User login
Study aims to validate AAD criteria for diagnosing AD and create usable form
CHICAGO – A streamlined set of diagnostic criteria from the American Academy of Dermatology’s most recent consensus criteria for diagnosing atopic dermatitis (AD) produced a specificity of more than 95%, and was also highly sensitive, a prospective analysis found.
“Atopic dermatitis typically presents in childhood and is associated with a worsened quality of life, with severe itch and lack of sleep, and substantial health care costs due to therapeutic management and increased hospitalizations,” study author Jeremy Udkoff said at the World Congress of Pediatric Dermatology. “We also know that in order to treat the disease and to learn more about it, we have to have a good tool for diagnosing it. When it comes to clinical studies and research, we require a systematic and refined set of criteria.”
The next set of commonly used criteria to appear were created by the U.K. working party, for which researchers used logistic regression to systematically create a minimum set of effective criteria for AD (Br J Dermatol. 1994;131[3]:383-96). For these guidelines, meeting a diagnosis of AD requires an itchy skin condition, followed by three or more of the following: a history of flexural involvement; a personal history of asthma or hay fever; a history of general dry skin in the last year; visible flexural eczema, and onset under the age of 2 years. “A subsequent validation trial found [the U.K. working party criteria] to have a low sensitivity, which as you can imagine, could be a very large problem,” he said (Arch Dermatol. 1999;135[5]:514-6).
In 2001, the AAD consensus conference created revised hierarchical criteria known as the AAD consensus criteria (J Am Acad Dermatol. 2003;49[6]:1088-95). “These were initially created for more of a gestalt-type picture of AD in the clinic, but because it flows so well, it’s currently being used in about one-third of clinical trials,” Mr. Udkoff said. “However, [the AAD criteria] have not been validated, so we didn’t know its sensitivity or specificity. In addition, we didn’t have a ‘checkbox’ form that tells us how many of each of the criteria are required to make the diagnosis. We didn’t know how many ‘essential,’ ‘important,’ or ‘associated’ features we need to make this diagnosis.”
For the current study, he and his associates set out to determine how many “essential,” “important,” and “associated” criteria are necessary to make the AAD consensus criteria work. They also set out to create a usable checkbox form, validate the criteria, and compare it to the Hanifin-Rajka (HR) and U.K. criteria. To accomplish this, they created a questionnaire comprised of HR, U.K., and AAD criteria, examined the criteria on 60 subjects with and without AD, and compared the diagnostic features of each of those criteria against a gold standard dermatology diagnosis from one of seven pediatric dermatologists. Next, they ranked all 56 possible AAD criterion combinations based on their overall sensitivity and specificity, and chose the most predictive combination. “Once we had the optimal set of criteria, we validated it on a new cohort to determine its sensitivity and specificity, and compared it with the classic HR and U.K. criteria,” Mr. Udkoff explained.
Overall, the researchers evaluated findings from 100 subjects: 58 with AD, and 42 controls. Those with AD were about 3 years younger, compared with controls (a mean age of 5 years vs. about 8 years, respectively). About 40% of patients were Hispanic and about 30% were white. Mr. Udkoff and his associates confirmed the hierarchical structure of the AAD criteria and found that individual “essential” AAD criteria of pruritus, typical AD pattern, and chronic/relapsing course each had a sensitivity that exceeded 96%. This was followed by the “important” criteria of early age of onset, atopy, and xerosis, which had a sensitivity that ranged between 88% and 95%, while the associated criteria had a sensitivity that ranged between 50% and 85%.
Next, the researchers systematically tested all combinations of the AAD criteria and found that three “essential” AAD criteria, two or more of the “important” criteria, and one or more of the “associated” criteria were optimal in diagnosing AD. Mr. Udkoff noted that the findings can be translated into a simple “3-2-1 rule” that “is both practical and pragmatic,” he said. Using this rule, sensitivity was 91.4% and specificity was 95.2%.
Currently, the researchers are working to validate this criteria in different subgroups of patients. To date, they have found that children younger than 1.5 years get one bonus “essential” criteria for being an infant, so for that population a 2-2-1 rule would apply.
Mr. Udkoff reported that the research was supported by a training grant from the National Institutes of Health. He reported having no financial disclosures.
CHICAGO – A streamlined set of diagnostic criteria from the American Academy of Dermatology’s most recent consensus criteria for diagnosing atopic dermatitis (AD) produced a specificity of more than 95%, and was also highly sensitive, a prospective analysis found.
“Atopic dermatitis typically presents in childhood and is associated with a worsened quality of life, with severe itch and lack of sleep, and substantial health care costs due to therapeutic management and increased hospitalizations,” study author Jeremy Udkoff said at the World Congress of Pediatric Dermatology. “We also know that in order to treat the disease and to learn more about it, we have to have a good tool for diagnosing it. When it comes to clinical studies and research, we require a systematic and refined set of criteria.”
The next set of commonly used criteria to appear were created by the U.K. working party, for which researchers used logistic regression to systematically create a minimum set of effective criteria for AD (Br J Dermatol. 1994;131[3]:383-96). For these guidelines, meeting a diagnosis of AD requires an itchy skin condition, followed by three or more of the following: a history of flexural involvement; a personal history of asthma or hay fever; a history of general dry skin in the last year; visible flexural eczema, and onset under the age of 2 years. “A subsequent validation trial found [the U.K. working party criteria] to have a low sensitivity, which as you can imagine, could be a very large problem,” he said (Arch Dermatol. 1999;135[5]:514-6).
In 2001, the AAD consensus conference created revised hierarchical criteria known as the AAD consensus criteria (J Am Acad Dermatol. 2003;49[6]:1088-95). “These were initially created for more of a gestalt-type picture of AD in the clinic, but because it flows so well, it’s currently being used in about one-third of clinical trials,” Mr. Udkoff said. “However, [the AAD criteria] have not been validated, so we didn’t know its sensitivity or specificity. In addition, we didn’t have a ‘checkbox’ form that tells us how many of each of the criteria are required to make the diagnosis. We didn’t know how many ‘essential,’ ‘important,’ or ‘associated’ features we need to make this diagnosis.”
For the current study, he and his associates set out to determine how many “essential,” “important,” and “associated” criteria are necessary to make the AAD consensus criteria work. They also set out to create a usable checkbox form, validate the criteria, and compare it to the Hanifin-Rajka (HR) and U.K. criteria. To accomplish this, they created a questionnaire comprised of HR, U.K., and AAD criteria, examined the criteria on 60 subjects with and without AD, and compared the diagnostic features of each of those criteria against a gold standard dermatology diagnosis from one of seven pediatric dermatologists. Next, they ranked all 56 possible AAD criterion combinations based on their overall sensitivity and specificity, and chose the most predictive combination. “Once we had the optimal set of criteria, we validated it on a new cohort to determine its sensitivity and specificity, and compared it with the classic HR and U.K. criteria,” Mr. Udkoff explained.
Overall, the researchers evaluated findings from 100 subjects: 58 with AD, and 42 controls. Those with AD were about 3 years younger, compared with controls (a mean age of 5 years vs. about 8 years, respectively). About 40% of patients were Hispanic and about 30% were white. Mr. Udkoff and his associates confirmed the hierarchical structure of the AAD criteria and found that individual “essential” AAD criteria of pruritus, typical AD pattern, and chronic/relapsing course each had a sensitivity that exceeded 96%. This was followed by the “important” criteria of early age of onset, atopy, and xerosis, which had a sensitivity that ranged between 88% and 95%, while the associated criteria had a sensitivity that ranged between 50% and 85%.
Next, the researchers systematically tested all combinations of the AAD criteria and found that three “essential” AAD criteria, two or more of the “important” criteria, and one or more of the “associated” criteria were optimal in diagnosing AD. Mr. Udkoff noted that the findings can be translated into a simple “3-2-1 rule” that “is both practical and pragmatic,” he said. Using this rule, sensitivity was 91.4% and specificity was 95.2%.
Currently, the researchers are working to validate this criteria in different subgroups of patients. To date, they have found that children younger than 1.5 years get one bonus “essential” criteria for being an infant, so for that population a 2-2-1 rule would apply.
Mr. Udkoff reported that the research was supported by a training grant from the National Institutes of Health. He reported having no financial disclosures.
CHICAGO – A streamlined set of diagnostic criteria from the American Academy of Dermatology’s most recent consensus criteria for diagnosing atopic dermatitis (AD) produced a specificity of more than 95%, and was also highly sensitive, a prospective analysis found.
“Atopic dermatitis typically presents in childhood and is associated with a worsened quality of life, with severe itch and lack of sleep, and substantial health care costs due to therapeutic management and increased hospitalizations,” study author Jeremy Udkoff said at the World Congress of Pediatric Dermatology. “We also know that in order to treat the disease and to learn more about it, we have to have a good tool for diagnosing it. When it comes to clinical studies and research, we require a systematic and refined set of criteria.”
The next set of commonly used criteria to appear were created by the U.K. working party, for which researchers used logistic regression to systematically create a minimum set of effective criteria for AD (Br J Dermatol. 1994;131[3]:383-96). For these guidelines, meeting a diagnosis of AD requires an itchy skin condition, followed by three or more of the following: a history of flexural involvement; a personal history of asthma or hay fever; a history of general dry skin in the last year; visible flexural eczema, and onset under the age of 2 years. “A subsequent validation trial found [the U.K. working party criteria] to have a low sensitivity, which as you can imagine, could be a very large problem,” he said (Arch Dermatol. 1999;135[5]:514-6).
In 2001, the AAD consensus conference created revised hierarchical criteria known as the AAD consensus criteria (J Am Acad Dermatol. 2003;49[6]:1088-95). “These were initially created for more of a gestalt-type picture of AD in the clinic, but because it flows so well, it’s currently being used in about one-third of clinical trials,” Mr. Udkoff said. “However, [the AAD criteria] have not been validated, so we didn’t know its sensitivity or specificity. In addition, we didn’t have a ‘checkbox’ form that tells us how many of each of the criteria are required to make the diagnosis. We didn’t know how many ‘essential,’ ‘important,’ or ‘associated’ features we need to make this diagnosis.”
For the current study, he and his associates set out to determine how many “essential,” “important,” and “associated” criteria are necessary to make the AAD consensus criteria work. They also set out to create a usable checkbox form, validate the criteria, and compare it to the Hanifin-Rajka (HR) and U.K. criteria. To accomplish this, they created a questionnaire comprised of HR, U.K., and AAD criteria, examined the criteria on 60 subjects with and without AD, and compared the diagnostic features of each of those criteria against a gold standard dermatology diagnosis from one of seven pediatric dermatologists. Next, they ranked all 56 possible AAD criterion combinations based on their overall sensitivity and specificity, and chose the most predictive combination. “Once we had the optimal set of criteria, we validated it on a new cohort to determine its sensitivity and specificity, and compared it with the classic HR and U.K. criteria,” Mr. Udkoff explained.
Overall, the researchers evaluated findings from 100 subjects: 58 with AD, and 42 controls. Those with AD were about 3 years younger, compared with controls (a mean age of 5 years vs. about 8 years, respectively). About 40% of patients were Hispanic and about 30% were white. Mr. Udkoff and his associates confirmed the hierarchical structure of the AAD criteria and found that individual “essential” AAD criteria of pruritus, typical AD pattern, and chronic/relapsing course each had a sensitivity that exceeded 96%. This was followed by the “important” criteria of early age of onset, atopy, and xerosis, which had a sensitivity that ranged between 88% and 95%, while the associated criteria had a sensitivity that ranged between 50% and 85%.
Next, the researchers systematically tested all combinations of the AAD criteria and found that three “essential” AAD criteria, two or more of the “important” criteria, and one or more of the “associated” criteria were optimal in diagnosing AD. Mr. Udkoff noted that the findings can be translated into a simple “3-2-1 rule” that “is both practical and pragmatic,” he said. Using this rule, sensitivity was 91.4% and specificity was 95.2%.
Currently, the researchers are working to validate this criteria in different subgroups of patients. To date, they have found that children younger than 1.5 years get one bonus “essential” criteria for being an infant, so for that population a 2-2-1 rule would apply.
Mr. Udkoff reported that the research was supported by a training grant from the National Institutes of Health. He reported having no financial disclosures.
AT WCPD 2017
Key clinical point:
Major finding: The “important” AD criteria of early age of onset, atopy, and xerosis had a sensitivity that ranged between 88% and 95%.
Data source: An analysis of optimal AAD criteria for AD that included 58 patients with AD and 42 controls.
Disclosures: Mr. Udkoff reported that the research was supported by a training grant from the National Institutes of Health. He reported having no financial disclosures.
ATO enables anthracycline reduction in pediatric APL
Consolidation therapy that includes arsenic trioxide (ATO) can decrease anthracycline dosing by about 40% in children and young adults with acute promyelocytic leukemia (APL), according to new research.
And it can accomplish this without compromising survival in standard-risk patients.
Outcomes for high-risk patients compared favorably to other pediatric APL trials, the research indicated.
Investigators compared ATO consolidation in the AAML0631 trial to the historic control trial AIDA0493 and reported the results in the Journal of Clinical Oncology.
The AAML0631 phase 3 trial, conducted by the Children’s Oncology Group, compared newly diagnosed pediatric APL patients receiving ATO consolidation to the benchmark of event-free survival (EFS) in standard-risk (SR) patients established by the AIDA0493 trial.
AIDA0493 enrolled patients between January 1993 and June 2000. The protocol involved treatment with all-trans retinoic acid (ATRA), anthracyclines, and high-dose cytarabine. The trial resulted in overall survival (OS) of approximately 90%.
AAML0631
AAML063 investigators defined SR as a white blood cell count (WBC) at presentation less than 10,000 cells/μL. They defined high risk (HR) as a WBC count of 10,000 cells/μL or more.
AAML0631 patients had to be at least 2 years old and younger than 22, and their de novo APL had to be confirmed by PML-RARα polymerase chain reaction.
The patients could have had no prior leukemia treatment, except for steroids, hydroxyurea, or leukapheresis.
AAML0631 did not exclude patients based on organ function or performance status. AIDA0493, however, excluded patients with performance status of 4 or liver function tests greater than 3 times the upper limit of normal.
Patients were excluded from AAML0631 if they had preexisting prolonged QT syndrome because of the risk of QT interval prolongation with ATO.
AAML0631 treatment protocol
All patients received ATRA during induction, each consolidation course, and maintenance.
Induction therapy consisted of ATRA and idarubicin.
All patients received 2 cycles of ATO during the first consolidation. SR patients received an additional 2 consolidation courses, and HR patients received 3 consolidation courses that included high-dose cytarabine and anthracycline.
Maintenance therapy consisted of ATRA, oral methotrexate, and 6-mercaptopurine for 2 years.
Patients also received prophylactic treatment with intrathecal cytarabine.
Patient demographics
Investigators enrolled 108 patients between March 2009 and November 2012, of which 101 (66 SR and 35 HR) were evaluable.
Patients were a median age of 15.04 years (range, 2.01 – 21.34), 56% were female, 80% were white, 10% black, 2% Native American, 3% Asian, and 5% unknown.
Three quarters of the patients had an ECOG score of 0 or 1, median WBC counts of 3.8 x 1000 cells/uL (range, 0.4 – 173.8), and median platelet counts of 21.5 x 1000/uL (range, 3 – 198).
Almost two-thirds of patients (63%) had the classic translocation (15;17), and 37% had an additional 1 or more cytogenetic abnormalities.
The SR patients in AAML0631 had similar characteristics to the patients in AIDA0493 except for the distribution of performance status scores and differences in racial/ethnic diversity.
Efficacy
After a median follow-up of 3.73 years (range, 0.003 – 5.97), the 3-year overall survival (OS) was 94% ± 5% and the 3-year EFS was 91% ± 6%.
For SR patients, the OS was 98% ± 3% and the EFS 95% ± 5%.
For HR patients, the OS was 86% ± 12% and the EFS was 83% ± 13%.
SR patients had a 2-year EFS of 97%. This compared with 91% for patients in the AIDA0493 trial, which means that therapy with ATO was not inferior to therapy in the historic comparator trial (P=0.93).
And these results were achieved with a cumulative anthracycline dosing of idarubicin at 51 mg/m2 (SR) and 61 mg/m2 (HR) and mitoxantrone at 20 mg/m2.
