User login
Actinomycetoma: An Update on Diagnosis and Treatment
Mycetoma is a subcutaneous disease that can be caused by aerobic bacteria (actinomycetoma) or fungi (eumycetoma). Diagnosis is based on clinical manifestations, including swelling and deformity of affected areas, as well as the presence of granulation tissue, scars, abscesses, sinus tracts, and a purulent exudate that contains the microorganisms.
The worldwide proportion of mycetomas is 60% actinomycetomas and 40% eumycetomas.1 The disease is endemic in tropical, subtropical, and temperate regions, predominating between latitudes 30°N and 15°S. Most cases occur in Africa, especially Sudan, Mauritania, and Senegal; India; Yemen; and Pakistan. In the Americas, the countries with the most reported cases are Mexico and Venezuela.1
Although mycetoma is rare in developed countries, migration of patients from endemic areas makes knowledge of this condition crucial for dermatologists worldwide. We present a review of the current concepts in the epidemiology, clinical presentation, diagnosis, and treatment of actinomycetoma.
Epidemiology
Actinomycetoma is more common in Latin America, with Mexico having the highest incidence. At last count, there were 2631 cases reported in Mexico.2 The majority of cases of mycetoma in Mexico are actinomycetoma (98%), including Nocardia (86%) and Actinomadura madurae (10%). Eumycetoma is rare in Mexico, constituting only 2% of cases.2 Worldwide, men are affected more commonly than women, which is thought to be related to a higher occupational risk during agricultural labor.
Clinical Features
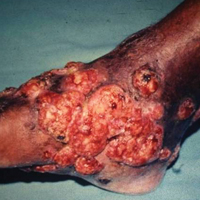
Mycetoma can affect the skin, subcutaneous tissue, bones, and occasionally the internal organs. It is characterized by swelling, deformation of the affected area, and fistulae that drain serosanguineous or purulent exudates.
In Mexico, 60% of cases of mycetoma affect the lower extremities; the feet are the most commonly affected area, followed by the trunk (back and chest), arms, forearms, legs, knees, and thighs.1 Other sites include the hands, shoulders, and abdominal wall. The head and neck area are seldom affected.3 Mycetoma lesions grow and disseminate locally. Bone lesions are possible depending on the osteophilic affinity of the etiological agent and on the interactions between the fungus and the host’s immune system. In severe advanced cases of mycetoma, the lesions may involve tendons and nerves. Dissemination via blood or lymphatics is extremely rare.4
Diagnosis
Diagnosis of actinomycetoma is suspected based on clinical features and confirmed by direct examination of exudates with Lugol iodine or saline solution. On direct microscopy, actinomycetes are recognized by the production of filaments with a width of 0.5 to 1 μm. On hematoxylin and eosin stain, the small grains of Nocardia appear eosinophilic with a blue center and pink filaments. On Gram stain, actinomycetoma grains show positive branching filaments. Culture of grains recovered from aspirated material or biopsy specimens provides specific etiologic diagnosis. Cultures should be held for at least 4 weeks. Additionally, there are some enzymatic, molecular, and serologic tests available for diagnosis.5-7 Serologic diagnosis is available in a few centers in Mexico and can be helpful in some cases for diagnosis or follow-up during treatment. Antibodies can be determined via enzyme-linked immunosorbent assay, Western blot analysis, immunodiffusion, or counterimmunoelectrophoresis.8
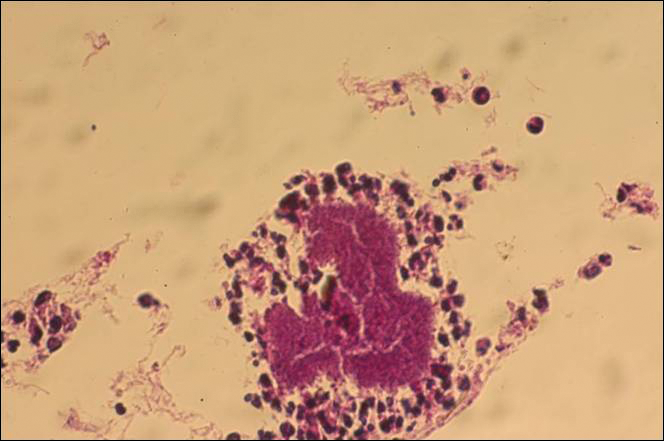
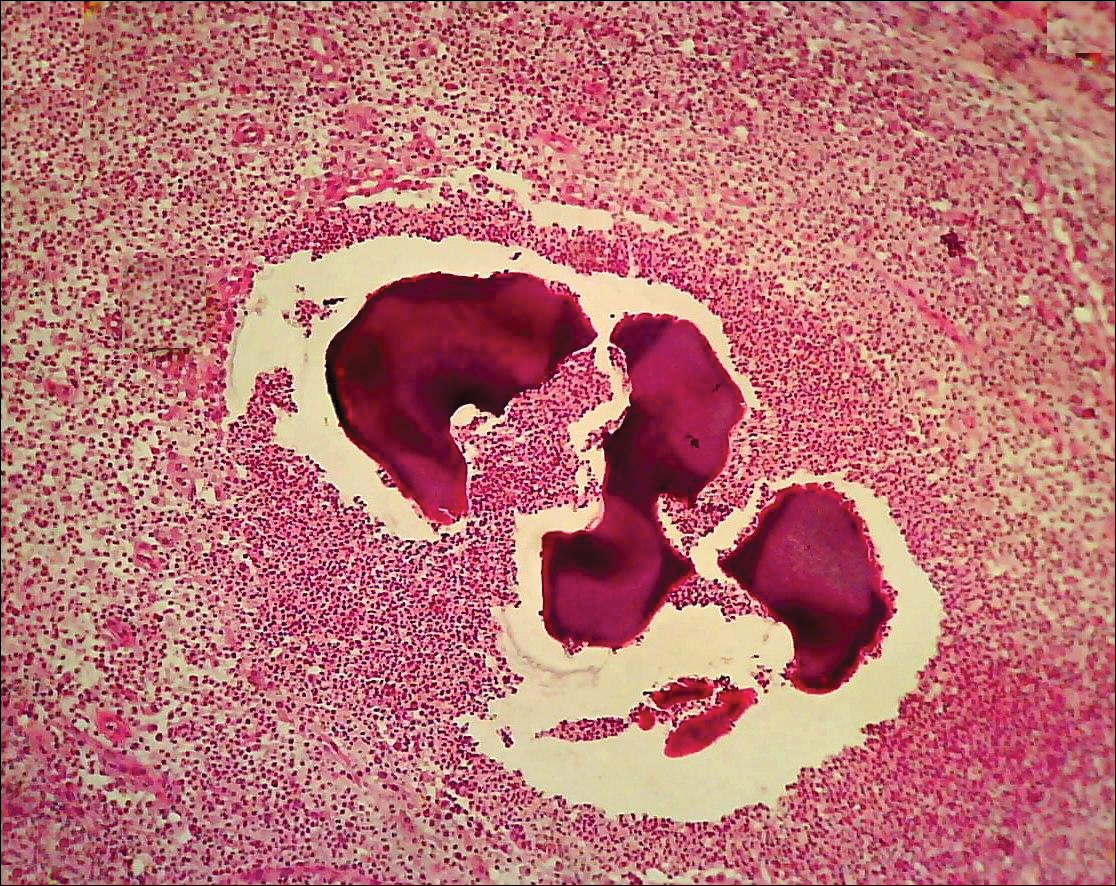
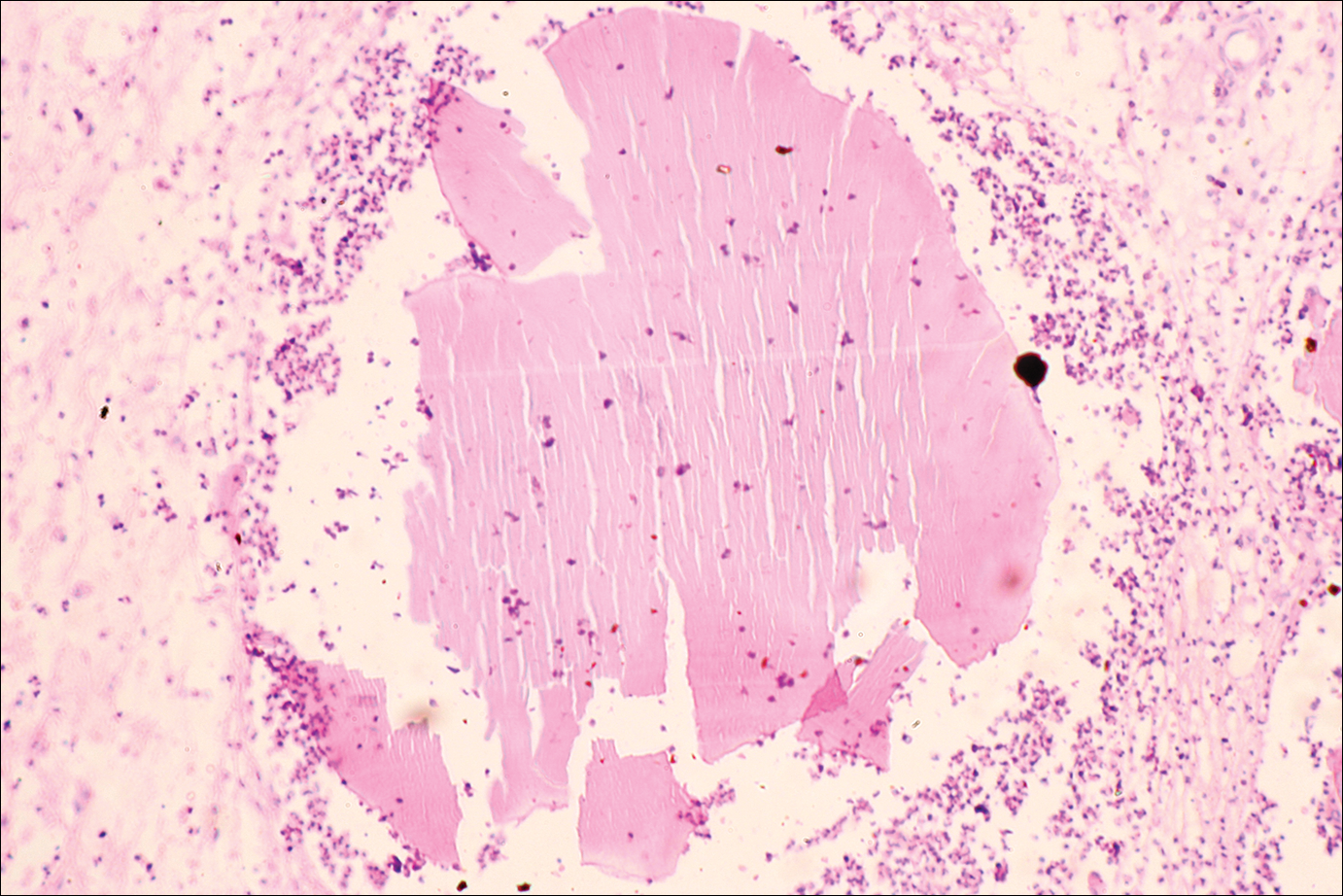
The causative agents of actinomycetoma can be isolated in Sabouraud dextrose agar. Deep wedge biopsies (or puncture aspiration) are useful in observing the diagnostic grains, which can be identified adequately with Gram stain. Grains usually are surrounded and/or infiltrated by neutrophils. The size, form, and color of grains can identify the causative agent.1 The granules of Nocardia are small (80–130 mm) and reniform or wormlike, with club structures in their periphery (Figure 1). Actinomadura madurae is characterized by large, white-yellow granules that can be seen with the naked eye (1–3 mm). On microscopic examination with hematoxylin and eosin stain, these grains are purple and exhibit peripheral pink pseudofilaments (Figure 2).2 The grains of Actinomadura pelletieri are large (1–3 mm) and red or violaceous. They fragment or break easily, giving the appearance of a broken dish (Figure 3). Streptomyces somaliensis forms round grains approximately 0.5 to 1 cm in diameter. These grains stain poorly and are extremely hard. Cutting the grains during processing results in striation, giving them the appearance of a potato chip (Figure 4).2

Treatment of Actinomycetoma
Precise identification of the etiologic agent is essential to provide effective treatment of actinomycetoma. Without treatment, or in resistant cases, progressive osseous and visceral involvement is inevitable.9 Actinomycetoma without osseous involvement usually responds well to medical treatment.
The treatment of choice for actinomycetoma involving Nocardia brasiliensis is a combination of dapsone 100 to 200 mg once daily and trimethoprim-sulfamethoxazole (TMP-SMX) 80/400 to 160/800 mg once daily for 2 to 3 years.10 Other treatments have included the following: (1) amikacin 15 mg/kg or 500 mg intramuscularly twice daily for 3 weeks plus dapsone 100 to 200 mg once daily plus TMP-SMX 80/400 to 160/800 mg daily for 2 to 3 years (amikacin, however, is expensive and potentially toxic [nephrotoxicity and ototoxicity] and therefore is used only in resistant cases); (2) dapsone 100 to 200 mg once daily or TMP-SMX 80/400 to 160/800 mg daily for 2 to 3 years plus intramuscular kanamycin 15 mg/kg once daily for 2 weeks at the beginning of treatment, alternating with rest periods to reduce the risk for nephrotoxicity and ototoxicity10; (3) dapsone 1.5 mg/kg orally twice daily plus phosphomycin 500 mg once daily; (4) dapsone 1.5 mg/kg orally twice daily plus streptomycin 1 g once daily (14 mg/kg/d) for 1 month, then the same dose every other day for 1 to 2 months monitoring for ototoxicity; and (5) TMP-SMX 80/400 to 160/800 mg once daily for 2 to 3 years or rifampicin (15–20 mg/kg/d) plus streptomycin 1 g once daily (14 mg/kg/d) for 1 month at the beginning of treatment, then the same dose every other day for 2 to 3 months until a total dose of 60 g is administered, monitoring for ototoxicity.11 Audiometric tests and creatinine levels must be performed every 5 weeks during the treatment to monitor toxicity.10
The best results for infections with A pelletieri, A madurae, and S somaliensis have been with streptomycin (1 g once daily in adults; 20 mg/kg once daily in children) until a total dose of 50 g is reached in combination with TMP-SMX or dapsone12 (Figure 5). Alternatives for A madurae infections include streptomycin plus oral clofazimine (100 mg once daily), oral rifampicin (300 mg twice daily), oral tetracycline (1 g once daily), oral isoniazid (300–600 mg once daily), or oral minocycline (100 mg twice daily; also effective for A pelletieri).

More recently, other drugs have been used such as carbapenems (eg, imipenem, meropenem), which have wide-spectrum efficacy and are resistant to β-lactamases. Patients should be hospitalized to receive intravenous therapy with imipenem.2 Carbapenems are effective against gram-positive and gram-negative as well as Nocardia species.13,14 Mycetoma that is resistant, severe, or has visceral involvement can be treated with a combination of amikacin and imipenem.15,16 Meropenem is a similar drug that is available as an oral formulation. Both imipenem and meropenem are recommended in cases with bone involvement.17,18 Alternatives for resistant cases include amoxicillin–clavulanic acid 500/125 mg orally 3 times daily for 3 to 6 months or intravenous cefotaxime 1 g every 8 hours plus intramuscular amikacin 500 mg twice daily plus oral levamisole 300 mg once weekly for 4 weeks.19-23
For resistant cases associated with Nocardia species, clindamycin plus quinolones (eg, ciprofloxacin, moxifloxacin, garenoxacin) at a dose of 25 mg/kg once daily for at least 3 months has been suggested in in vivo studies.23
Overall, the cure rate for actinomycetoma treated with any of the prior therapies ranges from 60% to 90%. Treatment must be modified or stopped if there is clinical or laboratory evidence of drug toxicity.13,24 Surgical treatment of actinomycetoma is contraindicated, as it may cause hematogenous dissemination.
Prognosis
Actinomycetomas of a few months’ duration and without bone involvement respond well to therapy. If no therapy is provided or if there is resistance, the functional and cosmetic prognosis is poor, mainly for the feet. There is a risk for spine involvement with mycetoma on the back and posterior head. Thoracic lesions may penetrate into the lungs. The muscular fascia impedes the penetration of abdominal lesions, but the inguinal canals can offer a path for intra-abdominal dissemination.4 Advanced cases lead to a poor general condition of patients, difficulty in using affected extremities, and in extreme cases even death.
The criteria used to guide the discontinuation of initial therapy for any mycetoma include a decrease in the volume of the lesion, closure of fistulae, 3 consecutive negative monthly cultures, imaging studies showing bone regeneration, lack of echoes and cavities on echography, and absence of grains on examination of fine-needle aspirates.11 After the initial treatment protocol is finished, most experts recommend continuing treatment with dapsone 100 to 300 mg once daily for several years to prevent recurrence.12
Prevention
Mycetoma is a disease associated with poverty. It could be prevented by improving living conditions and by regular use of shoes in rural populations.2
Conclusion
Mycetoma is a chronic infection that develops after traumatic inoculation of the skin with either true fungi or aerobic actinomycetes. The resultant infections are known as eumycetoma or actinomycetoma, respectively. The etiologic agents can be found in the so-called grains. Black grains suggest a fungal infection, minute white grains suggest Nocardia, and red grains are due to A pelletieri. Larger white grains or yellow-white grains may be fungal or actinomycotic in origin.
Specific diagnosis requires direct examination, culture, and biopsy. The treatment of choice for actinomycetoma by N brasiliensis is a combination of dapsone 100 to 200 mg once daily and TMP-SMX 80/400 to 160/800 mg once daily for 2 to 3 years. Other effective treatments include aminoglycosides (eg, amikacine, streptomycin) and quinolones. More recently, some other agents have been used such as carbapenems and natural products of Streptomyces cattleya (imipenem), which have wide-spectrum efficacy and are resistant to β-lactamases.
- Welsh O, Vera-Cabrera L, Welsh E, et al. Actinomycetoma and advances in its treatment. Clin Dermatol. 2012;30:372-381.
- Arenas R. Micología Medica Ilustrada. 4th ed. Mexico City, Mexico: McGraw-Hill Interamericana; 2011:125-146.
- McGinnis MR. Mycetoma. Dermatol Clin. 1996;14:97-104.
- Fahal AH. Mycetoma: Clinico-pathological Monograph. Khartoum, Sudan: University of Khartoum Press; 2006:20-23, 81-82.
- Estrada-Chavez GE, Vega-Memije ME, Arenas R, et al. Eumycotic mycetoma caused by Madurella mycetomatis successfully treated with antifungals, surgery, and topical negative pressure therapy. Int J Dermatol. 2009;48:401-403.
- Chávez G, Arenas R, Pérez-Polito A, et al. Eumycetic mycetoma due to Madurella mycetomatis. report of six cases. Rev Iberoam Micol. 1998;15:90-93.
- Vasquez del Mercado E, Arenas R, Moreno G. Sequelae and long-term consequences of systemic and subcutaneous mycoses. In: Fratamico PM, Smith JL, Brogden KA, eds. Sequelae and Long-term Consequences of Infectious Diseases. Washington, DC: ASM Press; 2009:415-420.
- Mancini N, Ossi CM, Perotti M, et al. Molecular mycological diagnosis and correct antimycotic treatments. J Clin Microbiol. 2005;43:3584-3585.
- Arenas R, Lavalle P. Micetoma (madura foot). In: Arenas R, Estrada R, eds. Tropical Dermatology. Austin, TX: Landes Bioscience; 2001:51-61.
- Welsh O, Sauceda E, González J, et al. Amikacin alone and in combination with trimethoprim-sulfamethoxazole in the treatment of actinomycotic mycetoma. J Am Acad Dermatol. 1987;17:443-448.
- Fahal AH. Mycetoma: clinico-pathological monograph. In: Fahal AH. Evidence Based Guidelines for the Management of Mycetoma Patients. Khartoum, Sudan: University of Khartoum Press; 2002:5-15.
- Welsh O, Salinas MC, Rodríguez MA. Treatment of eumycetoma and actinomycetoma. Curr Top Med Mycol. 1995;6:47-71.
- Valle ACF, Welsh O, Vera-Cabrera L. Subcutaneous mycoses—mycetoma. In: Tyring SK, Lupi O, Hengge UR, eds. Tropical Dermatology. Philadelphia, PA: Elsevier Churchill Livingstone; 2006:197-200.
- Fuentes A, Arenas R, Reyes M, et al. Actinomicetoma por Nocardia sp. Informe de cinco casos tratados con imipenem solo o combinado con amikacina. Gac Med Mex. 2006;142:247-252.
- Gombert ME, Aulicino TM, DuBouchet L, et al. Therapy of experimental cerebral nocardiosis with imipenem, amikacin, trimethoprim-sulfamethoxazole, and minocylina. Antimicrob Agents Chemother. 1986;30:270-273.
- Calandra GB, Ricci FM, Wang C, et al. Safety and tolerance comparison of imipenem-cilastatin to cephalotin and cefazolin. J Antimicrob Chemother. 1983;12:125-131.
