User login
Stevens-Johnson Syndrome Secondary to Isolated Albuterol Use
To the Editor:
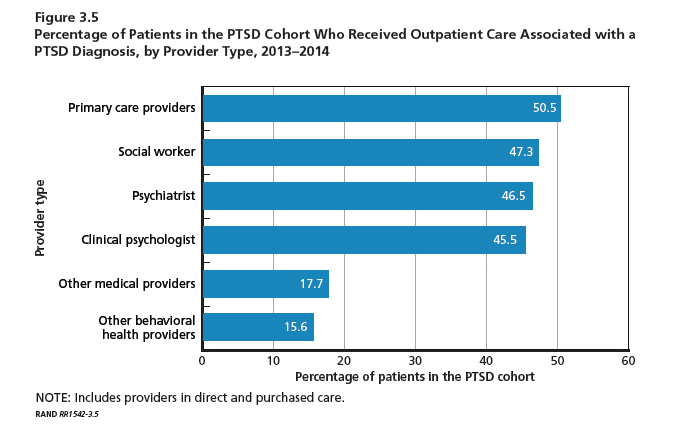
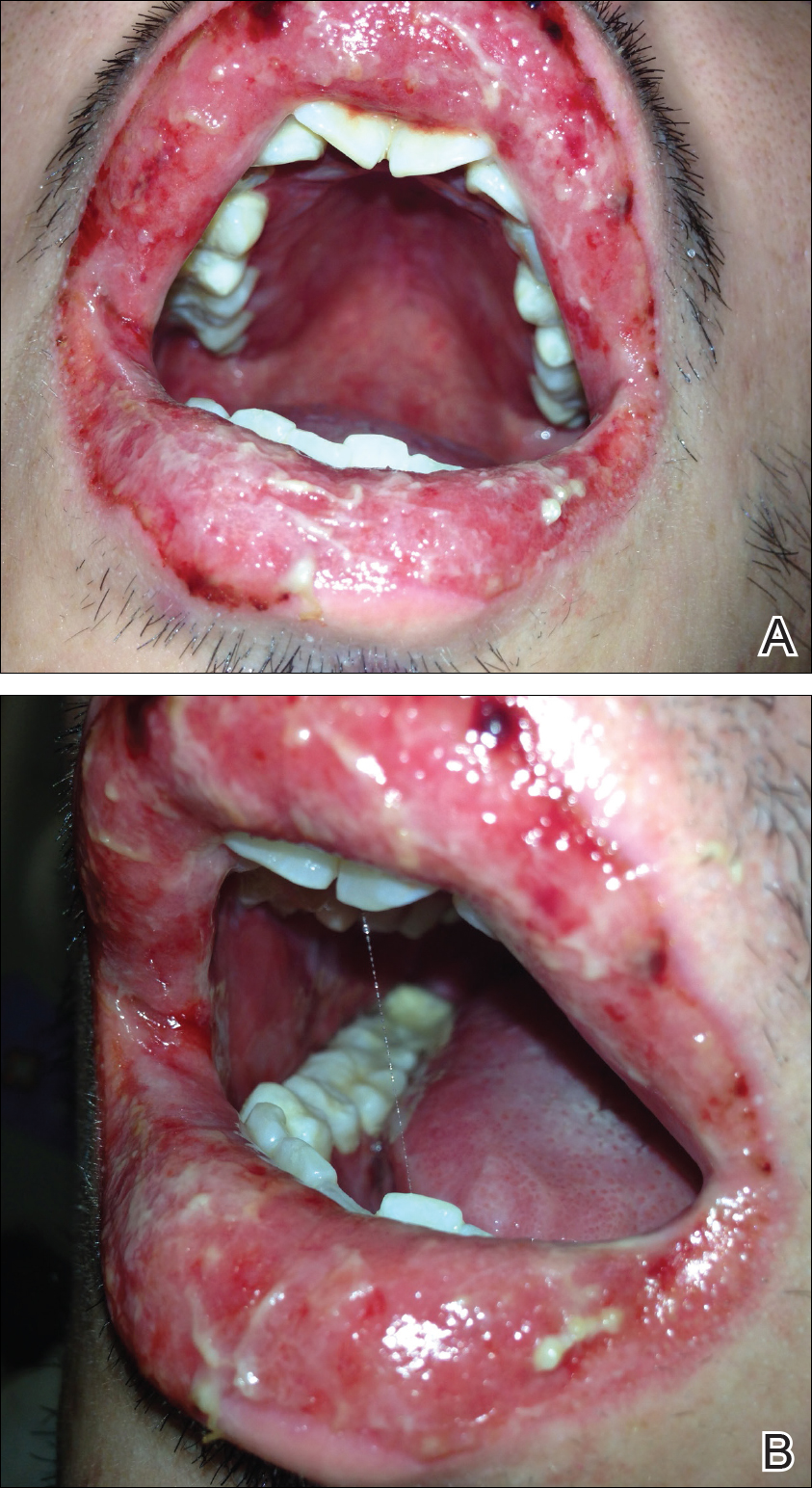
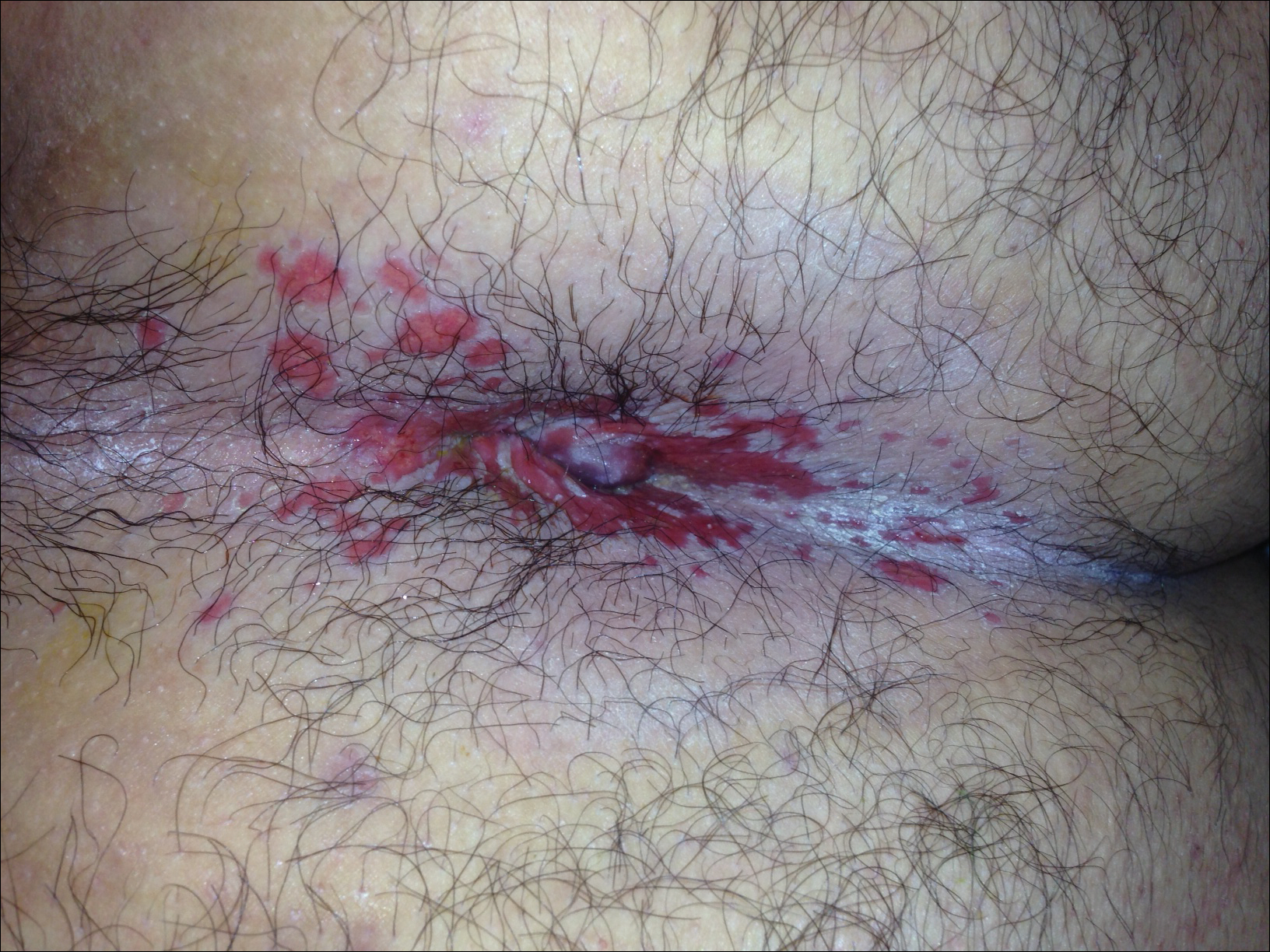
A 22-year-old obese man with untreated mild asthma diagnosed in childhood presented to the emergency department with cheilitis (Figure 1); conjunctivitis; and painful desquamation of the oral mucosa, penis (Figure 2), and perirectal area (Figure 3). Physical examination was notable for palpebral conjunctiva; mucosal involvement with stomatitis (Figure 1B); and isolated 0.5- to 2-cm erosions and ulcerations with positive Nikolsky sign of the scrotum (Figure 2), trunk, back, and arms and legs. Some areas had evidence of hemorrhagic crust, flaccid bullae, and denudation. Few scant targetoid lesions and dusky red macules on the trunk, face, palms, and soles also were present.

One week prior to presentation he had an episode of diarrhea and dyspnea with symptoms of mild heat stroke after working outdoors, and he self-treated with ibuprofen, which he had taken intermittently for years. He was subsequently seen at an outpatient clinic and was prescribed an albuterol inhaler for previously untreated childhood asthma. The patient stated that he inhaled 2 puffs every 6 hours for a total of 3 treatments. Shortly after the last dose, he noticed a tingling sensation of the oral mucosa that developed into a painful 2-cm bullous ulcer. Over the next 3 days, he developed several more oral ulcers and erosions. Three days before admission he developed dysuria and tense bullae at the glans penis. After admission, he developed cheilitis, conjunctivitis, dysuria, odynophagia, and dysphagia to solids. One day after admission, the patient had the onset of systemic symptoms, including cough with worsening dyspnea, fever, chills, hemoptysis, epistaxis, nausea, diarrhea, loss of appetite, joint pain, and myalgia. Review of systems was otherwise negative. A radiograph was performed at admission and was notable for mild atelectasis but was otherwise normal. The chest radiograph was negative for signs of perihilar lymphadenopathy, pleural effusion, pneumothorax, or lobar infiltrates suggestive of bacterial pneumonia. It also did not show signs of patchy opacities or air bronchograms suggestive of an interstitial pneumonia. On admission, he was started on acyclovir, fluconazole, methylprednisolone, nystatin, pantoprazole, acetaminophen, topical bacitracin, oxycodone, and topical silver nitrate.
At the time of admission our patient was afebrile with a normal heart rate, blood pressure, and respiratory rate. However, he was hypoxic, with a pulse oximetry of 86% on room air and 94% on 40% fraction of inspired oxygen. Complete blood cell count, electrolytes, and liver function tests were all within reference range. Urinalysis revealed evidence of scant red blood cells without pyuria, and the erythrocyte sedimentation rate and creatine kinase level were both elevated. Two blood cultures; sputum cultures; and polymerase chain reaction for Mycoplasma pneumoniae, herpes simplex virus, varicella-zoster virus, cytomegalovirus, and Epstein-Barr virus were negative. Human immunodeficiency virus panel, antinuclear antibody screen, and hepatitis B and C panels were all negative. Four punch biopsies were obtained showing full-thickness epidermal necrosis with neutrophils, few dyskeratotic cells, and sparse inflammatory infiltrate compatible with Stevens-Johnson syndrome (SJS).
After hospital admission, the patient’s mucosal desquamation progressively improved. By day 3, he required minimal supplemental oxygen with resolution of bowel symptoms and improved mucosal and skin findings. He was discharged on day 4 with supplemental oxygen and a 7-day course of prednisone, fluconazole, liquid oxycodone, pantoprazole, and acetaminophen. He showed continued improvement at a follow-up outpatient visit 2 days following discharge.
Stevens-Johnson syndrome is a rare severe drug reaction characterized by high fevers, mucosal erosions, tenderness, and skin detachment approximately 1 to 3 weeks after an inciting event.1,2 Although SJS has been linked to infections and less commonly to immunizations, in more than 80% of cases, SJS is strongly associated with a recent medication change.3 The classes of drugs that have been implicated in SJS most commonly include antibiotics, anticonvulsants, and nonsteroidal anti-inflammatory drugs.4 Stevens-Johnson syndrome from drug reactions is not uncommon; however, SJS secondary to isolated albuterol use is rare.
Although it is presumed that albuterol was the key inciting factor in our patient’s case of SJS, it also is recognized that mucosal SJS can be associated with M pneumoniae infection. For this reason, we performed polymerase chain reaction for M pneumoniae as well as a chest radiograph to rule out this possibility. In addition, our patient had denied prolonged respiratory symptoms suggestive of a mycoplasma pneumonia infection, such as a prodrome of cough, myalgia, headache, sore throat, or fever. A report of 8 patients with documented SJS and M pneumoniae as well as a review of the literature also demonstrated a mean of 10 days of prodromal symptoms prior to the onset of mucosal lesions and/or a rash.5 However, mucosal SJS associated with mycoplasma pneumonia is an important clinical entity that should not be forgotten during the workup of a young patient with mucosal lesions or rash suggestive of SJS.
The exact etiology and mechanism of drug-induced SJS is not well understood at this time; however, evidence suggests that SJS is strongly linked to the host’s inability to detoxify drug metabolites.6,7 It has been postulated that SJS occurs secondary to a cell-mediated immune response, which activates cytotoxic T lymphocytes and subsequently results in keratinocyte apoptosis. Keratinocyte apoptosis occurs via the CD95-CD95 death receptor and soluble or membrane-bound ligand interaction.3,8,9 Stevens-Johnson syndrome is thought to occur from an interaction involving an HLA antigen–restricted presentation of drug metabolites to cytotoxic T cells, which can be further supported by evidence of strong genetic associations with HLA antigen alleles B15.02 and B58.01 in the cases of carbamazepine- and allopurinol-induced SJS, respectively.6,7 However, the genetic associations of specific HLA antigen alleles and polymorphisms with SJS and other cutaneous reactions is thought to be drug specific and HLA antigen subtype specific.7 Therefore, it is difficult to determine or correlate the clinical outcomes and manifestations of drug reactions in individualized patients. The precise mechanism of antigenicity of albuterol in initiating this cascade has not yet been determined. However, these investigations provide strong evidence for a correlation between specific HLA antigen haplotypes and occurrence of drug antigenicity resulting in SJS and other cutaneous reactions in susceptible patient populations.
Although the specific molecular pathway and etiology of SJS is not well delineated, pathology in combination with clinical correlation allows for diagnosis. Early-stage biopsies in SJS typically show apoptotic keratinocytes throughout the epidermis. Late-stage biopsies exhibit subepidermal blisters and full-thickness epidermal necrosis.1 Histopathology was performed on 4-mm punch biopsies of the chest and back and demonstrated full-thickness epidermal necrosis with neutrophils and a few dyskeratotic cells, likely representing a late stage of epidermal involvement. Given the predominance of neutrophils, other diagnostic considerations based solely on the biopsy results included contact dermatitis or phototoxic dermatitis. The remaining inflammatory infiltrate was sparse. Immunofluorescence was pan-negative.
This report illustrates a rare case of SJS from isolated albuterol use. This adverse drug reaction has not been well reported in the literature and may be an important consideration in the management of a patient with asthma.
- Stern RS. Clinical practice. exanthematous drug eruptions. N Engl J Med. 2012;366:2492-2501.
- Tartarone A, Lerose R. Stevens-Johnson syndrome and toxic epidermal necrolysis: what do we know? Ther Drug Monit. 2010;32:669-672.
- Ferrandiz-Pulido C, Garcia-Patos V. A review of causes of Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Arch Dis Child. 2013;98:998-1003.
- Mockenhaupt M, Viboud C, Dunant A, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. the EuroSCAR-study. J Invest Dermatol. 2008;128:35-44.
- Levy M, Shear NH. Mycoplasma pneumoniae infections and Stevens-Johnson syndrome. report of eight cases and review of the literature. Clin Pediatr (Phila). 1991;30:42-49.
- Chung WH, Hung SI. Genetic markers and danger signals in Stevens-Johnson syndrome and toxic epidermal necrolysis [published online October 25, 2010]. Allergol Int. 2010;59:325-332.
- Chung WH, Hung SI. Recent advances in the genetics and immunology of Stevens-Johnson syndrome and toxic epidermal necrosis. J Dermatol Sci. 2012;66:190-196.
- Bharadwaj M, Illing P, Theodossis A, et al. Drug hypersensitivity and human leukocyte antigens of the major histocompatibility complex. Annu Rev Pharmacol Toxicol. 2012;52:401-431.
- Chessman D, Kostenko L, Lethborg T, et al. Human leukocyte antigen class I-restricted activation of CD8+ T cells provides the immunogenetic basis of a systemic drug hypersensitivity. Immunity. 2008;28:822-832.
To the Editor:
A 22-year-old obese man with untreated mild asthma diagnosed in childhood presented to the emergency department with cheilitis (Figure 1); conjunctivitis; and painful desquamation of the oral mucosa, penis (Figure 2), and perirectal area (Figure 3). Physical examination was notable for palpebral conjunctiva; mucosal involvement with stomatitis (Figure 1B); and isolated 0.5- to 2-cm erosions and ulcerations with positive Nikolsky sign of the scrotum (Figure 2), trunk, back, and arms and legs. Some areas had evidence of hemorrhagic crust, flaccid bullae, and denudation. Few scant targetoid lesions and dusky red macules on the trunk, face, palms, and soles also were present.



One week prior to presentation he had an episode of diarrhea and dyspnea with symptoms of mild heat stroke after working outdoors, and he self-treated with ibuprofen, which he had taken intermittently for years. He was subsequently seen at an outpatient clinic and was prescribed an albuterol inhaler for previously untreated childhood asthma. The patient stated that he inhaled 2 puffs every 6 hours for a total of 3 treatments. Shortly after the last dose, he noticed a tingling sensation of the oral mucosa that developed into a painful 2-cm bullous ulcer. Over the next 3 days, he developed several more oral ulcers and erosions. Three days before admission he developed dysuria and tense bullae at the glans penis. After admission, he developed cheilitis, conjunctivitis, dysuria, odynophagia, and dysphagia to solids. One day after admission, the patient had the onset of systemic symptoms, including cough with worsening dyspnea, fever, chills, hemoptysis, epistaxis, nausea, diarrhea, loss of appetite, joint pain, and myalgia. Review of systems was otherwise negative. A radiograph was performed at admission and was notable for mild atelectasis but was otherwise normal. The chest radiograph was negative for signs of perihilar lymphadenopathy, pleural effusion, pneumothorax, or lobar infiltrates suggestive of bacterial pneumonia. It also did not show signs of patchy opacities or air bronchograms suggestive of an interstitial pneumonia. On admission, he was started on acyclovir, fluconazole, methylprednisolone, nystatin, pantoprazole, acetaminophen, topical bacitracin, oxycodone, and topical silver nitrate.
At the time of admission our patient was afebrile with a normal heart rate, blood pressure, and respiratory rate. However, he was hypoxic, with a pulse oximetry of 86% on room air and 94% on 40% fraction of inspired oxygen. Complete blood cell count, electrolytes, and liver function tests were all within reference range. Urinalysis revealed evidence of scant red blood cells without pyuria, and the erythrocyte sedimentation rate and creatine kinase level were both elevated. Two blood cultures; sputum cultures; and polymerase chain reaction for Mycoplasma pneumoniae, herpes simplex virus, varicella-zoster virus, cytomegalovirus, and Epstein-Barr virus were negative. Human immunodeficiency virus panel, antinuclear antibody screen, and hepatitis B and C panels were all negative. Four punch biopsies were obtained showing full-thickness epidermal necrosis with neutrophils, few dyskeratotic cells, and sparse inflammatory infiltrate compatible with Stevens-Johnson syndrome (SJS).
After hospital admission, the patient’s mucosal desquamation progressively improved. By day 3, he required minimal supplemental oxygen with resolution of bowel symptoms and improved mucosal and skin findings. He was discharged on day 4 with supplemental oxygen and a 7-day course of prednisone, fluconazole, liquid oxycodone, pantoprazole, and acetaminophen. He showed continued improvement at a follow-up outpatient visit 2 days following discharge.
Stevens-Johnson syndrome is a rare severe drug reaction characterized by high fevers, mucosal erosions, tenderness, and skin detachment approximately 1 to 3 weeks after an inciting event.1,2 Although SJS has been linked to infections and less commonly to immunizations, in more than 80% of cases, SJS is strongly associated with a recent medication change.3 The classes of drugs that have been implicated in SJS most commonly include antibiotics, anticonvulsants, and nonsteroidal anti-inflammatory drugs.4 Stevens-Johnson syndrome from drug reactions is not uncommon; however, SJS secondary to isolated albuterol use is rare.
Although it is presumed that albuterol was the key inciting factor in our patient’s case of SJS, it also is recognized that mucosal SJS can be associated with M pneumoniae infection. For this reason, we performed polymerase chain reaction for M pneumoniae as well as a chest radiograph to rule out this possibility. In addition, our patient had denied prolonged respiratory symptoms suggestive of a mycoplasma pneumonia infection, such as a prodrome of cough, myalgia, headache, sore throat, or fever. A report of 8 patients with documented SJS and M pneumoniae as well as a review of the literature also demonstrated a mean of 10 days of prodromal symptoms prior to the onset of mucosal lesions and/or a rash.5 However, mucosal SJS associated with mycoplasma pneumonia is an important clinical entity that should not be forgotten during the workup of a young patient with mucosal lesions or rash suggestive of SJS.
