User login
Noninfectious Penile Lesions
1. A 26-year-old man presents with a penile lesion that has existed at least 10 years without significant change and with no attendant symptoms. The lesion consists of four 1-to-1.5-mm soft, compressible purple papules, in aggregate measuring about 8 mm. No other lesions are seen on the genitals or on the body.
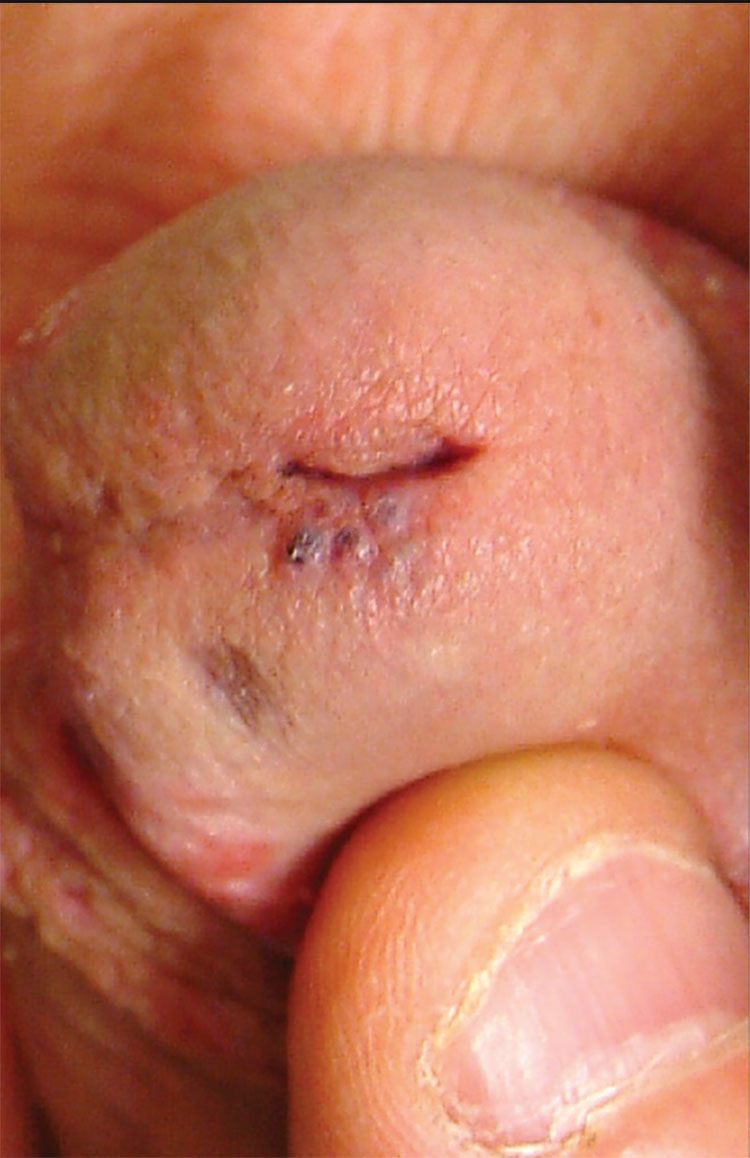
Diagnosis: The lesion proved to be angiokeratoma of Fordyce, a totally benign lesion. This type of angiokeratoma is ectatic, thin-walled vessels in the superficial dermis, with overlying epidermal hyperplasia that forms secondary to normal friction. When they are seen on the scrotum, vulva, or penis, they are usually referred to as angiokeratoma of Fordyce, a type of localized angiokeratoma, other types of which can appear on the legs or hands.
These totally benign lesions must be distinguished from generalized types of angiokeratomata, such as those seen in Fabry disease (angiokeratoma corporis diffusum), an inheritable metabolic disorder. With our patient’s history, his lesion was clearly benign.
Had his lesion been new or changing in any substantive way, additional testing, including a biopsy, might have been necessary to rule out entities such as squamous cell carcinoma (which is almost unknown in circumcised patients), condyloma, melanoma, the aforementioned Kaposi’s sarcoma, or even angiosarcoma.
For more information, see “Skin is skin, no matter the location.” Clinician Reviews. 2013 June;23(6):W5.
2. A 59-year-old uncircumcised man presents with a phimotic foreskin, which cannot be retracted without pain. Only a tiny opening remains through which the patient can urinate (with difficulty). The surface of the foreskin is atrophic, dry, and shiny with focal areas of purpura but little, if any, redness or swelling.
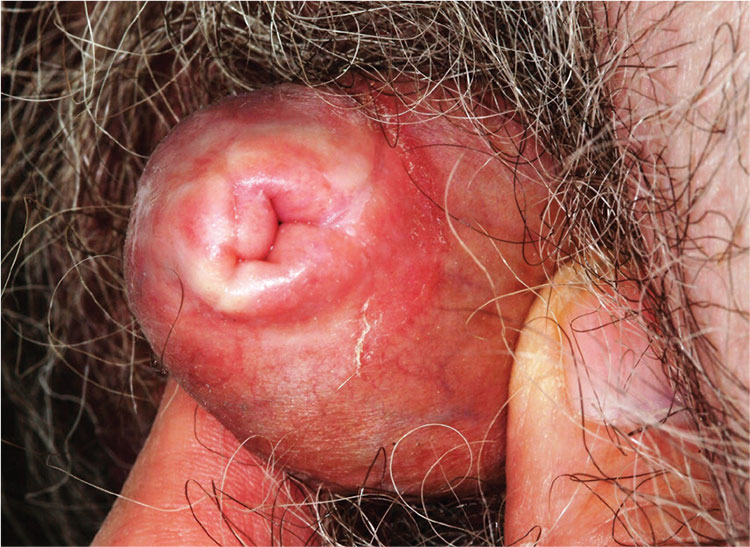
Diagnosis: This patient’s condition is lichen sclerosus, also called balanitis xerotica obliterans (BXO), a diagnosis of sufficient obscurity to almost guarantee initial misdiagnosis as “yeast infection” or “herpes.” One treatment failure after another eventually leads to referral to a provider familiar with BXO, which is the male version of lichen sclerosus et atrophicus (LS&A) and usually affects the glans, foreskin, and distal shaft.
The causes of these conditions are as yet unknown. However, much is known about how they present, how they look under a microscope, and how to treat them.
Treatment entails use of the most powerful topical steroid ointments, which are so effective that they have completely replaced previous treatment options (eg, testosterone ointment). While a cure is unlikely, control is a realistic goal.
BXO, as in this case, can also cause urinary obstruction, both from the overlying foreskin and from actual meatal stenosis. This is why advanced cases need referral to urology for possible circumcision. As with many penile diagnoses (eg, squamous cell carcinoma or condyloma), BXO is far more common in the uncircumcised.
For more information, see “Man has very uncomfortable problem.” Clinician Reviews. 2012 September;22(9):W4.
3. A 31-year-old man has a relatively asymptomatic penile rash that has repeatedly manifested and resolved over a period of months. A round, papulosquamous, bright pink patch on the distal right shaft of his circumcised penis measures > 3 cm in diameter and has a shiny appearance with slightly irregular margins. A similar rash is seen behind both ears, in the umbilicus, and patches of dandruff are noted. Despite good health, he has been under a great deal of stress recently.
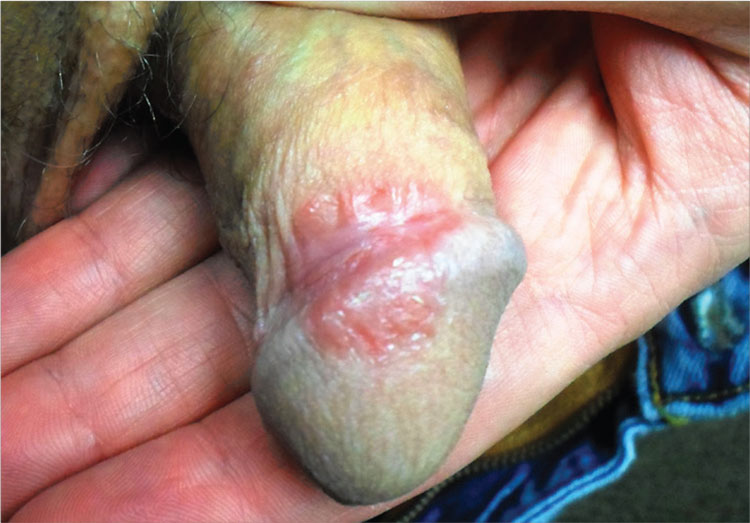
Diagnosis: Seborrheic dermatitis (SD), also known as seborrhea, is an extremely common chronic papulosquamous disorder patterned on the sebum-rich areas of the scalp, face, and trunk. Although not directly caused by the highly lipophilic commensal yeast Malassezia furfur, it does appear to be related to increases in the number of those organisms, as well as to immunologic abnormalities and increased production of sebum. It can range from a mild scaly rash to whole-body erythroderma and can affect an astonishing range of areas, including the genitals.
SD, especially in this case, represents the perfect example of the need to “look elsewhere” for clues when confronted with a mysterious rash. Patients can certainly have more than one dermatologic diagnosis at a time, but a single explanation is considerably more likely and should therefore be sought. In this case, corroboration for the diagnosis of SD was readily found by looking for it in its known locations.
In this case, treatment comprised a combination of oxiconazole lotion and 2.5% hydrocortisone cream. Many other combinations have been used successfully, including pimecrolimus or tacrolimus combined with ketoconazole cream.
Whatever is used, a cure will not be forthcoming, since the condition is almost always chronic. The main value of an accurate diagnosis in such a case lies in easing the patient’s mind regarding the terrible diseases he doesn’t have.
For more information, see “Relatively Asymptomatic, but Still Problematic." Clinician Reviews. 2014 April;24(4):15-16.
4. This circumscribed inflammatory plaque on the glans penis of allegedly > 20 years' duration was refractory to circumcision and local treatment. Because of unresponsiveness of the lesion to circumcision and focal steroid infiltration, repeated biopsies were performed.
