User login
Prospective Evaluation of Opioid Consumption After Distal Radius Fracture Repair Surgery
Take-Home Points
- Prescription opioid abuse and overdose-related deaths are on the rise in the United States.
- Following Open Reduction Internal Fixation (ORIF) of a distal radius fracture (DRF), patients consumed an average of 14.6 opioid pills. We recommend prescribing no more than 15-20 opioid pills after DRF ORIF.
- There was no difference in opioid consumption between patients who underwent general anesthesia vs regional anesthesia.
- There was a significant trend towards less opioid consumption with increasing age.
- There was a trend towards increased opioid consumption in patients with worsening fracture type as well as in self-pay/Medicaid patients.
Over the past 2 decades, prescription opioid abuse in the United States has risen steadily.1,2 Although use of opioid analgesics in the US far exceeds use in other countries, US patients do not report less pain or more satisfaction with pain relief.3-5 Between 1999 and 2002, oxycodone prescriptions increased by 50%, fentanyl prescriptions by 150%, and morphine prescriptions by 60%.6 Furthermore, the Centers for Disease Control and Prevention (CDC) reported in 2012 that, for every 100 people in the United States, US physicians wrote a mean of 82.5 opioid prescriptions and 37.6 benzodiazepine prescriptions; in total, US clinicians wrote 259 million opioid prescriptions in 2012, enough for every adult to have a bottle.7 The increase in prescription opioid abuse, not surprisingly, has paralleled a 124% increase in opioid overdose-related deaths.8 Cicero and colleagues2,9 recently found that, over the past 50 years, heroin use has dramatically shifted from being a problem mainly of urban centers and minorities toward one of older, suburban Caucasians with a previous history of prescription pain killer abuse. Deaths from prescription opioid overdoses now exceed deaths from heroin and cocaine overdoses combined.10 According to the CDC, emergency department visits related to nonmedical use of prescription opioid medications jumped 111% between 2004 and 2008.11
Opioid analgesics are often prescribed for the management of musculoskeletal pain and injuries.12-16 Orthopedic surgeons, who prescribe more opioids than physicians in any other surgical field, represent the third largest group of opioid prescribers, trailing only primary care physicians and internists, who far outnumber them.17 A study focused on opioid consumption after upper extremity surgery found that upper extremity surgeons tended to overprescribe opioids for postoperative analgesia.18 Many patients saved their remaining medication for later use and were never instructed on proper disposal. There is a developing consensus that opioid medication is not as safe and effective as once thought, and that a high-dose prescription or prolonged opioid therapy do not improve outcomes.19 In addition, patients may experience numerous opioid-associated adverse effects, including nausea, vomiting, constipation, lightheadedness, dizziness, blurred vision, headache, dry mouth, sweating, and itching.
In October 2012, patient satisfaction scores on the Hospital Consumer Assessment of Healthcare Providers and Systems started affecting Medicare reimbursements.20 By 2017, up to 6% of Medicare reimbursement will be at risk, given the poor outcomes caused by uncontrolled pain.21-24 The US healthcare culture has made it more important than ever for physicians to adequately manage postoperative pain while limiting opioid availability and the risk for abuse.
Distal radius fracture (DRF) open reduction and internal fixation (ORIF) is commonly performed by orthopedic surgeons and hand surgeons. Pain management and opioid consumption after DRF repair may be influenced by several variables. We conducted a study to investigate the impact of several clinical variables on postoperative opioid use; to test the hypothesis that post-DRF-ORIF opioid consumption would increase with worsening fracture classification and certain patient demographics; and to seek postoperative opioid consumption insights that would facilitate optimization of future opioid prescribing.
Materials and Methods
Institutional Review Board approval was obtained before initiation of the study. All outpatients who underwent DRF-ORIF (performed by 9 hand surgery fellowship-trained orthopedic surgeons) were consecutively enrolled over a 6-month period in 2014. All procedures were performed with a standard volar plating technique through a flexor carpi radialis approach. The postoperative rehabilitation protocol was standardized for all patients. Data collected on each patient included age, sex, payer type, fracture type, opioid prescribed, amount prescribed, amount consumed, reasons for stopping, adverse events, and any postoperative adjunctive pain medications. The data were taken from questionnaires completed by patients at their first visit within 2 weeks after surgery. Anesthesia type (general or regional) was noted as well. All fractures were classified by Dr. O’Neil using the AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification of long-bone fractures based on preoperative radiographs.
Amount of opioid analgesic consumed was converted into morphine equivalents to adjust for the different opioids prescribed after surgery: oxycodone/acetaminophen or oxycodone equivalent, hydrocodone/acetaminophen or hydrocodone equivalent, and acetaminophen/codeine.
Patients were excluded from the study if their procedure was performed on an inpatient basis, if they sustained other injuries or fractures from their trauma, or if an adjunctive procedure (including carpal tunnel release) was performed during the DRF repair.
We used the Spearman rank correlation coefficient and a count data model to examine the relationship between opioid use and age. The Kruskal-Wallis test was used to examine the relationships between opioid use and payer type, anesthesia type, and fracture type.
Results
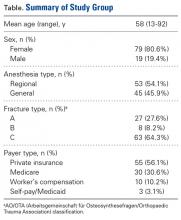
Of the 109 patients eligible for the study, 11 were excluded for incomplete postoperative questionnaires, leaving 98 patients (79 females, 19 males) for analysis. Mean age was 58 years (range, 13-92 years). Of the 98 patients, 45 received general anesthesia, and 53 received regional anesthesia with a single-shot peripheral nerve block before surgery and sedation perioperatively (Table).
Of the 98 study patients, 61 reported using over-the-counter adjunctive pain medications during the postoperative period, and 37 reported no use. Mean opioid consumption was 64.7 mg of morphine equivalents for the adjunctive medication users and 48.3 mg for the nonusers (P = .1947).
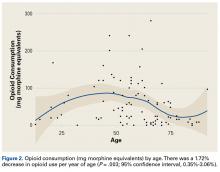
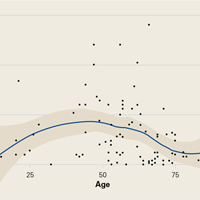
Demographic analysis revealed an inverse relationship between age and opioid use (Figure 2). The Spearman ρ between age and opioid consumption was –0.2958, which suggests decreased opioid use by older patients (P = .003).
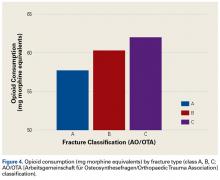
All fractures were graded with the AO/OTA long-bone fracture classification system. Mean opioid consumption for the 3 fracture-type groups was 57.7 mg (class A), 60.3 mg (class B), and 62.0 mg (class C) (Figure 4).
Discussion
The US healthcare culture has elevated physicians’ responsibility in adequately and aggressively managing their patients’ pain experience. Moreover, reimbursement may be affected by patient satisfaction scores, which are partly predicated on pain control.20-24 However, as rates of opioid use and abuse rise, it is important that physicians prescribe such medications judiciously. This is particularly germane to orthopedic surgeons, who prescribe more opioid analgesics than surgeons in any other field.17 Rodgers and colleagues18 found upper extremity surgeons, in particular, tended to overprescribe postoperative opioid analgesics. In the present study, we sought to identify the crucial risk factors that influence post-DRF-ORIF pain management and opioid consumption.
Mean postoperative opioid consumption (morphine equivalents) was 58.5 mg, roughly equivalent to 14.6 tabs of oxycodone/acetaminophen 5/325 mg, an opioid analgesic commonly used during the acute postoperative period. In addition, almost 70% of our patients required <75 mg of morphine equivalents, or <20 tabs of oxycodone/acetaminophen 5/325 mg. For upper extremity surgeons, these numbers may be better guides in determining the most appropriate amount of opioid to prescribe after DRF repair.
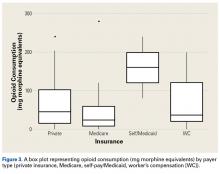
As for predicting levels of postoperative opioid medication, there was a significant trend toward less consumption with increasing age. Given this finding, surgeons prescribing for elderly patients should expect less opioid use. Regarding payer type, there was a trend toward more opioid use by self-pay/Medicaid patients; however, there were only 3 patients in this group. The situation in the study by Rodgers and colleagues18 is similar: Their finding that Medicaid patients consumed more pain pills after surgery was underpowered (only 5 patients in the group).
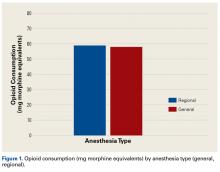
In the orthopedic community, support for use of regional anesthesia has been widespread for several reasons, including the belief that it reduces postoperative pain and therefore should reduce postoperative opioid consumption.25 However, we found no significant difference in postoperative opioid consumption between patients who received general anesthesia (with and without local anesthesia) and patients who received regional anesthesia (nerve block). Mean opioid consumption was 57.93 mg in the general anesthesia group and 58.98 mg in the regional anesthesia group. However, this finding could have been confounded by the variability in success and operator dependence inherent in regional anesthesia. In addition, the anatomical location for the peripheral nerve block and anesthetic could have affected the efficacy of the block and played a role in postoperative opioid consumption.
In this study, we tested the hypothesis that there would be more postoperative opioid consumption with worsening fracture type. Although our results did not reach statistical significance, there was a trend toward increased opioid consumption in patients with a complete intra-articular fracture (AO/OTA class C) vs patients with a partial articular fracture (class B) or an extra-articular fracture (class A). In addition, patients with a partial articular fracture tended to use more postoperative opioids than patients with an extra-articular fracture. In short, postoperative opioid consumption tended to be higher with increasing articular involvement of the fracture.
This study was limited in that it relied on patient self-reporting. Given the social stigma attached to opioid use, patients may have underreported their postoperative opioid consumption, been affected by recall bias, or both. The study also did not control for preoperative opioid use or history of opioid or substance abuse. Chronic preoperative opioid consumption may have affected postoperative opioid use. Other patient-related factors, such as body mass index (BMI) and hepatorenal dysfunction, can create tremendous variability in opioid metabolism across a population. Such factors were not controlled for in this study and therefore may have affected its results. That could help explain why older patients, who are more likely to have lower BMI and less efficient organ function for opioid metabolism, had lower postoperative opioid consumption. In addition, although we excluded patients with concomitant injuries and procedures, we did not screen patients for concomitant complex regional pain syndrome, fibromyalgia, or other medical conditions that might have had a significant impact on postoperative pain management needs. Last, some findings, such as the relationship between opioid use and payer type, were underpowered: Although self-pay/Medicaid patients had higher postoperative opioid consumption, they were few in number. The same was true of the Medicaid patients in the study by Rodgers and colleagues.18Our results demonstrated that post-DRF-ORIF opioid consumption decreased with age and was independent of type of perioperative anesthesia. There was a trend toward more opioid consumption with both self- and Medicaid payment and worsening fracture classification. It has become more important than ever for orthopedic surgeons to adequately manage postoperative pain while limiting opioid availability and the risk for abuse. Surgeons must remain aware of the variables in their patients’ postoperative pain experience in order to better optimize prescribing patterns and provide a safe and effective postoperative pain regimen.
Am J Orthop. 2017;46(1):E35-E40. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Kuehn BM. Opioid prescriptions soar: increase in legitimate use as well as abuse. JAMA. 2007;297(3):249-251.
2. Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71(7):821-826.
3. Helmerhorst GT, Lindenhovius AL, Vrahas M, Ring D, Kloen P. Satisfaction with pain relief after operative treatment of an ankle fracture. Injury. 2012;43(11):1958-1961.
4. Lindenhovius AL, Helmerhorst GT, Schnellen AC, Vrahas M, Ring D, Kloen P. Differences in prescription of narcotic pain medication after operative treatment of hip and ankle fractures in the United States and the Netherlands. J Trauma. 2009;67(1):160-164.
5. Seya MJ, Gelders SF, Achara OU, Milani B, Scholten WK. A first comparison between the consumption of and the need for opioid analgesics at country, regional, and global levels. J Pain Palliat Care Pharmacother. 2011;25(1):6-18.
6. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
7. Kuehn BM. CDC: major disparities in opioid prescribing among states: some states crack down on excess prescribing. JAMA. 2014;312(7):684-686.
8. Paulozzi LJ, Budnitz DS, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf. 2006;15(9):618-627.
9. Cicero TJ, Kuehn BM. Driven by prescription drug abuse, heroin use increases among suburban and rural whites. JAMA. 2014;312(2):118-119.
10. Painkillers fuel growth in drug addiction. Harvard Ment Health Lett. Harvard Medical School website. http://www.health.harvard.edu/newsletter_article/painkillers-fuel-growth-in-drug-addiction. Published January 2011. Accessed March 18, 2015.
11. Cai R, Crane E, Poneleit K, Paulozzi L. Emergency department visits involving nonmedical use of selected prescription drugs in the United States, 2004-2008. J Pain Palliat Care Pharmacother. 2010;24(3):293-297.
12. Armaghani SJ, Lee DS, Bible JE, et al. Preoperative narcotic use and its relation to depression and anxiety in patients undergoing spine surgery. Spine. 2013;38(25):2196-2200.
13. Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000. Pain. 2004;109(3):514-519.
14. Deyo RA, Mirza SK, Turner JA, Martin BI. Overtreating chronic back pain: time to back off? J Am Board Fam Med. 2009;22(1):62-68.
15. Lee D, Armaghani S, Archer KR, et al. Preoperative opioid use as a predictor of adverse postoperative self-reported outcomes in patients undergoing spine surgery. J Bone Joint Surg Am. 2014;96(11):e89.
16. Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine. 2007;32(19):2127-2132.
17. Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SR. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305(13):1299-1301.
18. Rodgers J, Cunningham K, Fitzgerald K, Finnerty E. Opioid consumption following outpatient upper extremity surgery. J Hand Surg Am. 2012;37(4):645-650.
19. Chen L, Vo T, Seefeld L, et al. Lack of correlation between opioid dose adjustment and pain score change in a group of chronic pain patients. J Pain. 2013;14(4):384-392.
20. Bush H. Doubling down on the patient experience. Hosp Health Netw. 2011;85(12):22-25, 1.
21. Centers for Medicare & Medicaid Services, US Department of Health and Human Services. Medicare program; hospital inpatient prospective payment systems for acute care hospitals and the long-term care hospital prospective payment system and fiscal year 2013 rates; hospitals’ resident caps for graduate medical education payment purposes; quality reporting requirements for specific providers and for ambulatory surgical centers. Final rule. Fed Regist. 2012;77(170):53257-53750.
22. Centers for Medicare & Medicaid Services, US Department of Health and Human Services. Hospital Value-Based Purchasing. http://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/Hospital_VBPurchasing_Fact_Sheet_ICN907664.pdf. Published September 2015. Accessed October 2015.
23. Manchikanti L, Singh V, Caraway DL, Benyamin RM, Falco FJ, Hirsch JA. Proposed physician payment schedule for 2013: guarded prognosis for interventional pain management. Pain Physician. 2012;15(5):E615-E627.
24. Bot AG, Bekkers S, Arnstein PM, Smith RM, Ring D. Opioid use after fracture surgery correlates with pain intensity and satisfaction with pain relief. Clin Orthop Relat Res. 2014;472(8):2542-2549.
25. Oldman M, McCartney CJ, Leung A, et al. A survey of orthopedic surgeons’ attitudes and knowledge regarding regional anesthesia. Anesth Analg. 2004;98(5):1486-1490.
Take-Home Points
- Prescription opioid abuse and overdose-related deaths are on the rise in the United States.
- Following Open Reduction Internal Fixation (ORIF) of a distal radius fracture (DRF), patients consumed an average of 14.6 opioid pills. We recommend prescribing no more than 15-20 opioid pills after DRF ORIF.
- There was no difference in opioid consumption between patients who underwent general anesthesia vs regional anesthesia.
- There was a significant trend towards less opioid consumption with increasing age.
- There was a trend towards increased opioid consumption in patients with worsening fracture type as well as in self-pay/Medicaid patients.
Over the past 2 decades, prescription opioid abuse in the United States has risen steadily.1,2 Although use of opioid analgesics in the US far exceeds use in other countries, US patients do not report less pain or more satisfaction with pain relief.3-5 Between 1999 and 2002, oxycodone prescriptions increased by 50%, fentanyl prescriptions by 150%, and morphine prescriptions by 60%.6 Furthermore, the Centers for Disease Control and Prevention (CDC) reported in 2012 that, for every 100 people in the United States, US physicians wrote a mean of 82.5 opioid prescriptions and 37.6 benzodiazepine prescriptions; in total, US clinicians wrote 259 million opioid prescriptions in 2012, enough for every adult to have a bottle.7 The increase in prescription opioid abuse, not surprisingly, has paralleled a 124% increase in opioid overdose-related deaths.8 Cicero and colleagues2,9 recently found that, over the past 50 years, heroin use has dramatically shifted from being a problem mainly of urban centers and minorities toward one of older, suburban Caucasians with a previous history of prescription pain killer abuse. Deaths from prescription opioid overdoses now exceed deaths from heroin and cocaine overdoses combined.10 According to the CDC, emergency department visits related to nonmedical use of prescription opioid medications jumped 111% between 2004 and 2008.11
Opioid analgesics are often prescribed for the management of musculoskeletal pain and injuries.12-16 Orthopedic surgeons, who prescribe more opioids than physicians in any other surgical field, represent the third largest group of opioid prescribers, trailing only primary care physicians and internists, who far outnumber them.17 A study focused on opioid consumption after upper extremity surgery found that upper extremity surgeons tended to overprescribe opioids for postoperative analgesia.18 Many patients saved their remaining medication for later use and were never instructed on proper disposal. There is a developing consensus that opioid medication is not as safe and effective as once thought, and that a high-dose prescription or prolonged opioid therapy do not improve outcomes.19 In addition, patients may experience numerous opioid-associated adverse effects, including nausea, vomiting, constipation, lightheadedness, dizziness, blurred vision, headache, dry mouth, sweating, and itching.
In October 2012, patient satisfaction scores on the Hospital Consumer Assessment of Healthcare Providers and Systems started affecting Medicare reimbursements.20 By 2017, up to 6% of Medicare reimbursement will be at risk, given the poor outcomes caused by uncontrolled pain.21-24 The US healthcare culture has made it more important than ever for physicians to adequately manage postoperative pain while limiting opioid availability and the risk for abuse.
Distal radius fracture (DRF) open reduction and internal fixation (ORIF) is commonly performed by orthopedic surgeons and hand surgeons. Pain management and opioid consumption after DRF repair may be influenced by several variables. We conducted a study to investigate the impact of several clinical variables on postoperative opioid use; to test the hypothesis that post-DRF-ORIF opioid consumption would increase with worsening fracture classification and certain patient demographics; and to seek postoperative opioid consumption insights that would facilitate optimization of future opioid prescribing.
Materials and Methods
Institutional Review Board approval was obtained before initiation of the study. All outpatients who underwent DRF-ORIF (performed by 9 hand surgery fellowship-trained orthopedic surgeons) were consecutively enrolled over a 6-month period in 2014. All procedures were performed with a standard volar plating technique through a flexor carpi radialis approach. The postoperative rehabilitation protocol was standardized for all patients. Data collected on each patient included age, sex, payer type, fracture type, opioid prescribed, amount prescribed, amount consumed, reasons for stopping, adverse events, and any postoperative adjunctive pain medications. The data were taken from questionnaires completed by patients at their first visit within 2 weeks after surgery. Anesthesia type (general or regional) was noted as well. All fractures were classified by Dr. O’Neil using the AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification of long-bone fractures based on preoperative radiographs.
Amount of opioid analgesic consumed was converted into morphine equivalents to adjust for the different opioids prescribed after surgery: oxycodone/acetaminophen or oxycodone equivalent, hydrocodone/acetaminophen or hydrocodone equivalent, and acetaminophen/codeine.
Patients were excluded from the study if their procedure was performed on an inpatient basis, if they sustained other injuries or fractures from their trauma, or if an adjunctive procedure (including carpal tunnel release) was performed during the DRF repair.
We used the Spearman rank correlation coefficient and a count data model to examine the relationship between opioid use and age. The Kruskal-Wallis test was used to examine the relationships between opioid use and payer type, anesthesia type, and fracture type.
Results
Of the 109 patients eligible for the study, 11 were excluded for incomplete postoperative questionnaires, leaving 98 patients (79 females, 19 males) for analysis. Mean age was 58 years (range, 13-92 years). Of the 98 patients, 45 received general anesthesia, and 53 received regional anesthesia with a single-shot peripheral nerve block before surgery and sedation perioperatively (Table).
Of the 98 study patients, 61 reported using over-the-counter adjunctive pain medications during the postoperative period, and 37 reported no use. Mean opioid consumption was 64.7 mg of morphine equivalents for the adjunctive medication users and 48.3 mg for the nonusers (P = .1947).
Demographic analysis revealed an inverse relationship between age and opioid use (Figure 2). The Spearman ρ between age and opioid consumption was –0.2958, which suggests decreased opioid use by older patients (P = .003).
All fractures were graded with the AO/OTA long-bone fracture classification system. Mean opioid consumption for the 3 fracture-type groups was 57.7 mg (class A), 60.3 mg (class B), and 62.0 mg (class C) (Figure 4).
Discussion
The US healthcare culture has elevated physicians’ responsibility in adequately and aggressively managing their patients’ pain experience. Moreover, reimbursement may be affected by patient satisfaction scores, which are partly predicated on pain control.20-24 However, as rates of opioid use and abuse rise, it is important that physicians prescribe such medications judiciously. This is particularly germane to orthopedic surgeons, who prescribe more opioid analgesics than surgeons in any other field.17 Rodgers and colleagues18 found upper extremity surgeons, in particular, tended to overprescribe postoperative opioid analgesics. In the present study, we sought to identify the crucial risk factors that influence post-DRF-ORIF pain management and opioid consumption.
Mean postoperative opioid consumption (morphine equivalents) was 58.5 mg, roughly equivalent to 14.6 tabs of oxycodone/acetaminophen 5/325 mg, an opioid analgesic commonly used during the acute postoperative period. In addition, almost 70% of our patients required <75 mg of morphine equivalents, or <20 tabs of oxycodone/acetaminophen 5/325 mg. For upper extremity surgeons, these numbers may be better guides in determining the most appropriate amount of opioid to prescribe after DRF repair.
As for predicting levels of postoperative opioid medication, there was a significant trend toward less consumption with increasing age. Given this finding, surgeons prescribing for elderly patients should expect less opioid use. Regarding payer type, there was a trend toward more opioid use by self-pay/Medicaid patients; however, there were only 3 patients in this group. The situation in the study by Rodgers and colleagues18 is similar: Their finding that Medicaid patients consumed more pain pills after surgery was underpowered (only 5 patients in the group).
In the orthopedic community, support for use of regional anesthesia has been widespread for several reasons, including the belief that it reduces postoperative pain and therefore should reduce postoperative opioid consumption.25 However, we found no significant difference in postoperative opioid consumption between patients who received general anesthesia (with and without local anesthesia) and patients who received regional anesthesia (nerve block). Mean opioid consumption was 57.93 mg in the general anesthesia group and 58.98 mg in the regional anesthesia group. However, this finding could have been confounded by the variability in success and operator dependence inherent in regional anesthesia. In addition, the anatomical location for the peripheral nerve block and anesthetic could have affected the efficacy of the block and played a role in postoperative opioid consumption.
In this study, we tested the hypothesis that there would be more postoperative opioid consumption with worsening fracture type. Although our results did not reach statistical significance, there was a trend toward increased opioid consumption in patients with a complete intra-articular fracture (AO/OTA class C) vs patients with a partial articular fracture (class B) or an extra-articular fracture (class A). In addition, patients with a partial articular fracture tended to use more postoperative opioids than patients with an extra-articular fracture. In short, postoperative opioid consumption tended to be higher with increasing articular involvement of the fracture.
This study was limited in that it relied on patient self-reporting. Given the social stigma attached to opioid use, patients may have underreported their postoperative opioid consumption, been affected by recall bias, or both. The study also did not control for preoperative opioid use or history of opioid or substance abuse. Chronic preoperative opioid consumption may have affected postoperative opioid use. Other patient-related factors, such as body mass index (BMI) and hepatorenal dysfunction, can create tremendous variability in opioid metabolism across a population. Such factors were not controlled for in this study and therefore may have affected its results. That could help explain why older patients, who are more likely to have lower BMI and less efficient organ function for opioid metabolism, had lower postoperative opioid consumption. In addition, although we excluded patients with concomitant injuries and procedures, we did not screen patients for concomitant complex regional pain syndrome, fibromyalgia, or other medical conditions that might have had a significant impact on postoperative pain management needs. Last, some findings, such as the relationship between opioid use and payer type, were underpowered: Although self-pay/Medicaid patients had higher postoperative opioid consumption, they were few in number. The same was true of the Medicaid patients in the study by Rodgers and colleagues.18Our results demonstrated that post-DRF-ORIF opioid consumption decreased with age and was independent of type of perioperative anesthesia. There was a trend toward more opioid consumption with both self- and Medicaid payment and worsening fracture classification. It has become more important than ever for orthopedic surgeons to adequately manage postoperative pain while limiting opioid availability and the risk for abuse. Surgeons must remain aware of the variables in their patients’ postoperative pain experience in order to better optimize prescribing patterns and provide a safe and effective postoperative pain regimen.
Am J Orthop. 2017;46(1):E35-E40. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Prescription opioid abuse and overdose-related deaths are on the rise in the United States.
- Following Open Reduction Internal Fixation (ORIF) of a distal radius fracture (DRF), patients consumed an average of 14.6 opioid pills. We recommend prescribing no more than 15-20 opioid pills after DRF ORIF.
- There was no difference in opioid consumption between patients who underwent general anesthesia vs regional anesthesia.
- There was a significant trend towards less opioid consumption with increasing age.
- There was a trend towards increased opioid consumption in patients with worsening fracture type as well as in self-pay/Medicaid patients.
Over the past 2 decades, prescription opioid abuse in the United States has risen steadily.1,2 Although use of opioid analgesics in the US far exceeds use in other countries, US patients do not report less pain or more satisfaction with pain relief.3-5 Between 1999 and 2002, oxycodone prescriptions increased by 50%, fentanyl prescriptions by 150%, and morphine prescriptions by 60%.6 Furthermore, the Centers for Disease Control and Prevention (CDC) reported in 2012 that, for every 100 people in the United States, US physicians wrote a mean of 82.5 opioid prescriptions and 37.6 benzodiazepine prescriptions; in total, US clinicians wrote 259 million opioid prescriptions in 2012, enough for every adult to have a bottle.7 The increase in prescription opioid abuse, not surprisingly, has paralleled a 124% increase in opioid overdose-related deaths.8 Cicero and colleagues2,9 recently found that, over the past 50 years, heroin use has dramatically shifted from being a problem mainly of urban centers and minorities toward one of older, suburban Caucasians with a previous history of prescription pain killer abuse. Deaths from prescription opioid overdoses now exceed deaths from heroin and cocaine overdoses combined.10 According to the CDC, emergency department visits related to nonmedical use of prescription opioid medications jumped 111% between 2004 and 2008.11
Opioid analgesics are often prescribed for the management of musculoskeletal pain and injuries.12-16 Orthopedic surgeons, who prescribe more opioids than physicians in any other surgical field, represent the third largest group of opioid prescribers, trailing only primary care physicians and internists, who far outnumber them.17 A study focused on opioid consumption after upper extremity surgery found that upper extremity surgeons tended to overprescribe opioids for postoperative analgesia.18 Many patients saved their remaining medication for later use and were never instructed on proper disposal. There is a developing consensus that opioid medication is not as safe and effective as once thought, and that a high-dose prescription or prolonged opioid therapy do not improve outcomes.19 In addition, patients may experience numerous opioid-associated adverse effects, including nausea, vomiting, constipation, lightheadedness, dizziness, blurred vision, headache, dry mouth, sweating, and itching.
In October 2012, patient satisfaction scores on the Hospital Consumer Assessment of Healthcare Providers and Systems started affecting Medicare reimbursements.20 By 2017, up to 6% of Medicare reimbursement will be at risk, given the poor outcomes caused by uncontrolled pain.21-24 The US healthcare culture has made it more important than ever for physicians to adequately manage postoperative pain while limiting opioid availability and the risk for abuse.
Distal radius fracture (DRF) open reduction and internal fixation (ORIF) is commonly performed by orthopedic surgeons and hand surgeons. Pain management and opioid consumption after DRF repair may be influenced by several variables. We conducted a study to investigate the impact of several clinical variables on postoperative opioid use; to test the hypothesis that post-DRF-ORIF opioid consumption would increase with worsening fracture classification and certain patient demographics; and to seek postoperative opioid consumption insights that would facilitate optimization of future opioid prescribing.
Materials and Methods
Institutional Review Board approval was obtained before initiation of the study. All outpatients who underwent DRF-ORIF (performed by 9 hand surgery fellowship-trained orthopedic surgeons) were consecutively enrolled over a 6-month period in 2014. All procedures were performed with a standard volar plating technique through a flexor carpi radialis approach. The postoperative rehabilitation protocol was standardized for all patients. Data collected on each patient included age, sex, payer type, fracture type, opioid prescribed, amount prescribed, amount consumed, reasons for stopping, adverse events, and any postoperative adjunctive pain medications. The data were taken from questionnaires completed by patients at their first visit within 2 weeks after surgery. Anesthesia type (general or regional) was noted as well. All fractures were classified by Dr. O’Neil using the AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification of long-bone fractures based on preoperative radiographs.
Amount of opioid analgesic consumed was converted into morphine equivalents to adjust for the different opioids prescribed after surgery: oxycodone/acetaminophen or oxycodone equivalent, hydrocodone/acetaminophen or hydrocodone equivalent, and acetaminophen/codeine.
Patients were excluded from the study if their procedure was performed on an inpatient basis, if they sustained other injuries or fractures from their trauma, or if an adjunctive procedure (including carpal tunnel release) was performed during the DRF repair.
We used the Spearman rank correlation coefficient and a count data model to examine the relationship between opioid use and age. The Kruskal-Wallis test was used to examine the relationships between opioid use and payer type, anesthesia type, and fracture type.
Results
Of the 109 patients eligible for the study, 11 were excluded for incomplete postoperative questionnaires, leaving 98 patients (79 females, 19 males) for analysis. Mean age was 58 years (range, 13-92 years). Of the 98 patients, 45 received general anesthesia, and 53 received regional anesthesia with a single-shot peripheral nerve block before surgery and sedation perioperatively (Table).
Of the 98 study patients, 61 reported using over-the-counter adjunctive pain medications during the postoperative period, and 37 reported no use. Mean opioid consumption was 64.7 mg of morphine equivalents for the adjunctive medication users and 48.3 mg for the nonusers (P = .1947).
Demographic analysis revealed an inverse relationship between age and opioid use (Figure 2). The Spearman ρ between age and opioid consumption was –0.2958, which suggests decreased opioid use by older patients (P = .003).
All fractures were graded with the AO/OTA long-bone fracture classification system. Mean opioid consumption for the 3 fracture-type groups was 57.7 mg (class A), 60.3 mg (class B), and 62.0 mg (class C) (Figure 4).
Discussion
The US healthcare culture has elevated physicians’ responsibility in adequately and aggressively managing their patients’ pain experience. Moreover, reimbursement may be affected by patient satisfaction scores, which are partly predicated on pain control.20-24 However, as rates of opioid use and abuse rise, it is important that physicians prescribe such medications judiciously. This is particularly germane to orthopedic surgeons, who prescribe more opioid analgesics than surgeons in any other field.17 Rodgers and colleagues18 found upper extremity surgeons, in particular, tended to overprescribe postoperative opioid analgesics. In the present study, we sought to identify the crucial risk factors that influence post-DRF-ORIF pain management and opioid consumption.
Mean postoperative opioid consumption (morphine equivalents) was 58.5 mg, roughly equivalent to 14.6 tabs of oxycodone/acetaminophen 5/325 mg, an opioid analgesic commonly used during the acute postoperative period. In addition, almost 70% of our patients required <75 mg of morphine equivalents, or <20 tabs of oxycodone/acetaminophen 5/325 mg. For upper extremity surgeons, these numbers may be better guides in determining the most appropriate amount of opioid to prescribe after DRF repair.
As for predicting levels of postoperative opioid medication, there was a significant trend toward less consumption with increasing age. Given this finding, surgeons prescribing for elderly patients should expect less opioid use. Regarding payer type, there was a trend toward more opioid use by self-pay/Medicaid patients; however, there were only 3 patients in this group. The situation in the study by Rodgers and colleagues18 is similar: Their finding that Medicaid patients consumed more pain pills after surgery was underpowered (only 5 patients in the group).
In the orthopedic community, support for use of regional anesthesia has been widespread for several reasons, including the belief that it reduces postoperative pain and therefore should reduce postoperative opioid consumption.25 However, we found no significant difference in postoperative opioid consumption between patients who received general anesthesia (with and without local anesthesia) and patients who received regional anesthesia (nerve block). Mean opioid consumption was 57.93 mg in the general anesthesia group and 58.98 mg in the regional anesthesia group. However, this finding could have been confounded by the variability in success and operator dependence inherent in regional anesthesia. In addition, the anatomical location for the peripheral nerve block and anesthetic could have affected the efficacy of the block and played a role in postoperative opioid consumption.
In this study, we tested the hypothesis that there would be more postoperative opioid consumption with worsening fracture type. Although our results did not reach statistical significance, there was a trend toward increased opioid consumption in patients with a complete intra-articular fracture (AO/OTA class C) vs patients with a partial articular fracture (class B) or an extra-articular fracture (class A). In addition, patients with a partial articular fracture tended to use more postoperative opioids than patients with an extra-articular fracture. In short, postoperative opioid consumption tended to be higher with increasing articular involvement of the fracture.
This study was limited in that it relied on patient self-reporting. Given the social stigma attached to opioid use, patients may have underreported their postoperative opioid consumption, been affected by recall bias, or both. The study also did not control for preoperative opioid use or history of opioid or substance abuse. Chronic preoperative opioid consumption may have affected postoperative opioid use. Other patient-related factors, such as body mass index (BMI) and hepatorenal dysfunction, can create tremendous variability in opioid metabolism across a population. Such factors were not controlled for in this study and therefore may have affected its results. That could help explain why older patients, who are more likely to have lower BMI and less efficient organ function for opioid metabolism, had lower postoperative opioid consumption. In addition, although we excluded patients with concomitant injuries and procedures, we did not screen patients for concomitant complex regional pain syndrome, fibromyalgia, or other medical conditions that might have had a significant impact on postoperative pain management needs. Last, some findings, such as the relationship between opioid use and payer type, were underpowered: Although self-pay/Medicaid patients had higher postoperative opioid consumption, they were few in number. The same was true of the Medicaid patients in the study by Rodgers and colleagues.18Our results demonstrated that post-DRF-ORIF opioid consumption decreased with age and was independent of type of perioperative anesthesia. There was a trend toward more opioid consumption with both self- and Medicaid payment and worsening fracture classification. It has become more important than ever for orthopedic surgeons to adequately manage postoperative pain while limiting opioid availability and the risk for abuse. Surgeons must remain aware of the variables in their patients’ postoperative pain experience in order to better optimize prescribing patterns and provide a safe and effective postoperative pain regimen.
Am J Orthop. 2017;46(1):E35-E40. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Kuehn BM. Opioid prescriptions soar: increase in legitimate use as well as abuse. JAMA. 2007;297(3):249-251.
2. Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71(7):821-826.
3. Helmerhorst GT, Lindenhovius AL, Vrahas M, Ring D, Kloen P. Satisfaction with pain relief after operative treatment of an ankle fracture. Injury. 2012;43(11):1958-1961.
4. Lindenhovius AL, Helmerhorst GT, Schnellen AC, Vrahas M, Ring D, Kloen P. Differences in prescription of narcotic pain medication after operative treatment of hip and ankle fractures in the United States and the Netherlands. J Trauma. 2009;67(1):160-164.
5. Seya MJ, Gelders SF, Achara OU, Milani B, Scholten WK. A first comparison between the consumption of and the need for opioid analgesics at country, regional, and global levels. J Pain Palliat Care Pharmacother. 2011;25(1):6-18.
6. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
7. Kuehn BM. CDC: major disparities in opioid prescribing among states: some states crack down on excess prescribing. JAMA. 2014;312(7):684-686.
8. Paulozzi LJ, Budnitz DS, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf. 2006;15(9):618-627.
9. Cicero TJ, Kuehn BM. Driven by prescription drug abuse, heroin use increases among suburban and rural whites. JAMA. 2014;312(2):118-119.
10. Painkillers fuel growth in drug addiction. Harvard Ment Health Lett. Harvard Medical School website. http://www.health.harvard.edu/newsletter_article/painkillers-fuel-growth-in-drug-addiction. Published January 2011. Accessed March 18, 2015.
11. Cai R, Crane E, Poneleit K, Paulozzi L. Emergency department visits involving nonmedical use of selected prescription drugs in the United States, 2004-2008. J Pain Palliat Care Pharmacother. 2010;24(3):293-297.
12. Armaghani SJ, Lee DS, Bible JE, et al. Preoperative narcotic use and its relation to depression and anxiety in patients undergoing spine surgery. Spine. 2013;38(25):2196-2200.
13. Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000. Pain. 2004;109(3):514-519.
14. Deyo RA, Mirza SK, Turner JA, Martin BI. Overtreating chronic back pain: time to back off? J Am Board Fam Med. 2009;22(1):62-68.
15. Lee D, Armaghani S, Archer KR, et al. Preoperative opioid use as a predictor of adverse postoperative self-reported outcomes in patients undergoing spine surgery. J Bone Joint Surg Am. 2014;96(11):e89.
16. Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine. 2007;32(19):2127-2132.
17. Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SR. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305(13):1299-1301.
18. Rodgers J, Cunningham K, Fitzgerald K, Finnerty E. Opioid consumption following outpatient upper extremity surgery. J Hand Surg Am. 2012;37(4):645-650.
19. Chen L, Vo T, Seefeld L, et al. Lack of correlation between opioid dose adjustment and pain score change in a group of chronic pain patients. J Pain. 2013;14(4):384-392.
20. Bush H. Doubling down on the patient experience. Hosp Health Netw. 2011;85(12):22-25, 1.
21. Centers for Medicare & Medicaid Services, US Department of Health and Human Services. Medicare program; hospital inpatient prospective payment systems for acute care hospitals and the long-term care hospital prospective payment system and fiscal year 2013 rates; hospitals’ resident caps for graduate medical education payment purposes; quality reporting requirements for specific providers and for ambulatory surgical centers. Final rule. Fed Regist. 2012;77(170):53257-53750.
22. Centers for Medicare & Medicaid Services, US Department of Health and Human Services. Hospital Value-Based Purchasing. http://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/Hospital_VBPurchasing_Fact_Sheet_ICN907664.pdf. Published September 2015. Accessed October 2015.
23. Manchikanti L, Singh V, Caraway DL, Benyamin RM, Falco FJ, Hirsch JA. Proposed physician payment schedule for 2013: guarded prognosis for interventional pain management. Pain Physician. 2012;15(5):E615-E627.
24. Bot AG, Bekkers S, Arnstein PM, Smith RM, Ring D. Opioid use after fracture surgery correlates with pain intensity and satisfaction with pain relief. Clin Orthop Relat Res. 2014;472(8):2542-2549.
25. Oldman M, McCartney CJ, Leung A, et al. A survey of orthopedic surgeons’ attitudes and knowledge regarding regional anesthesia. Anesth Analg. 2004;98(5):1486-1490.
1. Kuehn BM. Opioid prescriptions soar: increase in legitimate use as well as abuse. JAMA. 2007;297(3):249-251.
2. Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71(7):821-826.
3. Helmerhorst GT, Lindenhovius AL, Vrahas M, Ring D, Kloen P. Satisfaction with pain relief after operative treatment of an ankle fracture. Injury. 2012;43(11):1958-1961.
4. Lindenhovius AL, Helmerhorst GT, Schnellen AC, Vrahas M, Ring D, Kloen P. Differences in prescription of narcotic pain medication after operative treatment of hip and ankle fractures in the United States and the Netherlands. J Trauma. 2009;67(1):160-164.
5. Seya MJ, Gelders SF, Achara OU, Milani B, Scholten WK. A first comparison between the consumption of and the need for opioid analgesics at country, regional, and global levels. J Pain Palliat Care Pharmacother. 2011;25(1):6-18.
6. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
7. Kuehn BM. CDC: major disparities in opioid prescribing among states: some states crack down on excess prescribing. JAMA. 2014;312(7):684-686.
8. Paulozzi LJ, Budnitz DS, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf. 2006;15(9):618-627.
9. Cicero TJ, Kuehn BM. Driven by prescription drug abuse, heroin use increases among suburban and rural whites. JAMA. 2014;312(2):118-119.
10. Painkillers fuel growth in drug addiction. Harvard Ment Health Lett. Harvard Medical School website. http://www.health.harvard.edu/newsletter_article/painkillers-fuel-growth-in-drug-addiction. Published January 2011. Accessed March 18, 2015.
11. Cai R, Crane E, Poneleit K, Paulozzi L. Emergency department visits involving nonmedical use of selected prescription drugs in the United States, 2004-2008. J Pain Palliat Care Pharmacother. 2010;24(3):293-297.
12. Armaghani SJ, Lee DS, Bible JE, et al. Preoperative narcotic use and its relation to depression and anxiety in patients undergoing spine surgery. Spine. 2013;38(25):2196-2200.
13. Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000. Pain. 2004;109(3):514-519.
14. Deyo RA, Mirza SK, Turner JA, Martin BI. Overtreating chronic back pain: time to back off? J Am Board Fam Med. 2009;22(1):62-68.
15. Lee D, Armaghani S, Archer KR, et al. Preoperative opioid use as a predictor of adverse postoperative self-reported outcomes in patients undergoing spine surgery. J Bone Joint Surg Am. 2014;96(11):e89.
16. Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine. 2007;32(19):2127-2132.
17. Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SR. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305(13):1299-1301.
18. Rodgers J, Cunningham K, Fitzgerald K, Finnerty E. Opioid consumption following outpatient upper extremity surgery. J Hand Surg Am. 2012;37(4):645-650.
19. Chen L, Vo T, Seefeld L, et al. Lack of correlation between opioid dose adjustment and pain score change in a group of chronic pain patients. J Pain. 2013;14(4):384-392.
20. Bush H. Doubling down on the patient experience. Hosp Health Netw. 2011;85(12):22-25, 1.
21. Centers for Medicare & Medicaid Services, US Department of Health and Human Services. Medicare program; hospital inpatient prospective payment systems for acute care hospitals and the long-term care hospital prospective payment system and fiscal year 2013 rates; hospitals’ resident caps for graduate medical education payment purposes; quality reporting requirements for specific providers and for ambulatory surgical centers. Final rule. Fed Regist. 2012;77(170):53257-53750.
22. Centers for Medicare & Medicaid Services, US Department of Health and Human Services. Hospital Value-Based Purchasing. http://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/Hospital_VBPurchasing_Fact_Sheet_ICN907664.pdf. Published September 2015. Accessed October 2015.
23. Manchikanti L, Singh V, Caraway DL, Benyamin RM, Falco FJ, Hirsch JA. Proposed physician payment schedule for 2013: guarded prognosis for interventional pain management. Pain Physician. 2012;15(5):E615-E627.
24. Bot AG, Bekkers S, Arnstein PM, Smith RM, Ring D. Opioid use after fracture surgery correlates with pain intensity and satisfaction with pain relief. Clin Orthop Relat Res. 2014;472(8):2542-2549.
25. Oldman M, McCartney CJ, Leung A, et al. A survey of orthopedic surgeons’ attitudes and knowledge regarding regional anesthesia. Anesth Analg. 2004;98(5):1486-1490.
Progressive Widespread Warty Skin Growths
Epidermodysplasia Verruciformis
Epidermodysplasia verruciformis (EV) is a rare hereditary disorder that predisposes affected individuals to widespread infection with various forms of human papillomavirus (HPV). It is inherited in an autosomal-recessive pattern.1 The first manifestations generally are seen in childhood. The clinical appearance of lesions can vary, at times mimicking other disease processes. Patients can present with flat wartlike papules resembling verruca plana distributed in sun-exposed areas. Another distinct presentation is multiple salmon-colored, hyperpigmented, or hypopigmented macules, papules, or plaques with overlying scale that can resemble tinea versicolor.1,2 A large percentage of patients will go on to develop actinic keratosis and squamous cell carcinoma by 40 years of age.2 The malignancies most commonly develop in sun-exposed areas, suggesting UV radiation as an important contributor to development along with HPV infection. Mutations in the EVER1 and EVER2 genes that code for transmembrane proteins on the endoplasmic reticulum that are involved in zinc transport lead to EV. The mutations lead to decreased zinc movement into the cytoplasm, which is thought to play a role in preventing HPV infection. The decreased protection against HPV leads to infections from both common subtypes and those that immunocompetent individuals would be resistant to, namely the β-genus HPV-5, -8, -9 and -20.1,2 Immunosuppressed individuals, such as those with human immunodeficiency virus/AIDS, also are at an increased risk for infection with these HPV subtypes and generally have similar clinical and histological presentations.1 It is important to promote sunscreen use for preventive care in patients with EV due to the increased risk for squamous cell carcinoma.
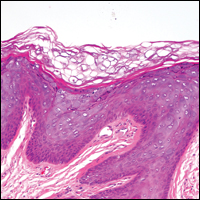
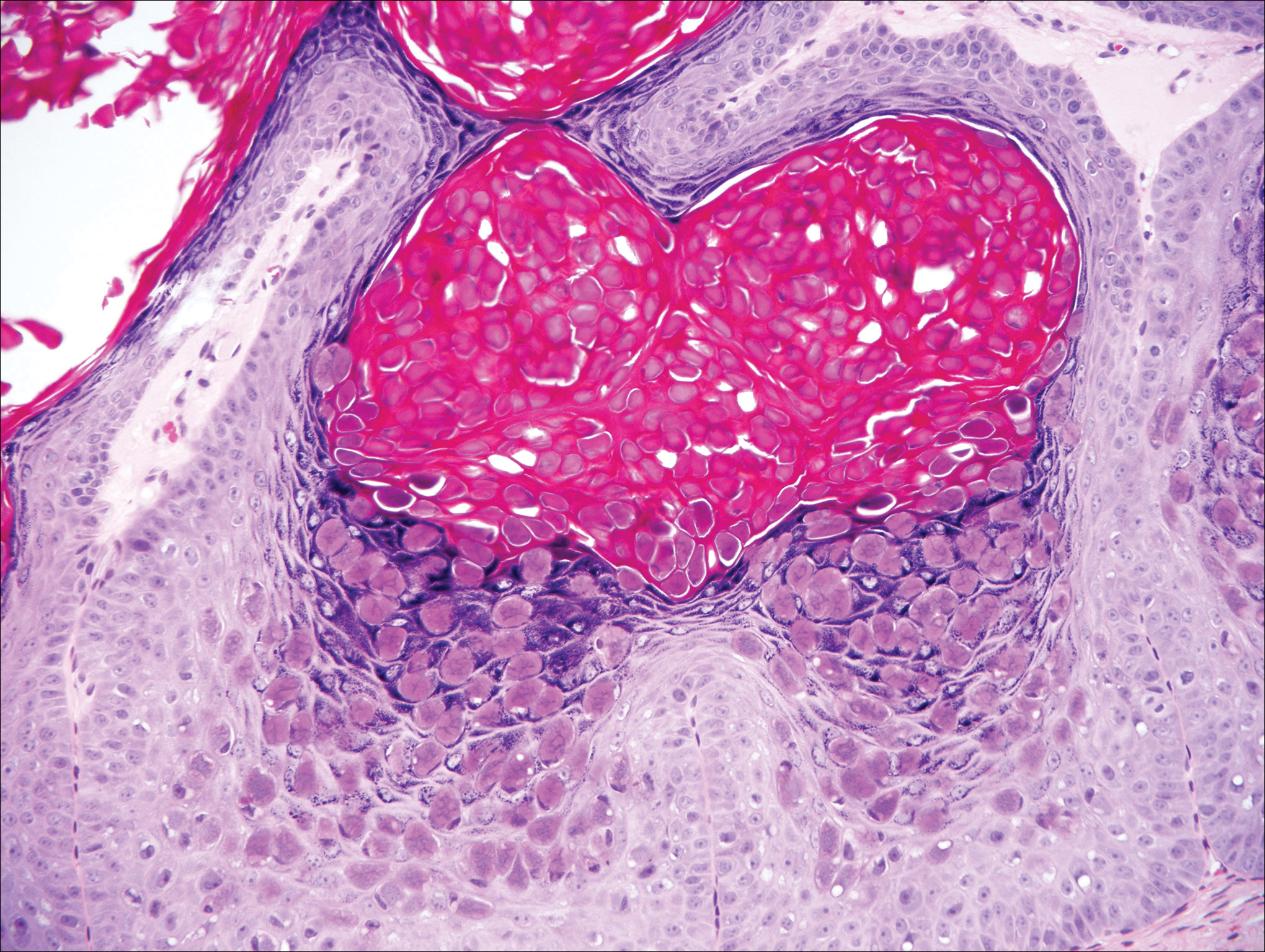
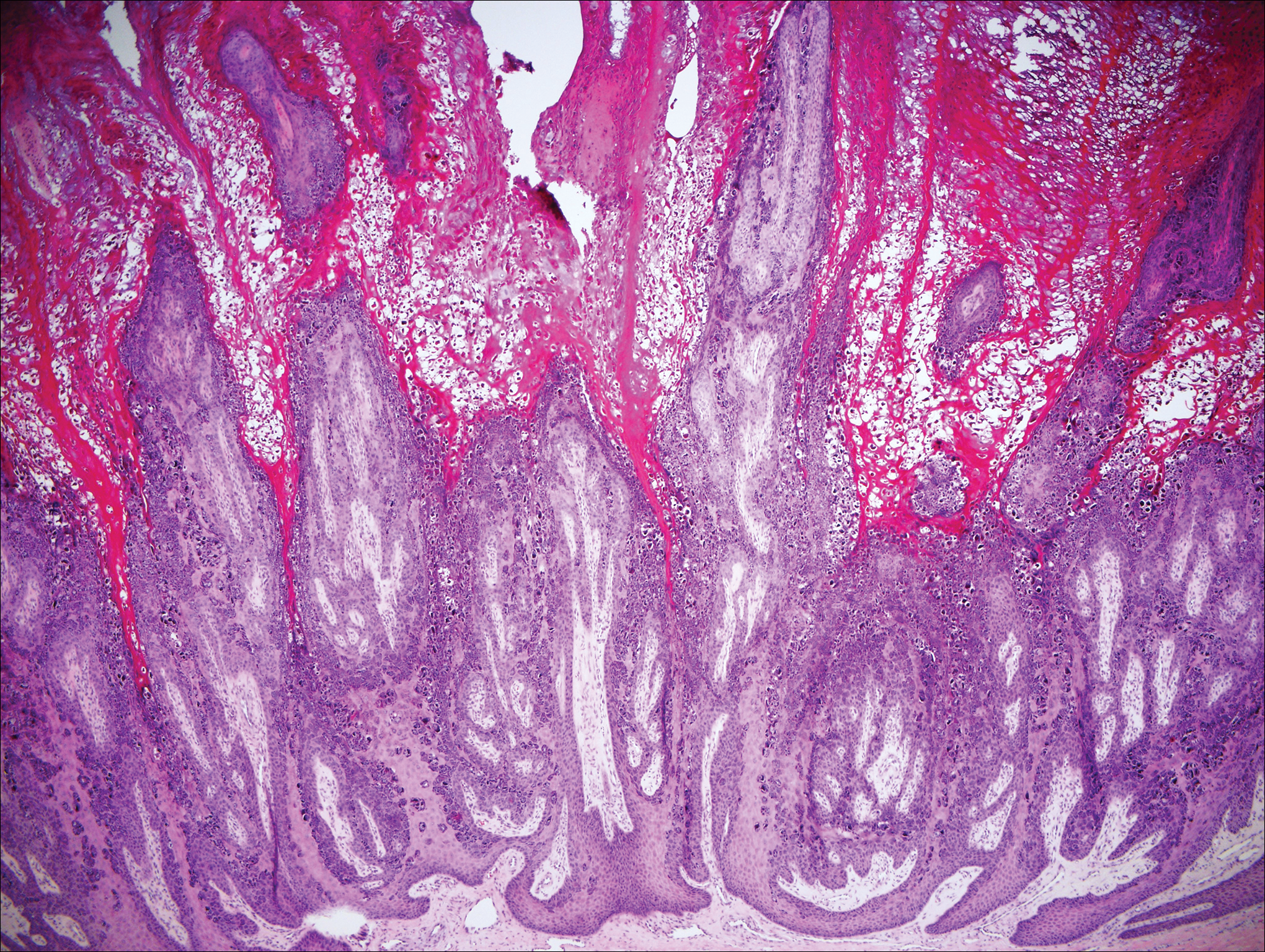
Histologically, the lesions in EV are composed of acanthosis and hyperkeratosis with keratinocytes arranged in clusters.1,3 There is orthokeratosis and parakeratosis.1 Scattered or clustered keratinocytes in the granular layer or upper stratum spinosum appear swollen with foamy blue-gray cytoplasm (quiz image and Figure 1).1,4 The keratinocytes may become atypical and progress to squamous cell carcinoma, particularly in sun-exposed regions. Cell differentiation becomes disorganized and nuclei become enlarged and hyperchromatic.1

