User login
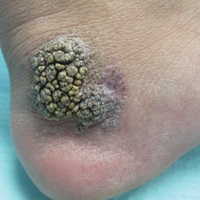
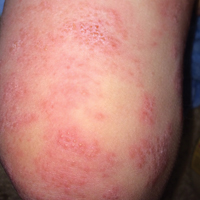
Friable Warty Plaque on the Heel
The Diagnosis: Verrucous Hemangioma
Verrucous hemangioma (VH) is a rare vascular anomaly that has not been definitively delineated as a malformation or a tumor, as it has features of both. Verrucous hemangioma presents at birth as a compressible soft mass with a red violaceous hue favoring the legs.1,2 Over time VH will develop a warty, friable, and keratotic surface that can begin to evolve as early as 6 months or as late as 34 years of age.3 Verrucous hemangioma does not involute and tends to grow proportionally with the patient. Thus, VH classically has been considered a vascular malformation.
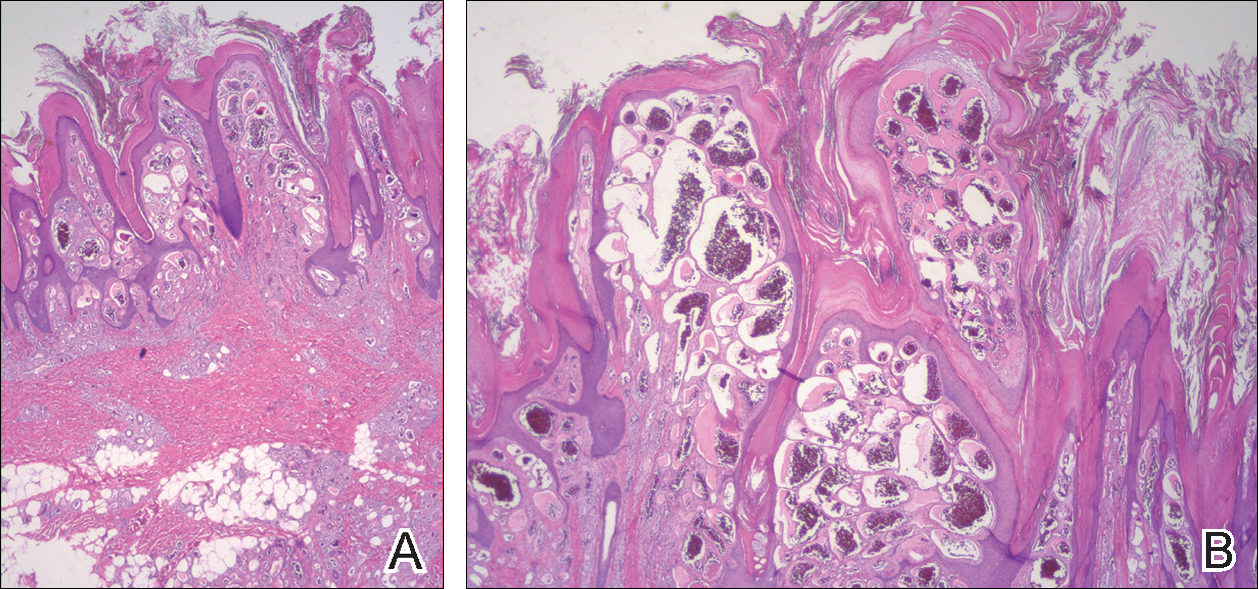
On histopathology VH shows collections of uniform, thin-walled vessels with a multilamellated basement membrane throughout the dermis, similar to an infantile hemangioma (IH). These lesions extend deep into the subcutaneous tissue and often involve the underlying fascia. The papillary dermis has large ectatic vessels, while the epidermis displays verrucous hyperkeratosis, papillomatosis, and irregular acanthosis without viral change (Figure).4,5 The superficial component can resemble an angiokeratoma; however, VH is differentiated by a deeper component that is often larger in size and has a more protracted clinical course.
Similar to IH, immunohistochemical studies have shown that VH expresses Wilms tumor 1 and glucose transporter 1 but is negative for D2-40.4 These findings suggest that VH is a vascular tumor rather than a vascular malformation, as was previously reported.6 Additional research has shown that the immunohistochemical staining profile of VH is nearly identical to IH, which has led to postulation that VH may be of placental mesodermal origin, as has been hypothesized for IH.5
Due to its deep infiltration and tendency for recurrence, VH is most effectively treated with wide local excision.3,6-8 Preoperative planning with magnetic resonance imaging may be indicated. Although laser monotherapy and other local destructive therapies have been largely unsuccessful, postsurgical laser therapy with CO2 lasers as well as dual pulsed dye laser and Nd:YAG laser have shown promise in preventing recurrence.3
- Tennant LB, Mulliken JB, Perez-Atayde AR, et al. Verrucous hemangioma revisited. Pediatr Dermatol. 2006;23:208-215.
- Koc M, Kavala M, Kocatür E, et al. An unusual vascular tumor: verrucous hemangioma. Dermatol Online J. 2009;15:7.
- Yang CH, Ohara K. Successful surgical treatment of verrucous hemangioma: a combined approach. Dermatol Surg. 2002;28:913-919; discussion 920.
- Trindade F, Torrelo A, Requena L, et al. An immunohistochemical study of verrucous hemangiomas. J Cutan Pathol. 2013;40:472-476.
- Laing EL, Brasch HD, Steel R, et al. Verrucous hemangioma expresses primitive markers. J Cutan Pathol. 2013;40:391-396.
- Mankani MH, Dufresne CR. Verrucous malformations: their presentation and management. Ann Plast Surg. 2000;45:31-36.
- Clairwood MQ, Bruckner AL, Dadras SS. Verrucous hemangioma: a report of two cases and review of the literature. J Cutan Pathol. 2011;38:740-746.
- Segura Palacios JM, Boixeda P, Rocha J, et al. Laser treatment for verrucous hemangioma. Laser Med Sci. 2012;27:681-684.
The Diagnosis: Verrucous Hemangioma
Verrucous hemangioma (VH) is a rare vascular anomaly that has not been definitively delineated as a malformation or a tumor, as it has features of both. Verrucous hemangioma presents at birth as a compressible soft mass with a red violaceous hue favoring the legs.1,2 Over time VH will develop a warty, friable, and keratotic surface that can begin to evolve as early as 6 months or as late as 34 years of age.3 Verrucous hemangioma does not involute and tends to grow proportionally with the patient. Thus, VH classically has been considered a vascular malformation.
On histopathology VH shows collections of uniform, thin-walled vessels with a multilamellated basement membrane throughout the dermis, similar to an infantile hemangioma (IH). These lesions extend deep into the subcutaneous tissue and often involve the underlying fascia. The papillary dermis has large ectatic vessels, while the epidermis displays verrucous hyperkeratosis, papillomatosis, and irregular acanthosis without viral change (Figure).4,5 The superficial component can resemble an angiokeratoma; however, VH is differentiated by a deeper component that is often larger in size and has a more protracted clinical course.

Similar to IH, immunohistochemical studies have shown that VH expresses Wilms tumor 1 and glucose transporter 1 but is negative for D2-40.4 These findings suggest that VH is a vascular tumor rather than a vascular malformation, as was previously reported.6 Additional research has shown that the immunohistochemical staining profile of VH is nearly identical to IH, which has led to postulation that VH may be of placental mesodermal origin, as has been hypothesized for IH.5
Due to its deep infiltration and tendency for recurrence, VH is most effectively treated with wide local excision.3,6-8 Preoperative planning with magnetic resonance imaging may be indicated. Although laser monotherapy and other local destructive therapies have been largely unsuccessful, postsurgical laser therapy with CO2 lasers as well as dual pulsed dye laser and Nd:YAG laser have shown promise in preventing recurrence.3
The Diagnosis: Verrucous Hemangioma
Verrucous hemangioma (VH) is a rare vascular anomaly that has not been definitively delineated as a malformation or a tumor, as it has features of both. Verrucous hemangioma presents at birth as a compressible soft mass with a red violaceous hue favoring the legs.1,2 Over time VH will develop a warty, friable, and keratotic surface that can begin to evolve as early as 6 months or as late as 34 years of age.3 Verrucous hemangioma does not involute and tends to grow proportionally with the patient. Thus, VH classically has been considered a vascular malformation.
On histopathology VH shows collections of uniform, thin-walled vessels with a multilamellated basement membrane throughout the dermis, similar to an infantile hemangioma (IH). These lesions extend deep into the subcutaneous tissue and often involve the underlying fascia. The papillary dermis has large ectatic vessels, while the epidermis displays verrucous hyperkeratosis, papillomatosis, and irregular acanthosis without viral change (Figure).4,5 The superficial component can resemble an angiokeratoma; however, VH is differentiated by a deeper component that is often larger in size and has a more protracted clinical course.

Similar to IH, immunohistochemical studies have shown that VH expresses Wilms tumor 1 and glucose transporter 1 but is negative for D2-40.4 These findings suggest that VH is a vascular tumor rather than a vascular malformation, as was previously reported.6 Additional research has shown that the immunohistochemical staining profile of VH is nearly identical to IH, which has led to postulation that VH may be of placental mesodermal origin, as has been hypothesized for IH.5
Due to its deep infiltration and tendency for recurrence, VH is most effectively treated with wide local excision.3,6-8 Preoperative planning with magnetic resonance imaging may be indicated. Although laser monotherapy and other local destructive therapies have been largely unsuccessful, postsurgical laser therapy with CO2 lasers as well as dual pulsed dye laser and Nd:YAG laser have shown promise in preventing recurrence.3
- Tennant LB, Mulliken JB, Perez-Atayde AR, et al. Verrucous hemangioma revisited. Pediatr Dermatol. 2006;23:208-215.
- Koc M, Kavala M, Kocatür E, et al. An unusual vascular tumor: verrucous hemangioma. Dermatol Online J. 2009;15:7.
- Yang CH, Ohara K. Successful surgical treatment of verrucous hemangioma: a combined approach. Dermatol Surg. 2002;28:913-919; discussion 920.
- Trindade F, Torrelo A, Requena L, et al. An immunohistochemical study of verrucous hemangiomas. J Cutan Pathol. 2013;40:472-476.
- Laing EL, Brasch HD, Steel R, et al. Verrucous hemangioma expresses primitive markers. J Cutan Pathol. 2013;40:391-396.
- Mankani MH, Dufresne CR. Verrucous malformations: their presentation and management. Ann Plast Surg. 2000;45:31-36.
- Clairwood MQ, Bruckner AL, Dadras SS. Verrucous hemangioma: a report of two cases and review of the literature. J Cutan Pathol. 2011;38:740-746.
- Segura Palacios JM, Boixeda P, Rocha J, et al. Laser treatment for verrucous hemangioma. Laser Med Sci. 2012;27:681-684.
- Tennant LB, Mulliken JB, Perez-Atayde AR, et al. Verrucous hemangioma revisited. Pediatr Dermatol. 2006;23:208-215.
- Koc M, Kavala M, Kocatür E, et al. An unusual vascular tumor: verrucous hemangioma. Dermatol Online J. 2009;15:7.
- Yang CH, Ohara K. Successful surgical treatment of verrucous hemangioma: a combined approach. Dermatol Surg. 2002;28:913-919; discussion 920.
- Trindade F, Torrelo A, Requena L, et al. An immunohistochemical study of verrucous hemangiomas. J Cutan Pathol. 2013;40:472-476.
- Laing EL, Brasch HD, Steel R, et al. Verrucous hemangioma expresses primitive markers. J Cutan Pathol. 2013;40:391-396.
- Mankani MH, Dufresne CR. Verrucous malformations: their presentation and management. Ann Plast Surg. 2000;45:31-36.
- Clairwood MQ, Bruckner AL, Dadras SS. Verrucous hemangioma: a report of two cases and review of the literature. J Cutan Pathol. 2011;38:740-746.
- Segura Palacios JM, Boixeda P, Rocha J, et al. Laser treatment for verrucous hemangioma. Laser Med Sci. 2012;27:681-684.
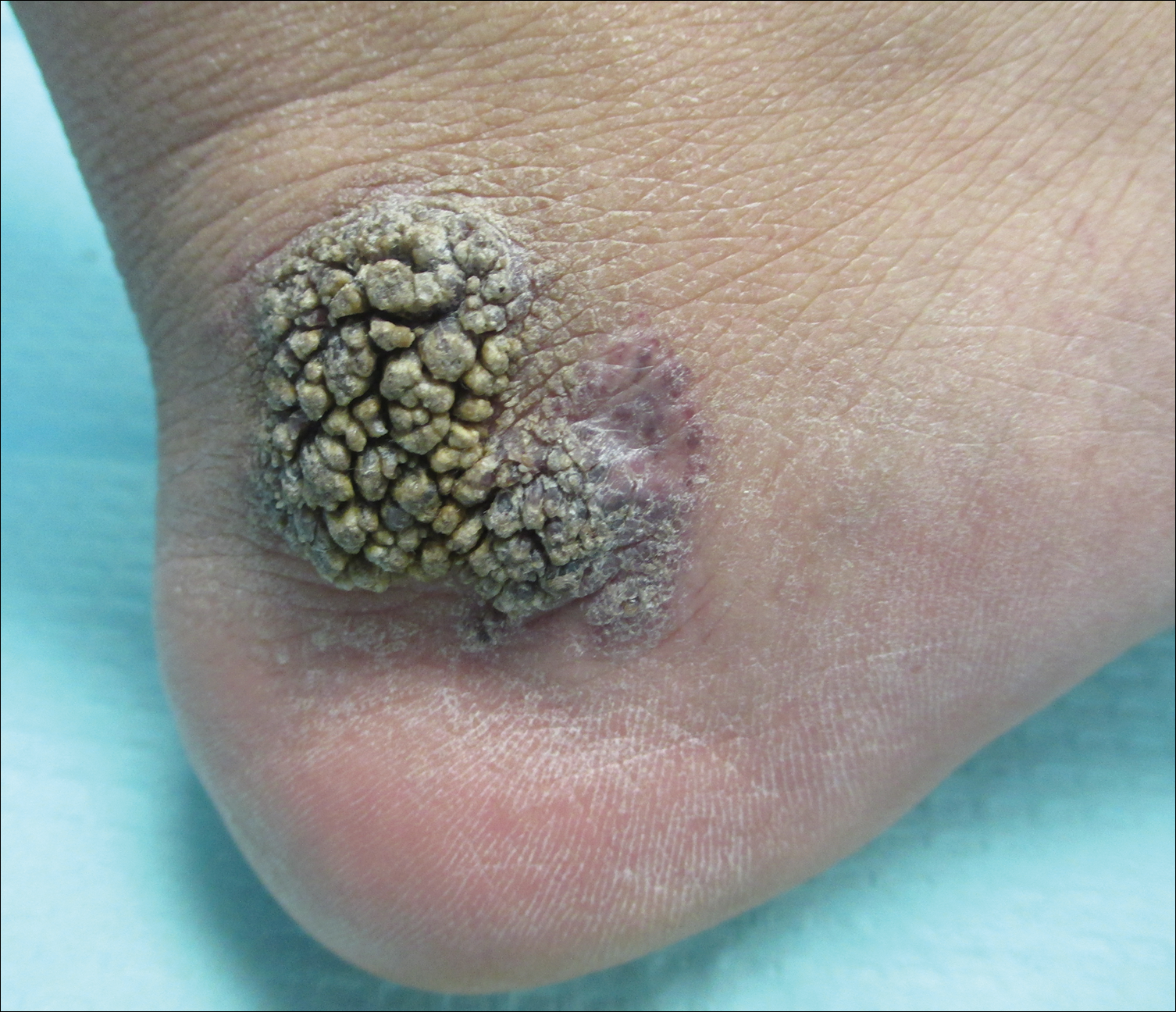
A 31-year-old man presented with a large friable and warty plaque on the left heel. He recalled that the lesion had been present since birth as a flat red birthmark that grew proportionally with him. Throughout his adolescence its surface became increasingly rough and bumpy. The patient described receiving laser treatment twice in his early 20s without notable improvement. He wanted the lesion removed because it was easily traumatized, resulting in bleeding, pain, and infection. The patient reported being otherwise healthy.
Willingness to Take Weight Loss Medication Among Obese Primary Care Patients
From Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA.
Abstracts
- Objective: To identify patient factors associated with willingness to take daily weight loss medication and weight loss expectations using these medications.
- Methods: A random sample of 331 primary care patients aged 18–65 years with a BMI ≥ 35 kg/m2 were recruited from 4 diverse primary care practices in Boston, MA. We conducted telephone interviews and chart reviews to assess patients’ willingness to take a weight loss medication and their expectations for weight loss. We used sequential logistic regression models to identify demographic, clinical, and quality of life (QOL) factors associated with this willingness.
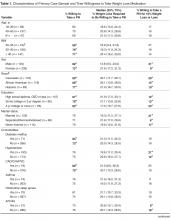
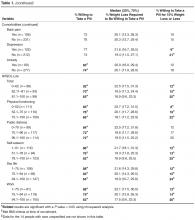
- Results: Of 331 subjects, 69% were women, 35% were white, 35% were black, and 25% were Hispanic; 249 (75%) of patients were willing to take a daily weight loss medication if recommended by their doctor but required a median weight loss of 15% to 24%; only 17% of patients were willing to take a medication for ≤ 10% weight loss. Men were significantly more willing than women (1.2 [95% CI 1.0–1.4]). Diabetes was the only comorbidity associated with willingness to consider pharmacotherapy (1.2 [1.0–1.3]) but only modestly improved model performance (C-statistic increased from 0.59 to 0.60). In contrast, lower QOL, especially low self-esteem and sex life, were stronger correlates (C-statistic 0.72).
- Conclusion: A majority of obese primary care patients were willing to take a daily weight loss pill; however, most required more than 10% weight loss to consider pharmacotherapy worthwhile. Poor QOL, especially low self-esteem and poor sex life, were stronger correlates than having diabetes.
Key words: obesity; primary care; weight loss medication.
In the United States, obesity continues to be unrelentingly prevalent, affecting more than one-third of adults (34.9%) [1]. This statistic has ominous implications when considering that obesity is a risk factor for numerous chronic diseases, such as coronary heart disease, diabetes, sleep apnea, osteoarthritis, and some types of cancers [2]. Moreover, it is associated with increased risk of all-cause and cardiovascular disease mortality. Promisingly, an initial 5% to 10% weight loss over 6 months has been associated with improvement in LDL, HDL, triglycerides, glucose, hemoglobin A1C, diabetes risk, blood pressure, and medication use [2]. Therefore, although patients may not be able to achieve their ideal body weight or normal BMI, modest weight loss can still have beneficial health effects.
Weight loss medications are effective adjunctive therapies in helping patients lose up to 10% of their body weight on average when combined with diet and exercise [3–5]. There are currently 5 medications approved by the Food and Drug Administration for long-term use for weight loss: orlistat, lorcaserin, phentermine-topiramate, bupropion-naltrexone, and liraglutide. Despite their proven efficacy, there are barriers to initiating a long-term weight loss medication. Insurance reimbursement is limited for these medications, thus resulting in high out-of-pocket cost for patients that they may be unable or unwilling to pay [6]. There may also be safety concerns given that several weight loss medications, including fenfluramine, sibutramine, and rimonabant, have been withdrawn from the market because of adverse effects [7]. Thus, in deciding whether to initiate a pharmacologic weight loss regimen, patients must believe that the weight loss benefits will exceed the potential risks.
Little is known, however, about patients’ willingness to take weight loss medications or the minimum weight loss they expect to lose to make pharmacotherapy worthwhile. Only a few studies have investigated patient willingness to adopt pharmacotherapy as part of a weight loss regimen, and only one investigated obese patients in the United States [8]. In this context, we surveyed a sociodemographically diverse group of primary care patients with moderate to severe obesity to examine patient characteristics associated with willingness to pursue weight loss pharmacotherapy. We also aimed to evaluate how much weight patients expected to lose in order to make taking a daily medication worth the effort. Characterizing patients seen in primary care who are willing to adopt pharmacotherapy to lose weight may guide weight loss counseling in the primary care setting. Furthermore, determining whether patients have realistic weight loss expectations can help clinicians better counsel their patients on weight loss goals.
Methods
Study Sample
We recruited 337 subjects from 4 diverse primary care practices in Boston, Massachusetts: a large hospital-based academic practice, a community practice in a working-class suburb, a community practice in an affluent suburb, and a health center serving a predominantly socially disadvantaged population. The primary goal of the parent study was to understand the preferences of patients for weight loss treatment, especially bariatric surgery. Therefore, to be included, patients needed to have a BMI ≥ 35 kg/m2 at the time of recruitment, been seen in clinic within the past year, be aged 18–65 years, and be English or Spanish speaking. By design, African-American and Hispanic patients were oversampled from an electronic list of potentially eligible patient groups so that we could examine for racial differences in treatment preferences. Study details have been previously described [9].
Data Collection and Measures
Trained interviewers conducted a 45- to 60-minute telephone interview with each participant in either English or Spanish. To assess willingness to use a daily weight loss medication, subjects were asked, “If your doctor recommended it, would you be willing to take a pill or medication every day in order to lose weight?” Those who answered affirmatively were then asked the minimum amount of weight they would have to lose to make taking a pill everyday worthwhile.
Subjects were also asked about demographic information (age, race, education, marital status) and comorbid health conditions commonly associated with obesity (diabetes mellitus, hypertension, asthma, obstructive sleep apnea, GERD, depression, anxiety, back pain, and cardiovascular problems). We assessed quality of life (QOL) using the Impact of Weight on Quality of Life-Lite (IWQOL-Lite), a 31-item instrument designed specifically to assess the impact of obesity on QOL capturing 5 domains (physical function, self-esteem, sexual life, public distress, and work). Subjects were asked to rate a series of statements beginning with “Because of my weight…” as “always true,” “usually true,” “sometimes true,” “rarely true,” or “never true.” Global and domain scores ranged from 0 to 100; higher scores reflected better QOL [10].
Data Analysis
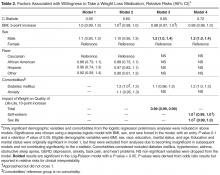
We used descriptive statistics to characterize the proportion of subjects willing to use a daily weight loss medication and the weight loss required for patients to be willing to consider pharmacotherapy. We used a stepwise logistic model to examine demographic, QOL, and clinical factors associated with the willingness to take a weight loss medication as the outcome, with an entry criteria of P value of 0.1 and an exit criteria of 0.05. Log-Poisson distribution using the sandwich estimator was used to obtain relative risks for each significant variable. Adjusted models included age, BMI, sex, and race and any significant comorbidities. We added overall QOL score and individual QOL scores in subsequent models to examine the relative influence of overall vs. domain-specific QOL. Statistical analyses were conducted with SAS (SAS Institute, Cary, NC). We considered the change in model C-statistic when specific variables were added to the model to determine the importance of these factors in contributing to patients’ willingness to consider pharmacotherapy; larger changes in model C-statistic signifies a greater contribution.
Results
Table 2 displays sequentially adjusted models examining various demographic, clinical, and QOL factors associated with willingness to take a weight loss medication.
Discussion
In our study, we found that a large proportion (75%) of primary care patients with at least moderate obesity were willing to take a daily weight loss medication if their doctor recommended it. After full adjustment, men, those with lower quality of life (QOL), and patients with diabetes were more likely to pursue weight loss pharmacotherapy than their counterparts. Moreover, QOL appeared more important than comorbid diagnoses in contributing to whether patients would consider taking a weight loss medication. Most patients expected to lose more than 10% of their weight to make taking a daily medication worthwhile.
Few studies have examined patients’ willingness to take a medication to lose weight. Tan et al [11] found that only about half of their surveyed outpatients were likely to take a medication to lose weight; however, approximately a quarter of the patients in that study were of normal BMI. In contrast, our study interviewed patients with at least a BMI of 35 kg/m2 and the majority of these patients reported a willingness to take a weight loss medication. Nevertheless, patients appear to have unrealistic expectations of the weight loss potential of pharmacotherapy. Only a minority of patients in our study would be willing to take a weight loss medication if the weight loss was no more than 10%, a level that is more consistent with the outcomes achievable in most clinical trials of weight loss medications [12]. Prior studies have also shown that patients often have unrealistic weight loss expectations and are unable to achieve their ideal body weight using diet, exercise, or pharmacotherapy [13,14]. Doyle et al found that percentage of weight loss was the most important treatment attribute when considering weight loss pharmacotherapy when compared to cost, health improvements, side effects, diet and exercise requirements, and method of medication administration [8]. Thus it is important to educate patients on realistic goal setting and the benefits of modest weight loss when considering pharmacotherapy. The weight loss preferences expressed in our study may also influence the weight loss outcomes targets pursued in pharmaceutical development. Interestingly, after full adjustment, BMI did not correlate with willingness to take a weight loss medication. Given that all patients in our study had a BMI of ≥ 35 kg/m2, this may imply that variations beyond this BMI threshold did not significantly affect a patient’s willingness to use pharmacotherapy. In contrast, weight-related QOL was an important correlate.
Men were slightly more likely than women to be willing to take a weight loss medication, which is interesting since men have been shown to be less likely to participate in behavioral weight loss programs and diets [15]. One reason may be that many weight loss programs are delivered in group settings which may deter men from participating. Whether this hypothetical willingness to undergo pharmacotherapy would translate to actual use is unclear, especially since there are barriers to pharmacotherapy including out-of-pocket costs. In a prior study in the United Kingdom, women were more likely to have reported prior weight loss medication use than men [16].
Our study did not find differences in willingness to pursue weight loss medication by race or educational attainment. This is consistent with our prior work demonstrating that racial and ethnic minorities were no less likely to consider bariatric surgery if the treatment were recommended by their doctor [9]. However, our other work did suggest that clinicians may be less likely to recommend bariatric surgery to their medically eligible minority patients as compared to their Caucasian patients. Whether this may be the case for pharmacotherapy is unclear since this was not explicitly queried in our current study [9].
Our study also found that patients with diabetes but not other comorbidities were more likely to consider weight loss medication after adjusting for QOL. This may reflect a stronger link between diabetes and obesity perceived by patients. Our result is consistent with our earlier data showing that diabetes but not other comorbid conditions was associated with a higher likelihood of considering weight loss surgery [9]. Nevertheless, having diabetes contributed only modestly to the variation in patient preferences regarding pharmacotherapy as reflected by the trivial change in model C-statistic when diabetes status was added to the model.
In contrast, lower QOL scores, especially in the domains of self-esteem and sex life, were associated with increased willingness to take a weight loss medication and appeared to be a stronger predictor than individual comorbidities. This is consistent with other studies showing that patients seeking treatment for obesity tend to have lower health-related QOL [9,17]. Our findings are also consistent with our previous research demonstrating that impairments in specific QOL domains are often more important to patients and stronger drivers of diminished well-being than measures of overall QOL [18]. Hence, given their importance to patients, clinicians need to consider QOL benefits when counseling patients about the risks and benefits of various obesity treatments.
This study is the first to our knowledge to systematically characterize demographic factors associated with the likelihood of primary care patients with obesity considering weight loss pharmacotherapy. This information may aid outpatient weight loss counseling by increasing awareness of gender and patient specific preferences. The fact that many patients with obesity appear to be interested in pursuing weight loss medication may also support public health initiatives in providing equitable access to weight loss pharmacotherapy. As our study characterizes patients who are willing to pursue weight loss medications, future studies may include retrospective analyses on actual use of weight loss medications among various demographic groups. Further investigation on specific reasons why patients choose whether or not to use weight loss medication may also be helpful.
This study has important limitations. The sample size was modest and potentially underpowered to detect small differences across different subgroups. Our sample was also limited to practices in Boston, which limits generalizability; although, by design, we oversampled racial and ethnic subjects to ensure diverse representation. Finally, our study examined patients’ hypothetical willingness to take weight loss medications rather than their actual adherence to treatment if offered.
Conclusion
In this sample of obese primary care patients, we found that the majority of patients were willing to take a daily medication to lose weight; however, patients had expectations for weight loss that far exceeded the level achievable by patients in pharmaceutical trials of these agents. Men and patients with diabetes were more likely to be willing to pursue weight loss medication; however, lower weight-related QOL, especially low self-esteem and impaired sexual function, appeared to be a stronger correlate of willingness to consider pharmacotherapy than comorbid diagnoses.
Corresponding author: Christina C. Wee, MD, MPH, Beth Israel Deaconess Medical Center, 330 Brookline Ave., Boston, MA 02215, [email protected].
Funding/support: This study was funded by the National Institute of Diabetes, Digestive and Kidney Diseases (R01 DK073302, PI Wee). Dr. Wee is also supported by an NIH midcareer mentorship award (K24DK087932). The sponsor had no role in the design or conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Financial disclosures: None reported.
1. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011–2012. NCHS Data Brief 2013;(131):1–8.
2. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014;63(25 Pt B):2985–3023.
3. Hauptman J, Lucas C, Boldrin MN, et al. Orlistat in the long-term treatment of obesity in primary care settings. Arch Fam Med 2000;9:160–7.
4. Kakkar AK, Dahiya N. Drug treatment of obesity: current status and future prospects. Eur J Intern Med 2015;26:89–94.
5. Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20:330–42.
6. Fabricatore AN, Wadden TA. Obesity. Annu Rev Clin Psychol 2006;2:357–77.
7. Cheung BM, Cheung TT, Samaranayake NR. Safety of antiobesity drugs. Ther Adv Drug Saf 2013;4:171–81.
8. Doyle S, Lloyd A, Birt J, et al. Willingness to pay for obesity pharmacotherapy. Obesity (Silver Spring) 2012;20:2019–26.
9. Wee CC HK, Bolcic-Jankovic D, Colten ME, et al. Sex, race, and consideration of bariatric surgery among primary care patients with moderate to severe obesity. J Gen Intern Med 2014;29:68–75.
10. Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life in obesity. Obes Res 2001;9:102–11.
11. Tan DZN, Dennis SM, Vagholkar S. Weight management in general practice: what do patients want? Med J Aust 2006;185:73–5.
12. Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA 2014;311:74–86.
13. Foster GD, Wadden TA, Vogt RA, Brewer G. What is a reasonable weight loss? Patients’ expectations and evaluations of obesity treatment outcomes. J Consult Clin Psychol 1997;65:79–85.
14. Fabricatore AN, Wadden TA, Womble LG, et al. The role of patients’ expectations and goals in the behavioral and pharmacological treatment of obesity. Int J Obes (Lond) 2007;31:1739–45.
15. Robertson C, Archibald D, Avenell A, et al. Systematic reviews of and integrated report on the quantitative, qualitative and economic evidence base for the management of obesity in men. Health Technol Assess 2014;18:1–424.
16. Thompson RL, Thomas DE. A cross-sectional survey of the opinions on weight loss treatments of adult obese patients attending a dietetic clinic. Int J Obes Relat Metab Disord 2000;24:164–70.
17. Kolotkin RL, Crosby RD, Williams GR. Health-related quality of life varies among obese subgroups. Obes Res 2002;10:748–56.
18. Wee C, Davis R, Chiodi S, et al. Sex, race, and the adverse effects of social stigma vs. other quality of life factors among primary care patients with moderate to severe obesity. J Gen Intern Med 2015;30:229–35.
From Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA.
Abstracts
- Objective: To identify patient factors associated with willingness to take daily weight loss medication and weight loss expectations using these medications.
- Methods: A random sample of 331 primary care patients aged 18–65 years with a BMI ≥ 35 kg/m2 were recruited from 4 diverse primary care practices in Boston, MA. We conducted telephone interviews and chart reviews to assess patients’ willingness to take a weight loss medication and their expectations for weight loss. We used sequential logistic regression models to identify demographic, clinical, and quality of life (QOL) factors associated with this willingness.
- Results: Of 331 subjects, 69% were women, 35% were white, 35% were black, and 25% were Hispanic; 249 (75%) of patients were willing to take a daily weight loss medication if recommended by their doctor but required a median weight loss of 15% to 24%; only 17% of patients were willing to take a medication for ≤ 10% weight loss. Men were significantly more willing than women (1.2 [95% CI 1.0–1.4]). Diabetes was the only comorbidity associated with willingness to consider pharmacotherapy (1.2 [1.0–1.3]) but only modestly improved model performance (C-statistic increased from 0.59 to 0.60). In contrast, lower QOL, especially low self-esteem and sex life, were stronger correlates (C-statistic 0.72).
- Conclusion: A majority of obese primary care patients were willing to take a daily weight loss pill; however, most required more than 10% weight loss to consider pharmacotherapy worthwhile. Poor QOL, especially low self-esteem and poor sex life, were stronger correlates than having diabetes.
Key words: obesity; primary care; weight loss medication.
In the United States, obesity continues to be unrelentingly prevalent, affecting more than one-third of adults (34.9%) [1]. This statistic has ominous implications when considering that obesity is a risk factor for numerous chronic diseases, such as coronary heart disease, diabetes, sleep apnea, osteoarthritis, and some types of cancers [2]. Moreover, it is associated with increased risk of all-cause and cardiovascular disease mortality. Promisingly, an initial 5% to 10% weight loss over 6 months has been associated with improvement in LDL, HDL, triglycerides, glucose, hemoglobin A1C, diabetes risk, blood pressure, and medication use [2]. Therefore, although patients may not be able to achieve their ideal body weight or normal BMI, modest weight loss can still have beneficial health effects.
Weight loss medications are effective adjunctive therapies in helping patients lose up to 10% of their body weight on average when combined with diet and exercise [3–5]. There are currently 5 medications approved by the Food and Drug Administration for long-term use for weight loss: orlistat, lorcaserin, phentermine-topiramate, bupropion-naltrexone, and liraglutide. Despite their proven efficacy, there are barriers to initiating a long-term weight loss medication. Insurance reimbursement is limited for these medications, thus resulting in high out-of-pocket cost for patients that they may be unable or unwilling to pay [6]. There may also be safety concerns given that several weight loss medications, including fenfluramine, sibutramine, and rimonabant, have been withdrawn from the market because of adverse effects [7]. Thus, in deciding whether to initiate a pharmacologic weight loss regimen, patients must believe that the weight loss benefits will exceed the potential risks.
Little is known, however, about patients’ willingness to take weight loss medications or the minimum weight loss they expect to lose to make pharmacotherapy worthwhile. Only a few studies have investigated patient willingness to adopt pharmacotherapy as part of a weight loss regimen, and only one investigated obese patients in the United States [8]. In this context, we surveyed a sociodemographically diverse group of primary care patients with moderate to severe obesity to examine patient characteristics associated with willingness to pursue weight loss pharmacotherapy. We also aimed to evaluate how much weight patients expected to lose in order to make taking a daily medication worth the effort. Characterizing patients seen in primary care who are willing to adopt pharmacotherapy to lose weight may guide weight loss counseling in the primary care setting. Furthermore, determining whether patients have realistic weight loss expectations can help clinicians better counsel their patients on weight loss goals.
Methods
Study Sample
We recruited 337 subjects from 4 diverse primary care practices in Boston, Massachusetts: a large hospital-based academic practice, a community practice in a working-class suburb, a community practice in an affluent suburb, and a health center serving a predominantly socially disadvantaged population. The primary goal of the parent study was to understand the preferences of patients for weight loss treatment, especially bariatric surgery. Therefore, to be included, patients needed to have a BMI ≥ 35 kg/m2 at the time of recruitment, been seen in clinic within the past year, be aged 18–65 years, and be English or Spanish speaking. By design, African-American and Hispanic patients were oversampled from an electronic list of potentially eligible patient groups so that we could examine for racial differences in treatment preferences. Study details have been previously described [9].
Data Collection and Measures
Trained interviewers conducted a 45- to 60-minute telephone interview with each participant in either English or Spanish. To assess willingness to use a daily weight loss medication, subjects were asked, “If your doctor recommended it, would you be willing to take a pill or medication every day in order to lose weight?” Those who answered affirmatively were then asked the minimum amount of weight they would have to lose to make taking a pill everyday worthwhile.
Subjects were also asked about demographic information (age, race, education, marital status) and comorbid health conditions commonly associated with obesity (diabetes mellitus, hypertension, asthma, obstructive sleep apnea, GERD, depression, anxiety, back pain, and cardiovascular problems). We assessed quality of life (QOL) using the Impact of Weight on Quality of Life-Lite (IWQOL-Lite), a 31-item instrument designed specifically to assess the impact of obesity on QOL capturing 5 domains (physical function, self-esteem, sexual life, public distress, and work). Subjects were asked to rate a series of statements beginning with “Because of my weight…” as “always true,” “usually true,” “sometimes true,” “rarely true,” or “never true.” Global and domain scores ranged from 0 to 100; higher scores reflected better QOL [10].
Data Analysis
We used descriptive statistics to characterize the proportion of subjects willing to use a daily weight loss medication and the weight loss required for patients to be willing to consider pharmacotherapy. We used a stepwise logistic model to examine demographic, QOL, and clinical factors associated with the willingness to take a weight loss medication as the outcome, with an entry criteria of P value of 0.1 and an exit criteria of 0.05. Log-Poisson distribution using the sandwich estimator was used to obtain relative risks for each significant variable. Adjusted models included age, BMI, sex, and race and any significant comorbidities. We added overall QOL score and individual QOL scores in subsequent models to examine the relative influence of overall vs. domain-specific QOL. Statistical analyses were conducted with SAS (SAS Institute, Cary, NC). We considered the change in model C-statistic when specific variables were added to the model to determine the importance of these factors in contributing to patients’ willingness to consider pharmacotherapy; larger changes in model C-statistic signifies a greater contribution.
Results
Table 2 displays sequentially adjusted models examining various demographic, clinical, and QOL factors associated with willingness to take a weight loss medication.
Discussion
In our study, we found that a large proportion (75%) of primary care patients with at least moderate obesity were willing to take a daily weight loss medication if their doctor recommended it. After full adjustment, men, those with lower quality of life (QOL), and patients with diabetes were more likely to pursue weight loss pharmacotherapy than their counterparts. Moreover, QOL appeared more important than comorbid diagnoses in contributing to whether patients would consider taking a weight loss medication. Most patients expected to lose more than 10% of their weight to make taking a daily medication worthwhile.
Few studies have examined patients’ willingness to take a medication to lose weight. Tan et al [11] found that only about half of their surveyed outpatients were likely to take a medication to lose weight; however, approximately a quarter of the patients in that study were of normal BMI. In contrast, our study interviewed patients with at least a BMI of 35 kg/m2 and the majority of these patients reported a willingness to take a weight loss medication. Nevertheless, patients appear to have unrealistic expectations of the weight loss potential of pharmacotherapy. Only a minority of patients in our study would be willing to take a weight loss medication if the weight loss was no more than 10%, a level that is more consistent with the outcomes achievable in most clinical trials of weight loss medications [12]. Prior studies have also shown that patients often have unrealistic weight loss expectations and are unable to achieve their ideal body weight using diet, exercise, or pharmacotherapy [13,14]. Doyle et al found that percentage of weight loss was the most important treatment attribute when considering weight loss pharmacotherapy when compared to cost, health improvements, side effects, diet and exercise requirements, and method of medication administration [8]. Thus it is important to educate patients on realistic goal setting and the benefits of modest weight loss when considering pharmacotherapy. The weight loss preferences expressed in our study may also influence the weight loss outcomes targets pursued in pharmaceutical development. Interestingly, after full adjustment, BMI did not correlate with willingness to take a weight loss medication. Given that all patients in our study had a BMI of ≥ 35 kg/m2, this may imply that variations beyond this BMI threshold did not significantly affect a patient’s willingness to use pharmacotherapy. In contrast, weight-related QOL was an important correlate.
Men were slightly more likely than women to be willing to take a weight loss medication, which is interesting since men have been shown to be less likely to participate in behavioral weight loss programs and diets [15]. One reason may be that many weight loss programs are delivered in group settings which may deter men from participating. Whether this hypothetical willingness to undergo pharmacotherapy would translate to actual use is unclear, especially since there are barriers to pharmacotherapy including out-of-pocket costs. In a prior study in the United Kingdom, women were more likely to have reported prior weight loss medication use than men [16].
Our study did not find differences in willingness to pursue weight loss medication by race or educational attainment. This is consistent with our prior work demonstrating that racial and ethnic minorities were no less likely to consider bariatric surgery if the treatment were recommended by their doctor [9]. However, our other work did suggest that clinicians may be less likely to recommend bariatric surgery to their medically eligible minority patients as compared to their Caucasian patients. Whether this may be the case for pharmacotherapy is unclear since this was not explicitly queried in our current study [9].
Our study also found that patients with diabetes but not other comorbidities were more likely to consider weight loss medication after adjusting for QOL. This may reflect a stronger link between diabetes and obesity perceived by patients. Our result is consistent with our earlier data showing that diabetes but not other comorbid conditions was associated with a higher likelihood of considering weight loss surgery [9]. Nevertheless, having diabetes contributed only modestly to the variation in patient preferences regarding pharmacotherapy as reflected by the trivial change in model C-statistic when diabetes status was added to the model.
In contrast, lower QOL scores, especially in the domains of self-esteem and sex life, were associated with increased willingness to take a weight loss medication and appeared to be a stronger predictor than individual comorbidities. This is consistent with other studies showing that patients seeking treatment for obesity tend to have lower health-related QOL [9,17]. Our findings are also consistent with our previous research demonstrating that impairments in specific QOL domains are often more important to patients and stronger drivers of diminished well-being than measures of overall QOL [18]. Hence, given their importance to patients, clinicians need to consider QOL benefits when counseling patients about the risks and benefits of various obesity treatments.
This study is the first to our knowledge to systematically characterize demographic factors associated with the likelihood of primary care patients with obesity considering weight loss pharmacotherapy. This information may aid outpatient weight loss counseling by increasing awareness of gender and patient specific preferences. The fact that many patients with obesity appear to be interested in pursuing weight loss medication may also support public health initiatives in providing equitable access to weight loss pharmacotherapy. As our study characterizes patients who are willing to pursue weight loss medications, future studies may include retrospective analyses on actual use of weight loss medications among various demographic groups. Further investigation on specific reasons why patients choose whether or not to use weight loss medication may also be helpful.
This study has important limitations. The sample size was modest and potentially underpowered to detect small differences across different subgroups. Our sample was also limited to practices in Boston, which limits generalizability; although, by design, we oversampled racial and ethnic subjects to ensure diverse representation. Finally, our study examined patients’ hypothetical willingness to take weight loss medications rather than their actual adherence to treatment if offered.
Conclusion
In this sample of obese primary care patients, we found that the majority of patients were willing to take a daily medication to lose weight; however, patients had expectations for weight loss that far exceeded the level achievable by patients in pharmaceutical trials of these agents. Men and patients with diabetes were more likely to be willing to pursue weight loss medication; however, lower weight-related QOL, especially low self-esteem and impaired sexual function, appeared to be a stronger correlate of willingness to consider pharmacotherapy than comorbid diagnoses.
Corresponding author: Christina C. Wee, MD, MPH, Beth Israel Deaconess Medical Center, 330 Brookline Ave., Boston, MA 02215, [email protected].
Funding/support: This study was funded by the National Institute of Diabetes, Digestive and Kidney Diseases (R01 DK073302, PI Wee). Dr. Wee is also supported by an NIH midcareer mentorship award (K24DK087932). The sponsor had no role in the design or conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Financial disclosures: None reported.
From Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA.
Abstracts
- Objective: To identify patient factors associated with willingness to take daily weight loss medication and weight loss expectations using these medications.
- Methods: A random sample of 331 primary care patients aged 18–65 years with a BMI ≥ 35 kg/m2 were recruited from 4 diverse primary care practices in Boston, MA. We conducted telephone interviews and chart reviews to assess patients’ willingness to take a weight loss medication and their expectations for weight loss. We used sequential logistic regression models to identify demographic, clinical, and quality of life (QOL) factors associated with this willingness.
- Results: Of 331 subjects, 69% were women, 35% were white, 35% were black, and 25% were Hispanic; 249 (75%) of patients were willing to take a daily weight loss medication if recommended by their doctor but required a median weight loss of 15% to 24%; only 17% of patients were willing to take a medication for ≤ 10% weight loss. Men were significantly more willing than women (1.2 [95% CI 1.0–1.4]). Diabetes was the only comorbidity associated with willingness to consider pharmacotherapy (1.2 [1.0–1.3]) but only modestly improved model performance (C-statistic increased from 0.59 to 0.60). In contrast, lower QOL, especially low self-esteem and sex life, were stronger correlates (C-statistic 0.72).
- Conclusion: A majority of obese primary care patients were willing to take a daily weight loss pill; however, most required more than 10% weight loss to consider pharmacotherapy worthwhile. Poor QOL, especially low self-esteem and poor sex life, were stronger correlates than having diabetes.
Key words: obesity; primary care; weight loss medication.
In the United States, obesity continues to be unrelentingly prevalent, affecting more than one-third of adults (34.9%) [1]. This statistic has ominous implications when considering that obesity is a risk factor for numerous chronic diseases, such as coronary heart disease, diabetes, sleep apnea, osteoarthritis, and some types of cancers [2]. Moreover, it is associated with increased risk of all-cause and cardiovascular disease mortality. Promisingly, an initial 5% to 10% weight loss over 6 months has been associated with improvement in LDL, HDL, triglycerides, glucose, hemoglobin A1C, diabetes risk, blood pressure, and medication use [2]. Therefore, although patients may not be able to achieve their ideal body weight or normal BMI, modest weight loss can still have beneficial health effects.
Weight loss medications are effective adjunctive therapies in helping patients lose up to 10% of their body weight on average when combined with diet and exercise [3–5]. There are currently 5 medications approved by the Food and Drug Administration for long-term use for weight loss: orlistat, lorcaserin, phentermine-topiramate, bupropion-naltrexone, and liraglutide. Despite their proven efficacy, there are barriers to initiating a long-term weight loss medication. Insurance reimbursement is limited for these medications, thus resulting in high out-of-pocket cost for patients that they may be unable or unwilling to pay [6]. There may also be safety concerns given that several weight loss medications, including fenfluramine, sibutramine, and rimonabant, have been withdrawn from the market because of adverse effects [7]. Thus, in deciding whether to initiate a pharmacologic weight loss regimen, patients must believe that the weight loss benefits will exceed the potential risks.
Little is known, however, about patients’ willingness to take weight loss medications or the minimum weight loss they expect to lose to make pharmacotherapy worthwhile. Only a few studies have investigated patient willingness to adopt pharmacotherapy as part of a weight loss regimen, and only one investigated obese patients in the United States [8]. In this context, we surveyed a sociodemographically diverse group of primary care patients with moderate to severe obesity to examine patient characteristics associated with willingness to pursue weight loss pharmacotherapy. We also aimed to evaluate how much weight patients expected to lose in order to make taking a daily medication worth the effort. Characterizing patients seen in primary care who are willing to adopt pharmacotherapy to lose weight may guide weight loss counseling in the primary care setting. Furthermore, determining whether patients have realistic weight loss expectations can help clinicians better counsel their patients on weight loss goals.
Methods
Study Sample
We recruited 337 subjects from 4 diverse primary care practices in Boston, Massachusetts: a large hospital-based academic practice, a community practice in a working-class suburb, a community practice in an affluent suburb, and a health center serving a predominantly socially disadvantaged population. The primary goal of the parent study was to understand the preferences of patients for weight loss treatment, especially bariatric surgery. Therefore, to be included, patients needed to have a BMI ≥ 35 kg/m2 at the time of recruitment, been seen in clinic within the past year, be aged 18–65 years, and be English or Spanish speaking. By design, African-American and Hispanic patients were oversampled from an electronic list of potentially eligible patient groups so that we could examine for racial differences in treatment preferences. Study details have been previously described [9].
Data Collection and Measures
Trained interviewers conducted a 45- to 60-minute telephone interview with each participant in either English or Spanish. To assess willingness to use a daily weight loss medication, subjects were asked, “If your doctor recommended it, would you be willing to take a pill or medication every day in order to lose weight?” Those who answered affirmatively were then asked the minimum amount of weight they would have to lose to make taking a pill everyday worthwhile.
Subjects were also asked about demographic information (age, race, education, marital status) and comorbid health conditions commonly associated with obesity (diabetes mellitus, hypertension, asthma, obstructive sleep apnea, GERD, depression, anxiety, back pain, and cardiovascular problems). We assessed quality of life (QOL) using the Impact of Weight on Quality of Life-Lite (IWQOL-Lite), a 31-item instrument designed specifically to assess the impact of obesity on QOL capturing 5 domains (physical function, self-esteem, sexual life, public distress, and work). Subjects were asked to rate a series of statements beginning with “Because of my weight…” as “always true,” “usually true,” “sometimes true,” “rarely true,” or “never true.” Global and domain scores ranged from 0 to 100; higher scores reflected better QOL [10].
Data Analysis
We used descriptive statistics to characterize the proportion of subjects willing to use a daily weight loss medication and the weight loss required for patients to be willing to consider pharmacotherapy. We used a stepwise logistic model to examine demographic, QOL, and clinical factors associated with the willingness to take a weight loss medication as the outcome, with an entry criteria of P value of 0.1 and an exit criteria of 0.05. Log-Poisson distribution using the sandwich estimator was used to obtain relative risks for each significant variable. Adjusted models included age, BMI, sex, and race and any significant comorbidities. We added overall QOL score and individual QOL scores in subsequent models to examine the relative influence of overall vs. domain-specific QOL. Statistical analyses were conducted with SAS (SAS Institute, Cary, NC). We considered the change in model C-statistic when specific variables were added to the model to determine the importance of these factors in contributing to patients’ willingness to consider pharmacotherapy; larger changes in model C-statistic signifies a greater contribution.
Results
Table 2 displays sequentially adjusted models examining various demographic, clinical, and QOL factors associated with willingness to take a weight loss medication.
Discussion
In our study, we found that a large proportion (75%) of primary care patients with at least moderate obesity were willing to take a daily weight loss medication if their doctor recommended it. After full adjustment, men, those with lower quality of life (QOL), and patients with diabetes were more likely to pursue weight loss pharmacotherapy than their counterparts. Moreover, QOL appeared more important than comorbid diagnoses in contributing to whether patients would consider taking a weight loss medication. Most patients expected to lose more than 10% of their weight to make taking a daily medication worthwhile.
Few studies have examined patients’ willingness to take a medication to lose weight. Tan et al [11] found that only about half of their surveyed outpatients were likely to take a medication to lose weight; however, approximately a quarter of the patients in that study were of normal BMI. In contrast, our study interviewed patients with at least a BMI of 35 kg/m2 and the majority of these patients reported a willingness to take a weight loss medication. Nevertheless, patients appear to have unrealistic expectations of the weight loss potential of pharmacotherapy. Only a minority of patients in our study would be willing to take a weight loss medication if the weight loss was no more than 10%, a level that is more consistent with the outcomes achievable in most clinical trials of weight loss medications [12]. Prior studies have also shown that patients often have unrealistic weight loss expectations and are unable to achieve their ideal body weight using diet, exercise, or pharmacotherapy [13,14]. Doyle et al found that percentage of weight loss was the most important treatment attribute when considering weight loss pharmacotherapy when compared to cost, health improvements, side effects, diet and exercise requirements, and method of medication administration [8]. Thus it is important to educate patients on realistic goal setting and the benefits of modest weight loss when considering pharmacotherapy. The weight loss preferences expressed in our study may also influence the weight loss outcomes targets pursued in pharmaceutical development. Interestingly, after full adjustment, BMI did not correlate with willingness to take a weight loss medication. Given that all patients in our study had a BMI of ≥ 35 kg/m2, this may imply that variations beyond this BMI threshold did not significantly affect a patient’s willingness to use pharmacotherapy. In contrast, weight-related QOL was an important correlate.
Men were slightly more likely than women to be willing to take a weight loss medication, which is interesting since men have been shown to be less likely to participate in behavioral weight loss programs and diets [15]. One reason may be that many weight loss programs are delivered in group settings which may deter men from participating. Whether this hypothetical willingness to undergo pharmacotherapy would translate to actual use is unclear, especially since there are barriers to pharmacotherapy including out-of-pocket costs. In a prior study in the United Kingdom, women were more likely to have reported prior weight loss medication use than men [16].
Our study did not find differences in willingness to pursue weight loss medication by race or educational attainment. This is consistent with our prior work demonstrating that racial and ethnic minorities were no less likely to consider bariatric surgery if the treatment were recommended by their doctor [9]. However, our other work did suggest that clinicians may be less likely to recommend bariatric surgery to their medically eligible minority patients as compared to their Caucasian patients. Whether this may be the case for pharmacotherapy is unclear since this was not explicitly queried in our current study [9].
Our study also found that patients with diabetes but not other comorbidities were more likely to consider weight loss medication after adjusting for QOL. This may reflect a stronger link between diabetes and obesity perceived by patients. Our result is consistent with our earlier data showing that diabetes but not other comorbid conditions was associated with a higher likelihood of considering weight loss surgery [9]. Nevertheless, having diabetes contributed only modestly to the variation in patient preferences regarding pharmacotherapy as reflected by the trivial change in model C-statistic when diabetes status was added to the model.
In contrast, lower QOL scores, especially in the domains of self-esteem and sex life, were associated with increased willingness to take a weight loss medication and appeared to be a stronger predictor than individual comorbidities. This is consistent with other studies showing that patients seeking treatment for obesity tend to have lower health-related QOL [9,17]. Our findings are also consistent with our previous research demonstrating that impairments in specific QOL domains are often more important to patients and stronger drivers of diminished well-being than measures of overall QOL [18]. Hence, given their importance to patients, clinicians need to consider QOL benefits when counseling patients about the risks and benefits of various obesity treatments.
This study is the first to our knowledge to systematically characterize demographic factors associated with the likelihood of primary care patients with obesity considering weight loss pharmacotherapy. This information may aid outpatient weight loss counseling by increasing awareness of gender and patient specific preferences. The fact that many patients with obesity appear to be interested in pursuing weight loss medication may also support public health initiatives in providing equitable access to weight loss pharmacotherapy. As our study characterizes patients who are willing to pursue weight loss medications, future studies may include retrospective analyses on actual use of weight loss medications among various demographic groups. Further investigation on specific reasons why patients choose whether or not to use weight loss medication may also be helpful.
This study has important limitations. The sample size was modest and potentially underpowered to detect small differences across different subgroups. Our sample was also limited to practices in Boston, which limits generalizability; although, by design, we oversampled racial and ethnic subjects to ensure diverse representation. Finally, our study examined patients’ hypothetical willingness to take weight loss medications rather than their actual adherence to treatment if offered.
Conclusion
In this sample of obese primary care patients, we found that the majority of patients were willing to take a daily medication to lose weight; however, patients had expectations for weight loss that far exceeded the level achievable by patients in pharmaceutical trials of these agents. Men and patients with diabetes were more likely to be willing to pursue weight loss medication; however, lower weight-related QOL, especially low self-esteem and impaired sexual function, appeared to be a stronger correlate of willingness to consider pharmacotherapy than comorbid diagnoses.
Corresponding author: Christina C. Wee, MD, MPH, Beth Israel Deaconess Medical Center, 330 Brookline Ave., Boston, MA 02215, [email protected].
Funding/support: This study was funded by the National Institute of Diabetes, Digestive and Kidney Diseases (R01 DK073302, PI Wee). Dr. Wee is also supported by an NIH midcareer mentorship award (K24DK087932). The sponsor had no role in the design or conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Financial disclosures: None reported.
1. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011–2012. NCHS Data Brief 2013;(131):1–8.
2. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014;63(25 Pt B):2985–3023.
3. Hauptman J, Lucas C, Boldrin MN, et al. Orlistat in the long-term treatment of obesity in primary care settings. Arch Fam Med 2000;9:160–7.
4. Kakkar AK, Dahiya N. Drug treatment of obesity: current status and future prospects. Eur J Intern Med 2015;26:89–94.
5. Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20:330–42.
6. Fabricatore AN, Wadden TA. Obesity. Annu Rev Clin Psychol 2006;2:357–77.
7. Cheung BM, Cheung TT, Samaranayake NR. Safety of antiobesity drugs. Ther Adv Drug Saf 2013;4:171–81.
8. Doyle S, Lloyd A, Birt J, et al. Willingness to pay for obesity pharmacotherapy. Obesity (Silver Spring) 2012;20:2019–26.
9. Wee CC HK, Bolcic-Jankovic D, Colten ME, et al. Sex, race, and consideration of bariatric surgery among primary care patients with moderate to severe obesity. J Gen Intern Med 2014;29:68–75.
10. Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life in obesity. Obes Res 2001;9:102–11.
11. Tan DZN, Dennis SM, Vagholkar S. Weight management in general practice: what do patients want? Med J Aust 2006;185:73–5.
12. Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA 2014;311:74–86.
13. Foster GD, Wadden TA, Vogt RA, Brewer G. What is a reasonable weight loss? Patients’ expectations and evaluations of obesity treatment outcomes. J Consult Clin Psychol 1997;65:79–85.
14. Fabricatore AN, Wadden TA, Womble LG, et al. The role of patients’ expectations and goals in the behavioral and pharmacological treatment of obesity. Int J Obes (Lond) 2007;31:1739–45.
15. Robertson C, Archibald D, Avenell A, et al. Systematic reviews of and integrated report on the quantitative, qualitative and economic evidence base for the management of obesity in men. Health Technol Assess 2014;18:1–424.
16. Thompson RL, Thomas DE. A cross-sectional survey of the opinions on weight loss treatments of adult obese patients attending a dietetic clinic. Int J Obes Relat Metab Disord 2000;24:164–70.
17. Kolotkin RL, Crosby RD, Williams GR. Health-related quality of life varies among obese subgroups. Obes Res 2002;10:748–56.
18. Wee C, Davis R, Chiodi S, et al. Sex, race, and the adverse effects of social stigma vs. other quality of life factors among primary care patients with moderate to severe obesity. J Gen Intern Med 2015;30:229–35.
1. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011–2012. NCHS Data Brief 2013;(131):1–8.
2. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014;63(25 Pt B):2985–3023.
3. Hauptman J, Lucas C, Boldrin MN, et al. Orlistat in the long-term treatment of obesity in primary care settings. Arch Fam Med 2000;9:160–7.
4. Kakkar AK, Dahiya N. Drug treatment of obesity: current status and future prospects. Eur J Intern Med 2015;26:89–94.
5. Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20:330–42.
6. Fabricatore AN, Wadden TA. Obesity. Annu Rev Clin Psychol 2006;2:357–77.
7. Cheung BM, Cheung TT, Samaranayake NR. Safety of antiobesity drugs. Ther Adv Drug Saf 2013;4:171–81.
8. Doyle S, Lloyd A, Birt J, et al. Willingness to pay for obesity pharmacotherapy. Obesity (Silver Spring) 2012;20:2019–26.
9. Wee CC HK, Bolcic-Jankovic D, Colten ME, et al. Sex, race, and consideration of bariatric surgery among primary care patients with moderate to severe obesity. J Gen Intern Med 2014;29:68–75.
10. Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life in obesity. Obes Res 2001;9:102–11.
