User login
Morbo Serpentino
A 58-year-old Danish man presented to an urgent care center due to several months of gradually worsening fatigue, weight loss, abdominal pain, and changes in vision . His abdominal pain was diffuse, constant, and moderate in severity. There was no association with meals, and he reported no nausea, vomiting, or change in bowel movements. He also said his vision in both eyes was blurry, but denied diplopia and said the blurring did not improve when either eye w as closed. He denied dysphagia, headache, focal weakness, or sensitivity to bright lights.
Fatigue and weight loss in a middle-aged man are nonspecific complaints that mainly help to alert the clinician that there may be a serious, systemic process lurking. Constant abdominal pain without nausea, vomiting, or change in bowel movements makes intestinal obstruction or a motility disorder less likely. Given that the pain is diffuse, it raises the possibility of an intraperitoneal process or a process within an organ that is irritating the peritoneum.
Worsening of vision can result from disorders anywhere along the visual pathway, including the cornea (keratitis or corneal edema from glaucoma), anterior chamber (uveitis or hyphema), lens (cataracts, dislocations, hyperglycemia), vitreous humor (uveitis), retina (infections, ischemia, detachment, diabetic retinopathy), macula (degenerative disease), optic nerve (optic neuritis), optic chiasm, and the visual projections through the hemispheres to the occipital lobes. To narrow the differential diagnosis, it would be important to inquire about prior eye problems, to measure visual acuity and intraocular pressure, to perform fundoscopic and slit-lamp exams to detect retinal and anterior chamber disorders, respectively, and to assess visual fields. An afferent pupillary defect would suggest optic nerve pathology.
Disorders that could unify the constitutional, abdominal, and visual symptoms include systemic inflammatory diseases, such as sarcoidosis (which has an increased incidence among Northern Europeans), tuberculosis, or cancer. While diabetes mellitus could explain his visual problems, weight loss, and fatigue, the absence of polyuria, polydipsia, or polyphagia argues against this possibility.
The patient had hypercholesterolemia and type 2 diabetes mellitus. Medications were metformin, atorvastatin, and glimepiride. He was a former smoker with 23 pack-years and had quit over 5 years prior. He had not traveled outside of Denmark in 2 years and had no pets at home. He reported being monogamous with his same-sex partner for the past 25 years. He had no significant family history, and he worked at a local hospital as a nurse. He denied any previous ocular history.
On examination, the pulse was 67 beats per minute, temperature was 36.7 degrees Celsius, respiratory rate was 16 breaths per minute, oxygen saturation was 99% while breathing ambient air, and blood pressure was 132/78. Oropharynx demonstrated no thrush or other lesions. The heart rhythm was regular and there were no murmurs. Lungs were clear to auscultation bilaterally. Abdominal exam was normal except for mild tenderness upon palpation in all quadrants, but no masses, organomegaly, rigidity, or rebound tenderness were present. Skin examination revealed several subcutaneous nodules measuring up to 0.5 cm in diameter overlying the right and left posterolateral chest walls. T he nodules were rubbery, pink, nontender, and not warm nor fluctuant. Visual acuity was reduced in both eyes. Extraocular movements were intact, and the pupils reacted to light and accommodated appropriately. The sclerae were injected bilaterally. The remainder of the cranial nerves and neurologic exam were normal. Due to the vision loss , the patient was referred to an ophthalmologist who diagnosed bilateral anterior uveitis.
Though monogamous with his male partner for many years, it is mandatory to consider complications of human immunodeficiency virus infection (HIV ). The absence of oral lesions indicative of a low CD4 count, such as oral hairy leukoplakia or thrush, does not rule out HIV disease. Additional history about his work as a nurse might shed light on his risk of infection, such as airborne exposure to tuberculosis or acquisition of blood-borne pathogens through a needle stick injury. His unremarkable vital signs support the chronicity of his medical condition.
Uveitis can result from numerous causes. When confined to the eye, uncommon hereditary and acquired causes are less likely . In many patients, uveitis arises in the setting of systemic infection or inflammation. The numerous infectious causes of uveitis include syphilis, tuberculosis, toxoplasmosis, cat scratch disease, and viruses such as HIV, West Nile, and Ebola. Among the inflammatory diseases that can cause uveitis are sarcoidosis, inflammatory bowel disease, systemic lupus erythematosus, Behçet disease, and Sjogren syndrome.
Several of these conditions, including tuberculosis and syphilis, may also cause subcutaneous nodules. Both tuberculosis and syphilis can cause skin and gastrointestinal disease. Sarcoidosis could involve the skin, peritoneum, and uvea, and is a possibility in this patient. The dermatologic conditions associated with sarcoidosis are protean and include granulomatous inflammation and nongranulomatous processes such as erythema nodosum. Usually the nodules of erythema nodosum are tender, red or purple, and located on the lower extremities. The lack of tenderness points away from erythema nodosum in this patient. Metastatic cancer can disseminate to the subcutaneous tissue, and the patient’s smoking history and age mandate we consider malignancy. However, skin metastases tend to be hard, not rubbery.
A cost-effective evaluation at this point would include syphilis serologies, HIV testing, testing for tuberculosis with either a purified protein derivative test or interferon gamma release assay, chest radiography, and biopsy of 1 of the lesions on his back.
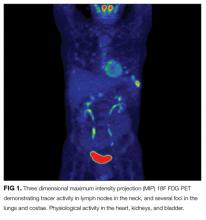
Laboratory data showed 12,400 white blood cells per cubic milliliter (64% neutrophils, 24% lymphocytes, 9% monocytes, 2% eosinophils, 1% basophils), hemoglobin 7.9 g/dL, mean corpuscular volume 85 fL, platelets 476,000 per cubic milliliter , C-reactive protein 43 mg/ d L (normal < 8 mg/L), gamma-glutamyl-transferase 554 IU/L (normal range 0-45), alkaline phosphatase 865 U/L (normal range 60-200), and erythrocyte sedimentation rate (ESR) 71 mm per hour. International normalized ratio was 1.0, albumin was 3.0 mg/dL, activated partial thromboplastin time was 32 seconds (normal 22 to 35 seconds), and bilirubin was 0.3 mg/dL. Antibodies to HIV , hepatitis C, and hepatitis B surface antigen were not detectable. Electrocardiography ( ECG ) was normal. Plain radiograph of the chest demonstrated multiple nodular lesions bilaterally measuring up to 1 cm with no cavitation. There was a left pleural effusion.
The history and exam findings indicate a serious inflammatory condition affecting his lungs, pleura, eyes, skin, liver, and possibly his peritoneum. In this context, the elevated C-reactive protein and ESR are not helpful in differentiating inflammatory from infectious causes. The constellation of uveitis, pulmonary and cutaneous nodules, and marked abnormalities of liver tests in a middle-aged man of Northern European origin points us toward sarcoidosis. Pleural effusions are not common with sarcoidosis but may occur. However, to avoid premature closure, it is important to consider other possibilities.
Metastatic cancer, including lymphoma, could cause pulmonary and cutaneous nodules and liver involvement, but the chronic time course and uveitis are not consistent with malignancy. Tuberculosis is still a consideration, though one would have expected him to report fevers, night sweats, and, perhaps, exposure to patients with pulmonary tuberculosis in his job as a nurse. Multiple solid pulmonary nodules are also uncommon with pulmonary tuberculosis. Fungal infections such as histoplasmosis can cause skin lesions and pulmonary nodules but do not fit well with uveitis.
At this point, “ tissue is the issue.” A skin nodule would be the easiest site to biopsy. If skin biopsy was not diagnostic, computed tomography (CT) of his chest and abdomen should be performed to identify the next most accessible site for biopsy.
Esophagogastroduodenoscopy (EGD) and colonoscopy showed normal findings, and random biopsies from the stomach and colon were normal. CT of the chest, abdomen, and pelvis performed with the administration of intravenous contrast showed multiple solid opacities in both lung fields up to 1 cm, with enlarged mediastinal and retroperitoneal lymph nodes measuring 1 to 3 cm in diameter, a left pleural effusion, wall thickening in the right colon, and several nonspecific hypodensities in the liver. A punch biopsy taken from the right chest wall lesion demonstrated chronic inflammation without granulomas. The patient underwent CT-guided biopsy of 1 of the right-sided lung nodules, which revealed noncaseating granulomatous inflammation, fibrosis, and necrosis. Neither biopsy contained malignant cells, and additional stains revealed no bacteria, fungi, or acid fast bacilli.
The retroperitoneal and mediastinal adenopathy are indicative of a widely disseminated inflammatory process. Lymphoma continues to be a concern, though uveitis as an initial presenting problem would very unusual. Although biopsy of the chest wall lesion failed to demonstrate granulomatous inflammation, the most parsimonious explanation is that the skin and lung nodules are both related to a single systemic process.
Granulomas form in an attempt to wall off offending agents, whether foreign antigens (talc, certain medications), infectious agents, or self-antigens. Review of histopathology and microbiologic studies are useful first steps. Stains for bacteria, fungi, or acid-fast organisms may diagnose an infectious cause, such as tuberculosis, leprosy, syphilis, fungi, or cat scratch disease. Granulomas in association with vascular inflammation would indicate vasculitis. Other autoimmune considerations include sarcoidosis and Crohn disease. Noncaseating granulomas are typically found in sarcoidosis, cat scratch disease, syphilis, leprosy, or Crohn disease, but do not entirely exclude tuberculosis.
The negative infectious studies and lack of classic features of Crohn disease or other autoimmune diseases further point to sarcoidosis as the etiology of this patient’s illness. A Norwegian dermatologist first described the pathology of sarcoidosis based upon specimens taken from skin nodules. He thought the lesions were sarcoma and described them as, “ multiple benign sarcoid of the skin,” which is where the name “ sarcoidosis” originated.
Diagnosing sarcoidosis requires excluding other mimickers. Additional testing should include syphilis serologies, rheumatoid factor, and antineutrophilic cytoplasmic antibodies. The latter is associated with granulomatosis with polyangiitis and eosinophilic granulomatosis with polyangiitis, either of which may produce granulomatous inflammation of the lungs, skin, and uvea.
At this juncture, PET-CT represents a costly and unnecessary test that does not narrow our diagnostic possibilities sufficiently to justify its use. Osteolytic lesions would be unusual in sarcoidosis and more likely in lymphoma or infectious processes such as tuberculosis. Tests for syphilis and tuberculosis are required, and are a fraction of the cost of a PET-CT.
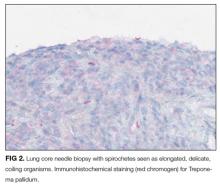
With the biopsy revealing spirochetes, and the positive results of a nontreponemal test (RPR) and confirmatory treponemal results, the diagnosis of syphilis is firmly established. Uveitis indicates neurosyphilis and warrants a longer course of intravenous penicillin. Lumbar puncture should be performed.
A lumbar puncture was performed. Cerebrospinal fluid (CSF) contained 9 white blood cells and 73 red blood cells per cubic milliliter; protein concentration was 73 mg/dL, and glucose was 116 mg/dL. Polymerase chain reaction for T. pallidum was negative. Transthoracic ECG and magnetic resonance imaging of the brain were normal. The patient was treated with intravenous penicillin G at 5 million units 4 times daily for 15 days. A PET-CT scan 3 months later revealed complete resolution of the subcutaneous, pulmonary, liver lesions, lymphadenopathy, and uveitis. Repeat treponemal serologies demonstrated a greater than 4-fold decline in titers.
DISCUSSION
Syphilis is a sexually transmitted disease with increasing incidence worldwide. Untreated infection progresses through 3 stages. The primary stage is characterized by the appearance of a painless chancre after an incubation period of 2 to 3 weeks. Four to 8 weeks later, the secondary stage emerges as a systemic infection, often heralded by a maculopapular rash with desquamation, frequently involving the soles and palms. Hepatitis, iridocyclitis, and early neurosyphilis may also be seen at this stage. Subsequently, syphilis becomes latent. One-third of patients with untreated latent syphilis will develop tertiary syphilis, typified by late neurosyphilis (tabes dorsalis and general paresis), cardiovascular disease (aortitis), or gummatous disease. 1
Gummas are destructive granulomatous lesions that typically present indolently, may occur singly or multiply, and may involve almost any organ. It has been suggested that gummas are the immune system’s defense to slow the bacteria after attempts to kill it have failed. Histologically, gummas are hyalinized nodules with surrounding granulomatous infiltrate of lymphocytes, plasma cells, and multinucleated giant cells with or without necrosis . In the preantibiotic era, gummas were seen in approximately 15% of infected patients, with a latency of 1 to 46 years after primary infection. 2 Penicillin led to a drastic reduction in gummas until the HIV epidemic, which led to the resurgence of gummas at a drastically shortened interval following primary syphilis. 3
Most commonly, gummas affect the skin and bones. In the skin , lesions may be superficial or deep and may progress into ulcerative nodules. In the bones, destructive gummas have a characteristic “moth-eaten” appearance. Less common sequelae of gummas incude gummatous hepatitis, perforated nasal septum (saddle nose deformity), or hard palate erosions. 2,4 R arely, syphilis involves the lungs, appearing as nodules, infiltrates, or pleural effusion. 5
Ocular manifestations occur in approximately 5% of patients with syphilis, more often in secondary and tertiary stages, and are strongly associated with a spread to the central nervous system. Syphilis may affect any structure of the eye, with anterior uveitis as the most frequent manifestation. Partial or complete vision loss is identified in approximately half of the patients with ocular syphilis and may be completely reversed by appropriate treatment. Ophthalmologic findings such as optic neuritis and papilledema imply advanced illness , as do Argyll-Robertson pupils (small pupils that are poorly reactive to light , but with preserved accommodation and convergence). 6,7 The treatment of ocular syphilis is identical to that of neurosyphilis. The Centers for Disease Control and Prevention recommends CSF analysis in any patient with ocular syphilis. Abnormal results should prompt repeat lumbar puncture every 3 to 6 months following treatment until the CSF results normalize. 8
The diagnosis of syphilis relies on indirect serologic tests. T. pallidum cannot be cultured in vitro, and techniques to identify spirochetes directly by using darkfield microscopy or DNA amplification via polymerase chain reaction are limited by availability or by poor sensitivity in advanced syphilis. 1 Imaging modalities including PET cannot reliably differentiate syphilis from other infectious and noninfectious mimickers. 9 F ortunately, syphilis infection can be diagnosed accurately based on reactive treponemal and nontreponemal serum tests. Nontreponemal tests, such as the RPR and Venereal Disease Research Laboratory, have traditionally been utilized as first-line evaluation, followed by a confirmatory treponemal test. However, nontreponemal tests may be nonreactive in a few settings: very early or very late in infection, and in individuals previously treated for syphilis. Thus, newer “reverse testing” algorithms utilize more sensitive and less expensive treponemal tests as the first test, followed by nontreponemal tests if the initial treponemal test is reactive. 8 Regardless of the testing sequence, in patients with no prior history of syphilis, reactive results on both treponemal and nontreponemal assays firmly establish a diagnosis of syphilis, obviating the need for more invasive and costly testing.
In patients with unexplained systemic illness, clinicians should have a low threshold to test for syphilis. Testing should be extended to certain asymptomatic individuals at higher risk of infection, including men who have sex with men, sexual partners of patients infected with syphilis, individuals with HIV or sexually-transmitted diseases, and others with high-risk sexual behavior or a history of sexually-transmitted diseases. 8 As the discussant points out, earlier consideration of and testing for syphilis would have spared the patient from unnecessary and costly EGD, colonoscopy, PET-CT scanning, and 3 biopsies.
Syphilis has been known to be a horribly destructive disease for centuries, earning the moniker “morbo serpentino” (serpentine disease) from the Spanish physician Ruiz Diaz de Isla in the 1500s. 10 In the modern era, physicians must remember to consider the diagnosis of syphilis in order to effectively mitigate the harm from this resurgent disease when it attacks our patients.
TEACHING POINTS
- Syphilis, the great imposter, is rising in incidence and should be on the differential diagnosis in all patients with unexplained multisystem inflammatory disease.
- A cost-effective diagnostic approach to syphilis entails serologic testing with treponemal and nontreponemal assays.
- Unexplained granulomas, especially in the skin, bone, or liver, should prompt consideration of gummatous syphilis.
- Ocular syphilis may involve any part of the visual tract and is treated the same as neurosyphilis.
Disclosure
Dr. Weinreich has received payment for lectures from Boehringer er Ingelheim, Astra Zeneca, TEVA and Novartis in 2016. All other contributors have nothing to report.
1. French P. Syphilis. BMJ. 2007;334:143-147. PubMed
2. Singh AE, Romanowski B. Syphilis: Review with emphasis on clinical, epidemiologic, and some biologic features. Clin Micriobio Rev. 1999;12(2):187-209. PubMed
3. Karp G, Schlaeffer F, Jotkowitz A, Riesenberg K. Syphilis and HIV co-infection. Eur J Int Med. 2009; 20:9-13. PubMed
4. Pilozzi-Edmonds L, Kong LY, Szabo J, Birnbaum LM. Rapid progression to gummatous syphilitic hepatitis and neurosyphilis in a patient with newly diagnosed HIV. Int J STD AIDS. 2014;26(13)985-987. PubMed
5. David G, Perpoint T, Boibieux A, et al. Secondary pulmonary syphilis: report of a likely case and literature review. Clin Infect Dis. 2006;42(3):e11-e15. PubMed
6. Moradi A, Salek S, Daniel E, et al. Clinical features and incidence rates of ocular complications in patients with ocular syphilis. Am J Ophthalmol. 2015;159:334-343. PubMed
7. Aldave AJ, King JA, Cunningham ET Jr. Ocular syphilis. Curr Opin Ophthalmol. 2001;12:433-441. PubMed
8. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137. PubMed
9. Lin M, Darwish B, Chu J. Neurosyphilitic gumma on F18-2-fluoro-2-deoxy-D-glucose (FDG) positron emission tomography: An old disease investigated with new technology. J Clin Neurosc. 2009;16:410-412. PubMed
10. de Ricon‐Ferraz A. Early work on syphilis: Diaz de Ysla’s treatise on the serpentine disease of Hispaniola Island. Int J Dermatol. 1999;38(3):222-227. PubMed
A 58-year-old Danish man presented to an urgent care center due to several months of gradually worsening fatigue, weight loss, abdominal pain, and changes in vision . His abdominal pain was diffuse, constant, and moderate in severity. There was no association with meals, and he reported no nausea, vomiting, or change in bowel movements. He also said his vision in both eyes was blurry, but denied diplopia and said the blurring did not improve when either eye w as closed. He denied dysphagia, headache, focal weakness, or sensitivity to bright lights.
Fatigue and weight loss in a middle-aged man are nonspecific complaints that mainly help to alert the clinician that there may be a serious, systemic process lurking. Constant abdominal pain without nausea, vomiting, or change in bowel movements makes intestinal obstruction or a motility disorder less likely. Given that the pain is diffuse, it raises the possibility of an intraperitoneal process or a process within an organ that is irritating the peritoneum.
Worsening of vision can result from disorders anywhere along the visual pathway, including the cornea (keratitis or corneal edema from glaucoma), anterior chamber (uveitis or hyphema), lens (cataracts, dislocations, hyperglycemia), vitreous humor (uveitis), retina (infections, ischemia, detachment, diabetic retinopathy), macula (degenerative disease), optic nerve (optic neuritis), optic chiasm, and the visual projections through the hemispheres to the occipital lobes. To narrow the differential diagnosis, it would be important to inquire about prior eye problems, to measure visual acuity and intraocular pressure, to perform fundoscopic and slit-lamp exams to detect retinal and anterior chamber disorders, respectively, and to assess visual fields. An afferent pupillary defect would suggest optic nerve pathology.
Disorders that could unify the constitutional, abdominal, and visual symptoms include systemic inflammatory diseases, such as sarcoidosis (which has an increased incidence among Northern Europeans), tuberculosis, or cancer. While diabetes mellitus could explain his visual problems, weight loss, and fatigue, the absence of polyuria, polydipsia, or polyphagia argues against this possibility.
The patient had hypercholesterolemia and type 2 diabetes mellitus. Medications were metformin, atorvastatin, and glimepiride. He was a former smoker with 23 pack-years and had quit over 5 years prior. He had not traveled outside of Denmark in 2 years and had no pets at home. He reported being monogamous with his same-sex partner for the past 25 years. He had no significant family history, and he worked at a local hospital as a nurse. He denied any previous ocular history.
On examination, the pulse was 67 beats per minute, temperature was 36.7 degrees Celsius, respiratory rate was 16 breaths per minute, oxygen saturation was 99% while breathing ambient air, and blood pressure was 132/78. Oropharynx demonstrated no thrush or other lesions. The heart rhythm was regular and there were no murmurs. Lungs were clear to auscultation bilaterally. Abdominal exam was normal except for mild tenderness upon palpation in all quadrants, but no masses, organomegaly, rigidity, or rebound tenderness were present. Skin examination revealed several subcutaneous nodules measuring up to 0.5 cm in diameter overlying the right and left posterolateral chest walls. T he nodules were rubbery, pink, nontender, and not warm nor fluctuant. Visual acuity was reduced in both eyes. Extraocular movements were intact, and the pupils reacted to light and accommodated appropriately. The sclerae were injected bilaterally. The remainder of the cranial nerves and neurologic exam were normal. Due to the vision loss , the patient was referred to an ophthalmologist who diagnosed bilateral anterior uveitis.
Though monogamous with his male partner for many years, it is mandatory to consider complications of human immunodeficiency virus infection (HIV ). The absence of oral lesions indicative of a low CD4 count, such as oral hairy leukoplakia or thrush, does not rule out HIV disease. Additional history about his work as a nurse might shed light on his risk of infection, such as airborne exposure to tuberculosis or acquisition of blood-borne pathogens through a needle stick injury. His unremarkable vital signs support the chronicity of his medical condition.
Uveitis can result from numerous causes. When confined to the eye, uncommon hereditary and acquired causes are less likely . In many patients, uveitis arises in the setting of systemic infection or inflammation. The numerous infectious causes of uveitis include syphilis, tuberculosis, toxoplasmosis, cat scratch disease, and viruses such as HIV, West Nile, and Ebola. Among the inflammatory diseases that can cause uveitis are sarcoidosis, inflammatory bowel disease, systemic lupus erythematosus, Behçet disease, and Sjogren syndrome.
Several of these conditions, including tuberculosis and syphilis, may also cause subcutaneous nodules. Both tuberculosis and syphilis can cause skin and gastrointestinal disease. Sarcoidosis could involve the skin, peritoneum, and uvea, and is a possibility in this patient. The dermatologic conditions associated with sarcoidosis are protean and include granulomatous inflammation and nongranulomatous processes such as erythema nodosum. Usually the nodules of erythema nodosum are tender, red or purple, and located on the lower extremities. The lack of tenderness points away from erythema nodosum in this patient. Metastatic cancer can disseminate to the subcutaneous tissue, and the patient’s smoking history and age mandate we consider malignancy. However, skin metastases tend to be hard, not rubbery.
A cost-effective evaluation at this point would include syphilis serologies, HIV testing, testing for tuberculosis with either a purified protein derivative test or interferon gamma release assay, chest radiography, and biopsy of 1 of the lesions on his back.
Laboratory data showed 12,400 white blood cells per cubic milliliter (64% neutrophils, 24% lymphocytes, 9% monocytes, 2% eosinophils, 1% basophils), hemoglobin 7.9 g/dL, mean corpuscular volume 85 fL, platelets 476,000 per cubic milliliter , C-reactive protein 43 mg/ d L (normal < 8 mg/L), gamma-glutamyl-transferase 554 IU/L (normal range 0-45), alkaline phosphatase 865 U/L (normal range 60-200), and erythrocyte sedimentation rate (ESR) 71 mm per hour. International normalized ratio was 1.0, albumin was 3.0 mg/dL, activated partial thromboplastin time was 32 seconds (normal 22 to 35 seconds), and bilirubin was 0.3 mg/dL. Antibodies to HIV , hepatitis C, and hepatitis B surface antigen were not detectable. Electrocardiography ( ECG ) was normal. Plain radiograph of the chest demonstrated multiple nodular lesions bilaterally measuring up to 1 cm with no cavitation. There was a left pleural effusion.
The history and exam findings indicate a serious inflammatory condition affecting his lungs, pleura, eyes, skin, liver, and possibly his peritoneum. In this context, the elevated C-reactive protein and ESR are not helpful in differentiating inflammatory from infectious causes. The constellation of uveitis, pulmonary and cutaneous nodules, and marked abnormalities of liver tests in a middle-aged man of Northern European origin points us toward sarcoidosis. Pleural effusions are not common with sarcoidosis but may occur. However, to avoid premature closure, it is important to consider other possibilities.
Metastatic cancer, including lymphoma, could cause pulmonary and cutaneous nodules and liver involvement, but the chronic time course and uveitis are not consistent with malignancy. Tuberculosis is still a consideration, though one would have expected him to report fevers, night sweats, and, perhaps, exposure to patients with pulmonary tuberculosis in his job as a nurse. Multiple solid pulmonary nodules are also uncommon with pulmonary tuberculosis. Fungal infections such as histoplasmosis can cause skin lesions and pulmonary nodules but do not fit well with uveitis.
At this point, “ tissue is the issue.” A skin nodule would be the easiest site to biopsy. If skin biopsy was not diagnostic, computed tomography (CT) of his chest and abdomen should be performed to identify the next most accessible site for biopsy.
Esophagogastroduodenoscopy (EGD) and colonoscopy showed normal findings, and random biopsies from the stomach and colon were normal. CT of the chest, abdomen, and pelvis performed with the administration of intravenous contrast showed multiple solid opacities in both lung fields up to 1 cm, with enlarged mediastinal and retroperitoneal lymph nodes measuring 1 to 3 cm in diameter, a left pleural effusion, wall thickening in the right colon, and several nonspecific hypodensities in the liver. A punch biopsy taken from the right chest wall lesion demonstrated chronic inflammation without granulomas. The patient underwent CT-guided biopsy of 1 of the right-sided lung nodules, which revealed noncaseating granulomatous inflammation, fibrosis, and necrosis. Neither biopsy contained malignant cells, and additional stains revealed no bacteria, fungi, or acid fast bacilli.
The retroperitoneal and mediastinal adenopathy are indicative of a widely disseminated inflammatory process. Lymphoma continues to be a concern, though uveitis as an initial presenting problem would very unusual. Although biopsy of the chest wall lesion failed to demonstrate granulomatous inflammation, the most parsimonious explanation is that the skin and lung nodules are both related to a single systemic process.
Granulomas form in an attempt to wall off offending agents, whether foreign antigens (talc, certain medications), infectious agents, or self-antigens. Review of histopathology and microbiologic studies are useful first steps. Stains for bacteria, fungi, or acid-fast organisms may diagnose an infectious cause, such as tuberculosis, leprosy, syphilis, fungi, or cat scratch disease. Granulomas in association with vascular inflammation would indicate vasculitis. Other autoimmune considerations include sarcoidosis and Crohn disease. Noncaseating granulomas are typically found in sarcoidosis, cat scratch disease, syphilis, leprosy, or Crohn disease, but do not entirely exclude tuberculosis.
The negative infectious studies and lack of classic features of Crohn disease or other autoimmune diseases further point to sarcoidosis as the etiology of this patient’s illness. A Norwegian dermatologist first described the pathology of sarcoidosis based upon specimens taken from skin nodules. He thought the lesions were sarcoma and described them as, “ multiple benign sarcoid of the skin,” which is where the name “ sarcoidosis” originated.
Diagnosing sarcoidosis requires excluding other mimickers. Additional testing should include syphilis serologies, rheumatoid factor, and antineutrophilic cytoplasmic antibodies. The latter is associated with granulomatosis with polyangiitis and eosinophilic granulomatosis with polyangiitis, either of which may produce granulomatous inflammation of the lungs, skin, and uvea.
At this juncture, PET-CT represents a costly and unnecessary test that does not narrow our diagnostic possibilities sufficiently to justify its use. Osteolytic lesions would be unusual in sarcoidosis and more likely in lymphoma or infectious processes such as tuberculosis. Tests for syphilis and tuberculosis are required, and are a fraction of the cost of a PET-CT.
With the biopsy revealing spirochetes, and the positive results of a nontreponemal test (RPR) and confirmatory treponemal results, the diagnosis of syphilis is firmly established. Uveitis indicates neurosyphilis and warrants a longer course of intravenous penicillin. Lumbar puncture should be performed.
A lumbar puncture was performed. Cerebrospinal fluid (CSF) contained 9 white blood cells and 73 red blood cells per cubic milliliter; protein concentration was 73 mg/dL, and glucose was 116 mg/dL. Polymerase chain reaction for T. pallidum was negative. Transthoracic ECG and magnetic resonance imaging of the brain were normal. The patient was treated with intravenous penicillin G at 5 million units 4 times daily for 15 days. A PET-CT scan 3 months later revealed complete resolution of the subcutaneous, pulmonary, liver lesions, lymphadenopathy, and uveitis. Repeat treponemal serologies demonstrated a greater than 4-fold decline in titers.
DISCUSSION
Syphilis is a sexually transmitted disease with increasing incidence worldwide. Untreated infection progresses through 3 stages. The primary stage is characterized by the appearance of a painless chancre after an incubation period of 2 to 3 weeks. Four to 8 weeks later, the secondary stage emerges as a systemic infection, often heralded by a maculopapular rash with desquamation, frequently involving the soles and palms. Hepatitis, iridocyclitis, and early neurosyphilis may also be seen at this stage. Subsequently, syphilis becomes latent. One-third of patients with untreated latent syphilis will develop tertiary syphilis, typified by late neurosyphilis (tabes dorsalis and general paresis), cardiovascular disease (aortitis), or gummatous disease. 1
Gummas are destructive granulomatous lesions that typically present indolently, may occur singly or multiply, and may involve almost any organ. It has been suggested that gummas are the immune system’s defense to slow the bacteria after attempts to kill it have failed. Histologically, gummas are hyalinized nodules with surrounding granulomatous infiltrate of lymphocytes, plasma cells, and multinucleated giant cells with or without necrosis . In the preantibiotic era, gummas were seen in approximately 15% of infected patients, with a latency of 1 to 46 years after primary infection. 2 Penicillin led to a drastic reduction in gummas until the HIV epidemic, which led to the resurgence of gummas at a drastically shortened interval following primary syphilis. 3
Most commonly, gummas affect the skin and bones. In the skin , lesions may be superficial or deep and may progress into ulcerative nodules. In the bones, destructive gummas have a characteristic “moth-eaten” appearance. Less common sequelae of gummas incude gummatous hepatitis, perforated nasal septum (saddle nose deformity), or hard palate erosions. 2,4 R arely, syphilis involves the lungs, appearing as nodules, infiltrates, or pleural effusion. 5
Ocular manifestations occur in approximately 5% of patients with syphilis, more often in secondary and tertiary stages, and are strongly associated with a spread to the central nervous system. Syphilis may affect any structure of the eye, with anterior uveitis as the most frequent manifestation. Partial or complete vision loss is identified in approximately half of the patients with ocular syphilis and may be completely reversed by appropriate treatment. Ophthalmologic findings such as optic neuritis and papilledema imply advanced illness , as do Argyll-Robertson pupils (small pupils that are poorly reactive to light , but with preserved accommodation and convergence). 6,7 The treatment of ocular syphilis is identical to that of neurosyphilis. The Centers for Disease Control and Prevention recommends CSF analysis in any patient with ocular syphilis. Abnormal results should prompt repeat lumbar puncture every 3 to 6 months following treatment until the CSF results normalize. 8
The diagnosis of syphilis relies on indirect serologic tests. T. pallidum cannot be cultured in vitro, and techniques to identify spirochetes directly by using darkfield microscopy or DNA amplification via polymerase chain reaction are limited by availability or by poor sensitivity in advanced syphilis. 1 Imaging modalities including PET cannot reliably differentiate syphilis from other infectious and noninfectious mimickers. 9 F ortunately, syphilis infection can be diagnosed accurately based on reactive treponemal and nontreponemal serum tests. Nontreponemal tests, such as the RPR and Venereal Disease Research Laboratory, have traditionally been utilized as first-line evaluation, followed by a confirmatory treponemal test. However, nontreponemal tests may be nonreactive in a few settings: very early or very late in infection, and in individuals previously treated for syphilis. Thus, newer “reverse testing” algorithms utilize more sensitive and less expensive treponemal tests as the first test, followed by nontreponemal tests if the initial treponemal test is reactive. 8 Regardless of the testing sequence, in patients with no prior history of syphilis, reactive results on both treponemal and nontreponemal assays firmly establish a diagnosis of syphilis, obviating the need for more invasive and costly testing.
In patients with unexplained systemic illness, clinicians should have a low threshold to test for syphilis. Testing should be extended to certain asymptomatic individuals at higher risk of infection, including men who have sex with men, sexual partners of patients infected with syphilis, individuals with HIV or sexually-transmitted diseases, and others with high-risk sexual behavior or a history of sexually-transmitted diseases. 8 As the discussant points out, earlier consideration of and testing for syphilis would have spared the patient from unnecessary and costly EGD, colonoscopy, PET-CT scanning, and 3 biopsies.
Syphilis has been known to be a horribly destructive disease for centuries, earning the moniker “morbo serpentino” (serpentine disease) from the Spanish physician Ruiz Diaz de Isla in the 1500s. 10 In the modern era, physicians must remember to consider the diagnosis of syphilis in order to effectively mitigate the harm from this resurgent disease when it attacks our patients.
TEACHING POINTS
- Syphilis, the great imposter, is rising in incidence and should be on the differential diagnosis in all patients with unexplained multisystem inflammatory disease.
- A cost-effective diagnostic approach to syphilis entails serologic testing with treponemal and nontreponemal assays.
- Unexplained granulomas, especially in the skin, bone, or liver, should prompt consideration of gummatous syphilis.
- Ocular syphilis may involve any part of the visual tract and is treated the same as neurosyphilis.
Disclosure
Dr. Weinreich has received payment for lectures from Boehringer er Ingelheim, Astra Zeneca, TEVA and Novartis in 2016. All other contributors have nothing to report.
A 58-year-old Danish man presented to an urgent care center due to several months of gradually worsening fatigue, weight loss, abdominal pain, and changes in vision . His abdominal pain was diffuse, constant, and moderate in severity. There was no association with meals, and he reported no nausea, vomiting, or change in bowel movements. He also said his vision in both eyes was blurry, but denied diplopia and said the blurring did not improve when either eye w as closed. He denied dysphagia, headache, focal weakness, or sensitivity to bright lights.
Fatigue and weight loss in a middle-aged man are nonspecific complaints that mainly help to alert the clinician that there may be a serious, systemic process lurking. Constant abdominal pain without nausea, vomiting, or change in bowel movements makes intestinal obstruction or a motility disorder less likely. Given that the pain is diffuse, it raises the possibility of an intraperitoneal process or a process within an organ that is irritating the peritoneum.
Worsening of vision can result from disorders anywhere along the visual pathway, including the cornea (keratitis or corneal edema from glaucoma), anterior chamber (uveitis or hyphema), lens (cataracts, dislocations, hyperglycemia), vitreous humor (uveitis), retina (infections, ischemia, detachment, diabetic retinopathy), macula (degenerative disease), optic nerve (optic neuritis), optic chiasm, and the visual projections through the hemispheres to the occipital lobes. To narrow the differential diagnosis, it would be important to inquire about prior eye problems, to measure visual acuity and intraocular pressure, to perform fundoscopic and slit-lamp exams to detect retinal and anterior chamber disorders, respectively, and to assess visual fields. An afferent pupillary defect would suggest optic nerve pathology.
Disorders that could unify the constitutional, abdominal, and visual symptoms include systemic inflammatory diseases, such as sarcoidosis (which has an increased incidence among Northern Europeans), tuberculosis, or cancer. While diabetes mellitus could explain his visual problems, weight loss, and fatigue, the absence of polyuria, polydipsia, or polyphagia argues against this possibility.
The patient had hypercholesterolemia and type 2 diabetes mellitus. Medications were metformin, atorvastatin, and glimepiride. He was a former smoker with 23 pack-years and had quit over 5 years prior. He had not traveled outside of Denmark in 2 years and had no pets at home. He reported being monogamous with his same-sex partner for the past 25 years. He had no significant family history, and he worked at a local hospital as a nurse. He denied any previous ocular history.
On examination, the pulse was 67 beats per minute, temperature was 36.7 degrees Celsius, respiratory rate was 16 breaths per minute, oxygen saturation was 99% while breathing ambient air, and blood pressure was 132/78. Oropharynx demonstrated no thrush or other lesions. The heart rhythm was regular and there were no murmurs. Lungs were clear to auscultation bilaterally. Abdominal exam was normal except for mild tenderness upon palpation in all quadrants, but no masses, organomegaly, rigidity, or rebound tenderness were present. Skin examination revealed several subcutaneous nodules measuring up to 0.5 cm in diameter overlying the right and left posterolateral chest walls. T he nodules were rubbery, pink, nontender, and not warm nor fluctuant. Visual acuity was reduced in both eyes. Extraocular movements were intact, and the pupils reacted to light and accommodated appropriately. The sclerae were injected bilaterally. The remainder of the cranial nerves and neurologic exam were normal. Due to the vision loss , the patient was referred to an ophthalmologist who diagnosed bilateral anterior uveitis.
Though monogamous with his male partner for many years, it is mandatory to consider complications of human immunodeficiency virus infection (HIV ). The absence of oral lesions indicative of a low CD4 count, such as oral hairy leukoplakia or thrush, does not rule out HIV disease. Additional history about his work as a nurse might shed light on his risk of infection, such as airborne exposure to tuberculosis or acquisition of blood-borne pathogens through a needle stick injury. His unremarkable vital signs support the chronicity of his medical condition.
Uveitis can result from numerous causes. When confined to the eye, uncommon hereditary and acquired causes are less likely . In many patients, uveitis arises in the setting of systemic infection or inflammation. The numerous infectious causes of uveitis include syphilis, tuberculosis, toxoplasmosis, cat scratch disease, and viruses such as HIV, West Nile, and Ebola. Among the inflammatory diseases that can cause uveitis are sarcoidosis, inflammatory bowel disease, systemic lupus erythematosus, Behçet disease, and Sjogren syndrome.
Several of these conditions, including tuberculosis and syphilis, may also cause subcutaneous nodules. Both tuberculosis and syphilis can cause skin and gastrointestinal disease. Sarcoidosis could involve the skin, peritoneum, and uvea, and is a possibility in this patient. The dermatologic conditions associated with sarcoidosis are protean and include granulomatous inflammation and nongranulomatous processes such as erythema nodosum. Usually the nodules of erythema nodosum are tender, red or purple, and located on the lower extremities. The lack of tenderness points away from erythema nodosum in this patient. Metastatic cancer can disseminate to the subcutaneous tissue, and the patient’s smoking history and age mandate we consider malignancy. However, skin metastases tend to be hard, not rubbery.
A cost-effective evaluation at this point would include syphilis serologies, HIV testing, testing for tuberculosis with either a purified protein derivative test or interferon gamma release assay, chest radiography, and biopsy of 1 of the lesions on his back.
Laboratory data showed 12,400 white blood cells per cubic milliliter (64% neutrophils, 24% lymphocytes, 9% monocytes, 2% eosinophils, 1% basophils), hemoglobin 7.9 g/dL, mean corpuscular volume 85 fL, platelets 476,000 per cubic milliliter , C-reactive protein 43 mg/ d L (normal < 8 mg/L), gamma-glutamyl-transferase 554 IU/L (normal range 0-45), alkaline phosphatase 865 U/L (normal range 60-200), and erythrocyte sedimentation rate (ESR) 71 mm per hour. International normalized ratio was 1.0, albumin was 3.0 mg/dL, activated partial thromboplastin time was 32 seconds (normal 22 to 35 seconds), and bilirubin was 0.3 mg/dL. Antibodies to HIV , hepatitis C, and hepatitis B surface antigen were not detectable. Electrocardiography ( ECG ) was normal. Plain radiograph of the chest demonstrated multiple nodular lesions bilaterally measuring up to 1 cm with no cavitation. There was a left pleural effusion.
The history and exam findings indicate a serious inflammatory condition affecting his lungs, pleura, eyes, skin, liver, and possibly his peritoneum. In this context, the elevated C-reactive protein and ESR are not helpful in differentiating inflammatory from infectious causes. The constellation of uveitis, pulmonary and cutaneous nodules, and marked abnormalities of liver tests in a middle-aged man of Northern European origin points us toward sarcoidosis. Pleural effusions are not common with sarcoidosis but may occur. However, to avoid premature closure, it is important to consider other possibilities.
Metastatic cancer, including lymphoma, could cause pulmonary and cutaneous nodules and liver involvement, but the chronic time course and uveitis are not consistent with malignancy. Tuberculosis is still a consideration, though one would have expected him to report fevers, night sweats, and, perhaps, exposure to patients with pulmonary tuberculosis in his job as a nurse. Multiple solid pulmonary nodules are also uncommon with pulmonary tuberculosis. Fungal infections such as histoplasmosis can cause skin lesions and pulmonary nodules but do not fit well with uveitis.
At this point, “ tissue is the issue.” A skin nodule would be the easiest site to biopsy. If skin biopsy was not diagnostic, computed tomography (CT) of his chest and abdomen should be performed to identify the next most accessible site for biopsy.
Esophagogastroduodenoscopy (EGD) and colonoscopy showed normal findings, and random biopsies from the stomach and colon were normal. CT of the chest, abdomen, and pelvis performed with the administration of intravenous contrast showed multiple solid opacities in both lung fields up to 1 cm, with enlarged mediastinal and retroperitoneal lymph nodes measuring 1 to 3 cm in diameter, a left pleural effusion, wall thickening in the right colon, and several nonspecific hypodensities in the liver. A punch biopsy taken from the right chest wall lesion demonstrated chronic inflammation without granulomas. The patient underwent CT-guided biopsy of 1 of the right-sided lung nodules, which revealed noncaseating granulomatous inflammation, fibrosis, and necrosis. Neither biopsy contained malignant cells, and additional stains revealed no bacteria, fungi, or acid fast bacilli.
The retroperitoneal and mediastinal adenopathy are indicative of a widely disseminated inflammatory process. Lymphoma continues to be a concern, though uveitis as an initial presenting problem would very unusual. Although biopsy of the chest wall lesion failed to demonstrate granulomatous inflammation, the most parsimonious explanation is that the skin and lung nodules are both related to a single systemic process.
Granulomas form in an attempt to wall off offending agents, whether foreign antigens (talc, certain medications), infectious agents, or self-antigens. Review of histopathology and microbiologic studies are useful first steps. Stains for bacteria, fungi, or acid-fast organisms may diagnose an infectious cause, such as tuberculosis, leprosy, syphilis, fungi, or cat scratch disease. Granulomas in association with vascular inflammation would indicate vasculitis. Other autoimmune considerations include sarcoidosis and Crohn disease. Noncaseating granulomas are typically found in sarcoidosis, cat scratch disease, syphilis, leprosy, or Crohn disease, but do not entirely exclude tuberculosis.
The negative infectious studies and lack of classic features of Crohn disease or other autoimmune diseases further point to sarcoidosis as the etiology of this patient’s illness. A Norwegian dermatologist first described the pathology of sarcoidosis based upon specimens taken from skin nodules. He thought the lesions were sarcoma and described them as, “ multiple benign sarcoid of the skin,” which is where the name “ sarcoidosis” originated.
Diagnosing sarcoidosis requires excluding other mimickers. Additional testing should include syphilis serologies, rheumatoid factor, and antineutrophilic cytoplasmic antibodies. The latter is associated with granulomatosis with polyangiitis and eosinophilic granulomatosis with polyangiitis, either of which may produce granulomatous inflammation of the lungs, skin, and uvea.
At this juncture, PET-CT represents a costly and unnecessary test that does not narrow our diagnostic possibilities sufficiently to justify its use. Osteolytic lesions would be unusual in sarcoidosis and more likely in lymphoma or infectious processes such as tuberculosis. Tests for syphilis and tuberculosis are required, and are a fraction of the cost of a PET-CT.
With the biopsy revealing spirochetes, and the positive results of a nontreponemal test (RPR) and confirmatory treponemal results, the diagnosis of syphilis is firmly established. Uveitis indicates neurosyphilis and warrants a longer course of intravenous penicillin. Lumbar puncture should be performed.
A lumbar puncture was performed. Cerebrospinal fluid (CSF) contained 9 white blood cells and 73 red blood cells per cubic milliliter; protein concentration was 73 mg/dL, and glucose was 116 mg/dL. Polymerase chain reaction for T. pallidum was negative. Transthoracic ECG and magnetic resonance imaging of the brain were normal. The patient was treated with intravenous penicillin G at 5 million units 4 times daily for 15 days. A PET-CT scan 3 months later revealed complete resolution of the subcutaneous, pulmonary, liver lesions, lymphadenopathy, and uveitis. Repeat treponemal serologies demonstrated a greater than 4-fold decline in titers.