Condyloma acuminatum will have pronounced acanthosis and hyperkeratosis with exophytic growth. Rounded parakeratosis is visible. The characteristic cell is the koilocyte, a keratinocyte that has an enlarged nucleus with areas of surrounding clearing, increased dark color in the nucleus, and wrinkled nuclear membrane (Figure 2).1,3 True koilocytes may be rare in condyloma acuminatum.4 Other distinct features include coarse hypergranulosis and a compact stratum corneum.

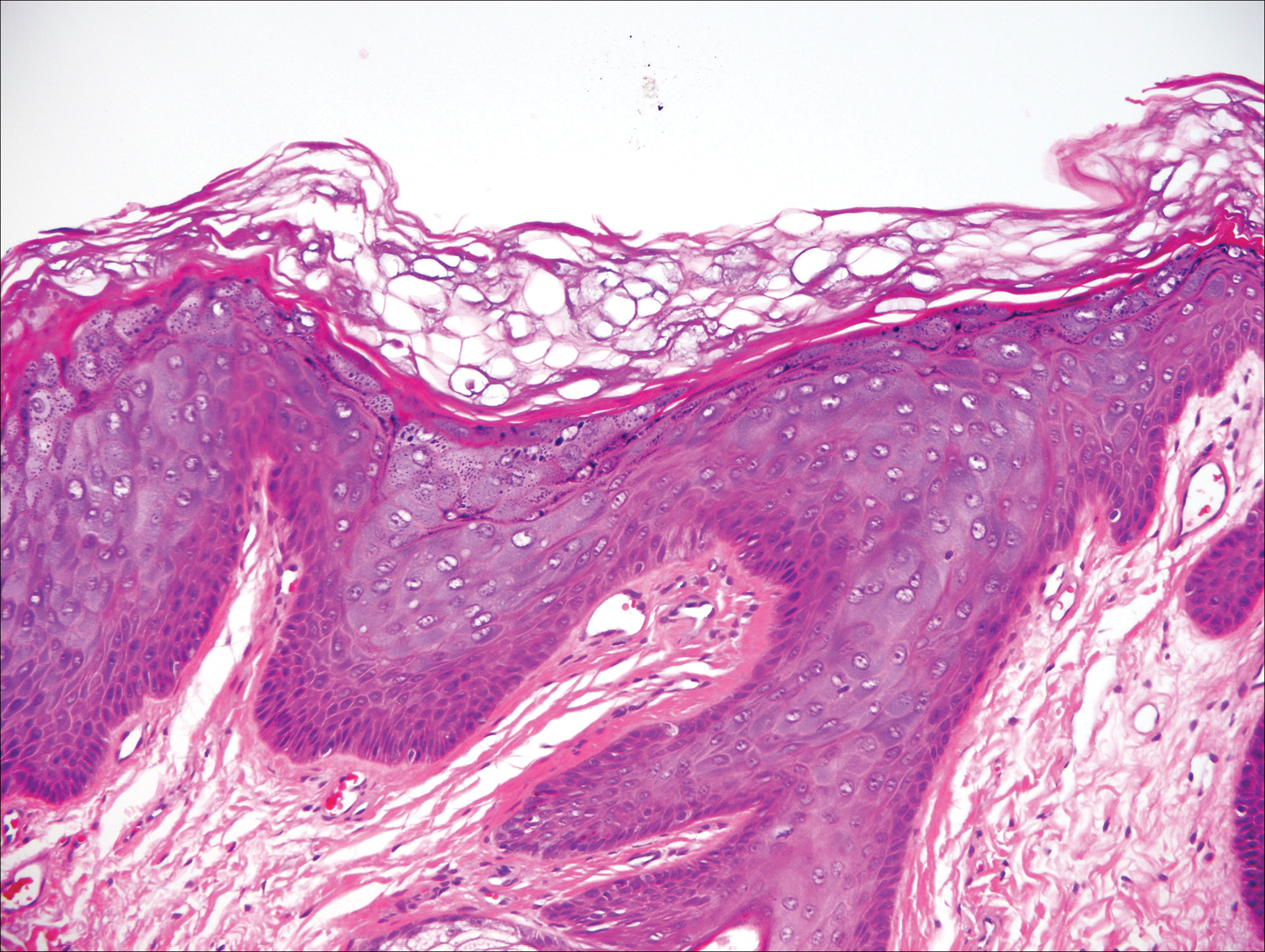
Herpesvirus lesions typically demonstrate ballooning degeneration of keratinocytes.1 They will become pale and fuse to form multinucleated giant cells, a feature not found in verruca. The nuclei will be slate gray with margination of the chromatin, which can be identified due to its increased basophilic appearance (Figure 3).1,4 Inclusion bodies can be found, but unlike molluscum contagiosum (MC), these bodies are intranuclear as opposed to cytoplasmic.1
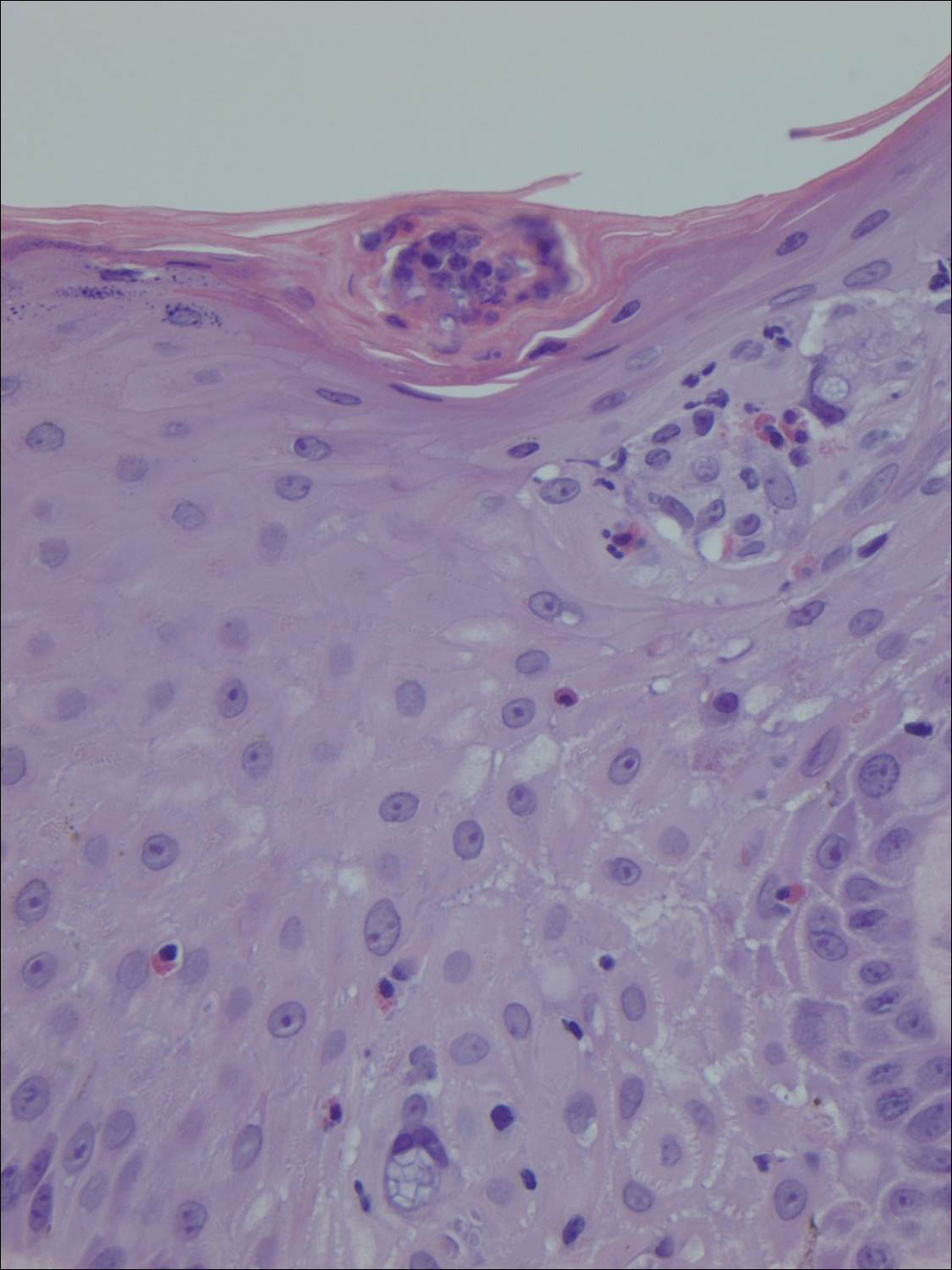
The telltale Henderson-Patterson (molluscum) bodies can identify MC histologically.4 Located within keratinocytes, these cytoplasmic inclusions can vary in both color and size as they mature. As the keratinocytes develop outward, the molluscum bodies grow larger and become more eosinophilic (Figure 4).1,4 Another feature of MC that can be used to differentiate it from EV is the scalloped borders located on lesions.4
On histology, verruca vulgaris will have pronounced acanthosis with orthokeratosis and vertical tiers of parakeratosis.3,4 Growth is exophytic. The granular layer will have large irregular basophilic granules. Koilocytes may be seen. A distinctive feature is the papillomatosis with inward bending of rete ridges.3,4 It is common to see invasion of tortuous blood vessels into the exophytic projections.3 In myrmecia (palmoplantar warts) it is common to see thrombosis of these vessels and inclusions of red cytoplasmic bodies (Figure 5).1,4
- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012.
- Hunzeker CM, Soldano AC, Prystowsky S. Epidermodysplasia verruciformis. Dermatology Online J. 2008;14:2.
- Calonje E, McKee PH. McKee's Pathology of the Skin. 4th ed. Edinburgh, Scotland: Elsevier/Saunders; 2012.
- Elston DM, Ko CJ, Ferringer T. Dermatopathology. Edinburgh, Scotland: Saunders/Elsevier; 2009.
Epidermodysplasia Verruciformis
Epidermodysplasia verruciformis (EV) is a rare hereditary disorder that predisposes affected individuals to widespread infection with various forms of human papillomavirus (HPV). It is inherited in an autosomal-recessive pattern.1 The first manifestations generally are seen in childhood. The clinical appearance of lesions can vary, at times mimicking other disease processes. Patients can present with flat wartlike papules resembling verruca plana distributed in sun-exposed areas. Another distinct presentation is multiple salmon-colored, hyperpigmented, or hypopigmented macules, papules, or plaques with overlying scale that can resemble tinea versicolor.1,2 A large percentage of patients will go on to develop actinic keratosis and squamous cell carcinoma by 40 years of age.2 The malignancies most commonly develop in sun-exposed areas, suggesting UV radiation as an important contributor to development along with HPV infection. Mutations in the EVER1 and EVER2 genes that code for transmembrane proteins on the endoplasmic reticulum that are involved in zinc transport lead to EV. The mutations lead to decreased zinc movement into the cytoplasm, which is thought to play a role in preventing HPV infection. The decreased protection against HPV leads to infections from both common subtypes and those that immunocompetent individuals would be resistant to, namely the β-genus HPV-5, -8, -9 and -20.1,2 Immunosuppressed individuals, such as those with human immunodeficiency virus/AIDS, also are at an increased risk for infection with these HPV subtypes and generally have similar clinical and histological presentations.1 It is important to promote sunscreen use for preventive care in patients with EV due to the increased risk for squamous cell carcinoma.
Histologically, the lesions in EV are composed of acanthosis and hyperkeratosis with keratinocytes arranged in clusters.1,3 There is orthokeratosis and parakeratosis.1 Scattered or clustered keratinocytes in the granular layer or upper stratum spinosum appear swollen with foamy blue-gray cytoplasm (quiz image and Figure 1).1,4 The keratinocytes may become atypical and progress to squamous cell carcinoma, particularly in sun-exposed regions. Cell differentiation becomes disorganized and nuclei become enlarged and hyperchromatic.1

Condyloma acuminatum will have pronounced acanthosis and hyperkeratosis with exophytic growth. Rounded parakeratosis is visible. The characteristic cell is the koilocyte, a keratinocyte that has an enlarged nucleus with areas of surrounding clearing, increased dark color in the nucleus, and wrinkled nuclear membrane (Figure 2).1,3 True koilocytes may be rare in condyloma acuminatum.4 Other distinct features include coarse hypergranulosis and a compact stratum corneum.

Herpesvirus lesions typically demonstrate ballooning degeneration of keratinocytes.1 They will become pale and fuse to form multinucleated giant cells, a feature not found in verruca. The nuclei will be slate gray with margination of the chromatin, which can be identified due to its increased basophilic appearance (Figure 3).1,4 Inclusion bodies can be found, but unlike molluscum contagiosum (MC), these bodies are intranuclear as opposed to cytoplasmic.1

The telltale Henderson-Patterson (molluscum) bodies can identify MC histologically.4 Located within keratinocytes, these cytoplasmic inclusions can vary in both color and size as they mature. As the keratinocytes develop outward, the molluscum bodies grow larger and become more eosinophilic (Figure 4).1,4 Another feature of MC that can be used to differentiate it from EV is the scalloped borders located on lesions.4

On histology, verruca vulgaris will have pronounced acanthosis with orthokeratosis and vertical tiers of parakeratosis.3,4 Growth is exophytic. The granular layer will have large irregular basophilic granules. Koilocytes may be seen. A distinctive feature is the papillomatosis with inward bending of rete ridges.3,4 It is common to see invasion of tortuous blood vessels into the exophytic projections.3 In myrmecia (palmoplantar warts) it is common to see thrombosis of these vessels and inclusions of red cytoplasmic bodies (Figure 5).1,4

Epidermodysplasia Verruciformis
Epidermodysplasia verruciformis (EV) is a rare hereditary disorder that predisposes affected individuals to widespread infection with various forms of human papillomavirus (HPV). It is inherited in an autosomal-recessive pattern.1 The first manifestations generally are seen in childhood. The clinical appearance of lesions can vary, at times mimicking other disease processes. Patients can present with flat wartlike papules resembling verruca plana distributed in sun-exposed areas. Another distinct presentation is multiple salmon-colored, hyperpigmented, or hypopigmented macules, papules, or plaques with overlying scale that can resemble tinea versicolor.1,2 A large percentage of patients will go on to develop actinic keratosis and squamous cell carcinoma by 40 years of age.2 The malignancies most commonly develop in sun-exposed areas, suggesting UV radiation as an important contributor to development along with HPV infection. Mutations in the EVER1 and EVER2 genes that code for transmembrane proteins on the endoplasmic reticulum that are involved in zinc transport lead to EV. The mutations lead to decreased zinc movement into the cytoplasm, which is thought to play a role in preventing HPV infection. The decreased protection against HPV leads to infections from both common subtypes and those that immunocompetent individuals would be resistant to, namely the β-genus HPV-5, -8, -9 and -20.1,2 Immunosuppressed individuals, such as those with human immunodeficiency virus/AIDS, also are at an increased risk for infection with these HPV subtypes and generally have similar clinical and histological presentations.1 It is important to promote sunscreen use for preventive care in patients with EV due to the increased risk for squamous cell carcinoma.
Histologically, the lesions in EV are composed of acanthosis and hyperkeratosis with keratinocytes arranged in clusters.1,3 There is orthokeratosis and parakeratosis.1 Scattered or clustered keratinocytes in the granular layer or upper stratum spinosum appear swollen with foamy blue-gray cytoplasm (quiz image and Figure 1).1,4 The keratinocytes may become atypical and progress to squamous cell carcinoma, particularly in sun-exposed regions. Cell differentiation becomes disorganized and nuclei become enlarged and hyperchromatic.1

Condyloma acuminatum will have pronounced acanthosis and hyperkeratosis with exophytic growth. Rounded parakeratosis is visible. The characteristic cell is the koilocyte, a keratinocyte that has an enlarged nucleus with areas of surrounding clearing, increased dark color in the nucleus, and wrinkled nuclear membrane (Figure 2).1,3 True koilocytes may be rare in condyloma acuminatum.4 Other distinct features include coarse hypergranulosis and a compact stratum corneum.