11. Tan DZN, Dennis SM, Vagholkar S. Weight management in general practice: what do patients want? Med J Aust 2006;185:73–5.
12. Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA 2014;311:74–86.
13. Foster GD, Wadden TA, Vogt RA, Brewer G. What is a reasonable weight loss? Patients’ expectations and evaluations of obesity treatment outcomes. J Consult Clin Psychol 1997;65:79–85.
14. Fabricatore AN, Wadden TA, Womble LG, et al. The role of patients’ expectations and goals in the behavioral and pharmacological treatment of obesity. Int J Obes (Lond) 2007;31:1739–45.
15. Robertson C, Archibald D, Avenell A, et al. Systematic reviews of and integrated report on the quantitative, qualitative and economic evidence base for the management of obesity in men. Health Technol Assess 2014;18:1–424.
16. Thompson RL, Thomas DE. A cross-sectional survey of the opinions on weight loss treatments of adult obese patients attending a dietetic clinic. Int J Obes Relat Metab Disord 2000;24:164–70.
17. Kolotkin RL, Crosby RD, Williams GR. Health-related quality of life varies among obese subgroups. Obes Res 2002;10:748–56.
18. Wee C, Davis R, Chiodi S, et al. Sex, race, and the adverse effects of social stigma vs. other quality of life factors among primary care patients with moderate to severe obesity. J Gen Intern Med 2015;30:229–35.
How to Treat Pediatric MS
VANCOUVER—Pediatric multiple sclerosis (MS) presents unique concerns, making appropriate treatment especially important, according to an overview presented at the 45th Annual Meeting of the Child Neurology Society. Currently, disease-modifying therapies with FDA approval in adult MS have not been approved for the treatment of pediatric MS. In addition, brain growth and cognition are adversely affected in pediatric MS. Furthermore, children with MS tend to become disabled at a younger age, compared with adults.
Diagnosis and Initiation of Therapy
Neurologists should be prepared to engage with children and their families to ensure the most efficacious treatment. “Whenever you are having that conversation or you are considering starting the disease-modifying therapy, you need to balance the need to treat the disease with the idea that you are going to be starting a therapy for a lifetime,” said Dr. Lotze.
Disease-modifying therapies target the inflammatory aspects of pediatric-onset MS and should be initiated in children with a confirmed diagnosis, said Dr. Lotze. Children with clinically isolated syndrome who appear to be at high risk for MS and children with positive oligoclonal bands or elevated IgG index may also need to begin a disease-modifying therapy. Distinguishing between MS, acute disseminated encephalomyelitis (ADEM), and neuromyelitis optica (NMO) poses challenges, especially in children under age 10. Certain interferons can exacerbate NMO and may be harmful in children with ADEM or multiphasic ADEM.
Setting Goals
Neurologists are encouraged to counsel patients about the purpose and side effects of treatment, as well as to establish reasonable expectations for treatment. Additionally, children and families should be aware that another attack may occur during treatment and should be informed about what to do if it does.
No therapy is 100% efficacious in pediatric or adult MS, said Dr. Lotze. Research suggests that 50% of adults have no evidence of disease activity (NEDA) at two years of the disease. After seven years, however, 7% of these adults meet NEDA criteria. As a result, minimal disease progression (defined as less than one attack per year, fewer than three new lesions on a yearly MRI, and no progression in disability) may be a more attainable goal. “We would like to see a drug in a single individual or patient that is effective in achieving NEDA. If that is not possible … you might find that ultimately what you can go for is minimal disease progression,” said Dr. Lotze. He added that there appears to be no clear difference in terms of long-term outcome between children with MS who achieve NEDA and those who achieve minimal disease progression.
First-Line Therapies and Follow-Up Appointments
First-line agents for pediatric MS are the injectable drugs known as platform therapies. The International Pediatric MS Society Group (IPMSSG) recommends that all patients start first-line therapy (ie, interferon β or glatiramer acetate) soon after diagnosis.
Positive results from phase IV observational studies suggest that interferon β and glatiramer acetate are safe and efficacious treatments in pediatric MS. When studied in populations ranging in age from 12 to 17, these therapies decreased relapse rate, stabilized disability, and reduced accrual of new lesions. However, injectable treatments are associated with flu-like symptoms and injection-site reactions. Neurologists should start pediatric patients on interferon β with 25% to 50% of full dosing and titrate to a full adult dosing over four to six weeks. When initiating glatiramer acetate, neurologists should start children with a full adult dosing, 20 mg subcutaneous daily or 40 mg subcutaneous three times per week.
Follow-up appointments should occur every three to six months to assess adherence and determine efficacy of treatment. Neurologists are advised to ask patients whether they are comfortable with taking shots. Patients with needle anxiety, a common problem in pediatric MS, may need assistance from a child psychologist or child life specialist. Asking patients how often the patient or family forgets to take the disease-modifying therapy is also necessary, said Dr. Lotze. In addition, the transition from pediatric to adult care should be addressed in follow-up discussions. Teens must understand that certain disease-modifying therapies are contraindicated for pregnant patients. They also should be aware of how alcohol and other drugs interact with treatment.
Treatment Failure
Research indicates that approximately 30% of patients with pediatric-onset MS will not respond to the first-line therapies, and numerous variables may explain why. Age and disease duration can influence treatment efficacy, as can the number of relapses and the level of disease activity before treatment initiation. Patients who are nonadherent because of side effects and who continue to struggle with needle anxiety may experience treatment failure. The IPMSSG defines treatment failure as adherence to treatment for at least six months with no reduction in relapse rate or in new MRI T2 or contrast-enhancing lesions, or with two or more relapses within a 12-month period.
Second-Line Therapies
Trials of several oral agents are currently under way. The IPMSSG, however, urges neurologists to use extreme caution when considering nonplatform therapies for pediatric patients.
Fingolimod is a second-line drug for pediatric MS that blocks the egress of lymphocytes from the lymph nodes. A small percentage of patients taking this oral agent may develop bradycardia. Monitoring is required for the first six hours of treatment to ensure that the patient has no side effects. Some adverse effects associated with the drug include: first dose bradycardia, macular edema, and herpetic infections.
Dimethyl fumarate is an Nrf2 antioxidant pathway modulator that is associated with adverse effects such as flushing and gastrointestinal upset, said Dr. Lotze. Low-dose aspirin may help with flushing, and a proton pump inhibitor can help to manage the gastrointestinal upset. This treatment requires patients to undergo monitoring for blood count and liver function, as does fingolimod.
Teriflunomide, a pyrimidine synthesis inhibitor, is a Pregnancy Category X drug because it increases the risk of birth defects. Rituximab, an anti-CD20 chimeric monoclonal antibody, is gaining popularity for treating MS. Studies suggest that ocrelizumab may be well tolerated in pediatric MS. Natlizumamb, cladribine, and alemtuzumab are typically used to treat more aggressive forms of MS.
Neurologists rarely prescribe cyclophosphamide or mitoxantrone in pediatric MS. Cyclophosphamide has no formal FDA approval for adult MS or pediatric-onset MS and is associated with increased risks of bladder cancer, secondary leukemia, and infertility. Mitoxantrone is FDA-approved for adults with aggressive relapsing-remitting MS and secondary progressive MS. It is associated with increased cancer risk, however, and is highly cardiotoxic.
“After you have initiated a second-line agent, you need to continue to monitor aspects of disease control, including relapse rate, disability, MRI changes, and other adverse events. If you continue to see breakthrough disease, then you may need to consider … changing to another agent or moving to a more aggressive therapy such as rituximab or natalizumab,” said Dr. Lotze.
—Erica Tricarico
Suggested Reading
Chitnis T, Ghezzi A, Bajer-Korneck B, et al. Pediatric multiple sclerosis: Escalation and emerging treatments. Neurology. 2016;87(9 Suppl 2):S10 3-S109.
Jancic J, Nikolic B, Ivancevic N, et al. Multiple sclerosis in pediatrics: Current concepts and treatment options. Neurol Ther. 2016:5(2):131-1
VANCOUVER—Pediatric multiple sclerosis (MS) presents unique concerns, making appropriate treatment especially important, according to an overview presented at the 45th Annual Meeting of the Child Neurology Society. Currently, disease-modifying therapies with FDA approval in adult MS have not been approved for the treatment of pediatric MS. In addition, brain growth and cognition are adversely affected in pediatric MS. Furthermore, children with MS tend to become disabled at a younger age, compared with adults.
Diagnosis and Initiation of Therapy
Neurologists should be prepared to engage with children and their families to ensure the most efficacious treatment. “Whenever you are having that conversation or you are considering starting the disease-modifying therapy, you need to balance the need to treat the disease with the idea that you are going to be starting a therapy for a lifetime,” said Dr. Lotze.
Disease-modifying therapies target the inflammatory aspects of pediatric-onset MS and should be initiated in children with a confirmed diagnosis, said Dr. Lotze. Children with clinically isolated syndrome who appear to be at high risk for MS and children with positive oligoclonal bands or elevated IgG index may also need to begin a disease-modifying therapy. Distinguishing between MS, acute disseminated encephalomyelitis (ADEM), and neuromyelitis optica (NMO) poses challenges, especially in children under age 10. Certain interferons can exacerbate NMO and may be harmful in children with ADEM or multiphasic ADEM.
Setting Goals
Neurologists are encouraged to counsel patients about the purpose and side effects of treatment, as well as to establish reasonable expectations for treatment. Additionally, children and families should be aware that another attack may occur during treatment and should be informed about what to do if it does.
No therapy is 100% efficacious in pediatric or adult MS, said Dr. Lotze. Research suggests that 50% of adults have no evidence of disease activity (NEDA) at two years of the disease. After seven years, however, 7% of these adults meet NEDA criteria. As a result, minimal disease progression (defined as less than one attack per year, fewer than three new lesions on a yearly MRI, and no progression in disability) may be a more attainable goal. “We would like to see a drug in a single individual or patient that is effective in achieving NEDA. If that is not possible … you might find that ultimately what you can go for is minimal disease progression,” said Dr. Lotze. He added that there appears to be no clear difference in terms of long-term outcome between children with MS who achieve NEDA and those who achieve minimal disease progression.
First-Line Therapies and Follow-Up Appointments
First-line agents for pediatric MS are the injectable drugs known as platform therapies. The International Pediatric MS Society Group (IPMSSG) recommends that all patients start first-line therapy (ie, interferon β or glatiramer acetate) soon after diagnosis.
Positive results from phase IV observational studies suggest that interferon β and glatiramer acetate are safe and efficacious treatments in pediatric MS. When studied in populations ranging in age from 12 to 17, these therapies decreased relapse rate, stabilized disability, and reduced accrual of new lesions. However, injectable treatments are associated with flu-like symptoms and injection-site reactions. Neurologists should start pediatric patients on interferon β with 25% to 50% of full dosing and titrate to a full adult dosing over four to six weeks. When initiating glatiramer acetate, neurologists should start children with a full adult dosing, 20 mg subcutaneous daily or 40 mg subcutaneous three times per week.
Follow-up appointments should occur every three to six months to assess adherence and determine efficacy of treatment. Neurologists are advised to ask patients whether they are comfortable with taking shots. Patients with needle anxiety, a common problem in pediatric MS, may need assistance from a child psychologist or child life specialist. Asking patients how often the patient or family forgets to take the disease-modifying therapy is also necessary, said Dr. Lotze. In addition, the transition from pediatric to adult care should be addressed in follow-up discussions. Teens must understand that certain disease-modifying therapies are contraindicated for pregnant patients. They also should be aware of how alcohol and other drugs interact with treatment.
Treatment Failure
Research indicates that approximately 30% of patients with pediatric-onset MS will not respond to the first-line therapies, and numerous variables may explain why. Age and disease duration can influence treatment efficacy, as can the number of relapses and the level of disease activity before treatment initiation. Patients who are nonadherent because of side effects and who continue to struggle with needle anxiety may experience treatment failure. The IPMSSG defines treatment failure as adherence to treatment for at least six months with no reduction in relapse rate or in new MRI T2 or contrast-enhancing lesions, or with two or more relapses within a 12-month period.
Second-Line Therapies
Trials of several oral agents are currently under way. The IPMSSG, however, urges neurologists to use extreme caution when considering nonplatform therapies for pediatric patients.
Fingolimod is a second-line drug for pediatric MS that blocks the egress of lymphocytes from the lymph nodes. A small percentage of patients taking this oral agent may develop bradycardia. Monitoring is required for the first six hours of treatment to ensure that the patient has no side effects. Some adverse effects associated with the drug include: first dose bradycardia, macular edema, and herpetic infections.
Dimethyl fumarate is an Nrf2 antioxidant pathway modulator that is associated with adverse effects such as flushing and gastrointestinal upset, said Dr. Lotze. Low-dose aspirin may help with flushing, and a proton pump inhibitor can help to manage the gastrointestinal upset. This treatment requires patients to undergo monitoring for blood count and liver function, as does fingolimod.
Teriflunomide, a pyrimidine synthesis inhibitor, is a Pregnancy Category X drug because it increases the risk of birth defects. Rituximab, an anti-CD20 chimeric monoclonal antibody, is gaining popularity for treating MS. Studies suggest that ocrelizumab may be well tolerated in pediatric MS. Natlizumamb, cladribine, and alemtuzumab are typically used to treat more aggressive forms of MS.
Neurologists rarely prescribe cyclophosphamide or mitoxantrone in pediatric MS. Cyclophosphamide has no formal FDA approval for adult MS or pediatric-onset MS and is associated with increased risks of bladder cancer, secondary leukemia, and infertility. Mitoxantrone is FDA-approved for adults with aggressive relapsing-remitting MS and secondary progressive MS. It is associated with increased cancer risk, however, and is highly cardiotoxic.
“After you have initiated a second-line agent, you need to continue to monitor aspects of disease control, including relapse rate, disability, MRI changes, and other adverse events. If you continue to see breakthrough disease, then you may need to consider … changing to another agent or moving to a more aggressive therapy such as rituximab or natalizumab,” said Dr. Lotze.
—Erica Tricarico
Suggested Reading
Chitnis T, Ghezzi A, Bajer-Korneck B, et al. Pediatric multiple sclerosis: Escalation and emerging treatments. Neurology. 2016;87(9 Suppl 2):S10 3-S109.
Jancic J, Nikolic B, Ivancevic N, et al. Multiple sclerosis in pediatrics: Current concepts and treatment options. Neurol Ther. 2016:5(2):131-1
VANCOUVER—Pediatric multiple sclerosis (MS) presents unique concerns, making appropriate treatment especially important, according to an overview presented at the 45th Annual Meeting of the Child Neurology Society. Currently, disease-modifying therapies with FDA approval in adult MS have not been approved for the treatment of pediatric MS. In addition, brain growth and cognition are adversely affected in pediatric MS. Furthermore, children with MS tend to become disabled at a younger age, compared with adults.
Diagnosis and Initiation of Therapy
Neurologists should be prepared to engage with children and their families to ensure the most efficacious treatment. “Whenever you are having that conversation or you are considering starting the disease-modifying therapy, you need to balance the need to treat the disease with the idea that you are going to be starting a therapy for a lifetime,” said Dr. Lotze.
Disease-modifying therapies target the inflammatory aspects of pediatric-onset MS and should be initiated in children with a confirmed diagnosis, said Dr. Lotze. Children with clinically isolated syndrome who appear to be at high risk for MS and children with positive oligoclonal bands or elevated IgG index may also need to begin a disease-modifying therapy. Distinguishing between MS, acute disseminated encephalomyelitis (ADEM), and neuromyelitis optica (NMO) poses challenges, especially in children under age 10. Certain interferons can exacerbate NMO and may be harmful in children with ADEM or multiphasic ADEM.
Setting Goals
Neurologists are encouraged to counsel patients about the purpose and side effects of treatment, as well as to establish reasonable expectations for treatment. Additionally, children and families should be aware that another attack may occur during treatment and should be informed about what to do if it does.
No therapy is 100% efficacious in pediatric or adult MS, said Dr. Lotze. Research suggests that 50% of adults have no evidence of disease activity (NEDA) at two years of the disease. After seven years, however, 7% of these adults meet NEDA criteria. As a result, minimal disease progression (defined as less than one attack per year, fewer than three new lesions on a yearly MRI, and no progression in disability) may be a more attainable goal. “We would like to see a drug in a single individual or patient that is effective in achieving NEDA. If that is not possible … you might find that ultimately what you can go for is minimal disease progression,” said Dr. Lotze. He added that there appears to be no clear difference in terms of long-term outcome between children with MS who achieve NEDA and those who achieve minimal disease progression.
First-Line Therapies and Follow-Up Appointments
First-line agents for pediatric MS are the injectable drugs known as platform therapies. The International Pediatric MS Society Group (IPMSSG) recommends that all patients start first-line therapy (ie, interferon β or glatiramer acetate) soon after diagnosis.
Positive results from phase IV observational studies suggest that interferon β and glatiramer acetate are safe and efficacious treatments in pediatric MS. When studied in populations ranging in age from 12 to 17, these therapies decreased relapse rate, stabilized disability, and reduced accrual of new lesions. However, injectable treatments are associated with flu-like symptoms and injection-site reactions. Neurologists should start pediatric patients on interferon β with 25% to 50% of full dosing and titrate to a full adult dosing over four to six weeks. When initiating glatiramer acetate, neurologists should start children with a full adult dosing, 20 mg subcutaneous daily or 40 mg subcutaneous three times per week.
Follow-up appointments should occur every three to six months to assess adherence and determine efficacy of treatment. Neurologists are advised to ask patients whether they are comfortable with taking shots. Patients with needle anxiety, a common problem in pediatric MS, may need assistance from a child psychologist or child life specialist. Asking patients how often the patient or family forgets to take the disease-modifying therapy is also necessary, said Dr. Lotze. In addition, the transition from pediatric to adult care should be addressed in follow-up discussions. Teens must understand that certain disease-modifying therapies are contraindicated for pregnant patients. They also should be aware of how alcohol and other drugs interact with treatment.
Treatment Failure
Research indicates that approximately 30% of patients with pediatric-onset MS will not respond to the first-line therapies, and numerous variables may explain why. Age and disease duration can influence treatment efficacy, as can the number of relapses and the level of disease activity before treatment initiation. Patients who are nonadherent because of side effects and who continue to struggle with needle anxiety may experience treatment failure. The IPMSSG defines treatment failure as adherence to treatment for at least six months with no reduction in relapse rate or in new MRI T2 or contrast-enhancing lesions, or with two or more relapses within a 12-month period.
Second-Line Therapies
Trials of several oral agents are currently under way. The IPMSSG, however, urges neurologists to use extreme caution when considering nonplatform therapies for pediatric patients.
Fingolimod is a second-line drug for pediatric MS that blocks the egress of lymphocytes from the lymph nodes. A small percentage of patients taking this oral agent may develop bradycardia. Monitoring is required for the first six hours of treatment to ensure that the patient has no side effects. Some adverse effects associated with the drug include: first dose bradycardia, macular edema, and herpetic infections.
Dimethyl fumarate is an Nrf2 antioxidant pathway modulator that is associated with adverse effects such as flushing and gastrointestinal upset, said Dr. Lotze. Low-dose aspirin may help with flushing, and a proton pump inhibitor can help to manage the gastrointestinal upset. This treatment requires patients to undergo monitoring for blood count and liver function, as does fingolimod.
Teriflunomide, a pyrimidine synthesis inhibitor, is a Pregnancy Category X drug because it increases the risk of birth defects. Rituximab, an anti-CD20 chimeric monoclonal antibody, is gaining popularity for treating MS. Studies suggest that ocrelizumab may be well tolerated in pediatric MS. Natlizumamb, cladribine, and alemtuzumab are typically used to treat more aggressive forms of MS.
Neurologists rarely prescribe cyclophosphamide or mitoxantrone in pediatric MS. Cyclophosphamide has no formal FDA approval for adult MS or pediatric-onset MS and is associated with increased risks of bladder cancer, secondary leukemia, and infertility. Mitoxantrone is FDA-approved for adults with aggressive relapsing-remitting MS and secondary progressive MS. It is associated with increased cancer risk, however, and is highly cardiotoxic.
“After you have initiated a second-line agent, you need to continue to monitor aspects of disease control, including relapse rate, disability, MRI changes, and other adverse events. If you continue to see breakthrough disease, then you may need to consider … changing to another agent or moving to a more aggressive therapy such as rituximab or natalizumab,” said Dr. Lotze.
—Erica Tricarico
Suggested Reading
Chitnis T, Ghezzi A, Bajer-Korneck B, et al. Pediatric multiple sclerosis: Escalation and emerging treatments. Neurology. 2016;87(9 Suppl 2):S10 3-S109.
Jancic J, Nikolic B, Ivancevic N, et al. Multiple sclerosis in pediatrics: Current concepts and treatment options. Neurol Ther. 2016:5(2):131-1
Herpes Zoster Following Varicella Vaccination in Children
Varicella-zoster virus (VZV) causes varicella as a primary infection. It is a highly contagious disease characterized by a widespread papulovesicular eruption with fever and malaise.1,2 After the primary infection, the virus remains latent within the sensory dorsal root ganglia and can reactivate as herpes zoster (HZ).1-5 Herpes zoster is characterized by unilateral radicular pain and a vesicular rash in a dermatomal pattern.1,2 It is most common in adults, especially elderly and immunocompromised patients, but rarely occurs in children. Herpes zoster is most often seen in individuals previously infected with VZV, but it also has occurred in individuals without known varicella infection,1-17 possibly because these individuals had a prior subclinical VZV infection.
A live attenuated VZV vaccine was created after isolation of the virus from a child in Japan.2 Since the introduction of the vaccine in 1995 in the United States, the incidence of VZV and HZ has declined.5 Herpes zoster rates after vaccination vary from 14 to 19 per 100,000 individuals.3,5 Breakthrough disease with the wild-type strain does occur in vaccinated children, but vaccine-strain HZ also has been reported.1-5 The risk for HZ caused by reactivated VZV vaccine in healthy children is unknown. We present a case of HZ in an otherwise healthy 19-month-old boy with no known varicella exposure who received the VZV vaccine at 13 months of age.
Case Report
An otherwise healthy 19-month-old boy presented to the dermatology clinic with a rash that began 2 days prior on the right groin and spread to the right leg. The patient’s mother denied that the child had been febrile and noted that the rash did not appear to bother him in any way. The patient was up-to-date on his vaccinations and received the first dose of the varicella series 6 months prior to presentation. He had no personal history of varicella, no exposure to sick contacts with varicella, and no known exposure to the virus. He was otherwise completely healthy with no signs or symptoms of immunocompromise.
Physical examination revealed grouped vesicles on an erythematous base on the right thigh, right sacrum, and lower abdomen that did not cross the midline (Figure). There were no other pertinent physical examination findings. The eruption was most consistent with HZ but concern remained for herpes simplex virus (HSV) or impetigo. A bacterial culture and polymerase chain reaction assay for VZV and HSV from skin swabs was ordered. The patient was prescribed acyclovir 20 mg/kg every 6 hours for 5 days. Laboratory testing revealed a positive result for VZV on polymerase chain reaction and a negative result for HSV. The majority of the patient’s lesions had crusted after 2 days of treatment with acyclovir, and the rash had nearly resolved 1 week after presentation. Subsequent evaluation with a complete blood cell count with differential and basic metabolic profile was normal. Levels of IgG, IgA, and IgM also were normal; IgE was slightly elevated.
Comment
Herpes zoster in children is an uncommon clinical entity. Most children with HZ are immunocompromised, have a history of varicella, or were exposed to varicella during gestation.8 With the introduction of the live VZV vaccine, the incidence of HZ has declined, but reactivation of the live vaccine leading to HZ infection is possible. The vaccine is 90% effective, and breakthrough varicella has been reported in 15% to 20% of vaccinated patients.1-17 The cause of HZ in vaccinated children is unclear due to the potential for either wild-type or vaccine-strain VZV to induce HZ.
Twenty-two cases of HZ in healthy children after vaccination were identified with a PubMed search of articles indexed for MEDLINE using the search terms herpes zoster infection after vaccination and herpes zoster infection AND immunocompetent AND vaccination in separate searches for all English-language studies (Table). The search was limited to immunocompetent children and adolescents who were 18 years or younger with no history of varicella or exposure to varicella during gestation.
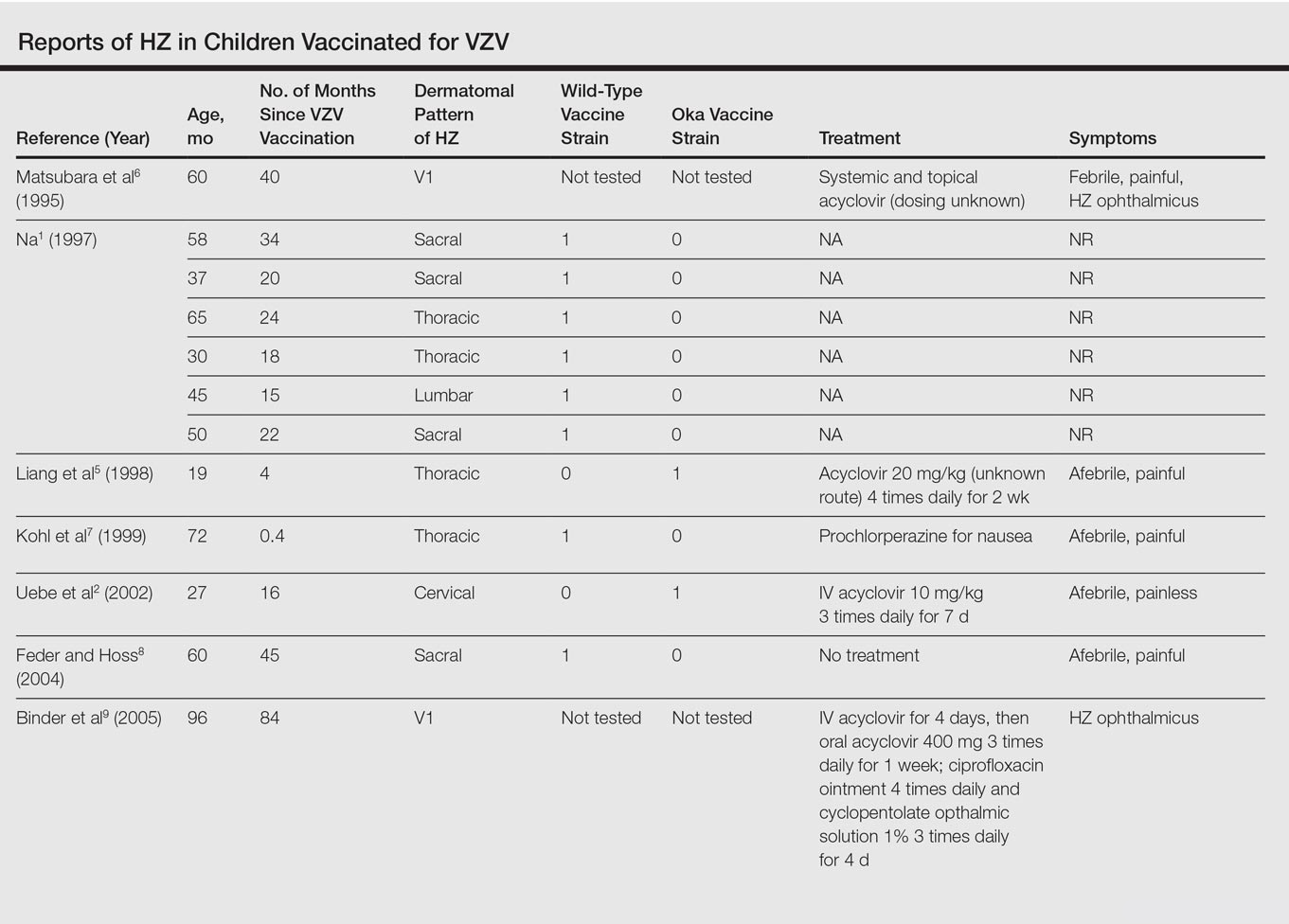

The mean age for HZ infection was 5.3 years. The average time between vaccination and HZ infection was 3.3 years. There was a spread of dermatomal patterns with cases in the first division of the trigeminal nerve, cervical, thoracic, lumbar, and sacral distributions. Of the 22 cases of HZ we reviewed, 16 underwent genotype testing to determine the source of the infection. The Oka vaccine strain virus was identified in 8 (50%) cases, while wild-type virus was found in 8 (50%) cases.1,2,4,5,7,8,10,11,13,14,16 Twelve cases were treated with acyclovir.2,3,5,6,9-12,14-17 The method of delivery, either oral or intravenous, and the length of treatment depended on the severity of the disease. Patients with meningoencephalitis and HZ ophthalmicus received intravenous acyclovir more often and also had a longer course of acyclovir compared to those individuals with involvement limited to the skin.
This review found HZ occurs from reactivation of wild-type or Oka vaccine-strain VZV in immunocompetent children.1-17 It shows that subclinical varicella infection is not the only explanation for HZ in a healthy vaccinated child. It is currently not clear why some healthy children experience HZ from vaccine-strain VZV. When HZ presents in a vaccinated immunocompetent child without a history of varicella infection or exposure, the possibility for vaccine strain–induced HZ should be considered.
- . Herpes zoster in three healthy children immunized with varicella vaccine (Oka/Biken); the causative virus differed from vaccine strain on PCR analysis of the IV variable region (R5) and of a PstI-site region. Br J Dermatol. 1997;137:255-258.
- Uebe B, Sauerbrei A, Burdach S, et al. Herpes zoster by reactivated vaccine varicella zoster virus in a healthy child [published online June 25, 2002]. Eur J Pediatr. 2002;161:442-444.
- Obieta MP, Jacinto SS. Herpes zoster after varicella vaccination in a healthy young child. Int J Dermatol. 2008;47:640-641.
- Ota K, Kim V, Lavi S, et al. Vaccine-strain varicella zoster virus causing recurrent herpes zoster in an immunocompetent 2-year-old. Pediatr Infect Dis J. 2008;27:847-848.
- Liang GL, Heidelberg KA, Jacobson RM, et al. Herpes zoster after varicella vaccination. J Am Acad Dermatol. 1998;38:761-763.
- Matsubara K, Nigami H, Harigaya H, et al. Herpes zoster in a normal child after varicella vaccination. Acta Paediatr Jpn. 1995;37:648-650.
- Kohl S, Rapp J, Larussa P, et al. Natural varicella-zoster virus reactivation shortly after varicella immunization in a child. Pediatr Infect Dis J. 1999;18:1112-1113.
- Feder HM Jr, Hoss DM. Herpes zoster in otherwise healthy children. Pediatr Infect Dis J. 2004;23:451-457; quiz 458-460.
- Binder NR, Holland GN, Hosea S, et al. Herpes zoster ophthalmicus in an otherwise-healthy child. J AAPOS. 2005;9:597-598.
- Levin MJ, DeBiasi RL, Bostik V, et al. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella-zoster virus vaccine. J Infect Dis. 2008;198:1444-1447.
- Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792-795.
- Lin P, Yoon MK, Chiu CS. Herpes zoster keratouveitis and inflammatory ocular hypertension 8 years after varicella vaccination. Ocul Immunol Inflamm. 2009;17:33-35.
- Chouliaras G, Spoulou V, Quinlivan M, et al. Vaccine-associated herpes zoster ophthalmicus [correction of opthalmicus] and encephalitis in an immunocompetent child [published online March 1, 2010]. Pediatrics. 2010;125:E969-E972.
- Han JY, Hanson DC, Way SS. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30:266-268.
- Ryu WY, Kim NY, Kwon YH, et al. Herpes zoster ophthalmicus with isolated trochlear nerve palsy in an otherwise healthy 13-year-old girl. J AAPOS. 2014;18:193-195.
- Iwasaki S, Motokura K, Honda Y, et al. Vaccine-strain herpes zoster found in the trigeminal nerve area in a healthy child: a case report [published online November 3, 2016]. J Clin Virol. 2016;85:44-47.
- Peterson N, Goodman S, Peterson M, et al. Herpes zoster in children. Cutis. 2016;98:94-95.
Varicella-zoster virus (VZV) causes varicella as a primary infection. It is a highly contagious disease characterized by a widespread papulovesicular eruption with fever and malaise.1,2 After the primary infection, the virus remains latent within the sensory dorsal root ganglia and can reactivate as herpes zoster (HZ).1-5 Herpes zoster is characterized by unilateral radicular pain and a vesicular rash in a dermatomal pattern.1,2 It is most common in adults, especially elderly and immunocompromised patients, but rarely occurs in children. Herpes zoster is most often seen in individuals previously infected with VZV, but it also has occurred in individuals without known varicella infection,1-17 possibly because these individuals had a prior subclinical VZV infection.
A live attenuated VZV vaccine was created after isolation of the virus from a child in Japan.2 Since the introduction of the vaccine in 1995 in the United States, the incidence of VZV and HZ has declined.5 Herpes zoster rates after vaccination vary from 14 to 19 per 100,000 individuals.3,5 Breakthrough disease with the wild-type strain does occur in vaccinated children, but vaccine-strain HZ also has been reported.1-5 The risk for HZ caused by reactivated VZV vaccine in healthy children is unknown. We present a case of HZ in an otherwise healthy 19-month-old boy with no known varicella exposure who received the VZV vaccine at 13 months of age.
Case Report
An otherwise healthy 19-month-old boy presented to the dermatology clinic with a rash that began 2 days prior on the right groin and spread to the right leg. The patient’s mother denied that the child had been febrile and noted that the rash did not appear to bother him in any way. The patient was up-to-date on his vaccinations and received the first dose of the varicella series 6 months prior to presentation. He had no personal history of varicella, no exposure to sick contacts with varicella, and no known exposure to the virus. He was otherwise completely healthy with no signs or symptoms of immunocompromise.
Physical examination revealed grouped vesicles on an erythematous base on the right thigh, right sacrum, and lower abdomen that did not cross the midline (Figure). There were no other pertinent physical examination findings. The eruption was most consistent with HZ but concern remained for herpes simplex virus (HSV) or impetigo. A bacterial culture and polymerase chain reaction assay for VZV and HSV from skin swabs was ordered. The patient was prescribed acyclovir 20 mg/kg every 6 hours for 5 days. Laboratory testing revealed a positive result for VZV on polymerase chain reaction and a negative result for HSV. The majority of the patient’s lesions had crusted after 2 days of treatment with acyclovir, and the rash had nearly resolved 1 week after presentation. Subsequent evaluation with a complete blood cell count with differential and basic metabolic profile was normal. Levels of IgG, IgA, and IgM also were normal; IgE was slightly elevated.

Comment
Herpes zoster in children is an uncommon clinical entity. Most children with HZ are immunocompromised, have a history of varicella, or were exposed to varicella during gestation.8 With the introduction of the live VZV vaccine, the incidence of HZ has declined, but reactivation of the live vaccine leading to HZ infection is possible. The vaccine is 90% effective, and breakthrough varicella has been reported in 15% to 20% of vaccinated patients.1-17 The cause of HZ in vaccinated children is unclear due to the potential for either wild-type or vaccine-strain VZV to induce HZ.
Twenty-two cases of HZ in healthy children after vaccination were identified with a PubMed search of articles indexed for MEDLINE using the search terms herpes zoster infection after vaccination and herpes zoster infection AND immunocompetent AND vaccination in separate searches for all English-language studies (Table). The search was limited to immunocompetent children and adolescents who were 18 years or younger with no history of varicella or exposure to varicella during gestation.


The mean age for HZ infection was 5.3 years. The average time between vaccination and HZ infection was 3.3 years. There was a spread of dermatomal patterns with cases in the first division of the trigeminal nerve, cervical, thoracic, lumbar, and sacral distributions. Of the 22 cases of HZ we reviewed, 16 underwent genotype testing to determine the source of the infection. The Oka vaccine strain virus was identified in 8 (50%) cases, while wild-type virus was found in 8 (50%) cases.1,2,4,5,7,8,10,11,13,14,16 Twelve cases were treated with acyclovir.2,3,5,6,9-12,14-17 The method of delivery, either oral or intravenous, and the length of treatment depended on the severity of the disease. Patients with meningoencephalitis and HZ ophthalmicus received intravenous acyclovir more often and also had a longer course of acyclovir compared to those individuals with involvement limited to the skin.
This review found HZ occurs from reactivation of wild-type or Oka vaccine-strain VZV in immunocompetent children.1-17 It shows that subclinical varicella infection is not the only explanation for HZ in a healthy vaccinated child. It is currently not clear why some healthy children experience HZ from vaccine-strain VZV. When HZ presents in a vaccinated immunocompetent child without a history of varicella infection or exposure, the possibility for vaccine strain–induced HZ should be considered.
Varicella-zoster virus (VZV) causes varicella as a primary infection. It is a highly contagious disease characterized by a widespread papulovesicular eruption with fever and malaise.1,2 After the primary infection, the virus remains latent within the sensory dorsal root ganglia and can reactivate as herpes zoster (HZ).1-5 Herpes zoster is characterized by unilateral radicular pain and a vesicular rash in a dermatomal pattern.1,2 It is most common in adults, especially elderly and immunocompromised patients, but rarely occurs in children. Herpes zoster is most often seen in individuals previously infected with VZV, but it also has occurred in individuals without known varicella infection,1-17 possibly because these individuals had a prior subclinical VZV infection.
A live attenuated VZV vaccine was created after isolation of the virus from a child in Japan.2 Since the introduction of the vaccine in 1995 in the United States, the incidence of VZV and HZ has declined.5 Herpes zoster rates after vaccination vary from 14 to 19 per 100,000 individuals.3,5 Breakthrough disease with the wild-type strain does occur in vaccinated children, but vaccine-strain HZ also has been reported.1-5 The risk for HZ caused by reactivated VZV vaccine in healthy children is unknown. We present a case of HZ in an otherwise healthy 19-month-old boy with no known varicella exposure who received the VZV vaccine at 13 months of age.
Case Report
An otherwise healthy 19-month-old boy presented to the dermatology clinic with a rash that began 2 days prior on the right groin and spread to the right leg. The patient’s mother denied that the child had been febrile and noted that the rash did not appear to bother him in any way. The patient was up-to-date on his vaccinations and received the first dose of the varicella series 6 months prior to presentation. He had no personal history of varicella, no exposure to sick contacts with varicella, and no known exposure to the virus. He was otherwise completely healthy with no signs or symptoms of immunocompromise.
Physical examination revealed grouped vesicles on an erythematous base on the right thigh, right sacrum, and lower abdomen that did not cross the midline (Figure). There were no other pertinent physical examination findings. The eruption was most consistent with HZ but concern remained for herpes simplex virus (HSV) or impetigo. A bacterial culture and polymerase chain reaction assay for VZV and HSV from skin swabs was ordered. The patient was prescribed acyclovir 20 mg/kg every 6 hours for 5 days. Laboratory testing revealed a positive result for VZV on polymerase chain reaction and a negative result for HSV. The majority of the patient’s lesions had crusted after 2 days of treatment with acyclovir, and the rash had nearly resolved 1 week after presentation. Subsequent evaluation with a complete blood cell count with differential and basic metabolic profile was normal. Levels of IgG, IgA, and IgM also were normal; IgE was slightly elevated.

Comment
Herpes zoster in children is an uncommon clinical entity. Most children with HZ are immunocompromised, have a history of varicella, or were exposed to varicella during gestation.8 With the introduction of the live VZV vaccine, the incidence of HZ has declined, but reactivation of the live vaccine leading to HZ infection is possible. The vaccine is 90% effective, and breakthrough varicella has been reported in 15% to 20% of vaccinated patients.1-17 The cause of HZ in vaccinated children is unclear due to the potential for either wild-type or vaccine-strain VZV to induce HZ.
Twenty-two cases of HZ in healthy children after vaccination were identified with a PubMed search of articles indexed for MEDLINE using the search terms herpes zoster infection after vaccination and herpes zoster infection AND immunocompetent AND vaccination in separate searches for all English-language studies (Table). The search was limited to immunocompetent children and adolescents who were 18 years or younger with no history of varicella or exposure to varicella during gestation.


The mean age for HZ infection was 5.3 years. The average time between vaccination and HZ infection was 3.3 years. There was a spread of dermatomal patterns with cases in the first division of the trigeminal nerve, cervical, thoracic, lumbar, and sacral distributions. Of the 22 cases of HZ we reviewed, 16 underwent genotype testing to determine the source of the infection. The Oka vaccine strain virus was identified in 8 (50%) cases, while wild-type virus was found in 8 (50%) cases.1,2,4,5,7,8,10,11,13,14,16 Twelve cases were treated with acyclovir.2,3,5,6,9-12,14-17 The method of delivery, either oral or intravenous, and the length of treatment depended on the severity of the disease. Patients with meningoencephalitis and HZ ophthalmicus received intravenous acyclovir more often and also had a longer course of acyclovir compared to those individuals with involvement limited to the skin.
This review found HZ occurs from reactivation of wild-type or Oka vaccine-strain VZV in immunocompetent children.1-17 It shows that subclinical varicella infection is not the only explanation for HZ in a healthy vaccinated child. It is currently not clear why some healthy children experience HZ from vaccine-strain VZV. When HZ presents in a vaccinated immunocompetent child without a history of varicella infection or exposure, the possibility for vaccine strain–induced HZ should be considered.
- . Herpes zoster in three healthy children immunized with varicella vaccine (Oka/Biken); the causative virus differed from vaccine strain on PCR analysis of the IV variable region (R5) and of a PstI-site region. Br J Dermatol. 1997;137:255-258.
- Uebe B, Sauerbrei A, Burdach S, et al. Herpes zoster by reactivated vaccine varicella zoster virus in a healthy child [published online June 25, 2002]. Eur J Pediatr. 2002;161:442-444.
- Obieta MP, Jacinto SS. Herpes zoster after varicella vaccination in a healthy young child. Int J Dermatol. 2008;47:640-641.
- Ota K, Kim V, Lavi S, et al. Vaccine-strain varicella zoster virus causing recurrent herpes zoster in an immunocompetent 2-year-old. Pediatr Infect Dis J. 2008;27:847-848.
- Liang GL, Heidelberg KA, Jacobson RM, et al. Herpes zoster after varicella vaccination. J Am Acad Dermatol. 1998;38:761-763.
- Matsubara K, Nigami H, Harigaya H, et al. Herpes zoster in a normal child after varicella vaccination. Acta Paediatr Jpn. 1995;37:648-650.
- Kohl S, Rapp J, Larussa P, et al. Natural varicella-zoster virus reactivation shortly after varicella immunization in a child. Pediatr Infect Dis J. 1999;18:1112-1113.
- Feder HM Jr, Hoss DM. Herpes zoster in otherwise healthy children. Pediatr Infect Dis J. 2004;23:451-457; quiz 458-460.
- Binder NR, Holland GN, Hosea S, et al. Herpes zoster ophthalmicus in an otherwise-healthy child. J AAPOS. 2005;9:597-598.
- Levin MJ, DeBiasi RL, Bostik V, et al. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella-zoster virus vaccine. J Infect Dis. 2008;198:1444-1447.
- Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792-795.
- Lin P, Yoon MK, Chiu CS. Herpes zoster keratouveitis and inflammatory ocular hypertension 8 years after varicella vaccination. Ocul Immunol Inflamm. 2009;17:33-35.
- Chouliaras G, Spoulou V, Quinlivan M, et al. Vaccine-associated herpes zoster ophthalmicus [correction of opthalmicus] and encephalitis in an immunocompetent child [published online March 1, 2010]. Pediatrics. 2010;125:E969-E972.
- Han JY, Hanson DC, Way SS. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30:266-268.
- Ryu WY, Kim NY, Kwon YH, et al. Herpes zoster ophthalmicus with isolated trochlear nerve palsy in an otherwise healthy 13-year-old girl. J AAPOS. 2014;18:193-195.
- Iwasaki S, Motokura K, Honda Y, et al. Vaccine-strain herpes zoster found in the trigeminal nerve area in a healthy child: a case report [published online November 3, 2016]. J Clin Virol. 2016;85:44-47.
- Peterson N, Goodman S, Peterson M, et al. Herpes zoster in children. Cutis. 2016;98:94-95.
- . Herpes zoster in three healthy children immunized with varicella vaccine (Oka/Biken); the causative virus differed from vaccine strain on PCR analysis of the IV variable region (R5) and of a PstI-site region. Br J Dermatol. 1997;137:255-258.
- Uebe B, Sauerbrei A, Burdach S, et al. Herpes zoster by reactivated vaccine varicella zoster virus in a healthy child [published online June 25, 2002]. Eur J Pediatr. 2002;161:442-444.
- Obieta MP, Jacinto SS. Herpes zoster after varicella vaccination in a healthy young child. Int J Dermatol. 2008;47:640-641.
- Ota K, Kim V, Lavi S, et al. Vaccine-strain varicella zoster virus causing recurrent herpes zoster in an immunocompetent 2-year-old. Pediatr Infect Dis J. 2008;27:847-848.
- Liang GL, Heidelberg KA, Jacobson RM, et al. Herpes zoster after varicella vaccination. J Am Acad Dermatol. 1998;38:761-763.
- Matsubara K, Nigami H, Harigaya H, et al. Herpes zoster in a normal child after varicella vaccination. Acta Paediatr Jpn. 1995;37:648-650.
- Kohl S, Rapp J, Larussa P, et al. Natural varicella-zoster virus reactivation shortly after varicella immunization in a child. Pediatr Infect Dis J. 1999;18:1112-1113.
- Feder HM Jr, Hoss DM. Herpes zoster in otherwise healthy children. Pediatr Infect Dis J. 2004;23:451-457; quiz 458-460.
- Binder NR, Holland GN, Hosea S, et al. Herpes zoster ophthalmicus in an otherwise-healthy child. J AAPOS. 2005;9:597-598.
- Levin MJ, DeBiasi RL, Bostik V, et al. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella-zoster virus vaccine. J Infect Dis. 2008;198:1444-1447.
- Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792-795.
- Lin P, Yoon MK, Chiu CS. Herpes zoster keratouveitis and inflammatory ocular hypertension 8 years after varicella vaccination. Ocul Immunol Inflamm. 2009;17:33-35.
- Chouliaras G, Spoulou V, Quinlivan M, et al. Vaccine-associated herpes zoster ophthalmicus [correction of opthalmicus] and encephalitis in an immunocompetent child [published online March 1, 2010]. Pediatrics. 2010;125:E969-E972.
- Han JY, Hanson DC, Way SS. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30:266-268.
- Ryu WY, Kim NY, Kwon YH, et al. Herpes zoster ophthalmicus with isolated trochlear nerve palsy in an otherwise healthy 13-year-old girl. J AAPOS. 2014;18:193-195.
- Iwasaki S, Motokura K, Honda Y, et al. Vaccine-strain herpes zoster found in the trigeminal nerve area in a healthy child: a case report [published online November 3, 2016]. J Clin Virol. 2016;85:44-47.
- Peterson N, Goodman S, Peterson M, et al. Herpes zoster in children. Cutis. 2016;98:94-95.
Practice Points
- Most children with herpes zoster are immunocompromised, have a history of varicella, or were exposed to varicella in utero.
- Herpes zoster has been reported in immunocompetent children due to either wild-type or vaccine-strain varicella-zoster virus.
Assessment and Treatment of Late-Life Depression
From the Department of Neuropsychiatry and Behavioral Sceince, University of South Carolina School of Medicine, Columbia, SC.
Abstract
- Objective: To review the identification, clinical assessment and treatment of patients with late-life depression.
- Methods: Review of the literature.
- Results: Depressive symptoms are present in up to 1 in 4 older adults. Comprehensive evaluation of depressive symptoms in this population often requires a multidisciplinary and collaborative approach between primary care, mental health, and other ancillary providers. Key aspects include a detailed history, physical and mental status examinations, cognitive and functional status assessment, and suicide risk assessment. Treatment options include anti-depressants, psychotherapy, and electroconvulsive therapy.
- Conclusion: A systematic approach to evaluating depressive symptoms in the elderly can enhance timely recognition and treatment.
Key words: Late-life depression; clinical assessment; antidepressants; psychotherapy; electroconvulsive therapy.
The U.S. population is aging, and with this comes the potential for increased health care needs. In 2014, there were over 46 million Americans age 65 and over (14.5% of the U.S. population). This number is projected to increase to 88 million by the year 2050 [1]. One in 4 older adults suffers with depressive symptoms that cause distress and functional impairment [2]. The World Health Organization Global Burden of Disease Study found depressive disorders to be the leading cause of disability-adjusted life years (DALYs) and the second leading cause of years lived with disability (YLDs). The burden of disease due to depressive disorders increased by 37.5% between 1990 and 2010, and 10.4% was attributable to aging [3]. These figures underscore the importance of accurate assessment and treatment of depression in the elderly. In this article, we review the identification, clinical assessment, and treatment of patients with late-life depression.
Diagnostic Criteria
Prevalence
It is estimated that 1% to 4% of community-dwelling adults age 65 and older suffer from MDD, with women more likely to be affected than men (prevalence of 4.4% vs. 2.7) [2,5–7]. This estimate is low compared with lifetime prevalence of almost 20% in the general adult population [8]. However, when depressive symptoms that do not meet criteria for MDD are considered, prevalence rates increase up to 25% [2,9]. These estimates also vary by clinical setting, with the highest rates (up to 40%) among elderly patients in long-term care facilities [10,11]. While individuals with subsyndromal depression may experience fewer symptoms than those with MDD, clinically significant distress persists, impacting health and functional status. Depression is associated with overall poor social or occupational functioning, cognitive decline, increased health care utilization and cost, increased morbidity and mortality from medical illness, and increased suicide mortality [5,9,10,12].
Identifying LLD
Accurate identification of LLD also requires recognition of the differences in the presentation of LLD compared with onset in earlier life. Depression in younger adults is often marked by depressed mood and loss of interest [18]. In contrast, older adults may present with increased anger or irritability [5]. Younger adults are more likely to report suicidal thoughts while older patients report feelings of hopelessness and thoughts of death [18]. LLD is often characterized by increased somatic complaints, hypochondriasis, or pain [5,18,19]. Another major difference lies in the presentation of cognitive difficulties. Younger patients typically complain of poor concentration or indecisiveness. Geriatric patients may present with cognitive changes including objective findings of slower processing speed and executive dysfunction on neuropsychological testing [17].
Depression rating scales may aid in identification of LLD. They are not a substitute for clinical diagnosis but can be useful as screening tools. Two commonly utilized depression rating scales are the Geriatric Depression Scale (GDS) and the Patient Health Questionnaire-9 (PHQ-9). GDS is a 30-item instrument developed specifically for older adults. Shorter 15-item, 5-item, and 4-item versions exist. The scale utilizes a Yes/No format and can be self- or clinician-administered [20]. One advantage of the GDS lies in its focus on psychological and cognitive aspects of depression rather than neurovegetative symptoms that may overlap with medical illnesses common in older adults [21]. The PHQ-9 is a 9-item self- or clinician-administered screening tool designed for use in primary care settings and has also been validated in geriatric populations [22,23]. The 9 items on this scale correspond to the DSM-5 criteria for major depression. A shorter 2-item version (PHQ-2) has also been validated, and a positive screen on this test should prompt administration of the full-length version. Both versions have approximately 80% sensitivity and specificity in detecting depression. An added advantage of PHQ-9 over GDS is that it can be useful in monitoring treatment response over time [22,23]
Comprehensive Assessment of LLD
The comprehensive assessment of patients with LLD can be carried out by health professionals in both mental health or primary care settings. In a multidisciplinary approach, psychiatrists and mental health professionals have collaborated with primary care providers using depression care managers with successful outcomes in managing depression in older adults [24,25]. Complete evaluation of a patient with suspected LLD begins with a history and physical and mental status examination. Other essential components of the evaluation include assessment of cognition, functional status, and suicide risk. Laboratory and neuroimaging studies may be necessary as well. Due to the comprehensive nature of this assessment, a multidisciplinary approach with collaboration between primary care, psychiatry, psychology, and ancillary services such as social work may be necessary. Multiple patient interactions may be required to complete a thorough evaluation.
History and Mental Status Examination
As with many other psychiatric illnesses, LLD is a clinical diagnosis. A careful history should be obtained initially utilizing open-ended questions. This should be followed by more directed questions as indicated to elicit the presence of depressive symptoms. The history should be obtained from the patient. A relevant collateral informant can be invaluable in the assessment, especially in cases where there is a comorbid neurocognitive disorder. However, the patient’s informed consent must be obtained prior to obtaining collateral information whenever possible. Psychosocial stressors that may have precipitated or may be perpetuating symptoms should be explored. Such stressors may include recent changes in living situation, loss of social support, recent deaths, or financial difficulties. Biological precipitants also need to be explored including presence of physical illness, depressogenic medications, and comorbid alcohol or other substance use. The patient’s past psychiatric history, psychiatric hospitalizations, and past medication trials should be ascertained. Any family history of depression, other psychiatric disorders, substance use disorders, and suicide attempts should be documented. A full mental status exam including cognitive assessment should be completed [21,26].
Cognitive Assessment
Cognitive impairment can be associated with LLD and may be due to the underlying depression or represent a comorbid neurocognitive disorder. Furthermore, the burden of medical illness as well as cerebrovascular and cardiovascular risk factors have been linked to executive dysfunction and reduced processing speed in individuals with LDD [27,28]. Distinguishing between these can be challenging; however, chronology of symptom onset is often helpful. Depression is more likely the etiology of cognitive impairment when depressive symptoms precede onset of cognitive deficits. This type of cognitive impairment is termed dementia syndrome of depression and may improve with treatment of depression [5]. Some patients may progress to develop major cognitive decline, and it remains unclear whether LLD represents a risk factor or prodrome to developing a major neurocognitive disorder [29]. On the other hand, if depression develops later in the course of cognitive decline, there may be an underlying neurocognitive disorder [17]. Up to 20% of individuals with major neurocognitive disorder due to Alzheimer’s disease also have major depression [11]. For these reasons, concomitant assessment of cognition is essential to the evaluation of the older adult presenting with depressive symptoms [30]. Cognitive domains that may be affected include learning and memory, language, attention, perceptual motor abilities, social cognition, and executive function [4]. Many of these domains can be assessed during the mental status examination, with brief cognitive screening tools, or with formal neuropsychological testing.
While there are numerous cognitive screening tools, some commonly used, brief tools include the Mini-Cog, the Folstein Mini-Mental State Exam (MMSE), and the Montreal Cognitive Assessment (MoCA). The Mini-Cog consists of a 3-item registration, delayed recall, and clock drawing test and has several advantages over other screening tools. It is a brief test (taking approximately 3 minutes to administer) with good sensitivity and specificity of 80% or greater. Compared with other cognitive screening tools, it is less influenced by level of education, language, or cultural background [31–33]. The MMSE is a longer screening tool consisting of 19 items and requires about 10 minutes to administer. Unlike the Mini-Cog, performance on the MMSE can be affected by level of education and cultural background. However, the MMSE can be administered serially to monitor changes in cognition over time [34,35]. The MoCA is a 10-minute cognitive screening tool first developed to detect mild cognitive impairment (MCI) [36]. The MoCA consists of 7 subscore sections covering visuospatial/executive function, naming, memory (delayed recall), attention, language, abstraction, and orientation. The total score is 30, and 1 point is added to the score if the testing subject has less than high school/12 years of education. The MoCA has demonstrated better sensitivity than the MMSE for the detection of MCI [36]. Elderly patients with depression often perform poorly on these cognitive screening tests due to apathy and poor effort.
Functional Assessment
The diagnosis of LLD requires that symptoms cause significant distress or interfere with functioning. A functional assessment is especially important in the evaluation of the older adult in that it allows clinicians to determine an individual’s ability to live independently and attend to daily needs. Basic activities of daily living (ADLs) include bathing, dressing, grooming, toileting, and self-transferring. Instrumental activities of daily living (IADLs) include more complex daily activities such as preparing meals, administering medications, driving, managing finances, and using simple electronics such as the telephone or remote control [26]. Impairment in IADLs is associated with increased depression severity. Conversely, the severity of depressive symptoms along with associated cognitive impairment predicts IADL impairment [37]. The Philadelphia Multilevel Assessment Instrument is a tool that can aid in the assessment of ADLs and IADLs and has been utilized in studies examining disability in depressed elderly patients [37,38]. Other available scales to quantify functional status include OARS Physical Activities of Daily Living, OARS Instrumental Activities of Daily Living Scale, and Direct Assessment of Functional Status Scale [26].
Suicide Assessment
Assessment for suicidality is an integral part of all psychiatric evaluations and is especially important in the evaluation of the depressed older adult. According to the Centers for Disease Control and Prevention, the suicide rate for individuals age 65 and older is 16.6 per 100,000, a figure that is comparable to that for individuals 18–64 years of age [39]. Non-Hispanic Caucasian males age 85 and older have the highest rate of completed suicide (56.5 per 100,000), underscoring the importance of a thorough suicide assessment [39]. Suicidality can range from passive thoughts of death and wishing that one were not alive, to active thoughts of self-harm with plan and intent. A Canadian study found 2% of community-dwelling adults age 55 and older had suicidal thoughts over a 12-month period and, of these, 28% had major depression [40]. A suicide assessment begins with inquiring about the presence of suicidal thoughts, plans, and intent. The 3 most frequently used methods of completed suicide in the elderly are firearms (28%), hanging (24%) and poisoning (21%) [41]. Access to weapons or other lethal means of self-harm such has hoarding of medications should be ascertained.
A complete suicide assessment requires attention to suicide risk factors, protective factors, and warning signs of impending suicide. Risk factors for suicide in the older adult include mood disorders, chronic medical illnesses and associated functional impairment, chronic pain, and psychosocial factors such as social isolation [42]. Mood disorders are present in 54% to 87% of cases of completed suicide, with major depression being the most common [42]. Chronic medical illness and pain can result in functional impairment leading to feelings of excessive guilt or being a burden to loved ones. Protective factors such as social connectedness, spirituality, religious beliefs, and cultural attitudes against suicide may serve as buffers against these risk factors [43]. Warning signs of impending suicide may indicate preparations for suicide and include feelings of hopelessness or lack of purpose, feeling trapped, talking about death, threatening suicide, agitation, social withdrawal, increased substance use and reckless behavior. Warning signs should prompt action to ensure the safety of the individual [44,45].
Physical Examination, Laboratory Studies, and Neuroimaging
Evaluation of LLD is not complete without a physical examination and ancillary studies to identify underlying medical conditions possibly contributing to or mimicking depressive symptoms. Routine laboratory studies include complete blood count, complete metabolic panel, thyroid studies, and urine drug screen. Signs and symptoms of underlying medical conditions may necessitate further laboratory studies [46]. Neuroimaging may reveal signs of cerebrovascular disease which can predispose, precipitate, or perpetuate depression in older adults [47].
