User login
Tracy E. Madsen, MD
The Core Competencies in Hospital Medicine – 2017 revision
“You must be the change you wish to see in the world.” This famous quote from Mahatma Gandhi has inspired many to transform their work and personal space into an eternal quest for improvement. We hospitalists are now well-recognized agents of change in our work environment, improving the quality and safety of inpatient care, striving to create increased value, and promoting the delivery of cost-effective care.
Much has changed in the U.S. health care and hospital practice environment over the past decade. The 2017 revision of the Core Competencies seeks to maintain its relevance, value and more importantly, highlight areas for future growth and innovation.
What does the “Core Competencies” represent and who should use it?
It comprises a set of competency-based learning objectives that present a shared understanding of the knowledge, skills, and attitudes expected of physicians practicing hospital medicine in the United States.
A common misconception is that every hospitalist can be expected to demonstrate proficiency in all topics in the Core Competencies. While every item in the compendium is highly relevant to the field as a whole, its significance for individual hospitalists will vary depending on their practice pattern, leadership role, and local culture.
It also is noteworthy to indicate that it is not a set of practice guidelines that provide recommendations based on the latest scientific evidence, nor does it represent any legal standard of care. Rather, the Core Competencies offers an agenda for curricular training and to broadly influence the direction of the field. It also is important to realize that the Core Competencies is not an all-inclusive list that restricts a hospitalist’s scope of practice. Instead, hospitalists should use the Core Competencies as an educational and professional benchmark with the ultimate goal of providing safe, efficient, and high-value care using interdisciplinary collaboration when necessary.
As a core set of attributes, all hospitalists can use it to reflect on their knowledge, skills, and attitudes, as well as those of their group or practice collectively. The Core Competencies highlights areas within the field that are prime for further research and quality improvement initiatives on a national, regional, and local level. Thus, they also should be of interest to health care administrators and a variety of stakeholders looking to support and fund such efforts in enhancing health care value and quality for all.
It is also a framework for the development of curricula for both education and professional development purposes for use by hospitalists, hospital medicine programs, and health care institutions. Course Directors of Continuing Medical Education programs can use the Core Competencies to identify learning objectives that fulfill the goal of the educational program. Similarly, residency and fellowship program directors and medical school clerkship directors can use it to develop course syllabi targeted to the needs of their learner groups.
The structure and format of the Core Competencies in Hospital Medicine
The 53 chapters in the 2017 revision are divided into three sections – Clinical Conditions, Procedures, and Healthcare Systems, all integral to the practice of hospital medicine. Each chapter starts with an introductory paragraph that discusses the relevance and importance of the subject. Each competency-based learning objective describes a particular concept coupled with an action verb that specifies an expected level of proficiency.
For example, the action verb “explain” that requires a mere description of a subject denotes a lower competency level, compared with the verb “evaluate,” which implies not only an understanding of the matter but also the ability to assess its value for a particular purpose. These learning objectives are further categorized into knowledge, skills, and attitudes subsections to reflect the cognitive, psychomotor, and affective domains of learning.
Because hospitalists are the experts in complex hospital systems, the clinical and procedural sections have an additional subsection, “System Organization and Improvement.” The objectives in this paragraph emphasize the critical role that hospitalists can play as leaders of multidisciplinary teams to improve the quality of care of all patients with a similar condition or undergoing the same procedure.
Examples of everyday use of the Core Competencies for practicing hospitalists
A hospitalist looking to improve her performance of bedside thoracentesis reviews the chapter on Thoracentesis. She then decides to enhance her skills by attending an educational workshop on the use of point-of-care ultrasonography.
A hospital medicine group interested in improving the rate of common hospital-acquired infections reviews the Urinary Tract Infection, Hospital-Acquired and Healthcare-Associated Pneumonia, and Prevention of Healthcare-Associated Infections and Antimicrobial Resistance chapters to identify possible gaps in practice patterns. The group also goes through the chapters on Quality Improvement, Practice-based Learning and Improvement, and Hospitalist as Educator, to further reflect upon the characteristics of their practice environment. The group then adopts a separate strategy to address identified gaps by finding suitable evidence-based content in a format that best fits their need.
An attending physician leading a team of medical residents and students reviews the chapter on Syncope to identify the teaching objectives for each learner. He decides that the medical student should be able to “define syncope” and “explain the physiologic mechanisms that lead to reflex or neurally mediated syncope.” He determines that the intern on the team should be able to “differentiate syncope from other causes of loss of consciousness,” and the senior resident should be able to “formulate a logical diagnostic plan to determine the cause of syncope while avoiding rarely indicated diagnostic tests … ”
New chapters in the 2017 revision
SHM’s Core Competencies Task Force (CCTF) considered several topics as potential new chapters for the 2017 Revision. The SHM Education Committee judged each for its value as a “core” subject by its relevance, intersection with other specialties, and its scope as a stand-alone chapter.
There are two new clinical conditions – hyponatremia and syncope – mainly chosen because of their clinical importance, the risk of complications, and management inconsistencies that offer hospitalists great opportunities for quality improvement initiatives. The CCTF also identified the use of point-of-care ultrasonography as a notable advancement in the field. A separate task force is working to evaluate best practices and develop a practice guideline that hospitalists can use. The CCTF expects to add more chapters as the field of hospital medicine continues to advance and transform the delivery of health care globally.
The 2017 Revision of the Core Competencies in Hospital Medicine is located online at www.journalofhospitalmedicine.com or using the URL shortener bit.ly/corecomp17.
Dr. Nichani is assistant professor of medicine and director of education for the division of hospital medicine at Michigan Medicine, University of Michigan, Ann Arbor. He serves as the chair of the SHM Education Committee.
“You must be the change you wish to see in the world.” This famous quote from Mahatma Gandhi has inspired many to transform their work and personal space into an eternal quest for improvement. We hospitalists are now well-recognized agents of change in our work environment, improving the quality and safety of inpatient care, striving to create increased value, and promoting the delivery of cost-effective care.
Much has changed in the U.S. health care and hospital practice environment over the past decade. The 2017 revision of the Core Competencies seeks to maintain its relevance, value and more importantly, highlight areas for future growth and innovation.
What does the “Core Competencies” represent and who should use it?
It comprises a set of competency-based learning objectives that present a shared understanding of the knowledge, skills, and attitudes expected of physicians practicing hospital medicine in the United States.
A common misconception is that every hospitalist can be expected to demonstrate proficiency in all topics in the Core Competencies. While every item in the compendium is highly relevant to the field as a whole, its significance for individual hospitalists will vary depending on their practice pattern, leadership role, and local culture.
It also is noteworthy to indicate that it is not a set of practice guidelines that provide recommendations based on the latest scientific evidence, nor does it represent any legal standard of care. Rather, the Core Competencies offers an agenda for curricular training and to broadly influence the direction of the field. It also is important to realize that the Core Competencies is not an all-inclusive list that restricts a hospitalist’s scope of practice. Instead, hospitalists should use the Core Competencies as an educational and professional benchmark with the ultimate goal of providing safe, efficient, and high-value care using interdisciplinary collaboration when necessary.
As a core set of attributes, all hospitalists can use it to reflect on their knowledge, skills, and attitudes, as well as those of their group or practice collectively. The Core Competencies highlights areas within the field that are prime for further research and quality improvement initiatives on a national, regional, and local level. Thus, they also should be of interest to health care administrators and a variety of stakeholders looking to support and fund such efforts in enhancing health care value and quality for all.
It is also a framework for the development of curricula for both education and professional development purposes for use by hospitalists, hospital medicine programs, and health care institutions. Course Directors of Continuing Medical Education programs can use the Core Competencies to identify learning objectives that fulfill the goal of the educational program. Similarly, residency and fellowship program directors and medical school clerkship directors can use it to develop course syllabi targeted to the needs of their learner groups.
The structure and format of the Core Competencies in Hospital Medicine
The 53 chapters in the 2017 revision are divided into three sections – Clinical Conditions, Procedures, and Healthcare Systems, all integral to the practice of hospital medicine. Each chapter starts with an introductory paragraph that discusses the relevance and importance of the subject. Each competency-based learning objective describes a particular concept coupled with an action verb that specifies an expected level of proficiency.
For example, the action verb “explain” that requires a mere description of a subject denotes a lower competency level, compared with the verb “evaluate,” which implies not only an understanding of the matter but also the ability to assess its value for a particular purpose. These learning objectives are further categorized into knowledge, skills, and attitudes subsections to reflect the cognitive, psychomotor, and affective domains of learning.
Because hospitalists are the experts in complex hospital systems, the clinical and procedural sections have an additional subsection, “System Organization and Improvement.” The objectives in this paragraph emphasize the critical role that hospitalists can play as leaders of multidisciplinary teams to improve the quality of care of all patients with a similar condition or undergoing the same procedure.
Examples of everyday use of the Core Competencies for practicing hospitalists
A hospitalist looking to improve her performance of bedside thoracentesis reviews the chapter on Thoracentesis. She then decides to enhance her skills by attending an educational workshop on the use of point-of-care ultrasonography.
A hospital medicine group interested in improving the rate of common hospital-acquired infections reviews the Urinary Tract Infection, Hospital-Acquired and Healthcare-Associated Pneumonia, and Prevention of Healthcare-Associated Infections and Antimicrobial Resistance chapters to identify possible gaps in practice patterns. The group also goes through the chapters on Quality Improvement, Practice-based Learning and Improvement, and Hospitalist as Educator, to further reflect upon the characteristics of their practice environment. The group then adopts a separate strategy to address identified gaps by finding suitable evidence-based content in a format that best fits their need.
An attending physician leading a team of medical residents and students reviews the chapter on Syncope to identify the teaching objectives for each learner. He decides that the medical student should be able to “define syncope” and “explain the physiologic mechanisms that lead to reflex or neurally mediated syncope.” He determines that the intern on the team should be able to “differentiate syncope from other causes of loss of consciousness,” and the senior resident should be able to “formulate a logical diagnostic plan to determine the cause of syncope while avoiding rarely indicated diagnostic tests … ”
New chapters in the 2017 revision
SHM’s Core Competencies Task Force (CCTF) considered several topics as potential new chapters for the 2017 Revision. The SHM Education Committee judged each for its value as a “core” subject by its relevance, intersection with other specialties, and its scope as a stand-alone chapter.
There are two new clinical conditions – hyponatremia and syncope – mainly chosen because of their clinical importance, the risk of complications, and management inconsistencies that offer hospitalists great opportunities for quality improvement initiatives. The CCTF also identified the use of point-of-care ultrasonography as a notable advancement in the field. A separate task force is working to evaluate best practices and develop a practice guideline that hospitalists can use. The CCTF expects to add more chapters as the field of hospital medicine continues to advance and transform the delivery of health care globally.
The 2017 Revision of the Core Competencies in Hospital Medicine is located online at www.journalofhospitalmedicine.com or using the URL shortener bit.ly/corecomp17.
Dr. Nichani is assistant professor of medicine and director of education for the division of hospital medicine at Michigan Medicine, University of Michigan, Ann Arbor. He serves as the chair of the SHM Education Committee.
“You must be the change you wish to see in the world.” This famous quote from Mahatma Gandhi has inspired many to transform their work and personal space into an eternal quest for improvement. We hospitalists are now well-recognized agents of change in our work environment, improving the quality and safety of inpatient care, striving to create increased value, and promoting the delivery of cost-effective care.
Much has changed in the U.S. health care and hospital practice environment over the past decade. The 2017 revision of the Core Competencies seeks to maintain its relevance, value and more importantly, highlight areas for future growth and innovation.
What does the “Core Competencies” represent and who should use it?
It comprises a set of competency-based learning objectives that present a shared understanding of the knowledge, skills, and attitudes expected of physicians practicing hospital medicine in the United States.
A common misconception is that every hospitalist can be expected to demonstrate proficiency in all topics in the Core Competencies. While every item in the compendium is highly relevant to the field as a whole, its significance for individual hospitalists will vary depending on their practice pattern, leadership role, and local culture.
It also is noteworthy to indicate that it is not a set of practice guidelines that provide recommendations based on the latest scientific evidence, nor does it represent any legal standard of care. Rather, the Core Competencies offers an agenda for curricular training and to broadly influence the direction of the field. It also is important to realize that the Core Competencies is not an all-inclusive list that restricts a hospitalist’s scope of practice. Instead, hospitalists should use the Core Competencies as an educational and professional benchmark with the ultimate goal of providing safe, efficient, and high-value care using interdisciplinary collaboration when necessary.
As a core set of attributes, all hospitalists can use it to reflect on their knowledge, skills, and attitudes, as well as those of their group or practice collectively. The Core Competencies highlights areas within the field that are prime for further research and quality improvement initiatives on a national, regional, and local level. Thus, they also should be of interest to health care administrators and a variety of stakeholders looking to support and fund such efforts in enhancing health care value and quality for all.
It is also a framework for the development of curricula for both education and professional development purposes for use by hospitalists, hospital medicine programs, and health care institutions. Course Directors of Continuing Medical Education programs can use the Core Competencies to identify learning objectives that fulfill the goal of the educational program. Similarly, residency and fellowship program directors and medical school clerkship directors can use it to develop course syllabi targeted to the needs of their learner groups.
The structure and format of the Core Competencies in Hospital Medicine
The 53 chapters in the 2017 revision are divided into three sections – Clinical Conditions, Procedures, and Healthcare Systems, all integral to the practice of hospital medicine. Each chapter starts with an introductory paragraph that discusses the relevance and importance of the subject. Each competency-based learning objective describes a particular concept coupled with an action verb that specifies an expected level of proficiency.
For example, the action verb “explain” that requires a mere description of a subject denotes a lower competency level, compared with the verb “evaluate,” which implies not only an understanding of the matter but also the ability to assess its value for a particular purpose. These learning objectives are further categorized into knowledge, skills, and attitudes subsections to reflect the cognitive, psychomotor, and affective domains of learning.
Because hospitalists are the experts in complex hospital systems, the clinical and procedural sections have an additional subsection, “System Organization and Improvement.” The objectives in this paragraph emphasize the critical role that hospitalists can play as leaders of multidisciplinary teams to improve the quality of care of all patients with a similar condition or undergoing the same procedure.
Examples of everyday use of the Core Competencies for practicing hospitalists
A hospitalist looking to improve her performance of bedside thoracentesis reviews the chapter on Thoracentesis. She then decides to enhance her skills by attending an educational workshop on the use of point-of-care ultrasonography.
A hospital medicine group interested in improving the rate of common hospital-acquired infections reviews the Urinary Tract Infection, Hospital-Acquired and Healthcare-Associated Pneumonia, and Prevention of Healthcare-Associated Infections and Antimicrobial Resistance chapters to identify possible gaps in practice patterns. The group also goes through the chapters on Quality Improvement, Practice-based Learning and Improvement, and Hospitalist as Educator, to further reflect upon the characteristics of their practice environment. The group then adopts a separate strategy to address identified gaps by finding suitable evidence-based content in a format that best fits their need.
An attending physician leading a team of medical residents and students reviews the chapter on Syncope to identify the teaching objectives for each learner. He decides that the medical student should be able to “define syncope” and “explain the physiologic mechanisms that lead to reflex or neurally mediated syncope.” He determines that the intern on the team should be able to “differentiate syncope from other causes of loss of consciousness,” and the senior resident should be able to “formulate a logical diagnostic plan to determine the cause of syncope while avoiding rarely indicated diagnostic tests … ”
New chapters in the 2017 revision
SHM’s Core Competencies Task Force (CCTF) considered several topics as potential new chapters for the 2017 Revision. The SHM Education Committee judged each for its value as a “core” subject by its relevance, intersection with other specialties, and its scope as a stand-alone chapter.
There are two new clinical conditions – hyponatremia and syncope – mainly chosen because of their clinical importance, the risk of complications, and management inconsistencies that offer hospitalists great opportunities for quality improvement initiatives. The CCTF also identified the use of point-of-care ultrasonography as a notable advancement in the field. A separate task force is working to evaluate best practices and develop a practice guideline that hospitalists can use. The CCTF expects to add more chapters as the field of hospital medicine continues to advance and transform the delivery of health care globally.
The 2017 Revision of the Core Competencies in Hospital Medicine is located online at www.journalofhospitalmedicine.com or using the URL shortener bit.ly/corecomp17.
Dr. Nichani is assistant professor of medicine and director of education for the division of hospital medicine at Michigan Medicine, University of Michigan, Ann Arbor. He serves as the chair of the SHM Education Committee.