- Ameen M, Arenas R, Vasquez del Mercado E, et al. Efficacy of imipenem therapy for Nocardia actinomycetomas refractory to sulfonamides. J Am Acad Dermatol. 2010;62:239-246.
- Ameen M, Vargas F, Vasquez del Mercado E, et al. Successful treatment of Nocardia actinomycetoma with meropenem and amikacin combination therapy. Int J Dermatol. 2011;50:443-445.
- Ameen M, Arenas R. Emerging therapeutic regimes for the management of mycetomas. Expert Opin Pharmacother. 2008;9:2077-2085.
- Vera-Cabrera L, Daw-Garza A, Said-Fernández S, et al. Therapeutic effect of a novel oxazolidinone, DA-7867 in BALB/c mice infected with Nocardia brasiliensis. PloS Negl Trop Dis. 2008;2:e289.
- Gómez A, Saúl A, Bonifaz A. Amoxicillin and clavulanic acid in the treatment of actinomicetoma. Int J Dermatol. 1993;32:218-220.
- Méndez-Tovar L, Serrano-Jaen L, Almeida-Arvizu VM. Cefotaxima mas amikacina asociadas a inmunomodulación en el tratamiento de actinomicetoma resistente a tratamiento convencional. Gac Med Mex. 1999;135:517-521.
- Chacon-Moreno BE, Welsh O, Cavazos-Rocha N, et al. Efficacy of ciprofloxacin and moxifloxacin against Nocardia brasiliensis in vitro in an experimental model of actinomycetoma in BALB/c mice. Antimicrob Agents Chemother. 2009;53:295-297.
- Welsh O. Treatment of actinomycetoma. Arch Med Res. 1993;24:413-415.
Mycetoma is a subcutaneous disease that can be caused by aerobic bacteria (actinomycetoma) or fungi (eumycetoma). Diagnosis is based on clinical manifestations, including swelling and deformity of affected areas, as well as the presence of granulation tissue, scars, abscesses, sinus tracts, and a purulent exudate that contains the microorganisms.
The worldwide proportion of mycetomas is 60% actinomycetomas and 40% eumycetomas.1 The disease is endemic in tropical, subtropical, and temperate regions, predominating between latitudes 30°N and 15°S. Most cases occur in Africa, especially Sudan, Mauritania, and Senegal; India; Yemen; and Pakistan. In the Americas, the countries with the most reported cases are Mexico and Venezuela.1
Although mycetoma is rare in developed countries, migration of patients from endemic areas makes knowledge of this condition crucial for dermatologists worldwide. We present a review of the current concepts in the epidemiology, clinical presentation, diagnosis, and treatment of actinomycetoma.
Epidemiology
Actinomycetoma is more common in Latin America, with Mexico having the highest incidence. At last count, there were 2631 cases reported in Mexico.2 The majority of cases of mycetoma in Mexico are actinomycetoma (98%), including Nocardia (86%) and Actinomadura madurae (10%). Eumycetoma is rare in Mexico, constituting only 2% of cases.2 Worldwide, men are affected more commonly than women, which is thought to be related to a higher occupational risk during agricultural labor.
Clinical Features
Mycetoma can affect the skin, subcutaneous tissue, bones, and occasionally the internal organs. It is characterized by swelling, deformation of the affected area, and fistulae that drain serosanguineous or purulent exudates.
In Mexico, 60% of cases of mycetoma affect the lower extremities; the feet are the most commonly affected area, followed by the trunk (back and chest), arms, forearms, legs, knees, and thighs.1 Other sites include the hands, shoulders, and abdominal wall. The head and neck area are seldom affected.3 Mycetoma lesions grow and disseminate locally. Bone lesions are possible depending on the osteophilic affinity of the etiological agent and on the interactions between the fungus and the host’s immune system. In severe advanced cases of mycetoma, the lesions may involve tendons and nerves. Dissemination via blood or lymphatics is extremely rare.4
Diagnosis
Diagnosis of actinomycetoma is suspected based on clinical features and confirmed by direct examination of exudates with Lugol iodine or saline solution. On direct microscopy, actinomycetes are recognized by the production of filaments with a width of 0.5 to 1 μm. On hematoxylin and eosin stain, the small grains of Nocardia appear eosinophilic with a blue center and pink filaments. On Gram stain, actinomycetoma grains show positive branching filaments. Culture of grains recovered from aspirated material or biopsy specimens provides specific etiologic diagnosis. Cultures should be held for at least 4 weeks. Additionally, there are some enzymatic, molecular, and serologic tests available for diagnosis.5-7 Serologic diagnosis is available in a few centers in Mexico and can be helpful in some cases for diagnosis or follow-up during treatment. Antibodies can be determined via enzyme-linked immunosorbent assay, Western blot analysis, immunodiffusion, or counterimmunoelectrophoresis.8
The causative agents of actinomycetoma can be isolated in Sabouraud dextrose agar. Deep wedge biopsies (or puncture aspiration) are useful in observing the diagnostic grains, which can be identified adequately with Gram stain. Grains usually are surrounded and/or infiltrated by neutrophils. The size, form, and color of grains can identify the causative agent.1 The granules of Nocardia are small (80–130 mm) and reniform or wormlike, with club structures in their periphery (Figure 1). Actinomadura madurae is characterized by large, white-yellow granules that can be seen with the naked eye (1–3 mm). On microscopic examination with hematoxylin and eosin stain, these grains are purple and exhibit peripheral pink pseudofilaments (Figure 2).2 The grains of Actinomadura pelletieri are large (1–3 mm) and red or violaceous. They fragment or break easily, giving the appearance of a broken dish (Figure 3). Streptomyces somaliensis forms round grains approximately 0.5 to 1 cm in diameter. These grains stain poorly and are extremely hard. Cutting the grains during processing results in striation, giving them the appearance of a potato chip (Figure 4).2




Treatment of Actinomycetoma
Precise identification of the etiologic agent is essential to provide effective treatment of actinomycetoma. Without treatment, or in resistant cases, progressive osseous and visceral involvement is inevitable.9 Actinomycetoma without osseous involvement usually responds well to medical treatment.
The treatment of choice for actinomycetoma involving Nocardia brasiliensis is a combination of dapsone 100 to 200 mg once daily and trimethoprim-sulfamethoxazole (TMP-SMX) 80/400 to 160/800 mg once daily for 2 to 3 years.10 Other treatments have included the following: (1) amikacin 15 mg/kg or 500 mg intramuscularly twice daily for 3 weeks plus dapsone 100 to 200 mg once daily plus TMP-SMX 80/400 to 160/800 mg daily for 2 to 3 years (amikacin, however, is expensive and potentially toxic [nephrotoxicity and ototoxicity] and therefore is used only in resistant cases); (2) dapsone 100 to 200 mg once daily or TMP-SMX 80/400 to 160/800 mg daily for 2 to 3 years plus intramuscular kanamycin 15 mg/kg once daily for 2 weeks at the beginning of treatment, alternating with rest periods to reduce the risk for nephrotoxicity and ototoxicity10; (3) dapsone 1.5 mg/kg orally twice daily plus phosphomycin 500 mg once daily; (4) dapsone 1.5 mg/kg orally twice daily plus streptomycin 1 g once daily (14 mg/kg/d) for 1 month, then the same dose every other day for 1 to 2 months monitoring for ototoxicity; and (5) TMP-SMX 80/400 to 160/800 mg once daily for 2 to 3 years or rifampicin (15–20 mg/kg/d) plus streptomycin 1 g once daily (14 mg/kg/d) for 1 month at the beginning of treatment, then the same dose every other day for 2 to 3 months until a total dose of 60 g is administered, monitoring for ototoxicity.11 Audiometric tests and creatinine levels must be performed every 5 weeks during the treatment to monitor toxicity.10
The best results for infections with A pelletieri, A madurae, and S somaliensis have been with streptomycin (1 g once daily in adults; 20 mg/kg once daily in children) until a total dose of 50 g is reached in combination with TMP-SMX or dapsone12 (Figure 5). Alternatives for A madurae infections include streptomycin plus oral clofazimine (100 mg once daily), oral rifampicin (300 mg twice daily), oral tetracycline (1 g once daily), oral isoniazid (300–600 mg once daily), or oral minocycline (100 mg twice daily; also effective for A pelletieri).

More recently, other drugs have been used such as carbapenems (eg, imipenem, meropenem), which have wide-spectrum efficacy and are resistant to β-lactamases. Patients should be hospitalized to receive intravenous therapy with imipenem.2 Carbapenems are effective against gram-positive and gram-negative as well as Nocardia species.13,14 Mycetoma that is resistant, severe, or has visceral involvement can be treated with a combination of amikacin and imipenem.15,16 Meropenem is a similar drug that is available as an oral formulation. Both imipenem and meropenem are recommended in cases with bone involvement.17,18 Alternatives for resistant cases include amoxicillin–clavulanic acid 500/125 mg orally 3 times daily for 3 to 6 months or intravenous cefotaxime 1 g every 8 hours plus intramuscular amikacin 500 mg twice daily plus oral levamisole 300 mg once weekly for 4 weeks.19-23
For resistant cases associated with Nocardia species, clindamycin plus quinolones (eg, ciprofloxacin, moxifloxacin, garenoxacin) at a dose of 25 mg/kg once daily for at least 3 months has been suggested in in vivo studies.23
Overall, the cure rate for actinomycetoma treated with any of the prior therapies ranges from 60% to 90%. Treatment must be modified or stopped if there is clinical or laboratory evidence of drug toxicity.13,24 Surgical treatment of actinomycetoma is contraindicated, as it may cause hematogenous dissemination.
Prognosis
Actinomycetomas of a few months’ duration and without bone involvement respond well to therapy. If no therapy is provided or if there is resistance, the functional and cosmetic prognosis is poor, mainly for the feet. There is a risk for spine involvement with mycetoma on the back and posterior head. Thoracic lesions may penetrate into the lungs. The muscular fascia impedes the penetration of abdominal lesions, but the inguinal canals can offer a path for intra-abdominal dissemination.4 Advanced cases lead to a poor general condition of patients, difficulty in using affected extremities, and in extreme cases even death.
The criteria used to guide the discontinuation of initial therapy for any mycetoma include a decrease in the volume of the lesion, closure of fistulae, 3 consecutive negative monthly cultures, imaging studies showing bone regeneration, lack of echoes and cavities on echography, and absence of grains on examination of fine-needle aspirates.11 After the initial treatment protocol is finished, most experts recommend continuing treatment with dapsone 100 to 300 mg once daily for several years to prevent recurrence.12
Prevention
Mycetoma is a disease associated with poverty. It could be prevented by improving living conditions and by regular use of shoes in rural populations.2
Conclusion
Mycetoma is a chronic infection that develops after traumatic inoculation of the skin with either true fungi or aerobic actinomycetes. The resultant infections are known as eumycetoma or actinomycetoma, respectively. The etiologic agents can be found in the so-called grains. Black grains suggest a fungal infection, minute white grains suggest Nocardia, and red grains are due to A pelletieri. Larger white grains or yellow-white grains may be fungal or actinomycotic in origin.
Specific diagnosis requires direct examination, culture, and biopsy. The treatment of choice for actinomycetoma by N brasiliensis is a combination of dapsone 100 to 200 mg once daily and TMP-SMX 80/400 to 160/800 mg once daily for 2 to 3 years. Other effective treatments include aminoglycosides (eg, amikacine, streptomycin) and quinolones. More recently, some other agents have been used such as carbapenems and natural products of Streptomyces cattleya (imipenem), which have wide-spectrum efficacy and are resistant to β-lactamases.
Mycetoma is a subcutaneous disease that can be caused by aerobic bacteria (actinomycetoma) or fungi (eumycetoma). Diagnosis is based on clinical manifestations, including swelling and deformity of affected areas, as well as the presence of granulation tissue, scars, abscesses, sinus tracts, and a purulent exudate that contains the microorganisms.
The worldwide proportion of mycetomas is 60% actinomycetomas and 40% eumycetomas.1 The disease is endemic in tropical, subtropical, and temperate regions, predominating between latitudes 30°N and 15°S. Most cases occur in Africa, especially Sudan, Mauritania, and Senegal; India; Yemen; and Pakistan. In the Americas, the countries with the most reported cases are Mexico and Venezuela.1
Although mycetoma is rare in developed countries, migration of patients from endemic areas makes knowledge of this condition crucial for dermatologists worldwide. We present a review of the current concepts in the epidemiology, clinical presentation, diagnosis, and treatment of actinomycetoma.
Epidemiology
Actinomycetoma is more common in Latin America, with Mexico having the highest incidence. At last count, there were 2631 cases reported in Mexico.2 The majority of cases of mycetoma in Mexico are actinomycetoma (98%), including Nocardia (86%) and Actinomadura madurae (10%). Eumycetoma is rare in Mexico, constituting only 2% of cases.2 Worldwide, men are affected more commonly than women, which is thought to be related to a higher occupational risk during agricultural labor.
Clinical Features
Mycetoma can affect the skin, subcutaneous tissue, bones, and occasionally the internal organs. It is characterized by swelling, deformation of the affected area, and fistulae that drain serosanguineous or purulent exudates.
In Mexico, 60% of cases of mycetoma affect the lower extremities; the feet are the most commonly affected area, followed by the trunk (back and chest), arms, forearms, legs, knees, and thighs.1 Other sites include the hands, shoulders, and abdominal wall. The head and neck area are seldom affected.3 Mycetoma lesions grow and disseminate locally. Bone lesions are possible depending on the osteophilic affinity of the etiological agent and on the interactions between the fungus and the host’s immune system. In severe advanced cases of mycetoma, the lesions may involve tendons and nerves. Dissemination via blood or lymphatics is extremely rare.4
Diagnosis
Diagnosis of actinomycetoma is suspected based on clinical features and confirmed by direct examination of exudates with Lugol iodine or saline solution. On direct microscopy, actinomycetes are recognized by the production of filaments with a width of 0.5 to 1 μm. On hematoxylin and eosin stain, the small grains of Nocardia appear eosinophilic with a blue center and pink filaments. On Gram stain, actinomycetoma grains show positive branching filaments. Culture of grains recovered from aspirated material or biopsy specimens provides specific etiologic diagnosis. Cultures should be held for at least 4 weeks. Additionally, there are some enzymatic, molecular, and serologic tests available for diagnosis.5-7 Serologic diagnosis is available in a few centers in Mexico and can be helpful in some cases for diagnosis or follow-up during treatment. Antibodies can be determined via enzyme-linked immunosorbent assay, Western blot analysis, immunodiffusion, or counterimmunoelectrophoresis.8
The causative agents of actinomycetoma can be isolated in Sabouraud dextrose agar. Deep wedge biopsies (or puncture aspiration) are useful in observing the diagnostic grains, which can be identified adequately with Gram stain. Grains usually are surrounded and/or infiltrated by neutrophils. The size, form, and color of grains can identify the causative agent.1 The granules of Nocardia are small (80–130 mm) and reniform or wormlike, with club structures in their periphery (Figure 1). Actinomadura madurae is characterized by large, white-yellow granules that can be seen with the naked eye (1–3 mm). On microscopic examination with hematoxylin and eosin stain, these grains are purple and exhibit peripheral pink pseudofilaments (Figure 2).2 The grains of Actinomadura pelletieri are large (1–3 mm) and red or violaceous. They fragment or break easily, giving the appearance of a broken dish (Figure 3). Streptomyces somaliensis forms round grains approximately 0.5 to 1 cm in diameter. These grains stain poorly and are extremely hard. Cutting the grains during processing results in striation, giving them the appearance of a potato chip (Figure 4).2




Treatment of Actinomycetoma
Precise identification of the etiologic agent is essential to provide effective treatment of actinomycetoma. Without treatment, or in resistant cases, progressive osseous and visceral involvement is inevitable.9 Actinomycetoma without osseous involvement usually responds well to medical treatment.
The treatment of choice for actinomycetoma involving Nocardia brasiliensis is a combination of dapsone 100 to 200 mg once daily and trimethoprim-sulfamethoxazole (TMP-SMX) 80/400 to 160/800 mg once daily for 2 to 3 years.10 Other treatments have included the following: (1) amikacin 15 mg/kg or 500 mg intramuscularly twice daily for 3 weeks plus dapsone 100 to 200 mg once daily plus TMP-SMX 80/400 to 160/800 mg daily for 2 to 3 years (amikacin, however, is expensive and potentially toxic [nephrotoxicity and ototoxicity] and therefore is used only in resistant cases); (2) dapsone 100 to 200 mg once daily or TMP-SMX 80/400 to 160/800 mg daily for 2 to 3 years plus intramuscular kanamycin 15 mg/kg once daily for 2 weeks at the beginning of treatment, alternating with rest periods to reduce the risk for nephrotoxicity and ototoxicity10; (3) dapsone 1.5 mg/kg orally twice daily plus phosphomycin 500 mg once daily; (4) dapsone 1.5 mg/kg orally twice daily plus streptomycin 1 g once daily (14 mg/kg/d) for 1 month, then the same dose every other day for 1 to 2 months monitoring for ototoxicity; and (5) TMP-SMX 80/400 to 160/800 mg once daily for 2 to 3 years or rifampicin (15–20 mg/kg/d) plus streptomycin 1 g once daily (14 mg/kg/d) for 1 month at the beginning of treatment, then the same dose every other day for 2 to 3 months until a total dose of 60 g is administered, monitoring for ototoxicity.11 Audiometric tests and creatinine levels must be performed every 5 weeks during the treatment to monitor toxicity.10
The best results for infections with A pelletieri, A madurae, and S somaliensis have been with streptomycin (1 g once daily in adults; 20 mg/kg once daily in children) until a total dose of 50 g is reached in combination with TMP-SMX or dapsone12 (Figure 5). Alternatives for A madurae infections include streptomycin plus oral clofazimine (100 mg once daily), oral rifampicin (300 mg twice daily), oral tetracycline (1 g once daily), oral isoniazid (300–600 mg once daily), or oral minocycline (100 mg twice daily; also effective for A pelletieri).

More recently, other drugs have been used such as carbapenems (eg, imipenem, meropenem), which have wide-spectrum efficacy and are resistant to β-lactamases. Patients should be hospitalized to receive intravenous therapy with imipenem.2 Carbapenems are effective against gram-positive and gram-negative as well as Nocardia species.13,14 Mycetoma that is resistant, severe, or has visceral involvement can be treated with a combination of amikacin and imipenem.15,16 Meropenem is a similar drug that is available as an oral formulation. Both imipenem and meropenem are recommended in cases with bone involvement.17,18 Alternatives for resistant cases include amoxicillin–clavulanic acid 500/125 mg orally 3 times daily for 3 to 6 months or intravenous cefotaxime 1 g every 8 hours plus intramuscular amikacin 500 mg twice daily plus oral levamisole 300 mg once weekly for 4 weeks.19-23
For resistant cases associated with Nocardia species, clindamycin plus quinolones (eg, ciprofloxacin, moxifloxacin, garenoxacin) at a dose of 25 mg/kg once daily for at least 3 months has been suggested in in vivo studies.23
Overall, the cure rate for actinomycetoma treated with any of the prior therapies ranges from 60% to 90%. Treatment must be modified or stopped if there is clinical or laboratory evidence of drug toxicity.13,24 Surgical treatment of actinomycetoma is contraindicated, as it may cause hematogenous dissemination.
Prognosis
Actinomycetomas of a few months’ duration and without bone involvement respond well to therapy. If no therapy is provided or if there is resistance, the functional and cosmetic prognosis is poor, mainly for the feet. There is a risk for spine involvement with mycetoma on the back and posterior head. Thoracic lesions may penetrate into the lungs. The muscular fascia impedes the penetration of abdominal lesions, but the inguinal canals can offer a path for intra-abdominal dissemination.4 Advanced cases lead to a poor general condition of patients, difficulty in using affected extremities, and in extreme cases even death.
The criteria used to guide the discontinuation of initial therapy for any mycetoma include a decrease in the volume of the lesion, closure of fistulae, 3 consecutive negative monthly cultures, imaging studies showing bone regeneration, lack of echoes and cavities on echography, and absence of grains on examination of fine-needle aspirates.11 After the initial treatment protocol is finished, most experts recommend continuing treatment with dapsone 100 to 300 mg once daily for several years to prevent recurrence.12
Prevention
Mycetoma is a disease associated with poverty. It could be prevented by improving living conditions and by regular use of shoes in rural populations.2
Conclusion
Mycetoma is a chronic infection that develops after traumatic inoculation of the skin with either true fungi or aerobic actinomycetes. The resultant infections are known as eumycetoma or actinomycetoma, respectively. The etiologic agents can be found in the so-called grains. Black grains suggest a fungal infection, minute white grains suggest Nocardia, and red grains are due to A pelletieri. Larger white grains or yellow-white grains may be fungal or actinomycotic in origin.
Specific diagnosis requires direct examination, culture, and biopsy. The treatment of choice for actinomycetoma by N brasiliensis is a combination of dapsone 100 to 200 mg once daily and TMP-SMX 80/400 to 160/800 mg once daily for 2 to 3 years. Other effective treatments include aminoglycosides (eg, amikacine, streptomycin) and quinolones. More recently, some other agents have been used such as carbapenems and natural products of Streptomyces cattleya (imipenem), which have wide-spectrum efficacy and are resistant to β-lactamases.