Treatment
Treatment of LLD can take many forms and occur in various settings. Geriatric psychiatrists have expertise in the assessment and treatment of mental illness in the elderly. Workforce estimates for 2010 revealed 1 geriatric psychiatrist per 10,000 adults age 75 and over. This figure is estimated to decrease to 0.5 per 10,000 by the year 2030, underscoring the importance of increasing the knowledge base of clinicians across specialties who provide care to the depressed elderly [48]. The primary care setting is often the locus of care for depression in older adults; however, studies suggest that patients are often left untreated or undertreated [49]. Collaborative care models whereby mental health care is integrated into primary care have been shown to improve outcomes. The Prevention of Suicide in Primary Care Elderly: Collaborative Trial (PROSPECT) study found that use of care managers to assist primary care providers in identification of depression, offer algorithm-based treatment recommendations, monitor symptoms and medication side effects, and provide follow-up yielded improvement in outcomes. Patients in the intervention group were more likely to receive pharmacotherapy or psychotherapy, achieve remission, and showed greater decline in suicidal ideation [50]. Similar results were found in the Improving Mood-Promoting Access to Collaborative Treatment (IMPACT) study in which intervention patients treated under a collaborative care model showed lower depression severity, less functional impairment, and greater reduction in depressive symptoms [25].
Just as a collaborative care model can lead to improved outcomes, the overall strategy of treating depression must be multifaceted. The biopsychosocial model of disease first described in the 1970s emphasizes biological and psychosocial determinants of illness that must be addressed when treatment is considered [51]. This includes nonmodifiable biological factors such as age, gender, and genetic predisposition that may affect treatment options, as well as modifiable biological factors such as comorbid medical illness, medications, or substance use disorders. Psychological factors that can affect depressive symptoms include coping skills and defense mechanisms in the face of stressful life events. Social factors including the role of culture, environment, and family dynamics in disease presentation must be considered as well [52].
Pharmacologic Treatment of LLD
The primary pharmacologic treatment for depression is antidepressants. Treatment consists of 3 phases—acute, continuation, and maintenance. In the acute phase, the goal is remission of current symptoms and restoration of function. The continuation phase, extending up to 6 months after remission, aims to prevent relapse back into a depressive episode. Maintenance therapy is geared at preventing recurrence of future depressive episodes [53]. Studies have found a 50% risk of relapse after 1 episode of depression and 80% after 2 episodes. Up to 20% will develop chronic symptoms. For this reason, maintenance therapy is often necessary for recurrent depression [54].
While cognitive impairment may affect antidepressant efficacy, age does not appear to be a determinant. Gildengers et al examined antidepressant response in young, middle, and older-old patients and found no significant difference in response rates [59]. Early onset versus late onset of first depressive episode also does not predict antidepressant response in patients age 55 and over [60]. There is scant evidence for efficacy of antidepressants in depressed patients with neurocognitive disorders. A 2002 Cochrane review with 4 studies in the meta-analysis (n = 137) concluded that there was weak support for antidepressant efficacy in this population [61]. A 2011 meta-analysis with 330 participants also yielded inconclusive results [62]. The paucity of evidence for antidepressant efficacy in depressed patients with neurocognitive disorders should prompt careful consideration of potential benefits versus adverse effects.
Antidepressants are generally well tolerated in older adults. Side effects vary by medication and contribute to discontinuation in up to 25% of new users (versus 22% for new users who discontinue for reasons other than side effects) [63]. Potential adverse effects shared by most SSRIs and SNRIs include GI disturbance (nausea, diarrhea or constipation), sexual dysfunction, headache, and sleep disturbance [64,65]. In addition, abrupt discontinuation can precipitate serotonin withdrawal syndrome characterized by sensory disturbance (paresthesia, tremor, and irritability) as well as headache, lightheadedness, diaphoresis, insomnia, and agitation. Other medication-specific side effects include risk of seizure with bupropion and sedation with mirtazapine [65].
Despite superiority of antidepressants to placebo in treating depression, up to one-third of patients may not respond to a trial of antidepressants. Sequential treatment protocols such as switching to a different antidepressant or augmentation can increase the proportion of antidepressant responders [66–68]. Studies have found particularly favorable response to augmentation with lithium, with one study achieving a 33% remission rate in treatment- resistant geriatric depression [67,69]. Other pharmacologic augmentation strategies include the addition of mood stabilizers such as lamotrigine, antipsychotics (aripiprazole, olanzapine, quetiapine, and risperidone), and psychostimulants [70–73]. Electroconvulsive therapy (ECT) is a nonpharmacologic option for treatment-resistant depression that will be reviewed later.
Psychotherapeutic and Psychosocial Interventions
Psychotherapeutic interventions have demonstrated efficacy in the treatment of geriatric depression, including but not limited to cognitive behavioral therapy (CBT), interpersonal therapy (IPT), problem-solving therapy (PST), reminiscence and life review, and brief psychodynamic psychotherapy [74]. Some older adults may prefer psychotherapy to pharmacologic treatment (57% vs. 43%) [75]. Potential benefits of psychotherapy include ability to directly address psychosocial stressors that may precipitate or perpetuate depressive symptoms. In addition, psychotherapy is associated with few to no side effects and avoids drug interactions. Barriers to employing psychotherapy may include cost and access to trained psychotherapists [76]. Efficacy of several psychotherapeutic approaches in the care of older depressed adults has been examined. CBT, brief psychodynamic psychotherapy, and IPT will be briefly reviewed here.
CBT. Cognitive therapy was first described by Aaron Beck in the 1960s [77]. It is a highly structured therapy built on the premise that beliefs and assumptions an individual holds can influence emotions and behavior. CBT aims to identify maladaptive belief systems, test the validity of these cognitive distortions, and help individuals formulate more realistic cognitions [78]. Symptom improvement results from addressing these cognitive aspects as well as integration of behavioral activation and skills training to overcome maladaptive behavioral patterns [78]. CBT approaches have been applied to older adults with depression and results show acceptability [79] and efficacy in this population [80–82]. A 2008 Cochrane review (n = 153) found CBT to be superior to waitlist controls [82].
Brief psychodynamic psychotherapy. Brief psychodynamic psychotherapy, unlike highly structured CBT, aims to alter behavior by examining how past experiences and unresolved conflicts influence current emotions and behavior. While studies on application to the treatment of geriatric depression are scarce, limited data demonstrate efficacy in treating geriatric depression [81] and no significant difference in outcomes when compared to CBT [82].
IPT. Like CBT, IPT is a structured time-limited psychotherapeutic treatment approach first developed in the late 1960s by Klerman and Weissman [83]. IPT focuses on the impact of interpersonal relationships on depressive symptoms and examines 4 domains: interpersonal conflict, interpersonal deficits, role transitions, and grief [74].
Studies have shown efficacy of IPT in reducing depressive symptoms in the elderly when compared to usual care [84]. Reynolds et al found IPT combined with nortriptyline (a tricyclic antidepressant) to be superior to either nortriptyline alone or IPT alone in preventing recurrent depressive episodes [85]. Interestingly, a similar study investigating the efficacy of IPT in combination with paroxetine (an SSRI) failed to show added benefit of IPT in preventing recurrence, suggesting that further studies are needed [86].
Psychosocial interventions are integral in the care of the elderly depressed patient. Studies have shown positive benefits of aerobic exercise on depressive symptoms [87]. Yoga, Tai Chi, and other mindfulness-based exercises can increase sense of emotional and physical wellbeing [88–90]. Spirituality, religious beliefs, and involvement with a faith group may be protective against development of mental illness while at the same time provide avenues for increased social connectedness [91]. These and other avenues for socialization should be encouraged as part of the treatment plan for older depressed patients [92]
Electroconvulsive Therapy
ECT is indicated for the treatment of mood and psychotic disorders and has demonstrated efficacy in the treatment of severe depression [93]. It is typically initiated when patients fail to respond to pharmacotherapy and psychotherapy. Circumstances in which ECT can be considered first-line treatment include situations that require a rapid response (severe inanition, weight loss, or suicidality), situations where risks of ECT are lower than that of alternative treatments, previous positive response to ECT, or strong patient preference [94]. ECT is performed under general anesthesia and involves the induction of a generalized tonic-clonic seizure, which is theorized to enhance serotonergic, noradrenergic, and dopaminergic neurotransmission. A typical course of ECT involves treatments 3 times a week for an average of 6 to 12 treatments in total [95]. Elderly patients and those suffering from severe depression with psychotic features respond more robustly to ECT [93,96]. Estimated remission rates after an ECT series have been higher than 80% [93], making this modality the most effective treatment for severe depression to date.
Conclusion
As the population continues to age, clinicians are increasingly likely to encounter patients with late-life depression. A thorough evaluation includes not only assessment of depressive symptoms, but also cognitive, functional, and suicide assessment. Treatment options include pharmaco-therapy, psychotherapy, and in some cases electroconvulsive therapy. Utilization of assessment and treatment nuances unique to the geriatric population, with a multidisciplinary and collaborative approach involving primary care, mental health, and other ancillary providers, will serve to ultimately enhance patient care.
Corresponding author: Corresponding author: Juliet Glover, MD, Dept. of Neuropsychiatry and Behavioral Science, Univ. of South Carolina School of Medicine, 15 Medical Park, Suite 301, Columbia, SC 29203, [email protected].
Financial disclosures: None reported.
Author contributions: conception and design, JAG, SS; drafting of article, JAG, SS; critical revision of the article, JAG, SS.
1. Vincent GK, Velkoff VA. The next four decades: the older population in the United States: 2010 to 2050. US Census Bureau: May 2010.
2. Koenig HG, Blazer DG. Epidemiology of geriatric affective disorders. Clinc Geriatr Med 1992; 8:235–51.
3. Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global gurden of disease study 2010. PLoS Med 2013;10:e1001547.
4 .American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
5 .Ellison JM, Kyomen HH, Harper DG. Depression in later life: an overview with treatment recommendations. Psychiatr Clin North Am 2012;35:203–29.
6. Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry 2005;62:1097–106.
7. Steffens DC, Skoog I, Norton MC, et al. Prevalence of depression and its treatment in an elderly population: the Cache County study. Arch Gen Psychiatry 2000;57:601–7.
8. Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003;289:3095–105.
9. McKinney BC, Sibille E. The age-by-disease interaction hypothesis of late-life depression. Am J Geriatr Psychiatry 2013;21:418–32.
10. Djernes JK. Prevalence and predictors of depression in populations of elderly: a review. Acta Psychiatr Scand 2006;113:372–87.
11. Blazer DG. Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci 2003;58:249–65.
12. Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry 2003;160:1147–56.
13. Ritchie K. Late-life depression. European Psychiatry 2014;29:577.
14. Hegeman JM, de Waal MW, Comijs HC, et al. Depression in later life: a more somatic presentation? J Affect Disord 2015;170:196–202.
15. Lackamp J, Schlachet R, Sajatovic M. Assessment and management of major depressive disorder in older adults. Psychiatria Danubina 2016;28(Suppl 1):95–98.
16. Morichi V, Dell’Aquila G, Trotta F, et al. Diagnosing and treating depression in older and oldest old. Curr Pharm Des 2015;21:1690–8.
17. Fiske AJ, Wetherell JL, Gatz M. Depression in older adults. Annu Rev Clin Psychol 2009;5:363–89.
18. Balsis S, Cully JA. Comparing depression diagnostic symptoms across younger and older adults. Aging Ment Health 2008;12:800–6.
19. Hegeman JM, Kok RM, van der Mast RC, Giltay EJ. Phenomenology of depression in older compared with younger adults: meta-analysis. Br J Psychiatry 2012;200:275–81.
20. Mitchell AJ, Bird V, Rizzo M, Meader N. Which version of the geriatric depression scale is most useful in medical settings and nursing homes? Diagnostic validity meta-analysis. Am J Geriatr Psychiatry 2010;18:1066–77.
21. Blazer DG. The psychiatric interview of older adults. In: Blazer D, Steffens D, Busse E, editors. Textbook of geriatric psychiatry. 3rd ed. Arlington, VA: American Psychiatric Publishing; 2004.
22. Spitzer RL, Kroenke K, Williams JW. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999;282:1737–44.
23. Richardson TM, He H, Podgorski C, et al. Screening for depression in aging services clients. Am J Geriatr Psychiatry 2010;18:1116–23.
24. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev 2012 Oct 17.
25. Unutzer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting a randomized controlled trial. JAMA 2002;288:2836–45.
26. Silver I, Herrmann N. Comprehensive psychiatric evaluation. In: Sadavoy J, Jarvik L, Grossberg G, Meyers B, editors. Comprehensive textbook of geriatric psychiatry. 3rd ed. New York: W.W. Norton; 2004.
27. Rapp MA, Dahlman K, Sano M, et al. Neuropsychological differences between late-onset and recurrent geriatric major depression. Am J Psychiatry 2005;162:691–8.
28. Sheline YI, Barch DM, Garcia K, et al. Cognitive function in late life depression: relationships to depression severity, cerebrovascular risk factors and processing speed. Biol Psychiatry 2006; 60:58–65.
29. Barnes DE, Yaffe K, Byers AL, et al. Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry 2012;69:493–8.
30. Morimoto SS, Kanellopoulos D, Manning KJ, Alexopoulos GS. Diagnosis and treatment of depression and cognitive impairment in late life. Ann NY Acad Sci 2015;1345:36–46.
31. Borson S, Scanlan J, Brush M, et al. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry 2000;15:1021–7.
32. Brodaty H, Low LF, Gibson L, Burns K. What is the best dementia screening instrument for general practitioners to use? Am J Geriatr Psychiatry 2006;14:391–400.
33. Ismail Z, Rajji TK, Shulman KI. Brief cognitive screening instruments: an update. Int J Geriatr Psychiatry 2010;25:111–20.
34. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98.
35. Vertesi A, Lever JA, Molloy DW, et al. Standardized Mini-Mental State Examination. Use and interpretation. Can Fam Physician 2001;47: 2018–23.
36. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–9.
37. Kiosses DN, Alexopoulos GS. IADL functions, cognitive deficits, and severity of depression: a preliminary study. Am J Geriatr Psychiatry 2005;13:244–9.
38. Alexopoulos GS, Vrontou C, Kakuma T, et al. Disability in geriatric depression. Am J Psychiatry 1996;153:877–85.
39. Centers for Disease Control and Prevention. National Center for Injury Prevention and Control. Web-Based Injury Statistics Query and Reporting System (WISQARS). Accessed 9 Feb 2016 at http://webappa.cdc.gov/sasweb/ncipc/dataRestriction_inj.html.
40. Corna LM, Cairney J, Streiner DL. Suicide ideation in older adults: relationship to mental health problems and service use. Gerontologist 2010;50:785–97.
41. Juurlink DN, Herrmann N, Szalai JP, et al. Medical illness and the risk of suicide in the elderly. Arch Intern Med 2004;164:1179–84.
42. Van Orden K, Conwell Y. Suicides in late life. Curr Psychiatry Rep 2011;13:234–41.
43. Conwell Y, Van Orden K, Caine ED. Suicide in older adults. Psychiatr Clin North Am 2011;34:451–68, ix.
44. Rudd MD, Berman AL, Joiner TE, et al. Warning signs for suicide: theory, research, and clinical applications. Suicide Life Threat Behav 2006;36:255–62.
45. Know the warning signs of suicide. American Association of Suicidology. Accessed 9 Feb 2016 at www.suicidology.org/resources/warning-signs.
46. Taylor W, Doraiswamy P. Use of the laboratory in the diagnostic workup of older adults. In: Blazer D, Steffen D, Busse E, editors. Textbook of geriatric psychiatry. 3rd ed. Arlington, VA: American Psychiatric Publishing; 2004.
47. Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry 1997;54:915–22.
48. ADGAP Status of Geriatrics Workforce Study. Accessed 26 Dec 2016 at www.americangeriatrics.org/files/documents/gwps/Table%201_29.pdf.
49. Alexopoulos G. Late-life mood disorders. In: Sadavoy J, Jarvik L, Grossberg G, Meyers B, editors. Comprehensive textbook of geriatric psychiatry. 3rd ed. New York: W.W. Norton; 2004.
50. Alexopoulos GS, Reynolds CF, Bruce ML, et al. Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. Am J Psychiatry 2009;166:882–90.
51. Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977;196:129–36.
52. Schotte CKW, Van den Bossche B, Doncker DD, et al. A biopsychosocial model as a guide for psychoeducation and treatment of depression. Depression Anxiety 2006;23:312–24.
53. Kupfer DJ, Frank E. The interaction of drug-and psychotherapy in the long-term treatment of depression. J Affect Disord 2001;62:131–7.
54. Katon W, Rutter C, Ludman EJ, et al. A randomized trial of relpase prevention of depression in primary care. Arch Gen Psychiatry 2001;58:241–7.
55. Kok RM, Nolen WA, Heeren TJ. Efficacy of treatment in older depressed patients: a systematic review and meta-analysis of double-blind randomized controlled trials with antidepressants. J Affect Disord 2012;141:103–15.
56. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62.
57. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382–9.
58. Mukai Y, Tampi RR. Treatment of depression in the elderly: a review of the recent literature on the efficacy of single- versus dual-action antidepressants. Clin Ther 2009;31:945–61.
59. Gildengers AG, Houck PR, Mulsant BH, et al. Course and rate of antidepressant response in the very old. J Affect Disord 2002;69:177–84.
60. Kozel FA, Trivedi MH, Wisniewski SR, et al. Treatment outcomes for older depressed patients with earlier versus late onset of first depressive episode. Am J Geriatr Psychiatry 2008;16:58–64.
61. Bains J, Birks J, Dening T. Antidepressants for treating depression in dementia. Cochrane Databse Syst Rev 2002;(4):CD003944.
62. Nelson JC, Devanand DP. A systematic review and meta-analysis of placebo-controlled antidepressant studies in people with depression and dementia. J Am Geriatr Soc 2011;59:577–85.
63. Mark TL, Joish VN, Hay JW, et al. Antidepressant use in geriatric populations: the burden of side effects and interactions and their impact on adherence and costs. Am J Geriatr Psychiatry 2011;19:211–21.
64. Frank C. Pharmacologic treatment of depression in the elderly. Can Fam Physician 2014;60:121–6.
65. Kennedy GJ, Marcus P. Use of antidepressants in older patients with co-morbid medical conditions: guidance from studies of depression in somatic illness. Drugs Aging 2005;22:273–87.
66. Sackheim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-tern outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006;163:1905–17.
67. Kok RM, Nolen WA, Hereen TJ. Outcome of late-life depression after 3 years of sequential treatment. Acta Psychiatr Scand 2009;119:274–81.
68. Whyte EM, Basinski J, Farhi P, et al. Geriatric depression treatment in nonresponders to selective serotonin reuptake inhibitors. J Clin Psychiatry 2004;65:1634–41.
69. Kok RM, Vink D, Hereen TJ, Nolen WA. Lithium augmentation compared with phenelzine in treatment-resistant depression in the elderly; an open, randomized, controlled trial. J Clin Psychiatry 2006;68:1177–85.
70. Kok RM. What is the role of medications in late life depression? Psychiatr Clin North Am 2003;36:597–605.
71. Wen XJ, Wang LM, Liu ZL,. Meta-analysis on the efficacy and tolerability of the augmentation of antidepressants with atypical antipsychotics in patients with major depressive disorder. Braz J Med Biol Res 2014;47:605–16.
72. Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised double-blind, placebo-controlled trial. Lancet 2015;386:2404–12.
73. Alexopoulos GS, Canuso CM, Gharabawi GM, et al. Placebo-controlled study of relapse prevention with risperidone augmentation in older patients with resistant depression. Am J Geriatr Psychiatry 2008;16:21–30.
74. Francis JL, Kumar A. Psychological treatment of late-life depression. Psychiatr Clin North Am 2013;36:561–75.
75. Gum AM, Arean PA, Hunkeler E, et al. Depression treatment preferences in older primary care patients. Gerontologist 2006;46:14–22.
76. Pinquart M, Duberstein PR, Lyness JM. Treatments for late-life depressive conditions: a meta-analytic comparison of pharmacotherapy and psychotherapy. Am J Psychiatry 2006;163:1493–501.
77. Beck Institute for Cognitive Behavior Therapy. Accessed 28 Dec 2016 at www.beckinstitute.org
78. Beck AT. Cognitive therapy: nature and relation to behavior therapy. Behav Ther 1970;1:184–200.
79. Landrevile P, Landry J, Baillargeon L, et al. Older adults’ acceptance of psychological and pharmacological treatments for depression. J Gerontology B Psychol Sci Soc Sci 2001;56:P285–91.
80. Thompson LW, Gallagher D, Breckenridge JS. Comparitive effectiveness of psychotherapies for depressed elders. J Consult Clin Psychol 1987;55:385–90.
81. Gallagher-Thompson D, Steffen AM. Comparative effects of cognitive-behavioral and brief psychodynamic psychotherapies for depressed family caregivers. J Consult Clin Psychol 1994;62:543–9.
82. Wilson KC, Mottram PG, Vassilas C. Psychotherapeutic treatments for older depressed people. Cochrane Database Syst Rev 2008;(1):CD004853
83. Markowitz JC, Weissman MM. Interpersonal psychotherapy: past, present and future. Clin Psychol Psychother 2012;19:99–105.
84. Van Schaik A, van Marwijk H, Ader H, et al. Interpersonal psychotherapy for elderly patients in primary care. Am J Geriatr Psychiatry 2006;14:777–86.
85. Reynolds CF 3rd, Frank E, Perel JM, et al. Nortriptyline and interpersonal psychotherapy as maintenance therapies for recurrent major depression: a randomized controlled trial in patients older than 59 years. JAMA 1999;281:39–45.
86. Reynolds CF 3rd, Dew MA, Pollock BG, et al. Maintenance treatment of major depression in old age. N Engl J Med 2006;354:1130–8.
87. Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med 1999;159:2349–56.
88. Krishnamurthy MN, Telles S. Assessing depression following two ancient Indian interventions: effects of yoga and ayurveda on older adults in a residential home. J Gerontol Nurs 2007;33:17–23.
89. Wang C, Bannuru R, Ramel J, et al. Tai Chi on psychological well-being: systematic review and meta-analysis. BMC Complement Altern Med 2010;10:23.
90. Cho KL. Effect of tai chi on depressive symptoms amongst Chinese older patients with depressive disorders: a randomized clinical trial. Med Sport Sci 2008;52:146–54.
91. Moritz S, Quan H, Rickhi B, et al. A home study-based spirituality education program decreases emotional distress and increases quality of life- a randomized, controlled trial. Altern Ther Health Med 2006;12:26–35.
92. Nyer M, Doorley J, Durham K, et al. What is the role of alternative treatments in late-life depression? Psychiatr Clin North Am 2013;36:577–96.
93. Petrides G, Fink M, Husain MM, et al. ECT remission rates in psychotic versus nonpsychotic depressed patients: a report from CORE. J ECT 2001;17:244–53.
94. Mankad MV, Beyer JL, Weiner RD, Krystal AD. Clinical manual of electroconvulsive therapy. American Psychiatric Publishing; 2010.
95. Kellner CH, Greenberg RM, Murrough JW, et al. ECT in treatment-resistant depression. Am J Psychiatry 2012;169:1238–44.
96. Flint AJ, Gagnon N. Effective use of electroconvulsive therapy in late-life depression. Can J Psychiatry 2002;47:734–41.
From the Department of Neuropsychiatry and Behavioral Sceince, University of South Carolina School of Medicine, Columbia, SC.
Abstract
- Objective: To review the identification, clinical assessment and treatment of patients with late-life depression.
- Methods: Review of the literature.
- Results: Depressive symptoms are present in up to 1 in 4 older adults. Comprehensive evaluation of depressive symptoms in this population often requires a multidisciplinary and collaborative approach between primary care, mental health, and other ancillary providers. Key aspects include a detailed history, physical and mental status examinations, cognitive and functional status assessment, and suicide risk assessment. Treatment options include anti-depressants, psychotherapy, and electroconvulsive therapy.
- Conclusion: A systematic approach to evaluating depressive symptoms in the elderly can enhance timely recognition and treatment.
Key words: Late-life depression; clinical assessment; antidepressants; psychotherapy; electroconvulsive therapy.
The U.S. population is aging, and with this comes the potential for increased health care needs. In 2014, there were over 46 million Americans age 65 and over (14.5% of the U.S. population). This number is projected to increase to 88 million by the year 2050 [1]. One in 4 older adults suffers with depressive symptoms that cause distress and functional impairment [2]. The World Health Organization Global Burden of Disease Study found depressive disorders to be the leading cause of disability-adjusted life years (DALYs) and the second leading cause of years lived with disability (YLDs). The burden of disease due to depressive disorders increased by 37.5% between 1990 and 2010, and 10.4% was attributable to aging [3]. These figures underscore the importance of accurate assessment and treatment of depression in the elderly. In this article, we review the identification, clinical assessment, and treatment of patients with late-life depression.
Diagnostic Criteria
Prevalence
It is estimated that 1% to 4% of community-dwelling adults age 65 and older suffer from MDD, with women more likely to be affected than men (prevalence of 4.4% vs. 2.7) [2,5–7]. This estimate is low compared with lifetime prevalence of almost 20% in the general adult population [8]. However, when depressive symptoms that do not meet criteria for MDD are considered, prevalence rates increase up to 25% [2,9]. These estimates also vary by clinical setting, with the highest rates (up to 40%) among elderly patients in long-term care facilities [10,11]. While individuals with subsyndromal depression may experience fewer symptoms than those with MDD, clinically significant distress persists, impacting health and functional status. Depression is associated with overall poor social or occupational functioning, cognitive decline, increased health care utilization and cost, increased morbidity and mortality from medical illness, and increased suicide mortality [5,9,10,12].
Identifying LLD
Accurate identification of LLD also requires recognition of the differences in the presentation of LLD compared with onset in earlier life. Depression in younger adults is often marked by depressed mood and loss of interest [18]. In contrast, older adults may present with increased anger or irritability [5]. Younger adults are more likely to report suicidal thoughts while older patients report feelings of hopelessness and thoughts of death [18]. LLD is often characterized by increased somatic complaints, hypochondriasis, or pain [5,18,19]. Another major difference lies in the presentation of cognitive difficulties. Younger patients typically complain of poor concentration or indecisiveness. Geriatric patients may present with cognitive changes including objective findings of slower processing speed and executive dysfunction on neuropsychological testing [17].
Depression rating scales may aid in identification of LLD. They are not a substitute for clinical diagnosis but can be useful as screening tools. Two commonly utilized depression rating scales are the Geriatric Depression Scale (GDS) and the Patient Health Questionnaire-9 (PHQ-9). GDS is a 30-item instrument developed specifically for older adults. Shorter 15-item, 5-item, and 4-item versions exist. The scale utilizes a Yes/No format and can be self- or clinician-administered [20]. One advantage of the GDS lies in its focus on psychological and cognitive aspects of depression rather than neurovegetative symptoms that may overlap with medical illnesses common in older adults [21]. The PHQ-9 is a 9-item self- or clinician-administered screening tool designed for use in primary care settings and has also been validated in geriatric populations [22,23]. The 9 items on this scale correspond to the DSM-5 criteria for major depression. A shorter 2-item version (PHQ-2) has also been validated, and a positive screen on this test should prompt administration of the full-length version. Both versions have approximately 80% sensitivity and specificity in detecting depression. An added advantage of PHQ-9 over GDS is that it can be useful in monitoring treatment response over time [22,23]
Comprehensive Assessment of LLD
The comprehensive assessment of patients with LLD can be carried out by health professionals in both mental health or primary care settings. In a multidisciplinary approach, psychiatrists and mental health professionals have collaborated with primary care providers using depression care managers with successful outcomes in managing depression in older adults [24,25]. Complete evaluation of a patient with suspected LLD begins with a history and physical and mental status examination. Other essential components of the evaluation include assessment of cognition, functional status, and suicide risk. Laboratory and neuroimaging studies may be necessary as well. Due to the comprehensive nature of this assessment, a multidisciplinary approach with collaboration between primary care, psychiatry, psychology, and ancillary services such as social work may be necessary. Multiple patient interactions may be required to complete a thorough evaluation.
History and Mental Status Examination
As with many other psychiatric illnesses, LLD is a clinical diagnosis. A careful history should be obtained initially utilizing open-ended questions. This should be followed by more directed questions as indicated to elicit the presence of depressive symptoms. The history should be obtained from the patient. A relevant collateral informant can be invaluable in the assessment, especially in cases where there is a comorbid neurocognitive disorder. However, the patient’s informed consent must be obtained prior to obtaining collateral information whenever possible. Psychosocial stressors that may have precipitated or may be perpetuating symptoms should be explored. Such stressors may include recent changes in living situation, loss of social support, recent deaths, or financial difficulties. Biological precipitants also need to be explored including presence of physical illness, depressogenic medications, and comorbid alcohol or other substance use. The patient’s past psychiatric history, psychiatric hospitalizations, and past medication trials should be ascertained. Any family history of depression, other psychiatric disorders, substance use disorders, and suicide attempts should be documented. A full mental status exam including cognitive assessment should be completed [21,26].
Cognitive Assessment
Cognitive impairment can be associated with LLD and may be due to the underlying depression or represent a comorbid neurocognitive disorder. Furthermore, the burden of medical illness as well as cerebrovascular and cardiovascular risk factors have been linked to executive dysfunction and reduced processing speed in individuals with LDD [27,28]. Distinguishing between these can be challenging; however, chronology of symptom onset is often helpful. Depression is more likely the etiology of cognitive impairment when depressive symptoms precede onset of cognitive deficits. This type of cognitive impairment is termed dementia syndrome of depression and may improve with treatment of depression [5]. Some patients may progress to develop major cognitive decline, and it remains unclear whether LLD represents a risk factor or prodrome to developing a major neurocognitive disorder [29]. On the other hand, if depression develops later in the course of cognitive decline, there may be an underlying neurocognitive disorder [17]. Up to 20% of individuals with major neurocognitive disorder due to Alzheimer’s disease also have major depression [11]. For these reasons, concomitant assessment of cognition is essential to the evaluation of the older adult presenting with depressive symptoms [30]. Cognitive domains that may be affected include learning and memory, language, attention, perceptual motor abilities, social cognition, and executive function [4]. Many of these domains can be assessed during the mental status examination, with brief cognitive screening tools, or with formal neuropsychological testing.
While there are numerous cognitive screening tools, some commonly used, brief tools include the Mini-Cog, the Folstein Mini-Mental State Exam (MMSE), and the Montreal Cognitive Assessment (MoCA). The Mini-Cog consists of a 3-item registration, delayed recall, and clock drawing test and has several advantages over other screening tools. It is a brief test (taking approximately 3 minutes to administer) with good sensitivity and specificity of 80% or greater. Compared with other cognitive screening tools, it is less influenced by level of education, language, or cultural background [31–33]. The MMSE is a longer screening tool consisting of 19 items and requires about 10 minutes to administer. Unlike the Mini-Cog, performance on the MMSE can be affected by level of education and cultural background. However, the MMSE can be administered serially to monitor changes in cognition over time [34,35]. The MoCA is a 10-minute cognitive screening tool first developed to detect mild cognitive impairment (MCI) [36]. The MoCA consists of 7 subscore sections covering visuospatial/executive function, naming, memory (delayed recall), attention, language, abstraction, and orientation. The total score is 30, and 1 point is added to the score if the testing subject has less than high school/12 years of education. The MoCA has demonstrated better sensitivity than the MMSE for the detection of MCI [36]. Elderly patients with depression often perform poorly on these cognitive screening tests due to apathy and poor effort.
Functional Assessment
The diagnosis of LLD requires that symptoms cause significant distress or interfere with functioning. A functional assessment is especially important in the evaluation of the older adult in that it allows clinicians to determine an individual’s ability to live independently and attend to daily needs. Basic activities of daily living (ADLs) include bathing, dressing, grooming, toileting, and self-transferring. Instrumental activities of daily living (IADLs) include more complex daily activities such as preparing meals, administering medications, driving, managing finances, and using simple electronics such as the telephone or remote control [26]. Impairment in IADLs is associated with increased depression severity. Conversely, the severity of depressive symptoms along with associated cognitive impairment predicts IADL impairment [37]. The Philadelphia Multilevel Assessment Instrument is a tool that can aid in the assessment of ADLs and IADLs and has been utilized in studies examining disability in depressed elderly patients [37,38]. Other available scales to quantify functional status include OARS Physical Activities of Daily Living, OARS Instrumental Activities of Daily Living Scale, and Direct Assessment of Functional Status Scale [26].
Suicide Assessment
Assessment for suicidality is an integral part of all psychiatric evaluations and is especially important in the evaluation of the depressed older adult. According to the Centers for Disease Control and Prevention, the suicide rate for individuals age 65 and older is 16.6 per 100,000, a figure that is comparable to that for individuals 18–64 years of age [39]. Non-Hispanic Caucasian males age 85 and older have the highest rate of completed suicide (56.5 per 100,000), underscoring the importance of a thorough suicide assessment [39]. Suicidality can range from passive thoughts of death and wishing that one were not alive, to active thoughts of self-harm with plan and intent. A Canadian study found 2% of community-dwelling adults age 55 and older had suicidal thoughts over a 12-month period and, of these, 28% had major depression [40]. A suicide assessment begins with inquiring about the presence of suicidal thoughts, plans, and intent. The 3 most frequently used methods of completed suicide in the elderly are firearms (28%), hanging (24%) and poisoning (21%) [41]. Access to weapons or other lethal means of self-harm such has hoarding of medications should be ascertained.
A complete suicide assessment requires attention to suicide risk factors, protective factors, and warning signs of impending suicide. Risk factors for suicide in the older adult include mood disorders, chronic medical illnesses and associated functional impairment, chronic pain, and psychosocial factors such as social isolation [42]. Mood disorders are present in 54% to 87% of cases of completed suicide, with major depression being the most common [42]. Chronic medical illness and pain can result in functional impairment leading to feelings of excessive guilt or being a burden to loved ones. Protective factors such as social connectedness, spirituality, religious beliefs, and cultural attitudes against suicide may serve as buffers against these risk factors [43]. Warning signs of impending suicide may indicate preparations for suicide and include feelings of hopelessness or lack of purpose, feeling trapped, talking about death, threatening suicide, agitation, social withdrawal, increased substance use and reckless behavior. Warning signs should prompt action to ensure the safety of the individual [44,45].
Physical Examination, Laboratory Studies, and Neuroimaging
Evaluation of LLD is not complete without a physical examination and ancillary studies to identify underlying medical conditions possibly contributing to or mimicking depressive symptoms. Routine laboratory studies include complete blood count, complete metabolic panel, thyroid studies, and urine drug screen. Signs and symptoms of underlying medical conditions may necessitate further laboratory studies [46]. Neuroimaging may reveal signs of cerebrovascular disease which can predispose, precipitate, or perpetuate depression in older adults [47].
Treatment
Treatment of LLD can take many forms and occur in various settings. Geriatric psychiatrists have expertise in the assessment and treatment of mental illness in the elderly. Workforce estimates for 2010 revealed 1 geriatric psychiatrist per 10,000 adults age 75 and over. This figure is estimated to decrease to 0.5 per 10,000 by the year 2030, underscoring the importance of increasing the knowledge base of clinicians across specialties who provide care to the depressed elderly [48]. The primary care setting is often the locus of care for depression in older adults; however, studies suggest that patients are often left untreated or undertreated [49]. Collaborative care models whereby mental health care is integrated into primary care have been shown to improve outcomes. The Prevention of Suicide in Primary Care Elderly: Collaborative Trial (PROSPECT) study found that use of care managers to assist primary care providers in identification of depression, offer algorithm-based treatment recommendations, monitor symptoms and medication side effects, and provide follow-up yielded improvement in outcomes. Patients in the intervention group were more likely to receive pharmacotherapy or psychotherapy, achieve remission, and showed greater decline in suicidal ideation [50]. Similar results were found in the Improving Mood-Promoting Access to Collaborative Treatment (IMPACT) study in which intervention patients treated under a collaborative care model showed lower depression severity, less functional impairment, and greater reduction in depressive symptoms [25].
Just as a collaborative care model can lead to improved outcomes, the overall strategy of treating depression must be multifaceted. The biopsychosocial model of disease first described in the 1970s emphasizes biological and psychosocial determinants of illness that must be addressed when treatment is considered [51]. This includes nonmodifiable biological factors such as age, gender, and genetic predisposition that may affect treatment options, as well as modifiable biological factors such as comorbid medical illness, medications, or substance use disorders. Psychological factors that can affect depressive symptoms include coping skills and defense mechanisms in the face of stressful life events. Social factors including the role of culture, environment, and family dynamics in disease presentation must be considered as well [52].
Pharmacologic Treatment of LLD
The primary pharmacologic treatment for depression is antidepressants. Treatment consists of 3 phases—acute, continuation, and maintenance. In the acute phase, the goal is remission of current symptoms and restoration of function. The continuation phase, extending up to 6 months after remission, aims to prevent relapse back into a depressive episode. Maintenance therapy is geared at preventing recurrence of future depressive episodes [53]. Studies have found a 50% risk of relapse after 1 episode of depression and 80% after 2 episodes. Up to 20% will develop chronic symptoms. For this reason, maintenance therapy is often necessary for recurrent depression [54].
While cognitive impairment may affect antidepressant efficacy, age does not appear to be a determinant. Gildengers et al examined antidepressant response in young, middle, and older-old patients and found no significant difference in response rates [59]. Early onset versus late onset of first depressive episode also does not predict antidepressant response in patients age 55 and over [60]. There is scant evidence for efficacy of antidepressants in depressed patients with neurocognitive disorders. A 2002 Cochrane review with 4 studies in the meta-analysis (n = 137) concluded that there was weak support for antidepressant efficacy in this population [61]. A 2011 meta-analysis with 330 participants also yielded inconclusive results [62]. The paucity of evidence for antidepressant efficacy in depressed patients with neurocognitive disorders should prompt careful consideration of potential benefits versus adverse effects.
Antidepressants are generally well tolerated in older adults. Side effects vary by medication and contribute to discontinuation in up to 25% of new users (versus 22% for new users who discontinue for reasons other than side effects) [63]. Potential adverse effects shared by most SSRIs and SNRIs include GI disturbance (nausea, diarrhea or constipation), sexual dysfunction, headache, and sleep disturbance [64,65]. In addition, abrupt discontinuation can precipitate serotonin withdrawal syndrome characterized by sensory disturbance (paresthesia, tremor, and irritability) as well as headache, lightheadedness, diaphoresis, insomnia, and agitation. Other medication-specific side effects include risk of seizure with bupropion and sedation with mirtazapine [65].
Despite superiority of antidepressants to placebo in treating depression, up to one-third of patients may not respond to a trial of antidepressants. Sequential treatment protocols such as switching to a different antidepressant or augmentation can increase the proportion of antidepressant responders [66–68]. Studies have found particularly favorable response to augmentation with lithium, with one study achieving a 33% remission rate in treatment- resistant geriatric depression [67,69]. Other pharmacologic augmentation strategies include the addition of mood stabilizers such as lamotrigine, antipsychotics (aripiprazole, olanzapine, quetiapine, and risperidone), and psychostimulants [70–73]. Electroconvulsive therapy (ECT) is a nonpharmacologic option for treatment-resistant depression that will be reviewed later.
Psychotherapeutic and Psychosocial Interventions
Psychotherapeutic interventions have demonstrated efficacy in the treatment of geriatric depression, including but not limited to cognitive behavioral therapy (CBT), interpersonal therapy (IPT), problem-solving therapy (PST), reminiscence and life review, and brief psychodynamic psychotherapy [74]. Some older adults may prefer psychotherapy to pharmacologic treatment (57% vs. 43%) [75]. Potential benefits of psychotherapy include ability to directly address psychosocial stressors that may precipitate or perpetuate depressive symptoms. In addition, psychotherapy is associated with few to no side effects and avoids drug interactions. Barriers to employing psychotherapy may include cost and access to trained psychotherapists [76]. Efficacy of several psychotherapeutic approaches in the care of older depressed adults has been examined. CBT, brief psychodynamic psychotherapy, and IPT will be briefly reviewed here.
CBT. Cognitive therapy was first described by Aaron Beck in the 1960s [77]. It is a highly structured therapy built on the premise that beliefs and assumptions an individual holds can influence emotions and behavior. CBT aims to identify maladaptive belief systems, test the validity of these cognitive distortions, and help individuals formulate more realistic cognitions [78]. Symptom improvement results from addressing these cognitive aspects as well as integration of behavioral activation and skills training to overcome maladaptive behavioral patterns [78]. CBT approaches have been applied to older adults with depression and results show acceptability [79] and efficacy in this population [80–82]. A 2008 Cochrane review (n = 153) found CBT to be superior to waitlist controls [82].
Brief psychodynamic psychotherapy. Brief psychodynamic psychotherapy, unlike highly structured CBT, aims to alter behavior by examining how past experiences and unresolved conflicts influence current emotions and behavior. While studies on application to the treatment of geriatric depression are scarce, limited data demonstrate efficacy in treating geriatric depression [81] and no significant difference in outcomes when compared to CBT [82].
IPT. Like CBT, IPT is a structured time-limited psychotherapeutic treatment approach first developed in the late 1960s by Klerman and Weissman [83]. IPT focuses on the impact of interpersonal relationships on depressive symptoms and examines 4 domains: interpersonal conflict, interpersonal deficits, role transitions, and grief [74].
Studies have shown efficacy of IPT in reducing depressive symptoms in the elderly when compared to usual care [84]. Reynolds et al found IPT combined with nortriptyline (a tricyclic antidepressant) to be superior to either nortriptyline alone or IPT alone in preventing recurrent depressive episodes [85]. Interestingly, a similar study investigating the efficacy of IPT in combination with paroxetine (an SSRI) failed to show added benefit of IPT in preventing recurrence, suggesting that further studies are needed [86].
Psychosocial interventions are integral in the care of the elderly depressed patient. Studies have shown positive benefits of aerobic exercise on depressive symptoms [87]. Yoga, Tai Chi, and other mindfulness-based exercises can increase sense of emotional and physical wellbeing [88–90]. Spirituality, religious beliefs, and involvement with a faith group may be protective against development of mental illness while at the same time provide avenues for increased social connectedness [91]. These and other avenues for socialization should be encouraged as part of the treatment plan for older depressed patients [92]
Electroconvulsive Therapy
ECT is indicated for the treatment of mood and psychotic disorders and has demonstrated efficacy in the treatment of severe depression [93]. It is typically initiated when patients fail to respond to pharmacotherapy and psychotherapy. Circumstances in which ECT can be considered first-line treatment include situations that require a rapid response (severe inanition, weight loss, or suicidality), situations where risks of ECT are lower than that of alternative treatments, previous positive response to ECT, or strong patient preference [94]. ECT is performed under general anesthesia and involves the induction of a generalized tonic-clonic seizure, which is theorized to enhance serotonergic, noradrenergic, and dopaminergic neurotransmission. A typical course of ECT involves treatments 3 times a week for an average of 6 to 12 treatments in total [95]. Elderly patients and those suffering from severe depression with psychotic features respond more robustly to ECT [93,96]. Estimated remission rates after an ECT series have been higher than 80% [93], making this modality the most effective treatment for severe depression to date.
Conclusion
As the population continues to age, clinicians are increasingly likely to encounter patients with late-life depression. A thorough evaluation includes not only assessment of depressive symptoms, but also cognitive, functional, and suicide assessment. Treatment options include pharmaco-therapy, psychotherapy, and in some cases electroconvulsive therapy. Utilization of assessment and treatment nuances unique to the geriatric population, with a multidisciplinary and collaborative approach involving primary care, mental health, and other ancillary providers, will serve to ultimately enhance patient care.
Corresponding author: Corresponding author: Juliet Glover, MD, Dept. of Neuropsychiatry and Behavioral Science, Univ. of South Carolina School of Medicine, 15 Medical Park, Suite 301, Columbia, SC 29203, [email protected].
Financial disclosures: None reported.
Author contributions: conception and design, JAG, SS; drafting of article, JAG, SS; critical revision of the article, JAG, SS.
From the Department of Neuropsychiatry and Behavioral Sceince, University of South Carolina School of Medicine, Columbia, SC.
Abstract
- Objective: To review the identification, clinical assessment and treatment of patients with late-life depression.
- Methods: Review of the literature.
- Results: Depressive symptoms are present in up to 1 in 4 older adults. Comprehensive evaluation of depressive symptoms in this population often requires a multidisciplinary and collaborative approach between primary care, mental health, and other ancillary providers. Key aspects include a detailed history, physical and mental status examinations, cognitive and functional status assessment, and suicide risk assessment. Treatment options include anti-depressants, psychotherapy, and electroconvulsive therapy.
- Conclusion: A systematic approach to evaluating depressive symptoms in the elderly can enhance timely recognition and treatment.
Key words: Late-life depression; clinical assessment; antidepressants; psychotherapy; electroconvulsive therapy.
The U.S. population is aging, and with this comes the potential for increased health care needs. In 2014, there were over 46 million Americans age 65 and over (14.5% of the U.S. population). This number is projected to increase to 88 million by the year 2050 [1]. One in 4 older adults suffers with depressive symptoms that cause distress and functional impairment [2]. The World Health Organization Global Burden of Disease Study found depressive disorders to be the leading cause of disability-adjusted life years (DALYs) and the second leading cause of years lived with disability (YLDs). The burden of disease due to depressive disorders increased by 37.5% between 1990 and 2010, and 10.4% was attributable to aging [3]. These figures underscore the importance of accurate assessment and treatment of depression in the elderly. In this article, we review the identification, clinical assessment, and treatment of patients with late-life depression.
Diagnostic Criteria
Prevalence
It is estimated that 1% to 4% of community-dwelling adults age 65 and older suffer from MDD, with women more likely to be affected than men (prevalence of 4.4% vs. 2.7) [2,5–7]. This estimate is low compared with lifetime prevalence of almost 20% in the general adult population [8]. However, when depressive symptoms that do not meet criteria for MDD are considered, prevalence rates increase up to 25% [2,9]. These estimates also vary by clinical setting, with the highest rates (up to 40%) among elderly patients in long-term care facilities [10,11]. While individuals with subsyndromal depression may experience fewer symptoms than those with MDD, clinically significant distress persists, impacting health and functional status. Depression is associated with overall poor social or occupational functioning, cognitive decline, increased health care utilization and cost, increased morbidity and mortality from medical illness, and increased suicide mortality [5,9,10,12].
Identifying LLD
Accurate identification of LLD also requires recognition of the differences in the presentation of LLD compared with onset in earlier life. Depression in younger adults is often marked by depressed mood and loss of interest [18]. In contrast, older adults may present with increased anger or irritability [5]. Younger adults are more likely to report suicidal thoughts while older patients report feelings of hopelessness and thoughts of death [18]. LLD is often characterized by increased somatic complaints, hypochondriasis, or pain [5,18,19]. Another major difference lies in the presentation of cognitive difficulties. Younger patients typically complain of poor concentration or indecisiveness. Geriatric patients may present with cognitive changes including objective findings of slower processing speed and executive dysfunction on neuropsychological testing [17].
Depression rating scales may aid in identification of LLD. They are not a substitute for clinical diagnosis but can be useful as screening tools. Two commonly utilized depression rating scales are the Geriatric Depression Scale (GDS) and the Patient Health Questionnaire-9 (PHQ-9). GDS is a 30-item instrument developed specifically for older adults. Shorter 15-item, 5-item, and 4-item versions exist. The scale utilizes a Yes/No format and can be self- or clinician-administered [20]. One advantage of the GDS lies in its focus on psychological and cognitive aspects of depression rather than neurovegetative symptoms that may overlap with medical illnesses common in older adults [21]. The PHQ-9 is a 9-item self- or clinician-administered screening tool designed for use in primary care settings and has also been validated in geriatric populations [22,23]. The 9 items on this scale correspond to the DSM-5 criteria for major depression. A shorter 2-item version (PHQ-2) has also been validated, and a positive screen on this test should prompt administration of the full-length version. Both versions have approximately 80% sensitivity and specificity in detecting depression. An added advantage of PHQ-9 over GDS is that it can be useful in monitoring treatment response over time [22,23]
Comprehensive Assessment of LLD
The comprehensive assessment of patients with LLD can be carried out by health professionals in both mental health or primary care settings. In a multidisciplinary approach, psychiatrists and mental health professionals have collaborated with primary care providers using depression care managers with successful outcomes in managing depression in older adults [24,25]. Complete evaluation of a patient with suspected LLD begins with a history and physical and mental status examination. Other essential components of the evaluation include assessment of cognition, functional status, and suicide risk. Laboratory and neuroimaging studies may be necessary as well. Due to the comprehensive nature of this assessment, a multidisciplinary approach with collaboration between primary care, psychiatry, psychology, and ancillary services such as social work may be necessary. Multiple patient interactions may be required to complete a thorough evaluation.
History and Mental Status Examination
As with many other psychiatric illnesses, LLD is a clinical diagnosis. A careful history should be obtained initially utilizing open-ended questions. This should be followed by more directed questions as indicated to elicit the presence of depressive symptoms. The history should be obtained from the patient. A relevant collateral informant can be invaluable in the assessment, especially in cases where there is a comorbid neurocognitive disorder. However, the patient’s informed consent must be obtained prior to obtaining collateral information whenever possible. Psychosocial stressors that may have precipitated or may be perpetuating symptoms should be explored. Such stressors may include recent changes in living situation, loss of social support, recent deaths, or financial difficulties. Biological precipitants also need to be explored including presence of physical illness, depressogenic medications, and comorbid alcohol or other substance use. The patient’s past psychiatric history, psychiatric hospitalizations, and past medication trials should be ascertained. Any family history of depression, other psychiatric disorders, substance use disorders, and suicide attempts should be documented. A full mental status exam including cognitive assessment should be completed [21,26].
Cognitive Assessment
Cognitive impairment can be associated with LLD and may be due to the underlying depression or represent a comorbid neurocognitive disorder. Furthermore, the burden of medical illness as well as cerebrovascular and cardiovascular risk factors have been linked to executive dysfunction and reduced processing speed in individuals with LDD [27,28]. Distinguishing between these can be challenging; however, chronology of symptom onset is often helpful. Depression is more likely the etiology of cognitive impairment when depressive symptoms precede onset of cognitive deficits. This type of cognitive impairment is termed dementia syndrome of depression and may improve with treatment of depression [5]. Some patients may progress to develop major cognitive decline, and it remains unclear whether LLD represents a risk factor or prodrome to developing a major neurocognitive disorder [29]. On the other hand, if depression develops later in the course of cognitive decline, there may be an underlying neurocognitive disorder [17]. Up to 20% of individuals with major neurocognitive disorder due to Alzheimer’s disease also have major depression [11]. For these reasons, concomitant assessment of cognition is essential to the evaluation of the older adult presenting with depressive symptoms [30]. Cognitive domains that may be affected include learning and memory, language, attention, perceptual motor abilities, social cognition, and executive function [4]. Many of these domains can be assessed during the mental status examination, with brief cognitive screening tools, or with formal neuropsychological testing.
While there are numerous cognitive screening tools, some commonly used, brief tools include the Mini-Cog, the Folstein Mini-Mental State Exam (MMSE), and the Montreal Cognitive Assessment (MoCA). The Mini-Cog consists of a 3-item registration, delayed recall, and clock drawing test and has several advantages over other screening tools. It is a brief test (taking approximately 3 minutes to administer) with good sensitivity and specificity of 80% or greater. Compared with other cognitive screening tools, it is less influenced by level of education, language, or cultural background [31–33]. The MMSE is a longer screening tool consisting of 19 items and requires about 10 minutes to administer. Unlike the Mini-Cog, performance on the MMSE can be affected by level of education and cultural background. However, the MMSE can be administered serially to monitor changes in cognition over time [34,35]. The MoCA is a 10-minute cognitive screening tool first developed to detect mild cognitive impairment (MCI) [36]. The MoCA consists of 7 subscore sections covering visuospatial/executive function, naming, memory (delayed recall), attention, language, abstraction, and orientation. The total score is 30, and 1 point is added to the score if the testing subject has less than high school/12 years of education. The MoCA has demonstrated better sensitivity than the MMSE for the detection of MCI [36]. Elderly patients with depression often perform poorly on these cognitive screening tests due to apathy and poor effort.
Functional Assessment
The diagnosis of LLD requires that symptoms cause significant distress or interfere with functioning. A functional assessment is especially important in the evaluation of the older adult in that it allows clinicians to determine an individual’s ability to live independently and attend to daily needs. Basic activities of daily living (ADLs) include bathing, dressing, grooming, toileting, and self-transferring. Instrumental activities of daily living (IADLs) include more complex daily activities such as preparing meals, administering medications, driving, managing finances, and using simple electronics such as the telephone or remote control [26]. Impairment in IADLs is associated with increased depression severity. Conversely, the severity of depressive symptoms along with associated cognitive impairment predicts IADL impairment [37]. The Philadelphia Multilevel Assessment Instrument is a tool that can aid in the assessment of ADLs and IADLs and has been utilized in studies examining disability in depressed elderly patients [37,38]. Other available scales to quantify functional status include OARS Physical Activities of Daily Living, OARS Instrumental Activities of Daily Living Scale, and Direct Assessment of Functional Status Scale [26].
Suicide Assessment
Assessment for suicidality is an integral part of all psychiatric evaluations and is especially important in the evaluation of the depressed older adult. According to the Centers for Disease Control and Prevention, the suicide rate for individuals age 65 and older is 16.6 per 100,000, a figure that is comparable to that for individuals 18–64 years of age [39]. Non-Hispanic Caucasian males age 85 and older have the highest rate of completed suicide (56.5 per 100,000), underscoring the importance of a thorough suicide assessment [39]. Suicidality can range from passive thoughts of death and wishing that one were not alive, to active thoughts of self-harm with plan and intent. A Canadian study found 2% of community-dwelling adults age 55 and older had suicidal thoughts over a 12-month period and, of these, 28% had major depression [40]. A suicide assessment begins with inquiring about the presence of suicidal thoughts, plans, and intent. The 3 most frequently used methods of completed suicide in the elderly are firearms (28%), hanging (24%) and poisoning (21%) [41]. Access to weapons or other lethal means of self-harm such has hoarding of medications should be ascertained.
A complete suicide assessment requires attention to suicide risk factors, protective factors, and warning signs of impending suicide. Risk factors for suicide in the older adult include mood disorders, chronic medical illnesses and associated functional impairment, chronic pain, and psychosocial factors such as social isolation [42]. Mood disorders are present in 54% to 87% of cases of completed suicide, with major depression being the most common [42]. Chronic medical illness and pain can result in functional impairment leading to feelings of excessive guilt or being a burden to loved ones. Protective factors such as social connectedness, spirituality, religious beliefs, and cultural attitudes against suicide may serve as buffers against these risk factors [43]. Warning signs of impending suicide may indicate preparations for suicide and include feelings of hopelessness or lack of purpose, feeling trapped, talking about death, threatening suicide, agitation, social withdrawal, increased substance use and reckless behavior. Warning signs should prompt action to ensure the safety of the individual [44,45].
Physical Examination, Laboratory Studies, and Neuroimaging
Evaluation of LLD is not complete without a physical examination and ancillary studies to identify underlying medical conditions possibly contributing to or mimicking depressive symptoms. Routine laboratory studies include complete blood count, complete metabolic panel, thyroid studies, and urine drug screen. Signs and symptoms of underlying medical conditions may necessitate further laboratory studies [46]. Neuroimaging may reveal signs of cerebrovascular disease which can predispose, precipitate, or perpetuate depression in older adults [47].
Treatment
Treatment of LLD can take many forms and occur in various settings. Geriatric psychiatrists have expertise in the assessment and treatment of mental illness in the elderly. Workforce estimates for 2010 revealed 1 geriatric psychiatrist per 10,000 adults age 75 and over. This figure is estimated to decrease to 0.5 per 10,000 by the year 2030, underscoring the importance of increasing the knowledge base of clinicians across specialties who provide care to the depressed elderly [48]. The primary care setting is often the locus of care for depression in older adults; however, studies suggest that patients are often left untreated or undertreated [49]. Collaborative care models whereby mental health care is integrated into primary care have been shown to improve outcomes. The Prevention of Suicide in Primary Care Elderly: Collaborative Trial (PROSPECT) study found that use of care managers to assist primary care providers in identification of depression, offer algorithm-based treatment recommendations, monitor symptoms and medication side effects, and provide follow-up yielded improvement in outcomes. Patients in the intervention group were more likely to receive pharmacotherapy or psychotherapy, achieve remission, and showed greater decline in suicidal ideation [50]. Similar results were found in the Improving Mood-Promoting Access to Collaborative Treatment (IMPACT) study in which intervention patients treated under a collaborative care model showed lower depression severity, less functional impairment, and greater reduction in depressive symptoms [25].
Just as a collaborative care model can lead to improved outcomes, the overall strategy of treating depression must be multifaceted. The biopsychosocial model of disease first described in the 1970s emphasizes biological and psychosocial determinants of illness that must be addressed when treatment is considered [51]. This includes nonmodifiable biological factors such as age, gender, and genetic predisposition that may affect treatment options, as well as modifiable biological factors such as comorbid medical illness, medications, or substance use disorders. Psychological factors that can affect depressive symptoms include coping skills and defense mechanisms in the face of stressful life events. Social factors including the role of culture, environment, and family dynamics in disease presentation must be considered as well [52].
Pharmacologic Treatment of LLD
The primary pharmacologic treatment for depression is antidepressants. Treatment consists of 3 phases—acute, continuation, and maintenance. In the acute phase, the goal is remission of current symptoms and restoration of function. The continuation phase, extending up to 6 months after remission, aims to prevent relapse back into a depressive episode. Maintenance therapy is geared at preventing recurrence of future depressive episodes [53]. Studies have found a 50% risk of relapse after 1 episode of depression and 80% after 2 episodes. Up to 20% will develop chronic symptoms. For this reason, maintenance therapy is often necessary for recurrent depression [54].
While cognitive impairment may affect antidepressant efficacy, age does not appear to be a determinant. Gildengers et al examined antidepressant response in young, middle, and older-old patients and found no significant difference in response rates [59]. Early onset versus late onset of first depressive episode also does not predict antidepressant response in patients age 55 and over [60]. There is scant evidence for efficacy of antidepressants in depressed patients with neurocognitive disorders. A 2002 Cochrane review with 4 studies in the meta-analysis (n = 137) concluded that there was weak support for antidepressant efficacy in this population [61]. A 2011 meta-analysis with 330 participants also yielded inconclusive results [62]. The paucity of evidence for antidepressant efficacy in depressed patients with neurocognitive disorders should prompt careful consideration of potential benefits versus adverse effects.
Antidepressants are generally well tolerated in older adults. Side effects vary by medication and contribute to discontinuation in up to 25% of new users (versus 22% for new users who discontinue for reasons other than side effects) [63]. Potential adverse effects shared by most SSRIs and SNRIs include GI disturbance (nausea, diarrhea or constipation), sexual dysfunction, headache, and sleep disturbance [64,65]. In addition, abrupt discontinuation can precipitate serotonin withdrawal syndrome characterized by sensory disturbance (paresthesia, tremor, and irritability) as well as headache, lightheadedness, diaphoresis, insomnia, and agitation. Other medication-specific side effects include risk of seizure with bupropion and sedation with mirtazapine [65].