This compared with the AIDA0493 cumulative anthracycline dosing of 80 mg/m2 of idarubicin and 50 mg/m2 of mitoxantrone.
The cumulative daunorubicin equivalent in the AAML0631 trial was 335 mg/m2 (SR) and 385 mg/m2 (HR) compared with 600 mg/m2 in the AIDA 0493 trial.
Toxicity
The percentage of patients with adverse events varied according to treatment cycle and was highest during induction and high-dose cytarabine-containing courses.
The most common adverse events were fever/neutropenia and infection.
Differentiation syndrome occurred in 20% of patients during induction, 31% in HR patients and 13% in SR patients. ATRA was held for 15 of these patients during induction. It was subsequently re-started at a lower dose and increased to the full dose.
QTc interval prolongation of grade 1 or 2 occurred in 16% (n=15) and 12% (n=11) during the ATO cycles.
One patient experienced grade 3 QTc interval prolongation during ATO consolidation. There were no grade 4 or 5 events for this toxicity.
One event of grade 1 ventricular arrhythmia and 1 event of grade 1 left ventricular systolic dysfunction occurred during ATO consolidation.
Two off-therapy cardiac events have been reported: a grade 1 QTc interval prolongation and a grade 2 ventricular arrhythmia.
No cardiac deaths have occurred, and liver toxicity was minimal during ATO cycles.
The investigators believe the favorable results of this study provide a new benchmark for outcomes in pediatric APL.
The Children’s Oncology Group is currently accruing pediatric APL patients to further investigate similar treatment approaches. ![]()
Consolidation therapy that includes arsenic trioxide (ATO) can decrease anthracycline dosing by about 40% in children and young adults with acute promyelocytic leukemia (APL), according to new research.
And it can accomplish this without compromising survival in standard-risk patients.
Outcomes for high-risk patients compared favorably to other pediatric APL trials, the research indicated.
Investigators compared ATO consolidation in the AAML0631 trial to the historic control trial AIDA0493 and reported the results in the Journal of Clinical Oncology.
The AAML0631 phase 3 trial, conducted by the Children’s Oncology Group, compared newly diagnosed pediatric APL patients receiving ATO consolidation to the benchmark of event-free survival (EFS) in standard-risk (SR) patients established by the AIDA0493 trial.
AIDA0493 enrolled patients between January 1993 and June 2000. The protocol involved treatment with all-trans retinoic acid (ATRA), anthracyclines, and high-dose cytarabine. The trial resulted in overall survival (OS) of approximately 90%.
AAML0631
AAML063 investigators defined SR as a white blood cell count (WBC) at presentation less than 10,000 cells/μL. They defined high risk (HR) as a WBC count of 10,000 cells/μL or more.
AAML0631 patients had to be at least 2 years old and younger than 22, and their de novo APL had to be confirmed by PML-RARα polymerase chain reaction.
The patients could have had no prior leukemia treatment, except for steroids, hydroxyurea, or leukapheresis.
AAML0631 did not exclude patients based on organ function or performance status. AIDA0493, however, excluded patients with performance status of 4 or liver function tests greater than 3 times the upper limit of normal.
Patients were excluded from AAML0631 if they had preexisting prolonged QT syndrome because of the risk of QT interval prolongation with ATO.
AAML0631 treatment protocol
All patients received ATRA during induction, each consolidation course, and maintenance.
Induction therapy consisted of ATRA and idarubicin.
All patients received 2 cycles of ATO during the first consolidation. SR patients received an additional 2 consolidation courses, and HR patients received 3 consolidation courses that included high-dose cytarabine and anthracycline.
Maintenance therapy consisted of ATRA, oral methotrexate, and 6-mercaptopurine for 2 years.
Patients also received prophylactic treatment with intrathecal cytarabine.
Patient demographics
Investigators enrolled 108 patients between March 2009 and November 2012, of which 101 (66 SR and 35 HR) were evaluable.
Patients were a median age of 15.04 years (range, 2.01 – 21.34), 56% were female, 80% were white, 10% black, 2% Native American, 3% Asian, and 5% unknown.
Three quarters of the patients had an ECOG score of 0 or 1, median WBC counts of 3.8 x 1000 cells/uL (range, 0.4 – 173.8), and median platelet counts of 21.5 x 1000/uL (range, 3 – 198).
Almost two-thirds of patients (63%) had the classic translocation (15;17), and 37% had an additional 1 or more cytogenetic abnormalities.
The SR patients in AAML0631 had similar characteristics to the patients in AIDA0493 except for the distribution of performance status scores and differences in racial/ethnic diversity.
Efficacy
After a median follow-up of 3.73 years (range, 0.003 – 5.97), the 3-year overall survival (OS) was 94% ± 5% and the 3-year EFS was 91% ± 6%.
For SR patients, the OS was 98% ± 3% and the EFS 95% ± 5%.
For HR patients, the OS was 86% ± 12% and the EFS was 83% ± 13%.
SR patients had a 2-year EFS of 97%. This compared with 91% for patients in the AIDA0493 trial, which means that therapy with ATO was not inferior to therapy in the historic comparator trial (P=0.93).
And these results were achieved with a cumulative anthracycline dosing of idarubicin at 51 mg/m2 (SR) and 61 mg/m2 (HR) and mitoxantrone at 20 mg/m2.
This compared with the AIDA0493 cumulative anthracycline dosing of 80 mg/m2 of idarubicin and 50 mg/m2 of mitoxantrone.
The cumulative daunorubicin equivalent in the AAML0631 trial was 335 mg/m2 (SR) and 385 mg/m2 (HR) compared with 600 mg/m2 in the AIDA 0493 trial.
Toxicity
The percentage of patients with adverse events varied according to treatment cycle and was highest during induction and high-dose cytarabine-containing courses.
The most common adverse events were fever/neutropenia and infection.
Differentiation syndrome occurred in 20% of patients during induction, 31% in HR patients and 13% in SR patients. ATRA was held for 15 of these patients during induction. It was subsequently re-started at a lower dose and increased to the full dose.
QTc interval prolongation of grade 1 or 2 occurred in 16% (n=15) and 12% (n=11) during the ATO cycles.
One patient experienced grade 3 QTc interval prolongation during ATO consolidation. There were no grade 4 or 5 events for this toxicity.
One event of grade 1 ventricular arrhythmia and 1 event of grade 1 left ventricular systolic dysfunction occurred during ATO consolidation.
Two off-therapy cardiac events have been reported: a grade 1 QTc interval prolongation and a grade 2 ventricular arrhythmia.
No cardiac deaths have occurred, and liver toxicity was minimal during ATO cycles.
The investigators believe the favorable results of this study provide a new benchmark for outcomes in pediatric APL.
The Children’s Oncology Group is currently accruing pediatric APL patients to further investigate similar treatment approaches. ![]()
Consolidation therapy that includes arsenic trioxide (ATO) can decrease anthracycline dosing by about 40% in children and young adults with acute promyelocytic leukemia (APL), according to new research.
And it can accomplish this without compromising survival in standard-risk patients.
Outcomes for high-risk patients compared favorably to other pediatric APL trials, the research indicated.
Investigators compared ATO consolidation in the AAML0631 trial to the historic control trial AIDA0493 and reported the results in the Journal of Clinical Oncology.
The AAML0631 phase 3 trial, conducted by the Children’s Oncology Group, compared newly diagnosed pediatric APL patients receiving ATO consolidation to the benchmark of event-free survival (EFS) in standard-risk (SR) patients established by the AIDA0493 trial.
AIDA0493 enrolled patients between January 1993 and June 2000. The protocol involved treatment with all-trans retinoic acid (ATRA), anthracyclines, and high-dose cytarabine. The trial resulted in overall survival (OS) of approximately 90%.
AAML0631
AAML063 investigators defined SR as a white blood cell count (WBC) at presentation less than 10,000 cells/μL. They defined high risk (HR) as a WBC count of 10,000 cells/μL or more.
AAML0631 patients had to be at least 2 years old and younger than 22, and their de novo APL had to be confirmed by PML-RARα polymerase chain reaction.
The patients could have had no prior leukemia treatment, except for steroids, hydroxyurea, or leukapheresis.
AAML0631 did not exclude patients based on organ function or performance status. AIDA0493, however, excluded patients with performance status of 4 or liver function tests greater than 3 times the upper limit of normal.
Patients were excluded from AAML0631 if they had preexisting prolonged QT syndrome because of the risk of QT interval prolongation with ATO.
AAML0631 treatment protocol
All patients received ATRA during induction, each consolidation course, and maintenance.
Induction therapy consisted of ATRA and idarubicin.
All patients received 2 cycles of ATO during the first consolidation. SR patients received an additional 2 consolidation courses, and HR patients received 3 consolidation courses that included high-dose cytarabine and anthracycline.
Maintenance therapy consisted of ATRA, oral methotrexate, and 6-mercaptopurine for 2 years.
Patients also received prophylactic treatment with intrathecal cytarabine.
Patient demographics
Investigators enrolled 108 patients between March 2009 and November 2012, of which 101 (66 SR and 35 HR) were evaluable.
Patients were a median age of 15.04 years (range, 2.01 – 21.34), 56% were female, 80% were white, 10% black, 2% Native American, 3% Asian, and 5% unknown.
Three quarters of the patients had an ECOG score of 0 or 1, median WBC counts of 3.8 x 1000 cells/uL (range, 0.4 – 173.8), and median platelet counts of 21.5 x 1000/uL (range, 3 – 198).
Almost two-thirds of patients (63%) had the classic translocation (15;17), and 37% had an additional 1 or more cytogenetic abnormalities.
The SR patients in AAML0631 had similar characteristics to the patients in AIDA0493 except for the distribution of performance status scores and differences in racial/ethnic diversity.
Efficacy
After a median follow-up of 3.73 years (range, 0.003 – 5.97), the 3-year overall survival (OS) was 94% ± 5% and the 3-year EFS was 91% ± 6%.
For SR patients, the OS was 98% ± 3% and the EFS 95% ± 5%.
For HR patients, the OS was 86% ± 12% and the EFS was 83% ± 13%.
SR patients had a 2-year EFS of 97%. This compared with 91% for patients in the AIDA0493 trial, which means that therapy with ATO was not inferior to therapy in the historic comparator trial (P=0.93).
And these results were achieved with a cumulative anthracycline dosing of idarubicin at 51 mg/m2 (SR) and 61 mg/m2 (HR) and mitoxantrone at 20 mg/m2.
This compared with the AIDA0493 cumulative anthracycline dosing of 80 mg/m2 of idarubicin and 50 mg/m2 of mitoxantrone.
The cumulative daunorubicin equivalent in the AAML0631 trial was 335 mg/m2 (SR) and 385 mg/m2 (HR) compared with 600 mg/m2 in the AIDA 0493 trial.
Toxicity
The percentage of patients with adverse events varied according to treatment cycle and was highest during induction and high-dose cytarabine-containing courses.
The most common adverse events were fever/neutropenia and infection.
Differentiation syndrome occurred in 20% of patients during induction, 31% in HR patients and 13% in SR patients. ATRA was held for 15 of these patients during induction. It was subsequently re-started at a lower dose and increased to the full dose.
QTc interval prolongation of grade 1 or 2 occurred in 16% (n=15) and 12% (n=11) during the ATO cycles.
One patient experienced grade 3 QTc interval prolongation during ATO consolidation. There were no grade 4 or 5 events for this toxicity.
One event of grade 1 ventricular arrhythmia and 1 event of grade 1 left ventricular systolic dysfunction occurred during ATO consolidation.
Two off-therapy cardiac events have been reported: a grade 1 QTc interval prolongation and a grade 2 ventricular arrhythmia.
No cardiac deaths have occurred, and liver toxicity was minimal during ATO cycles.
The investigators believe the favorable results of this study provide a new benchmark for outcomes in pediatric APL.
The Children’s Oncology Group is currently accruing pediatric APL patients to further investigate similar treatment approaches. ![]()
Incontinentia Pigmenti: Do You Know the Signs?
IN THIS ARTICLE
- Presenting stages
- Diagnostic criteria
- Management of IP
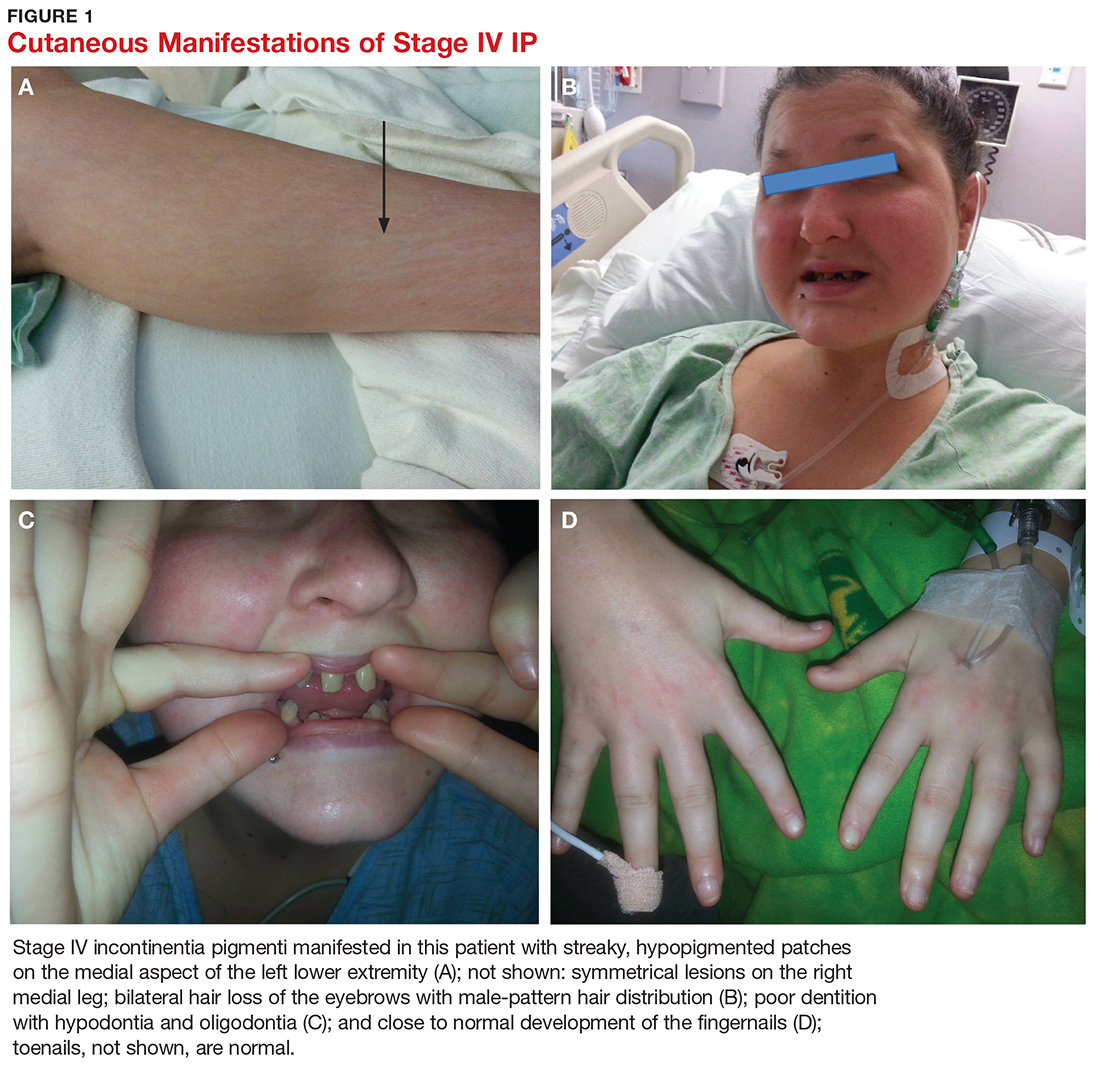
A 21-year-old woman with type 1 diabetes is admitted for recurrent diabetic ketoacidosis. Physical exam reveals hypopigmented, linear, streaky patches on the medial aspects of the bilateral lower legs (Figure 1A). The patient denies tenderness, pruritus, or paresthesia. There is obvious symmetrical hair loss on the lateral aspects of the eyebrows, as well as slightly wooly male-pattern hair distribution with patchy alopecia on the vertex of the head (Figure 1B). She has very poor dentition with hypodontia and malformed teeth (Figure 1C). Her fingernails and toenails appear normal, with no visible atrophy (Figure 1D). What explains her condition?
