User login
Senate confirms first mental health czar
The Senate has confirmed Elinore F. McCance-Katz, MD, PhD, as the nation’s first mental health czar.
Dr. McCance-Katz, a psychiatrist, has focused her career largely on addiction treatment, particularly opioid abuse. This expertise has helped her receive the backing of several mental health organizations, including the American Psychiatric Association, the National Alliance on Mental Illness, and the American Society of Addiction Medicine.
As leader of the Substance Abuse and Mental Health Services Administration, Dr. McCance-Katz is expected to streamline more than 100 federal mental health agencies into a more effective system and to oversee a more than $3.5 billion budget annually. Having previously served as SAMHSA’s chief medical officer, Dr. McCance-Katz once again enters the fray surrounding the agency’s historically poor record of serving people with serious mental illnesses such as schizophrenia and bipolar disorder. Dr. McCance-Katz stepped down from her chief medical officer role in 2015 after serving 2 years, later penning a piece criticizing the agency’s lack of commitment to understanding – much less implementing – psychiatric treatments based on a medical approach. Instead, she argued, SAMHSA favored non–evidence-based psychosocial interventions.
Despite this, Rep. Tim Murphy (R.-Penn.) – a clinical psychologist and the congressman most responsible for drafting the legislation that created the position – issued a highly critical statement when her nomination was announced earlier this year. He called into question Dr. McCance-Katz’s commitment to the medical model and blamed her in part for the agency’s previous failures.
Dr. McCance-Katz currently serves as the chief medical officer at the Rhode Island Department of Behavioral Healthcare, Developmental Disabilities, and Hospitals. She also is a professor of psychiatry and human behavior, and professor of behavioral and social sciences at Brown University, Providence, R.I. She is expected to take up her new post as U.S. assistant secretary for mental health and substance use by the fall, according to a SAMHSA spokeperson.
[email protected]
On Twitter @whitneymcknight
The Senate has confirmed Elinore F. McCance-Katz, MD, PhD, as the nation’s first mental health czar.
Dr. McCance-Katz, a psychiatrist, has focused her career largely on addiction treatment, particularly opioid abuse. This expertise has helped her receive the backing of several mental health organizations, including the American Psychiatric Association, the National Alliance on Mental Illness, and the American Society of Addiction Medicine.
As leader of the Substance Abuse and Mental Health Services Administration, Dr. McCance-Katz is expected to streamline more than 100 federal mental health agencies into a more effective system and to oversee a more than $3.5 billion budget annually. Having previously served as SAMHSA’s chief medical officer, Dr. McCance-Katz once again enters the fray surrounding the agency’s historically poor record of serving people with serious mental illnesses such as schizophrenia and bipolar disorder. Dr. McCance-Katz stepped down from her chief medical officer role in 2015 after serving 2 years, later penning a piece criticizing the agency’s lack of commitment to understanding – much less implementing – psychiatric treatments based on a medical approach. Instead, she argued, SAMHSA favored non–evidence-based psychosocial interventions.
Despite this, Rep. Tim Murphy (R.-Penn.) – a clinical psychologist and the congressman most responsible for drafting the legislation that created the position – issued a highly critical statement when her nomination was announced earlier this year. He called into question Dr. McCance-Katz’s commitment to the medical model and blamed her in part for the agency’s previous failures.
Dr. McCance-Katz currently serves as the chief medical officer at the Rhode Island Department of Behavioral Healthcare, Developmental Disabilities, and Hospitals. She also is a professor of psychiatry and human behavior, and professor of behavioral and social sciences at Brown University, Providence, R.I. She is expected to take up her new post as U.S. assistant secretary for mental health and substance use by the fall, according to a SAMHSA spokeperson.
[email protected]
On Twitter @whitneymcknight
The Senate has confirmed Elinore F. McCance-Katz, MD, PhD, as the nation’s first mental health czar.
Dr. McCance-Katz, a psychiatrist, has focused her career largely on addiction treatment, particularly opioid abuse. This expertise has helped her receive the backing of several mental health organizations, including the American Psychiatric Association, the National Alliance on Mental Illness, and the American Society of Addiction Medicine.
As leader of the Substance Abuse and Mental Health Services Administration, Dr. McCance-Katz is expected to streamline more than 100 federal mental health agencies into a more effective system and to oversee a more than $3.5 billion budget annually. Having previously served as SAMHSA’s chief medical officer, Dr. McCance-Katz once again enters the fray surrounding the agency’s historically poor record of serving people with serious mental illnesses such as schizophrenia and bipolar disorder. Dr. McCance-Katz stepped down from her chief medical officer role in 2015 after serving 2 years, later penning a piece criticizing the agency’s lack of commitment to understanding – much less implementing – psychiatric treatments based on a medical approach. Instead, she argued, SAMHSA favored non–evidence-based psychosocial interventions.
Despite this, Rep. Tim Murphy (R.-Penn.) – a clinical psychologist and the congressman most responsible for drafting the legislation that created the position – issued a highly critical statement when her nomination was announced earlier this year. He called into question Dr. McCance-Katz’s commitment to the medical model and blamed her in part for the agency’s previous failures.
Dr. McCance-Katz currently serves as the chief medical officer at the Rhode Island Department of Behavioral Healthcare, Developmental Disabilities, and Hospitals. She also is a professor of psychiatry and human behavior, and professor of behavioral and social sciences at Brown University, Providence, R.I. She is expected to take up her new post as U.S. assistant secretary for mental health and substance use by the fall, according to a SAMHSA spokeperson.
[email protected]
On Twitter @whitneymcknight
Vemurafenib granted sNDA, priority review for Erdheim-Chester disease
Vemurafenib (Zelboraf) has been granted a supplemental new drug application and priority review by the Food and Drug Administration for the treatment of Erdheim-Chester disease with BRAF V600 mutation, according to a press release issued by Genentech.
The FDA is expected to make a decision on the indication by Dec. 7, 2017. Vemurafenib is approved for the treatment of unresectable or metastatic melanoma with BRAF V600E mutation.
The supportive data for the application came from VE-BASKET, a phase 2, nonrandomized study investigating the use of vemurafenib for people with various BRAF V600 mutation–positive cancers and other diseases. Final results for the 22 people with Erdheim-Chester disease showed a best overall response rate of 54.5% by RECIST v1.1 criteria.
The median duration of response, progression-free survival, and overall survival were not reached at a median follow-up time of 26.6 months. The most common grade 3 or higher adverse events were new skin cancers, high blood pressure, rash, and joint pain. Initial study results were published in the New England Journal of Medicine in August 2015.
Based on available published data, there are fewer than 500 cases of Erdheim-Chester disease in the United States. More than half of affected people have BRAF V600 mutation–positive disease, and there are no approved treatments, according to the release.
Vemurafenib (Zelboraf) has been granted a supplemental new drug application and priority review by the Food and Drug Administration for the treatment of Erdheim-Chester disease with BRAF V600 mutation, according to a press release issued by Genentech.
The FDA is expected to make a decision on the indication by Dec. 7, 2017. Vemurafenib is approved for the treatment of unresectable or metastatic melanoma with BRAF V600E mutation.
The supportive data for the application came from VE-BASKET, a phase 2, nonrandomized study investigating the use of vemurafenib for people with various BRAF V600 mutation–positive cancers and other diseases. Final results for the 22 people with Erdheim-Chester disease showed a best overall response rate of 54.5% by RECIST v1.1 criteria.
The median duration of response, progression-free survival, and overall survival were not reached at a median follow-up time of 26.6 months. The most common grade 3 or higher adverse events were new skin cancers, high blood pressure, rash, and joint pain. Initial study results were published in the New England Journal of Medicine in August 2015.
Based on available published data, there are fewer than 500 cases of Erdheim-Chester disease in the United States. More than half of affected people have BRAF V600 mutation–positive disease, and there are no approved treatments, according to the release.
Vemurafenib (Zelboraf) has been granted a supplemental new drug application and priority review by the Food and Drug Administration for the treatment of Erdheim-Chester disease with BRAF V600 mutation, according to a press release issued by Genentech.
The FDA is expected to make a decision on the indication by Dec. 7, 2017. Vemurafenib is approved for the treatment of unresectable or metastatic melanoma with BRAF V600E mutation.
The supportive data for the application came from VE-BASKET, a phase 2, nonrandomized study investigating the use of vemurafenib for people with various BRAF V600 mutation–positive cancers and other diseases. Final results for the 22 people with Erdheim-Chester disease showed a best overall response rate of 54.5% by RECIST v1.1 criteria.
The median duration of response, progression-free survival, and overall survival were not reached at a median follow-up time of 26.6 months. The most common grade 3 or higher adverse events were new skin cancers, high blood pressure, rash, and joint pain. Initial study results were published in the New England Journal of Medicine in August 2015.
Based on available published data, there are fewer than 500 cases of Erdheim-Chester disease in the United States. More than half of affected people have BRAF V600 mutation–positive disease, and there are no approved treatments, according to the release.
Federal medical tort reform: Has its time come?
Question: Congressional proposals on medical tort reform can be expected to include the following, except:
A. A no-fault system akin to automobile no-fault insurance.
B. A cap on noneconomic damages.
C. “Safe-harbor” immunity against medical negligence.
D. Health courts in place of the judge/jury system to adjudicate claims.
E. Promotion of laws that encourage apologies and error disclosures.
Answer: A. Under the current Republican administration, one can expect legislative efforts at federal tort reform, especially given that Thomas E. Price, MD, the new secretary of the Department of Health & Human Services, is an orthopedic surgeon who has spoken passionately about defensive medicine, damage caps, health tribunals, and practice guidelines. As a former House representative for Georgia, Dr. Price has introduced several tort reform bills, so it is likely that any omnibus federal law will incorporate some of his proposals.1
Over the decades, many states have gone ahead in enacting their own statutes while awaiting federal action. Iowa is the latest example. It recently passed legislation that included a noneconomic damages cap of $250,000, stronger expert witness standards, a certificate of merit in all medical liability lawsuits, and an expansion of its “candor” protections.2 Additional reforms in other states include pretrial screening panels; arbitration; structured periodic payments in lieu of lump sum payments; penalties for frivolous suits; shortened statutes of limitations; making the loser bear all litigation costs; abolishing the collateral source rule, as well as joint and several liability; and limiting attorney contingency fees.
The best-known reform is a cap on noneconomic losses, such as pain and suffering, that doesn’t abridge compensation for economic losses, i.e., medical expenses and lost wages. This provides some predictability because noneconomic damages are difficult to quantify, and jury sympathy may result in unrealistically high payments.
Interestingly, Dr. Price himself has not pushed for a federal cap on noneconomic damages, but other Republican bills have proposed a cap of $250,000. Many states, such as California, Kansas, and Texas, have seen their cap statutes withstand constitutional challenge. However, other jurisdictions, notably Georgia, Illinois, and Missouri, have ruled them unconstitutional.
California’s law, popularly known as MICRA (Medical Injury Compensation Reform Act), came under renewed attack in 2015 with a wrongful death suit from hemorrhagic complications related to Coumadin (warfarin) use following heart surgery.3 The plaintiff’s constitutional challenges included violation of equal protection, due process, and the right to a jury trial, but these were essentially all grounded on an entitlement to recover additional noneconomic damages sufficient to cover attorney fees. The trial court had reduced her $1 million noneconomic damages to $250,000, as required under MICRA. A California court of appeal rejected her claim as being “contrary to many well-established legal principles.”
On the other hand, Florida’s Supreme Court recently held in a closely divided decision of 4-3 that the state’s caps were unconstitutional.4 The law limited noneconomic damages in malpractice cases to either $500,000 or $1 million if the injuries were catastrophic. The court ruled that the caps were arbitrary and unfairly hurt the most severely injured. It was unconvinced that they would reduce malpractice insurance rates; at any rate, there was no present crisis to justify the caps. The decision came 3 years after the court had struck down caps in a case of wrongful death.5
Three relative newcomers to the legal landscape – health courts, apology laws, and safe harbors – appear to be taking center stage in any forthcoming federal reform measures.