When patients get the travel bug, dermatologists should beware
NEW YORK – All dermatologists, including those who are office based, should know how to recognize and treat infectious diseases and infections from all over the world.
That was the unifying message put forth by dermatologists who spoke at the American Academy of Dermatology summer meeting during a session on infectious diseases and infestations in returned travelers.
Key to recognizing such diseases is knowing what questions to ask, said Vikash S. Oza, MD, director of pediatric dermatology at New York University.“It’s important to know where the patient went to understand the endemic issues,” as well as the purpose of the patient’s visit, said Dr. Oza. “Patients who travel to be with family come back with a higher burden of illness,” possibly because they are less likely to seek medical advice prior to travel and more likely to mingle with local populations, drink from local water supplies, and come into contact with livestock during travel, he added.
Watch out for children
Children are at particular risk: One analysis found that 25% of children suffer at least one skin disorder after international travel, he said.
In the United States, the spirochete infection tends to be caused by the bacterial species Borrelia burgdorferi, which is typically transmitted by a tick bite. Hosts include the white-footed mouse, chipmunks, and even robins. In the Northeastern United States, Lyme season peaks from June through August; children aged 5-10 years of age tend to be at highest risk.
Changes to the skin are an important part of the clinical spectrum, with erythema migrans developing 1-2 weeks after infection and continuing for months. It can affect the cranial nerves, causing Bell’s palsy, meningitis, and carditis. In the late stage, large joint arthritis can occur.
But doctors cannot depend on the classic bull’s eye associated with erythema migrans, since it occurs only rarely in the United States, Dr. Oza pointed out. “More often, it is a homogenous, expanding area.”
Only about one in four children who present with Lyme disease display multiple erythema migrans rashes, he said. And the vector is rarely noticed. “Twenty-five percent recall a tick bite,” he added.
Erythema migrans can also occur among people who do not live in areas where Lyme disease is endemic. So doctors should be alert to Southern Tick–Associated Rash Illness, which is endemic to much of the Southeast – caused by the bite of the Lone Star tick. Unlike Lyme, this disease tends to be self-limiting and does not tend to cause a late-stage illness to develop neurologic or joint-related problems, he said.
Prevention
The best defense is to prevent tick bites, and liberal use of DEET has proved to be effective as has permethrin-impregnated clothing, which kills the tick.
Ticks tend to be found on long blades of grass or in leaf debris. They neither jump nor fly, “but reach out in desperation,” said Dr. Oza, who urges hikers to take a shower after hiking, check the scalp and behind the ears, and place all clothing in a hot dryer for 10 minutes, which will kill any deer ticks.
Pets, too, should be checked – even on their eyelids, he added. If a tick is found and removed within 48 hours, it has little chance of infecting its host, he said.
Aedes aegypti mosquitoes pose multiple threats
Common causes of rash and fever in travelers include malaria, dengue, spotted fever, rickettsia, yellow fever, chikungunya, and Zika, said Jose Dario Martinez, MD, of the departments of internal medicine and dermatology, University Hospital, Monterrey, Mexico.
The latter has proved to be a major challenge. In just a few months, the Zika virus has swept across all of the Americas, with the exception of Canada and Chile. It is spread by Aedes aegypti, which thrives and breeds close to homes and is a difficult vector to eradicate, he said. The same mosquito also transmits yellow fever, dengue, and chikungunya.
This year, the Aedes aegypti mosquito has been disrupting tropical vacations because of its ability to transmit not only Zika but dengue, chikungunya, and yellow fever.
Again, the 60-year-old product DEET plays a major defensive role. It lasts the longest of any such products, repels a broad array of insects, and is recommended by the Centers for Disease Control and Prevention and the American Academy of Pediatrics, but it is not recommended for children younger than 2 months of age.
Picaridin, which has been available in the United States since 2005, is also recommended by AAP. It is odorless and does not irritate the skin. Oil of lemon eucalyptus is commonly used in China, but has not been tested for children under aged 3 years.
“If you’re going camping, probably the best thing you can do is wear permethrin-treated clothing and shoes,” Dr. Oza said.
Bedbugs
No discussion of infections among travelers would be complete without a discussion of bedbugs, whose numbers have rebounded since the 1950s, when DDT nearly wiped them out, said Theodore Rosen, MD, professor of dermatology, Baylor College of Medicine, Houston.
Mother Nature offers little help, since bedbugs can survive winters. And they are not always easy to notice, since their saliva contains an anesthetic, which can mask the feeling of a bite. “Insects can thus feed undetected for 5-10 minutes,” Dr. Rosen said. But, though experiments have shown them to be competent vectors at spreading disease, “in real life, they have not been demonstrated to be the purveyors of human disease,” he noted.
So far, the best way to get rid of them is “thermal remediation,” which entails heating infested areas to 120-140° F for 5-8 hours.
Also effective, but less practical, would be to set any infested structures ablaze.
Advice for the traveler: Keep your suitcases zipped in hotel rooms, and store them up high or in the shower, since bedbugs have a tough time jumping or gaining traction on porcelain. And make sure you launder your clothes once you get home.
Dr. Rosen, Dr. Martinez, and Dr. Oza had no disclosures.
NEW YORK – All dermatologists, including those who are office based, should know how to recognize and treat infectious diseases and infections from all over the world.
That was the unifying message put forth by dermatologists who spoke at the American Academy of Dermatology summer meeting during a session on infectious diseases and infestations in returned travelers.
Key to recognizing such diseases is knowing what questions to ask, said Vikash S. Oza, MD, director of pediatric dermatology at New York University.“It’s important to know where the patient went to understand the endemic issues,” as well as the purpose of the patient’s visit, said Dr. Oza. “Patients who travel to be with family come back with a higher burden of illness,” possibly because they are less likely to seek medical advice prior to travel and more likely to mingle with local populations, drink from local water supplies, and come into contact with livestock during travel, he added.
Watch out for children
Children are at particular risk: One analysis found that 25% of children suffer at least one skin disorder after international travel, he said.
In the United States, the spirochete infection tends to be caused by the bacterial species Borrelia burgdorferi, which is typically transmitted by a tick bite. Hosts include the white-footed mouse, chipmunks, and even robins. In the Northeastern United States, Lyme season peaks from June through August; children aged 5-10 years of age tend to be at highest risk.
Changes to the skin are an important part of the clinical spectrum, with erythema migrans developing 1-2 weeks after infection and continuing for months. It can affect the cranial nerves, causing Bell’s palsy, meningitis, and carditis. In the late stage, large joint arthritis can occur.
But doctors cannot depend on the classic bull’s eye associated with erythema migrans, since it occurs only rarely in the United States, Dr. Oza pointed out. “More often, it is a homogenous, expanding area.”
Only about one in four children who present with Lyme disease display multiple erythema migrans rashes, he said. And the vector is rarely noticed. “Twenty-five percent recall a tick bite,” he added.
Erythema migrans can also occur among people who do not live in areas where Lyme disease is endemic. So doctors should be alert to Southern Tick–Associated Rash Illness, which is endemic to much of the Southeast – caused by the bite of the Lone Star tick. Unlike Lyme, this disease tends to be self-limiting and does not tend to cause a late-stage illness to develop neurologic or joint-related problems, he said.
Prevention
The best defense is to prevent tick bites, and liberal use of DEET has proved to be effective as has permethrin-impregnated clothing, which kills the tick.
Ticks tend to be found on long blades of grass or in leaf debris. They neither jump nor fly, “but reach out in desperation,” said Dr. Oza, who urges hikers to take a shower after hiking, check the scalp and behind the ears, and place all clothing in a hot dryer for 10 minutes, which will kill any deer ticks.
Pets, too, should be checked – even on their eyelids, he added. If a tick is found and removed within 48 hours, it has little chance of infecting its host, he said.
Aedes aegypti mosquitoes pose multiple threats
Common causes of rash and fever in travelers include malaria, dengue, spotted fever, rickettsia, yellow fever, chikungunya, and Zika, said Jose Dario Martinez, MD, of the departments of internal medicine and dermatology, University Hospital, Monterrey, Mexico.
The latter has proved to be a major challenge. In just a few months, the Zika virus has swept across all of the Americas, with the exception of Canada and Chile. It is spread by Aedes aegypti, which thrives and breeds close to homes and is a difficult vector to eradicate, he said. The same mosquito also transmits yellow fever, dengue, and chikungunya.
This year, the Aedes aegypti mosquito has been disrupting tropical vacations because of its ability to transmit not only Zika but dengue, chikungunya, and yellow fever.
Again, the 60-year-old product DEET plays a major defensive role. It lasts the longest of any such products, repels a broad array of insects, and is recommended by the Centers for Disease Control and Prevention and the American Academy of Pediatrics, but it is not recommended for children younger than 2 months of age.
Picaridin, which has been available in the United States since 2005, is also recommended by AAP. It is odorless and does not irritate the skin. Oil of lemon eucalyptus is commonly used in China, but has not been tested for children under aged 3 years.
“If you’re going camping, probably the best thing you can do is wear permethrin-treated clothing and shoes,” Dr. Oza said.
Bedbugs
No discussion of infections among travelers would be complete without a discussion of bedbugs, whose numbers have rebounded since the 1950s, when DDT nearly wiped them out, said Theodore Rosen, MD, professor of dermatology, Baylor College of Medicine, Houston.
Mother Nature offers little help, since bedbugs can survive winters. And they are not always easy to notice, since their saliva contains an anesthetic, which can mask the feeling of a bite. “Insects can thus feed undetected for 5-10 minutes,” Dr. Rosen said. But, though experiments have shown them to be competent vectors at spreading disease, “in real life, they have not been demonstrated to be the purveyors of human disease,” he noted.
So far, the best way to get rid of them is “thermal remediation,” which entails heating infested areas to 120-140° F for 5-8 hours.
Also effective, but less practical, would be to set any infested structures ablaze.
Advice for the traveler: Keep your suitcases zipped in hotel rooms, and store them up high or in the shower, since bedbugs have a tough time jumping or gaining traction on porcelain. And make sure you launder your clothes once you get home.
Dr. Rosen, Dr. Martinez, and Dr. Oza had no disclosures.
NEW YORK – All dermatologists, including those who are office based, should know how to recognize and treat infectious diseases and infections from all over the world.
That was the unifying message put forth by dermatologists who spoke at the American Academy of Dermatology summer meeting during a session on infectious diseases and infestations in returned travelers.
Key to recognizing such diseases is knowing what questions to ask, said Vikash S. Oza, MD, director of pediatric dermatology at New York University.“It’s important to know where the patient went to understand the endemic issues,” as well as the purpose of the patient’s visit, said Dr. Oza. “Patients who travel to be with family come back with a higher burden of illness,” possibly because they are less likely to seek medical advice prior to travel and more likely to mingle with local populations, drink from local water supplies, and come into contact with livestock during travel, he added.
Watch out for children
Children are at particular risk: One analysis found that 25% of children suffer at least one skin disorder after international travel, he said.
In the United States, the spirochete infection tends to be caused by the bacterial species Borrelia burgdorferi, which is typically transmitted by a tick bite. Hosts include the white-footed mouse, chipmunks, and even robins. In the Northeastern United States, Lyme season peaks from June through August; children aged 5-10 years of age tend to be at highest risk.
Changes to the skin are an important part of the clinical spectrum, with erythema migrans developing 1-2 weeks after infection and continuing for months. It can affect the cranial nerves, causing Bell’s palsy, meningitis, and carditis. In the late stage, large joint arthritis can occur.
But doctors cannot depend on the classic bull’s eye associated with erythema migrans, since it occurs only rarely in the United States, Dr. Oza pointed out. “More often, it is a homogenous, expanding area.”
Only about one in four children who present with Lyme disease display multiple erythema migrans rashes, he said. And the vector is rarely noticed. “Twenty-five percent recall a tick bite,” he added.
Erythema migrans can also occur among people who do not live in areas where Lyme disease is endemic. So doctors should be alert to Southern Tick–Associated Rash Illness, which is endemic to much of the Southeast – caused by the bite of the Lone Star tick. Unlike Lyme, this disease tends to be self-limiting and does not tend to cause a late-stage illness to develop neurologic or joint-related problems, he said.
Prevention
The best defense is to prevent tick bites, and liberal use of DEET has proved to be effective as has permethrin-impregnated clothing, which kills the tick.
Ticks tend to be found on long blades of grass or in leaf debris. They neither jump nor fly, “but reach out in desperation,” said Dr. Oza, who urges hikers to take a shower after hiking, check the scalp and behind the ears, and place all clothing in a hot dryer for 10 minutes, which will kill any deer ticks.
Pets, too, should be checked – even on their eyelids, he added. If a tick is found and removed within 48 hours, it has little chance of infecting its host, he said.
Aedes aegypti mosquitoes pose multiple threats
Common causes of rash and fever in travelers include malaria, dengue, spotted fever, rickettsia, yellow fever, chikungunya, and Zika, said Jose Dario Martinez, MD, of the departments of internal medicine and dermatology, University Hospital, Monterrey, Mexico.
The latter has proved to be a major challenge. In just a few months, the Zika virus has swept across all of the Americas, with the exception of Canada and Chile. It is spread by Aedes aegypti, which thrives and breeds close to homes and is a difficult vector to eradicate, he said. The same mosquito also transmits yellow fever, dengue, and chikungunya.
This year, the Aedes aegypti mosquito has been disrupting tropical vacations because of its ability to transmit not only Zika but dengue, chikungunya, and yellow fever.
Again, the 60-year-old product DEET plays a major defensive role. It lasts the longest of any such products, repels a broad array of insects, and is recommended by the Centers for Disease Control and Prevention and the American Academy of Pediatrics, but it is not recommended for children younger than 2 months of age.
Picaridin, which has been available in the United States since 2005, is also recommended by AAP. It is odorless and does not irritate the skin. Oil of lemon eucalyptus is commonly used in China, but has not been tested for children under aged 3 years.
“If you’re going camping, probably the best thing you can do is wear permethrin-treated clothing and shoes,” Dr. Oza said.
Bedbugs
No discussion of infections among travelers would be complete without a discussion of bedbugs, whose numbers have rebounded since the 1950s, when DDT nearly wiped them out, said Theodore Rosen, MD, professor of dermatology, Baylor College of Medicine, Houston.
Mother Nature offers little help, since bedbugs can survive winters. And they are not always easy to notice, since their saliva contains an anesthetic, which can mask the feeling of a bite. “Insects can thus feed undetected for 5-10 minutes,” Dr. Rosen said. But, though experiments have shown them to be competent vectors at spreading disease, “in real life, they have not been demonstrated to be the purveyors of human disease,” he noted.
So far, the best way to get rid of them is “thermal remediation,” which entails heating infested areas to 120-140° F for 5-8 hours.
Also effective, but less practical, would be to set any infested structures ablaze.
Advice for the traveler: Keep your suitcases zipped in hotel rooms, and store them up high or in the shower, since bedbugs have a tough time jumping or gaining traction on porcelain. And make sure you launder your clothes once you get home.
Dr. Rosen, Dr. Martinez, and Dr. Oza had no disclosures.
AT THE 2017 AAD SUMMER MEETING
Working With Black Women to Tailor Weight-Loss Programs
For many black women, big is beautiful, and size is not only a cultural norm, but also an asset, say researchers from University of North Texas in Fort Worth. But African American women are nearly twice as likely to develop diabetes and more than twice as likely to develop end-stage kidney disease or die of complications of diabetes than are white women. They also have a higher prevalence of being overweight or obese.
Related: Genomic Variation May Reveal ‘Biological Pathway’ to Obesity
However, focusing on weight loss to reduce rates of chronic disease, disability, and premature death may be the wrong tack, the researchers say; weight-loss programs often don’t work well for black women. Even those programs that may have been tailored to the black woman through culturally appropriate artwork and language, African American media outlets, and “meaningful themes” (such as family and spirituality) tend not to produce sustainable results. Most programs aim for participants to lose 5% to 10% of body mass (or roughly 9 to 18 lb for a 185-lb woman) to reduce cardiovascular risk. On average, the researchers say, black women lose 4 to 10 lb—and they typically regain as much as 33% of the weight lost within the year.
The answer, the researchers say, is to not use body mass index (BMI) as a guide. Body mass index is a poor proxy, they say, for general health and health behaviors because it fails to account for differences in body composition, fitness levels, and nutritional differences that predict health and longevity. “BMI itself,” the researchers note, “as a measure of health is of limited value.” According to an analysis of National Health and Nutrition Examination Surveys data, using BMI to predict cardiometabolic risk misclassifies nearly 75 million U.S. adults.
