User login
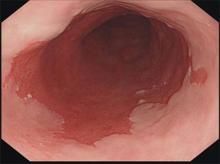
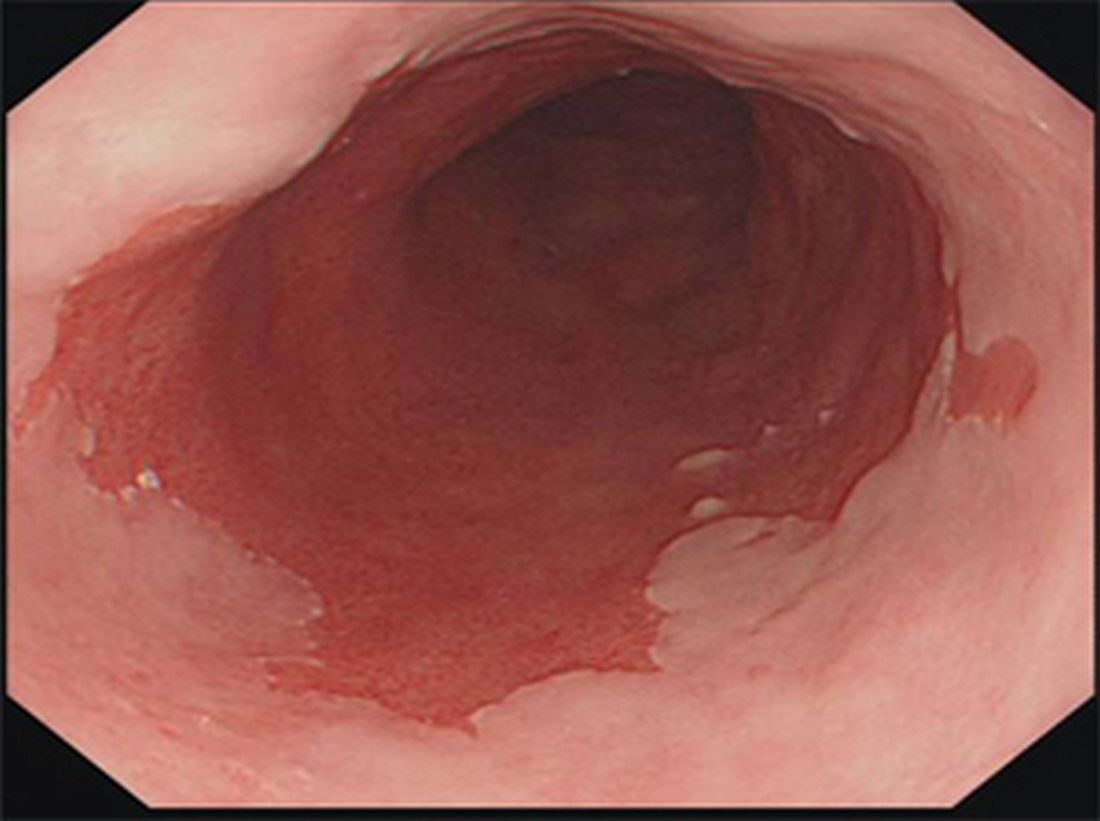
Barrett’s esophagus: Cancer risk is highest in first year after diagnosis
The highest risk of diagnosis with esophageal adenocarcinoma occurs in the first month after diagnosis with Barrett’s esophagus (BE).
Further, the risk of this type of esophageal cancer is lower than previously believed after that first year, according to a study of 7,932 Swedish residents who were diagnosed with BE from 2006 to 2013.
Esophageal adenocarcinoma (EA) is the most common type of esophageal cancer in the United States, and more than half of patients present with late-stage disease. “The connection between BE and EA is well known, and risk factors for both conditions remain vastly the same, including male sex, age over 50 years, central obesity, and symptomatic GERD [gastroesophageal reflux disease],” Dr. Holmberg said in an interview.
“In order to reduce mortality from esophageal cancer through surveillance and early treatment, we need to improve on BE screening programs,” he said. He also noted that, of all EA cases in Sweden, where screening for BE itself is not recommended in national guidelines, an “astonishing” 97% were diagnosed in patients with previously unknown BE.
Screening programs urge regular endoscopic surveillance in nondysplastic BE patients, but Dr. Holmberg said that their cost-effectiveness is questionable. Complicating matters, there has been conflicting research about the risk of EA that these patients actually face.
As the new study notes, patients with BE were previously thought to face a 30-125 times greater relative risk of EA than the general population did and a mean annual risk of 0.5%. But, recent studies in Europe and the U.S. have suggested that the risk is lower, with an estimated annual risk range of 0.12%-0.18%.
Dr. Holmberg said he and his colleagues launched the new study to better understand the risk in the first year after BE diagnosis.
The researchers used the Swedish National Patient Register to track 7,932 Swedish residents who were diagnosed with BE from 2006 to 2013. About two-thirds were male, and the median age at BE diagnosis was 66 years.
The findings appear in the European Journal of Cancer (2017;75:41-6). Of the patients with BE, 89 new cases of EA were diagnosed 62% of the time within a year of BE diagnosis.
Over a median follow-up of 2.13 person-years, the overall incidence of EA in the patients with BE was 1.47 (95% CI, 0.91-2.02) per 1,000 person-years. That is the equivalent of an annual risk of 0.15%.
The highest incidence rate by far was in the period of 7 to 30 days after diagnosis at 15.53 (95% CI, 4.77-26.29). The incident rates varied from 1.44 to 4.10 (95% CI, 0.00-7.38) for other periods (31-100 days, 101 days to 6 months, over 6 months to 1 year, over 1 year to 3 years, over 3 years to end of follow-up).
How should physicians interpret the study findings and look forward? “Nondysplastic BE represents almost 80% of all BE,” Dr. Holmberg said, but “nondiscriminatory long-term surveillance in nondysplastic BE is not cost-efficient.”
However, while this is “the group with the least risk of EA development, it still generates a large chunk of all EA cases. It is likely that future management of nondysplastic BE will include more risk factors than just degree of dysplasia.”
He added that “individuals with multiple risk factors, such as Caucasian males, age over 50 years, obesity, symptomatic reflux, and with long-segment nondysplastic BE would likely benefit more from more frequent surveillance or even prophylactic treatment with radio-frequency ablation compared to individuals without those risk factors.”
The study was funded by the Swedish Cancer Society and the Swedish Research Council. The authors report no relevant disclosures.
The highest risk of diagnosis with esophageal adenocarcinoma occurs in the first month after diagnosis with Barrett’s esophagus (BE).
Further, the risk of this type of esophageal cancer is lower than previously believed after that first year, according to a study of 7,932 Swedish residents who were diagnosed with BE from 2006 to 2013.
Esophageal adenocarcinoma (EA) is the most common type of esophageal cancer in the United States, and more than half of patients present with late-stage disease. “The connection between BE and EA is well known, and risk factors for both conditions remain vastly the same, including male sex, age over 50 years, central obesity, and symptomatic GERD [gastroesophageal reflux disease],” Dr. Holmberg said in an interview.
“In order to reduce mortality from esophageal cancer through surveillance and early treatment, we need to improve on BE screening programs,” he said. He also noted that, of all EA cases in Sweden, where screening for BE itself is not recommended in national guidelines, an “astonishing” 97% were diagnosed in patients with previously unknown BE.
Screening programs urge regular endoscopic surveillance in nondysplastic BE patients, but Dr. Holmberg said that their cost-effectiveness is questionable. Complicating matters, there has been conflicting research about the risk of EA that these patients actually face.
As the new study notes, patients with BE were previously thought to face a 30-125 times greater relative risk of EA than the general population did and a mean annual risk of 0.5%. But, recent studies in Europe and the U.S. have suggested that the risk is lower, with an estimated annual risk range of 0.12%-0.18%.
Dr. Holmberg said he and his colleagues launched the new study to better understand the risk in the first year after BE diagnosis.
The researchers used the Swedish National Patient Register to track 7,932 Swedish residents who were diagnosed with BE from 2006 to 2013. About two-thirds were male, and the median age at BE diagnosis was 66 years.
The findings appear in the European Journal of Cancer (2017;75:41-6). Of the patients with BE, 89 new cases of EA were diagnosed 62% of the time within a year of BE diagnosis.
Over a median follow-up of 2.13 person-years, the overall incidence of EA in the patients with BE was 1.47 (95% CI, 0.91-2.02) per 1,000 person-years. That is the equivalent of an annual risk of 0.15%.
The highest incidence rate by far was in the period of 7 to 30 days after diagnosis at 15.53 (95% CI, 4.77-26.29). The incident rates varied from 1.44 to 4.10 (95% CI, 0.00-7.38) for other periods (31-100 days, 101 days to 6 months, over 6 months to 1 year, over 1 year to 3 years, over 3 years to end of follow-up).
How should physicians interpret the study findings and look forward? “Nondysplastic BE represents almost 80% of all BE,” Dr. Holmberg said, but “nondiscriminatory long-term surveillance in nondysplastic BE is not cost-efficient.”
However, while this is “the group with the least risk of EA development, it still generates a large chunk of all EA cases. It is likely that future management of nondysplastic BE will include more risk factors than just degree of dysplasia.”
He added that “individuals with multiple risk factors, such as Caucasian males, age over 50 years, obesity, symptomatic reflux, and with long-segment nondysplastic BE would likely benefit more from more frequent surveillance or even prophylactic treatment with radio-frequency ablation compared to individuals without those risk factors.”
The study was funded by the Swedish Cancer Society and the Swedish Research Council. The authors report no relevant disclosures.
The highest risk of diagnosis with esophageal adenocarcinoma occurs in the first month after diagnosis with Barrett’s esophagus (BE).
Further, the risk of this type of esophageal cancer is lower than previously believed after that first year, according to a study of 7,932 Swedish residents who were diagnosed with BE from 2006 to 2013.
Esophageal adenocarcinoma (EA) is the most common type of esophageal cancer in the United States, and more than half of patients present with late-stage disease. “The connection between BE and EA is well known, and risk factors for both conditions remain vastly the same, including male sex, age over 50 years, central obesity, and symptomatic GERD [gastroesophageal reflux disease],” Dr. Holmberg said in an interview.
“In order to reduce mortality from esophageal cancer through surveillance and early treatment, we need to improve on BE screening programs,” he said. He also noted that, of all EA cases in Sweden, where screening for BE itself is not recommended in national guidelines, an “astonishing” 97% were diagnosed in patients with previously unknown BE.
Screening programs urge regular endoscopic surveillance in nondysplastic BE patients, but Dr. Holmberg said that their cost-effectiveness is questionable. Complicating matters, there has been conflicting research about the risk of EA that these patients actually face.
As the new study notes, patients with BE were previously thought to face a 30-125 times greater relative risk of EA than the general population did and a mean annual risk of 0.5%. But, recent studies in Europe and the U.S. have suggested that the risk is lower, with an estimated annual risk range of 0.12%-0.18%.
Dr. Holmberg said he and his colleagues launched the new study to better understand the risk in the first year after BE diagnosis.
The researchers used the Swedish National Patient Register to track 7,932 Swedish residents who were diagnosed with BE from 2006 to 2013. About two-thirds were male, and the median age at BE diagnosis was 66 years.
The findings appear in the European Journal of Cancer (2017;75:41-6). Of the patients with BE, 89 new cases of EA were diagnosed 62% of the time within a year of BE diagnosis.
Over a median follow-up of 2.13 person-years, the overall incidence of EA in the patients with BE was 1.47 (95% CI, 0.91-2.02) per 1,000 person-years. That is the equivalent of an annual risk of 0.15%.
The highest incidence rate by far was in the period of 7 to 30 days after diagnosis at 15.53 (95% CI, 4.77-26.29). The incident rates varied from 1.44 to 4.10 (95% CI, 0.00-7.38) for other periods (31-100 days, 101 days to 6 months, over 6 months to 1 year, over 1 year to 3 years, over 3 years to end of follow-up).
How should physicians interpret the study findings and look forward? “Nondysplastic BE represents almost 80% of all BE,” Dr. Holmberg said, but “nondiscriminatory long-term surveillance in nondysplastic BE is not cost-efficient.”
However, while this is “the group with the least risk of EA development, it still generates a large chunk of all EA cases. It is likely that future management of nondysplastic BE will include more risk factors than just degree of dysplasia.”
He added that “individuals with multiple risk factors, such as Caucasian males, age over 50 years, obesity, symptomatic reflux, and with long-segment nondysplastic BE would likely benefit more from more frequent surveillance or even prophylactic treatment with radio-frequency ablation compared to individuals without those risk factors.”
The study was funded by the Swedish Cancer Society and the Swedish Research Council. The authors report no relevant disclosures.
FROM THE EUROPEAN JOURNAL OF CANCER
Key clinical point: While their overall risk over the short term is low, patients with Barrett’s esophagus are especially likely to be diagnosed with esophageal adenocarcinoma in the first weeks after Barrett’s diagnosis.
Major finding: Over a median of 2.13 person-years, the annual risk of esophageal adenocarcinoma diagnosis was 0.15% in patients with Barrett’s esophagus, but the incidence rate was especially high (15.53; 95% confidence interval, 4.77-26.29) from 7 to 30 days after Barrett’s diagnosis, compared with rates of 1.44-4.10 (95% CI, 0.00-7.38) for later periods.
Data source: A population-centered analysis of 7,932 Swedish residents diagnosed with Barrett’s esophagus from 2006 to 2013.
Disclosures: The study was funded by the Swedish Cancer Society and the Swedish Research Council. The authors report no relevant disclosures.
Miami Breast Cancer Symposium to explore treatment controversies
FROM MBCC (FRONTLINE MEDICAL NEWS) – Oncology Practice will be on site this coming week at the Miami Breast Cancer Symposium in Miami Beach, reporting on the latest in multidisciplinary management of breast cancer patients. Sessions will highlight updates in systemic therapy, immunology and immunotherapy, and overcoming therapeutic resistance, as well as explore treatment controversies, such as omitting anthracyclines, extended-field radiation therapy, and who should be receiving extended hormonal therapy. The symposium, hosted by Physicians’ Education Resource, begins Thursday, March 9. Our team will provide daily coverage of the following presentations and more:
Overcoming Resistance to HER2 Targeted Therapy
Mark D. Pegram, MD
Overcoming Resistance to Endocrine Ablative Therapy
William J. Gradishar, MD
Medical Crossfire: Is Extended Field Radiation Therapy Ready for Prime Time?
Lawrence J. Solin, MD, & Thomas A. Buchholz, MD
Raising the Therapeutic Index for HER2+ Targeted Therapy: Can We Safely Omit
Anthracyclines?
Sara Hurvitz, MD
Update on Neoadjuvant Treatment Strategies in HER2+ Breast Cancer
Debu Tripathy, MD
Update on PARP Inhibitors in Breast Cancer
Kimberly L. Blackwell, MD
Update on Immunology and Immunotherapy in Breast Cancer
Elizabeth Mittendorf, MD, PhD
Medical Crossfire: Is Extended Hormonal Therapy Required for Everyone?
William J. Gradishar, MD, & Sara Hurvitz, MD
FROM MBCC (FRONTLINE MEDICAL NEWS) – Oncology Practice will be on site this coming week at the Miami Breast Cancer Symposium in Miami Beach, reporting on the latest in multidisciplinary management of breast cancer patients. Sessions will highlight updates in systemic therapy, immunology and immunotherapy, and overcoming therapeutic resistance, as well as explore treatment controversies, such as omitting anthracyclines, extended-field radiation therapy, and who should be receiving extended hormonal therapy. The symposium, hosted by Physicians’ Education Resource, begins Thursday, March 9. Our team will provide daily coverage of the following presentations and more:
Overcoming Resistance to HER2 Targeted Therapy
Mark D. Pegram, MD
Overcoming Resistance to Endocrine Ablative Therapy
William J. Gradishar, MD
Medical Crossfire: Is Extended Field Radiation Therapy Ready for Prime Time?
Lawrence J. Solin, MD, & Thomas A. Buchholz, MD
Raising the Therapeutic Index for HER2+ Targeted Therapy: Can We Safely Omit
Anthracyclines?
