User login
Hospitalization Risk With Benzodiazepine and Opioid Use in Veterans With Posttraumatic Stress Disorder (FULL)
Posttraumatic stress disorder (PTSD) is a mental health condition that may develop in response to a traumatic event, such as that experienced by a soldier during active combat duty. In 2009, more than 495,000 veterans within the VA health care system were treated for PTSD—nearly triple the number a decade earlier.1 Core symptoms of PTSD include alterations in arousal and reactivity, avoidant behaviors, negative alterations in mood and cognition, and intrusive thoughts and nightmares. All of the symptoms can be debilitating. First-line pharmacotherapy options that target these core symptoms include selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs).2
The anxiolytic and sedative effects of benzodiazepines may provide quick relief from many of the secondary symptoms of PTSD, including sleep disturbances, irritability, and panic attacks. However, benzodiazepines potentially interfere with the extinction of conditioned fear—a goal integral to certain types of psychotherapy, such as exposure therapy.3,4 In addition, the systematic review and meta-analysis by Guina and colleagues revealed that benzodiazepines are ineffective in the treatment of PTSD.5 The majority of the evaluated studies that used PTSD-specific measures (eg, Clinician-Administered PTSD Scale [CAPS]) found increased PTSD severity and worse prognosis with use of these medications.5 In 2010, the VA and the DoD released a joint guideline for PTSD management.2 According to the guideline, benzodiazepines cause harm when used in PTSD and are relatively contraindicated in combat veterans because of the higher incidence of comorbid substance use disorders (SUDs) in these veterans relative to the general population.2,6
Opioid use also has been linked to poor functional and clinical outcomes in veterans with PTSD. Among patients being treated for opioid use disorder, those with PTSD were less likely to endorse employment as a main source of income and had a higher incidence of recent attempted suicide.7 In a large retrospective cohort study, Operation Iraqi Freedom and Operation Enduring Freedom veterans with PTSD who were prescribed opioids were more likely to present to the emergency department (ED) and to be hospitalized for overdoses and injuries.8
Despite the risks of benzodiazepine and opioid use in this patient population, these medications are still often prescribed to veterans with PTSD for symptomatic relief. In fiscal year 2009, across the VHA system 37% of veterans diagnosed with PTSD were prescribed a benzodiazepine, 69% of the time by a mental health provider.9 Among Iraq and Afghanistan veterans, those with PTSD were significantly more likely to be prescribed an opioid for diagnosed pain—relative to those with a mental health disorder other than PTSD and those without a mental health disorder.8 Thus, there seems to be a disconnect between guideline recommendations and current practice.
The authors conducted a study to assess the potential risk of hospitalization for veterans with PTSD prescribed first-line pharmacotherapy and those also prescribed concurrent benzodiazepine and/or opioid therapy since the release of the PTSD guideline in 2010.2
Methods
In this retrospective cohort study, conducted at the Southern Arizona VA Health Care System (SAVAHCS), the authors analyzed electronic medical record data from November 1, 2009 to August 1, 2015. Study inclusion criteria were veteran, aged 18 to 89 years, diagnosis of PTSD (International Classification of Diseases, Ninth Revision, Clinical Modification code 309.81), and SSRI or SNRI newly prescribed between November 1, 2010 and August 1, 2013.
Any veteran prescribed at least one 30-day or longer supply of any benzodiazepine or opioid within 1 year before the SSRI/SNRI initial prescription date was excluded from the study. Also excluded was any patient treated for PTSD at a facility outside SAVAHCS or whose 2-year evaluation period extended past August 1, 2015.
Study Groups
An outpatient prescription was determined to be the initial SSRI/SNRI prescription for a patient who received less than a 30-day cumulative supply of any SSRI or SNRI within 1 year before that prescription date. Citalopram, desvenlafaxine, duloxetine, escitalopram, fluoxetine, fluvoxamine, levomilnacipran, milnacipran, paroxetine, sertraline, venlafaxine, vilazodone, and vortioxetine were the prespecified SSRI/SNRIs included in the study.
Patients who received at least 1 outpatient prescription for any benzodiazepine (minimum 30-day supply) within 1 year after the initial SSRI/SNRI prescription date were determined to be on concurrent SSRI/SNRI and benzodiazepine therapy. Alprazolam, chlordiazepoxide, clonazepam, clorazepate, diazepam, estazolam, flurazepam, lorazepam, oxazepam, temazepam, and triazolam were the prespecified benzodiazepines included in the study.
Patients who received at least 1 outpatient prescription for any opioid (minimum 30-day supply) within 1 year after the initial SSRI/SNRI prescription date were determined to be on concurrent SSRI/SNRI and opioid therapy. Codeine, fentanyl, hydrocodone, hydromorphone, levorphanol, meperidine, methadone, morphine, oxymorphone, pentazocine, propoxyphene, and tramadol were the prespecified opioids included in this study.
Patients who received at least 1 outpatient prescription for any benzodiazepine and any opioid (minimum 30-day supply) within 1 year after the initial SSRI/SNRI prescription date were determined to be on concurrent SSRI/SNRI, benzodiazepine, and opioid therapy.
The index date was defined as the first date of prescription overlap. If there was no benzodiazepine or opioid prescription within 1 year after the initial SSRI/SNRI prescription date, the patient was categorized as being on SSRI/SNRI monotherapy, and the index date was the date of the initial SSRI/SNRI prescription. For each patient, hospitalization data from the 2-year period after the index date were evaluated.
Outcomes and Data Collection
For evaluation of the primary outcome (2-year overall hospitalization risk), the number of unique mental health and medical/surgical hospitalizations was identified by the number of discharge summaries documented in the patient chart during the evaluation period. Time to first hospitalization was recorded for the survival data analysis. Secondary outcomes were mental health hospitalization risk, medical/surgical hospitalization risk, and all-cause mortality within 2 years.
Demographic data that were collected included age, sex, comorbid mental health disorders, comorbid SUDs, and concomitant use of psychotropic medications at index date (baseline). Select comorbid mental health disorders (anxiety, schizophrenia, depression, bipolar disorder) and substance use disorders (alcohol, opioid, illicit drug) also were identified. Data on insomnia and pain comorbidities (headaches or migraines; neuropathy; head, neck, back, arthritis, or joint pain) were collected, as these comorbidities could be indications for prescribing benzodiazepines and opioids. Concomitant baseline use of classes of psychotropic medications (antipsychotics, non-SSRI/SNRI antidepressants, mood stabilizers, anxiolytics, nonbenzodiazepine sedatives/hypnotics) also were documented. Last, hospitalizations within 6 months before the initial SSRI/SNRI prescription date were noted.
Statistical Analysis
Descriptive statistics were used to analyze all baseline demographic data. Continuous measures were evaluated with 1-way analyses of variance and post hoc Bonferroni-corrected pairwise comparisons, and categorical measures with contingency tables and χ2 tests or Fisher exact tests. When the overall χ2 test was significant across all 4 study groups, post hoc comparisons were performed between the SSRI/SNRI monotherapy group and each other group with Bonferroni adjusted for 3 comparisons.
Unadjusted and adjusted Weibull proportional hazard regression models were used to estimate hospitalization risk within 2 years after the index date for the different study groups with the SSRI/SNRI monotherapy group as the referent. Robust standard errors were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). The Weibull model (and not the Cox model) was used because it does not assume hazard remains constant over time, which is appropriate in this instance, as the risk of an adverse event (AE) may be higher when first starting a medication or combination of medications relative to when doses are stabilized. Models were adjusted for age, sex, baseline mental health disorders, and baseline psychotropic medications. As earlier hospitalizations showed evidence of effect modification when this covariate was tested, hazard analyses were limited to patients not previously hospitalized.
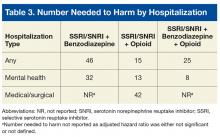
The effect size of differences in hospitalization risk meeting statistical significance was assessed by estimating the number needed to harm (NNH) and 95% CIs (not shown) to observe 1 additional hospitalization in each medication group relative to the SSRI/SNRI monotherapy group over a 90-day period. A 95% CI for NNH that did not include 0 indicated the NNH was significant at the .05 level.10 All-cause mortality was evaluated with the Fisher exact test with post hoc Bonferroni-corrected comparisons as appropriate.
Results
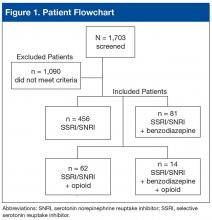
Of 1,703 patients screened, 613 met all study inclusion criteria (Figure 1).
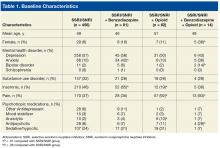
Baseline characteristics revealed no significant differences between groups in age or comorbid depression, schizophrenia, or SUDs (Table 1).
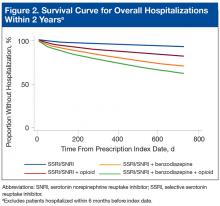
With the SSRI/SNRI monotherapy group as the referent, all concurrent therapy groups were at significantly increased risk for overall hospitalization within 2 years after the index date (Tables 2 & 3, Figure 2).
Risk for mental health hospitalization was significantly increased in all concurrent therapy groups relative to the referent group.
Although the risk for medical/surgical hospitalization was not significantly increased in the SSRI/SNRI and benzodiazepine therapy group (AHR, 1.9; 95% CI, 0.67-5.6), a significant difference was found in the SSRI/SNRI and opioid therapy group (AHR, 4.4; 95% CI, 1.6-12.0; NNH, 42).
Discussion
In 2013, Hawkins and colleagues evaluated hospitalization risk in veterans treated for PTSD within the Northwest VISN 20 between 2004 and 2010.11 Compared with patients treated with only an SSRI or SNRI, those treated with 1 of those medications and a benzodiazepine were at significantly higher risk for overall hospitalization (AHR, 1.79; 95% CI, 1.38-2.32; P < .001) and mental health hospitalization (AHR, 1.87; 95% CI, 1.37-2.53; P < .001). Furthermore, those prescribed a benzodiazepine and an opioid along with an SSRI or SNRI were at higher risk for overall hospitalization (AHR, 2.98; 95% CI, 2.22-4.00; P < .001), mental health hospitalization (AHR, 2.00; 95% CI, 1.35-2.98; P < .01), medical/surgical hospitalization (AHR, 4.86; 95% CI, 3.30-7.14; P < .001), and ED visits (AHR, 2.01; 95% CI, 1.53-2.65; P < .001).
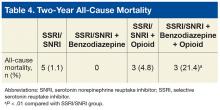
Findings from the present study, which covered a period after the newest PTSD guideline was released,support findings reported by Hawkins and colleagues in their retrospective cohort study covering an earlier period.2,11 In the present study, compared with the monotherapy group, the SSRI/SNRI and benzodiazepine therapy group and the SSRI/SNRI, benzodiazepine, and opioid therapy group were at higher risk for both overall hospitalization and mental health hospitalization within 2 years. However, in a subset of PTSD patients prescribed opioids along with first-line pharmacotherapy, this study found that overall, mental health, and medical/surgical hospitalizations were significantly increased as well. Furthermore, this study found 2-year mortality was significantly higher for the SSRI/SNRI, benzodiazepine, and opioid therapy group than for the SSRI/SNRI monotherapy group.
Adjusted hazard ratios were higher in the present study than those in the study by Hawkins and colleagues,but CIs were wider as well.11 These differences may be attributable to the relatively smaller sample size of the present study and may explain why the HR was higher for the SSRI/SNRI and opioid therapy group than for the SSRI/SNRI, benzodiazepine, and opioid therapy group.
Nevertheless, these results support the growing body of evidence establishing the many risks for AEs when benzodiazepines and opioids are prescribed in the setting of PTSD. Unfortunately, it seems that, against clear guideline recommendations and literature findings, these medications still are being prescribed to this vulnerable, high-risk population.
In the last few months of 2013, the VA health care system launched 2 important medication safety initiatives. The Psychotropic Drug Safety Initiative (PDSI) was established as a quality improvement initiative for evidence-based provision of psychotropic medications. One PDSI metric in particular focused on reducing the proportion of veterans with PTSD being treated with benzodiazepines. The Opioid Safety Initiative (OSI) came as a response to a dramatic increase in the number of fatal overdoses related to prescription opioids—an increase linked to an unprecedented jump in opioid use for nonmalignant pain. As the present study’s inclusion cutoff date of August 1, 2013, preceded the debut of both PDSI and OSI, the benzodiazepine and opioid prescription rates reported here might be higher than those currently being found under the 2 initiatives.
Limitations
This study had several limitations that might affect the interpretation or generalizability of findings. Requiring at least a 30-day supply for prescription eligibility was an attempt to focus on chronic use of medications rather than on, for example, onetime supplies of opioids for dental procedures. However, prescription fill history was not assessed. Therefore, patients could have been included in certain study groups even if their SSRI, SNRI, benzodiazepine, or opioid prescription was not refilled. Furthermore, only VA medical records were used; non-VA prescriptions were not captured.
In addition, this study was limited to patients who at bare minimum were prescribed an SSRI or an SNRI. Some patients may have been prescribed a benzodiazepine and/or an opioid but were not on appropriate first-line pharmacotherapy for PTSD. These patients were excluded from the study, and their relative hospitalization risk went unexplored. Therefore, the magnitude of the issue at hand might have been underestimated.
Although psychotherapy is a first-line treatment option for PTSD, the study did not assess the potential impact of psychotherapy on outcomes or the groups’ relative proportions of patients undergoing psychotherapy. It is unknown whether the groups were equivalent at baseline in regards to psychotherapy participation rates.
This study did not characterize the specific reasons for hospitalization beyond whether it was for a mental health or a medical/surgical issue; thus, no distinction was made between hospitalizations for an elective procedure and hospitalizations for a drug overdose or an injury. Investigators could characterize admission diagnoses to better assess whether hospitalizations are truly associated with study medications or whether patients are being hospitalized for unrelated reasons. In addition, they could elucidate the true nature of hospitalization risk associated with SSRI/SNRI, benzodiazepine, and opioid use by comparing admission diagnoses made before and after initiation of these pharmacologic therapies.
This study also could not assess outcomes for patients who presented to the ED but were not admitted. If the hospital’s floor and ED beds were at full capacity, some patients might have been transferred to an outside facility. However, this scenario is not common at SAVAHCS, where the study was conducted.
Although some comorbid conditions were noted, the study did not evaluate whether its patients had a compelling indication for benzodiazepines in particular. Opioid use is very limited to the treatment of pain, and the majority of the patients on opioid therapy in this study had a diagnosed pain syndrome.
Because of the study’s sample size and power limitations, patients were eligible to be included in a concurrent therapy group if a benzodiazepine, an opioid, or both were added no later than 1 year after SSRI/SNRI initiation. This gap of up to 1 year might have introduced some variability in exposure to risk from earlier prescribed medications. However, sensitivity analyses were performed with multiple constructed Weibull models of time to hospitalization based on subsets with varying overlapping medication gaps. Analyses revealed relatively stable HRs, suggesting that potential bias did not occur.
Future Directions
Investigators could explore the higher all-cause mortality rates in the SSRI/SNRI, benzodiazepine, and opioid therapy group, as this study did not assess cause of death in these patients. Whether any patients died of reasons directly attributable to benzodiazepines or opioids is unknown.
That SSRIs and SNRIs are the only established first-line pharmacologic treatment options for PTSD symptoms partly accounts for the widespread use of benzodiazepines in this population. For that reason, beyond characterizing the many risks associated with using benzodiazepines to manage these symptoms, there is a huge need to research the viability of other pharmacologic agents in treating PTSD. This is especially important given the slower onset to efficacy of the SSRIs and SNRIs; per estimates, only up to 60% of patients respond to SSRIs, and 20% to 30% achieve full remission of PTSD.12 Furthermore, these rates likely are even lower for combat veterans than those for the general population. Several trials discussed in a 2009 guideline review of the treatment of patients with acute stress disorder and PTSD have called into question the efficacy of SSRIs for combat-related PTSD.13 In these randomized, controlled trials, change in PTSD symptom severity as measured with CAPS was not significantly reduced with SSRIs compared with placebo.
A systematic review revealed that, of the nonantidepressants used as adjuncts in treating patients who do not achieve remission with SSRIs, the atypical antipsychotic risperidone may have the strongest supporting evidence.12 However, the present study found high rates of antipsychotic use in the SSRI/SNRI, benzodiazepine, and opioid therapy group, which also had the highest all-cause mortality rate. The safety of risperidone as an alternative treatment needs further evaluation.
Some prospective studies have suggested that the α1 blockers doxazosin and prazosin, the latter of which is commonly used for PTSD nightmares, also may improve PTSD symptoms as assessed by CAPS.14,15 Although these results are promising, the trials to date have been conducted with relatively small sample sizes.
With more veterans being treated for PTSD within the VA health care system, the central treatment goal remains: Adequately address the symptoms of PTSD while minimizing the harm caused by medications. Prescribers should limit benzodiazepine and opioid use in this population and consider safer nonpharmacologic and pharmacologic treatment options when possible.
Conclusion
Combat veterans with PTSD who are prescribed benzodiazepines and/or opioids in addition to first-line pharmacotherapy are at significantly increased risk for overall and mental health hospitalization.
Click here to read the digital edition.
1. Bernardy NC, Lund BC, Alexander B, Jenkyn AB, Schnurr PP, Friedman MJ. Gender differences in prescribing among veterans diagnosed with posttraumatic stress disorder. J Gen Intern Med. 2013;28(suppl 2):S542-S548.
2. Management of Post-Traumatic Stress Working Group, Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for Management of Post-Traumatic Stress. http://www.healthquality.va.gov/PTSD-full-2010c .pdf. Published October 2010. Accessed July 12, 2015.
3. Marks IM, Swinson RP, Baso˘glu M, et al. Alprazolam and exposure alone and combined in panic disorder with agoraphobia. A controlled study in London and Toronto. Br J Psychiatry. 1993;162:776-787.
4. Wilhelm FH, Roth WT. Acute and delayed effects of alprazolam on flight phobics during exposure. Behav Res Ther. 1997;35(9):831-841.
5. Guina J, Rossetter SR, DeRhodes BJ, Nahhas RW, Welton RS. Benzodiazepines for PTSD: a systematic review and meta-analysis. J Psychiatr Pract. 2015;21(4):281-303.
6. Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J Anxiety Disord. 2011;25(3):456-465.
7. Mills KL, Teesson M, Ross J, Darke S, Shanahan M. The costs and outcomes of treatment for opioid dependence associated with posttraumatic stress disorder. Psychiatr Serv. 2005;56(8):940-945.
8. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940-947.
9. Abrams TE, Lund BC, Bernardy NC, Friedman MJ. Aligning clinical practice to PTSD treatment guidelines: medication prescribing by provider type. Psychiatr Serv. 2013;64(2):142-148.
10. Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319(7223):1492-1495.
11. Hawkins EJ, Malte CA, Grossbard J, Saxon AJ, Imel ZE, Kivlahan DR. Comparative safety of benzodiazepines and opioids among Veterans Affairs patients with posttraumatic stress disorder. J Addict Med. 2013;7(5):354-362.
12. Berger W, Mendlowicz MV, Marques-Portella C, et al. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(2):169-180.
13. Benedek DM, Friedman MJ, Zatzick D, Ursano RJ. Guideline watch (March 2009): practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Focus. 2009;7(2):204-213.
14. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
15. Rodgman C, Verrico CD, Holst M, et al. Doxazosin XL reduces symptoms of posttraumatic stress disorder in veterans with PTSD: a pilot clinical trial. J Clin Psychiatry. 2016;77(5):e561-e565.
Posttraumatic stress disorder (PTSD) is a mental health condition that may develop in response to a traumatic event, such as that experienced by a soldier during active combat duty. In 2009, more than 495,000 veterans within the VA health care system were treated for PTSD—nearly triple the number a decade earlier.1 Core symptoms of PTSD include alterations in arousal and reactivity, avoidant behaviors, negative alterations in mood and cognition, and intrusive thoughts and nightmares. All of the symptoms can be debilitating. First-line pharmacotherapy options that target these core symptoms include selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs).2
The anxiolytic and sedative effects of benzodiazepines may provide quick relief from many of the secondary symptoms of PTSD, including sleep disturbances, irritability, and panic attacks. However, benzodiazepines potentially interfere with the extinction of conditioned fear—a goal integral to certain types of psychotherapy, such as exposure therapy.3,4 In addition, the systematic review and meta-analysis by Guina and colleagues revealed that benzodiazepines are ineffective in the treatment of PTSD.5 The majority of the evaluated studies that used PTSD-specific measures (eg, Clinician-Administered PTSD Scale [CAPS]) found increased PTSD severity and worse prognosis with use of these medications.5 In 2010, the VA and the DoD released a joint guideline for PTSD management.2 According to the guideline, benzodiazepines cause harm when used in PTSD and are relatively contraindicated in combat veterans because of the higher incidence of comorbid substance use disorders (SUDs) in these veterans relative to the general population.2,6
Opioid use also has been linked to poor functional and clinical outcomes in veterans with PTSD. Among patients being treated for opioid use disorder, those with PTSD were less likely to endorse employment as a main source of income and had a higher incidence of recent attempted suicide.7 In a large retrospective cohort study, Operation Iraqi Freedom and Operation Enduring Freedom veterans with PTSD who were prescribed opioids were more likely to present to the emergency department (ED) and to be hospitalized for overdoses and injuries.8
Despite the risks of benzodiazepine and opioid use in this patient population, these medications are still often prescribed to veterans with PTSD for symptomatic relief. In fiscal year 2009, across the VHA system 37% of veterans diagnosed with PTSD were prescribed a benzodiazepine, 69% of the time by a mental health provider.9 Among Iraq and Afghanistan veterans, those with PTSD were significantly more likely to be prescribed an opioid for diagnosed pain—relative to those with a mental health disorder other than PTSD and those without a mental health disorder.8 Thus, there seems to be a disconnect between guideline recommendations and current practice.
