User login
Potential Uses of Nonthermal Atmospheric Pressure Technology for Dermatologic Conditions in Children
Nonthermal atmospheric plasma (NTAP)(or cold atmospheric plasma [CAP]) is a rapidly developing treatment modality for a wide range of dermatologic conditions. Plasma (or ionized gas) refers to a state of matter composed of electrons, protons, and neutral atoms that generate reactive oxygen and nitrogen species.1 Plasma previously was created using thermal energy, but recent advances have allowed the creation of plasma using atmospheric pressure and room temperature; thus, NTAP can be used without causing damage to living tissue through heat.1 Plasma technology varies greatly, but it generally can be classified as either direct or indirect therapy; direct therapy uses the human body as an electrode, whereas indirect therapy creates plasma through the interaction between 2 electrode devices.1,2 When used on the skin, important dose-dependent relationships have been observed, with CAP application longer than 2 minutes being associated with increased keratinocyte and fibroblast apoptosis.2 Thus, CAP can cause diverse changes to the skin depending on application time and methodology. At adequate yet low concentrations, plasma can promote fibroblast proliferation and upregulate genes involved in collagen and transforming growth factor synthesis.1 Additionally, the reactive oxygen and nitrogen species created by NTAP have been shown to inactivate microorganisms through the destruction of biofilms, lead to diminished immune cell infiltration and cytokine release in autoimmune dermatologic conditions, and exert antitumor properties through cellular DNA damage.1-3 In dermatology, these properties can be harvested to promote wound healing at low doses and the treatment of proliferative skin conditions at high doses.1
Because of its novelty, the safety profile of NTAP is still under investigation, but preliminary studies are promising and show no damage to the skin barrier when excessive plasma exposure is avoided.4 However, dose- and time-dependent damage to cells has been shown. As a result, the exact dose of plasma considered safe is highly variable depending on the vessel, technique, and user, and future clinical research is needed to guide this methodology.4 Additionally, CAP has been shown to cause little pain at the skin surface and may lead to decreased levels of pain in healing wound sites.5 Given this promising safety profile and minimal discomfort to patients, NTAP technology remains promising for use in pediatric dermatology, but there are limited data to characterize its potential use in this population. In this systematic review, we aimed to elucidate reported applications of NTAP for skin conditions in children and discuss the trajectory of this technology in the future of pediatric dermatology.
Methodology
A comprehensive literature review was conducted to identify studies evaluating NTAP technology in pediatric populations using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines. A search of PubMed, Embase, and Web of Science articles was conducted in April 2023 using the terms nonthermal atmospheric plasma or cold atmospheric plasma. All English-language articles that described the use of NTAP as a treatment in pediatric populations or articles that described NTAP use in the treatment of common conditions in this patient group were included based on a review of the article titles and abstracts by 2 independent reviewers, followed by full-text review of relevant articles (M.G., C.L.). Any discrepancies in eligible articles were settled by a third independent researcher (M.V.). One hundred twenty studies were identified, and 95 were screened for inclusion; 9 studies met inclusion criteria and were summarized in this review.
Results
A total of 9 studies were included in this review: 3 describing the success of NTAP in pediatric populations6-8 and 6 describing the potential success of NTAP for dermatologic conditions commonly seen in children (Table).9-14
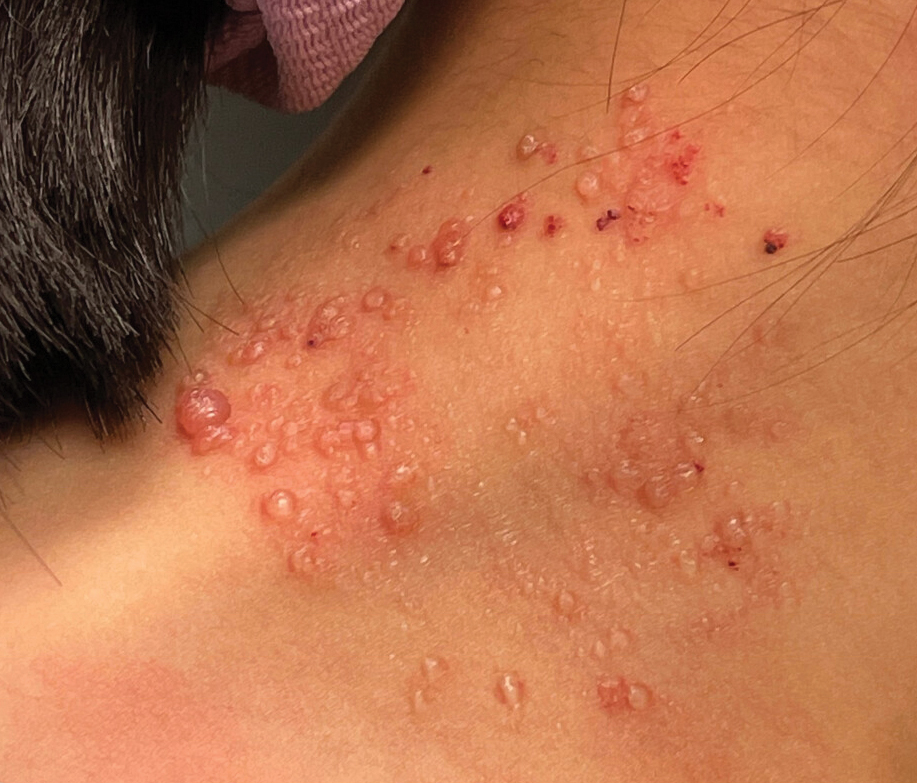
Studies Describing Success of NTAP—Three clinical reports described the efficacy of NTAP in pediatric dermatology. A case series from 2020 showed full clearance of warts in 100% of patients (n=5) with a 0% recurrence rate when NTAP treatment was applied for 2 minutes to each lesion during each treatment session with the electrode held 1 mm from the lesional surface.6 Each patient was followed up at 3 to 4 weeks, and treatment was repeated if lesions persisted. Patients reported no pain during the procedure, and no adverse effects were noted over the course of treatment.6 Second, a case report described full clearance of diaper dermatitis with no recurrence after 6 months following 6 treatments with NTAP in a 14-month-old girl.7 After treatment with econazole nitrate cream, oral antibiotics, and prednisone failed, CAP treatment was initiated. Each treatment lasted 15 minutes with 3-day time intervals between each of the 6 treatments. There were no adverse events or recurrence of rash at 6-month follow-up.7 A final case report described full clearance of molluscum contagiosum (MC), with no recurrence after 2 months following 4 treatments with NTAP in a 12-year-old boy.8 The patient had untreated MC on the face, neck, shoulder, and thighs. Lesions of the face were treated with CAP, while the other sites were treated with cantharidin using a 0.7% collodion-based solution. Four CAP treatments were performed at 1-month intervals, with CAP applied 1 mm from the lesional surfaces in a circular pattern for 2 minutes. At follow-up 2 months after the final treatment, the patient had no adverse effects and showed no pigmentary changes or scarring.8
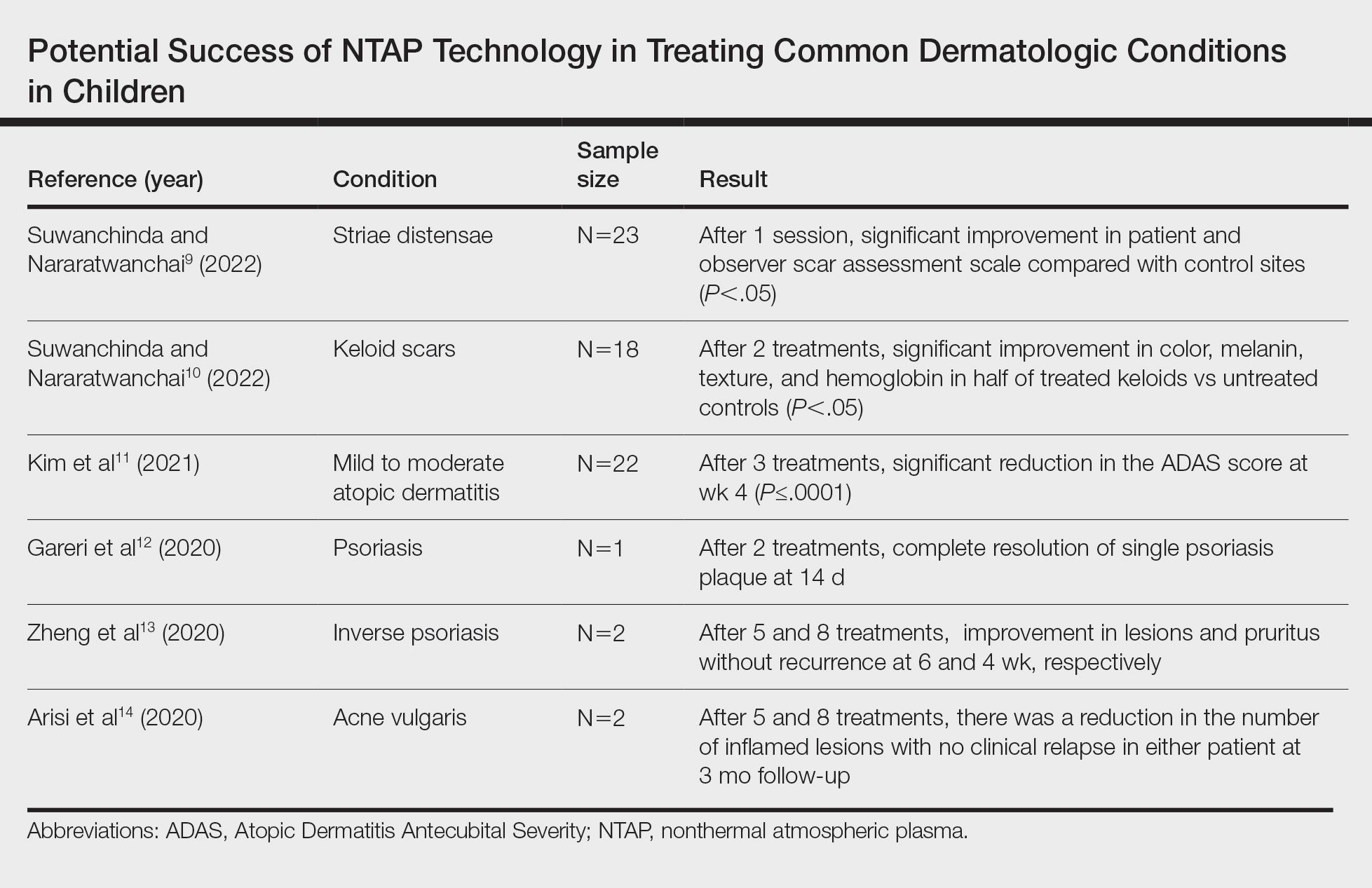
Studies Describing the Potential Success of NTAP—Beyond these studies, limited research has been done on NTAP in pediatric populations. The Table summarizes 6 additional studies completed with promising treatment results for dermatologic conditions commonly seen in children: striae distensae, keloids, atopic dermatitis, psoriasis, inverse psoriasis, and acne vulgaris. Across all reports and studies, patients showed significant improvement in their dermatologic conditions following the use of NTAP technology with limited adverse effects reported (P<.05). Suwanchinda and Nararatwanchai9 studied the use of CAP for the treatment of striae distensae. They recruited 23 patients and treated half the body with CAP biweekly for 5 sessions; the other half was left untreated. At follow-up 30 days after the final treatment, striae distensae had improved for both patient and observer assessment scores.9 Another study performed by Suwanchinda and Nararatwanchai10 looked at the efficacy of CAP in treating keloids. They recruited 18 patients, and keloid scars were treated in halves—one half treated with CAP biweekly for 5 sessions and the other left untreated. At follow-up 30 days after the final treatment, keloids significantly improved in color, melanin, texture, and hemoglobin based on assessment by the Antera 3D imaging system (Miravex Limited)(P<.05).10
Kim et al11 studied the efficacy of CAP for the treatment of atopic dermatitis in 22 patients. Each patient had mild to moderate atopic dermatitis that had not been treated with topical agents or antibiotics for at least 2 weeks prior to beginning the study. Additionally, only patients with symmetric lesions—meaning only patients with lesions on both sides of the anatomical extremities—were included. Each patient then received CAP on 1 symmetric lesion and placebo on the other. Cold atmospheric plasma treatment was done 5 mm away from the lesion, and each treatment lasted for 5 minutes. Treatments were done at weeks 0, 1, and 2, with follow-up 4 weeks after the final treatment. The clinical severity of disease was assessed at weeks 0, 1, 2, and 4. Results showed that at week 4, the mean (SD) modified Atopic Dermatitis Antecubital Severity score decreased from 33.73 (21.21) at week 0 to 13.12 (15.92). Additionally, the pruritic visual analog scale showed significant improvement with treatment vs baseline (P≤.0001).11
Two studies examined how NTAP can be used in the treatment of psoriasis. First, Gareri et al12 used CAP to treat a psoriatic plaque in a 20-year-old woman. These plaques on the left hand previously had been unresponsive to topical psoriasis treatments. The patient received 2 treatments with CAP on days 0 and 3; at 14 days, the plaque completely resolved with an itch score of 0.12 Next, Zheng et al13 treated 2 patients with NTAP for inverse psoriasis. The first patient was a 26-year-old woman with plaques in the axilla and buttocks as well as inframammary lesions that failed to respond to treatment with topicals and vitamin D analogues. She received CAP treatments 2 to 3 times weekly for 5 total treatments with application to each region occurring 1 mm from the skin surface. The lesions completely resolved with no recurrence at 6 weeks. The second patient was a 38-year-old woman with inverse psoriasis in the axilla and groin; she received treatment every 3 days for 8 total treatments, which led to complete remission, with no recurrence noted at 1 month.13
Arisi et al14 used NTAP to treat acne vulgaris in 2 patients. The first patient was a 24-year-old man with moderate acne on the face that did not improve with topicals or oral antibiotics. The patient received 5 CAP treatments with no adverse events noted. The patient discontinued treatment on his own, but the number of lesions decreased after the fifth treatment. The second patient was a 21-year-old woman with moderate facial acne that failed to respond to treatment with topicals and oral tetracycline. The patient received 8 CAP treatments and experienced a reduction in the number of lesions during treatment. There were no adverse events, and improvement was maintained at 3-month follow-up.14
Comment
Although the use of NTAP in pediatric dermatology is scarcely described in the literature, the technology will certainly have applications in the future treatment of a wide variety of pediatric disorders. In addition to the clinical success shown in several studies,6-14 this technology has been shown to cause minimal damage to skin when application time is minimized. One study conducted on ex vivo skin showed that NTAP technology can safely be used for up to 2 minutes without major DNA damage.15 Through its diverse mechanisms of action, NTAP can induce modification of proteins and cell membranes in a noninvasive manner.2 In conditions with impaired barrier function, such as atopic and diaper dermatitis, studies in mouse models have shown improvement in lesions via upregulation of mesencephalic astrocyte-derived neurotrophic factor that contributes to decreased inflammation and cell apoptosis.16 Additionally, the generation of reactive oxygen and nitrogen species has been shown to decrease Staphylococcus aureus colonization to improve atopic dermatitis lesions in patients.11
Many other proposed benefits of NTAP in dermatologic disease also have been proposed. Nonthermal atmospheric plasma has been shown to increase messenger RNA expression of proinflammatory cytokines (IL-1, IL-6) and upregulate type III collagen production in early stages of wound healing.17 Furthermore, NTAP has been shown to stimulate nuclear factor erythroid 2–related pathways involved in antioxidant production in keratinocytes, further promoting wound healing.18 Additionally, CAP has been shown to increase expression of caspases and induce mitochondrial dysfunction that promotes cell death in different cancer cell lines.19 It is clear that the exact breadth of NTAP’s biochemical effects are unknown, but the current literature shows promise for its use in cutaneous healing and cancer treatment.
Beyond its diverse applications, treatment with NTAP yields a unique advantage to pharmacologic therapies in that there is no risk for medication interactions or risk for pharmacologic adverse effects. Cantharidin is not approved by the US Food and Drug Administration but commonly is used to treat MC. It is a blister beetle extract that causes a blister to form when applied to the skin. When orally ingested, the drug is toxic to the gastrointestinal tract and kidneys because of its phosphodiesterase inhibition, a feared complication in pediatric patients who may inadvertently ingest it during treatment.20 This utility extends beyond MC, such as the beneficial outcomes described by Suwanchinda and Nararatwanchai10 in using NTAP for keloid scars. Treatment with NTAP may replace triamcinolone injections, which are commonly associated with skin atrophy and ulceration. In addition, NTAP application to the skin has been reported to be relatively painless.5 Thus, NTAP maintains a distinct advantage over other commonly used nonpharmacologic treatment options, including curettage and cryosurgery. Curettage has widely been noted to be traumatic for the patient, may be more likely to leave a mark, and is prone to user error.20 Cryosurgery is a common form of treatment for MC because it is cost-effective and has good cosmetic results; however, it is more painful than cantharidin or anesthetized curettage.21 Treatment with NTAP is an emerging therapeutic tool with an expanding role in the treatment of dermatologic patients because it provides advantages over many standard therapies due to its minimal side-effect profile involving pain and nonpharmacologic nature.
Limitations of this report include exclusion of non–English-language articles and lack of control or comparison groups to standard therapies across studies. Additionally, reports of NTAP success occurred in many conditions that are self-limited and may have resolved on their own. Regardless, we aimed to summarize how NTAP currently is being used in pediatric populations and highlight its potential uses moving forward. Given its promising safety profile and painless nature, future clinical trials should prioritize the investigation of NTAP use in common pediatric dermatologic conditions to determine if they are equal or superior to current standards of care.
- Gan L, Zhang S, Poorun D, et al. Medical applications of nonthermal atmospheric pressure plasma in dermatology. J Dtsch Dermatol Ges. 2018;16:7-13. doi:https://doi.org/10.1111/ddg.13373
- Gay-Mimbrera J, García MC, Isla-Tejera B, et al. Clinical and biological principles of cold atmospheric plasma application in skin cancer. Adv Ther. 2016;33:894-909. doi:10.1007/s12325-016-0338-1. Published correction appears in Adv Ther. 2017;34:280. doi:10.1007/s12325-016-0437-z
- Zhai SY, Kong MG, Xia YM. Cold atmospheric plasma ameliorates skin diseases involving reactive oxygen/nitrogen species-mediated functions. Front Immunol. 2022;13:868386. doi:10.3389/fimmu.2022.868386
- Tan F, Wang Y, Zhang S, et al. Plasma dermatology: skin therapy using cold atmospheric plasma. Front Oncol. 2022;12:918484. doi:10.3389/fonc.2022.918484
- van Welzen A, Hoch M, Wahl P, et al. The response and tolerability of a novel cold atmospheric plasma wound dressing for the healing of split skin graft donor sites: a controlled pilot study. Skin Pharmacol Physiol. 2021;34:328-336. doi:10.1159/000517524
- Friedman PC, Fridman G, Fridman A. Using cold plasma to treat warts in children: a case series. Pediatr Dermatol. 2020;37:706-709. doi:10.1111/pde.14180
- Zhang C, Zhao J, Gao Y, et al. Cold atmospheric plasma treatment for diaper dermatitis: a case report [published online January 27, 2021]. Dermatol Ther. 2021;34:E14739. doi:10.1111/dth.14739
- Friedman PC, Fridman G, Fridman A. Cold atmospheric pressure plasma clears molluscum contagiosum. Exp Dermatol. 2023;32:562-563. doi:10.1111/exd.14695
- Suwanchinda A, Nararatwanchai T. The efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of striae distensae: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6805-6814. doi:10.1111/jocd.15458
- Suwanchinda A, Nararatwanchai T. Efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of keloid: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6788-6797. doi:10.1111/jocd.15397
- Kim YJ, Lim DJ, Lee MY, et al. Prospective, comparative clinical pilot study of cold atmospheric plasma device in the treatment of atopic dermatitis. Sci Rep. 2021;11:14461. doi:10.1038/s41598-021-93941-y
- Gareri C, Bennardo L, De Masi G. Use of a new cold plasma tool for psoriasis treatment: a case report. SAGE Open Med Case Rep. 2020;8:2050313X20922709. doi:10.1177/2050313X20922709
- Zheng L, Gao J, Cao Y, et al. Two case reports of inverse psoriasis treated with cold atmospheric plasma. Dermatol Ther. 2020;33:E14257. doi:10.1111/dth.14257
- Arisi M, Venturuzzo A, Gelmetti A, et al. Cold atmospheric plasma (CAP) as a promising therapeutic option for mild to moderate acne vulgaris: clinical and non-invasive evaluation of two cases. Clin Plasma Med. 2020;19-20:100110.
- Isbary G, Köritzer J, Mitra A, et al. Ex vivo human skin experiments for the evaluation of safety of new cold atmospheric plasma devices. Clin Plasma Med. 2013;1:36-44.
- Sun T, Zhang X, Hou C, et al. Cold plasma irradiation attenuates atopic dermatitis via enhancing HIF-1α-induced MANF transcription expression. Front Immunol. 2022;13:941219. doi:10.3389/fimmu.2022.941219
- Eggers B, Marciniak J, Memmert S, et al. The beneficial effect of cold atmospheric plasma on parameters of molecules and cell function involved in wound healing in human osteoblast-like cells in vitro. Odontology. 2020;108:607-616. doi:10.1007/s10266-020-00487-y
- Conway GE, He Z, Hutanu AL, et al. Cold atmospheric plasma induces accumulation of lysosomes and caspase-independent cell death in U373MG glioblastoma multiforme cells. Sci Rep. 2019;9:12891. doi:10.1038/s41598-019-49013-3
- Schmidt A, Dietrich S, Steuer A, et al. Non-thermal plasma activates human keratinocytes by stimulation of antioxidant and phase II pathways. J Biol Chem. 2015;290:6731-6750. doi:10.1074/jbc.M114.603555
- Silverberg NB. Pediatric molluscum contagiosum. Pediatr Drugs. 2003;5:505-511. doi:10.2165/00148581-200305080-00001
- Cotton DW, Cooper C, Barrett DF, et al. Severe atypical molluscum contagiosum infection in an immunocompromised host. Br J Dermatol. 1987;116:871-876. doi:10.1111/j.1365-2133.1987.tb04908.x
Nonthermal atmospheric plasma (NTAP)(or cold atmospheric plasma [CAP]) is a rapidly developing treatment modality for a wide range of dermatologic conditions. Plasma (or ionized gas) refers to a state of matter composed of electrons, protons, and neutral atoms that generate reactive oxygen and nitrogen species.1 Plasma previously was created using thermal energy, but recent advances have allowed the creation of plasma using atmospheric pressure and room temperature; thus, NTAP can be used without causing damage to living tissue through heat.1 Plasma technology varies greatly, but it generally can be classified as either direct or indirect therapy; direct therapy uses the human body as an electrode, whereas indirect therapy creates plasma through the interaction between 2 electrode devices.1,2 When used on the skin, important dose-dependent relationships have been observed, with CAP application longer than 2 minutes being associated with increased keratinocyte and fibroblast apoptosis.2 Thus, CAP can cause diverse changes to the skin depending on application time and methodology. At adequate yet low concentrations, plasma can promote fibroblast proliferation and upregulate genes involved in collagen and transforming growth factor synthesis.1 Additionally, the reactive oxygen and nitrogen species created by NTAP have been shown to inactivate microorganisms through the destruction of biofilms, lead to diminished immune cell infiltration and cytokine release in autoimmune dermatologic conditions, and exert antitumor properties through cellular DNA damage.1-3 In dermatology, these properties can be harvested to promote wound healing at low doses and the treatment of proliferative skin conditions at high doses.1
Because of its novelty, the safety profile of NTAP is still under investigation, but preliminary studies are promising and show no damage to the skin barrier when excessive plasma exposure is avoided.4 However, dose- and time-dependent damage to cells has been shown. As a result, the exact dose of plasma considered safe is highly variable depending on the vessel, technique, and user, and future clinical research is needed to guide this methodology.4 Additionally, CAP has been shown to cause little pain at the skin surface and may lead to decreased levels of pain in healing wound sites.5 Given this promising safety profile and minimal discomfort to patients, NTAP technology remains promising for use in pediatric dermatology, but there are limited data to characterize its potential use in this population. In this systematic review, we aimed to elucidate reported applications of NTAP for skin conditions in children and discuss the trajectory of this technology in the future of pediatric dermatology.
Methodology
A comprehensive literature review was conducted to identify studies evaluating NTAP technology in pediatric populations using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines. A search of PubMed, Embase, and Web of Science articles was conducted in April 2023 using the terms nonthermal atmospheric plasma or cold atmospheric plasma. All English-language articles that described the use of NTAP as a treatment in pediatric populations or articles that described NTAP use in the treatment of common conditions in this patient group were included based on a review of the article titles and abstracts by 2 independent reviewers, followed by full-text review of relevant articles (M.G., C.L.). Any discrepancies in eligible articles were settled by a third independent researcher (M.V.). One hundred twenty studies were identified, and 95 were screened for inclusion; 9 studies met inclusion criteria and were summarized in this review.
