User login
Induction chemotherapy in first line improves survival for locally advanced cervical cancer
and should be considered the new standard of care, according to Mary McCormack, MBBS, PhD, a gynecologic and breast oncologist at the University College Hospital, London.
Dr. McCormack was the lead investigator on a phase 3 trial called INTERLACE that tested the approach against stand-alone chemoradiation – the current standard of care – in 500 women, majority in the United Kingdom and Mexico.
She made her comments after presenting the results at the annual meeting of the European Society for Medical Oncology.
The 250 women randomized to induction chemotherapy before chemoradiation (CRT) had a 35% improvement in progression-free survival (PFS), with a 5-year PFS of 73% versus 64% among 250 randomized to CRT alone. Likewise, overall survival (OS) improved 39% in the induction group, with a 5-year OS of 80% versus 72% among women who went straight to CRT.
Induction chemotherapy consisted of 6 weekly doses of carboplatin AUC2 and paclitaxel 80 mg/m2 followed by CRT within 7 days. CRT consisted of 5 weekly doses of cisplatin 40 mg/m2 plus external beam radiotherapy and brachytherapy. Compliance in both arms was high.
“Induction chemotherapy with weekly paclitaxel and carboplatin delivered immediately before chemoradiotherapy should be considered the new standard in locally advanced cervical cancer, and [it] is feasible across diverse healthcare settings,” Dr. McCormack said.
Study discussant Krishnansu Tewari, MD, a gynecologic oncologist at the University of California, Irvine, was impressed by the results.
“This is the first phase 3 randomized trial in locally advanced cervical cancer that has shown [an overall] survival benefit in over 2 decades. Physicians taking care of these patients could consider induction chemotherapy ... tomorrow morning,” he said.
Dr. Tewari brought up how to incorporate the findings with another trial presented earlier at the meeting, KEYNOTE-A18.
KEYNOTE-A18 added pembrolizumab to CRT, which resulted in substantially better PFS and a strong trend towards better OS that could reach statistical significance with additional follow-up.
Both trials are “practice changing” for locally advanced cervical cancer. “I think we are ready for a paradigm shift,” Dr. Tewari said.
He noted a limit in the INTERLACE presentation was that outcomes were not broken down by tumor stage.
Over three-quarters of the women had stage 2 disease; 9% had stage 1 disease, and only 14% had stage 3B or 4A tumors. Almost 60% of the women were node negative.
It’s unclear at this point if women who have node-negative stage 1B3 or stage 2A-B disease “really need induction chemotherapy. I would think that those patients are probably curable by standard chemoradiation plus brachytherapy, and that the real [benefit would be] for stage 3B and 4A patients,” he said.
The median age in the study was 46 years, and 82% of the women had squamous cell tumors.
Grade 3/4 adverse events were higher in the induction arm, 59% versus 48%, driven mostly by a higher incidence of neutropenia and other hematologic adverse events with induction.
One woman died of adverse events in the induction arm and two died in the CRT-alone arm.
Local and pelvic relapse rates were equal in both groups at 16%, but total distant relapses were lower with induction chemotherapy, 12% versus 20%, over a median follow-up of 64 months.
The work was funded by Cancer Research UK. Dr. McCormack is a consultant for AstraZeneca, Eisai, and GSK, and disclosed honoraria/meeting expenses from Daiicho Sankyo, Roche, and Medscape, the publisher of this article. Among other industry ties, Dr. Tewari is an advisor/consultant, researcher, and speaker for Merck, SeaGen, and AstraZeneca.
and should be considered the new standard of care, according to Mary McCormack, MBBS, PhD, a gynecologic and breast oncologist at the University College Hospital, London.
Dr. McCormack was the lead investigator on a phase 3 trial called INTERLACE that tested the approach against stand-alone chemoradiation – the current standard of care – in 500 women, majority in the United Kingdom and Mexico.
She made her comments after presenting the results at the annual meeting of the European Society for Medical Oncology.
The 250 women randomized to induction chemotherapy before chemoradiation (CRT) had a 35% improvement in progression-free survival (PFS), with a 5-year PFS of 73% versus 64% among 250 randomized to CRT alone. Likewise, overall survival (OS) improved 39% in the induction group, with a 5-year OS of 80% versus 72% among women who went straight to CRT.
Induction chemotherapy consisted of 6 weekly doses of carboplatin AUC2 and paclitaxel 80 mg/m2 followed by CRT within 7 days. CRT consisted of 5 weekly doses of cisplatin 40 mg/m2 plus external beam radiotherapy and brachytherapy. Compliance in both arms was high.
“Induction chemotherapy with weekly paclitaxel and carboplatin delivered immediately before chemoradiotherapy should be considered the new standard in locally advanced cervical cancer, and [it] is feasible across diverse healthcare settings,” Dr. McCormack said.
Study discussant Krishnansu Tewari, MD, a gynecologic oncologist at the University of California, Irvine, was impressed by the results.
“This is the first phase 3 randomized trial in locally advanced cervical cancer that has shown [an overall] survival benefit in over 2 decades. Physicians taking care of these patients could consider induction chemotherapy ... tomorrow morning,” he said.
Dr. Tewari brought up how to incorporate the findings with another trial presented earlier at the meeting, KEYNOTE-A18.
KEYNOTE-A18 added pembrolizumab to CRT, which resulted in substantially better PFS and a strong trend towards better OS that could reach statistical significance with additional follow-up.
Both trials are “practice changing” for locally advanced cervical cancer. “I think we are ready for a paradigm shift,” Dr. Tewari said.
He noted a limit in the INTERLACE presentation was that outcomes were not broken down by tumor stage.
Over three-quarters of the women had stage 2 disease; 9% had stage 1 disease, and only 14% had stage 3B or 4A tumors. Almost 60% of the women were node negative.
It’s unclear at this point if women who have node-negative stage 1B3 or stage 2A-B disease “really need induction chemotherapy. I would think that those patients are probably curable by standard chemoradiation plus brachytherapy, and that the real [benefit would be] for stage 3B and 4A patients,” he said.
The median age in the study was 46 years, and 82% of the women had squamous cell tumors.
Grade 3/4 adverse events were higher in the induction arm, 59% versus 48%, driven mostly by a higher incidence of neutropenia and other hematologic adverse events with induction.
One woman died of adverse events in the induction arm and two died in the CRT-alone arm.
Local and pelvic relapse rates were equal in both groups at 16%, but total distant relapses were lower with induction chemotherapy, 12% versus 20%, over a median follow-up of 64 months.
The work was funded by Cancer Research UK. Dr. McCormack is a consultant for AstraZeneca, Eisai, and GSK, and disclosed honoraria/meeting expenses from Daiicho Sankyo, Roche, and Medscape, the publisher of this article. Among other industry ties, Dr. Tewari is an advisor/consultant, researcher, and speaker for Merck, SeaGen, and AstraZeneca.
and should be considered the new standard of care, according to Mary McCormack, MBBS, PhD, a gynecologic and breast oncologist at the University College Hospital, London.
Dr. McCormack was the lead investigator on a phase 3 trial called INTERLACE that tested the approach against stand-alone chemoradiation – the current standard of care – in 500 women, majority in the United Kingdom and Mexico.
She made her comments after presenting the results at the annual meeting of the European Society for Medical Oncology.
The 250 women randomized to induction chemotherapy before chemoradiation (CRT) had a 35% improvement in progression-free survival (PFS), with a 5-year PFS of 73% versus 64% among 250 randomized to CRT alone. Likewise, overall survival (OS) improved 39% in the induction group, with a 5-year OS of 80% versus 72% among women who went straight to CRT.
Induction chemotherapy consisted of 6 weekly doses of carboplatin AUC2 and paclitaxel 80 mg/m2 followed by CRT within 7 days. CRT consisted of 5 weekly doses of cisplatin 40 mg/m2 plus external beam radiotherapy and brachytherapy. Compliance in both arms was high.
“Induction chemotherapy with weekly paclitaxel and carboplatin delivered immediately before chemoradiotherapy should be considered the new standard in locally advanced cervical cancer, and [it] is feasible across diverse healthcare settings,” Dr. McCormack said.
Study discussant Krishnansu Tewari, MD, a gynecologic oncologist at the University of California, Irvine, was impressed by the results.
“This is the first phase 3 randomized trial in locally advanced cervical cancer that has shown [an overall] survival benefit in over 2 decades. Physicians taking care of these patients could consider induction chemotherapy ... tomorrow morning,” he said.
Dr. Tewari brought up how to incorporate the findings with another trial presented earlier at the meeting, KEYNOTE-A18.
KEYNOTE-A18 added pembrolizumab to CRT, which resulted in substantially better PFS and a strong trend towards better OS that could reach statistical significance with additional follow-up.
Both trials are “practice changing” for locally advanced cervical cancer. “I think we are ready for a paradigm shift,” Dr. Tewari said.
He noted a limit in the INTERLACE presentation was that outcomes were not broken down by tumor stage.
Over three-quarters of the women had stage 2 disease; 9% had stage 1 disease, and only 14% had stage 3B or 4A tumors. Almost 60% of the women were node negative.
It’s unclear at this point if women who have node-negative stage 1B3 or stage 2A-B disease “really need induction chemotherapy. I would think that those patients are probably curable by standard chemoradiation plus brachytherapy, and that the real [benefit would be] for stage 3B and 4A patients,” he said.
The median age in the study was 46 years, and 82% of the women had squamous cell tumors.
Grade 3/4 adverse events were higher in the induction arm, 59% versus 48%, driven mostly by a higher incidence of neutropenia and other hematologic adverse events with induction.
One woman died of adverse events in the induction arm and two died in the CRT-alone arm.
Local and pelvic relapse rates were equal in both groups at 16%, but total distant relapses were lower with induction chemotherapy, 12% versus 20%, over a median follow-up of 64 months.
The work was funded by Cancer Research UK. Dr. McCormack is a consultant for AstraZeneca, Eisai, and GSK, and disclosed honoraria/meeting expenses from Daiicho Sankyo, Roche, and Medscape, the publisher of this article. Among other industry ties, Dr. Tewari is an advisor/consultant, researcher, and speaker for Merck, SeaGen, and AstraZeneca.
FROM ESMO CONGRESS 2023
Cutaneous Collagenous Vasculopathy With Ocular Involvement
To the Editor:
Cutaneous collagenous vasculopathy (CCV) is an uncommon microangiopathy that presents with progressive telangiectases on the lower extremities that can eventually spread to involve the upper extremities and trunk. Systemic involvement is uncommon. The diagnosis is confirmed by biopsy, which demonstrates dilated capillaries and postcapillary venules with eosinophilic hyalinized walls. Treatment generally has focused on the use of vascular lasers.1 We report a patient with advanced CCV and ocular involvement that responded to a combination of pulsed dye laser (PDL) therapy and sclerotherapy for cutaneous lesions.
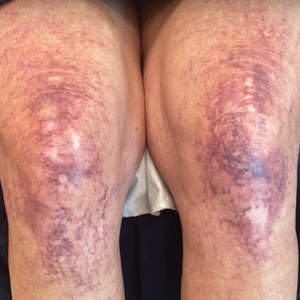
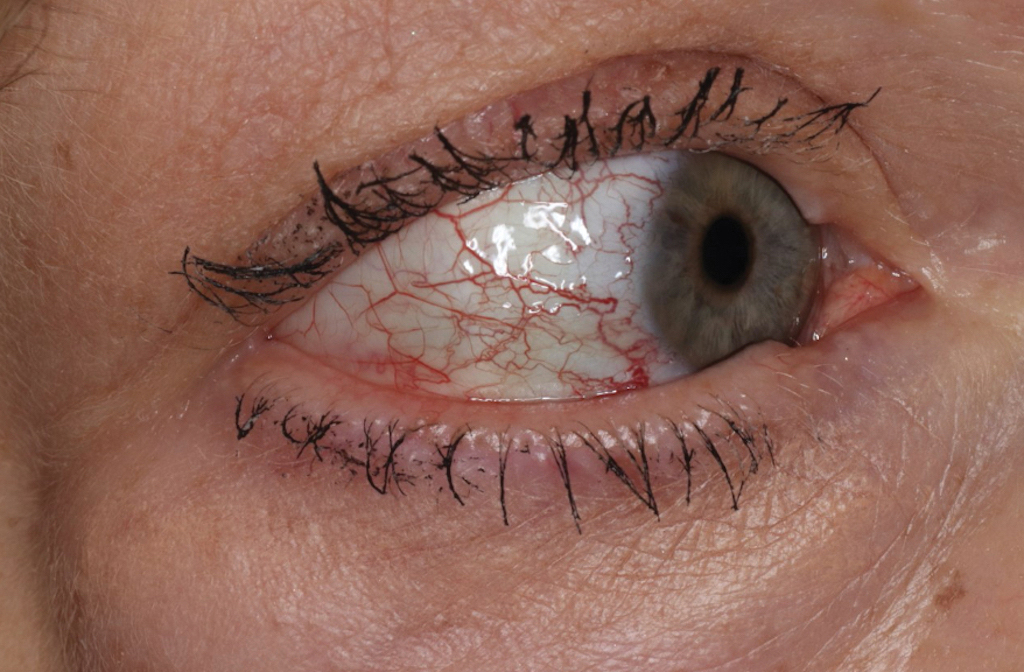
A 63-year-old woman presented with partially blanchable, purple-black patches on the lower extremities (Figure 1). The upper extremities had minimal involvement at the time of presentation. A medical history revealed the lesions presented on the legs 10 years prior but were beginning to form on the arms. She had a history of hypertension and bleeding in the retina.
Histopathology revealed prominent dilation of postcapillary venules with eosinophilic collagenous materials in the vessel walls that was positive on periodic acid–Schiff stain, confirming the diagnosis of CCV. The perivascular collagenous material failed to stain with Congo red. Laboratory testing for serum protein electrophoresis, antinuclear antibodies, and baseline hematologic and metabolic panels revealed no abnormalities.
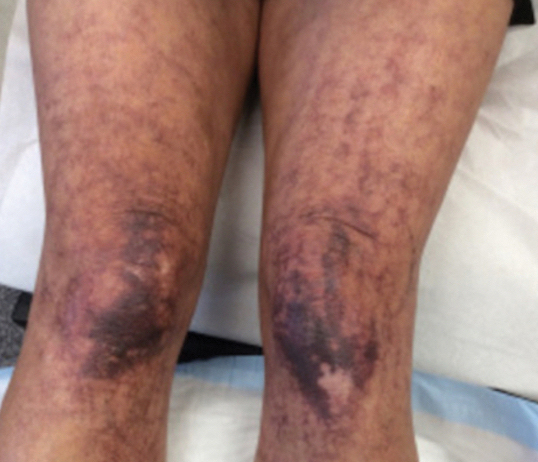
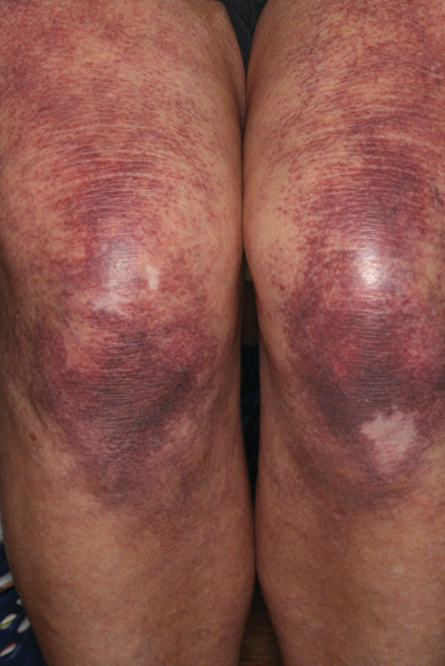
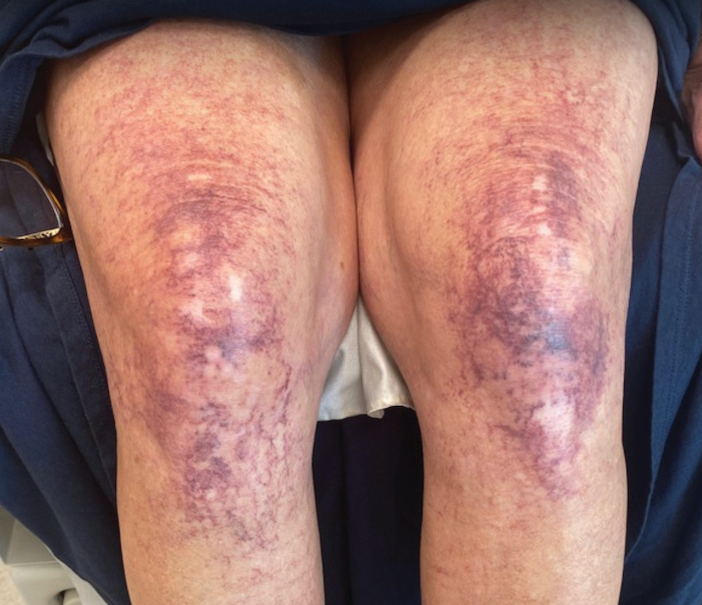
Over 3 years of treatment with PDL, most of the black patches resolved, but prominent telangiectatic vessels remained (Figure 2). Sclerotherapy with polidocanol (10 mg/mL) resulted in clearance of the majority of telangiectatic vessels. After each sclerotherapy treatment, Unna boots were applied for a minimum of 24 hours. The patient had no adverse effects from either PDL or sclerotherapy and was pleased with the results (Figure 3). An ophthalmologist had attributed the retinal bleeding to central serous chorioretinopathy, but tortuosity of superficial scleral and episcleral vessels progressed, suggesting CCV as the more likely cause (Figure 4). Currently, she is being followed for visual changes and further retinal bleeding.
