User login
HM17 session summary: Building a practice that people want to be part of
Presenters
Roberta Himebaugh MBA, SHM; John Nelson, MD, FACP, MHM; Jerome Siy, MD, SFHM
Session summary
Creating a “culture of ownership” by recruiting the right people, promoting physician leadership, and improving structural elements such as compensation model and schedule were topics discussed in this practice management precourse at HM17.
The presenters said leaders must reduce hierarchy and promote shared decision making among the group, while instilling a “thank you culture” that recognizes motivations such as autonomy, mastery, and purpose.
Leaders must also consider current changes in health care payment models, such as MIPS (Merit-based Incentive Payment System), bundled payments, and Hospital Value-based Purchasing. Hospitalist groups must be prepared for these changes by learning about them and looking for potential cost reduction opportunities (e.g., reducing the number of patients going to skilled nursing facilities after joint replacement by sending patients home whenever possible).
Promoting a culture of engagement might include the development of interpersonal support strategies (e.g., meditation and mindfulness), innovative staffing (is 7 on/7 off right for everyone?), and comprehensive support for career and leadership development.
Finally, hospitalists should give special attention to the value formula by focusing on improving patient outcomes and experience, but also reducing direct and indirect costs. This is crucial for the sustainability of any hospitalist group.
Key takeaways for HM
• Create a culture of ownership to promote engagement and job satisfaction.
• Make adjustments to schedule and workflow to improve efficiency.
• Prepare for evolving pay-for-performance programs.
• Demonstrate the value of the group by setting expectations with key stakeholders, developing a practice score, and providing effective feedback to providers.
Dr. Villagra is a chief hospitalist in Batesville, Ark., and an editorial board member of The Hospitalist.
Presenters
Roberta Himebaugh MBA, SHM; John Nelson, MD, FACP, MHM; Jerome Siy, MD, SFHM
Session summary
Creating a “culture of ownership” by recruiting the right people, promoting physician leadership, and improving structural elements such as compensation model and schedule were topics discussed in this practice management precourse at HM17.
The presenters said leaders must reduce hierarchy and promote shared decision making among the group, while instilling a “thank you culture” that recognizes motivations such as autonomy, mastery, and purpose.
Leaders must also consider current changes in health care payment models, such as MIPS (Merit-based Incentive Payment System), bundled payments, and Hospital Value-based Purchasing. Hospitalist groups must be prepared for these changes by learning about them and looking for potential cost reduction opportunities (e.g., reducing the number of patients going to skilled nursing facilities after joint replacement by sending patients home whenever possible).
Promoting a culture of engagement might include the development of interpersonal support strategies (e.g., meditation and mindfulness), innovative staffing (is 7 on/7 off right for everyone?), and comprehensive support for career and leadership development.
Finally, hospitalists should give special attention to the value formula by focusing on improving patient outcomes and experience, but also reducing direct and indirect costs. This is crucial for the sustainability of any hospitalist group.
Key takeaways for HM
• Create a culture of ownership to promote engagement and job satisfaction.
• Make adjustments to schedule and workflow to improve efficiency.
• Prepare for evolving pay-for-performance programs.
• Demonstrate the value of the group by setting expectations with key stakeholders, developing a practice score, and providing effective feedback to providers.
Dr. Villagra is a chief hospitalist in Batesville, Ark., and an editorial board member of The Hospitalist.
Presenters
Roberta Himebaugh MBA, SHM; John Nelson, MD, FACP, MHM; Jerome Siy, MD, SFHM
Session summary
Creating a “culture of ownership” by recruiting the right people, promoting physician leadership, and improving structural elements such as compensation model and schedule were topics discussed in this practice management precourse at HM17.
The presenters said leaders must reduce hierarchy and promote shared decision making among the group, while instilling a “thank you culture” that recognizes motivations such as autonomy, mastery, and purpose.
Leaders must also consider current changes in health care payment models, such as MIPS (Merit-based Incentive Payment System), bundled payments, and Hospital Value-based Purchasing. Hospitalist groups must be prepared for these changes by learning about them and looking for potential cost reduction opportunities (e.g., reducing the number of patients going to skilled nursing facilities after joint replacement by sending patients home whenever possible).
Promoting a culture of engagement might include the development of interpersonal support strategies (e.g., meditation and mindfulness), innovative staffing (is 7 on/7 off right for everyone?), and comprehensive support for career and leadership development.
Finally, hospitalists should give special attention to the value formula by focusing on improving patient outcomes and experience, but also reducing direct and indirect costs. This is crucial for the sustainability of any hospitalist group.
Key takeaways for HM
• Create a culture of ownership to promote engagement and job satisfaction.
• Make adjustments to schedule and workflow to improve efficiency.
• Prepare for evolving pay-for-performance programs.
• Demonstrate the value of the group by setting expectations with key stakeholders, developing a practice score, and providing effective feedback to providers.
Dr. Villagra is a chief hospitalist in Batesville, Ark., and an editorial board member of The Hospitalist.
The Design and Implementation of a Home-Based Cardiac Rehabilitation Program
Despite a 30% decline in heart disease mortality from 2001 to 2011, heart disease prevalence is on the rise, responsible for 1 of every 3 deaths in the U.S.1 Cardiac rehabilitation (CR) is an evidence-based, secondary prevention strategy that has been proven effective in preventing future cardiovascular events and decreasing heart disease mortality.2-4 The American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR) is the leading authority on CR and provides guidelines for CR programs. The AACVPR and the American Heart Association (AHA) published core components for CR programs deemed essential for all CR/secondary prevention programs, including evaluations, interventions, and expected outcomes.5 These core components are aimed at promoting a healthy lifestyle and increasing function and well-being while reducing injury, death, and the reoccurrence of disease.6
In a meta-analysis of 47 trials with 10,794 participants, CR reduced cardiovascular disease (CVD) mortality and hospital admissions by 26% and 18%, respectively.2 Performance measures (Class 1, Level A) recommend the following types of patients should be referred from the inpatient setting: “all patients hospitalized with a primary diagnosis of an acute myocardial infarction (MI) or chronic stable angina, or who during hospitalization have undergone coronary artery bypass graft (CABG) surgery, a percutaneous coronary intervention (PCI), cardiac valve surgery, or cardiac transplantation.”7 However, despite overwhelming evidence and widespread endorsement (Class 1, Level A), service utilization, uptake, and patient adherence to CR programs remain suboptimal. In a U.S. study of claims from > 250,000 Medicare beneficiaries, < 30% of eligible patients participated
This treatment gap is echoed throughout the VHA. Schopfer and colleagues found that only 28% of the 124 VAMCs that provide inpatient care also offer a supervised, facility-based CR program.10 Furthermore, only 10.3% of eligible veterans participated in at least 1 CR session (VA or non-VA). On a systemic level, low patient referral rates and inadequate third-party reimbursement were the most common barriers to participation in CR.10,11 On a patient level, distance was by far the largest barrier to veterans receiving CR. Currently, 74% of the 9.3 million VA-enrolled veterans live at least 1 hour by car from a VA facility that offers CR.9 Within some regions of the VHA, there are no VA facility-based CR programs. For example, VISN 21 has no facility-based CR programs. At the same time, referral of eligible veterans to facility-based CR outside the VA remains low. Prior to April 2013, < 2% of qualified patients residing in VISN 21 were being referred to Non-VA CR programs, making it the VISN with the lowest participation rate for CR.
One potential solution that addresses both systemic and patient barriers to CR utilization is home-based CR. Veterans within the wide geographic area of VISN 21 are referred to San Francisco VAMC (SFVAMC) for ischemic heart disease, cardiovascular revascularization, and cardiac valve surgeries. In 2013, a comprehensive home-based CR program named The Healthy Heart Program was developed based on a successful evidence-based CVD secondary prevention program. The Healthy Heart Program is designed to be a physician-directed, nurse case-managed, customized exercise and lifestyle program that provides a safe and convenient way for veterans to participate in CR. Exercise and disease self-management education are the cornerstones of the Healthy Heart Program. The program’s multidisciplinary team includes physicians, nurses, a dietician, an exercise physiologist, and a health behavior psychologist.
An Alternative Approach
DeBusk and colleagues demonstrated that a physician-directed, nurse-managed, home-based cardiac risk-factor modification program improved smoking cessation, reduced low-density lipoprotein cholesterol, and increased exercise capacity compared with usual care.12 The results of this study helped pave the way for one of the first CR programs with a strong home-based element. The MULTIFIT program was jointly developed by the Stanford Coronary Rehabilitation Program and Kaiser Permanente (Oakland, CA) in 1995. MULTIFIT is a nurse-based care model for CVD prevention.
Further research that evaluated other home-based programs showed similar promise. A Cochrane review demonstrated that home- and facility-based CR programs were equal in cardiac risk factor reduction, reduced hospital readmissions and mortality rates, and improved quality of life (QOL).13 Cost-effectiveness also seemed to be similar in both home- and hospital-based CR
Referrals
To address the problems with referrals that plague other CR programs, staff of the Healthy Heart Program worked closely with interventional cardiology and the cardiothoracic team, including the clinical informatics coordinators, to develop an automatic referral system for CR evaluation. Consults for CR evaluation were embedded within the post-CABG and PCI order sets in the electronic health record. Laboratory troponin alerts were created to alert CR staff of patients with elevated troponins, which identified patients admitted for acute MI. Healthy Heart Program staff members received the referrals once a patient was admitted to the unit following their heart procedure. Early referrals for evaluation allowed staff to begin a chart review of all eligible patients and to follow the patient’s course of recovery. Most consults were generated during hospitalization for one of the indications; however, a minority of consults come from both the cardiology and primary care clinics.
Three Phases of CR
The AACVPR describes the challenges and opportunities found throughout the CR continuum.5 Over the past several decades, the continuum of care was more program centered and service utilization was more isolated. Today, CR is viewed as more process oriented and coordinates care across many professionals and services. Phase 1 inpatient CR begins in the hospital and is a shared responsibility between several services. Shortened hospital stays have led to innovative solutions for early ambulation, risk factor education, and discharge planning, including enrollment into phase 2 CR. Phase 2, also known as early outpatient, should begin within 1 to 2 weeks postevent in healthier patients and can last between 6 and 12 weeks postdischarge. Phase 3 (maintenance phase) should begin immediately at the conclusion of phase 2.
Phase 1
Prior to the advent of the Healthy Heart Program, secondary prevention education was not done at the bedside for SFVAMC patients following cardiac revascularization. The AACVPR recommends patient assessment, mobilization, risk-factor identification and education, and facilitation into outpatient CR as essential components of phase 1 CR.5 The Healthy Heart Program clinician initiates phase 1 CR by examining cardiac risk factor management for all referred patients. Physical and cardiac risk factor assessments are accomplished by completing a detailed chart review and interview with the patient. During this interview with the patient, the clinician evaluates cognitive function and readiness to learn. Staff will interview the patient further to assess the overall patient needs, including availability of social support, resources to maintain optimal health, and the need for secondary preventive education. For the PCI patient, the interview may occur in the hours following their procedure; for the surgical patient, this bedside visit typically occurs postoperative day 3 or 4.
A standardized cardiac risk factor evaluation tool was designed, which also serves as an education form to help guide the conversation on risk factor management. The interactive, patient-centered form includes opportunities to review risk, discuss current laboratory values (eg, lipids and hemoglobin A1c), and establish individualized goals based on patient preference and recommended guidelines. Healthy Heart Program staff assist the patient in formulating achievable goals using the SMART (specific, measurable, attainable, realistic, and time-related) criteria.19 Immediately after a heart event or procedure, patients often feel highly motivated to initiate lifestyle changes.20 However, PCI patients may have a short window of opportunity for learning between their readiness to learn state and before the activities of discharge. Staff use these opportunities as a teachable moment and to increase enrollment into outpatient CR (phase 2).
The provider performs a thorough chart review and bedside consultation to determine whether home-based CR is indicated, feasible, and appropriate. Not every patient that is referred will be enrolled in CR. Patients have the option to opt out. In addition, clinical staff adhere to the program protocol’s exclusion criteria.
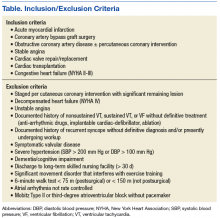
Absolute contraindications for home enrollment include unstable angina, staged cardiac procedure (PCI and surgery), complex ventricular arrhythmias, severe or symptomatic aortic stenosis, decompensated heart failure, and uncontrolled hypertension (Table). Patients deemed high risk for home-based CR may be referred to a non-VA facility-based CR program. Risk stratification, using the Canadian Cardiovascular Society Grading of Angina Pectoris, is a continuous process that is used to identify patients who may move from moderate to high risk, both before and during the program.21,22
Phases 2 and 3
Phase 2 of the Healthy Heart Program CR includes physical activity, risk-factor modification, nutritional guidance, psychosocial modification, a return to previous activities, and an improved QOL. Prior to entry into the program, a submaximal exercise test, the 6-minute walk test (6MWT), is used as both a qualifying test and for developing the initial exercise prescription.22 The minimum 6MWT distance needed to qualify is 75 m for postoperative and 150 m for nonsurgical patients. The 6MWT is performed in-hospital for patients who were admitted for stable angina, PCI, and are > 4 days following acute MI.23 Cardiothoracic surgery patients are tested at their first follow-up clinic visit (typically 2-3 weeks postoperatively). The clinician monitors the heart rate with either a wearable device or via inpatient telemetry monitors. This exercise testing also serves as a motivational tool for patients to gain confidence in their ability to begin to exercise at home.
Each participant receives a workbook and a DVD titled An Active Partnership for the Health of Your Heart. A personal health journal is provided for documenting vital signs, activity, and dietary intake. In addition, each participant receives equipment on an as-needed basis, including resistance bands, a weight scale, a blood pressure cuff, a pedometer/heart rate monitoring device, an exercise peddler or stationary bike, and a dietary video. Baseline assessments include the General Anxiety Disorder (GAD-7), Personal Health Questionnaire (PHQ-9) and a nutrition (Rate Your Plate) questionnaire. A cognitive function test (Montreal Cognitive Assessment) is used on an as-needed basis.
Nine 30-minute telephone follow-up sessions are scheduled within a 12-week period (weekly for the first 6 weeks, then biweekly). Topics covered are customized and include exercise; nutrition; medications; smoking cessation; and diabetes, hypertension, and weight management. Via a telephone follow-up session, the program nurses and patients codevelop an electronic individualized treatment plan that is tailored to the patient’s diagnosis, individual goals, and preferences. Clinicians teach participants how to self-monitor exercise, using a continuous heart rate monitoring device (Mio Alpha II or Fuse) and the 6-20 Borg dyspnea rating scale.24 Initially, moderate intensity exercise is prescribed with a target heart rate that is 60% to 75% of the 6MWT peak heart rate and an initial Borg scale target (11-14 on 20 point scale). The program physicians approve the treatment plan at the first patient visit and every 30 days until phase 2 is complete.
Patients who have completed early outpatient phase 2 CR can benefit from continuing to a phase 3 CR program.25 Participants of the Healthy Heart Program automatically are enrolled in phase 3, which is a long-term maintenance program that includes monthly or bimonthly phone calls for up to 1-year posthospital discharge. The goal is to support each veteran’s transition to a long-term healthy lifestyle that includes regular exercise.
Client-Clinician Partnership
The Healthy Heart Program establishes the client-clinician partnership prior to discharge for hospitalized patients. The nurse who initiates phase 1 at the bedside is the primary clinician throughout phases 2 and 3 with the exception of a dietician, psychologist, and/or exercise physiologist who provide follow-up calls as needed. Throughout these weekly follow-up phone sessions, the clinician gains an appreciation of the patient’s understanding of his or her disease, patterns of behavior, desire to change, confidence in being able to change, potential barriers, and responses to obstacles
Tailored Behavioral Change
The clinician’s responsibility is to listen to the patient’s concerns, assess their level of commitment for changing health behaviors, and provide guidance and support at the patient’s current level. The clinician applies the Transtheoretical Model founded on the Stages of Change principals to help understand and provide guidance based on the patient’s feelings about health behavior change.26 People are actively open to changing behaviors by only 20% at any given time.27 Therefore, action-oriented guidance for patients who are in the contemplative stage would not be helpful. This patient-centered approach promotes patients’ self-awareness, participation, and understanding of their decision-making role in their health management. Ultimately, individuals must take ownership of their health care maintenance for sustained behavioral change and medication management, and clinicians should facilitate that process.