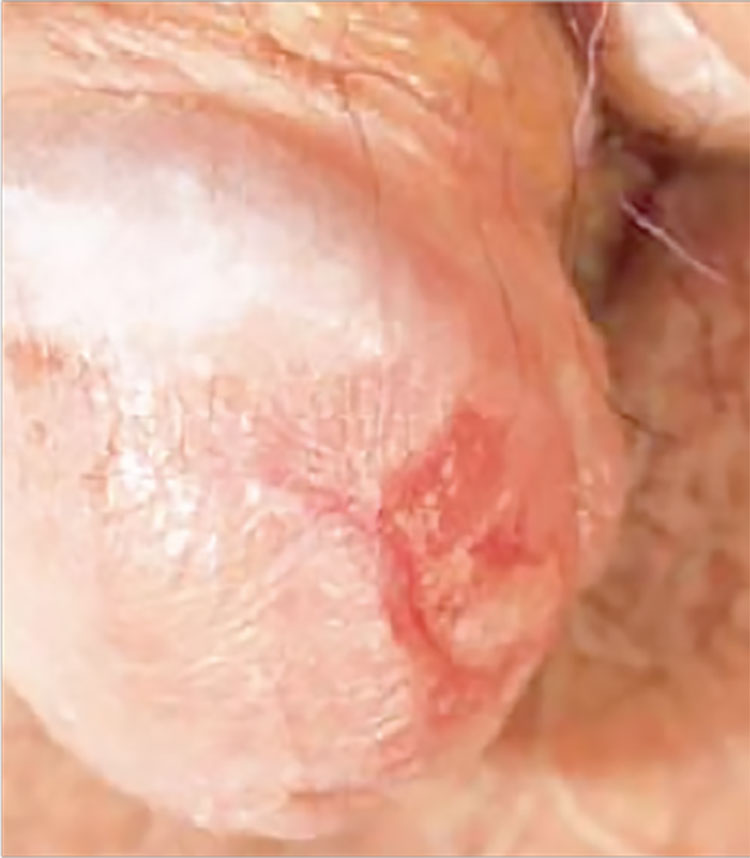
Diagnosis: One biopsy again showed the features of a plasmacellular inflammation, while the other finally revealed the histopathologic features of erythroplasia of Queyrat (carcinoma in situ or Bowen's disease of the glans penis). We assume that either the former biopsy specimens were taken from a plasma cell-rich reactive infiltrate around the neoplastic lesion, or that carcinoma in situ may have arisen due to the chronic inflammation of Zoon's balanitis plasmacellularis. Radiotherapy was performed with good clinical response and subsequent histopathologic proof of complete remission of the lesion.
For more information, see “Bowen's Disease of the Glans Penis (Erythroplasia of Queyrat) in Plasma Cell Balanitis.” Cutis. 2000 June;65(6):395-398.
1. A 26-year-old man presents with a penile lesion that has existed at least 10 years without significant change and with no attendant symptoms. The lesion consists of four 1-to-1.5-mm soft, compressible purple papules, in aggregate measuring about 8 mm. No other lesions are seen on the genitals or on the body.

Diagnosis: The lesion proved to be angiokeratoma of Fordyce, a totally benign lesion. This type of angiokeratoma is ectatic, thin-walled vessels in the superficial dermis, with overlying epidermal hyperplasia that forms secondary to normal friction. When they are seen on the scrotum, vulva, or penis, they are usually referred to as angiokeratoma of Fordyce, a type of localized angiokeratoma, other types of which can appear on the legs or hands.
These totally benign lesions must be distinguished from generalized types of angiokeratomata, such as those seen in Fabry disease (angiokeratoma corporis diffusum), an inheritable metabolic disorder. With our patient’s history, his lesion was clearly benign.
Had his lesion been new or changing in any substantive way, additional testing, including a biopsy, might have been necessary to rule out entities such as squamous cell carcinoma (which is almost unknown in circumcised patients), condyloma, melanoma, the aforementioned Kaposi’s sarcoma, or even angiosarcoma.
For more information, see “Skin is skin, no matter the location.” Clinician Reviews. 2013 June;23(6):W5.
2. A 59-year-old uncircumcised man presents with a phimotic foreskin, which cannot be retracted without pain. Only a tiny opening remains through which the patient can urinate (with difficulty). The surface of the foreskin is atrophic, dry, and shiny with focal areas of purpura but little, if any, redness or swelling.

Diagnosis: This patient’s condition is lichen sclerosus, also called balanitis xerotica obliterans (BXO), a diagnosis of sufficient obscurity to almost guarantee initial misdiagnosis as “yeast infection” or “herpes.” One treatment failure after another eventually leads to referral to a provider familiar with BXO, which is the male version of lichen sclerosus et atrophicus (LS&A) and usually affects the glans, foreskin, and distal shaft.
The causes of these conditions are as yet unknown. However, much is known about how they present, how they look under a microscope, and how to treat them.
Treatment entails use of the most powerful topical steroid ointments, which are so effective that they have completely replaced previous treatment options (eg, testosterone ointment). While a cure is unlikely, control is a realistic goal.
BXO, as in this case, can also cause urinary obstruction, both from the overlying foreskin and from actual meatal stenosis. This is why advanced cases need referral to urology for possible circumcision. As with many penile diagnoses (eg, squamous cell carcinoma or condyloma), BXO is far more common in the uncircumcised.
For more information, see “Man has very uncomfortable problem.” Clinician Reviews. 2012 September;22(9):W4.
3. A 31-year-old man has a relatively asymptomatic penile rash that has repeatedly manifested and resolved over a period of months. A round, papulosquamous, bright pink patch on the distal right shaft of his circumcised penis measures > 3 cm in diameter and has a shiny appearance with slightly irregular margins. A similar rash is seen behind both ears, in the umbilicus, and patches of dandruff are noted. Despite good health, he has been under a great deal of stress recently.

Diagnosis: Seborrheic dermatitis (SD), also known as seborrhea, is an extremely common chronic papulosquamous disorder patterned on the sebum-rich areas of the scalp, face, and trunk. Although not directly caused by the highly lipophilic commensal yeast Malassezia furfur, it does appear to be related to increases in the number of those organisms, as well as to immunologic abnormalities and increased production of sebum. It can range from a mild scaly rash to whole-body erythroderma and can affect an astonishing range of areas, including the genitals.
SD, especially in this case, represents the perfect example of the need to “look elsewhere” for clues when confronted with a mysterious rash. Patients can certainly have more than one dermatologic diagnosis at a time, but a single explanation is considerably more likely and should therefore be sought. In this case, corroboration for the diagnosis of SD was readily found by looking for it in its known locations.
In this case, treatment comprised a combination of oxiconazole lotion and 2.5% hydrocortisone cream. Many other combinations have been used successfully, including pimecrolimus or tacrolimus combined with ketoconazole cream.
Whatever is used, a cure will not be forthcoming, since the condition is almost always chronic. The main value of an accurate diagnosis in such a case lies in easing the patient’s mind regarding the terrible diseases he doesn’t have.
For more information, see “Relatively Asymptomatic, but Still Problematic." Clinician Reviews. 2014 April;24(4):15-16.
4. This circumscribed inflammatory plaque on the glans penis of allegedly > 20 years' duration was refractory to circumcision and local treatment. Because of unresponsiveness of the lesion to circumcision and focal steroid infiltration, repeated biopsies were performed.

Diagnosis: One biopsy again showed the features of a plasmacellular inflammation, while the other finally revealed the histopathologic features of erythroplasia of Queyrat (carcinoma in situ or Bowen's disease of the glans penis). We assume that either the former biopsy specimens were taken from a plasma cell-rich reactive infiltrate around the neoplastic lesion, or that carcinoma in situ may have arisen due to the chronic inflammation of Zoon's balanitis plasmacellularis. Radiotherapy was performed with good clinical response and subsequent histopathologic proof of complete remission of the lesion.
For more information, see “Bowen's Disease of the Glans Penis (Erythroplasia of Queyrat) in Plasma Cell Balanitis.” Cutis. 2000 June;65(6):395-398.
1. A 26-year-old man presents with a penile lesion that has existed at least 10 years without significant change and with no attendant symptoms. The lesion consists of four 1-to-1.5-mm soft, compressible purple papules, in aggregate measuring about 8 mm. No other lesions are seen on the genitals or on the body.

Diagnosis: The lesion proved to be angiokeratoma of Fordyce, a totally benign lesion. This type of angiokeratoma is ectatic, thin-walled vessels in the superficial dermis, with overlying epidermal hyperplasia that forms secondary to normal friction. When they are seen on the scrotum, vulva, or penis, they are usually referred to as angiokeratoma of Fordyce, a type of localized angiokeratoma, other types of which can appear on the legs or hands.
These totally benign lesions must be distinguished from generalized types of angiokeratomata, such as those seen in Fabry disease (angiokeratoma corporis diffusum), an inheritable metabolic disorder. With our patient’s history, his lesion was clearly benign.
Had his lesion been new or changing in any substantive way, additional testing, including a biopsy, might have been necessary to rule out entities such as squamous cell carcinoma (which is almost unknown in circumcised patients), condyloma, melanoma, the aforementioned Kaposi’s sarcoma, or even angiosarcoma.
For more information, see “Skin is skin, no matter the location.” Clinician Reviews. 2013 June;23(6):W5.
2. A 59-year-old uncircumcised man presents with a phimotic foreskin, which cannot be retracted without pain. Only a tiny opening remains through which the patient can urinate (with difficulty). The surface of the foreskin is atrophic, dry, and shiny with focal areas of purpura but little, if any, redness or swelling.

Diagnosis: This patient’s condition is lichen sclerosus, also called balanitis xerotica obliterans (BXO), a diagnosis of sufficient obscurity to almost guarantee initial misdiagnosis as “yeast infection” or “herpes.” One treatment failure after another eventually leads to referral to a provider familiar with BXO, which is the male version of lichen sclerosus et atrophicus (LS&A) and usually affects the glans, foreskin, and distal shaft.
The causes of these conditions are as yet unknown. However, much is known about how they present, how they look under a microscope, and how to treat them.
Treatment entails use of the most powerful topical steroid ointments, which are so effective that they have completely replaced previous treatment options (eg, testosterone ointment). While a cure is unlikely, control is a realistic goal.
BXO, as in this case, can also cause urinary obstruction, both from the overlying foreskin and from actual meatal stenosis. This is why advanced cases need referral to urology for possible circumcision. As with many penile diagnoses (eg, squamous cell carcinoma or condyloma), BXO is far more common in the uncircumcised.
For more information, see “Man has very uncomfortable problem.” Clinician Reviews. 2012 September;22(9):W4.
3. A 31-year-old man has a relatively asymptomatic penile rash that has repeatedly manifested and resolved over a period of months. A round, papulosquamous, bright pink patch on the distal right shaft of his circumcised penis measures > 3 cm in diameter and has a shiny appearance with slightly irregular margins. A similar rash is seen behind both ears, in the umbilicus, and patches of dandruff are noted. Despite good health, he has been under a great deal of stress recently.

Diagnosis: Seborrheic dermatitis (SD), also known as seborrhea, is an extremely common chronic papulosquamous disorder patterned on the sebum-rich areas of the scalp, face, and trunk. Although not directly caused by the highly lipophilic commensal yeast Malassezia furfur, it does appear to be related to increases in the number of those organisms, as well as to immunologic abnormalities and increased production of sebum. It can range from a mild scaly rash to whole-body erythroderma and can affect an astonishing range of areas, including the genitals.
SD, especially in this case, represents the perfect example of the need to “look elsewhere” for clues when confronted with a mysterious rash. Patients can certainly have more than one dermatologic diagnosis at a time, but a single explanation is considerably more likely and should therefore be sought. In this case, corroboration for the diagnosis of SD was readily found by looking for it in its known locations.
In this case, treatment comprised a combination of oxiconazole lotion and 2.5% hydrocortisone cream. Many other combinations have been used successfully, including pimecrolimus or tacrolimus combined with ketoconazole cream.
Whatever is used, a cure will not be forthcoming, since the condition is almost always chronic. The main value of an accurate diagnosis in such a case lies in easing the patient’s mind regarding the terrible diseases he doesn’t have.
For more information, see “Relatively Asymptomatic, but Still Problematic." Clinician Reviews. 2014 April;24(4):15-16.
