User login
Amitriptyline use nearly doubles symptom improvement in IBS
GLASGOW – in what the researchers call the largest randomized controlled trial (RCT) of a tricyclic antidepressant in the condition.
Patients who took low-dose amitriptyline were almost twice as likely to report an overall improvement in symptoms as those taking placebo, according to investigators of the Amitriptyline at Low-Dose and Titrated for Irritable Bowel Syndrome as Second-Line Treatment (ATLANTIS) trial. Low-dose amitriptyline appeared safe and well tolerated, they reported.
Hazel Everitt, PhD, professor of primary care research at the University of Southampton, England, presented the findings at the annual conference of the Royal College of General Practitioners.
The data were also published in The Lancet and were presented at the recent United European Gastroenterology Week 2023.
Clinicians “should offer low-dose amitriptyline to patients with IBS whose symptoms do not improve with first-line therapies, with appropriate support to guide patient-led dose titration,” the researchers wrote in the journal article.
Despite first-line treatments such as diet, fiber, and antispasmodics, many patients with IBS continue to have troublesome symptoms, Dr. Everitt said in an interview. “GPs haven’t often prescribed amitriptyline for IBS – probably because of the lack of research evidence for its use in primary care.”
Dr. Everitt added that primary care physicians and patients interviewed for the study welcomed low-dose amitriptyline as a potential additional option, especially with increased patient-led care. “The dose titration document that was developed with patients specifically for the trial enables patients to be more empowered to manage their IBS by helping them to titrate their dose up or down depending on their symptoms and side effects.”
Judith Danby, MBBS, a retired GP who moderated the session at which the ATLANTIS results were presented, said, “Self-titration of the dose equals patient empowerment, and if patients can be helped to manage their own medication, then they will also be more empowered to think about lifestyle change, too.”
RCT across 55 practices
The U.K.’s National Institute for Health and Care Excellence guidance for the management of IBS in primary care says clinicians should “consider” using low-dose tricyclic antidepressants as a second-line treatment but highlight the need for an RCT of these drugs carried out solely in primary care.
The ATLANTIS trial was conducted across 55 general practices in England and included adults with Rome IV IBS of any subtype and ongoing symptoms (IBS Severity Scoring System [IBS-SSS] score ≥ 75 points) despite dietary changes and first-line therapies. Participants had normal full blood counts and C-reactive protein measures, negative celiac serology, and no evidence of suicidal ideation. The mean age was 48.5 years, and 68% were female. The mean IBS-SSS score in all participants was 272.8 at baseline.
Patients were randomly assigned in a 1:1 ratio to receive either low-dose oral amitriptyline (10 mg once daily; n = 232) with dose titration over 3 weeks (up to a maximum dose of 30 mg once daily) as determined by a participant’s symptoms and tolerability; or placebo (n = 231). Both groups participated for 6 months. The primary outcome was the IBS-SSS score at 6 months.
Amitriptyline
Three-quarters of participants adhered to the therapy over the 6 months, which was particularly notable given that the trial was conducted during the COVID-19 pandemic, according to the researchers.
An intent-to-treat analysis found that at 6 months, amitriptyline was superior to placebo, with a significant mean difference in IBS-SSS score between groups of –27.0 (95% confidence interval, –46.9 to –7.1; P = .0079; mean IBS-SSS, 170.4 vs. 200.1 with amitriptyline and placebo). A secondary outcome showed an increased likelihood of relief of IBS symptoms by subjective global assessment (odds ratio, 1.78; 95% CI. 1.19-2.66; P = .0050).
At 3 months, the difference in mean change in IBS-SSS score between groups was also significant, at –23.3 (95% CI, –42.0 to –4.6; P = .014), the researchers reported.
People who took the drug were 70% more likely to report relief of symptoms on SGA than those who took placebo (P = .08), according to the researchers.
The researchers reported no effect of low-dose amitriptyline on psychiatric symptoms, such as distressing thoughts, anxiety, and depression, during the 6-month follow-up, nor was there any effect on ability to work or go about social activities.
“This was a pragmatic trial performed in a large number of participants with IBS, with an average duration of symptoms of 10 years and with 80% having moderate to severe symptoms at baseline,” Alexander Ford, MD, professor of gastroenterology at the University of Leeds, England, and a coinvestigator on the study, told this news organization. “The fact that amitriptyline showed such a strong effect over placebo in this group of patients, with a mean decrease in IBS-SSS of almost 100 points at both 3 and 6 months, is therefore all the more impressive.”
Mild adverse events, such as dry mouth and drowsiness, were more frequent with low-dose amitriptyline than with placebo,
Dr. Everitt said the ATLANTIS findings could change practice. Previous trials of low-dose amitriptyline for IBS had mostly been small and were conducted in secondary care settings such as gastroenterology clinics with relatively short follow-up times.
“This is a problem for a long-term condition that fluctuates over time and is diagnosed and managed mostly in primary care,” she said. “The ATLANTIS trial is the largest trial of low-dose amitriptyline for IBS undertaken worldwide and was rigorously conducted with 6 months follow-up, providing reliable results that can help inform GPs and patients’ treatment decision-making in usual clinical practice.”
On a pragmatic level, the research group developed a dose titration document for use by patients and GPs. “Both the GPs and participants found the ATLANTIS dose titration document acceptable and helpful,” Dr. Everitt pointed out. She noted, “We’ve made the dose titration document freely available to support patients and clinicians to try low-dose amitriptyline for IBS.”
Dr. Ford and Dr. Everitt received grant funding (institutional) from the National Institute for Health and Care Research.
A version of this article first appeared on Medscape.com.
GLASGOW – in what the researchers call the largest randomized controlled trial (RCT) of a tricyclic antidepressant in the condition.
Patients who took low-dose amitriptyline were almost twice as likely to report an overall improvement in symptoms as those taking placebo, according to investigators of the Amitriptyline at Low-Dose and Titrated for Irritable Bowel Syndrome as Second-Line Treatment (ATLANTIS) trial. Low-dose amitriptyline appeared safe and well tolerated, they reported.
Hazel Everitt, PhD, professor of primary care research at the University of Southampton, England, presented the findings at the annual conference of the Royal College of General Practitioners.
The data were also published in The Lancet and were presented at the recent United European Gastroenterology Week 2023.
Clinicians “should offer low-dose amitriptyline to patients with IBS whose symptoms do not improve with first-line therapies, with appropriate support to guide patient-led dose titration,” the researchers wrote in the journal article.
Despite first-line treatments such as diet, fiber, and antispasmodics, many patients with IBS continue to have troublesome symptoms, Dr. Everitt said in an interview. “GPs haven’t often prescribed amitriptyline for IBS – probably because of the lack of research evidence for its use in primary care.”
Dr. Everitt added that primary care physicians and patients interviewed for the study welcomed low-dose amitriptyline as a potential additional option, especially with increased patient-led care. “The dose titration document that was developed with patients specifically for the trial enables patients to be more empowered to manage their IBS by helping them to titrate their dose up or down depending on their symptoms and side effects.”
Judith Danby, MBBS, a retired GP who moderated the session at which the ATLANTIS results were presented, said, “Self-titration of the dose equals patient empowerment, and if patients can be helped to manage their own medication, then they will also be more empowered to think about lifestyle change, too.”
RCT across 55 practices
The U.K.’s National Institute for Health and Care Excellence guidance for the management of IBS in primary care says clinicians should “consider” using low-dose tricyclic antidepressants as a second-line treatment but highlight the need for an RCT of these drugs carried out solely in primary care.
The ATLANTIS trial was conducted across 55 general practices in England and included adults with Rome IV IBS of any subtype and ongoing symptoms (IBS Severity Scoring System [IBS-SSS] score ≥ 75 points) despite dietary changes and first-line therapies. Participants had normal full blood counts and C-reactive protein measures, negative celiac serology, and no evidence of suicidal ideation. The mean age was 48.5 years, and 68% were female. The mean IBS-SSS score in all participants was 272.8 at baseline.
Patients were randomly assigned in a 1:1 ratio to receive either low-dose oral amitriptyline (10 mg once daily; n = 232) with dose titration over 3 weeks (up to a maximum dose of 30 mg once daily) as determined by a participant’s symptoms and tolerability; or placebo (n = 231). Both groups participated for 6 months. The primary outcome was the IBS-SSS score at 6 months.
Amitriptyline
Three-quarters of participants adhered to the therapy over the 6 months, which was particularly notable given that the trial was conducted during the COVID-19 pandemic, according to the researchers.
An intent-to-treat analysis found that at 6 months, amitriptyline was superior to placebo, with a significant mean difference in IBS-SSS score between groups of –27.0 (95% confidence interval, –46.9 to –7.1; P = .0079; mean IBS-SSS, 170.4 vs. 200.1 with amitriptyline and placebo). A secondary outcome showed an increased likelihood of relief of IBS symptoms by subjective global assessment (odds ratio, 1.78; 95% CI. 1.19-2.66; P = .0050).
At 3 months, the difference in mean change in IBS-SSS score between groups was also significant, at –23.3 (95% CI, –42.0 to –4.6; P = .014), the researchers reported.
People who took the drug were 70% more likely to report relief of symptoms on SGA than those who took placebo (P = .08), according to the researchers.
The researchers reported no effect of low-dose amitriptyline on psychiatric symptoms, such as distressing thoughts, anxiety, and depression, during the 6-month follow-up, nor was there any effect on ability to work or go about social activities.
“This was a pragmatic trial performed in a large number of participants with IBS, with an average duration of symptoms of 10 years and with 80% having moderate to severe symptoms at baseline,” Alexander Ford, MD, professor of gastroenterology at the University of Leeds, England, and a coinvestigator on the study, told this news organization. “The fact that amitriptyline showed such a strong effect over placebo in this group of patients, with a mean decrease in IBS-SSS of almost 100 points at both 3 and 6 months, is therefore all the more impressive.”
Mild adverse events, such as dry mouth and drowsiness, were more frequent with low-dose amitriptyline than with placebo,
Dr. Everitt said the ATLANTIS findings could change practice. Previous trials of low-dose amitriptyline for IBS had mostly been small and were conducted in secondary care settings such as gastroenterology clinics with relatively short follow-up times.
“This is a problem for a long-term condition that fluctuates over time and is diagnosed and managed mostly in primary care,” she said. “The ATLANTIS trial is the largest trial of low-dose amitriptyline for IBS undertaken worldwide and was rigorously conducted with 6 months follow-up, providing reliable results that can help inform GPs and patients’ treatment decision-making in usual clinical practice.”
On a pragmatic level, the research group developed a dose titration document for use by patients and GPs. “Both the GPs and participants found the ATLANTIS dose titration document acceptable and helpful,” Dr. Everitt pointed out. She noted, “We’ve made the dose titration document freely available to support patients and clinicians to try low-dose amitriptyline for IBS.”
Dr. Ford and Dr. Everitt received grant funding (institutional) from the National Institute for Health and Care Research.
A version of this article first appeared on Medscape.com.
GLASGOW – in what the researchers call the largest randomized controlled trial (RCT) of a tricyclic antidepressant in the condition.
Patients who took low-dose amitriptyline were almost twice as likely to report an overall improvement in symptoms as those taking placebo, according to investigators of the Amitriptyline at Low-Dose and Titrated for Irritable Bowel Syndrome as Second-Line Treatment (ATLANTIS) trial. Low-dose amitriptyline appeared safe and well tolerated, they reported.
Hazel Everitt, PhD, professor of primary care research at the University of Southampton, England, presented the findings at the annual conference of the Royal College of General Practitioners.
The data were also published in The Lancet and were presented at the recent United European Gastroenterology Week 2023.
Clinicians “should offer low-dose amitriptyline to patients with IBS whose symptoms do not improve with first-line therapies, with appropriate support to guide patient-led dose titration,” the researchers wrote in the journal article.
Despite first-line treatments such as diet, fiber, and antispasmodics, many patients with IBS continue to have troublesome symptoms, Dr. Everitt said in an interview. “GPs haven’t often prescribed amitriptyline for IBS – probably because of the lack of research evidence for its use in primary care.”
Dr. Everitt added that primary care physicians and patients interviewed for the study welcomed low-dose amitriptyline as a potential additional option, especially with increased patient-led care. “The dose titration document that was developed with patients specifically for the trial enables patients to be more empowered to manage their IBS by helping them to titrate their dose up or down depending on their symptoms and side effects.”
Judith Danby, MBBS, a retired GP who moderated the session at which the ATLANTIS results were presented, said, “Self-titration of the dose equals patient empowerment, and if patients can be helped to manage their own medication, then they will also be more empowered to think about lifestyle change, too.”
RCT across 55 practices
The U.K.’s National Institute for Health and Care Excellence guidance for the management of IBS in primary care says clinicians should “consider” using low-dose tricyclic antidepressants as a second-line treatment but highlight the need for an RCT of these drugs carried out solely in primary care.
The ATLANTIS trial was conducted across 55 general practices in England and included adults with Rome IV IBS of any subtype and ongoing symptoms (IBS Severity Scoring System [IBS-SSS] score ≥ 75 points) despite dietary changes and first-line therapies. Participants had normal full blood counts and C-reactive protein measures, negative celiac serology, and no evidence of suicidal ideation. The mean age was 48.5 years, and 68% were female. The mean IBS-SSS score in all participants was 272.8 at baseline.
Patients were randomly assigned in a 1:1 ratio to receive either low-dose oral amitriptyline (10 mg once daily; n = 232) with dose titration over 3 weeks (up to a maximum dose of 30 mg once daily) as determined by a participant’s symptoms and tolerability; or placebo (n = 231). Both groups participated for 6 months. The primary outcome was the IBS-SSS score at 6 months.
Amitriptyline
Three-quarters of participants adhered to the therapy over the 6 months, which was particularly notable given that the trial was conducted during the COVID-19 pandemic, according to the researchers.
An intent-to-treat analysis found that at 6 months, amitriptyline was superior to placebo, with a significant mean difference in IBS-SSS score between groups of –27.0 (95% confidence interval, –46.9 to –7.1; P = .0079; mean IBS-SSS, 170.4 vs. 200.1 with amitriptyline and placebo). A secondary outcome showed an increased likelihood of relief of IBS symptoms by subjective global assessment (odds ratio, 1.78; 95% CI. 1.19-2.66; P = .0050).
At 3 months, the difference in mean change in IBS-SSS score between groups was also significant, at –23.3 (95% CI, –42.0 to –4.6; P = .014), the researchers reported.
People who took the drug were 70% more likely to report relief of symptoms on SGA than those who took placebo (P = .08), according to the researchers.
The researchers reported no effect of low-dose amitriptyline on psychiatric symptoms, such as distressing thoughts, anxiety, and depression, during the 6-month follow-up, nor was there any effect on ability to work or go about social activities.
“This was a pragmatic trial performed in a large number of participants with IBS, with an average duration of symptoms of 10 years and with 80% having moderate to severe symptoms at baseline,” Alexander Ford, MD, professor of gastroenterology at the University of Leeds, England, and a coinvestigator on the study, told this news organization. “The fact that amitriptyline showed such a strong effect over placebo in this group of patients, with a mean decrease in IBS-SSS of almost 100 points at both 3 and 6 months, is therefore all the more impressive.”
Mild adverse events, such as dry mouth and drowsiness, were more frequent with low-dose amitriptyline than with placebo,
Dr. Everitt said the ATLANTIS findings could change practice. Previous trials of low-dose amitriptyline for IBS had mostly been small and were conducted in secondary care settings such as gastroenterology clinics with relatively short follow-up times.
“This is a problem for a long-term condition that fluctuates over time and is diagnosed and managed mostly in primary care,” she said. “The ATLANTIS trial is the largest trial of low-dose amitriptyline for IBS undertaken worldwide and was rigorously conducted with 6 months follow-up, providing reliable results that can help inform GPs and patients’ treatment decision-making in usual clinical practice.”
On a pragmatic level, the research group developed a dose titration document for use by patients and GPs. “Both the GPs and participants found the ATLANTIS dose titration document acceptable and helpful,” Dr. Everitt pointed out. She noted, “We’ve made the dose titration document freely available to support patients and clinicians to try low-dose amitriptyline for IBS.”
Dr. Ford and Dr. Everitt received grant funding (institutional) from the National Institute for Health and Care Research.
A version of this article first appeared on Medscape.com.
AT RCGP 2023
Novel PET tracer for perfusion imaging: What’s the potential?
The emerging advantages of PET myocardial perfusion imaging (MPI) for coronary artery disease (CAD) diagnosis and assessment of cardiovascular event risk has prompted growing use of this technology as an alternative to the more commonly used single photon–emission CT (SPECT) MPI.
The advantages of PET MPI include better diagnostic performance and shorter acquisition times. , including consistent, high-quality images and low radiation exposure. It also allows quantification of myocardial blood flow, and it has “strong prognostic power.”