Despite superiority of antidepressants to placebo in treating depression, up to one-third of patients may not respond to a trial of antidepressants. Sequential treatment protocols such as switching to a different antidepressant or augmentation can increase the proportion of antidepressant responders [66–68]. Studies have found particularly favorable response to augmentation with lithium, with one study achieving a 33% remission rate in treatment- resistant geriatric depression [67,69]. Other pharmacologic augmentation strategies include the addition of mood stabilizers such as lamotrigine, antipsychotics (aripiprazole, olanzapine, quetiapine, and risperidone), and psychostimulants [70–73]. Electroconvulsive therapy (ECT) is a nonpharmacologic option for treatment-resistant depression that will be reviewed later.
Psychotherapeutic and Psychosocial Interventions
Psychotherapeutic interventions have demonstrated efficacy in the treatment of geriatric depression, including but not limited to cognitive behavioral therapy (CBT), interpersonal therapy (IPT), problem-solving therapy (PST), reminiscence and life review, and brief psychodynamic psychotherapy [74]. Some older adults may prefer psychotherapy to pharmacologic treatment (57% vs. 43%) [75]. Potential benefits of psychotherapy include ability to directly address psychosocial stressors that may precipitate or perpetuate depressive symptoms. In addition, psychotherapy is associated with few to no side effects and avoids drug interactions. Barriers to employing psychotherapy may include cost and access to trained psychotherapists [76]. Efficacy of several psychotherapeutic approaches in the care of older depressed adults has been examined. CBT, brief psychodynamic psychotherapy, and IPT will be briefly reviewed here.
CBT. Cognitive therapy was first described by Aaron Beck in the 1960s [77]. It is a highly structured therapy built on the premise that beliefs and assumptions an individual holds can influence emotions and behavior. CBT aims to identify maladaptive belief systems, test the validity of these cognitive distortions, and help individuals formulate more realistic cognitions [78]. Symptom improvement results from addressing these cognitive aspects as well as integration of behavioral activation and skills training to overcome maladaptive behavioral patterns [78]. CBT approaches have been applied to older adults with depression and results show acceptability [79] and efficacy in this population [80–82]. A 2008 Cochrane review (n = 153) found CBT to be superior to waitlist controls [82].
Brief psychodynamic psychotherapy. Brief psychodynamic psychotherapy, unlike highly structured CBT, aims to alter behavior by examining how past experiences and unresolved conflicts influence current emotions and behavior. While studies on application to the treatment of geriatric depression are scarce, limited data demonstrate efficacy in treating geriatric depression [81] and no significant difference in outcomes when compared to CBT [82].
IPT. Like CBT, IPT is a structured time-limited psychotherapeutic treatment approach first developed in the late 1960s by Klerman and Weissman [83]. IPT focuses on the impact of interpersonal relationships on depressive symptoms and examines 4 domains: interpersonal conflict, interpersonal deficits, role transitions, and grief [74].
Studies have shown efficacy of IPT in reducing depressive symptoms in the elderly when compared to usual care [84]. Reynolds et al found IPT combined with nortriptyline (a tricyclic antidepressant) to be superior to either nortriptyline alone or IPT alone in preventing recurrent depressive episodes [85]. Interestingly, a similar study investigating the efficacy of IPT in combination with paroxetine (an SSRI) failed to show added benefit of IPT in preventing recurrence, suggesting that further studies are needed [86].
Psychosocial interventions are integral in the care of the elderly depressed patient. Studies have shown positive benefits of aerobic exercise on depressive symptoms [87]. Yoga, Tai Chi, and other mindfulness-based exercises can increase sense of emotional and physical wellbeing [88–90]. Spirituality, religious beliefs, and involvement with a faith group may be protective against development of mental illness while at the same time provide avenues for increased social connectedness [91]. These and other avenues for socialization should be encouraged as part of the treatment plan for older depressed patients [92]
Electroconvulsive Therapy
ECT is indicated for the treatment of mood and psychotic disorders and has demonstrated efficacy in the treatment of severe depression [93]. It is typically initiated when patients fail to respond to pharmacotherapy and psychotherapy. Circumstances in which ECT can be considered first-line treatment include situations that require a rapid response (severe inanition, weight loss, or suicidality), situations where risks of ECT are lower than that of alternative treatments, previous positive response to ECT, or strong patient preference [94]. ECT is performed under general anesthesia and involves the induction of a generalized tonic-clonic seizure, which is theorized to enhance serotonergic, noradrenergic, and dopaminergic neurotransmission. A typical course of ECT involves treatments 3 times a week for an average of 6 to 12 treatments in total [95]. Elderly patients and those suffering from severe depression with psychotic features respond more robustly to ECT [93,96]. Estimated remission rates after an ECT series have been higher than 80% [93], making this modality the most effective treatment for severe depression to date.
Conclusion
As the population continues to age, clinicians are increasingly likely to encounter patients with late-life depression. A thorough evaluation includes not only assessment of depressive symptoms, but also cognitive, functional, and suicide assessment. Treatment options include pharmaco-therapy, psychotherapy, and in some cases electroconvulsive therapy. Utilization of assessment and treatment nuances unique to the geriatric population, with a multidisciplinary and collaborative approach involving primary care, mental health, and other ancillary providers, will serve to ultimately enhance patient care.
Corresponding author: Corresponding author: Juliet Glover, MD, Dept. of Neuropsychiatry and Behavioral Science, Univ. of South Carolina School of Medicine, 15 Medical Park, Suite 301, Columbia, SC 29203, [email protected].
Financial disclosures: None reported.
Author contributions: conception and design, JAG, SS; drafting of article, JAG, SS; critical revision of the article, JAG, SS.
1. Vincent GK, Velkoff VA. The next four decades: the older population in the United States: 2010 to 2050. US Census Bureau: May 2010.
2. Koenig HG, Blazer DG. Epidemiology of geriatric affective disorders. Clinc Geriatr Med 1992; 8:235–51.
3. Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global gurden of disease study 2010. PLoS Med 2013;10:e1001547.
4 .American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
5 .Ellison JM, Kyomen HH, Harper DG. Depression in later life: an overview with treatment recommendations. Psychiatr Clin North Am 2012;35:203–29.
6. Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry 2005;62:1097–106.
7. Steffens DC, Skoog I, Norton MC, et al. Prevalence of depression and its treatment in an elderly population: the Cache County study. Arch Gen Psychiatry 2000;57:601–7.
8. Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003;289:3095–105.
9. McKinney BC, Sibille E. The age-by-disease interaction hypothesis of late-life depression. Am J Geriatr Psychiatry 2013;21:418–32.
10. Djernes JK. Prevalence and predictors of depression in populations of elderly: a review. Acta Psychiatr Scand 2006;113:372–87.
11. Blazer DG. Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci 2003;58:249–65.
12. Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry 2003;160:1147–56.
13. Ritchie K. Late-life depression. European Psychiatry 2014;29:577.
14. Hegeman JM, de Waal MW, Comijs HC, et al. Depression in later life: a more somatic presentation? J Affect Disord 2015;170:196–202.
15. Lackamp J, Schlachet R, Sajatovic M. Assessment and management of major depressive disorder in older adults. Psychiatria Danubina 2016;28(Suppl 1):95–98.
16. Morichi V, Dell’Aquila G, Trotta F, et al. Diagnosing and treating depression in older and oldest old. Curr Pharm Des 2015;21:1690–8.
17. Fiske AJ, Wetherell JL, Gatz M. Depression in older adults. Annu Rev Clin Psychol 2009;5:363–89.
18. Balsis S, Cully JA. Comparing depression diagnostic symptoms across younger and older adults. Aging Ment Health 2008;12:800–6.
19. Hegeman JM, Kok RM, van der Mast RC, Giltay EJ. Phenomenology of depression in older compared with younger adults: meta-analysis. Br J Psychiatry 2012;200:275–81.
20. Mitchell AJ, Bird V, Rizzo M, Meader N. Which version of the geriatric depression scale is most useful in medical settings and nursing homes? Diagnostic validity meta-analysis. Am J Geriatr Psychiatry 2010;18:1066–77.
21. Blazer DG. The psychiatric interview of older adults. In: Blazer D, Steffens D, Busse E, editors. Textbook of geriatric psychiatry. 3rd ed. Arlington, VA: American Psychiatric Publishing; 2004.
22. Spitzer RL, Kroenke K, Williams JW. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999;282:1737–44.
23. Richardson TM, He H, Podgorski C, et al. Screening for depression in aging services clients. Am J Geriatr Psychiatry 2010;18:1116–23.
24. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev 2012 Oct 17.
25. Unutzer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting a randomized controlled trial. JAMA 2002;288:2836–45.
26. Silver I, Herrmann N. Comprehensive psychiatric evaluation. In: Sadavoy J, Jarvik L, Grossberg G, Meyers B, editors. Comprehensive textbook of geriatric psychiatry. 3rd ed. New York: W.W. Norton; 2004.
27. Rapp MA, Dahlman K, Sano M, et al. Neuropsychological differences between late-onset and recurrent geriatric major depression. Am J Psychiatry 2005;162:691–8.
28. Sheline YI, Barch DM, Garcia K, et al. Cognitive function in late life depression: relationships to depression severity, cerebrovascular risk factors and processing speed. Biol Psychiatry 2006; 60:58–65.
29. Barnes DE, Yaffe K, Byers AL, et al. Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry 2012;69:493–8.
30. Morimoto SS, Kanellopoulos D, Manning KJ, Alexopoulos GS. Diagnosis and treatment of depression and cognitive impairment in late life. Ann NY Acad Sci 2015;1345:36–46.
31. Borson S, Scanlan J, Brush M, et al. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry 2000;15:1021–7.
32. Brodaty H, Low LF, Gibson L, Burns K. What is the best dementia screening instrument for general practitioners to use? Am J Geriatr Psychiatry 2006;14:391–400.
33. Ismail Z, Rajji TK, Shulman KI. Brief cognitive screening instruments: an update. Int J Geriatr Psychiatry 2010;25:111–20.
34. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98.
35. Vertesi A, Lever JA, Molloy DW, et al. Standardized Mini-Mental State Examination. Use and interpretation. Can Fam Physician 2001;47: 2018–23.
36. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–9.
37. Kiosses DN, Alexopoulos GS. IADL functions, cognitive deficits, and severity of depression: a preliminary study. Am J Geriatr Psychiatry 2005;13:244–9.
38. Alexopoulos GS, Vrontou C, Kakuma T, et al. Disability in geriatric depression. Am J Psychiatry 1996;153:877–85.
39. Centers for Disease Control and Prevention. National Center for Injury Prevention and Control. Web-Based Injury Statistics Query and Reporting System (WISQARS). Accessed 9 Feb 2016 at http://webappa.cdc.gov/sasweb/ncipc/dataRestriction_inj.html.
40. Corna LM, Cairney J, Streiner DL. Suicide ideation in older adults: relationship to mental health problems and service use. Gerontologist 2010;50:785–97.
41. Juurlink DN, Herrmann N, Szalai JP, et al. Medical illness and the risk of suicide in the elderly. Arch Intern Med 2004;164:1179–84.
42. Van Orden K, Conwell Y. Suicides in late life. Curr Psychiatry Rep 2011;13:234–41.
43. Conwell Y, Van Orden K, Caine ED. Suicide in older adults. Psychiatr Clin North Am 2011;34:451–68, ix.
44. Rudd MD, Berman AL, Joiner TE, et al. Warning signs for suicide: theory, research, and clinical applications. Suicide Life Threat Behav 2006;36:255–62.
45. Know the warning signs of suicide. American Association of Suicidology. Accessed 9 Feb 2016 at www.suicidology.org/resources/warning-signs.
46. Taylor W, Doraiswamy P. Use of the laboratory in the diagnostic workup of older adults. In: Blazer D, Steffen D, Busse E, editors. Textbook of geriatric psychiatry. 3rd ed. Arlington, VA: American Psychiatric Publishing; 2004.
47. Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry 1997;54:915–22.
48. ADGAP Status of Geriatrics Workforce Study. Accessed 26 Dec 2016 at www.americangeriatrics.org/files/documents/gwps/Table%201_29.pdf.
49. Alexopoulos G. Late-life mood disorders. In: Sadavoy J, Jarvik L, Grossberg G, Meyers B, editors. Comprehensive textbook of geriatric psychiatry. 3rd ed. New York: W.W. Norton; 2004.
50. Alexopoulos GS, Reynolds CF, Bruce ML, et al. Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. Am J Psychiatry 2009;166:882–90.
51. Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977;196:129–36.
52. Schotte CKW, Van den Bossche B, Doncker DD, et al. A biopsychosocial model as a guide for psychoeducation and treatment of depression. Depression Anxiety 2006;23:312–24.
53. Kupfer DJ, Frank E. The interaction of drug-and psychotherapy in the long-term treatment of depression. J Affect Disord 2001;62:131–7.
54. Katon W, Rutter C, Ludman EJ, et al. A randomized trial of relpase prevention of depression in primary care. Arch Gen Psychiatry 2001;58:241–7.
55. Kok RM, Nolen WA, Heeren TJ. Efficacy of treatment in older depressed patients: a systematic review and meta-analysis of double-blind randomized controlled trials with antidepressants. J Affect Disord 2012;141:103–15.
56. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62.
57. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382–9.
58. Mukai Y, Tampi RR. Treatment of depression in the elderly: a review of the recent literature on the efficacy of single- versus dual-action antidepressants. Clin Ther 2009;31:945–61.
59. Gildengers AG, Houck PR, Mulsant BH, et al. Course and rate of antidepressant response in the very old. J Affect Disord 2002;69:177–84.
60. Kozel FA, Trivedi MH, Wisniewski SR, et al. Treatment outcomes for older depressed patients with earlier versus late onset of first depressive episode. Am J Geriatr Psychiatry 2008;16:58–64.
61. Bains J, Birks J, Dening T. Antidepressants for treating depression in dementia. Cochrane Databse Syst Rev 2002;(4):CD003944.
62. Nelson JC, Devanand DP. A systematic review and meta-analysis of placebo-controlled antidepressant studies in people with depression and dementia. J Am Geriatr Soc 2011;59:577–85.
63. Mark TL, Joish VN, Hay JW, et al. Antidepressant use in geriatric populations: the burden of side effects and interactions and their impact on adherence and costs. Am J Geriatr Psychiatry 2011;19:211–21.
64. Frank C. Pharmacologic treatment of depression in the elderly. Can Fam Physician 2014;60:121–6.
65. Kennedy GJ, Marcus P. Use of antidepressants in older patients with co-morbid medical conditions: guidance from studies of depression in somatic illness. Drugs Aging 2005;22:273–87.
66. Sackheim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-tern outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006;163:1905–17.
67. Kok RM, Nolen WA, Hereen TJ. Outcome of late-life depression after 3 years of sequential treatment. Acta Psychiatr Scand 2009;119:274–81.
68. Whyte EM, Basinski J, Farhi P, et al. Geriatric depression treatment in nonresponders to selective serotonin reuptake inhibitors. J Clin Psychiatry 2004;65:1634–41.
69. Kok RM, Vink D, Hereen TJ, Nolen WA. Lithium augmentation compared with phenelzine in treatment-resistant depression in the elderly; an open, randomized, controlled trial. J Clin Psychiatry 2006;68:1177–85.
70. Kok RM. What is the role of medications in late life depression? Psychiatr Clin North Am 2003;36:597–605.
71. Wen XJ, Wang LM, Liu ZL,. Meta-analysis on the efficacy and tolerability of the augmentation of antidepressants with atypical antipsychotics in patients with major depressive disorder. Braz J Med Biol Res 2014;47:605–16.
72. Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised double-blind, placebo-controlled trial. Lancet 2015;386:2404–12.
73. Alexopoulos GS, Canuso CM, Gharabawi GM, et al. Placebo-controlled study of relapse prevention with risperidone augmentation in older patients with resistant depression. Am J Geriatr Psychiatry 2008;16:21–30.
74. Francis JL, Kumar A. Psychological treatment of late-life depression. Psychiatr Clin North Am 2013;36:561–75.
75. Gum AM, Arean PA, Hunkeler E, et al. Depression treatment preferences in older primary care patients. Gerontologist 2006;46:14–22.
76. Pinquart M, Duberstein PR, Lyness JM. Treatments for late-life depressive conditions: a meta-analytic comparison of pharmacotherapy and psychotherapy. Am J Psychiatry 2006;163:1493–501.
77. Beck Institute for Cognitive Behavior Therapy. Accessed 28 Dec 2016 at www.beckinstitute.org
78. Beck AT. Cognitive therapy: nature and relation to behavior therapy. Behav Ther 1970;1:184–200.
79. Landrevile P, Landry J, Baillargeon L, et al. Older adults’ acceptance of psychological and pharmacological treatments for depression. J Gerontology B Psychol Sci Soc Sci 2001;56:P285–91.
80. Thompson LW, Gallagher D, Breckenridge JS. Comparitive effectiveness of psychotherapies for depressed elders. J Consult Clin Psychol 1987;55:385–90.
81. Gallagher-Thompson D, Steffen AM. Comparative effects of cognitive-behavioral and brief psychodynamic psychotherapies for depressed family caregivers. J Consult Clin Psychol 1994;62:543–9.
82. Wilson KC, Mottram PG, Vassilas C. Psychotherapeutic treatments for older depressed people. Cochrane Database Syst Rev 2008;(1):CD004853
83. Markowitz JC, Weissman MM. Interpersonal psychotherapy: past, present and future. Clin Psychol Psychother 2012;19:99–105.
84. Van Schaik A, van Marwijk H, Ader H, et al. Interpersonal psychotherapy for elderly patients in primary care. Am J Geriatr Psychiatry 2006;14:777–86.
85. Reynolds CF 3rd, Frank E, Perel JM, et al. Nortriptyline and interpersonal psychotherapy as maintenance therapies for recurrent major depression: a randomized controlled trial in patients older than 59 years. JAMA 1999;281:39–45.
86. Reynolds CF 3rd, Dew MA, Pollock BG, et al. Maintenance treatment of major depression in old age. N Engl J Med 2006;354:1130–8.
87. Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med 1999;159:2349–56.
88. Krishnamurthy MN, Telles S. Assessing depression following two ancient Indian interventions: effects of yoga and ayurveda on older adults in a residential home. J Gerontol Nurs 2007;33:17–23.
89. Wang C, Bannuru R, Ramel J, et al. Tai Chi on psychological well-being: systematic review and meta-analysis. BMC Complement Altern Med 2010;10:23.
90. Cho KL. Effect of tai chi on depressive symptoms amongst Chinese older patients with depressive disorders: a randomized clinical trial. Med Sport Sci 2008;52:146–54.
91. Moritz S, Quan H, Rickhi B, et al. A home study-based spirituality education program decreases emotional distress and increases quality of life- a randomized, controlled trial. Altern Ther Health Med 2006;12:26–35.
92. Nyer M, Doorley J, Durham K, et al. What is the role of alternative treatments in late-life depression? Psychiatr Clin North Am 2013;36:577–96.
93. Petrides G, Fink M, Husain MM, et al. ECT remission rates in psychotic versus nonpsychotic depressed patients: a report from CORE. J ECT 2001;17:244–53.
94. Mankad MV, Beyer JL, Weiner RD, Krystal AD. Clinical manual of electroconvulsive therapy. American Psychiatric Publishing; 2010.
95. Kellner CH, Greenberg RM, Murrough JW, et al. ECT in treatment-resistant depression. Am J Psychiatry 2012;169:1238–44.
96. Flint AJ, Gagnon N. Effective use of electroconvulsive therapy in late-life depression. Can J Psychiatry 2002;47:734–41.
1. Vincent GK, Velkoff VA. The next four decades: the older population in the United States: 2010 to 2050. US Census Bureau: May 2010.
2. Koenig HG, Blazer DG. Epidemiology of geriatric affective disorders. Clinc Geriatr Med 1992; 8:235–51.
3. Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global gurden of disease study 2010. PLoS Med 2013;10:e1001547.
4 .American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
5 .Ellison JM, Kyomen HH, Harper DG. Depression in later life: an overview with treatment recommendations. Psychiatr Clin North Am 2012;35:203–29.
6. Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry 2005;62:1097–106.
7. Steffens DC, Skoog I, Norton MC, et al. Prevalence of depression and its treatment in an elderly population: the Cache County study. Arch Gen Psychiatry 2000;57:601–7.
8. Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003;289:3095–105.
9. McKinney BC, Sibille E. The age-by-disease interaction hypothesis of late-life depression. Am J Geriatr Psychiatry 2013;21:418–32.
10. Djernes JK. Prevalence and predictors of depression in populations of elderly: a review. Acta Psychiatr Scand 2006;113:372–87.
11. Blazer DG. Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci 2003;58:249–65.
12. Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry 2003;160:1147–56.
13. Ritchie K. Late-life depression. European Psychiatry 2014;29:577.
14. Hegeman JM, de Waal MW, Comijs HC, et al. Depression in later life: a more somatic presentation? J Affect Disord 2015;170:196–202.
15. Lackamp J, Schlachet R, Sajatovic M. Assessment and management of major depressive disorder in older adults. Psychiatria Danubina 2016;28(Suppl 1):95–98.
16. Morichi V, Dell’Aquila G, Trotta F, et al. Diagnosing and treating depression in older and oldest old. Curr Pharm Des 2015;21:1690–8.
17. Fiske AJ, Wetherell JL, Gatz M. Depression in older adults. Annu Rev Clin Psychol 2009;5:363–89.
18. Balsis S, Cully JA. Comparing depression diagnostic symptoms across younger and older adults. Aging Ment Health 2008;12:800–6.
19. Hegeman JM, Kok RM, van der Mast RC, Giltay EJ. Phenomenology of depression in older compared with younger adults: meta-analysis. Br J Psychiatry 2012;200:275–81.
20. Mitchell AJ, Bird V, Rizzo M, Meader N. Which version of the geriatric depression scale is most useful in medical settings and nursing homes? Diagnostic validity meta-analysis. Am J Geriatr Psychiatry 2010;18:1066–77.
21. Blazer DG. The psychiatric interview of older adults. In: Blazer D, Steffens D, Busse E, editors. Textbook of geriatric psychiatry. 3rd ed. Arlington, VA: American Psychiatric Publishing; 2004.
22. Spitzer RL, Kroenke K, Williams JW. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999;282:1737–44.
23. Richardson TM, He H, Podgorski C, et al. Screening for depression in aging services clients. Am J Geriatr Psychiatry 2010;18:1116–23.
24. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev 2012 Oct 17.
25. Unutzer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting a randomized controlled trial. JAMA 2002;288:2836–45.
26. Silver I, Herrmann N. Comprehensive psychiatric evaluation. In: Sadavoy J, Jarvik L, Grossberg G, Meyers B, editors. Comprehensive textbook of geriatric psychiatry. 3rd ed. New York: W.W. Norton; 2004.
27. Rapp MA, Dahlman K, Sano M, et al. Neuropsychological differences between late-onset and recurrent geriatric major depression. Am J Psychiatry 2005;162:691–8.
28. Sheline YI, Barch DM, Garcia K, et al. Cognitive function in late life depression: relationships to depression severity, cerebrovascular risk factors and processing speed. Biol Psychiatry 2006; 60:58–65.
29. Barnes DE, Yaffe K, Byers AL, et al. Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry 2012;69:493–8.
30. Morimoto SS, Kanellopoulos D, Manning KJ, Alexopoulos GS. Diagnosis and treatment of depression and cognitive impairment in late life. Ann NY Acad Sci 2015;1345:36–46.
31. Borson S, Scanlan J, Brush M, et al. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry 2000;15:1021–7.
32. Brodaty H, Low LF, Gibson L, Burns K. What is the best dementia screening instrument for general practitioners to use? Am J Geriatr Psychiatry 2006;14:391–400.
33. Ismail Z, Rajji TK, Shulman KI. Brief cognitive screening instruments: an update. Int J Geriatr Psychiatry 2010;25:111–20.
34. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98.
35. Vertesi A, Lever JA, Molloy DW, et al. Standardized Mini-Mental State Examination. Use and interpretation. Can Fam Physician 2001;47: 2018–23.
36. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–9.
37. Kiosses DN, Alexopoulos GS. IADL functions, cognitive deficits, and severity of depression: a preliminary study. Am J Geriatr Psychiatry 2005;13:244–9.
38. Alexopoulos GS, Vrontou C, Kakuma T, et al. Disability in geriatric depression. Am J Psychiatry 1996;153:877–85.
39. Centers for Disease Control and Prevention. National Center for Injury Prevention and Control. Web-Based Injury Statistics Query and Reporting System (WISQARS). Accessed 9 Feb 2016 at http://webappa.cdc.gov/sasweb/ncipc/dataRestriction_inj.html.
40. Corna LM, Cairney J, Streiner DL. Suicide ideation in older adults: relationship to mental health problems and service use. Gerontologist 2010;50:785–97.
41. Juurlink DN, Herrmann N, Szalai JP, et al. Medical illness and the risk of suicide in the elderly. Arch Intern Med 2004;164:1179–84.
42. Van Orden K, Conwell Y. Suicides in late life. Curr Psychiatry Rep 2011;13:234–41.
43. Conwell Y, Van Orden K, Caine ED. Suicide in older adults. Psychiatr Clin North Am 2011;34:451–68, ix.
44. Rudd MD, Berman AL, Joiner TE, et al. Warning signs for suicide: theory, research, and clinical applications. Suicide Life Threat Behav 2006;36:255–62.
45. Know the warning signs of suicide. American Association of Suicidology. Accessed 9 Feb 2016 at www.suicidology.org/resources/warning-signs.
46. Taylor W, Doraiswamy P. Use of the laboratory in the diagnostic workup of older adults. In: Blazer D, Steffen D, Busse E, editors. Textbook of geriatric psychiatry. 3rd ed. Arlington, VA: American Psychiatric Publishing; 2004.
47. Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry 1997;54:915–22.
48. ADGAP Status of Geriatrics Workforce Study. Accessed 26 Dec 2016 at www.americangeriatrics.org/files/documents/gwps/Table%201_29.pdf.
49. Alexopoulos G. Late-life mood disorders. In: Sadavoy J, Jarvik L, Grossberg G, Meyers B, editors. Comprehensive textbook of geriatric psychiatry. 3rd ed. New York: W.W. Norton; 2004.
50. Alexopoulos GS, Reynolds CF, Bruce ML, et al. Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. Am J Psychiatry 2009;166:882–90.
51. Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977;196:129–36.
52. Schotte CKW, Van den Bossche B, Doncker DD, et al. A biopsychosocial model as a guide for psychoeducation and treatment of depression. Depression Anxiety 2006;23:312–24.
53. Kupfer DJ, Frank E. The interaction of drug-and psychotherapy in the long-term treatment of depression. J Affect Disord 2001;62:131–7.
54. Katon W, Rutter C, Ludman EJ, et al. A randomized trial of relpase prevention of depression in primary care. Arch Gen Psychiatry 2001;58:241–7.
55. Kok RM, Nolen WA, Heeren TJ. Efficacy of treatment in older depressed patients: a systematic review and meta-analysis of double-blind randomized controlled trials with antidepressants. J Affect Disord 2012;141:103–15.
56. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62.
57. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382–9.
58. Mukai Y, Tampi RR. Treatment of depression in the elderly: a review of the recent literature on the efficacy of single- versus dual-action antidepressants. Clin Ther 2009;31:945–61.
59. Gildengers AG, Houck PR, Mulsant BH, et al. Course and rate of antidepressant response in the very old. J Affect Disord 2002;69:177–84.
60. Kozel FA, Trivedi MH, Wisniewski SR, et al. Treatment outcomes for older depressed patients with earlier versus late onset of first depressive episode. Am J Geriatr Psychiatry 2008;16:58–64.
61. Bains J, Birks J, Dening T. Antidepressants for treating depression in dementia. Cochrane Databse Syst Rev 2002;(4):CD003944.
62. Nelson JC, Devanand DP. A systematic review and meta-analysis of placebo-controlled antidepressant studies in people with depression and dementia. J Am Geriatr Soc 2011;59:577–85.
63. Mark TL, Joish VN, Hay JW, et al. Antidepressant use in geriatric populations: the burden of side effects and interactions and their impact on adherence and costs. Am J Geriatr Psychiatry 2011;19:211–21.
64. Frank C. Pharmacologic treatment of depression in the elderly. Can Fam Physician 2014;60:121–6.
65. Kennedy GJ, Marcus P. Use of antidepressants in older patients with co-morbid medical conditions: guidance from studies of depression in somatic illness. Drugs Aging 2005;22:273–87.
66. Sackheim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-tern outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006;163:1905–17.
67. Kok RM, Nolen WA, Hereen TJ. Outcome of late-life depression after 3 years of sequential treatment. Acta Psychiatr Scand 2009;119:274–81.
68. Whyte EM, Basinski J, Farhi P, et al. Geriatric depression treatment in nonresponders to selective serotonin reuptake inhibitors. J Clin Psychiatry 2004;65:1634–41.
69. Kok RM, Vink D, Hereen TJ, Nolen WA. Lithium augmentation compared with phenelzine in treatment-resistant depression in the elderly; an open, randomized, controlled trial. J Clin Psychiatry 2006;68:1177–85.
70. Kok RM. What is the role of medications in late life depression? Psychiatr Clin North Am 2003;36:597–605.
71. Wen XJ, Wang LM, Liu ZL,. Meta-analysis on the efficacy and tolerability of the augmentation of antidepressants with atypical antipsychotics in patients with major depressive disorder. Braz J Med Biol Res 2014;47:605–16.
72. Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised double-blind, placebo-controlled trial. Lancet 2015;386:2404–12.
73. Alexopoulos GS, Canuso CM, Gharabawi GM, et al. Placebo-controlled study of relapse prevention with risperidone augmentation in older patients with resistant depression. Am J Geriatr Psychiatry 2008;16:21–30.
74. Francis JL, Kumar A. Psychological treatment of late-life depression. Psychiatr Clin North Am 2013;36:561–75.
75. Gum AM, Arean PA, Hunkeler E, et al. Depression treatment preferences in older primary care patients. Gerontologist 2006;46:14–22.
76. Pinquart M, Duberstein PR, Lyness JM. Treatments for late-life depressive conditions: a meta-analytic comparison of pharmacotherapy and psychotherapy. Am J Psychiatry 2006;163:1493–501.
77. Beck Institute for Cognitive Behavior Therapy. Accessed 28 Dec 2016 at www.beckinstitute.org
78. Beck AT. Cognitive therapy: nature and relation to behavior therapy. Behav Ther 1970;1:184–200.
79. Landrevile P, Landry J, Baillargeon L, et al. Older adults’ acceptance of psychological and pharmacological treatments for depression. J Gerontology B Psychol Sci Soc Sci 2001;56:P285–91.
80. Thompson LW, Gallagher D, Breckenridge JS. Comparitive effectiveness of psychotherapies for depressed elders. J Consult Clin Psychol 1987;55:385–90.
81. Gallagher-Thompson D, Steffen AM. Comparative effects of cognitive-behavioral and brief psychodynamic psychotherapies for depressed family caregivers. J Consult Clin Psychol 1994;62:543–9.
82. Wilson KC, Mottram PG, Vassilas C. Psychotherapeutic treatments for older depressed people. Cochrane Database Syst Rev 2008;(1):CD004853
83. Markowitz JC, Weissman MM. Interpersonal psychotherapy: past, present and future. Clin Psychol Psychother 2012;19:99–105.
84. Van Schaik A, van Marwijk H, Ader H, et al. Interpersonal psychotherapy for elderly patients in primary care. Am J Geriatr Psychiatry 2006;14:777–86.
85. Reynolds CF 3rd, Frank E, Perel JM, et al. Nortriptyline and interpersonal psychotherapy as maintenance therapies for recurrent major depression: a randomized controlled trial in patients older than 59 years. JAMA 1999;281:39–45.
86. Reynolds CF 3rd, Dew MA, Pollock BG, et al. Maintenance treatment of major depression in old age. N Engl J Med 2006;354:1130–8.
87. Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med 1999;159:2349–56.
88. Krishnamurthy MN, Telles S. Assessing depression following two ancient Indian interventions: effects of yoga and ayurveda on older adults in a residential home. J Gerontol Nurs 2007;33:17–23.
89. Wang C, Bannuru R, Ramel J, et al. Tai Chi on psychological well-being: systematic review and meta-analysis. BMC Complement Altern Med 2010;10:23.
90. Cho KL. Effect of tai chi on depressive symptoms amongst Chinese older patients with depressive disorders: a randomized clinical trial. Med Sport Sci 2008;52:146–54.
91. Moritz S, Quan H, Rickhi B, et al. A home study-based spirituality education program decreases emotional distress and increases quality of life- a randomized, controlled trial. Altern Ther Health Med 2006;12:26–35.
92. Nyer M, Doorley J, Durham K, et al. What is the role of alternative treatments in late-life depression? Psychiatr Clin North Am 2013;36:577–96.
93. Petrides G, Fink M, Husain MM, et al. ECT remission rates in psychotic versus nonpsychotic depressed patients: a report from CORE. J ECT 2001;17:244–53.
94. Mankad MV, Beyer JL, Weiner RD, Krystal AD. Clinical manual of electroconvulsive therapy. American Psychiatric Publishing; 2010.
95. Kellner CH, Greenberg RM, Murrough JW, et al. ECT in treatment-resistant depression. Am J Psychiatry 2012;169:1238–44.
96. Flint AJ, Gagnon N. Effective use of electroconvulsive therapy in late-life depression. Can J Psychiatry 2002;47:734–41.
Clindamycin Phosphate–Tretinoin Combination Gel Revisited: Status Report on a Specific Formulation Used for Acne Treatment
Topical management of acne vulgaris (AV) incorporates a variety of agents with diverse modes of action (MOAs), including retinoids and antibiotics.1-3 The first topical retinoid developed for acne therapy was tretinoin, available in the United States since 1971.2,4 Topical retinoids, including tretinoin, exhibit multiple pharmacologic effects that are believed to correlate with efficacy for acne treatment,1,2,4,5 such as the reduction of inflammatory and comedonal lesions and contribution to dermal matrix remodeling.1,2,4-9 The predominant topical antibiotic used for acne treatment, often in combination with benzoyl peroxide (BP) and/or a topical retinoid, is clindamycin. Clindamycin is a lincosamide antibiotic that is closely related to erythromycin, a member of the macrolide antibiotic category.1,3,10 Available data support that over time topical clindamycin has sustained greater efficacy in reducing AV lesions than topical erythromycin; the latter also has been shown to exhibit a greater prevalence of Propionibacterium acnes resistance than clindamycin.1,3,10-12
Combination gel formulations of clindamycin phosphate 1.2%–tretinoin 0.025% (CP-Tret) are approved by the US Food and Drug Administration and available in the United States for once-daily treatment of AV in patients 12 years of age and older.13-15 Large-scale randomized controlled trials (RCTs) have demonstrated both efficacy and safety for these formulations.16,17 This article reviews important considerations related to both individual active ingredients (clindamycin phosphate [CP] and tretinoin [Tret]), formulation characteristics, and data from pivotal RCTs with a CP-Tret gel that has more recently been reintroduced into the US marketplace for acne therapy (Veltin, Aqua Pharmaceuticals).
What is the rationale behind combining CP and Tret in a single combination formulation?
Clindamycin is a lincosamide antibiotic that has been used for the treatment of AV for approximately 5 decades.1,3,10,17 The main MOA of clindamycin in the treatment of AV is believed to be reduction of P acnes; however, anti-inflammatory effects maypotentially play some role in AV lesion reduction.3,10,12,17-19 Multiple RCTs completed over approximately 3 decades and inclusive of more than 2000 participants treated topically with clindamycin as monotherapy have shown that the efficacy of this agent in reducing AV lesions has remained consistent overall,3,20-24 unlike topical erythromycin, which did not sustain its efficacy over a similar comparative time period.20 Importantly, these data are based on RCTs designed to evaluate the efficacy and safety of individual agents, including topical clindamycin; however, topical antibiotic therapy is not recommended as monotherapy for AV treatment due to emergence of antibiotic-resistant bacterial strains.1,3,11,12,25-28 Although the prevalence of resistant strains of P acnes is lower in the United States and many other countries for clindamycin versus erythromycin, the magnitude of clindamycin-resistant P acnes strains increases and response to clindamycin therapy may decrease when this agent is used alone.12,25-27,29,30 Therefore, it is recommended that a BP formulation that exhibits the ability to adequately reduce P acnes counts be used concurrently with antibiotic therapy for AV to reduce the emergence and proliferation of antibiotic-resistant P acnes organisms; short-contact BP therapy using a high-concentration (9.8%) emollient foam formulation and sufficient contact time (ie, 2 minutes) prior to washing off also has been shown to markedly reduce truncal P acnes organism counts.1,3,10-12,25-33 The Table depicts the major characteristics of clindamycin related to its use for treatment of AV.
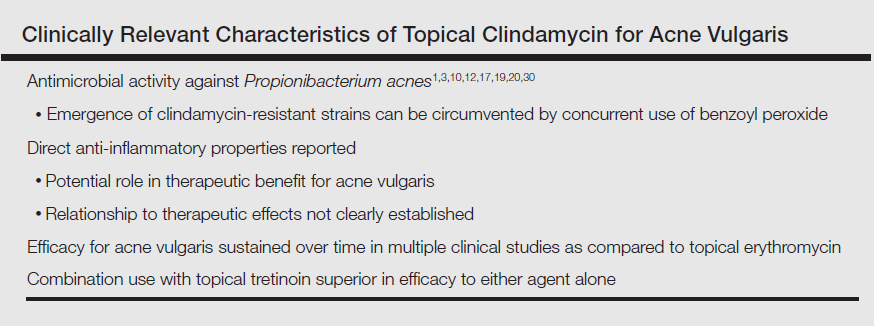
Tretinoin has been used extensively for the treatment of AV since its introduction in the United States in 1971.1,2,4,5 The proposed MOAs of topical retinoids, including tretinoin, based on available data include a variety of pharmacologic effects such as inhibition of follicular hyperkeratosis (decreased microcomedone formation), modulation of keratinocyte differentiation, anti-inflammatory properties, and inhibition of dermal matrix degradation (Figure).1,2,4,5,14,34,35 Topical retinoids, including tretinoin, have been shown to reduce both inflammatory and comedonal acne lesions, likely due to multiple MOAs, and are devoid of antibiotic properties.2,4-8,16 Available data support that topical combination therapy for AV with a retinoid and a topical antimicrobial agent augments the therapeutic benefit as compared to use of either agent alone.1-4,12,15,16,28,31,32
The rationale for incorporating both clindamycin and tretinoin together into one topical formulation includes combining different MOAs that appear to correlate with suppression of AV lesion formation and to improve patient adherence through once-daily application of a single topical product.16,31,32,36 Importantly, formulation researchers were able to combine CP-Tret into a specific aqueous gel formulation that maintained the stability of both active ingredients and proved to be effective and safe in preliminary studies completed in participants with AV.16,23,37-39 This aqueous formulation incorporated a limited number of excipients with low to negligible potential for cutaneous irritation or allergenicity, including anhydrous citric acid (chelating agent, preservative, emulsifier, acidulent), butylated hydroxytoluene (antioxidant), carbomer homopolymer type C (thickening agent, dispersing agent, biocompatible gel matrix), edetate disodium (chelating agent), laureth 4 (emulsifier, dissolution agent), methylparaben (preservative), propylene glycol (low-concentration humectant), purified water (diluent), and tromethamine (buffer, permeability enhancer).14
What are the clinical data evaluating the efficacy and tolerability/safety of the specific aqueous-based gel formulation of CP-Tret?
An aqueous-based gel formulation (referred to in the literature as a hydrogel) of CP-Tret is devoid of alcohol and contains the excipients described above.14 This formulation was shown to be efficacious, well tolerated, and safe in smaller clinical studies of participants with AV.23,37-39 Two large-scale phase 3 studies were completed (N=2219), powered to compare the efficacy and tolerability/safety of CP-Tret hydrogel (n=634) versus CP hydrogel (n=635), Tret hydrogel (n=635), and vehicle hydrogel (n=315) in participants with facial AV. All 4 study drug formulations in both studies—CP-Tret, CP, Tret, vehicle—used the same hydrogel vehicle, hereafter referred to simply as gel.16
In both trials, participants 12 years of age and older with AV were randomized to active drug groups versus vehicle (2:2:2:1 randomization), each applied once daily at bedtime for 12 weeks.16 The baseline demographics among all 4 study groups were well matched, with approximately two-thirds of white participants and one-third Asian (2%–3%), black (19%–21%), or Hispanic (9%–10%). Approximately half of enrolled participants were 16 years of age or younger (mean age [range], 19.0–20.2 years). Enrolled participants in each study group presented at baseline predominantly with facial AV of mild (grade 2 [20%–23% of enrolled participants]) or moderate (grade 3 [60%–62% of enrolled participants]) severity based on a protocol-mandated, 6-point investigator static global assessment scale. Investigator static global assessment scores and acne lesion counts, including noninflammatory (comedonal), inflammatory (papules, pustules), and total AV lesions, were evaluated at baseline and weeks 2, 4, 8, and 12 (end of study [EOS]). Among the 4 study groups at baseline, the range of mean lesion counts was 27.7 to 29.3 for noninflammatory lesions, 26.0 to 26.4 for inflammatory lesions, and 76.4 to 78.3 for total lesions. All enrolled participants met protocol-mandated, standardized, inclusion, exclusion, and prestudy washout period criteria.16
The primary efficacy end points determined based on intention-to-treat analysis were the percentage reduction in all 3 lesion counts at EOS compared to baseline and the proportion of participants who achieved scores of clear (grade 0) or almost clear (grade 1) at EOS. The secondary end point parameter was time to 50% reduction in total lesion counts.16
The study efficacy outcomes were as follows: The mean percentage reduction in inflammatory lesions at EOS versus baseline was significantly higher in the CP-Tret group than in each of the other 3 groups (CP-Tret, 53.4%; CP, 47.5%; Tret, 43.3%; vehicle, 30.3%)(P<.005).16 The mean percentage reduction in noninflammatory lesions at EOS versus baseline was significantly higher in the CP-Tret group than in each of the other 3 groups (CP-Tret, 45.2%; CP, 31.6%; Tret, 37.9%; vehicle, 18.5%)(P≤.0004). The mean percentage reduction in total AV lesions at EOS versus baseline was significantly higher in the CP-Tret group than in each of the other 3 groups (CP-Tret, 48.7%; CP, 38.3%; Tret, 40.3%; vehicle, 23.2%)(P≤.0001). The median time to 50% reduction in total AV lesion counts was significantly faster with CP-Tret (8 weeks) compared to the other 3 groups (CP, 12 weeks [P<.0001]; Tret, 12 weeks [P<.001]; vehicle, not reached by EOS [P<.0001]). The consistency of results, methodologies, and overall study characteristics between the 2 phase 3 RCTs allowed for accurate pooling of data.16
Tolerability and safety assessments were completed at each visit for all enrolled participants. No adverse events (AEs) were noted in approximately 90% of enrolled participants.16 The most common AEs noted over the course of the study were mild to moderate application-site reactions (eg, dryness, erythema, burning, pruritus, desquamation), mostly correlated with the 2 groups containing tretinoin—CP-Tret and Tret—which is not unanticipated with topical retinoid use; 1.2% of these participants withdrew from the study due to such application-site AEs. No serious AEs or systemic safety signals emerged during the study.16
What summarizing statements can be made about CP-Tret gel from these study results that may be helpful to clinicians treating patients with AV?
The gel formulation of CP-Tret incorporates active ingredients that target different pathophysiologic cascades in AV, providing antimicrobial, anti-inflammatory, and anticomedonal effects.
Applied once daily, CP-Tret gel demonstrated the ability to achieve complete or near-complete clearance of comedonal and papulopustular facial AV lesions of mild to moderate severity in approximately 40% of participants within 12 weeks of use in 2 large-scale RCTs.16 The ability to achieve a median 50% reduction in total lesions by 8 weeks of use provides relevant information for patients regarding reasonable expectations with therapy.
The favorable cutaneous tolerability profile and low number of AEs demonstrated with CP-Tret gel are major considerations, especially as skin tolerability reactions can impede patient adherence with treatment. Any issues that interfere with achieving a favorable therapeutic outcome can lead to patients giving up with their therapy.
The large number of patients with skin of color treated with CP-Tret gel (n=209) in the 2 phase 3 RCTs is important, as the spectrum of racial origins, skin types, and skin colors seen in dermatology practices is diversifying across the United States. Both clinicians and patients with skin of color are often concerned about the sequelae of medication-induced skin irritation, which can lead to ensuing dyschromia.
Concerns related to potential development of clindamycin-resistant P acnes with CP-Tret gel may be addressed by concurrent use of BP, including with leave-on or short-contact therapy.
Although phase 3 RCTs evaluate therapeutic agents as monotherapy, in real world clinical practice, combination topical regimens using different individual products are common to optimize therapeutic outcomes. Advantages of the CP-Tret gel formulation, if a clinician desires to use it along with another topical product, are once-daily use and the low risk for cutaneous irritation.
- Gollnick H, Cunliffe W, Berson D, et al. Management of acne: a report from the Global Alliance to improve outcomes in acne. J Am Acad Dermatol. 2003;49(suppl 1):S1-S37.
- Hui AM, Shalita AR. Topical retinoids. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:86-94.
- Del Rosso JQ. Topical antibiotics. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:95-104.
- Sami N. Topical retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2013:505-517.
- Baldwin HE, Nighland M, Kendall C, et al. 40 years of topical tretinoin use in review. J Drugs Dermatol. 2013;12:638-642.
- Retin-A Micro [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals; 2015.
- Tazorac [package insert]. Irvine, CA: Allergan, Inc; 2014.
- Differin [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2011.
- Kang S. The mechanism of action of topical retinoids. Cutis. 2005;75(suppl 2):10-13; discussion 13.
- Motaparthi K, Hsu S. Topical antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2013:445-459.
- Leyden JJ. The evolving role of Propionibacterium acnes in acne. Semin Cutan Med Surg. 2001;20:139-143.
- Leyden JJ, Del Rosso JQ, Webster GF. Clinical considerations in the treatment of acne vulgaris and other inflammatory skin disorders: focus on antibiotic resistance. Cutis. 2007;79(suppl 6):9-25.
- Ziana [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals; 2016.
- Veltin [package insert]. Exton, PA: Aqua Pharmaceuticals; 2015.
- Ochsendorf F. Clindamycin phosphate 1.2%/tretinoin 0.025%: a novel fixed-dose combination treatment for acne vulgaris. J Eur Acad Dermatol Venereol. 2015;29(suppl 5):8-13.
- Leyden JJ, Krochmal L, Yaroshinsky A. Two randomized, double-blind, controlled trials of 2219 subjects to compare the combination clindamycin/tretinoin hydrogel with each agent alone and vehicle for the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54:73-81.
- Del Rosso JQ. Topical and oral antibiotics for acne vulgaris. Semin Cutan Med Surg. 2016;35:57-61.
- Leyden JJ. Open-label evaluation of topical antimicrobial and anti-acne preparations for effectiveness versus Propionibacterium acnes in vivo. Cutis. 1992;49(suppl 6A):8-11.
- Del Rosso JQ, Schmidt NF. A review of the anti-inflammatory properties of clindamycin in the treatment of acne vulgaris. Cutis. 2010;85:15-24.
- Simonart T, Dramaix M. Treatment of acne with topical antibiotics: lessons from clinical studies. Br J Dermatol. 2005;153:395-403.
- Schlessinger J, Menter A, Gold M, et al. Clinical safety and efficacy studies of a novel formulation combining 1.2% clindamycin phosphate and 0.025% tretinoin for the treatment of acne vulgaris. J Drugs Dermatol. 2007;6:607-615.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Zouboulis CC, Derumeaux L, Decroix J, et al. A multicentre, single-blind, randomized comparison of a fixed clindamycin phosphate/tretinoin gel formulation (Velac) applied once daily and a clindamycin lotion formulation (Dalacin T) applied twice daily in the topical treatment of acne vulgaris. Br J Dermatol. 2000;143:498-505.
- Del Rosso JQ. Topical therapy for acne in women: is there a role for clindamycin phosphate-benzoyl peroxide gel? Cutis. 2014;94:177-182.
- Del Rosso JQ, Zeichner JA. The clinical relevance of antibiotic resistance: thirteen principles that every dermatologist needs to consider when prescribing antibiotic therapy. Dermatol Clin. 2016;34:167-173.
- Leyden JJ. Antibiotic resistance in the topical treatment of acne vulgaris. Cutis. 2004;73(6 suppl):6-10.
- Del Rosso JQ, Webster GF, Rosen T, et al. Status report from the Scientific Panel on Antibiotic Use in Dermatology of the American Acne and Rosacea Society: part 1: antibiotic prescribing patterns, sources of antibiotic exposure, antibiotic consumption and emergence of antibiotic resistance, impact of alterations in antibiotic prescribing, and clinical sequelae of antibiotic use. J Clin Aesthet Dermatol. 2016;9:18-24.
- Layton AM. Top ten list of clinical pearls in the treatment of acne vulgaris. Dermatol Clin. 2016;34:147-157.
- Leyden JJ. In vivo antibacterial effects of tretinoin-clindamycin and clindamycin alone on Propionibacterium acnes with varying clindamycin minimum inhibitory. J Drugs Dermatol. 2012;11:1434-1438.
- Cunliffe WJ, Holland KT, Bojar R, et al. A randomized, double-blind comparison of a clindamycin phosphate/benzoyl peroxide gel formulation and a matching clindamycin gel with respect to microbiologic activity and clinical efficacy in the topical treatment of acne vulgaris. Clin Ther. 2002;24:1117-1133.
- Villasenor J, Berson DS, Kroshinsky D. Combination therapy. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:105-112.
- Feneran A, Kaufman WS, Dabade TS, et al. Retinoid plus antimicrobial combination treatments for acne. Clin Cosmet Investig Dermatol. 2011;4:79-92.
- Leyden JJ, Del Rosso JQ. The effect of benzoyl peroxide 9.8% emollient foam on reduction of Propionibacterium acnes on the back using a short contact therapy approach. J Drugs Dermatol. 2012;11:830-833.
- Bikowski JB. Mechanisms of the comedolytic and anti-inflammatory properties of topical retinoids. J Drugs Dermatol. 2005;4:41-47.
- Del Rosso JQ. Retinoic acid receptors and topical acne therapy: establishing the link between gene expression and drug efficacy. Cutis. 2002;70:127-129.
- Zaghloul SS, Cunliffe WJ, Goodfield MJ. Objective assessment of compliance with treatments in acne. Br J Dermatol. 2005;152:1015-1021.
- Richter JR, Bousema MT, DeBoulle KLVM, et al. Efficacy of fixed clindamycin 1.2%, tretinoin 0.025% gel formulation (Velac) in topical control of facial acne lesions. J Dermatol Treat. 1998;9:81-90.
- Richter JR, Fӧrstrӧm LR, Kiistala UO, et al. Efficacy of fixed 1.2% clindamycin phosphate, 0.025% tretinoin gel formulation (Velac) and a proprietary 0.025% tretinoin gel formulation (Aberela) in the topical control of facial acne. J Eur Acad Dermatol Venereol. 1998;11:227-233.
- Cambazard F. Clinical efficacy of Velac, a new tretinoin and clindamycin gel in acne vulgaris. J Eur Acad Dermatol Venereol. 1998;11(suppl 1):S20-S27; discussion S28-S29.
Topical management of acne vulgaris (AV) incorporates a variety of agents with diverse modes of action (MOAs), including retinoids and antibiotics.1-3 The first topical retinoid developed for acne therapy was tretinoin, available in the United States since 1971.2,4 Topical retinoids, including tretinoin, exhibit multiple pharmacologic effects that are believed to correlate with efficacy for acne treatment,1,2,4,5 such as the reduction of inflammatory and comedonal lesions and contribution to dermal matrix remodeling.1,2,4-9 The predominant topical antibiotic used for acne treatment, often in combination with benzoyl peroxide (BP) and/or a topical retinoid, is clindamycin. Clindamycin is a lincosamide antibiotic that is closely related to erythromycin, a member of the macrolide antibiotic category.1,3,10 Available data support that over time topical clindamycin has sustained greater efficacy in reducing AV lesions than topical erythromycin; the latter also has been shown to exhibit a greater prevalence of Propionibacterium acnes resistance than clindamycin.1,3,10-12
Combination gel formulations of clindamycin phosphate 1.2%–tretinoin 0.025% (CP-Tret) are approved by the US Food and Drug Administration and available in the United States for once-daily treatment of AV in patients 12 years of age and older.13-15 Large-scale randomized controlled trials (RCTs) have demonstrated both efficacy and safety for these formulations.16,17 This article reviews important considerations related to both individual active ingredients (clindamycin phosphate [CP] and tretinoin [Tret]), formulation characteristics, and data from pivotal RCTs with a CP-Tret gel that has more recently been reintroduced into the US marketplace for acne therapy (Veltin, Aqua Pharmaceuticals).
What is the rationale behind combining CP and Tret in a single combination formulation?
Clindamycin is a lincosamide antibiotic that has been used for the treatment of AV for approximately 5 decades.1,3,10,17 The main MOA of clindamycin in the treatment of AV is believed to be reduction of P acnes; however, anti-inflammatory effects maypotentially play some role in AV lesion reduction.3,10,12,17-19 Multiple RCTs completed over approximately 3 decades and inclusive of more than 2000 participants treated topically with clindamycin as monotherapy have shown that the efficacy of this agent in reducing AV lesions has remained consistent overall,3,20-24 unlike topical erythromycin, which did not sustain its efficacy over a similar comparative time period.20 Importantly, these data are based on RCTs designed to evaluate the efficacy and safety of individual agents, including topical clindamycin; however, topical antibiotic therapy is not recommended as monotherapy for AV treatment due to emergence of antibiotic-resistant bacterial strains.1,3,11,12,25-28 Although the prevalence of resistant strains of P acnes is lower in the United States and many other countries for clindamycin versus erythromycin, the magnitude of clindamycin-resistant P acnes strains increases and response to clindamycin therapy may decrease when this agent is used alone.12,25-27,29,30 Therefore, it is recommended that a BP formulation that exhibits the ability to adequately reduce P acnes counts be used concurrently with antibiotic therapy for AV to reduce the emergence and proliferation of antibiotic-resistant P acnes organisms; short-contact BP therapy using a high-concentration (9.8%) emollient foam formulation and sufficient contact time (ie, 2 minutes) prior to washing off also has been shown to markedly reduce truncal P acnes organism counts.1,3,10-12,25-33 The Table depicts the major characteristics of clindamycin related to its use for treatment of AV.

Tretinoin has been used extensively for the treatment of AV since its introduction in the United States in 1971.1,2,4,5 The proposed MOAs of topical retinoids, including tretinoin, based on available data include a variety of pharmacologic effects such as inhibition of follicular hyperkeratosis (decreased microcomedone formation), modulation of keratinocyte differentiation, anti-inflammatory properties, and inhibition of dermal matrix degradation (Figure).1,2,4,5,14,34,35 Topical retinoids, including tretinoin, have been shown to reduce both inflammatory and comedonal acne lesions, likely due to multiple MOAs, and are devoid of antibiotic properties.2,4-8,16 Available data support that topical combination therapy for AV with a retinoid and a topical antimicrobial agent augments the therapeutic benefit as compared to use of either agent alone.1-4,12,15,16,28,31,32
The rationale for incorporating both clindamycin and tretinoin together into one topical formulation includes combining different MOAs that appear to correlate with suppression of AV lesion formation and to improve patient adherence through once-daily application of a single topical product.16,31,32,36 Importantly, formulation researchers were able to combine CP-Tret into a specific aqueous gel formulation that maintained the stability of both active ingredients and proved to be effective and safe in preliminary studies completed in participants with AV.16,23,37-39 This aqueous formulation incorporated a limited number of excipients with low to negligible potential for cutaneous irritation or allergenicity, including anhydrous citric acid (chelating agent, preservative, emulsifier, acidulent), butylated hydroxytoluene (antioxidant), carbomer homopolymer type C (thickening agent, dispersing agent, biocompatible gel matrix), edetate disodium (chelating agent), laureth 4 (emulsifier, dissolution agent), methylparaben (preservative), propylene glycol (low-concentration humectant), purified water (diluent), and tromethamine (buffer, permeability enhancer).14

What are the clinical data evaluating the efficacy and tolerability/safety of the specific aqueous-based gel formulation of CP-Tret?
An aqueous-based gel formulation (referred to in the literature as a hydrogel) of CP-Tret is devoid of alcohol and contains the excipients described above.14 This formulation was shown to be efficacious, well tolerated, and safe in smaller clinical studies of participants with AV.23,37-39 Two large-scale phase 3 studies were completed (N=2219), powered to compare the efficacy and tolerability/safety of CP-Tret hydrogel (n=634) versus CP hydrogel (n=635), Tret hydrogel (n=635), and vehicle hydrogel (n=315) in participants with facial AV. All 4 study drug formulations in both studies—CP-Tret, CP, Tret, vehicle—used the same hydrogel vehicle, hereafter referred to simply as gel.16
In both trials, participants 12 years of age and older with AV were randomized to active drug groups versus vehicle (2:2:2:1 randomization), each applied once daily at bedtime for 12 weeks.16 The baseline demographics among all 4 study groups were well matched, with approximately two-thirds of white participants and one-third Asian (2%–3%), black (19%–21%), or Hispanic (9%–10%). Approximately half of enrolled participants were 16 years of age or younger (mean age [range], 19.0–20.2 years). Enrolled participants in each study group presented at baseline predominantly with facial AV of mild (grade 2 [20%–23% of enrolled participants]) or moderate (grade 3 [60%–62% of enrolled participants]) severity based on a protocol-mandated, 6-point investigator static global assessment scale. Investigator static global assessment scores and acne lesion counts, including noninflammatory (comedonal), inflammatory (papules, pustules), and total AV lesions, were evaluated at baseline and weeks 2, 4, 8, and 12 (end of study [EOS]). Among the 4 study groups at baseline, the range of mean lesion counts was 27.7 to 29.3 for noninflammatory lesions, 26.0 to 26.4 for inflammatory lesions, and 76.4 to 78.3 for total lesions. All enrolled participants met protocol-mandated, standardized, inclusion, exclusion, and prestudy washout period criteria.16
The primary efficacy end points determined based on intention-to-treat analysis were the percentage reduction in all 3 lesion counts at EOS compared to baseline and the proportion of participants who achieved scores of clear (grade 0) or almost clear (grade 1) at EOS. The secondary end point parameter was time to 50% reduction in total lesion counts.16
The study efficacy outcomes were as follows: The mean percentage reduction in inflammatory lesions at EOS versus baseline was significantly higher in the CP-Tret group than in each of the other 3 groups (CP-Tret, 53.4%; CP, 47.5%; Tret, 43.3%; vehicle, 30.3%)(P<.005).16 The mean percentage reduction in noninflammatory lesions at EOS versus baseline was significantly higher in the CP-Tret group than in each of the other 3 groups (CP-Tret, 45.2%; CP, 31.6%; Tret, 37.9%; vehicle, 18.5%)(P≤.0004). The mean percentage reduction in total AV lesions at EOS versus baseline was significantly higher in the CP-Tret group than in each of the other 3 groups (CP-Tret, 48.7%; CP, 38.3%; Tret, 40.3%; vehicle, 23.2%)(P≤.0001). The median time to 50% reduction in total AV lesion counts was significantly faster with CP-Tret (8 weeks) compared to the other 3 groups (CP, 12 weeks [P<.0001]; Tret, 12 weeks [P<.001]; vehicle, not reached by EOS [P<.0001]). The consistency of results, methodologies, and overall study characteristics between the 2 phase 3 RCTs allowed for accurate pooling of data.16
Tolerability and safety assessments were completed at each visit for all enrolled participants. No adverse events (AEs) were noted in approximately 90% of enrolled participants.16 The most common AEs noted over the course of the study were mild to moderate application-site reactions (eg, dryness, erythema, burning, pruritus, desquamation), mostly correlated with the 2 groups containing tretinoin—CP-Tret and Tret—which is not unanticipated with topical retinoid use; 1.2% of these participants withdrew from the study due to such application-site AEs. No serious AEs or systemic safety signals emerged during the study.16
What summarizing statements can be made about CP-Tret gel from these study results that may be helpful to clinicians treating patients with AV?