Related: Some Patients With Diabetes Aren’t Getting Needed Weight Advice
The researchers suggest a collaborative approach may help: community-based participatory research. A key feature is bringing in the women as partners in research so that their subjective experiences inform the programs. The researchers cite a study in which the collaborative team ultimately “shifted its focus” from using weight loss alone as a metric to what they labeled as “a common-sense approach” more focused on physical activity and nutritional goals perceived by participants as relevant and valuable.
For many black women, big is beautiful, and size is not only a cultural norm, but also an asset, say researchers from University of North Texas in Fort Worth. But African American women are nearly twice as likely to develop diabetes and more than twice as likely to develop end-stage kidney disease or die of complications of diabetes than are white women. They also have a higher prevalence of being overweight or obese.
Related: Genomic Variation May Reveal ‘Biological Pathway’ to Obesity
However, focusing on weight loss to reduce rates of chronic disease, disability, and premature death may be the wrong tack, the researchers say; weight-loss programs often don’t work well for black women. Even those programs that may have been tailored to the black woman through culturally appropriate artwork and language, African American media outlets, and “meaningful themes” (such as family and spirituality) tend not to produce sustainable results. Most programs aim for participants to lose 5% to 10% of body mass (or roughly 9 to 18 lb for a 185-lb woman) to reduce cardiovascular risk. On average, the researchers say, black women lose 4 to 10 lb—and they typically regain as much as 33% of the weight lost within the year.
The answer, the researchers say, is to not use body mass index (BMI) as a guide. Body mass index is a poor proxy, they say, for general health and health behaviors because it fails to account for differences in body composition, fitness levels, and nutritional differences that predict health and longevity. “BMI itself,” the researchers note, “as a measure of health is of limited value.” According to an analysis of National Health and Nutrition Examination Surveys data, using BMI to predict cardiometabolic risk misclassifies nearly 75 million U.S. adults.
Related: Some Patients With Diabetes Aren’t Getting Needed Weight Advice
The researchers suggest a collaborative approach may help: community-based participatory research. A key feature is bringing in the women as partners in research so that their subjective experiences inform the programs. The researchers cite a study in which the collaborative team ultimately “shifted its focus” from using weight loss alone as a metric to what they labeled as “a common-sense approach” more focused on physical activity and nutritional goals perceived by participants as relevant and valuable.
For many black women, big is beautiful, and size is not only a cultural norm, but also an asset, say researchers from University of North Texas in Fort Worth. But African American women are nearly twice as likely to develop diabetes and more than twice as likely to develop end-stage kidney disease or die of complications of diabetes than are white women. They also have a higher prevalence of being overweight or obese.
Related: Genomic Variation May Reveal ‘Biological Pathway’ to Obesity
However, focusing on weight loss to reduce rates of chronic disease, disability, and premature death may be the wrong tack, the researchers say; weight-loss programs often don’t work well for black women. Even those programs that may have been tailored to the black woman through culturally appropriate artwork and language, African American media outlets, and “meaningful themes” (such as family and spirituality) tend not to produce sustainable results. Most programs aim for participants to lose 5% to 10% of body mass (or roughly 9 to 18 lb for a 185-lb woman) to reduce cardiovascular risk. On average, the researchers say, black women lose 4 to 10 lb—and they typically regain as much as 33% of the weight lost within the year.
The answer, the researchers say, is to not use body mass index (BMI) as a guide. Body mass index is a poor proxy, they say, for general health and health behaviors because it fails to account for differences in body composition, fitness levels, and nutritional differences that predict health and longevity. “BMI itself,” the researchers note, “as a measure of health is of limited value.” According to an analysis of National Health and Nutrition Examination Surveys data, using BMI to predict cardiometabolic risk misclassifies nearly 75 million U.S. adults.
Related: Some Patients With Diabetes Aren’t Getting Needed Weight Advice
The researchers suggest a collaborative approach may help: community-based participatory research. A key feature is bringing in the women as partners in research so that their subjective experiences inform the programs. The researchers cite a study in which the collaborative team ultimately “shifted its focus” from using weight loss alone as a metric to what they labeled as “a common-sense approach” more focused on physical activity and nutritional goals perceived by participants as relevant and valuable.
Targeting heme synthesis to treat AML
Researchers have found evidence to suggest that a type of acute myeloid leukemia (AML) depends on the production of heme.
The group’s work has revealed 2 ways to target heme synthesis that might be used to treat this type of AML, which is driven by the oncogene MYCN.
John Schuetz, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, and his colleague described this research in JCI Insight.
Previous research had suggested that heme production was affected in leukemia.
However, Dr Schuetz said, “Absolutely nothing was known about the role of heme biosynthesis [in AML] before our work.”
The researchers’ first clue regarding heme’s role in AML arose from a computer search. The team searched a genomic database for other genes that were abnormally switched on in MYCN-driven AML.
They found that UROD was highly activated and noted that UROD is part of the molecular machinery that synthesizes heme.
Especially significant, Dr Schuetz said, was the finding that MYCN-driven AML with the most over-activated UROD was far more lethal than other AMLs.
The researchers found that cells with over-activated MYCN consumed more oxygen and depended on the production of heme for self-renewal and oncogenic transformation. But the team was able to block cancer cell self-renewal in the MYCN cells by blocking heme synthesis.
The researchers also found they could suppress self-renewal by blocking ABCG2, a “relief-valve” molecule that rids the cells of porphyrin, a building-block molecule of heme.
Blocking ABCG2 caused the buildup of porphyrin, which is toxic to the leukemia cells. However, blocking ABCG2 in normal cells produced no ill effects.
In mouse models of MYCN leukemia, the researchers tested a strategy of knocking out ABCG2. These knockout mice had significantly slower disease progression and longer survival.
What’s more, the team found they could cure leukemia in these mice by inhibiting ABCG2 and ramping up the heme machinery.
“Our findings suggest 2 drug strategies to treat AML,” Dr Schuetz said. “One would be to target UROD, which would reduce heme biosynthesis. Such drugs would selectively affect leukemia cells because they are so dependent on heme.”
“The other strategy would be to use drugs to inhibit the relief-valve protein and, at the same time, administer a chemical that is a precursor of heme. This would cause a buildup of toxic molecules that are part of the heme synthesis pathway.”
Dr Schuetz said other cancers with an over-activated heme pathway might also be vulnerable to such a treatment strategy.
He and his colleagues plan to extend their understanding of the heme machinery in AML with further studies. For example, they don’t know whether heme’s role in cell respiration is the only important one in supporting AML progression, since heme plays a wide range of roles in cells.
The researchers are also planning to test whether drugs that suppress UROD function in the heme-production machinery can effectively battle AML. ![]()
Researchers have found evidence to suggest that a type of acute myeloid leukemia (AML) depends on the production of heme.
The group’s work has revealed 2 ways to target heme synthesis that might be used to treat this type of AML, which is driven by the oncogene MYCN.
John Schuetz, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, and his colleague described this research in JCI Insight.
Previous research had suggested that heme production was affected in leukemia.
However, Dr Schuetz said, “Absolutely nothing was known about the role of heme biosynthesis [in AML] before our work.”
The researchers’ first clue regarding heme’s role in AML arose from a computer search. The team searched a genomic database for other genes that were abnormally switched on in MYCN-driven AML.
They found that UROD was highly activated and noted that UROD is part of the molecular machinery that synthesizes heme.
Especially significant, Dr Schuetz said, was the finding that MYCN-driven AML with the most over-activated UROD was far more lethal than other AMLs.
The researchers found that cells with over-activated MYCN consumed more oxygen and depended on the production of heme for self-renewal and oncogenic transformation. But the team was able to block cancer cell self-renewal in the MYCN cells by blocking heme synthesis.
The researchers also found they could suppress self-renewal by blocking ABCG2, a “relief-valve” molecule that rids the cells of porphyrin, a building-block molecule of heme.
Blocking ABCG2 caused the buildup of porphyrin, which is toxic to the leukemia cells. However, blocking ABCG2 in normal cells produced no ill effects.
In mouse models of MYCN leukemia, the researchers tested a strategy of knocking out ABCG2. These knockout mice had significantly slower disease progression and longer survival.
What’s more, the team found they could cure leukemia in these mice by inhibiting ABCG2 and ramping up the heme machinery.
“Our findings suggest 2 drug strategies to treat AML,” Dr Schuetz said. “One would be to target UROD, which would reduce heme biosynthesis. Such drugs would selectively affect leukemia cells because they are so dependent on heme.”
“The other strategy would be to use drugs to inhibit the relief-valve protein and, at the same time, administer a chemical that is a precursor of heme. This would cause a buildup of toxic molecules that are part of the heme synthesis pathway.”
Dr Schuetz said other cancers with an over-activated heme pathway might also be vulnerable to such a treatment strategy.
He and his colleagues plan to extend their understanding of the heme machinery in AML with further studies. For example, they don’t know whether heme’s role in cell respiration is the only important one in supporting AML progression, since heme plays a wide range of roles in cells.
The researchers are also planning to test whether drugs that suppress UROD function in the heme-production machinery can effectively battle AML. ![]()
Researchers have found evidence to suggest that a type of acute myeloid leukemia (AML) depends on the production of heme.
The group’s work has revealed 2 ways to target heme synthesis that might be used to treat this type of AML, which is driven by the oncogene MYCN.
John Schuetz, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, and his colleague described this research in JCI Insight.
Previous research had suggested that heme production was affected in leukemia.
However, Dr Schuetz said, “Absolutely nothing was known about the role of heme biosynthesis [in AML] before our work.”
The researchers’ first clue regarding heme’s role in AML arose from a computer search. The team searched a genomic database for other genes that were abnormally switched on in MYCN-driven AML.
They found that UROD was highly activated and noted that UROD is part of the molecular machinery that synthesizes heme.
Especially significant, Dr Schuetz said, was the finding that MYCN-driven AML with the most over-activated UROD was far more lethal than other AMLs.
The researchers found that cells with over-activated MYCN consumed more oxygen and depended on the production of heme for self-renewal and oncogenic transformation. But the team was able to block cancer cell self-renewal in the MYCN cells by blocking heme synthesis.
The researchers also found they could suppress self-renewal by blocking ABCG2, a “relief-valve” molecule that rids the cells of porphyrin, a building-block molecule of heme.
Blocking ABCG2 caused the buildup of porphyrin, which is toxic to the leukemia cells. However, blocking ABCG2 in normal cells produced no ill effects.
In mouse models of MYCN leukemia, the researchers tested a strategy of knocking out ABCG2. These knockout mice had significantly slower disease progression and longer survival.
What’s more, the team found they could cure leukemia in these mice by inhibiting ABCG2 and ramping up the heme machinery.
“Our findings suggest 2 drug strategies to treat AML,” Dr Schuetz said. “One would be to target UROD, which would reduce heme biosynthesis. Such drugs would selectively affect leukemia cells because they are so dependent on heme.”
“The other strategy would be to use drugs to inhibit the relief-valve protein and, at the same time, administer a chemical that is a precursor of heme. This would cause a buildup of toxic molecules that are part of the heme synthesis pathway.”
Dr Schuetz said other cancers with an over-activated heme pathway might also be vulnerable to such a treatment strategy.
He and his colleagues plan to extend their understanding of the heme machinery in AML with further studies. For example, they don’t know whether heme’s role in cell respiration is the only important one in supporting AML progression, since heme plays a wide range of roles in cells.
The researchers are also planning to test whether drugs that suppress UROD function in the heme-production machinery can effectively battle AML. ![]()
Advanced cancer patients have lower survival after cardiac arrest
A new study suggests patients with advanced cancer who suffer cardiac arrest in the hospital have a survival rate of less than 10%, which is about half the rate of patients without cancer who suffer cardiac arrest.
This finding helps to clear up some myths about cardiac arrest survival and can be used as a guidepost when hospitalized cancer patients and their families consider do-not-resuscitate (DNR) orders, said Jeffrey T. Bruckel, MD, of the University of Rochester Medical Center in New York.
“We’re hopeful that our study, in some way, will help doctors and cancer patients make more informed decisions about the end of life,” Dr Bruckel said. “It’s very important to have early, frank discussions around the goals of care.”
Dr Bruckel and his colleagues published their study in the Journal of Oncology Practice.
The researchers used a US-wide resuscitation registry to evaluate survival after cardiac arrest in patients treated at 369 hospitals.
The study excluded patients with implantable defibrillators and those who were admitted for surgery, emergency room treatment, rehabilitation, or treatment from cardiac catheterization labs or interventional radiology.
Of the 47,157 eligible patients who experienced cardiac arrest, 14% (n=6585) had advanced cancer, including hematologic malignancies.
After cardiac arrest, 57.5% of the advanced cancer patients were resuscitated successfully, as were 63% of non-cancer patients (P<0.001).
After resuscitation, 9.6% of the cancer patients survived to be discharged, compared to 19.2% of non-cancer patients (P<0.001).
When the researchers adjusted their analysis for potential confounders, results were similar. The rate of successful resuscitation was 52.3% in advanced cancer patients and 56.6% in non-cancer patients (P<0.001). The rate of survival to discharge was 7.4% and 13.4%, respectively (P<0.001).
Dr Bruckel said there was no evidence to suggest that patients with advanced cancer received less aggressive resuscitation care.
However, there was a significant difference in the mean duration of resuscitation time among non-survivors with cancer—22.5 minutes—and non-survivors without cancer—24.2 minutes (P<0.001). After adjustment, the mean duration of resuscitation was 22.5 minutes and 24.1 minutes, respectively (P<0.001).
Cancer patients were more likely than those without cancer to sign DNR orders after resuscitation—55.6% and 43%, respectively (P<0.001). Results were similar after adjustment—50.4% and 41.6%, respectively (P<0.001).
Dr Bruckel and his colleagues said there were several limitations to this study, including a lack of detailed data on the types of advanced cancer and cancer treatments being given at the time of cardiac arrest.
Therefore, the next step in advancing this work is to gather data on the types of cancer diagnosis and treatment plans of patients who undergo in-hospital cardiac arrest.
Dr Bruckel also believes it is important to know how patients feel about this data and how both patients and physicians are using this data in decision-making.
“A large component of end-of-life care involves patient and family care decision-making, and a lot of that is driven by the routine discussions that we have,” Dr Bruckel said. “Not every patient is going to want detailed information, but for those that do, it’s important to have it. It’s important to tell them what we know.” ![]()
A new study suggests patients with advanced cancer who suffer cardiac arrest in the hospital have a survival rate of less than 10%, which is about half the rate of patients without cancer who suffer cardiac arrest.
This finding helps to clear up some myths about cardiac arrest survival and can be used as a guidepost when hospitalized cancer patients and their families consider do-not-resuscitate (DNR) orders, said Jeffrey T. Bruckel, MD, of the University of Rochester Medical Center in New York.
“We’re hopeful that our study, in some way, will help doctors and cancer patients make more informed decisions about the end of life,” Dr Bruckel said. “It’s very important to have early, frank discussions around the goals of care.”
Dr Bruckel and his colleagues published their study in the Journal of Oncology Practice.
The researchers used a US-wide resuscitation registry to evaluate survival after cardiac arrest in patients treated at 369 hospitals.
The study excluded patients with implantable defibrillators and those who were admitted for surgery, emergency room treatment, rehabilitation, or treatment from cardiac catheterization labs or interventional radiology.
Of the 47,157 eligible patients who experienced cardiac arrest, 14% (n=6585) had advanced cancer, including hematologic malignancies.
After cardiac arrest, 57.5% of the advanced cancer patients were resuscitated successfully, as were 63% of non-cancer patients (P<0.001).
After resuscitation, 9.6% of the cancer patients survived to be discharged, compared to 19.2% of non-cancer patients (P<0.001).
When the researchers adjusted their analysis for potential confounders, results were similar. The rate of successful resuscitation was 52.3% in advanced cancer patients and 56.6% in non-cancer patients (P<0.001). The rate of survival to discharge was 7.4% and 13.4%, respectively (P<0.001).
Dr Bruckel said there was no evidence to suggest that patients with advanced cancer received less aggressive resuscitation care.
However, there was a significant difference in the mean duration of resuscitation time among non-survivors with cancer—22.5 minutes—and non-survivors without cancer—24.2 minutes (P<0.001). After adjustment, the mean duration of resuscitation was 22.5 minutes and 24.1 minutes, respectively (P<0.001).
Cancer patients were more likely than those without cancer to sign DNR orders after resuscitation—55.6% and 43%, respectively (P<0.001). Results were similar after adjustment—50.4% and 41.6%, respectively (P<0.001).