Tracer availability
Despite these advantages, that position paper and subsequent studies note that PET MPI has been underutilized in the United States, largely owing to issues with the available tracers, which have characteristics that limit widespread use in the clinic.
Rubidium, arguably the most commonly used tracer for PET MPI, is not available in unit dosing and so can be expensive for low-volume centers, plus it also requires an on-site generator, Michael Salerno, MD, PhD, a member of the American College of Cardiology’s Imaging Council and section chief of cardiovascular imaging, Stanford (Calif.) University, told this news organization.
N-ammonia, the other U.S. Food and Drug Administration–approved tracer, is available in unit dosing, but its short half-life means that centers need an onsite cyclotron, Dr. Salerno said.
For cardiac perfusion imaging and myocardial blood flow (MBF) quantification, 15O-water is considered the gold standard, although it’s not approved by the FDA. This tracer also requires an on-site cyclotron and “is challenging to use,” Dr. Salerno said. Use has been largely restricted to research purposes, though efforts are underway to widen its availability.
Enter flurpiridaz F-18 (GE Healthcare), a novel PET MPI tracer labeled with fluorine-18. Its longer half-life – similar to that of fluorodeoxyglucose, a tracer used to detect various cancers – could broaden the number of sites that could perform perfusion PET studies, Dr. Salerno said.
“Flurpiridaz also is supposed to have a more linear relationship between flow and tracer uptake, which could improve the ability to perform quantification of perfusion,” he noted. “It also offers the ability to do exercise PET, which is impossible for rubidium and challenging for ammonia, given its 11-minute half-life.”
Flurpiridaz status
The FDA requires two phase-3 studies that show safety and sufficient diagnostic performance before it will approve a new tracer. The first required study, published in the Journal of the American College of Cardiology, showed that the tracer’s sensitivity for detection of greater than or equal to 50% stenosis by ICA was significantly higher than SPECT; however, the specificity did not meet the prespecified noninferiority criterion.
The second FDA-required study, published online recently, also in the Journal of the American College of Cardiology, was designed differently from the first in that only patients with suspected – not known – CAD were enrolled. The primary efficacy endpoint was sensitivity and specificity of flurpiridaz PET for overall detection of CAD, rather than comparing it to SPECT MPI (which became a secondary endpoint). PET and SPECT studies were both performed before invasive coronary angiography to minimize referral bias; SPECT studies included cadmium zinc telluride cameras.
In that study, which included 578 patients (mean age, 64; 32.5% women) from 48 centers in the United States, Canada, and Europe, flurpiridaz met the efficacy endpoints: Its sensitivity and specificity were significantly higher than the prespecified threshold value by two of the three readers; its sensitivity was higher than SPECT (80.3% vs. 68.7%); and its specificity was noninferior (63.8% vs. 61.7%).
PET areas under the receiver-operating characteristic curves were higher than SPECT in the overall population and in women and obese patients, at half the radiation dose of SPECT.
“Cardiac PET MPI is positioned to serve as the leading modality for the functional evaluation of suspected and known CAD,” Jamieson M. Bourque, MD, MHS, medical director of nuclear cardiology, echocardiography, and the Stress Laboratory, University of Virginia, Charlottesville, wrote in an editorial accompanying the second study . “18F-flurpiridaz will facilitate this upward progression with beneficial tracer characteristics that will increase access and availability, enable exercise stress, and optimize MBF quantification.”
At this point, FDA approval of flurpiridaz is expected sometime in 2024, said James E. Udelson, MD, principal investigator of the recent study, chief of the division of cardiology, and director of the Nuclear Cardiology Laboratory at Tufts University School of Medicine, Boston.
Learning curve
Flurpiridaz comes with “a really interesting and important” learning curve, Dr. Udelson said. “The images are really crisp, and they look very different from what most people are used to. The GE folks are going to have to make sure that the American Society of Nuclear Cardiology and other professional societies are tuned in to help in the education part, because it’s not an easy, automatic switch. Very good image readers can adapt, but it’s not just one day you do one, then switch to the other.”
A “somewhat apt” analogy would be the difference between an echocardiogram and an MRI, he explained. “The MRI is much crisper. You’re seeing edges more crisply. You’re seeing the difference between a thicker and a thinner segment of the wall more crisply, and that’s actually real. You can’t say the thinner segment is abnormal; it’s just that you’re seeing it better. So, with this tracer, normal differences in the thickness of a wall can almost look like a defect if you’re not used to knowing that’s the new normal.”
The expected approval of flurpiridaz “will be a win for cardiac PET, broadening the range of sites that could perform PET,” Dr. Salerno commented. “However, it is worth cautioning that all of the prior data with PET using different agents does not necessarily equate to the same performance with the new agent, given that the performance seems to be lower than that shown in prior PET studies using other agents.”
Dr. Salerno would like to see additional studies comparing flurpiridaz with rubidium or ammonia, as well as studies performing quantification with flurpiridaz, “which theoretically should have some advantages,” he said.
Dr. Udelson noted that MedTrace, a company in Denmark, is working on a radiolabeled water tracer based on 15-O-water that is just starting a pivotal trial. Dr. Udelson is a consultant to the company and is a steering committee member for the pivotal trial.
For now, “the big take-home is that there are a lot of ways these days to test people for CAD,” he said. “As the types of things we can do to test people expand, individuals and centers need to make sure they focus on providing any new service, however they do it, with really superb quality and experience.”
“You don’t just do something new because it’s new,” he added. “It has to be done really well. If you do the new thing badly, you’re not going to get better information.”
Dr. Udelson is a consultant and advisory board member for GE Healthcare, a consultant to MedTrace, and a steering committee member for MedTrace’s pivotal trial. Dr. Bourque has served on a GE Healthcare advisory board for amyloid imaging. Dr. Salerno reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The emerging advantages of PET myocardial perfusion imaging (MPI) for coronary artery disease (CAD) diagnosis and assessment of cardiovascular event risk has prompted growing use of this technology as an alternative to the more commonly used single photon–emission CT (SPECT) MPI.
The advantages of PET MPI include better diagnostic performance and shorter acquisition times. , including consistent, high-quality images and low radiation exposure. It also allows quantification of myocardial blood flow, and it has “strong prognostic power.”
Tracer availability
Despite these advantages, that position paper and subsequent studies note that PET MPI has been underutilized in the United States, largely owing to issues with the available tracers, which have characteristics that limit widespread use in the clinic.
Rubidium, arguably the most commonly used tracer for PET MPI, is not available in unit dosing and so can be expensive for low-volume centers, plus it also requires an on-site generator, Michael Salerno, MD, PhD, a member of the American College of Cardiology’s Imaging Council and section chief of cardiovascular imaging, Stanford (Calif.) University, told this news organization.
N-ammonia, the other U.S. Food and Drug Administration–approved tracer, is available in unit dosing, but its short half-life means that centers need an onsite cyclotron, Dr. Salerno said.
For cardiac perfusion imaging and myocardial blood flow (MBF) quantification, 15O-water is considered the gold standard, although it’s not approved by the FDA. This tracer also requires an on-site cyclotron and “is challenging to use,” Dr. Salerno said. Use has been largely restricted to research purposes, though efforts are underway to widen its availability.
Enter flurpiridaz F-18 (GE Healthcare), a novel PET MPI tracer labeled with fluorine-18. Its longer half-life – similar to that of fluorodeoxyglucose, a tracer used to detect various cancers – could broaden the number of sites that could perform perfusion PET studies, Dr. Salerno said.
“Flurpiridaz also is supposed to have a more linear relationship between flow and tracer uptake, which could improve the ability to perform quantification of perfusion,” he noted. “It also offers the ability to do exercise PET, which is impossible for rubidium and challenging for ammonia, given its 11-minute half-life.”
Flurpiridaz status
The FDA requires two phase-3 studies that show safety and sufficient diagnostic performance before it will approve a new tracer. The first required study, published in the Journal of the American College of Cardiology, showed that the tracer’s sensitivity for detection of greater than or equal to 50% stenosis by ICA was significantly higher than SPECT; however, the specificity did not meet the prespecified noninferiority criterion.
The second FDA-required study, published online recently, also in the Journal of the American College of Cardiology, was designed differently from the first in that only patients with suspected – not known – CAD were enrolled. The primary efficacy endpoint was sensitivity and specificity of flurpiridaz PET for overall detection of CAD, rather than comparing it to SPECT MPI (which became a secondary endpoint). PET and SPECT studies were both performed before invasive coronary angiography to minimize referral bias; SPECT studies included cadmium zinc telluride cameras.
In that study, which included 578 patients (mean age, 64; 32.5% women) from 48 centers in the United States, Canada, and Europe, flurpiridaz met the efficacy endpoints: Its sensitivity and specificity were significantly higher than the prespecified threshold value by two of the three readers; its sensitivity was higher than SPECT (80.3% vs. 68.7%); and its specificity was noninferior (63.8% vs. 61.7%).
PET areas under the receiver-operating characteristic curves were higher than SPECT in the overall population and in women and obese patients, at half the radiation dose of SPECT.
“Cardiac PET MPI is positioned to serve as the leading modality for the functional evaluation of suspected and known CAD,” Jamieson M. Bourque, MD, MHS, medical director of nuclear cardiology, echocardiography, and the Stress Laboratory, University of Virginia, Charlottesville, wrote in an editorial accompanying the second study . “18F-flurpiridaz will facilitate this upward progression with beneficial tracer characteristics that will increase access and availability, enable exercise stress, and optimize MBF quantification.”
At this point, FDA approval of flurpiridaz is expected sometime in 2024, said James E. Udelson, MD, principal investigator of the recent study, chief of the division of cardiology, and director of the Nuclear Cardiology Laboratory at Tufts University School of Medicine, Boston.
Learning curve
Flurpiridaz comes with “a really interesting and important” learning curve, Dr. Udelson said. “The images are really crisp, and they look very different from what most people are used to. The GE folks are going to have to make sure that the American Society of Nuclear Cardiology and other professional societies are tuned in to help in the education part, because it’s not an easy, automatic switch. Very good image readers can adapt, but it’s not just one day you do one, then switch to the other.”
A “somewhat apt” analogy would be the difference between an echocardiogram and an MRI, he explained. “The MRI is much crisper. You’re seeing edges more crisply. You’re seeing the difference between a thicker and a thinner segment of the wall more crisply, and that’s actually real. You can’t say the thinner segment is abnormal; it’s just that you’re seeing it better. So, with this tracer, normal differences in the thickness of a wall can almost look like a defect if you’re not used to knowing that’s the new normal.”
The expected approval of flurpiridaz “will be a win for cardiac PET, broadening the range of sites that could perform PET,” Dr. Salerno commented. “However, it is worth cautioning that all of the prior data with PET using different agents does not necessarily equate to the same performance with the new agent, given that the performance seems to be lower than that shown in prior PET studies using other agents.”
Dr. Salerno would like to see additional studies comparing flurpiridaz with rubidium or ammonia, as well as studies performing quantification with flurpiridaz, “which theoretically should have some advantages,” he said.
Dr. Udelson noted that MedTrace, a company in Denmark, is working on a radiolabeled water tracer based on 15-O-water that is just starting a pivotal trial. Dr. Udelson is a consultant to the company and is a steering committee member for the pivotal trial.
For now, “the big take-home is that there are a lot of ways these days to test people for CAD,” he said. “As the types of things we can do to test people expand, individuals and centers need to make sure they focus on providing any new service, however they do it, with really superb quality and experience.”
“You don’t just do something new because it’s new,” he added. “It has to be done really well. If you do the new thing badly, you’re not going to get better information.”
Dr. Udelson is a consultant and advisory board member for GE Healthcare, a consultant to MedTrace, and a steering committee member for MedTrace’s pivotal trial. Dr. Bourque has served on a GE Healthcare advisory board for amyloid imaging. Dr. Salerno reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The emerging advantages of PET myocardial perfusion imaging (MPI) for coronary artery disease (CAD) diagnosis and assessment of cardiovascular event risk has prompted growing use of this technology as an alternative to the more commonly used single photon–emission CT (SPECT) MPI.
The advantages of PET MPI include better diagnostic performance and shorter acquisition times. , including consistent, high-quality images and low radiation exposure. It also allows quantification of myocardial blood flow, and it has “strong prognostic power.”
Tracer availability
Despite these advantages, that position paper and subsequent studies note that PET MPI has been underutilized in the United States, largely owing to issues with the available tracers, which have characteristics that limit widespread use in the clinic.
Rubidium, arguably the most commonly used tracer for PET MPI, is not available in unit dosing and so can be expensive for low-volume centers, plus it also requires an on-site generator, Michael Salerno, MD, PhD, a member of the American College of Cardiology’s Imaging Council and section chief of cardiovascular imaging, Stanford (Calif.) University, told this news organization.
N-ammonia, the other U.S. Food and Drug Administration–approved tracer, is available in unit dosing, but its short half-life means that centers need an onsite cyclotron, Dr. Salerno said.
For cardiac perfusion imaging and myocardial blood flow (MBF) quantification, 15O-water is considered the gold standard, although it’s not approved by the FDA. This tracer also requires an on-site cyclotron and “is challenging to use,” Dr. Salerno said. Use has been largely restricted to research purposes, though efforts are underway to widen its availability.
Enter flurpiridaz F-18 (GE Healthcare), a novel PET MPI tracer labeled with fluorine-18. Its longer half-life – similar to that of fluorodeoxyglucose, a tracer used to detect various cancers – could broaden the number of sites that could perform perfusion PET studies, Dr. Salerno said.
“Flurpiridaz also is supposed to have a more linear relationship between flow and tracer uptake, which could improve the ability to perform quantification of perfusion,” he noted. “It also offers the ability to do exercise PET, which is impossible for rubidium and challenging for ammonia, given its 11-minute half-life.”
Flurpiridaz status
The FDA requires two phase-3 studies that show safety and sufficient diagnostic performance before it will approve a new tracer. The first required study, published in the Journal of the American College of Cardiology, showed that the tracer’s sensitivity for detection of greater than or equal to 50% stenosis by ICA was significantly higher than SPECT; however, the specificity did not meet the prespecified noninferiority criterion.
The second FDA-required study, published online recently, also in the Journal of the American College of Cardiology, was designed differently from the first in that only patients with suspected – not known – CAD were enrolled. The primary efficacy endpoint was sensitivity and specificity of flurpiridaz PET for overall detection of CAD, rather than comparing it to SPECT MPI (which became a secondary endpoint). PET and SPECT studies were both performed before invasive coronary angiography to minimize referral bias; SPECT studies included cadmium zinc telluride cameras.
In that study, which included 578 patients (mean age, 64; 32.5% women) from 48 centers in the United States, Canada, and Europe, flurpiridaz met the efficacy endpoints: Its sensitivity and specificity were significantly higher than the prespecified threshold value by two of the three readers; its sensitivity was higher than SPECT (80.3% vs. 68.7%); and its specificity was noninferior (63.8% vs. 61.7%).
PET areas under the receiver-operating characteristic curves were higher than SPECT in the overall population and in women and obese patients, at half the radiation dose of SPECT.
“Cardiac PET MPI is positioned to serve as the leading modality for the functional evaluation of suspected and known CAD,” Jamieson M. Bourque, MD, MHS, medical director of nuclear cardiology, echocardiography, and the Stress Laboratory, University of Virginia, Charlottesville, wrote in an editorial accompanying the second study . “18F-flurpiridaz will facilitate this upward progression with beneficial tracer characteristics that will increase access and availability, enable exercise stress, and optimize MBF quantification.”
At this point, FDA approval of flurpiridaz is expected sometime in 2024, said James E. Udelson, MD, principal investigator of the recent study, chief of the division of cardiology, and director of the Nuclear Cardiology Laboratory at Tufts University School of Medicine, Boston.
Learning curve
Flurpiridaz comes with “a really interesting and important” learning curve, Dr. Udelson said. “The images are really crisp, and they look very different from what most people are used to. The GE folks are going to have to make sure that the American Society of Nuclear Cardiology and other professional societies are tuned in to help in the education part, because it’s not an easy, automatic switch. Very good image readers can adapt, but it’s not just one day you do one, then switch to the other.”
A “somewhat apt” analogy would be the difference between an echocardiogram and an MRI, he explained. “The MRI is much crisper. You’re seeing edges more crisply. You’re seeing the difference between a thicker and a thinner segment of the wall more crisply, and that’s actually real. You can’t say the thinner segment is abnormal; it’s just that you’re seeing it better. So, with this tracer, normal differences in the thickness of a wall can almost look like a defect if you’re not used to knowing that’s the new normal.”
The expected approval of flurpiridaz “will be a win for cardiac PET, broadening the range of sites that could perform PET,” Dr. Salerno commented. “However, it is worth cautioning that all of the prior data with PET using different agents does not necessarily equate to the same performance with the new agent, given that the performance seems to be lower than that shown in prior PET studies using other agents.”
Dr. Salerno would like to see additional studies comparing flurpiridaz with rubidium or ammonia, as well as studies performing quantification with flurpiridaz, “which theoretically should have some advantages,” he said.