Herpesvirus lesions typically demonstrate ballooning degeneration of keratinocytes.1 They will become pale and fuse to form multinucleated giant cells, a feature not found in verruca. The nuclei will be slate gray with margination of the chromatin, which can be identified due to its increased basophilic appearance (Figure 3).1,4 Inclusion bodies can be found, but unlike molluscum contagiosum (MC), these bodies are intranuclear as opposed to cytoplasmic.1

The telltale Henderson-Patterson (molluscum) bodies can identify MC histologically.4 Located within keratinocytes, these cytoplasmic inclusions can vary in both color and size as they mature. As the keratinocytes develop outward, the molluscum bodies grow larger and become more eosinophilic (Figure 4).1,4 Another feature of MC that can be used to differentiate it from EV is the scalloped borders located on lesions.4

On histology, verruca vulgaris will have pronounced acanthosis with orthokeratosis and vertical tiers of parakeratosis.3,4 Growth is exophytic. The granular layer will have large irregular basophilic granules. Koilocytes may be seen. A distinctive feature is the papillomatosis with inward bending of rete ridges.3,4 It is common to see invasion of tortuous blood vessels into the exophytic projections.3 In myrmecia (palmoplantar warts) it is common to see thrombosis of these vessels and inclusions of red cytoplasmic bodies (Figure 5).1,4

- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012.
- Hunzeker CM, Soldano AC, Prystowsky S. Epidermodysplasia verruciformis. Dermatology Online J. 2008;14:2.
- Calonje E, McKee PH. McKee's Pathology of the Skin. 4th ed. Edinburgh, Scotland: Elsevier/Saunders; 2012.
- Elston DM, Ko CJ, Ferringer T. Dermatopathology. Edinburgh, Scotland: Saunders/Elsevier; 2009.
- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012.
- Hunzeker CM, Soldano AC, Prystowsky S. Epidermodysplasia verruciformis. Dermatology Online J. 2008;14:2.
- Calonje E, McKee PH. McKee's Pathology of the Skin. 4th ed. Edinburgh, Scotland: Elsevier/Saunders; 2012.
- Elston DM, Ko CJ, Ferringer T. Dermatopathology. Edinburgh, Scotland: Saunders/Elsevier; 2009.
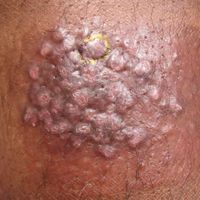
A 33-year-old man presented with progressive widespread warty skin growths that had been present since 6 years of age. Physical examination revealed numerous verrucous papules on the face and neck along with verrucous, tan-pink papules and plaques diffusely scattered on the trunk, arms, and legs. A biopsy of a lesion on the neck was performed.
Expanding Pruritic Plaque on the Forearm
The Diagnosis: Cutaneous Protothecosis
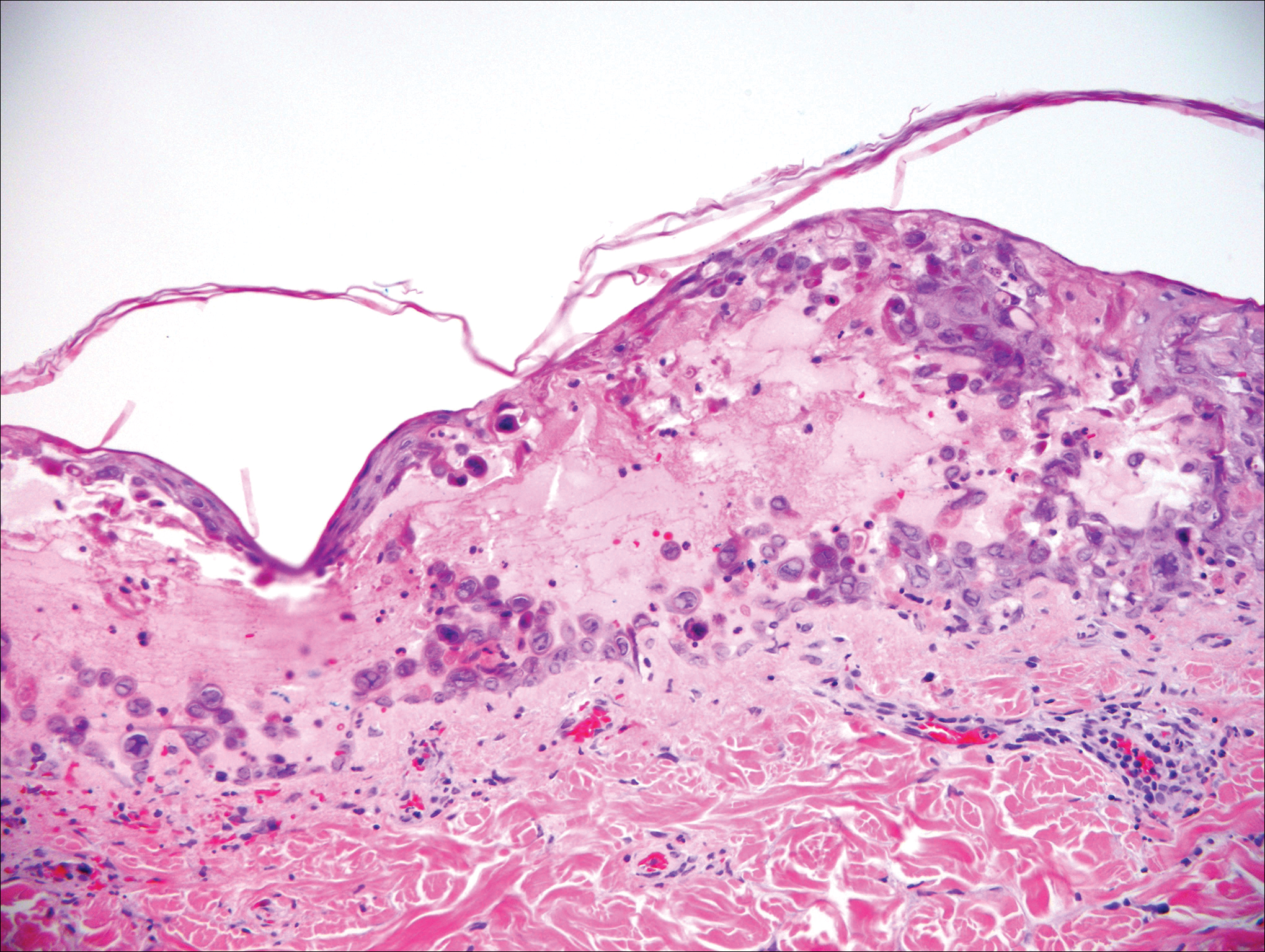
A 4-mm punch biopsy of the plaque on the right forearm was performed. The biopsy showed chronic inflammation with prominent histiocytes, foreign body giant cells, plasma cells, and abundant eosinophils (Figure 1). Grocott-Gomori methenamine-silver stain demonstrated abundant soccer ball-like or floretlike sporangia that were 3 to 11 μm, consistent with a diagnosis of protothecosis (Figure 2).

Cutaneous protothecosis is an infection caused by chlorophyll-lacking algae of the genus Prototheca.1 It is ubiquitous in nature and can be isolated from various reservoirs such as trees, grass, water, and food sources.2 Protothecosis is present worldwide and in the United States; it is most prevalent in the Southeast. Prototheca species are rare but often endemic in cattle and can cause bovine mastitis and enteritis.3 However, they are rare opportunistic infections in humans.
The pathogenesis of cutaneous protothecosis is largely unknown.4 However, most infections are thought to be caused by traumatic inoculation into subcutaneous tissues.1,2 The majority of cases occur in patients older than 30 years. To date, approximately 160 cases have been reported in the literature worldwide.5 There are 3 main species of Prototheca, but almost all human infections are caused by Prototheca wickerhamii.2 Clinically, most patients with protothecosis present with cutaneous findings, but olecranon bursitis and systemic forms also have been reported.1
Risk factors for protothecosis include immunosuppression, most often due to steroids, in addition to malignancies, diabetes mellitus, and certain occupations.1 The presentation can be variable from papules and plaques to even herpetiform appearances.4 Protothecosis usually affects the skin and soft tissues of exposed areas such as the extremities or the face.6 Diagnosis largely is made on detection of characteristic floretlike sporangia with a prominent cell wall on histopathological examination. Prototheca wickerhamii specifically produces a morula form of sporangia with endospores arranged symmetrically, giving it a characteristic soccer ball appearance.2
Treatment of protothecosis is difficult and remains controversial.1 There are no established protothecosis treatment protocols or guidelines due to the small number of cases.7 In vitro studies have demonstrated sensitivity to amphotericin B and various azoles as well as a wide range of antibiotics.1 Olecranon bursitis and small skin lesions can be treated by surgical excision. All other Prototheca infections require systemic treatment with azoles or intravenous amphotericin B for immunocompromised patients or those with disseminated disease.5 However, failure to respond to medical management often occurs, requiring surgical excision.1,6
Our patient was treated with a 3-month course of voriconazole but therapy failed and the plaque continued to expand. The patient underwent a wide excision that was repaired with a partial-thickness skin graft. Rebiopsy of the papule adjacent to the skin graft showed no further recurrence.
In conclusion, protothecosis generally is not clinically suspected and patients are subjected to various treatments without adequate results. A definitive diagnosis easily can be established with a skin biopsy, which can direct timely and appropriate treatment.
- Lass-Flörl C, Mary A. Human protothecosis. Clin Microbiol Rev. 2007;20:230-242.
- Mayorga J, Barba-Gómez JF, Verduzco-Martínez AP, et al. Protothecosis. Clin Dermatol. 2012;30:432-436.
- Jensen HE, Aalbaek B, Bloch B, et al. Bovine mammary protothecosis due to Prototheca zopfii. Med Mycol. 1998;36:89-95.
- Boyd AS, Langley M, King LE Jr. Cutaneous manifestations of Prototheca infections. J Am Acad Dermatol. 1995;32:758-764.
- Todd JR, King JW, Oberle A, et al. Protothecosis: report of a case with 20-year follow-up, and review of previously published cases. Med Mycol. 2012;50:673-689.
- Hightower KD, Messina JL. Cutaneous protothecosis: a case report and review of the literature. Cutis. 2007;80:129-131.
- Yamada N, Yoshida Y, Ohsawa T, et al. A case of cutaneous protothecosis successfully treated with local thermal therapy as an adjunct to itraconazole therapy in an immunocompromised host. Med Mycol. 2010;48:643-646.
The Diagnosis: Cutaneous Protothecosis
A 4-mm punch biopsy of the plaque on the right forearm was performed. The biopsy showed chronic inflammation with prominent histiocytes, foreign body giant cells, plasma cells, and abundant eosinophils (Figure 1). Grocott-Gomori methenamine-silver stain demonstrated abundant soccer ball-like or floretlike sporangia that were 3 to 11 μm, consistent with a diagnosis of protothecosis (Figure 2).


Cutaneous protothecosis is an infection caused by chlorophyll-lacking algae of the genus Prototheca.1 It is ubiquitous in nature and can be isolated from various reservoirs such as trees, grass, water, and food sources.2 Protothecosis is present worldwide and in the United States; it is most prevalent in the Southeast. Prototheca species are rare but often endemic in cattle and can cause bovine mastitis and enteritis.3 However, they are rare opportunistic infections in humans.
The pathogenesis of cutaneous protothecosis is largely unknown.4 However, most infections are thought to be caused by traumatic inoculation into subcutaneous tissues.1,2 The majority of cases occur in patients older than 30 years. To date, approximately 160 cases have been reported in the literature worldwide.5 There are 3 main species of Prototheca, but almost all human infections are caused by Prototheca wickerhamii.2 Clinically, most patients with protothecosis present with cutaneous findings, but olecranon bursitis and systemic forms also have been reported.1
Risk factors for protothecosis include immunosuppression, most often due to steroids, in addition to malignancies, diabetes mellitus, and certain occupations.1 The presentation can be variable from papules and plaques to even herpetiform appearances.4 Protothecosis usually affects the skin and soft tissues of exposed areas such as the extremities or the face.6 Diagnosis largely is made on detection of characteristic floretlike sporangia with a prominent cell wall on histopathological examination. Prototheca wickerhamii specifically produces a morula form of sporangia with endospores arranged symmetrically, giving it a characteristic soccer ball appearance.2
Treatment of protothecosis is difficult and remains controversial.1 There are no established protothecosis treatment protocols or guidelines due to the small number of cases.7 In vitro studies have demonstrated sensitivity to amphotericin B and various azoles as well as a wide range of antibiotics.1 Olecranon bursitis and small skin lesions can be treated by surgical excision. All other Prototheca infections require systemic treatment with azoles or intravenous amphotericin B for immunocompromised patients or those with disseminated disease.5 However, failure to respond to medical management often occurs, requiring surgical excision.1,6
Our patient was treated with a 3-month course of voriconazole but therapy failed and the plaque continued to expand. The patient underwent a wide excision that was repaired with a partial-thickness skin graft. Rebiopsy of the papule adjacent to the skin graft showed no further recurrence.
In conclusion, protothecosis generally is not clinically suspected and patients are subjected to various treatments without adequate results. A definitive diagnosis easily can be established with a skin biopsy, which can direct timely and appropriate treatment.
The Diagnosis: Cutaneous Protothecosis
A 4-mm punch biopsy of the plaque on the right forearm was performed. The biopsy showed chronic inflammation with prominent histiocytes, foreign body giant cells, plasma cells, and abundant eosinophils (Figure 1). Grocott-Gomori methenamine-silver stain demonstrated abundant soccer ball-like or floretlike sporangia that were 3 to 11 μm, consistent with a diagnosis of protothecosis (Figure 2).