Dr Bruckel and his colleagues said there were several limitations to this study, including a lack of detailed data on the types of advanced cancer and cancer treatments being given at the time of cardiac arrest.
Therefore, the next step in advancing this work is to gather data on the types of cancer diagnosis and treatment plans of patients who undergo in-hospital cardiac arrest.
Dr Bruckel also believes it is important to know how patients feel about this data and how both patients and physicians are using this data in decision-making.
“A large component of end-of-life care involves patient and family care decision-making, and a lot of that is driven by the routine discussions that we have,” Dr Bruckel said. “Not every patient is going to want detailed information, but for those that do, it’s important to have it. It’s important to tell them what we know.” ![]()
A new study suggests patients with advanced cancer who suffer cardiac arrest in the hospital have a survival rate of less than 10%, which is about half the rate of patients without cancer who suffer cardiac arrest.
This finding helps to clear up some myths about cardiac arrest survival and can be used as a guidepost when hospitalized cancer patients and their families consider do-not-resuscitate (DNR) orders, said Jeffrey T. Bruckel, MD, of the University of Rochester Medical Center in New York.
“We’re hopeful that our study, in some way, will help doctors and cancer patients make more informed decisions about the end of life,” Dr Bruckel said. “It’s very important to have early, frank discussions around the goals of care.”
Dr Bruckel and his colleagues published their study in the Journal of Oncology Practice.
The researchers used a US-wide resuscitation registry to evaluate survival after cardiac arrest in patients treated at 369 hospitals.
The study excluded patients with implantable defibrillators and those who were admitted for surgery, emergency room treatment, rehabilitation, or treatment from cardiac catheterization labs or interventional radiology.
Of the 47,157 eligible patients who experienced cardiac arrest, 14% (n=6585) had advanced cancer, including hematologic malignancies.
After cardiac arrest, 57.5% of the advanced cancer patients were resuscitated successfully, as were 63% of non-cancer patients (P<0.001).
After resuscitation, 9.6% of the cancer patients survived to be discharged, compared to 19.2% of non-cancer patients (P<0.001).
When the researchers adjusted their analysis for potential confounders, results were similar. The rate of successful resuscitation was 52.3% in advanced cancer patients and 56.6% in non-cancer patients (P<0.001). The rate of survival to discharge was 7.4% and 13.4%, respectively (P<0.001).
Dr Bruckel said there was no evidence to suggest that patients with advanced cancer received less aggressive resuscitation care.
However, there was a significant difference in the mean duration of resuscitation time among non-survivors with cancer—22.5 minutes—and non-survivors without cancer—24.2 minutes (P<0.001). After adjustment, the mean duration of resuscitation was 22.5 minutes and 24.1 minutes, respectively (P<0.001).
Cancer patients were more likely than those without cancer to sign DNR orders after resuscitation—55.6% and 43%, respectively (P<0.001). Results were similar after adjustment—50.4% and 41.6%, respectively (P<0.001).
Dr Bruckel and his colleagues said there were several limitations to this study, including a lack of detailed data on the types of advanced cancer and cancer treatments being given at the time of cardiac arrest.
Therefore, the next step in advancing this work is to gather data on the types of cancer diagnosis and treatment plans of patients who undergo in-hospital cardiac arrest.
Dr Bruckel also believes it is important to know how patients feel about this data and how both patients and physicians are using this data in decision-making.
“A large component of end-of-life care involves patient and family care decision-making, and a lot of that is driven by the routine discussions that we have,” Dr Bruckel said. “Not every patient is going to want detailed information, but for those that do, it’s important to have it. It’s important to tell them what we know.” ![]()
Database may provide insight into childhood cancers
A database containing information on more than 11,000 tumors is now available to researchers studying pediatric cancers.
The database was created as part of UC Santa Cruz Genomics Institute’s Treehouse Childhood Cancer Initiative.
The goal of this initiative is to allow researchers to analyze their patients’ data alongside data from thousands of patients with pediatric and adult cancers, including leukemias and lymphomas.
The intention is to help researchers find hidden causes of cancer that may be missed when they analyze a patient’s data in isolation.
The database, which is available at https://treehouse.xenahubs.net, contains RNA-sequencing gene expression data, as well as information on patients’ age, sex, and disease. ![]()
A database containing information on more than 11,000 tumors is now available to researchers studying pediatric cancers.
The database was created as part of UC Santa Cruz Genomics Institute’s Treehouse Childhood Cancer Initiative.
The goal of this initiative is to allow researchers to analyze their patients’ data alongside data from thousands of patients with pediatric and adult cancers, including leukemias and lymphomas.
The intention is to help researchers find hidden causes of cancer that may be missed when they analyze a patient’s data in isolation.
The database, which is available at https://treehouse.xenahubs.net, contains RNA-sequencing gene expression data, as well as information on patients’ age, sex, and disease. ![]()
A database containing information on more than 11,000 tumors is now available to researchers studying pediatric cancers.
The database was created as part of UC Santa Cruz Genomics Institute’s Treehouse Childhood Cancer Initiative.
The goal of this initiative is to allow researchers to analyze their patients’ data alongside data from thousands of patients with pediatric and adult cancers, including leukemias and lymphomas.
The intention is to help researchers find hidden causes of cancer that may be missed when they analyze a patient’s data in isolation.
The database, which is available at https://treehouse.xenahubs.net, contains RNA-sequencing gene expression data, as well as information on patients’ age, sex, and disease. ![]()
Recurrent UTIs in Women: How to Refine Your Care

For the third time in nine months, Joan, 28, presents with complaints of painful, frequent, and urgent urination. Joan is sexually active; her medical history is otherwise unremarkable. In each of the previous two episodes, her urine culture grew Escherichia coli, and she was treated with a five-day course of nitrofurantoin. Now, she asks about the need for additional workup and treatment, as well as whether there is a way to prevent further infections.
Urinary tract infections (UTIs) are the most common bacterial infection in women and account for an estimated 5.4 million primary care office visits and 2.3 million emergency department visits annually.1,2 For women, the lifetime risk for a UTI is greater than 50%.3 In one study of UTI in a primary care setting, 36% of women younger than 55 and 53% of women older than 55 had a recurrent infection within a year.4 Most women with UTI are treated as outpatients, but 16.7% require hospitalization.5 In the United States, direct costs for evaluation and treatment of UTI total $1.6 billion each year.5
Accurately characterizing recurrent UTI
Bacteriuria is defined as the presence of 100,000 colony-forming units (ie, viable bacteria) per milliliter of urine collected midstream on two consecutive urinations.6 UTIs are symptomatic infections of the urinary tract and may involve the urethra, bladder, ureters, or kidneys.7 Infections of the lower tract (bladder and urethra) are commonly referred to as cystitis; infections of the upper tract (kidney and ureters) are referred to as pyelonephritis.
Most UTIs are uncomplicated and do not progress to more serious infections. However, patients who are pregnant or who have chronic medical conditions (eg, renal insufficiency or use of immunosuppressant medications), urinary obstruction, or calculi may develop complicated UTIs.8
Recurrent UTI is an infection that follows resolution of bacteriuria and symptoms of a prior UTI; the term applies when such an infection occurs within six months of the previous UTI or when three or more UTIs occur within a year.7 Recurrent infection can be further characterized as relapse or reinfection. Relapse occurs when the patient has a second UTI caused by the same pathogen within two weeks of the original treatment.9 Reinfection is a UTI that occurs more than two weeks after completion of treatment for the original UTI. The pathogen in a reinfection may be the same one that caused the original UTI or it may be a different agent.9
It’s also important to differentiate between recurrent and resistant UTI. In resistant UTI, bacteriuria fails to resolve following seven to 14 days of appropriate antibiotic treatment.9
FACTORS THAT INCREASE RISK FOR RECURRENT UTI
Premenopausal women
Both modifiable and nonmodifiable factors (see Table 1) have been associated with increased risk for recurrent UTI in premenopausal women.10-21 Among those with specific blood group phenotypes (Lewis non-secretor, in particular), rates of UTI rise secondary to increased adherence of bacteria to epithelial cells in the urinary tract.10 Other nonmodifiable risk factors include congenital urinary tract anomalies, obstruction of the urinary tract, and a history of UTI.11,12 Women whose mothers had UTIs are at higher risk for recurrent UTI than are those whose mothers had no such history.13
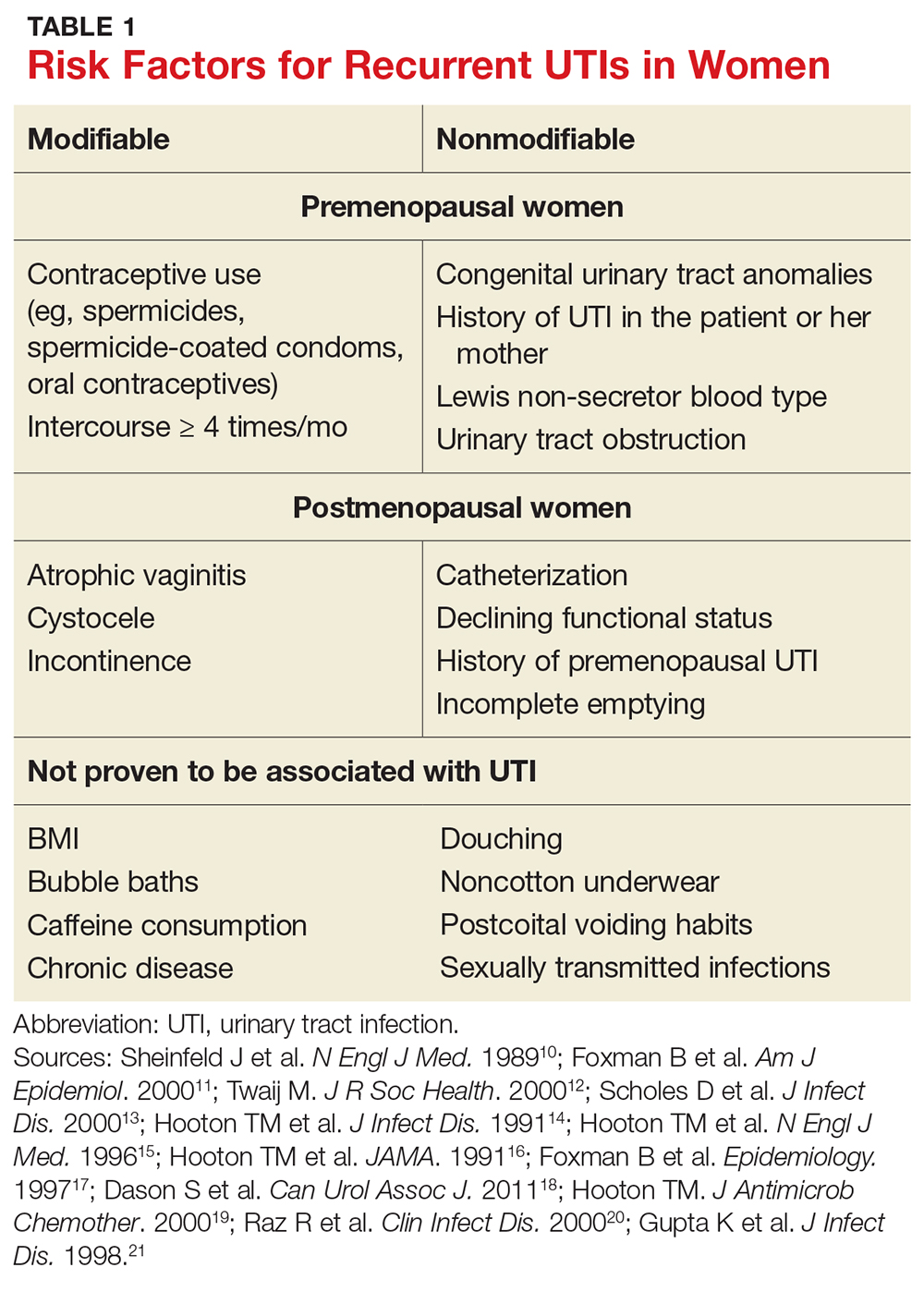
Modifiable risk factors for recurrent UTI include contraceptive use (spermicides, spermicide-coated condoms, and oral contraceptives) and frequency of intercourse (≥ 4 times/month).13 Spermicides alter the normal vaginal flora and lead to increased colonization of E coli, which increases the risk for UTI.14 Women with recurrent UTIs were 1.27 to 1.45 times more likely to use oral contraceptives than those without recurrent UTIs.13 Compared with college women who had not had intercourse, sexually active college women who had engaged in intercourse three times in a week had a 2.6-fold increase in relative risk for UTI.15 Those who had daily intercourse had a 9-fold increase in relative risk for UTI.15This elevated risk is due to trauma to the lower urogenital tract (urethra) and introduction of bacteria into the urethra via mechanical factors.16,17
Postmenopausal women
Atrophic vaginitis, catheterization, declining functional status, cystocele, incomplete emptying, incontinence, and history of premenopausal UTIs are all risk factors for recurrent UTI in postmenopausal women.19,20 Decreased estrogen and resulting vaginal atrophy appear to be associated with increased rates of UTI in these women. Additionally, postmenopausal women’s vaginas are more likely to be colonized with E coli and have fewer lactobacilli than those of premenopausal women, which is thought to predispose them to UTI.21 These risk factors are summarized in Table 1.10-21
INITIAL EVALUATION OF RECURRENT UTI
Patients with recurrent UTI experience signs and symptoms similar to those with isolated uncomplicated UTI: dysuria, frequency, urgency, and hematuria. Focus your history interview on potential causes of complicated UTI (see Table 2).18 Likewise, perform a pelvic exam to evaluate for predisposing anatomic abnormalities.22 Finally, obtain a urine culture with antibiotic sensitivities to ensure that previous treatment was appropriate and to rule out microbes associated with infected uroliths.18 Given the low probability of finding abnormalities on cystoscopy or imaging, neither one is routinely recommended for the evaluation of recurrent UTI.18

TREATMENT OPTIONS AND PRECAUTIONS
As with isolated UTI, E coli is the most common pathogen in recurrent UTI. However, recurrent UTI is more likely than isolated UTI to result from other pathogens (odds ratio [OR], 1.5), such as Klebsiella, Enterococcus, Proteus, and Citrobacter.23 Since a patient’s recurrent UTI most likely arises from the same pathogen that caused the prior infection, start an antibiotic you know is effective against it.8 Additionally, take into account local resistance rates; antibiotic availability, cost, and adverse effects; and a patient’s drug allergies.