Dr. Udelson noted that MedTrace, a company in Denmark, is working on a radiolabeled water tracer based on 15-O-water that is just starting a pivotal trial. Dr. Udelson is a consultant to the company and is a steering committee member for the pivotal trial.
For now, “the big take-home is that there are a lot of ways these days to test people for CAD,” he said. “As the types of things we can do to test people expand, individuals and centers need to make sure they focus on providing any new service, however they do it, with really superb quality and experience.”
“You don’t just do something new because it’s new,” he added. “It has to be done really well. If you do the new thing badly, you’re not going to get better information.”
Dr. Udelson is a consultant and advisory board member for GE Healthcare, a consultant to MedTrace, and a steering committee member for MedTrace’s pivotal trial. Dr. Bourque has served on a GE Healthcare advisory board for amyloid imaging. Dr. Salerno reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Stair climbing tied to reduced risk for heart disease
TOPLINE:
new observational data suggest.
METHODOLOGY:
- The prospective cohort study used data from 458,860 adults in the UK Biobank cohort who were 38-73 years old at baseline (2006-2010).
- Information about stair climbing, sociodemographic, and lifestyle factors was collected at baseline and 5 years later.
- Cases of ASCVD – defined as coronary artery disease (CAD), ischemic stroke, or acute complications – were identified via hospital records and death registry.
- Associations between stair climbing and ASCVD were examined as hazard ratios from Cox proportional hazards model. Analyses were stratified by susceptibility to ASCVD based on family history, genetic risk, and established risk factors.
TAKEAWAY:
- A total of 39,043 ASCVD, 30,718 CAD, and 10,521 ischemic stroke cases were recorded during a median follow-up of 12.5 years.
- Compared with no-stair climbing, climbing 6-10 flights of stairs daily was associated with a 7% lower ASCVD risk (multivariable-adjusted HR, 0.93; 95% confidence interval, 0.90-0.96) and climbing 16-20 flights daily was associated with a 10% lower risk (HR, 0.90; 95% CI, 0.85-0.94).
- The benefits plateaued at 20 flights daily; comparable results were obtained for CAD and ischemic stroke; the protective effect of stair climbing was attenuated by increasing levels of disease susceptibility.
- Adults who stopped climbing stairs daily during the study had a 32% higher risk of ASCVD (HR, 1.32; 95% CI,1.06-1.65), compared with peers who never reported stair climbing.
IN PRACTICE:
“These findings highlight the potential advantages of stair climbing as a primary preventive measure for ASCVD in the general population. Short bursts of high-intensity stair climbing are a time-efficient way to improve cardiorespiratory fitness and lipid profile, especially among those unable to achieve the current physical activity recommendations,” study author Lu Qi, with Tulane University, New Orleans, said in a news release.
SOURCE:
The study was published online in Atherosclerosis.
LIMITATIONS:
The observational design limits causal inferences. Stair climbing was self-reported via questionnaires and recall bias is a possibility. The UK Biobank participants do not represent the entire population of the country, with a healthy volunteer selection bias previously reported.
DISCLOSURES:
The study was supported by grants from the National Key R&D Program of China. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
new observational data suggest.
METHODOLOGY:
- The prospective cohort study used data from 458,860 adults in the UK Biobank cohort who were 38-73 years old at baseline (2006-2010).
- Information about stair climbing, sociodemographic, and lifestyle factors was collected at baseline and 5 years later.
- Cases of ASCVD – defined as coronary artery disease (CAD), ischemic stroke, or acute complications – were identified via hospital records and death registry.
- Associations between stair climbing and ASCVD were examined as hazard ratios from Cox proportional hazards model. Analyses were stratified by susceptibility to ASCVD based on family history, genetic risk, and established risk factors.
TAKEAWAY:
- A total of 39,043 ASCVD, 30,718 CAD, and 10,521 ischemic stroke cases were recorded during a median follow-up of 12.5 years.
- Compared with no-stair climbing, climbing 6-10 flights of stairs daily was associated with a 7% lower ASCVD risk (multivariable-adjusted HR, 0.93; 95% confidence interval, 0.90-0.96) and climbing 16-20 flights daily was associated with a 10% lower risk (HR, 0.90; 95% CI, 0.85-0.94).
- The benefits plateaued at 20 flights daily; comparable results were obtained for CAD and ischemic stroke; the protective effect of stair climbing was attenuated by increasing levels of disease susceptibility.
- Adults who stopped climbing stairs daily during the study had a 32% higher risk of ASCVD (HR, 1.32; 95% CI,1.06-1.65), compared with peers who never reported stair climbing.
IN PRACTICE:
“These findings highlight the potential advantages of stair climbing as a primary preventive measure for ASCVD in the general population. Short bursts of high-intensity stair climbing are a time-efficient way to improve cardiorespiratory fitness and lipid profile, especially among those unable to achieve the current physical activity recommendations,” study author Lu Qi, with Tulane University, New Orleans, said in a news release.
SOURCE:
The study was published online in Atherosclerosis.
LIMITATIONS:
The observational design limits causal inferences. Stair climbing was self-reported via questionnaires and recall bias is a possibility. The UK Biobank participants do not represent the entire population of the country, with a healthy volunteer selection bias previously reported.
DISCLOSURES:
The study was supported by grants from the National Key R&D Program of China. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
new observational data suggest.
METHODOLOGY:
- The prospective cohort study used data from 458,860 adults in the UK Biobank cohort who were 38-73 years old at baseline (2006-2010).
- Information about stair climbing, sociodemographic, and lifestyle factors was collected at baseline and 5 years later.
- Cases of ASCVD – defined as coronary artery disease (CAD), ischemic stroke, or acute complications – were identified via hospital records and death registry.
- Associations between stair climbing and ASCVD were examined as hazard ratios from Cox proportional hazards model. Analyses were stratified by susceptibility to ASCVD based on family history, genetic risk, and established risk factors.
TAKEAWAY:
- A total of 39,043 ASCVD, 30,718 CAD, and 10,521 ischemic stroke cases were recorded during a median follow-up of 12.5 years.
- Compared with no-stair climbing, climbing 6-10 flights of stairs daily was associated with a 7% lower ASCVD risk (multivariable-adjusted HR, 0.93; 95% confidence interval, 0.90-0.96) and climbing 16-20 flights daily was associated with a 10% lower risk (HR, 0.90; 95% CI, 0.85-0.94).
- The benefits plateaued at 20 flights daily; comparable results were obtained for CAD and ischemic stroke; the protective effect of stair climbing was attenuated by increasing levels of disease susceptibility.
- Adults who stopped climbing stairs daily during the study had a 32% higher risk of ASCVD (HR, 1.32; 95% CI,1.06-1.65), compared with peers who never reported stair climbing.
IN PRACTICE:
“These findings highlight the potential advantages of stair climbing as a primary preventive measure for ASCVD in the general population. Short bursts of high-intensity stair climbing are a time-efficient way to improve cardiorespiratory fitness and lipid profile, especially among those unable to achieve the current physical activity recommendations,” study author Lu Qi, with Tulane University, New Orleans, said in a news release.
SOURCE:
The study was published online in Atherosclerosis.
LIMITATIONS:
The observational design limits causal inferences. Stair climbing was self-reported via questionnaires and recall bias is a possibility. The UK Biobank participants do not represent the entire population of the country, with a healthy volunteer selection bias previously reported.
DISCLOSURES:
The study was supported by grants from the National Key R&D Program of China. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
EBV and MS: Just how deep is the link?
MILAN – in a joint presentation at the 9th Joint ECTRIMS-ACTRIMS meeting.
Armed with the findings of his own landmark 2022 study into EBV and MS, Harvard Medical School, Boston, professor of medicine Alberto Ascherio, MD, DrPH, argued that they’re tightly connected. But rheumatologist William H. Robinson, MD, PhD, of Stanford (Calif.) University, said that while he also believes EBV plays a significant role in MS, “there’s likely a role for a second hit” – some other factor. “Why are 95% of us EBV-infected, but only a small subset ultimately develop MS or ... other autoimmune diseases?”
As a 2023 review noted, researchers have puzzled over the connection between EBV and MS since the early 1980s. “Until that point, EBV was primarily viewed as a cancer-causing agent, but the culmination of evidence now shows that EBV has a pivotal role in development of MS.” But it’s not clear how EBV – which strikes more than an estimated 95% of humans and causes mononucleosis – manages to trigger MS.
A rare complication of EBV infection
In the 2022 study, Dr. Ascherio aimed to understand exactly how deeply EBV and MS are connected by analyzing serum data gathered from more than 10 million active-duty members of the U.S. military. Of those, 955 were diagnosed with MS.
The researchers focused on 801 subjects with MS and matched them to 1,566 controls. Only 1 of the 801 subjects with MS had a negative EBV test prior to diagnosis, a fact that researchers believe could be due to a factor such as a failure to seroconvert during infection. “At baseline, 35 MS cases and 107 controls were EBV-negative,” the study reported. “All but one of these 35 EBV-negative MS cases became infected with EBV during the follow-up.”
Overall, subjects who were positive for EBV were 32.4 times to develop MS than those who weren’t (95% confidence interval, 4.3-245.3; P < 0.001).
Is it possible that immune dysregulation from MS precedes EBV infection? The researchers analyzed viruses in 30 subjects with MS – before and after MS onset – and in 30 controls. The findings suggested that EBV was the major player, Dr. Ascherio said.
Researchers also focused on cytomegalovirus (CMV) infection, which is closely related to EBV and to the chicken pox virus. “CMV seroconversion is not associated with MS, and positivity for CMV at baseline was associated with a modestly lower risk of MS,” Dr. Ascherio said.
In the big picture, “this data establishes beyond reasonable doubt that MS is a rare complication of EBV infection,” Dr. Ascherio said. “The main question now is whether the virus triggers an immune process that then is self-maintained, or whether the presence of the infection keeps feeding the immune process.”
Inadequate evidence for causation
In his presentation, Dr. Robinson asked: “Does EBV cause MS? Really? All of MS? In humans [with MS], yes, we found monoclonal antibodies expressed by the B cells that bound to EBV. But we also found spinal fluid B cells and coding antibodies that bound to multiple other viruses, including rubella, VZV [varicella-zoster virus/chickenpox], CMV, and HSV [herpes simplex virus]. And there’s even a measles reactive antibody there.”
And there’s evidence that human herpes virus type 6 (HHV-6) and HHV-6A could be linked to MS: “Maybe HHV-6 or HHV-6A is the cause of MS in a subset of patients,” Dr. Robinson said. Research suggests that pox viruses could be another possible cause, he said.
He added: “I’m a rheumatologist, and I see patients in the clinic and in the hospital who have lupus, a disease highly associated with EBV infection. But they definitely do not have MS, nor do they have RA [rheumatoid arthritis], and likewise your MS patients don’t have lupus. What’s up with all these diseases potentially being linked to EBV?”
A missing piece of the puzzle?
In a discussion period, Dr. Ascherio responded to Dr. Robinson by saying he’s waiting to see evidence that patients with the other diseases linked to EBV don’t develop them if they’re EBV-negative. Dr. Ascherio added that it’s possible that there are different strains of EBV, and some may be more likely to cause MS.
What does this all mean for MS prevention? In a commentary published with Dr. Ascherio’s 2022 study, Dr. Robinson and a coauthor asked: “Would a vaccine against EBV protect against MS? Can the B cells that dwell in the CSF be killed or inactivated with therapeutics? Would antivirals that target EBV provide effective therapy, especially when given early in the course of disease? Now that the initial trigger for MS has been identified, perhaps MS could be eradicated.”
Dr. Ascherio discloses speaker/consultant relationships with Prada Foundation, WebMD, Biogen, Moderna, Merck, Roche, and GSK. Dr. Robinson discloses unspecified relationships with Altreca and Flatiron Bio, and he is a coinventor on a patent application filed by Stanford University that includes antibodies to EBV.
MILAN – in a joint presentation at the 9th Joint ECTRIMS-ACTRIMS meeting.
Armed with the findings of his own landmark 2022 study into EBV and MS, Harvard Medical School, Boston, professor of medicine Alberto Ascherio, MD, DrPH, argued that they’re tightly connected. But rheumatologist William H. Robinson, MD, PhD, of Stanford (Calif.) University, said that while he also believes EBV plays a significant role in MS, “there’s likely a role for a second hit” – some other factor. “Why are 95% of us EBV-infected, but only a small subset ultimately develop MS or ... other autoimmune diseases?”
As a 2023 review noted, researchers have puzzled over the connection between EBV and MS since the early 1980s. “Until that point, EBV was primarily viewed as a cancer-causing agent, but the culmination of evidence now shows that EBV has a pivotal role in development of MS.” But it’s not clear how EBV – which strikes more than an estimated 95% of humans and causes mononucleosis – manages to trigger MS.
A rare complication of EBV infection
In the 2022 study, Dr. Ascherio aimed to understand exactly how deeply EBV and MS are connected by analyzing serum data gathered from more than 10 million active-duty members of the U.S. military. Of those, 955 were diagnosed with MS.
The researchers focused on 801 subjects with MS and matched them to 1,566 controls. Only 1 of the 801 subjects with MS had a negative EBV test prior to diagnosis, a fact that researchers believe could be due to a factor such as a failure to seroconvert during infection. “At baseline, 35 MS cases and 107 controls were EBV-negative,” the study reported. “All but one of these 35 EBV-negative MS cases became infected with EBV during the follow-up.”
Overall, subjects who were positive for EBV were 32.4 times to develop MS than those who weren’t (95% confidence interval, 4.3-245.3; P < 0.001).
Is it possible that immune dysregulation from MS precedes EBV infection? The researchers analyzed viruses in 30 subjects with MS – before and after MS onset – and in 30 controls. The findings suggested that EBV was the major player, Dr. Ascherio said.
Researchers also focused on cytomegalovirus (CMV) infection, which is closely related to EBV and to the chicken pox virus. “CMV seroconversion is not associated with MS, and positivity for CMV at baseline was associated with a modestly lower risk of MS,” Dr. Ascherio said.
In the big picture, “this data establishes beyond reasonable doubt that MS is a rare complication of EBV infection,” Dr. Ascherio said. “The main question now is whether the virus triggers an immune process that then is self-maintained, or whether the presence of the infection keeps feeding the immune process.”
Inadequate evidence for causation
In his presentation, Dr. Robinson asked: “Does EBV cause MS? Really? All of MS? In humans [with MS], yes, we found monoclonal antibodies expressed by the B cells that bound to EBV. But we also found spinal fluid B cells and coding antibodies that bound to multiple other viruses, including rubella, VZV [varicella-zoster virus/chickenpox], CMV, and HSV [herpes simplex virus]. And there’s even a measles reactive antibody there.”
And there’s evidence that human herpes virus type 6 (HHV-6) and HHV-6A could be linked to MS: “Maybe HHV-6 or HHV-6A is the cause of MS in a subset of patients,” Dr. Robinson said. Research suggests that pox viruses could be another possible cause, he said.
He added: “I’m a rheumatologist, and I see patients in the clinic and in the hospital who have lupus, a disease highly associated with EBV infection. But they definitely do not have MS, nor do they have RA [rheumatoid arthritis], and likewise your MS patients don’t have lupus. What’s up with all these diseases potentially being linked to EBV?”
A missing piece of the puzzle?
In a discussion period, Dr. Ascherio responded to Dr. Robinson by saying he’s waiting to see evidence that patients with the other diseases linked to EBV don’t develop them if they’re EBV-negative. Dr. Ascherio added that it’s possible that there are different strains of EBV, and some may be more likely to cause MS.
What does this all mean for MS prevention? In a commentary published with Dr. Ascherio’s 2022 study, Dr. Robinson and a coauthor asked: “Would a vaccine against EBV protect against MS? Can the B cells that dwell in the CSF be killed or inactivated with therapeutics? Would antivirals that target EBV provide effective therapy, especially when given early in the course of disease? Now that the initial trigger for MS has been identified, perhaps MS could be eradicated.”
Dr. Ascherio discloses speaker/consultant relationships with Prada Foundation, WebMD, Biogen, Moderna, Merck, Roche, and GSK. Dr. Robinson discloses unspecified relationships with Altreca and Flatiron Bio, and he is a coinventor on a patent application filed by Stanford University that includes antibodies to EBV.
MILAN – in a joint presentation at the 9th Joint ECTRIMS-ACTRIMS meeting.
Armed with the findings of his own landmark 2022 study into EBV and MS, Harvard Medical School, Boston, professor of medicine Alberto Ascherio, MD, DrPH, argued that they’re tightly connected. But rheumatologist William H. Robinson, MD, PhD, of Stanford (Calif.) University, said that while he also believes EBV plays a significant role in MS, “there’s likely a role for a second hit” – some other factor. “Why are 95% of us EBV-infected, but only a small subset ultimately develop MS or ... other autoimmune diseases?”