Results
A total of 9 studies were included in this review: 3 describing the success of NTAP in pediatric populations6-8 and 6 describing the potential success of NTAP for dermatologic conditions commonly seen in children (Table).9-14

Studies Describing Success of NTAP—Three clinical reports described the efficacy of NTAP in pediatric dermatology. A case series from 2020 showed full clearance of warts in 100% of patients (n=5) with a 0% recurrence rate when NTAP treatment was applied for 2 minutes to each lesion during each treatment session with the electrode held 1 mm from the lesional surface.6 Each patient was followed up at 3 to 4 weeks, and treatment was repeated if lesions persisted. Patients reported no pain during the procedure, and no adverse effects were noted over the course of treatment.6 Second, a case report described full clearance of diaper dermatitis with no recurrence after 6 months following 6 treatments with NTAP in a 14-month-old girl.7 After treatment with econazole nitrate cream, oral antibiotics, and prednisone failed, CAP treatment was initiated. Each treatment lasted 15 minutes with 3-day time intervals between each of the 6 treatments. There were no adverse events or recurrence of rash at 6-month follow-up.7 A final case report described full clearance of molluscum contagiosum (MC), with no recurrence after 2 months following 4 treatments with NTAP in a 12-year-old boy.8 The patient had untreated MC on the face, neck, shoulder, and thighs. Lesions of the face were treated with CAP, while the other sites were treated with cantharidin using a 0.7% collodion-based solution. Four CAP treatments were performed at 1-month intervals, with CAP applied 1 mm from the lesional surfaces in a circular pattern for 2 minutes. At follow-up 2 months after the final treatment, the patient had no adverse effects and showed no pigmentary changes or scarring.8
Studies Describing the Potential Success of NTAP—Beyond these studies, limited research has been done on NTAP in pediatric populations. The Table summarizes 6 additional studies completed with promising treatment results for dermatologic conditions commonly seen in children: striae distensae, keloids, atopic dermatitis, psoriasis, inverse psoriasis, and acne vulgaris. Across all reports and studies, patients showed significant improvement in their dermatologic conditions following the use of NTAP technology with limited adverse effects reported (P<.05). Suwanchinda and Nararatwanchai9 studied the use of CAP for the treatment of striae distensae. They recruited 23 patients and treated half the body with CAP biweekly for 5 sessions; the other half was left untreated. At follow-up 30 days after the final treatment, striae distensae had improved for both patient and observer assessment scores.9 Another study performed by Suwanchinda and Nararatwanchai10 looked at the efficacy of CAP in treating keloids. They recruited 18 patients, and keloid scars were treated in halves—one half treated with CAP biweekly for 5 sessions and the other left untreated. At follow-up 30 days after the final treatment, keloids significantly improved in color, melanin, texture, and hemoglobin based on assessment by the Antera 3D imaging system (Miravex Limited)(P<.05).10
Kim et al11 studied the efficacy of CAP for the treatment of atopic dermatitis in 22 patients. Each patient had mild to moderate atopic dermatitis that had not been treated with topical agents or antibiotics for at least 2 weeks prior to beginning the study. Additionally, only patients with symmetric lesions—meaning only patients with lesions on both sides of the anatomical extremities—were included. Each patient then received CAP on 1 symmetric lesion and placebo on the other. Cold atmospheric plasma treatment was done 5 mm away from the lesion, and each treatment lasted for 5 minutes. Treatments were done at weeks 0, 1, and 2, with follow-up 4 weeks after the final treatment. The clinical severity of disease was assessed at weeks 0, 1, 2, and 4. Results showed that at week 4, the mean (SD) modified Atopic Dermatitis Antecubital Severity score decreased from 33.73 (21.21) at week 0 to 13.12 (15.92). Additionally, the pruritic visual analog scale showed significant improvement with treatment vs baseline (P≤.0001).11
Two studies examined how NTAP can be used in the treatment of psoriasis. First, Gareri et al12 used CAP to treat a psoriatic plaque in a 20-year-old woman. These plaques on the left hand previously had been unresponsive to topical psoriasis treatments. The patient received 2 treatments with CAP on days 0 and 3; at 14 days, the plaque completely resolved with an itch score of 0.12 Next, Zheng et al13 treated 2 patients with NTAP for inverse psoriasis. The first patient was a 26-year-old woman with plaques in the axilla and buttocks as well as inframammary lesions that failed to respond to treatment with topicals and vitamin D analogues. She received CAP treatments 2 to 3 times weekly for 5 total treatments with application to each region occurring 1 mm from the skin surface. The lesions completely resolved with no recurrence at 6 weeks. The second patient was a 38-year-old woman with inverse psoriasis in the axilla and groin; she received treatment every 3 days for 8 total treatments, which led to complete remission, with no recurrence noted at 1 month.13
Arisi et al14 used NTAP to treat acne vulgaris in 2 patients. The first patient was a 24-year-old man with moderate acne on the face that did not improve with topicals or oral antibiotics. The patient received 5 CAP treatments with no adverse events noted. The patient discontinued treatment on his own, but the number of lesions decreased after the fifth treatment. The second patient was a 21-year-old woman with moderate facial acne that failed to respond to treatment with topicals and oral tetracycline. The patient received 8 CAP treatments and experienced a reduction in the number of lesions during treatment. There were no adverse events, and improvement was maintained at 3-month follow-up.14
Comment
Although the use of NTAP in pediatric dermatology is scarcely described in the literature, the technology will certainly have applications in the future treatment of a wide variety of pediatric disorders. In addition to the clinical success shown in several studies,6-14 this technology has been shown to cause minimal damage to skin when application time is minimized. One study conducted on ex vivo skin showed that NTAP technology can safely be used for up to 2 minutes without major DNA damage.15 Through its diverse mechanisms of action, NTAP can induce modification of proteins and cell membranes in a noninvasive manner.2 In conditions with impaired barrier function, such as atopic and diaper dermatitis, studies in mouse models have shown improvement in lesions via upregulation of mesencephalic astrocyte-derived neurotrophic factor that contributes to decreased inflammation and cell apoptosis.16 Additionally, the generation of reactive oxygen and nitrogen species has been shown to decrease Staphylococcus aureus colonization to improve atopic dermatitis lesions in patients.11
Many other proposed benefits of NTAP in dermatologic disease also have been proposed. Nonthermal atmospheric plasma has been shown to increase messenger RNA expression of proinflammatory cytokines (IL-1, IL-6) and upregulate type III collagen production in early stages of wound healing.17 Furthermore, NTAP has been shown to stimulate nuclear factor erythroid 2–related pathways involved in antioxidant production in keratinocytes, further promoting wound healing.18 Additionally, CAP has been shown to increase expression of caspases and induce mitochondrial dysfunction that promotes cell death in different cancer cell lines.19 It is clear that the exact breadth of NTAP’s biochemical effects are unknown, but the current literature shows promise for its use in cutaneous healing and cancer treatment.
Beyond its diverse applications, treatment with NTAP yields a unique advantage to pharmacologic therapies in that there is no risk for medication interactions or risk for pharmacologic adverse effects. Cantharidin is not approved by the US Food and Drug Administration but commonly is used to treat MC. It is a blister beetle extract that causes a blister to form when applied to the skin. When orally ingested, the drug is toxic to the gastrointestinal tract and kidneys because of its phosphodiesterase inhibition, a feared complication in pediatric patients who may inadvertently ingest it during treatment.20 This utility extends beyond MC, such as the beneficial outcomes described by Suwanchinda and Nararatwanchai10 in using NTAP for keloid scars. Treatment with NTAP may replace triamcinolone injections, which are commonly associated with skin atrophy and ulceration. In addition, NTAP application to the skin has been reported to be relatively painless.5 Thus, NTAP maintains a distinct advantage over other commonly used nonpharmacologic treatment options, including curettage and cryosurgery. Curettage has widely been noted to be traumatic for the patient, may be more likely to leave a mark, and is prone to user error.20 Cryosurgery is a common form of treatment for MC because it is cost-effective and has good cosmetic results; however, it is more painful than cantharidin or anesthetized curettage.21 Treatment with NTAP is an emerging therapeutic tool with an expanding role in the treatment of dermatologic patients because it provides advantages over many standard therapies due to its minimal side-effect profile involving pain and nonpharmacologic nature.
Limitations of this report include exclusion of non–English-language articles and lack of control or comparison groups to standard therapies across studies. Additionally, reports of NTAP success occurred in many conditions that are self-limited and may have resolved on their own. Regardless, we aimed to summarize how NTAP currently is being used in pediatric populations and highlight its potential uses moving forward. Given its promising safety profile and painless nature, future clinical trials should prioritize the investigation of NTAP use in common pediatric dermatologic conditions to determine if they are equal or superior to current standards of care.
Nonthermal atmospheric plasma (NTAP)(or cold atmospheric plasma [CAP]) is a rapidly developing treatment modality for a wide range of dermatologic conditions. Plasma (or ionized gas) refers to a state of matter composed of electrons, protons, and neutral atoms that generate reactive oxygen and nitrogen species.1 Plasma previously was created using thermal energy, but recent advances have allowed the creation of plasma using atmospheric pressure and room temperature; thus, NTAP can be used without causing damage to living tissue through heat.1 Plasma technology varies greatly, but it generally can be classified as either direct or indirect therapy; direct therapy uses the human body as an electrode, whereas indirect therapy creates plasma through the interaction between 2 electrode devices.1,2 When used on the skin, important dose-dependent relationships have been observed, with CAP application longer than 2 minutes being associated with increased keratinocyte and fibroblast apoptosis.2 Thus, CAP can cause diverse changes to the skin depending on application time and methodology. At adequate yet low concentrations, plasma can promote fibroblast proliferation and upregulate genes involved in collagen and transforming growth factor synthesis.1 Additionally, the reactive oxygen and nitrogen species created by NTAP have been shown to inactivate microorganisms through the destruction of biofilms, lead to diminished immune cell infiltration and cytokine release in autoimmune dermatologic conditions, and exert antitumor properties through cellular DNA damage.1-3 In dermatology, these properties can be harvested to promote wound healing at low doses and the treatment of proliferative skin conditions at high doses.1
Because of its novelty, the safety profile of NTAP is still under investigation, but preliminary studies are promising and show no damage to the skin barrier when excessive plasma exposure is avoided.4 However, dose- and time-dependent damage to cells has been shown. As a result, the exact dose of plasma considered safe is highly variable depending on the vessel, technique, and user, and future clinical research is needed to guide this methodology.4 Additionally, CAP has been shown to cause little pain at the skin surface and may lead to decreased levels of pain in healing wound sites.5 Given this promising safety profile and minimal discomfort to patients, NTAP technology remains promising for use in pediatric dermatology, but there are limited data to characterize its potential use in this population. In this systematic review, we aimed to elucidate reported applications of NTAP for skin conditions in children and discuss the trajectory of this technology in the future of pediatric dermatology.
Methodology
A comprehensive literature review was conducted to identify studies evaluating NTAP technology in pediatric populations using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines. A search of PubMed, Embase, and Web of Science articles was conducted in April 2023 using the terms nonthermal atmospheric plasma or cold atmospheric plasma. All English-language articles that described the use of NTAP as a treatment in pediatric populations or articles that described NTAP use in the treatment of common conditions in this patient group were included based on a review of the article titles and abstracts by 2 independent reviewers, followed by full-text review of relevant articles (M.G., C.L.). Any discrepancies in eligible articles were settled by a third independent researcher (M.V.). One hundred twenty studies were identified, and 95 were screened for inclusion; 9 studies met inclusion criteria and were summarized in this review.
Results
A total of 9 studies were included in this review: 3 describing the success of NTAP in pediatric populations6-8 and 6 describing the potential success of NTAP for dermatologic conditions commonly seen in children (Table).9-14

Studies Describing Success of NTAP—Three clinical reports described the efficacy of NTAP in pediatric dermatology. A case series from 2020 showed full clearance of warts in 100% of patients (n=5) with a 0% recurrence rate when NTAP treatment was applied for 2 minutes to each lesion during each treatment session with the electrode held 1 mm from the lesional surface.6 Each patient was followed up at 3 to 4 weeks, and treatment was repeated if lesions persisted. Patients reported no pain during the procedure, and no adverse effects were noted over the course of treatment.6 Second, a case report described full clearance of diaper dermatitis with no recurrence after 6 months following 6 treatments with NTAP in a 14-month-old girl.7 After treatment with econazole nitrate cream, oral antibiotics, and prednisone failed, CAP treatment was initiated. Each treatment lasted 15 minutes with 3-day time intervals between each of the 6 treatments. There were no adverse events or recurrence of rash at 6-month follow-up.7 A final case report described full clearance of molluscum contagiosum (MC), with no recurrence after 2 months following 4 treatments with NTAP in a 12-year-old boy.8 The patient had untreated MC on the face, neck, shoulder, and thighs. Lesions of the face were treated with CAP, while the other sites were treated with cantharidin using a 0.7% collodion-based solution. Four CAP treatments were performed at 1-month intervals, with CAP applied 1 mm from the lesional surfaces in a circular pattern for 2 minutes. At follow-up 2 months after the final treatment, the patient had no adverse effects and showed no pigmentary changes or scarring.8
Studies Describing the Potential Success of NTAP—Beyond these studies, limited research has been done on NTAP in pediatric populations. The Table summarizes 6 additional studies completed with promising treatment results for dermatologic conditions commonly seen in children: striae distensae, keloids, atopic dermatitis, psoriasis, inverse psoriasis, and acne vulgaris. Across all reports and studies, patients showed significant improvement in their dermatologic conditions following the use of NTAP technology with limited adverse effects reported (P<.05). Suwanchinda and Nararatwanchai9 studied the use of CAP for the treatment of striae distensae. They recruited 23 patients and treated half the body with CAP biweekly for 5 sessions; the other half was left untreated. At follow-up 30 days after the final treatment, striae distensae had improved for both patient and observer assessment scores.9 Another study performed by Suwanchinda and Nararatwanchai10 looked at the efficacy of CAP in treating keloids. They recruited 18 patients, and keloid scars were treated in halves—one half treated with CAP biweekly for 5 sessions and the other left untreated. At follow-up 30 days after the final treatment, keloids significantly improved in color, melanin, texture, and hemoglobin based on assessment by the Antera 3D imaging system (Miravex Limited)(P<.05).10
Kim et al11 studied the efficacy of CAP for the treatment of atopic dermatitis in 22 patients. Each patient had mild to moderate atopic dermatitis that had not been treated with topical agents or antibiotics for at least 2 weeks prior to beginning the study. Additionally, only patients with symmetric lesions—meaning only patients with lesions on both sides of the anatomical extremities—were included. Each patient then received CAP on 1 symmetric lesion and placebo on the other. Cold atmospheric plasma treatment was done 5 mm away from the lesion, and each treatment lasted for 5 minutes. Treatments were done at weeks 0, 1, and 2, with follow-up 4 weeks after the final treatment. The clinical severity of disease was assessed at weeks 0, 1, 2, and 4. Results showed that at week 4, the mean (SD) modified Atopic Dermatitis Antecubital Severity score decreased from 33.73 (21.21) at week 0 to 13.12 (15.92). Additionally, the pruritic visual analog scale showed significant improvement with treatment vs baseline (P≤.0001).11
Two studies examined how NTAP can be used in the treatment of psoriasis. First, Gareri et al12 used CAP to treat a psoriatic plaque in a 20-year-old woman. These plaques on the left hand previously had been unresponsive to topical psoriasis treatments. The patient received 2 treatments with CAP on days 0 and 3; at 14 days, the plaque completely resolved with an itch score of 0.12 Next, Zheng et al13 treated 2 patients with NTAP for inverse psoriasis. The first patient was a 26-year-old woman with plaques in the axilla and buttocks as well as inframammary lesions that failed to respond to treatment with topicals and vitamin D analogues. She received CAP treatments 2 to 3 times weekly for 5 total treatments with application to each region occurring 1 mm from the skin surface. The lesions completely resolved with no recurrence at 6 weeks. The second patient was a 38-year-old woman with inverse psoriasis in the axilla and groin; she received treatment every 3 days for 8 total treatments, which led to complete remission, with no recurrence noted at 1 month.13
Arisi et al14 used NTAP to treat acne vulgaris in 2 patients. The first patient was a 24-year-old man with moderate acne on the face that did not improve with topicals or oral antibiotics. The patient received 5 CAP treatments with no adverse events noted. The patient discontinued treatment on his own, but the number of lesions decreased after the fifth treatment. The second patient was a 21-year-old woman with moderate facial acne that failed to respond to treatment with topicals and oral tetracycline. The patient received 8 CAP treatments and experienced a reduction in the number of lesions during treatment. There were no adverse events, and improvement was maintained at 3-month follow-up.14
Comment
Although the use of NTAP in pediatric dermatology is scarcely described in the literature, the technology will certainly have applications in the future treatment of a wide variety of pediatric disorders. In addition to the clinical success shown in several studies,6-14 this technology has been shown to cause minimal damage to skin when application time is minimized. One study conducted on ex vivo skin showed that NTAP technology can safely be used for up to 2 minutes without major DNA damage.15 Through its diverse mechanisms of action, NTAP can induce modification of proteins and cell membranes in a noninvasive manner.2 In conditions with impaired barrier function, such as atopic and diaper dermatitis, studies in mouse models have shown improvement in lesions via upregulation of mesencephalic astrocyte-derived neurotrophic factor that contributes to decreased inflammation and cell apoptosis.16 Additionally, the generation of reactive oxygen and nitrogen species has been shown to decrease Staphylococcus aureus colonization to improve atopic dermatitis lesions in patients.11
Many other proposed benefits of NTAP in dermatologic disease also have been proposed. Nonthermal atmospheric plasma has been shown to increase messenger RNA expression of proinflammatory cytokines (IL-1, IL-6) and upregulate type III collagen production in early stages of wound healing.17 Furthermore, NTAP has been shown to stimulate nuclear factor erythroid 2–related pathways involved in antioxidant production in keratinocytes, further promoting wound healing.18 Additionally, CAP has been shown to increase expression of caspases and induce mitochondrial dysfunction that promotes cell death in different cancer cell lines.19 It is clear that the exact breadth of NTAP’s biochemical effects are unknown, but the current literature shows promise for its use in cutaneous healing and cancer treatment.
Beyond its diverse applications, treatment with NTAP yields a unique advantage to pharmacologic therapies in that there is no risk for medication interactions or risk for pharmacologic adverse effects. Cantharidin is not approved by the US Food and Drug Administration but commonly is used to treat MC. It is a blister beetle extract that causes a blister to form when applied to the skin. When orally ingested, the drug is toxic to the gastrointestinal tract and kidneys because of its phosphodiesterase inhibition, a feared complication in pediatric patients who may inadvertently ingest it during treatment.20 This utility extends beyond MC, such as the beneficial outcomes described by Suwanchinda and Nararatwanchai10 in using NTAP for keloid scars. Treatment with NTAP may replace triamcinolone injections, which are commonly associated with skin atrophy and ulceration. In addition, NTAP application to the skin has been reported to be relatively painless.5 Thus, NTAP maintains a distinct advantage over other commonly used nonpharmacologic treatment options, including curettage and cryosurgery. Curettage has widely been noted to be traumatic for the patient, may be more likely to leave a mark, and is prone to user error.20 Cryosurgery is a common form of treatment for MC because it is cost-effective and has good cosmetic results; however, it is more painful than cantharidin or anesthetized curettage.21 Treatment with NTAP is an emerging therapeutic tool with an expanding role in the treatment of dermatologic patients because it provides advantages over many standard therapies due to its minimal side-effect profile involving pain and nonpharmacologic nature.
Limitations of this report include exclusion of non–English-language articles and lack of control or comparison groups to standard therapies across studies. Additionally, reports of NTAP success occurred in many conditions that are self-limited and may have resolved on their own. Regardless, we aimed to summarize how NTAP currently is being used in pediatric populations and highlight its potential uses moving forward. Given its promising safety profile and painless nature, future clinical trials should prioritize the investigation of NTAP use in common pediatric dermatologic conditions to determine if they are equal or superior to current standards of care.
- Gan L, Zhang S, Poorun D, et al. Medical applications of nonthermal atmospheric pressure plasma in dermatology. J Dtsch Dermatol Ges. 2018;16:7-13. doi:https://doi.org/10.1111/ddg.13373
- Gay-Mimbrera J, García MC, Isla-Tejera B, et al. Clinical and biological principles of cold atmospheric plasma application in skin cancer. Adv Ther. 2016;33:894-909. doi:10.1007/s12325-016-0338-1. Published correction appears in Adv Ther. 2017;34:280. doi:10.1007/s12325-016-0437-z
- Zhai SY, Kong MG, Xia YM. Cold atmospheric plasma ameliorates skin diseases involving reactive oxygen/nitrogen species-mediated functions. Front Immunol. 2022;13:868386. doi:10.3389/fimmu.2022.868386
- Tan F, Wang Y, Zhang S, et al. Plasma dermatology: skin therapy using cold atmospheric plasma. Front Oncol. 2022;12:918484. doi:10.3389/fonc.2022.918484
- van Welzen A, Hoch M, Wahl P, et al. The response and tolerability of a novel cold atmospheric plasma wound dressing for the healing of split skin graft donor sites: a controlled pilot study. Skin Pharmacol Physiol. 2021;34:328-336. doi:10.1159/000517524
- Friedman PC, Fridman G, Fridman A. Using cold plasma to treat warts in children: a case series. Pediatr Dermatol. 2020;37:706-709. doi:10.1111/pde.14180
- Zhang C, Zhao J, Gao Y, et al. Cold atmospheric plasma treatment for diaper dermatitis: a case report [published online January 27, 2021]. Dermatol Ther. 2021;34:E14739. doi:10.1111/dth.14739
- Friedman PC, Fridman G, Fridman A. Cold atmospheric pressure plasma clears molluscum contagiosum. Exp Dermatol. 2023;32:562-563. doi:10.1111/exd.14695
- Suwanchinda A, Nararatwanchai T. The efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of striae distensae: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6805-6814. doi:10.1111/jocd.15458
- Suwanchinda A, Nararatwanchai T. Efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of keloid: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6788-6797. doi:10.1111/jocd.15397
- Kim YJ, Lim DJ, Lee MY, et al. Prospective, comparative clinical pilot study of cold atmospheric plasma device in the treatment of atopic dermatitis. Sci Rep. 2021;11:14461. doi:10.1038/s41598-021-93941-y
- Gareri C, Bennardo L, De Masi G. Use of a new cold plasma tool for psoriasis treatment: a case report. SAGE Open Med Case Rep. 2020;8:2050313X20922709. doi:10.1177/2050313X20922709
- Zheng L, Gao J, Cao Y, et al. Two case reports of inverse psoriasis treated with cold atmospheric plasma. Dermatol Ther. 2020;33:E14257. doi:10.1111/dth.14257
- Arisi M, Venturuzzo A, Gelmetti A, et al. Cold atmospheric plasma (CAP) as a promising therapeutic option for mild to moderate acne vulgaris: clinical and non-invasive evaluation of two cases. Clin Plasma Med. 2020;19-20:100110.
- Isbary G, Köritzer J, Mitra A, et al. Ex vivo human skin experiments for the evaluation of safety of new cold atmospheric plasma devices. Clin Plasma Med. 2013;1:36-44.
- Sun T, Zhang X, Hou C, et al. Cold plasma irradiation attenuates atopic dermatitis via enhancing HIF-1α-induced MANF transcription expression. Front Immunol. 2022;13:941219. doi:10.3389/fimmu.2022.941219
- Eggers B, Marciniak J, Memmert S, et al. The beneficial effect of cold atmospheric plasma on parameters of molecules and cell function involved in wound healing in human osteoblast-like cells in vitro. Odontology. 2020;108:607-616. doi:10.1007/s10266-020-00487-y
- Conway GE, He Z, Hutanu AL, et al. Cold atmospheric plasma induces accumulation of lysosomes and caspase-independent cell death in U373MG glioblastoma multiforme cells. Sci Rep. 2019;9:12891. doi:10.1038/s41598-019-49013-3
- Schmidt A, Dietrich S, Steuer A, et al. Non-thermal plasma activates human keratinocytes by stimulation of antioxidant and phase II pathways. J Biol Chem. 2015;290:6731-6750. doi:10.1074/jbc.M114.603555
- Silverberg NB. Pediatric molluscum contagiosum. Pediatr Drugs. 2003;5:505-511. doi:10.2165/00148581-200305080-00001
- Cotton DW, Cooper C, Barrett DF, et al. Severe atypical molluscum contagiosum infection in an immunocompromised host. Br J Dermatol. 1987;116:871-876. doi:10.1111/j.1365-2133.1987.tb04908.x
- Gan L, Zhang S, Poorun D, et al. Medical applications of nonthermal atmospheric pressure plasma in dermatology. J Dtsch Dermatol Ges. 2018;16:7-13. doi:https://doi.org/10.1111/ddg.13373
- Gay-Mimbrera J, García MC, Isla-Tejera B, et al. Clinical and biological principles of cold atmospheric plasma application in skin cancer. Adv Ther. 2016;33:894-909. doi:10.1007/s12325-016-0338-1. Published correction appears in Adv Ther. 2017;34:280. doi:10.1007/s12325-016-0437-z
- Zhai SY, Kong MG, Xia YM. Cold atmospheric plasma ameliorates skin diseases involving reactive oxygen/nitrogen species-mediated functions. Front Immunol. 2022;13:868386. doi:10.3389/fimmu.2022.868386
- Tan F, Wang Y, Zhang S, et al. Plasma dermatology: skin therapy using cold atmospheric plasma. Front Oncol. 2022;12:918484. doi:10.3389/fonc.2022.918484
- van Welzen A, Hoch M, Wahl P, et al. The response and tolerability of a novel cold atmospheric plasma wound dressing for the healing of split skin graft donor sites: a controlled pilot study. Skin Pharmacol Physiol. 2021;34:328-336. doi:10.1159/000517524
- Friedman PC, Fridman G, Fridman A. Using cold plasma to treat warts in children: a case series. Pediatr Dermatol. 2020;37:706-709. doi:10.1111/pde.14180
- Zhang C, Zhao J, Gao Y, et al. Cold atmospheric plasma treatment for diaper dermatitis: a case report [published online January 27, 2021]. Dermatol Ther. 2021;34:E14739. doi:10.1111/dth.14739
- Friedman PC, Fridman G, Fridman A. Cold atmospheric pressure plasma clears molluscum contagiosum. Exp Dermatol. 2023;32:562-563. doi:10.1111/exd.14695
- Suwanchinda A, Nararatwanchai T. The efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of striae distensae: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6805-6814. doi:10.1111/jocd.15458
- Suwanchinda A, Nararatwanchai T. Efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of keloid: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6788-6797. doi:10.1111/jocd.15397
- Kim YJ, Lim DJ, Lee MY, et al. Prospective, comparative clinical pilot study of cold atmospheric plasma device in the treatment of atopic dermatitis. Sci Rep. 2021;11:14461. doi:10.1038/s41598-021-93941-y
- Gareri C, Bennardo L, De Masi G. Use of a new cold plasma tool for psoriasis treatment: a case report. SAGE Open Med Case Rep. 2020;8:2050313X20922709. doi:10.1177/2050313X20922709
- Zheng L, Gao J, Cao Y, et al. Two case reports of inverse psoriasis treated with cold atmospheric plasma. Dermatol Ther. 2020;33:E14257. doi:10.1111/dth.14257
- Arisi M, Venturuzzo A, Gelmetti A, et al. Cold atmospheric plasma (CAP) as a promising therapeutic option for mild to moderate acne vulgaris: clinical and non-invasive evaluation of two cases. Clin Plasma Med. 2020;19-20:100110.