Incontinentia pigmenti (IP), also known as Bloch-Sulzberger syndrome, is a rare, X-linked dominant genodermatosis involving the cutaneous, ophthalmic, neurologic, and dental systems.1-3 It results from X-inactivation due to mutations in the NF-kappaB essential modulator (NEMO) gene with deletion of exons 4-10 in most cases. The NEMO gene encodes a regulatory component of the IkappaB kinase complex required to activate the NF-kappa B pathway, which is important for many immune, inflammatory, and apoptotic processes.4-6 This deletional mutation is typically lethal in normal 46,XY male karyotypes. Male fetuses with this mutation usually die in utero, making the reported cases predominantly female.4,7
The estimated incidence of IP is between 1/10,000 and 1/100,000.4 Due to the rarity of the condition, IP may be underrecognized and underdiagnosed.
CLINICAL PRESENTATION
Characteristic skin lesions of IP begin to develop at birth or in utero, in an evolving pattern that consists of four stages:
- The vesicular stage (stage I) is characterized by linear erythematous papules and blisters that manifest in newborns.
- The verrucous stage (stage II) begins as the blisters start to heal—usually after several weeks—and is distinguished by hyperkeratotic warty papules in linear or swirling distribution. This stage resolves on its own within months.
- The hyperpigmentation stage (stage III) is when swirling macules or patches develop. This hallmark stage of IP tends to remain static until adolescence.
- The hypopigmentation stage (stage IV) manifests with faded streaky patches, which may be subtly atrophic. This final stage usually develops in the second or third decade of life.2,3
All these cutaneous lesions follow Blaschko lines—invisible lines believed to result from embryonic cell migration that become visible with the manifestation of cutaneous or mucous lesions.6
Other associated cutaneous findings include patchy alopecia, nail dystrophy, and oral/dental anomalies such as hypodontia, oligodontia, and tooth deformities. In addition, ophthalmologic involvement can result in strabismus, cataracts, and retinal vascular changes that can lead to blindness. Central nervous system manifestations include seizures, cognitive impairment, and spastic paralysis.3
DIFFERENTIAL DIAGNOSIS
Because IP is uncommon, it may be easily overlooked or misdiagnosed as another, similar cutaneous manifestation. Cutaneous sarcoidosis, for example, is a skin lesion of noncaseating granuloma. It can present as patches, papules, ulcers, scars, ichthyosis, and alopecia. The development of cutaneous sarcoidosis can be idiopathic or iatrogenic, particularly in patients using anti-TNF therapy. The diagnosis is made clinically and can be confirmed pathologically.8
Stage I IP can also be confused with neonatal herpes simplex virus-1 (HSV-1) infection, given the similarities in vesicular morphology and linear distribution. The diagnosis of HSV-1 can be made based on history, physical exam, and pathology. Given the serious sequelae of neonatal HSV-1 infection, antiviral therapy should not be delayed until confirmation of the diagnosis in infants with vesicular eruptions.9
Erythema multiforme (EM) is another dermatologic condition frequently encountered in children and young adults. Its characteristic round target lesion usually has two rings surrounding the dusky-appearing central zone. Atypical lesions can be bullous or crusty, mimicking the appearance of stage I or II IP. EM is usually a self-limiting condition, but specific treatment may be required if the infectious agent is identified.10
Vitiligo, the development of white patches due to the loss of melanocytes, is another item in the differential. Although it most commonly involves the skin, the hair may also be affected. The diagnosis is made clinically and can be confirmed with skin biopsy if needed.11
DIAGNOSIS
Diagnostic criteria for IP have been proposed, with family history playing a role (see Table).2,3,12 Results of a case-study series indicate that 28% of patients with IP have a family history involving at least one first-degree female relative. IP was considered “sporadic” in 62% of cases studied.3
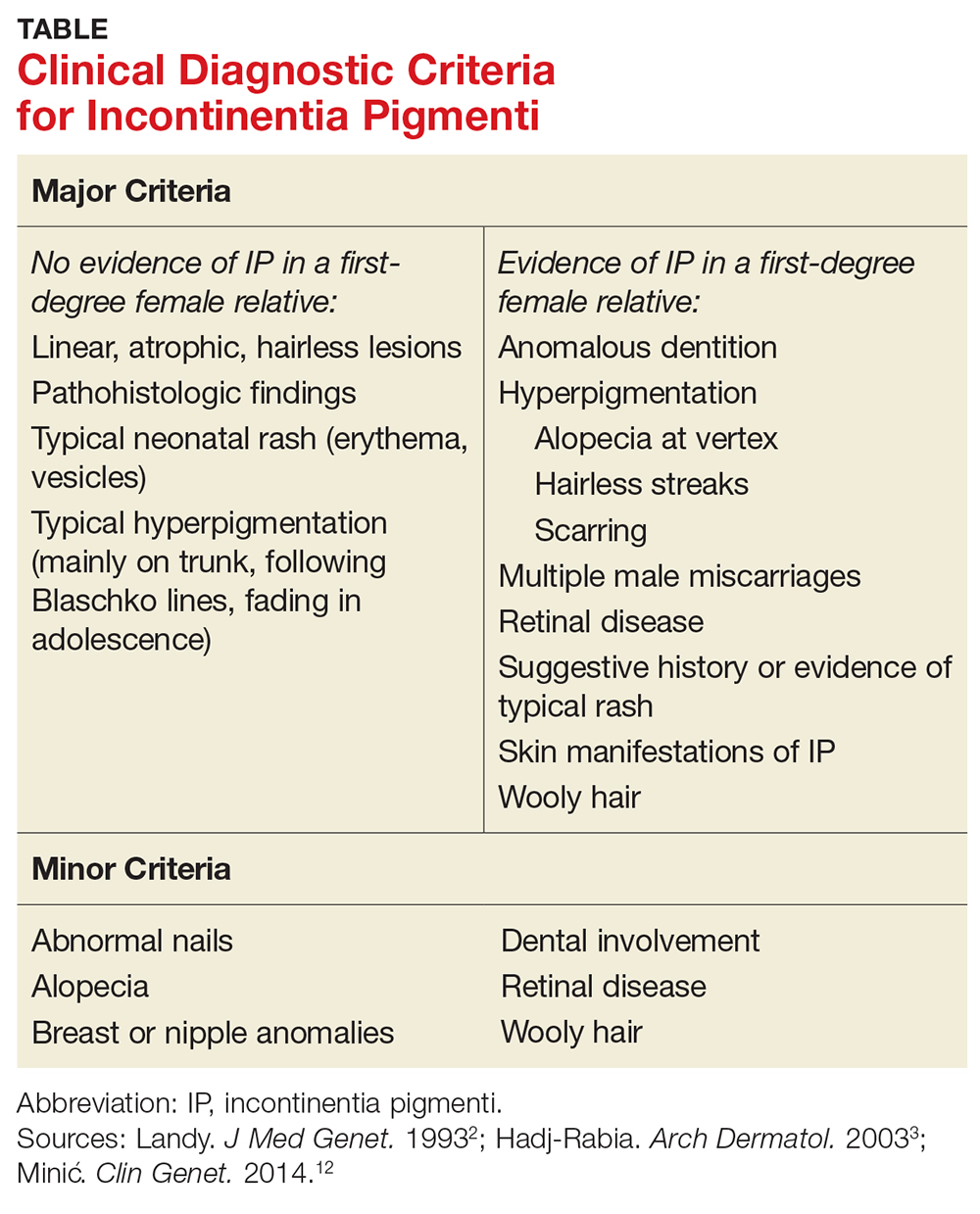
Without a family history of IP, at least one major criterion must be present to support the diagnosis. These include
- Neonatal rash (erythema, vesicles)
- Linear, atrophic, hairless lesions
- Hyperpigmentation (mainly on trunk, following Blaschko lines)
In a patient with a family history of IP, the presence of any major criterion strongly supports the diagnosis. These, as well as minor criteria, are outlined in the Table.2,3,12
In stages I and II of IP, pathologic features include spongiotic dermatitis with characteristic eosinophils and large dyskeratotic cells.3,13 In stage IV, skin biopsies may reveal slight atrophy and scattered apoptotic cells in the epidermis and epidermal hypopigmentation due to reduced melanocytes. The dermis typically appears thickened and is absent hair follicles and sweat glands.14 In a 2014 update, these pathologic features were proposed to be included in the major diagnostic criteria.12
TREATMENT/MANAGEMENT
Treatment of IP is centered on the involved organ systems. For cutaneous lesions, treatment is not usually necessary unless inflammation persists. In such cases, topical steroids or tacrolimus have been used with some success.15,16 In the vesicular stage, the patient should be monitored for bacterial infection, with appropriate prevention or treatment as necessary.
With other involved systems—such as dental, ophthalmologic, or neurologic (eg, seizures or other encephalopathy) anomalies—consultation and follow-up with the relevant specialist is warranted.
In this case, the patient denied family history of IP. She did have a history of infantile cataract and seizure. Her presenting signs were typical of stage IV IP: hypopigmented streaky patches on the skin of the lower legs, dental abnormalities, somewhat wooly hair, alopecia on the head, and loss of hair on the lateral aspects of the eyebrows. The uniqueness of this case is that the patient also had type 1 diabetes, a condition with a strong genetic predisposition. However, there is no evidence supporting an association between IP and either type of diabetes.
CONCLUSION
Although rare, when IP does occur, its manifestations are vast and severe enough to significantly reduce quality of life for patients; when it occurs in males, it is usually lethal. This genetic disorder can affect multiple body systems, making knowledge of its symptoms essential for proper diagnosis. Because its characteristic stages may be present at birth or in infancy, early identification and diagnosis of IP can help guide treatment intervention.
1. Roberts AP. Incontinentia pigmenti (Bloch-Sulzberger). Br Med. J. 1958;1(5079):1106-1107.
2. Landy SJ, Donnai D. Incontinentia pigmenti (Bloch-Sulzberger syndrome). J Med Genet. 1993;30(1):53-59.
3. Hadj-Rabia S, Froidevaux N, Bodak D, et al. Clinical study of 40 cases of incontinentia pigmenti. Arch Dermatol. 2003; 139(9):1163-1170.
4. Smahi A, Courtois G, Vabres P, et al. Genomic rearrangement in NEMO impairs NF-kappaB activation and is a cause of incontinentia pigmenti. Nature. 2000;405(6785):466-472.
5. Aradhya S, Courtois G, Rajkovic A, et al. Atypical forms of incontinentia pigmenti in male individuals result from mutations of a cytosine tract in exon 10 of NEMO (IKK-gamma). Am J Hum Genet. 2001;68(3):765-771.
6. Poziomczyk CS, Recuero JK, Bringhenti L, et al. Incontinentia pigmenti. An Bras Dermatol. 2014;89(1):26-36.
7. Kenwrick S, Woffendin H, Jakins T, et al. Survival of male patients with incontinentia pigmenti carrying a lethal mutation can be explained by somatic mosaicism or Klinefelter Syndrome. Am J Hum Genet. 2001;69(6):1210-1217.
8. Katta R. Cutaneous sarcoidosis: a dermatologic masquerader. Am Fam Physician. 2002;65(8):1581-1584.
9. Faloyin M, Levitt J, Bercowitz E, et al. All that is vesicular is not herpes: incontinentia pigmenti masquerading as herpes simplex virus in a newborn. Pediatrics. 2004;114(2):e270-272.
10. Siedner-Weintraub Y, Gross I, David A, et al. Paediatric erythema multiforme: epidemiological, clinical and laboratory characteristics. Acta Derm Venereol. 2016 Nov 10. doi: 10.2340/00015555-2569.
11. Gawkrodger DJ, Ormerod AD, Shaw L, et al. Guideline for the diagnosis and management of vitiligo. Br J Dermatol. 2008;159(5):1051-1076.
12. Minic´ S, Trpinac D, Obradovic´ M. Incontinentia pigmenti diagnostic criteria update. Clin Genet. 2014;85(6):536-542.
13. Jean-Baptiste S, O’Toole EA, Chen M, et al. Expression of eotaxin, an eosinophil-selective chemokine, parallels eosinophil accumulation in the vesiculobullous stage of incontinentia pigmenti. Clin Exp Immunol. 2002;127(3):470-478.
14. Hadj-Rabia S, Rimella A, Smahi A, et al. Clinical and histologic features of incontinentia pigmenti in adults with nuclear factor-κ B essential modulator gene mutations. J Am Acad Dermatol. 2011;64(3):508-515.
15. Kaya TI, Tursen U, Ikizoglu G. Therapeutic use of topical corticosteroids in the vesiculobullous lesions of incontinentia pigmenti. Clin Exp Dermatol. 2009;34(8):e611-613.
16. Jessup CJ, Morgan SC, Cohen LM, Viders DE. Incontinentia pigmenti: treatment of IP with topical tacrolimus. J Drugs Dermatol. 2009;8(10):944-946.
IN THIS ARTICLE
- Presenting stages
- Diagnostic criteria
- Management of IP
A 21-year-old woman with type 1 diabetes is admitted for recurrent diabetic ketoacidosis. Physical exam reveals hypopigmented, linear, streaky patches on the medial aspects of the bilateral lower legs (Figure 1A). The patient denies tenderness, pruritus, or paresthesia. There is obvious symmetrical hair loss on the lateral aspects of the eyebrows, as well as slightly wooly male-pattern hair distribution with patchy alopecia on the vertex of the head (Figure 1B). She has very poor dentition with hypodontia and malformed teeth (Figure 1C). Her fingernails and toenails appear normal, with no visible atrophy (Figure 1D). What explains her condition?

Incontinentia pigmenti (IP), also known as Bloch-Sulzberger syndrome, is a rare, X-linked dominant genodermatosis involving the cutaneous, ophthalmic, neurologic, and dental systems.1-3 It results from X-inactivation due to mutations in the NF-kappaB essential modulator (NEMO) gene with deletion of exons 4-10 in most cases. The NEMO gene encodes a regulatory component of the IkappaB kinase complex required to activate the NF-kappa B pathway, which is important for many immune, inflammatory, and apoptotic processes.4-6 This deletional mutation is typically lethal in normal 46,XY male karyotypes. Male fetuses with this mutation usually die in utero, making the reported cases predominantly female.4,7
The estimated incidence of IP is between 1/10,000 and 1/100,000.4 Due to the rarity of the condition, IP may be underrecognized and underdiagnosed.
CLINICAL PRESENTATION
Characteristic skin lesions of IP begin to develop at birth or in utero, in an evolving pattern that consists of four stages:
- The vesicular stage (stage I) is characterized by linear erythematous papules and blisters that manifest in newborns.
- The verrucous stage (stage II) begins as the blisters start to heal—usually after several weeks—and is distinguished by hyperkeratotic warty papules in linear or swirling distribution. This stage resolves on its own within months.
- The hyperpigmentation stage (stage III) is when swirling macules or patches develop. This hallmark stage of IP tends to remain static until adolescence.