As a 2023 review noted, researchers have puzzled over the connection between EBV and MS since the early 1980s. “Until that point, EBV was primarily viewed as a cancer-causing agent, but the culmination of evidence now shows that EBV has a pivotal role in development of MS.” But it’s not clear how EBV – which strikes more than an estimated 95% of humans and causes mononucleosis – manages to trigger MS.
A rare complication of EBV infection
In the 2022 study, Dr. Ascherio aimed to understand exactly how deeply EBV and MS are connected by analyzing serum data gathered from more than 10 million active-duty members of the U.S. military. Of those, 955 were diagnosed with MS.
The researchers focused on 801 subjects with MS and matched them to 1,566 controls. Only 1 of the 801 subjects with MS had a negative EBV test prior to diagnosis, a fact that researchers believe could be due to a factor such as a failure to seroconvert during infection. “At baseline, 35 MS cases and 107 controls were EBV-negative,” the study reported. “All but one of these 35 EBV-negative MS cases became infected with EBV during the follow-up.”
Overall, subjects who were positive for EBV were 32.4 times to develop MS than those who weren’t (95% confidence interval, 4.3-245.3; P < 0.001).
Is it possible that immune dysregulation from MS precedes EBV infection? The researchers analyzed viruses in 30 subjects with MS – before and after MS onset – and in 30 controls. The findings suggested that EBV was the major player, Dr. Ascherio said.
Researchers also focused on cytomegalovirus (CMV) infection, which is closely related to EBV and to the chicken pox virus. “CMV seroconversion is not associated with MS, and positivity for CMV at baseline was associated with a modestly lower risk of MS,” Dr. Ascherio said.
In the big picture, “this data establishes beyond reasonable doubt that MS is a rare complication of EBV infection,” Dr. Ascherio said. “The main question now is whether the virus triggers an immune process that then is self-maintained, or whether the presence of the infection keeps feeding the immune process.”
Inadequate evidence for causation
In his presentation, Dr. Robinson asked: “Does EBV cause MS? Really? All of MS? In humans [with MS], yes, we found monoclonal antibodies expressed by the B cells that bound to EBV. But we also found spinal fluid B cells and coding antibodies that bound to multiple other viruses, including rubella, VZV [varicella-zoster virus/chickenpox], CMV, and HSV [herpes simplex virus]. And there’s even a measles reactive antibody there.”
And there’s evidence that human herpes virus type 6 (HHV-6) and HHV-6A could be linked to MS: “Maybe HHV-6 or HHV-6A is the cause of MS in a subset of patients,” Dr. Robinson said. Research suggests that pox viruses could be another possible cause, he said.
He added: “I’m a rheumatologist, and I see patients in the clinic and in the hospital who have lupus, a disease highly associated with EBV infection. But they definitely do not have MS, nor do they have RA [rheumatoid arthritis], and likewise your MS patients don’t have lupus. What’s up with all these diseases potentially being linked to EBV?”
A missing piece of the puzzle?
In a discussion period, Dr. Ascherio responded to Dr. Robinson by saying he’s waiting to see evidence that patients with the other diseases linked to EBV don’t develop them if they’re EBV-negative. Dr. Ascherio added that it’s possible that there are different strains of EBV, and some may be more likely to cause MS.
What does this all mean for MS prevention? In a commentary published with Dr. Ascherio’s 2022 study, Dr. Robinson and a coauthor asked: “Would a vaccine against EBV protect against MS? Can the B cells that dwell in the CSF be killed or inactivated with therapeutics? Would antivirals that target EBV provide effective therapy, especially when given early in the course of disease? Now that the initial trigger for MS has been identified, perhaps MS could be eradicated.”
Dr. Ascherio discloses speaker/consultant relationships with Prada Foundation, WebMD, Biogen, Moderna, Merck, Roche, and GSK. Dr. Robinson discloses unspecified relationships with Altreca and Flatiron Bio, and he is a coinventor on a patent application filed by Stanford University that includes antibodies to EBV.
AT ECTRIMS 2023
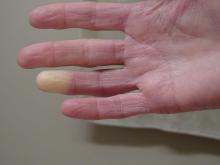
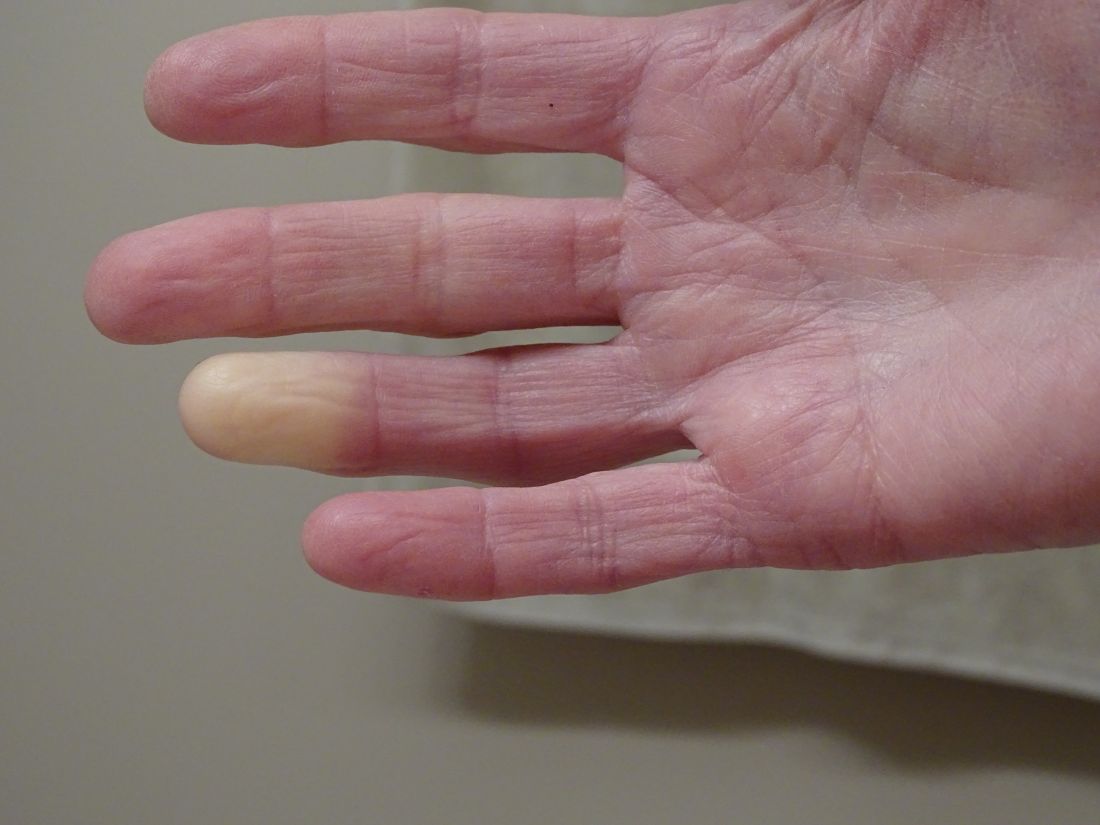
Researchers link two genes to Raynaud’s disease
Researchers have identified two genes that may contribute to Raynaud’s phenomenon, a condition where blood vessels in the extremities constrict and limit blood flow.
Raynaud’s is a relatively common condition, affecting 2%-5% of the general population. Though Raynaud’s can be an annoyance for some, it can also cause severe pain and can require medication.
These newly identified genes will hopefully lead to new therapeutic options, said Maik Pietzner, PhD, chair in health data modeling at Queen Mary University of London’s Precision Healthcare University Research Institute (PHURI) and group leader in the Computational Medicine Group at the Berlin Institute of Health at Charité – Universitätsmedizin Berlin, Germany.
Dr. Pietzner led the research along with Claudia Langenberg, MD, PhD, director of PHURI.
The study was published in Nature Communications.
Largest genomic study of Raynaud’s to date
The researchers looked through electronic medical records from the UK Biobank, a large-scale database that contains genetic and health information on half a million participants. They identified more than 5,100 individuals with Raynaud’s, of which 68% had primary Raynaud’s. These participants were compared with more than 439,000 controls who did not have Raynaud’s.
In a secondary analysis, the team also used health records from the Queen Mary University of London Genes & Health Study, which contains health information on individuals of South Asian ancestry.
The researchers identified two genes that are likely involved with Raynaud’s. The first, ADRA2A, encodes for the alpha-2A adrenergic receptor that can cause vasoconstriction of small blood vessels in response to stress hormones. Researchers have long suspected that this type of receptor could be involved with Raynaud’s, but there was debate over which receptor subtype was responsible.
“Our finding of alpha-2A receptors is quite interesting because the focus has always been on alpha-2C receptors,” said Dr. Pietzner. “It’s only a letter, but it’s a massive difference in terms of biology and physiology,” he said, and could be why therapies targeting 2C receptors have been ineffective.
The second strongest association was for the transcription factor IRX1. Less is known about this gene, but the data we do have suggest that it is involved with regulating the dilation of blood vessels, Dr. Pietzner noted.
“There might be balance between the ADRA2A finding being responsible for constriction and the IRX1 finding indirectly linked to the dilation of those vessels following constrictions. Having both may explain why these prolonged episodes of vasoconstriction lead to a loss of oxygen to the tissues,” so they turn white and then blue, he said.
Because the Biobank cohort was European-centric, Dr. Pietzner and colleagues also identified 400 cases of Raynaud’s in British individuals of Bangladeshi and Pakistani ancestry and were able to replicate the association between IRX1 and Raynaud’s. Data on ADRA2A were unavailable.
The genes identified are associated with primary Raynaud’s. Secondary Raynaud’s is a rarer type of the condition that occurs along with autoimmune disorders, such as scleroderma, and is generally more severe.
It’s long been suspected that Raynaud’s had some genetic component, because half of patients with Raynaud’s have another family member with the same condition, said Laura Hummers, MD, who codirects the John Hopkins Scleroderma Center in Baltimore. She was not involved with the study.
This is “the largest study of this kind that’s been done,” she said, and the first to show a potential mechanism behind this genetic association.
The main gene finding, ADRA2A, “points to a receptor on the cells that regulate the tone of these blood vessels,” she continued. “It suggests maybe there’s too many of these receptors or they’re overly sensitive; something about them is different that makes patients more susceptible to these cold triggers. Knowing that is potentially really important, because it could give you a direct way to intervene, if true.”
New therapeutic avenues
The first-line treatment for primary Raynaud’s is behavioral interventions, such as maintaining body and extremity warmth and avoiding certain vasoconstricting drugs, said Kimberly Lakin, MD, a rheumatologist at the Hospital for Special Surgery in New York, who not involved in the research. These drugs could include over-the-counter decongestants and certain medications for attention-deficit/hyperactivity disorder.
If these behavioral interventions are not enough, clinicians most commonly prescribe calcium channel blockers. These medications are vasodilators but can be a concern for people with normal or already low blood pressure, Dr. Lakin said. They can also cause symptoms such as headache, leg swelling, constipation, and other gastrointestinal symptoms.
Other medications, such as fluoxetine, may also be considered as a later-line therapy, “but the effectiveness is fairly limited in Raynaud’s,” she said. “Certainly, other medication options that would be helpful and driven by the mechanisms of Raynaud’s would add to our ability to help patients.”
As it turns out, one of the genes identified in the study, ADRA2A, “is actually one of the most commonly targeted genes by drugs,” said Dr. Pietzner. Because the findings suggest that ADRA2A is overexpressed in Raynaud, a selective inhibitor like the antidepressant mirtazapine could be a promising candidate to repurpose for treating Raynaud’s, he said.
Limitations to electronic medical record analyses
Both Dr. Hummers and Dr. Lakin noted that research using diagnostic codes from medical records to identify cases has some limitations. The study may have included patients misdiagnosed with Raynaud’s when perhaps they had another condition. Patients with milder Raynaud’s who have not sought medical attention for the condition would not be represented in the study, Dr. Lakin said.
The UK Biobank includes individuals of mostly European descent, so an analysis confirming these findings in a more diverse population would be helpful, she said.
However, both Dr. Lakin and Dr. Hummers agreed that the study contributes to the understanding of the mechanisms behind Raynaud’s. Although the two identified genes were tied to primary Raynaud’s, the study’s findings could potentially apply to secondary Raynaud’s as well, Dr. Hummers said.
“Anything we learn about primary Raynaud’s may have implication for Raynaud’s more broadly,” she noted.
Dr. Hummers and Dr. Lakin disclosed no relevant financial relationships. Dr. Pietzner has received partnership funding for the MRC Clinical Pharmacology Training Scheme (cofunded by MRC and Roche, UCB, Eli Lilly, and Novartis) and a PhD studentship jointly funded by the UK Engineering and Physical Sciences Research Council and AstraZeneca. Dr. Pietzner also has unrestricted educational grant support for the UK Pharmacogenetics and Stratified Medicine Network from Bristol-Myers Squibb.
A version of this article appeared on Medscape.com.
Researchers have identified two genes that may contribute to Raynaud’s phenomenon, a condition where blood vessels in the extremities constrict and limit blood flow.
Raynaud’s is a relatively common condition, affecting 2%-5% of the general population. Though Raynaud’s can be an annoyance for some, it can also cause severe pain and can require medication.
These newly identified genes will hopefully lead to new therapeutic options, said Maik Pietzner, PhD, chair in health data modeling at Queen Mary University of London’s Precision Healthcare University Research Institute (PHURI) and group leader in the Computational Medicine Group at the Berlin Institute of Health at Charité – Universitätsmedizin Berlin, Germany.
Dr. Pietzner led the research along with Claudia Langenberg, MD, PhD, director of PHURI.
The study was published in Nature Communications.
Largest genomic study of Raynaud’s to date
The researchers looked through electronic medical records from the UK Biobank, a large-scale database that contains genetic and health information on half a million participants. They identified more than 5,100 individuals with Raynaud’s, of which 68% had primary Raynaud’s. These participants were compared with more than 439,000 controls who did not have Raynaud’s.
In a secondary analysis, the team also used health records from the Queen Mary University of London Genes & Health Study, which contains health information on individuals of South Asian ancestry.
The researchers identified two genes that are likely involved with Raynaud’s. The first, ADRA2A, encodes for the alpha-2A adrenergic receptor that can cause vasoconstriction of small blood vessels in response to stress hormones. Researchers have long suspected that this type of receptor could be involved with Raynaud’s, but there was debate over which receptor subtype was responsible.
“Our finding of alpha-2A receptors is quite interesting because the focus has always been on alpha-2C receptors,” said Dr. Pietzner. “It’s only a letter, but it’s a massive difference in terms of biology and physiology,” he said, and could be why therapies targeting 2C receptors have been ineffective.
The second strongest association was for the transcription factor IRX1. Less is known about this gene, but the data we do have suggest that it is involved with regulating the dilation of blood vessels, Dr. Pietzner noted.
“There might be balance between the ADRA2A finding being responsible for constriction and the IRX1 finding indirectly linked to the dilation of those vessels following constrictions. Having both may explain why these prolonged episodes of vasoconstriction lead to a loss of oxygen to the tissues,” so they turn white and then blue, he said.
Because the Biobank cohort was European-centric, Dr. Pietzner and colleagues also identified 400 cases of Raynaud’s in British individuals of Bangladeshi and Pakistani ancestry and were able to replicate the association between IRX1 and Raynaud’s. Data on ADRA2A were unavailable.
The genes identified are associated with primary Raynaud’s. Secondary Raynaud’s is a rarer type of the condition that occurs along with autoimmune disorders, such as scleroderma, and is generally more severe.
It’s long been suspected that Raynaud’s had some genetic component, because half of patients with Raynaud’s have another family member with the same condition, said Laura Hummers, MD, who codirects the John Hopkins Scleroderma Center in Baltimore. She was not involved with the study.
This is “the largest study of this kind that’s been done,” she said, and the first to show a potential mechanism behind this genetic association.
The main gene finding, ADRA2A, “points to a receptor on the cells that regulate the tone of these blood vessels,” she continued. “It suggests maybe there’s too many of these receptors or they’re overly sensitive; something about them is different that makes patients more susceptible to these cold triggers. Knowing that is potentially really important, because it could give you a direct way to intervene, if true.”
New therapeutic avenues
The first-line treatment for primary Raynaud’s is behavioral interventions, such as maintaining body and extremity warmth and avoiding certain vasoconstricting drugs, said Kimberly Lakin, MD, a rheumatologist at the Hospital for Special Surgery in New York, who not involved in the research. These drugs could include over-the-counter decongestants and certain medications for attention-deficit/hyperactivity disorder.
If these behavioral interventions are not enough, clinicians most commonly prescribe calcium channel blockers. These medications are vasodilators but can be a concern for people with normal or already low blood pressure, Dr. Lakin said. They can also cause symptoms such as headache, leg swelling, constipation, and other gastrointestinal symptoms.
