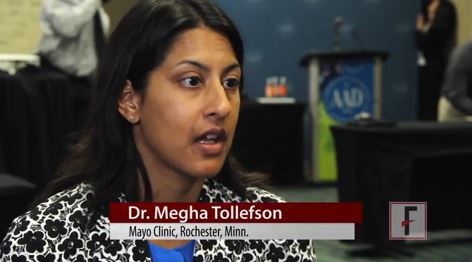User login
Long-term peanut sublingual immunotherapy found safe
ATLANTA – Peanut sublingual immunotherapy induces clinically significant desensitization in the majority of subjects and can induce sustained unresponsiveness in a subset of children treated for 36-60 months, results from a small study suggest.
“Sublingual immunotherapy [SLIT] is an easy-to-administer treatment that appears to be safe, and with extended treatment, may provide a clinically significant amount of protection with the potential for a lasting effect,” one of the study authors, Edwin H. Kim, MD, said in an interview in advance of the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
To find out, the researchers treated 37 patients with 2 mg of peanut SLIT for 36-60 months and then assessed a 5,000-mg peanut oral food challenge to further assess desensitization. Those who passed the challenge discontinued SLIT for 2-4 weeks and were then re-challenged with 5,000 mg of peanut protein to assess for sustained unresponsiveness.
“Existing data suggested that about 50% of patients on oral immunotherapy develop sustained unresponsiveness, which was defined by being able to tolerate the same full amount of peanut 1 month after stopping therapy,” Dr. Kim said. “As the assumption was that SLIT would have a more modest effect, it was unclear if any patients at all on SLIT would develop sustained unresponsiveness.”
Of the 37 subjects who completed the study, 32 (86%) safely ingested more than 300 mg of peanut and 12 (32%) passed the oral food challenge at the end of SLIT therapy. The median amount of peanut tolerated was 1,750 mg (compared with 1,710 mg in the original 12-month paper). The 12 subjects who passed the oral food challenge were re-challenged with 5,000 mg of peanut 2-4 weeks after discontinuing SLIT. Of these, 10 (27%) demonstrated sustained unresponsiveness. Dr. Kim characterized the results as “better than we would have expected.”
He acknowledged certain limitations to the study, including the lack of an entry food challenge to determine a baseline reaction threshold and the lack of a placebo arm for the study’s extended maintenance phase.
Dr. Kim reported having no financial disclosures.
[email protected]
ATLANTA – Peanut sublingual immunotherapy induces clinically significant desensitization in the majority of subjects and can induce sustained unresponsiveness in a subset of children treated for 36-60 months, results from a small study suggest.
“Sublingual immunotherapy [SLIT] is an easy-to-administer treatment that appears to be safe, and with extended treatment, may provide a clinically significant amount of protection with the potential for a lasting effect,” one of the study authors, Edwin H. Kim, MD, said in an interview in advance of the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
To find out, the researchers treated 37 patients with 2 mg of peanut SLIT for 36-60 months and then assessed a 5,000-mg peanut oral food challenge to further assess desensitization. Those who passed the challenge discontinued SLIT for 2-4 weeks and were then re-challenged with 5,000 mg of peanut protein to assess for sustained unresponsiveness.
“Existing data suggested that about 50% of patients on oral immunotherapy develop sustained unresponsiveness, which was defined by being able to tolerate the same full amount of peanut 1 month after stopping therapy,” Dr. Kim said. “As the assumption was that SLIT would have a more modest effect, it was unclear if any patients at all on SLIT would develop sustained unresponsiveness.”
Of the 37 subjects who completed the study, 32 (86%) safely ingested more than 300 mg of peanut and 12 (32%) passed the oral food challenge at the end of SLIT therapy. The median amount of peanut tolerated was 1,750 mg (compared with 1,710 mg in the original 12-month paper). The 12 subjects who passed the oral food challenge were re-challenged with 5,000 mg of peanut 2-4 weeks after discontinuing SLIT. Of these, 10 (27%) demonstrated sustained unresponsiveness. Dr. Kim characterized the results as “better than we would have expected.”
He acknowledged certain limitations to the study, including the lack of an entry food challenge to determine a baseline reaction threshold and the lack of a placebo arm for the study’s extended maintenance phase.
Dr. Kim reported having no financial disclosures.
[email protected]
ATLANTA – Peanut sublingual immunotherapy induces clinically significant desensitization in the majority of subjects and can induce sustained unresponsiveness in a subset of children treated for 36-60 months, results from a small study suggest.
“Sublingual immunotherapy [SLIT] is an easy-to-administer treatment that appears to be safe, and with extended treatment, may provide a clinically significant amount of protection with the potential for a lasting effect,” one of the study authors, Edwin H. Kim, MD, said in an interview in advance of the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
To find out, the researchers treated 37 patients with 2 mg of peanut SLIT for 36-60 months and then assessed a 5,000-mg peanut oral food challenge to further assess desensitization. Those who passed the challenge discontinued SLIT for 2-4 weeks and were then re-challenged with 5,000 mg of peanut protein to assess for sustained unresponsiveness.
“Existing data suggested that about 50% of patients on oral immunotherapy develop sustained unresponsiveness, which was defined by being able to tolerate the same full amount of peanut 1 month after stopping therapy,” Dr. Kim said. “As the assumption was that SLIT would have a more modest effect, it was unclear if any patients at all on SLIT would develop sustained unresponsiveness.”
Of the 37 subjects who completed the study, 32 (86%) safely ingested more than 300 mg of peanut and 12 (32%) passed the oral food challenge at the end of SLIT therapy. The median amount of peanut tolerated was 1,750 mg (compared with 1,710 mg in the original 12-month paper). The 12 subjects who passed the oral food challenge were re-challenged with 5,000 mg of peanut 2-4 weeks after discontinuing SLIT. Of these, 10 (27%) demonstrated sustained unresponsiveness. Dr. Kim characterized the results as “better than we would have expected.”
He acknowledged certain limitations to the study, including the lack of an entry food challenge to determine a baseline reaction threshold and the lack of a placebo arm for the study’s extended maintenance phase.
Dr. Kim reported having no financial disclosures.
[email protected]
AT THE 2017 AAAAI ANNUAL MEETING
Key clinical point:
Major finding: Of the children who completed the study, 86% safely ingested more than 300 mg of peanut and 32% passed the oral food challenge at the end of SLIT therapy.
Data source: A study of 37 patients who were treated with 2 mg of peanut SLIT for 36-60 months.
Disclosures: Dr. Kim reported having no financial disclosures.
CIMPASI-1 and -2 show certolizumab pegol benefits patients with severe plaque psoriasis
ORLANDO – Results from two phase III trials indicated the tumor necrosis factor alpha inhibitor certolizumab pegol led to significant improvements in moderate to severe chronic plaque psoriasis, compared with placebo, according to an oral presentation at this year’s annual Academy of Dermatology Meeting.
Across the CIMPASI-1 and CIMPASI-2 trials, at 16 weeks, both certolizumab pegol (Cimzia) 400 mg and 200 mg, administered subcutaneously every 2 weeks in groups of patients with moderate to severe chronic plaque psoriasis, demonstrated statistically significant, clinically meaningful improvements in Psoriasis Area and Severity Index (PASI) scores, and on the Physician Global Assessment (PGA) scores, when compared with placebo. The data were presented by Alice B. Gottlieb, MD, PhD, professor of dermatology at New York Medical College, Valhalla. Certolizumab pegol currently is approved in the United States for use in psoriatic arthritis, but not for psoriasis.
In CIMPASI-1, 234 mostly white male patients in their mid-40s who had moderate to severe chronic plaque psoriasis were randomly assigned to one of three groups. There were 88 participants in the arm given 400 mg every 2 weeks at weeks 0, 2, and 4; 95 given 200 mg at weeks 0, 2, and 4; and 51 given placebo. At week 16, the percentage of patients who achieved a PASI 75 was 75.8% in the first group, 66.5% in the second, and 6.5% for placebo. A PGA scale improvement of at least two points over baseline toward a final score of clear or almost clear skin at week 16 was 57.9% for group 1, 47.0% for the second group, and 4.2% for placebo.
Also at week 16, the percentage of patients who achieved a PASI 90 was 43.6% in the 400 mg dose group, 35.8% in the 200 mg dose group, and 0.4% in the placebo group.
In CIMPASI-2, 227 mostly white patients with moderate to severe chronic plaque psoriasis, most of whom were in their mid-40s, evenly distributed across the sexes, were randomly assigned to one of three groups. There were 87 participants in the 400 mg every 2 weeks at weeks 0, 2, and 4 group; 91 in the 200 mg every 2 weeks at weeks 0, 2, and 4 arm, and 49 in the placebo group. At week 16, 82.6% of patients in the first group and 81.4% in the second group had a PASI 75, compared with 11.6% of the placebo group. Also at 16 weeks, a PGA scale improvement of at least two points over baseline toward a final score of clear or almost clear skin at week 16 was 71.6% for group 1, 66.8% for the second group, and 2.0% for placebo. At week 16, 55.4% of patients receiving the 400 mg dose had a PASI 90, as did 52.6% of patients receiving the 200 mg dose every 2 weeks, compared with only 4.5% of the placebo group.
Additionally, Dr. Gottlieb said that at week 16, patients in the 400-mg and 200-mg groups showed significant improvements over baseline Dermatology Life Quality Index (DLQI) average scores, compared with placebo: a 10.2 decrease in the 400-mg group and a 9.3 decrease in the 200-mg group, compared with a 3.3 decrease in the placebo arm (P less than .001) in the CIMPASI-1 study. In the CIMPASI-2 study at week 16, there was a drop of 10.0 DLQI in average scores in the 400-mg group, 10.4 in the 200-mg group, and an average drop of only 3.8 in controls (P less than .001).
Dr. Gottlieb, who disclosed that her mother suffers from severe plaque psoriasis, told the audience that the patient improvements were on par with those seen with infliximab, which she said was “the best anti–TNF alpha in the pack to date, and that’s an intravenous drug. These are impressive drops in the DLQI.”
Adverse events were, said Dr. Gottlieb, “a whole lot of nothing. There’s nothing that stands out.” She said there were no cases of tuberculosis, one case of vulvovaginal candidiasis, and equal distribution of herpes zoster across the study.
[email protected]
On Twitter @whitneymcknight
ORLANDO – Results from two phase III trials indicated the tumor necrosis factor alpha inhibitor certolizumab pegol led to significant improvements in moderate to severe chronic plaque psoriasis, compared with placebo, according to an oral presentation at this year’s annual Academy of Dermatology Meeting.