The authors conducted a study to assess the potential risk of hospitalization for veterans with PTSD prescribed first-line pharmacotherapy and those also prescribed concurrent benzodiazepine and/or opioid therapy since the release of the PTSD guideline in 2010.2
Methods
In this retrospective cohort study, conducted at the Southern Arizona VA Health Care System (SAVAHCS), the authors analyzed electronic medical record data from November 1, 2009 to August 1, 2015. Study inclusion criteria were veteran, aged 18 to 89 years, diagnosis of PTSD (International Classification of Diseases, Ninth Revision, Clinical Modification code 309.81), and SSRI or SNRI newly prescribed between November 1, 2010 and August 1, 2013.
Any veteran prescribed at least one 30-day or longer supply of any benzodiazepine or opioid within 1 year before the SSRI/SNRI initial prescription date was excluded from the study. Also excluded was any patient treated for PTSD at a facility outside SAVAHCS or whose 2-year evaluation period extended past August 1, 2015.
Study Groups
An outpatient prescription was determined to be the initial SSRI/SNRI prescription for a patient who received less than a 30-day cumulative supply of any SSRI or SNRI within 1 year before that prescription date. Citalopram, desvenlafaxine, duloxetine, escitalopram, fluoxetine, fluvoxamine, levomilnacipran, milnacipran, paroxetine, sertraline, venlafaxine, vilazodone, and vortioxetine were the prespecified SSRI/SNRIs included in the study.
Patients who received at least 1 outpatient prescription for any benzodiazepine (minimum 30-day supply) within 1 year after the initial SSRI/SNRI prescription date were determined to be on concurrent SSRI/SNRI and benzodiazepine therapy. Alprazolam, chlordiazepoxide, clonazepam, clorazepate, diazepam, estazolam, flurazepam, lorazepam, oxazepam, temazepam, and triazolam were the prespecified benzodiazepines included in the study.
Patients who received at least 1 outpatient prescription for any opioid (minimum 30-day supply) within 1 year after the initial SSRI/SNRI prescription date were determined to be on concurrent SSRI/SNRI and opioid therapy. Codeine, fentanyl, hydrocodone, hydromorphone, levorphanol, meperidine, methadone, morphine, oxymorphone, pentazocine, propoxyphene, and tramadol were the prespecified opioids included in this study.
Patients who received at least 1 outpatient prescription for any benzodiazepine and any opioid (minimum 30-day supply) within 1 year after the initial SSRI/SNRI prescription date were determined to be on concurrent SSRI/SNRI, benzodiazepine, and opioid therapy.
The index date was defined as the first date of prescription overlap. If there was no benzodiazepine or opioid prescription within 1 year after the initial SSRI/SNRI prescription date, the patient was categorized as being on SSRI/SNRI monotherapy, and the index date was the date of the initial SSRI/SNRI prescription. For each patient, hospitalization data from the 2-year period after the index date were evaluated.
Outcomes and Data Collection
For evaluation of the primary outcome (2-year overall hospitalization risk), the number of unique mental health and medical/surgical hospitalizations was identified by the number of discharge summaries documented in the patient chart during the evaluation period. Time to first hospitalization was recorded for the survival data analysis. Secondary outcomes were mental health hospitalization risk, medical/surgical hospitalization risk, and all-cause mortality within 2 years.
Demographic data that were collected included age, sex, comorbid mental health disorders, comorbid SUDs, and concomitant use of psychotropic medications at index date (baseline). Select comorbid mental health disorders (anxiety, schizophrenia, depression, bipolar disorder) and substance use disorders (alcohol, opioid, illicit drug) also were identified. Data on insomnia and pain comorbidities (headaches or migraines; neuropathy; head, neck, back, arthritis, or joint pain) were collected, as these comorbidities could be indications for prescribing benzodiazepines and opioids. Concomitant baseline use of classes of psychotropic medications (antipsychotics, non-SSRI/SNRI antidepressants, mood stabilizers, anxiolytics, nonbenzodiazepine sedatives/hypnotics) also were documented. Last, hospitalizations within 6 months before the initial SSRI/SNRI prescription date were noted.
Statistical Analysis
Descriptive statistics were used to analyze all baseline demographic data. Continuous measures were evaluated with 1-way analyses of variance and post hoc Bonferroni-corrected pairwise comparisons, and categorical measures with contingency tables and χ2 tests or Fisher exact tests. When the overall χ2 test was significant across all 4 study groups, post hoc comparisons were performed between the SSRI/SNRI monotherapy group and each other group with Bonferroni adjusted for 3 comparisons.
Unadjusted and adjusted Weibull proportional hazard regression models were used to estimate hospitalization risk within 2 years after the index date for the different study groups with the SSRI/SNRI monotherapy group as the referent. Robust standard errors were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). The Weibull model (and not the Cox model) was used because it does not assume hazard remains constant over time, which is appropriate in this instance, as the risk of an adverse event (AE) may be higher when first starting a medication or combination of medications relative to when doses are stabilized. Models were adjusted for age, sex, baseline mental health disorders, and baseline psychotropic medications. As earlier hospitalizations showed evidence of effect modification when this covariate was tested, hazard analyses were limited to patients not previously hospitalized.
The effect size of differences in hospitalization risk meeting statistical significance was assessed by estimating the number needed to harm (NNH) and 95% CIs (not shown) to observe 1 additional hospitalization in each medication group relative to the SSRI/SNRI monotherapy group over a 90-day period. A 95% CI for NNH that did not include 0 indicated the NNH was significant at the .05 level.10 All-cause mortality was evaluated with the Fisher exact test with post hoc Bonferroni-corrected comparisons as appropriate.
Results
Of 1,703 patients screened, 613 met all study inclusion criteria (Figure 1).
Baseline characteristics revealed no significant differences between groups in age or comorbid depression, schizophrenia, or SUDs (Table 1).
With the SSRI/SNRI monotherapy group as the referent, all concurrent therapy groups were at significantly increased risk for overall hospitalization within 2 years after the index date (Tables 2 & 3, Figure 2).
Risk for mental health hospitalization was significantly increased in all concurrent therapy groups relative to the referent group.
Although the risk for medical/surgical hospitalization was not significantly increased in the SSRI/SNRI and benzodiazepine therapy group (AHR, 1.9; 95% CI, 0.67-5.6), a significant difference was found in the SSRI/SNRI and opioid therapy group (AHR, 4.4; 95% CI, 1.6-12.0; NNH, 42).
Discussion
In 2013, Hawkins and colleagues evaluated hospitalization risk in veterans treated for PTSD within the Northwest VISN 20 between 2004 and 2010.11 Compared with patients treated with only an SSRI or SNRI, those treated with 1 of those medications and a benzodiazepine were at significantly higher risk for overall hospitalization (AHR, 1.79; 95% CI, 1.38-2.32; P < .001) and mental health hospitalization (AHR, 1.87; 95% CI, 1.37-2.53; P < .001). Furthermore, those prescribed a benzodiazepine and an opioid along with an SSRI or SNRI were at higher risk for overall hospitalization (AHR, 2.98; 95% CI, 2.22-4.00; P < .001), mental health hospitalization (AHR, 2.00; 95% CI, 1.35-2.98; P < .01), medical/surgical hospitalization (AHR, 4.86; 95% CI, 3.30-7.14; P < .001), and ED visits (AHR, 2.01; 95% CI, 1.53-2.65; P < .001).
Findings from the present study, which covered a period after the newest PTSD guideline was released,support findings reported by Hawkins and colleagues in their retrospective cohort study covering an earlier period.2,11 In the present study, compared with the monotherapy group, the SSRI/SNRI and benzodiazepine therapy group and the SSRI/SNRI, benzodiazepine, and opioid therapy group were at higher risk for both overall hospitalization and mental health hospitalization within 2 years. However, in a subset of PTSD patients prescribed opioids along with first-line pharmacotherapy, this study found that overall, mental health, and medical/surgical hospitalizations were significantly increased as well. Furthermore, this study found 2-year mortality was significantly higher for the SSRI/SNRI, benzodiazepine, and opioid therapy group than for the SSRI/SNRI monotherapy group.
Adjusted hazard ratios were higher in the present study than those in the study by Hawkins and colleagues,but CIs were wider as well.11 These differences may be attributable to the relatively smaller sample size of the present study and may explain why the HR was higher for the SSRI/SNRI and opioid therapy group than for the SSRI/SNRI, benzodiazepine, and opioid therapy group.
Nevertheless, these results support the growing body of evidence establishing the many risks for AEs when benzodiazepines and opioids are prescribed in the setting of PTSD. Unfortunately, it seems that, against clear guideline recommendations and literature findings, these medications still are being prescribed to this vulnerable, high-risk population.
In the last few months of 2013, the VA health care system launched 2 important medication safety initiatives. The Psychotropic Drug Safety Initiative (PDSI) was established as a quality improvement initiative for evidence-based provision of psychotropic medications. One PDSI metric in particular focused on reducing the proportion of veterans with PTSD being treated with benzodiazepines. The Opioid Safety Initiative (OSI) came as a response to a dramatic increase in the number of fatal overdoses related to prescription opioids—an increase linked to an unprecedented jump in opioid use for nonmalignant pain. As the present study’s inclusion cutoff date of August 1, 2013, preceded the debut of both PDSI and OSI, the benzodiazepine and opioid prescription rates reported here might be higher than those currently being found under the 2 initiatives.
Limitations
This study had several limitations that might affect the interpretation or generalizability of findings. Requiring at least a 30-day supply for prescription eligibility was an attempt to focus on chronic use of medications rather than on, for example, onetime supplies of opioids for dental procedures. However, prescription fill history was not assessed. Therefore, patients could have been included in certain study groups even if their SSRI, SNRI, benzodiazepine, or opioid prescription was not refilled. Furthermore, only VA medical records were used; non-VA prescriptions were not captured.
In addition, this study was limited to patients who at bare minimum were prescribed an SSRI or an SNRI. Some patients may have been prescribed a benzodiazepine and/or an opioid but were not on appropriate first-line pharmacotherapy for PTSD. These patients were excluded from the study, and their relative hospitalization risk went unexplored. Therefore, the magnitude of the issue at hand might have been underestimated.
Although psychotherapy is a first-line treatment option for PTSD, the study did not assess the potential impact of psychotherapy on outcomes or the groups’ relative proportions of patients undergoing psychotherapy. It is unknown whether the groups were equivalent at baseline in regards to psychotherapy participation rates.
This study did not characterize the specific reasons for hospitalization beyond whether it was for a mental health or a medical/surgical issue; thus, no distinction was made between hospitalizations for an elective procedure and hospitalizations for a drug overdose or an injury. Investigators could characterize admission diagnoses to better assess whether hospitalizations are truly associated with study medications or whether patients are being hospitalized for unrelated reasons. In addition, they could elucidate the true nature of hospitalization risk associated with SSRI/SNRI, benzodiazepine, and opioid use by comparing admission diagnoses made before and after initiation of these pharmacologic therapies.
This study also could not assess outcomes for patients who presented to the ED but were not admitted. If the hospital’s floor and ED beds were at full capacity, some patients might have been transferred to an outside facility. However, this scenario is not common at SAVAHCS, where the study was conducted.
Although some comorbid conditions were noted, the study did not evaluate whether its patients had a compelling indication for benzodiazepines in particular. Opioid use is very limited to the treatment of pain, and the majority of the patients on opioid therapy in this study had a diagnosed pain syndrome.
Because of the study’s sample size and power limitations, patients were eligible to be included in a concurrent therapy group if a benzodiazepine, an opioid, or both were added no later than 1 year after SSRI/SNRI initiation. This gap of up to 1 year might have introduced some variability in exposure to risk from earlier prescribed medications. However, sensitivity analyses were performed with multiple constructed Weibull models of time to hospitalization based on subsets with varying overlapping medication gaps. Analyses revealed relatively stable HRs, suggesting that potential bias did not occur.
Future Directions
Investigators could explore the higher all-cause mortality rates in the SSRI/SNRI, benzodiazepine, and opioid therapy group, as this study did not assess cause of death in these patients. Whether any patients died of reasons directly attributable to benzodiazepines or opioids is unknown.
That SSRIs and SNRIs are the only established first-line pharmacologic treatment options for PTSD symptoms partly accounts for the widespread use of benzodiazepines in this population. For that reason, beyond characterizing the many risks associated with using benzodiazepines to manage these symptoms, there is a huge need to research the viability of other pharmacologic agents in treating PTSD. This is especially important given the slower onset to efficacy of the SSRIs and SNRIs; per estimates, only up to 60% of patients respond to SSRIs, and 20% to 30% achieve full remission of PTSD.12 Furthermore, these rates likely are even lower for combat veterans than those for the general population. Several trials discussed in a 2009 guideline review of the treatment of patients with acute stress disorder and PTSD have called into question the efficacy of SSRIs for combat-related PTSD.13 In these randomized, controlled trials, change in PTSD symptom severity as measured with CAPS was not significantly reduced with SSRIs compared with placebo.
A systematic review revealed that, of the nonantidepressants used as adjuncts in treating patients who do not achieve remission with SSRIs, the atypical antipsychotic risperidone may have the strongest supporting evidence.12 However, the present study found high rates of antipsychotic use in the SSRI/SNRI, benzodiazepine, and opioid therapy group, which also had the highest all-cause mortality rate. The safety of risperidone as an alternative treatment needs further evaluation.
Some prospective studies have suggested that the α1 blockers doxazosin and prazosin, the latter of which is commonly used for PTSD nightmares, also may improve PTSD symptoms as assessed by CAPS.14,15 Although these results are promising, the trials to date have been conducted with relatively small sample sizes.
With more veterans being treated for PTSD within the VA health care system, the central treatment goal remains: Adequately address the symptoms of PTSD while minimizing the harm caused by medications. Prescribers should limit benzodiazepine and opioid use in this population and consider safer nonpharmacologic and pharmacologic treatment options when possible.
Conclusion
Combat veterans with PTSD who are prescribed benzodiazepines and/or opioids in addition to first-line pharmacotherapy are at significantly increased risk for overall and mental health hospitalization.
Click here to read the digital edition.
Posttraumatic stress disorder (PTSD) is a mental health condition that may develop in response to a traumatic event, such as that experienced by a soldier during active combat duty. In 2009, more than 495,000 veterans within the VA health care system were treated for PTSD—nearly triple the number a decade earlier.1 Core symptoms of PTSD include alterations in arousal and reactivity, avoidant behaviors, negative alterations in mood and cognition, and intrusive thoughts and nightmares. All of the symptoms can be debilitating. First-line pharmacotherapy options that target these core symptoms include selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs).2
The anxiolytic and sedative effects of benzodiazepines may provide quick relief from many of the secondary symptoms of PTSD, including sleep disturbances, irritability, and panic attacks. However, benzodiazepines potentially interfere with the extinction of conditioned fear—a goal integral to certain types of psychotherapy, such as exposure therapy.3,4 In addition, the systematic review and meta-analysis by Guina and colleagues revealed that benzodiazepines are ineffective in the treatment of PTSD.5 The majority of the evaluated studies that used PTSD-specific measures (eg, Clinician-Administered PTSD Scale [CAPS]) found increased PTSD severity and worse prognosis with use of these medications.5 In 2010, the VA and the DoD released a joint guideline for PTSD management.2 According to the guideline, benzodiazepines cause harm when used in PTSD and are relatively contraindicated in combat veterans because of the higher incidence of comorbid substance use disorders (SUDs) in these veterans relative to the general population.2,6
Opioid use also has been linked to poor functional and clinical outcomes in veterans with PTSD. Among patients being treated for opioid use disorder, those with PTSD were less likely to endorse employment as a main source of income and had a higher incidence of recent attempted suicide.7 In a large retrospective cohort study, Operation Iraqi Freedom and Operation Enduring Freedom veterans with PTSD who were prescribed opioids were more likely to present to the emergency department (ED) and to be hospitalized for overdoses and injuries.8
Despite the risks of benzodiazepine and opioid use in this patient population, these medications are still often prescribed to veterans with PTSD for symptomatic relief. In fiscal year 2009, across the VHA system 37% of veterans diagnosed with PTSD were prescribed a benzodiazepine, 69% of the time by a mental health provider.9 Among Iraq and Afghanistan veterans, those with PTSD were significantly more likely to be prescribed an opioid for diagnosed pain—relative to those with a mental health disorder other than PTSD and those without a mental health disorder.8 Thus, there seems to be a disconnect between guideline recommendations and current practice.
The authors conducted a study to assess the potential risk of hospitalization for veterans with PTSD prescribed first-line pharmacotherapy and those also prescribed concurrent benzodiazepine and/or opioid therapy since the release of the PTSD guideline in 2010.2
Methods
In this retrospective cohort study, conducted at the Southern Arizona VA Health Care System (SAVAHCS), the authors analyzed electronic medical record data from November 1, 2009 to August 1, 2015. Study inclusion criteria were veteran, aged 18 to 89 years, diagnosis of PTSD (International Classification of Diseases, Ninth Revision, Clinical Modification code 309.81), and SSRI or SNRI newly prescribed between November 1, 2010 and August 1, 2013.
Any veteran prescribed at least one 30-day or longer supply of any benzodiazepine or opioid within 1 year before the SSRI/SNRI initial prescription date was excluded from the study. Also excluded was any patient treated for PTSD at a facility outside SAVAHCS or whose 2-year evaluation period extended past August 1, 2015.
Study Groups
An outpatient prescription was determined to be the initial SSRI/SNRI prescription for a patient who received less than a 30-day cumulative supply of any SSRI or SNRI within 1 year before that prescription date. Citalopram, desvenlafaxine, duloxetine, escitalopram, fluoxetine, fluvoxamine, levomilnacipran, milnacipran, paroxetine, sertraline, venlafaxine, vilazodone, and vortioxetine were the prespecified SSRI/SNRIs included in the study.
Patients who received at least 1 outpatient prescription for any benzodiazepine (minimum 30-day supply) within 1 year after the initial SSRI/SNRI prescription date were determined to be on concurrent SSRI/SNRI and benzodiazepine therapy. Alprazolam, chlordiazepoxide, clonazepam, clorazepate, diazepam, estazolam, flurazepam, lorazepam, oxazepam, temazepam, and triazolam were the prespecified benzodiazepines included in the study.
Patients who received at least 1 outpatient prescription for any opioid (minimum 30-day supply) within 1 year after the initial SSRI/SNRI prescription date were determined to be on concurrent SSRI/SNRI and opioid therapy. Codeine, fentanyl, hydrocodone, hydromorphone, levorphanol, meperidine, methadone, morphine, oxymorphone, pentazocine, propoxyphene, and tramadol were the prespecified opioids included in this study.
Patients who received at least 1 outpatient prescription for any benzodiazepine and any opioid (minimum 30-day supply) within 1 year after the initial SSRI/SNRI prescription date were determined to be on concurrent SSRI/SNRI, benzodiazepine, and opioid therapy.
The index date was defined as the first date of prescription overlap. If there was no benzodiazepine or opioid prescription within 1 year after the initial SSRI/SNRI prescription date, the patient was categorized as being on SSRI/SNRI monotherapy, and the index date was the date of the initial SSRI/SNRI prescription. For each patient, hospitalization data from the 2-year period after the index date were evaluated.
Outcomes and Data Collection
For evaluation of the primary outcome (2-year overall hospitalization risk), the number of unique mental health and medical/surgical hospitalizations was identified by the number of discharge summaries documented in the patient chart during the evaluation period. Time to first hospitalization was recorded for the survival data analysis. Secondary outcomes were mental health hospitalization risk, medical/surgical hospitalization risk, and all-cause mortality within 2 years.
