User login
Pre- and post-HCT MRD levels predict ALL survival
ORLANDO – Minimal residual disease (MRD) measured before and after allogeneic hematopoietic stem cell transplantation (HCT) is a powerful predictor of survival in children with acute lymphoblastic leukemia (ALL), according to a review of hundreds of cases from around the world.
The findings could have implications for using minimal residual disease measures to guide posttransplant interventions, Michael A. Pulsipher, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“MRD pretransplant was a very powerful predictor of outcomes. MRD posttransplant highlights individual patients at risk,” Dr. Pulsipher said. Results comparing reverse transcriptase–polymerase chain reaction with flow cytometry require validation by direct comparison in the same patient cohort, but “the new risk scores ... very nicely predict outcomes both pre- and post-transplant and can guide study planning and patient counseling.”
A total of 2,960 bone marrow MRD measurements were performed in the 747 patients included in the study. MRD was assessed prior to HCT and on or near days 30, 60, 100, 180, and 365 and beyond after HCT.
Patients were grouped for analysis according to MRD level: Group 1 had no detectable MRD, group 2 had low detectable MRD levels (less than 10E-4, or 0.01% by flow cytometry), and group 3 had high detectable MRD levels (10E-4 or higher). A second analysis compared findings in those tested by flow cytometry and those tested by real-time quantitative PCR (RQ-PCR), said Dr. Pulsipher of Children’s Hospital Los Angeles.
In 648 patients with pre-HCT MRD measurements available, the 4-year probability of event-free survival was 62%, 67%, and 37% for groups 1, 2, and 3, respectively. Group 3 – the high MRD level group – had 2.47 times the increased hazard ratio for relapse and 1.67 times the increased risk of treatment-related mortality, Dr. Pulsipher said, adding that pre-HCT MRD and remission status both significantly influenced survival, while age, sex, relapse site, cytogenetics, donor type, and stem cell source did not influence outcome.
Post-HCT MRD values were analyzed as time-dependent covariates.
“As time went by more and more, any detectable level of MRD led to a very poor prognosis, whereas patients arriving at day 365 with no detectable MRD had exceptional prognosis with survival approaching 90%,” he said.
Specifically, the 4-year probability of event-free survival for groups 1, 2, and 3, respectively, were 59%, 65%, and 43% at day 30; 64%, 47%, and 36% at day 60; 65%, 69%, and 44% at day 90; 79%, 40%, and 12% at day 180; and 87%, 36%, and 25% at day 365.
Of note, a very significant interaction was seen between acute graft-versus-host disease (GVHD) and MRD, Dr. Pulsipher said, explaining that patients who were MRD positive and had developed GVHD had a significant decrease in the cumulative incidence of relapse, compared with those with no GVHD.
“This translated into improved event-free survival with patients post transplant, who were MRD-positive [and] developing GVHD, still having a reasonable chance of survival, whereas patients post transplant who had MRD measured who did not develop GVHD had a very poor chance of survival,” he added.
Additionally, based on detailed multivariate analysis including a number of clinical factors, risk predictive scores were developed for event-free survival risk at 18 months or cumulative incidence of relapse at 18 months. Multiple scores were developed for each, but, as an example of factors that had an important effect on outcomes, patients with very early pretransplant relapse (those who went into remission but relapsed within 18 months) or with greater than 2nd relapse had a high risk for poor event-free survival. Mismatched donors and unrelated cord-blood stem cell transplant recipients also had high risk, he said, noting that, “of course, MRD had a significant effect” and was the most important factor prior to transplant.
These patients, who had a 4-point or greater risk score, were the poorest risk group, with survival that was less than 50%, as opposed to better risk groups that exceeded 90%, he said.
“A score of greater than 5 could identify 80% of patients who were going to relapse after transplant, and of course, event-free and overall survival in those patients were very poor,” he added.
As time went by, the early risk of GVHD diminished somewhat, as did the risk of mismatched donors.
“Most of the risk was associated with any MRD detection,” he said.
Flow cytometry and RQ-PCR levels of at least 10-4 were highly predictive of relapse at all pre- and post-HCT time points; however, RQ-PCR values between 10-4 and 10-3, in cases where adequate numbers were available for comparison, better predicted relapse as compared with flow cytometry results.
For example, before HCT, hazard ratios were 1.26 and 2.41 with flow cytometry vs. RQ-PCR. At day 30, the hazard ratios were 1.33 and 2.53, and at day 365, they were 3.54 and 31.84, Dr. Pulsipher reported.
The findings provide important information for improving outcomes in children with high-risk ALL undergoing HCT, he said.
“Older prognostic models for relapsed and refractory high-risk ALL have focused on timing and location of relapse, as well as disease phenotype. But it is clear that, in order to treat children with very high risk ALL with transplantation, MRD has become the most important thing to look at in the pretreatment setting. The challenges that we face in assessing MRD, however, have been hampered by the fact that we have differing MRD measurements,” he said, noting that RQ-PCR is often used in Europe, while flow cytometry is more often used in the United States. As such, direct comparisons are lacking, as are T-cell and posttransplant data.
The current study represents a “tremendous effort” by international collaborators to address these shortcoming, he said.
“This is a great opportunity, as our goal, of course, is to avoid futility in transplantation, but, more importantly, to find opportunities to identify groups for which we can improve our outcomes,” he added.
Patients included in the study were treated in Europe, North America, and Australia and were transplanted during Sept. 1999-May 2016. Most were in first or second remission, and most (586) had pre-B ALL. A notable 145 had T-cell ALL – “more than ever has been analyzed previously” – and 16 had B-lineage or biphenotypic ALL. About half were under age 10 years, 62% were boys, and stem cell sources were typical, although 20% received a cord blood transplant.
Dr. Pulsipher reported serving as an advisor and/or consultant for Chimerix, Novartis, Jazz Pharmaceutical, and receiving housing support from Medac Pharma for an educational meeting.
ORLANDO – Minimal residual disease (MRD) measured before and after allogeneic hematopoietic stem cell transplantation (HCT) is a powerful predictor of survival in children with acute lymphoblastic leukemia (ALL), according to a review of hundreds of cases from around the world.
The findings could have implications for using minimal residual disease measures to guide posttransplant interventions, Michael A. Pulsipher, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“MRD pretransplant was a very powerful predictor of outcomes. MRD posttransplant highlights individual patients at risk,” Dr. Pulsipher said. Results comparing reverse transcriptase–polymerase chain reaction with flow cytometry require validation by direct comparison in the same patient cohort, but “the new risk scores ... very nicely predict outcomes both pre- and post-transplant and can guide study planning and patient counseling.”
A total of 2,960 bone marrow MRD measurements were performed in the 747 patients included in the study. MRD was assessed prior to HCT and on or near days 30, 60, 100, 180, and 365 and beyond after HCT.
Patients were grouped for analysis according to MRD level: Group 1 had no detectable MRD, group 2 had low detectable MRD levels (less than 10E-4, or 0.01% by flow cytometry), and group 3 had high detectable MRD levels (10E-4 or higher). A second analysis compared findings in those tested by flow cytometry and those tested by real-time quantitative PCR (RQ-PCR), said Dr. Pulsipher of Children’s Hospital Los Angeles.
In 648 patients with pre-HCT MRD measurements available, the 4-year probability of event-free survival was 62%, 67%, and 37% for groups 1, 2, and 3, respectively. Group 3 – the high MRD level group – had 2.47 times the increased hazard ratio for relapse and 1.67 times the increased risk of treatment-related mortality, Dr. Pulsipher said, adding that pre-HCT MRD and remission status both significantly influenced survival, while age, sex, relapse site, cytogenetics, donor type, and stem cell source did not influence outcome.
Post-HCT MRD values were analyzed as time-dependent covariates.
“As time went by more and more, any detectable level of MRD led to a very poor prognosis, whereas patients arriving at day 365 with no detectable MRD had exceptional prognosis with survival approaching 90%,” he said.
Specifically, the 4-year probability of event-free survival for groups 1, 2, and 3, respectively, were 59%, 65%, and 43% at day 30; 64%, 47%, and 36% at day 60; 65%, 69%, and 44% at day 90; 79%, 40%, and 12% at day 180; and 87%, 36%, and 25% at day 365.
Of note, a very significant interaction was seen between acute graft-versus-host disease (GVHD) and MRD, Dr. Pulsipher said, explaining that patients who were MRD positive and had developed GVHD had a significant decrease in the cumulative incidence of relapse, compared with those with no GVHD.
“This translated into improved event-free survival with patients post transplant, who were MRD-positive [and] developing GVHD, still having a reasonable chance of survival, whereas patients post transplant who had MRD measured who did not develop GVHD had a very poor chance of survival,” he added.
Additionally, based on detailed multivariate analysis including a number of clinical factors, risk predictive scores were developed for event-free survival risk at 18 months or cumulative incidence of relapse at 18 months. Multiple scores were developed for each, but, as an example of factors that had an important effect on outcomes, patients with very early pretransplant relapse (those who went into remission but relapsed within 18 months) or with greater than 2nd relapse had a high risk for poor event-free survival. Mismatched donors and unrelated cord-blood stem cell transplant recipients also had high risk, he said, noting that, “of course, MRD had a significant effect” and was the most important factor prior to transplant.
These patients, who had a 4-point or greater risk score, were the poorest risk group, with survival that was less than 50%, as opposed to better risk groups that exceeded 90%, he said.
“A score of greater than 5 could identify 80% of patients who were going to relapse after transplant, and of course, event-free and overall survival in those patients were very poor,” he added.
As time went by, the early risk of GVHD diminished somewhat, as did the risk of mismatched donors.
“Most of the risk was associated with any MRD detection,” he said.
Flow cytometry and RQ-PCR levels of at least 10-4 were highly predictive of relapse at all pre- and post-HCT time points; however, RQ-PCR values between 10-4 and 10-3, in cases where adequate numbers were available for comparison, better predicted relapse as compared with flow cytometry results.
For example, before HCT, hazard ratios were 1.26 and 2.41 with flow cytometry vs. RQ-PCR. At day 30, the hazard ratios were 1.33 and 2.53, and at day 365, they were 3.54 and 31.84, Dr. Pulsipher reported.
The findings provide important information for improving outcomes in children with high-risk ALL undergoing HCT, he said.
“Older prognostic models for relapsed and refractory high-risk ALL have focused on timing and location of relapse, as well as disease phenotype. But it is clear that, in order to treat children with very high risk ALL with transplantation, MRD has become the most important thing to look at in the pretreatment setting. The challenges that we face in assessing MRD, however, have been hampered by the fact that we have differing MRD measurements,” he said, noting that RQ-PCR is often used in Europe, while flow cytometry is more often used in the United States. As such, direct comparisons are lacking, as are T-cell and posttransplant data.
The current study represents a “tremendous effort” by international collaborators to address these shortcoming, he said.
“This is a great opportunity, as our goal, of course, is to avoid futility in transplantation, but, more importantly, to find opportunities to identify groups for which we can improve our outcomes,” he added.
Patients included in the study were treated in Europe, North America, and Australia and were transplanted during Sept. 1999-May 2016. Most were in first or second remission, and most (586) had pre-B ALL. A notable 145 had T-cell ALL – “more than ever has been analyzed previously” – and 16 had B-lineage or biphenotypic ALL. About half were under age 10 years, 62% were boys, and stem cell sources were typical, although 20% received a cord blood transplant.
Dr. Pulsipher reported serving as an advisor and/or consultant for Chimerix, Novartis, Jazz Pharmaceutical, and receiving housing support from Medac Pharma for an educational meeting.
ORLANDO – Minimal residual disease (MRD) measured before and after allogeneic hematopoietic stem cell transplantation (HCT) is a powerful predictor of survival in children with acute lymphoblastic leukemia (ALL), according to a review of hundreds of cases from around the world.
The findings could have implications for using minimal residual disease measures to guide posttransplant interventions, Michael A. Pulsipher, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“MRD pretransplant was a very powerful predictor of outcomes. MRD posttransplant highlights individual patients at risk,” Dr. Pulsipher said. Results comparing reverse transcriptase–polymerase chain reaction with flow cytometry require validation by direct comparison in the same patient cohort, but “the new risk scores ... very nicely predict outcomes both pre- and post-transplant and can guide study planning and patient counseling.”
A total of 2,960 bone marrow MRD measurements were performed in the 747 patients included in the study. MRD was assessed prior to HCT and on or near days 30, 60, 100, 180, and 365 and beyond after HCT.
Patients were grouped for analysis according to MRD level: Group 1 had no detectable MRD, group 2 had low detectable MRD levels (less than 10E-4, or 0.01% by flow cytometry), and group 3 had high detectable MRD levels (10E-4 or higher). A second analysis compared findings in those tested by flow cytometry and those tested by real-time quantitative PCR (RQ-PCR), said Dr. Pulsipher of Children’s Hospital Los Angeles.
In 648 patients with pre-HCT MRD measurements available, the 4-year probability of event-free survival was 62%, 67%, and 37% for groups 1, 2, and 3, respectively. Group 3 – the high MRD level group – had 2.47 times the increased hazard ratio for relapse and 1.67 times the increased risk of treatment-related mortality, Dr. Pulsipher said, adding that pre-HCT MRD and remission status both significantly influenced survival, while age, sex, relapse site, cytogenetics, donor type, and stem cell source did not influence outcome.
Post-HCT MRD values were analyzed as time-dependent covariates.
“As time went by more and more, any detectable level of MRD led to a very poor prognosis, whereas patients arriving at day 365 with no detectable MRD had exceptional prognosis with survival approaching 90%,” he said.
Specifically, the 4-year probability of event-free survival for groups 1, 2, and 3, respectively, were 59%, 65%, and 43% at day 30; 64%, 47%, and 36% at day 60; 65%, 69%, and 44% at day 90; 79%, 40%, and 12% at day 180; and 87%, 36%, and 25% at day 365.
Of note, a very significant interaction was seen between acute graft-versus-host disease (GVHD) and MRD, Dr. Pulsipher said, explaining that patients who were MRD positive and had developed GVHD had a significant decrease in the cumulative incidence of relapse, compared with those with no GVHD.
