User login
Air pollution tied to postpartum depression
TOPLINE:
METHODOLOGY:
- Researchers analyzed data on 340,679 women who had singleton live births at Kaiser Permanente Southern California facilities between 2008 and 2016.
- Ambient air pollution exposures were assessed based on maternal residential addresses using monthly averages of particulate matter ≤ 2.5 mcm (PM2.5), PM ≤ 10 mcm (PM10), nitrogen dioxide, and ozone from Environmental Protection Agency monitoring stations.
- Constituents of PM2.5 (sulfate, nitrate, ammonium, organic matter, and black carbon) were obtained from models based on satellite, ground-based monitor, and chemical transport modeling data.
- Women with an Edinburgh Postnatal Depression Scale score of at least 10 during the first 6 months postpartum were referred for further assessment, including diagnosis and treatment.
TAKEAWAY:
- A total of 25,674 women had PPD (7.5%).
- Positive associations were observed between PPD ozone (adjusted odds ratio, 1.09), PM10 (aOR, 1.02), and PM2.5 (aOR, 1.02), with no statistically significant association with nitrogen dioxide.
- Among PM2.5 constituents, black carbon had the strongest association with PPD (OR 1.04).
- Overall, a higher risk of PPD was associated with ozone exposure during the entire pregnancy and postpartum periods and with PM exposure during the late pregnancy and postpartum periods.
IN PRACTICE:
“These findings suggest that long-term antepartum and postpartum air pollution exposure is a potentially modifiable environmental risk factor for PPD and an important public health issue to address for improved maternal mental health,” the authors wrote.
SOURCE:
The study, with first author Yi Sun, PhD, Chinese Academy of Medical Sciences and Peking Medical College, Beijing, was published online in JAMA Network Open.
LIMITATIONS:
Postpartum exposures were estimated using only maternal address at delivery, which may have led to exposure misclassification. Potential exposure misclassifications may also exist since indoor and personal exposure levels could not be estimated. Although several covariates were adjusted for, some residual or unmeasured covariates were inevitable due to data unavailability, such as psychiatric history, adverse life events, and marital status, which may affect mental health.
DISCLOSURES:
This study was supported by a grant from the National Institute of Environmental Health Sciences. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers analyzed data on 340,679 women who had singleton live births at Kaiser Permanente Southern California facilities between 2008 and 2016.
- Ambient air pollution exposures were assessed based on maternal residential addresses using monthly averages of particulate matter ≤ 2.5 mcm (PM2.5), PM ≤ 10 mcm (PM10), nitrogen dioxide, and ozone from Environmental Protection Agency monitoring stations.
- Constituents of PM2.5 (sulfate, nitrate, ammonium, organic matter, and black carbon) were obtained from models based on satellite, ground-based monitor, and chemical transport modeling data.
- Women with an Edinburgh Postnatal Depression Scale score of at least 10 during the first 6 months postpartum were referred for further assessment, including diagnosis and treatment.
TAKEAWAY:
- A total of 25,674 women had PPD (7.5%).
- Positive associations were observed between PPD ozone (adjusted odds ratio, 1.09), PM10 (aOR, 1.02), and PM2.5 (aOR, 1.02), with no statistically significant association with nitrogen dioxide.
- Among PM2.5 constituents, black carbon had the strongest association with PPD (OR 1.04).
- Overall, a higher risk of PPD was associated with ozone exposure during the entire pregnancy and postpartum periods and with PM exposure during the late pregnancy and postpartum periods.
IN PRACTICE:
“These findings suggest that long-term antepartum and postpartum air pollution exposure is a potentially modifiable environmental risk factor for PPD and an important public health issue to address for improved maternal mental health,” the authors wrote.
SOURCE:
The study, with first author Yi Sun, PhD, Chinese Academy of Medical Sciences and Peking Medical College, Beijing, was published online in JAMA Network Open.
LIMITATIONS:
Postpartum exposures were estimated using only maternal address at delivery, which may have led to exposure misclassification. Potential exposure misclassifications may also exist since indoor and personal exposure levels could not be estimated. Although several covariates were adjusted for, some residual or unmeasured covariates were inevitable due to data unavailability, such as psychiatric history, adverse life events, and marital status, which may affect mental health.
DISCLOSURES:
This study was supported by a grant from the National Institute of Environmental Health Sciences. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers analyzed data on 340,679 women who had singleton live births at Kaiser Permanente Southern California facilities between 2008 and 2016.
- Ambient air pollution exposures were assessed based on maternal residential addresses using monthly averages of particulate matter ≤ 2.5 mcm (PM2.5), PM ≤ 10 mcm (PM10), nitrogen dioxide, and ozone from Environmental Protection Agency monitoring stations.
- Constituents of PM2.5 (sulfate, nitrate, ammonium, organic matter, and black carbon) were obtained from models based on satellite, ground-based monitor, and chemical transport modeling data.
- Women with an Edinburgh Postnatal Depression Scale score of at least 10 during the first 6 months postpartum were referred for further assessment, including diagnosis and treatment.
TAKEAWAY:
- A total of 25,674 women had PPD (7.5%).
- Positive associations were observed between PPD ozone (adjusted odds ratio, 1.09), PM10 (aOR, 1.02), and PM2.5 (aOR, 1.02), with no statistically significant association with nitrogen dioxide.
- Among PM2.5 constituents, black carbon had the strongest association with PPD (OR 1.04).
- Overall, a higher risk of PPD was associated with ozone exposure during the entire pregnancy and postpartum periods and with PM exposure during the late pregnancy and postpartum periods.
IN PRACTICE:
“These findings suggest that long-term antepartum and postpartum air pollution exposure is a potentially modifiable environmental risk factor for PPD and an important public health issue to address for improved maternal mental health,” the authors wrote.
SOURCE:
The study, with first author Yi Sun, PhD, Chinese Academy of Medical Sciences and Peking Medical College, Beijing, was published online in JAMA Network Open.
LIMITATIONS:
Postpartum exposures were estimated using only maternal address at delivery, which may have led to exposure misclassification. Potential exposure misclassifications may also exist since indoor and personal exposure levels could not be estimated. Although several covariates were adjusted for, some residual or unmeasured covariates were inevitable due to data unavailability, such as psychiatric history, adverse life events, and marital status, which may affect mental health.
DISCLOSURES:
This study was supported by a grant from the National Institute of Environmental Health Sciences. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
No benefit of colchicine after stroke, TIA: CHANCE-3
The anti-inflammatory agent in the CHANCE-3 trial.
The results were presented by Yongjun Wang, MD, Beijing Tiantan Hospital, Capital Medical University, at the annual World Stroke Congress, sponsored by the World Stroke Organization.
Dr. Wang noted that inflammation may be a key factor involved in the residual risk for recurrent stroke, with data from previous CHANCE trials suggesting a higher stroke recurrence rate in patients with higher levels of high-sensitivity C-reactive protein (hsCRP), a key marker of inflammation.
Low-dose colchicine, which acts as an anti-inflammatory agent, has recently been approved in many countries for patients with established atherosclerotic disease or multiple risk factors for cardiovascular disease to reduce the risk for future cardiovascular events. This follows benefits seen in those populations in the LoDoCo-2 and COLCOT trials.
The CHANCE-3 study was conducted to evaluate whether similar benefits could be found in patients with acute ischemic stroke.
The trial involved 8,369 Chinese patients with minor to moderate ischemic stroke (National Institutes of Health Stroke Scale score ≤ 5) or high-risk TIA (ABCD2 score ≥ 4) who had an hsCRP level of at least 2 mg/L.
Patients were assigned within 24 hours after symptom onset, in a 1:1 ratio, to receive colchicine (1 mg daily on days 1-3, followed by 0.5 mg daily for a total of 90 days) or placebo, on a background of optimal medical therapy.
The primary outcome was any stroke within 90 days. The key secondary outcomes included a composite of stroke, TIA, myocardial infarction, and vascular death within 90 days, and Modified Rankin Scale score greater than 1 at 90 days.
Results showed that the primary outcome of any stroke at 90 days occurred in 6.3% of the colchicine group versus 6.5% of the placebo group, a nonsignificant difference (P = .79).
All secondary outcomes were also neutral, with no differences between the two groups.
Addressing the different results in CHANCE-3, compared with those of the cardiovascular trials of colchicine, Dr. Wang pointed out that the cardiovascular trials had a much longer treatment and follow-up time (an average of 22 months), compared with just 3 months in CHANCE-3.
“Clinical trials with longer treatment times are needed to further assess the effects of colchicine after cerebrovascular events, but it may be that ischemic cerebrovascular disease and ischemic heart disease respond differently to colchicine treatment,” he concluded.
Commenting on the study, cochair of the WSC session at which it was presented, Ashkan Shoamanesh, MD, associate professor of medicine at McMaster University, Hamilton, Ont., said CHANCE-3 was a well-designed large phase 3 randomized trial and the first such trial to test colchicine for secondary stroke prevention.
He agreed with Dr. Wang that the follow-up duration for this initial analysis of 3-month outcomes may have been too short to see an effect.
“So, we require randomized trials with longer follow-up prior to abandoning this potential treatment,” he added.
Dr. Shoamanesh noted that several additional trials are currently ongoing testing colchicine for secondary prevention in patients with stroke. These include the CONVINCE, CASPER, CoVasc-ICH, and RIISC-THETIS trials.
He also pointed out that, in contrast to ischemic heart disease, which results from atherosclerosis, the mechanisms underlying ischemic stroke are more heterogeneous and include various vascular and cardioembolic pathologies.
The CHANCE-3 study was funded by grants from the National Natural Science Foundation of China, the Ministry of Science and Technology of China, the Chinese Academy of Medical Sciences, and the Beijing Municipal Health Commission.
A version of this article first appeared on Medscape.com.
The anti-inflammatory agent in the CHANCE-3 trial.
The results were presented by Yongjun Wang, MD, Beijing Tiantan Hospital, Capital Medical University, at the annual World Stroke Congress, sponsored by the World Stroke Organization.
Dr. Wang noted that inflammation may be a key factor involved in the residual risk for recurrent stroke, with data from previous CHANCE trials suggesting a higher stroke recurrence rate in patients with higher levels of high-sensitivity C-reactive protein (hsCRP), a key marker of inflammation.
Low-dose colchicine, which acts as an anti-inflammatory agent, has recently been approved in many countries for patients with established atherosclerotic disease or multiple risk factors for cardiovascular disease to reduce the risk for future cardiovascular events. This follows benefits seen in those populations in the LoDoCo-2 and COLCOT trials.
The CHANCE-3 study was conducted to evaluate whether similar benefits could be found in patients with acute ischemic stroke.
The trial involved 8,369 Chinese patients with minor to moderate ischemic stroke (National Institutes of Health Stroke Scale score ≤ 5) or high-risk TIA (ABCD2 score ≥ 4) who had an hsCRP level of at least 2 mg/L.
Patients were assigned within 24 hours after symptom onset, in a 1:1 ratio, to receive colchicine (1 mg daily on days 1-3, followed by 0.5 mg daily for a total of 90 days) or placebo, on a background of optimal medical therapy.
The primary outcome was any stroke within 90 days. The key secondary outcomes included a composite of stroke, TIA, myocardial infarction, and vascular death within 90 days, and Modified Rankin Scale score greater than 1 at 90 days.
Results showed that the primary outcome of any stroke at 90 days occurred in 6.3% of the colchicine group versus 6.5% of the placebo group, a nonsignificant difference (P = .79).
All secondary outcomes were also neutral, with no differences between the two groups.
Addressing the different results in CHANCE-3, compared with those of the cardiovascular trials of colchicine, Dr. Wang pointed out that the cardiovascular trials had a much longer treatment and follow-up time (an average of 22 months), compared with just 3 months in CHANCE-3.
“Clinical trials with longer treatment times are needed to further assess the effects of colchicine after cerebrovascular events, but it may be that ischemic cerebrovascular disease and ischemic heart disease respond differently to colchicine treatment,” he concluded.
Commenting on the study, cochair of the WSC session at which it was presented, Ashkan Shoamanesh, MD, associate professor of medicine at McMaster University, Hamilton, Ont., said CHANCE-3 was a well-designed large phase 3 randomized trial and the first such trial to test colchicine for secondary stroke prevention.
He agreed with Dr. Wang that the follow-up duration for this initial analysis of 3-month outcomes may have been too short to see an effect.
“So, we require randomized trials with longer follow-up prior to abandoning this potential treatment,” he added.
Dr. Shoamanesh noted that several additional trials are currently ongoing testing colchicine for secondary prevention in patients with stroke. These include the CONVINCE, CASPER, CoVasc-ICH, and RIISC-THETIS trials.
He also pointed out that, in contrast to ischemic heart disease, which results from atherosclerosis, the mechanisms underlying ischemic stroke are more heterogeneous and include various vascular and cardioembolic pathologies.
The CHANCE-3 study was funded by grants from the National Natural Science Foundation of China, the Ministry of Science and Technology of China, the Chinese Academy of Medical Sciences, and the Beijing Municipal Health Commission.
A version of this article first appeared on Medscape.com.
The anti-inflammatory agent in the CHANCE-3 trial.
The results were presented by Yongjun Wang, MD, Beijing Tiantan Hospital, Capital Medical University, at the annual World Stroke Congress, sponsored by the World Stroke Organization.
Dr. Wang noted that inflammation may be a key factor involved in the residual risk for recurrent stroke, with data from previous CHANCE trials suggesting a higher stroke recurrence rate in patients with higher levels of high-sensitivity C-reactive protein (hsCRP), a key marker of inflammation.
Low-dose colchicine, which acts as an anti-inflammatory agent, has recently been approved in many countries for patients with established atherosclerotic disease or multiple risk factors for cardiovascular disease to reduce the risk for future cardiovascular events. This follows benefits seen in those populations in the LoDoCo-2 and COLCOT trials.
The CHANCE-3 study was conducted to evaluate whether similar benefits could be found in patients with acute ischemic stroke.
The trial involved 8,369 Chinese patients with minor to moderate ischemic stroke (National Institutes of Health Stroke Scale score ≤ 5) or high-risk TIA (ABCD2 score ≥ 4) who had an hsCRP level of at least 2 mg/L.
Patients were assigned within 24 hours after symptom onset, in a 1:1 ratio, to receive colchicine (1 mg daily on days 1-3, followed by 0.5 mg daily for a total of 90 days) or placebo, on a background of optimal medical therapy.
The primary outcome was any stroke within 90 days. The key secondary outcomes included a composite of stroke, TIA, myocardial infarction, and vascular death within 90 days, and Modified Rankin Scale score greater than 1 at 90 days.
Results showed that the primary outcome of any stroke at 90 days occurred in 6.3% of the colchicine group versus 6.5% of the placebo group, a nonsignificant difference (P = .79).
All secondary outcomes were also neutral, with no differences between the two groups.
Addressing the different results in CHANCE-3, compared with those of the cardiovascular trials of colchicine, Dr. Wang pointed out that the cardiovascular trials had a much longer treatment and follow-up time (an average of 22 months), compared with just 3 months in CHANCE-3.
“Clinical trials with longer treatment times are needed to further assess the effects of colchicine after cerebrovascular events, but it may be that ischemic cerebrovascular disease and ischemic heart disease respond differently to colchicine treatment,” he concluded.
Commenting on the study, cochair of the WSC session at which it was presented, Ashkan Shoamanesh, MD, associate professor of medicine at McMaster University, Hamilton, Ont., said CHANCE-3 was a well-designed large phase 3 randomized trial and the first such trial to test colchicine for secondary stroke prevention.
He agreed with Dr. Wang that the follow-up duration for this initial analysis of 3-month outcomes may have been too short to see an effect.
“So, we require randomized trials with longer follow-up prior to abandoning this potential treatment,” he added.
Dr. Shoamanesh noted that several additional trials are currently ongoing testing colchicine for secondary prevention in patients with stroke. These include the CONVINCE, CASPER, CoVasc-ICH, and RIISC-THETIS trials.
He also pointed out that, in contrast to ischemic heart disease, which results from atherosclerosis, the mechanisms underlying ischemic stroke are more heterogeneous and include various vascular and cardioembolic pathologies.
The CHANCE-3 study was funded by grants from the National Natural Science Foundation of China, the Ministry of Science and Technology of China, the Chinese Academy of Medical Sciences, and the Beijing Municipal Health Commission.
A version of this article first appeared on Medscape.com.
FROM WSC 2023
Ready to start engaging on social media? A dermatologist shares tips
CARLSBAD, CALIF. – In the opinion of Swati Kannan, MD, deciding whether or not to establish a presence on social media starts with a gut-check about your intentions.
“Why use it?” Dr. Kannan, a dermatologist and Mohs surgeon at the University of California, San Diego, asked attendees at the annual symposium of the California Society of Dermatology & Dermatologic Surgery. “Isn’t being an MD or DO enough? Not anymore. and differentiates us from other nondermatology providers.”
Her favorite part about using Instagram and other social media platforms, she said, is connecting with other dermatologists and other specialists. “I’ve learned a lot from communicating with other dermatologists on different platforms, not just for social media but for changing how I practice as well.”
Dr. Kannan offered the following tips and considerations for building and maintaining a presence on social media:
Know the demographics of your practice and your target audience. In general, individuals in their 20s have a presence on many platforms, mainly TikTok for entertainment. Those in their 30s and 40s mainly use Facebook, Instagram, and YouTube, and those in their 40s-60s primarily use Facebook and YouTube. “Men tend to use YouTube, Twitter (X), Reddit, and LinkedIn, while women prefer more photo or video content platforms like Instagram, TikTok, and Facebook,” she said. In addition, knowing your target audience will help select which social media platforms to be active on.
Think about your goal. Is it a side hustle? Is it to raise awareness of various dermatologic conditions? Is it to grow your business? “Knowing this goal will help you determine how much time you’re going to commit to it.”
Do you have the time? To be effective, being active on social media can take 10-15 hours a week, especially for beginners, “so it’s like another job,” she said.
Devise a social media strategy. “Ideally, pick one to three social media platforms that you are going to be active on,” Dr. Kannan advised. “I’m active on Instagram and YouTube, and I cross-post on TikTok and Facebook. That means when I’m making content, it’s geared toward the audience on Instagram. If it hits a few people on TikTok, that’s fine, too, but the TikTok audience is not my target.”
Stick to a posting schedule. Ideally, post three to five times per week.
Create a content strategy. This includes a variety of photos, diagrams, videos, “and you want to use relevant hashtags,” she said.
Find your niche and style. This comes with time. If you specialize in a specific dermatologic condition such as psoriasis, hair loss, or vitiligo, emphasize that in your content.
Find your voice. This also comes with time. But be a professional version of yourself.
Have a plan for how to handle complaints or bad comments. “Avoid posting content that would make you a target,” she advised. “When I get a rude comment, I delete it. If the comment is racist or sexist, I will report it.”
Learn how to review the stats on your accounts. This will provide information on which posts or videos are being well received, which can serve as the basis of creating content that’s similar going forward.
Follow certain social media strategists. This can help grow followers and learn how to find trending audio or music to accompany your content. On Instagram, for example, Dr. Kannan follows @creators and @instagramforbusiness. On YouTube, she follows the Think Media channel.
Avoid posting content that would make you a target. Limit photos about partying/alcohol consumption or anything considered unprofessional. “If you can’t say it or do it in front of a patient, then you shouldn’t post it on your professional social media page,” she said.
Protect yourself. Don’t provide individual medical advice. “All of my home pages contain the statement, ‘this page is not for medical advice,’” Dr. Kannan said. “Get photo and video consent from all patients, even if you’re posting a zoomed-in version of their face. Deidentify patients as much as possible, and watermark your before and after photos and videos so that they’re not easily used by others.”
Be consistent and patient as you engage on social media platforms. Being a good digital citizen includes networking with other creators by liking and commenting on their posts, and responding to and liking comments that people make to your posts. “Remember: it’s not just about the number of followers, but also about engagement,” she said.
Dr. Kannan reported having no relevant disclosures.
CARLSBAD, CALIF. – In the opinion of Swati Kannan, MD, deciding whether or not to establish a presence on social media starts with a gut-check about your intentions.
“Why use it?” Dr. Kannan, a dermatologist and Mohs surgeon at the University of California, San Diego, asked attendees at the annual symposium of the California Society of Dermatology & Dermatologic Surgery. “Isn’t being an MD or DO enough? Not anymore. and differentiates us from other nondermatology providers.”
Her favorite part about using Instagram and other social media platforms, she said, is connecting with other dermatologists and other specialists. “I’ve learned a lot from communicating with other dermatologists on different platforms, not just for social media but for changing how I practice as well.”
Dr. Kannan offered the following tips and considerations for building and maintaining a presence on social media:
Know the demographics of your practice and your target audience. In general, individuals in their 20s have a presence on many platforms, mainly TikTok for entertainment. Those in their 30s and 40s mainly use Facebook, Instagram, and YouTube, and those in their 40s-60s primarily use Facebook and YouTube. “Men tend to use YouTube, Twitter (X), Reddit, and LinkedIn, while women prefer more photo or video content platforms like Instagram, TikTok, and Facebook,” she said. In addition, knowing your target audience will help select which social media platforms to be active on.
Think about your goal. Is it a side hustle? Is it to raise awareness of various dermatologic conditions? Is it to grow your business? “Knowing this goal will help you determine how much time you’re going to commit to it.”
Do you have the time? To be effective, being active on social media can take 10-15 hours a week, especially for beginners, “so it’s like another job,” she said.
Devise a social media strategy. “Ideally, pick one to three social media platforms that you are going to be active on,” Dr. Kannan advised. “I’m active on Instagram and YouTube, and I cross-post on TikTok and Facebook. That means when I’m making content, it’s geared toward the audience on Instagram. If it hits a few people on TikTok, that’s fine, too, but the TikTok audience is not my target.”
Stick to a posting schedule. Ideally, post three to five times per week.
Create a content strategy. This includes a variety of photos, diagrams, videos, “and you want to use relevant hashtags,” she said.
Find your niche and style. This comes with time. If you specialize in a specific dermatologic condition such as psoriasis, hair loss, or vitiligo, emphasize that in your content.
Find your voice. This also comes with time. But be a professional version of yourself.
Have a plan for how to handle complaints or bad comments. “Avoid posting content that would make you a target,” she advised. “When I get a rude comment, I delete it. If the comment is racist or sexist, I will report it.”
Learn how to review the stats on your accounts. This will provide information on which posts or videos are being well received, which can serve as the basis of creating content that’s similar going forward.
Follow certain social media strategists. This can help grow followers and learn how to find trending audio or music to accompany your content. On Instagram, for example, Dr. Kannan follows @creators and @instagramforbusiness. On YouTube, she follows the Think Media channel.
Avoid posting content that would make you a target. Limit photos about partying/alcohol consumption or anything considered unprofessional. “If you can’t say it or do it in front of a patient, then you shouldn’t post it on your professional social media page,” she said.
Protect yourself. Don’t provide individual medical advice. “All of my home pages contain the statement, ‘this page is not for medical advice,’” Dr. Kannan said. “Get photo and video consent from all patients, even if you’re posting a zoomed-in version of their face. Deidentify patients as much as possible, and watermark your before and after photos and videos so that they’re not easily used by others.”
Be consistent and patient as you engage on social media platforms. Being a good digital citizen includes networking with other creators by liking and commenting on their posts, and responding to and liking comments that people make to your posts. “Remember: it’s not just about the number of followers, but also about engagement,” she said.
Dr. Kannan reported having no relevant disclosures.
CARLSBAD, CALIF. – In the opinion of Swati Kannan, MD, deciding whether or not to establish a presence on social media starts with a gut-check about your intentions.
“Why use it?” Dr. Kannan, a dermatologist and Mohs surgeon at the University of California, San Diego, asked attendees at the annual symposium of the California Society of Dermatology & Dermatologic Surgery. “Isn’t being an MD or DO enough? Not anymore. and differentiates us from other nondermatology providers.”
Her favorite part about using Instagram and other social media platforms, she said, is connecting with other dermatologists and other specialists. “I’ve learned a lot from communicating with other dermatologists on different platforms, not just for social media but for changing how I practice as well.”
Dr. Kannan offered the following tips and considerations for building and maintaining a presence on social media:
Know the demographics of your practice and your target audience. In general, individuals in their 20s have a presence on many platforms, mainly TikTok for entertainment. Those in their 30s and 40s mainly use Facebook, Instagram, and YouTube, and those in their 40s-60s primarily use Facebook and YouTube. “Men tend to use YouTube, Twitter (X), Reddit, and LinkedIn, while women prefer more photo or video content platforms like Instagram, TikTok, and Facebook,” she said. In addition, knowing your target audience will help select which social media platforms to be active on.
Think about your goal. Is it a side hustle? Is it to raise awareness of various dermatologic conditions? Is it to grow your business? “Knowing this goal will help you determine how much time you’re going to commit to it.”
Do you have the time? To be effective, being active on social media can take 10-15 hours a week, especially for beginners, “so it’s like another job,” she said.
Devise a social media strategy. “Ideally, pick one to three social media platforms that you are going to be active on,” Dr. Kannan advised. “I’m active on Instagram and YouTube, and I cross-post on TikTok and Facebook. That means when I’m making content, it’s geared toward the audience on Instagram. If it hits a few people on TikTok, that’s fine, too, but the TikTok audience is not my target.”
Stick to a posting schedule. Ideally, post three to five times per week.
Create a content strategy. This includes a variety of photos, diagrams, videos, “and you want to use relevant hashtags,” she said.
Find your niche and style. This comes with time. If you specialize in a specific dermatologic condition such as psoriasis, hair loss, or vitiligo, emphasize that in your content.
Find your voice. This also comes with time. But be a professional version of yourself.
Have a plan for how to handle complaints or bad comments. “Avoid posting content that would make you a target,” she advised. “When I get a rude comment, I delete it. If the comment is racist or sexist, I will report it.”
Learn how to review the stats on your accounts. This will provide information on which posts or videos are being well received, which can serve as the basis of creating content that’s similar going forward.
Follow certain social media strategists. This can help grow followers and learn how to find trending audio or music to accompany your content. On Instagram, for example, Dr. Kannan follows @creators and @instagramforbusiness. On YouTube, she follows the Think Media channel.
Avoid posting content that would make you a target. Limit photos about partying/alcohol consumption or anything considered unprofessional. “If you can’t say it or do it in front of a patient, then you shouldn’t post it on your professional social media page,” she said.
Protect yourself. Don’t provide individual medical advice. “All of my home pages contain the statement, ‘this page is not for medical advice,’” Dr. Kannan said. “Get photo and video consent from all patients, even if you’re posting a zoomed-in version of their face. Deidentify patients as much as possible, and watermark your before and after photos and videos so that they’re not easily used by others.”
Be consistent and patient as you engage on social media platforms. Being a good digital citizen includes networking with other creators by liking and commenting on their posts, and responding to and liking comments that people make to your posts. “Remember: it’s not just about the number of followers, but also about engagement,” she said.
Dr. Kannan reported having no relevant disclosures.
AT CALDERM 2023
Breastfeeding and colorectal cancer
I, like every pediatrician I know, believe that breast milk is the best nutrition for human newborns. Its balance of nutritive elements and its role in preventing of a wide range of illnesses are so great that we are still learning the extent of their magnitude. It just makes sense that a mother’s milk is most well suited for her baby.
I am a bit less unambiguous about breastfeeding. By that I mean the process of providing breast milk to an infant directly from its mother’s breast. Before you yank my AAP membership card, let me make it clear that I think every woman should consider breastfeeding her infant. But we must accept that in a few situations, even with help from caring and enlightened health care providers and family members, breastfeeding doesn’t work as well as we would have hoped. Fortunately, there are alternatives.
My reservations about the process are few, and until recently I have had an unwaveringly positive attitude toward the safety of breast milk. The cause of my little bit of uncertainty arrived in a recent study by two researchers at the Dana Farber Institute in Boston, in which the A younger cohort within that larger group had a dramatic 40% increased risk of developing high-risk cancer before reaching age 55.
The population the investigators studied came from the large Nurses’ Health Study II, a well-known repository of longitudinal health data. The researchers reported that they included biometric data and a large collection of lifestyle factors including smoking, alcohol intake, and diet in their calculations. However, breastfeeding continued to register the highest association. Interestingly, the investigators found that women who were breastfed for 9 months or longer had twice the risk of colorectal cancer as those who breastfed for from 4 to 8 months.
The study population was all women and predominantly white. However, in the general population it is the non-Hispanic white population that is experiencing the greatest increase in incidence. Of course, the study could not answer whether this association with breastfeeding also existed in minority populations.
The researchers suspect that what they are seeing is a reflection of the Westernization of the American lifestyle. One of the researchers is interested in the gut biome of infants and plans to further the investigation in that direction. Could some substance from the environment be concentrating in breast milk? Or is something missing in breast milk? She points out that, while formulas are generally fortified with vitamin D, breast milk is not.
As concerning as the results of this study may sound, the authors are very careful to urge mothers to continue to breastfeed and choose it as their first choice for feeding their babies. I have been pleasantly surprised that this study has not gotten widespread media attention because bad news travels fast. I have chosen to share it with you because at some point you may begin getting questions from concerned parents.
While apparently well done, this study is just the beginning. Like any good research, it poses more questions than it answers. For us as pediatricians it means we should continue to recommend breast milk as the first food. But, we must stay alert as further research looks deeper into this association.
We should also take advantage of our special access to young parents, a demographic that less frequently sees a physician for preventive care. For whatever reason colorectal cancer is occurring at younger ages. When we have the opportunity we should be reminding 40-year-olds not to wait until age 50 to screen for colorectal cancer, particularly if they have a family history of the disease.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I, like every pediatrician I know, believe that breast milk is the best nutrition for human newborns. Its balance of nutritive elements and its role in preventing of a wide range of illnesses are so great that we are still learning the extent of their magnitude. It just makes sense that a mother’s milk is most well suited for her baby.
I am a bit less unambiguous about breastfeeding. By that I mean the process of providing breast milk to an infant directly from its mother’s breast. Before you yank my AAP membership card, let me make it clear that I think every woman should consider breastfeeding her infant. But we must accept that in a few situations, even with help from caring and enlightened health care providers and family members, breastfeeding doesn’t work as well as we would have hoped. Fortunately, there are alternatives.
My reservations about the process are few, and until recently I have had an unwaveringly positive attitude toward the safety of breast milk. The cause of my little bit of uncertainty arrived in a recent study by two researchers at the Dana Farber Institute in Boston, in which the A younger cohort within that larger group had a dramatic 40% increased risk of developing high-risk cancer before reaching age 55.
The population the investigators studied came from the large Nurses’ Health Study II, a well-known repository of longitudinal health data. The researchers reported that they included biometric data and a large collection of lifestyle factors including smoking, alcohol intake, and diet in their calculations. However, breastfeeding continued to register the highest association. Interestingly, the investigators found that women who were breastfed for 9 months or longer had twice the risk of colorectal cancer as those who breastfed for from 4 to 8 months.
The study population was all women and predominantly white. However, in the general population it is the non-Hispanic white population that is experiencing the greatest increase in incidence. Of course, the study could not answer whether this association with breastfeeding also existed in minority populations.
The researchers suspect that what they are seeing is a reflection of the Westernization of the American lifestyle. One of the researchers is interested in the gut biome of infants and plans to further the investigation in that direction. Could some substance from the environment be concentrating in breast milk? Or is something missing in breast milk? She points out that, while formulas are generally fortified with vitamin D, breast milk is not.
As concerning as the results of this study may sound, the authors are very careful to urge mothers to continue to breastfeed and choose it as their first choice for feeding their babies. I have been pleasantly surprised that this study has not gotten widespread media attention because bad news travels fast. I have chosen to share it with you because at some point you may begin getting questions from concerned parents.
While apparently well done, this study is just the beginning. Like any good research, it poses more questions than it answers. For us as pediatricians it means we should continue to recommend breast milk as the first food. But, we must stay alert as further research looks deeper into this association.
We should also take advantage of our special access to young parents, a demographic that less frequently sees a physician for preventive care. For whatever reason colorectal cancer is occurring at younger ages. When we have the opportunity we should be reminding 40-year-olds not to wait until age 50 to screen for colorectal cancer, particularly if they have a family history of the disease.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I, like every pediatrician I know, believe that breast milk is the best nutrition for human newborns. Its balance of nutritive elements and its role in preventing of a wide range of illnesses are so great that we are still learning the extent of their magnitude. It just makes sense that a mother’s milk is most well suited for her baby.
I am a bit less unambiguous about breastfeeding. By that I mean the process of providing breast milk to an infant directly from its mother’s breast. Before you yank my AAP membership card, let me make it clear that I think every woman should consider breastfeeding her infant. But we must accept that in a few situations, even with help from caring and enlightened health care providers and family members, breastfeeding doesn’t work as well as we would have hoped. Fortunately, there are alternatives.
My reservations about the process are few, and until recently I have had an unwaveringly positive attitude toward the safety of breast milk. The cause of my little bit of uncertainty arrived in a recent study by two researchers at the Dana Farber Institute in Boston, in which the A younger cohort within that larger group had a dramatic 40% increased risk of developing high-risk cancer before reaching age 55.
The population the investigators studied came from the large Nurses’ Health Study II, a well-known repository of longitudinal health data. The researchers reported that they included biometric data and a large collection of lifestyle factors including smoking, alcohol intake, and diet in their calculations. However, breastfeeding continued to register the highest association. Interestingly, the investigators found that women who were breastfed for 9 months or longer had twice the risk of colorectal cancer as those who breastfed for from 4 to 8 months.
The study population was all women and predominantly white. However, in the general population it is the non-Hispanic white population that is experiencing the greatest increase in incidence. Of course, the study could not answer whether this association with breastfeeding also existed in minority populations.
The researchers suspect that what they are seeing is a reflection of the Westernization of the American lifestyle. One of the researchers is interested in the gut biome of infants and plans to further the investigation in that direction. Could some substance from the environment be concentrating in breast milk? Or is something missing in breast milk? She points out that, while formulas are generally fortified with vitamin D, breast milk is not.
As concerning as the results of this study may sound, the authors are very careful to urge mothers to continue to breastfeed and choose it as their first choice for feeding their babies. I have been pleasantly surprised that this study has not gotten widespread media attention because bad news travels fast. I have chosen to share it with you because at some point you may begin getting questions from concerned parents.
While apparently well done, this study is just the beginning. Like any good research, it poses more questions than it answers. For us as pediatricians it means we should continue to recommend breast milk as the first food. But, we must stay alert as further research looks deeper into this association.
We should also take advantage of our special access to young parents, a demographic that less frequently sees a physician for preventive care. For whatever reason colorectal cancer is occurring at younger ages. When we have the opportunity we should be reminding 40-year-olds not to wait until age 50 to screen for colorectal cancer, particularly if they have a family history of the disease.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Teens have easy online access to Delta-8 cannabinoid products
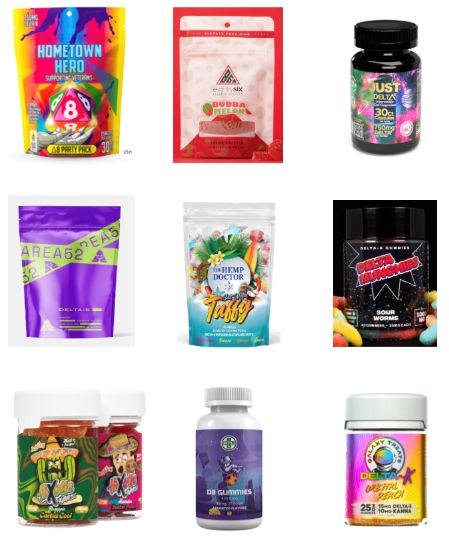
WASHINGTON – , researchers reported at the 2023 annual meeting of the American Academy of Pediatrics. Most of the products identified came in bright, colorful, kid-friendly packaging and cost less than $10, the researchers found, and only 2 out of 45 sites had a third-party age verification requirement for purchases.
Delta-8 THC, also called D8, is a synthetically produced cannabinoid whose chemical structure and effects are nearly identical to traditional THC, the authors explained, and past research has found that D8 products, such as e-cigarettes, can contain toxic byproducts and contaminants.
”Since D8 is not traditional THC, minors may underestimate its strength and potential danger,” wrote lead author Abhijeet Grewal, BS, a research assistant at Cohen Children’s Medical Center, New York, and senior author Ruth Milanaik, DO, director of the Neonatal Neurodevelopmental Program at Cohen Children’s and a developmental/behavioral pediatrician at Northwell Health, also in New York. “Although traditional THC is a federally banned substance, D8 is legal on a federal level and less restricted on a state by state basis, making it easier for individuals to acquire D8.”
Easily accessible
During the first seven moments of 2021, 77% of reports of accidental exposure occurred in people under age 18, including some children who required ICU admission. The U.S. Food and Drug Administration also received 104 reports of adverse events from products containing D8 between December 2020-February 2022, and more than half of those required medical intervention.
To better understand how easy it is to access D8, the authors collected data on 45 websites they identified that sold D8. The researchers looked for age verification questions for accessing the site, third-party age certification, what kinds of products (edibles, smoke products, or tinctures) were sold, the price and dosage of the cheapest product, and examples of packaging, flavors, marketing claims, and warning statements at each site.
More than a third of the sites (36%) did not ask for customers’ age and almost none of the sites asked for proof: 96% of the sites lacked formal third-party age verification procedures. All but one of the sites sold D8 edibles, and most (82%) sold D8 vaping or smoking products. Only 42% sold tinctures, a mix of concentrated D8 with oil that’s orally consumed.
The cheapest product was priced under $5 on one-third of the sites and under $10 on another third of the sites. The cheapest product was between $10-20 on 16% of the sites while the remaining nine sites’ cheapest product was more than $20. In assessing only the cheapest D8 products on each site, nearly half (47%) contained 51 mg or more of D8, and 20% of the products didn’t report the dosage. Another 22% contained 41-50 mg of D8, and the remaining five products contained 20-40 mg.
Kid-friendly D8
More than half of the D8 products were sold in kid-friendly packaging – packages with bright, colorful designs and fonts that resemble candy or snack food, sometimes cartoon characters or fun items like dice on the packaging. Further, 24% of the websites did not include any warnings or other health information about D8.
“The low prices, high dosages available, and eye-popping packaging make these products extremely attractive to teens who are looking for a high,” the researchers concluded. They advised clinicians to talk with teen patients about the dangers of D8 and advocated for policymakers to more strictly regulate online distributors of D8 products, particularly in requiring age verification procedures and prohibiting kid-friendly packaging.
Megan Moreno, MD, MSEd, MPH, an adolescent medicine physician and researcher at the University of Wisconsin, Madison, School of Medicine and Public Health and UWHealthKids, was particularly struck by how eye-catching the packaging was. “The bright colors and font choices are really designed to attract adolescents,” commented Dr. Moreno, who was not involved in the study. But she was not surprised overall by the findings.
“Other studies have found that the cannabis industry leverages online tools and social media, alongside youth-friendly packaging, to attract youth to their products,” she said. “What is disappointing is that these companies do not use industry standard approaches, such as the alcohol industry, to age-gate their websites.”
It’s important for providers who care for adolescents to ask about substance use but to especially include questions about substances that teens might not think of as “drugs,” such as Delta 8, Dr. Moreno said.
“Prior research on other types of substance such as these has found that teens can think these are less dangerous versions of cannabis, so providing accurate information and asking about these products can prevent harm to kids,” Dr. Moreno said. Although this study focused on websites that sell D8 products, she said that “another important area of influence to consider is social media messaging around these products, which may drive traffic to the purchasing site.” It’s clear this industry is not going to self-regulate without policy changes, Dr. Moreno added, so she noted the importance of advocating for policy that regulates these sites.
Mr. Grewal, Dr. Milanaik and Dr. Moreno had no disclosures. No external funding sources were noted.
WASHINGTON – , researchers reported at the 2023 annual meeting of the American Academy of Pediatrics. Most of the products identified came in bright, colorful, kid-friendly packaging and cost less than $10, the researchers found, and only 2 out of 45 sites had a third-party age verification requirement for purchases.