The gel formulation of CP-Tret incorporates active ingredients that target different pathophysiologic cascades in AV, providing antimicrobial, anti-inflammatory, and anticomedonal effects.
Applied once daily, CP-Tret gel demonstrated the ability to achieve complete or near-complete clearance of comedonal and papulopustular facial AV lesions of mild to moderate severity in approximately 40% of participants within 12 weeks of use in 2 large-scale RCTs.16 The ability to achieve a median 50% reduction in total lesions by 8 weeks of use provides relevant information for patients regarding reasonable expectations with therapy.
The favorable cutaneous tolerability profile and low number of AEs demonstrated with CP-Tret gel are major considerations, especially as skin tolerability reactions can impede patient adherence with treatment. Any issues that interfere with achieving a favorable therapeutic outcome can lead to patients giving up with their therapy.
The large number of patients with skin of color treated with CP-Tret gel (n=209) in the 2 phase 3 RCTs is important, as the spectrum of racial origins, skin types, and skin colors seen in dermatology practices is diversifying across the United States. Both clinicians and patients with skin of color are often concerned about the sequelae of medication-induced skin irritation, which can lead to ensuing dyschromia.
Concerns related to potential development of clindamycin-resistant P acnes with CP-Tret gel may be addressed by concurrent use of BP, including with leave-on or short-contact therapy.
Although phase 3 RCTs evaluate therapeutic agents as monotherapy, in real world clinical practice, combination topical regimens using different individual products are common to optimize therapeutic outcomes. Advantages of the CP-Tret gel formulation, if a clinician desires to use it along with another topical product, are once-daily use and the low risk for cutaneous irritation.
Topical management of acne vulgaris (AV) incorporates a variety of agents with diverse modes of action (MOAs), including retinoids and antibiotics.1-3 The first topical retinoid developed for acne therapy was tretinoin, available in the United States since 1971.2,4 Topical retinoids, including tretinoin, exhibit multiple pharmacologic effects that are believed to correlate with efficacy for acne treatment,1,2,4,5 such as the reduction of inflammatory and comedonal lesions and contribution to dermal matrix remodeling.1,2,4-9 The predominant topical antibiotic used for acne treatment, often in combination with benzoyl peroxide (BP) and/or a topical retinoid, is clindamycin. Clindamycin is a lincosamide antibiotic that is closely related to erythromycin, a member of the macrolide antibiotic category.1,3,10 Available data support that over time topical clindamycin has sustained greater efficacy in reducing AV lesions than topical erythromycin; the latter also has been shown to exhibit a greater prevalence of Propionibacterium acnes resistance than clindamycin.1,3,10-12
Combination gel formulations of clindamycin phosphate 1.2%–tretinoin 0.025% (CP-Tret) are approved by the US Food and Drug Administration and available in the United States for once-daily treatment of AV in patients 12 years of age and older.13-15 Large-scale randomized controlled trials (RCTs) have demonstrated both efficacy and safety for these formulations.16,17 This article reviews important considerations related to both individual active ingredients (clindamycin phosphate [CP] and tretinoin [Tret]), formulation characteristics, and data from pivotal RCTs with a CP-Tret gel that has more recently been reintroduced into the US marketplace for acne therapy (Veltin, Aqua Pharmaceuticals).
What is the rationale behind combining CP and Tret in a single combination formulation?
Clindamycin is a lincosamide antibiotic that has been used for the treatment of AV for approximately 5 decades.1,3,10,17 The main MOA of clindamycin in the treatment of AV is believed to be reduction of P acnes; however, anti-inflammatory effects maypotentially play some role in AV lesion reduction.3,10,12,17-19 Multiple RCTs completed over approximately 3 decades and inclusive of more than 2000 participants treated topically with clindamycin as monotherapy have shown that the efficacy of this agent in reducing AV lesions has remained consistent overall,3,20-24 unlike topical erythromycin, which did not sustain its efficacy over a similar comparative time period.20 Importantly, these data are based on RCTs designed to evaluate the efficacy and safety of individual agents, including topical clindamycin; however, topical antibiotic therapy is not recommended as monotherapy for AV treatment due to emergence of antibiotic-resistant bacterial strains.1,3,11,12,25-28 Although the prevalence of resistant strains of P acnes is lower in the United States and many other countries for clindamycin versus erythromycin, the magnitude of clindamycin-resistant P acnes strains increases and response to clindamycin therapy may decrease when this agent is used alone.12,25-27,29,30 Therefore, it is recommended that a BP formulation that exhibits the ability to adequately reduce P acnes counts be used concurrently with antibiotic therapy for AV to reduce the emergence and proliferation of antibiotic-resistant P acnes organisms; short-contact BP therapy using a high-concentration (9.8%) emollient foam formulation and sufficient contact time (ie, 2 minutes) prior to washing off also has been shown to markedly reduce truncal P acnes organism counts.1,3,10-12,25-33 The Table depicts the major characteristics of clindamycin related to its use for treatment of AV.

Tretinoin has been used extensively for the treatment of AV since its introduction in the United States in 1971.1,2,4,5 The proposed MOAs of topical retinoids, including tretinoin, based on available data include a variety of pharmacologic effects such as inhibition of follicular hyperkeratosis (decreased microcomedone formation), modulation of keratinocyte differentiation, anti-inflammatory properties, and inhibition of dermal matrix degradation (Figure).1,2,4,5,14,34,35 Topical retinoids, including tretinoin, have been shown to reduce both inflammatory and comedonal acne lesions, likely due to multiple MOAs, and are devoid of antibiotic properties.2,4-8,16 Available data support that topical combination therapy for AV with a retinoid and a topical antimicrobial agent augments the therapeutic benefit as compared to use of either agent alone.1-4,12,15,16,28,31,32
The rationale for incorporating both clindamycin and tretinoin together into one topical formulation includes combining different MOAs that appear to correlate with suppression of AV lesion formation and to improve patient adherence through once-daily application of a single topical product.16,31,32,36 Importantly, formulation researchers were able to combine CP-Tret into a specific aqueous gel formulation that maintained the stability of both active ingredients and proved to be effective and safe in preliminary studies completed in participants with AV.16,23,37-39 This aqueous formulation incorporated a limited number of excipients with low to negligible potential for cutaneous irritation or allergenicity, including anhydrous citric acid (chelating agent, preservative, emulsifier, acidulent), butylated hydroxytoluene (antioxidant), carbomer homopolymer type C (thickening agent, dispersing agent, biocompatible gel matrix), edetate disodium (chelating agent), laureth 4 (emulsifier, dissolution agent), methylparaben (preservative), propylene glycol (low-concentration humectant), purified water (diluent), and tromethamine (buffer, permeability enhancer).14

What are the clinical data evaluating the efficacy and tolerability/safety of the specific aqueous-based gel formulation of CP-Tret?
An aqueous-based gel formulation (referred to in the literature as a hydrogel) of CP-Tret is devoid of alcohol and contains the excipients described above.14 This formulation was shown to be efficacious, well tolerated, and safe in smaller clinical studies of participants with AV.23,37-39 Two large-scale phase 3 studies were completed (N=2219), powered to compare the efficacy and tolerability/safety of CP-Tret hydrogel (n=634) versus CP hydrogel (n=635), Tret hydrogel (n=635), and vehicle hydrogel (n=315) in participants with facial AV. All 4 study drug formulations in both studies—CP-Tret, CP, Tret, vehicle—used the same hydrogel vehicle, hereafter referred to simply as gel.16
In both trials, participants 12 years of age and older with AV were randomized to active drug groups versus vehicle (2:2:2:1 randomization), each applied once daily at bedtime for 12 weeks.16 The baseline demographics among all 4 study groups were well matched, with approximately two-thirds of white participants and one-third Asian (2%–3%), black (19%–21%), or Hispanic (9%–10%). Approximately half of enrolled participants were 16 years of age or younger (mean age [range], 19.0–20.2 years). Enrolled participants in each study group presented at baseline predominantly with facial AV of mild (grade 2 [20%–23% of enrolled participants]) or moderate (grade 3 [60%–62% of enrolled participants]) severity based on a protocol-mandated, 6-point investigator static global assessment scale. Investigator static global assessment scores and acne lesion counts, including noninflammatory (comedonal), inflammatory (papules, pustules), and total AV lesions, were evaluated at baseline and weeks 2, 4, 8, and 12 (end of study [EOS]). Among the 4 study groups at baseline, the range of mean lesion counts was 27.7 to 29.3 for noninflammatory lesions, 26.0 to 26.4 for inflammatory lesions, and 76.4 to 78.3 for total lesions. All enrolled participants met protocol-mandated, standardized, inclusion, exclusion, and prestudy washout period criteria.16
The primary efficacy end points determined based on intention-to-treat analysis were the percentage reduction in all 3 lesion counts at EOS compared to baseline and the proportion of participants who achieved scores of clear (grade 0) or almost clear (grade 1) at EOS. The secondary end point parameter was time to 50% reduction in total lesion counts.16
The study efficacy outcomes were as follows: The mean percentage reduction in inflammatory lesions at EOS versus baseline was significantly higher in the CP-Tret group than in each of the other 3 groups (CP-Tret, 53.4%; CP, 47.5%; Tret, 43.3%; vehicle, 30.3%)(P<.005).16 The mean percentage reduction in noninflammatory lesions at EOS versus baseline was significantly higher in the CP-Tret group than in each of the other 3 groups (CP-Tret, 45.2%; CP, 31.6%; Tret, 37.9%; vehicle, 18.5%)(P≤.0004). The mean percentage reduction in total AV lesions at EOS versus baseline was significantly higher in the CP-Tret group than in each of the other 3 groups (CP-Tret, 48.7%; CP, 38.3%; Tret, 40.3%; vehicle, 23.2%)(P≤.0001). The median time to 50% reduction in total AV lesion counts was significantly faster with CP-Tret (8 weeks) compared to the other 3 groups (CP, 12 weeks [P<.0001]; Tret, 12 weeks [P<.001]; vehicle, not reached by EOS [P<.0001]). The consistency of results, methodologies, and overall study characteristics between the 2 phase 3 RCTs allowed for accurate pooling of data.16
Tolerability and safety assessments were completed at each visit for all enrolled participants. No adverse events (AEs) were noted in approximately 90% of enrolled participants.16 The most common AEs noted over the course of the study were mild to moderate application-site reactions (eg, dryness, erythema, burning, pruritus, desquamation), mostly correlated with the 2 groups containing tretinoin—CP-Tret and Tret—which is not unanticipated with topical retinoid use; 1.2% of these participants withdrew from the study due to such application-site AEs. No serious AEs or systemic safety signals emerged during the study.16
What summarizing statements can be made about CP-Tret gel from these study results that may be helpful to clinicians treating patients with AV?
The gel formulation of CP-Tret incorporates active ingredients that target different pathophysiologic cascades in AV, providing antimicrobial, anti-inflammatory, and anticomedonal effects.
Applied once daily, CP-Tret gel demonstrated the ability to achieve complete or near-complete clearance of comedonal and papulopustular facial AV lesions of mild to moderate severity in approximately 40% of participants within 12 weeks of use in 2 large-scale RCTs.16 The ability to achieve a median 50% reduction in total lesions by 8 weeks of use provides relevant information for patients regarding reasonable expectations with therapy.
The favorable cutaneous tolerability profile and low number of AEs demonstrated with CP-Tret gel are major considerations, especially as skin tolerability reactions can impede patient adherence with treatment. Any issues that interfere with achieving a favorable therapeutic outcome can lead to patients giving up with their therapy.
The large number of patients with skin of color treated with CP-Tret gel (n=209) in the 2 phase 3 RCTs is important, as the spectrum of racial origins, skin types, and skin colors seen in dermatology practices is diversifying across the United States. Both clinicians and patients with skin of color are often concerned about the sequelae of medication-induced skin irritation, which can lead to ensuing dyschromia.
Concerns related to potential development of clindamycin-resistant P acnes with CP-Tret gel may be addressed by concurrent use of BP, including with leave-on or short-contact therapy.
Although phase 3 RCTs evaluate therapeutic agents as monotherapy, in real world clinical practice, combination topical regimens using different individual products are common to optimize therapeutic outcomes. Advantages of the CP-Tret gel formulation, if a clinician desires to use it along with another topical product, are once-daily use and the low risk for cutaneous irritation.
- Gollnick H, Cunliffe W, Berson D, et al. Management of acne: a report from the Global Alliance to improve outcomes in acne. J Am Acad Dermatol. 2003;49(suppl 1):S1-S37.
- Hui AM, Shalita AR. Topical retinoids. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:86-94.
- Del Rosso JQ. Topical antibiotics. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:95-104.
- Sami N. Topical retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2013:505-517.
- Baldwin HE, Nighland M, Kendall C, et al. 40 years of topical tretinoin use in review. J Drugs Dermatol. 2013;12:638-642.
- Retin-A Micro [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals; 2015.
- Tazorac [package insert]. Irvine, CA: Allergan, Inc; 2014.
- Differin [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2011.
- Kang S. The mechanism of action of topical retinoids. Cutis. 2005;75(suppl 2):10-13; discussion 13.
- Motaparthi K, Hsu S. Topical antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2013:445-459.
- Leyden JJ. The evolving role of Propionibacterium acnes in acne. Semin Cutan Med Surg. 2001;20:139-143.
- Leyden JJ, Del Rosso JQ, Webster GF. Clinical considerations in the treatment of acne vulgaris and other inflammatory skin disorders: focus on antibiotic resistance. Cutis. 2007;79(suppl 6):9-25.
- Ziana [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals; 2016.
- Veltin [package insert]. Exton, PA: Aqua Pharmaceuticals; 2015.
- Ochsendorf F. Clindamycin phosphate 1.2%/tretinoin 0.025%: a novel fixed-dose combination treatment for acne vulgaris. J Eur Acad Dermatol Venereol. 2015;29(suppl 5):8-13.
- Leyden JJ, Krochmal L, Yaroshinsky A. Two randomized, double-blind, controlled trials of 2219 subjects to compare the combination clindamycin/tretinoin hydrogel with each agent alone and vehicle for the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54:73-81.
- Del Rosso JQ. Topical and oral antibiotics for acne vulgaris. Semin Cutan Med Surg. 2016;35:57-61.
- Leyden JJ. Open-label evaluation of topical antimicrobial and anti-acne preparations for effectiveness versus Propionibacterium acnes in vivo. Cutis. 1992;49(suppl 6A):8-11.
- Del Rosso JQ, Schmidt NF. A review of the anti-inflammatory properties of clindamycin in the treatment of acne vulgaris. Cutis. 2010;85:15-24.
- Simonart T, Dramaix M. Treatment of acne with topical antibiotics: lessons from clinical studies. Br J Dermatol. 2005;153:395-403.
- Schlessinger J, Menter A, Gold M, et al. Clinical safety and efficacy studies of a novel formulation combining 1.2% clindamycin phosphate and 0.025% tretinoin for the treatment of acne vulgaris. J Drugs Dermatol. 2007;6:607-615.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Zouboulis CC, Derumeaux L, Decroix J, et al. A multicentre, single-blind, randomized comparison of a fixed clindamycin phosphate/tretinoin gel formulation (Velac) applied once daily and a clindamycin lotion formulation (Dalacin T) applied twice daily in the topical treatment of acne vulgaris. Br J Dermatol. 2000;143:498-505.
- Del Rosso JQ. Topical therapy for acne in women: is there a role for clindamycin phosphate-benzoyl peroxide gel? Cutis. 2014;94:177-182.
- Del Rosso JQ, Zeichner JA. The clinical relevance of antibiotic resistance: thirteen principles that every dermatologist needs to consider when prescribing antibiotic therapy. Dermatol Clin. 2016;34:167-173.
- Leyden JJ. Antibiotic resistance in the topical treatment of acne vulgaris. Cutis. 2004;73(6 suppl):6-10.
- Del Rosso JQ, Webster GF, Rosen T, et al. Status report from the Scientific Panel on Antibiotic Use in Dermatology of the American Acne and Rosacea Society: part 1: antibiotic prescribing patterns, sources of antibiotic exposure, antibiotic consumption and emergence of antibiotic resistance, impact of alterations in antibiotic prescribing, and clinical sequelae of antibiotic use. J Clin Aesthet Dermatol. 2016;9:18-24.
- Layton AM. Top ten list of clinical pearls in the treatment of acne vulgaris. Dermatol Clin. 2016;34:147-157.
- Leyden JJ. In vivo antibacterial effects of tretinoin-clindamycin and clindamycin alone on Propionibacterium acnes with varying clindamycin minimum inhibitory. J Drugs Dermatol. 2012;11:1434-1438.
- Cunliffe WJ, Holland KT, Bojar R, et al. A randomized, double-blind comparison of a clindamycin phosphate/benzoyl peroxide gel formulation and a matching clindamycin gel with respect to microbiologic activity and clinical efficacy in the topical treatment of acne vulgaris. Clin Ther. 2002;24:1117-1133.
- Villasenor J, Berson DS, Kroshinsky D. Combination therapy. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:105-112.
- Feneran A, Kaufman WS, Dabade TS, et al. Retinoid plus antimicrobial combination treatments for acne. Clin Cosmet Investig Dermatol. 2011;4:79-92.
- Leyden JJ, Del Rosso JQ. The effect of benzoyl peroxide 9.8% emollient foam on reduction of Propionibacterium acnes on the back using a short contact therapy approach. J Drugs Dermatol. 2012;11:830-833.
- Bikowski JB. Mechanisms of the comedolytic and anti-inflammatory properties of topical retinoids. J Drugs Dermatol. 2005;4:41-47.
- Del Rosso JQ. Retinoic acid receptors and topical acne therapy: establishing the link between gene expression and drug efficacy. Cutis. 2002;70:127-129.
- Zaghloul SS, Cunliffe WJ, Goodfield MJ. Objective assessment of compliance with treatments in acne. Br J Dermatol. 2005;152:1015-1021.
- Richter JR, Bousema MT, DeBoulle KLVM, et al. Efficacy of fixed clindamycin 1.2%, tretinoin 0.025% gel formulation (Velac) in topical control of facial acne lesions. J Dermatol Treat. 1998;9:81-90.
- Richter JR, Fӧrstrӧm LR, Kiistala UO, et al. Efficacy of fixed 1.2% clindamycin phosphate, 0.025% tretinoin gel formulation (Velac) and a proprietary 0.025% tretinoin gel formulation (Aberela) in the topical control of facial acne. J Eur Acad Dermatol Venereol. 1998;11:227-233.
- Cambazard F. Clinical efficacy of Velac, a new tretinoin and clindamycin gel in acne vulgaris. J Eur Acad Dermatol Venereol. 1998;11(suppl 1):S20-S27; discussion S28-S29.
- Gollnick H, Cunliffe W, Berson D, et al. Management of acne: a report from the Global Alliance to improve outcomes in acne. J Am Acad Dermatol. 2003;49(suppl 1):S1-S37.
- Hui AM, Shalita AR. Topical retinoids. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:86-94.
- Del Rosso JQ. Topical antibiotics. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:95-104.
- Sami N. Topical retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2013:505-517.
- Baldwin HE, Nighland M, Kendall C, et al. 40 years of topical tretinoin use in review. J Drugs Dermatol. 2013;12:638-642.
- Retin-A Micro [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals; 2015.
- Tazorac [package insert]. Irvine, CA: Allergan, Inc; 2014.
- Differin [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2011.
- Kang S. The mechanism of action of topical retinoids. Cutis. 2005;75(suppl 2):10-13; discussion 13.
- Motaparthi K, Hsu S. Topical antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2013:445-459.
- Leyden JJ. The evolving role of Propionibacterium acnes in acne. Semin Cutan Med Surg. 2001;20:139-143.
- Leyden JJ, Del Rosso JQ, Webster GF. Clinical considerations in the treatment of acne vulgaris and other inflammatory skin disorders: focus on antibiotic resistance. Cutis. 2007;79(suppl 6):9-25.
- Ziana [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals; 2016.
- Veltin [package insert]. Exton, PA: Aqua Pharmaceuticals; 2015.
- Ochsendorf F. Clindamycin phosphate 1.2%/tretinoin 0.025%: a novel fixed-dose combination treatment for acne vulgaris. J Eur Acad Dermatol Venereol. 2015;29(suppl 5):8-13.
- Leyden JJ, Krochmal L, Yaroshinsky A. Two randomized, double-blind, controlled trials of 2219 subjects to compare the combination clindamycin/tretinoin hydrogel with each agent alone and vehicle for the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54:73-81.
- Del Rosso JQ. Topical and oral antibiotics for acne vulgaris. Semin Cutan Med Surg. 2016;35:57-61.
- Leyden JJ. Open-label evaluation of topical antimicrobial and anti-acne preparations for effectiveness versus Propionibacterium acnes in vivo. Cutis. 1992;49(suppl 6A):8-11.
- Del Rosso JQ, Schmidt NF. A review of the anti-inflammatory properties of clindamycin in the treatment of acne vulgaris. Cutis. 2010;85:15-24.
- Simonart T, Dramaix M. Treatment of acne with topical antibiotics: lessons from clinical studies. Br J Dermatol. 2005;153:395-403.
- Schlessinger J, Menter A, Gold M, et al. Clinical safety and efficacy studies of a novel formulation combining 1.2% clindamycin phosphate and 0.025% tretinoin for the treatment of acne vulgaris. J Drugs Dermatol. 2007;6:607-615.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Zouboulis CC, Derumeaux L, Decroix J, et al. A multicentre, single-blind, randomized comparison of a fixed clindamycin phosphate/tretinoin gel formulation (Velac) applied once daily and a clindamycin lotion formulation (Dalacin T) applied twice daily in the topical treatment of acne vulgaris. Br J Dermatol. 2000;143:498-505.
- Del Rosso JQ. Topical therapy for acne in women: is there a role for clindamycin phosphate-benzoyl peroxide gel? Cutis. 2014;94:177-182.
- Del Rosso JQ, Zeichner JA. The clinical relevance of antibiotic resistance: thirteen principles that every dermatologist needs to consider when prescribing antibiotic therapy. Dermatol Clin. 2016;34:167-173.
- Leyden JJ. Antibiotic resistance in the topical treatment of acne vulgaris. Cutis. 2004;73(6 suppl):6-10.
- Del Rosso JQ, Webster GF, Rosen T, et al. Status report from the Scientific Panel on Antibiotic Use in Dermatology of the American Acne and Rosacea Society: part 1: antibiotic prescribing patterns, sources of antibiotic exposure, antibiotic consumption and emergence of antibiotic resistance, impact of alterations in antibiotic prescribing, and clinical sequelae of antibiotic use. J Clin Aesthet Dermatol. 2016;9:18-24.
- Layton AM. Top ten list of clinical pearls in the treatment of acne vulgaris. Dermatol Clin. 2016;34:147-157.
- Leyden JJ. In vivo antibacterial effects of tretinoin-clindamycin and clindamycin alone on Propionibacterium acnes with varying clindamycin minimum inhibitory. J Drugs Dermatol. 2012;11:1434-1438.
- Cunliffe WJ, Holland KT, Bojar R, et al. A randomized, double-blind comparison of a clindamycin phosphate/benzoyl peroxide gel formulation and a matching clindamycin gel with respect to microbiologic activity and clinical efficacy in the topical treatment of acne vulgaris. Clin Ther. 2002;24:1117-1133.
- Villasenor J, Berson DS, Kroshinsky D. Combination therapy. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:105-112.
- Feneran A, Kaufman WS, Dabade TS, et al. Retinoid plus antimicrobial combination treatments for acne. Clin Cosmet Investig Dermatol. 2011;4:79-92.
- Leyden JJ, Del Rosso JQ. The effect of benzoyl peroxide 9.8% emollient foam on reduction of Propionibacterium acnes on the back using a short contact therapy approach. J Drugs Dermatol. 2012;11:830-833.
- Bikowski JB. Mechanisms of the comedolytic and anti-inflammatory properties of topical retinoids. J Drugs Dermatol. 2005;4:41-47.
- Del Rosso JQ. Retinoic acid receptors and topical acne therapy: establishing the link between gene expression and drug efficacy. Cutis. 2002;70:127-129.
- Zaghloul SS, Cunliffe WJ, Goodfield MJ. Objective assessment of compliance with treatments in acne. Br J Dermatol. 2005;152:1015-1021.
- Richter JR, Bousema MT, DeBoulle KLVM, et al. Efficacy of fixed clindamycin 1.2%, tretinoin 0.025% gel formulation (Velac) in topical control of facial acne lesions. J Dermatol Treat. 1998;9:81-90.
- Richter JR, Fӧrstrӧm LR, Kiistala UO, et al. Efficacy of fixed 1.2% clindamycin phosphate, 0.025% tretinoin gel formulation (Velac) and a proprietary 0.025% tretinoin gel formulation (Aberela) in the topical control of facial acne. J Eur Acad Dermatol Venereol. 1998;11:227-233.
- Cambazard F. Clinical efficacy of Velac, a new tretinoin and clindamycin gel in acne vulgaris. J Eur Acad Dermatol Venereol. 1998;11(suppl 1):S20-S27; discussion S28-S29.
Practice Points
- Clindamycin phosphate (CP)–tretinoin (Tret) formulated in an aqueous gel is effective based on clinical trials of the management of acne vulgaris (AV).
- The favorable tolerability of CP-Tret gel is advantageous, as topical agents often are used in combination with other therapies to treat AV, especially with a benzoyl peroxide–containing product.
- The availability of 2 active agents in 1 formulation is likely to optimize compliance.
Genital Wart Treatment
What does your patient need to know?
When a patient presents with a history of genital warts (GWs), find out when and where the lesions started; where the lesions are currently located; what new lesions have developed; what treatments have been administered (eg, physician applied, prescription) and which one(s) worked; what side effects to treatments have been experienced and at what dose; does a partner(s) have similar lesions; is there a history of other sexually transmitted diseases or genital cancer; is he/she immunocompromised (eg, human immunodeficiency virus, transplant, medications); and what is his/her sexual orientation.
Once all of the information has been gathered and the entire anogenital region has been examined, a treatment plan can be formulated. If the patient is immunocompromised or is a man who has sex with men, the risk for anogenital malignancy due to human papillomavirus (HPV) is higher, and GWs, which can be coinfected with oncogenic HPV types, should be treated more aggressively. If the patient is still getting new lesions, use of only a destructive method such as cryotherapy will likely lead to suboptimal results.
Any patients with GWs in the anal region but particularly those in high-risk groups such as men who have sex with men and human immunodeficiency virus–infected patients should have an anoscopy to evaluate for lesions on the anal mucosa and in the rectum.
What are your go-to treatments?
Prior treatments need to be taken into account; make sure to understand any side effects and how he/she applied the prior treatment before eliminating it as a viable option. Treatment usually depends on the number of lesions, surface area, anatomic locations involved, and size of the lesions. I start with a 2-pronged approach—a debulking therapy and a patient-applied topical therapy—which allows me to physically remove some of the lesions, typically the larger ones, and then have the patient apply a topical medication at home that will treat the smaller lesions as well as help to clear or decrease the burden of HPV virus on the skin. I use cryotherapy as a debulking agent, but curettage or podophyllin 25% also can be used in the office. I use imiquimod cream 5% as a first-line topical agent at the recommended dose of 3 times weekly; however, if after the first 2 weeks the patient has little response or too much irritation, I titrate the dose so that the patient has mild inflammation on the skin. The dose ultimately can range from daily to once weekly. Some patients who can only tolerate imiquimod once or twice weekly may require zinc oxide paste for the inguinal folds and scrotum to protect from irritation. Alternate topical medications for GWs include sinecatechins ointment 15% or cidofovir ointment 2%.
How do you keep patients compliant?
Start the visit with open communication about the disease, where it came from, what the risks are if it is not treated, and how we can best treat it to make sure we minimize those risks. I explain all of the treatment options as well as our role in treating these lesions and minimizing the risk for disease progression.
What do you do if they refuse treatment?
Most patients with GWs are motivated to be treated. If pain is a concern, such as with cryotherapy, I recommend topical treatments.
What patient resources do you recommend?
The American Academy of Dermatology (https://www.aad.org/public/diseases/contagious-skin-diseases/genital-warts), Harvard Medical School patient education center (Boston, Massachusetts)(http://www.patienteducationcenter.org/articles/genital-warts/), and American Family Physician (http://www.aafp.org/afp/2004/1215/p2345.html) provide patient materials that I recommend.
What does your patient need to know?
When a patient presents with a history of genital warts (GWs), find out when and where the lesions started; where the lesions are currently located; what new lesions have developed; what treatments have been administered (eg, physician applied, prescription) and which one(s) worked; what side effects to treatments have been experienced and at what dose; does a partner(s) have similar lesions; is there a history of other sexually transmitted diseases or genital cancer; is he/she immunocompromised (eg, human immunodeficiency virus, transplant, medications); and what is his/her sexual orientation.
Once all of the information has been gathered and the entire anogenital region has been examined, a treatment plan can be formulated. If the patient is immunocompromised or is a man who has sex with men, the risk for anogenital malignancy due to human papillomavirus (HPV) is higher, and GWs, which can be coinfected with oncogenic HPV types, should be treated more aggressively. If the patient is still getting new lesions, use of only a destructive method such as cryotherapy will likely lead to suboptimal results.
Any patients with GWs in the anal region but particularly those in high-risk groups such as men who have sex with men and human immunodeficiency virus–infected patients should have an anoscopy to evaluate for lesions on the anal mucosa and in the rectum.
What are your go-to treatments?
Prior treatments need to be taken into account; make sure to understand any side effects and how he/she applied the prior treatment before eliminating it as a viable option. Treatment usually depends on the number of lesions, surface area, anatomic locations involved, and size of the lesions. I start with a 2-pronged approach—a debulking therapy and a patient-applied topical therapy—which allows me to physically remove some of the lesions, typically the larger ones, and then have the patient apply a topical medication at home that will treat the smaller lesions as well as help to clear or decrease the burden of HPV virus on the skin. I use cryotherapy as a debulking agent, but curettage or podophyllin 25% also can be used in the office. I use imiquimod cream 5% as a first-line topical agent at the recommended dose of 3 times weekly; however, if after the first 2 weeks the patient has little response or too much irritation, I titrate the dose so that the patient has mild inflammation on the skin. The dose ultimately can range from daily to once weekly. Some patients who can only tolerate imiquimod once or twice weekly may require zinc oxide paste for the inguinal folds and scrotum to protect from irritation. Alternate topical medications for GWs include sinecatechins ointment 15% or cidofovir ointment 2%.
How do you keep patients compliant?
Start the visit with open communication about the disease, where it came from, what the risks are if it is not treated, and how we can best treat it to make sure we minimize those risks. I explain all of the treatment options as well as our role in treating these lesions and minimizing the risk for disease progression.
What do you do if they refuse treatment?
Most patients with GWs are motivated to be treated. If pain is a concern, such as with cryotherapy, I recommend topical treatments.
What patient resources do you recommend?
The American Academy of Dermatology (https://www.aad.org/public/diseases/contagious-skin-diseases/genital-warts), Harvard Medical School patient education center (Boston, Massachusetts)(http://www.patienteducationcenter.org/articles/genital-warts/), and American Family Physician (http://www.aafp.org/afp/2004/1215/p2345.html) provide patient materials that I recommend.
What does your patient need to know?
When a patient presents with a history of genital warts (GWs), find out when and where the lesions started; where the lesions are currently located; what new lesions have developed; what treatments have been administered (eg, physician applied, prescription) and which one(s) worked; what side effects to treatments have been experienced and at what dose; does a partner(s) have similar lesions; is there a history of other sexually transmitted diseases or genital cancer; is he/she immunocompromised (eg, human immunodeficiency virus, transplant, medications); and what is his/her sexual orientation.
Once all of the information has been gathered and the entire anogenital region has been examined, a treatment plan can be formulated. If the patient is immunocompromised or is a man who has sex with men, the risk for anogenital malignancy due to human papillomavirus (HPV) is higher, and GWs, which can be coinfected with oncogenic HPV types, should be treated more aggressively. If the patient is still getting new lesions, use of only a destructive method such as cryotherapy will likely lead to suboptimal results.
Any patients with GWs in the anal region but particularly those in high-risk groups such as men who have sex with men and human immunodeficiency virus–infected patients should have an anoscopy to evaluate for lesions on the anal mucosa and in the rectum.
What are your go-to treatments?
Prior treatments need to be taken into account; make sure to understand any side effects and how he/she applied the prior treatment before eliminating it as a viable option. Treatment usually depends on the number of lesions, surface area, anatomic locations involved, and size of the lesions. I start with a 2-pronged approach—a debulking therapy and a patient-applied topical therapy—which allows me to physically remove some of the lesions, typically the larger ones, and then have the patient apply a topical medication at home that will treat the smaller lesions as well as help to clear or decrease the burden of HPV virus on the skin. I use cryotherapy as a debulking agent, but curettage or podophyllin 25% also can be used in the office. I use imiquimod cream 5% as a first-line topical agent at the recommended dose of 3 times weekly; however, if after the first 2 weeks the patient has little response or too much irritation, I titrate the dose so that the patient has mild inflammation on the skin. The dose ultimately can range from daily to once weekly. Some patients who can only tolerate imiquimod once or twice weekly may require zinc oxide paste for the inguinal folds and scrotum to protect from irritation. Alternate topical medications for GWs include sinecatechins ointment 15% or cidofovir ointment 2%.
How do you keep patients compliant?
Start the visit with open communication about the disease, where it came from, what the risks are if it is not treated, and how we can best treat it to make sure we minimize those risks. I explain all of the treatment options as well as our role in treating these lesions and minimizing the risk for disease progression.
What do you do if they refuse treatment?
Most patients with GWs are motivated to be treated. If pain is a concern, such as with cryotherapy, I recommend topical treatments.
What patient resources do you recommend?
The American Academy of Dermatology (https://www.aad.org/public/diseases/contagious-skin-diseases/genital-warts), Harvard Medical School patient education center (Boston, Massachusetts)(http://www.patienteducationcenter.org/articles/genital-warts/), and American Family Physician (http://www.aafp.org/afp/2004/1215/p2345.html) provide patient materials that I recommend.
One Diagnosis and Modifier -25: Appropriate or Audit Target?
An established patient comes into your office with a painful new lesion on the hand. He thinks it may be a wart. You take a focused history of the lesion, do a physical examination, and confirm the diagnosis of verruca vulgaris. You discuss treatment options, risks, and the benefits of treatment, as well as the pathophysiology of warts. The decision is made to proceed that same day with cryosurgical destruction, which is performed. You feel that billing both an office visit with an appended modifier -25 and the benign destruction code 17110 is warranted, but your biller says only the procedure should be reported. Who is correct?
Modifier -25 use has come under increased scrutiny by insurers and regulators. There is a perception that this modifier is frequently used inappropriately or unnecessarily. In fact, the Office of Inspector General reported that 35% of claims using modifier -25 that Medicare allowed did not meet the requirements. The Office of Inspector General has recommended that the “[Centers for Medicare & Medicaid Services] should work with carriers to reduce the number of claims submitted using modifier -25” and “include modifier -25 reviews in their medical review strategies.”1 Translation: More chart reviews and audits! In my discussions with insurer medical directors, they point to the single diagnosis modifier -25 as likely abused and feel that its use in this context is almost never appropriate. Their audits have been focused on this aspect of dermatologists’ coding. In addition, some private insurers have started to discount reimbursement for office visits billed with modifier -25 by 50% to account for the level of perceived overuse.2
The Current Procedural Terminology description of modifier -25 is relatively clear: Modifier -25 is used to facilitate billing of evaluation and management (E/M) services on the day of a procedure for which separate payment may be made.3 This modifier indicates that a significant, separately identifiable E/M service was performed by the same physician on the day of a procedure. To appropriately bill both the E/M service and the procedure, the physician must indicate that the patient’s condition required an E/M service “above and beyond the usual pre- and post-operative work of a procedure.”4 However, it is largely left up to the physician to decide what constitutes the significant, separately identifiable E/M service.
As dermatologists, we all report modifier -25 appropriately as part of our daily practice. Performance of a medically necessary procedure on the same day as an E/M service generally is done to facilitate a prompt diagnosis or streamline treatment of a complex condition. Providing distinct medically necessary services on the same date allows physicians to provide effective and efficient high-quality care, in many cases saving patients a return visit. The most common scenario for using modifier -25 involves multiple concerns and multiple diagnoses, some of which are not associated with a procedure(s) that is performed on the same date of service. With multiple diagnoses, it is straightforward to demonstrate the separate E/M service associated with the nonprocedure-related diagnosis code(s); however, with one diagnosis for both the office visit and the procedure, clear documentation of the separate and identifiable E/M service is critical and is dependent on understanding what is included in the global surgical package.
Insurer payment for procedures includes local or topical anesthesia, the surgical service/procedure itself, immediate postoperative care including dictating the operative note, meeting/discussing the patient’s procedure with family and other physicians, evaluating the patient in postanesthesia/recovery area, and writing orders for the patient. This group of services is called the global surgical package. For minor procedures (ie, those with either 0- or 10-day global periods), the surgical package also includes same-day E/M associated with the decision to perform surgery. An appropriate history and physical examination, as well as the discussion of differential diagnosis, treatment options, and risk and benefits of treatments, are all included in the payment of a minor procedure itself. Therefore, if an E/M service is performed on the same day as a minor procedure to decide whether to proceed with the minor surgical procedure, this E/M service cannot be separately reported. Moreover, the fact that the patient is new to the physician is not sufficient to allow reporting of an E/M service with such a minor procedure. For major procedures (ie, those with 90-day postoperative periods), the decision for surgery is excluded from the global surgical package.
Therefore, it is clear that the clinical scenario for verruca vulgaris treatment as described at the start of this article does not meet criteria for an office visit billed in addition to the destruction. The E/M services performed prior to the patient’s verruca vulgaris treatment are integral to and necessary for the decision to perform the procedure. Making and confirming the diagnosis of a condition or lesion prior to a procedure either by physical evaluation or by interpretation of a pathology report is part of the evaluation required to make the decision to proceed with a particular procedure.
There are clinical scenarios in which a physician can support additional E/M services beyond that of the procedure with just one diagnosis. If a patient presents with warts on the hand and face with resultant cryosurgical destruction done on the hand and a prescription for imiquimod to be used on the face to induce immunologic clearance of viral infection and decrease the risk of scarring, it is clear that a significant and separately identifiable E/M service exists. The evaluation of the facial warts and the prescription of medication and discussion of the risks, benefits, and therapeutic effects of that prescription is definitely distinct from the procedure. Similarly, if an evaluation of a patient with a rash results in only a diagnostic biopsy with no separate cognitive services other than the decision to perform the biopsy, an office visit charge in addition to the biopsy charge would not be warranted. However, if in addition to the biopsy the rash also is treated with topical or systemic steroids because of pruritus or a more extensive evaluation for systemic complications is required, an office visit charge is appropriate.
The frequent use of modifier -25 is a critical part of a high-quality and cost-effective dermatology practice. Same-day performance of E/M services and minor procedures allows for more rapid and efficient diagnosis and treatment of various conditions as well as minimizing unnecessary office visits. However, modifier -25 use, particularly in the context of the same diagnosis for the office visit and the procedure, is under intense insurer scrutiny. Careful and complete documentation of the additional E/M service provided, including the additional history, physical examination results, and treatment considerations above and beyond those typically required by the minor procedure, will reduce the likelihood of redeterminations from reviews and audits. Understanding Medicare guidelines and National Correct Coding Initiative recommendations will help keep the dermatologist out of hot water.5
- Levinson DR. Use of modifier 25. Office of Inspector General website. https://oig.hhs.gov/oei/reports/oei-07-03-00470.pdf. Published November 2005. Accessed January 31, 2017.
- Modifier tables. Tufts Health Plan website. https://tuftshealthplan.com/documents/providers/payment-policies/modifier-tables-payment-policy. Revised April 2016. Accessed February 24, 2017.
- Current Procedural Terminology 2017, Professional Edition. Chicago, IL: American Medical Association; 2016.
- Centers for Medicare & Medicaid Services. Payment for evaluation and management services provided during global period of surgery. MLN Matters. May 19, 2006. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/MM5025.pdf. Updated November 1, 2012. Accessed January 31, 2017.
- National Correct Coding Initiative Policy Manual for Medicare Services—Effective January 1, 2017. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Medicare/Coding/NationalCorrectCodInitEd/Downloads/2017-NCCI-Policy-Manual.zip. Accessed February 24, 2017.
An established patient comes into your office with a painful new lesion on the hand. He thinks it may be a wart. You take a focused history of the lesion, do a physical examination, and confirm the diagnosis of verruca vulgaris. You discuss treatment options, risks, and the benefits of treatment, as well as the pathophysiology of warts. The decision is made to proceed that same day with cryosurgical destruction, which is performed. You feel that billing both an office visit with an appended modifier -25 and the benign destruction code 17110 is warranted, but your biller says only the procedure should be reported. Who is correct?
Modifier -25 use has come under increased scrutiny by insurers and regulators. There is a perception that this modifier is frequently used inappropriately or unnecessarily. In fact, the Office of Inspector General reported that 35% of claims using modifier -25 that Medicare allowed did not meet the requirements. The Office of Inspector General has recommended that the “[Centers for Medicare & Medicaid Services] should work with carriers to reduce the number of claims submitted using modifier -25” and “include modifier -25 reviews in their medical review strategies.”1 Translation: More chart reviews and audits! In my discussions with insurer medical directors, they point to the single diagnosis modifier -25 as likely abused and feel that its use in this context is almost never appropriate. Their audits have been focused on this aspect of dermatologists’ coding. In addition, some private insurers have started to discount reimbursement for office visits billed with modifier -25 by 50% to account for the level of perceived overuse.2
The Current Procedural Terminology description of modifier -25 is relatively clear: Modifier -25 is used to facilitate billing of evaluation and management (E/M) services on the day of a procedure for which separate payment may be made.3 This modifier indicates that a significant, separately identifiable E/M service was performed by the same physician on the day of a procedure. To appropriately bill both the E/M service and the procedure, the physician must indicate that the patient’s condition required an E/M service “above and beyond the usual pre- and post-operative work of a procedure.”4 However, it is largely left up to the physician to decide what constitutes the significant, separately identifiable E/M service.
As dermatologists, we all report modifier -25 appropriately as part of our daily practice. Performance of a medically necessary procedure on the same day as an E/M service generally is done to facilitate a prompt diagnosis or streamline treatment of a complex condition. Providing distinct medically necessary services on the same date allows physicians to provide effective and efficient high-quality care, in many cases saving patients a return visit. The most common scenario for using modifier -25 involves multiple concerns and multiple diagnoses, some of which are not associated with a procedure(s) that is performed on the same date of service. With multiple diagnoses, it is straightforward to demonstrate the separate E/M service associated with the nonprocedure-related diagnosis code(s); however, with one diagnosis for both the office visit and the procedure, clear documentation of the separate and identifiable E/M service is critical and is dependent on understanding what is included in the global surgical package.
Insurer payment for procedures includes local or topical anesthesia, the surgical service/procedure itself, immediate postoperative care including dictating the operative note, meeting/discussing the patient’s procedure with family and other physicians, evaluating the patient in postanesthesia/recovery area, and writing orders for the patient. This group of services is called the global surgical package. For minor procedures (ie, those with either 0- or 10-day global periods), the surgical package also includes same-day E/M associated with the decision to perform surgery. An appropriate history and physical examination, as well as the discussion of differential diagnosis, treatment options, and risk and benefits of treatments, are all included in the payment of a minor procedure itself. Therefore, if an E/M service is performed on the same day as a minor procedure to decide whether to proceed with the minor surgical procedure, this E/M service cannot be separately reported. Moreover, the fact that the patient is new to the physician is not sufficient to allow reporting of an E/M service with such a minor procedure. For major procedures (ie, those with 90-day postoperative periods), the decision for surgery is excluded from the global surgical package.
Therefore, it is clear that the clinical scenario for verruca vulgaris treatment as described at the start of this article does not meet criteria for an office visit billed in addition to the destruction. The E/M services performed prior to the patient’s verruca vulgaris treatment are integral to and necessary for the decision to perform the procedure. Making and confirming the diagnosis of a condition or lesion prior to a procedure either by physical evaluation or by interpretation of a pathology report is part of the evaluation required to make the decision to proceed with a particular procedure.
There are clinical scenarios in which a physician can support additional E/M services beyond that of the procedure with just one diagnosis. If a patient presents with warts on the hand and face with resultant cryosurgical destruction done on the hand and a prescription for imiquimod to be used on the face to induce immunologic clearance of viral infection and decrease the risk of scarring, it is clear that a significant and separately identifiable E/M service exists. The evaluation of the facial warts and the prescription of medication and discussion of the risks, benefits, and therapeutic effects of that prescription is definitely distinct from the procedure. Similarly, if an evaluation of a patient with a rash results in only a diagnostic biopsy with no separate cognitive services other than the decision to perform the biopsy, an office visit charge in addition to the biopsy charge would not be warranted. However, if in addition to the biopsy the rash also is treated with topical or systemic steroids because of pruritus or a more extensive evaluation for systemic complications is required, an office visit charge is appropriate.
The frequent use of modifier -25 is a critical part of a high-quality and cost-effective dermatology practice. Same-day performance of E/M services and minor procedures allows for more rapid and efficient diagnosis and treatment of various conditions as well as minimizing unnecessary office visits. However, modifier -25 use, particularly in the context of the same diagnosis for the office visit and the procedure, is under intense insurer scrutiny. Careful and complete documentation of the additional E/M service provided, including the additional history, physical examination results, and treatment considerations above and beyond those typically required by the minor procedure, will reduce the likelihood of redeterminations from reviews and audits. Understanding Medicare guidelines and National Correct Coding Initiative recommendations will help keep the dermatologist out of hot water.5
An established patient comes into your office with a painful new lesion on the hand. He thinks it may be a wart. You take a focused history of the lesion, do a physical examination, and confirm the diagnosis of verruca vulgaris. You discuss treatment options, risks, and the benefits of treatment, as well as the pathophysiology of warts. The decision is made to proceed that same day with cryosurgical destruction, which is performed. You feel that billing both an office visit with an appended modifier -25 and the benign destruction code 17110 is warranted, but your biller says only the procedure should be reported. Who is correct?
Modifier -25 use has come under increased scrutiny by insurers and regulators. There is a perception that this modifier is frequently used inappropriately or unnecessarily. In fact, the Office of Inspector General reported that 35% of claims using modifier -25 that Medicare allowed did not meet the requirements. The Office of Inspector General has recommended that the “[Centers for Medicare & Medicaid Services] should work with carriers to reduce the number of claims submitted using modifier -25” and “include modifier -25 reviews in their medical review strategies.”1 Translation: More chart reviews and audits! In my discussions with insurer medical directors, they point to the single diagnosis modifier -25 as likely abused and feel that its use in this context is almost never appropriate. Their audits have been focused on this aspect of dermatologists’ coding. In addition, some private insurers have started to discount reimbursement for office visits billed with modifier -25 by 50% to account for the level of perceived overuse.2
The Current Procedural Terminology description of modifier -25 is relatively clear: Modifier -25 is used to facilitate billing of evaluation and management (E/M) services on the day of a procedure for which separate payment may be made.3 This modifier indicates that a significant, separately identifiable E/M service was performed by the same physician on the day of a procedure. To appropriately bill both the E/M service and the procedure, the physician must indicate that the patient’s condition required an E/M service “above and beyond the usual pre- and post-operative work of a procedure.”4 However, it is largely left up to the physician to decide what constitutes the significant, separately identifiable E/M service.
As dermatologists, we all report modifier -25 appropriately as part of our daily practice. Performance of a medically necessary procedure on the same day as an E/M service generally is done to facilitate a prompt diagnosis or streamline treatment of a complex condition. Providing distinct medically necessary services on the same date allows physicians to provide effective and efficient high-quality care, in many cases saving patients a return visit. The most common scenario for using modifier -25 involves multiple concerns and multiple diagnoses, some of which are not associated with a procedure(s) that is performed on the same date of service. With multiple diagnoses, it is straightforward to demonstrate the separate E/M service associated with the nonprocedure-related diagnosis code(s); however, with one diagnosis for both the office visit and the procedure, clear documentation of the separate and identifiable E/M service is critical and is dependent on understanding what is included in the global surgical package.
Insurer payment for procedures includes local or topical anesthesia, the surgical service/procedure itself, immediate postoperative care including dictating the operative note, meeting/discussing the patient’s procedure with family and other physicians, evaluating the patient in postanesthesia/recovery area, and writing orders for the patient. This group of services is called the global surgical package. For minor procedures (ie, those with either 0- or 10-day global periods), the surgical package also includes same-day E/M associated with the decision to perform surgery. An appropriate history and physical examination, as well as the discussion of differential diagnosis, treatment options, and risk and benefits of treatments, are all included in the payment of a minor procedure itself. Therefore, if an E/M service is performed on the same day as a minor procedure to decide whether to proceed with the minor surgical procedure, this E/M service cannot be separately reported. Moreover, the fact that the patient is new to the physician is not sufficient to allow reporting of an E/M service with such a minor procedure. For major procedures (ie, those with 90-day postoperative periods), the decision for surgery is excluded from the global surgical package.
Therefore, it is clear that the clinical scenario for verruca vulgaris treatment as described at the start of this article does not meet criteria for an office visit billed in addition to the destruction. The E/M services performed prior to the patient’s verruca vulgaris treatment are integral to and necessary for the decision to perform the procedure. Making and confirming the diagnosis of a condition or lesion prior to a procedure either by physical evaluation or by interpretation of a pathology report is part of the evaluation required to make the decision to proceed with a particular procedure.
There are clinical scenarios in which a physician can support additional E/M services beyond that of the procedure with just one diagnosis. If a patient presents with warts on the hand and face with resultant cryosurgical destruction done on the hand and a prescription for imiquimod to be used on the face to induce immunologic clearance of viral infection and decrease the risk of scarring, it is clear that a significant and separately identifiable E/M service exists. The evaluation of the facial warts and the prescription of medication and discussion of the risks, benefits, and therapeutic effects of that prescription is definitely distinct from the procedure. Similarly, if an evaluation of a patient with a rash results in only a diagnostic biopsy with no separate cognitive services other than the decision to perform the biopsy, an office visit charge in addition to the biopsy charge would not be warranted. However, if in addition to the biopsy the rash also is treated with topical or systemic steroids because of pruritus or a more extensive evaluation for systemic complications is required, an office visit charge is appropriate.
The frequent use of modifier -25 is a critical part of a high-quality and cost-effective dermatology practice. Same-day performance of E/M services and minor procedures allows for more rapid and efficient diagnosis and treatment of various conditions as well as minimizing unnecessary office visits. However, modifier -25 use, particularly in the context of the same diagnosis for the office visit and the procedure, is under intense insurer scrutiny. Careful and complete documentation of the additional E/M service provided, including the additional history, physical examination results, and treatment considerations above and beyond those typically required by the minor procedure, will reduce the likelihood of redeterminations from reviews and audits. Understanding Medicare guidelines and National Correct Coding Initiative recommendations will help keep the dermatologist out of hot water.5
- Levinson DR. Use of modifier 25. Office of Inspector General website. https://oig.hhs.gov/oei/reports/oei-07-03-00470.pdf. Published November 2005. Accessed January 31, 2017.
- Modifier tables. Tufts Health Plan website. https://tuftshealthplan.com/documents/providers/payment-policies/modifier-tables-payment-policy. Revised April 2016. Accessed February 24, 2017.
- Current Procedural Terminology 2017, Professional Edition. Chicago, IL: American Medical Association; 2016.
- Centers for Medicare & Medicaid Services. Payment for evaluation and management services provided during global period of surgery. MLN Matters. May 19, 2006. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/MM5025.pdf. Updated November 1, 2012. Accessed January 31, 2017.
- National Correct Coding Initiative Policy Manual for Medicare Services—Effective January 1, 2017. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Medicare/Coding/NationalCorrectCodInitEd/Downloads/2017-NCCI-Policy-Manual.zip. Accessed February 24, 2017.
- Levinson DR. Use of modifier 25. Office of Inspector General website. https://oig.hhs.gov/oei/reports/oei-07-03-00470.pdf. Published November 2005. Accessed January 31, 2017.
- Modifier tables. Tufts Health Plan website. https://tuftshealthplan.com/documents/providers/payment-policies/modifier-tables-payment-policy. Revised April 2016. Accessed February 24, 2017.
- Current Procedural Terminology 2017, Professional Edition. Chicago, IL: American Medical Association; 2016.
- Centers for Medicare & Medicaid Services. Payment for evaluation and management services provided during global period of surgery. MLN Matters. May 19, 2006. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/MM5025.pdf. Updated November 1, 2012. Accessed January 31, 2017.
- National Correct Coding Initiative Policy Manual for Medicare Services—Effective January 1, 2017. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Medicare/Coding/NationalCorrectCodInitEd/Downloads/2017-NCCI-Policy-Manual.zip. Accessed February 24, 2017.
Practice Points
- Modifier -25 use is appropriate and critical for high-quality, efficient dermatology care.
- Correct use and documentation can help avoid loss of audits associated with modifier -25.
Communicating with Families About HPV Vaccines
From the University of Colorado Denver, Aurora, CO.
Abstract
- Objective: To provide evidence-based guidance on strategies that are likely or unlikely to be successful in navigating HPV vaccine conversations with patients and parents.
- Methods: Nonsystematic review of the literature.
- Results: This review highlights some of the most recent innovations in provider HPV vaccine communication and describes provider communication strategies that have been found to improve adolescent vaccination rates in rigorous scientific studies. Promising strategies for which additional research is needed and strategies that probably do not work are also described.
- Conclusion: By understanding what works, what may work, and what not to do when it comes to communicating with families about HPV vaccines, providers can be better prepared for maximizing the impact they can have on adolescent HPV vaccination rates.
Key words: human papillomavirus; vaccine hesitancy; health communication; parents; immunization.
In the United States, more than 14 million people newly acquire genital human papillomavirus (HPV) annually, and 75 million Americans are infected at any given time [1]. As the most common sexually transmitted disease, more than 80% of sexually active U.S. adults are estimated to be infected with HPV by the age of 50 [1,2]. Although the majority of infections are “silent” and resolve without clinical sequelae, a proportion of infected individuals will go on to develop HPV-related diseases. In women, these include cervical cancer and pre-cancer (ie, abnormal Pap smears); cancers of the vagina, vulva, anus, and oropharynx; and genital warts [3]. Males also bear a high burden of HPV-related disease in the form of penile, anal, and oropharyngeal cancers, as well as genital warts [3]. While once thought of as primarily a “woman’s disease” [4], recent research demonstrates men are also significantly impacted by HPV—particularly in the form of oropharyngeal cancers, which are 2 to 3 times more common in men than in women [5]. In fact, it is estimated by the year 2020 more men will die of HPV-related oropharyngeal cancer than women will die of cervical cancer [6,7]. The combined cost of HPV-associated cancers and other conditions is estimated to be $8 billion per year in the United States [8–11].
HPV Vaccines
Effective HPV vaccines have been available for females aged 9 to 26 years since 2006 (bivalent and quadrivalent vaccines) and for males aged 9 to 26 since 2010 (quadrivalent vaccine only) [12]. These vaccines have been shown in clinical trials to be highly efficacious in preventing HPV infection, cervical pre-cancer, and anal, vaginal, penile, and vulvar cancers caused by the HPV types covered in the vaccine [2]. Although their effectiveness against head and neck cancer has not been studied in clinical trials, most experts believe that these vaccines will also provide protection against at least a proportion of these cancers [13,14]. In 2015 the U.S. Food and Drug Administration approved licensure of a 9-valent HPV vaccine that will soon replace the quadrivalent vaccine in the U.S. market [15]. The 9-valent vaccine is licensed for both males and females aged 9 to 26 and is expected to prevent an even higher proportion of HPV-related cancers than earlier HPV vaccines due to the protection against 5 additional oncogenic HPV types [15].
Despite the potential of HPV vaccines to drastically reduce the incidence of HPV-related cancers and other diseases, these vaccines are not being as widely used in the United States as was hoped. The most recent national data from 2015 demonstrates that only 41.9% of girls and 28.1% of boys have received all 3 doses recommended in the HPV vaccine series [16]. This level of vaccine utilization is significantly lower than the Healthy People 2020 goal of 80% coverage [17], and also significantly lower than that of other developed countries such as Australia and the United Kingdom, which have achieved vaccination levels of ~70% among their target adolescent populations [18,19]. In the future, these low vaccination levels will likely be mitigated somewhat by the recent approval from the FDA and recent recommendation from the Advisory Committee on Immunization Practices (ACIP) for only 2 doses of the 9-valent HPV vaccine (spaced 6 to 12 months apart) for adolescents less than 15 years of age [20,21]. Three doses are still recommended for those aged 15 to 26 years.
Provider Communication About HPV Vaccines
How providers communicate with parents and patients about HPV vaccines is a key influential factor driving current U.S. adolescent HPV vaccination levels [22,23]. Numerous studies demonstrate that a provider’s recommendation generally has the largest impact on whether or not an adolescent receives the vaccine, even above that of parent factors such as attitudes and beliefs about the vaccine and patient characteristics such as age and insurance status [23–31]. Moreover, parents consistently cite their adolescent’s provider as one of the most trusted and impactful resources for obtaining vaccine information [22,32].