Other medications, such as fluoxetine, may also be considered as a later-line therapy, “but the effectiveness is fairly limited in Raynaud’s,” she said. “Certainly, other medication options that would be helpful and driven by the mechanisms of Raynaud’s would add to our ability to help patients.”
As it turns out, one of the genes identified in the study, ADRA2A, “is actually one of the most commonly targeted genes by drugs,” said Dr. Pietzner. Because the findings suggest that ADRA2A is overexpressed in Raynaud, a selective inhibitor like the antidepressant mirtazapine could be a promising candidate to repurpose for treating Raynaud’s, he said.
Limitations to electronic medical record analyses
Both Dr. Hummers and Dr. Lakin noted that research using diagnostic codes from medical records to identify cases has some limitations. The study may have included patients misdiagnosed with Raynaud’s when perhaps they had another condition. Patients with milder Raynaud’s who have not sought medical attention for the condition would not be represented in the study, Dr. Lakin said.
The UK Biobank includes individuals of mostly European descent, so an analysis confirming these findings in a more diverse population would be helpful, she said.
However, both Dr. Lakin and Dr. Hummers agreed that the study contributes to the understanding of the mechanisms behind Raynaud’s. Although the two identified genes were tied to primary Raynaud’s, the study’s findings could potentially apply to secondary Raynaud’s as well, Dr. Hummers said.
“Anything we learn about primary Raynaud’s may have implication for Raynaud’s more broadly,” she noted.
Dr. Hummers and Dr. Lakin disclosed no relevant financial relationships. Dr. Pietzner has received partnership funding for the MRC Clinical Pharmacology Training Scheme (cofunded by MRC and Roche, UCB, Eli Lilly, and Novartis) and a PhD studentship jointly funded by the UK Engineering and Physical Sciences Research Council and AstraZeneca. Dr. Pietzner also has unrestricted educational grant support for the UK Pharmacogenetics and Stratified Medicine Network from Bristol-Myers Squibb.
A version of this article appeared on Medscape.com.
Researchers have identified two genes that may contribute to Raynaud’s phenomenon, a condition where blood vessels in the extremities constrict and limit blood flow.
Raynaud’s is a relatively common condition, affecting 2%-5% of the general population. Though Raynaud’s can be an annoyance for some, it can also cause severe pain and can require medication.
These newly identified genes will hopefully lead to new therapeutic options, said Maik Pietzner, PhD, chair in health data modeling at Queen Mary University of London’s Precision Healthcare University Research Institute (PHURI) and group leader in the Computational Medicine Group at the Berlin Institute of Health at Charité – Universitätsmedizin Berlin, Germany.
Dr. Pietzner led the research along with Claudia Langenberg, MD, PhD, director of PHURI.
The study was published in Nature Communications.
Largest genomic study of Raynaud’s to date
The researchers looked through electronic medical records from the UK Biobank, a large-scale database that contains genetic and health information on half a million participants. They identified more than 5,100 individuals with Raynaud’s, of which 68% had primary Raynaud’s. These participants were compared with more than 439,000 controls who did not have Raynaud’s.
In a secondary analysis, the team also used health records from the Queen Mary University of London Genes & Health Study, which contains health information on individuals of South Asian ancestry.
The researchers identified two genes that are likely involved with Raynaud’s. The first, ADRA2A, encodes for the alpha-2A adrenergic receptor that can cause vasoconstriction of small blood vessels in response to stress hormones. Researchers have long suspected that this type of receptor could be involved with Raynaud’s, but there was debate over which receptor subtype was responsible.
“Our finding of alpha-2A receptors is quite interesting because the focus has always been on alpha-2C receptors,” said Dr. Pietzner. “It’s only a letter, but it’s a massive difference in terms of biology and physiology,” he said, and could be why therapies targeting 2C receptors have been ineffective.
The second strongest association was for the transcription factor IRX1. Less is known about this gene, but the data we do have suggest that it is involved with regulating the dilation of blood vessels, Dr. Pietzner noted.
“There might be balance between the ADRA2A finding being responsible for constriction and the IRX1 finding indirectly linked to the dilation of those vessels following constrictions. Having both may explain why these prolonged episodes of vasoconstriction lead to a loss of oxygen to the tissues,” so they turn white and then blue, he said.
Because the Biobank cohort was European-centric, Dr. Pietzner and colleagues also identified 400 cases of Raynaud’s in British individuals of Bangladeshi and Pakistani ancestry and were able to replicate the association between IRX1 and Raynaud’s. Data on ADRA2A were unavailable.
The genes identified are associated with primary Raynaud’s. Secondary Raynaud’s is a rarer type of the condition that occurs along with autoimmune disorders, such as scleroderma, and is generally more severe.
It’s long been suspected that Raynaud’s had some genetic component, because half of patients with Raynaud’s have another family member with the same condition, said Laura Hummers, MD, who codirects the John Hopkins Scleroderma Center in Baltimore. She was not involved with the study.
This is “the largest study of this kind that’s been done,” she said, and the first to show a potential mechanism behind this genetic association.
The main gene finding, ADRA2A, “points to a receptor on the cells that regulate the tone of these blood vessels,” she continued. “It suggests maybe there’s too many of these receptors or they’re overly sensitive; something about them is different that makes patients more susceptible to these cold triggers. Knowing that is potentially really important, because it could give you a direct way to intervene, if true.”
New therapeutic avenues
The first-line treatment for primary Raynaud’s is behavioral interventions, such as maintaining body and extremity warmth and avoiding certain vasoconstricting drugs, said Kimberly Lakin, MD, a rheumatologist at the Hospital for Special Surgery in New York, who not involved in the research. These drugs could include over-the-counter decongestants and certain medications for attention-deficit/hyperactivity disorder.
If these behavioral interventions are not enough, clinicians most commonly prescribe calcium channel blockers. These medications are vasodilators but can be a concern for people with normal or already low blood pressure, Dr. Lakin said. They can also cause symptoms such as headache, leg swelling, constipation, and other gastrointestinal symptoms.
Other medications, such as fluoxetine, may also be considered as a later-line therapy, “but the effectiveness is fairly limited in Raynaud’s,” she said. “Certainly, other medication options that would be helpful and driven by the mechanisms of Raynaud’s would add to our ability to help patients.”
As it turns out, one of the genes identified in the study, ADRA2A, “is actually one of the most commonly targeted genes by drugs,” said Dr. Pietzner. Because the findings suggest that ADRA2A is overexpressed in Raynaud, a selective inhibitor like the antidepressant mirtazapine could be a promising candidate to repurpose for treating Raynaud’s, he said.
Limitations to electronic medical record analyses
Both Dr. Hummers and Dr. Lakin noted that research using diagnostic codes from medical records to identify cases has some limitations. The study may have included patients misdiagnosed with Raynaud’s when perhaps they had another condition. Patients with milder Raynaud’s who have not sought medical attention for the condition would not be represented in the study, Dr. Lakin said.
The UK Biobank includes individuals of mostly European descent, so an analysis confirming these findings in a more diverse population would be helpful, she said.
However, both Dr. Lakin and Dr. Hummers agreed that the study contributes to the understanding of the mechanisms behind Raynaud’s. Although the two identified genes were tied to primary Raynaud’s, the study’s findings could potentially apply to secondary Raynaud’s as well, Dr. Hummers said.
“Anything we learn about primary Raynaud’s may have implication for Raynaud’s more broadly,” she noted.
Dr. Hummers and Dr. Lakin disclosed no relevant financial relationships. Dr. Pietzner has received partnership funding for the MRC Clinical Pharmacology Training Scheme (cofunded by MRC and Roche, UCB, Eli Lilly, and Novartis) and a PhD studentship jointly funded by the UK Engineering and Physical Sciences Research Council and AstraZeneca. Dr. Pietzner also has unrestricted educational grant support for the UK Pharmacogenetics and Stratified Medicine Network from Bristol-Myers Squibb.
A version of this article appeared on Medscape.com.
FROM NATURE COMMUNICATIONS
Higher fracture risk not seen with SGLT2 inhibitors
VANCOUVER – In patients with type 2 diabetes, sodium-glucose cotransporter 2 (SGLT2) inhibitors as an adjunct to metformin were not associated with an increase in fracture risk, according to a new real-world study.
There have been some reports of an increase in fracture risk associated with SGLT2 inhibitors, and it was observed in the phase 3 CANVAS trial of canagliflozin (Invokana), which led to a Food and Drug Administration warning of fracture risks associated with canagliflozin use. Some ensuing studies did not show an increased risk, but these studies were generally less than a year in duration and may have missed longer-term risk, according to Veerle van Hulten, MSc.
“Fracture risk is something that takes a long time to develop, so we wanted to have a longer follow-up. We looked into the CPRD [Clinical Practice Research Datalink], which is a beautiful database containing real-world data from primary care practices,” said Ms. van Hulten, a PhD student at Maastricht (the Netherlands) University, who presented the study at the annual meeting of the American Society for Bone and Mineral Research.
Ms. van Hulten and colleagues compared SGLT2 inhibitors with dipeptidyl peptidase–4 (DPP-4) inhibitors because the latter are used in similar populations and have been shown to have no effect on fracture risk.
“What we found is that SGLT2 inhibitors are not associated with an increased fracture risk. Even with a duration of use of over 811 days, we did not observe an increased hazard ratio for fractures when compared DPP-4 inhibitor users,” Ms. van Hulten said.
SGLT2 inhibitors reduce blood sugar by increasing elimination of sugar in the urine. They also increase phosphate, reduce calcium, and increase parathyroid hormone, which could in turn negatively affect bone turnover, according to Ms. van Hulten.
In the new study, conducted between January 2013 and June 2020, the researchers used propensity score matching to compare adult patients, including 13,807 who were prescribed SGLT2 inhibitors and 28,524 who were prescribed DPP-4 inhibitors for the first time. They matched patients based on demographics, comorbidities, comedication, and lifestyle factors.
There was no association between SGLT2 inhibitor use and overall fracture risk or major osteoporotic, hip, vertebral, humerus, radius, or ulna fractures. There was no difference in risk for any duration of use, even with the longest duration of use of 811 days (adjusted hazard ratio, 1.0). There were no differences among specific SGLT2 inhibitors, including canagliflozin (aHR, 1.12; 95% confidence interval, 0.73-1.72). Analyses by sex and age also revealed no statistically significant differences between the two drug classes.
During the Q&A session after the presentation, Sarah Berry, MD, MPH, an associate professor of medicine at Harvard Medical School and a clinical researcher at the Marcus Institute for Aging Research, both in Boston, noted the trend toward an increase in fracture risk in the first 90 days. “It looked like there was something going on in the first 90 days, and then after that the results were much closer to the null. I would put out maybe another potential mechanism whereby the SGLT2 inhibitors might cause fracture, and that’s falls. They cause polyuria, and any drug you give that causes women to rush to the bathroom may well cause fractures, particularly in the short term,” Dr. Berry said.
Ms. van Hulten agreed, and also brought up that the drugs can cause osmotic diuresis. That can lead to hypovolemia, the symptoms of which include weakness, fatigue, and dizziness. “And increased falls, of course, increases fracture risk. We do not expect anything to happen to bone metabolism in the first 90 days. I think we can agree that there would be more time needed to alter the bone enough to increase fracture risk, so we expect that this trend toward an increased risk might be attributable to that increased fall risk that might occur with SGLT2 inhibitor use,” she said.
It’s possible that such a mechanism explains increased fracture risk seen in some earlier short-term studies, she added.
Overall, Ms. van Hulten said that the results should provide some confidence in SGLT2 inhibitors, though more work needs to be done. “I think we provide reassurance that SGLT2 inhibitors are safe to use. However, we still only have a median follow-up of 1.6 years. It’s not as long as we maybe would like, but it’s the best we can do with the data available, since the SGLT2 inhibitors have only been used since 2013. So maybe it’s best to prescribe it and keep [fall risk] in mind and look into the effects later on again, but it seems to be safe to use.”
The study received funding from the Novo Nordisk Foundation. Ms. van Hulten and Dr. Berry reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
VANCOUVER – In patients with type 2 diabetes, sodium-glucose cotransporter 2 (SGLT2) inhibitors as an adjunct to metformin were not associated with an increase in fracture risk, according to a new real-world study.
There have been some reports of an increase in fracture risk associated with SGLT2 inhibitors, and it was observed in the phase 3 CANVAS trial of canagliflozin (Invokana), which led to a Food and Drug Administration warning of fracture risks associated with canagliflozin use. Some ensuing studies did not show an increased risk, but these studies were generally less than a year in duration and may have missed longer-term risk, according to Veerle van Hulten, MSc.
“Fracture risk is something that takes a long time to develop, so we wanted to have a longer follow-up. We looked into the CPRD [Clinical Practice Research Datalink], which is a beautiful database containing real-world data from primary care practices,” said Ms. van Hulten, a PhD student at Maastricht (the Netherlands) University, who presented the study at the annual meeting of the American Society for Bone and Mineral Research.
Ms. van Hulten and colleagues compared SGLT2 inhibitors with dipeptidyl peptidase–4 (DPP-4) inhibitors because the latter are used in similar populations and have been shown to have no effect on fracture risk.
“What we found is that SGLT2 inhibitors are not associated with an increased fracture risk. Even with a duration of use of over 811 days, we did not observe an increased hazard ratio for fractures when compared DPP-4 inhibitor users,” Ms. van Hulten said.
SGLT2 inhibitors reduce blood sugar by increasing elimination of sugar in the urine. They also increase phosphate, reduce calcium, and increase parathyroid hormone, which could in turn negatively affect bone turnover, according to Ms. van Hulten.
In the new study, conducted between January 2013 and June 2020, the researchers used propensity score matching to compare adult patients, including 13,807 who were prescribed SGLT2 inhibitors and 28,524 who were prescribed DPP-4 inhibitors for the first time. They matched patients based on demographics, comorbidities, comedication, and lifestyle factors.
There was no association between SGLT2 inhibitor use and overall fracture risk or major osteoporotic, hip, vertebral, humerus, radius, or ulna fractures. There was no difference in risk for any duration of use, even with the longest duration of use of 811 days (adjusted hazard ratio, 1.0). There were no differences among specific SGLT2 inhibitors, including canagliflozin (aHR, 1.12; 95% confidence interval, 0.73-1.72). Analyses by sex and age also revealed no statistically significant differences between the two drug classes.
During the Q&A session after the presentation, Sarah Berry, MD, MPH, an associate professor of medicine at Harvard Medical School and a clinical researcher at the Marcus Institute for Aging Research, both in Boston, noted the trend toward an increase in fracture risk in the first 90 days. “It looked like there was something going on in the first 90 days, and then after that the results were much closer to the null. I would put out maybe another potential mechanism whereby the SGLT2 inhibitors might cause fracture, and that’s falls. They cause polyuria, and any drug you give that causes women to rush to the bathroom may well cause fractures, particularly in the short term,” Dr. Berry said.
Ms. van Hulten agreed, and also brought up that the drugs can cause osmotic diuresis. That can lead to hypovolemia, the symptoms of which include weakness, fatigue, and dizziness. “And increased falls, of course, increases fracture risk. We do not expect anything to happen to bone metabolism in the first 90 days. I think we can agree that there would be more time needed to alter the bone enough to increase fracture risk, so we expect that this trend toward an increased risk might be attributable to that increased fall risk that might occur with SGLT2 inhibitor use,” she said.
It’s possible that such a mechanism explains increased fracture risk seen in some earlier short-term studies, she added.
Overall, Ms. van Hulten said that the results should provide some confidence in SGLT2 inhibitors, though more work needs to be done. “I think we provide reassurance that SGLT2 inhibitors are safe to use. However, we still only have a median follow-up of 1.6 years. It’s not as long as we maybe would like, but it’s the best we can do with the data available, since the SGLT2 inhibitors have only been used since 2013. So maybe it’s best to prescribe it and keep [fall risk] in mind and look into the effects later on again, but it seems to be safe to use.”
The study received funding from the Novo Nordisk Foundation. Ms. van Hulten and Dr. Berry reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
VANCOUVER – In patients with type 2 diabetes, sodium-glucose cotransporter 2 (SGLT2) inhibitors as an adjunct to metformin were not associated with an increase in fracture risk, according to a new real-world study.
There have been some reports of an increase in fracture risk associated with SGLT2 inhibitors, and it was observed in the phase 3 CANVAS trial of canagliflozin (Invokana), which led to a Food and Drug Administration warning of fracture risks associated with canagliflozin use. Some ensuing studies did not show an increased risk, but these studies were generally less than a year in duration and may have missed longer-term risk, according to Veerle van Hulten, MSc.
“Fracture risk is something that takes a long time to develop, so we wanted to have a longer follow-up. We looked into the CPRD [Clinical Practice Research Datalink], which is a beautiful database containing real-world data from primary care practices,” said Ms. van Hulten, a PhD student at Maastricht (the Netherlands) University, who presented the study at the annual meeting of the American Society for Bone and Mineral Research.