Cutaneous protothecosis is an infection caused by chlorophyll-lacking algae of the genus Prototheca.1 It is ubiquitous in nature and can be isolated from various reservoirs such as trees, grass, water, and food sources.2 Protothecosis is present worldwide and in the United States; it is most prevalent in the Southeast. Prototheca species are rare but often endemic in cattle and can cause bovine mastitis and enteritis.3 However, they are rare opportunistic infections in humans.
The pathogenesis of cutaneous protothecosis is largely unknown.4 However, most infections are thought to be caused by traumatic inoculation into subcutaneous tissues.1,2 The majority of cases occur in patients older than 30 years. To date, approximately 160 cases have been reported in the literature worldwide.5 There are 3 main species of Prototheca, but almost all human infections are caused by Prototheca wickerhamii.2 Clinically, most patients with protothecosis present with cutaneous findings, but olecranon bursitis and systemic forms also have been reported.1
Risk factors for protothecosis include immunosuppression, most often due to steroids, in addition to malignancies, diabetes mellitus, and certain occupations.1 The presentation can be variable from papules and plaques to even herpetiform appearances.4 Protothecosis usually affects the skin and soft tissues of exposed areas such as the extremities or the face.6 Diagnosis largely is made on detection of characteristic floretlike sporangia with a prominent cell wall on histopathological examination. Prototheca wickerhamii specifically produces a morula form of sporangia with endospores arranged symmetrically, giving it a characteristic soccer ball appearance.2
Treatment of protothecosis is difficult and remains controversial.1 There are no established protothecosis treatment protocols or guidelines due to the small number of cases.7 In vitro studies have demonstrated sensitivity to amphotericin B and various azoles as well as a wide range of antibiotics.1 Olecranon bursitis and small skin lesions can be treated by surgical excision. All other Prototheca infections require systemic treatment with azoles or intravenous amphotericin B for immunocompromised patients or those with disseminated disease.5 However, failure to respond to medical management often occurs, requiring surgical excision.1,6
Our patient was treated with a 3-month course of voriconazole but therapy failed and the plaque continued to expand. The patient underwent a wide excision that was repaired with a partial-thickness skin graft. Rebiopsy of the papule adjacent to the skin graft showed no further recurrence.
In conclusion, protothecosis generally is not clinically suspected and patients are subjected to various treatments without adequate results. A definitive diagnosis easily can be established with a skin biopsy, which can direct timely and appropriate treatment.
- Lass-Flörl C, Mary A. Human protothecosis. Clin Microbiol Rev. 2007;20:230-242.
- Mayorga J, Barba-Gómez JF, Verduzco-Martínez AP, et al. Protothecosis. Clin Dermatol. 2012;30:432-436.
- Jensen HE, Aalbaek B, Bloch B, et al. Bovine mammary protothecosis due to Prototheca zopfii. Med Mycol. 1998;36:89-95.
- Boyd AS, Langley M, King LE Jr. Cutaneous manifestations of Prototheca infections. J Am Acad Dermatol. 1995;32:758-764.
- Todd JR, King JW, Oberle A, et al. Protothecosis: report of a case with 20-year follow-up, and review of previously published cases. Med Mycol. 2012;50:673-689.
- Hightower KD, Messina JL. Cutaneous protothecosis: a case report and review of the literature. Cutis. 2007;80:129-131.
- Yamada N, Yoshida Y, Ohsawa T, et al. A case of cutaneous protothecosis successfully treated with local thermal therapy as an adjunct to itraconazole therapy in an immunocompromised host. Med Mycol. 2010;48:643-646.
- Lass-Flörl C, Mary A. Human protothecosis. Clin Microbiol Rev. 2007;20:230-242.
- Mayorga J, Barba-Gómez JF, Verduzco-Martínez AP, et al. Protothecosis. Clin Dermatol. 2012;30:432-436.
- Jensen HE, Aalbaek B, Bloch B, et al. Bovine mammary protothecosis due to Prototheca zopfii. Med Mycol. 1998;36:89-95.
- Boyd AS, Langley M, King LE Jr. Cutaneous manifestations of Prototheca infections. J Am Acad Dermatol. 1995;32:758-764.
- Todd JR, King JW, Oberle A, et al. Protothecosis: report of a case with 20-year follow-up, and review of previously published cases. Med Mycol. 2012;50:673-689.
- Hightower KD, Messina JL. Cutaneous protothecosis: a case report and review of the literature. Cutis. 2007;80:129-131.
- Yamada N, Yoshida Y, Ohsawa T, et al. A case of cutaneous protothecosis successfully treated with local thermal therapy as an adjunct to itraconazole therapy in an immunocompromised host. Med Mycol. 2010;48:643-646.
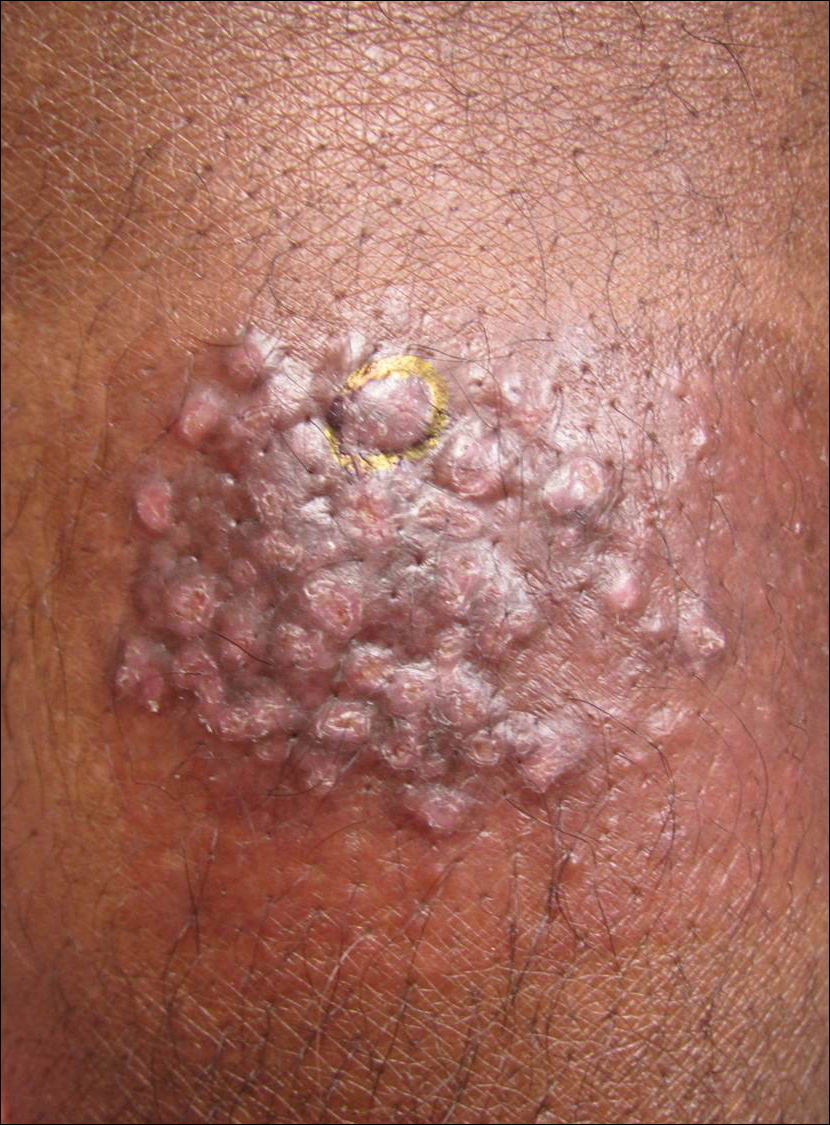
A 66-year-old male firefighter initially presented to the emergency department with an expanding pruritic plaque on the dorsal aspect of the right forearm. The patient recalled the appearance of a single 3-mm papule shortly after doing yardwork in Biloxi, Mississippi. He remembered getting wet grass on the arms, which he later washed off without any notable trauma. The single papule grew into a larger plaque over the next month. In the emergency department he was treated with sulfamethoxazole-trimethoprim, mupirocin, and clotrimazole without response. He was referred to the dermatology department 6 months later and was noted to have multiple 3- to 4-mm papules that coalesced into a 4-cm lichenified plaque with surrounding erythema on the right forearm. His medical history was notable for type 2 diabetes mellitus, hypertension, and hyperlipidemia. The remainder of the physical examination and review of systems was negative.
Proposed Alzheimer’s classification system relies solely on biomarkers
A proposed Alzheimer’s disease classification system eschews neurocognitive testing and relies instead on the presence or absence of known biomarkers.
These markers of Alzheimer’s pathophysiology fall into three distinct categories: amyloid, tau, and neuronal injury (A/T/N). By rating patients as positive or negative for each category, the criteria aim to allow researchers to differentiate true Alzheimer’s patients from those with suspected non-Alzheimer’s pathology (SNAP), a diverse group that experiences neurocognitive decline in the absence of amyloid (Neurology. 2016 Aug 2;87[5]:539-47).
The A/T/N system could be employed in three clinical groups: clinically normal patients, those who meet the clinical criteria for mild cognitive impairment (MCI), and patients with a probable Alzheimer’s disease diagnosis based on the 2011 criteria established by the National Institute on Aging–Alzheimer’s Association (NIA-AA) staging system (Alzheimers Dement. 2011 May;7[3]:257-62).
Both the NIA-AA and IWG systems comprise five biomarkers (low cerebrospinal fluid [CSF] amyloid-beta42; positive PET amyloid imaging; elevated CSF tau; hypometabolism in the temporoparietal cortex on 18F-fluorodeoxyglucose PET imaging; and atrophy in specific brain regions) plus cognitive testing.
The NIA-AA system defines three clinical stages of Alzheimer’s, each with separate classification criteria: stage 1, preclinical; stage 2, MCI; and stage 3, Alzheimer’s disease (AD) dementia. The IWG system is more complex than the NIA-AA system and takes into account biomarkers, cognition, and other clinical manifestations.
The A/T/N system doesn’t require cognitive testing, and includes additional biomarkers that should allow for a much more nuanced classification of patients. It also reflects the field’s growing confidence in objective markers that measure the pathophysiology of the disease, rather than the way it manifests symptomatically, said Dr. Jack, professor of radiology at the Mayo Clinic, Rochester, Minn.
He was the lead author on the August 2016 paper that provided a first look at the proposal. He presented a more mature version of the system at the Clinical Trials on Alzheimer’s Disease meeting in San Diego last fall. The committee is refining the document now, and intends to present a final draft at the Alzheimer’s Association International Conference in London this summer.
Would the A/T/N system benefit clinical research?
Researchers desperately need a better classification system, Dr. Jack said in an interview. The vast majority of AD drug trials have enrolled clinical cohorts that, by most estimates, are only 70% amyloid positive. The other 30% are patients whose neurocognitive deficits are due to other disorders. Many in the field believe this cohort impurity is directly tied to the consistently negative findings on anti-amyloid drugs: Simply put, anti-amyloid drugs won’t work in patients who don’t have amyloid brain plaques to begin with.
With the A/T/N system, “We take the position that cognitive impairment is a late occurrence,” in the Alzheimer’s disease trajectory, Dr. Jack said. “Clinical symptoms are not a good way to diagnose AD. This system does not require clinical symptoms for a diagnosis. In fact, the term ‘Alzheimer’s disease’ should refer only to the pathology in the brain, as it can exist in the absence of clinical symptoms or in the presence of atypical symptoms [such as language dysfunction], as well as in patients with the classic phenotype.”
The classification system comprises seven CSF and imaging biomarkers:
• A (amyloid) may be measured by an amyloid PET scan or by cerebrospinal fluid level of amyloid-beta42.
• T (tau) may be measured by CSF level of phosphorylated or total tau, or by tau PET imaging. Although tau imaging is still in the early phase, with no approved imaging agents, research is moving quickly and the committee wanted to be well positioned to incorporate it into the model as soon as an agent is approved.
• N (neurodegeneration/neuronal injury) may be measured by CSF level of total tau, hypometabolism on 18F-fluorodeoxyglucose PET imaging, or MRI that shows brain atrophy in regions characteristic of AD.
Since each component can be measured with CSF or imaging, the A/T/N system can be globally adopted despite different preferences for how biomarkers are acquired, Dr. Jack said.
“In the U.S., it’s much more common to use imaging, while in the E.U., it’s more common to use CSF for biomarkers. This is the beauty of the A/T/N system. With a single lumbar puncture or a series of imaging tests you can classify every research participant.”
In any of the patient groups, amyloid positivity is a key finding that an individual is probably on the path to Alzheimer’s disease. The biomarker permutations can be compared to the NIA-AA and IWG classifications as follows.
For clinically normal patients:
• A+/T-/N- is analogous to NIA-AA preclinical AD stage 1 and IWG asymptomatic at risk for AD (if A+ is established by amyloid PET).
• A+/T+/N- and A+/T+/N+ are analogous to NIA-AA preclinical AD stage 2/3 and IWG asymptomatic at risk for AD.
For MCI patients:
• A+/T-/N-, A+/T+/N-, and A+/T-/N+ are all analogous to the NIA-AA MCI core clinical criteria and IWG typical AD.
• A+/T+/N+ is analogous to NIA-AA MCI probably due to AD and IWG typical AD.
The A/T/N system offers the most useful details for patients who meet clinical criteria for probable AD dementia. Here, clinical criteria can be considered to help clarify the diagnostic picture:
• A-/T-/N-, analogous to NIA-AA dementia unlikely due to AD and is undefined by IWG.
• A+/T-/N-, analogous to NIA-AA intermediate likelihood of probable AD dementia, based on clinical criteria, and IWG typical AD (if A+ is established by amyloid PET).
• A+/T+/N-, analogous to NIA-AA high likelihood of probable AD dementia, based on clinical criteria, and IWG typical AD.
• A+/T-/N+, analogous to NIA-AA high likelihood of probable AD dementia, based on clinical criteria, and IWG typical AD (if A+ is established by amyloid PET).
• A+/T+/N+, analogous to NIA-AA high likelihood of AD pathophysiology and IWG typical AD.
• A-/T+/N+, analogous to NIA-AA probable AD dementia, based on clinical criteria and is undefined by IWG.
• A-/T-/N+, analogous to NIA-AA intermediate likelihood of probable AD dementia, based on clinical criteria, and is undefined by IWG.
• A-/T+/N+, analogous to NIA-AA intermediate likelihood of probable AD dementia, based on clinical criteria, and is undefined by IWG.
Would the A/T/N system benefit diagnostic accuracy?
The August paper sheds some additional light on how the system could hone diagnostic accuracy.
“For example, an A-/T-/N+ profile would be expected with pathologies such as ischemic cerebrovascular disease or hippocampal sclerosis, whereas an A-/T+/N+ profile would be expected with primary age-related tauopathy. An A+/T-/N+ profile might indicate an individual in the earliest stage of preclinical AD (accounting for the A+/T- status), who also has a non-AD pathology such as hippocampal sclerosis (accounting for the N+ status).”
Other typical profiles will certainly emerge as the A/T/N system is applied in large cohorts, the paper noted.
While the system is now aimed at building stronger research cohorts, it may eventually be adopted – and adapted – by clinicians.
“Right now, the reality is that most clinical practices don’t have access to getting these biomarkers,” Dr. Jack said. “Having said that, we also know that clinicians will vote with their feet. If they think this is useful they’ll end up adopting it, at least in the highly specialized centers that have access to these tests. The ultimate outcome someday will be a clinical practice guideline where people don’t get the label of AD unless they really have positive biomarkers.”
Dr. Jack has been a consultant for Eli Lilly.
[email protected]
On Twitter @alz_gal
A proposed Alzheimer’s disease classification system eschews neurocognitive testing and relies instead on the presence or absence of known biomarkers.
These markers of Alzheimer’s pathophysiology fall into three distinct categories: amyloid, tau, and neuronal injury (A/T/N). By rating patients as positive or negative for each category, the criteria aim to allow researchers to differentiate true Alzheimer’s patients from those with suspected non-Alzheimer’s pathology (SNAP), a diverse group that experiences neurocognitive decline in the absence of amyloid (Neurology. 2016 Aug 2;87[5]:539-47).
The A/T/N system could be employed in three clinical groups: clinically normal patients, those who meet the clinical criteria for mild cognitive impairment (MCI), and patients with a probable Alzheimer’s disease diagnosis based on the 2011 criteria established by the National Institute on Aging–Alzheimer’s Association (NIA-AA) staging system (Alzheimers Dement. 2011 May;7[3]:257-62).
Both the NIA-AA and IWG systems comprise five biomarkers (low cerebrospinal fluid [CSF] amyloid-beta42; positive PET amyloid imaging; elevated CSF tau; hypometabolism in the temporoparietal cortex on 18F-fluorodeoxyglucose PET imaging; and atrophy in specific brain regions) plus cognitive testing.
The NIA-AA system defines three clinical stages of Alzheimer’s, each with separate classification criteria: stage 1, preclinical; stage 2, MCI; and stage 3, Alzheimer’s disease (AD) dementia. The IWG system is more complex than the NIA-AA system and takes into account biomarkers, cognition, and other clinical manifestations.
The A/T/N system doesn’t require cognitive testing, and includes additional biomarkers that should allow for a much more nuanced classification of patients. It also reflects the field’s growing confidence in objective markers that measure the pathophysiology of the disease, rather than the way it manifests symptomatically, said Dr. Jack, professor of radiology at the Mayo Clinic, Rochester, Minn.
He was the lead author on the August 2016 paper that provided a first look at the proposal. He presented a more mature version of the system at the Clinical Trials on Alzheimer’s Disease meeting in San Diego last fall. The committee is refining the document now, and intends to present a final draft at the Alzheimer’s Association International Conference in London this summer.
Would the A/T/N system benefit clinical research?
Researchers desperately need a better classification system, Dr. Jack said in an interview. The vast majority of AD drug trials have enrolled clinical cohorts that, by most estimates, are only 70% amyloid positive. The other 30% are patients whose neurocognitive deficits are due to other disorders. Many in the field believe this cohort impurity is directly tied to the consistently negative findings on anti-amyloid drugs: Simply put, anti-amyloid drugs won’t work in patients who don’t have amyloid brain plaques to begin with.
With the A/T/N system, “We take the position that cognitive impairment is a late occurrence,” in the Alzheimer’s disease trajectory, Dr. Jack said. “Clinical symptoms are not a good way to diagnose AD. This system does not require clinical symptoms for a diagnosis. In fact, the term ‘Alzheimer’s disease’ should refer only to the pathology in the brain, as it can exist in the absence of clinical symptoms or in the presence of atypical symptoms [such as language dysfunction], as well as in patients with the classic phenotype.”
The classification system comprises seven CSF and imaging biomarkers:
• A (amyloid) may be measured by an amyloid PET scan or by cerebrospinal fluid level of amyloid-beta42.
• T (tau) may be measured by CSF level of phosphorylated or total tau, or by tau PET imaging. Although tau imaging is still in the early phase, with no approved imaging agents, research is moving quickly and the committee wanted to be well positioned to incorporate it into the model as soon as an agent is approved.
• N (neurodegeneration/neuronal injury) may be measured by CSF level of total tau, hypometabolism on 18F-fluorodeoxyglucose PET imaging, or MRI that shows brain atrophy in regions characteristic of AD.
Since each component can be measured with CSF or imaging, the A/T/N system can be globally adopted despite different preferences for how biomarkers are acquired, Dr. Jack said.
“In the U.S., it’s much more common to use imaging, while in the E.U., it’s more common to use CSF for biomarkers. This is the beauty of the A/T/N system. With a single lumbar puncture or a series of imaging tests you can classify every research participant.”
In any of the patient groups, amyloid positivity is a key finding that an individual is probably on the path to Alzheimer’s disease. The biomarker permutations can be compared to the NIA-AA and IWG classifications as follows.
For clinically normal patients:
• A+/T-/N- is analogous to NIA-AA preclinical AD stage 1 and IWG asymptomatic at risk for AD (if A+ is established by amyloid PET).
• A+/T+/N- and A+/T+/N+ are analogous to NIA-AA preclinical AD stage 2/3 and IWG asymptomatic at risk for AD.
For MCI patients:
• A+/T-/N-, A+/T+/N-, and A+/T-/N+ are all analogous to the NIA-AA MCI core clinical criteria and IWG typical AD.
• A+/T+/N+ is analogous to NIA-AA MCI probably due to AD and IWG typical AD.
The A/T/N system offers the most useful details for patients who meet clinical criteria for probable AD dementia. Here, clinical criteria can be considered to help clarify the diagnostic picture:
• A-/T-/N-, analogous to NIA-AA dementia unlikely due to AD and is undefined by IWG.
• A+/T-/N-, analogous to NIA-AA intermediate likelihood of probable AD dementia, based on clinical criteria, and IWG typical AD (if A+ is established by amyloid PET).
• A+/T+/N-, analogous to NIA-AA high likelihood of probable AD dementia, based on clinical criteria, and IWG typical AD.
• A+/T-/N+, analogous to NIA-AA high likelihood of probable AD dementia, based on clinical criteria, and IWG typical AD (if A+ is established by amyloid PET).
• A+/T+/N+, analogous to NIA-AA high likelihood of AD pathophysiology and IWG typical AD.
• A-/T+/N+, analogous to NIA-AA probable AD dementia, based on clinical criteria and is undefined by IWG.
• A-/T-/N+, analogous to NIA-AA intermediate likelihood of probable AD dementia, based on clinical criteria, and is undefined by IWG.
• A-/T+/N+, analogous to NIA-AA intermediate likelihood of probable AD dementia, based on clinical criteria, and is undefined by IWG.
Would the A/T/N system benefit diagnostic accuracy?
The August paper sheds some additional light on how the system could hone diagnostic accuracy.
“For example, an A-/T-/N+ profile would be expected with pathologies such as ischemic cerebrovascular disease or hippocampal sclerosis, whereas an A-/T+/N+ profile would be expected with primary age-related tauopathy. An A+/T-/N+ profile might indicate an individual in the earliest stage of preclinical AD (accounting for the A+/T- status), who also has a non-AD pathology such as hippocampal sclerosis (accounting for the N+ status).”
Other typical profiles will certainly emerge as the A/T/N system is applied in large cohorts, the paper noted.
While the system is now aimed at building stronger research cohorts, it may eventually be adopted – and adapted – by clinicians.
“Right now, the reality is that most clinical practices don’t have access to getting these biomarkers,” Dr. Jack said. “Having said that, we also know that clinicians will vote with their feet. If they think this is useful they’ll end up adopting it, at least in the highly specialized centers that have access to these tests. The ultimate outcome someday will be a clinical practice guideline where people don’t get the label of AD unless they really have positive biomarkers.”
Dr. Jack has been a consultant for Eli Lilly.
[email protected]
On Twitter @alz_gal
A proposed Alzheimer’s disease classification system eschews neurocognitive testing and relies instead on the presence or absence of known biomarkers.
These markers of Alzheimer’s pathophysiology fall into three distinct categories: amyloid, tau, and neuronal injury (A/T/N). By rating patients as positive or negative for each category, the criteria aim to allow researchers to differentiate true Alzheimer’s patients from those with suspected non-Alzheimer’s pathology (SNAP), a diverse group that experiences neurocognitive decline in the absence of amyloid (Neurology. 2016 Aug 2;87[5]:539-47).
The A/T/N system could be employed in three clinical groups: clinically normal patients, those who meet the clinical criteria for mild cognitive impairment (MCI), and patients with a probable Alzheimer’s disease diagnosis based on the 2011 criteria established by the National Institute on Aging–Alzheimer’s Association (NIA-AA) staging system (Alzheimers Dement. 2011 May;7[3]:257-62).
Both the NIA-AA and IWG systems comprise five biomarkers (low cerebrospinal fluid [CSF] amyloid-beta42; positive PET amyloid imaging; elevated CSF tau; hypometabolism in the temporoparietal cortex on 18F-fluorodeoxyglucose PET imaging; and atrophy in specific brain regions) plus cognitive testing.
The NIA-AA system defines three clinical stages of Alzheimer’s, each with separate classification criteria: stage 1, preclinical; stage 2, MCI; and stage 3, Alzheimer’s disease (AD) dementia. The IWG system is more complex than the NIA-AA system and takes into account biomarkers, cognition, and other clinical manifestations.
The A/T/N system doesn’t require cognitive testing, and includes additional biomarkers that should allow for a much more nuanced classification of patients. It also reflects the field’s growing confidence in objective markers that measure the pathophysiology of the disease, rather than the way it manifests symptomatically, said Dr. Jack, professor of radiology at the Mayo Clinic, Rochester, Minn.
He was the lead author on the August 2016 paper that provided a first look at the proposal. He presented a more mature version of the system at the Clinical Trials on Alzheimer’s Disease meeting in San Diego last fall. The committee is refining the document now, and intends to present a final draft at the Alzheimer’s Association International Conference in London this summer.
Would the A/T/N system benefit clinical research?
Researchers desperately need a better classification system, Dr. Jack said in an interview. The vast majority of AD drug trials have enrolled clinical cohorts that, by most estimates, are only 70% amyloid positive. The other 30% are patients whose neurocognitive deficits are due to other disorders. Many in the field believe this cohort impurity is directly tied to the consistently negative findings on anti-amyloid drugs: Simply put, anti-amyloid drugs won’t work in patients who don’t have amyloid brain plaques to begin with.
With the A/T/N system, “We take the position that cognitive impairment is a late occurrence,” in the Alzheimer’s disease trajectory, Dr. Jack said. “Clinical symptoms are not a good way to diagnose AD. This system does not require clinical symptoms for a diagnosis. In fact, the term ‘Alzheimer’s disease’ should refer only to the pathology in the brain, as it can exist in the absence of clinical symptoms or in the presence of atypical symptoms [such as language dysfunction], as well as in patients with the classic phenotype.”
The classification system comprises seven CSF and imaging biomarkers:
• A (amyloid) may be measured by an amyloid PET scan or by cerebrospinal fluid level of amyloid-beta42.
• T (tau) may be measured by CSF level of phosphorylated or total tau, or by tau PET imaging. Although tau imaging is still in the early phase, with no approved imaging agents, research is moving quickly and the committee wanted to be well positioned to incorporate it into the model as soon as an agent is approved.
• N (neurodegeneration/neuronal injury) may be measured by CSF level of total tau, hypometabolism on 18F-fluorodeoxyglucose PET imaging, or MRI that shows brain atrophy in regions characteristic of AD.
Since each component can be measured with CSF or imaging, the A/T/N system can be globally adopted despite different preferences for how biomarkers are acquired, Dr. Jack said.
“In the U.S., it’s much more common to use imaging, while in the E.U., it’s more common to use CSF for biomarkers. This is the beauty of the A/T/N system. With a single lumbar puncture or a series of imaging tests you can classify every research participant.”
In any of the patient groups, amyloid positivity is a key finding that an individual is probably on the path to Alzheimer’s disease. The biomarker permutations can be compared to the NIA-AA and IWG classifications as follows.
For clinically normal patients:
• A+/T-/N- is analogous to NIA-AA preclinical AD stage 1 and IWG asymptomatic at risk for AD (if A+ is established by amyloid PET).
• A+/T+/N- and A+/T+/N+ are analogous to NIA-AA preclinical AD stage 2/3 and IWG asymptomatic at risk for AD.
For MCI patients:
• A+/T-/N-, A+/T+/N-, and A+/T-/N+ are all analogous to the NIA-AA MCI core clinical criteria and IWG typical AD.
• A+/T+/N+ is analogous to NIA-AA MCI probably due to AD and IWG typical AD.
The A/T/N system offers the most useful details for patients who meet clinical criteria for probable AD dementia. Here, clinical criteria can be considered to help clarify the diagnostic picture:
• A-/T-/N-, analogous to NIA-AA dementia unlikely due to AD and is undefined by IWG.
• A+/T-/N-, analogous to NIA-AA intermediate likelihood of probable AD dementia, based on clinical criteria, and IWG typical AD (if A+ is established by amyloid PET).
• A+/T+/N-, analogous to NIA-AA high likelihood of probable AD dementia, based on clinical criteria, and IWG typical AD.
• A+/T-/N+, analogous to NIA-AA high likelihood of probable AD dementia, based on clinical criteria, and IWG typical AD (if A+ is established by amyloid PET).
• A+/T+/N+, analogous to NIA-AA high likelihood of AD pathophysiology and IWG typical AD.
• A-/T+/N+, analogous to NIA-AA probable AD dementia, based on clinical criteria and is undefined by IWG.
• A-/T-/N+, analogous to NIA-AA intermediate likelihood of probable AD dementia, based on clinical criteria, and is undefined by IWG.
• A-/T+/N+, analogous to NIA-AA intermediate likelihood of probable AD dementia, based on clinical criteria, and is undefined by IWG.
Would the A/T/N system benefit diagnostic accuracy?
The August paper sheds some additional light on how the system could hone diagnostic accuracy.
“For example, an A-/T-/N+ profile would be expected with pathologies such as ischemic cerebrovascular disease or hippocampal sclerosis, whereas an A-/T+/N+ profile would be expected with primary age-related tauopathy. An A+/T-/N+ profile might indicate an individual in the earliest stage of preclinical AD (accounting for the A+/T- status), who also has a non-AD pathology such as hippocampal sclerosis (accounting for the N+ status).”
Other typical profiles will certainly emerge as the A/T/N system is applied in large cohorts, the paper noted.
While the system is now aimed at building stronger research cohorts, it may eventually be adopted – and adapted – by clinicians.
“Right now, the reality is that most clinical practices don’t have access to getting these biomarkers,” Dr. Jack said. “Having said that, we also know that clinicians will vote with their feet. If they think this is useful they’ll end up adopting it, at least in the highly specialized centers that have access to these tests. The ultimate outcome someday will be a clinical practice guideline where people don’t get the label of AD unless they really have positive biomarkers.”
Dr. Jack has been a consultant for Eli Lilly.
[email protected]
On Twitter @alz_gal
Tele-rheumatology reaches patients who lack access to care
By the time Christine Peoples, MD, examined the patient, the middle-aged man had been treated inadequately for psoriatic arthritis for years.
Poor mobility made it difficult for him to travel and gaps in his care had exacerbated his condition. But a bidirectional video system at the University of Pittsburgh Medical Center (UPMC) allowed Dr. Peoples to assess the patient, correct his therapeutic regimen, and establish consistent follow up.
Without telemedicine, the patient would have likely continued to deteriorate, said Dr. Peoples, a rheumatologist with the UPMC Teleconsult Centers.
Across the country, more rheumatology practices are employing telemedicine to treat patients, particularly physicians at academic medicine centers that have the resources to launch comprehensive units. The technology is a way to bridge the shortage of rheumatologists in rural areas and reach patients who cannot access specialty care for rheumatic conditions, proponents say. A 2013 study in Arthritis & Rheumatism found that many towns and small cities with populations up to 50,000 have no practicing rheumatologists, with some patients having to travel more than 200 miles to get specialty care (2013 Dec;65[12]:3017-25).
“Telemedicine is expanding both in terms of the number of consultations and the breadth of services involved,” he said. “Tele-rheumatology was certainly not a big area to be looked at in years past, but it’s one more area that is starting to expand.”
Research is lacking regarding how prevalent tele-rheumatology has become in the United States and whether it’s as effective as face-to-face visits. In a recent analysis of 1,468 potentially eligible tele-rheumatology studies and literature, only 20 addressed direct provider to patient contact that influenced or had the potential to influence clinical care, according to a November 2016 review in Arthritis Care & Research (doi: 10.1002/acr.23153). Of the 20 studies, the majority of articles involved tele-rheumatology use in Europe or Great Britain, said study author John Allen McDougall, MD, a postdoctoral fellow at Yale University, New Haven, Conn. According to the literature, the most common condition treated through telemedicine was rheumatoid arthritis. Little information existed on telemedicine use for gout or the treatment of connective tissue diseases, Dr. McDougall said.
Increasing access, saving time
UPMC has operated a tele-rheumatology program for nearly 3 years that connects rural patients with their specialists. Patients present for video examination at teleconsult centers located in underserved areas within western and central Pennsylvania. On the other end of the screen, Dr. Peoples uses high-level audio and video technology to discuss the patient’s history, direct nurses, make diagnoses, answer questions, and prescribe treatment to patients.
“Patients comment about how much easier it is for them and what a difference this makes in their health care,” Dr. Peoples said. “[Tele-rheumatology also increases] the ability to have regular follow-up care. I see them every few months for their condition and manage it. You can really establish a long relationship with patients just as you would if you saw patients in person.”
Rheumatologists at Dartmouth–Hitchcock Medical Center (DHMC) in Lebanon, N.H., are achieving similar results.
The medical center’s tele-rheumatology unit began in 2011 with the aim of increasing specialty access for patients in northern New Hampshire. Dartmouth also employed a telemedicine clinic from 2012 to 2013 in southern Vermont while seeking to replace a well-established rheumatologist who had retired.
Between October 2011 and December 2014, 176 patients were seen via tele-rheumatology between the two sites over the course of 244 tele-rheumatology patient visits with Dartmouth providers, according to a study published in the June 2016 Seminars in Arthritis & Rheumatism (2016 Dec;46[3]:380-5). Patients lived on average 99 miles from DHMC and 22 miles from the remote clinic site, the study found. The top diagnosis was inflammatory arthritis.
For most of doctors and patients involved in the study, tele-rheumatology visits were positive and aided patient access, said Daniel Albert, MD, section chief of rheumatology at DHMC.
“There are some limitations, there’s no question about that,” he said. ... [However], right now there are way too few rheumatologists to care for patients with rheumatic disease. We’ve tried to improve that with [nurse practitioners and physician assistants], but this is another way of addressing that need.”
Pediatric rheumatologists are also using the benefits of telemedicine. Children’s Mercy in Kansas City, Mo., launched a pediatric rheumatology telemedicine clinic in 2014 that links children in Joplin, Mo., with rheumatologists at Children’s Mercy, about 160 miles away. Patients and their parents/guardians treated via telemedicine at the Joplin site missed less work and school and saved money on food, compared with traveling to Kansas City for treatment, according to a 2016 study published in Pediatric Rheumatology (doi: 10.1186/s12969-016-0116-2).
“This is much more convenient for patients,” said Elizabeth A. Kessler, MD, a pediatric rheumatologist at Children’s Mercy and coauthor of the study. “It saves them time and money. With the training of the tele-facilitator, I believe that we’re able to provide them with equivalent care compared with if they were in-person.”
Is tele-rheumatology right for all settings?
But tele-rheumatology may not be appropriate in all practice settings or the right method for all rheumatology patients, doctors say.
As the Dartmouth-Hitchcock study noted, patients with complex diseases or unclear underlying conditions do not make good telemedicine patients. In addition, opinions are mixed about whether physicians and patients should have an established relationship before telemedicine visits occur and what such visits should entail.
Anchorage, Alaska-based rheumatologist Elizabeth Ferucci, MD, uses telemedicine only for follow up. Like many rheumatologists in Alaska, she travels to field clinics in larger communities throughout the state twice a year to treat patients, said Dr. Ferucci, a rheumatologist for the Alaska Native Tribal Health Consortium. Because some patients need to be seen more than twice a year, Dr. Ferucci conducts follow-up visits every 2-3 months via video.
However, Dr. Ferucci notes that having a trained telemedicine presenter capable of performing a joint examination in the room with the patient is not always possible because of high staff turnover and the more than 200 villages served. A nurse or staff member remains in the room with the patient, but they are not always trained to perform joint exams.
At Dartmouth-Hitchcock, Dr. Albert’s practice accepts both initial and follow-up patient visits. He acknowledges that tele-rheumatology is best used for follow-up care, but explains that limiting the center’s telemedicine use would leave some patients without treatment.
“Because the constraints of getting to Dartmouth are so great, we don’t want to impose that restriction on patients,” Dr. Albert said. “However, in general, it is preferable to do an in-person consultation first to clarify the physical findings.”
Dartmouth has used both nurses and medical assistants as presenters during tele-rheumatology after developing a web-based video series to train presenters. Nurses at UPMC are also trained as telemedicine presenters, and telemedicine visits are only used for follow-up care, Dr. Peoples said.
At Children’s Mercy in Kansas City, telemedicine visits are restricted to follow-up care and include a nurse facilitator at the virtual site. However, the type of conditions and stages of disease examined through the technology has grown since the telemedicine services started, Dr. Kessler said.
“What we feel comfortable seeing has expanded since the time we initially started the clinic because some of the patients, when they were having flares, could not come to Kansas City for financial reasons,” she said, adding that she has since examined children with lupus, vasculitis, and active arthritis through telemedicine.
She stresses that in-person visits should depend on the patient, the circumstances, and the physician’s comfort level. ”You need to be realistic,” she said. “There are times when I’ve said to families, ‘I would like you to come to Kansas City because I’m a little unsure about your exam findings.’ There are definitely some patients who do more of a hybrid approach where they come to Kansas City and are also seen by telemedicine.”
Barriers slow telemedicine progress
Amid its benefits, challenges for tele-rheumatology, such as reimbursement and licensure obstacles, remain.
Commercial insurers pay for telemedicine at varying rates, making it difficult for doctors to consistently be paid, Dr. Peoples said.
“A lot of insurance companies do cover telemedicine visits, but still, a lot don’t,” she said. “I do think that in order to remain competitive, insurance companies overall are going to have to start covering these services.”
At least 32 states and the District of Columbia have parity laws that require commercial health insurers to cover services provided through telehealth to the same extent as those services are covered in person, according to an Aug. 15, 2016 Health Affairs policy brief. However, how private insurers pay and what services they cover vary widely.
Medicaid reimbursement for telehealth depends on individual state Medicaid programs. Only 9 states Medicaid programs reimburse for store-and-forward services, while at least 16 states have some sort of Medicaid reimbursement for remote patient monitoring, according to the Health Affairs report.
Medicare payment for telehealth is clouded in restrictions. The Centers for Medicare & Medicaid Services reimburses for synchronous communications only and does not cover store-and-forward services or remote patient monitoring for chronic diseases, except in Alaska and Hawaii. The patient must be present at an originating site for the visit and cannot be home to receive the services. In addition, CMS reimburses for telehealth only when the originating site is in a Health Professional Shortage Area or within a county outside a Metropolitan Statistical Area. In 2016, CMS expanded its coverage of telehealth to include several new codes including, end-stage renal disease–related services for dialysis, advance care planning services, and critical care consultations furnished via telehealth using new Medicare G-codes.
It’s worth noting that rheumatologists who are salaried employees of academic medical centers, as is the case for all rheumatologists interviewed here, don’t handle the billing for their telemedicine services, and so have not experienced the payment challenges that smaller practices may face.
Mr. Linkous of the American Telemedicine Association said that he believes that reimbursement for telemedicine will improve as health care heads toward value-based payment systems.
“In terms of reimbursement, with the move away from fee-based systems to value-based systems, it really opens the door because for many of the services, you no longer write out a bill with a code associated with it,” he said. “You are paid on the basis of keeping people well. That’s a great positive incentive for using telemedicine.”
Differing state licensure requirements also pose a challenge. The process to obtain licenses and navigate varying credentialing processes can be a headache for health providers and delay approval.
The barrier has led to model legislation by the Federation of State Medical Boards (FSMB) that aims to make it easier for telemedicine physicians to gain licenses in multiple states. Under the Interstate Medical Licensure Compact, physicians designate a member state as the state of principal licensure and select the other states in which they want to license. The state of principal licensure then verifies the physician’s eligibility and provides credential information to the interstate commission, which collects applicable fees and transmits the doctor’s information to the other states. Upon receipt in the additional states, the physician would be granted a license.
As of January, 18 states had enacted the compact legislation, and at least 4 states had introduced the legislation. In 2015, the Health Resources and Services Administration awarded the FSMB a grant to support establishment of the commission and aid with the compact’s infrastructure.
A new normal?
Dr. Albert notes that tele-rheumatology programs can succeed only with adequate resources, time, and staff.
“To set up Skype or Facetime is nothing; you just click on it,” he said. “To set up tele-rheumatology requires a fair amount of administrative substructure that has to be generated in terms of who’s going to do the documentation? Who is going to do the billing? Who is going to do the credentialing? Where is the credentialing going to be validated?”
The equipment required for telemedicine visits also requires a significant amount of time and regular maintenance, Dr. Peoples said. At UPMC for instance, the tele-rheumatology program has an information technology team that assists with technology glitches, changes, and updates, she said. That’s why it’s helpful to have the support of a large academic medical center with the sufficient resources to build a telemedicine program, she added.
In order for more practices to consider tele-rheumatology, more research about cost-effectiveness and best uses of the technology use would be useful, Dr. McDougall said.
“The main question that policy makers are going to want to answer is, ‘What’s the return on investment? Does this make sense for my practice?’ ” he said. “The methods reporting in tele-rheumatologist [literature] is lacking.” But regardless of barriers, telemedicine experts say the technology will likely continue to expand and transform the way rheumatologists are practicing and patients are receiving care.
“Tele-rheumatology will never replace an in-person exam,” Dr. Ferucci said. “But my vision is that it will be able to improve the quality of care for patients living in rural and remote locations, by allowing for more frequent visits and adjustment of medications, which are necessary to achieve the goal of treat-to-target for RA and other rheumatologic conditions.”
* This story was updated on 2/10/2017.
On Twitter @legal_med
By the time Christine Peoples, MD, examined the patient, the middle-aged man had been treated inadequately for psoriatic arthritis for years.
Poor mobility made it difficult for him to travel and gaps in his care had exacerbated his condition. But a bidirectional video system at the University of Pittsburgh Medical Center (UPMC) allowed Dr. Peoples to assess the patient, correct his therapeutic regimen, and establish consistent follow up.
Without telemedicine, the patient would have likely continued to deteriorate, said Dr. Peoples, a rheumatologist with the UPMC Teleconsult Centers.
Across the country, more rheumatology practices are employing telemedicine to treat patients, particularly physicians at academic medicine centers that have the resources to launch comprehensive units. The technology is a way to bridge the shortage of rheumatologists in rural areas and reach patients who cannot access specialty care for rheumatic conditions, proponents say. A 2013 study in Arthritis & Rheumatism found that many towns and small cities with populations up to 50,000 have no practicing rheumatologists, with some patients having to travel more than 200 miles to get specialty care (2013 Dec;65[12]:3017-25).
“Telemedicine is expanding both in terms of the number of consultations and the breadth of services involved,” he said. “Tele-rheumatology was certainly not a big area to be looked at in years past, but it’s one more area that is starting to expand.”
Research is lacking regarding how prevalent tele-rheumatology has become in the United States and whether it’s as effective as face-to-face visits. In a recent analysis of 1,468 potentially eligible tele-rheumatology studies and literature, only 20 addressed direct provider to patient contact that influenced or had the potential to influence clinical care, according to a November 2016 review in Arthritis Care & Research (doi: 10.1002/acr.23153). Of the 20 studies, the majority of articles involved tele-rheumatology use in Europe or Great Britain, said study author John Allen McDougall, MD, a postdoctoral fellow at Yale University, New Haven, Conn. According to the literature, the most common condition treated through telemedicine was rheumatoid arthritis. Little information existed on telemedicine use for gout or the treatment of connective tissue diseases, Dr. McDougall said.
Increasing access, saving time
UPMC has operated a tele-rheumatology program for nearly 3 years that connects rural patients with their specialists. Patients present for video examination at teleconsult centers located in underserved areas within western and central Pennsylvania. On the other end of the screen, Dr. Peoples uses high-level audio and video technology to discuss the patient’s history, direct nurses, make diagnoses, answer questions, and prescribe treatment to patients.
“Patients comment about how much easier it is for them and what a difference this makes in their health care,” Dr. Peoples said. “[Tele-rheumatology also increases] the ability to have regular follow-up care. I see them every few months for their condition and manage it. You can really establish a long relationship with patients just as you would if you saw patients in person.”
Rheumatologists at Dartmouth–Hitchcock Medical Center (DHMC) in Lebanon, N.H., are achieving similar results.
The medical center’s tele-rheumatology unit began in 2011 with the aim of increasing specialty access for patients in northern New Hampshire. Dartmouth also employed a telemedicine clinic from 2012 to 2013 in southern Vermont while seeking to replace a well-established rheumatologist who had retired.
Between October 2011 and December 2014, 176 patients were seen via tele-rheumatology between the two sites over the course of 244 tele-rheumatology patient visits with Dartmouth providers, according to a study published in the June 2016 Seminars in Arthritis & Rheumatism (2016 Dec;46[3]:380-5). Patients lived on average 99 miles from DHMC and 22 miles from the remote clinic site, the study found. The top diagnosis was inflammatory arthritis.