Early CCV typically appears as blanchable pink or red macules, telangiectases, or petechiae on the lower extremities, progressing to involve the trunk and upper extremity.1-3 In rare cases, CCV presents in a papular or annular variant instead of the typical telangiectatic form.4,5 As the lesions progress, they often darken in appearance. Bleeding can occur, and the progressive patches are disfiguring.6,7 Middle-aged to older adults typically present with CCV (range, 16–83 years), with a mean age of 62 years.1,2,6 This disease affects both males and females, predominantly in White individuals.1 Extracutaneous manifestations are rare.1,2,6 One case of mucosal involvement was described in a patient with glossitis and oral erosions.8 We found no prior reports of nail or eye changes.1,2
The etiology of CCV is unknown, but different theories have been proposed. One is that CCV is due to a genetic defect that changes collagen synthesis in the cutaneous microvasculature. Another more widely held belief is that CCV originates from an injury that occurs to the microvasculature endothelial cells. Regardless of the cause of the triggering injury, the result is induced intravascular occlusive microthrombi that cause perivascular fibrosis and endothelial hyperplasia.2,6,7,9
Cutaneous collagenous vasculopathy may be influenced by systemic diseases. The most common comorbidities are hypertension, cardiovascular disease, diabetes mellitus, and hyperlipidemia.1,3,6-8 The presentation of CCV with a malignancy is rare; 1 patient was diagnosed with multiple myeloma 18 months after CCV, and another patient’s cutaneous presentation led to discovery of pancreatic cancer with metastasis.8,10 In this setting, the increased growth factors or hypercoagulability of malignancy may play a role in endothelial cell damage and hyperplasia. Autoimmune vascular injury also has been suggested to trigger CCV; 1 case involved antiribonucleoprotein antibodies, while another case involved anti–endothelial cell antibody assays.11 In addition, CCV has been reported in hypercoagulable patients, demonstrating another route for endothelial damage, with 1 patient being heterozygous for prothrombin G20210A, a report of CCV in a patient with cryofibrinogenemia, and another patient being found positive for lupus anticoagulant.11,12 Drugs also have been thought to influence CCV, including corticosteroids, lithium, thiothixene, interferon, isotretinoin, calcium channel blockers, antibiotics, hydroxyurea, and antidepressants.7,11
The diagnosis of CCV is confirmed using light microscopy and collagen-specific immunostaining. Examination shows hyaline eosinophilic deposition of type IV collagen around the affected vessels, with the postcapillary venules showing characteristic duplication of the basal lamina.3,9 The material stains positive with periodic acid-Schiff and Masson trichrome.3
Underreporting may contribute to the low incidence of CCV. The clinical presentation of CCV is similar to generalized essential telangiectasia, with biopsy distinguishing the two. Other diagnoses in the differential include hereditary hemorrhagic telangiectasia, which typically would have mucosal involvement; radiating telangiectatic mats and a strong family history; and hereditary benign telangiectasia, which typically presents in younger patients aged 1 year to adolescence.1
Treatment with vascular lasers has been the main focus, using either the 595-nm PDL or the 1064-nm Nd:YAG laser.6,13 Pulsed dye laser or intense pulsed light devices can improve patient well-being1,2; intense pulsed light allows for a larger spot size and may be preferred in patients with a larger body surface area involved.13 However, a few other treatments have been proposed. One case report noted poor response to sclerotherapy.1 In another case, a patient treated with a chemotherapy agent, bortezomib, for their concurrent multiple myeloma showed notable CCV cutaneous improvement. The proposed mechanism for bortezomib improving CCV is through its antiproliferative effect on endothelial cells of the superficial dermal vessels.8 Our patient did not achieve an adequate response with PDL, but the addition of sclerotherapy with polidocanol induced a successful response.
Patients should be examined for evidence of ocular involvement and referred to an ophthalmologist for appropriate care. Although there is no definite association with systemic illnesses or mediation, recent associations with an autoimmune disorder or underlying malignancy have been noted.8,10,11 Age-appropriate cancer screening and attention to associated signs and symptoms are recommended.
- Brady BG, Ortleb M, Boyd AS, et al. Cutaneous collagenous vasculopathy. J Clin Aesthet Dermatol. 2015;8:49-52. https://doi.org/10.1097/dad.0000000000000194
- Castiñeiras-Mato I, Rodríguez-Lojo R, Fernández-Díaz ML, et al. Cutaneous collagenous vasculopathy: a case report and review of the literature. Actas Dermosifiliogr. 2016;107:444-447. https://doi.org/10.1016/j.ad.2015.11.006
- Rambhia KD, Hadawale SD, Khopkar US. Cutaneous collagenous vasculopathy: a rare case report. Indian Dermatol Online J. 2016;7:40-42. https://doi.org/10.4103/2229-5178.174327
- Conde-Ferreirós A, Roncero-Riesco M, Cañueto J, et al. Cutaneous collagenous vasculopathy: papular form [published online August 15, 2019]. Dermatol Online J. https://doi.org/10.5070/d3258045128
- García-Martínez P, Gomez-Martin I, Lloreta J, et al. Multiple progressive annular telangiectasias: a clinicopathological variant of cutaneous collagenous vasculopathy? J Cutan Pathol. 2017;44:982-985. https://doi.org/10.1111/cup.13029
- Sartori DS, de Almeida Jr HL, Dorn TV, et al. Cutaneous collagenous vasculopathy: light and transmission electron microscopy. An Bras Dermatol. 2019;94:211-213. https://doi.org/10.1590/abd1806-4841.20198166
- Basso D, Ribero S, Blazek C, et al. Cutaneous collagenous vasculopathy: a rare form of microangiopathy successfully treated with a combination of multiplex laser and optimized pulsed light with a review of the literature. Dermatology. 2016;232:107-111. https://doi.org/10.1159/000439126
- Dura M, Pock L, Cetkovska P, et al. A case of cutaneous collagenous vasculopathy associated with multiple myeloma and with a pathogenic variant of the glucocerebrosidase gene. J Cutan Pathol. 2022;49:717-721. https://doi.org/10.1111/cup.14227
- Salama S, Chorneyko K, Belovic B. Cutaneous collagenous vasculopathy associated with intravascular occlusive fibrin thrombi. J Cutan Pathol. 2014;41:386-393. https://doi.org/10.1111/cup.12285
- Holder E, Schreckenberg C, Lipsker D. Cutaneous collagenous vasculopathy leading to the diagnosis of an advanced pancreatic cancer. J Eur Acad Dermatol Venereol. 2022;36:E699-E701. https://doi.org/10.1111/jdv.18152
- Grossman ME, Cohen M, Ravits M, et al. Cutaneous collagenous vasculopathy: a report of three cases. J Cutan Pathol. 2022;49:491-495. https://doi.org/10.1111/cup.14192
- Eldik H, Leisenring NH, Al-Rohil RN, et al. Cutaneous collagenous vasculopathy in a middle-aged woman with a history of prothrombin G20210A thrombophilia. J Cutan Pathol. 2022;49:679-682. https://doi.org/10.1111/cup.13895
- Weiss E, Lazzara DR. Commentary on clinical improvement of cutaneous collagenous vasculopathy with intense pulsed light therapy. Dermatol Surg. 2021;47:1412. https://doi.org/10.1097/DSS.0000000000003209
To the Editor:
Cutaneous collagenous vasculopathy (CCV) is an uncommon microangiopathy that presents with progressive telangiectases on the lower extremities that can eventually spread to involve the upper extremities and trunk. Systemic involvement is uncommon. The diagnosis is confirmed by biopsy, which demonstrates dilated capillaries and postcapillary venules with eosinophilic hyalinized walls. Treatment generally has focused on the use of vascular lasers.1 We report a patient with advanced CCV and ocular involvement that responded to a combination of pulsed dye laser (PDL) therapy and sclerotherapy for cutaneous lesions.
A 63-year-old woman presented with partially blanchable, purple-black patches on the lower extremities (Figure 1). The upper extremities had minimal involvement at the time of presentation. A medical history revealed the lesions presented on the legs 10 years prior but were beginning to form on the arms. She had a history of hypertension and bleeding in the retina.

Histopathology revealed prominent dilation of postcapillary venules with eosinophilic collagenous materials in the vessel walls that was positive on periodic acid–Schiff stain, confirming the diagnosis of CCV. The perivascular collagenous material failed to stain with Congo red. Laboratory testing for serum protein electrophoresis, antinuclear antibodies, and baseline hematologic and metabolic panels revealed no abnormalities.
Over 3 years of treatment with PDL, most of the black patches resolved, but prominent telangiectatic vessels remained (Figure 2). Sclerotherapy with polidocanol (10 mg/mL) resulted in clearance of the majority of telangiectatic vessels. After each sclerotherapy treatment, Unna boots were applied for a minimum of 24 hours. The patient had no adverse effects from either PDL or sclerotherapy and was pleased with the results (Figure 3). An ophthalmologist had attributed the retinal bleeding to central serous chorioretinopathy, but tortuosity of superficial scleral and episcleral vessels progressed, suggesting CCV as the more likely cause (Figure 4). Currently, she is being followed for visual changes and further retinal bleeding.

Early CCV typically appears as blanchable pink or red macules, telangiectases, or petechiae on the lower extremities, progressing to involve the trunk and upper extremity.1-3 In rare cases, CCV presents in a papular or annular variant instead of the typical telangiectatic form.4,5 As the lesions progress, they often darken in appearance. Bleeding can occur, and the progressive patches are disfiguring.6,7 Middle-aged to older adults typically present with CCV (range, 16–83 years), with a mean age of 62 years.1,2,6 This disease affects both males and females, predominantly in White individuals.1 Extracutaneous manifestations are rare.1,2,6 One case of mucosal involvement was described in a patient with glossitis and oral erosions.8 We found no prior reports of nail or eye changes.1,2

The etiology of CCV is unknown, but different theories have been proposed. One is that CCV is due to a genetic defect that changes collagen synthesis in the cutaneous microvasculature. Another more widely held belief is that CCV originates from an injury that occurs to the microvasculature endothelial cells. Regardless of the cause of the triggering injury, the result is induced intravascular occlusive microthrombi that cause perivascular fibrosis and endothelial hyperplasia.2,6,7,9

Cutaneous collagenous vasculopathy may be influenced by systemic diseases. The most common comorbidities are hypertension, cardiovascular disease, diabetes mellitus, and hyperlipidemia.1,3,6-8 The presentation of CCV with a malignancy is rare; 1 patient was diagnosed with multiple myeloma 18 months after CCV, and another patient’s cutaneous presentation led to discovery of pancreatic cancer with metastasis.8,10 In this setting, the increased growth factors or hypercoagulability of malignancy may play a role in endothelial cell damage and hyperplasia. Autoimmune vascular injury also has been suggested to trigger CCV; 1 case involved antiribonucleoprotein antibodies, while another case involved anti–endothelial cell antibody assays.11 In addition, CCV has been reported in hypercoagulable patients, demonstrating another route for endothelial damage, with 1 patient being heterozygous for prothrombin G20210A, a report of CCV in a patient with cryofibrinogenemia, and another patient being found positive for lupus anticoagulant.11,12 Drugs also have been thought to influence CCV, including corticosteroids, lithium, thiothixene, interferon, isotretinoin, calcium channel blockers, antibiotics, hydroxyurea, and antidepressants.7,11
The diagnosis of CCV is confirmed using light microscopy and collagen-specific immunostaining. Examination shows hyaline eosinophilic deposition of type IV collagen around the affected vessels, with the postcapillary venules showing characteristic duplication of the basal lamina.3,9 The material stains positive with periodic acid-Schiff and Masson trichrome.3
Underreporting may contribute to the low incidence of CCV. The clinical presentation of CCV is similar to generalized essential telangiectasia, with biopsy distinguishing the two. Other diagnoses in the differential include hereditary hemorrhagic telangiectasia, which typically would have mucosal involvement; radiating telangiectatic mats and a strong family history; and hereditary benign telangiectasia, which typically presents in younger patients aged 1 year to adolescence.1
Treatment with vascular lasers has been the main focus, using either the 595-nm PDL or the 1064-nm Nd:YAG laser.6,13 Pulsed dye laser or intense pulsed light devices can improve patient well-being1,2; intense pulsed light allows for a larger spot size and may be preferred in patients with a larger body surface area involved.13 However, a few other treatments have been proposed. One case report noted poor response to sclerotherapy.1 In another case, a patient treated with a chemotherapy agent, bortezomib, for their concurrent multiple myeloma showed notable CCV cutaneous improvement. The proposed mechanism for bortezomib improving CCV is through its antiproliferative effect on endothelial cells of the superficial dermal vessels.8 Our patient did not achieve an adequate response with PDL, but the addition of sclerotherapy with polidocanol induced a successful response.
Patients should be examined for evidence of ocular involvement and referred to an ophthalmologist for appropriate care. Although there is no definite association with systemic illnesses or mediation, recent associations with an autoimmune disorder or underlying malignancy have been noted.8,10,11 Age-appropriate cancer screening and attention to associated signs and symptoms are recommended.
To the Editor:
Cutaneous collagenous vasculopathy (CCV) is an uncommon microangiopathy that presents with progressive telangiectases on the lower extremities that can eventually spread to involve the upper extremities and trunk. Systemic involvement is uncommon. The diagnosis is confirmed by biopsy, which demonstrates dilated capillaries and postcapillary venules with eosinophilic hyalinized walls. Treatment generally has focused on the use of vascular lasers.1 We report a patient with advanced CCV and ocular involvement that responded to a combination of pulsed dye laser (PDL) therapy and sclerotherapy for cutaneous lesions.
A 63-year-old woman presented with partially blanchable, purple-black patches on the lower extremities (Figure 1). The upper extremities had minimal involvement at the time of presentation. A medical history revealed the lesions presented on the legs 10 years prior but were beginning to form on the arms. She had a history of hypertension and bleeding in the retina.

Histopathology revealed prominent dilation of postcapillary venules with eosinophilic collagenous materials in the vessel walls that was positive on periodic acid–Schiff stain, confirming the diagnosis of CCV. The perivascular collagenous material failed to stain with Congo red. Laboratory testing for serum protein electrophoresis, antinuclear antibodies, and baseline hematologic and metabolic panels revealed no abnormalities.
Over 3 years of treatment with PDL, most of the black patches resolved, but prominent telangiectatic vessels remained (Figure 2). Sclerotherapy with polidocanol (10 mg/mL) resulted in clearance of the majority of telangiectatic vessels. After each sclerotherapy treatment, Unna boots were applied for a minimum of 24 hours. The patient had no adverse effects from either PDL or sclerotherapy and was pleased with the results (Figure 3). An ophthalmologist had attributed the retinal bleeding to central serous chorioretinopathy, but tortuosity of superficial scleral and episcleral vessels progressed, suggesting CCV as the more likely cause (Figure 4). Currently, she is being followed for visual changes and further retinal bleeding.

Early CCV typically appears as blanchable pink or red macules, telangiectases, or petechiae on the lower extremities, progressing to involve the trunk and upper extremity.1-3 In rare cases, CCV presents in a papular or annular variant instead of the typical telangiectatic form.4,5 As the lesions progress, they often darken in appearance. Bleeding can occur, and the progressive patches are disfiguring.6,7 Middle-aged to older adults typically present with CCV (range, 16–83 years), with a mean age of 62 years.1,2,6 This disease affects both males and females, predominantly in White individuals.1 Extracutaneous manifestations are rare.1,2,6 One case of mucosal involvement was described in a patient with glossitis and oral erosions.8 We found no prior reports of nail or eye changes.1,2

The etiology of CCV is unknown, but different theories have been proposed. One is that CCV is due to a genetic defect that changes collagen synthesis in the cutaneous microvasculature. Another more widely held belief is that CCV originates from an injury that occurs to the microvasculature endothelial cells. Regardless of the cause of the triggering injury, the result is induced intravascular occlusive microthrombi that cause perivascular fibrosis and endothelial hyperplasia.2,6,7,9

Cutaneous collagenous vasculopathy may be influenced by systemic diseases. The most common comorbidities are hypertension, cardiovascular disease, diabetes mellitus, and hyperlipidemia.1,3,6-8 The presentation of CCV with a malignancy is rare; 1 patient was diagnosed with multiple myeloma 18 months after CCV, and another patient’s cutaneous presentation led to discovery of pancreatic cancer with metastasis.8,10 In this setting, the increased growth factors or hypercoagulability of malignancy may play a role in endothelial cell damage and hyperplasia. Autoimmune vascular injury also has been suggested to trigger CCV; 1 case involved antiribonucleoprotein antibodies, while another case involved anti–endothelial cell antibody assays.11 In addition, CCV has been reported in hypercoagulable patients, demonstrating another route for endothelial damage, with 1 patient being heterozygous for prothrombin G20210A, a report of CCV in a patient with cryofibrinogenemia, and another patient being found positive for lupus anticoagulant.11,12 Drugs also have been thought to influence CCV, including corticosteroids, lithium, thiothixene, interferon, isotretinoin, calcium channel blockers, antibiotics, hydroxyurea, and antidepressants.7,11
The diagnosis of CCV is confirmed using light microscopy and collagen-specific immunostaining. Examination shows hyaline eosinophilic deposition of type IV collagen around the affected vessels, with the postcapillary venules showing characteristic duplication of the basal lamina.3,9 The material stains positive with periodic acid-Schiff and Masson trichrome.3
Underreporting may contribute to the low incidence of CCV. The clinical presentation of CCV is similar to generalized essential telangiectasia, with biopsy distinguishing the two. Other diagnoses in the differential include hereditary hemorrhagic telangiectasia, which typically would have mucosal involvement; radiating telangiectatic mats and a strong family history; and hereditary benign telangiectasia, which typically presents in younger patients aged 1 year to adolescence.1
Treatment with vascular lasers has been the main focus, using either the 595-nm PDL or the 1064-nm Nd:YAG laser.6,13 Pulsed dye laser or intense pulsed light devices can improve patient well-being1,2; intense pulsed light allows for a larger spot size and may be preferred in patients with a larger body surface area involved.13 However, a few other treatments have been proposed. One case report noted poor response to sclerotherapy.1 In another case, a patient treated with a chemotherapy agent, bortezomib, for their concurrent multiple myeloma showed notable CCV cutaneous improvement. The proposed mechanism for bortezomib improving CCV is through its antiproliferative effect on endothelial cells of the superficial dermal vessels.8 Our patient did not achieve an adequate response with PDL, but the addition of sclerotherapy with polidocanol induced a successful response.