DISCUSSION
Syphilis is a sexually transmitted disease with increasing incidence worldwide. Untreated infection progresses through 3 stages. The primary stage is characterized by the appearance of a painless chancre after an incubation period of 2 to 3 weeks. Four to 8 weeks later, the secondary stage emerges as a systemic infection, often heralded by a maculopapular rash with desquamation, frequently involving the soles and palms. Hepatitis, iridocyclitis, and early neurosyphilis may also be seen at this stage. Subsequently, syphilis becomes latent. One-third of patients with untreated latent syphilis will develop tertiary syphilis, typified by late neurosyphilis (tabes dorsalis and general paresis), cardiovascular disease (aortitis), or gummatous disease. 1
Gummas are destructive granulomatous lesions that typically present indolently, may occur singly or multiply, and may involve almost any organ. It has been suggested that gummas are the immune system’s defense to slow the bacteria after attempts to kill it have failed. Histologically, gummas are hyalinized nodules with surrounding granulomatous infiltrate of lymphocytes, plasma cells, and multinucleated giant cells with or without necrosis . In the preantibiotic era, gummas were seen in approximately 15% of infected patients, with a latency of 1 to 46 years after primary infection. 2 Penicillin led to a drastic reduction in gummas until the HIV epidemic, which led to the resurgence of gummas at a drastically shortened interval following primary syphilis. 3
Most commonly, gummas affect the skin and bones. In the skin , lesions may be superficial or deep and may progress into ulcerative nodules. In the bones, destructive gummas have a characteristic “moth-eaten” appearance. Less common sequelae of gummas incude gummatous hepatitis, perforated nasal septum (saddle nose deformity), or hard palate erosions. 2,4 R arely, syphilis involves the lungs, appearing as nodules, infiltrates, or pleural effusion. 5
Ocular manifestations occur in approximately 5% of patients with syphilis, more often in secondary and tertiary stages, and are strongly associated with a spread to the central nervous system. Syphilis may affect any structure of the eye, with anterior uveitis as the most frequent manifestation. Partial or complete vision loss is identified in approximately half of the patients with ocular syphilis and may be completely reversed by appropriate treatment. Ophthalmologic findings such as optic neuritis and papilledema imply advanced illness , as do Argyll-Robertson pupils (small pupils that are poorly reactive to light , but with preserved accommodation and convergence). 6,7 The treatment of ocular syphilis is identical to that of neurosyphilis. The Centers for Disease Control and Prevention recommends CSF analysis in any patient with ocular syphilis. Abnormal results should prompt repeat lumbar puncture every 3 to 6 months following treatment until the CSF results normalize. 8
The diagnosis of syphilis relies on indirect serologic tests. T. pallidum cannot be cultured in vitro, and techniques to identify spirochetes directly by using darkfield microscopy or DNA amplification via polymerase chain reaction are limited by availability or by poor sensitivity in advanced syphilis. 1 Imaging modalities including PET cannot reliably differentiate syphilis from other infectious and noninfectious mimickers. 9 F ortunately, syphilis infection can be diagnosed accurately based on reactive treponemal and nontreponemal serum tests. Nontreponemal tests, such as the RPR and Venereal Disease Research Laboratory, have traditionally been utilized as first-line evaluation, followed by a confirmatory treponemal test. However, nontreponemal tests may be nonreactive in a few settings: very early or very late in infection, and in individuals previously treated for syphilis. Thus, newer “reverse testing” algorithms utilize more sensitive and less expensive treponemal tests as the first test, followed by nontreponemal tests if the initial treponemal test is reactive. 8 Regardless of the testing sequence, in patients with no prior history of syphilis, reactive results on both treponemal and nontreponemal assays firmly establish a diagnosis of syphilis, obviating the need for more invasive and costly testing.
In patients with unexplained systemic illness, clinicians should have a low threshold to test for syphilis. Testing should be extended to certain asymptomatic individuals at higher risk of infection, including men who have sex with men, sexual partners of patients infected with syphilis, individuals with HIV or sexually-transmitted diseases, and others with high-risk sexual behavior or a history of sexually-transmitted diseases. 8 As the discussant points out, earlier consideration of and testing for syphilis would have spared the patient from unnecessary and costly EGD, colonoscopy, PET-CT scanning, and 3 biopsies.
Syphilis has been known to be a horribly destructive disease for centuries, earning the moniker “morbo serpentino” (serpentine disease) from the Spanish physician Ruiz Diaz de Isla in the 1500s. 10 In the modern era, physicians must remember to consider the diagnosis of syphilis in order to effectively mitigate the harm from this resurgent disease when it attacks our patients.
TEACHING POINTS
- Syphilis, the great imposter, is rising in incidence and should be on the differential diagnosis in all patients with unexplained multisystem inflammatory disease.
- A cost-effective diagnostic approach to syphilis entails serologic testing with treponemal and nontreponemal assays.
- Unexplained granulomas, especially in the skin, bone, or liver, should prompt consideration of gummatous syphilis.
- Ocular syphilis may involve any part of the visual tract and is treated the same as neurosyphilis.
Disclosure
Dr. Weinreich has received payment for lectures from Boehringer er Ingelheim, Astra Zeneca, TEVA and Novartis in 2016. All other contributors have nothing to report.
1. French P. Syphilis. BMJ. 2007;334:143-147. PubMed
2. Singh AE, Romanowski B. Syphilis: Review with emphasis on clinical, epidemiologic, and some biologic features. Clin Micriobio Rev. 1999;12(2):187-209. PubMed
3. Karp G, Schlaeffer F, Jotkowitz A, Riesenberg K. Syphilis and HIV co-infection. Eur J Int Med. 2009; 20:9-13. PubMed
4. Pilozzi-Edmonds L, Kong LY, Szabo J, Birnbaum LM. Rapid progression to gummatous syphilitic hepatitis and neurosyphilis in a patient with newly diagnosed HIV. Int J STD AIDS. 2014;26(13)985-987. PubMed
5. David G, Perpoint T, Boibieux A, et al. Secondary pulmonary syphilis: report of a likely case and literature review. Clin Infect Dis. 2006;42(3):e11-e15. PubMed
6. Moradi A, Salek S, Daniel E, et al. Clinical features and incidence rates of ocular complications in patients with ocular syphilis. Am J Ophthalmol. 2015;159:334-343. PubMed
7. Aldave AJ, King JA, Cunningham ET Jr. Ocular syphilis. Curr Opin Ophthalmol. 2001;12:433-441. PubMed
8. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137. PubMed
9. Lin M, Darwish B, Chu J. Neurosyphilitic gumma on F18-2-fluoro-2-deoxy-D-glucose (FDG) positron emission tomography: An old disease investigated with new technology. J Clin Neurosc. 2009;16:410-412. PubMed
10. de Ricon‐Ferraz A. Early work on syphilis: Diaz de Ysla’s treatise on the serpentine disease of Hispaniola Island. Int J Dermatol. 1999;38(3):222-227. PubMed
1. French P. Syphilis. BMJ. 2007;334:143-147. PubMed
2. Singh AE, Romanowski B. Syphilis: Review with emphasis on clinical, epidemiologic, and some biologic features. Clin Micriobio Rev. 1999;12(2):187-209. PubMed
3. Karp G, Schlaeffer F, Jotkowitz A, Riesenberg K. Syphilis and HIV co-infection. Eur J Int Med. 2009; 20:9-13. PubMed
4. Pilozzi-Edmonds L, Kong LY, Szabo J, Birnbaum LM. Rapid progression to gummatous syphilitic hepatitis and neurosyphilis in a patient with newly diagnosed HIV. Int J STD AIDS. 2014;26(13)985-987. PubMed
5. David G, Perpoint T, Boibieux A, et al. Secondary pulmonary syphilis: report of a likely case and literature review. Clin Infect Dis. 2006;42(3):e11-e15. PubMed
6. Moradi A, Salek S, Daniel E, et al. Clinical features and incidence rates of ocular complications in patients with ocular syphilis. Am J Ophthalmol. 2015;159:334-343. PubMed
7. Aldave AJ, King JA, Cunningham ET Jr. Ocular syphilis. Curr Opin Ophthalmol. 2001;12:433-441. PubMed
8. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137. PubMed
9. Lin M, Darwish B, Chu J. Neurosyphilitic gumma on F18-2-fluoro-2-deoxy-D-glucose (FDG) positron emission tomography: An old disease investigated with new technology. J Clin Neurosc. 2009;16:410-412. PubMed
10. de Ricon‐Ferraz A. Early work on syphilis: Diaz de Ysla’s treatise on the serpentine disease of Hispaniola Island. Int J Dermatol. 1999;38(3):222-227. PubMed
© 2017 Society of Hospital Medicine
The cost of experimental medicine
It has been a remarkable summer of milestones and crises for high-technology medicine.
An FDA panel has unanimously approved a gene therapy: The patient’s own immune cells are taken from his or her body, genetically modified, and reinfused to attack cancer. While the treatment has some dangers, it can be worth trying when conventional therapy has failed, and it appears to be curative when it works. Final FDA approval is expected in September.
While those breakthroughs were occurring, the parents of Charlie Gard, an infant in England with a very rare and devastating mitochondrial disease, were seeking experimental therapy for their child. The medical staff disagreed with the parents: They recommended that the best thing for Charlie would be to stop the ventilator and allow him to die, rather than let him to continue to suffer. Three British courts reviewed Charlie’s case and concurred with the medical staff; on appeal, the European Court of Human Rights also denied the parents’ wishes.
End of life cases similar to Charlie’s are not rare. In modern medicine, parents sometimes must make the heart-wrenching decision to stop aggressive therapies and accept that death is imminent and unavoidable. Many factors go into making that decision. Both the courts and medical staff presume that parents are the best decision makers. Generally, medical staff provide emotional and spiritual support to the parents, along with a tincture of time. In the vast majority of cases, parents and physicians come to agree on the course of care, but sometimes, there are irreconcilable disagreements.
It is rare for courts to overrule parents. The government typically intervenes only when the harm from a parent’s choice exceeds some threshold. For instance, it is not in a child’s best interest to be put in a car during a blizzard and driven to the store to get cigarettes. But neither is it wise to have an intrusive government reviewing every choice a parent makes. The potential harm must be large enough, likely enough, and imminent enough before most judges will intervene. The law will insist the child be in a car seat at least.
In Charlie’s case, the medical staff and the judges all explicitly said that the cost of therapy did not factor into their decision making; they looked solely at what was best for Charlie. The focus was on whether the unproven potential benefits of experimental therapy outweighed the risk of suffering caused by the therapy and continued intensive medical care.
Even when a bedside decision ignores the financial impact, money often structures which therapeutic choices are available. There are also issues of equitable access to be raised and weighed. Expenditures impact other social choices.
Money influenced the actions of Martin Shkreli, who is best known as the pharmacy company executive who markedly increased the price of a drug. Mr. Shkreli was recently convicted on three of eight charges for securities fraud, and sentencing is pending; the convictions were not related to the price increase.
The United States has created some amazing technologies to save individual, identifiable lives, but they come at a high price that often costs lives in ways more subtle than the incident in India. At some point, the government and the public are responsible for either financing or rationing care, but that doesn’t absolve the scientists completely. The Russell-Einstein (Pugwash) Manifesto established that scientists have a moral accountability for the negative consequences of creating new technology, and that includes the financial aspects.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected]
It has been a remarkable summer of milestones and crises for high-technology medicine.
An FDA panel has unanimously approved a gene therapy: The patient’s own immune cells are taken from his or her body, genetically modified, and reinfused to attack cancer. While the treatment has some dangers, it can be worth trying when conventional therapy has failed, and it appears to be curative when it works. Final FDA approval is expected in September.
While those breakthroughs were occurring, the parents of Charlie Gard, an infant in England with a very rare and devastating mitochondrial disease, were seeking experimental therapy for their child. The medical staff disagreed with the parents: They recommended that the best thing for Charlie would be to stop the ventilator and allow him to die, rather than let him to continue to suffer. Three British courts reviewed Charlie’s case and concurred with the medical staff; on appeal, the European Court of Human Rights also denied the parents’ wishes.
End of life cases similar to Charlie’s are not rare. In modern medicine, parents sometimes must make the heart-wrenching decision to stop aggressive therapies and accept that death is imminent and unavoidable. Many factors go into making that decision. Both the courts and medical staff presume that parents are the best decision makers. Generally, medical staff provide emotional and spiritual support to the parents, along with a tincture of time. In the vast majority of cases, parents and physicians come to agree on the course of care, but sometimes, there are irreconcilable disagreements.
It is rare for courts to overrule parents. The government typically intervenes only when the harm from a parent’s choice exceeds some threshold. For instance, it is not in a child’s best interest to be put in a car during a blizzard and driven to the store to get cigarettes. But neither is it wise to have an intrusive government reviewing every choice a parent makes. The potential harm must be large enough, likely enough, and imminent enough before most judges will intervene. The law will insist the child be in a car seat at least.
In Charlie’s case, the medical staff and the judges all explicitly said that the cost of therapy did not factor into their decision making; they looked solely at what was best for Charlie. The focus was on whether the unproven potential benefits of experimental therapy outweighed the risk of suffering caused by the therapy and continued intensive medical care.
Even when a bedside decision ignores the financial impact, money often structures which therapeutic choices are available. There are also issues of equitable access to be raised and weighed. Expenditures impact other social choices.
Money influenced the actions of Martin Shkreli, who is best known as the pharmacy company executive who markedly increased the price of a drug. Mr. Shkreli was recently convicted on three of eight charges for securities fraud, and sentencing is pending; the convictions were not related to the price increase.
The United States has created some amazing technologies to save individual, identifiable lives, but they come at a high price that often costs lives in ways more subtle than the incident in India. At some point, the government and the public are responsible for either financing or rationing care, but that doesn’t absolve the scientists completely. The Russell-Einstein (Pugwash) Manifesto established that scientists have a moral accountability for the negative consequences of creating new technology, and that includes the financial aspects.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected]
It has been a remarkable summer of milestones and crises for high-technology medicine.
An FDA panel has unanimously approved a gene therapy: The patient’s own immune cells are taken from his or her body, genetically modified, and reinfused to attack cancer. While the treatment has some dangers, it can be worth trying when conventional therapy has failed, and it appears to be curative when it works. Final FDA approval is expected in September.
While those breakthroughs were occurring, the parents of Charlie Gard, an infant in England with a very rare and devastating mitochondrial disease, were seeking experimental therapy for their child. The medical staff disagreed with the parents: They recommended that the best thing for Charlie would be to stop the ventilator and allow him to die, rather than let him to continue to suffer. Three British courts reviewed Charlie’s case and concurred with the medical staff; on appeal, the European Court of Human Rights also denied the parents’ wishes.
End of life cases similar to Charlie’s are not rare. In modern medicine, parents sometimes must make the heart-wrenching decision to stop aggressive therapies and accept that death is imminent and unavoidable. Many factors go into making that decision. Both the courts and medical staff presume that parents are the best decision makers. Generally, medical staff provide emotional and spiritual support to the parents, along with a tincture of time. In the vast majority of cases, parents and physicians come to agree on the course of care, but sometimes, there are irreconcilable disagreements.
It is rare for courts to overrule parents. The government typically intervenes only when the harm from a parent’s choice exceeds some threshold. For instance, it is not in a child’s best interest to be put in a car during a blizzard and driven to the store to get cigarettes. But neither is it wise to have an intrusive government reviewing every choice a parent makes. The potential harm must be large enough, likely enough, and imminent enough before most judges will intervene. The law will insist the child be in a car seat at least.
In Charlie’s case, the medical staff and the judges all explicitly said that the cost of therapy did not factor into their decision making; they looked solely at what was best for Charlie. The focus was on whether the unproven potential benefits of experimental therapy outweighed the risk of suffering caused by the therapy and continued intensive medical care.
Even when a bedside decision ignores the financial impact, money often structures which therapeutic choices are available. There are also issues of equitable access to be raised and weighed. Expenditures impact other social choices.
Money influenced the actions of Martin Shkreli, who is best known as the pharmacy company executive who markedly increased the price of a drug. Mr. Shkreli was recently convicted on three of eight charges for securities fraud, and sentencing is pending; the convictions were not related to the price increase.
The United States has created some amazing technologies to save individual, identifiable lives, but they come at a high price that often costs lives in ways more subtle than the incident in India. At some point, the government and the public are responsible for either financing or rationing care, but that doesn’t absolve the scientists completely. The Russell-Einstein (Pugwash) Manifesto established that scientists have a moral accountability for the negative consequences of creating new technology, and that includes the financial aspects.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected]
Mass Confusion
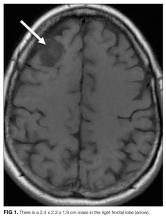
A 57-year-old woman presented to the emergency department of a community hospital with a 2-week history of dizziness, blurred vision, and poor coordination following a flu-like illness. Symptoms were initially attributed to complications from a presumed viral illness, but when they persisted for 2 weeks, she underwent magnetic resonance imaging (MRI) of the brain, which was reported as showing a 2.4 x 2.3 x 1.9 cm right frontal lobe mass with mild mass effect and contrast enhancement (Figure 1). She was discharged home at her request with plans for outpatient follow-up.
Brain masses are usually neoplastic, infectious, or less commonly, inflammatory. The isolated lesion in the right frontal lobe is unlikely to explain her symptoms, which are more suggestive of multifocal disease or elevated intracranial pressure. Although the frontal eye fields could be affected by the mass, such lesions usually cause tonic eye deviation, not blurry vision; furthermore, coordination, which is impaired here, is not governed by the frontal lobe.
Two weeks later, she returned to the same emergency department with worsening symptoms and new bilateral upper extremity dystonia, confusion, and visual hallucinations. Cerebrospinal fluid (CSF) analysis revealed clear, nonxanthochromic fluid with 4 nucleated cells (a differential was not performed), 113 red blood cells, glucose of 80 mg/dL (normal range, 50-80 mg/dL), and protein of 52 mg/dL (normal range, 15-45 mg/dL).
Confusion is generally caused by a metabolic, infectious, structural, or toxic etiology. Standard CSF test results are usually normal with most toxic or metabolic encephalopathies. The absence of significant CSF inflammation argues against infectious encephalitis; paraneoplastic and autoimmune encephalitis, however, are still possible. The CSF red blood cells were likely due to a mildly traumatic tap, but also may have arisen from the frontal lobe mass or a more diffuse invasive process, although the lack of xanthochromia argues against this. Delirium and red blood cells in the CSF should trigger consideration of herpes simplex virus (HSV) encephalitis, although the time course is a bit too protracted and the reported MRI findings do not suggest typical medial temporal lobe involvement.
The disparate neurologic findings suggest a multifocal process, perhaps embolic (eg, endocarditis), ischemic (eg, intravascular lymphoma), infiltrative (eg, malignancy, neurosarcoidosis), or demyelinating (eg, postinfectious acute disseminated encephalomyelitis, multiple sclerosis). However, most of these would have been detected on the initial MRI. Upper extremity dystonia would likely localize to the basal ganglia, whereas confusion and visual hallucinations are more global. The combination of a movement disorder and visual hallucinations is seen in Lewy body dementia, but this tempo is not typical.
Although the CSF does not have pleocytosis, her original symptoms were flu-like; therefore, CSF testing for viruses (eg, enterovirus) is reasonable. Bacterial, mycobacteria, and fungal studies are apt to be unrevealing, but CSF cytology, IgG index, and oligoclonal bands may be useful. Should the encephalopathy progress further and the general medical evaluation prove to be normal, then tests for autoimmune disorders (eg, antinuclear antibodies, NMDAR, paraneoplastic disorders) and rare causes of rapidly progressive dementias (eg, prion diseases) should be sent.
Additional CSF studies including HSV polymerase chain reaction (PCR), West Nile PCR, Lyme antibody, paraneoplastic antibodies, and cytology were sent. Intravenous acyclovir was administered. The above studies, as well as Gram stain, acid-fast bacillus stain, fungal stain, and cultures, were negative. She was started on levetiracetam for seizure prevention due to the mass lesion. An electroencephalogram (EEG) was reported as showing diffuse background slowing with superimposed semiperiodic sharp waves with a right hemispheric emphasis. Intravenous immunoglobulin (IVIG) 0.4 mg/kg/day over 5 days was administered with no improvement. The patient was transferred to an academic medical center for further evaluation.
The EEG reflects encephalopathy without pointing to a specific diagnosis. Prophylactic antiepileptic medications are not indicated for CNS mass lesions without clinical or electrophysiologic seizure activity. IVIG is often administered when an autoimmune encephalitis is suspected, but the lack of response does not rule out an autoimmune condition.
Her medical history included bilateral cataract extraction, right leg fracture, tonsillectomy, and total abdominal hysterectomy. She had a 25-year smoking history and a family history of lung cancer. She had no history of drug or alcohol use. On examination, her temperature was 37.9°C, blood pressure of 144/98 mm Hg, respiratory rate of 18 breaths per minute, a heart rate of 121 beats per minute, and oxygen saturation of 97% on ambient air. Her eyes were open but she was nonverbal. Her chest was clear to auscultation. Heart sounds were distinct and rhythm was regular. Abdomen was soft and nontender with no organomegaly. Skin examination revealed no rash. Her pupils were equal, round, and reactive to light. She did not follow verbal or gestural commands and intermittently tracked with her eyes, but not consistently enough to characterize extraocular movements. Her face was symmetric. She had a normal gag and blink reflex and an increased jaw jerk reflex. Her arms were flexed with increased tone. She had a positive palmo-mental reflex. She had spontaneous movement of all extremities. She had symmetric, 3+ reflexes of the patella and Achilles tendon with a bilateral Babinski’s sign. Sensation was intact only to withdrawal from noxious stimuli.
The physical exam does not localize to a specific brain region, but suggests a diffuse brain process. There are multiple signs of upper motor neuron involvement, including increased tone, hyperreflexia, and Babinski (plantar flexion) reflexes. A palmo-mental reflex signifies pathology in the cerebrum. Although cranial nerve testing is limited, there are no features of cranial neuropathy; similarly, no pyramidal weakness or sensory deficit has been demonstrated on limited testing. The differential diagnosis of her rapidly progressive encephalopathy includes autoimmune or paraneoplastic encephalitis, diffuse infiltrative malignancy, metabolic diseases (eg, porphyria, heavy metal intoxication), and prion disease.
Her family history of lung cancer and her smoking increases the possibility of paraneoplastic encephalitis, which often has subacute behavioral changes that precede complete neurologic impairment. Inflammatory or hemorrhagic CSF is seen with Balamuthia amoebic infection, which causes a granulomatous encephalitis and is characteristically associated with a mass lesion. Toxoplasmosis causes encephalitis that can be profound, but patients are usually immunocompromised and there are typically multiple lesions.
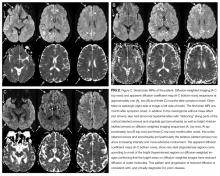
Laboratory results showed a normal white blood cell count and differential, basic metabolic profile and liver function tests, and C-reactive protein. Human immunodeficiency virus antibody testing was negative. Chest radiography and computed tomography of chest, abdomen, and pelvis were normal. A repeat MRI of the brain with contrast was reported as showing a 2.4 x 2.3 x 1.9 cm heterogeneously enhancing mass in the right frontal lobe with an enhancing dural tail and underlying hyperostosis consistent with a meningioma, and blooming within the mass consistent with prior hemorrhage. No mass effect was present.
The meningioma was resected 3 days after admission but her symptoms did not improve. Routine postoperative MRI was reported to show expected postsurgical changes but no infarct. Brain biopsy at the time of the operation was reported as meningioma and mild gliosis without encephalitis.
The reported MRI findings showing unchanged size and overall appearance of the mass, its connection to the dura and skull, and the pathology results all suggest that the mass is a meningioma. There is no evidence of disease outside of the CNS. Some cancers that provoke a paraneoplastic response can be quite small yet may incite an immune encephalitis; anti-NMDAR-mediated encephalitis can occur with malignancy (often ovarian), although it also arises in the absence of any tumor. Any inclination to definitively exclude conditions not seen on the brain biopsy must be tempered by the limited sensitivity of brain histology examination. Still, what was not seen warrants mention: vascular inflammation suggestive of CNS vasculitis, granulomas that might point to neurosarcoidosis, malignant cells of an infiltrating lymphoma or glioma, or inflammatory cells suggestive of encephalitis. Prion encephalopathy remains possible.
The patient remained unresponsive. A repeat EEG showed bilateral generalized periodic epileptiform discharges with accompanying twitching of the head, face, and left arm, which were suppressed with intravenous propofol and levetiracetam. Three weeks following meningioma resection, a new MRI was read as showing new abnormal signal in the right basal ganglia, abnormality of the cortex on the diffusion weighted images, and progressive generalized volume loss.
Among the aforementioned diagnoses, focal or diffuse periodic epileptiform discharges at 1-2 hertz are most characteristic of prion disease. Striatal and cortical transverse relaxation time (T2)-weighted and diffusion-weighted imaging (DWI) hyperintensities with corresponding restricted diffusion is characteristic of Creutzfeldt-Jakob disease (CJD), although metabolic disorders, seizures, and encephalitis can very rarely show similar MRI findings. The clinical course, the MRI and EEG findings, and nondiagnostic biopsy results, which were initially not assessed for prion disease, collectively point to prion disease. Detection of abnormal prion protein in the brain tissue by immunohistochemistry or molecular methods would confirm the diagnosis.
Review of the original right frontal cortex biopsy specimen at the National Prion Disease Pathology Surveillance Center, including immunostaining with 3F4, a monoclonal antibody to the prion protein, revealed granular deposits typical of prion disease. This finding established a diagnosis of prion disease, likely sporadic CJD. The patient was transitioned to palliative care and died shortly thereafter.
Brain autopsy showed regions with transcortical vacuolation (spongiform change), other cortical regions with varying degrees of vacuolation, abundant reactive astrocytes, paucity of neurons, and dark shrunken neurons. Vacuolation and gliosis were observed in the striatum and were most pronounced in the thalamus. There was no evidence of an inflammatory infiltrate or a neoplastic process. These findings with the positive 3F4 immunohistochemistry and positive Western blot from brain autopsy, as well as the absence of a mutation in the prion protein gene, were diagnostic for CJD.
An investigation was initiated to track the nondisposable surgical instruments used in the meningioma resection that may have been subsequently used in other patients. It was determined that 52 neurosurgical patients may have been exposed to prion-contaminated instruments. The instruments were subsequently processed specifically for prion decontamination. After 7 years, no cases of CJD have been diagnosed in the potentially exposed patients.
DISCUSSION
CJD is a rare neurodegenerative condition1 classified as one of the transmissible spongiform encephalopathies, so called because of the characteristic spongiform pattern (vacuolation) seen on histology, as well as the presence of neuronal loss, reactive gliosis in the gray matter, and the accumulation of the abnormal isoform of the cellular prion protein.2 It affects about one person in every one million people per year worldwide; in the United States there are about 300 cases per year. The most common form of human prion disease, sporadic CJD, is relentlessly progressive and invariably fatal, and in most cases, death occurs less than 5 months from onset.3 There is no cure, although temporizing treatments for symptoms can be helpful.
Sporadic CJD, which accounts for approximately 85% of all cases of prion disease in humans, typically manifests with rapidly progressive dementia and myoclonus after a prolonged incubation period in persons between 55 and 75 years of age. Genetic forms account for approximately 15% and acquired forms less than 1% of human prion diseases.1 Prion diseases have a broad spectrum of clinical manifestations, including dementia, ataxia, parkinsonism, myoclonus, insomnia, paresthesias, and abnormal or changed behavior.4 Given the protean clinical manifestations of prion diseases and rarity, the diagnosis is challenging to make antemortem. One recent study showed that most patients receive about 4 misdiagnoses and are often two-thirds of the way through their disease course before the correct diagnosis of sporadic CJD is made.5
Testing for protein markers of rapid neuronal injury8 in the CSF including 14-3-3, total tau, and neuron-specific enolase can increase suspicion for CJD, although there is a 10%-50% false positive rate with these markers.9 In this case, those tests were not performed; positive results would have been even more nonspecific in the setting of an enhancing brain mass and recent brain surgery.
Although not available at the time this patient was evaluated, the real-time quaking-induced conversion (RT-QuIC) test performed in CSF is diagnostically helpful, and, if positive, supportive of the MRI findings. The sensitivity and specificity of this test have been reported to be between 87%-91% and 98%-100%, respectively, albeit with limited data.10 Applying RT-QuIC to nasal mucosal brushings might lead to even higher sensitivity and specificity.11Seeking a premortem diagnosis for a rare disease with no known cure may seem superfluous, but it has important implications for establishing prognosis, limiting subsequent diagnostic and therapeutic measures, and safeguarding of other patients and operating room personnel. Iatrogenic CJD has occurred following invasive procedures involving neurosurgical instrumentation.12 CJD has been transmitted from grafts of dura mater, transplanted corneas, implantation of inadequately sterilized electrodes in the brain, and in the early 1980s, injections of contaminated pituitary hormones (particularly growth hormone) derived from human pituitary glands taken from cadavers. Since CJD was first described in the 1920s, less than 1% of human prion cases have been acquired iatrogenically.13In patients with rapidly progressive cognitive decline who warrant brain biopsy or surgery, the probability of prion diseases should be assessed based on clinical information and the results of MRI, EEG, and CSF testing. If prion disease is plausible, World Health Organization14 precautions should be employed for neuroinvasive procedures to reduce transmission risk. Disposable equipment should be used when possible, and nondisposable neurosurgical instruments should be quarantined until a nonprion disease diagnosis is identified, or should be regarded as contaminated and reprocessed using the aforementioned protocol.
This case highlights the challenges of seeking the correct diagnosis and its consequences, especially from an infection control perspective. The initial imaging finding of a mass lesion (a meningioma—which is a common incidental finding in older adults15) was a red herring that initially obscured the correct diagnosis. The patient’s progressive cognitive decline, EEG results, and evolving MRI findings, however, prompted further scrutiny of the brain biopsy specimen that eventually steered the clinicians away from mass confusion to diagnostic certainty.
TEACHING POINTS
- Rapidly progressive dementias (RPD) are characterized by cognitive decline over weeks to months. The RPD differential diagnosis includes fulminant forms of common neurodegenerative disorders (eg, Alzheimer’s disease, dementia with Lewy bodies, frontotemporal dementia spectrum), autoimmune encephalidites, CNS cancers, and prion disease.
- Sporadic CJD is the most common human prion disease. It is a rare neurodegenerative condition with onset usually between the ages of 50 and 70 years, and most commonly manifests with rapidly progressive dementia, ataxia, and myoclonus.
- Because of its protean manifestations, the diagnosis of CJD is difficult to make antemortem, and diagnosis is often delayed. Specialist evaluation of brain MRI DWI sequences and new CSF diagnostic tests may allow for earlier diagnosis, which has management and infection control implications.
Disclosure
Dr. Dhaliwal reports receiving honoraria from ISMIE Mutual Insurance Company and Physicians’ Reciprocal Insurers. Dr Geschwind’s institution has received R01 grant funding from NIH/NIA; and Alliance Biosecure and the Michael J Homer Family Fund as paid money to his institution, Dr Geschwind has received consulting fees or honoraria from Best Doctors, Kendall Brill & Kelly, CJD Foundation, and Tau Consortium; Dr Geschwind is a consultant for Gerson Lehrman Group, Biohaven Pharmaceuticals, and Advance Medical, outside the submitted work; has grants/grantspending with Quest, Cure PSP, and Tau Consortium, and received payment for lectures from Multiple Grand Rounds Lectures, outside the submitted work. Dr Saint is on a medical advisory board of Doximity, has received honorarium for being a member of the medical advisory board; he is also on the scientifice advisory board of Jvion. Dr Safdar’s institution has received a grant from the VA Patient Safety Center.
1. Brown P, Gibbs CJ, Jr., Rodgers-Johnson P, et al. Human spongiform encephalopathy: the National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann Neurol. 1994;35:513-529. PubMed
2. Kretzschmar HA, Ironside JW, DeArmond SJ, Tateishi J. Diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Arch Neurol. 1996;53:913-920. PubMed
3. Johnson RT, Gibbs CJ, Jr. Creutzfeldt-Jakob disease and related transmissible spongiform encephalopathies. N Engl J Med. 1998;339:1994-2004. PubMed
4. Will RG, Alpers MP, Dormont D, Schonberger LB. Infectious and sporadic prion diseases. In: Prusiner SB, ed. Prion biology and diseases. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1999:465-507. \
5. Paterson RW, Torres-Chae CC, Kuo AL, et al. Differential diagnosis of Jakob-Creutzfeldt disease. Arch Neurol. 2012;69:1578-1582. PubMed
6. Tschampa HJ, Kallenberg K, Kretzschmar HA, et al. Pattern of cortical changes in sporadic Creutzfeldt-Jakob disease. AJNR Am J Neuroradiol. 2007;28:1114-1118. PubMed
7. Carswell C, Thompson A, Lukic A, et al. MRI findings are often missed in the diagnosis of Creutzfeldt-Jakob disease. BMC Neurol. 2012;12:153. PubMed
8. Geschwind MD, Martindale J, Miller D, et al. Challenging the clinical utility of the 14-3-3 protein for the diagnosis of sporadic Creutzfeldt-Jakob disease. Arch Neurol. 2003;60:813-816. PubMed
9. Burkhard PR, Sanchez JC, Landis T, Hochstrasser DF. CSF detection of the 14-3-3 protein in unselected patients with dementia. Neurology. 2001;56:1528-1533. PubMed
10. Orrú CD, Groveman BR, Hughson AG, Zanusso G, Coulthart MB, Caughey B. Rapid and sensitive RT-QuIC detection of human Creutzfeldt-Jakob disease using cerebrospinal fluid. MBio. 2015;6:pii: e02451-14 PubMed
11. Orrú CD, Bongianni M, Tonoli G, et al. A test for Creutzfeldt-Jakob disease using nasal brushings. N Engl J Med. 2014;371:519-529. PubMed
12. Brown P, Preece M, Brandel JP, et al. Iatrogenic Creutzfeldt-Jakob disease at the millennium. Neurology. 2000;55:1075-1081. PubMed
13. Brown P, Brandel JP, Sato T, et al. Iatrogenic Creutzfeldt-Jakob disease, final assessment. Emerg Infect Dis. 2012;18:901-907. PubMed
14. WHO infection control guidelines for transmissible spongiform encephalopathies. Report of a WHO consultation, Geneva, Switzerland, 23-26 March 1999. http://www.who.int/csr/resources/publications/bse/whocdscsraph2003.pdf. Accessed on July 10, 2017.
15. Bondy M, Ligon BL. Epidemiology and etiology of intracranial meningiomas: a review. J Neurooncol. 1996;29:197-205. PubMed
A 57-year-old woman presented to the emergency department of a community hospital with a 2-week history of dizziness, blurred vision, and poor coordination following a flu-like illness. Symptoms were initially attributed to complications from a presumed viral illness, but when they persisted for 2 weeks, she underwent magnetic resonance imaging (MRI) of the brain, which was reported as showing a 2.4 x 2.3 x 1.9 cm right frontal lobe mass with mild mass effect and contrast enhancement (Figure 1). She was discharged home at her request with plans for outpatient follow-up.
Brain masses are usually neoplastic, infectious, or less commonly, inflammatory. The isolated lesion in the right frontal lobe is unlikely to explain her symptoms, which are more suggestive of multifocal disease or elevated intracranial pressure. Although the frontal eye fields could be affected by the mass, such lesions usually cause tonic eye deviation, not blurry vision; furthermore, coordination, which is impaired here, is not governed by the frontal lobe.
Two weeks later, she returned to the same emergency department with worsening symptoms and new bilateral upper extremity dystonia, confusion, and visual hallucinations. Cerebrospinal fluid (CSF) analysis revealed clear, nonxanthochromic fluid with 4 nucleated cells (a differential was not performed), 113 red blood cells, glucose of 80 mg/dL (normal range, 50-80 mg/dL), and protein of 52 mg/dL (normal range, 15-45 mg/dL).
Confusion is generally caused by a metabolic, infectious, structural, or toxic etiology. Standard CSF test results are usually normal with most toxic or metabolic encephalopathies. The absence of significant CSF inflammation argues against infectious encephalitis; paraneoplastic and autoimmune encephalitis, however, are still possible. The CSF red blood cells were likely due to a mildly traumatic tap, but also may have arisen from the frontal lobe mass or a more diffuse invasive process, although the lack of xanthochromia argues against this. Delirium and red blood cells in the CSF should trigger consideration of herpes simplex virus (HSV) encephalitis, although the time course is a bit too protracted and the reported MRI findings do not suggest typical medial temporal lobe involvement.
The disparate neurologic findings suggest a multifocal process, perhaps embolic (eg, endocarditis), ischemic (eg, intravascular lymphoma), infiltrative (eg, malignancy, neurosarcoidosis), or demyelinating (eg, postinfectious acute disseminated encephalomyelitis, multiple sclerosis). However, most of these would have been detected on the initial MRI. Upper extremity dystonia would likely localize to the basal ganglia, whereas confusion and visual hallucinations are more global. The combination of a movement disorder and visual hallucinations is seen in Lewy body dementia, but this tempo is not typical.
Although the CSF does not have pleocytosis, her original symptoms were flu-like; therefore, CSF testing for viruses (eg, enterovirus) is reasonable. Bacterial, mycobacteria, and fungal studies are apt to be unrevealing, but CSF cytology, IgG index, and oligoclonal bands may be useful. Should the encephalopathy progress further and the general medical evaluation prove to be normal, then tests for autoimmune disorders (eg, antinuclear antibodies, NMDAR, paraneoplastic disorders) and rare causes of rapidly progressive dementias (eg, prion diseases) should be sent.
Additional CSF studies including HSV polymerase chain reaction (PCR), West Nile PCR, Lyme antibody, paraneoplastic antibodies, and cytology were sent. Intravenous acyclovir was administered. The above studies, as well as Gram stain, acid-fast bacillus stain, fungal stain, and cultures, were negative. She was started on levetiracetam for seizure prevention due to the mass lesion. An electroencephalogram (EEG) was reported as showing diffuse background slowing with superimposed semiperiodic sharp waves with a right hemispheric emphasis. Intravenous immunoglobulin (IVIG) 0.4 mg/kg/day over 5 days was administered with no improvement. The patient was transferred to an academic medical center for further evaluation.
The EEG reflects encephalopathy without pointing to a specific diagnosis. Prophylactic antiepileptic medications are not indicated for CNS mass lesions without clinical or electrophysiologic seizure activity. IVIG is often administered when an autoimmune encephalitis is suspected, but the lack of response does not rule out an autoimmune condition.
Her medical history included bilateral cataract extraction, right leg fracture, tonsillectomy, and total abdominal hysterectomy. She had a 25-year smoking history and a family history of lung cancer. She had no history of drug or alcohol use. On examination, her temperature was 37.9°C, blood pressure of 144/98 mm Hg, respiratory rate of 18 breaths per minute, a heart rate of 121 beats per minute, and oxygen saturation of 97% on ambient air. Her eyes were open but she was nonverbal. Her chest was clear to auscultation. Heart sounds were distinct and rhythm was regular. Abdomen was soft and nontender with no organomegaly. Skin examination revealed no rash. Her pupils were equal, round, and reactive to light. She did not follow verbal or gestural commands and intermittently tracked with her eyes, but not consistently enough to characterize extraocular movements. Her face was symmetric. She had a normal gag and blink reflex and an increased jaw jerk reflex. Her arms were flexed with increased tone. She had a positive palmo-mental reflex. She had spontaneous movement of all extremities. She had symmetric, 3+ reflexes of the patella and Achilles tendon with a bilateral Babinski’s sign. Sensation was intact only to withdrawal from noxious stimuli.
The physical exam does not localize to a specific brain region, but suggests a diffuse brain process. There are multiple signs of upper motor neuron involvement, including increased tone, hyperreflexia, and Babinski (plantar flexion) reflexes. A palmo-mental reflex signifies pathology in the cerebrum. Although cranial nerve testing is limited, there are no features of cranial neuropathy; similarly, no pyramidal weakness or sensory deficit has been demonstrated on limited testing. The differential diagnosis of her rapidly progressive encephalopathy includes autoimmune or paraneoplastic encephalitis, diffuse infiltrative malignancy, metabolic diseases (eg, porphyria, heavy metal intoxication), and prion disease.
Her family history of lung cancer and her smoking increases the possibility of paraneoplastic encephalitis, which often has subacute behavioral changes that precede complete neurologic impairment. Inflammatory or hemorrhagic CSF is seen with Balamuthia amoebic infection, which causes a granulomatous encephalitis and is characteristically associated with a mass lesion. Toxoplasmosis causes encephalitis that can be profound, but patients are usually immunocompromised and there are typically multiple lesions.
Laboratory results showed a normal white blood cell count and differential, basic metabolic profile and liver function tests, and C-reactive protein. Human immunodeficiency virus antibody testing was negative. Chest radiography and computed tomography of chest, abdomen, and pelvis were normal. A repeat MRI of the brain with contrast was reported as showing a 2.4 x 2.3 x 1.9 cm heterogeneously enhancing mass in the right frontal lobe with an enhancing dural tail and underlying hyperostosis consistent with a meningioma, and blooming within the mass consistent with prior hemorrhage. No mass effect was present.
The meningioma was resected 3 days after admission but her symptoms did not improve. Routine postoperative MRI was reported to show expected postsurgical changes but no infarct. Brain biopsy at the time of the operation was reported as meningioma and mild gliosis without encephalitis.
The reported MRI findings showing unchanged size and overall appearance of the mass, its connection to the dura and skull, and the pathology results all suggest that the mass is a meningioma. There is no evidence of disease outside of the CNS. Some cancers that provoke a paraneoplastic response can be quite small yet may incite an immune encephalitis; anti-NMDAR-mediated encephalitis can occur with malignancy (often ovarian), although it also arises in the absence of any tumor. Any inclination to definitively exclude conditions not seen on the brain biopsy must be tempered by the limited sensitivity of brain histology examination. Still, what was not seen warrants mention: vascular inflammation suggestive of CNS vasculitis, granulomas that might point to neurosarcoidosis, malignant cells of an infiltrating lymphoma or glioma, or inflammatory cells suggestive of encephalitis. Prion encephalopathy remains possible.
The patient remained unresponsive. A repeat EEG showed bilateral generalized periodic epileptiform discharges with accompanying twitching of the head, face, and left arm, which were suppressed with intravenous propofol and levetiracetam. Three weeks following meningioma resection, a new MRI was read as showing new abnormal signal in the right basal ganglia, abnormality of the cortex on the diffusion weighted images, and progressive generalized volume loss.
Among the aforementioned diagnoses, focal or diffuse periodic epileptiform discharges at 1-2 hertz are most characteristic of prion disease. Striatal and cortical transverse relaxation time (T2)-weighted and diffusion-weighted imaging (DWI) hyperintensities with corresponding restricted diffusion is characteristic of Creutzfeldt-Jakob disease (CJD), although metabolic disorders, seizures, and encephalitis can very rarely show similar MRI findings. The clinical course, the MRI and EEG findings, and nondiagnostic biopsy results, which were initially not assessed for prion disease, collectively point to prion disease. Detection of abnormal prion protein in the brain tissue by immunohistochemistry or molecular methods would confirm the diagnosis.
Review of the original right frontal cortex biopsy specimen at the National Prion Disease Pathology Surveillance Center, including immunostaining with 3F4, a monoclonal antibody to the prion protein, revealed granular deposits typical of prion disease. This finding established a diagnosis of prion disease, likely sporadic CJD. The patient was transitioned to palliative care and died shortly thereafter.
Brain autopsy showed regions with transcortical vacuolation (spongiform change), other cortical regions with varying degrees of vacuolation, abundant reactive astrocytes, paucity of neurons, and dark shrunken neurons. Vacuolation and gliosis were observed in the striatum and were most pronounced in the thalamus. There was no evidence of an inflammatory infiltrate or a neoplastic process. These findings with the positive 3F4 immunohistochemistry and positive Western blot from brain autopsy, as well as the absence of a mutation in the prion protein gene, were diagnostic for CJD.
An investigation was initiated to track the nondisposable surgical instruments used in the meningioma resection that may have been subsequently used in other patients. It was determined that 52 neurosurgical patients may have been exposed to prion-contaminated instruments. The instruments were subsequently processed specifically for prion decontamination. After 7 years, no cases of CJD have been diagnosed in the potentially exposed patients.
DISCUSSION
CJD is a rare neurodegenerative condition1 classified as one of the transmissible spongiform encephalopathies, so called because of the characteristic spongiform pattern (vacuolation) seen on histology, as well as the presence of neuronal loss, reactive gliosis in the gray matter, and the accumulation of the abnormal isoform of the cellular prion protein.2 It affects about one person in every one million people per year worldwide; in the United States there are about 300 cases per year. The most common form of human prion disease, sporadic CJD, is relentlessly progressive and invariably fatal, and in most cases, death occurs less than 5 months from onset.3 There is no cure, although temporizing treatments for symptoms can be helpful.