Health courts
Under this proposal, so-called health panels and tribunals would now adjudicate malpractice claims. Such health courts would dispense with the jury; further, regular judges would be replaced with specialized judges who would make binding determinations. In one version, a panel of medical experts would initially screen the complaint, followed by an administrative health care tribunal that would feature judges with medical expertise. These tribunals would issue binding rulings, but either party could appeal to a state court for a reversal.
In countries such as Scandinavia and New Zealand, these administrative compensation approaches are coupled to a no-fault system and appear to work well. However, unlike auto no-fault and workers’ compensation, the notion of medical no-fault has never caught on in the United States.
As currently construed, health courts evince dramatic departures from traditional rules of civil procedure. For one, the panels may render decisions before discovery has occurred, which would substantially limit a patient’s ability to learn the facts of what had happened to cause the injury. The panel may rely on a standard of “gross negligence” instead of “ordinary negligence,” requiring evidence not merely of substandard care but of recklessness. This would be a heavier burden on the victim, and could be expected to generate stiff opposition from the plaintiff’s bar. In addition, evidentiary rules may be modified, requiring that an appeal show with clear and convincing proof that the tribunal had erred.
Apology law
Disclosure of medical errors to the injured patient is believed to serve as an ethical and effective way of thwarting potential malpractice claims. Many states have enacted so-called apology laws that disallow statements of sympathy from being admitted into evidence. In some cases, these laws may assist the physician.
For example, the Ohio Supreme Court ruled that a surgeon’s comments and alleged admission of guilt (“I take full responsibility for this” regarding accidentally sectioning the common bile duct) were properly shielded from discovery by the state’s apology statute.6 Apology laws vary from state to state, and some do not shield admissions regarding causation of error or fault.
However, it is unclear if apology laws work. A recent study from Vanderbilt University reported that, for physicians who do not regularly perform surgery, apology laws actually increased the probability of facing a lawsuit.7 And for surgeons, apology laws do not have a substantial effect on the probability of facing a claim or the average payment made to resolve a claim.
Safe harbors
A proposal released by U.S. House Speaker Paul Ryan (R-Wis.) in June 2016 made reference to “safe harbors” from liability for those adhering to clinical practice guidelines. The Institute of Medicine defines practice guidelines as “systematically developed statements to assist practitioner and patient decisions about appropriate health care for specific clinical circumstances.”
There are thousands of guidelines that have been developed by medical organizations and governmental agencies, as well as by insurance carriers, managed care organizations, and others. They purport to define the best evidence-based medicine, and if they were arrived at by the consensus of an authoritative body of experts, courts will tend to view them as reflective, though not necessarily dispositive, of customary medical standards.
Theoretically, adherence to guidelines could reduce the practice of defensive medicine and improve the quality of care. However, the available evidence does not indicate that guideline-based safe harbors will prove very effective in reducing malpractice claims: They are inapplicable in 85% of cases, and they have been estimated to eliminate defendants’ payments in less than 1% of claims.
Whether any form of tort reform emerges from the current Congress is as much about politics as it is about justice. It comes at an inopportune time, given the impasse over the health care debate. Still, on June 29, 2017, the U.S. House passed a medical liability reform bill with a vote of 218-210 along party lines that would cap noneconomic damages at $250,000, shorten the statute of limitations to 3 years after the date of injury, and abolish joint and several liability.8 The outlook in the U.S. Senate, however, is anything but certain.
References
1. N Engl J Med. 2017 May 11;376(19):1806-8.
2. “Sweeping new tort reforms will protect Iowa physicians” AMA Wire. June 1, 2017.
3. Chan v. Curran, 237 CA 4th 601 (2015).
4. N. Broward Hospital District v. Kalitan, (Florida Supreme Court, decided June 8, 2017).
5. Estate of Michelle Evette McCall v. U.S., 2014 Fla LEXIS 933 (No. SC 11-1148; March 13, 2014).
6. Estate of Johnson v. Randall Smith, Inc., 135 Ohio St.3d 440 (2013).
7. “Sorry is Never Enough: The Effect of State Apology Laws on Medical Malpractice Liability Risk” SSRN. 2016 Dec 10.
8. Protecting Access to Care Act of 2017 (H.R. 1215).
Question: Congressional proposals on medical tort reform can be expected to include the following, except:
A. A no-fault system akin to automobile no-fault insurance.
B. A cap on noneconomic damages.
C. “Safe-harbor” immunity against medical negligence.
D. Health courts in place of the judge/jury system to adjudicate claims.
E. Promotion of laws that encourage apologies and error disclosures.
Answer: A. Under the current Republican administration, one can expect legislative efforts at federal tort reform, especially given that Thomas E. Price, MD, the new secretary of the Department of Health & Human Services, is an orthopedic surgeon who has spoken passionately about defensive medicine, damage caps, health tribunals, and practice guidelines. As a former House representative for Georgia, Dr. Price has introduced several tort reform bills, so it is likely that any omnibus federal law will incorporate some of his proposals.1
Over the decades, many states have gone ahead in enacting their own statutes while awaiting federal action. Iowa is the latest example. It recently passed legislation that included a noneconomic damages cap of $250,000, stronger expert witness standards, a certificate of merit in all medical liability lawsuits, and an expansion of its “candor” protections.2 Additional reforms in other states include pretrial screening panels; arbitration; structured periodic payments in lieu of lump sum payments; penalties for frivolous suits; shortened statutes of limitations; making the loser bear all litigation costs; abolishing the collateral source rule, as well as joint and several liability; and limiting attorney contingency fees.
The best-known reform is a cap on noneconomic losses, such as pain and suffering, that doesn’t abridge compensation for economic losses, i.e., medical expenses and lost wages. This provides some predictability because noneconomic damages are difficult to quantify, and jury sympathy may result in unrealistically high payments.
Interestingly, Dr. Price himself has not pushed for a federal cap on noneconomic damages, but other Republican bills have proposed a cap of $250,000. Many states, such as California, Kansas, and Texas, have seen their cap statutes withstand constitutional challenge. However, other jurisdictions, notably Georgia, Illinois, and Missouri, have ruled them unconstitutional.
California’s law, popularly known as MICRA (Medical Injury Compensation Reform Act), came under renewed attack in 2015 with a wrongful death suit from hemorrhagic complications related to Coumadin (warfarin) use following heart surgery.3 The plaintiff’s constitutional challenges included violation of equal protection, due process, and the right to a jury trial, but these were essentially all grounded on an entitlement to recover additional noneconomic damages sufficient to cover attorney fees. The trial court had reduced her $1 million noneconomic damages to $250,000, as required under MICRA. A California court of appeal rejected her claim as being “contrary to many well-established legal principles.”
On the other hand, Florida’s Supreme Court recently held in a closely divided decision of 4-3 that the state’s caps were unconstitutional.4 The law limited noneconomic damages in malpractice cases to either $500,000 or $1 million if the injuries were catastrophic. The court ruled that the caps were arbitrary and unfairly hurt the most severely injured. It was unconvinced that they would reduce malpractice insurance rates; at any rate, there was no present crisis to justify the caps. The decision came 3 years after the court had struck down caps in a case of wrongful death.5
Three relative newcomers to the legal landscape – health courts, apology laws, and safe harbors – appear to be taking center stage in any forthcoming federal reform measures.
Health courts
Under this proposal, so-called health panels and tribunals would now adjudicate malpractice claims. Such health courts would dispense with the jury; further, regular judges would be replaced with specialized judges who would make binding determinations. In one version, a panel of medical experts would initially screen the complaint, followed by an administrative health care tribunal that would feature judges with medical expertise. These tribunals would issue binding rulings, but either party could appeal to a state court for a reversal.
In countries such as Scandinavia and New Zealand, these administrative compensation approaches are coupled to a no-fault system and appear to work well. However, unlike auto no-fault and workers’ compensation, the notion of medical no-fault has never caught on in the United States.
As currently construed, health courts evince dramatic departures from traditional rules of civil procedure. For one, the panels may render decisions before discovery has occurred, which would substantially limit a patient’s ability to learn the facts of what had happened to cause the injury. The panel may rely on a standard of “gross negligence” instead of “ordinary negligence,” requiring evidence not merely of substandard care but of recklessness. This would be a heavier burden on the victim, and could be expected to generate stiff opposition from the plaintiff’s bar. In addition, evidentiary rules may be modified, requiring that an appeal show with clear and convincing proof that the tribunal had erred.
Apology law
Disclosure of medical errors to the injured patient is believed to serve as an ethical and effective way of thwarting potential malpractice claims. Many states have enacted so-called apology laws that disallow statements of sympathy from being admitted into evidence. In some cases, these laws may assist the physician.
For example, the Ohio Supreme Court ruled that a surgeon’s comments and alleged admission of guilt (“I take full responsibility for this” regarding accidentally sectioning the common bile duct) were properly shielded from discovery by the state’s apology statute.6 Apology laws vary from state to state, and some do not shield admissions regarding causation of error or fault.
However, it is unclear if apology laws work. A recent study from Vanderbilt University reported that, for physicians who do not regularly perform surgery, apology laws actually increased the probability of facing a lawsuit.7 And for surgeons, apology laws do not have a substantial effect on the probability of facing a claim or the average payment made to resolve a claim.
Safe harbors
A proposal released by U.S. House Speaker Paul Ryan (R-Wis.) in June 2016 made reference to “safe harbors” from liability for those adhering to clinical practice guidelines. The Institute of Medicine defines practice guidelines as “systematically developed statements to assist practitioner and patient decisions about appropriate health care for specific clinical circumstances.”
There are thousands of guidelines that have been developed by medical organizations and governmental agencies, as well as by insurance carriers, managed care organizations, and others. They purport to define the best evidence-based medicine, and if they were arrived at by the consensus of an authoritative body of experts, courts will tend to view them as reflective, though not necessarily dispositive, of customary medical standards.
Theoretically, adherence to guidelines could reduce the practice of defensive medicine and improve the quality of care. However, the available evidence does not indicate that guideline-based safe harbors will prove very effective in reducing malpractice claims: They are inapplicable in 85% of cases, and they have been estimated to eliminate defendants’ payments in less than 1% of claims.
Whether any form of tort reform emerges from the current Congress is as much about politics as it is about justice. It comes at an inopportune time, given the impasse over the health care debate. Still, on June 29, 2017, the U.S. House passed a medical liability reform bill with a vote of 218-210 along party lines that would cap noneconomic damages at $250,000, shorten the statute of limitations to 3 years after the date of injury, and abolish joint and several liability.8 The outlook in the U.S. Senate, however, is anything but certain.
References
1. N Engl J Med. 2017 May 11;376(19):1806-8.
2. “Sweeping new tort reforms will protect Iowa physicians” AMA Wire. June 1, 2017.
3. Chan v. Curran, 237 CA 4th 601 (2015).
4. N. Broward Hospital District v. Kalitan, (Florida Supreme Court, decided June 8, 2017).
5. Estate of Michelle Evette McCall v. U.S., 2014 Fla LEXIS 933 (No. SC 11-1148; March 13, 2014).
6. Estate of Johnson v. Randall Smith, Inc., 135 Ohio St.3d 440 (2013).
7. “Sorry is Never Enough: The Effect of State Apology Laws on Medical Malpractice Liability Risk” SSRN. 2016 Dec 10.
8. Protecting Access to Care Act of 2017 (H.R. 1215).
Question: Congressional proposals on medical tort reform can be expected to include the following, except:
A. A no-fault system akin to automobile no-fault insurance.
B. A cap on noneconomic damages.
C. “Safe-harbor” immunity against medical negligence.
D. Health courts in place of the judge/jury system to adjudicate claims.
E. Promotion of laws that encourage apologies and error disclosures.
Answer: A. Under the current Republican administration, one can expect legislative efforts at federal tort reform, especially given that Thomas E. Price, MD, the new secretary of the Department of Health & Human Services, is an orthopedic surgeon who has spoken passionately about defensive medicine, damage caps, health tribunals, and practice guidelines. As a former House representative for Georgia, Dr. Price has introduced several tort reform bills, so it is likely that any omnibus federal law will incorporate some of his proposals.1
Over the decades, many states have gone ahead in enacting their own statutes while awaiting federal action. Iowa is the latest example. It recently passed legislation that included a noneconomic damages cap of $250,000, stronger expert witness standards, a certificate of merit in all medical liability lawsuits, and an expansion of its “candor” protections.2 Additional reforms in other states include pretrial screening panels; arbitration; structured periodic payments in lieu of lump sum payments; penalties for frivolous suits; shortened statutes of limitations; making the loser bear all litigation costs; abolishing the collateral source rule, as well as joint and several liability; and limiting attorney contingency fees.