- Welsh O, Vera-Cabrera L, Welsh E, et al. Actinomycetoma and advances in its treatment. Clin Dermatol. 2012;30:372-381.
- Arenas R. Micología Medica Ilustrada. 4th ed. Mexico City, Mexico: McGraw-Hill Interamericana; 2011:125-146.
- McGinnis MR. Mycetoma. Dermatol Clin. 1996;14:97-104.
- Fahal AH. Mycetoma: Clinico-pathological Monograph. Khartoum, Sudan: University of Khartoum Press; 2006:20-23, 81-82.
- Estrada-Chavez GE, Vega-Memije ME, Arenas R, et al. Eumycotic mycetoma caused by Madurella mycetomatis successfully treated with antifungals, surgery, and topical negative pressure therapy. Int J Dermatol. 2009;48:401-403.
- Chávez G, Arenas R, Pérez-Polito A, et al. Eumycetic mycetoma due to Madurella mycetomatis. report of six cases. Rev Iberoam Micol. 1998;15:90-93.
- Vasquez del Mercado E, Arenas R, Moreno G. Sequelae and long-term consequences of systemic and subcutaneous mycoses. In: Fratamico PM, Smith JL, Brogden KA, eds. Sequelae and Long-term Consequences of Infectious Diseases. Washington, DC: ASM Press; 2009:415-420.
- Mancini N, Ossi CM, Perotti M, et al. Molecular mycological diagnosis and correct antimycotic treatments. J Clin Microbiol. 2005;43:3584-3585.
- Arenas R, Lavalle P. Micetoma (madura foot). In: Arenas R, Estrada R, eds. Tropical Dermatology. Austin, TX: Landes Bioscience; 2001:51-61.
- Welsh O, Sauceda E, González J, et al. Amikacin alone and in combination with trimethoprim-sulfamethoxazole in the treatment of actinomycotic mycetoma. J Am Acad Dermatol. 1987;17:443-448.
- Fahal AH. Mycetoma: clinico-pathological monograph. In: Fahal AH. Evidence Based Guidelines for the Management of Mycetoma Patients. Khartoum, Sudan: University of Khartoum Press; 2002:5-15.
- Welsh O, Salinas MC, Rodríguez MA. Treatment of eumycetoma and actinomycetoma. Curr Top Med Mycol. 1995;6:47-71.
- Valle ACF, Welsh O, Vera-Cabrera L. Subcutaneous mycoses—mycetoma. In: Tyring SK, Lupi O, Hengge UR, eds. Tropical Dermatology. Philadelphia, PA: Elsevier Churchill Livingstone; 2006:197-200.
- Fuentes A, Arenas R, Reyes M, et al. Actinomicetoma por Nocardia sp. Informe de cinco casos tratados con imipenem solo o combinado con amikacina. Gac Med Mex. 2006;142:247-252.
- Gombert ME, Aulicino TM, DuBouchet L, et al. Therapy of experimental cerebral nocardiosis with imipenem, amikacin, trimethoprim-sulfamethoxazole, and minocylina. Antimicrob Agents Chemother. 1986;30:270-273.
- Calandra GB, Ricci FM, Wang C, et al. Safety and tolerance comparison of imipenem-cilastatin to cephalotin and cefazolin. J Antimicrob Chemother. 1983;12:125-131.
- Ameen M, Arenas R, Vasquez del Mercado E, et al. Efficacy of imipenem therapy for Nocardia actinomycetomas refractory to sulfonamides. J Am Acad Dermatol. 2010;62:239-246.
- Ameen M, Vargas F, Vasquez del Mercado E, et al. Successful treatment of Nocardia actinomycetoma with meropenem and amikacin combination therapy. Int J Dermatol. 2011;50:443-445.
- Ameen M, Arenas R. Emerging therapeutic regimes for the management of mycetomas. Expert Opin Pharmacother. 2008;9:2077-2085.
- Vera-Cabrera L, Daw-Garza A, Said-Fernández S, et al. Therapeutic effect of a novel oxazolidinone, DA-7867 in BALB/c mice infected with Nocardia brasiliensis. PloS Negl Trop Dis. 2008;2:e289.
- Gómez A, Saúl A, Bonifaz A. Amoxicillin and clavulanic acid in the treatment of actinomicetoma. Int J Dermatol. 1993;32:218-220.
- Méndez-Tovar L, Serrano-Jaen L, Almeida-Arvizu VM. Cefotaxima mas amikacina asociadas a inmunomodulación en el tratamiento de actinomicetoma resistente a tratamiento convencional. Gac Med Mex. 1999;135:517-521.
- Chacon-Moreno BE, Welsh O, Cavazos-Rocha N, et al. Efficacy of ciprofloxacin and moxifloxacin against Nocardia brasiliensis in vitro in an experimental model of actinomycetoma in BALB/c mice. Antimicrob Agents Chemother. 2009;53:295-297.
- Welsh O. Treatment of actinomycetoma. Arch Med Res. 1993;24:413-415.
- Welsh O, Vera-Cabrera L, Welsh E, et al. Actinomycetoma and advances in its treatment. Clin Dermatol. 2012;30:372-381.
- Arenas R. Micología Medica Ilustrada. 4th ed. Mexico City, Mexico: McGraw-Hill Interamericana; 2011:125-146.
- McGinnis MR. Mycetoma. Dermatol Clin. 1996;14:97-104.
- Fahal AH. Mycetoma: Clinico-pathological Monograph. Khartoum, Sudan: University of Khartoum Press; 2006:20-23, 81-82.
- Estrada-Chavez GE, Vega-Memije ME, Arenas R, et al. Eumycotic mycetoma caused by Madurella mycetomatis successfully treated with antifungals, surgery, and topical negative pressure therapy. Int J Dermatol. 2009;48:401-403.
- Chávez G, Arenas R, Pérez-Polito A, et al. Eumycetic mycetoma due to Madurella mycetomatis. report of six cases. Rev Iberoam Micol. 1998;15:90-93.
- Vasquez del Mercado E, Arenas R, Moreno G. Sequelae and long-term consequences of systemic and subcutaneous mycoses. In: Fratamico PM, Smith JL, Brogden KA, eds. Sequelae and Long-term Consequences of Infectious Diseases. Washington, DC: ASM Press; 2009:415-420.
- Mancini N, Ossi CM, Perotti M, et al. Molecular mycological diagnosis and correct antimycotic treatments. J Clin Microbiol. 2005;43:3584-3585.
- Arenas R, Lavalle P. Micetoma (madura foot). In: Arenas R, Estrada R, eds. Tropical Dermatology. Austin, TX: Landes Bioscience; 2001:51-61.
- Welsh O, Sauceda E, González J, et al. Amikacin alone and in combination with trimethoprim-sulfamethoxazole in the treatment of actinomycotic mycetoma. J Am Acad Dermatol. 1987;17:443-448.
- Fahal AH. Mycetoma: clinico-pathological monograph. In: Fahal AH. Evidence Based Guidelines for the Management of Mycetoma Patients. Khartoum, Sudan: University of Khartoum Press; 2002:5-15.
- Welsh O, Salinas MC, Rodríguez MA. Treatment of eumycetoma and actinomycetoma. Curr Top Med Mycol. 1995;6:47-71.
- Valle ACF, Welsh O, Vera-Cabrera L. Subcutaneous mycoses—mycetoma. In: Tyring SK, Lupi O, Hengge UR, eds. Tropical Dermatology. Philadelphia, PA: Elsevier Churchill Livingstone; 2006:197-200.
- Fuentes A, Arenas R, Reyes M, et al. Actinomicetoma por Nocardia sp. Informe de cinco casos tratados con imipenem solo o combinado con amikacina. Gac Med Mex. 2006;142:247-252.
- Gombert ME, Aulicino TM, DuBouchet L, et al. Therapy of experimental cerebral nocardiosis with imipenem, amikacin, trimethoprim-sulfamethoxazole, and minocylina. Antimicrob Agents Chemother. 1986;30:270-273.
- Calandra GB, Ricci FM, Wang C, et al. Safety and tolerance comparison of imipenem-cilastatin to cephalotin and cefazolin. J Antimicrob Chemother. 1983;12:125-131.
- Ameen M, Arenas R, Vasquez del Mercado E, et al. Efficacy of imipenem therapy for Nocardia actinomycetomas refractory to sulfonamides. J Am Acad Dermatol. 2010;62:239-246.
- Ameen M, Vargas F, Vasquez del Mercado E, et al. Successful treatment of Nocardia actinomycetoma with meropenem and amikacin combination therapy. Int J Dermatol. 2011;50:443-445.
- Ameen M, Arenas R. Emerging therapeutic regimes for the management of mycetomas. Expert Opin Pharmacother. 2008;9:2077-2085.
- Vera-Cabrera L, Daw-Garza A, Said-Fernández S, et al. Therapeutic effect of a novel oxazolidinone, DA-7867 in BALB/c mice infected with Nocardia brasiliensis. PloS Negl Trop Dis. 2008;2:e289.
- Gómez A, Saúl A, Bonifaz A. Amoxicillin and clavulanic acid in the treatment of actinomicetoma. Int J Dermatol. 1993;32:218-220.
- Méndez-Tovar L, Serrano-Jaen L, Almeida-Arvizu VM. Cefotaxima mas amikacina asociadas a inmunomodulación en el tratamiento de actinomicetoma resistente a tratamiento convencional. Gac Med Mex. 1999;135:517-521.
- Chacon-Moreno BE, Welsh O, Cavazos-Rocha N, et al. Efficacy of ciprofloxacin and moxifloxacin against Nocardia brasiliensis in vitro in an experimental model of actinomycetoma in BALB/c mice. Antimicrob Agents Chemother. 2009;53:295-297.
- Welsh O. Treatment of actinomycetoma. Arch Med Res. 1993;24:413-415.
Practice Points
- Diagnosis of actinomycetoma is based on clinical manifestations including increased swelling and deformity of affected areas, presence of granulation tissue, scars, abscesses, sinus tracts, and a purulent exudate containing microorganisms.
- The feet are the most commonly affected location, followed by the trunk (back and chest), arms, forearms, legs, knees, and thighs.
- Specific diagnosis of actinomycetoma requires clinical examination as well as direct examination of culture and biopsy results.
- Overall, the cure rate for actinomycetoma ranges from 60% to 90%.
AACE: Updated lipid guidelines include ‘extreme risk’ category
according to a summary by the American Association of Clinical Endocrinologists and American College of Endocrinology.
Achieving such a low LDL-cholesterol (LDL-C) level is possible with well-established medications such as a combination of simvastatin and ezetimibe and the newer, more clinically powerful agent PCSK9 inhibitors, Paul S. Jellinger, MD, chair of the guideline committee and professor of clinical medicine, University of Miami, said in an interview. The “extreme risk” category was derived largely from IMPROVE-IT and supporting meta-analyses.
The new category applies to patients with established atherosclerotic cardiovascular disease (ASCVD) who have any of the following risk factors:
• Progressive ASCVD including unstable angina after achieving an LDL-C level less than 70 mg/dL.
• Type 2 diabetes mellitus, chronic kidney disease stage 3 or 4, or heterozygous familial hypercholesterolemia.
• A history of premature ASCVD (before age 55 years in men, 65 years in women).
Heterozygous hypercholesterolemia is more common than many physicians realize, affecting as it does 1 in 250 people, Dr. Jellinger noted.
“Supporting evidence for the ‘extreme risk’ category with an LDL-C of less than 55 mg/dL was recently announced with the top line results from the FOURIER CVD outcome trial utilizing PCSK9-inhibitor therapy, which met both its primary and secondary endpoints,” he stressed.
PCSK9 inhibitors are extremely expensive, said Dr. Jellinger. “In my view, payers should not be denying patients who clearly meet the Food and Drug Administration approved indications for PCSK9 inhibitors and those within the extreme risk criteria. Yet, I have been made aware that 80% of insurance claims for PCSK9 inhibitors in these extremely high-risk and vulnerable patients are being denied.”
Inclusion in the updated AACE lipid management guidelines should strengthen PCSK9 inhibitors’ place among lipid-lowering drugs that are acceptable to insurance companies by making it clear that PCSK9 is appropriate and indicated for select patients, according to Dr. Jellinger.
Aside from creation of the extreme risk category, the AACE lipid guidelines discuss the available tools for making a global risk assessment of having a cardiac, vascular, or stroke event in the next 10 years, without favoring any one risk score. Among the systems discussed, with links, are the Framingham, MESA (Multi-Ethnic Study of Atherosclerosis), Reynolds, and United Kingdom Prospective Diabetes Study risk scores.
The guidelines provide separate approaches to management of people at various ages. Another change from the 2012 guidelines is the establishment of acceptable and worrisome LDL-C levels in children: Acceptable is a level under 100 mg/dL, borderline is 100-129 mg/dL, and high is LDL-C at or above 130 mg/dL.
The guidelines, which will be published in full in the April issue of Endocrine Practice, are based largely on numerous clinical trials and studies, and in the end produced 87 recommendations, of which 45 are Grade A, 18 are Grade B, 15 are Grade C, and 9 are Grade D.
These are the first guidelines on lipid management from AACE since 2012. The executive summary of the guidelines is available at www.aace.com.
Dr. Jellinger reported receiving speakers honoraria from Amgen, AstraZeneca, BI-Lilly, Merck, and Novo Nordisk.
according to a summary by the American Association of Clinical Endocrinologists and American College of Endocrinology.
Achieving such a low LDL-cholesterol (LDL-C) level is possible with well-established medications such as a combination of simvastatin and ezetimibe and the newer, more clinically powerful agent PCSK9 inhibitors, Paul S. Jellinger, MD, chair of the guideline committee and professor of clinical medicine, University of Miami, said in an interview. The “extreme risk” category was derived largely from IMPROVE-IT and supporting meta-analyses.
The new category applies to patients with established atherosclerotic cardiovascular disease (ASCVD) who have any of the following risk factors:
• Progressive ASCVD including unstable angina after achieving an LDL-C level less than 70 mg/dL.
• Type 2 diabetes mellitus, chronic kidney disease stage 3 or 4, or heterozygous familial hypercholesterolemia.
• A history of premature ASCVD (before age 55 years in men, 65 years in women).
Heterozygous hypercholesterolemia is more common than many physicians realize, affecting as it does 1 in 250 people, Dr. Jellinger noted.
“Supporting evidence for the ‘extreme risk’ category with an LDL-C of less than 55 mg/dL was recently announced with the top line results from the FOURIER CVD outcome trial utilizing PCSK9-inhibitor therapy, which met both its primary and secondary endpoints,” he stressed.
PCSK9 inhibitors are extremely expensive, said Dr. Jellinger. “In my view, payers should not be denying patients who clearly meet the Food and Drug Administration approved indications for PCSK9 inhibitors and those within the extreme risk criteria. Yet, I have been made aware that 80% of insurance claims for PCSK9 inhibitors in these extremely high-risk and vulnerable patients are being denied.”
Inclusion in the updated AACE lipid management guidelines should strengthen PCSK9 inhibitors’ place among lipid-lowering drugs that are acceptable to insurance companies by making it clear that PCSK9 is appropriate and indicated for select patients, according to Dr. Jellinger.
Aside from creation of the extreme risk category, the AACE lipid guidelines discuss the available tools for making a global risk assessment of having a cardiac, vascular, or stroke event in the next 10 years, without favoring any one risk score. Among the systems discussed, with links, are the Framingham, MESA (Multi-Ethnic Study of Atherosclerosis), Reynolds, and United Kingdom Prospective Diabetes Study risk scores.
The guidelines provide separate approaches to management of people at various ages. Another change from the 2012 guidelines is the establishment of acceptable and worrisome LDL-C levels in children: Acceptable is a level under 100 mg/dL, borderline is 100-129 mg/dL, and high is LDL-C at or above 130 mg/dL.
The guidelines, which will be published in full in the April issue of Endocrine Practice, are based largely on numerous clinical trials and studies, and in the end produced 87 recommendations, of which 45 are Grade A, 18 are Grade B, 15 are Grade C, and 9 are Grade D.
These are the first guidelines on lipid management from AACE since 2012. The executive summary of the guidelines is available at www.aace.com.
Dr. Jellinger reported receiving speakers honoraria from Amgen, AstraZeneca, BI-Lilly, Merck, and Novo Nordisk.
according to a summary by the American Association of Clinical Endocrinologists and American College of Endocrinology.
Achieving such a low LDL-cholesterol (LDL-C) level is possible with well-established medications such as a combination of simvastatin and ezetimibe and the newer, more clinically powerful agent PCSK9 inhibitors, Paul S. Jellinger, MD, chair of the guideline committee and professor of clinical medicine, University of Miami, said in an interview. The “extreme risk” category was derived largely from IMPROVE-IT and supporting meta-analyses.
The new category applies to patients with established atherosclerotic cardiovascular disease (ASCVD) who have any of the following risk factors:
• Progressive ASCVD including unstable angina after achieving an LDL-C level less than 70 mg/dL.
• Type 2 diabetes mellitus, chronic kidney disease stage 3 or 4, or heterozygous familial hypercholesterolemia.
• A history of premature ASCVD (before age 55 years in men, 65 years in women).
Heterozygous hypercholesterolemia is more common than many physicians realize, affecting as it does 1 in 250 people, Dr. Jellinger noted.
“Supporting evidence for the ‘extreme risk’ category with an LDL-C of less than 55 mg/dL was recently announced with the top line results from the FOURIER CVD outcome trial utilizing PCSK9-inhibitor therapy, which met both its primary and secondary endpoints,” he stressed.
PCSK9 inhibitors are extremely expensive, said Dr. Jellinger. “In my view, payers should not be denying patients who clearly meet the Food and Drug Administration approved indications for PCSK9 inhibitors and those within the extreme risk criteria. Yet, I have been made aware that 80% of insurance claims for PCSK9 inhibitors in these extremely high-risk and vulnerable patients are being denied.”
Inclusion in the updated AACE lipid management guidelines should strengthen PCSK9 inhibitors’ place among lipid-lowering drugs that are acceptable to insurance companies by making it clear that PCSK9 is appropriate and indicated for select patients, according to Dr. Jellinger.
Aside from creation of the extreme risk category, the AACE lipid guidelines discuss the available tools for making a global risk assessment of having a cardiac, vascular, or stroke event in the next 10 years, without favoring any one risk score. Among the systems discussed, with links, are the Framingham, MESA (Multi-Ethnic Study of Atherosclerosis), Reynolds, and United Kingdom Prospective Diabetes Study risk scores.
The guidelines provide separate approaches to management of people at various ages. Another change from the 2012 guidelines is the establishment of acceptable and worrisome LDL-C levels in children: Acceptable is a level under 100 mg/dL, borderline is 100-129 mg/dL, and high is LDL-C at or above 130 mg/dL.
The guidelines, which will be published in full in the April issue of Endocrine Practice, are based largely on numerous clinical trials and studies, and in the end produced 87 recommendations, of which 45 are Grade A, 18 are Grade B, 15 are Grade C, and 9 are Grade D.
These are the first guidelines on lipid management from AACE since 2012. The executive summary of the guidelines is available at www.aace.com.
Dr. Jellinger reported receiving speakers honoraria from Amgen, AstraZeneca, BI-Lilly, Merck, and Novo Nordisk.
Bronchiolitis pathway adherence tied to shorter LOS, lower costs
and lower health care costs, according to Mersine A. Bryan, MD, of the University of Washington, Seattle and her associates.
In a retrospective cohort study, researchers looked at 267 patients less than 24 months old diagnosed with bronchiolitis from December 2009 to July 2012. Levels of adherence were then categorized into low, middle, and high tertiles. Results show that adherence was highest for the inpatient quality indicators (mean score, 95) and lowest for the emergency department (ED) quality indicators (mean score, 79). The mean ED LOS was significantly shorter for cases with ED adherence scores in the highest versus the lowest tertile (90 vs. 140 minutes; P less than .05). There were no significant differences in mean inpatient LOS by inpatient adherence score tertiles. “However, the mean inpatient LOS was approximately 17 hours shorter for cases with combined ED/inpatient adherence scores in the highest, compared with the lowest tertile,” they said.
“Our study demonstrates that high adherence to evidence-based recommendations within a clinical pathway across the entire continuum of care, from the ED to the inpatient setting, is associated with lower costs and shorter LOS,” Dr. Bryan and associates concluded. “By improving adherence to evidence-based recommendations within a clinical pathway, we may be able to provide higher-value care by optimizing the quality of bronchiolitis care at lower costs and with shorter LOS.”
Read the full study in Pediatrics (doi: 10.1542/peds.2016-3432).
and lower health care costs, according to Mersine A. Bryan, MD, of the University of Washington, Seattle and her associates.
