User login
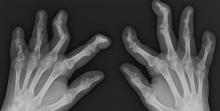
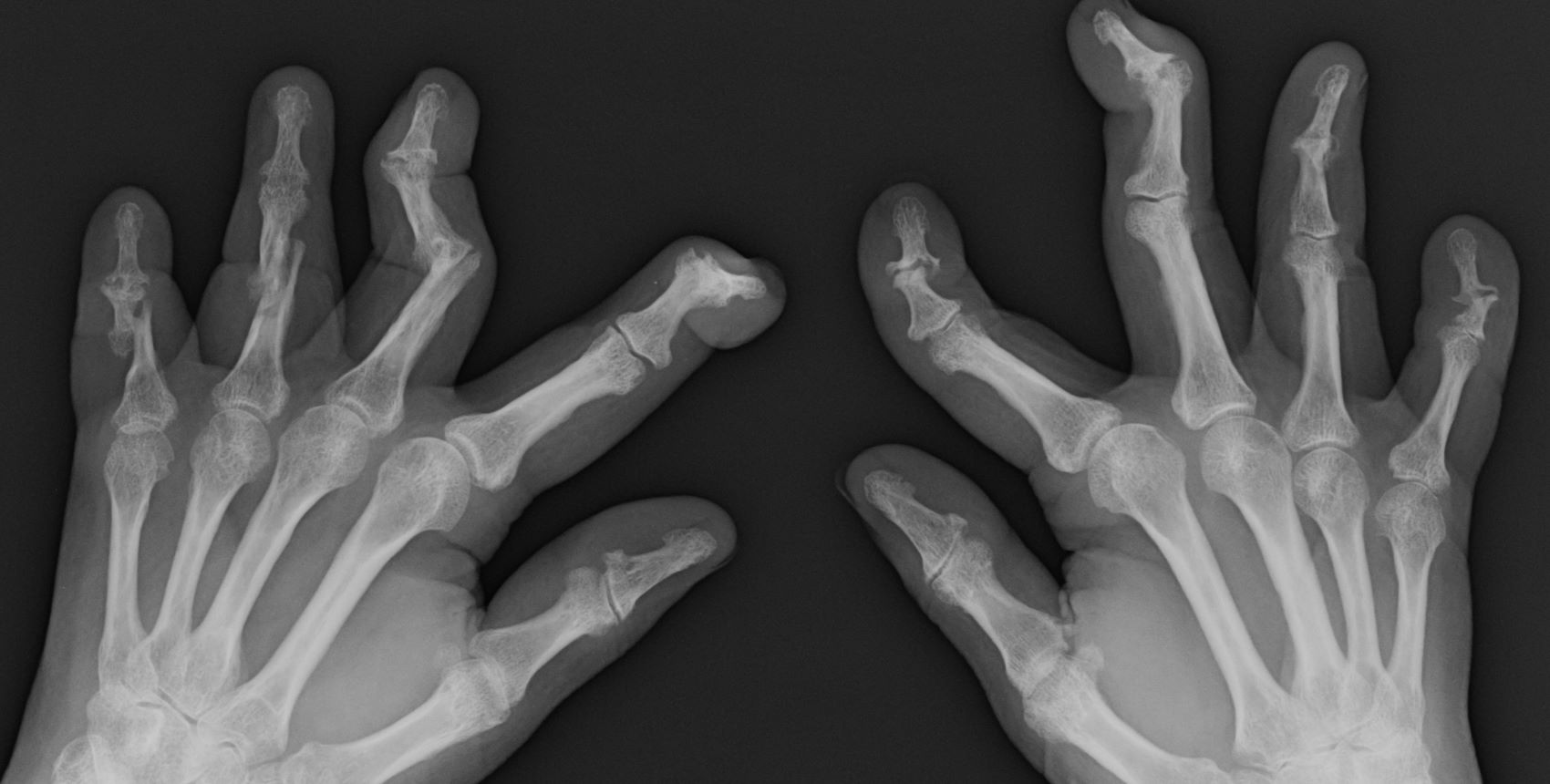
Remote symptom monitoring in advanced cancer improves quality of life
During treatment for metastatic cancer, remote monitoring of symptoms using electronic patient-reported outcomes (ePROs) reduced health care visits and improved patients’ physical function and quality of life, but did not impact overall survival, according to findings from the PRO-TECT trial.
said Ethan Basch, MD, University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill, who presented the findings at the annual meeting of the European Society for Medical Oncology.
Jiyoung Ahn, PhD, professor of population health at NYU Langone Health and associate director of population science, NYU Langone Perlmutter Cancer Center, both in New York, said this study “provides exciting scientific evidence” supporting real-time, remote monitoring of PROs. Dr. Ahn was not involved with the PRO-TECT trial.
Symptoms among patients with advanced cancer receiving treatment are “exceedingly common,” Dr. Basch explained, but “unfortunately, evidence demonstrates that we as clinicians miss up to 50% of our patients’ symptoms with potential serious downstream consequences.”
Remote monitoring with ePROs can help clinicians detect patients’ symptoms early so they can intervene early.
In the PRO-TECT cluster-randomized trial, 52 oncology practices in the United States were randomly assigned (1:1) to remote monitoring with ePRO surveys or usual care. The cohort included 1,191 patients with metastatic cancer – with 593 patients at PRO practices and 598 patients at control practices. Participating practices could enroll up to 50 patients with any type of metastatic cancer, except for indolent lymphoma or acute leukemia, who were receiving systemic treatment.
Patients in the ePRO practices completed weekly surveys either online or using an automated telephone system for up to 1 year. The survey included questions related to nine common symptoms, performance status, and falls.
For symptoms that are severe or worsening, a real-time alert goes to the care team through the electronic health record or by an email, Dr. Basch explained. Similarly, reports highlighting the longitudinal trajectory of symptoms can be generated at patient visits and reviewed by clinicians, which can bring “the patient and the care team closer together by elevating those issues that are particularly salient to the patient’s experience,” he noted.
Patients completed over 91% of the electronic symptom surveys. After 24 months, the team observed no significant difference in the primary outcome of overall survival – 42.0 months with ePRO vs. 43.5 months with usual care (hazard ratio, 0.99; P = .86).
Dr. Basch and colleagues did, however, observe a 6% reduction in emergency or hospital admissions in the ePRO group, compared with usual care. The ePRO group also had a significantly longer time to first emergency admission (HR, 0.84; P = .03) and a decreased average number of admissions per patient over 1 year (1.48 vs. 1.81; P = .006).
At multiple time points, the team also observed “clinically meaningful and statistically significant” benefits in physical functioning, symptom control, and health-related quality of life, Dr. Basch reported. More patients in the ePRO than the usual-care group experienced benefits in fatigue (odds ratio, 1.77; P < .001), anorexia (OR, 1.32; P = .03), nausea/vomiting (OR, 1.40; P = .01), and sleep (OR, 1.73; P < .001).
Patients’ impressions of the ePRO symptom monitoring system were also “overwhelmingly” positive, Dr. Basch said. Most found the questions relevant and easy to understand and felt that their care team used the information, which made patients feel more in control of their care.
Nurses generally had a favorable impression of the system, with the majority stating that the information was helpful for electronic health record documentation and that it improved discussions with their patients and improved their efficiency.
However, about one-quarter of the nurses expressed reluctance about continuing to use the system, citing the “added work of the ePROs, particularly alerts that were triggered that prompted them to call their patients, particularly during the pandemic when nurses in the United States were pulled in many directions,” Dr. Basch said.
He noted that future ePRO implementations should aim to integrate ePROs into care processes and adjust nurse responsibilities to allow time for ePRO work.
It will also be important to offer a variety of ePRO platforms that are easily accessible for different patient groups. “Notably,” said Dr. Basch, about one-third of the patients selected the automated telephone option. These were largely patients living in rural areas of the United States with lower socioeconomic status and lower health literacy, “suggesting that we need to think about our technologies to meet patients where they are,” he said.
Despite the positive outcomes, there are “challenges to widespread adoption,” agreed NYU’s Dr. Ahn.
These challenges include the need for physician adaptation to new technologies, data security, and ensuring patient engagement and compliance with remote monitoring systems.
“Successfully addressing these challenges is crucial for optimizing the integration of ePROs into cancer care,” Dr. Ahn said.
ESMO’s invited discussant, Anne Letsch, MD, noted that “cancer therapies are getting more complex, and it’s important that patients are well informed and empowered to get together with the treatment teams throughout therapy.”
The high completion rate with ePRO symptom surveys was “quite remarkable,” said Dr. Letsch, head of the Cancer Center at the University Hospital Schleswig Holstein, Kiel, Germany.
But, Dr. Letsch said, it’s “a pity” that there was no overall survival benefit among patients in the ePRO group. Perhaps overall survival is not what matters most in this context, she said. Instead, she asked, “are other outcomes, like health-related quality of life, symptom control and treatment safety, much more important?”
Dr. Basch also questioned whether the survival differences between the two groups may have been blunted because a substantial portion of the trial was conducted during the COVID-19 pandemic, when medical resources and treatments were delayed and diverted.
Dr. Basch pointed to a 2017 study he and colleagues conducted at a single tertiary care medical center, in which patients monitored with ePROs did demonstrate an overall survival benefit, compared with usual care.
Overall, though, the study demonstrated that “symptom monitoring with ePROs is feasible during routine treatment for advanced cancers across diverse practices in the U.S.” and improved patients’ quality of life, Dr. Basch said.
Funding for the study was provided by a grant from the Patient-Centered Outcomes Research Institute. Dr. Basch has disclosed relationships with Resilience Health, Sivan Health, Navigating Cancer, and AstraZeneca. Dr. Letsch and Dr. Ahn report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
During treatment for metastatic cancer, remote monitoring of symptoms using electronic patient-reported outcomes (ePROs) reduced health care visits and improved patients’ physical function and quality of life, but did not impact overall survival, according to findings from the PRO-TECT trial.
said Ethan Basch, MD, University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill, who presented the findings at the annual meeting of the European Society for Medical Oncology.
Jiyoung Ahn, PhD, professor of population health at NYU Langone Health and associate director of population science, NYU Langone Perlmutter Cancer Center, both in New York, said this study “provides exciting scientific evidence” supporting real-time, remote monitoring of PROs. Dr. Ahn was not involved with the PRO-TECT trial.
Symptoms among patients with advanced cancer receiving treatment are “exceedingly common,” Dr. Basch explained, but “unfortunately, evidence demonstrates that we as clinicians miss up to 50% of our patients’ symptoms with potential serious downstream consequences.”
Remote monitoring with ePROs can help clinicians detect patients’ symptoms early so they can intervene early.
In the PRO-TECT cluster-randomized trial, 52 oncology practices in the United States were randomly assigned (1:1) to remote monitoring with ePRO surveys or usual care. The cohort included 1,191 patients with metastatic cancer – with 593 patients at PRO practices and 598 patients at control practices. Participating practices could enroll up to 50 patients with any type of metastatic cancer, except for indolent lymphoma or acute leukemia, who were receiving systemic treatment.
Patients in the ePRO practices completed weekly surveys either online or using an automated telephone system for up to 1 year. The survey included questions related to nine common symptoms, performance status, and falls.
For symptoms that are severe or worsening, a real-time alert goes to the care team through the electronic health record or by an email, Dr. Basch explained. Similarly, reports highlighting the longitudinal trajectory of symptoms can be generated at patient visits and reviewed by clinicians, which can bring “the patient and the care team closer together by elevating those issues that are particularly salient to the patient’s experience,” he noted.
Patients completed over 91% of the electronic symptom surveys. After 24 months, the team observed no significant difference in the primary outcome of overall survival – 42.0 months with ePRO vs. 43.5 months with usual care (hazard ratio, 0.99; P = .86).
Dr. Basch and colleagues did, however, observe a 6% reduction in emergency or hospital admissions in the ePRO group, compared with usual care. The ePRO group also had a significantly longer time to first emergency admission (HR, 0.84; P = .03) and a decreased average number of admissions per patient over 1 year (1.48 vs. 1.81; P = .006).
At multiple time points, the team also observed “clinically meaningful and statistically significant” benefits in physical functioning, symptom control, and health-related quality of life, Dr. Basch reported. More patients in the ePRO than the usual-care group experienced benefits in fatigue (odds ratio, 1.77; P < .001), anorexia (OR, 1.32; P = .03), nausea/vomiting (OR, 1.40; P = .01), and sleep (OR, 1.73; P < .001).
Patients’ impressions of the ePRO symptom monitoring system were also “overwhelmingly” positive, Dr. Basch said. Most found the questions relevant and easy to understand and felt that their care team used the information, which made patients feel more in control of their care.
Nurses generally had a favorable impression of the system, with the majority stating that the information was helpful for electronic health record documentation and that it improved discussions with their patients and improved their efficiency.
However, about one-quarter of the nurses expressed reluctance about continuing to use the system, citing the “added work of the ePROs, particularly alerts that were triggered that prompted them to call their patients, particularly during the pandemic when nurses in the United States were pulled in many directions,” Dr. Basch said.
He noted that future ePRO implementations should aim to integrate ePROs into care processes and adjust nurse responsibilities to allow time for ePRO work.
It will also be important to offer a variety of ePRO platforms that are easily accessible for different patient groups. “Notably,” said Dr. Basch, about one-third of the patients selected the automated telephone option. These were largely patients living in rural areas of the United States with lower socioeconomic status and lower health literacy, “suggesting that we need to think about our technologies to meet patients where they are,” he said.
Despite the positive outcomes, there are “challenges to widespread adoption,” agreed NYU’s Dr. Ahn.
These challenges include the need for physician adaptation to new technologies, data security, and ensuring patient engagement and compliance with remote monitoring systems.
“Successfully addressing these challenges is crucial for optimizing the integration of ePROs into cancer care,” Dr. Ahn said.
ESMO’s invited discussant, Anne Letsch, MD, noted that “cancer therapies are getting more complex, and it’s important that patients are well informed and empowered to get together with the treatment teams throughout therapy.”
The high completion rate with ePRO symptom surveys was “quite remarkable,” said Dr. Letsch, head of the Cancer Center at the University Hospital Schleswig Holstein, Kiel, Germany.
But, Dr. Letsch said, it’s “a pity” that there was no overall survival benefit among patients in the ePRO group. Perhaps overall survival is not what matters most in this context, she said. Instead, she asked, “are other outcomes, like health-related quality of life, symptom control and treatment safety, much more important?”
Dr. Basch also questioned whether the survival differences between the two groups may have been blunted because a substantial portion of the trial was conducted during the COVID-19 pandemic, when medical resources and treatments were delayed and diverted.
Dr. Basch pointed to a 2017 study he and colleagues conducted at a single tertiary care medical center, in which patients monitored with ePROs did demonstrate an overall survival benefit, compared with usual care.
Overall, though, the study demonstrated that “symptom monitoring with ePROs is feasible during routine treatment for advanced cancers across diverse practices in the U.S.” and improved patients’ quality of life, Dr. Basch said.
Funding for the study was provided by a grant from the Patient-Centered Outcomes Research Institute. Dr. Basch has disclosed relationships with Resilience Health, Sivan Health, Navigating Cancer, and AstraZeneca. Dr. Letsch and Dr. Ahn report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
During treatment for metastatic cancer, remote monitoring of symptoms using electronic patient-reported outcomes (ePROs) reduced health care visits and improved patients’ physical function and quality of life, but did not impact overall survival, according to findings from the PRO-TECT trial.
said Ethan Basch, MD, University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill, who presented the findings at the annual meeting of the European Society for Medical Oncology.
Jiyoung Ahn, PhD, professor of population health at NYU Langone Health and associate director of population science, NYU Langone Perlmutter Cancer Center, both in New York, said this study “provides exciting scientific evidence” supporting real-time, remote monitoring of PROs. Dr. Ahn was not involved with the PRO-TECT trial.
Symptoms among patients with advanced cancer receiving treatment are “exceedingly common,” Dr. Basch explained, but “unfortunately, evidence demonstrates that we as clinicians miss up to 50% of our patients’ symptoms with potential serious downstream consequences.”
Remote monitoring with ePROs can help clinicians detect patients’ symptoms early so they can intervene early.
In the PRO-TECT cluster-randomized trial, 52 oncology practices in the United States were randomly assigned (1:1) to remote monitoring with ePRO surveys or usual care. The cohort included 1,191 patients with metastatic cancer – with 593 patients at PRO practices and 598 patients at control practices. Participating practices could enroll up to 50 patients with any type of metastatic cancer, except for indolent lymphoma or acute leukemia, who were receiving systemic treatment.
Patients in the ePRO practices completed weekly surveys either online or using an automated telephone system for up to 1 year. The survey included questions related to nine common symptoms, performance status, and falls.