Demographic data that were collected included age, sex, comorbid mental health disorders, comorbid SUDs, and concomitant use of psychotropic medications at index date (baseline). Select comorbid mental health disorders (anxiety, schizophrenia, depression, bipolar disorder) and substance use disorders (alcohol, opioid, illicit drug) also were identified. Data on insomnia and pain comorbidities (headaches or migraines; neuropathy; head, neck, back, arthritis, or joint pain) were collected, as these comorbidities could be indications for prescribing benzodiazepines and opioids. Concomitant baseline use of classes of psychotropic medications (antipsychotics, non-SSRI/SNRI antidepressants, mood stabilizers, anxiolytics, nonbenzodiazepine sedatives/hypnotics) also were documented. Last, hospitalizations within 6 months before the initial SSRI/SNRI prescription date were noted.
Statistical Analysis
Descriptive statistics were used to analyze all baseline demographic data. Continuous measures were evaluated with 1-way analyses of variance and post hoc Bonferroni-corrected pairwise comparisons, and categorical measures with contingency tables and χ2 tests or Fisher exact tests. When the overall χ2 test was significant across all 4 study groups, post hoc comparisons were performed between the SSRI/SNRI monotherapy group and each other group with Bonferroni adjusted for 3 comparisons.
Unadjusted and adjusted Weibull proportional hazard regression models were used to estimate hospitalization risk within 2 years after the index date for the different study groups with the SSRI/SNRI monotherapy group as the referent. Robust standard errors were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). The Weibull model (and not the Cox model) was used because it does not assume hazard remains constant over time, which is appropriate in this instance, as the risk of an adverse event (AE) may be higher when first starting a medication or combination of medications relative to when doses are stabilized. Models were adjusted for age, sex, baseline mental health disorders, and baseline psychotropic medications. As earlier hospitalizations showed evidence of effect modification when this covariate was tested, hazard analyses were limited to patients not previously hospitalized.
The effect size of differences in hospitalization risk meeting statistical significance was assessed by estimating the number needed to harm (NNH) and 95% CIs (not shown) to observe 1 additional hospitalization in each medication group relative to the SSRI/SNRI monotherapy group over a 90-day period. A 95% CI for NNH that did not include 0 indicated the NNH was significant at the .05 level.10 All-cause mortality was evaluated with the Fisher exact test with post hoc Bonferroni-corrected comparisons as appropriate.
Results
Of 1,703 patients screened, 613 met all study inclusion criteria (Figure 1).
Baseline characteristics revealed no significant differences between groups in age or comorbid depression, schizophrenia, or SUDs (Table 1).
With the SSRI/SNRI monotherapy group as the referent, all concurrent therapy groups were at significantly increased risk for overall hospitalization within 2 years after the index date (Tables 2 & 3, Figure 2).
Risk for mental health hospitalization was significantly increased in all concurrent therapy groups relative to the referent group.
Although the risk for medical/surgical hospitalization was not significantly increased in the SSRI/SNRI and benzodiazepine therapy group (AHR, 1.9; 95% CI, 0.67-5.6), a significant difference was found in the SSRI/SNRI and opioid therapy group (AHR, 4.4; 95% CI, 1.6-12.0; NNH, 42).
Discussion
In 2013, Hawkins and colleagues evaluated hospitalization risk in veterans treated for PTSD within the Northwest VISN 20 between 2004 and 2010.11 Compared with patients treated with only an SSRI or SNRI, those treated with 1 of those medications and a benzodiazepine were at significantly higher risk for overall hospitalization (AHR, 1.79; 95% CI, 1.38-2.32; P < .001) and mental health hospitalization (AHR, 1.87; 95% CI, 1.37-2.53; P < .001). Furthermore, those prescribed a benzodiazepine and an opioid along with an SSRI or SNRI were at higher risk for overall hospitalization (AHR, 2.98; 95% CI, 2.22-4.00; P < .001), mental health hospitalization (AHR, 2.00; 95% CI, 1.35-2.98; P < .01), medical/surgical hospitalization (AHR, 4.86; 95% CI, 3.30-7.14; P < .001), and ED visits (AHR, 2.01; 95% CI, 1.53-2.65; P < .001).
Findings from the present study, which covered a period after the newest PTSD guideline was released,support findings reported by Hawkins and colleagues in their retrospective cohort study covering an earlier period.2,11 In the present study, compared with the monotherapy group, the SSRI/SNRI and benzodiazepine therapy group and the SSRI/SNRI, benzodiazepine, and opioid therapy group were at higher risk for both overall hospitalization and mental health hospitalization within 2 years. However, in a subset of PTSD patients prescribed opioids along with first-line pharmacotherapy, this study found that overall, mental health, and medical/surgical hospitalizations were significantly increased as well. Furthermore, this study found 2-year mortality was significantly higher for the SSRI/SNRI, benzodiazepine, and opioid therapy group than for the SSRI/SNRI monotherapy group.
Adjusted hazard ratios were higher in the present study than those in the study by Hawkins and colleagues,but CIs were wider as well.11 These differences may be attributable to the relatively smaller sample size of the present study and may explain why the HR was higher for the SSRI/SNRI and opioid therapy group than for the SSRI/SNRI, benzodiazepine, and opioid therapy group.
Nevertheless, these results support the growing body of evidence establishing the many risks for AEs when benzodiazepines and opioids are prescribed in the setting of PTSD. Unfortunately, it seems that, against clear guideline recommendations and literature findings, these medications still are being prescribed to this vulnerable, high-risk population.
In the last few months of 2013, the VA health care system launched 2 important medication safety initiatives. The Psychotropic Drug Safety Initiative (PDSI) was established as a quality improvement initiative for evidence-based provision of psychotropic medications. One PDSI metric in particular focused on reducing the proportion of veterans with PTSD being treated with benzodiazepines. The Opioid Safety Initiative (OSI) came as a response to a dramatic increase in the number of fatal overdoses related to prescription opioids—an increase linked to an unprecedented jump in opioid use for nonmalignant pain. As the present study’s inclusion cutoff date of August 1, 2013, preceded the debut of both PDSI and OSI, the benzodiazepine and opioid prescription rates reported here might be higher than those currently being found under the 2 initiatives.
Limitations
This study had several limitations that might affect the interpretation or generalizability of findings. Requiring at least a 30-day supply for prescription eligibility was an attempt to focus on chronic use of medications rather than on, for example, onetime supplies of opioids for dental procedures. However, prescription fill history was not assessed. Therefore, patients could have been included in certain study groups even if their SSRI, SNRI, benzodiazepine, or opioid prescription was not refilled. Furthermore, only VA medical records were used; non-VA prescriptions were not captured.
In addition, this study was limited to patients who at bare minimum were prescribed an SSRI or an SNRI. Some patients may have been prescribed a benzodiazepine and/or an opioid but were not on appropriate first-line pharmacotherapy for PTSD. These patients were excluded from the study, and their relative hospitalization risk went unexplored. Therefore, the magnitude of the issue at hand might have been underestimated.
Although psychotherapy is a first-line treatment option for PTSD, the study did not assess the potential impact of psychotherapy on outcomes or the groups’ relative proportions of patients undergoing psychotherapy. It is unknown whether the groups were equivalent at baseline in regards to psychotherapy participation rates.
This study did not characterize the specific reasons for hospitalization beyond whether it was for a mental health or a medical/surgical issue; thus, no distinction was made between hospitalizations for an elective procedure and hospitalizations for a drug overdose or an injury. Investigators could characterize admission diagnoses to better assess whether hospitalizations are truly associated with study medications or whether patients are being hospitalized for unrelated reasons. In addition, they could elucidate the true nature of hospitalization risk associated with SSRI/SNRI, benzodiazepine, and opioid use by comparing admission diagnoses made before and after initiation of these pharmacologic therapies.
This study also could not assess outcomes for patients who presented to the ED but were not admitted. If the hospital’s floor and ED beds were at full capacity, some patients might have been transferred to an outside facility. However, this scenario is not common at SAVAHCS, where the study was conducted.
Although some comorbid conditions were noted, the study did not evaluate whether its patients had a compelling indication for benzodiazepines in particular. Opioid use is very limited to the treatment of pain, and the majority of the patients on opioid therapy in this study had a diagnosed pain syndrome.
Because of the study’s sample size and power limitations, patients were eligible to be included in a concurrent therapy group if a benzodiazepine, an opioid, or both were added no later than 1 year after SSRI/SNRI initiation. This gap of up to 1 year might have introduced some variability in exposure to risk from earlier prescribed medications. However, sensitivity analyses were performed with multiple constructed Weibull models of time to hospitalization based on subsets with varying overlapping medication gaps. Analyses revealed relatively stable HRs, suggesting that potential bias did not occur.
Future Directions
Investigators could explore the higher all-cause mortality rates in the SSRI/SNRI, benzodiazepine, and opioid therapy group, as this study did not assess cause of death in these patients. Whether any patients died of reasons directly attributable to benzodiazepines or opioids is unknown.
That SSRIs and SNRIs are the only established first-line pharmacologic treatment options for PTSD symptoms partly accounts for the widespread use of benzodiazepines in this population. For that reason, beyond characterizing the many risks associated with using benzodiazepines to manage these symptoms, there is a huge need to research the viability of other pharmacologic agents in treating PTSD. This is especially important given the slower onset to efficacy of the SSRIs and SNRIs; per estimates, only up to 60% of patients respond to SSRIs, and 20% to 30% achieve full remission of PTSD.12 Furthermore, these rates likely are even lower for combat veterans than those for the general population. Several trials discussed in a 2009 guideline review of the treatment of patients with acute stress disorder and PTSD have called into question the efficacy of SSRIs for combat-related PTSD.13 In these randomized, controlled trials, change in PTSD symptom severity as measured with CAPS was not significantly reduced with SSRIs compared with placebo.
A systematic review revealed that, of the nonantidepressants used as adjuncts in treating patients who do not achieve remission with SSRIs, the atypical antipsychotic risperidone may have the strongest supporting evidence.12 However, the present study found high rates of antipsychotic use in the SSRI/SNRI, benzodiazepine, and opioid therapy group, which also had the highest all-cause mortality rate. The safety of risperidone as an alternative treatment needs further evaluation.
Some prospective studies have suggested that the α1 blockers doxazosin and prazosin, the latter of which is commonly used for PTSD nightmares, also may improve PTSD symptoms as assessed by CAPS.14,15 Although these results are promising, the trials to date have been conducted with relatively small sample sizes.
With more veterans being treated for PTSD within the VA health care system, the central treatment goal remains: Adequately address the symptoms of PTSD while minimizing the harm caused by medications. Prescribers should limit benzodiazepine and opioid use in this population and consider safer nonpharmacologic and pharmacologic treatment options when possible.
Conclusion
Combat veterans with PTSD who are prescribed benzodiazepines and/or opioids in addition to first-line pharmacotherapy are at significantly increased risk for overall and mental health hospitalization.
Click here to read the digital edition.
1. Bernardy NC, Lund BC, Alexander B, Jenkyn AB, Schnurr PP, Friedman MJ. Gender differences in prescribing among veterans diagnosed with posttraumatic stress disorder. J Gen Intern Med. 2013;28(suppl 2):S542-S548.
2. Management of Post-Traumatic Stress Working Group, Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for Management of Post-Traumatic Stress. http://www.healthquality.va.gov/PTSD-full-2010c .pdf. Published October 2010. Accessed July 12, 2015.
3. Marks IM, Swinson RP, Baso˘glu M, et al. Alprazolam and exposure alone and combined in panic disorder with agoraphobia. A controlled study in London and Toronto. Br J Psychiatry. 1993;162:776-787.
4. Wilhelm FH, Roth WT. Acute and delayed effects of alprazolam on flight phobics during exposure. Behav Res Ther. 1997;35(9):831-841.
5. Guina J, Rossetter SR, DeRhodes BJ, Nahhas RW, Welton RS. Benzodiazepines for PTSD: a systematic review and meta-analysis. J Psychiatr Pract. 2015;21(4):281-303.
6. Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J Anxiety Disord. 2011;25(3):456-465.
7. Mills KL, Teesson M, Ross J, Darke S, Shanahan M. The costs and outcomes of treatment for opioid dependence associated with posttraumatic stress disorder. Psychiatr Serv. 2005;56(8):940-945.
8. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940-947.
9. Abrams TE, Lund BC, Bernardy NC, Friedman MJ. Aligning clinical practice to PTSD treatment guidelines: medication prescribing by provider type. Psychiatr Serv. 2013;64(2):142-148.
10. Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319(7223):1492-1495.
11. Hawkins EJ, Malte CA, Grossbard J, Saxon AJ, Imel ZE, Kivlahan DR. Comparative safety of benzodiazepines and opioids among Veterans Affairs patients with posttraumatic stress disorder. J Addict Med. 2013;7(5):354-362.
12. Berger W, Mendlowicz MV, Marques-Portella C, et al. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(2):169-180.
13. Benedek DM, Friedman MJ, Zatzick D, Ursano RJ. Guideline watch (March 2009): practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Focus. 2009;7(2):204-213.
14. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
15. Rodgman C, Verrico CD, Holst M, et al. Doxazosin XL reduces symptoms of posttraumatic stress disorder in veterans with PTSD: a pilot clinical trial. J Clin Psychiatry. 2016;77(5):e561-e565.
1. Bernardy NC, Lund BC, Alexander B, Jenkyn AB, Schnurr PP, Friedman MJ. Gender differences in prescribing among veterans diagnosed with posttraumatic stress disorder. J Gen Intern Med. 2013;28(suppl 2):S542-S548.
2. Management of Post-Traumatic Stress Working Group, Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for Management of Post-Traumatic Stress. http://www.healthquality.va.gov/PTSD-full-2010c .pdf. Published October 2010. Accessed July 12, 2015.
3. Marks IM, Swinson RP, Baso˘glu M, et al. Alprazolam and exposure alone and combined in panic disorder with agoraphobia. A controlled study in London and Toronto. Br J Psychiatry. 1993;162:776-787.
4. Wilhelm FH, Roth WT. Acute and delayed effects of alprazolam on flight phobics during exposure. Behav Res Ther. 1997;35(9):831-841.
5. Guina J, Rossetter SR, DeRhodes BJ, Nahhas RW, Welton RS. Benzodiazepines for PTSD: a systematic review and meta-analysis. J Psychiatr Pract. 2015;21(4):281-303.
6. Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J Anxiety Disord. 2011;25(3):456-465.
7. Mills KL, Teesson M, Ross J, Darke S, Shanahan M. The costs and outcomes of treatment for opioid dependence associated with posttraumatic stress disorder. Psychiatr Serv. 2005;56(8):940-945.
8. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940-947.
9. Abrams TE, Lund BC, Bernardy NC, Friedman MJ. Aligning clinical practice to PTSD treatment guidelines: medication prescribing by provider type. Psychiatr Serv. 2013;64(2):142-148.
10. Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319(7223):1492-1495.
11. Hawkins EJ, Malte CA, Grossbard J, Saxon AJ, Imel ZE, Kivlahan DR. Comparative safety of benzodiazepines and opioids among Veterans Affairs patients with posttraumatic stress disorder. J Addict Med. 2013;7(5):354-362.
12. Berger W, Mendlowicz MV, Marques-Portella C, et al. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(2):169-180.
13. Benedek DM, Friedman MJ, Zatzick D, Ursano RJ. Guideline watch (March 2009): practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Focus. 2009;7(2):204-213.
14. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
15. Rodgman C, Verrico CD, Holst M, et al. Doxazosin XL reduces symptoms of posttraumatic stress disorder in veterans with PTSD: a pilot clinical trial. J Clin Psychiatry. 2016;77(5):e561-e565.
How Do You Treat a Patient With Refractory Headache?
RIVIERA BEACH, FL—Neurologists sometimes encounter patients with headaches that have not responded to prior treatment. These patients may be demoralized, and neurologists may be at a loss for a way to relieve their pain. Effective treatment is possible for many of these patients, according to Thomas N. Ward, MD, Emeritus Professor of Neurology at Dartmouth College in Hanover, New Hampshire. He described the process of differential diagnosis, as well as outpatient and inpatient therapeutic options for refractory headache, at the 44th Annual Meeting of the Southern Clinical Neurological Society.
Confirm the Diagnosis
When faced with a patient with refractory headache, a neurologist should first verify the diagnosis and rule out the possibility of secondary headache. These steps will improve the likelihood of a positive outcome. “If you follow the fundamentals and treat the type of headache it is, you usually get a pretty good result,” said Dr. Ward.
A patient with headache on 15 days per month or more has chronic daily headache. The duration of the headaches can provide the basis for a more specific diagnosis. Headaches of short duration (ie, less than four hours) may be symptoms of cluster headache, chronic paroxysmal hemicrania, hypnic headache, or trigeminal neuralgia. Headaches of long duration (ie, more than four hours) may indicate chronic migraine, chronic tension-type headache, hemicrania continua, or new daily persistent headache.
A patient with headache on 15 or more days per month, and for whom headaches on at least eight days per month meet the criteria of migraine, has chronic migraine. The two best-supported treatments for chronic migraine are topiramate and onabotulinumtoxinA. In patients with chronic migraine, what appears to be a tension-type headache may eventually declare its true nature and become a migraine headache with accompanying pounding and photophobia. What looks like a tension-type headache in a migraineur may respond to a triptan, said Dr. Ward.
Stop Medication Overuse
Medication overuse can confound the diagnosis and alter the headache itself. Many patients with refractory headache overuse medication but may fail to mention this to a neurologist. The overused medication may be a prescription or an over-the-counter drug such as ibuprofen, acetaminophen, or a combination that includes caffeine. Drugs with short half-lives appear to be particularly likely to cause medication overuse headache.
Some patients may be overusing opioids for their headache. “Opioids for headache are not a good idea,” said Dr. Ward. “Nothing good will come of it.” These drugs may cause central sensitization and reduce the efficacy of other headache remedies.
The risk of medication overuse headache increases if the patient uses combination analgesics, ergotamine, or triptans on 10 or more days per month, or simple analgesics on more than 15 days per month. “The clinical question I always ask patients is, ‘Are you taking more pills and having more headaches?’ If the answer is ‘yes,’ then they have medication overuse headache,” said Dr. Ward.
If patients stop taking the overused medication, they may have a withdrawal headache that is worse than their normal headache. Medication overuse headache usually resolves itself after the overuse is stopped, and bridge therapies such as steroids, nonsteroidal anti-inflammatory drugs, or dihydroergotamine may alleviate pain during withdrawal. “If you can get the patient over that hump, which can be several days of bad headache, they often do remarkably better,” said Dr. Ward.
Get Back to Basics
Taking a careful history is essential to successful treatment. “If you do not get the original history, you could miss the diagnosis,” said Dr. Ward. The neurologist must know about the mode of onset of the patient’s headache, and also know all about his or her prior headaches.
A patient with refractory headache should undergo a thorough head and neck examination, but physicians sometimes neglect to perform it. An MRI of the brain with gadolinium generally is warranted. About 90% of patients with low CSF pressure have pachymeningeal enhancement, which is visible on MRI performed with gadolinium, said Dr. Ward. Blood work, however, usually reveals little and appears normal. Sometimes thyroid tests, a Lyme test, a blood count, and a serum creatinine test are helpful, and a serum erythrocyte sedimentation rate test in those over age 50 is important to obtain.
Lumbar punctures may be underused, said Dr. Ward. Although it is uncommon, some patients present with high intracranial pressure, but without papilledema. The correct diagnosis can lead to effective treatment for these patients.
Effective treatment also is more likely when the neurologist gets to know the patient. He or she can use preventive medications to reduce the number of headache days. The literature suggests that successful preventive therapy should achieve a target of four headache days or fewer per month.
Neurologists also should treat the patient’s comorbid conditions, which often are psychiatric in people with refractory headache. It is unusual to see a patient with chronic migraine who does not have anxiety and depression, said Dr. Ward. Patients with refractory headache also may have phobias, bipolar disorder, or posttraumatic stress disorder, which is a significant confounder.
To Admit or Not to Admit?
A neurologist may have to decide whether to admit to the hospital a patient with chronic headache who is not doing well. First, the neurologist and patient should agree on a therapeutic target. Outpatient treatment works well if the patient is motivated and compliant and does not have confounding conditions. If the therapeutic target cannot be met through outpatient treatment, the neurologist should consider hospital admission. Insurance companies generally will cover three days of inpatient treatment, said Dr. Ward.
Neurologists have many options for inpatient treatment of refractory headache. Repetitive dihydroergotamine, known as the Raskin protocol, is highly effective if administered correctly. Dihydroergotamine should be given three times per day. “If you order it q. 8 h., the nurse will wake your patient up in the middle of the night, and waking up a patient with benign headaches is not a good idea,” said Dr. Ward. The dose must not be sufficient to cause nausea, because nauseating the patient can exacerbate headaches. “We usually premedicate with metoclopramide or prochlorperazine for nausea, but both of those drugs … also are good headache remedies.”
The Raskin protocol requires the withdrawal of other analgesics. The protocol typically lasts for three days, and most patients have good outcomes at this point. Extending the protocol to six or seven days may increase the number of patients with good outcomes. The success rate for the Raskin protocol is between 60% and 70%, said Dr. Ward. Patients who are pregnant or who have coronary artery disease should not receive dihydroergotamine, however.