Discussion
Secondary prevention strategies for heart disease continue to be underutilized. The Healthy Heart Program aims to improve participation in CR, improve QOL, help patients understand their heart disease, and support these patients psychologically. An advantage of this program is that it begins inpatient CR immediately following the heart event, when many patients often are more receptive to behavioral change support and guidance. Another advantage is that the program breaks down barriers to access, which is especially important in the veteran population. The Healthy Heart Program provides support and guidance for exercise and cardiac risk factor management to patients who otherwise would have not participated in any type of CR program.
A home-based CR program can be adopted independently or in conjunction with a facility-based program to which patients lack access. Furthermore, home-based CR programs function well as a phase 3 maintenance program at the completion of a traditional CR program. Since its inception, the Healthy Heart Program has increased the number of veterans enrolled in cardiac rehabilitation at the SFVAMC dramatically, from < 1% in FY 2012 to > 40% in FY 2015.
Program Limitations
One potential disadvantage of a home-based CR program is patients’ fear of returning to an exercise routine following a cardiac event. In addition, a lack of in-person supervision in home-based CR can lead patients to engage in less intensive activity than in facility-based CR. Other disadvantages include a lack of social support, less patient accountability, and safety concerns for sicker patients. Staff have consulted on several patients who expressed a lack of confidence in their ability to do well in this type of program, where accountability for exercising is self-reported. Staff referred these patients, who had the means to travel, to a non-VA facility-based CR program of their choice. Ideally, patients would have the choice between facility- or home-based programs or be able to choose a hybrid program that would best meet their needs.
Another identified limitation of this program was the lack of group support and in-person interactions with rehabilitation staff. Finally, although this program uses mobile devices with heart rate monitoring technology, these devices currently lack the capability to remotely share data with clinicians. Clinicians are reliant on the patient’s use of a personal health journal and memory. Subjective patient reporting has been found to be overestimated; therefore, more objective methods to measure important clinical outcomes are necessary.28
Conclusion
Facility-based CR is effective but underutilized. Alternative secondary programs are needed to help meet patient needs and overcome patient barriers. One promising approach to increase participation is home-based CR. Home-based CR programs have the potential to increase CR uptake and adherence. Home-based CR optimizes enrollment through evidence-based alternative models due to improved access. The future of CR will become highly individualized and multifaceted as a result of available mobile technologies and Internet-based tools, which will help increase the number of participants and expand the reach of cardiac risk factor management programs beyond the facility-based setting. A home-based program will be a valuable addition to facility-based programs as a stand-alone program or adopted into a hybrid program.
Acknowledgments
This work was funded by the VA Quality Enhancement Research Initiative.
1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Cicrulation. 2017;135(10):e146-e603.
2. Anderson L, Oldridge N, Thompson DR, Zwisler A, Rees K, Martin N, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Systematic Review and Meta-analysis. J Am Coll Card. 2016;67:1-12.
3. Oldridge NB, Guyatt GH, Fischer ME, Rimm AA. Cardiac rehabilitation after myocardial infarction. Combined experience of randomized clinical trials. JAMA. 1988;260:940-950.
4. Taylor RS, Brown A, Ebrahim S, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116(10):682-692.
5. American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs. 5th ed. Champaign, IL: Human Kinetics; 2013.
6. Balady GJ, Williams MA, Ades PA, et al; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing; American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Nutrition, Physical Activity, and Metabolism; American Association of Cardiovascular and Pulmonary Rehabilitation. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2007;115(10):2675-2682.
7. Thomas R J, King M, Lui K, et al; Writing Committee Members. AACVPR/ACCF/AHA 2010 update: performance measures on cardiac rehabilitation for referral to cardiac rehabilitation/secondary prevention services: a report of the American Association of Cardiovascular and Pulmonary Rehabilitation and the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Clinical Performance Measures for Cardiac Rehabilitation). Circulation. 2010;122(13):1342-1350.
8. Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation. 2007;116(15):1653-1662.
9. Balady GJ, Ades PA, Bitner VA, et al; American Heart Association Science Advisory and Coordinating Committee. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: a presidential advisory from the American Heart Association. Circulation. 2011;124(25):2951-2960.
10. Schopfer DW, Takemoto S, Allsup K, et al. Notice of Retraction and Replacement. Schopfer DW, et al. Cardiac rehabilitation use among veterans with ischemic heart disease. JAMA Intern Med. 2014;174(10):1687-1689. JAMA Intern Med. 2016;176(11):1726-1727.
11. Ferguson EE. Cardiac rehabilitation—an effective and comprehensive but underutilized program to reduce cardiovascular risk in patients with CVD. US Cardiology. 2006;3(2):14-16.
12. DeBusk RF, Miller NH, Superko HR, et al. A case-management system for coronary risk factor modification after acute myocardial infarction. Ann Intern Med. 1994;120(9):721-729.
13. Buckingham SA, Taylor RS, Jolly K, et al. Home-based versus centre-based cardiac rehabilitation: abridged Cochrane systematic review and meta-analysis. Open Heart. 2016;3(2):e000463.
14. Taylor RS, Watt A, Dalal HM, et al. Home-based cardiac rehabilitation versus hospital-based rehabilitation: a cost effectiveness analysis. Int J Cardiol. 2007;119(2):196-201.
15. Kotb A, Hsieh S, Wells GA. The effect of telephone support interventions on coronary artery disease (CAD) patient outcomes during cardiac rehabilitation: a systematic review and meta-analysis. PLoS One. 2014;9(5):e96581.
16. Grace SL, McDonald J, Fishman D, Caruso V. Patient preferences for home-based versus hospital-based cardiac rehabilitation. J Cardiopulm Rehabil. 2005;25(1):24-29.
17. Wakefield B, Drwal K, Scherubel M, Klobucar T, Johnson S, Kaboli P. Feasibility and effectiveness of remote, telephone-based delivery of cardiac rehabilitation. Telemed J E Health. 2014;20(1):32-38.
18. Smith SC, Benjamin EJ, Bonow RO, et al; World Heart Federation and the Preventive Cardiovascular Nurses Association. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124(22):2458-2473.
19. Doran GT. There’s a S.M.A.R.T. way to write management’s goals and objectives. Manage Rev. 1981;70(11):35-36.
20. Dullaghan L, Lusk L, Donnelly P, McGeough M, Fitzsimons D. Communicating with people who have experienced heart attack. Emerg Nurse. 2013;21(6):33-36.
21. Campeau L. Letter: grading of angina pectoris. Circulation. 1976;54(3):522-523.
22. Fletcher GF, Balady GJ, Armstrong EA, et al. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104(14):1694-1740.
23. Gibbons RJ, Balady GJ, Bricker JT, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Committee to Update the 1997 Exercise Testing Guidelines. Committee to Update the 1997 Exercise Testing Guidelines. ACC/AHA 2002 guideline update for exercise testing: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). J Am Coll Cardiol. 2002;40(8):1531-1540.
24. Borg G. Borg’s Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998.
25. Seki E, Watanabe Y, Sunayama S, et al. Effects of phase III cardiac rehabilitation programs on health-related quality of life in elderly patients with coronary artery disease: Juntendo Cardiac Rehabilitation Program (J-CARP). Circ J. 2003;67(1):73-77.
26. The transtheoretical model. Pro-Change Behavior Systems, Inc. http://www.prochange.com/transtheoretical-model-of-behavior-change. Published 2016. Accessed April 6, 2017.
27. Prochaska JO, Ever KE, Castle PH, et al. Enhancing multiple domains of well-being by decreasing multiple health risk behaviors: a randomized clinical trial. Popul Health Manag. 2012;15(5):276-286.
28. Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56.
Despite a 30% decline in heart disease mortality from 2001 to 2011, heart disease prevalence is on the rise, responsible for 1 of every 3 deaths in the U.S.1 Cardiac rehabilitation (CR) is an evidence-based, secondary prevention strategy that has been proven effective in preventing future cardiovascular events and decreasing heart disease mortality.2-4 The American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR) is the leading authority on CR and provides guidelines for CR programs. The AACVPR and the American Heart Association (AHA) published core components for CR programs deemed essential for all CR/secondary prevention programs, including evaluations, interventions, and expected outcomes.5 These core components are aimed at promoting a healthy lifestyle and increasing function and well-being while reducing injury, death, and the reoccurrence of disease.6
In a meta-analysis of 47 trials with 10,794 participants, CR reduced cardiovascular disease (CVD) mortality and hospital admissions by 26% and 18%, respectively.2 Performance measures (Class 1, Level A) recommend the following types of patients should be referred from the inpatient setting: “all patients hospitalized with a primary diagnosis of an acute myocardial infarction (MI) or chronic stable angina, or who during hospitalization have undergone coronary artery bypass graft (CABG) surgery, a percutaneous coronary intervention (PCI), cardiac valve surgery, or cardiac transplantation.”7 However, despite overwhelming evidence and widespread endorsement (Class 1, Level A), service utilization, uptake, and patient adherence to CR programs remain suboptimal. In a U.S. study of claims from > 250,000 Medicare beneficiaries, < 30% of eligible patients participated
This treatment gap is echoed throughout the VHA. Schopfer and colleagues found that only 28% of the 124 VAMCs that provide inpatient care also offer a supervised, facility-based CR program.10 Furthermore, only 10.3% of eligible veterans participated in at least 1 CR session (VA or non-VA). On a systemic level, low patient referral rates and inadequate third-party reimbursement were the most common barriers to participation in CR.10,11 On a patient level, distance was by far the largest barrier to veterans receiving CR. Currently, 74% of the 9.3 million VA-enrolled veterans live at least 1 hour by car from a VA facility that offers CR.9 Within some regions of the VHA, there are no VA facility-based CR programs. For example, VISN 21 has no facility-based CR programs. At the same time, referral of eligible veterans to facility-based CR outside the VA remains low. Prior to April 2013, < 2% of qualified patients residing in VISN 21 were being referred to Non-VA CR programs, making it the VISN with the lowest participation rate for CR.
One potential solution that addresses both systemic and patient barriers to CR utilization is home-based CR. Veterans within the wide geographic area of VISN 21 are referred to San Francisco VAMC (SFVAMC) for ischemic heart disease, cardiovascular revascularization, and cardiac valve surgeries. In 2013, a comprehensive home-based CR program named The Healthy Heart Program was developed based on a successful evidence-based CVD secondary prevention program. The Healthy Heart Program is designed to be a physician-directed, nurse case-managed, customized exercise and lifestyle program that provides a safe and convenient way for veterans to participate in CR. Exercise and disease self-management education are the cornerstones of the Healthy Heart Program. The program’s multidisciplinary team includes physicians, nurses, a dietician, an exercise physiologist, and a health behavior psychologist.
An Alternative Approach
DeBusk and colleagues demonstrated that a physician-directed, nurse-managed, home-based cardiac risk-factor modification program improved smoking cessation, reduced low-density lipoprotein cholesterol, and increased exercise capacity compared with usual care.12 The results of this study helped pave the way for one of the first CR programs with a strong home-based element. The MULTIFIT program was jointly developed by the Stanford Coronary Rehabilitation Program and Kaiser Permanente (Oakland, CA) in 1995. MULTIFIT is a nurse-based care model for CVD prevention.
Further research that evaluated other home-based programs showed similar promise. A Cochrane review demonstrated that home- and facility-based CR programs were equal in cardiac risk factor reduction, reduced hospital readmissions and mortality rates, and improved quality of life (QOL).13 Cost-effectiveness also seemed to be similar in both home- and hospital-based CR
Referrals
To address the problems with referrals that plague other CR programs, staff of the Healthy Heart Program worked closely with interventional cardiology and the cardiothoracic team, including the clinical informatics coordinators, to develop an automatic referral system for CR evaluation. Consults for CR evaluation were embedded within the post-CABG and PCI order sets in the electronic health record. Laboratory troponin alerts were created to alert CR staff of patients with elevated troponins, which identified patients admitted for acute MI. Healthy Heart Program staff members received the referrals once a patient was admitted to the unit following their heart procedure. Early referrals for evaluation allowed staff to begin a chart review of all eligible patients and to follow the patient’s course of recovery. Most consults were generated during hospitalization for one of the indications; however, a minority of consults come from both the cardiology and primary care clinics.
Three Phases of CR
The AACVPR describes the challenges and opportunities found throughout the CR continuum.5 Over the past several decades, the continuum of care was more program centered and service utilization was more isolated. Today, CR is viewed as more process oriented and coordinates care across many professionals and services. Phase 1 inpatient CR begins in the hospital and is a shared responsibility between several services. Shortened hospital stays have led to innovative solutions for early ambulation, risk factor education, and discharge planning, including enrollment into phase 2 CR. Phase 2, also known as early outpatient, should begin within 1 to 2 weeks postevent in healthier patients and can last between 6 and 12 weeks postdischarge. Phase 3 (maintenance phase) should begin immediately at the conclusion of phase 2.
Phase 1
Prior to the advent of the Healthy Heart Program, secondary prevention education was not done at the bedside for SFVAMC patients following cardiac revascularization. The AACVPR recommends patient assessment, mobilization, risk-factor identification and education, and facilitation into outpatient CR as essential components of phase 1 CR.5 The Healthy Heart Program clinician initiates phase 1 CR by examining cardiac risk factor management for all referred patients. Physical and cardiac risk factor assessments are accomplished by completing a detailed chart review and interview with the patient. During this interview with the patient, the clinician evaluates cognitive function and readiness to learn. Staff will interview the patient further to assess the overall patient needs, including availability of social support, resources to maintain optimal health, and the need for secondary preventive education. For the PCI patient, the interview may occur in the hours following their procedure; for the surgical patient, this bedside visit typically occurs postoperative day 3 or 4.
A standardized cardiac risk factor evaluation tool was designed, which also serves as an education form to help guide the conversation on risk factor management. The interactive, patient-centered form includes opportunities to review risk, discuss current laboratory values (eg, lipids and hemoglobin A1c), and establish individualized goals based on patient preference and recommended guidelines. Healthy Heart Program staff assist the patient in formulating achievable goals using the SMART (specific, measurable, attainable, realistic, and time-related) criteria.19 Immediately after a heart event or procedure, patients often feel highly motivated to initiate lifestyle changes.20 However, PCI patients may have a short window of opportunity for learning between their readiness to learn state and before the activities of discharge. Staff use these opportunities as a teachable moment and to increase enrollment into outpatient CR (phase 2).
The provider performs a thorough chart review and bedside consultation to determine whether home-based CR is indicated, feasible, and appropriate. Not every patient that is referred will be enrolled in CR. Patients have the option to opt out. In addition, clinical staff adhere to the program protocol’s exclusion criteria.
Absolute contraindications for home enrollment include unstable angina, staged cardiac procedure (PCI and surgery), complex ventricular arrhythmias, severe or symptomatic aortic stenosis, decompensated heart failure, and uncontrolled hypertension (Table). Patients deemed high risk for home-based CR may be referred to a non-VA facility-based CR program. Risk stratification, using the Canadian Cardiovascular Society Grading of Angina Pectoris, is a continuous process that is used to identify patients who may move from moderate to high risk, both before and during the program.21,22
Phases 2 and 3
Phase 2 of the Healthy Heart Program CR includes physical activity, risk-factor modification, nutritional guidance, psychosocial modification, a return to previous activities, and an improved QOL. Prior to entry into the program, a submaximal exercise test, the 6-minute walk test (6MWT), is used as both a qualifying test and for developing the initial exercise prescription.22 The minimum 6MWT distance needed to qualify is 75 m for postoperative and 150 m for nonsurgical patients. The 6MWT is performed in-hospital for patients who were admitted for stable angina, PCI, and are > 4 days following acute MI.23 Cardiothoracic surgery patients are tested at their first follow-up clinic visit (typically 2-3 weeks postoperatively). The clinician monitors the heart rate with either a wearable device or via inpatient telemetry monitors. This exercise testing also serves as a motivational tool for patients to gain confidence in their ability to begin to exercise at home.
Each participant receives a workbook and a DVD titled An Active Partnership for the Health of Your Heart. A personal health journal is provided for documenting vital signs, activity, and dietary intake. In addition, each participant receives equipment on an as-needed basis, including resistance bands, a weight scale, a blood pressure cuff, a pedometer/heart rate monitoring device, an exercise peddler or stationary bike, and a dietary video. Baseline assessments include the General Anxiety Disorder (GAD-7), Personal Health Questionnaire (PHQ-9) and a nutrition (Rate Your Plate) questionnaire. A cognitive function test (Montreal Cognitive Assessment) is used on an as-needed basis.