For symptoms that are severe or worsening, a real-time alert goes to the care team through the electronic health record or by an email, Dr. Basch explained. Similarly, reports highlighting the longitudinal trajectory of symptoms can be generated at patient visits and reviewed by clinicians, which can bring “the patient and the care team closer together by elevating those issues that are particularly salient to the patient’s experience,” he noted.
Patients completed over 91% of the electronic symptom surveys. After 24 months, the team observed no significant difference in the primary outcome of overall survival – 42.0 months with ePRO vs. 43.5 months with usual care (hazard ratio, 0.99; P = .86).
Dr. Basch and colleagues did, however, observe a 6% reduction in emergency or hospital admissions in the ePRO group, compared with usual care. The ePRO group also had a significantly longer time to first emergency admission (HR, 0.84; P = .03) and a decreased average number of admissions per patient over 1 year (1.48 vs. 1.81; P = .006).
At multiple time points, the team also observed “clinically meaningful and statistically significant” benefits in physical functioning, symptom control, and health-related quality of life, Dr. Basch reported. More patients in the ePRO than the usual-care group experienced benefits in fatigue (odds ratio, 1.77; P < .001), anorexia (OR, 1.32; P = .03), nausea/vomiting (OR, 1.40; P = .01), and sleep (OR, 1.73; P < .001).
Patients’ impressions of the ePRO symptom monitoring system were also “overwhelmingly” positive, Dr. Basch said. Most found the questions relevant and easy to understand and felt that their care team used the information, which made patients feel more in control of their care.
Nurses generally had a favorable impression of the system, with the majority stating that the information was helpful for electronic health record documentation and that it improved discussions with their patients and improved their efficiency.
However, about one-quarter of the nurses expressed reluctance about continuing to use the system, citing the “added work of the ePROs, particularly alerts that were triggered that prompted them to call their patients, particularly during the pandemic when nurses in the United States were pulled in many directions,” Dr. Basch said.
He noted that future ePRO implementations should aim to integrate ePROs into care processes and adjust nurse responsibilities to allow time for ePRO work.
It will also be important to offer a variety of ePRO platforms that are easily accessible for different patient groups. “Notably,” said Dr. Basch, about one-third of the patients selected the automated telephone option. These were largely patients living in rural areas of the United States with lower socioeconomic status and lower health literacy, “suggesting that we need to think about our technologies to meet patients where they are,” he said.
Despite the positive outcomes, there are “challenges to widespread adoption,” agreed NYU’s Dr. Ahn.
These challenges include the need for physician adaptation to new technologies, data security, and ensuring patient engagement and compliance with remote monitoring systems.
“Successfully addressing these challenges is crucial for optimizing the integration of ePROs into cancer care,” Dr. Ahn said.
ESMO’s invited discussant, Anne Letsch, MD, noted that “cancer therapies are getting more complex, and it’s important that patients are well informed and empowered to get together with the treatment teams throughout therapy.”
The high completion rate with ePRO symptom surveys was “quite remarkable,” said Dr. Letsch, head of the Cancer Center at the University Hospital Schleswig Holstein, Kiel, Germany.
But, Dr. Letsch said, it’s “a pity” that there was no overall survival benefit among patients in the ePRO group. Perhaps overall survival is not what matters most in this context, she said. Instead, she asked, “are other outcomes, like health-related quality of life, symptom control and treatment safety, much more important?”
Dr. Basch also questioned whether the survival differences between the two groups may have been blunted because a substantial portion of the trial was conducted during the COVID-19 pandemic, when medical resources and treatments were delayed and diverted.
Dr. Basch pointed to a 2017 study he and colleagues conducted at a single tertiary care medical center, in which patients monitored with ePROs did demonstrate an overall survival benefit, compared with usual care.
Overall, though, the study demonstrated that “symptom monitoring with ePROs is feasible during routine treatment for advanced cancers across diverse practices in the U.S.” and improved patients’ quality of life, Dr. Basch said.
Funding for the study was provided by a grant from the Patient-Centered Outcomes Research Institute. Dr. Basch has disclosed relationships with Resilience Health, Sivan Health, Navigating Cancer, and AstraZeneca. Dr. Letsch and Dr. Ahn report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ESMO CONGRESS 2023
Fasting during breast cancer chemo improves quality of life
Short-term fasting during chemotherapy enhances health-related quality of life in patients with early breast cancer, with no untoward effects, according to late-breaking research presented on day 1 of the annual meeting of the European Society for Medical Oncology.
“Strikingly,” fasting also appeared to prevent fatigue, something patients with breast cancer struggle with, Daniela A. Koppold, MD, Charité University Medicine Berlin, noted in her oral presentation.
The invited discussant, Jann Arends, MD, with Freiburg (Germany) University Medical Center, said that the findings fit “very well” with previous observations. “Short-term fasting in subjects not at risk for malnutrition is feasible, well tolerated, and appears to improve several parameters of quality of life,” Dr. Arends said.
Promising supportive therapy
The randomized controlled trial assessed the feasibility and impact of short-term fasting on health-related quality of life, compared with a plant-based, low-sugar diet (active comparator) in 106 women with early breast cancer.
The chemotherapy regimens in the trial included four cycles of doxorubicin or epirubicin, followed by taxane therapy. The interventions for both groups occurred about 2 days before chemotherapy plus 24 hours after each cycle ended (about 60-72 hours total).
For the fasting group, this meant about 200 kcal/day through vegetable juices and vegetable broths. In between chemotherapy sessions, both groups were advised to eat a more vegetarian-focused diet, but that was not mandatory.
Health-related quality of life assessments occurred at baseline and after each chemotherapy session (cycle four at day 7) as well as after 4 and 6 months.
The investigators assessed health-related quality of life using the 27-item Functional Assessment of Cancer Therapy-General (FACT-G) that measured the domains of physical, social/familial, emotional, and functional well-being.
At baseline, the two groups had similar FACT-G scores (fasting, 82.9 vs. plant diet, 81.9; P = .523). By day 7, the short-term–fasting group had a significantly better FACT-G score, compared with the plant-based–diet group (fasting, 78.3 vs. plant, 69.5; P = .021).
Although the two groups “started out from the same point, the fasting group had an incremental effect, which quite startled us,” Dr. Koppold told the audience. “Over the course of the chemotherapies, [fasting] had additive effects” and by cycle four of chemotherapy, the difference became statistically and clinically significant, indicating “much better” quality of life in the short-term–fasting group.
What was “even more striking,” said Dr. Koppold, was the impact fasting had on the secondary outcome of fatigue (Functional Assessment of Chronic Illness Therapy–Fatigue).
“Short-term fasting not only had a protective effect on fatigue, compared to the control group, but the short-term–fasting group didn’t develop any clinically visible fatigue,” Dr. Koppold said. “They were in a normal range by cycle four while the control group developed fatigue as we would have expected.”
Importantly, she noted, fasting had no significant impact on weight. The study excluded women who were underweight or had a history of eating disorder or relevant psychopathology.
Summing up, Dr. Koppold said that short-term fasting represents a “promising” supportive therapy during breast cancer chemotherapy to enhance quality of life.
Commenting on the study, Rebecca Guterman, a registered dietitian at Perlmutter Cancer Center at NYU Langone Health, New York, said that it’s well known that a healthy diet plays “a key role during anticancer treatments.” Dietary changes can, for instance, help alleviate common chemotherapy side effects such as loss of appetite, nausea, fatigue, or diarrhea, she said.
These new findings support fasting for 60-72 hours around chemotherapy for some patients with breast cancer who may experience more rapid recovery and better quality of life, said Ms. Guterman.
However, she noted, the results should not be applied to patient populations outside of breast cancer or treatment regimens outside this study. And, she noted, “how the patient feels during the 60-72 hour fast also has to be considered.”
An individual’s “nutritional status must be considered. If a patient has poor appetite and loses weight between treatments, fasting should not be done before next treatment,” Ms. Guterman said.
The study was funded by a private sponsor (G. Müller, Munich, Germany) and a grant from the Günter and Regine KelmFoundation (Zurich). Dr. Koppold is a member of the steering board of ÄGHE e.V. (German-speaking Medical Association for Fasting and Nutrition); cofounder of the Academy for Integrative Fasting GbR; and consults for a mobile app on intermittent fasting (Fastic) as well as a company producing plant-based supplements (EVERYYIN). Dr. Arends has disclosed relationships with Baxter. Ms. Guterman has no relevant disclosures.
A version of this article first appeared on Medscape.com.
Short-term fasting during chemotherapy enhances health-related quality of life in patients with early breast cancer, with no untoward effects, according to late-breaking research presented on day 1 of the annual meeting of the European Society for Medical Oncology.
“Strikingly,” fasting also appeared to prevent fatigue, something patients with breast cancer struggle with, Daniela A. Koppold, MD, Charité University Medicine Berlin, noted in her oral presentation.
The invited discussant, Jann Arends, MD, with Freiburg (Germany) University Medical Center, said that the findings fit “very well” with previous observations. “Short-term fasting in subjects not at risk for malnutrition is feasible, well tolerated, and appears to improve several parameters of quality of life,” Dr. Arends said.
Promising supportive therapy
The randomized controlled trial assessed the feasibility and impact of short-term fasting on health-related quality of life, compared with a plant-based, low-sugar diet (active comparator) in 106 women with early breast cancer.
The chemotherapy regimens in the trial included four cycles of doxorubicin or epirubicin, followed by taxane therapy. The interventions for both groups occurred about 2 days before chemotherapy plus 24 hours after each cycle ended (about 60-72 hours total).
For the fasting group, this meant about 200 kcal/day through vegetable juices and vegetable broths. In between chemotherapy sessions, both groups were advised to eat a more vegetarian-focused diet, but that was not mandatory.
Health-related quality of life assessments occurred at baseline and after each chemotherapy session (cycle four at day 7) as well as after 4 and 6 months.
The investigators assessed health-related quality of life using the 27-item Functional Assessment of Cancer Therapy-General (FACT-G) that measured the domains of physical, social/familial, emotional, and functional well-being.
At baseline, the two groups had similar FACT-G scores (fasting, 82.9 vs. plant diet, 81.9; P = .523). By day 7, the short-term–fasting group had a significantly better FACT-G score, compared with the plant-based–diet group (fasting, 78.3 vs. plant, 69.5; P = .021).
Although the two groups “started out from the same point, the fasting group had an incremental effect, which quite startled us,” Dr. Koppold told the audience. “Over the course of the chemotherapies, [fasting] had additive effects” and by cycle four of chemotherapy, the difference became statistically and clinically significant, indicating “much better” quality of life in the short-term–fasting group.
What was “even more striking,” said Dr. Koppold, was the impact fasting had on the secondary outcome of fatigue (Functional Assessment of Chronic Illness Therapy–Fatigue).
“Short-term fasting not only had a protective effect on fatigue, compared to the control group, but the short-term–fasting group didn’t develop any clinically visible fatigue,” Dr. Koppold said. “They were in a normal range by cycle four while the control group developed fatigue as we would have expected.”
Importantly, she noted, fasting had no significant impact on weight. The study excluded women who were underweight or had a history of eating disorder or relevant psychopathology.
Summing up, Dr. Koppold said that short-term fasting represents a “promising” supportive therapy during breast cancer chemotherapy to enhance quality of life.
Commenting on the study, Rebecca Guterman, a registered dietitian at Perlmutter Cancer Center at NYU Langone Health, New York, said that it’s well known that a healthy diet plays “a key role during anticancer treatments.” Dietary changes can, for instance, help alleviate common chemotherapy side effects such as loss of appetite, nausea, fatigue, or diarrhea, she said.
These new findings support fasting for 60-72 hours around chemotherapy for some patients with breast cancer who may experience more rapid recovery and better quality of life, said Ms. Guterman.
However, she noted, the results should not be applied to patient populations outside of breast cancer or treatment regimens outside this study. And, she noted, “how the patient feels during the 60-72 hour fast also has to be considered.”
An individual’s “nutritional status must be considered. If a patient has poor appetite and loses weight between treatments, fasting should not be done before next treatment,” Ms. Guterman said.
The study was funded by a private sponsor (G. Müller, Munich, Germany) and a grant from the Günter and Regine KelmFoundation (Zurich). Dr. Koppold is a member of the steering board of ÄGHE e.V. (German-speaking Medical Association for Fasting and Nutrition); cofounder of the Academy for Integrative Fasting GbR; and consults for a mobile app on intermittent fasting (Fastic) as well as a company producing plant-based supplements (EVERYYIN). Dr. Arends has disclosed relationships with Baxter. Ms. Guterman has no relevant disclosures.
A version of this article first appeared on Medscape.com.
Short-term fasting during chemotherapy enhances health-related quality of life in patients with early breast cancer, with no untoward effects, according to late-breaking research presented on day 1 of the annual meeting of the European Society for Medical Oncology.
“Strikingly,” fasting also appeared to prevent fatigue, something patients with breast cancer struggle with, Daniela A. Koppold, MD, Charité University Medicine Berlin, noted in her oral presentation.
The invited discussant, Jann Arends, MD, with Freiburg (Germany) University Medical Center, said that the findings fit “very well” with previous observations. “Short-term fasting in subjects not at risk for malnutrition is feasible, well tolerated, and appears to improve several parameters of quality of life,” Dr. Arends said.
Promising supportive therapy
The randomized controlled trial assessed the feasibility and impact of short-term fasting on health-related quality of life, compared with a plant-based, low-sugar diet (active comparator) in 106 women with early breast cancer.
The chemotherapy regimens in the trial included four cycles of doxorubicin or epirubicin, followed by taxane therapy. The interventions for both groups occurred about 2 days before chemotherapy plus 24 hours after each cycle ended (about 60-72 hours total).
For the fasting group, this meant about 200 kcal/day through vegetable juices and vegetable broths. In between chemotherapy sessions, both groups were advised to eat a more vegetarian-focused diet, but that was not mandatory.
Health-related quality of life assessments occurred at baseline and after each chemotherapy session (cycle four at day 7) as well as after 4 and 6 months.
The investigators assessed health-related quality of life using the 27-item Functional Assessment of Cancer Therapy-General (FACT-G) that measured the domains of physical, social/familial, emotional, and functional well-being.
At baseline, the two groups had similar FACT-G scores (fasting, 82.9 vs. plant diet, 81.9; P = .523). By day 7, the short-term–fasting group had a significantly better FACT-G score, compared with the plant-based–diet group (fasting, 78.3 vs. plant, 69.5; P = .021).
Although the two groups “started out from the same point, the fasting group had an incremental effect, which quite startled us,” Dr. Koppold told the audience. “Over the course of the chemotherapies, [fasting] had additive effects” and by cycle four of chemotherapy, the difference became statistically and clinically significant, indicating “much better” quality of life in the short-term–fasting group.
What was “even more striking,” said Dr. Koppold, was the impact fasting had on the secondary outcome of fatigue (Functional Assessment of Chronic Illness Therapy–Fatigue).
“Short-term fasting not only had a protective effect on fatigue, compared to the control group, but the short-term–fasting group didn’t develop any clinically visible fatigue,” Dr. Koppold said. “They were in a normal range by cycle four while the control group developed fatigue as we would have expected.”
Importantly, she noted, fasting had no significant impact on weight. The study excluded women who were underweight or had a history of eating disorder or relevant psychopathology.
Summing up, Dr. Koppold said that short-term fasting represents a “promising” supportive therapy during breast cancer chemotherapy to enhance quality of life.
Commenting on the study, Rebecca Guterman, a registered dietitian at Perlmutter Cancer Center at NYU Langone Health, New York, said that it’s well known that a healthy diet plays “a key role during anticancer treatments.” Dietary changes can, for instance, help alleviate common chemotherapy side effects such as loss of appetite, nausea, fatigue, or diarrhea, she said.
These new findings support fasting for 60-72 hours around chemotherapy for some patients with breast cancer who may experience more rapid recovery and better quality of life, said Ms. Guterman.
However, she noted, the results should not be applied to patient populations outside of breast cancer or treatment regimens outside this study. And, she noted, “how the patient feels during the 60-72 hour fast also has to be considered.”
An individual’s “nutritional status must be considered. If a patient has poor appetite and loses weight between treatments, fasting should not be done before next treatment,” Ms. Guterman said.
The study was funded by a private sponsor (G. Müller, Munich, Germany) and a grant from the Günter and Regine KelmFoundation (Zurich). Dr. Koppold is a member of the steering board of ÄGHE e.V. (German-speaking Medical Association for Fasting and Nutrition); cofounder of the Academy for Integrative Fasting GbR; and consults for a mobile app on intermittent fasting (Fastic) as well as a company producing plant-based supplements (EVERYYIN). Dr. Arends has disclosed relationships with Baxter. Ms. Guterman has no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM ESMO CONGRESS 2023
Does first-line pembrolizumab add-on improve PFS in high-risk cervical cancer?
Pembrolizumab (Keytruda) improved progression-free survival when added to standard concurrent chemoradiotherapy in the first-line for newly diagnosed, locally advanced cervical cancer in the KEYNOTE-A18 trial, according to a study presented at the annual meeting of the European Society for Medical Oncology.