Sporadic CJD, which accounts for approximately 85% of all cases of prion disease in humans, typically manifests with rapidly progressive dementia and myoclonus after a prolonged incubation period in persons between 55 and 75 years of age. Genetic forms account for approximately 15% and acquired forms less than 1% of human prion diseases.1 Prion diseases have a broad spectrum of clinical manifestations, including dementia, ataxia, parkinsonism, myoclonus, insomnia, paresthesias, and abnormal or changed behavior.4 Given the protean clinical manifestations of prion diseases and rarity, the diagnosis is challenging to make antemortem. One recent study showed that most patients receive about 4 misdiagnoses and are often two-thirds of the way through their disease course before the correct diagnosis of sporadic CJD is made.5
Testing for protein markers of rapid neuronal injury8 in the CSF including 14-3-3, total tau, and neuron-specific enolase can increase suspicion for CJD, although there is a 10%-50% false positive rate with these markers.9 In this case, those tests were not performed; positive results would have been even more nonspecific in the setting of an enhancing brain mass and recent brain surgery.
Although not available at the time this patient was evaluated, the real-time quaking-induced conversion (RT-QuIC) test performed in CSF is diagnostically helpful, and, if positive, supportive of the MRI findings. The sensitivity and specificity of this test have been reported to be between 87%-91% and 98%-100%, respectively, albeit with limited data.10 Applying RT-QuIC to nasal mucosal brushings might lead to even higher sensitivity and specificity.11Seeking a premortem diagnosis for a rare disease with no known cure may seem superfluous, but it has important implications for establishing prognosis, limiting subsequent diagnostic and therapeutic measures, and safeguarding of other patients and operating room personnel. Iatrogenic CJD has occurred following invasive procedures involving neurosurgical instrumentation.12 CJD has been transmitted from grafts of dura mater, transplanted corneas, implantation of inadequately sterilized electrodes in the brain, and in the early 1980s, injections of contaminated pituitary hormones (particularly growth hormone) derived from human pituitary glands taken from cadavers. Since CJD was first described in the 1920s, less than 1% of human prion cases have been acquired iatrogenically.13In patients with rapidly progressive cognitive decline who warrant brain biopsy or surgery, the probability of prion diseases should be assessed based on clinical information and the results of MRI, EEG, and CSF testing. If prion disease is plausible, World Health Organization14 precautions should be employed for neuroinvasive procedures to reduce transmission risk. Disposable equipment should be used when possible, and nondisposable neurosurgical instruments should be quarantined until a nonprion disease diagnosis is identified, or should be regarded as contaminated and reprocessed using the aforementioned protocol.
This case highlights the challenges of seeking the correct diagnosis and its consequences, especially from an infection control perspective. The initial imaging finding of a mass lesion (a meningioma—which is a common incidental finding in older adults15) was a red herring that initially obscured the correct diagnosis. The patient’s progressive cognitive decline, EEG results, and evolving MRI findings, however, prompted further scrutiny of the brain biopsy specimen that eventually steered the clinicians away from mass confusion to diagnostic certainty.
TEACHING POINTS
- Rapidly progressive dementias (RPD) are characterized by cognitive decline over weeks to months. The RPD differential diagnosis includes fulminant forms of common neurodegenerative disorders (eg, Alzheimer’s disease, dementia with Lewy bodies, frontotemporal dementia spectrum), autoimmune encephalidites, CNS cancers, and prion disease.
- Sporadic CJD is the most common human prion disease. It is a rare neurodegenerative condition with onset usually between the ages of 50 and 70 years, and most commonly manifests with rapidly progressive dementia, ataxia, and myoclonus.
- Because of its protean manifestations, the diagnosis of CJD is difficult to make antemortem, and diagnosis is often delayed. Specialist evaluation of brain MRI DWI sequences and new CSF diagnostic tests may allow for earlier diagnosis, which has management and infection control implications.
Disclosure
Dr. Dhaliwal reports receiving honoraria from ISMIE Mutual Insurance Company and Physicians’ Reciprocal Insurers. Dr Geschwind’s institution has received R01 grant funding from NIH/NIA; and Alliance Biosecure and the Michael J Homer Family Fund as paid money to his institution, Dr Geschwind has received consulting fees or honoraria from Best Doctors, Kendall Brill & Kelly, CJD Foundation, and Tau Consortium; Dr Geschwind is a consultant for Gerson Lehrman Group, Biohaven Pharmaceuticals, and Advance Medical, outside the submitted work; has grants/grantspending with Quest, Cure PSP, and Tau Consortium, and received payment for lectures from Multiple Grand Rounds Lectures, outside the submitted work. Dr Saint is on a medical advisory board of Doximity, has received honorarium for being a member of the medical advisory board; he is also on the scientifice advisory board of Jvion. Dr Safdar’s institution has received a grant from the VA Patient Safety Center.
A 57-year-old woman presented to the emergency department of a community hospital with a 2-week history of dizziness, blurred vision, and poor coordination following a flu-like illness. Symptoms were initially attributed to complications from a presumed viral illness, but when they persisted for 2 weeks, she underwent magnetic resonance imaging (MRI) of the brain, which was reported as showing a 2.4 x 2.3 x 1.9 cm right frontal lobe mass with mild mass effect and contrast enhancement (Figure 1). She was discharged home at her request with plans for outpatient follow-up.
Brain masses are usually neoplastic, infectious, or less commonly, inflammatory. The isolated lesion in the right frontal lobe is unlikely to explain her symptoms, which are more suggestive of multifocal disease or elevated intracranial pressure. Although the frontal eye fields could be affected by the mass, such lesions usually cause tonic eye deviation, not blurry vision; furthermore, coordination, which is impaired here, is not governed by the frontal lobe.
Two weeks later, she returned to the same emergency department with worsening symptoms and new bilateral upper extremity dystonia, confusion, and visual hallucinations. Cerebrospinal fluid (CSF) analysis revealed clear, nonxanthochromic fluid with 4 nucleated cells (a differential was not performed), 113 red blood cells, glucose of 80 mg/dL (normal range, 50-80 mg/dL), and protein of 52 mg/dL (normal range, 15-45 mg/dL).
Confusion is generally caused by a metabolic, infectious, structural, or toxic etiology. Standard CSF test results are usually normal with most toxic or metabolic encephalopathies. The absence of significant CSF inflammation argues against infectious encephalitis; paraneoplastic and autoimmune encephalitis, however, are still possible. The CSF red blood cells were likely due to a mildly traumatic tap, but also may have arisen from the frontal lobe mass or a more diffuse invasive process, although the lack of xanthochromia argues against this. Delirium and red blood cells in the CSF should trigger consideration of herpes simplex virus (HSV) encephalitis, although the time course is a bit too protracted and the reported MRI findings do not suggest typical medial temporal lobe involvement.
The disparate neurologic findings suggest a multifocal process, perhaps embolic (eg, endocarditis), ischemic (eg, intravascular lymphoma), infiltrative (eg, malignancy, neurosarcoidosis), or demyelinating (eg, postinfectious acute disseminated encephalomyelitis, multiple sclerosis). However, most of these would have been detected on the initial MRI. Upper extremity dystonia would likely localize to the basal ganglia, whereas confusion and visual hallucinations are more global. The combination of a movement disorder and visual hallucinations is seen in Lewy body dementia, but this tempo is not typical.
Although the CSF does not have pleocytosis, her original symptoms were flu-like; therefore, CSF testing for viruses (eg, enterovirus) is reasonable. Bacterial, mycobacteria, and fungal studies are apt to be unrevealing, but CSF cytology, IgG index, and oligoclonal bands may be useful. Should the encephalopathy progress further and the general medical evaluation prove to be normal, then tests for autoimmune disorders (eg, antinuclear antibodies, NMDAR, paraneoplastic disorders) and rare causes of rapidly progressive dementias (eg, prion diseases) should be sent.
Additional CSF studies including HSV polymerase chain reaction (PCR), West Nile PCR, Lyme antibody, paraneoplastic antibodies, and cytology were sent. Intravenous acyclovir was administered. The above studies, as well as Gram stain, acid-fast bacillus stain, fungal stain, and cultures, were negative. She was started on levetiracetam for seizure prevention due to the mass lesion. An electroencephalogram (EEG) was reported as showing diffuse background slowing with superimposed semiperiodic sharp waves with a right hemispheric emphasis. Intravenous immunoglobulin (IVIG) 0.4 mg/kg/day over 5 days was administered with no improvement. The patient was transferred to an academic medical center for further evaluation.
The EEG reflects encephalopathy without pointing to a specific diagnosis. Prophylactic antiepileptic medications are not indicated for CNS mass lesions without clinical or electrophysiologic seizure activity. IVIG is often administered when an autoimmune encephalitis is suspected, but the lack of response does not rule out an autoimmune condition.
Her medical history included bilateral cataract extraction, right leg fracture, tonsillectomy, and total abdominal hysterectomy. She had a 25-year smoking history and a family history of lung cancer. She had no history of drug or alcohol use. On examination, her temperature was 37.9°C, blood pressure of 144/98 mm Hg, respiratory rate of 18 breaths per minute, a heart rate of 121 beats per minute, and oxygen saturation of 97% on ambient air. Her eyes were open but she was nonverbal. Her chest was clear to auscultation. Heart sounds were distinct and rhythm was regular. Abdomen was soft and nontender with no organomegaly. Skin examination revealed no rash. Her pupils were equal, round, and reactive to light. She did not follow verbal or gestural commands and intermittently tracked with her eyes, but not consistently enough to characterize extraocular movements. Her face was symmetric. She had a normal gag and blink reflex and an increased jaw jerk reflex. Her arms were flexed with increased tone. She had a positive palmo-mental reflex. She had spontaneous movement of all extremities. She had symmetric, 3+ reflexes of the patella and Achilles tendon with a bilateral Babinski’s sign. Sensation was intact only to withdrawal from noxious stimuli.
The physical exam does not localize to a specific brain region, but suggests a diffuse brain process. There are multiple signs of upper motor neuron involvement, including increased tone, hyperreflexia, and Babinski (plantar flexion) reflexes. A palmo-mental reflex signifies pathology in the cerebrum. Although cranial nerve testing is limited, there are no features of cranial neuropathy; similarly, no pyramidal weakness or sensory deficit has been demonstrated on limited testing. The differential diagnosis of her rapidly progressive encephalopathy includes autoimmune or paraneoplastic encephalitis, diffuse infiltrative malignancy, metabolic diseases (eg, porphyria, heavy metal intoxication), and prion disease.
Her family history of lung cancer and her smoking increases the possibility of paraneoplastic encephalitis, which often has subacute behavioral changes that precede complete neurologic impairment. Inflammatory or hemorrhagic CSF is seen with Balamuthia amoebic infection, which causes a granulomatous encephalitis and is characteristically associated with a mass lesion. Toxoplasmosis causes encephalitis that can be profound, but patients are usually immunocompromised and there are typically multiple lesions.
Laboratory results showed a normal white blood cell count and differential, basic metabolic profile and liver function tests, and C-reactive protein. Human immunodeficiency virus antibody testing was negative. Chest radiography and computed tomography of chest, abdomen, and pelvis were normal. A repeat MRI of the brain with contrast was reported as showing a 2.4 x 2.3 x 1.9 cm heterogeneously enhancing mass in the right frontal lobe with an enhancing dural tail and underlying hyperostosis consistent with a meningioma, and blooming within the mass consistent with prior hemorrhage. No mass effect was present.
The meningioma was resected 3 days after admission but her symptoms did not improve. Routine postoperative MRI was reported to show expected postsurgical changes but no infarct. Brain biopsy at the time of the operation was reported as meningioma and mild gliosis without encephalitis.
The reported MRI findings showing unchanged size and overall appearance of the mass, its connection to the dura and skull, and the pathology results all suggest that the mass is a meningioma. There is no evidence of disease outside of the CNS. Some cancers that provoke a paraneoplastic response can be quite small yet may incite an immune encephalitis; anti-NMDAR-mediated encephalitis can occur with malignancy (often ovarian), although it also arises in the absence of any tumor. Any inclination to definitively exclude conditions not seen on the brain biopsy must be tempered by the limited sensitivity of brain histology examination. Still, what was not seen warrants mention: vascular inflammation suggestive of CNS vasculitis, granulomas that might point to neurosarcoidosis, malignant cells of an infiltrating lymphoma or glioma, or inflammatory cells suggestive of encephalitis. Prion encephalopathy remains possible.
The patient remained unresponsive. A repeat EEG showed bilateral generalized periodic epileptiform discharges with accompanying twitching of the head, face, and left arm, which were suppressed with intravenous propofol and levetiracetam. Three weeks following meningioma resection, a new MRI was read as showing new abnormal signal in the right basal ganglia, abnormality of the cortex on the diffusion weighted images, and progressive generalized volume loss.
Among the aforementioned diagnoses, focal or diffuse periodic epileptiform discharges at 1-2 hertz are most characteristic of prion disease. Striatal and cortical transverse relaxation time (T2)-weighted and diffusion-weighted imaging (DWI) hyperintensities with corresponding restricted diffusion is characteristic of Creutzfeldt-Jakob disease (CJD), although metabolic disorders, seizures, and encephalitis can very rarely show similar MRI findings. The clinical course, the MRI and EEG findings, and nondiagnostic biopsy results, which were initially not assessed for prion disease, collectively point to prion disease. Detection of abnormal prion protein in the brain tissue by immunohistochemistry or molecular methods would confirm the diagnosis.
Review of the original right frontal cortex biopsy specimen at the National Prion Disease Pathology Surveillance Center, including immunostaining with 3F4, a monoclonal antibody to the prion protein, revealed granular deposits typical of prion disease. This finding established a diagnosis of prion disease, likely sporadic CJD. The patient was transitioned to palliative care and died shortly thereafter.
Brain autopsy showed regions with transcortical vacuolation (spongiform change), other cortical regions with varying degrees of vacuolation, abundant reactive astrocytes, paucity of neurons, and dark shrunken neurons. Vacuolation and gliosis were observed in the striatum and were most pronounced in the thalamus. There was no evidence of an inflammatory infiltrate or a neoplastic process. These findings with the positive 3F4 immunohistochemistry and positive Western blot from brain autopsy, as well as the absence of a mutation in the prion protein gene, were diagnostic for CJD.
An investigation was initiated to track the nondisposable surgical instruments used in the meningioma resection that may have been subsequently used in other patients. It was determined that 52 neurosurgical patients may have been exposed to prion-contaminated instruments. The instruments were subsequently processed specifically for prion decontamination. After 7 years, no cases of CJD have been diagnosed in the potentially exposed patients.
DISCUSSION
CJD is a rare neurodegenerative condition1 classified as one of the transmissible spongiform encephalopathies, so called because of the characteristic spongiform pattern (vacuolation) seen on histology, as well as the presence of neuronal loss, reactive gliosis in the gray matter, and the accumulation of the abnormal isoform of the cellular prion protein.2 It affects about one person in every one million people per year worldwide; in the United States there are about 300 cases per year. The most common form of human prion disease, sporadic CJD, is relentlessly progressive and invariably fatal, and in most cases, death occurs less than 5 months from onset.3 There is no cure, although temporizing treatments for symptoms can be helpful.
Sporadic CJD, which accounts for approximately 85% of all cases of prion disease in humans, typically manifests with rapidly progressive dementia and myoclonus after a prolonged incubation period in persons between 55 and 75 years of age. Genetic forms account for approximately 15% and acquired forms less than 1% of human prion diseases.1 Prion diseases have a broad spectrum of clinical manifestations, including dementia, ataxia, parkinsonism, myoclonus, insomnia, paresthesias, and abnormal or changed behavior.4 Given the protean clinical manifestations of prion diseases and rarity, the diagnosis is challenging to make antemortem. One recent study showed that most patients receive about 4 misdiagnoses and are often two-thirds of the way through their disease course before the correct diagnosis of sporadic CJD is made.5
Testing for protein markers of rapid neuronal injury8 in the CSF including 14-3-3, total tau, and neuron-specific enolase can increase suspicion for CJD, although there is a 10%-50% false positive rate with these markers.9 In this case, those tests were not performed; positive results would have been even more nonspecific in the setting of an enhancing brain mass and recent brain surgery.
Although not available at the time this patient was evaluated, the real-time quaking-induced conversion (RT-QuIC) test performed in CSF is diagnostically helpful, and, if positive, supportive of the MRI findings. The sensitivity and specificity of this test have been reported to be between 87%-91% and 98%-100%, respectively, albeit with limited data.10 Applying RT-QuIC to nasal mucosal brushings might lead to even higher sensitivity and specificity.11Seeking a premortem diagnosis for a rare disease with no known cure may seem superfluous, but it has important implications for establishing prognosis, limiting subsequent diagnostic and therapeutic measures, and safeguarding of other patients and operating room personnel. Iatrogenic CJD has occurred following invasive procedures involving neurosurgical instrumentation.12 CJD has been transmitted from grafts of dura mater, transplanted corneas, implantation of inadequately sterilized electrodes in the brain, and in the early 1980s, injections of contaminated pituitary hormones (particularly growth hormone) derived from human pituitary glands taken from cadavers. Since CJD was first described in the 1920s, less than 1% of human prion cases have been acquired iatrogenically.13In patients with rapidly progressive cognitive decline who warrant brain biopsy or surgery, the probability of prion diseases should be assessed based on clinical information and the results of MRI, EEG, and CSF testing. If prion disease is plausible, World Health Organization14 precautions should be employed for neuroinvasive procedures to reduce transmission risk. Disposable equipment should be used when possible, and nondisposable neurosurgical instruments should be quarantined until a nonprion disease diagnosis is identified, or should be regarded as contaminated and reprocessed using the aforementioned protocol.
This case highlights the challenges of seeking the correct diagnosis and its consequences, especially from an infection control perspective. The initial imaging finding of a mass lesion (a meningioma—which is a common incidental finding in older adults15) was a red herring that initially obscured the correct diagnosis. The patient’s progressive cognitive decline, EEG results, and evolving MRI findings, however, prompted further scrutiny of the brain biopsy specimen that eventually steered the clinicians away from mass confusion to diagnostic certainty.
TEACHING POINTS
- Rapidly progressive dementias (RPD) are characterized by cognitive decline over weeks to months. The RPD differential diagnosis includes fulminant forms of common neurodegenerative disorders (eg, Alzheimer’s disease, dementia with Lewy bodies, frontotemporal dementia spectrum), autoimmune encephalidites, CNS cancers, and prion disease.
- Sporadic CJD is the most common human prion disease. It is a rare neurodegenerative condition with onset usually between the ages of 50 and 70 years, and most commonly manifests with rapidly progressive dementia, ataxia, and myoclonus.
- Because of its protean manifestations, the diagnosis of CJD is difficult to make antemortem, and diagnosis is often delayed. Specialist evaluation of brain MRI DWI sequences and new CSF diagnostic tests may allow for earlier diagnosis, which has management and infection control implications.
Disclosure
Dr. Dhaliwal reports receiving honoraria from ISMIE Mutual Insurance Company and Physicians’ Reciprocal Insurers. Dr Geschwind’s institution has received R01 grant funding from NIH/NIA; and Alliance Biosecure and the Michael J Homer Family Fund as paid money to his institution, Dr Geschwind has received consulting fees or honoraria from Best Doctors, Kendall Brill & Kelly, CJD Foundation, and Tau Consortium; Dr Geschwind is a consultant for Gerson Lehrman Group, Biohaven Pharmaceuticals, and Advance Medical, outside the submitted work; has grants/grantspending with Quest, Cure PSP, and Tau Consortium, and received payment for lectures from Multiple Grand Rounds Lectures, outside the submitted work. Dr Saint is on a medical advisory board of Doximity, has received honorarium for being a member of the medical advisory board; he is also on the scientifice advisory board of Jvion. Dr Safdar’s institution has received a grant from the VA Patient Safety Center.
1. Brown P, Gibbs CJ, Jr., Rodgers-Johnson P, et al. Human spongiform encephalopathy: the National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann Neurol. 1994;35:513-529. PubMed
2. Kretzschmar HA, Ironside JW, DeArmond SJ, Tateishi J. Diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Arch Neurol. 1996;53:913-920. PubMed
3. Johnson RT, Gibbs CJ, Jr. Creutzfeldt-Jakob disease and related transmissible spongiform encephalopathies. N Engl J Med. 1998;339:1994-2004. PubMed
4. Will RG, Alpers MP, Dormont D, Schonberger LB. Infectious and sporadic prion diseases. In: Prusiner SB, ed. Prion biology and diseases. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1999:465-507. \
5. Paterson RW, Torres-Chae CC, Kuo AL, et al. Differential diagnosis of Jakob-Creutzfeldt disease. Arch Neurol. 2012;69:1578-1582. PubMed
6. Tschampa HJ, Kallenberg K, Kretzschmar HA, et al. Pattern of cortical changes in sporadic Creutzfeldt-Jakob disease. AJNR Am J Neuroradiol. 2007;28:1114-1118. PubMed
7. Carswell C, Thompson A, Lukic A, et al. MRI findings are often missed in the diagnosis of Creutzfeldt-Jakob disease. BMC Neurol. 2012;12:153. PubMed
8. Geschwind MD, Martindale J, Miller D, et al. Challenging the clinical utility of the 14-3-3 protein for the diagnosis of sporadic Creutzfeldt-Jakob disease. Arch Neurol. 2003;60:813-816. PubMed
9. Burkhard PR, Sanchez JC, Landis T, Hochstrasser DF. CSF detection of the 14-3-3 protein in unselected patients with dementia. Neurology. 2001;56:1528-1533. PubMed
10. Orrú CD, Groveman BR, Hughson AG, Zanusso G, Coulthart MB, Caughey B. Rapid and sensitive RT-QuIC detection of human Creutzfeldt-Jakob disease using cerebrospinal fluid. MBio. 2015;6:pii: e02451-14 PubMed
11. Orrú CD, Bongianni M, Tonoli G, et al. A test for Creutzfeldt-Jakob disease using nasal brushings. N Engl J Med. 2014;371:519-529. PubMed
12. Brown P, Preece M, Brandel JP, et al. Iatrogenic Creutzfeldt-Jakob disease at the millennium. Neurology. 2000;55:1075-1081. PubMed
13. Brown P, Brandel JP, Sato T, et al. Iatrogenic Creutzfeldt-Jakob disease, final assessment. Emerg Infect Dis. 2012;18:901-907. PubMed
14. WHO infection control guidelines for transmissible spongiform encephalopathies. Report of a WHO consultation, Geneva, Switzerland, 23-26 March 1999. http://www.who.int/csr/resources/publications/bse/whocdscsraph2003.pdf. Accessed on July 10, 2017.
15. Bondy M, Ligon BL. Epidemiology and etiology of intracranial meningiomas: a review. J Neurooncol. 1996;29:197-205. PubMed
1. Brown P, Gibbs CJ, Jr., Rodgers-Johnson P, et al. Human spongiform encephalopathy: the National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann Neurol. 1994;35:513-529. PubMed
2. Kretzschmar HA, Ironside JW, DeArmond SJ, Tateishi J. Diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Arch Neurol. 1996;53:913-920. PubMed
3. Johnson RT, Gibbs CJ, Jr. Creutzfeldt-Jakob disease and related transmissible spongiform encephalopathies. N Engl J Med. 1998;339:1994-2004. PubMed
4. Will RG, Alpers MP, Dormont D, Schonberger LB. Infectious and sporadic prion diseases. In: Prusiner SB, ed. Prion biology and diseases. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1999:465-507. \
5. Paterson RW, Torres-Chae CC, Kuo AL, et al. Differential diagnosis of Jakob-Creutzfeldt disease. Arch Neurol. 2012;69:1578-1582. PubMed
6. Tschampa HJ, Kallenberg K, Kretzschmar HA, et al. Pattern of cortical changes in sporadic Creutzfeldt-Jakob disease. AJNR Am J Neuroradiol. 2007;28:1114-1118. PubMed
7. Carswell C, Thompson A, Lukic A, et al. MRI findings are often missed in the diagnosis of Creutzfeldt-Jakob disease. BMC Neurol. 2012;12:153. PubMed
8. Geschwind MD, Martindale J, Miller D, et al. Challenging the clinical utility of the 14-3-3 protein for the diagnosis of sporadic Creutzfeldt-Jakob disease. Arch Neurol. 2003;60:813-816. PubMed
9. Burkhard PR, Sanchez JC, Landis T, Hochstrasser DF. CSF detection of the 14-3-3 protein in unselected patients with dementia. Neurology. 2001;56:1528-1533. PubMed
10. Orrú CD, Groveman BR, Hughson AG, Zanusso G, Coulthart MB, Caughey B. Rapid and sensitive RT-QuIC detection of human Creutzfeldt-Jakob disease using cerebrospinal fluid. MBio. 2015;6:pii: e02451-14 PubMed
11. Orrú CD, Bongianni M, Tonoli G, et al. A test for Creutzfeldt-Jakob disease using nasal brushings. N Engl J Med. 2014;371:519-529. PubMed
12. Brown P, Preece M, Brandel JP, et al. Iatrogenic Creutzfeldt-Jakob disease at the millennium. Neurology. 2000;55:1075-1081. PubMed
13. Brown P, Brandel JP, Sato T, et al. Iatrogenic Creutzfeldt-Jakob disease, final assessment. Emerg Infect Dis. 2012;18:901-907. PubMed
14. WHO infection control guidelines for transmissible spongiform encephalopathies. Report of a WHO consultation, Geneva, Switzerland, 23-26 March 1999. http://www.who.int/csr/resources/publications/bse/whocdscsraph2003.pdf. Accessed on July 10, 2017.
15. Bondy M, Ligon BL. Epidemiology and etiology of intracranial meningiomas: a review. J Neurooncol. 1996;29:197-205. PubMed
© 2017 Society of Hospital Medicine
Things We Do For No Reason: Two-Unit Red Cell Transfusions in Stable Anemic Patients
The “Things We Do for No Reason” (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

INTRODUCTION
Blood transfusion is not only the most common procedure performed in US hospitals but is also widely overused, according to The Joint Commission. Unnecessary transfusions can increase risks and costs, and now, multiple landmark trials support using restrictive transfusion strategies. This manuscript discusses the importance and potential impacts of giving single-unit red blood cell (RBC) transfusions in anemic patients who are not actively bleeding and are hemodynamically stable. The “thing we do for no reason” is giving 2-unit RBC transfusions when 1 unit would suffice. We call this the “Why give 2 when 1 will do?” campaign for RBC transfusion.
CASE PRESENTATION
A 74-year-old, 70-kg male with a known history of myelodysplastic syndrome is admitted for dizziness and shortness of breath. His hemoglobin (Hb) concentration is 6.2 g/dL (baseline Hb of 8 g/dL). The patient denies any hematuria, hematemesis, and melena. Physical examination is remarkable only for tachycardia—heart rate of 110. The admitting hospitalist ponders whether to order a 2-unit red blood cell (RBC) transfusion.
WHY YOU MIGHT THINK DOUBLE UNIT RED BLOOD CELL TRANSFUSIONS ARE HELPFUL
RBC transfusion is the most common procedure performed in US hospitals, with about 12 million RBC units given to patients in the United States each year.1 Based on an opinion paper published in 1942 by Adams and Lundy2 the “10/30 rule” set the standard that the ideal transfusion thresholds were an Hb of 10 g/dL or a hematocrit of 30%. Until human immunodeficiency virus (HIV) became a threat to the nation’s blood supply in the early 1980s, few questioned the 10/30 rule. There is no doubt that blood transfusions can be lifesaving in the presence of active bleeding or hemorrhagic shock; in fact, many hospitals have blood donation campaigns reminding us to “give blood—save a life.” To some, these messages may suggest that more blood is better. Prior to the 1990s, clinicians were taught that if the patient needed an RBC transfusion, 2 units was the optimal dose for adult patients. In fact, single-unit transfusions were strongly discouraged, and authorities on the risks of transfusion wrote that single-unit transfusions were acknowledged to be unnecessary.3
WHY THERE IS “NO REASON” TO ROUTINELY ORDER DOUBLE UNIT TRANSFUSIONS
According to a recent Joint Commission Overuse Summit, transfusion was identified as 1 of the top 5 overused medical procedures.4 Blood transfusions can cause complications such as transfusion-related acute lung injury and transfusion-associated circulatory overload, the number 1 and 2 causes of transfusion-related deaths, respectively,5 as well as other transfusion reactions (eg, allergic and hemolytic) and alloimmunization. Transfusion-related morbidity and mortality have been shown to be dose dependent,6 suggesting that the lowest effective number of units should be transfused. Although, with modern-day testing, the risks of HIV and viral hepatitis are exceedingly low, emerging infectious diseases such as the Zika virus and Babesiosis represent new threats to the nation’s blood supply, with potential transfusion-related transmission and severe consequences, especially for the immunosuppressed. As quality-improvement, patient safety, and cost-saving initiatives, many hospitals have implemented strategies to reduce unnecessary transfusions and decrease overall blood utilization.
The above-mentioned studies support the concept that oftentimes less is more for transfusions, which includes giving the lowest effective amount of transfused blood. These trials have enrolled multiple patient populations, such as critically ill patients in the intensive care unit,11,13 elderly orthopedic surgery patients,14 cardiac surgery patients,12 and patients with gastrointestinal hemorrhage,15 traumatic brain injury,17 and septicemia.16 Outcomes in the trials included mortality, serious infections, thrombotic and ischemic events, neurologic deficits, multiple-organ dysfunction, and inability to ambulate (Table). The findings in these studies suggest that we increase risks and cost without improving outcomes only by giving more blood than is necessary. Since most of these trials were published in the last decade, some very recently, clinicians have not fully adopted these newer, restrictive transfusion strategies.19
ARE THERE REASONS TO ORDER 2-UNIT TRANSFUSIONS IN CERTAIN CIRCUMSTANCES?
Perhaps the most common indication for ordering multiunit RBC transfusions is active bleeding, as it is clear that whatever Hb threshold is chosen, transfusion should be given in sufficient amounts to stay ahead of the bleeding.20 It is important to remember that we treat patients and their symptoms, not just their laboratory values. Good medical care adapts and/or modifies treatment protocols and guidelines according to the clinical situation. Intravascular volume is also important to consider because what really matters for oxygen content and delivery is the total red cell mass (ie, the Hb concentration times the blood volume). If a patient is hypovolemic and/or actively bleeding, the Hb transfusion trigger, as well as the dose of blood, may need to be adjusted upward, creating clinical scenarios in which 2-unit RBC transfusions may be appropriate. Other clinical settings for which multiunit RBC transfusions may be indicated include patients with severe anemia, for whom both the pretransfusion Hb (the trigger) and the posttransfusion Hb (the target) should be considered. Patients with hemoglobinopathies (eg, sickle cell or thalassemia) sometimes require multiunit transfusions or even exchange transfusions to improve oxygen delivery. Other patients who may benefit from higher Hb levels achieved by multiunit transfusions include those with acute coronary syndromes; however, the ideal Hb transfusion threshold in this setting has yet to be determined.21
WHAT YOU SHOULD DO INSTEAD
For hemodynamically stable patients and in the absence of active bleeding, single-unit RBC transfusions, followed by reassessment, should be the standard for most patients. The reassessment should include measuring the posttransfusion Hb level and checking for improvement in vital sign abnormalities and signs or symptoms of anemia or end-organ ischemia. A recent publication on our hospital-wide campaign called “Why give 2 when 1 will do?” showed a significant (35%) reduction in 2-unit transfusion orders along with an 18% overall decrease in RBC utilization and substantial cost savings (≈$600,000 per year).10 These findings demonstrate that there is a large opportunity to reduce transfusion overuse by encouraging single-unit transfusions.
RECOMMENDATIONS
- For nonbleeding, hemodynamically stable patients who require a transfusion, transfuse a single RBC unit and then reassess the Hb level before transfusing a second unit.
- The decision to transfuse RBCs should take into account the patient’s overall condition, including their symptoms, intravascular volume, and the occurrence and rate of active bleeding, not just the Hb value alone.
CONCLUSIONS
In stable patients, a single unit of RBCs often is adequate to raise the Hb to an acceptable level and relieve the signs and symptoms of anemia. Additional units should be prescribed only after reassessment of the patient and the Hb level. For our patient with symptomatic anemia, it is reasonable to transfuse 1 RBC unit, and then measure the Hb level, and reassess his symptoms before giving additional RBC units.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Acknowledgments
This publication is dedicated to our beloved colleague, Dr. Rajiv N. Thakkar, who recently and unexpectedly suffered a fatal cardiac event. We will miss him dearly.
Disclosure
S.M.F. has been on advisory boards for the Haemonetics Corporation (Braintree, MA), Medtronic Inc. (Minneapolis, MN), and Zimmer Biomet (Warsaw, IN). All other authors declare no competing interests.
1. Whitaker B, Rajbhandary S, Kleinman S, Harris A, Kamani N. Trends in United States blood collection and transfusion: results from the 2013 AABB Blood Collection, Utilization, and Patient Blood Management Survey. Transfusion. 2016;56:2173-2183. PubMed
2. Adams C, Lundy JS. Anesthesia in cases of poor surgical risk – Some suggestions for decreasing the risk. Surg Gynec Obstet. 1942;74:1011-1019.
3. Morton JH. An evaluation of blood-transfusion practices on a surgical service. N Engl J Med. 1960;263:1285-1287. PubMed
4. Pfunter A, Wier LM, Stocks C. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality, Rockville, MD. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb165.pdf. Accessed January 7, 2017.
5. Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113:3406-3417. PubMed
6. Koch CG, Li L, Duncan AI, et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608-1616. PubMed
7. Carson JL, Guyatt G, Heddle NM, et al. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA. 2016;316:2025-2035. PubMed
8. Ferraris VA, Brown JR, Despotis GJ, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91:944-982. PubMed
9. Callum JL, Waters JH, Shaz BH, et al. The AABB recommendations for the Choosing Wisely campaign of the American Board of Internal Medicine. Transfusion. 2014;54:2344-2352. PubMed
10. Podlasek SJ, Thakkar RN, Rotello LC, et al. Implementing a “Why give 2 when 1 will do?” Choosing Wisely campaign. Transfusion. 2016;56:2164. PubMed
11. Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409-417. PubMed
12. Hajjar LA, Vincent JL, Galas FR, et al. Transfusion requirements after cardiac surgery: The TRACS randomized controlled trial. JAMA. 2010;304:1559-1567. PubMed
13. Lacroix J, Hebert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609-1619. PubMed
14. Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453-2462. PubMed
15. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. New Engl J Med. 2013;368:11-21. PubMed
16. Holst LB, Haase N, Wetterslev J, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371:1381-1391. PubMed
17. Robertson CS, Hannay HJ, Yamal JM, et al. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: A randomized clinical trial. JAMA. 2014;312:36-47. PubMed
18. Murphy GJ, Pike K, Rogers CA, et al. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med. 2015;372:997-1008.
19. Meybohm P, Richards T, Isbister J, et al. Patient blood management bundles to facilitate implementation. Transfus Med Rev. 2017;31:62-71. PubMed
20. Frank SM, Resar LM, Rothschild JA, et al. A novel method of data analysis for utilization of red blood cell transfusion. Transfusion. 2013;53:3052-9. PubMed
21. Carson JL, Brooks MM, Abbott JD, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J. 2013;165:964.el-971.e1. PubMed
The “Things We Do for No Reason” (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

INTRODUCTION
Blood transfusion is not only the most common procedure performed in US hospitals but is also widely overused, according to The Joint Commission. Unnecessary transfusions can increase risks and costs, and now, multiple landmark trials support using restrictive transfusion strategies. This manuscript discusses the importance and potential impacts of giving single-unit red blood cell (RBC) transfusions in anemic patients who are not actively bleeding and are hemodynamically stable. The “thing we do for no reason” is giving 2-unit RBC transfusions when 1 unit would suffice. We call this the “Why give 2 when 1 will do?” campaign for RBC transfusion.
CASE PRESENTATION
A 74-year-old, 70-kg male with a known history of myelodysplastic syndrome is admitted for dizziness and shortness of breath. His hemoglobin (Hb) concentration is 6.2 g/dL (baseline Hb of 8 g/dL). The patient denies any hematuria, hematemesis, and melena. Physical examination is remarkable only for tachycardia—heart rate of 110. The admitting hospitalist ponders whether to order a 2-unit red blood cell (RBC) transfusion.
WHY YOU MIGHT THINK DOUBLE UNIT RED BLOOD CELL TRANSFUSIONS ARE HELPFUL
RBC transfusion is the most common procedure performed in US hospitals, with about 12 million RBC units given to patients in the United States each year.1 Based on an opinion paper published in 1942 by Adams and Lundy2 the “10/30 rule” set the standard that the ideal transfusion thresholds were an Hb of 10 g/dL or a hematocrit of 30%. Until human immunodeficiency virus (HIV) became a threat to the nation’s blood supply in the early 1980s, few questioned the 10/30 rule. There is no doubt that blood transfusions can be lifesaving in the presence of active bleeding or hemorrhagic shock; in fact, many hospitals have blood donation campaigns reminding us to “give blood—save a life.” To some, these messages may suggest that more blood is better. Prior to the 1990s, clinicians were taught that if the patient needed an RBC transfusion, 2 units was the optimal dose for adult patients. In fact, single-unit transfusions were strongly discouraged, and authorities on the risks of transfusion wrote that single-unit transfusions were acknowledged to be unnecessary.3
WHY THERE IS “NO REASON” TO ROUTINELY ORDER DOUBLE UNIT TRANSFUSIONS
According to a recent Joint Commission Overuse Summit, transfusion was identified as 1 of the top 5 overused medical procedures.4 Blood transfusions can cause complications such as transfusion-related acute lung injury and transfusion-associated circulatory overload, the number 1 and 2 causes of transfusion-related deaths, respectively,5 as well as other transfusion reactions (eg, allergic and hemolytic) and alloimmunization. Transfusion-related morbidity and mortality have been shown to be dose dependent,6 suggesting that the lowest effective number of units should be transfused. Although, with modern-day testing, the risks of HIV and viral hepatitis are exceedingly low, emerging infectious diseases such as the Zika virus and Babesiosis represent new threats to the nation’s blood supply, with potential transfusion-related transmission and severe consequences, especially for the immunosuppressed. As quality-improvement, patient safety, and cost-saving initiatives, many hospitals have implemented strategies to reduce unnecessary transfusions and decrease overall blood utilization.
The above-mentioned studies support the concept that oftentimes less is more for transfusions, which includes giving the lowest effective amount of transfused blood. These trials have enrolled multiple patient populations, such as critically ill patients in the intensive care unit,11,13 elderly orthopedic surgery patients,14 cardiac surgery patients,12 and patients with gastrointestinal hemorrhage,15 traumatic brain injury,17 and septicemia.16 Outcomes in the trials included mortality, serious infections, thrombotic and ischemic events, neurologic deficits, multiple-organ dysfunction, and inability to ambulate (Table). The findings in these studies suggest that we increase risks and cost without improving outcomes only by giving more blood than is necessary. Since most of these trials were published in the last decade, some very recently, clinicians have not fully adopted these newer, restrictive transfusion strategies.19
ARE THERE REASONS TO ORDER 2-UNIT TRANSFUSIONS IN CERTAIN CIRCUMSTANCES?
Perhaps the most common indication for ordering multiunit RBC transfusions is active bleeding, as it is clear that whatever Hb threshold is chosen, transfusion should be given in sufficient amounts to stay ahead of the bleeding.20 It is important to remember that we treat patients and their symptoms, not just their laboratory values. Good medical care adapts and/or modifies treatment protocols and guidelines according to the clinical situation. Intravascular volume is also important to consider because what really matters for oxygen content and delivery is the total red cell mass (ie, the Hb concentration times the blood volume). If a patient is hypovolemic and/or actively bleeding, the Hb transfusion trigger, as well as the dose of blood, may need to be adjusted upward, creating clinical scenarios in which 2-unit RBC transfusions may be appropriate. Other clinical settings for which multiunit RBC transfusions may be indicated include patients with severe anemia, for whom both the pretransfusion Hb (the trigger) and the posttransfusion Hb (the target) should be considered. Patients with hemoglobinopathies (eg, sickle cell or thalassemia) sometimes require multiunit transfusions or even exchange transfusions to improve oxygen delivery. Other patients who may benefit from higher Hb levels achieved by multiunit transfusions include those with acute coronary syndromes; however, the ideal Hb transfusion threshold in this setting has yet to be determined.21
WHAT YOU SHOULD DO INSTEAD
For hemodynamically stable patients and in the absence of active bleeding, single-unit RBC transfusions, followed by reassessment, should be the standard for most patients. The reassessment should include measuring the posttransfusion Hb level and checking for improvement in vital sign abnormalities and signs or symptoms of anemia or end-organ ischemia. A recent publication on our hospital-wide campaign called “Why give 2 when 1 will do?” showed a significant (35%) reduction in 2-unit transfusion orders along with an 18% overall decrease in RBC utilization and substantial cost savings (≈$600,000 per year).10 These findings demonstrate that there is a large opportunity to reduce transfusion overuse by encouraging single-unit transfusions.
RECOMMENDATIONS
- For nonbleeding, hemodynamically stable patients who require a transfusion, transfuse a single RBC unit and then reassess the Hb level before transfusing a second unit.
- The decision to transfuse RBCs should take into account the patient’s overall condition, including their symptoms, intravascular volume, and the occurrence and rate of active bleeding, not just the Hb value alone.
CONCLUSIONS
In stable patients, a single unit of RBCs often is adequate to raise the Hb to an acceptable level and relieve the signs and symptoms of anemia. Additional units should be prescribed only after reassessment of the patient and the Hb level. For our patient with symptomatic anemia, it is reasonable to transfuse 1 RBC unit, and then measure the Hb level, and reassess his symptoms before giving additional RBC units.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Acknowledgments
This publication is dedicated to our beloved colleague, Dr. Rajiv N. Thakkar, who recently and unexpectedly suffered a fatal cardiac event. We will miss him dearly.
Disclosure
S.M.F. has been on advisory boards for the Haemonetics Corporation (Braintree, MA), Medtronic Inc. (Minneapolis, MN), and Zimmer Biomet (Warsaw, IN). All other authors declare no competing interests.
The “Things We Do for No Reason” (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

INTRODUCTION
Blood transfusion is not only the most common procedure performed in US hospitals but is also widely overused, according to The Joint Commission. Unnecessary transfusions can increase risks and costs, and now, multiple landmark trials support using restrictive transfusion strategies. This manuscript discusses the importance and potential impacts of giving single-unit red blood cell (RBC) transfusions in anemic patients who are not actively bleeding and are hemodynamically stable. The “thing we do for no reason” is giving 2-unit RBC transfusions when 1 unit would suffice. We call this the “Why give 2 when 1 will do?” campaign for RBC transfusion.
CASE PRESENTATION
A 74-year-old, 70-kg male with a known history of myelodysplastic syndrome is admitted for dizziness and shortness of breath. His hemoglobin (Hb) concentration is 6.2 g/dL (baseline Hb of 8 g/dL). The patient denies any hematuria, hematemesis, and melena. Physical examination is remarkable only for tachycardia—heart rate of 110. The admitting hospitalist ponders whether to order a 2-unit red blood cell (RBC) transfusion.
WHY YOU MIGHT THINK DOUBLE UNIT RED BLOOD CELL TRANSFUSIONS ARE HELPFUL
RBC transfusion is the most common procedure performed in US hospitals, with about 12 million RBC units given to patients in the United States each year.1 Based on an opinion paper published in 1942 by Adams and Lundy2 the “10/30 rule” set the standard that the ideal transfusion thresholds were an Hb of 10 g/dL or a hematocrit of 30%. Until human immunodeficiency virus (HIV) became a threat to the nation’s blood supply in the early 1980s, few questioned the 10/30 rule. There is no doubt that blood transfusions can be lifesaving in the presence of active bleeding or hemorrhagic shock; in fact, many hospitals have blood donation campaigns reminding us to “give blood—save a life.” To some, these messages may suggest that more blood is better. Prior to the 1990s, clinicians were taught that if the patient needed an RBC transfusion, 2 units was the optimal dose for adult patients. In fact, single-unit transfusions were strongly discouraged, and authorities on the risks of transfusion wrote that single-unit transfusions were acknowledged to be unnecessary.3
WHY THERE IS “NO REASON” TO ROUTINELY ORDER DOUBLE UNIT TRANSFUSIONS
According to a recent Joint Commission Overuse Summit, transfusion was identified as 1 of the top 5 overused medical procedures.4 Blood transfusions can cause complications such as transfusion-related acute lung injury and transfusion-associated circulatory overload, the number 1 and 2 causes of transfusion-related deaths, respectively,5 as well as other transfusion reactions (eg, allergic and hemolytic) and alloimmunization. Transfusion-related morbidity and mortality have been shown to be dose dependent,6 suggesting that the lowest effective number of units should be transfused. Although, with modern-day testing, the risks of HIV and viral hepatitis are exceedingly low, emerging infectious diseases such as the Zika virus and Babesiosis represent new threats to the nation’s blood supply, with potential transfusion-related transmission and severe consequences, especially for the immunosuppressed. As quality-improvement, patient safety, and cost-saving initiatives, many hospitals have implemented strategies to reduce unnecessary transfusions and decrease overall blood utilization.