In a retrospective cohort study, researchers looked at 267 patients less than 24 months old diagnosed with bronchiolitis from December 2009 to July 2012. Levels of adherence were then categorized into low, middle, and high tertiles. Results show that adherence was highest for the inpatient quality indicators (mean score, 95) and lowest for the emergency department (ED) quality indicators (mean score, 79). The mean ED LOS was significantly shorter for cases with ED adherence scores in the highest versus the lowest tertile (90 vs. 140 minutes; P less than .05). There were no significant differences in mean inpatient LOS by inpatient adherence score tertiles. “However, the mean inpatient LOS was approximately 17 hours shorter for cases with combined ED/inpatient adherence scores in the highest, compared with the lowest tertile,” they said.
“Our study demonstrates that high adherence to evidence-based recommendations within a clinical pathway across the entire continuum of care, from the ED to the inpatient setting, is associated with lower costs and shorter LOS,” Dr. Bryan and associates concluded. “By improving adherence to evidence-based recommendations within a clinical pathway, we may be able to provide higher-value care by optimizing the quality of bronchiolitis care at lower costs and with shorter LOS.”
Read the full study in Pediatrics (doi: 10.1542/peds.2016-3432).
and lower health care costs, according to Mersine A. Bryan, MD, of the University of Washington, Seattle and her associates.
In a retrospective cohort study, researchers looked at 267 patients less than 24 months old diagnosed with bronchiolitis from December 2009 to July 2012. Levels of adherence were then categorized into low, middle, and high tertiles. Results show that adherence was highest for the inpatient quality indicators (mean score, 95) and lowest for the emergency department (ED) quality indicators (mean score, 79). The mean ED LOS was significantly shorter for cases with ED adherence scores in the highest versus the lowest tertile (90 vs. 140 minutes; P less than .05). There were no significant differences in mean inpatient LOS by inpatient adherence score tertiles. “However, the mean inpatient LOS was approximately 17 hours shorter for cases with combined ED/inpatient adherence scores in the highest, compared with the lowest tertile,” they said.
“Our study demonstrates that high adherence to evidence-based recommendations within a clinical pathway across the entire continuum of care, from the ED to the inpatient setting, is associated with lower costs and shorter LOS,” Dr. Bryan and associates concluded. “By improving adherence to evidence-based recommendations within a clinical pathway, we may be able to provide higher-value care by optimizing the quality of bronchiolitis care at lower costs and with shorter LOS.”
Read the full study in Pediatrics (doi: 10.1542/peds.2016-3432).
FROM PEDIATRICS
Ultrasound guidance of early RA therapy doesn’t improve outcomes
SNOWMASS, COLO. – Rheumatologists who use musculoskeletal ultrasound to help guide a treat-to-target strategy in early rheumatoid arthritis may want to reconsider that practice in light of the results of a recent randomized trial known as the TaSER study, Michael E. Weinblatt, MD, observed at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
In TaSER, Scottish investigators randomized 111 newly diagnosed early rheumatoid arthritis patients to an intensive treat-to-target strategy guided by 28-joint Disease Activity Score with erythrocyte sedimentation rate (DAS28-ESR) alone or in conjunction with musculoskeletal ultrasound assessment of disease activity in a limited joint set.
The stepped treatment escalation regimen began with methotrexate monotherapy, followed by the addition of sulfasalazine and hydroxychloroquine to methotrexate as triple therapy, then etanercept plus triple therapy.
The primary outcome was improvement in DAS44 at 18 months. Both groups experienced similarly robust improvements: a mean 2.69-point reduction in the ultrasound group and a 2.59-point decrease in the control arm. Nor were there any between-group differences in radiographic or MRI erosions. This was the case even though the ultrasound group received more intensive therapy (Ann Rheum Dis. 2016 Jun;75[6]:1043-50).
“This doesn’t mean that there’s not great value in ultrasound to evaluate disease activity, but I’m not convinced that using ultrasound to guide your treatment per se shows any advantages over a good clinical evaluation of the patient,” concluded Dr. Weinblatt, professor of medicine at Harvard Medical School in Boston.
He reported receiving research grants from half a dozen companies and serving as a consultant to more than two dozen.
SNOWMASS, COLO. – Rheumatologists who use musculoskeletal ultrasound to help guide a treat-to-target strategy in early rheumatoid arthritis may want to reconsider that practice in light of the results of a recent randomized trial known as the TaSER study, Michael E. Weinblatt, MD, observed at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
In TaSER, Scottish investigators randomized 111 newly diagnosed early rheumatoid arthritis patients to an intensive treat-to-target strategy guided by 28-joint Disease Activity Score with erythrocyte sedimentation rate (DAS28-ESR) alone or in conjunction with musculoskeletal ultrasound assessment of disease activity in a limited joint set.
The stepped treatment escalation regimen began with methotrexate monotherapy, followed by the addition of sulfasalazine and hydroxychloroquine to methotrexate as triple therapy, then etanercept plus triple therapy.
The primary outcome was improvement in DAS44 at 18 months. Both groups experienced similarly robust improvements: a mean 2.69-point reduction in the ultrasound group and a 2.59-point decrease in the control arm. Nor were there any between-group differences in radiographic or MRI erosions. This was the case even though the ultrasound group received more intensive therapy (Ann Rheum Dis. 2016 Jun;75[6]:1043-50).
“This doesn’t mean that there’s not great value in ultrasound to evaluate disease activity, but I’m not convinced that using ultrasound to guide your treatment per se shows any advantages over a good clinical evaluation of the patient,” concluded Dr. Weinblatt, professor of medicine at Harvard Medical School in Boston.
He reported receiving research grants from half a dozen companies and serving as a consultant to more than two dozen.
SNOWMASS, COLO. – Rheumatologists who use musculoskeletal ultrasound to help guide a treat-to-target strategy in early rheumatoid arthritis may want to reconsider that practice in light of the results of a recent randomized trial known as the TaSER study, Michael E. Weinblatt, MD, observed at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
In TaSER, Scottish investigators randomized 111 newly diagnosed early rheumatoid arthritis patients to an intensive treat-to-target strategy guided by 28-joint Disease Activity Score with erythrocyte sedimentation rate (DAS28-ESR) alone or in conjunction with musculoskeletal ultrasound assessment of disease activity in a limited joint set.
The stepped treatment escalation regimen began with methotrexate monotherapy, followed by the addition of sulfasalazine and hydroxychloroquine to methotrexate as triple therapy, then etanercept plus triple therapy.
The primary outcome was improvement in DAS44 at 18 months. Both groups experienced similarly robust improvements: a mean 2.69-point reduction in the ultrasound group and a 2.59-point decrease in the control arm. Nor were there any between-group differences in radiographic or MRI erosions. This was the case even though the ultrasound group received more intensive therapy (Ann Rheum Dis. 2016 Jun;75[6]:1043-50).
“This doesn’t mean that there’s not great value in ultrasound to evaluate disease activity, but I’m not convinced that using ultrasound to guide your treatment per se shows any advantages over a good clinical evaluation of the patient,” concluded Dr. Weinblatt, professor of medicine at Harvard Medical School in Boston.
He reported receiving research grants from half a dozen companies and serving as a consultant to more than two dozen.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Fecal microbiota transplantation a decolonization treatment option
The use of fecal microbiota transplantation is an option to eradicate highly drug-resistant enteric bacteria carriage, according to results from a small pilot study conducted by French investigators.
“A rapid and dramatic emergence of highly drug-resistant enteric bacteria (HDREB), i.e., carbapenem-resistant Enterobacteriaceae (CRE) and vancomycin-resistant enterococci (VRE), is occurring worldwide,” researchers led by Benjamin Davido, MD, of the infectious diseases unit at Raymond Poincaré Teaching Hospital, Garches, France, wrote in a study published online Feb. 1 in the Journal of Hospital Infection. “Patients carrying these bacteria are at risk of developing severe infections due to these bacteria; these infections are associated with a high mortality rate, partially because of inappropriate antimicrobial treatment.”
Citing recent studies that have demonstrated the efficacy of fecal microbiota transplant (FMT) as an accepted therapy to prevent recurrent Clostridium difficile infection, Dr. Davido and his associates prospectively identified eight different case reports of FMT used in adults for intestinal decolonization from extended-spectrum beta-lactamase (ESBL)–producing Enterobacteriaceae, VRE, or methicillin-resistant Staphylococcus aureus. Patients on immunosuppressive agents were excluded from the study, as were those taking antibiotics at the time of FMT (J Hosp Infect. 2017 Feb. 1. doi: 10.1016/j.jhin.2017.02.001).
The protocol for the procedure involved insertion of a nasoduodenal tube the day before FMT in order to perform a bowel lavage with XPrep solution. FMT was performed using a frozen preparation of fecal microbiota from a universal donor who was previously screened for potential diseases. The main outcome of interest was time to successful decolonization following FMT, which was determined by at least two consecutive negative rectal swabs at a 1-week interval during a follow-up of 3 months.
The mean age of the eight patients was 70 years, their median Charlson comorbidity index score was 5, and the median duration of carriage of HDREB before FMT was 83 days. The researchers observed that 1 month after FMT, two patients were free from CRE colonization, while one more patient was free from VRE after 3 months. Five patients received antibiotic during follow-up. Among them, one patient was decolonized at 1 month, while another was decolonized at 3 months. One patient with cirrhosis and persistent VRE carriage died 3 months after FMT from ascetic fluid infection and VRE bacteremia. No other adverse events were reported.
“In a context where no other efficient strategy is available, our first results show that FMT seems to be safe, with an impact on CRE decolonization at 1 month and on VRE decolonization at 3 months,” the researchers concluded. “Of particular importance, there was no recolonization after the intervention, which is contrary to decolonization with antibiotics. However, our study has important limitations in that the sample size was very small, it was nonrandomized, and follow-up was limited to a 3-month period.” They noted that at least five trials are underway to investigate the impact of FMT on multidrug resistant organism bacterial decolonization. The researchers reported having no financial disclosures.
The use of fecal microbiota transplantation is an option to eradicate highly drug-resistant enteric bacteria carriage, according to results from a small pilot study conducted by French investigators.
“A rapid and dramatic emergence of highly drug-resistant enteric bacteria (HDREB), i.e., carbapenem-resistant Enterobacteriaceae (CRE) and vancomycin-resistant enterococci (VRE), is occurring worldwide,” researchers led by Benjamin Davido, MD, of the infectious diseases unit at Raymond Poincaré Teaching Hospital, Garches, France, wrote in a study published online Feb. 1 in the Journal of Hospital Infection. “Patients carrying these bacteria are at risk of developing severe infections due to these bacteria; these infections are associated with a high mortality rate, partially because of inappropriate antimicrobial treatment.”
Citing recent studies that have demonstrated the efficacy of fecal microbiota transplant (FMT) as an accepted therapy to prevent recurrent Clostridium difficile infection, Dr. Davido and his associates prospectively identified eight different case reports of FMT used in adults for intestinal decolonization from extended-spectrum beta-lactamase (ESBL)–producing Enterobacteriaceae, VRE, or methicillin-resistant Staphylococcus aureus. Patients on immunosuppressive agents were excluded from the study, as were those taking antibiotics at the time of FMT (J Hosp Infect. 2017 Feb. 1. doi: 10.1016/j.jhin.2017.02.001).
The protocol for the procedure involved insertion of a nasoduodenal tube the day before FMT in order to perform a bowel lavage with XPrep solution. FMT was performed using a frozen preparation of fecal microbiota from a universal donor who was previously screened for potential diseases. The main outcome of interest was time to successful decolonization following FMT, which was determined by at least two consecutive negative rectal swabs at a 1-week interval during a follow-up of 3 months.
The mean age of the eight patients was 70 years, their median Charlson comorbidity index score was 5, and the median duration of carriage of HDREB before FMT was 83 days. The researchers observed that 1 month after FMT, two patients were free from CRE colonization, while one more patient was free from VRE after 3 months. Five patients received antibiotic during follow-up. Among them, one patient was decolonized at 1 month, while another was decolonized at 3 months. One patient with cirrhosis and persistent VRE carriage died 3 months after FMT from ascetic fluid infection and VRE bacteremia. No other adverse events were reported.
“In a context where no other efficient strategy is available, our first results show that FMT seems to be safe, with an impact on CRE decolonization at 1 month and on VRE decolonization at 3 months,” the researchers concluded. “Of particular importance, there was no recolonization after the intervention, which is contrary to decolonization with antibiotics. However, our study has important limitations in that the sample size was very small, it was nonrandomized, and follow-up was limited to a 3-month period.” They noted that at least five trials are underway to investigate the impact of FMT on multidrug resistant organism bacterial decolonization. The researchers reported having no financial disclosures.
The use of fecal microbiota transplantation is an option to eradicate highly drug-resistant enteric bacteria carriage, according to results from a small pilot study conducted by French investigators.
“A rapid and dramatic emergence of highly drug-resistant enteric bacteria (HDREB), i.e., carbapenem-resistant Enterobacteriaceae (CRE) and vancomycin-resistant enterococci (VRE), is occurring worldwide,” researchers led by Benjamin Davido, MD, of the infectious diseases unit at Raymond Poincaré Teaching Hospital, Garches, France, wrote in a study published online Feb. 1 in the Journal of Hospital Infection. “Patients carrying these bacteria are at risk of developing severe infections due to these bacteria; these infections are associated with a high mortality rate, partially because of inappropriate antimicrobial treatment.”
Citing recent studies that have demonstrated the efficacy of fecal microbiota transplant (FMT) as an accepted therapy to prevent recurrent Clostridium difficile infection, Dr. Davido and his associates prospectively identified eight different case reports of FMT used in adults for intestinal decolonization from extended-spectrum beta-lactamase (ESBL)–producing Enterobacteriaceae, VRE, or methicillin-resistant Staphylococcus aureus. Patients on immunosuppressive agents were excluded from the study, as were those taking antibiotics at the time of FMT (J Hosp Infect. 2017 Feb. 1. doi: 10.1016/j.jhin.2017.02.001).
The protocol for the procedure involved insertion of a nasoduodenal tube the day before FMT in order to perform a bowel lavage with XPrep solution. FMT was performed using a frozen preparation of fecal microbiota from a universal donor who was previously screened for potential diseases. The main outcome of interest was time to successful decolonization following FMT, which was determined by at least two consecutive negative rectal swabs at a 1-week interval during a follow-up of 3 months.
The mean age of the eight patients was 70 years, their median Charlson comorbidity index score was 5, and the median duration of carriage of HDREB before FMT was 83 days. The researchers observed that 1 month after FMT, two patients were free from CRE colonization, while one more patient was free from VRE after 3 months. Five patients received antibiotic during follow-up. Among them, one patient was decolonized at 1 month, while another was decolonized at 3 months. One patient with cirrhosis and persistent VRE carriage died 3 months after FMT from ascetic fluid infection and VRE bacteremia. No other adverse events were reported.
“In a context where no other efficient strategy is available, our first results show that FMT seems to be safe, with an impact on CRE decolonization at 1 month and on VRE decolonization at 3 months,” the researchers concluded. “Of particular importance, there was no recolonization after the intervention, which is contrary to decolonization with antibiotics. However, our study has important limitations in that the sample size was very small, it was nonrandomized, and follow-up was limited to a 3-month period.” They noted that at least five trials are underway to investigate the impact of FMT on multidrug resistant organism bacterial decolonization. The researchers reported having no financial disclosures.
FROM THE JOURNAL OF HOSPITAL INFECTION
Key clinical point:
Major finding: Of eight patients who underwent fecal microbiota transplantation (FMT) for carbapenem-resistant Enterobacteriaceae (CRE) and vancomycin-resistant enterococci (VRE) digestive tract decolonization, three achieved decolonization after 3 months.
Data source: A prospective study of eight cases of FMT used in adults for intestinal decolonization from drug-resistant enteric bacteria carriage.
Disclosures: The researchers reported having no financial disclosures.
Specific polymorphisms excluded in hemophilic arthropathy
Carriers of a hemochromatosis (HFE) gene mutation or a long (GT)n-repeat length within the HMOX1 promoter regions did not appear to have an increase in hemophilic arthropathy.
In 201 blood samples from patients with severe hemophilia A or B and 37 from patients with moderate disease, neither the presence of an HFE mutation nor a long (GT)n-repeat length was associated with an increase in joint damage. The assessment was based on Pettersson score after adjustment for disease severity, presence of inhibitors, annual joint bleeding rate (AJBR), age at Pettersson score and at clinic entrance, and birth cohort (standardized beta = 0.033 and -0.022, respectively), Lize F. van Vulpen, MD, of University Medical Center Utrecht, the Netherlands, reported at the annual meeting of the European Association of Haemophilia and Allied Disorders.
Study subjects had a median age of 43 years, median AJBRs of 2.5 and 0.5 for severe and moderate disease, respectively, and median Pettersson scores of 22 and 4 in the groups, respectively. An HFE mutation was detected in 91 patients, and their levels of ferritin, iron, and transferrin saturation were significantly increased, but other baseline characteristic were similar in those with and without HFE mutation, and regardless of (GT)n-repeat length.
The marked heterogeneity in joint damage seen among hemophilia patients was hypothesized in this study to be associated with differences in iron handling, but the findings failed to support that hypothesis, Dr. van Vulpen concluded.
Dr. van Vulpen reported having no disclosures.
Carriers of a hemochromatosis (HFE) gene mutation or a long (GT)n-repeat length within the HMOX1 promoter regions did not appear to have an increase in hemophilic arthropathy.
In 201 blood samples from patients with severe hemophilia A or B and 37 from patients with moderate disease, neither the presence of an HFE mutation nor a long (GT)n-repeat length was associated with an increase in joint damage. The assessment was based on Pettersson score after adjustment for disease severity, presence of inhibitors, annual joint bleeding rate (AJBR), age at Pettersson score and at clinic entrance, and birth cohort (standardized beta = 0.033 and -0.022, respectively), Lize F. van Vulpen, MD, of University Medical Center Utrecht, the Netherlands, reported at the annual meeting of the European Association of Haemophilia and Allied Disorders.
Study subjects had a median age of 43 years, median AJBRs of 2.5 and 0.5 for severe and moderate disease, respectively, and median Pettersson scores of 22 and 4 in the groups, respectively. An HFE mutation was detected in 91 patients, and their levels of ferritin, iron, and transferrin saturation were significantly increased, but other baseline characteristic were similar in those with and without HFE mutation, and regardless of (GT)n-repeat length.
The marked heterogeneity in joint damage seen among hemophilia patients was hypothesized in this study to be associated with differences in iron handling, but the findings failed to support that hypothesis, Dr. van Vulpen concluded.
Dr. van Vulpen reported having no disclosures.
Carriers of a hemochromatosis (HFE) gene mutation or a long (GT)n-repeat length within the HMOX1 promoter regions did not appear to have an increase in hemophilic arthropathy.
In 201 blood samples from patients with severe hemophilia A or B and 37 from patients with moderate disease, neither the presence of an HFE mutation nor a long (GT)n-repeat length was associated with an increase in joint damage. The assessment was based on Pettersson score after adjustment for disease severity, presence of inhibitors, annual joint bleeding rate (AJBR), age at Pettersson score and at clinic entrance, and birth cohort (standardized beta = 0.033 and -0.022, respectively), Lize F. van Vulpen, MD, of University Medical Center Utrecht, the Netherlands, reported at the annual meeting of the European Association of Haemophilia and Allied Disorders.
Study subjects had a median age of 43 years, median AJBRs of 2.5 and 0.5 for severe and moderate disease, respectively, and median Pettersson scores of 22 and 4 in the groups, respectively. An HFE mutation was detected in 91 patients, and their levels of ferritin, iron, and transferrin saturation were significantly increased, but other baseline characteristic were similar in those with and without HFE mutation, and regardless of (GT)n-repeat length.
The marked heterogeneity in joint damage seen among hemophilia patients was hypothesized in this study to be associated with differences in iron handling, but the findings failed to support that hypothesis, Dr. van Vulpen concluded.
Dr. van Vulpen reported having no disclosures.
FROM EAHAD 2017
Key clinical point:
Major finding: Neither the presence of an HFE mutation, nor a long (GT)n-repeat length was associated with an increase in joint damage as assessed by Pettersson score (standardized beta = 0.033 and –0.022, respectively).
Data source: An evaluation of blood samples from a cohort of 238 patients.
Disclosures: Dr. van Vulpen reported having no disclosures.
Low inhibitor incidence seen with new generation rhFVIII
Human-cl rhFVIII, a new generation recombinant factor VIII of human origin, appears to be associated with a low inhibitor incidence in patients with severe hemophilia A, according to interim findings from the ongoing NuProtect study.
In 66 evaluable previously untreated patients with at least 20 days of exposure to human-cl rhFVIII, the cumulative incidence of high-titer and low-titer inhibitor development was 12.8% and 8.4%, respectively, Ellis J. Neufeld, MD, reported at the annual meeting of the European Association for Haemophilia and Allied Disorders.
The median age at first treatment was 13 months (range of 3-135 months). Of 59 patients with available F8 gene analysis, 44 had high-risk mutations and 47 had null mutations; 1 had no mutation identified. After a median of 11.5 treatment exposure days, 8 of the 66 patients developed high-titer inhibitors and 5 developed low-titer inhibitors; 4 of those were transient, said Dr. Neufeld of Harvard Medical School, Boston.
Only two of the patients developed an inhibitor, including one high- and one low-titer inhibitor, after 20 exposure days. Among the 13 inhibitor patients, 12 had an F8 mutation identified; all were null, and 11 were high risk.