Across the CIMPASI-1 and CIMPASI-2 trials, at 16 weeks, both certolizumab pegol (Cimzia) 400 mg and 200 mg, administered subcutaneously every 2 weeks in groups of patients with moderate to severe chronic plaque psoriasis, demonstrated statistically significant, clinically meaningful improvements in Psoriasis Area and Severity Index (PASI) scores, and on the Physician Global Assessment (PGA) scores, when compared with placebo. The data were presented by Alice B. Gottlieb, MD, PhD, professor of dermatology at New York Medical College, Valhalla. Certolizumab pegol currently is approved in the United States for use in psoriatic arthritis, but not for psoriasis.
In CIMPASI-1, 234 mostly white male patients in their mid-40s who had moderate to severe chronic plaque psoriasis were randomly assigned to one of three groups. There were 88 participants in the arm given 400 mg every 2 weeks at weeks 0, 2, and 4; 95 given 200 mg at weeks 0, 2, and 4; and 51 given placebo. At week 16, the percentage of patients who achieved a PASI 75 was 75.8% in the first group, 66.5% in the second, and 6.5% for placebo. A PGA scale improvement of at least two points over baseline toward a final score of clear or almost clear skin at week 16 was 57.9% for group 1, 47.0% for the second group, and 4.2% for placebo.
Also at week 16, the percentage of patients who achieved a PASI 90 was 43.6% in the 400 mg dose group, 35.8% in the 200 mg dose group, and 0.4% in the placebo group.
In CIMPASI-2, 227 mostly white patients with moderate to severe chronic plaque psoriasis, most of whom were in their mid-40s, evenly distributed across the sexes, were randomly assigned to one of three groups. There were 87 participants in the 400 mg every 2 weeks at weeks 0, 2, and 4 group; 91 in the 200 mg every 2 weeks at weeks 0, 2, and 4 arm, and 49 in the placebo group. At week 16, 82.6% of patients in the first group and 81.4% in the second group had a PASI 75, compared with 11.6% of the placebo group. Also at 16 weeks, a PGA scale improvement of at least two points over baseline toward a final score of clear or almost clear skin at week 16 was 71.6% for group 1, 66.8% for the second group, and 2.0% for placebo. At week 16, 55.4% of patients receiving the 400 mg dose had a PASI 90, as did 52.6% of patients receiving the 200 mg dose every 2 weeks, compared with only 4.5% of the placebo group.
Additionally, Dr. Gottlieb said that at week 16, patients in the 400-mg and 200-mg groups showed significant improvements over baseline Dermatology Life Quality Index (DLQI) average scores, compared with placebo: a 10.2 decrease in the 400-mg group and a 9.3 decrease in the 200-mg group, compared with a 3.3 decrease in the placebo arm (P less than .001) in the CIMPASI-1 study. In the CIMPASI-2 study at week 16, there was a drop of 10.0 DLQI in average scores in the 400-mg group, 10.4 in the 200-mg group, and an average drop of only 3.8 in controls (P less than .001).
Dr. Gottlieb, who disclosed that her mother suffers from severe plaque psoriasis, told the audience that the patient improvements were on par with those seen with infliximab, which she said was “the best anti–TNF alpha in the pack to date, and that’s an intravenous drug. These are impressive drops in the DLQI.”
Adverse events were, said Dr. Gottlieb, “a whole lot of nothing. There’s nothing that stands out.” She said there were no cases of tuberculosis, one case of vulvovaginal candidiasis, and equal distribution of herpes zoster across the study.
[email protected]
On Twitter @whitneymcknight
ORLANDO – Results from two phase III trials indicated the tumor necrosis factor alpha inhibitor certolizumab pegol led to significant improvements in moderate to severe chronic plaque psoriasis, compared with placebo, according to an oral presentation at this year’s annual Academy of Dermatology Meeting.
Across the CIMPASI-1 and CIMPASI-2 trials, at 16 weeks, both certolizumab pegol (Cimzia) 400 mg and 200 mg, administered subcutaneously every 2 weeks in groups of patients with moderate to severe chronic plaque psoriasis, demonstrated statistically significant, clinically meaningful improvements in Psoriasis Area and Severity Index (PASI) scores, and on the Physician Global Assessment (PGA) scores, when compared with placebo. The data were presented by Alice B. Gottlieb, MD, PhD, professor of dermatology at New York Medical College, Valhalla. Certolizumab pegol currently is approved in the United States for use in psoriatic arthritis, but not for psoriasis.
In CIMPASI-1, 234 mostly white male patients in their mid-40s who had moderate to severe chronic plaque psoriasis were randomly assigned to one of three groups. There were 88 participants in the arm given 400 mg every 2 weeks at weeks 0, 2, and 4; 95 given 200 mg at weeks 0, 2, and 4; and 51 given placebo. At week 16, the percentage of patients who achieved a PASI 75 was 75.8% in the first group, 66.5% in the second, and 6.5% for placebo. A PGA scale improvement of at least two points over baseline toward a final score of clear or almost clear skin at week 16 was 57.9% for group 1, 47.0% for the second group, and 4.2% for placebo.
Also at week 16, the percentage of patients who achieved a PASI 90 was 43.6% in the 400 mg dose group, 35.8% in the 200 mg dose group, and 0.4% in the placebo group.
In CIMPASI-2, 227 mostly white patients with moderate to severe chronic plaque psoriasis, most of whom were in their mid-40s, evenly distributed across the sexes, were randomly assigned to one of three groups. There were 87 participants in the 400 mg every 2 weeks at weeks 0, 2, and 4 group; 91 in the 200 mg every 2 weeks at weeks 0, 2, and 4 arm, and 49 in the placebo group. At week 16, 82.6% of patients in the first group and 81.4% in the second group had a PASI 75, compared with 11.6% of the placebo group. Also at 16 weeks, a PGA scale improvement of at least two points over baseline toward a final score of clear or almost clear skin at week 16 was 71.6% for group 1, 66.8% for the second group, and 2.0% for placebo. At week 16, 55.4% of patients receiving the 400 mg dose had a PASI 90, as did 52.6% of patients receiving the 200 mg dose every 2 weeks, compared with only 4.5% of the placebo group.
Additionally, Dr. Gottlieb said that at week 16, patients in the 400-mg and 200-mg groups showed significant improvements over baseline Dermatology Life Quality Index (DLQI) average scores, compared with placebo: a 10.2 decrease in the 400-mg group and a 9.3 decrease in the 200-mg group, compared with a 3.3 decrease in the placebo arm (P less than .001) in the CIMPASI-1 study. In the CIMPASI-2 study at week 16, there was a drop of 10.0 DLQI in average scores in the 400-mg group, 10.4 in the 200-mg group, and an average drop of only 3.8 in controls (P less than .001).
Dr. Gottlieb, who disclosed that her mother suffers from severe plaque psoriasis, told the audience that the patient improvements were on par with those seen with infliximab, which she said was “the best anti–TNF alpha in the pack to date, and that’s an intravenous drug. These are impressive drops in the DLQI.”
Adverse events were, said Dr. Gottlieb, “a whole lot of nothing. There’s nothing that stands out.” She said there were no cases of tuberculosis, one case of vulvovaginal candidiasis, and equal distribution of herpes zoster across the study.
[email protected]
On Twitter @whitneymcknight
AT AAD 17
Key clinical point:
Major finding: In CIMPASI-1, at week 16, the response rate for patients who achieved a PASI 75 was 75.8% in the 400-mg group, 66.5% in the 200-mg group, and 6.5% in the placebo group.
Data source: 461 adults with moderate to severe plaque arthritis randomly assigned to either 400 mg certolizumab pegol, 200 mg of the treatment, or placebo every other week at weeks 0, 2, and 4.
Disclosures: Dr. Gottlieb had relevant disclosures, including with Dermira and UCB, the sponsors of this trial.
Uptick found in severe allergy shot reactions
ATLANTA – Systemic reactions against subcutaneous allergen immunotherapy have trended down overall in recent years, but there has been an uptick in reports of severe grade 4 reactions for reasons that are not yet clear, according to a review of 46.6 million injection office visits from 2008-2015.
The data come from annual surveys of members of the American Academy of Allergy, Asthma, and Immunology and of the American College of Allergy, Asthma, and Immunology, of whom 27%-51% responded each year.
However, on surveys from 2013 to 2015, one grade 4 reaction – hypotension or respiratory failure – was reported for every 160,000 injection visits, up from one per million office visits in previous years. Two-thirds (152/252) of grade 3/4 systemic reactions (SRs) for 2014-2015 occurred in asthmatics. Among the three fatalities directly linked to allergy shots since 2008, all of them occurred in adults; two patients had asthma. The findings highlight the need for good asthma control before shots begin, with a forced expiratory volume of at least 70% in 1 second (J Allergy Clin Immunol. 2017 Feb;139[2]:AB377).
“Asthma remains a major risk factor for severe SRs... [It’s something] allergists need to be vigilant about,” said lead investigator Tolly Epstein, MD, an assistant professor of immunology at the University of Cincinnati.
It’s unclear if there has been a true increase in severe reactions, or simply better reporting of them since the reaction grading system started being used a few years ago. “I was surprised a little bit to see the uptick in severe systemic reactions, but I am not sure if it’s real yet,” Dr. Epstein said. She and her colleagues are collecting more data on the reports of severe reactions.
Dr. Epstein and her colleagues found in previous work that higher maintenance doses may increase the risk of SRs. The right balance between efficacy and safety is still being worked out, she said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The surveys started collecting data on infections from allergy shots in 2014, amid concerns about contaminants in compounded medications. The fact that none were reported probably isn’t a surprise to allergists, but it might be news to others who have raised concerns about the possibility, Dr. Epstein said.
Overall safety was good for sublingual allergen immunotherapy, first approved by the Food and Drug Administration in 2014. One of the grade 3 reactions was pharyngeal edema; the other involved throat tightening and lower respiratory symptoms.
One of the asthma patients who died was morbidly obese and under treatment for weed allergies during pollen season, and died from overwhelming laryngeal edema. The patient’s weight made IV access difficult. The other asthma patient had severe disease, and was on the maximum dose of fluticasone/salmeterol. He was on an accelerated buildup for dust mites, pollen, mold, and other allergies, and apparently died of asthma complications triggered by the shots. There were no known risk factors in the third death.
Mortalities are lower than they used to be in the past with allergy shots, Dr. Epstein said.
She said she had no relevant disclosures.
[email protected]
ATLANTA – Systemic reactions against subcutaneous allergen immunotherapy have trended down overall in recent years, but there has been an uptick in reports of severe grade 4 reactions for reasons that are not yet clear, according to a review of 46.6 million injection office visits from 2008-2015.