“This translated into improved event-free survival with patients post transplant, who were MRD-positive [and] developing GVHD, still having a reasonable chance of survival, whereas patients post transplant who had MRD measured who did not develop GVHD had a very poor chance of survival,” he added.
Additionally, based on detailed multivariate analysis including a number of clinical factors, risk predictive scores were developed for event-free survival risk at 18 months or cumulative incidence of relapse at 18 months. Multiple scores were developed for each, but, as an example of factors that had an important effect on outcomes, patients with very early pretransplant relapse (those who went into remission but relapsed within 18 months) or with greater than 2nd relapse had a high risk for poor event-free survival. Mismatched donors and unrelated cord-blood stem cell transplant recipients also had high risk, he said, noting that, “of course, MRD had a significant effect” and was the most important factor prior to transplant.
These patients, who had a 4-point or greater risk score, were the poorest risk group, with survival that was less than 50%, as opposed to better risk groups that exceeded 90%, he said.
“A score of greater than 5 could identify 80% of patients who were going to relapse after transplant, and of course, event-free and overall survival in those patients were very poor,” he added.
As time went by, the early risk of GVHD diminished somewhat, as did the risk of mismatched donors.
“Most of the risk was associated with any MRD detection,” he said.
Flow cytometry and RQ-PCR levels of at least 10-4 were highly predictive of relapse at all pre- and post-HCT time points; however, RQ-PCR values between 10-4 and 10-3, in cases where adequate numbers were available for comparison, better predicted relapse as compared with flow cytometry results.
For example, before HCT, hazard ratios were 1.26 and 2.41 with flow cytometry vs. RQ-PCR. At day 30, the hazard ratios were 1.33 and 2.53, and at day 365, they were 3.54 and 31.84, Dr. Pulsipher reported.
The findings provide important information for improving outcomes in children with high-risk ALL undergoing HCT, he said.
“Older prognostic models for relapsed and refractory high-risk ALL have focused on timing and location of relapse, as well as disease phenotype. But it is clear that, in order to treat children with very high risk ALL with transplantation, MRD has become the most important thing to look at in the pretreatment setting. The challenges that we face in assessing MRD, however, have been hampered by the fact that we have differing MRD measurements,” he said, noting that RQ-PCR is often used in Europe, while flow cytometry is more often used in the United States. As such, direct comparisons are lacking, as are T-cell and posttransplant data.
The current study represents a “tremendous effort” by international collaborators to address these shortcoming, he said.
“This is a great opportunity, as our goal, of course, is to avoid futility in transplantation, but, more importantly, to find opportunities to identify groups for which we can improve our outcomes,” he added.
Patients included in the study were treated in Europe, North America, and Australia and were transplanted during Sept. 1999-May 2016. Most were in first or second remission, and most (586) had pre-B ALL. A notable 145 had T-cell ALL – “more than ever has been analyzed previously” – and 16 had B-lineage or biphenotypic ALL. About half were under age 10 years, 62% were boys, and stem cell sources were typical, although 20% received a cord blood transplant.
Dr. Pulsipher reported serving as an advisor and/or consultant for Chimerix, Novartis, Jazz Pharmaceutical, and receiving housing support from Medac Pharma for an educational meeting.
Key clinical point:
Major finding: Patients with high pretransplant MRD levels had a 2.47-fold increased hazard ratio for relapse and a 1.67-fold increased risk of treatment-related mortality.
Data source: A review of data from 747 pediatric high-risk ALL cases.
Disclosures: Dr. Pulsipher reported serving as an adviser and/or consultant for Chimerix, Novartis, and Jazz Pharmaceuticals and receiving housing support from Medac Pharma for an educational meeting.
The percutaneous mitral valve replacement pipe dream
SNOWMASS, COLO. – Percutaneous mitral valve replacement is unlikely to ever catch on in any way remotely approaching that of transcatheter aortic valve replacement for the treatment of aortic stenosis, Blase A. Carabello, MD, predicted at the Annual Cardiovascular Conference at Snowmass.
“We’ve spent $2 billion looking for methods of percutaneous mitral valve replacement, and yet, I have to wonder if that makes any sense,” said Dr. Carabello, professor of medicine and chief of cardiology at East Carolina University in Greenville, N.C.
“If repair is superior to replacement in primary MR [mitral regurgitation], which I think we all agree is true, and you don’t need to get rid of every last molecule of blood going backward across the mitral valve when you’ve got a good left ventricle, then a percutaneous replacement in primary MR would have only the niche of patients who are inoperable and whose leaflets can’t be grabbed by the MitraClip or some new percutaneous device down the road. And, in secondary MR, it doesn’t seem to matter whether you replace or repair the valve, so why not just repair it with a clip?” he argued.
Numerous nonrandomized studies have invariably demonstrated superior survival for surgical repair versus replacement in patients with primary MR.
“There’s never going to be a randomized controlled trial of repair versus replacement; there’s no equipoise there. We all believe that, in primary MR, repair is superior to replacement. There are no data anywhere to suggest the opposite. It’s essentially sacrosanct,” according to the cardiologist.
In contrast, a major randomized trial of surgical repair versus replacement has been conducted in patients with severe secondary MR. This NIH-funded study conducted by the Cardiothoracic Surgical Trials Network found no difference in survival between the two groups (N Engl J Med. 2016 Jan 28; 374[4]:344-53). That’s not a surprising result, Dr. Carabello said, since the underlying cause of this type of valve disease is a sick left ventricle. But, since surgical repair entails less morbidity than replacement – and a percutaneous repair with a leaflet-grasping device such as the MitraClip is simpler and safer than a surgical repair – it seems likely that the future treatment for secondary MR will be a percutaneous device, he said.
That future could depend upon the results of the ongoing COAPT trial (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy), in which the MitraClip is being studied as an alternative to surgical repair for significant secondary MR. The MitraClip, which doesn’t entail a concomitant annuloplasty, is currently approved by the Food and Drug Administration only for patients with primary, degenerative mitral regurgitation not amenable to surgical repair. But, if COAPT yields positive results, the role of the MitraClip will greatly expand.
An intriguing and poorly understood difference exists in the significance of residual mitral regurgitation following surgical repair as opposed to percutaneous MitraClip repair, Dr. Carabello observed.
“I go to the OR a lot, and I know of no surgeon [who] will leave 2+ MR behind. Most surgeons won’t leave 1+ MR behind. They’ll put the patient back on the pump to repair even mild residual MR, accepting only trace MR or zero before they leave the OR because they know that the best predictor of a failed mitral repair is the presence of residual MR in the OR,” he said.
In contrast, following successful deployment of the MitraClip most patients are left with 1-2+ MR. Yet, as was demonstrated in the 5-year results of the randomized EVEREST II trial (Endovascular Valve Edge-to-Edge Repair Study), this residual MR wasn’t a harbinger of poor outcomes long-term (J Am Coll Cardiol. 2015 Dec 29;66[25]:2844-54).
“You would have expected, with that much residual MR, there would be a perpetually increasing failure rate over time, but that didn’t happen. In Everest II, there was an early failure rate for percutaneous repair, where the MitraClip didn’t work and those patients required surgical mitral valve repair. But, after the first 6 months, the failure rate for the clip was exactly the same as the surgical failure rate, even though, with the clip, you start with more MR to begin with,” the cardiologist noted.
The MitraClip procedure is modeled after the surgical Alfieri double-orifice end-to-end stitch technique, which has been shown to have durable results when performed in conjunction with an annuloplasty ring for primary MR.
“The MitraClip essentially joins the valve in the middle the way the Alfieri stitch does, but it doesn’t appear to behave the same way. Why is that? Maybe the clip does something different than the Alfieri stitch on which it was modeled. Maybe that bar in the middle of the mitral valve does something in terms of scarring or stabilization that we don’t know about yet,” he speculated.
As for the prospects for percutaneous mitral valve replacement, Dr. Carabello said that this type of procedure “is a very difficult thing to do, and so far, has been met with a fair amount of failure. It’ll be very interesting to see what percentage of market share it gets 10 years down the road. My prediction is that, for mitral regurgitation, repair is always going to be it.”
Dr. Carabello reported serving on a data safety monitoring board for Edwards Lifesciences.
The author provides valuable insight into how the definition of “success” of a procedure can change depending on the approach to the problem. While the gold standard of open mitral valve repair is 1+ regurgitation or less, those promoting percutaneous valve replacement are willing to accept long term 1+ to 2+ regurgitation. New technology and innovation is critical in medicine, provided the results are at least equivalent or superior to the standard techniques.
The author provides valuable insight into how the definition of “success” of a procedure can change depending on the approach to the problem. While the gold standard of open mitral valve repair is 1+ regurgitation or less, those promoting percutaneous valve replacement are willing to accept long term 1+ to 2+ regurgitation. New technology and innovation is critical in medicine, provided the results are at least equivalent or superior to the standard techniques.
The author provides valuable insight into how the definition of “success” of a procedure can change depending on the approach to the problem. While the gold standard of open mitral valve repair is 1+ regurgitation or less, those promoting percutaneous valve replacement are willing to accept long term 1+ to 2+ regurgitation. New technology and innovation is critical in medicine, provided the results are at least equivalent or superior to the standard techniques.
SNOWMASS, COLO. – Percutaneous mitral valve replacement is unlikely to ever catch on in any way remotely approaching that of transcatheter aortic valve replacement for the treatment of aortic stenosis, Blase A. Carabello, MD, predicted at the Annual Cardiovascular Conference at Snowmass.
“We’ve spent $2 billion looking for methods of percutaneous mitral valve replacement, and yet, I have to wonder if that makes any sense,” said Dr. Carabello, professor of medicine and chief of cardiology at East Carolina University in Greenville, N.C.
“If repair is superior to replacement in primary MR [mitral regurgitation], which I think we all agree is true, and you don’t need to get rid of every last molecule of blood going backward across the mitral valve when you’ve got a good left ventricle, then a percutaneous replacement in primary MR would have only the niche of patients who are inoperable and whose leaflets can’t be grabbed by the MitraClip or some new percutaneous device down the road. And, in secondary MR, it doesn’t seem to matter whether you replace or repair the valve, so why not just repair it with a clip?” he argued.
Numerous nonrandomized studies have invariably demonstrated superior survival for surgical repair versus replacement in patients with primary MR.
“There’s never going to be a randomized controlled trial of repair versus replacement; there’s no equipoise there. We all believe that, in primary MR, repair is superior to replacement. There are no data anywhere to suggest the opposite. It’s essentially sacrosanct,” according to the cardiologist.
In contrast, a major randomized trial of surgical repair versus replacement has been conducted in patients with severe secondary MR. This NIH-funded study conducted by the Cardiothoracic Surgical Trials Network found no difference in survival between the two groups (N Engl J Med. 2016 Jan 28; 374[4]:344-53). That’s not a surprising result, Dr. Carabello said, since the underlying cause of this type of valve disease is a sick left ventricle. But, since surgical repair entails less morbidity than replacement – and a percutaneous repair with a leaflet-grasping device such as the MitraClip is simpler and safer than a surgical repair – it seems likely that the future treatment for secondary MR will be a percutaneous device, he said.
That future could depend upon the results of the ongoing COAPT trial (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy), in which the MitraClip is being studied as an alternative to surgical repair for significant secondary MR. The MitraClip, which doesn’t entail a concomitant annuloplasty, is currently approved by the Food and Drug Administration only for patients with primary, degenerative mitral regurgitation not amenable to surgical repair. But, if COAPT yields positive results, the role of the MitraClip will greatly expand.
An intriguing and poorly understood difference exists in the significance of residual mitral regurgitation following surgical repair as opposed to percutaneous MitraClip repair, Dr. Carabello observed.
“I go to the OR a lot, and I know of no surgeon [who] will leave 2+ MR behind. Most surgeons won’t leave 1+ MR behind. They’ll put the patient back on the pump to repair even mild residual MR, accepting only trace MR or zero before they leave the OR because they know that the best predictor of a failed mitral repair is the presence of residual MR in the OR,” he said.
In contrast, following successful deployment of the MitraClip most patients are left with 1-2+ MR. Yet, as was demonstrated in the 5-year results of the randomized EVEREST II trial (Endovascular Valve Edge-to-Edge Repair Study), this residual MR wasn’t a harbinger of poor outcomes long-term (J Am Coll Cardiol. 2015 Dec 29;66[25]:2844-54).
“You would have expected, with that much residual MR, there would be a perpetually increasing failure rate over time, but that didn’t happen. In Everest II, there was an early failure rate for percutaneous repair, where the MitraClip didn’t work and those patients required surgical mitral valve repair. But, after the first 6 months, the failure rate for the clip was exactly the same as the surgical failure rate, even though, with the clip, you start with more MR to begin with,” the cardiologist noted.
The MitraClip procedure is modeled after the surgical Alfieri double-orifice end-to-end stitch technique, which has been shown to have durable results when performed in conjunction with an annuloplasty ring for primary MR.
“The MitraClip essentially joins the valve in the middle the way the Alfieri stitch does, but it doesn’t appear to behave the same way. Why is that? Maybe the clip does something different than the Alfieri stitch on which it was modeled. Maybe that bar in the middle of the mitral valve does something in terms of scarring or stabilization that we don’t know about yet,” he speculated.
As for the prospects for percutaneous mitral valve replacement, Dr. Carabello said that this type of procedure “is a very difficult thing to do, and so far, has been met with a fair amount of failure. It’ll be very interesting to see what percentage of market share it gets 10 years down the road. My prediction is that, for mitral regurgitation, repair is always going to be it.”
Dr. Carabello reported serving on a data safety monitoring board for Edwards Lifesciences.
SNOWMASS, COLO. – Percutaneous mitral valve replacement is unlikely to ever catch on in any way remotely approaching that of transcatheter aortic valve replacement for the treatment of aortic stenosis, Blase A. Carabello, MD, predicted at the Annual Cardiovascular Conference at Snowmass.
“We’ve spent $2 billion looking for methods of percutaneous mitral valve replacement, and yet, I have to wonder if that makes any sense,” said Dr. Carabello, professor of medicine and chief of cardiology at East Carolina University in Greenville, N.C.