Preferred antibiotics. Trimethoprim-sulfamethoxazole (TMP-SMX; 160 mg/800 mg bid for 3 d) has long been the mainstay of treatment for uncomplicated UTI. In recent years, however, resistance to TMP-SMX has increased. While it is still appropriate for many situations as firstline treatment, it is not recommended for empiric treatment if local resistance rates are higher than 20%.24 Nitrofurantoin (100 mg bid for 5 d) has efficacy similar to that of TMP-SMX but without significant bacterial resistance. While fosfomycin (3 g as a single dose) is still recommended as firstline treatment, it is less effective than either TMP-SMX or nitrofurantoin. Table 3 summarizes these antibiotic choices and their efficacies.24
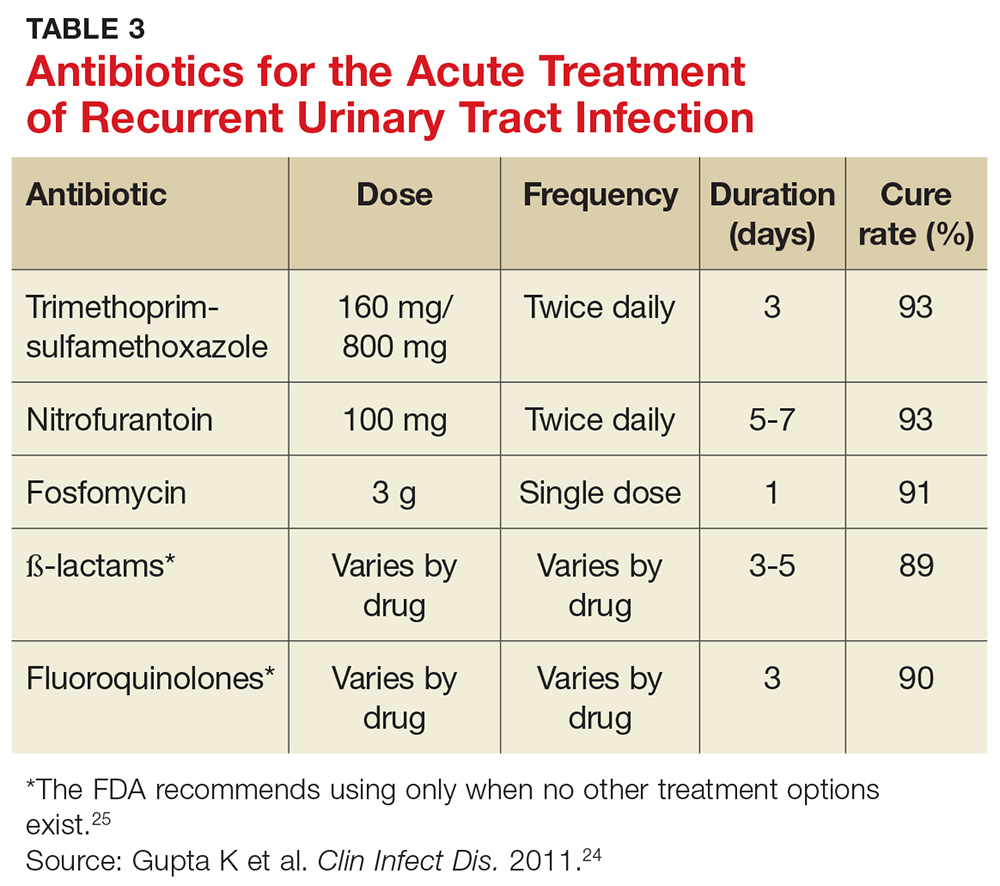
Agents to avoid or use only as a last resort. For patients who are unable to take any of the mentioned drugs, consider ß-lactam antibiotics—although they are typically less effective for this indication. While fluoroquinolones are very effective and have low (but rising) resistance rates, they are also associated with serious and potentially permanent adverse effects. As a result, on May 12, 2016, the FDA issued a Drug Safety Communication recommending that fluoroquinolones be used only in patients without other treatment options.24,25 Do not use ampicillin or amoxicillin, which lack effectiveness for this indication and are compromised by high levels of bacterial resistance.
Shorter course of treatment? When deciding on the length of treatment for recurrent UTI, remember that shorter antibiotic courses (3-5 d) are associated with similar rates of cure and progression to systemic infections as longer courses (7-10 d). Also, patients adhere better to the shorter treatment regimen and experience fewer adverse effects.26,27
Standing prescription? Studies have shown that women know when they have a UTI. Therefore, for those who experience recurrent UTI, consider giving them a standing prescription for antibiotics that they can initiate when symptoms arise (see Table 3).24 Patient-initiated treatment yields similar rates of efficacy as clinician-initiated treatment, while avoiding the adverse effects and costs associated with preventive strategies (see text).28
TIME FOR IMAGING AND REFERRAL?
For patients with a high risk for complicated UTI or a surgically amenable condition, either ultrasound or CT of the abdomen and pelvis with and without contrast is appropriate to evaluate for anatomic anomalies. While CT is the more sensitive imaging study to identify anomalies, ultrasound is less expensive and minimizes radiation exposure and is therefore also appropriate.18
Consider referring patients to a urologist if they have an underlying condition that may be amenable to surgery, such as bladder outlet obstruction, cystoceles, urinary tract diverticula, fistulae, pelvic floor dysfunction, ureteral stricture, urolithiasis, or vesicoureteral reflux.18 Additional risk factors for complicated UTI, which warrant referral as outlined by the Canadian Urologic Association, are summarized in Table 2.18
Two weeks later … and it’s back? Finally, for women who experience recurrent symptoms within two weeks of completing treatment, obtain a urine culture with antibiotic sensitivities to ensure that the infecting organism is not one typically associated with urolithiasis (Proteus and Yersinia) and that it is susceptible to planned antibiotic therapy.18Proteus and Yersinia are urease-positive bacteria that may cause stone formation in the urinary tract system. Evaluate any patient who has a UTI from either organism for urinary tract stones.
PREVENTION DOS AND DON’TS
Popular myth suggests that recurrent UTIs are more common in patients who do not void after intercourse or those who douche, consume caffeinated beverages, or wear noncotton underwear. Research, however, has failed to show a relationship between any of these factors and recurrent UTIs.13,18 Clinicians should therefore stop recommending that patients modify these behaviors to decrease recurrent infections.
Antibiotic prophylaxis decreases the rate of recurrent UTI by 95%.29 It has been recommended for women who have had two or more UTIs in the past six months or three or more UTIs in the past year. 29,30 Effective strategies to prevent recurrent UTI are low-dose continuous antibiotic prophylaxis or postcoital antibiotic prophylaxis.
While a test-of-cure culture is not typically recommended following treatment for uncomplicated UTI, you will want to obtain a confirmatory urine culture one to two weeks before starting low-dose antibiotic prophylaxis. Base your choice of antibiotic on known patient allergies and previous culture results. Agents typically used are trimethoprim, TMP-SMX, or nitrofurantoin (see Table 4), none of which demonstrated superiority in a Cochrane review.31-33 Although the same review showed no optimal duration of treatment, six to 24 months of treatment is usually recommended.29,33
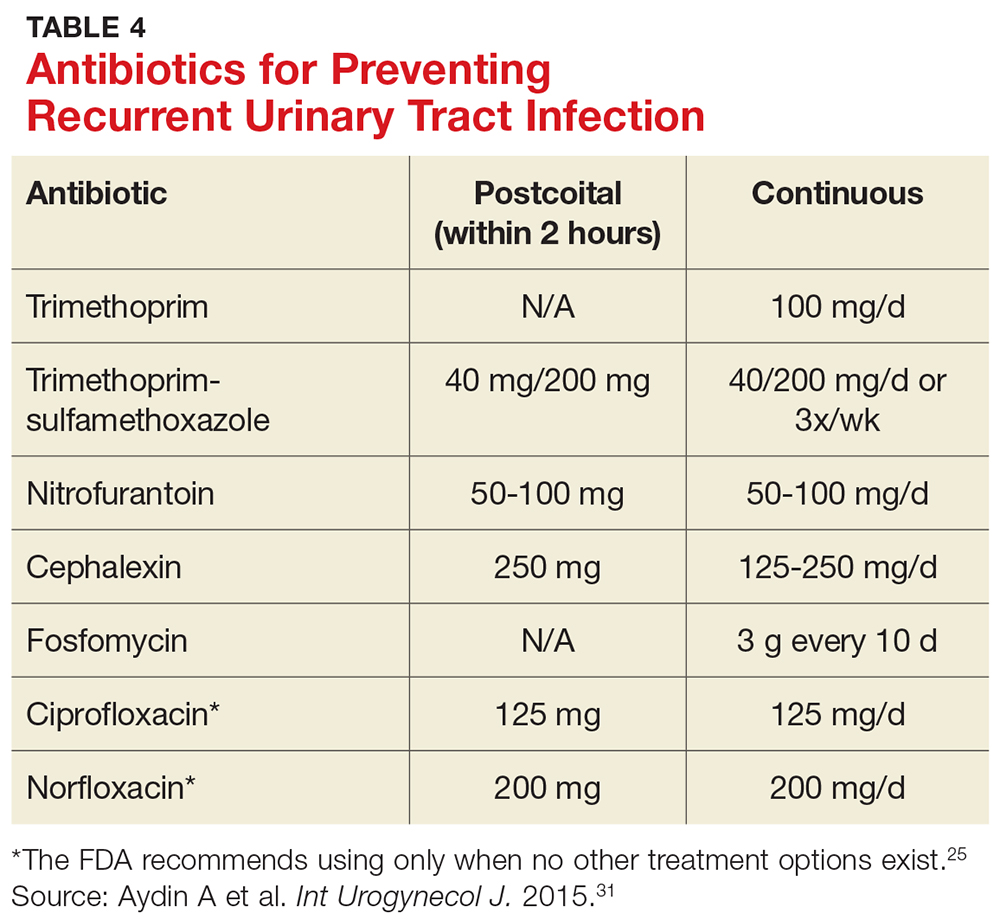
A single dose of antibiotic following intercourse may be as effective as daily low-dose prophylaxis for women whose UTIs are related to sexual activity.34 Studies have shown that single doses of TMP-SMX, nitrofurantoin, cephalexin, or a fluoroquinolone (see earlier notes about the FDA warning on fluoroquinolone use) are similarly effective in decreasing the rate of recurrence (see Table 4).31,35,36
Several nonpharmacologic strategies have been suggested for prevention of recurrent UTI. Among them are use of cranberry products, lactobacillus, vaginal estrogen in postmenopausal women, methenamine salts, and
A 2012 Cochrane review of 24 studies found that cranberry products were less effective in preventing recurrent UTIs than previously thought, with no statistically significant difference between women who took them and those who did not.37
Results have been mixed in using lactobacilli or probiotics to prevent recurrent UTIs. One study examining the use of lactobacilli to colonize the vaginal flora found a reduction in the number of recurrent infections in premenopausal women taking intravaginal lactobacillus over 12 months.38 A second study, involving postmenopausal women, found that those who were randomized to take lactobacillus tablets for 12 months had more frequent recurrences of UTIs than women randomized to take daily TMP-SMX.39 However, this last study was designed as a noninferiority trial, and its results do not negate the prior study’s findings. Additionally, vaginal estrogen, which is thought to work through colonization of the vagina with lactobacilli, has prevented recurrent UTIs in postmenopausal women.40
Ascorbic acid (which is bacteriostatic), methenamine salts (which are hydrolyzed to bactericidal ammonia and formaldehyde), and
As noted, the only behavioral modifications that have been shown to decrease the risk for recurrent UTI are discontinuing the use of spermicides/spermicide-coated condoms or oral contraceptives, and decreasing the frequency of intercourse.13
Joan is started on a three-day course of TMP-SMX. Further questioning reveals that each of her three UTIs followed sexual intercourse. Her clinician discusses the options of self-directed therapy using continuous prophylaxis or postcoital prophylaxis, either of which would be an appropriate evidence-based intervention for her. After engaging in shared decision-making, she is prescribed TMP-SMX to be taken as a single dose following intercourse in the future.
1. Nicolle LE. Epidemiology of urinary tract infections. Infect Med. 2001;18:153-162.
2. CDC. Annual number and percent distribution of ambulatory care visits by setting type according to diagnosis group: United States, 2009-2010. www.cdc.gov/nchs/data/ahcd/combined_tables/2009-2010_combined_web_table01.pdf. Accessed June 8, 2017.
3. Griebling TL. Urologic Diseases in America project: trends in resource use for urinary tract infections in women. J Urol. 2005;173:1281-1287.
4. Ikaheimo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;222:91-99.
5. Sammon JD, Sharma P, Rahbar H, et al. Predictors of admission in patients presenting to the emergency department with urinary tract infection. World J Urol. 2014;32:813-819.
6. Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643-654.
7. Barber AE, Norton JP, Spivak AM, et al. Urinary tract infections: current and emerging management strategies. Clin Infect Dis. 2013;57:719-724.
8. Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
9. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 91: treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111:785-794.
10. Sheinfeld J, Schaeffer AJ, Cordon-Cardo C, et al. Association of the Lewis blood group phenotype with recurrent urinary tract infections in women. N Engl J Med. 1989;320:773-777.
11. Foxman B, Gillespie B, Koopman J, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151:1194-1205.
12. Twaij M. Urinary tract infection in children: a review of its pathogenesis and risk factors. J R Soc Health. 2000;120:220-226.
13. Scholes D, Hooton TM, Roberts DL, et al. Risk factors for recurrent urinary tract infection in young women. J Infect Dis. 2000;182:1177-1182.
14. Hooton TM, Fennell CL, Clark AM, et al. Nonoxynol-9: differential antibacterial activity and enhancement of bacterial adherence to vaginal epithelial cells. J Infect Dis. 1991; 164: 1216-1219.
15. Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med. 1996;335:468-474.
16. Hooton TM, Hillier S, Johnson C, et al. Escherichia coli bacteriuria and contraceptive method. JAMA. 1991;265:64-69.
17. Foxman B, Marsh J, Gillespie B, et al. Condom use and first-time urinary tract infection. Epidemiology. 1997;8:637-641.
18. Dason S, Dason JT, Kapoor A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J. 2011;5:316-322.
19. Hooton TM. Pathogenesis of urinary tract infections: an update. J Antimicrob Chemother. 2000;46(suppl 1):1-7.
20. Raz R, Gennesin Y, Wasser J, et al. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis. 2000; 30:152-156.
21. Gupta K, Stapleton AE, Hooton TM, et al. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli in women with recurrent urinary tract infections. J Infect Dis. 1998;178:446-450.
22. Neal DE. Complicated urinary tract infections. Urol Clin North Am. 2008;35:13-22.
23. Amna MA, Chazan B, Raz R, et al. Risk factors for non-Escherichia coli community-acquired bacteriuria. Infection. 2013;41:473-477.
24. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
25. FDA. FDA drug safety communication. www.fda.gov/downloads/Drugs/DrugSafety/UCM500591.pdf. Accessed June 8, 2017.
26. Katchman EA, Milo G, Paul M, et al. Three-day vs longer duration of antibiotic treatment for cystitis in women: systematic review and meta-analysis. Am J Med. 2005;118:1196-1207.
27. Milo G, Katchman EA, Paul M, et al. Duration of antibacterial treatment for uncomplicated urinary tract infection in women. Cochrane Database Syst Rev. 2005;(2):CD004682.
28. Gupta K, Hooton TM, Roberts PL, et al. Patient-initiated treatment of uncomplicated recurrent urinary tract infections in young women. Ann Intern Med. 2001;135:9-16.
29. Nicolle LE, Ronald AR. Recurrent urinary tract infection in adult women: diagnosis and treatment. Infect Dis Clin North Am. 1987;1:793-806.
30. Ronald AR, Conway B. An approach to urinary tract infections in ambulatory women. Curr Clin Top Infect Dis. 1988; 9:76-125.
31. Aydin A, Ahmed K, Zaman I, et al. Recurrent urinary tract infections in women. Int Urogynecol J. 2015;26:795-804.
32. McLaughlin SP, Carson CC. Urinary tract infections in women. Med Clin North Am. 2004;88:417-429.
33. Albert X, Huertas I, Pereiro II, et al. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev. 2004;(3):CD001209.
34. Melekos MD, Asbach HW, Gerharz E, et al. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157: 935-939.
35. Chew LD, Fihn SD. Recurrent cystitis in nonpregnant women. West J Med. 1999;170:274-277.
36. Stapleton A, Latham RH, Johnson C, et al. Postcoital antimicrobial prophylaxis for recurrent urinary tract infection: A randomized, double-blind, placebo-controlled trial. JAMA. 1990;264:703-706.
37. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012; (10):CD001321.
38. Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52:1212-1217.
39. Beerepoot MA, ter Riet G, Nys S, et al. Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double-blind, noninferiority trial in postmenopausal women. Arch Intern Med. 2012;172:704-712.
40. Perrotta C, Aznar M, Mejia R, et al. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;(2):CD005131.
41. Foxman B, Chi JW. Health behavior and urinary tract infection in college-aged women. J Clin Epidemiol. 1990;43:329-337.
42. Lee BB, Simpson JM, Craig JC, et al. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003265.
43. Krancˇec B, Papeš D, Altarac S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: a randomized clinical trial. World J Urol. 2014;32:79-84.

For the third time in nine months, Joan, 28, presents with complaints of painful, frequent, and urgent urination. Joan is sexually active; her medical history is otherwise unremarkable. In each of the previous two episodes, her urine culture grew Escherichia coli, and she was treated with a five-day course of nitrofurantoin. Now, she asks about the need for additional workup and treatment, as well as whether there is a way to prevent further infections.
Urinary tract infections (UTIs) are the most common bacterial infection in women and account for an estimated 5.4 million primary care office visits and 2.3 million emergency department visits annually.1,2 For women, the lifetime risk for a UTI is greater than 50%.3 In one study of UTI in a primary care setting, 36% of women younger than 55 and 53% of women older than 55 had a recurrent infection within a year.4 Most women with UTI are treated as outpatients, but 16.7% require hospitalization.5 In the United States, direct costs for evaluation and treatment of UTI total $1.6 billion each year.5
Accurately characterizing recurrent UTI
Bacteriuria is defined as the presence of 100,000 colony-forming units (ie, viable bacteria) per milliliter of urine collected midstream on two consecutive urinations.6 UTIs are symptomatic infections of the urinary tract and may involve the urethra, bladder, ureters, or kidneys.7 Infections of the lower tract (bladder and urethra) are commonly referred to as cystitis; infections of the upper tract (kidney and ureters) are referred to as pyelonephritis.