Delta-8 THC, also called D8, is a synthetically produced cannabinoid whose chemical structure and effects are nearly identical to traditional THC, the authors explained, and past research has found that D8 products, such as e-cigarettes, can contain toxic byproducts and contaminants.
”Since D8 is not traditional THC, minors may underestimate its strength and potential danger,” wrote lead author Abhijeet Grewal, BS, a research assistant at Cohen Children’s Medical Center, New York, and senior author Ruth Milanaik, DO, director of the Neonatal Neurodevelopmental Program at Cohen Children’s and a developmental/behavioral pediatrician at Northwell Health, also in New York. “Although traditional THC is a federally banned substance, D8 is legal on a federal level and less restricted on a state by state basis, making it easier for individuals to acquire D8.”
Easily accessible
During the first seven moments of 2021, 77% of reports of accidental exposure occurred in people under age 18, including some children who required ICU admission. The U.S. Food and Drug Administration also received 104 reports of adverse events from products containing D8 between December 2020-February 2022, and more than half of those required medical intervention.
To better understand how easy it is to access D8, the authors collected data on 45 websites they identified that sold D8. The researchers looked for age verification questions for accessing the site, third-party age certification, what kinds of products (edibles, smoke products, or tinctures) were sold, the price and dosage of the cheapest product, and examples of packaging, flavors, marketing claims, and warning statements at each site.
More than a third of the sites (36%) did not ask for customers’ age and almost none of the sites asked for proof: 96% of the sites lacked formal third-party age verification procedures. All but one of the sites sold D8 edibles, and most (82%) sold D8 vaping or smoking products. Only 42% sold tinctures, a mix of concentrated D8 with oil that’s orally consumed.
The cheapest product was priced under $5 on one-third of the sites and under $10 on another third of the sites. The cheapest product was between $10-20 on 16% of the sites while the remaining nine sites’ cheapest product was more than $20. In assessing only the cheapest D8 products on each site, nearly half (47%) contained 51 mg or more of D8, and 20% of the products didn’t report the dosage. Another 22% contained 41-50 mg of D8, and the remaining five products contained 20-40 mg.
Kid-friendly D8
More than half of the D8 products were sold in kid-friendly packaging – packages with bright, colorful designs and fonts that resemble candy or snack food, sometimes cartoon characters or fun items like dice on the packaging. Further, 24% of the websites did not include any warnings or other health information about D8.
“The low prices, high dosages available, and eye-popping packaging make these products extremely attractive to teens who are looking for a high,” the researchers concluded. They advised clinicians to talk with teen patients about the dangers of D8 and advocated for policymakers to more strictly regulate online distributors of D8 products, particularly in requiring age verification procedures and prohibiting kid-friendly packaging.
Megan Moreno, MD, MSEd, MPH, an adolescent medicine physician and researcher at the University of Wisconsin, Madison, School of Medicine and Public Health and UWHealthKids, was particularly struck by how eye-catching the packaging was. “The bright colors and font choices are really designed to attract adolescents,” commented Dr. Moreno, who was not involved in the study. But she was not surprised overall by the findings.
“Other studies have found that the cannabis industry leverages online tools and social media, alongside youth-friendly packaging, to attract youth to their products,” she said. “What is disappointing is that these companies do not use industry standard approaches, such as the alcohol industry, to age-gate their websites.”
It’s important for providers who care for adolescents to ask about substance use but to especially include questions about substances that teens might not think of as “drugs,” such as Delta 8, Dr. Moreno said.
“Prior research on other types of substance such as these has found that teens can think these are less dangerous versions of cannabis, so providing accurate information and asking about these products can prevent harm to kids,” Dr. Moreno said. Although this study focused on websites that sell D8 products, she said that “another important area of influence to consider is social media messaging around these products, which may drive traffic to the purchasing site.” It’s clear this industry is not going to self-regulate without policy changes, Dr. Moreno added, so she noted the importance of advocating for policy that regulates these sites.
Mr. Grewal, Dr. Milanaik and Dr. Moreno had no disclosures. No external funding sources were noted.
WASHINGTON – , researchers reported at the 2023 annual meeting of the American Academy of Pediatrics. Most of the products identified came in bright, colorful, kid-friendly packaging and cost less than $10, the researchers found, and only 2 out of 45 sites had a third-party age verification requirement for purchases.