Patients should be examined for evidence of ocular involvement and referred to an ophthalmologist for appropriate care. Although there is no definite association with systemic illnesses or mediation, recent associations with an autoimmune disorder or underlying malignancy have been noted.8,10,11 Age-appropriate cancer screening and attention to associated signs and symptoms are recommended.
- Brady BG, Ortleb M, Boyd AS, et al. Cutaneous collagenous vasculopathy. J Clin Aesthet Dermatol. 2015;8:49-52. https://doi.org/10.1097/dad.0000000000000194
- Castiñeiras-Mato I, Rodríguez-Lojo R, Fernández-Díaz ML, et al. Cutaneous collagenous vasculopathy: a case report and review of the literature. Actas Dermosifiliogr. 2016;107:444-447. https://doi.org/10.1016/j.ad.2015.11.006
- Rambhia KD, Hadawale SD, Khopkar US. Cutaneous collagenous vasculopathy: a rare case report. Indian Dermatol Online J. 2016;7:40-42. https://doi.org/10.4103/2229-5178.174327
- Conde-Ferreirós A, Roncero-Riesco M, Cañueto J, et al. Cutaneous collagenous vasculopathy: papular form [published online August 15, 2019]. Dermatol Online J. https://doi.org/10.5070/d3258045128
- García-Martínez P, Gomez-Martin I, Lloreta J, et al. Multiple progressive annular telangiectasias: a clinicopathological variant of cutaneous collagenous vasculopathy? J Cutan Pathol. 2017;44:982-985. https://doi.org/10.1111/cup.13029
- Sartori DS, de Almeida Jr HL, Dorn TV, et al. Cutaneous collagenous vasculopathy: light and transmission electron microscopy. An Bras Dermatol. 2019;94:211-213. https://doi.org/10.1590/abd1806-4841.20198166
- Basso D, Ribero S, Blazek C, et al. Cutaneous collagenous vasculopathy: a rare form of microangiopathy successfully treated with a combination of multiplex laser and optimized pulsed light with a review of the literature. Dermatology. 2016;232:107-111. https://doi.org/10.1159/000439126
- Dura M, Pock L, Cetkovska P, et al. A case of cutaneous collagenous vasculopathy associated with multiple myeloma and with a pathogenic variant of the glucocerebrosidase gene. J Cutan Pathol. 2022;49:717-721. https://doi.org/10.1111/cup.14227
- Salama S, Chorneyko K, Belovic B. Cutaneous collagenous vasculopathy associated with intravascular occlusive fibrin thrombi. J Cutan Pathol. 2014;41:386-393. https://doi.org/10.1111/cup.12285
- Holder E, Schreckenberg C, Lipsker D. Cutaneous collagenous vasculopathy leading to the diagnosis of an advanced pancreatic cancer. J Eur Acad Dermatol Venereol. 2022;36:E699-E701. https://doi.org/10.1111/jdv.18152
- Grossman ME, Cohen M, Ravits M, et al. Cutaneous collagenous vasculopathy: a report of three cases. J Cutan Pathol. 2022;49:491-495. https://doi.org/10.1111/cup.14192
- Eldik H, Leisenring NH, Al-Rohil RN, et al. Cutaneous collagenous vasculopathy in a middle-aged woman with a history of prothrombin G20210A thrombophilia. J Cutan Pathol. 2022;49:679-682. https://doi.org/10.1111/cup.13895
- Weiss E, Lazzara DR. Commentary on clinical improvement of cutaneous collagenous vasculopathy with intense pulsed light therapy. Dermatol Surg. 2021;47:1412. https://doi.org/10.1097/DSS.0000000000003209
- Brady BG, Ortleb M, Boyd AS, et al. Cutaneous collagenous vasculopathy. J Clin Aesthet Dermatol. 2015;8:49-52. https://doi.org/10.1097/dad.0000000000000194
- Castiñeiras-Mato I, Rodríguez-Lojo R, Fernández-Díaz ML, et al. Cutaneous collagenous vasculopathy: a case report and review of the literature. Actas Dermosifiliogr. 2016;107:444-447. https://doi.org/10.1016/j.ad.2015.11.006
- Rambhia KD, Hadawale SD, Khopkar US. Cutaneous collagenous vasculopathy: a rare case report. Indian Dermatol Online J. 2016;7:40-42. https://doi.org/10.4103/2229-5178.174327
- Conde-Ferreirós A, Roncero-Riesco M, Cañueto J, et al. Cutaneous collagenous vasculopathy: papular form [published online August 15, 2019]. Dermatol Online J. https://doi.org/10.5070/d3258045128
- García-Martínez P, Gomez-Martin I, Lloreta J, et al. Multiple progressive annular telangiectasias: a clinicopathological variant of cutaneous collagenous vasculopathy? J Cutan Pathol. 2017;44:982-985. https://doi.org/10.1111/cup.13029
- Sartori DS, de Almeida Jr HL, Dorn TV, et al. Cutaneous collagenous vasculopathy: light and transmission electron microscopy. An Bras Dermatol. 2019;94:211-213. https://doi.org/10.1590/abd1806-4841.20198166
- Basso D, Ribero S, Blazek C, et al. Cutaneous collagenous vasculopathy: a rare form of microangiopathy successfully treated with a combination of multiplex laser and optimized pulsed light with a review of the literature. Dermatology. 2016;232:107-111. https://doi.org/10.1159/000439126
- Dura M, Pock L, Cetkovska P, et al. A case of cutaneous collagenous vasculopathy associated with multiple myeloma and with a pathogenic variant of the glucocerebrosidase gene. J Cutan Pathol. 2022;49:717-721. https://doi.org/10.1111/cup.14227
- Salama S, Chorneyko K, Belovic B. Cutaneous collagenous vasculopathy associated with intravascular occlusive fibrin thrombi. J Cutan Pathol. 2014;41:386-393. https://doi.org/10.1111/cup.12285
- Holder E, Schreckenberg C, Lipsker D. Cutaneous collagenous vasculopathy leading to the diagnosis of an advanced pancreatic cancer. J Eur Acad Dermatol Venereol. 2022;36:E699-E701. https://doi.org/10.1111/jdv.18152
- Grossman ME, Cohen M, Ravits M, et al. Cutaneous collagenous vasculopathy: a report of three cases. J Cutan Pathol. 2022;49:491-495. https://doi.org/10.1111/cup.14192
- Eldik H, Leisenring NH, Al-Rohil RN, et al. Cutaneous collagenous vasculopathy in a middle-aged woman with a history of prothrombin G20210A thrombophilia. J Cutan Pathol. 2022;49:679-682. https://doi.org/10.1111/cup.13895
- Weiss E, Lazzara DR. Commentary on clinical improvement of cutaneous collagenous vasculopathy with intense pulsed light therapy. Dermatol Surg. 2021;47:1412. https://doi.org/10.1097/DSS.0000000000003209
Practice Points
- Collagenous vasculopathy is an underrecognized entity.
- Although most patients exhibit only cutaneous disease, systemic involvement also should be assessed.
Right under our noses
Until a couple of weeks ago I considered myself a COVID virgin. I had navigated a full 36 months without a positive test, despite cohabiting with my wife in a 2,500-square-foot house during her bout with the SARS-CoV-2 virus last year. I have been reasonably careful, a situational mask wearer, and good about avoiding poorly ventilated crowded spaces. Of course I was fully vaccinated but was waiting until we had gotten closer to a December trip before getting the newest booster.
I had always been quietly smug about my good luck. And, I was pretty sure that luck had been the major contributor to my run of good health. Nonetheless, in my private moments I often wondered if I somehow had inherited or acquired an unusual defense against the virus that had been getting the best of my peers. One rather far-fetched explanation that kept popping out of my subconscious involved my profuse and persistent runny nose.
Like a fair number in my demographic, I have what I have self-diagnosed as vasomotor rhinitis. In the cooler months and particularly when I am active outdoors, my nose runs like a faucet. I half-jokingly told my wife after a particularly drippy bike ride on a frigid November afternoon that even the most robust virus couldn’t possibly have survived the swim upstream against torrent of mucus splashing onto the handlebars of my bike.
A recent study published in the journal Cell suggests that my off-the-wall explanation for my COVID resistance wasn’t quite so hair-brained. The investigators haven’t found that septuagenarian adults with high-volume runny noses are drowning the SARS-Co- 2 virus before it can do any damage. However, the researchers did discover that, This first line of defense seems to be more effective than in adults, where the virus can more easily slip through into the bloodstream, sometimes with a dramatic release of circulating cytokines, which occasionally create problems of their own. Children also release cytokines, but this is predominantly in their nose, where it appears to be less damaging. Interestingly, in children this initial response persists for around 300 days while in adults the immune response experiences a much more rapid decline. I guess this means we have to chalk one more up for snotty nose kids.
However, the results of this study also suggest that we should be giving more attention to the development of nasal vaccines. I recall that nearly 3 years ago, at the beginning of the pandemic, scientists using a ferret model had developed an effective nasal vaccine. I’m not sure why this faded out of the picture, but it feels like it’s time to turn the spotlight on this line of research again.
I suspect that in addition to being more effective, a nasal vaccine may gain more support among the antivaxxer population, many of whom I suspect are really needle phobics hiding behind a smoke screen of anti-science double talk.
At any rate, I will continue to search for articles that support my contention that my high-flow rhinorrhea is protecting me. I have always been told that a cold nose was the sign of a healthy dog. I’m just trying to prove that the same is true for us old guys with clear runny noses.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Until a couple of weeks ago I considered myself a COVID virgin. I had navigated a full 36 months without a positive test, despite cohabiting with my wife in a 2,500-square-foot house during her bout with the SARS-CoV-2 virus last year. I have been reasonably careful, a situational mask wearer, and good about avoiding poorly ventilated crowded spaces. Of course I was fully vaccinated but was waiting until we had gotten closer to a December trip before getting the newest booster.
I had always been quietly smug about my good luck. And, I was pretty sure that luck had been the major contributor to my run of good health. Nonetheless, in my private moments I often wondered if I somehow had inherited or acquired an unusual defense against the virus that had been getting the best of my peers. One rather far-fetched explanation that kept popping out of my subconscious involved my profuse and persistent runny nose.
Like a fair number in my demographic, I have what I have self-diagnosed as vasomotor rhinitis. In the cooler months and particularly when I am active outdoors, my nose runs like a faucet. I half-jokingly told my wife after a particularly drippy bike ride on a frigid November afternoon that even the most robust virus couldn’t possibly have survived the swim upstream against torrent of mucus splashing onto the handlebars of my bike.
A recent study published in the journal Cell suggests that my off-the-wall explanation for my COVID resistance wasn’t quite so hair-brained. The investigators haven’t found that septuagenarian adults with high-volume runny noses are drowning the SARS-Co- 2 virus before it can do any damage. However, the researchers did discover that, This first line of defense seems to be more effective than in adults, where the virus can more easily slip through into the bloodstream, sometimes with a dramatic release of circulating cytokines, which occasionally create problems of their own. Children also release cytokines, but this is predominantly in their nose, where it appears to be less damaging. Interestingly, in children this initial response persists for around 300 days while in adults the immune response experiences a much more rapid decline. I guess this means we have to chalk one more up for snotty nose kids.
However, the results of this study also suggest that we should be giving more attention to the development of nasal vaccines. I recall that nearly 3 years ago, at the beginning of the pandemic, scientists using a ferret model had developed an effective nasal vaccine. I’m not sure why this faded out of the picture, but it feels like it’s time to turn the spotlight on this line of research again.
I suspect that in addition to being more effective, a nasal vaccine may gain more support among the antivaxxer population, many of whom I suspect are really needle phobics hiding behind a smoke screen of anti-science double talk.
At any rate, I will continue to search for articles that support my contention that my high-flow rhinorrhea is protecting me. I have always been told that a cold nose was the sign of a healthy dog. I’m just trying to prove that the same is true for us old guys with clear runny noses.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Until a couple of weeks ago I considered myself a COVID virgin. I had navigated a full 36 months without a positive test, despite cohabiting with my wife in a 2,500-square-foot house during her bout with the SARS-CoV-2 virus last year. I have been reasonably careful, a situational mask wearer, and good about avoiding poorly ventilated crowded spaces. Of course I was fully vaccinated but was waiting until we had gotten closer to a December trip before getting the newest booster.
I had always been quietly smug about my good luck. And, I was pretty sure that luck had been the major contributor to my run of good health. Nonetheless, in my private moments I often wondered if I somehow had inherited or acquired an unusual defense against the virus that had been getting the best of my peers. One rather far-fetched explanation that kept popping out of my subconscious involved my profuse and persistent runny nose.
Like a fair number in my demographic, I have what I have self-diagnosed as vasomotor rhinitis. In the cooler months and particularly when I am active outdoors, my nose runs like a faucet. I half-jokingly told my wife after a particularly drippy bike ride on a frigid November afternoon that even the most robust virus couldn’t possibly have survived the swim upstream against torrent of mucus splashing onto the handlebars of my bike.
A recent study published in the journal Cell suggests that my off-the-wall explanation for my COVID resistance wasn’t quite so hair-brained. The investigators haven’t found that septuagenarian adults with high-volume runny noses are drowning the SARS-Co- 2 virus before it can do any damage. However, the researchers did discover that, This first line of defense seems to be more effective than in adults, where the virus can more easily slip through into the bloodstream, sometimes with a dramatic release of circulating cytokines, which occasionally create problems of their own. Children also release cytokines, but this is predominantly in their nose, where it appears to be less damaging. Interestingly, in children this initial response persists for around 300 days while in adults the immune response experiences a much more rapid decline. I guess this means we have to chalk one more up for snotty nose kids.
However, the results of this study also suggest that we should be giving more attention to the development of nasal vaccines. I recall that nearly 3 years ago, at the beginning of the pandemic, scientists using a ferret model had developed an effective nasal vaccine. I’m not sure why this faded out of the picture, but it feels like it’s time to turn the spotlight on this line of research again.
I suspect that in addition to being more effective, a nasal vaccine may gain more support among the antivaxxer population, many of whom I suspect are really needle phobics hiding behind a smoke screen of anti-science double talk.
At any rate, I will continue to search for articles that support my contention that my high-flow rhinorrhea is protecting me. I have always been told that a cold nose was the sign of a healthy dog. I’m just trying to prove that the same is true for us old guys with clear runny noses.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
FDA warns of hidden ingredients in arthritis, pain products
Some of these products contain active ingredients found in anti-inflammatory prescription medication.
“These products may cause potentially serious side effects and may interact with medications or dietary supplements a consumer is taking,” the FDA said in a statement. “It is clear from the results of our decade of testing that retailers and distributors, including online marketplaces, do not effectively prevent these types of potentially harmful products from being sold to consumers.”
Unlike prescription medication and over-the-counter drugs such as loratadine (Claritin) or acetaminophen (Tylenol), supplements do not need FDA approval before they can be sold. Only after a complaint is made or FDA testing reveals illegal or unsafe ingredients can the FDA get involved.
From August 2013 to September 2023, the FDA identified 22 arthritis and pain products with active ingredients not disclosed on the product label. The most common hidden ingredients detected in these supplements were prescription-only corticosteroids, nonsteroidal anti-inflammatory drugs (NSAIDs), and muscle relaxants, said Candy Tsourounis, PharmD, a professor in the department of clinical pharmacy at the University of California, San Francisco.
Kuka Flex Forte and Reumo Flex, both promoted for joint pain and arthritis, both contain the NSAID diclofenac. Tapee Tea, a product promoted for pain relief, contains dexamethasone and piroxicam. AK Forte, also sold for joint pain and arthritis, contains diclofenac, dexamethasone, and methocarbamol not disclosed on the label.
“It is interesting that these products have hidden ingredients that are used to reduce swelling and inflammation,” Dr. Tsourounis said. “I don’t know if this was intentional, but it seems suspicious that a product marketed to reduce joint pain and inflammation contains prescription-only ingredients that are used for this purpose.”
Certain products also contained antihistamines including cyproheptadine and chlorpheniramine.
These types of products are likely targeted toward underserved and immigrant communities, added Pieter Cohen, MD, a primary care physician and an assistant professor of medicine at Harvard Medical School, Boston, who studies dietary supplements. They might be sold in mom-and-pop shops or gas stations to individuals with limited access to health care or insurance, he noted.
The FDA warned that this list included “only a small fraction of the potentially dangerous products marketed to consumers online and in stores. Even if a product is not included in this list, consumers should exercise caution before using these types of arthritis and pain management products.”
Advising patients
Research suggests that most patients do not tell doctors about the supplements they are taking, and often, clinicians do not ask, said Dr. Cohen. “Most of the time it’s a total black box – we don’t know what’s going on,” he added.
He advised raising the subject of supplements in a very nonjudgmental way, particularly when treating patients in marginalized and immigrant communities. One approach he suggested was first mentioning that other patients in your care dealing with joint pain have bought remedies locally or have tried treatments that friends recommend. You can then ask a patient about their own use, framing it as a way to better help with treatment decisions.
Once a clinician understands what their patient is taking, they can then give advice and discuss if a product is safe to combine with prescription drugs, Dr. Cohen said. “If they come down too hard, I think the patients will just clam up and not talk about it anymore,” he said.
If a patient begins to experience side effects or gets sick, a clinician will already be informed of what their patient is taking and can ask that patient to bring the product or supplement in, so they can look over the product together, Dr. Cohen noted. Any side effects or other adverse events potentially related to the use of these products should then be reported to FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
Tips for safe shopping
To make sure supplements and other over-the-counter products are safe to use, Dr. Tsourounis recommends that consumers:
- Buy products from well-known retailers like Target or large pharmacies like CVS or Walgreens.