- Isbary G, Köritzer J, Mitra A, et al. Ex vivo human skin experiments for the evaluation of safety of new cold atmospheric plasma devices. Clin Plasma Med. 2013;1:36-44.
- Sun T, Zhang X, Hou C, et al. Cold plasma irradiation attenuates atopic dermatitis via enhancing HIF-1α-induced MANF transcription expression. Front Immunol. 2022;13:941219. doi:10.3389/fimmu.2022.941219
- Eggers B, Marciniak J, Memmert S, et al. The beneficial effect of cold atmospheric plasma on parameters of molecules and cell function involved in wound healing in human osteoblast-like cells in vitro. Odontology. 2020;108:607-616. doi:10.1007/s10266-020-00487-y
- Conway GE, He Z, Hutanu AL, et al. Cold atmospheric plasma induces accumulation of lysosomes and caspase-independent cell death in U373MG glioblastoma multiforme cells. Sci Rep. 2019;9:12891. doi:10.1038/s41598-019-49013-3
- Schmidt A, Dietrich S, Steuer A, et al. Non-thermal plasma activates human keratinocytes by stimulation of antioxidant and phase II pathways. J Biol Chem. 2015;290:6731-6750. doi:10.1074/jbc.M114.603555
- Silverberg NB. Pediatric molluscum contagiosum. Pediatr Drugs. 2003;5:505-511. doi:10.2165/00148581-200305080-00001
- Cotton DW, Cooper C, Barrett DF, et al. Severe atypical molluscum contagiosum infection in an immunocompromised host. Br J Dermatol. 1987;116:871-876. doi:10.1111/j.1365-2133.1987.tb04908.x
Practice Points
- Nonthermal atmospheric plasma (NTAP)(also known as cold atmospheric plasma) has been shown to cause minimal damage to skin when application time is minimized.
- Beyond its diverse applications, treatment with NTAP yields a unique advantage to pharmacologic therapies in that there is no risk for medication interactions or pharmacologic adverse effects.
- Although the use of NTAP in pediatric dermatology is scarcely described in the literature, the technology will certainly have applications in the future treatment of a wide variety of pediatric disorders.
Teledermatology: A Postpandemic Update
The rapid expansion of teledermatology in the United States due to the COVID-19 pandemic has been well documented, 1 but where do we stand now that health care and society as a whole are back to a new version of normal? It is important to consider why telemedicine was able to grow so quickly during that period—the Centers for Medicare & Medicaid Services (CMS) unilaterally changed policies related to provision of services and reimbursement thereof due to the public health emergency (PHE), which was declared by the Department of Health and Human Services in January 2020 to provide increased access to care for patients. Under the PHE, reimbursement rates for virtual visits improved, providers could care for patients from their homes and across state lines, and the use of video platforms that were not Health Insurance Portability and Accountability Act compliant was allowed. 2,3
The trajectory of teledermatology after the pandemic, however, remains unclear. In a survey assessing dermatologists’ perceptions of telemedicine (N=4356), 97% used telemedicine during the pandemic but only 58% reported that they intended to continue using teledermatology postpandemic,1 which is driven, at least in part, by the potential concern that dermatologists will again experience the same regulatory and logistical barriers that limited teledermatology utilization prepandemic.
What has changed in reimbursement for teledermatology since the PHE ended?
The PHE ended on May 11, 2023, and already video platforms that were used during the pandemic to provide telemedicine visits but are not Health Insurance Portability and Accountability Act compliant are now forbidden,2 Medicare virtual check-in appointments can only be conducted with established patients,4 and medical licensing requirements have been reinstated in most states such that patients must be located in the state where the provider is licensed to practice medicine at the time of a virtual visit.3 Although the CMS was granted wide freedoms to waive and suspend certain rules, this was only in the context of the PHE, and any lasting changes must be established by Congress.
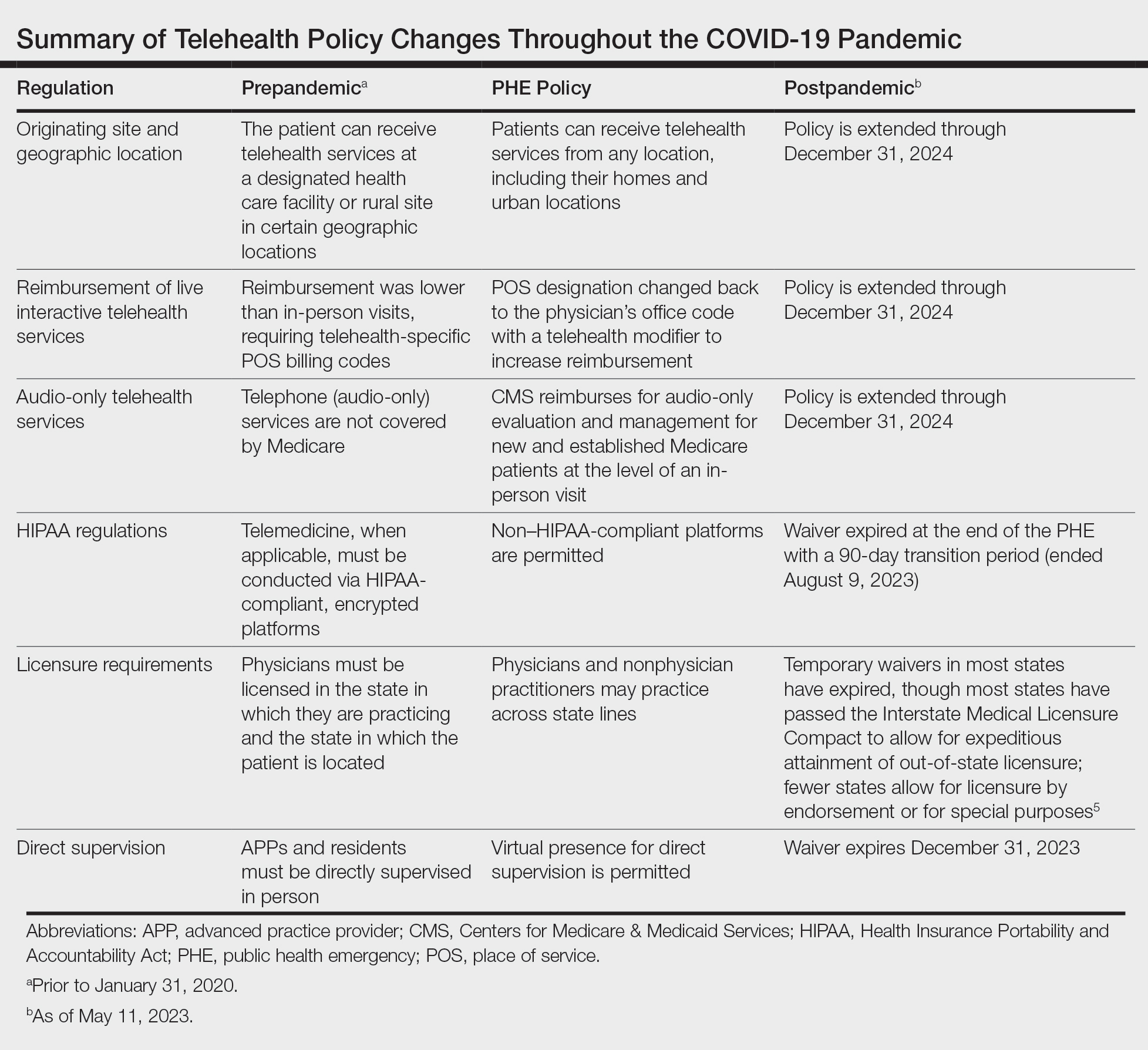
Reassuringly, recent legislation via the Consolidated Appropriations Act, 2023, authorized an extension of many of the CMS telehealth flexibilities that were in place during the PHE through December 31, 2024 (Table),2 such as allowing access to telehealth services in any geographic area in the United States rather than only rural areas, allowing patients to stay in their homes for telehealth visits rather than traveling to an approved health care facility, and allowing the delivery of telemedicine via audio-only technology if a patient is unable to use both audio and video. As of now, the place of service (POS) designation for telehealth visits will not revert back to the former code (POS 02) but will remain at POS 11 with the telehealth modifier -95 so physicians will be reimbursed at the full level of a non-facility physician’s office rate.4 The CMS has indicated that there will be no change in the reimbursement policy until after December 31, 20234; however, the sense of uncertainty around what happens after this date has made it hard for organizations and practices to fully commit to teledermatology services without knowing what the long-term financial impact may be. Some organizations have already noted that they plan to continue supporting telemedicine after the CMS flexibilities expire. Accountable Care Organizations have the ability to offer services that allow participating practitioners to continue the use of telemedicine visits to expand access to care. Medicaid and Children’s Health Insurance Program policies vary by state and private health insurance policies vary by individual plans, but it should be noted that commercial coverage for telemedicine visits was already strong prior to the pandemic.2
What medical licensing requirements are in place now for telehealth?
During the PHE, medical licensing requirements also were relaxed, enabling providers to deliver telemedicine service in states where they were not licensed.3 As the PHE orders ended, some states including New York discontinued cross-state licensing waivers altogether,6 whereas others have enacted legislation to make them permanent or extend them for brief periods of time.3,6 One potential solution is the Interstate Medical Licensure Compact (https://www.imlcc.org/), which includes 39 states as of October 2023. This program expedites the process for physicians already licensed in participating states to obtain their medical license in another participating state, though licensing fees are required for each state in which a physician wants to practice. Furthermore, some states such as North Dakota, Hawaii, and Virginia have licensure by endorsement policies, which enable licensed physicians with specific qualifications to provide telehealth services in the endorsing state. Other states such as Florida, New Jersey, Louisiana, Minnesota, Nevada, and New Mexico have special telehealth registries that allow physicians in good standing who are licensed in other states to deliver telehealth services to in-state residents barring they do not provide in-person, in-state services.6 Lastly, some states have temporary practice laws to allow existing patients who need medical attention while traveling out of state to see their home providers virtually or in person under certain circumstances for a limited period of time.3,5 In Hawaii and New Hampshire, physicians with out-of-state licenses can provide consultative services in some circumstances.5
What changes have been made to make it easier for patients to use telehealth?
As the legislation around telemedicine is shifting postpandemic, it is important to address additional logistical barriers to teledermatology on a larger scale if the discipline is to stay in practice. On November 15, 2021, the Infrastructure Investment and Jobs Act provided $65 billion in funding for broadband to expand access to high-speed internet. Some of this money was allocated to the Affordable Connectivity Program, which provides eligible households with a discount on broadband service and internet-connected devices. Eligible patrons can qualify for a discount of up to $75 per month for internet service and a one-time discount up to $100 on a laptop, desktop computer, or tablet purchased through a participating provider.6 Although a step in the right direction, the effects of this program on telemedicine encounters remains to be proven. Additionally, these programs do not address educational barriers to understanding how to utilize telemedicine platforms or provide incentives for practitioners to offer telemedicine services.
Final Thoughts
The pandemic taught our specialty a great deal about how to utilize telemedicine. For many dermatologists a return to in-person business as usual could not come fast enough; however, many practices have continued to offer at least some teledermatology services. Although the PHE waivers have ended, the extension of numerous CMS flexibilities through the end of 2024 allows us more time to develop sustainable policies to support the long-term health of telemedicine as a whole, both to sustain practices and to expand access to care in dermatology. The favorable attitudes of both patients and physicians about teledermatology have been clearly documented,1,7 and we should continue to safely expand the use of this technology.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- US Department of Health and Human Services. HHS fact sheet: telehealth flexibilities and resources and the COVID-19 public health emergency. Published May 10, 2023. Accessed October 18, 2023. https://www.hhs.gov/aboutnews/2023/05/10/hhs-fact-sheet-telehealth-flexibilities-resources-covid-19-public-health-emergency.html
- US Department of Health and Human Services. Licensing across state lines. Updated May 11, 2023. Accessed October 25, 2023. https://telehealth.hhs.gov/licensure/licensing-across-state-lines
- American Academy of Dermatology. Teledermatology and the COVID-19 pandemic. Accessed October 12, 2023. https://www.aad.org/member/practice/telederm/covid-19
- American Medical Association. Licensure & Telehealth. Accessed October 12, 2023. https://www.ama-assn.org/system/files/issue-brief-licensure-telehealth.pdf
- Federal Communications Commission. Affordable Connectivity Program. Updated June 29, 2023. Accessed October 12, 2023. https://www.fcc.gov/affordable-connectivity-program
- Tensen E, van der Heijden JP, Jaspers MWM, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
The rapid expansion of teledermatology in the United States due to the COVID-19 pandemic has been well documented, 1 but where do we stand now that health care and society as a whole are back to a new version of normal? It is important to consider why telemedicine was able to grow so quickly during that period—the Centers for Medicare & Medicaid Services (CMS) unilaterally changed policies related to provision of services and reimbursement thereof due to the public health emergency (PHE), which was declared by the Department of Health and Human Services in January 2020 to provide increased access to care for patients. Under the PHE, reimbursement rates for virtual visits improved, providers could care for patients from their homes and across state lines, and the use of video platforms that were not Health Insurance Portability and Accountability Act compliant was allowed. 2,3
The trajectory of teledermatology after the pandemic, however, remains unclear. In a survey assessing dermatologists’ perceptions of telemedicine (N=4356), 97% used telemedicine during the pandemic but only 58% reported that they intended to continue using teledermatology postpandemic,1 which is driven, at least in part, by the potential concern that dermatologists will again experience the same regulatory and logistical barriers that limited teledermatology utilization prepandemic.
What has changed in reimbursement for teledermatology since the PHE ended?
The PHE ended on May 11, 2023, and already video platforms that were used during the pandemic to provide telemedicine visits but are not Health Insurance Portability and Accountability Act compliant are now forbidden,2 Medicare virtual check-in appointments can only be conducted with established patients,4 and medical licensing requirements have been reinstated in most states such that patients must be located in the state where the provider is licensed to practice medicine at the time of a virtual visit.3 Although the CMS was granted wide freedoms to waive and suspend certain rules, this was only in the context of the PHE, and any lasting changes must be established by Congress.
Reassuringly, recent legislation via the Consolidated Appropriations Act, 2023, authorized an extension of many of the CMS telehealth flexibilities that were in place during the PHE through December 31, 2024 (Table),2 such as allowing access to telehealth services in any geographic area in the United States rather than only rural areas, allowing patients to stay in their homes for telehealth visits rather than traveling to an approved health care facility, and allowing the delivery of telemedicine via audio-only technology if a patient is unable to use both audio and video. As of now, the place of service (POS) designation for telehealth visits will not revert back to the former code (POS 02) but will remain at POS 11 with the telehealth modifier -95 so physicians will be reimbursed at the full level of a non-facility physician’s office rate.4 The CMS has indicated that there will be no change in the reimbursement policy until after December 31, 20234; however, the sense of uncertainty around what happens after this date has made it hard for organizations and practices to fully commit to teledermatology services without knowing what the long-term financial impact may be. Some organizations have already noted that they plan to continue supporting telemedicine after the CMS flexibilities expire. Accountable Care Organizations have the ability to offer services that allow participating practitioners to continue the use of telemedicine visits to expand access to care. Medicaid and Children’s Health Insurance Program policies vary by state and private health insurance policies vary by individual plans, but it should be noted that commercial coverage for telemedicine visits was already strong prior to the pandemic.2

What medical licensing requirements are in place now for telehealth?
During the PHE, medical licensing requirements also were relaxed, enabling providers to deliver telemedicine service in states where they were not licensed.3 As the PHE orders ended, some states including New York discontinued cross-state licensing waivers altogether,6 whereas others have enacted legislation to make them permanent or extend them for brief periods of time.3,6 One potential solution is the Interstate Medical Licensure Compact (https://www.imlcc.org/), which includes 39 states as of October 2023. This program expedites the process for physicians already licensed in participating states to obtain their medical license in another participating state, though licensing fees are required for each state in which a physician wants to practice. Furthermore, some states such as North Dakota, Hawaii, and Virginia have licensure by endorsement policies, which enable licensed physicians with specific qualifications to provide telehealth services in the endorsing state. Other states such as Florida, New Jersey, Louisiana, Minnesota, Nevada, and New Mexico have special telehealth registries that allow physicians in good standing who are licensed in other states to deliver telehealth services to in-state residents barring they do not provide in-person, in-state services.6 Lastly, some states have temporary practice laws to allow existing patients who need medical attention while traveling out of state to see their home providers virtually or in person under certain circumstances for a limited period of time.3,5 In Hawaii and New Hampshire, physicians with out-of-state licenses can provide consultative services in some circumstances.5
What changes have been made to make it easier for patients to use telehealth?
As the legislation around telemedicine is shifting postpandemic, it is important to address additional logistical barriers to teledermatology on a larger scale if the discipline is to stay in practice. On November 15, 2021, the Infrastructure Investment and Jobs Act provided $65 billion in funding for broadband to expand access to high-speed internet. Some of this money was allocated to the Affordable Connectivity Program, which provides eligible households with a discount on broadband service and internet-connected devices. Eligible patrons can qualify for a discount of up to $75 per month for internet service and a one-time discount up to $100 on a laptop, desktop computer, or tablet purchased through a participating provider.6 Although a step in the right direction, the effects of this program on telemedicine encounters remains to be proven. Additionally, these programs do not address educational barriers to understanding how to utilize telemedicine platforms or provide incentives for practitioners to offer telemedicine services.
Final Thoughts
The pandemic taught our specialty a great deal about how to utilize telemedicine. For many dermatologists a return to in-person business as usual could not come fast enough; however, many practices have continued to offer at least some teledermatology services. Although the PHE waivers have ended, the extension of numerous CMS flexibilities through the end of 2024 allows us more time to develop sustainable policies to support the long-term health of telemedicine as a whole, both to sustain practices and to expand access to care in dermatology. The favorable attitudes of both patients and physicians about teledermatology have been clearly documented,1,7 and we should continue to safely expand the use of this technology.
The rapid expansion of teledermatology in the United States due to the COVID-19 pandemic has been well documented, 1 but where do we stand now that health care and society as a whole are back to a new version of normal? It is important to consider why telemedicine was able to grow so quickly during that period—the Centers for Medicare & Medicaid Services (CMS) unilaterally changed policies related to provision of services and reimbursement thereof due to the public health emergency (PHE), which was declared by the Department of Health and Human Services in January 2020 to provide increased access to care for patients. Under the PHE, reimbursement rates for virtual visits improved, providers could care for patients from their homes and across state lines, and the use of video platforms that were not Health Insurance Portability and Accountability Act compliant was allowed. 2,3
The trajectory of teledermatology after the pandemic, however, remains unclear. In a survey assessing dermatologists’ perceptions of telemedicine (N=4356), 97% used telemedicine during the pandemic but only 58% reported that they intended to continue using teledermatology postpandemic,1 which is driven, at least in part, by the potential concern that dermatologists will again experience the same regulatory and logistical barriers that limited teledermatology utilization prepandemic.
What has changed in reimbursement for teledermatology since the PHE ended?
The PHE ended on May 11, 2023, and already video platforms that were used during the pandemic to provide telemedicine visits but are not Health Insurance Portability and Accountability Act compliant are now forbidden,2 Medicare virtual check-in appointments can only be conducted with established patients,4 and medical licensing requirements have been reinstated in most states such that patients must be located in the state where the provider is licensed to practice medicine at the time of a virtual visit.3 Although the CMS was granted wide freedoms to waive and suspend certain rules, this was only in the context of the PHE, and any lasting changes must be established by Congress.
Reassuringly, recent legislation via the Consolidated Appropriations Act, 2023, authorized an extension of many of the CMS telehealth flexibilities that were in place during the PHE through December 31, 2024 (Table),2 such as allowing access to telehealth services in any geographic area in the United States rather than only rural areas, allowing patients to stay in their homes for telehealth visits rather than traveling to an approved health care facility, and allowing the delivery of telemedicine via audio-only technology if a patient is unable to use both audio and video. As of now, the place of service (POS) designation for telehealth visits will not revert back to the former code (POS 02) but will remain at POS 11 with the telehealth modifier -95 so physicians will be reimbursed at the full level of a non-facility physician’s office rate.4 The CMS has indicated that there will be no change in the reimbursement policy until after December 31, 20234; however, the sense of uncertainty around what happens after this date has made it hard for organizations and practices to fully commit to teledermatology services without knowing what the long-term financial impact may be. Some organizations have already noted that they plan to continue supporting telemedicine after the CMS flexibilities expire. Accountable Care Organizations have the ability to offer services that allow participating practitioners to continue the use of telemedicine visits to expand access to care. Medicaid and Children’s Health Insurance Program policies vary by state and private health insurance policies vary by individual plans, but it should be noted that commercial coverage for telemedicine visits was already strong prior to the pandemic.2

What medical licensing requirements are in place now for telehealth?
During the PHE, medical licensing requirements also were relaxed, enabling providers to deliver telemedicine service in states where they were not licensed.3 As the PHE orders ended, some states including New York discontinued cross-state licensing waivers altogether,6 whereas others have enacted legislation to make them permanent or extend them for brief periods of time.3,6 One potential solution is the Interstate Medical Licensure Compact (https://www.imlcc.org/), which includes 39 states as of October 2023. This program expedites the process for physicians already licensed in participating states to obtain their medical license in another participating state, though licensing fees are required for each state in which a physician wants to practice. Furthermore, some states such as North Dakota, Hawaii, and Virginia have licensure by endorsement policies, which enable licensed physicians with specific qualifications to provide telehealth services in the endorsing state. Other states such as Florida, New Jersey, Louisiana, Minnesota, Nevada, and New Mexico have special telehealth registries that allow physicians in good standing who are licensed in other states to deliver telehealth services to in-state residents barring they do not provide in-person, in-state services.6 Lastly, some states have temporary practice laws to allow existing patients who need medical attention while traveling out of state to see their home providers virtually or in person under certain circumstances for a limited period of time.3,5 In Hawaii and New Hampshire, physicians with out-of-state licenses can provide consultative services in some circumstances.5
What changes have been made to make it easier for patients to use telehealth?
As the legislation around telemedicine is shifting postpandemic, it is important to address additional logistical barriers to teledermatology on a larger scale if the discipline is to stay in practice. On November 15, 2021, the Infrastructure Investment and Jobs Act provided $65 billion in funding for broadband to expand access to high-speed internet. Some of this money was allocated to the Affordable Connectivity Program, which provides eligible households with a discount on broadband service and internet-connected devices. Eligible patrons can qualify for a discount of up to $75 per month for internet service and a one-time discount up to $100 on a laptop, desktop computer, or tablet purchased through a participating provider.6 Although a step in the right direction, the effects of this program on telemedicine encounters remains to be proven. Additionally, these programs do not address educational barriers to understanding how to utilize telemedicine platforms or provide incentives for practitioners to offer telemedicine services.
Final Thoughts
The pandemic taught our specialty a great deal about how to utilize telemedicine. For many dermatologists a return to in-person business as usual could not come fast enough; however, many practices have continued to offer at least some teledermatology services. Although the PHE waivers have ended, the extension of numerous CMS flexibilities through the end of 2024 allows us more time to develop sustainable policies to support the long-term health of telemedicine as a whole, both to sustain practices and to expand access to care in dermatology. The favorable attitudes of both patients and physicians about teledermatology have been clearly documented,1,7 and we should continue to safely expand the use of this technology.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- US Department of Health and Human Services. HHS fact sheet: telehealth flexibilities and resources and the COVID-19 public health emergency. Published May 10, 2023. Accessed October 18, 2023. https://www.hhs.gov/aboutnews/2023/05/10/hhs-fact-sheet-telehealth-flexibilities-resources-covid-19-public-health-emergency.html
- US Department of Health and Human Services. Licensing across state lines. Updated May 11, 2023. Accessed October 25, 2023. https://telehealth.hhs.gov/licensure/licensing-across-state-lines
- American Academy of Dermatology. Teledermatology and the COVID-19 pandemic. Accessed October 12, 2023. https://www.aad.org/member/practice/telederm/covid-19
- American Medical Association. Licensure & Telehealth. Accessed October 12, 2023. https://www.ama-assn.org/system/files/issue-brief-licensure-telehealth.pdf
- Federal Communications Commission. Affordable Connectivity Program. Updated June 29, 2023. Accessed October 12, 2023. https://www.fcc.gov/affordable-connectivity-program
- Tensen E, van der Heijden JP, Jaspers MWM, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- US Department of Health and Human Services. HHS fact sheet: telehealth flexibilities and resources and the COVID-19 public health emergency. Published May 10, 2023. Accessed October 18, 2023. https://www.hhs.gov/aboutnews/2023/05/10/hhs-fact-sheet-telehealth-flexibilities-resources-covid-19-public-health-emergency.html
- US Department of Health and Human Services. Licensing across state lines. Updated May 11, 2023. Accessed October 25, 2023. https://telehealth.hhs.gov/licensure/licensing-across-state-lines
- American Academy of Dermatology. Teledermatology and the COVID-19 pandemic. Accessed October 12, 2023. https://www.aad.org/member/practice/telederm/covid-19
- American Medical Association. Licensure & Telehealth. Accessed October 12, 2023. https://www.ama-assn.org/system/files/issue-brief-licensure-telehealth.pdf
- Federal Communications Commission. Affordable Connectivity Program. Updated June 29, 2023. Accessed October 12, 2023. https://www.fcc.gov/affordable-connectivity-program
- Tensen E, van der Heijden JP, Jaspers MWM, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
Postmenopausal testosterone for low libido only, doctors say
Your patients may see ads claiming that testosterone replacement therapy (TRT) offers postmenopausal women health benefits beyond restored sex drive: that TRT can improve their mood, energy, and thinking and give them stronger bones and bigger muscles.