Ms. van Hulten and colleagues compared SGLT2 inhibitors with dipeptidyl peptidase–4 (DPP-4) inhibitors because the latter are used in similar populations and have been shown to have no effect on fracture risk.
“What we found is that SGLT2 inhibitors are not associated with an increased fracture risk. Even with a duration of use of over 811 days, we did not observe an increased hazard ratio for fractures when compared DPP-4 inhibitor users,” Ms. van Hulten said.
SGLT2 inhibitors reduce blood sugar by increasing elimination of sugar in the urine. They also increase phosphate, reduce calcium, and increase parathyroid hormone, which could in turn negatively affect bone turnover, according to Ms. van Hulten.
In the new study, conducted between January 2013 and June 2020, the researchers used propensity score matching to compare adult patients, including 13,807 who were prescribed SGLT2 inhibitors and 28,524 who were prescribed DPP-4 inhibitors for the first time. They matched patients based on demographics, comorbidities, comedication, and lifestyle factors.
There was no association between SGLT2 inhibitor use and overall fracture risk or major osteoporotic, hip, vertebral, humerus, radius, or ulna fractures. There was no difference in risk for any duration of use, even with the longest duration of use of 811 days (adjusted hazard ratio, 1.0). There were no differences among specific SGLT2 inhibitors, including canagliflozin (aHR, 1.12; 95% confidence interval, 0.73-1.72). Analyses by sex and age also revealed no statistically significant differences between the two drug classes.
During the Q&A session after the presentation, Sarah Berry, MD, MPH, an associate professor of medicine at Harvard Medical School and a clinical researcher at the Marcus Institute for Aging Research, both in Boston, noted the trend toward an increase in fracture risk in the first 90 days. “It looked like there was something going on in the first 90 days, and then after that the results were much closer to the null. I would put out maybe another potential mechanism whereby the SGLT2 inhibitors might cause fracture, and that’s falls. They cause polyuria, and any drug you give that causes women to rush to the bathroom may well cause fractures, particularly in the short term,” Dr. Berry said.
Ms. van Hulten agreed, and also brought up that the drugs can cause osmotic diuresis. That can lead to hypovolemia, the symptoms of which include weakness, fatigue, and dizziness. “And increased falls, of course, increases fracture risk. We do not expect anything to happen to bone metabolism in the first 90 days. I think we can agree that there would be more time needed to alter the bone enough to increase fracture risk, so we expect that this trend toward an increased risk might be attributable to that increased fall risk that might occur with SGLT2 inhibitor use,” she said.
It’s possible that such a mechanism explains increased fracture risk seen in some earlier short-term studies, she added.
Overall, Ms. van Hulten said that the results should provide some confidence in SGLT2 inhibitors, though more work needs to be done. “I think we provide reassurance that SGLT2 inhibitors are safe to use. However, we still only have a median follow-up of 1.6 years. It’s not as long as we maybe would like, but it’s the best we can do with the data available, since the SGLT2 inhibitors have only been used since 2013. So maybe it’s best to prescribe it and keep [fall risk] in mind and look into the effects later on again, but it seems to be safe to use.”
The study received funding from the Novo Nordisk Foundation. Ms. van Hulten and Dr. Berry reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
AT ASBMR 2023
My pet peeves about the current state of primary care
For this month’s column, I wanted to share some frustrations I have had about the current state of primary care. We all find those things that are going on in medicine that seem crazy and we just have to find a way to adapt to them. It is good to be able to share some of these thoughts with a community as distinguished as you readers. I know some of these are issues that you all struggle with and I wanted to give a voice to them. I wish I had answers to fix them.
Faxes from insurance companies
I find faxes from insurance companies immensely annoying. First, it takes time to go through lots of unwanted faxes but these faxes are extremely inaccurate. Today I received a fax telling me I might want to consider starting a statin in my 64-year-old HIV patient who has hypertension. He has been on a statin for 10 years.
Another fax warned me to not combine ACE inhibitors and angiotensin II receptor blockers (ARBs) in a patient who was switched from an ACE inhibitor in July to an ARB because of a cough. The fax that was sent to me has a documented end date for the ACE inhibitor before the start date of the ARB.
We only have so much time in the day and piles of faxes are not helpful.
Speaking of faxes: Why do physical therapy offices and nursing homes fax the same form every day? Physicians do not always work in clinic every single day and it increases the workload and burden when you have to sort through three copies of the same fax. I once worked in a world where these would be sent by mail, and mailed back a week later, which seemed to work just fine.
Misinformation
Our patients have many sources of health information. Much of the information they get comes from family, friends, social media posts, and Internet sites. The accuracy of the information is often questionable, and in some cases, they are victims of intentional misinformation.
It is frustrating and time consuming to counter the bogus, unsubstantiated information patients receive. It is especially difficult when patients have done their own research on proven therapies (such as statins) and do not want to use them because of the many websites they have looked at that make unscientific claims about the dangers of the proposed therapy. I share evidence-based websites with my patients for their research; my favorite is medlineplus.gov.
Access crisis
The availability of specialty care is extremely limited now. In my health care system, there is up to a 6-month wait for appointments in neurology, cardiology, and endocrinology. This puts the burden on the primary care professional to manage the patient’s health, even when the patient really needs specialty care. It also increases the calls we receive to interpret the echocardiograms, MRIs, or lab tests ordered by specialists who do not share the interpretation of the results with their patients.
What can be done to improve this situation? Automatic consults in the hospital should be limited. Every patient who has a transient ischemic attack with a negative workup does not need neurology follow-up. The same goes for patients who have chest pain but a negative cardiac workup in the hospital – they do not need follow-up by a cardiologist, nor do those who have stable, well-managed coronary disease. We have to find a way to keep our specialists seeing the patients whom they can help the most and available for consultation in a timely fashion.
Please share your pet peeves with me. I will try to give them voice in the future. Hang in there, you are the glue that keeps this flawed system together.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
For this month’s column, I wanted to share some frustrations I have had about the current state of primary care. We all find those things that are going on in medicine that seem crazy and we just have to find a way to adapt to them. It is good to be able to share some of these thoughts with a community as distinguished as you readers. I know some of these are issues that you all struggle with and I wanted to give a voice to them. I wish I had answers to fix them.
Faxes from insurance companies
I find faxes from insurance companies immensely annoying. First, it takes time to go through lots of unwanted faxes but these faxes are extremely inaccurate. Today I received a fax telling me I might want to consider starting a statin in my 64-year-old HIV patient who has hypertension. He has been on a statin for 10 years.
Another fax warned me to not combine ACE inhibitors and angiotensin II receptor blockers (ARBs) in a patient who was switched from an ACE inhibitor in July to an ARB because of a cough. The fax that was sent to me has a documented end date for the ACE inhibitor before the start date of the ARB.
We only have so much time in the day and piles of faxes are not helpful.
Speaking of faxes: Why do physical therapy offices and nursing homes fax the same form every day? Physicians do not always work in clinic every single day and it increases the workload and burden when you have to sort through three copies of the same fax. I once worked in a world where these would be sent by mail, and mailed back a week later, which seemed to work just fine.
Misinformation
Our patients have many sources of health information. Much of the information they get comes from family, friends, social media posts, and Internet sites. The accuracy of the information is often questionable, and in some cases, they are victims of intentional misinformation.
It is frustrating and time consuming to counter the bogus, unsubstantiated information patients receive. It is especially difficult when patients have done their own research on proven therapies (such as statins) and do not want to use them because of the many websites they have looked at that make unscientific claims about the dangers of the proposed therapy. I share evidence-based websites with my patients for their research; my favorite is medlineplus.gov.
Access crisis
The availability of specialty care is extremely limited now. In my health care system, there is up to a 6-month wait for appointments in neurology, cardiology, and endocrinology. This puts the burden on the primary care professional to manage the patient’s health, even when the patient really needs specialty care. It also increases the calls we receive to interpret the echocardiograms, MRIs, or lab tests ordered by specialists who do not share the interpretation of the results with their patients.
What can be done to improve this situation? Automatic consults in the hospital should be limited. Every patient who has a transient ischemic attack with a negative workup does not need neurology follow-up. The same goes for patients who have chest pain but a negative cardiac workup in the hospital – they do not need follow-up by a cardiologist, nor do those who have stable, well-managed coronary disease. We have to find a way to keep our specialists seeing the patients whom they can help the most and available for consultation in a timely fashion.
Please share your pet peeves with me. I will try to give them voice in the future. Hang in there, you are the glue that keeps this flawed system together.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
For this month’s column, I wanted to share some frustrations I have had about the current state of primary care. We all find those things that are going on in medicine that seem crazy and we just have to find a way to adapt to them. It is good to be able to share some of these thoughts with a community as distinguished as you readers. I know some of these are issues that you all struggle with and I wanted to give a voice to them. I wish I had answers to fix them.
Faxes from insurance companies
I find faxes from insurance companies immensely annoying. First, it takes time to go through lots of unwanted faxes but these faxes are extremely inaccurate. Today I received a fax telling me I might want to consider starting a statin in my 64-year-old HIV patient who has hypertension. He has been on a statin for 10 years.
Another fax warned me to not combine ACE inhibitors and angiotensin II receptor blockers (ARBs) in a patient who was switched from an ACE inhibitor in July to an ARB because of a cough. The fax that was sent to me has a documented end date for the ACE inhibitor before the start date of the ARB.
We only have so much time in the day and piles of faxes are not helpful.
Speaking of faxes: Why do physical therapy offices and nursing homes fax the same form every day? Physicians do not always work in clinic every single day and it increases the workload and burden when you have to sort through three copies of the same fax. I once worked in a world where these would be sent by mail, and mailed back a week later, which seemed to work just fine.
Misinformation
Our patients have many sources of health information. Much of the information they get comes from family, friends, social media posts, and Internet sites. The accuracy of the information is often questionable, and in some cases, they are victims of intentional misinformation.
It is frustrating and time consuming to counter the bogus, unsubstantiated information patients receive. It is especially difficult when patients have done their own research on proven therapies (such as statins) and do not want to use them because of the many websites they have looked at that make unscientific claims about the dangers of the proposed therapy. I share evidence-based websites with my patients for their research; my favorite is medlineplus.gov.
Access crisis
The availability of specialty care is extremely limited now. In my health care system, there is up to a 6-month wait for appointments in neurology, cardiology, and endocrinology. This puts the burden on the primary care professional to manage the patient’s health, even when the patient really needs specialty care. It also increases the calls we receive to interpret the echocardiograms, MRIs, or lab tests ordered by specialists who do not share the interpretation of the results with their patients.
What can be done to improve this situation? Automatic consults in the hospital should be limited. Every patient who has a transient ischemic attack with a negative workup does not need neurology follow-up. The same goes for patients who have chest pain but a negative cardiac workup in the hospital – they do not need follow-up by a cardiologist, nor do those who have stable, well-managed coronary disease. We have to find a way to keep our specialists seeing the patients whom they can help the most and available for consultation in a timely fashion.
Please share your pet peeves with me. I will try to give them voice in the future. Hang in there, you are the glue that keeps this flawed system together.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
COVID, no matter the severity, linked with urologic effects in men
SARS-CoV-2 infection is linked in men with increased incidence of urinary retention, urinary tract infection (UTI), and blood in the urine, a new study finds.
Authors of the study, led by Alex Qinyang Liu, of S.H. Ho Urology Centre, at The Chinese University of Hong Kong, highlighted the clinical implications.
“Clinicians should be aware of the significantly higher incidence of LUTS [lower urinary tract symptoms] complications with COVID-19 in this patient group and understand that these urological manifestations can occur regardless of COVID-19 severity,” the authors wrote.
Findings were published online in the Journal of Internal Medicine.
They explained that current literature has included only small case series and observational studies assessing the connection between COVID-19 and male LUTS.
Nearly 18,000 patients in study
Included in this study were all male patients who used the public health care system in Hong Kong who received alpha-blocker monotherapy for LUTS from 2021 to 2022. After propensity score matching, 17,986 patients were included. Half had polymerase chain reaction–confirmed SARS-CoV-2 infection (n = 8,993).
The retrospective study compared urologic outcomes, including male benign prostatic hyperplasia (BPH) complications, and changes in medical treatment in the two groups. They compared male patients with SARS-CoV-2 infection who were taking baseline alpha blocker monotherapy for LUTS with a control group who had no SARS-CoV-2 infection.
They found that, compared with controls, the SARS-CoV-2–infected group had significantly higher incidence of retention of urine (4.55% vs. 0.86%, P < .001), hematuria (1.36% vs. 0.41%, P < .001), clinical UTI (4.31% vs. 1.49%, P < .001), culture-proven bacteriuria (9.02% vs. 1.97%, P < .001), and addition of 5-alpha reductase inhibitors (0.50% vs. 0.02%, P < .001).
Similar side effects even with asymptomatic infection
The researchers pointed out that similar incidence of retention of urine, hematuria, and addition of medication were seen even when patients had asymptomatic infection.
They added that their findings have biological plausibility because the coexpression of the proteins ACE2 and TMPRSS2 in the prostate makes it a target for SARS-CoV-2, which leads to inflammation and may help explain the primary outcomes.
“Given the high infectivity and unprecedented scale of the COVID-19 pandemic, these urological symptoms and complications represent a significant clinical burden that clinicians and urologists should be aware of,” the authors wrote.
The authors noted that the prevalence of BPH and LUTS rises with age and are among the most common urologic conditions affecting older men. “Incidentally, male patients of advanced age are also more significantly affected by COVID-19.”
The authors declare no relevant financial relationships.
SARS-CoV-2 infection is linked in men with increased incidence of urinary retention, urinary tract infection (UTI), and blood in the urine, a new study finds.
Authors of the study, led by Alex Qinyang Liu, of S.H. Ho Urology Centre, at The Chinese University of Hong Kong, highlighted the clinical implications.
“Clinicians should be aware of the significantly higher incidence of LUTS [lower urinary tract symptoms] complications with COVID-19 in this patient group and understand that these urological manifestations can occur regardless of COVID-19 severity,” the authors wrote.
Findings were published online in the Journal of Internal Medicine.
They explained that current literature has included only small case series and observational studies assessing the connection between COVID-19 and male LUTS.
Nearly 18,000 patients in study
Included in this study were all male patients who used the public health care system in Hong Kong who received alpha-blocker monotherapy for LUTS from 2021 to 2022. After propensity score matching, 17,986 patients were included. Half had polymerase chain reaction–confirmed SARS-CoV-2 infection (n = 8,993).
The retrospective study compared urologic outcomes, including male benign prostatic hyperplasia (BPH) complications, and changes in medical treatment in the two groups. They compared male patients with SARS-CoV-2 infection who were taking baseline alpha blocker monotherapy for LUTS with a control group who had no SARS-CoV-2 infection.
They found that, compared with controls, the SARS-CoV-2–infected group had significantly higher incidence of retention of urine (4.55% vs. 0.86%, P < .001), hematuria (1.36% vs. 0.41%, P < .001), clinical UTI (4.31% vs. 1.49%, P < .001), culture-proven bacteriuria (9.02% vs. 1.97%, P < .001), and addition of 5-alpha reductase inhibitors (0.50% vs. 0.02%, P < .001).
Similar side effects even with asymptomatic infection
The researchers pointed out that similar incidence of retention of urine, hematuria, and addition of medication were seen even when patients had asymptomatic infection.
They added that their findings have biological plausibility because the coexpression of the proteins ACE2 and TMPRSS2 in the prostate makes it a target for SARS-CoV-2, which leads to inflammation and may help explain the primary outcomes.
“Given the high infectivity and unprecedented scale of the COVID-19 pandemic, these urological symptoms and complications represent a significant clinical burden that clinicians and urologists should be aware of,” the authors wrote.
The authors noted that the prevalence of BPH and LUTS rises with age and are among the most common urologic conditions affecting older men. “Incidentally, male patients of advanced age are also more significantly affected by COVID-19.”
The authors declare no relevant financial relationships.
SARS-CoV-2 infection is linked in men with increased incidence of urinary retention, urinary tract infection (UTI), and blood in the urine, a new study finds.
Authors of the study, led by Alex Qinyang Liu, of S.H. Ho Urology Centre, at The Chinese University of Hong Kong, highlighted the clinical implications.
“Clinicians should be aware of the significantly higher incidence of LUTS [lower urinary tract symptoms] complications with COVID-19 in this patient group and understand that these urological manifestations can occur regardless of COVID-19 severity,” the authors wrote.
Findings were published online in the Journal of Internal Medicine.
They explained that current literature has included only small case series and observational studies assessing the connection between COVID-19 and male LUTS.
Nearly 18,000 patients in study
Included in this study were all male patients who used the public health care system in Hong Kong who received alpha-blocker monotherapy for LUTS from 2021 to 2022. After propensity score matching, 17,986 patients were included. Half had polymerase chain reaction–confirmed SARS-CoV-2 infection (n = 8,993).