The best-known reform is a cap on noneconomic losses, such as pain and suffering, that doesn’t abridge compensation for economic losses, i.e., medical expenses and lost wages. This provides some predictability because noneconomic damages are difficult to quantify, and jury sympathy may result in unrealistically high payments.
Interestingly, Dr. Price himself has not pushed for a federal cap on noneconomic damages, but other Republican bills have proposed a cap of $250,000. Many states, such as California, Kansas, and Texas, have seen their cap statutes withstand constitutional challenge. However, other jurisdictions, notably Georgia, Illinois, and Missouri, have ruled them unconstitutional.
California’s law, popularly known as MICRA (Medical Injury Compensation Reform Act), came under renewed attack in 2015 with a wrongful death suit from hemorrhagic complications related to Coumadin (warfarin) use following heart surgery.3 The plaintiff’s constitutional challenges included violation of equal protection, due process, and the right to a jury trial, but these were essentially all grounded on an entitlement to recover additional noneconomic damages sufficient to cover attorney fees. The trial court had reduced her $1 million noneconomic damages to $250,000, as required under MICRA. A California court of appeal rejected her claim as being “contrary to many well-established legal principles.”
On the other hand, Florida’s Supreme Court recently held in a closely divided decision of 4-3 that the state’s caps were unconstitutional.4 The law limited noneconomic damages in malpractice cases to either $500,000 or $1 million if the injuries were catastrophic. The court ruled that the caps were arbitrary and unfairly hurt the most severely injured. It was unconvinced that they would reduce malpractice insurance rates; at any rate, there was no present crisis to justify the caps. The decision came 3 years after the court had struck down caps in a case of wrongful death.5
Three relative newcomers to the legal landscape – health courts, apology laws, and safe harbors – appear to be taking center stage in any forthcoming federal reform measures.
Health courts
Under this proposal, so-called health panels and tribunals would now adjudicate malpractice claims. Such health courts would dispense with the jury; further, regular judges would be replaced with specialized judges who would make binding determinations. In one version, a panel of medical experts would initially screen the complaint, followed by an administrative health care tribunal that would feature judges with medical expertise. These tribunals would issue binding rulings, but either party could appeal to a state court for a reversal.
In countries such as Scandinavia and New Zealand, these administrative compensation approaches are coupled to a no-fault system and appear to work well. However, unlike auto no-fault and workers’ compensation, the notion of medical no-fault has never caught on in the United States.
As currently construed, health courts evince dramatic departures from traditional rules of civil procedure. For one, the panels may render decisions before discovery has occurred, which would substantially limit a patient’s ability to learn the facts of what had happened to cause the injury. The panel may rely on a standard of “gross negligence” instead of “ordinary negligence,” requiring evidence not merely of substandard care but of recklessness. This would be a heavier burden on the victim, and could be expected to generate stiff opposition from the plaintiff’s bar. In addition, evidentiary rules may be modified, requiring that an appeal show with clear and convincing proof that the tribunal had erred.
Apology law
Disclosure of medical errors to the injured patient is believed to serve as an ethical and effective way of thwarting potential malpractice claims. Many states have enacted so-called apology laws that disallow statements of sympathy from being admitted into evidence. In some cases, these laws may assist the physician.
For example, the Ohio Supreme Court ruled that a surgeon’s comments and alleged admission of guilt (“I take full responsibility for this” regarding accidentally sectioning the common bile duct) were properly shielded from discovery by the state’s apology statute.6 Apology laws vary from state to state, and some do not shield admissions regarding causation of error or fault.
However, it is unclear if apology laws work. A recent study from Vanderbilt University reported that, for physicians who do not regularly perform surgery, apology laws actually increased the probability of facing a lawsuit.7 And for surgeons, apology laws do not have a substantial effect on the probability of facing a claim or the average payment made to resolve a claim.
Safe harbors
A proposal released by U.S. House Speaker Paul Ryan (R-Wis.) in June 2016 made reference to “safe harbors” from liability for those adhering to clinical practice guidelines. The Institute of Medicine defines practice guidelines as “systematically developed statements to assist practitioner and patient decisions about appropriate health care for specific clinical circumstances.”
There are thousands of guidelines that have been developed by medical organizations and governmental agencies, as well as by insurance carriers, managed care organizations, and others. They purport to define the best evidence-based medicine, and if they were arrived at by the consensus of an authoritative body of experts, courts will tend to view them as reflective, though not necessarily dispositive, of customary medical standards.
Theoretically, adherence to guidelines could reduce the practice of defensive medicine and improve the quality of care. However, the available evidence does not indicate that guideline-based safe harbors will prove very effective in reducing malpractice claims: They are inapplicable in 85% of cases, and they have been estimated to eliminate defendants’ payments in less than 1% of claims.
Whether any form of tort reform emerges from the current Congress is as much about politics as it is about justice. It comes at an inopportune time, given the impasse over the health care debate. Still, on June 29, 2017, the U.S. House passed a medical liability reform bill with a vote of 218-210 along party lines that would cap noneconomic damages at $250,000, shorten the statute of limitations to 3 years after the date of injury, and abolish joint and several liability.8 The outlook in the U.S. Senate, however, is anything but certain.
References
1. N Engl J Med. 2017 May 11;376(19):1806-8.
2. “Sweeping new tort reforms will protect Iowa physicians” AMA Wire. June 1, 2017.
3. Chan v. Curran, 237 CA 4th 601 (2015).
4. N. Broward Hospital District v. Kalitan, (Florida Supreme Court, decided June 8, 2017).
5. Estate of Michelle Evette McCall v. U.S., 2014 Fla LEXIS 933 (No. SC 11-1148; March 13, 2014).
6. Estate of Johnson v. Randall Smith, Inc., 135 Ohio St.3d 440 (2013).
7. “Sorry is Never Enough: The Effect of State Apology Laws on Medical Malpractice Liability Risk” SSRN. 2016 Dec 10.
8. Protecting Access to Care Act of 2017 (H.R. 1215).
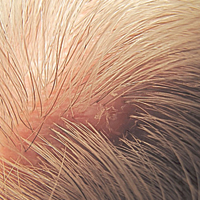
Screening MRI misses Sturge-Weber in babies with port-wine stain
CHICAGO – Screening infants with a port-wine stain for Sturge-Weber syndrome (SWS) with a magnetic resonance imaging brain scan had a 23% false-negative rate and actually delayed seizure detection, according to a recent study.
When infants with port-wine stains receive a dermatology consult, they may also be screened for SWS by means of MRI and by electroencephalography, particularly if their lesion phenotype puts them at higher risk for SWS. But the accuracy and benefit of the screenings has not been well established, said Michaela Zallmann, MD, the study’s first author. Hemifacial lesions that involve both the forehead and cheek and median lesions that are centered around the facial midline are both considered high-risk lesions.
Dr. Zallmann, a dermatologist at Monash University and the Royal Children’s Hospital, Melbourne, and her coinvestigators examined data on 126 patients with facial port-wine stains who came to a laser clinic over a 12-month period. Of these, 32 (25.4%) had a high-risk port-wine stain, and 9 of those 32 (28.1%) had a capillary-venous malformation characteristic of SWS. Of the high-risk patients, 14 received a screening MRI or EEG before having had a first seizure. Of those 14 scans, 1 resulted in a diagnosis of SWS; of the 13 patients with a negative MRI screen, 3 (23.1%) were later found to have SWS when their parents or caregivers detected seizures. Thus, a total of four of the high-risk infants who were screened eventually were diagnosed with SWS.
Of the 18 high-risk patients who did not receive a screening MRI, 3 (16.7%) developed seizures, while 2 (11.1%) were seizure free but developed glaucoma severe enough to require treatment. One patient who was also seizure free developed an autism spectrum disorder.
Two patients who were in the high-risk group received screening EEGs that detected abnormalities that were not yet clinically evident. These included sub-clinical seizures and posterior-quadrant focal slowing. Both of these patients had initial negative screening MRIs.
Scanning early in life, using inappropriate imaging protocols, and having an inexperienced radiologist were all factors associated with a higher probability of false negative screening MRI, according to the researchers’ analysis, which was presented in a poster session at the World Congress of Pediatric Dermatology.
All of the false negative MRIs in the study cohort were conducted in infants younger than 9 weeks old. But whether it is useful to reserve imaging for later in infancy is debatable. “While later imaging may have improved sensitivity, 75% of infants with SWS will have already had their first seizure by 12 months of age,” wrote Dr. Zallmann and her colleagues.
Of the infants involved in the study, two of the three patients with false negative scans did not receive a referral to a neurologist, nor did their parents receive seizure education. “False reassurance may delay seizure detection,” Dr. Zallmann said.
For infants with positive MRIs who went on to develop seizures, the mean age of when they experienced their first documented seizure was 10 weeks. For those who did not receive an MRI, the mean age was 14 months, compared with 28 months for patients who had received a false negative MRI.
In discussing the findings, Dr. Zallmann and her colleagues made the point that early referral to a pediatric neurologist is important, especially for infants with the higher-risk port-wine stain patterns of hemifacial and median lesions. Seizure education can help parents detect the often subtle signs of seizures in infants with SWS, which can include staring spells, subtle limb twitching, and lip smacking.
The fact that seizures were detected an average of 14 months later in patients with negative screening MRIs may mean that such subtle signs were missed. “False reassurance may delay the recognition and treatment of seizures and impact neurodevelopmental outcomes,” Dr. Zallmann and her colleagues wrote in the abstract that accompanied the poster.
The study, while small, helps fill in some knowledge gaps, the researchers pointed out; they noted that there is no consensus on what level or type of facial involvement warrants screening, which protocols are best for MRI and EEG, or even whether the screening will improve seizure detection or outcomes.
“Currently there is no evidence that screening improves neurodevelopmental outcomes,” they said. “Conversely, there is a role for early neurological referral, symptom education, and potentially of EEGs in the prevention of complications related to SWS.”
Dr. Zallmann reported no conflicts of interest.
[email protected]
On Twitter @karioakes
CHICAGO – Screening infants with a port-wine stain for Sturge-Weber syndrome (SWS) with a magnetic resonance imaging brain scan had a 23% false-negative rate and actually delayed seizure detection, according to a recent study.
When infants with port-wine stains receive a dermatology consult, they may also be screened for SWS by means of MRI and by electroencephalography, particularly if their lesion phenotype puts them at higher risk for SWS. But the accuracy and benefit of the screenings has not been well established, said Michaela Zallmann, MD, the study’s first author. Hemifacial lesions that involve both the forehead and cheek and median lesions that are centered around the facial midline are both considered high-risk lesions.
Dr. Zallmann, a dermatologist at Monash University and the Royal Children’s Hospital, Melbourne, and her coinvestigators examined data on 126 patients with facial port-wine stains who came to a laser clinic over a 12-month period. Of these, 32 (25.4%) had a high-risk port-wine stain, and 9 of those 32 (28.1%) had a capillary-venous malformation characteristic of SWS. Of the high-risk patients, 14 received a screening MRI or EEG before having had a first seizure. Of those 14 scans, 1 resulted in a diagnosis of SWS; of the 13 patients with a negative MRI screen, 3 (23.1%) were later found to have SWS when their parents or caregivers detected seizures. Thus, a total of four of the high-risk infants who were screened eventually were diagnosed with SWS.
Of the 18 high-risk patients who did not receive a screening MRI, 3 (16.7%) developed seizures, while 2 (11.1%) were seizure free but developed glaucoma severe enough to require treatment. One patient who was also seizure free developed an autism spectrum disorder.
Two patients who were in the high-risk group received screening EEGs that detected abnormalities that were not yet clinically evident. These included sub-clinical seizures and posterior-quadrant focal slowing. Both of these patients had initial negative screening MRIs.
Scanning early in life, using inappropriate imaging protocols, and having an inexperienced radiologist were all factors associated with a higher probability of false negative screening MRI, according to the researchers’ analysis, which was presented in a poster session at the World Congress of Pediatric Dermatology.