The exact etiology and mechanism of drug-induced SJS is not well understood at this time; however, evidence suggests that SJS is strongly linked to the host’s inability to detoxify drug metabolites.6,7 It has been postulated that SJS occurs secondary to a cell-mediated immune response, which activates cytotoxic T lymphocytes and subsequently results in keratinocyte apoptosis. Keratinocyte apoptosis occurs via the CD95-CD95 death receptor and soluble or membrane-bound ligand interaction.3,8,9 Stevens-Johnson syndrome is thought to occur from an interaction involving an HLA antigen–restricted presentation of drug metabolites to cytotoxic T cells, which can be further supported by evidence of strong genetic associations with HLA antigen alleles B15.02 and B58.01 in the cases of carbamazepine- and allopurinol-induced SJS, respectively.6,7 However, the genetic associations of specific HLA antigen alleles and polymorphisms with SJS and other cutaneous reactions is thought to be drug specific and HLA antigen subtype specific.7 Therefore, it is difficult to determine or correlate the clinical outcomes and manifestations of drug reactions in individualized patients. The precise mechanism of antigenicity of albuterol in initiating this cascade has not yet been determined. However, these investigations provide strong evidence for a correlation between specific HLA antigen haplotypes and occurrence of drug antigenicity resulting in SJS and other cutaneous reactions in susceptible patient populations.
Although the specific molecular pathway and etiology of SJS is not well delineated, pathology in combination with clinical correlation allows for diagnosis. Early-stage biopsies in SJS typically show apoptotic keratinocytes throughout the epidermis. Late-stage biopsies exhibit subepidermal blisters and full-thickness epidermal necrosis.1 Histopathology was performed on 4-mm punch biopsies of the chest and back and demonstrated full-thickness epidermal necrosis with neutrophils and a few dyskeratotic cells, likely representing a late stage of epidermal involvement. Given the predominance of neutrophils, other diagnostic considerations based solely on the biopsy results included contact dermatitis or phototoxic dermatitis. The remaining inflammatory infiltrate was sparse. Immunofluorescence was pan-negative.
This report illustrates a rare case of SJS from isolated albuterol use. This adverse drug reaction has not been well reported in the literature and may be an important consideration in the management of a patient with asthma.
To the Editor:
A 22-year-old obese man with untreated mild asthma diagnosed in childhood presented to the emergency department with cheilitis (Figure 1); conjunctivitis; and painful desquamation of the oral mucosa, penis (Figure 2), and perirectal area (Figure 3). Physical examination was notable for palpebral conjunctiva; mucosal involvement with stomatitis (Figure 1B); and isolated 0.5- to 2-cm erosions and ulcerations with positive Nikolsky sign of the scrotum (Figure 2), trunk, back, and arms and legs. Some areas had evidence of hemorrhagic crust, flaccid bullae, and denudation. Few scant targetoid lesions and dusky red macules on the trunk, face, palms, and soles also were present.



One week prior to presentation he had an episode of diarrhea and dyspnea with symptoms of mild heat stroke after working outdoors, and he self-treated with ibuprofen, which he had taken intermittently for years. He was subsequently seen at an outpatient clinic and was prescribed an albuterol inhaler for previously untreated childhood asthma. The patient stated that he inhaled 2 puffs every 6 hours for a total of 3 treatments. Shortly after the last dose, he noticed a tingling sensation of the oral mucosa that developed into a painful 2-cm bullous ulcer. Over the next 3 days, he developed several more oral ulcers and erosions. Three days before admission he developed dysuria and tense bullae at the glans penis. After admission, he developed cheilitis, conjunctivitis, dysuria, odynophagia, and dysphagia to solids. One day after admission, the patient had the onset of systemic symptoms, including cough with worsening dyspnea, fever, chills, hemoptysis, epistaxis, nausea, diarrhea, loss of appetite, joint pain, and myalgia. Review of systems was otherwise negative. A radiograph was performed at admission and was notable for mild atelectasis but was otherwise normal. The chest radiograph was negative for signs of perihilar lymphadenopathy, pleural effusion, pneumothorax, or lobar infiltrates suggestive of bacterial pneumonia. It also did not show signs of patchy opacities or air bronchograms suggestive of an interstitial pneumonia. On admission, he was started on acyclovir, fluconazole, methylprednisolone, nystatin, pantoprazole, acetaminophen, topical bacitracin, oxycodone, and topical silver nitrate.
At the time of admission our patient was afebrile with a normal heart rate, blood pressure, and respiratory rate. However, he was hypoxic, with a pulse oximetry of 86% on room air and 94% on 40% fraction of inspired oxygen. Complete blood cell count, electrolytes, and liver function tests were all within reference range. Urinalysis revealed evidence of scant red blood cells without pyuria, and the erythrocyte sedimentation rate and creatine kinase level were both elevated. Two blood cultures; sputum cultures; and polymerase chain reaction for Mycoplasma pneumoniae, herpes simplex virus, varicella-zoster virus, cytomegalovirus, and Epstein-Barr virus were negative. Human immunodeficiency virus panel, antinuclear antibody screen, and hepatitis B and C panels were all negative. Four punch biopsies were obtained showing full-thickness epidermal necrosis with neutrophils, few dyskeratotic cells, and sparse inflammatory infiltrate compatible with Stevens-Johnson syndrome (SJS).
After hospital admission, the patient’s mucosal desquamation progressively improved. By day 3, he required minimal supplemental oxygen with resolution of bowel symptoms and improved mucosal and skin findings. He was discharged on day 4 with supplemental oxygen and a 7-day course of prednisone, fluconazole, liquid oxycodone, pantoprazole, and acetaminophen. He showed continued improvement at a follow-up outpatient visit 2 days following discharge.
Stevens-Johnson syndrome is a rare severe drug reaction characterized by high fevers, mucosal erosions, tenderness, and skin detachment approximately 1 to 3 weeks after an inciting event.1,2 Although SJS has been linked to infections and less commonly to immunizations, in more than 80% of cases, SJS is strongly associated with a recent medication change.3 The classes of drugs that have been implicated in SJS most commonly include antibiotics, anticonvulsants, and nonsteroidal anti-inflammatory drugs.4 Stevens-Johnson syndrome from drug reactions is not uncommon; however, SJS secondary to isolated albuterol use is rare.
Although it is presumed that albuterol was the key inciting factor in our patient’s case of SJS, it also is recognized that mucosal SJS can be associated with M pneumoniae infection. For this reason, we performed polymerase chain reaction for M pneumoniae as well as a chest radiograph to rule out this possibility. In addition, our patient had denied prolonged respiratory symptoms suggestive of a mycoplasma pneumonia infection, such as a prodrome of cough, myalgia, headache, sore throat, or fever. A report of 8 patients with documented SJS and M pneumoniae as well as a review of the literature also demonstrated a mean of 10 days of prodromal symptoms prior to the onset of mucosal lesions and/or a rash.5 However, mucosal SJS associated with mycoplasma pneumonia is an important clinical entity that should not be forgotten during the workup of a young patient with mucosal lesions or rash suggestive of SJS.
The exact etiology and mechanism of drug-induced SJS is not well understood at this time; however, evidence suggests that SJS is strongly linked to the host’s inability to detoxify drug metabolites.6,7 It has been postulated that SJS occurs secondary to a cell-mediated immune response, which activates cytotoxic T lymphocytes and subsequently results in keratinocyte apoptosis. Keratinocyte apoptosis occurs via the CD95-CD95 death receptor and soluble or membrane-bound ligand interaction.3,8,9 Stevens-Johnson syndrome is thought to occur from an interaction involving an HLA antigen–restricted presentation of drug metabolites to cytotoxic T cells, which can be further supported by evidence of strong genetic associations with HLA antigen alleles B15.02 and B58.01 in the cases of carbamazepine- and allopurinol-induced SJS, respectively.6,7 However, the genetic associations of specific HLA antigen alleles and polymorphisms with SJS and other cutaneous reactions is thought to be drug specific and HLA antigen subtype specific.7 Therefore, it is difficult to determine or correlate the clinical outcomes and manifestations of drug reactions in individualized patients. The precise mechanism of antigenicity of albuterol in initiating this cascade has not yet been determined. However, these investigations provide strong evidence for a correlation between specific HLA antigen haplotypes and occurrence of drug antigenicity resulting in SJS and other cutaneous reactions in susceptible patient populations.
Although the specific molecular pathway and etiology of SJS is not well delineated, pathology in combination with clinical correlation allows for diagnosis. Early-stage biopsies in SJS typically show apoptotic keratinocytes throughout the epidermis. Late-stage biopsies exhibit subepidermal blisters and full-thickness epidermal necrosis.1 Histopathology was performed on 4-mm punch biopsies of the chest and back and demonstrated full-thickness epidermal necrosis with neutrophils and a few dyskeratotic cells, likely representing a late stage of epidermal involvement. Given the predominance of neutrophils, other diagnostic considerations based solely on the biopsy results included contact dermatitis or phototoxic dermatitis. The remaining inflammatory infiltrate was sparse. Immunofluorescence was pan-negative.
This report illustrates a rare case of SJS from isolated albuterol use. This adverse drug reaction has not been well reported in the literature and may be an important consideration in the management of a patient with asthma.
- Stern RS. Clinical practice. exanthematous drug eruptions. N Engl J Med. 2012;366:2492-2501.
- Tartarone A, Lerose R. Stevens-Johnson syndrome and toxic epidermal necrolysis: what do we know? Ther Drug Monit. 2010;32:669-672.
- Ferrandiz-Pulido C, Garcia-Patos V. A review of causes of Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Arch Dis Child. 2013;98:998-1003.
- Mockenhaupt M, Viboud C, Dunant A, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. the EuroSCAR-study. J Invest Dermatol. 2008;128:35-44.
- Levy M, Shear NH. Mycoplasma pneumoniae infections and Stevens-Johnson syndrome. report of eight cases and review of the literature. Clin Pediatr (Phila). 1991;30:42-49.
- Chung WH, Hung SI. Genetic markers and danger signals in Stevens-Johnson syndrome and toxic epidermal necrolysis [published online October 25, 2010]. Allergol Int. 2010;59:325-332.
- Chung WH, Hung SI. Recent advances in the genetics and immunology of Stevens-Johnson syndrome and toxic epidermal necrosis. J Dermatol Sci. 2012;66:190-196.
- Bharadwaj M, Illing P, Theodossis A, et al. Drug hypersensitivity and human leukocyte antigens of the major histocompatibility complex. Annu Rev Pharmacol Toxicol. 2012;52:401-431.
- Chessman D, Kostenko L, Lethborg T, et al. Human leukocyte antigen class I-restricted activation of CD8+ T cells provides the immunogenetic basis of a systemic drug hypersensitivity. Immunity. 2008;28:822-832.
- Stern RS. Clinical practice. exanthematous drug eruptions. N Engl J Med. 2012;366:2492-2501.
- Tartarone A, Lerose R. Stevens-Johnson syndrome and toxic epidermal necrolysis: what do we know? Ther Drug Monit. 2010;32:669-672.
- Ferrandiz-Pulido C, Garcia-Patos V. A review of causes of Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Arch Dis Child. 2013;98:998-1003.
- Mockenhaupt M, Viboud C, Dunant A, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. the EuroSCAR-study. J Invest Dermatol. 2008;128:35-44.
- Levy M, Shear NH. Mycoplasma pneumoniae infections and Stevens-Johnson syndrome. report of eight cases and review of the literature. Clin Pediatr (Phila). 1991;30:42-49.
- Chung WH, Hung SI. Genetic markers and danger signals in Stevens-Johnson syndrome and toxic epidermal necrolysis [published online October 25, 2010]. Allergol Int. 2010;59:325-332.
- Chung WH, Hung SI. Recent advances in the genetics and immunology of Stevens-Johnson syndrome and toxic epidermal necrosis. J Dermatol Sci. 2012;66:190-196.
- Bharadwaj M, Illing P, Theodossis A, et al. Drug hypersensitivity and human leukocyte antigens of the major histocompatibility complex. Annu Rev Pharmacol Toxicol. 2012;52:401-431.
- Chessman D, Kostenko L, Lethborg T, et al. Human leukocyte antigen class I-restricted activation of CD8+ T cells provides the immunogenetic basis of a systemic drug hypersensitivity. Immunity. 2008;28:822-832.
Practice Points
- Think of Stevens-Johnson syndrome when new skin lesions are seen after any new medication is started.
- Perform a full-body examination to assess the extent of skin eruptions.
- When a medication is atypical for skin eruption, it becomes necessary to assess for other systemic causes and confirm pathologic results on skin biopsy.
Award for best hospital goes to … the Mayo Clinic
For the second consecutive year, the Mayo Clinic was named the top hospital in the country by U.S. News & World Report.
Also for the second consecutive year, the Cleveland Clinic is ranked second, while Johns Hopkins Hospital in Baltimore and Massachusetts General Hospital in Boston finished third and fourth – switching their places from last year’s ranking – and UCSF Medical Center in San Francisco is fifth after ranking seventh last year, according to the 2017-2018 Best Hospitals ranking.
The Mayo Clinic is nationally ranked in 15 of the 16 specialties included in the overall process, which started with 4,658 community inpatient hospitals and finished with 152 ranking nationally in at least one specialty and 20 earning Honor Roll status with high rankings in multiple specialties. The specialties used in the ranking process include 12 that are data driven – cancer; cardiology and heart surgery; diabetes and endocrinology; otolaryngology; gastroenterology and gastrointestinal surgery; geriatrics; gynecology; nephrology; neurology and neurosurgery; orthopedics; pulmonology; and urology – and four rated by reputation only – ophthalmology; psychiatry; rehabilitation; and rheumatology.
The research organization RTI International conducted the physician survey and produced the Best Hospitals methodology and national rankings under contract with U.S. News. The launch of this year’s edition of Best Hospitals is sponsored by Fidelity Investments.
For the second consecutive year, the Mayo Clinic was named the top hospital in the country by U.S. News & World Report.
Also for the second consecutive year, the Cleveland Clinic is ranked second, while Johns Hopkins Hospital in Baltimore and Massachusetts General Hospital in Boston finished third and fourth – switching their places from last year’s ranking – and UCSF Medical Center in San Francisco is fifth after ranking seventh last year, according to the 2017-2018 Best Hospitals ranking.
The Mayo Clinic is nationally ranked in 15 of the 16 specialties included in the overall process, which started with 4,658 community inpatient hospitals and finished with 152 ranking nationally in at least one specialty and 20 earning Honor Roll status with high rankings in multiple specialties. The specialties used in the ranking process include 12 that are data driven – cancer; cardiology and heart surgery; diabetes and endocrinology; otolaryngology; gastroenterology and gastrointestinal surgery; geriatrics; gynecology; nephrology; neurology and neurosurgery; orthopedics; pulmonology; and urology – and four rated by reputation only – ophthalmology; psychiatry; rehabilitation; and rheumatology.
The research organization RTI International conducted the physician survey and produced the Best Hospitals methodology and national rankings under contract with U.S. News. The launch of this year’s edition of Best Hospitals is sponsored by Fidelity Investments.
For the second consecutive year, the Mayo Clinic was named the top hospital in the country by U.S. News & World Report.
Also for the second consecutive year, the Cleveland Clinic is ranked second, while Johns Hopkins Hospital in Baltimore and Massachusetts General Hospital in Boston finished third and fourth – switching their places from last year’s ranking – and UCSF Medical Center in San Francisco is fifth after ranking seventh last year, according to the 2017-2018 Best Hospitals ranking.
The Mayo Clinic is nationally ranked in 15 of the 16 specialties included in the overall process, which started with 4,658 community inpatient hospitals and finished with 152 ranking nationally in at least one specialty and 20 earning Honor Roll status with high rankings in multiple specialties. The specialties used in the ranking process include 12 that are data driven – cancer; cardiology and heart surgery; diabetes and endocrinology; otolaryngology; gastroenterology and gastrointestinal surgery; geriatrics; gynecology; nephrology; neurology and neurosurgery; orthopedics; pulmonology; and urology – and four rated by reputation only – ophthalmology; psychiatry; rehabilitation; and rheumatology.
The research organization RTI International conducted the physician survey and produced the Best Hospitals methodology and national rankings under contract with U.S. News. The launch of this year’s edition of Best Hospitals is sponsored by Fidelity Investments.
Space Heater–Induced Bullous Erythema Ab Igne
To the Editor:
Erythema ab igne (EAI) is a reticular erythematous hyperpigmentation of skin repeatedly exposed to moderate heat.1 It usually is asymptomatic, though some patients report itching or burning at the site.2 Historically caused by exposure to coal stoves or open fires, EAI has become increasingly common among individuals using space heaters, heating pads, or laptop computers near bare skin.2,3 Although EAI itself is benign and usually resolves with the removal of the exposure, it remains of clinical importance because of its association with underlying chronic disease, as chronic pain often is managed with frequent heating pad or hot water bottle use.2 Additionally, accurate diagnosis is important given the future risk for malignancy, as chronic changes of EAI have been reported to lead to squamous cell carcinoma or rarely Merkel cell carcinoma.2 Erythema ab igne is not traditionally associated with the formation of bullae; however, we present a case of bullous EAI that we believe highlights the importance of including this condition in the differential diagnosis of bullous disorders.
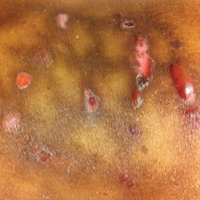
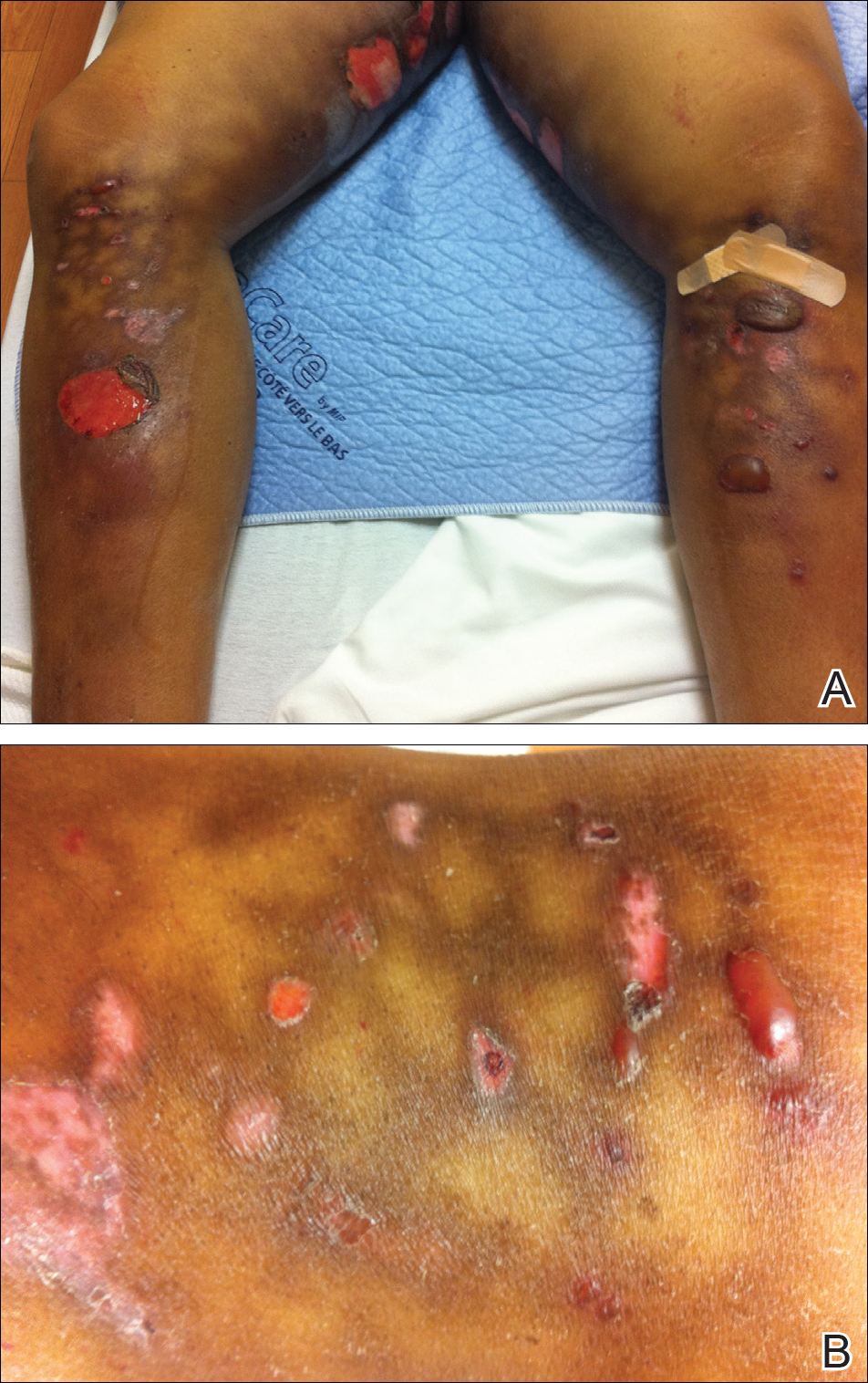
A 55-year-old man was admitted for presumed cellulitis of the bilateral legs. The patient had developed hyperpigmented discoloration of the medial surface of both legs with subsequent formation of tense bullae over the last 2 months. The dermatology department was consulted, as there was concern for bullous pemphigoid. The patient’s medical history was notable for hypertension, hyperlipidemia, diet-controlled type 2 diabetes mellitus, and hepatitis C virus with cirrhosis. The patient denied pruritus, pain, or known exposure of the legs to potential irritants prior to developing the lesions; however, with additional questioning he did report frequently sitting in front of a space heater with bare legs. Physical examination revealed multiple areas of reticulated erythematous hyperpigmentation with several overlying bullae (Figure 1). Many of the bullae were unroofed with full-thickness ulceration. Biopsies were taken for hematoxylin and eosin staining (Figure 2) and direct immunofluorescence.
Basic hematologic and metabolic laboratory test results as well as blood cultures were negative. Wound culture was positive for methicillin-resistant Staphylococcus aureus. Histologic examination showed interface dermatitis with subepidermal vesicle (Figure 2). Scattered necrotic keratinocytes were present in the adjacent epidermis, and focal subtle vacuolar alteration of the dermoepidermal junction was seen (Figure 3). Sparse perivascular mononuclear cells and scattered melanophages were present in the dermis. Direct immunofluorescence showed no diagnostic immunopathologic abnormality. Focal weak nonspecific vascular positivity for IgG and C3 was seen, but IgA and IgM were negative. Although not specific, these changes were compatible with EAI in the clinical context provided. The diagnosis of bullous EAI with superimposed staphylococcal infection was made.