- The hypopigmentation stage (stage IV) manifests with faded streaky patches, which may be subtly atrophic. This final stage usually develops in the second or third decade of life.2,3
All these cutaneous lesions follow Blaschko lines—invisible lines believed to result from embryonic cell migration that become visible with the manifestation of cutaneous or mucous lesions.6
Other associated cutaneous findings include patchy alopecia, nail dystrophy, and oral/dental anomalies such as hypodontia, oligodontia, and tooth deformities. In addition, ophthalmologic involvement can result in strabismus, cataracts, and retinal vascular changes that can lead to blindness. Central nervous system manifestations include seizures, cognitive impairment, and spastic paralysis.3
DIFFERENTIAL DIAGNOSIS
Because IP is uncommon, it may be easily overlooked or misdiagnosed as another, similar cutaneous manifestation. Cutaneous sarcoidosis, for example, is a skin lesion of noncaseating granuloma. It can present as patches, papules, ulcers, scars, ichthyosis, and alopecia. The development of cutaneous sarcoidosis can be idiopathic or iatrogenic, particularly in patients using anti-TNF therapy. The diagnosis is made clinically and can be confirmed pathologically.8
Stage I IP can also be confused with neonatal herpes simplex virus-1 (HSV-1) infection, given the similarities in vesicular morphology and linear distribution. The diagnosis of HSV-1 can be made based on history, physical exam, and pathology. Given the serious sequelae of neonatal HSV-1 infection, antiviral therapy should not be delayed until confirmation of the diagnosis in infants with vesicular eruptions.9
Erythema multiforme (EM) is another dermatologic condition frequently encountered in children and young adults. Its characteristic round target lesion usually has two rings surrounding the dusky-appearing central zone. Atypical lesions can be bullous or crusty, mimicking the appearance of stage I or II IP. EM is usually a self-limiting condition, but specific treatment may be required if the infectious agent is identified.10
Vitiligo, the development of white patches due to the loss of melanocytes, is another item in the differential. Although it most commonly involves the skin, the hair may also be affected. The diagnosis is made clinically and can be confirmed with skin biopsy if needed.11
DIAGNOSIS
Diagnostic criteria for IP have been proposed, with family history playing a role (see Table).2,3,12 Results of a case-study series indicate that 28% of patients with IP have a family history involving at least one first-degree female relative. IP was considered “sporadic” in 62% of cases studied.3

Without a family history of IP, at least one major criterion must be present to support the diagnosis. These include
- Neonatal rash (erythema, vesicles)
- Linear, atrophic, hairless lesions
- Hyperpigmentation (mainly on trunk, following Blaschko lines)
In a patient with a family history of IP, the presence of any major criterion strongly supports the diagnosis. These, as well as minor criteria, are outlined in the Table.2,3,12
In stages I and II of IP, pathologic features include spongiotic dermatitis with characteristic eosinophils and large dyskeratotic cells.3,13 In stage IV, skin biopsies may reveal slight atrophy and scattered apoptotic cells in the epidermis and epidermal hypopigmentation due to reduced melanocytes. The dermis typically appears thickened and is absent hair follicles and sweat glands.14 In a 2014 update, these pathologic features were proposed to be included in the major diagnostic criteria.12
TREATMENT/MANAGEMENT
Treatment of IP is centered on the involved organ systems. For cutaneous lesions, treatment is not usually necessary unless inflammation persists. In such cases, topical steroids or tacrolimus have been used with some success.15,16 In the vesicular stage, the patient should be monitored for bacterial infection, with appropriate prevention or treatment as necessary.
With other involved systems—such as dental, ophthalmologic, or neurologic (eg, seizures or other encephalopathy) anomalies—consultation and follow-up with the relevant specialist is warranted.
In this case, the patient denied family history of IP. She did have a history of infantile cataract and seizure. Her presenting signs were typical of stage IV IP: hypopigmented streaky patches on the skin of the lower legs, dental abnormalities, somewhat wooly hair, alopecia on the head, and loss of hair on the lateral aspects of the eyebrows. The uniqueness of this case is that the patient also had type 1 diabetes, a condition with a strong genetic predisposition. However, there is no evidence supporting an association between IP and either type of diabetes.
CONCLUSION
Although rare, when IP does occur, its manifestations are vast and severe enough to significantly reduce quality of life for patients; when it occurs in males, it is usually lethal. This genetic disorder can affect multiple body systems, making knowledge of its symptoms essential for proper diagnosis. Because its characteristic stages may be present at birth or in infancy, early identification and diagnosis of IP can help guide treatment intervention.
IN THIS ARTICLE
- Presenting stages
- Diagnostic criteria
- Management of IP
A 21-year-old woman with type 1 diabetes is admitted for recurrent diabetic ketoacidosis. Physical exam reveals hypopigmented, linear, streaky patches on the medial aspects of the bilateral lower legs (Figure 1A). The patient denies tenderness, pruritus, or paresthesia. There is obvious symmetrical hair loss on the lateral aspects of the eyebrows, as well as slightly wooly male-pattern hair distribution with patchy alopecia on the vertex of the head (Figure 1B). She has very poor dentition with hypodontia and malformed teeth (Figure 1C). Her fingernails and toenails appear normal, with no visible atrophy (Figure 1D). What explains her condition?

Incontinentia pigmenti (IP), also known as Bloch-Sulzberger syndrome, is a rare, X-linked dominant genodermatosis involving the cutaneous, ophthalmic, neurologic, and dental systems.1-3 It results from X-inactivation due to mutations in the NF-kappaB essential modulator (NEMO) gene with deletion of exons 4-10 in most cases. The NEMO gene encodes a regulatory component of the IkappaB kinase complex required to activate the NF-kappa B pathway, which is important for many immune, inflammatory, and apoptotic processes.4-6 This deletional mutation is typically lethal in normal 46,XY male karyotypes. Male fetuses with this mutation usually die in utero, making the reported cases predominantly female.4,7
The estimated incidence of IP is between 1/10,000 and 1/100,000.4 Due to the rarity of the condition, IP may be underrecognized and underdiagnosed.
CLINICAL PRESENTATION
Characteristic skin lesions of IP begin to develop at birth or in utero, in an evolving pattern that consists of four stages:
- The vesicular stage (stage I) is characterized by linear erythematous papules and blisters that manifest in newborns.
- The verrucous stage (stage II) begins as the blisters start to heal—usually after several weeks—and is distinguished by hyperkeratotic warty papules in linear or swirling distribution. This stage resolves on its own within months.
- The hyperpigmentation stage (stage III) is when swirling macules or patches develop. This hallmark stage of IP tends to remain static until adolescence.
- The hypopigmentation stage (stage IV) manifests with faded streaky patches, which may be subtly atrophic. This final stage usually develops in the second or third decade of life.2,3
All these cutaneous lesions follow Blaschko lines—invisible lines believed to result from embryonic cell migration that become visible with the manifestation of cutaneous or mucous lesions.6
Other associated cutaneous findings include patchy alopecia, nail dystrophy, and oral/dental anomalies such as hypodontia, oligodontia, and tooth deformities. In addition, ophthalmologic involvement can result in strabismus, cataracts, and retinal vascular changes that can lead to blindness. Central nervous system manifestations include seizures, cognitive impairment, and spastic paralysis.3
DIFFERENTIAL DIAGNOSIS
Because IP is uncommon, it may be easily overlooked or misdiagnosed as another, similar cutaneous manifestation. Cutaneous sarcoidosis, for example, is a skin lesion of noncaseating granuloma. It can present as patches, papules, ulcers, scars, ichthyosis, and alopecia. The development of cutaneous sarcoidosis can be idiopathic or iatrogenic, particularly in patients using anti-TNF therapy. The diagnosis is made clinically and can be confirmed pathologically.8
Stage I IP can also be confused with neonatal herpes simplex virus-1 (HSV-1) infection, given the similarities in vesicular morphology and linear distribution. The diagnosis of HSV-1 can be made based on history, physical exam, and pathology. Given the serious sequelae of neonatal HSV-1 infection, antiviral therapy should not be delayed until confirmation of the diagnosis in infants with vesicular eruptions.9
Erythema multiforme (EM) is another dermatologic condition frequently encountered in children and young adults. Its characteristic round target lesion usually has two rings surrounding the dusky-appearing central zone. Atypical lesions can be bullous or crusty, mimicking the appearance of stage I or II IP. EM is usually a self-limiting condition, but specific treatment may be required if the infectious agent is identified.10
Vitiligo, the development of white patches due to the loss of melanocytes, is another item in the differential. Although it most commonly involves the skin, the hair may also be affected. The diagnosis is made clinically and can be confirmed with skin biopsy if needed.11
DIAGNOSIS
Diagnostic criteria for IP have been proposed, with family history playing a role (see Table).2,3,12 Results of a case-study series indicate that 28% of patients with IP have a family history involving at least one first-degree female relative. IP was considered “sporadic” in 62% of cases studied.3

Without a family history of IP, at least one major criterion must be present to support the diagnosis. These include
- Neonatal rash (erythema, vesicles)
- Linear, atrophic, hairless lesions
- Hyperpigmentation (mainly on trunk, following Blaschko lines)
In a patient with a family history of IP, the presence of any major criterion strongly supports the diagnosis. These, as well as minor criteria, are outlined in the Table.2,3,12
In stages I and II of IP, pathologic features include spongiotic dermatitis with characteristic eosinophils and large dyskeratotic cells.3,13 In stage IV, skin biopsies may reveal slight atrophy and scattered apoptotic cells in the epidermis and epidermal hypopigmentation due to reduced melanocytes. The dermis typically appears thickened and is absent hair follicles and sweat glands.14 In a 2014 update, these pathologic features were proposed to be included in the major diagnostic criteria.12
TREATMENT/MANAGEMENT
Treatment of IP is centered on the involved organ systems. For cutaneous lesions, treatment is not usually necessary unless inflammation persists. In such cases, topical steroids or tacrolimus have been used with some success.15,16 In the vesicular stage, the patient should be monitored for bacterial infection, with appropriate prevention or treatment as necessary.
With other involved systems—such as dental, ophthalmologic, or neurologic (eg, seizures or other encephalopathy) anomalies—consultation and follow-up with the relevant specialist is warranted.
In this case, the patient denied family history of IP. She did have a history of infantile cataract and seizure. Her presenting signs were typical of stage IV IP: hypopigmented streaky patches on the skin of the lower legs, dental abnormalities, somewhat wooly hair, alopecia on the head, and loss of hair on the lateral aspects of the eyebrows. The uniqueness of this case is that the patient also had type 1 diabetes, a condition with a strong genetic predisposition. However, there is no evidence supporting an association between IP and either type of diabetes.
CONCLUSION
Although rare, when IP does occur, its manifestations are vast and severe enough to significantly reduce quality of life for patients; when it occurs in males, it is usually lethal. This genetic disorder can affect multiple body systems, making knowledge of its symptoms essential for proper diagnosis. Because its characteristic stages may be present at birth or in infancy, early identification and diagnosis of IP can help guide treatment intervention.
1. Roberts AP. Incontinentia pigmenti (Bloch-Sulzberger). Br Med. J. 1958;1(5079):1106-1107.
2. Landy SJ, Donnai D. Incontinentia pigmenti (Bloch-Sulzberger syndrome). J Med Genet. 1993;30(1):53-59.
3. Hadj-Rabia S, Froidevaux N, Bodak D, et al. Clinical study of 40 cases of incontinentia pigmenti. Arch Dermatol. 2003; 139(9):1163-1170.
4. Smahi A, Courtois G, Vabres P, et al. Genomic rearrangement in NEMO impairs NF-kappaB activation and is a cause of incontinentia pigmenti. Nature. 2000;405(6785):466-472.
5. Aradhya S, Courtois G, Rajkovic A, et al. Atypical forms of incontinentia pigmenti in male individuals result from mutations of a cytosine tract in exon 10 of NEMO (IKK-gamma). Am J Hum Genet. 2001;68(3):765-771.
6. Poziomczyk CS, Recuero JK, Bringhenti L, et al. Incontinentia pigmenti. An Bras Dermatol. 2014;89(1):26-36.
7. Kenwrick S, Woffendin H, Jakins T, et al. Survival of male patients with incontinentia pigmenti carrying a lethal mutation can be explained by somatic mosaicism or Klinefelter Syndrome. Am J Hum Genet. 2001;69(6):1210-1217.
8. Katta R. Cutaneous sarcoidosis: a dermatologic masquerader. Am Fam Physician. 2002;65(8):1581-1584.
9. Faloyin M, Levitt J, Bercowitz E, et al. All that is vesicular is not herpes: incontinentia pigmenti masquerading as herpes simplex virus in a newborn. Pediatrics. 2004;114(2):e270-272.
10. Siedner-Weintraub Y, Gross I, David A, et al. Paediatric erythema multiforme: epidemiological, clinical and laboratory characteristics. Acta Derm Venereol. 2016 Nov 10. doi: 10.2340/00015555-2569.
11. Gawkrodger DJ, Ormerod AD, Shaw L, et al. Guideline for the diagnosis and management of vitiligo. Br J Dermatol. 2008;159(5):1051-1076.
12. Minic´ S, Trpinac D, Obradovic´ M. Incontinentia pigmenti diagnostic criteria update. Clin Genet. 2014;85(6):536-542.
13. Jean-Baptiste S, O’Toole EA, Chen M, et al. Expression of eotaxin, an eosinophil-selective chemokine, parallels eosinophil accumulation in the vesiculobullous stage of incontinentia pigmenti. Clin Exp Immunol. 2002;127(3):470-478.
14. Hadj-Rabia S, Rimella A, Smahi A, et al. Clinical and histologic features of incontinentia pigmenti in adults with nuclear factor-κ B essential modulator gene mutations. J Am Acad Dermatol. 2011;64(3):508-515.
15. Kaya TI, Tursen U, Ikizoglu G. Therapeutic use of topical corticosteroids in the vesiculobullous lesions of incontinentia pigmenti. Clin Exp Dermatol. 2009;34(8):e611-613.
16. Jessup CJ, Morgan SC, Cohen LM, Viders DE. Incontinentia pigmenti: treatment of IP with topical tacrolimus. J Drugs Dermatol. 2009;8(10):944-946.
1. Roberts AP. Incontinentia pigmenti (Bloch-Sulzberger). Br Med. J. 1958;1(5079):1106-1107.
2. Landy SJ, Donnai D. Incontinentia pigmenti (Bloch-Sulzberger syndrome). J Med Genet. 1993;30(1):53-59.
3. Hadj-Rabia S, Froidevaux N, Bodak D, et al. Clinical study of 40 cases of incontinentia pigmenti. Arch Dermatol. 2003; 139(9):1163-1170.
4. Smahi A, Courtois G, Vabres P, et al. Genomic rearrangement in NEMO impairs NF-kappaB activation and is a cause of incontinentia pigmenti. Nature. 2000;405(6785):466-472.
5. Aradhya S, Courtois G, Rajkovic A, et al. Atypical forms of incontinentia pigmenti in male individuals result from mutations of a cytosine tract in exon 10 of NEMO (IKK-gamma). Am J Hum Genet. 2001;68(3):765-771.
6. Poziomczyk CS, Recuero JK, Bringhenti L, et al. Incontinentia pigmenti. An Bras Dermatol. 2014;89(1):26-36.
7. Kenwrick S, Woffendin H, Jakins T, et al. Survival of male patients with incontinentia pigmenti carrying a lethal mutation can be explained by somatic mosaicism or Klinefelter Syndrome. Am J Hum Genet. 2001;69(6):1210-1217.
8. Katta R. Cutaneous sarcoidosis: a dermatologic masquerader. Am Fam Physician. 2002;65(8):1581-1584.
9. Faloyin M, Levitt J, Bercowitz E, et al. All that is vesicular is not herpes: incontinentia pigmenti masquerading as herpes simplex virus in a newborn. Pediatrics. 2004;114(2):e270-272.
10. Siedner-Weintraub Y, Gross I, David A, et al. Paediatric erythema multiforme: epidemiological, clinical and laboratory characteristics. Acta Derm Venereol. 2016 Nov 10. doi: 10.2340/00015555-2569.
11. Gawkrodger DJ, Ormerod AD, Shaw L, et al. Guideline for the diagnosis and management of vitiligo. Br J Dermatol. 2008;159(5):1051-1076.
12. Minic´ S, Trpinac D, Obradovic´ M. Incontinentia pigmenti diagnostic criteria update. Clin Genet. 2014;85(6):536-542.
13. Jean-Baptiste S, O’Toole EA, Chen M, et al. Expression of eotaxin, an eosinophil-selective chemokine, parallels eosinophil accumulation in the vesiculobullous stage of incontinentia pigmenti. Clin Exp Immunol. 2002;127(3):470-478.
14. Hadj-Rabia S, Rimella A, Smahi A, et al. Clinical and histologic features of incontinentia pigmenti in adults with nuclear factor-κ B essential modulator gene mutations. J Am Acad Dermatol. 2011;64(3):508-515.
15. Kaya TI, Tursen U, Ikizoglu G. Therapeutic use of topical corticosteroids in the vesiculobullous lesions of incontinentia pigmenti. Clin Exp Dermatol. 2009;34(8):e611-613.
16. Jessup CJ, Morgan SC, Cohen LM, Viders DE. Incontinentia pigmenti: treatment of IP with topical tacrolimus. J Drugs Dermatol. 2009;8(10):944-946.
Program reduces transfusions in leukemia, HSCT patients
New research suggests a patient blood management (PBM) program can safely reduce transfusion use in patients with acute leukemia and those undergoing hematopoietic stem cell transplant (HSCT).