The retrospective study compared urologic outcomes, including male benign prostatic hyperplasia (BPH) complications, and changes in medical treatment in the two groups. They compared male patients with SARS-CoV-2 infection who were taking baseline alpha blocker monotherapy for LUTS with a control group who had no SARS-CoV-2 infection.
They found that, compared with controls, the SARS-CoV-2–infected group had significantly higher incidence of retention of urine (4.55% vs. 0.86%, P < .001), hematuria (1.36% vs. 0.41%, P < .001), clinical UTI (4.31% vs. 1.49%, P < .001), culture-proven bacteriuria (9.02% vs. 1.97%, P < .001), and addition of 5-alpha reductase inhibitors (0.50% vs. 0.02%, P < .001).
Similar side effects even with asymptomatic infection
The researchers pointed out that similar incidence of retention of urine, hematuria, and addition of medication were seen even when patients had asymptomatic infection.
They added that their findings have biological plausibility because the coexpression of the proteins ACE2 and TMPRSS2 in the prostate makes it a target for SARS-CoV-2, which leads to inflammation and may help explain the primary outcomes.
“Given the high infectivity and unprecedented scale of the COVID-19 pandemic, these urological symptoms and complications represent a significant clinical burden that clinicians and urologists should be aware of,” the authors wrote.
The authors noted that the prevalence of BPH and LUTS rises with age and are among the most common urologic conditions affecting older men. “Incidentally, male patients of advanced age are also more significantly affected by COVID-19.”
The authors declare no relevant financial relationships.
FROM THE JOURNAL OF INTERNAL MEDICINE
Fatigue and night sweats
Given the patient's presentation of generalized lymphadenopathy, B symptoms, fatigue (probably from anemia), hepatosplenomegaly, immunophenotyping results of flow cell cytometry, and central nervous system (CNS) involvement, blastoid mantle cell lymphoma (MCL) is the most likely diagnosis. Although small lymphocytic lymphoma (SLL)/chronic lymphocytic leukemia (CLL) and diffuse large B-cell lymphoma (DLBCL) most often occur in men 60-70 years old with similar clinical findings, an initial presentation with a stage IV involvement is rare; moreover, SLL/CLL and DLBCL are typically CD23 positive. Pleomorphic MCL displays larger and more pleomorphic cells with irregular nuclei, prominent nucleoli, and pale cytoplasm, resembling DLBCL.
MCL is a rare type of mature B-cell lymphoma that was first described in 1992 and was recognized by World Health Organization in 2001. MCL represents 3%-10% of all non-Hodgkin lymphoma cases, with an incidence between 0.50 and 1.0 per 100,000 population. Men are more likely than women to present with MCL by a ratio of 3:1, with a median age at presentation of 67 years. Clinical presentation includes advanced disease with B symptoms (eg, night sweats, fever, weight loss), generalized lymphadenopathy, abdominal distention associated with hepatosplenomegaly, and fatigue. MCL usually affects the lymph nodes, with the spleen and bone marrow being significant sites of the disease. Stage IV disease is present in 70% of patients; the gastrointestinal tract, lung, pleura, and CNS are also frequently affected.
Besides classic MCL, several variants have been described that exhibit specific morphologic features, including small cell variant mimicking SLL marginal zone-like MCL (resembling marginal zone lymphoma), in situ mantle cell neoplasia (associated with indolent course), and two aggressive variants, including blastoid and pleomorphic MCL. These blastoid and pleomorphic variants are defined by cytomorphologic features; the criteria are somewhat subjective, but both are characterized by highly aggressive features and a dismal clinical course. In clinical cohorts, the frequency of these subsets varies widely but probably represents ∼10% of all cases.
Diagnosing MCL requires a multipronged approach. Lymph node biopsy and aspiration with immunophenotyping in MCL reveal monoclonal B cells expressing surface immunoglobulin, immunoglobulin M, or immunoglobulin D that are characteristically CD5+ and pan B-cell antigen positive (eg, CD19, CD20, CD22) but lack expression of CD10 and CD23 and overexpress cyclin D1. Bone marrow aspirate and biopsy are used more for staging than diagnosis. Blood studies commonly reveal anemia and cytopenias secondary to bone marrow infiltration (with 20%-40% of cases showing lymphocytosis > 4000 cells/μL), abnormal liver function tests, and elevated lactate dehydrogenase when tumor burden is high. The term "blastoid mantle cell lymphoma" describes a morphologic subgroup of lymphomas with blastic features that morphologically resemble the lymphoblasts found in lymphoblastic lymphoma/leukemia (roundish nuclei, a narrow rim of cytoplasm, and finely dispersed chromatin).
MCL is associated with a poor prognosis; patients generally experience disease progression after chemotherapy, even with initial treatment response rates ranging from 50% to 70%. The 5-year survival rate is about 50% in the overall population, 75% in persons younger than 50 years, and 36% in those aged 75 years or older. A poorer prognosis is also associated with the presence of the blastoid variant, commonly associated with TP53 mutations. Median survival can vary by as much as 5 years, depending on the expression of cyclin D1 and other proliferation signature genes.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given the patient's presentation of generalized lymphadenopathy, B symptoms, fatigue (probably from anemia), hepatosplenomegaly, immunophenotyping results of flow cell cytometry, and central nervous system (CNS) involvement, blastoid mantle cell lymphoma (MCL) is the most likely diagnosis. Although small lymphocytic lymphoma (SLL)/chronic lymphocytic leukemia (CLL) and diffuse large B-cell lymphoma (DLBCL) most often occur in men 60-70 years old with similar clinical findings, an initial presentation with a stage IV involvement is rare; moreover, SLL/CLL and DLBCL are typically CD23 positive. Pleomorphic MCL displays larger and more pleomorphic cells with irregular nuclei, prominent nucleoli, and pale cytoplasm, resembling DLBCL.
MCL is a rare type of mature B-cell lymphoma that was first described in 1992 and was recognized by World Health Organization in 2001. MCL represents 3%-10% of all non-Hodgkin lymphoma cases, with an incidence between 0.50 and 1.0 per 100,000 population. Men are more likely than women to present with MCL by a ratio of 3:1, with a median age at presentation of 67 years. Clinical presentation includes advanced disease with B symptoms (eg, night sweats, fever, weight loss), generalized lymphadenopathy, abdominal distention associated with hepatosplenomegaly, and fatigue. MCL usually affects the lymph nodes, with the spleen and bone marrow being significant sites of the disease. Stage IV disease is present in 70% of patients; the gastrointestinal tract, lung, pleura, and CNS are also frequently affected.
Besides classic MCL, several variants have been described that exhibit specific morphologic features, including small cell variant mimicking SLL marginal zone-like MCL (resembling marginal zone lymphoma), in situ mantle cell neoplasia (associated with indolent course), and two aggressive variants, including blastoid and pleomorphic MCL. These blastoid and pleomorphic variants are defined by cytomorphologic features; the criteria are somewhat subjective, but both are characterized by highly aggressive features and a dismal clinical course. In clinical cohorts, the frequency of these subsets varies widely but probably represents ∼10% of all cases.
Diagnosing MCL requires a multipronged approach. Lymph node biopsy and aspiration with immunophenotyping in MCL reveal monoclonal B cells expressing surface immunoglobulin, immunoglobulin M, or immunoglobulin D that are characteristically CD5+ and pan B-cell antigen positive (eg, CD19, CD20, CD22) but lack expression of CD10 and CD23 and overexpress cyclin D1. Bone marrow aspirate and biopsy are used more for staging than diagnosis. Blood studies commonly reveal anemia and cytopenias secondary to bone marrow infiltration (with 20%-40% of cases showing lymphocytosis > 4000 cells/μL), abnormal liver function tests, and elevated lactate dehydrogenase when tumor burden is high. The term "blastoid mantle cell lymphoma" describes a morphologic subgroup of lymphomas with blastic features that morphologically resemble the lymphoblasts found in lymphoblastic lymphoma/leukemia (roundish nuclei, a narrow rim of cytoplasm, and finely dispersed chromatin).
MCL is associated with a poor prognosis; patients generally experience disease progression after chemotherapy, even with initial treatment response rates ranging from 50% to 70%. The 5-year survival rate is about 50% in the overall population, 75% in persons younger than 50 years, and 36% in those aged 75 years or older. A poorer prognosis is also associated with the presence of the blastoid variant, commonly associated with TP53 mutations. Median survival can vary by as much as 5 years, depending on the expression of cyclin D1 and other proliferation signature genes.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given the patient's presentation of generalized lymphadenopathy, B symptoms, fatigue (probably from anemia), hepatosplenomegaly, immunophenotyping results of flow cell cytometry, and central nervous system (CNS) involvement, blastoid mantle cell lymphoma (MCL) is the most likely diagnosis. Although small lymphocytic lymphoma (SLL)/chronic lymphocytic leukemia (CLL) and diffuse large B-cell lymphoma (DLBCL) most often occur in men 60-70 years old with similar clinical findings, an initial presentation with a stage IV involvement is rare; moreover, SLL/CLL and DLBCL are typically CD23 positive. Pleomorphic MCL displays larger and more pleomorphic cells with irregular nuclei, prominent nucleoli, and pale cytoplasm, resembling DLBCL.
MCL is a rare type of mature B-cell lymphoma that was first described in 1992 and was recognized by World Health Organization in 2001. MCL represents 3%-10% of all non-Hodgkin lymphoma cases, with an incidence between 0.50 and 1.0 per 100,000 population. Men are more likely than women to present with MCL by a ratio of 3:1, with a median age at presentation of 67 years. Clinical presentation includes advanced disease with B symptoms (eg, night sweats, fever, weight loss), generalized lymphadenopathy, abdominal distention associated with hepatosplenomegaly, and fatigue. MCL usually affects the lymph nodes, with the spleen and bone marrow being significant sites of the disease. Stage IV disease is present in 70% of patients; the gastrointestinal tract, lung, pleura, and CNS are also frequently affected.
Besides classic MCL, several variants have been described that exhibit specific morphologic features, including small cell variant mimicking SLL marginal zone-like MCL (resembling marginal zone lymphoma), in situ mantle cell neoplasia (associated with indolent course), and two aggressive variants, including blastoid and pleomorphic MCL. These blastoid and pleomorphic variants are defined by cytomorphologic features; the criteria are somewhat subjective, but both are characterized by highly aggressive features and a dismal clinical course. In clinical cohorts, the frequency of these subsets varies widely but probably represents ∼10% of all cases.
Diagnosing MCL requires a multipronged approach. Lymph node biopsy and aspiration with immunophenotyping in MCL reveal monoclonal B cells expressing surface immunoglobulin, immunoglobulin M, or immunoglobulin D that are characteristically CD5+ and pan B-cell antigen positive (eg, CD19, CD20, CD22) but lack expression of CD10 and CD23 and overexpress cyclin D1. Bone marrow aspirate and biopsy are used more for staging than diagnosis. Blood studies commonly reveal anemia and cytopenias secondary to bone marrow infiltration (with 20%-40% of cases showing lymphocytosis > 4000 cells/μL), abnormal liver function tests, and elevated lactate dehydrogenase when tumor burden is high. The term "blastoid mantle cell lymphoma" describes a morphologic subgroup of lymphomas with blastic features that morphologically resemble the lymphoblasts found in lymphoblastic lymphoma/leukemia (roundish nuclei, a narrow rim of cytoplasm, and finely dispersed chromatin).
MCL is associated with a poor prognosis; patients generally experience disease progression after chemotherapy, even with initial treatment response rates ranging from 50% to 70%. The 5-year survival rate is about 50% in the overall population, 75% in persons younger than 50 years, and 36% in those aged 75 years or older. A poorer prognosis is also associated with the presence of the blastoid variant, commonly associated with TP53 mutations. Median survival can vary by as much as 5 years, depending on the expression of cyclin D1 and other proliferation signature genes.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 65-year-old man presents to the oncology clinic with a 6-week history of fatigue, night sweats, and unintentional weight loss of 15 lb. He reports occasional fevers and generalized discomfort in his abdomen and has recently been experiencing painful headaches that are not relieved with nonsteroidal anti-inflammatory drugs. His medical history is otherwise unremarkable except for mild hypertension, for which he takes medication. His family history is unremarkable.
Physical examination reveals palpable lymph nodes in the neck, axilla, and inguinal regions; the spleen is palpable 3 cm below the left costal margin. A complete blood count shows anemia (hemoglobin level, 9.1g/dL) thrombocytopenia (platelet count, 90,000 cells/μL), and lymphocytosis (total leukocyte count, 5000 cells/μL); peripheral blood smear shows small, monomorphic lymphoid cells with oval-shaped nuclei and high nuclear-to-cytoplasmic ratio. Flow cytometry of lymph node biopsy is CD5-positive and pan B-cell antigen positive (eg, CD19, CD20, and CD22) but lacks expression of CD10 and CD23. A T2-weighted MRI is ordered.
A focus on women with diabetes and their offspring
In 2021, diabetes and related complications was the 8th leading cause of death in the United States.1 As of 2022, more than 11% of the U.S. population had diabetes and 38% of the adult U.S. population had prediabetes.2 Diabetes is the most expensive chronic condition in the United States, where $1 of every $4 in health care costs is spent on care.3
Where this is most concerning is diabetes in pregnancy. While childbirth rates in the United States have decreased since the 2007 high of 4.32 million births4 to 3.66 million in 2021,5 the incidence of diabetes in pregnancy – both pregestational and gestational – has increased. The rate of pregestational diabetes in 2021 was 10.9 per 1,000 births, a 27% increase from 2016 (8.6 per 1,000).6 The percentage of those giving birth who also were diagnosed with gestational diabetes mellitus (GDM) was 8.3% in 2021, up from 6.0% in 2016.7
Adverse outcomes for an infant born to a mother with diabetes include a higher risk of obesity and diabetes as adults, potentially leading to a forward-feeding cycle.
We and our colleagues established the Diabetes in Pregnancy Study Group of North America in 1997 because we had witnessed too frequently the devastating diabetes-induced pregnancy complications in our patients. The mission we set forth was to provide a forum for dialogue among maternal-fetal medicine subspecialists. The three main goals we set forth to support this mission were to provide a catalyst for research, contribute to the creation and refinement of medical policies, and influence professional practices in diabetes in pregnancy.8
In the last quarter century, DPSG-NA, through its annual and biennial meetings, has brought together several hundred practitioners that include physicians, nurses, statisticians, researchers, nutritionists, and allied health professionals, among others. As a group, it has improved the detection and management of diabetes in pregnant women and their offspring through knowledge sharing and influencing policies on GDM screening, diagnosis, management, and treatment. Our members have shown that preconceptional counseling for women with diabetes can significantly reduce congenital malformation and perinatal mortality compared with those women with pregestational diabetes who receive no counseling.9,10
We have addressed a wide variety of topics including the paucity of data in determining the timing of delivery for women with diabetes and the Institute of Medicine/National Academy of Medicine recommendations of gestational weight gain and risks of not adhering to them. We have learned about new scientific discoveries that reveal underlying mechanisms to diabetes-related birth defects and potential therapeutic targets; and we have discussed the health literacy requirements, ethics, and opportunities for lifestyle intervention.11-16
But we need to do more.
Two risk factors are at play: Women continue to choose to have babies at later ages and their pregnancies continue to be complicated by the rising incidence of obesity (see Figure 1 and Figure 2). 
The global obesity epidemic has become a significant concern for all aspects of health and particularly for diabetes in pregnancy. 
In 1990, 24.9% of women in the United States were obese; in 2010, 35.8%; and now more than 41%. Some experts project that by 2030 more than 80% of women in the United States will be overweight or obese.21
If we are to stop this cycle of diabetes begets more diabetes, now more than ever we need to come together and accelerate the research and education around the diabetes in pregnancy. Join us at this year’s DPSG-NA meeting Oct. 26-28 to take part in the knowledge sharing, discussions, and planning. More information can be found online at https://events.dpsg-na.com/home.
Dr. Miodovnik is adjunct professor of obstetrics, gynecology, and reproductive sciences at University of Maryland School of Medicine. Dr. Reece is professor of obstetrics, gynecology, and reproductive sciences and senior scientist at the Center for Birth Defects Research at University of Maryland School of Medicine.
References
1. Xu J et al. Mortality in the United States, 2021. NCHS Data Brief. 2022 Dec;(456):1-8. PMID: 36598387.
2. Centers for Disease Control and Prevention, diabetes data and statistics.
3. American Diabetes Association. The Cost of Diabetes.
4. Martin JA et al. Births: Final data for 2007. Natl Vital Stat Rep. 2010 Aug 9;58(24):1-85. PMID: 21254725.
5. Osterman MJK et al. Births: Final data for 2021. Natl Vital Stat Rep. 2023 Jan;72(1):1-53. PMID: 36723449.
6. Gregory ECW and Ely DM. Trends and characteristics in prepregnancy diabetes: United States, 2016-2021. Natl Vital Stat Rep. 2023 May;72(6):1-13. PMID: 37256333.
