acad Dermatol. 2010;62:751-756.
- Gerami P, Busam K, Cochran A, et al. Histomorphologic assessment and interobserver diagnostic reproducibility of atypical spitzoid melanocytic neoplasms with long-term follow-up. Am J Surg Pathol. 2014;38:934-940.
- Puri PK, Ferringer TC, Tyler WB, et al. Statistical analysis of the concordance of immunohistochemical stains with the final diagnosis in spitzoid neoplasms. Am J Dermatopathol. 2011;33:72-77.
- Braun RP, Gutkowicz-Krusin D, Rabinovitz H, et al. Agreement of dermatopathologists in the evaluation of clinically difficult melanocytic lesions: how golden is the ‘gold standard’? Dermatology. 2012;224:51-58.
- Urso C, Rongioletti F, Innocenzi D, et al. Interobserver reproducibility of histological features in cutaneous malignant melanoma. J Clin Pathol. 2005;58:1194-1198.
- Harvey NT, Budgeon CA, Leecy T, et al. Interobserver variability in the diagnosis of circumscribed sebaceous neoplasms of the skin. Pathology. 2013;45:581-586.
As employers search for ways to reduce the cost of providing health care to their employees, there is a growing trend toward narrowed provider networks and exclusive laboratory contracts. In the case of clinical pathology, some of these choices make sense from the employer’s perspective. A complete blood cell count or comprehensive metabolic panel is done on a machine and the result is much the same regardless of the laboratory. So why not have all laboratory tests performed by the lowest bidder?
Laboratories vary in quality and anatomic pathology services are different from blood tests. Each slide must be interpreted by a physician and skill in the interpretation of skin specimens varies widely. Dermatopathology was one of the first subspecialties to be recognized within pathology, as it requires a high level of expertise. Clinicopathological correlation often is key to the accurate interpretation of a specimen. The stakes are high, and a delay in diagnosis of melanoma remains one of the most serious errors in medicine and one of the most common causes for litigation in dermatology.
The accurate interpretation of skin biopsy specimens becomes especially difficult when inadequate or misleading clinical information accompanies the specimen. A study of 589 biopsies submitted by primary care physicians and reported by general pathologists demonstrated a 6.5% error rate. False-negative errors were the most common, but false-positives also were observed.1 A study of pigmented lesions referred to the University of California, San Francisco, demonstrated a discordance rate of 14.3%.2 The degree of discordance would be expected to vary based on the range of diagnoses included in each study.
Board-certified dermatopathologists have varying areas of expertise and there is notable subjectivity in the interpretation of biopsy specimens. In the case of problematic pigmented lesions such as atypical Spitz nevi, there can be low interobserver agreement even among the experts in categorizing lesions as malignant versus nonmalignant (κ=0.30).3 The low concordance among expert dermatopathologists demonstrates that light microscopic features alone often are inadequate for diagnosis. Advanced studies, including immunohistochemical stains, can help to clarify the diagnosis. In the case of atypical Spitz tumors, the contribution of special stains to the final diagnosis is statistically similar to that of hematoxylin and eosin sections and age, suggesting that nothing alone is sufficiently reliable to establish a definitive diagnosis in every case.4 Although helpful, these studies are costly, and savings obtained by sending cases to the lowest bidder can evaporate quickly. Costs are higher when factoring in molecular studies, which can run upwards of $3000 per slide; the cost of litigation related to incorrect diagnoses; or the human costs of an incorrect diagnosis.
As a group, dermatopathologists are highly skilled in the interpretation of skin specimens, but challenging lesions are common in the routine practice of dermatopathology. A study of 1249 pigmented melanocytic lesions demonstrated substantial agreement among expert dermatopathologists for less problematic lesions, though agreement was greater for patients 40 years and older (κ=0.67) than for younger patients (κ=0.49). Agreement was lower for patients with atypical mole syndrome (κ=0.31).5 These discrepancies occur despite the fact that there is good interobserver reproducibility for grading of individual histological features such as asymmetry, circumscription, irregular confluent nests, single melanocytes predominating, absence of maturation, suprabasal melanocytes, symmetrical melanin, deep melanin, cytological atypia, mitoses, dermal lymphocytic infiltrate, and necrosis.6 These results indicate that accurate diagnoses cannot be reliably established simply by grading a list of histological features. Accurate diagnosis requires complex pattern recognition and integration of findings. Conflicting criteria often are present and an accurate interpretation requires considerable judgment as to which features are significant and which are not.
Separation of sebaceous adenoma, sebaceoma, and well-differentiated sebaceous carcinoma is another challenging area, and interobserver consensus can be as low as 11%,7 which suggests notable subjectivity in the criteria for diagnosis of nonmelanocytic tumors and emphasizes the importance of communication between the dermatopathologist and clinician when determining how to manage an ambiguous lesion. The interpretation of inflammatory skin diseases, alopecia, and lymphoid proliferations also can be problematic, and expert consultation often is required.
All dermatologists receive substantial training in dermatopathology, which puts them in an excellent position to interpret ambiguous findings in the context of the clinical presentation. Sometimes the dermatologist who has seen the clinical presentation can be in the best position to make the diagnosis. All clinicians must be wary of bias and an objective set of eyes often can be helpful. Communication is crucial to ensure appropriate care for each patient, and policies that restrict the choice of pathologist can be damaging.
As employers search for ways to reduce the cost of providing health care to their employees, there is a growing trend toward narrowed provider networks and exclusive laboratory contracts. In the case of clinical pathology, some of these choices make sense from the employer’s perspective. A complete blood cell count or comprehensive metabolic panel is done on a machine and the result is much the same regardless of the laboratory. So why not have all laboratory tests performed by the lowest bidder?
Laboratories vary in quality and anatomic pathology services are different from blood tests. Each slide must be interpreted by a physician and skill in the interpretation of skin specimens varies widely. Dermatopathology was one of the first subspecialties to be recognized within pathology, as it requires a high level of expertise. Clinicopathological correlation often is key to the accurate interpretation of a specimen. The stakes are high, and a delay in diagnosis of melanoma remains one of the most serious errors in medicine and one of the most common causes for litigation in dermatology.
The accurate interpretation of skin biopsy specimens becomes especially difficult when inadequate or misleading clinical information accompanies the specimen. A study of 589 biopsies submitted by primary care physicians and reported by general pathologists demonstrated a 6.5% error rate. False-negative errors were the most common, but false-positives also were observed.1 A study of pigmented lesions referred to the University of California, San Francisco, demonstrated a discordance rate of 14.3%.2 The degree of discordance would be expected to vary based on the range of diagnoses included in each study.
Board-certified dermatopathologists have varying areas of expertise and there is notable subjectivity in the interpretation of biopsy specimens. In the case of problematic pigmented lesions such as atypical Spitz nevi, there can be low interobserver agreement even among the experts in categorizing lesions as malignant versus nonmalignant (κ=0.30).3 The low concordance among expert dermatopathologists demonstrates that light microscopic features alone often are inadequate for diagnosis. Advanced studies, including immunohistochemical stains, can help to clarify the diagnosis. In the case of atypical Spitz tumors, the contribution of special stains to the final diagnosis is statistically similar to that of hematoxylin and eosin sections and age, suggesting that nothing alone is sufficiently reliable to establish a definitive diagnosis in every case.4 Although helpful, these studies are costly, and savings obtained by sending cases to the lowest bidder can evaporate quickly. Costs are higher when factoring in molecular studies, which can run upwards of $3000 per slide; the cost of litigation related to incorrect diagnoses; or the human costs of an incorrect diagnosis.
As a group, dermatopathologists are highly skilled in the interpretation of skin specimens, but challenging lesions are common in the routine practice of dermatopathology. A study of 1249 pigmented melanocytic lesions demonstrated substantial agreement among expert dermatopathologists for less problematic lesions, though agreement was greater for patients 40 years and older (κ=0.67) than for younger patients (κ=0.49). Agreement was lower for patients with atypical mole syndrome (κ=0.31).5 These discrepancies occur despite the fact that there is good interobserver reproducibility for grading of individual histological features such as asymmetry, circumscription, irregular confluent nests, single melanocytes predominating, absence of maturation, suprabasal melanocytes, symmetrical melanin, deep melanin, cytological atypia, mitoses, dermal lymphocytic infiltrate, and necrosis.6 These results indicate that accurate diagnoses cannot be reliably established simply by grading a list of histological features. Accurate diagnosis requires complex pattern recognition and integration of findings. Conflicting criteria often are present and an accurate interpretation requires considerable judgment as to which features are significant and which are not.
Separation of sebaceous adenoma, sebaceoma, and well-differentiated sebaceous carcinoma is another challenging area, and interobserver consensus can be as low as 11%,7 which suggests notable subjectivity in the criteria for diagnosis of nonmelanocytic tumors and emphasizes the importance of communication between the dermatopathologist and clinician when determining how to manage an ambiguous lesion. The interpretation of inflammatory skin diseases, alopecia, and lymphoid proliferations also can be problematic, and expert consultation often is required.
All dermatologists receive substantial training in dermatopathology, which puts them in an excellent position to interpret ambiguous findings in the context of the clinical presentation. Sometimes the dermatologist who has seen the clinical presentation can be in the best position to make the diagnosis. All clinicians must be wary of bias and an objective set of eyes often can be helpful. Communication is crucial to ensure appropriate care for each patient, and policies that restrict the choice of pathologist can be damaging.
- Trotter MJ, Bruecks AK. Interpretation of skin biopsies by general pathologists: diagnostic discrepancy rate measured by blinded review. Arch Pathol Lab Med. 2003;127:1489-1492.
- Shoo BA, Sagebiel RW, Kashani-Sabet M. Discordance in the histopathologic diagnosis of melanoma at a melanoma referral center [published online March 19, 2010]. J Am Acad Dermatol. 2010;62:751-756.
- Gerami P, Busam K, Cochran A, et al. Histomorphologic assessment and interobserver diagnostic reproducibility of atypical spitzoid melanocytic neoplasms with long-term follow-up. Am J Surg Pathol. 2014;38:934-940.
- Puri PK, Ferringer TC, Tyler WB, et al. Statistical analysis of the concordance of immunohistochemical stains with the final diagnosis in spitzoid neoplasms. Am J Dermatopathol. 2011;33:72-77.
- Braun RP, Gutkowicz-Krusin D, Rabinovitz H, et al. Agreement of dermatopathologists in the evaluation of clinically difficult melanocytic lesions: how golden is the ‘gold standard’? Dermatology. 2012;224:51-58.
- Urso C, Rongioletti F, Innocenzi D, et al. Interobserver reproducibility of histological features in cutaneous malignant melanoma. J Clin Pathol. 2005;58:1194-1198.
- Harvey NT, Budgeon CA, Leecy T, et al. Interobserver variability in the diagnosis of circumscribed sebaceous neoplasms of the skin. Pathology. 2013;45:581-586.
- Trotter MJ, Bruecks AK. Interpretation of skin biopsies by general pathologists: diagnostic discrepancy rate measured by blinded review. Arch Pathol Lab Med. 2003;127:1489-1492.
- Shoo BA, Sagebiel RW, Kashani-Sabet M. Discordance in the histopathologic diagnosis of melanoma at a melanoma referral center [published online March 19, 2010]. J Am Acad Dermatol. 2010;62:751-756.
- Gerami P, Busam K, Cochran A, et al. Histomorphologic assessment and interobserver diagnostic reproducibility of atypical spitzoid melanocytic neoplasms with long-term follow-up. Am J Surg Pathol. 2014;38:934-940.
- Puri PK, Ferringer TC, Tyler WB, et al. Statistical analysis of the concordance of immunohistochemical stains with the final diagnosis in spitzoid neoplasms. Am J Dermatopathol. 2011;33:72-77.
- Braun RP, Gutkowicz-Krusin D, Rabinovitz H, et al. Agreement of dermatopathologists in the evaluation of clinically difficult melanocytic lesions: how golden is the ‘gold standard’? Dermatology. 2012;224:51-58.
- Urso C, Rongioletti F, Innocenzi D, et al. Interobserver reproducibility of histological features in cutaneous malignant melanoma. J Clin Pathol. 2005;58:1194-1198.
- Harvey NT, Budgeon CA, Leecy T, et al. Interobserver variability in the diagnosis of circumscribed sebaceous neoplasms of the skin. Pathology. 2013;45:581-586.
French psychiatrist condemned for society’s deficiency
On Dec. 14, 2016, a French psychiatrist was sentenced to an 18-month suspended prison sentence. Lekhraj Gujadhur, MD, was the supervisor of unit 101 at the Psychiatric Hospital Center of Saint-Egrève in France. In November 2008, he had approved the nonsupervised release of a schizophrenia patient, Jean-Pierre Guillaud, to outside of his unit but within the hospital facility. Mr. Guillaud, while outside supervision, escaped. He subsequently purchased a large knife and murdered a 26-year-old student, Luc Meunier, Le Monde reported.1
This is reminiscent of a similar case in 2012 in Marseille, where a psychiatrist received a suspended prison sentence after his patient committed murder. That prior case was later dismissed in appellate court. In my opinion, both trials point to a failure in psychiatry’s responsibility to educate the public in our limitations and roles. They also highlight the necessary discourse that society should have on the role of mental illness when it comes to crime.
Although I appreciate society’s concern about such crimes, I think that displacement of our anger onto Dr. Gujadhur is misguided, and instead, allows us to forget to look at our own poor judgment. Dr. Gujadhur, other psychiatrists, and mental hospitals do not have the responsibility to enact sentences for crimes; the legal system does. Law enforcement and prosecutors had numerous opportunities to charge and commit Mr. Guillaud over the years but chose not to do so, instead permitting him to stay within society under the care of the mental health system.
Asking Dr. Gujadhur to primarily focus on becoming an agent of the law, instead of treating his patient, is unfair. Schizophrenia, and in particular paranoia, are greatly worsened by social isolation. Confining Mr. Guillaud would be countertherapeutic and possibly lead to his suicide. Would Dr. Gujadhur have been responsible for the suicide? Mental health providers have to understand and support the psychological functioning of their patients. Creating a dual agency blurs and effaces the doctor-patient relationship, already so fragile in the treatment of paranoid patients.
The publicity of such cases, and of Mr. Guillaud’s mental illness, seems to go against current mental health research. Recent work has suggested that mental illness is not a significant risk factor for violence but rather a risk factor for being the victim of violence. Certainly, some patients with mental illness commit acts of violence, but studies suggest that this is mostly independent of their mental illness (Law Hum Behav. 2014 Oct;38[5];439-49).2 Our overemphasis on the mental status of criminals belittles their crimes and suggests that psychiatrists are responsible for the failings of our legal system.
As a supervising psychiatrist at one of the largest jail systems in America, I am familiar with the challenges in such cases. All of my patients are facing legal charges, and many suffer from severe mental illness like schizophrenia. As their treating psychiatrist, I am not asked to also sentence them for the charges they are facing. Simply working for the sheriff makes my ability to gain the trust of my patients much more difficult. Conspiring with the city or district attorney in an attempt to protect society would obliterate any chance at rapport building.
Working in corrections, I am deeply familiar with the current debate on the solitary confinement of our mentally ill offenders. Ironically, in that context, society has blamed the legal system for socially isolating our mentally ill offenders, especially ones with severe mental illness.3 In our jail, we meet regularly and discuss in an interdisciplinary fashion the role and consequences of social isolation. During our weekly sessions, a case involving stabbing someone 2 years prior would not have justified the punishment of social isolation and constant monitoring.
As a field, psychiatry must educate society on its ability to create a therapeutic environment and its ability to provide risk assessment of violence. We must also remind others of the impossibility of doing both simultaneously. Decisions on removing patients’ right to freedom can be informed by the mental health perspective but should be left to the courts. Society’s need to find a target after such tragedies is understandable, but blaming the treating psychiatrists will not help past or future victims.
References
1. Le psychiatre d’un schizophrène meurtrier condamné pour homicide involontaire, Le Monde, Dec. 14, 2016.
2. How often and how consistently do symptoms directly precede criminal behavior among offenders with mental illness? (Law Hum Behav. 2014 Oct;38[5]:439-49).
3. How to fix solitary confinement in American prisons, Los Angeles Times, Oct. 17, 2016.
Dr. Badre is a supervising psychiatric contractor at the San Diego Central Jail. He also holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches on medical education, psychopharmacology, ethics in psychiatry, and correctional care. He mentors several residents on projects, including reduction in the use of solitary confinement of patients with mental illness, reduction in the use of involuntary treatment of the mentally ill, and examination of the mentally ill offender.
On Dec. 14, 2016, a French psychiatrist was sentenced to an 18-month suspended prison sentence. Lekhraj Gujadhur, MD, was the supervisor of unit 101 at the Psychiatric Hospital Center of Saint-Egrève in France. In November 2008, he had approved the nonsupervised release of a schizophrenia patient, Jean-Pierre Guillaud, to outside of his unit but within the hospital facility. Mr. Guillaud, while outside supervision, escaped. He subsequently purchased a large knife and murdered a 26-year-old student, Luc Meunier, Le Monde reported.1
This is reminiscent of a similar case in 2012 in Marseille, where a psychiatrist received a suspended prison sentence after his patient committed murder. That prior case was later dismissed in appellate court. In my opinion, both trials point to a failure in psychiatry’s responsibility to educate the public in our limitations and roles. They also highlight the necessary discourse that society should have on the role of mental illness when it comes to crime.
Although I appreciate society’s concern about such crimes, I think that displacement of our anger onto Dr. Gujadhur is misguided, and instead, allows us to forget to look at our own poor judgment. Dr. Gujadhur, other psychiatrists, and mental hospitals do not have the responsibility to enact sentences for crimes; the legal system does. Law enforcement and prosecutors had numerous opportunities to charge and commit Mr. Guillaud over the years but chose not to do so, instead permitting him to stay within society under the care of the mental health system.
Asking Dr. Gujadhur to primarily focus on becoming an agent of the law, instead of treating his patient, is unfair. Schizophrenia, and in particular paranoia, are greatly worsened by social isolation. Confining Mr. Guillaud would be countertherapeutic and possibly lead to his suicide. Would Dr. Gujadhur have been responsible for the suicide? Mental health providers have to understand and support the psychological functioning of their patients. Creating a dual agency blurs and effaces the doctor-patient relationship, already so fragile in the treatment of paranoid patients.
The publicity of such cases, and of Mr. Guillaud’s mental illness, seems to go against current mental health research. Recent work has suggested that mental illness is not a significant risk factor for violence but rather a risk factor for being the victim of violence. Certainly, some patients with mental illness commit acts of violence, but studies suggest that this is mostly independent of their mental illness (Law Hum Behav. 2014 Oct;38[5];439-49).2 Our overemphasis on the mental status of criminals belittles their crimes and suggests that psychiatrists are responsible for the failings of our legal system.
As a supervising psychiatrist at one of the largest jail systems in America, I am familiar with the challenges in such cases. All of my patients are facing legal charges, and many suffer from severe mental illness like schizophrenia. As their treating psychiatrist, I am not asked to also sentence them for the charges they are facing. Simply working for the sheriff makes my ability to gain the trust of my patients much more difficult. Conspiring with the city or district attorney in an attempt to protect society would obliterate any chance at rapport building.
Working in corrections, I am deeply familiar with the current debate on the solitary confinement of our mentally ill offenders. Ironically, in that context, society has blamed the legal system for socially isolating our mentally ill offenders, especially ones with severe mental illness.3 In our jail, we meet regularly and discuss in an interdisciplinary fashion the role and consequences of social isolation. During our weekly sessions, a case involving stabbing someone 2 years prior would not have justified the punishment of social isolation and constant monitoring.