The program significantly reduced the use of red blood cell (RBC) and platelet transfusions without increasing morbidity or mortality in patients who were receiving intensive chemotherapy to treat acute leukemia and in patients receiving an allogeneic or autologous HSCT.
“There has been a long-standing belief among hematologists that patients with leukemia undergoing chemotherapy should have a transfusion of red blood cells if their hemoglobin level drops below about 9 g/dL to help avoid adverse outcomes,” said study author Michael Leahy, MB ChB, a consultant hematologist at the University of Western Australia in Perth.
“Findings in this real-world, non-clinical trial setting challenge that belief.”
Dr Leahy and his colleagues published their findings in Transfusion.
The researchers said the PBM program used in this study was built around the “3 pillars” concept of PBM, which are:
- Optimize the patient’s RBC mass
- Minimize blood loss
- Harness and optimize the patient’s physiologic anemia reserve.
No specific transfusion thresholds were established. However, the hospitals did adopt a single-unit RBC transfusion policy for symptomatic anemic patients who were not actively bleeding.
Results
The study included 695 admissions to 2 major hospitals in Western Australia. Patients were admitted between July 2010 and December 2014 for treatment of acute leukemia or for autologous or allogeneic HSCT.
During this time, the patients received 3384 RBC units and 3639 units of platelets.
The mean number of platelet units transfused per hospital admission decreased 35% from baseline to the end of the study period, from 6.3 to 4.1 units (P<0.001).
The mean number of RBC units transfused decreased 39%, from 6.1 to 3.7 (P<0.001). Meanwhile, the use of single-unit RBC transfusions increased from 39% to 67% (P<0.001).
And the mean hemoglobin level prior to RBC transfusion decreased from 8.0 g/dL to 6.8 g/dL (P<0.001).
“This study suggests that patients undergoing chemotherapy with hematological disease may tolerate much lower levels of hemoglobin than previously thought,” said Shannon Farmer, an adjunct research fellow at the University of Western Australia.
“The transfusion threshold, the hemoglobin value at which a transfusion is given, dropped significantly from 8.0 g/dL at the beginning of the study to 6.8 g/dL at the end. This was associated with significant reductions in transfusion and substantial costs savings without evidence of harm to the patients. In fact, it was associated with a trend toward improved survival.”
The reduction in blood products over the study period resulted in a cost savings of AU$694,886 (US$654,007)—AU$389,537 (US$364,177) for RBCs and AU$305,349 (US$289,830) for platelets.
There were no significant changes over the study period in length of hospital stay, serious bleeding events, or in-hospital mortality.
There was a non-significant reduction in the mean length of hospital stay, from 24.5 days to 22.6 days (P=0.338). The difference was still not significant after the researchers adjusted for patient age, patient group, and comorbidities (incident rate ratio=0.88; 95% CI, 0.75-1.04).
The rate of serious bleeding increased over the study period, from 5.3% to 7.0% (P=0.582). After adjustment, the odds ratio was 1.14 (95% CI, 0.38-3.44; P=0.811).
There was a non-significant decrease in in-hospital mortality, from 5.3% to 2.0% (P=0.218). After adjustment, the odds ratio was 0.31 (95% CI, 0.06-1.56; P=0.154).
Based on these results, the researchers concluded that PBM programs could have a substantial impact in this patient population, reducing blood utilization and healthcare costs. ![]()
New research suggests a patient blood management (PBM) program can safely reduce transfusion use in patients with acute leukemia and those undergoing hematopoietic stem cell transplant (HSCT).
The program significantly reduced the use of red blood cell (RBC) and platelet transfusions without increasing morbidity or mortality in patients who were receiving intensive chemotherapy to treat acute leukemia and in patients receiving an allogeneic or autologous HSCT.
“There has been a long-standing belief among hematologists that patients with leukemia undergoing chemotherapy should have a transfusion of red blood cells if their hemoglobin level drops below about 9 g/dL to help avoid adverse outcomes,” said study author Michael Leahy, MB ChB, a consultant hematologist at the University of Western Australia in Perth.
“Findings in this real-world, non-clinical trial setting challenge that belief.”
Dr Leahy and his colleagues published their findings in Transfusion.
The researchers said the PBM program used in this study was built around the “3 pillars” concept of PBM, which are:
- Optimize the patient’s RBC mass
- Minimize blood loss
- Harness and optimize the patient’s physiologic anemia reserve.
No specific transfusion thresholds were established. However, the hospitals did adopt a single-unit RBC transfusion policy for symptomatic anemic patients who were not actively bleeding.
Results
The study included 695 admissions to 2 major hospitals in Western Australia. Patients were admitted between July 2010 and December 2014 for treatment of acute leukemia or for autologous or allogeneic HSCT.
During this time, the patients received 3384 RBC units and 3639 units of platelets.
The mean number of platelet units transfused per hospital admission decreased 35% from baseline to the end of the study period, from 6.3 to 4.1 units (P<0.001).
The mean number of RBC units transfused decreased 39%, from 6.1 to 3.7 (P<0.001). Meanwhile, the use of single-unit RBC transfusions increased from 39% to 67% (P<0.001).
And the mean hemoglobin level prior to RBC transfusion decreased from 8.0 g/dL to 6.8 g/dL (P<0.001).
“This study suggests that patients undergoing chemotherapy with hematological disease may tolerate much lower levels of hemoglobin than previously thought,” said Shannon Farmer, an adjunct research fellow at the University of Western Australia.
“The transfusion threshold, the hemoglobin value at which a transfusion is given, dropped significantly from 8.0 g/dL at the beginning of the study to 6.8 g/dL at the end. This was associated with significant reductions in transfusion and substantial costs savings without evidence of harm to the patients. In fact, it was associated with a trend toward improved survival.”
The reduction in blood products over the study period resulted in a cost savings of AU$694,886 (US$654,007)—AU$389,537 (US$364,177) for RBCs and AU$305,349 (US$289,830) for platelets.
There were no significant changes over the study period in length of hospital stay, serious bleeding events, or in-hospital mortality.
There was a non-significant reduction in the mean length of hospital stay, from 24.5 days to 22.6 days (P=0.338). The difference was still not significant after the researchers adjusted for patient age, patient group, and comorbidities (incident rate ratio=0.88; 95% CI, 0.75-1.04).
The rate of serious bleeding increased over the study period, from 5.3% to 7.0% (P=0.582). After adjustment, the odds ratio was 1.14 (95% CI, 0.38-3.44; P=0.811).
There was a non-significant decrease in in-hospital mortality, from 5.3% to 2.0% (P=0.218). After adjustment, the odds ratio was 0.31 (95% CI, 0.06-1.56; P=0.154).
Based on these results, the researchers concluded that PBM programs could have a substantial impact in this patient population, reducing blood utilization and healthcare costs. ![]()
New research suggests a patient blood management (PBM) program can safely reduce transfusion use in patients with acute leukemia and those undergoing hematopoietic stem cell transplant (HSCT).
The program significantly reduced the use of red blood cell (RBC) and platelet transfusions without increasing morbidity or mortality in patients who were receiving intensive chemotherapy to treat acute leukemia and in patients receiving an allogeneic or autologous HSCT.
“There has been a long-standing belief among hematologists that patients with leukemia undergoing chemotherapy should have a transfusion of red blood cells if their hemoglobin level drops below about 9 g/dL to help avoid adverse outcomes,” said study author Michael Leahy, MB ChB, a consultant hematologist at the University of Western Australia in Perth.
“Findings in this real-world, non-clinical trial setting challenge that belief.”
Dr Leahy and his colleagues published their findings in Transfusion.
The researchers said the PBM program used in this study was built around the “3 pillars” concept of PBM, which are:
- Optimize the patient’s RBC mass
- Minimize blood loss
- Harness and optimize the patient’s physiologic anemia reserve.
No specific transfusion thresholds were established. However, the hospitals did adopt a single-unit RBC transfusion policy for symptomatic anemic patients who were not actively bleeding.
Results
The study included 695 admissions to 2 major hospitals in Western Australia. Patients were admitted between July 2010 and December 2014 for treatment of acute leukemia or for autologous or allogeneic HSCT.
During this time, the patients received 3384 RBC units and 3639 units of platelets.
The mean number of platelet units transfused per hospital admission decreased 35% from baseline to the end of the study period, from 6.3 to 4.1 units (P<0.001).
The mean number of RBC units transfused decreased 39%, from 6.1 to 3.7 (P<0.001). Meanwhile, the use of single-unit RBC transfusions increased from 39% to 67% (P<0.001).
And the mean hemoglobin level prior to RBC transfusion decreased from 8.0 g/dL to 6.8 g/dL (P<0.001).
“This study suggests that patients undergoing chemotherapy with hematological disease may tolerate much lower levels of hemoglobin than previously thought,” said Shannon Farmer, an adjunct research fellow at the University of Western Australia.
“The transfusion threshold, the hemoglobin value at which a transfusion is given, dropped significantly from 8.0 g/dL at the beginning of the study to 6.8 g/dL at the end. This was associated with significant reductions in transfusion and substantial costs savings without evidence of harm to the patients. In fact, it was associated with a trend toward improved survival.”
The reduction in blood products over the study period resulted in a cost savings of AU$694,886 (US$654,007)—AU$389,537 (US$364,177) for RBCs and AU$305,349 (US$289,830) for platelets.
There were no significant changes over the study period in length of hospital stay, serious bleeding events, or in-hospital mortality.
There was a non-significant reduction in the mean length of hospital stay, from 24.5 days to 22.6 days (P=0.338). The difference was still not significant after the researchers adjusted for patient age, patient group, and comorbidities (incident rate ratio=0.88; 95% CI, 0.75-1.04).
The rate of serious bleeding increased over the study period, from 5.3% to 7.0% (P=0.582). After adjustment, the odds ratio was 1.14 (95% CI, 0.38-3.44; P=0.811).
There was a non-significant decrease in in-hospital mortality, from 5.3% to 2.0% (P=0.218). After adjustment, the odds ratio was 0.31 (95% CI, 0.06-1.56; P=0.154).
Based on these results, the researchers concluded that PBM programs could have a substantial impact in this patient population, reducing blood utilization and healthcare costs. ![]()
Company resubmits BLA for andexanet alfa
Portola Pharmaceuticals Inc. has resubmitted its biologics license application (BLA) for andexanet alfa (AndexXa®) to the US Food and Drug Administration (FDA).
With this BLA, Portola is seeking approval of andexanet alfa for reversal of the anticoagulant effects of apixaban and rivaroxaban in patients experiencing uncontrolled or life-threatening bleeding.
The resubmission includes supplemental information requested by the FDA in a complete response letter issued in August 2016.
At that time, Portola was seeking approval for andexanet alfa in patients treated with apixaban, rivaroxaban, edoxaban, or enoxaparin when reversal of anticoagulation is needed.
However, the FDA said it could not approve andexanet alfa for that indication. The FDA requested that Portola provide information related to manufacturing of andexanet alfa as well as additional data to support the inclusion of edoxaban and enoxaparin on andexanet alfa’s label.
The initial BLA for andexanet alfa included data from a pair of phase 3 studies—ANNEXA-A and ANNEXA-R—which were designed to assess andexanet alfa’s ability to reverse the effects of apixaban and rivaroxaban—but not edoxaban or enoxaparin—in healthy volunteers.
Results from ANNEXA-A and ANNEXA-R were published in NEJM in 2015.
The BLA also included limited adjudicated efficacy and safety data from patients enrolled in the phase 3b/4 ANNEXA-4 study. This ongoing study is enrolling patients receiving apixaban, rivaroxaban, edoxaban, and enoxaparin who present with an acute major bleed.
Preliminary results from ANNEXA-4 were presented at ESC Congress 2016 and published in NEJM. ![]()
Portola Pharmaceuticals Inc. has resubmitted its biologics license application (BLA) for andexanet alfa (AndexXa®) to the US Food and Drug Administration (FDA).
With this BLA, Portola is seeking approval of andexanet alfa for reversal of the anticoagulant effects of apixaban and rivaroxaban in patients experiencing uncontrolled or life-threatening bleeding.
The resubmission includes supplemental information requested by the FDA in a complete response letter issued in August 2016.
At that time, Portola was seeking approval for andexanet alfa in patients treated with apixaban, rivaroxaban, edoxaban, or enoxaparin when reversal of anticoagulation is needed.
However, the FDA said it could not approve andexanet alfa for that indication. The FDA requested that Portola provide information related to manufacturing of andexanet alfa as well as additional data to support the inclusion of edoxaban and enoxaparin on andexanet alfa’s label.
The initial BLA for andexanet alfa included data from a pair of phase 3 studies—ANNEXA-A and ANNEXA-R—which were designed to assess andexanet alfa’s ability to reverse the effects of apixaban and rivaroxaban—but not edoxaban or enoxaparin—in healthy volunteers.
Results from ANNEXA-A and ANNEXA-R were published in NEJM in 2015.
The BLA also included limited adjudicated efficacy and safety data from patients enrolled in the phase 3b/4 ANNEXA-4 study. This ongoing study is enrolling patients receiving apixaban, rivaroxaban, edoxaban, and enoxaparin who present with an acute major bleed.
Preliminary results from ANNEXA-4 were presented at ESC Congress 2016 and published in NEJM. ![]()
Portola Pharmaceuticals Inc. has resubmitted its biologics license application (BLA) for andexanet alfa (AndexXa®) to the US Food and Drug Administration (FDA).
With this BLA, Portola is seeking approval of andexanet alfa for reversal of the anticoagulant effects of apixaban and rivaroxaban in patients experiencing uncontrolled or life-threatening bleeding.
The resubmission includes supplemental information requested by the FDA in a complete response letter issued in August 2016.
At that time, Portola was seeking approval for andexanet alfa in patients treated with apixaban, rivaroxaban, edoxaban, or enoxaparin when reversal of anticoagulation is needed.
However, the FDA said it could not approve andexanet alfa for that indication. The FDA requested that Portola provide information related to manufacturing of andexanet alfa as well as additional data to support the inclusion of edoxaban and enoxaparin on andexanet alfa’s label.
The initial BLA for andexanet alfa included data from a pair of phase 3 studies—ANNEXA-A and ANNEXA-R—which were designed to assess andexanet alfa’s ability to reverse the effects of apixaban and rivaroxaban—but not edoxaban or enoxaparin—in healthy volunteers.
Results from ANNEXA-A and ANNEXA-R were published in NEJM in 2015.
The BLA also included limited adjudicated efficacy and safety data from patients enrolled in the phase 3b/4 ANNEXA-4 study. This ongoing study is enrolling patients receiving apixaban, rivaroxaban, edoxaban, and enoxaparin who present with an acute major bleed.
Preliminary results from ANNEXA-4 were presented at ESC Congress 2016 and published in NEJM. ![]()
Less than half of office visits involve primary care
, according to the National Center for Health Statistics.
Primary care physicians’ share of office visits fell from 66.2% in 1980 to 49.1% in 2013, the NCHS reported in “Health, United States, 2016.” The corresponding increase among specialty care physicians gave them a total of 50.9% of all office visits in 2013, up from 33.8% in 1980.
The NCHS estimates are based on data collected by the National Ambulatory Medical Care Survey, which excluded Alaska and Hawaii in 1980.
, according to the National Center for Health Statistics.
Primary care physicians’ share of office visits fell from 66.2% in 1980 to 49.1% in 2013, the NCHS reported in “Health, United States, 2016.” The corresponding increase among specialty care physicians gave them a total of 50.9% of all office visits in 2013, up from 33.8% in 1980.
The NCHS estimates are based on data collected by the National Ambulatory Medical Care Survey, which excluded Alaska and Hawaii in 1980.
, according to the National Center for Health Statistics.
Primary care physicians’ share of office visits fell from 66.2% in 1980 to 49.1% in 2013, the NCHS reported in “Health, United States, 2016.” The corresponding increase among specialty care physicians gave them a total of 50.9% of all office visits in 2013, up from 33.8% in 1980.
The NCHS estimates are based on data collected by the National Ambulatory Medical Care Survey, which excluded Alaska and Hawaii in 1980.
Sodium fusidate noninferior to linezolid for acute skin infections
NEW ORLEANS – An oral antibiotic in development in the United States, fusidic acid (oral formulation, sodium fusidate) was noninferior to linezolid based on early clinical response in a randomized, double-blind, multicenter trial of 716 people with acute bacterial skin and skin structure infections (ABSSSI), including cellulitis, wound infection, and major cutaneous abscesses.