Delta-8 THC, also called D8, is a synthetically produced cannabinoid whose chemical structure and effects are nearly identical to traditional THC, the authors explained, and past research has found that D8 products, such as e-cigarettes, can contain toxic byproducts and contaminants.
”Since D8 is not traditional THC, minors may underestimate its strength and potential danger,” wrote lead author Abhijeet Grewal, BS, a research assistant at Cohen Children’s Medical Center, New York, and senior author Ruth Milanaik, DO, director of the Neonatal Neurodevelopmental Program at Cohen Children’s and a developmental/behavioral pediatrician at Northwell Health, also in New York. “Although traditional THC is a federally banned substance, D8 is legal on a federal level and less restricted on a state by state basis, making it easier for individuals to acquire D8.”
Easily accessible
During the first seven moments of 2021, 77% of reports of accidental exposure occurred in people under age 18, including some children who required ICU admission. The U.S. Food and Drug Administration also received 104 reports of adverse events from products containing D8 between December 2020-February 2022, and more than half of those required medical intervention.
To better understand how easy it is to access D8, the authors collected data on 45 websites they identified that sold D8. The researchers looked for age verification questions for accessing the site, third-party age certification, what kinds of products (edibles, smoke products, or tinctures) were sold, the price and dosage of the cheapest product, and examples of packaging, flavors, marketing claims, and warning statements at each site.
More than a third of the sites (36%) did not ask for customers’ age and almost none of the sites asked for proof: 96% of the sites lacked formal third-party age verification procedures. All but one of the sites sold D8 edibles, and most (82%) sold D8 vaping or smoking products. Only 42% sold tinctures, a mix of concentrated D8 with oil that’s orally consumed.
The cheapest product was priced under $5 on one-third of the sites and under $10 on another third of the sites. The cheapest product was between $10-20 on 16% of the sites while the remaining nine sites’ cheapest product was more than $20. In assessing only the cheapest D8 products on each site, nearly half (47%) contained 51 mg or more of D8, and 20% of the products didn’t report the dosage. Another 22% contained 41-50 mg of D8, and the remaining five products contained 20-40 mg.
Kid-friendly D8
More than half of the D8 products were sold in kid-friendly packaging – packages with bright, colorful designs and fonts that resemble candy or snack food, sometimes cartoon characters or fun items like dice on the packaging. Further, 24% of the websites did not include any warnings or other health information about D8.
“The low prices, high dosages available, and eye-popping packaging make these products extremely attractive to teens who are looking for a high,” the researchers concluded. They advised clinicians to talk with teen patients about the dangers of D8 and advocated for policymakers to more strictly regulate online distributors of D8 products, particularly in requiring age verification procedures and prohibiting kid-friendly packaging.
Megan Moreno, MD, MSEd, MPH, an adolescent medicine physician and researcher at the University of Wisconsin, Madison, School of Medicine and Public Health and UWHealthKids, was particularly struck by how eye-catching the packaging was. “The bright colors and font choices are really designed to attract adolescents,” commented Dr. Moreno, who was not involved in the study. But she was not surprised overall by the findings.
“Other studies have found that the cannabis industry leverages online tools and social media, alongside youth-friendly packaging, to attract youth to their products,” she said. “What is disappointing is that these companies do not use industry standard approaches, such as the alcohol industry, to age-gate their websites.”
It’s important for providers who care for adolescents to ask about substance use but to especially include questions about substances that teens might not think of as “drugs,” such as Delta 8, Dr. Moreno said.
“Prior research on other types of substance such as these has found that teens can think these are less dangerous versions of cannabis, so providing accurate information and asking about these products can prevent harm to kids,” Dr. Moreno said. Although this study focused on websites that sell D8 products, she said that “another important area of influence to consider is social media messaging around these products, which may drive traffic to the purchasing site.” It’s clear this industry is not going to self-regulate without policy changes, Dr. Moreno added, so she noted the importance of advocating for policy that regulates these sites.
Mr. Grewal, Dr. Milanaik and Dr. Moreno had no disclosures. No external funding sources were noted.
At AAP 2023
Tezepelumab effective in asthma with GERD
Among patients with asthma and comorbid gastroesophageal reflux disease (GERD), the , according to a new post-hoc analysis of the phase 2b PATHWAY and phase 3 NAVIGATOR clinical trials.
GERD occurs in about 60% of asthma patients, and the comorbidity is associated with a greater risk of asthma exacerbations. “As we start doing subgroup analyses, we are looking at different comorbidities and reflux is one that’s very common and very impactful on asthma outcomes in a negative way, so it became an area of interest,” said Njira Lugogo, MD, who presented the study during a poster session at the annual meeting of the American College of Chest Physicians (CHEST). She is a professor of internal medicine and pulmonary critical care at the University of Michigan, Ann Arbor.
The analysis confirmed other findings, with comorbid GERD associated with more exacerbations, use of maintenance steroids, and high-dose inhaled steroids. “They had more disease activity, and the effect [of tezepelumab treatment] was similar whether you had reflux or didn’t have reflux. It did seem like the people without reflux had a slightly higher reduction in exacerbations, so maybe there is a slight difference, but overall it looked like both groups were really improving,” said Dr. Lugogo.
Tezepelumab is a newer biologic, having received Food and Drug Administration approval in 2021. It targets the epithelial cytokine thymic stromal lymphopoietin (TSLP), which contributes allergic inflammatory responses by acting on various innate immune cells, including dendritic cells, mast cells, and CD34+ progenitor cells. It is upregulated in the airways of asthma patients, with higher levels linked to more severe disease. A single-nucleotide polymorphism in the gene that codes TSLP has also been found to be protective against asthma, atopic asthma, and airway hyper-responsiveness.
Dr. Lugogo noted that TSLP could be a factor in how GERD may worsen trigger or worsen asthma. It is produced in the epithelium of the upper airway in response to injury, which could include aspiration into bronchial tubes attributable to GERD, and this could lead to a downstream inflammatory and immune response. “Reducing the production of or at least blocking TSLP from an epithelium that’s being irritated by acid reflux could have potential benefits. On the reverse side, could the continued presence of reflux blunt the expected response [to tezepelumab]? If someone has very severe reflux, maybe you’ve treated their asthma with tezepelumab, and they’re still having symptoms. Could it be a masquerading issue [where] you have untreated reflux contributing to ongoing symptoms, which you’re attributing to not being related to asthma? So it’s looking at it in two different ways,” said Dr. Lugogo.
TSLP is the only biologic available to treat patients with non–type 2 inflammation, which includes about 10% of adult patients, according to Dr. Lugogo. Its mechanism also influences eosinophilic and allergic asthma. When tezepelumab first became available, Dr. Lugogo noticed that physicians tended to switch to it from another biologic rather than starting it up front, but that may be changing. “I feel like more and more people are starting it up front as a therapeutic intervention, so there seems to be more and more people embracing its use in the treatment of severe asthma,” she said.
The analysis included 294 patients with asthma and GERD and 1,040 with asthma alone. Patients in the GERD comorbidity group were older (55.0 versus 48.6 years), had a higher mean body mass index (30.8 versus 27.8), and were more likely to be female (67.3% versus 63.0%).
Maintenance oral corticosteroid use was higher in the GERD group (17.0% versus 6.9%), as was use of high inhaled corticosteroid dose (78.2% versus 67.0%), frequency of nasal polyps in the previous 2 years (21.4% versus 13.8%), and experience of more than two exacerbations in the previous year (42.2% versus 34.6%).
There was a 65% reduction (95% confidence interval, 50%-76%) in annualized asthma exacerbation rate versus placebo with tezepelumab treatment in the GERD group, compared with a 58% reduction in the asthma-only group (95% CI, 48%-66%). The drug led to a 0.10 increase in forced expiratory volume in 1 second versus placebo (95% CI, 0.00-0.19) at week 52 in the GERD group, versus 0.15 (95% CI, 0.10-0.20) in the asthma-only group. Tezepelumab also improved week 52 ACQ-6 scores in the GERD group (–0.39 versus placebo; 95% CI, –0.63 to –0.14) and the asthma-only group (–0.32 versus placebo; 95% CI, –0.45 to –0.19).
The study adds to the evidence supporting tezepelumab as a promising new therapy, according to Muhammad Adrish, MD, who attended the poster session and was asked to comment on the study. “I think that this is a very interesting analysis in the sense that gastric reflux disease is a frequent comorbid condition that we see in patients with asthma, and a lot of these patients can have poor outcomes. When you look at the results from the data, you see that regardless of how sick they were and how much medication utilization these patients have at baseline, they still had a pretty decent response to tezepelumab. That speaks to the efficacy of that drug along a wide spectrum of patients,” said Dr. Adrish, who is an associate professor of pulmonary, critical care, and sleep medicine at Baylor College of Medicine, Houston.
The PATHWAY and NAVIGATOR studies were funded by Amgen. Dr. Lugogo has advised or consulted for AstraZeneca, Amgen, Regeneron, TEVA, Avillion, Sanofi, Novartis, Genentech, GSK, and Janssen. Dr. Adrish has no relevant financial disclosures.
Among patients with asthma and comorbid gastroesophageal reflux disease (GERD), the , according to a new post-hoc analysis of the phase 2b PATHWAY and phase 3 NAVIGATOR clinical trials.
GERD occurs in about 60% of asthma patients, and the comorbidity is associated with a greater risk of asthma exacerbations. “As we start doing subgroup analyses, we are looking at different comorbidities and reflux is one that’s very common and very impactful on asthma outcomes in a negative way, so it became an area of interest,” said Njira Lugogo, MD, who presented the study during a poster session at the annual meeting of the American College of Chest Physicians (CHEST). She is a professor of internal medicine and pulmonary critical care at the University of Michigan, Ann Arbor.
The analysis confirmed other findings, with comorbid GERD associated with more exacerbations, use of maintenance steroids, and high-dose inhaled steroids. “They had more disease activity, and the effect [of tezepelumab treatment] was similar whether you had reflux or didn’t have reflux. It did seem like the people without reflux had a slightly higher reduction in exacerbations, so maybe there is a slight difference, but overall it looked like both groups were really improving,” said Dr. Lugogo.
Tezepelumab is a newer biologic, having received Food and Drug Administration approval in 2021. It targets the epithelial cytokine thymic stromal lymphopoietin (TSLP), which contributes allergic inflammatory responses by acting on various innate immune cells, including dendritic cells, mast cells, and CD34+ progenitor cells. It is upregulated in the airways of asthma patients, with higher levels linked to more severe disease. A single-nucleotide polymorphism in the gene that codes TSLP has also been found to be protective against asthma, atopic asthma, and airway hyper-responsiveness.
Dr. Lugogo noted that TSLP could be a factor in how GERD may worsen trigger or worsen asthma. It is produced in the epithelium of the upper airway in response to injury, which could include aspiration into bronchial tubes attributable to GERD, and this could lead to a downstream inflammatory and immune response. “Reducing the production of or at least blocking TSLP from an epithelium that’s being irritated by acid reflux could have potential benefits. On the reverse side, could the continued presence of reflux blunt the expected response [to tezepelumab]? If someone has very severe reflux, maybe you’ve treated their asthma with tezepelumab, and they’re still having symptoms. Could it be a masquerading issue [where] you have untreated reflux contributing to ongoing symptoms, which you’re attributing to not being related to asthma? So it’s looking at it in two different ways,” said Dr. Lugogo.
TSLP is the only biologic available to treat patients with non–type 2 inflammation, which includes about 10% of adult patients, according to Dr. Lugogo. Its mechanism also influences eosinophilic and allergic asthma. When tezepelumab first became available, Dr. Lugogo noticed that physicians tended to switch to it from another biologic rather than starting it up front, but that may be changing. “I feel like more and more people are starting it up front as a therapeutic intervention, so there seems to be more and more people embracing its use in the treatment of severe asthma,” she said.
The analysis included 294 patients with asthma and GERD and 1,040 with asthma alone. Patients in the GERD comorbidity group were older (55.0 versus 48.6 years), had a higher mean body mass index (30.8 versus 27.8), and were more likely to be female (67.3% versus 63.0%).
Maintenance oral corticosteroid use was higher in the GERD group (17.0% versus 6.9%), as was use of high inhaled corticosteroid dose (78.2% versus 67.0%), frequency of nasal polyps in the previous 2 years (21.4% versus 13.8%), and experience of more than two exacerbations in the previous year (42.2% versus 34.6%).
There was a 65% reduction (95% confidence interval, 50%-76%) in annualized asthma exacerbation rate versus placebo with tezepelumab treatment in the GERD group, compared with a 58% reduction in the asthma-only group (95% CI, 48%-66%). The drug led to a 0.10 increase in forced expiratory volume in 1 second versus placebo (95% CI, 0.00-0.19) at week 52 in the GERD group, versus 0.15 (95% CI, 0.10-0.20) in the asthma-only group. Tezepelumab also improved week 52 ACQ-6 scores in the GERD group (–0.39 versus placebo; 95% CI, –0.63 to –0.14) and the asthma-only group (–0.32 versus placebo; 95% CI, –0.45 to –0.19).
The study adds to the evidence supporting tezepelumab as a promising new therapy, according to Muhammad Adrish, MD, who attended the poster session and was asked to comment on the study. “I think that this is a very interesting analysis in the sense that gastric reflux disease is a frequent comorbid condition that we see in patients with asthma, and a lot of these patients can have poor outcomes. When you look at the results from the data, you see that regardless of how sick they were and how much medication utilization these patients have at baseline, they still had a pretty decent response to tezepelumab. That speaks to the efficacy of that drug along a wide spectrum of patients,” said Dr. Adrish, who is an associate professor of pulmonary, critical care, and sleep medicine at Baylor College of Medicine, Houston.
The PATHWAY and NAVIGATOR studies were funded by Amgen. Dr. Lugogo has advised or consulted for AstraZeneca, Amgen, Regeneron, TEVA, Avillion, Sanofi, Novartis, Genentech, GSK, and Janssen. Dr. Adrish has no relevant financial disclosures.
Among patients with asthma and comorbid gastroesophageal reflux disease (GERD), the , according to a new post-hoc analysis of the phase 2b PATHWAY and phase 3 NAVIGATOR clinical trials.
GERD occurs in about 60% of asthma patients, and the comorbidity is associated with a greater risk of asthma exacerbations. “As we start doing subgroup analyses, we are looking at different comorbidities and reflux is one that’s very common and very impactful on asthma outcomes in a negative way, so it became an area of interest,” said Njira Lugogo, MD, who presented the study during a poster session at the annual meeting of the American College of Chest Physicians (CHEST). She is a professor of internal medicine and pulmonary critical care at the University of Michigan, Ann Arbor.
The analysis confirmed other findings, with comorbid GERD associated with more exacerbations, use of maintenance steroids, and high-dose inhaled steroids. “They had more disease activity, and the effect [of tezepelumab treatment] was similar whether you had reflux or didn’t have reflux. It did seem like the people without reflux had a slightly higher reduction in exacerbations, so maybe there is a slight difference, but overall it looked like both groups were really improving,” said Dr. Lugogo.
Tezepelumab is a newer biologic, having received Food and Drug Administration approval in 2021. It targets the epithelial cytokine thymic stromal lymphopoietin (TSLP), which contributes allergic inflammatory responses by acting on various innate immune cells, including dendritic cells, mast cells, and CD34+ progenitor cells. It is upregulated in the airways of asthma patients, with higher levels linked to more severe disease. A single-nucleotide polymorphism in the gene that codes TSLP has also been found to be protective against asthma, atopic asthma, and airway hyper-responsiveness.
Dr. Lugogo noted that TSLP could be a factor in how GERD may worsen trigger or worsen asthma. It is produced in the epithelium of the upper airway in response to injury, which could include aspiration into bronchial tubes attributable to GERD, and this could lead to a downstream inflammatory and immune response. “Reducing the production of or at least blocking TSLP from an epithelium that’s being irritated by acid reflux could have potential benefits. On the reverse side, could the continued presence of reflux blunt the expected response [to tezepelumab]? If someone has very severe reflux, maybe you’ve treated their asthma with tezepelumab, and they’re still having symptoms. Could it be a masquerading issue [where] you have untreated reflux contributing to ongoing symptoms, which you’re attributing to not being related to asthma? So it’s looking at it in two different ways,” said Dr. Lugogo.
TSLP is the only biologic available to treat patients with non–type 2 inflammation, which includes about 10% of adult patients, according to Dr. Lugogo. Its mechanism also influences eosinophilic and allergic asthma. When tezepelumab first became available, Dr. Lugogo noticed that physicians tended to switch to it from another biologic rather than starting it up front, but that may be changing. “I feel like more and more people are starting it up front as a therapeutic intervention, so there seems to be more and more people embracing its use in the treatment of severe asthma,” she said.
The analysis included 294 patients with asthma and GERD and 1,040 with asthma alone. Patients in the GERD comorbidity group were older (55.0 versus 48.6 years), had a higher mean body mass index (30.8 versus 27.8), and were more likely to be female (67.3% versus 63.0%).
Maintenance oral corticosteroid use was higher in the GERD group (17.0% versus 6.9%), as was use of high inhaled corticosteroid dose (78.2% versus 67.0%), frequency of nasal polyps in the previous 2 years (21.4% versus 13.8%), and experience of more than two exacerbations in the previous year (42.2% versus 34.6%).
There was a 65% reduction (95% confidence interval, 50%-76%) in annualized asthma exacerbation rate versus placebo with tezepelumab treatment in the GERD group, compared with a 58% reduction in the asthma-only group (95% CI, 48%-66%). The drug led to a 0.10 increase in forced expiratory volume in 1 second versus placebo (95% CI, 0.00-0.19) at week 52 in the GERD group, versus 0.15 (95% CI, 0.10-0.20) in the asthma-only group. Tezepelumab also improved week 52 ACQ-6 scores in the GERD group (–0.39 versus placebo; 95% CI, –0.63 to –0.14) and the asthma-only group (–0.32 versus placebo; 95% CI, –0.45 to –0.19).
The study adds to the evidence supporting tezepelumab as a promising new therapy, according to Muhammad Adrish, MD, who attended the poster session and was asked to comment on the study. “I think that this is a very interesting analysis in the sense that gastric reflux disease is a frequent comorbid condition that we see in patients with asthma, and a lot of these patients can have poor outcomes. When you look at the results from the data, you see that regardless of how sick they were and how much medication utilization these patients have at baseline, they still had a pretty decent response to tezepelumab. That speaks to the efficacy of that drug along a wide spectrum of patients,” said Dr. Adrish, who is an associate professor of pulmonary, critical care, and sleep medicine at Baylor College of Medicine, Houston.
The PATHWAY and NAVIGATOR studies were funded by Amgen. Dr. Lugogo has advised or consulted for AstraZeneca, Amgen, Regeneron, TEVA, Avillion, Sanofi, Novartis, Genentech, GSK, and Janssen. Dr. Adrish has no relevant financial disclosures.
FROM CHEST 2023
Keratotic Nodules in a Patient With End-Stage Renal Disease
The Diagnosis: Reactive Perforating Collagenosis
Reactive perforating collagenosis (RPC) is the most common type of primary perforating dermatosis and is characterized by the transepithelial elimination of collagen from the dermis. Although familial RPC usually presents in infancy or early childhood, the acquired form has a strong association with type 2 diabetes mellitus and chronic renal disease. Up to 10% of hemodialysis patients develop RPC.1 Patients with RPC develop red-brown, umbilicated, papulonodular lesions, often with a central keratotic crust and erythematous halo. The lesions are variable in shape and size (typically up to 10 mm in diameter) and commonly are located on the trunk or extensor aspects of the limbs. Pruritus is the primary concern, and the Koebner phenomenon commonly is seen.2
Although the histopathology can vary depending on the stage of the lesion, an invaginating epidermal process with prominent epidermal hyperplasia surrounding a central plug of keratin, basophilic inflammatory debris, and degenerated collagen are findings indicative of RPC. At the base of the invagination, the altered collagen perforates through the epidermis by the process of transepidermal elimination.3 Trichrome stains can highlight the collagen, while Verhoeff–van Gieson staining is negative (no elastic fiber elimination). Anecdotal reports have described a variety of successful therapies including retinoids, allopurinol, doxycycline, dupilumab, and phototherapy, with phototherapy being especially effective in patients with coexistent renal disease.4-8 Our patient was started on dupilumab 300 mg every other week and triamcinolone cream 0.1% twice daily (Monday through Friday) for itchy areas. The efficacy of the treatment was to be assessed at the next visit.
Elastosis perforans serpiginosa (EPS) is a rare skin disease that presents as small papules arranged in serpiginous or annular patterns on the neck, face, arms, or other flexural areas in early adulthood. It more commonly is seen in males and can be associated with other inherited disorders such as Down syndrome, Ehlers-Danlos syndrome, and Marfan syndrome. In rare instances, EPS has been linked to D-penicillamine.9 Elastosis perforans serpiginosa is characterized by focal dermal elastosis and transepithelial elimination of abnormal elastic fibers instead of collagen. The formation of narrow channels extending upward from the dermis in straight or corkscrew patterns commonly is seen (Figure 1). The dermis also may contain a chronic inflammatory infiltrate consisting of lymphocytes, macrophages, or multinucleated giant cells.10 Verhoeff– van Gieson stain highlights the altered elastic fibers in the papillary dermis.
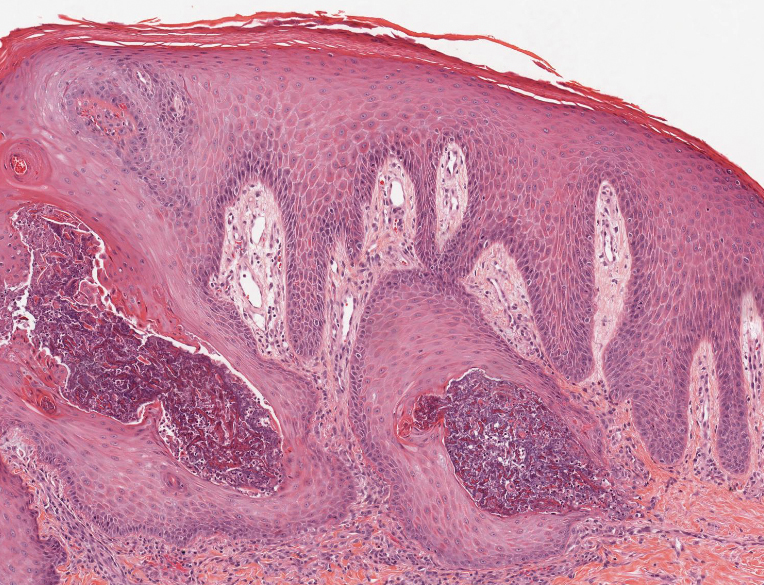
Prurigo nodularis involves chronic, intensely pruritic, lichenified, excoriated nodules that often present as grouped symmetric lesions predominantly on the extensor aspects of the distal extremities and occasionally the trunk. Histologically, prurigo nodularis appears similar to lichen simplex chronicus but in a nodular form with pronounced hyperkeratosis and acanthosis, sometimes to the degree of pseudoepitheliomatous hyperplasia (Figure 2).11 Its features may resemble chronic eczema with mild spongiosis and focal parakeratosis. In the dermis, there is vascular hyperplasia surrounded by perivascular inflammatory infiltrates. Immunohistochemical staining for calcitonin gene-related peptide and substance P may show a large increase of immunoreactive nerves in the lesional skin of nodular prurigo patients compared to the lichenified skin of eczema patients.12 However, neural hyperplasia is not a diagnostic prerequisite in prurigo nodularis.13 Rarely, hyperplasic nerve trunks associated with Schwann cell proliferation may give rise to small neuromata that can be detected on electron microscopy.14 Screening for underlying systemic disease is recommended to rule out cancer, liver disease, chronic kidney disease, thyroid disorders, or HIV.
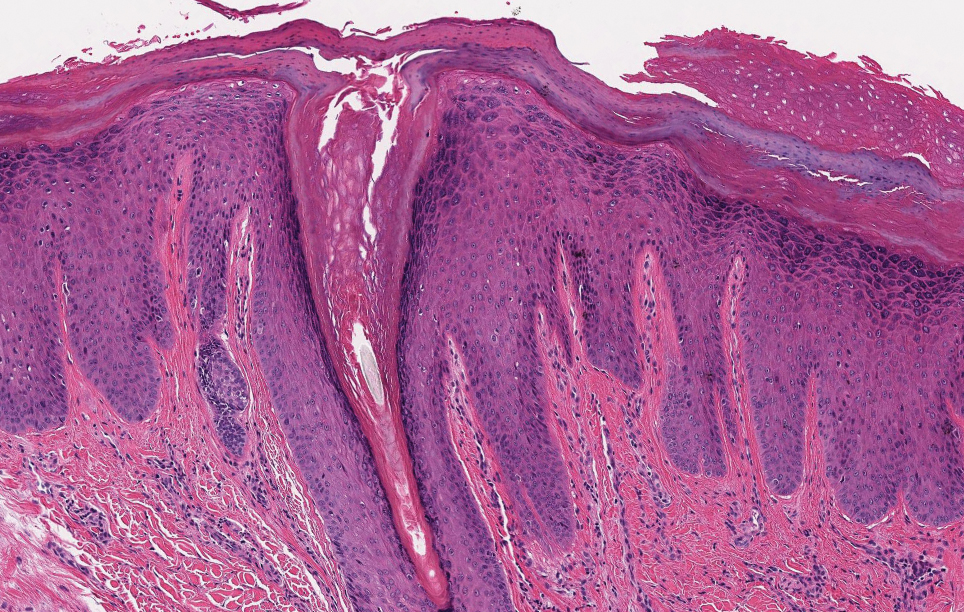
Ecthyma can affect children, adults, and especially immunocompromised patients at sites of trauma that allow entry of Streptococcus pyogenes or Staphylococcus aureus. Histologically, there is ulceration of the epidermis with a thick overlying inflammatory crust (Figure 3). The heavy infiltrate of neutrophils in the reticular dermis forms the base of the ulcer, and gram-positive cocci may be detected within the inflammatory crust. Ecthyma lesions may resemble the excoriations and shallow ulcers that are seen in a variety of other pruritic conditions.15
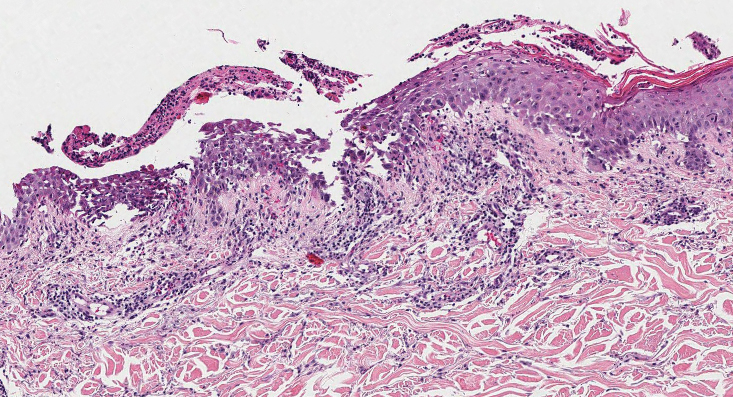
Pityriasis lichenoides et varioliformis acuta is a T-cell–mediated disease that is characterized by crops of lesions in varying sizes and stages including vesicular, hemorrhagic, ulcerated, and necrotic. It often results in varioliform scarring. Histologic findings can include parakeratosis, lichenoid inflammation, extravasation of red blood cells, vasculitis, and apoptotic keratinocytes (Figure 4).16
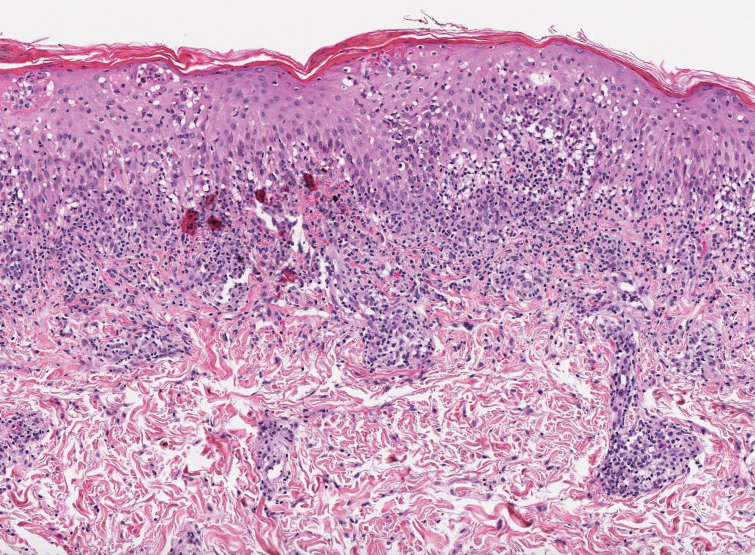
- Hong SB, Park JH, Ihm CG, et al. Acquired perforating dermatosis in patients with chronic renal failure and diabetes mellitus. J Korean Med Sci. 2004;19:283-288. doi:10.3346/jkms.2004.19.2.283
- Mullins TB, Sickinger M, Zito PM. Reactive perforating collagenosis. StatPearls [Internet]. StatPearls Publishing; 2022.
- Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritus! Am J Med. 2019;132:E658-E660. doi:10.1016/j.amjmed.2019.03.015
- Cullen SI. Successful treatment of reactive perforating collagenosis with tretinoin. Cutis. 1979;23:187-193.
- Tilz H, Becker JC, Legat F, et al. Allopurinol in the treatment of acquired reactive perforating collagenosis. An Bras Dermatol. 2013;88:94-97. doi:10.1590/s0365-05962013000100012
- Brinkmeier T, Schaller J, Herbst RA, et al. Successful treatment of acquired reactive perforating collagenosis with doxycycline. Acta Derm Venereol. 2002;82:393-395. doi:10.1080/000155502320624249
- Gil-Lianes J, Riquelme-McLoughlin C, Mascaró JM Jr. Reactive perforating collagenosis successfully treated with dupilumab. Australas J Dermatol. 2022;63:398-400. doi:10.1111/ajd.13874
- Gambichler T, Altmeyer P, Kreuter A. Treatment of acquired perforating dermatosis with narrowband ultraviolet B. J Am Acad Dermatol. 2005;52:363-364. doi:10.1016/j.jaad.2004.08.018
- Na SY, Choi M, Kim MJ, et al. Penicillamine-induced elastosis perforans serpiginosa and cutis laxa in a patient with Wilson’s disease. Ann Dermatol. 2010;22:468-471. doi:10.5021/ad.2010.22.4.468
- Lee SH, Choi Y, Kim SC. Elastosis perforans serpiginosa. Ann Dermatol. 2014;26:103-106. doi:10.5021/ad.2014.26.1.103
- Weigelt N, Metze D, Ständer S. Prurigo nodularis: systematic analysis of 58 histological criteria in 136 patients. J Cutan Pathol. 2010;37:578-586. doi:10.1111/j.1600-0560.2009.01484.x
- Abadía Molina F, Burrows NP, Jones RR, et al. Increased sensory neuropeptides in nodular prurigo: a quantitative immunohistochemical analysis. Br J Dermatol. 1992;127:344-351. doi:10.1111/j.1365-2133.1992.tb00452.x
- Lindley RP, Payne CM. Neural hyperplasia is not a diagnostic prerequisite in nodular prurigo. a controlled morphometric microscopic study of 26 biopsy specimens. J Cutan Pathol. 1989;16:14-18. doi:10.1111/j.1600-0560.1989.tb00003.x
- Feuerman EJ, Sandbank M. Prurigo nodularis. histological and electron microscopical study. Arch Dermatol. 1975;111:1472-1477. doi:10.1001/archderm.111.11.1472
- Weedon D, ed. Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone; 2010. 16. Clarey DD, Lauer SR, Trowbridge RM. Clinical, dermatoscopic, and histological findings in a diagnosis of pityriasis lichenoides [published online June 20, 2020]. Cureus. 2020;12:E8725. doi:10.7759 /cureus.8725
The Diagnosis: Reactive Perforating Collagenosis
Reactive perforating collagenosis (RPC) is the most common type of primary perforating dermatosis and is characterized by the transepithelial elimination of collagen from the dermis. Although familial RPC usually presents in infancy or early childhood, the acquired form has a strong association with type 2 diabetes mellitus and chronic renal disease. Up to 10% of hemodialysis patients develop RPC.1 Patients with RPC develop red-brown, umbilicated, papulonodular lesions, often with a central keratotic crust and erythematous halo. The lesions are variable in shape and size (typically up to 10 mm in diameter) and commonly are located on the trunk or extensor aspects of the limbs. Pruritus is the primary concern, and the Koebner phenomenon commonly is seen.2
Although the histopathology can vary depending on the stage of the lesion, an invaginating epidermal process with prominent epidermal hyperplasia surrounding a central plug of keratin, basophilic inflammatory debris, and degenerated collagen are findings indicative of RPC. At the base of the invagination, the altered collagen perforates through the epidermis by the process of transepidermal elimination.3 Trichrome stains can highlight the collagen, while Verhoeff–van Gieson staining is negative (no elastic fiber elimination). Anecdotal reports have described a variety of successful therapies including retinoids, allopurinol, doxycycline, dupilumab, and phototherapy, with phototherapy being especially effective in patients with coexistent renal disease.4-8 Our patient was started on dupilumab 300 mg every other week and triamcinolone cream 0.1% twice daily (Monday through Friday) for itchy areas. The efficacy of the treatment was to be assessed at the next visit.
Elastosis perforans serpiginosa (EPS) is a rare skin disease that presents as small papules arranged in serpiginous or annular patterns on the neck, face, arms, or other flexural areas in early adulthood. It more commonly is seen in males and can be associated with other inherited disorders such as Down syndrome, Ehlers-Danlos syndrome, and Marfan syndrome. In rare instances, EPS has been linked to D-penicillamine.9 Elastosis perforans serpiginosa is characterized by focal dermal elastosis and transepithelial elimination of abnormal elastic fibers instead of collagen. The formation of narrow channels extending upward from the dermis in straight or corkscrew patterns commonly is seen (Figure 1). The dermis also may contain a chronic inflammatory infiltrate consisting of lymphocytes, macrophages, or multinucleated giant cells.10 Verhoeff– van Gieson stain highlights the altered elastic fibers in the papillary dermis.