As a field, psychiatry must educate society on its ability to create a therapeutic environment and its ability to provide risk assessment of violence. We must also remind others of the impossibility of doing both simultaneously. Decisions on removing patients’ right to freedom can be informed by the mental health perspective but should be left to the courts. Society’s need to find a target after such tragedies is understandable, but blaming the treating psychiatrists will not help past or future victims.
References
1. Le psychiatre d’un schizophrène meurtrier condamné pour homicide involontaire, Le Monde, Dec. 14, 2016.
2. How often and how consistently do symptoms directly precede criminal behavior among offenders with mental illness? (Law Hum Behav. 2014 Oct;38[5]:439-49).
3. How to fix solitary confinement in American prisons, Los Angeles Times, Oct. 17, 2016.
Dr. Badre is a supervising psychiatric contractor at the San Diego Central Jail. He also holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches on medical education, psychopharmacology, ethics in psychiatry, and correctional care. He mentors several residents on projects, including reduction in the use of solitary confinement of patients with mental illness, reduction in the use of involuntary treatment of the mentally ill, and examination of the mentally ill offender.
On Dec. 14, 2016, a French psychiatrist was sentenced to an 18-month suspended prison sentence. Lekhraj Gujadhur, MD, was the supervisor of unit 101 at the Psychiatric Hospital Center of Saint-Egrève in France. In November 2008, he had approved the nonsupervised release of a schizophrenia patient, Jean-Pierre Guillaud, to outside of his unit but within the hospital facility. Mr. Guillaud, while outside supervision, escaped. He subsequently purchased a large knife and murdered a 26-year-old student, Luc Meunier, Le Monde reported.1
This is reminiscent of a similar case in 2012 in Marseille, where a psychiatrist received a suspended prison sentence after his patient committed murder. That prior case was later dismissed in appellate court. In my opinion, both trials point to a failure in psychiatry’s responsibility to educate the public in our limitations and roles. They also highlight the necessary discourse that society should have on the role of mental illness when it comes to crime.
Although I appreciate society’s concern about such crimes, I think that displacement of our anger onto Dr. Gujadhur is misguided, and instead, allows us to forget to look at our own poor judgment. Dr. Gujadhur, other psychiatrists, and mental hospitals do not have the responsibility to enact sentences for crimes; the legal system does. Law enforcement and prosecutors had numerous opportunities to charge and commit Mr. Guillaud over the years but chose not to do so, instead permitting him to stay within society under the care of the mental health system.
Asking Dr. Gujadhur to primarily focus on becoming an agent of the law, instead of treating his patient, is unfair. Schizophrenia, and in particular paranoia, are greatly worsened by social isolation. Confining Mr. Guillaud would be countertherapeutic and possibly lead to his suicide. Would Dr. Gujadhur have been responsible for the suicide? Mental health providers have to understand and support the psychological functioning of their patients. Creating a dual agency blurs and effaces the doctor-patient relationship, already so fragile in the treatment of paranoid patients.
The publicity of such cases, and of Mr. Guillaud’s mental illness, seems to go against current mental health research. Recent work has suggested that mental illness is not a significant risk factor for violence but rather a risk factor for being the victim of violence. Certainly, some patients with mental illness commit acts of violence, but studies suggest that this is mostly independent of their mental illness (Law Hum Behav. 2014 Oct;38[5];439-49).2 Our overemphasis on the mental status of criminals belittles their crimes and suggests that psychiatrists are responsible for the failings of our legal system.
As a supervising psychiatrist at one of the largest jail systems in America, I am familiar with the challenges in such cases. All of my patients are facing legal charges, and many suffer from severe mental illness like schizophrenia. As their treating psychiatrist, I am not asked to also sentence them for the charges they are facing. Simply working for the sheriff makes my ability to gain the trust of my patients much more difficult. Conspiring with the city or district attorney in an attempt to protect society would obliterate any chance at rapport building.
Working in corrections, I am deeply familiar with the current debate on the solitary confinement of our mentally ill offenders. Ironically, in that context, society has blamed the legal system for socially isolating our mentally ill offenders, especially ones with severe mental illness.3 In our jail, we meet regularly and discuss in an interdisciplinary fashion the role and consequences of social isolation. During our weekly sessions, a case involving stabbing someone 2 years prior would not have justified the punishment of social isolation and constant monitoring.
As a field, psychiatry must educate society on its ability to create a therapeutic environment and its ability to provide risk assessment of violence. We must also remind others of the impossibility of doing both simultaneously. Decisions on removing patients’ right to freedom can be informed by the mental health perspective but should be left to the courts. Society’s need to find a target after such tragedies is understandable, but blaming the treating psychiatrists will not help past or future victims.
References
1. Le psychiatre d’un schizophrène meurtrier condamné pour homicide involontaire, Le Monde, Dec. 14, 2016.
2. How often and how consistently do symptoms directly precede criminal behavior among offenders with mental illness? (Law Hum Behav. 2014 Oct;38[5]:439-49).
3. How to fix solitary confinement in American prisons, Los Angeles Times, Oct. 17, 2016.
Dr. Badre is a supervising psychiatric contractor at the San Diego Central Jail. He also holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches on medical education, psychopharmacology, ethics in psychiatry, and correctional care. He mentors several residents on projects, including reduction in the use of solitary confinement of patients with mental illness, reduction in the use of involuntary treatment of the mentally ill, and examination of the mentally ill offender.
Endoscopy during pregnancy increases risk of preterm, SGA birth
Women who undergo an endoscopy during pregnancy are increasing the chances that their baby will be born preterm, or be small for gestational age (SGA), according to research published in the February issue of Gastroenterology (doi: 10.1053/j.gastro.2016.10.016).
“Research in pregnancy outcome in women undergoing endoscopy during pregnancy is scarce,” wrote the authors, led by Jonas F. Ludvigsson, MD, of the Karolinska Institutet in Stockholm, adding that there are nine studies with original data on a total of 379 pregnant women undergoing endoscopy; two of these studies examined pregnancy outcome in upper endoscopy (n = 143), two examined pregnancy outcome in sigmoidoscopy or colonoscopy (n = 116), and four examined pregnancy outcome in endoscopic retrograde cholangiopancreatography (n = 120).
Additionally, the authors noted that, to their knowledge, there are no studies that offer data on the relative risk of endoscopy during pregnancy, and none that followed up subjects after birth. Of the few studies that do exist, a handful conclude that endoscopy during pregnancy is actually safe, but do not include data on stillbirths and neonatal deaths that did not occur immediately after patients underwent endoscopy, which could compromise that data.
To address the lack of reliable research on the effect of endoscopy on pregnancy, Dr. Ludvigsson and his coinvestigators launched a nationwide study of pregnancies in Sweden that occurred between 1992 and 2011, all of which were registered in the Swedish Medical Birth Registry and the Swedish Patient Registry. The databases revealed 2,025 upper endoscopies, 1,109 lower endoscopies, and 58 endoscopic retrograde cholangiopancreatographies, for a total of 3,052 pregnancies exposed to endoscopy over that time period.
The primary endpoint of the study was the frequency of preterm birth and stillbirth in this population. To measure this, the investigators used adjusted relative risk (ARR), calculated via Poisson regression by using data on 1,589,173 pregnancies that were not exposed to endoscopy as reference.
“Stillbirth is recorded from 22 completed gestational weeks since mid-2008, and before that from gestational week 28. Gestational age was determined using ultrasound, and when ultrasound data were missing, we used the first day of the last menstrual period for pregnancy start,” the authors wrote.
The results showed that mothers who had any kind of endoscopy during pregnancy were more likely to experience a preterm birth or give birth to a baby who was SGA, with the ARR being 1.54 (95% confidence interval, 1.36-1.75) and 1.30 (95% CI, 1.07-1.57), respectively. However, the risk of other adverse effects, such as stillbirth or congenital malformation, was not significant: Stillbirth ARR was 1.45 (95% CI, 0.87-2.40) and congenital malformation ARR was 1.00 (95% CI, 0.83-1.20).
Women who were exposed to endoscopy during pregnancy were more likely to have a preterm birth, compared with women who had endoscopy 1 year before or after pregnancy, but were not more highly predisposed to SGA, stillbirth, or congenital malformations. Additionally, when data on multiple pregnancies carried by the same mother were compared, no correlation was found between endoscopy and gestational age or birth weight, if the mother was exposed to endoscopy during only one of the pregnancies.
“Earlier recommendations suggest that endoscopy should only be performed during pregnancy if there are strong indications, and if so, not during the second trimester, [but] our study shows that endoscopy is unlikely to have a more than marginal influence on pregnancy outcome independently of trimester,” the authors concluded. “Neither does it seem that sigmoidoscopy is preferable to a full colonoscopy in the pregnant woman.”
Regarding the latter conclusion, the authors clarified that “it is possible that in women with particularly severe gastrointestinal disease where endoscopy is inevitable, the physician will prefer a sigmoidoscopy rather than a full colonoscopy, and under such circumstances the sigmoidoscopy will signal a more severe disease.”
The investigators also noted that their study had several limitations, including not knowing the length of time each endoscopy took, the sedatives and bowel preparations that were used, the patient’s position during the procedure, and the indication that prompted the endoscopy in the first place.
The study was funded by grants from the Swedish Society of Medicine and the Stockholm County Council, and the Swedish Research Council. Dr. Ludvigsson and his coauthors did not report any relevant financial disclosures.
Women who undergo an endoscopy during pregnancy are increasing the chances that their baby will be born preterm, or be small for gestational age (SGA), according to research published in the February issue of Gastroenterology (doi: 10.1053/j.gastro.2016.10.016).
“Research in pregnancy outcome in women undergoing endoscopy during pregnancy is scarce,” wrote the authors, led by Jonas F. Ludvigsson, MD, of the Karolinska Institutet in Stockholm, adding that there are nine studies with original data on a total of 379 pregnant women undergoing endoscopy; two of these studies examined pregnancy outcome in upper endoscopy (n = 143), two examined pregnancy outcome in sigmoidoscopy or colonoscopy (n = 116), and four examined pregnancy outcome in endoscopic retrograde cholangiopancreatography (n = 120).
Additionally, the authors noted that, to their knowledge, there are no studies that offer data on the relative risk of endoscopy during pregnancy, and none that followed up subjects after birth. Of the few studies that do exist, a handful conclude that endoscopy during pregnancy is actually safe, but do not include data on stillbirths and neonatal deaths that did not occur immediately after patients underwent endoscopy, which could compromise that data.
To address the lack of reliable research on the effect of endoscopy on pregnancy, Dr. Ludvigsson and his coinvestigators launched a nationwide study of pregnancies in Sweden that occurred between 1992 and 2011, all of which were registered in the Swedish Medical Birth Registry and the Swedish Patient Registry. The databases revealed 2,025 upper endoscopies, 1,109 lower endoscopies, and 58 endoscopic retrograde cholangiopancreatographies, for a total of 3,052 pregnancies exposed to endoscopy over that time period.
The primary endpoint of the study was the frequency of preterm birth and stillbirth in this population. To measure this, the investigators used adjusted relative risk (ARR), calculated via Poisson regression by using data on 1,589,173 pregnancies that were not exposed to endoscopy as reference.
“Stillbirth is recorded from 22 completed gestational weeks since mid-2008, and before that from gestational week 28. Gestational age was determined using ultrasound, and when ultrasound data were missing, we used the first day of the last menstrual period for pregnancy start,” the authors wrote.
The results showed that mothers who had any kind of endoscopy during pregnancy were more likely to experience a preterm birth or give birth to a baby who was SGA, with the ARR being 1.54 (95% confidence interval, 1.36-1.75) and 1.30 (95% CI, 1.07-1.57), respectively. However, the risk of other adverse effects, such as stillbirth or congenital malformation, was not significant: Stillbirth ARR was 1.45 (95% CI, 0.87-2.40) and congenital malformation ARR was 1.00 (95% CI, 0.83-1.20).
Women who were exposed to endoscopy during pregnancy were more likely to have a preterm birth, compared with women who had endoscopy 1 year before or after pregnancy, but were not more highly predisposed to SGA, stillbirth, or congenital malformations. Additionally, when data on multiple pregnancies carried by the same mother were compared, no correlation was found between endoscopy and gestational age or birth weight, if the mother was exposed to endoscopy during only one of the pregnancies.
“Earlier recommendations suggest that endoscopy should only be performed during pregnancy if there are strong indications, and if so, not during the second trimester, [but] our study shows that endoscopy is unlikely to have a more than marginal influence on pregnancy outcome independently of trimester,” the authors concluded. “Neither does it seem that sigmoidoscopy is preferable to a full colonoscopy in the pregnant woman.”
Regarding the latter conclusion, the authors clarified that “it is possible that in women with particularly severe gastrointestinal disease where endoscopy is inevitable, the physician will prefer a sigmoidoscopy rather than a full colonoscopy, and under such circumstances the sigmoidoscopy will signal a more severe disease.”
The investigators also noted that their study had several limitations, including not knowing the length of time each endoscopy took, the sedatives and bowel preparations that were used, the patient’s position during the procedure, and the indication that prompted the endoscopy in the first place.
The study was funded by grants from the Swedish Society of Medicine and the Stockholm County Council, and the Swedish Research Council. Dr. Ludvigsson and his coauthors did not report any relevant financial disclosures.
Women who undergo an endoscopy during pregnancy are increasing the chances that their baby will be born preterm, or be small for gestational age (SGA), according to research published in the February issue of Gastroenterology (doi: 10.1053/j.gastro.2016.10.016).
“Research in pregnancy outcome in women undergoing endoscopy during pregnancy is scarce,” wrote the authors, led by Jonas F. Ludvigsson, MD, of the Karolinska Institutet in Stockholm, adding that there are nine studies with original data on a total of 379 pregnant women undergoing endoscopy; two of these studies examined pregnancy outcome in upper endoscopy (n = 143), two examined pregnancy outcome in sigmoidoscopy or colonoscopy (n = 116), and four examined pregnancy outcome in endoscopic retrograde cholangiopancreatography (n = 120).
Additionally, the authors noted that, to their knowledge, there are no studies that offer data on the relative risk of endoscopy during pregnancy, and none that followed up subjects after birth. Of the few studies that do exist, a handful conclude that endoscopy during pregnancy is actually safe, but do not include data on stillbirths and neonatal deaths that did not occur immediately after patients underwent endoscopy, which could compromise that data.
To address the lack of reliable research on the effect of endoscopy on pregnancy, Dr. Ludvigsson and his coinvestigators launched a nationwide study of pregnancies in Sweden that occurred between 1992 and 2011, all of which were registered in the Swedish Medical Birth Registry and the Swedish Patient Registry. The databases revealed 2,025 upper endoscopies, 1,109 lower endoscopies, and 58 endoscopic retrograde cholangiopancreatographies, for a total of 3,052 pregnancies exposed to endoscopy over that time period.
The primary endpoint of the study was the frequency of preterm birth and stillbirth in this population. To measure this, the investigators used adjusted relative risk (ARR), calculated via Poisson regression by using data on 1,589,173 pregnancies that were not exposed to endoscopy as reference.
“Stillbirth is recorded from 22 completed gestational weeks since mid-2008, and before that from gestational week 28. Gestational age was determined using ultrasound, and when ultrasound data were missing, we used the first day of the last menstrual period for pregnancy start,” the authors wrote.
The results showed that mothers who had any kind of endoscopy during pregnancy were more likely to experience a preterm birth or give birth to a baby who was SGA, with the ARR being 1.54 (95% confidence interval, 1.36-1.75) and 1.30 (95% CI, 1.07-1.57), respectively. However, the risk of other adverse effects, such as stillbirth or congenital malformation, was not significant: Stillbirth ARR was 1.45 (95% CI, 0.87-2.40) and congenital malformation ARR was 1.00 (95% CI, 0.83-1.20).
Women who were exposed to endoscopy during pregnancy were more likely to have a preterm birth, compared with women who had endoscopy 1 year before or after pregnancy, but were not more highly predisposed to SGA, stillbirth, or congenital malformations. Additionally, when data on multiple pregnancies carried by the same mother were compared, no correlation was found between endoscopy and gestational age or birth weight, if the mother was exposed to endoscopy during only one of the pregnancies.
“Earlier recommendations suggest that endoscopy should only be performed during pregnancy if there are strong indications, and if so, not during the second trimester, [but] our study shows that endoscopy is unlikely to have a more than marginal influence on pregnancy outcome independently of trimester,” the authors concluded. “Neither does it seem that sigmoidoscopy is preferable to a full colonoscopy in the pregnant woman.”
Regarding the latter conclusion, the authors clarified that “it is possible that in women with particularly severe gastrointestinal disease where endoscopy is inevitable, the physician will prefer a sigmoidoscopy rather than a full colonoscopy, and under such circumstances the sigmoidoscopy will signal a more severe disease.”
The investigators also noted that their study had several limitations, including not knowing the length of time each endoscopy took, the sedatives and bowel preparations that were used, the patient’s position during the procedure, and the indication that prompted the endoscopy in the first place.
The study was funded by grants from the Swedish Society of Medicine and the Stockholm County Council, and the Swedish Research Council. Dr. Ludvigsson and his coauthors did not report any relevant financial disclosures.
FROM GASTROENTEROLOGY
Key clinical point:
Major finding: The adjusted relative risk of preterm birth was 1.54 (95% CI, 1.36-1.75) and was 1.30 (95% CI, 1.07-1.57) for SGA.
Data source: A population-based cohort study of 3,052 pregnancies in Sweden exposed to endoscopy from 1992 through 2011.
Disclosures: The study was funded by the Swedish Society of Medicine and the Stockholm County Council, and the Swedish Research Council. The authors did not report any relevant financial disclosures.
Recognizing Congenital Zika Syndrome
VANCOUVER—Infants infected with Zika virus in utero may develop a syndrome characterized by brain volume loss, intracerebral calcifications, and spasticity. They may develop dyskinesia or seizures after several months, and a subset of children has severe arthrogryposis.
And although microcephaly at birth is common, infants may have a normal head size at birth, but develop postnatal microcephaly or other neurologic symptoms at six months, according to research described at the 45th Annual Meeting of the Child Neurology Society.
“The spectrum of congenital Zika syndrome is expanding as we come to understand it better,” said William B. Dobyns, MD, Professor of Pediatrics at the University of Washington in Seattle and a faculty member at the Center for Integrative Brain Research at Seattle Children’s Research Institute. “We all need to stop calling this microcephaly. This is much more than that. This is the congenital Zika syndrome.”
Zika virus is trophic for neural stem cells, and the first reports of microcephaly associated with prenatal Zika virus infection came from Brazil in January 2016. In the US, mosquitoes that transmit Zika virus, Aedes aegypti and albopictus, are present year round in Florida and seasonally in about a quarter of the states. “It is pretty clear that it will be coming.… We need to take precautions until treatments or preventives are available,” he said. In addition, child neurologists need to be able to recognize congenital Zika syndrome. “It is entirely possible for us to do so,” Dr. Dobyns said. “You do not even need viral titers in the more classically affected children.”
A Case Series of 57 Children
Dr. Dobyns worked with André Pessoa, MD, a child neurologist at Hospital Infantil Albert Sabin in Fortaleza, Brazil, and other neurologists in the region to compile data on a series of 57 children with congenital microcephaly and presumed or proven Zika exposure of the mothers during pregnancy. Microcephaly was defined as occipitofrontal head circumference of at least two standard deviations below the mean.
About half of the children had a bony protuberance of the occipital bone, known as an occipital shelf, Dr. Dobyns said. This feature occurs when the fetal brain, instead of growing and pushing out the skull plates, is severely injured and shrinks. The frontal and parietal bones, but not the occipital bone, collapse over the injured brain.
Almost all of the children had prominent calcifications in the brain. Unlike in children infected with cytomegalovirus, periventricular calcifications are the exception in children infected with Zika virus. Researchers observed subcortical or cortical calcifications on CT in 51 of the 57 children infected with Zika virus and basal ganglia calcifications in 33 of the 57 children.