4. This circumscribed inflammatory plaque on the glans penis of allegedly > 20 years' duration was refractory to circumcision and local treatment. Because of unresponsiveness of the lesion to circumcision and focal steroid infiltration, repeated biopsies were performed.

Diagnosis: One biopsy again showed the features of a plasmacellular inflammation, while the other finally revealed the histopathologic features of erythroplasia of Queyrat (carcinoma in situ or Bowen's disease of the glans penis). We assume that either the former biopsy specimens were taken from a plasma cell-rich reactive infiltrate around the neoplastic lesion, or that carcinoma in situ may have arisen due to the chronic inflammation of Zoon's balanitis plasmacellularis. Radiotherapy was performed with good clinical response and subsequent histopathologic proof of complete remission of the lesion.
For more information, see “Bowen's Disease of the Glans Penis (Erythroplasia of Queyrat) in Plasma Cell Balanitis.” Cutis. 2000 June;65(6):395-398.
NSAIDs and cardiovascular risk
Clinical question: Which NSAID confers the least cardiovascular risk?
Background: Nonsteroidal anti-inflammatory drugs (NSAIDs) reduce pain and inflammation by decreasing prostaglandin production through cyclo-oxygenase (COX) inhibition. However, the COX enzyme stimulates protective prostaglandins for the GI mucosa. The development of cyclo-oxygenase-2 (COX-2) inhibitors did reduce the gastrointestinal side effects of NSAIDs, but subsequent trials pointed to increased cardiovascular events and resulted in rofecoxib being removed from the market. Celecoxib remained on the market as the only COX-2 inhibitor, but the Food and Drug Administration required a cardiovascular safety trial.
Study design: Prospective randomized, double-blind noninferiority study.
Setting: International (926 centers in 13 countries).
Synopsis: The PRECISION trial enrolled 24,081 patients who required NSAIDs for arthritis pain and who either had established cardiovascular disease or an increased risk of CV disease. Patients were randomized to celecoxib 100 mg twice a day, ibuprofen 600 mg three times a day, or naproxen 375 mg twice a day. Patients were allowed to continue taking low-dose aspirin (325 mg or less daily). The primary composite outcome was cardiovascular death (including hemorrhagic death), nonfatal myocardial infarction, or nonfatal stroke. A secondary composite outcome was major adverse CV events (the primary outcome plus coronary revascularization or hospitalization for unstable angina or transient ischemic attack) and significant gastrointestinal events.
The primary outcome occurred in 188 patients in the celecoxib group (2.3%), 201 in the naproxen group (2.5%), and 218 in the ibuprofen group (2.7%). Celecoxib, as compared with either naproxen or ibuprofen, met all four prespecified noninferiority requirements (P less than .01 for noninferiority in both comparisons. Ibuprofen, as compared with naproxen, just met the noninferiority criteria (P = .025).
Bottom line: The PRECISION trial provides statistically strong evidence that the cardiovascular risk associated with moderate doses of celecoxib is not greater than that associated with nonselective NSAIDs (ibuprofen and naproxen).
Citations: Nissen SE, Yeomans ND, Solomon DH, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis.” N Engl J M. 2016;375(26):2519-2529.
Dr. Cerceo is an assistant professor in the Division of Hospital Medicine, and associate director of the internal medicine residency program at Cooper Medical School of Rowan University, Camden, N.J.
Clinical question: Which NSAID confers the least cardiovascular risk?
Background: Nonsteroidal anti-inflammatory drugs (NSAIDs) reduce pain and inflammation by decreasing prostaglandin production through cyclo-oxygenase (COX) inhibition. However, the COX enzyme stimulates protective prostaglandins for the GI mucosa. The development of cyclo-oxygenase-2 (COX-2) inhibitors did reduce the gastrointestinal side effects of NSAIDs, but subsequent trials pointed to increased cardiovascular events and resulted in rofecoxib being removed from the market. Celecoxib remained on the market as the only COX-2 inhibitor, but the Food and Drug Administration required a cardiovascular safety trial.
Study design: Prospective randomized, double-blind noninferiority study.
Setting: International (926 centers in 13 countries).
Synopsis: The PRECISION trial enrolled 24,081 patients who required NSAIDs for arthritis pain and who either had established cardiovascular disease or an increased risk of CV disease. Patients were randomized to celecoxib 100 mg twice a day, ibuprofen 600 mg three times a day, or naproxen 375 mg twice a day. Patients were allowed to continue taking low-dose aspirin (325 mg or less daily). The primary composite outcome was cardiovascular death (including hemorrhagic death), nonfatal myocardial infarction, or nonfatal stroke. A secondary composite outcome was major adverse CV events (the primary outcome plus coronary revascularization or hospitalization for unstable angina or transient ischemic attack) and significant gastrointestinal events.
The primary outcome occurred in 188 patients in the celecoxib group (2.3%), 201 in the naproxen group (2.5%), and 218 in the ibuprofen group (2.7%). Celecoxib, as compared with either naproxen or ibuprofen, met all four prespecified noninferiority requirements (P less than .01 for noninferiority in both comparisons. Ibuprofen, as compared with naproxen, just met the noninferiority criteria (P = .025).
Bottom line: The PRECISION trial provides statistically strong evidence that the cardiovascular risk associated with moderate doses of celecoxib is not greater than that associated with nonselective NSAIDs (ibuprofen and naproxen).
Citations: Nissen SE, Yeomans ND, Solomon DH, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis.” N Engl J M. 2016;375(26):2519-2529.
Dr. Cerceo is an assistant professor in the Division of Hospital Medicine, and associate director of the internal medicine residency program at Cooper Medical School of Rowan University, Camden, N.J.
Clinical question: Which NSAID confers the least cardiovascular risk?
Background: Nonsteroidal anti-inflammatory drugs (NSAIDs) reduce pain and inflammation by decreasing prostaglandin production through cyclo-oxygenase (COX) inhibition. However, the COX enzyme stimulates protective prostaglandins for the GI mucosa. The development of cyclo-oxygenase-2 (COX-2) inhibitors did reduce the gastrointestinal side effects of NSAIDs, but subsequent trials pointed to increased cardiovascular events and resulted in rofecoxib being removed from the market. Celecoxib remained on the market as the only COX-2 inhibitor, but the Food and Drug Administration required a cardiovascular safety trial.
Study design: Prospective randomized, double-blind noninferiority study.
Setting: International (926 centers in 13 countries).
Synopsis: The PRECISION trial enrolled 24,081 patients who required NSAIDs for arthritis pain and who either had established cardiovascular disease or an increased risk of CV disease. Patients were randomized to celecoxib 100 mg twice a day, ibuprofen 600 mg three times a day, or naproxen 375 mg twice a day. Patients were allowed to continue taking low-dose aspirin (325 mg or less daily). The primary composite outcome was cardiovascular death (including hemorrhagic death), nonfatal myocardial infarction, or nonfatal stroke. A secondary composite outcome was major adverse CV events (the primary outcome plus coronary revascularization or hospitalization for unstable angina or transient ischemic attack) and significant gastrointestinal events.
The primary outcome occurred in 188 patients in the celecoxib group (2.3%), 201 in the naproxen group (2.5%), and 218 in the ibuprofen group (2.7%). Celecoxib, as compared with either naproxen or ibuprofen, met all four prespecified noninferiority requirements (P less than .01 for noninferiority in both comparisons. Ibuprofen, as compared with naproxen, just met the noninferiority criteria (P = .025).
Bottom line: The PRECISION trial provides statistically strong evidence that the cardiovascular risk associated with moderate doses of celecoxib is not greater than that associated with nonselective NSAIDs (ibuprofen and naproxen).
Citations: Nissen SE, Yeomans ND, Solomon DH, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis.” N Engl J M. 2016;375(26):2519-2529.
Dr. Cerceo is an assistant professor in the Division of Hospital Medicine, and associate director of the internal medicine residency program at Cooper Medical School of Rowan University, Camden, N.J.
Palliative care: a systematic review for patients and their caregivers
Clinical question: What is the association of palliative care programs on quality of life, symptoms, and survival for patients and their caregivers?
Background: Palliative care programs have expanded across the country: More than 65% of U.S. hospitals have such a program. Efforts have been made to assess their effectiveness for terminally ill patients and their caregivers.
Study design: Systematic review and meta-analysis of 43 randomized controlled trials.
Setting: Not applicable
Synopsis: Two reviewers independently assessed 43 trials (12,731 patients and 2,479 caregivers) with the main outcomes being quality of life, symptom burden, survival, mood, advance care planning, site of death, health care satisfaction, resource utilization, and health care expenditures. Estimates of QOL were translated to units of the Functional Assessment of Chronic Illness Therapy–palliative care scale (FACIT-Pal) instrument and symptom burden was translated into the Edmonton Symptom Assessment Scale (ESAS). Palliative care was associated with statistically and clinically significant improvements in patient QOL at the 1- to 3-month follow-up (standardized mean difference, 0.46; 95% CI, 0.08-0.83; FACIT-Pal mean difference, 11.36) and symptom burden at the 1- to 3-month follow-up (standardized mean difference, −0.66; 95% CI, −1.25 to −0.07; ESAS mean difference, −10.30).
When analyses were limited to trials at low risk of bias (n = 5), the association between palliative care and QOL was attenuated but remained statistically significant (standardized mean difference, 0.20; 95% CI, 0.06-0.34; FACIT-Pal mean difference, 4.94), whereas the association with symptom burden was no longer statistically significant (standardized mean difference, −0.21; 95% CI, −0.42-0.00; ESAS mean difference, −3.28). Caregiver outcomes were mixed but with limited quality of evidence.
Bottom line: Although there was no significant association between palliative care and survival, palliative interventions were associated with improved patient QOL, patient and caregiver satisfaction, lower health care utilization, and symptom burden.
Citations: Kavalieratos D, Corbelli J, Zhang D, et al. Association between palliative care and patient and caregiver outcomes: A systematic review and meta-analysis. JAMA. 2016;316(20):2104-2114. doi: 10.1001/jama.2016.16840
Dr. Cerceo is an assistant professor in the Division of Hospital Medicine, and associate director of the internal medicine residency program at Cooper Medical School of Rowan University, Camden, N.J.
Clinical question: What is the association of palliative care programs on quality of life, symptoms, and survival for patients and their caregivers?
Background: Palliative care programs have expanded across the country: More than 65% of U.S. hospitals have such a program. Efforts have been made to assess their effectiveness for terminally ill patients and their caregivers.
Study design: Systematic review and meta-analysis of 43 randomized controlled trials.
Setting: Not applicable
Synopsis: Two reviewers independently assessed 43 trials (12,731 patients and 2,479 caregivers) with the main outcomes being quality of life, symptom burden, survival, mood, advance care planning, site of death, health care satisfaction, resource utilization, and health care expenditures. Estimates of QOL were translated to units of the Functional Assessment of Chronic Illness Therapy–palliative care scale (FACIT-Pal) instrument and symptom burden was translated into the Edmonton Symptom Assessment Scale (ESAS). Palliative care was associated with statistically and clinically significant improvements in patient QOL at the 1- to 3-month follow-up (standardized mean difference, 0.46; 95% CI, 0.08-0.83; FACIT-Pal mean difference, 11.36) and symptom burden at the 1- to 3-month follow-up (standardized mean difference, −0.66; 95% CI, −1.25 to −0.07; ESAS mean difference, −10.30).