The data come from annual surveys of members of the American Academy of Allergy, Asthma, and Immunology and of the American College of Allergy, Asthma, and Immunology, of whom 27%-51% responded each year.
However, on surveys from 2013 to 2015, one grade 4 reaction – hypotension or respiratory failure – was reported for every 160,000 injection visits, up from one per million office visits in previous years. Two-thirds (152/252) of grade 3/4 systemic reactions (SRs) for 2014-2015 occurred in asthmatics. Among the three fatalities directly linked to allergy shots since 2008, all of them occurred in adults; two patients had asthma. The findings highlight the need for good asthma control before shots begin, with a forced expiratory volume of at least 70% in 1 second (J Allergy Clin Immunol. 2017 Feb;139[2]:AB377).
“Asthma remains a major risk factor for severe SRs... [It’s something] allergists need to be vigilant about,” said lead investigator Tolly Epstein, MD, an assistant professor of immunology at the University of Cincinnati.
It’s unclear if there has been a true increase in severe reactions, or simply better reporting of them since the reaction grading system started being used a few years ago. “I was surprised a little bit to see the uptick in severe systemic reactions, but I am not sure if it’s real yet,” Dr. Epstein said. She and her colleagues are collecting more data on the reports of severe reactions.
Dr. Epstein and her colleagues found in previous work that higher maintenance doses may increase the risk of SRs. The right balance between efficacy and safety is still being worked out, she said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The surveys started collecting data on infections from allergy shots in 2014, amid concerns about contaminants in compounded medications. The fact that none were reported probably isn’t a surprise to allergists, but it might be news to others who have raised concerns about the possibility, Dr. Epstein said.
Overall safety was good for sublingual allergen immunotherapy, first approved by the Food and Drug Administration in 2014. One of the grade 3 reactions was pharyngeal edema; the other involved throat tightening and lower respiratory symptoms.
One of the asthma patients who died was morbidly obese and under treatment for weed allergies during pollen season, and died from overwhelming laryngeal edema. The patient’s weight made IV access difficult. The other asthma patient had severe disease, and was on the maximum dose of fluticasone/salmeterol. He was on an accelerated buildup for dust mites, pollen, mold, and other allergies, and apparently died of asthma complications triggered by the shots. There were no known risk factors in the third death.
Mortalities are lower than they used to be in the past with allergy shots, Dr. Epstein said.
She said she had no relevant disclosures.
[email protected]
ATLANTA – Systemic reactions against subcutaneous allergen immunotherapy have trended down overall in recent years, but there has been an uptick in reports of severe grade 4 reactions for reasons that are not yet clear, according to a review of 46.6 million injection office visits from 2008-2015.
The data come from annual surveys of members of the American Academy of Allergy, Asthma, and Immunology and of the American College of Allergy, Asthma, and Immunology, of whom 27%-51% responded each year.
However, on surveys from 2013 to 2015, one grade 4 reaction – hypotension or respiratory failure – was reported for every 160,000 injection visits, up from one per million office visits in previous years. Two-thirds (152/252) of grade 3/4 systemic reactions (SRs) for 2014-2015 occurred in asthmatics. Among the three fatalities directly linked to allergy shots since 2008, all of them occurred in adults; two patients had asthma. The findings highlight the need for good asthma control before shots begin, with a forced expiratory volume of at least 70% in 1 second (J Allergy Clin Immunol. 2017 Feb;139[2]:AB377).
“Asthma remains a major risk factor for severe SRs... [It’s something] allergists need to be vigilant about,” said lead investigator Tolly Epstein, MD, an assistant professor of immunology at the University of Cincinnati.
It’s unclear if there has been a true increase in severe reactions, or simply better reporting of them since the reaction grading system started being used a few years ago. “I was surprised a little bit to see the uptick in severe systemic reactions, but I am not sure if it’s real yet,” Dr. Epstein said. She and her colleagues are collecting more data on the reports of severe reactions.
Dr. Epstein and her colleagues found in previous work that higher maintenance doses may increase the risk of SRs. The right balance between efficacy and safety is still being worked out, she said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The surveys started collecting data on infections from allergy shots in 2014, amid concerns about contaminants in compounded medications. The fact that none were reported probably isn’t a surprise to allergists, but it might be news to others who have raised concerns about the possibility, Dr. Epstein said.
Overall safety was good for sublingual allergen immunotherapy, first approved by the Food and Drug Administration in 2014. One of the grade 3 reactions was pharyngeal edema; the other involved throat tightening and lower respiratory symptoms.
One of the asthma patients who died was morbidly obese and under treatment for weed allergies during pollen season, and died from overwhelming laryngeal edema. The patient’s weight made IV access difficult. The other asthma patient had severe disease, and was on the maximum dose of fluticasone/salmeterol. He was on an accelerated buildup for dust mites, pollen, mold, and other allergies, and apparently died of asthma complications triggered by the shots. There were no known risk factors in the third death.
Mortalities are lower than they used to be in the past with allergy shots, Dr. Epstein said.
She said she had no relevant disclosures.
[email protected]
AT THE 2017 AAAAI ANNUAL MEETING
Key clinical point: for reasons that are not yet clear, according to a review of 46.6 million injection office visits from 2008-2015.
Major finding: On surveys from 2013 to 2015, one grade 4 reaction – hypotension or respiratory failure – was reported for every 160,000 injection visits, up from one per million office visits in previous years. Two-thirds (152/252) of grade 3/4 systemic reactions for 2014-2015 occurred in patients with asthma.
Data source: Allergist surveys from 2008 to 2015.
Disclosures: The lead investigator said she had no relevant disclosures.
VIDEO: Despite promises of abstinence, isotretinoin-exposed pregnancies still occur
ORLANDO – Pregnancy prevention remains a challenge when prescribing isotretinoin to women of childbearing years with acne, Megha M. Tollefson, MD, said at the annual meeting of the American Academy of Dermatology.
Like so many promises having to do with love, a promise of sexual abstinence, however earnest, may at some point fall by the wayside, said Dr. Tollefson of the Mayo Clinic, Rochester, Minn., in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Despite the promises, the education, and the allegiance to the iPLEDGE program, exposed pregnancies still occur. iPLEDGE, with a 2.67 rate of fetal exposure over 1,000 treatment courses, was no more effective than its predecessor, SMART, with a 3.11 exposure rate. Altogether, there are still about 150 exposed pregnancies every year; 18 babies were exposed to the drug during SMART and 11 during iPLEDGE.
An anonymous survey of 75 iPLEDGE participants disclosed that 19% of those who chose abstinence were not, and that 34% of those who were sexually active did not comply with the promise to use two forms of birth control.
For some patients, Dr. Tollefson said, the best choice is a patient-independent form of birth control. That’s not an easy conversation to have sometimes, especially when parents are involved, but it’s an important one to have.
Dr. Tollefson had no financial disclosures.
[email protected]
On Twitter @Alz_Gal
ORLANDO – Pregnancy prevention remains a challenge when prescribing isotretinoin to women of childbearing years with acne, Megha M. Tollefson, MD, said at the annual meeting of the American Academy of Dermatology.
Like so many promises having to do with love, a promise of sexual abstinence, however earnest, may at some point fall by the wayside, said Dr. Tollefson of the Mayo Clinic, Rochester, Minn., in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Despite the promises, the education, and the allegiance to the iPLEDGE program, exposed pregnancies still occur. iPLEDGE, with a 2.67 rate of fetal exposure over 1,000 treatment courses, was no more effective than its predecessor, SMART, with a 3.11 exposure rate. Altogether, there are still about 150 exposed pregnancies every year; 18 babies were exposed to the drug during SMART and 11 during iPLEDGE.
An anonymous survey of 75 iPLEDGE participants disclosed that 19% of those who chose abstinence were not, and that 34% of those who were sexually active did not comply with the promise to use two forms of birth control.
For some patients, Dr. Tollefson said, the best choice is a patient-independent form of birth control. That’s not an easy conversation to have sometimes, especially when parents are involved, but it’s an important one to have.
Dr. Tollefson had no financial disclosures.
[email protected]
On Twitter @Alz_Gal
ORLANDO – Pregnancy prevention remains a challenge when prescribing isotretinoin to women of childbearing years with acne, Megha M. Tollefson, MD, said at the annual meeting of the American Academy of Dermatology.
Like so many promises having to do with love, a promise of sexual abstinence, however earnest, may at some point fall by the wayside, said Dr. Tollefson of the Mayo Clinic, Rochester, Minn., in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Despite the promises, the education, and the allegiance to the iPLEDGE program, exposed pregnancies still occur. iPLEDGE, with a 2.67 rate of fetal exposure over 1,000 treatment courses, was no more effective than its predecessor, SMART, with a 3.11 exposure rate. Altogether, there are still about 150 exposed pregnancies every year; 18 babies were exposed to the drug during SMART and 11 during iPLEDGE.
An anonymous survey of 75 iPLEDGE participants disclosed that 19% of those who chose abstinence were not, and that 34% of those who were sexually active did not comply with the promise to use two forms of birth control.
For some patients, Dr. Tollefson said, the best choice is a patient-independent form of birth control. That’s not an easy conversation to have sometimes, especially when parents are involved, but it’s an important one to have.
Dr. Tollefson had no financial disclosures.
[email protected]
On Twitter @Alz_Gal
AT AAD 17
VIDEO: Gold nanoparticles target laser acne therapy
ORLANDO – There may soon be a new gold standard in acne therapy – gold nanoparticles.
Evidence is mounting that the combination of a laser and a topical suspension of gold- or silver-coated silica nanoparticles can ablate the inner lining of the pilosebaceous unit, dramatically reducing or eliminating acne.
The system is still investigational in the United States, but shows great promise, Gilly Munavalli, MD, said in a video interview at the annual meeting of the American Academy of Dermatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Munavalli, medical director and founder of Dermatology, Laser, and Vein Specialists of the Carolinas, Charlotte, N.C., described how the system works, and some of the evidence supporting it.
“Basically what we’re doing is creating a chromophore for the laser to target,” he said. Just as a laser won’t work on light hair, it can’t target the colorless sebum inside the gland. The colored particles absorb the laser energy, vibrate, and create the locally destructive heat.
The particles are about 150 nm in diameter. They are designed to absorb infrared and near-infrared irradiation. A nanoparticle solution is applied to the affected area. Dr. Munavalli uses an ultrasound paddle to drive the particles into the sebaceous gland, and then removes any solution left on the skin.