- Avoid buying products with labels in another language that you cannot read or products with no drug label.
- Be cautious of buying products online or from other countries.
- Look up suspicious products on the FDA’s health fraud database.
- Be wary of any product that offers miracle cures or relies on personal testimonies without evidence.
In general, do not base purchasing decisions on any health claims on a product label because companies selling supplements making these claims “don’t have to have any clinical data to back them up,” Dr. Cohen said.
Dr. Cohen also recommends sticking with individual ingredients. “If you want echinacea, buy echinacea. Don’t buy a complicated mix that is supposed to be good for arthritis with 10 different botanical [ingredients]. That’s more likely to run [you] into trouble,” he said.
Last, Dr. Cohen recommended buying supplements that are certified by NSF International or United States Pharmacopeia, both respected third-party testing organizations. “If it has an NSF International or USP stamp, that gives us more certainty that what’s in the bottle is going to be what’s listed on label,” he said.
Dr. Tsourounis noted that if you are skeptical of a product, you can also try calling the manufacturer number on the product label.
“I always encourage people to call that number to see if somebody answers,” she said. “Sometimes, you can tell a lot about that company just by calling that number.”
Dr. Cohen has received research support from the Consumers Union and PEW Charitable Trusts and royalties from UpToDate. He has collaborated in research with NSF International. Dr. Tsourounis disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Some of these products contain active ingredients found in anti-inflammatory prescription medication.
“These products may cause potentially serious side effects and may interact with medications or dietary supplements a consumer is taking,” the FDA said in a statement. “It is clear from the results of our decade of testing that retailers and distributors, including online marketplaces, do not effectively prevent these types of potentially harmful products from being sold to consumers.”
Unlike prescription medication and over-the-counter drugs such as loratadine (Claritin) or acetaminophen (Tylenol), supplements do not need FDA approval before they can be sold. Only after a complaint is made or FDA testing reveals illegal or unsafe ingredients can the FDA get involved.
From August 2013 to September 2023, the FDA identified 22 arthritis and pain products with active ingredients not disclosed on the product label. The most common hidden ingredients detected in these supplements were prescription-only corticosteroids, nonsteroidal anti-inflammatory drugs (NSAIDs), and muscle relaxants, said Candy Tsourounis, PharmD, a professor in the department of clinical pharmacy at the University of California, San Francisco.
Kuka Flex Forte and Reumo Flex, both promoted for joint pain and arthritis, both contain the NSAID diclofenac. Tapee Tea, a product promoted for pain relief, contains dexamethasone and piroxicam. AK Forte, also sold for joint pain and arthritis, contains diclofenac, dexamethasone, and methocarbamol not disclosed on the label.
“It is interesting that these products have hidden ingredients that are used to reduce swelling and inflammation,” Dr. Tsourounis said. “I don’t know if this was intentional, but it seems suspicious that a product marketed to reduce joint pain and inflammation contains prescription-only ingredients that are used for this purpose.”
Certain products also contained antihistamines including cyproheptadine and chlorpheniramine.
These types of products are likely targeted toward underserved and immigrant communities, added Pieter Cohen, MD, a primary care physician and an assistant professor of medicine at Harvard Medical School, Boston, who studies dietary supplements. They might be sold in mom-and-pop shops or gas stations to individuals with limited access to health care or insurance, he noted.
The FDA warned that this list included “only a small fraction of the potentially dangerous products marketed to consumers online and in stores. Even if a product is not included in this list, consumers should exercise caution before using these types of arthritis and pain management products.”
Advising patients
Research suggests that most patients do not tell doctors about the supplements they are taking, and often, clinicians do not ask, said Dr. Cohen. “Most of the time it’s a total black box – we don’t know what’s going on,” he added.
He advised raising the subject of supplements in a very nonjudgmental way, particularly when treating patients in marginalized and immigrant communities. One approach he suggested was first mentioning that other patients in your care dealing with joint pain have bought remedies locally or have tried treatments that friends recommend. You can then ask a patient about their own use, framing it as a way to better help with treatment decisions.
Once a clinician understands what their patient is taking, they can then give advice and discuss if a product is safe to combine with prescription drugs, Dr. Cohen said. “If they come down too hard, I think the patients will just clam up and not talk about it anymore,” he said.
If a patient begins to experience side effects or gets sick, a clinician will already be informed of what their patient is taking and can ask that patient to bring the product or supplement in, so they can look over the product together, Dr. Cohen noted. Any side effects or other adverse events potentially related to the use of these products should then be reported to FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
Tips for safe shopping
To make sure supplements and other over-the-counter products are safe to use, Dr. Tsourounis recommends that consumers:
- Buy products from well-known retailers like Target or large pharmacies like CVS or Walgreens.
- Avoid buying products with labels in another language that you cannot read or products with no drug label.
- Be cautious of buying products online or from other countries.
- Look up suspicious products on the FDA’s health fraud database.
- Be wary of any product that offers miracle cures or relies on personal testimonies without evidence.
In general, do not base purchasing decisions on any health claims on a product label because companies selling supplements making these claims “don’t have to have any clinical data to back them up,” Dr. Cohen said.
Dr. Cohen also recommends sticking with individual ingredients. “If you want echinacea, buy echinacea. Don’t buy a complicated mix that is supposed to be good for arthritis with 10 different botanical [ingredients]. That’s more likely to run [you] into trouble,” he said.
Last, Dr. Cohen recommended buying supplements that are certified by NSF International or United States Pharmacopeia, both respected third-party testing organizations. “If it has an NSF International or USP stamp, that gives us more certainty that what’s in the bottle is going to be what’s listed on label,” he said.
Dr. Tsourounis noted that if you are skeptical of a product, you can also try calling the manufacturer number on the product label.
“I always encourage people to call that number to see if somebody answers,” she said. “Sometimes, you can tell a lot about that company just by calling that number.”
Dr. Cohen has received research support from the Consumers Union and PEW Charitable Trusts and royalties from UpToDate. He has collaborated in research with NSF International. Dr. Tsourounis disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Some of these products contain active ingredients found in anti-inflammatory prescription medication.
“These products may cause potentially serious side effects and may interact with medications or dietary supplements a consumer is taking,” the FDA said in a statement. “It is clear from the results of our decade of testing that retailers and distributors, including online marketplaces, do not effectively prevent these types of potentially harmful products from being sold to consumers.”
Unlike prescription medication and over-the-counter drugs such as loratadine (Claritin) or acetaminophen (Tylenol), supplements do not need FDA approval before they can be sold. Only after a complaint is made or FDA testing reveals illegal or unsafe ingredients can the FDA get involved.
From August 2013 to September 2023, the FDA identified 22 arthritis and pain products with active ingredients not disclosed on the product label. The most common hidden ingredients detected in these supplements were prescription-only corticosteroids, nonsteroidal anti-inflammatory drugs (NSAIDs), and muscle relaxants, said Candy Tsourounis, PharmD, a professor in the department of clinical pharmacy at the University of California, San Francisco.
Kuka Flex Forte and Reumo Flex, both promoted for joint pain and arthritis, both contain the NSAID diclofenac. Tapee Tea, a product promoted for pain relief, contains dexamethasone and piroxicam. AK Forte, also sold for joint pain and arthritis, contains diclofenac, dexamethasone, and methocarbamol not disclosed on the label.
“It is interesting that these products have hidden ingredients that are used to reduce swelling and inflammation,” Dr. Tsourounis said. “I don’t know if this was intentional, but it seems suspicious that a product marketed to reduce joint pain and inflammation contains prescription-only ingredients that are used for this purpose.”
Certain products also contained antihistamines including cyproheptadine and chlorpheniramine.
These types of products are likely targeted toward underserved and immigrant communities, added Pieter Cohen, MD, a primary care physician and an assistant professor of medicine at Harvard Medical School, Boston, who studies dietary supplements. They might be sold in mom-and-pop shops or gas stations to individuals with limited access to health care or insurance, he noted.
The FDA warned that this list included “only a small fraction of the potentially dangerous products marketed to consumers online and in stores. Even if a product is not included in this list, consumers should exercise caution before using these types of arthritis and pain management products.”
Advising patients
Research suggests that most patients do not tell doctors about the supplements they are taking, and often, clinicians do not ask, said Dr. Cohen. “Most of the time it’s a total black box – we don’t know what’s going on,” he added.
He advised raising the subject of supplements in a very nonjudgmental way, particularly when treating patients in marginalized and immigrant communities. One approach he suggested was first mentioning that other patients in your care dealing with joint pain have bought remedies locally or have tried treatments that friends recommend. You can then ask a patient about their own use, framing it as a way to better help with treatment decisions.
Once a clinician understands what their patient is taking, they can then give advice and discuss if a product is safe to combine with prescription drugs, Dr. Cohen said. “If they come down too hard, I think the patients will just clam up and not talk about it anymore,” he said.
If a patient begins to experience side effects or gets sick, a clinician will already be informed of what their patient is taking and can ask that patient to bring the product or supplement in, so they can look over the product together, Dr. Cohen noted. Any side effects or other adverse events potentially related to the use of these products should then be reported to FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
Tips for safe shopping
To make sure supplements and other over-the-counter products are safe to use, Dr. Tsourounis recommends that consumers:
- Buy products from well-known retailers like Target or large pharmacies like CVS or Walgreens.
- Avoid buying products with labels in another language that you cannot read or products with no drug label.
- Be cautious of buying products online or from other countries.
- Look up suspicious products on the FDA’s health fraud database.
- Be wary of any product that offers miracle cures or relies on personal testimonies without evidence.
In general, do not base purchasing decisions on any health claims on a product label because companies selling supplements making these claims “don’t have to have any clinical data to back them up,” Dr. Cohen said.
Dr. Cohen also recommends sticking with individual ingredients. “If you want echinacea, buy echinacea. Don’t buy a complicated mix that is supposed to be good for arthritis with 10 different botanical [ingredients]. That’s more likely to run [you] into trouble,” he said.
Last, Dr. Cohen recommended buying supplements that are certified by NSF International or United States Pharmacopeia, both respected third-party testing organizations. “If it has an NSF International or USP stamp, that gives us more certainty that what’s in the bottle is going to be what’s listed on label,” he said.
Dr. Tsourounis noted that if you are skeptical of a product, you can also try calling the manufacturer number on the product label.
“I always encourage people to call that number to see if somebody answers,” she said. “Sometimes, you can tell a lot about that company just by calling that number.”
Dr. Cohen has received research support from the Consumers Union and PEW Charitable Trusts and royalties from UpToDate. He has collaborated in research with NSF International. Dr. Tsourounis disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Subcutaneous ocrelizumab, ofatumumab ‘reassuring’ in MS
MILAN – , suggest results from two clinical trials.
For OCARINA II, more than 325 patients with MS were randomly assigned to either subcutaneous or intravenous treatment with the anti-CD20 monoclonal antibody ocrelizumab (Ocrevus).
After 24 weeks, the presence of lesions on imaging and the occurrence of clinical remissions were almost completely suppressed by both treatments albeit with a higher rate of mild to moderate injection reactions with subcutaneous administration.
The study “makes me feel pretty comfortable that regardless of where you’re delivering the therapy, IV or subcutaneously, it’s getting in there and doing the job that we want it to do,” said lead author Scott D. Newsome, DO, director, Stiff Person Syndrome Center, Johns Hopkins University, Baltimore.
The second study, OLIKOS, involved just over 100 patients with relapsing MS who had previously been treated with an anti-CD20 monoclonal antibody and were switched to subcutaneous therapy with another: ofatumumab (Arzerra).
Le H. Hua, MD, Cleveland Clinic Lou Ruvo Center for Brain Health, Las Vegas, and colleagues report that the novel treatment maintained clinical efficacy in all patients, with no safety concerns and no changes in serum immunoglobulin levels.
The findings were presented at the 9th Joint ECTRIMS-ACTRIMS meeting.
Anti-CD20–naive
OCARINA II involved patients aged 18-65 years with relapsing or primary progressive MS who had never received ocrelizumab or any other anti-CD20 therapy and had an Expanded Disability Status Scale (EDSS) score of 0.0-6.5.
They were randomly assigned to subcutaneous or IV ocrelizumab as a first dose. At week 24, all patients were scheduled to receive subcutaneous ocrelizumab every 24 weeks up to week 96.
In all, 326 patients were randomly assigned to the two treatment arms. They had a mean age of approximately 40 years, and 59.3%-65.3% were women. The mean time since symptom onset was 6.8-7.7 years, and the mean EDSS score at baseline was 2.5-3.0. The majority (89.8%-89.0%) had relapsing MS.
The results showed that subcutaneous and IV administration led to similar exposure to ocrelizumab, and both resulted in rapid reduction in CD19+ B-cell counts.
By week 24, the mean number of lesions on MRI reduced to zero, resulting in “near-complete suppression” of disease activity, the team says, which was reflected in 99% of patients have no clinical evidence of relapse.
The overall adverse event rate was higher with subcutaneous vs. IV administration of ocrelizumab, at 73.7% vs. 45.8%, driven by both local and systemic injection reactions, which were mild to moderate in nature.
However, a similar proportion of patients in the subcutaneous and IV arms experienced serious adverse events, at 2.5% and 3.4%, respectively.
Crucially, the patients were “overwhelmingly positive” about the subcutaneous administration, Dr. Newsome said, and at his institution, “all the patients want to continue, if and when this gets approved.”
He said that, overall, he would like to have both routes available “because, coming down to patient preference, some prefer to have IV over subcutaneous in general, and that could be for a variety of reasons, so I would love to have as many different routes of administration as possible to offer.”
Efficacy maintained
The OLIKOS trial included patients aged 18-60 years with relapsing MS who had received at least two consecutive courses of anti-CD20 therapy with either ocrelizumab or rituximab and who had an EDSS score ≤ 5.5 and were neurologically stable.
After an initial loading regimen of subcutaneous ofatumumab on days 1, 7, and 14, the patients continued open-label subcutaneous ofatumumab once a month for 12 months, with assessments carried out at baseline and at 1, 6, and 12 months.
Of 142 patients assessed, 102 received treatment and were evaluated. Their mean age was 43.5 years, and 67.6% were women. The mean baseline EDSS score was 2.9, and the mean disease duration since diagnosis was 9.4 years.
The vast majority of patients (99.0%) had previously received ocrelizumab for an average duration of 26.7 months.
At this interim analysis, 100% of the 77 patients with follow-up MRI met the primary endpoint at month 6 of no change or a reduction in the number of lesions.
The team says there were “no new safety signals,” with 75.5% of patients experiencing a treatment-emergent adverse event, but only 1.0% having a serious adverse event. Injection site reactions occurred in 7.8%; 15.7% had a systemic injection reaction.
They also report that there were no changes in IgG and IgM concentrations between baseline and follow-up, which remained within normal reference ranges.
Reassuring results
“It’s exciting to see reassuring results from clinical studies of two high-efficacy therapies for multiple sclerosis, especially given their route of administration,” commented Julie Fiol, LMSW, BSN, RN, MSCN, associate vice president of Clinical Innovation and Strategy for the U.S. National MS Society.
“Subcutaneous injections allow people with multiple sclerosis more flexibility when selecting a therapy that matches their lifestyle and preferences,” she said in an interview.
“Adherence to therapy is critical in multiple sclerosis, and additional options for route of administration and site of care enhance the likelihood that someone with multiple sclerosis will find a medication that effectively manages their disease and fits into their lifestyle,” Dr. Fiol explained.
“Subcutaneous injections also have the potential to be more affordable as they could be administered at home or over a shorter duration than an infused medication,” she noted.
In terms of these two particular studies, she added, “it’s reassuring to see that the safety and efficacy of subcutaneous ocrelizumab was similar to intravenous. It was also reassuring to see those who switched from ocrelizumab and rituximab to ofatumumab remained clinically stable.”
OCARINA II was supported by F. Hoffmann-La Roche. OLIKOS was supported by Novartis. Dr. Newsome declares relationships with Biogen, Genentech, Bristol-Myers Squibb, EMD Serono, Greenwich Biosciences, Horizon Therapeutics, Novartis, Roche, and TG Therapeutics and institutional relationships with Biogen, Lundbeck, Roche, Genentech, National MS Society, The Stiff Person Syndrome Research Foundation, Department of Defense, and the Patient-Centered Outcomes Research Institute. Dr. Hua declares relationships with Alexion, Biogen, Bristol-Meyers Squibb, EMD Serono, Genentech, Genzyme, Greenwich Biosciences, Horizon Therapeutics, and Novartis. Other authors also declare relationships.
A version of this article first appeared on Medscape.com.
MILAN – , suggest results from two clinical trials.
For OCARINA II, more than 325 patients with MS were randomly assigned to either subcutaneous or intravenous treatment with the anti-CD20 monoclonal antibody ocrelizumab (Ocrevus).
After 24 weeks, the presence of lesions on imaging and the occurrence of clinical remissions were almost completely suppressed by both treatments albeit with a higher rate of mild to moderate injection reactions with subcutaneous administration.
The study “makes me feel pretty comfortable that regardless of where you’re delivering the therapy, IV or subcutaneously, it’s getting in there and doing the job that we want it to do,” said lead author Scott D. Newsome, DO, director, Stiff Person Syndrome Center, Johns Hopkins University, Baltimore.
The second study, OLIKOS, involved just over 100 patients with relapsing MS who had previously been treated with an anti-CD20 monoclonal antibody and were switched to subcutaneous therapy with another: ofatumumab (Arzerra).
Le H. Hua, MD, Cleveland Clinic Lou Ruvo Center for Brain Health, Las Vegas, and colleagues report that the novel treatment maintained clinical efficacy in all patients, with no safety concerns and no changes in serum immunoglobulin levels.