Nine 30-minute telephone follow-up sessions are scheduled within a 12-week period (weekly for the first 6 weeks, then biweekly). Topics covered are customized and include exercise; nutrition; medications; smoking cessation; and diabetes, hypertension, and weight management. Via a telephone follow-up session, the program nurses and patients codevelop an electronic individualized treatment plan that is tailored to the patient’s diagnosis, individual goals, and preferences. Clinicians teach participants how to self-monitor exercise, using a continuous heart rate monitoring device (Mio Alpha II or Fuse) and the 6-20 Borg dyspnea rating scale.24 Initially, moderate intensity exercise is prescribed with a target heart rate that is 60% to 75% of the 6MWT peak heart rate and an initial Borg scale target (11-14 on 20 point scale). The program physicians approve the treatment plan at the first patient visit and every 30 days until phase 2 is complete.
Patients who have completed early outpatient phase 2 CR can benefit from continuing to a phase 3 CR program.25 Participants of the Healthy Heart Program automatically are enrolled in phase 3, which is a long-term maintenance program that includes monthly or bimonthly phone calls for up to 1-year posthospital discharge. The goal is to support each veteran’s transition to a long-term healthy lifestyle that includes regular exercise.
Client-Clinician Partnership
The Healthy Heart Program establishes the client-clinician partnership prior to discharge for hospitalized patients. The nurse who initiates phase 1 at the bedside is the primary clinician throughout phases 2 and 3 with the exception of a dietician, psychologist, and/or exercise physiologist who provide follow-up calls as needed. Throughout these weekly follow-up phone sessions, the clinician gains an appreciation of the patient’s understanding of his or her disease, patterns of behavior, desire to change, confidence in being able to change, potential barriers, and responses to obstacles
Tailored Behavioral Change
The clinician’s responsibility is to listen to the patient’s concerns, assess their level of commitment for changing health behaviors, and provide guidance and support at the patient’s current level. The clinician applies the Transtheoretical Model founded on the Stages of Change principals to help understand and provide guidance based on the patient’s feelings about health behavior change.26 People are actively open to changing behaviors by only 20% at any given time.27 Therefore, action-oriented guidance for patients who are in the contemplative stage would not be helpful. This patient-centered approach promotes patients’ self-awareness, participation, and understanding of their decision-making role in their health management. Ultimately, individuals must take ownership of their health care maintenance for sustained behavioral change and medication management, and clinicians should facilitate that process.
Discussion
Secondary prevention strategies for heart disease continue to be underutilized. The Healthy Heart Program aims to improve participation in CR, improve QOL, help patients understand their heart disease, and support these patients psychologically. An advantage of this program is that it begins inpatient CR immediately following the heart event, when many patients often are more receptive to behavioral change support and guidance. Another advantage is that the program breaks down barriers to access, which is especially important in the veteran population. The Healthy Heart Program provides support and guidance for exercise and cardiac risk factor management to patients who otherwise would have not participated in any type of CR program.
A home-based CR program can be adopted independently or in conjunction with a facility-based program to which patients lack access. Furthermore, home-based CR programs function well as a phase 3 maintenance program at the completion of a traditional CR program. Since its inception, the Healthy Heart Program has increased the number of veterans enrolled in cardiac rehabilitation at the SFVAMC dramatically, from < 1% in FY 2012 to > 40% in FY 2015.
Program Limitations
One potential disadvantage of a home-based CR program is patients’ fear of returning to an exercise routine following a cardiac event. In addition, a lack of in-person supervision in home-based CR can lead patients to engage in less intensive activity than in facility-based CR. Other disadvantages include a lack of social support, less patient accountability, and safety concerns for sicker patients. Staff have consulted on several patients who expressed a lack of confidence in their ability to do well in this type of program, where accountability for exercising is self-reported. Staff referred these patients, who had the means to travel, to a non-VA facility-based CR program of their choice. Ideally, patients would have the choice between facility- or home-based programs or be able to choose a hybrid program that would best meet their needs.
Another identified limitation of this program was the lack of group support and in-person interactions with rehabilitation staff. Finally, although this program uses mobile devices with heart rate monitoring technology, these devices currently lack the capability to remotely share data with clinicians. Clinicians are reliant on the patient’s use of a personal health journal and memory. Subjective patient reporting has been found to be overestimated; therefore, more objective methods to measure important clinical outcomes are necessary.28
Conclusion
Facility-based CR is effective but underutilized. Alternative secondary programs are needed to help meet patient needs and overcome patient barriers. One promising approach to increase participation is home-based CR. Home-based CR programs have the potential to increase CR uptake and adherence. Home-based CR optimizes enrollment through evidence-based alternative models due to improved access. The future of CR will become highly individualized and multifaceted as a result of available mobile technologies and Internet-based tools, which will help increase the number of participants and expand the reach of cardiac risk factor management programs beyond the facility-based setting. A home-based program will be a valuable addition to facility-based programs as a stand-alone program or adopted into a hybrid program.
Acknowledgments
This work was funded by the VA Quality Enhancement Research Initiative.
Despite a 30% decline in heart disease mortality from 2001 to 2011, heart disease prevalence is on the rise, responsible for 1 of every 3 deaths in the U.S.1 Cardiac rehabilitation (CR) is an evidence-based, secondary prevention strategy that has been proven effective in preventing future cardiovascular events and decreasing heart disease mortality.2-4 The American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR) is the leading authority on CR and provides guidelines for CR programs. The AACVPR and the American Heart Association (AHA) published core components for CR programs deemed essential for all CR/secondary prevention programs, including evaluations, interventions, and expected outcomes.5 These core components are aimed at promoting a healthy lifestyle and increasing function and well-being while reducing injury, death, and the reoccurrence of disease.6
In a meta-analysis of 47 trials with 10,794 participants, CR reduced cardiovascular disease (CVD) mortality and hospital admissions by 26% and 18%, respectively.2 Performance measures (Class 1, Level A) recommend the following types of patients should be referred from the inpatient setting: “all patients hospitalized with a primary diagnosis of an acute myocardial infarction (MI) or chronic stable angina, or who during hospitalization have undergone coronary artery bypass graft (CABG) surgery, a percutaneous coronary intervention (PCI), cardiac valve surgery, or cardiac transplantation.”7 However, despite overwhelming evidence and widespread endorsement (Class 1, Level A), service utilization, uptake, and patient adherence to CR programs remain suboptimal. In a U.S. study of claims from > 250,000 Medicare beneficiaries, < 30% of eligible patients participated
This treatment gap is echoed throughout the VHA. Schopfer and colleagues found that only 28% of the 124 VAMCs that provide inpatient care also offer a supervised, facility-based CR program.10 Furthermore, only 10.3% of eligible veterans participated in at least 1 CR session (VA or non-VA). On a systemic level, low patient referral rates and inadequate third-party reimbursement were the most common barriers to participation in CR.10,11 On a patient level, distance was by far the largest barrier to veterans receiving CR. Currently, 74% of the 9.3 million VA-enrolled veterans live at least 1 hour by car from a VA facility that offers CR.9 Within some regions of the VHA, there are no VA facility-based CR programs. For example, VISN 21 has no facility-based CR programs. At the same time, referral of eligible veterans to facility-based CR outside the VA remains low. Prior to April 2013, < 2% of qualified patients residing in VISN 21 were being referred to Non-VA CR programs, making it the VISN with the lowest participation rate for CR.
One potential solution that addresses both systemic and patient barriers to CR utilization is home-based CR. Veterans within the wide geographic area of VISN 21 are referred to San Francisco VAMC (SFVAMC) for ischemic heart disease, cardiovascular revascularization, and cardiac valve surgeries. In 2013, a comprehensive home-based CR program named The Healthy Heart Program was developed based on a successful evidence-based CVD secondary prevention program. The Healthy Heart Program is designed to be a physician-directed, nurse case-managed, customized exercise and lifestyle program that provides a safe and convenient way for veterans to participate in CR. Exercise and disease self-management education are the cornerstones of the Healthy Heart Program. The program’s multidisciplinary team includes physicians, nurses, a dietician, an exercise physiologist, and a health behavior psychologist.
An Alternative Approach
DeBusk and colleagues demonstrated that a physician-directed, nurse-managed, home-based cardiac risk-factor modification program improved smoking cessation, reduced low-density lipoprotein cholesterol, and increased exercise capacity compared with usual care.12 The results of this study helped pave the way for one of the first CR programs with a strong home-based element. The MULTIFIT program was jointly developed by the Stanford Coronary Rehabilitation Program and Kaiser Permanente (Oakland, CA) in 1995. MULTIFIT is a nurse-based care model for CVD prevention.
Further research that evaluated other home-based programs showed similar promise. A Cochrane review demonstrated that home- and facility-based CR programs were equal in cardiac risk factor reduction, reduced hospital readmissions and mortality rates, and improved quality of life (QOL).13 Cost-effectiveness also seemed to be similar in both home- and hospital-based CR
Referrals
To address the problems with referrals that plague other CR programs, staff of the Healthy Heart Program worked closely with interventional cardiology and the cardiothoracic team, including the clinical informatics coordinators, to develop an automatic referral system for CR evaluation. Consults for CR evaluation were embedded within the post-CABG and PCI order sets in the electronic health record. Laboratory troponin alerts were created to alert CR staff of patients with elevated troponins, which identified patients admitted for acute MI. Healthy Heart Program staff members received the referrals once a patient was admitted to the unit following their heart procedure. Early referrals for evaluation allowed staff to begin a chart review of all eligible patients and to follow the patient’s course of recovery. Most consults were generated during hospitalization for one of the indications; however, a minority of consults come from both the cardiology and primary care clinics.
Three Phases of CR
The AACVPR describes the challenges and opportunities found throughout the CR continuum.5 Over the past several decades, the continuum of care was more program centered and service utilization was more isolated. Today, CR is viewed as more process oriented and coordinates care across many professionals and services. Phase 1 inpatient CR begins in the hospital and is a shared responsibility between several services. Shortened hospital stays have led to innovative solutions for early ambulation, risk factor education, and discharge planning, including enrollment into phase 2 CR. Phase 2, also known as early outpatient, should begin within 1 to 2 weeks postevent in healthier patients and can last between 6 and 12 weeks postdischarge. Phase 3 (maintenance phase) should begin immediately at the conclusion of phase 2.
Phase 1
Prior to the advent of the Healthy Heart Program, secondary prevention education was not done at the bedside for SFVAMC patients following cardiac revascularization. The AACVPR recommends patient assessment, mobilization, risk-factor identification and education, and facilitation into outpatient CR as essential components of phase 1 CR.5 The Healthy Heart Program clinician initiates phase 1 CR by examining cardiac risk factor management for all referred patients. Physical and cardiac risk factor assessments are accomplished by completing a detailed chart review and interview with the patient. During this interview with the patient, the clinician evaluates cognitive function and readiness to learn. Staff will interview the patient further to assess the overall patient needs, including availability of social support, resources to maintain optimal health, and the need for secondary preventive education. For the PCI patient, the interview may occur in the hours following their procedure; for the surgical patient, this bedside visit typically occurs postoperative day 3 or 4.
A standardized cardiac risk factor evaluation tool was designed, which also serves as an education form to help guide the conversation on risk factor management. The interactive, patient-centered form includes opportunities to review risk, discuss current laboratory values (eg, lipids and hemoglobin A1c), and establish individualized goals based on patient preference and recommended guidelines. Healthy Heart Program staff assist the patient in formulating achievable goals using the SMART (specific, measurable, attainable, realistic, and time-related) criteria.19 Immediately after a heart event or procedure, patients often feel highly motivated to initiate lifestyle changes.20 However, PCI patients may have a short window of opportunity for learning between their readiness to learn state and before the activities of discharge. Staff use these opportunities as a teachable moment and to increase enrollment into outpatient CR (phase 2).
The provider performs a thorough chart review and bedside consultation to determine whether home-based CR is indicated, feasible, and appropriate. Not every patient that is referred will be enrolled in CR. Patients have the option to opt out. In addition, clinical staff adhere to the program protocol’s exclusion criteria.
Absolute contraindications for home enrollment include unstable angina, staged cardiac procedure (PCI and surgery), complex ventricular arrhythmias, severe or symptomatic aortic stenosis, decompensated heart failure, and uncontrolled hypertension (Table). Patients deemed high risk for home-based CR may be referred to a non-VA facility-based CR program. Risk stratification, using the Canadian Cardiovascular Society Grading of Angina Pectoris, is a continuous process that is used to identify patients who may move from moderate to high risk, both before and during the program.21,22
Phases 2 and 3
Phase 2 of the Healthy Heart Program CR includes physical activity, risk-factor modification, nutritional guidance, psychosocial modification, a return to previous activities, and an improved QOL. Prior to entry into the program, a submaximal exercise test, the 6-minute walk test (6MWT), is used as both a qualifying test and for developing the initial exercise prescription.22 The minimum 6MWT distance needed to qualify is 75 m for postoperative and 150 m for nonsurgical patients. The 6MWT is performed in-hospital for patients who were admitted for stable angina, PCI, and are > 4 days following acute MI.23 Cardiothoracic surgery patients are tested at their first follow-up clinic visit (typically 2-3 weeks postoperatively). The clinician monitors the heart rate with either a wearable device or via inpatient telemetry monitors. This exercise testing also serves as a motivational tool for patients to gain confidence in their ability to begin to exercise at home.
Each participant receives a workbook and a DVD titled An Active Partnership for the Health of Your Heart. A personal health journal is provided for documenting vital signs, activity, and dietary intake. In addition, each participant receives equipment on an as-needed basis, including resistance bands, a weight scale, a blood pressure cuff, a pedometer/heart rate monitoring device, an exercise peddler or stationary bike, and a dietary video. Baseline assessments include the General Anxiety Disorder (GAD-7), Personal Health Questionnaire (PHQ-9) and a nutrition (Rate Your Plate) questionnaire. A cognitive function test (Montreal Cognitive Assessment) is used on an as-needed basis.
Nine 30-minute telephone follow-up sessions are scheduled within a 12-week period (weekly for the first 6 weeks, then biweekly). Topics covered are customized and include exercise; nutrition; medications; smoking cessation; and diabetes, hypertension, and weight management. Via a telephone follow-up session, the program nurses and patients codevelop an electronic individualized treatment plan that is tailored to the patient’s diagnosis, individual goals, and preferences. Clinicians teach participants how to self-monitor exercise, using a continuous heart rate monitoring device (Mio Alpha II or Fuse) and the 6-20 Borg dyspnea rating scale.24 Initially, moderate intensity exercise is prescribed with a target heart rate that is 60% to 75% of the 6MWT peak heart rate and an initial Borg scale target (11-14 on 20 point scale). The program physicians approve the treatment plan at the first patient visit and every 30 days until phase 2 is complete.
Patients who have completed early outpatient phase 2 CR can benefit from continuing to a phase 3 CR program.25 Participants of the Healthy Heart Program automatically are enrolled in phase 3, which is a long-term maintenance program that includes monthly or bimonthly phone calls for up to 1-year posthospital discharge. The goal is to support each veteran’s transition to a long-term healthy lifestyle that includes regular exercise.
Client-Clinician Partnership
The Healthy Heart Program establishes the client-clinician partnership prior to discharge for hospitalized patients. The nurse who initiates phase 1 at the bedside is the primary clinician throughout phases 2 and 3 with the exception of a dietician, psychologist, and/or exercise physiologist who provide follow-up calls as needed. Throughout these weekly follow-up phone sessions, the clinician gains an appreciation of the patient’s understanding of his or her disease, patterns of behavior, desire to change, confidence in being able to change, potential barriers, and responses to obstacles
Tailored Behavioral Change
The clinician’s responsibility is to listen to the patient’s concerns, assess their level of commitment for changing health behaviors, and provide guidance and support at the patient’s current level. The clinician applies the Transtheoretical Model founded on the Stages of Change principals to help understand and provide guidance based on the patient’s feelings about health behavior change.26 People are actively open to changing behaviors by only 20% at any given time.27 Therefore, action-oriented guidance for patients who are in the contemplative stage would not be helpful. This patient-centered approach promotes patients’ self-awareness, participation, and understanding of their decision-making role in their health management. Ultimately, individuals must take ownership of their health care maintenance for sustained behavioral change and medication management, and clinicians should facilitate that process.
Discussion
Secondary prevention strategies for heart disease continue to be underutilized. The Healthy Heart Program aims to improve participation in CR, improve QOL, help patients understand their heart disease, and support these patients psychologically. An advantage of this program is that it begins inpatient CR immediately following the heart event, when many patients often are more receptive to behavioral change support and guidance. Another advantage is that the program breaks down barriers to access, which is especially important in the veteran population. The Healthy Heart Program provides support and guidance for exercise and cardiac risk factor management to patients who otherwise would have not participated in any type of CR program.