Another option for inpatient treatment is IV chlorpromazine. The goal of this treatment is to induce a light sleep and maintain it for two or three days. The neurologist may start with a dose of 10 mg t.i.d. and monitor the patient’s response. The drug effectively suppresses narcotic withdrawal symptoms, so the neurologist may withdraw overused medications while the patient is asleep. Chlorpromazine may cause QT prolongation, so the patient should undergo cardiac monitoring. The drug also causes orthostatic hypotension, so patients should remain on bed rest and receive prophylaxis for deep venous thrombosis, said Dr. Ward.
IV valproate is an excellent choice if the patient has cardiac problems or bipolar disease, he added. The drug can be administered in a single dose of between 300 mg and 500 mg run in rapidly. “You can run in a whole loading dose in five or 10 minutes with virtually no side effects,” said Dr. Ward. Treatment can be administered b.i.d. or t.i.d. for two or three days. Pregnant patients should not receive valproate, however. Yet another option is IV magnesium, although the evidence for its efficacy is mostly anecdotal. A protocol of 1 to 2 g administered over 10 to 20 minutes, repeated several times per day, may be effective. It is advisable to monitor the patient’s serum magnesium levels to ensure that they do not become excessive. Magnesium may adversely affect fetal bone development, so neurologists should exercise caution when considering the drug for a pregnant patient. IV magnesium is “an excellent choice for hemiplegic migraine,” said Dr. Ward.
If the patient’s occipital nerves are tender, occipital nerve blockade may relieve pain. IV ketorolac, in 30-mg doses t.i.d. or q.i.d., may alleviate breakthrough headaches. Lidocaine patches can reduce back or neck pain for as long as 12 hours daily.
Abruptly withdrawing butalbital entails a risk of seizures and delirium. Neurologists may wish to administer phenobarbital in its place, as a single bedtime dose, while they are tapering or stopping butalbital. A 30-mg dose of phenobarbital may be substituted for every 100 mg of butalbital, said Dr. Ward.
Suggested Reading
Ford RG, Ford KT. Continuous intravenous dihydroergotamine in the treatment of intractable headache. Headache. 1997;37(3):129-136.
Lai TH, Wang SJ. Update of inpatient treatment for refractory chronic daily headache. Curr Pain Headache Rep. 2016;20(1):5.
Levin M. Opioids in headache. Headache. 2014;54(1):12-21.
Lipton RB, Silberstein SD, Saper JR, et al. Why headache treatment fails. Neurology. 2003;60(7):1064-1070.
RIVIERA BEACH, FL—Neurologists sometimes encounter patients with headaches that have not responded to prior treatment. These patients may be demoralized, and neurologists may be at a loss for a way to relieve their pain. Effective treatment is possible for many of these patients, according to Thomas N. Ward, MD, Emeritus Professor of Neurology at Dartmouth College in Hanover, New Hampshire. He described the process of differential diagnosis, as well as outpatient and inpatient therapeutic options for refractory headache, at the 44th Annual Meeting of the Southern Clinical Neurological Society.
Confirm the Diagnosis
When faced with a patient with refractory headache, a neurologist should first verify the diagnosis and rule out the possibility of secondary headache. These steps will improve the likelihood of a positive outcome. “If you follow the fundamentals and treat the type of headache it is, you usually get a pretty good result,” said Dr. Ward.
A patient with headache on 15 days per month or more has chronic daily headache. The duration of the headaches can provide the basis for a more specific diagnosis. Headaches of short duration (ie, less than four hours) may be symptoms of cluster headache, chronic paroxysmal hemicrania, hypnic headache, or trigeminal neuralgia. Headaches of long duration (ie, more than four hours) may indicate chronic migraine, chronic tension-type headache, hemicrania continua, or new daily persistent headache.
A patient with headache on 15 or more days per month, and for whom headaches on at least eight days per month meet the criteria of migraine, has chronic migraine. The two best-supported treatments for chronic migraine are topiramate and onabotulinumtoxinA. In patients with chronic migraine, what appears to be a tension-type headache may eventually declare its true nature and become a migraine headache with accompanying pounding and photophobia. What looks like a tension-type headache in a migraineur may respond to a triptan, said Dr. Ward.
Stop Medication Overuse
Medication overuse can confound the diagnosis and alter the headache itself. Many patients with refractory headache overuse medication but may fail to mention this to a neurologist. The overused medication may be a prescription or an over-the-counter drug such as ibuprofen, acetaminophen, or a combination that includes caffeine. Drugs with short half-lives appear to be particularly likely to cause medication overuse headache.
Some patients may be overusing opioids for their headache. “Opioids for headache are not a good idea,” said Dr. Ward. “Nothing good will come of it.” These drugs may cause central sensitization and reduce the efficacy of other headache remedies.
The risk of medication overuse headache increases if the patient uses combination analgesics, ergotamine, or triptans on 10 or more days per month, or simple analgesics on more than 15 days per month. “The clinical question I always ask patients is, ‘Are you taking more pills and having more headaches?’ If the answer is ‘yes,’ then they have medication overuse headache,” said Dr. Ward.
If patients stop taking the overused medication, they may have a withdrawal headache that is worse than their normal headache. Medication overuse headache usually resolves itself after the overuse is stopped, and bridge therapies such as steroids, nonsteroidal anti-inflammatory drugs, or dihydroergotamine may alleviate pain during withdrawal. “If you can get the patient over that hump, which can be several days of bad headache, they often do remarkably better,” said Dr. Ward.
Get Back to Basics
Taking a careful history is essential to successful treatment. “If you do not get the original history, you could miss the diagnosis,” said Dr. Ward. The neurologist must know about the mode of onset of the patient’s headache, and also know all about his or her prior headaches.
A patient with refractory headache should undergo a thorough head and neck examination, but physicians sometimes neglect to perform it. An MRI of the brain with gadolinium generally is warranted. About 90% of patients with low CSF pressure have pachymeningeal enhancement, which is visible on MRI performed with gadolinium, said Dr. Ward. Blood work, however, usually reveals little and appears normal. Sometimes thyroid tests, a Lyme test, a blood count, and a serum creatinine test are helpful, and a serum erythrocyte sedimentation rate test in those over age 50 is important to obtain.
Lumbar punctures may be underused, said Dr. Ward. Although it is uncommon, some patients present with high intracranial pressure, but without papilledema. The correct diagnosis can lead to effective treatment for these patients.
Effective treatment also is more likely when the neurologist gets to know the patient. He or she can use preventive medications to reduce the number of headache days. The literature suggests that successful preventive therapy should achieve a target of four headache days or fewer per month.
Neurologists also should treat the patient’s comorbid conditions, which often are psychiatric in people with refractory headache. It is unusual to see a patient with chronic migraine who does not have anxiety and depression, said Dr. Ward. Patients with refractory headache also may have phobias, bipolar disorder, or posttraumatic stress disorder, which is a significant confounder.
To Admit or Not to Admit?
A neurologist may have to decide whether to admit to the hospital a patient with chronic headache who is not doing well. First, the neurologist and patient should agree on a therapeutic target. Outpatient treatment works well if the patient is motivated and compliant and does not have confounding conditions. If the therapeutic target cannot be met through outpatient treatment, the neurologist should consider hospital admission. Insurance companies generally will cover three days of inpatient treatment, said Dr. Ward.
Neurologists have many options for inpatient treatment of refractory headache. Repetitive dihydroergotamine, known as the Raskin protocol, is highly effective if administered correctly. Dihydroergotamine should be given three times per day. “If you order it q. 8 h., the nurse will wake your patient up in the middle of the night, and waking up a patient with benign headaches is not a good idea,” said Dr. Ward. The dose must not be sufficient to cause nausea, because nauseating the patient can exacerbate headaches. “We usually premedicate with metoclopramide or prochlorperazine for nausea, but both of those drugs … also are good headache remedies.”
The Raskin protocol requires the withdrawal of other analgesics. The protocol typically lasts for three days, and most patients have good outcomes at this point. Extending the protocol to six or seven days may increase the number of patients with good outcomes. The success rate for the Raskin protocol is between 60% and 70%, said Dr. Ward. Patients who are pregnant or who have coronary artery disease should not receive dihydroergotamine, however.
Another option for inpatient treatment is IV chlorpromazine. The goal of this treatment is to induce a light sleep and maintain it for two or three days. The neurologist may start with a dose of 10 mg t.i.d. and monitor the patient’s response. The drug effectively suppresses narcotic withdrawal symptoms, so the neurologist may withdraw overused medications while the patient is asleep. Chlorpromazine may cause QT prolongation, so the patient should undergo cardiac monitoring. The drug also causes orthostatic hypotension, so patients should remain on bed rest and receive prophylaxis for deep venous thrombosis, said Dr. Ward.
IV valproate is an excellent choice if the patient has cardiac problems or bipolar disease, he added. The drug can be administered in a single dose of between 300 mg and 500 mg run in rapidly. “You can run in a whole loading dose in five or 10 minutes with virtually no side effects,” said Dr. Ward. Treatment can be administered b.i.d. or t.i.d. for two or three days. Pregnant patients should not receive valproate, however. Yet another option is IV magnesium, although the evidence for its efficacy is mostly anecdotal. A protocol of 1 to 2 g administered over 10 to 20 minutes, repeated several times per day, may be effective. It is advisable to monitor the patient’s serum magnesium levels to ensure that they do not become excessive. Magnesium may adversely affect fetal bone development, so neurologists should exercise caution when considering the drug for a pregnant patient. IV magnesium is “an excellent choice for hemiplegic migraine,” said Dr. Ward.
If the patient’s occipital nerves are tender, occipital nerve blockade may relieve pain. IV ketorolac, in 30-mg doses t.i.d. or q.i.d., may alleviate breakthrough headaches. Lidocaine patches can reduce back or neck pain for as long as 12 hours daily.
Abruptly withdrawing butalbital entails a risk of seizures and delirium. Neurologists may wish to administer phenobarbital in its place, as a single bedtime dose, while they are tapering or stopping butalbital. A 30-mg dose of phenobarbital may be substituted for every 100 mg of butalbital, said Dr. Ward.
Suggested Reading
Ford RG, Ford KT. Continuous intravenous dihydroergotamine in the treatment of intractable headache. Headache. 1997;37(3):129-136.
Lai TH, Wang SJ. Update of inpatient treatment for refractory chronic daily headache. Curr Pain Headache Rep. 2016;20(1):5.
Levin M. Opioids in headache. Headache. 2014;54(1):12-21.
Lipton RB, Silberstein SD, Saper JR, et al. Why headache treatment fails. Neurology. 2003;60(7):1064-1070.
RIVIERA BEACH, FL—Neurologists sometimes encounter patients with headaches that have not responded to prior treatment. These patients may be demoralized, and neurologists may be at a loss for a way to relieve their pain. Effective treatment is possible for many of these patients, according to Thomas N. Ward, MD, Emeritus Professor of Neurology at Dartmouth College in Hanover, New Hampshire. He described the process of differential diagnosis, as well as outpatient and inpatient therapeutic options for refractory headache, at the 44th Annual Meeting of the Southern Clinical Neurological Society.
Confirm the Diagnosis
When faced with a patient with refractory headache, a neurologist should first verify the diagnosis and rule out the possibility of secondary headache. These steps will improve the likelihood of a positive outcome. “If you follow the fundamentals and treat the type of headache it is, you usually get a pretty good result,” said Dr. Ward.
A patient with headache on 15 days per month or more has chronic daily headache. The duration of the headaches can provide the basis for a more specific diagnosis. Headaches of short duration (ie, less than four hours) may be symptoms of cluster headache, chronic paroxysmal hemicrania, hypnic headache, or trigeminal neuralgia. Headaches of long duration (ie, more than four hours) may indicate chronic migraine, chronic tension-type headache, hemicrania continua, or new daily persistent headache.
A patient with headache on 15 or more days per month, and for whom headaches on at least eight days per month meet the criteria of migraine, has chronic migraine. The two best-supported treatments for chronic migraine are topiramate and onabotulinumtoxinA. In patients with chronic migraine, what appears to be a tension-type headache may eventually declare its true nature and become a migraine headache with accompanying pounding and photophobia. What looks like a tension-type headache in a migraineur may respond to a triptan, said Dr. Ward.
Stop Medication Overuse
Medication overuse can confound the diagnosis and alter the headache itself. Many patients with refractory headache overuse medication but may fail to mention this to a neurologist. The overused medication may be a prescription or an over-the-counter drug such as ibuprofen, acetaminophen, or a combination that includes caffeine. Drugs with short half-lives appear to be particularly likely to cause medication overuse headache.
Some patients may be overusing opioids for their headache. “Opioids for headache are not a good idea,” said Dr. Ward. “Nothing good will come of it.” These drugs may cause central sensitization and reduce the efficacy of other headache remedies.
The risk of medication overuse headache increases if the patient uses combination analgesics, ergotamine, or triptans on 10 or more days per month, or simple analgesics on more than 15 days per month. “The clinical question I always ask patients is, ‘Are you taking more pills and having more headaches?’ If the answer is ‘yes,’ then they have medication overuse headache,” said Dr. Ward.
If patients stop taking the overused medication, they may have a withdrawal headache that is worse than their normal headache. Medication overuse headache usually resolves itself after the overuse is stopped, and bridge therapies such as steroids, nonsteroidal anti-inflammatory drugs, or dihydroergotamine may alleviate pain during withdrawal. “If you can get the patient over that hump, which can be several days of bad headache, they often do remarkably better,” said Dr. Ward.
Get Back to Basics
Taking a careful history is essential to successful treatment. “If you do not get the original history, you could miss the diagnosis,” said Dr. Ward. The neurologist must know about the mode of onset of the patient’s headache, and also know all about his or her prior headaches.
A patient with refractory headache should undergo a thorough head and neck examination, but physicians sometimes neglect to perform it. An MRI of the brain with gadolinium generally is warranted. About 90% of patients with low CSF pressure have pachymeningeal enhancement, which is visible on MRI performed with gadolinium, said Dr. Ward. Blood work, however, usually reveals little and appears normal. Sometimes thyroid tests, a Lyme test, a blood count, and a serum creatinine test are helpful, and a serum erythrocyte sedimentation rate test in those over age 50 is important to obtain.
Lumbar punctures may be underused, said Dr. Ward. Although it is uncommon, some patients present with high intracranial pressure, but without papilledema. The correct diagnosis can lead to effective treatment for these patients.
Effective treatment also is more likely when the neurologist gets to know the patient. He or she can use preventive medications to reduce the number of headache days. The literature suggests that successful preventive therapy should achieve a target of four headache days or fewer per month.
Neurologists also should treat the patient’s comorbid conditions, which often are psychiatric in people with refractory headache. It is unusual to see a patient with chronic migraine who does not have anxiety and depression, said Dr. Ward. Patients with refractory headache also may have phobias, bipolar disorder, or posttraumatic stress disorder, which is a significant confounder.
To Admit or Not to Admit?
A neurologist may have to decide whether to admit to the hospital a patient with chronic headache who is not doing well. First, the neurologist and patient should agree on a therapeutic target. Outpatient treatment works well if the patient is motivated and compliant and does not have confounding conditions. If the therapeutic target cannot be met through outpatient treatment, the neurologist should consider hospital admission. Insurance companies generally will cover three days of inpatient treatment, said Dr. Ward.
Neurologists have many options for inpatient treatment of refractory headache. Repetitive dihydroergotamine, known as the Raskin protocol, is highly effective if administered correctly. Dihydroergotamine should be given three times per day. “If you order it q. 8 h., the nurse will wake your patient up in the middle of the night, and waking up a patient with benign headaches is not a good idea,” said Dr. Ward. The dose must not be sufficient to cause nausea, because nauseating the patient can exacerbate headaches. “We usually premedicate with metoclopramide or prochlorperazine for nausea, but both of those drugs … also are good headache remedies.”
The Raskin protocol requires the withdrawal of other analgesics. The protocol typically lasts for three days, and most patients have good outcomes at this point. Extending the protocol to six or seven days may increase the number of patients with good outcomes. The success rate for the Raskin protocol is between 60% and 70%, said Dr. Ward. Patients who are pregnant or who have coronary artery disease should not receive dihydroergotamine, however.
Another option for inpatient treatment is IV chlorpromazine. The goal of this treatment is to induce a light sleep and maintain it for two or three days. The neurologist may start with a dose of 10 mg t.i.d. and monitor the patient’s response. The drug effectively suppresses narcotic withdrawal symptoms, so the neurologist may withdraw overused medications while the patient is asleep. Chlorpromazine may cause QT prolongation, so the patient should undergo cardiac monitoring. The drug also causes orthostatic hypotension, so patients should remain on bed rest and receive prophylaxis for deep venous thrombosis, said Dr. Ward.
IV valproate is an excellent choice if the patient has cardiac problems or bipolar disease, he added. The drug can be administered in a single dose of between 300 mg and 500 mg run in rapidly. “You can run in a whole loading dose in five or 10 minutes with virtually no side effects,” said Dr. Ward. Treatment can be administered b.i.d. or t.i.d. for two or three days. Pregnant patients should not receive valproate, however. Yet another option is IV magnesium, although the evidence for its efficacy is mostly anecdotal. A protocol of 1 to 2 g administered over 10 to 20 minutes, repeated several times per day, may be effective. It is advisable to monitor the patient’s serum magnesium levels to ensure that they do not become excessive. Magnesium may adversely affect fetal bone development, so neurologists should exercise caution when considering the drug for a pregnant patient. IV magnesium is “an excellent choice for hemiplegic migraine,” said Dr. Ward.
If the patient’s occipital nerves are tender, occipital nerve blockade may relieve pain. IV ketorolac, in 30-mg doses t.i.d. or q.i.d., may alleviate breakthrough headaches. Lidocaine patches can reduce back or neck pain for as long as 12 hours daily.
Abruptly withdrawing butalbital entails a risk of seizures and delirium. Neurologists may wish to administer phenobarbital in its place, as a single bedtime dose, while they are tapering or stopping butalbital. A 30-mg dose of phenobarbital may be substituted for every 100 mg of butalbital, said Dr. Ward.
Suggested Reading
Ford RG, Ford KT. Continuous intravenous dihydroergotamine in the treatment of intractable headache. Headache. 1997;37(3):129-136.
Lai TH, Wang SJ. Update of inpatient treatment for refractory chronic daily headache. Curr Pain Headache Rep. 2016;20(1):5.
Levin M. Opioids in headache. Headache. 2014;54(1):12-21.
Lipton RB, Silberstein SD, Saper JR, et al. Why headache treatment fails. Neurology. 2003;60(7):1064-1070.
Moving toward safer morcellation techniques
For minimally invasive surgeons throughout the world, particularly in the United States, as well as the patients we treat, April 17, 2014, is our day of infamy. It was on this day that the Food and Drug Administration recommended against the use of the electronic power morcellator. The basis of the agency’s decision was the concern about inadvertent spread of sarcomatous tissue. Many hospitals, medical centers, and hospital systems subsequently banned the use of power morcellation. With such bans, a subsequent study by Wright et al. noted a decrease in the percentage of both laparoscopic and vaginal hysterectomy (JAMA. 2016 Aug 23-30;316[8]:877-8). This is concerning when you consider that the complication rate for abdominal hysterectomy is around 17%, compared with about 4% for the minimally invasive procedure.
For this edition of the Master Class in Gynecologic Surgery, I have asked Tony Shibley, MD, to describe the PneumoLiner, the first FDA-approved bag for the purpose of contained laparoscopic morcellation. Dr. Shibley, who is in private practice in the Minneapolis area, first came to national attention because of his expertise in single-port surgery. He has been performing power morcellation in a contained system for 5 years and is the thought leader behind the design and creation of the PneumoLiner.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported receiving research funds from Espiner Medical Inc., and being a consultant to Olympus, which manufacturers the PneumoLiner.
For minimally invasive surgeons throughout the world, particularly in the United States, as well as the patients we treat, April 17, 2014, is our day of infamy. It was on this day that the Food and Drug Administration recommended against the use of the electronic power morcellator. The basis of the agency’s decision was the concern about inadvertent spread of sarcomatous tissue. Many hospitals, medical centers, and hospital systems subsequently banned the use of power morcellation. With such bans, a subsequent study by Wright et al. noted a decrease in the percentage of both laparoscopic and vaginal hysterectomy (JAMA. 2016 Aug 23-30;316[8]:877-8). This is concerning when you consider that the complication rate for abdominal hysterectomy is around 17%, compared with about 4% for the minimally invasive procedure.
For this edition of the Master Class in Gynecologic Surgery, I have asked Tony Shibley, MD, to describe the PneumoLiner, the first FDA-approved bag for the purpose of contained laparoscopic morcellation. Dr. Shibley, who is in private practice in the Minneapolis area, first came to national attention because of his expertise in single-port surgery. He has been performing power morcellation in a contained system for 5 years and is the thought leader behind the design and creation of the PneumoLiner.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported receiving research funds from Espiner Medical Inc., and being a consultant to Olympus, which manufacturers the PneumoLiner.