The above-mentioned studies support the concept that oftentimes less is more for transfusions, which includes giving the lowest effective amount of transfused blood. These trials have enrolled multiple patient populations, such as critically ill patients in the intensive care unit,11,13 elderly orthopedic surgery patients,14 cardiac surgery patients,12 and patients with gastrointestinal hemorrhage,15 traumatic brain injury,17 and septicemia.16 Outcomes in the trials included mortality, serious infections, thrombotic and ischemic events, neurologic deficits, multiple-organ dysfunction, and inability to ambulate (Table). The findings in these studies suggest that we increase risks and cost without improving outcomes only by giving more blood than is necessary. Since most of these trials were published in the last decade, some very recently, clinicians have not fully adopted these newer, restrictive transfusion strategies.19
ARE THERE REASONS TO ORDER 2-UNIT TRANSFUSIONS IN CERTAIN CIRCUMSTANCES?
Perhaps the most common indication for ordering multiunit RBC transfusions is active bleeding, as it is clear that whatever Hb threshold is chosen, transfusion should be given in sufficient amounts to stay ahead of the bleeding.20 It is important to remember that we treat patients and their symptoms, not just their laboratory values. Good medical care adapts and/or modifies treatment protocols and guidelines according to the clinical situation. Intravascular volume is also important to consider because what really matters for oxygen content and delivery is the total red cell mass (ie, the Hb concentration times the blood volume). If a patient is hypovolemic and/or actively bleeding, the Hb transfusion trigger, as well as the dose of blood, may need to be adjusted upward, creating clinical scenarios in which 2-unit RBC transfusions may be appropriate. Other clinical settings for which multiunit RBC transfusions may be indicated include patients with severe anemia, for whom both the pretransfusion Hb (the trigger) and the posttransfusion Hb (the target) should be considered. Patients with hemoglobinopathies (eg, sickle cell or thalassemia) sometimes require multiunit transfusions or even exchange transfusions to improve oxygen delivery. Other patients who may benefit from higher Hb levels achieved by multiunit transfusions include those with acute coronary syndromes; however, the ideal Hb transfusion threshold in this setting has yet to be determined.21
WHAT YOU SHOULD DO INSTEAD
For hemodynamically stable patients and in the absence of active bleeding, single-unit RBC transfusions, followed by reassessment, should be the standard for most patients. The reassessment should include measuring the posttransfusion Hb level and checking for improvement in vital sign abnormalities and signs or symptoms of anemia or end-organ ischemia. A recent publication on our hospital-wide campaign called “Why give 2 when 1 will do?” showed a significant (35%) reduction in 2-unit transfusion orders along with an 18% overall decrease in RBC utilization and substantial cost savings (≈$600,000 per year).10 These findings demonstrate that there is a large opportunity to reduce transfusion overuse by encouraging single-unit transfusions.
RECOMMENDATIONS
- For nonbleeding, hemodynamically stable patients who require a transfusion, transfuse a single RBC unit and then reassess the Hb level before transfusing a second unit.
- The decision to transfuse RBCs should take into account the patient’s overall condition, including their symptoms, intravascular volume, and the occurrence and rate of active bleeding, not just the Hb value alone.
CONCLUSIONS
In stable patients, a single unit of RBCs often is adequate to raise the Hb to an acceptable level and relieve the signs and symptoms of anemia. Additional units should be prescribed only after reassessment of the patient and the Hb level. For our patient with symptomatic anemia, it is reasonable to transfuse 1 RBC unit, and then measure the Hb level, and reassess his symptoms before giving additional RBC units.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Acknowledgments
This publication is dedicated to our beloved colleague, Dr. Rajiv N. Thakkar, who recently and unexpectedly suffered a fatal cardiac event. We will miss him dearly.
Disclosure
S.M.F. has been on advisory boards for the Haemonetics Corporation (Braintree, MA), Medtronic Inc. (Minneapolis, MN), and Zimmer Biomet (Warsaw, IN). All other authors declare no competing interests.
1. Whitaker B, Rajbhandary S, Kleinman S, Harris A, Kamani N. Trends in United States blood collection and transfusion: results from the 2013 AABB Blood Collection, Utilization, and Patient Blood Management Survey. Transfusion. 2016;56:2173-2183. PubMed
2. Adams C, Lundy JS. Anesthesia in cases of poor surgical risk – Some suggestions for decreasing the risk. Surg Gynec Obstet. 1942;74:1011-1019.
3. Morton JH. An evaluation of blood-transfusion practices on a surgical service. N Engl J Med. 1960;263:1285-1287. PubMed
4. Pfunter A, Wier LM, Stocks C. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality, Rockville, MD. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb165.pdf. Accessed January 7, 2017.
5. Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113:3406-3417. PubMed
6. Koch CG, Li L, Duncan AI, et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608-1616. PubMed
7. Carson JL, Guyatt G, Heddle NM, et al. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA. 2016;316:2025-2035. PubMed
8. Ferraris VA, Brown JR, Despotis GJ, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91:944-982. PubMed
9. Callum JL, Waters JH, Shaz BH, et al. The AABB recommendations for the Choosing Wisely campaign of the American Board of Internal Medicine. Transfusion. 2014;54:2344-2352. PubMed
10. Podlasek SJ, Thakkar RN, Rotello LC, et al. Implementing a “Why give 2 when 1 will do?” Choosing Wisely campaign. Transfusion. 2016;56:2164. PubMed
11. Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409-417. PubMed
12. Hajjar LA, Vincent JL, Galas FR, et al. Transfusion requirements after cardiac surgery: The TRACS randomized controlled trial. JAMA. 2010;304:1559-1567. PubMed
13. Lacroix J, Hebert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609-1619. PubMed
14. Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453-2462. PubMed
15. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. New Engl J Med. 2013;368:11-21. PubMed
16. Holst LB, Haase N, Wetterslev J, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371:1381-1391. PubMed
17. Robertson CS, Hannay HJ, Yamal JM, et al. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: A randomized clinical trial. JAMA. 2014;312:36-47. PubMed
18. Murphy GJ, Pike K, Rogers CA, et al. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med. 2015;372:997-1008.
19. Meybohm P, Richards T, Isbister J, et al. Patient blood management bundles to facilitate implementation. Transfus Med Rev. 2017;31:62-71. PubMed
20. Frank SM, Resar LM, Rothschild JA, et al. A novel method of data analysis for utilization of red blood cell transfusion. Transfusion. 2013;53:3052-9. PubMed
21. Carson JL, Brooks MM, Abbott JD, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J. 2013;165:964.el-971.e1. PubMed
1. Whitaker B, Rajbhandary S, Kleinman S, Harris A, Kamani N. Trends in United States blood collection and transfusion: results from the 2013 AABB Blood Collection, Utilization, and Patient Blood Management Survey. Transfusion. 2016;56:2173-2183. PubMed
2. Adams C, Lundy JS. Anesthesia in cases of poor surgical risk – Some suggestions for decreasing the risk. Surg Gynec Obstet. 1942;74:1011-1019.
3. Morton JH. An evaluation of blood-transfusion practices on a surgical service. N Engl J Med. 1960;263:1285-1287. PubMed
4. Pfunter A, Wier LM, Stocks C. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality, Rockville, MD. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb165.pdf. Accessed January 7, 2017.
5. Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113:3406-3417. PubMed
6. Koch CG, Li L, Duncan AI, et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608-1616. PubMed
7. Carson JL, Guyatt G, Heddle NM, et al. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA. 2016;316:2025-2035. PubMed
8. Ferraris VA, Brown JR, Despotis GJ, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91:944-982. PubMed
9. Callum JL, Waters JH, Shaz BH, et al. The AABB recommendations for the Choosing Wisely campaign of the American Board of Internal Medicine. Transfusion. 2014;54:2344-2352. PubMed
10. Podlasek SJ, Thakkar RN, Rotello LC, et al. Implementing a “Why give 2 when 1 will do?” Choosing Wisely campaign. Transfusion. 2016;56:2164. PubMed
11. Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409-417. PubMed
12. Hajjar LA, Vincent JL, Galas FR, et al. Transfusion requirements after cardiac surgery: The TRACS randomized controlled trial. JAMA. 2010;304:1559-1567. PubMed
13. Lacroix J, Hebert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609-1619. PubMed
14. Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453-2462. PubMed
15. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. New Engl J Med. 2013;368:11-21. PubMed
16. Holst LB, Haase N, Wetterslev J, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371:1381-1391. PubMed
17. Robertson CS, Hannay HJ, Yamal JM, et al. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: A randomized clinical trial. JAMA. 2014;312:36-47. PubMed
18. Murphy GJ, Pike K, Rogers CA, et al. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med. 2015;372:997-1008.
19. Meybohm P, Richards T, Isbister J, et al. Patient blood management bundles to facilitate implementation. Transfus Med Rev. 2017;31:62-71. PubMed
20. Frank SM, Resar LM, Rothschild JA, et al. A novel method of data analysis for utilization of red blood cell transfusion. Transfusion. 2013;53:3052-9. PubMed
21. Carson JL, Brooks MM, Abbott JD, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J. 2013;165:964.el-971.e1. PubMed
© 2017 Society of Hospital Medicine
Internal Medicine Resident Engagement with a Laboratory Utilization Dashboard: Mixed Methods Study
Recent efforts to reduce waste and overuse in healthcare include reforms, such as merit-based physician reimbursement for efficient resource use1 and the inclusion of cost-effective care as a competency for physician trainees.2 Focusing on resource use in physician training and reimbursement presumes that teaching and feedback about utilization can alter physician behavior. Early studies of social comparison feedback observed considerable variation in effectiveness, depending on the behavior targeted and how feedback was provided to physicians.3-5 The widespread adoption of electronic medical record (EMR) software enables the design of feedback interventions that provide continuous feedback in real-time via EMR-based practice dashboards. Currently, little is known about physician engagement with practice dashboards and, in particular, about trainee engagement with dashboards aimed to improve cost-effective care.
To inform future efforts in using social comparison feedback to teach cost-effective care in residency, we measured internal medicine resident engagement with an EMR-based utilization dashboard that provides feedback on their use of routine laboratory tests on an inpatient medicine service. Routine labs are often overused in the inpatient setting. In fact, one study reported that 68% of laboratory tests ordered in an academic hospital did not contribute to improving patient outcomes.6 To understand resident perceptions of the dashboards and identify barriers to their use, we conducted a mixed methods study tracking resident utilization of the dashboard over time and collecting qualitative data from 3 focus groups about resident attitudes toward the dashboards.
METHODS
From January 2016 to June 2016, resident-specific rates of routine lab orders (eg, complete blood count, basic metabolic panel, complete metabolic panel, liver function panel, and common coagulation tests) were synthesized continuously in a web-based dashboard. Laboratory orders could be placed either individually on a day-to-day basis or ordered on a recurrent basis (eg, daily morning labs ordered on admission). The dashboard contained an interactive graph, which plotted the average number of labs per patient-day ordered by each resident over the past week, along with an overall graph for all services for comparison (Appendix Figure). Residents could click on an individual day on the graph to review the labs they ordered for each patient. The dashboard also allowed the user to look up each patient’s medical record to obtain more detailed information.
All residents received an e-mail describing the study, including the purpose of the intervention, basic description of the feedback intervention (dashboard and e-mail), potential risks and benefits, duration and scope of data collection, and contact information of the principal investigator. One hundred and ninety-eight resident-blocks on 6 general medicine services at the Hospital of the University of Pennsylvania were cluster-randomized with an equal probability to 1 of 2 arms: (1) those e-mailed a snapshot of the personalized dashboard, a link to the online dashboard, and text containing resident and service utilization averages, and (2) those who did not receive the feedback intervention. Postgraduate year (PGY) 1 residents were attributed only orders by that resident. PGY2 and PGY3 residents were attributed orders for all patients assigned to the resident’s team.
The initial e-mails were timed to arrive in the middle of each resident’s 2-week service to allow for a baseline and follow-up period. The e-mail contained an attachment of a snapshot of the personalized graphic dashboard (Appendix Figure), a link to the online dashboard, and a few sentences summarizing the resident utilization average compared to the general medicine service overall, for the same time interval. They were followed by a reminder e-mail 24 hours later containing only the link to the report card. We measured resident engagement with the utilization dashboard by using e-mail read-receipts and a web-based tracking platform that recorded when the dashboard was opened and who logged on.
Following completion of the intervention, 3-hour-long focus groups were conducted with residents. These focus groups were guided with prescripted questions to prompt discussion on the advantages and drawbacks of the study intervention and the usage of dashboards in general. These sessions were digitally recorded and transcribed. The transcripts were reviewed by 2 authors (KR and GK) and analyzed to identify common themes by using a grounded theory approach.7 First, the transcripts were reviewed independently by each author, who each generated a broad list of themes across 3 domains: dashboard usability, barriers to use, and suggestions for the future. Next, the codebook was refined through an iterative series of discussions and transcript review, resulting in a unified codebook. Lastly, all transcripts were reviewed by using the final codebook definitions, resulting in a list of exemplary quotes and suggestions.
The study was approved by the University of Pennsylvania Institutional Review Board and registered on clinicaltrials.gov (NCT02330289).
RESULTS
Eighty unique residents participated in the intervention, including 51 PGY1s (64%) and 29 PGY2- or PGY3-level (36%) residents. Of these, 19/80 (24%) physicians participated more than once. 74% of participants opened the e-mail and 21% opened the link to the dashboard. The average elapsed time from receiving the initial e-mail to logging into the dashboard was 28.5 hours (standard deviation [SD] = 25.7, median = 25.5, interquartile range [IQR] = 40.5). On average, residents deviated from the service mean by 0.54 laboratory test orders (SD = 0.49, median = 0.40, IQR = 0.60). The mean baseline rate of targeted labs was 1.30 (SD 1.77) labs per physician per patient-day.8
We did not observe a statistically significant difference in routine laboratory ordering by dashboard use, although residents who opened the link to the dashboard ordered 0.26 fewer labs per doctor-patient-day than those who did not (95% CI, −0.77-0.25; P = 0.31). The greatest difference was observed on day 2 after the intervention, when lab orders were lower among dashboard users by 0.59 labs per doc-patient-day (95% CI, −1.41-0.24; P = 0.16) when compared with the residents who did not open the dashboard.
Third, participants identified barriers to using dashboards during training, including time constraints, insufficient patient volume, possible unanticipated consequences, and concerns regarding punitive action by the hospital administration or teaching supervisors. Suggestions to improve the uptake of practice feedback via dashboards included additional guidance for interpreting the data, exclusion of outlier cases or risk-adjustment, and ensuring ease of access to the data.
Last, participants also expressed enthusiasm toward receiving other types of individualized feedback data, including patient satisfaction, timing of discharges, readmission rates, utilization of consulting services, length of stay, antibiotic stewardship practices, costs and utilization data, and mortality or intensive care unit transfer rates (data not shown).
DISCUSSION
Overall, the engagement rates of internal medicine trainees with the online dashboard were low. Most residents did open the e-mails containing the link and basic information about their utilization rates, but less than a quarter of them accessed the dashboard containing real-time data. Additionally, on average, it took them more than a day to do so. However, there is some indication that residents who deviated further from the mean in either direction, which was described in the body of the e-mail, were more motivated to investigate further and click the link to access the dashboard. This suggests that providing practice feedback in this manner may be effective for a subset of residents who deviate from the “typical practice,” and as such, dashboards may represent a potential educational tool that could be aligned with practice-based learning competencies.
The focus groups provided important context about residents’ attitudes toward EMR-based dashboards. Overall, residents were enthusiastic about receiving information regarding their personal laboratory ordering, both in terms of preventing iatrogenic harm and waste of resources. This supports previous research that found that both medical students and residents overwhelmingly believe that the overuse of labs is a problem and that there may be insufficient focus on cost-conscious care during training.9,10 However, many residents questioned several aspects of the specific intervention used in this study and suggested that significant improvements would need to be made to future dashboards to increase their utility.
To our knowledge, this is the first attempt to evaluate resident engagement and attitudes toward receiving practice-based feedback via an EMR-based online dashboard. Previous efforts to influence resident laboratory ordering behavior have primarily focused on didactic sessions, financial incentives, price transparency, and repeated e-mail messaging containing summary statistics about ordering practices and peer comparisons.11-14 While some prior studies observed success in decreasing unnecessary use of laboratory tests, such efforts are challenging to implement routinely on a teaching service with multiple rotating providers and may be difficult to replicate. Future iterations of dashboards that incorporate focused curriculum design and active participation of teaching attendings require further study.
This study has several limitations. The sample size of physicians is relatively small and consists of residents at a single institution. This may limit the generalizability of the results. Additionally, the dashboard captured laboratory-ordering rates during a 2-week block on an inpatient medicine service and was not adjusted for factors such as patient case mix. However, the rates were adjusted for patient volume. In future iterations of utilization dashboards, residents’ concerns about small sample size and variability in clinical severity could be addressed through the adoption of risk-adjustment methodologies to balance out patient burden. This could be accomplished using currently available EMR data, such as diagnosis related groups or diagnoses codes to adjust for clinical complexity or report expected length of stay as a surrogate indicator of complexity.
Because residents are expected to be responsive to feedback, their use of the dashboards may represent an upper bound on physician responsiveness to social comparison feedback regarding utilization. However, e-mails alone may not be an effective way to provide feedback in areas that require additional engagement by the learner, especially given the volume of e-mails and alerts physicians receive. Future efforts to improve care efficiency may try to better capture baseline ordering rates, follow resident ordering over a longer period of time, encourage hospital staff to review utilization information with trainees, integrate dashboard information into regular performance reviews by the attendings, and provide more concrete feedback from attendings or senior residents for how this information can be used to adjust behavior.
Disclosure
Dr. Ryskina’s work on this study was supported by the Ruth L. Kirschstein National Research Service Award (T32-HP10026) and the NIA Career Development Award (K08AG052572). Dr. Patel reports board membership on the advisory board of and owning stock/stock options for Healthmine Services, and serving as a consultant and owning stock/stock options for Catalyst Health LLC. The authors declare no conflict of interest.
1. Clough JD, McClellan M. Implementing MACRA: Implications for Physicians and for Physician Leadership. JAMA. 2016;315(22):2397-2398. PubMed
2. The Internal Medicine Subspecialty Milestones Project. A Joint Initiative of the Accrediation Council for Graduate Medical Education and The American Board of Internal Medicine. http://www.acgme.org/portals/0/pdfs/milestones/internalmedicinesubspecialtymilestoint.pdf. Accessed July 6, 2016.
3. Meeker D, Linder JA, Fox CR, et al. Effect of Behavioral Interventions on Inappropriate Antibiotic Prescribing Among Primary Care Practices: A Randomized Clinical Trial. JAMA. 2016;315(6):562-570. PubMed
4. Jamtvedt G, Young JM, Kristoffersen DT, O’Brien MA, Oxman AD. Audit and feedback: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2006;2(2):CD000259. PubMed
5. Navathe AS, Emanuel EJ. Physician Peer Comparisons as a Nonfinancial Strategy to Improve the Value of Care. JAMA. 2016;316(17)1759-1760. PubMed
6. Miyakis S, Karamanof G, Liontos M, Mountokalakis TD. Factors contributing to inappropriate ordering of tests in an academic medical department and the effect of an educational feedback strategy. Postgrad Med J. 2006;82(974):823-829. PubMed
7. Glaser B, Strauss A. The Discovery of Grounded Theory. London: Weidenfeld and Nicholson; 1967.
8. Ryskina K, Dine J, Gitelman Y, et al. Effect of norms on laboratory and imaging testing (ENLITen): A Randomized Controlled Trial. Abstract presented at the Society of General Internal Medicine Conference; April 20, 2017; Washington, DC.
9. Sedrak MS, Patel MS, Ziemba JB, et al. Residents’ self-report on why they order perceived unnecessary inpatient laboratory tests. J Hosp Med. 2016;11(12):869-872. PubMed
10. Tartaglia KM, Kman N, Ledford C. Medical student perceptions of cost-conscious care in an internal medicine clerkship: a thematic analysis. J Gen Intern Med. 2015;30(10):1491-1496. PubMed
11. Iams W, Heck J, Kapp M, et al. A Multidisciplinary Housestaff-Led Initiative to Safely Reduce Daily Laboratory Testing. Acad Med. 2016;91(6):813-820. DOI:10.1097/ACM.0000000000001149. PubMed
12. Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: decreased frequency of common labs. J Hosp Med. 2015;10:390-395. PubMed
13. Yarbrough P, Kukhareva P, Horton D, Edholm K, Kawamoto K. Multifaceted Intervention including Education, Rounding Checklist Implementation, Cost Feedback, and Financial Incentives Reduces Inpatient Laboratory Costs. J Hosp Med. 2016;11(5):348-354. PubMed
14. Feldman LS, Shihab HM, Thiemann D, et al. Impact of Providing Fee Data on Laboratory Test Ordering: A Controlled Clinical Trial. JAMA Intern Med. 2013;173(10):903-908. PubMed
Recent efforts to reduce waste and overuse in healthcare include reforms, such as merit-based physician reimbursement for efficient resource use1 and the inclusion of cost-effective care as a competency for physician trainees.2 Focusing on resource use in physician training and reimbursement presumes that teaching and feedback about utilization can alter physician behavior. Early studies of social comparison feedback observed considerable variation in effectiveness, depending on the behavior targeted and how feedback was provided to physicians.3-5 The widespread adoption of electronic medical record (EMR) software enables the design of feedback interventions that provide continuous feedback in real-time via EMR-based practice dashboards. Currently, little is known about physician engagement with practice dashboards and, in particular, about trainee engagement with dashboards aimed to improve cost-effective care.
To inform future efforts in using social comparison feedback to teach cost-effective care in residency, we measured internal medicine resident engagement with an EMR-based utilization dashboard that provides feedback on their use of routine laboratory tests on an inpatient medicine service. Routine labs are often overused in the inpatient setting. In fact, one study reported that 68% of laboratory tests ordered in an academic hospital did not contribute to improving patient outcomes.6 To understand resident perceptions of the dashboards and identify barriers to their use, we conducted a mixed methods study tracking resident utilization of the dashboard over time and collecting qualitative data from 3 focus groups about resident attitudes toward the dashboards.
METHODS
From January 2016 to June 2016, resident-specific rates of routine lab orders (eg, complete blood count, basic metabolic panel, complete metabolic panel, liver function panel, and common coagulation tests) were synthesized continuously in a web-based dashboard. Laboratory orders could be placed either individually on a day-to-day basis or ordered on a recurrent basis (eg, daily morning labs ordered on admission). The dashboard contained an interactive graph, which plotted the average number of labs per patient-day ordered by each resident over the past week, along with an overall graph for all services for comparison (Appendix Figure). Residents could click on an individual day on the graph to review the labs they ordered for each patient. The dashboard also allowed the user to look up each patient’s medical record to obtain more detailed information.
All residents received an e-mail describing the study, including the purpose of the intervention, basic description of the feedback intervention (dashboard and e-mail), potential risks and benefits, duration and scope of data collection, and contact information of the principal investigator. One hundred and ninety-eight resident-blocks on 6 general medicine services at the Hospital of the University of Pennsylvania were cluster-randomized with an equal probability to 1 of 2 arms: (1) those e-mailed a snapshot of the personalized dashboard, a link to the online dashboard, and text containing resident and service utilization averages, and (2) those who did not receive the feedback intervention. Postgraduate year (PGY) 1 residents were attributed only orders by that resident. PGY2 and PGY3 residents were attributed orders for all patients assigned to the resident’s team.
The initial e-mails were timed to arrive in the middle of each resident’s 2-week service to allow for a baseline and follow-up period. The e-mail contained an attachment of a snapshot of the personalized graphic dashboard (Appendix Figure), a link to the online dashboard, and a few sentences summarizing the resident utilization average compared to the general medicine service overall, for the same time interval. They were followed by a reminder e-mail 24 hours later containing only the link to the report card. We measured resident engagement with the utilization dashboard by using e-mail read-receipts and a web-based tracking platform that recorded when the dashboard was opened and who logged on.
Following completion of the intervention, 3-hour-long focus groups were conducted with residents. These focus groups were guided with prescripted questions to prompt discussion on the advantages and drawbacks of the study intervention and the usage of dashboards in general. These sessions were digitally recorded and transcribed. The transcripts were reviewed by 2 authors (KR and GK) and analyzed to identify common themes by using a grounded theory approach.7 First, the transcripts were reviewed independently by each author, who each generated a broad list of themes across 3 domains: dashboard usability, barriers to use, and suggestions for the future. Next, the codebook was refined through an iterative series of discussions and transcript review, resulting in a unified codebook. Lastly, all transcripts were reviewed by using the final codebook definitions, resulting in a list of exemplary quotes and suggestions.
The study was approved by the University of Pennsylvania Institutional Review Board and registered on clinicaltrials.gov (NCT02330289).
RESULTS
Eighty unique residents participated in the intervention, including 51 PGY1s (64%) and 29 PGY2- or PGY3-level (36%) residents. Of these, 19/80 (24%) physicians participated more than once. 74% of participants opened the e-mail and 21% opened the link to the dashboard. The average elapsed time from receiving the initial e-mail to logging into the dashboard was 28.5 hours (standard deviation [SD] = 25.7, median = 25.5, interquartile range [IQR] = 40.5). On average, residents deviated from the service mean by 0.54 laboratory test orders (SD = 0.49, median = 0.40, IQR = 0.60). The mean baseline rate of targeted labs was 1.30 (SD 1.77) labs per physician per patient-day.8
We did not observe a statistically significant difference in routine laboratory ordering by dashboard use, although residents who opened the link to the dashboard ordered 0.26 fewer labs per doctor-patient-day than those who did not (95% CI, −0.77-0.25; P = 0.31). The greatest difference was observed on day 2 after the intervention, when lab orders were lower among dashboard users by 0.59 labs per doc-patient-day (95% CI, −1.41-0.24; P = 0.16) when compared with the residents who did not open the dashboard.
Third, participants identified barriers to using dashboards during training, including time constraints, insufficient patient volume, possible unanticipated consequences, and concerns regarding punitive action by the hospital administration or teaching supervisors. Suggestions to improve the uptake of practice feedback via dashboards included additional guidance for interpreting the data, exclusion of outlier cases or risk-adjustment, and ensuring ease of access to the data.
Last, participants also expressed enthusiasm toward receiving other types of individualized feedback data, including patient satisfaction, timing of discharges, readmission rates, utilization of consulting services, length of stay, antibiotic stewardship practices, costs and utilization data, and mortality or intensive care unit transfer rates (data not shown).
DISCUSSION
Overall, the engagement rates of internal medicine trainees with the online dashboard were low. Most residents did open the e-mails containing the link and basic information about their utilization rates, but less than a quarter of them accessed the dashboard containing real-time data. Additionally, on average, it took them more than a day to do so. However, there is some indication that residents who deviated further from the mean in either direction, which was described in the body of the e-mail, were more motivated to investigate further and click the link to access the dashboard. This suggests that providing practice feedback in this manner may be effective for a subset of residents who deviate from the “typical practice,” and as such, dashboards may represent a potential educational tool that could be aligned with practice-based learning competencies.
The focus groups provided important context about residents’ attitudes toward EMR-based dashboards. Overall, residents were enthusiastic about receiving information regarding their personal laboratory ordering, both in terms of preventing iatrogenic harm and waste of resources. This supports previous research that found that both medical students and residents overwhelmingly believe that the overuse of labs is a problem and that there may be insufficient focus on cost-conscious care during training.9,10 However, many residents questioned several aspects of the specific intervention used in this study and suggested that significant improvements would need to be made to future dashboards to increase their utility.
To our knowledge, this is the first attempt to evaluate resident engagement and attitudes toward receiving practice-based feedback via an EMR-based online dashboard. Previous efforts to influence resident laboratory ordering behavior have primarily focused on didactic sessions, financial incentives, price transparency, and repeated e-mail messaging containing summary statistics about ordering practices and peer comparisons.11-14 While some prior studies observed success in decreasing unnecessary use of laboratory tests, such efforts are challenging to implement routinely on a teaching service with multiple rotating providers and may be difficult to replicate. Future iterations of dashboards that incorporate focused curriculum design and active participation of teaching attendings require further study.
This study has several limitations. The sample size of physicians is relatively small and consists of residents at a single institution. This may limit the generalizability of the results. Additionally, the dashboard captured laboratory-ordering rates during a 2-week block on an inpatient medicine service and was not adjusted for factors such as patient case mix. However, the rates were adjusted for patient volume. In future iterations of utilization dashboards, residents’ concerns about small sample size and variability in clinical severity could be addressed through the adoption of risk-adjustment methodologies to balance out patient burden. This could be accomplished using currently available EMR data, such as diagnosis related groups or diagnoses codes to adjust for clinical complexity or report expected length of stay as a surrogate indicator of complexity.
Because residents are expected to be responsive to feedback, their use of the dashboards may represent an upper bound on physician responsiveness to social comparison feedback regarding utilization. However, e-mails alone may not be an effective way to provide feedback in areas that require additional engagement by the learner, especially given the volume of e-mails and alerts physicians receive. Future efforts to improve care efficiency may try to better capture baseline ordering rates, follow resident ordering over a longer period of time, encourage hospital staff to review utilization information with trainees, integrate dashboard information into regular performance reviews by the attendings, and provide more concrete feedback from attendings or senior residents for how this information can be used to adjust behavior.
Disclosure
Dr. Ryskina’s work on this study was supported by the Ruth L. Kirschstein National Research Service Award (T32-HP10026) and the NIA Career Development Award (K08AG052572). Dr. Patel reports board membership on the advisory board of and owning stock/stock options for Healthmine Services, and serving as a consultant and owning stock/stock options for Catalyst Health LLC. The authors declare no conflict of interest.
Recent efforts to reduce waste and overuse in healthcare include reforms, such as merit-based physician reimbursement for efficient resource use1 and the inclusion of cost-effective care as a competency for physician trainees.2 Focusing on resource use in physician training and reimbursement presumes that teaching and feedback about utilization can alter physician behavior. Early studies of social comparison feedback observed considerable variation in effectiveness, depending on the behavior targeted and how feedback was provided to physicians.3-5 The widespread adoption of electronic medical record (EMR) software enables the design of feedback interventions that provide continuous feedback in real-time via EMR-based practice dashboards. Currently, little is known about physician engagement with practice dashboards and, in particular, about trainee engagement with dashboards aimed to improve cost-effective care.
To inform future efforts in using social comparison feedback to teach cost-effective care in residency, we measured internal medicine resident engagement with an EMR-based utilization dashboard that provides feedback on their use of routine laboratory tests on an inpatient medicine service. Routine labs are often overused in the inpatient setting. In fact, one study reported that 68% of laboratory tests ordered in an academic hospital did not contribute to improving patient outcomes.6 To understand resident perceptions of the dashboards and identify barriers to their use, we conducted a mixed methods study tracking resident utilization of the dashboard over time and collecting qualitative data from 3 focus groups about resident attitudes toward the dashboards.
METHODS
From January 2016 to June 2016, resident-specific rates of routine lab orders (eg, complete blood count, basic metabolic panel, complete metabolic panel, liver function panel, and common coagulation tests) were synthesized continuously in a web-based dashboard. Laboratory orders could be placed either individually on a day-to-day basis or ordered on a recurrent basis (eg, daily morning labs ordered on admission). The dashboard contained an interactive graph, which plotted the average number of labs per patient-day ordered by each resident over the past week, along with an overall graph for all services for comparison (Appendix Figure). Residents could click on an individual day on the graph to review the labs they ordered for each patient. The dashboard also allowed the user to look up each patient’s medical record to obtain more detailed information.
All residents received an e-mail describing the study, including the purpose of the intervention, basic description of the feedback intervention (dashboard and e-mail), potential risks and benefits, duration and scope of data collection, and contact information of the principal investigator. One hundred and ninety-eight resident-blocks on 6 general medicine services at the Hospital of the University of Pennsylvania were cluster-randomized with an equal probability to 1 of 2 arms: (1) those e-mailed a snapshot of the personalized dashboard, a link to the online dashboard, and text containing resident and service utilization averages, and (2) those who did not receive the feedback intervention. Postgraduate year (PGY) 1 residents were attributed only orders by that resident. PGY2 and PGY3 residents were attributed orders for all patients assigned to the resident’s team.
The initial e-mails were timed to arrive in the middle of each resident’s 2-week service to allow for a baseline and follow-up period. The e-mail contained an attachment of a snapshot of the personalized graphic dashboard (Appendix Figure), a link to the online dashboard, and a few sentences summarizing the resident utilization average compared to the general medicine service overall, for the same time interval. They were followed by a reminder e-mail 24 hours later containing only the link to the report card. We measured resident engagement with the utilization dashboard by using e-mail read-receipts and a web-based tracking platform that recorded when the dashboard was opened and who logged on.
Following completion of the intervention, 3-hour-long focus groups were conducted with residents. These focus groups were guided with prescripted questions to prompt discussion on the advantages and drawbacks of the study intervention and the usage of dashboards in general. These sessions were digitally recorded and transcribed. The transcripts were reviewed by 2 authors (KR and GK) and analyzed to identify common themes by using a grounded theory approach.7 First, the transcripts were reviewed independently by each author, who each generated a broad list of themes across 3 domains: dashboard usability, barriers to use, and suggestions for the future. Next, the codebook was refined through an iterative series of discussions and transcript review, resulting in a unified codebook. Lastly, all transcripts were reviewed by using the final codebook definitions, resulting in a list of exemplary quotes and suggestions.
The study was approved by the University of Pennsylvania Institutional Review Board and registered on clinicaltrials.gov (NCT02330289).
RESULTS
Eighty unique residents participated in the intervention, including 51 PGY1s (64%) and 29 PGY2- or PGY3-level (36%) residents. Of these, 19/80 (24%) physicians participated more than once. 74% of participants opened the e-mail and 21% opened the link to the dashboard. The average elapsed time from receiving the initial e-mail to logging into the dashboard was 28.5 hours (standard deviation [SD] = 25.7, median = 25.5, interquartile range [IQR] = 40.5). On average, residents deviated from the service mean by 0.54 laboratory test orders (SD = 0.49, median = 0.40, IQR = 0.60). The mean baseline rate of targeted labs was 1.30 (SD 1.77) labs per physician per patient-day.8
We did not observe a statistically significant difference in routine laboratory ordering by dashboard use, although residents who opened the link to the dashboard ordered 0.26 fewer labs per doctor-patient-day than those who did not (95% CI, −0.77-0.25; P = 0.31). The greatest difference was observed on day 2 after the intervention, when lab orders were lower among dashboard users by 0.59 labs per doc-patient-day (95% CI, −1.41-0.24; P = 0.16) when compared with the residents who did not open the dashboard.
Third, participants identified barriers to using dashboards during training, including time constraints, insufficient patient volume, possible unanticipated consequences, and concerns regarding punitive action by the hospital administration or teaching supervisors. Suggestions to improve the uptake of practice feedback via dashboards included additional guidance for interpreting the data, exclusion of outlier cases or risk-adjustment, and ensuring ease of access to the data.
Last, participants also expressed enthusiasm toward receiving other types of individualized feedback data, including patient satisfaction, timing of discharges, readmission rates, utilization of consulting services, length of stay, antibiotic stewardship practices, costs and utilization data, and mortality or intensive care unit transfer rates (data not shown).
DISCUSSION
Overall, the engagement rates of internal medicine trainees with the online dashboard were low. Most residents did open the e-mails containing the link and basic information about their utilization rates, but less than a quarter of them accessed the dashboard containing real-time data. Additionally, on average, it took them more than a day to do so. However, there is some indication that residents who deviated further from the mean in either direction, which was described in the body of the e-mail, were more motivated to investigate further and click the link to access the dashboard. This suggests that providing practice feedback in this manner may be effective for a subset of residents who deviate from the “typical practice,” and as such, dashboards may represent a potential educational tool that could be aligned with practice-based learning competencies.
The focus groups provided important context about residents’ attitudes toward EMR-based dashboards. Overall, residents were enthusiastic about receiving information regarding their personal laboratory ordering, both in terms of preventing iatrogenic harm and waste of resources. This supports previous research that found that both medical students and residents overwhelmingly believe that the overuse of labs is a problem and that there may be insufficient focus on cost-conscious care during training.9,10 However, many residents questioned several aspects of the specific intervention used in this study and suggested that significant improvements would need to be made to future dashboards to increase their utility.
To our knowledge, this is the first attempt to evaluate resident engagement and attitudes toward receiving practice-based feedback via an EMR-based online dashboard. Previous efforts to influence resident laboratory ordering behavior have primarily focused on didactic sessions, financial incentives, price transparency, and repeated e-mail messaging containing summary statistics about ordering practices and peer comparisons.11-14 While some prior studies observed success in decreasing unnecessary use of laboratory tests, such efforts are challenging to implement routinely on a teaching service with multiple rotating providers and may be difficult to replicate. Future iterations of dashboards that incorporate focused curriculum design and active participation of teaching attendings require further study.
This study has several limitations. The sample size of physicians is relatively small and consists of residents at a single institution. This may limit the generalizability of the results. Additionally, the dashboard captured laboratory-ordering rates during a 2-week block on an inpatient medicine service and was not adjusted for factors such as patient case mix. However, the rates were adjusted for patient volume. In future iterations of utilization dashboards, residents’ concerns about small sample size and variability in clinical severity could be addressed through the adoption of risk-adjustment methodologies to balance out patient burden. This could be accomplished using currently available EMR data, such as diagnosis related groups or diagnoses codes to adjust for clinical complexity or report expected length of stay as a surrogate indicator of complexity.
Because residents are expected to be responsive to feedback, their use of the dashboards may represent an upper bound on physician responsiveness to social comparison feedback regarding utilization. However, e-mails alone may not be an effective way to provide feedback in areas that require additional engagement by the learner, especially given the volume of e-mails and alerts physicians receive. Future efforts to improve care efficiency may try to better capture baseline ordering rates, follow resident ordering over a longer period of time, encourage hospital staff to review utilization information with trainees, integrate dashboard information into regular performance reviews by the attendings, and provide more concrete feedback from attendings or senior residents for how this information can be used to adjust behavior.
Disclosure
Dr. Ryskina’s work on this study was supported by the Ruth L. Kirschstein National Research Service Award (T32-HP10026) and the NIA Career Development Award (K08AG052572). Dr. Patel reports board membership on the advisory board of and owning stock/stock options for Healthmine Services, and serving as a consultant and owning stock/stock options for Catalyst Health LLC. The authors declare no conflict of interest.
1. Clough JD, McClellan M. Implementing MACRA: Implications for Physicians and for Physician Leadership. JAMA. 2016;315(22):2397-2398. PubMed
2. The Internal Medicine Subspecialty Milestones Project. A Joint Initiative of the Accrediation Council for Graduate Medical Education and The American Board of Internal Medicine. http://www.acgme.org/portals/0/pdfs/milestones/internalmedicinesubspecialtymilestoint.pdf. Accessed July 6, 2016.
3. Meeker D, Linder JA, Fox CR, et al. Effect of Behavioral Interventions on Inappropriate Antibiotic Prescribing Among Primary Care Practices: A Randomized Clinical Trial. JAMA. 2016;315(6):562-570. PubMed
4. Jamtvedt G, Young JM, Kristoffersen DT, O’Brien MA, Oxman AD. Audit and feedback: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2006;2(2):CD000259. PubMed
5. Navathe AS, Emanuel EJ. Physician Peer Comparisons as a Nonfinancial Strategy to Improve the Value of Care. JAMA. 2016;316(17)1759-1760. PubMed
6. Miyakis S, Karamanof G, Liontos M, Mountokalakis TD. Factors contributing to inappropriate ordering of tests in an academic medical department and the effect of an educational feedback strategy. Postgrad Med J. 2006;82(974):823-829. PubMed
7. Glaser B, Strauss A. The Discovery of Grounded Theory. London: Weidenfeld and Nicholson; 1967.
8. Ryskina K, Dine J, Gitelman Y, et al. Effect of norms on laboratory and imaging testing (ENLITen): A Randomized Controlled Trial. Abstract presented at the Society of General Internal Medicine Conference; April 20, 2017; Washington, DC.
9. Sedrak MS, Patel MS, Ziemba JB, et al. Residents’ self-report on why they order perceived unnecessary inpatient laboratory tests. J Hosp Med. 2016;11(12):869-872. PubMed
10. Tartaglia KM, Kman N, Ledford C. Medical student perceptions of cost-conscious care in an internal medicine clerkship: a thematic analysis. J Gen Intern Med. 2015;30(10):1491-1496. PubMed
11. Iams W, Heck J, Kapp M, et al. A Multidisciplinary Housestaff-Led Initiative to Safely Reduce Daily Laboratory Testing. Acad Med. 2016;91(6):813-820. DOI:10.1097/ACM.0000000000001149. PubMed
12. Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: decreased frequency of common labs. J Hosp Med. 2015;10:390-395. PubMed
13. Yarbrough P, Kukhareva P, Horton D, Edholm K, Kawamoto K. Multifaceted Intervention including Education, Rounding Checklist Implementation, Cost Feedback, and Financial Incentives Reduces Inpatient Laboratory Costs. J Hosp Med. 2016;11(5):348-354. PubMed
14. Feldman LS, Shihab HM, Thiemann D, et al. Impact of Providing Fee Data on Laboratory Test Ordering: A Controlled Clinical Trial. JAMA Intern Med. 2013;173(10):903-908. PubMed
1. Clough JD, McClellan M. Implementing MACRA: Implications for Physicians and for Physician Leadership. JAMA. 2016;315(22):2397-2398. PubMed
2. The Internal Medicine Subspecialty Milestones Project. A Joint Initiative of the Accrediation Council for Graduate Medical Education and The American Board of Internal Medicine. http://www.acgme.org/portals/0/pdfs/milestones/internalmedicinesubspecialtymilestoint.pdf. Accessed July 6, 2016.
3. Meeker D, Linder JA, Fox CR, et al. Effect of Behavioral Interventions on Inappropriate Antibiotic Prescribing Among Primary Care Practices: A Randomized Clinical Trial. JAMA. 2016;315(6):562-570. PubMed
4. Jamtvedt G, Young JM, Kristoffersen DT, O’Brien MA, Oxman AD. Audit and feedback: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2006;2(2):CD000259. PubMed
5. Navathe AS, Emanuel EJ. Physician Peer Comparisons as a Nonfinancial Strategy to Improve the Value of Care. JAMA. 2016;316(17)1759-1760. PubMed
6. Miyakis S, Karamanof G, Liontos M, Mountokalakis TD. Factors contributing to inappropriate ordering of tests in an academic medical department and the effect of an educational feedback strategy. Postgrad Med J. 2006;82(974):823-829. PubMed
7. Glaser B, Strauss A. The Discovery of Grounded Theory. London: Weidenfeld and Nicholson; 1967.
8. Ryskina K, Dine J, Gitelman Y, et al. Effect of norms on laboratory and imaging testing (ENLITen): A Randomized Controlled Trial. Abstract presented at the Society of General Internal Medicine Conference; April 20, 2017; Washington, DC.
9. Sedrak MS, Patel MS, Ziemba JB, et al. Residents’ self-report on why they order perceived unnecessary inpatient laboratory tests. J Hosp Med. 2016;11(12):869-872. PubMed
10. Tartaglia KM, Kman N, Ledford C. Medical student perceptions of cost-conscious care in an internal medicine clerkship: a thematic analysis. J Gen Intern Med. 2015;30(10):1491-1496. PubMed
11. Iams W, Heck J, Kapp M, et al. A Multidisciplinary Housestaff-Led Initiative to Safely Reduce Daily Laboratory Testing. Acad Med. 2016;91(6):813-820. DOI:10.1097/ACM.0000000000001149. PubMed
12. Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: decreased frequency of common labs. J Hosp Med. 2015;10:390-395. PubMed
13. Yarbrough P, Kukhareva P, Horton D, Edholm K, Kawamoto K. Multifaceted Intervention including Education, Rounding Checklist Implementation, Cost Feedback, and Financial Incentives Reduces Inpatient Laboratory Costs. J Hosp Med. 2016;11(5):348-354. PubMed
14. Feldman LS, Shihab HM, Thiemann D, et al. Impact of Providing Fee Data on Laboratory Test Ordering: A Controlled Clinical Trial. JAMA Intern Med. 2013;173(10):903-908. PubMed
© 2017 Society of Hospital Medicine
Blood Products Provided to Patients Receiving Futile Critical Care
Critical care physicians frequently find themselves providing care that they find to be futile or inappropriate for hospitalized critically ill patients. A survey of physicians found that 87% felt that “futile” treatment was provided in their intensive care unit (ICU) in the past year.1 In a single-day cross-sectional study, 27% of ICU clinicians reported providing inappropriate care to at least 1 patient, most of which was excessive.2 In a 3-month study, 11% of all ICU patients were perceived by their physician as receiving futile treatment at some point during their ICU hospitalization.3 Given that more than 1 in 5 decedents die after an ICU stay during a terminal admission, there is increasing scrutiny of the ICU as a setting where potentially inappropriate resource-intensive treatment is provided.4-6 Blood is an especially valuable resource, not only because it exists in finite supply (and is sometimes in shortage) but also because it is donated in ways that arguably create special stewardship expectations and responsibilities for those trusted to make decisions about its use. The amount of blood products used for patients who are perceived to be receiving inappropriate critical care has not been quantified.