“If repair is superior to replacement in primary MR [mitral regurgitation], which I think we all agree is true, and you don’t need to get rid of every last molecule of blood going backward across the mitral valve when you’ve got a good left ventricle, then a percutaneous replacement in primary MR would have only the niche of patients who are inoperable and whose leaflets can’t be grabbed by the MitraClip or some new percutaneous device down the road. And, in secondary MR, it doesn’t seem to matter whether you replace or repair the valve, so why not just repair it with a clip?” he argued.
Numerous nonrandomized studies have invariably demonstrated superior survival for surgical repair versus replacement in patients with primary MR.
“There’s never going to be a randomized controlled trial of repair versus replacement; there’s no equipoise there. We all believe that, in primary MR, repair is superior to replacement. There are no data anywhere to suggest the opposite. It’s essentially sacrosanct,” according to the cardiologist.
In contrast, a major randomized trial of surgical repair versus replacement has been conducted in patients with severe secondary MR. This NIH-funded study conducted by the Cardiothoracic Surgical Trials Network found no difference in survival between the two groups (N Engl J Med. 2016 Jan 28; 374[4]:344-53). That’s not a surprising result, Dr. Carabello said, since the underlying cause of this type of valve disease is a sick left ventricle. But, since surgical repair entails less morbidity than replacement – and a percutaneous repair with a leaflet-grasping device such as the MitraClip is simpler and safer than a surgical repair – it seems likely that the future treatment for secondary MR will be a percutaneous device, he said.
That future could depend upon the results of the ongoing COAPT trial (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy), in which the MitraClip is being studied as an alternative to surgical repair for significant secondary MR. The MitraClip, which doesn’t entail a concomitant annuloplasty, is currently approved by the Food and Drug Administration only for patients with primary, degenerative mitral regurgitation not amenable to surgical repair. But, if COAPT yields positive results, the role of the MitraClip will greatly expand.
An intriguing and poorly understood difference exists in the significance of residual mitral regurgitation following surgical repair as opposed to percutaneous MitraClip repair, Dr. Carabello observed.
“I go to the OR a lot, and I know of no surgeon [who] will leave 2+ MR behind. Most surgeons won’t leave 1+ MR behind. They’ll put the patient back on the pump to repair even mild residual MR, accepting only trace MR or zero before they leave the OR because they know that the best predictor of a failed mitral repair is the presence of residual MR in the OR,” he said.
In contrast, following successful deployment of the MitraClip most patients are left with 1-2+ MR. Yet, as was demonstrated in the 5-year results of the randomized EVEREST II trial (Endovascular Valve Edge-to-Edge Repair Study), this residual MR wasn’t a harbinger of poor outcomes long-term (J Am Coll Cardiol. 2015 Dec 29;66[25]:2844-54).
“You would have expected, with that much residual MR, there would be a perpetually increasing failure rate over time, but that didn’t happen. In Everest II, there was an early failure rate for percutaneous repair, where the MitraClip didn’t work and those patients required surgical mitral valve repair. But, after the first 6 months, the failure rate for the clip was exactly the same as the surgical failure rate, even though, with the clip, you start with more MR to begin with,” the cardiologist noted.
The MitraClip procedure is modeled after the surgical Alfieri double-orifice end-to-end stitch technique, which has been shown to have durable results when performed in conjunction with an annuloplasty ring for primary MR.
“The MitraClip essentially joins the valve in the middle the way the Alfieri stitch does, but it doesn’t appear to behave the same way. Why is that? Maybe the clip does something different than the Alfieri stitch on which it was modeled. Maybe that bar in the middle of the mitral valve does something in terms of scarring or stabilization that we don’t know about yet,” he speculated.
As for the prospects for percutaneous mitral valve replacement, Dr. Carabello said that this type of procedure “is a very difficult thing to do, and so far, has been met with a fair amount of failure. It’ll be very interesting to see what percentage of market share it gets 10 years down the road. My prediction is that, for mitral regurgitation, repair is always going to be it.”
Dr. Carabello reported serving on a data safety monitoring board for Edwards Lifesciences.
Connective tissue diseases reported in patients receiving immune checkpoint inhibitors
For the first time, new-onset connective tissue disease has been reported in patients who were treated with anti-PD1/PDL-1 agents, according to findings published in the Annals of the Rheumatic Diseases.
In a cohort of 447 cancer patients who received therapy with immune checkpoint inhibitors (ICIs), Sébastien Le Burel, MD, of the Bicêtre Hospital in Le Kremlin-Bicêtre, France, and his colleagues described four patients who developed a connective tissue disease (CTD). There were two cases of Sjögren’s syndrome in patients taking an anti–programmed cell death 1 (anti-PD1) drug, one case of cryoglobulinemic vasculitis as a complication of suspected Sjögren’s syndrome in a patient taking an anti–programmed cell death ligand 1 (PDL-1) agent, and a case of a patient with antinuclear antibody positive myositis who was taking an anti-PDL-1 drug (Ann Rheum Dis. 2017 Feb 27. doi: 10.1136/annrheumdis-2016-210820).
“While the onset of systemic autoimmune disease after ICI treatment remains uncommon, greater awareness of these conditions should enable physicians to provide more effective patient care,” the investigators wrote. “This underlines the need for close collaboration within a network of oncologists and other specialist physicians in the new era of immunotherapy.”
The investigators discovered the cases by screening the French prospective, multicenter, academic REISAMIC registry for reports of CTD among patients being treated with anti-PD1 or anti-PDL-1 agents.
All four of the patients who developed a CTD had metastatic cancer, and their mean age was 62 years. Two patients had been treated with anti-PD1 agents and two with anti-PDL-1 agents. None of the four patients had presented with symptoms of CTD before they began treatment.
The mean time interval between the first treatment dose and the first symptom of CTD was 60 days (range, 24-72), and the mean time interval between the first symptom and subsequent diagnosis of CTD was 40 days (range, 10-74).
Three patients discontinued the ICI agent, and two patients were treated with steroids (1 mg/kg/day).
The estimated prevalence of CTD was 0.7% in the REISAMIC registry, and the authors emphasize that the high proportion of cases of Sjögren’s syndrome is noteworthy, with two of the patients fulfilling the recent American College of Rheumatology/European League Against Rheumatism criteria for Sjögren’s syndrome.
A limitation of the study is that some patients presenting with milder symptoms might not have been investigated by their oncologist.
The findings raise the question of whether asymptomatic patients taking ICIs who are at risk for immune-related adverse events should be screened and monitored closely, the authors explained.
One of the study authors received research funding from Novartis and Pfizer for the current paper. Several authors report relationships with industry.
For the first time, new-onset connective tissue disease has been reported in patients who were treated with anti-PD1/PDL-1 agents, according to findings published in the Annals of the Rheumatic Diseases.
In a cohort of 447 cancer patients who received therapy with immune checkpoint inhibitors (ICIs), Sébastien Le Burel, MD, of the Bicêtre Hospital in Le Kremlin-Bicêtre, France, and his colleagues described four patients who developed a connective tissue disease (CTD). There were two cases of Sjögren’s syndrome in patients taking an anti–programmed cell death 1 (anti-PD1) drug, one case of cryoglobulinemic vasculitis as a complication of suspected Sjögren’s syndrome in a patient taking an anti–programmed cell death ligand 1 (PDL-1) agent, and a case of a patient with antinuclear antibody positive myositis who was taking an anti-PDL-1 drug (Ann Rheum Dis. 2017 Feb 27. doi: 10.1136/annrheumdis-2016-210820).
“While the onset of systemic autoimmune disease after ICI treatment remains uncommon, greater awareness of these conditions should enable physicians to provide more effective patient care,” the investigators wrote. “This underlines the need for close collaboration within a network of oncologists and other specialist physicians in the new era of immunotherapy.”
The investigators discovered the cases by screening the French prospective, multicenter, academic REISAMIC registry for reports of CTD among patients being treated with anti-PD1 or anti-PDL-1 agents.
All four of the patients who developed a CTD had metastatic cancer, and their mean age was 62 years. Two patients had been treated with anti-PD1 agents and two with anti-PDL-1 agents. None of the four patients had presented with symptoms of CTD before they began treatment.
The mean time interval between the first treatment dose and the first symptom of CTD was 60 days (range, 24-72), and the mean time interval between the first symptom and subsequent diagnosis of CTD was 40 days (range, 10-74).
Three patients discontinued the ICI agent, and two patients were treated with steroids (1 mg/kg/day).
The estimated prevalence of CTD was 0.7% in the REISAMIC registry, and the authors emphasize that the high proportion of cases of Sjögren’s syndrome is noteworthy, with two of the patients fulfilling the recent American College of Rheumatology/European League Against Rheumatism criteria for Sjögren’s syndrome.
A limitation of the study is that some patients presenting with milder symptoms might not have been investigated by their oncologist.
The findings raise the question of whether asymptomatic patients taking ICIs who are at risk for immune-related adverse events should be screened and monitored closely, the authors explained.
One of the study authors received research funding from Novartis and Pfizer for the current paper. Several authors report relationships with industry.
For the first time, new-onset connective tissue disease has been reported in patients who were treated with anti-PD1/PDL-1 agents, according to findings published in the Annals of the Rheumatic Diseases.
In a cohort of 447 cancer patients who received therapy with immune checkpoint inhibitors (ICIs), Sébastien Le Burel, MD, of the Bicêtre Hospital in Le Kremlin-Bicêtre, France, and his colleagues described four patients who developed a connective tissue disease (CTD). There were two cases of Sjögren’s syndrome in patients taking an anti–programmed cell death 1 (anti-PD1) drug, one case of cryoglobulinemic vasculitis as a complication of suspected Sjögren’s syndrome in a patient taking an anti–programmed cell death ligand 1 (PDL-1) agent, and a case of a patient with antinuclear antibody positive myositis who was taking an anti-PDL-1 drug (Ann Rheum Dis. 2017 Feb 27. doi: 10.1136/annrheumdis-2016-210820).
“While the onset of systemic autoimmune disease after ICI treatment remains uncommon, greater awareness of these conditions should enable physicians to provide more effective patient care,” the investigators wrote. “This underlines the need for close collaboration within a network of oncologists and other specialist physicians in the new era of immunotherapy.”
The investigators discovered the cases by screening the French prospective, multicenter, academic REISAMIC registry for reports of CTD among patients being treated with anti-PD1 or anti-PDL-1 agents.
All four of the patients who developed a CTD had metastatic cancer, and their mean age was 62 years. Two patients had been treated with anti-PD1 agents and two with anti-PDL-1 agents. None of the four patients had presented with symptoms of CTD before they began treatment.
The mean time interval between the first treatment dose and the first symptom of CTD was 60 days (range, 24-72), and the mean time interval between the first symptom and subsequent diagnosis of CTD was 40 days (range, 10-74).
Three patients discontinued the ICI agent, and two patients were treated with steroids (1 mg/kg/day).
The estimated prevalence of CTD was 0.7% in the REISAMIC registry, and the authors emphasize that the high proportion of cases of Sjögren’s syndrome is noteworthy, with two of the patients fulfilling the recent American College of Rheumatology/European League Against Rheumatism criteria for Sjögren’s syndrome.
A limitation of the study is that some patients presenting with milder symptoms might not have been investigated by their oncologist.
The findings raise the question of whether asymptomatic patients taking ICIs who are at risk for immune-related adverse events should be screened and monitored closely, the authors explained.
One of the study authors received research funding from Novartis and Pfizer for the current paper. Several authors report relationships with industry.
Key clinical point:
Major finding: In a cohort of 447 patients, 4 with metastatic cancer developed connective tissue disease following anti-PD-1/PDL-1 treatment.
Data source: A prospective, multicenter, academic registry was screened for reports of CTD among patients being treated with anti-PD1/PDL-1 agents.
Disclosures: One of the study authors received research funding from Novartis and Pfizer for the current paper. Several authors report relationships with industry.
Skin disease costs $75 billion a year
The direct cost of treating skin diseases in the United States was almost $75 billion in 2013, according to a report on the burden of skin disease from the American Academy of Dermatology.
For the nearly 85 million Americans who were seen by a physician for a skin disease in 2013, about $45.9 billion was spent on medical costs such as office visits, procedures, and tests; $15 billion was spent on prescription drugs; and $4.1 billion went for vaccines and skin cancer screening. Another $10 billion was spent on OTC products, the AAD’s Burden of Skin Disease Work Group reported (J Am Acad Dermatol. 2017 Feb 24. doi: 10.1016/j.jaad.2016.12.043).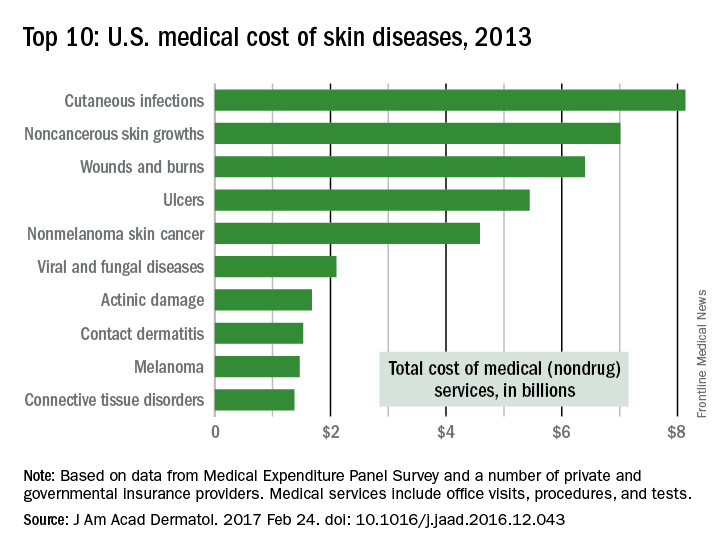
The per-capita cost of skin disease in 2013 was $213, compared with $950 per person for cardiovascular disease, $785 for diabetes, and $147 for end-stage renal disease, they noted.
In discussing the future burden of skin disease, the work group noted that the aging U.S. population and “the increased costs of currently in-use and newly developed dermatologic treatment options [need] to be addressed by an appropriate increase in dermatologic care providers.”
The direct cost of treating skin diseases in the United States was almost $75 billion in 2013, according to a report on the burden of skin disease from the American Academy of Dermatology.