For minimally invasive surgeons throughout the world, particularly in the United States, as well as the patients we treat, April 17, 2014, is our day of infamy. It was on this day that the Food and Drug Administration recommended against the use of the electronic power morcellator. The basis of the agency’s decision was the concern about inadvertent spread of sarcomatous tissue. Many hospitals, medical centers, and hospital systems subsequently banned the use of power morcellation. With such bans, a subsequent study by Wright et al. noted a decrease in the percentage of both laparoscopic and vaginal hysterectomy (JAMA. 2016 Aug 23-30;316[8]:877-8). This is concerning when you consider that the complication rate for abdominal hysterectomy is around 17%, compared with about 4% for the minimally invasive procedure.
For this edition of the Master Class in Gynecologic Surgery, I have asked Tony Shibley, MD, to describe the PneumoLiner, the first FDA-approved bag for the purpose of contained laparoscopic morcellation. Dr. Shibley, who is in private practice in the Minneapolis area, first came to national attention because of his expertise in single-port surgery. He has been performing power morcellation in a contained system for 5 years and is the thought leader behind the design and creation of the PneumoLiner.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported receiving research funds from Espiner Medical Inc., and being a consultant to Olympus, which manufacturers the PneumoLiner.
VIDEO: Tips for performing contained power morcellation
Experience with electromechanical power morcellation in a bag has advanced in the last several years in an effort to achieve safe tissue removal for minimally invasive procedures such as myomectomy, laparoscopic supracervical hysterectomy, or total hysterectomy of a large uterus.
Tissue extraction using contained power morcellation has become favored over contained morcellation using a scalpel – not only because the latter approach is cumbersome but because of the risk of bag puncture and subsequent organ injury. Surgeons have experimented with various sizes and types of retrieval bags and with various techniques for contained power morcellation.
The PneumoLiner carries the same restrictions as do other laparoscopic power morcellation systems – namely that it should not be used in surgery in which the tissue to be morcellated is known or suspected to contain malignancy, and that it should not be used in women who are peri- or postmenopausal. Moreover, to further enhance safety, physicians must have successfully completed the FDA-required validated training program run by Advanced Surgical Concepts and Olympus in order to use the device.
The FDA reviewed the PneumoLiner through a regulatory process known as the de novo classification process. This regulatory process is for first of its kind, low- to moderate-risk medical devices. The PneumoLiner was tested in laboratory conditions to ensure that it could withstand stress force in excess of the normal forces of surgery, and was found to be impervious to substances similar in molecular size to tissues, cells, and body fluids. There could be no cellular migration or leakage.
As surgeons were advancing the idea of inflated bag morcellation, one promising adaptation was to puncture the inflated bag to place accessory ports. However, recent research has shown that contained morcellation involving intentional bag puncture with a trocar may result in tissue or fluid leakage.
Spillage was noted in 7 of 76 cases (9.2%) in a multicenter prospective cohort of women who underwent hysterectomy or myomectomy using a contained power morcellation technique that involved perforation of the containment bag with a balloon-tipped lateral trocar. Investigators had injected blue dye into the bag prior to morcellation and examined the abdomen and pelvis after removing the bag for signs of spillage of dye, fluid, or tissue. In all cases, the containment bags were intact (Am J Obstet Gynecol. 2016 Feb;214[2]:257.e1-6).
The authors prematurely closed this study and recommended against this puncture technique. For complete containment, it appears to be important that we morcellate using a bag that has a single opening and is not punctured with accessory trocars.
The technique
The PneumoLiner comes loaded in an insertion tube for placement. It has a plunger to deploy the device and a retrieval lanyard that closes the bag around the specimen, enabling retrieval of the neck of the bag outside the abdomen.
Included with the PneumoLiner is a multi-instrument port that can be used during the laparoscopic procedure and then converted to the active port for morcellation. The port has an opening for the laparoscope (either a 5-mm 30-degree straight or a 5-mm articulating laparoscope) and an opening for the morcellator, as well as two small openings for insufflation and for smoke exhaustion.
Surgery may be performed using this single-port or a multiport laparoscopic or robotic approach. For morcellation, the approach converts to a single-site technique that involves only one entry point for all instruments and no perforation of the bag.
At the beginning of the procedure (or at the end of the case if preferred), a 25-mm incision is made in the umbilicus and the system’s port is inserted and trimmed. The port cap is placed, the abdomen is insufflated, and the laparoscope is inserted. If placed at the beginning of the case, this port can be used as a camera or accessory port.
Before deployment of the PneumoLiner, the uterus or target tissue is placed out of the way; I recommend the upper right quadrant. The PneumoLiner is then inserted with its directional tab pointing upward, and the system’s plunger is depressed while the sleeve is pulled back. In essence, the PneumoLiner is advanced while the sleeve is simultaneously withdrawn, laying it flat in the pelvis.
With an atraumatic grasper, the uterus is placed within the opening of the bag, and the bag is grasped at the collar and elevated up and around the specimen. When full containment of the specimen is visualized, the retrieval lanyard is withdrawn until an opening ring partially protrudes outside the port. All lateral trocars must have been withdrawn prior to inflation of the bag to prevent it from being damaged.
At this point, the port cap is removed and the PneumoLiner neck is withdrawn until a black grid pattern on the bag is visible. The surgeon should then ensure there are no twists in the bag before replacing the port cap and insufflating the bag to a pressure of 15 mm Hg.*
The bag must be correctly in place and fully insufflated before the laparoscope is inserted. The laparoscope must be inserted prior to the morcellator. When the morcellator is inserted, care must be taken to ensure that the morcellator probe is in place.
Once the morcellator is placed, the probe is withdrawn and a closed tenaculum is placed. With the closed tenaculum, the surgeon can manipulate tissue and gauge depth and bearings without inadvertently grabbing the bag. The black grid pattern on the bag assists with estimation of tissue fragment size; morcellation proceeds under direct vision until the tissue fragments are smaller than four printed grids.
Instrumentation is removed in a set order, with the morcellator first and the laparoscope last. The port cap is detached and the PneumoLiner is removed while allowing fumes to escape. The morcellator, camera, tenaculum, and port cap are considered contaminated at this point and should not re-enter the field.
Pearls for morcellation
- The single-site nature of the procedure can sometimes be challenging. If you’ve placed your laparoscope and are having difficulty locating the morcellator, bring your laparoscope and morcellator shaft in parallel to each other, and you’ll be able to better orient yourself.
- To enlarge your field of view after you’ve inflated the PneumoLiner and captured the tissue within the bag, level the patient a bit and move the tissue further away from the laparoscope.
- If the morcellator tube is limiting visualization of the tenaculum tip, slide the morcellator back while leaving the tenaculum in a fixed position.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Courtesy Dr. Tony Shibley and Olympus
Dr. Shibley is an ob.gyn. in private practice in the Minneapolis area. He receives royalties from Advanced Surgical Concepts and serves as a consultant for Olympus.
*Correction 3/8/17: An earlier version of this article misstated the name of the Pneumoliner device in a photo caption. The pressure of the morcellation bag also was misstated.
Experience with electromechanical power morcellation in a bag has advanced in the last several years in an effort to achieve safe tissue removal for minimally invasive procedures such as myomectomy, laparoscopic supracervical hysterectomy, or total hysterectomy of a large uterus.
Tissue extraction using contained power morcellation has become favored over contained morcellation using a scalpel – not only because the latter approach is cumbersome but because of the risk of bag puncture and subsequent organ injury. Surgeons have experimented with various sizes and types of retrieval bags and with various techniques for contained power morcellation.
The PneumoLiner carries the same restrictions as do other laparoscopic power morcellation systems – namely that it should not be used in surgery in which the tissue to be morcellated is known or suspected to contain malignancy, and that it should not be used in women who are peri- or postmenopausal. Moreover, to further enhance safety, physicians must have successfully completed the FDA-required validated training program run by Advanced Surgical Concepts and Olympus in order to use the device.
The FDA reviewed the PneumoLiner through a regulatory process known as the de novo classification process. This regulatory process is for first of its kind, low- to moderate-risk medical devices. The PneumoLiner was tested in laboratory conditions to ensure that it could withstand stress force in excess of the normal forces of surgery, and was found to be impervious to substances similar in molecular size to tissues, cells, and body fluids. There could be no cellular migration or leakage.
As surgeons were advancing the idea of inflated bag morcellation, one promising adaptation was to puncture the inflated bag to place accessory ports. However, recent research has shown that contained morcellation involving intentional bag puncture with a trocar may result in tissue or fluid leakage.
Spillage was noted in 7 of 76 cases (9.2%) in a multicenter prospective cohort of women who underwent hysterectomy or myomectomy using a contained power morcellation technique that involved perforation of the containment bag with a balloon-tipped lateral trocar. Investigators had injected blue dye into the bag prior to morcellation and examined the abdomen and pelvis after removing the bag for signs of spillage of dye, fluid, or tissue. In all cases, the containment bags were intact (Am J Obstet Gynecol. 2016 Feb;214[2]:257.e1-6).
The authors prematurely closed this study and recommended against this puncture technique. For complete containment, it appears to be important that we morcellate using a bag that has a single opening and is not punctured with accessory trocars.
The technique
The PneumoLiner comes loaded in an insertion tube for placement. It has a plunger to deploy the device and a retrieval lanyard that closes the bag around the specimen, enabling retrieval of the neck of the bag outside the abdomen.
Included with the PneumoLiner is a multi-instrument port that can be used during the laparoscopic procedure and then converted to the active port for morcellation. The port has an opening for the laparoscope (either a 5-mm 30-degree straight or a 5-mm articulating laparoscope) and an opening for the morcellator, as well as two small openings for insufflation and for smoke exhaustion.
Surgery may be performed using this single-port or a multiport laparoscopic or robotic approach. For morcellation, the approach converts to a single-site technique that involves only one entry point for all instruments and no perforation of the bag.
At the beginning of the procedure (or at the end of the case if preferred), a 25-mm incision is made in the umbilicus and the system’s port is inserted and trimmed. The port cap is placed, the abdomen is insufflated, and the laparoscope is inserted. If placed at the beginning of the case, this port can be used as a camera or accessory port.
Before deployment of the PneumoLiner, the uterus or target tissue is placed out of the way; I recommend the upper right quadrant. The PneumoLiner is then inserted with its directional tab pointing upward, and the system’s plunger is depressed while the sleeve is pulled back. In essence, the PneumoLiner is advanced while the sleeve is simultaneously withdrawn, laying it flat in the pelvis.
With an atraumatic grasper, the uterus is placed within the opening of the bag, and the bag is grasped at the collar and elevated up and around the specimen. When full containment of the specimen is visualized, the retrieval lanyard is withdrawn until an opening ring partially protrudes outside the port. All lateral trocars must have been withdrawn prior to inflation of the bag to prevent it from being damaged.
At this point, the port cap is removed and the PneumoLiner neck is withdrawn until a black grid pattern on the bag is visible. The surgeon should then ensure there are no twists in the bag before replacing the port cap and insufflating the bag to a pressure of 15 mm Hg.*
The bag must be correctly in place and fully insufflated before the laparoscope is inserted. The laparoscope must be inserted prior to the morcellator. When the morcellator is inserted, care must be taken to ensure that the morcellator probe is in place.
Once the morcellator is placed, the probe is withdrawn and a closed tenaculum is placed. With the closed tenaculum, the surgeon can manipulate tissue and gauge depth and bearings without inadvertently grabbing the bag. The black grid pattern on the bag assists with estimation of tissue fragment size; morcellation proceeds under direct vision until the tissue fragments are smaller than four printed grids.
Instrumentation is removed in a set order, with the morcellator first and the laparoscope last. The port cap is detached and the PneumoLiner is removed while allowing fumes to escape. The morcellator, camera, tenaculum, and port cap are considered contaminated at this point and should not re-enter the field.
Pearls for morcellation
- The single-site nature of the procedure can sometimes be challenging. If you’ve placed your laparoscope and are having difficulty locating the morcellator, bring your laparoscope and morcellator shaft in parallel to each other, and you’ll be able to better orient yourself.
- To enlarge your field of view after you’ve inflated the PneumoLiner and captured the tissue within the bag, level the patient a bit and move the tissue further away from the laparoscope.
- If the morcellator tube is limiting visualization of the tenaculum tip, slide the morcellator back while leaving the tenaculum in a fixed position.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Courtesy Dr. Tony Shibley and Olympus
Dr. Shibley is an ob.gyn. in private practice in the Minneapolis area. He receives royalties from Advanced Surgical Concepts and serves as a consultant for Olympus.
*Correction 3/8/17: An earlier version of this article misstated the name of the Pneumoliner device in a photo caption. The pressure of the morcellation bag also was misstated.
Experience with electromechanical power morcellation in a bag has advanced in the last several years in an effort to achieve safe tissue removal for minimally invasive procedures such as myomectomy, laparoscopic supracervical hysterectomy, or total hysterectomy of a large uterus.
Tissue extraction using contained power morcellation has become favored over contained morcellation using a scalpel – not only because the latter approach is cumbersome but because of the risk of bag puncture and subsequent organ injury. Surgeons have experimented with various sizes and types of retrieval bags and with various techniques for contained power morcellation.
The PneumoLiner carries the same restrictions as do other laparoscopic power morcellation systems – namely that it should not be used in surgery in which the tissue to be morcellated is known or suspected to contain malignancy, and that it should not be used in women who are peri- or postmenopausal. Moreover, to further enhance safety, physicians must have successfully completed the FDA-required validated training program run by Advanced Surgical Concepts and Olympus in order to use the device.
The FDA reviewed the PneumoLiner through a regulatory process known as the de novo classification process. This regulatory process is for first of its kind, low- to moderate-risk medical devices. The PneumoLiner was tested in laboratory conditions to ensure that it could withstand stress force in excess of the normal forces of surgery, and was found to be impervious to substances similar in molecular size to tissues, cells, and body fluids. There could be no cellular migration or leakage.
As surgeons were advancing the idea of inflated bag morcellation, one promising adaptation was to puncture the inflated bag to place accessory ports. However, recent research has shown that contained morcellation involving intentional bag puncture with a trocar may result in tissue or fluid leakage.
Spillage was noted in 7 of 76 cases (9.2%) in a multicenter prospective cohort of women who underwent hysterectomy or myomectomy using a contained power morcellation technique that involved perforation of the containment bag with a balloon-tipped lateral trocar. Investigators had injected blue dye into the bag prior to morcellation and examined the abdomen and pelvis after removing the bag for signs of spillage of dye, fluid, or tissue. In all cases, the containment bags were intact (Am J Obstet Gynecol. 2016 Feb;214[2]:257.e1-6).
The authors prematurely closed this study and recommended against this puncture technique. For complete containment, it appears to be important that we morcellate using a bag that has a single opening and is not punctured with accessory trocars.
The technique
The PneumoLiner comes loaded in an insertion tube for placement. It has a plunger to deploy the device and a retrieval lanyard that closes the bag around the specimen, enabling retrieval of the neck of the bag outside the abdomen.
Included with the PneumoLiner is a multi-instrument port that can be used during the laparoscopic procedure and then converted to the active port for morcellation. The port has an opening for the laparoscope (either a 5-mm 30-degree straight or a 5-mm articulating laparoscope) and an opening for the morcellator, as well as two small openings for insufflation and for smoke exhaustion.
Surgery may be performed using this single-port or a multiport laparoscopic or robotic approach. For morcellation, the approach converts to a single-site technique that involves only one entry point for all instruments and no perforation of the bag.
At the beginning of the procedure (or at the end of the case if preferred), a 25-mm incision is made in the umbilicus and the system’s port is inserted and trimmed. The port cap is placed, the abdomen is insufflated, and the laparoscope is inserted. If placed at the beginning of the case, this port can be used as a camera or accessory port.
Before deployment of the PneumoLiner, the uterus or target tissue is placed out of the way; I recommend the upper right quadrant. The PneumoLiner is then inserted with its directional tab pointing upward, and the system’s plunger is depressed while the sleeve is pulled back. In essence, the PneumoLiner is advanced while the sleeve is simultaneously withdrawn, laying it flat in the pelvis.
With an atraumatic grasper, the uterus is placed within the opening of the bag, and the bag is grasped at the collar and elevated up and around the specimen. When full containment of the specimen is visualized, the retrieval lanyard is withdrawn until an opening ring partially protrudes outside the port. All lateral trocars must have been withdrawn prior to inflation of the bag to prevent it from being damaged.
At this point, the port cap is removed and the PneumoLiner neck is withdrawn until a black grid pattern on the bag is visible. The surgeon should then ensure there are no twists in the bag before replacing the port cap and insufflating the bag to a pressure of 15 mm Hg.*
The bag must be correctly in place and fully insufflated before the laparoscope is inserted. The laparoscope must be inserted prior to the morcellator. When the morcellator is inserted, care must be taken to ensure that the morcellator probe is in place.
Once the morcellator is placed, the probe is withdrawn and a closed tenaculum is placed. With the closed tenaculum, the surgeon can manipulate tissue and gauge depth and bearings without inadvertently grabbing the bag. The black grid pattern on the bag assists with estimation of tissue fragment size; morcellation proceeds under direct vision until the tissue fragments are smaller than four printed grids.
Instrumentation is removed in a set order, with the morcellator first and the laparoscope last. The port cap is detached and the PneumoLiner is removed while allowing fumes to escape. The morcellator, camera, tenaculum, and port cap are considered contaminated at this point and should not re-enter the field.
Pearls for morcellation
- The single-site nature of the procedure can sometimes be challenging. If you’ve placed your laparoscope and are having difficulty locating the morcellator, bring your laparoscope and morcellator shaft in parallel to each other, and you’ll be able to better orient yourself.
- To enlarge your field of view after you’ve inflated the PneumoLiner and captured the tissue within the bag, level the patient a bit and move the tissue further away from the laparoscope.
- If the morcellator tube is limiting visualization of the tenaculum tip, slide the morcellator back while leaving the tenaculum in a fixed position.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Courtesy Dr. Tony Shibley and Olympus
Dr. Shibley is an ob.gyn. in private practice in the Minneapolis area. He receives royalties from Advanced Surgical Concepts and serves as a consultant for Olympus.
*Correction 3/8/17: An earlier version of this article misstated the name of the Pneumoliner device in a photo caption. The pressure of the morcellation bag also was misstated.
Breast cancer mortality mapped for 2017
U.S. breast cancer mortality will be an estimated 25.3 per 100,000 females in 2017, with the highest state rates in the East and the lowest in the West and Midwest.
Approximately 40,600 breast cancer deaths are predicted for the year in the United States by the American Cancer Society in its Cancer Facts & Figures 2017, based on 2000-2014 data from the National Center for Health Statistics. With the U.S. population currently around 321 million, that works out to a completely unadjusted death rate of 25.3 per 100,000 women. Doing a little more math puts the highest death rate (29.8) in West Virginia and the lowest (18.8) in Utah.
The incidence rate for female breast cancer was 123.3 per 100,000 for 2009-2013, with that figure age adjusted to the 2000 U.S. standard population, according to data from the North American Association of Central Cancer Registries, the ACS reported.
Wyoming had the lowest incidence over that time period (109.6 per 100,000), and New Hampshire had the highest at 138.1. Utah had the fifth-lowest incidence in that period at 112.7, but West Virginia, the state with the highest estimated mortality for 2017, had a relatively low incidence of 114.4 in 2009-2013, the ACS said.
U.S. breast cancer mortality will be an estimated 25.3 per 100,000 females in 2017, with the highest state rates in the East and the lowest in the West and Midwest.
Approximately 40,600 breast cancer deaths are predicted for the year in the United States by the American Cancer Society in its Cancer Facts & Figures 2017, based on 2000-2014 data from the National Center for Health Statistics. With the U.S. population currently around 321 million, that works out to a completely unadjusted death rate of 25.3 per 100,000 women. Doing a little more math puts the highest death rate (29.8) in West Virginia and the lowest (18.8) in Utah.
The incidence rate for female breast cancer was 123.3 per 100,000 for 2009-2013, with that figure age adjusted to the 2000 U.S. standard population, according to data from the North American Association of Central Cancer Registries, the ACS reported.
Wyoming had the lowest incidence over that time period (109.6 per 100,000), and New Hampshire had the highest at 138.1. Utah had the fifth-lowest incidence in that period at 112.7, but West Virginia, the state with the highest estimated mortality for 2017, had a relatively low incidence of 114.4 in 2009-2013, the ACS said.
U.S. breast cancer mortality will be an estimated 25.3 per 100,000 females in 2017, with the highest state rates in the East and the lowest in the West and Midwest.