Sara Hurvitz, MD
Update on Neoadjuvant Treatment Strategies in HER2+ Breast Cancer
Debu Tripathy, MD
Update on PARP Inhibitors in Breast Cancer
Kimberly L. Blackwell, MD
Update on Immunology and Immunotherapy in Breast Cancer
Elizabeth Mittendorf, MD, PhD
Medical Crossfire: Is Extended Hormonal Therapy Required for Everyone?
William J. Gradishar, MD, & Sara Hurvitz, MD
FROM MBCC (FRONTLINE MEDICAL NEWS) – Oncology Practice will be on site this coming week at the Miami Breast Cancer Symposium in Miami Beach, reporting on the latest in multidisciplinary management of breast cancer patients. Sessions will highlight updates in systemic therapy, immunology and immunotherapy, and overcoming therapeutic resistance, as well as explore treatment controversies, such as omitting anthracyclines, extended-field radiation therapy, and who should be receiving extended hormonal therapy. The symposium, hosted by Physicians’ Education Resource, begins Thursday, March 9. Our team will provide daily coverage of the following presentations and more:
Overcoming Resistance to HER2 Targeted Therapy
Mark D. Pegram, MD
Overcoming Resistance to Endocrine Ablative Therapy
William J. Gradishar, MD
Medical Crossfire: Is Extended Field Radiation Therapy Ready for Prime Time?
Lawrence J. Solin, MD, & Thomas A. Buchholz, MD
Raising the Therapeutic Index for HER2+ Targeted Therapy: Can We Safely Omit
Anthracyclines?
Sara Hurvitz, MD
Update on Neoadjuvant Treatment Strategies in HER2+ Breast Cancer
Debu Tripathy, MD
Update on PARP Inhibitors in Breast Cancer
Kimberly L. Blackwell, MD
Update on Immunology and Immunotherapy in Breast Cancer
Elizabeth Mittendorf, MD, PhD
Medical Crossfire: Is Extended Hormonal Therapy Required for Everyone?
William J. Gradishar, MD, & Sara Hurvitz, MD
Voclosporin linked to threefold increase in lupus nephritis remission
Treatment with the investigational calcineurin inhibitor voclosporin is associated with a significant, threefold-higher rate of complete remission for lupus nephritis, compared with the current standard of care, according to 48-week data from the AURA-LV (Aurinia Urinary Protein Reduction Active–Lupus With Voclosporin) study.
In a company release, manufacturer Aurinia Pharmaceuticals presented the results of the international phase IIb controlled trial involving 265 patients with active lupus nephritis from 20 countries, which they say has now met its primary and secondary endpoints.
After 48 weeks, 49% of patients in the low-dose group and 40% in the high-dose group had achieved complete remission, compared with 24% in the control group (P less than .001 for low-dose vs. control; P = .026 for high-dose vs. control). This represented a threefold-higher remission rate for the low-dose group (odds ratio, 3.21) and a twofold-higher rate for the high-dose group (OR, 2.1), compared with controls.
Complete remission was defined as a composite endpoint that included urine protein/creatinine ratio of 0.5 mg/mg or less; normal, stable renal function; presence of sustained, low dose steroids; and no administration of rescue medications.
Partial remission – defined as at least a 50% reduction in urine protein/creatinine ratio with no concomitant use of rescue medication – was seen in 68% of patients in the low-dose voclosporin group, 72% of patients in the high-dose group, and 48% of patients in the control arm (P = .007 for low-dose vs. control; P = .002 for high-dose vs. control).
Investigators said there were no unexpected safety signals from voclosporin, but there were three deaths and one malignancy reported in the control arm after the study treatment period ended.
Brad Rovin, MD, of Ohio State University in Columbus said in the company’s statement that current treatments for this severe complication of systemic lupus erythematosus are toxic, and complete renal response rates are low.
“The AURA trial’s long-term results convincingly demonstrate that the addition of voclosporin to standard of care treatment is superior to standard of care alone,” Dr. Rovin said. “This is an impressive renal response rate, and these results may shift the treatment paradigm of lupus nephritis.”
Aurinia’s chief medical officer, Neil Solomons, MD, said in the release that the results provide the company with confidence that they can now execute a successful phase III program.
The 24-week results of the study – in which all primary and the 24-week secondary endpoints were also met – were released in November 2016.
Treatment with the investigational calcineurin inhibitor voclosporin is associated with a significant, threefold-higher rate of complete remission for lupus nephritis, compared with the current standard of care, according to 48-week data from the AURA-LV (Aurinia Urinary Protein Reduction Active–Lupus With Voclosporin) study.
In a company release, manufacturer Aurinia Pharmaceuticals presented the results of the international phase IIb controlled trial involving 265 patients with active lupus nephritis from 20 countries, which they say has now met its primary and secondary endpoints.
After 48 weeks, 49% of patients in the low-dose group and 40% in the high-dose group had achieved complete remission, compared with 24% in the control group (P less than .001 for low-dose vs. control; P = .026 for high-dose vs. control). This represented a threefold-higher remission rate for the low-dose group (odds ratio, 3.21) and a twofold-higher rate for the high-dose group (OR, 2.1), compared with controls.
Complete remission was defined as a composite endpoint that included urine protein/creatinine ratio of 0.5 mg/mg or less; normal, stable renal function; presence of sustained, low dose steroids; and no administration of rescue medications.
Partial remission – defined as at least a 50% reduction in urine protein/creatinine ratio with no concomitant use of rescue medication – was seen in 68% of patients in the low-dose voclosporin group, 72% of patients in the high-dose group, and 48% of patients in the control arm (P = .007 for low-dose vs. control; P = .002 for high-dose vs. control).
Investigators said there were no unexpected safety signals from voclosporin, but there were three deaths and one malignancy reported in the control arm after the study treatment period ended.
Brad Rovin, MD, of Ohio State University in Columbus said in the company’s statement that current treatments for this severe complication of systemic lupus erythematosus are toxic, and complete renal response rates are low.
“The AURA trial’s long-term results convincingly demonstrate that the addition of voclosporin to standard of care treatment is superior to standard of care alone,” Dr. Rovin said. “This is an impressive renal response rate, and these results may shift the treatment paradigm of lupus nephritis.”
Aurinia’s chief medical officer, Neil Solomons, MD, said in the release that the results provide the company with confidence that they can now execute a successful phase III program.
The 24-week results of the study – in which all primary and the 24-week secondary endpoints were also met – were released in November 2016.
Treatment with the investigational calcineurin inhibitor voclosporin is associated with a significant, threefold-higher rate of complete remission for lupus nephritis, compared with the current standard of care, according to 48-week data from the AURA-LV (Aurinia Urinary Protein Reduction Active–Lupus With Voclosporin) study.
In a company release, manufacturer Aurinia Pharmaceuticals presented the results of the international phase IIb controlled trial involving 265 patients with active lupus nephritis from 20 countries, which they say has now met its primary and secondary endpoints.
After 48 weeks, 49% of patients in the low-dose group and 40% in the high-dose group had achieved complete remission, compared with 24% in the control group (P less than .001 for low-dose vs. control; P = .026 for high-dose vs. control). This represented a threefold-higher remission rate for the low-dose group (odds ratio, 3.21) and a twofold-higher rate for the high-dose group (OR, 2.1), compared with controls.
Complete remission was defined as a composite endpoint that included urine protein/creatinine ratio of 0.5 mg/mg or less; normal, stable renal function; presence of sustained, low dose steroids; and no administration of rescue medications.
Partial remission – defined as at least a 50% reduction in urine protein/creatinine ratio with no concomitant use of rescue medication – was seen in 68% of patients in the low-dose voclosporin group, 72% of patients in the high-dose group, and 48% of patients in the control arm (P = .007 for low-dose vs. control; P = .002 for high-dose vs. control).
Investigators said there were no unexpected safety signals from voclosporin, but there were three deaths and one malignancy reported in the control arm after the study treatment period ended.
Brad Rovin, MD, of Ohio State University in Columbus said in the company’s statement that current treatments for this severe complication of systemic lupus erythematosus are toxic, and complete renal response rates are low.
“The AURA trial’s long-term results convincingly demonstrate that the addition of voclosporin to standard of care treatment is superior to standard of care alone,” Dr. Rovin said. “This is an impressive renal response rate, and these results may shift the treatment paradigm of lupus nephritis.”
Aurinia’s chief medical officer, Neil Solomons, MD, said in the release that the results provide the company with confidence that they can now execute a successful phase III program.
The 24-week results of the study – in which all primary and the 24-week secondary endpoints were also met – were released in November 2016.
Key clinical point:
Major finding: Nearly half of patients treated with the lower dose of investigational calcineurin inhibitor voclosporin achieved complete remission compared to one-quarter of patients in the control group.
Data source: The AURA-LV phase IIb controlled study involving 265 patients with active lupus nephritis.
Disclosures: The results were presented in a company release from manufacturer Aurinia Pharmaceuticals.
Neuropsychiatric Strategies May Benefit Patients With Refractory Epilepsy
HOUSTON—Treating psychiatric symptoms in patients with refractory epilepsy is likely to improve the overall course of the disease, as well as patients’ quality of life, according to an overview provided at the 70th Annual Meeting of the American Epilepsy Society.
Epilepsy “is not necessarily a neurologic disease with psychiatric comorbidity. Maybe there is something in the interface … that is really our target,” he said.
Epilepsy and psychiatry have considerable symptom overlap, Dr. Salpekar noted. For example, someone with a seizure focus in the amygdala might have an aura of anxiety or overwhelming fear that resembles the early stages of a panic attack.
Neurologists should aim to treat seizures and psychiatric symptoms. “Think about psychiatric symptoms as clues that we have not treated the entire disease yet,” Dr. Salpekar said. “We want to use our anticonvulsants to treat the entire disease, and maybe that will lead to improved seizure control.”
A Bidirectional Relationship
Understanding the bidirectional relationship between neuropsychiatric disorders and epilepsy is key to treatment. “Not only are you more likely to be depressed if you have epilepsy, but you are more likely to have epilepsy or develop seizures if you are depressed,” Dr. Salpekar said. This finding represents “a paradigm shift … in how we interpret what this illness could be.”
In addition, ADHD increases the likelihood of seizures, and one study found that 28% of children with ADHD who underwent polysomnography had centrotemporal spikes. Other research suggests that patients with epilepsy may have structural changes and cognitive symptoms prior to their first identified seizures.
Antiepileptic drugs (AEDs) effectively treat psychiatric symptoms, including impulsivity, rage outbursts, and mood lability. “In the psychiatric world, we depend upon these [drugs] all the time,” Dr. Salpekar said. “Maybe there are relationships between seizure control and behavior control that are more clear-cut than we have thought.”
Treatment Options
Dr. Salpekar and colleagues in 2006 conducted a review of patients with epilepsy and comorbid bipolar spectrum disorder. They reviewed cases to see whether treatment with AEDs improved bipolar symptoms, such as mood lability, impulsivity, rage outbursts, and extreme irritability, as well as seizures. They found that carbamazepine, divalproex sodium, lamotrigine, and oxcarbazepine monotherapies were associated with better psychiatric symptom ratings, compared with other monotherapies. In many cases, the drugs appeared to treat epilepsy and mood disorder simultaneously. The findings suggest that AEDs can “bridge the gap between epilepsy or seizure counts and psychiatric symptoms” and that neurologists should “aim for broad-spectrum treatment,” he said.
Psychiatric symptoms sometimes may be related to AEDs. If an AED causes depressive symptoms or irritability, neurologists should try to remove it. In addition, benzodiazepine withdrawal may cause depression, and this effect may occur in certain patients due to their metabolism. “If someone is a fast metabolizer, and they are taking b.i.d. clonazepam, what happens if it wears off?” In those cases, behavior problems may occur late in the afternoon when serum drug concentrations are lowest.
Many GABAergic drugs have been reported to improve anxiety. Gabapentin also has been reported to improve social phobia and chronic pain. Potential negative effects of GABAergic drugs include depression, sedation, mood lability, and hyperactivity.
Among antiglutamatergic drugs, lamotrigine may be the best evidence-based option for improving mood or depression. Lamotrigine, however, may promote mania in patients with a propensity for bipolar spectrum disorder.
Among drugs with other mechanisms of action, carbamazepine is known to be a mood stabilizer, and eslicarbazepine may have the same effect. Levetiracetam may cause irritability, but the drug also may improve mood in some cases. “There are positives and negatives with any of our AEDs,” Dr. Salpekar said.
An Important Question to Ask Patients
Patients with severe refractory epilepsy often have depression, and treating depression may improve their quality of life dramatically. “When all else fails, at the very least, we want to identify if depression is present because we can do something about that most of the time,” Dr. Salpekar said.
Many depression screening tools are available, but the important thing is to broach the subject. “I would encourage all of you to ask one question of your patients. Just say, ‘Are you depressed?’ That might be all you have to do.”
Depression may be more common in patients with temporal lobe foci, and evidence suggests that adults with left foci have an increased likelihood of depression. Waxman and Geschwind observed that patients with temporal lobe epilepsy may have an increased preoccupation with philosophical, moral, or religious issues, which also can be characteristic of manic episodes, Dr. Salpekar said.
Dr. Salpekar and colleagues conducted a study of children with medically refractory epilepsy who presented for surgical evaluation to assess whether psychiatric symptoms differed according to seizure focus. Researchers reviewed case records for 40 patients. Patients with suspected temporal lobe foci had more behavioral problems and were more likely to have a diagnosis of depression, compared with children with extratemporal foci. This finding “lends some credence to our idea that temporal lobe foci are worse … in terms of depression,” he said.
Treating Depression
Neurologists can follow practical steps to address depression. First, determine whether symptoms are attributable to drug side effects. Then, address lifestyle factors, including sleep, exercise, and diet, and consider social stressors, such as family members or employers who do not appreciate the patient’s cognitive or physical limitations. Neurologists should identify targets that help measure whether treatment is working and consider whether low doses of adjunctive AEDs may help treat psychiatric symptoms. Finally, neurologists may consider prescribing an antidepressant. “If you are going to use an antidepressant, get comfortable, skilled, and knowledgeable about using one of them,” he said.
Studies have provided data regarding the use of antidepressants in patients with epilepsy. In an observational pediatric study, 36 patients who received fluoxetine or sertraline improved clinically, whereas two patients who received fluoxetine or sertraline had worsened seizures. Studies also have provided evidence for the use of citalopram and sertraline in adults with epilepsy. Other studies regarding the treatment of depression in epilepsy are ongoing.
Selective serotonin reuptake inhibitors (SSRIs) entail a low risk of lowering seizure threshold, as do certain antipsychotics (eg, haloperidol, risperidone, and aripiprazole). Methylphenidate is considered safe and is widely used for children with epilepsy and comorbid ADHD.
Nonmedical treatment also can benefit patients. Social skills groups, overnight camps, and cognitive behavioral therapy may be effective options. Vocational support also is important. “Meaningful activity can … tip the balance into more functionality,” he said. “Think about seizure control and behavior control as partners,” Dr. Salpekar concluded. “Think about treating both of these types of targets as working together to improve the entire illness.”
—Jake Remaly
Suggested Reading
Hermann BP, Dabbs K, Becker T, et al. Brain development in children with new onset epilepsy: a prospective controlled cohort investigation. Epilepsia. 2010;51(10):2038-2046.
Hesdorffer DC, Hauser WA, Olafsson E, et al. Depression and suicide attempt as risk factors for incident unprovoked seizures. Ann Neurol. 2006;59(1):35-41.
Salpekar JA. Mood disorders in epilepsy. Focus. 2016;14(4):465-472.
Salpekar JA, Berl MM, Havens K, et al. Psychiatric symptoms in children prior to epilepsy surgery differ according to suspected seizure focus. Epilepsia. 2013;54(6):1074-1082.
Salpekar JA, Conry JA, Doss W, et al. Clinical experience with anticonvulsant medication in pediatric epilepsy and comorbid bipolar spectrum disorder. Epilepsy Behav. 2006;9(2):327-334.
Silvestri R, Gagliano A, Calarese T, et al. Ictal and interictal EEG abnormalities in ADHD children recorded over night by video-polysomnography. Epilepsy Res. 2007;75(2-3):130-137.