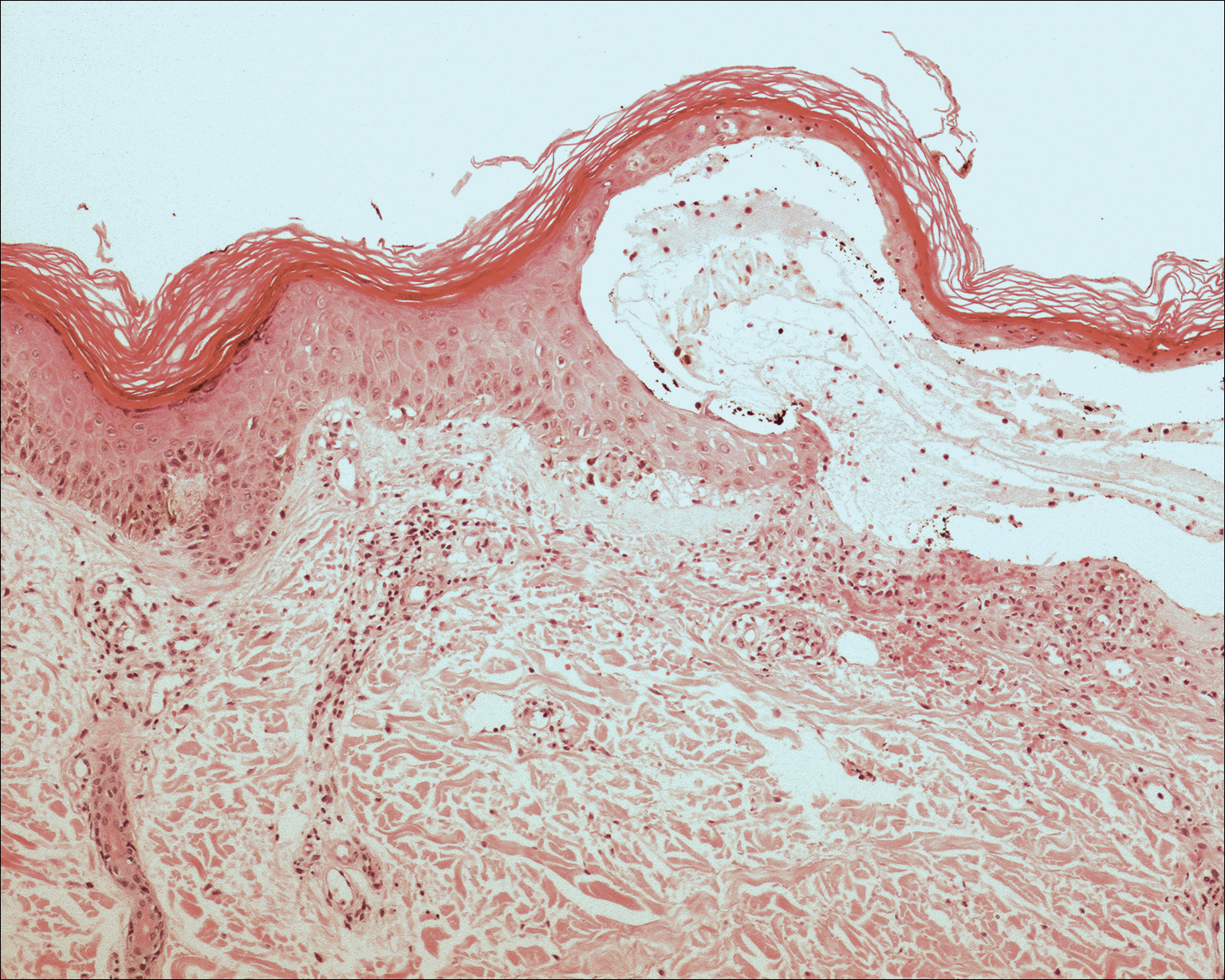
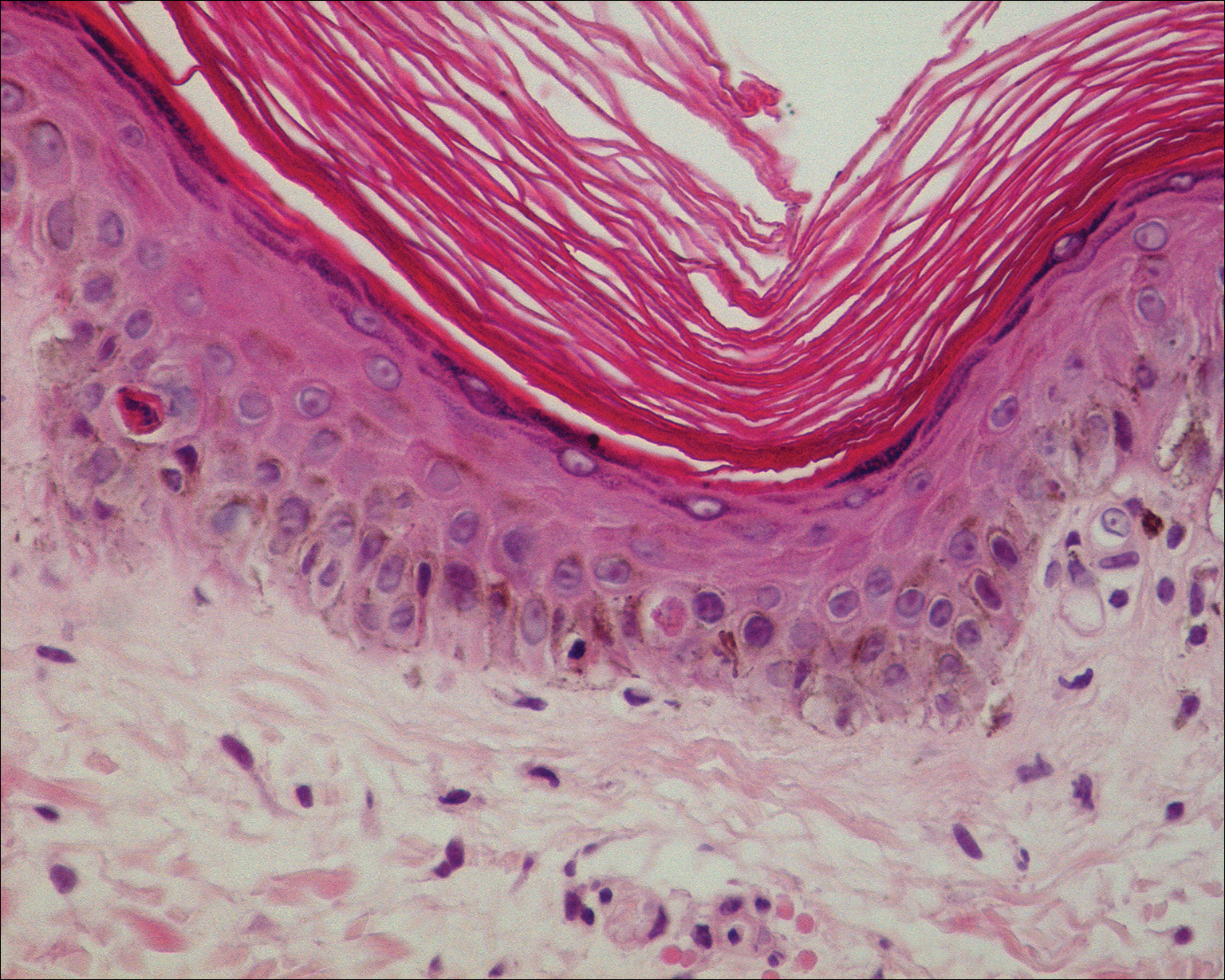
Although rare, there have been reports of a bullous variant of EAI. Flanagan et al4 described 3 cases of bullous EAI with histopathology similar to our case. All 3 biopsies showed subepidermal separation with a mild perivascular dermal lymphocytic infiltrate. Direct immunofluorescence was negative in 2 cases but showed nonspecific weak patchy deposition of IgM along the dermoepidermal junction.4 Although our case was negative for IgM, there was a similar weak nonspecific distribution of IgG. Kokturk et al5 described a case of bullous EAI in a man with repeated exposure to a space heater. The lesions showed subepidermal separation of the epidermis; increased elastic fibers; dilated dermal capillaries; melanophages in the upper dermis; and a mild, superficial, perivascular-lymphocytic infiltrate. Direct immunofluorescence showed no immune deposits.5 Several earlier cases of bullae associated with EAI have been reported in the literature but were thought to be bullous lichen planus superimposed on EAI.6 Our case, which exhibited similar historical, physical, and histopathologic findings, strengthens the argument for a defined bullous variant of EAI.
- Baruchin AM. Erythema ab igne—a neglected entity? Burns. 1994;20:460-462.
- Arnold AW, Itin PH. Laptop computer−induced erythema ab igne in a child and review of the literature [published online October 4, 2010]. Pediatrics. 2010;126:E1227-E1230.
- Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;162:77-78.
- Flanagan N, Watson R, Sweeney E, et al. Bullous erythema ab igne. Br J Dermatol. 1996;134:1159-1160.
- Kokturk A, Kaya TI, Baz K, et al. Bullous erythema ab igne. Dermatol Online J. 2003;9:18.
- Horio T, Imamura S. Bullous lichen planus developed on erythema ab igne. J Dermatol. 1986;13:203-207.
To the Editor:
Erythema ab igne (EAI) is a reticular erythematous hyperpigmentation of skin repeatedly exposed to moderate heat.1 It usually is asymptomatic, though some patients report itching or burning at the site.2 Historically caused by exposure to coal stoves or open fires, EAI has become increasingly common among individuals using space heaters, heating pads, or laptop computers near bare skin.2,3 Although EAI itself is benign and usually resolves with the removal of the exposure, it remains of clinical importance because of its association with underlying chronic disease, as chronic pain often is managed with frequent heating pad or hot water bottle use.2 Additionally, accurate diagnosis is important given the future risk for malignancy, as chronic changes of EAI have been reported to lead to squamous cell carcinoma or rarely Merkel cell carcinoma.2 Erythema ab igne is not traditionally associated with the formation of bullae; however, we present a case of bullous EAI that we believe highlights the importance of including this condition in the differential diagnosis of bullous disorders.
A 55-year-old man was admitted for presumed cellulitis of the bilateral legs. The patient had developed hyperpigmented discoloration of the medial surface of both legs with subsequent formation of tense bullae over the last 2 months. The dermatology department was consulted, as there was concern for bullous pemphigoid. The patient’s medical history was notable for hypertension, hyperlipidemia, diet-controlled type 2 diabetes mellitus, and hepatitis C virus with cirrhosis. The patient denied pruritus, pain, or known exposure of the legs to potential irritants prior to developing the lesions; however, with additional questioning he did report frequently sitting in front of a space heater with bare legs. Physical examination revealed multiple areas of reticulated erythematous hyperpigmentation with several overlying bullae (Figure 1). Many of the bullae were unroofed with full-thickness ulceration. Biopsies were taken for hematoxylin and eosin staining (Figure 2) and direct immunofluorescence.

Basic hematologic and metabolic laboratory test results as well as blood cultures were negative. Wound culture was positive for methicillin-resistant Staphylococcus aureus. Histologic examination showed interface dermatitis with subepidermal vesicle (Figure 2). Scattered necrotic keratinocytes were present in the adjacent epidermis, and focal subtle vacuolar alteration of the dermoepidermal junction was seen (Figure 3). Sparse perivascular mononuclear cells and scattered melanophages were present in the dermis. Direct immunofluorescence showed no diagnostic immunopathologic abnormality. Focal weak nonspecific vascular positivity for IgG and C3 was seen, but IgA and IgM were negative. Although not specific, these changes were compatible with EAI in the clinical context provided. The diagnosis of bullous EAI with superimposed staphylococcal infection was made.


Although rare, there have been reports of a bullous variant of EAI. Flanagan et al4 described 3 cases of bullous EAI with histopathology similar to our case. All 3 biopsies showed subepidermal separation with a mild perivascular dermal lymphocytic infiltrate. Direct immunofluorescence was negative in 2 cases but showed nonspecific weak patchy deposition of IgM along the dermoepidermal junction.4 Although our case was negative for IgM, there was a similar weak nonspecific distribution of IgG. Kokturk et al5 described a case of bullous EAI in a man with repeated exposure to a space heater. The lesions showed subepidermal separation of the epidermis; increased elastic fibers; dilated dermal capillaries; melanophages in the upper dermis; and a mild, superficial, perivascular-lymphocytic infiltrate. Direct immunofluorescence showed no immune deposits.5 Several earlier cases of bullae associated with EAI have been reported in the literature but were thought to be bullous lichen planus superimposed on EAI.6 Our case, which exhibited similar historical, physical, and histopathologic findings, strengthens the argument for a defined bullous variant of EAI.
To the Editor:
Erythema ab igne (EAI) is a reticular erythematous hyperpigmentation of skin repeatedly exposed to moderate heat.1 It usually is asymptomatic, though some patients report itching or burning at the site.2 Historically caused by exposure to coal stoves or open fires, EAI has become increasingly common among individuals using space heaters, heating pads, or laptop computers near bare skin.2,3 Although EAI itself is benign and usually resolves with the removal of the exposure, it remains of clinical importance because of its association with underlying chronic disease, as chronic pain often is managed with frequent heating pad or hot water bottle use.2 Additionally, accurate diagnosis is important given the future risk for malignancy, as chronic changes of EAI have been reported to lead to squamous cell carcinoma or rarely Merkel cell carcinoma.2 Erythema ab igne is not traditionally associated with the formation of bullae; however, we present a case of bullous EAI that we believe highlights the importance of including this condition in the differential diagnosis of bullous disorders.
A 55-year-old man was admitted for presumed cellulitis of the bilateral legs. The patient had developed hyperpigmented discoloration of the medial surface of both legs with subsequent formation of tense bullae over the last 2 months. The dermatology department was consulted, as there was concern for bullous pemphigoid. The patient’s medical history was notable for hypertension, hyperlipidemia, diet-controlled type 2 diabetes mellitus, and hepatitis C virus with cirrhosis. The patient denied pruritus, pain, or known exposure of the legs to potential irritants prior to developing the lesions; however, with additional questioning he did report frequently sitting in front of a space heater with bare legs. Physical examination revealed multiple areas of reticulated erythematous hyperpigmentation with several overlying bullae (Figure 1). Many of the bullae were unroofed with full-thickness ulceration. Biopsies were taken for hematoxylin and eosin staining (Figure 2) and direct immunofluorescence.

Basic hematologic and metabolic laboratory test results as well as blood cultures were negative. Wound culture was positive for methicillin-resistant Staphylococcus aureus. Histologic examination showed interface dermatitis with subepidermal vesicle (Figure 2). Scattered necrotic keratinocytes were present in the adjacent epidermis, and focal subtle vacuolar alteration of the dermoepidermal junction was seen (Figure 3). Sparse perivascular mononuclear cells and scattered melanophages were present in the dermis. Direct immunofluorescence showed no diagnostic immunopathologic abnormality. Focal weak nonspecific vascular positivity for IgG and C3 was seen, but IgA and IgM were negative. Although not specific, these changes were compatible with EAI in the clinical context provided. The diagnosis of bullous EAI with superimposed staphylococcal infection was made.


Although rare, there have been reports of a bullous variant of EAI. Flanagan et al4 described 3 cases of bullous EAI with histopathology similar to our case. All 3 biopsies showed subepidermal separation with a mild perivascular dermal lymphocytic infiltrate. Direct immunofluorescence was negative in 2 cases but showed nonspecific weak patchy deposition of IgM along the dermoepidermal junction.4 Although our case was negative for IgM, there was a similar weak nonspecific distribution of IgG. Kokturk et al5 described a case of bullous EAI in a man with repeated exposure to a space heater. The lesions showed subepidermal separation of the epidermis; increased elastic fibers; dilated dermal capillaries; melanophages in the upper dermis; and a mild, superficial, perivascular-lymphocytic infiltrate. Direct immunofluorescence showed no immune deposits.5 Several earlier cases of bullae associated with EAI have been reported in the literature but were thought to be bullous lichen planus superimposed on EAI.6 Our case, which exhibited similar historical, physical, and histopathologic findings, strengthens the argument for a defined bullous variant of EAI.
- Baruchin AM. Erythema ab igne—a neglected entity? Burns. 1994;20:460-462.
- Arnold AW, Itin PH. Laptop computer−induced erythema ab igne in a child and review of the literature [published online October 4, 2010]. Pediatrics. 2010;126:E1227-E1230.
- Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;162:77-78.
- Flanagan N, Watson R, Sweeney E, et al. Bullous erythema ab igne. Br J Dermatol. 1996;134:1159-1160.
- Kokturk A, Kaya TI, Baz K, et al. Bullous erythema ab igne. Dermatol Online J. 2003;9:18.
- Horio T, Imamura S. Bullous lichen planus developed on erythema ab igne. J Dermatol. 1986;13:203-207.
- Baruchin AM. Erythema ab igne—a neglected entity? Burns. 1994;20:460-462.
- Arnold AW, Itin PH. Laptop computer−induced erythema ab igne in a child and review of the literature [published online October 4, 2010]. Pediatrics. 2010;126:E1227-E1230.
- Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;162:77-78.
- Flanagan N, Watson R, Sweeney E, et al. Bullous erythema ab igne. Br J Dermatol. 1996;134:1159-1160.
- Kokturk A, Kaya TI, Baz K, et al. Bullous erythema ab igne. Dermatol Online J. 2003;9:18.
- Horio T, Imamura S. Bullous lichen planus developed on erythema ab igne. J Dermatol. 1986;13:203-207.
Practice Points
- Consider erythema ab igne (EAI) as a potential differential diagnosis in bullous eruptions.
- Space heaters, heating pads, and even laptop computers should be considered as potential causes of EAI.
Acne-associated hyperpigmentation an important consideration in patients with skin of color
NEW YORK – When treating patients with skin of color for acne, treatment goals may vary from those of patients with lighter skin, according to Andrew F. Alexis, MD.
For example, in patients with Fitzpatrick skin types V and VI, the desired treatment outcome is not only resolution of acne, but also resolution of hyperpigmentation, said Dr. Alexis, chairman of the department of dermatology at Mount Sinai St. Luke’s and Mount Sinai West, New York, N.Y.
“Postinflammatory hyperpigmentation is often the driving force for the dermatology consult” in individuals with skin of color, Dr. Alexis said at the summer meeting of the American Academy of Dermatology. “They may be just as concerned about their dark spots as underlying acne,” he noted, citing a study that he coauthored (J Clin Aesthet Dermatol. 2014 Jul;7[7]:19-31).
In the study – a survey of patients with acne to determine which treatment outcomes were most important – 41.6% of the nonwhite female patients reported that clearance of postinflammatory hyperpigmentation was the most important goal, compared with 8.4% of white female respondents (P less than .0001).
It’s important to avoid undertreating patients, especially darker-skinned patients, where ongoing subclinical inflammation may contribute to hyperpigmentation. Even in lesions that appear grossly noninflamed, biopsies may find histological evidence of inflammation, with increased T-cell infiltration of the pilosebaceous units, Dr. Alexis said.
However, there’s always a balancing act in determining how aggressively to treat patients, he added. Dermatologists have to be aware of the risk of hypertrophic scar formation in darker-skinned individuals, especially in truncal areas.
When addressing the acne, step one is to aggressively reduce acne-associated inflammation to reduce potential sequelae. This can be done with any of a number of agents, such as retinoids, benzoyl peroxide, dapsone, azelaic acid, and even intralesional corticosteroid injections, he said.
“All agents have been considered in darker skin types,” he said, noting that “retinoids are particularly important because they can also treat postinflammatory hyperpigmentation.” Tretinoin 0.1% cream and tazarotene 0.1% cream are both good choices, he added.
Adapalene in a fixed combination with benzoyl peroxide has been studied in darker-skinned patients, with no difference in tolerability or higher incidence of pigmentary sequelae than in lighter-skinned patients, he pointed out.
Dapsone 5% and 7.5% have also been studied in patients with darker skin, and both concentrations showed comparable results for safety and efficacy.
The thinking about second-line agents can shift a bit when treating acne in darker skin. For example, azelaic acid as a 20% cream or 15% gel can be a good choice, and can be helpful in treating postinflammatory hyperpigmentation, but azelaic acid is “not as good an antiacne agent as retinoids,” Dr. Alexis said.
Patients should understand that any of these choices are primarily acne-directed treatments, to be deployed over the first 3-6 months of treatment. Then, beginning at about the 3-month mark and continuing for up to a year, hyperpigmentation can be addressed. “Really emphasize the duration of treatment,” when treating hyperpigmentation, Dr. Alexis advised.
Once the acne is under control and hyperpigmentation can be assessed on its own, dermatologists can consider whether bleaching agents are appropriate. “Should they be used? If so, how?” he asked.
Bleaching agents can be effective, said Dr. Alexis, who recommends lesion-directed rather than broad-field therapy, unless there are many larger hyperpigmented macules. “The more common scenario is smaller, more distributed lesions,” he said. “Superficial chemical peels, if used with caution, can be a good adjunct,” to bleaching agents, he added.
Coming down the road are topical nitric oxide preparations, which he said are looking good for darker skin in clinical trials.
“The key to great outcomes is to initiate a combination regimen that targets inflammation and reduces hyperpigmentation,” said Dr. Alexis. Then, he advised, minimize irritation but don’t undertreat, consider adjunctive chemical peels, and above all, “set realistic timeline expectations.”
Dr. Alexis reported financial relationships with multiple pharmaceutical companies.
[email protected]
On Twitter @karioakes
NEW YORK – When treating patients with skin of color for acne, treatment goals may vary from those of patients with lighter skin, according to Andrew F. Alexis, MD.
For example, in patients with Fitzpatrick skin types V and VI, the desired treatment outcome is not only resolution of acne, but also resolution of hyperpigmentation, said Dr. Alexis, chairman of the department of dermatology at Mount Sinai St. Luke’s and Mount Sinai West, New York, N.Y.
“Postinflammatory hyperpigmentation is often the driving force for the dermatology consult” in individuals with skin of color, Dr. Alexis said at the summer meeting of the American Academy of Dermatology. “They may be just as concerned about their dark spots as underlying acne,” he noted, citing a study that he coauthored (J Clin Aesthet Dermatol. 2014 Jul;7[7]:19-31).
In the study – a survey of patients with acne to determine which treatment outcomes were most important – 41.6% of the nonwhite female patients reported that clearance of postinflammatory hyperpigmentation was the most important goal, compared with 8.4% of white female respondents (P less than .0001).