The study “supports pembrolizumab plus chemoradiotherapy as a new potential standard of care” in the first-line setting for high-risk, locally advanced cervical cancer, said lead investigator Domenica Lorusso, MD, PhD, a gynecologic oncologist at the Catholic University of Rome, who reported the findings at the meeting.
The results of the trial “are compelling, especially considering newly diagnosed patients with high-risk locally advanced cervical cancer have not seen an advance in treatment options in 20 years,” she said in a press release from pembrolizumab maker Merck.
Trial data are under review at the Food and Drug Administration as part of Merck’s application for a first-line indication for pembrolizumab added to concurrent chemoradiotherapy in newly diagnosed patients with high-risk, locally advanced cervical cancer; the agency’s decision is expected in Jan. 2024.
Pembrolizumab already carries indications for persistent, recurrent, or metastatic cervical cancer.
Women in the trial were new to treatment and had either stage 1B2-2B disease with lymph node involvement or stage 3-4A disease; almost 85% had squamous cell cancer. About half the women were White; 28% were Asian, and about 2% were Black. About 5% of subjects were PD-L1 negative.
Overall, 529 women were randomized to 200 mg pembrolizumab every 3 weeks for five cycles with concurrent chemoradiotherapy (CCRT); they then received pembrolizumab 400 mg every 6 weeks for 15 cycles; 531 were randomized to placebo with CCRT, followed by 15 6-week placebo cycles.
CCRT included five cycles of cisplatin 40 mg/m2 every week for 5-6 weeks plus external beam radiotherapy followed by brachytherapy.
Two-year progression-free survival was 57.3% with placebo but 67.8% with pembrolizumab add-on, a 30% reduction in the risk of progression (P = .002).
On subgroup analysis, pembrolizumab’s PFS benefit was not statistically significant for White women (hazard ratio, 0.83; 95% confidence interval, 0.59-1.15) and women with stage 1B2 to 2B disease (HR, 0.91; 95% CI, 0.63-1.31), among others.
Although OS is not yet mature, 80.8% of placebo subjects but 87.2% of pembrolizumab women were alive at 2 years, a 27% drop in the risk of death (95% CI, 0.49-1.07).
At the meeting, Bradley Monk, MD, a gynecologic oncologist at the University of Arizona, Phoenix, who was also the study discussant, noted that “the magnitude of the benefit here is difficult to interpret because 55% of the patients [were] still on treatment” in the interim analysis, but the difference “is substantial enough for us to have confidence.”
Rates of grade 3/4 treatment-related adverse events were 60.6% in the placebo group and 67% with pembrolizumab, with anemia, nausea, and diarrhea the most common.
Grade 3/4 immune-mediated adverse events occurred in 1.1% of placebo and 4.2% of pembrolizumab subjects; hypothyroidism was the most common with pembrolizumab.
Protocol amendments in the trial included a change from PFS assessment by blinded, independent, central review to investigator assessment.
In the press release, Dr. Monk, said the results “demonstrate that, by moving an immunotherapy regimen to earlier stages of cervical cancer, we have the potential to improve outcomes for these patients compared to the current standard of care.”
The study was funded by Merck, maker of pembrolizumab. Investigators reported wide-ranging ties to the company, including Dr. Lorusso, who reported honoraria from Merck as well as ties to other companies. Dr. Monk also had deep industry ties, including being a speaker and consultant for Merck and reporting honoraria from the company.
Pembrolizumab (Keytruda) improved progression-free survival when added to standard concurrent chemoradiotherapy in the first-line for newly diagnosed, locally advanced cervical cancer in the KEYNOTE-A18 trial, according to a study presented at the annual meeting of the European Society for Medical Oncology.
The study “supports pembrolizumab plus chemoradiotherapy as a new potential standard of care” in the first-line setting for high-risk, locally advanced cervical cancer, said lead investigator Domenica Lorusso, MD, PhD, a gynecologic oncologist at the Catholic University of Rome, who reported the findings at the meeting.
The results of the trial “are compelling, especially considering newly diagnosed patients with high-risk locally advanced cervical cancer have not seen an advance in treatment options in 20 years,” she said in a press release from pembrolizumab maker Merck.
Trial data are under review at the Food and Drug Administration as part of Merck’s application for a first-line indication for pembrolizumab added to concurrent chemoradiotherapy in newly diagnosed patients with high-risk, locally advanced cervical cancer; the agency’s decision is expected in Jan. 2024.
Pembrolizumab already carries indications for persistent, recurrent, or metastatic cervical cancer.
Women in the trial were new to treatment and had either stage 1B2-2B disease with lymph node involvement or stage 3-4A disease; almost 85% had squamous cell cancer. About half the women were White; 28% were Asian, and about 2% were Black. About 5% of subjects were PD-L1 negative.
Overall, 529 women were randomized to 200 mg pembrolizumab every 3 weeks for five cycles with concurrent chemoradiotherapy (CCRT); they then received pembrolizumab 400 mg every 6 weeks for 15 cycles; 531 were randomized to placebo with CCRT, followed by 15 6-week placebo cycles.
CCRT included five cycles of cisplatin 40 mg/m2 every week for 5-6 weeks plus external beam radiotherapy followed by brachytherapy.
Two-year progression-free survival was 57.3% with placebo but 67.8% with pembrolizumab add-on, a 30% reduction in the risk of progression (P = .002).
On subgroup analysis, pembrolizumab’s PFS benefit was not statistically significant for White women (hazard ratio, 0.83; 95% confidence interval, 0.59-1.15) and women with stage 1B2 to 2B disease (HR, 0.91; 95% CI, 0.63-1.31), among others.
Although OS is not yet mature, 80.8% of placebo subjects but 87.2% of pembrolizumab women were alive at 2 years, a 27% drop in the risk of death (95% CI, 0.49-1.07).
At the meeting, Bradley Monk, MD, a gynecologic oncologist at the University of Arizona, Phoenix, who was also the study discussant, noted that “the magnitude of the benefit here is difficult to interpret because 55% of the patients [were] still on treatment” in the interim analysis, but the difference “is substantial enough for us to have confidence.”
Rates of grade 3/4 treatment-related adverse events were 60.6% in the placebo group and 67% with pembrolizumab, with anemia, nausea, and diarrhea the most common.
Grade 3/4 immune-mediated adverse events occurred in 1.1% of placebo and 4.2% of pembrolizumab subjects; hypothyroidism was the most common with pembrolizumab.
Protocol amendments in the trial included a change from PFS assessment by blinded, independent, central review to investigator assessment.
In the press release, Dr. Monk, said the results “demonstrate that, by moving an immunotherapy regimen to earlier stages of cervical cancer, we have the potential to improve outcomes for these patients compared to the current standard of care.”
The study was funded by Merck, maker of pembrolizumab. Investigators reported wide-ranging ties to the company, including Dr. Lorusso, who reported honoraria from Merck as well as ties to other companies. Dr. Monk also had deep industry ties, including being a speaker and consultant for Merck and reporting honoraria from the company.
Pembrolizumab (Keytruda) improved progression-free survival when added to standard concurrent chemoradiotherapy in the first-line for newly diagnosed, locally advanced cervical cancer in the KEYNOTE-A18 trial, according to a study presented at the annual meeting of the European Society for Medical Oncology.
The study “supports pembrolizumab plus chemoradiotherapy as a new potential standard of care” in the first-line setting for high-risk, locally advanced cervical cancer, said lead investigator Domenica Lorusso, MD, PhD, a gynecologic oncologist at the Catholic University of Rome, who reported the findings at the meeting.
The results of the trial “are compelling, especially considering newly diagnosed patients with high-risk locally advanced cervical cancer have not seen an advance in treatment options in 20 years,” she said in a press release from pembrolizumab maker Merck.
Trial data are under review at the Food and Drug Administration as part of Merck’s application for a first-line indication for pembrolizumab added to concurrent chemoradiotherapy in newly diagnosed patients with high-risk, locally advanced cervical cancer; the agency’s decision is expected in Jan. 2024.
Pembrolizumab already carries indications for persistent, recurrent, or metastatic cervical cancer.
Women in the trial were new to treatment and had either stage 1B2-2B disease with lymph node involvement or stage 3-4A disease; almost 85% had squamous cell cancer. About half the women were White; 28% were Asian, and about 2% were Black. About 5% of subjects were PD-L1 negative.
Overall, 529 women were randomized to 200 mg pembrolizumab every 3 weeks for five cycles with concurrent chemoradiotherapy (CCRT); they then received pembrolizumab 400 mg every 6 weeks for 15 cycles; 531 were randomized to placebo with CCRT, followed by 15 6-week placebo cycles.
CCRT included five cycles of cisplatin 40 mg/m2 every week for 5-6 weeks plus external beam radiotherapy followed by brachytherapy.
Two-year progression-free survival was 57.3% with placebo but 67.8% with pembrolizumab add-on, a 30% reduction in the risk of progression (P = .002).
On subgroup analysis, pembrolizumab’s PFS benefit was not statistically significant for White women (hazard ratio, 0.83; 95% confidence interval, 0.59-1.15) and women with stage 1B2 to 2B disease (HR, 0.91; 95% CI, 0.63-1.31), among others.
Although OS is not yet mature, 80.8% of placebo subjects but 87.2% of pembrolizumab women were alive at 2 years, a 27% drop in the risk of death (95% CI, 0.49-1.07).
At the meeting, Bradley Monk, MD, a gynecologic oncologist at the University of Arizona, Phoenix, who was also the study discussant, noted that “the magnitude of the benefit here is difficult to interpret because 55% of the patients [were] still on treatment” in the interim analysis, but the difference “is substantial enough for us to have confidence.”
Rates of grade 3/4 treatment-related adverse events were 60.6% in the placebo group and 67% with pembrolizumab, with anemia, nausea, and diarrhea the most common.
Grade 3/4 immune-mediated adverse events occurred in 1.1% of placebo and 4.2% of pembrolizumab subjects; hypothyroidism was the most common with pembrolizumab.
Protocol amendments in the trial included a change from PFS assessment by blinded, independent, central review to investigator assessment.
In the press release, Dr. Monk, said the results “demonstrate that, by moving an immunotherapy regimen to earlier stages of cervical cancer, we have the potential to improve outcomes for these patients compared to the current standard of care.”
The study was funded by Merck, maker of pembrolizumab. Investigators reported wide-ranging ties to the company, including Dr. Lorusso, who reported honoraria from Merck as well as ties to other companies. Dr. Monk also had deep industry ties, including being a speaker and consultant for Merck and reporting honoraria from the company.
FROM ESMO CONGRESS 2023
Can some patients with esophageal cancer avoid surgery?
MADRID – , findings from the Dutch SANO trial suggest.
After 2 years, researchers found no significant differences in overall and disease-free survival between patients on active surveillance and those who received surgery either immediately following neoadjuvant chemoradiotherapy or who switched from active surveillance to surgery.
Overall, patients who underwent active surveillance had “noninferior overall survival at 2 years,” said Berend J. Van der Wilk, PhD candidate, Erasmus University, Rotterdam, the Netherlands, who presented the findings at the annual meeting of the European Society for Medical Oncology.
Over the 2-year follow-up, at least 35% of patients on active surveillance were spared surgery. Patients on active surveillance who experienced locoregional regrowth could still undergo surgery, Mr. Van der Wilk said.
Magnus Nilsson, MD, PhD, the invited discussant, who was not involved in the research, said performing such a conceptually important and complex trial was a “huge achievement.” However, Dr. Nilsson highlighted some “major concerns” with the trial design, which could affect the generalizability of the findings.
Avoiding surgery?
Esophagectomy remains the “keystone of curative treatment for esophageal cancer,” Mr. Van der Wilks explained. However, this operation is a “major surgical procedure” that comes with a mortality rate of up to 5%. As many as 59% of patients experience complications.
The CROSS trial, which included more than 360 patients with esophageal or esophagogastric junction cancer, found that neoadjuvant chemoradiotherapy improved survival among patients with potentially curable disease; 29% of patients achieved a pathologic complete response.
Mr. Van der Wilk said those strong outcomes create some uncertainty as to whether all patients need standard surgery after chemoradiotherapy.
In other words, Mr. Van der Wilk asked, “Should we be willing to follow an active surveillance, organ-sparing strategy for patients with a clinical response?”
An active surveillance strategy, he said, would require frequent evaluations of the patient’s clinical response. Surgery would be performed only in cases of proven residual tumor in which there were no distant metastases. The potential pitfall of an active surveillance approach is that patients may develop unresectable tumor regrowths, “possibly resulting in inferior overall survival.”
To compare active surveillance with standard surgery, the team conducted a phase 3 noninferiority stepped-wedge cluster randomized trial involving patients with locally advanced esophageal cancer.
Patients received neoadjuvant chemoradiotherapy with carboplatin and paclitaxel for 5 weeks. Concurrent radiotherapy was delivered at 41.4 Gy in 23 fractions, 5 days per week, as in the CROSS trial.
More than 300 patients who achieved a complete clinical response 12 weeks after completing chemoradiotherapy were randomly assigned to undergo standard surgery or active surveillance. Surgery was performed for those with subsequent tumor regrowth.
Overall, 198 patients underwent active surveillance, and 111 patients underwent standard surgery. The two groups were well balanced in terms of median age, sex distribution, proportion of adenocarcinomas, and World Health Organization performance scores. At the last patient assessment, on July 6, 2023, the median follow-up was 38 months.
Overall, 101 of 111 patients in the standard surgery arm and 83 of 198 (42%) in the active surveillance group had surgery. The time to surgery in the active surveillance arm was 5.9 months, compared with 0.7 months with standard surgery. For both groups, the R0 resection rate was 98%.
Mr. Van der Wilk reported no significant difference in overall survival between the active surveillance and standard surgery groups (hazard ratio for death, 1.14; 95% confidence interval, 0.74-0.78; P = .55). Overall survival in the active surveillance group was noninferior to that in the standard surgery group at 2 years. Noninferiority was defined as an overall survival difference between the two arms of less than 15%.
Mr. Van der Wilk also reported no significant difference in disease-free survival between the active surveillance and the standard surgery groups – 35 months with active surveillance, and 49 months with surgery (HR, 1.35; P = .15). At 30 months following neoadjuvant chemoradiotherapy, 43% of patients on active surveillance and 34% with standard surgery developed distant metastases, but the difference was not significant (odds ratio, 1.45; P = .18).
Among the patients in the active surveillance arm who had a complete response, 35% (n = 69) had a persistent clinical response, while 17% (33 patients) developed distant metastases, and 48% (n = 96) experienced locoregional growth. The postoperative 90-day mortality was 4% in the active surveillance group and 5% in the surgery group.
Health-related quality of life was significantly better at 6 and 9 months in the active surveillance group, Mr. Van der Wilk noted.
Although Dr. Nilsson, the invited discussant, highlighted the importance of the trial, he also expressed concern over the trial design.
The intention-to-treat analysis was contaminated, Dr. Nilsson said, because the trial design allowed for one crossover, but patients in the trial crossed over at two time points – 35 patients who were initially assigned to standard surgery crossed over to the active surveillance arm, and later, seven patients from a preSANO trial were included in the active surveillance arm.
Dr. Nilsson also expressed concern about mixing squamous cell carcinoma and adenocarcinoma histologies in the study. If the authors had distinguished patients with squamous cell carcinoma and those with adenocarcinoma in each arm, there may have a difference in overall survival, given that squamous cell carcinoma is much easier to treat.
The study also included some patients who did not have a complete clinical response and whose surgery was delayed by more than 10 weeks – a practice that, Dr. Nilsson said, “does not really seem to be safe.” A recent study led by Dr. Nilsson found that delaying surgery for 10-12 weeks in comparison with 4-6 weeks did not improve histologic complete response or other pathologic endpoints and may have led to worse survival.
“I’m afraid it’s not really certain that it’s safe to prolong surgery more than 10 weeks or longer in the clinical noncomplete responders,” said Dr. Nilsson, from the department of clinical science, intervention, and technology, Karolinska Institute, Stockholm.
Overall, he said, the study “suggests that survival may be noninferior” among patients on active surveillance in comparison with those who undergo immediate surgery, but the findings need to be confirmed in a trial with a more stringent intention-to-treat analysis that is stratified by histologic subtypes.
The study was funded by the Dutch Cancer Society and the Netherlands Organisation for Health Research and Development (ZonMw). Mr. Van der Wilk has disclosed no relevant financial relationships. Dr. Nilsson has relationships with Medtronic, Intuitive Surgical, Bristol-Myers Squibb, and Merck Sharp & Dohme, from which he received no personal financial benefit.
A version of this article first appeared on Medscape.com.
MADRID – , findings from the Dutch SANO trial suggest.
After 2 years, researchers found no significant differences in overall and disease-free survival between patients on active surveillance and those who received surgery either immediately following neoadjuvant chemoradiotherapy or who switched from active surveillance to surgery.