Most UTIs are uncomplicated and do not progress to more serious infections. However, patients who are pregnant or who have chronic medical conditions (eg, renal insufficiency or use of immunosuppressant medications), urinary obstruction, or calculi may develop complicated UTIs.8
Recurrent UTI is an infection that follows resolution of bacteriuria and symptoms of a prior UTI; the term applies when such an infection occurs within six months of the previous UTI or when three or more UTIs occur within a year.7 Recurrent infection can be further characterized as relapse or reinfection. Relapse occurs when the patient has a second UTI caused by the same pathogen within two weeks of the original treatment.9 Reinfection is a UTI that occurs more than two weeks after completion of treatment for the original UTI. The pathogen in a reinfection may be the same one that caused the original UTI or it may be a different agent.9
It’s also important to differentiate between recurrent and resistant UTI. In resistant UTI, bacteriuria fails to resolve following seven to 14 days of appropriate antibiotic treatment.9

FACTORS THAT INCREASE RISK FOR RECURRENT UTI
Premenopausal women
Both modifiable and nonmodifiable factors (see Table 1) have been associated with increased risk for recurrent UTI in premenopausal women.10-21 Among those with specific blood group phenotypes (Lewis non-secretor, in particular), rates of UTI rise secondary to increased adherence of bacteria to epithelial cells in the urinary tract.10 Other nonmodifiable risk factors include congenital urinary tract anomalies, obstruction of the urinary tract, and a history of UTI.11,12 Women whose mothers had UTIs are at higher risk for recurrent UTI than are those whose mothers had no such history.13

Modifiable risk factors for recurrent UTI include contraceptive use (spermicides, spermicide-coated condoms, and oral contraceptives) and frequency of intercourse (≥ 4 times/month).13 Spermicides alter the normal vaginal flora and lead to increased colonization of E coli, which increases the risk for UTI.14 Women with recurrent UTIs were 1.27 to 1.45 times more likely to use oral contraceptives than those without recurrent UTIs.13 Compared with college women who had not had intercourse, sexually active college women who had engaged in intercourse three times in a week had a 2.6-fold increase in relative risk for UTI.15 Those who had daily intercourse had a 9-fold increase in relative risk for UTI.15This elevated risk is due to trauma to the lower urogenital tract (urethra) and introduction of bacteria into the urethra via mechanical factors.16,17
Postmenopausal women
Atrophic vaginitis, catheterization, declining functional status, cystocele, incomplete emptying, incontinence, and history of premenopausal UTIs are all risk factors for recurrent UTI in postmenopausal women.19,20 Decreased estrogen and resulting vaginal atrophy appear to be associated with increased rates of UTI in these women. Additionally, postmenopausal women’s vaginas are more likely to be colonized with E coli and have fewer lactobacilli than those of premenopausal women, which is thought to predispose them to UTI.21 These risk factors are summarized in Table 1.10-21
INITIAL EVALUATION OF RECURRENT UTI
Patients with recurrent UTI experience signs and symptoms similar to those with isolated uncomplicated UTI: dysuria, frequency, urgency, and hematuria. Focus your history interview on potential causes of complicated UTI (see Table 2).18 Likewise, perform a pelvic exam to evaluate for predisposing anatomic abnormalities.22 Finally, obtain a urine culture with antibiotic sensitivities to ensure that previous treatment was appropriate and to rule out microbes associated with infected uroliths.18 Given the low probability of finding abnormalities on cystoscopy or imaging, neither one is routinely recommended for the evaluation of recurrent UTI.18

TREATMENT OPTIONS AND PRECAUTIONS
As with isolated UTI, E coli is the most common pathogen in recurrent UTI. However, recurrent UTI is more likely than isolated UTI to result from other pathogens (odds ratio [OR], 1.5), such as Klebsiella, Enterococcus, Proteus, and Citrobacter.23 Since a patient’s recurrent UTI most likely arises from the same pathogen that caused the prior infection, start an antibiotic you know is effective against it.8 Additionally, take into account local resistance rates; antibiotic availability, cost, and adverse effects; and a patient’s drug allergies.
Preferred antibiotics. Trimethoprim-sulfamethoxazole (TMP-SMX; 160 mg/800 mg bid for 3 d) has long been the mainstay of treatment for uncomplicated UTI. In recent years, however, resistance to TMP-SMX has increased. While it is still appropriate for many situations as firstline treatment, it is not recommended for empiric treatment if local resistance rates are higher than 20%.24 Nitrofurantoin (100 mg bid for 5 d) has efficacy similar to that of TMP-SMX but without significant bacterial resistance. While fosfomycin (3 g as a single dose) is still recommended as firstline treatment, it is less effective than either TMP-SMX or nitrofurantoin. Table 3 summarizes these antibiotic choices and their efficacies.24

Agents to avoid or use only as a last resort. For patients who are unable to take any of the mentioned drugs, consider ß-lactam antibiotics—although they are typically less effective for this indication. While fluoroquinolones are very effective and have low (but rising) resistance rates, they are also associated with serious and potentially permanent adverse effects. As a result, on May 12, 2016, the FDA issued a Drug Safety Communication recommending that fluoroquinolones be used only in patients without other treatment options.24,25 Do not use ampicillin or amoxicillin, which lack effectiveness for this indication and are compromised by high levels of bacterial resistance.
Shorter course of treatment? When deciding on the length of treatment for recurrent UTI, remember that shorter antibiotic courses (3-5 d) are associated with similar rates of cure and progression to systemic infections as longer courses (7-10 d). Also, patients adhere better to the shorter treatment regimen and experience fewer adverse effects.26,27
Standing prescription? Studies have shown that women know when they have a UTI. Therefore, for those who experience recurrent UTI, consider giving them a standing prescription for antibiotics that they can initiate when symptoms arise (see Table 3).24 Patient-initiated treatment yields similar rates of efficacy as clinician-initiated treatment, while avoiding the adverse effects and costs associated with preventive strategies (see text).28
TIME FOR IMAGING AND REFERRAL?
For patients with a high risk for complicated UTI or a surgically amenable condition, either ultrasound or CT of the abdomen and pelvis with and without contrast is appropriate to evaluate for anatomic anomalies. While CT is the more sensitive imaging study to identify anomalies, ultrasound is less expensive and minimizes radiation exposure and is therefore also appropriate.18
Consider referring patients to a urologist if they have an underlying condition that may be amenable to surgery, such as bladder outlet obstruction, cystoceles, urinary tract diverticula, fistulae, pelvic floor dysfunction, ureteral stricture, urolithiasis, or vesicoureteral reflux.18 Additional risk factors for complicated UTI, which warrant referral as outlined by the Canadian Urologic Association, are summarized in Table 2.18
Two weeks later … and it’s back? Finally, for women who experience recurrent symptoms within two weeks of completing treatment, obtain a urine culture with antibiotic sensitivities to ensure that the infecting organism is not one typically associated with urolithiasis (Proteus and Yersinia) and that it is susceptible to planned antibiotic therapy.18Proteus and Yersinia are urease-positive bacteria that may cause stone formation in the urinary tract system. Evaluate any patient who has a UTI from either organism for urinary tract stones.
PREVENTION DOS AND DON’TS
Popular myth suggests that recurrent UTIs are more common in patients who do not void after intercourse or those who douche, consume caffeinated beverages, or wear noncotton underwear. Research, however, has failed to show a relationship between any of these factors and recurrent UTIs.13,18 Clinicians should therefore stop recommending that patients modify these behaviors to decrease recurrent infections.
Antibiotic prophylaxis decreases the rate of recurrent UTI by 95%.29 It has been recommended for women who have had two or more UTIs in the past six months or three or more UTIs in the past year. 29,30 Effective strategies to prevent recurrent UTI are low-dose continuous antibiotic prophylaxis or postcoital antibiotic prophylaxis.
While a test-of-cure culture is not typically recommended following treatment for uncomplicated UTI, you will want to obtain a confirmatory urine culture one to two weeks before starting low-dose antibiotic prophylaxis. Base your choice of antibiotic on known patient allergies and previous culture results. Agents typically used are trimethoprim, TMP-SMX, or nitrofurantoin (see Table 4), none of which demonstrated superiority in a Cochrane review.31-33 Although the same review showed no optimal duration of treatment, six to 24 months of treatment is usually recommended.29,33

A single dose of antibiotic following intercourse may be as effective as daily low-dose prophylaxis for women whose UTIs are related to sexual activity.34 Studies have shown that single doses of TMP-SMX, nitrofurantoin, cephalexin, or a fluoroquinolone (see earlier notes about the FDA warning on fluoroquinolone use) are similarly effective in decreasing the rate of recurrence (see Table 4).31,35,36
Several nonpharmacologic strategies have been suggested for prevention of recurrent UTI. Among them are use of cranberry products, lactobacillus, vaginal estrogen in postmenopausal women, methenamine salts, and
A 2012 Cochrane review of 24 studies found that cranberry products were less effective in preventing recurrent UTIs than previously thought, with no statistically significant difference between women who took them and those who did not.37
Results have been mixed in using lactobacilli or probiotics to prevent recurrent UTIs. One study examining the use of lactobacilli to colonize the vaginal flora found a reduction in the number of recurrent infections in premenopausal women taking intravaginal lactobacillus over 12 months.38 A second study, involving postmenopausal women, found that those who were randomized to take lactobacillus tablets for 12 months had more frequent recurrences of UTIs than women randomized to take daily TMP-SMX.39 However, this last study was designed as a noninferiority trial, and its results do not negate the prior study’s findings. Additionally, vaginal estrogen, which is thought to work through colonization of the vagina with lactobacilli, has prevented recurrent UTIs in postmenopausal women.40
Ascorbic acid (which is bacteriostatic), methenamine salts (which are hydrolyzed to bactericidal ammonia and formaldehyde), and
As noted, the only behavioral modifications that have been shown to decrease the risk for recurrent UTI are discontinuing the use of spermicides/spermicide-coated condoms or oral contraceptives, and decreasing the frequency of intercourse.13
Joan is started on a three-day course of TMP-SMX. Further questioning reveals that each of her three UTIs followed sexual intercourse. Her clinician discusses the options of self-directed therapy using continuous prophylaxis or postcoital prophylaxis, either of which would be an appropriate evidence-based intervention for her. After engaging in shared decision-making, she is prescribed TMP-SMX to be taken as a single dose following intercourse in the future.

For the third time in nine months, Joan, 28, presents with complaints of painful, frequent, and urgent urination. Joan is sexually active; her medical history is otherwise unremarkable. In each of the previous two episodes, her urine culture grew Escherichia coli, and she was treated with a five-day course of nitrofurantoin. Now, she asks about the need for additional workup and treatment, as well as whether there is a way to prevent further infections.
Urinary tract infections (UTIs) are the most common bacterial infection in women and account for an estimated 5.4 million primary care office visits and 2.3 million emergency department visits annually.1,2 For women, the lifetime risk for a UTI is greater than 50%.3 In one study of UTI in a primary care setting, 36% of women younger than 55 and 53% of women older than 55 had a recurrent infection within a year.4 Most women with UTI are treated as outpatients, but 16.7% require hospitalization.5 In the United States, direct costs for evaluation and treatment of UTI total $1.6 billion each year.5
Accurately characterizing recurrent UTI
Bacteriuria is defined as the presence of 100,000 colony-forming units (ie, viable bacteria) per milliliter of urine collected midstream on two consecutive urinations.6 UTIs are symptomatic infections of the urinary tract and may involve the urethra, bladder, ureters, or kidneys.7 Infections of the lower tract (bladder and urethra) are commonly referred to as cystitis; infections of the upper tract (kidney and ureters) are referred to as pyelonephritis.
Most UTIs are uncomplicated and do not progress to more serious infections. However, patients who are pregnant or who have chronic medical conditions (eg, renal insufficiency or use of immunosuppressant medications), urinary obstruction, or calculi may develop complicated UTIs.8
Recurrent UTI is an infection that follows resolution of bacteriuria and symptoms of a prior UTI; the term applies when such an infection occurs within six months of the previous UTI or when three or more UTIs occur within a year.7 Recurrent infection can be further characterized as relapse or reinfection. Relapse occurs when the patient has a second UTI caused by the same pathogen within two weeks of the original treatment.9 Reinfection is a UTI that occurs more than two weeks after completion of treatment for the original UTI. The pathogen in a reinfection may be the same one that caused the original UTI or it may be a different agent.9
It’s also important to differentiate between recurrent and resistant UTI. In resistant UTI, bacteriuria fails to resolve following seven to 14 days of appropriate antibiotic treatment.9

FACTORS THAT INCREASE RISK FOR RECURRENT UTI
Premenopausal women
Both modifiable and nonmodifiable factors (see Table 1) have been associated with increased risk for recurrent UTI in premenopausal women.10-21 Among those with specific blood group phenotypes (Lewis non-secretor, in particular), rates of UTI rise secondary to increased adherence of bacteria to epithelial cells in the urinary tract.10 Other nonmodifiable risk factors include congenital urinary tract anomalies, obstruction of the urinary tract, and a history of UTI.11,12 Women whose mothers had UTIs are at higher risk for recurrent UTI than are those whose mothers had no such history.13

Modifiable risk factors for recurrent UTI include contraceptive use (spermicides, spermicide-coated condoms, and oral contraceptives) and frequency of intercourse (≥ 4 times/month).13 Spermicides alter the normal vaginal flora and lead to increased colonization of E coli, which increases the risk for UTI.14 Women with recurrent UTIs were 1.27 to 1.45 times more likely to use oral contraceptives than those without recurrent UTIs.13 Compared with college women who had not had intercourse, sexually active college women who had engaged in intercourse three times in a week had a 2.6-fold increase in relative risk for UTI.15 Those who had daily intercourse had a 9-fold increase in relative risk for UTI.15This elevated risk is due to trauma to the lower urogenital tract (urethra) and introduction of bacteria into the urethra via mechanical factors.16,17
Postmenopausal women
Atrophic vaginitis, catheterization, declining functional status, cystocele, incomplete emptying, incontinence, and history of premenopausal UTIs are all risk factors for recurrent UTI in postmenopausal women.19,20 Decreased estrogen and resulting vaginal atrophy appear to be associated with increased rates of UTI in these women. Additionally, postmenopausal women’s vaginas are more likely to be colonized with E coli and have fewer lactobacilli than those of premenopausal women, which is thought to predispose them to UTI.21 These risk factors are summarized in Table 1.10-21
INITIAL EVALUATION OF RECURRENT UTI
Patients with recurrent UTI experience signs and symptoms similar to those with isolated uncomplicated UTI: dysuria, frequency, urgency, and hematuria. Focus your history interview on potential causes of complicated UTI (see Table 2).18 Likewise, perform a pelvic exam to evaluate for predisposing anatomic abnormalities.22 Finally, obtain a urine culture with antibiotic sensitivities to ensure that previous treatment was appropriate and to rule out microbes associated with infected uroliths.18 Given the low probability of finding abnormalities on cystoscopy or imaging, neither one is routinely recommended for the evaluation of recurrent UTI.18

TREATMENT OPTIONS AND PRECAUTIONS
As with isolated UTI, E coli is the most common pathogen in recurrent UTI. However, recurrent UTI is more likely than isolated UTI to result from other pathogens (odds ratio [OR], 1.5), such as Klebsiella, Enterococcus, Proteus, and Citrobacter.23 Since a patient’s recurrent UTI most likely arises from the same pathogen that caused the prior infection, start an antibiotic you know is effective against it.8 Additionally, take into account local resistance rates; antibiotic availability, cost, and adverse effects; and a patient’s drug allergies.
Preferred antibiotics. Trimethoprim-sulfamethoxazole (TMP-SMX; 160 mg/800 mg bid for 3 d) has long been the mainstay of treatment for uncomplicated UTI. In recent years, however, resistance to TMP-SMX has increased. While it is still appropriate for many situations as firstline treatment, it is not recommended for empiric treatment if local resistance rates are higher than 20%.24 Nitrofurantoin (100 mg bid for 5 d) has efficacy similar to that of TMP-SMX but without significant bacterial resistance. While fosfomycin (3 g as a single dose) is still recommended as firstline treatment, it is less effective than either TMP-SMX or nitrofurantoin. Table 3 summarizes these antibiotic choices and their efficacies.24

Agents to avoid or use only as a last resort. For patients who are unable to take any of the mentioned drugs, consider ß-lactam antibiotics—although they are typically less effective for this indication. While fluoroquinolones are very effective and have low (but rising) resistance rates, they are also associated with serious and potentially permanent adverse effects. As a result, on May 12, 2016, the FDA issued a Drug Safety Communication recommending that fluoroquinolones be used only in patients without other treatment options.24,25 Do not use ampicillin or amoxicillin, which lack effectiveness for this indication and are compromised by high levels of bacterial resistance.