How accurate are these claims? According to six experts who talked with this news organization, not very.
“Right now in this country and around the world, testosterone’s only use in postmenopausal women is for libido,” said Adrian Sandra Dobs, MD, MHS, professor of medicine and director of the Johns Hopkins Clinical Research Network at Johns Hopkins Medicine, Baltimore.
“Treating postmenopausal women with testosterone is a rarity. Some physicians and some wellness centers make their money out of prescribing estrogen and testosterone to women in patches, gels, creams, capsules, pellets, and other forms. she added by phone.
“One has to be very careful about using testosterone in women,” Dr. Dobs cautioned. “There’s a lot of hype out there.”
Low testosterone in women has not been well studied, and no testosterone treatments for this condition have been approved by the U.S. Food and Drug Administration. Providers need to adjust male treatment data to their female patients, who require significantly lower doses than males. Contraindications and long-term side effects are poorly understood, said Mary Rosser, MD, PhD, assistant professor of women’s health and director of integrated women’s health at Columbia University Irving Medical Center, New York.
“Despite this preponderance of scientific evidence and recommendations, the myths about testosterone die hard, including that it improves women’s muscle function, endurance, and well-being,” Dr. Rosser said.
“Websites that use compounded products or pellets are not FDA-regulated; therefore, they have no responsibility to prove their claims. They can entice women into using this stuff with all kinds of promises about ‘hormone balancing’ and other meaningless terms. The Endocrine Society statement reviewed the clinical studies on testosterone for various indications surrounding physical endurance, well-being, and mental health – and the studies do not support its use,” Dr. Rosser added.
According to the Australasian Menopause Society, women’s blood testosterone levels tend to peak in their 20s, slowly decline to around 25% of peak levels at menopause, then rise again in later years.
Susan Davis, PhD, and her colleagues at Monash University, Melbourne, found in a study that TRT in postmenopausal women may improve sexual well-being and that side effects include acne and increased hair growth. But they found no benefits for cognition, bone mineral density, body composition, muscle strength, or psychological well-being, and they note that more data are needed on long-term safety.
Postmenopausal testosterone recommended for libido only
“Hypoactive sexual desire disorder (HSDD) is really the only indication for postmenopausal testosterone use,” Nanette F. Santoro, MD, professor and chair of obstetrics and gynecology at the University of Colorado School of Medicine, Aurora, noted by email. “In clinical studies using androgen gel containing testosterone, testosterone treatment has resulted in a mean of one more satisfying sexual encounter per month. Consensus statements issued by the Endocrine Society, The International Menopause Society, and the North American Menopause Society have come to similar conclusions: The only indication for androgen therapy for women is HSDD,” added Santoro, an author of the Endocrine Society statement.
“Sexual health and the sense of well-being are very much related,” Sandra Ann Carson, MD, professor of obstetrics and gynecology at Yale Medicine, New Haven, Conn., said by phone. “So we give testosterone to increase sexual desire. Testosterone is not a treatment for decreased sense of well-being alone. Women who lose their sense of well-being due to depression or other factors need to have a mental health evaluation, not testosterone.”
“Because no female product is presently approved by a national regulatory body, male formulations can be judiciously used in female doses and blood testosterone concentrations must be monitored regularly,” Dr. Rosser said. “The recommendation is for considering use of compounded testosterone for hypoactive sexual desire only; it is against use for overall health and wellness.”
“The real mischief occurs when women are exposed to doses that are supraphysiologic,” Dr. Rosser cautioned. “At high doses that approach and sometimes exceed men’s levels of testosterone, women can have deepening of the voice, adverse changes in cholesterol, and even breast atrophy. This can occur with bioidentical compounded testosterone and with testosterone pellets. The National Academies of Science, Engineering, and Medicine recommend unequivocally that such preparations not be used.”
Not all postmenopausal women should take TRT, said Meredith McClure, MD, assistant professor in the department of obstetrics and gynecology of UT Southwestern Medical School, Dallas, because it has only been shown in trials to help with HSDD.
She advised clinicians to avoid prescribing testosterone to patients who “can’t take estrogen, including if [they] have hormone-sensitive cancer, blood clot risk, liver disease, heart attack, stroke, or undiagnosed genital bleeding.”
TRT for non-libido issues may sometimes be appropriate
“Perhaps women with hip fracture or cancer cachexia could benefit from testosterone to build muscle mass,” said Dr. Dobbs, who is involved in an ongoing study of testosterone treatment in women with hip fracture. “But as yet, we have no proof that testosterone helps.”
In rare cases, Stanley G. Korenman, MD, a reproductive endocrinologist and associate dean for ethics at UCLA Health, treats postmenopausal patients with TRT for reasons other than low libido. “I have a very specialized practice in reproductive endocrinology and internal medicine and am one of very few people in the country who do this kind of management,” he said in an interview. “If my postmenopausal patients have low testosterone and lack energy, I’m willing to give them low doses. If they feel more energetic, we continue, but if they don’t, we stop. I don’t think there’s any risk whatsoever at the low level I prescribe.
“I prescribe standard gel that comes in a squirt bottle, and I suggest they take half a squirt every other day – about one-eighth of a male dose – on the sole of the foot, where hair does not grow.
“I would not prescribe testosterone for bone health. We have bisphosphonates and other much better treatments for that. And I would not prescribe it to someone who is seriously emotionally disturbed or seriously depressed. This is not a treatment for depression.”
“Postmenopausal testosterone is not ‘the latest greatest thing,’ but being very low risk, it’s worth trying once in a while, in the appropriate patient, at the right dose,” Dr. Korenman advised. He cautioned people to “avoid the longevity salespeople who sell all sorts of things in all sorts of doses to try to keep us alive forever.”
All contributors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Your patients may see ads claiming that testosterone replacement therapy (TRT) offers postmenopausal women health benefits beyond restored sex drive: that TRT can improve their mood, energy, and thinking and give them stronger bones and bigger muscles.
How accurate are these claims? According to six experts who talked with this news organization, not very.
“Right now in this country and around the world, testosterone’s only use in postmenopausal women is for libido,” said Adrian Sandra Dobs, MD, MHS, professor of medicine and director of the Johns Hopkins Clinical Research Network at Johns Hopkins Medicine, Baltimore.
“Treating postmenopausal women with testosterone is a rarity. Some physicians and some wellness centers make their money out of prescribing estrogen and testosterone to women in patches, gels, creams, capsules, pellets, and other forms. she added by phone.
“One has to be very careful about using testosterone in women,” Dr. Dobs cautioned. “There’s a lot of hype out there.”
Low testosterone in women has not been well studied, and no testosterone treatments for this condition have been approved by the U.S. Food and Drug Administration. Providers need to adjust male treatment data to their female patients, who require significantly lower doses than males. Contraindications and long-term side effects are poorly understood, said Mary Rosser, MD, PhD, assistant professor of women’s health and director of integrated women’s health at Columbia University Irving Medical Center, New York.
“Despite this preponderance of scientific evidence and recommendations, the myths about testosterone die hard, including that it improves women’s muscle function, endurance, and well-being,” Dr. Rosser said.
“Websites that use compounded products or pellets are not FDA-regulated; therefore, they have no responsibility to prove their claims. They can entice women into using this stuff with all kinds of promises about ‘hormone balancing’ and other meaningless terms. The Endocrine Society statement reviewed the clinical studies on testosterone for various indications surrounding physical endurance, well-being, and mental health – and the studies do not support its use,” Dr. Rosser added.
According to the Australasian Menopause Society, women’s blood testosterone levels tend to peak in their 20s, slowly decline to around 25% of peak levels at menopause, then rise again in later years.
Susan Davis, PhD, and her colleagues at Monash University, Melbourne, found in a study that TRT in postmenopausal women may improve sexual well-being and that side effects include acne and increased hair growth. But they found no benefits for cognition, bone mineral density, body composition, muscle strength, or psychological well-being, and they note that more data are needed on long-term safety.
Postmenopausal testosterone recommended for libido only
“Hypoactive sexual desire disorder (HSDD) is really the only indication for postmenopausal testosterone use,” Nanette F. Santoro, MD, professor and chair of obstetrics and gynecology at the University of Colorado School of Medicine, Aurora, noted by email. “In clinical studies using androgen gel containing testosterone, testosterone treatment has resulted in a mean of one more satisfying sexual encounter per month. Consensus statements issued by the Endocrine Society, The International Menopause Society, and the North American Menopause Society have come to similar conclusions: The only indication for androgen therapy for women is HSDD,” added Santoro, an author of the Endocrine Society statement.
“Sexual health and the sense of well-being are very much related,” Sandra Ann Carson, MD, professor of obstetrics and gynecology at Yale Medicine, New Haven, Conn., said by phone. “So we give testosterone to increase sexual desire. Testosterone is not a treatment for decreased sense of well-being alone. Women who lose their sense of well-being due to depression or other factors need to have a mental health evaluation, not testosterone.”
“Because no female product is presently approved by a national regulatory body, male formulations can be judiciously used in female doses and blood testosterone concentrations must be monitored regularly,” Dr. Rosser said. “The recommendation is for considering use of compounded testosterone for hypoactive sexual desire only; it is against use for overall health and wellness.”
“The real mischief occurs when women are exposed to doses that are supraphysiologic,” Dr. Rosser cautioned. “At high doses that approach and sometimes exceed men’s levels of testosterone, women can have deepening of the voice, adverse changes in cholesterol, and even breast atrophy. This can occur with bioidentical compounded testosterone and with testosterone pellets. The National Academies of Science, Engineering, and Medicine recommend unequivocally that such preparations not be used.”
Not all postmenopausal women should take TRT, said Meredith McClure, MD, assistant professor in the department of obstetrics and gynecology of UT Southwestern Medical School, Dallas, because it has only been shown in trials to help with HSDD.
She advised clinicians to avoid prescribing testosterone to patients who “can’t take estrogen, including if [they] have hormone-sensitive cancer, blood clot risk, liver disease, heart attack, stroke, or undiagnosed genital bleeding.”
TRT for non-libido issues may sometimes be appropriate
“Perhaps women with hip fracture or cancer cachexia could benefit from testosterone to build muscle mass,” said Dr. Dobbs, who is involved in an ongoing study of testosterone treatment in women with hip fracture. “But as yet, we have no proof that testosterone helps.”
In rare cases, Stanley G. Korenman, MD, a reproductive endocrinologist and associate dean for ethics at UCLA Health, treats postmenopausal patients with TRT for reasons other than low libido. “I have a very specialized practice in reproductive endocrinology and internal medicine and am one of very few people in the country who do this kind of management,” he said in an interview. “If my postmenopausal patients have low testosterone and lack energy, I’m willing to give them low doses. If they feel more energetic, we continue, but if they don’t, we stop. I don’t think there’s any risk whatsoever at the low level I prescribe.
“I prescribe standard gel that comes in a squirt bottle, and I suggest they take half a squirt every other day – about one-eighth of a male dose – on the sole of the foot, where hair does not grow.
“I would not prescribe testosterone for bone health. We have bisphosphonates and other much better treatments for that. And I would not prescribe it to someone who is seriously emotionally disturbed or seriously depressed. This is not a treatment for depression.”
“Postmenopausal testosterone is not ‘the latest greatest thing,’ but being very low risk, it’s worth trying once in a while, in the appropriate patient, at the right dose,” Dr. Korenman advised. He cautioned people to “avoid the longevity salespeople who sell all sorts of things in all sorts of doses to try to keep us alive forever.”
All contributors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Your patients may see ads claiming that testosterone replacement therapy (TRT) offers postmenopausal women health benefits beyond restored sex drive: that TRT can improve their mood, energy, and thinking and give them stronger bones and bigger muscles.
How accurate are these claims? According to six experts who talked with this news organization, not very.
“Right now in this country and around the world, testosterone’s only use in postmenopausal women is for libido,” said Adrian Sandra Dobs, MD, MHS, professor of medicine and director of the Johns Hopkins Clinical Research Network at Johns Hopkins Medicine, Baltimore.
“Treating postmenopausal women with testosterone is a rarity. Some physicians and some wellness centers make their money out of prescribing estrogen and testosterone to women in patches, gels, creams, capsules, pellets, and other forms. she added by phone.
“One has to be very careful about using testosterone in women,” Dr. Dobs cautioned. “There’s a lot of hype out there.”
Low testosterone in women has not been well studied, and no testosterone treatments for this condition have been approved by the U.S. Food and Drug Administration. Providers need to adjust male treatment data to their female patients, who require significantly lower doses than males. Contraindications and long-term side effects are poorly understood, said Mary Rosser, MD, PhD, assistant professor of women’s health and director of integrated women’s health at Columbia University Irving Medical Center, New York.
“Despite this preponderance of scientific evidence and recommendations, the myths about testosterone die hard, including that it improves women’s muscle function, endurance, and well-being,” Dr. Rosser said.
“Websites that use compounded products or pellets are not FDA-regulated; therefore, they have no responsibility to prove their claims. They can entice women into using this stuff with all kinds of promises about ‘hormone balancing’ and other meaningless terms. The Endocrine Society statement reviewed the clinical studies on testosterone for various indications surrounding physical endurance, well-being, and mental health – and the studies do not support its use,” Dr. Rosser added.
According to the Australasian Menopause Society, women’s blood testosterone levels tend to peak in their 20s, slowly decline to around 25% of peak levels at menopause, then rise again in later years.
Susan Davis, PhD, and her colleagues at Monash University, Melbourne, found in a study that TRT in postmenopausal women may improve sexual well-being and that side effects include acne and increased hair growth. But they found no benefits for cognition, bone mineral density, body composition, muscle strength, or psychological well-being, and they note that more data are needed on long-term safety.
Postmenopausal testosterone recommended for libido only
“Hypoactive sexual desire disorder (HSDD) is really the only indication for postmenopausal testosterone use,” Nanette F. Santoro, MD, professor and chair of obstetrics and gynecology at the University of Colorado School of Medicine, Aurora, noted by email. “In clinical studies using androgen gel containing testosterone, testosterone treatment has resulted in a mean of one more satisfying sexual encounter per month. Consensus statements issued by the Endocrine Society, The International Menopause Society, and the North American Menopause Society have come to similar conclusions: The only indication for androgen therapy for women is HSDD,” added Santoro, an author of the Endocrine Society statement.
“Sexual health and the sense of well-being are very much related,” Sandra Ann Carson, MD, professor of obstetrics and gynecology at Yale Medicine, New Haven, Conn., said by phone. “So we give testosterone to increase sexual desire. Testosterone is not a treatment for decreased sense of well-being alone. Women who lose their sense of well-being due to depression or other factors need to have a mental health evaluation, not testosterone.”
“Because no female product is presently approved by a national regulatory body, male formulations can be judiciously used in female doses and blood testosterone concentrations must be monitored regularly,” Dr. Rosser said. “The recommendation is for considering use of compounded testosterone for hypoactive sexual desire only; it is against use for overall health and wellness.”
“The real mischief occurs when women are exposed to doses that are supraphysiologic,” Dr. Rosser cautioned. “At high doses that approach and sometimes exceed men’s levels of testosterone, women can have deepening of the voice, adverse changes in cholesterol, and even breast atrophy. This can occur with bioidentical compounded testosterone and with testosterone pellets. The National Academies of Science, Engineering, and Medicine recommend unequivocally that such preparations not be used.”
Not all postmenopausal women should take TRT, said Meredith McClure, MD, assistant professor in the department of obstetrics and gynecology of UT Southwestern Medical School, Dallas, because it has only been shown in trials to help with HSDD.
She advised clinicians to avoid prescribing testosterone to patients who “can’t take estrogen, including if [they] have hormone-sensitive cancer, blood clot risk, liver disease, heart attack, stroke, or undiagnosed genital bleeding.”
TRT for non-libido issues may sometimes be appropriate
“Perhaps women with hip fracture or cancer cachexia could benefit from testosterone to build muscle mass,” said Dr. Dobbs, who is involved in an ongoing study of testosterone treatment in women with hip fracture. “But as yet, we have no proof that testosterone helps.”
In rare cases, Stanley G. Korenman, MD, a reproductive endocrinologist and associate dean for ethics at UCLA Health, treats postmenopausal patients with TRT for reasons other than low libido. “I have a very specialized practice in reproductive endocrinology and internal medicine and am one of very few people in the country who do this kind of management,” he said in an interview. “If my postmenopausal patients have low testosterone and lack energy, I’m willing to give them low doses. If they feel more energetic, we continue, but if they don’t, we stop. I don’t think there’s any risk whatsoever at the low level I prescribe.
“I prescribe standard gel that comes in a squirt bottle, and I suggest they take half a squirt every other day – about one-eighth of a male dose – on the sole of the foot, where hair does not grow.
“I would not prescribe testosterone for bone health. We have bisphosphonates and other much better treatments for that. And I would not prescribe it to someone who is seriously emotionally disturbed or seriously depressed. This is not a treatment for depression.”
“Postmenopausal testosterone is not ‘the latest greatest thing,’ but being very low risk, it’s worth trying once in a while, in the appropriate patient, at the right dose,” Dr. Korenman advised. He cautioned people to “avoid the longevity salespeople who sell all sorts of things in all sorts of doses to try to keep us alive forever.”
All contributors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Low-risk TAVR studies: Divergent long-term results
The PARTNER-3 and Evolut trials were heralded as a landmark advance in medicine when the 1-year results from the two studies were presented back in 2019. Both trials suggested benefits of the less-invasive TAVR approach over surgery.
But because these low-surgical-risk patients are younger and will likely have a longer lifespan than will higher risk patients for whom the TAVR technique was first established, patient outcomes and information on how the TAVR devices hold up over the long-term are critical to inform clinical decision-making.
Latest results from the two trials show that the initial benefits of TAVR over surgery seen in PARTNER-3 seem to have attenuated over the longer-term, with main outcomes looking very similar in both groups after 5 years.
However, in the Evolut trial, the early benefit in all-cause mortality or disabling stroke seen in the TAVR group is continuing to increase, with current results showing a 26% relative reduction in this endpoint with TAVR vs. surgery at 4 years.
The 5-year results of the PARTNER-3 trial and the 4-year results of the Evolut study were presented Transcatheter Cardiovascular Therapeutics annual meeting sponsored by the Cardiovascular Research Foundation. Both sets of results were simultaneously published online: PARTNER-3 in The New England Journal of Medicine and Evolut in JACC.
Marty Leon, MD, of NewYork-Presbyterian Columbia University Irving Medical Center, who presented the PARTNER-3 results, said in an interview that both trials are good news for TAVR:
“Both trials have clearly reaffirmed clinical and echocardiographic benefits of TAVR as a meaningful alternative therapy to surgery in low-risk severe symptomatic aortic stenosis patients.” Michael Reardon, MD, Houston Methodist Debakey Heart & Vascular Center, who presented the Evolut results, agreed that both trials were positive for TAVR “as TAVR just has to be as good as surgery to be a winner because clearly it is a lot less invasive.”
But Dr. Dr. Reardon added that, “In making that decision for younger lower-risk patients, then the Evolut valve is the only TAVR valve that has shown superior hemodynamics and durability at all time points with excellent outcomes and widening benefits compared with surgery over the first 4 years.”
PARTNER-3
The PARTNER-3 trial randomly assigned 1,000 patients with severe symptomatic aortic stenosis and low surgical risk to undergo either TAVR with the SAPIEN 3 transcatheter heart valve or surgery.
The results at 5 years show no difference in the two primary composite outcomes between TAVR and surgery patients.
The incidence of the composite end point of death, stroke, or rehospitalization related to the valve, the procedure, or heart failure was similar in the TAVR group and the surgery group, occurring in 22.8% of patients in the TAVR group and 27.2% in the surgery group, which is a nonsignificant difference (P = .07).
The incidence of the individual components of the composite end point were also similar in the two groups. Death occurred in 10.0% in the TAVR group and 8.2% in the surgery group; stroke in 5.8% of the TAVR group and 6.4% of the surgery group; and rehospitalization in 13.7% and 17.4%, respectively.
Aortic-valve durability also looked similar in the two groups. The hemodynamic performance of the valve, assessed according to the mean valve gradient, was 12.8 mm Hg in the TAVR group and 11.7 mm Hg in the surgery group. Bioprosthetic-valve failure occurred in 3.3% of the patients in the TAVR group and in 3.8% of those in the surgery group.
Among the secondary end points, atrial fibrillation and bleeding appeared to be less frequent in the TAVR group than in the surgery group, whereas paravalvular aortic regurgitation, valve thrombosis, and pacemaker implantation appeared to be less frequent in the surgery group.
Functional and health-status outcomes assessed according to New York Heart Association class, quality of life scores, and the percentage of patients who were alive and well at 5 years appeared to be similar in the two groups.
“These data are reassuring,” Dr. Leon said. “Cardiovascular mortality occurred at a rate of about 1% per year with both therapies, strokes at the rate of 1% per year with both therapies, and hospitalization for cardiovascular reasons at about 3% per year with both therapies. For patients in their 70s, these are very good numbers.”
Along with showing similar outcomes for TAVR and surgery at 5 years, he added, “the need for re-intervention was particularly low (2%-3%) and equivalent for both approaches. And structural valve deterioration was also very low and equivalent in both groups.”
Evolut low-risk trial
The Evolut trial enrolled 1,414 patients with low surgical risk who were randomly assigned to TAVR, a self-expanding supra-annular CoreValve Evolut R PRO, or surgery.
By 4 years, the primary endpoint of all-cause mortality or disabling stroke had occurred in 10.7% of the TAVR group and 14.1% in the surgery group (hazard ratio, 0.74; P = .05), representing a 26% relative reduction with TAVR.
The absolute difference between treatment arms for the primary endpoint continued to increase over time: 1.8% at 1 year, 2.0% at 2 years, 2.9% at 3 years, and 3.4% at 4 years.
Rates of the primary endpoint components were all-cause mortality 9.0% with TAVR vs. 12.1% with surgery (P = .07); and disabling stroke was 2.9% with TAVR) vs. 3.8% for surgery (P = .32). Aortic valve rehospitalization was 10.3% with TAVR vs. 12.1% with surgery (P = .27).
The composite of all-cause mortality, disabling stroke, or aortic valve rehospitalization was significantly lower with TAVR, compared with surgery (18.0% vs. 22.4%; HR, 0.78; P = .04).
New permanent pacemaker implantation was significantly higher in the TAVR group (24.6% vs. 9.9%).
Indicators of valve performance including aortic valve reintervention (1.3% TAVR vs. 1.7% surgery); clinical or subclinical valve thrombosis (0.7% TAVR vs. 0.6% surgery); and valve endocarditis (0.9% TAVR vs. 2.2% surgery) were similarly low between groups, the authors report.
TAVR patients had sustained improvement in hemodynamics as measured by echocardiography, with significantly lower aortic valve mean gradients (9.8 mm Hg TAVR vs. 12.1 mm Hg surgery) and greater effective orifice area (2.1 cm2 TAVR vs. 2.0 cm2 surgery).
At 4 years, 84.7% of TAVR patients and 98.4% of surgery patients had no or trace paravalvular regurgitation, and there was no difference between groups in moderate or greater paravalvular regurgitation (0.4% TAVR vs. 0.0% surgery).
“The Evolut valve has shown a superior performance to surgery,” Dr. Reardon concluded. “It has less structural valve deterioration, less severe patient prosthetic mismatch, and superior hemodynamics, compared to surgery. All these factors are translating into a widening difference in clinical event curves year on year with the Evolut valve vs. surgery.”
Why the difference between trials?
The big question is why the early benefit seen with TAVR vs. surgery in both trials was attenuated by 5 years in PARTNER-3 but seemed to become greater each year in the Evolut trial. There were no definite explanations for these observations, but several possibilities were suggested.
Dr. Leon noted that with trials of intervention vs. surgery, it is common for the intervention group to do better in the beginning and for surgery to catch up a bit in later years. “So, it is not that much of a surprise to see outcomes plateauing in PARTNER-3.”
But he also suggested some other factors that may have played a role, one of which was the COVID pandemic.
“During the 2-year COVID period more than 75% of the deaths and strokes in the trial occurred in the TAVR patients,” he said. “Surgery patients were getting more anticoagulation because they had more paroxysmal [atrial fibrillation]. We know that COVID is a stimulus of thrombogenic events so in an odd way we think there may have been some cardioprotective effects from anticoagulation therapy in the surgery group.”
He also pointed out that though hospitalization and strokes were slightly lower with TAVR vs. surgery in the PARTNER-3 trial, mortality was slightly greater in the TAVR group.
“There was a 2:1 ratio in the TAVR and surgery groups in noncardiovascular deaths which influenced the all-cause mortality numbers,” he noted.
Mortality rates in the surgery groups
Dr. Leon also pointed out differences in the mortality rates in the surgery groups in the two trials, which he suggested may contribute to the explanation for the different longer-term results.
“The baseline characteristics for patients in these two trials were almost identical, and results at 1 year were very similar, but for whatever reason, over the course of a few years, the outcomes in the Evolut trial were different to those in PARTNER-3, and in particular the difference was in the surgical arms, with a higher event rate in the surgical arm in Evolut than in the surgery arm in PARTNER-3,” he said. “When the control does not perform well it is a lot easier to show that the experimental arm is better.”