Waxman SG, Geschwind N. The interictal behavior syndrome of temporal lobe epilepsy. Arch Gen Psychiatry. 1975;32(12):1580-1586.
HOUSTON—Treating psychiatric symptoms in patients with refractory epilepsy is likely to improve the overall course of the disease, as well as patients’ quality of life, according to an overview provided at the 70th Annual Meeting of the American Epilepsy Society.
Epilepsy “is not necessarily a neurologic disease with psychiatric comorbidity. Maybe there is something in the interface … that is really our target,” he said.
Epilepsy and psychiatry have considerable symptom overlap, Dr. Salpekar noted. For example, someone with a seizure focus in the amygdala might have an aura of anxiety or overwhelming fear that resembles the early stages of a panic attack.
Neurologists should aim to treat seizures and psychiatric symptoms. “Think about psychiatric symptoms as clues that we have not treated the entire disease yet,” Dr. Salpekar said. “We want to use our anticonvulsants to treat the entire disease, and maybe that will lead to improved seizure control.”
A Bidirectional Relationship
Understanding the bidirectional relationship between neuropsychiatric disorders and epilepsy is key to treatment. “Not only are you more likely to be depressed if you have epilepsy, but you are more likely to have epilepsy or develop seizures if you are depressed,” Dr. Salpekar said. This finding represents “a paradigm shift … in how we interpret what this illness could be.”
In addition, ADHD increases the likelihood of seizures, and one study found that 28% of children with ADHD who underwent polysomnography had centrotemporal spikes. Other research suggests that patients with epilepsy may have structural changes and cognitive symptoms prior to their first identified seizures.
Antiepileptic drugs (AEDs) effectively treat psychiatric symptoms, including impulsivity, rage outbursts, and mood lability. “In the psychiatric world, we depend upon these [drugs] all the time,” Dr. Salpekar said. “Maybe there are relationships between seizure control and behavior control that are more clear-cut than we have thought.”
Treatment Options
Dr. Salpekar and colleagues in 2006 conducted a review of patients with epilepsy and comorbid bipolar spectrum disorder. They reviewed cases to see whether treatment with AEDs improved bipolar symptoms, such as mood lability, impulsivity, rage outbursts, and extreme irritability, as well as seizures. They found that carbamazepine, divalproex sodium, lamotrigine, and oxcarbazepine monotherapies were associated with better psychiatric symptom ratings, compared with other monotherapies. In many cases, the drugs appeared to treat epilepsy and mood disorder simultaneously. The findings suggest that AEDs can “bridge the gap between epilepsy or seizure counts and psychiatric symptoms” and that neurologists should “aim for broad-spectrum treatment,” he said.
Psychiatric symptoms sometimes may be related to AEDs. If an AED causes depressive symptoms or irritability, neurologists should try to remove it. In addition, benzodiazepine withdrawal may cause depression, and this effect may occur in certain patients due to their metabolism. “If someone is a fast metabolizer, and they are taking b.i.d. clonazepam, what happens if it wears off?” In those cases, behavior problems may occur late in the afternoon when serum drug concentrations are lowest.
Many GABAergic drugs have been reported to improve anxiety. Gabapentin also has been reported to improve social phobia and chronic pain. Potential negative effects of GABAergic drugs include depression, sedation, mood lability, and hyperactivity.
Among antiglutamatergic drugs, lamotrigine may be the best evidence-based option for improving mood or depression. Lamotrigine, however, may promote mania in patients with a propensity for bipolar spectrum disorder.
Among drugs with other mechanisms of action, carbamazepine is known to be a mood stabilizer, and eslicarbazepine may have the same effect. Levetiracetam may cause irritability, but the drug also may improve mood in some cases. “There are positives and negatives with any of our AEDs,” Dr. Salpekar said.
An Important Question to Ask Patients
Patients with severe refractory epilepsy often have depression, and treating depression may improve their quality of life dramatically. “When all else fails, at the very least, we want to identify if depression is present because we can do something about that most of the time,” Dr. Salpekar said.
Many depression screening tools are available, but the important thing is to broach the subject. “I would encourage all of you to ask one question of your patients. Just say, ‘Are you depressed?’ That might be all you have to do.”
Depression may be more common in patients with temporal lobe foci, and evidence suggests that adults with left foci have an increased likelihood of depression. Waxman and Geschwind observed that patients with temporal lobe epilepsy may have an increased preoccupation with philosophical, moral, or religious issues, which also can be characteristic of manic episodes, Dr. Salpekar said.
Dr. Salpekar and colleagues conducted a study of children with medically refractory epilepsy who presented for surgical evaluation to assess whether psychiatric symptoms differed according to seizure focus. Researchers reviewed case records for 40 patients. Patients with suspected temporal lobe foci had more behavioral problems and were more likely to have a diagnosis of depression, compared with children with extratemporal foci. This finding “lends some credence to our idea that temporal lobe foci are worse … in terms of depression,” he said.
Treating Depression
Neurologists can follow practical steps to address depression. First, determine whether symptoms are attributable to drug side effects. Then, address lifestyle factors, including sleep, exercise, and diet, and consider social stressors, such as family members or employers who do not appreciate the patient’s cognitive or physical limitations. Neurologists should identify targets that help measure whether treatment is working and consider whether low doses of adjunctive AEDs may help treat psychiatric symptoms. Finally, neurologists may consider prescribing an antidepressant. “If you are going to use an antidepressant, get comfortable, skilled, and knowledgeable about using one of them,” he said.
Studies have provided data regarding the use of antidepressants in patients with epilepsy. In an observational pediatric study, 36 patients who received fluoxetine or sertraline improved clinically, whereas two patients who received fluoxetine or sertraline had worsened seizures. Studies also have provided evidence for the use of citalopram and sertraline in adults with epilepsy. Other studies regarding the treatment of depression in epilepsy are ongoing.
Selective serotonin reuptake inhibitors (SSRIs) entail a low risk of lowering seizure threshold, as do certain antipsychotics (eg, haloperidol, risperidone, and aripiprazole). Methylphenidate is considered safe and is widely used for children with epilepsy and comorbid ADHD.
Nonmedical treatment also can benefit patients. Social skills groups, overnight camps, and cognitive behavioral therapy may be effective options. Vocational support also is important. “Meaningful activity can … tip the balance into more functionality,” he said. “Think about seizure control and behavior control as partners,” Dr. Salpekar concluded. “Think about treating both of these types of targets as working together to improve the entire illness.”
—Jake Remaly
Suggested Reading
Hermann BP, Dabbs K, Becker T, et al. Brain development in children with new onset epilepsy: a prospective controlled cohort investigation. Epilepsia. 2010;51(10):2038-2046.
Hesdorffer DC, Hauser WA, Olafsson E, et al. Depression and suicide attempt as risk factors for incident unprovoked seizures. Ann Neurol. 2006;59(1):35-41.
Salpekar JA. Mood disorders in epilepsy. Focus. 2016;14(4):465-472.
Salpekar JA, Berl MM, Havens K, et al. Psychiatric symptoms in children prior to epilepsy surgery differ according to suspected seizure focus. Epilepsia. 2013;54(6):1074-1082.
Salpekar JA, Conry JA, Doss W, et al. Clinical experience with anticonvulsant medication in pediatric epilepsy and comorbid bipolar spectrum disorder. Epilepsy Behav. 2006;9(2):327-334.
Silvestri R, Gagliano A, Calarese T, et al. Ictal and interictal EEG abnormalities in ADHD children recorded over night by video-polysomnography. Epilepsy Res. 2007;75(2-3):130-137.
Waxman SG, Geschwind N. The interictal behavior syndrome of temporal lobe epilepsy. Arch Gen Psychiatry. 1975;32(12):1580-1586.
HOUSTON—Treating psychiatric symptoms in patients with refractory epilepsy is likely to improve the overall course of the disease, as well as patients’ quality of life, according to an overview provided at the 70th Annual Meeting of the American Epilepsy Society.
Epilepsy “is not necessarily a neurologic disease with psychiatric comorbidity. Maybe there is something in the interface … that is really our target,” he said.
Epilepsy and psychiatry have considerable symptom overlap, Dr. Salpekar noted. For example, someone with a seizure focus in the amygdala might have an aura of anxiety or overwhelming fear that resembles the early stages of a panic attack.
Neurologists should aim to treat seizures and psychiatric symptoms. “Think about psychiatric symptoms as clues that we have not treated the entire disease yet,” Dr. Salpekar said. “We want to use our anticonvulsants to treat the entire disease, and maybe that will lead to improved seizure control.”
A Bidirectional Relationship
Understanding the bidirectional relationship between neuropsychiatric disorders and epilepsy is key to treatment. “Not only are you more likely to be depressed if you have epilepsy, but you are more likely to have epilepsy or develop seizures if you are depressed,” Dr. Salpekar said. This finding represents “a paradigm shift … in how we interpret what this illness could be.”
In addition, ADHD increases the likelihood of seizures, and one study found that 28% of children with ADHD who underwent polysomnography had centrotemporal spikes. Other research suggests that patients with epilepsy may have structural changes and cognitive symptoms prior to their first identified seizures.
Antiepileptic drugs (AEDs) effectively treat psychiatric symptoms, including impulsivity, rage outbursts, and mood lability. “In the psychiatric world, we depend upon these [drugs] all the time,” Dr. Salpekar said. “Maybe there are relationships between seizure control and behavior control that are more clear-cut than we have thought.”
Treatment Options
Dr. Salpekar and colleagues in 2006 conducted a review of patients with epilepsy and comorbid bipolar spectrum disorder. They reviewed cases to see whether treatment with AEDs improved bipolar symptoms, such as mood lability, impulsivity, rage outbursts, and extreme irritability, as well as seizures. They found that carbamazepine, divalproex sodium, lamotrigine, and oxcarbazepine monotherapies were associated with better psychiatric symptom ratings, compared with other monotherapies. In many cases, the drugs appeared to treat epilepsy and mood disorder simultaneously. The findings suggest that AEDs can “bridge the gap between epilepsy or seizure counts and psychiatric symptoms” and that neurologists should “aim for broad-spectrum treatment,” he said.
Psychiatric symptoms sometimes may be related to AEDs. If an AED causes depressive symptoms or irritability, neurologists should try to remove it. In addition, benzodiazepine withdrawal may cause depression, and this effect may occur in certain patients due to their metabolism. “If someone is a fast metabolizer, and they are taking b.i.d. clonazepam, what happens if it wears off?” In those cases, behavior problems may occur late in the afternoon when serum drug concentrations are lowest.
Many GABAergic drugs have been reported to improve anxiety. Gabapentin also has been reported to improve social phobia and chronic pain. Potential negative effects of GABAergic drugs include depression, sedation, mood lability, and hyperactivity.
Among antiglutamatergic drugs, lamotrigine may be the best evidence-based option for improving mood or depression. Lamotrigine, however, may promote mania in patients with a propensity for bipolar spectrum disorder.
Among drugs with other mechanisms of action, carbamazepine is known to be a mood stabilizer, and eslicarbazepine may have the same effect. Levetiracetam may cause irritability, but the drug also may improve mood in some cases. “There are positives and negatives with any of our AEDs,” Dr. Salpekar said.
An Important Question to Ask Patients
Patients with severe refractory epilepsy often have depression, and treating depression may improve their quality of life dramatically. “When all else fails, at the very least, we want to identify if depression is present because we can do something about that most of the time,” Dr. Salpekar said.
Many depression screening tools are available, but the important thing is to broach the subject. “I would encourage all of you to ask one question of your patients. Just say, ‘Are you depressed?’ That might be all you have to do.”
Depression may be more common in patients with temporal lobe foci, and evidence suggests that adults with left foci have an increased likelihood of depression. Waxman and Geschwind observed that patients with temporal lobe epilepsy may have an increased preoccupation with philosophical, moral, or religious issues, which also can be characteristic of manic episodes, Dr. Salpekar said.
Dr. Salpekar and colleagues conducted a study of children with medically refractory epilepsy who presented for surgical evaluation to assess whether psychiatric symptoms differed according to seizure focus. Researchers reviewed case records for 40 patients. Patients with suspected temporal lobe foci had more behavioral problems and were more likely to have a diagnosis of depression, compared with children with extratemporal foci. This finding “lends some credence to our idea that temporal lobe foci are worse … in terms of depression,” he said.
Treating Depression
Neurologists can follow practical steps to address depression. First, determine whether symptoms are attributable to drug side effects. Then, address lifestyle factors, including sleep, exercise, and diet, and consider social stressors, such as family members or employers who do not appreciate the patient’s cognitive or physical limitations. Neurologists should identify targets that help measure whether treatment is working and consider whether low doses of adjunctive AEDs may help treat psychiatric symptoms. Finally, neurologists may consider prescribing an antidepressant. “If you are going to use an antidepressant, get comfortable, skilled, and knowledgeable about using one of them,” he said.
Studies have provided data regarding the use of antidepressants in patients with epilepsy. In an observational pediatric study, 36 patients who received fluoxetine or sertraline improved clinically, whereas two patients who received fluoxetine or sertraline had worsened seizures. Studies also have provided evidence for the use of citalopram and sertraline in adults with epilepsy. Other studies regarding the treatment of depression in epilepsy are ongoing.
Selective serotonin reuptake inhibitors (SSRIs) entail a low risk of lowering seizure threshold, as do certain antipsychotics (eg, haloperidol, risperidone, and aripiprazole). Methylphenidate is considered safe and is widely used for children with epilepsy and comorbid ADHD.
Nonmedical treatment also can benefit patients. Social skills groups, overnight camps, and cognitive behavioral therapy may be effective options. Vocational support also is important. “Meaningful activity can … tip the balance into more functionality,” he said. “Think about seizure control and behavior control as partners,” Dr. Salpekar concluded. “Think about treating both of these types of targets as working together to improve the entire illness.”
—Jake Remaly
Suggested Reading
Hermann BP, Dabbs K, Becker T, et al. Brain development in children with new onset epilepsy: a prospective controlled cohort investigation. Epilepsia. 2010;51(10):2038-2046.
Hesdorffer DC, Hauser WA, Olafsson E, et al. Depression and suicide attempt as risk factors for incident unprovoked seizures. Ann Neurol. 2006;59(1):35-41.
Salpekar JA. Mood disorders in epilepsy. Focus. 2016;14(4):465-472.
Salpekar JA, Berl MM, Havens K, et al. Psychiatric symptoms in children prior to epilepsy surgery differ according to suspected seizure focus. Epilepsia. 2013;54(6):1074-1082.
Salpekar JA, Conry JA, Doss W, et al. Clinical experience with anticonvulsant medication in pediatric epilepsy and comorbid bipolar spectrum disorder. Epilepsy Behav. 2006;9(2):327-334.
Silvestri R, Gagliano A, Calarese T, et al. Ictal and interictal EEG abnormalities in ADHD children recorded over night by video-polysomnography. Epilepsy Res. 2007;75(2-3):130-137.
Waxman SG, Geschwind N. The interictal behavior syndrome of temporal lobe epilepsy. Arch Gen Psychiatry. 1975;32(12):1580-1586.
Outcomes of neoadjuvant therapy vary by subtype in bladder cancer
Clinical outcomes for neoadjuvant chemotherapy (NAC) vary by molecular subtype in muscle-invasive bladder cancer, investigators report.
Researchers classified the subtypes in 223 patients with muscle-invasive bladder cancer (luminal, basal, claudin-low, and luminal-infiltrated) and found response to neoadjuvant cisplatin-based chemotherapy varied by subtype (P = .0001).
“Neoadjuvant chemotherapy improves outcomes in muscle-invasive bladder cancer, but only at 5%-7% for overall survival at 5 years,” study author Roland Seiler, MD, of the University of British Columbia, Vancouver, said in a press briefing held at the 2017 genitourinary cancers symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology. “About 60% of patients still have invasive disease at cystectomy so they are therefore nonresponders, but they still suffer from unnecessary side effects.”
Dr. Seiler explained that molecular subtypes of muscle-invasive bladder cancers had been identified that were based on gene expression. They decided to investigate the impact of different subtyping methods on patient response to NAC with the goal of developing a single sample model for subtyping.