A home-based CR program can be adopted independently or in conjunction with a facility-based program to which patients lack access. Furthermore, home-based CR programs function well as a phase 3 maintenance program at the completion of a traditional CR program. Since its inception, the Healthy Heart Program has increased the number of veterans enrolled in cardiac rehabilitation at the SFVAMC dramatically, from < 1% in FY 2012 to > 40% in FY 2015.
Program Limitations
One potential disadvantage of a home-based CR program is patients’ fear of returning to an exercise routine following a cardiac event. In addition, a lack of in-person supervision in home-based CR can lead patients to engage in less intensive activity than in facility-based CR. Other disadvantages include a lack of social support, less patient accountability, and safety concerns for sicker patients. Staff have consulted on several patients who expressed a lack of confidence in their ability to do well in this type of program, where accountability for exercising is self-reported. Staff referred these patients, who had the means to travel, to a non-VA facility-based CR program of their choice. Ideally, patients would have the choice between facility- or home-based programs or be able to choose a hybrid program that would best meet their needs.
Another identified limitation of this program was the lack of group support and in-person interactions with rehabilitation staff. Finally, although this program uses mobile devices with heart rate monitoring technology, these devices currently lack the capability to remotely share data with clinicians. Clinicians are reliant on the patient’s use of a personal health journal and memory. Subjective patient reporting has been found to be overestimated; therefore, more objective methods to measure important clinical outcomes are necessary.28
Conclusion
Facility-based CR is effective but underutilized. Alternative secondary programs are needed to help meet patient needs and overcome patient barriers. One promising approach to increase participation is home-based CR. Home-based CR programs have the potential to increase CR uptake and adherence. Home-based CR optimizes enrollment through evidence-based alternative models due to improved access. The future of CR will become highly individualized and multifaceted as a result of available mobile technologies and Internet-based tools, which will help increase the number of participants and expand the reach of cardiac risk factor management programs beyond the facility-based setting. A home-based program will be a valuable addition to facility-based programs as a stand-alone program or adopted into a hybrid program.
Acknowledgments
This work was funded by the VA Quality Enhancement Research Initiative.
1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Cicrulation. 2017;135(10):e146-e603.
2. Anderson L, Oldridge N, Thompson DR, Zwisler A, Rees K, Martin N, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Systematic Review and Meta-analysis. J Am Coll Card. 2016;67:1-12.
3. Oldridge NB, Guyatt GH, Fischer ME, Rimm AA. Cardiac rehabilitation after myocardial infarction. Combined experience of randomized clinical trials. JAMA. 1988;260:940-950.
4. Taylor RS, Brown A, Ebrahim S, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116(10):682-692.
5. American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs. 5th ed. Champaign, IL: Human Kinetics; 2013.
6. Balady GJ, Williams MA, Ades PA, et al; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing; American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Nutrition, Physical Activity, and Metabolism; American Association of Cardiovascular and Pulmonary Rehabilitation. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2007;115(10):2675-2682.
7. Thomas R J, King M, Lui K, et al; Writing Committee Members. AACVPR/ACCF/AHA 2010 update: performance measures on cardiac rehabilitation for referral to cardiac rehabilitation/secondary prevention services: a report of the American Association of Cardiovascular and Pulmonary Rehabilitation and the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Clinical Performance Measures for Cardiac Rehabilitation). Circulation. 2010;122(13):1342-1350.
8. Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation. 2007;116(15):1653-1662.
9. Balady GJ, Ades PA, Bitner VA, et al; American Heart Association Science Advisory and Coordinating Committee. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: a presidential advisory from the American Heart Association. Circulation. 2011;124(25):2951-2960.
10. Schopfer DW, Takemoto S, Allsup K, et al. Notice of Retraction and Replacement. Schopfer DW, et al. Cardiac rehabilitation use among veterans with ischemic heart disease. JAMA Intern Med. 2014;174(10):1687-1689. JAMA Intern Med. 2016;176(11):1726-1727.
11. Ferguson EE. Cardiac rehabilitation—an effective and comprehensive but underutilized program to reduce cardiovascular risk in patients with CVD. US Cardiology. 2006;3(2):14-16.
12. DeBusk RF, Miller NH, Superko HR, et al. A case-management system for coronary risk factor modification after acute myocardial infarction. Ann Intern Med. 1994;120(9):721-729.
13. Buckingham SA, Taylor RS, Jolly K, et al. Home-based versus centre-based cardiac rehabilitation: abridged Cochrane systematic review and meta-analysis. Open Heart. 2016;3(2):e000463.
14. Taylor RS, Watt A, Dalal HM, et al. Home-based cardiac rehabilitation versus hospital-based rehabilitation: a cost effectiveness analysis. Int J Cardiol. 2007;119(2):196-201.
15. Kotb A, Hsieh S, Wells GA. The effect of telephone support interventions on coronary artery disease (CAD) patient outcomes during cardiac rehabilitation: a systematic review and meta-analysis. PLoS One. 2014;9(5):e96581.
16. Grace SL, McDonald J, Fishman D, Caruso V. Patient preferences for home-based versus hospital-based cardiac rehabilitation. J Cardiopulm Rehabil. 2005;25(1):24-29.
17. Wakefield B, Drwal K, Scherubel M, Klobucar T, Johnson S, Kaboli P. Feasibility and effectiveness of remote, telephone-based delivery of cardiac rehabilitation. Telemed J E Health. 2014;20(1):32-38.
18. Smith SC, Benjamin EJ, Bonow RO, et al; World Heart Federation and the Preventive Cardiovascular Nurses Association. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124(22):2458-2473.
19. Doran GT. There’s a S.M.A.R.T. way to write management’s goals and objectives. Manage Rev. 1981;70(11):35-36.
20. Dullaghan L, Lusk L, Donnelly P, McGeough M, Fitzsimons D. Communicating with people who have experienced heart attack. Emerg Nurse. 2013;21(6):33-36.
21. Campeau L. Letter: grading of angina pectoris. Circulation. 1976;54(3):522-523.
22. Fletcher GF, Balady GJ, Armstrong EA, et al. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104(14):1694-1740.
23. Gibbons RJ, Balady GJ, Bricker JT, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Committee to Update the 1997 Exercise Testing Guidelines. Committee to Update the 1997 Exercise Testing Guidelines. ACC/AHA 2002 guideline update for exercise testing: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). J Am Coll Cardiol. 2002;40(8):1531-1540.
24. Borg G. Borg’s Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998.
25. Seki E, Watanabe Y, Sunayama S, et al. Effects of phase III cardiac rehabilitation programs on health-related quality of life in elderly patients with coronary artery disease: Juntendo Cardiac Rehabilitation Program (J-CARP). Circ J. 2003;67(1):73-77.
26. The transtheoretical model. Pro-Change Behavior Systems, Inc. http://www.prochange.com/transtheoretical-model-of-behavior-change. Published 2016. Accessed April 6, 2017.
27. Prochaska JO, Ever KE, Castle PH, et al. Enhancing multiple domains of well-being by decreasing multiple health risk behaviors: a randomized clinical trial. Popul Health Manag. 2012;15(5):276-286.
28. Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56.
1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Cicrulation. 2017;135(10):e146-e603.
2. Anderson L, Oldridge N, Thompson DR, Zwisler A, Rees K, Martin N, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Systematic Review and Meta-analysis. J Am Coll Card. 2016;67:1-12.
3. Oldridge NB, Guyatt GH, Fischer ME, Rimm AA. Cardiac rehabilitation after myocardial infarction. Combined experience of randomized clinical trials. JAMA. 1988;260:940-950.
4. Taylor RS, Brown A, Ebrahim S, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116(10):682-692.
5. American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs. 5th ed. Champaign, IL: Human Kinetics; 2013.
6. Balady GJ, Williams MA, Ades PA, et al; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing; American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Nutrition, Physical Activity, and Metabolism; American Association of Cardiovascular and Pulmonary Rehabilitation. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2007;115(10):2675-2682.
7. Thomas R J, King M, Lui K, et al; Writing Committee Members. AACVPR/ACCF/AHA 2010 update: performance measures on cardiac rehabilitation for referral to cardiac rehabilitation/secondary prevention services: a report of the American Association of Cardiovascular and Pulmonary Rehabilitation and the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Clinical Performance Measures for Cardiac Rehabilitation). Circulation. 2010;122(13):1342-1350.
8. Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation. 2007;116(15):1653-1662.
9. Balady GJ, Ades PA, Bitner VA, et al; American Heart Association Science Advisory and Coordinating Committee. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: a presidential advisory from the American Heart Association. Circulation. 2011;124(25):2951-2960.
10. Schopfer DW, Takemoto S, Allsup K, et al. Notice of Retraction and Replacement. Schopfer DW, et al. Cardiac rehabilitation use among veterans with ischemic heart disease. JAMA Intern Med. 2014;174(10):1687-1689. JAMA Intern Med. 2016;176(11):1726-1727.
11. Ferguson EE. Cardiac rehabilitation—an effective and comprehensive but underutilized program to reduce cardiovascular risk in patients with CVD. US Cardiology. 2006;3(2):14-16.
12. DeBusk RF, Miller NH, Superko HR, et al. A case-management system for coronary risk factor modification after acute myocardial infarction. Ann Intern Med. 1994;120(9):721-729.
13. Buckingham SA, Taylor RS, Jolly K, et al. Home-based versus centre-based cardiac rehabilitation: abridged Cochrane systematic review and meta-analysis. Open Heart. 2016;3(2):e000463.
14. Taylor RS, Watt A, Dalal HM, et al. Home-based cardiac rehabilitation versus hospital-based rehabilitation: a cost effectiveness analysis. Int J Cardiol. 2007;119(2):196-201.
15. Kotb A, Hsieh S, Wells GA. The effect of telephone support interventions on coronary artery disease (CAD) patient outcomes during cardiac rehabilitation: a systematic review and meta-analysis. PLoS One. 2014;9(5):e96581.
16. Grace SL, McDonald J, Fishman D, Caruso V. Patient preferences for home-based versus hospital-based cardiac rehabilitation. J Cardiopulm Rehabil. 2005;25(1):24-29.
17. Wakefield B, Drwal K, Scherubel M, Klobucar T, Johnson S, Kaboli P. Feasibility and effectiveness of remote, telephone-based delivery of cardiac rehabilitation. Telemed J E Health. 2014;20(1):32-38.
18. Smith SC, Benjamin EJ, Bonow RO, et al; World Heart Federation and the Preventive Cardiovascular Nurses Association. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124(22):2458-2473.
19. Doran GT. There’s a S.M.A.R.T. way to write management’s goals and objectives. Manage Rev. 1981;70(11):35-36.
20. Dullaghan L, Lusk L, Donnelly P, McGeough M, Fitzsimons D. Communicating with people who have experienced heart attack. Emerg Nurse. 2013;21(6):33-36.
21. Campeau L. Letter: grading of angina pectoris. Circulation. 1976;54(3):522-523.
22. Fletcher GF, Balady GJ, Armstrong EA, et al. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104(14):1694-1740.
23. Gibbons RJ, Balady GJ, Bricker JT, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Committee to Update the 1997 Exercise Testing Guidelines. Committee to Update the 1997 Exercise Testing Guidelines. ACC/AHA 2002 guideline update for exercise testing: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). J Am Coll Cardiol. 2002;40(8):1531-1540.
24. Borg G. Borg’s Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998.
25. Seki E, Watanabe Y, Sunayama S, et al. Effects of phase III cardiac rehabilitation programs on health-related quality of life in elderly patients with coronary artery disease: Juntendo Cardiac Rehabilitation Program (J-CARP). Circ J. 2003;67(1):73-77.
26. The transtheoretical model. Pro-Change Behavior Systems, Inc. http://www.prochange.com/transtheoretical-model-of-behavior-change. Published 2016. Accessed April 6, 2017.
27. Prochaska JO, Ever KE, Castle PH, et al. Enhancing multiple domains of well-being by decreasing multiple health risk behaviors: a randomized clinical trial. Popul Health Manag. 2012;15(5):276-286.
28. Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56.
Severe hospital-acquired anemia linked to readmission, death
Severe hospital-acquired anemia (HAA) may increase a person’s risk of hospital readmission and death, a new study suggests.
Researchers studied more than 11,000 patients admitted to 6 Texas hospitals and found that a third of the patients developed HAA.
The team also found that severe HAA was associated with a higher risk of death or readmission, even after the researchers adjusted for other factors.
They reported these findings in the Journal of Hospital Medicine.
“This study shines a spotlight on a very common but underappreciated risk of hospitalization, hospital-acquired anemia, which has traditionally been viewed as an incidental change in the red blood count of no significance,” said study author Ethan Halm, MD, of the University of Texas Southwestern Medical Center in Dallas.
“However, our results showed that hospital-acquired anemia was associated with worse clinical outcomes after leaving the hospital, so it needs to be taken more seriously.”
Dr Halm and his colleagues looked at consecutive medicine discharges between November 1, 2009, and October 30, 2010, from 6 hospitals in Texas (safety-net, teaching, and nonteaching).
Of the 11,309 patients studied, 33.1% developed HAA. Most (21.6%) had mild HAA, followed by moderate HAA (10.1%), and severe HAA (1.4%).
The study’s primary outcome was a composite of 30-day mortality and nonelective readmission. This occurred in 9.7% of patients without HAA and 16.4% of those with severe HAA.
The researchers found that severe HAA was independently associated with a 39% increase in the odds of meeting the primary outcome (readmission or 30-day mortality).
The team noted that 85% of patients with severe HAA underwent a major procedure, had a discharge diagnosis of hemorrhage, and/or a discharge diagnosis of hemorrhagic disorder.
The researchers identified 2 potentially modifiable predictors of moderate or severe HAA. These were length of hospital stay (adjusted odds ratio=1.26 per day) and undergoing a major procedure (adjusted odds ratio=5.09).
“Our findings suggest that reducing blood loss during major surgeries and reducing unnecessary testing during hospital stays may lower a patient’s risk of developing severe hospital-acquired anemia, and potentially improve their recovery,” said Anil N. Makam, MD, of the University of Texas Southwestern Medical Center.
In the future, the researchers hope to examine other patient-centered outcomes that may be related to HAA, such as fatigue, functional impairment, and the trajectory of post-hospital recovery. ![]()
Severe hospital-acquired anemia (HAA) may increase a person’s risk of hospital readmission and death, a new study suggests.
Researchers studied more than 11,000 patients admitted to 6 Texas hospitals and found that a third of the patients developed HAA.
The team also found that severe HAA was associated with a higher risk of death or readmission, even after the researchers adjusted for other factors.
They reported these findings in the Journal of Hospital Medicine.
“This study shines a spotlight on a very common but underappreciated risk of hospitalization, hospital-acquired anemia, which has traditionally been viewed as an incidental change in the red blood count of no significance,” said study author Ethan Halm, MD, of the University of Texas Southwestern Medical Center in Dallas.
“However, our results showed that hospital-acquired anemia was associated with worse clinical outcomes after leaving the hospital, so it needs to be taken more seriously.”
Dr Halm and his colleagues looked at consecutive medicine discharges between November 1, 2009, and October 30, 2010, from 6 hospitals in Texas (safety-net, teaching, and nonteaching).
Of the 11,309 patients studied, 33.1% developed HAA. Most (21.6%) had mild HAA, followed by moderate HAA (10.1%), and severe HAA (1.4%).
The study’s primary outcome was a composite of 30-day mortality and nonelective readmission. This occurred in 9.7% of patients without HAA and 16.4% of those with severe HAA.
The researchers found that severe HAA was independently associated with a 39% increase in the odds of meeting the primary outcome (readmission or 30-day mortality).
The team noted that 85% of patients with severe HAA underwent a major procedure, had a discharge diagnosis of hemorrhage, and/or a discharge diagnosis of hemorrhagic disorder.
The researchers identified 2 potentially modifiable predictors of moderate or severe HAA. These were length of hospital stay (adjusted odds ratio=1.26 per day) and undergoing a major procedure (adjusted odds ratio=5.09).
“Our findings suggest that reducing blood loss during major surgeries and reducing unnecessary testing during hospital stays may lower a patient’s risk of developing severe hospital-acquired anemia, and potentially improve their recovery,” said Anil N. Makam, MD, of the University of Texas Southwestern Medical Center.
In the future, the researchers hope to examine other patient-centered outcomes that may be related to HAA, such as fatigue, functional impairment, and the trajectory of post-hospital recovery. ![]()
Severe hospital-acquired anemia (HAA) may increase a person’s risk of hospital readmission and death, a new study suggests.
Researchers studied more than 11,000 patients admitted to 6 Texas hospitals and found that a third of the patients developed HAA.