In a prior study of 201 patients with previously treated severe hemophilia A, no inhibitors were reported with human-cl rhFVIII – which is produced in human cells without chemical modification or protein fusion. The NuProtect study is looking at immunogenicity, efficacy, and safety in previously untreated patients. Final data are expected in 2018, Dr. Neufeld noted.
Dr. Neufeld disclosed financial ties to Octapharma, the sponsor of NuProtect, and numerous other pharmaceutical companies.
Human-cl rhFVIII, a new generation recombinant factor VIII of human origin, appears to be associated with a low inhibitor incidence in patients with severe hemophilia A, according to interim findings from the ongoing NuProtect study.
In 66 evaluable previously untreated patients with at least 20 days of exposure to human-cl rhFVIII, the cumulative incidence of high-titer and low-titer inhibitor development was 12.8% and 8.4%, respectively, Ellis J. Neufeld, MD, reported at the annual meeting of the European Association for Haemophilia and Allied Disorders.
The median age at first treatment was 13 months (range of 3-135 months). Of 59 patients with available F8 gene analysis, 44 had high-risk mutations and 47 had null mutations; 1 had no mutation identified. After a median of 11.5 treatment exposure days, 8 of the 66 patients developed high-titer inhibitors and 5 developed low-titer inhibitors; 4 of those were transient, said Dr. Neufeld of Harvard Medical School, Boston.
Only two of the patients developed an inhibitor, including one high- and one low-titer inhibitor, after 20 exposure days. Among the 13 inhibitor patients, 12 had an F8 mutation identified; all were null, and 11 were high risk.
In a prior study of 201 patients with previously treated severe hemophilia A, no inhibitors were reported with human-cl rhFVIII – which is produced in human cells without chemical modification or protein fusion. The NuProtect study is looking at immunogenicity, efficacy, and safety in previously untreated patients. Final data are expected in 2018, Dr. Neufeld noted.
Dr. Neufeld disclosed financial ties to Octapharma, the sponsor of NuProtect, and numerous other pharmaceutical companies.
Human-cl rhFVIII, a new generation recombinant factor VIII of human origin, appears to be associated with a low inhibitor incidence in patients with severe hemophilia A, according to interim findings from the ongoing NuProtect study.
In 66 evaluable previously untreated patients with at least 20 days of exposure to human-cl rhFVIII, the cumulative incidence of high-titer and low-titer inhibitor development was 12.8% and 8.4%, respectively, Ellis J. Neufeld, MD, reported at the annual meeting of the European Association for Haemophilia and Allied Disorders.
The median age at first treatment was 13 months (range of 3-135 months). Of 59 patients with available F8 gene analysis, 44 had high-risk mutations and 47 had null mutations; 1 had no mutation identified. After a median of 11.5 treatment exposure days, 8 of the 66 patients developed high-titer inhibitors and 5 developed low-titer inhibitors; 4 of those were transient, said Dr. Neufeld of Harvard Medical School, Boston.
Only two of the patients developed an inhibitor, including one high- and one low-titer inhibitor, after 20 exposure days. Among the 13 inhibitor patients, 12 had an F8 mutation identified; all were null, and 11 were high risk.
In a prior study of 201 patients with previously treated severe hemophilia A, no inhibitors were reported with human-cl rhFVIII – which is produced in human cells without chemical modification or protein fusion. The NuProtect study is looking at immunogenicity, efficacy, and safety in previously untreated patients. Final data are expected in 2018, Dr. Neufeld noted.
Dr. Neufeld disclosed financial ties to Octapharma, the sponsor of NuProtect, and numerous other pharmaceutical companies.
Key clinical point:
Major finding: The cumulative incidence of high-titer and low-titer inhibitor development was 12.8% and 8.4%, respectively.
Data source: The ongoing NuProtect study of 66 patients.
Disclosures: Dr. Neufeld disclosed financial ties to Octapharma, the sponsor of NuProtect, and numerous other pharmaceutical companies.
ACR guidelines on HBV screening called inadequate
SNOWMASS, COLO. – The ACR guidelines for hepatitis B virus screening prior to starting immunosuppressive therapy are sorely in need of an overhaul, Leonard H. Calabrese, MD, asserted at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“We need to do better,” declared Dr. Calabrese, professor of medicine and head of the section of clinical immunology at the Cleveland Clinic.
The current ACR guidelines on hepatitis B virus (HBV) screening in rheumatoid arthritis patients (Arthritis Rheumatol. 2016 Jan;68[1]:1-26) are unchanged since 2008. The recommendation is for selective screening confined to patients who are going to go on methotrexate or leflunomide or who state they have an HBV risk factor.
He recommended that his fellow rheumatologists skip the ACR guidance and instead do what he does, which is to follow the American Association for the Study of Liver Diseases (AASLD) guidelines that have been in place for the past 8 years (Hepatology. 2009 Sep;50[3]:661-2).
The AASLD screening algorithm is simple and straightforward: Screen everyone who is going to go on any form of immunosuppressive therapy or cancer therapy by testing for hepatitis B surface antigen (HBsAg) and hepatitis B core antibodies (anti-HBc).
The highest-risk group for HBV reactivation are patients who are HBsAg positive. They have an active infection and should be referred to a hepatologist for antiviral therapy. Depending upon the patient’s HBV DNA level and liver function test results, the antiviral therapy will be started either prior to or simultaneously with the immunosuppressive therapy.
The AASLD guidelines rank immunosuppressive agents in terms of their associated risk of HBV reactivation based upon the best available evidence. It’s a tricky business. Patients with a history of HBV are excluded from clinical trials of immunosuppressants, so the evidence is sketchy.
Nevertheless, the consensus is that among HBsAg-positive patients the risk associated with methotrexate and other antimetabolites is considered low. The tumor necrosis factor inhibitors are deemed to pose a moderate risk, as does cyclosporine. The risk of other cytokine inhibitors, including ustekinumab (Stelara) and abatacept (Orencia), is uncertain, but thought to be moderate to high. The use of corticosteroids at more than 20 mg/day for longer than 4 weeks is considered high-risk therapy. And rituximab (Rituxan), which carries a black box warning regarding HBV reactivation, is rated very-high-risk, as is cancer chemotherapy and hematopoietic stem cell transplantation.
An HBV screen that comes back HBsAg negative but anti-HBc positive indicates past HBV infection. This places a patient at intermediate risk for reactivation during immunosuppressive therapy. If Dr. Calabrese plans to put that patient on rituximab, he’ll make a referral to a hepatologist for consideration of antiviral therapy. If he’s going to use any other form of immunosuppressive therapy in a patient who is HBsAg negative/anti-HBc positive, he tests for HBV DNA every 1-3 months while the patient is on treatment. If HBV DNA becomes detectable, it’s time to start antiviral therapy.
No further action is required if a rheumatology patient’s HBV screen comes back negative for both HBsAg and anti-HBc.
“There are six approved drugs for HBV. They don’t have the power of the direct-acting antiviral agents used for hepatitis C. Cures are very infrequent. But lifelong viral suppression is readily achievable with these drugs, which are well tolerated and have low potential for drug-drug interactions. If patients have undetectable HBV DNA on antiviral therapy, I believe we can treat them with any of our rheumatologic regimens, including rituximab,” Dr. Calabrese said.
He reported having no financial conflicts of interest regarding his presentation.
SNOWMASS, COLO. – The ACR guidelines for hepatitis B virus screening prior to starting immunosuppressive therapy are sorely in need of an overhaul, Leonard H. Calabrese, MD, asserted at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“We need to do better,” declared Dr. Calabrese, professor of medicine and head of the section of clinical immunology at the Cleveland Clinic.
The current ACR guidelines on hepatitis B virus (HBV) screening in rheumatoid arthritis patients (Arthritis Rheumatol. 2016 Jan;68[1]:1-26) are unchanged since 2008. The recommendation is for selective screening confined to patients who are going to go on methotrexate or leflunomide or who state they have an HBV risk factor.
He recommended that his fellow rheumatologists skip the ACR guidance and instead do what he does, which is to follow the American Association for the Study of Liver Diseases (AASLD) guidelines that have been in place for the past 8 years (Hepatology. 2009 Sep;50[3]:661-2).
The AASLD screening algorithm is simple and straightforward: Screen everyone who is going to go on any form of immunosuppressive therapy or cancer therapy by testing for hepatitis B surface antigen (HBsAg) and hepatitis B core antibodies (anti-HBc).
The highest-risk group for HBV reactivation are patients who are HBsAg positive. They have an active infection and should be referred to a hepatologist for antiviral therapy. Depending upon the patient’s HBV DNA level and liver function test results, the antiviral therapy will be started either prior to or simultaneously with the immunosuppressive therapy.
The AASLD guidelines rank immunosuppressive agents in terms of their associated risk of HBV reactivation based upon the best available evidence. It’s a tricky business. Patients with a history of HBV are excluded from clinical trials of immunosuppressants, so the evidence is sketchy.
Nevertheless, the consensus is that among HBsAg-positive patients the risk associated with methotrexate and other antimetabolites is considered low. The tumor necrosis factor inhibitors are deemed to pose a moderate risk, as does cyclosporine. The risk of other cytokine inhibitors, including ustekinumab (Stelara) and abatacept (Orencia), is uncertain, but thought to be moderate to high. The use of corticosteroids at more than 20 mg/day for longer than 4 weeks is considered high-risk therapy. And rituximab (Rituxan), which carries a black box warning regarding HBV reactivation, is rated very-high-risk, as is cancer chemotherapy and hematopoietic stem cell transplantation.
An HBV screen that comes back HBsAg negative but anti-HBc positive indicates past HBV infection. This places a patient at intermediate risk for reactivation during immunosuppressive therapy. If Dr. Calabrese plans to put that patient on rituximab, he’ll make a referral to a hepatologist for consideration of antiviral therapy. If he’s going to use any other form of immunosuppressive therapy in a patient who is HBsAg negative/anti-HBc positive, he tests for HBV DNA every 1-3 months while the patient is on treatment. If HBV DNA becomes detectable, it’s time to start antiviral therapy.
No further action is required if a rheumatology patient’s HBV screen comes back negative for both HBsAg and anti-HBc.
“There are six approved drugs for HBV. They don’t have the power of the direct-acting antiviral agents used for hepatitis C. Cures are very infrequent. But lifelong viral suppression is readily achievable with these drugs, which are well tolerated and have low potential for drug-drug interactions. If patients have undetectable HBV DNA on antiviral therapy, I believe we can treat them with any of our rheumatologic regimens, including rituximab,” Dr. Calabrese said.
He reported having no financial conflicts of interest regarding his presentation.
SNOWMASS, COLO. – The ACR guidelines for hepatitis B virus screening prior to starting immunosuppressive therapy are sorely in need of an overhaul, Leonard H. Calabrese, MD, asserted at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“We need to do better,” declared Dr. Calabrese, professor of medicine and head of the section of clinical immunology at the Cleveland Clinic.
The current ACR guidelines on hepatitis B virus (HBV) screening in rheumatoid arthritis patients (Arthritis Rheumatol. 2016 Jan;68[1]:1-26) are unchanged since 2008. The recommendation is for selective screening confined to patients who are going to go on methotrexate or leflunomide or who state they have an HBV risk factor.
He recommended that his fellow rheumatologists skip the ACR guidance and instead do what he does, which is to follow the American Association for the Study of Liver Diseases (AASLD) guidelines that have been in place for the past 8 years (Hepatology. 2009 Sep;50[3]:661-2).
The AASLD screening algorithm is simple and straightforward: Screen everyone who is going to go on any form of immunosuppressive therapy or cancer therapy by testing for hepatitis B surface antigen (HBsAg) and hepatitis B core antibodies (anti-HBc).
The highest-risk group for HBV reactivation are patients who are HBsAg positive. They have an active infection and should be referred to a hepatologist for antiviral therapy. Depending upon the patient’s HBV DNA level and liver function test results, the antiviral therapy will be started either prior to or simultaneously with the immunosuppressive therapy.
The AASLD guidelines rank immunosuppressive agents in terms of their associated risk of HBV reactivation based upon the best available evidence. It’s a tricky business. Patients with a history of HBV are excluded from clinical trials of immunosuppressants, so the evidence is sketchy.
Nevertheless, the consensus is that among HBsAg-positive patients the risk associated with methotrexate and other antimetabolites is considered low. The tumor necrosis factor inhibitors are deemed to pose a moderate risk, as does cyclosporine. The risk of other cytokine inhibitors, including ustekinumab (Stelara) and abatacept (Orencia), is uncertain, but thought to be moderate to high. The use of corticosteroids at more than 20 mg/day for longer than 4 weeks is considered high-risk therapy. And rituximab (Rituxan), which carries a black box warning regarding HBV reactivation, is rated very-high-risk, as is cancer chemotherapy and hematopoietic stem cell transplantation.
An HBV screen that comes back HBsAg negative but anti-HBc positive indicates past HBV infection. This places a patient at intermediate risk for reactivation during immunosuppressive therapy. If Dr. Calabrese plans to put that patient on rituximab, he’ll make a referral to a hepatologist for consideration of antiviral therapy. If he’s going to use any other form of immunosuppressive therapy in a patient who is HBsAg negative/anti-HBc positive, he tests for HBV DNA every 1-3 months while the patient is on treatment. If HBV DNA becomes detectable, it’s time to start antiviral therapy.
No further action is required if a rheumatology patient’s HBV screen comes back negative for both HBsAg and anti-HBc.
“There are six approved drugs for HBV. They don’t have the power of the direct-acting antiviral agents used for hepatitis C. Cures are very infrequent. But lifelong viral suppression is readily achievable with these drugs, which are well tolerated and have low potential for drug-drug interactions. If patients have undetectable HBV DNA on antiviral therapy, I believe we can treat them with any of our rheumatologic regimens, including rituximab,” Dr. Calabrese said.
He reported having no financial conflicts of interest regarding his presentation.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Review of the Long-Term Effects of Proton Pump Inhibitors
Proton pump inhibitors (PPIs) are one of the most frequently used drug classes, given that they are readily accessible over-the-counter as well as via prescription. About 100 million PPI prescriptions dispensed an
The human stomach uses 3 primary neurotransmitters that regulate gastric acid secretion: acetylcholine (ACh), histamine (H), and gastrin (G). The interactions between these neurotransmitters promote and inhibit hydrogen ion (H+) generation. Stimulation of their corresponding receptors draws H+ into parietal cells that line the stomach. Once in the cell, a H+-K+-ATPase (more commonly known as the proton pump) actively transports H+ into the lumen of the stomach. The H+ bind with chlorine ions to form hydrochloric acid, which increases stomach acidity.5 Histamine receptors were thought to be responsible for the greatest degree of stimulation. Hence, histamine type 2-receptor antagonists (H2RAs) became a novel means of therapy to reduce stomach acidity. While utilizing H2RAs was effective, it was theorized the downstream inhibition of the action of all 3 neurotransmitters would serve as a more successful therapy. Therefore, PPIs were developed to target the H+-K+-ATPase Over the past decade, many studies have evaluated the long-term PPI adverse effects (AEs). These include calcium and magnesium malabsorption, vitamin B12 deficiency, Clostridium difficile (C difficile) associated disease (CDAD), and community-acquired pneumonia (CAP). Within the past year, data have become available linking PPI use to dementia and chronic kidney disease (CKD).3,4 The following article reviews literature on the safety of long-term PPI use and proposes recommendations for proper use for their most common indications.
Malabsorption
Calcium & Long-Term Fracture Risk
Calcium is an essential component in bone health and formation. In fact, 99% of all calcium found in the body is stored in bones.6 The primary source of calcium is through diet and oral supplements. After it is ingested, calcium is absorbed from the stomach into the blood in a pH dependent manner. If the pH of the stomach is too high (ie, too basic) calcium is not absorbed into blood and remains in the gastrointestinal (GI) tract for fecal excretion. Without sufficient calcium, the body’s osteoclasts and osteoblasts remain inactive, which hinders proper bone turnover.7
The decrease in acidity leads to calcium malabsorption and increases fracture risk long- term.8 Khalili and colleagues surveyed 80,000 postmenopausal women to measure the incidence of hip fracture in women taking PPIs. The study found that there was a 35% increase in risk of hip fracture among women who regularly used PPIs for at least 2 years (age-adjusted hazard ratio [HR] 1.35; 95% confidence interval [CI], 1.13 -1.62). Adjusted HRs for 4-year and 6- to 8-year use of a PPI was 1.42 (95% CI, 1.05-1.93) and 1.55 (95% CI, 1.03-2.32), respectively, indicating that the longer women were on PPI therapy, the higher the risk of hip fracture. The study also evaluated the time since stopping PPI and the risk of hip fracture. Women who stopped PPI use more than 2 years prior had a similar risk to that of women who never used a PPI, indicating that the effect was reversible.9
Magnesium
Magnesium is an important intracellular ion that has a number of key functions in metabolism and ion transport in the human body. Once ingested, magnesium is absorbed into the bloodstream from the small and large intestines via passive and active transport. Transient receptor potential melastatin 6 (TRPM6) is one of the essential proteins that serve as a transporter for magnesium.10 The high affinity for magnesium of these transporters allows them to maintain adequate levels of magnesium in the blood. In states of low magnesium (hypomagnesemia), the body is at risk for many AEs including seizures, arrhythmias, tetany, and hypotension.11
Proton pump inhibitors have been linked to hypomagnesemia, and recent evaluation has clarified a potential mechanism.12 TRPM6 activity is increased in an acidic environment. When a PPI increases the pH of the stomach, TRPM6 and magnesium levels decrease.12 Luk and colleagues identified 66,102 subjects experiencing AEs while taking a PPI. Hypomagnesemia had a prevalence rate of 1% in these patients. According to the researchers, PPIs were associated with hypomagnesemia and that pantoprazole had the highest incidence among all other PPIs studied (OR, 4.3; 95% CI, 3.3 – 5.7; P < .001).13
Vitamin B12
In recent years, vitamin B12 has been the subject of many studies. An area of concern is vitamin B12’s neurologic effect, as it has been successfully demonstrated that vitamin B12 is essential for proper cognitive function.14 Some data suggest that degeneration is present in parts of the spinal column in patients with cognitive decline or neurologic problems. These lesions are due to improper myelin formation and are specific to vitamin B12 deficiency.15 In 2013 the CDC published the Healthy Brain Initiative, which stated cognitive impairment can be caused by vitamin B12 deficiency.16
Similar to calcium, vitamin B12 needs an acidic environment to be digested and absorbed.17 Vitamin B12 is released from food proteins via gastric acid and pepsin. Once free, the vitamin B12 pairs with R-binders secreted in the stomach. Pancreatic enzymes then degrade this complex into a form that can be absorbed into circulation by the intestine. Given that PPIs reduce the acidity of the stomach, they also reduce the body’s ability to release vitamin B12 from food proteins and be paired with the R-binders.18
In 2013, Lam and colleagues evaluated the association between vitamin B12 deficiency and the use of PPIs and H2RAs. An extensive evaluation was performed on 25,956 patients with a diagnosis of vitamin B12 deficiency and 184,199 patients without. About 12% of patients with vitamin B12 deficiency had received more than a 2-year supply of a PPI, whereas only 7.2% of the patients without vitamin B12 deficiency received a 2-year supply of a PPI. Four point 3 percent of patients with vitamin B12 deficiency received more than a 2-year supply of an H2RA. Only 3.2% of patients without vitamin B12 deficiency received more than a 2-year supply of H2RA. The study concluded that a 2-year or greater history of PPI (OR, 1.65; 95% CI, 1.58-1.73) or H2RA (OR, 1.25; 95% CI, 1.17-1.34) use was associated with vitamin B12 deficiency.19
PPIs and Infections
Clostridium difficile-associated disease
Nationwide CDAD has become a prevalent infection nationwide. In 2011, C difficile caused nearly 500,000 infections and was associated with 29,000 deaths in the U.S.20 One study stated that C difficile is the third most common cause of infectious diarrhea in people aged >75 years.21
C difficile is part of the body’s normal flora in the large intestine. It grows and colonizes in an environment of low acidity. Therefore, in the stomach, where the pH is relatively low, C difficile is unable to colonize.22 When a PPI is introduced, the increased gastric pH increases the risk for CDAD.