“When you look at the TAVR arms in both studies at each time point they are either similar or PARTNER-3 is actually lower,” he added. “That is why it is so difficult to compare these two trials.”
But Dr. Reardon dismissed this argument.
“What determines long-term survival after a procedure is the intrinsic risk level of the patients,” he said. “Overall mortality rates differ between the two trials because the PARTNER-3 trial enrolled a lower end of a low-risk population while Evolut enrolled an upper end of a low-risk population. You cannot look at absolute numbers between trials. That is intellectually and scientifically invalid.”
“It is the relative difference between surgery and TAVR that we are interested in, and we see in Evolut that the relative difference between the two procedures in terms of benefit with TAVR is widening every year,” he added. “That is because the superior valve performance and hemodynamics of the Evolut valve compared to surgery has translated into excellent clinical outcomes.
“In the PARTNER-3 trial – their curves are coming together. I think that is worrisome, but I don’t want to draw conclusions about their trial,” Dr. Reardon said. “All I know is that in our trial, we have excellent outcomes that are getting better year after year.”
Competition between valves
The different results of the two trials is inevitably producing some competition between the two products.
Dr. Reardon said: “In terms of which valve to use, clinicians will want to choose a valve that has the best durability and shows the best survival vs. surgery and that is clearly the Evolut valve. I think the writing is on the wall. Some clinicians are going to wait for longer term data, but the question is do we have enough long-term data now.”
But Dr. Leon countered: “There’s never been a head-to-head device to suggest that the self- expanding device performs better than the balloon expanding device. We always think about them as being similar in terms of performance. There is an aggressive effort to suggest that by virtue of the current trial results there was a superior outcome with the Medtronic device, but it’s hard to explain why that would be the case, and we should not compare between the two trials.”
Both Dr. Leon and Dr. Reardon stressed that longer-term follow-up is critical because some surgical valves are known to fail between 5-10 years, and it is not known how the TAVR valves will perform over that period.
Both the PARTNER-3 and Evolut trials are planning to keep following patients out to 10 years.
For the time being though, both Dr. Leon and Dr. Reardon agreed that these current results will probably accelerate the already rapid transition from surgery to TAVR in low-risk patients.
“TAVR will be the default therapy,” Dr. Leon commented. “It will be the first choice for patients. Whether TAVR is superior to surgery in terms of outcomes or just the same, there are sufficient benefits from a logistic and patient perspective that most people would prefer to have the less invasive therapy. TAVR is a one-day procedure, there is no need for general anesthetic, a lot of the secondary outcomes that are so problematic with surgery don’t exist, and the ability to be in a symptom-free state is dramatically accelerated.”
“This was the first serious foray into the low-risk population with TAVR,” he added. “We had an age cut of 65 years, but the vast majority of patients in both trials were over 70. We could now start looking at younger patient populations.”
But Dr. Reardon said that these younger patients are already being given TAVR, and future trials randomizing between TAVR and surgery may not be possible.
“Even though US guidelines still recommend surgery for patients under 65 years, patients want TAVR, and they get TAVR,” he said. “Recent data shows that in 2021, use of TAVR rose to 47.5% in patients under 65 needing isolated aortic valve replacement. That doesn’t meet the guidelines but there’s clearly a big shift going on. These results will just keep that momentum going.”
The PARTNER-3 trial was funded by Edwards Lifesciences. The Evolut study was funded by Medtronic. Dr. Leon reports grant support from Edwards Lifesciences and Medtronic. Dr. Reardon receives research grants from Medtronic.
A version of this article first appeared on Medscape.com.
The PARTNER-3 and Evolut trials were heralded as a landmark advance in medicine when the 1-year results from the two studies were presented back in 2019. Both trials suggested benefits of the less-invasive TAVR approach over surgery.
But because these low-surgical-risk patients are younger and will likely have a longer lifespan than will higher risk patients for whom the TAVR technique was first established, patient outcomes and information on how the TAVR devices hold up over the long-term are critical to inform clinical decision-making.
Latest results from the two trials show that the initial benefits of TAVR over surgery seen in PARTNER-3 seem to have attenuated over the longer-term, with main outcomes looking very similar in both groups after 5 years.
However, in the Evolut trial, the early benefit in all-cause mortality or disabling stroke seen in the TAVR group is continuing to increase, with current results showing a 26% relative reduction in this endpoint with TAVR vs. surgery at 4 years.
The 5-year results of the PARTNER-3 trial and the 4-year results of the Evolut study were presented Transcatheter Cardiovascular Therapeutics annual meeting sponsored by the Cardiovascular Research Foundation. Both sets of results were simultaneously published online: PARTNER-3 in The New England Journal of Medicine and Evolut in JACC.
Marty Leon, MD, of NewYork-Presbyterian Columbia University Irving Medical Center, who presented the PARTNER-3 results, said in an interview that both trials are good news for TAVR:
“Both trials have clearly reaffirmed clinical and echocardiographic benefits of TAVR as a meaningful alternative therapy to surgery in low-risk severe symptomatic aortic stenosis patients.” Michael Reardon, MD, Houston Methodist Debakey Heart & Vascular Center, who presented the Evolut results, agreed that both trials were positive for TAVR “as TAVR just has to be as good as surgery to be a winner because clearly it is a lot less invasive.”
But Dr. Dr. Reardon added that, “In making that decision for younger lower-risk patients, then the Evolut valve is the only TAVR valve that has shown superior hemodynamics and durability at all time points with excellent outcomes and widening benefits compared with surgery over the first 4 years.”
PARTNER-3
The PARTNER-3 trial randomly assigned 1,000 patients with severe symptomatic aortic stenosis and low surgical risk to undergo either TAVR with the SAPIEN 3 transcatheter heart valve or surgery.
The results at 5 years show no difference in the two primary composite outcomes between TAVR and surgery patients.
The incidence of the composite end point of death, stroke, or rehospitalization related to the valve, the procedure, or heart failure was similar in the TAVR group and the surgery group, occurring in 22.8% of patients in the TAVR group and 27.2% in the surgery group, which is a nonsignificant difference (P = .07).
The incidence of the individual components of the composite end point were also similar in the two groups. Death occurred in 10.0% in the TAVR group and 8.2% in the surgery group; stroke in 5.8% of the TAVR group and 6.4% of the surgery group; and rehospitalization in 13.7% and 17.4%, respectively.
Aortic-valve durability also looked similar in the two groups. The hemodynamic performance of the valve, assessed according to the mean valve gradient, was 12.8 mm Hg in the TAVR group and 11.7 mm Hg in the surgery group. Bioprosthetic-valve failure occurred in 3.3% of the patients in the TAVR group and in 3.8% of those in the surgery group.
Among the secondary end points, atrial fibrillation and bleeding appeared to be less frequent in the TAVR group than in the surgery group, whereas paravalvular aortic regurgitation, valve thrombosis, and pacemaker implantation appeared to be less frequent in the surgery group.
Functional and health-status outcomes assessed according to New York Heart Association class, quality of life scores, and the percentage of patients who were alive and well at 5 years appeared to be similar in the two groups.
“These data are reassuring,” Dr. Leon said. “Cardiovascular mortality occurred at a rate of about 1% per year with both therapies, strokes at the rate of 1% per year with both therapies, and hospitalization for cardiovascular reasons at about 3% per year with both therapies. For patients in their 70s, these are very good numbers.”
Along with showing similar outcomes for TAVR and surgery at 5 years, he added, “the need for re-intervention was particularly low (2%-3%) and equivalent for both approaches. And structural valve deterioration was also very low and equivalent in both groups.”
Evolut low-risk trial
The Evolut trial enrolled 1,414 patients with low surgical risk who were randomly assigned to TAVR, a self-expanding supra-annular CoreValve Evolut R PRO, or surgery.
By 4 years, the primary endpoint of all-cause mortality or disabling stroke had occurred in 10.7% of the TAVR group and 14.1% in the surgery group (hazard ratio, 0.74; P = .05), representing a 26% relative reduction with TAVR.
The absolute difference between treatment arms for the primary endpoint continued to increase over time: 1.8% at 1 year, 2.0% at 2 years, 2.9% at 3 years, and 3.4% at 4 years.
Rates of the primary endpoint components were all-cause mortality 9.0% with TAVR vs. 12.1% with surgery (P = .07); and disabling stroke was 2.9% with TAVR) vs. 3.8% for surgery (P = .32). Aortic valve rehospitalization was 10.3% with TAVR vs. 12.1% with surgery (P = .27).
The composite of all-cause mortality, disabling stroke, or aortic valve rehospitalization was significantly lower with TAVR, compared with surgery (18.0% vs. 22.4%; HR, 0.78; P = .04).
New permanent pacemaker implantation was significantly higher in the TAVR group (24.6% vs. 9.9%).
Indicators of valve performance including aortic valve reintervention (1.3% TAVR vs. 1.7% surgery); clinical or subclinical valve thrombosis (0.7% TAVR vs. 0.6% surgery); and valve endocarditis (0.9% TAVR vs. 2.2% surgery) were similarly low between groups, the authors report.
TAVR patients had sustained improvement in hemodynamics as measured by echocardiography, with significantly lower aortic valve mean gradients (9.8 mm Hg TAVR vs. 12.1 mm Hg surgery) and greater effective orifice area (2.1 cm2 TAVR vs. 2.0 cm2 surgery).
At 4 years, 84.7% of TAVR patients and 98.4% of surgery patients had no or trace paravalvular regurgitation, and there was no difference between groups in moderate or greater paravalvular regurgitation (0.4% TAVR vs. 0.0% surgery).
“The Evolut valve has shown a superior performance to surgery,” Dr. Reardon concluded. “It has less structural valve deterioration, less severe patient prosthetic mismatch, and superior hemodynamics, compared to surgery. All these factors are translating into a widening difference in clinical event curves year on year with the Evolut valve vs. surgery.”
Why the difference between trials?
The big question is why the early benefit seen with TAVR vs. surgery in both trials was attenuated by 5 years in PARTNER-3 but seemed to become greater each year in the Evolut trial. There were no definite explanations for these observations, but several possibilities were suggested.
Dr. Leon noted that with trials of intervention vs. surgery, it is common for the intervention group to do better in the beginning and for surgery to catch up a bit in later years. “So, it is not that much of a surprise to see outcomes plateauing in PARTNER-3.”
But he also suggested some other factors that may have played a role, one of which was the COVID pandemic.
“During the 2-year COVID period more than 75% of the deaths and strokes in the trial occurred in the TAVR patients,” he said. “Surgery patients were getting more anticoagulation because they had more paroxysmal [atrial fibrillation]. We know that COVID is a stimulus of thrombogenic events so in an odd way we think there may have been some cardioprotective effects from anticoagulation therapy in the surgery group.”
He also pointed out that though hospitalization and strokes were slightly lower with TAVR vs. surgery in the PARTNER-3 trial, mortality was slightly greater in the TAVR group.
“There was a 2:1 ratio in the TAVR and surgery groups in noncardiovascular deaths which influenced the all-cause mortality numbers,” he noted.
Mortality rates in the surgery groups
Dr. Leon also pointed out differences in the mortality rates in the surgery groups in the two trials, which he suggested may contribute to the explanation for the different longer-term results.
“The baseline characteristics for patients in these two trials were almost identical, and results at 1 year were very similar, but for whatever reason, over the course of a few years, the outcomes in the Evolut trial were different to those in PARTNER-3, and in particular the difference was in the surgical arms, with a higher event rate in the surgical arm in Evolut than in the surgery arm in PARTNER-3,” he said. “When the control does not perform well it is a lot easier to show that the experimental arm is better.”
“When you look at the TAVR arms in both studies at each time point they are either similar or PARTNER-3 is actually lower,” he added. “That is why it is so difficult to compare these two trials.”
But Dr. Reardon dismissed this argument.
“What determines long-term survival after a procedure is the intrinsic risk level of the patients,” he said. “Overall mortality rates differ between the two trials because the PARTNER-3 trial enrolled a lower end of a low-risk population while Evolut enrolled an upper end of a low-risk population. You cannot look at absolute numbers between trials. That is intellectually and scientifically invalid.”
“It is the relative difference between surgery and TAVR that we are interested in, and we see in Evolut that the relative difference between the two procedures in terms of benefit with TAVR is widening every year,” he added. “That is because the superior valve performance and hemodynamics of the Evolut valve compared to surgery has translated into excellent clinical outcomes.
“In the PARTNER-3 trial – their curves are coming together. I think that is worrisome, but I don’t want to draw conclusions about their trial,” Dr. Reardon said. “All I know is that in our trial, we have excellent outcomes that are getting better year after year.”
Competition between valves
The different results of the two trials is inevitably producing some competition between the two products.
Dr. Reardon said: “In terms of which valve to use, clinicians will want to choose a valve that has the best durability and shows the best survival vs. surgery and that is clearly the Evolut valve. I think the writing is on the wall. Some clinicians are going to wait for longer term data, but the question is do we have enough long-term data now.”
But Dr. Leon countered: “There’s never been a head-to-head device to suggest that the self- expanding device performs better than the balloon expanding device. We always think about them as being similar in terms of performance. There is an aggressive effort to suggest that by virtue of the current trial results there was a superior outcome with the Medtronic device, but it’s hard to explain why that would be the case, and we should not compare between the two trials.”
Both Dr. Leon and Dr. Reardon stressed that longer-term follow-up is critical because some surgical valves are known to fail between 5-10 years, and it is not known how the TAVR valves will perform over that period.
Both the PARTNER-3 and Evolut trials are planning to keep following patients out to 10 years.
For the time being though, both Dr. Leon and Dr. Reardon agreed that these current results will probably accelerate the already rapid transition from surgery to TAVR in low-risk patients.
“TAVR will be the default therapy,” Dr. Leon commented. “It will be the first choice for patients. Whether TAVR is superior to surgery in terms of outcomes or just the same, there are sufficient benefits from a logistic and patient perspective that most people would prefer to have the less invasive therapy. TAVR is a one-day procedure, there is no need for general anesthetic, a lot of the secondary outcomes that are so problematic with surgery don’t exist, and the ability to be in a symptom-free state is dramatically accelerated.”
“This was the first serious foray into the low-risk population with TAVR,” he added. “We had an age cut of 65 years, but the vast majority of patients in both trials were over 70. We could now start looking at younger patient populations.”
But Dr. Reardon said that these younger patients are already being given TAVR, and future trials randomizing between TAVR and surgery may not be possible.
“Even though US guidelines still recommend surgery for patients under 65 years, patients want TAVR, and they get TAVR,” he said. “Recent data shows that in 2021, use of TAVR rose to 47.5% in patients under 65 needing isolated aortic valve replacement. That doesn’t meet the guidelines but there’s clearly a big shift going on. These results will just keep that momentum going.”
The PARTNER-3 trial was funded by Edwards Lifesciences. The Evolut study was funded by Medtronic. Dr. Leon reports grant support from Edwards Lifesciences and Medtronic. Dr. Reardon receives research grants from Medtronic.
A version of this article first appeared on Medscape.com.
The PARTNER-3 and Evolut trials were heralded as a landmark advance in medicine when the 1-year results from the two studies were presented back in 2019. Both trials suggested benefits of the less-invasive TAVR approach over surgery.
But because these low-surgical-risk patients are younger and will likely have a longer lifespan than will higher risk patients for whom the TAVR technique was first established, patient outcomes and information on how the TAVR devices hold up over the long-term are critical to inform clinical decision-making.
Latest results from the two trials show that the initial benefits of TAVR over surgery seen in PARTNER-3 seem to have attenuated over the longer-term, with main outcomes looking very similar in both groups after 5 years.
However, in the Evolut trial, the early benefit in all-cause mortality or disabling stroke seen in the TAVR group is continuing to increase, with current results showing a 26% relative reduction in this endpoint with TAVR vs. surgery at 4 years.
The 5-year results of the PARTNER-3 trial and the 4-year results of the Evolut study were presented Transcatheter Cardiovascular Therapeutics annual meeting sponsored by the Cardiovascular Research Foundation. Both sets of results were simultaneously published online: PARTNER-3 in The New England Journal of Medicine and Evolut in JACC.
Marty Leon, MD, of NewYork-Presbyterian Columbia University Irving Medical Center, who presented the PARTNER-3 results, said in an interview that both trials are good news for TAVR:
“Both trials have clearly reaffirmed clinical and echocardiographic benefits of TAVR as a meaningful alternative therapy to surgery in low-risk severe symptomatic aortic stenosis patients.” Michael Reardon, MD, Houston Methodist Debakey Heart & Vascular Center, who presented the Evolut results, agreed that both trials were positive for TAVR “as TAVR just has to be as good as surgery to be a winner because clearly it is a lot less invasive.”
But Dr. Dr. Reardon added that, “In making that decision for younger lower-risk patients, then the Evolut valve is the only TAVR valve that has shown superior hemodynamics and durability at all time points with excellent outcomes and widening benefits compared with surgery over the first 4 years.”
PARTNER-3
The PARTNER-3 trial randomly assigned 1,000 patients with severe symptomatic aortic stenosis and low surgical risk to undergo either TAVR with the SAPIEN 3 transcatheter heart valve or surgery.
The results at 5 years show no difference in the two primary composite outcomes between TAVR and surgery patients.
The incidence of the composite end point of death, stroke, or rehospitalization related to the valve, the procedure, or heart failure was similar in the TAVR group and the surgery group, occurring in 22.8% of patients in the TAVR group and 27.2% in the surgery group, which is a nonsignificant difference (P = .07).
The incidence of the individual components of the composite end point were also similar in the two groups. Death occurred in 10.0% in the TAVR group and 8.2% in the surgery group; stroke in 5.8% of the TAVR group and 6.4% of the surgery group; and rehospitalization in 13.7% and 17.4%, respectively.
Aortic-valve durability also looked similar in the two groups. The hemodynamic performance of the valve, assessed according to the mean valve gradient, was 12.8 mm Hg in the TAVR group and 11.7 mm Hg in the surgery group. Bioprosthetic-valve failure occurred in 3.3% of the patients in the TAVR group and in 3.8% of those in the surgery group.
Among the secondary end points, atrial fibrillation and bleeding appeared to be less frequent in the TAVR group than in the surgery group, whereas paravalvular aortic regurgitation, valve thrombosis, and pacemaker implantation appeared to be less frequent in the surgery group.
Functional and health-status outcomes assessed according to New York Heart Association class, quality of life scores, and the percentage of patients who were alive and well at 5 years appeared to be similar in the two groups.
“These data are reassuring,” Dr. Leon said. “Cardiovascular mortality occurred at a rate of about 1% per year with both therapies, strokes at the rate of 1% per year with both therapies, and hospitalization for cardiovascular reasons at about 3% per year with both therapies. For patients in their 70s, these are very good numbers.”
Along with showing similar outcomes for TAVR and surgery at 5 years, he added, “the need for re-intervention was particularly low (2%-3%) and equivalent for both approaches. And structural valve deterioration was also very low and equivalent in both groups.”
Evolut low-risk trial
The Evolut trial enrolled 1,414 patients with low surgical risk who were randomly assigned to TAVR, a self-expanding supra-annular CoreValve Evolut R PRO, or surgery.
By 4 years, the primary endpoint of all-cause mortality or disabling stroke had occurred in 10.7% of the TAVR group and 14.1% in the surgery group (hazard ratio, 0.74; P = .05), representing a 26% relative reduction with TAVR.
The absolute difference between treatment arms for the primary endpoint continued to increase over time: 1.8% at 1 year, 2.0% at 2 years, 2.9% at 3 years, and 3.4% at 4 years.
Rates of the primary endpoint components were all-cause mortality 9.0% with TAVR vs. 12.1% with surgery (P = .07); and disabling stroke was 2.9% with TAVR) vs. 3.8% for surgery (P = .32). Aortic valve rehospitalization was 10.3% with TAVR vs. 12.1% with surgery (P = .27).
The composite of all-cause mortality, disabling stroke, or aortic valve rehospitalization was significantly lower with TAVR, compared with surgery (18.0% vs. 22.4%; HR, 0.78; P = .04).
New permanent pacemaker implantation was significantly higher in the TAVR group (24.6% vs. 9.9%).
Indicators of valve performance including aortic valve reintervention (1.3% TAVR vs. 1.7% surgery); clinical or subclinical valve thrombosis (0.7% TAVR vs. 0.6% surgery); and valve endocarditis (0.9% TAVR vs. 2.2% surgery) were similarly low between groups, the authors report.
TAVR patients had sustained improvement in hemodynamics as measured by echocardiography, with significantly lower aortic valve mean gradients (9.8 mm Hg TAVR vs. 12.1 mm Hg surgery) and greater effective orifice area (2.1 cm2 TAVR vs. 2.0 cm2 surgery).
At 4 years, 84.7% of TAVR patients and 98.4% of surgery patients had no or trace paravalvular regurgitation, and there was no difference between groups in moderate or greater paravalvular regurgitation (0.4% TAVR vs. 0.0% surgery).
“The Evolut valve has shown a superior performance to surgery,” Dr. Reardon concluded. “It has less structural valve deterioration, less severe patient prosthetic mismatch, and superior hemodynamics, compared to surgery. All these factors are translating into a widening difference in clinical event curves year on year with the Evolut valve vs. surgery.”
Why the difference between trials?
The big question is why the early benefit seen with TAVR vs. surgery in both trials was attenuated by 5 years in PARTNER-3 but seemed to become greater each year in the Evolut trial. There were no definite explanations for these observations, but several possibilities were suggested.
Dr. Leon noted that with trials of intervention vs. surgery, it is common for the intervention group to do better in the beginning and for surgery to catch up a bit in later years. “So, it is not that much of a surprise to see outcomes plateauing in PARTNER-3.”
But he also suggested some other factors that may have played a role, one of which was the COVID pandemic.
“During the 2-year COVID period more than 75% of the deaths and strokes in the trial occurred in the TAVR patients,” he said. “Surgery patients were getting more anticoagulation because they had more paroxysmal [atrial fibrillation]. We know that COVID is a stimulus of thrombogenic events so in an odd way we think there may have been some cardioprotective effects from anticoagulation therapy in the surgery group.”
He also pointed out that though hospitalization and strokes were slightly lower with TAVR vs. surgery in the PARTNER-3 trial, mortality was slightly greater in the TAVR group.
“There was a 2:1 ratio in the TAVR and surgery groups in noncardiovascular deaths which influenced the all-cause mortality numbers,” he noted.
Mortality rates in the surgery groups
Dr. Leon also pointed out differences in the mortality rates in the surgery groups in the two trials, which he suggested may contribute to the explanation for the different longer-term results.
“The baseline characteristics for patients in these two trials were almost identical, and results at 1 year were very similar, but for whatever reason, over the course of a few years, the outcomes in the Evolut trial were different to those in PARTNER-3, and in particular the difference was in the surgical arms, with a higher event rate in the surgical arm in Evolut than in the surgery arm in PARTNER-3,” he said. “When the control does not perform well it is a lot easier to show that the experimental arm is better.”
“When you look at the TAVR arms in both studies at each time point they are either similar or PARTNER-3 is actually lower,” he added. “That is why it is so difficult to compare these two trials.”
But Dr. Reardon dismissed this argument.
“What determines long-term survival after a procedure is the intrinsic risk level of the patients,” he said. “Overall mortality rates differ between the two trials because the PARTNER-3 trial enrolled a lower end of a low-risk population while Evolut enrolled an upper end of a low-risk population. You cannot look at absolute numbers between trials. That is intellectually and scientifically invalid.”
“It is the relative difference between surgery and TAVR that we are interested in, and we see in Evolut that the relative difference between the two procedures in terms of benefit with TAVR is widening every year,” he added. “That is because the superior valve performance and hemodynamics of the Evolut valve compared to surgery has translated into excellent clinical outcomes.
“In the PARTNER-3 trial – their curves are coming together. I think that is worrisome, but I don’t want to draw conclusions about their trial,” Dr. Reardon said. “All I know is that in our trial, we have excellent outcomes that are getting better year after year.”
Competition between valves
The different results of the two trials is inevitably producing some competition between the two products.
Dr. Reardon said: “In terms of which valve to use, clinicians will want to choose a valve that has the best durability and shows the best survival vs. surgery and that is clearly the Evolut valve. I think the writing is on the wall. Some clinicians are going to wait for longer term data, but the question is do we have enough long-term data now.”
But Dr. Leon countered: “There’s never been a head-to-head device to suggest that the self- expanding device performs better than the balloon expanding device. We always think about them as being similar in terms of performance. There is an aggressive effort to suggest that by virtue of the current trial results there was a superior outcome with the Medtronic device, but it’s hard to explain why that would be the case, and we should not compare between the two trials.”
Both Dr. Leon and Dr. Reardon stressed that longer-term follow-up is critical because some surgical valves are known to fail between 5-10 years, and it is not known how the TAVR valves will perform over that period.
Both the PARTNER-3 and Evolut trials are planning to keep following patients out to 10 years.
For the time being though, both Dr. Leon and Dr. Reardon agreed that these current results will probably accelerate the already rapid transition from surgery to TAVR in low-risk patients.
“TAVR will be the default therapy,” Dr. Leon commented. “It will be the first choice for patients. Whether TAVR is superior to surgery in terms of outcomes or just the same, there are sufficient benefits from a logistic and patient perspective that most people would prefer to have the less invasive therapy. TAVR is a one-day procedure, there is no need for general anesthetic, a lot of the secondary outcomes that are so problematic with surgery don’t exist, and the ability to be in a symptom-free state is dramatically accelerated.”
“This was the first serious foray into the low-risk population with TAVR,” he added. “We had an age cut of 65 years, but the vast majority of patients in both trials were over 70. We could now start looking at younger patient populations.”
But Dr. Reardon said that these younger patients are already being given TAVR, and future trials randomizing between TAVR and surgery may not be possible.
“Even though US guidelines still recommend surgery for patients under 65 years, patients want TAVR, and they get TAVR,” he said. “Recent data shows that in 2021, use of TAVR rose to 47.5% in patients under 65 needing isolated aortic valve replacement. That doesn’t meet the guidelines but there’s clearly a big shift going on. These results will just keep that momentum going.”