The findings were presented at the 9th Joint ECTRIMS-ACTRIMS meeting.
Anti-CD20–naive
OCARINA II involved patients aged 18-65 years with relapsing or primary progressive MS who had never received ocrelizumab or any other anti-CD20 therapy and had an Expanded Disability Status Scale (EDSS) score of 0.0-6.5.
They were randomly assigned to subcutaneous or IV ocrelizumab as a first dose. At week 24, all patients were scheduled to receive subcutaneous ocrelizumab every 24 weeks up to week 96.
In all, 326 patients were randomly assigned to the two treatment arms. They had a mean age of approximately 40 years, and 59.3%-65.3% were women. The mean time since symptom onset was 6.8-7.7 years, and the mean EDSS score at baseline was 2.5-3.0. The majority (89.8%-89.0%) had relapsing MS.
The results showed that subcutaneous and IV administration led to similar exposure to ocrelizumab, and both resulted in rapid reduction in CD19+ B-cell counts.
By week 24, the mean number of lesions on MRI reduced to zero, resulting in “near-complete suppression” of disease activity, the team says, which was reflected in 99% of patients have no clinical evidence of relapse.
The overall adverse event rate was higher with subcutaneous vs. IV administration of ocrelizumab, at 73.7% vs. 45.8%, driven by both local and systemic injection reactions, which were mild to moderate in nature.
However, a similar proportion of patients in the subcutaneous and IV arms experienced serious adverse events, at 2.5% and 3.4%, respectively.
Crucially, the patients were “overwhelmingly positive” about the subcutaneous administration, Dr. Newsome said, and at his institution, “all the patients want to continue, if and when this gets approved.”
He said that, overall, he would like to have both routes available “because, coming down to patient preference, some prefer to have IV over subcutaneous in general, and that could be for a variety of reasons, so I would love to have as many different routes of administration as possible to offer.”
Efficacy maintained
The OLIKOS trial included patients aged 18-60 years with relapsing MS who had received at least two consecutive courses of anti-CD20 therapy with either ocrelizumab or rituximab and who had an EDSS score ≤ 5.5 and were neurologically stable.
After an initial loading regimen of subcutaneous ofatumumab on days 1, 7, and 14, the patients continued open-label subcutaneous ofatumumab once a month for 12 months, with assessments carried out at baseline and at 1, 6, and 12 months.
Of 142 patients assessed, 102 received treatment and were evaluated. Their mean age was 43.5 years, and 67.6% were women. The mean baseline EDSS score was 2.9, and the mean disease duration since diagnosis was 9.4 years.
The vast majority of patients (99.0%) had previously received ocrelizumab for an average duration of 26.7 months.
At this interim analysis, 100% of the 77 patients with follow-up MRI met the primary endpoint at month 6 of no change or a reduction in the number of lesions.
The team says there were “no new safety signals,” with 75.5% of patients experiencing a treatment-emergent adverse event, but only 1.0% having a serious adverse event. Injection site reactions occurred in 7.8%; 15.7% had a systemic injection reaction.
They also report that there were no changes in IgG and IgM concentrations between baseline and follow-up, which remained within normal reference ranges.
Reassuring results
“It’s exciting to see reassuring results from clinical studies of two high-efficacy therapies for multiple sclerosis, especially given their route of administration,” commented Julie Fiol, LMSW, BSN, RN, MSCN, associate vice president of Clinical Innovation and Strategy for the U.S. National MS Society.
“Subcutaneous injections allow people with multiple sclerosis more flexibility when selecting a therapy that matches their lifestyle and preferences,” she said in an interview.
“Adherence to therapy is critical in multiple sclerosis, and additional options for route of administration and site of care enhance the likelihood that someone with multiple sclerosis will find a medication that effectively manages their disease and fits into their lifestyle,” Dr. Fiol explained.
“Subcutaneous injections also have the potential to be more affordable as they could be administered at home or over a shorter duration than an infused medication,” she noted.
In terms of these two particular studies, she added, “it’s reassuring to see that the safety and efficacy of subcutaneous ocrelizumab was similar to intravenous. It was also reassuring to see those who switched from ocrelizumab and rituximab to ofatumumab remained clinically stable.”
OCARINA II was supported by F. Hoffmann-La Roche. OLIKOS was supported by Novartis. Dr. Newsome declares relationships with Biogen, Genentech, Bristol-Myers Squibb, EMD Serono, Greenwich Biosciences, Horizon Therapeutics, Novartis, Roche, and TG Therapeutics and institutional relationships with Biogen, Lundbeck, Roche, Genentech, National MS Society, The Stiff Person Syndrome Research Foundation, Department of Defense, and the Patient-Centered Outcomes Research Institute. Dr. Hua declares relationships with Alexion, Biogen, Bristol-Meyers Squibb, EMD Serono, Genentech, Genzyme, Greenwich Biosciences, Horizon Therapeutics, and Novartis. Other authors also declare relationships.
A version of this article first appeared on Medscape.com.
MILAN – , suggest results from two clinical trials.
For OCARINA II, more than 325 patients with MS were randomly assigned to either subcutaneous or intravenous treatment with the anti-CD20 monoclonal antibody ocrelizumab (Ocrevus).
After 24 weeks, the presence of lesions on imaging and the occurrence of clinical remissions were almost completely suppressed by both treatments albeit with a higher rate of mild to moderate injection reactions with subcutaneous administration.
The study “makes me feel pretty comfortable that regardless of where you’re delivering the therapy, IV or subcutaneously, it’s getting in there and doing the job that we want it to do,” said lead author Scott D. Newsome, DO, director, Stiff Person Syndrome Center, Johns Hopkins University, Baltimore.
The second study, OLIKOS, involved just over 100 patients with relapsing MS who had previously been treated with an anti-CD20 monoclonal antibody and were switched to subcutaneous therapy with another: ofatumumab (Arzerra).
Le H. Hua, MD, Cleveland Clinic Lou Ruvo Center for Brain Health, Las Vegas, and colleagues report that the novel treatment maintained clinical efficacy in all patients, with no safety concerns and no changes in serum immunoglobulin levels.
The findings were presented at the 9th Joint ECTRIMS-ACTRIMS meeting.
Anti-CD20–naive
OCARINA II involved patients aged 18-65 years with relapsing or primary progressive MS who had never received ocrelizumab or any other anti-CD20 therapy and had an Expanded Disability Status Scale (EDSS) score of 0.0-6.5.
They were randomly assigned to subcutaneous or IV ocrelizumab as a first dose. At week 24, all patients were scheduled to receive subcutaneous ocrelizumab every 24 weeks up to week 96.
In all, 326 patients were randomly assigned to the two treatment arms. They had a mean age of approximately 40 years, and 59.3%-65.3% were women. The mean time since symptom onset was 6.8-7.7 years, and the mean EDSS score at baseline was 2.5-3.0. The majority (89.8%-89.0%) had relapsing MS.
The results showed that subcutaneous and IV administration led to similar exposure to ocrelizumab, and both resulted in rapid reduction in CD19+ B-cell counts.
By week 24, the mean number of lesions on MRI reduced to zero, resulting in “near-complete suppression” of disease activity, the team says, which was reflected in 99% of patients have no clinical evidence of relapse.
The overall adverse event rate was higher with subcutaneous vs. IV administration of ocrelizumab, at 73.7% vs. 45.8%, driven by both local and systemic injection reactions, which were mild to moderate in nature.
However, a similar proportion of patients in the subcutaneous and IV arms experienced serious adverse events, at 2.5% and 3.4%, respectively.
Crucially, the patients were “overwhelmingly positive” about the subcutaneous administration, Dr. Newsome said, and at his institution, “all the patients want to continue, if and when this gets approved.”
He said that, overall, he would like to have both routes available “because, coming down to patient preference, some prefer to have IV over subcutaneous in general, and that could be for a variety of reasons, so I would love to have as many different routes of administration as possible to offer.”
Efficacy maintained
The OLIKOS trial included patients aged 18-60 years with relapsing MS who had received at least two consecutive courses of anti-CD20 therapy with either ocrelizumab or rituximab and who had an EDSS score ≤ 5.5 and were neurologically stable.
After an initial loading regimen of subcutaneous ofatumumab on days 1, 7, and 14, the patients continued open-label subcutaneous ofatumumab once a month for 12 months, with assessments carried out at baseline and at 1, 6, and 12 months.
Of 142 patients assessed, 102 received treatment and were evaluated. Their mean age was 43.5 years, and 67.6% were women. The mean baseline EDSS score was 2.9, and the mean disease duration since diagnosis was 9.4 years.
The vast majority of patients (99.0%) had previously received ocrelizumab for an average duration of 26.7 months.
At this interim analysis, 100% of the 77 patients with follow-up MRI met the primary endpoint at month 6 of no change or a reduction in the number of lesions.
The team says there were “no new safety signals,” with 75.5% of patients experiencing a treatment-emergent adverse event, but only 1.0% having a serious adverse event. Injection site reactions occurred in 7.8%; 15.7% had a systemic injection reaction.
They also report that there were no changes in IgG and IgM concentrations between baseline and follow-up, which remained within normal reference ranges.
Reassuring results
“It’s exciting to see reassuring results from clinical studies of two high-efficacy therapies for multiple sclerosis, especially given their route of administration,” commented Julie Fiol, LMSW, BSN, RN, MSCN, associate vice president of Clinical Innovation and Strategy for the U.S. National MS Society.
“Subcutaneous injections allow people with multiple sclerosis more flexibility when selecting a therapy that matches their lifestyle and preferences,” she said in an interview.
“Adherence to therapy is critical in multiple sclerosis, and additional options for route of administration and site of care enhance the likelihood that someone with multiple sclerosis will find a medication that effectively manages their disease and fits into their lifestyle,” Dr. Fiol explained.
“Subcutaneous injections also have the potential to be more affordable as they could be administered at home or over a shorter duration than an infused medication,” she noted.
In terms of these two particular studies, she added, “it’s reassuring to see that the safety and efficacy of subcutaneous ocrelizumab was similar to intravenous. It was also reassuring to see those who switched from ocrelizumab and rituximab to ofatumumab remained clinically stable.”
OCARINA II was supported by F. Hoffmann-La Roche. OLIKOS was supported by Novartis. Dr. Newsome declares relationships with Biogen, Genentech, Bristol-Myers Squibb, EMD Serono, Greenwich Biosciences, Horizon Therapeutics, Novartis, Roche, and TG Therapeutics and institutional relationships with Biogen, Lundbeck, Roche, Genentech, National MS Society, The Stiff Person Syndrome Research Foundation, Department of Defense, and the Patient-Centered Outcomes Research Institute. Dr. Hua declares relationships with Alexion, Biogen, Bristol-Meyers Squibb, EMD Serono, Genentech, Genzyme, Greenwich Biosciences, Horizon Therapeutics, and Novartis. Other authors also declare relationships.
A version of this article first appeared on Medscape.com.
AT ECTRIMS 2023
Higher weight loss on tirzepatide links to seven factors
TOPLINE:
Among the 3,188 people with type 2 diabetes who were adherent to their tirzepatide (Mounjaro, Lilly) regimen in four pivotal trials of the agent, a quarter achieved at least a 15% cut from their baseline body weight after 40-42 weeks of treatment, and researchers found seven baseline variables that were significantly linked with a higher incidence of this level of weight loss.
say the authors.
METHODOLOGY:
- Investigators conducted a post hoc analysis of data collected from a total of 3,188 people with type 2 diabetes who had been adherent to their assigned tirzepatide regimen for 40-42 weeks in any one of four pivotal trials of the agent.
- The researchers aimed to identify predictors of a reduction in body weight of at least 15% with tirzepatide treatment at any of the three tested doses – 5 mg, 10 mg, or 15 mg – which were administered by subcutaneous injection once a week.
- All four trials that provided data prohibited concurrent therapy that would promote weight loss, and the people included in the analysis did not receive any rescue medications for controlling glycemia.
- The primary efficacy measure in all four studies was the ability of tirzepatide to improve glycemic control (measured by A1c level), compared with placebo, semaglutide (Ozempic) 1 mg SC once weekly, insulin degludec (Tresiba, Novo Nordisk), or insulin glargine (Basaglar, Lilly).
TAKEAWAY:
- Among the 3,188 people who remained adherent to their tirzepatide regimen for 40-42 weeks, 792 (25%) experienced a weight reduction of at least 15% from baseline.
- Multivariate analysis of baseline covariates showed that these seven factors were significantly linked with greater than or equal to 15% weight loss: higher tirzepatide dose, being female, being of White or Asian race, being of younger age, undergoing treatment with metformin, having better glycemic control (based on lower A1c and lower fasting serum glucose), and having lower non–high-density lipoprotein cholesterol level.
- During follow-up, achievement of at least a 15% cut in baseline body weight was significantly associated with greater reductions in A1c, fasting serum glucose level, waist circumference, blood pressure, serum triglyceride level, and serum level of the liver enzyme alanine transaminase.
IN PRACTICE:
“These findings may provide valuable information to clinicians and people with type 2 diabetes regarding the likelihood of achieving substantial body weight reduction with tirzepatide and also help to signal likely improvements to be seen in a range of cardiometabolic risk parameters with tirzepatide-induced weight loss,” the authors concluded in their report.
SOURCE:
The study was largely run by researchers who are employees of Lilly, the company that markets tirzepatide (Mounjaro). It was published in Diabetes Care.
LIMITATIONS:
- The analysis was post hoc.
- The follow-up was limited.
- The analysis focused entirely on baseline parameters as potential predictors of weight loss magnitude.
DISCLOSURES:
The study was funded by Eli Lilly, the company that markets tirzepatide (Mounjaro) and that sponsored the SURPASS trials. Six authors are employees of Lilly, one is a contractor for Lilly, and the two remaining authors have had financial relationships with Lilly and with several other companies.
A version of this article first appeared on Medscape.com.
TOPLINE:
Among the 3,188 people with type 2 diabetes who were adherent to their tirzepatide (Mounjaro, Lilly) regimen in four pivotal trials of the agent, a quarter achieved at least a 15% cut from their baseline body weight after 40-42 weeks of treatment, and researchers found seven baseline variables that were significantly linked with a higher incidence of this level of weight loss.
say the authors.
METHODOLOGY:
- Investigators conducted a post hoc analysis of data collected from a total of 3,188 people with type 2 diabetes who had been adherent to their assigned tirzepatide regimen for 40-42 weeks in any one of four pivotal trials of the agent.
- The researchers aimed to identify predictors of a reduction in body weight of at least 15% with tirzepatide treatment at any of the three tested doses – 5 mg, 10 mg, or 15 mg – which were administered by subcutaneous injection once a week.
- All four trials that provided data prohibited concurrent therapy that would promote weight loss, and the people included in the analysis did not receive any rescue medications for controlling glycemia.
- The primary efficacy measure in all four studies was the ability of tirzepatide to improve glycemic control (measured by A1c level), compared with placebo, semaglutide (Ozempic) 1 mg SC once weekly, insulin degludec (Tresiba, Novo Nordisk), or insulin glargine (Basaglar, Lilly).
TAKEAWAY:
- Among the 3,188 people who remained adherent to their tirzepatide regimen for 40-42 weeks, 792 (25%) experienced a weight reduction of at least 15% from baseline.
- Multivariate analysis of baseline covariates showed that these seven factors were significantly linked with greater than or equal to 15% weight loss: higher tirzepatide dose, being female, being of White or Asian race, being of younger age, undergoing treatment with metformin, having better glycemic control (based on lower A1c and lower fasting serum glucose), and having lower non–high-density lipoprotein cholesterol level.
- During follow-up, achievement of at least a 15% cut in baseline body weight was significantly associated with greater reductions in A1c, fasting serum glucose level, waist circumference, blood pressure, serum triglyceride level, and serum level of the liver enzyme alanine transaminase.
IN PRACTICE:
“These findings may provide valuable information to clinicians and people with type 2 diabetes regarding the likelihood of achieving substantial body weight reduction with tirzepatide and also help to signal likely improvements to be seen in a range of cardiometabolic risk parameters with tirzepatide-induced weight loss,” the authors concluded in their report.
SOURCE:
The study was largely run by researchers who are employees of Lilly, the company that markets tirzepatide (Mounjaro). It was published in Diabetes Care.
LIMITATIONS:
- The analysis was post hoc.
- The follow-up was limited.
- The analysis focused entirely on baseline parameters as potential predictors of weight loss magnitude.
DISCLOSURES:
The study was funded by Eli Lilly, the company that markets tirzepatide (Mounjaro) and that sponsored the SURPASS trials. Six authors are employees of Lilly, one is a contractor for Lilly, and the two remaining authors have had financial relationships with Lilly and with several other companies.
A version of this article first appeared on Medscape.com.
TOPLINE:
Among the 3,188 people with type 2 diabetes who were adherent to their tirzepatide (Mounjaro, Lilly) regimen in four pivotal trials of the agent, a quarter achieved at least a 15% cut from their baseline body weight after 40-42 weeks of treatment, and researchers found seven baseline variables that were significantly linked with a higher incidence of this level of weight loss.
say the authors.
METHODOLOGY:
- Investigators conducted a post hoc analysis of data collected from a total of 3,188 people with type 2 diabetes who had been adherent to their assigned tirzepatide regimen for 40-42 weeks in any one of four pivotal trials of the agent.
- The researchers aimed to identify predictors of a reduction in body weight of at least 15% with tirzepatide treatment at any of the three tested doses – 5 mg, 10 mg, or 15 mg – which were administered by subcutaneous injection once a week.
- All four trials that provided data prohibited concurrent therapy that would promote weight loss, and the people included in the analysis did not receive any rescue medications for controlling glycemia.
- The primary efficacy measure in all four studies was the ability of tirzepatide to improve glycemic control (measured by A1c level), compared with placebo, semaglutide (Ozempic) 1 mg SC once weekly, insulin degludec (Tresiba, Novo Nordisk), or insulin glargine (Basaglar, Lilly).