Overall, patients who underwent active surveillance had “noninferior overall survival at 2 years,” said Berend J. Van der Wilk, PhD candidate, Erasmus University, Rotterdam, the Netherlands, who presented the findings at the annual meeting of the European Society for Medical Oncology.
Over the 2-year follow-up, at least 35% of patients on active surveillance were spared surgery. Patients on active surveillance who experienced locoregional regrowth could still undergo surgery, Mr. Van der Wilk said.
Magnus Nilsson, MD, PhD, the invited discussant, who was not involved in the research, said performing such a conceptually important and complex trial was a “huge achievement.” However, Dr. Nilsson highlighted some “major concerns” with the trial design, which could affect the generalizability of the findings.
Avoiding surgery?
Esophagectomy remains the “keystone of curative treatment for esophageal cancer,” Mr. Van der Wilks explained. However, this operation is a “major surgical procedure” that comes with a mortality rate of up to 5%. As many as 59% of patients experience complications.
The CROSS trial, which included more than 360 patients with esophageal or esophagogastric junction cancer, found that neoadjuvant chemoradiotherapy improved survival among patients with potentially curable disease; 29% of patients achieved a pathologic complete response.
Mr. Van der Wilk said those strong outcomes create some uncertainty as to whether all patients need standard surgery after chemoradiotherapy.
In other words, Mr. Van der Wilk asked, “Should we be willing to follow an active surveillance, organ-sparing strategy for patients with a clinical response?”
An active surveillance strategy, he said, would require frequent evaluations of the patient’s clinical response. Surgery would be performed only in cases of proven residual tumor in which there were no distant metastases. The potential pitfall of an active surveillance approach is that patients may develop unresectable tumor regrowths, “possibly resulting in inferior overall survival.”
To compare active surveillance with standard surgery, the team conducted a phase 3 noninferiority stepped-wedge cluster randomized trial involving patients with locally advanced esophageal cancer.
Patients received neoadjuvant chemoradiotherapy with carboplatin and paclitaxel for 5 weeks. Concurrent radiotherapy was delivered at 41.4 Gy in 23 fractions, 5 days per week, as in the CROSS trial.
More than 300 patients who achieved a complete clinical response 12 weeks after completing chemoradiotherapy were randomly assigned to undergo standard surgery or active surveillance. Surgery was performed for those with subsequent tumor regrowth.
Overall, 198 patients underwent active surveillance, and 111 patients underwent standard surgery. The two groups were well balanced in terms of median age, sex distribution, proportion of adenocarcinomas, and World Health Organization performance scores. At the last patient assessment, on July 6, 2023, the median follow-up was 38 months.
Overall, 101 of 111 patients in the standard surgery arm and 83 of 198 (42%) in the active surveillance group had surgery. The time to surgery in the active surveillance arm was 5.9 months, compared with 0.7 months with standard surgery. For both groups, the R0 resection rate was 98%.
Mr. Van der Wilk reported no significant difference in overall survival between the active surveillance and standard surgery groups (hazard ratio for death, 1.14; 95% confidence interval, 0.74-0.78; P = .55). Overall survival in the active surveillance group was noninferior to that in the standard surgery group at 2 years. Noninferiority was defined as an overall survival difference between the two arms of less than 15%.
Mr. Van der Wilk also reported no significant difference in disease-free survival between the active surveillance and the standard surgery groups – 35 months with active surveillance, and 49 months with surgery (HR, 1.35; P = .15). At 30 months following neoadjuvant chemoradiotherapy, 43% of patients on active surveillance and 34% with standard surgery developed distant metastases, but the difference was not significant (odds ratio, 1.45; P = .18).
Among the patients in the active surveillance arm who had a complete response, 35% (n = 69) had a persistent clinical response, while 17% (33 patients) developed distant metastases, and 48% (n = 96) experienced locoregional growth. The postoperative 90-day mortality was 4% in the active surveillance group and 5% in the surgery group.
Health-related quality of life was significantly better at 6 and 9 months in the active surveillance group, Mr. Van der Wilk noted.
Although Dr. Nilsson, the invited discussant, highlighted the importance of the trial, he also expressed concern over the trial design.
The intention-to-treat analysis was contaminated, Dr. Nilsson said, because the trial design allowed for one crossover, but patients in the trial crossed over at two time points – 35 patients who were initially assigned to standard surgery crossed over to the active surveillance arm, and later, seven patients from a preSANO trial were included in the active surveillance arm.
Dr. Nilsson also expressed concern about mixing squamous cell carcinoma and adenocarcinoma histologies in the study. If the authors had distinguished patients with squamous cell carcinoma and those with adenocarcinoma in each arm, there may have a difference in overall survival, given that squamous cell carcinoma is much easier to treat.
The study also included some patients who did not have a complete clinical response and whose surgery was delayed by more than 10 weeks – a practice that, Dr. Nilsson said, “does not really seem to be safe.” A recent study led by Dr. Nilsson found that delaying surgery for 10-12 weeks in comparison with 4-6 weeks did not improve histologic complete response or other pathologic endpoints and may have led to worse survival.
“I’m afraid it’s not really certain that it’s safe to prolong surgery more than 10 weeks or longer in the clinical noncomplete responders,” said Dr. Nilsson, from the department of clinical science, intervention, and technology, Karolinska Institute, Stockholm.
Overall, he said, the study “suggests that survival may be noninferior” among patients on active surveillance in comparison with those who undergo immediate surgery, but the findings need to be confirmed in a trial with a more stringent intention-to-treat analysis that is stratified by histologic subtypes.
The study was funded by the Dutch Cancer Society and the Netherlands Organisation for Health Research and Development (ZonMw). Mr. Van der Wilk has disclosed no relevant financial relationships. Dr. Nilsson has relationships with Medtronic, Intuitive Surgical, Bristol-Myers Squibb, and Merck Sharp & Dohme, from which he received no personal financial benefit.
A version of this article first appeared on Medscape.com.
MADRID – , findings from the Dutch SANO trial suggest.
After 2 years, researchers found no significant differences in overall and disease-free survival between patients on active surveillance and those who received surgery either immediately following neoadjuvant chemoradiotherapy or who switched from active surveillance to surgery.
Overall, patients who underwent active surveillance had “noninferior overall survival at 2 years,” said Berend J. Van der Wilk, PhD candidate, Erasmus University, Rotterdam, the Netherlands, who presented the findings at the annual meeting of the European Society for Medical Oncology.
Over the 2-year follow-up, at least 35% of patients on active surveillance were spared surgery. Patients on active surveillance who experienced locoregional regrowth could still undergo surgery, Mr. Van der Wilk said.
Magnus Nilsson, MD, PhD, the invited discussant, who was not involved in the research, said performing such a conceptually important and complex trial was a “huge achievement.” However, Dr. Nilsson highlighted some “major concerns” with the trial design, which could affect the generalizability of the findings.
Avoiding surgery?
Esophagectomy remains the “keystone of curative treatment for esophageal cancer,” Mr. Van der Wilks explained. However, this operation is a “major surgical procedure” that comes with a mortality rate of up to 5%. As many as 59% of patients experience complications.
The CROSS trial, which included more than 360 patients with esophageal or esophagogastric junction cancer, found that neoadjuvant chemoradiotherapy improved survival among patients with potentially curable disease; 29% of patients achieved a pathologic complete response.
Mr. Van der Wilk said those strong outcomes create some uncertainty as to whether all patients need standard surgery after chemoradiotherapy.
In other words, Mr. Van der Wilk asked, “Should we be willing to follow an active surveillance, organ-sparing strategy for patients with a clinical response?”
An active surveillance strategy, he said, would require frequent evaluations of the patient’s clinical response. Surgery would be performed only in cases of proven residual tumor in which there were no distant metastases. The potential pitfall of an active surveillance approach is that patients may develop unresectable tumor regrowths, “possibly resulting in inferior overall survival.”
To compare active surveillance with standard surgery, the team conducted a phase 3 noninferiority stepped-wedge cluster randomized trial involving patients with locally advanced esophageal cancer.
Patients received neoadjuvant chemoradiotherapy with carboplatin and paclitaxel for 5 weeks. Concurrent radiotherapy was delivered at 41.4 Gy in 23 fractions, 5 days per week, as in the CROSS trial.
More than 300 patients who achieved a complete clinical response 12 weeks after completing chemoradiotherapy were randomly assigned to undergo standard surgery or active surveillance. Surgery was performed for those with subsequent tumor regrowth.
Overall, 198 patients underwent active surveillance, and 111 patients underwent standard surgery. The two groups were well balanced in terms of median age, sex distribution, proportion of adenocarcinomas, and World Health Organization performance scores. At the last patient assessment, on July 6, 2023, the median follow-up was 38 months.
Overall, 101 of 111 patients in the standard surgery arm and 83 of 198 (42%) in the active surveillance group had surgery. The time to surgery in the active surveillance arm was 5.9 months, compared with 0.7 months with standard surgery. For both groups, the R0 resection rate was 98%.
Mr. Van der Wilk reported no significant difference in overall survival between the active surveillance and standard surgery groups (hazard ratio for death, 1.14; 95% confidence interval, 0.74-0.78; P = .55). Overall survival in the active surveillance group was noninferior to that in the standard surgery group at 2 years. Noninferiority was defined as an overall survival difference between the two arms of less than 15%.
Mr. Van der Wilk also reported no significant difference in disease-free survival between the active surveillance and the standard surgery groups – 35 months with active surveillance, and 49 months with surgery (HR, 1.35; P = .15). At 30 months following neoadjuvant chemoradiotherapy, 43% of patients on active surveillance and 34% with standard surgery developed distant metastases, but the difference was not significant (odds ratio, 1.45; P = .18).
Among the patients in the active surveillance arm who had a complete response, 35% (n = 69) had a persistent clinical response, while 17% (33 patients) developed distant metastases, and 48% (n = 96) experienced locoregional growth. The postoperative 90-day mortality was 4% in the active surveillance group and 5% in the surgery group.
Health-related quality of life was significantly better at 6 and 9 months in the active surveillance group, Mr. Van der Wilk noted.
Although Dr. Nilsson, the invited discussant, highlighted the importance of the trial, he also expressed concern over the trial design.
The intention-to-treat analysis was contaminated, Dr. Nilsson said, because the trial design allowed for one crossover, but patients in the trial crossed over at two time points – 35 patients who were initially assigned to standard surgery crossed over to the active surveillance arm, and later, seven patients from a preSANO trial were included in the active surveillance arm.
Dr. Nilsson also expressed concern about mixing squamous cell carcinoma and adenocarcinoma histologies in the study. If the authors had distinguished patients with squamous cell carcinoma and those with adenocarcinoma in each arm, there may have a difference in overall survival, given that squamous cell carcinoma is much easier to treat.
The study also included some patients who did not have a complete clinical response and whose surgery was delayed by more than 10 weeks – a practice that, Dr. Nilsson said, “does not really seem to be safe.” A recent study led by Dr. Nilsson found that delaying surgery for 10-12 weeks in comparison with 4-6 weeks did not improve histologic complete response or other pathologic endpoints and may have led to worse survival.
“I’m afraid it’s not really certain that it’s safe to prolong surgery more than 10 weeks or longer in the clinical noncomplete responders,” said Dr. Nilsson, from the department of clinical science, intervention, and technology, Karolinska Institute, Stockholm.
Overall, he said, the study “suggests that survival may be noninferior” among patients on active surveillance in comparison with those who undergo immediate surgery, but the findings need to be confirmed in a trial with a more stringent intention-to-treat analysis that is stratified by histologic subtypes.
The study was funded by the Dutch Cancer Society and the Netherlands Organisation for Health Research and Development (ZonMw). Mr. Van der Wilk has disclosed no relevant financial relationships. Dr. Nilsson has relationships with Medtronic, Intuitive Surgical, Bristol-Myers Squibb, and Merck Sharp & Dohme, from which he received no personal financial benefit.
A version of this article first appeared on Medscape.com.
Adjuvant abemaciclib-ET combo shows long-term benefit in high-risk early breast cancer
MADRID – Five years on, the addition of the CDK4/6 inhibitor abemaciclib (Verzenio) to endocrine therapy for women with high-risk hormone receptor–positive, HER2-negative (HR+/HER2–) early breast cancer continues to show modest but clinically significant benefits, compared with endocrine therapy alone.
Results of a planned 5-year efficacy analysis of the monarchE trial showed that , reported Nadia Harbeck, MD, from the Breast Center at Ludwig Maximilians University Hospital in Munich.
“The data are consistent with a carryover effect and further support the addition of adjuvant abemaciclib to endocrine therapy for patients with hormone receptor–positive, HER2-negative, node-positive high-risk early breast cancer,” she said at the 2023 European Society for Medical Oncology Congress.
High recurrence risk
Although HR+/HER2– breast cancer, the most common subtype of breast cancer, is generally associated with better outcomes than other subtypes, patients with node-positive early disease are at high risk for early recurrence and need treatment intensification, Dr. Harbeck said.
The monarchE trial included two cohorts: a primary cohort consisting of patients deemed at high risk based on clinical pathological features such as the number of involved axillary nodes, grade 3 disease, and tumors 5 cm or larger, and a second cohort of patients with lower disease grade and smaller tumors but with high levels of the proliferation marker Ki-67.
A total of 5,637 patients were randomized to receive either 2 years of abemaciclib 150 mg twice daily plus endocrine therapy, or endocrine therapy alone, followed by 3-8 years of additional endocrine as clinically indicated in each study arm.
An earlier preplanned interim analysis of the phase 3 trial of more than 5,600 patients was presented at the ESMO Virtual Congress 2020, and simultaneously published in the Journal of Clinical Oncology.
As that analysis showed, at a median follow-up of 15.5 months abemaciclib plus endocrine therapy was associated with a 25% relative risk reduction in the primary endpoint of IDFS vs. endocrine therapy alone.
At the time, the findings were hailed as practice-changing and, once approved for high-risk HR+/HER2-negative early breast cancer, as the new standard of care.
In the current analysis, Dr. Harbeck and colleagues looked at 5-year outcomes from a prespecified analysis, with a data cutoff of July 3, 2023.
All patients originally assigned to abemaciclib are now off the drug, and more than 80% have been followed for a minimum of 2 year since completing therapy with the CDK4/6 inhibitor.
Results
At 5 years there were cumulative totals of 407 IDFS events in the combination arm, compared with 585 in the endocrine therapy alone arm, a difference that translated into a hazard ratio of 0.68 favoring abemaciclib (P < .001).
The IDFS benefit with the combination was consistent across most subgroups, including older patients, perimenopausal and postmenopausal patients, those who had received prior neoadjuvant or adjuvant chemotherapy, all tumor sizes, number of positive lymph nodes, less favorable tumor stage or grade, and order of endocrine therapy (tamoxifen or aromatase inhibitor as first drug).
As noted before, DRFS, a secondary endpoint, also favored abemaciclib, with 345 events occurring over 5 years in the combination arm, compared with 501 in the endocrine therapy arm alone. This translated into a HR with the combination of 0.68 (P < .001).
There were fewer deaths in the abemaciclib arm (208 vs. 234), but this difference was not statistically significant.
The proportions of patients with treatment-emergent adverse events and serious adverse events (SAEs) were higher in the combination arm than in the endocrine therapy alone arm in all previous analyses of the trial data.
In the current analysis, “I would say it’s reassuring to see that the SAEs reported in the follow-up period, after the study treatment had been completed, are quite similar between the endocrine therapy alone arm and the abemaciclib plus endocrine therapy arm,” Dr. Harbeck said.
Changing road map
Invited discussant Kevin Kalinsky, MD, MS, from the Winship Cancer Institute at Emory University, Atlanta, commented that CDK4/6 inhibitors “have changed the road map for treating hormone receptor–positive, HER2-negative disease.”
To put the monarchE results in context, he compared them with those of the NATALEE trial, in which patients were randomized to endocrine therapy with or without the CDK4/6 inhibitor ribociclib (Kisqali). That combination was previously shown to provide a significant survival advantage for women with metastatic breast cancer.
In NATALEE, which included both high-risk and intermediate-risk patients with early breast cancer, the absolute difference in 3-year IDFS rates between the combination group and endocrine monotherapy groups was 3.3%.
To determine the ultimate value of combining a CDK4/6 inhibitor with endocrine therapy in early breast cancer, longer follow-up of both trials will be necessary, Dr. Kalinsky said.
“The reason that follow-up is critical for both of these studies is that for this subtype of breast cancer, based upon data including from the Early Breast Cancer Trialists Group, we can see approximately 50% of recurrences after the first 5 years, and we think of cytotoxic chemotherapy as benefiting patients within those first 5 years. And while we think of CDK4/6 inhibitors as being cytostatic drugs, we are seeing a carryover effect in which 2 years of abemaciclib is improving outcome at the 5-year landmark,” he said.