The PARTNER-3 trial was funded by Edwards Lifesciences. The Evolut study was funded by Medtronic. Dr. Leon reports grant support from Edwards Lifesciences and Medtronic. Dr. Reardon receives research grants from Medtronic.
A version of this article first appeared on Medscape.com.
FROM TCT 2023
Trilogy TAVR safe, effective in aortic regurgitation
SAN FRANCISCO – , achieving a 1-year all-cause mortality rate of 7.8%.
New pacemaker implantation was 24%, similar to previously reported outcomes.
Vinod Thourani, MD, Piedmont Heart Institute, Atlanta, presented initial outcome results of the ALIGN-AR trial at the Transcatheter Cardiovascular Therapeutics annual meeting.
Dr. Thourani concluded that the Trilogy system provides the first dedicated transcatheter aortic valve replacement options “for symptomatic patients with moderate to severe or severe aortic regurgitation or at high risk for surgery and is well positioned to become the preferred therapy upon approval for this population.”
Currently, Trilogy is not approved by the U.S. Food and Drug Administration in the United States and is for investigational use only.
Untreated, severe symptomatic aortic regurgitation (AR) is associated with high mortality, especially for those with NYHA class 3 or 4 symptoms, Dr. Thourani explained. “While surgery remains the only recommended intervention for patients with native severe AR, there are a multitude of high-risk patients who are not offered therapy.”
Off-label use of transcatheter valves for AR has been associated with “higher rates of complications, including paravalvular regurgitation and embolization,” he noted.
Dr. Thourani described the unique features of the JenaValve Trilogy valve. The system has a set of three “locators” in its own sheath that allows it to be rotated to align with the three cusps of the native aortic valve, falling into the sinuses and securely anchored to the native valve leaflets – then the valve is deployed. Inside a self-expanding nitinol frame is porcine pericardial tissue. A sealing ring provides sufficient anchoring while conforming to the annulus.
ALIGN-AR was a multicenter, single arm, non-blinded trial with follow-up out to 5 years involving patients with 3-plus or greater AR at high risk for surgical aortic valve replacement. Exclusion criteria included an aortic root diameter greater than 5 cm, a previous prosthetic aortic valve, mitral regurgitation greater than moderate, or coronary artery disease requiring revascularization.
After Trilogy valve implantation, patients were followed for 1, 6, and 12 months, as well as annually out to 5 years. Safety and efficacy endpoints were compared with prespecified performance goals. Of 180 patients enrolled, 177 were successfully implanted with the Trilogy device.
Patients had an average age of 75.5 years, 47.2% were women, 67.2% were in NYHA class III/IV, 82.8% were hypertensive, and one-third were frail. Severe AR was present in 62.4%, and 31.7% had moderate to severe AR.
The primary composite safety endpoint included all-cause mortality, any stroke, major vascular complication, major bleeding, a new pacemaker, acute kidney injury, valve dysfunction, or any intervention related to the device. The primary efficacy endpoint was all-cause mortality at 12 months.
The performance goal for primary efficacy was a weighted average of 25%, derived mainly from 1-year mortality figures for NYHA class I/II and class II/IV with conservative management.
Non-inferiority margin met
With a 25% prespecified non-inferiority margin for the primary efficacy endpoint, “We have observed a rate of 7.8%,” Dr. Thourani reported during a late-breaking clinical trials session. “The non-inferiority margin was met for the primary efficacy endpoint with a P value of less than .0001.”
“With a 40.5% prespecified non-inferiority margin of our primary safety endpoint, with a Trilogy [heart valve] we have observed a rate of 26.7%,” he said. “At 30 days there was a 2.2% mortality and a 2.2% stroke rate. There was a 26.7% primary safety endpoint, mainly driven by the 24% new pacemaker implantation rate. Without pacemaker implantation, the rate of safety events was less than 8%,” (P noninferiority < .0001).
Procedure technical success was 95%, device success 96.7%, and procedure success 92.8%. There was one ascending aortic dissection (0.6%). Moderate or greater paravalvular regurgitation also occurred in one patient. There were four cases of valve embolization.
Pacemaker implants occurred in 30% of patients in the first tercile enrolled and decreased to 14% for the third tercile enrolled. “Lower rates are most likely due to the change in the insertion technique, placing locators above the nadir of the native cusps, reduction in oversizing, and also evolution in the management of periprocedural conduction abnormalities,” Dr. Thourani proposed.
The hemodynamics of the valve improved from a gradient of 8.7 mm Hg at baseline to 3.9 mm Hg at 30 days and remained fairly stable out to 1 year. Paravalvular regurgitation was absent in 80.8% of patients at 30 days and mild in 18%. It improved over time, being absent in 93.5% at 6 months and in 92.2% at 1 year.
Left ventricular (LV) remodeling occurred over one year, with LV end systolic diameter, LV end systolic volume, LV mass, and LV mass index all decreasing significantly from baseline to one year (all P < .0001). Importantly to patients, NYHA class improved, from 32% class II, 63% class III, and 5% class IV at baseline to 54% class I, 37% class II, and 10% class III at 30 days and improving slightly out to 1 year.
These improvements resulted in better quality of life, as reflected in a 21.8-point improvement in the self-reported Kansas City Cardiomyopathy Questionnaire Overall Summary Score, with a score of 77.6 at 1 year, indicating self-perceived good health.
Encouraging data
During the session, Robert Bonow, MD, of Northwestern University Feinberg School of Medicine, Chicago, commented that Dr. Thourani presented very encouraging data from the ALIGN-AR trial of high-risk surgical patients with significant aortic regurgitation. However, he had a couple of questions for Dr. Thourani.
One related to the efficacy data being compared with historical survival data. “So, are you planning to do a randomized study of these patients? You could argue, unlike aortic stenosis, where there’s no medical therapy, there could be medical therapies for the patients.” He noted that one-third of the patients are only in NYHA functional class II, so those patients “might do well over the long haul, with medical therapy as an agent.”
Dr. Thourani said it was an excellent question. “Doing a randomized trial with a high-risk patient [is] probably less likely,” he said. “I think there is a lot of interest among physicians on the cardiology side and on the surgery side of looking at lower-risk patients, and this could include those that are intermediate and or low risk.”
He said he believes investigators and leadership of the ALIGN-AR trial have conceived of such a trial involving all comers. “I think that’s warranted if we go into younger patients,” he said.
Dr. Bonow then asked if there was significant aortic valve calcification in the study population, because it is common with regurgitation, “which is why standard approaches are not effective ... And how does this device behave in calcified valves?” But Dr. Thourani said calcification was an exclusion criterion for this trial.
He said, “deep dives are going to come,” looking at the ventricular outcomes and also looking at a lot of the echocardiographic parameters.
Dr. Bonow related this study’s findings on ventricular remodeling to what is seen with surgical aortic valve replacement, where the ventricle decompresses within days. “And that’s predictive of good outcome if you have early data and these patients show how the ventricle remodels quickly,” he said.
The trial was supported by JenaValve. Dr. Thourani has received grant/research support from Abbott Vascular, Artivion, Atricure, Boston Scientific, CroiValve, Edwards Lifesciences, JenaValve, Medtronic, and Trisol; consultant fees/honoraria from Abbott Vascular, Artivion, Atricure, Boston Scientific, Croivalve, and Edwards Lifesciences; and has an executive role/ownership interest in DASI Simulations. Dr. Bonow had no disclosures.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – , achieving a 1-year all-cause mortality rate of 7.8%.
New pacemaker implantation was 24%, similar to previously reported outcomes.
Vinod Thourani, MD, Piedmont Heart Institute, Atlanta, presented initial outcome results of the ALIGN-AR trial at the Transcatheter Cardiovascular Therapeutics annual meeting.
Dr. Thourani concluded that the Trilogy system provides the first dedicated transcatheter aortic valve replacement options “for symptomatic patients with moderate to severe or severe aortic regurgitation or at high risk for surgery and is well positioned to become the preferred therapy upon approval for this population.”
Currently, Trilogy is not approved by the U.S. Food and Drug Administration in the United States and is for investigational use only.
Untreated, severe symptomatic aortic regurgitation (AR) is associated with high mortality, especially for those with NYHA class 3 or 4 symptoms, Dr. Thourani explained. “While surgery remains the only recommended intervention for patients with native severe AR, there are a multitude of high-risk patients who are not offered therapy.”
Off-label use of transcatheter valves for AR has been associated with “higher rates of complications, including paravalvular regurgitation and embolization,” he noted.
Dr. Thourani described the unique features of the JenaValve Trilogy valve. The system has a set of three “locators” in its own sheath that allows it to be rotated to align with the three cusps of the native aortic valve, falling into the sinuses and securely anchored to the native valve leaflets – then the valve is deployed. Inside a self-expanding nitinol frame is porcine pericardial tissue. A sealing ring provides sufficient anchoring while conforming to the annulus.
ALIGN-AR was a multicenter, single arm, non-blinded trial with follow-up out to 5 years involving patients with 3-plus or greater AR at high risk for surgical aortic valve replacement. Exclusion criteria included an aortic root diameter greater than 5 cm, a previous prosthetic aortic valve, mitral regurgitation greater than moderate, or coronary artery disease requiring revascularization.
After Trilogy valve implantation, patients were followed for 1, 6, and 12 months, as well as annually out to 5 years. Safety and efficacy endpoints were compared with prespecified performance goals. Of 180 patients enrolled, 177 were successfully implanted with the Trilogy device.
Patients had an average age of 75.5 years, 47.2% were women, 67.2% were in NYHA class III/IV, 82.8% were hypertensive, and one-third were frail. Severe AR was present in 62.4%, and 31.7% had moderate to severe AR.
The primary composite safety endpoint included all-cause mortality, any stroke, major vascular complication, major bleeding, a new pacemaker, acute kidney injury, valve dysfunction, or any intervention related to the device. The primary efficacy endpoint was all-cause mortality at 12 months.
The performance goal for primary efficacy was a weighted average of 25%, derived mainly from 1-year mortality figures for NYHA class I/II and class II/IV with conservative management.
Non-inferiority margin met
With a 25% prespecified non-inferiority margin for the primary efficacy endpoint, “We have observed a rate of 7.8%,” Dr. Thourani reported during a late-breaking clinical trials session. “The non-inferiority margin was met for the primary efficacy endpoint with a P value of less than .0001.”
“With a 40.5% prespecified non-inferiority margin of our primary safety endpoint, with a Trilogy [heart valve] we have observed a rate of 26.7%,” he said. “At 30 days there was a 2.2% mortality and a 2.2% stroke rate. There was a 26.7% primary safety endpoint, mainly driven by the 24% new pacemaker implantation rate. Without pacemaker implantation, the rate of safety events was less than 8%,” (P noninferiority < .0001).
Procedure technical success was 95%, device success 96.7%, and procedure success 92.8%. There was one ascending aortic dissection (0.6%). Moderate or greater paravalvular regurgitation also occurred in one patient. There were four cases of valve embolization.
Pacemaker implants occurred in 30% of patients in the first tercile enrolled and decreased to 14% for the third tercile enrolled. “Lower rates are most likely due to the change in the insertion technique, placing locators above the nadir of the native cusps, reduction in oversizing, and also evolution in the management of periprocedural conduction abnormalities,” Dr. Thourani proposed.
The hemodynamics of the valve improved from a gradient of 8.7 mm Hg at baseline to 3.9 mm Hg at 30 days and remained fairly stable out to 1 year. Paravalvular regurgitation was absent in 80.8% of patients at 30 days and mild in 18%. It improved over time, being absent in 93.5% at 6 months and in 92.2% at 1 year.
Left ventricular (LV) remodeling occurred over one year, with LV end systolic diameter, LV end systolic volume, LV mass, and LV mass index all decreasing significantly from baseline to one year (all P < .0001). Importantly to patients, NYHA class improved, from 32% class II, 63% class III, and 5% class IV at baseline to 54% class I, 37% class II, and 10% class III at 30 days and improving slightly out to 1 year.
These improvements resulted in better quality of life, as reflected in a 21.8-point improvement in the self-reported Kansas City Cardiomyopathy Questionnaire Overall Summary Score, with a score of 77.6 at 1 year, indicating self-perceived good health.
Encouraging data
During the session, Robert Bonow, MD, of Northwestern University Feinberg School of Medicine, Chicago, commented that Dr. Thourani presented very encouraging data from the ALIGN-AR trial of high-risk surgical patients with significant aortic regurgitation. However, he had a couple of questions for Dr. Thourani.
One related to the efficacy data being compared with historical survival data. “So, are you planning to do a randomized study of these patients? You could argue, unlike aortic stenosis, where there’s no medical therapy, there could be medical therapies for the patients.” He noted that one-third of the patients are only in NYHA functional class II, so those patients “might do well over the long haul, with medical therapy as an agent.”
Dr. Thourani said it was an excellent question. “Doing a randomized trial with a high-risk patient [is] probably less likely,” he said. “I think there is a lot of interest among physicians on the cardiology side and on the surgery side of looking at lower-risk patients, and this could include those that are intermediate and or low risk.”
He said he believes investigators and leadership of the ALIGN-AR trial have conceived of such a trial involving all comers. “I think that’s warranted if we go into younger patients,” he said.
Dr. Bonow then asked if there was significant aortic valve calcification in the study population, because it is common with regurgitation, “which is why standard approaches are not effective ... And how does this device behave in calcified valves?” But Dr. Thourani said calcification was an exclusion criterion for this trial.
He said, “deep dives are going to come,” looking at the ventricular outcomes and also looking at a lot of the echocardiographic parameters.
Dr. Bonow related this study’s findings on ventricular remodeling to what is seen with surgical aortic valve replacement, where the ventricle decompresses within days. “And that’s predictive of good outcome if you have early data and these patients show how the ventricle remodels quickly,” he said.
The trial was supported by JenaValve. Dr. Thourani has received grant/research support from Abbott Vascular, Artivion, Atricure, Boston Scientific, CroiValve, Edwards Lifesciences, JenaValve, Medtronic, and Trisol; consultant fees/honoraria from Abbott Vascular, Artivion, Atricure, Boston Scientific, Croivalve, and Edwards Lifesciences; and has an executive role/ownership interest in DASI Simulations. Dr. Bonow had no disclosures.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – , achieving a 1-year all-cause mortality rate of 7.8%.
New pacemaker implantation was 24%, similar to previously reported outcomes.
Vinod Thourani, MD, Piedmont Heart Institute, Atlanta, presented initial outcome results of the ALIGN-AR trial at the Transcatheter Cardiovascular Therapeutics annual meeting.
Dr. Thourani concluded that the Trilogy system provides the first dedicated transcatheter aortic valve replacement options “for symptomatic patients with moderate to severe or severe aortic regurgitation or at high risk for surgery and is well positioned to become the preferred therapy upon approval for this population.”
Currently, Trilogy is not approved by the U.S. Food and Drug Administration in the United States and is for investigational use only.
Untreated, severe symptomatic aortic regurgitation (AR) is associated with high mortality, especially for those with NYHA class 3 or 4 symptoms, Dr. Thourani explained. “While surgery remains the only recommended intervention for patients with native severe AR, there are a multitude of high-risk patients who are not offered therapy.”
Off-label use of transcatheter valves for AR has been associated with “higher rates of complications, including paravalvular regurgitation and embolization,” he noted.
Dr. Thourani described the unique features of the JenaValve Trilogy valve. The system has a set of three “locators” in its own sheath that allows it to be rotated to align with the three cusps of the native aortic valve, falling into the sinuses and securely anchored to the native valve leaflets – then the valve is deployed. Inside a self-expanding nitinol frame is porcine pericardial tissue. A sealing ring provides sufficient anchoring while conforming to the annulus.
ALIGN-AR was a multicenter, single arm, non-blinded trial with follow-up out to 5 years involving patients with 3-plus or greater AR at high risk for surgical aortic valve replacement. Exclusion criteria included an aortic root diameter greater than 5 cm, a previous prosthetic aortic valve, mitral regurgitation greater than moderate, or coronary artery disease requiring revascularization.
After Trilogy valve implantation, patients were followed for 1, 6, and 12 months, as well as annually out to 5 years. Safety and efficacy endpoints were compared with prespecified performance goals. Of 180 patients enrolled, 177 were successfully implanted with the Trilogy device.
Patients had an average age of 75.5 years, 47.2% were women, 67.2% were in NYHA class III/IV, 82.8% were hypertensive, and one-third were frail. Severe AR was present in 62.4%, and 31.7% had moderate to severe AR.
The primary composite safety endpoint included all-cause mortality, any stroke, major vascular complication, major bleeding, a new pacemaker, acute kidney injury, valve dysfunction, or any intervention related to the device. The primary efficacy endpoint was all-cause mortality at 12 months.
The performance goal for primary efficacy was a weighted average of 25%, derived mainly from 1-year mortality figures for NYHA class I/II and class II/IV with conservative management.
Non-inferiority margin met
With a 25% prespecified non-inferiority margin for the primary efficacy endpoint, “We have observed a rate of 7.8%,” Dr. Thourani reported during a late-breaking clinical trials session. “The non-inferiority margin was met for the primary efficacy endpoint with a P value of less than .0001.”
“With a 40.5% prespecified non-inferiority margin of our primary safety endpoint, with a Trilogy [heart valve] we have observed a rate of 26.7%,” he said. “At 30 days there was a 2.2% mortality and a 2.2% stroke rate. There was a 26.7% primary safety endpoint, mainly driven by the 24% new pacemaker implantation rate. Without pacemaker implantation, the rate of safety events was less than 8%,” (P noninferiority < .0001).
Procedure technical success was 95%, device success 96.7%, and procedure success 92.8%. There was one ascending aortic dissection (0.6%). Moderate or greater paravalvular regurgitation also occurred in one patient. There were four cases of valve embolization.
Pacemaker implants occurred in 30% of patients in the first tercile enrolled and decreased to 14% for the third tercile enrolled. “Lower rates are most likely due to the change in the insertion technique, placing locators above the nadir of the native cusps, reduction in oversizing, and also evolution in the management of periprocedural conduction abnormalities,” Dr. Thourani proposed.
The hemodynamics of the valve improved from a gradient of 8.7 mm Hg at baseline to 3.9 mm Hg at 30 days and remained fairly stable out to 1 year. Paravalvular regurgitation was absent in 80.8% of patients at 30 days and mild in 18%. It improved over time, being absent in 93.5% at 6 months and in 92.2% at 1 year.
Left ventricular (LV) remodeling occurred over one year, with LV end systolic diameter, LV end systolic volume, LV mass, and LV mass index all decreasing significantly from baseline to one year (all P < .0001). Importantly to patients, NYHA class improved, from 32% class II, 63% class III, and 5% class IV at baseline to 54% class I, 37% class II, and 10% class III at 30 days and improving slightly out to 1 year.
These improvements resulted in better quality of life, as reflected in a 21.8-point improvement in the self-reported Kansas City Cardiomyopathy Questionnaire Overall Summary Score, with a score of 77.6 at 1 year, indicating self-perceived good health.
Encouraging data
During the session, Robert Bonow, MD, of Northwestern University Feinberg School of Medicine, Chicago, commented that Dr. Thourani presented very encouraging data from the ALIGN-AR trial of high-risk surgical patients with significant aortic regurgitation. However, he had a couple of questions for Dr. Thourani.
One related to the efficacy data being compared with historical survival data. “So, are you planning to do a randomized study of these patients? You could argue, unlike aortic stenosis, where there’s no medical therapy, there could be medical therapies for the patients.” He noted that one-third of the patients are only in NYHA functional class II, so those patients “might do well over the long haul, with medical therapy as an agent.”
Dr. Thourani said it was an excellent question. “Doing a randomized trial with a high-risk patient [is] probably less likely,” he said. “I think there is a lot of interest among physicians on the cardiology side and on the surgery side of looking at lower-risk patients, and this could include those that are intermediate and or low risk.”
He said he believes investigators and leadership of the ALIGN-AR trial have conceived of such a trial involving all comers. “I think that’s warranted if we go into younger patients,” he said.
Dr. Bonow then asked if there was significant aortic valve calcification in the study population, because it is common with regurgitation, “which is why standard approaches are not effective ... And how does this device behave in calcified valves?” But Dr. Thourani said calcification was an exclusion criterion for this trial.
He said, “deep dives are going to come,” looking at the ventricular outcomes and also looking at a lot of the echocardiographic parameters.
Dr. Bonow related this study’s findings on ventricular remodeling to what is seen with surgical aortic valve replacement, where the ventricle decompresses within days. “And that’s predictive of good outcome if you have early data and these patients show how the ventricle remodels quickly,” he said.
The trial was supported by JenaValve. Dr. Thourani has received grant/research support from Abbott Vascular, Artivion, Atricure, Boston Scientific, CroiValve, Edwards Lifesciences, JenaValve, Medtronic, and Trisol; consultant fees/honoraria from Abbott Vascular, Artivion, Atricure, Boston Scientific, Croivalve, and Edwards Lifesciences; and has an executive role/ownership interest in DASI Simulations. Dr. Bonow had no disclosures.
A version of this article first appeared on Medscape.com.
AT TCT 2023
Obesity boosts gestational diabetes risk in women with PCOS
In a population-based cohort study that included more than 1.2 million hospital live births, PCOS was associated with a 5% increase in risk for gestational diabetes. Almost 90% of this association was mediated by obesity.
“Women with PCOS are at higher risk, but it’s only 5% higher than the general population. However, that risk rises substantially with obesity,” senior author Maria P. Velez, MD, PhD, clinician-scientist and associate professor of obstetrics and gynecology at Queen’s University, Kingston, Ont., said in an interview. “Our study highlights the need for counseling our patients about the importance of weight optimization, ideally starting with lifestyle changes like diet and exercise.”The findings were published in the Journal of Obstetrics and Gynaecology Canada.
Major mediator
The estimated prevalence of PCOS is 8%-13%, and affected patients often present with anovulation, hyperandrogenism, obesity, metabolic syndrome, and infertility. Prepregnancy insulin resistance is common among women with PCOS and may play a major part in the pathogenesis of gestational diabetes. In addition, PCOS is often accompanied by excess weight gain; about 60% of women with PCOS are overweight or obese.
Previous research has shown that PCOS is a risk factor for gestational diabetes independent of obesity, while other research has shown that obesity has an important effect on this risk.
For the current study, the researchers used causal mediation analysis to elucidate more clearly the effect of obesity on the development of gestational diabetes among patients with PCOS. No previous study has used causal mediation analysis to examine this relationship.
Using data from linked universal health databases in Ontario, the researchers analyzed data on 1,268,901 births between 2006 and 2018. Of these births, 386,748 were associated with maternal PCOS.
The rate of gestational diabetes was higher among women with PCOS (60.2 per 1000 births), compared with women without PCOS (48.6 per 1,000 births). The finding resulted in an adjusted relative risk of 1.05. Obesity mediated 89.7% of this association.
“We hope that these data will inform preconception counseling and gestational diabetes screening in pregnant women with PCOS,” said Dr. Velez. “We have the data now to counsel our patients on the importance of weight management before pregnancy. But we need more resources, such as specialized clinics, to help these patients cope with managing their weight. We can tell our patients to work on their weight management, but they need much more support from the health care system.”
Results ‘not surprising’
Commenting on the study, Francine Hippolyte, MD, vice chair of obstetrics and gynecology at Long Island Jewish Medical Center, Katz Women’s Hospital, New Hyde Park, N.Y., said that the results are “not at all surprising.” Dr. Hippolyte was not involved in the research.
“We do know that PCOS is and should be treated as a metabolic syndrome. It’s a lot more than just infertility or changes or abnormalities with one’s menstrual cycle. It impacts a woman’s risk for diabetes, prediabetes, and abnormal lipid profile, regardless of whether or not she is obese,” said Dr. Hippolyte.
She agrees with the need for specialized clinics to help such vulnerable patients manage their weight.
“It would be great if insurances would cover things like nutritional counseling or have nutritionists on their roster so that patients can easily access that service. Many patients want to do right, especially preconceptually, but it is difficult without having access to resources. Unfortunately, as clinicians, we’re not as well versed in nutrition as we would like to be or should be, so we need a multidisciplinary approach. We need nutrition and weight loss clinics and proper services to really help these patients.”
The study was supported by the Canadian Institute of Health Research and ICES. Dr. Velez and Dr. Hippolyte reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a population-based cohort study that included more than 1.2 million hospital live births, PCOS was associated with a 5% increase in risk for gestational diabetes. Almost 90% of this association was mediated by obesity.
“Women with PCOS are at higher risk, but it’s only 5% higher than the general population. However, that risk rises substantially with obesity,” senior author Maria P. Velez, MD, PhD, clinician-scientist and associate professor of obstetrics and gynecology at Queen’s University, Kingston, Ont., said in an interview. “Our study highlights the need for counseling our patients about the importance of weight optimization, ideally starting with lifestyle changes like diet and exercise.”The findings were published in the Journal of Obstetrics and Gynaecology Canada.
Major mediator
The estimated prevalence of PCOS is 8%-13%, and affected patients often present with anovulation, hyperandrogenism, obesity, metabolic syndrome, and infertility. Prepregnancy insulin resistance is common among women with PCOS and may play a major part in the pathogenesis of gestational diabetes. In addition, PCOS is often accompanied by excess weight gain; about 60% of women with PCOS are overweight or obese.
Previous research has shown that PCOS is a risk factor for gestational diabetes independent of obesity, while other research has shown that obesity has an important effect on this risk.
For the current study, the researchers used causal mediation analysis to elucidate more clearly the effect of obesity on the development of gestational diabetes among patients with PCOS. No previous study has used causal mediation analysis to examine this relationship.
Using data from linked universal health databases in Ontario, the researchers analyzed data on 1,268,901 births between 2006 and 2018. Of these births, 386,748 were associated with maternal PCOS.
The rate of gestational diabetes was higher among women with PCOS (60.2 per 1000 births), compared with women without PCOS (48.6 per 1,000 births). The finding resulted in an adjusted relative risk of 1.05. Obesity mediated 89.7% of this association.