A transcriptome-wide microarray analysis was conducted, using bladder tumor transurethral resection specimens that were obtained from 223 patients prior to receipt of NAC and cystectomy. The specimens were then classified according to four published methods for molecular subtype: University of North Carolina (UNC) dataset, MD Anderson (MDA) dataset, The Cancer Genome Atlas (TCGA) dataset, and the Lund dataset.
A validation set of 82 pre-NAC specimens also was done, with the results compared to non-NAC cohorts in the public domain including 179 cases from TCGA dataset, 107 from the MDA dataset, and 190 from the Lund dataset.
Finally, a genomic classifier was trained to predict the different subtypes in a single sample model and was then validated in both independent NAC and non-NAC datasets.
Investigators found that patients with luminal tumors had the best overall survival and it was independent of NAC. Patients with tumors classified as UNC basal, MDA basal, and TCGA cluster III achieved the greatest improvement in overall survival following NAC as compared with cystectomy alone.
Patients with tumors that were classified as UNC basal, MDA basal and TCGA cluster III achieved the greatest survival benefit following NAC, compared to surgery alone. Tumors that were assigned as UNC claudin-low had the worst overall survival regardless of the type of treatment regimen (P = .005).
“Basal tumors showed the most improvement with neoadjuvant therapy and should be prioritized with this treatment,” said Dr. Seiler. “Whereas for other subtypes we may need novel therapies.”
As discussant for the presentation, Jonathan Rosenberg, MD, of Memorial Sloan Kettering Cancer Center in New York, said that cisplatin “is a toxic therapy and we would love to have a great biomarker to select the right group of patients. Currently using present data, anywhere from 10 to 20 patients need to be treated to save one life.”
He reiterated that these findings confirm that basal tumors appear to benefit significantly from platinum-based chemotherapy and this benefit appears to exist regardless of downstaging.
“However it does not automatically follow that other subtypes do not benefit, and we are not ready to change clinical practice just yet,” Dr. Rosenberg said. “We need to prospectively validate these findings before we implement them into clinical practice.”
Clinical outcomes for neoadjuvant chemotherapy (NAC) vary by molecular subtype in muscle-invasive bladder cancer, investigators report.
Researchers classified the subtypes in 223 patients with muscle-invasive bladder cancer (luminal, basal, claudin-low, and luminal-infiltrated) and found response to neoadjuvant cisplatin-based chemotherapy varied by subtype (P = .0001).
“Neoadjuvant chemotherapy improves outcomes in muscle-invasive bladder cancer, but only at 5%-7% for overall survival at 5 years,” study author Roland Seiler, MD, of the University of British Columbia, Vancouver, said in a press briefing held at the 2017 genitourinary cancers symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology. “About 60% of patients still have invasive disease at cystectomy so they are therefore nonresponders, but they still suffer from unnecessary side effects.”
Dr. Seiler explained that molecular subtypes of muscle-invasive bladder cancers had been identified that were based on gene expression. They decided to investigate the impact of different subtyping methods on patient response to NAC with the goal of developing a single sample model for subtyping.
A transcriptome-wide microarray analysis was conducted, using bladder tumor transurethral resection specimens that were obtained from 223 patients prior to receipt of NAC and cystectomy. The specimens were then classified according to four published methods for molecular subtype: University of North Carolina (UNC) dataset, MD Anderson (MDA) dataset, The Cancer Genome Atlas (TCGA) dataset, and the Lund dataset.
A validation set of 82 pre-NAC specimens also was done, with the results compared to non-NAC cohorts in the public domain including 179 cases from TCGA dataset, 107 from the MDA dataset, and 190 from the Lund dataset.
Finally, a genomic classifier was trained to predict the different subtypes in a single sample model and was then validated in both independent NAC and non-NAC datasets.
Investigators found that patients with luminal tumors had the best overall survival and it was independent of NAC. Patients with tumors classified as UNC basal, MDA basal, and TCGA cluster III achieved the greatest improvement in overall survival following NAC as compared with cystectomy alone.
Patients with tumors that were classified as UNC basal, MDA basal and TCGA cluster III achieved the greatest survival benefit following NAC, compared to surgery alone. Tumors that were assigned as UNC claudin-low had the worst overall survival regardless of the type of treatment regimen (P = .005).
“Basal tumors showed the most improvement with neoadjuvant therapy and should be prioritized with this treatment,” said Dr. Seiler. “Whereas for other subtypes we may need novel therapies.”
As discussant for the presentation, Jonathan Rosenberg, MD, of Memorial Sloan Kettering Cancer Center in New York, said that cisplatin “is a toxic therapy and we would love to have a great biomarker to select the right group of patients. Currently using present data, anywhere from 10 to 20 patients need to be treated to save one life.”
He reiterated that these findings confirm that basal tumors appear to benefit significantly from platinum-based chemotherapy and this benefit appears to exist regardless of downstaging.
“However it does not automatically follow that other subtypes do not benefit, and we are not ready to change clinical practice just yet,” Dr. Rosenberg said. “We need to prospectively validate these findings before we implement them into clinical practice.”
Clinical outcomes for neoadjuvant chemotherapy (NAC) vary by molecular subtype in muscle-invasive bladder cancer, investigators report.
Researchers classified the subtypes in 223 patients with muscle-invasive bladder cancer (luminal, basal, claudin-low, and luminal-infiltrated) and found response to neoadjuvant cisplatin-based chemotherapy varied by subtype (P = .0001).
“Neoadjuvant chemotherapy improves outcomes in muscle-invasive bladder cancer, but only at 5%-7% for overall survival at 5 years,” study author Roland Seiler, MD, of the University of British Columbia, Vancouver, said in a press briefing held at the 2017 genitourinary cancers symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology. “About 60% of patients still have invasive disease at cystectomy so they are therefore nonresponders, but they still suffer from unnecessary side effects.”
Dr. Seiler explained that molecular subtypes of muscle-invasive bladder cancers had been identified that were based on gene expression. They decided to investigate the impact of different subtyping methods on patient response to NAC with the goal of developing a single sample model for subtyping.
A transcriptome-wide microarray analysis was conducted, using bladder tumor transurethral resection specimens that were obtained from 223 patients prior to receipt of NAC and cystectomy. The specimens were then classified according to four published methods for molecular subtype: University of North Carolina (UNC) dataset, MD Anderson (MDA) dataset, The Cancer Genome Atlas (TCGA) dataset, and the Lund dataset.
A validation set of 82 pre-NAC specimens also was done, with the results compared to non-NAC cohorts in the public domain including 179 cases from TCGA dataset, 107 from the MDA dataset, and 190 from the Lund dataset.
Finally, a genomic classifier was trained to predict the different subtypes in a single sample model and was then validated in both independent NAC and non-NAC datasets.
Investigators found that patients with luminal tumors had the best overall survival and it was independent of NAC. Patients with tumors classified as UNC basal, MDA basal, and TCGA cluster III achieved the greatest improvement in overall survival following NAC as compared with cystectomy alone.
Patients with tumors that were classified as UNC basal, MDA basal and TCGA cluster III achieved the greatest survival benefit following NAC, compared to surgery alone. Tumors that were assigned as UNC claudin-low had the worst overall survival regardless of the type of treatment regimen (P = .005).
“Basal tumors showed the most improvement with neoadjuvant therapy and should be prioritized with this treatment,” said Dr. Seiler. “Whereas for other subtypes we may need novel therapies.”
As discussant for the presentation, Jonathan Rosenberg, MD, of Memorial Sloan Kettering Cancer Center in New York, said that cisplatin “is a toxic therapy and we would love to have a great biomarker to select the right group of patients. Currently using present data, anywhere from 10 to 20 patients need to be treated to save one life.”
He reiterated that these findings confirm that basal tumors appear to benefit significantly from platinum-based chemotherapy and this benefit appears to exist regardless of downstaging.
“However it does not automatically follow that other subtypes do not benefit, and we are not ready to change clinical practice just yet,” Dr. Rosenberg said. “We need to prospectively validate these findings before we implement them into clinical practice.”
FROM THE GENITOURINARY CANCERS SYMPOSIUM
Key clinical point: Clinical outcomes for neoadjuvant chemotherapy (NAC) vary by molecular subtype in muscle-invasive bladder cancer.
Major finding: Luminal tumors had the best overall survival independent of NAC and basal tumors the best response to NAC.
Data source: Experimental study that evaluated the efficacy of NAC in the molecular subtypes of muscle invasive bladder cancer.
Disclosures: The funding source is not disclosed. Dr Seiler has no disclosures. Several of his coauthors report relationships with multiple pharmaceutical companies. Dr Rosenberg reports financial ties to multiple pharmaceutical companies.
Does Patient History Affect the Treatment of Pediatric Refractory Status Epilepticus?
Children with refractory status epilepticus do not receive more timely treatment if they have a prior diagnosis of epilepsy, according to an investigation published January 24 in Neurology. A history of status epilepticus, however, is associated with more timely administration of abortive medication.
Most episodes of pediatric status epilepticus occur in children with no history of seizures. Investigators had not examined whether having a history of seizures or status epilepticus leads to more timely treatment, including escalation of therapy when indicated, and better outcomes.
Iván Sánchez Fernández, MD, Clinical Fellow in Neurology at Boston Children’s Hospital, and colleagues conducted a prospective observational study to compare the management and outcomes of refractory status epilepticus in children with and without a prior diagnosis of epilepsy and with and without a history of status epilepticus. Eligible patients were between ages one month and 21 years and had convulsive seizures at onset that continued after administration of at least two antiepileptic drugs (AEDs).
The investigators enrolled 189 participants (53% male) with a median age of 4.2 years. Eighty-nine (47%) patients had a prior diagnosis of epilepsy. Thirty-four (18%) patients had a history of status epilepticus.
The time to first benzodiazepine was similar in participants with and without a diagnosis of epilepsy (15 minutes vs 16.5 minutes). Patients with a diagnosis of epilepsy received their first nonbenzodiazepine AED later (93 minutes vs 50.5 minutes) and were less likely to receive at least one continuous infusion (39.3% vs 57%). The time to the first continuous infusion, however, was similar in patients with and without a diagnosis of epilepsy (258.5 vs 149 minutes)
Patients with a diagnosis of epilepsy were less likely to be intubated (66.3% vs 83%), had a longer duration of status epilepticus (174 minutes vs 120 minutes), and had a shorter ICU stay (3.9 days vs 5 days). At hospital discharge, patients with a diagnosis of epilepsy were more likely to return to baseline (75% vs 65%) and had lower mortality (0% vs 7%).
Time to the first benzodiazepine was shorter for patients with a history of status epilepticus, compared with patients without (8 minutes vs 20 minutes). The time to first nonbenzodiazepine AED was similar in patients with and without a history of status epilepticus (76.5 minutes vs 65 minutes). Patients with and without a history of status epilepticus were as likely to receive continuous infusions (52.9% vs 47.7%), had a similar time to the first continuous infusion (182 vs 180 minutes), and were as likely to be intubated (82.4% vs 73.5%). Patients with a history of status epilepticus had a longer duration of status epilepticus (150 minutes vs 124.5 minutes) and a shorter ICU stay (5 vs 4.3 days).
At hospital discharge, return to baseline function was similar between patients with and without a history of status epilepticus (76.5% vs 68.4%). The difference between groups in mortality rate was not statistically significant (0% vs 4.5%). The more timely administration of the first benzodiazepine in patients with a history of status epilepticus mainly resulted from cases with onset outside a hospital, while management of status epilepticus with in-hospital onset was similar in patients with and without a history of status epilepticus.
—Erik Greb
Suggested Reading
Sánchez Fernández I, Jackson MC, Abend NS, et al. Refractory status epilepticus in children with and without prior epilepsy or status epilepticus. Neurology. 2017;88(4):386-394.
Children with refractory status epilepticus do not receive more timely treatment if they have a prior diagnosis of epilepsy, according to an investigation published January 24 in Neurology. A history of status epilepticus, however, is associated with more timely administration of abortive medication.
Most episodes of pediatric status epilepticus occur in children with no history of seizures. Investigators had not examined whether having a history of seizures or status epilepticus leads to more timely treatment, including escalation of therapy when indicated, and better outcomes.
Iván Sánchez Fernández, MD, Clinical Fellow in Neurology at Boston Children’s Hospital, and colleagues conducted a prospective observational study to compare the management and outcomes of refractory status epilepticus in children with and without a prior diagnosis of epilepsy and with and without a history of status epilepticus. Eligible patients were between ages one month and 21 years and had convulsive seizures at onset that continued after administration of at least two antiepileptic drugs (AEDs).
The investigators enrolled 189 participants (53% male) with a median age of 4.2 years. Eighty-nine (47%) patients had a prior diagnosis of epilepsy. Thirty-four (18%) patients had a history of status epilepticus.
The time to first benzodiazepine was similar in participants with and without a diagnosis of epilepsy (15 minutes vs 16.5 minutes). Patients with a diagnosis of epilepsy received their first nonbenzodiazepine AED later (93 minutes vs 50.5 minutes) and were less likely to receive at least one continuous infusion (39.3% vs 57%). The time to the first continuous infusion, however, was similar in patients with and without a diagnosis of epilepsy (258.5 vs 149 minutes)
Patients with a diagnosis of epilepsy were less likely to be intubated (66.3% vs 83%), had a longer duration of status epilepticus (174 minutes vs 120 minutes), and had a shorter ICU stay (3.9 days vs 5 days). At hospital discharge, patients with a diagnosis of epilepsy were more likely to return to baseline (75% vs 65%) and had lower mortality (0% vs 7%).
Time to the first benzodiazepine was shorter for patients with a history of status epilepticus, compared with patients without (8 minutes vs 20 minutes). The time to first nonbenzodiazepine AED was similar in patients with and without a history of status epilepticus (76.5 minutes vs 65 minutes). Patients with and without a history of status epilepticus were as likely to receive continuous infusions (52.9% vs 47.7%), had a similar time to the first continuous infusion (182 vs 180 minutes), and were as likely to be intubated (82.4% vs 73.5%). Patients with a history of status epilepticus had a longer duration of status epilepticus (150 minutes vs 124.5 minutes) and a shorter ICU stay (5 vs 4.3 days).
At hospital discharge, return to baseline function was similar between patients with and without a history of status epilepticus (76.5% vs 68.4%). The difference between groups in mortality rate was not statistically significant (0% vs 4.5%). The more timely administration of the first benzodiazepine in patients with a history of status epilepticus mainly resulted from cases with onset outside a hospital, while management of status epilepticus with in-hospital onset was similar in patients with and without a history of status epilepticus.
—Erik Greb
Suggested Reading
Sánchez Fernández I, Jackson MC, Abend NS, et al. Refractory status epilepticus in children with and without prior epilepsy or status epilepticus. Neurology. 2017;88(4):386-394.
Children with refractory status epilepticus do not receive more timely treatment if they have a prior diagnosis of epilepsy, according to an investigation published January 24 in Neurology. A history of status epilepticus, however, is associated with more timely administration of abortive medication.
Most episodes of pediatric status epilepticus occur in children with no history of seizures. Investigators had not examined whether having a history of seizures or status epilepticus leads to more timely treatment, including escalation of therapy when indicated, and better outcomes.
Iván Sánchez Fernández, MD, Clinical Fellow in Neurology at Boston Children’s Hospital, and colleagues conducted a prospective observational study to compare the management and outcomes of refractory status epilepticus in children with and without a prior diagnosis of epilepsy and with and without a history of status epilepticus. Eligible patients were between ages one month and 21 years and had convulsive seizures at onset that continued after administration of at least two antiepileptic drugs (AEDs).
The investigators enrolled 189 participants (53% male) with a median age of 4.2 years. Eighty-nine (47%) patients had a prior diagnosis of epilepsy. Thirty-four (18%) patients had a history of status epilepticus.