Questions that still need to be answered include the optimal duration of CDK4/6 inhibitor therapy, whether adjuvant therapy should be resumed when there are signs of renewed proliferation, and whether there would be a benefit to restarting CDK4/6 inhibitors when metastasis occurs.
The monarchE trial was sponsored by Eli Lilly and Co. Dr. Harbeck disclosed research funding and speaker’s bureau activity for Lilly and others, and a consulting or advisory role with Gilead, Roche, Sanofi, Sandoz, and Seagen. Dr. Kalinsky disclosed a consulting or advisory role with multiple companies, not including Lilly.
MADRID – Five years on, the addition of the CDK4/6 inhibitor abemaciclib (Verzenio) to endocrine therapy for women with high-risk hormone receptor–positive, HER2-negative (HR+/HER2–) early breast cancer continues to show modest but clinically significant benefits, compared with endocrine therapy alone.
Results of a planned 5-year efficacy analysis of the monarchE trial showed that , reported Nadia Harbeck, MD, from the Breast Center at Ludwig Maximilians University Hospital in Munich.
“The data are consistent with a carryover effect and further support the addition of adjuvant abemaciclib to endocrine therapy for patients with hormone receptor–positive, HER2-negative, node-positive high-risk early breast cancer,” she said at the 2023 European Society for Medical Oncology Congress.
High recurrence risk
Although HR+/HER2– breast cancer, the most common subtype of breast cancer, is generally associated with better outcomes than other subtypes, patients with node-positive early disease are at high risk for early recurrence and need treatment intensification, Dr. Harbeck said.
The monarchE trial included two cohorts: a primary cohort consisting of patients deemed at high risk based on clinical pathological features such as the number of involved axillary nodes, grade 3 disease, and tumors 5 cm or larger, and a second cohort of patients with lower disease grade and smaller tumors but with high levels of the proliferation marker Ki-67.
A total of 5,637 patients were randomized to receive either 2 years of abemaciclib 150 mg twice daily plus endocrine therapy, or endocrine therapy alone, followed by 3-8 years of additional endocrine as clinically indicated in each study arm.
An earlier preplanned interim analysis of the phase 3 trial of more than 5,600 patients was presented at the ESMO Virtual Congress 2020, and simultaneously published in the Journal of Clinical Oncology.
As that analysis showed, at a median follow-up of 15.5 months abemaciclib plus endocrine therapy was associated with a 25% relative risk reduction in the primary endpoint of IDFS vs. endocrine therapy alone.
At the time, the findings were hailed as practice-changing and, once approved for high-risk HR+/HER2-negative early breast cancer, as the new standard of care.
In the current analysis, Dr. Harbeck and colleagues looked at 5-year outcomes from a prespecified analysis, with a data cutoff of July 3, 2023.
All patients originally assigned to abemaciclib are now off the drug, and more than 80% have been followed for a minimum of 2 year since completing therapy with the CDK4/6 inhibitor.
Results
At 5 years there were cumulative totals of 407 IDFS events in the combination arm, compared with 585 in the endocrine therapy alone arm, a difference that translated into a hazard ratio of 0.68 favoring abemaciclib (P < .001).
The IDFS benefit with the combination was consistent across most subgroups, including older patients, perimenopausal and postmenopausal patients, those who had received prior neoadjuvant or adjuvant chemotherapy, all tumor sizes, number of positive lymph nodes, less favorable tumor stage or grade, and order of endocrine therapy (tamoxifen or aromatase inhibitor as first drug).
As noted before, DRFS, a secondary endpoint, also favored abemaciclib, with 345 events occurring over 5 years in the combination arm, compared with 501 in the endocrine therapy arm alone. This translated into a HR with the combination of 0.68 (P < .001).
There were fewer deaths in the abemaciclib arm (208 vs. 234), but this difference was not statistically significant.
The proportions of patients with treatment-emergent adverse events and serious adverse events (SAEs) were higher in the combination arm than in the endocrine therapy alone arm in all previous analyses of the trial data.
In the current analysis, “I would say it’s reassuring to see that the SAEs reported in the follow-up period, after the study treatment had been completed, are quite similar between the endocrine therapy alone arm and the abemaciclib plus endocrine therapy arm,” Dr. Harbeck said.
Changing road map
Invited discussant Kevin Kalinsky, MD, MS, from the Winship Cancer Institute at Emory University, Atlanta, commented that CDK4/6 inhibitors “have changed the road map for treating hormone receptor–positive, HER2-negative disease.”
To put the monarchE results in context, he compared them with those of the NATALEE trial, in which patients were randomized to endocrine therapy with or without the CDK4/6 inhibitor ribociclib (Kisqali). That combination was previously shown to provide a significant survival advantage for women with metastatic breast cancer.
In NATALEE, which included both high-risk and intermediate-risk patients with early breast cancer, the absolute difference in 3-year IDFS rates between the combination group and endocrine monotherapy groups was 3.3%.
To determine the ultimate value of combining a CDK4/6 inhibitor with endocrine therapy in early breast cancer, longer follow-up of both trials will be necessary, Dr. Kalinsky said.
“The reason that follow-up is critical for both of these studies is that for this subtype of breast cancer, based upon data including from the Early Breast Cancer Trialists Group, we can see approximately 50% of recurrences after the first 5 years, and we think of cytotoxic chemotherapy as benefiting patients within those first 5 years. And while we think of CDK4/6 inhibitors as being cytostatic drugs, we are seeing a carryover effect in which 2 years of abemaciclib is improving outcome at the 5-year landmark,” he said.
Questions that still need to be answered include the optimal duration of CDK4/6 inhibitor therapy, whether adjuvant therapy should be resumed when there are signs of renewed proliferation, and whether there would be a benefit to restarting CDK4/6 inhibitors when metastasis occurs.
The monarchE trial was sponsored by Eli Lilly and Co. Dr. Harbeck disclosed research funding and speaker’s bureau activity for Lilly and others, and a consulting or advisory role with Gilead, Roche, Sanofi, Sandoz, and Seagen. Dr. Kalinsky disclosed a consulting or advisory role with multiple companies, not including Lilly.
MADRID – Five years on, the addition of the CDK4/6 inhibitor abemaciclib (Verzenio) to endocrine therapy for women with high-risk hormone receptor–positive, HER2-negative (HR+/HER2–) early breast cancer continues to show modest but clinically significant benefits, compared with endocrine therapy alone.
Results of a planned 5-year efficacy analysis of the monarchE trial showed that , reported Nadia Harbeck, MD, from the Breast Center at Ludwig Maximilians University Hospital in Munich.
“The data are consistent with a carryover effect and further support the addition of adjuvant abemaciclib to endocrine therapy for patients with hormone receptor–positive, HER2-negative, node-positive high-risk early breast cancer,” she said at the 2023 European Society for Medical Oncology Congress.
High recurrence risk
Although HR+/HER2– breast cancer, the most common subtype of breast cancer, is generally associated with better outcomes than other subtypes, patients with node-positive early disease are at high risk for early recurrence and need treatment intensification, Dr. Harbeck said.
The monarchE trial included two cohorts: a primary cohort consisting of patients deemed at high risk based on clinical pathological features such as the number of involved axillary nodes, grade 3 disease, and tumors 5 cm or larger, and a second cohort of patients with lower disease grade and smaller tumors but with high levels of the proliferation marker Ki-67.
A total of 5,637 patients were randomized to receive either 2 years of abemaciclib 150 mg twice daily plus endocrine therapy, or endocrine therapy alone, followed by 3-8 years of additional endocrine as clinically indicated in each study arm.
An earlier preplanned interim analysis of the phase 3 trial of more than 5,600 patients was presented at the ESMO Virtual Congress 2020, and simultaneously published in the Journal of Clinical Oncology.
As that analysis showed, at a median follow-up of 15.5 months abemaciclib plus endocrine therapy was associated with a 25% relative risk reduction in the primary endpoint of IDFS vs. endocrine therapy alone.
At the time, the findings were hailed as practice-changing and, once approved for high-risk HR+/HER2-negative early breast cancer, as the new standard of care.
In the current analysis, Dr. Harbeck and colleagues looked at 5-year outcomes from a prespecified analysis, with a data cutoff of July 3, 2023.
All patients originally assigned to abemaciclib are now off the drug, and more than 80% have been followed for a minimum of 2 year since completing therapy with the CDK4/6 inhibitor.
Results
At 5 years there were cumulative totals of 407 IDFS events in the combination arm, compared with 585 in the endocrine therapy alone arm, a difference that translated into a hazard ratio of 0.68 favoring abemaciclib (P < .001).
The IDFS benefit with the combination was consistent across most subgroups, including older patients, perimenopausal and postmenopausal patients, those who had received prior neoadjuvant or adjuvant chemotherapy, all tumor sizes, number of positive lymph nodes, less favorable tumor stage or grade, and order of endocrine therapy (tamoxifen or aromatase inhibitor as first drug).
As noted before, DRFS, a secondary endpoint, also favored abemaciclib, with 345 events occurring over 5 years in the combination arm, compared with 501 in the endocrine therapy arm alone. This translated into a HR with the combination of 0.68 (P < .001).
There were fewer deaths in the abemaciclib arm (208 vs. 234), but this difference was not statistically significant.
The proportions of patients with treatment-emergent adverse events and serious adverse events (SAEs) were higher in the combination arm than in the endocrine therapy alone arm in all previous analyses of the trial data.
In the current analysis, “I would say it’s reassuring to see that the SAEs reported in the follow-up period, after the study treatment had been completed, are quite similar between the endocrine therapy alone arm and the abemaciclib plus endocrine therapy arm,” Dr. Harbeck said.
Changing road map
Invited discussant Kevin Kalinsky, MD, MS, from the Winship Cancer Institute at Emory University, Atlanta, commented that CDK4/6 inhibitors “have changed the road map for treating hormone receptor–positive, HER2-negative disease.”
To put the monarchE results in context, he compared them with those of the NATALEE trial, in which patients were randomized to endocrine therapy with or without the CDK4/6 inhibitor ribociclib (Kisqali). That combination was previously shown to provide a significant survival advantage for women with metastatic breast cancer.
In NATALEE, which included both high-risk and intermediate-risk patients with early breast cancer, the absolute difference in 3-year IDFS rates between the combination group and endocrine monotherapy groups was 3.3%.
To determine the ultimate value of combining a CDK4/6 inhibitor with endocrine therapy in early breast cancer, longer follow-up of both trials will be necessary, Dr. Kalinsky said.
“The reason that follow-up is critical for both of these studies is that for this subtype of breast cancer, based upon data including from the Early Breast Cancer Trialists Group, we can see approximately 50% of recurrences after the first 5 years, and we think of cytotoxic chemotherapy as benefiting patients within those first 5 years. And while we think of CDK4/6 inhibitors as being cytostatic drugs, we are seeing a carryover effect in which 2 years of abemaciclib is improving outcome at the 5-year landmark,” he said.
Questions that still need to be answered include the optimal duration of CDK4/6 inhibitor therapy, whether adjuvant therapy should be resumed when there are signs of renewed proliferation, and whether there would be a benefit to restarting CDK4/6 inhibitors when metastasis occurs.
The monarchE trial was sponsored by Eli Lilly and Co. Dr. Harbeck disclosed research funding and speaker’s bureau activity for Lilly and others, and a consulting or advisory role with Gilead, Roche, Sanofi, Sandoz, and Seagen. Dr. Kalinsky disclosed a consulting or advisory role with multiple companies, not including Lilly.
FROM ESMO 2023
FDA proposes ban on hair straightener ingredients
The
The proposal specifies that formaldehyde would be banned, as well as other chemicals that release formaldehyde, such as methylene glycol. Using hair smoothing products containing formaldehyde and formaldehyde-releasing chemicals “is linked to short-term adverse health effects, such as sensitization reactions and breathing problems, and long-term adverse health effects, including an increased risk of certain cancers,” the proposal states.
One study published last year showed that repeated use of hair straightening products, also called relaxers, could more than double the risk of uterine cancer. Although that study didn’t find that the uterine cancer risk varied based on a person’s race, the researchers noted that women who are Black are among the most likely to use the products and tend to start using them at younger ages, compared with people of other races and ethnicities.
Hair straightening products have also been linked to elevated risks of hormone-sensitive cancers, such as breast cancer and ovarian cancer.
Rep. Ayanna Pressley (D-Mass.) and Rep. Shontel Brown (D-Ohio) applauded the proposed rule in a statement issued jointly on Oct. 6. “The FDA’s proposal to ban these harmful chemicals in hair straighteners and relaxers is a win for public health – especially the health of Black women who are disproportionately put at risk by these products as a result of systemic racism and anti–Black hair sentiment,” Rep. Pressley said The two congresswomen wrote a letter to the FDA earlier this year requesting the topic be investigated.
“Regardless of how we wear our hair, we should be allowed to show up in the world without putting our health at risk. I applaud the FDA for being responsive to our calls and advancing a rule that will help prevent manufacturers from making a profit at the expense of our health,” Rep. Pressley said in the statement. “The administration should finalize this rule without delay.”
A version of this article appeared on WebMD.com
The
The proposal specifies that formaldehyde would be banned, as well as other chemicals that release formaldehyde, such as methylene glycol. Using hair smoothing products containing formaldehyde and formaldehyde-releasing chemicals “is linked to short-term adverse health effects, such as sensitization reactions and breathing problems, and long-term adverse health effects, including an increased risk of certain cancers,” the proposal states.
One study published last year showed that repeated use of hair straightening products, also called relaxers, could more than double the risk of uterine cancer. Although that study didn’t find that the uterine cancer risk varied based on a person’s race, the researchers noted that women who are Black are among the most likely to use the products and tend to start using them at younger ages, compared with people of other races and ethnicities.
Hair straightening products have also been linked to elevated risks of hormone-sensitive cancers, such as breast cancer and ovarian cancer.
Rep. Ayanna Pressley (D-Mass.) and Rep. Shontel Brown (D-Ohio) applauded the proposed rule in a statement issued jointly on Oct. 6. “The FDA’s proposal to ban these harmful chemicals in hair straighteners and relaxers is a win for public health – especially the health of Black women who are disproportionately put at risk by these products as a result of systemic racism and anti–Black hair sentiment,” Rep. Pressley said The two congresswomen wrote a letter to the FDA earlier this year requesting the topic be investigated.
“Regardless of how we wear our hair, we should be allowed to show up in the world without putting our health at risk. I applaud the FDA for being responsive to our calls and advancing a rule that will help prevent manufacturers from making a profit at the expense of our health,” Rep. Pressley said in the statement. “The administration should finalize this rule without delay.”
A version of this article appeared on WebMD.com
The
The proposal specifies that formaldehyde would be banned, as well as other chemicals that release formaldehyde, such as methylene glycol. Using hair smoothing products containing formaldehyde and formaldehyde-releasing chemicals “is linked to short-term adverse health effects, such as sensitization reactions and breathing problems, and long-term adverse health effects, including an increased risk of certain cancers,” the proposal states.
One study published last year showed that repeated use of hair straightening products, also called relaxers, could more than double the risk of uterine cancer. Although that study didn’t find that the uterine cancer risk varied based on a person’s race, the researchers noted that women who are Black are among the most likely to use the products and tend to start using them at younger ages, compared with people of other races and ethnicities.
Hair straightening products have also been linked to elevated risks of hormone-sensitive cancers, such as breast cancer and ovarian cancer.
Rep. Ayanna Pressley (D-Mass.) and Rep. Shontel Brown (D-Ohio) applauded the proposed rule in a statement issued jointly on Oct. 6. “The FDA’s proposal to ban these harmful chemicals in hair straighteners and relaxers is a win for public health – especially the health of Black women who are disproportionately put at risk by these products as a result of systemic racism and anti–Black hair sentiment,” Rep. Pressley said The two congresswomen wrote a letter to the FDA earlier this year requesting the topic be investigated.
“Regardless of how we wear our hair, we should be allowed to show up in the world without putting our health at risk. I applaud the FDA for being responsive to our calls and advancing a rule that will help prevent manufacturers from making a profit at the expense of our health,” Rep. Pressley said in the statement. “The administration should finalize this rule without delay.”
A version of this article appeared on WebMD.com
Pain in fingers for several months
Psoriatic arthritis (PsA) is an immune-mediated arthritis that is almost always associated with plaque psoriasis. PsA is diagnosed in about 20% of patients with plaque psoriasis, and, in most patients, a diagnosis of plaque psoriasis precedes PsA development. However, in this patient, PsA appears to have become symptomatic at about the same time as the skin symptoms behind his ears, which is consistent with studies showing that PsA co-occurs with plaque psoriasis in 15% of patients and precedes plaque psoriasis diagnosis in 17% of patients. Environmental risk factors for PsA in genetically susceptible patients with plaque psoriasis include joint trauma, streptococcal infection, and certain antibiotics. Smoking appears to have a protective effect in PsA. PsA is more highly associated with severe plaque psoriasis than with mild plaque psoriasis.