Furthermore, calcifications with Zika virus infection tend to be diffuse and bilateral, whereas calcifications with cytomegalovirus infection tend to be patchy, Dr. Dobyns said.
All patients had the same general pattern of enlarged extra-axial space, ventriculomegaly, or both, indicating brain volume loss.
About 20% of patients had severe arthrogryposis multiplex congenita, and all of these children had abnormally positioned proximal joints.
Twenty of the children underwent brain MRI. MRI showed an abnormal cortex in all 20 children. The patients appear to have a diffuse cortical malformation that is most consistent with polymicrogyria, Dr. Dobyns said.
Nearly 20% of children in the series had microcephaly between two and three standard deviations below the mean. But 81% had microcephaly of three or more standard deviations below the mean. The mean occipitofrontal head circumference was four standard deviations below the mean.
Neurologic features included spasticity in 94% of the children and severe irritability or tremor in 64% of the children. About 20% had seizures after several months. Some patients had eye abnormalities, including optic nerve pallor, macular atrophy, and strabismus.
“The exam is characteristic,” Dr. Dobyns said. “They all develop a dyskinesia later in the first year of life. They have spastic quadriparesis. They frequently have tremors at birth. They feed poorly. They tend to be irritable and scream all the time. They are starting to have seizures as they get past six months of age.”
As in other studies, data from this series suggest that children whose mothers have a symptomatic illness or are infected earlier in pregnancy may be at higher risk of congenital Zika syndrome.
Infants Without Microcephaly at Birth
Dr. Dobyns presented preliminary data from children who were exposed to Zika virus but did not have microcephaly at birth. These children had most of the same features on exam as children with microcephaly, although the features tended to be less severe. The children started to have seizures after several months. When their head size was measured at six months or older, it fell below the second percentile, meaning that these children had postnatal microcephaly. The children did not have congenital contractures, Dr. Dobyns said.
Vanessa van der Linden, MD, a pediatric neurologist at the Association for Assistance of Disabled Children in Recife, Brazil, Dr. Pessoa, Dr. Dobyns, and colleagues on November 22, 2016, published a description of 13 infants who had evidence of congenital Zika infection but did not have microcephaly at birth. Their report was published online in the CDC’s Morbidity and Mortality Weekly Report. The researchers found that head growth decelerated in all 13 of the infants by as early as age 5 months, and 11 of the infants had microcephaly. The findings suggest that infants exposed to Zika virus prenatally should receive comprehensive medical and developmental follow-up, even in the absence of microcephaly at birth, the investigators said.
That infants with prenatal Zika infection may develop postnatal microcephaly is not surprising, Dr. Dobyns said. Microcephaly, however, remains only one possible symptom of congenital Zika syndrome. “It is a pattern of features seen clinically, on CT scans, and behaviorally that will mark this syndrome,” he said.
—Jake Remaly
Suggested Reading
Moore CA, Staples JE, Dobyns WB, et al. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr. 2016 Nov 3 [Epub ahead of print].
van der Linden V, Pessoa A, Dobyns W, et al. Description of 13 infants born during October 2015–January 2016 with congenital Zika virus infection without microcephaly at birth — Brazil. MMWR Morb Mortal Wkly Rep. 22 Nov 2016 [Epub ahead of print].
VANCOUVER—Infants infected with Zika virus in utero may develop a syndrome characterized by brain volume loss, intracerebral calcifications, and spasticity. They may develop dyskinesia or seizures after several months, and a subset of children has severe arthrogryposis.
And although microcephaly at birth is common, infants may have a normal head size at birth, but develop postnatal microcephaly or other neurologic symptoms at six months, according to research described at the 45th Annual Meeting of the Child Neurology Society.
“The spectrum of congenital Zika syndrome is expanding as we come to understand it better,” said William B. Dobyns, MD, Professor of Pediatrics at the University of Washington in Seattle and a faculty member at the Center for Integrative Brain Research at Seattle Children’s Research Institute. “We all need to stop calling this microcephaly. This is much more than that. This is the congenital Zika syndrome.”
Zika virus is trophic for neural stem cells, and the first reports of microcephaly associated with prenatal Zika virus infection came from Brazil in January 2016. In the US, mosquitoes that transmit Zika virus, Aedes aegypti and albopictus, are present year round in Florida and seasonally in about a quarter of the states. “It is pretty clear that it will be coming.… We need to take precautions until treatments or preventives are available,” he said. In addition, child neurologists need to be able to recognize congenital Zika syndrome. “It is entirely possible for us to do so,” Dr. Dobyns said. “You do not even need viral titers in the more classically affected children.”
A Case Series of 57 Children
Dr. Dobyns worked with André Pessoa, MD, a child neurologist at Hospital Infantil Albert Sabin in Fortaleza, Brazil, and other neurologists in the region to compile data on a series of 57 children with congenital microcephaly and presumed or proven Zika exposure of the mothers during pregnancy. Microcephaly was defined as occipitofrontal head circumference of at least two standard deviations below the mean.
About half of the children had a bony protuberance of the occipital bone, known as an occipital shelf, Dr. Dobyns said. This feature occurs when the fetal brain, instead of growing and pushing out the skull plates, is severely injured and shrinks. The frontal and parietal bones, but not the occipital bone, collapse over the injured brain.
Almost all of the children had prominent calcifications in the brain. Unlike in children infected with cytomegalovirus, periventricular calcifications are the exception in children infected with Zika virus. Researchers observed subcortical or cortical calcifications on CT in 51 of the 57 children infected with Zika virus and basal ganglia calcifications in 33 of the 57 children.
Furthermore, calcifications with Zika virus infection tend to be diffuse and bilateral, whereas calcifications with cytomegalovirus infection tend to be patchy, Dr. Dobyns said.
All patients had the same general pattern of enlarged extra-axial space, ventriculomegaly, or both, indicating brain volume loss.
About 20% of patients had severe arthrogryposis multiplex congenita, and all of these children had abnormally positioned proximal joints.
Twenty of the children underwent brain MRI. MRI showed an abnormal cortex in all 20 children. The patients appear to have a diffuse cortical malformation that is most consistent with polymicrogyria, Dr. Dobyns said.
Nearly 20% of children in the series had microcephaly between two and three standard deviations below the mean. But 81% had microcephaly of three or more standard deviations below the mean. The mean occipitofrontal head circumference was four standard deviations below the mean.
Neurologic features included spasticity in 94% of the children and severe irritability or tremor in 64% of the children. About 20% had seizures after several months. Some patients had eye abnormalities, including optic nerve pallor, macular atrophy, and strabismus.
“The exam is characteristic,” Dr. Dobyns said. “They all develop a dyskinesia later in the first year of life. They have spastic quadriparesis. They frequently have tremors at birth. They feed poorly. They tend to be irritable and scream all the time. They are starting to have seizures as they get past six months of age.”
As in other studies, data from this series suggest that children whose mothers have a symptomatic illness or are infected earlier in pregnancy may be at higher risk of congenital Zika syndrome.
Infants Without Microcephaly at Birth
Dr. Dobyns presented preliminary data from children who were exposed to Zika virus but did not have microcephaly at birth. These children had most of the same features on exam as children with microcephaly, although the features tended to be less severe. The children started to have seizures after several months. When their head size was measured at six months or older, it fell below the second percentile, meaning that these children had postnatal microcephaly. The children did not have congenital contractures, Dr. Dobyns said.
Vanessa van der Linden, MD, a pediatric neurologist at the Association for Assistance of Disabled Children in Recife, Brazil, Dr. Pessoa, Dr. Dobyns, and colleagues on November 22, 2016, published a description of 13 infants who had evidence of congenital Zika infection but did not have microcephaly at birth. Their report was published online in the CDC’s Morbidity and Mortality Weekly Report. The researchers found that head growth decelerated in all 13 of the infants by as early as age 5 months, and 11 of the infants had microcephaly. The findings suggest that infants exposed to Zika virus prenatally should receive comprehensive medical and developmental follow-up, even in the absence of microcephaly at birth, the investigators said.
That infants with prenatal Zika infection may develop postnatal microcephaly is not surprising, Dr. Dobyns said. Microcephaly, however, remains only one possible symptom of congenital Zika syndrome. “It is a pattern of features seen clinically, on CT scans, and behaviorally that will mark this syndrome,” he said.
—Jake Remaly
Suggested Reading
Moore CA, Staples JE, Dobyns WB, et al. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr. 2016 Nov 3 [Epub ahead of print].
van der Linden V, Pessoa A, Dobyns W, et al. Description of 13 infants born during October 2015–January 2016 with congenital Zika virus infection without microcephaly at birth — Brazil. MMWR Morb Mortal Wkly Rep. 22 Nov 2016 [Epub ahead of print].
VANCOUVER—Infants infected with Zika virus in utero may develop a syndrome characterized by brain volume loss, intracerebral calcifications, and spasticity. They may develop dyskinesia or seizures after several months, and a subset of children has severe arthrogryposis.
And although microcephaly at birth is common, infants may have a normal head size at birth, but develop postnatal microcephaly or other neurologic symptoms at six months, according to research described at the 45th Annual Meeting of the Child Neurology Society.
“The spectrum of congenital Zika syndrome is expanding as we come to understand it better,” said William B. Dobyns, MD, Professor of Pediatrics at the University of Washington in Seattle and a faculty member at the Center for Integrative Brain Research at Seattle Children’s Research Institute. “We all need to stop calling this microcephaly. This is much more than that. This is the congenital Zika syndrome.”
Zika virus is trophic for neural stem cells, and the first reports of microcephaly associated with prenatal Zika virus infection came from Brazil in January 2016. In the US, mosquitoes that transmit Zika virus, Aedes aegypti and albopictus, are present year round in Florida and seasonally in about a quarter of the states. “It is pretty clear that it will be coming.… We need to take precautions until treatments or preventives are available,” he said. In addition, child neurologists need to be able to recognize congenital Zika syndrome. “It is entirely possible for us to do so,” Dr. Dobyns said. “You do not even need viral titers in the more classically affected children.”
A Case Series of 57 Children
Dr. Dobyns worked with André Pessoa, MD, a child neurologist at Hospital Infantil Albert Sabin in Fortaleza, Brazil, and other neurologists in the region to compile data on a series of 57 children with congenital microcephaly and presumed or proven Zika exposure of the mothers during pregnancy. Microcephaly was defined as occipitofrontal head circumference of at least two standard deviations below the mean.
About half of the children had a bony protuberance of the occipital bone, known as an occipital shelf, Dr. Dobyns said. This feature occurs when the fetal brain, instead of growing and pushing out the skull plates, is severely injured and shrinks. The frontal and parietal bones, but not the occipital bone, collapse over the injured brain.
Almost all of the children had prominent calcifications in the brain. Unlike in children infected with cytomegalovirus, periventricular calcifications are the exception in children infected with Zika virus. Researchers observed subcortical or cortical calcifications on CT in 51 of the 57 children infected with Zika virus and basal ganglia calcifications in 33 of the 57 children.
Furthermore, calcifications with Zika virus infection tend to be diffuse and bilateral, whereas calcifications with cytomegalovirus infection tend to be patchy, Dr. Dobyns said.
All patients had the same general pattern of enlarged extra-axial space, ventriculomegaly, or both, indicating brain volume loss.
About 20% of patients had severe arthrogryposis multiplex congenita, and all of these children had abnormally positioned proximal joints.
Twenty of the children underwent brain MRI. MRI showed an abnormal cortex in all 20 children. The patients appear to have a diffuse cortical malformation that is most consistent with polymicrogyria, Dr. Dobyns said.
Nearly 20% of children in the series had microcephaly between two and three standard deviations below the mean. But 81% had microcephaly of three or more standard deviations below the mean. The mean occipitofrontal head circumference was four standard deviations below the mean.
Neurologic features included spasticity in 94% of the children and severe irritability or tremor in 64% of the children. About 20% had seizures after several months. Some patients had eye abnormalities, including optic nerve pallor, macular atrophy, and strabismus.
“The exam is characteristic,” Dr. Dobyns said. “They all develop a dyskinesia later in the first year of life. They have spastic quadriparesis. They frequently have tremors at birth. They feed poorly. They tend to be irritable and scream all the time. They are starting to have seizures as they get past six months of age.”
As in other studies, data from this series suggest that children whose mothers have a symptomatic illness or are infected earlier in pregnancy may be at higher risk of congenital Zika syndrome.
Infants Without Microcephaly at Birth
Dr. Dobyns presented preliminary data from children who were exposed to Zika virus but did not have microcephaly at birth. These children had most of the same features on exam as children with microcephaly, although the features tended to be less severe. The children started to have seizures after several months. When their head size was measured at six months or older, it fell below the second percentile, meaning that these children had postnatal microcephaly. The children did not have congenital contractures, Dr. Dobyns said.
Vanessa van der Linden, MD, a pediatric neurologist at the Association for Assistance of Disabled Children in Recife, Brazil, Dr. Pessoa, Dr. Dobyns, and colleagues on November 22, 2016, published a description of 13 infants who had evidence of congenital Zika infection but did not have microcephaly at birth. Their report was published online in the CDC’s Morbidity and Mortality Weekly Report. The researchers found that head growth decelerated in all 13 of the infants by as early as age 5 months, and 11 of the infants had microcephaly. The findings suggest that infants exposed to Zika virus prenatally should receive comprehensive medical and developmental follow-up, even in the absence of microcephaly at birth, the investigators said.
That infants with prenatal Zika infection may develop postnatal microcephaly is not surprising, Dr. Dobyns said. Microcephaly, however, remains only one possible symptom of congenital Zika syndrome. “It is a pattern of features seen clinically, on CT scans, and behaviorally that will mark this syndrome,” he said.
—Jake Remaly
Suggested Reading
Moore CA, Staples JE, Dobyns WB, et al. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr. 2016 Nov 3 [Epub ahead of print].
van der Linden V, Pessoa A, Dobyns W, et al. Description of 13 infants born during October 2015–January 2016 with congenital Zika virus infection without microcephaly at birth — Brazil. MMWR Morb Mortal Wkly Rep. 22 Nov 2016 [Epub ahead of print].
IV Ketamine May Be Effective as Subacute Treatment for Refractory Chronic Migraine
Ketamine may help to treat pain in patients with refractory chronic migraine, according to a case series published in the December 2016 Journal of Headache and Pain. IV ketamine treatment was associated with short-term improvement in pain severity in six of six patients with refractory chronic migraine.
“This study highlights the need for further research regarding new treatment options for patients who suffer daily consequences of refractory migraine and have failed many abortive and preventive medications,” said Clinton Lauritsen, MD, a Headache Fellow at Thomas Jefferson University Hospital in Philadelphia.
Ketamine is a dissociative anesthetic that acts on glutamate binding sites at the N-methyl-D-aspartate (NMDA) receptor, as well as at opioid, monoaminergic, cholinergic, nicotinic, and muscarinic receptors. IV ketamine was previously studied in several refractory pain conditions, including complex regional pain. While intranasal ketamine reduced the severity of migraine aura in a small randomized trial, the use of IV ketamine has only been reported in case series. Krusz et al showed improvement in pain scores in patients who used IV ketamine for refractory migraine; few side effects were reported.
Inpatient IV Ketamine
To further investigate the effect of IV ketamine in patients with intractable migraine, Dr. Lauritsen and colleagues conducted a retrospective chart review study. The researchers identified six patients with refractory chronic migraine admitted to Mount Sinai Beth Israel Hospital in New York from 2010 through 2014 for treatment with continuous IV ketamine.
Patients were given a starting dose of 0.1 mg/kg/h that was increased by 0.1 mg/kg/h every three to four hours as tolerated until the target pain score of 3 out of 10 was achieved and maintained for at least eight hours. Subsequently, the infusion was decreased by 0.2 mg/kg/h every three to four hours until the infusion rate reached 0 mg/kg/h.
The dose of ketamine was increased until maximum response was achieved or undesirable side effects, including psychomimetic and dysphoric effects, developed. Researchers used the Visual Analogue Score (VAS) at admission and during follow-up. VAS scores at different ketamine infusion rates were assessed from nursing and infusion records. Pain response was defined as a reduction in the initial VAS to a score of 3 or less. In addition, researchers attempted to contact patients for a telephone follow-up; however, they were only able to reach two of the six patients. During the telephone interview, researchers administered a questionnaire.
Pain Relief Achieved
Results from the data revealed a median age of 36.5 years; 83% of the patients were women. All of the patients were Caucasian, and the median age of migraine onset was 17. The median duration of the disease was 17 years. The mean number of failed acute migraine treatments was 18, and the mean number of failed preventive medications was 25. Pre-treatment pain scores ranged from 9 to 10.
In this small case series, all six patients with refractory migraine met
Overall, IV ketamine relieved pain in patients with chronic migraine without substantial adverse effects. “However, future study of this benefit on short-term headache relief needs to be conducted in a placebo-controlled fashion,” said Dr. Lauritsen.
“It is biologically plausible that ketamine could be an effective treatment for intractable headache,” the researchers said. “Ketamine is an antagonist at NMDA receptors, blocking the excitatory action of glutamate, a neurotransmitter long implicated in the pathophysiology of migraine. Glutamate has been … implicated in induction of cortical spreading depression [and] activation of trigeminal nociceptive neurons [and may] play a role in central sensitization.”
—Erica Tricarico
Suggested Reading
Lauritsen C, Mazuera S, Lipton RB, Ashina S. Intravenous ketamine for subacute treatment of refractory chronic migraine: a case series. J Headache Pain. 2016;17(1)106-110.
Ketamine may help to treat pain in patients with refractory chronic migraine, according to a case series published in the December 2016 Journal of Headache and Pain. IV ketamine treatment was associated with short-term improvement in pain severity in six of six patients with refractory chronic migraine.
“This study highlights the need for further research regarding new treatment options for patients who suffer daily consequences of refractory migraine and have failed many abortive and preventive medications,” said Clinton Lauritsen, MD, a Headache Fellow at Thomas Jefferson University Hospital in Philadelphia.
Ketamine is a dissociative anesthetic that acts on glutamate binding sites at the N-methyl-D-aspartate (NMDA) receptor, as well as at opioid, monoaminergic, cholinergic, nicotinic, and muscarinic receptors. IV ketamine was previously studied in several refractory pain conditions, including complex regional pain. While intranasal ketamine reduced the severity of migraine aura in a small randomized trial, the use of IV ketamine has only been reported in case series. Krusz et al showed improvement in pain scores in patients who used IV ketamine for refractory migraine; few side effects were reported.
Inpatient IV Ketamine
To further investigate the effect of IV ketamine in patients with intractable migraine, Dr. Lauritsen and colleagues conducted a retrospective chart review study. The researchers identified six patients with refractory chronic migraine admitted to Mount Sinai Beth Israel Hospital in New York from 2010 through 2014 for treatment with continuous IV ketamine.
Patients were given a starting dose of 0.1 mg/kg/h that was increased by 0.1 mg/kg/h every three to four hours as tolerated until the target pain score of 3 out of 10 was achieved and maintained for at least eight hours. Subsequently, the infusion was decreased by 0.2 mg/kg/h every three to four hours until the infusion rate reached 0 mg/kg/h.
The dose of ketamine was increased until maximum response was achieved or undesirable side effects, including psychomimetic and dysphoric effects, developed. Researchers used the Visual Analogue Score (VAS) at admission and during follow-up. VAS scores at different ketamine infusion rates were assessed from nursing and infusion records. Pain response was defined as a reduction in the initial VAS to a score of 3 or less. In addition, researchers attempted to contact patients for a telephone follow-up; however, they were only able to reach two of the six patients. During the telephone interview, researchers administered a questionnaire.