Early clinical response was defined as a 20% or greater reduction from baseline in the surface area of redness, edema, or induration at 48-72 hours after starting treatment with the study drugs. In an intent-to-treat analysis, 87.2% of patients randomized to fusidic acid and 86.6% of the linezolid group met this primary endpoint of the phase 3 study.
“Fusidic acid showed similar efficacy and comparable safety” that persisted through treatment, said Andy Strayer, PharmD, vice president of clinical programs at Cempra Pharmaceuticals, which is developing sodium fusidate as an oral agent to treat ABSSSI patients in the United States. Leo Pharmaceuticals has marketed sodium fusidate outside the United States in various formulations for decades.
Fusidic acid has potent activity against gram-positive aerobic organisms, including methicillin-resistant Staphylococcus aureus (MRSA). “Strikingly, fusidic acid showed 100% success in patients with MRSA in the microbiologically evaluable population at the end of treatment and posttherapy evaluation time points,” Dr. Strayer said at the annual meeting of the American Society for Microbiology. “Fusidic acid may offer an important oral therapy alternative for MRSA infection.”
“Fusidic acid, a drug long used in other parts of the world, has been demonstrated in this first phase 3 trial, to be a potential new option for the treatment of MRSA skin and skin structure infections in the U.S.,” said Carrie Cardenas, MD, lead study author and a principal investigator at eStudySite, San Diego, and an internist in private practice in La Mesa, California.
There was a microbiological diagnosis established in 75% of patients. S. aureus was the most commonly detected pathogen (422 patients; 59%), and the study included 235 patients diagnosed with MRSA infection.
About two-thirds, 65%, of participants were men. Mean age was 45 years. Infections were classified as wounds in 61%, cellulitis in 26%, and abscess in 13%. Notably, 68% of the recruited participants had ABSSSI associated with intravenous drug use, a “sometimes overlooked consequence of the ongoing epidemic of IV drug use in the U.S.,” Dr. Strayer said.
In terms of safety, treatment-emergent adverse event rates were comparable between the two groups (37.9% with fusidic acid versus 36.1% with linezolid). Gastrointestinal events were the most common adverse events, 22.8% versus 18.2%, respectively.
“Considering complicated skin infections are one of the most rapidly growing reasons for hospitalizations and emergency department visits each year, we anticipate that fusidic acid, if approved, may help clinicians decrease the length of inpatient stay or avoid hospitalization altogether,” Dr. Strayer said.
Cempra sponsored the study. Dr. Strayer is a Cempra employee and shareholder. Dr. Carrie Cardenas is a principal investigator at eStudySite, San Diego, and performs research for Cempra, Paratek, Debiopharm, Motif, Durata, MicuRx, Bristol-Myers Squibb, and Bayer.
NEW ORLEANS – An oral antibiotic in development in the United States, fusidic acid (oral formulation, sodium fusidate) was noninferior to linezolid based on early clinical response in a randomized, double-blind, multicenter trial of 716 people with acute bacterial skin and skin structure infections (ABSSSI), including cellulitis, wound infection, and major cutaneous abscesses.
Early clinical response was defined as a 20% or greater reduction from baseline in the surface area of redness, edema, or induration at 48-72 hours after starting treatment with the study drugs. In an intent-to-treat analysis, 87.2% of patients randomized to fusidic acid and 86.6% of the linezolid group met this primary endpoint of the phase 3 study.
“Fusidic acid showed similar efficacy and comparable safety” that persisted through treatment, said Andy Strayer, PharmD, vice president of clinical programs at Cempra Pharmaceuticals, which is developing sodium fusidate as an oral agent to treat ABSSSI patients in the United States. Leo Pharmaceuticals has marketed sodium fusidate outside the United States in various formulations for decades.
Fusidic acid has potent activity against gram-positive aerobic organisms, including methicillin-resistant Staphylococcus aureus (MRSA). “Strikingly, fusidic acid showed 100% success in patients with MRSA in the microbiologically evaluable population at the end of treatment and posttherapy evaluation time points,” Dr. Strayer said at the annual meeting of the American Society for Microbiology. “Fusidic acid may offer an important oral therapy alternative for MRSA infection.”
“Fusidic acid, a drug long used in other parts of the world, has been demonstrated in this first phase 3 trial, to be a potential new option for the treatment of MRSA skin and skin structure infections in the U.S.,” said Carrie Cardenas, MD, lead study author and a principal investigator at eStudySite, San Diego, and an internist in private practice in La Mesa, California.
There was a microbiological diagnosis established in 75% of patients. S. aureus was the most commonly detected pathogen (422 patients; 59%), and the study included 235 patients diagnosed with MRSA infection.
About two-thirds, 65%, of participants were men. Mean age was 45 years. Infections were classified as wounds in 61%, cellulitis in 26%, and abscess in 13%. Notably, 68% of the recruited participants had ABSSSI associated with intravenous drug use, a “sometimes overlooked consequence of the ongoing epidemic of IV drug use in the U.S.,” Dr. Strayer said.
In terms of safety, treatment-emergent adverse event rates were comparable between the two groups (37.9% with fusidic acid versus 36.1% with linezolid). Gastrointestinal events were the most common adverse events, 22.8% versus 18.2%, respectively.
“Considering complicated skin infections are one of the most rapidly growing reasons for hospitalizations and emergency department visits each year, we anticipate that fusidic acid, if approved, may help clinicians decrease the length of inpatient stay or avoid hospitalization altogether,” Dr. Strayer said.
Cempra sponsored the study. Dr. Strayer is a Cempra employee and shareholder. Dr. Carrie Cardenas is a principal investigator at eStudySite, San Diego, and performs research for Cempra, Paratek, Debiopharm, Motif, Durata, MicuRx, Bristol-Myers Squibb, and Bayer.
NEW ORLEANS – An oral antibiotic in development in the United States, fusidic acid (oral formulation, sodium fusidate) was noninferior to linezolid based on early clinical response in a randomized, double-blind, multicenter trial of 716 people with acute bacterial skin and skin structure infections (ABSSSI), including cellulitis, wound infection, and major cutaneous abscesses.
Early clinical response was defined as a 20% or greater reduction from baseline in the surface area of redness, edema, or induration at 48-72 hours after starting treatment with the study drugs. In an intent-to-treat analysis, 87.2% of patients randomized to fusidic acid and 86.6% of the linezolid group met this primary endpoint of the phase 3 study.
“Fusidic acid showed similar efficacy and comparable safety” that persisted through treatment, said Andy Strayer, PharmD, vice president of clinical programs at Cempra Pharmaceuticals, which is developing sodium fusidate as an oral agent to treat ABSSSI patients in the United States. Leo Pharmaceuticals has marketed sodium fusidate outside the United States in various formulations for decades.
Fusidic acid has potent activity against gram-positive aerobic organisms, including methicillin-resistant Staphylococcus aureus (MRSA). “Strikingly, fusidic acid showed 100% success in patients with MRSA in the microbiologically evaluable population at the end of treatment and posttherapy evaluation time points,” Dr. Strayer said at the annual meeting of the American Society for Microbiology. “Fusidic acid may offer an important oral therapy alternative for MRSA infection.”
“Fusidic acid, a drug long used in other parts of the world, has been demonstrated in this first phase 3 trial, to be a potential new option for the treatment of MRSA skin and skin structure infections in the U.S.,” said Carrie Cardenas, MD, lead study author and a principal investigator at eStudySite, San Diego, and an internist in private practice in La Mesa, California.
There was a microbiological diagnosis established in 75% of patients. S. aureus was the most commonly detected pathogen (422 patients; 59%), and the study included 235 patients diagnosed with MRSA infection.
About two-thirds, 65%, of participants were men. Mean age was 45 years. Infections were classified as wounds in 61%, cellulitis in 26%, and abscess in 13%. Notably, 68% of the recruited participants had ABSSSI associated with intravenous drug use, a “sometimes overlooked consequence of the ongoing epidemic of IV drug use in the U.S.,” Dr. Strayer said.
In terms of safety, treatment-emergent adverse event rates were comparable between the two groups (37.9% with fusidic acid versus 36.1% with linezolid). Gastrointestinal events were the most common adverse events, 22.8% versus 18.2%, respectively.
“Considering complicated skin infections are one of the most rapidly growing reasons for hospitalizations and emergency department visits each year, we anticipate that fusidic acid, if approved, may help clinicians decrease the length of inpatient stay or avoid hospitalization altogether,” Dr. Strayer said.
Cempra sponsored the study. Dr. Strayer is a Cempra employee and shareholder. Dr. Carrie Cardenas is a principal investigator at eStudySite, San Diego, and performs research for Cempra, Paratek, Debiopharm, Motif, Durata, MicuRx, Bristol-Myers Squibb, and Bayer.
AT ASM MICROBE 2017
Key clinical point: Sodium fusidate, active as fusidic acid, showed noninferiority to linezolid for early clinical response in ABSSI patients.
Major finding: 87.2% of patients given sodium fusidate and 86.6% of those receiving linezolid achieved an early clinical response.
Data source: Randomized, controlled, double-blind, phase 3 study with 716 participants.
Disclosures: Cempra sponsored the study. Dr. Carrier Cardenas is a researcher for Cempra, Paratek, Debiopharm, Motif, Durata, MicuRx, Bristol-Myers Squibb, and Bayer. Dr. Strayer is a Cempra employee and shareholder.
For acute gout, corticosteroids look safer than NSAIDs
For the treatment of acute gout, corticosteroids may be as effective as nonsteroidal anti-inflammatory drugs but with fewer side effects, based on findings from a meta-analysis of six randomized, controlled trials.
“There is insufficient information to determine the comparative efficacy of corticosteroids and NSAID[s] to treat acute gout, while corticosteroids appear to have a more favorable safety profile for selected [adverse events],” Christy A. Billy, MD, of the University of Sydney and her coauthors wrote in their report published online in the Journal of Rheumatology (J Rheumatol. 2017 Aug. doi: 10.3899/jrheum.170137).
Two previous systematic reviews also suggested that corticosteroids may be therapeutically equivalent to but safer than NSAIDs, but both were based on a very small number of available studies and suffered from statistical between-trial heterogeneity.
The meta-analysis of six trials included a total of 817 patients. The trials had a mean follow-up of 15 days. Two trials were in hospitalized patients, two involved patients in the emergency department, one included outpatients, and one did not disclose the location of clinical presentation. Mean age of participants ranged from 44 years to 65.9 years, and the proportion of men ranged from 70% to 100%.
With respect to pain scores, the researchers found no significant difference between corticosteroid and NSAIDs within 7 days of treatment based on moderate-quality evidence from two randomized, controlled trials (RCTs) involving 534 patients (standardized mean difference = –0.09; 95% confidence interval, –0.26 to 0.08). There was also no difference between the two on pain after 7 or more days based on low-quality evidence from two RCTs of 506 patients (SMD = 0.32; 95% CI, –0.27 to 0.92). There was no evidence of statistical heterogeneity in the short-term trials, but there was evidence of significant heterogeneity in trials measuring treatment effects for 7 days or longer (P = .01; I2 [heterogeneity] = 85%).
Two RCTs of 173 patients gave low-quality evidence to show no difference between corticosteroids and NSAIDs in the rate of treatment response in the short term (relative risk, 1.07; 95% CI, 0.80-1.44; moderate heterogeneity, P = .15, I2 = 53%). One long-term study of the rate of treatment response provided similar results. There were also no between-group differences in joint swelling, erythema, tenderness, or activity limitations.
The investigators discovered that patients who took corticosteroids had a lower risk of indigestion in three RCTs with 526 patients (RR, 0.50; 95% CI, 0.27-0.92), nausea in three RCTs of 566 patients (RR, 0.25; 95% CI, 0.11-0.54), and vomiting in two RCTs totaling 506 patients (RR, 0.11; 95% CI, 0.02-0.56).
Patients taking corticosteroids had a higher risk of rash in two RCTs of 506 patients (RR, 4.62; 95% CI, 1.34-15.97). There was statistically significant heterogeneity in summary effects of treatment for total adverse events across all six studies (P = .04; I2 = 56%).
This meta-analysis was limited by the small number of clinical trials available for inclusion, which prevented the estimate of a number of outcomes and subgroup analyses. There was also a high risk of bias in many of the studies. Only half of the studies confirmed the diagnosis of gout by the presence of monosodium urate crystals within joint spaces. No studies reported on the effects of treatment on kidney function or injury.
The authors disclosed no source of funding or financial relationships.
For the treatment of acute gout, corticosteroids may be as effective as nonsteroidal anti-inflammatory drugs but with fewer side effects, based on findings from a meta-analysis of six randomized, controlled trials.
“There is insufficient information to determine the comparative efficacy of corticosteroids and NSAID[s] to treat acute gout, while corticosteroids appear to have a more favorable safety profile for selected [adverse events],” Christy A. Billy, MD, of the University of Sydney and her coauthors wrote in their report published online in the Journal of Rheumatology (J Rheumatol. 2017 Aug. doi: 10.3899/jrheum.170137).
Two previous systematic reviews also suggested that corticosteroids may be therapeutically equivalent to but safer than NSAIDs, but both were based on a very small number of available studies and suffered from statistical between-trial heterogeneity.
The meta-analysis of six trials included a total of 817 patients. The trials had a mean follow-up of 15 days. Two trials were in hospitalized patients, two involved patients in the emergency department, one included outpatients, and one did not disclose the location of clinical presentation. Mean age of participants ranged from 44 years to 65.9 years, and the proportion of men ranged from 70% to 100%.
With respect to pain scores, the researchers found no significant difference between corticosteroid and NSAIDs within 7 days of treatment based on moderate-quality evidence from two randomized, controlled trials (RCTs) involving 534 patients (standardized mean difference = –0.09; 95% confidence interval, –0.26 to 0.08). There was also no difference between the two on pain after 7 or more days based on low-quality evidence from two RCTs of 506 patients (SMD = 0.32; 95% CI, –0.27 to 0.92). There was no evidence of statistical heterogeneity in the short-term trials, but there was evidence of significant heterogeneity in trials measuring treatment effects for 7 days or longer (P = .01; I2 [heterogeneity] = 85%).
Two RCTs of 173 patients gave low-quality evidence to show no difference between corticosteroids and NSAIDs in the rate of treatment response in the short term (relative risk, 1.07; 95% CI, 0.80-1.44; moderate heterogeneity, P = .15, I2 = 53%). One long-term study of the rate of treatment response provided similar results. There were also no between-group differences in joint swelling, erythema, tenderness, or activity limitations.
The investigators discovered that patients who took corticosteroids had a lower risk of indigestion in three RCTs with 526 patients (RR, 0.50; 95% CI, 0.27-0.92), nausea in three RCTs of 566 patients (RR, 0.25; 95% CI, 0.11-0.54), and vomiting in two RCTs totaling 506 patients (RR, 0.11; 95% CI, 0.02-0.56).
Patients taking corticosteroids had a higher risk of rash in two RCTs of 506 patients (RR, 4.62; 95% CI, 1.34-15.97). There was statistically significant heterogeneity in summary effects of treatment for total adverse events across all six studies (P = .04; I2 = 56%).
This meta-analysis was limited by the small number of clinical trials available for inclusion, which prevented the estimate of a number of outcomes and subgroup analyses. There was also a high risk of bias in many of the studies. Only half of the studies confirmed the diagnosis of gout by the presence of monosodium urate crystals within joint spaces. No studies reported on the effects of treatment on kidney function or injury.
The authors disclosed no source of funding or financial relationships.
For the treatment of acute gout, corticosteroids may be as effective as nonsteroidal anti-inflammatory drugs but with fewer side effects, based on findings from a meta-analysis of six randomized, controlled trials.
“There is insufficient information to determine the comparative efficacy of corticosteroids and NSAID[s] to treat acute gout, while corticosteroids appear to have a more favorable safety profile for selected [adverse events],” Christy A. Billy, MD, of the University of Sydney and her coauthors wrote in their report published online in the Journal of Rheumatology (J Rheumatol. 2017 Aug. doi: 10.3899/jrheum.170137).
Two previous systematic reviews also suggested that corticosteroids may be therapeutically equivalent to but safer than NSAIDs, but both were based on a very small number of available studies and suffered from statistical between-trial heterogeneity.