When analyses were limited to trials at low risk of bias (n = 5), the association between palliative care and QOL was attenuated but remained statistically significant (standardized mean difference, 0.20; 95% CI, 0.06-0.34; FACIT-Pal mean difference, 4.94), whereas the association with symptom burden was no longer statistically significant (standardized mean difference, −0.21; 95% CI, −0.42-0.00; ESAS mean difference, −3.28). Caregiver outcomes were mixed but with limited quality of evidence.
Bottom line: Although there was no significant association between palliative care and survival, palliative interventions were associated with improved patient QOL, patient and caregiver satisfaction, lower health care utilization, and symptom burden.
Citations: Kavalieratos D, Corbelli J, Zhang D, et al. Association between palliative care and patient and caregiver outcomes: A systematic review and meta-analysis. JAMA. 2016;316(20):2104-2114. doi: 10.1001/jama.2016.16840
Dr. Cerceo is an assistant professor in the Division of Hospital Medicine, and associate director of the internal medicine residency program at Cooper Medical School of Rowan University, Camden, N.J.
Clinical question: What is the association of palliative care programs on quality of life, symptoms, and survival for patients and their caregivers?
Background: Palliative care programs have expanded across the country: More than 65% of U.S. hospitals have such a program. Efforts have been made to assess their effectiveness for terminally ill patients and their caregivers.
Study design: Systematic review and meta-analysis of 43 randomized controlled trials.
Setting: Not applicable
Synopsis: Two reviewers independently assessed 43 trials (12,731 patients and 2,479 caregivers) with the main outcomes being quality of life, symptom burden, survival, mood, advance care planning, site of death, health care satisfaction, resource utilization, and health care expenditures. Estimates of QOL were translated to units of the Functional Assessment of Chronic Illness Therapy–palliative care scale (FACIT-Pal) instrument and symptom burden was translated into the Edmonton Symptom Assessment Scale (ESAS). Palliative care was associated with statistically and clinically significant improvements in patient QOL at the 1- to 3-month follow-up (standardized mean difference, 0.46; 95% CI, 0.08-0.83; FACIT-Pal mean difference, 11.36) and symptom burden at the 1- to 3-month follow-up (standardized mean difference, −0.66; 95% CI, −1.25 to −0.07; ESAS mean difference, −10.30).
When analyses were limited to trials at low risk of bias (n = 5), the association between palliative care and QOL was attenuated but remained statistically significant (standardized mean difference, 0.20; 95% CI, 0.06-0.34; FACIT-Pal mean difference, 4.94), whereas the association with symptom burden was no longer statistically significant (standardized mean difference, −0.21; 95% CI, −0.42-0.00; ESAS mean difference, −3.28). Caregiver outcomes were mixed but with limited quality of evidence.
Bottom line: Although there was no significant association between palliative care and survival, palliative interventions were associated with improved patient QOL, patient and caregiver satisfaction, lower health care utilization, and symptom burden.
Citations: Kavalieratos D, Corbelli J, Zhang D, et al. Association between palliative care and patient and caregiver outcomes: A systematic review and meta-analysis. JAMA. 2016;316(20):2104-2114. doi: 10.1001/jama.2016.16840
Dr. Cerceo is an assistant professor in the Division of Hospital Medicine, and associate director of the internal medicine residency program at Cooper Medical School of Rowan University, Camden, N.J.
Esophageal retractor found handy tool in AF ablation
ORLANDO – A relatively simple mechanical tool to move the esophagus away from the energy delivered during ablation of atrial fibrillation (AF) does what it is supposed to do, according to data from a multicenter observational study presented at the AF Symposium 2017.
When esophageal temperature during the ablation procedure was monitored in 101 consecutive cases, no recording exceeded 38° C, according to Valay Parikh, MD, a clinical cardiac electrophysiology fellow working under Dhanunjaya Lakkireddy, MD, at the Kansas University Medical Center, Kansas City.
The tool is a stylet constructed from a nickel-titanium (nitinol) alloy. Malleable at room temperature, the stylet is inserted into an 18 Fr orogastric (OG) tube. Firmer at body temperature, the stylet within the OG tube is maneuvered to displace the esophagus away from the adjacent left atrium when radiofrequency ablation (RFA) is being administered.
The tool, marketed under the brand name EsoSure, was first made available almost 2 years ago, but the recently completed multicenter observational study was conducted to provide a more systematic evaluation of its safety and efficacy in routine use. In this study, 101 consecutive patients scheduled for RFA for AF had their esophagus displaced by the stylet during the procedure. The temperature of the esophagus as well as any adverse events involving the upper gastrointestinal tract were evaluated during the procedure. Patients were then followed for at least 6 months.
“The principal finding of our study is that mechanical displacement of the esophagus with the help of the EsoSure device is safe and provides sufficient room to deliver the intended energy at the site of ablation without any rise in temperature over 38° C,” Dr. Parikh reported.
The mean age of the 101 patients who participated in this study was 65 years. About half were female. The mean body mass index (BMI) was 32 kg/m2. The mean CHA2DS2-VASc score was 2.4. Barium x-rays were used to confirm esophageal displacement.
After the procedure, patients were discharged on a proton pump inhibitor and sucralfate, which inhibits pepsin activity and protects against ulceration. Follow-up endoscopy was performed only when medically indicated, but all patients were evaluated over the course of follow-up for odynophagia, dysphagia, hematemesis, dyspepsia, and other GI symptoms.
Pulmonary vein isolation (PVI) was achieved successfully in all cases. Although there was a rapid temperature rise at the site of ablation over the course of RFA, the esophagus was adequately displaced from the source of energy, as confirmed with the absence of significant temperature rises in this tissue. The mean esophageal displacement was 2.53 cm.
The only complication in this series, occurring in 7% of patients, was dysphagia. All cases of dysphagia developed immediately or soon after the procedure. All were mild, and all resolved within several days. There were no late GI complications observed, although Dr. Parikh acknowledged that no follow-up endoscopy was performed to rule out any esophageal injury.
In contrast, without the deviation permitted by the esophageal retractor, the rapid rise in temperature “could have precluded PVI,” Dr. Parikh maintained. He said that the retractor permitted the esophagus to be cleared from potential injury, as indicated by the lack of a temperature rise in esophageal tissue, in 100% of the cases. He noted that the tool is now in routine use at his center.
According to Dr. Lakkireddy, this tool is already in routine use at several centers across the country. The goal of this study was to provide an objective documentation of the ability of the device to enable successful posterior wall isolation during PVI without esophageal injury.
“It helped us get to the endpoint without a problem 100% of the time,” Dr. Lakkireddy reported.
Dr. Parikh has no industry relationships relevant to this study.
ORLANDO – A relatively simple mechanical tool to move the esophagus away from the energy delivered during ablation of atrial fibrillation (AF) does what it is supposed to do, according to data from a multicenter observational study presented at the AF Symposium 2017.
When esophageal temperature during the ablation procedure was monitored in 101 consecutive cases, no recording exceeded 38° C, according to Valay Parikh, MD, a clinical cardiac electrophysiology fellow working under Dhanunjaya Lakkireddy, MD, at the Kansas University Medical Center, Kansas City.
The tool is a stylet constructed from a nickel-titanium (nitinol) alloy. Malleable at room temperature, the stylet is inserted into an 18 Fr orogastric (OG) tube. Firmer at body temperature, the stylet within the OG tube is maneuvered to displace the esophagus away from the adjacent left atrium when radiofrequency ablation (RFA) is being administered.
The tool, marketed under the brand name EsoSure, was first made available almost 2 years ago, but the recently completed multicenter observational study was conducted to provide a more systematic evaluation of its safety and efficacy in routine use. In this study, 101 consecutive patients scheduled for RFA for AF had their esophagus displaced by the stylet during the procedure. The temperature of the esophagus as well as any adverse events involving the upper gastrointestinal tract were evaluated during the procedure. Patients were then followed for at least 6 months.
“The principal finding of our study is that mechanical displacement of the esophagus with the help of the EsoSure device is safe and provides sufficient room to deliver the intended energy at the site of ablation without any rise in temperature over 38° C,” Dr. Parikh reported.
The mean age of the 101 patients who participated in this study was 65 years. About half were female. The mean body mass index (BMI) was 32 kg/m2. The mean CHA2DS2-VASc score was 2.4. Barium x-rays were used to confirm esophageal displacement.
After the procedure, patients were discharged on a proton pump inhibitor and sucralfate, which inhibits pepsin activity and protects against ulceration. Follow-up endoscopy was performed only when medically indicated, but all patients were evaluated over the course of follow-up for odynophagia, dysphagia, hematemesis, dyspepsia, and other GI symptoms.
Pulmonary vein isolation (PVI) was achieved successfully in all cases. Although there was a rapid temperature rise at the site of ablation over the course of RFA, the esophagus was adequately displaced from the source of energy, as confirmed with the absence of significant temperature rises in this tissue. The mean esophageal displacement was 2.53 cm.
The only complication in this series, occurring in 7% of patients, was dysphagia. All cases of dysphagia developed immediately or soon after the procedure. All were mild, and all resolved within several days. There were no late GI complications observed, although Dr. Parikh acknowledged that no follow-up endoscopy was performed to rule out any esophageal injury.
In contrast, without the deviation permitted by the esophageal retractor, the rapid rise in temperature “could have precluded PVI,” Dr. Parikh maintained. He said that the retractor permitted the esophagus to be cleared from potential injury, as indicated by the lack of a temperature rise in esophageal tissue, in 100% of the cases. He noted that the tool is now in routine use at his center.
According to Dr. Lakkireddy, this tool is already in routine use at several centers across the country. The goal of this study was to provide an objective documentation of the ability of the device to enable successful posterior wall isolation during PVI without esophageal injury.
“It helped us get to the endpoint without a problem 100% of the time,” Dr. Lakkireddy reported.
Dr. Parikh has no industry relationships relevant to this study.
ORLANDO – A relatively simple mechanical tool to move the esophagus away from the energy delivered during ablation of atrial fibrillation (AF) does what it is supposed to do, according to data from a multicenter observational study presented at the AF Symposium 2017.
When esophageal temperature during the ablation procedure was monitored in 101 consecutive cases, no recording exceeded 38° C, according to Valay Parikh, MD, a clinical cardiac electrophysiology fellow working under Dhanunjaya Lakkireddy, MD, at the Kansas University Medical Center, Kansas City.
The tool is a stylet constructed from a nickel-titanium (nitinol) alloy. Malleable at room temperature, the stylet is inserted into an 18 Fr orogastric (OG) tube. Firmer at body temperature, the stylet within the OG tube is maneuvered to displace the esophagus away from the adjacent left atrium when radiofrequency ablation (RFA) is being administered.
The tool, marketed under the brand name EsoSure, was first made available almost 2 years ago, but the recently completed multicenter observational study was conducted to provide a more systematic evaluation of its safety and efficacy in routine use. In this study, 101 consecutive patients scheduled for RFA for AF had their esophagus displaced by the stylet during the procedure. The temperature of the esophagus as well as any adverse events involving the upper gastrointestinal tract were evaluated during the procedure. Patients were then followed for at least 6 months.