He passes a hand-held, 800-nm laser over the skin. The laser activates the particles, generating heat that disrupts the lining of the pilosebaceous unit.
In 2014, Sebacia (Duluth, Ga.), which is developing the technology, reported positive results from two small European trials enrolling a total of 97 patients with acne. The patients were randomized to the gold nanoparticles or to a control group of, in the first study, an over-the-counter face wash and later, the vehicle solution minus the gold nanoparticles.
At 12 weeks’ follow-up, there was a significant reduction in inflammatory lesions and in the Investigator’s Global Assessment score. Treatment was well tolerated and results were durable at 6 months. At that time, the inflammatory lesion count was still 60% below baseline. Side effects were transient erythema and mild edema.
Dr. Munavalli serves as a scientific advisor for Sebacia.
[email protected]
On Twitter @Alz_Gal
ORLANDO – There may soon be a new gold standard in acne therapy – gold nanoparticles.
Evidence is mounting that the combination of a laser and a topical suspension of gold- or silver-coated silica nanoparticles can ablate the inner lining of the pilosebaceous unit, dramatically reducing or eliminating acne.
The system is still investigational in the United States, but shows great promise, Gilly Munavalli, MD, said in a video interview at the annual meeting of the American Academy of Dermatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Munavalli, medical director and founder of Dermatology, Laser, and Vein Specialists of the Carolinas, Charlotte, N.C., described how the system works, and some of the evidence supporting it.
“Basically what we’re doing is creating a chromophore for the laser to target,” he said. Just as a laser won’t work on light hair, it can’t target the colorless sebum inside the gland. The colored particles absorb the laser energy, vibrate, and create the locally destructive heat.
The particles are about 150 nm in diameter. They are designed to absorb infrared and near-infrared irradiation. A nanoparticle solution is applied to the affected area. Dr. Munavalli uses an ultrasound paddle to drive the particles into the sebaceous gland, and then removes any solution left on the skin.
He passes a hand-held, 800-nm laser over the skin. The laser activates the particles, generating heat that disrupts the lining of the pilosebaceous unit.
In 2014, Sebacia (Duluth, Ga.), which is developing the technology, reported positive results from two small European trials enrolling a total of 97 patients with acne. The patients were randomized to the gold nanoparticles or to a control group of, in the first study, an over-the-counter face wash and later, the vehicle solution minus the gold nanoparticles.
At 12 weeks’ follow-up, there was a significant reduction in inflammatory lesions and in the Investigator’s Global Assessment score. Treatment was well tolerated and results were durable at 6 months. At that time, the inflammatory lesion count was still 60% below baseline. Side effects were transient erythema and mild edema.
Dr. Munavalli serves as a scientific advisor for Sebacia.
[email protected]
On Twitter @Alz_Gal
ORLANDO – There may soon be a new gold standard in acne therapy – gold nanoparticles.
Evidence is mounting that the combination of a laser and a topical suspension of gold- or silver-coated silica nanoparticles can ablate the inner lining of the pilosebaceous unit, dramatically reducing or eliminating acne.
The system is still investigational in the United States, but shows great promise, Gilly Munavalli, MD, said in a video interview at the annual meeting of the American Academy of Dermatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Munavalli, medical director and founder of Dermatology, Laser, and Vein Specialists of the Carolinas, Charlotte, N.C., described how the system works, and some of the evidence supporting it.
“Basically what we’re doing is creating a chromophore for the laser to target,” he said. Just as a laser won’t work on light hair, it can’t target the colorless sebum inside the gland. The colored particles absorb the laser energy, vibrate, and create the locally destructive heat.
The particles are about 150 nm in diameter. They are designed to absorb infrared and near-infrared irradiation. A nanoparticle solution is applied to the affected area. Dr. Munavalli uses an ultrasound paddle to drive the particles into the sebaceous gland, and then removes any solution left on the skin.
He passes a hand-held, 800-nm laser over the skin. The laser activates the particles, generating heat that disrupts the lining of the pilosebaceous unit.
In 2014, Sebacia (Duluth, Ga.), which is developing the technology, reported positive results from two small European trials enrolling a total of 97 patients with acne. The patients were randomized to the gold nanoparticles or to a control group of, in the first study, an over-the-counter face wash and later, the vehicle solution minus the gold nanoparticles.
At 12 weeks’ follow-up, there was a significant reduction in inflammatory lesions and in the Investigator’s Global Assessment score. Treatment was well tolerated and results were durable at 6 months. At that time, the inflammatory lesion count was still 60% below baseline. Side effects were transient erythema and mild edema.
Dr. Munavalli serves as a scientific advisor for Sebacia.
[email protected]
On Twitter @Alz_Gal
AT AAD 17
VIDEO: Updating the rule of threes for melanoma gene testing
ORLANDO – The rule of threes that has been used to identify patients at risk of hereditary melanoma who may be candidates for genetic testing may be modified soon, according to Sancy Leachman, MD, PhD, professor and chair of the department of dermatology, Oregon Health and Science University, Portland.
Dr. Leachman was the author of a 2009 study that listed three factors as criteria for identifying melanoma: a personal history of at least three invasive melanomas, a combination of at least three melanomas in the individual and in first-degree and second-degree blood relatives – or, in first- or second-degree relatives, a total of at least three diagnoses of melanoma or pancreatic cancer or astrocytoma, which also have been associated with a known susceptibility gene, p16 (J Am Acad Dermatol. 2009 Oct;61[4]:677.e1-14).
But with more genetic testing, it is becoming clear that there are other cancers associated with an increased risk of hereditary melanoma, she explained in a video interview at the annual meeting of the American Academy of Dermatology. “The genes are a little bit different, but if you could identify those patients, you could potentially then screen them for those other cancers,” said Dr. Leachman, who is also director of the melanoma research program at Knight Cancer Institute at OHSU.
In the interview, she discussed a soon-to-be-published literature review that builds upon the rule of threes and suggests a strategy for deciding which patients should be considered for genetic testing, and includes “a suggested list of genes” that should be used in these different subsets of patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Leachman had no relevant disclosures.
ORLANDO – The rule of threes that has been used to identify patients at risk of hereditary melanoma who may be candidates for genetic testing may be modified soon, according to Sancy Leachman, MD, PhD, professor and chair of the department of dermatology, Oregon Health and Science University, Portland.
Dr. Leachman was the author of a 2009 study that listed three factors as criteria for identifying melanoma: a personal history of at least three invasive melanomas, a combination of at least three melanomas in the individual and in first-degree and second-degree blood relatives – or, in first- or second-degree relatives, a total of at least three diagnoses of melanoma or pancreatic cancer or astrocytoma, which also have been associated with a known susceptibility gene, p16 (J Am Acad Dermatol. 2009 Oct;61[4]:677.e1-14).
But with more genetic testing, it is becoming clear that there are other cancers associated with an increased risk of hereditary melanoma, she explained in a video interview at the annual meeting of the American Academy of Dermatology. “The genes are a little bit different, but if you could identify those patients, you could potentially then screen them for those other cancers,” said Dr. Leachman, who is also director of the melanoma research program at Knight Cancer Institute at OHSU.
In the interview, she discussed a soon-to-be-published literature review that builds upon the rule of threes and suggests a strategy for deciding which patients should be considered for genetic testing, and includes “a suggested list of genes” that should be used in these different subsets of patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Leachman had no relevant disclosures.
ORLANDO – The rule of threes that has been used to identify patients at risk of hereditary melanoma who may be candidates for genetic testing may be modified soon, according to Sancy Leachman, MD, PhD, professor and chair of the department of dermatology, Oregon Health and Science University, Portland.
Dr. Leachman was the author of a 2009 study that listed three factors as criteria for identifying melanoma: a personal history of at least three invasive melanomas, a combination of at least three melanomas in the individual and in first-degree and second-degree blood relatives – or, in first- or second-degree relatives, a total of at least three diagnoses of melanoma or pancreatic cancer or astrocytoma, which also have been associated with a known susceptibility gene, p16 (J Am Acad Dermatol. 2009 Oct;61[4]:677.e1-14).
But with more genetic testing, it is becoming clear that there are other cancers associated with an increased risk of hereditary melanoma, she explained in a video interview at the annual meeting of the American Academy of Dermatology. “The genes are a little bit different, but if you could identify those patients, you could potentially then screen them for those other cancers,” said Dr. Leachman, who is also director of the melanoma research program at Knight Cancer Institute at OHSU.
In the interview, she discussed a soon-to-be-published literature review that builds upon the rule of threes and suggests a strategy for deciding which patients should be considered for genetic testing, and includes “a suggested list of genes” that should be used in these different subsets of patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Leachman had no relevant disclosures.
AT AAD 17
Blinatumomab bests chemo in rel/ref B-ALL
Blinatumomab proved more effective than chemotherapy in a phase 3 trial of adults with Ph-negative, relapsed/refractory B-cell precursor acute lymphoblastic leukemia (B-ALL).
Blinatumomab produced higher remission rates and nearly doubled overall survival (OS) when compared to standard care, which encompassed 4 different chemotherapy regimens (investigator’s choice).
The incidence of grade 3 or higher adverse events (AEs) was higher for patients who received chemotherapy, but the incidence of serious AEs was higher in the blinatumomab arm.
These results were published in NEJM. The trial, known as TOWER, was sponsored by Amgen, the company developing blinatumomab.
“Results from the TOWER study reinforce the potential of this single-agent, bispecific T-cell engager immunotherapy, which helped a higher percentage of patients achieve minimal residual disease response versus standard of care chemotherapy, highlighting the depth and quality of remissions achieved,” said study author Hagop M. Kantarjian, MD, of The University of Texas MD Anderson Cancer Center in Houston.
Treatment
TOWER enrolled 405 patients with Ph-negative, relapsed/refractory B-ALL, 376 of whom ultimately received treatment.
The patients received blinatumomab (n=267) or investigator’s choice of 1 of 4 protocol-defined standard chemotherapy regimens (n=109):
- FLAG (fludarabine, high-dose cytarabine arabinoside, and granulocyte-colony stimulating factor), with or without an anthracycline (n=49, 45%)
- A high-dose cytarabine arabinoside-based regimen (n=19, 17%)
- A high-dose methotrexate-based regimen (n=22, 20%)
- A clofarabine-based regimen (n=19, 17%).
Patients who received blinatumomab received it as a continuous infusion, 4 weeks on and 2 weeks off, at 9 µg/day for 7 days, then 28 µg/day on weeks 2-4. They received 2 cycles of induction, which was followed by 3 cycles of consolidation if they had ≤5% blasts.