Shorter course of treatment? When deciding on the length of treatment for recurrent UTI, remember that shorter antibiotic courses (3-5 d) are associated with similar rates of cure and progression to systemic infections as longer courses (7-10 d). Also, patients adhere better to the shorter treatment regimen and experience fewer adverse effects.26,27
Standing prescription? Studies have shown that women know when they have a UTI. Therefore, for those who experience recurrent UTI, consider giving them a standing prescription for antibiotics that they can initiate when symptoms arise (see Table 3).24 Patient-initiated treatment yields similar rates of efficacy as clinician-initiated treatment, while avoiding the adverse effects and costs associated with preventive strategies (see text).28
TIME FOR IMAGING AND REFERRAL?
For patients with a high risk for complicated UTI or a surgically amenable condition, either ultrasound or CT of the abdomen and pelvis with and without contrast is appropriate to evaluate for anatomic anomalies. While CT is the more sensitive imaging study to identify anomalies, ultrasound is less expensive and minimizes radiation exposure and is therefore also appropriate.18
Consider referring patients to a urologist if they have an underlying condition that may be amenable to surgery, such as bladder outlet obstruction, cystoceles, urinary tract diverticula, fistulae, pelvic floor dysfunction, ureteral stricture, urolithiasis, or vesicoureteral reflux.18 Additional risk factors for complicated UTI, which warrant referral as outlined by the Canadian Urologic Association, are summarized in Table 2.18
Two weeks later … and it’s back? Finally, for women who experience recurrent symptoms within two weeks of completing treatment, obtain a urine culture with antibiotic sensitivities to ensure that the infecting organism is not one typically associated with urolithiasis (Proteus and Yersinia) and that it is susceptible to planned antibiotic therapy.18Proteus and Yersinia are urease-positive bacteria that may cause stone formation in the urinary tract system. Evaluate any patient who has a UTI from either organism for urinary tract stones.
PREVENTION DOS AND DON’TS
Popular myth suggests that recurrent UTIs are more common in patients who do not void after intercourse or those who douche, consume caffeinated beverages, or wear noncotton underwear. Research, however, has failed to show a relationship between any of these factors and recurrent UTIs.13,18 Clinicians should therefore stop recommending that patients modify these behaviors to decrease recurrent infections.
Antibiotic prophylaxis decreases the rate of recurrent UTI by 95%.29 It has been recommended for women who have had two or more UTIs in the past six months or three or more UTIs in the past year. 29,30 Effective strategies to prevent recurrent UTI are low-dose continuous antibiotic prophylaxis or postcoital antibiotic prophylaxis.
While a test-of-cure culture is not typically recommended following treatment for uncomplicated UTI, you will want to obtain a confirmatory urine culture one to two weeks before starting low-dose antibiotic prophylaxis. Base your choice of antibiotic on known patient allergies and previous culture results. Agents typically used are trimethoprim, TMP-SMX, or nitrofurantoin (see Table 4), none of which demonstrated superiority in a Cochrane review.31-33 Although the same review showed no optimal duration of treatment, six to 24 months of treatment is usually recommended.29,33

A single dose of antibiotic following intercourse may be as effective as daily low-dose prophylaxis for women whose UTIs are related to sexual activity.34 Studies have shown that single doses of TMP-SMX, nitrofurantoin, cephalexin, or a fluoroquinolone (see earlier notes about the FDA warning on fluoroquinolone use) are similarly effective in decreasing the rate of recurrence (see Table 4).31,35,36
Several nonpharmacologic strategies have been suggested for prevention of recurrent UTI. Among them are use of cranberry products, lactobacillus, vaginal estrogen in postmenopausal women, methenamine salts, and
A 2012 Cochrane review of 24 studies found that cranberry products were less effective in preventing recurrent UTIs than previously thought, with no statistically significant difference between women who took them and those who did not.37
Results have been mixed in using lactobacilli or probiotics to prevent recurrent UTIs. One study examining the use of lactobacilli to colonize the vaginal flora found a reduction in the number of recurrent infections in premenopausal women taking intravaginal lactobacillus over 12 months.38 A second study, involving postmenopausal women, found that those who were randomized to take lactobacillus tablets for 12 months had more frequent recurrences of UTIs than women randomized to take daily TMP-SMX.39 However, this last study was designed as a noninferiority trial, and its results do not negate the prior study’s findings. Additionally, vaginal estrogen, which is thought to work through colonization of the vagina with lactobacilli, has prevented recurrent UTIs in postmenopausal women.40
Ascorbic acid (which is bacteriostatic), methenamine salts (which are hydrolyzed to bactericidal ammonia and formaldehyde), and
As noted, the only behavioral modifications that have been shown to decrease the risk for recurrent UTI are discontinuing the use of spermicides/spermicide-coated condoms or oral contraceptives, and decreasing the frequency of intercourse.13
Joan is started on a three-day course of TMP-SMX. Further questioning reveals that each of her three UTIs followed sexual intercourse. Her clinician discusses the options of self-directed therapy using continuous prophylaxis or postcoital prophylaxis, either of which would be an appropriate evidence-based intervention for her. After engaging in shared decision-making, she is prescribed TMP-SMX to be taken as a single dose following intercourse in the future.
1. Nicolle LE. Epidemiology of urinary tract infections. Infect Med. 2001;18:153-162.
2. CDC. Annual number and percent distribution of ambulatory care visits by setting type according to diagnosis group: United States, 2009-2010. www.cdc.gov/nchs/data/ahcd/combined_tables/2009-2010_combined_web_table01.pdf. Accessed June 8, 2017.
3. Griebling TL. Urologic Diseases in America project: trends in resource use for urinary tract infections in women. J Urol. 2005;173:1281-1287.
4. Ikaheimo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;222:91-99.
5. Sammon JD, Sharma P, Rahbar H, et al. Predictors of admission in patients presenting to the emergency department with urinary tract infection. World J Urol. 2014;32:813-819.
6. Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643-654.
7. Barber AE, Norton JP, Spivak AM, et al. Urinary tract infections: current and emerging management strategies. Clin Infect Dis. 2013;57:719-724.
8. Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
9. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 91: treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111:785-794.
10. Sheinfeld J, Schaeffer AJ, Cordon-Cardo C, et al. Association of the Lewis blood group phenotype with recurrent urinary tract infections in women. N Engl J Med. 1989;320:773-777.
11. Foxman B, Gillespie B, Koopman J, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151:1194-1205.
12. Twaij M. Urinary tract infection in children: a review of its pathogenesis and risk factors. J R Soc Health. 2000;120:220-226.
13. Scholes D, Hooton TM, Roberts DL, et al. Risk factors for recurrent urinary tract infection in young women. J Infect Dis. 2000;182:1177-1182.
14. Hooton TM, Fennell CL, Clark AM, et al. Nonoxynol-9: differential antibacterial activity and enhancement of bacterial adherence to vaginal epithelial cells. J Infect Dis. 1991; 164: 1216-1219.
15. Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med. 1996;335:468-474.
16. Hooton TM, Hillier S, Johnson C, et al. Escherichia coli bacteriuria and contraceptive method. JAMA. 1991;265:64-69.
17. Foxman B, Marsh J, Gillespie B, et al. Condom use and first-time urinary tract infection. Epidemiology. 1997;8:637-641.
18. Dason S, Dason JT, Kapoor A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J. 2011;5:316-322.
19. Hooton TM. Pathogenesis of urinary tract infections: an update. J Antimicrob Chemother. 2000;46(suppl 1):1-7.
20. Raz R, Gennesin Y, Wasser J, et al. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis. 2000; 30:152-156.
21. Gupta K, Stapleton AE, Hooton TM, et al. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli in women with recurrent urinary tract infections. J Infect Dis. 1998;178:446-450.
22. Neal DE. Complicated urinary tract infections. Urol Clin North Am. 2008;35:13-22.
23. Amna MA, Chazan B, Raz R, et al. Risk factors for non-Escherichia coli community-acquired bacteriuria. Infection. 2013;41:473-477.
24. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
25. FDA. FDA drug safety communication. www.fda.gov/downloads/Drugs/DrugSafety/UCM500591.pdf. Accessed June 8, 2017.
26. Katchman EA, Milo G, Paul M, et al. Three-day vs longer duration of antibiotic treatment for cystitis in women: systematic review and meta-analysis. Am J Med. 2005;118:1196-1207.
27. Milo G, Katchman EA, Paul M, et al. Duration of antibacterial treatment for uncomplicated urinary tract infection in women. Cochrane Database Syst Rev. 2005;(2):CD004682.
28. Gupta K, Hooton TM, Roberts PL, et al. Patient-initiated treatment of uncomplicated recurrent urinary tract infections in young women. Ann Intern Med. 2001;135:9-16.
29. Nicolle LE, Ronald AR. Recurrent urinary tract infection in adult women: diagnosis and treatment. Infect Dis Clin North Am. 1987;1:793-806.
30. Ronald AR, Conway B. An approach to urinary tract infections in ambulatory women. Curr Clin Top Infect Dis. 1988; 9:76-125.
31. Aydin A, Ahmed K, Zaman I, et al. Recurrent urinary tract infections in women. Int Urogynecol J. 2015;26:795-804.
32. McLaughlin SP, Carson CC. Urinary tract infections in women. Med Clin North Am. 2004;88:417-429.
33. Albert X, Huertas I, Pereiro II, et al. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev. 2004;(3):CD001209.
34. Melekos MD, Asbach HW, Gerharz E, et al. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157: 935-939.
35. Chew LD, Fihn SD. Recurrent cystitis in nonpregnant women. West J Med. 1999;170:274-277.
36. Stapleton A, Latham RH, Johnson C, et al. Postcoital antimicrobial prophylaxis for recurrent urinary tract infection: A randomized, double-blind, placebo-controlled trial. JAMA. 1990;264:703-706.
37. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012; (10):CD001321.
38. Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52:1212-1217.
39. Beerepoot MA, ter Riet G, Nys S, et al. Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double-blind, noninferiority trial in postmenopausal women. Arch Intern Med. 2012;172:704-712.
40. Perrotta C, Aznar M, Mejia R, et al. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;(2):CD005131.
41. Foxman B, Chi JW. Health behavior and urinary tract infection in college-aged women. J Clin Epidemiol. 1990;43:329-337.
42. Lee BB, Simpson JM, Craig JC, et al. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003265.
43. Krancˇec B, Papeš D, Altarac S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: a randomized clinical trial. World J Urol. 2014;32:79-84.
1. Nicolle LE. Epidemiology of urinary tract infections. Infect Med. 2001;18:153-162.
2. CDC. Annual number and percent distribution of ambulatory care visits by setting type according to diagnosis group: United States, 2009-2010. www.cdc.gov/nchs/data/ahcd/combined_tables/2009-2010_combined_web_table01.pdf. Accessed June 8, 2017.
3. Griebling TL. Urologic Diseases in America project: trends in resource use for urinary tract infections in women. J Urol. 2005;173:1281-1287.
4. Ikaheimo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;222:91-99.
5. Sammon JD, Sharma P, Rahbar H, et al. Predictors of admission in patients presenting to the emergency department with urinary tract infection. World J Urol. 2014;32:813-819.
6. Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643-654.
7. Barber AE, Norton JP, Spivak AM, et al. Urinary tract infections: current and emerging management strategies. Clin Infect Dis. 2013;57:719-724.
8. Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
9. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 91: treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111:785-794.
10. Sheinfeld J, Schaeffer AJ, Cordon-Cardo C, et al. Association of the Lewis blood group phenotype with recurrent urinary tract infections in women. N Engl J Med. 1989;320:773-777.
11. Foxman B, Gillespie B, Koopman J, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151:1194-1205.
12. Twaij M. Urinary tract infection in children: a review of its pathogenesis and risk factors. J R Soc Health. 2000;120:220-226.
13. Scholes D, Hooton TM, Roberts DL, et al. Risk factors for recurrent urinary tract infection in young women. J Infect Dis. 2000;182:1177-1182.
14. Hooton TM, Fennell CL, Clark AM, et al. Nonoxynol-9: differential antibacterial activity and enhancement of bacterial adherence to vaginal epithelial cells. J Infect Dis. 1991; 164: 1216-1219.
15. Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med. 1996;335:468-474.
16. Hooton TM, Hillier S, Johnson C, et al. Escherichia coli bacteriuria and contraceptive method. JAMA. 1991;265:64-69.
17. Foxman B, Marsh J, Gillespie B, et al. Condom use and first-time urinary tract infection. Epidemiology. 1997;8:637-641.
18. Dason S, Dason JT, Kapoor A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J. 2011;5:316-322.
19. Hooton TM. Pathogenesis of urinary tract infections: an update. J Antimicrob Chemother. 2000;46(suppl 1):1-7.
20. Raz R, Gennesin Y, Wasser J, et al. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis. 2000; 30:152-156.
21. Gupta K, Stapleton AE, Hooton TM, et al. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli in women with recurrent urinary tract infections. J Infect Dis. 1998;178:446-450.
22. Neal DE. Complicated urinary tract infections. Urol Clin North Am. 2008;35:13-22.
23. Amna MA, Chazan B, Raz R, et al. Risk factors for non-Escherichia coli community-acquired bacteriuria. Infection. 2013;41:473-477.
24. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
25. FDA. FDA drug safety communication. www.fda.gov/downloads/Drugs/DrugSafety/UCM500591.pdf. Accessed June 8, 2017.
26. Katchman EA, Milo G, Paul M, et al. Three-day vs longer duration of antibiotic treatment for cystitis in women: systematic review and meta-analysis. Am J Med. 2005;118:1196-1207.
27. Milo G, Katchman EA, Paul M, et al. Duration of antibacterial treatment for uncomplicated urinary tract infection in women. Cochrane Database Syst Rev. 2005;(2):CD004682.
28. Gupta K, Hooton TM, Roberts PL, et al. Patient-initiated treatment of uncomplicated recurrent urinary tract infections in young women. Ann Intern Med. 2001;135:9-16.
29. Nicolle LE, Ronald AR. Recurrent urinary tract infection in adult women: diagnosis and treatment. Infect Dis Clin North Am. 1987;1:793-806.
30. Ronald AR, Conway B. An approach to urinary tract infections in ambulatory women. Curr Clin Top Infect Dis. 1988; 9:76-125.
31. Aydin A, Ahmed K, Zaman I, et al. Recurrent urinary tract infections in women. Int Urogynecol J. 2015;26:795-804.
32. McLaughlin SP, Carson CC. Urinary tract infections in women. Med Clin North Am. 2004;88:417-429.
33. Albert X, Huertas I, Pereiro II, et al. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev. 2004;(3):CD001209.
34. Melekos MD, Asbach HW, Gerharz E, et al. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157: 935-939.
35. Chew LD, Fihn SD. Recurrent cystitis in nonpregnant women. West J Med. 1999;170:274-277.
36. Stapleton A, Latham RH, Johnson C, et al. Postcoital antimicrobial prophylaxis for recurrent urinary tract infection: A randomized, double-blind, placebo-controlled trial. JAMA. 1990;264:703-706.
37. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012; (10):CD001321.
38. Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52:1212-1217.
39. Beerepoot MA, ter Riet G, Nys S, et al. Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double-blind, noninferiority trial in postmenopausal women. Arch Intern Med. 2012;172:704-712.
40. Perrotta C, Aznar M, Mejia R, et al. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;(2):CD005131.
41. Foxman B, Chi JW. Health behavior and urinary tract infection in college-aged women. J Clin Epidemiol. 1990;43:329-337.
42. Lee BB, Simpson JM, Craig JC, et al. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003265.
43. Krancˇec B, Papeš D, Altarac S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: a randomized clinical trial. World J Urol. 2014;32:79-84.
E-cigarettes: A health threat or cessation tool?
DENVER –
“So far, the evidence regarding e-cigarettes’ effectiveness for smoking cessation is equivocal at best,” Alison Breland, PhD, said at the annual meeting of the Teratology Society.