Prurigo nodularis involves chronic, intensely pruritic, lichenified, excoriated nodules that often present as grouped symmetric lesions predominantly on the extensor aspects of the distal extremities and occasionally the trunk. Histologically, prurigo nodularis appears similar to lichen simplex chronicus but in a nodular form with pronounced hyperkeratosis and acanthosis, sometimes to the degree of pseudoepitheliomatous hyperplasia (Figure 2).11 Its features may resemble chronic eczema with mild spongiosis and focal parakeratosis. In the dermis, there is vascular hyperplasia surrounded by perivascular inflammatory infiltrates. Immunohistochemical staining for calcitonin gene-related peptide and substance P may show a large increase of immunoreactive nerves in the lesional skin of nodular prurigo patients compared to the lichenified skin of eczema patients.12 However, neural hyperplasia is not a diagnostic prerequisite in prurigo nodularis.13 Rarely, hyperplasic nerve trunks associated with Schwann cell proliferation may give rise to small neuromata that can be detected on electron microscopy.14 Screening for underlying systemic disease is recommended to rule out cancer, liver disease, chronic kidney disease, thyroid disorders, or HIV.

Ecthyma can affect children, adults, and especially immunocompromised patients at sites of trauma that allow entry of Streptococcus pyogenes or Staphylococcus aureus. Histologically, there is ulceration of the epidermis with a thick overlying inflammatory crust (Figure 3). The heavy infiltrate of neutrophils in the reticular dermis forms the base of the ulcer, and gram-positive cocci may be detected within the inflammatory crust. Ecthyma lesions may resemble the excoriations and shallow ulcers that are seen in a variety of other pruritic conditions.15

Pityriasis lichenoides et varioliformis acuta is a T-cell–mediated disease that is characterized by crops of lesions in varying sizes and stages including vesicular, hemorrhagic, ulcerated, and necrotic. It often results in varioliform scarring. Histologic findings can include parakeratosis, lichenoid inflammation, extravasation of red blood cells, vasculitis, and apoptotic keratinocytes (Figure 4).16

The Diagnosis: Reactive Perforating Collagenosis
Reactive perforating collagenosis (RPC) is the most common type of primary perforating dermatosis and is characterized by the transepithelial elimination of collagen from the dermis. Although familial RPC usually presents in infancy or early childhood, the acquired form has a strong association with type 2 diabetes mellitus and chronic renal disease. Up to 10% of hemodialysis patients develop RPC.1 Patients with RPC develop red-brown, umbilicated, papulonodular lesions, often with a central keratotic crust and erythematous halo. The lesions are variable in shape and size (typically up to 10 mm in diameter) and commonly are located on the trunk or extensor aspects of the limbs. Pruritus is the primary concern, and the Koebner phenomenon commonly is seen.2
Although the histopathology can vary depending on the stage of the lesion, an invaginating epidermal process with prominent epidermal hyperplasia surrounding a central plug of keratin, basophilic inflammatory debris, and degenerated collagen are findings indicative of RPC. At the base of the invagination, the altered collagen perforates through the epidermis by the process of transepidermal elimination.3 Trichrome stains can highlight the collagen, while Verhoeff–van Gieson staining is negative (no elastic fiber elimination). Anecdotal reports have described a variety of successful therapies including retinoids, allopurinol, doxycycline, dupilumab, and phototherapy, with phototherapy being especially effective in patients with coexistent renal disease.4-8 Our patient was started on dupilumab 300 mg every other week and triamcinolone cream 0.1% twice daily (Monday through Friday) for itchy areas. The efficacy of the treatment was to be assessed at the next visit.
Elastosis perforans serpiginosa (EPS) is a rare skin disease that presents as small papules arranged in serpiginous or annular patterns on the neck, face, arms, or other flexural areas in early adulthood. It more commonly is seen in males and can be associated with other inherited disorders such as Down syndrome, Ehlers-Danlos syndrome, and Marfan syndrome. In rare instances, EPS has been linked to D-penicillamine.9 Elastosis perforans serpiginosa is characterized by focal dermal elastosis and transepithelial elimination of abnormal elastic fibers instead of collagen. The formation of narrow channels extending upward from the dermis in straight or corkscrew patterns commonly is seen (Figure 1). The dermis also may contain a chronic inflammatory infiltrate consisting of lymphocytes, macrophages, or multinucleated giant cells.10 Verhoeff– van Gieson stain highlights the altered elastic fibers in the papillary dermis.

Prurigo nodularis involves chronic, intensely pruritic, lichenified, excoriated nodules that often present as grouped symmetric lesions predominantly on the extensor aspects of the distal extremities and occasionally the trunk. Histologically, prurigo nodularis appears similar to lichen simplex chronicus but in a nodular form with pronounced hyperkeratosis and acanthosis, sometimes to the degree of pseudoepitheliomatous hyperplasia (Figure 2).11 Its features may resemble chronic eczema with mild spongiosis and focal parakeratosis. In the dermis, there is vascular hyperplasia surrounded by perivascular inflammatory infiltrates. Immunohistochemical staining for calcitonin gene-related peptide and substance P may show a large increase of immunoreactive nerves in the lesional skin of nodular prurigo patients compared to the lichenified skin of eczema patients.12 However, neural hyperplasia is not a diagnostic prerequisite in prurigo nodularis.13 Rarely, hyperplasic nerve trunks associated with Schwann cell proliferation may give rise to small neuromata that can be detected on electron microscopy.14 Screening for underlying systemic disease is recommended to rule out cancer, liver disease, chronic kidney disease, thyroid disorders, or HIV.

Ecthyma can affect children, adults, and especially immunocompromised patients at sites of trauma that allow entry of Streptococcus pyogenes or Staphylococcus aureus. Histologically, there is ulceration of the epidermis with a thick overlying inflammatory crust (Figure 3). The heavy infiltrate of neutrophils in the reticular dermis forms the base of the ulcer, and gram-positive cocci may be detected within the inflammatory crust. Ecthyma lesions may resemble the excoriations and shallow ulcers that are seen in a variety of other pruritic conditions.15

Pityriasis lichenoides et varioliformis acuta is a T-cell–mediated disease that is characterized by crops of lesions in varying sizes and stages including vesicular, hemorrhagic, ulcerated, and necrotic. It often results in varioliform scarring. Histologic findings can include parakeratosis, lichenoid inflammation, extravasation of red blood cells, vasculitis, and apoptotic keratinocytes (Figure 4).16