Dial and colleagues conducted a multicenter case control study to determine whether gastric acid suppression increases the risk of CDAD. Compared with patients who did not take a gastric acid suppressant, those taking a PPI had a 2.9-fold increase in developing CDAD (95% CI, 2.4-3.4). Comparatively, H2RAs had a 2.0-fold increase for CDAD (95% CI, 1.6 to 2.7). These results correlated with the fact that PPIs have a greater impact on gastric pH than do H2RAs.23
Community-Acquired Pneumonia
Community-acquired pneumonia (CAP) has become a growing concern in the U.S. According to the Infectious Disease Society of America (IDSA) and American Thoracic clinical consensus guidelines, CAP remains one of the top reasons for hospitalizations in the U.S., and about 10% of patients admitted to the hospital for CAP end up in the intensive care unit (ICU).24 In the past, PPIs have been linked to patients’ predisposal for developingCAP.25 Although controversial, available evidence suggests a direct association. In 2008 Sarker and colleagues theorized a mechanism that the acid reduction of the gastric lumen allows for increased bacterial colonization in the upper part of the GI tract.26 Since the acidity of the stomach serves as a defense mechanism against many ingested bacteria, many pathogens will be able to survive in the more basic environment.25
Sarkar and colleagues went on to evaluate 80,000 cases over 15 years. The objective was to examine the association between PPI use and the date of diagnosis of the CAP infection, known as the index date. The study demonstrated that PPI use was not associated with increased CAP risk in the long-term (adjusted odds ratio (OR), 1.02; 95% CI, 0.97-1.08). The study did find a strong increase in the risk of CAP if a PPI was started within 2 days (adjusted OR, 6.53; 95% CI, 3.95-10.80), 7 days (adjusted OR, 3.79; 95% CI, 2.66-5.42), and 14 days (adjusted OR, 3.21; 95% CI, 2.46-4.18) of the index date.26
Four years later, de Jagar and colleagues examined the differences in microbial etiology in CAP patients with and without an active PPI. Over a 4-year study period, 463 individuals were selected with clinical suspicion of CAP. The microbial etiology could be determined in 70% of those patients. The remaining 30% were excluded due to an alternative diagnosis. One of the most likely pathogens to cause a CAP infection is Streptococcus pneumoniae (S pneumonia).27 Patients prescribed a PPI were significantly more likely to be infected with S pneumoniae than those not prescribed a PPI (28% vs 11%). The study concluded that the risk of S pneumoniae in patients taking a PPI was 2.23 times more likely (95% CI, 1.28-3.75).28
Dementia
In 2040, it is estimated that more than 80 million people will have from dementia.29 This is expected to become a large fiscal burden on the health care system. In 2010, about $604 billion was spent on therapy for dementia worldwide.30 Although no cure for dementia exists, it is more feasible than in previous years to prevent its occurrence. However, many medications, including PPIs, are associated with the development of dementia; therefore, it is important to minimize their use when possible.
As noted earlier vitamin B12 deficiency may lead to cognitive decline. Due to the malabsorption of vitamin B12 that results from PPI use, it is hypothesized that PPIs may be associated with incidence of dementia. Badiola and colleagues discovered that in the brains of mice given a PPI, levels of β-amyloid increased significantly affecting enzymes responsible for cognition.31 In a February 2016, JAMA article, researchers conducted a prospective cohort study evaluating 73,679 patients aged ≥75 years with no dementia at baseline. They went on to assess regular use of a PPI, defined as at least 1 PPI prescription every 3 months, and the incidence of dementia. Patients with regular use of a PPI (≥ 1 PPI prescription every 3 months) had a 44% increase risk of incident dementia (HR, 1.44; 95% CI, 1.36-1.52; P < .001).3 Therefore, it is theorized that avoiding PPI use in the elderly may prevent the development of dementia.
Chronic Kidney Disease
The prevalence of CKD has drastically increased in recent decades. It is estimated that up to 13% of people in the U.S. are affected by CKD.32 Some studies suggest that dosing errors occur at much higher rates in patients with declined glomerular filtration rate (GFR).33 The correct utilization use of medications becomes especially pertinent to this population. Several studies have already linked PPI use to acute interstitial nephritis (AIN) and acute kidney injury (AKI).34-36
Lazarus and colleagues evaluated the association between PPI use and the incidence of CKD. Their analysis was performed in a long-term running population-based cohort and replicated in a separate health care system. In the running cohort, patients receiving a PPI had a 1.45-fold greater chance of developing CKD (95% CI, 1.11-1.90; P = .006). In that same cohort, patients on a PPI had a 1.72-fold increase risk of AKI (95% CI, 1.28-2.30; P < .001).4 Similar outcomes were seen in the replicated cohort. However, the replicated cohort did observe that twice daily dosing of a PPI (adjusted HR, 1.46; CI, 1.28-1.67; P < .001) had a stronger association with CKD than once- daily dosing (adjusted HR, 1.15; 95% CI, 1.09-1.21; P < .001). H2RAs exhibited no association with CKD in the running cohort (HR, 1.15; 97% CI, 0.98-1.36; P = .10) or the replication cohort (HR, 0.93; 95% CI, 0.88-0.99; P = .03).4
Clinical PPI Recommendations
There are several FDA-approved and unapproved indications that warrant PPI therapy. Proton pump inhibitor indications include gastroesophageal reflux disease (GERD), peptic ulcer disease (PUD), Helicobacter pylori, and ulcers associated with the use of nonsteroidal anti-inflammatory drugs (NSAIDs).
GERD Recommendations
Optimal dosing and duration is important with all medications to maximize efficacy and minimize toxicity. In the case of PPIs, dosing and duration are of particularly concern due to the aforementioned AEs. Table illustrates manufacturer-recommended dosing and duration for the most commonly prescribed PPIs. Although these dosing regimens are based on clinical studies, PPIs are commonly prescribed at higher doses and for longer durations. By extending the duration of therapy, the risk of potential long-term AEs increases dramatically. If durations are limited to the recommended window, risk of AEs can be reduced.
Alternative Therapies
There are several strategies that exist to limit the use of PPIs, including lifestyle modifications to prevent GERD, supplementation of an alternative agent to prevent high doses of the PPI, or discontinuing PPI therapy all together. Lifestyle modifications provide additional benefit as monotherapy or to supplement a pharmacologic regimen.
The American Journal of Gastroenterology promoted lifestyle modifications that include:
- Weight loss for patients with GERD who are overweight and had a recent weight gain;
- Elevation of the head of the bed (if nighttime symptoms present);
- Elimination of dietary triggers;
- Fatty foods, caffeine, chocolate, spicy food, food with high fat content, carbonated beverages, and peppermint;
- Avoiding tight fitting garments to prevent increase in gastric pressure;
- Promote salivation through oral lozenges or chewing gum to neutralize refluxed acid;
- Avoidance of tobacco and alcohol; and
- Abdominal breathing exercise to strengthen the barrier of the lower esophageal sphincter.37
The above modifications may reduce the need for pharmacologic therapy, thereby reducing possible of long-term AEs.
If lifestyle modifications alone are not enough, it is reasonable to use a H2RA for acute symptom relief or reduce high doses and frequencies of a PPI. H2RAs are well studied and effective in the management of GERD. According to the American College of Gastroenterology 2013 clinical practice guidelines, H2RAs can serve as an effective maintenance medication to relieve heartburn in patients without erosive disease. The guideline also states that a bedtime H2RA can be used to supplement a once- daily daytime PPI if nighttime reflux exists. This can eliminate the need to exceed manufacturer-recommended doses.37
One of the final challenges to overcome is a patient that has been maintained on chronic PPI therapy. However, caution should be exercised if choosing to discontinue a PPI. In a study by Niklesson and colleagues, after a 4-week course of pantoprazole given to healthy volunteers, those patients with no preexisting symptoms developed dyspeptic symptoms of GERD, such as heartburn, indigestion, and stomach discomfort. This correlation suggests that a rebound hypersecretion occurs after prolonged suppression of the proton pump, and therefore a gradual taper should be used.38 Although no definitive national recommendations on how to taper a patient off of a PPI exist, one suggestion is a 2- to 3-week taper by using a half-dose once daily or full dose on alternate days.39 This strategy has exhibited moderate success rates when used. Oral and written education on symptom management and the administration of H2RAs for infrequent breakthrough symptoms supplemented the reduction of the PPI.
Conclusion
Proton pump inhibitors have become a popular and effective drug class for a multitude of indications. However, it is crucial to recognize the risk of long-term use. It is important to properly assess the need for a PPI and to use appropriate dosing and duration, since prolonged durations and doses above the manufacturer’s recommendations is a primary contributor to long-term consequences. Both package inserts and clinical guidelines serve as valuable resources to help balance the risks and benefits of this medication class and can help guide therapeutic decisions.
1. U.S. Food and Drug Administration. FDA Drug Safety Communication: Low magnesium levels can be associated with long-term use of Proton Pump Inhibitor drugs (PPIs). http://www.fda.gov/Drugs/DrugSafety/ucm245011.htm. Updated April 7, 2016. Accessed January 12, 2017.
2. Forgacs I. Overprescribing proton pump inhibitors. BMJ. 2008;336(7634):2-3.
3. Gomm W, von Holt K, Thome F, et al. Association of proton pump inhibitors with risk of dementia. JAMA Neurol. 2016;73(4):410-416.
4. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176(2):238-246.
5. Wolfe MM, Soll AH. The physiology of gastric acid secretion. N Engl J Med. 1988;319(26):1707-1715.
6. Flynn A. The role of dietary calcium in bone health. Proc Nutr Soc. 2003;62(4):851-858.
7. Mizunashi K, Furukawa Y, Katano K, Abe K. Effect of omeprazole, an inhibitor of H+, K(+)-ATPase, on bone resorption in humans. Calcif Tissue Int. 1993;53(1):21-25.
8. O’Connell MB, Darren DM, Murray AM, Heaney RP, Kerzner LJ. Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am J Med. 2005;118(7):778-781.
9. Khalili H, Huang ES, Jacobson BC, Camargo CA Jr, Feskanich D, Chan AT. Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: a prospective cohort study. BMJ. 2012;344:e372.
10. Schweigel M, Martens H. Magnesium transport in the gastrointestinal tract. Front Biosci. 2000;5:D666-D677.
11. Hess MW, Hoenderop JG, Bindels RJ, Drenth JP. Systematic review: hypomagnesaemia induced by proton pump inhibition. Aliment Pharmacol Ther. 2012;36(5):405-413.
12. William JH, Danziger J. Proton-pump inhibitor-induced hypomagnesemia: current research and proposed mechanisms. World J Nephrol. 2016;5(2):152-157.
13. Luk CP, Parsons R, Lee YP, Hughes JD. Proton pump inhibitor-associated hypomagnesemia: what do FDA data tell us? Ann Pharmacother. 2013;47(6):773-780.
14. Health Quality Ontario. Vitamin B12 and cognitive function: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13(23):1-45.
15. Green R, Kinsella LJ. Current concepts in the diagnosis of cobalamin deficiency. Neurology. 1995;45(8):1435-1440.
16. Centers for Disease Control and Prevention. The Healthy Brain Initiative. https://www.cdc.gov/aging/pdf/2013-healthy-brain-initiative.pdf. Accessed January 17, 2017.
17. Toh BH, van Driel IR, Gleeson PA. Pernicious anemia. N Engl J Med. 1997;337(20):1441-1448.
18. Tefferi A, Pruthi RK. The biochemical basis of cobalamin deficiency. Mayo Clin Proc. 1994;69(2):181-186.
19. Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310(22):2435-2442.
20. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825-834.
21. National Clostridium difficile Standards Group. National Clostridium difficile Standards Group: report to the Department of Health. J Hosp Infect. 2004;56(suppl 1):1-38.
22. Thorens J, Frohlich F, Schwizer W, et al. Bacterial overgrowth during treatment with omeprazole compared with cimetidine. Gut. 1996;39(1):54-59.
23. Dial S, Delaney JAC, Barkun AN, et al. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294(23):2989-2995
24. Mandell LA, Wunderink RG, Anzueto A, et al; Infectious Diseases Society of America; and American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
25. Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA. 2004;292(16):1955-1960.
26. Sarkar M, Hennessy S, Yang Y. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med. 2008;149(6):391-398.
27. Waterer GW, Wunderink RG. The influence of the severity of community-acquired pneumonia on the usefulness of blood cultures. Respir Med. 2001;95(1):78-82.
28. de Jagar CP, Wever PC, Gemen EF, et al. Proton pump inhibitor therapy predisposes to community-acquired Streptococcus pneumoniae pneumonia. Aliment Pharmacol Ther. 2012;36(10):941-949.
29. Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7(3):137-152.
30. Wimo A, Jönsson L, Bond J, Prince M, Winblad B; Alzheimer Disease International. The worldwide economic impact of dementia 2010. Alzheimers Dement. 2013;9(1):1-11.
31. Badiola N, Alcalde V, Pujol A, et al. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One. 2013;8(3):e58537.
32. Stevens LA, Li S, Wang C, et al. Prevalence of CKD and comorbid illness in elderly patients in the United States: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2010;55(3)(suppl 2):S23-S33.
33. Weir MR, Fink JC. Safety of medical therapy in patients with chronic kidney disease and end-stage renal disease. Curr Opin Nephrol Hypertens 2014;23(3):306-313.
34. Blank ML, Parkin L, Paul C, Herbison P. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86(4):837-844.
35. Antoniou T, Macdonald EM, Holland S, et al. Proton pump inhibitors and the risk of acute kidney injury in older patients: a population-based cohort study. CMAJ Open. 2015;3(2):E166-E171.
36. Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrol. 2013;14:150.
37. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108(3):308-328.
38. Niklasson A, Lindström L, Simrén M, Lindberg G, Björnsson E. Dyspeptic symptom development after discontinuation of a proton pump inhibitor: a double-blind placebo-controlled trial. Am J Gastroenterol. 2010;105(7):1531-1537.
39. Haastrup P, Paulsen MS, Begtrup LM, Hansen JM, Jarbøl DE. Strategies for discontinuation of proton pump inhibitors: a systematic review. Fam Pract. 2014;31(6):625-630.
40. Nexium [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2012.
41. Prevacid [package insert]. Deerfield, IL: Takeda Pharmaceuticals; 2012.
42. Prilosec [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2012.
43. Protonix [package insert]. Konstanz, Germany: Pfizer; 2012.
Proton pump inhibitors (PPIs) are one of the most frequently used drug classes, given that they are readily accessible over-the-counter as well as via prescription. About 100 million PPI prescriptions dispensed an
The human stomach uses 3 primary neurotransmitters that regulate gastric acid secretion: acetylcholine (ACh), histamine (H), and gastrin (G). The interactions between these neurotransmitters promote and inhibit hydrogen ion (H+) generation. Stimulation of their corresponding receptors draws H+ into parietal cells that line the stomach. Once in the cell, a H+-K+-ATPase (more commonly known as the proton pump) actively transports H+ into the lumen of the stomach. The H+ bind with chlorine ions to form hydrochloric acid, which increases stomach acidity.5 Histamine receptors were thought to be responsible for the greatest degree of stimulation. Hence, histamine type 2-receptor antagonists (H2RAs) became a novel means of therapy to reduce stomach acidity. While utilizing H2RAs was effective, it was theorized the downstream inhibition of the action of all 3 neurotransmitters would serve as a more successful therapy. Therefore, PPIs were developed to target the H+-K+-ATPase Over the past decade, many studies have evaluated the long-term PPI adverse effects (AEs). These include calcium and magnesium malabsorption, vitamin B12 deficiency, Clostridium difficile (C difficile) associated disease (CDAD), and community-acquired pneumonia (CAP). Within the past year, data have become available linking PPI use to dementia and chronic kidney disease (CKD).3,4 The following article reviews literature on the safety of long-term PPI use and proposes recommendations for proper use for their most common indications.
Malabsorption
Calcium & Long-Term Fracture Risk
Calcium is an essential component in bone health and formation. In fact, 99% of all calcium found in the body is stored in bones.6 The primary source of calcium is through diet and oral supplements. After it is ingested, calcium is absorbed from the stomach into the blood in a pH dependent manner. If the pH of the stomach is too high (ie, too basic) calcium is not absorbed into blood and remains in the gastrointestinal (GI) tract for fecal excretion. Without sufficient calcium, the body’s osteoclasts and osteoblasts remain inactive, which hinders proper bone turnover.7
The decrease in acidity leads to calcium malabsorption and increases fracture risk long- term.8 Khalili and colleagues surveyed 80,000 postmenopausal women to measure the incidence of hip fracture in women taking PPIs. The study found that there was a 35% increase in risk of hip fracture among women who regularly used PPIs for at least 2 years (age-adjusted hazard ratio [HR] 1.35; 95% confidence interval [CI], 1.13 -1.62). Adjusted HRs for 4-year and 6- to 8-year use of a PPI was 1.42 (95% CI, 1.05-1.93) and 1.55 (95% CI, 1.03-2.32), respectively, indicating that the longer women were on PPI therapy, the higher the risk of hip fracture. The study also evaluated the time since stopping PPI and the risk of hip fracture. Women who stopped PPI use more than 2 years prior had a similar risk to that of women who never used a PPI, indicating that the effect was reversible.9
Magnesium
Magnesium is an important intracellular ion that has a number of key functions in metabolism and ion transport in the human body. Once ingested, magnesium is absorbed into the bloodstream from the small and large intestines via passive and active transport. Transient receptor potential melastatin 6 (TRPM6) is one of the essential proteins that serve as a transporter for magnesium.10 The high affinity for magnesium of these transporters allows them to maintain adequate levels of magnesium in the blood. In states of low magnesium (hypomagnesemia), the body is at risk for many AEs including seizures, arrhythmias, tetany, and hypotension.11
Proton pump inhibitors have been linked to hypomagnesemia, and recent evaluation has clarified a potential mechanism.12 TRPM6 activity is increased in an acidic environment. When a PPI increases the pH of the stomach, TRPM6 and magnesium levels decrease.12 Luk and colleagues identified 66,102 subjects experiencing AEs while taking a PPI. Hypomagnesemia had a prevalence rate of 1% in these patients. According to the researchers, PPIs were associated with hypomagnesemia and that pantoprazole had the highest incidence among all other PPIs studied (OR, 4.3; 95% CI, 3.3 – 5.7; P < .001).13
Vitamin B12
In recent years, vitamin B12 has been the subject of many studies. An area of concern is vitamin B12’s neurologic effect, as it has been successfully demonstrated that vitamin B12 is essential for proper cognitive function.14 Some data suggest that degeneration is present in parts of the spinal column in patients with cognitive decline or neurologic problems. These lesions are due to improper myelin formation and are specific to vitamin B12 deficiency.15 In 2013 the CDC published the Healthy Brain Initiative, which stated cognitive impairment can be caused by vitamin B12 deficiency.16
Similar to calcium, vitamin B12 needs an acidic environment to be digested and absorbed.17 Vitamin B12 is released from food proteins via gastric acid and pepsin. Once free, the vitamin B12 pairs with R-binders secreted in the stomach. Pancreatic enzymes then degrade this complex into a form that can be absorbed into circulation by the intestine. Given that PPIs reduce the acidity of the stomach, they also reduce the body’s ability to release vitamin B12 from food proteins and be paired with the R-binders.18
In 2013, Lam and colleagues evaluated the association between vitamin B12 deficiency and the use of PPIs and H2RAs. An extensive evaluation was performed on 25,956 patients with a diagnosis of vitamin B12 deficiency and 184,199 patients without. About 12% of patients with vitamin B12 deficiency had received more than a 2-year supply of a PPI, whereas only 7.2% of the patients without vitamin B12 deficiency received a 2-year supply of a PPI. Four point 3 percent of patients with vitamin B12 deficiency received more than a 2-year supply of an H2RA. Only 3.2% of patients without vitamin B12 deficiency received more than a 2-year supply of H2RA. The study concluded that a 2-year or greater history of PPI (OR, 1.65; 95% CI, 1.58-1.73) or H2RA (OR, 1.25; 95% CI, 1.17-1.34) use was associated with vitamin B12 deficiency.19
PPIs and Infections
Clostridium difficile-associated disease
Nationwide CDAD has become a prevalent infection nationwide. In 2011, C difficile caused nearly 500,000 infections and was associated with 29,000 deaths in the U.S.20 One study stated that C difficile is the third most common cause of infectious diarrhea in people aged >75 years.21
C difficile is part of the body’s normal flora in the large intestine. It grows and colonizes in an environment of low acidity. Therefore, in the stomach, where the pH is relatively low, C difficile is unable to colonize.22 When a PPI is introduced, the increased gastric pH increases the risk for CDAD.