Pain Relief Achieved
Results from the data revealed a median age of 36.5 years; 83% of the patients were women. All of the patients were Caucasian, and the median age of migraine onset was 17. The median duration of the disease was 17 years. The mean number of failed acute migraine treatments was 18, and the mean number of failed preventive medications was 25. Pre-treatment pain scores ranged from 9 to 10.
In this small case series, all six patients with refractory migraine met
Overall, IV ketamine relieved pain in patients with chronic migraine without substantial adverse effects. “However, future study of this benefit on short-term headache relief needs to be conducted in a placebo-controlled fashion,” said Dr. Lauritsen.
“It is biologically plausible that ketamine could be an effective treatment for intractable headache,” the researchers said. “Ketamine is an antagonist at NMDA receptors, blocking the excitatory action of glutamate, a neurotransmitter long implicated in the pathophysiology of migraine. Glutamate has been … implicated in induction of cortical spreading depression [and] activation of trigeminal nociceptive neurons [and may] play a role in central sensitization.”
—Erica Tricarico
Suggested Reading
Lauritsen C, Mazuera S, Lipton RB, Ashina S. Intravenous ketamine for subacute treatment of refractory chronic migraine: a case series. J Headache Pain. 2016;17(1)106-110.
Ketamine may help to treat pain in patients with refractory chronic migraine, according to a case series published in the December 2016 Journal of Headache and Pain. IV ketamine treatment was associated with short-term improvement in pain severity in six of six patients with refractory chronic migraine.
“This study highlights the need for further research regarding new treatment options for patients who suffer daily consequences of refractory migraine and have failed many abortive and preventive medications,” said Clinton Lauritsen, MD, a Headache Fellow at Thomas Jefferson University Hospital in Philadelphia.
Ketamine is a dissociative anesthetic that acts on glutamate binding sites at the N-methyl-D-aspartate (NMDA) receptor, as well as at opioid, monoaminergic, cholinergic, nicotinic, and muscarinic receptors. IV ketamine was previously studied in several refractory pain conditions, including complex regional pain. While intranasal ketamine reduced the severity of migraine aura in a small randomized trial, the use of IV ketamine has only been reported in case series. Krusz et al showed improvement in pain scores in patients who used IV ketamine for refractory migraine; few side effects were reported.
Inpatient IV Ketamine
To further investigate the effect of IV ketamine in patients with intractable migraine, Dr. Lauritsen and colleagues conducted a retrospective chart review study. The researchers identified six patients with refractory chronic migraine admitted to Mount Sinai Beth Israel Hospital in New York from 2010 through 2014 for treatment with continuous IV ketamine.
Patients were given a starting dose of 0.1 mg/kg/h that was increased by 0.1 mg/kg/h every three to four hours as tolerated until the target pain score of 3 out of 10 was achieved and maintained for at least eight hours. Subsequently, the infusion was decreased by 0.2 mg/kg/h every three to four hours until the infusion rate reached 0 mg/kg/h.
The dose of ketamine was increased until maximum response was achieved or undesirable side effects, including psychomimetic and dysphoric effects, developed. Researchers used the Visual Analogue Score (VAS) at admission and during follow-up. VAS scores at different ketamine infusion rates were assessed from nursing and infusion records. Pain response was defined as a reduction in the initial VAS to a score of 3 or less. In addition, researchers attempted to contact patients for a telephone follow-up; however, they were only able to reach two of the six patients. During the telephone interview, researchers administered a questionnaire.
Pain Relief Achieved
Results from the data revealed a median age of 36.5 years; 83% of the patients were women. All of the patients were Caucasian, and the median age of migraine onset was 17. The median duration of the disease was 17 years. The mean number of failed acute migraine treatments was 18, and the mean number of failed preventive medications was 25. Pre-treatment pain scores ranged from 9 to 10.
In this small case series, all six patients with refractory migraine met
Overall, IV ketamine relieved pain in patients with chronic migraine without substantial adverse effects. “However, future study of this benefit on short-term headache relief needs to be conducted in a placebo-controlled fashion,” said Dr. Lauritsen.
“It is biologically plausible that ketamine could be an effective treatment for intractable headache,” the researchers said. “Ketamine is an antagonist at NMDA receptors, blocking the excitatory action of glutamate, a neurotransmitter long implicated in the pathophysiology of migraine. Glutamate has been … implicated in induction of cortical spreading depression [and] activation of trigeminal nociceptive neurons [and may] play a role in central sensitization.”
—Erica Tricarico
Suggested Reading
Lauritsen C, Mazuera S, Lipton RB, Ashina S. Intravenous ketamine for subacute treatment of refractory chronic migraine: a case series. J Headache Pain. 2016;17(1)106-110.
Propofol safety similar to that of traditional sedatives used in endoscopy
For doctors performing gastrointestinal endoscopic procedures, use of propofol as a sedative instead of the more commonly used drugs carries about the same risk of causing cardiopulmonary adverse events, according to a study published in the February issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2016.07.013).
“Because of its popularity, propofol is being used for both simple endoscopic procedures such as esophagogastroduodenoscopy and colonoscopy, and advanced endoscopic procedures [but] despite the widespread use of propofol, significant concerns remain regarding its safety profile,” according to the authors of the study, led by Vaibhav Wadhwa, MD, of Fairview Hospital in Cleveland.
The use of propofol as a sedative in gastrointestinal endoscopic procedures has increased in recent years, but because of an increasing number of advanced and, therefore, more complicated procedures being performed, the safety of sedatives has come into question because of their more prolonged use. Before use of propofol became prevalent, the more traditionally used sedative was a combination of benzodiazepine with an opioid. While still used today, this combination has seen a dramatic decline in usage because of its longer recovery time and lower rates of satisfaction among both patients and doctors, according to the authors. Combinations including midazolam, meperidine, pethidine, remifentanil, and fentanyl have also been used.
To compare the safety of propofol and a more traditional sedative combination, Dr. Wadhwa and his coauthors conducted a meta-analysis of published studies in the Medline (Ovid), EMBASE, and the Cochrane controlled trials registry databases. All searches were for research conducted through September of 2014, with the Medline database search starting in 1960, and the EMBASE and Cochrane searches starting in 1980, yielding a total of 2,117 studies eligible for inclusion.
Of those, 1,568 remained after duplicates were removed, then 136 were screened after removal of those deemed irrelevant or otherwise unsuitable. From those 136, 83 were excluded for various reasons – because they featured either ineligible populations, or were retrospective studies, single-arm studies, or conference abstracts – leaving 53 full-text articles to be evaluated for inclusion in the study. Of those, 27 were deemed eligible and were ultimately included.
“The primary outcomes measured were cardiopulmonary complications such as hypoxia, if oxygen saturation decreased to less than 90%; hypotension, if systolic blood pressure decreased to less than 90 mm Hg; arrhythmias, including bradycardia, supraventricular and ventricular arrhythmias, and ectopy,” Dr. Wadhwa and his coauthors wrote. “A subgroup analysis also was performed to assess studies in which sedation was directed by gastroenterologists and was compared with nongastroenterologists.” Apnea was not measured because of the lack of studies that assessed it qualitatively.
Pooled odds ratios were used to measure and compare results. The 27 included studies featured data on a total of 2,518 patients. Traditional sedatives were used on 1,194 of these subjects, while the remaining 1,324 received propofol. Regarding hypoxia, 26 of the 27 studies addressed this, of which 13 concluded that propofol was safer and 9 found that traditional sedatives were safer, with a pooled OR for propofol of 0.82 (95% confidence interval [CI] 0.63-1.07).
Twenty-five studies examined hypotension, of which 9 favored propofol and 10 favored traditional sedatives, for an OR of 0.92 (95% CI, 0.64-1.32). Of the 20 studies that included arrhythmia, 8 favored propofol and 7 favored traditional sedatives, for an OR of 1.07 (95% CI, 0.68-1.68).
“Our results showed that propofol sedation for gastrointestinal endoscopic procedures, whether simple or advanced, did not increase the cardiopulmonary adverse event rate when compared with traditional sedative agents,” the authors concluded.
In terms of the risk of developing any of the aforementioned complications, of the 20 relevant studies, 9 found propofol to be safer versus 6 that found traditional sedatives to be the better option, yielding an overall OR of 0.77 (95% CI, 0.56-1.07) for propofol. For the subanalysis regarding which type of clinician administered each sedative, 25 studies contained relevant data, of which 9 studies reported gastroenterologists administering sedatives, 5 studies reported endoscopy nurses administering sedatives under the supervision of the gastroenterologist, and 11 studies reported either an anesthesiologist, intensive care unit physician, or critical care physician administering sedatives.
“Gastroenterologist-directed sedation with propofol was noninferior to nongastroenterologist sedation,” Dr. Wadhwa and his coinvestigators wrote. “The risk of complications was similar to [that of traditional sedatives] both during simple and advanced endoscopic procedures.”
While the authors point to the sheer size of the study population as a huge strength of these results, they also note that because this is a study-level analysis rather than one conducted on an individual level, there is an inherent limitation to this study. Furthermore, variations from study to study in how propofol was administered to each patient may have caused heterogeneity with the findings of the meta-analysis. A large clinical trial would be the next logical step to affirm what this analysis has found.
“Because it may not be feasible to perform such a study, this meta-analysis should provide a rough idea of the possible associations,” the authors wrote. “However, the difference in complications between propofol and other agents might not be clinically relevant owing to the lack of any serious complications such as intubations or deaths in the studies used in this meta-analysis.”
No funding source was reported for this study. Dr. Wadhwa and his coauthors reported no relevant financial disclosures.
The use of propofol-mediated sedation and, in particular, anesthetist-directed sedation has become a hot-button item in the landscape of gastrointestinal endoscopy by virtue of its overall cost. Some experts place the cost of this at over $1.1 billion annually. Recent studies stemming from a large administrative database question the safety of propofol-mediated sedation when compared to the standard combination of a benzodiazepine and opioid. Still other studies have found that anesthesiologist-directed sedation did not improve the rate of polyp detection or polypectomy. Given these findings, our research group decided to embark upon a meta-analysis to further study the safety profile of propofol when compared to the combination of a benzodiazepine and opioid. We found that when compared to the traditional sedation agents, the pooled odds ratio of propofol-mediated sedation was not associated with a safety benefit in terms of the development of hypoxia or hypotension. We also found that the safety profile of propofol-mediated sedation was equivalent whether it was administered by a gastroenterologist or nongastroenterologist.
John Vargo, MD, MPH, is the department chair of gastroenterology and hepatology at Cleveland Clinic as well as vice chairman of Cleveland Clinic’s Digestive Disease Institute.
The use of propofol-mediated sedation and, in particular, anesthetist-directed sedation has become a hot-button item in the landscape of gastrointestinal endoscopy by virtue of its overall cost. Some experts place the cost of this at over $1.1 billion annually. Recent studies stemming from a large administrative database question the safety of propofol-mediated sedation when compared to the standard combination of a benzodiazepine and opioid. Still other studies have found that anesthesiologist-directed sedation did not improve the rate of polyp detection or polypectomy. Given these findings, our research group decided to embark upon a meta-analysis to further study the safety profile of propofol when compared to the combination of a benzodiazepine and opioid. We found that when compared to the traditional sedation agents, the pooled odds ratio of propofol-mediated sedation was not associated with a safety benefit in terms of the development of hypoxia or hypotension. We also found that the safety profile of propofol-mediated sedation was equivalent whether it was administered by a gastroenterologist or nongastroenterologist.
John Vargo, MD, MPH, is the department chair of gastroenterology and hepatology at Cleveland Clinic as well as vice chairman of Cleveland Clinic’s Digestive Disease Institute.
The use of propofol-mediated sedation and, in particular, anesthetist-directed sedation has become a hot-button item in the landscape of gastrointestinal endoscopy by virtue of its overall cost. Some experts place the cost of this at over $1.1 billion annually. Recent studies stemming from a large administrative database question the safety of propofol-mediated sedation when compared to the standard combination of a benzodiazepine and opioid. Still other studies have found that anesthesiologist-directed sedation did not improve the rate of polyp detection or polypectomy. Given these findings, our research group decided to embark upon a meta-analysis to further study the safety profile of propofol when compared to the combination of a benzodiazepine and opioid. We found that when compared to the traditional sedation agents, the pooled odds ratio of propofol-mediated sedation was not associated with a safety benefit in terms of the development of hypoxia or hypotension. We also found that the safety profile of propofol-mediated sedation was equivalent whether it was administered by a gastroenterologist or nongastroenterologist.
John Vargo, MD, MPH, is the department chair of gastroenterology and hepatology at Cleveland Clinic as well as vice chairman of Cleveland Clinic’s Digestive Disease Institute.
For doctors performing gastrointestinal endoscopic procedures, use of propofol as a sedative instead of the more commonly used drugs carries about the same risk of causing cardiopulmonary adverse events, according to a study published in the February issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2016.07.013).
“Because of its popularity, propofol is being used for both simple endoscopic procedures such as esophagogastroduodenoscopy and colonoscopy, and advanced endoscopic procedures [but] despite the widespread use of propofol, significant concerns remain regarding its safety profile,” according to the authors of the study, led by Vaibhav Wadhwa, MD, of Fairview Hospital in Cleveland.
The use of propofol as a sedative in gastrointestinal endoscopic procedures has increased in recent years, but because of an increasing number of advanced and, therefore, more complicated procedures being performed, the safety of sedatives has come into question because of their more prolonged use. Before use of propofol became prevalent, the more traditionally used sedative was a combination of benzodiazepine with an opioid. While still used today, this combination has seen a dramatic decline in usage because of its longer recovery time and lower rates of satisfaction among both patients and doctors, according to the authors. Combinations including midazolam, meperidine, pethidine, remifentanil, and fentanyl have also been used.
To compare the safety of propofol and a more traditional sedative combination, Dr. Wadhwa and his coauthors conducted a meta-analysis of published studies in the Medline (Ovid), EMBASE, and the Cochrane controlled trials registry databases. All searches were for research conducted through September of 2014, with the Medline database search starting in 1960, and the EMBASE and Cochrane searches starting in 1980, yielding a total of 2,117 studies eligible for inclusion.
Of those, 1,568 remained after duplicates were removed, then 136 were screened after removal of those deemed irrelevant or otherwise unsuitable. From those 136, 83 were excluded for various reasons – because they featured either ineligible populations, or were retrospective studies, single-arm studies, or conference abstracts – leaving 53 full-text articles to be evaluated for inclusion in the study. Of those, 27 were deemed eligible and were ultimately included.
“The primary outcomes measured were cardiopulmonary complications such as hypoxia, if oxygen saturation decreased to less than 90%; hypotension, if systolic blood pressure decreased to less than 90 mm Hg; arrhythmias, including bradycardia, supraventricular and ventricular arrhythmias, and ectopy,” Dr. Wadhwa and his coauthors wrote. “A subgroup analysis also was performed to assess studies in which sedation was directed by gastroenterologists and was compared with nongastroenterologists.” Apnea was not measured because of the lack of studies that assessed it qualitatively.
Pooled odds ratios were used to measure and compare results. The 27 included studies featured data on a total of 2,518 patients. Traditional sedatives were used on 1,194 of these subjects, while the remaining 1,324 received propofol. Regarding hypoxia, 26 of the 27 studies addressed this, of which 13 concluded that propofol was safer and 9 found that traditional sedatives were safer, with a pooled OR for propofol of 0.82 (95% confidence interval [CI] 0.63-1.07).
Twenty-five studies examined hypotension, of which 9 favored propofol and 10 favored traditional sedatives, for an OR of 0.92 (95% CI, 0.64-1.32). Of the 20 studies that included arrhythmia, 8 favored propofol and 7 favored traditional sedatives, for an OR of 1.07 (95% CI, 0.68-1.68).
“Our results showed that propofol sedation for gastrointestinal endoscopic procedures, whether simple or advanced, did not increase the cardiopulmonary adverse event rate when compared with traditional sedative agents,” the authors concluded.
In terms of the risk of developing any of the aforementioned complications, of the 20 relevant studies, 9 found propofol to be safer versus 6 that found traditional sedatives to be the better option, yielding an overall OR of 0.77 (95% CI, 0.56-1.07) for propofol. For the subanalysis regarding which type of clinician administered each sedative, 25 studies contained relevant data, of which 9 studies reported gastroenterologists administering sedatives, 5 studies reported endoscopy nurses administering sedatives under the supervision of the gastroenterologist, and 11 studies reported either an anesthesiologist, intensive care unit physician, or critical care physician administering sedatives.
“Gastroenterologist-directed sedation with propofol was noninferior to nongastroenterologist sedation,” Dr. Wadhwa and his coinvestigators wrote. “The risk of complications was similar to [that of traditional sedatives] both during simple and advanced endoscopic procedures.”
While the authors point to the sheer size of the study population as a huge strength of these results, they also note that because this is a study-level analysis rather than one conducted on an individual level, there is an inherent limitation to this study. Furthermore, variations from study to study in how propofol was administered to each patient may have caused heterogeneity with the findings of the meta-analysis. A large clinical trial would be the next logical step to affirm what this analysis has found.
“Because it may not be feasible to perform such a study, this meta-analysis should provide a rough idea of the possible associations,” the authors wrote. “However, the difference in complications between propofol and other agents might not be clinically relevant owing to the lack of any serious complications such as intubations or deaths in the studies used in this meta-analysis.”
No funding source was reported for this study. Dr. Wadhwa and his coauthors reported no relevant financial disclosures.
For doctors performing gastrointestinal endoscopic procedures, use of propofol as a sedative instead of the more commonly used drugs carries about the same risk of causing cardiopulmonary adverse events, according to a study published in the February issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2016.07.013).
“Because of its popularity, propofol is being used for both simple endoscopic procedures such as esophagogastroduodenoscopy and colonoscopy, and advanced endoscopic procedures [but] despite the widespread use of propofol, significant concerns remain regarding its safety profile,” according to the authors of the study, led by Vaibhav Wadhwa, MD, of Fairview Hospital in Cleveland.
The use of propofol as a sedative in gastrointestinal endoscopic procedures has increased in recent years, but because of an increasing number of advanced and, therefore, more complicated procedures being performed, the safety of sedatives has come into question because of their more prolonged use. Before use of propofol became prevalent, the more traditionally used sedative was a combination of benzodiazepine with an opioid. While still used today, this combination has seen a dramatic decline in usage because of its longer recovery time and lower rates of satisfaction among both patients and doctors, according to the authors. Combinations including midazolam, meperidine, pethidine, remifentanil, and fentanyl have also been used.
To compare the safety of propofol and a more traditional sedative combination, Dr. Wadhwa and his coauthors conducted a meta-analysis of published studies in the Medline (Ovid), EMBASE, and the Cochrane controlled trials registry databases. All searches were for research conducted through September of 2014, with the Medline database search starting in 1960, and the EMBASE and Cochrane searches starting in 1980, yielding a total of 2,117 studies eligible for inclusion.
Of those, 1,568 remained after duplicates were removed, then 136 were screened after removal of those deemed irrelevant or otherwise unsuitable. From those 136, 83 were excluded for various reasons – because they featured either ineligible populations, or were retrospective studies, single-arm studies, or conference abstracts – leaving 53 full-text articles to be evaluated for inclusion in the study. Of those, 27 were deemed eligible and were ultimately included.
“The primary outcomes measured were cardiopulmonary complications such as hypoxia, if oxygen saturation decreased to less than 90%; hypotension, if systolic blood pressure decreased to less than 90 mm Hg; arrhythmias, including bradycardia, supraventricular and ventricular arrhythmias, and ectopy,” Dr. Wadhwa and his coauthors wrote. “A subgroup analysis also was performed to assess studies in which sedation was directed by gastroenterologists and was compared with nongastroenterologists.” Apnea was not measured because of the lack of studies that assessed it qualitatively.