“The principal finding of our study is that mechanical displacement of the esophagus with the help of the EsoSure device is safe and provides sufficient room to deliver the intended energy at the site of ablation without any rise in temperature over 38° C,” Dr. Parikh reported.
The mean age of the 101 patients who participated in this study was 65 years. About half were female. The mean body mass index (BMI) was 32 kg/m2. The mean CHA2DS2-VASc score was 2.4. Barium x-rays were used to confirm esophageal displacement.
After the procedure, patients were discharged on a proton pump inhibitor and sucralfate, which inhibits pepsin activity and protects against ulceration. Follow-up endoscopy was performed only when medically indicated, but all patients were evaluated over the course of follow-up for odynophagia, dysphagia, hematemesis, dyspepsia, and other GI symptoms.
Pulmonary vein isolation (PVI) was achieved successfully in all cases. Although there was a rapid temperature rise at the site of ablation over the course of RFA, the esophagus was adequately displaced from the source of energy, as confirmed with the absence of significant temperature rises in this tissue. The mean esophageal displacement was 2.53 cm.
The only complication in this series, occurring in 7% of patients, was dysphagia. All cases of dysphagia developed immediately or soon after the procedure. All were mild, and all resolved within several days. There were no late GI complications observed, although Dr. Parikh acknowledged that no follow-up endoscopy was performed to rule out any esophageal injury.
In contrast, without the deviation permitted by the esophageal retractor, the rapid rise in temperature “could have precluded PVI,” Dr. Parikh maintained. He said that the retractor permitted the esophagus to be cleared from potential injury, as indicated by the lack of a temperature rise in esophageal tissue, in 100% of the cases. He noted that the tool is now in routine use at his center.
According to Dr. Lakkireddy, this tool is already in routine use at several centers across the country. The goal of this study was to provide an objective documentation of the ability of the device to enable successful posterior wall isolation during PVI without esophageal injury.
“It helped us get to the endpoint without a problem 100% of the time,” Dr. Lakkireddy reported.
Dr. Parikh has no industry relationships relevant to this study.
AT THE INTERNATIONAL AF SYMPOSIUM
Key clinical point: The efficacy of a tool to move the esophagus out of the way when performing ablation for atrial fibrillation is supported by a multicenter study.
Major finding: In 101 consecutive cases at four centers, all ablations were completed successfully with no esophageal temperature rise.
Data source: Prospective observational study.
Disclosures: Dr. Parikh has no industry relationships relevant to this study.
C. auris forms biofilms, enhancing its virulence, resistance
The emerging multidrug-resistant yeast organism Candida auris forms biofilms that enhance both its resistance and its virulence, according to in vitro analyses.
C. auris first attracted attention in 2009 because of its resistance to azoles and amphotericin B. Since then, it has been identified as the cause of life-threatening invasive infections worldwide, including hospital outbreaks across Asia and South America, wrote Leighann Sherry, PhD, a medical mycologist at the University of Glasgow, and her associates.
To determine whether the pathogen has the capacity to form biofilms, the investigators propagated several different strains in the laboratory and examined their development. In three separate trials, eight samples of each strain grew biofilms, which constitute “a key driver of candida pathogenicity.” In antifungal susceptibility tests, caspofungin was completely ineffective, an unexpected finding because the agent usually is highly effective against other candida species. Amphotericin B, liposomal amphotericin B, and fluconazole also were ineffective; micafungin and chlorhexidine were the most effective at clearing C. auris.
Biofilm formation “contributes not only to C. auris virulence but also to its [resistance] in hospital environments, increasing its ability to cause outbreaks,” Dr. Sherry and her associates said (Emerg. Infect. Dis. 2017 Feb;23[2]:328-31).
“Our findings suggest it is improbable that the spread and prevalence of C. auris can be controlled with antifungal stewardship approaches alone,” they noted, adding that “infection-prevention measures targeting C. auris biofilms in patients, on medical devices, and in the hospital environment will be required,” they noted.
The emerging multidrug-resistant yeast organism Candida auris forms biofilms that enhance both its resistance and its virulence, according to in vitro analyses.
C. auris first attracted attention in 2009 because of its resistance to azoles and amphotericin B. Since then, it has been identified as the cause of life-threatening invasive infections worldwide, including hospital outbreaks across Asia and South America, wrote Leighann Sherry, PhD, a medical mycologist at the University of Glasgow, and her associates.
To determine whether the pathogen has the capacity to form biofilms, the investigators propagated several different strains in the laboratory and examined their development. In three separate trials, eight samples of each strain grew biofilms, which constitute “a key driver of candida pathogenicity.” In antifungal susceptibility tests, caspofungin was completely ineffective, an unexpected finding because the agent usually is highly effective against other candida species. Amphotericin B, liposomal amphotericin B, and fluconazole also were ineffective; micafungin and chlorhexidine were the most effective at clearing C. auris.
Biofilm formation “contributes not only to C. auris virulence but also to its [resistance] in hospital environments, increasing its ability to cause outbreaks,” Dr. Sherry and her associates said (Emerg. Infect. Dis. 2017 Feb;23[2]:328-31).
“Our findings suggest it is improbable that the spread and prevalence of C. auris can be controlled with antifungal stewardship approaches alone,” they noted, adding that “infection-prevention measures targeting C. auris biofilms in patients, on medical devices, and in the hospital environment will be required,” they noted.
The emerging multidrug-resistant yeast organism Candida auris forms biofilms that enhance both its resistance and its virulence, according to in vitro analyses.
C. auris first attracted attention in 2009 because of its resistance to azoles and amphotericin B. Since then, it has been identified as the cause of life-threatening invasive infections worldwide, including hospital outbreaks across Asia and South America, wrote Leighann Sherry, PhD, a medical mycologist at the University of Glasgow, and her associates.
To determine whether the pathogen has the capacity to form biofilms, the investigators propagated several different strains in the laboratory and examined their development. In three separate trials, eight samples of each strain grew biofilms, which constitute “a key driver of candida pathogenicity.” In antifungal susceptibility tests, caspofungin was completely ineffective, an unexpected finding because the agent usually is highly effective against other candida species. Amphotericin B, liposomal amphotericin B, and fluconazole also were ineffective; micafungin and chlorhexidine were the most effective at clearing C. auris.
Biofilm formation “contributes not only to C. auris virulence but also to its [resistance] in hospital environments, increasing its ability to cause outbreaks,” Dr. Sherry and her associates said (Emerg. Infect. Dis. 2017 Feb;23[2]:328-31).
“Our findings suggest it is improbable that the spread and prevalence of C. auris can be controlled with antifungal stewardship approaches alone,” they noted, adding that “infection-prevention measures targeting C. auris biofilms in patients, on medical devices, and in the hospital environment will be required,” they noted.
FROM EMERGING INFECTIOUS DISEASES
Key clinical point: The emerging multidrug-resistant yeast organism Candida auris forms biofilms that enhance both its resistance and its virulence.
Major finding: In three separate trials, eight samples of each strain of C. auris grew biofilms that constitute “a key driver of Candida pathogenicity.”
Data source: In-vitro analyses of C. auris growth.
Disclosures: No funding source(s) or financial disclosures were provided.
VIDEO: Dual antibiotic prophylaxis cuts cesarean SSIs
LAS VEGAS – Two days of prophylaxis with two oral antibiotics cut the surgical site infection rate by more than half in a randomized trial with more than 400 obese women who had cesarean deliveries.
The protective effect from combined treatment with cephalexin and metronidazole was especially powerful in the most at-risk patients, women with ruptured membranes before cesarean surgery. In this subgroup prophylaxis with the two antibiotics for 2 days cut surgical site infections (SSIs) during the 30 days after surgery, from a rate of 33% in control women who received placebo to a 10% rate, a 77% relative risk reduction that was statistically significant, Carri R. Warshak, MD, said at the annual Pregnancy Meeting sponsored by the Society for Maternal and Fetal Medicine.
“I am very excited that we found a way to help the kinds of women in the study, very-high-risk women, with an effective way to reduce their risk of infection,” Dr. Warshak of the University of Cincinnati said in a video interview. The obese women enrolled in the study, especially those with ruptured membranes, “have a very high risk of morbidity, so it’s very exciting that we found a way to help prevent” SSIs.
“Our study is the first to target postpartum interventions to reduce SSIs specifically in this high-risk population” of obese mothers, said Amy M. Valent, DO, a maternal fetal medicine clinician at Oregon Health & Science University in Portland, who ran the trial with Dr. Warshak.
The trial randomized women with a body mass index of at least 30 kg/m2 who underwent a planned or unplanned cesarean delivery at the University of Cincinnati during 2010-2015. Following standard management during cesarean delivery, the women received either 500 mg oral cephalexin and 500 mg oral metronidazole or placebo every 8 hours for 48 hours following delivery. The primary outcome was the incidence of SSIs, and randomization was stratified so that similar numbers of women with ruptured membranes got into each treatment arm. The enrolled women averaged 28 years of age, and average BMI was about 40 kg/m2. Nearly a third of the women had ruptured membranes at the time of surgery, more than a quarter of the enrolled women used tobacco, and more than a fifth had preeclampsia.
Additional analyses reported at the meeting by Dr. Valent showed that other risk factors that significantly boosted the rate of SSIs were labor prior to delivery, use of internal monitoring, and operative time of more than 90 minutes. Antibiotic prophylaxis was able to significantly reduce SSI rates in women with any of these additional risk factors, compared with placebo. A cost effectiveness analysis she ran estimated that if the antibiotic prophylaxis tested in the study were used on the roughly 460,000 obese U.S. women having cesarean deliveries annually, it would be cost saving as long as the antibiotic regimen cost no more than $357 a person. Factoring in the SSIs and long-term morbidity that prophylaxis would prevent, and the quality-adjusted life-years it would add, showed that prophylaxis would be cost-effective up to a cost of $33,557 per woman.
The prophylaxis carries a “relatively low cost and is easy to use,” Dr. Valent said.
Safety of the antibiotic combination was a question raised by Laura E. Riley, MD, director of ob.gyn. infectious disease and labor and delivery at Massachusetts General Hospital in Boston. “My biggest concern is 48 hours of these antibiotics,” and whether prophylaxis could be achieved with fewer doses, she said in an interview. “I’d want to minimize the dosage, and also try other, nondrug approaches to minimizing SSI risk in obese women.”
“I wouldn’t say that universally, every obstetrical program should do this, but clinicians should look at the comorbidities their mothers have and their SSI rates. There are populations out there at lower risk, but there are also populations like ours, with a SSI rate of 10%-20%,” Dr. Warshak said.
She also acknowledged that even her own obstetrical group in Cincinnati needs to now reach a consensus on an appropriate strategy for expanded cesarean-delivery prophylaxis. That’s because a 2016 report from a large, randomized trial documented another successful strategy for limiting infections following cesarean delivery: a preoperative intravenous dose of azithromycin as a supplement to standard cefazolin. The Cesarean Section Optimal Antibiotic Prophylaxis (C/SOAP) trial, done in women with any BMI but specifically nonelective cesarean deliveries, showed a significant reduction in the combined rate of SSIs, endometritis, or any other infection during 6 weeks of follow-up among women who received azithromycin on top of standard prophylaxis (N Engl J Med. 2016 Sept 29;375[13]:1231-41).