Dial and colleagues conducted a multicenter case control study to determine whether gastric acid suppression increases the risk of CDAD. Compared with patients who did not take a gastric acid suppressant, those taking a PPI had a 2.9-fold increase in developing CDAD (95% CI, 2.4-3.4). Comparatively, H2RAs had a 2.0-fold increase for CDAD (95% CI, 1.6 to 2.7). These results correlated with the fact that PPIs have a greater impact on gastric pH than do H2RAs.23
Community-Acquired Pneumonia
Community-acquired pneumonia (CAP) has become a growing concern in the U.S. According to the Infectious Disease Society of America (IDSA) and American Thoracic clinical consensus guidelines, CAP remains one of the top reasons for hospitalizations in the U.S., and about 10% of patients admitted to the hospital for CAP end up in the intensive care unit (ICU).24 In the past, PPIs have been linked to patients’ predisposal for developingCAP.25 Although controversial, available evidence suggests a direct association. In 2008 Sarker and colleagues theorized a mechanism that the acid reduction of the gastric lumen allows for increased bacterial colonization in the upper part of the GI tract.26 Since the acidity of the stomach serves as a defense mechanism against many ingested bacteria, many pathogens will be able to survive in the more basic environment.25
Sarkar and colleagues went on to evaluate 80,000 cases over 15 years. The objective was to examine the association between PPI use and the date of diagnosis of the CAP infection, known as the index date. The study demonstrated that PPI use was not associated with increased CAP risk in the long-term (adjusted odds ratio (OR), 1.02; 95% CI, 0.97-1.08). The study did find a strong increase in the risk of CAP if a PPI was started within 2 days (adjusted OR, 6.53; 95% CI, 3.95-10.80), 7 days (adjusted OR, 3.79; 95% CI, 2.66-5.42), and 14 days (adjusted OR, 3.21; 95% CI, 2.46-4.18) of the index date.26
Four years later, de Jagar and colleagues examined the differences in microbial etiology in CAP patients with and without an active PPI. Over a 4-year study period, 463 individuals were selected with clinical suspicion of CAP. The microbial etiology could be determined in 70% of those patients. The remaining 30% were excluded due to an alternative diagnosis. One of the most likely pathogens to cause a CAP infection is Streptococcus pneumoniae (S pneumonia).27 Patients prescribed a PPI were significantly more likely to be infected with S pneumoniae than those not prescribed a PPI (28% vs 11%). The study concluded that the risk of S pneumoniae in patients taking a PPI was 2.23 times more likely (95% CI, 1.28-3.75).28
Dementia
In 2040, it is estimated that more than 80 million people will have from dementia.29 This is expected to become a large fiscal burden on the health care system. In 2010, about $604 billion was spent on therapy for dementia worldwide.30 Although no cure for dementia exists, it is more feasible than in previous years to prevent its occurrence. However, many medications, including PPIs, are associated with the development of dementia; therefore, it is important to minimize their use when possible.
As noted earlier vitamin B12 deficiency may lead to cognitive decline. Due to the malabsorption of vitamin B12 that results from PPI use, it is hypothesized that PPIs may be associated with incidence of dementia. Badiola and colleagues discovered that in the brains of mice given a PPI, levels of β-amyloid increased significantly affecting enzymes responsible for cognition.31 In a February 2016, JAMA article, researchers conducted a prospective cohort study evaluating 73,679 patients aged ≥75 years with no dementia at baseline. They went on to assess regular use of a PPI, defined as at least 1 PPI prescription every 3 months, and the incidence of dementia. Patients with regular use of a PPI (≥ 1 PPI prescription every 3 months) had a 44% increase risk of incident dementia (HR, 1.44; 95% CI, 1.36-1.52; P < .001).3 Therefore, it is theorized that avoiding PPI use in the elderly may prevent the development of dementia.
Chronic Kidney Disease
The prevalence of CKD has drastically increased in recent decades. It is estimated that up to 13% of people in the U.S. are affected by CKD.32 Some studies suggest that dosing errors occur at much higher rates in patients with declined glomerular filtration rate (GFR).33 The correct utilization use of medications becomes especially pertinent to this population. Several studies have already linked PPI use to acute interstitial nephritis (AIN) and acute kidney injury (AKI).34-36
Lazarus and colleagues evaluated the association between PPI use and the incidence of CKD. Their analysis was performed in a long-term running population-based cohort and replicated in a separate health care system. In the running cohort, patients receiving a PPI had a 1.45-fold greater chance of developing CKD (95% CI, 1.11-1.90; P = .006). In that same cohort, patients on a PPI had a 1.72-fold increase risk of AKI (95% CI, 1.28-2.30; P < .001).4 Similar outcomes were seen in the replicated cohort. However, the replicated cohort did observe that twice daily dosing of a PPI (adjusted HR, 1.46; CI, 1.28-1.67; P < .001) had a stronger association with CKD than once- daily dosing (adjusted HR, 1.15; 95% CI, 1.09-1.21; P < .001). H2RAs exhibited no association with CKD in the running cohort (HR, 1.15; 97% CI, 0.98-1.36; P = .10) or the replication cohort (HR, 0.93; 95% CI, 0.88-0.99; P = .03).4
Clinical PPI Recommendations
There are several FDA-approved and unapproved indications that warrant PPI therapy. Proton pump inhibitor indications include gastroesophageal reflux disease (GERD), peptic ulcer disease (PUD), Helicobacter pylori, and ulcers associated with the use of nonsteroidal anti-inflammatory drugs (NSAIDs).
GERD Recommendations
Optimal dosing and duration is important with all medications to maximize efficacy and minimize toxicity. In the case of PPIs, dosing and duration are of particularly concern due to the aforementioned AEs. Table illustrates manufacturer-recommended dosing and duration for the most commonly prescribed PPIs. Although these dosing regimens are based on clinical studies, PPIs are commonly prescribed at higher doses and for longer durations. By extending the duration of therapy, the risk of potential long-term AEs increases dramatically. If durations are limited to the recommended window, risk of AEs can be reduced.
Alternative Therapies
There are several strategies that exist to limit the use of PPIs, including lifestyle modifications to prevent GERD, supplementation of an alternative agent to prevent high doses of the PPI, or discontinuing PPI therapy all together. Lifestyle modifications provide additional benefit as monotherapy or to supplement a pharmacologic regimen.
The American Journal of Gastroenterology promoted lifestyle modifications that include:
- Weight loss for patients with GERD who are overweight and had a recent weight gain;
- Elevation of the head of the bed (if nighttime symptoms present);
- Elimination of dietary triggers;
- Fatty foods, caffeine, chocolate, spicy food, food with high fat content, carbonated beverages, and peppermint;
- Avoiding tight fitting garments to prevent increase in gastric pressure;
- Promote salivation through oral lozenges or chewing gum to neutralize refluxed acid;
- Avoidance of tobacco and alcohol; and
- Abdominal breathing exercise to strengthen the barrier of the lower esophageal sphincter.37
The above modifications may reduce the need for pharmacologic therapy, thereby reducing possible of long-term AEs.
If lifestyle modifications alone are not enough, it is reasonable to use a H2RA for acute symptom relief or reduce high doses and frequencies of a PPI. H2RAs are well studied and effective in the management of GERD. According to the American College of Gastroenterology 2013 clinical practice guidelines, H2RAs can serve as an effective maintenance medication to relieve heartburn in patients without erosive disease. The guideline also states that a bedtime H2RA can be used to supplement a once- daily daytime PPI if nighttime reflux exists. This can eliminate the need to exceed manufacturer-recommended doses.37
One of the final challenges to overcome is a patient that has been maintained on chronic PPI therapy. However, caution should be exercised if choosing to discontinue a PPI. In a study by Niklesson and colleagues, after a 4-week course of pantoprazole given to healthy volunteers, those patients with no preexisting symptoms developed dyspeptic symptoms of GERD, such as heartburn, indigestion, and stomach discomfort. This correlation suggests that a rebound hypersecretion occurs after prolonged suppression of the proton pump, and therefore a gradual taper should be used.38 Although no definitive national recommendations on how to taper a patient off of a PPI exist, one suggestion is a 2- to 3-week taper by using a half-dose once daily or full dose on alternate days.39 This strategy has exhibited moderate success rates when used. Oral and written education on symptom management and the administration of H2RAs for infrequent breakthrough symptoms supplemented the reduction of the PPI.
Conclusion
Proton pump inhibitors have become a popular and effective drug class for a multitude of indications. However, it is crucial to recognize the risk of long-term use. It is important to properly assess the need for a PPI and to use appropriate dosing and duration, since prolonged durations and doses above the manufacturer’s recommendations is a primary contributor to long-term consequences. Both package inserts and clinical guidelines serve as valuable resources to help balance the risks and benefits of this medication class and can help guide therapeutic decisions.
Proton pump inhibitors (PPIs) are one of the most frequently used drug classes, given that they are readily accessible over-the-counter as well as via prescription. About 100 million PPI prescriptions dispensed an
The human stomach uses 3 primary neurotransmitters that regulate gastric acid secretion: acetylcholine (ACh), histamine (H), and gastrin (G). The interactions between these neurotransmitters promote and inhibit hydrogen ion (H+) generation. Stimulation of their corresponding receptors draws H+ into parietal cells that line the stomach. Once in the cell, a H+-K+-ATPase (more commonly known as the proton pump) actively transports H+ into the lumen of the stomach. The H+ bind with chlorine ions to form hydrochloric acid, which increases stomach acidity.5 Histamine receptors were thought to be responsible for the greatest degree of stimulation. Hence, histamine type 2-receptor antagonists (H2RAs) became a novel means of therapy to reduce stomach acidity. While utilizing H2RAs was effective, it was theorized the downstream inhibition of the action of all 3 neurotransmitters would serve as a more successful therapy. Therefore, PPIs were developed to target the H+-K+-ATPase Over the past decade, many studies have evaluated the long-term PPI adverse effects (AEs). These include calcium and magnesium malabsorption, vitamin B12 deficiency, Clostridium difficile (C difficile) associated disease (CDAD), and community-acquired pneumonia (CAP). Within the past year, data have become available linking PPI use to dementia and chronic kidney disease (CKD).3,4 The following article reviews literature on the safety of long-term PPI use and proposes recommendations for proper use for their most common indications.
Malabsorption
Calcium & Long-Term Fracture Risk
Calcium is an essential component in bone health and formation. In fact, 99% of all calcium found in the body is stored in bones.6 The primary source of calcium is through diet and oral supplements. After it is ingested, calcium is absorbed from the stomach into the blood in a pH dependent manner. If the pH of the stomach is too high (ie, too basic) calcium is not absorbed into blood and remains in the gastrointestinal (GI) tract for fecal excretion. Without sufficient calcium, the body’s osteoclasts and osteoblasts remain inactive, which hinders proper bone turnover.7
The decrease in acidity leads to calcium malabsorption and increases fracture risk long- term.8 Khalili and colleagues surveyed 80,000 postmenopausal women to measure the incidence of hip fracture in women taking PPIs. The study found that there was a 35% increase in risk of hip fracture among women who regularly used PPIs for at least 2 years (age-adjusted hazard ratio [HR] 1.35; 95% confidence interval [CI], 1.13 -1.62). Adjusted HRs for 4-year and 6- to 8-year use of a PPI was 1.42 (95% CI, 1.05-1.93) and 1.55 (95% CI, 1.03-2.32), respectively, indicating that the longer women were on PPI therapy, the higher the risk of hip fracture. The study also evaluated the time since stopping PPI and the risk of hip fracture. Women who stopped PPI use more than 2 years prior had a similar risk to that of women who never used a PPI, indicating that the effect was reversible.9
Magnesium
Magnesium is an important intracellular ion that has a number of key functions in metabolism and ion transport in the human body. Once ingested, magnesium is absorbed into the bloodstream from the small and large intestines via passive and active transport. Transient receptor potential melastatin 6 (TRPM6) is one of the essential proteins that serve as a transporter for magnesium.10 The high affinity for magnesium of these transporters allows them to maintain adequate levels of magnesium in the blood. In states of low magnesium (hypomagnesemia), the body is at risk for many AEs including seizures, arrhythmias, tetany, and hypotension.11
Proton pump inhibitors have been linked to hypomagnesemia, and recent evaluation has clarified a potential mechanism.12 TRPM6 activity is increased in an acidic environment. When a PPI increases the pH of the stomach, TRPM6 and magnesium levels decrease.12 Luk and colleagues identified 66,102 subjects experiencing AEs while taking a PPI. Hypomagnesemia had a prevalence rate of 1% in these patients. According to the researchers, PPIs were associated with hypomagnesemia and that pantoprazole had the highest incidence among all other PPIs studied (OR, 4.3; 95% CI, 3.3 – 5.7; P < .001).13
Vitamin B12
In recent years, vitamin B12 has been the subject of many studies. An area of concern is vitamin B12’s neurologic effect, as it has been successfully demonstrated that vitamin B12 is essential for proper cognitive function.14 Some data suggest that degeneration is present in parts of the spinal column in patients with cognitive decline or neurologic problems. These lesions are due to improper myelin formation and are specific to vitamin B12 deficiency.15 In 2013 the CDC published the Healthy Brain Initiative, which stated cognitive impairment can be caused by vitamin B12 deficiency.16
Similar to calcium, vitamin B12 needs an acidic environment to be digested and absorbed.17 Vitamin B12 is released from food proteins via gastric acid and pepsin. Once free, the vitamin B12 pairs with R-binders secreted in the stomach. Pancreatic enzymes then degrade this complex into a form that can be absorbed into circulation by the intestine. Given that PPIs reduce the acidity of the stomach, they also reduce the body’s ability to release vitamin B12 from food proteins and be paired with the R-binders.18
In 2013, Lam and colleagues evaluated the association between vitamin B12 deficiency and the use of PPIs and H2RAs. An extensive evaluation was performed on 25,956 patients with a diagnosis of vitamin B12 deficiency and 184,199 patients without. About 12% of patients with vitamin B12 deficiency had received more than a 2-year supply of a PPI, whereas only 7.2% of the patients without vitamin B12 deficiency received a 2-year supply of a PPI. Four point 3 percent of patients with vitamin B12 deficiency received more than a 2-year supply of an H2RA. Only 3.2% of patients without vitamin B12 deficiency received more than a 2-year supply of H2RA. The study concluded that a 2-year or greater history of PPI (OR, 1.65; 95% CI, 1.58-1.73) or H2RA (OR, 1.25; 95% CI, 1.17-1.34) use was associated with vitamin B12 deficiency.19
PPIs and Infections
Clostridium difficile-associated disease
Nationwide CDAD has become a prevalent infection nationwide. In 2011, C difficile caused nearly 500,000 infections and was associated with 29,000 deaths in the U.S.20 One study stated that C difficile is the third most common cause of infectious diarrhea in people aged >75 years.21
C difficile is part of the body’s normal flora in the large intestine. It grows and colonizes in an environment of low acidity. Therefore, in the stomach, where the pH is relatively low, C difficile is unable to colonize.22 When a PPI is introduced, the increased gastric pH increases the risk for CDAD.
Dial and colleagues conducted a multicenter case control study to determine whether gastric acid suppression increases the risk of CDAD. Compared with patients who did not take a gastric acid suppressant, those taking a PPI had a 2.9-fold increase in developing CDAD (95% CI, 2.4-3.4). Comparatively, H2RAs had a 2.0-fold increase for CDAD (95% CI, 1.6 to 2.7). These results correlated with the fact that PPIs have a greater impact on gastric pH than do H2RAs.23
Community-Acquired Pneumonia
Community-acquired pneumonia (CAP) has become a growing concern in the U.S. According to the Infectious Disease Society of America (IDSA) and American Thoracic clinical consensus guidelines, CAP remains one of the top reasons for hospitalizations in the U.S., and about 10% of patients admitted to the hospital for CAP end up in the intensive care unit (ICU).24 In the past, PPIs have been linked to patients’ predisposal for developingCAP.25 Although controversial, available evidence suggests a direct association. In 2008 Sarker and colleagues theorized a mechanism that the acid reduction of the gastric lumen allows for increased bacterial colonization in the upper part of the GI tract.26 Since the acidity of the stomach serves as a defense mechanism against many ingested bacteria, many pathogens will be able to survive in the more basic environment.25
Sarkar and colleagues went on to evaluate 80,000 cases over 15 years. The objective was to examine the association between PPI use and the date of diagnosis of the CAP infection, known as the index date. The study demonstrated that PPI use was not associated with increased CAP risk in the long-term (adjusted odds ratio (OR), 1.02; 95% CI, 0.97-1.08). The study did find a strong increase in the risk of CAP if a PPI was started within 2 days (adjusted OR, 6.53; 95% CI, 3.95-10.80), 7 days (adjusted OR, 3.79; 95% CI, 2.66-5.42), and 14 days (adjusted OR, 3.21; 95% CI, 2.46-4.18) of the index date.26
Four years later, de Jagar and colleagues examined the differences in microbial etiology in CAP patients with and without an active PPI. Over a 4-year study period, 463 individuals were selected with clinical suspicion of CAP. The microbial etiology could be determined in 70% of those patients. The remaining 30% were excluded due to an alternative diagnosis. One of the most likely pathogens to cause a CAP infection is Streptococcus pneumoniae (S pneumonia).27 Patients prescribed a PPI were significantly more likely to be infected with S pneumoniae than those not prescribed a PPI (28% vs 11%). The study concluded that the risk of S pneumoniae in patients taking a PPI was 2.23 times more likely (95% CI, 1.28-3.75).28
Dementia
In 2040, it is estimated that more than 80 million people will have from dementia.29 This is expected to become a large fiscal burden on the health care system. In 2010, about $604 billion was spent on therapy for dementia worldwide.30 Although no cure for dementia exists, it is more feasible than in previous years to prevent its occurrence. However, many medications, including PPIs, are associated with the development of dementia; therefore, it is important to minimize their use when possible.
As noted earlier vitamin B12 deficiency may lead to cognitive decline. Due to the malabsorption of vitamin B12 that results from PPI use, it is hypothesized that PPIs may be associated with incidence of dementia. Badiola and colleagues discovered that in the brains of mice given a PPI, levels of β-amyloid increased significantly affecting enzymes responsible for cognition.31 In a February 2016, JAMA article, researchers conducted a prospective cohort study evaluating 73,679 patients aged ≥75 years with no dementia at baseline. They went on to assess regular use of a PPI, defined as at least 1 PPI prescription every 3 months, and the incidence of dementia. Patients with regular use of a PPI (≥ 1 PPI prescription every 3 months) had a 44% increase risk of incident dementia (HR, 1.44; 95% CI, 1.36-1.52; P < .001).3 Therefore, it is theorized that avoiding PPI use in the elderly may prevent the development of dementia.
Chronic Kidney Disease
The prevalence of CKD has drastically increased in recent decades. It is estimated that up to 13% of people in the U.S. are affected by CKD.32 Some studies suggest that dosing errors occur at much higher rates in patients with declined glomerular filtration rate (GFR).33 The correct utilization use of medications becomes especially pertinent to this population. Several studies have already linked PPI use to acute interstitial nephritis (AIN) and acute kidney injury (AKI).34-36
Lazarus and colleagues evaluated the association between PPI use and the incidence of CKD. Their analysis was performed in a long-term running population-based cohort and replicated in a separate health care system. In the running cohort, patients receiving a PPI had a 1.45-fold greater chance of developing CKD (95% CI, 1.11-1.90; P = .006). In that same cohort, patients on a PPI had a 1.72-fold increase risk of AKI (95% CI, 1.28-2.30; P < .001).4 Similar outcomes were seen in the replicated cohort. However, the replicated cohort did observe that twice daily dosing of a PPI (adjusted HR, 1.46; CI, 1.28-1.67; P < .001) had a stronger association with CKD than once- daily dosing (adjusted HR, 1.15; 95% CI, 1.09-1.21; P < .001). H2RAs exhibited no association with CKD in the running cohort (HR, 1.15; 97% CI, 0.98-1.36; P = .10) or the replication cohort (HR, 0.93; 95% CI, 0.88-0.99; P = .03).4
Clinical PPI Recommendations
There are several FDA-approved and unapproved indications that warrant PPI therapy. Proton pump inhibitor indications include gastroesophageal reflux disease (GERD), peptic ulcer disease (PUD), Helicobacter pylori, and ulcers associated with the use of nonsteroidal anti-inflammatory drugs (NSAIDs).
GERD Recommendations
Optimal dosing and duration is important with all medications to maximize efficacy and minimize toxicity. In the case of PPIs, dosing and duration are of particularly concern due to the aforementioned AEs. Table illustrates manufacturer-recommended dosing and duration for the most commonly prescribed PPIs. Although these dosing regimens are based on clinical studies, PPIs are commonly prescribed at higher doses and for longer durations. By extending the duration of therapy, the risk of potential long-term AEs increases dramatically. If durations are limited to the recommended window, risk of AEs can be reduced.
Alternative Therapies
There are several strategies that exist to limit the use of PPIs, including lifestyle modifications to prevent GERD, supplementation of an alternative agent to prevent high doses of the PPI, or discontinuing PPI therapy all together. Lifestyle modifications provide additional benefit as monotherapy or to supplement a pharmacologic regimen.
The American Journal of Gastroenterology promoted lifestyle modifications that include:
- Weight loss for patients with GERD who are overweight and had a recent weight gain;
- Elevation of the head of the bed (if nighttime symptoms present);
- Elimination of dietary triggers;
- Fatty foods, caffeine, chocolate, spicy food, food with high fat content, carbonated beverages, and peppermint;
- Avoiding tight fitting garments to prevent increase in gastric pressure;
- Promote salivation through oral lozenges or chewing gum to neutralize refluxed acid;
- Avoidance of tobacco and alcohol; and
- Abdominal breathing exercise to strengthen the barrier of the lower esophageal sphincter.37
The above modifications may reduce the need for pharmacologic therapy, thereby reducing possible of long-term AEs.