For the nearly 85 million Americans who were seen by a physician for a skin disease in 2013, about $45.9 billion was spent on medical costs such as office visits, procedures, and tests; $15 billion was spent on prescription drugs; and $4.1 billion went for vaccines and skin cancer screening. Another $10 billion was spent on OTC products, the AAD’s Burden of Skin Disease Work Group reported (J Am Acad Dermatol. 2017 Feb 24. doi: 10.1016/j.jaad.2016.12.043).
The per-capita cost of skin disease in 2013 was $213, compared with $950 per person for cardiovascular disease, $785 for diabetes, and $147 for end-stage renal disease, they noted.
In discussing the future burden of skin disease, the work group noted that the aging U.S. population and “the increased costs of currently in-use and newly developed dermatologic treatment options [need] to be addressed by an appropriate increase in dermatologic care providers.”
The direct cost of treating skin diseases in the United States was almost $75 billion in 2013, according to a report on the burden of skin disease from the American Academy of Dermatology.
For the nearly 85 million Americans who were seen by a physician for a skin disease in 2013, about $45.9 billion was spent on medical costs such as office visits, procedures, and tests; $15 billion was spent on prescription drugs; and $4.1 billion went for vaccines and skin cancer screening. Another $10 billion was spent on OTC products, the AAD’s Burden of Skin Disease Work Group reported (J Am Acad Dermatol. 2017 Feb 24. doi: 10.1016/j.jaad.2016.12.043).
The per-capita cost of skin disease in 2013 was $213, compared with $950 per person for cardiovascular disease, $785 for diabetes, and $147 for end-stage renal disease, they noted.
In discussing the future burden of skin disease, the work group noted that the aging U.S. population and “the increased costs of currently in-use and newly developed dermatologic treatment options [need] to be addressed by an appropriate increase in dermatologic care providers.”
Family-based treatment of anorexia promising
LAS VEGAS – Mounting evidence demonstrates that a family-based approach to treating adolescents with anorexia nervosa usually is more effective than hospitalization.
“In the history of psychiatric literature, families have been blamed a lot for psychiatric problems in their children,” James Lock, MD, PhD, said at the annual psychopharmacology update held by the Nevada Psychiatric Association. “Parents are put off by anorexia nervosa, because it’s a very confusing illness and terrifying for them to have their children radically change their behavior. These are kids who are often high achieving and have generally been easy to manage: self-starters, self-directors. Then, suddenly, over a period of 6-7 months, they go from being very well to being at death’s door.”
Enter family-based treatment (FBT) for anorexia nervosa, an outpatient intervention appropriate for children and adolescents who are medically stable. Developed around 2001, FBT is designed to restore weight and put the patient’s development back on track. It’s a team approach consisting of a primary therapist, a pediatrician, and a child and adolescent psychiatrist. Brief hospitalization sometimes is used to resolve medical concerns. Dr. Lock, professor of psychiatry and behavioral sciences at Stanford (Calif.) University, described FBT as “a highly focused staged treatment that emphasizes behavioral recovery rather than insight and understanding or cognitive change. This approach might indirectly improve family functioning and reduce eating-related cognitive distortions for the adolescent.”
According to Dr. Lock, coauthor with Daniel Le Grange, PhD, of “Treatment Manual for Anorexia Nervosa: A Family-Based Approach” second edition, (New York: Guilford Press, 2015), the current evidence base for FBT is limited, consisting of 879 patients enrolled in 11 studies at 12 different sites in four countries, as well as one meta-analysis. “We have not done the kind of research in anorexia nervosa that we need to do,” he said. “Parents ask me this every time I sit down with them: ‘Why don’t we know more? Why don’t we have more clinical guidance?’ It’s not been a priority despite the seriousness of this problem [and the] lifelong impact it has.”
Interest in FBT emerged in part because of studies demonstrating the limitations of hospitalizing patients with the disorder. One trial from the United Kingdom found that, among 90 children who were randomized to one of two outpatient treatments, to an inpatient arm, or to no treatment, no differences in outcomes were observed among the treatment groups (Br J Psychiatry. 1991;159:325-33). Similar results were found in a trial of 167 children who were randomized to either inpatient psychiatric treatment or two forms of outpatient management (Br J Psychiatry. 2007;191[5]:427-35). “If you think hospitalization will cure kids with anorexia, this study tells you that isn’t true,” Dr. Lock said. “It doesn’t say that it doesn’t benefit some people. What it says is that, on average, it’s not better than outpatient treatment for adolescents with anorexia nervosa. It’s important to build systems of care around that knowledge.”
Dr. Lock has his own opinion as to why inpatient psychiatric treatment alone usually doesn’t help anorexia nervosa patients in the long-term. “Learning in an inpatient setting is not generalizable,” he said. “You cannot learn and apply the learning that you get in the hospital to your real life: in your family and your school and your social processes. You can dress up psychotherapy any way you want to, but ultimately, it’s about learning. Can parents learn to be effective at helping their children with anorexia nervosa recover?”
In general, FBT is delivered in three phases over the course of 6-12 months. Phase I involves helping parents assume control of weight restoration in their child. “It tries to accomplish at home what could have been accomplished at a hospital by a nursing staff who are trained and able to disrupt and manage destructive behaviors that lead to weight loss and reinforce cognitive distortions,” Dr. Lock said. In phase II, parents gradually hand control over eating back to their child, while phase III involves shifting the family back to discussing adolescent issues without anorexia nervosa at the center of their concern. One fundamental assumption of FBT, he continued, is that it takes an agnostic view as to the cause of anorexia.
“We don’t have to address cause in order to have an effective treatment,” said Dr. Lock, also a professor of pediatrics at the university. “We are going to try to help patients and parents feel valued, not blamed. Secondly, we need to engage parents in a consultative way, recognizing their skills around their family, and help them apply that to anorexia nervosa.” The expected outcome should be a healthy weight, based on the child’s age. According to Dr. Lock, 79% of patients who have gained at least 4 pounds after 4 weeks of FBT will have a favorable treatment response, while 71% of those who don’t meet that benchmark are likely to fail treatment. “The therapeutic alliance is important in treatment outcome, but our studies suggest it is not enough,” he said. “You have to engage people in treatment to get them started, but if you don’t help the parents be effective in promoting weight gain, your therapeutic alliance will diminish.”
In a randomized trial that compared FBT with adolescent-focused individual therapy (AFT) for adolescents with anorexia nervosa, Dr. Lock and his associates found that, at both 6- and 12-month follow-up, FBT was significantly superior to AFT in helping patients achieve full remission, which was defined as normal weight for age and a mean global Eating Disorder Examination score within one standard deviation of published means (Arch Gen Psychiatry. 2010;67[10]:1025-32). A separate trial found that, after FBT was implemented at the Royal Children’s Hospital, Melbourne, admissions decreased by 50%, readmissions decreased by 75%, and the overall number of days patients spent in the hospital fell by 51% (J Pediatr Health Care. 2014 Jul-Aug 28[4]:322-30).
During the first FBT meeting with patients and their families, Dr. Lock discusses the hazards of anorexia nervosa, including the increased risk of death by cardiac arrest and suicide. “I instill the fact that we have a crisis on our hands, and we need to block the development of anorexia nervosa,” he said. “We have no evidence-based treatments for anorexia nervosa once it becomes chronic, and that occurs after about 5 years. The onset is about age 14. At age 19, on average, your chances of complete recovery are greatly diminished. Of course, you can still be of help by supporting improvement in the quality of their lives; you may help improve their thinking and you may help them restore weight, but many will live with the ongoing anorexia nervosa. So, our greatest chance to be effective is early intervention and the window of opportunity is 3-4 years.” Emphasizing these realities “stops people,” he said. “It’s meant to bring them into clear awareness of what they’re facing.”
Dr. Lock disclosed that he has received grant or research support from the National Institute of Mental Health. He also is a consultant for the Training Institute for Child and Adolescent Eating Disorders and has received royalties from Guilford Press and Oxford Press.
LAS VEGAS – Mounting evidence demonstrates that a family-based approach to treating adolescents with anorexia nervosa usually is more effective than hospitalization.
“In the history of psychiatric literature, families have been blamed a lot for psychiatric problems in their children,” James Lock, MD, PhD, said at the annual psychopharmacology update held by the Nevada Psychiatric Association. “Parents are put off by anorexia nervosa, because it’s a very confusing illness and terrifying for them to have their children radically change their behavior. These are kids who are often high achieving and have generally been easy to manage: self-starters, self-directors. Then, suddenly, over a period of 6-7 months, they go from being very well to being at death’s door.”
Enter family-based treatment (FBT) for anorexia nervosa, an outpatient intervention appropriate for children and adolescents who are medically stable. Developed around 2001, FBT is designed to restore weight and put the patient’s development back on track. It’s a team approach consisting of a primary therapist, a pediatrician, and a child and adolescent psychiatrist. Brief hospitalization sometimes is used to resolve medical concerns. Dr. Lock, professor of psychiatry and behavioral sciences at Stanford (Calif.) University, described FBT as “a highly focused staged treatment that emphasizes behavioral recovery rather than insight and understanding or cognitive change. This approach might indirectly improve family functioning and reduce eating-related cognitive distortions for the adolescent.”
According to Dr. Lock, coauthor with Daniel Le Grange, PhD, of “Treatment Manual for Anorexia Nervosa: A Family-Based Approach” second edition, (New York: Guilford Press, 2015), the current evidence base for FBT is limited, consisting of 879 patients enrolled in 11 studies at 12 different sites in four countries, as well as one meta-analysis. “We have not done the kind of research in anorexia nervosa that we need to do,” he said. “Parents ask me this every time I sit down with them: ‘Why don’t we know more? Why don’t we have more clinical guidance?’ It’s not been a priority despite the seriousness of this problem [and the] lifelong impact it has.”
Interest in FBT emerged in part because of studies demonstrating the limitations of hospitalizing patients with the disorder. One trial from the United Kingdom found that, among 90 children who were randomized to one of two outpatient treatments, to an inpatient arm, or to no treatment, no differences in outcomes were observed among the treatment groups (Br J Psychiatry. 1991;159:325-33). Similar results were found in a trial of 167 children who were randomized to either inpatient psychiatric treatment or two forms of outpatient management (Br J Psychiatry. 2007;191[5]:427-35). “If you think hospitalization will cure kids with anorexia, this study tells you that isn’t true,” Dr. Lock said. “It doesn’t say that it doesn’t benefit some people. What it says is that, on average, it’s not better than outpatient treatment for adolescents with anorexia nervosa. It’s important to build systems of care around that knowledge.”
Dr. Lock has his own opinion as to why inpatient psychiatric treatment alone usually doesn’t help anorexia nervosa patients in the long-term. “Learning in an inpatient setting is not generalizable,” he said. “You cannot learn and apply the learning that you get in the hospital to your real life: in your family and your school and your social processes. You can dress up psychotherapy any way you want to, but ultimately, it’s about learning. Can parents learn to be effective at helping their children with anorexia nervosa recover?”
In general, FBT is delivered in three phases over the course of 6-12 months. Phase I involves helping parents assume control of weight restoration in their child. “It tries to accomplish at home what could have been accomplished at a hospital by a nursing staff who are trained and able to disrupt and manage destructive behaviors that lead to weight loss and reinforce cognitive distortions,” Dr. Lock said. In phase II, parents gradually hand control over eating back to their child, while phase III involves shifting the family back to discussing adolescent issues without anorexia nervosa at the center of their concern. One fundamental assumption of FBT, he continued, is that it takes an agnostic view as to the cause of anorexia.
“We don’t have to address cause in order to have an effective treatment,” said Dr. Lock, also a professor of pediatrics at the university. “We are going to try to help patients and parents feel valued, not blamed. Secondly, we need to engage parents in a consultative way, recognizing their skills around their family, and help them apply that to anorexia nervosa.” The expected outcome should be a healthy weight, based on the child’s age. According to Dr. Lock, 79% of patients who have gained at least 4 pounds after 4 weeks of FBT will have a favorable treatment response, while 71% of those who don’t meet that benchmark are likely to fail treatment. “The therapeutic alliance is important in treatment outcome, but our studies suggest it is not enough,” he said. “You have to engage people in treatment to get them started, but if you don’t help the parents be effective in promoting weight gain, your therapeutic alliance will diminish.”
In a randomized trial that compared FBT with adolescent-focused individual therapy (AFT) for adolescents with anorexia nervosa, Dr. Lock and his associates found that, at both 6- and 12-month follow-up, FBT was significantly superior to AFT in helping patients achieve full remission, which was defined as normal weight for age and a mean global Eating Disorder Examination score within one standard deviation of published means (Arch Gen Psychiatry. 2010;67[10]:1025-32). A separate trial found that, after FBT was implemented at the Royal Children’s Hospital, Melbourne, admissions decreased by 50%, readmissions decreased by 75%, and the overall number of days patients spent in the hospital fell by 51% (J Pediatr Health Care. 2014 Jul-Aug 28[4]:322-30).
During the first FBT meeting with patients and their families, Dr. Lock discusses the hazards of anorexia nervosa, including the increased risk of death by cardiac arrest and suicide. “I instill the fact that we have a crisis on our hands, and we need to block the development of anorexia nervosa,” he said. “We have no evidence-based treatments for anorexia nervosa once it becomes chronic, and that occurs after about 5 years. The onset is about age 14. At age 19, on average, your chances of complete recovery are greatly diminished. Of course, you can still be of help by supporting improvement in the quality of their lives; you may help improve their thinking and you may help them restore weight, but many will live with the ongoing anorexia nervosa. So, our greatest chance to be effective is early intervention and the window of opportunity is 3-4 years.” Emphasizing these realities “stops people,” he said. “It’s meant to bring them into clear awareness of what they’re facing.”
Dr. Lock disclosed that he has received grant or research support from the National Institute of Mental Health. He also is a consultant for the Training Institute for Child and Adolescent Eating Disorders and has received royalties from Guilford Press and Oxford Press.
LAS VEGAS – Mounting evidence demonstrates that a family-based approach to treating adolescents with anorexia nervosa usually is more effective than hospitalization.
“In the history of psychiatric literature, families have been blamed a lot for psychiatric problems in their children,” James Lock, MD, PhD, said at the annual psychopharmacology update held by the Nevada Psychiatric Association. “Parents are put off by anorexia nervosa, because it’s a very confusing illness and terrifying for them to have their children radically change their behavior. These are kids who are often high achieving and have generally been easy to manage: self-starters, self-directors. Then, suddenly, over a period of 6-7 months, they go from being very well to being at death’s door.”