“The bottom line is that, a couple of grams of cefazolin [administered before the incision] isn’t enough, especially for women with risk factors for infection. We see infection rates of more than 10% because cefazolin alone is simply inadequate. The results from both our study and the 2016 study show we can do better to reduce morbidity,” said Dr. Warshak.
“In high-risk women, such as those who are obese, we probably need to expand the spectrum and duration of prophylaxis,” agreed Dr. Main. “Obesity is one high-risk group, but there are others.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
LAS VEGAS – Two days of prophylaxis with two oral antibiotics cut the surgical site infection rate by more than half in a randomized trial with more than 400 obese women who had cesarean deliveries.
The protective effect from combined treatment with cephalexin and metronidazole was especially powerful in the most at-risk patients, women with ruptured membranes before cesarean surgery. In this subgroup prophylaxis with the two antibiotics for 2 days cut surgical site infections (SSIs) during the 30 days after surgery, from a rate of 33% in control women who received placebo to a 10% rate, a 77% relative risk reduction that was statistically significant, Carri R. Warshak, MD, said at the annual Pregnancy Meeting sponsored by the Society for Maternal and Fetal Medicine.
“I am very excited that we found a way to help the kinds of women in the study, very-high-risk women, with an effective way to reduce their risk of infection,” Dr. Warshak of the University of Cincinnati said in a video interview. The obese women enrolled in the study, especially those with ruptured membranes, “have a very high risk of morbidity, so it’s very exciting that we found a way to help prevent” SSIs.
“Our study is the first to target postpartum interventions to reduce SSIs specifically in this high-risk population” of obese mothers, said Amy M. Valent, DO, a maternal fetal medicine clinician at Oregon Health & Science University in Portland, who ran the trial with Dr. Warshak.
The trial randomized women with a body mass index of at least 30 kg/m2 who underwent a planned or unplanned cesarean delivery at the University of Cincinnati during 2010-2015. Following standard management during cesarean delivery, the women received either 500 mg oral cephalexin and 500 mg oral metronidazole or placebo every 8 hours for 48 hours following delivery. The primary outcome was the incidence of SSIs, and randomization was stratified so that similar numbers of women with ruptured membranes got into each treatment arm. The enrolled women averaged 28 years of age, and average BMI was about 40 kg/m2. Nearly a third of the women had ruptured membranes at the time of surgery, more than a quarter of the enrolled women used tobacco, and more than a fifth had preeclampsia.
Additional analyses reported at the meeting by Dr. Valent showed that other risk factors that significantly boosted the rate of SSIs were labor prior to delivery, use of internal monitoring, and operative time of more than 90 minutes. Antibiotic prophylaxis was able to significantly reduce SSI rates in women with any of these additional risk factors, compared with placebo. A cost effectiveness analysis she ran estimated that if the antibiotic prophylaxis tested in the study were used on the roughly 460,000 obese U.S. women having cesarean deliveries annually, it would be cost saving as long as the antibiotic regimen cost no more than $357 a person. Factoring in the SSIs and long-term morbidity that prophylaxis would prevent, and the quality-adjusted life-years it would add, showed that prophylaxis would be cost-effective up to a cost of $33,557 per woman.
The prophylaxis carries a “relatively low cost and is easy to use,” Dr. Valent said.
Safety of the antibiotic combination was a question raised by Laura E. Riley, MD, director of ob.gyn. infectious disease and labor and delivery at Massachusetts General Hospital in Boston. “My biggest concern is 48 hours of these antibiotics,” and whether prophylaxis could be achieved with fewer doses, she said in an interview. “I’d want to minimize the dosage, and also try other, nondrug approaches to minimizing SSI risk in obese women.”
“I wouldn’t say that universally, every obstetrical program should do this, but clinicians should look at the comorbidities their mothers have and their SSI rates. There are populations out there at lower risk, but there are also populations like ours, with a SSI rate of 10%-20%,” Dr. Warshak said.
She also acknowledged that even her own obstetrical group in Cincinnati needs to now reach a consensus on an appropriate strategy for expanded cesarean-delivery prophylaxis. That’s because a 2016 report from a large, randomized trial documented another successful strategy for limiting infections following cesarean delivery: a preoperative intravenous dose of azithromycin as a supplement to standard cefazolin. The Cesarean Section Optimal Antibiotic Prophylaxis (C/SOAP) trial, done in women with any BMI but specifically nonelective cesarean deliveries, showed a significant reduction in the combined rate of SSIs, endometritis, or any other infection during 6 weeks of follow-up among women who received azithromycin on top of standard prophylaxis (N Engl J Med. 2016 Sept 29;375[13]:1231-41).
“The bottom line is that, a couple of grams of cefazolin [administered before the incision] isn’t enough, especially for women with risk factors for infection. We see infection rates of more than 10% because cefazolin alone is simply inadequate. The results from both our study and the 2016 study show we can do better to reduce morbidity,” said Dr. Warshak.
“In high-risk women, such as those who are obese, we probably need to expand the spectrum and duration of prophylaxis,” agreed Dr. Main. “Obesity is one high-risk group, but there are others.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
LAS VEGAS – Two days of prophylaxis with two oral antibiotics cut the surgical site infection rate by more than half in a randomized trial with more than 400 obese women who had cesarean deliveries.
The protective effect from combined treatment with cephalexin and metronidazole was especially powerful in the most at-risk patients, women with ruptured membranes before cesarean surgery. In this subgroup prophylaxis with the two antibiotics for 2 days cut surgical site infections (SSIs) during the 30 days after surgery, from a rate of 33% in control women who received placebo to a 10% rate, a 77% relative risk reduction that was statistically significant, Carri R. Warshak, MD, said at the annual Pregnancy Meeting sponsored by the Society for Maternal and Fetal Medicine.
“I am very excited that we found a way to help the kinds of women in the study, very-high-risk women, with an effective way to reduce their risk of infection,” Dr. Warshak of the University of Cincinnati said in a video interview. The obese women enrolled in the study, especially those with ruptured membranes, “have a very high risk of morbidity, so it’s very exciting that we found a way to help prevent” SSIs.
“Our study is the first to target postpartum interventions to reduce SSIs specifically in this high-risk population” of obese mothers, said Amy M. Valent, DO, a maternal fetal medicine clinician at Oregon Health & Science University in Portland, who ran the trial with Dr. Warshak.
The trial randomized women with a body mass index of at least 30 kg/m2 who underwent a planned or unplanned cesarean delivery at the University of Cincinnati during 2010-2015. Following standard management during cesarean delivery, the women received either 500 mg oral cephalexin and 500 mg oral metronidazole or placebo every 8 hours for 48 hours following delivery. The primary outcome was the incidence of SSIs, and randomization was stratified so that similar numbers of women with ruptured membranes got into each treatment arm. The enrolled women averaged 28 years of age, and average BMI was about 40 kg/m2. Nearly a third of the women had ruptured membranes at the time of surgery, more than a quarter of the enrolled women used tobacco, and more than a fifth had preeclampsia.
Additional analyses reported at the meeting by Dr. Valent showed that other risk factors that significantly boosted the rate of SSIs were labor prior to delivery, use of internal monitoring, and operative time of more than 90 minutes. Antibiotic prophylaxis was able to significantly reduce SSI rates in women with any of these additional risk factors, compared with placebo. A cost effectiveness analysis she ran estimated that if the antibiotic prophylaxis tested in the study were used on the roughly 460,000 obese U.S. women having cesarean deliveries annually, it would be cost saving as long as the antibiotic regimen cost no more than $357 a person. Factoring in the SSIs and long-term morbidity that prophylaxis would prevent, and the quality-adjusted life-years it would add, showed that prophylaxis would be cost-effective up to a cost of $33,557 per woman.
The prophylaxis carries a “relatively low cost and is easy to use,” Dr. Valent said.
Safety of the antibiotic combination was a question raised by Laura E. Riley, MD, director of ob.gyn. infectious disease and labor and delivery at Massachusetts General Hospital in Boston. “My biggest concern is 48 hours of these antibiotics,” and whether prophylaxis could be achieved with fewer doses, she said in an interview. “I’d want to minimize the dosage, and also try other, nondrug approaches to minimizing SSI risk in obese women.”
“I wouldn’t say that universally, every obstetrical program should do this, but clinicians should look at the comorbidities their mothers have and their SSI rates. There are populations out there at lower risk, but there are also populations like ours, with a SSI rate of 10%-20%,” Dr. Warshak said.
She also acknowledged that even her own obstetrical group in Cincinnati needs to now reach a consensus on an appropriate strategy for expanded cesarean-delivery prophylaxis. That’s because a 2016 report from a large, randomized trial documented another successful strategy for limiting infections following cesarean delivery: a preoperative intravenous dose of azithromycin as a supplement to standard cefazolin. The Cesarean Section Optimal Antibiotic Prophylaxis (C/SOAP) trial, done in women with any BMI but specifically nonelective cesarean deliveries, showed a significant reduction in the combined rate of SSIs, endometritis, or any other infection during 6 weeks of follow-up among women who received azithromycin on top of standard prophylaxis (N Engl J Med. 2016 Sept 29;375[13]:1231-41).
“The bottom line is that, a couple of grams of cefazolin [administered before the incision] isn’t enough, especially for women with risk factors for infection. We see infection rates of more than 10% because cefazolin alone is simply inadequate. The results from both our study and the 2016 study show we can do better to reduce morbidity,” said Dr. Warshak.
“In high-risk women, such as those who are obese, we probably need to expand the spectrum and duration of prophylaxis,” agreed Dr. Main. “Obesity is one high-risk group, but there are others.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
AT THE PREGNANCY MEETING
Key clinical point:
Major finding: Surgical site infections occurred in 7% of women who received oral prophylaxis and 16% of controls during 30-day follow-up.
Data source: A single-center randomized trial with 382 evaluable women.
Disclosures: Dr. Warshak had no relevant disclosures.
Eradicate HCV in patients with HIV, regardless of fibrosis stage
Eradicating hepatitis C virus in HIV-coinfected patients was associated with a significantly lower risk of diabetes mellitus and possibly chronic renal failure, along with lower rates of deaths, HIV progression, and liver-related events, according to an observational study of 1,625 patients.
“These findings argue for the prescription of HCV therapy regardless of liver fibrosis stage in coinfected patients,” Juan Berenguer, MD, PhD, and his associates at Hospital General Universitario Gregario Marañón, Madrid. Extrahepatic manifestations of HCV infection are numerous and contribute substantially to morbidity and mortality. “To the best of our knowledge, the effect of eradication of HCV on extrahepatic manifestations of HCV has not been systematically studied in HIV/HCV-coinfected patients,” the researchers wrote in Hepatology (Hepatology 2017 Jan 21:doi:10.1002/hep.29071 [Epub ahead of print]).