The team also found that severe HAA was associated with a higher risk of death or readmission, even after the researchers adjusted for other factors.
They reported these findings in the Journal of Hospital Medicine.
“This study shines a spotlight on a very common but underappreciated risk of hospitalization, hospital-acquired anemia, which has traditionally been viewed as an incidental change in the red blood count of no significance,” said study author Ethan Halm, MD, of the University of Texas Southwestern Medical Center in Dallas.
“However, our results showed that hospital-acquired anemia was associated with worse clinical outcomes after leaving the hospital, so it needs to be taken more seriously.”
Dr Halm and his colleagues looked at consecutive medicine discharges between November 1, 2009, and October 30, 2010, from 6 hospitals in Texas (safety-net, teaching, and nonteaching).
Of the 11,309 patients studied, 33.1% developed HAA. Most (21.6%) had mild HAA, followed by moderate HAA (10.1%), and severe HAA (1.4%).
The study’s primary outcome was a composite of 30-day mortality and nonelective readmission. This occurred in 9.7% of patients without HAA and 16.4% of those with severe HAA.
The researchers found that severe HAA was independently associated with a 39% increase in the odds of meeting the primary outcome (readmission or 30-day mortality).
The team noted that 85% of patients with severe HAA underwent a major procedure, had a discharge diagnosis of hemorrhage, and/or a discharge diagnosis of hemorrhagic disorder.
The researchers identified 2 potentially modifiable predictors of moderate or severe HAA. These were length of hospital stay (adjusted odds ratio=1.26 per day) and undergoing a major procedure (adjusted odds ratio=5.09).
“Our findings suggest that reducing blood loss during major surgeries and reducing unnecessary testing during hospital stays may lower a patient’s risk of developing severe hospital-acquired anemia, and potentially improve their recovery,” said Anil N. Makam, MD, of the University of Texas Southwestern Medical Center.
In the future, the researchers hope to examine other patient-centered outcomes that may be related to HAA, such as fatigue, functional impairment, and the trajectory of post-hospital recovery. ![]()
Declines in frequent binge drinking vary in some teen subgroups
The drop in frequent binge drinking (FBD) among adolescents can be attributed to age, period, and cohort effects, but there are variations in certain subgroups of teens, said Joy Bohyun Jang, PhD, and her associates.
The decline in FBD is not as great in teen girls, African American youth, and youth from low socioeconomic backgrounds, so those groups deserve close attention by researchers and clinicians, they said.
The study used an age-period-cohort analysis to examine how these variables affected drinking trends among adolescents, with a particular focus on FBD, which was defined as two or more occasions of consuming at least five alcoholic drinks in a row in the past 2 weeks.
FBD decreased in recent years in all ages during adolescence, suggesting that declines in teen FBD in the past 25 years were “driven by factors influencing all age groups simultaneously as well as influences on particular birth cohorts,” the researchers said. These factors might include greater public efforts to lessen the risk of underage drinking and disapproval of heavy alcohol use among the recent cohorts of teens. “Those born around 1990 had the highest decline of FBD compared with those in the preceding and subsequent cohorts of adolescents.”
But there are variations in FBD among teens by demographics. Boys and those of higher socioeconomic status (SES) showed rapid increases in FBD by age, compared with girls and teens of lower SES, respectively. However, there also has been a convergence in FBD by sex in the more recent time periods because of greater declines in FBD among boys than in girls. Likewise, there is a growing discrepancy by SES in FBD in U.S. teens because higher SES teens were less likely than those from a lower SES to engage in FBD and “the strength of the association is growing in more recent time periods.”
African American youth had the lowest rates of FBD for all the racial groups, yet declines in FBD have been slower among African American youth, compared with white adolescents, since 2007, reported Dr. Jang of the University of Michigan, Ann Arbor, and her associates.
The study was supported by grants from the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, and was funded by the National Institutes of Health. Dr. Jang and her associates said they had no relevant financial disclosures.
[email protected]
Teens who drink heavily are more likely to have unprotected sex, perform poorly at school or work, and have problems with their parents. Monitoring the Future data previously have shown that since the late 1990s, the prevalence of binge drinking has dropped to lows of 3%, 10%, and 16% among 8th, 10th, and 12th graders, respectively.
Dr. Jang et al. took a look at Monitoring the Future data to see how age, period, and cohort effects might alter FBD patterns among teens. There was an overall decrease in FBD since the 1990s, with the greatest decline among teens born between 1985 and 1990. It appeared that period and cohort effects drove this decline.
However, some subgroups exhibited differences. “The decline in frequent heavy drinking is not uniform, with female adolescents, black youth, and youth from low-SES backgrounds experiencing a less steep decline.”
“Pediatric primary care providers have an opportunity to screen all adolescents for alcohol use as part of routine annual care and to provide brief prevention and early intervention strategies. Despite the reassuring decline in frequent heavy drinking, it is critical that ongoing efforts address differences in declining rates to avoid exacerbating disparities.”
Justine Wittenauer Welsh, MD, of Emory Adolescent Substance Abuse Treatment Services, Emory University, Atlanta; John Rogers Knight, MD, at the Center for Adolescent Substance Abuse Research, Boston Children’s Hospital; and Scott Evan Hadland, MD, MPH, of Boston University, made these comments in an accompanying editorial (Pediatrics. 2017 May 22;139[6]:e20170932). The authors said they received no funding and have no relevant financial disclosures.
Teens who drink heavily are more likely to have unprotected sex, perform poorly at school or work, and have problems with their parents. Monitoring the Future data previously have shown that since the late 1990s, the prevalence of binge drinking has dropped to lows of 3%, 10%, and 16% among 8th, 10th, and 12th graders, respectively.
Dr. Jang et al. took a look at Monitoring the Future data to see how age, period, and cohort effects might alter FBD patterns among teens. There was an overall decrease in FBD since the 1990s, with the greatest decline among teens born between 1985 and 1990. It appeared that period and cohort effects drove this decline.
However, some subgroups exhibited differences. “The decline in frequent heavy drinking is not uniform, with female adolescents, black youth, and youth from low-SES backgrounds experiencing a less steep decline.”
“Pediatric primary care providers have an opportunity to screen all adolescents for alcohol use as part of routine annual care and to provide brief prevention and early intervention strategies. Despite the reassuring decline in frequent heavy drinking, it is critical that ongoing efforts address differences in declining rates to avoid exacerbating disparities.”
Justine Wittenauer Welsh, MD, of Emory Adolescent Substance Abuse Treatment Services, Emory University, Atlanta; John Rogers Knight, MD, at the Center for Adolescent Substance Abuse Research, Boston Children’s Hospital; and Scott Evan Hadland, MD, MPH, of Boston University, made these comments in an accompanying editorial (Pediatrics. 2017 May 22;139[6]:e20170932). The authors said they received no funding and have no relevant financial disclosures.
Teens who drink heavily are more likely to have unprotected sex, perform poorly at school or work, and have problems with their parents. Monitoring the Future data previously have shown that since the late 1990s, the prevalence of binge drinking has dropped to lows of 3%, 10%, and 16% among 8th, 10th, and 12th graders, respectively.
Dr. Jang et al. took a look at Monitoring the Future data to see how age, period, and cohort effects might alter FBD patterns among teens. There was an overall decrease in FBD since the 1990s, with the greatest decline among teens born between 1985 and 1990. It appeared that period and cohort effects drove this decline.
However, some subgroups exhibited differences. “The decline in frequent heavy drinking is not uniform, with female adolescents, black youth, and youth from low-SES backgrounds experiencing a less steep decline.”
“Pediatric primary care providers have an opportunity to screen all adolescents for alcohol use as part of routine annual care and to provide brief prevention and early intervention strategies. Despite the reassuring decline in frequent heavy drinking, it is critical that ongoing efforts address differences in declining rates to avoid exacerbating disparities.”
Justine Wittenauer Welsh, MD, of Emory Adolescent Substance Abuse Treatment Services, Emory University, Atlanta; John Rogers Knight, MD, at the Center for Adolescent Substance Abuse Research, Boston Children’s Hospital; and Scott Evan Hadland, MD, MPH, of Boston University, made these comments in an accompanying editorial (Pediatrics. 2017 May 22;139[6]:e20170932). The authors said they received no funding and have no relevant financial disclosures.
The drop in frequent binge drinking (FBD) among adolescents can be attributed to age, period, and cohort effects, but there are variations in certain subgroups of teens, said Joy Bohyun Jang, PhD, and her associates.
The decline in FBD is not as great in teen girls, African American youth, and youth from low socioeconomic backgrounds, so those groups deserve close attention by researchers and clinicians, they said.
The study used an age-period-cohort analysis to examine how these variables affected drinking trends among adolescents, with a particular focus on FBD, which was defined as two or more occasions of consuming at least five alcoholic drinks in a row in the past 2 weeks.
FBD decreased in recent years in all ages during adolescence, suggesting that declines in teen FBD in the past 25 years were “driven by factors influencing all age groups simultaneously as well as influences on particular birth cohorts,” the researchers said. These factors might include greater public efforts to lessen the risk of underage drinking and disapproval of heavy alcohol use among the recent cohorts of teens. “Those born around 1990 had the highest decline of FBD compared with those in the preceding and subsequent cohorts of adolescents.”
But there are variations in FBD among teens by demographics. Boys and those of higher socioeconomic status (SES) showed rapid increases in FBD by age, compared with girls and teens of lower SES, respectively. However, there also has been a convergence in FBD by sex in the more recent time periods because of greater declines in FBD among boys than in girls. Likewise, there is a growing discrepancy by SES in FBD in U.S. teens because higher SES teens were less likely than those from a lower SES to engage in FBD and “the strength of the association is growing in more recent time periods.”
African American youth had the lowest rates of FBD for all the racial groups, yet declines in FBD have been slower among African American youth, compared with white adolescents, since 2007, reported Dr. Jang of the University of Michigan, Ann Arbor, and her associates.
The study was supported by grants from the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, and was funded by the National Institutes of Health. Dr. Jang and her associates said they had no relevant financial disclosures.
[email protected]
The drop in frequent binge drinking (FBD) among adolescents can be attributed to age, period, and cohort effects, but there are variations in certain subgroups of teens, said Joy Bohyun Jang, PhD, and her associates.
The decline in FBD is not as great in teen girls, African American youth, and youth from low socioeconomic backgrounds, so those groups deserve close attention by researchers and clinicians, they said.
The study used an age-period-cohort analysis to examine how these variables affected drinking trends among adolescents, with a particular focus on FBD, which was defined as two or more occasions of consuming at least five alcoholic drinks in a row in the past 2 weeks.
FBD decreased in recent years in all ages during adolescence, suggesting that declines in teen FBD in the past 25 years were “driven by factors influencing all age groups simultaneously as well as influences on particular birth cohorts,” the researchers said. These factors might include greater public efforts to lessen the risk of underage drinking and disapproval of heavy alcohol use among the recent cohorts of teens. “Those born around 1990 had the highest decline of FBD compared with those in the preceding and subsequent cohorts of adolescents.”
But there are variations in FBD among teens by demographics. Boys and those of higher socioeconomic status (SES) showed rapid increases in FBD by age, compared with girls and teens of lower SES, respectively. However, there also has been a convergence in FBD by sex in the more recent time periods because of greater declines in FBD among boys than in girls. Likewise, there is a growing discrepancy by SES in FBD in U.S. teens because higher SES teens were less likely than those from a lower SES to engage in FBD and “the strength of the association is growing in more recent time periods.”
African American youth had the lowest rates of FBD for all the racial groups, yet declines in FBD have been slower among African American youth, compared with white adolescents, since 2007, reported Dr. Jang of the University of Michigan, Ann Arbor, and her associates.
The study was supported by grants from the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, and was funded by the National Institutes of Health. Dr. Jang and her associates said they had no relevant financial disclosures.
[email protected]
FROM PEDIATRICS
Key clinical point: The drop in frequent binge drinking (FBD) among U.S. teens can be attributed to age, period, and cohort effects, but there are variations in certain teen subgroups.
Major finding: FBD decreased in recent years among all ages during adolescence, suggesting that decreases in teen FBD in the past 25 years were “driven by factors influencing all age groups simultaneously as well as influences on particular birth cohorts.”
Data source: A Monitoring the Future study involved 1,065,022 student responses on self-administered questionnaires during 1991-2015 regarding binge drinking.
Disclosures: The study was supported by grants from the National Institutes of Health. Dr. Jang and her associates said they had no relevant financial disclosures.
Conflicts and the ethical practice of surgery
Once a month in my department, we focus on a case with challenging ethical considerations for part of the discussion at the M & M conference.
Earlier this week, my colleagues and I heard about an unfortunate 87-year-old man, who had been living independently when he developed a partial bowel obstruction. His wife had died over 10 years earlier and, although he lived alone, he had two sons and a daughter who lived close to him and regularly looked in on him and helped him to keep up his small home.
The ethical challenge presented at M & M was the difficulty of determining what was the “best” treatment for this patient and how that decision was reached. The surgical team explained to the patient and his family that there were two broad possibilities for his treatment: definitive resection of the primary tumor or palliative options. In order for him to have a colon resection, the cardiologists felt that he would need coronary artery bypass grafting before surgery. To pursue this course of treatment, they wanted him to have a diverting colostomy before the heart surgery. Then, after a period of recovery from the heart surgery, he could have a colon resection with takedown of the colostomy.
Alternatively, the palliative option of a colonic stent followed by external beam radiation to the lesion was offered. The surgical team tried to present the options in an evenhanded manner so as not to paint either option as being significantly worse. However, even with a definitive resection, the surgeons did not believe that they could cure the patient and they explained this to him and his family.
The patient seemed to have the capacity to make the choice and, although he had originally wanted “everything” done, when he was transferred to our hospital and when presented with these choices, he stated that the palliative option seemed better for him. He told the surgical team that he did not want to have heart surgery, and he did not want to risk dying with a colostomy.
At the end of the family meeting, the surgical team felt that the patient had made a reasonable decision, and they were comfortable with his choice. However, the following day, the patient’s daughter called demanding another meeting with the surgical team. She had been at the family meeting the prior day and stated that, in her opinion, the surgical team had “pushed” the patient to accept the palliative option and that she was not certain that he really had the capacity to make decisions for himself.
During the subsequent meeting with the family, the daughter was the primary spokesperson, but the two sons also seemed in agreement with her assessment that the patient lacked capacity. She stated that the patient was transferred to our medical center in order to allow him to get the treatments that he needed, and now, in her opinion, the surgical team was not pursuing the “best” treatment. She was upset and repeatedly expressed this sentiment.
The surgery team was understandably concerned with this turn of events. They had undertaken their evaluation with constant reassessment of the likely impact of the treatment options on the patient’s quality of life. They had tried to explain the options fully to the patient and involved his family in the discussion. In short, the surgical team had done their best to pursue high-quality ethical care by utilizing shared decision making. Despite spending significant time with the patient and his family, there was now conflict. The patient wanted to pursue a course of treatment that the surgical team felt was appropriate, but the family disagreed and wanted to make the decisions for the patient and pursue a more aggressive approach.
For many physicians, especially the residents who were actively involved in caring for this patient, this outcome – namely, significant conflict with the family and the family feeling that the patient should not be allowed to make his own decisions – seemed to be exactly what the careful attention to the ethical dimension of surgical practice tries to avoid.
Even though most of us try to avoid conflicts with patients and their families, optimal ethical practice does not always result in a consensus of opinions and that lack of conflict. As physicians, we can try to follow all of the ethical guidelines of extensive communication and shared decision making, yet we may still wind up with unhappy patients and families.
The goal of ethical practice should not be to avoid conflicts, but, rather, to treat patients in the manner that helps them to achieve what they value most.
In this present case, what could the surgical team do moving forward? Sometimes conflicts can be solved with additional information. A psychiatry consultation might be helpful to gain an opinion on whether the patient has the capacity to make decisions. Additionally, an ethics consultation might be valuable to gain an outside view to help the family understand the potential merits of a palliative approach. Although this case raises ethical concerns for the surgical team, the conflicts that resulted ought not be seen as a failure of the discussions surrounding the patient’s goals for his treatment.
Most of us prefer to avoid conflicts with patients and their families, but our ultimate goal in the ethical practice of surgery cannot be consensus. Rather, it should be to do the best we can to provide care that helps the patient achieve his or her goals. Unfortunately, we may do everything possible to provide high quality ethical care to patients and conflict still result. However, we cannot use resulting conflict as a reason to avoid the many discussions needed to communicate the options accurately to our patients and their families.
Dr. Angelos is the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, chief of endocrine surgery, and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
Once a month in my department, we focus on a case with challenging ethical considerations for part of the discussion at the M & M conference.