All of the false negative MRIs in the study cohort were conducted in infants younger than 9 weeks old. But whether it is useful to reserve imaging for later in infancy is debatable. “While later imaging may have improved sensitivity, 75% of infants with SWS will have already had their first seizure by 12 months of age,” wrote Dr. Zallmann and her colleagues.
Of the infants involved in the study, two of the three patients with false negative scans did not receive a referral to a neurologist, nor did their parents receive seizure education. “False reassurance may delay seizure detection,” Dr. Zallmann said.
For infants with positive MRIs who went on to develop seizures, the mean age of when they experienced their first documented seizure was 10 weeks. For those who did not receive an MRI, the mean age was 14 months, compared with 28 months for patients who had received a false negative MRI.
In discussing the findings, Dr. Zallmann and her colleagues made the point that early referral to a pediatric neurologist is important, especially for infants with the higher-risk port-wine stain patterns of hemifacial and median lesions. Seizure education can help parents detect the often subtle signs of seizures in infants with SWS, which can include staring spells, subtle limb twitching, and lip smacking.
The fact that seizures were detected an average of 14 months later in patients with negative screening MRIs may mean that such subtle signs were missed. “False reassurance may delay the recognition and treatment of seizures and impact neurodevelopmental outcomes,” Dr. Zallmann and her colleagues wrote in the abstract that accompanied the poster.
The study, while small, helps fill in some knowledge gaps, the researchers pointed out; they noted that there is no consensus on what level or type of facial involvement warrants screening, which protocols are best for MRI and EEG, or even whether the screening will improve seizure detection or outcomes.
“Currently there is no evidence that screening improves neurodevelopmental outcomes,” they said. “Conversely, there is a role for early neurological referral, symptom education, and potentially of EEGs in the prevention of complications related to SWS.”
Dr. Zallmann reported no conflicts of interest.
[email protected]
On Twitter @karioakes
CHICAGO – Screening infants with a port-wine stain for Sturge-Weber syndrome (SWS) with a magnetic resonance imaging brain scan had a 23% false-negative rate and actually delayed seizure detection, according to a recent study.
When infants with port-wine stains receive a dermatology consult, they may also be screened for SWS by means of MRI and by electroencephalography, particularly if their lesion phenotype puts them at higher risk for SWS. But the accuracy and benefit of the screenings has not been well established, said Michaela Zallmann, MD, the study’s first author. Hemifacial lesions that involve both the forehead and cheek and median lesions that are centered around the facial midline are both considered high-risk lesions.
Dr. Zallmann, a dermatologist at Monash University and the Royal Children’s Hospital, Melbourne, and her coinvestigators examined data on 126 patients with facial port-wine stains who came to a laser clinic over a 12-month period. Of these, 32 (25.4%) had a high-risk port-wine stain, and 9 of those 32 (28.1%) had a capillary-venous malformation characteristic of SWS. Of the high-risk patients, 14 received a screening MRI or EEG before having had a first seizure. Of those 14 scans, 1 resulted in a diagnosis of SWS; of the 13 patients with a negative MRI screen, 3 (23.1%) were later found to have SWS when their parents or caregivers detected seizures. Thus, a total of four of the high-risk infants who were screened eventually were diagnosed with SWS.
Of the 18 high-risk patients who did not receive a screening MRI, 3 (16.7%) developed seizures, while 2 (11.1%) were seizure free but developed glaucoma severe enough to require treatment. One patient who was also seizure free developed an autism spectrum disorder.
Two patients who were in the high-risk group received screening EEGs that detected abnormalities that were not yet clinically evident. These included sub-clinical seizures and posterior-quadrant focal slowing. Both of these patients had initial negative screening MRIs.
Scanning early in life, using inappropriate imaging protocols, and having an inexperienced radiologist were all factors associated with a higher probability of false negative screening MRI, according to the researchers’ analysis, which was presented in a poster session at the World Congress of Pediatric Dermatology.
All of the false negative MRIs in the study cohort were conducted in infants younger than 9 weeks old. But whether it is useful to reserve imaging for later in infancy is debatable. “While later imaging may have improved sensitivity, 75% of infants with SWS will have already had their first seizure by 12 months of age,” wrote Dr. Zallmann and her colleagues.
Of the infants involved in the study, two of the three patients with false negative scans did not receive a referral to a neurologist, nor did their parents receive seizure education. “False reassurance may delay seizure detection,” Dr. Zallmann said.
For infants with positive MRIs who went on to develop seizures, the mean age of when they experienced their first documented seizure was 10 weeks. For those who did not receive an MRI, the mean age was 14 months, compared with 28 months for patients who had received a false negative MRI.
In discussing the findings, Dr. Zallmann and her colleagues made the point that early referral to a pediatric neurologist is important, especially for infants with the higher-risk port-wine stain patterns of hemifacial and median lesions. Seizure education can help parents detect the often subtle signs of seizures in infants with SWS, which can include staring spells, subtle limb twitching, and lip smacking.
The fact that seizures were detected an average of 14 months later in patients with negative screening MRIs may mean that such subtle signs were missed. “False reassurance may delay the recognition and treatment of seizures and impact neurodevelopmental outcomes,” Dr. Zallmann and her colleagues wrote in the abstract that accompanied the poster.
The study, while small, helps fill in some knowledge gaps, the researchers pointed out; they noted that there is no consensus on what level or type of facial involvement warrants screening, which protocols are best for MRI and EEG, or even whether the screening will improve seizure detection or outcomes.
“Currently there is no evidence that screening improves neurodevelopmental outcomes,” they said. “Conversely, there is a role for early neurological referral, symptom education, and potentially of EEGs in the prevention of complications related to SWS.”
Dr. Zallmann reported no conflicts of interest.
[email protected]
On Twitter @karioakes
AT WCPD 2017
Key clinical point:
Major finding: Magnetic resonance imaging screening for Sturge-Weber syndrome resulted in a 23.2% false negative rate in babies with port-wine stain.
Data source: A review of screening and outcomes for 126 infants with port-wine stain seen in a laser clinic over a 12-month period. Disclosures: The lead author reported no disclosures.
Wide variability found in invasive mediastinal staging rates for lung cancer
COLORADO SPRINGS – Significant variability exists between hospitals in Washington state in their rates of invasive mediastinal staging for lung cancer, Farhood Farjah, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
“We found evidence of a fivefold variation in hospital-level rates of invasive mediastinal staging not explained by chance or case mix,” according to Dr. Farjah of the University of Washington, Seattle.
“This has led to substantial concerns about quality of thoracic surgical care in the community at large,” he noted.
The Washington study is the first to show hospital-by-hospital variation in rates of invasive mediastinal staging.
Invasive mediastinal staging for lung cancer is considered important because imaging is known to have a substantial false-negative rate, and staging results have a profound impact on treatment recommendations, which can range from surgery alone to additional chemoradiation therapy.
Yet the meaning of the hospital-level huge variability in practice observed in the Washington study remains unclear.
“Our understanding of the underutilization of invasive mediastinal staging is further complicated by the fact that patterns of invasive mediastinal staging are highly variable across hospitals staffed by at least one board-certified thoracic surgeon with a noncardiac practice,” Dr. Farjah explained. “This variability could be a marker of poor-quality care. However, because the guidelines are not supported by level 1 evidence, it’s equally plausible that this variability might represent uncertainty or even disagreement with the practice guidelines – and specifically about the appropriate indication for invasive staging.”
He presented a retrospective cohort study of 406 patients whose non–small cell lung cancer was resected during July 2011–December 2013 at one of five Washington hospitals, each with at least one board-certified thoracic surgeon with a noncardiac practice on staff. The four participating community hospitals and one academic medical center were involved in a National Cancer Institute–funded, physician-led quality improvement initiative.
Overall, 66% of the 406 patients underwent any form of invasive mediastinal staging: 85% by mediastinoscopy only; 12% by mediastinoscopy plus endobronchial ultrasound-guided nodal aspiration (EBUS); 3% by EBUS only; and the remaining handful by mediastinoscopy, EBUS, and esophageal ultrasound-guided nodal aspiration. The invasive staging was performed at the time of resection in 64% of cases. A median of three nodal stations were sampled.
After statistical adjustment for random variation and between-hospital differences in clinical stage, rates of invasive staging were all over the map. While an overall mean of 66% of the lung cancer patients underwent invasive mediastinal staging, the rates at the five hospitals were 94%, 84%, 31%, 80%, and 17%.
Dr. Farjah and his coinvestigators are now conducting provider interviews and focus groups in an effort to understand what drove the participating surgeons’ wide variability in performing invasive mediastinal staging.
Discussant Jane Yanagawa, MD, of the University of California, Los Angeles, commented, “I think this is a really interesting study because, historically, lower rates of mediastinoscopy are assumed to be a reflection of low-quality care – and you suggest that might not be the case, that it might be more complicated than that.”
Dr. Yanagawa sketched one fairly common scenario that might represent a surgeon’s reasonable avoidance of guideline-recommended invasive mediastinal staging: a patient who by all preoperative imaging appears to have stage IA lung cancer and wishes to avoid the morbidity, time, and cost of needle biopsy, instead choosing to go straight to the operating room for a diagnosis by wedge resection, followed by a completion lobectomy based upon the frozen section results. Could such a pathway account for the variability seen in the Washington study?
“I think it could have,” Dr. Farjah replied. “I would say that’s probably one driver of variability.”
As for the generalizability of the findings of a five-hospital study carried out in a single state, Dr. Farjah said he thinks the results are applicable to any academic or community hospital with at least one board-certified thoracic surgeon with a noncardiac practice.
He reported having no financial conflicts of interest regarding the study.
M. Patricia Rivera, MD, FCCP, comments: Staging of lung cancer is essential to select the best treatment strategy for a given patient. However, despite multiple guideline recommendations
M. Patricia Rivera, MD, FCCP, comments: Staging of lung cancer is essential to select the best treatment strategy for a given patient. However, despite multiple guideline recommendations
M. Patricia Rivera, MD, FCCP, comments: Staging of lung cancer is essential to select the best treatment strategy for a given patient. However, despite multiple guideline recommendations
COLORADO SPRINGS – Significant variability exists between hospitals in Washington state in their rates of invasive mediastinal staging for lung cancer, Farhood Farjah, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
“We found evidence of a fivefold variation in hospital-level rates of invasive mediastinal staging not explained by chance or case mix,” according to Dr. Farjah of the University of Washington, Seattle.
“This has led to substantial concerns about quality of thoracic surgical care in the community at large,” he noted.
The Washington study is the first to show hospital-by-hospital variation in rates of invasive mediastinal staging.
Invasive mediastinal staging for lung cancer is considered important because imaging is known to have a substantial false-negative rate, and staging results have a profound impact on treatment recommendations, which can range from surgery alone to additional chemoradiation therapy.
Yet the meaning of the hospital-level huge variability in practice observed in the Washington study remains unclear.
“Our understanding of the underutilization of invasive mediastinal staging is further complicated by the fact that patterns of invasive mediastinal staging are highly variable across hospitals staffed by at least one board-certified thoracic surgeon with a noncardiac practice,” Dr. Farjah explained. “This variability could be a marker of poor-quality care. However, because the guidelines are not supported by level 1 evidence, it’s equally plausible that this variability might represent uncertainty or even disagreement with the practice guidelines – and specifically about the appropriate indication for invasive staging.”
He presented a retrospective cohort study of 406 patients whose non–small cell lung cancer was resected during July 2011–December 2013 at one of five Washington hospitals, each with at least one board-certified thoracic surgeon with a noncardiac practice on staff. The four participating community hospitals and one academic medical center were involved in a National Cancer Institute–funded, physician-led quality improvement initiative.
Overall, 66% of the 406 patients underwent any form of invasive mediastinal staging: 85% by mediastinoscopy only; 12% by mediastinoscopy plus endobronchial ultrasound-guided nodal aspiration (EBUS); 3% by EBUS only; and the remaining handful by mediastinoscopy, EBUS, and esophageal ultrasound-guided nodal aspiration. The invasive staging was performed at the time of resection in 64% of cases. A median of three nodal stations were sampled.