It’s important to avoid undertreating patients, especially darker-skinned patients, where ongoing subclinical inflammation may contribute to hyperpigmentation. Even in lesions that appear grossly noninflamed, biopsies may find histological evidence of inflammation, with increased T-cell infiltration of the pilosebaceous units, Dr. Alexis said.
However, there’s always a balancing act in determining how aggressively to treat patients, he added. Dermatologists have to be aware of the risk of hypertrophic scar formation in darker-skinned individuals, especially in truncal areas.
When addressing the acne, step one is to aggressively reduce acne-associated inflammation to reduce potential sequelae. This can be done with any of a number of agents, such as retinoids, benzoyl peroxide, dapsone, azelaic acid, and even intralesional corticosteroid injections, he said.
“All agents have been considered in darker skin types,” he said, noting that “retinoids are particularly important because they can also treat postinflammatory hyperpigmentation.” Tretinoin 0.1% cream and tazarotene 0.1% cream are both good choices, he added.
Adapalene in a fixed combination with benzoyl peroxide has been studied in darker-skinned patients, with no difference in tolerability or higher incidence of pigmentary sequelae than in lighter-skinned patients, he pointed out.
Dapsone 5% and 7.5% have also been studied in patients with darker skin, and both concentrations showed comparable results for safety and efficacy.
The thinking about second-line agents can shift a bit when treating acne in darker skin. For example, azelaic acid as a 20% cream or 15% gel can be a good choice, and can be helpful in treating postinflammatory hyperpigmentation, but azelaic acid is “not as good an antiacne agent as retinoids,” Dr. Alexis said.
Patients should understand that any of these choices are primarily acne-directed treatments, to be deployed over the first 3-6 months of treatment. Then, beginning at about the 3-month mark and continuing for up to a year, hyperpigmentation can be addressed. “Really emphasize the duration of treatment,” when treating hyperpigmentation, Dr. Alexis advised.
Once the acne is under control and hyperpigmentation can be assessed on its own, dermatologists can consider whether bleaching agents are appropriate. “Should they be used? If so, how?” he asked.
Bleaching agents can be effective, said Dr. Alexis, who recommends lesion-directed rather than broad-field therapy, unless there are many larger hyperpigmented macules. “The more common scenario is smaller, more distributed lesions,” he said. “Superficial chemical peels, if used with caution, can be a good adjunct,” to bleaching agents, he added.
Coming down the road are topical nitric oxide preparations, which he said are looking good for darker skin in clinical trials.
“The key to great outcomes is to initiate a combination regimen that targets inflammation and reduces hyperpigmentation,” said Dr. Alexis. Then, he advised, minimize irritation but don’t undertreat, consider adjunctive chemical peels, and above all, “set realistic timeline expectations.”
Dr. Alexis reported financial relationships with multiple pharmaceutical companies.
[email protected]
On Twitter @karioakes
NEW YORK – When treating patients with skin of color for acne, treatment goals may vary from those of patients with lighter skin, according to Andrew F. Alexis, MD.
For example, in patients with Fitzpatrick skin types V and VI, the desired treatment outcome is not only resolution of acne, but also resolution of hyperpigmentation, said Dr. Alexis, chairman of the department of dermatology at Mount Sinai St. Luke’s and Mount Sinai West, New York, N.Y.
“Postinflammatory hyperpigmentation is often the driving force for the dermatology consult” in individuals with skin of color, Dr. Alexis said at the summer meeting of the American Academy of Dermatology. “They may be just as concerned about their dark spots as underlying acne,” he noted, citing a study that he coauthored (J Clin Aesthet Dermatol. 2014 Jul;7[7]:19-31).
In the study – a survey of patients with acne to determine which treatment outcomes were most important – 41.6% of the nonwhite female patients reported that clearance of postinflammatory hyperpigmentation was the most important goal, compared with 8.4% of white female respondents (P less than .0001).
It’s important to avoid undertreating patients, especially darker-skinned patients, where ongoing subclinical inflammation may contribute to hyperpigmentation. Even in lesions that appear grossly noninflamed, biopsies may find histological evidence of inflammation, with increased T-cell infiltration of the pilosebaceous units, Dr. Alexis said.
However, there’s always a balancing act in determining how aggressively to treat patients, he added. Dermatologists have to be aware of the risk of hypertrophic scar formation in darker-skinned individuals, especially in truncal areas.
When addressing the acne, step one is to aggressively reduce acne-associated inflammation to reduce potential sequelae. This can be done with any of a number of agents, such as retinoids, benzoyl peroxide, dapsone, azelaic acid, and even intralesional corticosteroid injections, he said.
“All agents have been considered in darker skin types,” he said, noting that “retinoids are particularly important because they can also treat postinflammatory hyperpigmentation.” Tretinoin 0.1% cream and tazarotene 0.1% cream are both good choices, he added.
Adapalene in a fixed combination with benzoyl peroxide has been studied in darker-skinned patients, with no difference in tolerability or higher incidence of pigmentary sequelae than in lighter-skinned patients, he pointed out.
Dapsone 5% and 7.5% have also been studied in patients with darker skin, and both concentrations showed comparable results for safety and efficacy.
The thinking about second-line agents can shift a bit when treating acne in darker skin. For example, azelaic acid as a 20% cream or 15% gel can be a good choice, and can be helpful in treating postinflammatory hyperpigmentation, but azelaic acid is “not as good an antiacne agent as retinoids,” Dr. Alexis said.
Patients should understand that any of these choices are primarily acne-directed treatments, to be deployed over the first 3-6 months of treatment. Then, beginning at about the 3-month mark and continuing for up to a year, hyperpigmentation can be addressed. “Really emphasize the duration of treatment,” when treating hyperpigmentation, Dr. Alexis advised.
Once the acne is under control and hyperpigmentation can be assessed on its own, dermatologists can consider whether bleaching agents are appropriate. “Should they be used? If so, how?” he asked.
Bleaching agents can be effective, said Dr. Alexis, who recommends lesion-directed rather than broad-field therapy, unless there are many larger hyperpigmented macules. “The more common scenario is smaller, more distributed lesions,” he said. “Superficial chemical peels, if used with caution, can be a good adjunct,” to bleaching agents, he added.
Coming down the road are topical nitric oxide preparations, which he said are looking good for darker skin in clinical trials.
“The key to great outcomes is to initiate a combination regimen that targets inflammation and reduces hyperpigmentation,” said Dr. Alexis. Then, he advised, minimize irritation but don’t undertreat, consider adjunctive chemical peels, and above all, “set realistic timeline expectations.”
Dr. Alexis reported financial relationships with multiple pharmaceutical companies.
[email protected]
On Twitter @karioakes
EXPERT ANALYSIS FROM THE 2017 SUMMER AAD MEETING
Cardiovascular risk factors more common in girls
Girls were more likely than boys to have cardiovascular disease risk factors such as poor aerobic capacity and a high percentage of body fat in a study involving almost 1,800 elementary school students.
Using the FITNESSGRAM Healthy Fitness Zone (HFZ) standards in 1,775 fourth- and fifth-grade students (879 boys, 896 girls) from an urban, predominantly black population, investigators found that significantly more girls than boys did not meet the HFZ for aerobic capacity (71% vs. 42%), percent body fat (53% vs. 30%), and body mass index (44% vs. 34%). Those who did not meet the HFZ for each measure were classified as “needs improvement” or “needs improvement – health risk.”
Overall, more than 66% of the study sample “had metabolic syndrome risk factors based on FITNESSGRAM assessments,” said Susan B. Racette, PhD, of Washington University, St. Louis, and her associates (Prev Med. 2017. doi: 10.1016/j.ypmed.2017.07.032).
More than half of the girls (51%) didn’t meet the HFZ for aerobic capacity and one of the body composition measures, compared with 32% of the boys, and 38% of the girls failed to meet the HFZ for all three measures, compared with 21% of the boys. The reverse side of that coin – those who did meet the HFZ for all three – favored the boys, 46% to 21%, the investigators reported.
“The presence of these cardiovascular disease risk factors in girls, in particular, highlights an important need for creative strategies to mitigate the long-term health and societal consequences of these modifiable risk factors,” Dr. Racette and her associates wrote.
This work was supported by the U.S. Department of Education and the Washington University division of biostatistics. The investigators said that they had no conflicts of interest.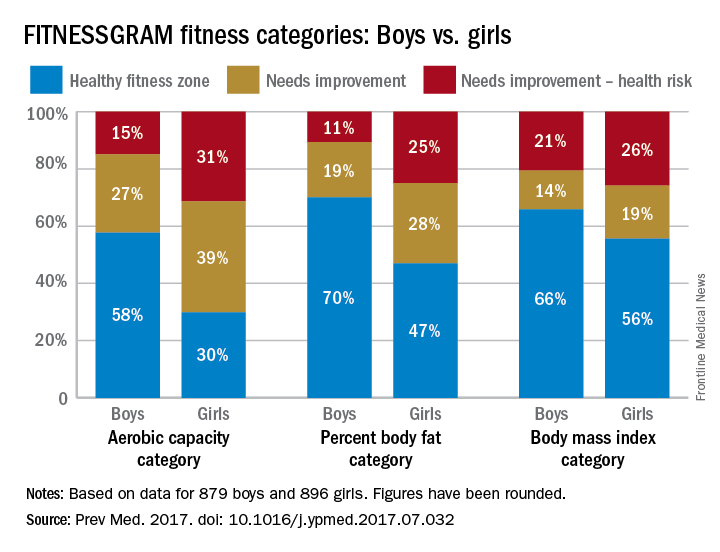
Girls were more likely than boys to have cardiovascular disease risk factors such as poor aerobic capacity and a high percentage of body fat in a study involving almost 1,800 elementary school students.
Using the FITNESSGRAM Healthy Fitness Zone (HFZ) standards in 1,775 fourth- and fifth-grade students (879 boys, 896 girls) from an urban, predominantly black population, investigators found that significantly more girls than boys did not meet the HFZ for aerobic capacity (71% vs. 42%), percent body fat (53% vs. 30%), and body mass index (44% vs. 34%). Those who did not meet the HFZ for each measure were classified as “needs improvement” or “needs improvement – health risk.”
Overall, more than 66% of the study sample “had metabolic syndrome risk factors based on FITNESSGRAM assessments,” said Susan B. Racette, PhD, of Washington University, St. Louis, and her associates (Prev Med. 2017. doi: 10.1016/j.ypmed.2017.07.032).
More than half of the girls (51%) didn’t meet the HFZ for aerobic capacity and one of the body composition measures, compared with 32% of the boys, and 38% of the girls failed to meet the HFZ for all three measures, compared with 21% of the boys. The reverse side of that coin – those who did meet the HFZ for all three – favored the boys, 46% to 21%, the investigators reported.
“The presence of these cardiovascular disease risk factors in girls, in particular, highlights an important need for creative strategies to mitigate the long-term health and societal consequences of these modifiable risk factors,” Dr. Racette and her associates wrote.
This work was supported by the U.S. Department of Education and the Washington University division of biostatistics. The investigators said that they had no conflicts of interest.
Girls were more likely than boys to have cardiovascular disease risk factors such as poor aerobic capacity and a high percentage of body fat in a study involving almost 1,800 elementary school students.
Using the FITNESSGRAM Healthy Fitness Zone (HFZ) standards in 1,775 fourth- and fifth-grade students (879 boys, 896 girls) from an urban, predominantly black population, investigators found that significantly more girls than boys did not meet the HFZ for aerobic capacity (71% vs. 42%), percent body fat (53% vs. 30%), and body mass index (44% vs. 34%). Those who did not meet the HFZ for each measure were classified as “needs improvement” or “needs improvement – health risk.”
Overall, more than 66% of the study sample “had metabolic syndrome risk factors based on FITNESSGRAM assessments,” said Susan B. Racette, PhD, of Washington University, St. Louis, and her associates (Prev Med. 2017. doi: 10.1016/j.ypmed.2017.07.032).
More than half of the girls (51%) didn’t meet the HFZ for aerobic capacity and one of the body composition measures, compared with 32% of the boys, and 38% of the girls failed to meet the HFZ for all three measures, compared with 21% of the boys. The reverse side of that coin – those who did meet the HFZ for all three – favored the boys, 46% to 21%, the investigators reported.
“The presence of these cardiovascular disease risk factors in girls, in particular, highlights an important need for creative strategies to mitigate the long-term health and societal consequences of these modifiable risk factors,” Dr. Racette and her associates wrote.
This work was supported by the U.S. Department of Education and the Washington University division of biostatistics. The investigators said that they had no conflicts of interest.
FROM PREVENTIVE MEDICINE
Alcohol use, high-risk drinking increases in U.S. to ‘crisis’ levels
Nearly one in eight adults in the United States had been diagnosed with alcohol use disorder in 2012-2013, a nearly 50% increase from a decade earlier, according to a study published Aug. 9. Other substantial increases occurring across virtually all demographic groups included overall 12-month alcohol consumption and high-risk drinking, particularly among adults aged 65 and older, racial/ethnic minorities, women, and those with lower education and incomes.
“The marked increases in high-risk drinking and DSM-IV [alcohol use disorder] between 2001-2002 and 2012-2013 also mirror recent sharp increases in morbidity and mortality from diseases and injuries in which alcohol use has a substantial role or deceleration of previously seen declines,” wrote Bridget F. Grant, PhD, of the National Institute on Alcohol Abuse and Alcoholism (NIAAA) in Rockville, Md., and her associates. High-risk drinking was defined as five drinks (14 g of ethanol each) per occasion for men and four drinks per occasion for women at least weekly.
“Mortality among alcohol-affected drivers who were simultaneously distracted also increased between 2005 and 2009 by 63%,” they wrote (JAMA Psychiatry. 2017 Aug 9. doi: 10.1001/jamapsychiatry.2017.2161).
The researchers analyzed data from 43,093 respondents to the National Epidemiologic Survey on Alcohol and Related Conditions (April 2001-June 2002) and from 36,309 respondents to the National Epidemiologic Survey on Alcohol and Related Conditions III (April 2012-June 2013). Both surveys involved face-to-face interviews with a nationally representative sample of U.S. adults.
The findings showed that 12-month alcohol use had increased 11.2% between 2001-2002 and 2012-2013, from 65.4% to 72.7%. A substantial increase also occurred in high-risk drinking and alcohol use disorder as defined in the DSM-IV. High-risk drinking increased 29.9% during that time, from 9.7% to 12.6%, representing an increase of approximately 9.4 million Americans engaging in high-risk drinking.
Alcohol use disorder (AUD) increased 49.4%, from 8.5% to 12.7% – a percentage that accounts for an additional 12.3 million Americans with the diagnosis. That increase dwarfs the 14.8% increase in alcohol use disorder that was seen between 1991-1992 and 2001-2002, the authors pointed out. The prevalence of 12-month AUD rose significantly among adults aged 65 and older (106.7%), African American individuals (92.8%), and women (83.7%). Interestingly, all subgroups reported significant increases in AUD except for Native Americans and people living in rural areas. By comparison, the 12-month prevalence of AUD among men increased by a third (34.7%), and their high-risk drinking increased 15.5%.
“Drinking norms and values have become more permissive among women, along with increases in educational and occupational opportunities and rising numbers of women in the workforce, all of which may have contributed to increased high-risk drinking and AUD in women during the past decade,” the authors wrote. “Stress associated with pursuing a career and raising a family may lead to increases in high-risk drinking and AUD among women.”
These increases indicate potential future increases among women in alcohol-related conditions, such as breast cancer and liver cirrhosis. Increases may also occur in fetal alcohol spectrum disorder and exposure to violence, the authors wrote.
The increases in alcohol use, high-risk drinking, and AUD found among minorities may be related to increased stress and demoralization as wealth inequality widened between minorities and whites in the wake of the 2008 recession. Other inequalities, such as income and educational disparities, unemployment, residential segregation, discrimination, and less health care access may also play a role in those increases, the authors wrote.
One limitation of the study is that certain populations were not surveyed, such as homeless individuals and people who are incarcerated. This means that the prevalence of alcohol use, high-risk drinking, and AUD could be underestimated, Dr. Grant and her colleagues said. However, they said, the large sample sizes of the surveys might balance out that limitation and others.
Nevertheless, the increases found in alcohol use, high-risk drinking, and AUD “constitute a public health crisis that may be overshadowed by increases in much less prevalent substance use (marijuana, opiates, and heroin) during the same period,” Dr. Grant and her colleagues wrote. “The findings herein highlight the urgency of educating the public, policymakers, and health care professionals about high-risk drinking and AUD.” In addition, they called for broader effors to address the “individual, biological, environmental, and societal factors” influencing high-risk drinking and AUD.
The research was sponsored by the NIAAA, and funded by the National Institutes of Health. The authors reported having no disclosures.
“This timely article by Grant et al. ... makes a compelling case that the United States is facing a crisis with alcohol use, one that is currently costly and about to get worse,” Marc A. Schuckit, MD, wrote in an accompanying editorial (JAMA Psychiatry. 2017 Aug 9. doi: 10.1001/jamapsychiatry.2017.1981). However, he said, several studies show that lowering the risk for future alcohol-related problems in 18 year olds is possible.
He said his group delivered an intervention to 500 college freshmen using Internet-based videos aimed at helping them “recognize their vulnerability toward heavy drinking.” Six and 12 months after watching four 50-minute videos, the effects on how much the students drank remained significant, he wrote. In addition, other studies have identified programs that help lower drinking during pregnancy. “These are only a few examples of ongoing hopeful developments,” Dr. Schuckit wrote.
The number in the study that is especially concerning for him, Dr. Schuckit said, is the 106% increase in AUDs among older individuals because of the many preexisting medical disorders “that can be exacerbated by heavier drinking. These drinkers are also likely to be taking multiple medications that can interact adversely with alcohol, with resulting significant and costly health consequences,” according to Dr. Schuckit.
“There is also some disturbing news,” he wrote. “The proposed cuts to the National Institutes of Health budget being considered in Washington in 2017 are potentially disastrous for future efforts to decrease alcohol problems and are likely to result in higher costs for us all. Efforts to identify risk factors for substance-related problems and to test prevention approaches take time and money and are less likely to be funded in the current financial atmosphere. … If we ignore these problems, they will come back to us at much higher costs through emergency department visits, impaired children … and higher costs for jails and prisons that are the last resort for help for many.”
Dr. Schuckit is affiliated with the department of psychiatry at the University of California, San Diego. He reported having no disclosures.
“This timely article by Grant et al. ... makes a compelling case that the United States is facing a crisis with alcohol use, one that is currently costly and about to get worse,” Marc A. Schuckit, MD, wrote in an accompanying editorial (JAMA Psychiatry. 2017 Aug 9. doi: 10.1001/jamapsychiatry.2017.1981). However, he said, several studies show that lowering the risk for future alcohol-related problems in 18 year olds is possible.
He said his group delivered an intervention to 500 college freshmen using Internet-based videos aimed at helping them “recognize their vulnerability toward heavy drinking.” Six and 12 months after watching four 50-minute videos, the effects on how much the students drank remained significant, he wrote. In addition, other studies have identified programs that help lower drinking during pregnancy. “These are only a few examples of ongoing hopeful developments,” Dr. Schuckit wrote.
The number in the study that is especially concerning for him, Dr. Schuckit said, is the 106% increase in AUDs among older individuals because of the many preexisting medical disorders “that can be exacerbated by heavier drinking. These drinkers are also likely to be taking multiple medications that can interact adversely with alcohol, with resulting significant and costly health consequences,” according to Dr. Schuckit.
“There is also some disturbing news,” he wrote. “The proposed cuts to the National Institutes of Health budget being considered in Washington in 2017 are potentially disastrous for future efforts to decrease alcohol problems and are likely to result in higher costs for us all. Efforts to identify risk factors for substance-related problems and to test prevention approaches take time and money and are less likely to be funded in the current financial atmosphere. … If we ignore these problems, they will come back to us at much higher costs through emergency department visits, impaired children … and higher costs for jails and prisons that are the last resort for help for many.”
Dr. Schuckit is affiliated with the department of psychiatry at the University of California, San Diego. He reported having no disclosures.
“This timely article by Grant et al. ... makes a compelling case that the United States is facing a crisis with alcohol use, one that is currently costly and about to get worse,” Marc A. Schuckit, MD, wrote in an accompanying editorial (JAMA Psychiatry. 2017 Aug 9. doi: 10.1001/jamapsychiatry.2017.1981). However, he said, several studies show that lowering the risk for future alcohol-related problems in 18 year olds is possible.
He said his group delivered an intervention to 500 college freshmen using Internet-based videos aimed at helping them “recognize their vulnerability toward heavy drinking.” Six and 12 months after watching four 50-minute videos, the effects on how much the students drank remained significant, he wrote. In addition, other studies have identified programs that help lower drinking during pregnancy. “These are only a few examples of ongoing hopeful developments,” Dr. Schuckit wrote.
The number in the study that is especially concerning for him, Dr. Schuckit said, is the 106% increase in AUDs among older individuals because of the many preexisting medical disorders “that can be exacerbated by heavier drinking. These drinkers are also likely to be taking multiple medications that can interact adversely with alcohol, with resulting significant and costly health consequences,” according to Dr. Schuckit.
“There is also some disturbing news,” he wrote. “The proposed cuts to the National Institutes of Health budget being considered in Washington in 2017 are potentially disastrous for future efforts to decrease alcohol problems and are likely to result in higher costs for us all. Efforts to identify risk factors for substance-related problems and to test prevention approaches take time and money and are less likely to be funded in the current financial atmosphere. … If we ignore these problems, they will come back to us at much higher costs through emergency department visits, impaired children … and higher costs for jails and prisons that are the last resort for help for many.”
Dr. Schuckit is affiliated with the department of psychiatry at the University of California, San Diego. He reported having no disclosures.
Nearly one in eight adults in the United States had been diagnosed with alcohol use disorder in 2012-2013, a nearly 50% increase from a decade earlier, according to a study published Aug. 9. Other substantial increases occurring across virtually all demographic groups included overall 12-month alcohol consumption and high-risk drinking, particularly among adults aged 65 and older, racial/ethnic minorities, women, and those with lower education and incomes.
“The marked increases in high-risk drinking and DSM-IV [alcohol use disorder] between 2001-2002 and 2012-2013 also mirror recent sharp increases in morbidity and mortality from diseases and injuries in which alcohol use has a substantial role or deceleration of previously seen declines,” wrote Bridget F. Grant, PhD, of the National Institute on Alcohol Abuse and Alcoholism (NIAAA) in Rockville, Md., and her associates. High-risk drinking was defined as five drinks (14 g of ethanol each) per occasion for men and four drinks per occasion for women at least weekly.