For most of doctors and patients involved in the study, tele-rheumatology visits were positive and aided patient access, said Daniel Albert, MD, section chief of rheumatology at DHMC.
“There are some limitations, there’s no question about that,” he said. ... [However], right now there are way too few rheumatologists to care for patients with rheumatic disease. We’ve tried to improve that with [nurse practitioners and physician assistants], but this is another way of addressing that need.”
Pediatric rheumatologists are also using the benefits of telemedicine. Children’s Mercy in Kansas City, Mo., launched a pediatric rheumatology telemedicine clinic in 2014 that links children in Joplin, Mo., with rheumatologists at Children’s Mercy, about 160 miles away. Patients and their parents/guardians treated via telemedicine at the Joplin site missed less work and school and saved money on food, compared with traveling to Kansas City for treatment, according to a 2016 study published in Pediatric Rheumatology (doi: 10.1186/s12969-016-0116-2).
“This is much more convenient for patients,” said Elizabeth A. Kessler, MD, a pediatric rheumatologist at Children’s Mercy and coauthor of the study. “It saves them time and money. With the training of the tele-facilitator, I believe that we’re able to provide them with equivalent care compared with if they were in-person.”
Is tele-rheumatology right for all settings?
But tele-rheumatology may not be appropriate in all practice settings or the right method for all rheumatology patients, doctors say.
As the Dartmouth-Hitchcock study noted, patients with complex diseases or unclear underlying conditions do not make good telemedicine patients. In addition, opinions are mixed about whether physicians and patients should have an established relationship before telemedicine visits occur and what such visits should entail.
Anchorage, Alaska-based rheumatologist Elizabeth Ferucci, MD, uses telemedicine only for follow up. Like many rheumatologists in Alaska, she travels to field clinics in larger communities throughout the state twice a year to treat patients, said Dr. Ferucci, a rheumatologist for the Alaska Native Tribal Health Consortium. Because some patients need to be seen more than twice a year, Dr. Ferucci conducts follow-up visits every 2-3 months via video.
However, Dr. Ferucci notes that having a trained telemedicine presenter capable of performing a joint examination in the room with the patient is not always possible because of high staff turnover and the more than 200 villages served. A nurse or staff member remains in the room with the patient, but they are not always trained to perform joint exams.
At Dartmouth-Hitchcock, Dr. Albert’s practice accepts both initial and follow-up patient visits. He acknowledges that tele-rheumatology is best used for follow-up care, but explains that limiting the center’s telemedicine use would leave some patients without treatment.
“Because the constraints of getting to Dartmouth are so great, we don’t want to impose that restriction on patients,” Dr. Albert said. “However, in general, it is preferable to do an in-person consultation first to clarify the physical findings.”
Dartmouth has used both nurses and medical assistants as presenters during tele-rheumatology after developing a web-based video series to train presenters. Nurses at UPMC are also trained as telemedicine presenters, and telemedicine visits are only used for follow-up care, Dr. Peoples said.
At Children’s Mercy in Kansas City, telemedicine visits are restricted to follow-up care and include a nurse facilitator at the virtual site. However, the type of conditions and stages of disease examined through the technology has grown since the telemedicine services started, Dr. Kessler said.
“What we feel comfortable seeing has expanded since the time we initially started the clinic because some of the patients, when they were having flares, could not come to Kansas City for financial reasons,” she said, adding that she has since examined children with lupus, vasculitis, and active arthritis through telemedicine.
She stresses that in-person visits should depend on the patient, the circumstances, and the physician’s comfort level. ”You need to be realistic,” she said. “There are times when I’ve said to families, ‘I would like you to come to Kansas City because I’m a little unsure about your exam findings.’ There are definitely some patients who do more of a hybrid approach where they come to Kansas City and are also seen by telemedicine.”
Barriers slow telemedicine progress
Amid its benefits, challenges for tele-rheumatology, such as reimbursement and licensure obstacles, remain.
Commercial insurers pay for telemedicine at varying rates, making it difficult for doctors to consistently be paid, Dr. Peoples said.
“A lot of insurance companies do cover telemedicine visits, but still, a lot don’t,” she said. “I do think that in order to remain competitive, insurance companies overall are going to have to start covering these services.”
At least 32 states and the District of Columbia have parity laws that require commercial health insurers to cover services provided through telehealth to the same extent as those services are covered in person, according to an Aug. 15, 2016 Health Affairs policy brief. However, how private insurers pay and what services they cover vary widely.
Medicaid reimbursement for telehealth depends on individual state Medicaid programs. Only 9 states Medicaid programs reimburse for store-and-forward services, while at least 16 states have some sort of Medicaid reimbursement for remote patient monitoring, according to the Health Affairs report.
Medicare payment for telehealth is clouded in restrictions. The Centers for Medicare & Medicaid Services reimburses for synchronous communications only and does not cover store-and-forward services or remote patient monitoring for chronic diseases, except in Alaska and Hawaii. The patient must be present at an originating site for the visit and cannot be home to receive the services. In addition, CMS reimburses for telehealth only when the originating site is in a Health Professional Shortage Area or within a county outside a Metropolitan Statistical Area. In 2016, CMS expanded its coverage of telehealth to include several new codes including, end-stage renal disease–related services for dialysis, advance care planning services, and critical care consultations furnished via telehealth using new Medicare G-codes.
It’s worth noting that rheumatologists who are salaried employees of academic medical centers, as is the case for all rheumatologists interviewed here, don’t handle the billing for their telemedicine services, and so have not experienced the payment challenges that smaller practices may face.
Mr. Linkous of the American Telemedicine Association said that he believes that reimbursement for telemedicine will improve as health care heads toward value-based payment systems.
“In terms of reimbursement, with the move away from fee-based systems to value-based systems, it really opens the door because for many of the services, you no longer write out a bill with a code associated with it,” he said. “You are paid on the basis of keeping people well. That’s a great positive incentive for using telemedicine.”
Differing state licensure requirements also pose a challenge. The process to obtain licenses and navigate varying credentialing processes can be a headache for health providers and delay approval.
The barrier has led to model legislation by the Federation of State Medical Boards (FSMB) that aims to make it easier for telemedicine physicians to gain licenses in multiple states. Under the Interstate Medical Licensure Compact, physicians designate a member state as the state of principal licensure and select the other states in which they want to license. The state of principal licensure then verifies the physician’s eligibility and provides credential information to the interstate commission, which collects applicable fees and transmits the doctor’s information to the other states. Upon receipt in the additional states, the physician would be granted a license.
As of January, 18 states had enacted the compact legislation, and at least 4 states had introduced the legislation. In 2015, the Health Resources and Services Administration awarded the FSMB a grant to support establishment of the commission and aid with the compact’s infrastructure.
A new normal?
Dr. Albert notes that tele-rheumatology programs can succeed only with adequate resources, time, and staff.
“To set up Skype or Facetime is nothing; you just click on it,” he said. “To set up tele-rheumatology requires a fair amount of administrative substructure that has to be generated in terms of who’s going to do the documentation? Who is going to do the billing? Who is going to do the credentialing? Where is the credentialing going to be validated?”
The equipment required for telemedicine visits also requires a significant amount of time and regular maintenance, Dr. Peoples said. At UPMC for instance, the tele-rheumatology program has an information technology team that assists with technology glitches, changes, and updates, she said. That’s why it’s helpful to have the support of a large academic medical center with the sufficient resources to build a telemedicine program, she added.
In order for more practices to consider tele-rheumatology, more research about cost-effectiveness and best uses of the technology use would be useful, Dr. McDougall said.
“The main question that policy makers are going to want to answer is, ‘What’s the return on investment? Does this make sense for my practice?’ ” he said. “The methods reporting in tele-rheumatologist [literature] is lacking.” But regardless of barriers, telemedicine experts say the technology will likely continue to expand and transform the way rheumatologists are practicing and patients are receiving care.
“Tele-rheumatology will never replace an in-person exam,” Dr. Ferucci said. “But my vision is that it will be able to improve the quality of care for patients living in rural and remote locations, by allowing for more frequent visits and adjustment of medications, which are necessary to achieve the goal of treat-to-target for RA and other rheumatologic conditions.”
* This story was updated on 2/10/2017.
On Twitter @legal_med
By the time Christine Peoples, MD, examined the patient, the middle-aged man had been treated inadequately for psoriatic arthritis for years.
Poor mobility made it difficult for him to travel and gaps in his care had exacerbated his condition. But a bidirectional video system at the University of Pittsburgh Medical Center (UPMC) allowed Dr. Peoples to assess the patient, correct his therapeutic regimen, and establish consistent follow up.
Without telemedicine, the patient would have likely continued to deteriorate, said Dr. Peoples, a rheumatologist with the UPMC Teleconsult Centers.
Across the country, more rheumatology practices are employing telemedicine to treat patients, particularly physicians at academic medicine centers that have the resources to launch comprehensive units. The technology is a way to bridge the shortage of rheumatologists in rural areas and reach patients who cannot access specialty care for rheumatic conditions, proponents say. A 2013 study in Arthritis & Rheumatism found that many towns and small cities with populations up to 50,000 have no practicing rheumatologists, with some patients having to travel more than 200 miles to get specialty care (2013 Dec;65[12]:3017-25).
“Telemedicine is expanding both in terms of the number of consultations and the breadth of services involved,” he said. “Tele-rheumatology was certainly not a big area to be looked at in years past, but it’s one more area that is starting to expand.”
Research is lacking regarding how prevalent tele-rheumatology has become in the United States and whether it’s as effective as face-to-face visits. In a recent analysis of 1,468 potentially eligible tele-rheumatology studies and literature, only 20 addressed direct provider to patient contact that influenced or had the potential to influence clinical care, according to a November 2016 review in Arthritis Care & Research (doi: 10.1002/acr.23153). Of the 20 studies, the majority of articles involved tele-rheumatology use in Europe or Great Britain, said study author John Allen McDougall, MD, a postdoctoral fellow at Yale University, New Haven, Conn. According to the literature, the most common condition treated through telemedicine was rheumatoid arthritis. Little information existed on telemedicine use for gout or the treatment of connective tissue diseases, Dr. McDougall said.
Increasing access, saving time
UPMC has operated a tele-rheumatology program for nearly 3 years that connects rural patients with their specialists. Patients present for video examination at teleconsult centers located in underserved areas within western and central Pennsylvania. On the other end of the screen, Dr. Peoples uses high-level audio and video technology to discuss the patient’s history, direct nurses, make diagnoses, answer questions, and prescribe treatment to patients.
“Patients comment about how much easier it is for them and what a difference this makes in their health care,” Dr. Peoples said. “[Tele-rheumatology also increases] the ability to have regular follow-up care. I see them every few months for their condition and manage it. You can really establish a long relationship with patients just as you would if you saw patients in person.”
Rheumatologists at Dartmouth–Hitchcock Medical Center (DHMC) in Lebanon, N.H., are achieving similar results.
The medical center’s tele-rheumatology unit began in 2011 with the aim of increasing specialty access for patients in northern New Hampshire. Dartmouth also employed a telemedicine clinic from 2012 to 2013 in southern Vermont while seeking to replace a well-established rheumatologist who had retired.
Between October 2011 and December 2014, 176 patients were seen via tele-rheumatology between the two sites over the course of 244 tele-rheumatology patient visits with Dartmouth providers, according to a study published in the June 2016 Seminars in Arthritis & Rheumatism (2016 Dec;46[3]:380-5). Patients lived on average 99 miles from DHMC and 22 miles from the remote clinic site, the study found. The top diagnosis was inflammatory arthritis.
For most of doctors and patients involved in the study, tele-rheumatology visits were positive and aided patient access, said Daniel Albert, MD, section chief of rheumatology at DHMC.
“There are some limitations, there’s no question about that,” he said. ... [However], right now there are way too few rheumatologists to care for patients with rheumatic disease. We’ve tried to improve that with [nurse practitioners and physician assistants], but this is another way of addressing that need.”
Pediatric rheumatologists are also using the benefits of telemedicine. Children’s Mercy in Kansas City, Mo., launched a pediatric rheumatology telemedicine clinic in 2014 that links children in Joplin, Mo., with rheumatologists at Children’s Mercy, about 160 miles away. Patients and their parents/guardians treated via telemedicine at the Joplin site missed less work and school and saved money on food, compared with traveling to Kansas City for treatment, according to a 2016 study published in Pediatric Rheumatology (doi: 10.1186/s12969-016-0116-2).
“This is much more convenient for patients,” said Elizabeth A. Kessler, MD, a pediatric rheumatologist at Children’s Mercy and coauthor of the study. “It saves them time and money. With the training of the tele-facilitator, I believe that we’re able to provide them with equivalent care compared with if they were in-person.”
Is tele-rheumatology right for all settings?
But tele-rheumatology may not be appropriate in all practice settings or the right method for all rheumatology patients, doctors say.
As the Dartmouth-Hitchcock study noted, patients with complex diseases or unclear underlying conditions do not make good telemedicine patients. In addition, opinions are mixed about whether physicians and patients should have an established relationship before telemedicine visits occur and what such visits should entail.
Anchorage, Alaska-based rheumatologist Elizabeth Ferucci, MD, uses telemedicine only for follow up. Like many rheumatologists in Alaska, she travels to field clinics in larger communities throughout the state twice a year to treat patients, said Dr. Ferucci, a rheumatologist for the Alaska Native Tribal Health Consortium. Because some patients need to be seen more than twice a year, Dr. Ferucci conducts follow-up visits every 2-3 months via video.
However, Dr. Ferucci notes that having a trained telemedicine presenter capable of performing a joint examination in the room with the patient is not always possible because of high staff turnover and the more than 200 villages served. A nurse or staff member remains in the room with the patient, but they are not always trained to perform joint exams.
At Dartmouth-Hitchcock, Dr. Albert’s practice accepts both initial and follow-up patient visits. He acknowledges that tele-rheumatology is best used for follow-up care, but explains that limiting the center’s telemedicine use would leave some patients without treatment.
“Because the constraints of getting to Dartmouth are so great, we don’t want to impose that restriction on patients,” Dr. Albert said. “However, in general, it is preferable to do an in-person consultation first to clarify the physical findings.”
Dartmouth has used both nurses and medical assistants as presenters during tele-rheumatology after developing a web-based video series to train presenters. Nurses at UPMC are also trained as telemedicine presenters, and telemedicine visits are only used for follow-up care, Dr. Peoples said.
At Children’s Mercy in Kansas City, telemedicine visits are restricted to follow-up care and include a nurse facilitator at the virtual site. However, the type of conditions and stages of disease examined through the technology has grown since the telemedicine services started, Dr. Kessler said.
“What we feel comfortable seeing has expanded since the time we initially started the clinic because some of the patients, when they were having flares, could not come to Kansas City for financial reasons,” she said, adding that she has since examined children with lupus, vasculitis, and active arthritis through telemedicine.
She stresses that in-person visits should depend on the patient, the circumstances, and the physician’s comfort level. ”You need to be realistic,” she said. “There are times when I’ve said to families, ‘I would like you to come to Kansas City because I’m a little unsure about your exam findings.’ There are definitely some patients who do more of a hybrid approach where they come to Kansas City and are also seen by telemedicine.”
Barriers slow telemedicine progress
Amid its benefits, challenges for tele-rheumatology, such as reimbursement and licensure obstacles, remain.
Commercial insurers pay for telemedicine at varying rates, making it difficult for doctors to consistently be paid, Dr. Peoples said.
“A lot of insurance companies do cover telemedicine visits, but still, a lot don’t,” she said. “I do think that in order to remain competitive, insurance companies overall are going to have to start covering these services.”
At least 32 states and the District of Columbia have parity laws that require commercial health insurers to cover services provided through telehealth to the same extent as those services are covered in person, according to an Aug. 15, 2016 Health Affairs policy brief. However, how private insurers pay and what services they cover vary widely.
Medicaid reimbursement for telehealth depends on individual state Medicaid programs. Only 9 states Medicaid programs reimburse for store-and-forward services, while at least 16 states have some sort of Medicaid reimbursement for remote patient monitoring, according to the Health Affairs report.
Medicare payment for telehealth is clouded in restrictions. The Centers for Medicare & Medicaid Services reimburses for synchronous communications only and does not cover store-and-forward services or remote patient monitoring for chronic diseases, except in Alaska and Hawaii. The patient must be present at an originating site for the visit and cannot be home to receive the services. In addition, CMS reimburses for telehealth only when the originating site is in a Health Professional Shortage Area or within a county outside a Metropolitan Statistical Area. In 2016, CMS expanded its coverage of telehealth to include several new codes including, end-stage renal disease–related services for dialysis, advance care planning services, and critical care consultations furnished via telehealth using new Medicare G-codes.
It’s worth noting that rheumatologists who are salaried employees of academic medical centers, as is the case for all rheumatologists interviewed here, don’t handle the billing for their telemedicine services, and so have not experienced the payment challenges that smaller practices may face.
Mr. Linkous of the American Telemedicine Association said that he believes that reimbursement for telemedicine will improve as health care heads toward value-based payment systems.
“In terms of reimbursement, with the move away from fee-based systems to value-based systems, it really opens the door because for many of the services, you no longer write out a bill with a code associated with it,” he said. “You are paid on the basis of keeping people well. That’s a great positive incentive for using telemedicine.”
Differing state licensure requirements also pose a challenge. The process to obtain licenses and navigate varying credentialing processes can be a headache for health providers and delay approval.
The barrier has led to model legislation by the Federation of State Medical Boards (FSMB) that aims to make it easier for telemedicine physicians to gain licenses in multiple states. Under the Interstate Medical Licensure Compact, physicians designate a member state as the state of principal licensure and select the other states in which they want to license. The state of principal licensure then verifies the physician’s eligibility and provides credential information to the interstate commission, which collects applicable fees and transmits the doctor’s information to the other states. Upon receipt in the additional states, the physician would be granted a license.
As of January, 18 states had enacted the compact legislation, and at least 4 states had introduced the legislation. In 2015, the Health Resources and Services Administration awarded the FSMB a grant to support establishment of the commission and aid with the compact’s infrastructure.
A new normal?
Dr. Albert notes that tele-rheumatology programs can succeed only with adequate resources, time, and staff.
“To set up Skype or Facetime is nothing; you just click on it,” he said. “To set up tele-rheumatology requires a fair amount of administrative substructure that has to be generated in terms of who’s going to do the documentation? Who is going to do the billing? Who is going to do the credentialing? Where is the credentialing going to be validated?”
The equipment required for telemedicine visits also requires a significant amount of time and regular maintenance, Dr. Peoples said. At UPMC for instance, the tele-rheumatology program has an information technology team that assists with technology glitches, changes, and updates, she said. That’s why it’s helpful to have the support of a large academic medical center with the sufficient resources to build a telemedicine program, she added.
In order for more practices to consider tele-rheumatology, more research about cost-effectiveness and best uses of the technology use would be useful, Dr. McDougall said.
“The main question that policy makers are going to want to answer is, ‘What’s the return on investment? Does this make sense for my practice?’ ” he said. “The methods reporting in tele-rheumatologist [literature] is lacking.” But regardless of barriers, telemedicine experts say the technology will likely continue to expand and transform the way rheumatologists are practicing and patients are receiving care.
“Tele-rheumatology will never replace an in-person exam,” Dr. Ferucci said. “But my vision is that it will be able to improve the quality of care for patients living in rural and remote locations, by allowing for more frequent visits and adjustment of medications, which are necessary to achieve the goal of treat-to-target for RA and other rheumatologic conditions.”
* This story was updated on 2/10/2017.
On Twitter @legal_med
Recent FDA Boxed Warnings
The FDA’s MedWatch program safety labeling changes for boxed warnings are compiled quarterly for drugs and therapeutic biologics where important changes have been made to the safety information. You can search these and other label changes in the Drug Safety Labeling Changes (SLC) database, where data are available to the public in downloadable and searchable formats. Boxed warnings are ordinarily used to highlight either adverse reactions so serious in proportion to the potential bene t from the drug that it is essential that it be considered in assessing the risks and bene ts of using the drug; or serious adverse reactions that can be prevented/reduced in frequency or severity by appropriate use of the drug; or FDA approved the drug with restrictions to ensure safe use because FDA concluded that the drug can be safely used only if distribution or use is restricted.
QUINOLONE:
- Edited and updated warning September 2016
WARNING: SERIOUS ADVERSE REACTIONS INCLUDING TENDINITIS, TENDON RUPTURE, PERIPHERAL NEUROPATHY, CENTRAL NERVOUS SYSTEM EFFECTS AND EXACERBATION OF MYASTHENIA GRAVIS
Fluoroquinolones have been associated with disabling and potentially irreversible serious adverse reactions that have occurred together including:
- Tendinitis and tendon rupture
- Peripheral neuropathy
- Central nervous system effects
Discontinue immediately and avoid the use of fluoroquinolones in patients who experience any of these serious adverse reactions. Fluoroquinolones may exacerbate muscle weakness in patients with myasthenia gravis. Avoid quinolones in patients with known history of myasthenia gravis. Because fluoroquinolones
have been associated with serious adverse reactions, reserve quinolones for use in patients who have no alternative treatment options for the following
indications:
Avelox (moxifloxacin hydrochloride): Avelox in sodium chloride 0.8% in plastic container; moxifloxacin hydrochloride; Cipro in dextrose 5% in plastic container):
Acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis.
Cipro (ciprofloxacin; ciprofloxacin hydrochloride): Acute exacerbation of chronic bronchitis, acute uncomplicated cystitis, and acute sinusitis.
Cipro XR; Noroxin (norfloxacin): Uncomplicated urinary tract infections.
Factive (gemifloxacin mesylate): Acute bacterial exacerbation of chronic bronchitis.
Levaquin (levofloxacin): Uncomplicated urinary tract infection, acute bacterial exacerbation of chronic bronchitis, and acute bacterial sinusitis.
KRYSTEXXA (PEGLOTICASE):
- Added section to warning September 2016
WARNING: ANAPHYLAXIS AND INFUSION REACTIONS; G6PD DEFICIENCY ASSOCIATED HEMOLYSIS AND METHEMOGLOBINEMIA (Title Updated)
Addition of: Screen patients at risk for G6PD deficiency prior to starting Krystexxa. Hemolysis and methemoglobinemia have been reported with Krystexxa in patients with G6PD deficiency. Do not administer Krystexxa to patients with G6PD deficiency.
PLAVIX (CLOPIDOGREL BISULFATE):
- Edited and updated warning September 2016
WARNING: DIMINISHED ANTIPLATELET EFFECT IN PATIENTS WITH TWO LOSS-OF-FUNCTION ALLELES OF THE CYP2C19 GENE
The effectiveness of Plavix results from its antiplatelet activity, which is dependent on its conversion to an active metabolite by the cytochrome P450 (CYP) system, principally CYP2C19. Plavix at recommended doses forms less of the active metabolite and so has a reduced effect on platelet activity in patients who are homozygous for nonfunctional alleles of the CYP2C19 gene, (termed “CYP2C19 poor metabolizers”). Tests are available to identify patients who are CYP2C19 poor metabolizers. Consider use of another platelet P2Y12 inhibitor in patients identified as CYP2C19 poor metabolizers.
SYNJARDY (EMPAGLIFLOZIN; METFORMIN HYDROCHLORIDE):
- Edited and updated warning September 2016
Postmarketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metformin-associated lactic acidosis is often subtle, accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metforminassociated lactic acidosis was characterized by elevated blood lactate levels (> 5 mmol/Liter), anion gap acidosis (without evidence of ketonuria or ketonemia), an increased lactate/pyruvate ratio; and metformin plasma levels generally > 5 mcg/mL.
Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (e.g., carbonic anhydrase inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (e.g., acute congestive heart failure), excessive alcohol intake, and hepatic impairment.
Steps to reduce the risk of and manage metformin-associated lactic acidosis in these high-risk groups are provided in the full prescribing information.
If metformin-associated lactic acidosis is suspected, immediately discontinue Synjardy and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended.
ZYDELIG (IDELALISIB)
- Edited and updated warning September 2016
WARNING: FATAL AND SERIOUS TOXICITIES: HEPATIC, SEVERE DIARRHEA, COLITIS, PNEUMONITIS, INFECTIONS, AND INTESTINAL PERFORATION
- Fatal and/or serious hepatotoxicity occurred in 11 % to 18% of Zydelig-treated patients. Monitor hepatic function prior to and during treatment. Interrupt and then reduce or discontinue Zydelig as recommended.
- Fatal and/or serious and severe diarrhea or colitis occurred in 14% to 19% of Zydelig-treated patients. Monitor for the development of severe diarrhea or colitis. Interrupt and then reduce or discontinue Zydelig as recommended.
- Fatal and/or serious pneumonitis occurred in 4% of Zydelig-treated patients. Monitor for pulmonary symptoms and bilateral interstitial infiltrates. Interrupt or discontinue Zydelig as recommended.
- Fatal and/or serious infections occurred in 21% to 36% of Zydelig-treated patients. Monitor for signs and symptoms of infection. Interrupt Zydelig if infection is suspected.
- Fatal and serious intestinal perforation can occur in Zydelig-treated patients across clinical trials. Discontinue Zydelig for intestinal perforation.
The FDA’s MedWatch program safety labeling changes for boxed warnings are compiled quarterly for drugs and therapeutic biologics where important changes have been made to the safety information. You can search these and other label changes in the Drug Safety Labeling Changes (SLC) database, where data are available to the public in downloadable and searchable formats. Boxed warnings are ordinarily used to highlight either adverse reactions so serious in proportion to the potential bene t from the drug that it is essential that it be considered in assessing the risks and bene ts of using the drug; or serious adverse reactions that can be prevented/reduced in frequency or severity by appropriate use of the drug; or FDA approved the drug with restrictions to ensure safe use because FDA concluded that the drug can be safely used only if distribution or use is restricted.
QUINOLONE:
- Edited and updated warning September 2016
WARNING: SERIOUS ADVERSE REACTIONS INCLUDING TENDINITIS, TENDON RUPTURE, PERIPHERAL NEUROPATHY, CENTRAL NERVOUS SYSTEM EFFECTS AND EXACERBATION OF MYASTHENIA GRAVIS
Fluoroquinolones have been associated with disabling and potentially irreversible serious adverse reactions that have occurred together including:
- Tendinitis and tendon rupture
- Peripheral neuropathy
- Central nervous system effects
Discontinue immediately and avoid the use of fluoroquinolones in patients who experience any of these serious adverse reactions. Fluoroquinolones may exacerbate muscle weakness in patients with myasthenia gravis. Avoid quinolones in patients with known history of myasthenia gravis. Because fluoroquinolones
have been associated with serious adverse reactions, reserve quinolones for use in patients who have no alternative treatment options for the following
indications:
Avelox (moxifloxacin hydrochloride): Avelox in sodium chloride 0.8% in plastic container; moxifloxacin hydrochloride; Cipro in dextrose 5% in plastic container):
Acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis.
Cipro (ciprofloxacin; ciprofloxacin hydrochloride): Acute exacerbation of chronic bronchitis, acute uncomplicated cystitis, and acute sinusitis.
Cipro XR; Noroxin (norfloxacin): Uncomplicated urinary tract infections.
Factive (gemifloxacin mesylate): Acute bacterial exacerbation of chronic bronchitis.
Levaquin (levofloxacin): Uncomplicated urinary tract infection, acute bacterial exacerbation of chronic bronchitis, and acute bacterial sinusitis.
KRYSTEXXA (PEGLOTICASE):
- Added section to warning September 2016
WARNING: ANAPHYLAXIS AND INFUSION REACTIONS; G6PD DEFICIENCY ASSOCIATED HEMOLYSIS AND METHEMOGLOBINEMIA (Title Updated)
Addition of: Screen patients at risk for G6PD deficiency prior to starting Krystexxa. Hemolysis and methemoglobinemia have been reported with Krystexxa in patients with G6PD deficiency. Do not administer Krystexxa to patients with G6PD deficiency.
PLAVIX (CLOPIDOGREL BISULFATE):
- Edited and updated warning September 2016
WARNING: DIMINISHED ANTIPLATELET EFFECT IN PATIENTS WITH TWO LOSS-OF-FUNCTION ALLELES OF THE CYP2C19 GENE
The effectiveness of Plavix results from its antiplatelet activity, which is dependent on its conversion to an active metabolite by the cytochrome P450 (CYP) system, principally CYP2C19. Plavix at recommended doses forms less of the active metabolite and so has a reduced effect on platelet activity in patients who are homozygous for nonfunctional alleles of the CYP2C19 gene, (termed “CYP2C19 poor metabolizers”). Tests are available to identify patients who are CYP2C19 poor metabolizers. Consider use of another platelet P2Y12 inhibitor in patients identified as CYP2C19 poor metabolizers.
SYNJARDY (EMPAGLIFLOZIN; METFORMIN HYDROCHLORIDE):
- Edited and updated warning September 2016
Postmarketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metformin-associated lactic acidosis is often subtle, accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metforminassociated lactic acidosis was characterized by elevated blood lactate levels (> 5 mmol/Liter), anion gap acidosis (without evidence of ketonuria or ketonemia), an increased lactate/pyruvate ratio; and metformin plasma levels generally > 5 mcg/mL.
Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (e.g., carbonic anhydrase inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (e.g., acute congestive heart failure), excessive alcohol intake, and hepatic impairment.
Steps to reduce the risk of and manage metformin-associated lactic acidosis in these high-risk groups are provided in the full prescribing information.
If metformin-associated lactic acidosis is suspected, immediately discontinue Synjardy and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended.
ZYDELIG (IDELALISIB)
- Edited and updated warning September 2016
WARNING: FATAL AND SERIOUS TOXICITIES: HEPATIC, SEVERE DIARRHEA, COLITIS, PNEUMONITIS, INFECTIONS, AND INTESTINAL PERFORATION
- Fatal and/or serious hepatotoxicity occurred in 11 % to 18% of Zydelig-treated patients. Monitor hepatic function prior to and during treatment. Interrupt and then reduce or discontinue Zydelig as recommended.
- Fatal and/or serious and severe diarrhea or colitis occurred in 14% to 19% of Zydelig-treated patients. Monitor for the development of severe diarrhea or colitis. Interrupt and then reduce or discontinue Zydelig as recommended.
- Fatal and/or serious pneumonitis occurred in 4% of Zydelig-treated patients. Monitor for pulmonary symptoms and bilateral interstitial infiltrates. Interrupt or discontinue Zydelig as recommended.
- Fatal and/or serious infections occurred in 21% to 36% of Zydelig-treated patients. Monitor for signs and symptoms of infection. Interrupt Zydelig if infection is suspected.
- Fatal and serious intestinal perforation can occur in Zydelig-treated patients across clinical trials. Discontinue Zydelig for intestinal perforation.
The FDA’s MedWatch program safety labeling changes for boxed warnings are compiled quarterly for drugs and therapeutic biologics where important changes have been made to the safety information. You can search these and other label changes in the Drug Safety Labeling Changes (SLC) database, where data are available to the public in downloadable and searchable formats. Boxed warnings are ordinarily used to highlight either adverse reactions so serious in proportion to the potential bene t from the drug that it is essential that it be considered in assessing the risks and bene ts of using the drug; or serious adverse reactions that can be prevented/reduced in frequency or severity by appropriate use of the drug; or FDA approved the drug with restrictions to ensure safe use because FDA concluded that the drug can be safely used only if distribution or use is restricted.
QUINOLONE:
- Edited and updated warning September 2016
WARNING: SERIOUS ADVERSE REACTIONS INCLUDING TENDINITIS, TENDON RUPTURE, PERIPHERAL NEUROPATHY, CENTRAL NERVOUS SYSTEM EFFECTS AND EXACERBATION OF MYASTHENIA GRAVIS
Fluoroquinolones have been associated with disabling and potentially irreversible serious adverse reactions that have occurred together including:
- Tendinitis and tendon rupture
- Peripheral neuropathy
- Central nervous system effects
Discontinue immediately and avoid the use of fluoroquinolones in patients who experience any of these serious adverse reactions. Fluoroquinolones may exacerbate muscle weakness in patients with myasthenia gravis. Avoid quinolones in patients with known history of myasthenia gravis. Because fluoroquinolones
have been associated with serious adverse reactions, reserve quinolones for use in patients who have no alternative treatment options for the following
indications:
Avelox (moxifloxacin hydrochloride): Avelox in sodium chloride 0.8% in plastic container; moxifloxacin hydrochloride; Cipro in dextrose 5% in plastic container):
Acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis.
Cipro (ciprofloxacin; ciprofloxacin hydrochloride): Acute exacerbation of chronic bronchitis, acute uncomplicated cystitis, and acute sinusitis.
Cipro XR; Noroxin (norfloxacin): Uncomplicated urinary tract infections.
Factive (gemifloxacin mesylate): Acute bacterial exacerbation of chronic bronchitis.
Levaquin (levofloxacin): Uncomplicated urinary tract infection, acute bacterial exacerbation of chronic bronchitis, and acute bacterial sinusitis.
KRYSTEXXA (PEGLOTICASE):
- Added section to warning September 2016
WARNING: ANAPHYLAXIS AND INFUSION REACTIONS; G6PD DEFICIENCY ASSOCIATED HEMOLYSIS AND METHEMOGLOBINEMIA (Title Updated)
Addition of: Screen patients at risk for G6PD deficiency prior to starting Krystexxa. Hemolysis and methemoglobinemia have been reported with Krystexxa in patients with G6PD deficiency. Do not administer Krystexxa to patients with G6PD deficiency.
PLAVIX (CLOPIDOGREL BISULFATE):
- Edited and updated warning September 2016
WARNING: DIMINISHED ANTIPLATELET EFFECT IN PATIENTS WITH TWO LOSS-OF-FUNCTION ALLELES OF THE CYP2C19 GENE
The effectiveness of Plavix results from its antiplatelet activity, which is dependent on its conversion to an active metabolite by the cytochrome P450 (CYP) system, principally CYP2C19. Plavix at recommended doses forms less of the active metabolite and so has a reduced effect on platelet activity in patients who are homozygous for nonfunctional alleles of the CYP2C19 gene, (termed “CYP2C19 poor metabolizers”). Tests are available to identify patients who are CYP2C19 poor metabolizers. Consider use of another platelet P2Y12 inhibitor in patients identified as CYP2C19 poor metabolizers.
SYNJARDY (EMPAGLIFLOZIN; METFORMIN HYDROCHLORIDE):
- Edited and updated warning September 2016
Postmarketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metformin-associated lactic acidosis is often subtle, accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metforminassociated lactic acidosis was characterized by elevated blood lactate levels (> 5 mmol/Liter), anion gap acidosis (without evidence of ketonuria or ketonemia), an increased lactate/pyruvate ratio; and metformin plasma levels generally > 5 mcg/mL.
Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (e.g., carbonic anhydrase inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (e.g., acute congestive heart failure), excessive alcohol intake, and hepatic impairment.
Steps to reduce the risk of and manage metformin-associated lactic acidosis in these high-risk groups are provided in the full prescribing information.
If metformin-associated lactic acidosis is suspected, immediately discontinue Synjardy and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended.
ZYDELIG (IDELALISIB)
- Edited and updated warning September 2016
WARNING: FATAL AND SERIOUS TOXICITIES: HEPATIC, SEVERE DIARRHEA, COLITIS, PNEUMONITIS, INFECTIONS, AND INTESTINAL PERFORATION
- Fatal and/or serious hepatotoxicity occurred in 11 % to 18% of Zydelig-treated patients. Monitor hepatic function prior to and during treatment. Interrupt and then reduce or discontinue Zydelig as recommended.
- Fatal and/or serious and severe diarrhea or colitis occurred in 14% to 19% of Zydelig-treated patients. Monitor for the development of severe diarrhea or colitis. Interrupt and then reduce or discontinue Zydelig as recommended.
- Fatal and/or serious pneumonitis occurred in 4% of Zydelig-treated patients. Monitor for pulmonary symptoms and bilateral interstitial infiltrates. Interrupt or discontinue Zydelig as recommended.
- Fatal and/or serious infections occurred in 21% to 36% of Zydelig-treated patients. Monitor for signs and symptoms of infection. Interrupt Zydelig if infection is suspected.
- Fatal and serious intestinal perforation can occur in Zydelig-treated patients across clinical trials. Discontinue Zydelig for intestinal perforation.
Hearing Loss Is Less Common in Adults
The number of older Americans is growing, but the number of those with hearing loss is declining, according to data from the National Health and Nutrition Examination Survey. Researchers compared 2 time periods (1999-2004 and 2011-2012) and found overall annual prevalence of hearing loss dropped from 16% to 14% in 1999-2004, to 28 million adults, then dropped further to 27.7 million in 2011-2012.
Age was the greatest predictor of hearing loss; people in the oldest age group surveyed (aged 60 to 69) had the most loss. Although not included in the study, people aged ≥ 70 years have the highest prevalence of hearing loss of any age group, the authors say. Men of all ages were twice as likely as women to have hearing loss. Non-Hispanic white adults were more likely to have hearing loss than were adults in other ethnic groups. Non-Hispanic black adults had the lowest risk.
The researchers don’t know why hearing loss is becoming less prevalent but suggest reasons include fewer manufacturing jobs, increased use of hearing protectors, less smoking, and better medical care to manage risk factors associated with hearing loss. They did find that lower education level and heavy use of firearms were associated with hearing loss.
“Our findings show a promising trend of better hearing among adults that spans more than half a century,” says Howard Hoffman, MA, first author on the paper and director of the National Institute on Deafness and Other Communication Disorders Epidemiology and Statistics Program. “The decline in hearing loss rates among adults aged < 70 years suggests that age-related hearing loss may be delayed until later in life. This is good news because for those who do develop hearing loss, they will have experienced more quality years of life with better hearing than earlier generations."
The number of older Americans is growing, but the number of those with hearing loss is declining, according to data from the National Health and Nutrition Examination Survey. Researchers compared 2 time periods (1999-2004 and 2011-2012) and found overall annual prevalence of hearing loss dropped from 16% to 14% in 1999-2004, to 28 million adults, then dropped further to 27.7 million in 2011-2012.
Age was the greatest predictor of hearing loss; people in the oldest age group surveyed (aged 60 to 69) had the most loss. Although not included in the study, people aged ≥ 70 years have the highest prevalence of hearing loss of any age group, the authors say. Men of all ages were twice as likely as women to have hearing loss. Non-Hispanic white adults were more likely to have hearing loss than were adults in other ethnic groups. Non-Hispanic black adults had the lowest risk.
The researchers don’t know why hearing loss is becoming less prevalent but suggest reasons include fewer manufacturing jobs, increased use of hearing protectors, less smoking, and better medical care to manage risk factors associated with hearing loss. They did find that lower education level and heavy use of firearms were associated with hearing loss.
“Our findings show a promising trend of better hearing among adults that spans more than half a century,” says Howard Hoffman, MA, first author on the paper and director of the National Institute on Deafness and Other Communication Disorders Epidemiology and Statistics Program. “The decline in hearing loss rates among adults aged < 70 years suggests that age-related hearing loss may be delayed until later in life. This is good news because for those who do develop hearing loss, they will have experienced more quality years of life with better hearing than earlier generations."
The number of older Americans is growing, but the number of those with hearing loss is declining, according to data from the National Health and Nutrition Examination Survey. Researchers compared 2 time periods (1999-2004 and 2011-2012) and found overall annual prevalence of hearing loss dropped from 16% to 14% in 1999-2004, to 28 million adults, then dropped further to 27.7 million in 2011-2012.
Age was the greatest predictor of hearing loss; people in the oldest age group surveyed (aged 60 to 69) had the most loss. Although not included in the study, people aged ≥ 70 years have the highest prevalence of hearing loss of any age group, the authors say. Men of all ages were twice as likely as women to have hearing loss. Non-Hispanic white adults were more likely to have hearing loss than were adults in other ethnic groups. Non-Hispanic black adults had the lowest risk.
The researchers don’t know why hearing loss is becoming less prevalent but suggest reasons include fewer manufacturing jobs, increased use of hearing protectors, less smoking, and better medical care to manage risk factors associated with hearing loss. They did find that lower education level and heavy use of firearms were associated with hearing loss.
“Our findings show a promising trend of better hearing among adults that spans more than half a century,” says Howard Hoffman, MA, first author on the paper and director of the National Institute on Deafness and Other Communication Disorders Epidemiology and Statistics Program. “The decline in hearing loss rates among adults aged < 70 years suggests that age-related hearing loss may be delayed until later in life. This is good news because for those who do develop hearing loss, they will have experienced more quality years of life with better hearing than earlier generations."
Third-hand smoke affects blood cell development in mice