After statistical adjustment for random variation and between-hospital differences in clinical stage, rates of invasive staging were all over the map. While an overall mean of 66% of the lung cancer patients underwent invasive mediastinal staging, the rates at the five hospitals were 94%, 84%, 31%, 80%, and 17%.
Dr. Farjah and his coinvestigators are now conducting provider interviews and focus groups in an effort to understand what drove the participating surgeons’ wide variability in performing invasive mediastinal staging.
Discussant Jane Yanagawa, MD, of the University of California, Los Angeles, commented, “I think this is a really interesting study because, historically, lower rates of mediastinoscopy are assumed to be a reflection of low-quality care – and you suggest that might not be the case, that it might be more complicated than that.”
Dr. Yanagawa sketched one fairly common scenario that might represent a surgeon’s reasonable avoidance of guideline-recommended invasive mediastinal staging: a patient who by all preoperative imaging appears to have stage IA lung cancer and wishes to avoid the morbidity, time, and cost of needle biopsy, instead choosing to go straight to the operating room for a diagnosis by wedge resection, followed by a completion lobectomy based upon the frozen section results. Could such a pathway account for the variability seen in the Washington study?
“I think it could have,” Dr. Farjah replied. “I would say that’s probably one driver of variability.”
As for the generalizability of the findings of a five-hospital study carried out in a single state, Dr. Farjah said he thinks the results are applicable to any academic or community hospital with at least one board-certified thoracic surgeon with a noncardiac practice.
He reported having no financial conflicts of interest regarding the study.
COLORADO SPRINGS – Significant variability exists between hospitals in Washington state in their rates of invasive mediastinal staging for lung cancer, Farhood Farjah, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
“We found evidence of a fivefold variation in hospital-level rates of invasive mediastinal staging not explained by chance or case mix,” according to Dr. Farjah of the University of Washington, Seattle.
“This has led to substantial concerns about quality of thoracic surgical care in the community at large,” he noted.
The Washington study is the first to show hospital-by-hospital variation in rates of invasive mediastinal staging.
Invasive mediastinal staging for lung cancer is considered important because imaging is known to have a substantial false-negative rate, and staging results have a profound impact on treatment recommendations, which can range from surgery alone to additional chemoradiation therapy.
Yet the meaning of the hospital-level huge variability in practice observed in the Washington study remains unclear.
“Our understanding of the underutilization of invasive mediastinal staging is further complicated by the fact that patterns of invasive mediastinal staging are highly variable across hospitals staffed by at least one board-certified thoracic surgeon with a noncardiac practice,” Dr. Farjah explained. “This variability could be a marker of poor-quality care. However, because the guidelines are not supported by level 1 evidence, it’s equally plausible that this variability might represent uncertainty or even disagreement with the practice guidelines – and specifically about the appropriate indication for invasive staging.”
He presented a retrospective cohort study of 406 patients whose non–small cell lung cancer was resected during July 2011–December 2013 at one of five Washington hospitals, each with at least one board-certified thoracic surgeon with a noncardiac practice on staff. The four participating community hospitals and one academic medical center were involved in a National Cancer Institute–funded, physician-led quality improvement initiative.
Overall, 66% of the 406 patients underwent any form of invasive mediastinal staging: 85% by mediastinoscopy only; 12% by mediastinoscopy plus endobronchial ultrasound-guided nodal aspiration (EBUS); 3% by EBUS only; and the remaining handful by mediastinoscopy, EBUS, and esophageal ultrasound-guided nodal aspiration. The invasive staging was performed at the time of resection in 64% of cases. A median of three nodal stations were sampled.
After statistical adjustment for random variation and between-hospital differences in clinical stage, rates of invasive staging were all over the map. While an overall mean of 66% of the lung cancer patients underwent invasive mediastinal staging, the rates at the five hospitals were 94%, 84%, 31%, 80%, and 17%.
Dr. Farjah and his coinvestigators are now conducting provider interviews and focus groups in an effort to understand what drove the participating surgeons’ wide variability in performing invasive mediastinal staging.
Discussant Jane Yanagawa, MD, of the University of California, Los Angeles, commented, “I think this is a really interesting study because, historically, lower rates of mediastinoscopy are assumed to be a reflection of low-quality care – and you suggest that might not be the case, that it might be more complicated than that.”
Dr. Yanagawa sketched one fairly common scenario that might represent a surgeon’s reasonable avoidance of guideline-recommended invasive mediastinal staging: a patient who by all preoperative imaging appears to have stage IA lung cancer and wishes to avoid the morbidity, time, and cost of needle biopsy, instead choosing to go straight to the operating room for a diagnosis by wedge resection, followed by a completion lobectomy based upon the frozen section results. Could such a pathway account for the variability seen in the Washington study?
“I think it could have,” Dr. Farjah replied. “I would say that’s probably one driver of variability.”
As for the generalizability of the findings of a five-hospital study carried out in a single state, Dr. Farjah said he thinks the results are applicable to any academic or community hospital with at least one board-certified thoracic surgeon with a noncardiac practice.
He reported having no financial conflicts of interest regarding the study.
AT THE WTSA ANNUAL MEETING
Key clinical point:
Major finding: Rates of invasive mediastinal staging after adjustment for clinical stage ranged from a low of 17% at one hospital to as high as 94% at another.
Data source: This retrospective cohort study included 406 patients.
Disclosures: Dr. Farjah reported having no financial conflicts of interest.
TAVR for failed surgical valves: the VIVA study
PARIS – Transcatheter aortic valve replacement using the self-expanding Evolut R device in high-surgical-risk patients with failing surgical aortic bioprostheses showed promising 30-day safety and effectiveness results in the ongoing VIVA study, Ran Kornowski, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“We had a lot of patients with small, failing valves in this study. Despite this, our valve gradients postprocedure were very, very low. This means to me that this platform is very well suited to deal with valve-in-valve procedures in general and with small bioprosthetic valves in particular,” observed Dr. Kornowski, chairman of the department of cardiology at Rabin Medical Center in Petah Tikva, Israel, and president of the Israel Heart Society.
The participants’ last surgical aortic valve replacement had been a mean of 9.3 years earlier. Seventy-one percent of subjects were New York Heart Association class III or IV. The mode of bioprosthetic failure was stenosis in 56% of cases, regurgitation in 23%, and both in the remainder. Ninety-three percent of their failing biosprothetic valves were stented devices. Forty-one percent of the devices were up to 21 mm and another 33% were more than 21 but less than 25 mm.
TAVR procedural access was by the iliofemoral route in 97% of cases. Local anesthesia was used in 42% of cases and conscious sedation in 35%. Fourteen percent of patients underwent preimplantation valvuloplasty; 21% postimplantation valvuloplasty. The procedural success rate was 98.5%.
The primary safety endpoint was 30-day cardiovascular mortality. The 2.0% rate was far lower than the prespecified cutoff which defined a positive outcome as less than a 10% rate in this high-surgical-risk population.
Other key 30-day outcomes:
• All-cause mortality occurred in 2.5% of patients.
• The 30-day stroke rate was 3%, with no disabling strokes.
• Major vascular complications occurred in 6.5% of the VIVA patients.
• Major bleeding occurred in 7%, minor bleeding in 7.9%. There were no cases of life-threatening bleeding.
• The incidence of Stage I acute kidney injury was rare, at 0.5%.
• Seven percent of patients received a permanent pacemaker.
• Eighty-seven percent of patients had no postprocedure paravalvular regurgitation (PVR), 11.4 had mild PVR, and 1.5% had moderate PVR.
• NYHA classification improved from baseline to 30 days in 81% of patients. At 30 days, 93% of participants were NYHA class I or II.
Turning to echocardiographic findings, the mean gradient improved from a mean baseline of 31.8 mm Hg to 12.6 mm Hg, while the effective orifice area rose from 1.0 to 1.5 cm2. The magnitude of both improvements was greater for patients with stenosis as their mode of valve failure.
“With the Evolut R, we aim for higher implantations – not more than about 4 mm below the ring – because going deeper could bring about higher gradients and functional deterioration later on,” the cardiologist explained.
The 1-year primary efficacy endpoint – lack of significant aortic stenosis as defined by a mean gradient less than 40 mm Hg – will be reported soon. The VIVA study is sponsored by Medtronic. Dr. Kornowski reported serving as a consultant to the company.
PARIS – Transcatheter aortic valve replacement using the self-expanding Evolut R device in high-surgical-risk patients with failing surgical aortic bioprostheses showed promising 30-day safety and effectiveness results in the ongoing VIVA study, Ran Kornowski, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“We had a lot of patients with small, failing valves in this study. Despite this, our valve gradients postprocedure were very, very low. This means to me that this platform is very well suited to deal with valve-in-valve procedures in general and with small bioprosthetic valves in particular,” observed Dr. Kornowski, chairman of the department of cardiology at Rabin Medical Center in Petah Tikva, Israel, and president of the Israel Heart Society.
The participants’ last surgical aortic valve replacement had been a mean of 9.3 years earlier. Seventy-one percent of subjects were New York Heart Association class III or IV. The mode of bioprosthetic failure was stenosis in 56% of cases, regurgitation in 23%, and both in the remainder. Ninety-three percent of their failing biosprothetic valves were stented devices. Forty-one percent of the devices were up to 21 mm and another 33% were more than 21 but less than 25 mm.
TAVR procedural access was by the iliofemoral route in 97% of cases. Local anesthesia was used in 42% of cases and conscious sedation in 35%. Fourteen percent of patients underwent preimplantation valvuloplasty; 21% postimplantation valvuloplasty. The procedural success rate was 98.5%.
The primary safety endpoint was 30-day cardiovascular mortality. The 2.0% rate was far lower than the prespecified cutoff which defined a positive outcome as less than a 10% rate in this high-surgical-risk population.
Other key 30-day outcomes:
• All-cause mortality occurred in 2.5% of patients.
• The 30-day stroke rate was 3%, with no disabling strokes.
• Major vascular complications occurred in 6.5% of the VIVA patients.
• Major bleeding occurred in 7%, minor bleeding in 7.9%. There were no cases of life-threatening bleeding.
• The incidence of Stage I acute kidney injury was rare, at 0.5%.
• Seven percent of patients received a permanent pacemaker.
• Eighty-seven percent of patients had no postprocedure paravalvular regurgitation (PVR), 11.4 had mild PVR, and 1.5% had moderate PVR.
• NYHA classification improved from baseline to 30 days in 81% of patients. At 30 days, 93% of participants were NYHA class I or II.
Turning to echocardiographic findings, the mean gradient improved from a mean baseline of 31.8 mm Hg to 12.6 mm Hg, while the effective orifice area rose from 1.0 to 1.5 cm2. The magnitude of both improvements was greater for patients with stenosis as their mode of valve failure.
“With the Evolut R, we aim for higher implantations – not more than about 4 mm below the ring – because going deeper could bring about higher gradients and functional deterioration later on,” the cardiologist explained.
The 1-year primary efficacy endpoint – lack of significant aortic stenosis as defined by a mean gradient less than 40 mm Hg – will be reported soon. The VIVA study is sponsored by Medtronic. Dr. Kornowski reported serving as a consultant to the company.
PARIS – Transcatheter aortic valve replacement using the self-expanding Evolut R device in high-surgical-risk patients with failing surgical aortic bioprostheses showed promising 30-day safety and effectiveness results in the ongoing VIVA study, Ran Kornowski, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“We had a lot of patients with small, failing valves in this study. Despite this, our valve gradients postprocedure were very, very low. This means to me that this platform is very well suited to deal with valve-in-valve procedures in general and with small bioprosthetic valves in particular,” observed Dr. Kornowski, chairman of the department of cardiology at Rabin Medical Center in Petah Tikva, Israel, and president of the Israel Heart Society.
The participants’ last surgical aortic valve replacement had been a mean of 9.3 years earlier. Seventy-one percent of subjects were New York Heart Association class III or IV. The mode of bioprosthetic failure was stenosis in 56% of cases, regurgitation in 23%, and both in the remainder. Ninety-three percent of their failing biosprothetic valves were stented devices. Forty-one percent of the devices were up to 21 mm and another 33% were more than 21 but less than 25 mm.
TAVR procedural access was by the iliofemoral route in 97% of cases. Local anesthesia was used in 42% of cases and conscious sedation in 35%. Fourteen percent of patients underwent preimplantation valvuloplasty; 21% postimplantation valvuloplasty. The procedural success rate was 98.5%.