“Mortality among alcohol-affected drivers who were simultaneously distracted also increased between 2005 and 2009 by 63%,” they wrote (JAMA Psychiatry. 2017 Aug 9. doi: 10.1001/jamapsychiatry.2017.2161).
The researchers analyzed data from 43,093 respondents to the National Epidemiologic Survey on Alcohol and Related Conditions (April 2001-June 2002) and from 36,309 respondents to the National Epidemiologic Survey on Alcohol and Related Conditions III (April 2012-June 2013). Both surveys involved face-to-face interviews with a nationally representative sample of U.S. adults.
The findings showed that 12-month alcohol use had increased 11.2% between 2001-2002 and 2012-2013, from 65.4% to 72.7%. A substantial increase also occurred in high-risk drinking and alcohol use disorder as defined in the DSM-IV. High-risk drinking increased 29.9% during that time, from 9.7% to 12.6%, representing an increase of approximately 9.4 million Americans engaging in high-risk drinking.
Alcohol use disorder (AUD) increased 49.4%, from 8.5% to 12.7% – a percentage that accounts for an additional 12.3 million Americans with the diagnosis. That increase dwarfs the 14.8% increase in alcohol use disorder that was seen between 1991-1992 and 2001-2002, the authors pointed out. The prevalence of 12-month AUD rose significantly among adults aged 65 and older (106.7%), African American individuals (92.8%), and women (83.7%). Interestingly, all subgroups reported significant increases in AUD except for Native Americans and people living in rural areas. By comparison, the 12-month prevalence of AUD among men increased by a third (34.7%), and their high-risk drinking increased 15.5%.
“Drinking norms and values have become more permissive among women, along with increases in educational and occupational opportunities and rising numbers of women in the workforce, all of which may have contributed to increased high-risk drinking and AUD in women during the past decade,” the authors wrote. “Stress associated with pursuing a career and raising a family may lead to increases in high-risk drinking and AUD among women.”
These increases indicate potential future increases among women in alcohol-related conditions, such as breast cancer and liver cirrhosis. Increases may also occur in fetal alcohol spectrum disorder and exposure to violence, the authors wrote.
The increases in alcohol use, high-risk drinking, and AUD found among minorities may be related to increased stress and demoralization as wealth inequality widened between minorities and whites in the wake of the 2008 recession. Other inequalities, such as income and educational disparities, unemployment, residential segregation, discrimination, and less health care access may also play a role in those increases, the authors wrote.
One limitation of the study is that certain populations were not surveyed, such as homeless individuals and people who are incarcerated. This means that the prevalence of alcohol use, high-risk drinking, and AUD could be underestimated, Dr. Grant and her colleagues said. However, they said, the large sample sizes of the surveys might balance out that limitation and others.
Nevertheless, the increases found in alcohol use, high-risk drinking, and AUD “constitute a public health crisis that may be overshadowed by increases in much less prevalent substance use (marijuana, opiates, and heroin) during the same period,” Dr. Grant and her colleagues wrote. “The findings herein highlight the urgency of educating the public, policymakers, and health care professionals about high-risk drinking and AUD.” In addition, they called for broader effors to address the “individual, biological, environmental, and societal factors” influencing high-risk drinking and AUD.
The research was sponsored by the NIAAA, and funded by the National Institutes of Health. The authors reported having no disclosures.
Nearly one in eight adults in the United States had been diagnosed with alcohol use disorder in 2012-2013, a nearly 50% increase from a decade earlier, according to a study published Aug. 9. Other substantial increases occurring across virtually all demographic groups included overall 12-month alcohol consumption and high-risk drinking, particularly among adults aged 65 and older, racial/ethnic minorities, women, and those with lower education and incomes.
“The marked increases in high-risk drinking and DSM-IV [alcohol use disorder] between 2001-2002 and 2012-2013 also mirror recent sharp increases in morbidity and mortality from diseases and injuries in which alcohol use has a substantial role or deceleration of previously seen declines,” wrote Bridget F. Grant, PhD, of the National Institute on Alcohol Abuse and Alcoholism (NIAAA) in Rockville, Md., and her associates. High-risk drinking was defined as five drinks (14 g of ethanol each) per occasion for men and four drinks per occasion for women at least weekly.
“Mortality among alcohol-affected drivers who were simultaneously distracted also increased between 2005 and 2009 by 63%,” they wrote (JAMA Psychiatry. 2017 Aug 9. doi: 10.1001/jamapsychiatry.2017.2161).
The researchers analyzed data from 43,093 respondents to the National Epidemiologic Survey on Alcohol and Related Conditions (April 2001-June 2002) and from 36,309 respondents to the National Epidemiologic Survey on Alcohol and Related Conditions III (April 2012-June 2013). Both surveys involved face-to-face interviews with a nationally representative sample of U.S. adults.
The findings showed that 12-month alcohol use had increased 11.2% between 2001-2002 and 2012-2013, from 65.4% to 72.7%. A substantial increase also occurred in high-risk drinking and alcohol use disorder as defined in the DSM-IV. High-risk drinking increased 29.9% during that time, from 9.7% to 12.6%, representing an increase of approximately 9.4 million Americans engaging in high-risk drinking.
Alcohol use disorder (AUD) increased 49.4%, from 8.5% to 12.7% – a percentage that accounts for an additional 12.3 million Americans with the diagnosis. That increase dwarfs the 14.8% increase in alcohol use disorder that was seen between 1991-1992 and 2001-2002, the authors pointed out. The prevalence of 12-month AUD rose significantly among adults aged 65 and older (106.7%), African American individuals (92.8%), and women (83.7%). Interestingly, all subgroups reported significant increases in AUD except for Native Americans and people living in rural areas. By comparison, the 12-month prevalence of AUD among men increased by a third (34.7%), and their high-risk drinking increased 15.5%.
“Drinking norms and values have become more permissive among women, along with increases in educational and occupational opportunities and rising numbers of women in the workforce, all of which may have contributed to increased high-risk drinking and AUD in women during the past decade,” the authors wrote. “Stress associated with pursuing a career and raising a family may lead to increases in high-risk drinking and AUD among women.”
These increases indicate potential future increases among women in alcohol-related conditions, such as breast cancer and liver cirrhosis. Increases may also occur in fetal alcohol spectrum disorder and exposure to violence, the authors wrote.
The increases in alcohol use, high-risk drinking, and AUD found among minorities may be related to increased stress and demoralization as wealth inequality widened between minorities and whites in the wake of the 2008 recession. Other inequalities, such as income and educational disparities, unemployment, residential segregation, discrimination, and less health care access may also play a role in those increases, the authors wrote.
One limitation of the study is that certain populations were not surveyed, such as homeless individuals and people who are incarcerated. This means that the prevalence of alcohol use, high-risk drinking, and AUD could be underestimated, Dr. Grant and her colleagues said. However, they said, the large sample sizes of the surveys might balance out that limitation and others.
Nevertheless, the increases found in alcohol use, high-risk drinking, and AUD “constitute a public health crisis that may be overshadowed by increases in much less prevalent substance use (marijuana, opiates, and heroin) during the same period,” Dr. Grant and her colleagues wrote. “The findings herein highlight the urgency of educating the public, policymakers, and health care professionals about high-risk drinking and AUD.” In addition, they called for broader effors to address the “individual, biological, environmental, and societal factors” influencing high-risk drinking and AUD.
The research was sponsored by the NIAAA, and funded by the National Institutes of Health. The authors reported having no disclosures.
FROM JAMA PSYCHIATRY
Key clinical point: Drinking and alcohol use disorder have substantially increased across virtually all demographic groups, particularly racial/ethnic minorities, adults aged 65 and older, and women.
Major finding: High-risk drinking increased 9.7%, and alcohol use disorder increased 49.4% among U.S. adults between 2001-2002 and 2012-2013.
Data source: The findings are based on data from 43,093 U.S. adults in 2001-2002 and 36,309 U.S. adults in 2012-2013.
Disclosures: The research was sponsored by the National Institute on Alcohol Abuse and Alcoholism, and funded by the National Institutes of Health. The authors reported having no disclosures.
Study finds secukinumab effective for moderate to severe scalp psoriasis
Secukinumab is a safe and effective treatment option for patients with moderate to severe scalp psoriasis, according to Dr. Jerry Bagel of the Psoriasis Treatment Center of Central New Jersey, and his associates.
After 12 weeks of treatment with 300 mg of secukinumab, administered subcutaneously, 52.9% of the 50 patients who received secukinumab achieved a 90% reduction in their Psoriasis Scalp Severity Index score, and 35.3% achieved complete clearance of scalp psoriasis. Only 2% of the 47 patients in the placebo group achieved PSSI 90, and no one achieved complete clearance, in the phase 3b study.
“These promising results demonstrate the possibility of establishing PSSI 90 as a new benchmark for scalp psoriasis treatment outcome,” the investigators noted. Secukinumab (Cosentyx) is approved for moderate to severe plaque psoriasis, active psoriatic arthritis, and in adult patients who are candidates for systemic therapy or phototherapy, and ankylosing spondylitis.
Find the study in the Journal of the American Academy of Dermatology (doi: 10.1016/j.jaad.2017.05.033).
Secukinumab is a safe and effective treatment option for patients with moderate to severe scalp psoriasis, according to Dr. Jerry Bagel of the Psoriasis Treatment Center of Central New Jersey, and his associates.
After 12 weeks of treatment with 300 mg of secukinumab, administered subcutaneously, 52.9% of the 50 patients who received secukinumab achieved a 90% reduction in their Psoriasis Scalp Severity Index score, and 35.3% achieved complete clearance of scalp psoriasis. Only 2% of the 47 patients in the placebo group achieved PSSI 90, and no one achieved complete clearance, in the phase 3b study.
“These promising results demonstrate the possibility of establishing PSSI 90 as a new benchmark for scalp psoriasis treatment outcome,” the investigators noted. Secukinumab (Cosentyx) is approved for moderate to severe plaque psoriasis, active psoriatic arthritis, and in adult patients who are candidates for systemic therapy or phototherapy, and ankylosing spondylitis.
Find the study in the Journal of the American Academy of Dermatology (doi: 10.1016/j.jaad.2017.05.033).
Secukinumab is a safe and effective treatment option for patients with moderate to severe scalp psoriasis, according to Dr. Jerry Bagel of the Psoriasis Treatment Center of Central New Jersey, and his associates.
After 12 weeks of treatment with 300 mg of secukinumab, administered subcutaneously, 52.9% of the 50 patients who received secukinumab achieved a 90% reduction in their Psoriasis Scalp Severity Index score, and 35.3% achieved complete clearance of scalp psoriasis. Only 2% of the 47 patients in the placebo group achieved PSSI 90, and no one achieved complete clearance, in the phase 3b study.
“These promising results demonstrate the possibility of establishing PSSI 90 as a new benchmark for scalp psoriasis treatment outcome,” the investigators noted. Secukinumab (Cosentyx) is approved for moderate to severe plaque psoriasis, active psoriatic arthritis, and in adult patients who are candidates for systemic therapy or phototherapy, and ankylosing spondylitis.
Find the study in the Journal of the American Academy of Dermatology (doi: 10.1016/j.jaad.2017.05.033).
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Acute otitis media: Which children to treat
Children whose ear infections involve severe bulging of the eardrum are likely to benefit from antibiotic treatment, while children with a peaked tympanogram pattern are likely to recover from the infections without the use of antibiotics, according to results from a new study.
The findings, published online Aug. 8 (Pediatrics. 2017), may help restrict pediatricians’ use of antibiotics to the patients who are most likely to benefit. For their research, Paula A. Tähtinen, MD, PhD, of Turku (Finland) University Hospital, and her colleagues, analyzed results from a previous placebo-controlled trial of 319 children in Finland with acute otitis media. Subjects were aged 6-35 months (median age 14 months; 57% boys; 99% white). The children in the cohort were evenly randomized to treatment with amoxicillin or placebo for 7 days. In the antibiotic arm, about 19% of patients failed to respond to treatment, while about 45% in the placebo arm did.
Dr. Tähtinen and her colleagues noted that in their study, severe bulging was an important prognostic factor irrespective of the number of ears affected or the severity of other common symptoms such as pain or fever. In this study, involvement of both ears and severity of other symptoms such as pain or fever were not seen affecting risk of treatment failure. Though only a minority of children in the study had a peaked tympanogram, failure rates for these were low in both treatment and placebo groups, and the number needed to treat to prevent treatment failure was 1 in 29.
“The evaluation of otoscopic signs is always subjective and prone to interobserver bias,” the researchers wrote. “Severe bulging is, however, a sign that is difficult to miss even for a less experienced otoscopist, and therefore this prognostic factor as an indication for antimicrobial treatment could be easily applied into clinical practice.”
Dr. Tähtinen and her colleagues’ research was supported by grants from the European Society for Pediatric Infectious Diseases and several other foundations. The study authors disclosed no financial conflicts of interest.
Children whose ear infections involve severe bulging of the eardrum are likely to benefit from antibiotic treatment, while children with a peaked tympanogram pattern are likely to recover from the infections without the use of antibiotics, according to results from a new study.
The findings, published online Aug. 8 (Pediatrics. 2017), may help restrict pediatricians’ use of antibiotics to the patients who are most likely to benefit. For their research, Paula A. Tähtinen, MD, PhD, of Turku (Finland) University Hospital, and her colleagues, analyzed results from a previous placebo-controlled trial of 319 children in Finland with acute otitis media. Subjects were aged 6-35 months (median age 14 months; 57% boys; 99% white). The children in the cohort were evenly randomized to treatment with amoxicillin or placebo for 7 days. In the antibiotic arm, about 19% of patients failed to respond to treatment, while about 45% in the placebo arm did.
Dr. Tähtinen and her colleagues noted that in their study, severe bulging was an important prognostic factor irrespective of the number of ears affected or the severity of other common symptoms such as pain or fever. In this study, involvement of both ears and severity of other symptoms such as pain or fever were not seen affecting risk of treatment failure. Though only a minority of children in the study had a peaked tympanogram, failure rates for these were low in both treatment and placebo groups, and the number needed to treat to prevent treatment failure was 1 in 29.
“The evaluation of otoscopic signs is always subjective and prone to interobserver bias,” the researchers wrote. “Severe bulging is, however, a sign that is difficult to miss even for a less experienced otoscopist, and therefore this prognostic factor as an indication for antimicrobial treatment could be easily applied into clinical practice.”
Dr. Tähtinen and her colleagues’ research was supported by grants from the European Society for Pediatric Infectious Diseases and several other foundations. The study authors disclosed no financial conflicts of interest.
Children whose ear infections involve severe bulging of the eardrum are likely to benefit from antibiotic treatment, while children with a peaked tympanogram pattern are likely to recover from the infections without the use of antibiotics, according to results from a new study.
The findings, published online Aug. 8 (Pediatrics. 2017), may help restrict pediatricians’ use of antibiotics to the patients who are most likely to benefit. For their research, Paula A. Tähtinen, MD, PhD, of Turku (Finland) University Hospital, and her colleagues, analyzed results from a previous placebo-controlled trial of 319 children in Finland with acute otitis media. Subjects were aged 6-35 months (median age 14 months; 57% boys; 99% white). The children in the cohort were evenly randomized to treatment with amoxicillin or placebo for 7 days. In the antibiotic arm, about 19% of patients failed to respond to treatment, while about 45% in the placebo arm did.
Dr. Tähtinen and her colleagues noted that in their study, severe bulging was an important prognostic factor irrespective of the number of ears affected or the severity of other common symptoms such as pain or fever. In this study, involvement of both ears and severity of other symptoms such as pain or fever were not seen affecting risk of treatment failure. Though only a minority of children in the study had a peaked tympanogram, failure rates for these were low in both treatment and placebo groups, and the number needed to treat to prevent treatment failure was 1 in 29.
“The evaluation of otoscopic signs is always subjective and prone to interobserver bias,” the researchers wrote. “Severe bulging is, however, a sign that is difficult to miss even for a less experienced otoscopist, and therefore this prognostic factor as an indication for antimicrobial treatment could be easily applied into clinical practice.”
Dr. Tähtinen and her colleagues’ research was supported by grants from the European Society for Pediatric Infectious Diseases and several other foundations. The study authors disclosed no financial conflicts of interest.
FROM PEDIATRICS
Key clinical point: Severe bulging of the eardrum, or a peaked tympanogram pattern, can inform the administration or withholding of antibiotic treatment in AOM.
Major finding: The number needed to treat using antibiotics among children presenting with severe eardrum bulging was 1.9.
Data source: Analysis of results from a randomized, placebo controlled trial (n = 319) of children younger than 3 with AOM conducted in Finland during 2006-2008.
Disclosures: Study authors disclosed research support from several foundations but no commercial conflicts of interest.
Perceived financial hardship among patients with advanced cancer
The American Cancer Society has identified a disparity in cancer death rates, noting that persons with lower socioeconomic status have higher rates of mortality.1 This is attributed to many factors, but it is largely owing to the higher burden of disease among lower-income individuals.1 A component of this disease burden is measured by assessing the patient-reported outcome of cancer-related distress. The National Comprehensive Cancer Network (NCCN) Distress Management Guidelines have defined distress as “a multifactorial unpleasant emotional experience of a psychological (cognitive, behavioral, emotional), social and/or spiritual nature that may interfere with the ability to cope with cancer, its physical symptoms and its treatment.”2
Financial hardship related to cancer diagnosis and treatment is increasingly being recognized as an important component of disease burden and distress. The advancements in costly cancer treatments have produced burdensome direct medical costs as well as numerous indirect costs that contribute to perceived financial hardship.3,4 These indirect costs include nonmedical expenses such as increased transportation needs or childcare, loss of earnings, or loss of household income due to caregiving needs.3 Moreover, indirect costs are often managed by patients and families through their use of savings, borrowing, reducing leisure activities, and selling possessions.3 Even though efforts to increase health coverage, such as the Affordable Care Act, have reduced the rates of individuals who are uninsured, persons with cancer who have insurance also face challenges because they cannot afford copays, monthly premiums, deductibles, and other high out-of-pocket expenses related to cancer treatment that are not covered by their insurance such as out-of-network services or providers.5-7
Thus, financial hardship may have an impact on several areas of a patient’s life and well-being, but the effects are commonly undetected.8-10 Research has established that financial strain can influence treatment choices and adherence to therapy.11 Furthermore, the effects of financial strain have been identified across the cancer care continuum, from diagnosis through survivorship, suggesting a bidirectional relationship between financial strain and well-being.11 Financial strain may reduce patient quality of life and worsen symptom burden because of the patient’s inability to access needed care, poor social supports, and/or increased stress.11-12 These worsening outcomes may also increase the use of financial reserves and affect their ability to work.7,11 Financial difficulties may also be associated with anxiety and depression, leading to worse quality of life and greater distress and symptom burden.12 Identifying groups at high risk for financial strain is crucial to ensure that resources are available to assist these populations.13 This burden can be even more pronounced in minority and underserved patients with cancer.7 Patients with advanced cancer are especially vulnerable to the burden of increased costs because of the use of expensive targeted therapies; their improved survival, which extends the time of expenditure; and increased use of financial reserves.9 Financial hardship in patients with advanced cancer is not well understood or characterized,9 which is why this study aimed to better quantify distress in advanced stage cancers by describing :
▪ A cohort of patients with advanced cancer and their levels of quality of life, symptom distress, cancer-related distress and perceived financial hardship;
▪ The relationship between perceived financial hardship, quality of life, symptom distress and overall cancer-related distress; and
▪ Quality of life, symptom distress, and overall cancer-related distress according to level of perceived financial hardship.
Methods
This study is a cross-sectional, descriptive, comparative study of distress, including perceived financial hardship, among patients with advanced cancer who were receiving palliative care treatment in two outpatient medical oncology clinics in Western Pennsylvania. The data were collected during May 2013-November 2014. The study protocol was approved by the Institutional Review Board at the University of Pittsburgh. Eligible participants had to be 18 years or older and have an advanced solid tumor of any kind, with a prognosis of 1 year or less confirmed by a physician or clinic nurse practitioner/physician assistant, and be able to read and understand English at the fourth-grade level. The sample was recruited from two clinics at the University of Pittsburgh Cancer Institute, a National Cancer Institute-designated Comprehensive Cancer Program.
Measurements
Sociodemographic factors. These were measured using an investigator-derived Sociodemographic Questionnaire, a 12-item form that includes variables such as age, race, marital status, cancer type, religion and spirituality, employment status, years of education, health insurance status, and income level.
Cancer-related distress. The NCCN Distress Thermometer is a self-report visual analog scale (0, no distress; 10, great distress) formed in the shape of a thermometer combined with a problem list that is often used in outpatient cancer settings for reporting of cancer-related distress.14-16 The sensitivity, specificity and convergent validity with the Brief Symptom Inventory and the Hospital Anxiety and Depression Scale have been established and appropriate cut-off score of the distress thermometer identified.14-16 A score of 4 or above indicates a clinically significant level of distress.14-16
Symptom distress. The McCorkle Symptom Distress Scale was developed in 1977 based on interviews that focused on the symptom experiences of patients. Psychometric testing among patients with cancer using the modified Symptom Distress Scale revealed high reliability (Cronbach alpha, 0.97).17 The instrument is a 13-item Likert scale (1-5) assessing the severity of distress experienced by a symptom. Total scores range from 13 to 65, where a higher score indicates greater distress. Moderate distress is indicated with a score of 25-33, and a score above 33 indicates severe distress, identifying the need for immediate intervention.17
Quality of life and spiritual well-being. The Functional Assessment of Cancer Therapy (FACT-G) is used to assess general cancer-related quality of life. It has four subscales: physical, emotional, social and family, and functional well-being, with a total score that ranges from 0-112, where higher scores show higher quality of life. The Spiritual Distress Well-Being questionnaire was used alongside the valid FACT-G assessment.18,19 The Spiritual Well-Being Short Form was developed with an ethnically diverse population and adds 12 items to the FACT-G. The items do not necessarily assume a faith in God, allowing a wide flexibility in application and tapping into issues such as faith, meaning, and finding peace and comfort despite advanced illness. Higher scores on the Spiritual Well-Being subscore (range, 0-48) are correlated with higher scores of quality of life. The possible scores for the combined FACT-G and Spiritual Well-Being assessment range from 0-160, with higher scores showing higher quality of life.