The meta-analysis of six trials included a total of 817 patients. The trials had a mean follow-up of 15 days. Two trials were in hospitalized patients, two involved patients in the emergency department, one included outpatients, and one did not disclose the location of clinical presentation. Mean age of participants ranged from 44 years to 65.9 years, and the proportion of men ranged from 70% to 100%.
With respect to pain scores, the researchers found no significant difference between corticosteroid and NSAIDs within 7 days of treatment based on moderate-quality evidence from two randomized, controlled trials (RCTs) involving 534 patients (standardized mean difference = –0.09; 95% confidence interval, –0.26 to 0.08). There was also no difference between the two on pain after 7 or more days based on low-quality evidence from two RCTs of 506 patients (SMD = 0.32; 95% CI, –0.27 to 0.92). There was no evidence of statistical heterogeneity in the short-term trials, but there was evidence of significant heterogeneity in trials measuring treatment effects for 7 days or longer (P = .01; I2 [heterogeneity] = 85%).
Two RCTs of 173 patients gave low-quality evidence to show no difference between corticosteroids and NSAIDs in the rate of treatment response in the short term (relative risk, 1.07; 95% CI, 0.80-1.44; moderate heterogeneity, P = .15, I2 = 53%). One long-term study of the rate of treatment response provided similar results. There were also no between-group differences in joint swelling, erythema, tenderness, or activity limitations.
The investigators discovered that patients who took corticosteroids had a lower risk of indigestion in three RCTs with 526 patients (RR, 0.50; 95% CI, 0.27-0.92), nausea in three RCTs of 566 patients (RR, 0.25; 95% CI, 0.11-0.54), and vomiting in two RCTs totaling 506 patients (RR, 0.11; 95% CI, 0.02-0.56).
Patients taking corticosteroids had a higher risk of rash in two RCTs of 506 patients (RR, 4.62; 95% CI, 1.34-15.97). There was statistically significant heterogeneity in summary effects of treatment for total adverse events across all six studies (P = .04; I2 = 56%).
This meta-analysis was limited by the small number of clinical trials available for inclusion, which prevented the estimate of a number of outcomes and subgroup analyses. There was also a high risk of bias in many of the studies. Only half of the studies confirmed the diagnosis of gout by the presence of monosodium urate crystals within joint spaces. No studies reported on the effects of treatment on kidney function or injury.
The authors disclosed no source of funding or financial relationships.
FROM JOURNAL OF RHEUMATOLOGY
Key clinical point: Corticosteroids had a similar efficacy but a better safety profile than NSAIDs.
Major finding: Corticosteroids had efficacy similar to NSAIDs in the treatment of acute gout.
Data source: Meta-analysis of six randomized, controlled trials (n = 817).
Disclosures: The authors disclosed no source of funding or financial relationships.
Depression, PTSD double risk of dementia for older female veterans
LONDON – Women veterans with either depression or post-traumatic stress disorder face a doubling in their risk of dementia – and having both increases the risk even more, Dr. Kristine Yaffe reported at the Alzheimer’s Association International Conference.*
The risk ratios for incident dementia that Dr. Yaffe of the University of California, San Francisco, and her colleagues calculated from their analysis of a cohort of 149,000 older female veterans in the national Veterans Health Administration (VHA) database remained unchanged even when they adjusted for age, education, medical comorbidities, and other confounders.
Not only are older women veterans a growing group; they are frequently diagnosed with mental health disorders. In 2012, 45% of women veteran patients in the VHA had a mental health condition, Dr. Yaffe noted.
“Over 9% of all veterans in the U.S. are women, accounting for more than 2 million women veterans. And 30% of those are more than 55 years old. Additionally, the number of women utilizing the Veterans Healthcare Administration system has nearly doubled in the last decade.”
The study of the impact of depression and PTSD on incident dementia is the first of its kind, Dr. Yaffe noted. The cohort comprised women without dementia who had at least two VHA visits during 2005-2015. They were followed for a mean of 5 years. A diagnosis of depression or PTSD had to occur during a 2-year baseline period. Confounders considered in the analysis were demographics, medical comorbidities, and health habits, including alcohol and tobacco use. The primary outcome was time to incident dementia.
At baseline, the group was a mean of 67 years old. Most subjects (70%) were white. Hypertension was common (46%), as was diabetes (16%). About 6% had cardiovascular disease. Depression was present in 18% and PTSD in 4%.
When parsed by diagnosis, there were some significant between-group differences at baseline. Women with depression or PTSD were younger than those without (65 and 63 vs. 67 years). Women who had both disorders were the youngest group, at 62 years.
Hypertension was least common in women without depression or PTSD (41%), and most common among those with depression (65%). Diabetes was almost more common among women with depression than among those without (24% vs. 14%).
Dr. Yaffe created two regression analyses. Model 1 controlled for age, race, and education. Model 2 controlled for the factors in Model 1, plus diabetes, hypertension, and cardiovascular disease.
By the end of follow-up, 4% of the group had developed dementia. The presence of depression approximately doubled the risk of dementia (hazard ratio, 2.14), compared with women who had neither depression nor PTSD. This risk was virtually unchanged in both Model 1 and Model 2 (HRs, 2.12 and 2.00).
The risk associated with PTSD was quite similar, increasing the risk of dementia twofold (HR, 2.19). Again, this was similar after controlling for the confounders in both Model 1 (HR, 2.20) and Model 2 (HR, 2.16).
Women with both depression and PTSD had almost a tripling of risk for dementia (HR, 2.71). Adjustment for confounders did not significantly alter this risk, either in Model 1 (HR, 2.59) or Model 2 (HR, 2.42).
“A question that often comes up in these types of studies is, ‘Is this a reverse causation?’ ” Dr. Yaffe said. “In other words, are people with dementia somehow getting more depression? We conducted a lag-time analysis that allowed a 2-year lag time for dementia, and also adjusted for the number of clinic visits. The results were almost identical.”
“This consistent doubling of risk is quite high,” Dr. Yaffe said. “In our prior work with male veterans, we didn’t see this robust an association.”
The study was funded by the Department of Defense and the National Institutes of Health. Dr. Yaffe had no financial disclosures.
Correction, 8/7/17: An earlier version of this article misstated Dr. Kristine Yaffe's degree.
[email protected]
On Twitter @alz_gal
LONDON – Women veterans with either depression or post-traumatic stress disorder face a doubling in their risk of dementia – and having both increases the risk even more, Dr. Kristine Yaffe reported at the Alzheimer’s Association International Conference.*
The risk ratios for incident dementia that Dr. Yaffe of the University of California, San Francisco, and her colleagues calculated from their analysis of a cohort of 149,000 older female veterans in the national Veterans Health Administration (VHA) database remained unchanged even when they adjusted for age, education, medical comorbidities, and other confounders.
Not only are older women veterans a growing group; they are frequently diagnosed with mental health disorders. In 2012, 45% of women veteran patients in the VHA had a mental health condition, Dr. Yaffe noted.
“Over 9% of all veterans in the U.S. are women, accounting for more than 2 million women veterans. And 30% of those are more than 55 years old. Additionally, the number of women utilizing the Veterans Healthcare Administration system has nearly doubled in the last decade.”
The study of the impact of depression and PTSD on incident dementia is the first of its kind, Dr. Yaffe noted. The cohort comprised women without dementia who had at least two VHA visits during 2005-2015. They were followed for a mean of 5 years. A diagnosis of depression or PTSD had to occur during a 2-year baseline period. Confounders considered in the analysis were demographics, medical comorbidities, and health habits, including alcohol and tobacco use. The primary outcome was time to incident dementia.
At baseline, the group was a mean of 67 years old. Most subjects (70%) were white. Hypertension was common (46%), as was diabetes (16%). About 6% had cardiovascular disease. Depression was present in 18% and PTSD in 4%.
When parsed by diagnosis, there were some significant between-group differences at baseline. Women with depression or PTSD were younger than those without (65 and 63 vs. 67 years). Women who had both disorders were the youngest group, at 62 years.
Hypertension was least common in women without depression or PTSD (41%), and most common among those with depression (65%). Diabetes was almost more common among women with depression than among those without (24% vs. 14%).
Dr. Yaffe created two regression analyses. Model 1 controlled for age, race, and education. Model 2 controlled for the factors in Model 1, plus diabetes, hypertension, and cardiovascular disease.
By the end of follow-up, 4% of the group had developed dementia. The presence of depression approximately doubled the risk of dementia (hazard ratio, 2.14), compared with women who had neither depression nor PTSD. This risk was virtually unchanged in both Model 1 and Model 2 (HRs, 2.12 and 2.00).
The risk associated with PTSD was quite similar, increasing the risk of dementia twofold (HR, 2.19). Again, this was similar after controlling for the confounders in both Model 1 (HR, 2.20) and Model 2 (HR, 2.16).
Women with both depression and PTSD had almost a tripling of risk for dementia (HR, 2.71). Adjustment for confounders did not significantly alter this risk, either in Model 1 (HR, 2.59) or Model 2 (HR, 2.42).
“A question that often comes up in these types of studies is, ‘Is this a reverse causation?’ ” Dr. Yaffe said. “In other words, are people with dementia somehow getting more depression? We conducted a lag-time analysis that allowed a 2-year lag time for dementia, and also adjusted for the number of clinic visits. The results were almost identical.”
“This consistent doubling of risk is quite high,” Dr. Yaffe said. “In our prior work with male veterans, we didn’t see this robust an association.”
The study was funded by the Department of Defense and the National Institutes of Health. Dr. Yaffe had no financial disclosures.
Correction, 8/7/17: An earlier version of this article misstated Dr. Kristine Yaffe's degree.
[email protected]
On Twitter @alz_gal
LONDON – Women veterans with either depression or post-traumatic stress disorder face a doubling in their risk of dementia – and having both increases the risk even more, Dr. Kristine Yaffe reported at the Alzheimer’s Association International Conference.*
The risk ratios for incident dementia that Dr. Yaffe of the University of California, San Francisco, and her colleagues calculated from their analysis of a cohort of 149,000 older female veterans in the national Veterans Health Administration (VHA) database remained unchanged even when they adjusted for age, education, medical comorbidities, and other confounders.
Not only are older women veterans a growing group; they are frequently diagnosed with mental health disorders. In 2012, 45% of women veteran patients in the VHA had a mental health condition, Dr. Yaffe noted.
“Over 9% of all veterans in the U.S. are women, accounting for more than 2 million women veterans. And 30% of those are more than 55 years old. Additionally, the number of women utilizing the Veterans Healthcare Administration system has nearly doubled in the last decade.”
The study of the impact of depression and PTSD on incident dementia is the first of its kind, Dr. Yaffe noted. The cohort comprised women without dementia who had at least two VHA visits during 2005-2015. They were followed for a mean of 5 years. A diagnosis of depression or PTSD had to occur during a 2-year baseline period. Confounders considered in the analysis were demographics, medical comorbidities, and health habits, including alcohol and tobacco use. The primary outcome was time to incident dementia.
At baseline, the group was a mean of 67 years old. Most subjects (70%) were white. Hypertension was common (46%), as was diabetes (16%). About 6% had cardiovascular disease. Depression was present in 18% and PTSD in 4%.
When parsed by diagnosis, there were some significant between-group differences at baseline. Women with depression or PTSD were younger than those without (65 and 63 vs. 67 years). Women who had both disorders were the youngest group, at 62 years.
Hypertension was least common in women without depression or PTSD (41%), and most common among those with depression (65%). Diabetes was almost more common among women with depression than among those without (24% vs. 14%).
Dr. Yaffe created two regression analyses. Model 1 controlled for age, race, and education. Model 2 controlled for the factors in Model 1, plus diabetes, hypertension, and cardiovascular disease.
By the end of follow-up, 4% of the group had developed dementia. The presence of depression approximately doubled the risk of dementia (hazard ratio, 2.14), compared with women who had neither depression nor PTSD. This risk was virtually unchanged in both Model 1 and Model 2 (HRs, 2.12 and 2.00).
The risk associated with PTSD was quite similar, increasing the risk of dementia twofold (HR, 2.19). Again, this was similar after controlling for the confounders in both Model 1 (HR, 2.20) and Model 2 (HR, 2.16).
Women with both depression and PTSD had almost a tripling of risk for dementia (HR, 2.71). Adjustment for confounders did not significantly alter this risk, either in Model 1 (HR, 2.59) or Model 2 (HR, 2.42).
“A question that often comes up in these types of studies is, ‘Is this a reverse causation?’ ” Dr. Yaffe said. “In other words, are people with dementia somehow getting more depression? We conducted a lag-time analysis that allowed a 2-year lag time for dementia, and also adjusted for the number of clinic visits. The results were almost identical.”
“This consistent doubling of risk is quite high,” Dr. Yaffe said. “In our prior work with male veterans, we didn’t see this robust an association.”
The study was funded by the Department of Defense and the National Institutes of Health. Dr. Yaffe had no financial disclosures.
Correction, 8/7/17: An earlier version of this article misstated Dr. Kristine Yaffe's degree.
[email protected]
On Twitter @alz_gal
AT AAIC 2017
Key clinical point:
Major finding: Depression or PTSD both doubled the risk of dementia; both conditions together increased the risk by almost 2.5 times.
Data source: The retrospective cohort study comprised 149,000 women in the national Veterans Health Administration database.
Disclosures: The Department of Defense and National Institutes of Health Funded the study. The presenter had no financial disclosures.
Under Trump, hospitals face same penalties embraced by Obama
Amid all the turbulence over the future of the Affordable Care Act, one facet continues unchanged: President Trump’s administration is penalizing more than half the nation’s hospitals for having too many patients return within a month.
Medicare is punishing 2,573 hospitals, just two dozen short of what it did last year under President Obama, according to federal records released Aug. 2. Starting in October, the federal government will cut those hospitals’ payments by as much as 3% for a year.
Medicare docked all but 174 of those hospitals last year as well. The $564 million the government projects to save also is roughly the same as it was last year under Obama.
High rates of readmissions have been a safety concern for decades, with one in five Medicare patients historically ending up back in the hospital within 30 days. In 2011, 3.3 million adults returned to the hospital, running up medical costs estimated at $41 billion, according to the Agency for Healthcare Research and Quality.
The penalties, which begin their sixth year in October, have coincided with a nationwide decrease in hospital repeat patients. Between 2007 and 2015, the frequency of readmissions for conditions targeted by Medicare dropped from 21.5% to 17.8%, with the majority of the decrease occurring shortly after the ACA passed in 2010, according to a study conducted by Obama administration health policy experts and published in 2016 in the New England Journal of Medicine.
Some hospitals began giving impoverished patients free medications that they prescribed for their recovery, while others sent nurses to check up on patients seen as most likely to relapse in their homes. Readmissions dropped more quickly at hospitals potentially subject to the penalty than at other hospitals, another study found.
“The sum of the evidence really suggests that this program is helping people,” said Susannah Bernheim, MD, the director of quality measurement at the Yale/Yale-New Haven Hospital Center for Outcomes Research and Evaluation.
But the pace of these reductions has been leveling off in the past few years, indicating that the penalties’ ability to induce improvements may be waning.
“Presumably, hospitals made substantial changes during the implementation period but could not sustain such a high rate of reductions in the long term,” the New England Journal article said.
An analysis by Dr. Bernheim’s group found no decrease in the overall rate of readmissions between 2012 and 2015, although small drops in the medical conditions targeted by the penalties continued.
“We have indeed reached the limits of what changes in how we deliver care will allow us to do,” said Nancy Foster, vice president for quality at the American Hospital Association. “We can’t prevent every readmission. It could be that there is further room for improvement, but we just don’t know what the technique is to make that happen.”
The Hospital Readmissions Reduction Program was designed to use the purchasing power of Medicare to reward hospitals for higher quality. Those penalties, along with other ones aimed at improving hospital care, have been spared the partisan rancor over the law, and they would have continued under the GOP repeal proposals that stalled in Congress. But they have also been largely ignored.
Ashish Jha, MD, a professor at the Harvard T.H. Chan School of Public Health, Boston, said the fight over abolishing the ACA has drowned out talk about how to make the health care system more effective.
“We’ve spent the last 6 months fighting about how we’re going to pay for health insurance, which is one part of the ACA,” he said. “There’s been almost no discussion of the underlying health care delivery system changes that the ACA ushered in, and that is more important in the long run to be discussing because that’s what’s going to determine the underlying costs and outcomes of the health system.”