Approximately 40,600 breast cancer deaths are predicted for the year in the United States by the American Cancer Society in its Cancer Facts & Figures 2017, based on 2000-2014 data from the National Center for Health Statistics. With the U.S. population currently around 321 million, that works out to a completely unadjusted death rate of 25.3 per 100,000 women. Doing a little more math puts the highest death rate (29.8) in West Virginia and the lowest (18.8) in Utah.
The incidence rate for female breast cancer was 123.3 per 100,000 for 2009-2013, with that figure age adjusted to the 2000 U.S. standard population, according to data from the North American Association of Central Cancer Registries, the ACS reported.
Wyoming had the lowest incidence over that time period (109.6 per 100,000), and New Hampshire had the highest at 138.1. Utah had the fifth-lowest incidence in that period at 112.7, but West Virginia, the state with the highest estimated mortality for 2017, had a relatively low incidence of 114.4 in 2009-2013, the ACS said.
ACOG stresses widespread prepregnancy carrier screening
The American College of Obstetricians and Gynecologists is calling on ob.gyns. to establish a standard carrier screening process that is consistently offered to all patients before pregnancy.
This a shift from previous ACOG policy, which recommended carrier screening based mainly on ethnicity.
In a pair of opinions from ACOG’s Committee on Genetics, they highlighted three acceptable screening methods: ethnic-specific screening, panethnic screening, and expanded-carrier screening (Obstet Gynecol. 2017;129:e35-40/Obstet Gynecol. 2017;129:e41-55).
Panethnic and expanded-carrier screening are especially helpful for patients with parents of different ethnic backgrounds or those who do not know their family history, situations that have become more common.
“In reality, over the last 5-7 years, the amount an obstetrician has to counsel patients on carrier screening and prenatal screening has grown immensely,” Dr. Biggio said in an interview. “Trying to find the time to do it and do it well, is a challenge. What is important is all practitioners have a way to approach offering carrier screening in their practice setting.”
While the committee advises crafting a process that fits individual practice needs, there are some general recommendations:
- Test only for diseases with a carrier frequency of 1 in 100 or greater, have a well-defined phenotype, have a detrimental effect on quality of life, cause cognitive or physical impairment, require surgical or medical intervention, or have an onset early in life.
- All patients, regardless of screening strategy and ethnicity, should be checked for cystic fibrosis and spinal muscular atrophy, and also undergo a complete blood count and screening for thalassemias and hemoglobinopathies.
- Prenatal carrier screening does not replace newborn screening, and at the same time, newborn screening does not diminish the potential benefits of prenatal carrier screening.
“Practitioners should be testing patients for these diseases as early as possible,” Dr. Biggio said. “A consistent approach to screening consultation will help with that immensely.”
[email protected]
On Twitter @EAZTweets
The American College of Obstetricians and Gynecologists is calling on ob.gyns. to establish a standard carrier screening process that is consistently offered to all patients before pregnancy.
This a shift from previous ACOG policy, which recommended carrier screening based mainly on ethnicity.
In a pair of opinions from ACOG’s Committee on Genetics, they highlighted three acceptable screening methods: ethnic-specific screening, panethnic screening, and expanded-carrier screening (Obstet Gynecol. 2017;129:e35-40/Obstet Gynecol. 2017;129:e41-55).
Panethnic and expanded-carrier screening are especially helpful for patients with parents of different ethnic backgrounds or those who do not know their family history, situations that have become more common.
“In reality, over the last 5-7 years, the amount an obstetrician has to counsel patients on carrier screening and prenatal screening has grown immensely,” Dr. Biggio said in an interview. “Trying to find the time to do it and do it well, is a challenge. What is important is all practitioners have a way to approach offering carrier screening in their practice setting.”
While the committee advises crafting a process that fits individual practice needs, there are some general recommendations:
- Test only for diseases with a carrier frequency of 1 in 100 or greater, have a well-defined phenotype, have a detrimental effect on quality of life, cause cognitive or physical impairment, require surgical or medical intervention, or have an onset early in life.
- All patients, regardless of screening strategy and ethnicity, should be checked for cystic fibrosis and spinal muscular atrophy, and also undergo a complete blood count and screening for thalassemias and hemoglobinopathies.
- Prenatal carrier screening does not replace newborn screening, and at the same time, newborn screening does not diminish the potential benefits of prenatal carrier screening.
“Practitioners should be testing patients for these diseases as early as possible,” Dr. Biggio said. “A consistent approach to screening consultation will help with that immensely.”
[email protected]
On Twitter @EAZTweets
The American College of Obstetricians and Gynecologists is calling on ob.gyns. to establish a standard carrier screening process that is consistently offered to all patients before pregnancy.
This a shift from previous ACOG policy, which recommended carrier screening based mainly on ethnicity.
In a pair of opinions from ACOG’s Committee on Genetics, they highlighted three acceptable screening methods: ethnic-specific screening, panethnic screening, and expanded-carrier screening (Obstet Gynecol. 2017;129:e35-40/Obstet Gynecol. 2017;129:e41-55).
Panethnic and expanded-carrier screening are especially helpful for patients with parents of different ethnic backgrounds or those who do not know their family history, situations that have become more common.
“In reality, over the last 5-7 years, the amount an obstetrician has to counsel patients on carrier screening and prenatal screening has grown immensely,” Dr. Biggio said in an interview. “Trying to find the time to do it and do it well, is a challenge. What is important is all practitioners have a way to approach offering carrier screening in their practice setting.”
While the committee advises crafting a process that fits individual practice needs, there are some general recommendations:
- Test only for diseases with a carrier frequency of 1 in 100 or greater, have a well-defined phenotype, have a detrimental effect on quality of life, cause cognitive or physical impairment, require surgical or medical intervention, or have an onset early in life.
- All patients, regardless of screening strategy and ethnicity, should be checked for cystic fibrosis and spinal muscular atrophy, and also undergo a complete blood count and screening for thalassemias and hemoglobinopathies.
- Prenatal carrier screening does not replace newborn screening, and at the same time, newborn screening does not diminish the potential benefits of prenatal carrier screening.
“Practitioners should be testing patients for these diseases as early as possible,” Dr. Biggio said. “A consistent approach to screening consultation will help with that immensely.”
[email protected]
On Twitter @EAZTweets
FROM OBSTETRICS & GYNECOLOGY
Postoperative pain in women with preexisting chronic pain
Chronic pain disorders have reached epidemic levels in the United States, with the Institute of Medicine reporting more than 100 million Americans affected and health care costs more than $500 billion annually.1 Although many pain disorders are confined to the abdomen or pelvis (chronic pelvic pain, vulvodynia, irritable bowel syndrome, and bladder pain syndrome), others present with global symptoms (fibromyalgia and chronic fatigue syndrome). Women are more likely to be diagnosed with a chronic pain condition and more likely to seek treatment for chronic pain, including undergoing a surgical intervention. In fact, chronic pelvic pain alone affects upward of 20% of women in the United States, and, of the 400,000 hysterectomies performed each year (54.2%, abdominal; 16.7%, vaginal; and 16.8%, laparoscopic/robotic assisted), approximately 15% are for chronic pain.2
Neurobiology of pain
Perioperative pain control, specifically in women with preexisting pain disorders, can provide an additional challenge. Unlike acute pain, chronic pain (lasting more than 6 months) is associated with an amplified pain response of the central nervous system. This abnormal pain processing, known as centralization of pain, may result in a decrease of the inhibitory pain pathways and/or an increase of the amplification pathways, often augmenting the pain response of the original peripheral insult, specifically surgery. Because of these physiologic changes, a multimodal approach to perioperative pain should be offered, especially in women with preexisting pain. The approach ideally ought to target the different mechanisms of actions in both the peripheral and central nervous systems to provide an overall reduction in pain perception.
Preoperative visit
Perhaps the most underutilized opportunity to optimize postoperative pain is a proactive, preoperative approach. Preoperative education, including goal setting of postoperative pain expectations, has been associated with a significant reduction in postoperative opioid use, less preoperative anxiety, and a decreased length of surgical stay.3 While it is unknown exactly when this should be provided to the patient in the treatment course, it should occur prior to the day of surgery to allow for appropriate intervention.
The use of a shared decision-making model between the clinician and the chronic pain patient in the development of a pain management plan has been highly successful in improving pain outcomes in the outpatient setting.4 A similar method can be applied to the preoperative course as well. A detailed history (including the use of an opioid risk assessment tool) allows the clinician to identify patients at risk for opioid misuse and abuse. This is also an opportunity to review a plan for an opioid taper with the patient and the prescriber, if the postoperative plan includes opioid reduction/cessation. The preoperative visit may be an opportunity to adjust centrally acting medications (antidepressants, anticonvulsants) before surgery or to reduce the dose or frequency of high-risk medications, such as benzodiazepines.
Perioperative strategy
One of the most impactful ways for us, as surgeons, to reduce tissue injury and decrease pain from surgery is by offering a minimally invasive approach. The benefits of minimally invasive surgery are well established, resulting in improved perioperative pain control, decreased blood loss, lower infection rates, decreased length of hospital stay, and a faster recovery, compared with laparotomy. Because patients with chronic pain disorders are at increased risk of greater acute postoperative pain and have an elevated risk for the development of chronic postsurgical pain, a minimally invasive surgical approach should be prioritized, when available.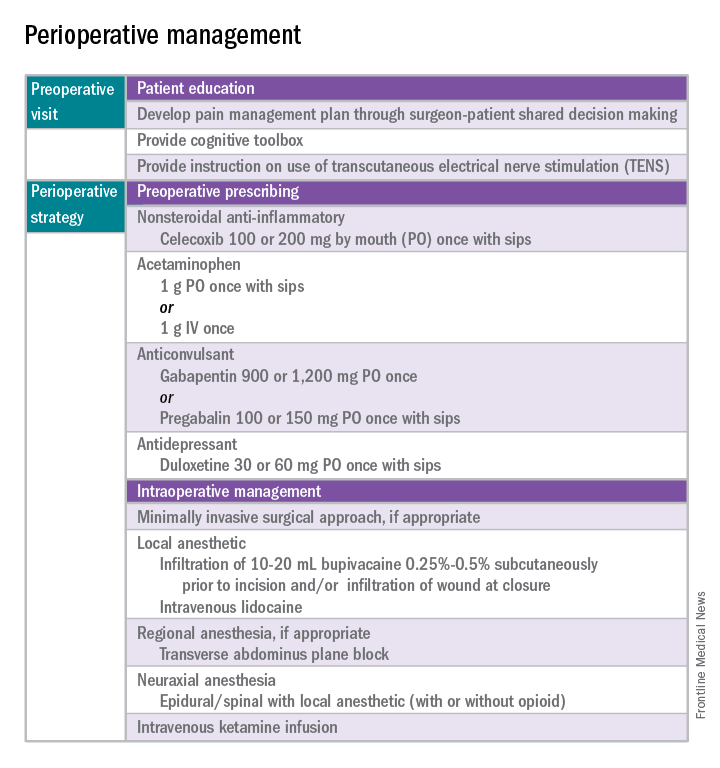
Perioperative multimodal drug therapy is associated with significant decreases in opioid consumption and reductions in acute postoperative pain.6 Recently, a multidisciplinary expert panel from the American Pain Society devised an evidence-based clinical practice guideline for postoperative pain.7 While there is no consensus as to the best regimen specific to gynecologic surgery, the general principles are similar across disciplines.
The postoperative period
Opioid-tolerant patients may experience greater pain during the first 24 hours postoperatively and require an increase in opioids, compared with opioid-naive patients.8 In the event that a postoperative patient does not respond as expected to the usual course, that patient should be evaluated for barriers to routine postoperative care, such as a surgical complication, opioid tolerance, or psychological distress. Surgeons should be aggressive with pain management immediately after surgery, even in the opioid-tolerant patient, and make short-term adjustments as needed based on the pain response. These patients will require pain medications beyond their baseline dose. Additionally, if an opioid taper is not planned in a chronic opioid user, work with the patient and the long-term opioid prescriber in restarting baseline opioid therapy outside of the acute surgical window. 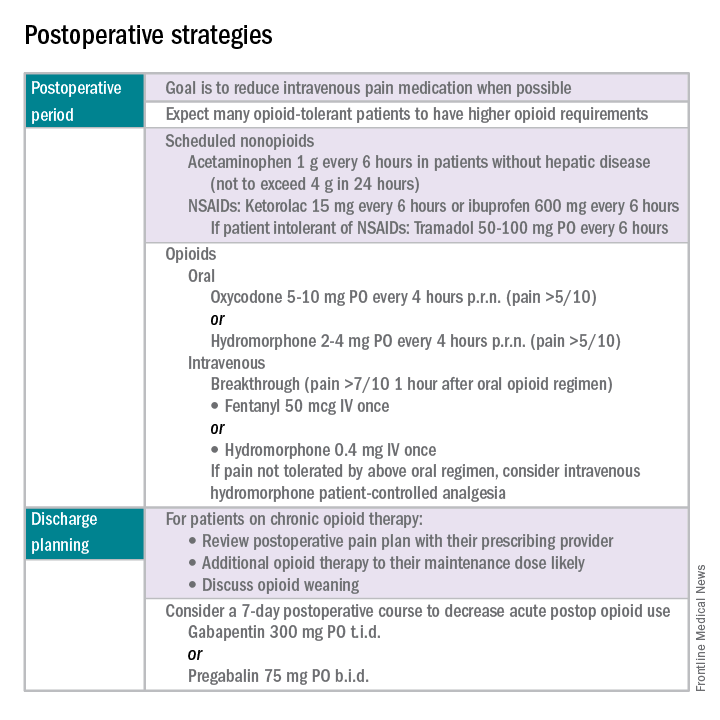
References
1. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press, 2011.
2. Obstet Gynecol. 2013 Aug;122(2 Pt 1):233-41.
3. N Engl J Med. 1964 Apr 16;270:825-7.
4. J Pain Symptom Manage. 1999 Jul;18(1):38-48.
5. Pain. 2010 Dec;151(3):694-702.
6. Anesthesiology. 2005 Dec;103(6):1296-304.
7. J Pain. 2016 Feb;17(2):131-57.
8. Pharmacotherapy. 2008 Dec;28(12):1453-60.
Dr. Carey is the director of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, and specializes in the medical and surgical management of pelvic pain disorders. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC–Chapel Hill. They reported having no relevant financial disclosures.
Chronic pain disorders have reached epidemic levels in the United States, with the Institute of Medicine reporting more than 100 million Americans affected and health care costs more than $500 billion annually.1 Although many pain disorders are confined to the abdomen or pelvis (chronic pelvic pain, vulvodynia, irritable bowel syndrome, and bladder pain syndrome), others present with global symptoms (fibromyalgia and chronic fatigue syndrome). Women are more likely to be diagnosed with a chronic pain condition and more likely to seek treatment for chronic pain, including undergoing a surgical intervention. In fact, chronic pelvic pain alone affects upward of 20% of women in the United States, and, of the 400,000 hysterectomies performed each year (54.2%, abdominal; 16.7%, vaginal; and 16.8%, laparoscopic/robotic assisted), approximately 15% are for chronic pain.2
Neurobiology of pain
Perioperative pain control, specifically in women with preexisting pain disorders, can provide an additional challenge. Unlike acute pain, chronic pain (lasting more than 6 months) is associated with an amplified pain response of the central nervous system. This abnormal pain processing, known as centralization of pain, may result in a decrease of the inhibitory pain pathways and/or an increase of the amplification pathways, often augmenting the pain response of the original peripheral insult, specifically surgery. Because of these physiologic changes, a multimodal approach to perioperative pain should be offered, especially in women with preexisting pain. The approach ideally ought to target the different mechanisms of actions in both the peripheral and central nervous systems to provide an overall reduction in pain perception.
Preoperative visit
Perhaps the most underutilized opportunity to optimize postoperative pain is a proactive, preoperative approach. Preoperative education, including goal setting of postoperative pain expectations, has been associated with a significant reduction in postoperative opioid use, less preoperative anxiety, and a decreased length of surgical stay.3 While it is unknown exactly when this should be provided to the patient in the treatment course, it should occur prior to the day of surgery to allow for appropriate intervention.
The use of a shared decision-making model between the clinician and the chronic pain patient in the development of a pain management plan has been highly successful in improving pain outcomes in the outpatient setting.4 A similar method can be applied to the preoperative course as well. A detailed history (including the use of an opioid risk assessment tool) allows the clinician to identify patients at risk for opioid misuse and abuse. This is also an opportunity to review a plan for an opioid taper with the patient and the prescriber, if the postoperative plan includes opioid reduction/cessation. The preoperative visit may be an opportunity to adjust centrally acting medications (antidepressants, anticonvulsants) before surgery or to reduce the dose or frequency of high-risk medications, such as benzodiazepines.
Perioperative strategy
One of the most impactful ways for us, as surgeons, to reduce tissue injury and decrease pain from surgery is by offering a minimally invasive approach. The benefits of minimally invasive surgery are well established, resulting in improved perioperative pain control, decreased blood loss, lower infection rates, decreased length of hospital stay, and a faster recovery, compared with laparotomy. Because patients with chronic pain disorders are at increased risk of greater acute postoperative pain and have an elevated risk for the development of chronic postsurgical pain, a minimally invasive surgical approach should be prioritized, when available.
Perioperative multimodal drug therapy is associated with significant decreases in opioid consumption and reductions in acute postoperative pain.6 Recently, a multidisciplinary expert panel from the American Pain Society devised an evidence-based clinical practice guideline for postoperative pain.7 While there is no consensus as to the best regimen specific to gynecologic surgery, the general principles are similar across disciplines.
The postoperative period
Opioid-tolerant patients may experience greater pain during the first 24 hours postoperatively and require an increase in opioids, compared with opioid-naive patients.8 In the event that a postoperative patient does not respond as expected to the usual course, that patient should be evaluated for barriers to routine postoperative care, such as a surgical complication, opioid tolerance, or psychological distress. Surgeons should be aggressive with pain management immediately after surgery, even in the opioid-tolerant patient, and make short-term adjustments as needed based on the pain response. These patients will require pain medications beyond their baseline dose. Additionally, if an opioid taper is not planned in a chronic opioid user, work with the patient and the long-term opioid prescriber in restarting baseline opioid therapy outside of the acute surgical window. 
References
1. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press, 2011.
2. Obstet Gynecol. 2013 Aug;122(2 Pt 1):233-41.
3. N Engl J Med. 1964 Apr 16;270:825-7.
4. J Pain Symptom Manage. 1999 Jul;18(1):38-48.
5. Pain. 2010 Dec;151(3):694-702.
6. Anesthesiology. 2005 Dec;103(6):1296-304.
7. J Pain. 2016 Feb;17(2):131-57.
8. Pharmacotherapy. 2008 Dec;28(12):1453-60.
Dr. Carey is the director of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, and specializes in the medical and surgical management of pelvic pain disorders. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC–Chapel Hill. They reported having no relevant financial disclosures.
Chronic pain disorders have reached epidemic levels in the United States, with the Institute of Medicine reporting more than 100 million Americans affected and health care costs more than $500 billion annually.1 Although many pain disorders are confined to the abdomen or pelvis (chronic pelvic pain, vulvodynia, irritable bowel syndrome, and bladder pain syndrome), others present with global symptoms (fibromyalgia and chronic fatigue syndrome). Women are more likely to be diagnosed with a chronic pain condition and more likely to seek treatment for chronic pain, including undergoing a surgical intervention. In fact, chronic pelvic pain alone affects upward of 20% of women in the United States, and, of the 400,000 hysterectomies performed each year (54.2%, abdominal; 16.7%, vaginal; and 16.8%, laparoscopic/robotic assisted), approximately 15% are for chronic pain.2
Neurobiology of pain
Perioperative pain control, specifically in women with preexisting pain disorders, can provide an additional challenge. Unlike acute pain, chronic pain (lasting more than 6 months) is associated with an amplified pain response of the central nervous system. This abnormal pain processing, known as centralization of pain, may result in a decrease of the inhibitory pain pathways and/or an increase of the amplification pathways, often augmenting the pain response of the original peripheral insult, specifically surgery. Because of these physiologic changes, a multimodal approach to perioperative pain should be offered, especially in women with preexisting pain. The approach ideally ought to target the different mechanisms of actions in both the peripheral and central nervous systems to provide an overall reduction in pain perception.
Preoperative visit
Perhaps the most underutilized opportunity to optimize postoperative pain is a proactive, preoperative approach. Preoperative education, including goal setting of postoperative pain expectations, has been associated with a significant reduction in postoperative opioid use, less preoperative anxiety, and a decreased length of surgical stay.3 While it is unknown exactly when this should be provided to the patient in the treatment course, it should occur prior to the day of surgery to allow for appropriate intervention.
The use of a shared decision-making model between the clinician and the chronic pain patient in the development of a pain management plan has been highly successful in improving pain outcomes in the outpatient setting.4 A similar method can be applied to the preoperative course as well. A detailed history (including the use of an opioid risk assessment tool) allows the clinician to identify patients at risk for opioid misuse and abuse. This is also an opportunity to review a plan for an opioid taper with the patient and the prescriber, if the postoperative plan includes opioid reduction/cessation. The preoperative visit may be an opportunity to adjust centrally acting medications (antidepressants, anticonvulsants) before surgery or to reduce the dose or frequency of high-risk medications, such as benzodiazepines.