PsA has a heterogeneous presentation, which may challenge diagnosis. It is classified by the degree of joint involvement as oligoarticular (four or fewer joints) or polyarticular (five or more joints). Radiographically, there are five main types of PsA, one of which is predominant involvement of the distal interphalangeal joint (DIP) joints — the form seen in this patient. The other four types of PsA are symmetrical peripheral polyarthritis, asymmetrical mono- or oligoarthritis, axial spondyloarthropathy, and arthritis mutilans. DIP joint involvement with proliferative bone changes suggests a diagnosis of PsA over rheumatoid arthritis.
PsA is diagnosed using radiography, skin biopsy of affected skin areas, and complete blood and metabolic assessments. With advanced PsA, radiographs typically reveal bone destruction and disease-related bone formation (juxta-articular bone formation), resulting in erosions, joint destruction, and joint-space narrowing. Juxta-articular bone formation (presenting poorly defined ossification adjacent to the joint margin) is often the earliest radiographic clue before erosions may occur. Enthesitis in multiple entheses is typical of PsA vs osteoarthritis or mechanical injury. There are no specific tests to confirm PsA. Patients with PsA usually are rheumatoid factor negative. Inflammatory biomarkers, such as C-reactive protein and erythrocyte sedimentation rate, are elevated in about 40% of patients with PsA.
In addition to plaques, extra-articular manifestations of PsA may include nail changes and chronic bilateral ocular disease. PsA is an articular manifestation of psoriasis but is still a systemic disease with deleterious effects on cardiometabolic factors; increased mortality with myocardial infarction; and possible involvement of other immune-mediated diseases, such as inflammatory bowel disease, that share a common pathway of tumor necrosis factor (TNF) alpha overexpression.
Early treatment with disease-modifying drugs plus nonpharmacologic interventions is crucial to minimizing disability progression and optimizing patients' quality of life. Nonpharmacologic interventions include physical and occupational therapy and exercise. Symptomatic therapies (nonsteroidal anti-inflammatory drugs or steroids) can be used to relieve mild symptoms. Assessment for cardiometabolic, renal, and other systemic impacts of PsA is essential.
Biologic therapies are key to preventing disease progression. The most broadly targeted are the TNF-alpha inhibitors, which are commonly recommended as first-line therapy in moderate to severe PsA or in patients with radiographic damage. The interleukin (IL)-17 inhibitors ixekizumab and secukinumab and the IL-12/23 inhibitor ustekinumab have targets downstream of TNF-alpha. Treatment options also include oral small molecule inhibitors of phosphodiesterase 4 (apremilast) or Janus kinase (tofacitinib, upadacitinib). Recognition of the role of IL-23 as a key cytokine in PsA development through promotion of Th17 differentiation has led to availability of drugs specifically targeting IL-23(p19) (guselkumab, tildrakizumab, and risankizumab). All have been studied, but only guselkumab and risankizumab currently are approved for treatment of PsA. Each class of drugs has specific benefits and risks, which should be discussed with patients before treatment initiation. Patient comorbidities should also be considered in choosing treatment.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Psoriatic arthritis (PsA) is an immune-mediated arthritis that is almost always associated with plaque psoriasis. PsA is diagnosed in about 20% of patients with plaque psoriasis, and, in most patients, a diagnosis of plaque psoriasis precedes PsA development. However, in this patient, PsA appears to have become symptomatic at about the same time as the skin symptoms behind his ears, which is consistent with studies showing that PsA co-occurs with plaque psoriasis in 15% of patients and precedes plaque psoriasis diagnosis in 17% of patients. Environmental risk factors for PsA in genetically susceptible patients with plaque psoriasis include joint trauma, streptococcal infection, and certain antibiotics. Smoking appears to have a protective effect in PsA. PsA is more highly associated with severe plaque psoriasis than with mild plaque psoriasis.
PsA has a heterogeneous presentation, which may challenge diagnosis. It is classified by the degree of joint involvement as oligoarticular (four or fewer joints) or polyarticular (five or more joints). Radiographically, there are five main types of PsA, one of which is predominant involvement of the distal interphalangeal joint (DIP) joints — the form seen in this patient. The other four types of PsA are symmetrical peripheral polyarthritis, asymmetrical mono- or oligoarthritis, axial spondyloarthropathy, and arthritis mutilans. DIP joint involvement with proliferative bone changes suggests a diagnosis of PsA over rheumatoid arthritis.
PsA is diagnosed using radiography, skin biopsy of affected skin areas, and complete blood and metabolic assessments. With advanced PsA, radiographs typically reveal bone destruction and disease-related bone formation (juxta-articular bone formation), resulting in erosions, joint destruction, and joint-space narrowing. Juxta-articular bone formation (presenting poorly defined ossification adjacent to the joint margin) is often the earliest radiographic clue before erosions may occur. Enthesitis in multiple entheses is typical of PsA vs osteoarthritis or mechanical injury. There are no specific tests to confirm PsA. Patients with PsA usually are rheumatoid factor negative. Inflammatory biomarkers, such as C-reactive protein and erythrocyte sedimentation rate, are elevated in about 40% of patients with PsA.
In addition to plaques, extra-articular manifestations of PsA may include nail changes and chronic bilateral ocular disease. PsA is an articular manifestation of psoriasis but is still a systemic disease with deleterious effects on cardiometabolic factors; increased mortality with myocardial infarction; and possible involvement of other immune-mediated diseases, such as inflammatory bowel disease, that share a common pathway of tumor necrosis factor (TNF) alpha overexpression.
Early treatment with disease-modifying drugs plus nonpharmacologic interventions is crucial to minimizing disability progression and optimizing patients' quality of life. Nonpharmacologic interventions include physical and occupational therapy and exercise. Symptomatic therapies (nonsteroidal anti-inflammatory drugs or steroids) can be used to relieve mild symptoms. Assessment for cardiometabolic, renal, and other systemic impacts of PsA is essential.
Biologic therapies are key to preventing disease progression. The most broadly targeted are the TNF-alpha inhibitors, which are commonly recommended as first-line therapy in moderate to severe PsA or in patients with radiographic damage. The interleukin (IL)-17 inhibitors ixekizumab and secukinumab and the IL-12/23 inhibitor ustekinumab have targets downstream of TNF-alpha. Treatment options also include oral small molecule inhibitors of phosphodiesterase 4 (apremilast) or Janus kinase (tofacitinib, upadacitinib). Recognition of the role of IL-23 as a key cytokine in PsA development through promotion of Th17 differentiation has led to availability of drugs specifically targeting IL-23(p19) (guselkumab, tildrakizumab, and risankizumab). All have been studied, but only guselkumab and risankizumab currently are approved for treatment of PsA. Each class of drugs has specific benefits and risks, which should be discussed with patients before treatment initiation. Patient comorbidities should also be considered in choosing treatment.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Psoriatic arthritis (PsA) is an immune-mediated arthritis that is almost always associated with plaque psoriasis. PsA is diagnosed in about 20% of patients with plaque psoriasis, and, in most patients, a diagnosis of plaque psoriasis precedes PsA development. However, in this patient, PsA appears to have become symptomatic at about the same time as the skin symptoms behind his ears, which is consistent with studies showing that PsA co-occurs with plaque psoriasis in 15% of patients and precedes plaque psoriasis diagnosis in 17% of patients. Environmental risk factors for PsA in genetically susceptible patients with plaque psoriasis include joint trauma, streptococcal infection, and certain antibiotics. Smoking appears to have a protective effect in PsA. PsA is more highly associated with severe plaque psoriasis than with mild plaque psoriasis.
PsA has a heterogeneous presentation, which may challenge diagnosis. It is classified by the degree of joint involvement as oligoarticular (four or fewer joints) or polyarticular (five or more joints). Radiographically, there are five main types of PsA, one of which is predominant involvement of the distal interphalangeal joint (DIP) joints — the form seen in this patient. The other four types of PsA are symmetrical peripheral polyarthritis, asymmetrical mono- or oligoarthritis, axial spondyloarthropathy, and arthritis mutilans. DIP joint involvement with proliferative bone changes suggests a diagnosis of PsA over rheumatoid arthritis.
PsA is diagnosed using radiography, skin biopsy of affected skin areas, and complete blood and metabolic assessments. With advanced PsA, radiographs typically reveal bone destruction and disease-related bone formation (juxta-articular bone formation), resulting in erosions, joint destruction, and joint-space narrowing. Juxta-articular bone formation (presenting poorly defined ossification adjacent to the joint margin) is often the earliest radiographic clue before erosions may occur. Enthesitis in multiple entheses is typical of PsA vs osteoarthritis or mechanical injury. There are no specific tests to confirm PsA. Patients with PsA usually are rheumatoid factor negative. Inflammatory biomarkers, such as C-reactive protein and erythrocyte sedimentation rate, are elevated in about 40% of patients with PsA.
In addition to plaques, extra-articular manifestations of PsA may include nail changes and chronic bilateral ocular disease. PsA is an articular manifestation of psoriasis but is still a systemic disease with deleterious effects on cardiometabolic factors; increased mortality with myocardial infarction; and possible involvement of other immune-mediated diseases, such as inflammatory bowel disease, that share a common pathway of tumor necrosis factor (TNF) alpha overexpression.
Early treatment with disease-modifying drugs plus nonpharmacologic interventions is crucial to minimizing disability progression and optimizing patients' quality of life. Nonpharmacologic interventions include physical and occupational therapy and exercise. Symptomatic therapies (nonsteroidal anti-inflammatory drugs or steroids) can be used to relieve mild symptoms. Assessment for cardiometabolic, renal, and other systemic impacts of PsA is essential.
Biologic therapies are key to preventing disease progression. The most broadly targeted are the TNF-alpha inhibitors, which are commonly recommended as first-line therapy in moderate to severe PsA or in patients with radiographic damage. The interleukin (IL)-17 inhibitors ixekizumab and secukinumab and the IL-12/23 inhibitor ustekinumab have targets downstream of TNF-alpha. Treatment options also include oral small molecule inhibitors of phosphodiesterase 4 (apremilast) or Janus kinase (tofacitinib, upadacitinib). Recognition of the role of IL-23 as a key cytokine in PsA development through promotion of Th17 differentiation has led to availability of drugs specifically targeting IL-23(p19) (guselkumab, tildrakizumab, and risankizumab). All have been studied, but only guselkumab and risankizumab currently are approved for treatment of PsA. Each class of drugs has specific benefits and risks, which should be discussed with patients before treatment initiation. Patient comorbidities should also be considered in choosing treatment.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 58-year-old man presents with pain in fingers of several months' duration, which is moderately relieved with over-the-counter naproxen. He is concerned about "crooked" fingers and is worried that his work will be affected. He is overweight (BMI, 28.8), hypertensive, hypercholesterolemic, and a nonsmoker. The patient also reports a 6-month history of itchy "scalp" behind the ears, not relieved with dandruff shampoos. Physical exam reveals advanced deformity in index and middle fingers of both hands and no evident deformity in the wrists or metacarpophalangeal joints. Nails are pitted and discolored. Scalp behind ears shows well-demarcated, scaly patches. Lab work, radiography, and biopsy of the retroauricular area are ordered.
Medicare Advantage: The good, the bad, and the ugly
As of 2023, most people eligible for Medicare are enrolled in Medicare Advantage plans administered by commercial insurers, rather than traditional Medicare plans sponsored by the federal government. The Kaiser Family Foundation reports that 31 million people are now enrolled in a Medicare Advantage plan, with almost half of them (47%) served by United Healthcare or Humana.
This is 51% of all people eligible for Medicare, compared with 19% in 2007. The Congressional Budget Office projects that 62% of Medicare participants will be in Medicare Advantage plans by 2033.
Given the explosive growth in Medicare Advantage participation, many readers have likely seen patients served by Medicare Advantage or will soon. Below is information about the program’s purpose, strengths, limitations, and effect on physicians.
How does Medicare Advantage differ from traditional Medicare?
A Medicare Advantage plan is approved by the U.S. Centers for Medicare & Medicaid Services and competes for customers by offering lower premiums and/or more benefits. Traditional Medicare plans are unified contracts across the country, with the same fees for the same services paid nationwide.
CMS pays Medicare Advantage plans a per-member rate, which can be increased for people who seem sicker than other plan participants – for example, someone with uncontrolled diabetes and multiple comorbidities. This so-called “risk adjustment” doesn’t exist in traditional Medicare.
CMS also gives incentive payments to Medicare Advantage plans whose members receive better care, measured by such metrics as lower unnecessary hospital admissions. There are some analogues to this in traditional Medicare, such as the Making Care Primary program, but value-based care is a larger component of Medicare Advantage.
“Being paid for outcomes is what we as physicians always thought we went into medicine to do,” said Sarah Candler, MD, an internist in Houston. She most recently worked for One Medical and her experiences with Veterans Affairs and One Medical focused on value-based contracts, including for Medicare Advantage plans.
How do patients benefit from Medicare Advantage?
“Honestly the financial benefits to patients are what’s really driving the rise in Medicare Advantage,” said
Claire Ankuda, MD, MPH, is a geriatrician at the Icahn School of Medicine at Mt. Sinai in New York who has published extensively about Medicare Advantage.
A spokesperson for Highmark Health, which provides Medicare Advantage plans, told us that “Medicare Advantage plans are offered by private health insurers, such as Highmark, and typically offer benefits that support members’ total health, such as low-cost access to doctors and preventive care, and cover things like prescription drugs, vision, and hearing services, dental, and chiropractic care. Medicare Advantage plans also protect members from unforeseen costs like hospitalizations, surgery, or an expensive drug. And unlike traditional Medicare, Medicare Advantage plans can offer set copays for doctor’s visits (rather than coinsurance) to help members budget for their costs.”
Dr. Ankuda said hospitalization costs are sometimes higher for Medicare Advantage but agreed that costs for doctor visits are often lower with Medicare Advantage plans than with traditional Medicare.
So while the overarching goal of Medicare Advantage makes sense, Dr. Candler said, the actual physician experience of working with Medicare Advantage can be challenging.
What challenges might physicians experience when treating patients in a Medicare Advantage plan?
“The plan itself has control over what it will pay for and they’re much more aggressive about it than traditional Medicare,” Dr. Candler said. Medicare Advantage plans are often structured as health maintenance organizations, with narrow provider networks and extensive prior authorization requirements.
Dr. Candler gave an example of a plan that offers transportation to medical appointments – a seemingly great benefit. But what if someone needs to see a cardiologist and the only cardiologist within the plan is 100 miles away? That’s too far for the transportation benefit, it turns out.
Or a Medicare Advantage plan requires a physician to first do a physical exam before ordering an MRI, even though in the physician’s judgment only the MRI will have diagnostic value. Or the plan denies coverage for a service that’s already occurred. These practices aim to weed out unnecessary care but at the cost of patient confusion or physician time in arguing why something should be covered.
“The argument from us as physicians would be, ‘Just trust us to practice good medicine,’” Dr. Candler said.
Beside these concerns at the physician level, the regulations surrounding Medicare Advantage plans may open the door to billing fraud.
How do Medicare Advantage Plans interact with diagnosis codes?
“I just can’t stand when I see fraud in the health care system,” said Nancy Keating, MD, MPH, an internist at Brigham and Women’s Hospital and a health policy professor at Harvard Medical School, both in Boston.
This July, Dr. Keating published a report in the Annals of Internal Medicine about a patient of hers whose health insurer – a Medicare Advantage plan – claimed had diabetes with comorbidities and was morbidly obese. None of this was true. But submitting such diagnoses to CMS would suggest that Dr. Keating’s patient was especially ill, leading to greater reimbursements from CMS for covering her care.
“I’m not averse to paying plans that are taking care of sicker patients more, but we need to figure out who those patients truly are,” Dr. Keating said.
“It is absolutely true and widely proven that Medicare Advantage plans do a lot of clever things” to inflate diagnoses, added Dr. Ankuda. Also, she said that Medicare Advantage plan representatives would say this is legitimate work, as the entire point of Medicare Advantage is to pay more for caring for sicker patients.
“I don’t think anyone here is acting in bad faith. It’s just that [there are] very different incentives,” Dr. Ankuda said.
How can Medicare Advantage be improved?
Dr. Keating believes that CMS should reduce the number of diagnosis codes allowed within Medicare Advantage to thwart the potential for upcoding. Dr. Ankuda thinks the biggest problem is that there is no good way for patients to choose among Medicare Advantage plans.
“I don’t see it as Medicare Advantage is bad or Medicare Advantage is good. MA plans are incredibly diverse,” Dr. Ankuda said. The problem is that it’s very hard for patients to tell which plans are delivering the best care, what their out-of-pocket costs will actually be, or how often a plan denies payment.
Dr. Ankuda argues for much more data transparency around such plan factors.