Unfortunately, research also shows that providers often fail to adequately recommend the HPV vaccine for their patients, especially for 11 to 12 year olds for whom the vaccine is preferentially recommended [33,34]. For example, in a national study of parents done in 2013, not being recommended by a provider was one of the top 5 reasons parents of males and of females aged 11 to 17 gave for not getting their adolescent vaccinated against HPV [35]. Supporting this also is a 2014 study of 776 pediatricians and family medicine providers nationally, in which Gilkey and colleagues found that more than 1 out of 4 providers did not highly endorse the HPV vaccine for 11 to 12 year olds despite this having been the recommended practice from ACIP for the prior 8 years for girls and 4 years for boys. This is in comparison to the other adolescents vaccines that were reported in the same study as being endorsed highly by these providers > 95% of the time [36].
Recognizing that providers’ HPV vaccine recommendations are often suboptimal, researchers have begun to define what components comprise “high-quality” HPV vaccine recommendations. This has been operationalized by one research group as (1) timeliness—routinely recommending the vaccine starting when the patient is ≤ 12 years; (2) consistency—recommending the vaccine for all eligible adolescents as opposed to an approach based on providers’ perception of their patients’ risk for HPV infection; (3) urgency—recommending that the vaccine be given on the same day the vaccine is being discussed, rather than offering the option of getting it at a future visit; and (4) strength—using language that clearly conveys that the provider believes the vaccine is very important for the adolescent to receive. A national study of primary care providers done in 2014 examined how frequently these quality components were implemented [37]. The results were startling and discouraging. Nearly half of providers (49%) reported they usually recommended that 11 to 12 year olds get the vaccine at a later visit, 41% used a risk-based approach for deciding when to recommend the vaccine, 27% did not tell the parents the vaccine was “very or extremely important,” and a large proportion did not start routinely recommending the vaccine before the age of 13 (39% for male patients and 25% for females) [37].
Much research has now accumulated to explain the underlying reasons why providers may not give consistent and high-quality HPV vaccine recommendations to all eligible adolescents [22]. Issues such as providers’ own knowledge about HPV-related diseases, personal beliefs about the vaccine’s safety and necessity, concern that a discussion about the vaccine will necessitate a discussion about adolescent sexuality with the parent, belief that parents will not want their child vaccinated if asked, perceptions that a provider can adequately select those patients most “in need” of HPV vaccination, and concern that raising the vaccine discussion with vaccine-hesitant parents will result in prolonged discussions have been shown to impact whether and how providers communicate about HPV vaccination during clinical visits [22,36–45]. Now that these barriers have been defined and described, there is a great need to use this knowledge to develop and evaluate interventions that help to mitigate these barriers and improve providers’ vaccine communication abilities. Such interventions are needed not only for HPV, but for all vaccines [46,47].
Possible Strategies for Helping Providers Communicate About HPV Vaccines
Before discussing these interventions, it is worth noting that several of the passive and active strategies have been shown in clinical trials to improve adolescent HPV vaccination rates. Although these are beyond the scope of this article, inclusion of these strategies should certainly be considered by any practice as a mechanism to increase vaccination levels, especially given that the most successful interventions to improve vaccination levels consist of multiple components [48]. Also useful is a recently described “taxonomy of vaccine communication interventions” that provides additional perspective on the scope and complexity of interventions to improve vaccine delivery [49]. There are several well-written review articles that describe interventions that focus on passive and active strategies at the practice or community level [50–52].
Interpersonal Communication Strategies Shown to Increase HPV Vaccination
Presumptive Communication
One of the first studies to examine the specific “way” in which providers communicate about vaccines focused not on HPV but rather on young childhood vaccines. In 2013 Opel and colleagues performed a study in which they taped clinical encounters between a pediatrician and a parent of a child aged 1 to 19 months [53]. Of the 111 encounters recorded, 50% of parents were classified as vaccine hesitant. Parents were aware they were being taped but not aware that the overall purpose of the study was to examine providers’ communication related to vaccination. The researchers found that providers generally used one of 2 communication styles to introduce the vaccine discussion. The first, called the “presumptive” style, assumed that parents would agree to vaccination and presented the vaccines as routine (ie, “We have to do some shots today”). The second style, called “participatory,” was more parent-oriented and used language suggesting shared decision-making (ie, “So what do you want to do about shots today?”). The study showed that the odds of resisting the provider’s vaccine recommendations were significantly higher when providers used a participatory approach than a presumptive one, suggesting that even small changes in language can have a major impact on the likelihood of vaccination. However, given the study design, causality between providers’ recommendation style and parents vaccination decisions could not be delineated.
In 2015 Moss and colleagues performed a study that examined the use of these 2 communication styles with regard to HPV vaccination [54]. This study used data from the 2010 National Immunization Survey–Teen, a national survey on childhood vaccination that includes provider verification of vaccines given [16]. Researchers categorized provider vaccine communication styles into “provider-driven,” which was similar to the presumptive style described Opel, and “patient-driven,” which was similar to Opel’s permissive style. Parents who received a more provider-driven style of HPV vaccine recommendation were far more likely to have allowed their adolescent to be vaccinated than those receiving patient-driven recommendations [54]. Further supporting this communication approach are results from a qualitative study done by Hughes and colleagues in which triads of mothers, adolescents, and providers were interviewed after a preventive care visit to assess the communication that occurred regarding HPV vaccination [39]. Providers’ communication style was categorized into 1 of 3 groups: paternalistic (clinician makes the vaccination decision and communicates this to the family); informed (patient and family gathers information from the clinician and other sources to reach a vaccination decision); and shared (medical and personal information is exchanged between the provider and family and then a decision is reached jointly). Providers who typically adopted the paternalistic approach perceived that they had the highest success in convincing parents to vaccinate—a perception that was confirmed in quantitative assessments of vaccination status among adolescents in the study sample [39]. Our own research demonstrates that learning and implementing a presumptive/paternalistic HPV vaccine recommendation style is easy for primary care providers to do and is perceived as often shortening the time taken during clinical visits to discuss the vaccine [55,56]. Thus, providers should consider opening the HPV vaccine conversation using this approach, and then turning to some of the other communication techniques described below when met with parental resistance or questions.
Motivational Interviewing
A second communication technique that seems effective for promoting HPV vaccination, especially for vaccine hesitant parents, is motivational interviewing. Motivational interviewing describes a communication technique in which the provider leverages a parents’ or patients’ intrinsic motivation to engage in a preferred health behavior [57]. Motivational interviewing was originally developed to combat substance abuse [58,59] but has subsequently been successfully applied to a number of other health issues [60–64]. There is growing interest from public health and medical providers in using this technique for increasing vaccination [39,65–68]. Our research group performed a large, cluster-randomized controlled trial of 16 pediatric and family medicine clinics to examine the impact of a provider communication “toolkit” on adolescent HPV vaccine series initiation and completion [50,69]. The toolkit consisted of motivational interviewing training for providers related to HPV vaccination and training on 3 tangible resources providers could also use with parents—an HPV fact sheet, an HPV vaccine decision aid, and an educational website. Results from the study demonstrated that motivational interviewing was the toolkit component most widely utilized by providers and was also perceived as being the most useful. More importantly, HPV vaccine series initiation levels were significantly higher among adolescents in practices receiving the toolkit than in control practices. There was no impact on HPV vaccine series completion (unpublished results). The usefulness of motivational interviewing for vaccination is further supported by a small study in which community pharmacists receiving motivational interviewing training for adult vaccination reported significantly higher patient readiness to receive vaccines following their interaction with the pharmacist than those who did not receive the training [70]. Finally, Perkins et al performed a cluster randomized controlled trial that evaluated the impact of a provider-focused intervention on adolescent HPV vaccination rates. The intervention included frequent provider support meetings, education on HPV infection and vaccination, feedback on providers’ individual HPV vaccination rates, provider incentives, and “basic motivational interviewing principles with vaccine-hesitant parents.” HPV vaccination series initiation and completion rates were significantly higher in intervention practices than controls, and this effect was sustained for at least 6 months after the active intervention period was over [67]. However, it was unknown how much the motivational interviewing contributed to these results. Based on the above information, and the long history of success of motivational interviewing for improving patient compliance with other recommended health behaviors, this technique appears to have a reasonable evidence base and should be considered for communicating with families that express resistance to HPV vaccination.
Personalized Communication
Parents’ reasons for not having their adolescent vaccinated against HPV are often complex and multifactorial [71,72]. Personalized approaches are needed to account for each parent’s unique informational needs, beliefs, and prior experiences [65]. Unfortunately, given the short amount of time allotted for clinical visits, it is often difficult to provide adequate information to parents during these encounters [73–75]. Indeed, concern about prolonged HPV vaccine discussions has been identified as an important barrier for providers that cause some to forgo recommending the vaccine [36,75].
One potential solution to this issue is to leverage technology in the form of web-based interventions that use software to tailor materials to each individual’s unique informational needs. Feasibility for this idea comes from the knowledge that many parents already use the web to research health issues related to their children [76], and that doctors’ offices are increasingly using patient portals and other web-based resources to help parents prepare for upcoming visits, especially those focused on health maintenance [77,78]. Tailored messaging interventions have been shown across populations and health issues to generally result in superior adherence with health behaviors when compared to untailored controls [79–82]. Several researchers have thus begun exploring whether such a personalized communication strategy may be similarly effective for adolescent HPV vaccination [50,83–85]. As an example, Maertens and colleagues used community-based participatory research techniques to develop a web-based tailored messaging intervention for Latinos regarding HPV vaccination [86]. A subsequent randomized controlled trial of the intervention in over 1200 parents of adolescents and young adults demonstrated that the intervention improved participants’ intentions to vaccinate compared to usual care [87], and among adolescents, higher HPV vaccine series initiation levels (unpublished data). Although additional work is needed to understand the feasibility of implementing such an intervention more broadly, additional support for the usefulness of a tailored messaging approach comes from a study of female university students that demonstrated higher HPV vaccination intentions after exposure to tailored information compared to untailored information. However, the impact on actual HPV vaccine utilization was not measured in the study [84]. Contrasting results were found in a different study of university students where researchers failed to find an impact of message tailoring on HPV vaccination utilization. However, this study was limited by a low response rate (~50%) to the follow up survey where vaccination status was assessed, and also by overall low levels of HPV vaccine initiation among the entire study sample (8%) [85]. Given the low number of studies in this area, and some conflicting data, additional research is needed to better understand the impact of personalized communication on HPV vaccination levels. However, results from these studies suggest that a modest benefit may be achieved with this approach, especially if coupled with other, evidence-based, clinic-level interventions to promote vaccination (eg, vaccine reminders, extended office hours), as is suggested by the Task Force on Community Preventive Services [48].
Focusing Communication on Cancer Prevention
HPV vaccines are unique in that they are only 1 of 2 vaccines for cancer prevention (the other being hepatitis B). Provider and parent surveys suggest that while most providers do mention cancer prevention when discussing HPV vaccines [40,88,89], this may be more commonly done with female patients than males [22]. Focusing on cancer prevention rather than sexual transmissibility is a communication technique suggested by the Centers for Disease Control and Prevention (CDC) as many parents cite this aspect of the vaccine as one of the most compelling reasons for vaccinating [45,90]. CDC’s “You are the Key” program [91] uses cancer prevention as a central theme in their physician and patient communication materials, based on significant prior market research on the acceptability and impact of such messages among parents and providers. In 2016 Malo and colleagues tested the potential impact of brief messages related to HPV vaccination, including cancer prevention messages, among a national sample of 776 medical providers and 1504 parents of adolescents [92]. In addition to their potential to motivate parents to vaccination, associations between parental endorsement of each message and their adolescent’s vaccination status were also examined. The cancer prevention messages were among those most highly endorsed by both parents and providers as being motivating for parents to get their adolescent vaccinated. More importantly, among parents these endorsements were associated with a significantly higher likelihood of the adolescent having been vaccinated against HPV. Interestingly, one of the briefest messages in the study, “I [the physician] strongly believe in the importance of this cancer preventing vaccine for [child’s name],” was perceived as the most persuasive message by parents.
Further support for the positive impact of framing HPV vaccines primarily as cancer prevention comes from another national study of 1495 parents of 11 to 17 year olds that examined 3 measures of quality of their adolescent provider’s HPV vaccine recommendation, and the relationship between recommendation quality and likelihood of adolescent HPV vaccination [40]. The 3 quality indicators assessed included providing information about cancer prevention, encouraging the vaccine “strongly,” and recommending it be given on the same day as it was being discussed. While 49% of parents reported receiving no HPV vaccine recommendation from their adolescents’ provider, of those that did, 86% received a cancer prevention message. Parents who had been given high quality recommendations that included either 2 or 3 of the quality indicator measures had over 9 times the odds of vaccine series initiation and 3 times the odds of vaccine series follow through than those who had not received any recommendation, and also significantly higher odds of vaccination than parents who had received low quality recommendations (ie, included only 1 indicator). Taken together, these results suggest that focusing discussions about HPV vaccines on their ability to prevent cancer is likely to be persuasive for some parents.
Strategies That Are Promising But Not Thoroughly Tested
Helping Parents Create Vaccination Plans
A recent commentary suggested that instead of focusing on changing beliefs or “educating” parents and patients about the need for a given vaccine, perhaps a better way to craft interventions for increasing vaccination is to focus on structuring the environment to make vaccination “easy” [93,94]. Examples of this include strategies such as extended office hours and making the vaccine available in other locations such as schools and pharmacies, both of which have been shown in some populations and settings to improve vaccine utilization [48,95]. One aspect of structuring a vaccine-conducive environment that relates to provider communication is helping parents create “implementation intentions” for future vaccination visits. In its most obvious form, this would mean providers provide office resources that facilitate making an appointment for the next dose in the HPV vaccine series during a clinic visit where the first dose was provided. But such an approach could also potentially extend to parents who are on the fence about the vaccine—to make an appointment before the parent leaves the office with an unvaccinated child to either re-discuss the vaccine in the future or to actually start the vaccine series. Support for such a strategy comes primarily from the social sciences, which suggest that implementation intentions work by increasing attention to specific cues to action, making it more likely that that the cue will be acted upon [96–98]. Creating implementation intentions has been shown to be helpful for improving adherence with a variety of health behaviors [99–105], and there is a growing evidence base related to how implementation intentions may facilitate vaccination specifically. For example Vet and colleagues performed a randomized controlled trial among 616 men who have sex with men with either strong or weak intentions to receive the hepatitis B vaccine [106]. Half of the participants were asked to create an implementation intention plan where they described when, where and how they would obtain the vaccine. Those in the control arm were not given this prompt. Regardless of whether their initial vaccination intention was weak or strong, those who had been asked to create an implementation plan had more than double the likelihood of actually getting the vaccine than participants who did not receive the implementation plan prompt. Similarly, a study of influenza vaccination rates among corporate employees found that those who were asked to write down the day and time they planned to go to employee health to get the free vaccine were somewhat more likely (4% higher) to be vaccinated than those who did not receive this prompt [107]. In addition, a study of elderly individuals found that influenza vaccination rates were significantly higher among those who had received “action instructions” on how, when and where to get the vaccine than those who did not [108]. These studies suggest that helping parents craft a definitive follow-up plan regarding vaccination could have a significant impact on vaccination rates—particularly for vaccines like HPV that require multiple doses.
Treating all Adolescent Vaccines the Same
Prior research has demonstrated that providers often communicate differently about HPV vaccines than other adolescent vaccines such as the tetanus-diphtheria-pertussis (Tdap) and meningococcal (MCV) vaccines [22,36]. Providers often tend to discuss the HPV vaccine last among these 3 vaccines, provide weaker endorsements of the vaccine, and pre-emptively give much more detail about the HPV compared to the other vaccines, even in the absence of a parent’s request for additional information [36,39,41]. The CDC and the American Academy of Pediatrics now suggest putting HPV at the beginning or middle of the list of vaccines recommended to the adolescent (ie, “HPV, Tdap and MCV”), and treating all recommended vaccines equivalently in terms of the level of detail provided to parents in the absence of a parent’s request for more information [109,110]. Through these suggestions have face validity, their specific impact on HPV vaccination rates, and on patient and provider satisfaction with the visit have yet to be evaluated.
Strategies that Probably Don’t Work
Presenting Myths and Facts
Research related to promoting other vaccines provides insight into communication activities that probably would not work well for promoting HPV vaccination. A 2012 study by Nyhan and colleagues examined the impact of 2 different messages related to influenza vaccines on participants’ beliefs about the vaccine’s safety and intentions to get vaccinated [111]. One group received information to correct the commonly held belief that influenza vaccine can cause the flu while the other received information about the risks associated with contracting an influenza infection. While the correction of myths did improve participants’ perceptions of the vaccine’s safety, information about influenza dangers did not. Neither message impacted intentions to vaccinate in the study subjects overall. However, in sub-analyses the correction of myths actually decreased intentions to vaccinate among those with high baseline levels of concern about the vaccine’s side effects—that is, among those most concerned that the flu vaccine can give someone the flu, correcting this myth actually decreased the likelihood that they would receive the vaccine. Similar findings have been reported in other studies related to vaccination [112–114], and suggest that the “threat” generated by providing information opposing a person’s beliefs may actually entrench these beliefs further as part of the threat response—a phenomenon known as attitude polarization [115]. These results also are consistent with the concept of negativity bias, which posits that negative information influences people’s risk perceptions more than positive information, and that the more strongly a risk is attempted to be negated, the lower the effectiveness and perceived trust of the information [116].
Using Fear Appeals
One tactic that has been suggested by some as a way to promote vaccination is to provide graphic depictions of the possible sequelae of vaccine-preventable diseases. The thought behind this idea is that because vaccination is so successful, most parents will have never experienced significant impacts from vaccine preventable diseases that, in the past, had been a major motivator for parents to vaccinate. Thus, in order to counter beliefs about “controversial” issues like vaccination, highly emotionally compelling and engaging information may be especially useful. This is a common tactic used by anti-vaccination groups to spread their own messages [117]. However, several studies suggested that using “fear appeals” (aka scare tactics) such as this to promote vaccination can actually have a negative effect on vaccination intentions. For example, in a 2011 study of a nationally representative sample of parents of children < 18 years, 4 different message formats were tested for their impact on parental intentions to vaccinate a future child with the measles-mumps-rubella vaccine (MMR) [113]. Message formats included correcting the misinformation that MMR causes autism, presenting information on MMR-related disease risk, providing a dramatic narrative about a child endangered by measles, and showing pictures of infants affected by these diseases. Counter to the study’s hypotheses, the dramatic narrative message actually increased parents’ perceptions that MMR vaccines had serious side effects, and the pictures increased parents’ belief that the MMR vaccine could cause autism. These counter-intuitive results are consistent with other studies that have examined the impact of message framing on adults’ vaccination intentions for HPV and influenza [108,118,119]. Taken together, fear appeals seem unlikely to sway many hesitant parents towards HPV vaccination.
Looking Into the Future
Moving forward, additional interventions to improve providers’ ability to communicate with families about HPV vaccination will undoubtedly be developed. A major area of interest in this regard is leveraging the power of technology and the internet, including using social media, mobile technologies, and online interventions to augment the provider/parent interaction that occurs during the clinical visit [50,120]. Web-based approaches have the benefit of generally being low cost and easy to disseminate to large populations. Such interventions have already been developed for a number of other health issues, some of which have proven effective [121,122]. However, use of the internet to promote healthy behaviors in general, and vaccination specifically, is still in its infancy. There is still much to be learned about how to create effective web-based tools, how to engage patients with them, and how to assess their impact on health outcomes [123].
Another interesting area for future research is identifying psychological “levers” to motivate parents’ vaccination intentions [94]. One example is focusing on using parents’ values (ie, protecting my child from harm) as an intervention target rather than beliefs or attitudes. This is because values tend to be inherent and static over time, compared to beliefs and attitudes, which are subject to change depending on the context [124]. Prior research has shown that interventions that leverage values rather than facts can be an effective way to overcome beliefs that are highly emotional or controversial, and that individuals are more likely to trust sources and individuals with shared values than those without [125], suggesting that this may be a useful way to motivate parents toward vaccinating their children. Self-affirmation is another example of a psychological lever that has a significant evidence base from the social science literature as a helpful tool for moving patients towards a desired health behavior [126,127], but it has not been extensively applied to the field of vaccination. Researchers in the field of vaccine delivery are increasingly recognizing the potential value of these unique intervention approaches [101,128–134], and it may be fruitful in the future to more closely examine the efficacy of interventions that target things like values, self-affirmation or other psychological levers to change parents’ HPV vaccination behaviors.
A final notable area for intervention research related to HPV vaccination is the use of video games. Although not likely to be used directly during patient visits, this strategy could be conceptualized as a potential way to augment the information conveyed to a parent by a provider directly during a clinical encounter. A meta-analysis from 2016 identified 16 different “serious” video games that were used to train and educate users about specific vaccine preventable diseases (usually influenza, none for HPV) and the need for vaccination [135]. In many of them, the objective of the game was to protect a virtual community from a vaccine preventable disease and/or manage outbreaks. Only 2 of the games evaluated outcomes in the short term (ie, at the time the game was being played). None have evaluated longer-term impacts such as vaccination intention or utilization. In the era of “plugged in” parents and adolescents, video games represent a unique but understudied mechanism for helping providers “communicate,” albeit indirectly, with families about the need for vaccination. Imagine providing a prescription to an HPV-vaccine hesitant family to “go play Zombie Wars HPV!” One would expect the curiosity factor alone would result in significant engagement with this intervention tool.
Conclusion
With persistently lagging HPV vaccination rates among U.S. adolescents, there is a growing need for effective interventions to improve adolescent HPV vaccine utilization. How providers communicate with families is one of the most influential factors in parents’ vaccination decisions. Emerging research is beginning to delineate potentially effective communication techniques such as presumptive approaches to making the vaccine recommendation, framing the vaccine as cancer preventing, and using motivational interviewing and personalized messaging when met with parental vaccine resistance. Moving forward the list of evidence-based interventions to improve providers’ HPV vaccine communication is likely to grow, and to increasingly leverage technology based solutions. However, given the complexities of the vaccination decision [136] and the ever growing spread of vaccine hesitancy [137], it is unlikely that a single intervention approach will be effective for getting adolescent HPV vaccine levels up to the national goal of 80% coverage. As has been recognized in the past, the most effective interventions for HPV vaccination in the future are likely to be multicomponent, including not only provider communication strategies but also clinic-, community-, and parent-level interventions [48].
Corresponding author: Amanda Dempsey, MD, PhD, MPH, 13199 East Montview Blvd, Suite 300, Aurora, CO 80045, [email protected].
Financial disclosures: None reported.
1. Centers for Disease Control and Prevention. Genital HPV infection - CDC fact sheet. 2012. Accessed 26 Jun 2012 at www.cdc.gov/std/HPV/STDFact-HPV.htm.
2. Markowitz LE, Dunne EF, Saraiya M, et al. Quadrivalent human papillomavirus vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007;56(RR-2):1–24.
3. Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers - United States, 2008-2012. MMWR Morb Mortal Wkly Rep 2016;65:661–6.
4. Daley EM, Vamos CA, Zimet GD, et al. The feminization of HPV: Reversing gender biases in US human papillomavirus vaccine policy. Am J Public Health 2016;106:983–4.
5. Rettig EM, D’Souza G. Epidemiology of head and neck cancer. Surg Oncol Clin North Am 2015;24:379–96.
6. Louie KS, Mehanna H, Sasieni P. Trends in head and neck cancers in England from 1995 to 2011 and projections up to 2025. Oral Oncol 2015;51:341–8.
7. Mehanna H, Beech T, Nicholson T, et al. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer--systematic review and meta-analysis of trends by time and region. Head Neck 2013;35:747–55.
8. Chesson HW, Markowitz LE, Hariri S, et al. The impact and cost-effectiveness of nonavalent HPV vaccination in the United States: Estimates from a simplified transmission model. Hum Vaccin Immunother 2016;12:1363–72.
9. Insinga RP, Glass AG, Rush BB, et al. The health care costs of cervical human papillomavirus-related disease. Am J Obstet Gynecol 2004;191:114–20.
10. Chesson HW, Blandford JM, Gift TL, et al. The estimated direct medical cost of sexually transmitted diseases among American youth, 2000. Perspect Sex Reprod Health 2004;36:11–9.
11. Chesson HW, Ekwueme DU, Saraiya M, et al. Estimates of the annual direct medical costs of the prevention and treatment of disease associated with human papillomavirus in the United States. Vaccine 2012;30:6016–9.
12. Food and Drug Administration. Product approval information - licensing action, package insert: Gardasil. 2010. Accessed at www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM111263.pdf.
13. Ward G, Mehta V, Moore M. Morbidity, mortality and cost from HPV-related oropharyngeal cancer: Impact of 2-, 4- and 9-valent vaccines. Hum Vaccin Immunother 2016;12:1343–7.
14. Guo T, Eisele DW, Fakhry C. The potential impact of prophylactic human papillomavirus vaccination on oropharyngeal cancer. Cancer 2016;122:2313–23.
15. Food and Drug Administration. Product approval information - licensing action, package insert: Gardasil 9. 2014. Accessed at www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm426445.htm.
16. Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 Years - United States, 2015. MMWR Morb Mortal Wkly Rep 2016;65:850–8.
17. Healthy People 2020. Accessed at www.healthypeople.gov/2020/topics-objectives/national-snapshot/hpv-vaccine-adolescents-2008%E2%80%932012.
18. Garland SM. The Australian experience with the human papillomavirus vaccine. Clin Therapeut 2014;36:17–23.
19. Brabin L, Roberts SA, Stretch R, et al. Uptake of first two doses of human papillomavirus vaccine by adolescent schoolgirls in Manchester: prospective cohort study. BMJ 2008;336:1056–8.
20. Food and Drug Administration. Prescribing information [package insert]. Gardasil 9. 2016. Accessed at www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM426457.pdf
21. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination - updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2016;65:1405–8.
22. Gilkey MB, McRee AL. Provider communication about HPV vaccination: a systematic review. Hum Vaccin Immunother 2016;12:1454–68.
23. Clark SJ, Cowan AE, Filipp SL, et al. Parent perception of provider interactions influences hpv vaccination status of adolescent females. Clin Pediatr (Phila) 2016;55:701–6.
24. Ferrer HB, Trotter C, Hickman M, Audrey S. Barriers and facilitators to HPV vaccination of young women in high-income countries: a qualitative systematic review and evidence synthesis. BMC Public Health 2014;14:700.
25. Clark SJ, Cowan AE, Filipp SL, et al. Parent HPV vaccine perspectives and the likelihood of HPV vaccination of adolescent males. Hum Vaccin Immunother 2016;12:47–51.
26. Darden PM, Jacobson RM. Impact of a physician recommendation. Hum Vaccin Immunother 2014;10:2632–5.
27. Darden PM, Thompson DM, Roberts JR, et al. Reasons for not vaccinating adolescents: national immunization survey of teens, 2008-2010. Pediatrics 2013;131:645–51.
28. Gargano LM, Herbert NL, Painter JE, et al. Impact of a physician recommendation and parental immunization attitudes on receipt or intention to receive adolescent vaccines. Hum Vaccin Immunother 2013;9:2627–33.
29. Ylitalo KR, Lee H, Mehta NK. Health care provider recommendation, human papillomavirus vaccination, and race/ethnicity in the US National Immunization Survey. Am J Public Health 2013;103:164–9.
30. Brewer NT, Gottlieb SL, Reiter PL, et al. Longitudinal predictors of human papillomavirus vaccine initiation among adolescent girls in a high-risk geographic area. Sex Transm Dis 2011;38:197–204.
31. Reiter PL, McRee AL, Pepper JK, et al. Longitudinal predictors of human papillomavirus vaccination among a national sample of adolescent males. Am J Public Health 2013;103:1419–27.
32. Gottvall M, Grandahl M, Hoglund AT, et al. Trust versus concerns-how parents reason when they accept HPV vaccination for their young daughter. Ups J Med Sci 2013;118:263–70.
33. Vadaparampil ST, Malo TL, Kahn JA, et al. Physicians’ human papillomavirus vaccine recommendations, 2009 and 2011. Am J Prev Med 2014;46:80–4.
34. Vadaparampil ST, Malo TL, Sutton SK, et al. Missing the target for routine human papillomavirus vaccination: consistent and strong physician recommendations are lacking for 11- to 12-year-old males. Cancer Epidemiol Biomarkers Prev 2016;25:1435–46.
35. Stokley S, Jeyarajah J, Yankey D, et al. Human papillomavirus vaccination coverage among adolescents, 2007-2013, and postlicensure vaccine safety monitoring, 2006-2014 - United States. MMWR Morb Mortal Wkly Rep 2014;63:620–4.
36. Gilkey MB, Moss JL, Coyne-Beasley T, et al. Physician communication about adolescent vaccination: How is human papillomavirus vaccine different? Prev Med 2015;77:181–5.
37. Gilkey MB, Malo TL, Shah PD, et al. Quality of physician communication about human papillomavirus vaccine: findings from a national survey. Cancer Epidemiol Biomarkers Prev 2015;24:1673–9.
38. Herzog R, Alvarez-Pasquin MJ, Diaz C, et al. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Pub Health 2013;13:154.
39. Hughes CC, Jones AL, Feemster KA, Fiks AG. HPV vaccine decision making in pediatric primary care: a semi-structured interview study. BMC Pediatr 2011;11:74.
40. Gilkey MB, Calo WA, Moss JL, et al. Provider communication and HPV vaccination: the impact of recommendation quality. Vaccine 2016;34:1187–92.
41. Malo TL, Ali KN, Sutton SK, et al. The content and context of physicians’ communication with males about human papillomavirus vaccination. Hum Vaccin Immunother 2016;12:1511–8.
42. Moss JL, Gilkey MB, Rimer BK, Brewer NT. Disparities in collaborative patient-provider communication about human papillomavirus (HPV) vaccination. Hum Vaccin Immunother 2016:0.
43. Suryadevara M, Handel A, Bonville CA, et al. Pediatric provider vaccine hesitancy: an under-recognized obstacle to immunizing children. Vaccine 2015;33:6629–34.
44. Mohammed KA, Geneus CJ, Osazuwa-Peters N, et al. Disparities in provider recommendation of human papillomavirus vaccination for U.S. adolescents. J Adolesc Health 2016;59:592–8.
45. Perkins RB, Clark JA. Providers’ perceptions of parental concerns about HPV vaccination. J Health Care Poor Underserved 2013;24:828–39.
46. Bloom BR, Marcuse E, Mnookin S. Addressing vaccine hesitancy. Science 2014;344:339.
47. Zimet GD. Health care professionals and adolescent vaccination. A call for intervention research. Hum Vaccin Immunother 2014;10:2629–30.
48. Task Force on Community Preventive Services. The guide to community preventive services: increasing appropriate vaccination. 2014. Accessed at www.thecommunityguide.org/vaccines/index.html.
49. Willis N, Hill S, Kaufman J, et al. “Communicate to vaccinate”: the development of a taxonomy of communication interventions to improve routine childhood vaccination. BMC Int Health Hum Rights 2013;13:23.
50. Dempsey AF, Zimet GD. Interventions to improve adolescent vaccination: what may work and what still needs to be tested. Vaccine 2015;33 Suppl 4:D106–113.
51. Walling EB, Benzoni N, Dornfeld J, et al. Interventions to improve HPV vaccine uptake: a systematic review. Pediatrics 2016;138(1).
52. Smulian EA, Mitchell KR, Stokley S. Interventions to increase HPV vaccination coverage: a systematic review. Hum Vaccin Immunother 2016;12:1566–88.
53. Opel DJ, Heritage J, Taylor JA, et al. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics 2013;132:1037–46.
54. Moss JL, Reiter PL, Rimer BK, Brewer NT. Collaborative patient-provider communication and uptake of adolescent vaccines. Soc Sci Med 2016;159:100–7.
55. Lockhart S, Barnard J, O’Leary ST, et al. Exploring the feasibility of implementing a multifaceted toolkit to improve HPV vaccine provider communication in primary care settings. Presented at: Annual Meeting of the American Public Health Association; 2016; Denver, CO.
56. Reno J, O’Leary ST, Lockhart S, et al. Assessment of a communication toolkit for healcare providers about adolescent HPV vaccination. Presented at: Pediatric Academic Societies Annual Meeting; 2015; Baltimore, MD.
57. www.motivationalinterviewing.org
58. Foxcroft DR, Coombes L, Wood S, et al. Motivational interviewing for the prevention of alcohol misuse in young adults. Cochrane Database Syst Rev 2016;(7):CD007025.
59. Lindson-Hawley N, Thompson TP, Begh R. Motivational interviewing for smoking cessation. Cochrane Database Syst Rev 2015(3):CD006936.
60. McCain J. To heal the body, get into the patient’s head: motivational interviewing: to improve adherence. Biotechnol Healthc 2012;9:10–2.
61. Boutin-Foster C, Scott E, Rodriguez A, et al. The trial using motivational interviewing and positive affect and self-affirmation in african-americans with hypertension (TRIUMPH): from theory to clinical trial implementation. Contemp Clin Trials 2013;35:8–14.
62. Gance-Cleveland B. Motivational interviewing for adolescent obesity. Am J Nurs 2013;113:11.
63. Hides L, Carroll S, Scott R, et al. Quik fix: a randomized controlled trial of an enhanced brief motivational interviewing intervention for alcohol/cannabis and psychological distress in young people. Psychother Psychosom 2013;82:122–4.
64. Rongkavilit C, Naar-King S, Wang B, et al. Motivational interviewing targeting risk behaviors for youth living with HIV in Thailand. AIDS Behav 2013;17:2063–74.
65. Leask J, Kinnersley P, Jackson C, et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr 2012;12:154.
66. Zimet GD, Perkins SM, Sturm LA, et al. Predictors of STI vaccine acceptability among parents and their adolescent children. J Adolesc Health 2005;37:179–86.
67. Perkins RB, Zisblatt L, Legler A, et al. Effectiveness of a provider-focused intervention to improve HPV vaccination rates in boys and girls. Vaccine 2015;33:1223–9.
68. MacDonald N, Finlay JC. Working with vaccine-hesitant parents. Paediatr Child Health 2013;18:265–7.
69. Dempsey A, Lockhart S, Pyrzanowski J, et al. Impact of motivational interviewing training on providers communication about adolescent HPV vaccination. Presented at: Pediatric Academic Societies Annual Meeting; 2015; Baltimore, MD.
70. Brackett A, Butler M, Chapman L. Using motivational interviewing in the community pharmacy to increase adult immunization readiness: A pilot evaluation. J Am Pharm Assoc (2003) 2015;55:182–6.
71. Holman DM, Benard V, Roland KB, et al. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatrics 2014;168:76–82.
72. Rambout L, Tashkandi M, Hopkins L, Tricco AC. Self-reported barriers and facilitators to preventive human papillomavirus vaccination among adolescent girls and young women: a systematic review. Prev Med 2014;58:22–32.
73. McSherry LA, Dombrowski SU, Francis JJ, et al. ‘It’s a can of worms’: understanding primary care practitioners’ behaviours in relation to HPV using the Theoretical Domains Framework. Implement Sci 2012;7:73.
74. Palmer J, Carrico C, Coxtanzo C. Identifying and overcoming perceived barriers of providers towards HPV vaccination: A literature review. J Vaccines 2015:7.
75. Gowda C, Schaffer SE, Dombkowski KJ, Dempsey AF. Understanding attitudes toward adolescent vaccination and the decision-making dynamic among adolescents, parents and providers. BMC Public Health 2012;12:509.
76. Plantin L, Daneback K. Parenthood, information and support on the internet. A literature review of research on parents and professionals online. BMC Fam Pract 2009;10:34.
77. Irizarry T, DeVito Dabbs A, Curran CR. Patient portals and patient engagement: a state of the science review. J Med Internet Res 2015;17:e148.
78. Clark SJ, Costello LE, Gebremariam A, Dombkowski KJ. A national survey of parent perspectives on use of patient portals for their children’s health care. Appl Clin Inform 2015;6:110–9.
79. Noar SM, Benac CN, Harris MS. Does tailoring matter? Meta-analytic review of tailored print health behavior change interventions. Psychol Bull 2007;133:673–93.
80. Kreuter MW, Strecher VJ, Glassman B. One size does not fit all: the case for tailoring print materials. Ann Behav Med 1999;21:276–83.
81. Hawkins RP, Kreuter M, Resnicow K, et al. Understanding tailoring in communicating about health. Health Educ Res 2008;23:454–66.
82. Lustria ML, Noar SM, Cortese J, et al. A meta-analysis of web-delivered tailored health behavior change interventions. J Health Commun 2013;18:1039–69.
83. Paiva AL, Lipschitz JM, Fernandez AC, et al. Evaluation of the acceptability and feasibility of a computer-tailored intervention to increase human papillomavirus vaccination among young adult women. J Am College Health 2014;62:32–8.
84. Gerend MA, Shepherd MA, Lustria ML. Increasing human papillomavirus vaccine acceptability by tailoring messages to young adult women’s perceived barriers. Sex Transm Dis 2013;40:401–5.
85. Bennett AT, Patel DA, Carlos RC, et al. Human papillomavirus vaccine uptake after a tailored, online educational intervention for female university students: a randomized controlled trial. J Womens Health (Larchmt) 2015;24:950–7.
86. Maertens J, Zambrano-Jiminez A, Dempsey AF. Using community engagement to develop a web-based intervention for Latinas about the HPV vaccine. J Health Commun 2017. Forthcoming.
87. Reno J, Maertens J, Jimenez-Zambrano A, et al. Effectiveness of a tailored intervention on changing hpv vaccination intention among Hispanics. Presented at: Pediatric Academic Societies Meeting; 2016; Baltimore, MD.
88. Daley MF, Liddon N, Crane LA, et al. A national survey of pediatrician knowledge and attitudes regarding human papillomavirus vaccination. Pediatrics 2006;118:2280–9.
89. Sussman AL, Helitzer D, Sanders M,et al. HPV and cervical cancer prevention counseling with younger adolescents: implications for primary care. Ann Fam Med 2007;5:298–304.
90. Perkins RB, Chigurupati NL, Apte G, et al. Why don’t adolescents finish the HPV vaccine series? A qualitative study of parents and providers. Hum Vaccin Immunother 2016;12:1528–35.
91. Centers for Disease Control and Prevention. Immunization Education & Training. You are the key to HPV cancer prevention. Accessed at www.cdc.gov/vaccines/ed/hpv/index.html.
92. Malo TL, Gilkey MB, Hall ME, et al. Messages to motivate human papillomavirus vaccination: national studies of parents and physicians. Cancer Epidemiol Biomarkers Prev 2016;25:1383–91.
93. Chapman G. Increasing vaccination without changing beliefs. 2016. Accessed at http://catalyst.nejm.org/increasing-vaccination-without-changing-beliefs/.
94. Rossen I, Hurlstone MJ, Lawrence C. Going with the grain of cognition: applying insights from psychology to build support for childhood vaccination. Front Psychol 2016;7:1483.
95. Shah PD, Gilkey MB, Pepper JK, et al. Promising alternative settings for HPV vaccination of US adolescents. Exp Rev Vaccine 2014;13:235–46.
96. Webb TL, Sheeran P. Does changing behavioral intentions engender behavior change? A meta-analysis of the experimental evidence. Psychol Bull 2006;132:249–68.
97. Janczyk M, Dambacher M, Bieleke M, Gollwitzer PM. The benefit of no choice: goal-directed plans enhance perceptual processing. Psychol Res 2015;79:206–20.
98. Wieber F, Thurmer JL, Gollwitzer PM. Promoting the translation of intentions into action by implementation intentions: behavioral effects and physiological correlates. Front Hum Neurosci 2015;9:395.
99. Chapman J, Armitage CJ, Norman P. Comparing implementation intention interventions in relation to young adults’ intake of fruit and vegetables. Psychol Health 2009;24:317–32.
100. Martin J, Sheeran P, Slade P, et al. Implementation intention formation reduces consultations for emergency contraception and pregnancy testing among teenage women. Health Psychol 2009;28:762–9.
101. Chapman J, Armitage CJ. Do techniques that increase fruit intake also increase vegetable intake? Evidence from a comparison of two implementation intention interventions. Appetite 2012;58:28–33.
102. Armitage CJ, Arden MA. Enhancing the effectiveness of alcohol warning labels with a self-affirming implementation intention. Health Psychol 2016;35:1159–63.
103. Bradbury D, Upsher R, Chilcot J. A pilot randomised test of a self-affirmation implementation intention intervention to reduce dietary salt intake. J Health Psychol 2016 May 22.
104. Chen XJ, Liu LL, Cui JF, et al. The effect and mechanisms of implementation intention in improving prospective memory performance in schizophrenia patients. Psychiatry Res 2016;244:86–93.
105. Hagger MS, Luszczynska A, de Wit J, et al. Implementation intention and planning interventions in health psychology: recommendations from the Synergy Expert Group for research and practice. Psychol Health 2016;31:814–39.
106. Vet R, de Wit JB, Das E. The role of implementation intention formation in promoting hepatitis B vaccination uptake among men who have sex with men. Int J STD AIDS 2014;25:122–9.
107. Milkman KL, Beshears J, Choi JJ, et al. Using implementation intention prompts to enhance influenza vaccination rates. Proc Natl Acad Sci U S A 2011;108:10415–20.
108. McCaul KD, Johnson RJ, Rothman AJ. The effects of framing and action instructions on whether older adults obtain flu shots. Health Psychol 2002;21:624–8.
109. Centers for Disease Control and Prevention. You are the key: health care providers’ tip sheet. 2015. Accessed at www.cdc.gov/vaccines/who/teens/for-hcp-tipsheet-hpv.pdf.
110. American Academy of Pediatrics. HPV vaccine champion toolkit. 2015. Accessed at www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/Pages/HPV-Champion-Toolkit.aspx.
111. Nyhan B, Reifler J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine 2015;33:459–64.
112. Betsch C, Sachse K. Debunking vaccination myths: strong risk negations can increase perceived vaccination risks. Health Psychol 2013;32:146–55.
113. Nyhan B, Reifler J, Richey S, Freed GL. Effective messages in vaccine promotion: a randomized trial. Pediatrics 2014;133:e835–842.
114. Schwartz N, Sanna LJ, Skurnik I, Yoon C. Metacognitive expriences and the intericacies of setting people straight: Implications for debiasing and public information campaigns. Adv Exper Social Psychol 2007;39:127–161.
115. Lord C, Ross L, Lepper MR. Biased assimilation and attitude polarization: the effects of prior theories on subsequently considered evidence. J Personality Soc Pscyhol 1979;37:2098–109.
116. Siegrist M, Cvetkovich G. Better negative than positive? Evidence of a bias for negative information about possible health dangers. Risk Analysis 2001;21:199–206.
117. Witte K, Allen M. A meta-analysis of fear appeals: implications for effective public health campaigns. Health Educ Behav 2000;27:591–615.
118. Gerend MA, Shepherd JE. Using message framing to promote acceptance of the human papillomavirus vaccine. Health Psychol 2007;26:745–52.
119. Ferguson E, Gallagher L. Message framing with respect to decisions about vaccination: the roles of frame valence, frame method and perceived risk. Br J Psychol (London:1953) 2007;98(Pt 4):667–80.
120. Jarrett C, Wilson R, O’Leary M, et al. Strategies for addressing vaccine hesitancy - A systematic review. Vaccine 2015;33:4180–90.
121. Laranjo L, Arguel A, Neves AL, et al. The influence of social networking sites on health behavior change: a systematic review and meta-analysis. J Am Med Inform Assoc 2015;22:243–56.
122. Maher CA, Lewis LK, Ferrar K, et al. Are health behavior change interventions that use online social networks effective? A systematic review. J Med Internet Res 2014;16:e40.
123. Betsch C, Brewer NT, Brocard P, et al. Opportunities and challenges of Web 2.0 for vaccination decisions. Vaccine 2012;30:3727–33.
124. Barrett Values Center. Values versus beliefs. 2016. Accessed 31 Oct 2016 at www.valuescentre.com/mapping-values/values/values-vs-beliefs.
125. Dore RA, Stone ER, Buchanan CM. A social values analysis of parental decision making. J Psychol 2014;148:477–504.
126. Cohen GL, Sherman DK. The psychology of change: self-affirmation and social psychological intervention. Ann Rev Psychol 2014;65:333–71.
127. Sweeney AM, Moyer A. Self-affirmation and responses to health messages: a meta-analysis on intentions and behavior. Health Psychol 2015;34:149–59.
128. Tiro JA, Lee SC, Marks EG, et al. Developing a tablet-based self-persuasion intervention promoting adolescent hpv vaccination: protocol for a three-stage mixed-methods study. JMIR Res Protoc 2016;5:e19.
129. Leask J, Macartney K. Parental decisions about vaccination: collective values are important. J Paediatr Child Health 2008;44:534–5.
130. Salmon DA, Omer SB. Individual freedoms versus collective responsibility: immunization decision-making in the face of occasionally competing values. Emerg Themes Epidemiol 2006;3:13.
131. Gidengil C, Lieu TA, Payne K, et al. Parental and societal values for the risks and benefits of childhood combination vaccines. Vaccine 2012;30:3445–52.
132. Attwell K, Freeman M. I immunise: an evaluation of a values-based campaign to change attitudes and beliefs. Vaccine 2015;33:6235–40.
133. Witteman HO. Addressing vaccine hesitancy with values. Pediatrics 2015;136:215–7.
134. Witteman HO, Chipenda Dansokho S, Exe N, et al. Risk Communication, values clarification, and vaccination decisions. Risk Anal 2015;35:1801–19.
135. Ohannessian R, Yaghobian S, Verger P, Vanhems P. A systematic review of serious video games used for vaccination. Vaccine 2016.
136. National Vaccine Advisory Committee. Assessing the state of vaccine confidence in the United States: recommendations from the National Vaccine Advisory Committee. Pub Health Rep 2015;130:573–95.
137. Dube E, Vivion M, MacDonald NE. Vaccine hesitancy, vaccine refusal and the anti-vaccine movement: influence, impact and implications. Exp Rev Vaccine 2015;14:99–117.
From the University of Colorado Denver, Aurora, CO.
Abstract
- Objective: To provide evidence-based guidance on strategies that are likely or unlikely to be successful in navigating HPV vaccine conversations with patients and parents.
- Methods: Nonsystematic review of the literature.
- Results: This review highlights some of the most recent innovations in provider HPV vaccine communication and describes provider communication strategies that have been found to improve adolescent vaccination rates in rigorous scientific studies. Promising strategies for which additional research is needed and strategies that probably do not work are also described.
- Conclusion: By understanding what works, what may work, and what not to do when it comes to communicating with families about HPV vaccines, providers can be better prepared for maximizing the impact they can have on adolescent HPV vaccination rates.
Key words: human papillomavirus; vaccine hesitancy; health communication; parents; immunization.
In the United States, more than 14 million people newly acquire genital human papillomavirus (HPV) annually, and 75 million Americans are infected at any given time [1]. As the most common sexually transmitted disease, more than 80% of sexually active U.S. adults are estimated to be infected with HPV by the age of 50 [1,2]. Although the majority of infections are “silent” and resolve without clinical sequelae, a proportion of infected individuals will go on to develop HPV-related diseases. In women, these include cervical cancer and pre-cancer (ie, abnormal Pap smears); cancers of the vagina, vulva, anus, and oropharynx; and genital warts [3]. Males also bear a high burden of HPV-related disease in the form of penile, anal, and oropharyngeal cancers, as well as genital warts [3]. While once thought of as primarily a “woman’s disease” [4], recent research demonstrates men are also significantly impacted by HPV—particularly in the form of oropharyngeal cancers, which are 2 to 3 times more common in men than in women [5]. In fact, it is estimated by the year 2020 more men will die of HPV-related oropharyngeal cancer than women will die of cervical cancer [6,7]. The combined cost of HPV-associated cancers and other conditions is estimated to be $8 billion per year in the United States [8–11].
HPV Vaccines
Effective HPV vaccines have been available for females aged 9 to 26 years since 2006 (bivalent and quadrivalent vaccines) and for males aged 9 to 26 since 2010 (quadrivalent vaccine only) [12]. These vaccines have been shown in clinical trials to be highly efficacious in preventing HPV infection, cervical pre-cancer, and anal, vaginal, penile, and vulvar cancers caused by the HPV types covered in the vaccine [2]. Although their effectiveness against head and neck cancer has not been studied in clinical trials, most experts believe that these vaccines will also provide protection against at least a proportion of these cancers [13,14]. In 2015 the U.S. Food and Drug Administration approved licensure of a 9-valent HPV vaccine that will soon replace the quadrivalent vaccine in the U.S. market [15]. The 9-valent vaccine is licensed for both males and females aged 9 to 26 and is expected to prevent an even higher proportion of HPV-related cancers than earlier HPV vaccines due to the protection against 5 additional oncogenic HPV types [15].
Despite the potential of HPV vaccines to drastically reduce the incidence of HPV-related cancers and other diseases, these vaccines are not being as widely used in the United States as was hoped. The most recent national data from 2015 demonstrates that only 41.9% of girls and 28.1% of boys have received all 3 doses recommended in the HPV vaccine series [16]. This level of vaccine utilization is significantly lower than the Healthy People 2020 goal of 80% coverage [17], and also significantly lower than that of other developed countries such as Australia and the United Kingdom, which have achieved vaccination levels of ~70% among their target adolescent populations [18,19]. In the future, these low vaccination levels will likely be mitigated somewhat by the recent approval from the FDA and recent recommendation from the Advisory Committee on Immunization Practices (ACIP) for only 2 doses of the 9-valent HPV vaccine (spaced 6 to 12 months apart) for adolescents less than 15 years of age [20,21]. Three doses are still recommended for those aged 15 to 26 years.
Provider Communication About HPV Vaccines
How providers communicate with parents and patients about HPV vaccines is a key influential factor driving current U.S. adolescent HPV vaccination levels [22,23]. Numerous studies demonstrate that a provider’s recommendation generally has the largest impact on whether or not an adolescent receives the vaccine, even above that of parent factors such as attitudes and beliefs about the vaccine and patient characteristics such as age and insurance status [23–31]. Moreover, parents consistently cite their adolescent’s provider as one of the most trusted and impactful resources for obtaining vaccine information [22,32].
Unfortunately, research also shows that providers often fail to adequately recommend the HPV vaccine for their patients, especially for 11 to 12 year olds for whom the vaccine is preferentially recommended [33,34]. For example, in a national study of parents done in 2013, not being recommended by a provider was one of the top 5 reasons parents of males and of females aged 11 to 17 gave for not getting their adolescent vaccinated against HPV [35]. Supporting this also is a 2014 study of 776 pediatricians and family medicine providers nationally, in which Gilkey and colleagues found that more than 1 out of 4 providers did not highly endorse the HPV vaccine for 11 to 12 year olds despite this having been the recommended practice from ACIP for the prior 8 years for girls and 4 years for boys. This is in comparison to the other adolescents vaccines that were reported in the same study as being endorsed highly by these providers > 95% of the time [36].
Recognizing that providers’ HPV vaccine recommendations are often suboptimal, researchers have begun to define what components comprise “high-quality” HPV vaccine recommendations. This has been operationalized by one research group as (1) timeliness—routinely recommending the vaccine starting when the patient is ≤ 12 years; (2) consistency—recommending the vaccine for all eligible adolescents as opposed to an approach based on providers’ perception of their patients’ risk for HPV infection; (3) urgency—recommending that the vaccine be given on the same day the vaccine is being discussed, rather than offering the option of getting it at a future visit; and (4) strength—using language that clearly conveys that the provider believes the vaccine is very important for the adolescent to receive. A national study of primary care providers done in 2014 examined how frequently these quality components were implemented [37]. The results were startling and discouraging. Nearly half of providers (49%) reported they usually recommended that 11 to 12 year olds get the vaccine at a later visit, 41% used a risk-based approach for deciding when to recommend the vaccine, 27% did not tell the parents the vaccine was “very or extremely important,” and a large proportion did not start routinely recommending the vaccine before the age of 13 (39% for male patients and 25% for females) [37].
Much research has now accumulated to explain the underlying reasons why providers may not give consistent and high-quality HPV vaccine recommendations to all eligible adolescents [22]. Issues such as providers’ own knowledge about HPV-related diseases, personal beliefs about the vaccine’s safety and necessity, concern that a discussion about the vaccine will necessitate a discussion about adolescent sexuality with the parent, belief that parents will not want their child vaccinated if asked, perceptions that a provider can adequately select those patients most “in need” of HPV vaccination, and concern that raising the vaccine discussion with vaccine-hesitant parents will result in prolonged discussions have been shown to impact whether and how providers communicate about HPV vaccination during clinical visits [22,36–45]. Now that these barriers have been defined and described, there is a great need to use this knowledge to develop and evaluate interventions that help to mitigate these barriers and improve providers’ vaccine communication abilities. Such interventions are needed not only for HPV, but for all vaccines [46,47].
Possible Strategies for Helping Providers Communicate About HPV Vaccines
Before discussing these interventions, it is worth noting that several of the passive and active strategies have been shown in clinical trials to improve adolescent HPV vaccination rates. Although these are beyond the scope of this article, inclusion of these strategies should certainly be considered by any practice as a mechanism to increase vaccination levels, especially given that the most successful interventions to improve vaccination levels consist of multiple components [48]. Also useful is a recently described “taxonomy of vaccine communication interventions” that provides additional perspective on the scope and complexity of interventions to improve vaccine delivery [49]. There are several well-written review articles that describe interventions that focus on passive and active strategies at the practice or community level [50–52].
Interpersonal Communication Strategies Shown to Increase HPV Vaccination
Presumptive Communication
One of the first studies to examine the specific “way” in which providers communicate about vaccines focused not on HPV but rather on young childhood vaccines. In 2013 Opel and colleagues performed a study in which they taped clinical encounters between a pediatrician and a parent of a child aged 1 to 19 months [53]. Of the 111 encounters recorded, 50% of parents were classified as vaccine hesitant. Parents were aware they were being taped but not aware that the overall purpose of the study was to examine providers’ communication related to vaccination. The researchers found that providers generally used one of 2 communication styles to introduce the vaccine discussion. The first, called the “presumptive” style, assumed that parents would agree to vaccination and presented the vaccines as routine (ie, “We have to do some shots today”). The second style, called “participatory,” was more parent-oriented and used language suggesting shared decision-making (ie, “So what do you want to do about shots today?”). The study showed that the odds of resisting the provider’s vaccine recommendations were significantly higher when providers used a participatory approach than a presumptive one, suggesting that even small changes in language can have a major impact on the likelihood of vaccination. However, given the study design, causality between providers’ recommendation style and parents vaccination decisions could not be delineated.
In 2015 Moss and colleagues performed a study that examined the use of these 2 communication styles with regard to HPV vaccination [54]. This study used data from the 2010 National Immunization Survey–Teen, a national survey on childhood vaccination that includes provider verification of vaccines given [16]. Researchers categorized provider vaccine communication styles into “provider-driven,” which was similar to the presumptive style described Opel, and “patient-driven,” which was similar to Opel’s permissive style. Parents who received a more provider-driven style of HPV vaccine recommendation were far more likely to have allowed their adolescent to be vaccinated than those receiving patient-driven recommendations [54]. Further supporting this communication approach are results from a qualitative study done by Hughes and colleagues in which triads of mothers, adolescents, and providers were interviewed after a preventive care visit to assess the communication that occurred regarding HPV vaccination [39]. Providers’ communication style was categorized into 1 of 3 groups: paternalistic (clinician makes the vaccination decision and communicates this to the family); informed (patient and family gathers information from the clinician and other sources to reach a vaccination decision); and shared (medical and personal information is exchanged between the provider and family and then a decision is reached jointly). Providers who typically adopted the paternalistic approach perceived that they had the highest success in convincing parents to vaccinate—a perception that was confirmed in quantitative assessments of vaccination status among adolescents in the study sample [39]. Our own research demonstrates that learning and implementing a presumptive/paternalistic HPV vaccine recommendation style is easy for primary care providers to do and is perceived as often shortening the time taken during clinical visits to discuss the vaccine [55,56]. Thus, providers should consider opening the HPV vaccine conversation using this approach, and then turning to some of the other communication techniques described below when met with parental resistance or questions.
Motivational Interviewing
A second communication technique that seems effective for promoting HPV vaccination, especially for vaccine hesitant parents, is motivational interviewing. Motivational interviewing describes a communication technique in which the provider leverages a parents’ or patients’ intrinsic motivation to engage in a preferred health behavior [57]. Motivational interviewing was originally developed to combat substance abuse [58,59] but has subsequently been successfully applied to a number of other health issues [60–64]. There is growing interest from public health and medical providers in using this technique for increasing vaccination [39,65–68]. Our research group performed a large, cluster-randomized controlled trial of 16 pediatric and family medicine clinics to examine the impact of a provider communication “toolkit” on adolescent HPV vaccine series initiation and completion [50,69]. The toolkit consisted of motivational interviewing training for providers related to HPV vaccination and training on 3 tangible resources providers could also use with parents—an HPV fact sheet, an HPV vaccine decision aid, and an educational website. Results from the study demonstrated that motivational interviewing was the toolkit component most widely utilized by providers and was also perceived as being the most useful. More importantly, HPV vaccine series initiation levels were significantly higher among adolescents in practices receiving the toolkit than in control practices. There was no impact on HPV vaccine series completion (unpublished results). The usefulness of motivational interviewing for vaccination is further supported by a small study in which community pharmacists receiving motivational interviewing training for adult vaccination reported significantly higher patient readiness to receive vaccines following their interaction with the pharmacist than those who did not receive the training [70]. Finally, Perkins et al performed a cluster randomized controlled trial that evaluated the impact of a provider-focused intervention on adolescent HPV vaccination rates. The intervention included frequent provider support meetings, education on HPV infection and vaccination, feedback on providers’ individual HPV vaccination rates, provider incentives, and “basic motivational interviewing principles with vaccine-hesitant parents.” HPV vaccination series initiation and completion rates were significantly higher in intervention practices than controls, and this effect was sustained for at least 6 months after the active intervention period was over [67]. However, it was unknown how much the motivational interviewing contributed to these results. Based on the above information, and the long history of success of motivational interviewing for improving patient compliance with other recommended health behaviors, this technique appears to have a reasonable evidence base and should be considered for communicating with families that express resistance to HPV vaccination.
Personalized Communication
Parents’ reasons for not having their adolescent vaccinated against HPV are often complex and multifactorial [71,72]. Personalized approaches are needed to account for each parent’s unique informational needs, beliefs, and prior experiences [65]. Unfortunately, given the short amount of time allotted for clinical visits, it is often difficult to provide adequate information to parents during these encounters [73–75]. Indeed, concern about prolonged HPV vaccine discussions has been identified as an important barrier for providers that cause some to forgo recommending the vaccine [36,75].