Perioperative strategy
One of the most impactful ways for us, as surgeons, to reduce tissue injury and decrease pain from surgery is by offering a minimally invasive approach. The benefits of minimally invasive surgery are well established, resulting in improved perioperative pain control, decreased blood loss, lower infection rates, decreased length of hospital stay, and a faster recovery, compared with laparotomy. Because patients with chronic pain disorders are at increased risk of greater acute postoperative pain and have an elevated risk for the development of chronic postsurgical pain, a minimally invasive surgical approach should be prioritized, when available.
Perioperative multimodal drug therapy is associated with significant decreases in opioid consumption and reductions in acute postoperative pain.6 Recently, a multidisciplinary expert panel from the American Pain Society devised an evidence-based clinical practice guideline for postoperative pain.7 While there is no consensus as to the best regimen specific to gynecologic surgery, the general principles are similar across disciplines.
The postoperative period
Opioid-tolerant patients may experience greater pain during the first 24 hours postoperatively and require an increase in opioids, compared with opioid-naive patients.8 In the event that a postoperative patient does not respond as expected to the usual course, that patient should be evaluated for barriers to routine postoperative care, such as a surgical complication, opioid tolerance, or psychological distress. Surgeons should be aggressive with pain management immediately after surgery, even in the opioid-tolerant patient, and make short-term adjustments as needed based on the pain response. These patients will require pain medications beyond their baseline dose. Additionally, if an opioid taper is not planned in a chronic opioid user, work with the patient and the long-term opioid prescriber in restarting baseline opioid therapy outside of the acute surgical window. 
References
1. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press, 2011.
2. Obstet Gynecol. 2013 Aug;122(2 Pt 1):233-41.
3. N Engl J Med. 1964 Apr 16;270:825-7.
4. J Pain Symptom Manage. 1999 Jul;18(1):38-48.
5. Pain. 2010 Dec;151(3):694-702.
6. Anesthesiology. 2005 Dec;103(6):1296-304.
7. J Pain. 2016 Feb;17(2):131-57.
8. Pharmacotherapy. 2008 Dec;28(12):1453-60.
Dr. Carey is the director of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, and specializes in the medical and surgical management of pelvic pain disorders. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC–Chapel Hill. They reported having no relevant financial disclosures.
Treating gonococcal infections
Editor’s Note: This is the third installment of a six-part monthly series that will review key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte Inc. The series will cover issues in reproductive endocrinology and infertility, maternal-fetal medicine, gynecologic oncology, and female pelvic medicine, as well as general test-taking and study tips.
Management of sexually transmitted infections will always be tested on any ob.gyn. examination. Since the American College of Obstetricians and Gynecologists issued two new committee opinions in 2015 focusing on the dual treatment for gonococcal infections and expedited partner therapy in the management of gonorrhea and chlamydial infection, it is wise to review this topic.1,2
A. Ciprofloxacin
B. Ciprofloxacin plus azithromycin
C. Cefixime plus azithromycin
D. Doxycycline plus azithromycin
E. Gentamicin plus azithromycin
The correct answer is E.
Dual therapy with gentamicin (240 mg single-intramuscular dose) and azithromycin (2 grams single-oral dose) is the treatment of choice for pregnant patients with severe penicillin or cephalosporin allergy. Gentamicin is safe during pregnancy. An alternative regimen is a single dose of azithromycin, if the patient is allergic to gentamicin and cephalosporin. For this patient, the physician needs to perform a test-of-cure 1 week after treatment.
Answer A is incorrect because neither doxycycline nor quinolones are recommended during pregnancy.
Answer B and C are incorrect because the patient is allergic to cephalosporin (ceftriaxone and cefixime).
Answer D in incorrect because neither doxycycline nor quinolones are recommended during pregnancy.
Key points
The key points to remember are:
- Ceftriaxone and azithromycin must be started on the same day.
- In patients with HIV, the treatment is same: Ceftriaxone 250-mg single-intramuscular dose plus azithromycin 1-g single-oral dose.
- The Centers for Disease Control and Prevention and state health departments will periodically update the most current information on gonococcal susceptibility.
- Despite nucleic acid amplification test (NAAT) being negative for Chlamydia trachomatis, dual treatment with a cephalosporin plus either azithromycin or doxycycline is recommended by ACOG to prevent resistance to cephalosporins.
- ACOG does not recommend test-of-cure after treatment of uncomplicated gonorrhea.
- ACOG does not recommend test-of-cure after treatment of pregnant patients.
- ACOG recommends test-of-cure for pharyngeal gonorrhea only if the patient received an alternative regimen treatment after 14 days.
- ACOG accepts both culture or NAAT as a test-of-cure.
- Cefixime is not a first-line regimen treatment for gonorrhea and dual therapy with ceftriaxone and azithromycin is the only recommended first-line regimen.
Literature summary
The guidelines from the ACOG Committee on Gynecologic Practice outline recommended antibiotic treatment regimens, considerations when treating special populations, and advice for expedited therapy for sexual partners.
- The first-line regimen is ceftriaxone 250-mg, single-intramuscular dose plus azithromycin 1-g, single-oral dose.
- The second-line regimen takes into account allergies and shortages. In the case of a severe penicillin allergy, use gemifloxacin 320-mg, single-oral dose plus azithromycin 2-g, single-oral dose. Another option is gentamicin, 240-mg, single-intramuscular dose plus azithromycin 2-g, single-oral dose. When ceftriaxone is not available, ACOG recommends cefixime, 400-mg, single-oral dose plus azithromycin 1-g, single-oral dose.
- In pregnancy, ACOG recommends the same treatment, with no need for test-of-cure if treated properly. For severe penicillin or cephalosporin allergy in pregnancy, use gentamicin 240-mg, single-intramuscular dose plus azithromycin 2-g, single-oral dose. For allergy to gentamicin and cephalosporins, use azithromycin 2-g single-oral dose and send a test-of-cure in 1 week. Do not use doxycycline or quinolones during pregnancy.
- In HIV infection, use the general recommended treatment regimen and there is no need for a test-of-cure if treated properly.
- For sex partners, ACOG recommends evaluation and treatment for partners in the last 60 days. They should abstain from sex for 7 days after treatment. Expedited partner therapy with cefixime and azithromycin is recommended, but review the legal the status of expedited partner therapy in your state before prescribing.
References
1. Obstet Gynecol. 2015 Nov;126(5):e95-9.
2. Obstet Gynecol. 2015 Jun;125(6):1526-8.
Dr. Siddighi is editor-in-chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health in California. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.
Editor’s Note: This is the third installment of a six-part monthly series that will review key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte Inc. The series will cover issues in reproductive endocrinology and infertility, maternal-fetal medicine, gynecologic oncology, and female pelvic medicine, as well as general test-taking and study tips.
Management of sexually transmitted infections will always be tested on any ob.gyn. examination. Since the American College of Obstetricians and Gynecologists issued two new committee opinions in 2015 focusing on the dual treatment for gonococcal infections and expedited partner therapy in the management of gonorrhea and chlamydial infection, it is wise to review this topic.1,2
A. Ciprofloxacin
B. Ciprofloxacin plus azithromycin
C. Cefixime plus azithromycin
D. Doxycycline plus azithromycin
E. Gentamicin plus azithromycin
The correct answer is E.
Dual therapy with gentamicin (240 mg single-intramuscular dose) and azithromycin (2 grams single-oral dose) is the treatment of choice for pregnant patients with severe penicillin or cephalosporin allergy. Gentamicin is safe during pregnancy. An alternative regimen is a single dose of azithromycin, if the patient is allergic to gentamicin and cephalosporin. For this patient, the physician needs to perform a test-of-cure 1 week after treatment.
Answer A is incorrect because neither doxycycline nor quinolones are recommended during pregnancy.
Answer B and C are incorrect because the patient is allergic to cephalosporin (ceftriaxone and cefixime).
Answer D in incorrect because neither doxycycline nor quinolones are recommended during pregnancy.
Key points
The key points to remember are:
- Ceftriaxone and azithromycin must be started on the same day.
- In patients with HIV, the treatment is same: Ceftriaxone 250-mg single-intramuscular dose plus azithromycin 1-g single-oral dose.
- The Centers for Disease Control and Prevention and state health departments will periodically update the most current information on gonococcal susceptibility.
- Despite nucleic acid amplification test (NAAT) being negative for Chlamydia trachomatis, dual treatment with a cephalosporin plus either azithromycin or doxycycline is recommended by ACOG to prevent resistance to cephalosporins.
- ACOG does not recommend test-of-cure after treatment of uncomplicated gonorrhea.
- ACOG does not recommend test-of-cure after treatment of pregnant patients.
- ACOG recommends test-of-cure for pharyngeal gonorrhea only if the patient received an alternative regimen treatment after 14 days.
- ACOG accepts both culture or NAAT as a test-of-cure.
- Cefixime is not a first-line regimen treatment for gonorrhea and dual therapy with ceftriaxone and azithromycin is the only recommended first-line regimen.
Literature summary
The guidelines from the ACOG Committee on Gynecologic Practice outline recommended antibiotic treatment regimens, considerations when treating special populations, and advice for expedited therapy for sexual partners.
- The first-line regimen is ceftriaxone 250-mg, single-intramuscular dose plus azithromycin 1-g, single-oral dose.
- The second-line regimen takes into account allergies and shortages. In the case of a severe penicillin allergy, use gemifloxacin 320-mg, single-oral dose plus azithromycin 2-g, single-oral dose. Another option is gentamicin, 240-mg, single-intramuscular dose plus azithromycin 2-g, single-oral dose. When ceftriaxone is not available, ACOG recommends cefixime, 400-mg, single-oral dose plus azithromycin 1-g, single-oral dose.
- In pregnancy, ACOG recommends the same treatment, with no need for test-of-cure if treated properly. For severe penicillin or cephalosporin allergy in pregnancy, use gentamicin 240-mg, single-intramuscular dose plus azithromycin 2-g, single-oral dose. For allergy to gentamicin and cephalosporins, use azithromycin 2-g single-oral dose and send a test-of-cure in 1 week. Do not use doxycycline or quinolones during pregnancy.
- In HIV infection, use the general recommended treatment regimen and there is no need for a test-of-cure if treated properly.
- For sex partners, ACOG recommends evaluation and treatment for partners in the last 60 days. They should abstain from sex for 7 days after treatment. Expedited partner therapy with cefixime and azithromycin is recommended, but review the legal the status of expedited partner therapy in your state before prescribing.
References
1. Obstet Gynecol. 2015 Nov;126(5):e95-9.
2. Obstet Gynecol. 2015 Jun;125(6):1526-8.
Dr. Siddighi is editor-in-chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health in California. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.
Editor’s Note: This is the third installment of a six-part monthly series that will review key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte Inc. The series will cover issues in reproductive endocrinology and infertility, maternal-fetal medicine, gynecologic oncology, and female pelvic medicine, as well as general test-taking and study tips.
Management of sexually transmitted infections will always be tested on any ob.gyn. examination. Since the American College of Obstetricians and Gynecologists issued two new committee opinions in 2015 focusing on the dual treatment for gonococcal infections and expedited partner therapy in the management of gonorrhea and chlamydial infection, it is wise to review this topic.1,2
A. Ciprofloxacin
B. Ciprofloxacin plus azithromycin
C. Cefixime plus azithromycin
D. Doxycycline plus azithromycin
E. Gentamicin plus azithromycin
The correct answer is E.
Dual therapy with gentamicin (240 mg single-intramuscular dose) and azithromycin (2 grams single-oral dose) is the treatment of choice for pregnant patients with severe penicillin or cephalosporin allergy. Gentamicin is safe during pregnancy. An alternative regimen is a single dose of azithromycin, if the patient is allergic to gentamicin and cephalosporin. For this patient, the physician needs to perform a test-of-cure 1 week after treatment.
Answer A is incorrect because neither doxycycline nor quinolones are recommended during pregnancy.
Answer B and C are incorrect because the patient is allergic to cephalosporin (ceftriaxone and cefixime).
Answer D in incorrect because neither doxycycline nor quinolones are recommended during pregnancy.
Key points
The key points to remember are:
- Ceftriaxone and azithromycin must be started on the same day.
- In patients with HIV, the treatment is same: Ceftriaxone 250-mg single-intramuscular dose plus azithromycin 1-g single-oral dose.
- The Centers for Disease Control and Prevention and state health departments will periodically update the most current information on gonococcal susceptibility.
- Despite nucleic acid amplification test (NAAT) being negative for Chlamydia trachomatis, dual treatment with a cephalosporin plus either azithromycin or doxycycline is recommended by ACOG to prevent resistance to cephalosporins.
- ACOG does not recommend test-of-cure after treatment of uncomplicated gonorrhea.
- ACOG does not recommend test-of-cure after treatment of pregnant patients.
- ACOG recommends test-of-cure for pharyngeal gonorrhea only if the patient received an alternative regimen treatment after 14 days.
- ACOG accepts both culture or NAAT as a test-of-cure.
- Cefixime is not a first-line regimen treatment for gonorrhea and dual therapy with ceftriaxone and azithromycin is the only recommended first-line regimen.
Literature summary
The guidelines from the ACOG Committee on Gynecologic Practice outline recommended antibiotic treatment regimens, considerations when treating special populations, and advice for expedited therapy for sexual partners.
- The first-line regimen is ceftriaxone 250-mg, single-intramuscular dose plus azithromycin 1-g, single-oral dose.
- The second-line regimen takes into account allergies and shortages. In the case of a severe penicillin allergy, use gemifloxacin 320-mg, single-oral dose plus azithromycin 2-g, single-oral dose. Another option is gentamicin, 240-mg, single-intramuscular dose plus azithromycin 2-g, single-oral dose. When ceftriaxone is not available, ACOG recommends cefixime, 400-mg, single-oral dose plus azithromycin 1-g, single-oral dose.
- In pregnancy, ACOG recommends the same treatment, with no need for test-of-cure if treated properly. For severe penicillin or cephalosporin allergy in pregnancy, use gentamicin 240-mg, single-intramuscular dose plus azithromycin 2-g, single-oral dose. For allergy to gentamicin and cephalosporins, use azithromycin 2-g single-oral dose and send a test-of-cure in 1 week. Do not use doxycycline or quinolones during pregnancy.
- In HIV infection, use the general recommended treatment regimen and there is no need for a test-of-cure if treated properly.
- For sex partners, ACOG recommends evaluation and treatment for partners in the last 60 days. They should abstain from sex for 7 days after treatment. Expedited partner therapy with cefixime and azithromycin is recommended, but review the legal the status of expedited partner therapy in your state before prescribing.
References
1. Obstet Gynecol. 2015 Nov;126(5):e95-9.
2. Obstet Gynecol. 2015 Jun;125(6):1526-8.
Dr. Siddighi is editor-in-chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health in California. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.
Perinatal depression screening is just the start
Over the last decade, appreciation of the prevalence of perinatal depression – depression during pregnancy and/or the postpartum period – along with interest and willingness to diagnose and to treat these disorders across primary care, obstetric, and psychiatric clinical settings – has grown.
The passage of the Affordable Care Act in 2010 included the Melanie Blocker Stokes MOTHERS Act, which provides federal funding for programs to enhance awareness of postpartum depression and conduct research into its causes and treatment. At the same time, there has been increasing destigmatization associated with perinatal mood and anxiety disorders across many communities, and enhanced knowledge among clinicians and the public regarding evidence-based treatments, which mitigate suffering from untreated perinatal psychiatric illness.
The importance of identification of perinatal depression cannot be overestimated given the impact of untreated perinatal mood and anxiety disorders on women and families. Unfortunately, data describing the outcomes of these screening initiatives have been profoundly lacking.
There are many unanswered questions. What proportion of women get screened from state to state? What are the obstacles to screening across different sociodemographic populations? If screened, what proportion of women are referred for treatment and receive appropriate treatment? Of those who receive treatment, how many recover emotional well-being? These are all critically relevant questions and one has to wonder if they would be the same from other nonpsychiatric disease states. For example, would one screen for HIV or cervical cancer and not know the number of women who screened positive but failed to go on to receive referral or frank treatment?
This knowledge gap with respect to outcome of screening for perinatal depression was highlighted in one of the few studies that addresses this specific question. Published in 2016, the systematic review describes the so-called “perinatal depression treatment cascade” – the cumulative shortfalls in clinical recognition, initiation of treatment, adequacy of treatment, and treatment response among women with either depression during pregnancy or postpartum depression (J Clin Psychiatry. 2016 Sep;77[9]:1189-1200).
The investigators included 32 studies where they were able to look specifically at this question of what happens to women who are identified as having either antenatal depression or postpartum depression. In total, six studies examined the rate of treatment of women who had been diagnosed with antenatal depression, resulting in a weighted mean treatment rate of 13.6%. For women identified as having postpartum depression, four studies examined showed a weighted mean treatment rate of 15.8%. What that means is that even if we have a sensitive and specific screening tool and we look only at women who have screened positive, we still have just 14% and 16% of women receiving treatment of any kind.
Drilling down to the issue of treatment adequacy – defined in the review as at least 6 weeks of daily use of antidepressants or at least 6 weeks of psychotherapy – the picture is unfortunately worse. Among the entire population of women with diagnosed antenatal depression, 8.6% received an adequate trial of treatment. Similarly, 6.3% of women with diagnosed postpartum depression received an adequate trial of treatment.
Continuing down the treatment cascade, remission rates also were extremely low. The overall weighted mean remission rate – reflecting the percentage of women who actually ended up getting well – was just 4.8% for women with antenatal depression and 3.2% for women with postpartum depression. These are striking, although perhaps not surprising, data. It suggests, at least in part, the fundamental absence of adequate referral networks and systems for follow-up for those women who suffer from perinatal depression.
It is well established that postpartum depression is the most common complication in modern obstetrics. The data presented in this paper suggest that most women identified with perinatal depressive illness are not getting well. Assuming a prevalence of 10% for antenatal depression and 13% for postpartum depression, there are about 657,000 women with antenatal depression and about 550,000 women with postpartum depression in the United States. If this review is correct, more than 31,000 women with antenatal depression and almost 18,000 women with postpartum depression achieved remission. That leaves more than 600,000 women with undermanaged depression in pregnancy and more than 500,000 women with incompletely treated postpartum depression.
This is a wake-up call to consider a refocusing of effort. The importance of identification of women suffering from postpartum depression is clear and intuitive. We should certainly not abandon screening, but perhaps there has been an overemphasis on identification and incomplete attention to ensuring that referral networks and opportunities for clinical follow-up are in place following positive screening. There also has been inadequate focus on the obstacles to getting women in to see clinicians and getting those clinicians up to speed on the evidence base that supports treatment, both pharmacologic or nonpharmacologic.
Right now, we don’t even know for sure what obstacles exist to referral and treatment. Surveys of community clinicians suggest that collaborative care in managing reproductive-age women or pregnant and postpartum women has not evolved to the point where we have a clear, user-friendly system for getting patients referred and treated. In Massachusetts, where I practice, we have a state-funded effort (MCPAP [Massachusetts Child Psychiatry Access Program] for Moms) to train colleagues in obstetrics about how to identify and treat perinatal depression; perinatal psychiatrists also are available to consult with community-based clinicians. However, we do not have data to tell us if these efforts and the resources used to support them have yielded improvement in the overall symptom burden associated with perinatal mood disorders.
The bottom line is that even after identification of perinatal depression through screening programs, we still have women suffering in silence. It is so easy to get on the bandwagon regarding screening, but it seems even more challenging to design the systems that will accommodate the volume of women who are being identified. The fact that we do not have parallel efforts focusing on getting these women referred and treated, and a system to monitor improvement, conjures the image of setting off to sail without checking whether the boat is equipped with life preservers.
Dr. Cohen is the director of the Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications.
Over the last decade, appreciation of the prevalence of perinatal depression – depression during pregnancy and/or the postpartum period – along with interest and willingness to diagnose and to treat these disorders across primary care, obstetric, and psychiatric clinical settings – has grown.
The passage of the Affordable Care Act in 2010 included the Melanie Blocker Stokes MOTHERS Act, which provides federal funding for programs to enhance awareness of postpartum depression and conduct research into its causes and treatment. At the same time, there has been increasing destigmatization associated with perinatal mood and anxiety disorders across many communities, and enhanced knowledge among clinicians and the public regarding evidence-based treatments, which mitigate suffering from untreated perinatal psychiatric illness.
The importance of identification of perinatal depression cannot be overestimated given the impact of untreated perinatal mood and anxiety disorders on women and families. Unfortunately, data describing the outcomes of these screening initiatives have been profoundly lacking.