Dr. Candler and Dr. Ankuda had no relevant conflicts. Dr. Keating is a consultant to the Research Triangle Institute, which advises CMS about Medicare Advantage billing codes.
As of 2023, most people eligible for Medicare are enrolled in Medicare Advantage plans administered by commercial insurers, rather than traditional Medicare plans sponsored by the federal government. The Kaiser Family Foundation reports that 31 million people are now enrolled in a Medicare Advantage plan, with almost half of them (47%) served by United Healthcare or Humana.
This is 51% of all people eligible for Medicare, compared with 19% in 2007. The Congressional Budget Office projects that 62% of Medicare participants will be in Medicare Advantage plans by 2033.
Given the explosive growth in Medicare Advantage participation, many readers have likely seen patients served by Medicare Advantage or will soon. Below is information about the program’s purpose, strengths, limitations, and effect on physicians.
How does Medicare Advantage differ from traditional Medicare?
A Medicare Advantage plan is approved by the U.S. Centers for Medicare & Medicaid Services and competes for customers by offering lower premiums and/or more benefits. Traditional Medicare plans are unified contracts across the country, with the same fees for the same services paid nationwide.
CMS pays Medicare Advantage plans a per-member rate, which can be increased for people who seem sicker than other plan participants – for example, someone with uncontrolled diabetes and multiple comorbidities. This so-called “risk adjustment” doesn’t exist in traditional Medicare.
CMS also gives incentive payments to Medicare Advantage plans whose members receive better care, measured by such metrics as lower unnecessary hospital admissions. There are some analogues to this in traditional Medicare, such as the Making Care Primary program, but value-based care is a larger component of Medicare Advantage.
“Being paid for outcomes is what we as physicians always thought we went into medicine to do,” said Sarah Candler, MD, an internist in Houston. She most recently worked for One Medical and her experiences with Veterans Affairs and One Medical focused on value-based contracts, including for Medicare Advantage plans.
How do patients benefit from Medicare Advantage?
“Honestly the financial benefits to patients are what’s really driving the rise in Medicare Advantage,” said
Claire Ankuda, MD, MPH, is a geriatrician at the Icahn School of Medicine at Mt. Sinai in New York who has published extensively about Medicare Advantage.
A spokesperson for Highmark Health, which provides Medicare Advantage plans, told us that “Medicare Advantage plans are offered by private health insurers, such as Highmark, and typically offer benefits that support members’ total health, such as low-cost access to doctors and preventive care, and cover things like prescription drugs, vision, and hearing services, dental, and chiropractic care. Medicare Advantage plans also protect members from unforeseen costs like hospitalizations, surgery, or an expensive drug. And unlike traditional Medicare, Medicare Advantage plans can offer set copays for doctor’s visits (rather than coinsurance) to help members budget for their costs.”
Dr. Ankuda said hospitalization costs are sometimes higher for Medicare Advantage but agreed that costs for doctor visits are often lower with Medicare Advantage plans than with traditional Medicare.
So while the overarching goal of Medicare Advantage makes sense, Dr. Candler said, the actual physician experience of working with Medicare Advantage can be challenging.
What challenges might physicians experience when treating patients in a Medicare Advantage plan?
“The plan itself has control over what it will pay for and they’re much more aggressive about it than traditional Medicare,” Dr. Candler said. Medicare Advantage plans are often structured as health maintenance organizations, with narrow provider networks and extensive prior authorization requirements.
Dr. Candler gave an example of a plan that offers transportation to medical appointments – a seemingly great benefit. But what if someone needs to see a cardiologist and the only cardiologist within the plan is 100 miles away? That’s too far for the transportation benefit, it turns out.
Or a Medicare Advantage plan requires a physician to first do a physical exam before ordering an MRI, even though in the physician’s judgment only the MRI will have diagnostic value. Or the plan denies coverage for a service that’s already occurred. These practices aim to weed out unnecessary care but at the cost of patient confusion or physician time in arguing why something should be covered.
“The argument from us as physicians would be, ‘Just trust us to practice good medicine,’” Dr. Candler said.
Beside these concerns at the physician level, the regulations surrounding Medicare Advantage plans may open the door to billing fraud.
How do Medicare Advantage Plans interact with diagnosis codes?
“I just can’t stand when I see fraud in the health care system,” said Nancy Keating, MD, MPH, an internist at Brigham and Women’s Hospital and a health policy professor at Harvard Medical School, both in Boston.
This July, Dr. Keating published a report in the Annals of Internal Medicine about a patient of hers whose health insurer – a Medicare Advantage plan – claimed had diabetes with comorbidities and was morbidly obese. None of this was true. But submitting such diagnoses to CMS would suggest that Dr. Keating’s patient was especially ill, leading to greater reimbursements from CMS for covering her care.
“I’m not averse to paying plans that are taking care of sicker patients more, but we need to figure out who those patients truly are,” Dr. Keating said.
“It is absolutely true and widely proven that Medicare Advantage plans do a lot of clever things” to inflate diagnoses, added Dr. Ankuda. Also, she said that Medicare Advantage plan representatives would say this is legitimate work, as the entire point of Medicare Advantage is to pay more for caring for sicker patients.
“I don’t think anyone here is acting in bad faith. It’s just that [there are] very different incentives,” Dr. Ankuda said.
How can Medicare Advantage be improved?
Dr. Keating believes that CMS should reduce the number of diagnosis codes allowed within Medicare Advantage to thwart the potential for upcoding. Dr. Ankuda thinks the biggest problem is that there is no good way for patients to choose among Medicare Advantage plans.
“I don’t see it as Medicare Advantage is bad or Medicare Advantage is good. MA plans are incredibly diverse,” Dr. Ankuda said. The problem is that it’s very hard for patients to tell which plans are delivering the best care, what their out-of-pocket costs will actually be, or how often a plan denies payment.
Dr. Ankuda argues for much more data transparency around such plan factors.
Dr. Candler and Dr. Ankuda had no relevant conflicts. Dr. Keating is a consultant to the Research Triangle Institute, which advises CMS about Medicare Advantage billing codes.
As of 2023, most people eligible for Medicare are enrolled in Medicare Advantage plans administered by commercial insurers, rather than traditional Medicare plans sponsored by the federal government. The Kaiser Family Foundation reports that 31 million people are now enrolled in a Medicare Advantage plan, with almost half of them (47%) served by United Healthcare or Humana.
This is 51% of all people eligible for Medicare, compared with 19% in 2007. The Congressional Budget Office projects that 62% of Medicare participants will be in Medicare Advantage plans by 2033.
Given the explosive growth in Medicare Advantage participation, many readers have likely seen patients served by Medicare Advantage or will soon. Below is information about the program’s purpose, strengths, limitations, and effect on physicians.
How does Medicare Advantage differ from traditional Medicare?
A Medicare Advantage plan is approved by the U.S. Centers for Medicare & Medicaid Services and competes for customers by offering lower premiums and/or more benefits. Traditional Medicare plans are unified contracts across the country, with the same fees for the same services paid nationwide.
CMS pays Medicare Advantage plans a per-member rate, which can be increased for people who seem sicker than other plan participants – for example, someone with uncontrolled diabetes and multiple comorbidities. This so-called “risk adjustment” doesn’t exist in traditional Medicare.
CMS also gives incentive payments to Medicare Advantage plans whose members receive better care, measured by such metrics as lower unnecessary hospital admissions. There are some analogues to this in traditional Medicare, such as the Making Care Primary program, but value-based care is a larger component of Medicare Advantage.
“Being paid for outcomes is what we as physicians always thought we went into medicine to do,” said Sarah Candler, MD, an internist in Houston. She most recently worked for One Medical and her experiences with Veterans Affairs and One Medical focused on value-based contracts, including for Medicare Advantage plans.
How do patients benefit from Medicare Advantage?
“Honestly the financial benefits to patients are what’s really driving the rise in Medicare Advantage,” said
Claire Ankuda, MD, MPH, is a geriatrician at the Icahn School of Medicine at Mt. Sinai in New York who has published extensively about Medicare Advantage.
A spokesperson for Highmark Health, which provides Medicare Advantage plans, told us that “Medicare Advantage plans are offered by private health insurers, such as Highmark, and typically offer benefits that support members’ total health, such as low-cost access to doctors and preventive care, and cover things like prescription drugs, vision, and hearing services, dental, and chiropractic care. Medicare Advantage plans also protect members from unforeseen costs like hospitalizations, surgery, or an expensive drug. And unlike traditional Medicare, Medicare Advantage plans can offer set copays for doctor’s visits (rather than coinsurance) to help members budget for their costs.”
Dr. Ankuda said hospitalization costs are sometimes higher for Medicare Advantage but agreed that costs for doctor visits are often lower with Medicare Advantage plans than with traditional Medicare.
So while the overarching goal of Medicare Advantage makes sense, Dr. Candler said, the actual physician experience of working with Medicare Advantage can be challenging.
What challenges might physicians experience when treating patients in a Medicare Advantage plan?
“The plan itself has control over what it will pay for and they’re much more aggressive about it than traditional Medicare,” Dr. Candler said. Medicare Advantage plans are often structured as health maintenance organizations, with narrow provider networks and extensive prior authorization requirements.
Dr. Candler gave an example of a plan that offers transportation to medical appointments – a seemingly great benefit. But what if someone needs to see a cardiologist and the only cardiologist within the plan is 100 miles away? That’s too far for the transportation benefit, it turns out.
Or a Medicare Advantage plan requires a physician to first do a physical exam before ordering an MRI, even though in the physician’s judgment only the MRI will have diagnostic value. Or the plan denies coverage for a service that’s already occurred. These practices aim to weed out unnecessary care but at the cost of patient confusion or physician time in arguing why something should be covered.
“The argument from us as physicians would be, ‘Just trust us to practice good medicine,’” Dr. Candler said.
Beside these concerns at the physician level, the regulations surrounding Medicare Advantage plans may open the door to billing fraud.
How do Medicare Advantage Plans interact with diagnosis codes?
“I just can’t stand when I see fraud in the health care system,” said Nancy Keating, MD, MPH, an internist at Brigham and Women’s Hospital and a health policy professor at Harvard Medical School, both in Boston.
This July, Dr. Keating published a report in the Annals of Internal Medicine about a patient of hers whose health insurer – a Medicare Advantage plan – claimed had diabetes with comorbidities and was morbidly obese. None of this was true. But submitting such diagnoses to CMS would suggest that Dr. Keating’s patient was especially ill, leading to greater reimbursements from CMS for covering her care.
“I’m not averse to paying plans that are taking care of sicker patients more, but we need to figure out who those patients truly are,” Dr. Keating said.
“It is absolutely true and widely proven that Medicare Advantage plans do a lot of clever things” to inflate diagnoses, added Dr. Ankuda. Also, she said that Medicare Advantage plan representatives would say this is legitimate work, as the entire point of Medicare Advantage is to pay more for caring for sicker patients.
“I don’t think anyone here is acting in bad faith. It’s just that [there are] very different incentives,” Dr. Ankuda said.
How can Medicare Advantage be improved?
Dr. Keating believes that CMS should reduce the number of diagnosis codes allowed within Medicare Advantage to thwart the potential for upcoding. Dr. Ankuda thinks the biggest problem is that there is no good way for patients to choose among Medicare Advantage plans.
“I don’t see it as Medicare Advantage is bad or Medicare Advantage is good. MA plans are incredibly diverse,” Dr. Ankuda said. The problem is that it’s very hard for patients to tell which plans are delivering the best care, what their out-of-pocket costs will actually be, or how often a plan denies payment.
Dr. Ankuda argues for much more data transparency around such plan factors.
Dr. Candler and Dr. Ankuda had no relevant conflicts. Dr. Keating is a consultant to the Research Triangle Institute, which advises CMS about Medicare Advantage billing codes.
Not another emergency
This country faces a broad and frightening rogues’ gallery of challenges to its health. From the recent revelation that gunshots are the leading cause of death in children to the opioid epidemic to the overworked and discouraged health care providers, the crises are so numerous it is hard to choose where we should be investing what little political will we can muster. And, where do these disasters fit against a landscape raked by natural and climate change–triggered catastrophes? How do we even begin to triage our vocabulary as we are trying to label them?
The lead article in October’s journal Pediatrics makes a heroic effort to place pediatric obesity into this pantheon of health disasters. The authors of this Pediatrics Perspective ask a simple question: Should the United States declare pediatric obesity a public health emergency? They have wisely chosen to narrow the question to the pediatric population as being a more realistic target and one that is more likely to pay bigger dividends over time.
While acknowledging that obesity prevention strategies have been largely ineffective to this point, the authors are also concerned that despite the promising development of treatment strategies, the rollout of these therapies is likely to be uneven because of funding and disparities in health care delivery.
After reviewing pros and cons for an emergency declaration, they came to the conclusion that despite the scope of the problem and the fact that health emergencies have been declared for conditions effecting fewer individuals, now is not the time. The authors observed that a declaration may serve only to hype “the problem without offering tangible solutions.” Even when as yet to be discovered effective therapies become available, the time lag before measurable improvement is likely to be so delayed that “catastrophizing” pediatric obesity may be just another exercise in wolf-crying.
A closer look
While I applaud the authors for their courage in addressing this question and their decision to discourage an emergency declaration, a few of their observations deserve a closer look. First, they are legitimately concerned that any health policy must be careful not to further perpetuate the stigmatization of children with obesity. However, they feel the recognition by all stakeholders “that obesity is a genetically and biologically driven disease are essential.” While I have supported the disease designation as a pragmatic strategy to move things forward, I would prefer their statement to read “obesity can be ... “ I don’t think we have mined the data deep enough to determine how many out of a cohort of a million obese children from across a wide span of socioeconomic strata have become obese primarily as a result of decisions made by school departments, parents, and governmental entities – all of which had the resources to make healthier decisions but failed to do so.
While a majority of the population may believe that obesity is a “condition of choice,” I think they would be more likely to support the political will for action if they saw data that acknowledges that yes, obesity can be a condition of choice, but here are the circumstances in which choice can and can’t make a difference. Language must always be chosen carefully to minimize stigmatization. However, remember we are not pointing fingers at victims; we are instead looking for teaching moments in which adults can learn to make better choices for the children under their care who are too young to make their own.
Finally, as the authors of this Pediatric Perspectives considered cons of a declaration of health care emergency, they raised the peculiarly American concern of personal autonomy. As they pointed out, there are unfortunate examples in this country in which efforts to limit personal choice have backfired and well-meaning and potentially effective methods for limiting unhealthy behaviors have been eliminated in the name of personal freedom. I’m not sure how we manage this except to wait and be judicious as we move forward addressing pediatric obesity on a national scale. I urge you to take a few minutes to read this perspective. It is a topic worth considering.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
This country faces a broad and frightening rogues’ gallery of challenges to its health. From the recent revelation that gunshots are the leading cause of death in children to the opioid epidemic to the overworked and discouraged health care providers, the crises are so numerous it is hard to choose where we should be investing what little political will we can muster. And, where do these disasters fit against a landscape raked by natural and climate change–triggered catastrophes? How do we even begin to triage our vocabulary as we are trying to label them?
The lead article in October’s journal Pediatrics makes a heroic effort to place pediatric obesity into this pantheon of health disasters. The authors of this Pediatrics Perspective ask a simple question: Should the United States declare pediatric obesity a public health emergency? They have wisely chosen to narrow the question to the pediatric population as being a more realistic target and one that is more likely to pay bigger dividends over time.
While acknowledging that obesity prevention strategies have been largely ineffective to this point, the authors are also concerned that despite the promising development of treatment strategies, the rollout of these therapies is likely to be uneven because of funding and disparities in health care delivery.
After reviewing pros and cons for an emergency declaration, they came to the conclusion that despite the scope of the problem and the fact that health emergencies have been declared for conditions effecting fewer individuals, now is not the time. The authors observed that a declaration may serve only to hype “the problem without offering tangible solutions.” Even when as yet to be discovered effective therapies become available, the time lag before measurable improvement is likely to be so delayed that “catastrophizing” pediatric obesity may be just another exercise in wolf-crying.
A closer look
While I applaud the authors for their courage in addressing this question and their decision to discourage an emergency declaration, a few of their observations deserve a closer look. First, they are legitimately concerned that any health policy must be careful not to further perpetuate the stigmatization of children with obesity. However, they feel the recognition by all stakeholders “that obesity is a genetically and biologically driven disease are essential.” While I have supported the disease designation as a pragmatic strategy to move things forward, I would prefer their statement to read “obesity can be ... “ I don’t think we have mined the data deep enough to determine how many out of a cohort of a million obese children from across a wide span of socioeconomic strata have become obese primarily as a result of decisions made by school departments, parents, and governmental entities – all of which had the resources to make healthier decisions but failed to do so.