“We hope that these data will inform preconception counseling and gestational diabetes screening in pregnant women with PCOS,” said Dr. Velez. “We have the data now to counsel our patients on the importance of weight management before pregnancy. But we need more resources, such as specialized clinics, to help these patients cope with managing their weight. We can tell our patients to work on their weight management, but they need much more support from the health care system.”
Results ‘not surprising’
Commenting on the study, Francine Hippolyte, MD, vice chair of obstetrics and gynecology at Long Island Jewish Medical Center, Katz Women’s Hospital, New Hyde Park, N.Y., said that the results are “not at all surprising.” Dr. Hippolyte was not involved in the research.
“We do know that PCOS is and should be treated as a metabolic syndrome. It’s a lot more than just infertility or changes or abnormalities with one’s menstrual cycle. It impacts a woman’s risk for diabetes, prediabetes, and abnormal lipid profile, regardless of whether or not she is obese,” said Dr. Hippolyte.
She agrees with the need for specialized clinics to help such vulnerable patients manage their weight.
“It would be great if insurances would cover things like nutritional counseling or have nutritionists on their roster so that patients can easily access that service. Many patients want to do right, especially preconceptually, but it is difficult without having access to resources. Unfortunately, as clinicians, we’re not as well versed in nutrition as we would like to be or should be, so we need a multidisciplinary approach. We need nutrition and weight loss clinics and proper services to really help these patients.”
The study was supported by the Canadian Institute of Health Research and ICES. Dr. Velez and Dr. Hippolyte reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a population-based cohort study that included more than 1.2 million hospital live births, PCOS was associated with a 5% increase in risk for gestational diabetes. Almost 90% of this association was mediated by obesity.
“Women with PCOS are at higher risk, but it’s only 5% higher than the general population. However, that risk rises substantially with obesity,” senior author Maria P. Velez, MD, PhD, clinician-scientist and associate professor of obstetrics and gynecology at Queen’s University, Kingston, Ont., said in an interview. “Our study highlights the need for counseling our patients about the importance of weight optimization, ideally starting with lifestyle changes like diet and exercise.”The findings were published in the Journal of Obstetrics and Gynaecology Canada.
Major mediator
The estimated prevalence of PCOS is 8%-13%, and affected patients often present with anovulation, hyperandrogenism, obesity, metabolic syndrome, and infertility. Prepregnancy insulin resistance is common among women with PCOS and may play a major part in the pathogenesis of gestational diabetes. In addition, PCOS is often accompanied by excess weight gain; about 60% of women with PCOS are overweight or obese.
Previous research has shown that PCOS is a risk factor for gestational diabetes independent of obesity, while other research has shown that obesity has an important effect on this risk.
For the current study, the researchers used causal mediation analysis to elucidate more clearly the effect of obesity on the development of gestational diabetes among patients with PCOS. No previous study has used causal mediation analysis to examine this relationship.
Using data from linked universal health databases in Ontario, the researchers analyzed data on 1,268,901 births between 2006 and 2018. Of these births, 386,748 were associated with maternal PCOS.
The rate of gestational diabetes was higher among women with PCOS (60.2 per 1000 births), compared with women without PCOS (48.6 per 1,000 births). The finding resulted in an adjusted relative risk of 1.05. Obesity mediated 89.7% of this association.
“We hope that these data will inform preconception counseling and gestational diabetes screening in pregnant women with PCOS,” said Dr. Velez. “We have the data now to counsel our patients on the importance of weight management before pregnancy. But we need more resources, such as specialized clinics, to help these patients cope with managing their weight. We can tell our patients to work on their weight management, but they need much more support from the health care system.”
Results ‘not surprising’
Commenting on the study, Francine Hippolyte, MD, vice chair of obstetrics and gynecology at Long Island Jewish Medical Center, Katz Women’s Hospital, New Hyde Park, N.Y., said that the results are “not at all surprising.” Dr. Hippolyte was not involved in the research.
“We do know that PCOS is and should be treated as a metabolic syndrome. It’s a lot more than just infertility or changes or abnormalities with one’s menstrual cycle. It impacts a woman’s risk for diabetes, prediabetes, and abnormal lipid profile, regardless of whether or not she is obese,” said Dr. Hippolyte.
She agrees with the need for specialized clinics to help such vulnerable patients manage their weight.
“It would be great if insurances would cover things like nutritional counseling or have nutritionists on their roster so that patients can easily access that service. Many patients want to do right, especially preconceptually, but it is difficult without having access to resources. Unfortunately, as clinicians, we’re not as well versed in nutrition as we would like to be or should be, so we need a multidisciplinary approach. We need nutrition and weight loss clinics and proper services to really help these patients.”
The study was supported by the Canadian Institute of Health Research and ICES. Dr. Velez and Dr. Hippolyte reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF OBSTETRICS AND GYNAECOLOGY CANADA
Lack of racial, ethnic diversity in cryopreserved donor sperm in the U.S.
, according to a study presented at the American Society for Reproductive Medicine’s 2023 meeting.
“This really highlights the need to identify barriers to increase recruitment of these donors so that we can support family-building for all populations,” said Lauren Gibbs, MD, a resident in the department of obstetrics and gynecology at the Morehouse School of Medicine in Atlanta.
Dr. Gibbs and her colleagues compared the racial and ethnic makeup of sperm donors from online and self-reported profiles at 14 of the largest donor banks in the United States for March and April of 2023. Historical data were pulled from two large, national banks. The investigators compared these data to census estimates from 2021 for men between the ages of 18 and 44 years.
Donors who identified as Hispanic (10.9%) or Black (3.3%) were significantly underrepresented as compared to the U.S. population, of which Hispanic men compose 22% and Black men make up 13.3%.
Asian donors were overrepresented, making up 21.9% of the donors but only 6.5% of the U.S. population. White donors were proportionately represented in relation to national demographics, making up 56.6% of the donors and representing 55% of the U.S. population, according to the researchers. None of the donors identified as Native/Hawaiian/Pacific Islander or American Indian/Alaskan Natives; these groups represent 0.22% and 0.79% of the U.S. population, respectively.
“Next steps will be figuring out why this is happening and how to address it,” said Valerie L Baker, MD, director in the division of reproductive endocrinology and infertility at Johns Hopkins Medicine in Lutherville, Md., who was not involved in the study.
The study sheds light on the need to identify and address the barriers that discourage potential donors from underrepresented groups from participating in sperm donation, according to Kimball Pomeroy, PhD, scientific director at the World Egg and Sperm Bank in Scottsdale, Ariz.
“Sometimes there are inhibitors of different ethnic groups to want to act as sperm or egg donors, so trying to understand if that’s the case is important; but I’m sure a lot of it is also related to access,” Dr. Pomeroy, who was not part of the study team, said in an interview.
Longitudinal data from the two national donor banks did not indicate any significant increase or decrease in donation trends across the 5-year period from 2018 to 2022, highlighting the persisting issue of representation disparities. Dr. Gibbs said strategies need to be developed to increase recruitment of donors from underrepresented groups. Increasing the diversity of the donor pool will ultimately support family-building options for all patients, according to Dr. Gibbs.
Funding for the study was provided by the EMD Serono REI Diversity Fellowship Grant. Dr. Gibbs reports no relevant financial relationships.
, according to a study presented at the American Society for Reproductive Medicine’s 2023 meeting.
“This really highlights the need to identify barriers to increase recruitment of these donors so that we can support family-building for all populations,” said Lauren Gibbs, MD, a resident in the department of obstetrics and gynecology at the Morehouse School of Medicine in Atlanta.
Dr. Gibbs and her colleagues compared the racial and ethnic makeup of sperm donors from online and self-reported profiles at 14 of the largest donor banks in the United States for March and April of 2023. Historical data were pulled from two large, national banks. The investigators compared these data to census estimates from 2021 for men between the ages of 18 and 44 years.
Donors who identified as Hispanic (10.9%) or Black (3.3%) were significantly underrepresented as compared to the U.S. population, of which Hispanic men compose 22% and Black men make up 13.3%.
Asian donors were overrepresented, making up 21.9% of the donors but only 6.5% of the U.S. population. White donors were proportionately represented in relation to national demographics, making up 56.6% of the donors and representing 55% of the U.S. population, according to the researchers. None of the donors identified as Native/Hawaiian/Pacific Islander or American Indian/Alaskan Natives; these groups represent 0.22% and 0.79% of the U.S. population, respectively.
“Next steps will be figuring out why this is happening and how to address it,” said Valerie L Baker, MD, director in the division of reproductive endocrinology and infertility at Johns Hopkins Medicine in Lutherville, Md., who was not involved in the study.
The study sheds light on the need to identify and address the barriers that discourage potential donors from underrepresented groups from participating in sperm donation, according to Kimball Pomeroy, PhD, scientific director at the World Egg and Sperm Bank in Scottsdale, Ariz.
“Sometimes there are inhibitors of different ethnic groups to want to act as sperm or egg donors, so trying to understand if that’s the case is important; but I’m sure a lot of it is also related to access,” Dr. Pomeroy, who was not part of the study team, said in an interview.
Longitudinal data from the two national donor banks did not indicate any significant increase or decrease in donation trends across the 5-year period from 2018 to 2022, highlighting the persisting issue of representation disparities. Dr. Gibbs said strategies need to be developed to increase recruitment of donors from underrepresented groups. Increasing the diversity of the donor pool will ultimately support family-building options for all patients, according to Dr. Gibbs.
Funding for the study was provided by the EMD Serono REI Diversity Fellowship Grant. Dr. Gibbs reports no relevant financial relationships.
, according to a study presented at the American Society for Reproductive Medicine’s 2023 meeting.
“This really highlights the need to identify barriers to increase recruitment of these donors so that we can support family-building for all populations,” said Lauren Gibbs, MD, a resident in the department of obstetrics and gynecology at the Morehouse School of Medicine in Atlanta.
Dr. Gibbs and her colleagues compared the racial and ethnic makeup of sperm donors from online and self-reported profiles at 14 of the largest donor banks in the United States for March and April of 2023. Historical data were pulled from two large, national banks. The investigators compared these data to census estimates from 2021 for men between the ages of 18 and 44 years.
Donors who identified as Hispanic (10.9%) or Black (3.3%) were significantly underrepresented as compared to the U.S. population, of which Hispanic men compose 22% and Black men make up 13.3%.
Asian donors were overrepresented, making up 21.9% of the donors but only 6.5% of the U.S. population. White donors were proportionately represented in relation to national demographics, making up 56.6% of the donors and representing 55% of the U.S. population, according to the researchers. None of the donors identified as Native/Hawaiian/Pacific Islander or American Indian/Alaskan Natives; these groups represent 0.22% and 0.79% of the U.S. population, respectively.
“Next steps will be figuring out why this is happening and how to address it,” said Valerie L Baker, MD, director in the division of reproductive endocrinology and infertility at Johns Hopkins Medicine in Lutherville, Md., who was not involved in the study.
The study sheds light on the need to identify and address the barriers that discourage potential donors from underrepresented groups from participating in sperm donation, according to Kimball Pomeroy, PhD, scientific director at the World Egg and Sperm Bank in Scottsdale, Ariz.
“Sometimes there are inhibitors of different ethnic groups to want to act as sperm or egg donors, so trying to understand if that’s the case is important; but I’m sure a lot of it is also related to access,” Dr. Pomeroy, who was not part of the study team, said in an interview.
Longitudinal data from the two national donor banks did not indicate any significant increase or decrease in donation trends across the 5-year period from 2018 to 2022, highlighting the persisting issue of representation disparities. Dr. Gibbs said strategies need to be developed to increase recruitment of donors from underrepresented groups. Increasing the diversity of the donor pool will ultimately support family-building options for all patients, according to Dr. Gibbs.
Funding for the study was provided by the EMD Serono REI Diversity Fellowship Grant. Dr. Gibbs reports no relevant financial relationships.
FROM ASRM 2023
Tech encourages HIV prevention among women
Access to technology, particularly cellphones, is tied to a higher awareness of pre-exposure prophylaxis (PrEP) in women, according to survey results presented at the Association of Nurses in AIDS Care 2023 Annual Meeting.
Those with limited access to technology, older women, and women who had been incarcerated were also less likely to be aware of their medication options.
Researchers collected responses from 206 women in New York and Philadelphia by computer survey. The women were HIV negative and eligible to receive medication but were not currently taking any.
Most participants were Black (61%) or Hispanic (24%), and the average age of participants was 39 years. Nearly 60% of the group reported they were not aware of PrEP.
Younger women, Hispanic women, women who had not been incarcerated, and women with access to technology were most likely to be aware that they could take medication to prevent HIV.
“Women who utilized their cell phones for activities such as texting, emailing, watching videos, playing games, downloading apps, and accessing social media were more likely to be aware of PrEP,” point out the researchers led by Su Kyung Kim, PhD, WHNP-BC, an assistant professor at Thomas Jefferson University, Philadelphia.
These findings could help direct efforts to increase awareness among women where uptake has remained low, the researchers report. “Mobile technologies, in particular, offer a nimble, customizable, and accessible way to reach this target population and increase awareness of PrEP.”
A version of this article first appeared on Medscape.com.
Access to technology, particularly cellphones, is tied to a higher awareness of pre-exposure prophylaxis (PrEP) in women, according to survey results presented at the Association of Nurses in AIDS Care 2023 Annual Meeting.
Those with limited access to technology, older women, and women who had been incarcerated were also less likely to be aware of their medication options.
Researchers collected responses from 206 women in New York and Philadelphia by computer survey. The women were HIV negative and eligible to receive medication but were not currently taking any.
Most participants were Black (61%) or Hispanic (24%), and the average age of participants was 39 years. Nearly 60% of the group reported they were not aware of PrEP.
Younger women, Hispanic women, women who had not been incarcerated, and women with access to technology were most likely to be aware that they could take medication to prevent HIV.
“Women who utilized their cell phones for activities such as texting, emailing, watching videos, playing games, downloading apps, and accessing social media were more likely to be aware of PrEP,” point out the researchers led by Su Kyung Kim, PhD, WHNP-BC, an assistant professor at Thomas Jefferson University, Philadelphia.
These findings could help direct efforts to increase awareness among women where uptake has remained low, the researchers report. “Mobile technologies, in particular, offer a nimble, customizable, and accessible way to reach this target population and increase awareness of PrEP.”
A version of this article first appeared on Medscape.com.
Access to technology, particularly cellphones, is tied to a higher awareness of pre-exposure prophylaxis (PrEP) in women, according to survey results presented at the Association of Nurses in AIDS Care 2023 Annual Meeting.
Those with limited access to technology, older women, and women who had been incarcerated were also less likely to be aware of their medication options.
Researchers collected responses from 206 women in New York and Philadelphia by computer survey. The women were HIV negative and eligible to receive medication but were not currently taking any.
Most participants were Black (61%) or Hispanic (24%), and the average age of participants was 39 years. Nearly 60% of the group reported they were not aware of PrEP.
Younger women, Hispanic women, women who had not been incarcerated, and women with access to technology were most likely to be aware that they could take medication to prevent HIV.
“Women who utilized their cell phones for activities such as texting, emailing, watching videos, playing games, downloading apps, and accessing social media were more likely to be aware of PrEP,” point out the researchers led by Su Kyung Kim, PhD, WHNP-BC, an assistant professor at Thomas Jefferson University, Philadelphia.
These findings could help direct efforts to increase awareness among women where uptake has remained low, the researchers report. “Mobile technologies, in particular, offer a nimble, customizable, and accessible way to reach this target population and increase awareness of PrEP.”
A version of this article first appeared on Medscape.com.
Dupilumab promising for children aged 1-11 with EoE
VANCOUVER –
High exposure to dupilumab was associated with significantly improved histologic, endoscopic, and transcriptomic improvements, compared with placebo at week 16. Sustained response or improvements continued to week 52 with continued treatment in the high-exposure dupilumab group. Children in the high-exposure dupilumab group also gained more weight during the study than those initially assigned to placebo.
“Eosinophilic esophagitis is a chronic, aggressive, type 2 inflammatory disease that has a substantial impact on quality of life,” said Mirna Chehade, MD, MPH, of the Mount Sinai Center for Eosinophilic Disorders, Icahn School of Medicine at Mount Sinai in New York. And the incidence and prevalence of the disease is increasing.
Dupilumab is already indicated for treating EoE in adolescents aged 12 or older as well as adults, but “there are no approved treatments for EoE in children under 12,” said Dr. Chehade, who presented the results of the late-breaking abstract at the ACG: American College of Gastroenterology 2023 annual scientific meeting.
She and her colleagues randomly assigned 102 children aged 1-11 years with active EoE to three groups for the first 16 weeks of the study: 37 to high-exposure dupilumab; 31 to low-exposure dupilumab; and 34 others to placebo, followed by either high- or low-dose dupilumab. Baseline demographics and disease characteristics were comparable between groups.
During an active 36-week extension period, the 37 participants who were initially assigned to receive high-exposure dupilumab continued the same treatment up to week 52. A total of 29 participants initially assigned to receive low-exposure dupilumab continues their regimen as well. Those initially assigned to receive placebo switched to a preassigned active treatment group; 18 children started to take high-exposure dupilumab, and 14 began to take low-exposure dupilumab.
The children in the study had a high burden of disease, as reflected by the duration of EoE as well as histologic, endoscopic, and clinical scores. The mean age was 7.2 years in the placebo group and 6.8 years in the dupilumab group. They were mostly White boys, Dr. Chehade said.
Key outcomes
At week 16, the high-exposure dupilumab group met the primary study endpoint with a peak esophageal intraepithelial eosinophil count ≤ 6 on high-power field assessment. This was significantly different from the placebo group (least squares mean difference, 64.5; 95% confidence interval, 48.19-80.85; P < .0001).
At week 52, 63% of children who remained on high-exposure dupilumab and 53% of those who switched from placebo to high-exposure dupilumab achieved a peak eosinophil count ≤ 6.
The study included multiple secondary outcomes. For example, at week 16, the following measures improved from baseline with high-exposure dupilumab, compared with placebo:
- EoE-Histologic Scoring System grade and stage scores (–0.88 and –0.84 vs. +0.02 and +0.05; both P < .0001).
- EoE-Endoscopic Reference Score (–3.5 vs. +0.3; P < .0001).
- Change in body weight for age percentile (+3.09 vs. +0.29).
- Numeric improvement in caregiver-reported proportion of days experiencing one or more EoE sign (–0.28 vs. –0.17).
At week 52, these outcomes were sustained or improved with continued high-exposure dupilumab. The researchers also saw improvements among the placebo recipients who switched to high-exposure dupilumab.
The reason the children were randomly assigned to high-exposure or low-exposure groups instead of high-dose and low-dose cohorts is because the children grew during the study, Dr. Chehade explained. “As you can see, there was a nice change in weight, and at specific time periods the doses were adjusted to match.”
‘Good safety profile’
Dupilumab was well tolerated. “The safety profile is very similar to what has been so far described and published for dupilumab in adults,” said Dr. Chehade. At week 16, adverse events that were more frequent with dupilumab vs. placebo included COVID-19, rash, headache, and injection-site erythema, for example. Similar safety results were seen up to week 52.
“I think it’s promising as we wait for the actual study to be published,” said Asmeen Bhatt, MD, PhD, co-moderator of the session and assistant professor of medicine at University of Texas Health Science Center, Houston. “The drug was recently approved for adult EOE use, just last year, and it has been shown to be effective.”
“There are a lot of adult drugs that are now being tested in the pediatric population, and this is one of them,” Dr. Bhatt added. “It has a very good safety profile. I’m not a pediatric gastroenterologist but I expect that it will have a lot of utility.”
The study was funded by Regeneron and Sanofi. Dr. Chehade is a consultant for Sanofi and Regeneron and receives research funding from Regeneron. Dr. Bhatt had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
VANCOUVER –
High exposure to dupilumab was associated with significantly improved histologic, endoscopic, and transcriptomic improvements, compared with placebo at week 16. Sustained response or improvements continued to week 52 with continued treatment in the high-exposure dupilumab group. Children in the high-exposure dupilumab group also gained more weight during the study than those initially assigned to placebo.
“Eosinophilic esophagitis is a chronic, aggressive, type 2 inflammatory disease that has a substantial impact on quality of life,” said Mirna Chehade, MD, MPH, of the Mount Sinai Center for Eosinophilic Disorders, Icahn School of Medicine at Mount Sinai in New York. And the incidence and prevalence of the disease is increasing.
Dupilumab is already indicated for treating EoE in adolescents aged 12 or older as well as adults, but “there are no approved treatments for EoE in children under 12,” said Dr. Chehade, who presented the results of the late-breaking abstract at the ACG: American College of Gastroenterology 2023 annual scientific meeting.
She and her colleagues randomly assigned 102 children aged 1-11 years with active EoE to three groups for the first 16 weeks of the study: 37 to high-exposure dupilumab; 31 to low-exposure dupilumab; and 34 others to placebo, followed by either high- or low-dose dupilumab. Baseline demographics and disease characteristics were comparable between groups.
During an active 36-week extension period, the 37 participants who were initially assigned to receive high-exposure dupilumab continued the same treatment up to week 52. A total of 29 participants initially assigned to receive low-exposure dupilumab continues their regimen as well. Those initially assigned to receive placebo switched to a preassigned active treatment group; 18 children started to take high-exposure dupilumab, and 14 began to take low-exposure dupilumab.
The children in the study had a high burden of disease, as reflected by the duration of EoE as well as histologic, endoscopic, and clinical scores. The mean age was 7.2 years in the placebo group and 6.8 years in the dupilumab group. They were mostly White boys, Dr. Chehade said.
Key outcomes
At week 16, the high-exposure dupilumab group met the primary study endpoint with a peak esophageal intraepithelial eosinophil count ≤ 6 on high-power field assessment. This was significantly different from the placebo group (least squares mean difference, 64.5; 95% confidence interval, 48.19-80.85; P < .0001).
At week 52, 63% of children who remained on high-exposure dupilumab and 53% of those who switched from placebo to high-exposure dupilumab achieved a peak eosinophil count ≤ 6.
The study included multiple secondary outcomes. For example, at week 16, the following measures improved from baseline with high-exposure dupilumab, compared with placebo:
- EoE-Histologic Scoring System grade and stage scores (–0.88 and –0.84 vs. +0.02 and +0.05; both P < .0001).
- EoE-Endoscopic Reference Score (–3.5 vs. +0.3; P < .0001).
- Change in body weight for age percentile (+3.09 vs. +0.29).
- Numeric improvement in caregiver-reported proportion of days experiencing one or more EoE sign (–0.28 vs. –0.17).
At week 52, these outcomes were sustained or improved with continued high-exposure dupilumab. The researchers also saw improvements among the placebo recipients who switched to high-exposure dupilumab.
The reason the children were randomly assigned to high-exposure or low-exposure groups instead of high-dose and low-dose cohorts is because the children grew during the study, Dr. Chehade explained. “As you can see, there was a nice change in weight, and at specific time periods the doses were adjusted to match.”
‘Good safety profile’
Dupilumab was well tolerated. “The safety profile is very similar to what has been so far described and published for dupilumab in adults,” said Dr. Chehade. At week 16, adverse events that were more frequent with dupilumab vs. placebo included COVID-19, rash, headache, and injection-site erythema, for example. Similar safety results were seen up to week 52.
“I think it’s promising as we wait for the actual study to be published,” said Asmeen Bhatt, MD, PhD, co-moderator of the session and assistant professor of medicine at University of Texas Health Science Center, Houston. “The drug was recently approved for adult EOE use, just last year, and it has been shown to be effective.”
“There are a lot of adult drugs that are now being tested in the pediatric population, and this is one of them,” Dr. Bhatt added. “It has a very good safety profile. I’m not a pediatric gastroenterologist but I expect that it will have a lot of utility.”
The study was funded by Regeneron and Sanofi. Dr. Chehade is a consultant for Sanofi and Regeneron and receives research funding from Regeneron. Dr. Bhatt had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
VANCOUVER –
High exposure to dupilumab was associated with significantly improved histologic, endoscopic, and transcriptomic improvements, compared with placebo at week 16. Sustained response or improvements continued to week 52 with continued treatment in the high-exposure dupilumab group. Children in the high-exposure dupilumab group also gained more weight during the study than those initially assigned to placebo.
“Eosinophilic esophagitis is a chronic, aggressive, type 2 inflammatory disease that has a substantial impact on quality of life,” said Mirna Chehade, MD, MPH, of the Mount Sinai Center for Eosinophilic Disorders, Icahn School of Medicine at Mount Sinai in New York. And the incidence and prevalence of the disease is increasing.
Dupilumab is already indicated for treating EoE in adolescents aged 12 or older as well as adults, but “there are no approved treatments for EoE in children under 12,” said Dr. Chehade, who presented the results of the late-breaking abstract at the ACG: American College of Gastroenterology 2023 annual scientific meeting.
She and her colleagues randomly assigned 102 children aged 1-11 years with active EoE to three groups for the first 16 weeks of the study: 37 to high-exposure dupilumab; 31 to low-exposure dupilumab; and 34 others to placebo, followed by either high- or low-dose dupilumab. Baseline demographics and disease characteristics were comparable between groups.
During an active 36-week extension period, the 37 participants who were initially assigned to receive high-exposure dupilumab continued the same treatment up to week 52. A total of 29 participants initially assigned to receive low-exposure dupilumab continues their regimen as well. Those initially assigned to receive placebo switched to a preassigned active treatment group; 18 children started to take high-exposure dupilumab, and 14 began to take low-exposure dupilumab.
The children in the study had a high burden of disease, as reflected by the duration of EoE as well as histologic, endoscopic, and clinical scores. The mean age was 7.2 years in the placebo group and 6.8 years in the dupilumab group. They were mostly White boys, Dr. Chehade said.