The time to first benzodiazepine was similar in participants with and without a diagnosis of epilepsy (15 minutes vs 16.5 minutes). Patients with a diagnosis of epilepsy received their first nonbenzodiazepine AED later (93 minutes vs 50.5 minutes) and were less likely to receive at least one continuous infusion (39.3% vs 57%). The time to the first continuous infusion, however, was similar in patients with and without a diagnosis of epilepsy (258.5 vs 149 minutes)
Patients with a diagnosis of epilepsy were less likely to be intubated (66.3% vs 83%), had a longer duration of status epilepticus (174 minutes vs 120 minutes), and had a shorter ICU stay (3.9 days vs 5 days). At hospital discharge, patients with a diagnosis of epilepsy were more likely to return to baseline (75% vs 65%) and had lower mortality (0% vs 7%).
Time to the first benzodiazepine was shorter for patients with a history of status epilepticus, compared with patients without (8 minutes vs 20 minutes). The time to first nonbenzodiazepine AED was similar in patients with and without a history of status epilepticus (76.5 minutes vs 65 minutes). Patients with and without a history of status epilepticus were as likely to receive continuous infusions (52.9% vs 47.7%), had a similar time to the first continuous infusion (182 vs 180 minutes), and were as likely to be intubated (82.4% vs 73.5%). Patients with a history of status epilepticus had a longer duration of status epilepticus (150 minutes vs 124.5 minutes) and a shorter ICU stay (5 vs 4.3 days).
At hospital discharge, return to baseline function was similar between patients with and without a history of status epilepticus (76.5% vs 68.4%). The difference between groups in mortality rate was not statistically significant (0% vs 4.5%). The more timely administration of the first benzodiazepine in patients with a history of status epilepticus mainly resulted from cases with onset outside a hospital, while management of status epilepticus with in-hospital onset was similar in patients with and without a history of status epilepticus.
—Erik Greb
Suggested Reading
Sánchez Fernández I, Jackson MC, Abend NS, et al. Refractory status epilepticus in children with and without prior epilepsy or status epilepticus. Neurology. 2017;88(4):386-394.
When atopic dermatitis is really contact dermatitis
ATLANTA – When patients present with atopic dermatitis that worsens, changes distribution, fails to improve, or immediately rebounds, think contact dermatitis, Luz Fonacier, MD, advised at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Clinical signs of contact dermatitis include lesions with an atypical distribution/pattern, such as head, eyelid, or cheilitis/perioral predominance, or lesions on the hand or foot. Also elevate your suspicion in patients with therapy-resistant hand eczema, adult- or childhood-onset atopic dermatitis without childhood eczema, as well as in cases of severe or widespread dermatitis prior to initiating a systemic immunosuppressant. The list of potential allergens to consider includes metal (especially nickel, cobalt, and potassium dichromate), fragrances such as formaldehyde and balsam of Peru, preservatives, as well as topical emollients, corticosteroids, antibiotics, and antiseptics.
Dr. Fonacier, professor of medicine at the State University of New York at Stony Brook and section head of allergy at Winthrop University Hospital, Mineola, N.Y., recommends loading acrylates, fragrances, and allergens in an aqueous vehicle immediately before application. She noted that delayed patch test readings are common to metals, topical antibiotics, and topical corticosteroids, and that positive reactions to gold are often not clinically relevant. “The patch test positivity of gold can be as high as 30% in adults and a little bit less in children, but results from two large studies show clinical relevance in only 10%-15% of cases,” she said. A trial of gold avoidance may be warranted in patients with suspected jewelry allergy, facial or eyelid dermatitis, or exposure through gold dental restorations.
She went on to share tips for reading skin patch tests. The first reading should be done after 48 hours, while the second should be done 3, 4, or 7 days after application. “The second reading helps distinguish irritant from allergic responses,” she said. “Thirty percent of negative tests at 48 hours may be positive on delayed readings.” Most true allergic reactions occur between 72 and 96 hours. Allergens that may peak early include thiuram mix, carba mix, and balsam of Peru. Those that disappear after 5 days include balsam of Peru, benzoic acid, disperse blue #124, fragrance mix, mercury, methyldibromo glutaronitrile, phenoxyethanol, and octyl gallate. Delayed patch test reactions after five days include metals (gold potassium dichromate, nickel, and cobalt), topical antibiotics (neomycin and bacitracin) as well as topic corticosteroids.
Resources she recommended to attendees include the American Contact Dermatitis Society and the Contact Dermatitis Institute. Health and safety information about household products can be found here.
Dr. Fonacier disclosed that she has received research and educational grants from Baxter and Genentech. She is also a consultant to Church and Dwight and Regeneron.
ATLANTA – When patients present with atopic dermatitis that worsens, changes distribution, fails to improve, or immediately rebounds, think contact dermatitis, Luz Fonacier, MD, advised at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Clinical signs of contact dermatitis include lesions with an atypical distribution/pattern, such as head, eyelid, or cheilitis/perioral predominance, or lesions on the hand or foot. Also elevate your suspicion in patients with therapy-resistant hand eczema, adult- or childhood-onset atopic dermatitis without childhood eczema, as well as in cases of severe or widespread dermatitis prior to initiating a systemic immunosuppressant. The list of potential allergens to consider includes metal (especially nickel, cobalt, and potassium dichromate), fragrances such as formaldehyde and balsam of Peru, preservatives, as well as topical emollients, corticosteroids, antibiotics, and antiseptics.
Dr. Fonacier, professor of medicine at the State University of New York at Stony Brook and section head of allergy at Winthrop University Hospital, Mineola, N.Y., recommends loading acrylates, fragrances, and allergens in an aqueous vehicle immediately before application. She noted that delayed patch test readings are common to metals, topical antibiotics, and topical corticosteroids, and that positive reactions to gold are often not clinically relevant. “The patch test positivity of gold can be as high as 30% in adults and a little bit less in children, but results from two large studies show clinical relevance in only 10%-15% of cases,” she said. A trial of gold avoidance may be warranted in patients with suspected jewelry allergy, facial or eyelid dermatitis, or exposure through gold dental restorations.
She went on to share tips for reading skin patch tests. The first reading should be done after 48 hours, while the second should be done 3, 4, or 7 days after application. “The second reading helps distinguish irritant from allergic responses,” she said. “Thirty percent of negative tests at 48 hours may be positive on delayed readings.” Most true allergic reactions occur between 72 and 96 hours. Allergens that may peak early include thiuram mix, carba mix, and balsam of Peru. Those that disappear after 5 days include balsam of Peru, benzoic acid, disperse blue #124, fragrance mix, mercury, methyldibromo glutaronitrile, phenoxyethanol, and octyl gallate. Delayed patch test reactions after five days include metals (gold potassium dichromate, nickel, and cobalt), topical antibiotics (neomycin and bacitracin) as well as topic corticosteroids.
Resources she recommended to attendees include the American Contact Dermatitis Society and the Contact Dermatitis Institute. Health and safety information about household products can be found here.
Dr. Fonacier disclosed that she has received research and educational grants from Baxter and Genentech. She is also a consultant to Church and Dwight and Regeneron.
ATLANTA – When patients present with atopic dermatitis that worsens, changes distribution, fails to improve, or immediately rebounds, think contact dermatitis, Luz Fonacier, MD, advised at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Clinical signs of contact dermatitis include lesions with an atypical distribution/pattern, such as head, eyelid, or cheilitis/perioral predominance, or lesions on the hand or foot. Also elevate your suspicion in patients with therapy-resistant hand eczema, adult- or childhood-onset atopic dermatitis without childhood eczema, as well as in cases of severe or widespread dermatitis prior to initiating a systemic immunosuppressant. The list of potential allergens to consider includes metal (especially nickel, cobalt, and potassium dichromate), fragrances such as formaldehyde and balsam of Peru, preservatives, as well as topical emollients, corticosteroids, antibiotics, and antiseptics.
Dr. Fonacier, professor of medicine at the State University of New York at Stony Brook and section head of allergy at Winthrop University Hospital, Mineola, N.Y., recommends loading acrylates, fragrances, and allergens in an aqueous vehicle immediately before application. She noted that delayed patch test readings are common to metals, topical antibiotics, and topical corticosteroids, and that positive reactions to gold are often not clinically relevant. “The patch test positivity of gold can be as high as 30% in adults and a little bit less in children, but results from two large studies show clinical relevance in only 10%-15% of cases,” she said. A trial of gold avoidance may be warranted in patients with suspected jewelry allergy, facial or eyelid dermatitis, or exposure through gold dental restorations.
She went on to share tips for reading skin patch tests. The first reading should be done after 48 hours, while the second should be done 3, 4, or 7 days after application. “The second reading helps distinguish irritant from allergic responses,” she said. “Thirty percent of negative tests at 48 hours may be positive on delayed readings.” Most true allergic reactions occur between 72 and 96 hours. Allergens that may peak early include thiuram mix, carba mix, and balsam of Peru. Those that disappear after 5 days include balsam of Peru, benzoic acid, disperse blue #124, fragrance mix, mercury, methyldibromo glutaronitrile, phenoxyethanol, and octyl gallate. Delayed patch test reactions after five days include metals (gold potassium dichromate, nickel, and cobalt), topical antibiotics (neomycin and bacitracin) as well as topic corticosteroids.
Resources she recommended to attendees include the American Contact Dermatitis Society and the Contact Dermatitis Institute. Health and safety information about household products can be found here.
Dr. Fonacier disclosed that she has received research and educational grants from Baxter and Genentech. She is also a consultant to Church and Dwight and Regeneron.
EXPERT ANALYSIS AT THE 2017 AAAAI ANNUAL MEETING
Online Algorithm Identifies People at Risk of Parkinson’s Disease
The online PREDICT-PD algorithm effectively identifies people at increased risk of Parkinson’s disease, according to research published online ahead of print January 16 in Movement Disorders. The risk scores that the algorithm assigns are significantly associated with intermediate markers of Parkinson’s disease. Higher PREDICT-PD risk score at baseline also is associated with an increased rate of incident Parkinson’s disease.
Previous research with the goal of identifying patients at risk of Parkinson’s disease mainly has focused on people with a family history of the disease, asymptomatic carriers of genes associated with the disease, or people with imaging abnormalities associated with the disease (eg, hyperechogenicity on transcranial sonography). The rarity, the representativeness, and the feasibility of identifying these factors have limited these studies, however.
Internet-Based Testing
To overcome these limitations, Anette-Eleonore Schrag, PhD, Professor of Clinical Neurosciences at University College London, and colleagues developed an online, evidence-based algorithm to identify risk indicators of Parkinson’s disease in the UK population. The investigators used a study website to recruit participants between ages 60 and 80 who did not have Parkinson’s disease, any other movement disorder, or stroke. Participants were prompted to return to the website yearly and complete tests.
Among the tests was a survey that included demographic questions and validated questionnaires about early nonmotor features of and risk factors for Parkinson’s disease (eg, the Hospital Anxiety Depression Scale and the REM sleep behavior disorder Screening Questionnaire [RBDSQ]). Participants also took the Bradykinesia Akinesia Incoordination (BRAIN) test, an online keyboard-tapping task. At baseline and at year three, participants took the University of Pennsylvania Smell Identification Test (UPSIT). The annual survey included a question about whether the participants had received any new diagnoses, including Parkinson’s disease or movement disorder. At year three, participants also gave saliva samples that were genotyped for mutations in exons 8 to 11 of glucocerebrosidase (GBA) and exon 41 in the LRRK2 gene.
The investigators used participants’ early features and risk factors to calculate their risk scores and rank participants according to their risk. Dr. Schrag and colleagues also tested support for enrichment of the population at risk of Parkinson’s disease by examining associations between risk scores and the following intermediate markers of Parkinson’s disease: reduced sense of smell, presence of subjective RBD, and slowing of finger tapping speed.
Risk Scores Were Associated With Incident Disease
The researchers recruited 1,323 eligible volunteers. At baseline, their mean age was 66, and approximately 61% of the population was female. In all, 1,040 of the participants completed follow-up testing at year 1, 939 completed year 2 follow-up, and 846 completed year 3 follow-up. A total of 223 participants completed the baseline assessment only.
Baseline risk scores were associated with significantly higher rates of all intermediate markers of Parkinson’s disease during each year of follow-up. The group of patients at higher risk (ie, those above the 15th percentile) had significantly worse UPSIT score, RBDSQ score, and finger tapping in all years, compared with patients at lower risk (ie, those below the 85th percentile).
Risk scores in the entire sample were strongly associated with intermediate markers of Parkinson’s disease each year. Higher- and lower-risk groups differed significantly in median UPSIT score, RBDSQ score, and mean finger tapping speed in all years of follow-up.
At year 1, three patients had been newly diagnosed with Parkinson’s disease. An additional participant received the diagnosis in year 2, and three other participants received the diagnosis in year 3. All of the participants with newly diagnosed Parkinson’s disease at year 1 were in the higher risk group, and two had also been in the higher risk group at baseline. The participant diagnosed at year 2 was in the higher risk group at baseline, year 1, and year 2. All of the three participants diagnosed by year 3 were in the middle risk group at baseline. “There was substantial heterogeneity in the occurrence of intermediate markers in these individuals,” said the researchers.
The incidence of independently diagnosed Parkinson’s disease in participants who had been in the higher risk group during three years of follow-up was 1.6% per year and 0.2% across the whole population. Exploratory Cox regression analysis using incident Parkinson’s disease over three years as the outcome indicated an association with baseline risk estimate. In participants for whom GBA and LRRK2 status was known, the association between baseline risk and incident Parkinson’s disease was weaker. But the addition of GBA and LRRK2 variants in the algorithm improved the strength of association between baseline risk and incident Parkinson’s disease. Among the intermediate markers of disease, baseline finger tapping was associated with incident Parkinson’s disease at three years.
Results Could Reflect Selection Bias
“Although only a small number of individuals have been independently diagnosed with Parkinson’s disease during follow-up so far, the overall incidence of 0.2% is consistent with the expected incidence rate in the age group of 60 to 80 from the general population (one to three per 1,000 per year) and supports the representativeness of our sample,” said Dr. Schrag.
The recruitment of participants may have introduced selection bias into the study, she acknowledged. “However, we did not use family history as an entry criterion, and the proportion with a positive family history was lower than in other landmark studies.” The fact that 17% of participants only completed the baseline assessment also may have introduced selection bias, “although there were no differences between those with or without follow-up, meaning that bias as a result of loss to follow-up was less likely.
“This Internet-based approach may be useful for population screening because it can easily be scaled upward,” added Dr. Schrag. “It will allow larger numbers of Parkinson’s disease cases that represent the spectrum of the disease to be identified, rather than what would be possible from cohorts of carriers of specific risk factors.”
—Erik Greb
Suggested Readings
Noyce AJ, R’Bibo L, Peress L, et al. PREDICT-PD: An online approach to prospectively identify risk indicators of Parkinson’s disease. Mov Disord. 2017 Jan 16 [Epub ahead of print].
The online PREDICT-PD algorithm effectively identifies people at increased risk of Parkinson’s disease, according to research published online ahead of print January 16 in Movement Disorders. The risk scores that the algorithm assigns are significantly associated with intermediate markers of Parkinson’s disease. Higher PREDICT-PD risk score at baseline also is associated with an increased rate of incident Parkinson’s disease.
Previous research with the goal of identifying patients at risk of Parkinson’s disease mainly has focused on people with a family history of the disease, asymptomatic carriers of genes associated with the disease, or people with imaging abnormalities associated with the disease (eg, hyperechogenicity on transcranial sonography). The rarity, the representativeness, and the feasibility of identifying these factors have limited these studies, however.
Internet-Based Testing
To overcome these limitations, Anette-Eleonore Schrag, PhD, Professor of Clinical Neurosciences at University College London, and colleagues developed an online, evidence-based algorithm to identify risk indicators of Parkinson’s disease in the UK population. The investigators used a study website to recruit participants between ages 60 and 80 who did not have Parkinson’s disease, any other movement disorder, or stroke. Participants were prompted to return to the website yearly and complete tests.
Among the tests was a survey that included demographic questions and validated questionnaires about early nonmotor features of and risk factors for Parkinson’s disease (eg, the Hospital Anxiety Depression Scale and the REM sleep behavior disorder Screening Questionnaire [RBDSQ]). Participants also took the Bradykinesia Akinesia Incoordination (BRAIN) test, an online keyboard-tapping task. At baseline and at year three, participants took the University of Pennsylvania Smell Identification Test (UPSIT). The annual survey included a question about whether the participants had received any new diagnoses, including Parkinson’s disease or movement disorder. At year three, participants also gave saliva samples that were genotyped for mutations in exons 8 to 11 of glucocerebrosidase (GBA) and exon 41 in the LRRK2 gene.