If patients still had ≤5% blasts after consolidation, they received up to 12 months of blinatumomab maintenance. Maintenance was a continuous infusion, 4 weeks on and 8 weeks off, at 28 µg/day.
Patients in the blinatumomab arm received a median of 2 cycles of therapy (range, 1-9). And patients in the chemotherapy arm received a median of 1 cycle (range, 1 to 4).
Thirty-two percent of patients in the blinatumomab arm received consolidation, as did 3% of patients in the chemotherapy arm.
Patients
Patient characteristics were similar between the treatment arms. The mean age was 41 in both arms (range, 18-80). Nearly 60% of patients in both arms were male.
About 40% of patients in the blinatumomab arm and 50% in the chemotherapy arm had not received any prior salvage regimens.
Thirty-five percent of patients in the blinatumomab arm and 34% in the chemotherapy arm had an allogeneic hematopoietic stem cell transplant (allo-HSCT) prior to study enrollment. Seventeen percent and 20%, respectively, relapsed after HSCT.
Forty-two percent and 40%, respectively, were refractory to primary or salvage therapy. Twenty-eight percent of patients in both arms were in their first relapse, and their first remission had lasted less than 12 months. Twelve percent of patients in both arms had an untreated second or greater relapse.
Remission
Within 12 weeks of treatment initiation, complete remission (CR) rates were significantly higher in the blinatumomab arm than the chemotherapy arm. (This was in the intent-to-treat population, which included 271 patients in the blinatumomab arm and 134 patients in the chemotherapy arm.)
The rate of CR with full hematologic recovery was 34% in the blinatumomab arm and 16% in the chemotherapy arm (P<0.001). The rate of CR with full, partial, or incomplete hematologic recovery was 44% and 25%, respectively (P<0.001).
Among patients who achieved a CR with full, partial, or incomplete hematologic recovery, 76% of those in the blinatumomab arm and 48% of those in the chemotherapy arm were negative for minimal residual disease.
Survival
At a median follow-up of 11.7 months for the blinatumomab arm and 11.8 months for the chemotherapy arm, the OS was significantly longer in the blinatumomab arm.
The median OS was 7.7 months and 4.0 months, respectively (hazard ratio for death=0.71, P=0.01).
The improvement in OS with blinatumomab was consistent across subgroups, regardless of age, prior salvage therapy, or prior allo-HSCT.
The investigators also considered the effect that post-treatment allo-HSCT might have on OS. Sixty-five patients in the blinatumomab arm and 32 in the chemotherapy arm went on to receive an allo-HSCT (24% of patients in both arms).
When the investigators censored for post-treatment allo-HSCT, the median OS was 6.9 months in the blinatumomab arm and 3.9 months in the chemotherapy arm (hazard ratio=0.66, P=0.004).
Safety
Nearly all patients in both arms (99%) experienced AEs. Grade 3 or higher AEs occurred in 87% of patients in the blinatumomab arm and 92% of those in the chemotherapy arm. Serious AEs occurred in 62% and 45%, respectively.
Grade 3 or higher AEs of interest, according to the researchers, were infection (34% with blinatumomab and 52% with chemotherapy), neutropenia (38% and 58%, respectively), elevated liver enzymes (13% and 15%, respectively), neurologic events (9% and 8%, respectively), cytokine release syndrome (5% and 0%, respectively), infusion reactions (3% and 1%, respectively), and lymphopenia (2% and 4%, respectively).
Fatal AEs occurred in 19% of patients in the blinatumomab arm and 17% of those in the chemotherapy arm.
Fatal AEs that occurred in at least 1% of patients in either arm (blinatumomab and chemotherapy, respectively) were sepsis (3% and 4%), septic shock (2% and 0%), multiorgan failure (1% and 0%), respiratory failure (<1% and 2%), and bacteremia (0% and 2%).
Blinatumomab proved more effective than chemotherapy in a phase 3 trial of adults with Ph-negative, relapsed/refractory B-cell precursor acute lymphoblastic leukemia (B-ALL).
Blinatumomab produced higher remission rates and nearly doubled overall survival (OS) when compared to standard care, which encompassed 4 different chemotherapy regimens (investigator’s choice).
The incidence of grade 3 or higher adverse events (AEs) was higher for patients who received chemotherapy, but the incidence of serious AEs was higher in the blinatumomab arm.
These results were published in NEJM. The trial, known as TOWER, was sponsored by Amgen, the company developing blinatumomab.
“Results from the TOWER study reinforce the potential of this single-agent, bispecific T-cell engager immunotherapy, which helped a higher percentage of patients achieve minimal residual disease response versus standard of care chemotherapy, highlighting the depth and quality of remissions achieved,” said study author Hagop M. Kantarjian, MD, of The University of Texas MD Anderson Cancer Center in Houston.
Treatment
TOWER enrolled 405 patients with Ph-negative, relapsed/refractory B-ALL, 376 of whom ultimately received treatment.
The patients received blinatumomab (n=267) or investigator’s choice of 1 of 4 protocol-defined standard chemotherapy regimens (n=109):
- FLAG (fludarabine, high-dose cytarabine arabinoside, and granulocyte-colony stimulating factor), with or without an anthracycline (n=49, 45%)
- A high-dose cytarabine arabinoside-based regimen (n=19, 17%)
- A high-dose methotrexate-based regimen (n=22, 20%)
- A clofarabine-based regimen (n=19, 17%).
Patients who received blinatumomab received it as a continuous infusion, 4 weeks on and 2 weeks off, at 9 µg/day for 7 days, then 28 µg/day on weeks 2-4. They received 2 cycles of induction, which was followed by 3 cycles of consolidation if they had ≤5% blasts.
If patients still had ≤5% blasts after consolidation, they received up to 12 months of blinatumomab maintenance. Maintenance was a continuous infusion, 4 weeks on and 8 weeks off, at 28 µg/day.
Patients in the blinatumomab arm received a median of 2 cycles of therapy (range, 1-9). And patients in the chemotherapy arm received a median of 1 cycle (range, 1 to 4).
Thirty-two percent of patients in the blinatumomab arm received consolidation, as did 3% of patients in the chemotherapy arm.
Patients
Patient characteristics were similar between the treatment arms. The mean age was 41 in both arms (range, 18-80). Nearly 60% of patients in both arms were male.
About 40% of patients in the blinatumomab arm and 50% in the chemotherapy arm had not received any prior salvage regimens.
Thirty-five percent of patients in the blinatumomab arm and 34% in the chemotherapy arm had an allogeneic hematopoietic stem cell transplant (allo-HSCT) prior to study enrollment. Seventeen percent and 20%, respectively, relapsed after HSCT.
Forty-two percent and 40%, respectively, were refractory to primary or salvage therapy. Twenty-eight percent of patients in both arms were in their first relapse, and their first remission had lasted less than 12 months. Twelve percent of patients in both arms had an untreated second or greater relapse.
Remission
Within 12 weeks of treatment initiation, complete remission (CR) rates were significantly higher in the blinatumomab arm than the chemotherapy arm. (This was in the intent-to-treat population, which included 271 patients in the blinatumomab arm and 134 patients in the chemotherapy arm.)
The rate of CR with full hematologic recovery was 34% in the blinatumomab arm and 16% in the chemotherapy arm (P<0.001). The rate of CR with full, partial, or incomplete hematologic recovery was 44% and 25%, respectively (P<0.001).
Among patients who achieved a CR with full, partial, or incomplete hematologic recovery, 76% of those in the blinatumomab arm and 48% of those in the chemotherapy arm were negative for minimal residual disease.
Survival
At a median follow-up of 11.7 months for the blinatumomab arm and 11.8 months for the chemotherapy arm, the OS was significantly longer in the blinatumomab arm.
The median OS was 7.7 months and 4.0 months, respectively (hazard ratio for death=0.71, P=0.01).
The improvement in OS with blinatumomab was consistent across subgroups, regardless of age, prior salvage therapy, or prior allo-HSCT.
The investigators also considered the effect that post-treatment allo-HSCT might have on OS. Sixty-five patients in the blinatumomab arm and 32 in the chemotherapy arm went on to receive an allo-HSCT (24% of patients in both arms).
When the investigators censored for post-treatment allo-HSCT, the median OS was 6.9 months in the blinatumomab arm and 3.9 months in the chemotherapy arm (hazard ratio=0.66, P=0.004).
Safety
Nearly all patients in both arms (99%) experienced AEs. Grade 3 or higher AEs occurred in 87% of patients in the blinatumomab arm and 92% of those in the chemotherapy arm. Serious AEs occurred in 62% and 45%, respectively.
Grade 3 or higher AEs of interest, according to the researchers, were infection (34% with blinatumomab and 52% with chemotherapy), neutropenia (38% and 58%, respectively), elevated liver enzymes (13% and 15%, respectively), neurologic events (9% and 8%, respectively), cytokine release syndrome (5% and 0%, respectively), infusion reactions (3% and 1%, respectively), and lymphopenia (2% and 4%, respectively).
Fatal AEs occurred in 19% of patients in the blinatumomab arm and 17% of those in the chemotherapy arm.
Fatal AEs that occurred in at least 1% of patients in either arm (blinatumomab and chemotherapy, respectively) were sepsis (3% and 4%), septic shock (2% and 0%), multiorgan failure (1% and 0%), respiratory failure (<1% and 2%), and bacteremia (0% and 2%).
Blinatumomab proved more effective than chemotherapy in a phase 3 trial of adults with Ph-negative, relapsed/refractory B-cell precursor acute lymphoblastic leukemia (B-ALL).
Blinatumomab produced higher remission rates and nearly doubled overall survival (OS) when compared to standard care, which encompassed 4 different chemotherapy regimens (investigator’s choice).
The incidence of grade 3 or higher adverse events (AEs) was higher for patients who received chemotherapy, but the incidence of serious AEs was higher in the blinatumomab arm.
These results were published in NEJM. The trial, known as TOWER, was sponsored by Amgen, the company developing blinatumomab.
“Results from the TOWER study reinforce the potential of this single-agent, bispecific T-cell engager immunotherapy, which helped a higher percentage of patients achieve minimal residual disease response versus standard of care chemotherapy, highlighting the depth and quality of remissions achieved,” said study author Hagop M. Kantarjian, MD, of The University of Texas MD Anderson Cancer Center in Houston.
Treatment
TOWER enrolled 405 patients with Ph-negative, relapsed/refractory B-ALL, 376 of whom ultimately received treatment.