Key outcomes
At week 16, the high-exposure dupilumab group met the primary study endpoint with a peak esophageal intraepithelial eosinophil count ≤ 6 on high-power field assessment. This was significantly different from the placebo group (least squares mean difference, 64.5; 95% confidence interval, 48.19-80.85; P < .0001).
At week 52, 63% of children who remained on high-exposure dupilumab and 53% of those who switched from placebo to high-exposure dupilumab achieved a peak eosinophil count ≤ 6.
The study included multiple secondary outcomes. For example, at week 16, the following measures improved from baseline with high-exposure dupilumab, compared with placebo:
- EoE-Histologic Scoring System grade and stage scores (–0.88 and –0.84 vs. +0.02 and +0.05; both P < .0001).
- EoE-Endoscopic Reference Score (–3.5 vs. +0.3; P < .0001).
- Change in body weight for age percentile (+3.09 vs. +0.29).
- Numeric improvement in caregiver-reported proportion of days experiencing one or more EoE sign (–0.28 vs. –0.17).
At week 52, these outcomes were sustained or improved with continued high-exposure dupilumab. The researchers also saw improvements among the placebo recipients who switched to high-exposure dupilumab.
The reason the children were randomly assigned to high-exposure or low-exposure groups instead of high-dose and low-dose cohorts is because the children grew during the study, Dr. Chehade explained. “As you can see, there was a nice change in weight, and at specific time periods the doses were adjusted to match.”
‘Good safety profile’
Dupilumab was well tolerated. “The safety profile is very similar to what has been so far described and published for dupilumab in adults,” said Dr. Chehade. At week 16, adverse events that were more frequent with dupilumab vs. placebo included COVID-19, rash, headache, and injection-site erythema, for example. Similar safety results were seen up to week 52.
“I think it’s promising as we wait for the actual study to be published,” said Asmeen Bhatt, MD, PhD, co-moderator of the session and assistant professor of medicine at University of Texas Health Science Center, Houston. “The drug was recently approved for adult EOE use, just last year, and it has been shown to be effective.”
“There are a lot of adult drugs that are now being tested in the pediatric population, and this is one of them,” Dr. Bhatt added. “It has a very good safety profile. I’m not a pediatric gastroenterologist but I expect that it will have a lot of utility.”
The study was funded by Regeneron and Sanofi. Dr. Chehade is a consultant for Sanofi and Regeneron and receives research funding from Regeneron. Dr. Bhatt had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
AT ACG 2023
Clustered Vesicles on the Neck
The Diagnosis: Microcystic Lymphatic Malformation
A punch biopsy demonstrated anastomosing fluidfilled spaces within the papillary and reticular dermal layers (Figure), confirming the diagnosis of microcystic lymphatic malformation (LM)(formerly known as lymphangioma circumscriptum), a congenital vascular malformation composed of slow-flow lymphatic channels.1 The patient underwent serial excisions with improvement of the LM, though the treatment course was complicated by hypertrophic scar formation.
The classic clinical presentation of microcystic LM includes a crop of vesicles containing clear or hemorrhagic fluid with associated oozing or bleeding.2 When cutaneous lesions resembling microcystic LM develop in response to lymphatic damage and resulting stasis, such as from prior radiotherapy or surgery, the term lymphangiectasia is used to distinguish this entity from congenital microcystic LM.3
Microcystic LMs are histologically indistinguishable from macrocystic LMs; however, macrocystic LMs typically are clinically evident at birth as ill-defined subcutaneous masses.2,4-6 Dermatitis herpetiformis, a dermatologic manifestation of gluten sensitivity, causes intensely pruritic vesicles in a symmetric distribution on the elbows, knees, and buttocks. Histopathology shows neutrophilic microabscesses in the dermal papillae with subepidermal blistering. Direct immunofluorescence demonstrates the deposition of IgA along the basement membrane with dermal papillae aggregates.6 The underlying dermis also may contain a lymphohistiocytic infiltrate rich in neutrophils. The vesicles of herpes zoster virus are painful and present in a dermatomal distribution. A viral cytopathic effect often is observed in keratinocytes, specifically with multinucleation, molding, and margination of chromatin material. The lesions are accompanied by variable lymphocytic inflammation and epithelial necrosis resulting in intraepidermal blistering.7 Extragenital lichen sclerosus presents as polygonal white papules merging to form plaques and may include hemorrhagic blisters in some instances. Histopathology shows hyperkeratosis, epidermal atrophy with flattened rete ridges, vacuolar interface changes, loss of elastic fibers, and hyalinization of the lamina propria with lymphocytic infiltrate.8
Endothelial cells in LM exhibit activating mutations in the phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha gene, PIK3CA, which may lead to proliferation and overgrowth of the lymphatic vasculature, as well as increased production of cyclic guanosine monophosphate.9,10 Phosphodiesterase 5 (PDE5) is expressed in the perivascular smooth muscle adjacent to lymphatic spaces in LMs but not in the their vasculature. 10 This pattern of PDE5 expression may cause perilesional vasculature to constrict, preventing lymphatic fluid from draining into the veins.11 It is theorized that the PDE5 inhibitor sildenafil leads to relaxation of the vasculature adjacent to LMs, allowing the outflow of the accumulated lymphatic fluid and thus decompression.11-13
Management of LM should not only take into account the depth and location of involvement but also any associated symptoms or complications, such as pruritus, pain, bleeding, or secondary infections. Magnetic resonance imaging (MRI) typically has been considered the gold standard for determining the size and depth of involvement of the malformation.1,3,4 However, ultrasonography with Doppler flow may be considered an initial diagnostic and screening test, as it can distinguish between macrocystic and microcystic components and provide superior images of microcystic lesions, which are below the resolution capacity of MRI.4 Notably, our patient’s LM was undetectable on ultrasonography and was found to be largely superficial in nature on MRI.
Serial excision of the microcystic LM was conducted in our patient, but there currently is no consensus on optimal treatment of LM, and many treatment options are complicated by high recurrence rates or complications.5 Procedural approaches may include excision, cryotherapy, radiotherapy, sclerotherapy, or laser therapy, while pharmacologic approaches may include sildenafil for its inhibition of PDE5 or sirolimus (oral or topical) for its inhibition of mammalian target of rapamycin.5,12-14 Because recurrence is highly likely, patients may require repeat treatments or a combination approach to therapy.1,5 The development of targeted therapies may lead to a shift in management of LMs in the future, as successful use of the PIK3CA inhibitor alpelisib recently has been reported to lead to clinical improvement of PIK3CA-related LMs, including in patients with PIK3CA-related overgrowth syndromes.15
- Garzon MC, Huang JT, Enjolras O, et al. Vascular malformations: part I. J Am Acad Dermatol. 2007;56:353-374. doi:10.1016/j.jaad.2006.05.069
- Alrashdan MS, Hammad HM, Alzumaili BAI, et al. Lymphangioma circumscriptum of the tongue: a case with marked hemorrhagic component. J Cutan Pathol. 2018;45:278-281. doi:10.1111/cup.13101
- Osborne GE, Chinn RJ, Francis ND, et al. Magnetic resonance imaging in the investigation of penile lymphangioma circumscriptum. Br J Dermatol. 2000;143:467-468. doi:10.1046/j.1365-2133.2000.03695.x
- Davies D, Rogers M, Lam A, et al. Localized microcystic lymphatic malformations—ultrasound diagnosis. Pediatr Dermatol. 1999;16: 423-429. doi:10.1046/j.1525-1470.1999.00110.x
- García-Montero P, Del Boz J, Baselga-Torres E, et al. Use of topical rapamycin in the treatment of superficial lymphatic malformations. J Am Acad Dermatol. 2019;80:508-515. doi:10.1016/j.jaad.2018.09.050
- Clarindo MV, Possebon AT, Soligo EM, et al. Dermatitis herpetiformis: pathophysiology, clinical presentation, diagnosis and treatment. An Bras Dermatol. 2014;89:865-875; quiz 876-877. doi:10.1590/abd1806-4841.20142966
- Leinweber B, Kerl H, Cerroni L. Histopathologic features of cutaneous herpes virus infections (herpes simplex, herpes varicella/zoster): a broad spectrum of presentations with common pseudolymphomatous aspects. Am J Surg Pathol. 2006;30:50-58.
- Shiver M, Papasakelariou C, Brown JA, et al. Extragenital bullous lichen sclerosus in a pediatric patient: a case report and literature review. Pediatr Dermatol. 2014;31:383-385. doi:10.1111 /pde.12025
- Blesinger H, Kaulfuß S, Aung T, et al. PIK3CA mutations are specifically localized to lymphatic endothelial cells of lymphatic malformations [published online July 9, 2018]. PLoS One. 2018;13:E0200343. doi:10.1371/journal.pone.0200343
- Green JS, Prok L, Bruckner AL. Expression of phosphodiesterase-5 in lymphatic malformation tissue. JAMA Dermatol. 2014;150:455-456. doi:10.1001/jamadermatol.2013.7002
- Swetman GL, Berk DR, Vasanawala SS, et al. Sildenafil for severe lymphatic malformations. N Engl J Med. 2012;366:384-386. doi:10.1056 /NEJMc1112482
- Tu JH, Tafoya E, Jeng M, et al. Long-term follow-up of lymphatic malformations in children treated with sildenafil. Pediatr Dermatol. 2017;34:559-565. doi:10.1111/pde.13237
- Maruani A, Tavernier E, Boccara O, et al. Sirolimus (rapamycin) for slow-flow malformations in children: the Observational-Phase Randomized Clinical PERFORMUS Trial. JAMA Dermatol. 2021;157:1289-1298. doi:10.1001/jamadermatol.2021.3459
- Delestre F, Venot Q, Bayard C, et al. Alpelisib administration reduced lymphatic malformations in a mouse model and in patients. Sci Transl Med. 2021;13:eabg0809. doi:10.1126/scitranslmed .abg0809
- Garreta Fontelles G, Pardo Pastor J, Grande Moreillo C. Alpelisib to treat CLOVES syndrome, a member of the PIK3CA-related overgrowth syndrome spectrum [published online February 21, 2022]. Br J Clin Pharmacol. 2022;88:3891-3895. doi:10.1111/bcp.15270
The Diagnosis: Microcystic Lymphatic Malformation
A punch biopsy demonstrated anastomosing fluidfilled spaces within the papillary and reticular dermal layers (Figure), confirming the diagnosis of microcystic lymphatic malformation (LM)(formerly known as lymphangioma circumscriptum), a congenital vascular malformation composed of slow-flow lymphatic channels.1 The patient underwent serial excisions with improvement of the LM, though the treatment course was complicated by hypertrophic scar formation.

The classic clinical presentation of microcystic LM includes a crop of vesicles containing clear or hemorrhagic fluid with associated oozing or bleeding.2 When cutaneous lesions resembling microcystic LM develop in response to lymphatic damage and resulting stasis, such as from prior radiotherapy or surgery, the term lymphangiectasia is used to distinguish this entity from congenital microcystic LM.3
Microcystic LMs are histologically indistinguishable from macrocystic LMs; however, macrocystic LMs typically are clinically evident at birth as ill-defined subcutaneous masses.2,4-6 Dermatitis herpetiformis, a dermatologic manifestation of gluten sensitivity, causes intensely pruritic vesicles in a symmetric distribution on the elbows, knees, and buttocks. Histopathology shows neutrophilic microabscesses in the dermal papillae with subepidermal blistering. Direct immunofluorescence demonstrates the deposition of IgA along the basement membrane with dermal papillae aggregates.6 The underlying dermis also may contain a lymphohistiocytic infiltrate rich in neutrophils. The vesicles of herpes zoster virus are painful and present in a dermatomal distribution. A viral cytopathic effect often is observed in keratinocytes, specifically with multinucleation, molding, and margination of chromatin material. The lesions are accompanied by variable lymphocytic inflammation and epithelial necrosis resulting in intraepidermal blistering.7 Extragenital lichen sclerosus presents as polygonal white papules merging to form plaques and may include hemorrhagic blisters in some instances. Histopathology shows hyperkeratosis, epidermal atrophy with flattened rete ridges, vacuolar interface changes, loss of elastic fibers, and hyalinization of the lamina propria with lymphocytic infiltrate.8
Endothelial cells in LM exhibit activating mutations in the phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha gene, PIK3CA, which may lead to proliferation and overgrowth of the lymphatic vasculature, as well as increased production of cyclic guanosine monophosphate.9,10 Phosphodiesterase 5 (PDE5) is expressed in the perivascular smooth muscle adjacent to lymphatic spaces in LMs but not in the their vasculature. 10 This pattern of PDE5 expression may cause perilesional vasculature to constrict, preventing lymphatic fluid from draining into the veins.11 It is theorized that the PDE5 inhibitor sildenafil leads to relaxation of the vasculature adjacent to LMs, allowing the outflow of the accumulated lymphatic fluid and thus decompression.11-13
Management of LM should not only take into account the depth and location of involvement but also any associated symptoms or complications, such as pruritus, pain, bleeding, or secondary infections. Magnetic resonance imaging (MRI) typically has been considered the gold standard for determining the size and depth of involvement of the malformation.1,3,4 However, ultrasonography with Doppler flow may be considered an initial diagnostic and screening test, as it can distinguish between macrocystic and microcystic components and provide superior images of microcystic lesions, which are below the resolution capacity of MRI.4 Notably, our patient’s LM was undetectable on ultrasonography and was found to be largely superficial in nature on MRI.
Serial excision of the microcystic LM was conducted in our patient, but there currently is no consensus on optimal treatment of LM, and many treatment options are complicated by high recurrence rates or complications.5 Procedural approaches may include excision, cryotherapy, radiotherapy, sclerotherapy, or laser therapy, while pharmacologic approaches may include sildenafil for its inhibition of PDE5 or sirolimus (oral or topical) for its inhibition of mammalian target of rapamycin.5,12-14 Because recurrence is highly likely, patients may require repeat treatments or a combination approach to therapy.1,5 The development of targeted therapies may lead to a shift in management of LMs in the future, as successful use of the PIK3CA inhibitor alpelisib recently has been reported to lead to clinical improvement of PIK3CA-related LMs, including in patients with PIK3CA-related overgrowth syndromes.15
The Diagnosis: Microcystic Lymphatic Malformation
A punch biopsy demonstrated anastomosing fluidfilled spaces within the papillary and reticular dermal layers (Figure), confirming the diagnosis of microcystic lymphatic malformation (LM)(formerly known as lymphangioma circumscriptum), a congenital vascular malformation composed of slow-flow lymphatic channels.1 The patient underwent serial excisions with improvement of the LM, though the treatment course was complicated by hypertrophic scar formation.

The classic clinical presentation of microcystic LM includes a crop of vesicles containing clear or hemorrhagic fluid with associated oozing or bleeding.2 When cutaneous lesions resembling microcystic LM develop in response to lymphatic damage and resulting stasis, such as from prior radiotherapy or surgery, the term lymphangiectasia is used to distinguish this entity from congenital microcystic LM.3
Microcystic LMs are histologically indistinguishable from macrocystic LMs; however, macrocystic LMs typically are clinically evident at birth as ill-defined subcutaneous masses.2,4-6 Dermatitis herpetiformis, a dermatologic manifestation of gluten sensitivity, causes intensely pruritic vesicles in a symmetric distribution on the elbows, knees, and buttocks. Histopathology shows neutrophilic microabscesses in the dermal papillae with subepidermal blistering. Direct immunofluorescence demonstrates the deposition of IgA along the basement membrane with dermal papillae aggregates.6 The underlying dermis also may contain a lymphohistiocytic infiltrate rich in neutrophils. The vesicles of herpes zoster virus are painful and present in a dermatomal distribution. A viral cytopathic effect often is observed in keratinocytes, specifically with multinucleation, molding, and margination of chromatin material. The lesions are accompanied by variable lymphocytic inflammation and epithelial necrosis resulting in intraepidermal blistering.7 Extragenital lichen sclerosus presents as polygonal white papules merging to form plaques and may include hemorrhagic blisters in some instances. Histopathology shows hyperkeratosis, epidermal atrophy with flattened rete ridges, vacuolar interface changes, loss of elastic fibers, and hyalinization of the lamina propria with lymphocytic infiltrate.8
Endothelial cells in LM exhibit activating mutations in the phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha gene, PIK3CA, which may lead to proliferation and overgrowth of the lymphatic vasculature, as well as increased production of cyclic guanosine monophosphate.9,10 Phosphodiesterase 5 (PDE5) is expressed in the perivascular smooth muscle adjacent to lymphatic spaces in LMs but not in the their vasculature. 10 This pattern of PDE5 expression may cause perilesional vasculature to constrict, preventing lymphatic fluid from draining into the veins.11 It is theorized that the PDE5 inhibitor sildenafil leads to relaxation of the vasculature adjacent to LMs, allowing the outflow of the accumulated lymphatic fluid and thus decompression.11-13
Management of LM should not only take into account the depth and location of involvement but also any associated symptoms or complications, such as pruritus, pain, bleeding, or secondary infections. Magnetic resonance imaging (MRI) typically has been considered the gold standard for determining the size and depth of involvement of the malformation.1,3,4 However, ultrasonography with Doppler flow may be considered an initial diagnostic and screening test, as it can distinguish between macrocystic and microcystic components and provide superior images of microcystic lesions, which are below the resolution capacity of MRI.4 Notably, our patient’s LM was undetectable on ultrasonography and was found to be largely superficial in nature on MRI.
Serial excision of the microcystic LM was conducted in our patient, but there currently is no consensus on optimal treatment of LM, and many treatment options are complicated by high recurrence rates or complications.5 Procedural approaches may include excision, cryotherapy, radiotherapy, sclerotherapy, or laser therapy, while pharmacologic approaches may include sildenafil for its inhibition of PDE5 or sirolimus (oral or topical) for its inhibition of mammalian target of rapamycin.5,12-14 Because recurrence is highly likely, patients may require repeat treatments or a combination approach to therapy.1,5 The development of targeted therapies may lead to a shift in management of LMs in the future, as successful use of the PIK3CA inhibitor alpelisib recently has been reported to lead to clinical improvement of PIK3CA-related LMs, including in patients with PIK3CA-related overgrowth syndromes.15
- Garzon MC, Huang JT, Enjolras O, et al. Vascular malformations: part I. J Am Acad Dermatol. 2007;56:353-374. doi:10.1016/j.jaad.2006.05.069
- Alrashdan MS, Hammad HM, Alzumaili BAI, et al. Lymphangioma circumscriptum of the tongue: a case with marked hemorrhagic component. J Cutan Pathol. 2018;45:278-281. doi:10.1111/cup.13101
- Osborne GE, Chinn RJ, Francis ND, et al. Magnetic resonance imaging in the investigation of penile lymphangioma circumscriptum. Br J Dermatol. 2000;143:467-468. doi:10.1046/j.1365-2133.2000.03695.x
- Davies D, Rogers M, Lam A, et al. Localized microcystic lymphatic malformations—ultrasound diagnosis. Pediatr Dermatol. 1999;16: 423-429. doi:10.1046/j.1525-1470.1999.00110.x
- García-Montero P, Del Boz J, Baselga-Torres E, et al. Use of topical rapamycin in the treatment of superficial lymphatic malformations. J Am Acad Dermatol. 2019;80:508-515. doi:10.1016/j.jaad.2018.09.050
- Clarindo MV, Possebon AT, Soligo EM, et al. Dermatitis herpetiformis: pathophysiology, clinical presentation, diagnosis and treatment. An Bras Dermatol. 2014;89:865-875; quiz 876-877. doi:10.1590/abd1806-4841.20142966
- Leinweber B, Kerl H, Cerroni L. Histopathologic features of cutaneous herpes virus infections (herpes simplex, herpes varicella/zoster): a broad spectrum of presentations with common pseudolymphomatous aspects. Am J Surg Pathol. 2006;30:50-58.
- Shiver M, Papasakelariou C, Brown JA, et al. Extragenital bullous lichen sclerosus in a pediatric patient: a case report and literature review. Pediatr Dermatol. 2014;31:383-385. doi:10.1111 /pde.12025
- Blesinger H, Kaulfuß S, Aung T, et al. PIK3CA mutations are specifically localized to lymphatic endothelial cells of lymphatic malformations [published online July 9, 2018]. PLoS One. 2018;13:E0200343. doi:10.1371/journal.pone.0200343
- Green JS, Prok L, Bruckner AL. Expression of phosphodiesterase-5 in lymphatic malformation tissue. JAMA Dermatol. 2014;150:455-456. doi:10.1001/jamadermatol.2013.7002
- Swetman GL, Berk DR, Vasanawala SS, et al. Sildenafil for severe lymphatic malformations. N Engl J Med. 2012;366:384-386. doi:10.1056 /NEJMc1112482
- Tu JH, Tafoya E, Jeng M, et al. Long-term follow-up of lymphatic malformations in children treated with sildenafil. Pediatr Dermatol. 2017;34:559-565. doi:10.1111/pde.13237
- Maruani A, Tavernier E, Boccara O, et al. Sirolimus (rapamycin) for slow-flow malformations in children: the Observational-Phase Randomized Clinical PERFORMUS Trial. JAMA Dermatol. 2021;157:1289-1298. doi:10.1001/jamadermatol.2021.3459
- Delestre F, Venot Q, Bayard C, et al. Alpelisib administration reduced lymphatic malformations in a mouse model and in patients. Sci Transl Med. 2021;13:eabg0809. doi:10.1126/scitranslmed .abg0809
- Garreta Fontelles G, Pardo Pastor J, Grande Moreillo C. Alpelisib to treat CLOVES syndrome, a member of the PIK3CA-related overgrowth syndrome spectrum [published online February 21, 2022]. Br J Clin Pharmacol. 2022;88:3891-3895. doi:10.1111/bcp.15270
- Garzon MC, Huang JT, Enjolras O, et al. Vascular malformations: part I. J Am Acad Dermatol. 2007;56:353-374. doi:10.1016/j.jaad.2006.05.069
- Alrashdan MS, Hammad HM, Alzumaili BAI, et al. Lymphangioma circumscriptum of the tongue: a case with marked hemorrhagic component. J Cutan Pathol. 2018;45:278-281. doi:10.1111/cup.13101
- Osborne GE, Chinn RJ, Francis ND, et al. Magnetic resonance imaging in the investigation of penile lymphangioma circumscriptum. Br J Dermatol. 2000;143:467-468. doi:10.1046/j.1365-2133.2000.03695.x
- Davies D, Rogers M, Lam A, et al. Localized microcystic lymphatic malformations—ultrasound diagnosis. Pediatr Dermatol. 1999;16: 423-429. doi:10.1046/j.1525-1470.1999.00110.x
- García-Montero P, Del Boz J, Baselga-Torres E, et al. Use of topical rapamycin in the treatment of superficial lymphatic malformations. J Am Acad Dermatol. 2019;80:508-515. doi:10.1016/j.jaad.2018.09.050
- Clarindo MV, Possebon AT, Soligo EM, et al. Dermatitis herpetiformis: pathophysiology, clinical presentation, diagnosis and treatment. An Bras Dermatol. 2014;89:865-875; quiz 876-877. doi:10.1590/abd1806-4841.20142966
- Leinweber B, Kerl H, Cerroni L. Histopathologic features of cutaneous herpes virus infections (herpes simplex, herpes varicella/zoster): a broad spectrum of presentations with common pseudolymphomatous aspects. Am J Surg Pathol. 2006;30:50-58.
- Shiver M, Papasakelariou C, Brown JA, et al. Extragenital bullous lichen sclerosus in a pediatric patient: a case report and literature review. Pediatr Dermatol. 2014;31:383-385. doi:10.1111 /pde.12025
- Blesinger H, Kaulfuß S, Aung T, et al. PIK3CA mutations are specifically localized to lymphatic endothelial cells of lymphatic malformations [published online July 9, 2018]. PLoS One. 2018;13:E0200343. doi:10.1371/journal.pone.0200343
- Green JS, Prok L, Bruckner AL. Expression of phosphodiesterase-5 in lymphatic malformation tissue. JAMA Dermatol. 2014;150:455-456. doi:10.1001/jamadermatol.2013.7002
- Swetman GL, Berk DR, Vasanawala SS, et al. Sildenafil for severe lymphatic malformations. N Engl J Med. 2012;366:384-386. doi:10.1056 /NEJMc1112482
- Tu JH, Tafoya E, Jeng M, et al. Long-term follow-up of lymphatic malformations in children treated with sildenafil. Pediatr Dermatol. 2017;34:559-565. doi:10.1111/pde.13237
- Maruani A, Tavernier E, Boccara O, et al. Sirolimus (rapamycin) for slow-flow malformations in children: the Observational-Phase Randomized Clinical PERFORMUS Trial. JAMA Dermatol. 2021;157:1289-1298. doi:10.1001/jamadermatol.2021.3459
- Delestre F, Venot Q, Bayard C, et al. Alpelisib administration reduced lymphatic malformations in a mouse model and in patients. Sci Transl Med. 2021;13:eabg0809. doi:10.1126/scitranslmed .abg0809
- Garreta Fontelles G, Pardo Pastor J, Grande Moreillo C. Alpelisib to treat CLOVES syndrome, a member of the PIK3CA-related overgrowth syndrome spectrum [published online February 21, 2022]. Br J Clin Pharmacol. 2022;88:3891-3895. doi:10.1111/bcp.15270
A 6-year-old girl presented to the dermatology clinic with a rash on the right side of the neck that was noted at birth as a small raised lesion but slowly increased over time in size and number of lesions. She reported pruritus and irritation, particularly when rubbed or scratched. There was no family history of similar skin abnormalities. Her medical history was notable for a left-sided cholesteatoma on tympanomastoidectomy. Physical examination revealed clustered vesicles on the right side of the neck with underlying erythema. The vesicles contained mostly clear fluid with a few focal areas of hemorrhagic fluid. Ultrasonography was unremarkable, and magnetic resonance imaging revealed superficial T2 hyperintense nonenhancing cutaneous and subcutaneous lesions overlying the right lateral neck with minimal extension into the superficial right supraclavicular soft tissues.