Pooled odds ratios were used to measure and compare results. The 27 included studies featured data on a total of 2,518 patients. Traditional sedatives were used on 1,194 of these subjects, while the remaining 1,324 received propofol. Regarding hypoxia, 26 of the 27 studies addressed this, of which 13 concluded that propofol was safer and 9 found that traditional sedatives were safer, with a pooled OR for propofol of 0.82 (95% confidence interval [CI] 0.63-1.07).
Twenty-five studies examined hypotension, of which 9 favored propofol and 10 favored traditional sedatives, for an OR of 0.92 (95% CI, 0.64-1.32). Of the 20 studies that included arrhythmia, 8 favored propofol and 7 favored traditional sedatives, for an OR of 1.07 (95% CI, 0.68-1.68).
“Our results showed that propofol sedation for gastrointestinal endoscopic procedures, whether simple or advanced, did not increase the cardiopulmonary adverse event rate when compared with traditional sedative agents,” the authors concluded.
In terms of the risk of developing any of the aforementioned complications, of the 20 relevant studies, 9 found propofol to be safer versus 6 that found traditional sedatives to be the better option, yielding an overall OR of 0.77 (95% CI, 0.56-1.07) for propofol. For the subanalysis regarding which type of clinician administered each sedative, 25 studies contained relevant data, of which 9 studies reported gastroenterologists administering sedatives, 5 studies reported endoscopy nurses administering sedatives under the supervision of the gastroenterologist, and 11 studies reported either an anesthesiologist, intensive care unit physician, or critical care physician administering sedatives.
“Gastroenterologist-directed sedation with propofol was noninferior to nongastroenterologist sedation,” Dr. Wadhwa and his coinvestigators wrote. “The risk of complications was similar to [that of traditional sedatives] both during simple and advanced endoscopic procedures.”
While the authors point to the sheer size of the study population as a huge strength of these results, they also note that because this is a study-level analysis rather than one conducted on an individual level, there is an inherent limitation to this study. Furthermore, variations from study to study in how propofol was administered to each patient may have caused heterogeneity with the findings of the meta-analysis. A large clinical trial would be the next logical step to affirm what this analysis has found.
“Because it may not be feasible to perform such a study, this meta-analysis should provide a rough idea of the possible associations,” the authors wrote. “However, the difference in complications between propofol and other agents might not be clinically relevant owing to the lack of any serious complications such as intubations or deaths in the studies used in this meta-analysis.”
No funding source was reported for this study. Dr. Wadhwa and his coauthors reported no relevant financial disclosures.
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point:
Major finding: Pooled odds ratio for propofol was 0.82 for hypoxia (95% CI, 0.63-1.07), 0.92 for hypotension (95% CI, 0.64-1.32), and 0.86 (95% CI, 0.56-1.34) for complication rate in advanced endoscopic procedures; subjects who received propofol were 39% less likely to have complications than were those who received traditional sedatives.
Data source: Retrospective meta-analysis of 27 studies involving 2,518 patients from 1966 through 2014.
Disclosures: The authors reported no relevant financial disclosures.
How Does Cannabidiol Interact With Antiepileptic Drugs?
HOUSTON—Cannabidiol (CBD) interacts significantly with clobazam, rufinamide, topiramate, zonisamide, and eslicarbazepine, researchers said at the 70th Annual Meeting of the American Epilepsy Society. The study results underscore the importance of monitoring levels of antiepileptic drugs (AEDs) during treatment with CBD. “In the future, these data will need to be correlated with reported side effects or laboratory abnormalities to determine whether they are clinically significant,” said Jerzy P. Szaflarski, MD, PhD, Director of the University of Alabama at Birmingham Epilepsy Center.
Dr. Szaflarski and colleagues monitored serum AED levels during active titration of pharmaceutical grade CBD in patients with refractory epilepsy who were enrolled in an open-label safety study. The study was intended to investigate CBD as a potential adjunctive therapy. As part of the study protocol, researchers checked serum AED levels frequently to identify interactions between CBD and AEDs. Based on previous data and anecdotal observations, Dr. Szaflarski and colleagues hypothesized that they would find interactions between CBD and clobazam and valproate.
Participants received an initial CBD dose of 5 mg/kg/day. The dose was increased by 5 mg/kg/day every two weeks, provided that tolerability was maintained, to a maximum of 50 mg/kg/day. Baseline AED levels were drawn, and AEDs were required to have been at a stable dose for one month before enrollment. The researchers obtained AED levels at almost all study visits during dose titration and maintenance. AED doses were adjusted at the investigators’ discretion if an adverse effect, laboratory abnormality, or drug level change was considered related to a potential interaction between CBD and the AED. The researchers frequently adjusted doses of clobazam and valproate because of complaints of sedation and alteration in liver function tests, respectively. At the time of Dr. Szaflarski’s analysis, 81 participants were enrolled in the study (39 adults and 42 children). There were sufficient data to analyze potential interactions between CBD and 19 AEDs.
The researchers found increases in serum levels of topiramate, rufinamide, and desmethylclobazam (an active metabolite of clobazam), and a decrease in levels of clobazam, with increasing CBD dose in the pediatric and adult arms. In addition, they noted significant increases in serum levels of zonisamide and eslicarbazepine with increasing CBD dose in the adult arm. Dr. Szaflarski and colleagues observed no significant interactions between CBD and the other AEDs investigated, which included valproate, levetiracetam, lacosamide, and perampanel.
—Erik Greb
HOUSTON—Cannabidiol (CBD) interacts significantly with clobazam, rufinamide, topiramate, zonisamide, and eslicarbazepine, researchers said at the 70th Annual Meeting of the American Epilepsy Society. The study results underscore the importance of monitoring levels of antiepileptic drugs (AEDs) during treatment with CBD. “In the future, these data will need to be correlated with reported side effects or laboratory abnormalities to determine whether they are clinically significant,” said Jerzy P. Szaflarski, MD, PhD, Director of the University of Alabama at Birmingham Epilepsy Center.
Dr. Szaflarski and colleagues monitored serum AED levels during active titration of pharmaceutical grade CBD in patients with refractory epilepsy who were enrolled in an open-label safety study. The study was intended to investigate CBD as a potential adjunctive therapy. As part of the study protocol, researchers checked serum AED levels frequently to identify interactions between CBD and AEDs. Based on previous data and anecdotal observations, Dr. Szaflarski and colleagues hypothesized that they would find interactions between CBD and clobazam and valproate.
Participants received an initial CBD dose of 5 mg/kg/day. The dose was increased by 5 mg/kg/day every two weeks, provided that tolerability was maintained, to a maximum of 50 mg/kg/day. Baseline AED levels were drawn, and AEDs were required to have been at a stable dose for one month before enrollment. The researchers obtained AED levels at almost all study visits during dose titration and maintenance. AED doses were adjusted at the investigators’ discretion if an adverse effect, laboratory abnormality, or drug level change was considered related to a potential interaction between CBD and the AED. The researchers frequently adjusted doses of clobazam and valproate because of complaints of sedation and alteration in liver function tests, respectively. At the time of Dr. Szaflarski’s analysis, 81 participants were enrolled in the study (39 adults and 42 children). There were sufficient data to analyze potential interactions between CBD and 19 AEDs.
The researchers found increases in serum levels of topiramate, rufinamide, and desmethylclobazam (an active metabolite of clobazam), and a decrease in levels of clobazam, with increasing CBD dose in the pediatric and adult arms. In addition, they noted significant increases in serum levels of zonisamide and eslicarbazepine with increasing CBD dose in the adult arm. Dr. Szaflarski and colleagues observed no significant interactions between CBD and the other AEDs investigated, which included valproate, levetiracetam, lacosamide, and perampanel.
—Erik Greb
HOUSTON—Cannabidiol (CBD) interacts significantly with clobazam, rufinamide, topiramate, zonisamide, and eslicarbazepine, researchers said at the 70th Annual Meeting of the American Epilepsy Society. The study results underscore the importance of monitoring levels of antiepileptic drugs (AEDs) during treatment with CBD. “In the future, these data will need to be correlated with reported side effects or laboratory abnormalities to determine whether they are clinically significant,” said Jerzy P. Szaflarski, MD, PhD, Director of the University of Alabama at Birmingham Epilepsy Center.
Dr. Szaflarski and colleagues monitored serum AED levels during active titration of pharmaceutical grade CBD in patients with refractory epilepsy who were enrolled in an open-label safety study. The study was intended to investigate CBD as a potential adjunctive therapy. As part of the study protocol, researchers checked serum AED levels frequently to identify interactions between CBD and AEDs. Based on previous data and anecdotal observations, Dr. Szaflarski and colleagues hypothesized that they would find interactions between CBD and clobazam and valproate.
Participants received an initial CBD dose of 5 mg/kg/day. The dose was increased by 5 mg/kg/day every two weeks, provided that tolerability was maintained, to a maximum of 50 mg/kg/day. Baseline AED levels were drawn, and AEDs were required to have been at a stable dose for one month before enrollment. The researchers obtained AED levels at almost all study visits during dose titration and maintenance. AED doses were adjusted at the investigators’ discretion if an adverse effect, laboratory abnormality, or drug level change was considered related to a potential interaction between CBD and the AED. The researchers frequently adjusted doses of clobazam and valproate because of complaints of sedation and alteration in liver function tests, respectively. At the time of Dr. Szaflarski’s analysis, 81 participants were enrolled in the study (39 adults and 42 children). There were sufficient data to analyze potential interactions between CBD and 19 AEDs.
The researchers found increases in serum levels of topiramate, rufinamide, and desmethylclobazam (an active metabolite of clobazam), and a decrease in levels of clobazam, with increasing CBD dose in the pediatric and adult arms. In addition, they noted significant increases in serum levels of zonisamide and eslicarbazepine with increasing CBD dose in the adult arm. Dr. Szaflarski and colleagues observed no significant interactions between CBD and the other AEDs investigated, which included valproate, levetiracetam, lacosamide, and perampanel.
—Erik Greb
VIDEO: Protein-rich diet can help manage type 2 diabetes, NAFLD
Patients with type 2 diabetes should be put on diets rich in either animal or plant protein to reduce not only liver fat, but insulin resistance and hepatic necroinflammation markers as well, according to a study published in the February issue of Gastroenterology (doi: 10.1053/j.gastro.2016.10.007).
“High-protein diets have shown variable and sometimes even favorable effects on glucose metabolism and insulin sensitivity in people with type 2 diabetes and it is unclear which metabolic pathways are involved,” wrote the authors of the study, led by Mariya Markova, MD, of the German Institute of Human Nutrition Potsdam-Rehbrücke in Nuthetal, Germany.
SOURCE: American Gastroenterological Association
Obesity and insulin resistance have long been linked to liver fat, with excessive amounts of the latter causing nonalcoholic fatty liver disease (NAFLD), with a significant risk of nonalcoholic steatohepatitis (NASH) developing as well. Compounding this issue, at least in the United States, are widespread dietary and nutritional habits that promote consumption of animal protein, carbohydrates, and saturated fats. This “hypercaloric Western style diet,” as the authors call it, exacerbates the accumulation of fat deposits in the liver and complicates the health of patients across the country, regardless of weight.
“Remarkably, diets restricted in methionine were shown to prevent the development of insulin resistance and of the metabolic syndrome in animal models [so] the type of protein may elicit different metabolic responses depending on the amino acid composition,” Dr. Markova and her coinvestigators noted. “It is therefore hypothesized that high-plant-protein diets exert favorable effects on hepatic fat content and metabolic responses as compared to high intake of animal protein rich in BCAA [branched-chain amino acids] and methionine,” both of which can be found in suitably low levels via plant protein.
Dr. Markova and her team devised a prospective, randomized, open-label clinical trial involving 44 patients with type 2 diabetes and NAFLD, all of whom were recruited at the department of clinical nutrition of the German Institute of Human Nutrition Potsdam-Rehbrücke between June 2013 and March 2015. Subjects were randomized into one of two cohorts, each of which were assigned a diet rich in either animal protein (AP) or plant protein (PP) for a period of 6 weeks. Median body mass index in the AP cohort was 31.0 ± 0.8, and was 29.4 ± 1.0 in the PP cohort.
The AP cohort diet consisted mainly of meat and dairy products, while legumes constituted the bulk of the PP cohort diet. Both diets were isocaloric and had the same macronutrient makeup: 30% protein, 40% carbohydrates, and 30% fat. Seven subjects dropped out prior to completion of the study; of the 37 that remained all the way through – 19 in the AP cohort, 18 in the PP cohort – the age range was 49-78 years. Subjects maintained the same physical exercise regimens throughout the study that they had beforehand, and were asked not to alter them. Hemoglobin A1c levels ranged from 5.8% to 8.8% at baseline, and evaluations were carried out at fasting levels for each subject.
Patients in both cohorts saw significant decreases in intrahepatic fat content by the end of the trial period. Those in the AP cohort saw decreases of 48.0% (P = .0002), while those in the PP cohort saw a decrease of 35.7% (P = .001). Perhaps most importantly, the reductions in both cohorts were not correlated to body weight. In addition, levels of fibroblast growth factor 21 (FGF21), which has been shown to be a predictive marker of NAFLD, decreased by nearly 50% for both AP and PP cohorts (P less than .0002 for both).
“Despite the elevated intake and postprandial uptake of methionine and BCAA in the AP group, there was no indication of negative effects of these components,” the authors stated in the study. “The origin of protein – animal or plant – did not play a major role. Both high-protein diets unexpectedly induced strong reductions of FGF21, which was associated with metabolic improvements and the decrease of IHL.”
Despite these findings, however, the 6-week time span used here is not sufficient to determine just how viable this diet may be in the long term, according to the authors. Further studies will be needed, and will need to take place over longer periods of time, to “show the durability of the responses and eventual adverse effects of the diets.” Furthermore, different age groups must be examined to find out if the benefits observed by Dr. Markova and her coinvestigators were somehow related to the age of these subjects.
The study was funded by grants from German Federal Ministry of Food and Agriculture and German Center for Diabetes Research. Dr. Markova and her coauthors did not report any financial disclosures.
Human studies to assess the effects of isocaloric macronutrient substitution are fraught with difficulty. If one macronutrient is increased, what happens to the others? If you observe an effect, is it the phenomenon you were seeking due to the macronutrient you altered, or an epiphenomenon due to changes in the others?
Markova et al. attempted to study a 6-week “isocaloric” increase of animal vs. plant protein (from 17% to 30% of calories as protein). However, a decrease of percent fat from 41% to 30%, and a reduction in carbohydrate from 42% to 40% occurred commensurately. This brings up three concerns. First, despite the diet’s being “isocaloric,” weight and body mass index decreased by 2 kg and 0.8 kg/m2, respectively. Reductions in intrahepatic, visceral, and subcutaneous fat, and an increase in lean body mass were noted. So was the diet isocaloric? Protein reduces plasma ghrelin levels and is more satiating. Furthermore, metabolism of protein to ATP is inefficient compared to that of carbohydrate or fat. The authors say only that calories were “unrestricted.” These issues do not engender “isocaloric” confidence.
Lastly, the type of carbohydrate was not controlled for. Fructose is significantly more lipogenic than glucose. Yet they were lumped together as “carbohydrate,” and were uncontrolled. So what macronutrient really caused the reduction in liver fat? These methodological issues detract from the author’s message, and this study must be considered preliminary.
Robert H. Lustig, MD, MSL, is in the division of pediatric endocrinology, UCSF Benioff Children’s Hospital, San Francisco; member, UCSF Institute for Health Policy Studies. Dr. Lustig declared no conflicts of interest.
Human studies to assess the effects of isocaloric macronutrient substitution are fraught with difficulty. If one macronutrient is increased, what happens to the others? If you observe an effect, is it the phenomenon you were seeking due to the macronutrient you altered, or an epiphenomenon due to changes in the others?
Markova et al. attempted to study a 6-week “isocaloric” increase of animal vs. plant protein (from 17% to 30% of calories as protein). However, a decrease of percent fat from 41% to 30%, and a reduction in carbohydrate from 42% to 40% occurred commensurately. This brings up three concerns. First, despite the diet’s being “isocaloric,” weight and body mass index decreased by 2 kg and 0.8 kg/m2, respectively. Reductions in intrahepatic, visceral, and subcutaneous fat, and an increase in lean body mass were noted. So was the diet isocaloric? Protein reduces plasma ghrelin levels and is more satiating. Furthermore, metabolism of protein to ATP is inefficient compared to that of carbohydrate or fat. The authors say only that calories were “unrestricted.” These issues do not engender “isocaloric” confidence.
Lastly, the type of carbohydrate was not controlled for. Fructose is significantly more lipogenic than glucose. Yet they were lumped together as “carbohydrate,” and were uncontrolled. So what macronutrient really caused the reduction in liver fat? These methodological issues detract from the author’s message, and this study must be considered preliminary.
Robert H. Lustig, MD, MSL, is in the division of pediatric endocrinology, UCSF Benioff Children’s Hospital, San Francisco; member, UCSF Institute for Health Policy Studies. Dr. Lustig declared no conflicts of interest.
Human studies to assess the effects of isocaloric macronutrient substitution are fraught with difficulty. If one macronutrient is increased, what happens to the others? If you observe an effect, is it the phenomenon you were seeking due to the macronutrient you altered, or an epiphenomenon due to changes in the others?
Markova et al. attempted to study a 6-week “isocaloric” increase of animal vs. plant protein (from 17% to 30% of calories as protein). However, a decrease of percent fat from 41% to 30%, and a reduction in carbohydrate from 42% to 40% occurred commensurately. This brings up three concerns. First, despite the diet’s being “isocaloric,” weight and body mass index decreased by 2 kg and 0.8 kg/m2, respectively. Reductions in intrahepatic, visceral, and subcutaneous fat, and an increase in lean body mass were noted. So was the diet isocaloric? Protein reduces plasma ghrelin levels and is more satiating. Furthermore, metabolism of protein to ATP is inefficient compared to that of carbohydrate or fat. The authors say only that calories were “unrestricted.” These issues do not engender “isocaloric” confidence.
Lastly, the type of carbohydrate was not controlled for. Fructose is significantly more lipogenic than glucose. Yet they were lumped together as “carbohydrate,” and were uncontrolled. So what macronutrient really caused the reduction in liver fat? These methodological issues detract from the author’s message, and this study must be considered preliminary.
Robert H. Lustig, MD, MSL, is in the division of pediatric endocrinology, UCSF Benioff Children’s Hospital, San Francisco; member, UCSF Institute for Health Policy Studies. Dr. Lustig declared no conflicts of interest.
Patients with type 2 diabetes should be put on diets rich in either animal or plant protein to reduce not only liver fat, but insulin resistance and hepatic necroinflammation markers as well, according to a study published in the February issue of Gastroenterology (doi: 10.1053/j.gastro.2016.10.007).
“High-protein diets have shown variable and sometimes even favorable effects on glucose metabolism and insulin sensitivity in people with type 2 diabetes and it is unclear which metabolic pathways are involved,” wrote the authors of the study, led by Mariya Markova, MD, of the German Institute of Human Nutrition Potsdam-Rehbrücke in Nuthetal, Germany.
SOURCE: American Gastroenterological Association
Obesity and insulin resistance have long been linked to liver fat, with excessive amounts of the latter causing nonalcoholic fatty liver disease (NAFLD), with a significant risk of nonalcoholic steatohepatitis (NASH) developing as well. Compounding this issue, at least in the United States, are widespread dietary and nutritional habits that promote consumption of animal protein, carbohydrates, and saturated fats. This “hypercaloric Western style diet,” as the authors call it, exacerbates the accumulation of fat deposits in the liver and complicates the health of patients across the country, regardless of weight.