The primary safety endpoint was 30-day cardiovascular mortality. The 2.0% rate was far lower than the prespecified cutoff which defined a positive outcome as less than a 10% rate in this high-surgical-risk population.
Other key 30-day outcomes:
• All-cause mortality occurred in 2.5% of patients.
• The 30-day stroke rate was 3%, with no disabling strokes.
• Major vascular complications occurred in 6.5% of the VIVA patients.
• Major bleeding occurred in 7%, minor bleeding in 7.9%. There were no cases of life-threatening bleeding.
• The incidence of Stage I acute kidney injury was rare, at 0.5%.
• Seven percent of patients received a permanent pacemaker.
• Eighty-seven percent of patients had no postprocedure paravalvular regurgitation (PVR), 11.4 had mild PVR, and 1.5% had moderate PVR.
• NYHA classification improved from baseline to 30 days in 81% of patients. At 30 days, 93% of participants were NYHA class I or II.
Turning to echocardiographic findings, the mean gradient improved from a mean baseline of 31.8 mm Hg to 12.6 mm Hg, while the effective orifice area rose from 1.0 to 1.5 cm2. The magnitude of both improvements was greater for patients with stenosis as their mode of valve failure.
“With the Evolut R, we aim for higher implantations – not more than about 4 mm below the ring – because going deeper could bring about higher gradients and functional deterioration later on,” the cardiologist explained.
The 1-year primary efficacy endpoint – lack of significant aortic stenosis as defined by a mean gradient less than 40 mm Hg – will be reported soon. The VIVA study is sponsored by Medtronic. Dr. Kornowski reported serving as a consultant to the company.
AT EuroPCR
Key clinical point:
Major finding: The 30-day cardiovascular mortality rate after transcatheter aortic valve replacement via a valve-in-valve procedure in patients with a failing surgically implanted bioprosthesis was 2%, compared with a projected rate of at least 10% with redo surgery.
Data source: VIVA, a prospective observational registry of 202 high-surgical-risk patients at 23 centers in four countries who underwent valve-in-valve transcatheter aortic valve replacement because of a failing surgically implanted aortic bioprosthesis.
Disclosures: VIVA is sponsored by Medtronic. The presenter reported serving as a consultant to the company.
Now boarding: How we can skip coach and bump our patients up to first class
Cruising above the earth at 37,000 feet on the way back from vacation, my mind starts wandering. The impending reality of returning to work is setting in, and I can’t help but reflect on how the experience of a weary traveler trying to get home is like that of a weary patient trying to navigate modern health care. As it turns out, there are more than a few similarities, and that is not necessarily a good thing.
The modern airline industry is often cited by experts as a model for safety, efficiency, and innovation, though just a few decades ago this wasn’t the case. Several factors (for example, catastrophic crashes; the events of September 11th, 2001; the economic downturn) forced airlines to make radical improvements in how they operated – many of which I am quite thankful for as I gaze down upon America’s heartland from my window seat. Still, there are many who would say that in spite of (and sometimes because of) these improvements, air travel is the worst it’s ever been; airport lines are longer than ever, costs have steadily increased, and customer service has become little more than a quaint idea from a bygone era.
Most people deride the frustrations of air travel yet accept them as normal. The same expectations have unfortunately been set in health care. Patients wait, though waiting only contributes to anxiety and leads them to question the quality of their care. They also expect their journey to have many layover stops, though these involve even more waiting and often unnecessary redundancy. We need to streamline the care delivery process, and this is where technology can help.
First of all, we need to address the waiting. In health care, we tend to call this “access,” an ever-present problem for patients and providers. Thankfully, some recent innovations have helped significantly. The first of these innovations is online scheduling, which allows patients to find openings and schedule visits without the need to pick up the phone. Much like the ability to book a dinner reservation online, this is becoming an expectation for health care consumers. Participating practices and health systems can also use it as a marketing advantage; it is a fantastic way to recruit new patients as they search for a new provider online (that is, seeing that a physician has immediate openings may make the decision easier).
There are several companies providing third-party online scheduling services, and many of these can interface directly with electronic health records. EHR vendors themselves also provide this functionality to existing patients through an online web portal or mobile app. Either way, if you haven’t considered it yet, you should. It’s a great way to fill last-minute schedule openings and increase your patient base, all while improving access and patient satisfaction.
Another way to improve access is through telemedicine. We’ve written about this in prior columns, but it has certainly become more prevalent and available since then. Now more insurers are reimbursing for telemedicine services, and consumers are starting to embrace it as well. Consider some advantages: it’s more convenient for patients and often less expensive for those without insurance – cash prices tend to be in the $50-$75 range. It can also be more convenient for providers, as the typical telemedicine visit lasts only about 10 minutes and can be easily fit in last-minute. Better still, telemedicine can be a way for providers to now be paid for services they might have previously provided for free by telephone. It is critical to choose patients and conditions appropriate for these “virtual visits.” Medication checks, lab follow-ups, or rash evaluations are just a few examples, but with a little bit of thought it is easy to find dozens of other opportunities to use telemedicine to improve access.
In addition to access, we need to look for ways to improve efficiency and decrease redundancy when sending patients for testing and consultations. Recently, I had the experience of visiting a specialist for a minor medical issue. In spite of the fact that the specialist was a member of the same health system as my PCP, I still spent the first 15 minutes of my visit filling out paperwork that requested information easily available from my health record. There must be a better way.
Patients are beginning to question why, in the world of ubiquitous social media and connectivity, our computerized medical records can’t communicate. This is especially true when they are seeing physicians who are part of the same health system (as in my case). Thankfully, vendors have gotten the message and have begun allowing providers to collaborate, not only with physicians using the same software, but also with those using other EHRs through Health Information Exchanges (HIEs). Unfortunately, this alone won’t be enough. We must continue to promote the notion of patient-owned medical records, as that will be the only way to ensure true patient-centered care. In a future column, we’ll explore this concept in greater detail, but for now we’ll confirm our belief that universal interoperability is reasonable and possible.
As we are getting ready to land, I reflect on the wonderful vacation I just had and the tasks ahead at home, most of which I enjoy. Patients aren’t always as lucky; they are accessing medical care because they have to, not because they want to. Their “destination” is all too often an unfortunate diagnosis, unexpected surgical procedure, or lifetime of chronic discomfort. It is therefore incumbent on us, their care providers, to use the tools at our disposal to offer them the most efficient, most comfortable, and most connected journey possible.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington Jefferson Health.
Cruising above the earth at 37,000 feet on the way back from vacation, my mind starts wandering. The impending reality of returning to work is setting in, and I can’t help but reflect on how the experience of a weary traveler trying to get home is like that of a weary patient trying to navigate modern health care. As it turns out, there are more than a few similarities, and that is not necessarily a good thing.
The modern airline industry is often cited by experts as a model for safety, efficiency, and innovation, though just a few decades ago this wasn’t the case. Several factors (for example, catastrophic crashes; the events of September 11th, 2001; the economic downturn) forced airlines to make radical improvements in how they operated – many of which I am quite thankful for as I gaze down upon America’s heartland from my window seat. Still, there are many who would say that in spite of (and sometimes because of) these improvements, air travel is the worst it’s ever been; airport lines are longer than ever, costs have steadily increased, and customer service has become little more than a quaint idea from a bygone era.
Most people deride the frustrations of air travel yet accept them as normal. The same expectations have unfortunately been set in health care. Patients wait, though waiting only contributes to anxiety and leads them to question the quality of their care. They also expect their journey to have many layover stops, though these involve even more waiting and often unnecessary redundancy. We need to streamline the care delivery process, and this is where technology can help.
First of all, we need to address the waiting. In health care, we tend to call this “access,” an ever-present problem for patients and providers. Thankfully, some recent innovations have helped significantly. The first of these innovations is online scheduling, which allows patients to find openings and schedule visits without the need to pick up the phone. Much like the ability to book a dinner reservation online, this is becoming an expectation for health care consumers. Participating practices and health systems can also use it as a marketing advantage; it is a fantastic way to recruit new patients as they search for a new provider online (that is, seeing that a physician has immediate openings may make the decision easier).
There are several companies providing third-party online scheduling services, and many of these can interface directly with electronic health records. EHR vendors themselves also provide this functionality to existing patients through an online web portal or mobile app. Either way, if you haven’t considered it yet, you should. It’s a great way to fill last-minute schedule openings and increase your patient base, all while improving access and patient satisfaction.
Another way to improve access is through telemedicine. We’ve written about this in prior columns, but it has certainly become more prevalent and available since then. Now more insurers are reimbursing for telemedicine services, and consumers are starting to embrace it as well. Consider some advantages: it’s more convenient for patients and often less expensive for those without insurance – cash prices tend to be in the $50-$75 range. It can also be more convenient for providers, as the typical telemedicine visit lasts only about 10 minutes and can be easily fit in last-minute. Better still, telemedicine can be a way for providers to now be paid for services they might have previously provided for free by telephone. It is critical to choose patients and conditions appropriate for these “virtual visits.” Medication checks, lab follow-ups, or rash evaluations are just a few examples, but with a little bit of thought it is easy to find dozens of other opportunities to use telemedicine to improve access.
In addition to access, we need to look for ways to improve efficiency and decrease redundancy when sending patients for testing and consultations. Recently, I had the experience of visiting a specialist for a minor medical issue. In spite of the fact that the specialist was a member of the same health system as my PCP, I still spent the first 15 minutes of my visit filling out paperwork that requested information easily available from my health record. There must be a better way.
Patients are beginning to question why, in the world of ubiquitous social media and connectivity, our computerized medical records can’t communicate. This is especially true when they are seeing physicians who are part of the same health system (as in my case). Thankfully, vendors have gotten the message and have begun allowing providers to collaborate, not only with physicians using the same software, but also with those using other EHRs through Health Information Exchanges (HIEs). Unfortunately, this alone won’t be enough. We must continue to promote the notion of patient-owned medical records, as that will be the only way to ensure true patient-centered care. In a future column, we’ll explore this concept in greater detail, but for now we’ll confirm our belief that universal interoperability is reasonable and possible.
As we are getting ready to land, I reflect on the wonderful vacation I just had and the tasks ahead at home, most of which I enjoy. Patients aren’t always as lucky; they are accessing medical care because they have to, not because they want to. Their “destination” is all too often an unfortunate diagnosis, unexpected surgical procedure, or lifetime of chronic discomfort. It is therefore incumbent on us, their care providers, to use the tools at our disposal to offer them the most efficient, most comfortable, and most connected journey possible.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington Jefferson Health.
Cruising above the earth at 37,000 feet on the way back from vacation, my mind starts wandering. The impending reality of returning to work is setting in, and I can’t help but reflect on how the experience of a weary traveler trying to get home is like that of a weary patient trying to navigate modern health care. As it turns out, there are more than a few similarities, and that is not necessarily a good thing.
The modern airline industry is often cited by experts as a model for safety, efficiency, and innovation, though just a few decades ago this wasn’t the case. Several factors (for example, catastrophic crashes; the events of September 11th, 2001; the economic downturn) forced airlines to make radical improvements in how they operated – many of which I am quite thankful for as I gaze down upon America’s heartland from my window seat. Still, there are many who would say that in spite of (and sometimes because of) these improvements, air travel is the worst it’s ever been; airport lines are longer than ever, costs have steadily increased, and customer service has become little more than a quaint idea from a bygone era.
Most people deride the frustrations of air travel yet accept them as normal. The same expectations have unfortunately been set in health care. Patients wait, though waiting only contributes to anxiety and leads them to question the quality of their care. They also expect their journey to have many layover stops, though these involve even more waiting and often unnecessary redundancy. We need to streamline the care delivery process, and this is where technology can help.
First of all, we need to address the waiting. In health care, we tend to call this “access,” an ever-present problem for patients and providers. Thankfully, some recent innovations have helped significantly. The first of these innovations is online scheduling, which allows patients to find openings and schedule visits without the need to pick up the phone. Much like the ability to book a dinner reservation online, this is becoming an expectation for health care consumers. Participating practices and health systems can also use it as a marketing advantage; it is a fantastic way to recruit new patients as they search for a new provider online (that is, seeing that a physician has immediate openings may make the decision easier).