and Antoine Snijders analyze
blood cells collected from mice
exposed to third-hand smoke.
Photo courtesy of
Marilyn Chung/Berkeley Lab
Exposure to third-hand smoke leads to biological effects on weight and blood cell development, according to preclinical research published in Scientific Reports.
Researchers found that newborn mice housed with smoke-treated cloths for 3 weeks weighed significantly less than mice in a control group.
Moreover, newborn and adult mice exposed to third-hand smoke experienced persistent changes in blood cell counts.
The blood cell count changes are associated with inflammatory and allergic reactions upon exposure to third-hand smoke, the researchers said.
For this study, the team set out to characterize the biological effects of exposure to third-hand smoke by placing 5-square-centimeter pieces of smoke-contaminated cotton cloth in cages with mice.
The researchers then compared smoke-exposed mice to control mice. The team assessed changes to body weight and the hematopoietic system after 3 weeks of exposure (or no exposure) for mice belonging to 2 age groups: birth to 3 weeks (neonatal) and 12 to 15 weeks (young adult).
The results showed that smoke exposure temporarily inhibited weight gain in the neonatal mice. There was no effect on weight gain in the young adult mice.
In addition, smoke exposure produced changes in blood cell populations that persisted over time and were evident in mice from both age groups.
In general, there were lower levels of platelets and specific types of white blood cells in the smoke-exposed mice.
For example, neonatal mice exposed to third-hand smoke had higher levels of eosinophils, female mice had higher levels of neutrophils, males had higher levels of basophils, and all mice had higher levels of B cells.
“Those are all types of white blood cells associated with inflammation and allergic reactions,” said study author Jian-Hua Mao, PhD, of Lawrence Berkeley National Laboratory in Berkeley, California.
“And the effects on blood cell count persisted even after exposure ended. Changes remained at least 14 weeks after exposure ended for the neonatal group and 2 weeks after it ended for the adults.”
The researchers pointed out that they did not study whether the observed biological changes led to specific diseases or other health outcomes, but other studies suggest links to adverse health effects.
“Third-hand smoke is an underappreciated risk factor in health,” said study author Antoine Snijders, PhD, of Lawrence Berkeley National Laboratory.
“It’s clear that more and bigger studies are needed, particularly in humans, so we can support policy decisions on third-hand smoke.” ![]()