The patients received blinatumomab (n=267) or investigator’s choice of 1 of 4 protocol-defined standard chemotherapy regimens (n=109):
- FLAG (fludarabine, high-dose cytarabine arabinoside, and granulocyte-colony stimulating factor), with or without an anthracycline (n=49, 45%)
- A high-dose cytarabine arabinoside-based regimen (n=19, 17%)
- A high-dose methotrexate-based regimen (n=22, 20%)
- A clofarabine-based regimen (n=19, 17%).
Patients who received blinatumomab received it as a continuous infusion, 4 weeks on and 2 weeks off, at 9 µg/day for 7 days, then 28 µg/day on weeks 2-4. They received 2 cycles of induction, which was followed by 3 cycles of consolidation if they had ≤5% blasts.
If patients still had ≤5% blasts after consolidation, they received up to 12 months of blinatumomab maintenance. Maintenance was a continuous infusion, 4 weeks on and 8 weeks off, at 28 µg/day.
Patients in the blinatumomab arm received a median of 2 cycles of therapy (range, 1-9). And patients in the chemotherapy arm received a median of 1 cycle (range, 1 to 4).
Thirty-two percent of patients in the blinatumomab arm received consolidation, as did 3% of patients in the chemotherapy arm.
Patients
Patient characteristics were similar between the treatment arms. The mean age was 41 in both arms (range, 18-80). Nearly 60% of patients in both arms were male.
About 40% of patients in the blinatumomab arm and 50% in the chemotherapy arm had not received any prior salvage regimens.
Thirty-five percent of patients in the blinatumomab arm and 34% in the chemotherapy arm had an allogeneic hematopoietic stem cell transplant (allo-HSCT) prior to study enrollment. Seventeen percent and 20%, respectively, relapsed after HSCT.
Forty-two percent and 40%, respectively, were refractory to primary or salvage therapy. Twenty-eight percent of patients in both arms were in their first relapse, and their first remission had lasted less than 12 months. Twelve percent of patients in both arms had an untreated second or greater relapse.
Remission
Within 12 weeks of treatment initiation, complete remission (CR) rates were significantly higher in the blinatumomab arm than the chemotherapy arm. (This was in the intent-to-treat population, which included 271 patients in the blinatumomab arm and 134 patients in the chemotherapy arm.)
The rate of CR with full hematologic recovery was 34% in the blinatumomab arm and 16% in the chemotherapy arm (P<0.001). The rate of CR with full, partial, or incomplete hematologic recovery was 44% and 25%, respectively (P<0.001).
Among patients who achieved a CR with full, partial, or incomplete hematologic recovery, 76% of those in the blinatumomab arm and 48% of those in the chemotherapy arm were negative for minimal residual disease.
Survival
At a median follow-up of 11.7 months for the blinatumomab arm and 11.8 months for the chemotherapy arm, the OS was significantly longer in the blinatumomab arm.
The median OS was 7.7 months and 4.0 months, respectively (hazard ratio for death=0.71, P=0.01).
The improvement in OS with blinatumomab was consistent across subgroups, regardless of age, prior salvage therapy, or prior allo-HSCT.
The investigators also considered the effect that post-treatment allo-HSCT might have on OS. Sixty-five patients in the blinatumomab arm and 32 in the chemotherapy arm went on to receive an allo-HSCT (24% of patients in both arms).
When the investigators censored for post-treatment allo-HSCT, the median OS was 6.9 months in the blinatumomab arm and 3.9 months in the chemotherapy arm (hazard ratio=0.66, P=0.004).
Safety
Nearly all patients in both arms (99%) experienced AEs. Grade 3 or higher AEs occurred in 87% of patients in the blinatumomab arm and 92% of those in the chemotherapy arm. Serious AEs occurred in 62% and 45%, respectively.
Grade 3 or higher AEs of interest, according to the researchers, were infection (34% with blinatumomab and 52% with chemotherapy), neutropenia (38% and 58%, respectively), elevated liver enzymes (13% and 15%, respectively), neurologic events (9% and 8%, respectively), cytokine release syndrome (5% and 0%, respectively), infusion reactions (3% and 1%, respectively), and lymphopenia (2% and 4%, respectively).
Fatal AEs occurred in 19% of patients in the blinatumomab arm and 17% of those in the chemotherapy arm.
Fatal AEs that occurred in at least 1% of patients in either arm (blinatumomab and chemotherapy, respectively) were sepsis (3% and 4%), septic shock (2% and 0%), multiorgan failure (1% and 0%), respiratory failure (<1% and 2%), and bacteremia (0% and 2%).
Arteriovenous coupler for structural hypertension poised for sham-controlled study
WASHINGTON – A new device may be the best opportunity yet to achieve sustained blood pressure control in individuals aged 60 years and older, according to Paul Sobotka, MD, Chief Scientific Officer at ROX Medical, the maker of the device.
The experimental device is an arteriovenous coupler that provides an anastomosis between artery and vein to lower arterial volume and reduce blood pressure due to a structural cause. The device has already performed well in initial clinical studies, including a controlled, open-label trial, he reported at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
While neurohormonally driven elevations drive increases in diastolic and systolic blood pressures in younger patients, aortic stiffness and loss of vascular elasticity characterize the structurally driven hypertension of older patients, he said. While diuretics can lower arterial volume to achieve reductions in structurally related systolic hypertension, large doses are typically required to have meaningful results and are likely to be accompanied by unacceptable side effects in a large proportion of patients, he said.
“Somewhere between the age of 50 and 60 years, the majority of hypertensive patients will no longer be principally responsive to drugs acting on neurohormonal pathways or to renal denervation strategies.” In the elderly, cardiovascular risk is largely driven by hypertension principally related to the loss of aortic elasticity, which does not respond to most antihypertensive drugs.
The investigational arteriovenous coupler being developed by ROX Medical is intended to lower systolic blood pressure by reducing vascular resistance and therefore arterial volume. The coupler can be placed during cardiac catheterization, does not require sedation, takes about 1 hour to insert, and can be removed if necessary.
In a randomized, open-label study (Lancet. 2015 Apr;385:1634-41), mean systolic blood pressure was reduced by 26.9 mmHg on average in the 44 patients who received the arteriovenous coupler (P less than .001 vs. baseline) and by 3.7 mmHg (P = .31 vs. baseline) in the 39 patients who were maintained on antihypertensive medication, said Dr. Sobotka, who was a coauthor on this and several other clinical studies testing the efficacy and safety of the coupler. Systolic blood pressure falls almost immediately after the device is positioned, and blood pressure control has been sustained for up to 2 years of follow-up so far.
The procedure has been effective in patients resistant to antihypertensive medications and in those who have failed renal denervation, he said.
“The most significant adverse event observed has been venous stenosis related to turbulence, which occurs within in the first 12 months” after device placement, Dr. Sobotka reported. He said that venous stenting has resolved the problem in all affected patients. “Adverse events beyond that have been trivial.”
WASHINGTON – A new device may be the best opportunity yet to achieve sustained blood pressure control in individuals aged 60 years and older, according to Paul Sobotka, MD, Chief Scientific Officer at ROX Medical, the maker of the device.
The experimental device is an arteriovenous coupler that provides an anastomosis between artery and vein to lower arterial volume and reduce blood pressure due to a structural cause. The device has already performed well in initial clinical studies, including a controlled, open-label trial, he reported at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
While neurohormonally driven elevations drive increases in diastolic and systolic blood pressures in younger patients, aortic stiffness and loss of vascular elasticity characterize the structurally driven hypertension of older patients, he said. While diuretics can lower arterial volume to achieve reductions in structurally related systolic hypertension, large doses are typically required to have meaningful results and are likely to be accompanied by unacceptable side effects in a large proportion of patients, he said.
“Somewhere between the age of 50 and 60 years, the majority of hypertensive patients will no longer be principally responsive to drugs acting on neurohormonal pathways or to renal denervation strategies.” In the elderly, cardiovascular risk is largely driven by hypertension principally related to the loss of aortic elasticity, which does not respond to most antihypertensive drugs.
The investigational arteriovenous coupler being developed by ROX Medical is intended to lower systolic blood pressure by reducing vascular resistance and therefore arterial volume. The coupler can be placed during cardiac catheterization, does not require sedation, takes about 1 hour to insert, and can be removed if necessary.
In a randomized, open-label study (Lancet. 2015 Apr;385:1634-41), mean systolic blood pressure was reduced by 26.9 mmHg on average in the 44 patients who received the arteriovenous coupler (P less than .001 vs. baseline) and by 3.7 mmHg (P = .31 vs. baseline) in the 39 patients who were maintained on antihypertensive medication, said Dr. Sobotka, who was a coauthor on this and several other clinical studies testing the efficacy and safety of the coupler. Systolic blood pressure falls almost immediately after the device is positioned, and blood pressure control has been sustained for up to 2 years of follow-up so far.
The procedure has been effective in patients resistant to antihypertensive medications and in those who have failed renal denervation, he said.
“The most significant adverse event observed has been venous stenosis related to turbulence, which occurs within in the first 12 months” after device placement, Dr. Sobotka reported. He said that venous stenting has resolved the problem in all affected patients. “Adverse events beyond that have been trivial.”
WASHINGTON – A new device may be the best opportunity yet to achieve sustained blood pressure control in individuals aged 60 years and older, according to Paul Sobotka, MD, Chief Scientific Officer at ROX Medical, the maker of the device.
The experimental device is an arteriovenous coupler that provides an anastomosis between artery and vein to lower arterial volume and reduce blood pressure due to a structural cause. The device has already performed well in initial clinical studies, including a controlled, open-label trial, he reported at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
While neurohormonally driven elevations drive increases in diastolic and systolic blood pressures in younger patients, aortic stiffness and loss of vascular elasticity characterize the structurally driven hypertension of older patients, he said. While diuretics can lower arterial volume to achieve reductions in structurally related systolic hypertension, large doses are typically required to have meaningful results and are likely to be accompanied by unacceptable side effects in a large proportion of patients, he said.
“Somewhere between the age of 50 and 60 years, the majority of hypertensive patients will no longer be principally responsive to drugs acting on neurohormonal pathways or to renal denervation strategies.” In the elderly, cardiovascular risk is largely driven by hypertension principally related to the loss of aortic elasticity, which does not respond to most antihypertensive drugs.
The investigational arteriovenous coupler being developed by ROX Medical is intended to lower systolic blood pressure by reducing vascular resistance and therefore arterial volume. The coupler can be placed during cardiac catheterization, does not require sedation, takes about 1 hour to insert, and can be removed if necessary.