The investigators used participants’ early features and risk factors to calculate their risk scores and rank participants according to their risk. Dr. Schrag and colleagues also tested support for enrichment of the population at risk of Parkinson’s disease by examining associations between risk scores and the following intermediate markers of Parkinson’s disease: reduced sense of smell, presence of subjective RBD, and slowing of finger tapping speed.
Risk Scores Were Associated With Incident Disease
The researchers recruited 1,323 eligible volunteers. At baseline, their mean age was 66, and approximately 61% of the population was female. In all, 1,040 of the participants completed follow-up testing at year 1, 939 completed year 2 follow-up, and 846 completed year 3 follow-up. A total of 223 participants completed the baseline assessment only.
Baseline risk scores were associated with significantly higher rates of all intermediate markers of Parkinson’s disease during each year of follow-up. The group of patients at higher risk (ie, those above the 15th percentile) had significantly worse UPSIT score, RBDSQ score, and finger tapping in all years, compared with patients at lower risk (ie, those below the 85th percentile).
Risk scores in the entire sample were strongly associated with intermediate markers of Parkinson’s disease each year. Higher- and lower-risk groups differed significantly in median UPSIT score, RBDSQ score, and mean finger tapping speed in all years of follow-up.
At year 1, three patients had been newly diagnosed with Parkinson’s disease. An additional participant received the diagnosis in year 2, and three other participants received the diagnosis in year 3. All of the participants with newly diagnosed Parkinson’s disease at year 1 were in the higher risk group, and two had also been in the higher risk group at baseline. The participant diagnosed at year 2 was in the higher risk group at baseline, year 1, and year 2. All of the three participants diagnosed by year 3 were in the middle risk group at baseline. “There was substantial heterogeneity in the occurrence of intermediate markers in these individuals,” said the researchers.
The incidence of independently diagnosed Parkinson’s disease in participants who had been in the higher risk group during three years of follow-up was 1.6% per year and 0.2% across the whole population. Exploratory Cox regression analysis using incident Parkinson’s disease over three years as the outcome indicated an association with baseline risk estimate. In participants for whom GBA and LRRK2 status was known, the association between baseline risk and incident Parkinson’s disease was weaker. But the addition of GBA and LRRK2 variants in the algorithm improved the strength of association between baseline risk and incident Parkinson’s disease. Among the intermediate markers of disease, baseline finger tapping was associated with incident Parkinson’s disease at three years.
Results Could Reflect Selection Bias
“Although only a small number of individuals have been independently diagnosed with Parkinson’s disease during follow-up so far, the overall incidence of 0.2% is consistent with the expected incidence rate in the age group of 60 to 80 from the general population (one to three per 1,000 per year) and supports the representativeness of our sample,” said Dr. Schrag.
The recruitment of participants may have introduced selection bias into the study, she acknowledged. “However, we did not use family history as an entry criterion, and the proportion with a positive family history was lower than in other landmark studies.” The fact that 17% of participants only completed the baseline assessment also may have introduced selection bias, “although there were no differences between those with or without follow-up, meaning that bias as a result of loss to follow-up was less likely.
“This Internet-based approach may be useful for population screening because it can easily be scaled upward,” added Dr. Schrag. “It will allow larger numbers of Parkinson’s disease cases that represent the spectrum of the disease to be identified, rather than what would be possible from cohorts of carriers of specific risk factors.”
—Erik Greb
Suggested Readings
Noyce AJ, R’Bibo L, Peress L, et al. PREDICT-PD: An online approach to prospectively identify risk indicators of Parkinson’s disease. Mov Disord. 2017 Jan 16 [Epub ahead of print].
The online PREDICT-PD algorithm effectively identifies people at increased risk of Parkinson’s disease, according to research published online ahead of print January 16 in Movement Disorders. The risk scores that the algorithm assigns are significantly associated with intermediate markers of Parkinson’s disease. Higher PREDICT-PD risk score at baseline also is associated with an increased rate of incident Parkinson’s disease.
Previous research with the goal of identifying patients at risk of Parkinson’s disease mainly has focused on people with a family history of the disease, asymptomatic carriers of genes associated with the disease, or people with imaging abnormalities associated with the disease (eg, hyperechogenicity on transcranial sonography). The rarity, the representativeness, and the feasibility of identifying these factors have limited these studies, however.
Internet-Based Testing
To overcome these limitations, Anette-Eleonore Schrag, PhD, Professor of Clinical Neurosciences at University College London, and colleagues developed an online, evidence-based algorithm to identify risk indicators of Parkinson’s disease in the UK population. The investigators used a study website to recruit participants between ages 60 and 80 who did not have Parkinson’s disease, any other movement disorder, or stroke. Participants were prompted to return to the website yearly and complete tests.
Among the tests was a survey that included demographic questions and validated questionnaires about early nonmotor features of and risk factors for Parkinson’s disease (eg, the Hospital Anxiety Depression Scale and the REM sleep behavior disorder Screening Questionnaire [RBDSQ]). Participants also took the Bradykinesia Akinesia Incoordination (BRAIN) test, an online keyboard-tapping task. At baseline and at year three, participants took the University of Pennsylvania Smell Identification Test (UPSIT). The annual survey included a question about whether the participants had received any new diagnoses, including Parkinson’s disease or movement disorder. At year three, participants also gave saliva samples that were genotyped for mutations in exons 8 to 11 of glucocerebrosidase (GBA) and exon 41 in the LRRK2 gene.
The investigators used participants’ early features and risk factors to calculate their risk scores and rank participants according to their risk. Dr. Schrag and colleagues also tested support for enrichment of the population at risk of Parkinson’s disease by examining associations between risk scores and the following intermediate markers of Parkinson’s disease: reduced sense of smell, presence of subjective RBD, and slowing of finger tapping speed.
Risk Scores Were Associated With Incident Disease
The researchers recruited 1,323 eligible volunteers. At baseline, their mean age was 66, and approximately 61% of the population was female. In all, 1,040 of the participants completed follow-up testing at year 1, 939 completed year 2 follow-up, and 846 completed year 3 follow-up. A total of 223 participants completed the baseline assessment only.
Baseline risk scores were associated with significantly higher rates of all intermediate markers of Parkinson’s disease during each year of follow-up. The group of patients at higher risk (ie, those above the 15th percentile) had significantly worse UPSIT score, RBDSQ score, and finger tapping in all years, compared with patients at lower risk (ie, those below the 85th percentile).
Risk scores in the entire sample were strongly associated with intermediate markers of Parkinson’s disease each year. Higher- and lower-risk groups differed significantly in median UPSIT score, RBDSQ score, and mean finger tapping speed in all years of follow-up.
At year 1, three patients had been newly diagnosed with Parkinson’s disease. An additional participant received the diagnosis in year 2, and three other participants received the diagnosis in year 3. All of the participants with newly diagnosed Parkinson’s disease at year 1 were in the higher risk group, and two had also been in the higher risk group at baseline. The participant diagnosed at year 2 was in the higher risk group at baseline, year 1, and year 2. All of the three participants diagnosed by year 3 were in the middle risk group at baseline. “There was substantial heterogeneity in the occurrence of intermediate markers in these individuals,” said the researchers.
The incidence of independently diagnosed Parkinson’s disease in participants who had been in the higher risk group during three years of follow-up was 1.6% per year and 0.2% across the whole population. Exploratory Cox regression analysis using incident Parkinson’s disease over three years as the outcome indicated an association with baseline risk estimate. In participants for whom GBA and LRRK2 status was known, the association between baseline risk and incident Parkinson’s disease was weaker. But the addition of GBA and LRRK2 variants in the algorithm improved the strength of association between baseline risk and incident Parkinson’s disease. Among the intermediate markers of disease, baseline finger tapping was associated with incident Parkinson’s disease at three years.
Results Could Reflect Selection Bias
“Although only a small number of individuals have been independently diagnosed with Parkinson’s disease during follow-up so far, the overall incidence of 0.2% is consistent with the expected incidence rate in the age group of 60 to 80 from the general population (one to three per 1,000 per year) and supports the representativeness of our sample,” said Dr. Schrag.
The recruitment of participants may have introduced selection bias into the study, she acknowledged. “However, we did not use family history as an entry criterion, and the proportion with a positive family history was lower than in other landmark studies.” The fact that 17% of participants only completed the baseline assessment also may have introduced selection bias, “although there were no differences between those with or without follow-up, meaning that bias as a result of loss to follow-up was less likely.
“This Internet-based approach may be useful for population screening because it can easily be scaled upward,” added Dr. Schrag. “It will allow larger numbers of Parkinson’s disease cases that represent the spectrum of the disease to be identified, rather than what would be possible from cohorts of carriers of specific risk factors.”
—Erik Greb
Suggested Readings
Noyce AJ, R’Bibo L, Peress L, et al. PREDICT-PD: An online approach to prospectively identify risk indicators of Parkinson’s disease. Mov Disord. 2017 Jan 16 [Epub ahead of print].
Pulmonary embolism common in patients with AE-COPD
About 16% of patients with unexplained chronic obstructive pulmonary disease (COPD) acute exacerbations (AE-COPD) had an accompanying pulmonary embolism (PE), usually in regions that could be targeted with anticoagulants, according to a new systematic review and meta-analysis.
About 70% of the time an AE is a response to infection, but about 30% of the time, an AE has no clear cause, the authors said in a report on their research (CHEST. 2017 March;151[3]:544-54). There is a known biological link between inflammation and coagulation, which suggests that patients experiencing AE-COPD may be at increased risk of PE.
The researchers reviewed and analyzed seven studies, comprising 880 patients. Among the authors’ reasons for conducting this research was to update the pooled prevalence of PE in AE-COPD from a previous systematic review published in CHEST in 2009.
The meta-analysis revealed that 16.1% of patients with AE-COPD were also diagnosed with PE (95% confidence interval 8.3%-25.8%). There was a wide range of variation between individual studies (prevalence 3.3%-29.1%). In six studies that reported on deep vein thrombosis, the pooled prevalence of DVT was 10.5% (95% CI 4.3%-19.0%).
Five of the studies identified the PE location. An analysis of those studies showed that 35.0% were in the main pulmonary artery, and 31.7% were in the lobar and inter-lobar arteries. Such findings “[suggest] that the majority of these embolisms have important clinical consequences,” the authors wrote.
The researchers also looked at clinical markers that accompanied AE-COPD and found a potential signal with respect to pleuritic chest pain. One study found a strong association between pleuritic chest pain and AE-COPD patients with PE (81.0% versus 40.0% in those without PE). A second study showed a similar association (24.0% in PE versus 11.5% in non-PE patients), and a third study found no significant difference.
The presence of PE was also linked to hypotension, syncope, and acute right failure on ultrasonography, suggesting that PE may be associated with heart failure.
Patients with PE were less likely to have symptoms consistent with a respiratory tract infection. They also tended to have higher mortality rates and longer hospitalization rates compared with those without PE.
The meta-analysis had some limitations, including the heterogeneity of findings in the included studies, as well as the potential for publication bias, since reports showing unusually low or high rates may be more likely to be published, the researchers noted. There was also a high proportion of male subjects in the included studies.
Overall, the researchers concluded that PE is more likely in patients with pleuritic chest pain and signs of heart failure, and less likely in patients with signs of a respiratory infection. That information “might add to the clinical decision-making in patients with an AE-COPD, because it would be undesirable to perform [computed tomography pulmonary angiography] in every patient with an AE-COPD,” the researchers wrote.
“Early identification of these noninfectious events is important as standard antiexacerbation therapies including systemic corticosteroids and antibiotics are unlikely to be clinically useful for these etiologies and, importantly, may result in delays in the diagnosis and treatment of noninfectious causes of exacerbation such as acute coronary syndromes or congestive heart failure, leading to poor clinical outcomes.
“There is a clear and compelling need for more high quality evidence to determine the value of detecting PEs in patients with acute COPD exacerbations. There is an urgent need to understand the risks as well as the benefits of using CTPA [computed tomography pulmonary angiography] in the evaluation of acute COPD exacerbations. A Spanish group is currently conducting a randomized clinical trial to examine the clinical benefits and the safety of “routinely” deploying CTPA in the evaluation of hospitalized COPD patients with acute exacerbations (NCT02238639).
“What should clinicians do until high quality data from these and other studies are available? We suggest that in patients with typical infectious symptoms (e.g. increased cough, change in sputum volume or colour), CTPA is probably not required. CTPA may be considered for those who present with ‘atypical’ exacerbation symptoms (e.g. pleuritic chest pain, signs of cardiac failure, no clear identification of infectious origin) and in those with a prior history of thromboembolic disease. While we agree with Aleva and colleagues that the prevalence of PE is common (approximately 20%-25%) in unexplained COPD exacerbations, we remain unconvinced that all of these events require active treatment with anticoagulant therapy. Until compelling data from well-conducted randomized controlled trials are available, we suggest a conservative [first, no harm] approach to the management of acute exacerbations of COPD and [using] CTPA judiciously.”
Seung Won Ra, MD, PhD is with the Centre for Heart Lung Innovation, St. Paul’s Hospital and the department of medicine (respiratory division) at the University of British Columbia, Vancouver, as well as Ulsan (South Korea) University Hospital, University of Ulsan College of Medicine. Don D. Sin, MD, PhD is with the Centre for Heart Lung Innovation, St. Paul’s Hospital and the department of medicine (respiratory division) at the University of British Columbia, Vancouver. They had no relevant disclosures and made these remarks in an editorial (Chest. 2017;151[3]:523-4) that accompanied the published study.
“Early identification of these noninfectious events is important as standard antiexacerbation therapies including systemic corticosteroids and antibiotics are unlikely to be clinically useful for these etiologies and, importantly, may result in delays in the diagnosis and treatment of noninfectious causes of exacerbation such as acute coronary syndromes or congestive heart failure, leading to poor clinical outcomes.
“There is a clear and compelling need for more high quality evidence to determine the value of detecting PEs in patients with acute COPD exacerbations. There is an urgent need to understand the risks as well as the benefits of using CTPA [computed tomography pulmonary angiography] in the evaluation of acute COPD exacerbations. A Spanish group is currently conducting a randomized clinical trial to examine the clinical benefits and the safety of “routinely” deploying CTPA in the evaluation of hospitalized COPD patients with acute exacerbations (NCT02238639).
“What should clinicians do until high quality data from these and other studies are available? We suggest that in patients with typical infectious symptoms (e.g. increased cough, change in sputum volume or colour), CTPA is probably not required. CTPA may be considered for those who present with ‘atypical’ exacerbation symptoms (e.g. pleuritic chest pain, signs of cardiac failure, no clear identification of infectious origin) and in those with a prior history of thromboembolic disease. While we agree with Aleva and colleagues that the prevalence of PE is common (approximately 20%-25%) in unexplained COPD exacerbations, we remain unconvinced that all of these events require active treatment with anticoagulant therapy. Until compelling data from well-conducted randomized controlled trials are available, we suggest a conservative [first, no harm] approach to the management of acute exacerbations of COPD and [using] CTPA judiciously.”
Seung Won Ra, MD, PhD is with the Centre for Heart Lung Innovation, St. Paul’s Hospital and the department of medicine (respiratory division) at the University of British Columbia, Vancouver, as well as Ulsan (South Korea) University Hospital, University of Ulsan College of Medicine. Don D. Sin, MD, PhD is with the Centre for Heart Lung Innovation, St. Paul’s Hospital and the department of medicine (respiratory division) at the University of British Columbia, Vancouver. They had no relevant disclosures and made these remarks in an editorial (Chest. 2017;151[3]:523-4) that accompanied the published study.
“Early identification of these noninfectious events is important as standard antiexacerbation therapies including systemic corticosteroids and antibiotics are unlikely to be clinically useful for these etiologies and, importantly, may result in delays in the diagnosis and treatment of noninfectious causes of exacerbation such as acute coronary syndromes or congestive heart failure, leading to poor clinical outcomes.