and Antoine Snijders analyze
blood cells collected from mice
exposed to third-hand smoke.
Photo courtesy of
Marilyn Chung/Berkeley Lab
Exposure to third-hand smoke leads to biological effects on weight and blood cell development, according to preclinical research published in Scientific Reports.
Researchers found that newborn mice housed with smoke-treated cloths for 3 weeks weighed significantly less than mice in a control group.
Moreover, newborn and adult mice exposed to third-hand smoke experienced persistent changes in blood cell counts.
The blood cell count changes are associated with inflammatory and allergic reactions upon exposure to third-hand smoke, the researchers said.
For this study, the team set out to characterize the biological effects of exposure to third-hand smoke by placing 5-square-centimeter pieces of smoke-contaminated cotton cloth in cages with mice.
The researchers then compared smoke-exposed mice to control mice. The team assessed changes to body weight and the hematopoietic system after 3 weeks of exposure (or no exposure) for mice belonging to 2 age groups: birth to 3 weeks (neonatal) and 12 to 15 weeks (young adult).
The results showed that smoke exposure temporarily inhibited weight gain in the neonatal mice. There was no effect on weight gain in the young adult mice.
In addition, smoke exposure produced changes in blood cell populations that persisted over time and were evident in mice from both age groups.
In general, there were lower levels of platelets and specific types of white blood cells in the smoke-exposed mice.
For example, neonatal mice exposed to third-hand smoke had higher levels of eosinophils, female mice had higher levels of neutrophils, males had higher levels of basophils, and all mice had higher levels of B cells.
“Those are all types of white blood cells associated with inflammation and allergic reactions,” said study author Jian-Hua Mao, PhD, of Lawrence Berkeley National Laboratory in Berkeley, California.
“And the effects on blood cell count persisted even after exposure ended. Changes remained at least 14 weeks after exposure ended for the neonatal group and 2 weeks after it ended for the adults.”
The researchers pointed out that they did not study whether the observed biological changes led to specific diseases or other health outcomes, but other studies suggest links to adverse health effects.
“Third-hand smoke is an underappreciated risk factor in health,” said study author Antoine Snijders, PhD, of Lawrence Berkeley National Laboratory.
“It’s clear that more and bigger studies are needed, particularly in humans, so we can support policy decisions on third-hand smoke.” ![]()