In a randomized, open-label study (Lancet. 2015 Apr;385:1634-41), mean systolic blood pressure was reduced by 26.9 mmHg on average in the 44 patients who received the arteriovenous coupler (P less than .001 vs. baseline) and by 3.7 mmHg (P = .31 vs. baseline) in the 39 patients who were maintained on antihypertensive medication, said Dr. Sobotka, who was a coauthor on this and several other clinical studies testing the efficacy and safety of the coupler. Systolic blood pressure falls almost immediately after the device is positioned, and blood pressure control has been sustained for up to 2 years of follow-up so far.
The procedure has been effective in patients resistant to antihypertensive medications and in those who have failed renal denervation, he said.
“The most significant adverse event observed has been venous stenosis related to turbulence, which occurs within in the first 12 months” after device placement, Dr. Sobotka reported. He said that venous stenting has resolved the problem in all affected patients. “Adverse events beyond that have been trivial.”
EXPERT ANALYSIS AT CRT 2017
Chronic rhinosinusitis exacerbation factors identified
ATLANTA – Clinical factors associated with acute exacerbations of chronic rhinosinusitis include female sex, nonwhite race, and a higher body mass index, results from a retrospective study suggest.
“Previous research has shown associations between various risk factors and the development of chronic rhinosinusitis,” study author Jason H. Kwah, MD, said in an interview in advance of the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “However, there is minimal data regarding risk factor associations with frequent acute exacerbations of chronic rhinosinusitis. This is an important question as much of the morbidity and financial burden of this disease stems from costs and symptoms related to the management of acute exacerbations with antibiotics and steroids. If associations can be identified, the hope would be that modification of some of these risk factors could lead to improved control of the condition.”
Of the 3,109 CRS patients, 936 were frequent exacerbators while 2,173 were infrequent exacerbators. Univariate analysis revealed that the following factors were associated with frequent exacerbations, compared with infrequent exacerbations: female sex, nonwhite race, higher body mass index, having any drug allergy, allergic rhinitis, peripheral eosinophilia, asthma, lower forced expiratory volume in 1 second, previous sinus surgery, severe sinusitis on CT, one or more steroid prescriptions, and the presence of autoimmune disease. Multivariate analysis adjusted for race and sex revealed that allergic rhinitis (odds ratio, 1.48), peripheral eosinophilia (OR, 1.30), asthma (OR, 1.90), and autoimmune disease (OR, 1.61) remained significantly associated with frequent CRS exacerbations.
“We found it surprising that there were so many risk factors associated with frequent acute exacerbations of CRS, as well as the strengths of these associations,” Dr. Kwah said. “Acute exacerbations of CRS cause significant morbidity and financial burden, and based on the results of our study, are likely associated with many clinically relevant risk factors. Further study is needed to confirm the presence of these associations, study the mechanism of disease, and ultimately establish treatment protocols that can improve the control of frequent acute exacerbations of CRS.”
He acknowledged certain limitations of the study, including the challenges of establishing an accurate definition for acute exacerbations of CRS in a retrospective study. “While we felt our protocol for retrospective analysis was sufficient given our inclusion criteria, a prospective trial would be helpful in further delineating the presence of acute exacerbations of CRS and how they are associated with clinical risk factors,” he said.
The study was supported in part by the NIH National Center for Advancing Translational Sciences, the Chronic Rhinosinusitis Integrative Studies Program, the Ernest Bazley Foundation, and the Division of Allergy and Immunology at the Northwestern Feinberg School of Medicine. Dr. Kwah reported having no financial disclosures.
[email protected]
ATLANTA – Clinical factors associated with acute exacerbations of chronic rhinosinusitis include female sex, nonwhite race, and a higher body mass index, results from a retrospective study suggest.
“Previous research has shown associations between various risk factors and the development of chronic rhinosinusitis,” study author Jason H. Kwah, MD, said in an interview in advance of the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “However, there is minimal data regarding risk factor associations with frequent acute exacerbations of chronic rhinosinusitis. This is an important question as much of the morbidity and financial burden of this disease stems from costs and symptoms related to the management of acute exacerbations with antibiotics and steroids. If associations can be identified, the hope would be that modification of some of these risk factors could lead to improved control of the condition.”
Of the 3,109 CRS patients, 936 were frequent exacerbators while 2,173 were infrequent exacerbators. Univariate analysis revealed that the following factors were associated with frequent exacerbations, compared with infrequent exacerbations: female sex, nonwhite race, higher body mass index, having any drug allergy, allergic rhinitis, peripheral eosinophilia, asthma, lower forced expiratory volume in 1 second, previous sinus surgery, severe sinusitis on CT, one or more steroid prescriptions, and the presence of autoimmune disease. Multivariate analysis adjusted for race and sex revealed that allergic rhinitis (odds ratio, 1.48), peripheral eosinophilia (OR, 1.30), asthma (OR, 1.90), and autoimmune disease (OR, 1.61) remained significantly associated with frequent CRS exacerbations.
“We found it surprising that there were so many risk factors associated with frequent acute exacerbations of CRS, as well as the strengths of these associations,” Dr. Kwah said. “Acute exacerbations of CRS cause significant morbidity and financial burden, and based on the results of our study, are likely associated with many clinically relevant risk factors. Further study is needed to confirm the presence of these associations, study the mechanism of disease, and ultimately establish treatment protocols that can improve the control of frequent acute exacerbations of CRS.”
He acknowledged certain limitations of the study, including the challenges of establishing an accurate definition for acute exacerbations of CRS in a retrospective study. “While we felt our protocol for retrospective analysis was sufficient given our inclusion criteria, a prospective trial would be helpful in further delineating the presence of acute exacerbations of CRS and how they are associated with clinical risk factors,” he said.
The study was supported in part by the NIH National Center for Advancing Translational Sciences, the Chronic Rhinosinusitis Integrative Studies Program, the Ernest Bazley Foundation, and the Division of Allergy and Immunology at the Northwestern Feinberg School of Medicine. Dr. Kwah reported having no financial disclosures.
[email protected]
ATLANTA – Clinical factors associated with acute exacerbations of chronic rhinosinusitis include female sex, nonwhite race, and a higher body mass index, results from a retrospective study suggest.
“Previous research has shown associations between various risk factors and the development of chronic rhinosinusitis,” study author Jason H. Kwah, MD, said in an interview in advance of the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “However, there is minimal data regarding risk factor associations with frequent acute exacerbations of chronic rhinosinusitis. This is an important question as much of the morbidity and financial burden of this disease stems from costs and symptoms related to the management of acute exacerbations with antibiotics and steroids. If associations can be identified, the hope would be that modification of some of these risk factors could lead to improved control of the condition.”
Of the 3,109 CRS patients, 936 were frequent exacerbators while 2,173 were infrequent exacerbators. Univariate analysis revealed that the following factors were associated with frequent exacerbations, compared with infrequent exacerbations: female sex, nonwhite race, higher body mass index, having any drug allergy, allergic rhinitis, peripheral eosinophilia, asthma, lower forced expiratory volume in 1 second, previous sinus surgery, severe sinusitis on CT, one or more steroid prescriptions, and the presence of autoimmune disease. Multivariate analysis adjusted for race and sex revealed that allergic rhinitis (odds ratio, 1.48), peripheral eosinophilia (OR, 1.30), asthma (OR, 1.90), and autoimmune disease (OR, 1.61) remained significantly associated with frequent CRS exacerbations.
“We found it surprising that there were so many risk factors associated with frequent acute exacerbations of CRS, as well as the strengths of these associations,” Dr. Kwah said. “Acute exacerbations of CRS cause significant morbidity and financial burden, and based on the results of our study, are likely associated with many clinically relevant risk factors. Further study is needed to confirm the presence of these associations, study the mechanism of disease, and ultimately establish treatment protocols that can improve the control of frequent acute exacerbations of CRS.”
He acknowledged certain limitations of the study, including the challenges of establishing an accurate definition for acute exacerbations of CRS in a retrospective study. “While we felt our protocol for retrospective analysis was sufficient given our inclusion criteria, a prospective trial would be helpful in further delineating the presence of acute exacerbations of CRS and how they are associated with clinical risk factors,” he said.
The study was supported in part by the NIH National Center for Advancing Translational Sciences, the Chronic Rhinosinusitis Integrative Studies Program, the Ernest Bazley Foundation, and the Division of Allergy and Immunology at the Northwestern Feinberg School of Medicine. Dr. Kwah reported having no financial disclosures.
[email protected]
AT THE 2017 AAAAI ANNUAL MEETING
Key clinical point:
Major finding: On multivariate analysis, allergic rhinitis (odds ratio, 1.48), peripheral eosinophilia (OR, 1.30), asthma (OR, 1.90), and autoimmune disease (OR, 1.61) remained significantly associated with frequent CRS exacerbations.
Data source: A retrospective review of 3,109 patients who were treated for CRS from January 2014 to December 2015.
Disclosures: The study was supported in part by the NIH National Center for Advancing Translational Sciences, the Chronic Rhinosinusitis Integrative Studies Program, the Ernest Bazley Foundation, and the Division of Allergy and Immunology at the Northwestern Feinberg School of Medicine. Dr. Kwah reported having no financial disclosures.
When STEMI door-to-balloon times deteriorate
WASHINGTON – The interventional cardiology team at Geisinger Medical Center, Danville, Pa., reorganized their care for ST segment elevation MI (STEMI) in 2004 to improve their door-to-balloon angioplasty times. Their highly successful efforts soon streamlined the process, placing Geisinger in the top 10% nationally for low average door-to-balloon (DTB) times.
Then, Geisinger began to see their DTB times creep up.
After witnessing a 5- to 10-minute increase in DTB times for STEMI patients – whether picked up by ambulance, appearing in the emergency department (ED), or referred from neighboring hospitals – James Blankenship, MD, director of the division of cardiology, and fellow Geisinger researchers took on an institutional analysis, which was presented at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
Their analysis failed to identify a single explanation, but it did reinforce the importance of constantly reinvigorating processes, Dr. Blankenship said. And their analysis indicated that delays don’t necessarily translate into poorer outcomes.
One proposed explanation was that delays had been caused by an increased reliance on radial rather than femoral catheter access. Dr. Blankenship cited studies suggesting that radial access increases DTB times by 1 to 12 minutes. The proportion of percutaneous coronary interventions performed radially at Geisinger had risen from 2% to 85% over the time period that DTB times had risen.
“There is evidence of benefit from radial access, so even if it slows you down a few minutes, it may be worth doing,” Dr. Blankenship noted. However, when this variable was evaluated, the DTB times were, if anything, slightly faster with radial relative to femoral access.
Another theory was that the decision to provide fellows with a greater role in STEMI management had produced treatment delays. In the cath lab at Geisinger, the increased fellow participation “correlated perfectly” with the decline in DTB times, according to Dr. Blankenship. However, a close look at this variable failed to show any meaningful impact on DTB times.