Enter family-based treatment (FBT) for anorexia nervosa, an outpatient intervention appropriate for children and adolescents who are medically stable. Developed around 2001, FBT is designed to restore weight and put the patient’s development back on track. It’s a team approach consisting of a primary therapist, a pediatrician, and a child and adolescent psychiatrist. Brief hospitalization sometimes is used to resolve medical concerns. Dr. Lock, professor of psychiatry and behavioral sciences at Stanford (Calif.) University, described FBT as “a highly focused staged treatment that emphasizes behavioral recovery rather than insight and understanding or cognitive change. This approach might indirectly improve family functioning and reduce eating-related cognitive distortions for the adolescent.”
According to Dr. Lock, coauthor with Daniel Le Grange, PhD, of “Treatment Manual for Anorexia Nervosa: A Family-Based Approach” second edition, (New York: Guilford Press, 2015), the current evidence base for FBT is limited, consisting of 879 patients enrolled in 11 studies at 12 different sites in four countries, as well as one meta-analysis. “We have not done the kind of research in anorexia nervosa that we need to do,” he said. “Parents ask me this every time I sit down with them: ‘Why don’t we know more? Why don’t we have more clinical guidance?’ It’s not been a priority despite the seriousness of this problem [and the] lifelong impact it has.”
Interest in FBT emerged in part because of studies demonstrating the limitations of hospitalizing patients with the disorder. One trial from the United Kingdom found that, among 90 children who were randomized to one of two outpatient treatments, to an inpatient arm, or to no treatment, no differences in outcomes were observed among the treatment groups (Br J Psychiatry. 1991;159:325-33). Similar results were found in a trial of 167 children who were randomized to either inpatient psychiatric treatment or two forms of outpatient management (Br J Psychiatry. 2007;191[5]:427-35). “If you think hospitalization will cure kids with anorexia, this study tells you that isn’t true,” Dr. Lock said. “It doesn’t say that it doesn’t benefit some people. What it says is that, on average, it’s not better than outpatient treatment for adolescents with anorexia nervosa. It’s important to build systems of care around that knowledge.”
Dr. Lock has his own opinion as to why inpatient psychiatric treatment alone usually doesn’t help anorexia nervosa patients in the long-term. “Learning in an inpatient setting is not generalizable,” he said. “You cannot learn and apply the learning that you get in the hospital to your real life: in your family and your school and your social processes. You can dress up psychotherapy any way you want to, but ultimately, it’s about learning. Can parents learn to be effective at helping their children with anorexia nervosa recover?”
In general, FBT is delivered in three phases over the course of 6-12 months. Phase I involves helping parents assume control of weight restoration in their child. “It tries to accomplish at home what could have been accomplished at a hospital by a nursing staff who are trained and able to disrupt and manage destructive behaviors that lead to weight loss and reinforce cognitive distortions,” Dr. Lock said. In phase II, parents gradually hand control over eating back to their child, while phase III involves shifting the family back to discussing adolescent issues without anorexia nervosa at the center of their concern. One fundamental assumption of FBT, he continued, is that it takes an agnostic view as to the cause of anorexia.
“We don’t have to address cause in order to have an effective treatment,” said Dr. Lock, also a professor of pediatrics at the university. “We are going to try to help patients and parents feel valued, not blamed. Secondly, we need to engage parents in a consultative way, recognizing their skills around their family, and help them apply that to anorexia nervosa.” The expected outcome should be a healthy weight, based on the child’s age. According to Dr. Lock, 79% of patients who have gained at least 4 pounds after 4 weeks of FBT will have a favorable treatment response, while 71% of those who don’t meet that benchmark are likely to fail treatment. “The therapeutic alliance is important in treatment outcome, but our studies suggest it is not enough,” he said. “You have to engage people in treatment to get them started, but if you don’t help the parents be effective in promoting weight gain, your therapeutic alliance will diminish.”
In a randomized trial that compared FBT with adolescent-focused individual therapy (AFT) for adolescents with anorexia nervosa, Dr. Lock and his associates found that, at both 6- and 12-month follow-up, FBT was significantly superior to AFT in helping patients achieve full remission, which was defined as normal weight for age and a mean global Eating Disorder Examination score within one standard deviation of published means (Arch Gen Psychiatry. 2010;67[10]:1025-32). A separate trial found that, after FBT was implemented at the Royal Children’s Hospital, Melbourne, admissions decreased by 50%, readmissions decreased by 75%, and the overall number of days patients spent in the hospital fell by 51% (J Pediatr Health Care. 2014 Jul-Aug 28[4]:322-30).
During the first FBT meeting with patients and their families, Dr. Lock discusses the hazards of anorexia nervosa, including the increased risk of death by cardiac arrest and suicide. “I instill the fact that we have a crisis on our hands, and we need to block the development of anorexia nervosa,” he said. “We have no evidence-based treatments for anorexia nervosa once it becomes chronic, and that occurs after about 5 years. The onset is about age 14. At age 19, on average, your chances of complete recovery are greatly diminished. Of course, you can still be of help by supporting improvement in the quality of their lives; you may help improve their thinking and you may help them restore weight, but many will live with the ongoing anorexia nervosa. So, our greatest chance to be effective is early intervention and the window of opportunity is 3-4 years.” Emphasizing these realities “stops people,” he said. “It’s meant to bring them into clear awareness of what they’re facing.”
Dr. Lock disclosed that he has received grant or research support from the National Institute of Mental Health. He also is a consultant for the Training Institute for Child and Adolescent Eating Disorders and has received royalties from Guilford Press and Oxford Press.
MCI May Predict Dementia in Patients With Parkinson’s Disease
Mild cognitive impairment (MCI) in early Parkinson’s disease may predict subsequent dementia, whether or not the person reverts to normal cognition, according to research published online ahead of print January 20 in Neurology.
There is limited information about the incidence, persistence, and outcome of MCI due to Parkinson’s disease (PD-MCI) over time. A previous study suggested that PD-MCI in patients with newly diagnosed Parkinson’s disease predicts a highly increased risk for dementia within three years of diagnosis. Many patients with PD-MCI reverted to normal cognition during the investigation, however.
A Population-Based Cohort
Dr. Pedersen and his colleagues conducted a study to examine the incidence, progression, and reversion of MCI in patients with Parkinson’s disease over a five-year period. Patients included in the study were all participants in the Norwegian ParkWest project, an ongoing, prospective, population-based cohort study of the incidence, neurobiology, and prognosis of Parkinson’s disease. All participants were white and met diagnostic criteria for Parkinson’s disease.
Of the 212 patients recruited for the study, 196 were drug-naïve and without major depression or dementia at baseline. Among these 196 patients, 18 were rediagnosed during follow-up; as a result, 178 patients were eligible for the study.
After baseline examinations, researchers initiated dopaminergic medication and assessed patients clinically every six months. In addition, the investigators conducted standardized examinations of motor, neuropsychiatric, and cognitive function at study entry and after one, three, and five years of follow-up.
Diagnosing Parkinson’s Disease Dementia
Investigators identified patients with PD-MCI and determined dementia status using the Movement Disorder Society criteria. In addition, researchers made a diagnosis of Parkinson’s disease-associated dementia if they found evidence of cognitive decline during follow-up. Parkinson’s disease dementia exclusion criteria included comorbid conditions or disease that could cause or contribute to mental impairments. None of the participants met diagnostic criteria for dementia with Lewy bodies, Alzheimer dementia, or other dementia syndromes.
In all, 36 patients fulfilled criteria for PD-MCI at baseline. Among participants who were cognitively normal at baseline, the cumulative incidence of PD-MCI was 9.9% after one year of follow-up, 23.2% after three years, and 28.9% after five years. Also, 39.1% of patients with baseline or incident PD-MCI progressed to dementia during the five-year study period. A 59.1% rate of conversion to dementia was observed in patients with persistent PD-MCI at one year. Investigators also found that 27.8% of patients with baseline PD-MCI had reverted to normal cognition by the end of the study. Finally, PD-MCI reverters within the first three years of follow-up were at an increased risk of subsequently developing dementia, compared with cognitively normal patients.
One of the investigation’s limitations was that it had a short duration, considering the typically slow progression of Parkinson’s disease. In addition, the number of PD-MCI reverters was limited, and the cognitive test battery did not include language tests. Despite these limitations, the findings overall were valid and representative of the general population of patients with Parkinson’s disease, said the researchers.
“A remarkable observation in our study is the increased risk of dementia even in PD-MCI reverters. This [result] suggests that PD-MCI has clinical implications once diagnosed in early Parkinson’s disease,” said Dr. Pedersen and colleagues.
“This [finding] is important because patients with PD-MCI who revert to normal cognition may be excellent candidates for timely interventions to prevent progressive cognitive decline when they become available in the future.”
—Erica Tricarico
Suggested Reading
Pedersen KF, Larsen JP, Tysnes OB, Alves G. Natural course of mild cognitive impairment in Parkinson disease: A 5-year population-based study. Neurology. 2017 Jan 20 [Epub ahead of print].
Mild cognitive impairment (MCI) in early Parkinson’s disease may predict subsequent dementia, whether or not the person reverts to normal cognition, according to research published online ahead of print January 20 in Neurology.
There is limited information about the incidence, persistence, and outcome of MCI due to Parkinson’s disease (PD-MCI) over time. A previous study suggested that PD-MCI in patients with newly diagnosed Parkinson’s disease predicts a highly increased risk for dementia within three years of diagnosis. Many patients with PD-MCI reverted to normal cognition during the investigation, however.
A Population-Based Cohort
Dr. Pedersen and his colleagues conducted a study to examine the incidence, progression, and reversion of MCI in patients with Parkinson’s disease over a five-year period. Patients included in the study were all participants in the Norwegian ParkWest project, an ongoing, prospective, population-based cohort study of the incidence, neurobiology, and prognosis of Parkinson’s disease. All participants were white and met diagnostic criteria for Parkinson’s disease.
Of the 212 patients recruited for the study, 196 were drug-naïve and without major depression or dementia at baseline. Among these 196 patients, 18 were rediagnosed during follow-up; as a result, 178 patients were eligible for the study.
After baseline examinations, researchers initiated dopaminergic medication and assessed patients clinically every six months. In addition, the investigators conducted standardized examinations of motor, neuropsychiatric, and cognitive function at study entry and after one, three, and five years of follow-up.
Diagnosing Parkinson’s Disease Dementia
Investigators identified patients with PD-MCI and determined dementia status using the Movement Disorder Society criteria. In addition, researchers made a diagnosis of Parkinson’s disease-associated dementia if they found evidence of cognitive decline during follow-up. Parkinson’s disease dementia exclusion criteria included comorbid conditions or disease that could cause or contribute to mental impairments. None of the participants met diagnostic criteria for dementia with Lewy bodies, Alzheimer dementia, or other dementia syndromes.
In all, 36 patients fulfilled criteria for PD-MCI at baseline. Among participants who were cognitively normal at baseline, the cumulative incidence of PD-MCI was 9.9% after one year of follow-up, 23.2% after three years, and 28.9% after five years. Also, 39.1% of patients with baseline or incident PD-MCI progressed to dementia during the five-year study period. A 59.1% rate of conversion to dementia was observed in patients with persistent PD-MCI at one year. Investigators also found that 27.8% of patients with baseline PD-MCI had reverted to normal cognition by the end of the study. Finally, PD-MCI reverters within the first three years of follow-up were at an increased risk of subsequently developing dementia, compared with cognitively normal patients.
One of the investigation’s limitations was that it had a short duration, considering the typically slow progression of Parkinson’s disease. In addition, the number of PD-MCI reverters was limited, and the cognitive test battery did not include language tests. Despite these limitations, the findings overall were valid and representative of the general population of patients with Parkinson’s disease, said the researchers.
“A remarkable observation in our study is the increased risk of dementia even in PD-MCI reverters. This [result] suggests that PD-MCI has clinical implications once diagnosed in early Parkinson’s disease,” said Dr. Pedersen and colleagues.
“This [finding] is important because patients with PD-MCI who revert to normal cognition may be excellent candidates for timely interventions to prevent progressive cognitive decline when they become available in the future.”
—Erica Tricarico
Suggested Reading
Pedersen KF, Larsen JP, Tysnes OB, Alves G. Natural course of mild cognitive impairment in Parkinson disease: A 5-year population-based study. Neurology. 2017 Jan 20 [Epub ahead of print].
Mild cognitive impairment (MCI) in early Parkinson’s disease may predict subsequent dementia, whether or not the person reverts to normal cognition, according to research published online ahead of print January 20 in Neurology.
There is limited information about the incidence, persistence, and outcome of MCI due to Parkinson’s disease (PD-MCI) over time. A previous study suggested that PD-MCI in patients with newly diagnosed Parkinson’s disease predicts a highly increased risk for dementia within three years of diagnosis. Many patients with PD-MCI reverted to normal cognition during the investigation, however.
A Population-Based Cohort
Dr. Pedersen and his colleagues conducted a study to examine the incidence, progression, and reversion of MCI in patients with Parkinson’s disease over a five-year period. Patients included in the study were all participants in the Norwegian ParkWest project, an ongoing, prospective, population-based cohort study of the incidence, neurobiology, and prognosis of Parkinson’s disease. All participants were white and met diagnostic criteria for Parkinson’s disease.
Of the 212 patients recruited for the study, 196 were drug-naïve and without major depression or dementia at baseline. Among these 196 patients, 18 were rediagnosed during follow-up; as a result, 178 patients were eligible for the study.
After baseline examinations, researchers initiated dopaminergic medication and assessed patients clinically every six months. In addition, the investigators conducted standardized examinations of motor, neuropsychiatric, and cognitive function at study entry and after one, three, and five years of follow-up.
Diagnosing Parkinson’s Disease Dementia
Investigators identified patients with PD-MCI and determined dementia status using the Movement Disorder Society criteria. In addition, researchers made a diagnosis of Parkinson’s disease-associated dementia if they found evidence of cognitive decline during follow-up. Parkinson’s disease dementia exclusion criteria included comorbid conditions or disease that could cause or contribute to mental impairments. None of the participants met diagnostic criteria for dementia with Lewy bodies, Alzheimer dementia, or other dementia syndromes.