TAKEAWAY:
- Among the 3,188 people who remained adherent to their tirzepatide regimen for 40-42 weeks, 792 (25%) experienced a weight reduction of at least 15% from baseline.
- Multivariate analysis of baseline covariates showed that these seven factors were significantly linked with greater than or equal to 15% weight loss: higher tirzepatide dose, being female, being of White or Asian race, being of younger age, undergoing treatment with metformin, having better glycemic control (based on lower A1c and lower fasting serum glucose), and having lower non–high-density lipoprotein cholesterol level.
- During follow-up, achievement of at least a 15% cut in baseline body weight was significantly associated with greater reductions in A1c, fasting serum glucose level, waist circumference, blood pressure, serum triglyceride level, and serum level of the liver enzyme alanine transaminase.
IN PRACTICE:
“These findings may provide valuable information to clinicians and people with type 2 diabetes regarding the likelihood of achieving substantial body weight reduction with tirzepatide and also help to signal likely improvements to be seen in a range of cardiometabolic risk parameters with tirzepatide-induced weight loss,” the authors concluded in their report.
SOURCE:
The study was largely run by researchers who are employees of Lilly, the company that markets tirzepatide (Mounjaro). It was published in Diabetes Care.
LIMITATIONS:
- The analysis was post hoc.
- The follow-up was limited.
- The analysis focused entirely on baseline parameters as potential predictors of weight loss magnitude.
DISCLOSURES:
The study was funded by Eli Lilly, the company that markets tirzepatide (Mounjaro) and that sponsored the SURPASS trials. Six authors are employees of Lilly, one is a contractor for Lilly, and the two remaining authors have had financial relationships with Lilly and with several other companies.
A version of this article first appeared on Medscape.com.
Chinese medicine improves outcomes in STEMI patients
TOPLINE:
Tongxinluo, a traditional Chinese medicine made from extracts of multiple plants and insects, significantly reduced myocardial infarction (MI), stroke, and related events when used alongside guideline-directed treatments in patients with ST-segment elevation myocardial infarction (STEMI), results of a large trial suggest.
METHODOLOGY:
- The double-blind China Tongxinluo Study for Myocardial Protection in Patients With Acute Myocardial Infarction (CTS-AMI) included 3,777 adult patients, mean age 61 years, 76.9% men, with STEMI at 124 centers in China.
- Researchers randomly assigned patients to receive oral Tongxinluo using a loading dose of eight capsules (2.08 g) followed by a maintenance dose of four capsules (1.04 g) or oral placebo three times a day for 12 months.
- Doctors were instructed to also provide STEMI guideline-directed treatments, which include dual antiplatelet therapy and coronary reperfusion (percutaneous coronary intervention or thrombolysis).
- The primary endpoint was major adverse cardiac and cerebrovascular events (MACCEs) at 30 days, a composite of cardiac death, myocardial reinfarction, emergent coronary revascularization, and stroke.
TAKEAWAY:
- (relative risk, 0.64; 95% confidence interval, 0.47-0.88; risk difference, –1.8%; 95% CI, –3.2% to –0.6%; P = .006).
- Individual components of MACCEs were also significantly lower in the Tongxinluo group, including 30-day cardiac death (3.0% vs. 4.2%; RR, 0.70; 95% CI, 0.50-0.99; P = .04) and myocardial reinfarction (0% vs. 0.5%; RR, 0.35; 95% CI, 0.13-0.99; P = .003), but there was no significant difference in 30-day stroke rate.
- At 1 year, the Tongxinluo group had a lower MACCE rate than did the placebo group (5.3% vs. 8.3%; hazard ratio, 0.64; 95% CI, 0.49-0.82; P = .001), an almost significant lower rate of all-cause death (5.1% vs. 6.6%; HR, 0.77; 95% CI, 0.59-1.01; P = .06), and lower rates of other outcomes.
- Rates of nonfatal serious adverse events were similar (2.2% in the Tongxinluo and 2.8% in the placebo groups; P = .25), but the Tongxinluo group had more adverse drug reactions (2.1% vs 1.1%; P = .02), which were mainly driven by symptoms in the digestive system such as stomach discomfort and nausea.
IN PRACTICE:
Unlike most traditional Chinese medicine research, the design of this study incorporated all key elements of randomized clinical trials, including placebo control and blinding in addition to randomization, so it “may serve as a model for future clinical trials to evaluate the safety and efficacy of traditional Chinese medicine,” the authors conclude.
In an accompanying editorial, Richard G. Bach, MD, cardiovascular division, Washington University School of Medicine, St. Louis, expressed skepticism about whether the benefits of Tongxinluo can be extrapolated to populations outside China that have distinct genetic backgrounds, lipid profiles, and diets. He also stressed that the active ingredients and mechanisms of action of Tongxinluo are unknown and noted reports suggesting that traditional Chinese medicines can contain undeclared material, heavy metals, and other adulterants associated with potentially toxic effects.
In an Editor’s Note, Gregory Curfman, MD, said in making a judgment about the validity of this research, “the editors were faced with the task of walking a fine line between skepticism and plausibility.” Though all patients should receive beta-blockers and an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker after STEMI, use of these drugs was suboptimal in these patients. Still, the benefits of Chinese medicine are plausible, said Dr. Curfman, noting research on the malaria drug artemisinin, which was isolated from a traditional Chinese medicine, was awarded the Nobel Prize.
SOURCE:
The research was led by Yuejin Yang, MD, PhD, department of cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, and colleagues. It was published online on Oct. 24 in JAMA. The results were previously reported at the American Heart Association Scientific Sessions 2022.
LIMITATIONS:
Despite the demonstrated clinical benefit of Tongxinluo, its active ingredients and the exact mechanisms of action have not been established. Use of guideline-directed medical therapy was suboptimal, with only 64% of patients prescribed beta-blockers and 51%-52% prescribed an ACE inhibitor or ARB during hospitalization, which may have affected the magnitude of the benefit of Tongxinluo. As study patients were all Chinese, the generalizability to other populations, especially in countries with higher adherence to guideline-directed medical therapy, is unknown.
DISCLOSURES:
The study received funding from the National Key Research and Development Program of China and a research grant from Shijiazhuang Yiling Pharmaceutical. Yuejin Yang reported receiving grants from Shijiazhuang Yiling Pharmaceutical and the National Key Research and Development Program of China; in addition, he has a patent related to the mechanisms of Tongxinluo in alleviating rat myocardial reperfusion injury and a patent related to the mechanisms of Tongxinluo on enhancing the protective effects of exosomes derived from mesenchymal stem cells in rat acute myocardial infarction. See paper for disclosures of other authors.
A version of this article first appeared on Medscape.com.
TOPLINE:
Tongxinluo, a traditional Chinese medicine made from extracts of multiple plants and insects, significantly reduced myocardial infarction (MI), stroke, and related events when used alongside guideline-directed treatments in patients with ST-segment elevation myocardial infarction (STEMI), results of a large trial suggest.
METHODOLOGY:
- The double-blind China Tongxinluo Study for Myocardial Protection in Patients With Acute Myocardial Infarction (CTS-AMI) included 3,777 adult patients, mean age 61 years, 76.9% men, with STEMI at 124 centers in China.
- Researchers randomly assigned patients to receive oral Tongxinluo using a loading dose of eight capsules (2.08 g) followed by a maintenance dose of four capsules (1.04 g) or oral placebo three times a day for 12 months.
- Doctors were instructed to also provide STEMI guideline-directed treatments, which include dual antiplatelet therapy and coronary reperfusion (percutaneous coronary intervention or thrombolysis).
- The primary endpoint was major adverse cardiac and cerebrovascular events (MACCEs) at 30 days, a composite of cardiac death, myocardial reinfarction, emergent coronary revascularization, and stroke.
TAKEAWAY:
- (relative risk, 0.64; 95% confidence interval, 0.47-0.88; risk difference, –1.8%; 95% CI, –3.2% to –0.6%; P = .006).
- Individual components of MACCEs were also significantly lower in the Tongxinluo group, including 30-day cardiac death (3.0% vs. 4.2%; RR, 0.70; 95% CI, 0.50-0.99; P = .04) and myocardial reinfarction (0% vs. 0.5%; RR, 0.35; 95% CI, 0.13-0.99; P = .003), but there was no significant difference in 30-day stroke rate.
- At 1 year, the Tongxinluo group had a lower MACCE rate than did the placebo group (5.3% vs. 8.3%; hazard ratio, 0.64; 95% CI, 0.49-0.82; P = .001), an almost significant lower rate of all-cause death (5.1% vs. 6.6%; HR, 0.77; 95% CI, 0.59-1.01; P = .06), and lower rates of other outcomes.
- Rates of nonfatal serious adverse events were similar (2.2% in the Tongxinluo and 2.8% in the placebo groups; P = .25), but the Tongxinluo group had more adverse drug reactions (2.1% vs 1.1%; P = .02), which were mainly driven by symptoms in the digestive system such as stomach discomfort and nausea.
IN PRACTICE:
Unlike most traditional Chinese medicine research, the design of this study incorporated all key elements of randomized clinical trials, including placebo control and blinding in addition to randomization, so it “may serve as a model for future clinical trials to evaluate the safety and efficacy of traditional Chinese medicine,” the authors conclude.
In an accompanying editorial, Richard G. Bach, MD, cardiovascular division, Washington University School of Medicine, St. Louis, expressed skepticism about whether the benefits of Tongxinluo can be extrapolated to populations outside China that have distinct genetic backgrounds, lipid profiles, and diets. He also stressed that the active ingredients and mechanisms of action of Tongxinluo are unknown and noted reports suggesting that traditional Chinese medicines can contain undeclared material, heavy metals, and other adulterants associated with potentially toxic effects.
In an Editor’s Note, Gregory Curfman, MD, said in making a judgment about the validity of this research, “the editors were faced with the task of walking a fine line between skepticism and plausibility.” Though all patients should receive beta-blockers and an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker after STEMI, use of these drugs was suboptimal in these patients. Still, the benefits of Chinese medicine are plausible, said Dr. Curfman, noting research on the malaria drug artemisinin, which was isolated from a traditional Chinese medicine, was awarded the Nobel Prize.
SOURCE:
The research was led by Yuejin Yang, MD, PhD, department of cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, and colleagues. It was published online on Oct. 24 in JAMA. The results were previously reported at the American Heart Association Scientific Sessions 2022.
LIMITATIONS:
Despite the demonstrated clinical benefit of Tongxinluo, its active ingredients and the exact mechanisms of action have not been established. Use of guideline-directed medical therapy was suboptimal, with only 64% of patients prescribed beta-blockers and 51%-52% prescribed an ACE inhibitor or ARB during hospitalization, which may have affected the magnitude of the benefit of Tongxinluo. As study patients were all Chinese, the generalizability to other populations, especially in countries with higher adherence to guideline-directed medical therapy, is unknown.
DISCLOSURES:
The study received funding from the National Key Research and Development Program of China and a research grant from Shijiazhuang Yiling Pharmaceutical. Yuejin Yang reported receiving grants from Shijiazhuang Yiling Pharmaceutical and the National Key Research and Development Program of China; in addition, he has a patent related to the mechanisms of Tongxinluo in alleviating rat myocardial reperfusion injury and a patent related to the mechanisms of Tongxinluo on enhancing the protective effects of exosomes derived from mesenchymal stem cells in rat acute myocardial infarction. See paper for disclosures of other authors.
A version of this article first appeared on Medscape.com.
TOPLINE:
Tongxinluo, a traditional Chinese medicine made from extracts of multiple plants and insects, significantly reduced myocardial infarction (MI), stroke, and related events when used alongside guideline-directed treatments in patients with ST-segment elevation myocardial infarction (STEMI), results of a large trial suggest.
METHODOLOGY:
- The double-blind China Tongxinluo Study for Myocardial Protection in Patients With Acute Myocardial Infarction (CTS-AMI) included 3,777 adult patients, mean age 61 years, 76.9% men, with STEMI at 124 centers in China.
- Researchers randomly assigned patients to receive oral Tongxinluo using a loading dose of eight capsules (2.08 g) followed by a maintenance dose of four capsules (1.04 g) or oral placebo three times a day for 12 months.
- Doctors were instructed to also provide STEMI guideline-directed treatments, which include dual antiplatelet therapy and coronary reperfusion (percutaneous coronary intervention or thrombolysis).
- The primary endpoint was major adverse cardiac and cerebrovascular events (MACCEs) at 30 days, a composite of cardiac death, myocardial reinfarction, emergent coronary revascularization, and stroke.
TAKEAWAY:
- (relative risk, 0.64; 95% confidence interval, 0.47-0.88; risk difference, –1.8%; 95% CI, –3.2% to –0.6%; P = .006).
- Individual components of MACCEs were also significantly lower in the Tongxinluo group, including 30-day cardiac death (3.0% vs. 4.2%; RR, 0.70; 95% CI, 0.50-0.99; P = .04) and myocardial reinfarction (0% vs. 0.5%; RR, 0.35; 95% CI, 0.13-0.99; P = .003), but there was no significant difference in 30-day stroke rate.
- At 1 year, the Tongxinluo group had a lower MACCE rate than did the placebo group (5.3% vs. 8.3%; hazard ratio, 0.64; 95% CI, 0.49-0.82; P = .001), an almost significant lower rate of all-cause death (5.1% vs. 6.6%; HR, 0.77; 95% CI, 0.59-1.01; P = .06), and lower rates of other outcomes.
- Rates of nonfatal serious adverse events were similar (2.2% in the Tongxinluo and 2.8% in the placebo groups; P = .25), but the Tongxinluo group had more adverse drug reactions (2.1% vs 1.1%; P = .02), which were mainly driven by symptoms in the digestive system such as stomach discomfort and nausea.
IN PRACTICE:
Unlike most traditional Chinese medicine research, the design of this study incorporated all key elements of randomized clinical trials, including placebo control and blinding in addition to randomization, so it “may serve as a model for future clinical trials to evaluate the safety and efficacy of traditional Chinese medicine,” the authors conclude.
In an accompanying editorial, Richard G. Bach, MD, cardiovascular division, Washington University School of Medicine, St. Louis, expressed skepticism about whether the benefits of Tongxinluo can be extrapolated to populations outside China that have distinct genetic backgrounds, lipid profiles, and diets. He also stressed that the active ingredients and mechanisms of action of Tongxinluo are unknown and noted reports suggesting that traditional Chinese medicines can contain undeclared material, heavy metals, and other adulterants associated with potentially toxic effects.
In an Editor’s Note, Gregory Curfman, MD, said in making a judgment about the validity of this research, “the editors were faced with the task of walking a fine line between skepticism and plausibility.” Though all patients should receive beta-blockers and an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker after STEMI, use of these drugs was suboptimal in these patients. Still, the benefits of Chinese medicine are plausible, said Dr. Curfman, noting research on the malaria drug artemisinin, which was isolated from a traditional Chinese medicine, was awarded the Nobel Prize.
SOURCE:
The research was led by Yuejin Yang, MD, PhD, department of cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, and colleagues. It was published online on Oct. 24 in JAMA. The results were previously reported at the American Heart Association Scientific Sessions 2022.
LIMITATIONS:
Despite the demonstrated clinical benefit of Tongxinluo, its active ingredients and the exact mechanisms of action have not been established. Use of guideline-directed medical therapy was suboptimal, with only 64% of patients prescribed beta-blockers and 51%-52% prescribed an ACE inhibitor or ARB during hospitalization, which may have affected the magnitude of the benefit of Tongxinluo. As study patients were all Chinese, the generalizability to other populations, especially in countries with higher adherence to guideline-directed medical therapy, is unknown.
DISCLOSURES:
The study received funding from the National Key Research and Development Program of China and a research grant from Shijiazhuang Yiling Pharmaceutical. Yuejin Yang reported receiving grants from Shijiazhuang Yiling Pharmaceutical and the National Key Research and Development Program of China; in addition, he has a patent related to the mechanisms of Tongxinluo in alleviating rat myocardial reperfusion injury and a patent related to the mechanisms of Tongxinluo on enhancing the protective effects of exosomes derived from mesenchymal stem cells in rat acute myocardial infarction. See paper for disclosures of other authors.
A version of this article first appeared on Medscape.com.
Promising topline phase 2 results for novel oral Alzheimer’s drug
Topline results provide “clinical evidence of a modification of multiple AD pathologies associated with amyloid plaque burden,” said John Didsbury, PhD, chief executive officer of T3D Therapeutics Inc., the company developing the drug.
While the primary cognitive endpoints were not met in the overall study population, the data suggest that a “high plasma pTau-217/non–pTau-217 ratio, a marker of AD pathology, likely defines an AD population responsive to T3D-959 therapy,” Dr. Didsbury said.
He said it’s important to note that no PET imaging (amyloid/tau) or biomarkers were used as entry criteria and, in hindsight, some participants likely did not have AD, which likely played a role in the negative primary outcome.
The findings from the PIONEER study were presented at the annual Clinical Trials on Alzheimer’s Disease conference.
‘Surprised and shocked’
The PPAR family of proteins helps regulate blood sugar and triglyceride levels. The rationale for evaluating PPAR agonists in AD is based on the hypothesis that sporadic AD is fundamentally an age-related metabolic disease.
T3D-959 is the first PPAR delta-activating compound to be developed for the treatment of AD. Uniquely, this drug also activates PPAR gamma, which may provide potential additive or synergistic effects in regulating dysfunctional brain glucose energy and lipid metabolism in AD.
The PIONEER tested three doses of T3D-959 (15 mg, 30 mg, and 45 mg) vs. placebo in 250 adults with mild to moderate AD (Mini-Mental State Examination [MMSE] 14-26, Clinical Dementia Rating (CDR)-Global 0.5-2.0, and Sum of Boxes [CDR-SB] ≥ 3.0). T3D-959 or placebo was taken once daily for 24 weeks.