“There is a clear and compelling need for more high quality evidence to determine the value of detecting PEs in patients with acute COPD exacerbations. There is an urgent need to understand the risks as well as the benefits of using CTPA [computed tomography pulmonary angiography] in the evaluation of acute COPD exacerbations. A Spanish group is currently conducting a randomized clinical trial to examine the clinical benefits and the safety of “routinely” deploying CTPA in the evaluation of hospitalized COPD patients with acute exacerbations (NCT02238639).
“What should clinicians do until high quality data from these and other studies are available? We suggest that in patients with typical infectious symptoms (e.g. increased cough, change in sputum volume or colour), CTPA is probably not required. CTPA may be considered for those who present with ‘atypical’ exacerbation symptoms (e.g. pleuritic chest pain, signs of cardiac failure, no clear identification of infectious origin) and in those with a prior history of thromboembolic disease. While we agree with Aleva and colleagues that the prevalence of PE is common (approximately 20%-25%) in unexplained COPD exacerbations, we remain unconvinced that all of these events require active treatment with anticoagulant therapy. Until compelling data from well-conducted randomized controlled trials are available, we suggest a conservative [first, no harm] approach to the management of acute exacerbations of COPD and [using] CTPA judiciously.”
Seung Won Ra, MD, PhD is with the Centre for Heart Lung Innovation, St. Paul’s Hospital and the department of medicine (respiratory division) at the University of British Columbia, Vancouver, as well as Ulsan (South Korea) University Hospital, University of Ulsan College of Medicine. Don D. Sin, MD, PhD is with the Centre for Heart Lung Innovation, St. Paul’s Hospital and the department of medicine (respiratory division) at the University of British Columbia, Vancouver. They had no relevant disclosures and made these remarks in an editorial (Chest. 2017;151[3]:523-4) that accompanied the published study.
About 16% of patients with unexplained chronic obstructive pulmonary disease (COPD) acute exacerbations (AE-COPD) had an accompanying pulmonary embolism (PE), usually in regions that could be targeted with anticoagulants, according to a new systematic review and meta-analysis.
About 70% of the time an AE is a response to infection, but about 30% of the time, an AE has no clear cause, the authors said in a report on their research (CHEST. 2017 March;151[3]:544-54). There is a known biological link between inflammation and coagulation, which suggests that patients experiencing AE-COPD may be at increased risk of PE.
The researchers reviewed and analyzed seven studies, comprising 880 patients. Among the authors’ reasons for conducting this research was to update the pooled prevalence of PE in AE-COPD from a previous systematic review published in CHEST in 2009.
The meta-analysis revealed that 16.1% of patients with AE-COPD were also diagnosed with PE (95% confidence interval 8.3%-25.8%). There was a wide range of variation between individual studies (prevalence 3.3%-29.1%). In six studies that reported on deep vein thrombosis, the pooled prevalence of DVT was 10.5% (95% CI 4.3%-19.0%).
Five of the studies identified the PE location. An analysis of those studies showed that 35.0% were in the main pulmonary artery, and 31.7% were in the lobar and inter-lobar arteries. Such findings “[suggest] that the majority of these embolisms have important clinical consequences,” the authors wrote.
The researchers also looked at clinical markers that accompanied AE-COPD and found a potential signal with respect to pleuritic chest pain. One study found a strong association between pleuritic chest pain and AE-COPD patients with PE (81.0% versus 40.0% in those without PE). A second study showed a similar association (24.0% in PE versus 11.5% in non-PE patients), and a third study found no significant difference.
The presence of PE was also linked to hypotension, syncope, and acute right failure on ultrasonography, suggesting that PE may be associated with heart failure.
Patients with PE were less likely to have symptoms consistent with a respiratory tract infection. They also tended to have higher mortality rates and longer hospitalization rates compared with those without PE.
The meta-analysis had some limitations, including the heterogeneity of findings in the included studies, as well as the potential for publication bias, since reports showing unusually low or high rates may be more likely to be published, the researchers noted. There was also a high proportion of male subjects in the included studies.
Overall, the researchers concluded that PE is more likely in patients with pleuritic chest pain and signs of heart failure, and less likely in patients with signs of a respiratory infection. That information “might add to the clinical decision-making in patients with an AE-COPD, because it would be undesirable to perform [computed tomography pulmonary angiography] in every patient with an AE-COPD,” the researchers wrote.
About 16% of patients with unexplained chronic obstructive pulmonary disease (COPD) acute exacerbations (AE-COPD) had an accompanying pulmonary embolism (PE), usually in regions that could be targeted with anticoagulants, according to a new systematic review and meta-analysis.
About 70% of the time an AE is a response to infection, but about 30% of the time, an AE has no clear cause, the authors said in a report on their research (CHEST. 2017 March;151[3]:544-54). There is a known biological link between inflammation and coagulation, which suggests that patients experiencing AE-COPD may be at increased risk of PE.
The researchers reviewed and analyzed seven studies, comprising 880 patients. Among the authors’ reasons for conducting this research was to update the pooled prevalence of PE in AE-COPD from a previous systematic review published in CHEST in 2009.
The meta-analysis revealed that 16.1% of patients with AE-COPD were also diagnosed with PE (95% confidence interval 8.3%-25.8%). There was a wide range of variation between individual studies (prevalence 3.3%-29.1%). In six studies that reported on deep vein thrombosis, the pooled prevalence of DVT was 10.5% (95% CI 4.3%-19.0%).
Five of the studies identified the PE location. An analysis of those studies showed that 35.0% were in the main pulmonary artery, and 31.7% were in the lobar and inter-lobar arteries. Such findings “[suggest] that the majority of these embolisms have important clinical consequences,” the authors wrote.
The researchers also looked at clinical markers that accompanied AE-COPD and found a potential signal with respect to pleuritic chest pain. One study found a strong association between pleuritic chest pain and AE-COPD patients with PE (81.0% versus 40.0% in those without PE). A second study showed a similar association (24.0% in PE versus 11.5% in non-PE patients), and a third study found no significant difference.
The presence of PE was also linked to hypotension, syncope, and acute right failure on ultrasonography, suggesting that PE may be associated with heart failure.
Patients with PE were less likely to have symptoms consistent with a respiratory tract infection. They also tended to have higher mortality rates and longer hospitalization rates compared with those without PE.
The meta-analysis had some limitations, including the heterogeneity of findings in the included studies, as well as the potential for publication bias, since reports showing unusually low or high rates may be more likely to be published, the researchers noted. There was also a high proportion of male subjects in the included studies.
Overall, the researchers concluded that PE is more likely in patients with pleuritic chest pain and signs of heart failure, and less likely in patients with signs of a respiratory infection. That information “might add to the clinical decision-making in patients with an AE-COPD, because it would be undesirable to perform [computed tomography pulmonary angiography] in every patient with an AE-COPD,” the researchers wrote.
FROM CHEST
Key clinical point: Pulmonary embolisms are often present in unexplained acute exacerbations of COPD.
Major finding: About 16% of unexplained exacerbations occurred in patients who had a pulmonary embolism.
Data source: Systematic review and meta-analysis of seven studies (880 patients).
Disclosures: The study received no funding. The authors reported having no financial disclosures.
When to Suspect a CSF Leak in Patients With Headache
OJAI, CA—Spontaneous CSF leaks are treatable, often misdiagnosed, and can cause a neurologic syndrome that may include headache, nausea, and tinnitus. Spinal fluid leaks also can lead to serious complications, including seizures. Patients may have a CSF leak for years or decades before it is diagnosed.
Although CSF leaks may not be readily apparent on imaging, a suspected CSF leak is important to consider because it is fixable, said Dr. Carroll, Assistant Professor of Anesthesiology and Perioperative and Pain Medicine at Stanford University Medical Center in California and a member of the Stanford CSF leak program.
Postdural Puncture Headache Versus Spontaneous CSF Leaks
A spontaneous CSF leak and the clinical syndrome that it causes may be confused with a postdural puncture headache.
With a postdural puncture headache, a patient usually has a single leak site in the dorsal dura. “There is up to a 90% response to a single epidural blood patch. Its natural history is generally well understood and benign. It is rarely mysterious, and it is ultimately fixable,” said Dr. Carroll. In contrast, “a spontaneous CSF leak is often mysterious in terms of the onset, cause, and diagnosis.”
The natural history of CSF leaks is poorly understood. The percentage of patients whose spontaneous leaks seal on their own or whose leaks cause a catastrophe (eg, coma, seizures, or hematomas) is not known. Between 30% and 40% of patients with spontaneous leaks have leaks from multiple sites at diagnosis. “With a spontaneous leak a dural rent is more likely in the ventral dura, anterior to the spinal cord or at the nerve root, making the dura less amenable to patching. A single patch at the correct spinal level will fix the problem only 30% of the time. With multiple patches, the success rate can approach 65% to 75%.” If the first epidural blood patch fails, it should be repeated. Directed epidural blood patches, placement of fibrin sealant, and surgical treatment are other treatment options.
Headaches
Most patients with post-puncture CSF leaks have classic orthostatic headaches. The orthostatic headaches from spontaneous leaks are often atypical, however, in that patients may not feel better immediately when they lie down and worse when they stand, Dr. Carroll said. Headaches may occur late in the day after prolonged upright time or with exertion. In addition, patients may “go from having an orthostatic headache to having a terrible headache all the time, regardless of what position they are in.”
Nausea and Vomiting
Nausea and vomiting can be the main symptom of a CSF leak. Dr. Carroll described a patient with complex regional pain syndrome who underwent a spinal cord stimulation trial. Afterward, she had a postdural puncture headache and received an epidural blood patch. “After that, she developed vomiting up to nine times a day.” A CSF leak was not visible on the first CT myelogram but was apparent on the second. The leak was “so small it was missed by the slice thickness” of the first CT myelogram, said Dr. Carroll. She ultimately required surgery to fix the leak. The patient improved, although she continued vomiting three times per day.
Tinnitus
CSF leaks may cause tinnitus. “You can get ringing in the ears when you have migraine,” Dr. Carroll said. But if patients have tinnitus when they are not having headaches, “you should be thinking that there is something else going on.” Data suggest that CSF fluid is connected to inner ear fluid so a change of pressure in CSF changes inner ear pressure, and patients with high or low CSF pressure may get tinnitus.
Other symptoms may include neck pain and fatigue. “I have had the parents of patients tell me that the most remarkable thing that they see when we patch their sons or daughters is how they are bouncing around the house,” he said. Many patients complain of difficulty with concentrating, task persistence, and other nondescript, nonfocal neurologic symptoms.
Imaging Limitations
Imaging of patients with CSF leaks often initially is read as normal, and MRI is not an adequate evaluation in the high clinical suspicion of a leak, Dr. Carroll said. “It is a good place to start, because if you see a leak on your MRI, maybe you do not have to get a CT myelogram,” he said. “But if you have a clinical suspicion of a leak … you should pursue that in the face of your radiologist telling you that there is nothing.”
Schievink et al in 2007 looked at several years of data from an emergency department to assess how often imaging findings consistent with CSF leaks were missed. They reviewed MRIs of patients with headache to look for evidence of intracranial hypotension, and then compared the number of CSF leaks with the number of subarachnoid hemorrhages seen during the same time. They found that for every subarachnoid hemorrhage, there were approximately 0.5 CSF leaks (23 subarachnoid hemorrhages and 11 CSF leaks). The results suggested that spontaneous intracranial hypotension is more common than previously thought and its diagnosis in emergency departments is problematic. The 11 people with MRI evidence of intracranial hypotension subsequently were diagnosed with CSF leaks and treated. None were diagnosed at the time of the MRI while in the emergency department.
Causes of Leaks
The four main causes of CSF leaks are medical procedures; whiplash; bony, sharp calcifications penetrating the dura; and genetic disorders of connective tissue.
Webb et al conducted a study to evaluate headaches in patients who had a known wet tap (ie, unintentional dural puncture) after a labor epidural. The researchers reviewed quality assurance data in an obstetrics anesthesia division and identified 40 patients who had known wet taps and 40 controls who had received an epidural without a wet tap during the same week and were matched for age and weight. Investigators contacted patients between 12 and 24 months later (mean, 18 months) and asked them about the incidence of chronic headache. The incidence of chronic headache in controls was 5% versus nearly 30% in patients who had had a wet tap. The investigators then compared patients who were managed conservatively (ie, they did not receive an epidural blood patch) versus patients who were managed with a blood patch. “If you got a blood patch, your risk of having a chronic headache 18 months later was only half as much as if you did not get a blood patch,” he said.
Connective Tissue Disorders and Calcifications
Because connective tissue disorders are associated with CSF leaks, headache physicians should determine patients’ Beighton Hypermobility Scores, Dr. Carroll said. The score is derived from a simple test that assesses joint hypermobility. For instance, patients receive a point if they can touch their thumb to their wrist or straighten their elbow more than 10° beyond 180°. A score between 3 and 5 raises suspicion that the patient might have a hereditary disorder of connective tissue. Cataracts at an early age, being unusually tall or short, degenerative disc disease, and history of aneurysm also are associated with an increased risk of CSF leaks.
With regard to calcifications, Dr. Carroll described a patient whose main complaint was confusion upon standing too long. The patient also had neck pain. They determined that he had a calcified bone spur that was puncturing the spinal cord, causing a leak.
Whiplash
Trauma and whiplash can cause leaks. Researchers in Japan studied 66 patients with chronic whiplash-associated disorders (ie, they had a whiplash accident and complained of neck and head pain, as well as difficulty with fatigue or memory). The patients were given a radionuclide cisternogram. Thirty-seven of the 66 patients had imaging that was positive for a CSF leak. “After being patched, roughly half the people who were found to have a leak went back to work, whereas they had not been working before,” he said. Another study found that 10% of people with brachial plexus injuries have spinal fluid leaks.
Overlap With POTS
The fact that Ehlers-Danlos also is associated with postural orthostatic tachycardia syndrome (POTS) raises the possibility that patients with CSF leaks may be misdiagnosed as having POTS.
“Why should a hereditary disorder of connective tissue be associated with the only two known conditions that are associated with feeling worse when you are up?” Dr. Carroll asked. Among patients with POTS, 60% have headaches, and many have dizziness and nausea. Dr. Carroll asked the POTS clinics at Stanford to refer patients with POTS, headache, and Ehlers-Danlos syndrome to him. The first referred patient’s history was consistent with a CSF leak. She had been passing out and had severe headaches for more than 10 years. Although her initial imaging was read as negative for CSF leaks, and an MRI showed no signs of intracranial hypotension, “when we patched her, in fact, she got better.” Subsequently, more patients diagnosed with POTS have been referred to the CSF leak program.
Patients initially may be diagnosed as having chronic fatigue syndrome, fibromyalgia, or irritable bowel syndrome when a CSF leak is causing their symptoms. It is a tragedy when patients “have a fixable leak and … nothing is done to treat that underlying problem,” Dr. Carroll said.
—Jake Remaly
Suggested Reading
Ishikawa S, Yokoyama M, Mizobuchi S, et al. Epidural blood patch therapy for chronic whiplash-associated disorder. Anesth Analg. 2007;105(3):809-814.
Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006;295(19):2286-2296.
Schievink WI, Maya MM, Moser F, et al. Frequency of spontaneous intracranial hypotension in the emergency department. J Headache Pain. 2007;8(6):325-328.
Webb CA, Weyker PD, Zhang L, et al. Unintentional dural puncture with a Tuohy needle increases risk of chronic headache. Anesth Analg. 2012;115(1):124-132.
OJAI, CA—Spontaneous CSF leaks are treatable, often misdiagnosed, and can cause a neurologic syndrome that may include headache, nausea, and tinnitus. Spinal fluid leaks also can lead to serious complications, including seizures. Patients may have a CSF leak for years or decades before it is diagnosed.
Although CSF leaks may not be readily apparent on imaging, a suspected CSF leak is important to consider because it is fixable, said Dr. Carroll, Assistant Professor of Anesthesiology and Perioperative and Pain Medicine at Stanford University Medical Center in California and a member of the Stanford CSF leak program.