There are several companies providing third-party online scheduling services, and many of these can interface directly with electronic health records. EHR vendors themselves also provide this functionality to existing patients through an online web portal or mobile app. Either way, if you haven’t considered it yet, you should. It’s a great way to fill last-minute schedule openings and increase your patient base, all while improving access and patient satisfaction.
Another way to improve access is through telemedicine. We’ve written about this in prior columns, but it has certainly become more prevalent and available since then. Now more insurers are reimbursing for telemedicine services, and consumers are starting to embrace it as well. Consider some advantages: it’s more convenient for patients and often less expensive for those without insurance – cash prices tend to be in the $50-$75 range. It can also be more convenient for providers, as the typical telemedicine visit lasts only about 10 minutes and can be easily fit in last-minute. Better still, telemedicine can be a way for providers to now be paid for services they might have previously provided for free by telephone. It is critical to choose patients and conditions appropriate for these “virtual visits.” Medication checks, lab follow-ups, or rash evaluations are just a few examples, but with a little bit of thought it is easy to find dozens of other opportunities to use telemedicine to improve access.
In addition to access, we need to look for ways to improve efficiency and decrease redundancy when sending patients for testing and consultations. Recently, I had the experience of visiting a specialist for a minor medical issue. In spite of the fact that the specialist was a member of the same health system as my PCP, I still spent the first 15 minutes of my visit filling out paperwork that requested information easily available from my health record. There must be a better way.
Patients are beginning to question why, in the world of ubiquitous social media and connectivity, our computerized medical records can’t communicate. This is especially true when they are seeing physicians who are part of the same health system (as in my case). Thankfully, vendors have gotten the message and have begun allowing providers to collaborate, not only with physicians using the same software, but also with those using other EHRs through Health Information Exchanges (HIEs). Unfortunately, this alone won’t be enough. We must continue to promote the notion of patient-owned medical records, as that will be the only way to ensure true patient-centered care. In a future column, we’ll explore this concept in greater detail, but for now we’ll confirm our belief that universal interoperability is reasonable and possible.
As we are getting ready to land, I reflect on the wonderful vacation I just had and the tasks ahead at home, most of which I enjoy. Patients aren’t always as lucky; they are accessing medical care because they have to, not because they want to. Their “destination” is all too often an unfortunate diagnosis, unexpected surgical procedure, or lifetime of chronic discomfort. It is therefore incumbent on us, their care providers, to use the tools at our disposal to offer them the most efficient, most comfortable, and most connected journey possible.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington Jefferson Health.
Solitary Nodule With White Hairs
The Diagnosis: Trichofolliculoma
Microscopic examination revealed a dilated cystic follicle that communicated with the skin surface (Figure). The follicle was lined with squamous epithelium and surrounded by numerous secondary follicles, many of which contained a hair shaft. A diagnosis of trichofolliculoma was made.
Clinically, the differential diagnosis of a flesh-colored papule on the scalp with prominent follicle includes dilated pore of Winer, epidermoid cyst, pilar sheath acanthoma, and trichoepithelioma.1,2 Multiple hair shafts present in a single follicle may be seen in pili multigemini, tufted folliculitis, trichostasis spinulosa, and trichofolliculoma. On histopathologic examination, a dilated central follicle surrounded with smaller secondary follicles was identified, consistent with trichofolliculoma.
Trichofolliculoma is a rare follicular hamartoma typically occurring on the face, scalp, or trunk as a solitary papule or nodule due to the proliferation of abnormal hair follicle stem cells.3,4 It may present as a flesh-colored nodule with a central pore that may drain sebum or contain white vellus hairs. Trichofolliculoma is considered a benign entity, despite one case report of malignant transformation.5 Biopsy is diagnostic and no further treatment is needed. Recurrence rarely occurs at the primary site after surgical excision, which may be performed for cosmetic purposes or to alleviate functional impairment.
- Ghosh SK, Bandyopadhyay D, Barma KD. Perifollicular nodule on the face of a young man. Indian J Dermatol Venereol Leprol. 2011;77:531-533.
- Gokalp H, Gurer MA, Alan S. Trichofolliculoma: a rare variant of hair follicle hamartoma. Dermatol Online J. 2013;19:19264.
- Choi CM, Lew BL, Sim WY. Multiple trichofolliculomas on unusual sites: a case report and review of the literature. Int J Dermatol. 2013;52:87-89.
- Misago N, Kimura T, Toda S, et al. A revaluation of trichofolliculoma: the histopathological and immunohistochemical features. Am J Dermatopathol. 2010;32:35-43.
- Stem JB, Stout DA. Trichofolliculoma showing perineural invasion. trichofolliculocarcinoma? Arch Dermatol. 1979;115:1003-1004.
The Diagnosis: Trichofolliculoma
Microscopic examination revealed a dilated cystic follicle that communicated with the skin surface (Figure). The follicle was lined with squamous epithelium and surrounded by numerous secondary follicles, many of which contained a hair shaft. A diagnosis of trichofolliculoma was made.

Clinically, the differential diagnosis of a flesh-colored papule on the scalp with prominent follicle includes dilated pore of Winer, epidermoid cyst, pilar sheath acanthoma, and trichoepithelioma.1,2 Multiple hair shafts present in a single follicle may be seen in pili multigemini, tufted folliculitis, trichostasis spinulosa, and trichofolliculoma. On histopathologic examination, a dilated central follicle surrounded with smaller secondary follicles was identified, consistent with trichofolliculoma.
Trichofolliculoma is a rare follicular hamartoma typically occurring on the face, scalp, or trunk as a solitary papule or nodule due to the proliferation of abnormal hair follicle stem cells.3,4 It may present as a flesh-colored nodule with a central pore that may drain sebum or contain white vellus hairs. Trichofolliculoma is considered a benign entity, despite one case report of malignant transformation.5 Biopsy is diagnostic and no further treatment is needed. Recurrence rarely occurs at the primary site after surgical excision, which may be performed for cosmetic purposes or to alleviate functional impairment.
The Diagnosis: Trichofolliculoma
Microscopic examination revealed a dilated cystic follicle that communicated with the skin surface (Figure). The follicle was lined with squamous epithelium and surrounded by numerous secondary follicles, many of which contained a hair shaft. A diagnosis of trichofolliculoma was made.

Clinically, the differential diagnosis of a flesh-colored papule on the scalp with prominent follicle includes dilated pore of Winer, epidermoid cyst, pilar sheath acanthoma, and trichoepithelioma.1,2 Multiple hair shafts present in a single follicle may be seen in pili multigemini, tufted folliculitis, trichostasis spinulosa, and trichofolliculoma. On histopathologic examination, a dilated central follicle surrounded with smaller secondary follicles was identified, consistent with trichofolliculoma.
Trichofolliculoma is a rare follicular hamartoma typically occurring on the face, scalp, or trunk as a solitary papule or nodule due to the proliferation of abnormal hair follicle stem cells.3,4 It may present as a flesh-colored nodule with a central pore that may drain sebum or contain white vellus hairs. Trichofolliculoma is considered a benign entity, despite one case report of malignant transformation.5 Biopsy is diagnostic and no further treatment is needed. Recurrence rarely occurs at the primary site after surgical excision, which may be performed for cosmetic purposes or to alleviate functional impairment.
- Ghosh SK, Bandyopadhyay D, Barma KD. Perifollicular nodule on the face of a young man. Indian J Dermatol Venereol Leprol. 2011;77:531-533.
- Gokalp H, Gurer MA, Alan S. Trichofolliculoma: a rare variant of hair follicle hamartoma. Dermatol Online J. 2013;19:19264.
- Choi CM, Lew BL, Sim WY. Multiple trichofolliculomas on unusual sites: a case report and review of the literature. Int J Dermatol. 2013;52:87-89.
- Misago N, Kimura T, Toda S, et al. A revaluation of trichofolliculoma: the histopathological and immunohistochemical features. Am J Dermatopathol. 2010;32:35-43.
- Stem JB, Stout DA. Trichofolliculoma showing perineural invasion. trichofolliculocarcinoma? Arch Dermatol. 1979;115:1003-1004.
- Ghosh SK, Bandyopadhyay D, Barma KD. Perifollicular nodule on the face of a young man. Indian J Dermatol Venereol Leprol. 2011;77:531-533.
- Gokalp H, Gurer MA, Alan S. Trichofolliculoma: a rare variant of hair follicle hamartoma. Dermatol Online J. 2013;19:19264.
- Choi CM, Lew BL, Sim WY. Multiple trichofolliculomas on unusual sites: a case report and review of the literature. Int J Dermatol. 2013;52:87-89.
- Misago N, Kimura T, Toda S, et al. A revaluation of trichofolliculoma: the histopathological and immunohistochemical features. Am J Dermatopathol. 2010;32:35-43.
- Stem JB, Stout DA. Trichofolliculoma showing perineural invasion. trichofolliculocarcinoma? Arch Dermatol. 1979;115:1003-1004.
A 72-year-old man presented with a new asymptomatic 0.7-cm flesh-colored papule with a central tuft of white hairs on the posterior scalp. The remainder of the physical examination was unremarkable. Biopsy for histopathologic examination was performed to confirm diagnosis.
Campylobacteriosis incidence rises in U.S. from 2004 to 2012
Incidence of campylobacteriosis increased significantly in the United States from 2004 to 2012, according to Aimee Geissler, PhD, and her associates.
A total of 303,520 cases of campylobacteriosis were reported during the study period, with the average incidence rate growing from 10.5 cases per 100,000 persons during 2004-2006 to 12.7 cases per 100,000 persons during 2010-2012, an increase of 21%. The median number of Camplyobacter outbreaks doubled from 28 during 2004-2006 to 56 during 2010-2012; in total, 347 outbreaks were reported. Campylobacteriosis is the nation’s most common bacterial diarrheal illness.
The study findings “underscore the importance of standardizing national surveillance for campylobacteriosis, which is important in understanding the burden of infection, better describing geographic variations and differences among species, elucidating risk factors, and targeting prevention and control measures,” the investigators concluded.
Find the full study in Clinical Infectious Diseases (2017 Jul 20. doi: 10.1093/cid/cix624).
Incidence of campylobacteriosis increased significantly in the United States from 2004 to 2012, according to Aimee Geissler, PhD, and her associates.
A total of 303,520 cases of campylobacteriosis were reported during the study period, with the average incidence rate growing from 10.5 cases per 100,000 persons during 2004-2006 to 12.7 cases per 100,000 persons during 2010-2012, an increase of 21%. The median number of Camplyobacter outbreaks doubled from 28 during 2004-2006 to 56 during 2010-2012; in total, 347 outbreaks were reported. Campylobacteriosis is the nation’s most common bacterial diarrheal illness.
The study findings “underscore the importance of standardizing national surveillance for campylobacteriosis, which is important in understanding the burden of infection, better describing geographic variations and differences among species, elucidating risk factors, and targeting prevention and control measures,” the investigators concluded.
Find the full study in Clinical Infectious Diseases (2017 Jul 20. doi: 10.1093/cid/cix624).
Incidence of campylobacteriosis increased significantly in the United States from 2004 to 2012, according to Aimee Geissler, PhD, and her associates.
A total of 303,520 cases of campylobacteriosis were reported during the study period, with the average incidence rate growing from 10.5 cases per 100,000 persons during 2004-2006 to 12.7 cases per 100,000 persons during 2010-2012, an increase of 21%. The median number of Camplyobacter outbreaks doubled from 28 during 2004-2006 to 56 during 2010-2012; in total, 347 outbreaks were reported. Campylobacteriosis is the nation’s most common bacterial diarrheal illness.
The study findings “underscore the importance of standardizing national surveillance for campylobacteriosis, which is important in understanding the burden of infection, better describing geographic variations and differences among species, elucidating risk factors, and targeting prevention and control measures,” the investigators concluded.
Find the full study in Clinical Infectious Diseases (2017 Jul 20. doi: 10.1093/cid/cix624).
FROM CLINICAL INFECTIOUS DISEASES
Crisis in psychiatry: Top 5 problems, many solutions
Lack of access to psychiatric services has been a challenge for decades, resulting in significant delays to treatment with associated consequences in reduced quality of care, low patient satisfaction, poor patient outcomes, and higher costs.