- Hong SB, Park JH, Ihm CG, et al. Acquired perforating dermatosis in patients with chronic renal failure and diabetes mellitus. J Korean Med Sci. 2004;19:283-288. doi:10.3346/jkms.2004.19.2.283
- Mullins TB, Sickinger M, Zito PM. Reactive perforating collagenosis. StatPearls [Internet]. StatPearls Publishing; 2022.
- Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritus! Am J Med. 2019;132:E658-E660. doi:10.1016/j.amjmed.2019.03.015
- Cullen SI. Successful treatment of reactive perforating collagenosis with tretinoin. Cutis. 1979;23:187-193.
- Tilz H, Becker JC, Legat F, et al. Allopurinol in the treatment of acquired reactive perforating collagenosis. An Bras Dermatol. 2013;88:94-97. doi:10.1590/s0365-05962013000100012
- Brinkmeier T, Schaller J, Herbst RA, et al. Successful treatment of acquired reactive perforating collagenosis with doxycycline. Acta Derm Venereol. 2002;82:393-395. doi:10.1080/000155502320624249
- Gil-Lianes J, Riquelme-McLoughlin C, Mascaró JM Jr. Reactive perforating collagenosis successfully treated with dupilumab. Australas J Dermatol. 2022;63:398-400. doi:10.1111/ajd.13874
- Gambichler T, Altmeyer P, Kreuter A. Treatment of acquired perforating dermatosis with narrowband ultraviolet B. J Am Acad Dermatol. 2005;52:363-364. doi:10.1016/j.jaad.2004.08.018
- Na SY, Choi M, Kim MJ, et al. Penicillamine-induced elastosis perforans serpiginosa and cutis laxa in a patient with Wilson’s disease. Ann Dermatol. 2010;22:468-471. doi:10.5021/ad.2010.22.4.468
- Lee SH, Choi Y, Kim SC. Elastosis perforans serpiginosa. Ann Dermatol. 2014;26:103-106. doi:10.5021/ad.2014.26.1.103
- Weigelt N, Metze D, Ständer S. Prurigo nodularis: systematic analysis of 58 histological criteria in 136 patients. J Cutan Pathol. 2010;37:578-586. doi:10.1111/j.1600-0560.2009.01484.x
- Abadía Molina F, Burrows NP, Jones RR, et al. Increased sensory neuropeptides in nodular prurigo: a quantitative immunohistochemical analysis. Br J Dermatol. 1992;127:344-351. doi:10.1111/j.1365-2133.1992.tb00452.x
- Lindley RP, Payne CM. Neural hyperplasia is not a diagnostic prerequisite in nodular prurigo. a controlled morphometric microscopic study of 26 biopsy specimens. J Cutan Pathol. 1989;16:14-18. doi:10.1111/j.1600-0560.1989.tb00003.x
- Feuerman EJ, Sandbank M. Prurigo nodularis. histological and electron microscopical study. Arch Dermatol. 1975;111:1472-1477. doi:10.1001/archderm.111.11.1472
- Weedon D, ed. Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone; 2010. 16. Clarey DD, Lauer SR, Trowbridge RM. Clinical, dermatoscopic, and histological findings in a diagnosis of pityriasis lichenoides [published online June 20, 2020]. Cureus. 2020;12:E8725. doi:10.7759 /cureus.8725
- Hong SB, Park JH, Ihm CG, et al. Acquired perforating dermatosis in patients with chronic renal failure and diabetes mellitus. J Korean Med Sci. 2004;19:283-288. doi:10.3346/jkms.2004.19.2.283
- Mullins TB, Sickinger M, Zito PM. Reactive perforating collagenosis. StatPearls [Internet]. StatPearls Publishing; 2022.
- Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritus! Am J Med. 2019;132:E658-E660. doi:10.1016/j.amjmed.2019.03.015
- Cullen SI. Successful treatment of reactive perforating collagenosis with tretinoin. Cutis. 1979;23:187-193.
- Tilz H, Becker JC, Legat F, et al. Allopurinol in the treatment of acquired reactive perforating collagenosis. An Bras Dermatol. 2013;88:94-97. doi:10.1590/s0365-05962013000100012
- Brinkmeier T, Schaller J, Herbst RA, et al. Successful treatment of acquired reactive perforating collagenosis with doxycycline. Acta Derm Venereol. 2002;82:393-395. doi:10.1080/000155502320624249
- Gil-Lianes J, Riquelme-McLoughlin C, Mascaró JM Jr. Reactive perforating collagenosis successfully treated with dupilumab. Australas J Dermatol. 2022;63:398-400. doi:10.1111/ajd.13874
- Gambichler T, Altmeyer P, Kreuter A. Treatment of acquired perforating dermatosis with narrowband ultraviolet B. J Am Acad Dermatol. 2005;52:363-364. doi:10.1016/j.jaad.2004.08.018
- Na SY, Choi M, Kim MJ, et al. Penicillamine-induced elastosis perforans serpiginosa and cutis laxa in a patient with Wilson’s disease. Ann Dermatol. 2010;22:468-471. doi:10.5021/ad.2010.22.4.468
- Lee SH, Choi Y, Kim SC. Elastosis perforans serpiginosa. Ann Dermatol. 2014;26:103-106. doi:10.5021/ad.2014.26.1.103
- Weigelt N, Metze D, Ständer S. Prurigo nodularis: systematic analysis of 58 histological criteria in 136 patients. J Cutan Pathol. 2010;37:578-586. doi:10.1111/j.1600-0560.2009.01484.x
- Abadía Molina F, Burrows NP, Jones RR, et al. Increased sensory neuropeptides in nodular prurigo: a quantitative immunohistochemical analysis. Br J Dermatol. 1992;127:344-351. doi:10.1111/j.1365-2133.1992.tb00452.x
- Lindley RP, Payne CM. Neural hyperplasia is not a diagnostic prerequisite in nodular prurigo. a controlled morphometric microscopic study of 26 biopsy specimens. J Cutan Pathol. 1989;16:14-18. doi:10.1111/j.1600-0560.1989.tb00003.x
- Feuerman EJ, Sandbank M. Prurigo nodularis. histological and electron microscopical study. Arch Dermatol. 1975;111:1472-1477. doi:10.1001/archderm.111.11.1472
- Weedon D, ed. Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone; 2010. 16. Clarey DD, Lauer SR, Trowbridge RM. Clinical, dermatoscopic, and histological findings in a diagnosis of pityriasis lichenoides [published online June 20, 2020]. Cureus. 2020;12:E8725. doi:10.7759 /cureus.8725
A 42-year-old man with end-stage renal disease on hemodialysis presented with generalized body itching and nodules on the scalp and back of 1 year’s duration. Physical examination revealed diffuse, hyperpigmented, pruritic, keratotic nodules and macules on the scalp and back (top). A punch biopsy was performed (bottom).
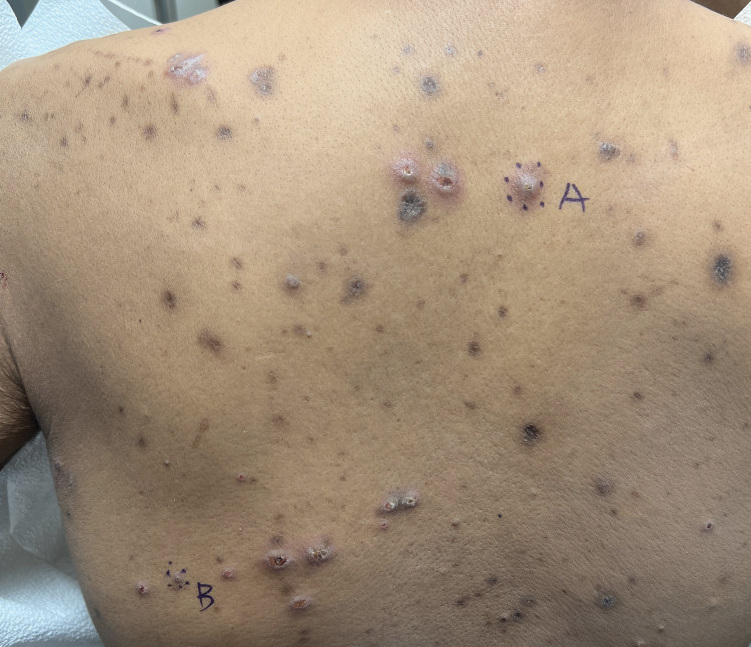
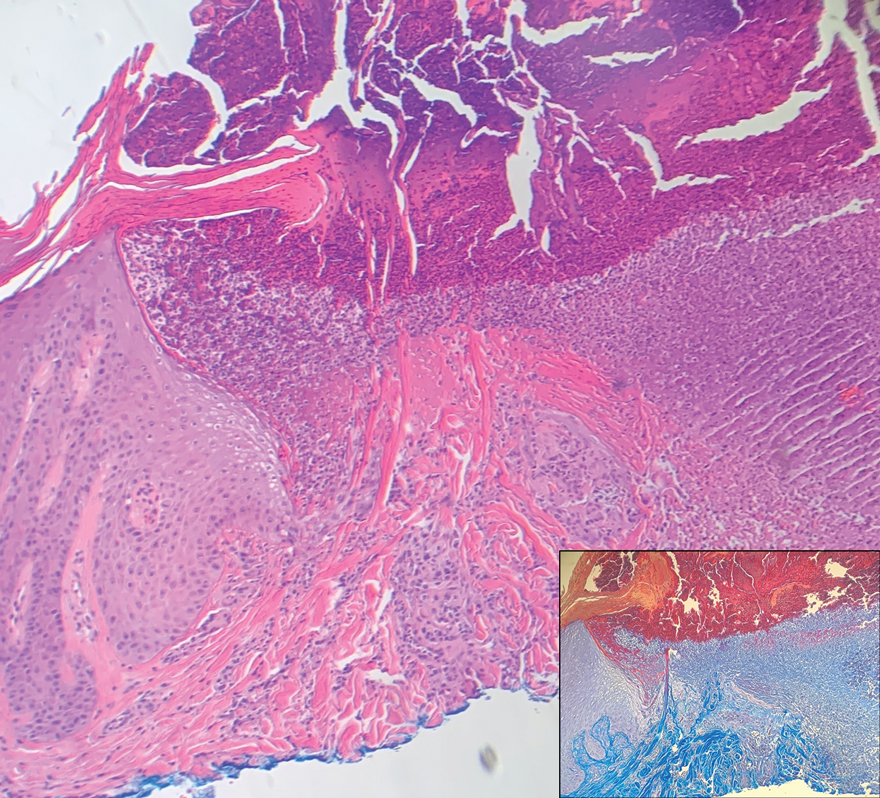
Is it time to scrap ultraprocessed foods?
Ultraprocessed foods (UPFs) make up nearly three-quarters of the entire U.S. food supply and about 60% of Americans’ daily caloric intake. A significant body of research has tied consumption of these foods – awash in added sugar, salt, fat, artificial colors, or preservatives – to cancer, diabetes, and heart disease.
Now, a growing number of studies also link them to poor brain health, including an increased risk of dementia, depression, and anxiety, and some experts are calling for public health policies aimed at reducing UPF consumption.
Under srutiny
A mainstay of diets in countries around the world, UPFs have come under increasing scrutiny because of their link to major diseases. The ingredients in UPFs add little or no nutritional value. Their primary function is to increase a product’s shelf life and palatability. Some recent evidence suggests these foods may be as addictive as tobacco. In addition, two pooled analysis studies using the Yale Food Addiction Scale showed that 14% of adults and 12% of children in the United States may have a UPF addiction.
The most widely used measure of what is, and what is not, a UPF was developed in 2009 by researchers in Brazil. The NOVA food classification system assigns food and beverages to one of four groups:
- Unprocessed and minimally processed foods, such as fruits, vegetables, milk, and meat.
- Processed culinary ingredients, including white sugar, butter, and oils derived from seeds, nuts, and fruits.
- Processed foods, such as tomato paste, bacon, canned tuna, and wine.
- Ultraprocessed foods, such as soda, ice cream, breakfast cereal, and prepackaged meals.
Those sounding the alarm about the potential harmful effects of UPFs are particularly concerned about their consumption by young people. The National Health and Nutrition Examination Survey showed that from 1999 to 2018, highly processed foods accounted for the majority of energy intake in those aged 2-19 years.
One of the most commonly used additives in UPFs, the artificial sweetener aspartame, garnered headlines this summer when the World Health Organization classified it as a likely carcinogen in humans. Aspartame is used in thousands of products, from soda to chewing gum to chewable vitamins.
The U.S. Food and Drug Administration strongly disagreed with the WHO’s position and is sticking by its recommended daily limit of 50 mg/kg of body weight – equivalent to 75 packets of the sweetener Equal – as safe for human consumption.
“Aspartame is one of the most studied food additives in the human food supply,” FDA officials said in a statement, adding that the agency found “significant shortcomings” in the studies the WHO used to justify the new classification. “FDA scientists do not have safety concerns when aspartame is used under the approved conditions.”
Increased attention to consumption of UPFs in general and aspartame particularly in recent years has yielded several studies pointing to the foods’ association with compromised brain health.
Link to depression, dementia
A recent report on UPF consumption and mental well-being among nearly 300,000 people across 70 countries showed that 53% of those who consumed UPFs several times a day were distressed or were struggling with their mental well-being, compared with 18% of those who rarely or never consumed UPFs.
Part of the Global Mind Project run by the nonprofit Sapien Labs in Arlington, Va., the report also showed that individuals with the highest rates of UPF consumption reported higher levels of confusion, slowed thinking, unwanted or obsessive thoughts, irritability, and feelings of sadness.
“There seems to be a much broader effect than just depression symptoms,” Tara Thiagarajan, PhD, founder and chief scientist of Sapien Labs and coauthor of the report, said in an interview.
The report, which has not been peer reviewed, comes on the heels of several other studies, including one from the Nurses Health Study II that showed that participants who consumed more than eight servings of UPFs daily had about a 50% higher depression risk, compared with those who consumed half that much.
“We found that UPFs in general, and artificial sweeteners and beverages in particular, were associated with increased risk,” said lead investigator Andrew T. Chan, MD, MPH, professor of medicine at Harvard Medical School and chief of the clinical and translational epidemiology unit, Massachusetts General Hospital, both in Boston.
“This was an interesting finding that correlates with data from animal studies that artificial sweeteners may trigger the transmission of particular signaling molecules in the brain that are important for mood,” he told this news organization.
Cognition may also be affected. An analysis of more than 72,000 people in the UK Biobank showed that those who consumed a high levels of UPFs were 50% more likely to develop dementia than those who consumed fewer processed foods. For every 10% increase in UPF consumption, the odds of developing any kind of dementia increased by 25%.
Another study of nearly 11,000 people showed that higher UPF consumption was associated with a significantly faster decline in executive and global cognitive function.
Epigenetic changes
While these and other studies suggest a link between UPF consumption and brain health, they are designed to demonstrate correlation. To date, no human study has proven that eating highly processed foods directly causes a decline in mental health or cognition.
Animal studies could provide that causal link. Earlier this year, researchers at Florida State University in Tallahassee reported learning and memory deficits in two groups of male mice that completed a maze test after being fed water mixed with aspartame for about 20% of their adult lives, compared with a group of mice that drank water only. Animals that ingested aspartame could finish the test, but it took them longer, and they needed help.
The amount of aspartame used in the study was just 7% and 15% of the FDA’s recommended maximum intake of aspartame (equivalent to two to four 8-ounce diet sodas daily).
Most intriguing was that offspring of the mice in the aspartame groups demonstrated the same levels of cognitive decline and anxiety as their fathers, even though they had never ingested the artificial sweetener. Researchers theorize that in addition to changes in brain gene expression, aspartame also caused epigenetic changes in germ cells.
“Epigenetic changes in germ cells due to environmental exposures are both good and bad,” lead investigator Pradeep G. Bhide, PhD, professor of developmental neuroscience and director of the Center for Brain Repair at FSU, told this news organization. “They are bad because the next generation is affected. But they’re good because as long as the exposure no longer occurs, 2 or 3 generations later, that’s gone.”
The mice, which lacked taste receptors for aspartame, were the same age and weight in all three groups. Because the only difference was exposure to the artificial sweetener, Dr. Bhide says it suggests a causal link.
“Extrapolation of data from well-controlled laboratory experiments in mice to humans is always risky,” Dr. Bhide said. “The extrapolations give us insights into what could happen rather than what will happen.”
Potential mechanisms
Although scientists can’t say for certain how UPFs affect brain health, there are several theories. UPFs may influence an inflammatory immune response, which has been linked to depression and dementia. Consumption of highly processed foods may also disrupt the gut microbiome, Dr. Chan said, which, in turn, may increase depression risk.
“This is an important potential mechanism linking ultraprocessed food to depression since there is emerging evidence that microbes in the gut have been linked with mood through their role in metabolizing and producing proteins that have activity in the brain,” he said.
In addition, with UPFs that contain aspartame, there could be a more direct link to brain function. In the gastrointestinal track, the sweetener is quickly broken down into methanol, aspartic acid, and phenylalanine. All three enter the bloodstream, cross the blood-brain barrier, and are neuroactive.
“Phenylalanine is a precursor for neurotransmitters in the brain, and aspartic acid activates the glutamate excitatory neurotransmitter receptor,” Dr. Bhide said. “The effects we’ve seen could be due to these metabolites that have a direct effect on the brain function.”
Time to act?
Some researchers are building a case for classifying UPFs as addictive substances. Others are calling for additional research on UPF safety that is conducted outside the food industry.
There has also been some discussion of placing warning labels on UPFs. However, there is disagreement about what information should be included and how consumers might interpret it. The question of which food products are UPFs and which are not also isn’t settled. The NOVA system may be widely used, but it still has its detractors who believe it misclassifies some healthy foods as ultraprocessed.
Dr. Chan and other experts say the research conducted thus far requires additional corroboration to inform appropriate public health interventions. That would likely take the form of a large, randomized trial with one group of participants eating a healthy diet and the other consuming large amounts of UPFs.
“This type of study is extremely challenging given the number of people that would have to be willing to participate and be willing to eat a very specific diet over a long period of time,” Dr. Chan said. “I am also not sure it would be ethical to assign people to such a diet, given what we already know about the potential health effects of UPFs.”
Dr. Thiagarajan and others have called on funding agencies to direct more grant monies toward studies of UPFs to better understand their effect on brain health.
“Given the magnitude of the problem and given that there is a fair bit of evidence that points to a potential causal link, then we damn well better put money into this and get to the bottom of it,” she said.
Others are looking to the FDA to increase the agency’s scrutiny of food additives. While some additives such as artificial sweeteners have a place in diets of people with diabetes or obesity, Dr. Bhide suggests it may be wise for healthy individuals to reduce their daily intake of UPFs.
“Our data raise this to a different level because of the transgenerational transmission, which has never been shown before,” he said. “We are saying that the FDA should look in preclinical models at germ cells and maybe transgenerational transmission before approving any food additive.”
A version of this article first appeared on Medscape.com.
Ultraprocessed foods (UPFs) make up nearly three-quarters of the entire U.S. food supply and about 60% of Americans’ daily caloric intake. A significant body of research has tied consumption of these foods – awash in added sugar, salt, fat, artificial colors, or preservatives – to cancer, diabetes, and heart disease.
Now, a growing number of studies also link them to poor brain health, including an increased risk of dementia, depression, and anxiety, and some experts are calling for public health policies aimed at reducing UPF consumption.
Under srutiny
A mainstay of diets in countries around the world, UPFs have come under increasing scrutiny because of their link to major diseases. The ingredients in UPFs add little or no nutritional value. Their primary function is to increase a product’s shelf life and palatability. Some recent evidence suggests these foods may be as addictive as tobacco. In addition, two pooled analysis studies using the Yale Food Addiction Scale showed that 14% of adults and 12% of children in the United States may have a UPF addiction.
The most widely used measure of what is, and what is not, a UPF was developed in 2009 by researchers in Brazil. The NOVA food classification system assigns food and beverages to one of four groups:
- Unprocessed and minimally processed foods, such as fruits, vegetables, milk, and meat.
- Processed culinary ingredients, including white sugar, butter, and oils derived from seeds, nuts, and fruits.
- Processed foods, such as tomato paste, bacon, canned tuna, and wine.
- Ultraprocessed foods, such as soda, ice cream, breakfast cereal, and prepackaged meals.
Those sounding the alarm about the potential harmful effects of UPFs are particularly concerned about their consumption by young people. The National Health and Nutrition Examination Survey showed that from 1999 to 2018, highly processed foods accounted for the majority of energy intake in those aged 2-19 years.
One of the most commonly used additives in UPFs, the artificial sweetener aspartame, garnered headlines this summer when the World Health Organization classified it as a likely carcinogen in humans. Aspartame is used in thousands of products, from soda to chewing gum to chewable vitamins.
The U.S. Food and Drug Administration strongly disagreed with the WHO’s position and is sticking by its recommended daily limit of 50 mg/kg of body weight – equivalent to 75 packets of the sweetener Equal – as safe for human consumption.
“Aspartame is one of the most studied food additives in the human food supply,” FDA officials said in a statement, adding that the agency found “significant shortcomings” in the studies the WHO used to justify the new classification. “FDA scientists do not have safety concerns when aspartame is used under the approved conditions.”
Increased attention to consumption of UPFs in general and aspartame particularly in recent years has yielded several studies pointing to the foods’ association with compromised brain health.
Link to depression, dementia
A recent report on UPF consumption and mental well-being among nearly 300,000 people across 70 countries showed that 53% of those who consumed UPFs several times a day were distressed or were struggling with their mental well-being, compared with 18% of those who rarely or never consumed UPFs.
Part of the Global Mind Project run by the nonprofit Sapien Labs in Arlington, Va., the report also showed that individuals with the highest rates of UPF consumption reported higher levels of confusion, slowed thinking, unwanted or obsessive thoughts, irritability, and feelings of sadness.
“There seems to be a much broader effect than just depression symptoms,” Tara Thiagarajan, PhD, founder and chief scientist of Sapien Labs and coauthor of the report, said in an interview.
The report, which has not been peer reviewed, comes on the heels of several other studies, including one from the Nurses Health Study II that showed that participants who consumed more than eight servings of UPFs daily had about a 50% higher depression risk, compared with those who consumed half that much.
“We found that UPFs in general, and artificial sweeteners and beverages in particular, were associated with increased risk,” said lead investigator Andrew T. Chan, MD, MPH, professor of medicine at Harvard Medical School and chief of the clinical and translational epidemiology unit, Massachusetts General Hospital, both in Boston.
“This was an interesting finding that correlates with data from animal studies that artificial sweeteners may trigger the transmission of particular signaling molecules in the brain that are important for mood,” he told this news organization.
Cognition may also be affected. An analysis of more than 72,000 people in the UK Biobank showed that those who consumed a high levels of UPFs were 50% more likely to develop dementia than those who consumed fewer processed foods. For every 10% increase in UPF consumption, the odds of developing any kind of dementia increased by 25%.
Another study of nearly 11,000 people showed that higher UPF consumption was associated with a significantly faster decline in executive and global cognitive function.
Epigenetic changes
While these and other studies suggest a link between UPF consumption and brain health, they are designed to demonstrate correlation. To date, no human study has proven that eating highly processed foods directly causes a decline in mental health or cognition.
Animal studies could provide that causal link. Earlier this year, researchers at Florida State University in Tallahassee reported learning and memory deficits in two groups of male mice that completed a maze test after being fed water mixed with aspartame for about 20% of their adult lives, compared with a group of mice that drank water only. Animals that ingested aspartame could finish the test, but it took them longer, and they needed help.
The amount of aspartame used in the study was just 7% and 15% of the FDA’s recommended maximum intake of aspartame (equivalent to two to four 8-ounce diet sodas daily).
Most intriguing was that offspring of the mice in the aspartame groups demonstrated the same levels of cognitive decline and anxiety as their fathers, even though they had never ingested the artificial sweetener. Researchers theorize that in addition to changes in brain gene expression, aspartame also caused epigenetic changes in germ cells.
“Epigenetic changes in germ cells due to environmental exposures are both good and bad,” lead investigator Pradeep G. Bhide, PhD, professor of developmental neuroscience and director of the Center for Brain Repair at FSU, told this news organization. “They are bad because the next generation is affected. But they’re good because as long as the exposure no longer occurs, 2 or 3 generations later, that’s gone.”
The mice, which lacked taste receptors for aspartame, were the same age and weight in all three groups. Because the only difference was exposure to the artificial sweetener, Dr. Bhide says it suggests a causal link.
“Extrapolation of data from well-controlled laboratory experiments in mice to humans is always risky,” Dr. Bhide said. “The extrapolations give us insights into what could happen rather than what will happen.”
Potential mechanisms
Although scientists can’t say for certain how UPFs affect brain health, there are several theories. UPFs may influence an inflammatory immune response, which has been linked to depression and dementia. Consumption of highly processed foods may also disrupt the gut microbiome, Dr. Chan said, which, in turn, may increase depression risk.
“This is an important potential mechanism linking ultraprocessed food to depression since there is emerging evidence that microbes in the gut have been linked with mood through their role in metabolizing and producing proteins that have activity in the brain,” he said.
In addition, with UPFs that contain aspartame, there could be a more direct link to brain function. In the gastrointestinal track, the sweetener is quickly broken down into methanol, aspartic acid, and phenylalanine. All three enter the bloodstream, cross the blood-brain barrier, and are neuroactive.
“Phenylalanine is a precursor for neurotransmitters in the brain, and aspartic acid activates the glutamate excitatory neurotransmitter receptor,” Dr. Bhide said. “The effects we’ve seen could be due to these metabolites that have a direct effect on the brain function.”
Time to act?
Some researchers are building a case for classifying UPFs as addictive substances. Others are calling for additional research on UPF safety that is conducted outside the food industry.
There has also been some discussion of placing warning labels on UPFs. However, there is disagreement about what information should be included and how consumers might interpret it. The question of which food products are UPFs and which are not also isn’t settled. The NOVA system may be widely used, but it still has its detractors who believe it misclassifies some healthy foods as ultraprocessed.
Dr. Chan and other experts say the research conducted thus far requires additional corroboration to inform appropriate public health interventions. That would likely take the form of a large, randomized trial with one group of participants eating a healthy diet and the other consuming large amounts of UPFs.
“This type of study is extremely challenging given the number of people that would have to be willing to participate and be willing to eat a very specific diet over a long period of time,” Dr. Chan said. “I am also not sure it would be ethical to assign people to such a diet, given what we already know about the potential health effects of UPFs.”
Dr. Thiagarajan and others have called on funding agencies to direct more grant monies toward studies of UPFs to better understand their effect on brain health.
“Given the magnitude of the problem and given that there is a fair bit of evidence that points to a potential causal link, then we damn well better put money into this and get to the bottom of it,” she said.
Others are looking to the FDA to increase the agency’s scrutiny of food additives. While some additives such as artificial sweeteners have a place in diets of people with diabetes or obesity, Dr. Bhide suggests it may be wise for healthy individuals to reduce their daily intake of UPFs.
“Our data raise this to a different level because of the transgenerational transmission, which has never been shown before,” he said. “We are saying that the FDA should look in preclinical models at germ cells and maybe transgenerational transmission before approving any food additive.”
A version of this article first appeared on Medscape.com.
Ultraprocessed foods (UPFs) make up nearly three-quarters of the entire U.S. food supply and about 60% of Americans’ daily caloric intake. A significant body of research has tied consumption of these foods – awash in added sugar, salt, fat, artificial colors, or preservatives – to cancer, diabetes, and heart disease.
Now, a growing number of studies also link them to poor brain health, including an increased risk of dementia, depression, and anxiety, and some experts are calling for public health policies aimed at reducing UPF consumption.
Under srutiny
A mainstay of diets in countries around the world, UPFs have come under increasing scrutiny because of their link to major diseases. The ingredients in UPFs add little or no nutritional value. Their primary function is to increase a product’s shelf life and palatability. Some recent evidence suggests these foods may be as addictive as tobacco. In addition, two pooled analysis studies using the Yale Food Addiction Scale showed that 14% of adults and 12% of children in the United States may have a UPF addiction.
The most widely used measure of what is, and what is not, a UPF was developed in 2009 by researchers in Brazil. The NOVA food classification system assigns food and beverages to one of four groups:
- Unprocessed and minimally processed foods, such as fruits, vegetables, milk, and meat.
- Processed culinary ingredients, including white sugar, butter, and oils derived from seeds, nuts, and fruits.
- Processed foods, such as tomato paste, bacon, canned tuna, and wine.
- Ultraprocessed foods, such as soda, ice cream, breakfast cereal, and prepackaged meals.
Those sounding the alarm about the potential harmful effects of UPFs are particularly concerned about their consumption by young people. The National Health and Nutrition Examination Survey showed that from 1999 to 2018, highly processed foods accounted for the majority of energy intake in those aged 2-19 years.
One of the most commonly used additives in UPFs, the artificial sweetener aspartame, garnered headlines this summer when the World Health Organization classified it as a likely carcinogen in humans. Aspartame is used in thousands of products, from soda to chewing gum to chewable vitamins.
The U.S. Food and Drug Administration strongly disagreed with the WHO’s position and is sticking by its recommended daily limit of 50 mg/kg of body weight – equivalent to 75 packets of the sweetener Equal – as safe for human consumption.
“Aspartame is one of the most studied food additives in the human food supply,” FDA officials said in a statement, adding that the agency found “significant shortcomings” in the studies the WHO used to justify the new classification. “FDA scientists do not have safety concerns when aspartame is used under the approved conditions.”
Increased attention to consumption of UPFs in general and aspartame particularly in recent years has yielded several studies pointing to the foods’ association with compromised brain health.
Link to depression, dementia
A recent report on UPF consumption and mental well-being among nearly 300,000 people across 70 countries showed that 53% of those who consumed UPFs several times a day were distressed or were struggling with their mental well-being, compared with 18% of those who rarely or never consumed UPFs.