In all, 36 patients fulfilled criteria for PD-MCI at baseline. Among participants who were cognitively normal at baseline, the cumulative incidence of PD-MCI was 9.9% after one year of follow-up, 23.2% after three years, and 28.9% after five years. Also, 39.1% of patients with baseline or incident PD-MCI progressed to dementia during the five-year study period. A 59.1% rate of conversion to dementia was observed in patients with persistent PD-MCI at one year. Investigators also found that 27.8% of patients with baseline PD-MCI had reverted to normal cognition by the end of the study. Finally, PD-MCI reverters within the first three years of follow-up were at an increased risk of subsequently developing dementia, compared with cognitively normal patients.
One of the investigation’s limitations was that it had a short duration, considering the typically slow progression of Parkinson’s disease. In addition, the number of PD-MCI reverters was limited, and the cognitive test battery did not include language tests. Despite these limitations, the findings overall were valid and representative of the general population of patients with Parkinson’s disease, said the researchers.
“A remarkable observation in our study is the increased risk of dementia even in PD-MCI reverters. This [result] suggests that PD-MCI has clinical implications once diagnosed in early Parkinson’s disease,” said Dr. Pedersen and colleagues.
“This [finding] is important because patients with PD-MCI who revert to normal cognition may be excellent candidates for timely interventions to prevent progressive cognitive decline when they become available in the future.”
—Erica Tricarico
Suggested Reading
Pedersen KF, Larsen JP, Tysnes OB, Alves G. Natural course of mild cognitive impairment in Parkinson disease: A 5-year population-based study. Neurology. 2017 Jan 20 [Epub ahead of print].
Blood NfL Accurately Differentiates Parkinson’s Disease From Atypical Parkinsonian Disorders
Quantification of blood neurofilament light chain (NfL) concentration effectively distinguishes Parkinson’s disease from atypical parkinsonian disorders (APD), according to research published online ahead of print February 8 in Neurology. In addition, NfL in blood may also improve the diagnostic examination of patients with parkinsonian symptoms in primary and specialized settings, as well as improve treatment of axonal degeneration. It can be challenging to differentiate Parkinson’s disease from APD, especially during the early stages of the diseases. Although there are no established diagnostic methods to accurately distinguish Parkinson’s disease from APD, previous studies suggest that the NfL protein in CSF may be a reliable biomarker for APD. Several studies also indicate that CSF concentration of NfL is increased in APD, but not in Parkinson’s disease. The diagnostic utility of blood NfL had not been studied previously, however.
The investigators found a significant correlation between blood and CSF concentrations of NfL. In addition, they observed in all cohorts that blood NfL was increased in patients with MSA, PSP, and CBS, when compared with patients with Parkinson’s disease and healthy controls. Researchers concluded that blood NfL accurately differentiated Parkinson’s disease from APD in all three cohorts.
“Development of a fully automated clinical-grade assay and establishment of cutoff points would be necessary for implementation of blood-based NfL measurements in clinical practice,” said the researchers.
—Erica Tricarico
Suggested Reading
Hansson O, Janelidze S, Hall S, et al. Blood-based NfL: A biomarker for differential diagnosis of parkinsonian disorder. Neurology. 2017 Feb 8 [Epub ahead of print].
Quantification of blood neurofilament light chain (NfL) concentration effectively distinguishes Parkinson’s disease from atypical parkinsonian disorders (APD), according to research published online ahead of print February 8 in Neurology. In addition, NfL in blood may also improve the diagnostic examination of patients with parkinsonian symptoms in primary and specialized settings, as well as improve treatment of axonal degeneration. It can be challenging to differentiate Parkinson’s disease from APD, especially during the early stages of the diseases. Although there are no established diagnostic methods to accurately distinguish Parkinson’s disease from APD, previous studies suggest that the NfL protein in CSF may be a reliable biomarker for APD. Several studies also indicate that CSF concentration of NfL is increased in APD, but not in Parkinson’s disease. The diagnostic utility of blood NfL had not been studied previously, however.
The investigators found a significant correlation between blood and CSF concentrations of NfL. In addition, they observed in all cohorts that blood NfL was increased in patients with MSA, PSP, and CBS, when compared with patients with Parkinson’s disease and healthy controls. Researchers concluded that blood NfL accurately differentiated Parkinson’s disease from APD in all three cohorts.
“Development of a fully automated clinical-grade assay and establishment of cutoff points would be necessary for implementation of blood-based NfL measurements in clinical practice,” said the researchers.
—Erica Tricarico
Suggested Reading
Hansson O, Janelidze S, Hall S, et al. Blood-based NfL: A biomarker for differential diagnosis of parkinsonian disorder. Neurology. 2017 Feb 8 [Epub ahead of print].
Quantification of blood neurofilament light chain (NfL) concentration effectively distinguishes Parkinson’s disease from atypical parkinsonian disorders (APD), according to research published online ahead of print February 8 in Neurology. In addition, NfL in blood may also improve the diagnostic examination of patients with parkinsonian symptoms in primary and specialized settings, as well as improve treatment of axonal degeneration. It can be challenging to differentiate Parkinson’s disease from APD, especially during the early stages of the diseases. Although there are no established diagnostic methods to accurately distinguish Parkinson’s disease from APD, previous studies suggest that the NfL protein in CSF may be a reliable biomarker for APD. Several studies also indicate that CSF concentration of NfL is increased in APD, but not in Parkinson’s disease. The diagnostic utility of blood NfL had not been studied previously, however.
The investigators found a significant correlation between blood and CSF concentrations of NfL. In addition, they observed in all cohorts that blood NfL was increased in patients with MSA, PSP, and CBS, when compared with patients with Parkinson’s disease and healthy controls. Researchers concluded that blood NfL accurately differentiated Parkinson’s disease from APD in all three cohorts.
“Development of a fully automated clinical-grade assay and establishment of cutoff points would be necessary for implementation of blood-based NfL measurements in clinical practice,” said the researchers.
—Erica Tricarico
Suggested Reading
Hansson O, Janelidze S, Hall S, et al. Blood-based NfL: A biomarker for differential diagnosis of parkinsonian disorder. Neurology. 2017 Feb 8 [Epub ahead of print].
Pediatric Dermatology Consult - March 2017
By Jusleen Ahluwalia, MD, and Lawrence F. Eichenfield, MD
Subcutaneous fat necrosis of the newborn
The clinical history and morphology of our patient’s cutaneous manifestation is highly suggestive of subcutaneous fat necrosis of the newborn (SFN). SFN is a self-limited, lobular form of panniculitis that typically affects newborns at term or post term until the first 6 weeks of life.1 Delivery complications resulting in perinatal stress – including perinatal hypothermia, hypoxia, and birth trauma – have been associated with the development of SFN.2 Other risk factors include maternal disorders during pregnancy, such as diabetes, hypertension, exposure to tobacco, and thrombotic events.1,2 SFN has been noted after therapeutic hypothermia that is utilized to minimize neurologic effects of neonatal hypoxic ischemic encephalopathy.1
It has been hypothesized that SFN follows hypoxic injury to fat caused by local trauma, while in some cases it is proposed that SFN results from an imbalance of saturated and monounsaturated fats leading to crystallization at certain temperatures in the neonatal subcutis.1
The clinical presentation of SFN is characterized by the development of one to several erythematous violaceous subcutaneous nodules and plaques that can evolve into firm calcifications, and may be tender to palpation. They are characteristically located on the shoulders, back, and upper limbs. Spontaneous regression has been observed without scarring within 2-5 months; however, cutaneous atrophy of the affected areas can follow recovery from the condition.1-3
Hypercalcemia is a potentially fatal complication of SFN that infrequently occurs in a subset of patients. Its risk increases with the extent of perinatal injury and degree of fat necrosis.1 Several studies have documented a prevalence of hypercalcemia in 36%-56% of infants with SFN; however, most of these cases were mild and conservatively managed.2,4 Hypercalcemia may manifest without symptoms or present with vomiting, irritability, and seizures.1 Nephrocalcinosis can complicate high calcium levels and is detected by abdominal ultrasonography. Other complications that can accompany the development of SFN include thrombocytopenia, hyperglycemia, and hypertriglyceridemia, although this relationship is controversial as these conditions can be attributed to other neonatal disease or maternal factors.5
Differential diagnosis
Although SFN is generally a transient, self-limited condition, recognition of SFN is critical to monitor and avoid metabolic alterations associated with SFN. Sclerema neonatorum is another rare condition characterized by diffuse hardening of the skin affecting infants up to 4 months of age with severe underlying disease and systemic symptoms.1
Deep soft tissue infections, such as cellulitis, are usually accompanied with fever and other signs of infection.1 Mechanical trauma may induce firm, subcutaneous nodules in areas where fat is adjacent to bone and should be considered in any infant or child with subcutaneous nodules over areas prone to injury.1 Sudden withdrawal of systemic steroids can cause subcutaneous nodules typically located on cheeks, arms, and trunk.1
Management
Most infants with SFN are managed conservatively.1 Recently proposed guidelines for the management of SFN include weekly monitoring of calcium levels until 1 month of age and monthly until 6 months of age or after resolution of the cutaneous lesion, and more frequently if hypercalcemia is documented. Platelet count and creatinine, glucose, and triglyceride levels also should be assessed.5 At-risk infants should be evaluated for nephrocalcinosis with abdominal ultrasonagraphy.1,6 Treatment of hypercalcemia can consist of modification of diet with low levels of calcium and vitamin D, intravenous saline, calcium-wasting diuretics, or occasionally corticosteroids. Bisphosphonates also have been reported to successfully treat hypercalcemia in the setting of SFN.1,6
References
1. Disorders of the subcutaneous tissue, in “Neonatal and Infant Dermatology,” 3rd ed. (Philadelphia: Saunders, 2015, p. 443-55).
2. Br J Dermatol. 2007 Apr;156(4):709-15.
3. An Bras Dermatol. 2013 Nov-Dec;88(6 Suppl 1):154-7.
4. Pediatr Dermatol. 1999 Sep-Oct;16(5):384-7.
5. Pediatr Dermatol. 2016 Nov;33(6):e353-5.
6. Arch Dis Child Fetal Neonatal Ed. 2014 Sep;99(5):F419-21.
Dr. Ahluwalia and Dr. Eichenfield are in the division of pediatric and adolescent dermatology, Rady Children’s Hospital, San Diego, and the departments of dermatology and pediatrics, University of California, San Diego. They said they had no relevant financial disclosures. Email them at [email protected].
By Jusleen Ahluwalia, MD, and Lawrence F. Eichenfield, MD
Subcutaneous fat necrosis of the newborn
The clinical history and morphology of our patient’s cutaneous manifestation is highly suggestive of subcutaneous fat necrosis of the newborn (SFN). SFN is a self-limited, lobular form of panniculitis that typically affects newborns at term or post term until the first 6 weeks of life.1 Delivery complications resulting in perinatal stress – including perinatal hypothermia, hypoxia, and birth trauma – have been associated with the development of SFN.2 Other risk factors include maternal disorders during pregnancy, such as diabetes, hypertension, exposure to tobacco, and thrombotic events.1,2 SFN has been noted after therapeutic hypothermia that is utilized to minimize neurologic effects of neonatal hypoxic ischemic encephalopathy.1
It has been hypothesized that SFN follows hypoxic injury to fat caused by local trauma, while in some cases it is proposed that SFN results from an imbalance of saturated and monounsaturated fats leading to crystallization at certain temperatures in the neonatal subcutis.1
The clinical presentation of SFN is characterized by the development of one to several erythematous violaceous subcutaneous nodules and plaques that can evolve into firm calcifications, and may be tender to palpation. They are characteristically located on the shoulders, back, and upper limbs. Spontaneous regression has been observed without scarring within 2-5 months; however, cutaneous atrophy of the affected areas can follow recovery from the condition.1-3
Hypercalcemia is a potentially fatal complication of SFN that infrequently occurs in a subset of patients. Its risk increases with the extent of perinatal injury and degree of fat necrosis.1 Several studies have documented a prevalence of hypercalcemia in 36%-56% of infants with SFN; however, most of these cases were mild and conservatively managed.2,4 Hypercalcemia may manifest without symptoms or present with vomiting, irritability, and seizures.1 Nephrocalcinosis can complicate high calcium levels and is detected by abdominal ultrasonography. Other complications that can accompany the development of SFN include thrombocytopenia, hyperglycemia, and hypertriglyceridemia, although this relationship is controversial as these conditions can be attributed to other neonatal disease or maternal factors.5
Differential diagnosis
Although SFN is generally a transient, self-limited condition, recognition of SFN is critical to monitor and avoid metabolic alterations associated with SFN. Sclerema neonatorum is another rare condition characterized by diffuse hardening of the skin affecting infants up to 4 months of age with severe underlying disease and systemic symptoms.1
Deep soft tissue infections, such as cellulitis, are usually accompanied with fever and other signs of infection.1 Mechanical trauma may induce firm, subcutaneous nodules in areas where fat is adjacent to bone and should be considered in any infant or child with subcutaneous nodules over areas prone to injury.1 Sudden withdrawal of systemic steroids can cause subcutaneous nodules typically located on cheeks, arms, and trunk.1
Management
Most infants with SFN are managed conservatively.1 Recently proposed guidelines for the management of SFN include weekly monitoring of calcium levels until 1 month of age and monthly until 6 months of age or after resolution of the cutaneous lesion, and more frequently if hypercalcemia is documented. Platelet count and creatinine, glucose, and triglyceride levels also should be assessed.5 At-risk infants should be evaluated for nephrocalcinosis with abdominal ultrasonagraphy.1,6 Treatment of hypercalcemia can consist of modification of diet with low levels of calcium and vitamin D, intravenous saline, calcium-wasting diuretics, or occasionally corticosteroids. Bisphosphonates also have been reported to successfully treat hypercalcemia in the setting of SFN.1,6
References
1. Disorders of the subcutaneous tissue, in “Neonatal and Infant Dermatology,” 3rd ed. (Philadelphia: Saunders, 2015, p. 443-55).