Earlier this week, my colleagues and I heard about an unfortunate 87-year-old man, who had been living independently when he developed a partial bowel obstruction. His wife had died over 10 years earlier and, although he lived alone, he had two sons and a daughter who lived close to him and regularly looked in on him and helped him to keep up his small home.
The ethical challenge presented at M & M was the difficulty of determining what was the “best” treatment for this patient and how that decision was reached. The surgical team explained to the patient and his family that there were two broad possibilities for his treatment: definitive resection of the primary tumor or palliative options. In order for him to have a colon resection, the cardiologists felt that he would need coronary artery bypass grafting before surgery. To pursue this course of treatment, they wanted him to have a diverting colostomy before the heart surgery. Then, after a period of recovery from the heart surgery, he could have a colon resection with takedown of the colostomy.
Alternatively, the palliative option of a colonic stent followed by external beam radiation to the lesion was offered. The surgical team tried to present the options in an evenhanded manner so as not to paint either option as being significantly worse. However, even with a definitive resection, the surgeons did not believe that they could cure the patient and they explained this to him and his family.
The patient seemed to have the capacity to make the choice and, although he had originally wanted “everything” done, when he was transferred to our hospital and when presented with these choices, he stated that the palliative option seemed better for him. He told the surgical team that he did not want to have heart surgery, and he did not want to risk dying with a colostomy.
At the end of the family meeting, the surgical team felt that the patient had made a reasonable decision, and they were comfortable with his choice. However, the following day, the patient’s daughter called demanding another meeting with the surgical team. She had been at the family meeting the prior day and stated that, in her opinion, the surgical team had “pushed” the patient to accept the palliative option and that she was not certain that he really had the capacity to make decisions for himself.
During the subsequent meeting with the family, the daughter was the primary spokesperson, but the two sons also seemed in agreement with her assessment that the patient lacked capacity. She stated that the patient was transferred to our medical center in order to allow him to get the treatments that he needed, and now, in her opinion, the surgical team was not pursuing the “best” treatment. She was upset and repeatedly expressed this sentiment.
The surgery team was understandably concerned with this turn of events. They had undertaken their evaluation with constant reassessment of the likely impact of the treatment options on the patient’s quality of life. They had tried to explain the options fully to the patient and involved his family in the discussion. In short, the surgical team had done their best to pursue high-quality ethical care by utilizing shared decision making. Despite spending significant time with the patient and his family, there was now conflict. The patient wanted to pursue a course of treatment that the surgical team felt was appropriate, but the family disagreed and wanted to make the decisions for the patient and pursue a more aggressive approach.
For many physicians, especially the residents who were actively involved in caring for this patient, this outcome – namely, significant conflict with the family and the family feeling that the patient should not be allowed to make his own decisions – seemed to be exactly what the careful attention to the ethical dimension of surgical practice tries to avoid.
Even though most of us try to avoid conflicts with patients and their families, optimal ethical practice does not always result in a consensus of opinions and that lack of conflict. As physicians, we can try to follow all of the ethical guidelines of extensive communication and shared decision making, yet we may still wind up with unhappy patients and families.
The goal of ethical practice should not be to avoid conflicts, but, rather, to treat patients in the manner that helps them to achieve what they value most.
In this present case, what could the surgical team do moving forward? Sometimes conflicts can be solved with additional information. A psychiatry consultation might be helpful to gain an opinion on whether the patient has the capacity to make decisions. Additionally, an ethics consultation might be valuable to gain an outside view to help the family understand the potential merits of a palliative approach. Although this case raises ethical concerns for the surgical team, the conflicts that resulted ought not be seen as a failure of the discussions surrounding the patient’s goals for his treatment.
Most of us prefer to avoid conflicts with patients and their families, but our ultimate goal in the ethical practice of surgery cannot be consensus. Rather, it should be to do the best we can to provide care that helps the patient achieve his or her goals. Unfortunately, we may do everything possible to provide high quality ethical care to patients and conflict still result. However, we cannot use resulting conflict as a reason to avoid the many discussions needed to communicate the options accurately to our patients and their families.
Dr. Angelos is the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, chief of endocrine surgery, and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
Once a month in my department, we focus on a case with challenging ethical considerations for part of the discussion at the M & M conference.
Earlier this week, my colleagues and I heard about an unfortunate 87-year-old man, who had been living independently when he developed a partial bowel obstruction. His wife had died over 10 years earlier and, although he lived alone, he had two sons and a daughter who lived close to him and regularly looked in on him and helped him to keep up his small home.
The ethical challenge presented at M & M was the difficulty of determining what was the “best” treatment for this patient and how that decision was reached. The surgical team explained to the patient and his family that there were two broad possibilities for his treatment: definitive resection of the primary tumor or palliative options. In order for him to have a colon resection, the cardiologists felt that he would need coronary artery bypass grafting before surgery. To pursue this course of treatment, they wanted him to have a diverting colostomy before the heart surgery. Then, after a period of recovery from the heart surgery, he could have a colon resection with takedown of the colostomy.
Alternatively, the palliative option of a colonic stent followed by external beam radiation to the lesion was offered. The surgical team tried to present the options in an evenhanded manner so as not to paint either option as being significantly worse. However, even with a definitive resection, the surgeons did not believe that they could cure the patient and they explained this to him and his family.
The patient seemed to have the capacity to make the choice and, although he had originally wanted “everything” done, when he was transferred to our hospital and when presented with these choices, he stated that the palliative option seemed better for him. He told the surgical team that he did not want to have heart surgery, and he did not want to risk dying with a colostomy.
At the end of the family meeting, the surgical team felt that the patient had made a reasonable decision, and they were comfortable with his choice. However, the following day, the patient’s daughter called demanding another meeting with the surgical team. She had been at the family meeting the prior day and stated that, in her opinion, the surgical team had “pushed” the patient to accept the palliative option and that she was not certain that he really had the capacity to make decisions for himself.
During the subsequent meeting with the family, the daughter was the primary spokesperson, but the two sons also seemed in agreement with her assessment that the patient lacked capacity. She stated that the patient was transferred to our medical center in order to allow him to get the treatments that he needed, and now, in her opinion, the surgical team was not pursuing the “best” treatment. She was upset and repeatedly expressed this sentiment.
The surgery team was understandably concerned with this turn of events. They had undertaken their evaluation with constant reassessment of the likely impact of the treatment options on the patient’s quality of life. They had tried to explain the options fully to the patient and involved his family in the discussion. In short, the surgical team had done their best to pursue high-quality ethical care by utilizing shared decision making. Despite spending significant time with the patient and his family, there was now conflict. The patient wanted to pursue a course of treatment that the surgical team felt was appropriate, but the family disagreed and wanted to make the decisions for the patient and pursue a more aggressive approach.
For many physicians, especially the residents who were actively involved in caring for this patient, this outcome – namely, significant conflict with the family and the family feeling that the patient should not be allowed to make his own decisions – seemed to be exactly what the careful attention to the ethical dimension of surgical practice tries to avoid.
Even though most of us try to avoid conflicts with patients and their families, optimal ethical practice does not always result in a consensus of opinions and that lack of conflict. As physicians, we can try to follow all of the ethical guidelines of extensive communication and shared decision making, yet we may still wind up with unhappy patients and families.
The goal of ethical practice should not be to avoid conflicts, but, rather, to treat patients in the manner that helps them to achieve what they value most.
In this present case, what could the surgical team do moving forward? Sometimes conflicts can be solved with additional information. A psychiatry consultation might be helpful to gain an opinion on whether the patient has the capacity to make decisions. Additionally, an ethics consultation might be valuable to gain an outside view to help the family understand the potential merits of a palliative approach. Although this case raises ethical concerns for the surgical team, the conflicts that resulted ought not be seen as a failure of the discussions surrounding the patient’s goals for his treatment.
Most of us prefer to avoid conflicts with patients and their families, but our ultimate goal in the ethical practice of surgery cannot be consensus. Rather, it should be to do the best we can to provide care that helps the patient achieve his or her goals. Unfortunately, we may do everything possible to provide high quality ethical care to patients and conflict still result. However, we cannot use resulting conflict as a reason to avoid the many discussions needed to communicate the options accurately to our patients and their families.
Dr. Angelos is the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, chief of endocrine surgery, and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
Doing a lot with little – health care in Cuba
Although Cuba lies less than 100 miles from the United States, we Americans tend to know far less about the island nation than about almost any other country in our hemisphere. Only since 2014 has the United States begun to allow its citizens to travel directly to Cuba and has opened official diplomatic relations, although direct trade still remains blocked.
Cuba’s health care system has been touted as providing universal access to primary care services, whose goals are promoting health and preventing disease as well as providing free medical education to a veritable army of health care workers. Less well known are the quality and standards of their surgical services.
Although the Cuban government is a centralized, one-party state that follows the Marxist-Leninist ideology, every individual with whom we met answered our many questions with apparent candor. Perhaps our easy rapport was based to some degree on our common profession and our shared commitment to patient care. Although they were clearly proud of the quality of their free education and medical care, they were also quick to admit the shortcomings in their system: widespread poverty, shortages of food and advanced pharmaceuticals, and old medical facilities. We were not restricted in any way from moving around Havana or speaking with anyone, although our free time was admittedly limited because our busy schedule was crammed with at least two visits per day with the groups listed above.
We were interested in looking at how primary care was delivered in Cuba. We met with a primary care doctor in her office, which was situated on the ground floor of the apartment complex in which she and her patients lived. We also visited a polyclinic, two blocks from the primary care doctor’s office that serves as the next step up the chain and is the site where medical and surgical specialists come to consult with patients from 40-60 primary care practices clustered around the polyclinic. The walls of the polyclinic have posters that educate the patients about the importance of handwashing and prevention of hypertension and cancer. The polyclinic also has an epidemiologist who monitors such basic preventive services as immunizations and prenatal care, both of which achieve nearly 100% compliance in a society in which acceptance of these services is not optional. Pap smears are performed in the primary care clinics, as is comprehensive medical care.
As interesting and impressive as we found the primary care clinics, it was the visits with the surgeons in their hospitals that intrigued us the most. The surgeons we met were modest and collegial, yet proud of what they had accomplished under challenging resource constraints. The hospitals that we visited were reminiscent of the city and county hospitals in the United States in which many of us on the trip had trained in the 1970s: older facilities that were clean and serviceable, but with older, basic equipment. Nevertheless, C. Julian F. Ruiz Torres, MD, has developed minimally invasive surgery in a hospital dedicated to such technological advances. Although he is 72 years old, he still works tirelessly to obtain the resources to build a state-of-the-art surgical simulation center, now under construction. Basic minimally invasive surgical procedures are available in most hospitals, although advanced procedures are restricted to centers such as Dr. Ruiz Torres’ facility, Centro Nacional De Cirugia De Minimo Acceso, of which he is justifiably proud.
In the short time that we were in Cuba, we obviously could observe only a fraction of their entire system. We were unable to determine how representative the health care workers we met were; those we did meet, however, were committed, hard working, and idealistic. Their rewards are clearly not financial, as they are equally (and poorly) paid, earning the same $70/month, no matter what their “rank” in the system.
Whatever the political realities of life in Cuba may be outside of the medical setting, we connected with our fellow physicians and bonded over our shared passion for patient care. This trip was about meeting them and gaining some understanding of their professional challenges and their efforts to work with what they have. Their system has evolved in the unique cultural, political, and economic circumstances of Cuba, and so of course, such a system could never work here. And yet, it was refreshing and inspiring to see medical professionals dedicated to the ideals of our profession – serving the people by delivering the best care they could for all of their patients. My hat is off to them for accomplishing so much despite their limited resources.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the coeditor of ACS Surgery News.
Although Cuba lies less than 100 miles from the United States, we Americans tend to know far less about the island nation than about almost any other country in our hemisphere. Only since 2014 has the United States begun to allow its citizens to travel directly to Cuba and has opened official diplomatic relations, although direct trade still remains blocked.
Cuba’s health care system has been touted as providing universal access to primary care services, whose goals are promoting health and preventing disease as well as providing free medical education to a veritable army of health care workers. Less well known are the quality and standards of their surgical services.
Although the Cuban government is a centralized, one-party state that follows the Marxist-Leninist ideology, every individual with whom we met answered our many questions with apparent candor. Perhaps our easy rapport was based to some degree on our common profession and our shared commitment to patient care. Although they were clearly proud of the quality of their free education and medical care, they were also quick to admit the shortcomings in their system: widespread poverty, shortages of food and advanced pharmaceuticals, and old medical facilities. We were not restricted in any way from moving around Havana or speaking with anyone, although our free time was admittedly limited because our busy schedule was crammed with at least two visits per day with the groups listed above.
We were interested in looking at how primary care was delivered in Cuba. We met with a primary care doctor in her office, which was situated on the ground floor of the apartment complex in which she and her patients lived. We also visited a polyclinic, two blocks from the primary care doctor’s office that serves as the next step up the chain and is the site where medical and surgical specialists come to consult with patients from 40-60 primary care practices clustered around the polyclinic. The walls of the polyclinic have posters that educate the patients about the importance of handwashing and prevention of hypertension and cancer. The polyclinic also has an epidemiologist who monitors such basic preventive services as immunizations and prenatal care, both of which achieve nearly 100% compliance in a society in which acceptance of these services is not optional. Pap smears are performed in the primary care clinics, as is comprehensive medical care.
As interesting and impressive as we found the primary care clinics, it was the visits with the surgeons in their hospitals that intrigued us the most. The surgeons we met were modest and collegial, yet proud of what they had accomplished under challenging resource constraints. The hospitals that we visited were reminiscent of the city and county hospitals in the United States in which many of us on the trip had trained in the 1970s: older facilities that were clean and serviceable, but with older, basic equipment. Nevertheless, C. Julian F. Ruiz Torres, MD, has developed minimally invasive surgery in a hospital dedicated to such technological advances. Although he is 72 years old, he still works tirelessly to obtain the resources to build a state-of-the-art surgical simulation center, now under construction. Basic minimally invasive surgical procedures are available in most hospitals, although advanced procedures are restricted to centers such as Dr. Ruiz Torres’ facility, Centro Nacional De Cirugia De Minimo Acceso, of which he is justifiably proud.
In the short time that we were in Cuba, we obviously could observe only a fraction of their entire system. We were unable to determine how representative the health care workers we met were; those we did meet, however, were committed, hard working, and idealistic. Their rewards are clearly not financial, as they are equally (and poorly) paid, earning the same $70/month, no matter what their “rank” in the system.
Whatever the political realities of life in Cuba may be outside of the medical setting, we connected with our fellow physicians and bonded over our shared passion for patient care. This trip was about meeting them and gaining some understanding of their professional challenges and their efforts to work with what they have. Their system has evolved in the unique cultural, political, and economic circumstances of Cuba, and so of course, such a system could never work here. And yet, it was refreshing and inspiring to see medical professionals dedicated to the ideals of our profession – serving the people by delivering the best care they could for all of their patients. My hat is off to them for accomplishing so much despite their limited resources.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the coeditor of ACS Surgery News.
Although Cuba lies less than 100 miles from the United States, we Americans tend to know far less about the island nation than about almost any other country in our hemisphere. Only since 2014 has the United States begun to allow its citizens to travel directly to Cuba and has opened official diplomatic relations, although direct trade still remains blocked.
Cuba’s health care system has been touted as providing universal access to primary care services, whose goals are promoting health and preventing disease as well as providing free medical education to a veritable army of health care workers. Less well known are the quality and standards of their surgical services.
Although the Cuban government is a centralized, one-party state that follows the Marxist-Leninist ideology, every individual with whom we met answered our many questions with apparent candor. Perhaps our easy rapport was based to some degree on our common profession and our shared commitment to patient care. Although they were clearly proud of the quality of their free education and medical care, they were also quick to admit the shortcomings in their system: widespread poverty, shortages of food and advanced pharmaceuticals, and old medical facilities. We were not restricted in any way from moving around Havana or speaking with anyone, although our free time was admittedly limited because our busy schedule was crammed with at least two visits per day with the groups listed above.
We were interested in looking at how primary care was delivered in Cuba. We met with a primary care doctor in her office, which was situated on the ground floor of the apartment complex in which she and her patients lived. We also visited a polyclinic, two blocks from the primary care doctor’s office that serves as the next step up the chain and is the site where medical and surgical specialists come to consult with patients from 40-60 primary care practices clustered around the polyclinic. The walls of the polyclinic have posters that educate the patients about the importance of handwashing and prevention of hypertension and cancer. The polyclinic also has an epidemiologist who monitors such basic preventive services as immunizations and prenatal care, both of which achieve nearly 100% compliance in a society in which acceptance of these services is not optional. Pap smears are performed in the primary care clinics, as is comprehensive medical care.