Part of the Global Mind Project run by the nonprofit Sapien Labs in Arlington, Va., the report also showed that individuals with the highest rates of UPF consumption reported higher levels of confusion, slowed thinking, unwanted or obsessive thoughts, irritability, and feelings of sadness.
“There seems to be a much broader effect than just depression symptoms,” Tara Thiagarajan, PhD, founder and chief scientist of Sapien Labs and coauthor of the report, said in an interview.
The report, which has not been peer reviewed, comes on the heels of several other studies, including one from the Nurses Health Study II that showed that participants who consumed more than eight servings of UPFs daily had about a 50% higher depression risk, compared with those who consumed half that much.
“We found that UPFs in general, and artificial sweeteners and beverages in particular, were associated with increased risk,” said lead investigator Andrew T. Chan, MD, MPH, professor of medicine at Harvard Medical School and chief of the clinical and translational epidemiology unit, Massachusetts General Hospital, both in Boston.
“This was an interesting finding that correlates with data from animal studies that artificial sweeteners may trigger the transmission of particular signaling molecules in the brain that are important for mood,” he told this news organization.
Cognition may also be affected. An analysis of more than 72,000 people in the UK Biobank showed that those who consumed a high levels of UPFs were 50% more likely to develop dementia than those who consumed fewer processed foods. For every 10% increase in UPF consumption, the odds of developing any kind of dementia increased by 25%.
Another study of nearly 11,000 people showed that higher UPF consumption was associated with a significantly faster decline in executive and global cognitive function.
Epigenetic changes
While these and other studies suggest a link between UPF consumption and brain health, they are designed to demonstrate correlation. To date, no human study has proven that eating highly processed foods directly causes a decline in mental health or cognition.
Animal studies could provide that causal link. Earlier this year, researchers at Florida State University in Tallahassee reported learning and memory deficits in two groups of male mice that completed a maze test after being fed water mixed with aspartame for about 20% of their adult lives, compared with a group of mice that drank water only. Animals that ingested aspartame could finish the test, but it took them longer, and they needed help.
The amount of aspartame used in the study was just 7% and 15% of the FDA’s recommended maximum intake of aspartame (equivalent to two to four 8-ounce diet sodas daily).
Most intriguing was that offspring of the mice in the aspartame groups demonstrated the same levels of cognitive decline and anxiety as their fathers, even though they had never ingested the artificial sweetener. Researchers theorize that in addition to changes in brain gene expression, aspartame also caused epigenetic changes in germ cells.
“Epigenetic changes in germ cells due to environmental exposures are both good and bad,” lead investigator Pradeep G. Bhide, PhD, professor of developmental neuroscience and director of the Center for Brain Repair at FSU, told this news organization. “They are bad because the next generation is affected. But they’re good because as long as the exposure no longer occurs, 2 or 3 generations later, that’s gone.”
The mice, which lacked taste receptors for aspartame, were the same age and weight in all three groups. Because the only difference was exposure to the artificial sweetener, Dr. Bhide says it suggests a causal link.
“Extrapolation of data from well-controlled laboratory experiments in mice to humans is always risky,” Dr. Bhide said. “The extrapolations give us insights into what could happen rather than what will happen.”
Potential mechanisms
Although scientists can’t say for certain how UPFs affect brain health, there are several theories. UPFs may influence an inflammatory immune response, which has been linked to depression and dementia. Consumption of highly processed foods may also disrupt the gut microbiome, Dr. Chan said, which, in turn, may increase depression risk.
“This is an important potential mechanism linking ultraprocessed food to depression since there is emerging evidence that microbes in the gut have been linked with mood through their role in metabolizing and producing proteins that have activity in the brain,” he said.
In addition, with UPFs that contain aspartame, there could be a more direct link to brain function. In the gastrointestinal track, the sweetener is quickly broken down into methanol, aspartic acid, and phenylalanine. All three enter the bloodstream, cross the blood-brain barrier, and are neuroactive.
“Phenylalanine is a precursor for neurotransmitters in the brain, and aspartic acid activates the glutamate excitatory neurotransmitter receptor,” Dr. Bhide said. “The effects we’ve seen could be due to these metabolites that have a direct effect on the brain function.”
Time to act?
Some researchers are building a case for classifying UPFs as addictive substances. Others are calling for additional research on UPF safety that is conducted outside the food industry.
There has also been some discussion of placing warning labels on UPFs. However, there is disagreement about what information should be included and how consumers might interpret it. The question of which food products are UPFs and which are not also isn’t settled. The NOVA system may be widely used, but it still has its detractors who believe it misclassifies some healthy foods as ultraprocessed.
Dr. Chan and other experts say the research conducted thus far requires additional corroboration to inform appropriate public health interventions. That would likely take the form of a large, randomized trial with one group of participants eating a healthy diet and the other consuming large amounts of UPFs.
“This type of study is extremely challenging given the number of people that would have to be willing to participate and be willing to eat a very specific diet over a long period of time,” Dr. Chan said. “I am also not sure it would be ethical to assign people to such a diet, given what we already know about the potential health effects of UPFs.”
Dr. Thiagarajan and others have called on funding agencies to direct more grant monies toward studies of UPFs to better understand their effect on brain health.
“Given the magnitude of the problem and given that there is a fair bit of evidence that points to a potential causal link, then we damn well better put money into this and get to the bottom of it,” she said.
Others are looking to the FDA to increase the agency’s scrutiny of food additives. While some additives such as artificial sweeteners have a place in diets of people with diabetes or obesity, Dr. Bhide suggests it may be wise for healthy individuals to reduce their daily intake of UPFs.
“Our data raise this to a different level because of the transgenerational transmission, which has never been shown before,” he said. “We are saying that the FDA should look in preclinical models at germ cells and maybe transgenerational transmission before approving any food additive.”
A version of this article first appeared on Medscape.com.
Attitudes Toward Utilization of Minimally Invasive Cosmetic Procedures in Black Women: Results of a Cross-sectional Survey
Beauty has been a topic of interest for centuries. Treatments and technologies have advanced, and more women are utilizing cosmetic procedures than ever before, especially neuromodulators, minimally invasive procedures, and topical treatments.1 Over the last decade, there was a 99% increase in minimally invasive cosmetic procedures in the United States.2 There also has been an observable increase in the utilization of cosmetic procedures by Black patients in recent years; the American Society of Plastic Surgeons reported that the number of cosmetic plastic surgery procedures performed on “ethnic patients” (referring to Asian, Black, or Hispanic patients) increased 243% from 2000 to 2013,3 possibly attributed to increased accessibility, awareness of procedures due to social media, cultural acceptance, and affordability. Minimally invasive procedures are considerably less expensive than major surgical procedures and are becoming progressively more affordable, with numerous financing options available.2 Additionally, neuromodulators and fillers are now commonly administered by nonaesthetic health professionals including dentists and nurses, which has increased accessibility of these procedures among patients who typically may not seek out a consultation with a plastic surgeon or dermatologist.4
When examining the most common cosmetic procedures collectively sought out by patients with skin of color (SOC), it has been found that an even skin tone is a highly desirable feature that impacts the selection of products and procedures in this particular patient population.5 Black, Hispanic, and Asian women report fewer signs of facial aging compared to White women in the glabellar lines, crow’s-feet, oral commissures, perioral lines, and lips.6 Increased melanocytes in darker skin types help prevent photoaging but also increase susceptibility to dyschromia. Prior studies have reported the most common concerns by patients with SOC are dyschromic disorders such as postinflammatory hyperpigmentation, postinflammatory hypopigmentation, and melasma.7 Common minimally invasive cosmetic procedures utilized by the SOC population include chemical peels, laser treatments, and injectables. Fillers are utilized more for volume loss in SOC patients rather than for the deep furrows and rhytides commonly seen in the lower face of White patients.8
We conducted a survey among Black women currently residing in the United States to better understand attitudes toward beauty and aging as well as the utilization of minimally invasive cosmetic procedures in this patient population.
Methods
An in-depth questionnaire comprised of 17 questions was created for this cross-sectional observational study. The study was submitted to and deemed exempt by the institutional review board at the University of Miami (Miami, Florida)(IRB #20211184). Survey participants primarily were recruited via social media posts on personal profiles of Black dermatologists, medical residents, and medicalstudents, including the authors, targeting Black women in the United States. Utilizing a method called snowball sampling, whereby study participants are used to recruit future participants, individuals were instructed to share the survey with their social network to assist with survey distribution. After participants provided informed consent, data were captured using the REDCap secure online data collection software. The questionnaire was structured to include a sociodemographic profile of respondents, attitudes toward beauty and aging, current usage of beauty products, prior utilization of cosmetic procedures, and intentions to use cosmetic procedures in the future. Surveys with incomplete consent forms, incomplete responses, and duplicate responses, as well as surveys from participants who were not residing in the United States at the time of survey completion, were excluded.
Data characteristics were summarized by frequency and percentage. A χ2 test was performed to compare participants’ age demographics with their attitudes toward beauty and aging, utilization of cosmetic procedures, and intention to try cosmetic procedures in the future. The Fisher exact test was used instead of the χ2 test when the expected cell count was less than 5. For all tests, P<.05 was considered statistically significant. All statistical analyses were performed using SPSS software version 28.
Results
General Characteristics of Participants—A sample of 475 self-identified Black women aged 21 to 70 years participated in the study, and 352 eligible participants were included in the final analysis. Of the 352 eligible participants, 48.3% were aged 21 to 30 years, 47.2% were aged 31 to 40 years, and 4.5% were aged 41 to 50 years. All survey participants identified their race as Black; among them, 4% specified Hispanic or Latino ethnicity, and 9% indicated that they held multiracial identities including White/Caucasian, Asian, and Native American backgrounds. Regarding the participants’ citizenship status, 54.3% reported that both they and their parents were born in the United States; 2.3% were not US citizens or permanent residents, 13.1% identified as first-generation Americans (born outside of the United States), and 30.4% identified as second-generation Americans (one or both parents born outside of the United States). Participant education levels (based on highest level) varied greatly: 4.5% were high school graduates, 1.1% attended trade or technical schools, 3.4% had associate’s degrees, 39.8% had bachelor’s degrees, 35.2% had master’s degrees, and 15.9% had doctorate degrees. Regarding household income, 6.3% earned less than $25,000 per year, 16.8% earned from $25,000 to $99,999, 75.6% earned from $100,000 to $499,999, and 1.4% earned $500,000 or more. Patient demographics are provided in Table 1.
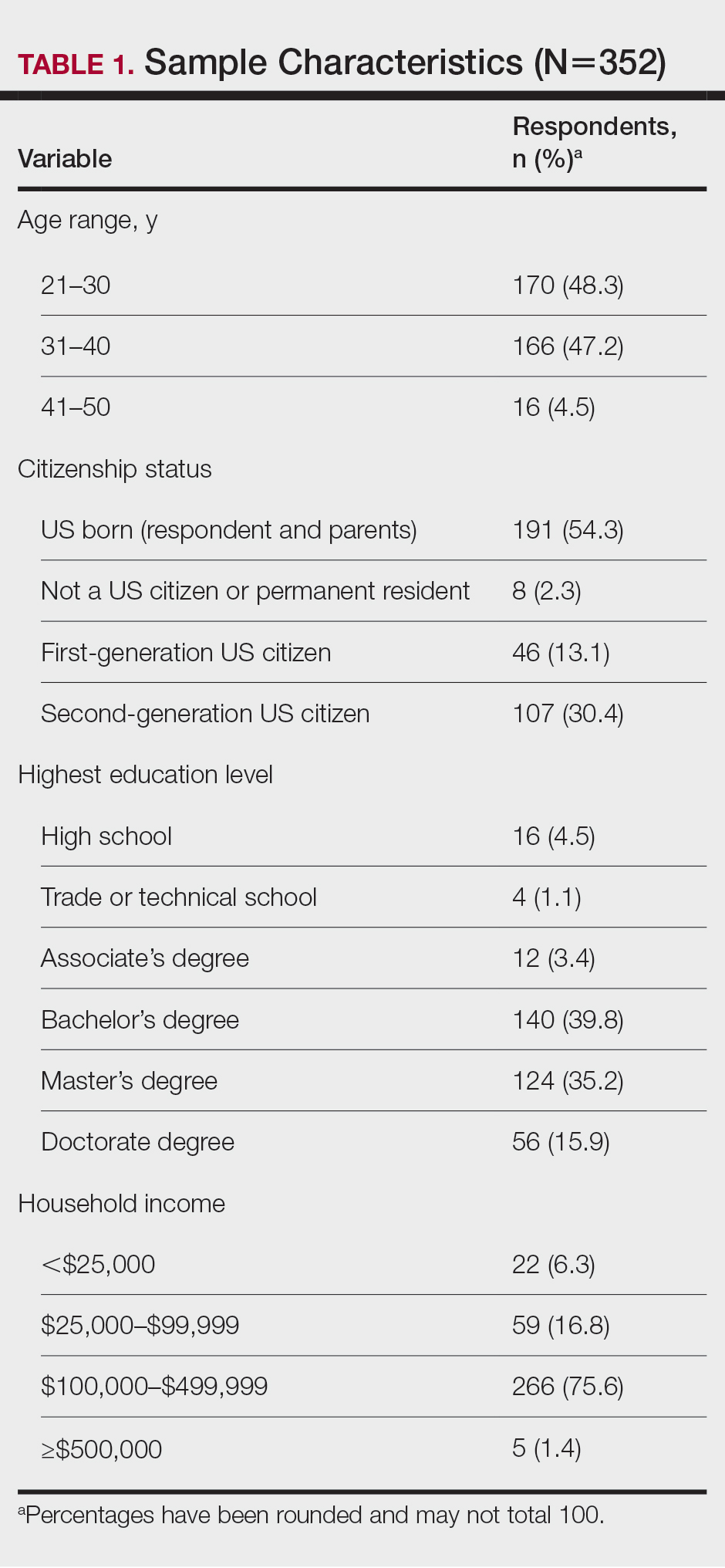
Cosmetic Skin Concerns—The top 3 aging skin concerns among participants were fine lines and wrinkles (51.9%), dark circles (33.8%), and uneven skin tone (31.8%) (Table 2). Approximately 5.4% of participants reported no desire to avoid the natural aging process. Among age groups, fine lines and wrinkles were a major concern for 51.7% of 21- to 30-year-olds, 47.6% of 31- to 40-year-olds, and 43.5% of 41- to 50-year-olds. Dark circles were a major concern for 61.3% of 21- to 30-year-olds, 44.4% of 31- to 40-year-olds, and 46.8% of 41- to 50-year-olds. Uneven skin tone was a major concern for 56.2% of 21- to 30-year-olds, 46.5% of 31- to 40-year-olds, and 31.2% of 41- to 50-year-olds. There was no statistically significant association between participants’ age and their concern with aging skin concerns.
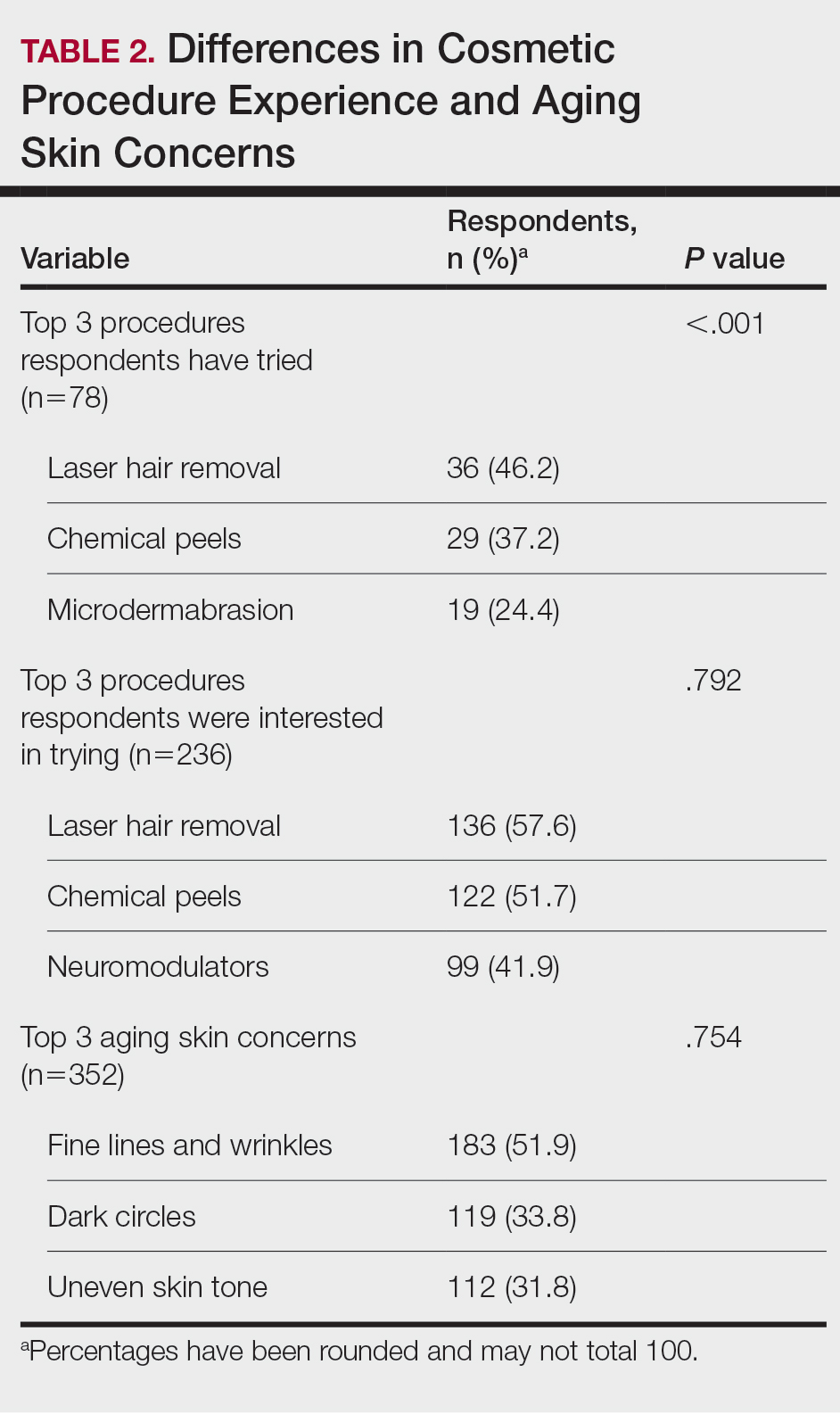
Differences in Experience and Acceptance of Cosmetic Procedures—Regarding participants’ prior experience with cosmetic procedures, 22.3% had tried 1 or more procedures. Additionally, 67.0% reported having intentions of trying cosmetic procedures in the future, while 10.8% reported no intentions. Of those who were uninterested in trying cosmetic procedures, 78.9% (30/38) believed it unnecessary while 47.3% (18/38) reported a fear of looking unnatural. Other perceived deterrents to cosmetic procedures among this subset of participants were the need to repeat treatment for lasting results (28.9% [11/38]), too expensive (31.6% [12/38]), and fear of side effects (39.5% [15/38]). A significant difference was found between participants’ age and their experience with cosmetic procedures (P=.020). Participants aged 21 to 30 years reported they were more likely to want to try cosmetic procedures in the future. Participants aged 31 to 40 years were more likely to have already tried a cosmetic procedure. Participants aged 41 to 50 years were more likely to report no desire to try cosmetic procedures in the future. There was no significant difference in cosmetic procedure acceptance according to citizenship status, education level, or household income.
Differences in Cosmetic Procedure Experience—Study participants indicated awareness of typically practiced cosmetic procedures. Of the 78 participants who have tried cosmetic procedures (Figure 1), the most common were laser hair removal (46.2% [36/78]), chemical peels (37.2% [29/78]), and microdermabrasion (24.4% [19/78])(Table 2). Age significantly influenced the type of cosmetic procedures utilized by participants (P<.001). Laser hair removal was the most common cosmetic procedure utilized by participants aged 21 to 30 years (64.7%) and chemical peels in participants aged 31 to 40 years (47.8%); participants aged 41 to 50 years reported equal use of chemical peels (50.0%) and microdermabrasion (50.0%).
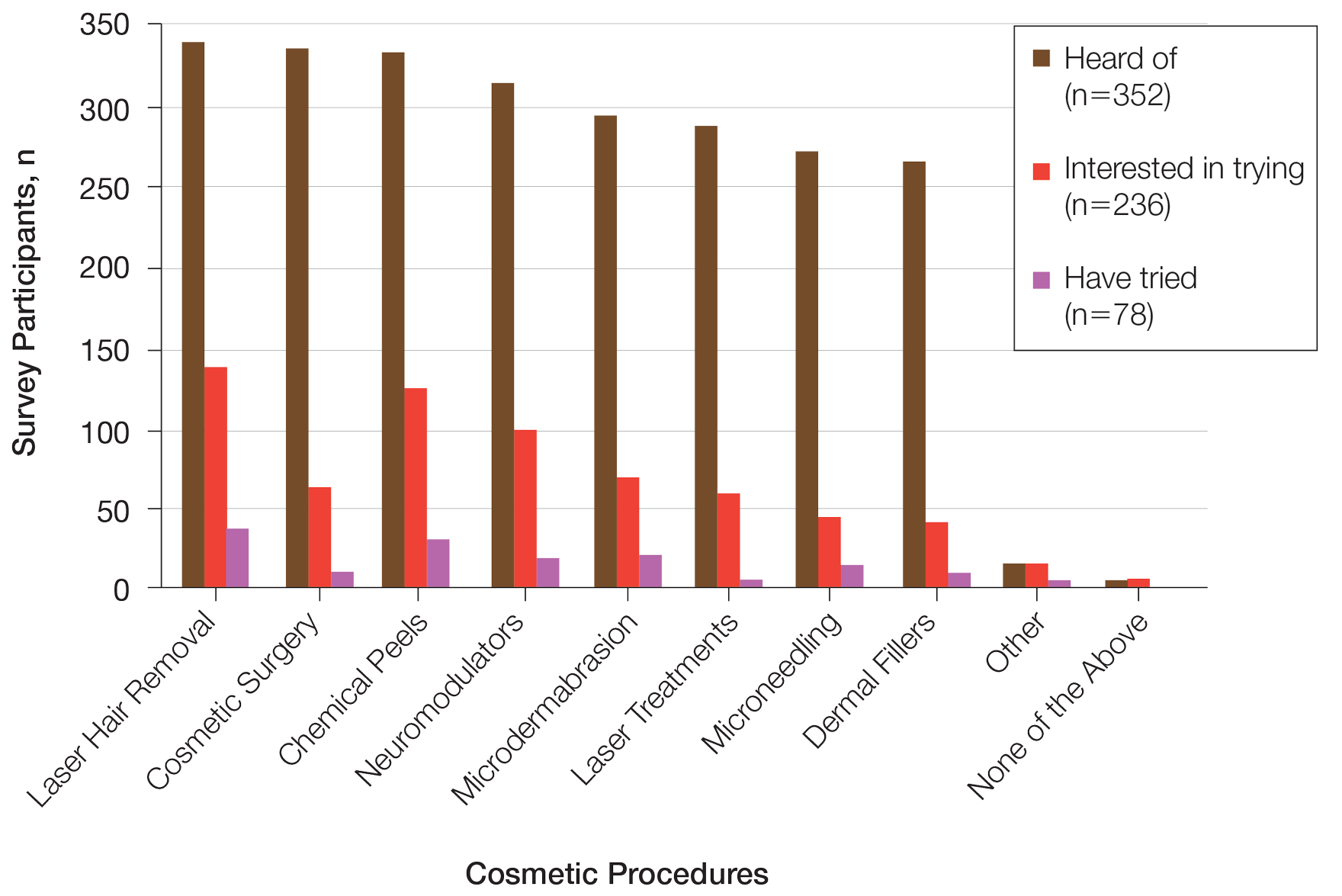
Two hundred thirty-six participants reported interest in trying cosmetic procedures, specifically laser hair removal (57.6%), chemical peels (51.7%), and neuromodulators (41.9%)(Table 2). Although not statistically significant, age appeared to influence interest levels in cosmetic procedures. Participants aged 21 to 30 years and 31 to 40 years were most interested in trying laser hair removal (60.7% and 58.3%, respectively). Participants aged 41 to 50 years were most interested in trying neuromodulators (36.4%). There was no significant association between age and intention to try neuromodulators, chemical peels, or laser hair removal.
Attitudes Toward Beauty—Approximately 40.6% of participants believed that peak beauty occurs when women reach their 20s, and 38.6% believed that peak beauty occurs when women reach their 30s. Participants’ strategies for maintaining beauty were assessed through their regular use of certain skin care products. The most frequently used skin care products were face wash or cleanser (92.6%), moisturizer (90.1%), lip balm (76.1%), and facial sunscreen (62.2%). Other commonly used items were serum (34.7%), toner (34.9%), topical vitamin C (33.2%), and retinol/retinoid products (33.0%). Only 2.3% of participants reported not using any skin care products regularly.
Perceptions of Aging—Concerning perceived external age, most respondents believed they looked younger than their true age (69.9%); 24.4% believed they looked their true age, and 5.7% believed they looked older. Perception of age also varied considerably by age group, though most believed they looked younger than their true age.
Comment
This survey helped to identify trends in cosmetic procedure acceptance and utilization in Black women. As expected, younger Black women were more receptive to cosmetic procedures, which was consistent with a recent finding that cosmetic procedures tend to be more widely accepted among younger generations overall.8 Participants aged 21 to 30 years had greater intentions to try a cosmetic procedure, while those aged 31 to 40 years were more likely to have tried 1 or more cosmetic procedures already, which may be because they are just beginning to see the signs of aging and are motivated to address these concerns. Additionally, women in this age group may be more likely to have a stable source of income and be able to afford these procedures. It is important to note that the population surveyed had a much higher reported household income than the average Black household income, with most respondents reporting an average annual income of $100,000 to $499,000. Our data also showed a trend toward greater acceptance and utilization of cosmetic procedures in those with higher levels of income, though the results were not statistically significant.
Respondents were most concerned about fine lines and wrinkles, followed by dark circles and uneven skin tone. One report in the literature (N=2000) indicated that the most common cosmetic concerns in women with SOC were hyperpigmentation/dark spots (86%) and blotchy or uneven skin (80%).9 Interestingly, sunscreen was one of the more commonly used products in our survey, which historically has not been the case among individuals with SOC10 and suggests that the attitudes and perceptions of SOC patients are changing to favor more frequent sunscreen use, at least among the younger generations. Because we did not specify moisturizer vs moisturizer with sun protection factor, the use of facial sunscreen may even be underestimated in our survey.
Compared to cosmetic surgery or dermal fillers, the procedures found to be most frequently utilized in our study population—microdermabrasion, chemical peels, and laser hair removal—are less invasive and fairly accessible with minimal downtime. An interesting topic for further research would be to investigate how the willingness of women to openly share their cosmetic procedure usage has changed over time. The rise of social media and influencer culture has undoubtedly had an impact on the sharing of such information. It also would have been interesting to ask participants where they receive the majority of their health/beauty information.
All skin types are susceptible to photoaging; however, melanin is known to have a natural photoprotective effect, resulting in a lesser degree and later onset of photoaging in patients with darker vs lighter skin.11 It has been reported that individuals with SOC show signs of facial aging on average a decade later than those with lighter skin tones,12 which may be why the majority of participants believed they look younger than they truly are. As expected, dyspigmentation was among the top skin concerns in our study population. Although melanin does offer some degree of protection against UVA and UVB, melanocyte lability with inflammation may make darker skin types more susceptible to pigmentary issues.13
Study Limitations—The income levels of our study population were not representative of typical Black American households, which is a limitation. Seventy-seven percent of our study population earned more than $100,000 annually, while only 18% of Black American households earned more than $100,000 in 2019.14 Another major limitation of our study was the lack of representation from older generations, as most participants were aged 21 to 40 years, which was expected, as it is the younger generation who typically is targeted by a snowball sampling method primarily shared through social media. Additionally, because participants were recruited from the social media profiles of medical professionals, followers of these accounts may be more interested in cosmetic procedures, skewing the study results. Finally, because geographic location was not captured in our initial data collection, we were unable to determine if results from a particular location within the United States were overrepresented in the data set.
Conclusion
Although the discourse around beauty and antiaging is constantly evolving, data about Black women frequently are underrepresented in the literature. The results of this study highlight the changing attitudes and perceptions of Black women regarding beauty, aging, and minimally invasive cosmetic procedures. Dermatologists should stay abreast of current trends in this population to be able to make appropriate, culturally sensitive recommendations to their Black patients—for example, pointing them to sunscreen brands that are best suited for darker skin.
- Ahn CS, Suchonwanit P, Foy CG, et al. Hair and scalp care in African American women who exercise. JAMA Dermatol. 2016;152:579-580.
- Prendergast TI, Ong’uti SK, Ortega G, et al. Differential trends in racial preferences for cosmetic surgery procedures. Am Surg. 2011;77:1081-1085.
- American Society of Plastic Surgeons. Briefing paper: plastic surgeryfor ethnic patients. Accessed October 20, 2023. https://www.plasticsurgery.org/news/briefing-papers/briefing-paper-plastic-surgery-for-ethnic-patients
- Small K, Kelly KM, Spinelli HM. Are nurse injectors the new norm? Aesthetic Plast Surg. 2014;38:946-955.
- Quiñonez RL, Agbai ON, Burgess CM, et al. An update on cosmetic procedures in people of color. part 1: scientific background, assessment, preprocedure preparation. J Am Acad Dermatol. 2022;86:715-725.
- Alexis AF, Grimes P, Boyd C, et al. Racial and ethnic differences in self-assessed facial aging in women: results from a multinational study. Dermatol Surg. 2019;45:1635-1648.
- Talakoub L, Wesley NO. Differences in perceptions of beauty and cosmetic procedures performed in ethnic patients. Semin Cutan Med Surg. 2009;28:115-129.
- Alotaibi AS. Demographic and cultural differences in the acceptance and pursuit of cosmetic surgery: a systematic literature review. Plast Reconstr Surg Glob Open. 2021;9:E3501.
- Grimes PE. Skin and hair cosmetic issues in women of color. Dermatol Clin. 2000;18:659-665.
- Buchanan Lunsford N, Berktold J, Holman DM, et al. Skin cancer knowledge, awareness, beliefs and preventive behaviors among black and Hispanic men and women. Prev Med Rep. 2018;12:203-209.
- Alexis AF, Rossi, A. Photoaging in skin of color. Cosmet Dermatol. 2011;24:367-370.
- Vashi NA, de Castro Maymone MB, Kundu RV. Aging differences in ethnic skin. J Clin Aesthet Dermatol. 2016;9:31-38.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Tamir C, Budiman A, Noe-Bustamante L, et al. Facts about the U.S. Black population. Pew Research Center website. Published March 2, 2023. Accessed October 20, 2023. https://www.pewresearch.org/social-trends/fact-sheet/facts-about-the-us-black-population/
Beauty has been a topic of interest for centuries. Treatments and technologies have advanced, and more women are utilizing cosmetic procedures than ever before, especially neuromodulators, minimally invasive procedures, and topical treatments.1 Over the last decade, there was a 99% increase in minimally invasive cosmetic procedures in the United States.2 There also has been an observable increase in the utilization of cosmetic procedures by Black patients in recent years; the American Society of Plastic Surgeons reported that the number of cosmetic plastic surgery procedures performed on “ethnic patients” (referring to Asian, Black, or Hispanic patients) increased 243% from 2000 to 2013,3 possibly attributed to increased accessibility, awareness of procedures due to social media, cultural acceptance, and affordability. Minimally invasive procedures are considerably less expensive than major surgical procedures and are becoming progressively more affordable, with numerous financing options available.2 Additionally, neuromodulators and fillers are now commonly administered by nonaesthetic health professionals including dentists and nurses, which has increased accessibility of these procedures among patients who typically may not seek out a consultation with a plastic surgeon or dermatologist.4
When examining the most common cosmetic procedures collectively sought out by patients with skin of color (SOC), it has been found that an even skin tone is a highly desirable feature that impacts the selection of products and procedures in this particular patient population.5 Black, Hispanic, and Asian women report fewer signs of facial aging compared to White women in the glabellar lines, crow’s-feet, oral commissures, perioral lines, and lips.6 Increased melanocytes in darker skin types help prevent photoaging but also increase susceptibility to dyschromia. Prior studies have reported the most common concerns by patients with SOC are dyschromic disorders such as postinflammatory hyperpigmentation, postinflammatory hypopigmentation, and melasma.7 Common minimally invasive cosmetic procedures utilized by the SOC population include chemical peels, laser treatments, and injectables. Fillers are utilized more for volume loss in SOC patients rather than for the deep furrows and rhytides commonly seen in the lower face of White patients.8
We conducted a survey among Black women currently residing in the United States to better understand attitudes toward beauty and aging as well as the utilization of minimally invasive cosmetic procedures in this patient population.
Methods
An in-depth questionnaire comprised of 17 questions was created for this cross-sectional observational study. The study was submitted to and deemed exempt by the institutional review board at the University of Miami (Miami, Florida)(IRB #20211184). Survey participants primarily were recruited via social media posts on personal profiles of Black dermatologists, medical residents, and medicalstudents, including the authors, targeting Black women in the United States. Utilizing a method called snowball sampling, whereby study participants are used to recruit future participants, individuals were instructed to share the survey with their social network to assist with survey distribution. After participants provided informed consent, data were captured using the REDCap secure online data collection software. The questionnaire was structured to include a sociodemographic profile of respondents, attitudes toward beauty and aging, current usage of beauty products, prior utilization of cosmetic procedures, and intentions to use cosmetic procedures in the future. Surveys with incomplete consent forms, incomplete responses, and duplicate responses, as well as surveys from participants who were not residing in the United States at the time of survey completion, were excluded.
Data characteristics were summarized by frequency and percentage. A χ2 test was performed to compare participants’ age demographics with their attitudes toward beauty and aging, utilization of cosmetic procedures, and intention to try cosmetic procedures in the future. The Fisher exact test was used instead of the χ2 test when the expected cell count was less than 5. For all tests, P<.05 was considered statistically significant. All statistical analyses were performed using SPSS software version 28.
Results
General Characteristics of Participants—A sample of 475 self-identified Black women aged 21 to 70 years participated in the study, and 352 eligible participants were included in the final analysis. Of the 352 eligible participants, 48.3% were aged 21 to 30 years, 47.2% were aged 31 to 40 years, and 4.5% were aged 41 to 50 years. All survey participants identified their race as Black; among them, 4% specified Hispanic or Latino ethnicity, and 9% indicated that they held multiracial identities including White/Caucasian, Asian, and Native American backgrounds. Regarding the participants’ citizenship status, 54.3% reported that both they and their parents were born in the United States; 2.3% were not US citizens or permanent residents, 13.1% identified as first-generation Americans (born outside of the United States), and 30.4% identified as second-generation Americans (one or both parents born outside of the United States). Participant education levels (based on highest level) varied greatly: 4.5% were high school graduates, 1.1% attended trade or technical schools, 3.4% had associate’s degrees, 39.8% had bachelor’s degrees, 35.2% had master’s degrees, and 15.9% had doctorate degrees. Regarding household income, 6.3% earned less than $25,000 per year, 16.8% earned from $25,000 to $99,999, 75.6% earned from $100,000 to $499,999, and 1.4% earned $500,000 or more. Patient demographics are provided in Table 1.