Economic hardship. Perceived financial hardship was measured using Barrera and colleagues’ Psychological Sense of Economic Hardship Scale. 20 The scale consists of 20-items broken down into 4 subscales: financial strain, inability to make ends meet, not enough money for necessities, and economic adjustments.20 Economic adjustments in the 3 months before administration of the questionnaire were assessed with 9 Yes or No items, such as added another job, received government assistance, or sold possessions to increase income. The subscale of not enough money for necessities was assessed with seven 5-point scale items in which respondents noted whether they felt they had enough money for housing, clothing, home furnishings, and a car over the previous 3 months. Inability to make ends meet included two 5-point scale items that assessed the difficulty in meeting financial demands in the previous 3 months. Financial strain consisted of two 5-point scale items concerned with expecting financial hardships in the coming 3 months. Scores can range from 20-73, with a higher score indicating worse economic hardship.
Data collection and analysis
In-person data collection occurred in the clinical waiting area before the clinician visit or in the treatment room with the patient using a consecutive, convenience sample. The nursing staff checked the clinic lists daily for possible patient participants. Patients with metastatic cancer were identified and then approached for consent. After we had received the patient’s consent, the administration of the instruments took about 20 minutes to complete. The data were then entered and verified in REDCap (Research Electronic Data Capture), which is hosted at the University of Pittsburgh.21The levels of symptom distress, quality of life, perceived financial hardship, and cancer-related distress were described through continuously measured variables. Descriptive statistics, measures of central tendency (mean and median), and dispersion (standard deviation and range), were obtained for the subscales and total scores. Correlation analysis was used to describe the relationship between perceived financial hardship and quality of life, symptom distress, and cancer-related distress. These primary outcome variables were further explored according to the level of dichotomized perceived financial hardship using mean score as the cut point. Independent sample t tests were used to compare patients experiencing high perceived financial hardship with those experiencing low perceived financial hardship.
Results
In all, 100 patients participated in the study. Any missing data points were replaced with the mean score for that variable, although this was minimal in this study. Most of the participants were women (67%), and the average age of the participants was 63.43 years (SD, 13.05; Table 1). Of the total number of participants, 73% were white, 26% were black, and 1% were Asian. Most of the participants were either retired and not working (39%) or disabled or unable to work (34%). Almost all of the participants had some form of insurance, with 99% having either private or public health insurance. A variety of cancer types were represented in this patient population, with higher percentages of breast (25%), gynecologic (10%), lung (19%), and colon/rectal cancer (15%). Of the total number of participants, 35% had annual household incomes below $20,000, and 50% had annual household incomes of more than $20,000. On average, participants had 13.48 years (SD, 2.78) of formal education.
Descriptive statistics for the primary outcome variables can be found in Table 2. The average score for cancer-related distress based on the NCCN Distress Thermometer tool was 4.16 (SD, 3.26). The average score for the McCorkle Symptom Distress measurement was 25.45 (SD, 9.34). For quality of life, the average FACT-G total score was 73.77 (SD, 19.40). Of the FACT-G subscale average scores, physical well-being was 17.35 (SD, 7.50), social/family well-being 24.21 (SD, 5.25), emotional well-being 16.34 (SD, 5.42), and functional well-being 15.87 (SD, 6.78). Participants’ average score for the spiritual well-being measure was 35.20 (SD, 9.25) and the combined FACT-G and spiritual well-being average score was 108.97 (SD, 26.07). The total average score for perceived financial hardship was 35.70 (SD, 13.87), with subscale average scores of 3.44 (SD, 2.36) for financial strain, 5.73 (SD, 1.91) for inability to make ends meet, 16.43 (SD, 8.92) for not enough money for necessities, and 10.63 (SD, 2.70) for economic adjustments.
We conducted a bivariate correlation analysis to assess the relationship between perceived financial hardship and three other primary outcome variables (Table 3). These analyses showed significant low to moderate correlations with overall cancer-related distress (r, 0.439; P < .001), symptom distress (r, 0.409; P < .001) and overall quality of life scores (FACT-G and spiritual well-being combined score: r, -0.323; P < .001).
Forty-three participants reporting high perceived financial hardship experienced worse quality of life overall (FACT-G and spiritual well-being; P = .002), worse FACT-G total scores (P < .001), worse physical well-being (P < .001), worse social/family well-being (P = .029), worse emotional well-being, and no significant difference for functional (P = .082) or spiritual well-being (P = .453), compared with those with lower economic hardship. In overall cancer-related distress, participants with higher perceived financial hardship reported higher levels of cancer-related distress (P < .001) than those with lower perceived financial hardship. For those participants reporting higher perceived financial hardship there was also worse symptom distress (P < .001), compared with those with lower economic hardship (Table 4).
Discussion
Overall, this report provides data to illuminate our understanding of disparities in well-being that may be present in patients with advanced cancer. Our analysis found that patients with advanced cancer who have higher perceived financial hardship have significantly higher overall cancer-related distress, symptom distress, and poorer overall quality of life. In this study’s population of patients with advanced cancer, the most notable areas of economic hardship identified by participants were: not having enough money for necessities in the 3 months before the survey and the inability to make ends meet during the same time span, with difficulty paying bills and not having enough money left at the end of the month being most noteworthy among this study’s patient population. Financial strain and making economic adjustment were not as notable in the category of perceived financial hardship.
In regard to not having enough money, participants most commonly cited not being able to afford everyday necessities such as food, clothing, medical care, or a home, as well as leisure and recreational activities. These findings are further supported with the positive, moderate associations between perceived financial hardship and symptom distress and overall cancer-related distress found in this cohort of patients with advanced cancer and the negative, moderately associated relationship between perceived financial hardship and overall quality of life in this study’s sample.
Although these findings have been confirmed in the literature on cancer-related distress, our findings add to our knowledge on both economic and cancer-related distress exclusively in patients with advanced cancer.9,22 The broader cancer-related distress literature has also found an association between being younger and having a lower household income as risk factors for increased financial hardship; however, the perception of financial strain and magnitude was a more significant predictor of quality of life and perception of overall well-being.6,8-9,12,22-23 Furthermore, patients with cancer who noted having higher financial distress typically reported decreased satisfaction with cancer care which also influenced their adherence to treatment and quality of life.24
Our work now adds the important element of perceived financial hardship to the advanced cancer-related distress puzzle. We should consider integrating a financial distress assessment into routine cancer care, particularly with patients and families with advanced cancer, to proactively and routinely assess and intervene with available distress mitigating resources. Therefore, understanding the patients most likely to experience financial distress will help personalize supportive therapy.
This study’s results as well as the existing literature describing financial distress support the use of comprehensive screening instruments to capture elements of financial burden beyond out-of-pocket costs.8,25 This screening is particularly relevant because we are increasingly recognizing that gross annual household income does not always reflect financial hardship or distress. The instrument we used for this analysis, the Psychological Sense of Economic Hardship, provides a broad view of financial toxicity including the specific components of financial strain, the inability to make ends meet, not having enough money for necessities, and economic adjustments experienced by patients with advanced cancer.20 Another measure to evaluate financial toxicity among patients with cancer includes the Comprehensive Score for Financial Toxicity (COST), which is a widely used patient-reported outcome measure. It was developed with input from both patients and oncology experts.25 Use of a financial toxicity assessment tool adds to our understanding of the economic financial burden experienced by patients with cancer, specifically those with advanced cancer.
Tucker-Seeley and Yabroff have identified several areas in which the research agenda for financial toxicity should focus, including: documentation of the socioeconomic context among patients across all areas of the cancer care continuum, further identification and characterization of at risk populations to address health disparities, and the inclusion of cost discussions in the health care context.26 Furthermore, research is needed to identify key areas to target for interventions addressing financial toxicity, such as addressing lack of financial resources to cover the cost of cancer care, focusing on managing or preventing the distress that results from a lack of financial resources, or addressing coping behaviors used by families to manage the financial burden of cancer care.26 Although cost discussions between health care providers and patients have been identified as important in reducing the financial burden of cancer care, the content, timing, and goals of those discussions still need to be better articulated for different patient populations, including patients with advanced cancer.3,27-28 In addition, resources such as social workers, patient navigators, or financial counselors have been identified as effective in assisting patients with financial planning and accessing community resources to address financial burden and assistance.4
Design considerations
This study has limitations that need to be noted. Its cross-sectional design does not allow for the analysis of causal inferences. In addition, certain groups were underrepresented in this study’s sample, including uninsured patients, men, and some minority groups, which may have underestimated the amount of financial burden experienced by patients with advanced cancer. The lack of representativeness of uninsured individuals may be a result of the eligibility of persons with advanced cancer for Medicaid. However, a strength of this study is its ability to increase the representativeness of African American/black patients in the study of advanced cancer and financial hardship. In our study, just over a quarter of the participants (26 of 100; 26%) were black/African American, compared with the US Census Bureau’s national census level of 13.3% and 13.4% in Allegheny County, Pennsylvania .29
The lack of employed participants in this study could be because many were not able to work because of the advanced stage of their disease. The low level of partnered status is a limitation, although one study site was a low-income hospital where one generally tends to see higher levels of unpartnered status. This study did not control for demographic information such as gender or age, thus, the relationships between the primary outcome variables and financial hardship may be overestimated. Moreover, this analysis of financial distress is limited to the context of the United States due to our lack of universal health care and unique payment system. Although we included only patients who were in the palliative phase of cancer treatment, no medical record review was conducted to determine previous cancer history and treatments, which might have provided more insight into other financial loss or cost of cancer treatment. Furthermore, we note that it can be difficult to prognosticate with accuracy and identify that some patients with advanced cancer may have been excluded from the study due to the inclusion criteria of less than 1 year of survival.
Conclusion
Perceived financial hardship is an important assessment of the burden placed on patients due to the cost of disease; and is a good start in assessing indirect costs that patients take on when coping with advanced stages of cancer and can shed light on an aspect of distress experienced by this patient population that is not commonly addressed. Subjective measures of perceived financial hardship complement objective measures that are commonly indicative of economic resources and can further our understanding of the impact of financial distress experienced by patients with cancer. Further study of financial impacts of advanced cancer as well as predictors of financial distress are essential to the early identification of financial hardship and the development of interventions to support those at high risk or experiencing financial distress.
Acknowledgments
The authors acknowledge the patients and staff at the UPMC Mercy Cancer Center in Pittsburgh, Pennsylvania, who made this study possible, and Peggy Tate for her role in data collection. They also recognize the support of the Robert Wood Johnson Foundation through the Future Nursing Scholars program. They would also like to acknowledge that permission was granted for the use of the Psychological Sense of Economic Hardship study instrument.
1. American Cancer Society. Cancer Facts & Figures 2015. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2015/cancer-facts-and-figures-2015.pdf. Atlanta, GA: American Cancer Society. 2015. Accessed January 16, 2016.
2. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology distress management version 1.2014. http://williams.medicine.wisc.edu/distress.pdf. Updated May 2014. Accessed January 16, 2016.
3. de Souza JA, Wong Y-N. Financial distress in cancer patients. J Med Person. 2014;11(2):13-15.
4. Mcdougall JA, Ramsey SD, Hutchinson F, Shih Y-CT. Financial toxicity: a growing concern among cancer patients in the United States. ISPOR Connect. 2014;20(2):10-11.
5. Sharpe K, Shaw B, Seiler MB. Practical solutions when facing cost sharing: the American Cancer Society’s health insurance assistance service. Am J Manag Care. 2016;22(4):92-94.
6. Shankaran V, Jolly S, Blough D, Ramsey SD. Risk factors for financial hardship in patients receiving adjuvant chemotherapy for colon cancer : a population-based exploratory analysis. J Clin Oncol. 2012;30(14):1608-1614.
7. Meneses K, Azuero A, Hassey L, Mcnees P, Pisu M. Does economic burden influence quality of life in breast cancer survivors? Gynecol Oncol. 2012;124(3):437-443.
8. Catt S, Starkings R, Shilling V, Fallowfield L. Patient-reported outcome measures of the impact of cancer on patients’ everyday lives: a systematic review. J Cancer Surviv. 2017;11(2):211-232.
9. Delgado-Guay M, Ferrer J, Rieber AG, et al. Financial distress and its associations with physical and emotional symptoms and quality of life among advanced cancer patients. Oncologist. 2015;20:1092-1098.
10. Kale HP, Carroll N V. Self-reported financial burden of cancer care and its effect on physical and mental health-related quality of life among US cancer survivors. Cancer. 2016;122:1283-1289.
11. Lathan CS, Cronin A, Tucker-Seeley R, Zafar SY, Ayanian JZ, Schrag D. Association of financial strain with symptom burden and quality of life for patients with lung or colorectal cancer. J Clin Oncol. 2016;34(15):1732-1740.
12. Fenn KM, Evans SB, Mccorkle R, et al. Impact of financial burden of cancer on survivors’ quality of life. J Onocol Pract. 2014;10(5):332-339.
13. Azzani M, Roslani AC, Su TT. The perceived cancer-related financial hardship among patients and their families: a systematic review. Support Cancer Care. 2015;23:889-898.
14. Jacobsen PB, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients a multicenter evaluation of the distress thermometer. Cancer. 2005;103:1494-1502.
15. Ransom S, Jacobsen PB, Booth-Jones M. Validation of the distress thermometer with bone marrow. Psychooncology. 2006;15:604-612.
16. Vodermaier A, Linden W, Siu C. Screening for emotional distress in cancer patients: a systematic review of assessment instruments. J Natl Cancer Inst. 2009;101:1464-1488.
17. McCorkle R, Quint-Benoliel J. Symptom distress, current concerns and mood disturbance after diagnosis of life-threatening disease. Soc Sci Med. 1983;17(7):431–8.
18. Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570-579.
19. Peterman AH, Fitchett G, Brady MJ, Hernandez L, Cella D. Measuring spiritual well-being in people with cancer: the functional assessment of chronic illness therapy – Spiritual Well-being Scale (FACIT-Sp). Ann Behav Med. 2002;24:49–58.
20. Barrera M, Caples H, Tein J. The psychological sense of economic hardship: measurement models, validity, and cross-ethnic equivalence for urban families. Am J Community Psychol. 2001;29:493-517.
21. Harris PA, Taylor R, Thielke R. Research electronic data capture (REDCap): A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381.
22. Zafar SY, McNeil RB, Thomas CM, Lathan CS, Ayanian JZ, Provenzale D. Population-based assessment of cancer survivors’ financial burden and quality of life: a prospective cohort study. J Oncol Pract. 2015;11(2):145-152.
23. Kent EE, Forsythe LP, Yabroff KR, et al. Are survivors who report cancer-related financial problems more likely to forgo or delay medical care? Cancer. 2013;119:3710-3717.
24. Chino F, Peppercorn J, Taylor Jr. DH, et al. Self-reported financial burden and satisfaction with care among patients with cancer. Oncologist. 2014;19:414-420.
25. De Souza JA, Yap BJ, Hlubocky FJ, et al. The development of a financial toxicity patient-reported outcome in cancer. Cancer. 2014;120:3245-3253.
26. Tucker-Seeley RD, Yabroff KR. Minimizing the “financial toxicity” associated with cancer care : advancing the research agenda. J Natl Cancer Inst. 2016;108(5):1-3.
27. Bestvina CM, Zullig LL, Rushing C, et al. Patient-oncologist cost communication, financial distress, and medication adherence. J Oncol Pract. 2014;10(3):162-168.
28. Irwin B, Kimmick G, Altomare I, et al. Patient experience and attitudes toward addressing the cost of breast cancer care. Oncologist. 2014;19:1135-1140.
29. US Census Bureau. United States. https://www.census.gov/quickfacts/. 2015.
The American Cancer Society has identified a disparity in cancer death rates, noting that persons with lower socioeconomic status have higher rates of mortality.1 This is attributed to many factors, but it is largely owing to the higher burden of disease among lower-income individuals.1 A component of this disease burden is measured by assessing the patient-reported outcome of cancer-related distress. The National Comprehensive Cancer Network (NCCN) Distress Management Guidelines have defined distress as “a multifactorial unpleasant emotional experience of a psychological (cognitive, behavioral, emotional), social and/or spiritual nature that may interfere with the ability to cope with cancer, its physical symptoms and its treatment.”2
Financial hardship related to cancer diagnosis and treatment is increasingly being recognized as an important component of disease burden and distress. The advancements in costly cancer treatments have produced burdensome direct medical costs as well as numerous indirect costs that contribute to perceived financial hardship.3,4 These indirect costs include nonmedical expenses such as increased transportation needs or childcare, loss of earnings, or loss of household income due to caregiving needs.3 Moreover, indirect costs are often managed by patients and families through their use of savings, borrowing, reducing leisure activities, and selling possessions.3 Even though efforts to increase health coverage, such as the Affordable Care Act, have reduced the rates of individuals who are uninsured, persons with cancer who have insurance also face challenges because they cannot afford copays, monthly premiums, deductibles, and other high out-of-pocket expenses related to cancer treatment that are not covered by their insurance such as out-of-network services or providers.5-7
Thus, financial hardship may have an impact on several areas of a patient’s life and well-being, but the effects are commonly undetected.8-10 Research has established that financial strain can influence treatment choices and adherence to therapy.11 Furthermore, the effects of financial strain have been identified across the cancer care continuum, from diagnosis through survivorship, suggesting a bidirectional relationship between financial strain and well-being.11 Financial strain may reduce patient quality of life and worsen symptom burden because of the patient’s inability to access needed care, poor social supports, and/or increased stress.11-12 These worsening outcomes may also increase the use of financial reserves and affect their ability to work.7,11 Financial difficulties may also be associated with anxiety and depression, leading to worse quality of life and greater distress and symptom burden.12 Identifying groups at high risk for financial strain is crucial to ensure that resources are available to assist these populations.13 This burden can be even more pronounced in minority and underserved patients with cancer.7 Patients with advanced cancer are especially vulnerable to the burden of increased costs because of the use of expensive targeted therapies; their improved survival, which extends the time of expenditure; and increased use of financial reserves.9 Financial hardship in patients with advanced cancer is not well understood or characterized,9 which is why this study aimed to better quantify distress in advanced stage cancers by describing :
▪ A cohort of patients with advanced cancer and their levels of quality of life, symptom distress, cancer-related distress and perceived financial hardship;
▪ The relationship between perceived financial hardship, quality of life, symptom distress and overall cancer-related distress; and
▪ Quality of life, symptom distress, and overall cancer-related distress according to level of perceived financial hardship.
Methods
This study is a cross-sectional, descriptive, comparative study of distress, including perceived financial hardship, among patients with advanced cancer who were receiving palliative care treatment in two outpatient medical oncology clinics in Western Pennsylvania. The data were collected during May 2013-November 2014. The study protocol was approved by the Institutional Review Board at the University of Pittsburgh. Eligible participants had to be 18 years or older and have an advanced solid tumor of any kind, with a prognosis of 1 year or less confirmed by a physician or clinic nurse practitioner/physician assistant, and be able to read and understand English at the fourth-grade level. The sample was recruited from two clinics at the University of Pittsburgh Cancer Institute, a National Cancer Institute-designated Comprehensive Cancer Program.
Measurements
Sociodemographic factors. These were measured using an investigator-derived Sociodemographic Questionnaire, a 12-item form that includes variables such as age, race, marital status, cancer type, religion and spirituality, employment status, years of education, health insurance status, and income level.
Cancer-related distress. The NCCN Distress Thermometer is a self-report visual analog scale (0, no distress; 10, great distress) formed in the shape of a thermometer combined with a problem list that is often used in outpatient cancer settings for reporting of cancer-related distress.14-16 The sensitivity, specificity and convergent validity with the Brief Symptom Inventory and the Hospital Anxiety and Depression Scale have been established and appropriate cut-off score of the distress thermometer identified.14-16 A score of 4 or above indicates a clinically significant level of distress.14-16
Symptom distress. The McCorkle Symptom Distress Scale was developed in 1977 based on interviews that focused on the symptom experiences of patients. Psychometric testing among patients with cancer using the modified Symptom Distress Scale revealed high reliability (Cronbach alpha, 0.97).17 The instrument is a 13-item Likert scale (1-5) assessing the severity of distress experienced by a symptom. Total scores range from 13 to 65, where a higher score indicates greater distress. Moderate distress is indicated with a score of 25-33, and a score above 33 indicates severe distress, identifying the need for immediate intervention.17
Quality of life and spiritual well-being. The Functional Assessment of Cancer Therapy (FACT-G) is used to assess general cancer-related quality of life. It has four subscales: physical, emotional, social and family, and functional well-being, with a total score that ranges from 0-112, where higher scores show higher quality of life. The Spiritual Distress Well-Being questionnaire was used alongside the valid FACT-G assessment.18,19 The Spiritual Well-Being Short Form was developed with an ethnically diverse population and adds 12 items to the FACT-G. The items do not necessarily assume a faith in God, allowing a wide flexibility in application and tapping into issues such as faith, meaning, and finding peace and comfort despite advanced illness. Higher scores on the Spiritual Well-Being subscore (range, 0-48) are correlated with higher scores of quality of life. The possible scores for the combined FACT-G and Spiritual Well-Being assessment range from 0-160, with higher scores showing higher quality of life.
Economic hardship. Perceived financial hardship was measured using Barrera and colleagues’ Psychological Sense of Economic Hardship Scale. 20 The scale consists of 20-items broken down into 4 subscales: financial strain, inability to make ends meet, not enough money for necessities, and economic adjustments.20 Economic adjustments in the 3 months before administration of the questionnaire were assessed with 9 Yes or No items, such as added another job, received government assistance, or sold possessions to increase income. The subscale of not enough money for necessities was assessed with seven 5-point scale items in which respondents noted whether they felt they had enough money for housing, clothing, home furnishings, and a car over the previous 3 months. Inability to make ends meet included two 5-point scale items that assessed the difficulty in meeting financial demands in the previous 3 months. Financial strain consisted of two 5-point scale items concerned with expecting financial hardships in the coming 3 months. Scores can range from 20-73, with a higher score indicating worse economic hardship.