As interesting and impressive as we found the primary care clinics, it was the visits with the surgeons in their hospitals that intrigued us the most. The surgeons we met were modest and collegial, yet proud of what they had accomplished under challenging resource constraints. The hospitals that we visited were reminiscent of the city and county hospitals in the United States in which many of us on the trip had trained in the 1970s: older facilities that were clean and serviceable, but with older, basic equipment. Nevertheless, C. Julian F. Ruiz Torres, MD, has developed minimally invasive surgery in a hospital dedicated to such technological advances. Although he is 72 years old, he still works tirelessly to obtain the resources to build a state-of-the-art surgical simulation center, now under construction. Basic minimally invasive surgical procedures are available in most hospitals, although advanced procedures are restricted to centers such as Dr. Ruiz Torres’ facility, Centro Nacional De Cirugia De Minimo Acceso, of which he is justifiably proud.
In the short time that we were in Cuba, we obviously could observe only a fraction of their entire system. We were unable to determine how representative the health care workers we met were; those we did meet, however, were committed, hard working, and idealistic. Their rewards are clearly not financial, as they are equally (and poorly) paid, earning the same $70/month, no matter what their “rank” in the system.
Whatever the political realities of life in Cuba may be outside of the medical setting, we connected with our fellow physicians and bonded over our shared passion for patient care. This trip was about meeting them and gaining some understanding of their professional challenges and their efforts to work with what they have. Their system has evolved in the unique cultural, political, and economic circumstances of Cuba, and so of course, such a system could never work here. And yet, it was refreshing and inspiring to see medical professionals dedicated to the ideals of our profession – serving the people by delivering the best care they could for all of their patients. My hat is off to them for accomplishing so much despite their limited resources.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the coeditor of ACS Surgery News.
From the Washington Office: Reporting global codes data in 2017
On July 1, 2017, the Centers for Medicare and Medicaid Services (CMS) will begin requiring practitioners in nine states who are part of groups of 10 or more to report data on the services that they provide for select 10- and 90-day global surgical codes. The data collected will be used to improve the accuracy of global codes starting in 2019.
Which states are impacted by the requirement to report global codes data?
What data must be reported?
Health care practitioners who meet claims-based data collection requirements will be required to report American Medical Association Current Procedure Terminology (CPT)* code 99024, Postoperative follow-up visit, normally included in the surgical package, to indicate that an evaluation and management service was performed during a postoperative period for a reason(s) related to the original procedure, for every postoperative visit they provide within the global period of a select list of 10- or 90-day global codes. CMS selected 293 services, which are provided to Medicare patients by more than 100 practitioners per year and are either furnished more than 10,000 times or have allowed charges of more than $10 million annually. The agency estimates that these 293 codes describe approximately 87 percent of all furnished 10- and 90-day global services, and approximately 77 percent of all Medicare expenditures for 10- and 90-day global services under the physician fee schedule.
Is claims-based data reporting mandatory? Is there a penalty for failure to report?
Reporting is mandatory. CMS’ goal is to gather data on postoperative visits as part of its effort to improve the accuracy of global code values starting in 2019. CMS has the authority to implement a 5 percent withhold in payment for global services for health care professionals who fail to report but has not implemented the withhold at this time. Although there is no penalty or withhold of payment for failure to report, ACS urges all surgeons required to report data to comply. Failure to report will result in incomplete data and should data analysis of both inpatient and outpatient postsurgical visits not reflect existing global code definitions, new assumptions may be created to redefine postoperative care.
Why is CMS requiring the reporting of global codes data?
For several years, CMS has communicated its concerns about the accuracy of the values assigned to 10- and 90-day global codes. In 2014, CMS proposed to transition all 10- and 90-day global codes to 0-day, with the requirement that postoperative visits would be reported separately. The ACS argued against this transition because it would have resulted in a reduction in surgeons’ reimbursement for 10- and 90-day global services.
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, DC.
On July 1, 2017, the Centers for Medicare and Medicaid Services (CMS) will begin requiring practitioners in nine states who are part of groups of 10 or more to report data on the services that they provide for select 10- and 90-day global surgical codes. The data collected will be used to improve the accuracy of global codes starting in 2019.
Which states are impacted by the requirement to report global codes data?
What data must be reported?
Health care practitioners who meet claims-based data collection requirements will be required to report American Medical Association Current Procedure Terminology (CPT)* code 99024, Postoperative follow-up visit, normally included in the surgical package, to indicate that an evaluation and management service was performed during a postoperative period for a reason(s) related to the original procedure, for every postoperative visit they provide within the global period of a select list of 10- or 90-day global codes. CMS selected 293 services, which are provided to Medicare patients by more than 100 practitioners per year and are either furnished more than 10,000 times or have allowed charges of more than $10 million annually. The agency estimates that these 293 codes describe approximately 87 percent of all furnished 10- and 90-day global services, and approximately 77 percent of all Medicare expenditures for 10- and 90-day global services under the physician fee schedule.
Is claims-based data reporting mandatory? Is there a penalty for failure to report?
Reporting is mandatory. CMS’ goal is to gather data on postoperative visits as part of its effort to improve the accuracy of global code values starting in 2019. CMS has the authority to implement a 5 percent withhold in payment for global services for health care professionals who fail to report but has not implemented the withhold at this time. Although there is no penalty or withhold of payment for failure to report, ACS urges all surgeons required to report data to comply. Failure to report will result in incomplete data and should data analysis of both inpatient and outpatient postsurgical visits not reflect existing global code definitions, new assumptions may be created to redefine postoperative care.
Why is CMS requiring the reporting of global codes data?
For several years, CMS has communicated its concerns about the accuracy of the values assigned to 10- and 90-day global codes. In 2014, CMS proposed to transition all 10- and 90-day global codes to 0-day, with the requirement that postoperative visits would be reported separately. The ACS argued against this transition because it would have resulted in a reduction in surgeons’ reimbursement for 10- and 90-day global services.
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, DC.
On July 1, 2017, the Centers for Medicare and Medicaid Services (CMS) will begin requiring practitioners in nine states who are part of groups of 10 or more to report data on the services that they provide for select 10- and 90-day global surgical codes. The data collected will be used to improve the accuracy of global codes starting in 2019.
Which states are impacted by the requirement to report global codes data?
What data must be reported?
Health care practitioners who meet claims-based data collection requirements will be required to report American Medical Association Current Procedure Terminology (CPT)* code 99024, Postoperative follow-up visit, normally included in the surgical package, to indicate that an evaluation and management service was performed during a postoperative period for a reason(s) related to the original procedure, for every postoperative visit they provide within the global period of a select list of 10- or 90-day global codes. CMS selected 293 services, which are provided to Medicare patients by more than 100 practitioners per year and are either furnished more than 10,000 times or have allowed charges of more than $10 million annually. The agency estimates that these 293 codes describe approximately 87 percent of all furnished 10- and 90-day global services, and approximately 77 percent of all Medicare expenditures for 10- and 90-day global services under the physician fee schedule.
Is claims-based data reporting mandatory? Is there a penalty for failure to report?
Reporting is mandatory. CMS’ goal is to gather data on postoperative visits as part of its effort to improve the accuracy of global code values starting in 2019. CMS has the authority to implement a 5 percent withhold in payment for global services for health care professionals who fail to report but has not implemented the withhold at this time. Although there is no penalty or withhold of payment for failure to report, ACS urges all surgeons required to report data to comply. Failure to report will result in incomplete data and should data analysis of both inpatient and outpatient postsurgical visits not reflect existing global code definitions, new assumptions may be created to redefine postoperative care.
Why is CMS requiring the reporting of global codes data?
For several years, CMS has communicated its concerns about the accuracy of the values assigned to 10- and 90-day global codes. In 2014, CMS proposed to transition all 10- and 90-day global codes to 0-day, with the requirement that postoperative visits would be reported separately. The ACS argued against this transition because it would have resulted in a reduction in surgeons’ reimbursement for 10- and 90-day global services.
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, DC.
ACS Board of Regents approves new Foundation officers teaser
The American College of Surgeons (ACS) Board of Regents approved new officers of the ACS Foundation Board of Directors at the Regents’ February meeting in Chicago, IL. The new officers, who began their two-year terms in February, are Chair Mary H. McGrath, MD, MPH, FACS, professor of surgery, University of California, San Francisco (UCSF); Vice-Chair Charles E. Lucas, MD, FACS, professor of surgery, Wayne State University, Detroit, MI; and Secretary Ruth L. Bush, MD, JD, MPH, FACS, deputy director, the Center for Innovations in Quality, Effectiveness and Safety, a partnership between the Baylor College of Medicine and the Michael E. DeBakey Veterans Affairs (VA) Medical Center, Houston, TX.
Mary H. McGrath, MD, MPH, FACS
Dr. McGrath is a graduate of St. Louis University School of Medicine, MO, and completed her general surgery residency at the University of Colorado Medical Center, Denver. She then trained in plastic surgery at Yale University School of Medicine, New Haven, CT, and has made outstanding clinical and academic contributions to the field of plastic surgery, especially in the areas of breast and hand surgery, wound healing, introduction of new technology, and workforce issues.
Dr. McGrath’s career as an academic surgeon started at Yale in 1978 with a position as assistant professor of surgery in the school of medicine’s division of plastic and reconstructive surgery. In 1980, she attained the position of assistant professor of surgery, division of plastic and reconstructive surgery, Columbia University College of Physicians and Surgeons, New York, NY. In 1984, she moved to the George Washington University Medical Center, Washington, DC, where she began as chief, division of plastic and reconstructive surgery, and director, residency training program, ultimately ascending to professor of surgery. She has held her present position at UCSF since 2003. She has served in many national positions in plastic surgery and is the present president of the American Association of Plastic Surgeons.
A Fellow of the College since 1983, Dr. McGrath has provided exceptional service to the ACS and has served for 25 years in leadership roles, including First Vice-President (2007–2008); Vice-Chair, Board of Regents (2005–2006); member, Executive Committee, Board of Regents (2002–2006); Regent (1997– 2006); and Chair, Committee on Ethics (2003–2006). She served on the Board of Governors (B/G) Executive Committee and as a Governor-at-Large representing the District of Columbia.
In 2009, the ACS appointed her to serve on the Board of Commissioners of The Joint Commission. She is currently serving her third term in this capacity.
For this remarkable record of service, Dr. McGrath received the College’s highest honor, the Distinguished Service Award, in 2011. She received the ACS Foundation’s Distinguished Philanthropist Award in 2016 for her generous contributions to the College and service to the larger philanthropic community.
Charles E. Lucas, MD, FACS
A native of Detroit, Dr. Lucas has dedicated his surgical career to his hometown. He earned his undergraduate degree from the University of Detroit and his doctor of medicine degree from Wayne State University (WSU). After completing his residency at WSU, he accepted a position on the WSU surgical faculty, specializing in trauma and surgical critical care.
Dr. Lucas conducted his clinical and academic activities at Detroit General Hospital until its closure in 1980. He then moved to the Detroit Medical Center (DMC), practicing at the new Detroit Receiving Hospital (DRH), the Harper University Hospital (HUH), the Hutzel Hospital, and the Karmanos Cancer Hospital. A renowned trauma surgeon, Dr. Lucas was instrumental in securing DRH as the first ACS-verified Level I trauma center in the U.S. He has trained hundreds of medical students and residents, and his research efforts have resulted in the publication of more than 400 peer-reviewed articles, books, and book chapters.
An ACS Fellow since 1970, Dr. Lucas has served in several ACS volunteer roles for more than 35 years, including member, B/G (1984–1990, 1998–2004); President, ACS Michigan Chapter, (1981–1983); and member, Committee on Trauma (1993–2003). He has also been active in a number of surgical organizations, including the American Association for the Surgery of Trauma, American Surgical Association, Central Surgical Association, Midwest Surgical Association, Society of University Surgeons, and Western Surgical Association.
Ruth L. Bush, MD, JD, MPH, FACS
Dr. Bush received her doctor of medicine degree from the University of North Carolina School of Medicine, Chapel Hill, in 1992. She completed her general surgery residency at the University of California, Davis, Medical Center, where she also spent two additional years as a vascular research fellow. She then finished her vascular surgery fellowship at Emory University Hospital, Atlanta, GA. Serving in several academic positions over her career, Dr. Bush was most recently vice-dean and professor of surgery at Texas A&M College of Medicine, Bryan, TX.
Dr. Bush has received several research support funding awards to conduct studies on vascular disease and improved treatment and has published broadly on an array of topics. In addition to service on the ACS Foundation Board, Dr. Bush has been a member of the ACS B/G (2007–2010). She is active in several surgical associations including American Venous Forum, Association of Women Surgeons, and the Society for Vascular Surgery. She has been honored with a number of awards, including Distinguished Fellow (2007) and Presidential Citation (2014), Society of Vascular Surgery; Distinguished Fellow (2015), American Venous Forum; and Outstanding Faculty Teaching Awards, Texas A&M College of Medicine in 2014 and 2015. She continues to practice vascular surgery at the Michael E. DeBakey VA Medical Center.
For more information on the ACS Foundation, contact Shane Hollett, ACS Foundation Executive Director, at 312-202-5506 or [email protected], and visit facs.org/acsfoundation.
Ms. Klein is Director, Donor Relations and Communications, ACS Foundation, Chicago, IL.
The American College of Surgeons (ACS) Board of Regents approved new officers of the ACS Foundation Board of Directors at the Regents’ February meeting in Chicago, IL. The new officers, who began their two-year terms in February, are Chair Mary H. McGrath, MD, MPH, FACS, professor of surgery, University of California, San Francisco (UCSF); Vice-Chair Charles E. Lucas, MD, FACS, professor of surgery, Wayne State University, Detroit, MI; and Secretary Ruth L. Bush, MD, JD, MPH, FACS, deputy director, the Center for Innovations in Quality, Effectiveness and Safety, a partnership between the Baylor College of Medicine and the Michael E. DeBakey Veterans Affairs (VA) Medical Center, Houston, TX.
Mary H. McGrath, MD, MPH, FACS
Dr. McGrath is a graduate of St. Louis University School of Medicine, MO, and completed her general surgery residency at the University of Colorado Medical Center, Denver. She then trained in plastic surgery at Yale University School of Medicine, New Haven, CT, and has made outstanding clinical and academic contributions to the field of plastic surgery, especially in the areas of breast and hand surgery, wound healing, introduction of new technology, and workforce issues.
Dr. McGrath’s career as an academic surgeon started at Yale in 1978 with a position as assistant professor of surgery in the school of medicine’s division of plastic and reconstructive surgery. In 1980, she attained the position of assistant professor of surgery, division of plastic and reconstructive surgery, Columbia University College of Physicians and Surgeons, New York, NY. In 1984, she moved to the George Washington University Medical Center, Washington, DC, where she began as chief, division of plastic and reconstructive surgery, and director, residency training program, ultimately ascending to professor of surgery. She has held her present position at UCSF since 2003. She has served in many national positions in plastic surgery and is the present president of the American Association of Plastic Surgeons.
A Fellow of the College since 1983, Dr. McGrath has provided exceptional service to the ACS and has served for 25 years in leadership roles, including First Vice-President (2007–2008); Vice-Chair, Board of Regents (2005–2006); member, Executive Committee, Board of Regents (2002–2006); Regent (1997– 2006); and Chair, Committee on Ethics (2003–2006). She served on the Board of Governors (B/G) Executive Committee and as a Governor-at-Large representing the District of Columbia.
In 2009, the ACS appointed her to serve on the Board of Commissioners of The Joint Commission. She is currently serving her third term in this capacity.
For this remarkable record of service, Dr. McGrath received the College’s highest honor, the Distinguished Service Award, in 2011. She received the ACS Foundation’s Distinguished Philanthropist Award in 2016 for her generous contributions to the College and service to the larger philanthropic community.
Charles E. Lucas, MD, FACS
A native of Detroit, Dr. Lucas has dedicated his surgical career to his hometown. He earned his undergraduate degree from the University of Detroit and his doctor of medicine degree from Wayne State University (WSU). After completing his residency at WSU, he accepted a position on the WSU surgical faculty, specializing in trauma and surgical critical care.
Dr. Lucas conducted his clinical and academic activities at Detroit General Hospital until its closure in 1980. He then moved to the Detroit Medical Center (DMC), practicing at the new Detroit Receiving Hospital (DRH), the Harper University Hospital (HUH), the Hutzel Hospital, and the Karmanos Cancer Hospital. A renowned trauma surgeon, Dr. Lucas was instrumental in securing DRH as the first ACS-verified Level I trauma center in the U.S. He has trained hundreds of medical students and residents, and his research efforts have resulted in the publication of more than 400 peer-reviewed articles, books, and book chapters.