There are many unanswered questions. What proportion of women get screened from state to state? What are the obstacles to screening across different sociodemographic populations? If screened, what proportion of women are referred for treatment and receive appropriate treatment? Of those who receive treatment, how many recover emotional well-being? These are all critically relevant questions and one has to wonder if they would be the same from other nonpsychiatric disease states. For example, would one screen for HIV or cervical cancer and not know the number of women who screened positive but failed to go on to receive referral or frank treatment?
This knowledge gap with respect to outcome of screening for perinatal depression was highlighted in one of the few studies that addresses this specific question. Published in 2016, the systematic review describes the so-called “perinatal depression treatment cascade” – the cumulative shortfalls in clinical recognition, initiation of treatment, adequacy of treatment, and treatment response among women with either depression during pregnancy or postpartum depression (J Clin Psychiatry. 2016 Sep;77[9]:1189-1200).
The investigators included 32 studies where they were able to look specifically at this question of what happens to women who are identified as having either antenatal depression or postpartum depression. In total, six studies examined the rate of treatment of women who had been diagnosed with antenatal depression, resulting in a weighted mean treatment rate of 13.6%. For women identified as having postpartum depression, four studies examined showed a weighted mean treatment rate of 15.8%. What that means is that even if we have a sensitive and specific screening tool and we look only at women who have screened positive, we still have just 14% and 16% of women receiving treatment of any kind.
Drilling down to the issue of treatment adequacy – defined in the review as at least 6 weeks of daily use of antidepressants or at least 6 weeks of psychotherapy – the picture is unfortunately worse. Among the entire population of women with diagnosed antenatal depression, 8.6% received an adequate trial of treatment. Similarly, 6.3% of women with diagnosed postpartum depression received an adequate trial of treatment.
Continuing down the treatment cascade, remission rates also were extremely low. The overall weighted mean remission rate – reflecting the percentage of women who actually ended up getting well – was just 4.8% for women with antenatal depression and 3.2% for women with postpartum depression. These are striking, although perhaps not surprising, data. It suggests, at least in part, the fundamental absence of adequate referral networks and systems for follow-up for those women who suffer from perinatal depression.
It is well established that postpartum depression is the most common complication in modern obstetrics. The data presented in this paper suggest that most women identified with perinatal depressive illness are not getting well. Assuming a prevalence of 10% for antenatal depression and 13% for postpartum depression, there are about 657,000 women with antenatal depression and about 550,000 women with postpartum depression in the United States. If this review is correct, more than 31,000 women with antenatal depression and almost 18,000 women with postpartum depression achieved remission. That leaves more than 600,000 women with undermanaged depression in pregnancy and more than 500,000 women with incompletely treated postpartum depression.
This is a wake-up call to consider a refocusing of effort. The importance of identification of women suffering from postpartum depression is clear and intuitive. We should certainly not abandon screening, but perhaps there has been an overemphasis on identification and incomplete attention to ensuring that referral networks and opportunities for clinical follow-up are in place following positive screening. There also has been inadequate focus on the obstacles to getting women in to see clinicians and getting those clinicians up to speed on the evidence base that supports treatment, both pharmacologic or nonpharmacologic.
Right now, we don’t even know for sure what obstacles exist to referral and treatment. Surveys of community clinicians suggest that collaborative care in managing reproductive-age women or pregnant and postpartum women has not evolved to the point where we have a clear, user-friendly system for getting patients referred and treated. In Massachusetts, where I practice, we have a state-funded effort (MCPAP [Massachusetts Child Psychiatry Access Program] for Moms) to train colleagues in obstetrics about how to identify and treat perinatal depression; perinatal psychiatrists also are available to consult with community-based clinicians. However, we do not have data to tell us if these efforts and the resources used to support them have yielded improvement in the overall symptom burden associated with perinatal mood disorders.
The bottom line is that even after identification of perinatal depression through screening programs, we still have women suffering in silence. It is so easy to get on the bandwagon regarding screening, but it seems even more challenging to design the systems that will accommodate the volume of women who are being identified. The fact that we do not have parallel efforts focusing on getting these women referred and treated, and a system to monitor improvement, conjures the image of setting off to sail without checking whether the boat is equipped with life preservers.
Dr. Cohen is the director of the Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications.
Over the last decade, appreciation of the prevalence of perinatal depression – depression during pregnancy and/or the postpartum period – along with interest and willingness to diagnose and to treat these disorders across primary care, obstetric, and psychiatric clinical settings – has grown.
The passage of the Affordable Care Act in 2010 included the Melanie Blocker Stokes MOTHERS Act, which provides federal funding for programs to enhance awareness of postpartum depression and conduct research into its causes and treatment. At the same time, there has been increasing destigmatization associated with perinatal mood and anxiety disorders across many communities, and enhanced knowledge among clinicians and the public regarding evidence-based treatments, which mitigate suffering from untreated perinatal psychiatric illness.
The importance of identification of perinatal depression cannot be overestimated given the impact of untreated perinatal mood and anxiety disorders on women and families. Unfortunately, data describing the outcomes of these screening initiatives have been profoundly lacking.
There are many unanswered questions. What proportion of women get screened from state to state? What are the obstacles to screening across different sociodemographic populations? If screened, what proportion of women are referred for treatment and receive appropriate treatment? Of those who receive treatment, how many recover emotional well-being? These are all critically relevant questions and one has to wonder if they would be the same from other nonpsychiatric disease states. For example, would one screen for HIV or cervical cancer and not know the number of women who screened positive but failed to go on to receive referral or frank treatment?
This knowledge gap with respect to outcome of screening for perinatal depression was highlighted in one of the few studies that addresses this specific question. Published in 2016, the systematic review describes the so-called “perinatal depression treatment cascade” – the cumulative shortfalls in clinical recognition, initiation of treatment, adequacy of treatment, and treatment response among women with either depression during pregnancy or postpartum depression (J Clin Psychiatry. 2016 Sep;77[9]:1189-1200).
The investigators included 32 studies where they were able to look specifically at this question of what happens to women who are identified as having either antenatal depression or postpartum depression. In total, six studies examined the rate of treatment of women who had been diagnosed with antenatal depression, resulting in a weighted mean treatment rate of 13.6%. For women identified as having postpartum depression, four studies examined showed a weighted mean treatment rate of 15.8%. What that means is that even if we have a sensitive and specific screening tool and we look only at women who have screened positive, we still have just 14% and 16% of women receiving treatment of any kind.
Drilling down to the issue of treatment adequacy – defined in the review as at least 6 weeks of daily use of antidepressants or at least 6 weeks of psychotherapy – the picture is unfortunately worse. Among the entire population of women with diagnosed antenatal depression, 8.6% received an adequate trial of treatment. Similarly, 6.3% of women with diagnosed postpartum depression received an adequate trial of treatment.
Continuing down the treatment cascade, remission rates also were extremely low. The overall weighted mean remission rate – reflecting the percentage of women who actually ended up getting well – was just 4.8% for women with antenatal depression and 3.2% for women with postpartum depression. These are striking, although perhaps not surprising, data. It suggests, at least in part, the fundamental absence of adequate referral networks and systems for follow-up for those women who suffer from perinatal depression.
It is well established that postpartum depression is the most common complication in modern obstetrics. The data presented in this paper suggest that most women identified with perinatal depressive illness are not getting well. Assuming a prevalence of 10% for antenatal depression and 13% for postpartum depression, there are about 657,000 women with antenatal depression and about 550,000 women with postpartum depression in the United States. If this review is correct, more than 31,000 women with antenatal depression and almost 18,000 women with postpartum depression achieved remission. That leaves more than 600,000 women with undermanaged depression in pregnancy and more than 500,000 women with incompletely treated postpartum depression.
This is a wake-up call to consider a refocusing of effort. The importance of identification of women suffering from postpartum depression is clear and intuitive. We should certainly not abandon screening, but perhaps there has been an overemphasis on identification and incomplete attention to ensuring that referral networks and opportunities for clinical follow-up are in place following positive screening. There also has been inadequate focus on the obstacles to getting women in to see clinicians and getting those clinicians up to speed on the evidence base that supports treatment, both pharmacologic or nonpharmacologic.
Right now, we don’t even know for sure what obstacles exist to referral and treatment. Surveys of community clinicians suggest that collaborative care in managing reproductive-age women or pregnant and postpartum women has not evolved to the point where we have a clear, user-friendly system for getting patients referred and treated. In Massachusetts, where I practice, we have a state-funded effort (MCPAP [Massachusetts Child Psychiatry Access Program] for Moms) to train colleagues in obstetrics about how to identify and treat perinatal depression; perinatal psychiatrists also are available to consult with community-based clinicians. However, we do not have data to tell us if these efforts and the resources used to support them have yielded improvement in the overall symptom burden associated with perinatal mood disorders.
The bottom line is that even after identification of perinatal depression through screening programs, we still have women suffering in silence. It is so easy to get on the bandwagon regarding screening, but it seems even more challenging to design the systems that will accommodate the volume of women who are being identified. The fact that we do not have parallel efforts focusing on getting these women referred and treated, and a system to monitor improvement, conjures the image of setting off to sail without checking whether the boat is equipped with life preservers.
Dr. Cohen is the director of the Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications.
Celebrating Federal Social Work!
March is National Professional Social Work Month, so it is an apt time to celebrate social workers’ contributions to our respective health care organizations. Military social workers are members of all 3 major federal practice organizations—DoD, VA, and the PHS—and fill a plethora of roles and positions, including active duty in all military branches
We all intuitively grasp that military service places immense stress and strain not only on soldiers, airmen, sailors, and marines, but also on their spouses and children. This is especially true during times of conflict and in theaters of combat. Social workers in the DoD provide consolation and consultation to the family unit of those who have been wounded in body or mind in Iraq, Afghanistan, and other war-torn areas.
In an article describing military social work, Nikki R. Wooten, PhD, offers this description of the profession: “Military social work is a specialized practice area that differs from generalized practice with civilians in that military personnel, veterans, and their families live, work, and receive health care and social benefits in a hierarchical, sociopolitical environment within a structured military organization.”1
Unfortunately, as with other mental health specialties in federal practice, a shortage of social workers exists. In order to publicize the need and promote the education and training of social workers who specialize in the care of military members, their families, and veterans, Former First Lady Michelle Obama and former Second Lady Jill Biden, PhD, created Joining Forces. The program is a national effort to galvanize public support for all aspects of social and economic life for military service members and veterans. The National Association of Social Workers has been part of Joining Forces since 2011.
The VA employs more than 12,000 social workers, making the agency the largest employer of social workers in the U.S. Last year, the VA commemorated 90 years of social work excellence. Social workers are the front line for many of the most innovative social programs in the VA, such as the outreach to homeless veterans to locate and support housing; the medical foster home program for veterans who need assistance with activities of daily living that enables them to live with families in their home; the caregiver support program that assists friends and family to provide care for veterans who might otherwise not be able to live outside a facility; and the mental health intensive case management program that empowers veterans with serious mental illness to function as independently as possible and reduce the need for hospitalization.
Social workers also are part of the USPHS Commissioned Corps as allied health professionals. As crucial participants in multidisciplinary teams, social workers in the PHS respond to fill basic needs of people who are displaced by national disasters. They also provide mental health and clinical social work care in the clinics and hospitals of the IHS and other facilities that offer medical treatment and psychosocial intervention to disadvantaged populations and underserved regions. Social workers also offer public health education, social services, and administrative leadership.Another vital function that social workers perform in federal health care is facilitating the difficult transition of men and women from uniform to civilian life. A young person leaving the services needs the help of military social workers to negotiate the complexities of the VA health and education benefits application processes. Like runners in a relay, military and attached civilian social workers coordinate with VA social workers toward a smooth transition from one organization and way of life to another.
Social workers inhabit almost every corner of the federal health care world. Here are just a few examples from my own experience:
- The social worker is the first professional encounter for a service member returning from deployment and having difficulty adjusting, resulting in family dysfunction. Whether it is substance use treatment, marital counseling, or intimate partner violence, the social worker will be integral in coordinating the care of the service member and family.• The social worker is the professional who will arrange the discharge plan for an elderly veteran who has been hospitalized for cardiac surgery in a VAMC and requires a brief stay in a rehabilitation facility and then aid and assistance to return home to his wife of 40 years.
- The social worker is the professional at a vet center who provides confidential counseling to a veteran with posttraumatic stress disorder who does not feel safe or comfortable at a VAMC but who needs a therapist who has knowledge of the military and specialized trauma skills to help and heal. I suspect that if most readers of this column reflect on their federal career, they will remember an action of a social worker who smoothed their life path at a rough spot. Take a moment in this month to thank a social worker for giving help and hope to service members, veterans, and their families.
For more information
You can learn more about federal social workers by visiting the following organizations: National Association of Social Workers (https://www.socialworkers.org/military.asp), VA Social Work (http://www.socialwork.va.gov), Joining Forces (https://obamawhitehouse.archives.gov/joiningforces), and Social Work Today (http://www.socialworktoday.com/archive/031513p12.shtml).
1. Wooten NR. Military social work: opportunities and challenges for social work education. J Soc Work Educ. 2015;51(suppl 1):S6-S25.
March is National Professional Social Work Month, so it is an apt time to celebrate social workers’ contributions to our respective health care organizations. Military social workers are members of all 3 major federal practice organizations—DoD, VA, and the PHS—and fill a plethora of roles and positions, including active duty in all military branches
We all intuitively grasp that military service places immense stress and strain not only on soldiers, airmen, sailors, and marines, but also on their spouses and children. This is especially true during times of conflict and in theaters of combat. Social workers in the DoD provide consolation and consultation to the family unit of those who have been wounded in body or mind in Iraq, Afghanistan, and other war-torn areas.
In an article describing military social work, Nikki R. Wooten, PhD, offers this description of the profession: “Military social work is a specialized practice area that differs from generalized practice with civilians in that military personnel, veterans, and their families live, work, and receive health care and social benefits in a hierarchical, sociopolitical environment within a structured military organization.”1
Unfortunately, as with other mental health specialties in federal practice, a shortage of social workers exists. In order to publicize the need and promote the education and training of social workers who specialize in the care of military members, their families, and veterans, Former First Lady Michelle Obama and former Second Lady Jill Biden, PhD, created Joining Forces. The program is a national effort to galvanize public support for all aspects of social and economic life for military service members and veterans. The National Association of Social Workers has been part of Joining Forces since 2011.
The VA employs more than 12,000 social workers, making the agency the largest employer of social workers in the U.S. Last year, the VA commemorated 90 years of social work excellence. Social workers are the front line for many of the most innovative social programs in the VA, such as the outreach to homeless veterans to locate and support housing; the medical foster home program for veterans who need assistance with activities of daily living that enables them to live with families in their home; the caregiver support program that assists friends and family to provide care for veterans who might otherwise not be able to live outside a facility; and the mental health intensive case management program that empowers veterans with serious mental illness to function as independently as possible and reduce the need for hospitalization.
Social workers also are part of the USPHS Commissioned Corps as allied health professionals. As crucial participants in multidisciplinary teams, social workers in the PHS respond to fill basic needs of people who are displaced by national disasters. They also provide mental health and clinical social work care in the clinics and hospitals of the IHS and other facilities that offer medical treatment and psychosocial intervention to disadvantaged populations and underserved regions. Social workers also offer public health education, social services, and administrative leadership.Another vital function that social workers perform in federal health care is facilitating the difficult transition of men and women from uniform to civilian life. A young person leaving the services needs the help of military social workers to negotiate the complexities of the VA health and education benefits application processes. Like runners in a relay, military and attached civilian social workers coordinate with VA social workers toward a smooth transition from one organization and way of life to another.
Social workers inhabit almost every corner of the federal health care world. Here are just a few examples from my own experience:
- The social worker is the first professional encounter for a service member returning from deployment and having difficulty adjusting, resulting in family dysfunction. Whether it is substance use treatment, marital counseling, or intimate partner violence, the social worker will be integral in coordinating the care of the service member and family.• The social worker is the professional who will arrange the discharge plan for an elderly veteran who has been hospitalized for cardiac surgery in a VAMC and requires a brief stay in a rehabilitation facility and then aid and assistance to return home to his wife of 40 years.
- The social worker is the professional at a vet center who provides confidential counseling to a veteran with posttraumatic stress disorder who does not feel safe or comfortable at a VAMC but who needs a therapist who has knowledge of the military and specialized trauma skills to help and heal. I suspect that if most readers of this column reflect on their federal career, they will remember an action of a social worker who smoothed their life path at a rough spot. Take a moment in this month to thank a social worker for giving help and hope to service members, veterans, and their families.
For more information
You can learn more about federal social workers by visiting the following organizations: National Association of Social Workers (https://www.socialworkers.org/military.asp), VA Social Work (http://www.socialwork.va.gov), Joining Forces (https://obamawhitehouse.archives.gov/joiningforces), and Social Work Today (http://www.socialworktoday.com/archive/031513p12.shtml).
March is National Professional Social Work Month, so it is an apt time to celebrate social workers’ contributions to our respective health care organizations. Military social workers are members of all 3 major federal practice organizations—DoD, VA, and the PHS—and fill a plethora of roles and positions, including active duty in all military branches
We all intuitively grasp that military service places immense stress and strain not only on soldiers, airmen, sailors, and marines, but also on their spouses and children. This is especially true during times of conflict and in theaters of combat. Social workers in the DoD provide consolation and consultation to the family unit of those who have been wounded in body or mind in Iraq, Afghanistan, and other war-torn areas.
In an article describing military social work, Nikki R. Wooten, PhD, offers this description of the profession: “Military social work is a specialized practice area that differs from generalized practice with civilians in that military personnel, veterans, and their families live, work, and receive health care and social benefits in a hierarchical, sociopolitical environment within a structured military organization.”1
Unfortunately, as with other mental health specialties in federal practice, a shortage of social workers exists. In order to publicize the need and promote the education and training of social workers who specialize in the care of military members, their families, and veterans, Former First Lady Michelle Obama and former Second Lady Jill Biden, PhD, created Joining Forces. The program is a national effort to galvanize public support for all aspects of social and economic life for military service members and veterans. The National Association of Social Workers has been part of Joining Forces since 2011.
The VA employs more than 12,000 social workers, making the agency the largest employer of social workers in the U.S. Last year, the VA commemorated 90 years of social work excellence. Social workers are the front line for many of the most innovative social programs in the VA, such as the outreach to homeless veterans to locate and support housing; the medical foster home program for veterans who need assistance with activities of daily living that enables them to live with families in their home; the caregiver support program that assists friends and family to provide care for veterans who might otherwise not be able to live outside a facility; and the mental health intensive case management program that empowers veterans with serious mental illness to function as independently as possible and reduce the need for hospitalization.
Social workers also are part of the USPHS Commissioned Corps as allied health professionals. As crucial participants in multidisciplinary teams, social workers in the PHS respond to fill basic needs of people who are displaced by national disasters. They also provide mental health and clinical social work care in the clinics and hospitals of the IHS and other facilities that offer medical treatment and psychosocial intervention to disadvantaged populations and underserved regions. Social workers also offer public health education, social services, and administrative leadership.Another vital function that social workers perform in federal health care is facilitating the difficult transition of men and women from uniform to civilian life. A young person leaving the services needs the help of military social workers to negotiate the complexities of the VA health and education benefits application processes. Like runners in a relay, military and attached civilian social workers coordinate with VA social workers toward a smooth transition from one organization and way of life to another.
Social workers inhabit almost every corner of the federal health care world. Here are just a few examples from my own experience:
- The social worker is the first professional encounter for a service member returning from deployment and having difficulty adjusting, resulting in family dysfunction. Whether it is substance use treatment, marital counseling, or intimate partner violence, the social worker will be integral in coordinating the care of the service member and family.• The social worker is the professional who will arrange the discharge plan for an elderly veteran who has been hospitalized for cardiac surgery in a VAMC and requires a brief stay in a rehabilitation facility and then aid and assistance to return home to his wife of 40 years.
- The social worker is the professional at a vet center who provides confidential counseling to a veteran with posttraumatic stress disorder who does not feel safe or comfortable at a VAMC but who needs a therapist who has knowledge of the military and specialized trauma skills to help and heal. I suspect that if most readers of this column reflect on their federal career, they will remember an action of a social worker who smoothed their life path at a rough spot. Take a moment in this month to thank a social worker for giving help and hope to service members, veterans, and their families.
For more information
You can learn more about federal social workers by visiting the following organizations: National Association of Social Workers (https://www.socialworkers.org/military.asp), VA Social Work (http://www.socialwork.va.gov), Joining Forces (https://obamawhitehouse.archives.gov/joiningforces), and Social Work Today (http://www.socialworktoday.com/archive/031513p12.shtml).
1. Wooten NR. Military social work: opportunities and challenges for social work education. J Soc Work Educ. 2015;51(suppl 1):S6-S25.
1. Wooten NR. Military social work: opportunities and challenges for social work education. J Soc Work Educ. 2015;51(suppl 1):S6-S25.