2. Br J Dermatol. 2007 Apr;156(4):709-15.
3. An Bras Dermatol. 2013 Nov-Dec;88(6 Suppl 1):154-7.
4. Pediatr Dermatol. 1999 Sep-Oct;16(5):384-7.
5. Pediatr Dermatol. 2016 Nov;33(6):e353-5.
6. Arch Dis Child Fetal Neonatal Ed. 2014 Sep;99(5):F419-21.
Dr. Ahluwalia and Dr. Eichenfield are in the division of pediatric and adolescent dermatology, Rady Children’s Hospital, San Diego, and the departments of dermatology and pediatrics, University of California, San Diego. They said they had no relevant financial disclosures. Email them at [email protected].
By Jusleen Ahluwalia, MD, and Lawrence F. Eichenfield, MD
Subcutaneous fat necrosis of the newborn
The clinical history and morphology of our patient’s cutaneous manifestation is highly suggestive of subcutaneous fat necrosis of the newborn (SFN). SFN is a self-limited, lobular form of panniculitis that typically affects newborns at term or post term until the first 6 weeks of life.1 Delivery complications resulting in perinatal stress – including perinatal hypothermia, hypoxia, and birth trauma – have been associated with the development of SFN.2 Other risk factors include maternal disorders during pregnancy, such as diabetes, hypertension, exposure to tobacco, and thrombotic events.1,2 SFN has been noted after therapeutic hypothermia that is utilized to minimize neurologic effects of neonatal hypoxic ischemic encephalopathy.1
It has been hypothesized that SFN follows hypoxic injury to fat caused by local trauma, while in some cases it is proposed that SFN results from an imbalance of saturated and monounsaturated fats leading to crystallization at certain temperatures in the neonatal subcutis.1
The clinical presentation of SFN is characterized by the development of one to several erythematous violaceous subcutaneous nodules and plaques that can evolve into firm calcifications, and may be tender to palpation. They are characteristically located on the shoulders, back, and upper limbs. Spontaneous regression has been observed without scarring within 2-5 months; however, cutaneous atrophy of the affected areas can follow recovery from the condition.1-3
Hypercalcemia is a potentially fatal complication of SFN that infrequently occurs in a subset of patients. Its risk increases with the extent of perinatal injury and degree of fat necrosis.1 Several studies have documented a prevalence of hypercalcemia in 36%-56% of infants with SFN; however, most of these cases were mild and conservatively managed.2,4 Hypercalcemia may manifest without symptoms or present with vomiting, irritability, and seizures.1 Nephrocalcinosis can complicate high calcium levels and is detected by abdominal ultrasonography. Other complications that can accompany the development of SFN include thrombocytopenia, hyperglycemia, and hypertriglyceridemia, although this relationship is controversial as these conditions can be attributed to other neonatal disease or maternal factors.5
Differential diagnosis
Although SFN is generally a transient, self-limited condition, recognition of SFN is critical to monitor and avoid metabolic alterations associated with SFN. Sclerema neonatorum is another rare condition characterized by diffuse hardening of the skin affecting infants up to 4 months of age with severe underlying disease and systemic symptoms.1
Deep soft tissue infections, such as cellulitis, are usually accompanied with fever and other signs of infection.1 Mechanical trauma may induce firm, subcutaneous nodules in areas where fat is adjacent to bone and should be considered in any infant or child with subcutaneous nodules over areas prone to injury.1 Sudden withdrawal of systemic steroids can cause subcutaneous nodules typically located on cheeks, arms, and trunk.1
Management
Most infants with SFN are managed conservatively.1 Recently proposed guidelines for the management of SFN include weekly monitoring of calcium levels until 1 month of age and monthly until 6 months of age or after resolution of the cutaneous lesion, and more frequently if hypercalcemia is documented. Platelet count and creatinine, glucose, and triglyceride levels also should be assessed.5 At-risk infants should be evaluated for nephrocalcinosis with abdominal ultrasonagraphy.1,6 Treatment of hypercalcemia can consist of modification of diet with low levels of calcium and vitamin D, intravenous saline, calcium-wasting diuretics, or occasionally corticosteroids. Bisphosphonates also have been reported to successfully treat hypercalcemia in the setting of SFN.1,6
References
1. Disorders of the subcutaneous tissue, in “Neonatal and Infant Dermatology,” 3rd ed. (Philadelphia: Saunders, 2015, p. 443-55).
2. Br J Dermatol. 2007 Apr;156(4):709-15.
3. An Bras Dermatol. 2013 Nov-Dec;88(6 Suppl 1):154-7.
4. Pediatr Dermatol. 1999 Sep-Oct;16(5):384-7.
5. Pediatr Dermatol. 2016 Nov;33(6):e353-5.
6. Arch Dis Child Fetal Neonatal Ed. 2014 Sep;99(5):F419-21.
Dr. Ahluwalia and Dr. Eichenfield are in the division of pediatric and adolescent dermatology, Rady Children’s Hospital, San Diego, and the departments of dermatology and pediatrics, University of California, San Diego. They said they had no relevant financial disclosures. Email them at [email protected].
An 8.8 pound boy was born to a 29-year-old healthy mother at full term by emergency cesarean section secondary to fetal distress. Apgar scores were 4 and 6 at 1 and 5 minutes, respectively. Pregnancy was otherwise uncomplicated. The infant’s postnatal course was complicated by sepsis requiring intravenous antibiotics. The infant was discharged home after 1 week of hospitalization. At 3 weeks of life, the infant developed a red, firm, woody area on the back that did not appear painful and did not appear to spread in 2 days of observation. There was no fever. He was referred to the dermatology clinic for evaluation.
Physical exam showed an afebrile, well-appearing infant with an 11.5-cm by 7-cm red, indurated, ill-defined plaque overlying his back. The area was nontender and nonfluctuant. Complete blood count, comprehensive metabolic panel, and inflammatory markers were within normal limits.
Soluble PD-L1 correlates with melanoma outcomes
ORLANDO – Patients with metastatic melanoma who have high blood levels of the soluble form of the programmed death-ligand 1 (sPD-L1) have poor clinical outcomes, decreased overall survival, and disease that is resistant to PD-L1 checkpoint inhibitors, compared with patients with low levels of sPD-L1, investigators have found.
High sPD-L1 levels are also associated with an immunosuppressive disease phenotype and with higher levels of pro-inflammatory cytokines, said Roxana S. Dronca, MD, from the Mayo Clinic in Rochester, Minn.
Tumor-induced immune suppression
Membrane-bound, tumor associated PD-L1 has been shown to play a key role in tumor-induced immunosuppression in melanoma and many other malignancies. Expression of PD-L1 on tumors has been shown to be associated with more aggressive tumor biology and with decreased survival in various tumor types, and it was previously thought to be prognostic, she said.
“However, other investigators more recently have found that expression of PD-L1, for instance in metastatic melanoma, is associated with improved survival, possibly reflective of endogenous anti-tumor immunity. So, therefore, the prognostic role of tumor associated PD-L1 is unclear. And also, PD-L1 has been found to be a suboptimal predictive biomarker for response to PD-1 blockade, likely due to heterogeneous and dynamic expression in the tumor tissues, which really cannot be captured with a single-time-point, random tumor biopsy,” she added.
In 2011, Mayo investigators reported on the presence of sPD-L1 (then called B7-H1) in the sera of patients with advanced renal-cell carcinoma and that it was associated with advanced tumor stage and negative clinicopathologic tumor characteristics.
“It seems that the molecule is biologically able to engage PD-1 on circulating T cells, and therefore, it may represent an unanticipated contributing factor to immune homeostasis beyond the tumor microenvironment,” Dr. Dronca said.
Higher levels correlate with outcomes
To see whether sPD-L1 levels are related to outcome and response to immune checkpoint inhibitor therapy in patients with metastatic melanoma, the investigators collected baseline peripheral blood samples from 276 patients with advanced melanoma prior to enrollment in nonimmunotherapy clinical trials, as well as samples from 36 healthy blood donors at their center.
They also evaluated samples from 80 patients who were undergoing anti-PD-1 based immunotherapy, with peripheral blood collected at baseline and each subsequent radiographic tumor evaluation, and serial monthly blood samples from healthy pregnant women (number not specified), with samples taken at 2 hours and at 6 weeks post delivery. Levels of PD-L1 were measured by enzyme-linked immunosorbent assay.
The investigators first observed that sPD-L1 levels rose steadily during pregnancy then fell sharply after delivery, showing the presence of PD-L1 levels in healthy subjects and in a normal model of immune tolerance (that is, pregnancy). This finding is not especially surprising given that PD-L1 was first cloned from human placentas, where it is present in abundant levels and forms a barrier at the fetal-maternal interface, Dr. Dronca said.
They also found that sPD-L1 was significantly higher among melanoma patients than among controls, with a mean level of 1.73 ng/mL, compared with 0.77 ng/mL in controls.
Using receiver operating characteristic analysis, the researchers determined a cutoff value of 0.239 ng/mL to distinguish between low and high levels of sPD-L1.
They found that melanoma patients with levels above 0.293 ng/mL had a median overall survival of 11.3 months, compared with 14.8 months for those with levels of 0.293 ng/mL or lower (P = .04).
They also found that high sPD-L1 levels were associated with resistance to anti-PD-1 therapy. Patients who had complete or partial objective responses had a mean level of 0.3 ng/mL, whereas patients who had unequivocal disease progression at 12 weeks had levels 7.5 times higher.
“Interestingly, at 12 weeks the levels were actually quite stable, both in responders and progressors, suggesting that, maybe, soluble PD-L1 is not only a direct reflection of the tumor load, but as mentioned, it can be released by other immune cells and is possibly a more global marker of immune dysfunction,” Dr. Dronca said.
‘A little bit curious’
Douglas G. McNeel, MD, PhD, from the University of Wisconsin–Madison, the invited discussant, commended the authors for their study and noted that it raises important questions about the role of PD-L1 in healthy and malignant cells.
He added that it’s still unclear, but worth pursuing, whether measuring sPD-L1 levels can identify patients who may benefit from anti-PD1 monotherapy versus combinatorial strategies and agrees with the authors’ conclusion that larger studies are needed to establish whether sPD-L1 can be a prognostic or predictive biomarker.
The study was supported by grants from the National Institutes of Health, Mayo Clinic, and Fraternal Order of Eagles Cancer Research Fund. Dr. Dronca disclosed institution research funding from Merck Sharp & Dohme, and other financial relationship with Elsevier. Dr. McNeel disclosed leadership, stock ownership, and consulting with Madison Vaccines, and consulting and/or institutional research funding from Bristol-Myers Squibb, Dendreon, Janssen, Madison Vaccines, and Medivation.
ORLANDO – Patients with metastatic melanoma who have high blood levels of the soluble form of the programmed death-ligand 1 (sPD-L1) have poor clinical outcomes, decreased overall survival, and disease that is resistant to PD-L1 checkpoint inhibitors, compared with patients with low levels of sPD-L1, investigators have found.
High sPD-L1 levels are also associated with an immunosuppressive disease phenotype and with higher levels of pro-inflammatory cytokines, said Roxana S. Dronca, MD, from the Mayo Clinic in Rochester, Minn.
Tumor-induced immune suppression
Membrane-bound, tumor associated PD-L1 has been shown to play a key role in tumor-induced immunosuppression in melanoma and many other malignancies. Expression of PD-L1 on tumors has been shown to be associated with more aggressive tumor biology and with decreased survival in various tumor types, and it was previously thought to be prognostic, she said.
“However, other investigators more recently have found that expression of PD-L1, for instance in metastatic melanoma, is associated with improved survival, possibly reflective of endogenous anti-tumor immunity. So, therefore, the prognostic role of tumor associated PD-L1 is unclear. And also, PD-L1 has been found to be a suboptimal predictive biomarker for response to PD-1 blockade, likely due to heterogeneous and dynamic expression in the tumor tissues, which really cannot be captured with a single-time-point, random tumor biopsy,” she added.
In 2011, Mayo investigators reported on the presence of sPD-L1 (then called B7-H1) in the sera of patients with advanced renal-cell carcinoma and that it was associated with advanced tumor stage and negative clinicopathologic tumor characteristics.
“It seems that the molecule is biologically able to engage PD-1 on circulating T cells, and therefore, it may represent an unanticipated contributing factor to immune homeostasis beyond the tumor microenvironment,” Dr. Dronca said.
Higher levels correlate with outcomes
To see whether sPD-L1 levels are related to outcome and response to immune checkpoint inhibitor therapy in patients with metastatic melanoma, the investigators collected baseline peripheral blood samples from 276 patients with advanced melanoma prior to enrollment in nonimmunotherapy clinical trials, as well as samples from 36 healthy blood donors at their center.
They also evaluated samples from 80 patients who were undergoing anti-PD-1 based immunotherapy, with peripheral blood collected at baseline and each subsequent radiographic tumor evaluation, and serial monthly blood samples from healthy pregnant women (number not specified), with samples taken at 2 hours and at 6 weeks post delivery. Levels of PD-L1 were measured by enzyme-linked immunosorbent assay.
The investigators first observed that sPD-L1 levels rose steadily during pregnancy then fell sharply after delivery, showing the presence of PD-L1 levels in healthy subjects and in a normal model of immune tolerance (that is, pregnancy). This finding is not especially surprising given that PD-L1 was first cloned from human placentas, where it is present in abundant levels and forms a barrier at the fetal-maternal interface, Dr. Dronca said.
They also found that sPD-L1 was significantly higher among melanoma patients than among controls, with a mean level of 1.73 ng/mL, compared with 0.77 ng/mL in controls.
Using receiver operating characteristic analysis, the researchers determined a cutoff value of 0.239 ng/mL to distinguish between low and high levels of sPD-L1.
They found that melanoma patients with levels above 0.293 ng/mL had a median overall survival of 11.3 months, compared with 14.8 months for those with levels of 0.293 ng/mL or lower (P = .04).
They also found that high sPD-L1 levels were associated with resistance to anti-PD-1 therapy. Patients who had complete or partial objective responses had a mean level of 0.3 ng/mL, whereas patients who had unequivocal disease progression at 12 weeks had levels 7.5 times higher.