Blood transfusion is the most frequently performed inpatient procedure, occurring in more than 10% of hospital admissions that involve a procedure.7 When used appropriately, the transfusion of blood products can be lifesaving; however, studies show that some transfused blood might not be needed and efforts are afoot to improve the match between transfusion and transfusion need.8,9 These efforts largely focus on generating guidelines based on physiologic benefit and aim mainly at promoting a restrictive transfusion protocol by avoiding blood product use for patients who will likely do well even without transfusion.8,10-12 The guiding principle behind efforts to improve the stewardship of scarce blood products is that they should only be used if they will make a difference in patient outcomes. Unlike prior studies, the goal of this study is to quantify the amount of blood products administered to patients who would do poorly with or without receipt of blood products, that is, patients perceived by their physicians as receiving futile critical care.
MATERIALS AND METHODS
Based on a focus group discussion with physicians who cared for critically ill patients, a questionnaire was developed to identify patients perceived as receiving futile critical care. Details of the definition of futile treatment and the core data collection are described in detail elsewhere.3
For each ICU patient under the physician’s care, the attending physician completed a daily questionnaire asking whether the patient was receiving futile treatment, probably futile treatment, or nonfutile treatment. These surveys were administered every day from December 15, 2011, through March 15, 2012, to each critical care specialist providing care in 5 ICUs (medical ICU, neurocritical care ICU, cardiac care unit, cardiothoracic ICU, and a mixed medical-surgical ICU) in 1 academic health system. All clinicians provided informed consent.
Patients were categorized into the following 3 groups: patients for whom treatment was never perceived as futile; patients with at least 1 assessment that treatment was probably futile, but no futile treatment assessments; and patients who had at least 1 assessment of futile treatment. Hospital and 6-month mortality was abstracted for all patients.
The Division of Transfusion Medicine provided a database of all adult patients during the 3-month study period who received a transfusion of packed red blood cells (PRBCs), apheresis platelets, plasma, or cryoprecipitate (5 unit prepooled units). This database was merged with the daily assessments of the appropriateness of critical care. To determine the proportion of blood products that was utilized for patients receiving inappropriate treatment, we tallied the blood products infused to these patients after the day the patient was assessed as receiving probably inappropriate or inappropriate treatment. The denominator was the total amount of blood products used by all assessed patients during the 3-month study period.
This study was approved by the University of California Los Angeles Institutional Review Board.
RESULTS
During the 3-month study period, 36 critical care clinicians in 5 ICUs provided care to 1193 adult patients. After excluding boarders in the ICUs and missed and invalid assessments, 6916 assessments were made on 1136 patients. Of these 1136 patients, 98 (8.6%) patients received probably futile treatment and 123 (11%) patients received futile treatment according to the physicians caring for them.
For patients who were never rated as receiving futile treatment, the in-hospital mortality was 4.6% and the 6-month mortality was 7.3%. On the contrary, 68% of the patients who were perceived to receive futile ICU treatment died before hospital discharge and 85% died within 6 months; survivors remained in severely compromised health states.3
DISCUSSION
Blood and blood products are donated resources. These biological products are altruistically given with the expectation that they will be used to benefit others.13 It is the clinicians’ responsibility to use these precious gifts to achieve the goals of medicine, which include curing, preserving function, and preventing clinical deterioration that has meaning to the patient. Our study shows that a small, but not insignificant, proportion of these donated resources are provided to hospitalized patients who are perceived as receiving futile critical care. That means that these transfusions are used as part of the critical care interventions that prolong the dying process and achieve outcomes, such as existence in coma, which few, if any, patients would desire. However, it should be noted that some of the health states preserved, such as neurological devastation or multi-organ failure with an inability to survive outside an ICU, were likely desired by patients’ families and might even have been desired by patients themselves. Whether blood donors would wish to donate blood to preserve life in such compromised health states is testable. This proportion of blood provided to ICU patients perceived as receiving futile treatment (7.6%) is similar to or greater than that lost due to wastage, which ranges from 0.1% to 6.7%.14 While the loss of this small proportion of blood products due to expiration or procedural issues is probably unavoidable, but should be minimized as much as possible, the provision of blood products to patients receiving futile critical care is under the control of the healthcare team. This raises the question of how altruistic blood donors would feel about donating if they were aware that 1 of every 13 units transfused in the ICU would be given to a patient that the physician feels will not benefit. In turn, it raises the question of whether the physician should refrain from using these blood products for patients who will not benefit in accordance with principles of evidence-based medicine, in order to ensure their availability for patients that will benefit.
This study has several limitations. Family/patient perspectives were not included in the assessment of futile treatment. It should also be recognized that the percentage of blood products provided to patients receiving inappropriate critical care is likely an underestimate as only blood product use during the 3-month study period was included, as many of these patients were admitted to the ICU prior the study period, and/or remained in the ICU or hospital after this window.
CONCLUSIONS
Similar to other treatments provided to patients who are perceived to receive futile critical care, blood products represent a healthcare resource that has the potential to be used without achieving the goals of medicine. But unlike many other medical treatments, the ability to maintain an adequate blood supply for transfusion relies on altruistic blood donors, individuals who are simply motivated by a desire to achieve a healthcare good.13 Explicit guidelines on the use of blood products should be developed to ensure that the use of this precious resource achieves meaningful goals. These goals need to be transparently defined such that a physician’s decision to not transfuse is expected as part of evidence-based medicine. Empiric research, educational interventions, and clearly delineated conflict-resolution processes may improve clinicians’ ability to handle these difficult cases.15
Disclosure
T. Neville was supported by the UCLA CTSI KL2 UL1TR000124, the NIH-NIA 1K23AG047900 - 01A1, and the NIH Loan Repayment Program grant. This project was supported by a donation from Mary Kay Farley to RAND Health. The funder played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. The authors have no conflicts of interest to disclose.
1. Palda VA, Bowman KW, McLean RF, Chapman MG. “Futile” care: do we provide it? Why? A semistructured, Canada-wide survey of intensive care unit doctors and nurses. J Crit Care. 2005;20:207-213. PubMed
2. Piers RD, Azoulay E, Ricou B, et al. Perceptions of appropriateness of care among European and Israeli intensive care unit nurses and physicians. JAMA. 2011;306:2694-2703. PubMed
3. Huang S, Dang H, Huynh W, Sambrook PJ, Goss AN. The healing of dental extraction sockets in patients with Type 2 diabetes on oral hypoglycaemics: a prospective cohort. Aust Dent J. 2013;58:89-93. PubMed
4. Angus DC, Barnato AE, Linde-Zwirble WT, et al. Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32:638-643. PubMed
5. Wunsch H, Linde-Zwirble WT, Harrison DA, Barnato AE, Rowan KM, Angus DC. Use of intensive care services during terminal hospitalizations in England and the United States. Am J Respir Crit Care Med. 2009;180:875-880. PubMed
6. Esserman L, Belkora J, Lenert L. Potentially ineffective care. A new outcome to assess the limits of critical care. JAMA. 1995;274:1544-1551. PubMed
7. Agency for Healthcare Research and Quality: HCUP facts and figures: statistics on hospital-based care in the United States. 2009. https://www.hcup-us.ahrq.gov/reports/factsandfigures/2009/TOC_2009.jsp. Accessed July 15, 2016.
8. Goodnough LT, Maggio P, Hadhazy E, et al. Restrictive blood transfusion practices are associated with improved patient outcomes. Transfusion. 2014;54:2753-2759. PubMed
9. Shander AS, Goodnough LT. Blood transfusion as a quality indicator in cardiac surgery. JAMA. 2010;304:1610-1611. PubMed
10. Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409-417. PubMed
11. Morton J, Anastassopoulos KP, Patel ST, et al. Frequency and outcomes of blood products transfusion across procedures and clinical conditions warranting inpatient care: an analysis of the 2004 healthcare cost and utilization project nationwide inpatient sample database. Am J Med Qual. 2010;25:289-296. PubMed
12. Shander A, Fink A, Javidroozi M, et al. Appropriateness of allogeneic red blood cell transfusion: the international consensus conference on transfusion outcomes. Transfus Med Rev. 2011;25:232-246 e53. PubMed
13. Bednall TC, Bove LL. Donating blood: a meta-analytic review of self-reported motivators and deterrents. Transfus Med Rev. 2011;25:317-334. PubMed
14. Heitmiller ES, Hill RB, Marshall CE, et al. Blood wastage reduction using Lean Sigma methodology. Transfusion. 2010;50:1887-1896. PubMed
15. Bosslet GT, Pope TM, Rubenfeld GD, et al. An Official ATS/AACN/ACCP/ESICM/SCCM Policy Statement: Responding to Requests for Potentially Inappropriate Treatments in Intensive Care Units. Am J Respir Crit Care Med. 2015;191:1318-1330. PubMed
Critical care physicians frequently find themselves providing care that they find to be futile or inappropriate for hospitalized critically ill patients. A survey of physicians found that 87% felt that “futile” treatment was provided in their intensive care unit (ICU) in the past year.1 In a single-day cross-sectional study, 27% of ICU clinicians reported providing inappropriate care to at least 1 patient, most of which was excessive.2 In a 3-month study, 11% of all ICU patients were perceived by their physician as receiving futile treatment at some point during their ICU hospitalization.3 Given that more than 1 in 5 decedents die after an ICU stay during a terminal admission, there is increasing scrutiny of the ICU as a setting where potentially inappropriate resource-intensive treatment is provided.4-6 Blood is an especially valuable resource, not only because it exists in finite supply (and is sometimes in shortage) but also because it is donated in ways that arguably create special stewardship expectations and responsibilities for those trusted to make decisions about its use. The amount of blood products used for patients who are perceived to be receiving inappropriate critical care has not been quantified.
Blood transfusion is the most frequently performed inpatient procedure, occurring in more than 10% of hospital admissions that involve a procedure.7 When used appropriately, the transfusion of blood products can be lifesaving; however, studies show that some transfused blood might not be needed and efforts are afoot to improve the match between transfusion and transfusion need.8,9 These efforts largely focus on generating guidelines based on physiologic benefit and aim mainly at promoting a restrictive transfusion protocol by avoiding blood product use for patients who will likely do well even without transfusion.8,10-12 The guiding principle behind efforts to improve the stewardship of scarce blood products is that they should only be used if they will make a difference in patient outcomes. Unlike prior studies, the goal of this study is to quantify the amount of blood products administered to patients who would do poorly with or without receipt of blood products, that is, patients perceived by their physicians as receiving futile critical care.
MATERIALS AND METHODS
Based on a focus group discussion with physicians who cared for critically ill patients, a questionnaire was developed to identify patients perceived as receiving futile critical care. Details of the definition of futile treatment and the core data collection are described in detail elsewhere.3
For each ICU patient under the physician’s care, the attending physician completed a daily questionnaire asking whether the patient was receiving futile treatment, probably futile treatment, or nonfutile treatment. These surveys were administered every day from December 15, 2011, through March 15, 2012, to each critical care specialist providing care in 5 ICUs (medical ICU, neurocritical care ICU, cardiac care unit, cardiothoracic ICU, and a mixed medical-surgical ICU) in 1 academic health system. All clinicians provided informed consent.
Patients were categorized into the following 3 groups: patients for whom treatment was never perceived as futile; patients with at least 1 assessment that treatment was probably futile, but no futile treatment assessments; and patients who had at least 1 assessment of futile treatment. Hospital and 6-month mortality was abstracted for all patients.
The Division of Transfusion Medicine provided a database of all adult patients during the 3-month study period who received a transfusion of packed red blood cells (PRBCs), apheresis platelets, plasma, or cryoprecipitate (5 unit prepooled units). This database was merged with the daily assessments of the appropriateness of critical care. To determine the proportion of blood products that was utilized for patients receiving inappropriate treatment, we tallied the blood products infused to these patients after the day the patient was assessed as receiving probably inappropriate or inappropriate treatment. The denominator was the total amount of blood products used by all assessed patients during the 3-month study period.
This study was approved by the University of California Los Angeles Institutional Review Board.
RESULTS
During the 3-month study period, 36 critical care clinicians in 5 ICUs provided care to 1193 adult patients. After excluding boarders in the ICUs and missed and invalid assessments, 6916 assessments were made on 1136 patients. Of these 1136 patients, 98 (8.6%) patients received probably futile treatment and 123 (11%) patients received futile treatment according to the physicians caring for them.
For patients who were never rated as receiving futile treatment, the in-hospital mortality was 4.6% and the 6-month mortality was 7.3%. On the contrary, 68% of the patients who were perceived to receive futile ICU treatment died before hospital discharge and 85% died within 6 months; survivors remained in severely compromised health states.3
DISCUSSION
Blood and blood products are donated resources. These biological products are altruistically given with the expectation that they will be used to benefit others.13 It is the clinicians’ responsibility to use these precious gifts to achieve the goals of medicine, which include curing, preserving function, and preventing clinical deterioration that has meaning to the patient. Our study shows that a small, but not insignificant, proportion of these donated resources are provided to hospitalized patients who are perceived as receiving futile critical care. That means that these transfusions are used as part of the critical care interventions that prolong the dying process and achieve outcomes, such as existence in coma, which few, if any, patients would desire. However, it should be noted that some of the health states preserved, such as neurological devastation or multi-organ failure with an inability to survive outside an ICU, were likely desired by patients’ families and might even have been desired by patients themselves. Whether blood donors would wish to donate blood to preserve life in such compromised health states is testable. This proportion of blood provided to ICU patients perceived as receiving futile treatment (7.6%) is similar to or greater than that lost due to wastage, which ranges from 0.1% to 6.7%.14 While the loss of this small proportion of blood products due to expiration or procedural issues is probably unavoidable, but should be minimized as much as possible, the provision of blood products to patients receiving futile critical care is under the control of the healthcare team. This raises the question of how altruistic blood donors would feel about donating if they were aware that 1 of every 13 units transfused in the ICU would be given to a patient that the physician feels will not benefit. In turn, it raises the question of whether the physician should refrain from using these blood products for patients who will not benefit in accordance with principles of evidence-based medicine, in order to ensure their availability for patients that will benefit.
This study has several limitations. Family/patient perspectives were not included in the assessment of futile treatment. It should also be recognized that the percentage of blood products provided to patients receiving inappropriate critical care is likely an underestimate as only blood product use during the 3-month study period was included, as many of these patients were admitted to the ICU prior the study period, and/or remained in the ICU or hospital after this window.
CONCLUSIONS
Similar to other treatments provided to patients who are perceived to receive futile critical care, blood products represent a healthcare resource that has the potential to be used without achieving the goals of medicine. But unlike many other medical treatments, the ability to maintain an adequate blood supply for transfusion relies on altruistic blood donors, individuals who are simply motivated by a desire to achieve a healthcare good.13 Explicit guidelines on the use of blood products should be developed to ensure that the use of this precious resource achieves meaningful goals. These goals need to be transparently defined such that a physician’s decision to not transfuse is expected as part of evidence-based medicine. Empiric research, educational interventions, and clearly delineated conflict-resolution processes may improve clinicians’ ability to handle these difficult cases.15
Disclosure
T. Neville was supported by the UCLA CTSI KL2 UL1TR000124, the NIH-NIA 1K23AG047900 - 01A1, and the NIH Loan Repayment Program grant. This project was supported by a donation from Mary Kay Farley to RAND Health. The funder played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. The authors have no conflicts of interest to disclose.
Critical care physicians frequently find themselves providing care that they find to be futile or inappropriate for hospitalized critically ill patients. A survey of physicians found that 87% felt that “futile” treatment was provided in their intensive care unit (ICU) in the past year.1 In a single-day cross-sectional study, 27% of ICU clinicians reported providing inappropriate care to at least 1 patient, most of which was excessive.2 In a 3-month study, 11% of all ICU patients were perceived by their physician as receiving futile treatment at some point during their ICU hospitalization.3 Given that more than 1 in 5 decedents die after an ICU stay during a terminal admission, there is increasing scrutiny of the ICU as a setting where potentially inappropriate resource-intensive treatment is provided.4-6 Blood is an especially valuable resource, not only because it exists in finite supply (and is sometimes in shortage) but also because it is donated in ways that arguably create special stewardship expectations and responsibilities for those trusted to make decisions about its use. The amount of blood products used for patients who are perceived to be receiving inappropriate critical care has not been quantified.
Blood transfusion is the most frequently performed inpatient procedure, occurring in more than 10% of hospital admissions that involve a procedure.7 When used appropriately, the transfusion of blood products can be lifesaving; however, studies show that some transfused blood might not be needed and efforts are afoot to improve the match between transfusion and transfusion need.8,9 These efforts largely focus on generating guidelines based on physiologic benefit and aim mainly at promoting a restrictive transfusion protocol by avoiding blood product use for patients who will likely do well even without transfusion.8,10-12 The guiding principle behind efforts to improve the stewardship of scarce blood products is that they should only be used if they will make a difference in patient outcomes. Unlike prior studies, the goal of this study is to quantify the amount of blood products administered to patients who would do poorly with or without receipt of blood products, that is, patients perceived by their physicians as receiving futile critical care.
MATERIALS AND METHODS
Based on a focus group discussion with physicians who cared for critically ill patients, a questionnaire was developed to identify patients perceived as receiving futile critical care. Details of the definition of futile treatment and the core data collection are described in detail elsewhere.3
For each ICU patient under the physician’s care, the attending physician completed a daily questionnaire asking whether the patient was receiving futile treatment, probably futile treatment, or nonfutile treatment. These surveys were administered every day from December 15, 2011, through March 15, 2012, to each critical care specialist providing care in 5 ICUs (medical ICU, neurocritical care ICU, cardiac care unit, cardiothoracic ICU, and a mixed medical-surgical ICU) in 1 academic health system. All clinicians provided informed consent.
Patients were categorized into the following 3 groups: patients for whom treatment was never perceived as futile; patients with at least 1 assessment that treatment was probably futile, but no futile treatment assessments; and patients who had at least 1 assessment of futile treatment. Hospital and 6-month mortality was abstracted for all patients.
The Division of Transfusion Medicine provided a database of all adult patients during the 3-month study period who received a transfusion of packed red blood cells (PRBCs), apheresis platelets, plasma, or cryoprecipitate (5 unit prepooled units). This database was merged with the daily assessments of the appropriateness of critical care. To determine the proportion of blood products that was utilized for patients receiving inappropriate treatment, we tallied the blood products infused to these patients after the day the patient was assessed as receiving probably inappropriate or inappropriate treatment. The denominator was the total amount of blood products used by all assessed patients during the 3-month study period.
This study was approved by the University of California Los Angeles Institutional Review Board.
RESULTS
During the 3-month study period, 36 critical care clinicians in 5 ICUs provided care to 1193 adult patients. After excluding boarders in the ICUs and missed and invalid assessments, 6916 assessments were made on 1136 patients. Of these 1136 patients, 98 (8.6%) patients received probably futile treatment and 123 (11%) patients received futile treatment according to the physicians caring for them.
For patients who were never rated as receiving futile treatment, the in-hospital mortality was 4.6% and the 6-month mortality was 7.3%. On the contrary, 68% of the patients who were perceived to receive futile ICU treatment died before hospital discharge and 85% died within 6 months; survivors remained in severely compromised health states.3
DISCUSSION
Blood and blood products are donated resources. These biological products are altruistically given with the expectation that they will be used to benefit others.13 It is the clinicians’ responsibility to use these precious gifts to achieve the goals of medicine, which include curing, preserving function, and preventing clinical deterioration that has meaning to the patient. Our study shows that a small, but not insignificant, proportion of these donated resources are provided to hospitalized patients who are perceived as receiving futile critical care. That means that these transfusions are used as part of the critical care interventions that prolong the dying process and achieve outcomes, such as existence in coma, which few, if any, patients would desire. However, it should be noted that some of the health states preserved, such as neurological devastation or multi-organ failure with an inability to survive outside an ICU, were likely desired by patients’ families and might even have been desired by patients themselves. Whether blood donors would wish to donate blood to preserve life in such compromised health states is testable. This proportion of blood provided to ICU patients perceived as receiving futile treatment (7.6%) is similar to or greater than that lost due to wastage, which ranges from 0.1% to 6.7%.14 While the loss of this small proportion of blood products due to expiration or procedural issues is probably unavoidable, but should be minimized as much as possible, the provision of blood products to patients receiving futile critical care is under the control of the healthcare team. This raises the question of how altruistic blood donors would feel about donating if they were aware that 1 of every 13 units transfused in the ICU would be given to a patient that the physician feels will not benefit. In turn, it raises the question of whether the physician should refrain from using these blood products for patients who will not benefit in accordance with principles of evidence-based medicine, in order to ensure their availability for patients that will benefit.
This study has several limitations. Family/patient perspectives were not included in the assessment of futile treatment. It should also be recognized that the percentage of blood products provided to patients receiving inappropriate critical care is likely an underestimate as only blood product use during the 3-month study period was included, as many of these patients were admitted to the ICU prior the study period, and/or remained in the ICU or hospital after this window.
CONCLUSIONS
Similar to other treatments provided to patients who are perceived to receive futile critical care, blood products represent a healthcare resource that has the potential to be used without achieving the goals of medicine. But unlike many other medical treatments, the ability to maintain an adequate blood supply for transfusion relies on altruistic blood donors, individuals who are simply motivated by a desire to achieve a healthcare good.13 Explicit guidelines on the use of blood products should be developed to ensure that the use of this precious resource achieves meaningful goals. These goals need to be transparently defined such that a physician’s decision to not transfuse is expected as part of evidence-based medicine. Empiric research, educational interventions, and clearly delineated conflict-resolution processes may improve clinicians’ ability to handle these difficult cases.15
Disclosure
T. Neville was supported by the UCLA CTSI KL2 UL1TR000124, the NIH-NIA 1K23AG047900 - 01A1, and the NIH Loan Repayment Program grant. This project was supported by a donation from Mary Kay Farley to RAND Health. The funder played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. The authors have no conflicts of interest to disclose.
1. Palda VA, Bowman KW, McLean RF, Chapman MG. “Futile” care: do we provide it? Why? A semistructured, Canada-wide survey of intensive care unit doctors and nurses. J Crit Care. 2005;20:207-213. PubMed
2. Piers RD, Azoulay E, Ricou B, et al. Perceptions of appropriateness of care among European and Israeli intensive care unit nurses and physicians. JAMA. 2011;306:2694-2703. PubMed
3. Huang S, Dang H, Huynh W, Sambrook PJ, Goss AN. The healing of dental extraction sockets in patients with Type 2 diabetes on oral hypoglycaemics: a prospective cohort. Aust Dent J. 2013;58:89-93. PubMed
4. Angus DC, Barnato AE, Linde-Zwirble WT, et al. Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32:638-643. PubMed
5. Wunsch H, Linde-Zwirble WT, Harrison DA, Barnato AE, Rowan KM, Angus DC. Use of intensive care services during terminal hospitalizations in England and the United States. Am J Respir Crit Care Med. 2009;180:875-880. PubMed
6. Esserman L, Belkora J, Lenert L. Potentially ineffective care. A new outcome to assess the limits of critical care. JAMA. 1995;274:1544-1551. PubMed
7. Agency for Healthcare Research and Quality: HCUP facts and figures: statistics on hospital-based care in the United States. 2009. https://www.hcup-us.ahrq.gov/reports/factsandfigures/2009/TOC_2009.jsp. Accessed July 15, 2016.
8. Goodnough LT, Maggio P, Hadhazy E, et al. Restrictive blood transfusion practices are associated with improved patient outcomes. Transfusion. 2014;54:2753-2759. PubMed
9. Shander AS, Goodnough LT. Blood transfusion as a quality indicator in cardiac surgery. JAMA. 2010;304:1610-1611. PubMed
10. Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409-417. PubMed
11. Morton J, Anastassopoulos KP, Patel ST, et al. Frequency and outcomes of blood products transfusion across procedures and clinical conditions warranting inpatient care: an analysis of the 2004 healthcare cost and utilization project nationwide inpatient sample database. Am J Med Qual. 2010;25:289-296. PubMed
12. Shander A, Fink A, Javidroozi M, et al. Appropriateness of allogeneic red blood cell transfusion: the international consensus conference on transfusion outcomes. Transfus Med Rev. 2011;25:232-246 e53. PubMed
13. Bednall TC, Bove LL. Donating blood: a meta-analytic review of self-reported motivators and deterrents. Transfus Med Rev. 2011;25:317-334. PubMed
14. Heitmiller ES, Hill RB, Marshall CE, et al. Blood wastage reduction using Lean Sigma methodology. Transfusion. 2010;50:1887-1896. PubMed
15. Bosslet GT, Pope TM, Rubenfeld GD, et al. An Official ATS/AACN/ACCP/ESICM/SCCM Policy Statement: Responding to Requests for Potentially Inappropriate Treatments in Intensive Care Units. Am J Respir Crit Care Med. 2015;191:1318-1330. PubMed
1. Palda VA, Bowman KW, McLean RF, Chapman MG. “Futile” care: do we provide it? Why? A semistructured, Canada-wide survey of intensive care unit doctors and nurses. J Crit Care. 2005;20:207-213. PubMed
2. Piers RD, Azoulay E, Ricou B, et al. Perceptions of appropriateness of care among European and Israeli intensive care unit nurses and physicians. JAMA. 2011;306:2694-2703. PubMed
3. Huang S, Dang H, Huynh W, Sambrook PJ, Goss AN. The healing of dental extraction sockets in patients with Type 2 diabetes on oral hypoglycaemics: a prospective cohort. Aust Dent J. 2013;58:89-93. PubMed
4. Angus DC, Barnato AE, Linde-Zwirble WT, et al. Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32:638-643. PubMed
5. Wunsch H, Linde-Zwirble WT, Harrison DA, Barnato AE, Rowan KM, Angus DC. Use of intensive care services during terminal hospitalizations in England and the United States. Am J Respir Crit Care Med. 2009;180:875-880. PubMed
6. Esserman L, Belkora J, Lenert L. Potentially ineffective care. A new outcome to assess the limits of critical care. JAMA. 1995;274:1544-1551. PubMed
7. Agency for Healthcare Research and Quality: HCUP facts and figures: statistics on hospital-based care in the United States. 2009. https://www.hcup-us.ahrq.gov/reports/factsandfigures/2009/TOC_2009.jsp. Accessed July 15, 2016.
8. Goodnough LT, Maggio P, Hadhazy E, et al. Restrictive blood transfusion practices are associated with improved patient outcomes. Transfusion. 2014;54:2753-2759. PubMed
9. Shander AS, Goodnough LT. Blood transfusion as a quality indicator in cardiac surgery. JAMA. 2010;304:1610-1611. PubMed
10. Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409-417. PubMed
11. Morton J, Anastassopoulos KP, Patel ST, et al. Frequency and outcomes of blood products transfusion across procedures and clinical conditions warranting inpatient care: an analysis of the 2004 healthcare cost and utilization project nationwide inpatient sample database. Am J Med Qual. 2010;25:289-296. PubMed
12. Shander A, Fink A, Javidroozi M, et al. Appropriateness of allogeneic red blood cell transfusion: the international consensus conference on transfusion outcomes. Transfus Med Rev. 2011;25:232-246 e53. PubMed
13. Bednall TC, Bove LL. Donating blood: a meta-analytic review of self-reported motivators and deterrents. Transfus Med Rev. 2011;25:317-334. PubMed
14. Heitmiller ES, Hill RB, Marshall CE, et al. Blood wastage reduction using Lean Sigma methodology. Transfusion. 2010;50:1887-1896. PubMed
15. Bosslet GT, Pope TM, Rubenfeld GD, et al. An Official ATS/AACN/ACCP/ESICM/SCCM Policy Statement: Responding to Requests for Potentially Inappropriate Treatments in Intensive Care Units. Am J Respir Crit Care Med. 2015;191:1318-1330. PubMed
© 2017 Society of Hospital Medicine
Magnitude of Potentially Inappropriate Thrombophilia Testing in the Inpatient Hospital Setting
Venous thromboembolism (VTE) affects more than 1 million patients and costs the US healthcare system more than $1.5 billion annually.1 Inherited and acquired thrombophilias have been perceived as important risk factors in assessing the risk of VTE recurrence and guiding the duration of anticoagulation.
Thrombophilias increase the risk of a first thrombotic event, but existing data have failed to demonstrate the usefulness of routine thrombophilia screening on subsequent management.2,3 Moreover, thrombophilia testing ordered in the context of an inpatient hospitalization is limited by confounding factors, especially during an acute thrombotic event or in the setting of concurrent anticoagulation.4
Recognizing the costliness of routine thrombophilia testing, The American Society of Hematology introduced its Choosing Wisely campaign in 2013 in an effort to reduce test ordering in the setting of provoked VTEs with a major transient risk factor.5 In order to define current practice behavior at our institution, we conducted a retrospective study to determine the magnitude and financial impact of potentially inappropriate thrombophilia testing in the inpatient setting.
METHODS
We performed a retrospective analysis of thrombophilia testing across all inpatient services at a large, quaternary-care academic institution over a 2-year period. Electronic medical record data containing all thrombophilia tests ordered on inpatients from June 2013 to June 2015 were obtained. This study was exempt from institutional review board approval.
Inclusion criteria included any inpatient for which thrombophilia testing occurred. Patients were excluded if testing was ordered in the absence of VTE or arterial thrombosis or if it was ordered as part of a work-up for another medical condition (see Supplementary Material).
Thrombophilia testing was defined as any of the following: inherited thrombophilias (Factor V Leiden or prothrombin 20210 gene mutations, antithrombin, or protein C or S activity levels) or acquired thrombophilias (lupus anticoagulant [Testing refers to the activated partial thromboplastin time lupus assay.], beta-2 glycoprotein 1 immunoglobulins M and G, anticardiolipin immunoglobulins M and G, dilute Russell’s viper venom time, or JAK2 V617F mutations).
Extracted data included patient age, sex, type of thrombophilia test ordered, ordering primary service, admission diagnosis, and objective confirmation of thrombotic events. The indication for test ordering was determined via medical record review of the patient’s corresponding hospitalization. Each test was evaluated in the context of the patient’s presenting history, hospital course, active medications, accompanying laboratory and radiographic studies, and consultant recommendations to arrive at a conclusion regarding both the test’s reason for ordering and whether its indication was “inappropriate,” “appropriate,” or “equivocal.” Cost data were obtained through the Centers for Medicare & Medicaid Services (CMS) Clinical Laboratory Fee Schedule for 2016 (see Supplementary Material).6
The criteria for defining test appropriateness were formulated by utilizing a combination of major society guidelines and literature review.5,7-10 The criteria placed emphasis upon the ordered tests’ clinical relevance and reliability and were subsequently reviewed by a senior hematologist with specific expertise in thrombosis (see Supplementary Material).
Two internal medicine resident physician data reviewers independently evaluated the ordered tests. To ensure consistency between reviewers, a sample of identical test orders was compared for concordance, and a Cohen’s kappa coefficient was calculated. For purposes of analysis, equivocal orders were included under the appropriate category, as this study focused on the quantification of potentially inappropriate ordering practices. Pearson chi-square testing was performed in order to compare ordering practices between services using Stata.11
RESULTS
In total, we reviewed 2179 individual tests, of which 362 (16.6%) were excluded. The remaining 1817 tests involved 299 patients across 26 primary specialties. Fifty-two (2.9% of orders) were ultimately deemed equivocal. The Table illustrates the overall proportion and cost of inappropriate test ordering as well as testing characteristics of the most commonly encountered thrombotic diagnoses. The Figure illustrates the proportion of potentially inappropriate test ordering with its associated cost by test type.
Orders for Factor V Leiden, prothrombin 20210, and protein C and S activity levels were most commonly deemed inappropriate due to the test results’ failure to alter clinical management (97.3%, 99.2%, 99.4% of their inappropriate orders, respectively). Antithrombin testing (59.4%) was deemed inappropriate most commonly in the setting of acute thrombosis. The lupus anticoagulant (82.8%) was inappropriately ordered most frequently in the setting of concurrent anticoagulation.
Ordering practices were then compared between nonteaching and teaching inpatient general medicine services. We observed a higher proportion of inappropriate tests ordered by the nonteaching services as compared to the teaching services (120 of 173 orders [69.4%] versus 125 of 320 [39.1%], respectively; P < 0.001).
The interreviewer kappa coefficient was 0.82 (P < 0.0001).
DISCUSSION
This retrospective analysis represents one of the largest examinations of inpatient thrombophilia testing practices to date. Our results illustrate the high prevalence and significant financial impact of potentially inappropriate thrombophilia testing conducted in the inpatient setting. The data confirm that, per our defined criteria, more than 90% of inherited thrombophilia testing was potentially inappropriate while the majority of acquired thrombophilia testing was appropriate, with the exception of the lupus anticoagulant.
Even when appropriately ordered, studies suggest that positive thrombophilia screening results fail to impact outcomes in most patients with VTE. In an effort to evaluate positive results’ potential to provide a basis from which to extend the duration of anticoagulation, and therefore reduce the risk of a recurrent VTE, a case-control analysis was performed on a series of patients with a first-VTE event (Multiple Environmental and Genetic Assessment of risk factors for venous thrombosis [MEGA] study).3 In examining the odds ratio (OR) for recurrence between patients who did or did not undergo testing for Factor V Leiden, antithrombin, or protein C or S activity, the data failed to show an impact of testing on the risk of VTE recurrence (OR 1.2; confidence interval, 0.8-1.8). In fact, decision making has increasingly relied on patients’ clinical characteristics rather than thrombophilia test results to guide anticoagulation duration after incident VTEs. A 2017 study illustrated that when using a clinical decision rule (Clinical Decision Rule Validation Study to Predict Low Recurrent Risk in Patients With Unprovoked Venous Thromboembolism [REVERSE criteria]) in patients with a first, unprovoked VTE, routine thrombophilia screening added little to determining the need for prolonged anticoagulation.12 These findings support the limited clinical utility of current test ordering practices for the prediction and management of recurrent venous thrombosis.
Regarding the acquired thrombophilias, antiphospholipid antibody testing was predominantly ordered in a justified manner, which is consistent with the notion that test results could affect clinical management, such as anticoagulation duration or choice of anticoagulant.13 However, the validity of lupus anticoagulant testing was limited by the frequency of patients on concurrent anticoagulation.
Financially, the cumulative cost associated with inappropriate ordering was substantial, regardless of the thrombotic event in question. Moreover, our calculated costs are derived from CMS reimbursement rates and likely underestimate the true financial impact of errant testing given that commercial laboratories frequently charge at rates several-fold higher. On a national scale, prior analyses have suggested that the annual cost of thrombophilia testing, based on typical commercial rates, ranges from $300 million to $672 million.14
Researchers in prior studies have similarly examined the frequency of inappropriate thrombophilia testing and methods to reduce it. Researchers in a 2014 study demonstrated initially high rates of inappropriate inherited thrombophilia testing, and then showed marked reductions in testing and cost savings across multiple specialties following the introduction of a flowchart on a preprinted order form.15 Our findings provide motivation to perform similar endeavors.
The proportional difference of inappropriate ordering observed between nonteaching- and teaching-medicine services indicates a potential role for educational interventions. We recently completed a series of lectures on high-value thrombophilia ordering for residents and are actively analyzing its impact on subsequent ordering practices. We are also piloting an electronic best practice advisory for thrombophilia test ordering. Though the advisory may be overridden, providers are asked to provide justification for doing so on a voluntary basis. We plan to evaluate its effect on our findings reported in this study.
We acknowledge that our exclusion criteria resulted in the omission of testing across a spectrum of nonthrombotic clinical conditions, raising the question of selection bias. Because there are no established guidelines to determine the appropriateness of testing in these scenarios, we chose to limit the analysis of errant ordering to the context of thrombotic events. Other limitations of this study include the analysis of equivocal orders as appropriate. However, because equivocal ordering represented less than 3% of all analyzed orders, including these as inappropriate would not have significantly altered our findings.
CONCLUSIONS
A review of thrombophilia testing practices at our institution demonstrated that inappropriate testing in the inpatient setting is a frequent phenomenon associated with a significant financial impact. This effect was more pronounced in inherited versus acquired thrombophilia testing. Testing was frequently confounded and often failed to impact patients’ short- or long-term clinical management, regardless of the result.
These findings serve as a strong impetus to reduce the burden of routine thrombophilia testing during hospital admissions. Our data demonstrate a need for institution-wide changes such as implementing best practice advisories, introducing ordering restrictions, and conducting educational interventions in order to reduce unnecessary expenditures and improve patient care.
Disclosure
The authors have nothing to disclose.
1. Dobesh PP. Economic burden of venous thromboembolism in hospitalized patients. Pharmacotherapy. 2009;29(8):943-953. PubMed
2. Cohn DM, Vansenne F, de Borgie CA, Middeldorp S. Thrombophilia testing for prevention of recurrent venous thromboembolism. Cochrane Database Syst Rev. 2012;12:Cd007069. PubMed
3. Coppens M, Reijnders JH, Middeldorp S, Doggen CJ, Rosendaal FR. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6(9):1474-1477. PubMed
4. Somma J, Sussman, II, Rand JH. An evaluation of thrombophilia screening in an urban tertiary care medical center: A “real world” experience. Am J Clin Pathol. 2006;126(1):120-127. PubMed
5. Hicks LK, Bering H, Carson KR, et al. The ASH Choosing Wisely® campaign: five hematologic tests and treatments to question. Blood. 2013;122(24):3879-3883. PubMed
6. Centers for Medicare & Medicaid Services: Clinical Laboratory Fee Schedule Files. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files.html. Accessed October 2016
7. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41(1):154-164. PubMed
8. Moll S. Thrombophilia: clinical-practical aspects. J Thromb Thrombolysis. 2015;39(3):367-378. PubMed
9. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for vte disease: Chest guideline and expert panel report. Chest. 2016;149(2):315-352. PubMed
10. Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149(2):209-220. PubMed
11. Stata Statistical Software [computer program]. Version Release 14. College Station, TX: StataCorp LP; 2015.
12. Garcia-Horton A, Kovacs MJ, Abdulrehman J, Taylor JE, Sharma S, Lazo-Langner A. Impact of thrombophilia screening on venous thromboembolism management practices. Thromb Res.149:76-80. PubMed
13. Schulman S, Svenungsson E, Granqvist S. Anticardiolipin antibodies predict early recurrence of thromboembolism and death among patients with venous thromboembolism following anticoagulant therapy. Duration of Anticoagulation Study Group. Am J Med. 1998;104(4):332-338. PubMed
14. Petrilli CM, Heidemann L, Mack M, Durance P, Chopra V. Inpatient inherited thrombophilia testing. J Hosp Med. 2016;11(11):801-804. PubMed
15. Smith TW, Pi D, Hudoba M, Lee AY. Reducing inpatient heritable thrombophilia testing using a clinical decision-making tool. J Clin Pathol. 2014;67(4):345-349. PubMed
Venous thromboembolism (VTE) affects more than 1 million patients and costs the US healthcare system more than $1.5 billion annually.1 Inherited and acquired thrombophilias have been perceived as important risk factors in assessing the risk of VTE recurrence and guiding the duration of anticoagulation.
Thrombophilias increase the risk of a first thrombotic event, but existing data have failed to demonstrate the usefulness of routine thrombophilia screening on subsequent management.2,3 Moreover, thrombophilia testing ordered in the context of an inpatient hospitalization is limited by confounding factors, especially during an acute thrombotic event or in the setting of concurrent anticoagulation.4
Recognizing the costliness of routine thrombophilia testing, The American Society of Hematology introduced its Choosing Wisely campaign in 2013 in an effort to reduce test ordering in the setting of provoked VTEs with a major transient risk factor.5 In order to define current practice behavior at our institution, we conducted a retrospective study to determine the magnitude and financial impact of potentially inappropriate thrombophilia testing in the inpatient setting.
METHODS
We performed a retrospective analysis of thrombophilia testing across all inpatient services at a large, quaternary-care academic institution over a 2-year period. Electronic medical record data containing all thrombophilia tests ordered on inpatients from June 2013 to June 2015 were obtained. This study was exempt from institutional review board approval.
Inclusion criteria included any inpatient for which thrombophilia testing occurred. Patients were excluded if testing was ordered in the absence of VTE or arterial thrombosis or if it was ordered as part of a work-up for another medical condition (see Supplementary Material).
Thrombophilia testing was defined as any of the following: inherited thrombophilias (Factor V Leiden or prothrombin 20210 gene mutations, antithrombin, or protein C or S activity levels) or acquired thrombophilias (lupus anticoagulant [Testing refers to the activated partial thromboplastin time lupus assay.], beta-2 glycoprotein 1 immunoglobulins M and G, anticardiolipin immunoglobulins M and G, dilute Russell’s viper venom time, or JAK2 V617F mutations).
Extracted data included patient age, sex, type of thrombophilia test ordered, ordering primary service, admission diagnosis, and objective confirmation of thrombotic events. The indication for test ordering was determined via medical record review of the patient’s corresponding hospitalization. Each test was evaluated in the context of the patient’s presenting history, hospital course, active medications, accompanying laboratory and radiographic studies, and consultant recommendations to arrive at a conclusion regarding both the test’s reason for ordering and whether its indication was “inappropriate,” “appropriate,” or “equivocal.” Cost data were obtained through the Centers for Medicare & Medicaid Services (CMS) Clinical Laboratory Fee Schedule for 2016 (see Supplementary Material).6
The criteria for defining test appropriateness were formulated by utilizing a combination of major society guidelines and literature review.5,7-10 The criteria placed emphasis upon the ordered tests’ clinical relevance and reliability and were subsequently reviewed by a senior hematologist with specific expertise in thrombosis (see Supplementary Material).
Two internal medicine resident physician data reviewers independently evaluated the ordered tests. To ensure consistency between reviewers, a sample of identical test orders was compared for concordance, and a Cohen’s kappa coefficient was calculated. For purposes of analysis, equivocal orders were included under the appropriate category, as this study focused on the quantification of potentially inappropriate ordering practices. Pearson chi-square testing was performed in order to compare ordering practices between services using Stata.11
RESULTS
In total, we reviewed 2179 individual tests, of which 362 (16.6%) were excluded. The remaining 1817 tests involved 299 patients across 26 primary specialties. Fifty-two (2.9% of orders) were ultimately deemed equivocal. The Table illustrates the overall proportion and cost of inappropriate test ordering as well as testing characteristics of the most commonly encountered thrombotic diagnoses. The Figure illustrates the proportion of potentially inappropriate test ordering with its associated cost by test type.
Orders for Factor V Leiden, prothrombin 20210, and protein C and S activity levels were most commonly deemed inappropriate due to the test results’ failure to alter clinical management (97.3%, 99.2%, 99.4% of their inappropriate orders, respectively). Antithrombin testing (59.4%) was deemed inappropriate most commonly in the setting of acute thrombosis. The lupus anticoagulant (82.8%) was inappropriately ordered most frequently in the setting of concurrent anticoagulation.
Ordering practices were then compared between nonteaching and teaching inpatient general medicine services. We observed a higher proportion of inappropriate tests ordered by the nonteaching services as compared to the teaching services (120 of 173 orders [69.4%] versus 125 of 320 [39.1%], respectively; P < 0.001).
The interreviewer kappa coefficient was 0.82 (P < 0.0001).
DISCUSSION
This retrospective analysis represents one of the largest examinations of inpatient thrombophilia testing practices to date. Our results illustrate the high prevalence and significant financial impact of potentially inappropriate thrombophilia testing conducted in the inpatient setting. The data confirm that, per our defined criteria, more than 90% of inherited thrombophilia testing was potentially inappropriate while the majority of acquired thrombophilia testing was appropriate, with the exception of the lupus anticoagulant.
Even when appropriately ordered, studies suggest that positive thrombophilia screening results fail to impact outcomes in most patients with VTE. In an effort to evaluate positive results’ potential to provide a basis from which to extend the duration of anticoagulation, and therefore reduce the risk of a recurrent VTE, a case-control analysis was performed on a series of patients with a first-VTE event (Multiple Environmental and Genetic Assessment of risk factors for venous thrombosis [MEGA] study).3 In examining the odds ratio (OR) for recurrence between patients who did or did not undergo testing for Factor V Leiden, antithrombin, or protein C or S activity, the data failed to show an impact of testing on the risk of VTE recurrence (OR 1.2; confidence interval, 0.8-1.8). In fact, decision making has increasingly relied on patients’ clinical characteristics rather than thrombophilia test results to guide anticoagulation duration after incident VTEs. A 2017 study illustrated that when using a clinical decision rule (Clinical Decision Rule Validation Study to Predict Low Recurrent Risk in Patients With Unprovoked Venous Thromboembolism [REVERSE criteria]) in patients with a first, unprovoked VTE, routine thrombophilia screening added little to determining the need for prolonged anticoagulation.12 These findings support the limited clinical utility of current test ordering practices for the prediction and management of recurrent venous thrombosis.
Regarding the acquired thrombophilias, antiphospholipid antibody testing was predominantly ordered in a justified manner, which is consistent with the notion that test results could affect clinical management, such as anticoagulation duration or choice of anticoagulant.13 However, the validity of lupus anticoagulant testing was limited by the frequency of patients on concurrent anticoagulation.