One potential solution to this issue is to leverage technology in the form of web-based interventions that use software to tailor materials to each individual’s unique informational needs. Feasibility for this idea comes from the knowledge that many parents already use the web to research health issues related to their children [76], and that doctors’ offices are increasingly using patient portals and other web-based resources to help parents prepare for upcoming visits, especially those focused on health maintenance [77,78]. Tailored messaging interventions have been shown across populations and health issues to generally result in superior adherence with health behaviors when compared to untailored controls [79–82]. Several researchers have thus begun exploring whether such a personalized communication strategy may be similarly effective for adolescent HPV vaccination [50,83–85]. As an example, Maertens and colleagues used community-based participatory research techniques to develop a web-based tailored messaging intervention for Latinos regarding HPV vaccination [86]. A subsequent randomized controlled trial of the intervention in over 1200 parents of adolescents and young adults demonstrated that the intervention improved participants’ intentions to vaccinate compared to usual care [87], and among adolescents, higher HPV vaccine series initiation levels (unpublished data). Although additional work is needed to understand the feasibility of implementing such an intervention more broadly, additional support for the usefulness of a tailored messaging approach comes from a study of female university students that demonstrated higher HPV vaccination intentions after exposure to tailored information compared to untailored information. However, the impact on actual HPV vaccine utilization was not measured in the study [84]. Contrasting results were found in a different study of university students where researchers failed to find an impact of message tailoring on HPV vaccination utilization. However, this study was limited by a low response rate (~50%) to the follow up survey where vaccination status was assessed, and also by overall low levels of HPV vaccine initiation among the entire study sample (8%) [85]. Given the low number of studies in this area, and some conflicting data, additional research is needed to better understand the impact of personalized communication on HPV vaccination levels. However, results from these studies suggest that a modest benefit may be achieved with this approach, especially if coupled with other, evidence-based, clinic-level interventions to promote vaccination (eg, vaccine reminders, extended office hours), as is suggested by the Task Force on Community Preventive Services [48].
Focusing Communication on Cancer Prevention
HPV vaccines are unique in that they are only 1 of 2 vaccines for cancer prevention (the other being hepatitis B). Provider and parent surveys suggest that while most providers do mention cancer prevention when discussing HPV vaccines [40,88,89], this may be more commonly done with female patients than males [22]. Focusing on cancer prevention rather than sexual transmissibility is a communication technique suggested by the Centers for Disease Control and Prevention (CDC) as many parents cite this aspect of the vaccine as one of the most compelling reasons for vaccinating [45,90]. CDC’s “You are the Key” program [91] uses cancer prevention as a central theme in their physician and patient communication materials, based on significant prior market research on the acceptability and impact of such messages among parents and providers. In 2016 Malo and colleagues tested the potential impact of brief messages related to HPV vaccination, including cancer prevention messages, among a national sample of 776 medical providers and 1504 parents of adolescents [92]. In addition to their potential to motivate parents to vaccination, associations between parental endorsement of each message and their adolescent’s vaccination status were also examined. The cancer prevention messages were among those most highly endorsed by both parents and providers as being motivating for parents to get their adolescent vaccinated. More importantly, among parents these endorsements were associated with a significantly higher likelihood of the adolescent having been vaccinated against HPV. Interestingly, one of the briefest messages in the study, “I [the physician] strongly believe in the importance of this cancer preventing vaccine for [child’s name],” was perceived as the most persuasive message by parents.
Further support for the positive impact of framing HPV vaccines primarily as cancer prevention comes from another national study of 1495 parents of 11 to 17 year olds that examined 3 measures of quality of their adolescent provider’s HPV vaccine recommendation, and the relationship between recommendation quality and likelihood of adolescent HPV vaccination [40]. The 3 quality indicators assessed included providing information about cancer prevention, encouraging the vaccine “strongly,” and recommending it be given on the same day as it was being discussed. While 49% of parents reported receiving no HPV vaccine recommendation from their adolescents’ provider, of those that did, 86% received a cancer prevention message. Parents who had been given high quality recommendations that included either 2 or 3 of the quality indicator measures had over 9 times the odds of vaccine series initiation and 3 times the odds of vaccine series follow through than those who had not received any recommendation, and also significantly higher odds of vaccination than parents who had received low quality recommendations (ie, included only 1 indicator). Taken together, these results suggest that focusing discussions about HPV vaccines on their ability to prevent cancer is likely to be persuasive for some parents.
Strategies That Are Promising But Not Thoroughly Tested
Helping Parents Create Vaccination Plans
A recent commentary suggested that instead of focusing on changing beliefs or “educating” parents and patients about the need for a given vaccine, perhaps a better way to craft interventions for increasing vaccination is to focus on structuring the environment to make vaccination “easy” [93,94]. Examples of this include strategies such as extended office hours and making the vaccine available in other locations such as schools and pharmacies, both of which have been shown in some populations and settings to improve vaccine utilization [48,95]. One aspect of structuring a vaccine-conducive environment that relates to provider communication is helping parents create “implementation intentions” for future vaccination visits. In its most obvious form, this would mean providers provide office resources that facilitate making an appointment for the next dose in the HPV vaccine series during a clinic visit where the first dose was provided. But such an approach could also potentially extend to parents who are on the fence about the vaccine—to make an appointment before the parent leaves the office with an unvaccinated child to either re-discuss the vaccine in the future or to actually start the vaccine series. Support for such a strategy comes primarily from the social sciences, which suggest that implementation intentions work by increasing attention to specific cues to action, making it more likely that that the cue will be acted upon [96–98]. Creating implementation intentions has been shown to be helpful for improving adherence with a variety of health behaviors [99–105], and there is a growing evidence base related to how implementation intentions may facilitate vaccination specifically. For example Vet and colleagues performed a randomized controlled trial among 616 men who have sex with men with either strong or weak intentions to receive the hepatitis B vaccine [106]. Half of the participants were asked to create an implementation intention plan where they described when, where and how they would obtain the vaccine. Those in the control arm were not given this prompt. Regardless of whether their initial vaccination intention was weak or strong, those who had been asked to create an implementation plan had more than double the likelihood of actually getting the vaccine than participants who did not receive the implementation plan prompt. Similarly, a study of influenza vaccination rates among corporate employees found that those who were asked to write down the day and time they planned to go to employee health to get the free vaccine were somewhat more likely (4% higher) to be vaccinated than those who did not receive this prompt [107]. In addition, a study of elderly individuals found that influenza vaccination rates were significantly higher among those who had received “action instructions” on how, when and where to get the vaccine than those who did not [108]. These studies suggest that helping parents craft a definitive follow-up plan regarding vaccination could have a significant impact on vaccination rates—particularly for vaccines like HPV that require multiple doses.
Treating all Adolescent Vaccines the Same
Prior research has demonstrated that providers often communicate differently about HPV vaccines than other adolescent vaccines such as the tetanus-diphtheria-pertussis (Tdap) and meningococcal (MCV) vaccines [22,36]. Providers often tend to discuss the HPV vaccine last among these 3 vaccines, provide weaker endorsements of the vaccine, and pre-emptively give much more detail about the HPV compared to the other vaccines, even in the absence of a parent’s request for additional information [36,39,41]. The CDC and the American Academy of Pediatrics now suggest putting HPV at the beginning or middle of the list of vaccines recommended to the adolescent (ie, “HPV, Tdap and MCV”), and treating all recommended vaccines equivalently in terms of the level of detail provided to parents in the absence of a parent’s request for more information [109,110]. Through these suggestions have face validity, their specific impact on HPV vaccination rates, and on patient and provider satisfaction with the visit have yet to be evaluated.
Strategies that Probably Don’t Work
Presenting Myths and Facts
Research related to promoting other vaccines provides insight into communication activities that probably would not work well for promoting HPV vaccination. A 2012 study by Nyhan and colleagues examined the impact of 2 different messages related to influenza vaccines on participants’ beliefs about the vaccine’s safety and intentions to get vaccinated [111]. One group received information to correct the commonly held belief that influenza vaccine can cause the flu while the other received information about the risks associated with contracting an influenza infection. While the correction of myths did improve participants’ perceptions of the vaccine’s safety, information about influenza dangers did not. Neither message impacted intentions to vaccinate in the study subjects overall. However, in sub-analyses the correction of myths actually decreased intentions to vaccinate among those with high baseline levels of concern about the vaccine’s side effects—that is, among those most concerned that the flu vaccine can give someone the flu, correcting this myth actually decreased the likelihood that they would receive the vaccine. Similar findings have been reported in other studies related to vaccination [112–114], and suggest that the “threat” generated by providing information opposing a person’s beliefs may actually entrench these beliefs further as part of the threat response—a phenomenon known as attitude polarization [115]. These results also are consistent with the concept of negativity bias, which posits that negative information influences people’s risk perceptions more than positive information, and that the more strongly a risk is attempted to be negated, the lower the effectiveness and perceived trust of the information [116].
Using Fear Appeals
One tactic that has been suggested by some as a way to promote vaccination is to provide graphic depictions of the possible sequelae of vaccine-preventable diseases. The thought behind this idea is that because vaccination is so successful, most parents will have never experienced significant impacts from vaccine preventable diseases that, in the past, had been a major motivator for parents to vaccinate. Thus, in order to counter beliefs about “controversial” issues like vaccination, highly emotionally compelling and engaging information may be especially useful. This is a common tactic used by anti-vaccination groups to spread their own messages [117]. However, several studies suggested that using “fear appeals” (aka scare tactics) such as this to promote vaccination can actually have a negative effect on vaccination intentions. For example, in a 2011 study of a nationally representative sample of parents of children < 18 years, 4 different message formats were tested for their impact on parental intentions to vaccinate a future child with the measles-mumps-rubella vaccine (MMR) [113]. Message formats included correcting the misinformation that MMR causes autism, presenting information on MMR-related disease risk, providing a dramatic narrative about a child endangered by measles, and showing pictures of infants affected by these diseases. Counter to the study’s hypotheses, the dramatic narrative message actually increased parents’ perceptions that MMR vaccines had serious side effects, and the pictures increased parents’ belief that the MMR vaccine could cause autism. These counter-intuitive results are consistent with other studies that have examined the impact of message framing on adults’ vaccination intentions for HPV and influenza [108,118,119]. Taken together, fear appeals seem unlikely to sway many hesitant parents towards HPV vaccination.
Looking Into the Future
Moving forward, additional interventions to improve providers’ ability to communicate with families about HPV vaccination will undoubtedly be developed. A major area of interest in this regard is leveraging the power of technology and the internet, including using social media, mobile technologies, and online interventions to augment the provider/parent interaction that occurs during the clinical visit [50,120]. Web-based approaches have the benefit of generally being low cost and easy to disseminate to large populations. Such interventions have already been developed for a number of other health issues, some of which have proven effective [121,122]. However, use of the internet to promote healthy behaviors in general, and vaccination specifically, is still in its infancy. There is still much to be learned about how to create effective web-based tools, how to engage patients with them, and how to assess their impact on health outcomes [123].
Another interesting area for future research is identifying psychological “levers” to motivate parents’ vaccination intentions [94]. One example is focusing on using parents’ values (ie, protecting my child from harm) as an intervention target rather than beliefs or attitudes. This is because values tend to be inherent and static over time, compared to beliefs and attitudes, which are subject to change depending on the context [124]. Prior research has shown that interventions that leverage values rather than facts can be an effective way to overcome beliefs that are highly emotional or controversial, and that individuals are more likely to trust sources and individuals with shared values than those without [125], suggesting that this may be a useful way to motivate parents toward vaccinating their children. Self-affirmation is another example of a psychological lever that has a significant evidence base from the social science literature as a helpful tool for moving patients towards a desired health behavior [126,127], but it has not been extensively applied to the field of vaccination. Researchers in the field of vaccine delivery are increasingly recognizing the potential value of these unique intervention approaches [101,128–134], and it may be fruitful in the future to more closely examine the efficacy of interventions that target things like values, self-affirmation or other psychological levers to change parents’ HPV vaccination behaviors.
A final notable area for intervention research related to HPV vaccination is the use of video games. Although not likely to be used directly during patient visits, this strategy could be conceptualized as a potential way to augment the information conveyed to a parent by a provider directly during a clinical encounter. A meta-analysis from 2016 identified 16 different “serious” video games that were used to train and educate users about specific vaccine preventable diseases (usually influenza, none for HPV) and the need for vaccination [135]. In many of them, the objective of the game was to protect a virtual community from a vaccine preventable disease and/or manage outbreaks. Only 2 of the games evaluated outcomes in the short term (ie, at the time the game was being played). None have evaluated longer-term impacts such as vaccination intention or utilization. In the era of “plugged in” parents and adolescents, video games represent a unique but understudied mechanism for helping providers “communicate,” albeit indirectly, with families about the need for vaccination. Imagine providing a prescription to an HPV-vaccine hesitant family to “go play Zombie Wars HPV!” One would expect the curiosity factor alone would result in significant engagement with this intervention tool.
Conclusion
With persistently lagging HPV vaccination rates among U.S. adolescents, there is a growing need for effective interventions to improve adolescent HPV vaccine utilization. How providers communicate with families is one of the most influential factors in parents’ vaccination decisions. Emerging research is beginning to delineate potentially effective communication techniques such as presumptive approaches to making the vaccine recommendation, framing the vaccine as cancer preventing, and using motivational interviewing and personalized messaging when met with parental vaccine resistance. Moving forward the list of evidence-based interventions to improve providers’ HPV vaccine communication is likely to grow, and to increasingly leverage technology based solutions. However, given the complexities of the vaccination decision [136] and the ever growing spread of vaccine hesitancy [137], it is unlikely that a single intervention approach will be effective for getting adolescent HPV vaccine levels up to the national goal of 80% coverage. As has been recognized in the past, the most effective interventions for HPV vaccination in the future are likely to be multicomponent, including not only provider communication strategies but also clinic-, community-, and parent-level interventions [48].
Corresponding author: Amanda Dempsey, MD, PhD, MPH, 13199 East Montview Blvd, Suite 300, Aurora, CO 80045, [email protected].
Financial disclosures: None reported.
From the University of Colorado Denver, Aurora, CO.
Abstract
- Objective: To provide evidence-based guidance on strategies that are likely or unlikely to be successful in navigating HPV vaccine conversations with patients and parents.
- Methods: Nonsystematic review of the literature.
- Results: This review highlights some of the most recent innovations in provider HPV vaccine communication and describes provider communication strategies that have been found to improve adolescent vaccination rates in rigorous scientific studies. Promising strategies for which additional research is needed and strategies that probably do not work are also described.
- Conclusion: By understanding what works, what may work, and what not to do when it comes to communicating with families about HPV vaccines, providers can be better prepared for maximizing the impact they can have on adolescent HPV vaccination rates.
Key words: human papillomavirus; vaccine hesitancy; health communication; parents; immunization.
In the United States, more than 14 million people newly acquire genital human papillomavirus (HPV) annually, and 75 million Americans are infected at any given time [1]. As the most common sexually transmitted disease, more than 80% of sexually active U.S. adults are estimated to be infected with HPV by the age of 50 [1,2]. Although the majority of infections are “silent” and resolve without clinical sequelae, a proportion of infected individuals will go on to develop HPV-related diseases. In women, these include cervical cancer and pre-cancer (ie, abnormal Pap smears); cancers of the vagina, vulva, anus, and oropharynx; and genital warts [3]. Males also bear a high burden of HPV-related disease in the form of penile, anal, and oropharyngeal cancers, as well as genital warts [3]. While once thought of as primarily a “woman’s disease” [4], recent research demonstrates men are also significantly impacted by HPV—particularly in the form of oropharyngeal cancers, which are 2 to 3 times more common in men than in women [5]. In fact, it is estimated by the year 2020 more men will die of HPV-related oropharyngeal cancer than women will die of cervical cancer [6,7]. The combined cost of HPV-associated cancers and other conditions is estimated to be $8 billion per year in the United States [8–11].
HPV Vaccines
Effective HPV vaccines have been available for females aged 9 to 26 years since 2006 (bivalent and quadrivalent vaccines) and for males aged 9 to 26 since 2010 (quadrivalent vaccine only) [12]. These vaccines have been shown in clinical trials to be highly efficacious in preventing HPV infection, cervical pre-cancer, and anal, vaginal, penile, and vulvar cancers caused by the HPV types covered in the vaccine [2]. Although their effectiveness against head and neck cancer has not been studied in clinical trials, most experts believe that these vaccines will also provide protection against at least a proportion of these cancers [13,14]. In 2015 the U.S. Food and Drug Administration approved licensure of a 9-valent HPV vaccine that will soon replace the quadrivalent vaccine in the U.S. market [15]. The 9-valent vaccine is licensed for both males and females aged 9 to 26 and is expected to prevent an even higher proportion of HPV-related cancers than earlier HPV vaccines due to the protection against 5 additional oncogenic HPV types [15].
Despite the potential of HPV vaccines to drastically reduce the incidence of HPV-related cancers and other diseases, these vaccines are not being as widely used in the United States as was hoped. The most recent national data from 2015 demonstrates that only 41.9% of girls and 28.1% of boys have received all 3 doses recommended in the HPV vaccine series [16]. This level of vaccine utilization is significantly lower than the Healthy People 2020 goal of 80% coverage [17], and also significantly lower than that of other developed countries such as Australia and the United Kingdom, which have achieved vaccination levels of ~70% among their target adolescent populations [18,19]. In the future, these low vaccination levels will likely be mitigated somewhat by the recent approval from the FDA and recent recommendation from the Advisory Committee on Immunization Practices (ACIP) for only 2 doses of the 9-valent HPV vaccine (spaced 6 to 12 months apart) for adolescents less than 15 years of age [20,21]. Three doses are still recommended for those aged 15 to 26 years.
Provider Communication About HPV Vaccines
How providers communicate with parents and patients about HPV vaccines is a key influential factor driving current U.S. adolescent HPV vaccination levels [22,23]. Numerous studies demonstrate that a provider’s recommendation generally has the largest impact on whether or not an adolescent receives the vaccine, even above that of parent factors such as attitudes and beliefs about the vaccine and patient characteristics such as age and insurance status [23–31]. Moreover, parents consistently cite their adolescent’s provider as one of the most trusted and impactful resources for obtaining vaccine information [22,32].
Unfortunately, research also shows that providers often fail to adequately recommend the HPV vaccine for their patients, especially for 11 to 12 year olds for whom the vaccine is preferentially recommended [33,34]. For example, in a national study of parents done in 2013, not being recommended by a provider was one of the top 5 reasons parents of males and of females aged 11 to 17 gave for not getting their adolescent vaccinated against HPV [35]. Supporting this also is a 2014 study of 776 pediatricians and family medicine providers nationally, in which Gilkey and colleagues found that more than 1 out of 4 providers did not highly endorse the HPV vaccine for 11 to 12 year olds despite this having been the recommended practice from ACIP for the prior 8 years for girls and 4 years for boys. This is in comparison to the other adolescents vaccines that were reported in the same study as being endorsed highly by these providers > 95% of the time [36].
Recognizing that providers’ HPV vaccine recommendations are often suboptimal, researchers have begun to define what components comprise “high-quality” HPV vaccine recommendations. This has been operationalized by one research group as (1) timeliness—routinely recommending the vaccine starting when the patient is ≤ 12 years; (2) consistency—recommending the vaccine for all eligible adolescents as opposed to an approach based on providers’ perception of their patients’ risk for HPV infection; (3) urgency—recommending that the vaccine be given on the same day the vaccine is being discussed, rather than offering the option of getting it at a future visit; and (4) strength—using language that clearly conveys that the provider believes the vaccine is very important for the adolescent to receive. A national study of primary care providers done in 2014 examined how frequently these quality components were implemented [37]. The results were startling and discouraging. Nearly half of providers (49%) reported they usually recommended that 11 to 12 year olds get the vaccine at a later visit, 41% used a risk-based approach for deciding when to recommend the vaccine, 27% did not tell the parents the vaccine was “very or extremely important,” and a large proportion did not start routinely recommending the vaccine before the age of 13 (39% for male patients and 25% for females) [37].
Much research has now accumulated to explain the underlying reasons why providers may not give consistent and high-quality HPV vaccine recommendations to all eligible adolescents [22]. Issues such as providers’ own knowledge about HPV-related diseases, personal beliefs about the vaccine’s safety and necessity, concern that a discussion about the vaccine will necessitate a discussion about adolescent sexuality with the parent, belief that parents will not want their child vaccinated if asked, perceptions that a provider can adequately select those patients most “in need” of HPV vaccination, and concern that raising the vaccine discussion with vaccine-hesitant parents will result in prolonged discussions have been shown to impact whether and how providers communicate about HPV vaccination during clinical visits [22,36–45]. Now that these barriers have been defined and described, there is a great need to use this knowledge to develop and evaluate interventions that help to mitigate these barriers and improve providers’ vaccine communication abilities. Such interventions are needed not only for HPV, but for all vaccines [46,47].
Possible Strategies for Helping Providers Communicate About HPV Vaccines
Before discussing these interventions, it is worth noting that several of the passive and active strategies have been shown in clinical trials to improve adolescent HPV vaccination rates. Although these are beyond the scope of this article, inclusion of these strategies should certainly be considered by any practice as a mechanism to increase vaccination levels, especially given that the most successful interventions to improve vaccination levels consist of multiple components [48]. Also useful is a recently described “taxonomy of vaccine communication interventions” that provides additional perspective on the scope and complexity of interventions to improve vaccine delivery [49]. There are several well-written review articles that describe interventions that focus on passive and active strategies at the practice or community level [50–52].
Interpersonal Communication Strategies Shown to Increase HPV Vaccination
Presumptive Communication
One of the first studies to examine the specific “way” in which providers communicate about vaccines focused not on HPV but rather on young childhood vaccines. In 2013 Opel and colleagues performed a study in which they taped clinical encounters between a pediatrician and a parent of a child aged 1 to 19 months [53]. Of the 111 encounters recorded, 50% of parents were classified as vaccine hesitant. Parents were aware they were being taped but not aware that the overall purpose of the study was to examine providers’ communication related to vaccination. The researchers found that providers generally used one of 2 communication styles to introduce the vaccine discussion. The first, called the “presumptive” style, assumed that parents would agree to vaccination and presented the vaccines as routine (ie, “We have to do some shots today”). The second style, called “participatory,” was more parent-oriented and used language suggesting shared decision-making (ie, “So what do you want to do about shots today?”). The study showed that the odds of resisting the provider’s vaccine recommendations were significantly higher when providers used a participatory approach than a presumptive one, suggesting that even small changes in language can have a major impact on the likelihood of vaccination. However, given the study design, causality between providers’ recommendation style and parents vaccination decisions could not be delineated.
In 2015 Moss and colleagues performed a study that examined the use of these 2 communication styles with regard to HPV vaccination [54]. This study used data from the 2010 National Immunization Survey–Teen, a national survey on childhood vaccination that includes provider verification of vaccines given [16]. Researchers categorized provider vaccine communication styles into “provider-driven,” which was similar to the presumptive style described Opel, and “patient-driven,” which was similar to Opel’s permissive style. Parents who received a more provider-driven style of HPV vaccine recommendation were far more likely to have allowed their adolescent to be vaccinated than those receiving patient-driven recommendations [54]. Further supporting this communication approach are results from a qualitative study done by Hughes and colleagues in which triads of mothers, adolescents, and providers were interviewed after a preventive care visit to assess the communication that occurred regarding HPV vaccination [39]. Providers’ communication style was categorized into 1 of 3 groups: paternalistic (clinician makes the vaccination decision and communicates this to the family); informed (patient and family gathers information from the clinician and other sources to reach a vaccination decision); and shared (medical and personal information is exchanged between the provider and family and then a decision is reached jointly). Providers who typically adopted the paternalistic approach perceived that they had the highest success in convincing parents to vaccinate—a perception that was confirmed in quantitative assessments of vaccination status among adolescents in the study sample [39]. Our own research demonstrates that learning and implementing a presumptive/paternalistic HPV vaccine recommendation style is easy for primary care providers to do and is perceived as often shortening the time taken during clinical visits to discuss the vaccine [55,56]. Thus, providers should consider opening the HPV vaccine conversation using this approach, and then turning to some of the other communication techniques described below when met with parental resistance or questions.
Motivational Interviewing
A second communication technique that seems effective for promoting HPV vaccination, especially for vaccine hesitant parents, is motivational interviewing. Motivational interviewing describes a communication technique in which the provider leverages a parents’ or patients’ intrinsic motivation to engage in a preferred health behavior [57]. Motivational interviewing was originally developed to combat substance abuse [58,59] but has subsequently been successfully applied to a number of other health issues [60–64]. There is growing interest from public health and medical providers in using this technique for increasing vaccination [39,65–68]. Our research group performed a large, cluster-randomized controlled trial of 16 pediatric and family medicine clinics to examine the impact of a provider communication “toolkit” on adolescent HPV vaccine series initiation and completion [50,69]. The toolkit consisted of motivational interviewing training for providers related to HPV vaccination and training on 3 tangible resources providers could also use with parents—an HPV fact sheet, an HPV vaccine decision aid, and an educational website. Results from the study demonstrated that motivational interviewing was the toolkit component most widely utilized by providers and was also perceived as being the most useful. More importantly, HPV vaccine series initiation levels were significantly higher among adolescents in practices receiving the toolkit than in control practices. There was no impact on HPV vaccine series completion (unpublished results). The usefulness of motivational interviewing for vaccination is further supported by a small study in which community pharmacists receiving motivational interviewing training for adult vaccination reported significantly higher patient readiness to receive vaccines following their interaction with the pharmacist than those who did not receive the training [70]. Finally, Perkins et al performed a cluster randomized controlled trial that evaluated the impact of a provider-focused intervention on adolescent HPV vaccination rates. The intervention included frequent provider support meetings, education on HPV infection and vaccination, feedback on providers’ individual HPV vaccination rates, provider incentives, and “basic motivational interviewing principles with vaccine-hesitant parents.” HPV vaccination series initiation and completion rates were significantly higher in intervention practices than controls, and this effect was sustained for at least 6 months after the active intervention period was over [67]. However, it was unknown how much the motivational interviewing contributed to these results. Based on the above information, and the long history of success of motivational interviewing for improving patient compliance with other recommended health behaviors, this technique appears to have a reasonable evidence base and should be considered for communicating with families that express resistance to HPV vaccination.
Personalized Communication
Parents’ reasons for not having their adolescent vaccinated against HPV are often complex and multifactorial [71,72]. Personalized approaches are needed to account for each parent’s unique informational needs, beliefs, and prior experiences [65]. Unfortunately, given the short amount of time allotted for clinical visits, it is often difficult to provide adequate information to parents during these encounters [73–75]. Indeed, concern about prolonged HPV vaccine discussions has been identified as an important barrier for providers that cause some to forgo recommending the vaccine [36,75].
One potential solution to this issue is to leverage technology in the form of web-based interventions that use software to tailor materials to each individual’s unique informational needs. Feasibility for this idea comes from the knowledge that many parents already use the web to research health issues related to their children [76], and that doctors’ offices are increasingly using patient portals and other web-based resources to help parents prepare for upcoming visits, especially those focused on health maintenance [77,78]. Tailored messaging interventions have been shown across populations and health issues to generally result in superior adherence with health behaviors when compared to untailored controls [79–82]. Several researchers have thus begun exploring whether such a personalized communication strategy may be similarly effective for adolescent HPV vaccination [50,83–85]. As an example, Maertens and colleagues used community-based participatory research techniques to develop a web-based tailored messaging intervention for Latinos regarding HPV vaccination [86]. A subsequent randomized controlled trial of the intervention in over 1200 parents of adolescents and young adults demonstrated that the intervention improved participants’ intentions to vaccinate compared to usual care [87], and among adolescents, higher HPV vaccine series initiation levels (unpublished data). Although additional work is needed to understand the feasibility of implementing such an intervention more broadly, additional support for the usefulness of a tailored messaging approach comes from a study of female university students that demonstrated higher HPV vaccination intentions after exposure to tailored information compared to untailored information. However, the impact on actual HPV vaccine utilization was not measured in the study [84]. Contrasting results were found in a different study of university students where researchers failed to find an impact of message tailoring on HPV vaccination utilization. However, this study was limited by a low response rate (~50%) to the follow up survey where vaccination status was assessed, and also by overall low levels of HPV vaccine initiation among the entire study sample (8%) [85]. Given the low number of studies in this area, and some conflicting data, additional research is needed to better understand the impact of personalized communication on HPV vaccination levels. However, results from these studies suggest that a modest benefit may be achieved with this approach, especially if coupled with other, evidence-based, clinic-level interventions to promote vaccination (eg, vaccine reminders, extended office hours), as is suggested by the Task Force on Community Preventive Services [48].
Focusing Communication on Cancer Prevention
HPV vaccines are unique in that they are only 1 of 2 vaccines for cancer prevention (the other being hepatitis B). Provider and parent surveys suggest that while most providers do mention cancer prevention when discussing HPV vaccines [40,88,89], this may be more commonly done with female patients than males [22]. Focusing on cancer prevention rather than sexual transmissibility is a communication technique suggested by the Centers for Disease Control and Prevention (CDC) as many parents cite this aspect of the vaccine as one of the most compelling reasons for vaccinating [45,90]. CDC’s “You are the Key” program [91] uses cancer prevention as a central theme in their physician and patient communication materials, based on significant prior market research on the acceptability and impact of such messages among parents and providers. In 2016 Malo and colleagues tested the potential impact of brief messages related to HPV vaccination, including cancer prevention messages, among a national sample of 776 medical providers and 1504 parents of adolescents [92]. In addition to their potential to motivate parents to vaccination, associations between parental endorsement of each message and their adolescent’s vaccination status were also examined. The cancer prevention messages were among those most highly endorsed by both parents and providers as being motivating for parents to get their adolescent vaccinated. More importantly, among parents these endorsements were associated with a significantly higher likelihood of the adolescent having been vaccinated against HPV. Interestingly, one of the briefest messages in the study, “I [the physician] strongly believe in the importance of this cancer preventing vaccine for [child’s name],” was perceived as the most persuasive message by parents.
Further support for the positive impact of framing HPV vaccines primarily as cancer prevention comes from another national study of 1495 parents of 11 to 17 year olds that examined 3 measures of quality of their adolescent provider’s HPV vaccine recommendation, and the relationship between recommendation quality and likelihood of adolescent HPV vaccination [40]. The 3 quality indicators assessed included providing information about cancer prevention, encouraging the vaccine “strongly,” and recommending it be given on the same day as it was being discussed. While 49% of parents reported receiving no HPV vaccine recommendation from their adolescents’ provider, of those that did, 86% received a cancer prevention message. Parents who had been given high quality recommendations that included either 2 or 3 of the quality indicator measures had over 9 times the odds of vaccine series initiation and 3 times the odds of vaccine series follow through than those who had not received any recommendation, and also significantly higher odds of vaccination than parents who had received low quality recommendations (ie, included only 1 indicator). Taken together, these results suggest that focusing discussions about HPV vaccines on their ability to prevent cancer is likely to be persuasive for some parents.
Strategies That Are Promising But Not Thoroughly Tested
Helping Parents Create Vaccination Plans
A recent commentary suggested that instead of focusing on changing beliefs or “educating” parents and patients about the need for a given vaccine, perhaps a better way to craft interventions for increasing vaccination is to focus on structuring the environment to make vaccination “easy” [93,94]. Examples of this include strategies such as extended office hours and making the vaccine available in other locations such as schools and pharmacies, both of which have been shown in some populations and settings to improve vaccine utilization [48,95]. One aspect of structuring a vaccine-conducive environment that relates to provider communication is helping parents create “implementation intentions” for future vaccination visits. In its most obvious form, this would mean providers provide office resources that facilitate making an appointment for the next dose in the HPV vaccine series during a clinic visit where the first dose was provided. But such an approach could also potentially extend to parents who are on the fence about the vaccine—to make an appointment before the parent leaves the office with an unvaccinated child to either re-discuss the vaccine in the future or to actually start the vaccine series. Support for such a strategy comes primarily from the social sciences, which suggest that implementation intentions work by increasing attention to specific cues to action, making it more likely that that the cue will be acted upon [96–98]. Creating implementation intentions has been shown to be helpful for improving adherence with a variety of health behaviors [99–105], and there is a growing evidence base related to how implementation intentions may facilitate vaccination specifically. For example Vet and colleagues performed a randomized controlled trial among 616 men who have sex with men with either strong or weak intentions to receive the hepatitis B vaccine [106]. Half of the participants were asked to create an implementation intention plan where they described when, where and how they would obtain the vaccine. Those in the control arm were not given this prompt. Regardless of whether their initial vaccination intention was weak or strong, those who had been asked to create an implementation plan had more than double the likelihood of actually getting the vaccine than participants who did not receive the implementation plan prompt. Similarly, a study of influenza vaccination rates among corporate employees found that those who were asked to write down the day and time they planned to go to employee health to get the free vaccine were somewhat more likely (4% higher) to be vaccinated than those who did not receive this prompt [107]. In addition, a study of elderly individuals found that influenza vaccination rates were significantly higher among those who had received “action instructions” on how, when and where to get the vaccine than those who did not [108]. These studies suggest that helping parents craft a definitive follow-up plan regarding vaccination could have a significant impact on vaccination rates—particularly for vaccines like HPV that require multiple doses.
Treating all Adolescent Vaccines the Same
Prior research has demonstrated that providers often communicate differently about HPV vaccines than other adolescent vaccines such as the tetanus-diphtheria-pertussis (Tdap) and meningococcal (MCV) vaccines [22,36]. Providers often tend to discuss the HPV vaccine last among these 3 vaccines, provide weaker endorsements of the vaccine, and pre-emptively give much more detail about the HPV compared to the other vaccines, even in the absence of a parent’s request for additional information [36,39,41]. The CDC and the American Academy of Pediatrics now suggest putting HPV at the beginning or middle of the list of vaccines recommended to the adolescent (ie, “HPV, Tdap and MCV”), and treating all recommended vaccines equivalently in terms of the level of detail provided to parents in the absence of a parent’s request for more information [109,110]. Through these suggestions have face validity, their specific impact on HPV vaccination rates, and on patient and provider satisfaction with the visit have yet to be evaluated.
Strategies that Probably Don’t Work
Presenting Myths and Facts
Research related to promoting other vaccines provides insight into communication activities that probably would not work well for promoting HPV vaccination. A 2012 study by Nyhan and colleagues examined the impact of 2 different messages related to influenza vaccines on participants’ beliefs about the vaccine’s safety and intentions to get vaccinated [111]. One group received information to correct the commonly held belief that influenza vaccine can cause the flu while the other received information about the risks associated with contracting an influenza infection. While the correction of myths did improve participants’ perceptions of the vaccine’s safety, information about influenza dangers did not. Neither message impacted intentions to vaccinate in the study subjects overall. However, in sub-analyses the correction of myths actually decreased intentions to vaccinate among those with high baseline levels of concern about the vaccine’s side effects—that is, among those most concerned that the flu vaccine can give someone the flu, correcting this myth actually decreased the likelihood that they would receive the vaccine. Similar findings have been reported in other studies related to vaccination [112–114], and suggest that the “threat” generated by providing information opposing a person’s beliefs may actually entrench these beliefs further as part of the threat response—a phenomenon known as attitude polarization [115]. These results also are consistent with the concept of negativity bias, which posits that negative information influences people’s risk perceptions more than positive information, and that the more strongly a risk is attempted to be negated, the lower the effectiveness and perceived trust of the information [116].
Using Fear Appeals
One tactic that has been suggested by some as a way to promote vaccination is to provide graphic depictions of the possible sequelae of vaccine-preventable diseases. The thought behind this idea is that because vaccination is so successful, most parents will have never experienced significant impacts from vaccine preventable diseases that, in the past, had been a major motivator for parents to vaccinate. Thus, in order to counter beliefs about “controversial” issues like vaccination, highly emotionally compelling and engaging information may be especially useful. This is a common tactic used by anti-vaccination groups to spread their own messages [117]. However, several studies suggested that using “fear appeals” (aka scare tactics) such as this to promote vaccination can actually have a negative effect on vaccination intentions. For example, in a 2011 study of a nationally representative sample of parents of children < 18 years, 4 different message formats were tested for their impact on parental intentions to vaccinate a future child with the measles-mumps-rubella vaccine (MMR) [113]. Message formats included correcting the misinformation that MMR causes autism, presenting information on MMR-related disease risk, providing a dramatic narrative about a child endangered by measles, and showing pictures of infants affected by these diseases. Counter to the study’s hypotheses, the dramatic narrative message actually increased parents’ perceptions that MMR vaccines had serious side effects, and the pictures increased parents’ belief that the MMR vaccine could cause autism. These counter-intuitive results are consistent with other studies that have examined the impact of message framing on adults’ vaccination intentions for HPV and influenza [108,118,119]. Taken together, fear appeals seem unlikely to sway many hesitant parents towards HPV vaccination.
Looking Into the Future
Moving forward, additional interventions to improve providers’ ability to communicate with families about HPV vaccination will undoubtedly be developed. A major area of interest in this regard is leveraging the power of technology and the internet, including using social media, mobile technologies, and online interventions to augment the provider/parent interaction that occurs during the clinical visit [50,120]. Web-based approaches have the benefit of generally being low cost and easy to disseminate to large populations. Such interventions have already been developed for a number of other health issues, some of which have proven effective [121,122]. However, use of the internet to promote healthy behaviors in general, and vaccination specifically, is still in its infancy. There is still much to be learned about how to create effective web-based tools, how to engage patients with them, and how to assess their impact on health outcomes [123].
Another interesting area for future research is identifying psychological “levers” to motivate parents’ vaccination intentions [94]. One example is focusing on using parents’ values (ie, protecting my child from harm) as an intervention target rather than beliefs or attitudes. This is because values tend to be inherent and static over time, compared to beliefs and attitudes, which are subject to change depending on the context [124]. Prior research has shown that interventions that leverage values rather than facts can be an effective way to overcome beliefs that are highly emotional or controversial, and that individuals are more likely to trust sources and individuals with shared values than those without [125], suggesting that this may be a useful way to motivate parents toward vaccinating their children. Self-affirmation is another example of a psychological lever that has a significant evidence base from the social science literature as a helpful tool for moving patients towards a desired health behavior [126,127], but it has not been extensively applied to the field of vaccination. Researchers in the field of vaccine delivery are increasingly recognizing the potential value of these unique intervention approaches [101,128–134], and it may be fruitful in the future to more closely examine the efficacy of interventions that target things like values, self-affirmation or other psychological levers to change parents’ HPV vaccination behaviors.
A final notable area for intervention research related to HPV vaccination is the use of video games. Although not likely to be used directly during patient visits, this strategy could be conceptualized as a potential way to augment the information conveyed to a parent by a provider directly during a clinical encounter. A meta-analysis from 2016 identified 16 different “serious” video games that were used to train and educate users about specific vaccine preventable diseases (usually influenza, none for HPV) and the need for vaccination [135]. In many of them, the objective of the game was to protect a virtual community from a vaccine preventable disease and/or manage outbreaks. Only 2 of the games evaluated outcomes in the short term (ie, at the time the game was being played). None have evaluated longer-term impacts such as vaccination intention or utilization. In the era of “plugged in” parents and adolescents, video games represent a unique but understudied mechanism for helping providers “communicate,” albeit indirectly, with families about the need for vaccination. Imagine providing a prescription to an HPV-vaccine hesitant family to “go play Zombie Wars HPV!” One would expect the curiosity factor alone would result in significant engagement with this intervention tool.
Conclusion
With persistently lagging HPV vaccination rates among U.S. adolescents, there is a growing need for effective interventions to improve adolescent HPV vaccine utilization. How providers communicate with families is one of the most influential factors in parents’ vaccination decisions. Emerging research is beginning to delineate potentially effective communication techniques such as presumptive approaches to making the vaccine recommendation, framing the vaccine as cancer preventing, and using motivational interviewing and personalized messaging when met with parental vaccine resistance. Moving forward the list of evidence-based interventions to improve providers’ HPV vaccine communication is likely to grow, and to increasingly leverage technology based solutions. However, given the complexities of the vaccination decision [136] and the ever growing spread of vaccine hesitancy [137], it is unlikely that a single intervention approach will be effective for getting adolescent HPV vaccine levels up to the national goal of 80% coverage. As has been recognized in the past, the most effective interventions for HPV vaccination in the future are likely to be multicomponent, including not only provider communication strategies but also clinic-, community-, and parent-level interventions [48].
Corresponding author: Amanda Dempsey, MD, PhD, MPH, 13199 East Montview Blvd, Suite 300, Aurora, CO 80045, [email protected].
Financial disclosures: None reported.
1. Centers for Disease Control and Prevention. Genital HPV infection - CDC fact sheet. 2012. Accessed 26 Jun 2012 at www.cdc.gov/std/HPV/STDFact-HPV.htm.
2. Markowitz LE, Dunne EF, Saraiya M, et al. Quadrivalent human papillomavirus vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007;56(RR-2):1–24.
3. Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers - United States, 2008-2012. MMWR Morb Mortal Wkly Rep 2016;65:661–6.
4. Daley EM, Vamos CA, Zimet GD, et al. The feminization of HPV: Reversing gender biases in US human papillomavirus vaccine policy. Am J Public Health 2016;106:983–4.
5. Rettig EM, D’Souza G. Epidemiology of head and neck cancer. Surg Oncol Clin North Am 2015;24:379–96.
6. Louie KS, Mehanna H, Sasieni P. Trends in head and neck cancers in England from 1995 to 2011 and projections up to 2025. Oral Oncol 2015;51:341–8.
7. Mehanna H, Beech T, Nicholson T, et al. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer--systematic review and meta-analysis of trends by time and region. Head Neck 2013;35:747–55.
8. Chesson HW, Markowitz LE, Hariri S, et al. The impact and cost-effectiveness of nonavalent HPV vaccination in the United States: Estimates from a simplified transmission model. Hum Vaccin Immunother 2016;12:1363–72.
9. Insinga RP, Glass AG, Rush BB, et al. The health care costs of cervical human papillomavirus-related disease. Am J Obstet Gynecol 2004;191:114–20.
10. Chesson HW, Blandford JM, Gift TL, et al. The estimated direct medical cost of sexually transmitted diseases among American youth, 2000. Perspect Sex Reprod Health 2004;36:11–9.
11. Chesson HW, Ekwueme DU, Saraiya M, et al. Estimates of the annual direct medical costs of the prevention and treatment of disease associated with human papillomavirus in the United States. Vaccine 2012;30:6016–9.
12. Food and Drug Administration. Product approval information - licensing action, package insert: Gardasil. 2010. Accessed at www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM111263.pdf.
13. Ward G, Mehta V, Moore M. Morbidity, mortality and cost from HPV-related oropharyngeal cancer: Impact of 2-, 4- and 9-valent vaccines. Hum Vaccin Immunother 2016;12:1343–7.
14. Guo T, Eisele DW, Fakhry C. The potential impact of prophylactic human papillomavirus vaccination on oropharyngeal cancer. Cancer 2016;122:2313–23.
15. Food and Drug Administration. Product approval information - licensing action, package insert: Gardasil 9. 2014. Accessed at www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm426445.htm.
16. Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 Years - United States, 2015. MMWR Morb Mortal Wkly Rep 2016;65:850–8.
17. Healthy People 2020. Accessed at www.healthypeople.gov/2020/topics-objectives/national-snapshot/hpv-vaccine-adolescents-2008%E2%80%932012.
18. Garland SM. The Australian experience with the human papillomavirus vaccine. Clin Therapeut 2014;36:17–23.
19. Brabin L, Roberts SA, Stretch R, et al. Uptake of first two doses of human papillomavirus vaccine by adolescent schoolgirls in Manchester: prospective cohort study. BMJ 2008;336:1056–8.
20. Food and Drug Administration. Prescribing information [package insert]. Gardasil 9. 2016. Accessed at www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM426457.pdf
21. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination - updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2016;65:1405–8.
22. Gilkey MB, McRee AL. Provider communication about HPV vaccination: a systematic review. Hum Vaccin Immunother 2016;12:1454–68.
23. Clark SJ, Cowan AE, Filipp SL, et al. Parent perception of provider interactions influences hpv vaccination status of adolescent females. Clin Pediatr (Phila) 2016;55:701–6.
24. Ferrer HB, Trotter C, Hickman M, Audrey S. Barriers and facilitators to HPV vaccination of young women in high-income countries: a qualitative systematic review and evidence synthesis. BMC Public Health 2014;14:700.
25. Clark SJ, Cowan AE, Filipp SL, et al. Parent HPV vaccine perspectives and the likelihood of HPV vaccination of adolescent males. Hum Vaccin Immunother 2016;12:47–51.
26. Darden PM, Jacobson RM. Impact of a physician recommendation. Hum Vaccin Immunother 2014;10:2632–5.
27. Darden PM, Thompson DM, Roberts JR, et al. Reasons for not vaccinating adolescents: national immunization survey of teens, 2008-2010. Pediatrics 2013;131:645–51.
28. Gargano LM, Herbert NL, Painter JE, et al. Impact of a physician recommendation and parental immunization attitudes on receipt or intention to receive adolescent vaccines. Hum Vaccin Immunother 2013;9:2627–33.
29. Ylitalo KR, Lee H, Mehta NK. Health care provider recommendation, human papillomavirus vaccination, and race/ethnicity in the US National Immunization Survey. Am J Public Health 2013;103:164–9.
30. Brewer NT, Gottlieb SL, Reiter PL, et al. Longitudinal predictors of human papillomavirus vaccine initiation among adolescent girls in a high-risk geographic area. Sex Transm Dis 2011;38:197–204.
31. Reiter PL, McRee AL, Pepper JK, et al. Longitudinal predictors of human papillomavirus vaccination among a national sample of adolescent males. Am J Public Health 2013;103:1419–27.
32. Gottvall M, Grandahl M, Hoglund AT, et al. Trust versus concerns-how parents reason when they accept HPV vaccination for their young daughter. Ups J Med Sci 2013;118:263–70.
33. Vadaparampil ST, Malo TL, Kahn JA, et al. Physicians’ human papillomavirus vaccine recommendations, 2009 and 2011. Am J Prev Med 2014;46:80–4.
34. Vadaparampil ST, Malo TL, Sutton SK, et al. Missing the target for routine human papillomavirus vaccination: consistent and strong physician recommendations are lacking for 11- to 12-year-old males. Cancer Epidemiol Biomarkers Prev 2016;25:1435–46.
35. Stokley S, Jeyarajah J, Yankey D, et al. Human papillomavirus vaccination coverage among adolescents, 2007-2013, and postlicensure vaccine safety monitoring, 2006-2014 - United States. MMWR Morb Mortal Wkly Rep 2014;63:620–4.
36. Gilkey MB, Moss JL, Coyne-Beasley T, et al. Physician communication about adolescent vaccination: How is human papillomavirus vaccine different? Prev Med 2015;77:181–5.
37. Gilkey MB, Malo TL, Shah PD, et al. Quality of physician communication about human papillomavirus vaccine: findings from a national survey. Cancer Epidemiol Biomarkers Prev 2015;24:1673–9.
38. Herzog R, Alvarez-Pasquin MJ, Diaz C, et al. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Pub Health 2013;13:154.
39. Hughes CC, Jones AL, Feemster KA, Fiks AG. HPV vaccine decision making in pediatric primary care: a semi-structured interview study. BMC Pediatr 2011;11:74.
40. Gilkey MB, Calo WA, Moss JL, et al. Provider communication and HPV vaccination: the impact of recommendation quality. Vaccine 2016;34:1187–92.
41. Malo TL, Ali KN, Sutton SK, et al. The content and context of physicians’ communication with males about human papillomavirus vaccination. Hum Vaccin Immunother 2016;12:1511–8.
42. Moss JL, Gilkey MB, Rimer BK, Brewer NT. Disparities in collaborative patient-provider communication about human papillomavirus (HPV) vaccination. Hum Vaccin Immunother 2016:0.
43. Suryadevara M, Handel A, Bonville CA, et al. Pediatric provider vaccine hesitancy: an under-recognized obstacle to immunizing children. Vaccine 2015;33:6629–34.
44. Mohammed KA, Geneus CJ, Osazuwa-Peters N, et al. Disparities in provider recommendation of human papillomavirus vaccination for U.S. adolescents. J Adolesc Health 2016;59:592–8.
45. Perkins RB, Clark JA. Providers’ perceptions of parental concerns about HPV vaccination. J Health Care Poor Underserved 2013;24:828–39.
46. Bloom BR, Marcuse E, Mnookin S. Addressing vaccine hesitancy. Science 2014;344:339.
47. Zimet GD. Health care professionals and adolescent vaccination. A call for intervention research. Hum Vaccin Immunother 2014;10:2629–30.
48. Task Force on Community Preventive Services. The guide to community preventive services: increasing appropriate vaccination. 2014. Accessed at www.thecommunityguide.org/vaccines/index.html.
49. Willis N, Hill S, Kaufman J, et al. “Communicate to vaccinate”: the development of a taxonomy of communication interventions to improve routine childhood vaccination. BMC Int Health Hum Rights 2013;13:23.
50. Dempsey AF, Zimet GD. Interventions to improve adolescent vaccination: what may work and what still needs to be tested. Vaccine 2015;33 Suppl 4:D106–113.
51. Walling EB, Benzoni N, Dornfeld J, et al. Interventions to improve HPV vaccine uptake: a systematic review. Pediatrics 2016;138(1).
52. Smulian EA, Mitchell KR, Stokley S. Interventions to increase HPV vaccination coverage: a systematic review. Hum Vaccin Immunother 2016;12:1566–88.
53. Opel DJ, Heritage J, Taylor JA, et al. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics 2013;132:1037–46.
54. Moss JL, Reiter PL, Rimer BK, Brewer NT. Collaborative patient-provider communication and uptake of adolescent vaccines. Soc Sci Med 2016;159:100–7.
55. Lockhart S, Barnard J, O’Leary ST, et al. Exploring the feasibility of implementing a multifaceted toolkit to improve HPV vaccine provider communication in primary care settings. Presented at: Annual Meeting of the American Public Health Association; 2016; Denver, CO.
56. Reno J, O’Leary ST, Lockhart S, et al. Assessment of a communication toolkit for healcare providers about adolescent HPV vaccination. Presented at: Pediatric Academic Societies Annual Meeting; 2015; Baltimore, MD.
57. www.motivationalinterviewing.org
58. Foxcroft DR, Coombes L, Wood S, et al. Motivational interviewing for the prevention of alcohol misuse in young adults. Cochrane Database Syst Rev 2016;(7):CD007025.
59. Lindson-Hawley N, Thompson TP, Begh R. Motivational interviewing for smoking cessation. Cochrane Database Syst Rev 2015(3):CD006936.
60. McCain J. To heal the body, get into the patient’s head: motivational interviewing: to improve adherence. Biotechnol Healthc 2012;9:10–2.
61. Boutin-Foster C, Scott E, Rodriguez A, et al. The trial using motivational interviewing and positive affect and self-affirmation in african-americans with hypertension (TRIUMPH): from theory to clinical trial implementation. Contemp Clin Trials 2013;35:8–14.
62. Gance-Cleveland B. Motivational interviewing for adolescent obesity. Am J Nurs 2013;113:11.
63. Hides L, Carroll S, Scott R, et al. Quik fix: a randomized controlled trial of an enhanced brief motivational interviewing intervention for alcohol/cannabis and psychological distress in young people. Psychother Psychosom 2013;82:122–4.
64. Rongkavilit C, Naar-King S, Wang B, et al. Motivational interviewing targeting risk behaviors for youth living with HIV in Thailand. AIDS Behav 2013;17:2063–74.
65. Leask J, Kinnersley P, Jackson C, et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr 2012;12:154.
66. Zimet GD, Perkins SM, Sturm LA, et al. Predictors of STI vaccine acceptability among parents and their adolescent children. J Adolesc Health 2005;37:179–86.
67. Perkins RB, Zisblatt L, Legler A, et al. Effectiveness of a provider-focused intervention to improve HPV vaccination rates in boys and girls. Vaccine 2015;33:1223–9.
68. MacDonald N, Finlay JC. Working with vaccine-hesitant parents. Paediatr Child Health 2013;18:265–7.
69. Dempsey A, Lockhart S, Pyrzanowski J, et al. Impact of motivational interviewing training on providers communication about adolescent HPV vaccination. Presented at: Pediatric Academic Societies Annual Meeting; 2015; Baltimore, MD.
70. Brackett A, Butler M, Chapman L. Using motivational interviewing in the community pharmacy to increase adult immunization readiness: A pilot evaluation. J Am Pharm Assoc (2003) 2015;55:182–6.
71. Holman DM, Benard V, Roland KB, et al. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatrics 2014;168:76–82.
72. Rambout L, Tashkandi M, Hopkins L, Tricco AC. Self-reported barriers and facilitators to preventive human papillomavirus vaccination among adolescent girls and young women: a systematic review. Prev Med 2014;58:22–32.
73. McSherry LA, Dombrowski SU, Francis JJ, et al. ‘It’s a can of worms’: understanding primary care practitioners’ behaviours in relation to HPV using the Theoretical Domains Framework. Implement Sci 2012;7:73.
74. Palmer J, Carrico C, Coxtanzo C. Identifying and overcoming perceived barriers of providers towards HPV vaccination: A literature review. J Vaccines 2015:7.
75. Gowda C, Schaffer SE, Dombkowski KJ, Dempsey AF. Understanding attitudes toward adolescent vaccination and the decision-making dynamic among adolescents, parents and providers. BMC Public Health 2012;12:509.
76. Plantin L, Daneback K. Parenthood, information and support on the internet. A literature review of research on parents and professionals online. BMC Fam Pract 2009;10:34.
77. Irizarry T, DeVito Dabbs A, Curran CR. Patient portals and patient engagement: a state of the science review. J Med Internet Res 2015;17:e148.
78. Clark SJ, Costello LE, Gebremariam A, Dombkowski KJ. A national survey of parent perspectives on use of patient portals for their children’s health care. Appl Clin Inform 2015;6:110–9.
79. Noar SM, Benac CN, Harris MS. Does tailoring matter? Meta-analytic review of tailored print health behavior change interventions. Psychol Bull 2007;133:673–93.
80. Kreuter MW, Strecher VJ, Glassman B. One size does not fit all: the case for tailoring print materials. Ann Behav Med 1999;21:276–83.
81. Hawkins RP, Kreuter M, Resnicow K, et al. Understanding tailoring in communicating about health. Health Educ Res 2008;23:454–66.
82. Lustria ML, Noar SM, Cortese J, et al. A meta-analysis of web-delivered tailored health behavior change interventions. J Health Commun 2013;18:1039–69.
83. Paiva AL, Lipschitz JM, Fernandez AC, et al. Evaluation of the acceptability and feasibility of a computer-tailored intervention to increase human papillomavirus vaccination among young adult women. J Am College Health 2014;62:32–8.
84. Gerend MA, Shepherd MA, Lustria ML. Increasing human papillomavirus vaccine acceptability by tailoring messages to young adult women’s perceived barriers. Sex Transm Dis 2013;40:401–5.
85. Bennett AT, Patel DA, Carlos RC, et al. Human papillomavirus vaccine uptake after a tailored, online educational intervention for female university students: a randomized controlled trial. J Womens Health (Larchmt) 2015;24:950–7.
86. Maertens J, Zambrano-Jiminez A, Dempsey AF. Using community engagement to develop a web-based intervention for Latinas about the HPV vaccine. J Health Commun 2017. Forthcoming.
87. Reno J, Maertens J, Jimenez-Zambrano A, et al. Effectiveness of a tailored intervention on changing hpv vaccination intention among Hispanics. Presented at: Pediatric Academic Societies Meeting; 2016; Baltimore, MD.
88. Daley MF, Liddon N, Crane LA, et al. A national survey of pediatrician knowledge and attitudes regarding human papillomavirus vaccination. Pediatrics 2006;118:2280–9.
89. Sussman AL, Helitzer D, Sanders M,et al. HPV and cervical cancer prevention counseling with younger adolescents: implications for primary care. Ann Fam Med 2007;5:298–304.
90. Perkins RB, Chigurupati NL, Apte G, et al. Why don’t adolescents finish the HPV vaccine series? A qualitative study of parents and providers. Hum Vaccin Immunother 2016;12:1528–35.
91. Centers for Disease Control and Prevention. Immunization Education & Training. You are the key to HPV cancer prevention. Accessed at www.cdc.gov/vaccines/ed/hpv/index.html.
92. Malo TL, Gilkey MB, Hall ME, et al. Messages to motivate human papillomavirus vaccination: national studies of parents and physicians. Cancer Epidemiol Biomarkers Prev 2016;25:1383–91.
93. Chapman G. Increasing vaccination without changing beliefs. 2016. Accessed at http://catalyst.nejm.org/increasing-vaccination-without-changing-beliefs/.
94. Rossen I, Hurlstone MJ, Lawrence C. Going with the grain of cognition: applying insights from psychology to build support for childhood vaccination. Front Psychol 2016;7:1483.
95. Shah PD, Gilkey MB, Pepper JK, et al. Promising alternative settings for HPV vaccination of US adolescents. Exp Rev Vaccine 2014;13:235–46.
96. Webb TL, Sheeran P. Does changing behavioral intentions engender behavior change? A meta-analysis of the experimental evidence. Psychol Bull 2006;132:249–68.
97. Janczyk M, Dambacher M, Bieleke M, Gollwitzer PM. The benefit of no choice: goal-directed plans enhance perceptual processing. Psychol Res 2015;79:206–20.
98. Wieber F, Thurmer JL, Gollwitzer PM. Promoting the translation of intentions into action by implementation intentions: behavioral effects and physiological correlates. Front Hum Neurosci 2015;9:395.
99. Chapman J, Armitage CJ, Norman P. Comparing implementation intention interventions in relation to young adults’ intake of fruit and vegetables. Psychol Health 2009;24:317–32.
100. Martin J, Sheeran P, Slade P, et al. Implementation intention formation reduces consultations for emergency contraception and pregnancy testing among teenage women. Health Psychol 2009;28:762–9.
101. Chapman J, Armitage CJ. Do techniques that increase fruit intake also increase vegetable intake? Evidence from a comparison of two implementation intention interventions. Appetite 2012;58:28–33.
102. Armitage CJ, Arden MA. Enhancing the effectiveness of alcohol warning labels with a self-affirming implementation intention. Health Psychol 2016;35:1159–63.
103. Bradbury D, Upsher R, Chilcot J. A pilot randomised test of a self-affirmation implementation intention intervention to reduce dietary salt intake. J Health Psychol 2016 May 22.
104. Chen XJ, Liu LL, Cui JF, et al. The effect and mechanisms of implementation intention in improving prospective memory performance in schizophrenia patients. Psychiatry Res 2016;244:86–93.
105. Hagger MS, Luszczynska A, de Wit J, et al. Implementation intention and planning interventions in health psychology: recommendations from the Synergy Expert Group for research and practice. Psychol Health 2016;31:814–39.
106. Vet R, de Wit JB, Das E. The role of implementation intention formation in promoting hepatitis B vaccination uptake among men who have sex with men. Int J STD AIDS 2014;25:122–9.