Postdural Puncture Headache Versus Spontaneous CSF Leaks
A spontaneous CSF leak and the clinical syndrome that it causes may be confused with a postdural puncture headache.
With a postdural puncture headache, a patient usually has a single leak site in the dorsal dura. “There is up to a 90% response to a single epidural blood patch. Its natural history is generally well understood and benign. It is rarely mysterious, and it is ultimately fixable,” said Dr. Carroll. In contrast, “a spontaneous CSF leak is often mysterious in terms of the onset, cause, and diagnosis.”
The natural history of CSF leaks is poorly understood. The percentage of patients whose spontaneous leaks seal on their own or whose leaks cause a catastrophe (eg, coma, seizures, or hematomas) is not known. Between 30% and 40% of patients with spontaneous leaks have leaks from multiple sites at diagnosis. “With a spontaneous leak a dural rent is more likely in the ventral dura, anterior to the spinal cord or at the nerve root, making the dura less amenable to patching. A single patch at the correct spinal level will fix the problem only 30% of the time. With multiple patches, the success rate can approach 65% to 75%.” If the first epidural blood patch fails, it should be repeated. Directed epidural blood patches, placement of fibrin sealant, and surgical treatment are other treatment options.
Headaches
Most patients with post-puncture CSF leaks have classic orthostatic headaches. The orthostatic headaches from spontaneous leaks are often atypical, however, in that patients may not feel better immediately when they lie down and worse when they stand, Dr. Carroll said. Headaches may occur late in the day after prolonged upright time or with exertion. In addition, patients may “go from having an orthostatic headache to having a terrible headache all the time, regardless of what position they are in.”
Nausea and Vomiting
Nausea and vomiting can be the main symptom of a CSF leak. Dr. Carroll described a patient with complex regional pain syndrome who underwent a spinal cord stimulation trial. Afterward, she had a postdural puncture headache and received an epidural blood patch. “After that, she developed vomiting up to nine times a day.” A CSF leak was not visible on the first CT myelogram but was apparent on the second. The leak was “so small it was missed by the slice thickness” of the first CT myelogram, said Dr. Carroll. She ultimately required surgery to fix the leak. The patient improved, although she continued vomiting three times per day.
Tinnitus
CSF leaks may cause tinnitus. “You can get ringing in the ears when you have migraine,” Dr. Carroll said. But if patients have tinnitus when they are not having headaches, “you should be thinking that there is something else going on.” Data suggest that CSF fluid is connected to inner ear fluid so a change of pressure in CSF changes inner ear pressure, and patients with high or low CSF pressure may get tinnitus.
Other symptoms may include neck pain and fatigue. “I have had the parents of patients tell me that the most remarkable thing that they see when we patch their sons or daughters is how they are bouncing around the house,” he said. Many patients complain of difficulty with concentrating, task persistence, and other nondescript, nonfocal neurologic symptoms.
Imaging Limitations
Imaging of patients with CSF leaks often initially is read as normal, and MRI is not an adequate evaluation in the high clinical suspicion of a leak, Dr. Carroll said. “It is a good place to start, because if you see a leak on your MRI, maybe you do not have to get a CT myelogram,” he said. “But if you have a clinical suspicion of a leak … you should pursue that in the face of your radiologist telling you that there is nothing.”
Schievink et al in 2007 looked at several years of data from an emergency department to assess how often imaging findings consistent with CSF leaks were missed. They reviewed MRIs of patients with headache to look for evidence of intracranial hypotension, and then compared the number of CSF leaks with the number of subarachnoid hemorrhages seen during the same time. They found that for every subarachnoid hemorrhage, there were approximately 0.5 CSF leaks (23 subarachnoid hemorrhages and 11 CSF leaks). The results suggested that spontaneous intracranial hypotension is more common than previously thought and its diagnosis in emergency departments is problematic. The 11 people with MRI evidence of intracranial hypotension subsequently were diagnosed with CSF leaks and treated. None were diagnosed at the time of the MRI while in the emergency department.
Causes of Leaks
The four main causes of CSF leaks are medical procedures; whiplash; bony, sharp calcifications penetrating the dura; and genetic disorders of connective tissue.
Webb et al conducted a study to evaluate headaches in patients who had a known wet tap (ie, unintentional dural puncture) after a labor epidural. The researchers reviewed quality assurance data in an obstetrics anesthesia division and identified 40 patients who had known wet taps and 40 controls who had received an epidural without a wet tap during the same week and were matched for age and weight. Investigators contacted patients between 12 and 24 months later (mean, 18 months) and asked them about the incidence of chronic headache. The incidence of chronic headache in controls was 5% versus nearly 30% in patients who had had a wet tap. The investigators then compared patients who were managed conservatively (ie, they did not receive an epidural blood patch) versus patients who were managed with a blood patch. “If you got a blood patch, your risk of having a chronic headache 18 months later was only half as much as if you did not get a blood patch,” he said.
Connective Tissue Disorders and Calcifications
Because connective tissue disorders are associated with CSF leaks, headache physicians should determine patients’ Beighton Hypermobility Scores, Dr. Carroll said. The score is derived from a simple test that assesses joint hypermobility. For instance, patients receive a point if they can touch their thumb to their wrist or straighten their elbow more than 10° beyond 180°. A score between 3 and 5 raises suspicion that the patient might have a hereditary disorder of connective tissue. Cataracts at an early age, being unusually tall or short, degenerative disc disease, and history of aneurysm also are associated with an increased risk of CSF leaks.
With regard to calcifications, Dr. Carroll described a patient whose main complaint was confusion upon standing too long. The patient also had neck pain. They determined that he had a calcified bone spur that was puncturing the spinal cord, causing a leak.
Whiplash
Trauma and whiplash can cause leaks. Researchers in Japan studied 66 patients with chronic whiplash-associated disorders (ie, they had a whiplash accident and complained of neck and head pain, as well as difficulty with fatigue or memory). The patients were given a radionuclide cisternogram. Thirty-seven of the 66 patients had imaging that was positive for a CSF leak. “After being patched, roughly half the people who were found to have a leak went back to work, whereas they had not been working before,” he said. Another study found that 10% of people with brachial plexus injuries have spinal fluid leaks.
Overlap With POTS
The fact that Ehlers-Danlos also is associated with postural orthostatic tachycardia syndrome (POTS) raises the possibility that patients with CSF leaks may be misdiagnosed as having POTS.
“Why should a hereditary disorder of connective tissue be associated with the only two known conditions that are associated with feeling worse when you are up?” Dr. Carroll asked. Among patients with POTS, 60% have headaches, and many have dizziness and nausea. Dr. Carroll asked the POTS clinics at Stanford to refer patients with POTS, headache, and Ehlers-Danlos syndrome to him. The first referred patient’s history was consistent with a CSF leak. She had been passing out and had severe headaches for more than 10 years. Although her initial imaging was read as negative for CSF leaks, and an MRI showed no signs of intracranial hypotension, “when we patched her, in fact, she got better.” Subsequently, more patients diagnosed with POTS have been referred to the CSF leak program.
Patients initially may be diagnosed as having chronic fatigue syndrome, fibromyalgia, or irritable bowel syndrome when a CSF leak is causing their symptoms. It is a tragedy when patients “have a fixable leak and … nothing is done to treat that underlying problem,” Dr. Carroll said.
—Jake Remaly
Suggested Reading
Ishikawa S, Yokoyama M, Mizobuchi S, et al. Epidural blood patch therapy for chronic whiplash-associated disorder. Anesth Analg. 2007;105(3):809-814.
Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006;295(19):2286-2296.
Schievink WI, Maya MM, Moser F, et al. Frequency of spontaneous intracranial hypotension in the emergency department. J Headache Pain. 2007;8(6):325-328.
Webb CA, Weyker PD, Zhang L, et al. Unintentional dural puncture with a Tuohy needle increases risk of chronic headache. Anesth Analg. 2012;115(1):124-132.
OJAI, CA—Spontaneous CSF leaks are treatable, often misdiagnosed, and can cause a neurologic syndrome that may include headache, nausea, and tinnitus. Spinal fluid leaks also can lead to serious complications, including seizures. Patients may have a CSF leak for years or decades before it is diagnosed.
Although CSF leaks may not be readily apparent on imaging, a suspected CSF leak is important to consider because it is fixable, said Dr. Carroll, Assistant Professor of Anesthesiology and Perioperative and Pain Medicine at Stanford University Medical Center in California and a member of the Stanford CSF leak program.
Postdural Puncture Headache Versus Spontaneous CSF Leaks
A spontaneous CSF leak and the clinical syndrome that it causes may be confused with a postdural puncture headache.
With a postdural puncture headache, a patient usually has a single leak site in the dorsal dura. “There is up to a 90% response to a single epidural blood patch. Its natural history is generally well understood and benign. It is rarely mysterious, and it is ultimately fixable,” said Dr. Carroll. In contrast, “a spontaneous CSF leak is often mysterious in terms of the onset, cause, and diagnosis.”
The natural history of CSF leaks is poorly understood. The percentage of patients whose spontaneous leaks seal on their own or whose leaks cause a catastrophe (eg, coma, seizures, or hematomas) is not known. Between 30% and 40% of patients with spontaneous leaks have leaks from multiple sites at diagnosis. “With a spontaneous leak a dural rent is more likely in the ventral dura, anterior to the spinal cord or at the nerve root, making the dura less amenable to patching. A single patch at the correct spinal level will fix the problem only 30% of the time. With multiple patches, the success rate can approach 65% to 75%.” If the first epidural blood patch fails, it should be repeated. Directed epidural blood patches, placement of fibrin sealant, and surgical treatment are other treatment options.
Headaches
Most patients with post-puncture CSF leaks have classic orthostatic headaches. The orthostatic headaches from spontaneous leaks are often atypical, however, in that patients may not feel better immediately when they lie down and worse when they stand, Dr. Carroll said. Headaches may occur late in the day after prolonged upright time or with exertion. In addition, patients may “go from having an orthostatic headache to having a terrible headache all the time, regardless of what position they are in.”
Nausea and Vomiting
Nausea and vomiting can be the main symptom of a CSF leak. Dr. Carroll described a patient with complex regional pain syndrome who underwent a spinal cord stimulation trial. Afterward, she had a postdural puncture headache and received an epidural blood patch. “After that, she developed vomiting up to nine times a day.” A CSF leak was not visible on the first CT myelogram but was apparent on the second. The leak was “so small it was missed by the slice thickness” of the first CT myelogram, said Dr. Carroll. She ultimately required surgery to fix the leak. The patient improved, although she continued vomiting three times per day.
Tinnitus
CSF leaks may cause tinnitus. “You can get ringing in the ears when you have migraine,” Dr. Carroll said. But if patients have tinnitus when they are not having headaches, “you should be thinking that there is something else going on.” Data suggest that CSF fluid is connected to inner ear fluid so a change of pressure in CSF changes inner ear pressure, and patients with high or low CSF pressure may get tinnitus.
Other symptoms may include neck pain and fatigue. “I have had the parents of patients tell me that the most remarkable thing that they see when we patch their sons or daughters is how they are bouncing around the house,” he said. Many patients complain of difficulty with concentrating, task persistence, and other nondescript, nonfocal neurologic symptoms.
Imaging Limitations
Imaging of patients with CSF leaks often initially is read as normal, and MRI is not an adequate evaluation in the high clinical suspicion of a leak, Dr. Carroll said. “It is a good place to start, because if you see a leak on your MRI, maybe you do not have to get a CT myelogram,” he said. “But if you have a clinical suspicion of a leak … you should pursue that in the face of your radiologist telling you that there is nothing.”
Schievink et al in 2007 looked at several years of data from an emergency department to assess how often imaging findings consistent with CSF leaks were missed. They reviewed MRIs of patients with headache to look for evidence of intracranial hypotension, and then compared the number of CSF leaks with the number of subarachnoid hemorrhages seen during the same time. They found that for every subarachnoid hemorrhage, there were approximately 0.5 CSF leaks (23 subarachnoid hemorrhages and 11 CSF leaks). The results suggested that spontaneous intracranial hypotension is more common than previously thought and its diagnosis in emergency departments is problematic. The 11 people with MRI evidence of intracranial hypotension subsequently were diagnosed with CSF leaks and treated. None were diagnosed at the time of the MRI while in the emergency department.
Causes of Leaks
The four main causes of CSF leaks are medical procedures; whiplash; bony, sharp calcifications penetrating the dura; and genetic disorders of connective tissue.
Webb et al conducted a study to evaluate headaches in patients who had a known wet tap (ie, unintentional dural puncture) after a labor epidural. The researchers reviewed quality assurance data in an obstetrics anesthesia division and identified 40 patients who had known wet taps and 40 controls who had received an epidural without a wet tap during the same week and were matched for age and weight. Investigators contacted patients between 12 and 24 months later (mean, 18 months) and asked them about the incidence of chronic headache. The incidence of chronic headache in controls was 5% versus nearly 30% in patients who had had a wet tap. The investigators then compared patients who were managed conservatively (ie, they did not receive an epidural blood patch) versus patients who were managed with a blood patch. “If you got a blood patch, your risk of having a chronic headache 18 months later was only half as much as if you did not get a blood patch,” he said.
Connective Tissue Disorders and Calcifications
Because connective tissue disorders are associated with CSF leaks, headache physicians should determine patients’ Beighton Hypermobility Scores, Dr. Carroll said. The score is derived from a simple test that assesses joint hypermobility. For instance, patients receive a point if they can touch their thumb to their wrist or straighten their elbow more than 10° beyond 180°. A score between 3 and 5 raises suspicion that the patient might have a hereditary disorder of connective tissue. Cataracts at an early age, being unusually tall or short, degenerative disc disease, and history of aneurysm also are associated with an increased risk of CSF leaks.
With regard to calcifications, Dr. Carroll described a patient whose main complaint was confusion upon standing too long. The patient also had neck pain. They determined that he had a calcified bone spur that was puncturing the spinal cord, causing a leak.
Whiplash
Trauma and whiplash can cause leaks. Researchers in Japan studied 66 patients with chronic whiplash-associated disorders (ie, they had a whiplash accident and complained of neck and head pain, as well as difficulty with fatigue or memory). The patients were given a radionuclide cisternogram. Thirty-seven of the 66 patients had imaging that was positive for a CSF leak. “After being patched, roughly half the people who were found to have a leak went back to work, whereas they had not been working before,” he said. Another study found that 10% of people with brachial plexus injuries have spinal fluid leaks.
Overlap With POTS
The fact that Ehlers-Danlos also is associated with postural orthostatic tachycardia syndrome (POTS) raises the possibility that patients with CSF leaks may be misdiagnosed as having POTS.
“Why should a hereditary disorder of connective tissue be associated with the only two known conditions that are associated with feeling worse when you are up?” Dr. Carroll asked. Among patients with POTS, 60% have headaches, and many have dizziness and nausea. Dr. Carroll asked the POTS clinics at Stanford to refer patients with POTS, headache, and Ehlers-Danlos syndrome to him. The first referred patient’s history was consistent with a CSF leak. She had been passing out and had severe headaches for more than 10 years. Although her initial imaging was read as negative for CSF leaks, and an MRI showed no signs of intracranial hypotension, “when we patched her, in fact, she got better.” Subsequently, more patients diagnosed with POTS have been referred to the CSF leak program.
Patients initially may be diagnosed as having chronic fatigue syndrome, fibromyalgia, or irritable bowel syndrome when a CSF leak is causing their symptoms. It is a tragedy when patients “have a fixable leak and … nothing is done to treat that underlying problem,” Dr. Carroll said.
—Jake Remaly
Suggested Reading
Ishikawa S, Yokoyama M, Mizobuchi S, et al. Epidural blood patch therapy for chronic whiplash-associated disorder. Anesth Analg. 2007;105(3):809-814.
Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006;295(19):2286-2296.
Schievink WI, Maya MM, Moser F, et al. Frequency of spontaneous intracranial hypotension in the emergency department. J Headache Pain. 2007;8(6):325-328.
Webb CA, Weyker PD, Zhang L, et al. Unintentional dural puncture with a Tuohy needle increases risk of chronic headache. Anesth Analg. 2012;115(1):124-132.