While a majority of the population may believe that obesity is a “condition of choice,” I think they would be more likely to support the political will for action if they saw data that acknowledges that yes, obesity can be a condition of choice, but here are the circumstances in which choice can and can’t make a difference. Language must always be chosen carefully to minimize stigmatization. However, remember we are not pointing fingers at victims; we are instead looking for teaching moments in which adults can learn to make better choices for the children under their care who are too young to make their own.
Finally, as the authors of this Pediatric Perspectives considered cons of a declaration of health care emergency, they raised the peculiarly American concern of personal autonomy. As they pointed out, there are unfortunate examples in this country in which efforts to limit personal choice have backfired and well-meaning and potentially effective methods for limiting unhealthy behaviors have been eliminated in the name of personal freedom. I’m not sure how we manage this except to wait and be judicious as we move forward addressing pediatric obesity on a national scale. I urge you to take a few minutes to read this perspective. It is a topic worth considering.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
This country faces a broad and frightening rogues’ gallery of challenges to its health. From the recent revelation that gunshots are the leading cause of death in children to the opioid epidemic to the overworked and discouraged health care providers, the crises are so numerous it is hard to choose where we should be investing what little political will we can muster. And, where do these disasters fit against a landscape raked by natural and climate change–triggered catastrophes? How do we even begin to triage our vocabulary as we are trying to label them?
The lead article in October’s journal Pediatrics makes a heroic effort to place pediatric obesity into this pantheon of health disasters. The authors of this Pediatrics Perspective ask a simple question: Should the United States declare pediatric obesity a public health emergency? They have wisely chosen to narrow the question to the pediatric population as being a more realistic target and one that is more likely to pay bigger dividends over time.
While acknowledging that obesity prevention strategies have been largely ineffective to this point, the authors are also concerned that despite the promising development of treatment strategies, the rollout of these therapies is likely to be uneven because of funding and disparities in health care delivery.
After reviewing pros and cons for an emergency declaration, they came to the conclusion that despite the scope of the problem and the fact that health emergencies have been declared for conditions effecting fewer individuals, now is not the time. The authors observed that a declaration may serve only to hype “the problem without offering tangible solutions.” Even when as yet to be discovered effective therapies become available, the time lag before measurable improvement is likely to be so delayed that “catastrophizing” pediatric obesity may be just another exercise in wolf-crying.
A closer look
While I applaud the authors for their courage in addressing this question and their decision to discourage an emergency declaration, a few of their observations deserve a closer look. First, they are legitimately concerned that any health policy must be careful not to further perpetuate the stigmatization of children with obesity. However, they feel the recognition by all stakeholders “that obesity is a genetically and biologically driven disease are essential.” While I have supported the disease designation as a pragmatic strategy to move things forward, I would prefer their statement to read “obesity can be ... “ I don’t think we have mined the data deep enough to determine how many out of a cohort of a million obese children from across a wide span of socioeconomic strata have become obese primarily as a result of decisions made by school departments, parents, and governmental entities – all of which had the resources to make healthier decisions but failed to do so.
While a majority of the population may believe that obesity is a “condition of choice,” I think they would be more likely to support the political will for action if they saw data that acknowledges that yes, obesity can be a condition of choice, but here are the circumstances in which choice can and can’t make a difference. Language must always be chosen carefully to minimize stigmatization. However, remember we are not pointing fingers at victims; we are instead looking for teaching moments in which adults can learn to make better choices for the children under their care who are too young to make their own.
Finally, as the authors of this Pediatric Perspectives considered cons of a declaration of health care emergency, they raised the peculiarly American concern of personal autonomy. As they pointed out, there are unfortunate examples in this country in which efforts to limit personal choice have backfired and well-meaning and potentially effective methods for limiting unhealthy behaviors have been eliminated in the name of personal freedom. I’m not sure how we manage this except to wait and be judicious as we move forward addressing pediatric obesity on a national scale. I urge you to take a few minutes to read this perspective. It is a topic worth considering.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
NMO: Study says double diagnoses with MS are common
MILAN – , according to a poster presented at the 9th Joint ECTRIMS-ACTRIMS meeting.
“There is a lack of education in differentiating between MS and NMO even in the medical community, which may result in a high misdiagnosis rate,” said study lead author Ka-Ho Wong, MBA, of the University of Utah, Salt Lake City, in an interview.
“NMO was recognized in the late 1800s and was historically thought to be a variant of MS until 1999,” said Michael Levy, MD, PhD, of Harvard Medical School and Massachusetts General Hospital, both in Boston, in an interview.
“They are both relapsing inflammatory disorders of the central nervous system with similarities in symptoms of weakness, numbness, mobility problem, vision defects, pain and fatigue,” said Dr. Levy, who did not take part in the new study. “A blood test for NMO was developed in 2004 and improved over time to the point that it can now reliably distinguish NMO from MS.”
As for therapy, “recent research has confirmed the two conditions are immunologically different and respond to different treatment,” Dr. Levy said. “The treatments developed for MS, especially from the 1990s, are harmful in NMO so it is important to make the diagnosis correctly.”
He added that “we do not recognize overlap between NMO or MS – it’s one or the other.”
Exploring the reasons for misdiagnosis
Mr. Wong, the present study’s lead author, said he and a research team launched the new study to better understand who gets misdiagnosed. “We know that almost 50% of the individuals get misdiagnosed at some point. However, what we don’t know yet is if the influencing factors are social determinants of health or if there are other causes.”
For the study, Mr. Wong and colleagues analyzed data from TriNetX, a health research network with access to medical records from 61 U.S. health care organizations. providing access to electronic medical records that includes sixty-one health care organizations (HCOs) in the United States.
ICD-10 coding statistics from 2008 to 2022 identified 7,657 patients with diagnoses for NMO. Of those, 4,040 (53%) only had diagnoses for NMO, and the rest (3,617, 47%) had diagnoses for both NMO and MS.
The researchers focused on 1,265 patients who had been coded for both diagnoses and had at least three clinical visits. They determined that a patient was misdiagnosed when they had three consecutive diagnoses of the same type. “For example, if they had MS but got misdiagnosed as NMO, once they are confirmed as MS they must have three or more consecutive diagnosis of MS to be considered as misdiagnosed,” Mr. Wong said.
Of the 1,265 subjects, the researchers determined that 308 (24%) had NMO but had been misdiagnosed as having MS, 189 (15%) had MS but were misdiagnosed as having NMO, and 768 (61%) were interchangeably diagnosed with the two conditions over time.
Among these three groups, 70.8%, 73.1%, and 78.4% were female, respectively; and 59.4%, 52.9%, and 53.0% were White, respectively. The percentages of Black patients were 17.2%, 24.3%, and 28.9%, respectively. Information about statistical significance was not provided in the poster.
Dr. Levy said he would “expect most NMO patients to initially be diagnosed with MS. It’s unusual to start with a diagnosis of NMO and then figure out it’s MS.”
As for the larger number of people with interchangeable diagnoses, Dr. Levy said that likely “reflects the messiness of billing codes.” For his part, Mr. Wong said there could be multiple causes for the interchangeable diagnoses: lack of disease knowledge, miscoding, lack of Food and Drug Administration–approved treatment for NMO at the time, and potentially other factors.
What does it all mean?
As for the study’s significance, Mr. Wong said a full workup should be performed before diagnosis, “and a neurologist should never prescribe disease-modifying therapies prior to a confirmation of diagnosis.”
Indeed, some disease-modifying therapies for MS are inappropriate for patients with NMO, Dr. Levy said. “The older medications, including beta-interferons, are among the most harmful to NMO patients. But they are not commonly used as first line for MS as they used to be. In contrast, B cell–depleting medications like ocrelizumab may be helpful in NMO.”
In regards to diagnosis, Dr. Levy noted that the NMO aquaporin-4 (AQP4) antibody test is “extremely specific and reliable.”
“A positive test result in the context of a clinical presentation of central nervous system inflammation allows for the diagnosis of NMO,” he said. “A negative test result is more complicated and may require some expertise to sort out after a careful review of the history, neurological exam, MRI features, central nervous system testing and other blood test results.”
The study was funded by the Sumaira Foundation. The authors did not provide information about relevant disclosures. Dr. Levy reports personal compensation for advisory board activities from Roche, Genentech, Chugai, Horizon, Alexion and Mitsubishi and grant support from Genentech, Horizon, Alexion, Sanofi, and UCB.
MILAN – , according to a poster presented at the 9th Joint ECTRIMS-ACTRIMS meeting.
“There is a lack of education in differentiating between MS and NMO even in the medical community, which may result in a high misdiagnosis rate,” said study lead author Ka-Ho Wong, MBA, of the University of Utah, Salt Lake City, in an interview.
“NMO was recognized in the late 1800s and was historically thought to be a variant of MS until 1999,” said Michael Levy, MD, PhD, of Harvard Medical School and Massachusetts General Hospital, both in Boston, in an interview.
“They are both relapsing inflammatory disorders of the central nervous system with similarities in symptoms of weakness, numbness, mobility problem, vision defects, pain and fatigue,” said Dr. Levy, who did not take part in the new study. “A blood test for NMO was developed in 2004 and improved over time to the point that it can now reliably distinguish NMO from MS.”
As for therapy, “recent research has confirmed the two conditions are immunologically different and respond to different treatment,” Dr. Levy said. “The treatments developed for MS, especially from the 1990s, are harmful in NMO so it is important to make the diagnosis correctly.”
He added that “we do not recognize overlap between NMO or MS – it’s one or the other.”
Exploring the reasons for misdiagnosis
Mr. Wong, the present study’s lead author, said he and a research team launched the new study to better understand who gets misdiagnosed. “We know that almost 50% of the individuals get misdiagnosed at some point. However, what we don’t know yet is if the influencing factors are social determinants of health or if there are other causes.”
For the study, Mr. Wong and colleagues analyzed data from TriNetX, a health research network with access to medical records from 61 U.S. health care organizations. providing access to electronic medical records that includes sixty-one health care organizations (HCOs) in the United States.
ICD-10 coding statistics from 2008 to 2022 identified 7,657 patients with diagnoses for NMO. Of those, 4,040 (53%) only had diagnoses for NMO, and the rest (3,617, 47%) had diagnoses for both NMO and MS.
The researchers focused on 1,265 patients who had been coded for both diagnoses and had at least three clinical visits. They determined that a patient was misdiagnosed when they had three consecutive diagnoses of the same type. “For example, if they had MS but got misdiagnosed as NMO, once they are confirmed as MS they must have three or more consecutive diagnosis of MS to be considered as misdiagnosed,” Mr. Wong said.
Of the 1,265 subjects, the researchers determined that 308 (24%) had NMO but had been misdiagnosed as having MS, 189 (15%) had MS but were misdiagnosed as having NMO, and 768 (61%) were interchangeably diagnosed with the two conditions over time.
Among these three groups, 70.8%, 73.1%, and 78.4% were female, respectively; and 59.4%, 52.9%, and 53.0% were White, respectively. The percentages of Black patients were 17.2%, 24.3%, and 28.9%, respectively. Information about statistical significance was not provided in the poster.
Dr. Levy said he would “expect most NMO patients to initially be diagnosed with MS. It’s unusual to start with a diagnosis of NMO and then figure out it’s MS.”
As for the larger number of people with interchangeable diagnoses, Dr. Levy said that likely “reflects the messiness of billing codes.” For his part, Mr. Wong said there could be multiple causes for the interchangeable diagnoses: lack of disease knowledge, miscoding, lack of Food and Drug Administration–approved treatment for NMO at the time, and potentially other factors.
What does it all mean?
As for the study’s significance, Mr. Wong said a full workup should be performed before diagnosis, “and a neurologist should never prescribe disease-modifying therapies prior to a confirmation of diagnosis.”
Indeed, some disease-modifying therapies for MS are inappropriate for patients with NMO, Dr. Levy said. “The older medications, including beta-interferons, are among the most harmful to NMO patients. But they are not commonly used as first line for MS as they used to be. In contrast, B cell–depleting medications like ocrelizumab may be helpful in NMO.”
In regards to diagnosis, Dr. Levy noted that the NMO aquaporin-4 (AQP4) antibody test is “extremely specific and reliable.”
“A positive test result in the context of a clinical presentation of central nervous system inflammation allows for the diagnosis of NMO,” he said. “A negative test result is more complicated and may require some expertise to sort out after a careful review of the history, neurological exam, MRI features, central nervous system testing and other blood test results.”
The study was funded by the Sumaira Foundation. The authors did not provide information about relevant disclosures. Dr. Levy reports personal compensation for advisory board activities from Roche, Genentech, Chugai, Horizon, Alexion and Mitsubishi and grant support from Genentech, Horizon, Alexion, Sanofi, and UCB.
MILAN – , according to a poster presented at the 9th Joint ECTRIMS-ACTRIMS meeting.
“There is a lack of education in differentiating between MS and NMO even in the medical community, which may result in a high misdiagnosis rate,” said study lead author Ka-Ho Wong, MBA, of the University of Utah, Salt Lake City, in an interview.
“NMO was recognized in the late 1800s and was historically thought to be a variant of MS until 1999,” said Michael Levy, MD, PhD, of Harvard Medical School and Massachusetts General Hospital, both in Boston, in an interview.
“They are both relapsing inflammatory disorders of the central nervous system with similarities in symptoms of weakness, numbness, mobility problem, vision defects, pain and fatigue,” said Dr. Levy, who did not take part in the new study. “A blood test for NMO was developed in 2004 and improved over time to the point that it can now reliably distinguish NMO from MS.”
As for therapy, “recent research has confirmed the two conditions are immunologically different and respond to different treatment,” Dr. Levy said. “The treatments developed for MS, especially from the 1990s, are harmful in NMO so it is important to make the diagnosis correctly.”
He added that “we do not recognize overlap between NMO or MS – it’s one or the other.”
Exploring the reasons for misdiagnosis
Mr. Wong, the present study’s lead author, said he and a research team launched the new study to better understand who gets misdiagnosed. “We know that almost 50% of the individuals get misdiagnosed at some point. However, what we don’t know yet is if the influencing factors are social determinants of health or if there are other causes.”
For the study, Mr. Wong and colleagues analyzed data from TriNetX, a health research network with access to medical records from 61 U.S. health care organizations. providing access to electronic medical records that includes sixty-one health care organizations (HCOs) in the United States.
ICD-10 coding statistics from 2008 to 2022 identified 7,657 patients with diagnoses for NMO. Of those, 4,040 (53%) only had diagnoses for NMO, and the rest (3,617, 47%) had diagnoses for both NMO and MS.
The researchers focused on 1,265 patients who had been coded for both diagnoses and had at least three clinical visits. They determined that a patient was misdiagnosed when they had three consecutive diagnoses of the same type. “For example, if they had MS but got misdiagnosed as NMO, once they are confirmed as MS they must have three or more consecutive diagnosis of MS to be considered as misdiagnosed,” Mr. Wong said.
Of the 1,265 subjects, the researchers determined that 308 (24%) had NMO but had been misdiagnosed as having MS, 189 (15%) had MS but were misdiagnosed as having NMO, and 768 (61%) were interchangeably diagnosed with the two conditions over time.
Among these three groups, 70.8%, 73.1%, and 78.4% were female, respectively; and 59.4%, 52.9%, and 53.0% were White, respectively. The percentages of Black patients were 17.2%, 24.3%, and 28.9%, respectively. Information about statistical significance was not provided in the poster.
Dr. Levy said he would “expect most NMO patients to initially be diagnosed with MS. It’s unusual to start with a diagnosis of NMO and then figure out it’s MS.”
As for the larger number of people with interchangeable diagnoses, Dr. Levy said that likely “reflects the messiness of billing codes.” For his part, Mr. Wong said there could be multiple causes for the interchangeable diagnoses: lack of disease knowledge, miscoding, lack of Food and Drug Administration–approved treatment for NMO at the time, and potentially other factors.
What does it all mean?
As for the study’s significance, Mr. Wong said a full workup should be performed before diagnosis, “and a neurologist should never prescribe disease-modifying therapies prior to a confirmation of diagnosis.”
Indeed, some disease-modifying therapies for MS are inappropriate for patients with NMO, Dr. Levy said. “The older medications, including beta-interferons, are among the most harmful to NMO patients. But they are not commonly used as first line for MS as they used to be. In contrast, B cell–depleting medications like ocrelizumab may be helpful in NMO.”
In regards to diagnosis, Dr. Levy noted that the NMO aquaporin-4 (AQP4) antibody test is “extremely specific and reliable.”
“A positive test result in the context of a clinical presentation of central nervous system inflammation allows for the diagnosis of NMO,” he said. “A negative test result is more complicated and may require some expertise to sort out after a careful review of the history, neurological exam, MRI features, central nervous system testing and other blood test results.”
The study was funded by the Sumaira Foundation. The authors did not provide information about relevant disclosures. Dr. Levy reports personal compensation for advisory board activities from Roche, Genentech, Chugai, Horizon, Alexion and Mitsubishi and grant support from Genentech, Horizon, Alexion, Sanofi, and UCB.
AT ECTRIMS 2023