7. QuickStats: Percentage of mothers with gestational diabetes, by maternal age – National Vital Statistics System, United States, 2016 and 2021. MMWR Morb Mortal Wkly Rep. 2023 Jan 6;72(1):16. doi: 10.15585/mmwr.mm7201a4.
8. Langer O et al. The Diabetes in Pregnancy Study Group of North America – Introduction and summary statement. Prenat Neonat Med. 1998;3(6):514-6.
9. Willhoite MB et al. The impact of preconception counseling on pregnancy outcomes. The experience of the Maine Diabetes in Pregnancy Program. Diabetes Care. 1993 Feb;16(2):450-5. doi: 10.2337/diacare.16.2.450.
10. McElvy SS et al. A focused preconceptional and early pregnancy program in women with type 1 diabetes reduces perinatal mortality and malformation rates to general population levels. J Matern Fetal Med. 2000 Jan-Feb;9(1):14-20. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<14::AID-MFM5>3.0.CO;2-K.
11. Rosen JA et al. The history and contributions of the Diabetes in Pregnancy Study Group of North America (1997-2015). Am J Perinatol. 2016 Nov;33(13):1223-6. doi: 10.1055/s-0036-1585082.
12. Driggers RW and Baschat A. The 12th meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA): Introduction and overview. J Matern Fetal Neonatal Med. 2012 Jan;25(1):3-4. doi: 10.3109/14767058.2012.626917.
13. Langer O et al. The proceedings of the Diabetes in Pregnancy Study Group of North America 2009 conference. J Matern Fetal Neonatal Med. 2010 Mar;23(3):196-8. doi: 10.3109/14767050903550634.
14. Reece EA et al. A consensus report of the Diabetes in Pregnancy Study Group of North America Conference, Little Rock, Ark., May 2002. J Matern Fetal Neonatal Med. 2002 Dec;12(6):362-4. doi: 10.1080/jmf.12.6.362.364.
15. Reece EA and Maulik D. A consensus conference of the Diabetes in Pregnancy Study Group of North America. J Matern Fetal Neonatal Med. 2002 Dec;12(6):361. doi: 10.1080/jmf.12.6.361.361.
16. Gabbe SG. Summation of the second meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA). J Matern Fetal Med. 2000 Jan-Feb;9(1):3-9.
17. Vital Statistics of the United States 1990: Volume I – Natality.
18. Martin JA et al. Births: final data for 2000. Natl Vital Stat Rep. 2002 Feb 12;50(5):1-101. PMID: 11876093.
19. Martin JA et al. Births: final data for 2010. Natl Vital Stat Rep. 2012 Aug 28;61(1):1-72. PMID: 24974589.
20. CDC Website. Normal weight, overweight, and obesity among adults aged 20 and over, by selected characteristics: United States.
21. Wang Y et al. Has the prevalence of overweight, obesity, and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int J Epidemiol. 2020 Jun 1;49(3):810-23. doi: 10.1093/ije/dyz273.
In 2021, diabetes and related complications was the 8th leading cause of death in the United States.1 As of 2022, more than 11% of the U.S. population had diabetes and 38% of the adult U.S. population had prediabetes.2 Diabetes is the most expensive chronic condition in the United States, where $1 of every $4 in health care costs is spent on care.3
Where this is most concerning is diabetes in pregnancy. While childbirth rates in the United States have decreased since the 2007 high of 4.32 million births4 to 3.66 million in 2021,5 the incidence of diabetes in pregnancy – both pregestational and gestational – has increased. The rate of pregestational diabetes in 2021 was 10.9 per 1,000 births, a 27% increase from 2016 (8.6 per 1,000).6 The percentage of those giving birth who also were diagnosed with gestational diabetes mellitus (GDM) was 8.3% in 2021, up from 6.0% in 2016.7
Adverse outcomes for an infant born to a mother with diabetes include a higher risk of obesity and diabetes as adults, potentially leading to a forward-feeding cycle.
We and our colleagues established the Diabetes in Pregnancy Study Group of North America in 1997 because we had witnessed too frequently the devastating diabetes-induced pregnancy complications in our patients. The mission we set forth was to provide a forum for dialogue among maternal-fetal medicine subspecialists. The three main goals we set forth to support this mission were to provide a catalyst for research, contribute to the creation and refinement of medical policies, and influence professional practices in diabetes in pregnancy.8
In the last quarter century, DPSG-NA, through its annual and biennial meetings, has brought together several hundred practitioners that include physicians, nurses, statisticians, researchers, nutritionists, and allied health professionals, among others. As a group, it has improved the detection and management of diabetes in pregnant women and their offspring through knowledge sharing and influencing policies on GDM screening, diagnosis, management, and treatment. Our members have shown that preconceptional counseling for women with diabetes can significantly reduce congenital malformation and perinatal mortality compared with those women with pregestational diabetes who receive no counseling.9,10
We have addressed a wide variety of topics including the paucity of data in determining the timing of delivery for women with diabetes and the Institute of Medicine/National Academy of Medicine recommendations of gestational weight gain and risks of not adhering to them. We have learned about new scientific discoveries that reveal underlying mechanisms to diabetes-related birth defects and potential therapeutic targets; and we have discussed the health literacy requirements, ethics, and opportunities for lifestyle intervention.11-16
But we need to do more.
Two risk factors are at play: Women continue to choose to have babies at later ages and their pregnancies continue to be complicated by the rising incidence of obesity (see Figure 1 and Figure 2). 
The global obesity epidemic has become a significant concern for all aspects of health and particularly for diabetes in pregnancy. 
In 1990, 24.9% of women in the United States were obese; in 2010, 35.8%; and now more than 41%. Some experts project that by 2030 more than 80% of women in the United States will be overweight or obese.21
If we are to stop this cycle of diabetes begets more diabetes, now more than ever we need to come together and accelerate the research and education around the diabetes in pregnancy. Join us at this year’s DPSG-NA meeting Oct. 26-28 to take part in the knowledge sharing, discussions, and planning. More information can be found online at https://events.dpsg-na.com/home.
Dr. Miodovnik is adjunct professor of obstetrics, gynecology, and reproductive sciences at University of Maryland School of Medicine. Dr. Reece is professor of obstetrics, gynecology, and reproductive sciences and senior scientist at the Center for Birth Defects Research at University of Maryland School of Medicine.
References
1. Xu J et al. Mortality in the United States, 2021. NCHS Data Brief. 2022 Dec;(456):1-8. PMID: 36598387.
2. Centers for Disease Control and Prevention, diabetes data and statistics.
3. American Diabetes Association. The Cost of Diabetes.
4. Martin JA et al. Births: Final data for 2007. Natl Vital Stat Rep. 2010 Aug 9;58(24):1-85. PMID: 21254725.
5. Osterman MJK et al. Births: Final data for 2021. Natl Vital Stat Rep. 2023 Jan;72(1):1-53. PMID: 36723449.
6. Gregory ECW and Ely DM. Trends and characteristics in prepregnancy diabetes: United States, 2016-2021. Natl Vital Stat Rep. 2023 May;72(6):1-13. PMID: 37256333.
7. QuickStats: Percentage of mothers with gestational diabetes, by maternal age – National Vital Statistics System, United States, 2016 and 2021. MMWR Morb Mortal Wkly Rep. 2023 Jan 6;72(1):16. doi: 10.15585/mmwr.mm7201a4.
8. Langer O et al. The Diabetes in Pregnancy Study Group of North America – Introduction and summary statement. Prenat Neonat Med. 1998;3(6):514-6.
9. Willhoite MB et al. The impact of preconception counseling on pregnancy outcomes. The experience of the Maine Diabetes in Pregnancy Program. Diabetes Care. 1993 Feb;16(2):450-5. doi: 10.2337/diacare.16.2.450.
10. McElvy SS et al. A focused preconceptional and early pregnancy program in women with type 1 diabetes reduces perinatal mortality and malformation rates to general population levels. J Matern Fetal Med. 2000 Jan-Feb;9(1):14-20. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<14::AID-MFM5>3.0.CO;2-K.
11. Rosen JA et al. The history and contributions of the Diabetes in Pregnancy Study Group of North America (1997-2015). Am J Perinatol. 2016 Nov;33(13):1223-6. doi: 10.1055/s-0036-1585082.
12. Driggers RW and Baschat A. The 12th meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA): Introduction and overview. J Matern Fetal Neonatal Med. 2012 Jan;25(1):3-4. doi: 10.3109/14767058.2012.626917.
13. Langer O et al. The proceedings of the Diabetes in Pregnancy Study Group of North America 2009 conference. J Matern Fetal Neonatal Med. 2010 Mar;23(3):196-8. doi: 10.3109/14767050903550634.
14. Reece EA et al. A consensus report of the Diabetes in Pregnancy Study Group of North America Conference, Little Rock, Ark., May 2002. J Matern Fetal Neonatal Med. 2002 Dec;12(6):362-4. doi: 10.1080/jmf.12.6.362.364.
15. Reece EA and Maulik D. A consensus conference of the Diabetes in Pregnancy Study Group of North America. J Matern Fetal Neonatal Med. 2002 Dec;12(6):361. doi: 10.1080/jmf.12.6.361.361.
16. Gabbe SG. Summation of the second meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA). J Matern Fetal Med. 2000 Jan-Feb;9(1):3-9.
17. Vital Statistics of the United States 1990: Volume I – Natality.
18. Martin JA et al. Births: final data for 2000. Natl Vital Stat Rep. 2002 Feb 12;50(5):1-101. PMID: 11876093.
19. Martin JA et al. Births: final data for 2010. Natl Vital Stat Rep. 2012 Aug 28;61(1):1-72. PMID: 24974589.
20. CDC Website. Normal weight, overweight, and obesity among adults aged 20 and over, by selected characteristics: United States.
21. Wang Y et al. Has the prevalence of overweight, obesity, and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int J Epidemiol. 2020 Jun 1;49(3):810-23. doi: 10.1093/ije/dyz273.
In 2021, diabetes and related complications was the 8th leading cause of death in the United States.1 As of 2022, more than 11% of the U.S. population had diabetes and 38% of the adult U.S. population had prediabetes.2 Diabetes is the most expensive chronic condition in the United States, where $1 of every $4 in health care costs is spent on care.3
Where this is most concerning is diabetes in pregnancy. While childbirth rates in the United States have decreased since the 2007 high of 4.32 million births4 to 3.66 million in 2021,5 the incidence of diabetes in pregnancy – both pregestational and gestational – has increased. The rate of pregestational diabetes in 2021 was 10.9 per 1,000 births, a 27% increase from 2016 (8.6 per 1,000).6 The percentage of those giving birth who also were diagnosed with gestational diabetes mellitus (GDM) was 8.3% in 2021, up from 6.0% in 2016.7
Adverse outcomes for an infant born to a mother with diabetes include a higher risk of obesity and diabetes as adults, potentially leading to a forward-feeding cycle.
We and our colleagues established the Diabetes in Pregnancy Study Group of North America in 1997 because we had witnessed too frequently the devastating diabetes-induced pregnancy complications in our patients. The mission we set forth was to provide a forum for dialogue among maternal-fetal medicine subspecialists. The three main goals we set forth to support this mission were to provide a catalyst for research, contribute to the creation and refinement of medical policies, and influence professional practices in diabetes in pregnancy.8
In the last quarter century, DPSG-NA, through its annual and biennial meetings, has brought together several hundred practitioners that include physicians, nurses, statisticians, researchers, nutritionists, and allied health professionals, among others. As a group, it has improved the detection and management of diabetes in pregnant women and their offspring through knowledge sharing and influencing policies on GDM screening, diagnosis, management, and treatment. Our members have shown that preconceptional counseling for women with diabetes can significantly reduce congenital malformation and perinatal mortality compared with those women with pregestational diabetes who receive no counseling.9,10
We have addressed a wide variety of topics including the paucity of data in determining the timing of delivery for women with diabetes and the Institute of Medicine/National Academy of Medicine recommendations of gestational weight gain and risks of not adhering to them. We have learned about new scientific discoveries that reveal underlying mechanisms to diabetes-related birth defects and potential therapeutic targets; and we have discussed the health literacy requirements, ethics, and opportunities for lifestyle intervention.11-16
But we need to do more.
Two risk factors are at play: Women continue to choose to have babies at later ages and their pregnancies continue to be complicated by the rising incidence of obesity (see Figure 1 and Figure 2). 
The global obesity epidemic has become a significant concern for all aspects of health and particularly for diabetes in pregnancy. 
In 1990, 24.9% of women in the United States were obese; in 2010, 35.8%; and now more than 41%. Some experts project that by 2030 more than 80% of women in the United States will be overweight or obese.21
If we are to stop this cycle of diabetes begets more diabetes, now more than ever we need to come together and accelerate the research and education around the diabetes in pregnancy. Join us at this year’s DPSG-NA meeting Oct. 26-28 to take part in the knowledge sharing, discussions, and planning. More information can be found online at https://events.dpsg-na.com/home.
Dr. Miodovnik is adjunct professor of obstetrics, gynecology, and reproductive sciences at University of Maryland School of Medicine. Dr. Reece is professor of obstetrics, gynecology, and reproductive sciences and senior scientist at the Center for Birth Defects Research at University of Maryland School of Medicine.
References
1. Xu J et al. Mortality in the United States, 2021. NCHS Data Brief. 2022 Dec;(456):1-8. PMID: 36598387.
2. Centers for Disease Control and Prevention, diabetes data and statistics.
3. American Diabetes Association. The Cost of Diabetes.
4. Martin JA et al. Births: Final data for 2007. Natl Vital Stat Rep. 2010 Aug 9;58(24):1-85. PMID: 21254725.
5. Osterman MJK et al. Births: Final data for 2021. Natl Vital Stat Rep. 2023 Jan;72(1):1-53. PMID: 36723449.
6. Gregory ECW and Ely DM. Trends and characteristics in prepregnancy diabetes: United States, 2016-2021. Natl Vital Stat Rep. 2023 May;72(6):1-13. PMID: 37256333.
7. QuickStats: Percentage of mothers with gestational diabetes, by maternal age – National Vital Statistics System, United States, 2016 and 2021. MMWR Morb Mortal Wkly Rep. 2023 Jan 6;72(1):16. doi: 10.15585/mmwr.mm7201a4.
8. Langer O et al. The Diabetes in Pregnancy Study Group of North America – Introduction and summary statement. Prenat Neonat Med. 1998;3(6):514-6.
9. Willhoite MB et al. The impact of preconception counseling on pregnancy outcomes. The experience of the Maine Diabetes in Pregnancy Program. Diabetes Care. 1993 Feb;16(2):450-5. doi: 10.2337/diacare.16.2.450.
10. McElvy SS et al. A focused preconceptional and early pregnancy program in women with type 1 diabetes reduces perinatal mortality and malformation rates to general population levels. J Matern Fetal Med. 2000 Jan-Feb;9(1):14-20. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<14::AID-MFM5>3.0.CO;2-K.
11. Rosen JA et al. The history and contributions of the Diabetes in Pregnancy Study Group of North America (1997-2015). Am J Perinatol. 2016 Nov;33(13):1223-6. doi: 10.1055/s-0036-1585082.
12. Driggers RW and Baschat A. The 12th meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA): Introduction and overview. J Matern Fetal Neonatal Med. 2012 Jan;25(1):3-4. doi: 10.3109/14767058.2012.626917.
13. Langer O et al. The proceedings of the Diabetes in Pregnancy Study Group of North America 2009 conference. J Matern Fetal Neonatal Med. 2010 Mar;23(3):196-8. doi: 10.3109/14767050903550634.
14. Reece EA et al. A consensus report of the Diabetes in Pregnancy Study Group of North America Conference, Little Rock, Ark., May 2002. J Matern Fetal Neonatal Med. 2002 Dec;12(6):362-4. doi: 10.1080/jmf.12.6.362.364.
15. Reece EA and Maulik D. A consensus conference of the Diabetes in Pregnancy Study Group of North America. J Matern Fetal Neonatal Med. 2002 Dec;12(6):361. doi: 10.1080/jmf.12.6.361.361.
16. Gabbe SG. Summation of the second meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA). J Matern Fetal Med. 2000 Jan-Feb;9(1):3-9.
17. Vital Statistics of the United States 1990: Volume I – Natality.
18. Martin JA et al. Births: final data for 2000. Natl Vital Stat Rep. 2002 Feb 12;50(5):1-101. PMID: 11876093.
19. Martin JA et al. Births: final data for 2010. Natl Vital Stat Rep. 2012 Aug 28;61(1):1-72. PMID: 24974589.
20. CDC Website. Normal weight, overweight, and obesity among adults aged 20 and over, by selected characteristics: United States.
21. Wang Y et al. Has the prevalence of overweight, obesity, and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int J Epidemiol. 2020 Jun 1;49(3):810-23. doi: 10.1093/ije/dyz273.