Cosmetic Skin Concerns—The top 3 aging skin concerns among participants were fine lines and wrinkles (51.9%), dark circles (33.8%), and uneven skin tone (31.8%) (Table 2). Approximately 5.4% of participants reported no desire to avoid the natural aging process. Among age groups, fine lines and wrinkles were a major concern for 51.7% of 21- to 30-year-olds, 47.6% of 31- to 40-year-olds, and 43.5% of 41- to 50-year-olds. Dark circles were a major concern for 61.3% of 21- to 30-year-olds, 44.4% of 31- to 40-year-olds, and 46.8% of 41- to 50-year-olds. Uneven skin tone was a major concern for 56.2% of 21- to 30-year-olds, 46.5% of 31- to 40-year-olds, and 31.2% of 41- to 50-year-olds. There was no statistically significant association between participants’ age and their concern with aging skin concerns.

Differences in Experience and Acceptance of Cosmetic Procedures—Regarding participants’ prior experience with cosmetic procedures, 22.3% had tried 1 or more procedures. Additionally, 67.0% reported having intentions of trying cosmetic procedures in the future, while 10.8% reported no intentions. Of those who were uninterested in trying cosmetic procedures, 78.9% (30/38) believed it unnecessary while 47.3% (18/38) reported a fear of looking unnatural. Other perceived deterrents to cosmetic procedures among this subset of participants were the need to repeat treatment for lasting results (28.9% [11/38]), too expensive (31.6% [12/38]), and fear of side effects (39.5% [15/38]). A significant difference was found between participants’ age and their experience with cosmetic procedures (P=.020). Participants aged 21 to 30 years reported they were more likely to want to try cosmetic procedures in the future. Participants aged 31 to 40 years were more likely to have already tried a cosmetic procedure. Participants aged 41 to 50 years were more likely to report no desire to try cosmetic procedures in the future. There was no significant difference in cosmetic procedure acceptance according to citizenship status, education level, or household income.
Differences in Cosmetic Procedure Experience—Study participants indicated awareness of typically practiced cosmetic procedures. Of the 78 participants who have tried cosmetic procedures (Figure 1), the most common were laser hair removal (46.2% [36/78]), chemical peels (37.2% [29/78]), and microdermabrasion (24.4% [19/78])(Table 2). Age significantly influenced the type of cosmetic procedures utilized by participants (P<.001). Laser hair removal was the most common cosmetic procedure utilized by participants aged 21 to 30 years (64.7%) and chemical peels in participants aged 31 to 40 years (47.8%); participants aged 41 to 50 years reported equal use of chemical peels (50.0%) and microdermabrasion (50.0%).