“Remarkably, diets restricted in methionine were shown to prevent the development of insulin resistance and of the metabolic syndrome in animal models [so] the type of protein may elicit different metabolic responses depending on the amino acid composition,” Dr. Markova and her coinvestigators noted. “It is therefore hypothesized that high-plant-protein diets exert favorable effects on hepatic fat content and metabolic responses as compared to high intake of animal protein rich in BCAA [branched-chain amino acids] and methionine,” both of which can be found in suitably low levels via plant protein.
Dr. Markova and her team devised a prospective, randomized, open-label clinical trial involving 44 patients with type 2 diabetes and NAFLD, all of whom were recruited at the department of clinical nutrition of the German Institute of Human Nutrition Potsdam-Rehbrücke between June 2013 and March 2015. Subjects were randomized into one of two cohorts, each of which were assigned a diet rich in either animal protein (AP) or plant protein (PP) for a period of 6 weeks. Median body mass index in the AP cohort was 31.0 ± 0.8, and was 29.4 ± 1.0 in the PP cohort.
The AP cohort diet consisted mainly of meat and dairy products, while legumes constituted the bulk of the PP cohort diet. Both diets were isocaloric and had the same macronutrient makeup: 30% protein, 40% carbohydrates, and 30% fat. Seven subjects dropped out prior to completion of the study; of the 37 that remained all the way through – 19 in the AP cohort, 18 in the PP cohort – the age range was 49-78 years. Subjects maintained the same physical exercise regimens throughout the study that they had beforehand, and were asked not to alter them. Hemoglobin A1c levels ranged from 5.8% to 8.8% at baseline, and evaluations were carried out at fasting levels for each subject.
Patients in both cohorts saw significant decreases in intrahepatic fat content by the end of the trial period. Those in the AP cohort saw decreases of 48.0% (P = .0002), while those in the PP cohort saw a decrease of 35.7% (P = .001). Perhaps most importantly, the reductions in both cohorts were not correlated to body weight. In addition, levels of fibroblast growth factor 21 (FGF21), which has been shown to be a predictive marker of NAFLD, decreased by nearly 50% for both AP and PP cohorts (P less than .0002 for both).
“Despite the elevated intake and postprandial uptake of methionine and BCAA in the AP group, there was no indication of negative effects of these components,” the authors stated in the study. “The origin of protein – animal or plant – did not play a major role. Both high-protein diets unexpectedly induced strong reductions of FGF21, which was associated with metabolic improvements and the decrease of IHL.”
Despite these findings, however, the 6-week time span used here is not sufficient to determine just how viable this diet may be in the long term, according to the authors. Further studies will be needed, and will need to take place over longer periods of time, to “show the durability of the responses and eventual adverse effects of the diets.” Furthermore, different age groups must be examined to find out if the benefits observed by Dr. Markova and her coinvestigators were somehow related to the age of these subjects.
The study was funded by grants from German Federal Ministry of Food and Agriculture and German Center for Diabetes Research. Dr. Markova and her coauthors did not report any financial disclosures.
Patients with type 2 diabetes should be put on diets rich in either animal or plant protein to reduce not only liver fat, but insulin resistance and hepatic necroinflammation markers as well, according to a study published in the February issue of Gastroenterology (doi: 10.1053/j.gastro.2016.10.007).
“High-protein diets have shown variable and sometimes even favorable effects on glucose metabolism and insulin sensitivity in people with type 2 diabetes and it is unclear which metabolic pathways are involved,” wrote the authors of the study, led by Mariya Markova, MD, of the German Institute of Human Nutrition Potsdam-Rehbrücke in Nuthetal, Germany.
SOURCE: American Gastroenterological Association
Obesity and insulin resistance have long been linked to liver fat, with excessive amounts of the latter causing nonalcoholic fatty liver disease (NAFLD), with a significant risk of nonalcoholic steatohepatitis (NASH) developing as well. Compounding this issue, at least in the United States, are widespread dietary and nutritional habits that promote consumption of animal protein, carbohydrates, and saturated fats. This “hypercaloric Western style diet,” as the authors call it, exacerbates the accumulation of fat deposits in the liver and complicates the health of patients across the country, regardless of weight.
“Remarkably, diets restricted in methionine were shown to prevent the development of insulin resistance and of the metabolic syndrome in animal models [so] the type of protein may elicit different metabolic responses depending on the amino acid composition,” Dr. Markova and her coinvestigators noted. “It is therefore hypothesized that high-plant-protein diets exert favorable effects on hepatic fat content and metabolic responses as compared to high intake of animal protein rich in BCAA [branched-chain amino acids] and methionine,” both of which can be found in suitably low levels via plant protein.
Dr. Markova and her team devised a prospective, randomized, open-label clinical trial involving 44 patients with type 2 diabetes and NAFLD, all of whom were recruited at the department of clinical nutrition of the German Institute of Human Nutrition Potsdam-Rehbrücke between June 2013 and March 2015. Subjects were randomized into one of two cohorts, each of which were assigned a diet rich in either animal protein (AP) or plant protein (PP) for a period of 6 weeks. Median body mass index in the AP cohort was 31.0 ± 0.8, and was 29.4 ± 1.0 in the PP cohort.
The AP cohort diet consisted mainly of meat and dairy products, while legumes constituted the bulk of the PP cohort diet. Both diets were isocaloric and had the same macronutrient makeup: 30% protein, 40% carbohydrates, and 30% fat. Seven subjects dropped out prior to completion of the study; of the 37 that remained all the way through – 19 in the AP cohort, 18 in the PP cohort – the age range was 49-78 years. Subjects maintained the same physical exercise regimens throughout the study that they had beforehand, and were asked not to alter them. Hemoglobin A1c levels ranged from 5.8% to 8.8% at baseline, and evaluations were carried out at fasting levels for each subject.
Patients in both cohorts saw significant decreases in intrahepatic fat content by the end of the trial period. Those in the AP cohort saw decreases of 48.0% (P = .0002), while those in the PP cohort saw a decrease of 35.7% (P = .001). Perhaps most importantly, the reductions in both cohorts were not correlated to body weight. In addition, levels of fibroblast growth factor 21 (FGF21), which has been shown to be a predictive marker of NAFLD, decreased by nearly 50% for both AP and PP cohorts (P less than .0002 for both).
“Despite the elevated intake and postprandial uptake of methionine and BCAA in the AP group, there was no indication of negative effects of these components,” the authors stated in the study. “The origin of protein – animal or plant – did not play a major role. Both high-protein diets unexpectedly induced strong reductions of FGF21, which was associated with metabolic improvements and the decrease of IHL.”
Despite these findings, however, the 6-week time span used here is not sufficient to determine just how viable this diet may be in the long term, according to the authors. Further studies will be needed, and will need to take place over longer periods of time, to “show the durability of the responses and eventual adverse effects of the diets.” Furthermore, different age groups must be examined to find out if the benefits observed by Dr. Markova and her coinvestigators were somehow related to the age of these subjects.
The study was funded by grants from German Federal Ministry of Food and Agriculture and German Center for Diabetes Research. Dr. Markova and her coauthors did not report any financial disclosures.
FROM GASTROENTEROLOGY
Key clinical point:
Major finding: Animal- and plant-protein diets reduced liver fat for type 2 diabetes patients by 36%-48% over the course of 6 months (P = .0002 and P = .001, respectively).
Data source: Prospective study of 37 type 2 diabetes patients from June 2013 to March 2015.
Disclosures: The German Federal Ministry of Food and Agriculture and German Center for Diabetes Research supported the study. The authors did not report any financial disclosures.
Papillary Transitional Cell Bladder Carcinoma and Systematized Epidermal Nevus Syndrome
Epidermal nevi can occur in isolation or in association with internal abnormalities. Epidermal nevus syndrome (ENS) is a heterogeneous group of neurocutaneous disorders characterized by mosaicism and epidermal nevi found in association with various systemic abnormalities.1-4 There are many possible associated systemic findings, including abnormalities of the central nervous, musculoskeletal, renal, and hematologic systems. Epidermal nevi have been associated with internal malignancies. We present the case of a patient with epidermal nevi associated with papillary transitional cell bladder carcinoma. According to a PubMed search of articles indexed for MEDLINE using the search terms transitional cell bladder carcinoma and epidermal nevus, there have only been 4 other cases of transitional cell bladder carcinoma and ENS reported in the literature,5-8 2 of which were reports of papillary transitional cell bladder carcinoma.5,6
Case Report
A 29-year-old woman presented to our clinic with a rash that had been present since 3 years of age. The emergency department consulted dermatology for evaluation of what was believed to be contact dermatitis; however, upon questioning the patient, it was revealed that the rash was chronic and persistent.
The rash was nonpruritic and was located on the face, hands (Figure 1), chest, buttocks, thighs, legs, and back (Figure 2). Although asymptomatic, the appearance of the skin caused the patient some emotional distress. As a child she had been evaluated by a dermatologist and a biopsy was performed, but she did not recall the results or have any records. She had been prescribed an oral medication by the dermatologist, but treatment was terminated early due to nausea. The skin lesions did not improve with the short course of treatment.
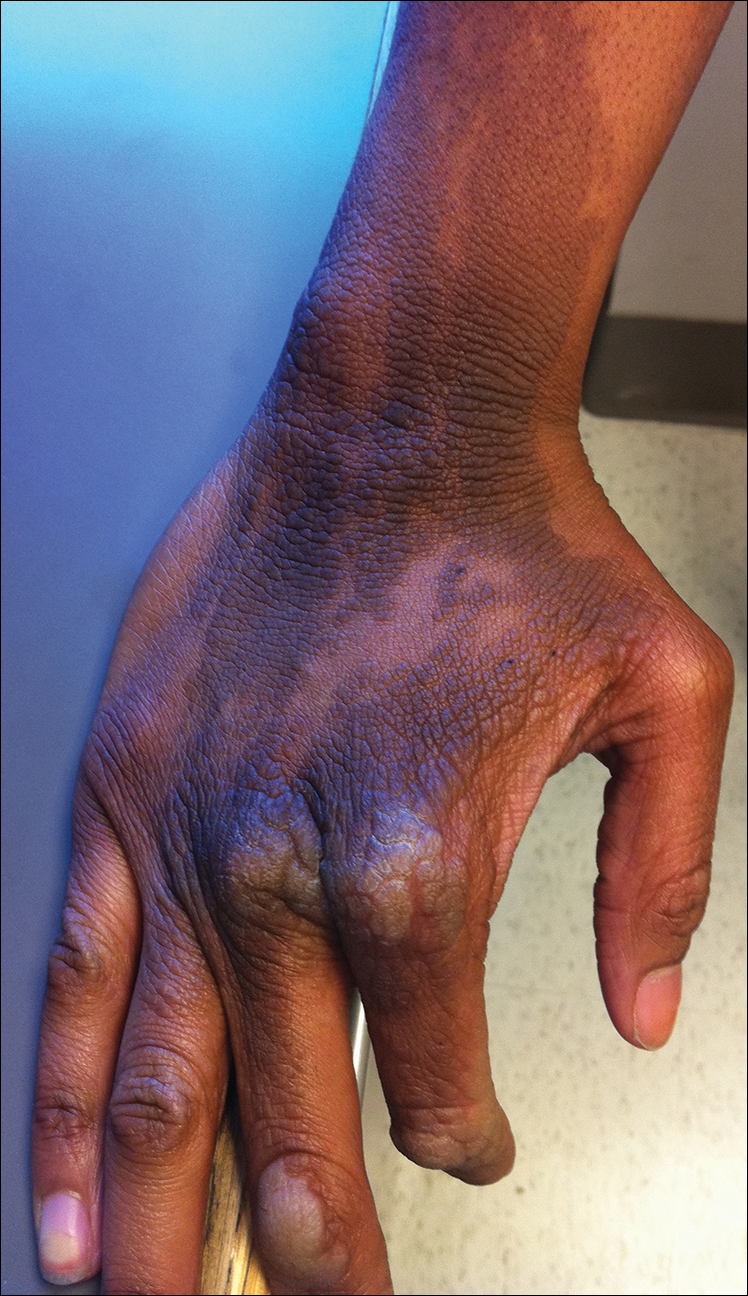
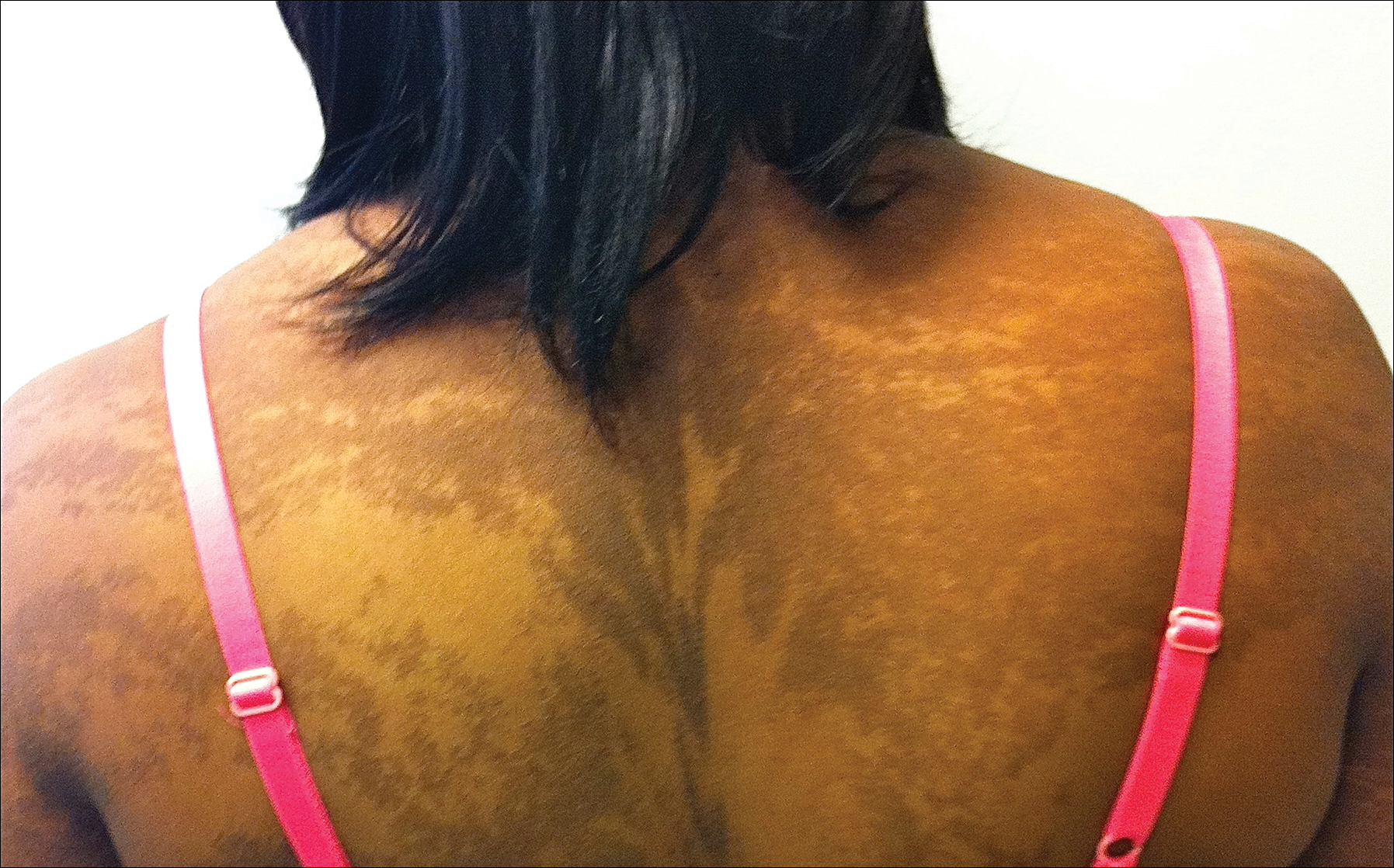
Eighteen months prior to presentation to our clinic, the patient was discovered to have hematuria on routine examination by her primary care physician. At that time, the patient underwent a workup for hematuria and a mass was discovered in the bladder via cystoscopy. A diagnosis of low-grade papillary transitional cell bladder carcinoma was made, and she underwent a partial cystectomy. No radiation or chemotherapy was required. The remainder of her medical history was only remarkable for asthma, which was well controlled with albuterol. On examination, generalized, hyperpigmented, reticulated patches, macules, and hyperpigmented verrucous plaques were distributed along the Blaschko lines, sparing the face. No limb abnormalities or dental or nail abnormalities were noted. Examination of the axillary and cervical lymph nodes was unremarkable, and no neurological abnormalities were noted. A 3-mm punch biopsy of the mid upper back was performed. Histopathology revealed papillomatous, nonorganoid, nonepidermolytic hyperplasia of the epidermis with elongated rete ridges (Figure 3), which was diagnosed as a nonorganoid nonepidermolytic epidermal nevus.