Changes in process were also examined. For one example, a form must now be completed documenting airway assessment. However, Dr. Blankenship found that filling out this form only takes about a minute and could account for only a small part of the observed loss.
The most significant cause for the increased DTB times among STEMI patients presenting in the ED may well have been a 2012 change in the configuration of the hospital. Prior to 2012, the distance from the ED to the cath lab was less than 100 yards and a 1-minute walk. After the change in the configuration, the cath lab was approximately 7 minutes away, a change that “correlated somewhat” to a prolongation in DTB times.
Similarly, regional hospital STEMI referral patterns changed when a hospital in relatively close proximity opened a cath lab. Up until that time, most referrals had been a 5-minute helicopter transfer, according to Dr. Blankenship. Afterwards, some helicopter transfer times rose to 25 minutes.
Yet, no explanation seemed to be more important than simply ensuring that new staff understand and adhere to the processes. Recounting his experience with a “secret shopper” approach in which he called Geisinger posing as a referring physician to report a potential STEMI, he was disappointed to reach a staff member uncertain of the meaning of the term STEMI.
“STEMI systems need a lot of maintenance,” Dr. Blankenship said. He cautioned that staff changes are common and frequent, making it important to continually assess whether all the participants in delivering STEMI care understand their role.
Some published studies challenge the importance of small changes in DTB times as a predictor of STEMI survival, according to Dr. Blankenship, citing one national survey unable to link a reduction in DTB times with a change in in-hospital mortality (N Engl J Med. 2013;369:901-09). However, he has been more convinced by those studies that do show a relationship. He cited one large study that found an 8% loss in the mortality benefit for each 10-minute delay in DTB (Lancet. 2015;385:1114-22).
Rapid DTB times cannot be divorced from quality of care, Dr. Blankenship said. He cited an experience at one center in which an aggressive program for reducing DTB times led to an increase in mortality among false-positive patients. “It is okay to sacrifice time for quality.”
It was at the 2016 CRT meeting that Dr. Blankenship first provided data showing the decline in DTB times at his institution. At that time and prior to the more comprehensive evaluation of the reasons for the delay presented at this year’s meeting, he speculated that it may be just a question of fatigue from sustaining a rapid response posture over several years.
“My original observation, that after a while you just get tired, is probably still true,” he said.
WASHINGTON – The interventional cardiology team at Geisinger Medical Center, Danville, Pa., reorganized their care for ST segment elevation MI (STEMI) in 2004 to improve their door-to-balloon angioplasty times. Their highly successful efforts soon streamlined the process, placing Geisinger in the top 10% nationally for low average door-to-balloon (DTB) times.
Then, Geisinger began to see their DTB times creep up.
After witnessing a 5- to 10-minute increase in DTB times for STEMI patients – whether picked up by ambulance, appearing in the emergency department (ED), or referred from neighboring hospitals – James Blankenship, MD, director of the division of cardiology, and fellow Geisinger researchers took on an institutional analysis, which was presented at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
Their analysis failed to identify a single explanation, but it did reinforce the importance of constantly reinvigorating processes, Dr. Blankenship said. And their analysis indicated that delays don’t necessarily translate into poorer outcomes.
One proposed explanation was that delays had been caused by an increased reliance on radial rather than femoral catheter access. Dr. Blankenship cited studies suggesting that radial access increases DTB times by 1 to 12 minutes. The proportion of percutaneous coronary interventions performed radially at Geisinger had risen from 2% to 85% over the time period that DTB times had risen.
“There is evidence of benefit from radial access, so even if it slows you down a few minutes, it may be worth doing,” Dr. Blankenship noted. However, when this variable was evaluated, the DTB times were, if anything, slightly faster with radial relative to femoral access.
Another theory was that the decision to provide fellows with a greater role in STEMI management had produced treatment delays. In the cath lab at Geisinger, the increased fellow participation “correlated perfectly” with the decline in DTB times, according to Dr. Blankenship. However, a close look at this variable failed to show any meaningful impact on DTB times.
Changes in process were also examined. For one example, a form must now be completed documenting airway assessment. However, Dr. Blankenship found that filling out this form only takes about a minute and could account for only a small part of the observed loss.
The most significant cause for the increased DTB times among STEMI patients presenting in the ED may well have been a 2012 change in the configuration of the hospital. Prior to 2012, the distance from the ED to the cath lab was less than 100 yards and a 1-minute walk. After the change in the configuration, the cath lab was approximately 7 minutes away, a change that “correlated somewhat” to a prolongation in DTB times.
Similarly, regional hospital STEMI referral patterns changed when a hospital in relatively close proximity opened a cath lab. Up until that time, most referrals had been a 5-minute helicopter transfer, according to Dr. Blankenship. Afterwards, some helicopter transfer times rose to 25 minutes.
Yet, no explanation seemed to be more important than simply ensuring that new staff understand and adhere to the processes. Recounting his experience with a “secret shopper” approach in which he called Geisinger posing as a referring physician to report a potential STEMI, he was disappointed to reach a staff member uncertain of the meaning of the term STEMI.
“STEMI systems need a lot of maintenance,” Dr. Blankenship said. He cautioned that staff changes are common and frequent, making it important to continually assess whether all the participants in delivering STEMI care understand their role.
Some published studies challenge the importance of small changes in DTB times as a predictor of STEMI survival, according to Dr. Blankenship, citing one national survey unable to link a reduction in DTB times with a change in in-hospital mortality (N Engl J Med. 2013;369:901-09). However, he has been more convinced by those studies that do show a relationship. He cited one large study that found an 8% loss in the mortality benefit for each 10-minute delay in DTB (Lancet. 2015;385:1114-22).
Rapid DTB times cannot be divorced from quality of care, Dr. Blankenship said. He cited an experience at one center in which an aggressive program for reducing DTB times led to an increase in mortality among false-positive patients. “It is okay to sacrifice time for quality.”
It was at the 2016 CRT meeting that Dr. Blankenship first provided data showing the decline in DTB times at his institution. At that time and prior to the more comprehensive evaluation of the reasons for the delay presented at this year’s meeting, he speculated that it may be just a question of fatigue from sustaining a rapid response posture over several years.
“My original observation, that after a while you just get tired, is probably still true,” he said.
WASHINGTON – The interventional cardiology team at Geisinger Medical Center, Danville, Pa., reorganized their care for ST segment elevation MI (STEMI) in 2004 to improve their door-to-balloon angioplasty times. Their highly successful efforts soon streamlined the process, placing Geisinger in the top 10% nationally for low average door-to-balloon (DTB) times.
Then, Geisinger began to see their DTB times creep up.
After witnessing a 5- to 10-minute increase in DTB times for STEMI patients – whether picked up by ambulance, appearing in the emergency department (ED), or referred from neighboring hospitals – James Blankenship, MD, director of the division of cardiology, and fellow Geisinger researchers took on an institutional analysis, which was presented at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
Their analysis failed to identify a single explanation, but it did reinforce the importance of constantly reinvigorating processes, Dr. Blankenship said. And their analysis indicated that delays don’t necessarily translate into poorer outcomes.
One proposed explanation was that delays had been caused by an increased reliance on radial rather than femoral catheter access. Dr. Blankenship cited studies suggesting that radial access increases DTB times by 1 to 12 minutes. The proportion of percutaneous coronary interventions performed radially at Geisinger had risen from 2% to 85% over the time period that DTB times had risen.
“There is evidence of benefit from radial access, so even if it slows you down a few minutes, it may be worth doing,” Dr. Blankenship noted. However, when this variable was evaluated, the DTB times were, if anything, slightly faster with radial relative to femoral access.
Another theory was that the decision to provide fellows with a greater role in STEMI management had produced treatment delays. In the cath lab at Geisinger, the increased fellow participation “correlated perfectly” with the decline in DTB times, according to Dr. Blankenship. However, a close look at this variable failed to show any meaningful impact on DTB times.
Changes in process were also examined. For one example, a form must now be completed documenting airway assessment. However, Dr. Blankenship found that filling out this form only takes about a minute and could account for only a small part of the observed loss.
The most significant cause for the increased DTB times among STEMI patients presenting in the ED may well have been a 2012 change in the configuration of the hospital. Prior to 2012, the distance from the ED to the cath lab was less than 100 yards and a 1-minute walk. After the change in the configuration, the cath lab was approximately 7 minutes away, a change that “correlated somewhat” to a prolongation in DTB times.
Similarly, regional hospital STEMI referral patterns changed when a hospital in relatively close proximity opened a cath lab. Up until that time, most referrals had been a 5-minute helicopter transfer, according to Dr. Blankenship. Afterwards, some helicopter transfer times rose to 25 minutes.
Yet, no explanation seemed to be more important than simply ensuring that new staff understand and adhere to the processes. Recounting his experience with a “secret shopper” approach in which he called Geisinger posing as a referring physician to report a potential STEMI, he was disappointed to reach a staff member uncertain of the meaning of the term STEMI.
“STEMI systems need a lot of maintenance,” Dr. Blankenship said. He cautioned that staff changes are common and frequent, making it important to continually assess whether all the participants in delivering STEMI care understand their role.
Some published studies challenge the importance of small changes in DTB times as a predictor of STEMI survival, according to Dr. Blankenship, citing one national survey unable to link a reduction in DTB times with a change in in-hospital mortality (N Engl J Med. 2013;369:901-09). However, he has been more convinced by those studies that do show a relationship. He cited one large study that found an 8% loss in the mortality benefit for each 10-minute delay in DTB (Lancet. 2015;385:1114-22).
Rapid DTB times cannot be divorced from quality of care, Dr. Blankenship said. He cited an experience at one center in which an aggressive program for reducing DTB times led to an increase in mortality among false-positive patients. “It is okay to sacrifice time for quality.”
It was at the 2016 CRT meeting that Dr. Blankenship first provided data showing the decline in DTB times at his institution. At that time and prior to the more comprehensive evaluation of the reasons for the delay presented at this year’s meeting, he speculated that it may be just a question of fatigue from sustaining a rapid response posture over several years.
“My original observation, that after a while you just get tired, is probably still true,” he said.
AT CRT 2017
Key clinical point: With small deviations in process over time, delays build in the emergency treatment of ST segment elevation MI (STEMI).
Major finding: Processes must be regularly reinvigorated, a systematic evaluation of deteriorating door-to-balloon times for STEMI care suggests.
Data source: Nonrandomized retrospective analysis.
Disclosures: Dr. Blankenship reported no financial relationships to disclose.