Financially, the cumulative cost associated with inappropriate ordering was substantial, regardless of the thrombotic event in question. Moreover, our calculated costs are derived from CMS reimbursement rates and likely underestimate the true financial impact of errant testing given that commercial laboratories frequently charge at rates several-fold higher. On a national scale, prior analyses have suggested that the annual cost of thrombophilia testing, based on typical commercial rates, ranges from $300 million to $672 million.14
Researchers in prior studies have similarly examined the frequency of inappropriate thrombophilia testing and methods to reduce it. Researchers in a 2014 study demonstrated initially high rates of inappropriate inherited thrombophilia testing, and then showed marked reductions in testing and cost savings across multiple specialties following the introduction of a flowchart on a preprinted order form.15 Our findings provide motivation to perform similar endeavors.
The proportional difference of inappropriate ordering observed between nonteaching- and teaching-medicine services indicates a potential role for educational interventions. We recently completed a series of lectures on high-value thrombophilia ordering for residents and are actively analyzing its impact on subsequent ordering practices. We are also piloting an electronic best practice advisory for thrombophilia test ordering. Though the advisory may be overridden, providers are asked to provide justification for doing so on a voluntary basis. We plan to evaluate its effect on our findings reported in this study.
We acknowledge that our exclusion criteria resulted in the omission of testing across a spectrum of nonthrombotic clinical conditions, raising the question of selection bias. Because there are no established guidelines to determine the appropriateness of testing in these scenarios, we chose to limit the analysis of errant ordering to the context of thrombotic events. Other limitations of this study include the analysis of equivocal orders as appropriate. However, because equivocal ordering represented less than 3% of all analyzed orders, including these as inappropriate would not have significantly altered our findings.
CONCLUSIONS
A review of thrombophilia testing practices at our institution demonstrated that inappropriate testing in the inpatient setting is a frequent phenomenon associated with a significant financial impact. This effect was more pronounced in inherited versus acquired thrombophilia testing. Testing was frequently confounded and often failed to impact patients’ short- or long-term clinical management, regardless of the result.
These findings serve as a strong impetus to reduce the burden of routine thrombophilia testing during hospital admissions. Our data demonstrate a need for institution-wide changes such as implementing best practice advisories, introducing ordering restrictions, and conducting educational interventions in order to reduce unnecessary expenditures and improve patient care.
Disclosure
The authors have nothing to disclose.
Venous thromboembolism (VTE) affects more than 1 million patients and costs the US healthcare system more than $1.5 billion annually.1 Inherited and acquired thrombophilias have been perceived as important risk factors in assessing the risk of VTE recurrence and guiding the duration of anticoagulation.
Thrombophilias increase the risk of a first thrombotic event, but existing data have failed to demonstrate the usefulness of routine thrombophilia screening on subsequent management.2,3 Moreover, thrombophilia testing ordered in the context of an inpatient hospitalization is limited by confounding factors, especially during an acute thrombotic event or in the setting of concurrent anticoagulation.4
Recognizing the costliness of routine thrombophilia testing, The American Society of Hematology introduced its Choosing Wisely campaign in 2013 in an effort to reduce test ordering in the setting of provoked VTEs with a major transient risk factor.5 In order to define current practice behavior at our institution, we conducted a retrospective study to determine the magnitude and financial impact of potentially inappropriate thrombophilia testing in the inpatient setting.
METHODS
We performed a retrospective analysis of thrombophilia testing across all inpatient services at a large, quaternary-care academic institution over a 2-year period. Electronic medical record data containing all thrombophilia tests ordered on inpatients from June 2013 to June 2015 were obtained. This study was exempt from institutional review board approval.
Inclusion criteria included any inpatient for which thrombophilia testing occurred. Patients were excluded if testing was ordered in the absence of VTE or arterial thrombosis or if it was ordered as part of a work-up for another medical condition (see Supplementary Material).
Thrombophilia testing was defined as any of the following: inherited thrombophilias (Factor V Leiden or prothrombin 20210 gene mutations, antithrombin, or protein C or S activity levels) or acquired thrombophilias (lupus anticoagulant [Testing refers to the activated partial thromboplastin time lupus assay.], beta-2 glycoprotein 1 immunoglobulins M and G, anticardiolipin immunoglobulins M and G, dilute Russell’s viper venom time, or JAK2 V617F mutations).
Extracted data included patient age, sex, type of thrombophilia test ordered, ordering primary service, admission diagnosis, and objective confirmation of thrombotic events. The indication for test ordering was determined via medical record review of the patient’s corresponding hospitalization. Each test was evaluated in the context of the patient’s presenting history, hospital course, active medications, accompanying laboratory and radiographic studies, and consultant recommendations to arrive at a conclusion regarding both the test’s reason for ordering and whether its indication was “inappropriate,” “appropriate,” or “equivocal.” Cost data were obtained through the Centers for Medicare & Medicaid Services (CMS) Clinical Laboratory Fee Schedule for 2016 (see Supplementary Material).6
The criteria for defining test appropriateness were formulated by utilizing a combination of major society guidelines and literature review.5,7-10 The criteria placed emphasis upon the ordered tests’ clinical relevance and reliability and were subsequently reviewed by a senior hematologist with specific expertise in thrombosis (see Supplementary Material).
Two internal medicine resident physician data reviewers independently evaluated the ordered tests. To ensure consistency between reviewers, a sample of identical test orders was compared for concordance, and a Cohen’s kappa coefficient was calculated. For purposes of analysis, equivocal orders were included under the appropriate category, as this study focused on the quantification of potentially inappropriate ordering practices. Pearson chi-square testing was performed in order to compare ordering practices between services using Stata.11
RESULTS
In total, we reviewed 2179 individual tests, of which 362 (16.6%) were excluded. The remaining 1817 tests involved 299 patients across 26 primary specialties. Fifty-two (2.9% of orders) were ultimately deemed equivocal. The Table illustrates the overall proportion and cost of inappropriate test ordering as well as testing characteristics of the most commonly encountered thrombotic diagnoses. The Figure illustrates the proportion of potentially inappropriate test ordering with its associated cost by test type.
Orders for Factor V Leiden, prothrombin 20210, and protein C and S activity levels were most commonly deemed inappropriate due to the test results’ failure to alter clinical management (97.3%, 99.2%, 99.4% of their inappropriate orders, respectively). Antithrombin testing (59.4%) was deemed inappropriate most commonly in the setting of acute thrombosis. The lupus anticoagulant (82.8%) was inappropriately ordered most frequently in the setting of concurrent anticoagulation.
Ordering practices were then compared between nonteaching and teaching inpatient general medicine services. We observed a higher proportion of inappropriate tests ordered by the nonteaching services as compared to the teaching services (120 of 173 orders [69.4%] versus 125 of 320 [39.1%], respectively; P < 0.001).
The interreviewer kappa coefficient was 0.82 (P < 0.0001).
DISCUSSION
This retrospective analysis represents one of the largest examinations of inpatient thrombophilia testing practices to date. Our results illustrate the high prevalence and significant financial impact of potentially inappropriate thrombophilia testing conducted in the inpatient setting. The data confirm that, per our defined criteria, more than 90% of inherited thrombophilia testing was potentially inappropriate while the majority of acquired thrombophilia testing was appropriate, with the exception of the lupus anticoagulant.
Even when appropriately ordered, studies suggest that positive thrombophilia screening results fail to impact outcomes in most patients with VTE. In an effort to evaluate positive results’ potential to provide a basis from which to extend the duration of anticoagulation, and therefore reduce the risk of a recurrent VTE, a case-control analysis was performed on a series of patients with a first-VTE event (Multiple Environmental and Genetic Assessment of risk factors for venous thrombosis [MEGA] study).3 In examining the odds ratio (OR) for recurrence between patients who did or did not undergo testing for Factor V Leiden, antithrombin, or protein C or S activity, the data failed to show an impact of testing on the risk of VTE recurrence (OR 1.2; confidence interval, 0.8-1.8). In fact, decision making has increasingly relied on patients’ clinical characteristics rather than thrombophilia test results to guide anticoagulation duration after incident VTEs. A 2017 study illustrated that when using a clinical decision rule (Clinical Decision Rule Validation Study to Predict Low Recurrent Risk in Patients With Unprovoked Venous Thromboembolism [REVERSE criteria]) in patients with a first, unprovoked VTE, routine thrombophilia screening added little to determining the need for prolonged anticoagulation.12 These findings support the limited clinical utility of current test ordering practices for the prediction and management of recurrent venous thrombosis.
Regarding the acquired thrombophilias, antiphospholipid antibody testing was predominantly ordered in a justified manner, which is consistent with the notion that test results could affect clinical management, such as anticoagulation duration or choice of anticoagulant.13 However, the validity of lupus anticoagulant testing was limited by the frequency of patients on concurrent anticoagulation.
Financially, the cumulative cost associated with inappropriate ordering was substantial, regardless of the thrombotic event in question. Moreover, our calculated costs are derived from CMS reimbursement rates and likely underestimate the true financial impact of errant testing given that commercial laboratories frequently charge at rates several-fold higher. On a national scale, prior analyses have suggested that the annual cost of thrombophilia testing, based on typical commercial rates, ranges from $300 million to $672 million.14
Researchers in prior studies have similarly examined the frequency of inappropriate thrombophilia testing and methods to reduce it. Researchers in a 2014 study demonstrated initially high rates of inappropriate inherited thrombophilia testing, and then showed marked reductions in testing and cost savings across multiple specialties following the introduction of a flowchart on a preprinted order form.15 Our findings provide motivation to perform similar endeavors.
The proportional difference of inappropriate ordering observed between nonteaching- and teaching-medicine services indicates a potential role for educational interventions. We recently completed a series of lectures on high-value thrombophilia ordering for residents and are actively analyzing its impact on subsequent ordering practices. We are also piloting an electronic best practice advisory for thrombophilia test ordering. Though the advisory may be overridden, providers are asked to provide justification for doing so on a voluntary basis. We plan to evaluate its effect on our findings reported in this study.
We acknowledge that our exclusion criteria resulted in the omission of testing across a spectrum of nonthrombotic clinical conditions, raising the question of selection bias. Because there are no established guidelines to determine the appropriateness of testing in these scenarios, we chose to limit the analysis of errant ordering to the context of thrombotic events. Other limitations of this study include the analysis of equivocal orders as appropriate. However, because equivocal ordering represented less than 3% of all analyzed orders, including these as inappropriate would not have significantly altered our findings.
CONCLUSIONS
A review of thrombophilia testing practices at our institution demonstrated that inappropriate testing in the inpatient setting is a frequent phenomenon associated with a significant financial impact. This effect was more pronounced in inherited versus acquired thrombophilia testing. Testing was frequently confounded and often failed to impact patients’ short- or long-term clinical management, regardless of the result.
These findings serve as a strong impetus to reduce the burden of routine thrombophilia testing during hospital admissions. Our data demonstrate a need for institution-wide changes such as implementing best practice advisories, introducing ordering restrictions, and conducting educational interventions in order to reduce unnecessary expenditures and improve patient care.
Disclosure
The authors have nothing to disclose.
1. Dobesh PP. Economic burden of venous thromboembolism in hospitalized patients. Pharmacotherapy. 2009;29(8):943-953. PubMed
2. Cohn DM, Vansenne F, de Borgie CA, Middeldorp S. Thrombophilia testing for prevention of recurrent venous thromboembolism. Cochrane Database Syst Rev. 2012;12:Cd007069. PubMed
3. Coppens M, Reijnders JH, Middeldorp S, Doggen CJ, Rosendaal FR. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6(9):1474-1477. PubMed
4. Somma J, Sussman, II, Rand JH. An evaluation of thrombophilia screening in an urban tertiary care medical center: A “real world” experience. Am J Clin Pathol. 2006;126(1):120-127. PubMed
5. Hicks LK, Bering H, Carson KR, et al. The ASH Choosing Wisely® campaign: five hematologic tests and treatments to question. Blood. 2013;122(24):3879-3883. PubMed
6. Centers for Medicare & Medicaid Services: Clinical Laboratory Fee Schedule Files. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files.html. Accessed October 2016
7. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41(1):154-164. PubMed
8. Moll S. Thrombophilia: clinical-practical aspects. J Thromb Thrombolysis. 2015;39(3):367-378. PubMed
9. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for vte disease: Chest guideline and expert panel report. Chest. 2016;149(2):315-352. PubMed
10. Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149(2):209-220. PubMed
11. Stata Statistical Software [computer program]. Version Release 14. College Station, TX: StataCorp LP; 2015.
12. Garcia-Horton A, Kovacs MJ, Abdulrehman J, Taylor JE, Sharma S, Lazo-Langner A. Impact of thrombophilia screening on venous thromboembolism management practices. Thromb Res.149:76-80. PubMed
13. Schulman S, Svenungsson E, Granqvist S. Anticardiolipin antibodies predict early recurrence of thromboembolism and death among patients with venous thromboembolism following anticoagulant therapy. Duration of Anticoagulation Study Group. Am J Med. 1998;104(4):332-338. PubMed
14. Petrilli CM, Heidemann L, Mack M, Durance P, Chopra V. Inpatient inherited thrombophilia testing. J Hosp Med. 2016;11(11):801-804. PubMed
15. Smith TW, Pi D, Hudoba M, Lee AY. Reducing inpatient heritable thrombophilia testing using a clinical decision-making tool. J Clin Pathol. 2014;67(4):345-349. PubMed
1. Dobesh PP. Economic burden of venous thromboembolism in hospitalized patients. Pharmacotherapy. 2009;29(8):943-953. PubMed
2. Cohn DM, Vansenne F, de Borgie CA, Middeldorp S. Thrombophilia testing for prevention of recurrent venous thromboembolism. Cochrane Database Syst Rev. 2012;12:Cd007069. PubMed
3. Coppens M, Reijnders JH, Middeldorp S, Doggen CJ, Rosendaal FR. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6(9):1474-1477. PubMed
4. Somma J, Sussman, II, Rand JH. An evaluation of thrombophilia screening in an urban tertiary care medical center: A “real world” experience. Am J Clin Pathol. 2006;126(1):120-127. PubMed
5. Hicks LK, Bering H, Carson KR, et al. The ASH Choosing Wisely® campaign: five hematologic tests and treatments to question. Blood. 2013;122(24):3879-3883. PubMed
6. Centers for Medicare & Medicaid Services: Clinical Laboratory Fee Schedule Files. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files.html. Accessed October 2016
7. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41(1):154-164. PubMed
8. Moll S. Thrombophilia: clinical-practical aspects. J Thromb Thrombolysis. 2015;39(3):367-378. PubMed
9. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for vte disease: Chest guideline and expert panel report. Chest. 2016;149(2):315-352. PubMed
10. Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149(2):209-220. PubMed
11. Stata Statistical Software [computer program]. Version Release 14. College Station, TX: StataCorp LP; 2015.
12. Garcia-Horton A, Kovacs MJ, Abdulrehman J, Taylor JE, Sharma S, Lazo-Langner A. Impact of thrombophilia screening on venous thromboembolism management practices. Thromb Res.149:76-80. PubMed
13. Schulman S, Svenungsson E, Granqvist S. Anticardiolipin antibodies predict early recurrence of thromboembolism and death among patients with venous thromboembolism following anticoagulant therapy. Duration of Anticoagulation Study Group. Am J Med. 1998;104(4):332-338. PubMed
14. Petrilli CM, Heidemann L, Mack M, Durance P, Chopra V. Inpatient inherited thrombophilia testing. J Hosp Med. 2016;11(11):801-804. PubMed
15. Smith TW, Pi D, Hudoba M, Lee AY. Reducing inpatient heritable thrombophilia testing using a clinical decision-making tool. J Clin Pathol. 2014;67(4):345-349. PubMed
© 2017 Society of Hospital Medicine
Antidepressant Use and Depressive Symptoms in Intensive Care Unit Survivors
As the number of intensive care unit (ICU) survivors has steadily increased over the past few decades, there is growing awareness of the long-term physical, cognitive, and psychological impairments after ICU hospitalization, collectively known as post–intensive care syndrome (PICS).1 Systematic reviews based mostly on research studies suggest that the prevalence of depressive symptoms 2-12 months after ICU discharge is nearly 30%.2-5 Due to the scarcity of established models of care for ICU survivors, there is limited characterization of depressive symptoms and antidepressant regimens in this clinical population. The Critical Care Recovery Center (CCRC) at Eskenazi Hospital is one of the first ICU survivor clinics in the United States and targets a racially diverse, underserved population in the Indianapolis metropolitan area.6 In this study, we examined whether patients had depressive symptoms at their initial CCRC visit, and whether the risk factors for depressive symptoms differed if they were on an antidepressant at their initial CCRC visit.
METHODS
Referral criteria to the CCRC were 18 years or older, admitted to the Eskenazi ICU, were on mechanical ventilation or delirious for ≥48 hours (major risk factors for the development of PICS), and recommended for follow-up by a critical care physician. The exclusion criterion included was enrollment in hospice or palliative care services. Institutional review board approval was obtained to conduct retrospective analyses of de-identified clinical data. Medical history and medication lists were collected from patients, informal caregivers, and electronic medical records.
Two hundred thirty-three patients were seen in the CCRC from July 2011 to August 2016. Two hundred four patients rated symptoms of depression with either the Patient Health Questionnaire (PHQ-9; N = 99) or Geriatric Depression Scale (GDS-30; N = 105) at their initial visit to the CCRC prior to receiving any treatment at the CCRC. Twenty-nine patients who did not complete depression questionnaires were excluded from the analyses. Patients with PHQ-9 score ≥10 or GDS score ≥20 were categorized as having moderate to severe depressive symptoms.7,8
Electronic medical records were reviewed to determine whether patients were on an antidepressant at hospital admission, hospital discharge, and the initial CCRC visit prior to any treatment in the CCRC. Patients who were on a tricyclic antidepressant, selective serotonin reuptake inhibitor, selective serotonin-norepinephrine reuptake inhibitor, noradrenergic and specific serotonergic antidepressant (eg, mirtazapine), or norepinephrine and dopaminergic reuptake inhibitor (eg, bupropion) at any dose were designated as being on an antidepressant. Prescribers of antidepressants included primary care providers, clinical providers during their hospital stay, and various outpatient subspecialists other than those in the CCRC.
We then examined whether the risk factors for depressive symptoms differed if patients were on an antidepressant at their initial CCRC visit. We compared demographic and clinical characteristics between depressed and nondepressed patients not on an antidepressant. We repeated these analyses for those on an antidepressant. Dichotomous outcomes were compared using chi-square testing, and two-way Student t tests for continuous outcomes. Demographic and clinical variables with P < 0.1 were included as covariates in a logistic regression model for depressive symptoms separately for those not an antidepressant and those on an antidepressant. History of depression was not included as a covariate because it is highly collinear with post-ICU depression.
RESULTS
Two hundred four ICU survivors in this study reflected a racially diverse and underserved population (monthly income $745.3 ± $931.5). Although most had respiratory failure and/or delirium during their hospital stay, 94.1% (N = 160) mostly lived independently after discharge. Nearly one-third of patients (N = 69) were on at least 1 antidepressant at their initial CCRC visit. Of these 69 patients, 60.9% (N = 42) had an antidepressant prescription on hospital admission, and 60.9% (N = 42) had an antidepressant prescription on hospital discharge.
We first compared the demographic and clinical characteristics of patients with and without depressive symptoms at their initial CCRC visit. Patients with depressive symptoms were younger, less likely to have cardiac disease, more likely to have a history of depression, more likely to have been prescribed an antidepressant on hospital admission, more likely to be prescribed an antidepressant on hospital discharge, and more likely to be on an antidepressant at their initial CCRC visit (Table 1).
Patients with depressive symptoms on an antidepressant (n = 65) were younger and more likely to be African American (borderline significance; Supplementary Table 2). Multivariate logistic regression showed that both younger age (OR = 0.92 per year, P = 0.003) and African American race (OR = 4.3, P = 0.024) remained significantly associated with depressive symptoms (Table 2).
DISCUSSION
Our study demonstrated that about one-third of our ICU survivor clinical cohort had untreated or inadequately treated depressive symptoms at their CCRC initial visit. Many patients with depressive symptoms had a history of depression and/or antidepressant prescription on hospital admission. This suggests that pre-ICU depression is a major contributor to post-ICU depression. These findings are consistent with the results of a large retrospective analysis of Danish ICU survivors that found that patients were more likely to have premorbid psychiatric diagnoses, compared with the general population.9 Another ICU survivor research study that excluded patients who were on antidepressants prior to ICU hospitalization found that 49% of these patients were on an antidepressant after their ICU stay.10 Our much lower rate of patients on an antidepressant after their ICU stay may reflect the differences between patient populations, differences in healthcare systems, and differences in clinician prescribing practices.
Younger age was associated with a higher likelihood of depressive symptoms independent of antidepressant status. Findings about the relationship between age and post-ICU depression have varied. The Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors group found that older age was associated with more depressive symptoms at 12 months postdischarge.11 On the other hand, a systematic review of post-ICU depression did not find any relationship between age and post-ICU depression.2,3 These differences may be due in part to demographic variations in cohorts.
Our logistic regression models suggest that there may also be different risk factors in patients who had untreated vs inadequately treated depressive symptoms. Patients who were not on an antidepressant at their initial CCRC visit were more likely to have a lower level of education. This is consistent with the Medical Expenditure Panel Surveys study, which showed that adults with less than a high school education were less likely to receive depression treatment.12 In patients who were on antidepressants at their initial CCRC visit, African Americans were more likely to have depressive symptoms. Possible reasons may include differences in receiving guideline-concordant antidepressant medication treatment, access to mental health subspecialty services, higher prevalence of treatment refractory depression, and differences in responses to antidepressant treatments.13,14
Strengths of our study include detailed characterization for a fairly large ICU survivor clinic population and a racially diverse cohort. To the best of our knowledge, our study is also the first to examine whether there may be different risk factors for depressive symptoms based on antidepressant status. Limitations include the lack of information about nonpharmacologic antidepressant treatment and the inability to assess whether noncompliance, insufficient dose, or insufficient time on antidepressants contributed to inadequate antidepressant treatment. Antidepressants may have also been prescribed for other purposes such as smoking cessation, neuropathic pain, and migraine headaches. However, because 72.4% of patients on antidepressants had a history of depression, it is likely that most of them were on antidepressants to treat depression.
Other limitations include potential biases in our clinical cohort. Over the last 5 years, the CCRC has provided care to more than 200 ICU survivors. With 1100 mechanically ventilated admissions per year, only 1.8% of survivors are seen. The referral criteria for the CCRC is a major source of selection bias, which likely overrepresents PICS. Because patients are seen in the CCRC about 3 months after hospital discharge, there is also informant censoring due to death. Physically sicker survivors in nursing home facilities were less likely to be included. Finally, the small cohort size may have resulted in an underpowered study.
Future studies will need to confirm our findings about the high prevalence of post-ICU depression and different responses to antidepressant medications by certain groups. Pre-ICU depression, lack of antidepressant treatment, and inadequate antidepressant treatment are major causes of post-ICU depression. Currently, the CCRC offers pharmacotherapy, problem-solving therapy, or referral to mental health specialists to treat patients with depressive symptoms. ICU survivor clinics, such as the CCRC, may become important settings that allow for increased access to depression treatment for those at higher risk for post-ICU depression as well as the testing of new antidepressant regimens for those with inadequately treated depression.
Acknowledgments
The authors thank Dr. Adil Sheikh for assistance with data entry and Cynthia Reynolds for her clinical services. Grant support: The Critical Care Recovery Center (CCRC) is supported by Eskenazi Health Services. SW is supported by NIA 2P30AG010133. SG is supported by NIA 2P30AG010133 and NIA 5R01AG045350. SK is supported by NHBLI 5T32HL091816-07. MB is supported by NIA R01 AG040220-05, AHRQ P30 HS024384-02, CMS 1 L1 CMS331444-02-00 and NIA R01 AG030618-05A1. BK is supported by NIA K23-AG043476 and NHLBI R01HL131730.
Disclosure
There are no conflicts of interest. None of the above NIH grants supported the CCRC or this work.
1. Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40:502-509. PubMed
2. Davydow DS, Gifford JM, Desai SV, Bienvenu OJ, Needham DM. Depression in general intensive care unit survivors: a systematic review. Intensive Care Med. 2009;35:796-809. PubMed
3. Rabiee A, Nikayin S, Hashem MD, et al. Depressive symptoms after critical illness: a systematic review and meta-analysis. Crit Care Med. 2016;44(9):1744-1753. PubMed
4. Huang M, Parker AM, Bienvenu OJ, et al. Psychiatric symptoms in acute respiratory distress syndrome survivors: A 1-year national multicenter study. Crit Care Med 2016;44:954-965. PubMed
5. Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, et al. Cooccurrence of and remission from general anxiety, depression, and posttraumatic stress disorder symptoms after acute lung injury: a 2-year longitudinal study. Crit Care Med. 2015;43:642-653. PubMed
6. Khan BA, Lasiter S, Boustani MA. CE: critical care recovery center: an innovative collaborative care model for ICU survivors. Am J Nurs. 2015;115:24-31. PubMed
7. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613. PubMed
8. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982-1983;17:37-49. PubMed
9. Wuns ch H, Christiansen CF, Johansen MB, et al. Psychiatric diagnoses and psychoactive medication use among nonsurgical critically ill patients receiving mechanical ventilation. JAMA. 2014;311:1133-1142. PubMed
10. Weinert C, Meller W. Epidemiology of depression and antidepressant therapy after acute respiratory failure. Psychosomatics. 2006;47(5):399-407. PubMed
11. Jackson JC, Pandharipande PP, Girard TD, et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2:369-379. PubMed
12. Olfson M, Blanco C, Marcus SC. Treatment of adult depression in the United States. JAMA Intern Med. 2016;176:1482-1491. PubMed
13. González HM, Vega WA, Williams DR, Tarraf W, West BT, Neighbors HW. Depression care in the United States: too little for too few. Arch Gen Psychiatry. 2010;67:37-46. PubMed
14. Bailey RK, Patel M, Barker NC, Ali S, Jabeen S. Major depressive disorder in the African American population. J Natl Med Assoc. 2011;103:548-557. PubMed
As the number of intensive care unit (ICU) survivors has steadily increased over the past few decades, there is growing awareness of the long-term physical, cognitive, and psychological impairments after ICU hospitalization, collectively known as post–intensive care syndrome (PICS).1 Systematic reviews based mostly on research studies suggest that the prevalence of depressive symptoms 2-12 months after ICU discharge is nearly 30%.2-5 Due to the scarcity of established models of care for ICU survivors, there is limited characterization of depressive symptoms and antidepressant regimens in this clinical population. The Critical Care Recovery Center (CCRC) at Eskenazi Hospital is one of the first ICU survivor clinics in the United States and targets a racially diverse, underserved population in the Indianapolis metropolitan area.6 In this study, we examined whether patients had depressive symptoms at their initial CCRC visit, and whether the risk factors for depressive symptoms differed if they were on an antidepressant at their initial CCRC visit.
METHODS
Referral criteria to the CCRC were 18 years or older, admitted to the Eskenazi ICU, were on mechanical ventilation or delirious for ≥48 hours (major risk factors for the development of PICS), and recommended for follow-up by a critical care physician. The exclusion criterion included was enrollment in hospice or palliative care services. Institutional review board approval was obtained to conduct retrospective analyses of de-identified clinical data. Medical history and medication lists were collected from patients, informal caregivers, and electronic medical records.
Two hundred thirty-three patients were seen in the CCRC from July 2011 to August 2016. Two hundred four patients rated symptoms of depression with either the Patient Health Questionnaire (PHQ-9; N = 99) or Geriatric Depression Scale (GDS-30; N = 105) at their initial visit to the CCRC prior to receiving any treatment at the CCRC. Twenty-nine patients who did not complete depression questionnaires were excluded from the analyses. Patients with PHQ-9 score ≥10 or GDS score ≥20 were categorized as having moderate to severe depressive symptoms.7,8
Electronic medical records were reviewed to determine whether patients were on an antidepressant at hospital admission, hospital discharge, and the initial CCRC visit prior to any treatment in the CCRC. Patients who were on a tricyclic antidepressant, selective serotonin reuptake inhibitor, selective serotonin-norepinephrine reuptake inhibitor, noradrenergic and specific serotonergic antidepressant (eg, mirtazapine), or norepinephrine and dopaminergic reuptake inhibitor (eg, bupropion) at any dose were designated as being on an antidepressant. Prescribers of antidepressants included primary care providers, clinical providers during their hospital stay, and various outpatient subspecialists other than those in the CCRC.
We then examined whether the risk factors for depressive symptoms differed if patients were on an antidepressant at their initial CCRC visit. We compared demographic and clinical characteristics between depressed and nondepressed patients not on an antidepressant. We repeated these analyses for those on an antidepressant. Dichotomous outcomes were compared using chi-square testing, and two-way Student t tests for continuous outcomes. Demographic and clinical variables with P < 0.1 were included as covariates in a logistic regression model for depressive symptoms separately for those not an antidepressant and those on an antidepressant. History of depression was not included as a covariate because it is highly collinear with post-ICU depression.
RESULTS
Two hundred four ICU survivors in this study reflected a racially diverse and underserved population (monthly income $745.3 ± $931.5). Although most had respiratory failure and/or delirium during their hospital stay, 94.1% (N = 160) mostly lived independently after discharge. Nearly one-third of patients (N = 69) were on at least 1 antidepressant at their initial CCRC visit. Of these 69 patients, 60.9% (N = 42) had an antidepressant prescription on hospital admission, and 60.9% (N = 42) had an antidepressant prescription on hospital discharge.
We first compared the demographic and clinical characteristics of patients with and without depressive symptoms at their initial CCRC visit. Patients with depressive symptoms were younger, less likely to have cardiac disease, more likely to have a history of depression, more likely to have been prescribed an antidepressant on hospital admission, more likely to be prescribed an antidepressant on hospital discharge, and more likely to be on an antidepressant at their initial CCRC visit (Table 1).
Patients with depressive symptoms on an antidepressant (n = 65) were younger and more likely to be African American (borderline significance; Supplementary Table 2). Multivariate logistic regression showed that both younger age (OR = 0.92 per year, P = 0.003) and African American race (OR = 4.3, P = 0.024) remained significantly associated with depressive symptoms (Table 2).
DISCUSSION
Our study demonstrated that about one-third of our ICU survivor clinical cohort had untreated or inadequately treated depressive symptoms at their CCRC initial visit. Many patients with depressive symptoms had a history of depression and/or antidepressant prescription on hospital admission. This suggests that pre-ICU depression is a major contributor to post-ICU depression. These findings are consistent with the results of a large retrospective analysis of Danish ICU survivors that found that patients were more likely to have premorbid psychiatric diagnoses, compared with the general population.9 Another ICU survivor research study that excluded patients who were on antidepressants prior to ICU hospitalization found that 49% of these patients were on an antidepressant after their ICU stay.10 Our much lower rate of patients on an antidepressant after their ICU stay may reflect the differences between patient populations, differences in healthcare systems, and differences in clinician prescribing practices.
Younger age was associated with a higher likelihood of depressive symptoms independent of antidepressant status. Findings about the relationship between age and post-ICU depression have varied. The Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors group found that older age was associated with more depressive symptoms at 12 months postdischarge.11 On the other hand, a systematic review of post-ICU depression did not find any relationship between age and post-ICU depression.2,3 These differences may be due in part to demographic variations in cohorts.
Our logistic regression models suggest that there may also be different risk factors in patients who had untreated vs inadequately treated depressive symptoms. Patients who were not on an antidepressant at their initial CCRC visit were more likely to have a lower level of education. This is consistent with the Medical Expenditure Panel Surveys study, which showed that adults with less than a high school education were less likely to receive depression treatment.12 In patients who were on antidepressants at their initial CCRC visit, African Americans were more likely to have depressive symptoms. Possible reasons may include differences in receiving guideline-concordant antidepressant medication treatment, access to mental health subspecialty services, higher prevalence of treatment refractory depression, and differences in responses to antidepressant treatments.13,14
Strengths of our study include detailed characterization for a fairly large ICU survivor clinic population and a racially diverse cohort. To the best of our knowledge, our study is also the first to examine whether there may be different risk factors for depressive symptoms based on antidepressant status. Limitations include the lack of information about nonpharmacologic antidepressant treatment and the inability to assess whether noncompliance, insufficient dose, or insufficient time on antidepressants contributed to inadequate antidepressant treatment. Antidepressants may have also been prescribed for other purposes such as smoking cessation, neuropathic pain, and migraine headaches. However, because 72.4% of patients on antidepressants had a history of depression, it is likely that most of them were on antidepressants to treat depression.
Other limitations include potential biases in our clinical cohort. Over the last 5 years, the CCRC has provided care to more than 200 ICU survivors. With 1100 mechanically ventilated admissions per year, only 1.8% of survivors are seen. The referral criteria for the CCRC is a major source of selection bias, which likely overrepresents PICS. Because patients are seen in the CCRC about 3 months after hospital discharge, there is also informant censoring due to death. Physically sicker survivors in nursing home facilities were less likely to be included. Finally, the small cohort size may have resulted in an underpowered study.
Future studies will need to confirm our findings about the high prevalence of post-ICU depression and different responses to antidepressant medications by certain groups. Pre-ICU depression, lack of antidepressant treatment, and inadequate antidepressant treatment are major causes of post-ICU depression. Currently, the CCRC offers pharmacotherapy, problem-solving therapy, or referral to mental health specialists to treat patients with depressive symptoms. ICU survivor clinics, such as the CCRC, may become important settings that allow for increased access to depression treatment for those at higher risk for post-ICU depression as well as the testing of new antidepressant regimens for those with inadequately treated depression.
Acknowledgments
The authors thank Dr. Adil Sheikh for assistance with data entry and Cynthia Reynolds for her clinical services. Grant support: The Critical Care Recovery Center (CCRC) is supported by Eskenazi Health Services. SW is supported by NIA 2P30AG010133. SG is supported by NIA 2P30AG010133 and NIA 5R01AG045350. SK is supported by NHBLI 5T32HL091816-07. MB is supported by NIA R01 AG040220-05, AHRQ P30 HS024384-02, CMS 1 L1 CMS331444-02-00 and NIA R01 AG030618-05A1. BK is supported by NIA K23-AG043476 and NHLBI R01HL131730.
Disclosure
There are no conflicts of interest. None of the above NIH grants supported the CCRC or this work.
As the number of intensive care unit (ICU) survivors has steadily increased over the past few decades, there is growing awareness of the long-term physical, cognitive, and psychological impairments after ICU hospitalization, collectively known as post–intensive care syndrome (PICS).1 Systematic reviews based mostly on research studies suggest that the prevalence of depressive symptoms 2-12 months after ICU discharge is nearly 30%.2-5 Due to the scarcity of established models of care for ICU survivors, there is limited characterization of depressive symptoms and antidepressant regimens in this clinical population. The Critical Care Recovery Center (CCRC) at Eskenazi Hospital is one of the first ICU survivor clinics in the United States and targets a racially diverse, underserved population in the Indianapolis metropolitan area.6 In this study, we examined whether patients had depressive symptoms at their initial CCRC visit, and whether the risk factors for depressive symptoms differed if they were on an antidepressant at their initial CCRC visit.
METHODS
Referral criteria to the CCRC were 18 years or older, admitted to the Eskenazi ICU, were on mechanical ventilation or delirious for ≥48 hours (major risk factors for the development of PICS), and recommended for follow-up by a critical care physician. The exclusion criterion included was enrollment in hospice or palliative care services. Institutional review board approval was obtained to conduct retrospective analyses of de-identified clinical data. Medical history and medication lists were collected from patients, informal caregivers, and electronic medical records.
Two hundred thirty-three patients were seen in the CCRC from July 2011 to August 2016. Two hundred four patients rated symptoms of depression with either the Patient Health Questionnaire (PHQ-9; N = 99) or Geriatric Depression Scale (GDS-30; N = 105) at their initial visit to the CCRC prior to receiving any treatment at the CCRC. Twenty-nine patients who did not complete depression questionnaires were excluded from the analyses. Patients with PHQ-9 score ≥10 or GDS score ≥20 were categorized as having moderate to severe depressive symptoms.7,8
Electronic medical records were reviewed to determine whether patients were on an antidepressant at hospital admission, hospital discharge, and the initial CCRC visit prior to any treatment in the CCRC. Patients who were on a tricyclic antidepressant, selective serotonin reuptake inhibitor, selective serotonin-norepinephrine reuptake inhibitor, noradrenergic and specific serotonergic antidepressant (eg, mirtazapine), or norepinephrine and dopaminergic reuptake inhibitor (eg, bupropion) at any dose were designated as being on an antidepressant. Prescribers of antidepressants included primary care providers, clinical providers during their hospital stay, and various outpatient subspecialists other than those in the CCRC.
We then examined whether the risk factors for depressive symptoms differed if patients were on an antidepressant at their initial CCRC visit. We compared demographic and clinical characteristics between depressed and nondepressed patients not on an antidepressant. We repeated these analyses for those on an antidepressant. Dichotomous outcomes were compared using chi-square testing, and two-way Student t tests for continuous outcomes. Demographic and clinical variables with P < 0.1 were included as covariates in a logistic regression model for depressive symptoms separately for those not an antidepressant and those on an antidepressant. History of depression was not included as a covariate because it is highly collinear with post-ICU depression.
RESULTS
Two hundred four ICU survivors in this study reflected a racially diverse and underserved population (monthly income $745.3 ± $931.5). Although most had respiratory failure and/or delirium during their hospital stay, 94.1% (N = 160) mostly lived independently after discharge. Nearly one-third of patients (N = 69) were on at least 1 antidepressant at their initial CCRC visit. Of these 69 patients, 60.9% (N = 42) had an antidepressant prescription on hospital admission, and 60.9% (N = 42) had an antidepressant prescription on hospital discharge.
We first compared the demographic and clinical characteristics of patients with and without depressive symptoms at their initial CCRC visit. Patients with depressive symptoms were younger, less likely to have cardiac disease, more likely to have a history of depression, more likely to have been prescribed an antidepressant on hospital admission, more likely to be prescribed an antidepressant on hospital discharge, and more likely to be on an antidepressant at their initial CCRC visit (Table 1).
Patients with depressive symptoms on an antidepressant (n = 65) were younger and more likely to be African American (borderline significance; Supplementary Table 2). Multivariate logistic regression showed that both younger age (OR = 0.92 per year, P = 0.003) and African American race (OR = 4.3, P = 0.024) remained significantly associated with depressive symptoms (Table 2).
DISCUSSION
Our study demonstrated that about one-third of our ICU survivor clinical cohort had untreated or inadequately treated depressive symptoms at their CCRC initial visit. Many patients with depressive symptoms had a history of depression and/or antidepressant prescription on hospital admission. This suggests that pre-ICU depression is a major contributor to post-ICU depression. These findings are consistent with the results of a large retrospective analysis of Danish ICU survivors that found that patients were more likely to have premorbid psychiatric diagnoses, compared with the general population.9 Another ICU survivor research study that excluded patients who were on antidepressants prior to ICU hospitalization found that 49% of these patients were on an antidepressant after their ICU stay.10 Our much lower rate of patients on an antidepressant after their ICU stay may reflect the differences between patient populations, differences in healthcare systems, and differences in clinician prescribing practices.
Younger age was associated with a higher likelihood of depressive symptoms independent of antidepressant status. Findings about the relationship between age and post-ICU depression have varied. The Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors group found that older age was associated with more depressive symptoms at 12 months postdischarge.11 On the other hand, a systematic review of post-ICU depression did not find any relationship between age and post-ICU depression.2,3 These differences may be due in part to demographic variations in cohorts.
Our logistic regression models suggest that there may also be different risk factors in patients who had untreated vs inadequately treated depressive symptoms. Patients who were not on an antidepressant at their initial CCRC visit were more likely to have a lower level of education. This is consistent with the Medical Expenditure Panel Surveys study, which showed that adults with less than a high school education were less likely to receive depression treatment.12 In patients who were on antidepressants at their initial CCRC visit, African Americans were more likely to have depressive symptoms. Possible reasons may include differences in receiving guideline-concordant antidepressant medication treatment, access to mental health subspecialty services, higher prevalence of treatment refractory depression, and differences in responses to antidepressant treatments.13,14
Strengths of our study include detailed characterization for a fairly large ICU survivor clinic population and a racially diverse cohort. To the best of our knowledge, our study is also the first to examine whether there may be different risk factors for depressive symptoms based on antidepressant status. Limitations include the lack of information about nonpharmacologic antidepressant treatment and the inability to assess whether noncompliance, insufficient dose, or insufficient time on antidepressants contributed to inadequate antidepressant treatment. Antidepressants may have also been prescribed for other purposes such as smoking cessation, neuropathic pain, and migraine headaches. However, because 72.4% of patients on antidepressants had a history of depression, it is likely that most of them were on antidepressants to treat depression.
Other limitations include potential biases in our clinical cohort. Over the last 5 years, the CCRC has provided care to more than 200 ICU survivors. With 1100 mechanically ventilated admissions per year, only 1.8% of survivors are seen. The referral criteria for the CCRC is a major source of selection bias, which likely overrepresents PICS. Because patients are seen in the CCRC about 3 months after hospital discharge, there is also informant censoring due to death. Physically sicker survivors in nursing home facilities were less likely to be included. Finally, the small cohort size may have resulted in an underpowered study.
Future studies will need to confirm our findings about the high prevalence of post-ICU depression and different responses to antidepressant medications by certain groups. Pre-ICU depression, lack of antidepressant treatment, and inadequate antidepressant treatment are major causes of post-ICU depression. Currently, the CCRC offers pharmacotherapy, problem-solving therapy, or referral to mental health specialists to treat patients with depressive symptoms. ICU survivor clinics, such as the CCRC, may become important settings that allow for increased access to depression treatment for those at higher risk for post-ICU depression as well as the testing of new antidepressant regimens for those with inadequately treated depression.
Acknowledgments
The authors thank Dr. Adil Sheikh for assistance with data entry and Cynthia Reynolds for her clinical services. Grant support: The Critical Care Recovery Center (CCRC) is supported by Eskenazi Health Services. SW is supported by NIA 2P30AG010133. SG is supported by NIA 2P30AG010133 and NIA 5R01AG045350. SK is supported by NHBLI 5T32HL091816-07. MB is supported by NIA R01 AG040220-05, AHRQ P30 HS024384-02, CMS 1 L1 CMS331444-02-00 and NIA R01 AG030618-05A1. BK is supported by NIA K23-AG043476 and NHLBI R01HL131730.
Disclosure
There are no conflicts of interest. None of the above NIH grants supported the CCRC or this work.
1. Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40:502-509. PubMed
2. Davydow DS, Gifford JM, Desai SV, Bienvenu OJ, Needham DM. Depression in general intensive care unit survivors: a systematic review. Intensive Care Med. 2009;35:796-809. PubMed
3. Rabiee A, Nikayin S, Hashem MD, et al. Depressive symptoms after critical illness: a systematic review and meta-analysis. Crit Care Med. 2016;44(9):1744-1753. PubMed
4. Huang M, Parker AM, Bienvenu OJ, et al. Psychiatric symptoms in acute respiratory distress syndrome survivors: A 1-year national multicenter study. Crit Care Med 2016;44:954-965. PubMed
5. Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, et al. Cooccurrence of and remission from general anxiety, depression, and posttraumatic stress disorder symptoms after acute lung injury: a 2-year longitudinal study. Crit Care Med. 2015;43:642-653. PubMed
6. Khan BA, Lasiter S, Boustani MA. CE: critical care recovery center: an innovative collaborative care model for ICU survivors. Am J Nurs. 2015;115:24-31. PubMed
7. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613. PubMed
8. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982-1983;17:37-49. PubMed
9. Wuns ch H, Christiansen CF, Johansen MB, et al. Psychiatric diagnoses and psychoactive medication use among nonsurgical critically ill patients receiving mechanical ventilation. JAMA. 2014;311:1133-1142. PubMed
10. Weinert C, Meller W. Epidemiology of depression and antidepressant therapy after acute respiratory failure. Psychosomatics. 2006;47(5):399-407. PubMed
11. Jackson JC, Pandharipande PP, Girard TD, et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2:369-379. PubMed
12. Olfson M, Blanco C, Marcus SC. Treatment of adult depression in the United States. JAMA Intern Med. 2016;176:1482-1491. PubMed
13. González HM, Vega WA, Williams DR, Tarraf W, West BT, Neighbors HW. Depression care in the United States: too little for too few. Arch Gen Psychiatry. 2010;67:37-46. PubMed
14. Bailey RK, Patel M, Barker NC, Ali S, Jabeen S. Major depressive disorder in the African American population. J Natl Med Assoc. 2011;103:548-557. PubMed
1. Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40:502-509. PubMed
2. Davydow DS, Gifford JM, Desai SV, Bienvenu OJ, Needham DM. Depression in general intensive care unit survivors: a systematic review. Intensive Care Med. 2009;35:796-809. PubMed
3. Rabiee A, Nikayin S, Hashem MD, et al. Depressive symptoms after critical illness: a systematic review and meta-analysis. Crit Care Med. 2016;44(9):1744-1753. PubMed
4. Huang M, Parker AM, Bienvenu OJ, et al. Psychiatric symptoms in acute respiratory distress syndrome survivors: A 1-year national multicenter study. Crit Care Med 2016;44:954-965. PubMed
5. Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, et al. Cooccurrence of and remission from general anxiety, depression, and posttraumatic stress disorder symptoms after acute lung injury: a 2-year longitudinal study. Crit Care Med. 2015;43:642-653. PubMed
6. Khan BA, Lasiter S, Boustani MA. CE: critical care recovery center: an innovative collaborative care model for ICU survivors. Am J Nurs. 2015;115:24-31. PubMed
7. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613. PubMed
8. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982-1983;17:37-49. PubMed
9. Wuns ch H, Christiansen CF, Johansen MB, et al. Psychiatric diagnoses and psychoactive medication use among nonsurgical critically ill patients receiving mechanical ventilation. JAMA. 2014;311:1133-1142. PubMed
10. Weinert C, Meller W. Epidemiology of depression and antidepressant therapy after acute respiratory failure. Psychosomatics. 2006;47(5):399-407. PubMed
11. Jackson JC, Pandharipande PP, Girard TD, et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2:369-379. PubMed
12. Olfson M, Blanco C, Marcus SC. Treatment of adult depression in the United States. JAMA Intern Med. 2016;176:1482-1491. PubMed
13. González HM, Vega WA, Williams DR, Tarraf W, West BT, Neighbors HW. Depression care in the United States: too little for too few. Arch Gen Psychiatry. 2010;67:37-46. PubMed
14. Bailey RK, Patel M, Barker NC, Ali S, Jabeen S. Major depressive disorder in the African American population. J Natl Med Assoc. 2011;103:548-557. PubMed
© 2017 Society of Hospital Medicine
Appropriate Reconciliation of Cardiovascular Medications After Elective Surgery and Postdischarge Acute Hospital and Ambulatory Visits
Medication reconciliation at hospital discharge is a critical component of the posthospital transition of care.1 Effective reconciliation involves a clear process for documenting a current medication list, identifying and resolving discrepancies, and then documenting decisions and instructions around which medications should be continued, modified, or stopped.2 Existing studies3-5 suggest that medication discrepancies are common during hospital discharge transitions of care and lead to preventable adverse drug events, patient disability, and increased healthcare utilization following hospital discharge, including physician office visits, emergency department (ED) visits, and hospitalizations.6-8
While the majority of studies of medication discrepancies have been conducted in general medical patients, few have examined how gaps in discharge medication reconciliation might affect surgical patients.9,10 Two prior studies9,10 suggest that medication discrepancies may occur more frequently for surgical patients, compared with medical patients, particularly discrepancies in reordering home medications postoperatively, raising patient safety concerns for more than 50 million patients hospitalized for surgery each year.11 In particular, little is known about the appropriate discharge reconciliation of chronic cardiovascular medications, such as beta-blockers, renin-angiotensin system inhibitors, and statins in surgical patients, despite perioperative practice guidelines recommending continuation or rapid reinitiation of these medications after noncardiac surgery.12 Problems with chronic cardiovascular medications have been implicated as major contributors to ED visits and hospitalizations for adverse drug events,13,14 further highlighting the importance of safe and appropriate management of these medications.