Data collection and analysis
In-person data collection occurred in the clinical waiting area before the clinician visit or in the treatment room with the patient using a consecutive, convenience sample. The nursing staff checked the clinic lists daily for possible patient participants. Patients with metastatic cancer were identified and then approached for consent. After we had received the patient’s consent, the administration of the instruments took about 20 minutes to complete. The data were then entered and verified in REDCap (Research Electronic Data Capture), which is hosted at the University of Pittsburgh.21The levels of symptom distress, quality of life, perceived financial hardship, and cancer-related distress were described through continuously measured variables. Descriptive statistics, measures of central tendency (mean and median), and dispersion (standard deviation and range), were obtained for the subscales and total scores. Correlation analysis was used to describe the relationship between perceived financial hardship and quality of life, symptom distress, and cancer-related distress. These primary outcome variables were further explored according to the level of dichotomized perceived financial hardship using mean score as the cut point. Independent sample t tests were used to compare patients experiencing high perceived financial hardship with those experiencing low perceived financial hardship.
Results
In all, 100 patients participated in the study. Any missing data points were replaced with the mean score for that variable, although this was minimal in this study. Most of the participants were women (67%), and the average age of the participants was 63.43 years (SD, 13.05; Table 1). Of the total number of participants, 73% were white, 26% were black, and 1% were Asian. Most of the participants were either retired and not working (39%) or disabled or unable to work (34%). Almost all of the participants had some form of insurance, with 99% having either private or public health insurance. A variety of cancer types were represented in this patient population, with higher percentages of breast (25%), gynecologic (10%), lung (19%), and colon/rectal cancer (15%). Of the total number of participants, 35% had annual household incomes below $20,000, and 50% had annual household incomes of more than $20,000. On average, participants had 13.48 years (SD, 2.78) of formal education.
Descriptive statistics for the primary outcome variables can be found in Table 2. The average score for cancer-related distress based on the NCCN Distress Thermometer tool was 4.16 (SD, 3.26). The average score for the McCorkle Symptom Distress measurement was 25.45 (SD, 9.34). For quality of life, the average FACT-G total score was 73.77 (SD, 19.40). Of the FACT-G subscale average scores, physical well-being was 17.35 (SD, 7.50), social/family well-being 24.21 (SD, 5.25), emotional well-being 16.34 (SD, 5.42), and functional well-being 15.87 (SD, 6.78). Participants’ average score for the spiritual well-being measure was 35.20 (SD, 9.25) and the combined FACT-G and spiritual well-being average score was 108.97 (SD, 26.07). The total average score for perceived financial hardship was 35.70 (SD, 13.87), with subscale average scores of 3.44 (SD, 2.36) for financial strain, 5.73 (SD, 1.91) for inability to make ends meet, 16.43 (SD, 8.92) for not enough money for necessities, and 10.63 (SD, 2.70) for economic adjustments.
We conducted a bivariate correlation analysis to assess the relationship between perceived financial hardship and three other primary outcome variables (Table 3). These analyses showed significant low to moderate correlations with overall cancer-related distress (r, 0.439; P < .001), symptom distress (r, 0.409; P < .001) and overall quality of life scores (FACT-G and spiritual well-being combined score: r, -0.323; P < .001).
Forty-three participants reporting high perceived financial hardship experienced worse quality of life overall (FACT-G and spiritual well-being; P = .002), worse FACT-G total scores (P < .001), worse physical well-being (P < .001), worse social/family well-being (P = .029), worse emotional well-being, and no significant difference for functional (P = .082) or spiritual well-being (P = .453), compared with those with lower economic hardship. In overall cancer-related distress, participants with higher perceived financial hardship reported higher levels of cancer-related distress (P < .001) than those with lower perceived financial hardship. For those participants reporting higher perceived financial hardship there was also worse symptom distress (P < .001), compared with those with lower economic hardship (Table 4).
Discussion
Overall, this report provides data to illuminate our understanding of disparities in well-being that may be present in patients with advanced cancer. Our analysis found that patients with advanced cancer who have higher perceived financial hardship have significantly higher overall cancer-related distress, symptom distress, and poorer overall quality of life. In this study’s population of patients with advanced cancer, the most notable areas of economic hardship identified by participants were: not having enough money for necessities in the 3 months before the survey and the inability to make ends meet during the same time span, with difficulty paying bills and not having enough money left at the end of the month being most noteworthy among this study’s patient population. Financial strain and making economic adjustment were not as notable in the category of perceived financial hardship.
In regard to not having enough money, participants most commonly cited not being able to afford everyday necessities such as food, clothing, medical care, or a home, as well as leisure and recreational activities. These findings are further supported with the positive, moderate associations between perceived financial hardship and symptom distress and overall cancer-related distress found in this cohort of patients with advanced cancer and the negative, moderately associated relationship between perceived financial hardship and overall quality of life in this study’s sample.
Although these findings have been confirmed in the literature on cancer-related distress, our findings add to our knowledge on both economic and cancer-related distress exclusively in patients with advanced cancer.9,22 The broader cancer-related distress literature has also found an association between being younger and having a lower household income as risk factors for increased financial hardship; however, the perception of financial strain and magnitude was a more significant predictor of quality of life and perception of overall well-being.6,8-9,12,22-23 Furthermore, patients with cancer who noted having higher financial distress typically reported decreased satisfaction with cancer care which also influenced their adherence to treatment and quality of life.24
Our work now adds the important element of perceived financial hardship to the advanced cancer-related distress puzzle. We should consider integrating a financial distress assessment into routine cancer care, particularly with patients and families with advanced cancer, to proactively and routinely assess and intervene with available distress mitigating resources. Therefore, understanding the patients most likely to experience financial distress will help personalize supportive therapy.
This study’s results as well as the existing literature describing financial distress support the use of comprehensive screening instruments to capture elements of financial burden beyond out-of-pocket costs.8,25 This screening is particularly relevant because we are increasingly recognizing that gross annual household income does not always reflect financial hardship or distress. The instrument we used for this analysis, the Psychological Sense of Economic Hardship, provides a broad view of financial toxicity including the specific components of financial strain, the inability to make ends meet, not having enough money for necessities, and economic adjustments experienced by patients with advanced cancer.20 Another measure to evaluate financial toxicity among patients with cancer includes the Comprehensive Score for Financial Toxicity (COST), which is a widely used patient-reported outcome measure. It was developed with input from both patients and oncology experts.25 Use of a financial toxicity assessment tool adds to our understanding of the economic financial burden experienced by patients with cancer, specifically those with advanced cancer.
Tucker-Seeley and Yabroff have identified several areas in which the research agenda for financial toxicity should focus, including: documentation of the socioeconomic context among patients across all areas of the cancer care continuum, further identification and characterization of at risk populations to address health disparities, and the inclusion of cost discussions in the health care context.26 Furthermore, research is needed to identify key areas to target for interventions addressing financial toxicity, such as addressing lack of financial resources to cover the cost of cancer care, focusing on managing or preventing the distress that results from a lack of financial resources, or addressing coping behaviors used by families to manage the financial burden of cancer care.26 Although cost discussions between health care providers and patients have been identified as important in reducing the financial burden of cancer care, the content, timing, and goals of those discussions still need to be better articulated for different patient populations, including patients with advanced cancer.3,27-28 In addition, resources such as social workers, patient navigators, or financial counselors have been identified as effective in assisting patients with financial planning and accessing community resources to address financial burden and assistance.4
Design considerations
This study has limitations that need to be noted. Its cross-sectional design does not allow for the analysis of causal inferences. In addition, certain groups were underrepresented in this study’s sample, including uninsured patients, men, and some minority groups, which may have underestimated the amount of financial burden experienced by patients with advanced cancer. The lack of representativeness of uninsured individuals may be a result of the eligibility of persons with advanced cancer for Medicaid. However, a strength of this study is its ability to increase the representativeness of African American/black patients in the study of advanced cancer and financial hardship. In our study, just over a quarter of the participants (26 of 100; 26%) were black/African American, compared with the US Census Bureau’s national census level of 13.3% and 13.4% in Allegheny County, Pennsylvania .29
The lack of employed participants in this study could be because many were not able to work because of the advanced stage of their disease. The low level of partnered status is a limitation, although one study site was a low-income hospital where one generally tends to see higher levels of unpartnered status. This study did not control for demographic information such as gender or age, thus, the relationships between the primary outcome variables and financial hardship may be overestimated. Moreover, this analysis of financial distress is limited to the context of the United States due to our lack of universal health care and unique payment system. Although we included only patients who were in the palliative phase of cancer treatment, no medical record review was conducted to determine previous cancer history and treatments, which might have provided more insight into other financial loss or cost of cancer treatment. Furthermore, we note that it can be difficult to prognosticate with accuracy and identify that some patients with advanced cancer may have been excluded from the study due to the inclusion criteria of less than 1 year of survival.
Conclusion
Perceived financial hardship is an important assessment of the burden placed on patients due to the cost of disease; and is a good start in assessing indirect costs that patients take on when coping with advanced stages of cancer and can shed light on an aspect of distress experienced by this patient population that is not commonly addressed. Subjective measures of perceived financial hardship complement objective measures that are commonly indicative of economic resources and can further our understanding of the impact of financial distress experienced by patients with cancer. Further study of financial impacts of advanced cancer as well as predictors of financial distress are essential to the early identification of financial hardship and the development of interventions to support those at high risk or experiencing financial distress.
Acknowledgments
The authors acknowledge the patients and staff at the UPMC Mercy Cancer Center in Pittsburgh, Pennsylvania, who made this study possible, and Peggy Tate for her role in data collection. They also recognize the support of the Robert Wood Johnson Foundation through the Future Nursing Scholars program. They would also like to acknowledge that permission was granted for the use of the Psychological Sense of Economic Hardship study instrument.
The American Cancer Society has identified a disparity in cancer death rates, noting that persons with lower socioeconomic status have higher rates of mortality.1 This is attributed to many factors, but it is largely owing to the higher burden of disease among lower-income individuals.1 A component of this disease burden is measured by assessing the patient-reported outcome of cancer-related distress. The National Comprehensive Cancer Network (NCCN) Distress Management Guidelines have defined distress as “a multifactorial unpleasant emotional experience of a psychological (cognitive, behavioral, emotional), social and/or spiritual nature that may interfere with the ability to cope with cancer, its physical symptoms and its treatment.”2
Financial hardship related to cancer diagnosis and treatment is increasingly being recognized as an important component of disease burden and distress. The advancements in costly cancer treatments have produced burdensome direct medical costs as well as numerous indirect costs that contribute to perceived financial hardship.3,4 These indirect costs include nonmedical expenses such as increased transportation needs or childcare, loss of earnings, or loss of household income due to caregiving needs.3 Moreover, indirect costs are often managed by patients and families through their use of savings, borrowing, reducing leisure activities, and selling possessions.3 Even though efforts to increase health coverage, such as the Affordable Care Act, have reduced the rates of individuals who are uninsured, persons with cancer who have insurance also face challenges because they cannot afford copays, monthly premiums, deductibles, and other high out-of-pocket expenses related to cancer treatment that are not covered by their insurance such as out-of-network services or providers.5-7
Thus, financial hardship may have an impact on several areas of a patient’s life and well-being, but the effects are commonly undetected.8-10 Research has established that financial strain can influence treatment choices and adherence to therapy.11 Furthermore, the effects of financial strain have been identified across the cancer care continuum, from diagnosis through survivorship, suggesting a bidirectional relationship between financial strain and well-being.11 Financial strain may reduce patient quality of life and worsen symptom burden because of the patient’s inability to access needed care, poor social supports, and/or increased stress.11-12 These worsening outcomes may also increase the use of financial reserves and affect their ability to work.7,11 Financial difficulties may also be associated with anxiety and depression, leading to worse quality of life and greater distress and symptom burden.12 Identifying groups at high risk for financial strain is crucial to ensure that resources are available to assist these populations.13 This burden can be even more pronounced in minority and underserved patients with cancer.7 Patients with advanced cancer are especially vulnerable to the burden of increased costs because of the use of expensive targeted therapies; their improved survival, which extends the time of expenditure; and increased use of financial reserves.9 Financial hardship in patients with advanced cancer is not well understood or characterized,9 which is why this study aimed to better quantify distress in advanced stage cancers by describing :
▪ A cohort of patients with advanced cancer and their levels of quality of life, symptom distress, cancer-related distress and perceived financial hardship;
▪ The relationship between perceived financial hardship, quality of life, symptom distress and overall cancer-related distress; and
▪ Quality of life, symptom distress, and overall cancer-related distress according to level of perceived financial hardship.
Methods
This study is a cross-sectional, descriptive, comparative study of distress, including perceived financial hardship, among patients with advanced cancer who were receiving palliative care treatment in two outpatient medical oncology clinics in Western Pennsylvania. The data were collected during May 2013-November 2014. The study protocol was approved by the Institutional Review Board at the University of Pittsburgh. Eligible participants had to be 18 years or older and have an advanced solid tumor of any kind, with a prognosis of 1 year or less confirmed by a physician or clinic nurse practitioner/physician assistant, and be able to read and understand English at the fourth-grade level. The sample was recruited from two clinics at the University of Pittsburgh Cancer Institute, a National Cancer Institute-designated Comprehensive Cancer Program.
Measurements
Sociodemographic factors. These were measured using an investigator-derived Sociodemographic Questionnaire, a 12-item form that includes variables such as age, race, marital status, cancer type, religion and spirituality, employment status, years of education, health insurance status, and income level.
Cancer-related distress. The NCCN Distress Thermometer is a self-report visual analog scale (0, no distress; 10, great distress) formed in the shape of a thermometer combined with a problem list that is often used in outpatient cancer settings for reporting of cancer-related distress.14-16 The sensitivity, specificity and convergent validity with the Brief Symptom Inventory and the Hospital Anxiety and Depression Scale have been established and appropriate cut-off score of the distress thermometer identified.14-16 A score of 4 or above indicates a clinically significant level of distress.14-16
Symptom distress. The McCorkle Symptom Distress Scale was developed in 1977 based on interviews that focused on the symptom experiences of patients. Psychometric testing among patients with cancer using the modified Symptom Distress Scale revealed high reliability (Cronbach alpha, 0.97).17 The instrument is a 13-item Likert scale (1-5) assessing the severity of distress experienced by a symptom. Total scores range from 13 to 65, where a higher score indicates greater distress. Moderate distress is indicated with a score of 25-33, and a score above 33 indicates severe distress, identifying the need for immediate intervention.17
Quality of life and spiritual well-being. The Functional Assessment of Cancer Therapy (FACT-G) is used to assess general cancer-related quality of life. It has four subscales: physical, emotional, social and family, and functional well-being, with a total score that ranges from 0-112, where higher scores show higher quality of life. The Spiritual Distress Well-Being questionnaire was used alongside the valid FACT-G assessment.18,19 The Spiritual Well-Being Short Form was developed with an ethnically diverse population and adds 12 items to the FACT-G. The items do not necessarily assume a faith in God, allowing a wide flexibility in application and tapping into issues such as faith, meaning, and finding peace and comfort despite advanced illness. Higher scores on the Spiritual Well-Being subscore (range, 0-48) are correlated with higher scores of quality of life. The possible scores for the combined FACT-G and Spiritual Well-Being assessment range from 0-160, with higher scores showing higher quality of life.
Economic hardship. Perceived financial hardship was measured using Barrera and colleagues’ Psychological Sense of Economic Hardship Scale. 20 The scale consists of 20-items broken down into 4 subscales: financial strain, inability to make ends meet, not enough money for necessities, and economic adjustments.20 Economic adjustments in the 3 months before administration of the questionnaire were assessed with 9 Yes or No items, such as added another job, received government assistance, or sold possessions to increase income. The subscale of not enough money for necessities was assessed with seven 5-point scale items in which respondents noted whether they felt they had enough money for housing, clothing, home furnishings, and a car over the previous 3 months. Inability to make ends meet included two 5-point scale items that assessed the difficulty in meeting financial demands in the previous 3 months. Financial strain consisted of two 5-point scale items concerned with expecting financial hardships in the coming 3 months. Scores can range from 20-73, with a higher score indicating worse economic hardship.
Data collection and analysis
In-person data collection occurred in the clinical waiting area before the clinician visit or in the treatment room with the patient using a consecutive, convenience sample. The nursing staff checked the clinic lists daily for possible patient participants. Patients with metastatic cancer were identified and then approached for consent. After we had received the patient’s consent, the administration of the instruments took about 20 minutes to complete. The data were then entered and verified in REDCap (Research Electronic Data Capture), which is hosted at the University of Pittsburgh.21The levels of symptom distress, quality of life, perceived financial hardship, and cancer-related distress were described through continuously measured variables. Descriptive statistics, measures of central tendency (mean and median), and dispersion (standard deviation and range), were obtained for the subscales and total scores. Correlation analysis was used to describe the relationship between perceived financial hardship and quality of life, symptom distress, and cancer-related distress. These primary outcome variables were further explored according to the level of dichotomized perceived financial hardship using mean score as the cut point. Independent sample t tests were used to compare patients experiencing high perceived financial hardship with those experiencing low perceived financial hardship.
Results
In all, 100 patients participated in the study. Any missing data points were replaced with the mean score for that variable, although this was minimal in this study. Most of the participants were women (67%), and the average age of the participants was 63.43 years (SD, 13.05; Table 1). Of the total number of participants, 73% were white, 26% were black, and 1% were Asian. Most of the participants were either retired and not working (39%) or disabled or unable to work (34%). Almost all of the participants had some form of insurance, with 99% having either private or public health insurance. A variety of cancer types were represented in this patient population, with higher percentages of breast (25%), gynecologic (10%), lung (19%), and colon/rectal cancer (15%). Of the total number of participants, 35% had annual household incomes below $20,000, and 50% had annual household incomes of more than $20,000. On average, participants had 13.48 years (SD, 2.78) of formal education.
Descriptive statistics for the primary outcome variables can be found in Table 2. The average score for cancer-related distress based on the NCCN Distress Thermometer tool was 4.16 (SD, 3.26). The average score for the McCorkle Symptom Distress measurement was 25.45 (SD, 9.34). For quality of life, the average FACT-G total score was 73.77 (SD, 19.40). Of the FACT-G subscale average scores, physical well-being was 17.35 (SD, 7.50), social/family well-being 24.21 (SD, 5.25), emotional well-being 16.34 (SD, 5.42), and functional well-being 15.87 (SD, 6.78). Participants’ average score for the spiritual well-being measure was 35.20 (SD, 9.25) and the combined FACT-G and spiritual well-being average score was 108.97 (SD, 26.07). The total average score for perceived financial hardship was 35.70 (SD, 13.87), with subscale average scores of 3.44 (SD, 2.36) for financial strain, 5.73 (SD, 1.91) for inability to make ends meet, 16.43 (SD, 8.92) for not enough money for necessities, and 10.63 (SD, 2.70) for economic adjustments.
We conducted a bivariate correlation analysis to assess the relationship between perceived financial hardship and three other primary outcome variables (Table 3). These analyses showed significant low to moderate correlations with overall cancer-related distress (r, 0.439; P < .001), symptom distress (r, 0.409; P < .001) and overall quality of life scores (FACT-G and spiritual well-being combined score: r, -0.323; P < .001).
Forty-three participants reporting high perceived financial hardship experienced worse quality of life overall (FACT-G and spiritual well-being; P = .002), worse FACT-G total scores (P < .001), worse physical well-being (P < .001), worse social/family well-being (P = .029), worse emotional well-being, and no significant difference for functional (P = .082) or spiritual well-being (P = .453), compared with those with lower economic hardship. In overall cancer-related distress, participants with higher perceived financial hardship reported higher levels of cancer-related distress (P < .001) than those with lower perceived financial hardship. For those participants reporting higher perceived financial hardship there was also worse symptom distress (P < .001), compared with those with lower economic hardship (Table 4).
Discussion
Overall, this report provides data to illuminate our understanding of disparities in well-being that may be present in patients with advanced cancer. Our analysis found that patients with advanced cancer who have higher perceived financial hardship have significantly higher overall cancer-related distress, symptom distress, and poorer overall quality of life. In this study’s population of patients with advanced cancer, the most notable areas of economic hardship identified by participants were: not having enough money for necessities in the 3 months before the survey and the inability to make ends meet during the same time span, with difficulty paying bills and not having enough money left at the end of the month being most noteworthy among this study’s patient population. Financial strain and making economic adjustment were not as notable in the category of perceived financial hardship.
In regard to not having enough money, participants most commonly cited not being able to afford everyday necessities such as food, clothing, medical care, or a home, as well as leisure and recreational activities. These findings are further supported with the positive, moderate associations between perceived financial hardship and symptom distress and overall cancer-related distress found in this cohort of patients with advanced cancer and the negative, moderately associated relationship between perceived financial hardship and overall quality of life in this study’s sample.
Although these findings have been confirmed in the literature on cancer-related distress, our findings add to our knowledge on both economic and cancer-related distress exclusively in patients with advanced cancer.9,22 The broader cancer-related distress literature has also found an association between being younger and having a lower household income as risk factors for increased financial hardship; however, the perception of financial strain and magnitude was a more significant predictor of quality of life and perception of overall well-being.6,8-9,12,22-23 Furthermore, patients with cancer who noted having higher financial distress typically reported decreased satisfaction with cancer care which also influenced their adherence to treatment and quality of life.24
Our work now adds the important element of perceived financial hardship to the advanced cancer-related distress puzzle. We should consider integrating a financial distress assessment into routine cancer care, particularly with patients and families with advanced cancer, to proactively and routinely assess and intervene with available distress mitigating resources. Therefore, understanding the patients most likely to experience financial distress will help personalize supportive therapy.
This study’s results as well as the existing literature describing financial distress support the use of comprehensive screening instruments to capture elements of financial burden beyond out-of-pocket costs.8,25 This screening is particularly relevant because we are increasingly recognizing that gross annual household income does not always reflect financial hardship or distress. The instrument we used for this analysis, the Psychological Sense of Economic Hardship, provides a broad view of financial toxicity including the specific components of financial strain, the inability to make ends meet, not having enough money for necessities, and economic adjustments experienced by patients with advanced cancer.20 Another measure to evaluate financial toxicity among patients with cancer includes the Comprehensive Score for Financial Toxicity (COST), which is a widely used patient-reported outcome measure. It was developed with input from both patients and oncology experts.25 Use of a financial toxicity assessment tool adds to our understanding of the economic financial burden experienced by patients with cancer, specifically those with advanced cancer.