But Dr. Breland noted that there is significant controversy around this topic. “I can tell you that, at the conferences I go to, where there are lots of people studying nicotine and tobacco, scientists are fighting with each other over this question,” said Dr. Breland, a psychologist and project director at the Center for the Study of Tobacco Products at Virginia Commonwealth University in Richmond.
That being said, she noted that this meta-analysis has generated unusually harsh printed comments from its critics.
“We could argue about the methodology of the studies all day. If you think all the studies are garbage then you won’t believe the odds ratio, either. But I think right now the evidence shows that e-cigarettes don’t seem to help people quit,” she said. “That may change in the future with testing of different kinds of devices.”
To be useful for smoking cessation, she explained, a device would need to consistently deliver enough nicotine to enable the smoker to fend off withdrawal symptoms but not so much that the wish to quit evaporates. It’s a matter of finding the sweet spot in what is technically termed device nicotine flux.
There is a great deal of misconception about e-cigarettes, Dr. Breland said, some of it promoted through misleading product advertising. She sought to set the record straight.
How e-cigarettes work
What are e-cigarettes? They are basically nicotine delivery devices. They use electricity to power a heating element that aerosolizes a liquid containing varying concentrations of nicotine; solvents, such as propylene glycol and vegetable glycerins; and flavorants. As a class, e-cigarettes are rapidly evolving. A vast array of devices are marketed with wide differences in design, materials, construction, amount of nicotine delivered, and electrical power – which, along with puff duration, is a key factor in how much nicotine gets into a user’s blood.
“Most of the devices have a battery, but it’s important to know that some of them can be plugged directly into a USB port on a computer,” Dr. Breland said.
E-cigarettes don’t generate a vapor, as is widely believed. It’s an aerosol, and it contains toxic byproducts. On the plus side, unlike combustible cigarettes, e-cigarettes don’t deliver carbon monoxide.
A vast array of flavorant mixtures are sold, including some that are clearly designed to be attractive to children, with names like “blue cotton candy” and “Apple Jacks.”
User demographics
Who is using e-cigarettes? Primarily adolescents and young adults in prime reproductive age. National surveys indicate e-cigarettes are now the most widely used tobacco product among U.S. high school students, well ahead of combustible cigarettes.
Of particular concern, data from the Centers for Disease Control and Prevention’s National Health Interview Survey indicate that, among 18- to 24-year-olds who use e-cigarettes, about 40% also currently use conventional cigarettes, about 20% are former cigarette smokers, and about 40% are never smokers – that is, have never smoked combustible cigarettes (MMWR Morb Mortal Wkly Rep. 2016;65:1177. doi: 10.15585/mmwr.mm6542a7).
“We don’t know what’s going to happen to these never smokers who are currently using e-cigarettes. Are they starting on a lifetime of nicotine dependence via e-cigarettes, or perhaps even worse, are they going to transition to combustible cigarettes? There’s more and more evidence showing that’s happening,” Dr. Breland said.
The CDC survey also showed that 59% of adult users of e-cigarettes are what Dr. Breland called “dualies,” individuals who also smoke conventional cigarettes.
“That really diminishes any potential benefit of e-cigarettes,” she said.
Impact on pregnancy
What is known about the impact of e-cigarettes on pregnancy and birth outcomes? Almost nothing at this point. E-cigarettes deliver nicotine to the bloodstream, and nicotine is known to cause unwelcome, long-term changes in fetal brain development and in that of adolescents as well. The other aerosolized toxicants have not been well studied. A few small surveys conducted in obstetric practices indicate some pregnant women perceive e-cigarettes as posing only minor health risks and safer than combustible cigarettes. And some pregnant women are using e-cigarettes.
“I think it’s notable that we’re not finding exclusive e-cigarette users. It’s early in the study, but so far the dual users are smoking the same number of cigarettes per day as cigarette-only users, and they have the same expired carbon monoxide levels. It makes me feel concerned in particular about dual use in pregnancy,” she said.
Regulation
One audience member asked what the point of allowing e-cigarettes is since, under a best-case scenario, their effectiveness as a smoking cessation tool is similar to a nicotine patch, and smokers already have access to the patch as well as nicotine gum.
Dr. Breland replied that the patch and gum deliver nicotine very slowly, so they are not as satisfying as smoking.
“The hope with e-cigarettes is that, since they get nicotine into your blood pretty fast – similar to a cigarette – they can more effectively suppress your withdrawal,” she said. “Whether or not that’s true isn’t known yet.”
The Food and Drug Administration has the authority to regulate e-cigarettes through several different mechanisms but, in late July 2017, announced a delay in issuing new regulations that would likely have removed many of the devices and flavorings from the marketplace.
Dr. Breland’s research is supported by the National Institute on Drug Abuse and the Food and Drug Administration. She reported having no financial conflicts of interest.
DENVER –
“So far, the evidence regarding e-cigarettes’ effectiveness for smoking cessation is equivocal at best,” Alison Breland, PhD, said at the annual meeting of the Teratology Society.
But Dr. Breland noted that there is significant controversy around this topic. “I can tell you that, at the conferences I go to, where there are lots of people studying nicotine and tobacco, scientists are fighting with each other over this question,” said Dr. Breland, a psychologist and project director at the Center for the Study of Tobacco Products at Virginia Commonwealth University in Richmond.
That being said, she noted that this meta-analysis has generated unusually harsh printed comments from its critics.
“We could argue about the methodology of the studies all day. If you think all the studies are garbage then you won’t believe the odds ratio, either. But I think right now the evidence shows that e-cigarettes don’t seem to help people quit,” she said. “That may change in the future with testing of different kinds of devices.”
To be useful for smoking cessation, she explained, a device would need to consistently deliver enough nicotine to enable the smoker to fend off withdrawal symptoms but not so much that the wish to quit evaporates. It’s a matter of finding the sweet spot in what is technically termed device nicotine flux.
There is a great deal of misconception about e-cigarettes, Dr. Breland said, some of it promoted through misleading product advertising. She sought to set the record straight.
How e-cigarettes work
What are e-cigarettes? They are basically nicotine delivery devices. They use electricity to power a heating element that aerosolizes a liquid containing varying concentrations of nicotine; solvents, such as propylene glycol and vegetable glycerins; and flavorants. As a class, e-cigarettes are rapidly evolving. A vast array of devices are marketed with wide differences in design, materials, construction, amount of nicotine delivered, and electrical power – which, along with puff duration, is a key factor in how much nicotine gets into a user’s blood.
“Most of the devices have a battery, but it’s important to know that some of them can be plugged directly into a USB port on a computer,” Dr. Breland said.
E-cigarettes don’t generate a vapor, as is widely believed. It’s an aerosol, and it contains toxic byproducts. On the plus side, unlike combustible cigarettes, e-cigarettes don’t deliver carbon monoxide.
A vast array of flavorant mixtures are sold, including some that are clearly designed to be attractive to children, with names like “blue cotton candy” and “Apple Jacks.”
User demographics
Who is using e-cigarettes? Primarily adolescents and young adults in prime reproductive age. National surveys indicate e-cigarettes are now the most widely used tobacco product among U.S. high school students, well ahead of combustible cigarettes.
Of particular concern, data from the Centers for Disease Control and Prevention’s National Health Interview Survey indicate that, among 18- to 24-year-olds who use e-cigarettes, about 40% also currently use conventional cigarettes, about 20% are former cigarette smokers, and about 40% are never smokers – that is, have never smoked combustible cigarettes (MMWR Morb Mortal Wkly Rep. 2016;65:1177. doi: 10.15585/mmwr.mm6542a7).
“We don’t know what’s going to happen to these never smokers who are currently using e-cigarettes. Are they starting on a lifetime of nicotine dependence via e-cigarettes, or perhaps even worse, are they going to transition to combustible cigarettes? There’s more and more evidence showing that’s happening,” Dr. Breland said.
The CDC survey also showed that 59% of adult users of e-cigarettes are what Dr. Breland called “dualies,” individuals who also smoke conventional cigarettes.
“That really diminishes any potential benefit of e-cigarettes,” she said.
Impact on pregnancy
What is known about the impact of e-cigarettes on pregnancy and birth outcomes? Almost nothing at this point. E-cigarettes deliver nicotine to the bloodstream, and nicotine is known to cause unwelcome, long-term changes in fetal brain development and in that of adolescents as well. The other aerosolized toxicants have not been well studied. A few small surveys conducted in obstetric practices indicate some pregnant women perceive e-cigarettes as posing only minor health risks and safer than combustible cigarettes. And some pregnant women are using e-cigarettes.
“I think it’s notable that we’re not finding exclusive e-cigarette users. It’s early in the study, but so far the dual users are smoking the same number of cigarettes per day as cigarette-only users, and they have the same expired carbon monoxide levels. It makes me feel concerned in particular about dual use in pregnancy,” she said.
Regulation
One audience member asked what the point of allowing e-cigarettes is since, under a best-case scenario, their effectiveness as a smoking cessation tool is similar to a nicotine patch, and smokers already have access to the patch as well as nicotine gum.
Dr. Breland replied that the patch and gum deliver nicotine very slowly, so they are not as satisfying as smoking.
“The hope with e-cigarettes is that, since they get nicotine into your blood pretty fast – similar to a cigarette – they can more effectively suppress your withdrawal,” she said. “Whether or not that’s true isn’t known yet.”
The Food and Drug Administration has the authority to regulate e-cigarettes through several different mechanisms but, in late July 2017, announced a delay in issuing new regulations that would likely have removed many of the devices and flavorings from the marketplace.
Dr. Breland’s research is supported by the National Institute on Drug Abuse and the Food and Drug Administration. She reported having no financial conflicts of interest.
DENVER –
“So far, the evidence regarding e-cigarettes’ effectiveness for smoking cessation is equivocal at best,” Alison Breland, PhD, said at the annual meeting of the Teratology Society.
But Dr. Breland noted that there is significant controversy around this topic. “I can tell you that, at the conferences I go to, where there are lots of people studying nicotine and tobacco, scientists are fighting with each other over this question,” said Dr. Breland, a psychologist and project director at the Center for the Study of Tobacco Products at Virginia Commonwealth University in Richmond.
That being said, she noted that this meta-analysis has generated unusually harsh printed comments from its critics.
“We could argue about the methodology of the studies all day. If you think all the studies are garbage then you won’t believe the odds ratio, either. But I think right now the evidence shows that e-cigarettes don’t seem to help people quit,” she said. “That may change in the future with testing of different kinds of devices.”
To be useful for smoking cessation, she explained, a device would need to consistently deliver enough nicotine to enable the smoker to fend off withdrawal symptoms but not so much that the wish to quit evaporates. It’s a matter of finding the sweet spot in what is technically termed device nicotine flux.
There is a great deal of misconception about e-cigarettes, Dr. Breland said, some of it promoted through misleading product advertising. She sought to set the record straight.
How e-cigarettes work
What are e-cigarettes? They are basically nicotine delivery devices. They use electricity to power a heating element that aerosolizes a liquid containing varying concentrations of nicotine; solvents, such as propylene glycol and vegetable glycerins; and flavorants. As a class, e-cigarettes are rapidly evolving. A vast array of devices are marketed with wide differences in design, materials, construction, amount of nicotine delivered, and electrical power – which, along with puff duration, is a key factor in how much nicotine gets into a user’s blood.
“Most of the devices have a battery, but it’s important to know that some of them can be plugged directly into a USB port on a computer,” Dr. Breland said.
E-cigarettes don’t generate a vapor, as is widely believed. It’s an aerosol, and it contains toxic byproducts. On the plus side, unlike combustible cigarettes, e-cigarettes don’t deliver carbon monoxide.
A vast array of flavorant mixtures are sold, including some that are clearly designed to be attractive to children, with names like “blue cotton candy” and “Apple Jacks.”
User demographics
Who is using e-cigarettes? Primarily adolescents and young adults in prime reproductive age. National surveys indicate e-cigarettes are now the most widely used tobacco product among U.S. high school students, well ahead of combustible cigarettes.
Of particular concern, data from the Centers for Disease Control and Prevention’s National Health Interview Survey indicate that, among 18- to 24-year-olds who use e-cigarettes, about 40% also currently use conventional cigarettes, about 20% are former cigarette smokers, and about 40% are never smokers – that is, have never smoked combustible cigarettes (MMWR Morb Mortal Wkly Rep. 2016;65:1177. doi: 10.15585/mmwr.mm6542a7).
“We don’t know what’s going to happen to these never smokers who are currently using e-cigarettes. Are they starting on a lifetime of nicotine dependence via e-cigarettes, or perhaps even worse, are they going to transition to combustible cigarettes? There’s more and more evidence showing that’s happening,” Dr. Breland said.
The CDC survey also showed that 59% of adult users of e-cigarettes are what Dr. Breland called “dualies,” individuals who also smoke conventional cigarettes.
“That really diminishes any potential benefit of e-cigarettes,” she said.
Impact on pregnancy
What is known about the impact of e-cigarettes on pregnancy and birth outcomes? Almost nothing at this point. E-cigarettes deliver nicotine to the bloodstream, and nicotine is known to cause unwelcome, long-term changes in fetal brain development and in that of adolescents as well. The other aerosolized toxicants have not been well studied. A few small surveys conducted in obstetric practices indicate some pregnant women perceive e-cigarettes as posing only minor health risks and safer than combustible cigarettes. And some pregnant women are using e-cigarettes.
“I think it’s notable that we’re not finding exclusive e-cigarette users. It’s early in the study, but so far the dual users are smoking the same number of cigarettes per day as cigarette-only users, and they have the same expired carbon monoxide levels. It makes me feel concerned in particular about dual use in pregnancy,” she said.
Regulation
One audience member asked what the point of allowing e-cigarettes is since, under a best-case scenario, their effectiveness as a smoking cessation tool is similar to a nicotine patch, and smokers already have access to the patch as well as nicotine gum.
Dr. Breland replied that the patch and gum deliver nicotine very slowly, so they are not as satisfying as smoking.
“The hope with e-cigarettes is that, since they get nicotine into your blood pretty fast – similar to a cigarette – they can more effectively suppress your withdrawal,” she said. “Whether or not that’s true isn’t known yet.”
The Food and Drug Administration has the authority to regulate e-cigarettes through several different mechanisms but, in late July 2017, announced a delay in issuing new regulations that would likely have removed many of the devices and flavorings from the marketplace.
Dr. Breland’s research is supported by the National Institute on Drug Abuse and the Food and Drug Administration. She reported having no financial conflicts of interest.
EXPERT ANALYSIS FROM TERATOLOGY SOCIETY 2017
FDA extends Liletta IUD duration of use to 4 years
The Food and Drug Administration has approved a supplemental New Drug Application to extend the duration of use for Liletta (levonorgestrel-releasing intrauterine system) 52 mg, for up to 4 years.
The approval, issued Aug. 3, adds 1 year to the duration of use on the drug label. It is based on additional efficacy and safety data from ACCESS IUS (A Comprehensive Contraceptive Efficacy & Safety Study of an Intrauterine System), an ongoing phase 3 trial with 1,751 U.S. women.
There are three other levonorgestrel-releasing IUDs currently on the market: Mirena and Kyleena, which are both approved for up to 5 years of use; and Skyla, which is approved for up to 3 years of use.
[email protected]
On Twitter @maryellenny
The Food and Drug Administration has approved a supplemental New Drug Application to extend the duration of use for Liletta (levonorgestrel-releasing intrauterine system) 52 mg, for up to 4 years.
The approval, issued Aug. 3, adds 1 year to the duration of use on the drug label. It is based on additional efficacy and safety data from ACCESS IUS (A Comprehensive Contraceptive Efficacy & Safety Study of an Intrauterine System), an ongoing phase 3 trial with 1,751 U.S. women.
There are three other levonorgestrel-releasing IUDs currently on the market: Mirena and Kyleena, which are both approved for up to 5 years of use; and Skyla, which is approved for up to 3 years of use.
[email protected]
On Twitter @maryellenny
The Food and Drug Administration has approved a supplemental New Drug Application to extend the duration of use for Liletta (levonorgestrel-releasing intrauterine system) 52 mg, for up to 4 years.
The approval, issued Aug. 3, adds 1 year to the duration of use on the drug label. It is based on additional efficacy and safety data from ACCESS IUS (A Comprehensive Contraceptive Efficacy & Safety Study of an Intrauterine System), an ongoing phase 3 trial with 1,751 U.S. women.
There are three other levonorgestrel-releasing IUDs currently on the market: Mirena and Kyleena, which are both approved for up to 5 years of use; and Skyla, which is approved for up to 3 years of use.
[email protected]
On Twitter @maryellenny