To better understand the current state and impact of postoperative discharge medication reconciliation of chronic cardiovascular medications in surgical patients, we examined (1) the appropriate discharge reconciliation of 4 cardiovascular medication classes, and (2) the associations between the appropriate discharge reconciliation of these medication classes and postdischarge acute hospital and ambulatory visits in patients hospitalized for elective noncardiac surgery at an academic medical center.
METHODS
Study Design and Patient Selection
We performed a retrospective analysis of data collected as part of a cohort study of hospitalized surgical patients admitted between January 2007 and December 2011. The original study was designed to assess the impact of a social marketing intervention on guideline-appropriate perioperative beta-blocker use in surgical patients. The study was conducted at 1 academic medical center that had 2 campuses with full noncardiac operative facilities and a 600-bed total capacity. Both sites had preoperative clinics, and patients were recruited by review of preoperative clinic records. Institutional review boards responsible for all sites approved the study.
For this analysis, we included adults (age >18 years) undergoing elective noncardiac surgery, who were expected to remain hospitalized for at least 1 day and were taking antiplatelet agents (aspirin, aspirin-dipyridamole, or clopidogrel), beta-blockers, renin-angiotensin system inhibitors (angiotensin-converting-enzyme inhibitors or angiotensin-receptor blockers), or statin lipid-lowering agents.
Data Collection
Data Sources. We collected data from a structured review of medical records as well as from discharge abstract information obtained from administrative data systems. Data regarding patient demographics (age, sex, and race/ethnicity), medical history, preoperative cardiovascular medications, surgical procedure and service, and attending surgeon were obtained from a medical record review of comprehensive preoperative clinic evaluations. Data regarding complications during hospitalization were obtained from medical record review and administrative data (Supplement for International Classification of Diseases, Ninth Revision codes).15 Research assistants abstracting data were trained by using a comprehensive reference manual providing specific criteria for classifying chart abstraction data. Research assistants also were directly observed during initial chart abstractions and underwent random chart validation audits by a senior investigator to ensure consistency. Any discrepancies in coding were resolved by consensus among senior investigators.
Definition of Key Predictor: Appropriate Reconciliation. We abstracted discharge medication lists from the electronic medical record. We defined the appropriate reconciliation of cardiovascular medications at discharge as documentation in discharge instructions, medication reconciliation tools, or discharge summaries that a preadmission cardiovascular medication was being continued at discharge, or, if the medication was not continued, documentation of a new contraindication to the medication or complication precluding its use during hospitalization. Medication continuity was considered appropriate if it was continued at discharge irrespective of changes in dosage. By using this measure for individual medications, we also assessed appropriate reconciliation as an “all-or-none” complete versus incomplete measure (appropriate reconciliation of all preoperative cardiovascular medication classes the patient was taking).16
Definition of Outcomes. Our coprimary outcomes were acute hospital visits (ED visits or hospitalizations) and unplanned ambulatory visits (primary care or surgical) at 30 days after surgery. Postoperative ambulatory visits that were not planned prior to surgery were defined as unplanned. Outcomes were ascertained by patient reports during follow-up telephone questionnaires administered by trained research staff and verified by medical record review.
Definition of Covariates. Using these data, we calculated a Revised Cardiac Risk Index (RCRI) score,17 which estimates the risk of perioperative cardiac complications in patients undergoing surgery. Through chart abstraction data supplemented by diagnosis codes from administrative data, we also constructed variables indicating occurrences of postoperative complications anytime during hospitalization that might pose contraindications to continuation of the 4 cardiovascular medication classes studied. For example, if a chart indicated that the patient had an acute rise in creatinine (elevation of baseline creatinine by 50% or absolute rise of 1 mg/dL in patients with baseline creatinine greater than 3 mg/dL) during hospitalization and a preoperative renin-angiotensin system inhibitor was not prescribed at discharge, we would have considered discontinuation appropriate. Other complications we abstracted were hypotension (systolic blood pressure less than 90 mmHg) for beta-blockers and renin-angiotensin system inhibitors, bradycardia (heart rate less than 50 bpm) for beta-blockers, acute kidney injury (defined above) and hyperkalemia for renin-angiotensin system inhibitors, and bleeding (any site) for antiplatelet agents.
Statistical Analysis
We used χ2 and Kruskal-Wallis tests to compare baseline patient characteristics. To assess associations between appropriate medication reconciliation and patient outcomes, we used multilevel mixed-effects logistic regression to account for the clustering of patients by the attending surgeon. We adjusted for baseline patient demographics, surgical service, the number of baseline cardiovascular medications, and individual RCRI criteria. We constructed separate models for all-or-none appropriate reconciliation and for each individual medication class.
As a sensitivity analysis, we constructed similar models by using a simplified definition of appropriate reconciliation based entirely on medication continuity (continued or not continued at discharge) without taking potential contraindications during hospitalization into account. For complete versus incomplete reconciliation, we also constructed models with an interaction term between the number of baseline cardiovascular medications and appropriate medication reconciliation to test the hypothesis that inappropriate reconciliation would be more likely with an increasing number of preoperative cardiovascular medications. Because this interaction term was not statistically significant, we did not include it in the final models for ease of reporting and interpretability. We performed all statistical analyses using Stata 14 (StataCorp, LLC, College Station, Texas), and used 2-sided statistical tests and a P value of less than .05 to define statistical significance.
RESULTS
Patient Characteristics
A total of 849 patients were enrolled, of which 752 (88.6%) were taking at least 1 of the specified cardiovascular medications in the preoperative period. Their mean age was 61.5; 50.9% were male, 72.6% were non-Hispanic white, and 89.4% had RCRI scores of 0 or 1 (Table 1). The majority (63.8%) were undergoing general surgery, orthopedic surgery, or neurosurgery procedures. In the preoperative period, 327 (43.5%) patients were taking antiplatelet agents, 624 (83.0%) were taking beta-blockers, 361 (48.0%) were taking renin-angiotensin system inhibitors, and 406 (54.0%) were taking statins (Table 2). Among patients taking antiplatelet agents, 271 (82.9%) were taking aspirin alone, 21 (6.4%) were taking clopidogrel alone, and 35 (10.7%) were taking dual antiplatelet therapy with aspirin and clopidogrel. Nearly three-quarters of the patients (551, 73.3%) were taking medications from 2 or more classes, and the proportion of patients with inappropriate reconciliation increased with the number of preoperative cardiovascular medications.
Patients with and without appropriate reconciliation of all preoperative cardiovascular medications were similar in age, sex, and race/ethnicity (Table 1). Patients with inappropriate reconciliation of at least 1 medication were more likely to be on the urology and renal/liver transplant surgical services, have higher RCRI scores, and be taking antiplatelet agents, statins, renin-angiotensin system inhibitors, and 3 or more cardiovascular medications in the preoperative period.
Appropriate Medication Reconciliation
Four hundred thirty-six patients (58.0%) had their baseline cardiovascular medications appropriately reconciled. Among all patients with appropriately reconciled medications, 1 (0.2%) had beta-blockers discontinued due to a documented episode of hypotension; 17 (3.9%) had renin-angiotensin system inhibitors discontinued due to episodes of acute kidney injury, hypotension, or hyperkalemia; and 1 (0.2%) had antiplatelet agents discontinued due to bleeding. For individual medications, appropriate reconciliation between the preoperative and discharge periods occurred for 156 of the 327 patients on antiplatelet agents (47.7%), 507 of the 624 patients on beta-blockers (81.3%), 259 of the 361 patients on renin-angiotensin system inhibitors (71.8%), and 302 of the 406 patients on statins (74.4%; Table 2).
Associations Between Medication Reconciliation and Outcomes
Thirty-day outcome data on acute hospital visits were available for 679 (90.3%) patients. Of these, 146 (21.5%) were seen in the ED or were hospitalized, and 111 (16.3%) were seen for an unplanned primary care or surgical outpatient visit at 30 days after surgery. Patients with incomplete outcome data were more likely to have complete medication reconciliation compared with those with complete outcome data (71.2% vs 56.6%, P = 0.02). As shown in Table 3, the proportion of patients with 30-day acute hospital visits was nonstatistically significantly lower in patients with complete medication reconciliation (20.8% vs 22.4%, P = 0.63) and the appropriate reconciliation of beta-blockers (21.9% vs 23.6%, P = 0.71) and renin-angiotensin system inhibitors (19.6% vs 20.0%, P = 0.93), and nonsignificantly higher with the appropriate reconciliation of antiplatelet agents (23.9% vs 19.9%, P = 0.40). Acute hospital visits were statistically significantly lower with the appropriate reconciliation of statins (17.9% vs 31.9%, P = 0.004).
Sensitivity Analysis
Overall, 430 (57.2%) patients had complete cardiovascular medication continuity without considering potential contraindications during hospitalization. Associations between medication continuity and acute hospital and ambulatory visits were similar to the primary analyses.
DISCUSSION
In this study of 752 patients hospitalized for elective noncardiac surgery, we found significant gaps in the appropriate reconciliation of commonly prescribed cardiovascular medications, with inappropriate discontinuation ranging from 18.8% to 52.3% for individual medications. Unplanned postdischarge healthcare utilization was high, with acute hospital visits documented in 21.5% of patients and unplanned ambulatory visits in 16.3% at 30 days after surgery. However, medication reconciliation gaps were not consistently associated with ED visits, hospitalizations, or unplanned ambulatory visits.
Our finding of large gaps in postoperative medication reconciliation is consistent with existing studies of medication reconciliation in surgical patients.9,10,18 One study found medication discrepancies in 40.2% of postoperative patients receiving usual care and discrepancies judged to have the potential to cause harm (such as the omission of beta-blockers) in 29.9%.9 Consistent with our findings, this study also found that most postoperative medication discrepancies were omissions in reordering home medications, though at a rate somewhat higher than those seen in medical patients at discharge.5 While hospitalization by itself increases the risk of unintentional discontinuation of chronic medications,3 our results, along with existing literature, suggest that the risk for omission of chronic medications is unacceptably high.
We also found significant variation in reconciliation among cardiovascular medications, with appropriate reconciliation occurring least frequently for antiplatelet agents and most frequently for beta-blockers. The low rates of appropriate reconciliation for antiplatelet agents may be attributable to deliberate withholding of antiplatelet therapy in the postoperative period based on clinical assessments of surgical bleeding risk in the absence of active bleeding. Perioperative management of antiplatelet agents for noncardiac surgery remains an unclear and controversial topic, which may also contribute to the variation noted.19 Conversely, beta-blockers demonstrated high rates of preoperative use (over 80% of patients) and appropriate reconciliation. Both findings are likely attributable in part to the timing of the study, which began prior to the publication of the Perioperative Ischemic Evaluation trial, which more definitively demonstrated the potential harms of perioperative beta-blocker therapy.20
Despite a high proportion of patients with discontinuous medications at discharge, we found no associations between the appropriate reconciliation of beta-blockers, renin-angiotensin system inhibitors, and antiplatelet agents and acute hospital or ambulatory visits in the first 30 days after discharge. One explanation for this discrepancy is that, although we focused on cardiovascular medications commonly implicated in acute hospital visits, the vast majority of patients in our study had low perioperative cardiovascular risk as assessed by the RCRI. Previous studies have demonstrated that the benefit of perioperative beta-blocker therapy is predominantly in patients with moderate to high perioperative cardiovascular risk.21,22 It is possible that the detrimental effects of the discontinuation of chronic cardiovascular medications are more prominent in populations at a higher risk of perioperative cardiovascular complications or that complications will occur later than 30 days after discharge. Similarly, while the benefits of continuation of renin-angiotensin system inhibitors are less clear,23 few patients in our cohort had a history of congestive heart failure (6.3%) or coronary artery disease (13.0%), 2 conditions in which the impact of perioperative discontinuation of renin-angiotensin inhibitor or beta-blocker therapy would likely be more pronounced.24,25 An additional explanation for the lack of associations is that, while multiple studies have demonstrated that medication errors are common, the proportion of errors with the potential for harm is much lower, and the proportion that causes actual harm is lower still.5,26,27 Thus, while we likely captured high-severity medication errors leading to acute hospital or unplanned ambulatory visits, we would not have captured medication errors with lower severity clinical consequences that did not result in medical encounters.
We did find an association between the continuation of statin therapy and reduced ED visits and hospitalizations. This finding is supported by previous studies of patients undergoing noncardiac surgery, including 1 demonstrating an association between immediate postoperative statin therapy and reduced in-hospital mortality28 and another study demonstrating an association between postoperative statin therapy and reductions in a composite endpoint of 30-day mortality, atrial fibrillation, and nonfatal myocardial infarction.29 Alternatively, this finding could reflect the effects of unaddressed confounding by factors contributing to statin discontinuation and poor health outcomes leading to acute hospital visits, such as acute elevations in liver enzymes.
Our study has important implications for patients undergoing elective noncardiac surgery and the healthcare providers caring for them. First, inappropriate omissions of chronic cardiovascular medications at discharge are common; clinicians should increase their general awareness and focus on appropriately reconciling these medications, for even if our results do not connect medication discontinuity to readmissions or unexpected clinical encounters, their impact on patients’ understanding of their medications remains a potential concern. Second, the overall high rates of unplanned postdischarge healthcare utilization in this study highlight the need for close postdischarge monitoring of patients undergoing elective surgical procedures and for further research to identify preventable etiologies of postdischarge healthcare utilization in this population. Third, further study is needed to identify specific patient populations and medication classes, in which appropriate reconciliation is associated with patient outcomes that may benefit from more intensive discharge medication reconciliation interventions.
Our study has limitations. First, the majority of patients in this single-center study were at low risk of perioperative cardiovascular events, and our results may not be generalizable to higher-risk patients undergoing elective surgery. Second, discharge reconciliation was based on documentation of medication reconciliation and not on patient-reported medication adherence. In addition, the ability to judge the accuracy of discharge medication reconciliation is in part dependent on the accuracy of the admission medication reconciliation. Thus, although we used preoperative medication regimens documented during preadmission visits to comprehensive preoperative clinics for comparison, discrepancies in these preoperative regimens could have affected our analysis of appropriate discharge reconciliation. Third, inadequate documentation of clinical reasons for discontinuing medications may have led to residual confounding by indication in our observational study. Finally, the outcomes available to us may have been relatively insensitive to other adverse effects of medication discontinuity, such as patient symptoms (eg, angina severity), patient awareness of medications, or work placed on primary care physicians needing to “clean up” erroneous medication lists.
In conclusion, gaps in appropriate discharge reconciliation of chronic cardiovascular medications were common but not consistently associated with postdischarge acute hospital or unplanned ambulatory visits in a relatively low-risk cohort of patients undergoing elective surgery. While appropriate medication reconciliation should always be a priority, further study is needed to identify medication reconciliation approaches associated with postdischarge healthcare utilization and other patient outcomes.
Disclosure
Dr. Lee reports receiving grant support from the Health Resources and Services Administration (T32HP19025). Dr. Vittinghoff reports receiving grant support from the Agency for Healthcare Research and Quality. Dr. Auerbach and Dr. Fleischmann report receiving grant support from the National Institutes of Health. Dr. Auerbach also reports receiving honorarium as Editor-in-Chief of the Journal of Hospital Medicine. Dr. Corbett reports receiving grant and travel support from Simon Fraser University. The remaining authors have no disclosures to report.
1. The Joint Commission. National Patient Safety Goals. 2016; https://www.jointcommission.org/standards_information/npsgs.aspx. Accessed June 21, 2016.
2. Institute for Healthcare Improvement. Medication Reconciliation to Prevent Adverse Drug Events. 2016; http://www.ihi.org/topics/ADEsMedicationReconciliation/Pages/default.aspx. Accessed June 24, 2016.
3. Bell CM, Brener SS, Gunraj N, et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 2011;306(8):840-847. PubMed
4. Coleman EA, Smith JD, Raha D, Min SJ. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med. 2005;165(16):1842-1847. PubMed
5. Wong JD, Bajcar JM, Wong GG, et al. Medication reconciliation at hospital discharge: evaluating discrepancies. Ann Pharmacother. 2008;42(10):1373-1379. PubMed
6. Forster AJ, Clark HD, Menard A, et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345-349. PubMed
7. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. PubMed
8. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. Adverse drug events occurring following hospital discharge. JGIM. 2005;20(4):317-323. PubMed
9. Kwan Y, Fernandes OA, Nagge JJ, et al. Pharmacist medication assessments in a surgical preadmission clinic. Arch Intern Med. 2007;167(10):1034-1040. PubMed
10. Unroe KT, Pfeiffenberger T, Riegelhaupt S, Jastrzembski J, Lokhnygina Y, Colon-Emeric C. Inpatient Medication Reconciliation at Admission and Discharge: A Retrospective Cohort Study of Age and Other Risk Factors for Medication Discrepancies. Am J Geriatr Pharmacother. 2010;8(2):115-126. PubMed
11. CDC - National Center for Health Statistics. Fast Stats: Inpatient Surgery. http://www.cdc.gov/nchs/fastats/inpatient-surgery.htm. Accessed on June 24, 2016.
12. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(24):e278-e333. PubMed
13. Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365(21):2002-2012. PubMed
14. Budnitz DS, Pollock DA, Weidenbach KN, Mendelsohn AB, Schroeder TJ, Annest JL. National surveillance of emergency department visits for outpatient adverse drug events. JAMA. 2006;296(15):1858-1866. PubMed
15. Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92(16):2643-2652. PubMed
16. Nolan T, Berwick DM. All-or-none measurement raises the bar on performance. JAMA. 2006;295(10):1168-1170. PubMed
17. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043-1049. PubMed
18. Gonzalez-Garcia L, Salmeron-Garcia A, Garcia-Lirola MA, Moya-Roldan S, Belda-Rustarazo S, Cabeza-Barrera J. Medication reconciliation at admission to surgical departments. J Eval Clin Pract. 2016;22(1):20-25. PubMed
19. Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1494-1503. PubMed
20. Devereaux PJ, Yang H, Yusuf S, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839-1847. PubMed
21. Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349-361. PubMed
22. London MJ, Hur K, Schwartz GG, Henderson WG. Association of perioperative beta-blockade with mortality and cardiovascular morbidity following major noncardiac surgery. JAMA. 2013;309(16):1704-1713. PubMed
23. Rosenman DJ, McDonald FS, Ebbert JO, Erwin PJ, LaBella M, Montori VM. Clinical consequences of withholding versus administering renin-angiotensin-aldosterone system antagonists in the preoperative period. J Hosp Med. 2008;3(4):319-325. PubMed
24. Andersson C, Merie C, Jorgensen M, et al. Association of beta-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing noncardiac surgery: a Danish nationwide cohort study. JAMA Intern Med. 2014;174(3):336-344. PubMed
25. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128(16):E240-E327. PubMed
26. Kwan JL, Lo L, Sampson M, Shojania KG. Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 Pt 2):397-403. PubMed
27. Tam VC, Knowles SR, Cornish PL, Fine N, Marchesano R, Etchells EE. Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. CMAJ. 2005;173(5):510-515. PubMed
28. Lindenauer PK, Pekow P, Wang K, Gutierrez B, Benjamin EM. Lipid-lowering therapy and in-hospital mortality following major noncardiac surgery. JAMA. 2004;291(17):2092-2099. PubMed
29. Raju MG, Pachika A, Punnam SR, et al. Statin Therapy in the Reduction of Cardiovascular Events in Patients Undergoing Intermediate-Risk Noncardiac, Nonvascular Surgery. Clin Cardiol. 2013;36(8):456-461. PubMed
Medication reconciliation at hospital discharge is a critical component of the posthospital transition of care.1 Effective reconciliation involves a clear process for documenting a current medication list, identifying and resolving discrepancies, and then documenting decisions and instructions around which medications should be continued, modified, or stopped.2 Existing studies3-5 suggest that medication discrepancies are common during hospital discharge transitions of care and lead to preventable adverse drug events, patient disability, and increased healthcare utilization following hospital discharge, including physician office visits, emergency department (ED) visits, and hospitalizations.6-8
While the majority of studies of medication discrepancies have been conducted in general medical patients, few have examined how gaps in discharge medication reconciliation might affect surgical patients.9,10 Two prior studies9,10 suggest that medication discrepancies may occur more frequently for surgical patients, compared with medical patients, particularly discrepancies in reordering home medications postoperatively, raising patient safety concerns for more than 50 million patients hospitalized for surgery each year.11 In particular, little is known about the appropriate discharge reconciliation of chronic cardiovascular medications, such as beta-blockers, renin-angiotensin system inhibitors, and statins in surgical patients, despite perioperative practice guidelines recommending continuation or rapid reinitiation of these medications after noncardiac surgery.12 Problems with chronic cardiovascular medications have been implicated as major contributors to ED visits and hospitalizations for adverse drug events,13,14 further highlighting the importance of safe and appropriate management of these medications.
To better understand the current state and impact of postoperative discharge medication reconciliation of chronic cardiovascular medications in surgical patients, we examined (1) the appropriate discharge reconciliation of 4 cardiovascular medication classes, and (2) the associations between the appropriate discharge reconciliation of these medication classes and postdischarge acute hospital and ambulatory visits in patients hospitalized for elective noncardiac surgery at an academic medical center.
METHODS
Study Design and Patient Selection
We performed a retrospective analysis of data collected as part of a cohort study of hospitalized surgical patients admitted between January 2007 and December 2011. The original study was designed to assess the impact of a social marketing intervention on guideline-appropriate perioperative beta-blocker use in surgical patients. The study was conducted at 1 academic medical center that had 2 campuses with full noncardiac operative facilities and a 600-bed total capacity. Both sites had preoperative clinics, and patients were recruited by review of preoperative clinic records. Institutional review boards responsible for all sites approved the study.
For this analysis, we included adults (age >18 years) undergoing elective noncardiac surgery, who were expected to remain hospitalized for at least 1 day and were taking antiplatelet agents (aspirin, aspirin-dipyridamole, or clopidogrel), beta-blockers, renin-angiotensin system inhibitors (angiotensin-converting-enzyme inhibitors or angiotensin-receptor blockers), or statin lipid-lowering agents.
Data Collection
Data Sources. We collected data from a structured review of medical records as well as from discharge abstract information obtained from administrative data systems. Data regarding patient demographics (age, sex, and race/ethnicity), medical history, preoperative cardiovascular medications, surgical procedure and service, and attending surgeon were obtained from a medical record review of comprehensive preoperative clinic evaluations. Data regarding complications during hospitalization were obtained from medical record review and administrative data (Supplement for International Classification of Diseases, Ninth Revision codes).15 Research assistants abstracting data were trained by using a comprehensive reference manual providing specific criteria for classifying chart abstraction data. Research assistants also were directly observed during initial chart abstractions and underwent random chart validation audits by a senior investigator to ensure consistency. Any discrepancies in coding were resolved by consensus among senior investigators.
Definition of Key Predictor: Appropriate Reconciliation. We abstracted discharge medication lists from the electronic medical record. We defined the appropriate reconciliation of cardiovascular medications at discharge as documentation in discharge instructions, medication reconciliation tools, or discharge summaries that a preadmission cardiovascular medication was being continued at discharge, or, if the medication was not continued, documentation of a new contraindication to the medication or complication precluding its use during hospitalization. Medication continuity was considered appropriate if it was continued at discharge irrespective of changes in dosage. By using this measure for individual medications, we also assessed appropriate reconciliation as an “all-or-none” complete versus incomplete measure (appropriate reconciliation of all preoperative cardiovascular medication classes the patient was taking).16
Definition of Outcomes. Our coprimary outcomes were acute hospital visits (ED visits or hospitalizations) and unplanned ambulatory visits (primary care or surgical) at 30 days after surgery. Postoperative ambulatory visits that were not planned prior to surgery were defined as unplanned. Outcomes were ascertained by patient reports during follow-up telephone questionnaires administered by trained research staff and verified by medical record review.
Definition of Covariates. Using these data, we calculated a Revised Cardiac Risk Index (RCRI) score,17 which estimates the risk of perioperative cardiac complications in patients undergoing surgery. Through chart abstraction data supplemented by diagnosis codes from administrative data, we also constructed variables indicating occurrences of postoperative complications anytime during hospitalization that might pose contraindications to continuation of the 4 cardiovascular medication classes studied. For example, if a chart indicated that the patient had an acute rise in creatinine (elevation of baseline creatinine by 50% or absolute rise of 1 mg/dL in patients with baseline creatinine greater than 3 mg/dL) during hospitalization and a preoperative renin-angiotensin system inhibitor was not prescribed at discharge, we would have considered discontinuation appropriate. Other complications we abstracted were hypotension (systolic blood pressure less than 90 mmHg) for beta-blockers and renin-angiotensin system inhibitors, bradycardia (heart rate less than 50 bpm) for beta-blockers, acute kidney injury (defined above) and hyperkalemia for renin-angiotensin system inhibitors, and bleeding (any site) for antiplatelet agents.
Statistical Analysis
We used χ2 and Kruskal-Wallis tests to compare baseline patient characteristics. To assess associations between appropriate medication reconciliation and patient outcomes, we used multilevel mixed-effects logistic regression to account for the clustering of patients by the attending surgeon. We adjusted for baseline patient demographics, surgical service, the number of baseline cardiovascular medications, and individual RCRI criteria. We constructed separate models for all-or-none appropriate reconciliation and for each individual medication class.
As a sensitivity analysis, we constructed similar models by using a simplified definition of appropriate reconciliation based entirely on medication continuity (continued or not continued at discharge) without taking potential contraindications during hospitalization into account. For complete versus incomplete reconciliation, we also constructed models with an interaction term between the number of baseline cardiovascular medications and appropriate medication reconciliation to test the hypothesis that inappropriate reconciliation would be more likely with an increasing number of preoperative cardiovascular medications. Because this interaction term was not statistically significant, we did not include it in the final models for ease of reporting and interpretability. We performed all statistical analyses using Stata 14 (StataCorp, LLC, College Station, Texas), and used 2-sided statistical tests and a P value of less than .05 to define statistical significance.
RESULTS
Patient Characteristics
A total of 849 patients were enrolled, of which 752 (88.6%) were taking at least 1 of the specified cardiovascular medications in the preoperative period. Their mean age was 61.5; 50.9% were male, 72.6% were non-Hispanic white, and 89.4% had RCRI scores of 0 or 1 (Table 1). The majority (63.8%) were undergoing general surgery, orthopedic surgery, or neurosurgery procedures. In the preoperative period, 327 (43.5%) patients were taking antiplatelet agents, 624 (83.0%) were taking beta-blockers, 361 (48.0%) were taking renin-angiotensin system inhibitors, and 406 (54.0%) were taking statins (Table 2). Among patients taking antiplatelet agents, 271 (82.9%) were taking aspirin alone, 21 (6.4%) were taking clopidogrel alone, and 35 (10.7%) were taking dual antiplatelet therapy with aspirin and clopidogrel. Nearly three-quarters of the patients (551, 73.3%) were taking medications from 2 or more classes, and the proportion of patients with inappropriate reconciliation increased with the number of preoperative cardiovascular medications.
Patients with and without appropriate reconciliation of all preoperative cardiovascular medications were similar in age, sex, and race/ethnicity (Table 1). Patients with inappropriate reconciliation of at least 1 medication were more likely to be on the urology and renal/liver transplant surgical services, have higher RCRI scores, and be taking antiplatelet agents, statins, renin-angiotensin system inhibitors, and 3 or more cardiovascular medications in the preoperative period.
Appropriate Medication Reconciliation
Four hundred thirty-six patients (58.0%) had their baseline cardiovascular medications appropriately reconciled. Among all patients with appropriately reconciled medications, 1 (0.2%) had beta-blockers discontinued due to a documented episode of hypotension; 17 (3.9%) had renin-angiotensin system inhibitors discontinued due to episodes of acute kidney injury, hypotension, or hyperkalemia; and 1 (0.2%) had antiplatelet agents discontinued due to bleeding. For individual medications, appropriate reconciliation between the preoperative and discharge periods occurred for 156 of the 327 patients on antiplatelet agents (47.7%), 507 of the 624 patients on beta-blockers (81.3%), 259 of the 361 patients on renin-angiotensin system inhibitors (71.8%), and 302 of the 406 patients on statins (74.4%; Table 2).
Associations Between Medication Reconciliation and Outcomes
Thirty-day outcome data on acute hospital visits were available for 679 (90.3%) patients. Of these, 146 (21.5%) were seen in the ED or were hospitalized, and 111 (16.3%) were seen for an unplanned primary care or surgical outpatient visit at 30 days after surgery. Patients with incomplete outcome data were more likely to have complete medication reconciliation compared with those with complete outcome data (71.2% vs 56.6%, P = 0.02). As shown in Table 3, the proportion of patients with 30-day acute hospital visits was nonstatistically significantly lower in patients with complete medication reconciliation (20.8% vs 22.4%, P = 0.63) and the appropriate reconciliation of beta-blockers (21.9% vs 23.6%, P = 0.71) and renin-angiotensin system inhibitors (19.6% vs 20.0%, P = 0.93), and nonsignificantly higher with the appropriate reconciliation of antiplatelet agents (23.9% vs 19.9%, P = 0.40). Acute hospital visits were statistically significantly lower with the appropriate reconciliation of statins (17.9% vs 31.9%, P = 0.004).
Sensitivity Analysis
Overall, 430 (57.2%) patients had complete cardiovascular medication continuity without considering potential contraindications during hospitalization. Associations between medication continuity and acute hospital and ambulatory visits were similar to the primary analyses.
DISCUSSION
In this study of 752 patients hospitalized for elective noncardiac surgery, we found significant gaps in the appropriate reconciliation of commonly prescribed cardiovascular medications, with inappropriate discontinuation ranging from 18.8% to 52.3% for individual medications. Unplanned postdischarge healthcare utilization was high, with acute hospital visits documented in 21.5% of patients and unplanned ambulatory visits in 16.3% at 30 days after surgery. However, medication reconciliation gaps were not consistently associated with ED visits, hospitalizations, or unplanned ambulatory visits.
Our finding of large gaps in postoperative medication reconciliation is consistent with existing studies of medication reconciliation in surgical patients.9,10,18 One study found medication discrepancies in 40.2% of postoperative patients receiving usual care and discrepancies judged to have the potential to cause harm (such as the omission of beta-blockers) in 29.9%.9 Consistent with our findings, this study also found that most postoperative medication discrepancies were omissions in reordering home medications, though at a rate somewhat higher than those seen in medical patients at discharge.5 While hospitalization by itself increases the risk of unintentional discontinuation of chronic medications,3 our results, along with existing literature, suggest that the risk for omission of chronic medications is unacceptably high.
We also found significant variation in reconciliation among cardiovascular medications, with appropriate reconciliation occurring least frequently for antiplatelet agents and most frequently for beta-blockers. The low rates of appropriate reconciliation for antiplatelet agents may be attributable to deliberate withholding of antiplatelet therapy in the postoperative period based on clinical assessments of surgical bleeding risk in the absence of active bleeding. Perioperative management of antiplatelet agents for noncardiac surgery remains an unclear and controversial topic, which may also contribute to the variation noted.19 Conversely, beta-blockers demonstrated high rates of preoperative use (over 80% of patients) and appropriate reconciliation. Both findings are likely attributable in part to the timing of the study, which began prior to the publication of the Perioperative Ischemic Evaluation trial, which more definitively demonstrated the potential harms of perioperative beta-blocker therapy.20
Despite a high proportion of patients with discontinuous medications at discharge, we found no associations between the appropriate reconciliation of beta-blockers, renin-angiotensin system inhibitors, and antiplatelet agents and acute hospital or ambulatory visits in the first 30 days after discharge. One explanation for this discrepancy is that, although we focused on cardiovascular medications commonly implicated in acute hospital visits, the vast majority of patients in our study had low perioperative cardiovascular risk as assessed by the RCRI. Previous studies have demonstrated that the benefit of perioperative beta-blocker therapy is predominantly in patients with moderate to high perioperative cardiovascular risk.21,22 It is possible that the detrimental effects of the discontinuation of chronic cardiovascular medications are more prominent in populations at a higher risk of perioperative cardiovascular complications or that complications will occur later than 30 days after discharge. Similarly, while the benefits of continuation of renin-angiotensin system inhibitors are less clear,23 few patients in our cohort had a history of congestive heart failure (6.3%) or coronary artery disease (13.0%), 2 conditions in which the impact of perioperative discontinuation of renin-angiotensin inhibitor or beta-blocker therapy would likely be more pronounced.24,25 An additional explanation for the lack of associations is that, while multiple studies have demonstrated that medication errors are common, the proportion of errors with the potential for harm is much lower, and the proportion that causes actual harm is lower still.5,26,27 Thus, while we likely captured high-severity medication errors leading to acute hospital or unplanned ambulatory visits, we would not have captured medication errors with lower severity clinical consequences that did not result in medical encounters.
We did find an association between the continuation of statin therapy and reduced ED visits and hospitalizations. This finding is supported by previous studies of patients undergoing noncardiac surgery, including 1 demonstrating an association between immediate postoperative statin therapy and reduced in-hospital mortality28 and another study demonstrating an association between postoperative statin therapy and reductions in a composite endpoint of 30-day mortality, atrial fibrillation, and nonfatal myocardial infarction.29 Alternatively, this finding could reflect the effects of unaddressed confounding by factors contributing to statin discontinuation and poor health outcomes leading to acute hospital visits, such as acute elevations in liver enzymes.
Our study has important implications for patients undergoing elective noncardiac surgery and the healthcare providers caring for them. First, inappropriate omissions of chronic cardiovascular medications at discharge are common; clinicians should increase their general awareness and focus on appropriately reconciling these medications, for even if our results do not connect medication discontinuity to readmissions or unexpected clinical encounters, their impact on patients’ understanding of their medications remains a potential concern. Second, the overall high rates of unplanned postdischarge healthcare utilization in this study highlight the need for close postdischarge monitoring of patients undergoing elective surgical procedures and for further research to identify preventable etiologies of postdischarge healthcare utilization in this population. Third, further study is needed to identify specific patient populations and medication classes, in which appropriate reconciliation is associated with patient outcomes that may benefit from more intensive discharge medication reconciliation interventions.
Our study has limitations. First, the majority of patients in this single-center study were at low risk of perioperative cardiovascular events, and our results may not be generalizable to higher-risk patients undergoing elective surgery. Second, discharge reconciliation was based on documentation of medication reconciliation and not on patient-reported medication adherence. In addition, the ability to judge the accuracy of discharge medication reconciliation is in part dependent on the accuracy of the admission medication reconciliation. Thus, although we used preoperative medication regimens documented during preadmission visits to comprehensive preoperative clinics for comparison, discrepancies in these preoperative regimens could have affected our analysis of appropriate discharge reconciliation. Third, inadequate documentation of clinical reasons for discontinuing medications may have led to residual confounding by indication in our observational study. Finally, the outcomes available to us may have been relatively insensitive to other adverse effects of medication discontinuity, such as patient symptoms (eg, angina severity), patient awareness of medications, or work placed on primary care physicians needing to “clean up” erroneous medication lists.
In conclusion, gaps in appropriate discharge reconciliation of chronic cardiovascular medications were common but not consistently associated with postdischarge acute hospital or unplanned ambulatory visits in a relatively low-risk cohort of patients undergoing elective surgery. While appropriate medication reconciliation should always be a priority, further study is needed to identify medication reconciliation approaches associated with postdischarge healthcare utilization and other patient outcomes.
Disclosure
Dr. Lee reports receiving grant support from the Health Resources and Services Administration (T32HP19025). Dr. Vittinghoff reports receiving grant support from the Agency for Healthcare Research and Quality. Dr. Auerbach and Dr. Fleischmann report receiving grant support from the National Institutes of Health. Dr. Auerbach also reports receiving honorarium as Editor-in-Chief of the Journal of Hospital Medicine. Dr. Corbett reports receiving grant and travel support from Simon Fraser University. The remaining authors have no disclosures to report.
Medication reconciliation at hospital discharge is a critical component of the posthospital transition of care.1 Effective reconciliation involves a clear process for documenting a current medication list, identifying and resolving discrepancies, and then documenting decisions and instructions around which medications should be continued, modified, or stopped.2 Existing studies3-5 suggest that medication discrepancies are common during hospital discharge transitions of care and lead to preventable adverse drug events, patient disability, and increased healthcare utilization following hospital discharge, including physician office visits, emergency department (ED) visits, and hospitalizations.6-8
While the majority of studies of medication discrepancies have been conducted in general medical patients, few have examined how gaps in discharge medication reconciliation might affect surgical patients.9,10 Two prior studies9,10 suggest that medication discrepancies may occur more frequently for surgical patients, compared with medical patients, particularly discrepancies in reordering home medications postoperatively, raising patient safety concerns for more than 50 million patients hospitalized for surgery each year.11 In particular, little is known about the appropriate discharge reconciliation of chronic cardiovascular medications, such as beta-blockers, renin-angiotensin system inhibitors, and statins in surgical patients, despite perioperative practice guidelines recommending continuation or rapid reinitiation of these medications after noncardiac surgery.12 Problems with chronic cardiovascular medications have been implicated as major contributors to ED visits and hospitalizations for adverse drug events,13,14 further highlighting the importance of safe and appropriate management of these medications.
To better understand the current state and impact of postoperative discharge medication reconciliation of chronic cardiovascular medications in surgical patients, we examined (1) the appropriate discharge reconciliation of 4 cardiovascular medication classes, and (2) the associations between the appropriate discharge reconciliation of these medication classes and postdischarge acute hospital and ambulatory visits in patients hospitalized for elective noncardiac surgery at an academic medical center.
METHODS
Study Design and Patient Selection
We performed a retrospective analysis of data collected as part of a cohort study of hospitalized surgical patients admitted between January 2007 and December 2011. The original study was designed to assess the impact of a social marketing intervention on guideline-appropriate perioperative beta-blocker use in surgical patients. The study was conducted at 1 academic medical center that had 2 campuses with full noncardiac operative facilities and a 600-bed total capacity. Both sites had preoperative clinics, and patients were recruited by review of preoperative clinic records. Institutional review boards responsible for all sites approved the study.
For this analysis, we included adults (age >18 years) undergoing elective noncardiac surgery, who were expected to remain hospitalized for at least 1 day and were taking antiplatelet agents (aspirin, aspirin-dipyridamole, or clopidogrel), beta-blockers, renin-angiotensin system inhibitors (angiotensin-converting-enzyme inhibitors or angiotensin-receptor blockers), or statin lipid-lowering agents.
Data Collection
Data Sources. We collected data from a structured review of medical records as well as from discharge abstract information obtained from administrative data systems. Data regarding patient demographics (age, sex, and race/ethnicity), medical history, preoperative cardiovascular medications, surgical procedure and service, and attending surgeon were obtained from a medical record review of comprehensive preoperative clinic evaluations. Data regarding complications during hospitalization were obtained from medical record review and administrative data (Supplement for International Classification of Diseases, Ninth Revision codes).15 Research assistants abstracting data were trained by using a comprehensive reference manual providing specific criteria for classifying chart abstraction data. Research assistants also were directly observed during initial chart abstractions and underwent random chart validation audits by a senior investigator to ensure consistency. Any discrepancies in coding were resolved by consensus among senior investigators.
Definition of Key Predictor: Appropriate Reconciliation. We abstracted discharge medication lists from the electronic medical record. We defined the appropriate reconciliation of cardiovascular medications at discharge as documentation in discharge instructions, medication reconciliation tools, or discharge summaries that a preadmission cardiovascular medication was being continued at discharge, or, if the medication was not continued, documentation of a new contraindication to the medication or complication precluding its use during hospitalization. Medication continuity was considered appropriate if it was continued at discharge irrespective of changes in dosage. By using this measure for individual medications, we also assessed appropriate reconciliation as an “all-or-none” complete versus incomplete measure (appropriate reconciliation of all preoperative cardiovascular medication classes the patient was taking).16
Definition of Outcomes. Our coprimary outcomes were acute hospital visits (ED visits or hospitalizations) and unplanned ambulatory visits (primary care or surgical) at 30 days after surgery. Postoperative ambulatory visits that were not planned prior to surgery were defined as unplanned. Outcomes were ascertained by patient reports during follow-up telephone questionnaires administered by trained research staff and verified by medical record review.
Definition of Covariates. Using these data, we calculated a Revised Cardiac Risk Index (RCRI) score,17 which estimates the risk of perioperative cardiac complications in patients undergoing surgery. Through chart abstraction data supplemented by diagnosis codes from administrative data, we also constructed variables indicating occurrences of postoperative complications anytime during hospitalization that might pose contraindications to continuation of the 4 cardiovascular medication classes studied. For example, if a chart indicated that the patient had an acute rise in creatinine (elevation of baseline creatinine by 50% or absolute rise of 1 mg/dL in patients with baseline creatinine greater than 3 mg/dL) during hospitalization and a preoperative renin-angiotensin system inhibitor was not prescribed at discharge, we would have considered discontinuation appropriate. Other complications we abstracted were hypotension (systolic blood pressure less than 90 mmHg) for beta-blockers and renin-angiotensin system inhibitors, bradycardia (heart rate less than 50 bpm) for beta-blockers, acute kidney injury (defined above) and hyperkalemia for renin-angiotensin system inhibitors, and bleeding (any site) for antiplatelet agents.
Statistical Analysis
We used χ2 and Kruskal-Wallis tests to compare baseline patient characteristics. To assess associations between appropriate medication reconciliation and patient outcomes, we used multilevel mixed-effects logistic regression to account for the clustering of patients by the attending surgeon. We adjusted for baseline patient demographics, surgical service, the number of baseline cardiovascular medications, and individual RCRI criteria. We constructed separate models for all-or-none appropriate reconciliation and for each individual medication class.
As a sensitivity analysis, we constructed similar models by using a simplified definition of appropriate reconciliation based entirely on medication continuity (continued or not continued at discharge) without taking potential contraindications during hospitalization into account. For complete versus incomplete reconciliation, we also constructed models with an interaction term between the number of baseline cardiovascular medications and appropriate medication reconciliation to test the hypothesis that inappropriate reconciliation would be more likely with an increasing number of preoperative cardiovascular medications. Because this interaction term was not statistically significant, we did not include it in the final models for ease of reporting and interpretability. We performed all statistical analyses using Stata 14 (StataCorp, LLC, College Station, Texas), and used 2-sided statistical tests and a P value of less than .05 to define statistical significance.