Two hundred thirty-six participants reported interest in trying cosmetic procedures, specifically laser hair removal (57.6%), chemical peels (51.7%), and neuromodulators (41.9%)(Table 2). Although not statistically significant, age appeared to influence interest levels in cosmetic procedures. Participants aged 21 to 30 years and 31 to 40 years were most interested in trying laser hair removal (60.7% and 58.3%, respectively). Participants aged 41 to 50 years were most interested in trying neuromodulators (36.4%). There was no significant association between age and intention to try neuromodulators, chemical peels, or laser hair removal.
Attitudes Toward Beauty—Approximately 40.6% of participants believed that peak beauty occurs when women reach their 20s, and 38.6% believed that peak beauty occurs when women reach their 30s. Participants’ strategies for maintaining beauty were assessed through their regular use of certain skin care products. The most frequently used skin care products were face wash or cleanser (92.6%), moisturizer (90.1%), lip balm (76.1%), and facial sunscreen (62.2%). Other commonly used items were serum (34.7%), toner (34.9%), topical vitamin C (33.2%), and retinol/retinoid products (33.0%). Only 2.3% of participants reported not using any skin care products regularly.
Perceptions of Aging—Concerning perceived external age, most respondents believed they looked younger than their true age (69.9%); 24.4% believed they looked their true age, and 5.7% believed they looked older. Perception of age also varied considerably by age group, though most believed they looked younger than their true age.
Comment
This survey helped to identify trends in cosmetic procedure acceptance and utilization in Black women. As expected, younger Black women were more receptive to cosmetic procedures, which was consistent with a recent finding that cosmetic procedures tend to be more widely accepted among younger generations overall.8 Participants aged 21 to 30 years had greater intentions to try a cosmetic procedure, while those aged 31 to 40 years were more likely to have tried 1 or more cosmetic procedures already, which may be because they are just beginning to see the signs of aging and are motivated to address these concerns. Additionally, women in this age group may be more likely to have a stable source of income and be able to afford these procedures. It is important to note that the population surveyed had a much higher reported household income than the average Black household income, with most respondents reporting an average annual income of $100,000 to $499,000. Our data also showed a trend toward greater acceptance and utilization of cosmetic procedures in those with higher levels of income, though the results were not statistically significant.
Respondents were most concerned about fine lines and wrinkles, followed by dark circles and uneven skin tone. One report in the literature (N=2000) indicated that the most common cosmetic concerns in women with SOC were hyperpigmentation/dark spots (86%) and blotchy or uneven skin (80%).9 Interestingly, sunscreen was one of the more commonly used products in our survey, which historically has not been the case among individuals with SOC10 and suggests that the attitudes and perceptions of SOC patients are changing to favor more frequent sunscreen use, at least among the younger generations. Because we did not specify moisturizer vs moisturizer with sun protection factor, the use of facial sunscreen may even be underestimated in our survey.
Compared to cosmetic surgery or dermal fillers, the procedures found to be most frequently utilized in our study population—microdermabrasion, chemical peels, and laser hair removal—are less invasive and fairly accessible with minimal downtime. An interesting topic for further research would be to investigate how the willingness of women to openly share their cosmetic procedure usage has changed over time. The rise of social media and influencer culture has undoubtedly had an impact on the sharing of such information. It also would have been interesting to ask participants where they receive the majority of their health/beauty information.
All skin types are susceptible to photoaging; however, melanin is known to have a natural photoprotective effect, resulting in a lesser degree and later onset of photoaging in patients with darker vs lighter skin.11 It has been reported that individuals with SOC show signs of facial aging on average a decade later than those with lighter skin tones,12 which may be why the majority of participants believed they look younger than they truly are. As expected, dyspigmentation was among the top skin concerns in our study population. Although melanin does offer some degree of protection against UVA and UVB, melanocyte lability with inflammation may make darker skin types more susceptible to pigmentary issues.13
Study Limitations—The income levels of our study population were not representative of typical Black American households, which is a limitation. Seventy-seven percent of our study population earned more than $100,000 annually, while only 18% of Black American households earned more than $100,000 in 2019.14 Another major limitation of our study was the lack of representation from older generations, as most participants were aged 21 to 40 years, which was expected, as it is the younger generation who typically is targeted by a snowball sampling method primarily shared through social media. Additionally, because participants were recruited from the social media profiles of medical professionals, followers of these accounts may be more interested in cosmetic procedures, skewing the study results. Finally, because geographic location was not captured in our initial data collection, we were unable to determine if results from a particular location within the United States were overrepresented in the data set.
Conclusion
Although the discourse around beauty and antiaging is constantly evolving, data about Black women frequently are underrepresented in the literature. The results of this study highlight the changing attitudes and perceptions of Black women regarding beauty, aging, and minimally invasive cosmetic procedures. Dermatologists should stay abreast of current trends in this population to be able to make appropriate, culturally sensitive recommendations to their Black patients—for example, pointing them to sunscreen brands that are best suited for darker skin.
Beauty has been a topic of interest for centuries. Treatments and technologies have advanced, and more women are utilizing cosmetic procedures than ever before, especially neuromodulators, minimally invasive procedures, and topical treatments.1 Over the last decade, there was a 99% increase in minimally invasive cosmetic procedures in the United States.2 There also has been an observable increase in the utilization of cosmetic procedures by Black patients in recent years; the American Society of Plastic Surgeons reported that the number of cosmetic plastic surgery procedures performed on “ethnic patients” (referring to Asian, Black, or Hispanic patients) increased 243% from 2000 to 2013,3 possibly attributed to increased accessibility, awareness of procedures due to social media, cultural acceptance, and affordability. Minimally invasive procedures are considerably less expensive than major surgical procedures and are becoming progressively more affordable, with numerous financing options available.2 Additionally, neuromodulators and fillers are now commonly administered by nonaesthetic health professionals including dentists and nurses, which has increased accessibility of these procedures among patients who typically may not seek out a consultation with a plastic surgeon or dermatologist.4
When examining the most common cosmetic procedures collectively sought out by patients with skin of color (SOC), it has been found that an even skin tone is a highly desirable feature that impacts the selection of products and procedures in this particular patient population.5 Black, Hispanic, and Asian women report fewer signs of facial aging compared to White women in the glabellar lines, crow’s-feet, oral commissures, perioral lines, and lips.6 Increased melanocytes in darker skin types help prevent photoaging but also increase susceptibility to dyschromia. Prior studies have reported the most common concerns by patients with SOC are dyschromic disorders such as postinflammatory hyperpigmentation, postinflammatory hypopigmentation, and melasma.7 Common minimally invasive cosmetic procedures utilized by the SOC population include chemical peels, laser treatments, and injectables. Fillers are utilized more for volume loss in SOC patients rather than for the deep furrows and rhytides commonly seen in the lower face of White patients.8
We conducted a survey among Black women currently residing in the United States to better understand attitudes toward beauty and aging as well as the utilization of minimally invasive cosmetic procedures in this patient population.
Methods
An in-depth questionnaire comprised of 17 questions was created for this cross-sectional observational study. The study was submitted to and deemed exempt by the institutional review board at the University of Miami (Miami, Florida)(IRB #20211184). Survey participants primarily were recruited via social media posts on personal profiles of Black dermatologists, medical residents, and medicalstudents, including the authors, targeting Black women in the United States. Utilizing a method called snowball sampling, whereby study participants are used to recruit future participants, individuals were instructed to share the survey with their social network to assist with survey distribution. After participants provided informed consent, data were captured using the REDCap secure online data collection software. The questionnaire was structured to include a sociodemographic profile of respondents, attitudes toward beauty and aging, current usage of beauty products, prior utilization of cosmetic procedures, and intentions to use cosmetic procedures in the future. Surveys with incomplete consent forms, incomplete responses, and duplicate responses, as well as surveys from participants who were not residing in the United States at the time of survey completion, were excluded.
Data characteristics were summarized by frequency and percentage. A χ2 test was performed to compare participants’ age demographics with their attitudes toward beauty and aging, utilization of cosmetic procedures, and intention to try cosmetic procedures in the future. The Fisher exact test was used instead of the χ2 test when the expected cell count was less than 5. For all tests, P<.05 was considered statistically significant. All statistical analyses were performed using SPSS software version 28.
Results
General Characteristics of Participants—A sample of 475 self-identified Black women aged 21 to 70 years participated in the study, and 352 eligible participants were included in the final analysis. Of the 352 eligible participants, 48.3% were aged 21 to 30 years, 47.2% were aged 31 to 40 years, and 4.5% were aged 41 to 50 years. All survey participants identified their race as Black; among them, 4% specified Hispanic or Latino ethnicity, and 9% indicated that they held multiracial identities including White/Caucasian, Asian, and Native American backgrounds. Regarding the participants’ citizenship status, 54.3% reported that both they and their parents were born in the United States; 2.3% were not US citizens or permanent residents, 13.1% identified as first-generation Americans (born outside of the United States), and 30.4% identified as second-generation Americans (one or both parents born outside of the United States). Participant education levels (based on highest level) varied greatly: 4.5% were high school graduates, 1.1% attended trade or technical schools, 3.4% had associate’s degrees, 39.8% had bachelor’s degrees, 35.2% had master’s degrees, and 15.9% had doctorate degrees. Regarding household income, 6.3% earned less than $25,000 per year, 16.8% earned from $25,000 to $99,999, 75.6% earned from $100,000 to $499,999, and 1.4% earned $500,000 or more. Patient demographics are provided in Table 1.

Cosmetic Skin Concerns—The top 3 aging skin concerns among participants were fine lines and wrinkles (51.9%), dark circles (33.8%), and uneven skin tone (31.8%) (Table 2). Approximately 5.4% of participants reported no desire to avoid the natural aging process. Among age groups, fine lines and wrinkles were a major concern for 51.7% of 21- to 30-year-olds, 47.6% of 31- to 40-year-olds, and 43.5% of 41- to 50-year-olds. Dark circles were a major concern for 61.3% of 21- to 30-year-olds, 44.4% of 31- to 40-year-olds, and 46.8% of 41- to 50-year-olds. Uneven skin tone was a major concern for 56.2% of 21- to 30-year-olds, 46.5% of 31- to 40-year-olds, and 31.2% of 41- to 50-year-olds. There was no statistically significant association between participants’ age and their concern with aging skin concerns.

Differences in Experience and Acceptance of Cosmetic Procedures—Regarding participants’ prior experience with cosmetic procedures, 22.3% had tried 1 or more procedures. Additionally, 67.0% reported having intentions of trying cosmetic procedures in the future, while 10.8% reported no intentions. Of those who were uninterested in trying cosmetic procedures, 78.9% (30/38) believed it unnecessary while 47.3% (18/38) reported a fear of looking unnatural. Other perceived deterrents to cosmetic procedures among this subset of participants were the need to repeat treatment for lasting results (28.9% [11/38]), too expensive (31.6% [12/38]), and fear of side effects (39.5% [15/38]). A significant difference was found between participants’ age and their experience with cosmetic procedures (P=.020). Participants aged 21 to 30 years reported they were more likely to want to try cosmetic procedures in the future. Participants aged 31 to 40 years were more likely to have already tried a cosmetic procedure. Participants aged 41 to 50 years were more likely to report no desire to try cosmetic procedures in the future. There was no significant difference in cosmetic procedure acceptance according to citizenship status, education level, or household income.
Differences in Cosmetic Procedure Experience—Study participants indicated awareness of typically practiced cosmetic procedures. Of the 78 participants who have tried cosmetic procedures (Figure 1), the most common were laser hair removal (46.2% [36/78]), chemical peels (37.2% [29/78]), and microdermabrasion (24.4% [19/78])(Table 2). Age significantly influenced the type of cosmetic procedures utilized by participants (P<.001). Laser hair removal was the most common cosmetic procedure utilized by participants aged 21 to 30 years (64.7%) and chemical peels in participants aged 31 to 40 years (47.8%); participants aged 41 to 50 years reported equal use of chemical peels (50.0%) and microdermabrasion (50.0%).

Two hundred thirty-six participants reported interest in trying cosmetic procedures, specifically laser hair removal (57.6%), chemical peels (51.7%), and neuromodulators (41.9%)(Table 2). Although not statistically significant, age appeared to influence interest levels in cosmetic procedures. Participants aged 21 to 30 years and 31 to 40 years were most interested in trying laser hair removal (60.7% and 58.3%, respectively). Participants aged 41 to 50 years were most interested in trying neuromodulators (36.4%). There was no significant association between age and intention to try neuromodulators, chemical peels, or laser hair removal.
Attitudes Toward Beauty—Approximately 40.6% of participants believed that peak beauty occurs when women reach their 20s, and 38.6% believed that peak beauty occurs when women reach their 30s. Participants’ strategies for maintaining beauty were assessed through their regular use of certain skin care products. The most frequently used skin care products were face wash or cleanser (92.6%), moisturizer (90.1%), lip balm (76.1%), and facial sunscreen (62.2%). Other commonly used items were serum (34.7%), toner (34.9%), topical vitamin C (33.2%), and retinol/retinoid products (33.0%). Only 2.3% of participants reported not using any skin care products regularly.
Perceptions of Aging—Concerning perceived external age, most respondents believed they looked younger than their true age (69.9%); 24.4% believed they looked their true age, and 5.7% believed they looked older. Perception of age also varied considerably by age group, though most believed they looked younger than their true age.
Comment
This survey helped to identify trends in cosmetic procedure acceptance and utilization in Black women. As expected, younger Black women were more receptive to cosmetic procedures, which was consistent with a recent finding that cosmetic procedures tend to be more widely accepted among younger generations overall.8 Participants aged 21 to 30 years had greater intentions to try a cosmetic procedure, while those aged 31 to 40 years were more likely to have tried 1 or more cosmetic procedures already, which may be because they are just beginning to see the signs of aging and are motivated to address these concerns. Additionally, women in this age group may be more likely to have a stable source of income and be able to afford these procedures. It is important to note that the population surveyed had a much higher reported household income than the average Black household income, with most respondents reporting an average annual income of $100,000 to $499,000. Our data also showed a trend toward greater acceptance and utilization of cosmetic procedures in those with higher levels of income, though the results were not statistically significant.
Respondents were most concerned about fine lines and wrinkles, followed by dark circles and uneven skin tone. One report in the literature (N=2000) indicated that the most common cosmetic concerns in women with SOC were hyperpigmentation/dark spots (86%) and blotchy or uneven skin (80%).9 Interestingly, sunscreen was one of the more commonly used products in our survey, which historically has not been the case among individuals with SOC10 and suggests that the attitudes and perceptions of SOC patients are changing to favor more frequent sunscreen use, at least among the younger generations. Because we did not specify moisturizer vs moisturizer with sun protection factor, the use of facial sunscreen may even be underestimated in our survey.
Compared to cosmetic surgery or dermal fillers, the procedures found to be most frequently utilized in our study population—microdermabrasion, chemical peels, and laser hair removal—are less invasive and fairly accessible with minimal downtime. An interesting topic for further research would be to investigate how the willingness of women to openly share their cosmetic procedure usage has changed over time. The rise of social media and influencer culture has undoubtedly had an impact on the sharing of such information. It also would have been interesting to ask participants where they receive the majority of their health/beauty information.
All skin types are susceptible to photoaging; however, melanin is known to have a natural photoprotective effect, resulting in a lesser degree and later onset of photoaging in patients with darker vs lighter skin.11 It has been reported that individuals with SOC show signs of facial aging on average a decade later than those with lighter skin tones,12 which may be why the majority of participants believed they look younger than they truly are. As expected, dyspigmentation was among the top skin concerns in our study population. Although melanin does offer some degree of protection against UVA and UVB, melanocyte lability with inflammation may make darker skin types more susceptible to pigmentary issues.13
Study Limitations—The income levels of our study population were not representative of typical Black American households, which is a limitation. Seventy-seven percent of our study population earned more than $100,000 annually, while only 18% of Black American households earned more than $100,000 in 2019.14 Another major limitation of our study was the lack of representation from older generations, as most participants were aged 21 to 40 years, which was expected, as it is the younger generation who typically is targeted by a snowball sampling method primarily shared through social media. Additionally, because participants were recruited from the social media profiles of medical professionals, followers of these accounts may be more interested in cosmetic procedures, skewing the study results. Finally, because geographic location was not captured in our initial data collection, we were unable to determine if results from a particular location within the United States were overrepresented in the data set.
Conclusion
Although the discourse around beauty and antiaging is constantly evolving, data about Black women frequently are underrepresented in the literature. The results of this study highlight the changing attitudes and perceptions of Black women regarding beauty, aging, and minimally invasive cosmetic procedures. Dermatologists should stay abreast of current trends in this population to be able to make appropriate, culturally sensitive recommendations to their Black patients—for example, pointing them to sunscreen brands that are best suited for darker skin.
- Ahn CS, Suchonwanit P, Foy CG, et al. Hair and scalp care in African American women who exercise. JAMA Dermatol. 2016;152:579-580.
- Prendergast TI, Ong’uti SK, Ortega G, et al. Differential trends in racial preferences for cosmetic surgery procedures. Am Surg. 2011;77:1081-1085.
- American Society of Plastic Surgeons. Briefing paper: plastic surgeryfor ethnic patients. Accessed October 20, 2023. https://www.plasticsurgery.org/news/briefing-papers/briefing-paper-plastic-surgery-for-ethnic-patients
- Small K, Kelly KM, Spinelli HM. Are nurse injectors the new norm? Aesthetic Plast Surg. 2014;38:946-955.
- Quiñonez RL, Agbai ON, Burgess CM, et al. An update on cosmetic procedures in people of color. part 1: scientific background, assessment, preprocedure preparation. J Am Acad Dermatol. 2022;86:715-725.
- Alexis AF, Grimes P, Boyd C, et al. Racial and ethnic differences in self-assessed facial aging in women: results from a multinational study. Dermatol Surg. 2019;45:1635-1648.
- Talakoub L, Wesley NO. Differences in perceptions of beauty and cosmetic procedures performed in ethnic patients. Semin Cutan Med Surg. 2009;28:115-129.
- Alotaibi AS. Demographic and cultural differences in the acceptance and pursuit of cosmetic surgery: a systematic literature review. Plast Reconstr Surg Glob Open. 2021;9:E3501.
- Grimes PE. Skin and hair cosmetic issues in women of color. Dermatol Clin. 2000;18:659-665.
- Buchanan Lunsford N, Berktold J, Holman DM, et al. Skin cancer knowledge, awareness, beliefs and preventive behaviors among black and Hispanic men and women. Prev Med Rep. 2018;12:203-209.
- Alexis AF, Rossi, A. Photoaging in skin of color. Cosmet Dermatol. 2011;24:367-370.
- Vashi NA, de Castro Maymone MB, Kundu RV. Aging differences in ethnic skin. J Clin Aesthet Dermatol. 2016;9:31-38.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Tamir C, Budiman A, Noe-Bustamante L, et al. Facts about the U.S. Black population. Pew Research Center website. Published March 2, 2023. Accessed October 20, 2023. https://www.pewresearch.org/social-trends/fact-sheet/facts-about-the-us-black-population/
- Ahn CS, Suchonwanit P, Foy CG, et al. Hair and scalp care in African American women who exercise. JAMA Dermatol. 2016;152:579-580.
- Prendergast TI, Ong’uti SK, Ortega G, et al. Differential trends in racial preferences for cosmetic surgery procedures. Am Surg. 2011;77:1081-1085.
- American Society of Plastic Surgeons. Briefing paper: plastic surgeryfor ethnic patients. Accessed October 20, 2023. https://www.plasticsurgery.org/news/briefing-papers/briefing-paper-plastic-surgery-for-ethnic-patients
- Small K, Kelly KM, Spinelli HM. Are nurse injectors the new norm? Aesthetic Plast Surg. 2014;38:946-955.
- Quiñonez RL, Agbai ON, Burgess CM, et al. An update on cosmetic procedures in people of color. part 1: scientific background, assessment, preprocedure preparation. J Am Acad Dermatol. 2022;86:715-725.
- Alexis AF, Grimes P, Boyd C, et al. Racial and ethnic differences in self-assessed facial aging in women: results from a multinational study. Dermatol Surg. 2019;45:1635-1648.
- Talakoub L, Wesley NO. Differences in perceptions of beauty and cosmetic procedures performed in ethnic patients. Semin Cutan Med Surg. 2009;28:115-129.
- Alotaibi AS. Demographic and cultural differences in the acceptance and pursuit of cosmetic surgery: a systematic literature review. Plast Reconstr Surg Glob Open. 2021;9:E3501.
- Grimes PE. Skin and hair cosmetic issues in women of color. Dermatol Clin. 2000;18:659-665.
- Buchanan Lunsford N, Berktold J, Holman DM, et al. Skin cancer knowledge, awareness, beliefs and preventive behaviors among black and Hispanic men and women. Prev Med Rep. 2018;12:203-209.
- Alexis AF, Rossi, A. Photoaging in skin of color. Cosmet Dermatol. 2011;24:367-370.
- Vashi NA, de Castro Maymone MB, Kundu RV. Aging differences in ethnic skin. J Clin Aesthet Dermatol. 2016;9:31-38.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Tamir C, Budiman A, Noe-Bustamante L, et al. Facts about the U.S. Black population. Pew Research Center website. Published March 2, 2023. Accessed October 20, 2023. https://www.pewresearch.org/social-trends/fact-sheet/facts-about-the-us-black-population/
Practice Points
- Cosmetic procedures may be more widely accepted among younger Black women than older Black women.
- Age has a considerable influence on the types of cosmetic procedures that Black women are interested in trying.
- Microdermabrasion, chemical peels, and laser hair removal were the most frequently utilized procedures in this study population.
- As attitudes and perceptions of young Black women are changing and favoring more frequent sunscreen use, dermatologists should remain on top of current trends to provide culturally sensitive and relevant recommendations to patients with darker skin tones.
FDA okays drug for Duchenne muscular dystrophy
Duchenne muscular dystrophy (DMD) in patients as young as age 2 years, the company has announced Vamorolone is a structurally unique steroidal anti-inflammatory drug that potently inhibits proinflammatory NFkB pathways via high-affinity binding to the glucocorticoid receptor.
“Corticosteroids have been a first line treatment for DMD for many years, but their utility has always been limited by the side effect profile, which includes weight gain, short stature, and decreased bone density, among others,” Sharon Hesterlee, PhD, chief research officer for the Muscular Dystrophy Association, said in a statement.
The approval of vamorolone “provides people living with Duchenne, and their families, a powerful tool to treat the disease, while limiting some negative side effects associated with corticosteroids,” Dr. Hesterlee added.
The approval was based on data from the phase 2b VISION-DMD study, supplemented with safety information collected from three open-label studies.
Vamorolone was administered at doses ranging from 2-6 mg/kg/d for a period of up to 48 months.
Vamorolone demonstrated efficacy similar to that of traditional corticosteroids, with data suggesting a reduction in adverse events – notably related to bone health, growth trajectory, and behavior.
Vamorolone had received orphan drug status for DMD, as well as fast track and rare pediatric disease designations. It will be made available in the United States by Catalyst Pharmaceuticals.
A version of this article first appeared on Medscape.com .
Duchenne muscular dystrophy (DMD) in patients as young as age 2 years, the company has announced Vamorolone is a structurally unique steroidal anti-inflammatory drug that potently inhibits proinflammatory NFkB pathways via high-affinity binding to the glucocorticoid receptor.
“Corticosteroids have been a first line treatment for DMD for many years, but their utility has always been limited by the side effect profile, which includes weight gain, short stature, and decreased bone density, among others,” Sharon Hesterlee, PhD, chief research officer for the Muscular Dystrophy Association, said in a statement.
The approval of vamorolone “provides people living with Duchenne, and their families, a powerful tool to treat the disease, while limiting some negative side effects associated with corticosteroids,” Dr. Hesterlee added.
The approval was based on data from the phase 2b VISION-DMD study, supplemented with safety information collected from three open-label studies.
Vamorolone was administered at doses ranging from 2-6 mg/kg/d for a period of up to 48 months.
Vamorolone demonstrated efficacy similar to that of traditional corticosteroids, with data suggesting a reduction in adverse events – notably related to bone health, growth trajectory, and behavior.
Vamorolone had received orphan drug status for DMD, as well as fast track and rare pediatric disease designations. It will be made available in the United States by Catalyst Pharmaceuticals.
A version of this article first appeared on Medscape.com .
Duchenne muscular dystrophy (DMD) in patients as young as age 2 years, the company has announced Vamorolone is a structurally unique steroidal anti-inflammatory drug that potently inhibits proinflammatory NFkB pathways via high-affinity binding to the glucocorticoid receptor.
“Corticosteroids have been a first line treatment for DMD for many years, but their utility has always been limited by the side effect profile, which includes weight gain, short stature, and decreased bone density, among others,” Sharon Hesterlee, PhD, chief research officer for the Muscular Dystrophy Association, said in a statement.
The approval of vamorolone “provides people living with Duchenne, and their families, a powerful tool to treat the disease, while limiting some negative side effects associated with corticosteroids,” Dr. Hesterlee added.
The approval was based on data from the phase 2b VISION-DMD study, supplemented with safety information collected from three open-label studies.
Vamorolone was administered at doses ranging from 2-6 mg/kg/d for a period of up to 48 months.
Vamorolone demonstrated efficacy similar to that of traditional corticosteroids, with data suggesting a reduction in adverse events – notably related to bone health, growth trajectory, and behavior.
Vamorolone had received orphan drug status for DMD, as well as fast track and rare pediatric disease designations. It will be made available in the United States by Catalyst Pharmaceuticals.
A version of this article first appeared on Medscape.com .