If lifestyle modifications alone are not enough, it is reasonable to use a H2RA for acute symptom relief or reduce high doses and frequencies of a PPI. H2RAs are well studied and effective in the management of GERD. According to the American College of Gastroenterology 2013 clinical practice guidelines, H2RAs can serve as an effective maintenance medication to relieve heartburn in patients without erosive disease. The guideline also states that a bedtime H2RA can be used to supplement a once- daily daytime PPI if nighttime reflux exists. This can eliminate the need to exceed manufacturer-recommended doses.37
One of the final challenges to overcome is a patient that has been maintained on chronic PPI therapy. However, caution should be exercised if choosing to discontinue a PPI. In a study by Niklesson and colleagues, after a 4-week course of pantoprazole given to healthy volunteers, those patients with no preexisting symptoms developed dyspeptic symptoms of GERD, such as heartburn, indigestion, and stomach discomfort. This correlation suggests that a rebound hypersecretion occurs after prolonged suppression of the proton pump, and therefore a gradual taper should be used.38 Although no definitive national recommendations on how to taper a patient off of a PPI exist, one suggestion is a 2- to 3-week taper by using a half-dose once daily or full dose on alternate days.39 This strategy has exhibited moderate success rates when used. Oral and written education on symptom management and the administration of H2RAs for infrequent breakthrough symptoms supplemented the reduction of the PPI.
Conclusion
Proton pump inhibitors have become a popular and effective drug class for a multitude of indications. However, it is crucial to recognize the risk of long-term use. It is important to properly assess the need for a PPI and to use appropriate dosing and duration, since prolonged durations and doses above the manufacturer’s recommendations is a primary contributor to long-term consequences. Both package inserts and clinical guidelines serve as valuable resources to help balance the risks and benefits of this medication class and can help guide therapeutic decisions.
1. U.S. Food and Drug Administration. FDA Drug Safety Communication: Low magnesium levels can be associated with long-term use of Proton Pump Inhibitor drugs (PPIs). http://www.fda.gov/Drugs/DrugSafety/ucm245011.htm. Updated April 7, 2016. Accessed January 12, 2017.
2. Forgacs I. Overprescribing proton pump inhibitors. BMJ. 2008;336(7634):2-3.
3. Gomm W, von Holt K, Thome F, et al. Association of proton pump inhibitors with risk of dementia. JAMA Neurol. 2016;73(4):410-416.
4. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176(2):238-246.
5. Wolfe MM, Soll AH. The physiology of gastric acid secretion. N Engl J Med. 1988;319(26):1707-1715.
6. Flynn A. The role of dietary calcium in bone health. Proc Nutr Soc. 2003;62(4):851-858.
7. Mizunashi K, Furukawa Y, Katano K, Abe K. Effect of omeprazole, an inhibitor of H+, K(+)-ATPase, on bone resorption in humans. Calcif Tissue Int. 1993;53(1):21-25.
8. O’Connell MB, Darren DM, Murray AM, Heaney RP, Kerzner LJ. Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am J Med. 2005;118(7):778-781.
9. Khalili H, Huang ES, Jacobson BC, Camargo CA Jr, Feskanich D, Chan AT. Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: a prospective cohort study. BMJ. 2012;344:e372.
10. Schweigel M, Martens H. Magnesium transport in the gastrointestinal tract. Front Biosci. 2000;5:D666-D677.
11. Hess MW, Hoenderop JG, Bindels RJ, Drenth JP. Systematic review: hypomagnesaemia induced by proton pump inhibition. Aliment Pharmacol Ther. 2012;36(5):405-413.
12. William JH, Danziger J. Proton-pump inhibitor-induced hypomagnesemia: current research and proposed mechanisms. World J Nephrol. 2016;5(2):152-157.
13. Luk CP, Parsons R, Lee YP, Hughes JD. Proton pump inhibitor-associated hypomagnesemia: what do FDA data tell us? Ann Pharmacother. 2013;47(6):773-780.
14. Health Quality Ontario. Vitamin B12 and cognitive function: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13(23):1-45.
15. Green R, Kinsella LJ. Current concepts in the diagnosis of cobalamin deficiency. Neurology. 1995;45(8):1435-1440.
16. Centers for Disease Control and Prevention. The Healthy Brain Initiative. https://www.cdc.gov/aging/pdf/2013-healthy-brain-initiative.pdf. Accessed January 17, 2017.
17. Toh BH, van Driel IR, Gleeson PA. Pernicious anemia. N Engl J Med. 1997;337(20):1441-1448.
18. Tefferi A, Pruthi RK. The biochemical basis of cobalamin deficiency. Mayo Clin Proc. 1994;69(2):181-186.
19. Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310(22):2435-2442.
20. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825-834.
21. National Clostridium difficile Standards Group. National Clostridium difficile Standards Group: report to the Department of Health. J Hosp Infect. 2004;56(suppl 1):1-38.
22. Thorens J, Frohlich F, Schwizer W, et al. Bacterial overgrowth during treatment with omeprazole compared with cimetidine. Gut. 1996;39(1):54-59.
23. Dial S, Delaney JAC, Barkun AN, et al. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294(23):2989-2995
24. Mandell LA, Wunderink RG, Anzueto A, et al; Infectious Diseases Society of America; and American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
25. Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA. 2004;292(16):1955-1960.
26. Sarkar M, Hennessy S, Yang Y. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med. 2008;149(6):391-398.
27. Waterer GW, Wunderink RG. The influence of the severity of community-acquired pneumonia on the usefulness of blood cultures. Respir Med. 2001;95(1):78-82.
28. de Jagar CP, Wever PC, Gemen EF, et al. Proton pump inhibitor therapy predisposes to community-acquired Streptococcus pneumoniae pneumonia. Aliment Pharmacol Ther. 2012;36(10):941-949.
29. Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7(3):137-152.
30. Wimo A, Jönsson L, Bond J, Prince M, Winblad B; Alzheimer Disease International. The worldwide economic impact of dementia 2010. Alzheimers Dement. 2013;9(1):1-11.
31. Badiola N, Alcalde V, Pujol A, et al. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One. 2013;8(3):e58537.
32. Stevens LA, Li S, Wang C, et al. Prevalence of CKD and comorbid illness in elderly patients in the United States: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2010;55(3)(suppl 2):S23-S33.
33. Weir MR, Fink JC. Safety of medical therapy in patients with chronic kidney disease and end-stage renal disease. Curr Opin Nephrol Hypertens 2014;23(3):306-313.
34. Blank ML, Parkin L, Paul C, Herbison P. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86(4):837-844.
35. Antoniou T, Macdonald EM, Holland S, et al. Proton pump inhibitors and the risk of acute kidney injury in older patients: a population-based cohort study. CMAJ Open. 2015;3(2):E166-E171.
36. Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrol. 2013;14:150.
37. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108(3):308-328.
38. Niklasson A, Lindström L, Simrén M, Lindberg G, Björnsson E. Dyspeptic symptom development after discontinuation of a proton pump inhibitor: a double-blind placebo-controlled trial. Am J Gastroenterol. 2010;105(7):1531-1537.
39. Haastrup P, Paulsen MS, Begtrup LM, Hansen JM, Jarbøl DE. Strategies for discontinuation of proton pump inhibitors: a systematic review. Fam Pract. 2014;31(6):625-630.
40. Nexium [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2012.
41. Prevacid [package insert]. Deerfield, IL: Takeda Pharmaceuticals; 2012.
42. Prilosec [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2012.
43. Protonix [package insert]. Konstanz, Germany: Pfizer; 2012.
1. U.S. Food and Drug Administration. FDA Drug Safety Communication: Low magnesium levels can be associated with long-term use of Proton Pump Inhibitor drugs (PPIs). http://www.fda.gov/Drugs/DrugSafety/ucm245011.htm. Updated April 7, 2016. Accessed January 12, 2017.
2. Forgacs I. Overprescribing proton pump inhibitors. BMJ. 2008;336(7634):2-3.
3. Gomm W, von Holt K, Thome F, et al. Association of proton pump inhibitors with risk of dementia. JAMA Neurol. 2016;73(4):410-416.
4. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176(2):238-246.
5. Wolfe MM, Soll AH. The physiology of gastric acid secretion. N Engl J Med. 1988;319(26):1707-1715.
6. Flynn A. The role of dietary calcium in bone health. Proc Nutr Soc. 2003;62(4):851-858.
7. Mizunashi K, Furukawa Y, Katano K, Abe K. Effect of omeprazole, an inhibitor of H+, K(+)-ATPase, on bone resorption in humans. Calcif Tissue Int. 1993;53(1):21-25.
8. O’Connell MB, Darren DM, Murray AM, Heaney RP, Kerzner LJ. Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am J Med. 2005;118(7):778-781.
9. Khalili H, Huang ES, Jacobson BC, Camargo CA Jr, Feskanich D, Chan AT. Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: a prospective cohort study. BMJ. 2012;344:e372.
10. Schweigel M, Martens H. Magnesium transport in the gastrointestinal tract. Front Biosci. 2000;5:D666-D677.
11. Hess MW, Hoenderop JG, Bindels RJ, Drenth JP. Systematic review: hypomagnesaemia induced by proton pump inhibition. Aliment Pharmacol Ther. 2012;36(5):405-413.
12. William JH, Danziger J. Proton-pump inhibitor-induced hypomagnesemia: current research and proposed mechanisms. World J Nephrol. 2016;5(2):152-157.
13. Luk CP, Parsons R, Lee YP, Hughes JD. Proton pump inhibitor-associated hypomagnesemia: what do FDA data tell us? Ann Pharmacother. 2013;47(6):773-780.
14. Health Quality Ontario. Vitamin B12 and cognitive function: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13(23):1-45.
15. Green R, Kinsella LJ. Current concepts in the diagnosis of cobalamin deficiency. Neurology. 1995;45(8):1435-1440.
16. Centers for Disease Control and Prevention. The Healthy Brain Initiative. https://www.cdc.gov/aging/pdf/2013-healthy-brain-initiative.pdf. Accessed January 17, 2017.
17. Toh BH, van Driel IR, Gleeson PA. Pernicious anemia. N Engl J Med. 1997;337(20):1441-1448.
18. Tefferi A, Pruthi RK. The biochemical basis of cobalamin deficiency. Mayo Clin Proc. 1994;69(2):181-186.
19. Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310(22):2435-2442.
20. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825-834.
21. National Clostridium difficile Standards Group. National Clostridium difficile Standards Group: report to the Department of Health. J Hosp Infect. 2004;56(suppl 1):1-38.
22. Thorens J, Frohlich F, Schwizer W, et al. Bacterial overgrowth during treatment with omeprazole compared with cimetidine. Gut. 1996;39(1):54-59.
23. Dial S, Delaney JAC, Barkun AN, et al. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294(23):2989-2995
24. Mandell LA, Wunderink RG, Anzueto A, et al; Infectious Diseases Society of America; and American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
25. Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA. 2004;292(16):1955-1960.
26. Sarkar M, Hennessy S, Yang Y. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med. 2008;149(6):391-398.
27. Waterer GW, Wunderink RG. The influence of the severity of community-acquired pneumonia on the usefulness of blood cultures. Respir Med. 2001;95(1):78-82.
28. de Jagar CP, Wever PC, Gemen EF, et al. Proton pump inhibitor therapy predisposes to community-acquired Streptococcus pneumoniae pneumonia. Aliment Pharmacol Ther. 2012;36(10):941-949.
29. Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7(3):137-152.
30. Wimo A, Jönsson L, Bond J, Prince M, Winblad B; Alzheimer Disease International. The worldwide economic impact of dementia 2010. Alzheimers Dement. 2013;9(1):1-11.
31. Badiola N, Alcalde V, Pujol A, et al. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One. 2013;8(3):e58537.
32. Stevens LA, Li S, Wang C, et al. Prevalence of CKD and comorbid illness in elderly patients in the United States: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2010;55(3)(suppl 2):S23-S33.
33. Weir MR, Fink JC. Safety of medical therapy in patients with chronic kidney disease and end-stage renal disease. Curr Opin Nephrol Hypertens 2014;23(3):306-313.
34. Blank ML, Parkin L, Paul C, Herbison P. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86(4):837-844.
35. Antoniou T, Macdonald EM, Holland S, et al. Proton pump inhibitors and the risk of acute kidney injury in older patients: a population-based cohort study. CMAJ Open. 2015;3(2):E166-E171.
36. Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrol. 2013;14:150.
37. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108(3):308-328.
38. Niklasson A, Lindström L, Simrén M, Lindberg G, Björnsson E. Dyspeptic symptom development after discontinuation of a proton pump inhibitor: a double-blind placebo-controlled trial. Am J Gastroenterol. 2010;105(7):1531-1537.
39. Haastrup P, Paulsen MS, Begtrup LM, Hansen JM, Jarbøl DE. Strategies for discontinuation of proton pump inhibitors: a systematic review. Fam Pract. 2014;31(6):625-630.
40. Nexium [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2012.
41. Prevacid [package insert]. Deerfield, IL: Takeda Pharmaceuticals; 2012.
42. Prilosec [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2012.
43. Protonix [package insert]. Konstanz, Germany: Pfizer; 2012.
Team creates online database of cancer mutations

Image by Spencer Phillips
Researchers have developed an online “knowledgebase” called CIViC, an open access resource for collecting and interpreting information from scientific publications on cancer genetics.
“CIViC” stands for Clinical Interpretations of Variants in Cancer, and the researchers liken it to a Wikipedia of cancer genetics.
Anyone can create an account and contribute information. That information is then curated by editors and moderators who are considered experts in the field.
The researchers described the resource in Nature Genetics.
“It’s relatively easy now to sequence the DNA of tumors—to gather the raw information—but there’s a big interpretation problem,” said study author Obi L. Griffith, PhD, of Washington University School of Medicine in St Louis, Missouri.
“What do these hundreds or thousands of mutations mean for this patient? There are a lot of studies being done to answer these questions. But oncologists trying to interpret the raw data are faced with an overwhelming task of plumbing the literature, reading papers, trying to understand what the latest studies tell them about these mutations and how they may or may not be important.”
The CIViC knowledgebase is an attempt to solve this problem. The researchers said this is one of many efforts to collect and interpret such information, but, to their knowledge, CIViC is the only one that is entirely open access. Anyone is free to contribute and use the content as well as the source code.
“We are committed to keeping this resource open and available to anyone who wants to contribute or make use of the information,” said Malachi Griffith, PhD, of Washington University School of Medicine.
“We would like it to be a community exercise and public resource. The information is in the public domain. There are no restrictions on its use, academic or commercial.”
Though anyone can submit a new piece of information or suggest edits to existing data, at least 2 independent contributors must agree that the new information should be incorporated, and 1 of those users must be an “expert editor.”
Expert editors are not permitted to approve their own submissions. Information on the CIViC website provides details about how new users may be promoted to expert editors and administrators.
To date, the site has seen over 17,500 users from academic institutions, governmental organizations, and commercial entities around the world.
Since CIViC’s launch, 59 users have volunteered their time to contribute their knowledge to CIViC, including descriptions of the clinical relevance of 732 mutations from 285 genes for 203 types of cancer, all gleaned from reviewing 1090 scientific and medical publications.
Despite the fact that there are many groups attempting to collect and interpret genomic variants in cancer, the researchers said the sheer volume of information has resulted in relatively little overlap in data gathered so far.
“While we believe this is the only such open access knowledgebase, there are other large research centers with similar resources,” Malachi Griffith said. “We did an analysis to compare the big ones.”
“Even though we all have access to the same published literature, if you look at the overlap of the information mined by each of these resources, it’s remarkably small. We’re all approaching the same problem, and, just by chance—and probably because of the amount of information out there—we haven’t duplicated our efforts very much yet.”
Obi and Malachi Griffith said finding a way to combine these resources is the primary goal of an international group they are helping lead called the Variant Interpretation for Cancer Consortium, which is a part of the Global Alliance for Genomics and Health (GA4GH).
“We’re just scratching the surface of the potential this holds for precision medicine,” Obi Griffith said. “There’s a lot of work to do.” ![]()

Image by Spencer Phillips
Researchers have developed an online “knowledgebase” called CIViC, an open access resource for collecting and interpreting information from scientific publications on cancer genetics.
“CIViC” stands for Clinical Interpretations of Variants in Cancer, and the researchers liken it to a Wikipedia of cancer genetics.
Anyone can create an account and contribute information. That information is then curated by editors and moderators who are considered experts in the field.
The researchers described the resource in Nature Genetics.
“It’s relatively easy now to sequence the DNA of tumors—to gather the raw information—but there’s a big interpretation problem,” said study author Obi L. Griffith, PhD, of Washington University School of Medicine in St Louis, Missouri.
“What do these hundreds or thousands of mutations mean for this patient? There are a lot of studies being done to answer these questions. But oncologists trying to interpret the raw data are faced with an overwhelming task of plumbing the literature, reading papers, trying to understand what the latest studies tell them about these mutations and how they may or may not be important.”
The CIViC knowledgebase is an attempt to solve this problem. The researchers said this is one of many efforts to collect and interpret such information, but, to their knowledge, CIViC is the only one that is entirely open access. Anyone is free to contribute and use the content as well as the source code.
“We are committed to keeping this resource open and available to anyone who wants to contribute or make use of the information,” said Malachi Griffith, PhD, of Washington University School of Medicine.
“We would like it to be a community exercise and public resource. The information is in the public domain. There are no restrictions on its use, academic or commercial.”
Though anyone can submit a new piece of information or suggest edits to existing data, at least 2 independent contributors must agree that the new information should be incorporated, and 1 of those users must be an “expert editor.”
Expert editors are not permitted to approve their own submissions. Information on the CIViC website provides details about how new users may be promoted to expert editors and administrators.
To date, the site has seen over 17,500 users from academic institutions, governmental organizations, and commercial entities around the world.
Since CIViC’s launch, 59 users have volunteered their time to contribute their knowledge to CIViC, including descriptions of the clinical relevance of 732 mutations from 285 genes for 203 types of cancer, all gleaned from reviewing 1090 scientific and medical publications.
Despite the fact that there are many groups attempting to collect and interpret genomic variants in cancer, the researchers said the sheer volume of information has resulted in relatively little overlap in data gathered so far.
“While we believe this is the only such open access knowledgebase, there are other large research centers with similar resources,” Malachi Griffith said. “We did an analysis to compare the big ones.”
“Even though we all have access to the same published literature, if you look at the overlap of the information mined by each of these resources, it’s remarkably small. We’re all approaching the same problem, and, just by chance—and probably because of the amount of information out there—we haven’t duplicated our efforts very much yet.”
Obi and Malachi Griffith said finding a way to combine these resources is the primary goal of an international group they are helping lead called the Variant Interpretation for Cancer Consortium, which is a part of the Global Alliance for Genomics and Health (GA4GH).
“We’re just scratching the surface of the potential this holds for precision medicine,” Obi Griffith said. “There’s a lot of work to do.” ![]()

Image by Spencer Phillips
Researchers have developed an online “knowledgebase” called CIViC, an open access resource for collecting and interpreting information from scientific publications on cancer genetics.
“CIViC” stands for Clinical Interpretations of Variants in Cancer, and the researchers liken it to a Wikipedia of cancer genetics.
Anyone can create an account and contribute information. That information is then curated by editors and moderators who are considered experts in the field.
The researchers described the resource in Nature Genetics.
“It’s relatively easy now to sequence the DNA of tumors—to gather the raw information—but there’s a big interpretation problem,” said study author Obi L. Griffith, PhD, of Washington University School of Medicine in St Louis, Missouri.
“What do these hundreds or thousands of mutations mean for this patient? There are a lot of studies being done to answer these questions. But oncologists trying to interpret the raw data are faced with an overwhelming task of plumbing the literature, reading papers, trying to understand what the latest studies tell them about these mutations and how they may or may not be important.”
The CIViC knowledgebase is an attempt to solve this problem. The researchers said this is one of many efforts to collect and interpret such information, but, to their knowledge, CIViC is the only one that is entirely open access. Anyone is free to contribute and use the content as well as the source code.
“We are committed to keeping this resource open and available to anyone who wants to contribute or make use of the information,” said Malachi Griffith, PhD, of Washington University School of Medicine.
“We would like it to be a community exercise and public resource. The information is in the public domain. There are no restrictions on its use, academic or commercial.”
Though anyone can submit a new piece of information or suggest edits to existing data, at least 2 independent contributors must agree that the new information should be incorporated, and 1 of those users must be an “expert editor.”
Expert editors are not permitted to approve their own submissions. Information on the CIViC website provides details about how new users may be promoted to expert editors and administrators.
To date, the site has seen over 17,500 users from academic institutions, governmental organizations, and commercial entities around the world.
Since CIViC’s launch, 59 users have volunteered their time to contribute their knowledge to CIViC, including descriptions of the clinical relevance of 732 mutations from 285 genes for 203 types of cancer, all gleaned from reviewing 1090 scientific and medical publications.
Despite the fact that there are many groups attempting to collect and interpret genomic variants in cancer, the researchers said the sheer volume of information has resulted in relatively little overlap in data gathered so far.
“While we believe this is the only such open access knowledgebase, there are other large research centers with similar resources,” Malachi Griffith said. “We did an analysis to compare the big ones.”
“Even though we all have access to the same published literature, if you look at the overlap of the information mined by each of these resources, it’s remarkably small. We’re all approaching the same problem, and, just by chance—and probably because of the amount of information out there—we haven’t duplicated our efforts very much yet.”
Obi and Malachi Griffith said finding a way to combine these resources is the primary goal of an international group they are helping lead called the Variant Interpretation for Cancer Consortium, which is a part of the Global Alliance for Genomics and Health (GA4GH).
“We’re just scratching the surface of the potential this holds for precision medicine,” Obi Griffith said. “There’s a lot of work to do.” ![]()