The problem is exacerbated by a growing shortage of psychiatrists, an increased demand for psychiatric services, and inadequate payment rates. The result is a crisis that is resonating throughout the U.S. health care system.
As many know, a few months ago, the Medical Director Institute of the National Council for Behavioral Health, in partnership with the American Psychiatric Association, convened an expert panel to develop a report responding to this evolving quandary. The findings of our 60-page report, “The Psychiatric Shortage: Causes and Solutions,” suggest that psychiatry is uniquely positioned to address the issues that face our specialty.
The institute identified five areas of critical concern: workforce development, improved efficiency of service delivery, reducing burdensome regulations and confidentiality restrictions, broader implementation of innovative models, and adoption of novel reimbursement methods.
1. Workforce development
Psychiatrists come out of residency training without the skills they need to practice in today’s rapidly evolving health care environment. We need better preparation in measurement-based care, telepsychiatry, collaborative care, and other methods of efficient team collaboration with primary care.
Funding for graduate medical education and training programs must be expanded with an infusion of new funding – not only for psychiatrists – but also for psychiatric nurse practitioners and psychiatric physician assistants.
2. Improved efficiency of service delivery
Providers of psychiatric services in outpatient psychiatric programs face a cramped daily routine with increasingly briefer appointments scheduled back-to-back that limit in-depth clinical assessment, collaboration with other members of the treatment team, and consultation with primary care providers outside of the program. Such a schedule leads to lower-quality care.
Psychiatrists must have the same level of nurse and paraprofessional assistance and support provided to other medical specialties. In addition, regulations that prevent the broader use of telepsychiatry must be revised. All behavioral health providers should implement open access scheduling, a proven modality for reducing missed appointments.
3. Reducing burdensome regulations and confidentiality restrictions
Excessive documentation requirements, especially necessarily detailed, lengthy assessments and treatment must be revised and 42 CFR Part 2 must be made consistent with HIPAA requirements.
4. Broader implementation of innovative models
The shortage of psychiatrists will only worsen with the integration of primary care and behavioral health, and the shift to Accountable Care Organizations as part of health care reform (which, as of this writing, faces much uncertainty). Thanks to more efficient screening for mental health and substance use disorders now occurring in primary care, there will be growing demand for access to psychiatric services.
The collaborative care model for providing psychiatric services should be implemented throughout primary care. Behavioral health organizations must develop their own version of collaborative care that targets the limited psychiatric resource where it is most needed by using measurement-based care and collaborating more effectively with other team members.
5. Adoption of novel reimbursement methods
Inappropriately low rates limit access to care. Today, 40% of psychiatrists choose cash-only private practices, the second-highest among medical specialties after dermatologists, and 75% of provider organizations employing psychiatrists report that they lose money on their psychiatric services. At the same time, the shrinking number of inpatient psychiatric services has become a significant obstacle to improved access. Beds have been eliminated because of lower rates of reimbursement, compared with other medical-surgical procedures and difficulty in recruiting psychiatrists to staff inpatient units.
Psychiatric service rates must be reset to be consistent with the actual cost of providing care. Prospective payment models like Certified Community Behavioral Health Clinics should be expanded, and bundled payments for services like collaborative care and complex care should be covered by payers.
The Medical Director Institute recommends these solutions so that access to psychiatric services does not remain a barrier to the overall success of health care reform and service delivery improving the health of Americans. Multiple stakeholders – federal and state governments, payers, providers, provider trade associations, and advocates – must take action within their spheres of influence in the design, funding, regulation, and delivery of behavioral health care to improve access to psychiatric services. Such broad collaboration is imperative for our patients to get the care they need.
Dr. Parks is the medical director for the National Council for Behavioral Health. He practices psychiatry on an outpatient basis at Family Health Center, a federally funded community health center established to expand services to uninsured and underinsured patients in central Missouri. He also holds the position of Distinguished Research Professor of Science at the University of Missouri–St. Louis and is a clinical assistant professor of psychiatry at the University of Missouri–Columbia.
Lack of access to psychiatric services has been a challenge for decades, resulting in significant delays to treatment with associated consequences in reduced quality of care, low patient satisfaction, poor patient outcomes, and higher costs.
The problem is exacerbated by a growing shortage of psychiatrists, an increased demand for psychiatric services, and inadequate payment rates. The result is a crisis that is resonating throughout the U.S. health care system.
As many know, a few months ago, the Medical Director Institute of the National Council for Behavioral Health, in partnership with the American Psychiatric Association, convened an expert panel to develop a report responding to this evolving quandary. The findings of our 60-page report, “The Psychiatric Shortage: Causes and Solutions,” suggest that psychiatry is uniquely positioned to address the issues that face our specialty.
The institute identified five areas of critical concern: workforce development, improved efficiency of service delivery, reducing burdensome regulations and confidentiality restrictions, broader implementation of innovative models, and adoption of novel reimbursement methods.
1. Workforce development
Psychiatrists come out of residency training without the skills they need to practice in today’s rapidly evolving health care environment. We need better preparation in measurement-based care, telepsychiatry, collaborative care, and other methods of efficient team collaboration with primary care.
Funding for graduate medical education and training programs must be expanded with an infusion of new funding – not only for psychiatrists – but also for psychiatric nurse practitioners and psychiatric physician assistants.
2. Improved efficiency of service delivery
Providers of psychiatric services in outpatient psychiatric programs face a cramped daily routine with increasingly briefer appointments scheduled back-to-back that limit in-depth clinical assessment, collaboration with other members of the treatment team, and consultation with primary care providers outside of the program. Such a schedule leads to lower-quality care.
Psychiatrists must have the same level of nurse and paraprofessional assistance and support provided to other medical specialties. In addition, regulations that prevent the broader use of telepsychiatry must be revised. All behavioral health providers should implement open access scheduling, a proven modality for reducing missed appointments.
3. Reducing burdensome regulations and confidentiality restrictions
Excessive documentation requirements, especially necessarily detailed, lengthy assessments and treatment must be revised and 42 CFR Part 2 must be made consistent with HIPAA requirements.
4. Broader implementation of innovative models
The shortage of psychiatrists will only worsen with the integration of primary care and behavioral health, and the shift to Accountable Care Organizations as part of health care reform (which, as of this writing, faces much uncertainty). Thanks to more efficient screening for mental health and substance use disorders now occurring in primary care, there will be growing demand for access to psychiatric services.
The collaborative care model for providing psychiatric services should be implemented throughout primary care. Behavioral health organizations must develop their own version of collaborative care that targets the limited psychiatric resource where it is most needed by using measurement-based care and collaborating more effectively with other team members.
5. Adoption of novel reimbursement methods
Inappropriately low rates limit access to care. Today, 40% of psychiatrists choose cash-only private practices, the second-highest among medical specialties after dermatologists, and 75% of provider organizations employing psychiatrists report that they lose money on their psychiatric services. At the same time, the shrinking number of inpatient psychiatric services has become a significant obstacle to improved access. Beds have been eliminated because of lower rates of reimbursement, compared with other medical-surgical procedures and difficulty in recruiting psychiatrists to staff inpatient units.
Psychiatric service rates must be reset to be consistent with the actual cost of providing care. Prospective payment models like Certified Community Behavioral Health Clinics should be expanded, and bundled payments for services like collaborative care and complex care should be covered by payers.
The Medical Director Institute recommends these solutions so that access to psychiatric services does not remain a barrier to the overall success of health care reform and service delivery improving the health of Americans. Multiple stakeholders – federal and state governments, payers, providers, provider trade associations, and advocates – must take action within their spheres of influence in the design, funding, regulation, and delivery of behavioral health care to improve access to psychiatric services. Such broad collaboration is imperative for our patients to get the care they need.
Dr. Parks is the medical director for the National Council for Behavioral Health. He practices psychiatry on an outpatient basis at Family Health Center, a federally funded community health center established to expand services to uninsured and underinsured patients in central Missouri. He also holds the position of Distinguished Research Professor of Science at the University of Missouri–St. Louis and is a clinical assistant professor of psychiatry at the University of Missouri–Columbia.
Lack of access to psychiatric services has been a challenge for decades, resulting in significant delays to treatment with associated consequences in reduced quality of care, low patient satisfaction, poor patient outcomes, and higher costs.
The problem is exacerbated by a growing shortage of psychiatrists, an increased demand for psychiatric services, and inadequate payment rates. The result is a crisis that is resonating throughout the U.S. health care system.
As many know, a few months ago, the Medical Director Institute of the National Council for Behavioral Health, in partnership with the American Psychiatric Association, convened an expert panel to develop a report responding to this evolving quandary. The findings of our 60-page report, “The Psychiatric Shortage: Causes and Solutions,” suggest that psychiatry is uniquely positioned to address the issues that face our specialty.
The institute identified five areas of critical concern: workforce development, improved efficiency of service delivery, reducing burdensome regulations and confidentiality restrictions, broader implementation of innovative models, and adoption of novel reimbursement methods.
1. Workforce development
Psychiatrists come out of residency training without the skills they need to practice in today’s rapidly evolving health care environment. We need better preparation in measurement-based care, telepsychiatry, collaborative care, and other methods of efficient team collaboration with primary care.
Funding for graduate medical education and training programs must be expanded with an infusion of new funding – not only for psychiatrists – but also for psychiatric nurse practitioners and psychiatric physician assistants.
2. Improved efficiency of service delivery
Providers of psychiatric services in outpatient psychiatric programs face a cramped daily routine with increasingly briefer appointments scheduled back-to-back that limit in-depth clinical assessment, collaboration with other members of the treatment team, and consultation with primary care providers outside of the program. Such a schedule leads to lower-quality care.
Psychiatrists must have the same level of nurse and paraprofessional assistance and support provided to other medical specialties. In addition, regulations that prevent the broader use of telepsychiatry must be revised. All behavioral health providers should implement open access scheduling, a proven modality for reducing missed appointments.
3. Reducing burdensome regulations and confidentiality restrictions
Excessive documentation requirements, especially necessarily detailed, lengthy assessments and treatment must be revised and 42 CFR Part 2 must be made consistent with HIPAA requirements.
4. Broader implementation of innovative models
The shortage of psychiatrists will only worsen with the integration of primary care and behavioral health, and the shift to Accountable Care Organizations as part of health care reform (which, as of this writing, faces much uncertainty). Thanks to more efficient screening for mental health and substance use disorders now occurring in primary care, there will be growing demand for access to psychiatric services.
The collaborative care model for providing psychiatric services should be implemented throughout primary care. Behavioral health organizations must develop their own version of collaborative care that targets the limited psychiatric resource where it is most needed by using measurement-based care and collaborating more effectively with other team members.
5. Adoption of novel reimbursement methods
Inappropriately low rates limit access to care. Today, 40% of psychiatrists choose cash-only private practices, the second-highest among medical specialties after dermatologists, and 75% of provider organizations employing psychiatrists report that they lose money on their psychiatric services. At the same time, the shrinking number of inpatient psychiatric services has become a significant obstacle to improved access. Beds have been eliminated because of lower rates of reimbursement, compared with other medical-surgical procedures and difficulty in recruiting psychiatrists to staff inpatient units.
Psychiatric service rates must be reset to be consistent with the actual cost of providing care. Prospective payment models like Certified Community Behavioral Health Clinics should be expanded, and bundled payments for services like collaborative care and complex care should be covered by payers.
The Medical Director Institute recommends these solutions so that access to psychiatric services does not remain a barrier to the overall success of health care reform and service delivery improving the health of Americans. Multiple stakeholders – federal and state governments, payers, providers, provider trade associations, and advocates – must take action within their spheres of influence in the design, funding, regulation, and delivery of behavioral health care to improve access to psychiatric services. Such broad collaboration is imperative for our patients to get the care they need.
Dr. Parks is the medical director for the National Council for Behavioral Health. He practices psychiatry on an outpatient basis at Family Health Center, a federally funded community health center established to expand services to uninsured and underinsured patients in central Missouri. He also holds the position of Distinguished Research Professor of Science at the University of Missouri–St. Louis and is a clinical assistant professor of psychiatry at the University of Missouri–Columbia.