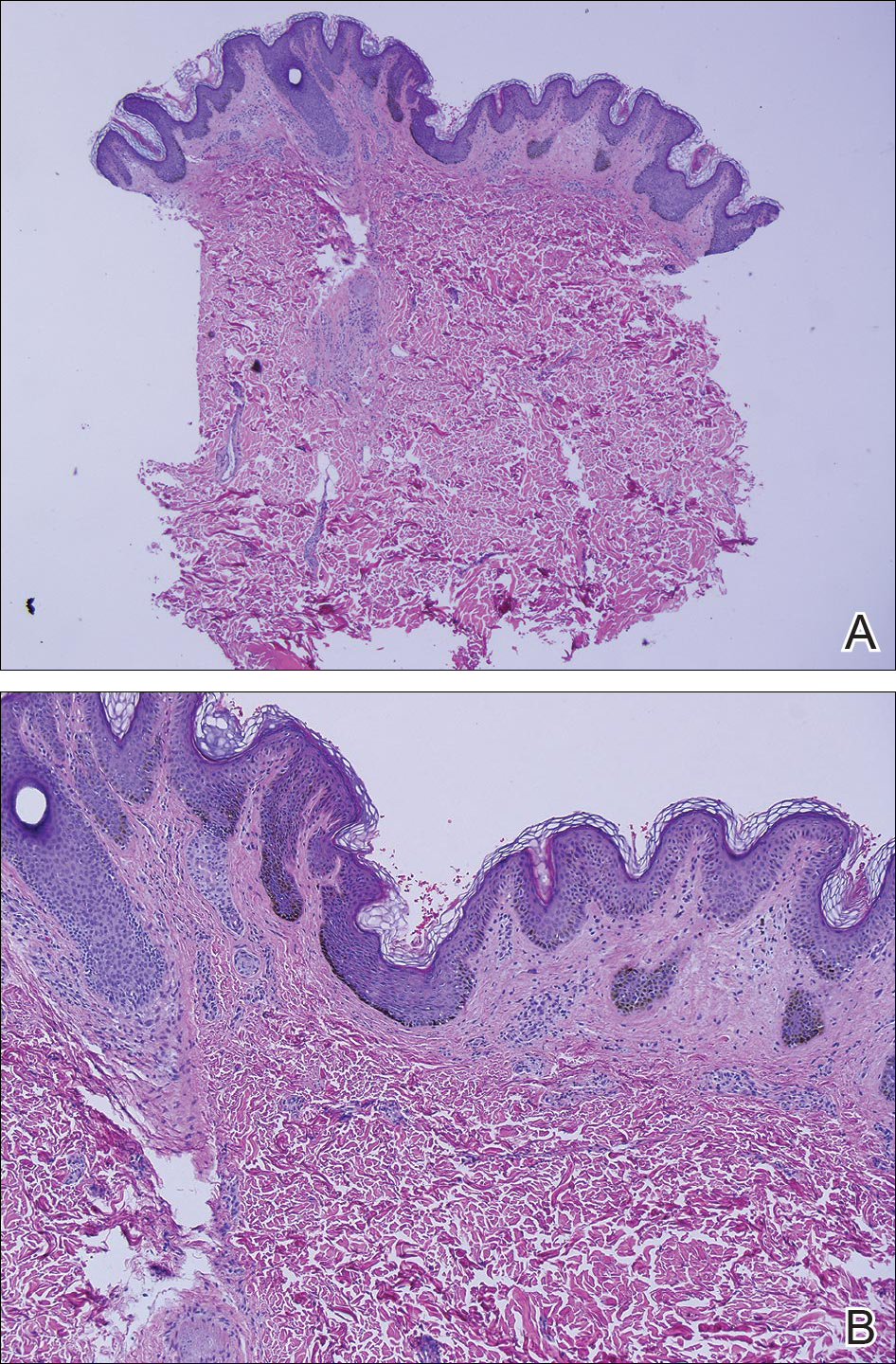
Comment
Epidermal nevus syndrome is a group of disorders characterized by both local or systematized epidermal nevi and systemic findings. Solomon et al4 first coined the term epidermal nevus syndrome more than 40 years ago; however, since then there has been confusion about how to define ENS. Epidermal nevus syndrome has been considered an umbrella term that includes more specific syndromes involving epidermal nevi, such as Proteus syndrome and Schimmelpenning syndrome; conversely, it also has been considered a term for those who do not meet the criteria for more specific syndromes.1,9 Happle1 discussed that the genetic variations found in ENS warrant recognition. Simply put, ENS is a heterogeneous group of syndromes that are similar in that they involve epidermal nevi and internal abnormalities but are genetically distinct. The list of definitive ENSs, as suggested by Happle1 and others, will likely continue to grow.3,5
The exact pathomechanism of ENS is unknown, but the clinical presentation most likely represents a lethal disorder mitigated by mosaicism.2,9 Gene defects vary depending on the specific ENS. For instance, the phosphatase and tensin homolog gene, PTEN, mutations have been associated with type 2 segmental Cowden disease. Fibroblast growth factor receptor 3, FGFR3, mutations have been linked to Garcia-Hafner-Happle syndrome.3FGFR3 mutations have been found in nonepidermolytic epidermal nevi, and some suggest that the majority of epidermal nevi exhibit mutations in FGFR3.5,10,11 On the other hand, other gene defects have not been elucidated, such as in Schimmelpenning syndrome.3
Clinically, ENS may involve nonepidermolytic verrucous nevi, sebaceous nevi, organoid nevi, linear Cowden nevi, and woolly hair nevi. Lesions may be flesh-colored, pink, yellow, or hyperpigmented plaques in a blaschkoid distribution and may be localized or systematized. Nevi typically are present at birth or develop within the first year of life.9,12,13 Other cutaneous findings may be noted apart from epidermal nevi, including melanocytic nevi, aplasia cutis congenita, and hemangiomas.13,14
Extracutaneous findings include central nervous system, skeletal, ocular, cardiac, and genitourinary defects, which are often observed in these patients.3,9,13,14 Central nervous system findings are seen in 50% to 70% of cases, with seizures and mental retardation among the most common.13-15 Genitourinary abnormalities associated with epidermal nevi, including horseshoe kidney, cystic kidney, duplicated collecting system, testicular and paratesticular tumors, and hypospadias have been documented in the literature.16 Our patient had a history of papillary transitional cell bladder carcinoma, which is rare for a patient younger than 30 years. The overall median age of diagnosis of bladder cancer is 65 years, and it is more common in men than in women.17 Transitional cell carcinomas account for approximately 90% of all bladder cancers in the United States. Other common types of bladder cancer include squamous cell carcinoma, adenocarcinoma, and rhabdomyosarcoma.16 Typically, transitional cell carcinoma is associated with smoking, exposure to aniline dyes, cyclophosphamide, and living in industrialized areas.16,17 Individuals who work with textiles, dyes, leather, tires, rubber, and/or petroleum; painters; truck drivers; drill press operators; and hairdressers are at an increased risk for development of bladder cancer.16
Interestingly, it has been shown in some studies that papillary transitional cell bladder carcinoma frequently is associated with FGFR3 mutations, which may be the missing link in the rare finding of papillary transitional cell bladder carcinoma and epidermal nevi.5,18,19 In addition, PTEN mutations also have been identified in low-grade papillary transitional cell carcinomas of the bladder, another gene linked to an ENS with type 2 segmental Cowden disease.3,20
Histopathologically, epidermal nevi have 10 different descriptions. Our patient had a nonorganoid nonepidermolytic epidermal nevus characterized by hyperkeratosis, acanthosis, papillomatosis, and elongated rete ridges. Focal acantholysis and epidermolytic hyperkeratosis also is seen in some epidermal nevi but was not seen in this case.9,21
Simple epidermal nevi occur in approximately 1 in 1000 newborns; however, when a child presents with multiple or systematized epidermal nevi, investigation should be undertaken for other possible associations.13,14 Of note, there have been several cases of squamous cell, verrucous, basal cell, and adnexal carcinomas arising in linear epidermal nevi.22-24
Epidermal nevi can be difficult to treat. Some patients are troubled by the appearance of these nevi, especially those with systematized disease. Unfortunately, for patients with multiple nevi or systematized disease, there are no consistently effective treatment options; however, there are case reports25,26 in the literature citing improvement or cure of epidermal nevi with full-thickness excision, continuous and pulsed CO2 laser, pulsed dye laser, and erbium-doped YAG laser.25 Other therapies that have been purported to help improve epidermal nevi are topical and oral retinoids, corticosteroids, topical 5-fluorouracil, anthralin, and podophyllin.26
Conclusion
Transitional cell bladder carcinoma is rare in patients in the third decade of life and younger. Given the age of our patient and her concomitant lack of risk factors, such as older age, history of smoking, and exposure to certain chemicals (eg, aniline dyes) and medications (eg, cyclophosphamide), it is more likely that the finding of papillary transitional cell bladder carcinoma and ENS are related. A clear genetic link between ENS and transitional cell papillary bladder carcinoma has yet to be elucidated, but the FGFR3 gene is promising.
- Happle R. What is a nevus? a proposed definition of a common medical term. Dermatology. 1995;191:1-5.
- Gonzalez ME, Jabbari A, Tlougan BE, et al. Epidermal nevus. Dermatol Online J. 2010;16:12.
- Happle R. The group of epidermal nevus syndromes. part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22.
- Solomon LM, Fretzin DF, Dewald RL. The epidermal nevus syndrome. Arch Dermatol. 1968;97:273-285.
- Flosadottir E, Bjarnason B. A non-epidermolytic epidermal nevus of a soft, papillomatous type with transitional cell cancer of the bladder: a case report and review of non-cutaneous cancers associated with epidermal naevi. Acta Derm Venerol. 2008;88:173-175.
- Rosenthal D, Fretzin DF. Epidermal nevus syndrome: report of association with transitional cell carcinoma of the bladder. Pediatr Dermatol. 1986;3:455-458.
- Garcia de Jalon A, Azua-Romea J, Trivez MA, et al. Epidermal naevus syndrome (Solomon’s syndrome) associated with bladder cancer in a 20-year-old female. Scand J Urol Nephrol. 2004;38:85-87.
- Rongioletti F, Rebora A. Epidermal nevus with transitional cell carcinomas of the urinary tract. J Am Acad Dermatol. 1991;25:856-858.
- Moss C. Mosacism and linear lesions. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. St. Louis, MO: Mosby/Elsevier; 2012:943-962.
- Hafner C, van Oers JM, Vogt T, et al. Mosaicisim of activating FGFR3 mutations in human skin causes epidermal nevi. J Clin Invest. 2006;116:2201-2207.
- Bygum A, Fagerberg CR, Clemmensen OJ, et al. Systemic epidermal nevus with involvement of the oral mucosa due to FGFR3 mutation. BMC Med Genet. 2011;12:79.
- Happle R. Linear Cowden nevus: a new distinct epidermal nevus. Eur J Dermatol. 2007;17:133-136.
- Vujevich JJ, Mancini AJ. The epidermal nevus syndromes: multisystem disorders. J Am Acad Dermatol. 2004;50:957-961.
- Solomon L, Esterly N. Epidermal and other congenital organoid nevi. Curr Probl Pediatr. 1975;6:1-56.
- Grebe TA, Rimsa ME, Richter SF, et al. Further delineation of the epidermal nevus syndrome: two cases with new findings and literature review. Am J Med Genet. 1993;47:24-30.
- Lamm DL, Torti FM. Bladder cancer, 1996. Ca Cancer J Clin. 1996;46:93-112.
- Metts MC, Metts JC, Milito SJ, et al. Bladder cancer: a review of diagnosis and management. J Natl Med Assoc. 2000;92:285-294.
- Kimura T, Suzuki H, Ohashi T, et al. The incidence of thanatophoric dysplasia mutations in FGFR3 gene is higher in low-grade or superficial bladder carcinomas. Cancer. 2001;92:2555-2561.
- Cappellen D, DeOliveira C, Ricol D, et al. Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat Genet. 1999;23:18-20.
- Knowles MA, Platt FM, Ross RL, et al. Phosphatidylinositol 3-kinase (PI3K) pathway activation in bladder cancer. Cancer Metastasis Rev. 2009;28:305-316.
- Luzar B, Calonje E, Bastian B. Tumors of the surface epithelium. In: Calonje JE, Breen T, McKee PH, eds. McKee’s Pathology of the Skin. 4th ed. Edinburgh, Scotland: Elsevier/Saunders; 2012:1076-1149.
- Masood Q, Narayan D. Squamous cell carcinoma in a linear epidermal nevus. J Plast Reconstr Aesthet Surg. 2009;62:693-694.
- Cramer SF, Mandel MA, Hauler R, et al. Squamous cell carcinoma arising in a linear epidermal nevus. Arch Dermatol. 1981;117:222-224.
- Affleck AG, Leach IJ, Varma S. Two squamous cell carcinomas arising in a linear epidermal nevus in a 28-year-old female. Clin Exp Dermatol. 2005;30:382-384.
- Alam M, Arndt KA. A method for pulsed carbon dioxide laser treatment of epidermal nevi. J Am Acad Dermatol. 2002;46:554-556.
- Requena L, Requena C, Cockerell CJ. Benign epidermal tumors and proliferations. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. St. Louis, MO: Mosby/Elsevier; 2012:1809-1810.
Epidermal nevi can occur in isolation or in association with internal abnormalities. Epidermal nevus syndrome (ENS) is a heterogeneous group of neurocutaneous disorders characterized by mosaicism and epidermal nevi found in association with various systemic abnormalities.1-4 There are many possible associated systemic findings, including abnormalities of the central nervous, musculoskeletal, renal, and hematologic systems. Epidermal nevi have been associated with internal malignancies. We present the case of a patient with epidermal nevi associated with papillary transitional cell bladder carcinoma. According to a PubMed search of articles indexed for MEDLINE using the search terms transitional cell bladder carcinoma and epidermal nevus, there have only been 4 other cases of transitional cell bladder carcinoma and ENS reported in the literature,5-8 2 of which were reports of papillary transitional cell bladder carcinoma.5,6
Case Report
A 29-year-old woman presented to our clinic with a rash that had been present since 3 years of age. The emergency department consulted dermatology for evaluation of what was believed to be contact dermatitis; however, upon questioning the patient, it was revealed that the rash was chronic and persistent.
The rash was nonpruritic and was located on the face, hands (Figure 1), chest, buttocks, thighs, legs, and back (Figure 2). Although asymptomatic, the appearance of the skin caused the patient some emotional distress. As a child she had been evaluated by a dermatologist and a biopsy was performed, but she did not recall the results or have any records. She had been prescribed an oral medication by the dermatologist, but treatment was terminated early due to nausea. The skin lesions did not improve with the short course of treatment.


Eighteen months prior to presentation to our clinic, the patient was discovered to have hematuria on routine examination by her primary care physician. At that time, the patient underwent a workup for hematuria and a mass was discovered in the bladder via cystoscopy. A diagnosis of low-grade papillary transitional cell bladder carcinoma was made, and she underwent a partial cystectomy. No radiation or chemotherapy was required. The remainder of her medical history was only remarkable for asthma, which was well controlled with albuterol. On examination, generalized, hyperpigmented, reticulated patches, macules, and hyperpigmented verrucous plaques were distributed along the Blaschko lines, sparing the face. No limb abnormalities or dental or nail abnormalities were noted. Examination of the axillary and cervical lymph nodes was unremarkable, and no neurological abnormalities were noted. A 3-mm punch biopsy of the mid upper back was performed. Histopathology revealed papillomatous, nonorganoid, nonepidermolytic hyperplasia of the epidermis with elongated rete ridges (Figure 3), which was diagnosed as a nonorganoid nonepidermolytic epidermal nevus.

Comment
Epidermal nevus syndrome is a group of disorders characterized by both local or systematized epidermal nevi and systemic findings. Solomon et al4 first coined the term epidermal nevus syndrome more than 40 years ago; however, since then there has been confusion about how to define ENS. Epidermal nevus syndrome has been considered an umbrella term that includes more specific syndromes involving epidermal nevi, such as Proteus syndrome and Schimmelpenning syndrome; conversely, it also has been considered a term for those who do not meet the criteria for more specific syndromes.1,9 Happle1 discussed that the genetic variations found in ENS warrant recognition. Simply put, ENS is a heterogeneous group of syndromes that are similar in that they involve epidermal nevi and internal abnormalities but are genetically distinct. The list of definitive ENSs, as suggested by Happle1 and others, will likely continue to grow.3,5
The exact pathomechanism of ENS is unknown, but the clinical presentation most likely represents a lethal disorder mitigated by mosaicism.2,9 Gene defects vary depending on the specific ENS. For instance, the phosphatase and tensin homolog gene, PTEN, mutations have been associated with type 2 segmental Cowden disease. Fibroblast growth factor receptor 3, FGFR3, mutations have been linked to Garcia-Hafner-Happle syndrome.3FGFR3 mutations have been found in nonepidermolytic epidermal nevi, and some suggest that the majority of epidermal nevi exhibit mutations in FGFR3.5,10,11 On the other hand, other gene defects have not been elucidated, such as in Schimmelpenning syndrome.3
Clinically, ENS may involve nonepidermolytic verrucous nevi, sebaceous nevi, organoid nevi, linear Cowden nevi, and woolly hair nevi. Lesions may be flesh-colored, pink, yellow, or hyperpigmented plaques in a blaschkoid distribution and may be localized or systematized. Nevi typically are present at birth or develop within the first year of life.9,12,13 Other cutaneous findings may be noted apart from epidermal nevi, including melanocytic nevi, aplasia cutis congenita, and hemangiomas.13,14
Extracutaneous findings include central nervous system, skeletal, ocular, cardiac, and genitourinary defects, which are often observed in these patients.3,9,13,14 Central nervous system findings are seen in 50% to 70% of cases, with seizures and mental retardation among the most common.13-15 Genitourinary abnormalities associated with epidermal nevi, including horseshoe kidney, cystic kidney, duplicated collecting system, testicular and paratesticular tumors, and hypospadias have been documented in the literature.16 Our patient had a history of papillary transitional cell bladder carcinoma, which is rare for a patient younger than 30 years. The overall median age of diagnosis of bladder cancer is 65 years, and it is more common in men than in women.17 Transitional cell carcinomas account for approximately 90% of all bladder cancers in the United States. Other common types of bladder cancer include squamous cell carcinoma, adenocarcinoma, and rhabdomyosarcoma.16 Typically, transitional cell carcinoma is associated with smoking, exposure to aniline dyes, cyclophosphamide, and living in industrialized areas.16,17 Individuals who work with textiles, dyes, leather, tires, rubber, and/or petroleum; painters; truck drivers; drill press operators; and hairdressers are at an increased risk for development of bladder cancer.16
Interestingly, it has been shown in some studies that papillary transitional cell bladder carcinoma frequently is associated with FGFR3 mutations, which may be the missing link in the rare finding of papillary transitional cell bladder carcinoma and epidermal nevi.5,18,19 In addition, PTEN mutations also have been identified in low-grade papillary transitional cell carcinomas of the bladder, another gene linked to an ENS with type 2 segmental Cowden disease.3,20
Histopathologically, epidermal nevi have 10 different descriptions. Our patient had a nonorganoid nonepidermolytic epidermal nevus characterized by hyperkeratosis, acanthosis, papillomatosis, and elongated rete ridges. Focal acantholysis and epidermolytic hyperkeratosis also is seen in some epidermal nevi but was not seen in this case.9,21
Simple epidermal nevi occur in approximately 1 in 1000 newborns; however, when a child presents with multiple or systematized epidermal nevi, investigation should be undertaken for other possible associations.13,14 Of note, there have been several cases of squamous cell, verrucous, basal cell, and adnexal carcinomas arising in linear epidermal nevi.22-24
Epidermal nevi can be difficult to treat. Some patients are troubled by the appearance of these nevi, especially those with systematized disease. Unfortunately, for patients with multiple nevi or systematized disease, there are no consistently effective treatment options; however, there are case reports25,26 in the literature citing improvement or cure of epidermal nevi with full-thickness excision, continuous and pulsed CO2 laser, pulsed dye laser, and erbium-doped YAG laser.25 Other therapies that have been purported to help improve epidermal nevi are topical and oral retinoids, corticosteroids, topical 5-fluorouracil, anthralin, and podophyllin.26
Conclusion
Transitional cell bladder carcinoma is rare in patients in the third decade of life and younger. Given the age of our patient and her concomitant lack of risk factors, such as older age, history of smoking, and exposure to certain chemicals (eg, aniline dyes) and medications (eg, cyclophosphamide), it is more likely that the finding of papillary transitional cell bladder carcinoma and ENS are related. A clear genetic link between ENS and transitional cell papillary bladder carcinoma has yet to be elucidated, but the FGFR3 gene is promising.
Epidermal nevi can occur in isolation or in association with internal abnormalities. Epidermal nevus syndrome (ENS) is a heterogeneous group of neurocutaneous disorders characterized by mosaicism and epidermal nevi found in association with various systemic abnormalities.1-4 There are many possible associated systemic findings, including abnormalities of the central nervous, musculoskeletal, renal, and hematologic systems. Epidermal nevi have been associated with internal malignancies. We present the case of a patient with epidermal nevi associated with papillary transitional cell bladder carcinoma. According to a PubMed search of articles indexed for MEDLINE using the search terms transitional cell bladder carcinoma and epidermal nevus, there have only been 4 other cases of transitional cell bladder carcinoma and ENS reported in the literature,5-8 2 of which were reports of papillary transitional cell bladder carcinoma.5,6
Case Report
A 29-year-old woman presented to our clinic with a rash that had been present since 3 years of age. The emergency department consulted dermatology for evaluation of what was believed to be contact dermatitis; however, upon questioning the patient, it was revealed that the rash was chronic and persistent.
The rash was nonpruritic and was located on the face, hands (Figure 1), chest, buttocks, thighs, legs, and back (Figure 2). Although asymptomatic, the appearance of the skin caused the patient some emotional distress. As a child she had been evaluated by a dermatologist and a biopsy was performed, but she did not recall the results or have any records. She had been prescribed an oral medication by the dermatologist, but treatment was terminated early due to nausea. The skin lesions did not improve with the short course of treatment.


Eighteen months prior to presentation to our clinic, the patient was discovered to have hematuria on routine examination by her primary care physician. At that time, the patient underwent a workup for hematuria and a mass was discovered in the bladder via cystoscopy. A diagnosis of low-grade papillary transitional cell bladder carcinoma was made, and she underwent a partial cystectomy. No radiation or chemotherapy was required. The remainder of her medical history was only remarkable for asthma, which was well controlled with albuterol. On examination, generalized, hyperpigmented, reticulated patches, macules, and hyperpigmented verrucous plaques were distributed along the Blaschko lines, sparing the face. No limb abnormalities or dental or nail abnormalities were noted. Examination of the axillary and cervical lymph nodes was unremarkable, and no neurological abnormalities were noted. A 3-mm punch biopsy of the mid upper back was performed. Histopathology revealed papillomatous, nonorganoid, nonepidermolytic hyperplasia of the epidermis with elongated rete ridges (Figure 3), which was diagnosed as a nonorganoid nonepidermolytic epidermal nevus.