After a median of 5 years of follow-up, SVR was associated with a 43% decrease in the likelihood of developing diabetes, even after controlling for a host of potential confounders, including Fibrosis-4 score (using 3.25 as the cutoff value), age, sex, history of AIDS, HIV-transmission category, nadir CD4+ T-cell count, antiretroviral therapy, HIV-RNA, HCV genotype, and exposure to specific anti-HIV drugs (adjusted hazard ratio, 0.57; 95% confidence interval, 0.35 to 0.93; P = .02). Sustained viral response also was associated with a lower likelihood of chronic renal failure with borderline statistical significance (aHR, 0.43; 95% CI, 0.17 to 1.09; P = .075).
“In agreement with previous reports from this cohort, we found that treatment response was associated with a decreased hazard of overall and liver-related death, all types of liver-related events, and new AIDS-related conditions,” the researchers noted. These findings underscore the vital importance of HCV therapy for HIV-coinfected patients, regardless of liver fibrosis stage, they emphasized.
The study was funded by Spanish Health Research Funds and AIDS Research Network. The investigators reported having no relevant conflicts of interest.
Eradicating hepatitis C virus in HIV-coinfected patients was associated with a significantly lower risk of diabetes mellitus and possibly chronic renal failure, along with lower rates of deaths, HIV progression, and liver-related events, according to an observational study of 1,625 patients.
“These findings argue for the prescription of HCV therapy regardless of liver fibrosis stage in coinfected patients,” Juan Berenguer, MD, PhD, and his associates at Hospital General Universitario Gregario Marañón, Madrid. Extrahepatic manifestations of HCV infection are numerous and contribute substantially to morbidity and mortality. “To the best of our knowledge, the effect of eradication of HCV on extrahepatic manifestations of HCV has not been systematically studied in HIV/HCV-coinfected patients,” the researchers wrote in Hepatology (Hepatology 2017 Jan 21:doi:10.1002/hep.29071 [Epub ahead of print]).
After a median of 5 years of follow-up, SVR was associated with a 43% decrease in the likelihood of developing diabetes, even after controlling for a host of potential confounders, including Fibrosis-4 score (using 3.25 as the cutoff value), age, sex, history of AIDS, HIV-transmission category, nadir CD4+ T-cell count, antiretroviral therapy, HIV-RNA, HCV genotype, and exposure to specific anti-HIV drugs (adjusted hazard ratio, 0.57; 95% confidence interval, 0.35 to 0.93; P = .02). Sustained viral response also was associated with a lower likelihood of chronic renal failure with borderline statistical significance (aHR, 0.43; 95% CI, 0.17 to 1.09; P = .075).
“In agreement with previous reports from this cohort, we found that treatment response was associated with a decreased hazard of overall and liver-related death, all types of liver-related events, and new AIDS-related conditions,” the researchers noted. These findings underscore the vital importance of HCV therapy for HIV-coinfected patients, regardless of liver fibrosis stage, they emphasized.
The study was funded by Spanish Health Research Funds and AIDS Research Network. The investigators reported having no relevant conflicts of interest.
Eradicating hepatitis C virus in HIV-coinfected patients was associated with a significantly lower risk of diabetes mellitus and possibly chronic renal failure, along with lower rates of deaths, HIV progression, and liver-related events, according to an observational study of 1,625 patients.
“These findings argue for the prescription of HCV therapy regardless of liver fibrosis stage in coinfected patients,” Juan Berenguer, MD, PhD, and his associates at Hospital General Universitario Gregario Marañón, Madrid. Extrahepatic manifestations of HCV infection are numerous and contribute substantially to morbidity and mortality. “To the best of our knowledge, the effect of eradication of HCV on extrahepatic manifestations of HCV has not been systematically studied in HIV/HCV-coinfected patients,” the researchers wrote in Hepatology (Hepatology 2017 Jan 21:doi:10.1002/hep.29071 [Epub ahead of print]).
After a median of 5 years of follow-up, SVR was associated with a 43% decrease in the likelihood of developing diabetes, even after controlling for a host of potential confounders, including Fibrosis-4 score (using 3.25 as the cutoff value), age, sex, history of AIDS, HIV-transmission category, nadir CD4+ T-cell count, antiretroviral therapy, HIV-RNA, HCV genotype, and exposure to specific anti-HIV drugs (adjusted hazard ratio, 0.57; 95% confidence interval, 0.35 to 0.93; P = .02). Sustained viral response also was associated with a lower likelihood of chronic renal failure with borderline statistical significance (aHR, 0.43; 95% CI, 0.17 to 1.09; P = .075).
“In agreement with previous reports from this cohort, we found that treatment response was associated with a decreased hazard of overall and liver-related death, all types of liver-related events, and new AIDS-related conditions,” the researchers noted. These findings underscore the vital importance of HCV therapy for HIV-coinfected patients, regardless of liver fibrosis stage, they emphasized.
The study was funded by Spanish Health Research Funds and AIDS Research Network. The investigators reported having no relevant conflicts of interest.
FROM HEPATOLOGY
Key clinical point. Eradicating hepatitis C virus infection in HIV-coinfected patients is crucial, regardless of fibrosis stage.
Major finding: After a median of 5 years of follow-up, sustained viral response to HCV treatment was associated with a 43% decrease in the likelihood of developing diabetes, even after controlling for fibrosis stage and other potential confounders. Reaching SVR also was associated with decreased rates of renal failure, HIV progression, and mortality.
Data source: An observational study of 1,625 patients with HIV and hepatitis C virus coinfection.
Disclosures: The study was funded by Spanish Health Research Funds and AIDS Research Network. The investigators reported having no relevant conflicts of interest.
Study highlights importance of genotyping in von Willebrand disease
Patients with genetically confirmed von Willebrand disease (VWD) type 2M have a relatively mild clinical phenotype, according to findings from a retrospective cross-sectional study.
In fact, three of 31 patients included in the study had a near normal laboratory phenotype. Additionally, patients with the p.Val1360Ala mutation had significantly higher values of all von Willebrand factor (VWF)-related laboratory parameters, compared with those with the p.Arg1374Cys or p.Phe1293Leu mutation, Dominique Maas reported at the European Association for Haemophilia and Allied Disorders annual meeting.
The findings underscore the importance of genotyping for making a correct diagnosis of VWD type 2M, said Ms. Maas of Radboud University Medical Center, Nijmegen, The Netherlands.
The study subjects, who had a least one VWD type 2M mutation, had a median age of 34 years and a median bleeding score of 6. Most suffered from mucocutaneous bleeding, but with low frequency compared with other VWD subtypes. The incidence of muscle hematomas, postpartum hemorrhage, and postsurgery bleeding were relatively high, however. Age and bleeding score were strongly positively correlated.
Subjects had a median VWF antigen level of 24 IU dL-1, median VWF ristocetin cofactor activity of 6 IU dL-1, and median VSF:RCo/VWF:Ag ratio of 0.29. The VSF collagen binding activity and VWF:Ag ratio was normal, she said.
Genotyping is a powerful diagnostic tool for making an appropriate diagnosis and classification of VWD. The current findings are important because understanding the correlation between genotype and phenotype improves the understanding of VWD pathogenesis and has important implications for treatment, follow-up, and genetic counseling, but has not been throughly investigated in fully genotyped patients with VWD type 2M, she said.
Ms. Maas reported having no disclosures.
Patients with genetically confirmed von Willebrand disease (VWD) type 2M have a relatively mild clinical phenotype, according to findings from a retrospective cross-sectional study.
In fact, three of 31 patients included in the study had a near normal laboratory phenotype. Additionally, patients with the p.Val1360Ala mutation had significantly higher values of all von Willebrand factor (VWF)-related laboratory parameters, compared with those with the p.Arg1374Cys or p.Phe1293Leu mutation, Dominique Maas reported at the European Association for Haemophilia and Allied Disorders annual meeting.
The findings underscore the importance of genotyping for making a correct diagnosis of VWD type 2M, said Ms. Maas of Radboud University Medical Center, Nijmegen, The Netherlands.
The study subjects, who had a least one VWD type 2M mutation, had a median age of 34 years and a median bleeding score of 6. Most suffered from mucocutaneous bleeding, but with low frequency compared with other VWD subtypes. The incidence of muscle hematomas, postpartum hemorrhage, and postsurgery bleeding were relatively high, however. Age and bleeding score were strongly positively correlated.
Subjects had a median VWF antigen level of 24 IU dL-1, median VWF ristocetin cofactor activity of 6 IU dL-1, and median VSF:RCo/VWF:Ag ratio of 0.29. The VSF collagen binding activity and VWF:Ag ratio was normal, she said.
Genotyping is a powerful diagnostic tool for making an appropriate diagnosis and classification of VWD. The current findings are important because understanding the correlation between genotype and phenotype improves the understanding of VWD pathogenesis and has important implications for treatment, follow-up, and genetic counseling, but has not been throughly investigated in fully genotyped patients with VWD type 2M, she said.
Ms. Maas reported having no disclosures.
Patients with genetically confirmed von Willebrand disease (VWD) type 2M have a relatively mild clinical phenotype, according to findings from a retrospective cross-sectional study.
In fact, three of 31 patients included in the study had a near normal laboratory phenotype. Additionally, patients with the p.Val1360Ala mutation had significantly higher values of all von Willebrand factor (VWF)-related laboratory parameters, compared with those with the p.Arg1374Cys or p.Phe1293Leu mutation, Dominique Maas reported at the European Association for Haemophilia and Allied Disorders annual meeting.
The findings underscore the importance of genotyping for making a correct diagnosis of VWD type 2M, said Ms. Maas of Radboud University Medical Center, Nijmegen, The Netherlands.
The study subjects, who had a least one VWD type 2M mutation, had a median age of 34 years and a median bleeding score of 6. Most suffered from mucocutaneous bleeding, but with low frequency compared with other VWD subtypes. The incidence of muscle hematomas, postpartum hemorrhage, and postsurgery bleeding were relatively high, however. Age and bleeding score were strongly positively correlated.
Subjects had a median VWF antigen level of 24 IU dL-1, median VWF ristocetin cofactor activity of 6 IU dL-1, and median VSF:RCo/VWF:Ag ratio of 0.29. The VSF collagen binding activity and VWF:Ag ratio was normal, she said.
Genotyping is a powerful diagnostic tool for making an appropriate diagnosis and classification of VWD. The current findings are important because understanding the correlation between genotype and phenotype improves the understanding of VWD pathogenesis and has important implications for treatment, follow-up, and genetic counseling, but has not been throughly investigated in fully genotyped patients with VWD type 2M, she said.
Ms. Maas reported having no disclosures.
FROM EAHAD 2017
Key clinical point:
Major finding: Three of 31 patients included in the study had a near normal laboratory phenotype.
Data source: A retrospective cross-sectional study of 31 patients.
Disclosures: Ms. Maas reported having no disclosures.
Solulin variants activate TAFI in vitro
A new generation of solulin variants is showing promise for the treatment of severe hemophilia A.
The in vitro production and characterization of these solulin variants – F376A-, M388A-, and F376A/M388A-solulin – showed that while they lost their abilities to activate protein C (an inhibitor of thrombin generation), they were still capable of activating thrombin activatable fibrinolysis inhibitor (TAFI), Yohann Repesse, PhD reported at the annual meeting of the European Association for Haemophilia and Allied Disorders.