In the overall population, the primary endpoints – Alzheimer Disease Assessment Scale-Cognitive subscale (ADAS-Cog11) and Clinical Global Impression of Change (CGIC) – were not met.
“Plain and simple, when we saw this data, we were surprised and shocked,” said Dr. Didsbury, and wondered, “How can placebo be doing so well on a 6-month AD trial?”
“We suspect the presence of non-AD subjects in the trial based on the lower-than-typical number of ApoE4 positive subjects, increased cognitive performance and learning effects observed, and only 45% of trial subjects having a low pTau-217 ratio, a biomarker indicating that they would have no AD pathology plasma,” he explained.
Plasma baseline pTau-217 ratio correlates with AD risk and severity and is a marker of AD pathology; in the subgroup with high pTau-217 ratio, the ADAS-Cog11 endpoint was met in the 30-mg T3D-959 group vs. the placebo group (–0.74 vs. 1.27; P = .112), “consistent with clinical benefit,” Dr. Didsbury noted.
The secondary endpoint of change in plasma amyloid-beta (Ab)42/40 ratio was also met in the 30-mg T3D-959 group – increasing at week 24 with T3D-959 vs. decreasing with placebo (P = .0206), with even greater improvement in the high pTau-217 ratio group. In this group, improvement of Ab42/40 ratio was nearly twofold greater than the overall group.
T3D-959 had a similar magnitude of effect on Ab42/40 as lecanemab (Leqembi) at 6 months, the researchers point out in their late-breaking abstract.
Biomarkers of all three AD diagnostic criteria (amyloid/tau/neurodegeneration) were improved, as well as markers of inflammation, insulin resistance, and dysfunctional lipid metabolism – results that demonstrate “peripheral targeted engagement,” Dr. Didsbury said.
“Along with the strong safety profile of T3D-959, the evidence supports a larger study evaluating T3D-959 30 mg/day in patients with mild to moderate AD and a baseline plasma p-Tau-217/non–pTau-217 ratio of ≥ 0.015,” the researchers conclude in their abstract.
Lessons learned
Commenting on the research for this article, Rebecca Edelmayer, PhD, senior director of scientific engagement for the Alzheimer’s Association, noted that “the idea behind this treatment is that impaired glucose metabolism in the brain leads to toxic misfolded proteins, including amyloid and tau in people with Alzheimer’s disease.”
“The treatment focuses on improving regulation of glucose and lipid metabolism in the brain. This is one of more than 140 approaches that are being tested today to target the biological drivers and contributors to Alzheimer’s disease,” Dr. Edelmayer said.
Because biomarkers were not used to enroll participants, “there was a high population of people in the trial who did not have Alzheimer’s. This very likely contributed to the negative result,” she noted.
However, the results also suggest that those taking the drug who had a high pTau217 ratio – and are likely to have brain amyloid plaques – had less cognitive decline, she noted.
Lessons learned from this negative trial include “the proper dose to balance efficacy and safety, and how to screen participants for their next study,” Dr. Edelmayer said.
Funding for the study was provided by the National Institute on Aging/National Institutes of Health and the Alzheimer’s Association. Dr. Didsbury and Dr. Edelmayer report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Topline results provide “clinical evidence of a modification of multiple AD pathologies associated with amyloid plaque burden,” said John Didsbury, PhD, chief executive officer of T3D Therapeutics Inc., the company developing the drug.
While the primary cognitive endpoints were not met in the overall study population, the data suggest that a “high plasma pTau-217/non–pTau-217 ratio, a marker of AD pathology, likely defines an AD population responsive to T3D-959 therapy,” Dr. Didsbury said.
He said it’s important to note that no PET imaging (amyloid/tau) or biomarkers were used as entry criteria and, in hindsight, some participants likely did not have AD, which likely played a role in the negative primary outcome.
The findings from the PIONEER study were presented at the annual Clinical Trials on Alzheimer’s Disease conference.
‘Surprised and shocked’
The PPAR family of proteins helps regulate blood sugar and triglyceride levels. The rationale for evaluating PPAR agonists in AD is based on the hypothesis that sporadic AD is fundamentally an age-related metabolic disease.
T3D-959 is the first PPAR delta-activating compound to be developed for the treatment of AD. Uniquely, this drug also activates PPAR gamma, which may provide potential additive or synergistic effects in regulating dysfunctional brain glucose energy and lipid metabolism in AD.
The PIONEER tested three doses of T3D-959 (15 mg, 30 mg, and 45 mg) vs. placebo in 250 adults with mild to moderate AD (Mini-Mental State Examination [MMSE] 14-26, Clinical Dementia Rating (CDR)-Global 0.5-2.0, and Sum of Boxes [CDR-SB] ≥ 3.0). T3D-959 or placebo was taken once daily for 24 weeks.
In the overall population, the primary endpoints – Alzheimer Disease Assessment Scale-Cognitive subscale (ADAS-Cog11) and Clinical Global Impression of Change (CGIC) – were not met.
“Plain and simple, when we saw this data, we were surprised and shocked,” said Dr. Didsbury, and wondered, “How can placebo be doing so well on a 6-month AD trial?”
“We suspect the presence of non-AD subjects in the trial based on the lower-than-typical number of ApoE4 positive subjects, increased cognitive performance and learning effects observed, and only 45% of trial subjects having a low pTau-217 ratio, a biomarker indicating that they would have no AD pathology plasma,” he explained.
Plasma baseline pTau-217 ratio correlates with AD risk and severity and is a marker of AD pathology; in the subgroup with high pTau-217 ratio, the ADAS-Cog11 endpoint was met in the 30-mg T3D-959 group vs. the placebo group (–0.74 vs. 1.27; P = .112), “consistent with clinical benefit,” Dr. Didsbury noted.
The secondary endpoint of change in plasma amyloid-beta (Ab)42/40 ratio was also met in the 30-mg T3D-959 group – increasing at week 24 with T3D-959 vs. decreasing with placebo (P = .0206), with even greater improvement in the high pTau-217 ratio group. In this group, improvement of Ab42/40 ratio was nearly twofold greater than the overall group.
T3D-959 had a similar magnitude of effect on Ab42/40 as lecanemab (Leqembi) at 6 months, the researchers point out in their late-breaking abstract.
Biomarkers of all three AD diagnostic criteria (amyloid/tau/neurodegeneration) were improved, as well as markers of inflammation, insulin resistance, and dysfunctional lipid metabolism – results that demonstrate “peripheral targeted engagement,” Dr. Didsbury said.
“Along with the strong safety profile of T3D-959, the evidence supports a larger study evaluating T3D-959 30 mg/day in patients with mild to moderate AD and a baseline plasma p-Tau-217/non–pTau-217 ratio of ≥ 0.015,” the researchers conclude in their abstract.
Lessons learned
Commenting on the research for this article, Rebecca Edelmayer, PhD, senior director of scientific engagement for the Alzheimer’s Association, noted that “the idea behind this treatment is that impaired glucose metabolism in the brain leads to toxic misfolded proteins, including amyloid and tau in people with Alzheimer’s disease.”
“The treatment focuses on improving regulation of glucose and lipid metabolism in the brain. This is one of more than 140 approaches that are being tested today to target the biological drivers and contributors to Alzheimer’s disease,” Dr. Edelmayer said.
Because biomarkers were not used to enroll participants, “there was a high population of people in the trial who did not have Alzheimer’s. This very likely contributed to the negative result,” she noted.
However, the results also suggest that those taking the drug who had a high pTau217 ratio – and are likely to have brain amyloid plaques – had less cognitive decline, she noted.
Lessons learned from this negative trial include “the proper dose to balance efficacy and safety, and how to screen participants for their next study,” Dr. Edelmayer said.
Funding for the study was provided by the National Institute on Aging/National Institutes of Health and the Alzheimer’s Association. Dr. Didsbury and Dr. Edelmayer report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Topline results provide “clinical evidence of a modification of multiple AD pathologies associated with amyloid plaque burden,” said John Didsbury, PhD, chief executive officer of T3D Therapeutics Inc., the company developing the drug.
While the primary cognitive endpoints were not met in the overall study population, the data suggest that a “high plasma pTau-217/non–pTau-217 ratio, a marker of AD pathology, likely defines an AD population responsive to T3D-959 therapy,” Dr. Didsbury said.
He said it’s important to note that no PET imaging (amyloid/tau) or biomarkers were used as entry criteria and, in hindsight, some participants likely did not have AD, which likely played a role in the negative primary outcome.
The findings from the PIONEER study were presented at the annual Clinical Trials on Alzheimer’s Disease conference.
‘Surprised and shocked’
The PPAR family of proteins helps regulate blood sugar and triglyceride levels. The rationale for evaluating PPAR agonists in AD is based on the hypothesis that sporadic AD is fundamentally an age-related metabolic disease.
T3D-959 is the first PPAR delta-activating compound to be developed for the treatment of AD. Uniquely, this drug also activates PPAR gamma, which may provide potential additive or synergistic effects in regulating dysfunctional brain glucose energy and lipid metabolism in AD.
The PIONEER tested three doses of T3D-959 (15 mg, 30 mg, and 45 mg) vs. placebo in 250 adults with mild to moderate AD (Mini-Mental State Examination [MMSE] 14-26, Clinical Dementia Rating (CDR)-Global 0.5-2.0, and Sum of Boxes [CDR-SB] ≥ 3.0). T3D-959 or placebo was taken once daily for 24 weeks.
In the overall population, the primary endpoints – Alzheimer Disease Assessment Scale-Cognitive subscale (ADAS-Cog11) and Clinical Global Impression of Change (CGIC) – were not met.
“Plain and simple, when we saw this data, we were surprised and shocked,” said Dr. Didsbury, and wondered, “How can placebo be doing so well on a 6-month AD trial?”
“We suspect the presence of non-AD subjects in the trial based on the lower-than-typical number of ApoE4 positive subjects, increased cognitive performance and learning effects observed, and only 45% of trial subjects having a low pTau-217 ratio, a biomarker indicating that they would have no AD pathology plasma,” he explained.
Plasma baseline pTau-217 ratio correlates with AD risk and severity and is a marker of AD pathology; in the subgroup with high pTau-217 ratio, the ADAS-Cog11 endpoint was met in the 30-mg T3D-959 group vs. the placebo group (–0.74 vs. 1.27; P = .112), “consistent with clinical benefit,” Dr. Didsbury noted.
The secondary endpoint of change in plasma amyloid-beta (Ab)42/40 ratio was also met in the 30-mg T3D-959 group – increasing at week 24 with T3D-959 vs. decreasing with placebo (P = .0206), with even greater improvement in the high pTau-217 ratio group. In this group, improvement of Ab42/40 ratio was nearly twofold greater than the overall group.
T3D-959 had a similar magnitude of effect on Ab42/40 as lecanemab (Leqembi) at 6 months, the researchers point out in their late-breaking abstract.
Biomarkers of all three AD diagnostic criteria (amyloid/tau/neurodegeneration) were improved, as well as markers of inflammation, insulin resistance, and dysfunctional lipid metabolism – results that demonstrate “peripheral targeted engagement,” Dr. Didsbury said.
“Along with the strong safety profile of T3D-959, the evidence supports a larger study evaluating T3D-959 30 mg/day in patients with mild to moderate AD and a baseline plasma p-Tau-217/non–pTau-217 ratio of ≥ 0.015,” the researchers conclude in their abstract.
Lessons learned
Commenting on the research for this article, Rebecca Edelmayer, PhD, senior director of scientific engagement for the Alzheimer’s Association, noted that “the idea behind this treatment is that impaired glucose metabolism in the brain leads to toxic misfolded proteins, including amyloid and tau in people with Alzheimer’s disease.”
“The treatment focuses on improving regulation of glucose and lipid metabolism in the brain. This is one of more than 140 approaches that are being tested today to target the biological drivers and contributors to Alzheimer’s disease,” Dr. Edelmayer said.
Because biomarkers were not used to enroll participants, “there was a high population of people in the trial who did not have Alzheimer’s. This very likely contributed to the negative result,” she noted.
However, the results also suggest that those taking the drug who had a high pTau217 ratio – and are likely to have brain amyloid plaques – had less cognitive decline, she noted.
Lessons learned from this negative trial include “the proper dose to balance efficacy and safety, and how to screen participants for their next study,” Dr. Edelmayer said.
Funding for the study was provided by the National Institute on Aging/National Institutes of Health and the Alzheimer’s Association. Dr. Didsbury and Dr. Edelmayer report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CTAD 2023
Adolescents with atopic dermatitis more likely to have experienced bullying, study finds
TOPLINE:
METHODOLOGY:
- Adolescents with AD have reported appearance-based bullying.
- To evaluate the association between AD and the prevalence and frequency of bullying, researchers analyzed cross-sectional data from adult caregivers of U.S. adolescents aged 12-17 years who participated in the 2021 National Health Interview Survey.
- Logistic regression and ordinal logistic regression were used to compare the prevalence of experiencing one or more bullying encounters during the previous 12 months and the frequency of bullying between adolescents with and those without AD.
TAKEAWAY:
- A total of 3,207 adolescents were included in the analysis. The mean age of the participants was 14.5 years, and 11.9% currently had AD. The prevalence of experiencing bullying was significantly higher among adolescents with AD, compared with those without AD (33.2% vs. 19%; P < .001), as was the prevalence of cyberbullying (9.1% vs. 5.8%; P = .04).
- Following adjustment for demographics and atopic comorbidities, adolescents with AD were at increased odds of bullying, compared with their peers without AD (adjusted odds ratio, 1.99; 95% confidence interval, 1.45-2.73).
- Following adjustment for demographics, adolescents with AD were also at increased odds of cyberbullying, compared with their peers without AD (AOR, 1.65; 95% CI, 1.04-2.62), but no association was observed following adjustment for atopic comorbidities (AOR, 1.27; 95% CI, 0.82-1.96).
- Following ordinal logistic regression that was adjusted for demographics and atopic comorbidities, adolescents with AD were at greater odds of being bullied at a higher frequency, compared with their peers without AD (AOR, 1.97; 95% CI, 1.44-2.68).
IN PRACTICE:
“Larger, future studies using clinical AD diagnoses and adolescent self-report can advance understanding of bullying and AD,” the researchers wrote. “Clinicians, families, and schools should address and monitor bullying among adolescents.”
SOURCE:
Howa Yeung, MD, of the department of dermatology at Emory University School of Medicine, Atlanta, led the research. The study was published online in JAMA Dermatology.
LIMITATIONS:
Limitations include the study’s cross-sectional design. In addition, the investigators could not directly attribute bullying to skin-specific findings, and it was a caregiver report.
DISCLOSURES:
The study was supported by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. One of the authors, Joy Wan, MD, received grants from Pfizer and personal fees from Janssen and Sun Pharmaceuticals outside of the submitted work.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Adolescents with AD have reported appearance-based bullying.
- To evaluate the association between AD and the prevalence and frequency of bullying, researchers analyzed cross-sectional data from adult caregivers of U.S. adolescents aged 12-17 years who participated in the 2021 National Health Interview Survey.
- Logistic regression and ordinal logistic regression were used to compare the prevalence of experiencing one or more bullying encounters during the previous 12 months and the frequency of bullying between adolescents with and those without AD.
TAKEAWAY:
- A total of 3,207 adolescents were included in the analysis. The mean age of the participants was 14.5 years, and 11.9% currently had AD. The prevalence of experiencing bullying was significantly higher among adolescents with AD, compared with those without AD (33.2% vs. 19%; P < .001), as was the prevalence of cyberbullying (9.1% vs. 5.8%; P = .04).
- Following adjustment for demographics and atopic comorbidities, adolescents with AD were at increased odds of bullying, compared with their peers without AD (adjusted odds ratio, 1.99; 95% confidence interval, 1.45-2.73).
- Following adjustment for demographics, adolescents with AD were also at increased odds of cyberbullying, compared with their peers without AD (AOR, 1.65; 95% CI, 1.04-2.62), but no association was observed following adjustment for atopic comorbidities (AOR, 1.27; 95% CI, 0.82-1.96).
- Following ordinal logistic regression that was adjusted for demographics and atopic comorbidities, adolescents with AD were at greater odds of being bullied at a higher frequency, compared with their peers without AD (AOR, 1.97; 95% CI, 1.44-2.68).
IN PRACTICE:
“Larger, future studies using clinical AD diagnoses and adolescent self-report can advance understanding of bullying and AD,” the researchers wrote. “Clinicians, families, and schools should address and monitor bullying among adolescents.”
SOURCE:
Howa Yeung, MD, of the department of dermatology at Emory University School of Medicine, Atlanta, led the research. The study was published online in JAMA Dermatology.
LIMITATIONS:
Limitations include the study’s cross-sectional design. In addition, the investigators could not directly attribute bullying to skin-specific findings, and it was a caregiver report.
DISCLOSURES:
The study was supported by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. One of the authors, Joy Wan, MD, received grants from Pfizer and personal fees from Janssen and Sun Pharmaceuticals outside of the submitted work.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Adolescents with AD have reported appearance-based bullying.
- To evaluate the association between AD and the prevalence and frequency of bullying, researchers analyzed cross-sectional data from adult caregivers of U.S. adolescents aged 12-17 years who participated in the 2021 National Health Interview Survey.
- Logistic regression and ordinal logistic regression were used to compare the prevalence of experiencing one or more bullying encounters during the previous 12 months and the frequency of bullying between adolescents with and those without AD.
TAKEAWAY:
- A total of 3,207 adolescents were included in the analysis. The mean age of the participants was 14.5 years, and 11.9% currently had AD. The prevalence of experiencing bullying was significantly higher among adolescents with AD, compared with those without AD (33.2% vs. 19%; P < .001), as was the prevalence of cyberbullying (9.1% vs. 5.8%; P = .04).
- Following adjustment for demographics and atopic comorbidities, adolescents with AD were at increased odds of bullying, compared with their peers without AD (adjusted odds ratio, 1.99; 95% confidence interval, 1.45-2.73).