An ACS Fellow since 1970, Dr. Lucas has served in several ACS volunteer roles for more than 35 years, including member, B/G (1984–1990, 1998–2004); President, ACS Michigan Chapter, (1981–1983); and member, Committee on Trauma (1993–2003). He has also been active in a number of surgical organizations, including the American Association for the Surgery of Trauma, American Surgical Association, Central Surgical Association, Midwest Surgical Association, Society of University Surgeons, and Western Surgical Association.
Ruth L. Bush, MD, JD, MPH, FACS
Dr. Bush received her doctor of medicine degree from the University of North Carolina School of Medicine, Chapel Hill, in 1992. She completed her general surgery residency at the University of California, Davis, Medical Center, where she also spent two additional years as a vascular research fellow. She then finished her vascular surgery fellowship at Emory University Hospital, Atlanta, GA. Serving in several academic positions over her career, Dr. Bush was most recently vice-dean and professor of surgery at Texas A&M College of Medicine, Bryan, TX.
Dr. Bush has received several research support funding awards to conduct studies on vascular disease and improved treatment and has published broadly on an array of topics. In addition to service on the ACS Foundation Board, Dr. Bush has been a member of the ACS B/G (2007–2010). She is active in several surgical associations including American Venous Forum, Association of Women Surgeons, and the Society for Vascular Surgery. She has been honored with a number of awards, including Distinguished Fellow (2007) and Presidential Citation (2014), Society of Vascular Surgery; Distinguished Fellow (2015), American Venous Forum; and Outstanding Faculty Teaching Awards, Texas A&M College of Medicine in 2014 and 2015. She continues to practice vascular surgery at the Michael E. DeBakey VA Medical Center.
For more information on the ACS Foundation, contact Shane Hollett, ACS Foundation Executive Director, at 312-202-5506 or [email protected], and visit facs.org/acsfoundation.
Ms. Klein is Director, Donor Relations and Communications, ACS Foundation, Chicago, IL.
The American College of Surgeons (ACS) Board of Regents approved new officers of the ACS Foundation Board of Directors at the Regents’ February meeting in Chicago, IL. The new officers, who began their two-year terms in February, are Chair Mary H. McGrath, MD, MPH, FACS, professor of surgery, University of California, San Francisco (UCSF); Vice-Chair Charles E. Lucas, MD, FACS, professor of surgery, Wayne State University, Detroit, MI; and Secretary Ruth L. Bush, MD, JD, MPH, FACS, deputy director, the Center for Innovations in Quality, Effectiveness and Safety, a partnership between the Baylor College of Medicine and the Michael E. DeBakey Veterans Affairs (VA) Medical Center, Houston, TX.
Mary H. McGrath, MD, MPH, FACS
Dr. McGrath is a graduate of St. Louis University School of Medicine, MO, and completed her general surgery residency at the University of Colorado Medical Center, Denver. She then trained in plastic surgery at Yale University School of Medicine, New Haven, CT, and has made outstanding clinical and academic contributions to the field of plastic surgery, especially in the areas of breast and hand surgery, wound healing, introduction of new technology, and workforce issues.
Dr. McGrath’s career as an academic surgeon started at Yale in 1978 with a position as assistant professor of surgery in the school of medicine’s division of plastic and reconstructive surgery. In 1980, she attained the position of assistant professor of surgery, division of plastic and reconstructive surgery, Columbia University College of Physicians and Surgeons, New York, NY. In 1984, she moved to the George Washington University Medical Center, Washington, DC, where she began as chief, division of plastic and reconstructive surgery, and director, residency training program, ultimately ascending to professor of surgery. She has held her present position at UCSF since 2003. She has served in many national positions in plastic surgery and is the present president of the American Association of Plastic Surgeons.
A Fellow of the College since 1983, Dr. McGrath has provided exceptional service to the ACS and has served for 25 years in leadership roles, including First Vice-President (2007–2008); Vice-Chair, Board of Regents (2005–2006); member, Executive Committee, Board of Regents (2002–2006); Regent (1997– 2006); and Chair, Committee on Ethics (2003–2006). She served on the Board of Governors (B/G) Executive Committee and as a Governor-at-Large representing the District of Columbia.
In 2009, the ACS appointed her to serve on the Board of Commissioners of The Joint Commission. She is currently serving her third term in this capacity.
For this remarkable record of service, Dr. McGrath received the College’s highest honor, the Distinguished Service Award, in 2011. She received the ACS Foundation’s Distinguished Philanthropist Award in 2016 for her generous contributions to the College and service to the larger philanthropic community.
Charles E. Lucas, MD, FACS
A native of Detroit, Dr. Lucas has dedicated his surgical career to his hometown. He earned his undergraduate degree from the University of Detroit and his doctor of medicine degree from Wayne State University (WSU). After completing his residency at WSU, he accepted a position on the WSU surgical faculty, specializing in trauma and surgical critical care.
Dr. Lucas conducted his clinical and academic activities at Detroit General Hospital until its closure in 1980. He then moved to the Detroit Medical Center (DMC), practicing at the new Detroit Receiving Hospital (DRH), the Harper University Hospital (HUH), the Hutzel Hospital, and the Karmanos Cancer Hospital. A renowned trauma surgeon, Dr. Lucas was instrumental in securing DRH as the first ACS-verified Level I trauma center in the U.S. He has trained hundreds of medical students and residents, and his research efforts have resulted in the publication of more than 400 peer-reviewed articles, books, and book chapters.
An ACS Fellow since 1970, Dr. Lucas has served in several ACS volunteer roles for more than 35 years, including member, B/G (1984–1990, 1998–2004); President, ACS Michigan Chapter, (1981–1983); and member, Committee on Trauma (1993–2003). He has also been active in a number of surgical organizations, including the American Association for the Surgery of Trauma, American Surgical Association, Central Surgical Association, Midwest Surgical Association, Society of University Surgeons, and Western Surgical Association.
Ruth L. Bush, MD, JD, MPH, FACS
Dr. Bush received her doctor of medicine degree from the University of North Carolina School of Medicine, Chapel Hill, in 1992. She completed her general surgery residency at the University of California, Davis, Medical Center, where she also spent two additional years as a vascular research fellow. She then finished her vascular surgery fellowship at Emory University Hospital, Atlanta, GA. Serving in several academic positions over her career, Dr. Bush was most recently vice-dean and professor of surgery at Texas A&M College of Medicine, Bryan, TX.
Dr. Bush has received several research support funding awards to conduct studies on vascular disease and improved treatment and has published broadly on an array of topics. In addition to service on the ACS Foundation Board, Dr. Bush has been a member of the ACS B/G (2007–2010). She is active in several surgical associations including American Venous Forum, Association of Women Surgeons, and the Society for Vascular Surgery. She has been honored with a number of awards, including Distinguished Fellow (2007) and Presidential Citation (2014), Society of Vascular Surgery; Distinguished Fellow (2015), American Venous Forum; and Outstanding Faculty Teaching Awards, Texas A&M College of Medicine in 2014 and 2015. She continues to practice vascular surgery at the Michael E. DeBakey VA Medical Center.
For more information on the ACS Foundation, contact Shane Hollett, ACS Foundation Executive Director, at 312-202-5506 or [email protected], and visit facs.org/acsfoundation.
Ms. Klein is Director, Donor Relations and Communications, ACS Foundation, Chicago, IL.
CQGS releases preliminary standards for quality surgical care of older adults
The Coalition for Quality in Geriatric Surgery (CQGS), a multidisciplinary coalition representing the ACS and 58 other stakeholder organizations, has released the first comprehensive set of hospital-level surgical care standards for older adults.
The Coalition for Quality in Geriatric Surgery (CQGS), a multidisciplinary coalition representing the American College of Surgeons (ACS) and 58 other stakeholder organizations, has released the first comprehensive set of hospital-level surgical care standards for older adults. The standards have been published on the Annals of Surgery website ahead of print publication in a report titled “Hospital Standards to Promote Optimal Surgical Care of the Older Adult.” (See the article at bit.ly/2p1TmNC.)
These preliminary standards reflect the shift toward interdisciplinary care of surgical patients, while taking into account the unique physiologic changes related to aging and chronic diseases that can leave older patients at risk for postoperative complications. The standards build upon existing quality indicators, National Quality Forum-endorsed quality measures, and previous work by the ACS, American Geriatrics Society, and John A. Hartford Foundation, which have previously collaborated to develop and release two sets of perioperative guidelines. Standards, however, differ from guidelines, as Julia Berian, MD, lead author and ACS Clinical Scholar, notes. “Standards, as they exist in ACS Quality Programs, are more than recommendations—they are elevated to the level of care practices that are expected to be completed,” Dr. Berian said.
The standards are divided into four categories—continuum of care, clinical care, program management, and patient outcomes and follow-up. CQGS stakeholders rated 308 proposed standards of care for whether they are validated means of improving quality of geriatric surgery and can feasibly be implemented. The reviewers rated 306 of the standards as valid and 304 as feasible.
The preliminary standards are undergoing a two-phase pilot process. The alpha phase, which involved engaging 15 end-user hospitals to gain their insight on the possibility of implementing these standards, is nearing completion. The beta phase, which involves actual implementation of the standards in six hospitals, will occur later this year.
Read the full ACS press release at facs.org/media/press-releases/2017/olderadults040617.
The Coalition for Quality in Geriatric Surgery (CQGS), a multidisciplinary coalition representing the ACS and 58 other stakeholder organizations, has released the first comprehensive set of hospital-level surgical care standards for older adults.
The Coalition for Quality in Geriatric Surgery (CQGS), a multidisciplinary coalition representing the American College of Surgeons (ACS) and 58 other stakeholder organizations, has released the first comprehensive set of hospital-level surgical care standards for older adults. The standards have been published on the Annals of Surgery website ahead of print publication in a report titled “Hospital Standards to Promote Optimal Surgical Care of the Older Adult.” (See the article at bit.ly/2p1TmNC.)
These preliminary standards reflect the shift toward interdisciplinary care of surgical patients, while taking into account the unique physiologic changes related to aging and chronic diseases that can leave older patients at risk for postoperative complications. The standards build upon existing quality indicators, National Quality Forum-endorsed quality measures, and previous work by the ACS, American Geriatrics Society, and John A. Hartford Foundation, which have previously collaborated to develop and release two sets of perioperative guidelines. Standards, however, differ from guidelines, as Julia Berian, MD, lead author and ACS Clinical Scholar, notes. “Standards, as they exist in ACS Quality Programs, are more than recommendations—they are elevated to the level of care practices that are expected to be completed,” Dr. Berian said.
The standards are divided into four categories—continuum of care, clinical care, program management, and patient outcomes and follow-up. CQGS stakeholders rated 308 proposed standards of care for whether they are validated means of improving quality of geriatric surgery and can feasibly be implemented. The reviewers rated 306 of the standards as valid and 304 as feasible.
The preliminary standards are undergoing a two-phase pilot process. The alpha phase, which involved engaging 15 end-user hospitals to gain their insight on the possibility of implementing these standards, is nearing completion. The beta phase, which involves actual implementation of the standards in six hospitals, will occur later this year.
Read the full ACS press release at facs.org/media/press-releases/2017/olderadults040617.
The Coalition for Quality in Geriatric Surgery (CQGS), a multidisciplinary coalition representing the ACS and 58 other stakeholder organizations, has released the first comprehensive set of hospital-level surgical care standards for older adults.
The Coalition for Quality in Geriatric Surgery (CQGS), a multidisciplinary coalition representing the American College of Surgeons (ACS) and 58 other stakeholder organizations, has released the first comprehensive set of hospital-level surgical care standards for older adults. The standards have been published on the Annals of Surgery website ahead of print publication in a report titled “Hospital Standards to Promote Optimal Surgical Care of the Older Adult.” (See the article at bit.ly/2p1TmNC.)
These preliminary standards reflect the shift toward interdisciplinary care of surgical patients, while taking into account the unique physiologic changes related to aging and chronic diseases that can leave older patients at risk for postoperative complications. The standards build upon existing quality indicators, National Quality Forum-endorsed quality measures, and previous work by the ACS, American Geriatrics Society, and John A. Hartford Foundation, which have previously collaborated to develop and release two sets of perioperative guidelines. Standards, however, differ from guidelines, as Julia Berian, MD, lead author and ACS Clinical Scholar, notes. “Standards, as they exist in ACS Quality Programs, are more than recommendations—they are elevated to the level of care practices that are expected to be completed,” Dr. Berian said.
The standards are divided into four categories—continuum of care, clinical care, program management, and patient outcomes and follow-up. CQGS stakeholders rated 308 proposed standards of care for whether they are validated means of improving quality of geriatric surgery and can feasibly be implemented. The reviewers rated 306 of the standards as valid and 304 as feasible.
The preliminary standards are undergoing a two-phase pilot process. The alpha phase, which involved engaging 15 end-user hospitals to gain their insight on the possibility of implementing these standards, is nearing completion. The beta phase, which involves actual implementation of the standards in six hospitals, will occur later this year.
Read the full ACS press release at facs.org/media/press-releases/2017/olderadults040617.
Consider applying for two ACS scholarships
The American College of Surgeons (ACS) International Relations Committee has announced the availability of the following scholarships for 2018.
Community Surgeon Travel Awards
The International Relations Committee of the ACS has announced the availability of Community Surgeon Travel Awards. This new program provides a stipend of $4,000 to enable international surgeons ages 30–50 in regional or community hospitals/clinics or financially challenged surgical departments to attend the ACS Clinical Congress 2018.
The complete requirements and the online application form are posted on the College website at facs.org/member-services/scholarships/international/communitytravel. Applications and all related documents are due July 3. For more information, contact [email protected].
International Guest Scholarships
The International Relations Committee of the ACS has announced the availability of International Guest Scholarships for 2018 to young surgeons from countries other than the U.S. or Canada. The scholarships, in the amount of $10,000 each, provide the scholars with an opportunity to visit clinical, teaching, and research activities in the U.S. or Canada, and to attend and participate fully in the educational opportunities and activities of the ACS Clinical Congress.
The complete requirements and the link to the direct online application form appear online on the College’s website at facs.org/member-services/scholarships/international/igs. Applications and all related documents are due July 3. For more information, contact [email protected].
The American College of Surgeons (ACS) International Relations Committee has announced the availability of the following scholarships for 2018.
Community Surgeon Travel Awards
The International Relations Committee of the ACS has announced the availability of Community Surgeon Travel Awards. This new program provides a stipend of $4,000 to enable international surgeons ages 30–50 in regional or community hospitals/clinics or financially challenged surgical departments to attend the ACS Clinical Congress 2018.
The complete requirements and the online application form are posted on the College website at facs.org/member-services/scholarships/international/communitytravel. Applications and all related documents are due July 3. For more information, contact [email protected].
International Guest Scholarships
The International Relations Committee of the ACS has announced the availability of International Guest Scholarships for 2018 to young surgeons from countries other than the U.S. or Canada. The scholarships, in the amount of $10,000 each, provide the scholars with an opportunity to visit clinical, teaching, and research activities in the U.S. or Canada, and to attend and participate fully in the educational opportunities and activities of the ACS Clinical Congress.
The complete requirements and the link to the direct online application form appear online on the College’s website at facs.org/member-services/scholarships/international/igs. Applications and all related documents are due July 3. For more information, contact [email protected].
The American College of Surgeons (ACS) International Relations Committee has announced the availability of the following scholarships for 2018.
Community Surgeon Travel Awards
The International Relations Committee of the ACS has announced the availability of Community Surgeon Travel Awards. This new program provides a stipend of $4,000 to enable international surgeons ages 30–50 in regional or community hospitals/clinics or financially challenged surgical departments to attend the ACS Clinical Congress 2018.
The complete requirements and the online application form are posted on the College website at facs.org/member-services/scholarships/international/communitytravel. Applications and all related documents are due July 3. For more information, contact [email protected].
International Guest Scholarships
The International Relations Committee of the ACS has announced the availability of International Guest Scholarships for 2018 to young surgeons from countries other than the U.S. or Canada. The scholarships, in the amount of $10,000 each, provide the scholars with an opportunity to visit clinical, teaching, and research activities in the U.S. or Canada, and to attend and participate fully in the educational opportunities and activities of the ACS Clinical Congress.
The complete requirements and the link to the direct online application form appear online on the College’s website at facs.org/member-services/scholarships/international/igs. Applications and all related documents are due July 3. For more information, contact [email protected].