Additionally, the double mutant F376A/M388A-solulin, which was tested ex vivo using blood samples from hemophilic A patients, was found on thromboelastography to increase clot firmness and stability. As opposed to wild type solulin, the effects were maintained even at high concentrations of F376A/M388A-solulin, said Dr. Repesse of the National Institute of Health and Medical Research, Caen, France.
An important aggravating factor when it comes to treating hemophilia involves premature fibrinolysis, which means that antifibrinolytics are of interest, he explained. Thrombomodulin is a key player in the coagulation cascade, because it activates protein C, and also in the fibrinolytic cascade, as it activates TAFI. Solulin, a soluble form of thrombomodulin, has both capabilities.
The findings with respect to the new generation of solulin variants tested in this study open new opportunities for the development of specific medications for hemophilia patients, he concluded.
Dr. Repesse reported having no disclosures.
A new generation of solulin variants is showing promise for the treatment of severe hemophilia A.
The in vitro production and characterization of these solulin variants – F376A-, M388A-, and F376A/M388A-solulin – showed that while they lost their abilities to activate protein C (an inhibitor of thrombin generation), they were still capable of activating thrombin activatable fibrinolysis inhibitor (TAFI), Yohann Repesse, PhD reported at the annual meeting of the European Association for Haemophilia and Allied Disorders.
Additionally, the double mutant F376A/M388A-solulin, which was tested ex vivo using blood samples from hemophilic A patients, was found on thromboelastography to increase clot firmness and stability. As opposed to wild type solulin, the effects were maintained even at high concentrations of F376A/M388A-solulin, said Dr. Repesse of the National Institute of Health and Medical Research, Caen, France.
An important aggravating factor when it comes to treating hemophilia involves premature fibrinolysis, which means that antifibrinolytics are of interest, he explained. Thrombomodulin is a key player in the coagulation cascade, because it activates protein C, and also in the fibrinolytic cascade, as it activates TAFI. Solulin, a soluble form of thrombomodulin, has both capabilities.
The findings with respect to the new generation of solulin variants tested in this study open new opportunities for the development of specific medications for hemophilia patients, he concluded.
Dr. Repesse reported having no disclosures.
A new generation of solulin variants is showing promise for the treatment of severe hemophilia A.
The in vitro production and characterization of these solulin variants – F376A-, M388A-, and F376A/M388A-solulin – showed that while they lost their abilities to activate protein C (an inhibitor of thrombin generation), they were still capable of activating thrombin activatable fibrinolysis inhibitor (TAFI), Yohann Repesse, PhD reported at the annual meeting of the European Association for Haemophilia and Allied Disorders.
Additionally, the double mutant F376A/M388A-solulin, which was tested ex vivo using blood samples from hemophilic A patients, was found on thromboelastography to increase clot firmness and stability. As opposed to wild type solulin, the effects were maintained even at high concentrations of F376A/M388A-solulin, said Dr. Repesse of the National Institute of Health and Medical Research, Caen, France.
An important aggravating factor when it comes to treating hemophilia involves premature fibrinolysis, which means that antifibrinolytics are of interest, he explained. Thrombomodulin is a key player in the coagulation cascade, because it activates protein C, and also in the fibrinolytic cascade, as it activates TAFI. Solulin, a soluble form of thrombomodulin, has both capabilities.
The findings with respect to the new generation of solulin variants tested in this study open new opportunities for the development of specific medications for hemophilia patients, he concluded.
Dr. Repesse reported having no disclosures.
FROM EAHAD 2017
Key clinical point:
Major finding: F376A-, M388A-, and F376A/M388A-solulin lost their abilities to activate protein C but were still capable of activating TAFI.
Data source: An in vitro and ex vivo evaluation of solulin variants.
Disclosures: Dr. Repesse reported having no disclosures.
Boys with severe hemophilia have good physical function
Children with severe hemophilia in a U.K. study had generally good self-reported health-related quality of life and physical functioning, but impairment on both measures was greater in those who reported pain.
Of 123 boys with a mean age of 12 years who were included in the multicenter SO-FIT study, 110 had hemophilia A, 25 had a past inhibitor, and 9 had a current inhibitor. Prophylactic treatment was given in 120 of the boys, Kate Khair, PhD of Great Ormond Street Hospital for Children, London, reported at the annual meeting of the European Association for Haemophilia and Allied Disorders.
In the preceding 6 months, participants experienced a median of 0 joint bleeds (range of 0-8), and a median Hemophilia Joint Health Score (HJHS) of 1 (range, 0-37). Physical function scores were good; the Haemophilia & Exercise Project questionnaire (HEP-Test-Q) mean score was 80.82, with the highest impairments seen in the domain of “endurance” (mean, 72.21), and the Paediatric Haemophilia Activities List (PedHAL) mean score was 85.13, with the highest impairments seen in the domains of “leisure activities and sports” and “lying/sitting/kneeling/standing” (mean, 82.04 and 82.97, respectively).
Health-related quality of life as assessed by the Haemo-QoL Short Form was also generally good (mean, 23.07), with the highest impairments seen in the domains of “friends” and “family” (mean, 27.08 and 26.59, respectively).
In the 27% of participants with pain, a high correlation was seen between the HEP-Test-Q and Haemo-QoL, and moderate correlation was seen between the PedHAL and Haemo-QoL, which implies that good physical functioning is related to good health-related quality of life, Dr. Khair explained.
In fact, patients who said they often or always had pain reported significantly worse subjective physical functioning in all HEP-Test-Q and PedHAL domains, as well as higher impairments in their HRQoL vs. those who said they sometimes, rarely, or never experienced pain, she said.
Interestingly, data completion by study participants was improved with use of an iPad vs. “paper and pencil” for questionnaire completion, Dr. Khair said in an interview.
“They preferred this method – it was more in keeping with how they communicate and learn at school,” she said, noting that the iPad database was set up to give real time scores, which could then be used to tailor assessments.
This is an approach that could be undertaken at home pre-clinic visits, she said.
“Gaining the views of children and young adults in patient-reported outcomes is complex, but reveals their views of their care and their lives – an area we should address more,” she added.
Dr. Khair received grant or research support from Pfizer ASPIRE.
Children with severe hemophilia in a U.K. study had generally good self-reported health-related quality of life and physical functioning, but impairment on both measures was greater in those who reported pain.
Of 123 boys with a mean age of 12 years who were included in the multicenter SO-FIT study, 110 had hemophilia A, 25 had a past inhibitor, and 9 had a current inhibitor. Prophylactic treatment was given in 120 of the boys, Kate Khair, PhD of Great Ormond Street Hospital for Children, London, reported at the annual meeting of the European Association for Haemophilia and Allied Disorders.
In the preceding 6 months, participants experienced a median of 0 joint bleeds (range of 0-8), and a median Hemophilia Joint Health Score (HJHS) of 1 (range, 0-37). Physical function scores were good; the Haemophilia & Exercise Project questionnaire (HEP-Test-Q) mean score was 80.82, with the highest impairments seen in the domain of “endurance” (mean, 72.21), and the Paediatric Haemophilia Activities List (PedHAL) mean score was 85.13, with the highest impairments seen in the domains of “leisure activities and sports” and “lying/sitting/kneeling/standing” (mean, 82.04 and 82.97, respectively).
Health-related quality of life as assessed by the Haemo-QoL Short Form was also generally good (mean, 23.07), with the highest impairments seen in the domains of “friends” and “family” (mean, 27.08 and 26.59, respectively).
In the 27% of participants with pain, a high correlation was seen between the HEP-Test-Q and Haemo-QoL, and moderate correlation was seen between the PedHAL and Haemo-QoL, which implies that good physical functioning is related to good health-related quality of life, Dr. Khair explained.
In fact, patients who said they often or always had pain reported significantly worse subjective physical functioning in all HEP-Test-Q and PedHAL domains, as well as higher impairments in their HRQoL vs. those who said they sometimes, rarely, or never experienced pain, she said.
Interestingly, data completion by study participants was improved with use of an iPad vs. “paper and pencil” for questionnaire completion, Dr. Khair said in an interview.
“They preferred this method – it was more in keeping with how they communicate and learn at school,” she said, noting that the iPad database was set up to give real time scores, which could then be used to tailor assessments.
This is an approach that could be undertaken at home pre-clinic visits, she said.
“Gaining the views of children and young adults in patient-reported outcomes is complex, but reveals their views of their care and their lives – an area we should address more,” she added.
Dr. Khair received grant or research support from Pfizer ASPIRE.
Children with severe hemophilia in a U.K. study had generally good self-reported health-related quality of life and physical functioning, but impairment on both measures was greater in those who reported pain.
Of 123 boys with a mean age of 12 years who were included in the multicenter SO-FIT study, 110 had hemophilia A, 25 had a past inhibitor, and 9 had a current inhibitor. Prophylactic treatment was given in 120 of the boys, Kate Khair, PhD of Great Ormond Street Hospital for Children, London, reported at the annual meeting of the European Association for Haemophilia and Allied Disorders.
In the preceding 6 months, participants experienced a median of 0 joint bleeds (range of 0-8), and a median Hemophilia Joint Health Score (HJHS) of 1 (range, 0-37). Physical function scores were good; the Haemophilia & Exercise Project questionnaire (HEP-Test-Q) mean score was 80.82, with the highest impairments seen in the domain of “endurance” (mean, 72.21), and the Paediatric Haemophilia Activities List (PedHAL) mean score was 85.13, with the highest impairments seen in the domains of “leisure activities and sports” and “lying/sitting/kneeling/standing” (mean, 82.04 and 82.97, respectively).
Health-related quality of life as assessed by the Haemo-QoL Short Form was also generally good (mean, 23.07), with the highest impairments seen in the domains of “friends” and “family” (mean, 27.08 and 26.59, respectively).
In the 27% of participants with pain, a high correlation was seen between the HEP-Test-Q and Haemo-QoL, and moderate correlation was seen between the PedHAL and Haemo-QoL, which implies that good physical functioning is related to good health-related quality of life, Dr. Khair explained.
In fact, patients who said they often or always had pain reported significantly worse subjective physical functioning in all HEP-Test-Q and PedHAL domains, as well as higher impairments in their HRQoL vs. those who said they sometimes, rarely, or never experienced pain, she said.
Interestingly, data completion by study participants was improved with use of an iPad vs. “paper and pencil” for questionnaire completion, Dr. Khair said in an interview.
“They preferred this method – it was more in keeping with how they communicate and learn at school,” she said, noting that the iPad database was set up to give real time scores, which could then be used to tailor assessments.
This is an approach that could be undertaken at home pre-clinic visits, she said.
“Gaining the views of children and young adults in patient-reported outcomes is complex, but reveals their views of their care and their lives – an area we should address more,” she added.
Dr. Khair received grant or research support from Pfizer ASPIRE.
FROM EAHAD 2017
Key clinical point:
Major finding: Physical function score (HEP-Test-Q) was a mean of 80.82, with the highest impairments seen in the domain of “endurance” (mean, 72.21).
Data source: The SO-FIT study of 123 boys with severe hemophilia.
Disclosures: Dr. Khair received grant or research support from Pfizer ASPIRE.