“Interestingly, at 12 weeks the levels were actually quite stable, both in responders and progressors, suggesting that, maybe, soluble PD-L1 is not only a direct reflection of the tumor load, but as mentioned, it can be released by other immune cells and is possibly a more global marker of immune dysfunction,” Dr. Dronca said.
‘A little bit curious’
Douglas G. McNeel, MD, PhD, from the University of Wisconsin–Madison, the invited discussant, commended the authors for their study and noted that it raises important questions about the role of PD-L1 in healthy and malignant cells.
He added that it’s still unclear, but worth pursuing, whether measuring sPD-L1 levels can identify patients who may benefit from anti-PD1 monotherapy versus combinatorial strategies and agrees with the authors’ conclusion that larger studies are needed to establish whether sPD-L1 can be a prognostic or predictive biomarker.
The study was supported by grants from the National Institutes of Health, Mayo Clinic, and Fraternal Order of Eagles Cancer Research Fund. Dr. Dronca disclosed institution research funding from Merck Sharp & Dohme, and other financial relationship with Elsevier. Dr. McNeel disclosed leadership, stock ownership, and consulting with Madison Vaccines, and consulting and/or institutional research funding from Bristol-Myers Squibb, Dendreon, Janssen, Madison Vaccines, and Medivation.
ORLANDO – Patients with metastatic melanoma who have high blood levels of the soluble form of the programmed death-ligand 1 (sPD-L1) have poor clinical outcomes, decreased overall survival, and disease that is resistant to PD-L1 checkpoint inhibitors, compared with patients with low levels of sPD-L1, investigators have found.
High sPD-L1 levels are also associated with an immunosuppressive disease phenotype and with higher levels of pro-inflammatory cytokines, said Roxana S. Dronca, MD, from the Mayo Clinic in Rochester, Minn.
Tumor-induced immune suppression
Membrane-bound, tumor associated PD-L1 has been shown to play a key role in tumor-induced immunosuppression in melanoma and many other malignancies. Expression of PD-L1 on tumors has been shown to be associated with more aggressive tumor biology and with decreased survival in various tumor types, and it was previously thought to be prognostic, she said.
“However, other investigators more recently have found that expression of PD-L1, for instance in metastatic melanoma, is associated with improved survival, possibly reflective of endogenous anti-tumor immunity. So, therefore, the prognostic role of tumor associated PD-L1 is unclear. And also, PD-L1 has been found to be a suboptimal predictive biomarker for response to PD-1 blockade, likely due to heterogeneous and dynamic expression in the tumor tissues, which really cannot be captured with a single-time-point, random tumor biopsy,” she added.
In 2011, Mayo investigators reported on the presence of sPD-L1 (then called B7-H1) in the sera of patients with advanced renal-cell carcinoma and that it was associated with advanced tumor stage and negative clinicopathologic tumor characteristics.
“It seems that the molecule is biologically able to engage PD-1 on circulating T cells, and therefore, it may represent an unanticipated contributing factor to immune homeostasis beyond the tumor microenvironment,” Dr. Dronca said.
Higher levels correlate with outcomes
To see whether sPD-L1 levels are related to outcome and response to immune checkpoint inhibitor therapy in patients with metastatic melanoma, the investigators collected baseline peripheral blood samples from 276 patients with advanced melanoma prior to enrollment in nonimmunotherapy clinical trials, as well as samples from 36 healthy blood donors at their center.
They also evaluated samples from 80 patients who were undergoing anti-PD-1 based immunotherapy, with peripheral blood collected at baseline and each subsequent radiographic tumor evaluation, and serial monthly blood samples from healthy pregnant women (number not specified), with samples taken at 2 hours and at 6 weeks post delivery. Levels of PD-L1 were measured by enzyme-linked immunosorbent assay.
The investigators first observed that sPD-L1 levels rose steadily during pregnancy then fell sharply after delivery, showing the presence of PD-L1 levels in healthy subjects and in a normal model of immune tolerance (that is, pregnancy). This finding is not especially surprising given that PD-L1 was first cloned from human placentas, where it is present in abundant levels and forms a barrier at the fetal-maternal interface, Dr. Dronca said.
They also found that sPD-L1 was significantly higher among melanoma patients than among controls, with a mean level of 1.73 ng/mL, compared with 0.77 ng/mL in controls.
Using receiver operating characteristic analysis, the researchers determined a cutoff value of 0.239 ng/mL to distinguish between low and high levels of sPD-L1.
They found that melanoma patients with levels above 0.293 ng/mL had a median overall survival of 11.3 months, compared with 14.8 months for those with levels of 0.293 ng/mL or lower (P = .04).
They also found that high sPD-L1 levels were associated with resistance to anti-PD-1 therapy. Patients who had complete or partial objective responses had a mean level of 0.3 ng/mL, whereas patients who had unequivocal disease progression at 12 weeks had levels 7.5 times higher.
“Interestingly, at 12 weeks the levels were actually quite stable, both in responders and progressors, suggesting that, maybe, soluble PD-L1 is not only a direct reflection of the tumor load, but as mentioned, it can be released by other immune cells and is possibly a more global marker of immune dysfunction,” Dr. Dronca said.
‘A little bit curious’
Douglas G. McNeel, MD, PhD, from the University of Wisconsin–Madison, the invited discussant, commended the authors for their study and noted that it raises important questions about the role of PD-L1 in healthy and malignant cells.
He added that it’s still unclear, but worth pursuing, whether measuring sPD-L1 levels can identify patients who may benefit from anti-PD1 monotherapy versus combinatorial strategies and agrees with the authors’ conclusion that larger studies are needed to establish whether sPD-L1 can be a prognostic or predictive biomarker.
The study was supported by grants from the National Institutes of Health, Mayo Clinic, and Fraternal Order of Eagles Cancer Research Fund. Dr. Dronca disclosed institution research funding from Merck Sharp & Dohme, and other financial relationship with Elsevier. Dr. McNeel disclosed leadership, stock ownership, and consulting with Madison Vaccines, and consulting and/or institutional research funding from Bristol-Myers Squibb, Dendreon, Janssen, Madison Vaccines, and Medivation.
Key clinical point: Soluble PD-L1 may be a predictive or prognostic biomarker for malignant melanoma outcomes.
Major finding: Patients with high levels of sPD-L1 had a median overall survival of 11.3 months, compared with 14.8 months for those with levels below a specified cutoff.
Data source: Prospective study of sPD-L1 in 276 patients with metastatic melanoma, 36 healthy volunteers, and 80 patients who were undergoing anti-PD-1 based immunotherapy.
Disclosures: The study was supported by grants from the National Institutes of Health, Mayo Clinic, and Fraternal Order of Eagles Cancer Research Fund. Dr. Dronca disclosed institution research funding from Merck Sharp & Dohme and another financial relationship with Elsevier. Dr. McNeel disclosed leadership, stock ownership, and consulting with Madison Vaccines and consulting and/or institutional research funding from Bristol-Myers Squibb, Dendreon, Janssen, Madison Vaccines, and Medivation.
Stroke Rates Are High When Catheter Ablation of Atrial Fibrillation Fails
ORLANDO—In patients with atrial fibrillation (AF) who fail to achieve rhythm control after catheter ablation, the risk of ischemic stroke may approach 30% over five or more years of follow-up, despite optimized anticoagulation therapy, according to data from 1,002 consecutive patients presented at the 22nd Annual International AF Symposium.
The retrospective analysis was conducted in 1,002 patients who underwent catheter ablation after failing pharmacologic treatment of AF. Of these, 169 (17%) failed the ablation, but the focus of this study was on the subgroup of 67 catheter ablation treatment failures that have been followed for at least five years. All had been maintained on anticoagulation therapy.
Within this group, 18 (27%) had an ischemic stroke during follow-up. The average time to stroke after the first ablation procedure was 3.9 years.
Prior to being declared catheter ablation failures, the average number of ablation procedures in this long-term follow-up group was 1.7. In 55.2% of patients, the first ablation was performed with a cryoballoon. The remaining first ablations were delivered with radiofrequency. For a second or third ablation, the same techniques were commonly repeated, but 25% received a cavotricuspid isthmus ablation, and 12% underwent a VATS-Maze procedure.
There were no deaths in this series, in which the average patient age was 66. The average duration of AF was 12 years, the mean left atrial size was 45 mm, and the average left ventricular ejection fraction was 55%.
In this study, researchers defined catheter ablation failure as inability to regain rhythm control despite repeated ablation procedures. However, many patients who initially achieve rhythm control after catheter ablation have recurrence of AF over time. It is unclear whether patients who initially achieve but then lose rhythm control face the same high risk for stroke as seen in the Dutch series if followed long-term.
One study suggests that they may not. In a study by Bottoni et al of 631 consecutive patients who underwent a mean 1.5 catheter ablations before achieving rhythm control, 34% had an AF recurrence at one year. When followed for a mean 4.1 years of additional follow-up (5.1 years from the initial ablation), only 10% had a serious adverse event, such as heart failure or hemorrhage, and only 2% had a cerebrovascular event.
Numerous clinical studies have shown that catheter ablation is more effective than pharmacologic therapy for both regaining rhythm control in AF patients and reducing symptoms, according to Dr. Martirosyan, but these long-term follow-up data confirm that the risk of thromboembolic complications remains high in those who fail the initial catheter ablation. Of the 18 strokes, only four occurred in the first year of follow-up. The remaining strokes accrued slowly over time. Strokes were recorded up until 10 years after the ablation, the longest period that any patient was followed.
—Ted Bosworth
ORLANDO—In patients with atrial fibrillation (AF) who fail to achieve rhythm control after catheter ablation, the risk of ischemic stroke may approach 30% over five or more years of follow-up, despite optimized anticoagulation therapy, according to data from 1,002 consecutive patients presented at the 22nd Annual International AF Symposium.
The retrospective analysis was conducted in 1,002 patients who underwent catheter ablation after failing pharmacologic treatment of AF. Of these, 169 (17%) failed the ablation, but the focus of this study was on the subgroup of 67 catheter ablation treatment failures that have been followed for at least five years. All had been maintained on anticoagulation therapy.
Within this group, 18 (27%) had an ischemic stroke during follow-up. The average time to stroke after the first ablation procedure was 3.9 years.
Prior to being declared catheter ablation failures, the average number of ablation procedures in this long-term follow-up group was 1.7. In 55.2% of patients, the first ablation was performed with a cryoballoon. The remaining first ablations were delivered with radiofrequency. For a second or third ablation, the same techniques were commonly repeated, but 25% received a cavotricuspid isthmus ablation, and 12% underwent a VATS-Maze procedure.
There were no deaths in this series, in which the average patient age was 66. The average duration of AF was 12 years, the mean left atrial size was 45 mm, and the average left ventricular ejection fraction was 55%.
In this study, researchers defined catheter ablation failure as inability to regain rhythm control despite repeated ablation procedures. However, many patients who initially achieve rhythm control after catheter ablation have recurrence of AF over time. It is unclear whether patients who initially achieve but then lose rhythm control face the same high risk for stroke as seen in the Dutch series if followed long-term.
One study suggests that they may not. In a study by Bottoni et al of 631 consecutive patients who underwent a mean 1.5 catheter ablations before achieving rhythm control, 34% had an AF recurrence at one year. When followed for a mean 4.1 years of additional follow-up (5.1 years from the initial ablation), only 10% had a serious adverse event, such as heart failure or hemorrhage, and only 2% had a cerebrovascular event.
Numerous clinical studies have shown that catheter ablation is more effective than pharmacologic therapy for both regaining rhythm control in AF patients and reducing symptoms, according to Dr. Martirosyan, but these long-term follow-up data confirm that the risk of thromboembolic complications remains high in those who fail the initial catheter ablation. Of the 18 strokes, only four occurred in the first year of follow-up. The remaining strokes accrued slowly over time. Strokes were recorded up until 10 years after the ablation, the longest period that any patient was followed.
—Ted Bosworth
ORLANDO—In patients with atrial fibrillation (AF) who fail to achieve rhythm control after catheter ablation, the risk of ischemic stroke may approach 30% over five or more years of follow-up, despite optimized anticoagulation therapy, according to data from 1,002 consecutive patients presented at the 22nd Annual International AF Symposium.
The retrospective analysis was conducted in 1,002 patients who underwent catheter ablation after failing pharmacologic treatment of AF. Of these, 169 (17%) failed the ablation, but the focus of this study was on the subgroup of 67 catheter ablation treatment failures that have been followed for at least five years. All had been maintained on anticoagulation therapy.
Within this group, 18 (27%) had an ischemic stroke during follow-up. The average time to stroke after the first ablation procedure was 3.9 years.
Prior to being declared catheter ablation failures, the average number of ablation procedures in this long-term follow-up group was 1.7. In 55.2% of patients, the first ablation was performed with a cryoballoon. The remaining first ablations were delivered with radiofrequency. For a second or third ablation, the same techniques were commonly repeated, but 25% received a cavotricuspid isthmus ablation, and 12% underwent a VATS-Maze procedure.
There were no deaths in this series, in which the average patient age was 66. The average duration of AF was 12 years, the mean left atrial size was 45 mm, and the average left ventricular ejection fraction was 55%.
In this study, researchers defined catheter ablation failure as inability to regain rhythm control despite repeated ablation procedures. However, many patients who initially achieve rhythm control after catheter ablation have recurrence of AF over time. It is unclear whether patients who initially achieve but then lose rhythm control face the same high risk for stroke as seen in the Dutch series if followed long-term.
One study suggests that they may not. In a study by Bottoni et al of 631 consecutive patients who underwent a mean 1.5 catheter ablations before achieving rhythm control, 34% had an AF recurrence at one year. When followed for a mean 4.1 years of additional follow-up (5.1 years from the initial ablation), only 10% had a serious adverse event, such as heart failure or hemorrhage, and only 2% had a cerebrovascular event.
Numerous clinical studies have shown that catheter ablation is more effective than pharmacologic therapy for both regaining rhythm control in AF patients and reducing symptoms, according to Dr. Martirosyan, but these long-term follow-up data confirm that the risk of thromboembolic complications remains high in those who fail the initial catheter ablation. Of the 18 strokes, only four occurred in the first year of follow-up. The remaining strokes accrued slowly over time. Strokes were recorded up until 10 years after the ablation, the longest period that any patient was followed.
—Ted Bosworth