- Following adjustment for demographics, adolescents with AD were also at increased odds of cyberbullying, compared with their peers without AD (AOR, 1.65; 95% CI, 1.04-2.62), but no association was observed following adjustment for atopic comorbidities (AOR, 1.27; 95% CI, 0.82-1.96).
- Following ordinal logistic regression that was adjusted for demographics and atopic comorbidities, adolescents with AD were at greater odds of being bullied at a higher frequency, compared with their peers without AD (AOR, 1.97; 95% CI, 1.44-2.68).
IN PRACTICE:
“Larger, future studies using clinical AD diagnoses and adolescent self-report can advance understanding of bullying and AD,” the researchers wrote. “Clinicians, families, and schools should address and monitor bullying among adolescents.”
SOURCE:
Howa Yeung, MD, of the department of dermatology at Emory University School of Medicine, Atlanta, led the research. The study was published online in JAMA Dermatology.
LIMITATIONS:
Limitations include the study’s cross-sectional design. In addition, the investigators could not directly attribute bullying to skin-specific findings, and it was a caregiver report.
DISCLOSURES:
The study was supported by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. One of the authors, Joy Wan, MD, received grants from Pfizer and personal fees from Janssen and Sun Pharmaceuticals outside of the submitted work.
A version of this article first appeared on Medscape.com.
Heart rate variability: Are we ignoring a harbinger of health?
A very long time ago, when I ran clinical labs, one of the most ordered tests was the “sed rate” (aka ESR, the erythrocyte sedimentation rate). Easy, quick, and low cost, with high sensitivity but very low specificity. If the sed rate was normal, the patient probably did not have an infectious or inflammatory disease. If it was elevated, they probably did, but no telling what. Later, the C-reactive protein (CRP) test came into common use. Same general inferences: If the CRP was low, the patient was unlikely to have an inflammatory process; if high, they were sick, but we didn’t know what with.
Could the heart rate variability (HRV) score come to be thought of similarly? Much as the sed rate and CRP are sensitivity indicators of infectious or inflammatory diseases, might the HRV score be a sensitivity indicator for nervous system (central and autonomic) and cardiovascular (especially heart rhythm) malfunctions?
A substantial and relatively old body of heart rhythm literature ties HRV alterations to posttraumatic stress disorder, physician occupational stress, sleep disorders, depression, autonomic nervous system derangements, various cardiac arrhythmias, fatigue, overexertion, medications, and age itself.
More than 100 million Americans are now believed to use smartwatches or personal fitness monitors. Some 30%-40% of these devices measure HRV. So what? Credible research about this huge mass of accumulating data from “wearables” is lacking.
What is HRV?
HRV is the variation in time between each heartbeat, in milliseconds. HRV is influenced by the autonomic nervous system, perhaps reflecting sympathetic-parasympathetic balance. Some devices measure HRV 24/7. My Fitbit Inspire 2 reports only nighttime measures during 3 hours of sustained sleep. Most trackers report averages; some calculate the root mean squares; others calculate standard deviations. All fitness trackers warn not to use the data for medical purposes.
Normal values (reference ranges) for HRV begin at an average of 100 msec in the first decade of life and decline by approximately 10 msec per decade lived. At age 30-40, the average is 70 msec; age 60-70, it’s 40 msec; and at age 90-100, it’s 10 msec.
As a long-time lab guy, I used to teach proper use of lab tests. Fitness trackers are “lab tests” of a sort. We taught never to do a lab test unless you know what you are going to do with the result, no matter what it is. We also taught “never do anything just because you can.” Curiosity, we know, is a frequent driver of lab test ordering.
That underlying philosophy gives me a hard time when it comes to wearables. I have been enamored of watching my step count, active zone minutes, resting heart rate, active heart rate, various sleep scores, and breathing rate (and, of course, a manually entered early morning daily body weight) for several years. I even check my “readiness score” (a calculation using resting heart rate, recent sleep, recent active zone minutes, and perhaps HRV) each morning and adjust my behaviors accordingly.
Why monitor HRV?
But what should we do with HRV scores? Ignore them? Try to understand them, perhaps as a screening tool? Or monitor HRV for consistency or change? “Monitoring” is a proper and common use of lab tests.
Some say we should improve the HRV score by managing stress, getting regular exercise, eating a healthy diet, getting enough sleep, and not smoking or consuming excess alcohol. Duh! I do all of that anyway.
The claims that HRV is a “simple but powerful tool that can be used to track overall health and well-being” might turn out to be true. Proper study and sharing of data will enable that determination.
To advance understanding, I offer an n-of-1, a real-world personal anecdote about HRV.
I did not request the HRV function on my Fitbit Inspire 2. It simply appeared, and I ignored it for some time.
A year or two ago, I started noticing my HRV score every morning. Initially, I did not like to see my “low” score, until I learned that the reference range was dramatically affected by age and I was in my late 80s at the time. The vast majority of my HRV readings were in the range of 17 msec to 27 msec.
Last week, I was administered the new Moderna COVID-19 Spikevax vaccine and the old folks’ influenza vaccine simultaneously. In my case, side effects from each vaccine have been modest in the past, but I never previously had both administered at the same time. My immune response was, shall we say, robust. Chills, muscle aches, headache, fatigue, deltoid swelling, fitful sleep, and increased resting heart rate.
My nightly average HRV had been running between 17 msec and 35 msec for many months. WHOA! After the shots, my overnight HRV score plummeted from 24 msec to 10 msec, my lowest ever. Instant worry. The next day, it rebounded to 28 msec, and it has been in the high teens or low 20s since then.
Off to PubMed. A recent study of HRV on the second and 10th days after administering the Pfizer mRNA vaccine to 75 healthy volunteers found that the HRV on day 2 was dramatically lower than prevaccination levels and by day 10, it had returned to prevaccination levels. Some comfort there.
Another review article has reported a rapid fall and rapid rebound of HRV after COVID-19 vaccination. A 2010 report demonstrated a significant but not dramatic short-term lowering of HRV after influenza A vaccination and correlated it with CRP changes.
Some believe that the decline in HRV after vaccination reflects an increased immune response and sympathetic nervous activity.
I don’t plan to receive my flu and COVID vaccines on the same day again.
So, I went back to review what happened to my HRV when I had COVID in 2023. My HRV was 14 msec and 12 msec on the first 2 days of symptoms, and then returned to the 20 msec range.
I received the RSV vaccine this year without adverse effects, and my HRV scores were 29 msec, 33 msec, and 32 msec on the first 3 days after vaccination. Finally, after receiving a pneumococcal vaccine in 2023, I had no adverse effects, and my HRV scores on the 5 days after vaccination were indeterminate: 19 msec, 14 msec, 18 msec, 13 msec, and 17 msec.
Of course, correlation is not causation. Cause and effect remain undetermined. But I find these observations interesting for a potentially useful screening test.
George D. Lundberg, MD, is the Editor in Chief of Cancer Commons.
A version of this article first appeared on Medscape.com.
A very long time ago, when I ran clinical labs, one of the most ordered tests was the “sed rate” (aka ESR, the erythrocyte sedimentation rate). Easy, quick, and low cost, with high sensitivity but very low specificity. If the sed rate was normal, the patient probably did not have an infectious or inflammatory disease. If it was elevated, they probably did, but no telling what. Later, the C-reactive protein (CRP) test came into common use. Same general inferences: If the CRP was low, the patient was unlikely to have an inflammatory process; if high, they were sick, but we didn’t know what with.
Could the heart rate variability (HRV) score come to be thought of similarly? Much as the sed rate and CRP are sensitivity indicators of infectious or inflammatory diseases, might the HRV score be a sensitivity indicator for nervous system (central and autonomic) and cardiovascular (especially heart rhythm) malfunctions?
A substantial and relatively old body of heart rhythm literature ties HRV alterations to posttraumatic stress disorder, physician occupational stress, sleep disorders, depression, autonomic nervous system derangements, various cardiac arrhythmias, fatigue, overexertion, medications, and age itself.
More than 100 million Americans are now believed to use smartwatches or personal fitness monitors. Some 30%-40% of these devices measure HRV. So what? Credible research about this huge mass of accumulating data from “wearables” is lacking.
What is HRV?
HRV is the variation in time between each heartbeat, in milliseconds. HRV is influenced by the autonomic nervous system, perhaps reflecting sympathetic-parasympathetic balance. Some devices measure HRV 24/7. My Fitbit Inspire 2 reports only nighttime measures during 3 hours of sustained sleep. Most trackers report averages; some calculate the root mean squares; others calculate standard deviations. All fitness trackers warn not to use the data for medical purposes.
Normal values (reference ranges) for HRV begin at an average of 100 msec in the first decade of life and decline by approximately 10 msec per decade lived. At age 30-40, the average is 70 msec; age 60-70, it’s 40 msec; and at age 90-100, it’s 10 msec.
As a long-time lab guy, I used to teach proper use of lab tests. Fitness trackers are “lab tests” of a sort. We taught never to do a lab test unless you know what you are going to do with the result, no matter what it is. We also taught “never do anything just because you can.” Curiosity, we know, is a frequent driver of lab test ordering.
That underlying philosophy gives me a hard time when it comes to wearables. I have been enamored of watching my step count, active zone minutes, resting heart rate, active heart rate, various sleep scores, and breathing rate (and, of course, a manually entered early morning daily body weight) for several years. I even check my “readiness score” (a calculation using resting heart rate, recent sleep, recent active zone minutes, and perhaps HRV) each morning and adjust my behaviors accordingly.
Why monitor HRV?
But what should we do with HRV scores? Ignore them? Try to understand them, perhaps as a screening tool? Or monitor HRV for consistency or change? “Monitoring” is a proper and common use of lab tests.
Some say we should improve the HRV score by managing stress, getting regular exercise, eating a healthy diet, getting enough sleep, and not smoking or consuming excess alcohol. Duh! I do all of that anyway.
The claims that HRV is a “simple but powerful tool that can be used to track overall health and well-being” might turn out to be true. Proper study and sharing of data will enable that determination.
To advance understanding, I offer an n-of-1, a real-world personal anecdote about HRV.
I did not request the HRV function on my Fitbit Inspire 2. It simply appeared, and I ignored it for some time.
A year or two ago, I started noticing my HRV score every morning. Initially, I did not like to see my “low” score, until I learned that the reference range was dramatically affected by age and I was in my late 80s at the time. The vast majority of my HRV readings were in the range of 17 msec to 27 msec.
Last week, I was administered the new Moderna COVID-19 Spikevax vaccine and the old folks’ influenza vaccine simultaneously. In my case, side effects from each vaccine have been modest in the past, but I never previously had both administered at the same time. My immune response was, shall we say, robust. Chills, muscle aches, headache, fatigue, deltoid swelling, fitful sleep, and increased resting heart rate.
My nightly average HRV had been running between 17 msec and 35 msec for many months. WHOA! After the shots, my overnight HRV score plummeted from 24 msec to 10 msec, my lowest ever. Instant worry. The next day, it rebounded to 28 msec, and it has been in the high teens or low 20s since then.
Off to PubMed. A recent study of HRV on the second and 10th days after administering the Pfizer mRNA vaccine to 75 healthy volunteers found that the HRV on day 2 was dramatically lower than prevaccination levels and by day 10, it had returned to prevaccination levels. Some comfort there.
Another review article has reported a rapid fall and rapid rebound of HRV after COVID-19 vaccination. A 2010 report demonstrated a significant but not dramatic short-term lowering of HRV after influenza A vaccination and correlated it with CRP changes.
Some believe that the decline in HRV after vaccination reflects an increased immune response and sympathetic nervous activity.
I don’t plan to receive my flu and COVID vaccines on the same day again.
So, I went back to review what happened to my HRV when I had COVID in 2023. My HRV was 14 msec and 12 msec on the first 2 days of symptoms, and then returned to the 20 msec range.
I received the RSV vaccine this year without adverse effects, and my HRV scores were 29 msec, 33 msec, and 32 msec on the first 3 days after vaccination. Finally, after receiving a pneumococcal vaccine in 2023, I had no adverse effects, and my HRV scores on the 5 days after vaccination were indeterminate: 19 msec, 14 msec, 18 msec, 13 msec, and 17 msec.
Of course, correlation is not causation. Cause and effect remain undetermined. But I find these observations interesting for a potentially useful screening test.
George D. Lundberg, MD, is the Editor in Chief of Cancer Commons.
A version of this article first appeared on Medscape.com.
A very long time ago, when I ran clinical labs, one of the most ordered tests was the “sed rate” (aka ESR, the erythrocyte sedimentation rate). Easy, quick, and low cost, with high sensitivity but very low specificity. If the sed rate was normal, the patient probably did not have an infectious or inflammatory disease. If it was elevated, they probably did, but no telling what. Later, the C-reactive protein (CRP) test came into common use. Same general inferences: If the CRP was low, the patient was unlikely to have an inflammatory process; if high, they were sick, but we didn’t know what with.
Could the heart rate variability (HRV) score come to be thought of similarly? Much as the sed rate and CRP are sensitivity indicators of infectious or inflammatory diseases, might the HRV score be a sensitivity indicator for nervous system (central and autonomic) and cardiovascular (especially heart rhythm) malfunctions?
A substantial and relatively old body of heart rhythm literature ties HRV alterations to posttraumatic stress disorder, physician occupational stress, sleep disorders, depression, autonomic nervous system derangements, various cardiac arrhythmias, fatigue, overexertion, medications, and age itself.
More than 100 million Americans are now believed to use smartwatches or personal fitness monitors. Some 30%-40% of these devices measure HRV. So what? Credible research about this huge mass of accumulating data from “wearables” is lacking.
What is HRV?
HRV is the variation in time between each heartbeat, in milliseconds. HRV is influenced by the autonomic nervous system, perhaps reflecting sympathetic-parasympathetic balance. Some devices measure HRV 24/7. My Fitbit Inspire 2 reports only nighttime measures during 3 hours of sustained sleep. Most trackers report averages; some calculate the root mean squares; others calculate standard deviations. All fitness trackers warn not to use the data for medical purposes.
Normal values (reference ranges) for HRV begin at an average of 100 msec in the first decade of life and decline by approximately 10 msec per decade lived. At age 30-40, the average is 70 msec; age 60-70, it’s 40 msec; and at age 90-100, it’s 10 msec.
As a long-time lab guy, I used to teach proper use of lab tests. Fitness trackers are “lab tests” of a sort. We taught never to do a lab test unless you know what you are going to do with the result, no matter what it is. We also taught “never do anything just because you can.” Curiosity, we know, is a frequent driver of lab test ordering.
That underlying philosophy gives me a hard time when it comes to wearables. I have been enamored of watching my step count, active zone minutes, resting heart rate, active heart rate, various sleep scores, and breathing rate (and, of course, a manually entered early morning daily body weight) for several years. I even check my “readiness score” (a calculation using resting heart rate, recent sleep, recent active zone minutes, and perhaps HRV) each morning and adjust my behaviors accordingly.
Why monitor HRV?
But what should we do with HRV scores? Ignore them? Try to understand them, perhaps as a screening tool? Or monitor HRV for consistency or change? “Monitoring” is a proper and common use of lab tests.
Some say we should improve the HRV score by managing stress, getting regular exercise, eating a healthy diet, getting enough sleep, and not smoking or consuming excess alcohol. Duh! I do all of that anyway.
The claims that HRV is a “simple but powerful tool that can be used to track overall health and well-being” might turn out to be true. Proper study and sharing of data will enable that determination.
To advance understanding, I offer an n-of-1, a real-world personal anecdote about HRV.
I did not request the HRV function on my Fitbit Inspire 2. It simply appeared, and I ignored it for some time.
A year or two ago, I started noticing my HRV score every morning. Initially, I did not like to see my “low” score, until I learned that the reference range was dramatically affected by age and I was in my late 80s at the time. The vast majority of my HRV readings were in the range of 17 msec to 27 msec.
Last week, I was administered the new Moderna COVID-19 Spikevax vaccine and the old folks’ influenza vaccine simultaneously. In my case, side effects from each vaccine have been modest in the past, but I never previously had both administered at the same time. My immune response was, shall we say, robust. Chills, muscle aches, headache, fatigue, deltoid swelling, fitful sleep, and increased resting heart rate.
My nightly average HRV had been running between 17 msec and 35 msec for many months. WHOA! After the shots, my overnight HRV score plummeted from 24 msec to 10 msec, my lowest ever. Instant worry. The next day, it rebounded to 28 msec, and it has been in the high teens or low 20s since then.
Off to PubMed. A recent study of HRV on the second and 10th days after administering the Pfizer mRNA vaccine to 75 healthy volunteers found that the HRV on day 2 was dramatically lower than prevaccination levels and by day 10, it had returned to prevaccination levels. Some comfort there.
Another review article has reported a rapid fall and rapid rebound of HRV after COVID-19 vaccination. A 2010 report demonstrated a significant but not dramatic short-term lowering of HRV after influenza A vaccination and correlated it with CRP changes.
Some believe that the decline in HRV after vaccination reflects an increased immune response and sympathetic nervous activity.
I don’t plan to receive my flu and COVID vaccines on the same day again.
So, I went back to review what happened to my HRV when I had COVID in 2023. My HRV was 14 msec and 12 msec on the first 2 days of symptoms, and then returned to the 20 msec range.
I received the RSV vaccine this year without adverse effects, and my HRV scores were 29 msec, 33 msec, and 32 msec on the first 3 days after vaccination. Finally, after receiving a pneumococcal vaccine in 2023, I had no adverse effects, and my HRV scores on the 5 days after vaccination were indeterminate: 19 msec, 14 msec, 18 msec, 13 msec, and 17 msec.
Of course, correlation is not causation. Cause and effect remain undetermined. But I find these observations interesting for a potentially useful screening test.
George D. Lundberg, MD, is the Editor in Chief of Cancer Commons.
A version of this article first appeared on Medscape.com.