and Antoine Snijders analyze
blood cells collected from mice
exposed to third-hand smoke.
Photo courtesy of
Marilyn Chung/Berkeley Lab
Exposure to third-hand smoke leads to biological effects on weight and blood cell development, according to preclinical research published in Scientific Reports.
Researchers found that newborn mice housed with smoke-treated cloths for 3 weeks weighed significantly less than mice in a control group.
Moreover, newborn and adult mice exposed to third-hand smoke experienced persistent changes in blood cell counts.
The blood cell count changes are associated with inflammatory and allergic reactions upon exposure to third-hand smoke, the researchers said.
For this study, the team set out to characterize the biological effects of exposure to third-hand smoke by placing 5-square-centimeter pieces of smoke-contaminated cotton cloth in cages with mice.
The researchers then compared smoke-exposed mice to control mice. The team assessed changes to body weight and the hematopoietic system after 3 weeks of exposure (or no exposure) for mice belonging to 2 age groups: birth to 3 weeks (neonatal) and 12 to 15 weeks (young adult).
The results showed that smoke exposure temporarily inhibited weight gain in the neonatal mice. There was no effect on weight gain in the young adult mice.
In addition, smoke exposure produced changes in blood cell populations that persisted over time and were evident in mice from both age groups.
In general, there were lower levels of platelets and specific types of white blood cells in the smoke-exposed mice.
For example, neonatal mice exposed to third-hand smoke had higher levels of eosinophils, female mice had higher levels of neutrophils, males had higher levels of basophils, and all mice had higher levels of B cells.
“Those are all types of white blood cells associated with inflammation and allergic reactions,” said study author Jian-Hua Mao, PhD, of Lawrence Berkeley National Laboratory in Berkeley, California.
“And the effects on blood cell count persisted even after exposure ended. Changes remained at least 14 weeks after exposure ended for the neonatal group and 2 weeks after it ended for the adults.”
The researchers pointed out that they did not study whether the observed biological changes led to specific diseases or other health outcomes, but other studies suggest links to adverse health effects.
“Third-hand smoke is an underappreciated risk factor in health,” said study author Antoine Snijders, PhD, of Lawrence Berkeley National Laboratory.
“It’s clear that more and bigger studies are needed, particularly in humans, so we can support policy decisions on third-hand smoke.” ![]()
NCCN releases patient guidelines for WM/LPL
The National Comprehensive Cancer Network® (NCCN) has published a set of
guidelines for patients with
Waldenström’s macroglobulinemia/ lymphoplasmacytic lymphoma (WM/LPL).
This resource describes what WM is and how it develops,
explains testing for WM, and provides information on the treatment of
primary, relapsed, and refractory WM.
NCCN Guidelines for Patients are adaptations of the
NCCN Clinical Practice Guidelines in Oncology.
The NCCN also publishes Quick Guide™ sheets, which are 1-page summaries of key points in the patient guidelines.
Both patient resources are available free of charge at NCCN.org/patients as well as on the NCCN Patient Guides for Cancer mobile app.
“The treatment approach to patients with Waldenström’s macroglobulinemia has significantly changed in the recent years with better understanding of the disease biology and its natural history and availability of new drugs, allowing for a more individualized approach,” said Shaji Kumar, MD, of the Mayo Clinic in Rochester, Minnesota.
“The revised guidelines reflect these changes and will be a valuable guide for patients in shared decision-making with their oncologists.”
NCCN Guidelines for Patients are based on the same clinical practice guidelines used by healthcare professionals to determine the best way to treat a patient with cancer.
Each resource features expert guidance from US cancer centers designed to help people living with cancer talk to their physicians about the best treatment options for their disease.
The Guidelines for Patients and Quick Guide sheets are written in plain language and include patient-friendly elements, such as “questions to ask your doctor,” a glossary of terms, and medical illustrations of anatomy, tests, and treatment.
NCCN currently offers NCCN Guidelines for Patients covering the following topics: brain, breast, colon esophageal, kidney, non-small cell lung, ovarian, pancreatic, prostate, and stomach cancers; acute lymphoblastic leukemia; adolescents and young adults with cancer; chronic lymphocytic leukemia; chronic myelogenous leukemia; Hodgkin lymphoma; lung cancer screening; malignant pleural mesothelioma; melanoma; multiple myeloma; nausea and vomiting; non-Hodgkin lymphomas; soft tissue sarcoma; and WM/LPL. ![]()

The National Comprehensive Cancer Network® (NCCN) has published a set of
guidelines for patients with
Waldenström’s macroglobulinemia/ lymphoplasmacytic lymphoma (WM/LPL).
This resource describes what WM is and how it develops,
explains testing for WM, and provides information on the treatment of
primary, relapsed, and refractory WM.
NCCN Guidelines for Patients are adaptations of the
NCCN Clinical Practice Guidelines in Oncology.
The NCCN also publishes Quick Guide™ sheets, which are 1-page summaries of key points in the patient guidelines.
Both patient resources are available free of charge at NCCN.org/patients as well as on the NCCN Patient Guides for Cancer mobile app.
“The treatment approach to patients with Waldenström’s macroglobulinemia has significantly changed in the recent years with better understanding of the disease biology and its natural history and availability of new drugs, allowing for a more individualized approach,” said Shaji Kumar, MD, of the Mayo Clinic in Rochester, Minnesota.
“The revised guidelines reflect these changes and will be a valuable guide for patients in shared decision-making with their oncologists.”
NCCN Guidelines for Patients are based on the same clinical practice guidelines used by healthcare professionals to determine the best way to treat a patient with cancer.
Each resource features expert guidance from US cancer centers designed to help people living with cancer talk to their physicians about the best treatment options for their disease.
The Guidelines for Patients and Quick Guide sheets are written in plain language and include patient-friendly elements, such as “questions to ask your doctor,” a glossary of terms, and medical illustrations of anatomy, tests, and treatment.
NCCN currently offers NCCN Guidelines for Patients covering the following topics: brain, breast, colon esophageal, kidney, non-small cell lung, ovarian, pancreatic, prostate, and stomach cancers; acute lymphoblastic leukemia; adolescents and young adults with cancer; chronic lymphocytic leukemia; chronic myelogenous leukemia; Hodgkin lymphoma; lung cancer screening; malignant pleural mesothelioma; melanoma; multiple myeloma; nausea and vomiting; non-Hodgkin lymphomas; soft tissue sarcoma; and WM/LPL. ![]()

The National Comprehensive Cancer Network® (NCCN) has published a set of
guidelines for patients with
Waldenström’s macroglobulinemia/ lymphoplasmacytic lymphoma (WM/LPL).
This resource describes what WM is and how it develops,
explains testing for WM, and provides information on the treatment of
primary, relapsed, and refractory WM.
NCCN Guidelines for Patients are adaptations of the
NCCN Clinical Practice Guidelines in Oncology.
The NCCN also publishes Quick Guide™ sheets, which are 1-page summaries of key points in the patient guidelines.
Both patient resources are available free of charge at NCCN.org/patients as well as on the NCCN Patient Guides for Cancer mobile app.
“The treatment approach to patients with Waldenström’s macroglobulinemia has significantly changed in the recent years with better understanding of the disease biology and its natural history and availability of new drugs, allowing for a more individualized approach,” said Shaji Kumar, MD, of the Mayo Clinic in Rochester, Minnesota.
“The revised guidelines reflect these changes and will be a valuable guide for patients in shared decision-making with their oncologists.”
NCCN Guidelines for Patients are based on the same clinical practice guidelines used by healthcare professionals to determine the best way to treat a patient with cancer.
Each resource features expert guidance from US cancer centers designed to help people living with cancer talk to their physicians about the best treatment options for their disease.
The Guidelines for Patients and Quick Guide sheets are written in plain language and include patient-friendly elements, such as “questions to ask your doctor,” a glossary of terms, and medical illustrations of anatomy, tests, and treatment.
NCCN currently offers NCCN Guidelines for Patients covering the following topics: brain, breast, colon esophageal, kidney, non-small cell lung, ovarian, pancreatic, prostate, and stomach cancers; acute lymphoblastic leukemia; adolescents and young adults with cancer; chronic lymphocytic leukemia; chronic myelogenous leukemia; Hodgkin lymphoma; lung cancer screening; malignant pleural mesothelioma; melanoma; multiple myeloma; nausea and vomiting; non-Hodgkin lymphomas; soft tissue sarcoma; and WM/LPL. ![]()
Azathioprine may increase risk of MDS, AML

Results of a large, retrospective study suggest that taking azathioprine, a drug commonly used to treat autoimmune disease, may increase a person’s risk of developing myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML).
Researchers analyzed data on more than 40,000 patients with 27 common autoimmune diseases and found that azathioprine use was significantly associated with an increased risk of MDS and AML.
“Similar associations were already documented in case reports and case series but have never been evaluated in a broad spectrum of autoimmune diseases in that many patients and in context of individual medications,” said study author Raoul Tibes, MD, PhD, of the Mayo Clinic in Phoenix, Arizona.
“Interestingly, there was no association with length of time on therapy and resulting myeloid neoplasm.”
Dr Tibes and his colleagues reported these findings in JAMA Oncology.
The researchers reviewed data on 40,011 patients with primary autoimmune disorders, such as lupus and rheumatoid arthritis, who were seen at 2 centers from January 1, 2004, to December 31, 2014.
There were 311 patients with MDS or AML, but only 86 met strict inclusion criteria. Fifty-five patients had MDS, 21 had de novo AML, and 10 had AML and a history of MDS.
The researchers collected detailed data on each patient’s drug exposures, treatment duration, and disease characteristics and compared this information to data from patients with autoimmune disorders who did not have MDS or AML.
This revealed that use of azathioprine sodium was more frequent in cases than controls, and azathioprine was significantly associated with an increased risk of MDS and AML. The odds ratio was 7.05 (P<0.001).
Other agents used showed a similar trend, but the results were not statistically significant. The odds ratios were 3.58 for cyclophosphamide and 2.73 for mitoxantrone hydrochloride.
The researchers said that, while these results are intriguing, they should not change or replace the clinical judgments, monitoring, and current standard treatments for patients with autoimmune diseases.
Despite its large size, this study had limitations, including its retrospective nature, the fact that many different autoimmune diseases were analyzed, and that the researchers only looked at cases of MDS and AML.
No definitive causal association was made between taking a particular drug and MDS or AML. The number of patients with autoimmune disease developing MDS or AML is still low overall, and no prediction for individual patients can be concluded from the study.
The researchers plan to perform molecular investigations into the genetic susceptibility for therapy-related myeloid neoplasms as the next phase of this research. ![]()

Results of a large, retrospective study suggest that taking azathioprine, a drug commonly used to treat autoimmune disease, may increase a person’s risk of developing myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML).
Researchers analyzed data on more than 40,000 patients with 27 common autoimmune diseases and found that azathioprine use was significantly associated with an increased risk of MDS and AML.
“Similar associations were already documented in case reports and case series but have never been evaluated in a broad spectrum of autoimmune diseases in that many patients and in context of individual medications,” said study author Raoul Tibes, MD, PhD, of the Mayo Clinic in Phoenix, Arizona.
“Interestingly, there was no association with length of time on therapy and resulting myeloid neoplasm.”
Dr Tibes and his colleagues reported these findings in JAMA Oncology.
The researchers reviewed data on 40,011 patients with primary autoimmune disorders, such as lupus and rheumatoid arthritis, who were seen at 2 centers from January 1, 2004, to December 31, 2014.
There were 311 patients with MDS or AML, but only 86 met strict inclusion criteria. Fifty-five patients had MDS, 21 had de novo AML, and 10 had AML and a history of MDS.
The researchers collected detailed data on each patient’s drug exposures, treatment duration, and disease characteristics and compared this information to data from patients with autoimmune disorders who did not have MDS or AML.
This revealed that use of azathioprine sodium was more frequent in cases than controls, and azathioprine was significantly associated with an increased risk of MDS and AML. The odds ratio was 7.05 (P<0.001).
Other agents used showed a similar trend, but the results were not statistically significant. The odds ratios were 3.58 for cyclophosphamide and 2.73 for mitoxantrone hydrochloride.
The researchers said that, while these results are intriguing, they should not change or replace the clinical judgments, monitoring, and current standard treatments for patients with autoimmune diseases.
Despite its large size, this study had limitations, including its retrospective nature, the fact that many different autoimmune diseases were analyzed, and that the researchers only looked at cases of MDS and AML.
No definitive causal association was made between taking a particular drug and MDS or AML. The number of patients with autoimmune disease developing MDS or AML is still low overall, and no prediction for individual patients can be concluded from the study.
The researchers plan to perform molecular investigations into the genetic susceptibility for therapy-related myeloid neoplasms as the next phase of this research. ![]()

Results of a large, retrospective study suggest that taking azathioprine, a drug commonly used to treat autoimmune disease, may increase a person’s risk of developing myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML).
Researchers analyzed data on more than 40,000 patients with 27 common autoimmune diseases and found that azathioprine use was significantly associated with an increased risk of MDS and AML.
“Similar associations were already documented in case reports and case series but have never been evaluated in a broad spectrum of autoimmune diseases in that many patients and in context of individual medications,” said study author Raoul Tibes, MD, PhD, of the Mayo Clinic in Phoenix, Arizona.
“Interestingly, there was no association with length of time on therapy and resulting myeloid neoplasm.”
Dr Tibes and his colleagues reported these findings in JAMA Oncology.
The researchers reviewed data on 40,011 patients with primary autoimmune disorders, such as lupus and rheumatoid arthritis, who were seen at 2 centers from January 1, 2004, to December 31, 2014.
There were 311 patients with MDS or AML, but only 86 met strict inclusion criteria. Fifty-five patients had MDS, 21 had de novo AML, and 10 had AML and a history of MDS.
The researchers collected detailed data on each patient’s drug exposures, treatment duration, and disease characteristics and compared this information to data from patients with autoimmune disorders who did not have MDS or AML.
This revealed that use of azathioprine sodium was more frequent in cases than controls, and azathioprine was significantly associated with an increased risk of MDS and AML. The odds ratio was 7.05 (P<0.001).
Other agents used showed a similar trend, but the results were not statistically significant. The odds ratios were 3.58 for cyclophosphamide and 2.73 for mitoxantrone hydrochloride.
The researchers said that, while these results are intriguing, they should not change or replace the clinical judgments, monitoring, and current standard treatments for patients with autoimmune diseases.
Despite its large size, this study had limitations, including its retrospective nature, the fact that many different autoimmune diseases were analyzed, and that the researchers only looked at cases of MDS and AML.
No definitive causal association was made between taking a particular drug and MDS or AML. The number of patients with autoimmune disease developing MDS or AML is still low overall, and no prediction for individual patients can be concluded from the study.
The researchers plan to perform molecular investigations into the genetic susceptibility for therapy-related myeloid neoplasms as the next phase of this research. ![]()