The readmission penalties are intended to neutralize an unintended incentive in the way Medicare pays hospitals that had profited from return patients. Medicare pays hospitals a lump sum for a patient’s stay based on the nature of the admission and other factors. Since hospitals generally are not paid extra if patients remain longer, they seek to discharge patients as soon as is medically feasible. If the patient ends up back in the hospital, it becomes a financial benefit as the hospital is paid for that second stay, filling a bed that would not have generated income if the patient had remained there continuously.
Because of the way the readmission penalty program was designed, it is not surprising that the new results are so similar to last year’s. As before, Medicare determined the penalties based on readmissions of the same six types of patients: those admitted for heart attacks, heart failure, pneumonia, chronic lung disease, hip or knee replacements, or coronary artery bypass graft surgery. Hospitals were judged on patients discharged between July 2013 and June 2016. Because the government looks at a 3-year period, 2 of those years were also examined in determining last year’s penalties.
This year, the average penalty will be 0.73% of each payment Medicare makes for a patient between Oct. 1 and Sept. 30, 2018, according to a Kaiser Health News analysis. That too was practically the same as last year. Forty-eight hospitals received the maximum punishment of a 3% reduction. Medicare did not release hospital-specific estimates for how much lost money these penalties would translate to.
More than 1,500 hospitals treating veterans, children, and psychiatric patients were exempted from penalties this year as required by law. Critical access hospitals, which Medicare also pays differently, also were excluded. So were Maryland hospitals because Congress has given that state extra leeway in how it distributes Medicare money.
Of the 3,241 hospitals whose readmissions were evaluated, Medicare penalized four out of five, the KHN analysis found. That is because the program’s methods are not very forgiving: A hospital can be penalized even if it has higher than expected readmission rates for only one of the six conditions that are targeted. Every nonexcluded hospital in Delaware and West Virginia will have their reimbursements reduced. Ninety percent or more will be punished in Arizona, Connecticut, Florida, Kentucky, Massachusetts, Minnesota, New Jersey, New York, and Virginia. Sixty percent or fewer will be penalized in Colorado, Kansas, Idaho, Montana, Oregon, South Dakota, and Utah.
Since the readmission program’s structure is set by law, the administration cannot make major changes unilaterally, even if it wanted to.
Congress last year instructed Medicare to make one future alteration in response to complaints from safety-net hospitals and major academic medical centers.
They have objected that their patients tended to be lower income than other hospitals and were more likely to return to the hospital, sometimes because they didn’t have a primary care doctor and other times because they could not afford the right medication or diet. Those hospitals argued that this was a disadvantage for them since Medicare bases its readmission targets on industry-wide trends and that it hurt them financially, depriving them of resources they could use to help those same patients.
Dr. Bernheim noted that, despite those complaints, safety-net hospitals have shown some of the greatest drops in readmission rates. In October 2018, Medicare will begin basing the penalties on how hospitals compared with their peer groups with similar numbers of poor patients. Akin Demehin, director of policy at the hospital association, said, “We expect the adjustment will provide some relief for safety-net hospitals.”
Medicare is planning to release two other rounds of recurring quality incentives for hospitals later this year. One gives out bonuses and penalties based on a mix of measures, with Medicare redistributing $1.9 billion based on how hospitals perform and improve. The other, the Hospital-Acquired Condition Reduction Program, cuts payments to roughly 750 hospitals with the highest rates of infections and other patient injuries by 1%.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation. KHN’s coverage related to aging & improving care of older adults is supported by The John A. Hartford Foundation.
Amid all the turbulence over the future of the Affordable Care Act, one facet continues unchanged: President Trump’s administration is penalizing more than half the nation’s hospitals for having too many patients return within a month.
Medicare is punishing 2,573 hospitals, just two dozen short of what it did last year under President Obama, according to federal records released Aug. 2. Starting in October, the federal government will cut those hospitals’ payments by as much as 3% for a year.
Medicare docked all but 174 of those hospitals last year as well. The $564 million the government projects to save also is roughly the same as it was last year under Obama.
High rates of readmissions have been a safety concern for decades, with one in five Medicare patients historically ending up back in the hospital within 30 days. In 2011, 3.3 million adults returned to the hospital, running up medical costs estimated at $41 billion, according to the Agency for Healthcare Research and Quality.
The penalties, which begin their sixth year in October, have coincided with a nationwide decrease in hospital repeat patients. Between 2007 and 2015, the frequency of readmissions for conditions targeted by Medicare dropped from 21.5% to 17.8%, with the majority of the decrease occurring shortly after the ACA passed in 2010, according to a study conducted by Obama administration health policy experts and published in 2016 in the New England Journal of Medicine.
Some hospitals began giving impoverished patients free medications that they prescribed for their recovery, while others sent nurses to check up on patients seen as most likely to relapse in their homes. Readmissions dropped more quickly at hospitals potentially subject to the penalty than at other hospitals, another study found.
“The sum of the evidence really suggests that this program is helping people,” said Susannah Bernheim, MD, the director of quality measurement at the Yale/Yale-New Haven Hospital Center for Outcomes Research and Evaluation.
But the pace of these reductions has been leveling off in the past few years, indicating that the penalties’ ability to induce improvements may be waning.
“Presumably, hospitals made substantial changes during the implementation period but could not sustain such a high rate of reductions in the long term,” the New England Journal article said.
An analysis by Dr. Bernheim’s group found no decrease in the overall rate of readmissions between 2012 and 2015, although small drops in the medical conditions targeted by the penalties continued.
“We have indeed reached the limits of what changes in how we deliver care will allow us to do,” said Nancy Foster, vice president for quality at the American Hospital Association. “We can’t prevent every readmission. It could be that there is further room for improvement, but we just don’t know what the technique is to make that happen.”
The Hospital Readmissions Reduction Program was designed to use the purchasing power of Medicare to reward hospitals for higher quality. Those penalties, along with other ones aimed at improving hospital care, have been spared the partisan rancor over the law, and they would have continued under the GOP repeal proposals that stalled in Congress. But they have also been largely ignored.
Ashish Jha, MD, a professor at the Harvard T.H. Chan School of Public Health, Boston, said the fight over abolishing the ACA has drowned out talk about how to make the health care system more effective.
“We’ve spent the last 6 months fighting about how we’re going to pay for health insurance, which is one part of the ACA,” he said. “There’s been almost no discussion of the underlying health care delivery system changes that the ACA ushered in, and that is more important in the long run to be discussing because that’s what’s going to determine the underlying costs and outcomes of the health system.”
The readmission penalties are intended to neutralize an unintended incentive in the way Medicare pays hospitals that had profited from return patients. Medicare pays hospitals a lump sum for a patient’s stay based on the nature of the admission and other factors. Since hospitals generally are not paid extra if patients remain longer, they seek to discharge patients as soon as is medically feasible. If the patient ends up back in the hospital, it becomes a financial benefit as the hospital is paid for that second stay, filling a bed that would not have generated income if the patient had remained there continuously.
Because of the way the readmission penalty program was designed, it is not surprising that the new results are so similar to last year’s. As before, Medicare determined the penalties based on readmissions of the same six types of patients: those admitted for heart attacks, heart failure, pneumonia, chronic lung disease, hip or knee replacements, or coronary artery bypass graft surgery. Hospitals were judged on patients discharged between July 2013 and June 2016. Because the government looks at a 3-year period, 2 of those years were also examined in determining last year’s penalties.
This year, the average penalty will be 0.73% of each payment Medicare makes for a patient between Oct. 1 and Sept. 30, 2018, according to a Kaiser Health News analysis. That too was practically the same as last year. Forty-eight hospitals received the maximum punishment of a 3% reduction. Medicare did not release hospital-specific estimates for how much lost money these penalties would translate to.
More than 1,500 hospitals treating veterans, children, and psychiatric patients were exempted from penalties this year as required by law. Critical access hospitals, which Medicare also pays differently, also were excluded. So were Maryland hospitals because Congress has given that state extra leeway in how it distributes Medicare money.
Of the 3,241 hospitals whose readmissions were evaluated, Medicare penalized four out of five, the KHN analysis found. That is because the program’s methods are not very forgiving: A hospital can be penalized even if it has higher than expected readmission rates for only one of the six conditions that are targeted. Every nonexcluded hospital in Delaware and West Virginia will have their reimbursements reduced. Ninety percent or more will be punished in Arizona, Connecticut, Florida, Kentucky, Massachusetts, Minnesota, New Jersey, New York, and Virginia. Sixty percent or fewer will be penalized in Colorado, Kansas, Idaho, Montana, Oregon, South Dakota, and Utah.
Since the readmission program’s structure is set by law, the administration cannot make major changes unilaterally, even if it wanted to.
Congress last year instructed Medicare to make one future alteration in response to complaints from safety-net hospitals and major academic medical centers.
They have objected that their patients tended to be lower income than other hospitals and were more likely to return to the hospital, sometimes because they didn’t have a primary care doctor and other times because they could not afford the right medication or diet. Those hospitals argued that this was a disadvantage for them since Medicare bases its readmission targets on industry-wide trends and that it hurt them financially, depriving them of resources they could use to help those same patients.
Dr. Bernheim noted that, despite those complaints, safety-net hospitals have shown some of the greatest drops in readmission rates. In October 2018, Medicare will begin basing the penalties on how hospitals compared with their peer groups with similar numbers of poor patients. Akin Demehin, director of policy at the hospital association, said, “We expect the adjustment will provide some relief for safety-net hospitals.”
Medicare is planning to release two other rounds of recurring quality incentives for hospitals later this year. One gives out bonuses and penalties based on a mix of measures, with Medicare redistributing $1.9 billion based on how hospitals perform and improve. The other, the Hospital-Acquired Condition Reduction Program, cuts payments to roughly 750 hospitals with the highest rates of infections and other patient injuries by 1%.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation. KHN’s coverage related to aging & improving care of older adults is supported by The John A. Hartford Foundation.
Amid all the turbulence over the future of the Affordable Care Act, one facet continues unchanged: President Trump’s administration is penalizing more than half the nation’s hospitals for having too many patients return within a month.
Medicare is punishing 2,573 hospitals, just two dozen short of what it did last year under President Obama, according to federal records released Aug. 2. Starting in October, the federal government will cut those hospitals’ payments by as much as 3% for a year.
Medicare docked all but 174 of those hospitals last year as well. The $564 million the government projects to save also is roughly the same as it was last year under Obama.
High rates of readmissions have been a safety concern for decades, with one in five Medicare patients historically ending up back in the hospital within 30 days. In 2011, 3.3 million adults returned to the hospital, running up medical costs estimated at $41 billion, according to the Agency for Healthcare Research and Quality.
The penalties, which begin their sixth year in October, have coincided with a nationwide decrease in hospital repeat patients. Between 2007 and 2015, the frequency of readmissions for conditions targeted by Medicare dropped from 21.5% to 17.8%, with the majority of the decrease occurring shortly after the ACA passed in 2010, according to a study conducted by Obama administration health policy experts and published in 2016 in the New England Journal of Medicine.
Some hospitals began giving impoverished patients free medications that they prescribed for their recovery, while others sent nurses to check up on patients seen as most likely to relapse in their homes. Readmissions dropped more quickly at hospitals potentially subject to the penalty than at other hospitals, another study found.
“The sum of the evidence really suggests that this program is helping people,” said Susannah Bernheim, MD, the director of quality measurement at the Yale/Yale-New Haven Hospital Center for Outcomes Research and Evaluation.
But the pace of these reductions has been leveling off in the past few years, indicating that the penalties’ ability to induce improvements may be waning.
“Presumably, hospitals made substantial changes during the implementation period but could not sustain such a high rate of reductions in the long term,” the New England Journal article said.
An analysis by Dr. Bernheim’s group found no decrease in the overall rate of readmissions between 2012 and 2015, although small drops in the medical conditions targeted by the penalties continued.
“We have indeed reached the limits of what changes in how we deliver care will allow us to do,” said Nancy Foster, vice president for quality at the American Hospital Association. “We can’t prevent every readmission. It could be that there is further room for improvement, but we just don’t know what the technique is to make that happen.”
The Hospital Readmissions Reduction Program was designed to use the purchasing power of Medicare to reward hospitals for higher quality. Those penalties, along with other ones aimed at improving hospital care, have been spared the partisan rancor over the law, and they would have continued under the GOP repeal proposals that stalled in Congress. But they have also been largely ignored.
Ashish Jha, MD, a professor at the Harvard T.H. Chan School of Public Health, Boston, said the fight over abolishing the ACA has drowned out talk about how to make the health care system more effective.
“We’ve spent the last 6 months fighting about how we’re going to pay for health insurance, which is one part of the ACA,” he said. “There’s been almost no discussion of the underlying health care delivery system changes that the ACA ushered in, and that is more important in the long run to be discussing because that’s what’s going to determine the underlying costs and outcomes of the health system.”
The readmission penalties are intended to neutralize an unintended incentive in the way Medicare pays hospitals that had profited from return patients. Medicare pays hospitals a lump sum for a patient’s stay based on the nature of the admission and other factors. Since hospitals generally are not paid extra if patients remain longer, they seek to discharge patients as soon as is medically feasible. If the patient ends up back in the hospital, it becomes a financial benefit as the hospital is paid for that second stay, filling a bed that would not have generated income if the patient had remained there continuously.
Because of the way the readmission penalty program was designed, it is not surprising that the new results are so similar to last year’s. As before, Medicare determined the penalties based on readmissions of the same six types of patients: those admitted for heart attacks, heart failure, pneumonia, chronic lung disease, hip or knee replacements, or coronary artery bypass graft surgery. Hospitals were judged on patients discharged between July 2013 and June 2016. Because the government looks at a 3-year period, 2 of those years were also examined in determining last year’s penalties.
This year, the average penalty will be 0.73% of each payment Medicare makes for a patient between Oct. 1 and Sept. 30, 2018, according to a Kaiser Health News analysis. That too was practically the same as last year. Forty-eight hospitals received the maximum punishment of a 3% reduction. Medicare did not release hospital-specific estimates for how much lost money these penalties would translate to.
More than 1,500 hospitals treating veterans, children, and psychiatric patients were exempted from penalties this year as required by law. Critical access hospitals, which Medicare also pays differently, also were excluded. So were Maryland hospitals because Congress has given that state extra leeway in how it distributes Medicare money.
Of the 3,241 hospitals whose readmissions were evaluated, Medicare penalized four out of five, the KHN analysis found. That is because the program’s methods are not very forgiving: A hospital can be penalized even if it has higher than expected readmission rates for only one of the six conditions that are targeted. Every nonexcluded hospital in Delaware and West Virginia will have their reimbursements reduced. Ninety percent or more will be punished in Arizona, Connecticut, Florida, Kentucky, Massachusetts, Minnesota, New Jersey, New York, and Virginia. Sixty percent or fewer will be penalized in Colorado, Kansas, Idaho, Montana, Oregon, South Dakota, and Utah.
Since the readmission program’s structure is set by law, the administration cannot make major changes unilaterally, even if it wanted to.
Congress last year instructed Medicare to make one future alteration in response to complaints from safety-net hospitals and major academic medical centers.
They have objected that their patients tended to be lower income than other hospitals and were more likely to return to the hospital, sometimes because they didn’t have a primary care doctor and other times because they could not afford the right medication or diet. Those hospitals argued that this was a disadvantage for them since Medicare bases its readmission targets on industry-wide trends and that it hurt them financially, depriving them of resources they could use to help those same patients.
Dr. Bernheim noted that, despite those complaints, safety-net hospitals have shown some of the greatest drops in readmission rates. In October 2018, Medicare will begin basing the penalties on how hospitals compared with their peer groups with similar numbers of poor patients. Akin Demehin, director of policy at the hospital association, said, “We expect the adjustment will provide some relief for safety-net hospitals.”
Medicare is planning to release two other rounds of recurring quality incentives for hospitals later this year. One gives out bonuses and penalties based on a mix of measures, with Medicare redistributing $1.9 billion based on how hospitals perform and improve. The other, the Hospital-Acquired Condition Reduction Program, cuts payments to roughly 750 hospitals with the highest rates of infections and other patient injuries by 1%.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation. KHN’s coverage related to aging & improving care of older adults is supported by The John A. Hartford Foundation.