Comment
Epidermal nevus syndrome is a group of disorders characterized by both local or systematized epidermal nevi and systemic findings. Solomon et al4 first coined the term epidermal nevus syndrome more than 40 years ago; however, since then there has been confusion about how to define ENS. Epidermal nevus syndrome has been considered an umbrella term that includes more specific syndromes involving epidermal nevi, such as Proteus syndrome and Schimmelpenning syndrome; conversely, it also has been considered a term for those who do not meet the criteria for more specific syndromes.1,9 Happle1 discussed that the genetic variations found in ENS warrant recognition. Simply put, ENS is a heterogeneous group of syndromes that are similar in that they involve epidermal nevi and internal abnormalities but are genetically distinct. The list of definitive ENSs, as suggested by Happle1 and others, will likely continue to grow.3,5
The exact pathomechanism of ENS is unknown, but the clinical presentation most likely represents a lethal disorder mitigated by mosaicism.2,9 Gene defects vary depending on the specific ENS. For instance, the phosphatase and tensin homolog gene, PTEN, mutations have been associated with type 2 segmental Cowden disease. Fibroblast growth factor receptor 3, FGFR3, mutations have been linked to Garcia-Hafner-Happle syndrome.3FGFR3 mutations have been found in nonepidermolytic epidermal nevi, and some suggest that the majority of epidermal nevi exhibit mutations in FGFR3.5,10,11 On the other hand, other gene defects have not been elucidated, such as in Schimmelpenning syndrome.3
Clinically, ENS may involve nonepidermolytic verrucous nevi, sebaceous nevi, organoid nevi, linear Cowden nevi, and woolly hair nevi. Lesions may be flesh-colored, pink, yellow, or hyperpigmented plaques in a blaschkoid distribution and may be localized or systematized. Nevi typically are present at birth or develop within the first year of life.9,12,13 Other cutaneous findings may be noted apart from epidermal nevi, including melanocytic nevi, aplasia cutis congenita, and hemangiomas.13,14
Extracutaneous findings include central nervous system, skeletal, ocular, cardiac, and genitourinary defects, which are often observed in these patients.3,9,13,14 Central nervous system findings are seen in 50% to 70% of cases, with seizures and mental retardation among the most common.13-15 Genitourinary abnormalities associated with epidermal nevi, including horseshoe kidney, cystic kidney, duplicated collecting system, testicular and paratesticular tumors, and hypospadias have been documented in the literature.16 Our patient had a history of papillary transitional cell bladder carcinoma, which is rare for a patient younger than 30 years. The overall median age of diagnosis of bladder cancer is 65 years, and it is more common in men than in women.17 Transitional cell carcinomas account for approximately 90% of all bladder cancers in the United States. Other common types of bladder cancer include squamous cell carcinoma, adenocarcinoma, and rhabdomyosarcoma.16 Typically, transitional cell carcinoma is associated with smoking, exposure to aniline dyes, cyclophosphamide, and living in industrialized areas.16,17 Individuals who work with textiles, dyes, leather, tires, rubber, and/or petroleum; painters; truck drivers; drill press operators; and hairdressers are at an increased risk for development of bladder cancer.16
Interestingly, it has been shown in some studies that papillary transitional cell bladder carcinoma frequently is associated with FGFR3 mutations, which may be the missing link in the rare finding of papillary transitional cell bladder carcinoma and epidermal nevi.5,18,19 In addition, PTEN mutations also have been identified in low-grade papillary transitional cell carcinomas of the bladder, another gene linked to an ENS with type 2 segmental Cowden disease.3,20
Histopathologically, epidermal nevi have 10 different descriptions. Our patient had a nonorganoid nonepidermolytic epidermal nevus characterized by hyperkeratosis, acanthosis, papillomatosis, and elongated rete ridges. Focal acantholysis and epidermolytic hyperkeratosis also is seen in some epidermal nevi but was not seen in this case.9,21
Simple epidermal nevi occur in approximately 1 in 1000 newborns; however, when a child presents with multiple or systematized epidermal nevi, investigation should be undertaken for other possible associations.13,14 Of note, there have been several cases of squamous cell, verrucous, basal cell, and adnexal carcinomas arising in linear epidermal nevi.22-24
Epidermal nevi can be difficult to treat. Some patients are troubled by the appearance of these nevi, especially those with systematized disease. Unfortunately, for patients with multiple nevi or systematized disease, there are no consistently effective treatment options; however, there are case reports25,26 in the literature citing improvement or cure of epidermal nevi with full-thickness excision, continuous and pulsed CO2 laser, pulsed dye laser, and erbium-doped YAG laser.25 Other therapies that have been purported to help improve epidermal nevi are topical and oral retinoids, corticosteroids, topical 5-fluorouracil, anthralin, and podophyllin.26
Conclusion
Transitional cell bladder carcinoma is rare in patients in the third decade of life and younger. Given the age of our patient and her concomitant lack of risk factors, such as older age, history of smoking, and exposure to certain chemicals (eg, aniline dyes) and medications (eg, cyclophosphamide), it is more likely that the finding of papillary transitional cell bladder carcinoma and ENS are related. A clear genetic link between ENS and transitional cell papillary bladder carcinoma has yet to be elucidated, but the FGFR3 gene is promising.
- Happle R. What is a nevus? a proposed definition of a common medical term. Dermatology. 1995;191:1-5.
- Gonzalez ME, Jabbari A, Tlougan BE, et al. Epidermal nevus. Dermatol Online J. 2010;16:12.
- Happle R. The group of epidermal nevus syndromes. part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22.
- Solomon LM, Fretzin DF, Dewald RL. The epidermal nevus syndrome. Arch Dermatol. 1968;97:273-285.
- Flosadottir E, Bjarnason B. A non-epidermolytic epidermal nevus of a soft, papillomatous type with transitional cell cancer of the bladder: a case report and review of non-cutaneous cancers associated with epidermal naevi. Acta Derm Venerol. 2008;88:173-175.
- Rosenthal D, Fretzin DF. Epidermal nevus syndrome: report of association with transitional cell carcinoma of the bladder. Pediatr Dermatol. 1986;3:455-458.
- Garcia de Jalon A, Azua-Romea J, Trivez MA, et al. Epidermal naevus syndrome (Solomon’s syndrome) associated with bladder cancer in a 20-year-old female. Scand J Urol Nephrol. 2004;38:85-87.
- Rongioletti F, Rebora A. Epidermal nevus with transitional cell carcinomas of the urinary tract. J Am Acad Dermatol. 1991;25:856-858.
- Moss C. Mosacism and linear lesions. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. St. Louis, MO: Mosby/Elsevier; 2012:943-962.
- Hafner C, van Oers JM, Vogt T, et al. Mosaicisim of activating FGFR3 mutations in human skin causes epidermal nevi. J Clin Invest. 2006;116:2201-2207.
- Bygum A, Fagerberg CR, Clemmensen OJ, et al. Systemic epidermal nevus with involvement of the oral mucosa due to FGFR3 mutation. BMC Med Genet. 2011;12:79.
- Happle R. Linear Cowden nevus: a new distinct epidermal nevus. Eur J Dermatol. 2007;17:133-136.
- Vujevich JJ, Mancini AJ. The epidermal nevus syndromes: multisystem disorders. J Am Acad Dermatol. 2004;50:957-961.
- Solomon L, Esterly N. Epidermal and other congenital organoid nevi. Curr Probl Pediatr. 1975;6:1-56.
- Grebe TA, Rimsa ME, Richter SF, et al. Further delineation of the epidermal nevus syndrome: two cases with new findings and literature review. Am J Med Genet. 1993;47:24-30.
- Lamm DL, Torti FM. Bladder cancer, 1996. Ca Cancer J Clin. 1996;46:93-112.
- Metts MC, Metts JC, Milito SJ, et al. Bladder cancer: a review of diagnosis and management. J Natl Med Assoc. 2000;92:285-294.
- Kimura T, Suzuki H, Ohashi T, et al. The incidence of thanatophoric dysplasia mutations in FGFR3 gene is higher in low-grade or superficial bladder carcinomas. Cancer. 2001;92:2555-2561.
- Cappellen D, DeOliveira C, Ricol D, et al. Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat Genet. 1999;23:18-20.
- Knowles MA, Platt FM, Ross RL, et al. Phosphatidylinositol 3-kinase (PI3K) pathway activation in bladder cancer. Cancer Metastasis Rev. 2009;28:305-316.
- Luzar B, Calonje E, Bastian B. Tumors of the surface epithelium. In: Calonje JE, Breen T, McKee PH, eds. McKee’s Pathology of the Skin. 4th ed. Edinburgh, Scotland: Elsevier/Saunders; 2012:1076-1149.
- Masood Q, Narayan D. Squamous cell carcinoma in a linear epidermal nevus. J Plast Reconstr Aesthet Surg. 2009;62:693-694.
- Cramer SF, Mandel MA, Hauler R, et al. Squamous cell carcinoma arising in a linear epidermal nevus. Arch Dermatol. 1981;117:222-224.
- Affleck AG, Leach IJ, Varma S. Two squamous cell carcinomas arising in a linear epidermal nevus in a 28-year-old female. Clin Exp Dermatol. 2005;30:382-384.
- Alam M, Arndt KA. A method for pulsed carbon dioxide laser treatment of epidermal nevi. J Am Acad Dermatol. 2002;46:554-556.
- Requena L, Requena C, Cockerell CJ. Benign epidermal tumors and proliferations. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. St. Louis, MO: Mosby/Elsevier; 2012:1809-1810.
- Happle R. What is a nevus? a proposed definition of a common medical term. Dermatology. 1995;191:1-5.
- Gonzalez ME, Jabbari A, Tlougan BE, et al. Epidermal nevus. Dermatol Online J. 2010;16:12.
- Happle R. The group of epidermal nevus syndromes. part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22.
- Solomon LM, Fretzin DF, Dewald RL. The epidermal nevus syndrome. Arch Dermatol. 1968;97:273-285.
- Flosadottir E, Bjarnason B. A non-epidermolytic epidermal nevus of a soft, papillomatous type with transitional cell cancer of the bladder: a case report and review of non-cutaneous cancers associated with epidermal naevi. Acta Derm Venerol. 2008;88:173-175.
- Rosenthal D, Fretzin DF. Epidermal nevus syndrome: report of association with transitional cell carcinoma of the bladder. Pediatr Dermatol. 1986;3:455-458.
- Garcia de Jalon A, Azua-Romea J, Trivez MA, et al. Epidermal naevus syndrome (Solomon’s syndrome) associated with bladder cancer in a 20-year-old female. Scand J Urol Nephrol. 2004;38:85-87.
- Rongioletti F, Rebora A. Epidermal nevus with transitional cell carcinomas of the urinary tract. J Am Acad Dermatol. 1991;25:856-858.
- Moss C. Mosacism and linear lesions. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. St. Louis, MO: Mosby/Elsevier; 2012:943-962.
- Hafner C, van Oers JM, Vogt T, et al. Mosaicisim of activating FGFR3 mutations in human skin causes epidermal nevi. J Clin Invest. 2006;116:2201-2207.
- Bygum A, Fagerberg CR, Clemmensen OJ, et al. Systemic epidermal nevus with involvement of the oral mucosa due to FGFR3 mutation. BMC Med Genet. 2011;12:79.
- Happle R. Linear Cowden nevus: a new distinct epidermal nevus. Eur J Dermatol. 2007;17:133-136.
- Vujevich JJ, Mancini AJ. The epidermal nevus syndromes: multisystem disorders. J Am Acad Dermatol. 2004;50:957-961.
- Solomon L, Esterly N. Epidermal and other congenital organoid nevi. Curr Probl Pediatr. 1975;6:1-56.
- Grebe TA, Rimsa ME, Richter SF, et al. Further delineation of the epidermal nevus syndrome: two cases with new findings and literature review. Am J Med Genet. 1993;47:24-30.
- Lamm DL, Torti FM. Bladder cancer, 1996. Ca Cancer J Clin. 1996;46:93-112.
- Metts MC, Metts JC, Milito SJ, et al. Bladder cancer: a review of diagnosis and management. J Natl Med Assoc. 2000;92:285-294.
- Kimura T, Suzuki H, Ohashi T, et al. The incidence of thanatophoric dysplasia mutations in FGFR3 gene is higher in low-grade or superficial bladder carcinomas. Cancer. 2001;92:2555-2561.
- Cappellen D, DeOliveira C, Ricol D, et al. Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat Genet. 1999;23:18-20.
- Knowles MA, Platt FM, Ross RL, et al. Phosphatidylinositol 3-kinase (PI3K) pathway activation in bladder cancer. Cancer Metastasis Rev. 2009;28:305-316.
- Luzar B, Calonje E, Bastian B. Tumors of the surface epithelium. In: Calonje JE, Breen T, McKee PH, eds. McKee’s Pathology of the Skin. 4th ed. Edinburgh, Scotland: Elsevier/Saunders; 2012:1076-1149.
- Masood Q, Narayan D. Squamous cell carcinoma in a linear epidermal nevus. J Plast Reconstr Aesthet Surg. 2009;62:693-694.
- Cramer SF, Mandel MA, Hauler R, et al. Squamous cell carcinoma arising in a linear epidermal nevus. Arch Dermatol. 1981;117:222-224.
- Affleck AG, Leach IJ, Varma S. Two squamous cell carcinomas arising in a linear epidermal nevus in a 28-year-old female. Clin Exp Dermatol. 2005;30:382-384.
- Alam M, Arndt KA. A method for pulsed carbon dioxide laser treatment of epidermal nevi. J Am Acad Dermatol. 2002;46:554-556.
- Requena L, Requena C, Cockerell CJ. Benign epidermal tumors and proliferations. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. St. Louis, MO: Mosby/Elsevier; 2012:1809-1810.
Practice Points
- Epidermal nevi are common benign cutaneous neoplasms.
- Extensive systematized epidermal nevi can be a sign of internal disease.
Team characterizes therapy-resistant ALL cells

Image by Vashi Donsk
Researchers say they have characterized a subpopulation of leukemia cells that are responsible for relapse in acute lymphoblastic leukemia (ALL).
The team identified these dormant, therapy-resistant cells in mouse models of ALL and found that removing the cells from their environment makes them sensitive to treatment.
The researchers believe these findings could pave the way to better relapse prevention in patients with ALL.
“Previously, the biological principles responsible for a relapse in leukemia were not fully understood,” said study author Irmela Jeremias, MD, PhD, of Helmholtz Zentrum München in Munich, Germany.
“Our new approach is to isolate dormant cells, which gives us the first possibility of developing therapies that switch off these cells.”
Dr Jeremias and colleagues described this approach in Cancer Cell.
First, the researchers created mouse models recapitulating minimal residual disease (MRD) and relapse in ALL patients.
The team then used genetic engineering and proliferation-sensitive dyes to isolate and characterize relapse-inducing cells.
This revealed a subpopulation of leukemia cells that exhibited long-term dormancy, treatment resistance, and stemness. These cells were similar to primary ALL cells isolated from pediatric and adult patients with MRD.
However, the dormant leukemia cells found in the mice changed once they were removed from the in vivo environment. They began to proliferate and became sensitive to ex vivo treatment with chemotherapy drugs.
“[T]hese cells, once they have been dissolved out of their surroundings, are indeed susceptible to therapy and react well to therapeutics,” said study author Erbey Özdemir, a doctoral candidate at Helmholtz Zentrum München.
The researchers therefore believe that therapeutic strategies aimed at dissociating dormant leukemia cells from their protective niche might prevent relapse in ALL patients.
“This has brought us a small step closer to the global goal of preventing disease relapse in patients suffering from leukemia,” Dr Jeremias said. “It might serve as basis for new therapies that destroy resistant leukemia cells before they induce relapse.” ![]()

Image by Vashi Donsk
Researchers say they have characterized a subpopulation of leukemia cells that are responsible for relapse in acute lymphoblastic leukemia (ALL).
The team identified these dormant, therapy-resistant cells in mouse models of ALL and found that removing the cells from their environment makes them sensitive to treatment.
The researchers believe these findings could pave the way to better relapse prevention in patients with ALL.
“Previously, the biological principles responsible for a relapse in leukemia were not fully understood,” said study author Irmela Jeremias, MD, PhD, of Helmholtz Zentrum München in Munich, Germany.
“Our new approach is to isolate dormant cells, which gives us the first possibility of developing therapies that switch off these cells.”
Dr Jeremias and colleagues described this approach in Cancer Cell.
First, the researchers created mouse models recapitulating minimal residual disease (MRD) and relapse in ALL patients.
The team then used genetic engineering and proliferation-sensitive dyes to isolate and characterize relapse-inducing cells.
This revealed a subpopulation of leukemia cells that exhibited long-term dormancy, treatment resistance, and stemness. These cells were similar to primary ALL cells isolated from pediatric and adult patients with MRD.
However, the dormant leukemia cells found in the mice changed once they were removed from the in vivo environment. They began to proliferate and became sensitive to ex vivo treatment with chemotherapy drugs.
“[T]hese cells, once they have been dissolved out of their surroundings, are indeed susceptible to therapy and react well to therapeutics,” said study author Erbey Özdemir, a doctoral candidate at Helmholtz Zentrum München.
The researchers therefore believe that therapeutic strategies aimed at dissociating dormant leukemia cells from their protective niche might prevent relapse in ALL patients.
“This has brought us a small step closer to the global goal of preventing disease relapse in patients suffering from leukemia,” Dr Jeremias said. “It might serve as basis for new therapies that destroy resistant leukemia cells before they induce relapse.” ![]()

Image by Vashi Donsk
Researchers say they have characterized a subpopulation of leukemia cells that are responsible for relapse in acute lymphoblastic leukemia (ALL).
The team identified these dormant, therapy-resistant cells in mouse models of ALL and found that removing the cells from their environment makes them sensitive to treatment.
The researchers believe these findings could pave the way to better relapse prevention in patients with ALL.
“Previously, the biological principles responsible for a relapse in leukemia were not fully understood,” said study author Irmela Jeremias, MD, PhD, of Helmholtz Zentrum München in Munich, Germany.
“Our new approach is to isolate dormant cells, which gives us the first possibility of developing therapies that switch off these cells.”
Dr Jeremias and colleagues described this approach in Cancer Cell.
First, the researchers created mouse models recapitulating minimal residual disease (MRD) and relapse in ALL patients.
The team then used genetic engineering and proliferation-sensitive dyes to isolate and characterize relapse-inducing cells.
This revealed a subpopulation of leukemia cells that exhibited long-term dormancy, treatment resistance, and stemness. These cells were similar to primary ALL cells isolated from pediatric and adult patients with MRD.
However, the dormant leukemia cells found in the mice changed once they were removed from the in vivo environment. They began to proliferate and became sensitive to ex vivo treatment with chemotherapy drugs.
“[T]hese cells, once they have been dissolved out of their surroundings, are indeed susceptible to therapy and react well to therapeutics,” said study author Erbey Özdemir, a doctoral candidate at Helmholtz Zentrum München.
The researchers therefore believe that therapeutic strategies aimed at dissociating dormant leukemia cells from their protective niche might prevent relapse in ALL patients.
“This has brought us a small step closer to the global goal of preventing disease relapse in patients suffering from leukemia,” Dr Jeremias said. “It might serve as basis for new therapies that destroy resistant leukemia cells before they induce relapse.” ![]()