107. Milkman KL, Beshears J, Choi JJ, et al. Using implementation intention prompts to enhance influenza vaccination rates. Proc Natl Acad Sci U S A 2011;108:10415–20.
108. McCaul KD, Johnson RJ, Rothman AJ. The effects of framing and action instructions on whether older adults obtain flu shots. Health Psychol 2002;21:624–8.
109. Centers for Disease Control and Prevention. You are the key: health care providers’ tip sheet. 2015. Accessed at www.cdc.gov/vaccines/who/teens/for-hcp-tipsheet-hpv.pdf.
110. American Academy of Pediatrics. HPV vaccine champion toolkit. 2015. Accessed at www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/Pages/HPV-Champion-Toolkit.aspx.
111. Nyhan B, Reifler J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine 2015;33:459–64.
112. Betsch C, Sachse K. Debunking vaccination myths: strong risk negations can increase perceived vaccination risks. Health Psychol 2013;32:146–55.
113. Nyhan B, Reifler J, Richey S, Freed GL. Effective messages in vaccine promotion: a randomized trial. Pediatrics 2014;133:e835–842.
114. Schwartz N, Sanna LJ, Skurnik I, Yoon C. Metacognitive expriences and the intericacies of setting people straight: Implications for debiasing and public information campaigns. Adv Exper Social Psychol 2007;39:127–161.
115. Lord C, Ross L, Lepper MR. Biased assimilation and attitude polarization: the effects of prior theories on subsequently considered evidence. J Personality Soc Pscyhol 1979;37:2098–109.
116. Siegrist M, Cvetkovich G. Better negative than positive? Evidence of a bias for negative information about possible health dangers. Risk Analysis 2001;21:199–206.
117. Witte K, Allen M. A meta-analysis of fear appeals: implications for effective public health campaigns. Health Educ Behav 2000;27:591–615.
118. Gerend MA, Shepherd JE. Using message framing to promote acceptance of the human papillomavirus vaccine. Health Psychol 2007;26:745–52.
119. Ferguson E, Gallagher L. Message framing with respect to decisions about vaccination: the roles of frame valence, frame method and perceived risk. Br J Psychol (London:1953) 2007;98(Pt 4):667–80.
120. Jarrett C, Wilson R, O’Leary M, et al. Strategies for addressing vaccine hesitancy - A systematic review. Vaccine 2015;33:4180–90.
121. Laranjo L, Arguel A, Neves AL, et al. The influence of social networking sites on health behavior change: a systematic review and meta-analysis. J Am Med Inform Assoc 2015;22:243–56.
122. Maher CA, Lewis LK, Ferrar K, et al. Are health behavior change interventions that use online social networks effective? A systematic review. J Med Internet Res 2014;16:e40.
123. Betsch C, Brewer NT, Brocard P, et al. Opportunities and challenges of Web 2.0 for vaccination decisions. Vaccine 2012;30:3727–33.
124. Barrett Values Center. Values versus beliefs. 2016. Accessed 31 Oct 2016 at www.valuescentre.com/mapping-values/values/values-vs-beliefs.
125. Dore RA, Stone ER, Buchanan CM. A social values analysis of parental decision making. J Psychol 2014;148:477–504.
126. Cohen GL, Sherman DK. The psychology of change: self-affirmation and social psychological intervention. Ann Rev Psychol 2014;65:333–71.
127. Sweeney AM, Moyer A. Self-affirmation and responses to health messages: a meta-analysis on intentions and behavior. Health Psychol 2015;34:149–59.
128. Tiro JA, Lee SC, Marks EG, et al. Developing a tablet-based self-persuasion intervention promoting adolescent hpv vaccination: protocol for a three-stage mixed-methods study. JMIR Res Protoc 2016;5:e19.
129. Leask J, Macartney K. Parental decisions about vaccination: collective values are important. J Paediatr Child Health 2008;44:534–5.
130. Salmon DA, Omer SB. Individual freedoms versus collective responsibility: immunization decision-making in the face of occasionally competing values. Emerg Themes Epidemiol 2006;3:13.
131. Gidengil C, Lieu TA, Payne K, et al. Parental and societal values for the risks and benefits of childhood combination vaccines. Vaccine 2012;30:3445–52.
132. Attwell K, Freeman M. I immunise: an evaluation of a values-based campaign to change attitudes and beliefs. Vaccine 2015;33:6235–40.
133. Witteman HO. Addressing vaccine hesitancy with values. Pediatrics 2015;136:215–7.
134. Witteman HO, Chipenda Dansokho S, Exe N, et al. Risk Communication, values clarification, and vaccination decisions. Risk Anal 2015;35:1801–19.
135. Ohannessian R, Yaghobian S, Verger P, Vanhems P. A systematic review of serious video games used for vaccination. Vaccine 2016.
136. National Vaccine Advisory Committee. Assessing the state of vaccine confidence in the United States: recommendations from the National Vaccine Advisory Committee. Pub Health Rep 2015;130:573–95.
137. Dube E, Vivion M, MacDonald NE. Vaccine hesitancy, vaccine refusal and the anti-vaccine movement: influence, impact and implications. Exp Rev Vaccine 2015;14:99–117.
1. Centers for Disease Control and Prevention. Genital HPV infection - CDC fact sheet. 2012. Accessed 26 Jun 2012 at www.cdc.gov/std/HPV/STDFact-HPV.htm.
2. Markowitz LE, Dunne EF, Saraiya M, et al. Quadrivalent human papillomavirus vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007;56(RR-2):1–24.
3. Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers - United States, 2008-2012. MMWR Morb Mortal Wkly Rep 2016;65:661–6.
4. Daley EM, Vamos CA, Zimet GD, et al. The feminization of HPV: Reversing gender biases in US human papillomavirus vaccine policy. Am J Public Health 2016;106:983–4.
5. Rettig EM, D’Souza G. Epidemiology of head and neck cancer. Surg Oncol Clin North Am 2015;24:379–96.
6. Louie KS, Mehanna H, Sasieni P. Trends in head and neck cancers in England from 1995 to 2011 and projections up to 2025. Oral Oncol 2015;51:341–8.
7. Mehanna H, Beech T, Nicholson T, et al. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer--systematic review and meta-analysis of trends by time and region. Head Neck 2013;35:747–55.
8. Chesson HW, Markowitz LE, Hariri S, et al. The impact and cost-effectiveness of nonavalent HPV vaccination in the United States: Estimates from a simplified transmission model. Hum Vaccin Immunother 2016;12:1363–72.
9. Insinga RP, Glass AG, Rush BB, et al. The health care costs of cervical human papillomavirus-related disease. Am J Obstet Gynecol 2004;191:114–20.
10. Chesson HW, Blandford JM, Gift TL, et al. The estimated direct medical cost of sexually transmitted diseases among American youth, 2000. Perspect Sex Reprod Health 2004;36:11–9.
11. Chesson HW, Ekwueme DU, Saraiya M, et al. Estimates of the annual direct medical costs of the prevention and treatment of disease associated with human papillomavirus in the United States. Vaccine 2012;30:6016–9.
12. Food and Drug Administration. Product approval information - licensing action, package insert: Gardasil. 2010. Accessed at www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM111263.pdf.
13. Ward G, Mehta V, Moore M. Morbidity, mortality and cost from HPV-related oropharyngeal cancer: Impact of 2-, 4- and 9-valent vaccines. Hum Vaccin Immunother 2016;12:1343–7.
14. Guo T, Eisele DW, Fakhry C. The potential impact of prophylactic human papillomavirus vaccination on oropharyngeal cancer. Cancer 2016;122:2313–23.
15. Food and Drug Administration. Product approval information - licensing action, package insert: Gardasil 9. 2014. Accessed at www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm426445.htm.
16. Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 Years - United States, 2015. MMWR Morb Mortal Wkly Rep 2016;65:850–8.
17. Healthy People 2020. Accessed at www.healthypeople.gov/2020/topics-objectives/national-snapshot/hpv-vaccine-adolescents-2008%E2%80%932012.
18. Garland SM. The Australian experience with the human papillomavirus vaccine. Clin Therapeut 2014;36:17–23.
19. Brabin L, Roberts SA, Stretch R, et al. Uptake of first two doses of human papillomavirus vaccine by adolescent schoolgirls in Manchester: prospective cohort study. BMJ 2008;336:1056–8.
20. Food and Drug Administration. Prescribing information [package insert]. Gardasil 9. 2016. Accessed at www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM426457.pdf
21. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination - updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2016;65:1405–8.
22. Gilkey MB, McRee AL. Provider communication about HPV vaccination: a systematic review. Hum Vaccin Immunother 2016;12:1454–68.
23. Clark SJ, Cowan AE, Filipp SL, et al. Parent perception of provider interactions influences hpv vaccination status of adolescent females. Clin Pediatr (Phila) 2016;55:701–6.
24. Ferrer HB, Trotter C, Hickman M, Audrey S. Barriers and facilitators to HPV vaccination of young women in high-income countries: a qualitative systematic review and evidence synthesis. BMC Public Health 2014;14:700.
25. Clark SJ, Cowan AE, Filipp SL, et al. Parent HPV vaccine perspectives and the likelihood of HPV vaccination of adolescent males. Hum Vaccin Immunother 2016;12:47–51.
26. Darden PM, Jacobson RM. Impact of a physician recommendation. Hum Vaccin Immunother 2014;10:2632–5.
27. Darden PM, Thompson DM, Roberts JR, et al. Reasons for not vaccinating adolescents: national immunization survey of teens, 2008-2010. Pediatrics 2013;131:645–51.
28. Gargano LM, Herbert NL, Painter JE, et al. Impact of a physician recommendation and parental immunization attitudes on receipt or intention to receive adolescent vaccines. Hum Vaccin Immunother 2013;9:2627–33.
29. Ylitalo KR, Lee H, Mehta NK. Health care provider recommendation, human papillomavirus vaccination, and race/ethnicity in the US National Immunization Survey. Am J Public Health 2013;103:164–9.
30. Brewer NT, Gottlieb SL, Reiter PL, et al. Longitudinal predictors of human papillomavirus vaccine initiation among adolescent girls in a high-risk geographic area. Sex Transm Dis 2011;38:197–204.
31. Reiter PL, McRee AL, Pepper JK, et al. Longitudinal predictors of human papillomavirus vaccination among a national sample of adolescent males. Am J Public Health 2013;103:1419–27.
32. Gottvall M, Grandahl M, Hoglund AT, et al. Trust versus concerns-how parents reason when they accept HPV vaccination for their young daughter. Ups J Med Sci 2013;118:263–70.
33. Vadaparampil ST, Malo TL, Kahn JA, et al. Physicians’ human papillomavirus vaccine recommendations, 2009 and 2011. Am J Prev Med 2014;46:80–4.
34. Vadaparampil ST, Malo TL, Sutton SK, et al. Missing the target for routine human papillomavirus vaccination: consistent and strong physician recommendations are lacking for 11- to 12-year-old males. Cancer Epidemiol Biomarkers Prev 2016;25:1435–46.
35. Stokley S, Jeyarajah J, Yankey D, et al. Human papillomavirus vaccination coverage among adolescents, 2007-2013, and postlicensure vaccine safety monitoring, 2006-2014 - United States. MMWR Morb Mortal Wkly Rep 2014;63:620–4.
36. Gilkey MB, Moss JL, Coyne-Beasley T, et al. Physician communication about adolescent vaccination: How is human papillomavirus vaccine different? Prev Med 2015;77:181–5.
37. Gilkey MB, Malo TL, Shah PD, et al. Quality of physician communication about human papillomavirus vaccine: findings from a national survey. Cancer Epidemiol Biomarkers Prev 2015;24:1673–9.
38. Herzog R, Alvarez-Pasquin MJ, Diaz C, et al. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Pub Health 2013;13:154.
39. Hughes CC, Jones AL, Feemster KA, Fiks AG. HPV vaccine decision making in pediatric primary care: a semi-structured interview study. BMC Pediatr 2011;11:74.
40. Gilkey MB, Calo WA, Moss JL, et al. Provider communication and HPV vaccination: the impact of recommendation quality. Vaccine 2016;34:1187–92.
41. Malo TL, Ali KN, Sutton SK, et al. The content and context of physicians’ communication with males about human papillomavirus vaccination. Hum Vaccin Immunother 2016;12:1511–8.
42. Moss JL, Gilkey MB, Rimer BK, Brewer NT. Disparities in collaborative patient-provider communication about human papillomavirus (HPV) vaccination. Hum Vaccin Immunother 2016:0.
43. Suryadevara M, Handel A, Bonville CA, et al. Pediatric provider vaccine hesitancy: an under-recognized obstacle to immunizing children. Vaccine 2015;33:6629–34.
44. Mohammed KA, Geneus CJ, Osazuwa-Peters N, et al. Disparities in provider recommendation of human papillomavirus vaccination for U.S. adolescents. J Adolesc Health 2016;59:592–8.
45. Perkins RB, Clark JA. Providers’ perceptions of parental concerns about HPV vaccination. J Health Care Poor Underserved 2013;24:828–39.
46. Bloom BR, Marcuse E, Mnookin S. Addressing vaccine hesitancy. Science 2014;344:339.
47. Zimet GD. Health care professionals and adolescent vaccination. A call for intervention research. Hum Vaccin Immunother 2014;10:2629–30.
48. Task Force on Community Preventive Services. The guide to community preventive services: increasing appropriate vaccination. 2014. Accessed at www.thecommunityguide.org/vaccines/index.html.
49. Willis N, Hill S, Kaufman J, et al. “Communicate to vaccinate”: the development of a taxonomy of communication interventions to improve routine childhood vaccination. BMC Int Health Hum Rights 2013;13:23.
50. Dempsey AF, Zimet GD. Interventions to improve adolescent vaccination: what may work and what still needs to be tested. Vaccine 2015;33 Suppl 4:D106–113.
51. Walling EB, Benzoni N, Dornfeld J, et al. Interventions to improve HPV vaccine uptake: a systematic review. Pediatrics 2016;138(1).
52. Smulian EA, Mitchell KR, Stokley S. Interventions to increase HPV vaccination coverage: a systematic review. Hum Vaccin Immunother 2016;12:1566–88.
53. Opel DJ, Heritage J, Taylor JA, et al. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics 2013;132:1037–46.
54. Moss JL, Reiter PL, Rimer BK, Brewer NT. Collaborative patient-provider communication and uptake of adolescent vaccines. Soc Sci Med 2016;159:100–7.
55. Lockhart S, Barnard J, O’Leary ST, et al. Exploring the feasibility of implementing a multifaceted toolkit to improve HPV vaccine provider communication in primary care settings. Presented at: Annual Meeting of the American Public Health Association; 2016; Denver, CO.
56. Reno J, O’Leary ST, Lockhart S, et al. Assessment of a communication toolkit for healcare providers about adolescent HPV vaccination. Presented at: Pediatric Academic Societies Annual Meeting; 2015; Baltimore, MD.
57. www.motivationalinterviewing.org
58. Foxcroft DR, Coombes L, Wood S, et al. Motivational interviewing for the prevention of alcohol misuse in young adults. Cochrane Database Syst Rev 2016;(7):CD007025.
59. Lindson-Hawley N, Thompson TP, Begh R. Motivational interviewing for smoking cessation. Cochrane Database Syst Rev 2015(3):CD006936.
60. McCain J. To heal the body, get into the patient’s head: motivational interviewing: to improve adherence. Biotechnol Healthc 2012;9:10–2.
61. Boutin-Foster C, Scott E, Rodriguez A, et al. The trial using motivational interviewing and positive affect and self-affirmation in african-americans with hypertension (TRIUMPH): from theory to clinical trial implementation. Contemp Clin Trials 2013;35:8–14.
62. Gance-Cleveland B. Motivational interviewing for adolescent obesity. Am J Nurs 2013;113:11.
63. Hides L, Carroll S, Scott R, et al. Quik fix: a randomized controlled trial of an enhanced brief motivational interviewing intervention for alcohol/cannabis and psychological distress in young people. Psychother Psychosom 2013;82:122–4.
64. Rongkavilit C, Naar-King S, Wang B, et al. Motivational interviewing targeting risk behaviors for youth living with HIV in Thailand. AIDS Behav 2013;17:2063–74.
65. Leask J, Kinnersley P, Jackson C, et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr 2012;12:154.
66. Zimet GD, Perkins SM, Sturm LA, et al. Predictors of STI vaccine acceptability among parents and their adolescent children. J Adolesc Health 2005;37:179–86.
67. Perkins RB, Zisblatt L, Legler A, et al. Effectiveness of a provider-focused intervention to improve HPV vaccination rates in boys and girls. Vaccine 2015;33:1223–9.
68. MacDonald N, Finlay JC. Working with vaccine-hesitant parents. Paediatr Child Health 2013;18:265–7.
69. Dempsey A, Lockhart S, Pyrzanowski J, et al. Impact of motivational interviewing training on providers communication about adolescent HPV vaccination. Presented at: Pediatric Academic Societies Annual Meeting; 2015; Baltimore, MD.
70. Brackett A, Butler M, Chapman L. Using motivational interviewing in the community pharmacy to increase adult immunization readiness: A pilot evaluation. J Am Pharm Assoc (2003) 2015;55:182–6.
71. Holman DM, Benard V, Roland KB, et al. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatrics 2014;168:76–82.
72. Rambout L, Tashkandi M, Hopkins L, Tricco AC. Self-reported barriers and facilitators to preventive human papillomavirus vaccination among adolescent girls and young women: a systematic review. Prev Med 2014;58:22–32.
73. McSherry LA, Dombrowski SU, Francis JJ, et al. ‘It’s a can of worms’: understanding primary care practitioners’ behaviours in relation to HPV using the Theoretical Domains Framework. Implement Sci 2012;7:73.
74. Palmer J, Carrico C, Coxtanzo C. Identifying and overcoming perceived barriers of providers towards HPV vaccination: A literature review. J Vaccines 2015:7.
75. Gowda C, Schaffer SE, Dombkowski KJ, Dempsey AF. Understanding attitudes toward adolescent vaccination and the decision-making dynamic among adolescents, parents and providers. BMC Public Health 2012;12:509.
76. Plantin L, Daneback K. Parenthood, information and support on the internet. A literature review of research on parents and professionals online. BMC Fam Pract 2009;10:34.
77. Irizarry T, DeVito Dabbs A, Curran CR. Patient portals and patient engagement: a state of the science review. J Med Internet Res 2015;17:e148.
78. Clark SJ, Costello LE, Gebremariam A, Dombkowski KJ. A national survey of parent perspectives on use of patient portals for their children’s health care. Appl Clin Inform 2015;6:110–9.
79. Noar SM, Benac CN, Harris MS. Does tailoring matter? Meta-analytic review of tailored print health behavior change interventions. Psychol Bull 2007;133:673–93.
80. Kreuter MW, Strecher VJ, Glassman B. One size does not fit all: the case for tailoring print materials. Ann Behav Med 1999;21:276–83.
81. Hawkins RP, Kreuter M, Resnicow K, et al. Understanding tailoring in communicating about health. Health Educ Res 2008;23:454–66.
82. Lustria ML, Noar SM, Cortese J, et al. A meta-analysis of web-delivered tailored health behavior change interventions. J Health Commun 2013;18:1039–69.
83. Paiva AL, Lipschitz JM, Fernandez AC, et al. Evaluation of the acceptability and feasibility of a computer-tailored intervention to increase human papillomavirus vaccination among young adult women. J Am College Health 2014;62:32–8.
84. Gerend MA, Shepherd MA, Lustria ML. Increasing human papillomavirus vaccine acceptability by tailoring messages to young adult women’s perceived barriers. Sex Transm Dis 2013;40:401–5.
85. Bennett AT, Patel DA, Carlos RC, et al. Human papillomavirus vaccine uptake after a tailored, online educational intervention for female university students: a randomized controlled trial. J Womens Health (Larchmt) 2015;24:950–7.
86. Maertens J, Zambrano-Jiminez A, Dempsey AF. Using community engagement to develop a web-based intervention for Latinas about the HPV vaccine. J Health Commun 2017. Forthcoming.
87. Reno J, Maertens J, Jimenez-Zambrano A, et al. Effectiveness of a tailored intervention on changing hpv vaccination intention among Hispanics. Presented at: Pediatric Academic Societies Meeting; 2016; Baltimore, MD.
88. Daley MF, Liddon N, Crane LA, et al. A national survey of pediatrician knowledge and attitudes regarding human papillomavirus vaccination. Pediatrics 2006;118:2280–9.
89. Sussman AL, Helitzer D, Sanders M,et al. HPV and cervical cancer prevention counseling with younger adolescents: implications for primary care. Ann Fam Med 2007;5:298–304.
90. Perkins RB, Chigurupati NL, Apte G, et al. Why don’t adolescents finish the HPV vaccine series? A qualitative study of parents and providers. Hum Vaccin Immunother 2016;12:1528–35.
91. Centers for Disease Control and Prevention. Immunization Education & Training. You are the key to HPV cancer prevention. Accessed at www.cdc.gov/vaccines/ed/hpv/index.html.
92. Malo TL, Gilkey MB, Hall ME, et al. Messages to motivate human papillomavirus vaccination: national studies of parents and physicians. Cancer Epidemiol Biomarkers Prev 2016;25:1383–91.
93. Chapman G. Increasing vaccination without changing beliefs. 2016. Accessed at http://catalyst.nejm.org/increasing-vaccination-without-changing-beliefs/.
94. Rossen I, Hurlstone MJ, Lawrence C. Going with the grain of cognition: applying insights from psychology to build support for childhood vaccination. Front Psychol 2016;7:1483.
95. Shah PD, Gilkey MB, Pepper JK, et al. Promising alternative settings for HPV vaccination of US adolescents. Exp Rev Vaccine 2014;13:235–46.
96. Webb TL, Sheeran P. Does changing behavioral intentions engender behavior change? A meta-analysis of the experimental evidence. Psychol Bull 2006;132:249–68.
97. Janczyk M, Dambacher M, Bieleke M, Gollwitzer PM. The benefit of no choice: goal-directed plans enhance perceptual processing. Psychol Res 2015;79:206–20.
98. Wieber F, Thurmer JL, Gollwitzer PM. Promoting the translation of intentions into action by implementation intentions: behavioral effects and physiological correlates. Front Hum Neurosci 2015;9:395.
99. Chapman J, Armitage CJ, Norman P. Comparing implementation intention interventions in relation to young adults’ intake of fruit and vegetables. Psychol Health 2009;24:317–32.
100. Martin J, Sheeran P, Slade P, et al. Implementation intention formation reduces consultations for emergency contraception and pregnancy testing among teenage women. Health Psychol 2009;28:762–9.
101. Chapman J, Armitage CJ. Do techniques that increase fruit intake also increase vegetable intake? Evidence from a comparison of two implementation intention interventions. Appetite 2012;58:28–33.
102. Armitage CJ, Arden MA. Enhancing the effectiveness of alcohol warning labels with a self-affirming implementation intention. Health Psychol 2016;35:1159–63.
103. Bradbury D, Upsher R, Chilcot J. A pilot randomised test of a self-affirmation implementation intention intervention to reduce dietary salt intake. J Health Psychol 2016 May 22.
104. Chen XJ, Liu LL, Cui JF, et al. The effect and mechanisms of implementation intention in improving prospective memory performance in schizophrenia patients. Psychiatry Res 2016;244:86–93.
105. Hagger MS, Luszczynska A, de Wit J, et al. Implementation intention and planning interventions in health psychology: recommendations from the Synergy Expert Group for research and practice. Psychol Health 2016;31:814–39.
106. Vet R, de Wit JB, Das E. The role of implementation intention formation in promoting hepatitis B vaccination uptake among men who have sex with men. Int J STD AIDS 2014;25:122–9.
107. Milkman KL, Beshears J, Choi JJ, et al. Using implementation intention prompts to enhance influenza vaccination rates. Proc Natl Acad Sci U S A 2011;108:10415–20.
108. McCaul KD, Johnson RJ, Rothman AJ. The effects of framing and action instructions on whether older adults obtain flu shots. Health Psychol 2002;21:624–8.
109. Centers for Disease Control and Prevention. You are the key: health care providers’ tip sheet. 2015. Accessed at www.cdc.gov/vaccines/who/teens/for-hcp-tipsheet-hpv.pdf.
110. American Academy of Pediatrics. HPV vaccine champion toolkit. 2015. Accessed at www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/Pages/HPV-Champion-Toolkit.aspx.
111. Nyhan B, Reifler J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine 2015;33:459–64.
112. Betsch C, Sachse K. Debunking vaccination myths: strong risk negations can increase perceived vaccination risks. Health Psychol 2013;32:146–55.
113. Nyhan B, Reifler J, Richey S, Freed GL. Effective messages in vaccine promotion: a randomized trial. Pediatrics 2014;133:e835–842.
114. Schwartz N, Sanna LJ, Skurnik I, Yoon C. Metacognitive expriences and the intericacies of setting people straight: Implications for debiasing and public information campaigns. Adv Exper Social Psychol 2007;39:127–161.
115. Lord C, Ross L, Lepper MR. Biased assimilation and attitude polarization: the effects of prior theories on subsequently considered evidence. J Personality Soc Pscyhol 1979;37:2098–109.
116. Siegrist M, Cvetkovich G. Better negative than positive? Evidence of a bias for negative information about possible health dangers. Risk Analysis 2001;21:199–206.
117. Witte K, Allen M. A meta-analysis of fear appeals: implications for effective public health campaigns. Health Educ Behav 2000;27:591–615.
118. Gerend MA, Shepherd JE. Using message framing to promote acceptance of the human papillomavirus vaccine. Health Psychol 2007;26:745–52.
119. Ferguson E, Gallagher L. Message framing with respect to decisions about vaccination: the roles of frame valence, frame method and perceived risk. Br J Psychol (London:1953) 2007;98(Pt 4):667–80.
120. Jarrett C, Wilson R, O’Leary M, et al. Strategies for addressing vaccine hesitancy - A systematic review. Vaccine 2015;33:4180–90.
121. Laranjo L, Arguel A, Neves AL, et al. The influence of social networking sites on health behavior change: a systematic review and meta-analysis. J Am Med Inform Assoc 2015;22:243–56.
122. Maher CA, Lewis LK, Ferrar K, et al. Are health behavior change interventions that use online social networks effective? A systematic review. J Med Internet Res 2014;16:e40.
123. Betsch C, Brewer NT, Brocard P, et al. Opportunities and challenges of Web 2.0 for vaccination decisions. Vaccine 2012;30:3727–33.
124. Barrett Values Center. Values versus beliefs. 2016. Accessed 31 Oct 2016 at www.valuescentre.com/mapping-values/values/values-vs-beliefs.
125. Dore RA, Stone ER, Buchanan CM. A social values analysis of parental decision making. J Psychol 2014;148:477–504.
126. Cohen GL, Sherman DK. The psychology of change: self-affirmation and social psychological intervention. Ann Rev Psychol 2014;65:333–71.
127. Sweeney AM, Moyer A. Self-affirmation and responses to health messages: a meta-analysis on intentions and behavior. Health Psychol 2015;34:149–59.
128. Tiro JA, Lee SC, Marks EG, et al. Developing a tablet-based self-persuasion intervention promoting adolescent hpv vaccination: protocol for a three-stage mixed-methods study. JMIR Res Protoc 2016;5:e19.
129. Leask J, Macartney K. Parental decisions about vaccination: collective values are important. J Paediatr Child Health 2008;44:534–5.
130. Salmon DA, Omer SB. Individual freedoms versus collective responsibility: immunization decision-making in the face of occasionally competing values. Emerg Themes Epidemiol 2006;3:13.
131. Gidengil C, Lieu TA, Payne K, et al. Parental and societal values for the risks and benefits of childhood combination vaccines. Vaccine 2012;30:3445–52.
132. Attwell K, Freeman M. I immunise: an evaluation of a values-based campaign to change attitudes and beliefs. Vaccine 2015;33:6235–40.
133. Witteman HO. Addressing vaccine hesitancy with values. Pediatrics 2015;136:215–7.
134. Witteman HO, Chipenda Dansokho S, Exe N, et al. Risk Communication, values clarification, and vaccination decisions. Risk Anal 2015;35:1801–19.
135. Ohannessian R, Yaghobian S, Verger P, Vanhems P. A systematic review of serious video games used for vaccination. Vaccine 2016.
136. National Vaccine Advisory Committee. Assessing the state of vaccine confidence in the United States: recommendations from the National Vaccine Advisory Committee. Pub Health Rep 2015;130:573–95.
137. Dube E, Vivion M, MacDonald NE. Vaccine hesitancy, vaccine refusal and the anti-vaccine movement: influence, impact and implications. Exp Rev Vaccine 2015;14:99–117.
Treatment of Biochemical Recurrence After Prostatectomy: A Step Forward
Study Overview
Objective. To evaluate the impact on overall survival of adding antiandrogen (bicalutamide) therapy to radiation in patients with prostate cancer who have an elevated prostate-specific antigen (PSA) after radical prostatectomy (either as persistence or as a relapse) and no evidence of metastatic disease.
Design. Phase III, randomized, double-blind, placebo-controlled trial.
Setting and participants. The trial was designed by NRG Oncology (Philadelphia, PA), sponsored by the National Cancer Institute, and conducted at NRG Oncology member sites, which included community-based hospitals. Eligible patients had undergone radical prostatectomy and had disease that was originally assessed, on the basis of pathological testing, as tumor stage T2 (confined to the prostate but with a positive surgical margin) or T3 (with histologic extension of tumor beyond the prostatic capsule) without nodal involvement. Patients also had to have a detectable PSA level between 0.2 and 4.0 ng/mL at least 8 weeks after surgery. All the patients underwent abdominal and pelvic computed tomography (CT) and bone scans to rule out metastatic disease. Patients who received prior chemotherapy or radiation therapy for prostate cancer were excluded. Most patients had not received prior hormonal therapy for prostate cancer.
Intervention. All eligible patients received salvage radiation therapy within 12 weeks after randomization. Radiation was directed to the original prostatic site, the tumor resection bed, and the membranous urethra at a total dose of 64.8 Gy given in 36 daily fractions. In addition to the radiation therapy, patients were randomly assigned to receive either 150 mg of bicalutamide or 1 placebo tablet daily, beginning at the initiation of radiation therapy and continuing for 24 months. Tablets were administered in a double blind fashion. Follow-up evaluations occurred every 3 months for 2 years, then every 6 months for 3 years, and then yearly. Bone and CT scans were performed either at biochemical recurrence or as indicated clinically. If metastatic disease was detected on follow up or if the serum PSA level rose to more than 4.0 ng/mL, maximum androgen blockade was recommended.
Main outcome measure. The main outcome was overall survival rate at 12 years. Secondary end points were disease-specific death, distant metastases, local disease progression, non–disease-specific death, any prostate cancer progression including a second biochemical recurrence, and adverse events.
Main results. 840 patients were randomized between March 1998 and March 2003, with 760 patients eligible for evaluation (384 patients in bicalutamide group and 376 in placebo group). Demographic and tumor-related characteristics of the 2 groups were similar. In both groups the majority of patients were white (89.6% in bicalutamide arm; 86.2 in placebo), had Karnofsky performance status score of 100% (77.1 % in bicalutamide arm; 74.5% in placebo), and had positive surgical margin (75% in bicalutamide arm; 74.7% in placebo). Median age was 65 years, and median PSA level at trial entry was 0.6 ng/mL. The median follow-up among the surviving patients was 13 years.
A total of 21 patients in the bicalutamide group and 46 patients in the placebo group died from prostate cancer. The actuarial rate of overall survival at 12 years was 76.3% in the bicalutamide group and 71.3% in the placebo group (hazard ratio [HR] for death 0.77 [95% confidence interval {CI} 0.59 to 0.99; P = 0.04), resulting in a 23% relative reduction in the risk of death in patients who received bicalutamide. The 12-year incidence of death from prostate cancer was 5.8% in the bicalutamide group versus 13.4% in the placebo group (HR 0.49 [95% CI 0.32 to 0.74]; P < 0.001), resulting in a 51% lower rate of death from prostate cancer in bicalutamide patients. Post hoc subgroup analyses showed that the greatest overall survival benefit was seen in subgroups of patients with more aggressive prostate cancer, such as those with high PSA level at trial entry (1.5 to 4.0 ng/mL) or Gleason score of 7. There were too few patients with Gleason scores of 8, 9, or 10 to draw meaningful conclusions about this subgroup. There appeared to be a larger benefit in patients with positive surgical margins than in those with negative surgical margins.
Adherence to radiation therapy was similar between the 2 trial groups, and addition of bicalutamide to radiation therapy did not result in an increase in adverse events associated with radiation therapy, such as cystitis, colitis, or sexual dysfunction. The rates of hot flashes and cardiovascular deaths were not significantly higher in the bicalutamide group than in the placebo group. However, gynecomastia was reported significantly more frequently in the bicalutamide group (69.7%) than in the placebo group (10.9%, P < 0.001).
Conclusion. The addition of 24 months of antiandrogen therapy with daily bicalutamide to salvage radiation therapy resulted in significantly higher rates of long-term overall survival and lower incidence of death from prostate cancer as compared to the addition of placebo. This benefit appeared to be without a significant cost in terms of toxicity.
Commentary
Prostate cancer is the second most common cancer in men worldwide, with an estimated 1,618,000 cases and 366,000 deaths in 2015 [1]. The current lifetime risk of developing prostate cancer for men living in the United States is estimated to be 1 in 6 [2]. Most prostate cancers are diagnosed in the localized stage, which is often treated with radical prostatectomy. All prostate tissue is removed during a successful radical prostatectomy. Postoperatively, detectable serum PSA is indicative of residual prostatic tissue, which presumably represents disease recurrence. This elevation in PSA after surgery in the absence of systemic metastatic disease is termed bio-chemical recurrence. The current standard of care for patients who develop biochemical recurrence is salvage radiation therapy. The prognosis for these patients is related to initial tumor characteristics—grade, volume and local stage. However, approximately 50% of the patients who are treated with salvage radiation therapy for biochemical recurrence will have further disease progression and may ultimately die from prostate cancer [3,4]. This is especially true when aggressive disease features are present. Radiation therapy combined with androgen-deprivation therapy using GnRH agonists or antiandrogen therapy (bicalutamide, flutamide) prolongs survival among some men with an intact prostate. This combination represents a rationale approach to prolong survival among men who develop non-metastatic biochemical relapse after radical prostatectomy.
The study by Shipley and colleagues reports the long-term outcomes of a randomized trial comparing salvage radiation plus 2 years of antiandrogen therapy to salvage radiation and placebo. Starting daily bicalutamide 150 mg orally with salvage radiation and continuing for 2 years was associated with a 23% improvement in overall survival and a 51% lower rate of death from prostate cancer, as compared to the placebo group. The number needed to treat (NNT) with bicalutamide to prevent one death from prostate cancer over 12 years was 20. By comparison, standard treatment of prostate cancer with surgery or radiation has an NNT of 27, which demonstrates the magnitude of the benefit of addition of antiandrogen therapy to salvage radiation. The benefit appears to be greatest in patients with poor prognostic factors such as higher Gleason scores (8 to 10), a higher PSA level at entry (0.7 to 4.0 ng/mL), or positive surgical margins. In contrast, patients with lower Gleason score or negative margins seemed to benefit less from the addition of antiandrogen therapy to salvage radiation. Two years of bicalutamide was not associated with increased incidence of radiation-related toxicities or cardiovascular death. As expected, the primary adverse effect of bicalutamide was gynecomastia, which was seen in 70% of the men treated. This adverse effect can be distressing but can be mitigated by prophylactic radiation of the breast or by the administration of tamoxifen, which were not done as preventative measures in this trial.
While the addition of bicalutamide to radiation did show a clear benefit to overall survival, questions remain about whether bicalutamide is the best drug to use. As the authors note, at present GnRH agonists such as leuprolide are considered first-line hormonal therapy with radiation for most patients with prostate cancer, and bicalutamide at the dose used in this study (150 mg) is not approved. We do not know how GnRH agonists will perform either as a single agent or in combination with antiandrogen for patients who develop biochemical relapse, as the use of GnRH agonists with radiation therapy has not been evaluated in patients who develop biochemical relapse in randomized clinical trials. Two trials exploring the use of androgen deprivation therapy with salvage radiation therapy in patients with biochemical recurrence (Radiotherapy and Androgen Deprivation in Combination After Local Surgery–Hormone Duration [RADICALS-HD] and the Groupe d’Etude des Tumeurs Uro-Génitales [GETUG]-16 trial) have finished enrollment; however, we will have to wait until the overall survival data matures before drawing any meaningful conclusions from them [5,6]. We know that patients with certain aggressive disease features based on tumor stage, grade, and volume are more likely to develop biochemical recurrence. As such, it is logical to consider evaluating the role of androgen deprivation therapy with adjuvant radiation therapy in patients who are at high risk of biochemical relapse with the goal of prolonging survival and reducing the risk of metastases. The RADICALS (Radiation Therapy and Androgen Deprivation Therapy in Treating Patients Who Have Undergone Surgery for Prostate Cancer) trial, which is evaluating the role of androgen deprivation therapy after adjuvant radiation therapy in patients who are at high risk of developing biochemical relapse, will help to address this issue.
Applications for Clinical Practice
Adding an antiandrogen agent (bicalutamide) to salvage radiation therapy in this randomized, double-blind, placebo-controlled trial resulted in higher rates of overall survival, disease-specific survival, and metastasis-free interval than radiation therapy alone for patients who developed biochemical relapse after radical prostatectomy for pathological T2/T3 and node-negative prostate cancer. We eagerly await the results of clinical trials evaluating the role of GnRH agonists in combination with salvage radiation therapy in patients in this setting. Given the long natural history of prostate cancer and the relatively low event rate, such studies can take over a decade to show differences in overall survival. Thus, until such data is available, 24 months of bicalutamide in combination with salvage radiation should be considered the new standard of care for patients (especially those at high risk) who develop non-metastatic biochemical relapse after prostatectomy.
—Devalkumar Rajyaguru, MD, and Lori Rosenstein, MD,
Gundersen Health System, La Crosse, WI
1. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol 2016 Dec 3.
2. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011;61:212–36.
3. Trock BJ, Han M, Freedland SJ, et al. Prostate cancer–specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA 2008;299:2760–9.
4. Stephenson AJ, Scardino PT, Kattan WW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol 2007;25:2035–41.
5. Parker C, Clarke N, Logue J, et al. RADICALS (Radiotherapy and Androgen Deprivation in Combination after Local Surgery). Clin Oncol (R Coll Radiol) 2007;19:167–71.
6. Carrie C, Hasbini A, De Laroche G, et al. Interest of short hormonotherapy associated with radiotherapy as salvage treatment for biological relapse after radical prostatectomy: results of the GETUG-AFU 16 phase III randomized trial. J Clin Oncol 2015;33:Suppl:5006 [abstract].
Study Overview
Objective. To evaluate the impact on overall survival of adding antiandrogen (bicalutamide) therapy to radiation in patients with prostate cancer who have an elevated prostate-specific antigen (PSA) after radical prostatectomy (either as persistence or as a relapse) and no evidence of metastatic disease.
Design. Phase III, randomized, double-blind, placebo-controlled trial.
Setting and participants. The trial was designed by NRG Oncology (Philadelphia, PA), sponsored by the National Cancer Institute, and conducted at NRG Oncology member sites, which included community-based hospitals. Eligible patients had undergone radical prostatectomy and had disease that was originally assessed, on the basis of pathological testing, as tumor stage T2 (confined to the prostate but with a positive surgical margin) or T3 (with histologic extension of tumor beyond the prostatic capsule) without nodal involvement. Patients also had to have a detectable PSA level between 0.2 and 4.0 ng/mL at least 8 weeks after surgery. All the patients underwent abdominal and pelvic computed tomography (CT) and bone scans to rule out metastatic disease. Patients who received prior chemotherapy or radiation therapy for prostate cancer were excluded. Most patients had not received prior hormonal therapy for prostate cancer.
Intervention. All eligible patients received salvage radiation therapy within 12 weeks after randomization. Radiation was directed to the original prostatic site, the tumor resection bed, and the membranous urethra at a total dose of 64.8 Gy given in 36 daily fractions. In addition to the radiation therapy, patients were randomly assigned to receive either 150 mg of bicalutamide or 1 placebo tablet daily, beginning at the initiation of radiation therapy and continuing for 24 months. Tablets were administered in a double blind fashion. Follow-up evaluations occurred every 3 months for 2 years, then every 6 months for 3 years, and then yearly. Bone and CT scans were performed either at biochemical recurrence or as indicated clinically. If metastatic disease was detected on follow up or if the serum PSA level rose to more than 4.0 ng/mL, maximum androgen blockade was recommended.
Main outcome measure. The main outcome was overall survival rate at 12 years. Secondary end points were disease-specific death, distant metastases, local disease progression, non–disease-specific death, any prostate cancer progression including a second biochemical recurrence, and adverse events.
Main results. 840 patients were randomized between March 1998 and March 2003, with 760 patients eligible for evaluation (384 patients in bicalutamide group and 376 in placebo group). Demographic and tumor-related characteristics of the 2 groups were similar. In both groups the majority of patients were white (89.6% in bicalutamide arm; 86.2 in placebo), had Karnofsky performance status score of 100% (77.1 % in bicalutamide arm; 74.5% in placebo), and had positive surgical margin (75% in bicalutamide arm; 74.7% in placebo). Median age was 65 years, and median PSA level at trial entry was 0.6 ng/mL. The median follow-up among the surviving patients was 13 years.
A total of 21 patients in the bicalutamide group and 46 patients in the placebo group died from prostate cancer. The actuarial rate of overall survival at 12 years was 76.3% in the bicalutamide group and 71.3% in the placebo group (hazard ratio [HR] for death 0.77 [95% confidence interval {CI} 0.59 to 0.99; P = 0.04), resulting in a 23% relative reduction in the risk of death in patients who received bicalutamide. The 12-year incidence of death from prostate cancer was 5.8% in the bicalutamide group versus 13.4% in the placebo group (HR 0.49 [95% CI 0.32 to 0.74]; P < 0.001), resulting in a 51% lower rate of death from prostate cancer in bicalutamide patients. Post hoc subgroup analyses showed that the greatest overall survival benefit was seen in subgroups of patients with more aggressive prostate cancer, such as those with high PSA level at trial entry (1.5 to 4.0 ng/mL) or Gleason score of 7. There were too few patients with Gleason scores of 8, 9, or 10 to draw meaningful conclusions about this subgroup. There appeared to be a larger benefit in patients with positive surgical margins than in those with negative surgical margins.
Adherence to radiation therapy was similar between the 2 trial groups, and addition of bicalutamide to radiation therapy did not result in an increase in adverse events associated with radiation therapy, such as cystitis, colitis, or sexual dysfunction. The rates of hot flashes and cardiovascular deaths were not significantly higher in the bicalutamide group than in the placebo group. However, gynecomastia was reported significantly more frequently in the bicalutamide group (69.7%) than in the placebo group (10.9%, P < 0.001).
Conclusion. The addition of 24 months of antiandrogen therapy with daily bicalutamide to salvage radiation therapy resulted in significantly higher rates of long-term overall survival and lower incidence of death from prostate cancer as compared to the addition of placebo. This benefit appeared to be without a significant cost in terms of toxicity.
Commentary
Prostate cancer is the second most common cancer in men worldwide, with an estimated 1,618,000 cases and 366,000 deaths in 2015 [1]. The current lifetime risk of developing prostate cancer for men living in the United States is estimated to be 1 in 6 [2]. Most prostate cancers are diagnosed in the localized stage, which is often treated with radical prostatectomy. All prostate tissue is removed during a successful radical prostatectomy. Postoperatively, detectable serum PSA is indicative of residual prostatic tissue, which presumably represents disease recurrence. This elevation in PSA after surgery in the absence of systemic metastatic disease is termed bio-chemical recurrence. The current standard of care for patients who develop biochemical recurrence is salvage radiation therapy. The prognosis for these patients is related to initial tumor characteristics—grade, volume and local stage. However, approximately 50% of the patients who are treated with salvage radiation therapy for biochemical recurrence will have further disease progression and may ultimately die from prostate cancer [3,4]. This is especially true when aggressive disease features are present. Radiation therapy combined with androgen-deprivation therapy using GnRH agonists or antiandrogen therapy (bicalutamide, flutamide) prolongs survival among some men with an intact prostate. This combination represents a rationale approach to prolong survival among men who develop non-metastatic biochemical relapse after radical prostatectomy.
The study by Shipley and colleagues reports the long-term outcomes of a randomized trial comparing salvage radiation plus 2 years of antiandrogen therapy to salvage radiation and placebo. Starting daily bicalutamide 150 mg orally with salvage radiation and continuing for 2 years was associated with a 23% improvement in overall survival and a 51% lower rate of death from prostate cancer, as compared to the placebo group. The number needed to treat (NNT) with bicalutamide to prevent one death from prostate cancer over 12 years was 20. By comparison, standard treatment of prostate cancer with surgery or radiation has an NNT of 27, which demonstrates the magnitude of the benefit of addition of antiandrogen therapy to salvage radiation. The benefit appears to be greatest in patients with poor prognostic factors such as higher Gleason scores (8 to 10), a higher PSA level at entry (0.7 to 4.0 ng/mL), or positive surgical margins. In contrast, patients with lower Gleason score or negative margins seemed to benefit less from the addition of antiandrogen therapy to salvage radiation. Two years of bicalutamide was not associated with increased incidence of radiation-related toxicities or cardiovascular death. As expected, the primary adverse effect of bicalutamide was gynecomastia, which was seen in 70% of the men treated. This adverse effect can be distressing but can be mitigated by prophylactic radiation of the breast or by the administration of tamoxifen, which were not done as preventative measures in this trial.
While the addition of bicalutamide to radiation did show a clear benefit to overall survival, questions remain about whether bicalutamide is the best drug to use. As the authors note, at present GnRH agonists such as leuprolide are considered first-line hormonal therapy with radiation for most patients with prostate cancer, and bicalutamide at the dose used in this study (150 mg) is not approved. We do not know how GnRH agonists will perform either as a single agent or in combination with antiandrogen for patients who develop biochemical relapse, as the use of GnRH agonists with radiation therapy has not been evaluated in patients who develop biochemical relapse in randomized clinical trials. Two trials exploring the use of androgen deprivation therapy with salvage radiation therapy in patients with biochemical recurrence (Radiotherapy and Androgen Deprivation in Combination After Local Surgery–Hormone Duration [RADICALS-HD] and the Groupe d’Etude des Tumeurs Uro-Génitales [GETUG]-16 trial) have finished enrollment; however, we will have to wait until the overall survival data matures before drawing any meaningful conclusions from them [5,6]. We know that patients with certain aggressive disease features based on tumor stage, grade, and volume are more likely to develop biochemical recurrence. As such, it is logical to consider evaluating the role of androgen deprivation therapy with adjuvant radiation therapy in patients who are at high risk of biochemical relapse with the goal of prolonging survival and reducing the risk of metastases. The RADICALS (Radiation Therapy and Androgen Deprivation Therapy in Treating Patients Who Have Undergone Surgery for Prostate Cancer) trial, which is evaluating the role of androgen deprivation therapy after adjuvant radiation therapy in patients who are at high risk of developing biochemical relapse, will help to address this issue.
Applications for Clinical Practice
Adding an antiandrogen agent (bicalutamide) to salvage radiation therapy in this randomized, double-blind, placebo-controlled trial resulted in higher rates of overall survival, disease-specific survival, and metastasis-free interval than radiation therapy alone for patients who developed biochemical relapse after radical prostatectomy for pathological T2/T3 and node-negative prostate cancer. We eagerly await the results of clinical trials evaluating the role of GnRH agonists in combination with salvage radiation therapy in patients in this setting. Given the long natural history of prostate cancer and the relatively low event rate, such studies can take over a decade to show differences in overall survival. Thus, until such data is available, 24 months of bicalutamide in combination with salvage radiation should be considered the new standard of care for patients (especially those at high risk) who develop non-metastatic biochemical relapse after prostatectomy.
—Devalkumar Rajyaguru, MD, and Lori Rosenstein, MD,
Gundersen Health System, La Crosse, WI
Study Overview
Objective. To evaluate the impact on overall survival of adding antiandrogen (bicalutamide) therapy to radiation in patients with prostate cancer who have an elevated prostate-specific antigen (PSA) after radical prostatectomy (either as persistence or as a relapse) and no evidence of metastatic disease.
Design. Phase III, randomized, double-blind, placebo-controlled trial.
Setting and participants. The trial was designed by NRG Oncology (Philadelphia, PA), sponsored by the National Cancer Institute, and conducted at NRG Oncology member sites, which included community-based hospitals. Eligible patients had undergone radical prostatectomy and had disease that was originally assessed, on the basis of pathological testing, as tumor stage T2 (confined to the prostate but with a positive surgical margin) or T3 (with histologic extension of tumor beyond the prostatic capsule) without nodal involvement. Patients also had to have a detectable PSA level between 0.2 and 4.0 ng/mL at least 8 weeks after surgery. All the patients underwent abdominal and pelvic computed tomography (CT) and bone scans to rule out metastatic disease. Patients who received prior chemotherapy or radiation therapy for prostate cancer were excluded. Most patients had not received prior hormonal therapy for prostate cancer.
Intervention. All eligible patients received salvage radiation therapy within 12 weeks after randomization. Radiation was directed to the original prostatic site, the tumor resection bed, and the membranous urethra at a total dose of 64.8 Gy given in 36 daily fractions. In addition to the radiation therapy, patients were randomly assigned to receive either 150 mg of bicalutamide or 1 placebo tablet daily, beginning at the initiation of radiation therapy and continuing for 24 months. Tablets were administered in a double blind fashion. Follow-up evaluations occurred every 3 months for 2 years, then every 6 months for 3 years, and then yearly. Bone and CT scans were performed either at biochemical recurrence or as indicated clinically. If metastatic disease was detected on follow up or if the serum PSA level rose to more than 4.0 ng/mL, maximum androgen blockade was recommended.
Main outcome measure. The main outcome was overall survival rate at 12 years. Secondary end points were disease-specific death, distant metastases, local disease progression, non–disease-specific death, any prostate cancer progression including a second biochemical recurrence, and adverse events.
Main results. 840 patients were randomized between March 1998 and March 2003, with 760 patients eligible for evaluation (384 patients in bicalutamide group and 376 in placebo group). Demographic and tumor-related characteristics of the 2 groups were similar. In both groups the majority of patients were white (89.6% in bicalutamide arm; 86.2 in placebo), had Karnofsky performance status score of 100% (77.1 % in bicalutamide arm; 74.5% in placebo), and had positive surgical margin (75% in bicalutamide arm; 74.7% in placebo). Median age was 65 years, and median PSA level at trial entry was 0.6 ng/mL. The median follow-up among the surviving patients was 13 years.
A total of 21 patients in the bicalutamide group and 46 patients in the placebo group died from prostate cancer. The actuarial rate of overall survival at 12 years was 76.3% in the bicalutamide group and 71.3% in the placebo group (hazard ratio [HR] for death 0.77 [95% confidence interval {CI} 0.59 to 0.99; P = 0.04), resulting in a 23% relative reduction in the risk of death in patients who received bicalutamide. The 12-year incidence of death from prostate cancer was 5.8% in the bicalutamide group versus 13.4% in the placebo group (HR 0.49 [95% CI 0.32 to 0.74]; P < 0.001), resulting in a 51% lower rate of death from prostate cancer in bicalutamide patients. Post hoc subgroup analyses showed that the greatest overall survival benefit was seen in subgroups of patients with more aggressive prostate cancer, such as those with high PSA level at trial entry (1.5 to 4.0 ng/mL) or Gleason score of 7. There were too few patients with Gleason scores of 8, 9, or 10 to draw meaningful conclusions about this subgroup. There appeared to be a larger benefit in patients with positive surgical margins than in those with negative surgical margins.
Adherence to radiation therapy was similar between the 2 trial groups, and addition of bicalutamide to radiation therapy did not result in an increase in adverse events associated with radiation therapy, such as cystitis, colitis, or sexual dysfunction. The rates of hot flashes and cardiovascular deaths were not significantly higher in the bicalutamide group than in the placebo group. However, gynecomastia was reported significantly more frequently in the bicalutamide group (69.7%) than in the placebo group (10.9%, P < 0.001).
Conclusion. The addition of 24 months of antiandrogen therapy with daily bicalutamide to salvage radiation therapy resulted in significantly higher rates of long-term overall survival and lower incidence of death from prostate cancer as compared to the addition of placebo. This benefit appeared to be without a significant cost in terms of toxicity.
Commentary
Prostate cancer is the second most common cancer in men worldwide, with an estimated 1,618,000 cases and 366,000 deaths in 2015 [1]. The current lifetime risk of developing prostate cancer for men living in the United States is estimated to be 1 in 6 [2]. Most prostate cancers are diagnosed in the localized stage, which is often treated with radical prostatectomy. All prostate tissue is removed during a successful radical prostatectomy. Postoperatively, detectable serum PSA is indicative of residual prostatic tissue, which presumably represents disease recurrence. This elevation in PSA after surgery in the absence of systemic metastatic disease is termed bio-chemical recurrence. The current standard of care for patients who develop biochemical recurrence is salvage radiation therapy. The prognosis for these patients is related to initial tumor characteristics—grade, volume and local stage. However, approximately 50% of the patients who are treated with salvage radiation therapy for biochemical recurrence will have further disease progression and may ultimately die from prostate cancer [3,4]. This is especially true when aggressive disease features are present. Radiation therapy combined with androgen-deprivation therapy using GnRH agonists or antiandrogen therapy (bicalutamide, flutamide) prolongs survival among some men with an intact prostate. This combination represents a rationale approach to prolong survival among men who develop non-metastatic biochemical relapse after radical prostatectomy.
The study by Shipley and colleagues reports the long-term outcomes of a randomized trial comparing salvage radiation plus 2 years of antiandrogen therapy to salvage radiation and placebo. Starting daily bicalutamide 150 mg orally with salvage radiation and continuing for 2 years was associated with a 23% improvement in overall survival and a 51% lower rate of death from prostate cancer, as compared to the placebo group. The number needed to treat (NNT) with bicalutamide to prevent one death from prostate cancer over 12 years was 20. By comparison, standard treatment of prostate cancer with surgery or radiation has an NNT of 27, which demonstrates the magnitude of the benefit of addition of antiandrogen therapy to salvage radiation. The benefit appears to be greatest in patients with poor prognostic factors such as higher Gleason scores (8 to 10), a higher PSA level at entry (0.7 to 4.0 ng/mL), or positive surgical margins. In contrast, patients with lower Gleason score or negative margins seemed to benefit less from the addition of antiandrogen therapy to salvage radiation. Two years of bicalutamide was not associated with increased incidence of radiation-related toxicities or cardiovascular death. As expected, the primary adverse effect of bicalutamide was gynecomastia, which was seen in 70% of the men treated. This adverse effect can be distressing but can be mitigated by prophylactic radiation of the breast or by the administration of tamoxifen, which were not done as preventative measures in this trial.
While the addition of bicalutamide to radiation did show a clear benefit to overall survival, questions remain about whether bicalutamide is the best drug to use. As the authors note, at present GnRH agonists such as leuprolide are considered first-line hormonal therapy with radiation for most patients with prostate cancer, and bicalutamide at the dose used in this study (150 mg) is not approved. We do not know how GnRH agonists will perform either as a single agent or in combination with antiandrogen for patients who develop biochemical relapse, as the use of GnRH agonists with radiation therapy has not been evaluated in patients who develop biochemical relapse in randomized clinical trials. Two trials exploring the use of androgen deprivation therapy with salvage radiation therapy in patients with biochemical recurrence (Radiotherapy and Androgen Deprivation in Combination After Local Surgery–Hormone Duration [RADICALS-HD] and the Groupe d’Etude des Tumeurs Uro-Génitales [GETUG]-16 trial) have finished enrollment; however, we will have to wait until the overall survival data matures before drawing any meaningful conclusions from them [5,6]. We know that patients with certain aggressive disease features based on tumor stage, grade, and volume are more likely to develop biochemical recurrence. As such, it is logical to consider evaluating the role of androgen deprivation therapy with adjuvant radiation therapy in patients who are at high risk of biochemical relapse with the goal of prolonging survival and reducing the risk of metastases. The RADICALS (Radiation Therapy and Androgen Deprivation Therapy in Treating Patients Who Have Undergone Surgery for Prostate Cancer) trial, which is evaluating the role of androgen deprivation therapy after adjuvant radiation therapy in patients who are at high risk of developing biochemical relapse, will help to address this issue.
Applications for Clinical Practice
Adding an antiandrogen agent (bicalutamide) to salvage radiation therapy in this randomized, double-blind, placebo-controlled trial resulted in higher rates of overall survival, disease-specific survival, and metastasis-free interval than radiation therapy alone for patients who developed biochemical relapse after radical prostatectomy for pathological T2/T3 and node-negative prostate cancer. We eagerly await the results of clinical trials evaluating the role of GnRH agonists in combination with salvage radiation therapy in patients in this setting. Given the long natural history of prostate cancer and the relatively low event rate, such studies can take over a decade to show differences in overall survival. Thus, until such data is available, 24 months of bicalutamide in combination with salvage radiation should be considered the new standard of care for patients (especially those at high risk) who develop non-metastatic biochemical relapse after prostatectomy.
—Devalkumar Rajyaguru, MD, and Lori Rosenstein, MD,
Gundersen Health System, La Crosse, WI
1. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol 2016 Dec 3.
2. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011;61:212–36.
3. Trock BJ, Han M, Freedland SJ, et al. Prostate cancer–specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA 2008;299:2760–9.
4. Stephenson AJ, Scardino PT, Kattan WW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol 2007;25:2035–41.
5. Parker C, Clarke N, Logue J, et al. RADICALS (Radiotherapy and Androgen Deprivation in Combination after Local Surgery). Clin Oncol (R Coll Radiol) 2007;19:167–71.
6. Carrie C, Hasbini A, De Laroche G, et al. Interest of short hormonotherapy associated with radiotherapy as salvage treatment for biological relapse after radical prostatectomy: results of the GETUG-AFU 16 phase III randomized trial. J Clin Oncol 2015;33:Suppl:5006 [abstract].
1. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol 2016 Dec 3.
2. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011;61:212–36.
3. Trock BJ, Han M, Freedland SJ, et al. Prostate cancer–specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA 2008;299:2760–9.
4. Stephenson AJ, Scardino PT, Kattan WW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol 2007;25:2035–41.
5. Parker C, Clarke N, Logue J, et al. RADICALS (Radiotherapy and Androgen Deprivation in Combination after Local Surgery). Clin Oncol (R Coll Radiol) 2007;19:167–71.
6. Carrie C, Hasbini A, De Laroche G, et al. Interest of short hormonotherapy associated with radiotherapy as salvage treatment for biological relapse after radical prostatectomy: results of the GETUG-AFU 16 phase III randomized trial. J Clin Oncol 2015;33:Suppl:5006 [abstract].