Tucker-Seeley and Yabroff have identified several areas in which the research agenda for financial toxicity should focus, including: documentation of the socioeconomic context among patients across all areas of the cancer care continuum, further identification and characterization of at risk populations to address health disparities, and the inclusion of cost discussions in the health care context.26 Furthermore, research is needed to identify key areas to target for interventions addressing financial toxicity, such as addressing lack of financial resources to cover the cost of cancer care, focusing on managing or preventing the distress that results from a lack of financial resources, or addressing coping behaviors used by families to manage the financial burden of cancer care.26 Although cost discussions between health care providers and patients have been identified as important in reducing the financial burden of cancer care, the content, timing, and goals of those discussions still need to be better articulated for different patient populations, including patients with advanced cancer.3,27-28 In addition, resources such as social workers, patient navigators, or financial counselors have been identified as effective in assisting patients with financial planning and accessing community resources to address financial burden and assistance.4
Design considerations
This study has limitations that need to be noted. Its cross-sectional design does not allow for the analysis of causal inferences. In addition, certain groups were underrepresented in this study’s sample, including uninsured patients, men, and some minority groups, which may have underestimated the amount of financial burden experienced by patients with advanced cancer. The lack of representativeness of uninsured individuals may be a result of the eligibility of persons with advanced cancer for Medicaid. However, a strength of this study is its ability to increase the representativeness of African American/black patients in the study of advanced cancer and financial hardship. In our study, just over a quarter of the participants (26 of 100; 26%) were black/African American, compared with the US Census Bureau’s national census level of 13.3% and 13.4% in Allegheny County, Pennsylvania .29
The lack of employed participants in this study could be because many were not able to work because of the advanced stage of their disease. The low level of partnered status is a limitation, although one study site was a low-income hospital where one generally tends to see higher levels of unpartnered status. This study did not control for demographic information such as gender or age, thus, the relationships between the primary outcome variables and financial hardship may be overestimated. Moreover, this analysis of financial distress is limited to the context of the United States due to our lack of universal health care and unique payment system. Although we included only patients who were in the palliative phase of cancer treatment, no medical record review was conducted to determine previous cancer history and treatments, which might have provided more insight into other financial loss or cost of cancer treatment. Furthermore, we note that it can be difficult to prognosticate with accuracy and identify that some patients with advanced cancer may have been excluded from the study due to the inclusion criteria of less than 1 year of survival.
Conclusion
Perceived financial hardship is an important assessment of the burden placed on patients due to the cost of disease; and is a good start in assessing indirect costs that patients take on when coping with advanced stages of cancer and can shed light on an aspect of distress experienced by this patient population that is not commonly addressed. Subjective measures of perceived financial hardship complement objective measures that are commonly indicative of economic resources and can further our understanding of the impact of financial distress experienced by patients with cancer. Further study of financial impacts of advanced cancer as well as predictors of financial distress are essential to the early identification of financial hardship and the development of interventions to support those at high risk or experiencing financial distress.
Acknowledgments
The authors acknowledge the patients and staff at the UPMC Mercy Cancer Center in Pittsburgh, Pennsylvania, who made this study possible, and Peggy Tate for her role in data collection. They also recognize the support of the Robert Wood Johnson Foundation through the Future Nursing Scholars program. They would also like to acknowledge that permission was granted for the use of the Psychological Sense of Economic Hardship study instrument.
1. American Cancer Society. Cancer Facts & Figures 2015. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2015/cancer-facts-and-figures-2015.pdf. Atlanta, GA: American Cancer Society. 2015. Accessed January 16, 2016.
2. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology distress management version 1.2014. http://williams.medicine.wisc.edu/distress.pdf. Updated May 2014. Accessed January 16, 2016.
3. de Souza JA, Wong Y-N. Financial distress in cancer patients. J Med Person. 2014;11(2):13-15.
4. Mcdougall JA, Ramsey SD, Hutchinson F, Shih Y-CT. Financial toxicity: a growing concern among cancer patients in the United States. ISPOR Connect. 2014;20(2):10-11.
5. Sharpe K, Shaw B, Seiler MB. Practical solutions when facing cost sharing: the American Cancer Society’s health insurance assistance service. Am J Manag Care. 2016;22(4):92-94.
6. Shankaran V, Jolly S, Blough D, Ramsey SD. Risk factors for financial hardship in patients receiving adjuvant chemotherapy for colon cancer : a population-based exploratory analysis. J Clin Oncol. 2012;30(14):1608-1614.
7. Meneses K, Azuero A, Hassey L, Mcnees P, Pisu M. Does economic burden influence quality of life in breast cancer survivors? Gynecol Oncol. 2012;124(3):437-443.
8. Catt S, Starkings R, Shilling V, Fallowfield L. Patient-reported outcome measures of the impact of cancer on patients’ everyday lives: a systematic review. J Cancer Surviv. 2017;11(2):211-232.
9. Delgado-Guay M, Ferrer J, Rieber AG, et al. Financial distress and its associations with physical and emotional symptoms and quality of life among advanced cancer patients. Oncologist. 2015;20:1092-1098.
10. Kale HP, Carroll N V. Self-reported financial burden of cancer care and its effect on physical and mental health-related quality of life among US cancer survivors. Cancer. 2016;122:1283-1289.
11. Lathan CS, Cronin A, Tucker-Seeley R, Zafar SY, Ayanian JZ, Schrag D. Association of financial strain with symptom burden and quality of life for patients with lung or colorectal cancer. J Clin Oncol. 2016;34(15):1732-1740.
12. Fenn KM, Evans SB, Mccorkle R, et al. Impact of financial burden of cancer on survivors’ quality of life. J Onocol Pract. 2014;10(5):332-339.
13. Azzani M, Roslani AC, Su TT. The perceived cancer-related financial hardship among patients and their families: a systematic review. Support Cancer Care. 2015;23:889-898.
14. Jacobsen PB, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients a multicenter evaluation of the distress thermometer. Cancer. 2005;103:1494-1502.
15. Ransom S, Jacobsen PB, Booth-Jones M. Validation of the distress thermometer with bone marrow. Psychooncology. 2006;15:604-612.
16. Vodermaier A, Linden W, Siu C. Screening for emotional distress in cancer patients: a systematic review of assessment instruments. J Natl Cancer Inst. 2009;101:1464-1488.
17. McCorkle R, Quint-Benoliel J. Symptom distress, current concerns and mood disturbance after diagnosis of life-threatening disease. Soc Sci Med. 1983;17(7):431–8.
18. Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570-579.
19. Peterman AH, Fitchett G, Brady MJ, Hernandez L, Cella D. Measuring spiritual well-being in people with cancer: the functional assessment of chronic illness therapy – Spiritual Well-being Scale (FACIT-Sp). Ann Behav Med. 2002;24:49–58.
20. Barrera M, Caples H, Tein J. The psychological sense of economic hardship: measurement models, validity, and cross-ethnic equivalence for urban families. Am J Community Psychol. 2001;29:493-517.
21. Harris PA, Taylor R, Thielke R. Research electronic data capture (REDCap): A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381.
22. Zafar SY, McNeil RB, Thomas CM, Lathan CS, Ayanian JZ, Provenzale D. Population-based assessment of cancer survivors’ financial burden and quality of life: a prospective cohort study. J Oncol Pract. 2015;11(2):145-152.
23. Kent EE, Forsythe LP, Yabroff KR, et al. Are survivors who report cancer-related financial problems more likely to forgo or delay medical care? Cancer. 2013;119:3710-3717.
24. Chino F, Peppercorn J, Taylor Jr. DH, et al. Self-reported financial burden and satisfaction with care among patients with cancer. Oncologist. 2014;19:414-420.
25. De Souza JA, Yap BJ, Hlubocky FJ, et al. The development of a financial toxicity patient-reported outcome in cancer. Cancer. 2014;120:3245-3253.
26. Tucker-Seeley RD, Yabroff KR. Minimizing the “financial toxicity” associated with cancer care : advancing the research agenda. J Natl Cancer Inst. 2016;108(5):1-3.
27. Bestvina CM, Zullig LL, Rushing C, et al. Patient-oncologist cost communication, financial distress, and medication adherence. J Oncol Pract. 2014;10(3):162-168.
28. Irwin B, Kimmick G, Altomare I, et al. Patient experience and attitudes toward addressing the cost of breast cancer care. Oncologist. 2014;19:1135-1140.
29. US Census Bureau. United States. https://www.census.gov/quickfacts/. 2015.
1. American Cancer Society. Cancer Facts & Figures 2015. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2015/cancer-facts-and-figures-2015.pdf. Atlanta, GA: American Cancer Society. 2015. Accessed January 16, 2016.
2. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology distress management version 1.2014. http://williams.medicine.wisc.edu/distress.pdf. Updated May 2014. Accessed January 16, 2016.
3. de Souza JA, Wong Y-N. Financial distress in cancer patients. J Med Person. 2014;11(2):13-15.
4. Mcdougall JA, Ramsey SD, Hutchinson F, Shih Y-CT. Financial toxicity: a growing concern among cancer patients in the United States. ISPOR Connect. 2014;20(2):10-11.
5. Sharpe K, Shaw B, Seiler MB. Practical solutions when facing cost sharing: the American Cancer Society’s health insurance assistance service. Am J Manag Care. 2016;22(4):92-94.
6. Shankaran V, Jolly S, Blough D, Ramsey SD. Risk factors for financial hardship in patients receiving adjuvant chemotherapy for colon cancer : a population-based exploratory analysis. J Clin Oncol. 2012;30(14):1608-1614.
7. Meneses K, Azuero A, Hassey L, Mcnees P, Pisu M. Does economic burden influence quality of life in breast cancer survivors? Gynecol Oncol. 2012;124(3):437-443.
8. Catt S, Starkings R, Shilling V, Fallowfield L. Patient-reported outcome measures of the impact of cancer on patients’ everyday lives: a systematic review. J Cancer Surviv. 2017;11(2):211-232.
9. Delgado-Guay M, Ferrer J, Rieber AG, et al. Financial distress and its associations with physical and emotional symptoms and quality of life among advanced cancer patients. Oncologist. 2015;20:1092-1098.
10. Kale HP, Carroll N V. Self-reported financial burden of cancer care and its effect on physical and mental health-related quality of life among US cancer survivors. Cancer. 2016;122:1283-1289.
11. Lathan CS, Cronin A, Tucker-Seeley R, Zafar SY, Ayanian JZ, Schrag D. Association of financial strain with symptom burden and quality of life for patients with lung or colorectal cancer. J Clin Oncol. 2016;34(15):1732-1740.
12. Fenn KM, Evans SB, Mccorkle R, et al. Impact of financial burden of cancer on survivors’ quality of life. J Onocol Pract. 2014;10(5):332-339.
13. Azzani M, Roslani AC, Su TT. The perceived cancer-related financial hardship among patients and their families: a systematic review. Support Cancer Care. 2015;23:889-898.
14. Jacobsen PB, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients a multicenter evaluation of the distress thermometer. Cancer. 2005;103:1494-1502.
15. Ransom S, Jacobsen PB, Booth-Jones M. Validation of the distress thermometer with bone marrow. Psychooncology. 2006;15:604-612.
16. Vodermaier A, Linden W, Siu C. Screening for emotional distress in cancer patients: a systematic review of assessment instruments. J Natl Cancer Inst. 2009;101:1464-1488.
17. McCorkle R, Quint-Benoliel J. Symptom distress, current concerns and mood disturbance after diagnosis of life-threatening disease. Soc Sci Med. 1983;17(7):431–8.
18. Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570-579.
19. Peterman AH, Fitchett G, Brady MJ, Hernandez L, Cella D. Measuring spiritual well-being in people with cancer: the functional assessment of chronic illness therapy – Spiritual Well-being Scale (FACIT-Sp). Ann Behav Med. 2002;24:49–58.
20. Barrera M, Caples H, Tein J. The psychological sense of economic hardship: measurement models, validity, and cross-ethnic equivalence for urban families. Am J Community Psychol. 2001;29:493-517.
21. Harris PA, Taylor R, Thielke R. Research electronic data capture (REDCap): A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381.
22. Zafar SY, McNeil RB, Thomas CM, Lathan CS, Ayanian JZ, Provenzale D. Population-based assessment of cancer survivors’ financial burden and quality of life: a prospective cohort study. J Oncol Pract. 2015;11(2):145-152.
23. Kent EE, Forsythe LP, Yabroff KR, et al. Are survivors who report cancer-related financial problems more likely to forgo or delay medical care? Cancer. 2013;119:3710-3717.
24. Chino F, Peppercorn J, Taylor Jr. DH, et al. Self-reported financial burden and satisfaction with care among patients with cancer. Oncologist. 2014;19:414-420.
25. De Souza JA, Yap BJ, Hlubocky FJ, et al. The development of a financial toxicity patient-reported outcome in cancer. Cancer. 2014;120:3245-3253.
26. Tucker-Seeley RD, Yabroff KR. Minimizing the “financial toxicity” associated with cancer care : advancing the research agenda. J Natl Cancer Inst. 2016;108(5):1-3.
27. Bestvina CM, Zullig LL, Rushing C, et al. Patient-oncologist cost communication, financial distress, and medication adherence. J Oncol Pract. 2014;10(3):162-168.
28. Irwin B, Kimmick G, Altomare I, et al. Patient experience and attitudes toward addressing the cost of breast cancer care. Oncologist. 2014;19:1135-1140.
29. US Census Bureau. United States. https://www.census.gov/quickfacts/. 2015.
RAND Study Provides Report Card for MHS PTSD and Depression Care
Deck: Study finds overall high quality of care but identifies important gaps in assessment and treatment.
Active-duty service members with posttraumatic stress disorder (PTSD) or depression are frequent users of both inpatient and outpatient Military Health System (MHS) care, but there were significant variations in the quality and type of care they received, according to a recent RAND Corporation study. According to the authors, the MHS “performed well in providing initial screening for suicide and substance abuse but needs to improve at providing adequate follow-up to service members with suicide risk.”
The Quality of Care for PTSD and Depression in the Military Health System study is the third in a series of reports on the quality of depression and PTSD care for active-duty service members. The study examined data for 2013-2014 and included 14,654 service members with PTSD and 30,496 with depression; 6,322 service members were in both groups. The goal of the report was to determine whether the service members with PTSD or depression receive evidence-based care and whether there were disparities in care quality by branch of service, geographic region, and other characteristics, such as gender, age, pay grade, race/ethnicity, or deployment history.
Related: New Center of Excellence to Lead Research of “Signature Wounds”
In both the PTSD and depression cohorts, the majority of active-component service members were enlisted soldiers and had 1 or more deployments. A large number of patients were in both the PTSD and depression cohorts. The study noted that more than half of patients in the PTSD cohort had a diagnosis of depression, and more than one-fourth of those in the depression cohort received a PTSD diagnosis during the 12-month observation period.
Service members with PTSD or depression were willing to engage the health system with medians of 40 and 31 visits for PTSD and depression, respectively, during the 1-year observation period. Most of these visits were for unrelated conditions. Importantly, more than half of patients received their care from primary care providers. Social workers, psychiatrists, and clinical psychologists provided care for slightly less than half of the PTSD cohort. These mental health provider groups saw between 33% and 40% of the depression cohort. The majority of patients in both the PTSD and depression cohorts received care for their cohort diagnosis solely at military treatment facilities
“The high utilization for both medical and psychological conditions combined with the high number of different providers raise questions about the extent of coordination vs fragmentation of care for all the care these service members received,” the study reported. “The high number of psychotherapy visits received by members of these cohorts suggests that the MHS may be more successful than the civilian sector in engaging patients with PTSD or depression in psychosocial interventions.”
The study also highlighted the following important gaps in treatment, assessment, and follow-up:
- Assessment of baseline symptom severity of PTSD for a new treatment episode with the PCL (PTSD Checklist) was not as frequent, though current efforts are under way within the MHS to increase the regular use of the PCL to monitor PTSD patient symptoms.
- Standardized tools were used in less than half of the assessments for depression, suicide risk, and recent substance use and almost never used for assessment of function.
- Appropriate minimal care for patients with suicidal ideation was less than optimal (54%), primarily due to a lack of documentation regarding addressing access to lethal means. A Safety Plan Worksheet has recently been added to the clinical guideline for assessment and management of suicide risk which may improve this performance in the future.
- Most service members with PTSD or depression received at least some psychotherapy, but the MHS could increase delivery of evidence-based psychotherapy.
- Improvements are still needed in rates of outcome monitoring and performance on outcome measures.
- Multiple “statistically significant and clinically meaningful differences” were found in measure scores by service branch, TRICARE region, and service member.
Deck: Study finds overall high quality of care but identifies important gaps in assessment and treatment.
Active-duty service members with posttraumatic stress disorder (PTSD) or depression are frequent users of both inpatient and outpatient Military Health System (MHS) care, but there were significant variations in the quality and type of care they received, according to a recent RAND Corporation study. According to the authors, the MHS “performed well in providing initial screening for suicide and substance abuse but needs to improve at providing adequate follow-up to service members with suicide risk.”
The Quality of Care for PTSD and Depression in the Military Health System study is the third in a series of reports on the quality of depression and PTSD care for active-duty service members. The study examined data for 2013-2014 and included 14,654 service members with PTSD and 30,496 with depression; 6,322 service members were in both groups. The goal of the report was to determine whether the service members with PTSD or depression receive evidence-based care and whether there were disparities in care quality by branch of service, geographic region, and other characteristics, such as gender, age, pay grade, race/ethnicity, or deployment history.
Related: New Center of Excellence to Lead Research of “Signature Wounds”
In both the PTSD and depression cohorts, the majority of active-component service members were enlisted soldiers and had 1 or more deployments. A large number of patients were in both the PTSD and depression cohorts. The study noted that more than half of patients in the PTSD cohort had a diagnosis of depression, and more than one-fourth of those in the depression cohort received a PTSD diagnosis during the 12-month observation period.
Service members with PTSD or depression were willing to engage the health system with medians of 40 and 31 visits for PTSD and depression, respectively, during the 1-year observation period. Most of these visits were for unrelated conditions. Importantly, more than half of patients received their care from primary care providers. Social workers, psychiatrists, and clinical psychologists provided care for slightly less than half of the PTSD cohort. These mental health provider groups saw between 33% and 40% of the depression cohort. The majority of patients in both the PTSD and depression cohorts received care for their cohort diagnosis solely at military treatment facilities
“The high utilization for both medical and psychological conditions combined with the high number of different providers raise questions about the extent of coordination vs fragmentation of care for all the care these service members received,” the study reported. “The high number of psychotherapy visits received by members of these cohorts suggests that the MHS may be more successful than the civilian sector in engaging patients with PTSD or depression in psychosocial interventions.”
The study also highlighted the following important gaps in treatment, assessment, and follow-up:
- Assessment of baseline symptom severity of PTSD for a new treatment episode with the PCL (PTSD Checklist) was not as frequent, though current efforts are under way within the MHS to increase the regular use of the PCL to monitor PTSD patient symptoms.
- Standardized tools were used in less than half of the assessments for depression, suicide risk, and recent substance use and almost never used for assessment of function.
- Appropriate minimal care for patients with suicidal ideation was less than optimal (54%), primarily due to a lack of documentation regarding addressing access to lethal means. A Safety Plan Worksheet has recently been added to the clinical guideline for assessment and management of suicide risk which may improve this performance in the future.
- Most service members with PTSD or depression received at least some psychotherapy, but the MHS could increase delivery of evidence-based psychotherapy.
- Improvements are still needed in rates of outcome monitoring and performance on outcome measures.
- Multiple “statistically significant and clinically meaningful differences” were found in measure scores by service branch, TRICARE region, and service member.
Deck: Study finds overall high quality of care but identifies important gaps in assessment and treatment.
Active-duty service members with posttraumatic stress disorder (PTSD) or depression are frequent users of both inpatient and outpatient Military Health System (MHS) care, but there were significant variations in the quality and type of care they received, according to a recent RAND Corporation study. According to the authors, the MHS “performed well in providing initial screening for suicide and substance abuse but needs to improve at providing adequate follow-up to service members with suicide risk.”
The Quality of Care for PTSD and Depression in the Military Health System study is the third in a series of reports on the quality of depression and PTSD care for active-duty service members. The study examined data for 2013-2014 and included 14,654 service members with PTSD and 30,496 with depression; 6,322 service members were in both groups. The goal of the report was to determine whether the service members with PTSD or depression receive evidence-based care and whether there were disparities in care quality by branch of service, geographic region, and other characteristics, such as gender, age, pay grade, race/ethnicity, or deployment history.
Related: New Center of Excellence to Lead Research of “Signature Wounds”
In both the PTSD and depression cohorts, the majority of active-component service members were enlisted soldiers and had 1 or more deployments. A large number of patients were in both the PTSD and depression cohorts. The study noted that more than half of patients in the PTSD cohort had a diagnosis of depression, and more than one-fourth of those in the depression cohort received a PTSD diagnosis during the 12-month observation period.
Service members with PTSD or depression were willing to engage the health system with medians of 40 and 31 visits for PTSD and depression, respectively, during the 1-year observation period. Most of these visits were for unrelated conditions. Importantly, more than half of patients received their care from primary care providers. Social workers, psychiatrists, and clinical psychologists provided care for slightly less than half of the PTSD cohort. These mental health provider groups saw between 33% and 40% of the depression cohort. The majority of patients in both the PTSD and depression cohorts received care for their cohort diagnosis solely at military treatment facilities
“The high utilization for both medical and psychological conditions combined with the high number of different providers raise questions about the extent of coordination vs fragmentation of care for all the care these service members received,” the study reported. “The high number of psychotherapy visits received by members of these cohorts suggests that the MHS may be more successful than the civilian sector in engaging patients with PTSD or depression in psychosocial interventions.”
The study also highlighted the following important gaps in treatment, assessment, and follow-up:
- Assessment of baseline symptom severity of PTSD for a new treatment episode with the PCL (PTSD Checklist) was not as frequent, though current efforts are under way within the MHS to increase the regular use of the PCL to monitor PTSD patient symptoms.
- Standardized tools were used in less than half of the assessments for depression, suicide risk, and recent substance use and almost never used for assessment of function.
- Appropriate minimal care for patients with suicidal ideation was less than optimal (54%), primarily due to a lack of documentation regarding addressing access to lethal means. A Safety Plan Worksheet has recently been added to the clinical guideline for assessment and management of suicide risk which may improve this performance in the future.
- Most service members with PTSD or depression received at least some psychotherapy, but the MHS could increase delivery of evidence-based psychotherapy.
- Improvements are still needed in rates of outcome monitoring and performance on outcome measures.
- Multiple “statistically significant and clinically meaningful differences” were found in measure scores by service branch, TRICARE region, and service member.