RESULTS
Patient Characteristics
A total of 849 patients were enrolled, of which 752 (88.6%) were taking at least 1 of the specified cardiovascular medications in the preoperative period. Their mean age was 61.5; 50.9% were male, 72.6% were non-Hispanic white, and 89.4% had RCRI scores of 0 or 1 (Table 1). The majority (63.8%) were undergoing general surgery, orthopedic surgery, or neurosurgery procedures. In the preoperative period, 327 (43.5%) patients were taking antiplatelet agents, 624 (83.0%) were taking beta-blockers, 361 (48.0%) were taking renin-angiotensin system inhibitors, and 406 (54.0%) were taking statins (Table 2). Among patients taking antiplatelet agents, 271 (82.9%) were taking aspirin alone, 21 (6.4%) were taking clopidogrel alone, and 35 (10.7%) were taking dual antiplatelet therapy with aspirin and clopidogrel. Nearly three-quarters of the patients (551, 73.3%) were taking medications from 2 or more classes, and the proportion of patients with inappropriate reconciliation increased with the number of preoperative cardiovascular medications.
Patients with and without appropriate reconciliation of all preoperative cardiovascular medications were similar in age, sex, and race/ethnicity (Table 1). Patients with inappropriate reconciliation of at least 1 medication were more likely to be on the urology and renal/liver transplant surgical services, have higher RCRI scores, and be taking antiplatelet agents, statins, renin-angiotensin system inhibitors, and 3 or more cardiovascular medications in the preoperative period.
Appropriate Medication Reconciliation
Four hundred thirty-six patients (58.0%) had their baseline cardiovascular medications appropriately reconciled. Among all patients with appropriately reconciled medications, 1 (0.2%) had beta-blockers discontinued due to a documented episode of hypotension; 17 (3.9%) had renin-angiotensin system inhibitors discontinued due to episodes of acute kidney injury, hypotension, or hyperkalemia; and 1 (0.2%) had antiplatelet agents discontinued due to bleeding. For individual medications, appropriate reconciliation between the preoperative and discharge periods occurred for 156 of the 327 patients on antiplatelet agents (47.7%), 507 of the 624 patients on beta-blockers (81.3%), 259 of the 361 patients on renin-angiotensin system inhibitors (71.8%), and 302 of the 406 patients on statins (74.4%; Table 2).
Associations Between Medication Reconciliation and Outcomes
Thirty-day outcome data on acute hospital visits were available for 679 (90.3%) patients. Of these, 146 (21.5%) were seen in the ED or were hospitalized, and 111 (16.3%) were seen for an unplanned primary care or surgical outpatient visit at 30 days after surgery. Patients with incomplete outcome data were more likely to have complete medication reconciliation compared with those with complete outcome data (71.2% vs 56.6%, P = 0.02). As shown in Table 3, the proportion of patients with 30-day acute hospital visits was nonstatistically significantly lower in patients with complete medication reconciliation (20.8% vs 22.4%, P = 0.63) and the appropriate reconciliation of beta-blockers (21.9% vs 23.6%, P = 0.71) and renin-angiotensin system inhibitors (19.6% vs 20.0%, P = 0.93), and nonsignificantly higher with the appropriate reconciliation of antiplatelet agents (23.9% vs 19.9%, P = 0.40). Acute hospital visits were statistically significantly lower with the appropriate reconciliation of statins (17.9% vs 31.9%, P = 0.004).
Sensitivity Analysis
Overall, 430 (57.2%) patients had complete cardiovascular medication continuity without considering potential contraindications during hospitalization. Associations between medication continuity and acute hospital and ambulatory visits were similar to the primary analyses.
DISCUSSION
In this study of 752 patients hospitalized for elective noncardiac surgery, we found significant gaps in the appropriate reconciliation of commonly prescribed cardiovascular medications, with inappropriate discontinuation ranging from 18.8% to 52.3% for individual medications. Unplanned postdischarge healthcare utilization was high, with acute hospital visits documented in 21.5% of patients and unplanned ambulatory visits in 16.3% at 30 days after surgery. However, medication reconciliation gaps were not consistently associated with ED visits, hospitalizations, or unplanned ambulatory visits.
Our finding of large gaps in postoperative medication reconciliation is consistent with existing studies of medication reconciliation in surgical patients.9,10,18 One study found medication discrepancies in 40.2% of postoperative patients receiving usual care and discrepancies judged to have the potential to cause harm (such as the omission of beta-blockers) in 29.9%.9 Consistent with our findings, this study also found that most postoperative medication discrepancies were omissions in reordering home medications, though at a rate somewhat higher than those seen in medical patients at discharge.5 While hospitalization by itself increases the risk of unintentional discontinuation of chronic medications,3 our results, along with existing literature, suggest that the risk for omission of chronic medications is unacceptably high.
We also found significant variation in reconciliation among cardiovascular medications, with appropriate reconciliation occurring least frequently for antiplatelet agents and most frequently for beta-blockers. The low rates of appropriate reconciliation for antiplatelet agents may be attributable to deliberate withholding of antiplatelet therapy in the postoperative period based on clinical assessments of surgical bleeding risk in the absence of active bleeding. Perioperative management of antiplatelet agents for noncardiac surgery remains an unclear and controversial topic, which may also contribute to the variation noted.19 Conversely, beta-blockers demonstrated high rates of preoperative use (over 80% of patients) and appropriate reconciliation. Both findings are likely attributable in part to the timing of the study, which began prior to the publication of the Perioperative Ischemic Evaluation trial, which more definitively demonstrated the potential harms of perioperative beta-blocker therapy.20
Despite a high proportion of patients with discontinuous medications at discharge, we found no associations between the appropriate reconciliation of beta-blockers, renin-angiotensin system inhibitors, and antiplatelet agents and acute hospital or ambulatory visits in the first 30 days after discharge. One explanation for this discrepancy is that, although we focused on cardiovascular medications commonly implicated in acute hospital visits, the vast majority of patients in our study had low perioperative cardiovascular risk as assessed by the RCRI. Previous studies have demonstrated that the benefit of perioperative beta-blocker therapy is predominantly in patients with moderate to high perioperative cardiovascular risk.21,22 It is possible that the detrimental effects of the discontinuation of chronic cardiovascular medications are more prominent in populations at a higher risk of perioperative cardiovascular complications or that complications will occur later than 30 days after discharge. Similarly, while the benefits of continuation of renin-angiotensin system inhibitors are less clear,23 few patients in our cohort had a history of congestive heart failure (6.3%) or coronary artery disease (13.0%), 2 conditions in which the impact of perioperative discontinuation of renin-angiotensin inhibitor or beta-blocker therapy would likely be more pronounced.24,25 An additional explanation for the lack of associations is that, while multiple studies have demonstrated that medication errors are common, the proportion of errors with the potential for harm is much lower, and the proportion that causes actual harm is lower still.5,26,27 Thus, while we likely captured high-severity medication errors leading to acute hospital or unplanned ambulatory visits, we would not have captured medication errors with lower severity clinical consequences that did not result in medical encounters.
We did find an association between the continuation of statin therapy and reduced ED visits and hospitalizations. This finding is supported by previous studies of patients undergoing noncardiac surgery, including 1 demonstrating an association between immediate postoperative statin therapy and reduced in-hospital mortality28 and another study demonstrating an association between postoperative statin therapy and reductions in a composite endpoint of 30-day mortality, atrial fibrillation, and nonfatal myocardial infarction.29 Alternatively, this finding could reflect the effects of unaddressed confounding by factors contributing to statin discontinuation and poor health outcomes leading to acute hospital visits, such as acute elevations in liver enzymes.
Our study has important implications for patients undergoing elective noncardiac surgery and the healthcare providers caring for them. First, inappropriate omissions of chronic cardiovascular medications at discharge are common; clinicians should increase their general awareness and focus on appropriately reconciling these medications, for even if our results do not connect medication discontinuity to readmissions or unexpected clinical encounters, their impact on patients’ understanding of their medications remains a potential concern. Second, the overall high rates of unplanned postdischarge healthcare utilization in this study highlight the need for close postdischarge monitoring of patients undergoing elective surgical procedures and for further research to identify preventable etiologies of postdischarge healthcare utilization in this population. Third, further study is needed to identify specific patient populations and medication classes, in which appropriate reconciliation is associated with patient outcomes that may benefit from more intensive discharge medication reconciliation interventions.
Our study has limitations. First, the majority of patients in this single-center study were at low risk of perioperative cardiovascular events, and our results may not be generalizable to higher-risk patients undergoing elective surgery. Second, discharge reconciliation was based on documentation of medication reconciliation and not on patient-reported medication adherence. In addition, the ability to judge the accuracy of discharge medication reconciliation is in part dependent on the accuracy of the admission medication reconciliation. Thus, although we used preoperative medication regimens documented during preadmission visits to comprehensive preoperative clinics for comparison, discrepancies in these preoperative regimens could have affected our analysis of appropriate discharge reconciliation. Third, inadequate documentation of clinical reasons for discontinuing medications may have led to residual confounding by indication in our observational study. Finally, the outcomes available to us may have been relatively insensitive to other adverse effects of medication discontinuity, such as patient symptoms (eg, angina severity), patient awareness of medications, or work placed on primary care physicians needing to “clean up” erroneous medication lists.
In conclusion, gaps in appropriate discharge reconciliation of chronic cardiovascular medications were common but not consistently associated with postdischarge acute hospital or unplanned ambulatory visits in a relatively low-risk cohort of patients undergoing elective surgery. While appropriate medication reconciliation should always be a priority, further study is needed to identify medication reconciliation approaches associated with postdischarge healthcare utilization and other patient outcomes.
Disclosure
Dr. Lee reports receiving grant support from the Health Resources and Services Administration (T32HP19025). Dr. Vittinghoff reports receiving grant support from the Agency for Healthcare Research and Quality. Dr. Auerbach and Dr. Fleischmann report receiving grant support from the National Institutes of Health. Dr. Auerbach also reports receiving honorarium as Editor-in-Chief of the Journal of Hospital Medicine. Dr. Corbett reports receiving grant and travel support from Simon Fraser University. The remaining authors have no disclosures to report.
1. The Joint Commission. National Patient Safety Goals. 2016; https://www.jointcommission.org/standards_information/npsgs.aspx. Accessed June 21, 2016.
2. Institute for Healthcare Improvement. Medication Reconciliation to Prevent Adverse Drug Events. 2016; http://www.ihi.org/topics/ADEsMedicationReconciliation/Pages/default.aspx. Accessed June 24, 2016.
3. Bell CM, Brener SS, Gunraj N, et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 2011;306(8):840-847. PubMed
4. Coleman EA, Smith JD, Raha D, Min SJ. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med. 2005;165(16):1842-1847. PubMed
5. Wong JD, Bajcar JM, Wong GG, et al. Medication reconciliation at hospital discharge: evaluating discrepancies. Ann Pharmacother. 2008;42(10):1373-1379. PubMed
6. Forster AJ, Clark HD, Menard A, et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345-349. PubMed
7. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. PubMed
8. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. Adverse drug events occurring following hospital discharge. JGIM. 2005;20(4):317-323. PubMed
9. Kwan Y, Fernandes OA, Nagge JJ, et al. Pharmacist medication assessments in a surgical preadmission clinic. Arch Intern Med. 2007;167(10):1034-1040. PubMed
10. Unroe KT, Pfeiffenberger T, Riegelhaupt S, Jastrzembski J, Lokhnygina Y, Colon-Emeric C. Inpatient Medication Reconciliation at Admission and Discharge: A Retrospective Cohort Study of Age and Other Risk Factors for Medication Discrepancies. Am J Geriatr Pharmacother. 2010;8(2):115-126. PubMed
11. CDC - National Center for Health Statistics. Fast Stats: Inpatient Surgery. http://www.cdc.gov/nchs/fastats/inpatient-surgery.htm. Accessed on June 24, 2016.
12. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(24):e278-e333. PubMed
13. Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365(21):2002-2012. PubMed
14. Budnitz DS, Pollock DA, Weidenbach KN, Mendelsohn AB, Schroeder TJ, Annest JL. National surveillance of emergency department visits for outpatient adverse drug events. JAMA. 2006;296(15):1858-1866. PubMed
15. Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92(16):2643-2652. PubMed
16. Nolan T, Berwick DM. All-or-none measurement raises the bar on performance. JAMA. 2006;295(10):1168-1170. PubMed
17. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043-1049. PubMed
18. Gonzalez-Garcia L, Salmeron-Garcia A, Garcia-Lirola MA, Moya-Roldan S, Belda-Rustarazo S, Cabeza-Barrera J. Medication reconciliation at admission to surgical departments. J Eval Clin Pract. 2016;22(1):20-25. PubMed
19. Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1494-1503. PubMed
20. Devereaux PJ, Yang H, Yusuf S, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839-1847. PubMed
21. Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349-361. PubMed
22. London MJ, Hur K, Schwartz GG, Henderson WG. Association of perioperative beta-blockade with mortality and cardiovascular morbidity following major noncardiac surgery. JAMA. 2013;309(16):1704-1713. PubMed
23. Rosenman DJ, McDonald FS, Ebbert JO, Erwin PJ, LaBella M, Montori VM. Clinical consequences of withholding versus administering renin-angiotensin-aldosterone system antagonists in the preoperative period. J Hosp Med. 2008;3(4):319-325. PubMed
24. Andersson C, Merie C, Jorgensen M, et al. Association of beta-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing noncardiac surgery: a Danish nationwide cohort study. JAMA Intern Med. 2014;174(3):336-344. PubMed
25. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128(16):E240-E327. PubMed
26. Kwan JL, Lo L, Sampson M, Shojania KG. Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 Pt 2):397-403. PubMed
27. Tam VC, Knowles SR, Cornish PL, Fine N, Marchesano R, Etchells EE. Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. CMAJ. 2005;173(5):510-515. PubMed
28. Lindenauer PK, Pekow P, Wang K, Gutierrez B, Benjamin EM. Lipid-lowering therapy and in-hospital mortality following major noncardiac surgery. JAMA. 2004;291(17):2092-2099. PubMed
29. Raju MG, Pachika A, Punnam SR, et al. Statin Therapy in the Reduction of Cardiovascular Events in Patients Undergoing Intermediate-Risk Noncardiac, Nonvascular Surgery. Clin Cardiol. 2013;36(8):456-461. PubMed
1. The Joint Commission. National Patient Safety Goals. 2016; https://www.jointcommission.org/standards_information/npsgs.aspx. Accessed June 21, 2016.
2. Institute for Healthcare Improvement. Medication Reconciliation to Prevent Adverse Drug Events. 2016; http://www.ihi.org/topics/ADEsMedicationReconciliation/Pages/default.aspx. Accessed June 24, 2016.
3. Bell CM, Brener SS, Gunraj N, et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 2011;306(8):840-847. PubMed
4. Coleman EA, Smith JD, Raha D, Min SJ. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med. 2005;165(16):1842-1847. PubMed
5. Wong JD, Bajcar JM, Wong GG, et al. Medication reconciliation at hospital discharge: evaluating discrepancies. Ann Pharmacother. 2008;42(10):1373-1379. PubMed
6. Forster AJ, Clark HD, Menard A, et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345-349. PubMed
7. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. PubMed
8. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. Adverse drug events occurring following hospital discharge. JGIM. 2005;20(4):317-323. PubMed
9. Kwan Y, Fernandes OA, Nagge JJ, et al. Pharmacist medication assessments in a surgical preadmission clinic. Arch Intern Med. 2007;167(10):1034-1040. PubMed
10. Unroe KT, Pfeiffenberger T, Riegelhaupt S, Jastrzembski J, Lokhnygina Y, Colon-Emeric C. Inpatient Medication Reconciliation at Admission and Discharge: A Retrospective Cohort Study of Age and Other Risk Factors for Medication Discrepancies. Am J Geriatr Pharmacother. 2010;8(2):115-126. PubMed
11. CDC - National Center for Health Statistics. Fast Stats: Inpatient Surgery. http://www.cdc.gov/nchs/fastats/inpatient-surgery.htm. Accessed on June 24, 2016.
12. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(24):e278-e333. PubMed
13. Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365(21):2002-2012. PubMed
14. Budnitz DS, Pollock DA, Weidenbach KN, Mendelsohn AB, Schroeder TJ, Annest JL. National surveillance of emergency department visits for outpatient adverse drug events. JAMA. 2006;296(15):1858-1866. PubMed
15. Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92(16):2643-2652. PubMed
16. Nolan T, Berwick DM. All-or-none measurement raises the bar on performance. JAMA. 2006;295(10):1168-1170. PubMed
17. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043-1049. PubMed
18. Gonzalez-Garcia L, Salmeron-Garcia A, Garcia-Lirola MA, Moya-Roldan S, Belda-Rustarazo S, Cabeza-Barrera J. Medication reconciliation at admission to surgical departments. J Eval Clin Pract. 2016;22(1):20-25. PubMed
19. Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1494-1503. PubMed
20. Devereaux PJ, Yang H, Yusuf S, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839-1847. PubMed
21. Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349-361. PubMed
22. London MJ, Hur K, Schwartz GG, Henderson WG. Association of perioperative beta-blockade with mortality and cardiovascular morbidity following major noncardiac surgery. JAMA. 2013;309(16):1704-1713. PubMed
23. Rosenman DJ, McDonald FS, Ebbert JO, Erwin PJ, LaBella M, Montori VM. Clinical consequences of withholding versus administering renin-angiotensin-aldosterone system antagonists in the preoperative period. J Hosp Med. 2008;3(4):319-325. PubMed
24. Andersson C, Merie C, Jorgensen M, et al. Association of beta-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing noncardiac surgery: a Danish nationwide cohort study. JAMA Intern Med. 2014;174(3):336-344. PubMed
25. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128(16):E240-E327. PubMed
26. Kwan JL, Lo L, Sampson M, Shojania KG. Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 Pt 2):397-403. PubMed
27. Tam VC, Knowles SR, Cornish PL, Fine N, Marchesano R, Etchells EE. Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. CMAJ. 2005;173(5):510-515. PubMed
28. Lindenauer PK, Pekow P, Wang K, Gutierrez B, Benjamin EM. Lipid-lowering therapy and in-hospital mortality following major noncardiac surgery. JAMA. 2004;291(17):2092-2099. PubMed
29. Raju MG, Pachika A, Punnam SR, et al. Statin Therapy in the Reduction of Cardiovascular Events in Patients Undergoing Intermediate-Risk Noncardiac, Nonvascular Surgery. Clin Cardiol. 2013;36(8):456-461. PubMed
© 2017 Society of Hospital Medicine
Influenza Season Hospitalization Trends in Israel: A Multi-Year Comparative Analysis 2005/2006 Through 2012/2013
Influenza-associated morbidity poses a significant hospital burden.1 A study from the United States estimated that seasonal influenza is responsible for 3.1 million hospitalization days per year.2
Assessment of hospital burden during influenza seasons presents a challenge due to several possible factors, such as inaccurate recording of diagnosis3 and incomplete age group data. Although great emphasis has historically been placed on older age groups, a study from England and Wales showed that the number of hospitalizations and deaths resulting from influenza was significantly higher in children as compared with adults.4 Moreover, excess visits to emergency departments in New York City because of fever and respiratory morbidity during influenza seasons were found mostly among school-age children, whereas in adults, the surplus was small to nonexistent.5
Studies examining influenza-related hospitalizations evaluated numbers and rates of hospitalization.6-11 However, information regarding length of hospitalizations, hospitalizations during the influenza season that were not influenza related, or comparisons between influenza seasons and summer seasons is scarce. These determinants are of great importance for hospital preparedness towards influenza seasons. The aim of the current study was to estimate excess hospitalizations and length of hospitalization during influenza seasons, as compared with the summer, in different age groups and selected diagnoses in Israel.
METHODS
Data Sources
Hospitalization data of internal medicine and pediatric departments in 28 acute care hospitals in Israel between 2005 and 2013 were obtained from the National Hospital Discharges Database managed by the Health Information Division (HID) in the Israel Ministry of Health (MOH). The information included number of discharges (including in-hospital deaths), number of hospitalization days, and the mean length of stay (LOS) per discharge for all diagnoses and for primary or secondary diagnoses of respiratory/cardiovascular disease (ICD9 390-519) and influenza/pneumonia (ICD9 480-487).
Bed occupancy rates for internal medicine and pediatric departments were based on the National Patient Flow Database managed by the HID.
The 2009-2010 pandemic influenza season was excluded from analysis due to different morbidity patterns and timing (April 2009 until August 2010) as compared with seasonal influenza.
Data Classification
Hospitalizations data were analyzed for all ages, for specific age groups (the first year of life [0], ages 1-4, 5-14, 15-24, 25-34, 35-44, 45-54, 55-64, 65-74, 75-84, and 85 years and older), for all diagnoses, and for primary or secondary discharge diagnosis of respiratory/cardiovascular disease (ICD9 390-519) and influenza/pneumonia (ICD9 480-487).
Duration of Influenza Season
The beginning and the end of the influenza season were determined by the National Influenza surveillance program, which includes on average 22 community sentinel clinics, throughout Israel, each influenza season. These clinics send nose-throat samples from a convenience sample of patients with influenza-like illness (ILI), from week 40 of each year until the end of the influenza season in the subsequent year. These samples are analyzed for the presence of influenza virus by real-time reverse transcription polymerase chain reaction (RT-PCR) at the Central Virology Laboratory of Israel. Based on influenza virus detection in nose-throat samples from patients with ILI attending the community sentinel clinics, we determined the first and last month of each influenza season. The first month in which positive influenza samples were identified in sequence was defined as the first month of the season. The month in which the sequence of positive influenza samples stopped was defined as the last month of the season.
The 2009-2010 pandemic influenza season was excluded from analysis due to different morbidity patterns and timing (April 2009 until August 2010) as compared with seasonal influenza.
Data Analysis
Rates. Rates of monthly hospitalizations and monthly hospitalization days were calculated per 100,000 residents for all ages and for the specific age groups. Estimated average population sizes in different years for all ages and for specific age groups were obtained from the Central Bureau of Statistics (http://www.cbs.gov.il/reader/shnaton/templ_shnaton.html?num_tab=st02_01&CYear=2014). Monthly LOS was not converted to rates.
Hospitalizations. Mean monthly rate of hospitalizations during influenza and summer seasons was calculated by dividing the sum of hospital discharge rates during influenza/summer seasons of the entire evaluation period (2005/2006 to 2012/2013) by the number of influenza/summer activity months of that period.
Hospitalization Days. The measure “hospitalization days” refers to the hospitalization days of all patients who were discharged during influenza seasons. Mean monthly rate of hospitalization days during the influenza season and summer season was calculated using the procedure described for monthly mean rate of hospitalizations.
Length of Stay. The measure “length of stay” refers to the number of days that individual patients stayed in the hospital during an admission in the evaluated seasons.
Mean monthly LOS during the influenza and summer seasons for all patients (in both internal medicine and pediatric departments) and by age group was calculated by dividing the sum of monthly LOS during influenza seasons/summer season of the entire evaluation period (2005/2006 to 2012/2013 except for the 2009/2010 season) by the number of influenza/summer activity months of that period.
LOS for each specific month of the evaluation period for a single patient was calculated by dividing the number of hospitalization days of all patients that were discharged that month (stratified by age group) by the number of discharges in the same month.
Bed Occupancy. Bed occupancy rates for internal medicine and pediatric departments of the seasons evaluated were computed as a weighted rate based on the hospitalization days and licensed inpatient beds for the period of each influenza and summer season. The calculation took into account the number of days of each month and was based on the monthly reporting of hospital inpatient days in these departments and on the number of inpatient beds according to standard license documents issued by the MOH for each hospital.
Difference Between Influenza and Summer Seasons. Differences in mean monthly rates of hospitalizations, mean monthly rate of hospitalization days, and LOS during influenza seasons and the preceding summer were calculated as absolute numbers per month and as a percentage. The difference between bed occupancy during the influenza seasons and the preceding summers was expressed in percentage. Differences were computed for all diagnoses and for ICD9 480-487 and 390-519.
Statistical Analysis
Mean and standard deviation for monthly hospitalization rates, rates of monthly hospitalization days, and for LOS were calculated for all the influenza and summer seasons that were evaluated. Differences and statistical significance for these parameters were evaluated using a two-tailed Wilcoxon-Mann-Whitney test adjusted for ties, with 95% confidence interval for mean locations. The null hypothesis of the Wilcoxon test used was that the mean ranks of the influenza and summer season observations were equal.
Mean of bed occupancy percentage was calculated for influenza and summer seasons, with the difference and statistical significance being evaluated using a χ2 test. P value of < 0.05 was considered statistically significant. SAS Version 9.1 and R program version 3.3.1 software were used for analysis.
RESULTS
Influenza Seasons
The length of influenza seasons varied, with the shortest season lasting 3 months (2006-2007) and the longest season lasting six months (2010-2011 and 2011-2012; Table 1). Of the 14 first and last months of the 7 influenza seasons, 9 had influenza activity throughout the month, 2 had 3 weeks of influenza activity, and 3 had 2 weeks of influenza activity (Table 1).
Hospitalizations
A total of 452,209 hospital discharges occurred in pediatric and internal medicine departments during the influenza seasons that were evaluated. The mean monthly rate of hospitalizations (as defined in M
The mean monthly rate of hospitalizations for all ages due to the diagnosis of respiratory/cardiovascular diseases and influenza/pneumonia was 18.6% and 60.8% higher, respectively, during influenza seasons compared with the preceding summers (panels B and C in Figure). These differences were statistically significant (panels B and C in Figure; Supplementary Table 1).
The increase in mean monthly hospitalization rates for patients with a diagnosis of respiratory/cardiovascular diseases and pneumonia/influenza was highest among infants <1 year and children aged 1-4 years (panels B and C in Figure; Supplementary Table 1). Increases were also observed among other age groups. However, they were more modest and reached statistical significance for respiratory/cardiovascular diseases in the age groups of ≤34 years and ≥75 years (panel B in Figure; Supplementary Table 1). The increases in mean monthly hospitalization rates for pneumonia/influenza were statistically significant in all age groups and were greater than 40% among adults ≥55 years (panel C in Figure; Supplementary Table 1).
Statistically significant decreases in mean monthly hospitalization rates during influenza seasons were observed for all diagnoses in the 5-54 age groups (panel A in Figure; Supplementary Table 1). Decreases were not seen for the diagnoses of respiratory/cardiovascular diseases or pneumonia/influenza (panels B and C in Figure; Supplementary Table 1).
Hospitalization Days
The mean monthly rate of hospitalization days per 100,000 residents showed a similar trend to that of the hospitalization rates (panels A, B, and C in Figure; Supplementary Table 2), with the most prominent increases observed among infants and children <5 years and adults ≥65 years.
The mean monthly rate of hospitalization days per 100,000 during influenza seasons for all ages due to all diagnoses was 8% higher (P < 0.001) as compared with the summer seasons (panel A in Figure; Supplementary Table 2). Statistically significant increases were also found among patients diagnosed with respiratory/cardiovascular diseases and for influenza/pneumonia (panels B and C in Figure; Supplementary Table 2).
Children <5 years of age showed the largest increases during the influenza season as compared with the summer, with an up to 155.9% increase in the mean monthly rate of hospitalization days due to influenza/pneumonia (panel C in Figure; Supplementary Table 2), and an up to 206.6% increase for respiratory/cardiovascular diseases in infants <1 year of age (panel B in Figure; Supplementary Table 2). In adults, the largest increases were observed among those ≥75 years; the rates for influenza/pneumonia increased by about 40% (panel C in Figure; Supplementary Table 2), and the rates for respiratory/cardiovascular diseases increased by 14.8%-20.7% as compared with the summer months (panel B in Figure; Supplementary Table 2).
Statistically significant decreases in monthly mean rate of hospitalization days during influenza seasons were observed for all diagnoses in the 5-54 age groups (panel A in Figure; Supplementary Table 2). Decreases were not seen for the diagnoses of respiratory/cardiovascular diseases or influenza/pneumonia (panels B and C in Figure; Supplementary Table 2).
Hospital Length of Stay
The longest mean monthly LOS due to all diagnoses (for both influenza and summer seasons) was observed in adults ≥65 years of age (Table 2). The longest mean monthly LOS due to influenza/pneumonia (for both influenza and summer seasons) was observed in adults ≥55 years or older, and for the diagnosis of respiratory/cardiovascular diseases, infants <1 year and adults ≥55 years had the longest LOS.
The differences between influenza and summer seasons in mean monthly LOS were mostly small or not observed in any of the diagnostic categories examined. The mean monthly LOS due to a diagnosis of influenza/pneumonia was shorter during the influenza seasons than summer seasons in most age groups. These differences were statistically significant in children <5 years and adults ≥45 years (Table 2).
The mean LOS due to respiratory/cardiovascular diseases was significantly shorter during influenza seasons than summer seasons in children under 5.
Bed Occupancy
Mean bed occupancy was significantly higher during influenza seasons compared with the preceding summer seasons, both in internal medicine and pediatric departments (Table 3). The differences were higher in pediatric departments as compared with internal medicine departments for most years evaluated.
DISCUSSION
Our study demonstrates trends of excess hospitalizations during influenza as compared with summer seasons and identifies patient groups that contribute mostly to changes in hospital burden between these seasons.
Overall, the present study demonstrates differences between influenza and summer seasons for all measures tested: hospitalizations, hospitalization days, LOS, and bed occupancy. These differences were due primarily to excess number of hospitalizations and hospitalization days, rather than to longer LOS.
Our results concerning hospitalizations for all diagnoses are consistent with a United States report showing about 5% more hospitalizations following emergency department visits during winter compared with summer.12
The increase in hospitalizations and total hospitalization days in older age groups reflects the probability of severe diseases in a population with multiple comorbidities, and is consistent with a 90% influenza-related mortality due to respiratory and cardiovascular diseases reported in patients 65 and older.13 The increase in hospitalization and total hospitalization days in the age groups <5 years during influenza seasons are consistent with studies showing that the risk of children to contract influenza is higher than that of adults surrounding them. In this regard, outbreak investigations during the 2009 influenza pandemic showed that influenza attack rates in children were higher than those of adults.14
Nationwide studies from Singapore and Taiwan also showed more hospitalizations related to influenza in young children and older adults.15,16
The increase in hospitalization days for all patients should be interpreted while taking into account the mean monthly LOS per patient (Table 2). In most age groups, a small decrease in the mean LOS for individual patients with the diagnosis of influenza/pneumonia was observed (Table 2). This decrease may suggest a need to shorten hospitalization slightly in order to accommodate new patients. Similarly, the decrease in hospitalization rates from all diagnoses during influenza seasons in the 5-54 years age groups (Figure) may stem, at least in part, from the shortage of available hospital beds due to patient overload. Additional study is required to further explore these decreases and their possible effects on morbidity and mortality.
Influenza vaccine guidelines in Israel following the 2009 influenza pandemic recommend influenza vaccination for all individuals age 6 months and older. However, influenza vaccination in Israel has remained low. Specifically, vaccination rates among children below the age of 5 years have been approximately 21%, as compared with 60%-65% in adults 65 years and over.17 Given the low rate of vaccination in children, we believe that there would be minimal or no difference in hospitalization of children under the age of 5 years, between the pre- and postpandemic years. Israel has started a school-based influenza vaccination program for the 2016-2017 influenza season in an effort to increase childhood influenza vaccination. It would be important to see if the expansion and continuation of the program would have an effect on influenza season hospitalizations.
Our study has several advantages. To the best of our knowledge, it is the first study examining differences in hospital burden between influenza and summer seasons on a national level. As such, it constitutes one of the largest studies on the subject. In addition, our study relies on original data, rather than estimates. Analysis of specific months of each year in which influenza virus circulates provides a targeted analysis of influenza seasons, rather than the entire winter season. The comparison with summer months is of great importance for preparatory plans by health systems, as it takes into account the degree of variation between the seasons. The analysis of 6 influenza seasons in our study intended to take into account season-to-season disease variability. Such variability among influenza seasons has been described previously due to changes in the virus itself, the population immune status, and the weather.18
We used several diagnosis categories to evaluate different aspects of hospital burden. Although the category of “all diagnoses” provided a broad assessment of hospital burden, influenza/pneumonia or pulmonary/cardiovascular disease constituted a more specific measure of influenza-associated burden.
Evaluating LOS added to the accuracy of hospital burden estimates, and our age-group analysis highlighted the specific age groups responsible for changes in hospital burden. Thus, the use of several measures to assess influenza season morbidity provides a comprehensive picture of the hospitalization dynamics between influenza and summer seasons. In this regard, the trends observed in our study for hospitalizations and total hospitalization days correspond to those observed in bed occupancy, especially for hospitalization rates due to all causes.
Our study has several limitations. We did not rely on laboratory diagnosis of influenza to determine burden. Because obtaining specimens for viral detection is usually based on individual clinical judgement, and patients hospitalized with influenza-related complications can often test negative for the virus due to time elapsed from disease onset, relying on a laboratory-based analysis may lead to underestimation of hospital burden. On the other hand, it is possible that patients with morbidity not specifically related to influenza were included in our analysis. Respiratory syncytial virus (RSV), for example, can also cause respiratory illness during the fall and winter.19 However, in Israel, RSV epidemic usually occurs before the influenza epidemic.17,20 Thus, it is expected that only a small percentage of hospital admissions due to RSV would occur during the influenza season. Another limitation of our study relates to the small number of months in the beginning and end of influenza seasons in which influenza activity was recorded only during part of the month. Thus, hospital burden may have been underestimated during these “incomplete” months. Future studies using time series analysis methods will contribute to a more accurate estimation of such differences, as well as account for variability in influenza activity.
Our results clearly highlight the issues that challenge hospitals in Israel, and possibly other countries, during influenza seasons, such as the most affected age groups and the shortening of hospital stay. Thus, our findings are most relevant for hospital preparedness towards influenza seasons, particularly in terms of the need for additional hospital beds and personnel.
Acknowledgment
We would like to thank Anneke ifrah for English language editing
Disclosure
All authors report no conflict of interest relevant to this article. No financial support was provided relevant to this article.
1. Bromberg M, Kaufman Z, Mandelboim M, et al. [Clinical and virological surveillance of influenza in Israel--implementation during pandemic influenza]. Harefuah. 2009;148(9):577-582, 659. PubMed
2. Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25(27):5086-5096. Epub 2007/06/05. doi: 10.1016/j.vaccine.2007.03.046. PubMed
3. De Pascale G, Bittner EA. Influenza-associated critical illness: estimating the burden and the burden of estimation. Crit Care Med. 2014;42(11):2441-2442. Epub 2014/10/17. doi: 10.1097/ccm.0000000000000589. PubMed
4. Pitman RJ, Melegaro A, Gelb D, Siddiqui MR, Gay NJ, Edmunds WJ. Assessing the burden of influenza and other respiratory infections in England and Wales. J Infect. 2007;54(6):530-538. Epub 2006/11/14. doi: 10.1016/j.jinf.2006.09.017. PubMed
5. Olson DR, Heffernan RT, Paladini M, Konty K, Weiss D, Mostashari F. Monitoring the impact of influenza by age: emergency department fever and respiratory complaint surveillance in New York City. PLoS Med. 2007;4(8):e247. doi: 10.1371/journal.pmed.0040247. PubMed
6. Sheu SM, Tsai CF, Yang HY, Pai HW, Chen SC. Comparison of age-specific hospitalization during pandemic and seasonal influenza periods from 2009 to 2012 in Taiwan: a nationwide population-based study. BMC Infect Dis. 2016;16:88. doi: 10.1186/s12879-016-1438-x. PubMed
7. Goldstein E, Greene SK, Olson DR, Hanage WP, Lipsitch M. Estimating the hospitalization burden associated with influenza and respiratory syncytial virus in New York City, 2003-2011. Influenza Other Respir Viruses. 2015;9(5):225-233. doi: 10.1111/irv.12325. PubMed
8. Matias G, Taylor RJ, Haguinet F, Schuck-Paim C, Lustig RL, Fleming DM. Modelling estimates of age-specific influenza-related hospitalisation and mortality in the United Kingdom. BMC Public Health. 2016;16:481. doi: 10.1186/s12889-016-3128-4. PubMed
9. Reed C, Chaves SS, Daily Kirley P, et al. Estimating influenza disease burden from population-based surveillance data in the United States. PLoS One. 2015;10(3):e0118369. doi: 10.1371/journal.pone.0118369. PubMed
10. Hirve S, Krishnan A, Dawood FS, et al. Incidence of influenza-associated hospitalization in rural communities in western and northern India, 2010-2012: a multi-site population-based study. J Infect. 2015;70(2):160-170. doi: 10.1016/j.jinf.2014.08.015. PubMed
11. Chaves SS, Perez A, Farley MM, et al. The burden of influenza hospitalizations in infants from 2003 to 2012, United States. Pediatr Infect Dis J. 2014;33(9):912-919. doi: 10.1097/inf.0000000000000321. PubMed
12. Pitts SR, Niska RW, Xu J, Burt CW. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. Natl Health Stat Report. 2008;(7):1-38. PubMed
13. Linhart Y, Shohat T, Bromberg M, Mendelson E, Dictiar R, Green MS. Excess mortality from seasonal influenza is negligible below the age of 50 in Israel: implications for vaccine policy. Infection. 2011;39(5):399-404. doi: 10.1007/s15010-011-0153-1. PubMed
14. Glatman-Freedman A, Portelli I, Jacobs SK, et al. Attack rates assessment of the 2009 pandemic H1N1 influenza A in children and their contacts: a systematic review and meta-analysis. PLoS One. 2012;7(11):e50228. doi: 10.1371/journal.pone.0050228. PubMed
15. Ang LW, Lim C, Lee VJ, et al. Influenza-associated hospitalizations, Singapore, 2004-2008 and 2010-2012. Emerg Infect Dis. 2014;20(10). doi: 10.3201/eid2010.131768. PubMed
16. Sheu SM, Tsai CF, Yang HY, Pai HW, Chen SC. Comparison of age-specific hospitalization during pandemic and seasonal influenza periods from 2009 to 2012 in Taiwan: a nationwide population-based study. BMC Infect Dis. 2016;16(1):88. doi: 10.1186/s12879-016-1438-x. PubMed
17. Israel Center for Disease Control. Summary Report - The 2015/2016 Influenza Season. https://www.health.gov.il/PublicationsFiles/flu2015-2016e.pdf. Accessed July 18, 2017.
18. Yaari R, Katriel G, Huppert A, Axelsen JB, Stone L. Modelling seasonal influenza: the role of weather and punctuated antigenic drift. J R Soc Interface. 2013;10(84):20130298. doi: 10.1098/rsif.2013.0298. PubMed
19. Hirsh S, Hindiyeh M, Kolet L, et al. Epidemiological changes of respiratory syncytial virus (RSV) infections in Israel. PLoS One. 2014;9(3):e90515. doi: 10.1371/journal.pone.0090515. PubMed
20. Miguez A, Iftimi A, Montes F. Temporal association between the influenza virus and respiratory syncytial virus (RSV): RSV as a predictor of seasonal influenza. Epidemiol Infect. 2016;144(12):2621-32. doi: 10.1017/s095026881600090x. PubMed
Influenza-associated morbidity poses a significant hospital burden.1 A study from the United States estimated that seasonal influenza is responsible for 3.1 million hospitalization days per year.2
Assessment of hospital burden during influenza seasons presents a challenge due to several possible factors, such as inaccurate recording of diagnosis3 and incomplete age group data. Although great emphasis has historically been placed on older age groups, a study from England and Wales showed that the number of hospitalizations and deaths resulting from influenza was significantly higher in children as compared with adults.4 Moreover, excess visits to emergency departments in New York City because of fever and respiratory morbidity during influenza seasons were found mostly among school-age children, whereas in adults, the surplus was small to nonexistent.5
Studies examining influenza-related hospitalizations evaluated numbers and rates of hospitalization.6-11 However, information regarding length of hospitalizations, hospitalizations during the influenza season that were not influenza related, or comparisons between influenza seasons and summer seasons is scarce. These determinants are of great importance for hospital preparedness towards influenza seasons. The aim of the current study was to estimate excess hospitalizations and length of hospitalization during influenza seasons, as compared with the summer, in different age groups and selected diagnoses in Israel.
METHODS
Data Sources
Hospitalization data of internal medicine and pediatric departments in 28 acute care hospitals in Israel between 2005 and 2013 were obtained from the National Hospital Discharges Database managed by the Health Information Division (HID) in the Israel Ministry of Health (MOH). The information included number of discharges (including in-hospital deaths), number of hospitalization days, and the mean length of stay (LOS) per discharge for all diagnoses and for primary or secondary diagnoses of respiratory/cardiovascular disease (ICD9 390-519) and influenza/pneumonia (ICD9 480-487).
Bed occupancy rates for internal medicine and pediatric departments were based on the National Patient Flow Database managed by the HID.
The 2009-2010 pandemic influenza season was excluded from analysis due to different morbidity patterns and timing (April 2009 until August 2010) as compared with seasonal influenza.
Data Classification
Hospitalizations data were analyzed for all ages, for specific age groups (the first year of life [0], ages 1-4, 5-14, 15-24, 25-34, 35-44, 45-54, 55-64, 65-74, 75-84, and 85 years and older), for all diagnoses, and for primary or secondary discharge diagnosis of respiratory/cardiovascular disease (ICD9 390-519) and influenza/pneumonia (ICD9 480-487).
Duration of Influenza Season
The beginning and the end of the influenza season were determined by the National Influenza surveillance program, which includes on average 22 community sentinel clinics, throughout Israel, each influenza season. These clinics send nose-throat samples from a convenience sample of patients with influenza-like illness (ILI), from week 40 of each year until the end of the influenza season in the subsequent year. These samples are analyzed for the presence of influenza virus by real-time reverse transcription polymerase chain reaction (RT-PCR) at the Central Virology Laboratory of Israel. Based on influenza virus detection in nose-throat samples from patients with ILI attending the community sentinel clinics, we determined the first and last month of each influenza season. The first month in which positive influenza samples were identified in sequence was defined as the first month of the season. The month in which the sequence of positive influenza samples stopped was defined as the last month of the season.
The 2009-2010 pandemic influenza season was excluded from analysis due to different morbidity patterns and timing (April 2009 until August 2010) as compared with seasonal influenza.
Data Analysis
Rates. Rates of monthly hospitalizations and monthly hospitalization days were calculated per 100,000 residents for all ages and for the specific age groups. Estimated average population sizes in different years for all ages and for specific age groups were obtained from the Central Bureau of Statistics (http://www.cbs.gov.il/reader/shnaton/templ_shnaton.html?num_tab=st02_01&CYear=2014). Monthly LOS was not converted to rates.
Hospitalizations. Mean monthly rate of hospitalizations during influenza and summer seasons was calculated by dividing the sum of hospital discharge rates during influenza/summer seasons of the entire evaluation period (2005/2006 to 2012/2013) by the number of influenza/summer activity months of that period.
Hospitalization Days. The measure “hospitalization days” refers to the hospitalization days of all patients who were discharged during influenza seasons. Mean monthly rate of hospitalization days during the influenza season and summer season was calculated using the procedure described for monthly mean rate of hospitalizations.
Length of Stay. The measure “length of stay” refers to the number of days that individual patients stayed in the hospital during an admission in the evaluated seasons.
Mean monthly LOS during the influenza and summer seasons for all patients (in both internal medicine and pediatric departments) and by age group was calculated by dividing the sum of monthly LOS during influenza seasons/summer season of the entire evaluation period (2005/2006 to 2012/2013 except for the 2009/2010 season) by the number of influenza/summer activity months of that period.
LOS for each specific month of the evaluation period for a single patient was calculated by dividing the number of hospitalization days of all patients that were discharged that month (stratified by age group) by the number of discharges in the same month.
Bed Occupancy. Bed occupancy rates for internal medicine and pediatric departments of the seasons evaluated were computed as a weighted rate based on the hospitalization days and licensed inpatient beds for the period of each influenza and summer season. The calculation took into account the number of days of each month and was based on the monthly reporting of hospital inpatient days in these departments and on the number of inpatient beds according to standard license documents issued by the MOH for each hospital.
Difference Between Influenza and Summer Seasons. Differences in mean monthly rates of hospitalizations, mean monthly rate of hospitalization days, and LOS during influenza seasons and the preceding summer were calculated as absolute numbers per month and as a percentage. The difference between bed occupancy during the influenza seasons and the preceding summers was expressed in percentage. Differences were computed for all diagnoses and for ICD9 480-487 and 390-519.
Statistical Analysis
Mean and standard deviation for monthly hospitalization rates, rates of monthly hospitalization days, and for LOS were calculated for all the influenza and summer seasons that were evaluated. Differences and statistical significance for these parameters were evaluated using a two-tailed Wilcoxon-Mann-Whitney test adjusted for ties, with 95% confidence interval for mean locations. The null hypothesis of the Wilcoxon test used was that the mean ranks of the influenza and summer season observations were equal.
Mean of bed occupancy percentage was calculated for influenza and summer seasons, with the difference and statistical significance being evaluated using a χ2 test. P value of < 0.05 was considered statistically significant. SAS Version 9.1 and R program version 3.3.1 software were used for analysis.
RESULTS
Influenza Seasons
The length of influenza seasons varied, with the shortest season lasting 3 months (2006-2007) and the longest season lasting six months (2010-2011 and 2011-2012; Table 1). Of the 14 first and last months of the 7 influenza seasons, 9 had influenza activity throughout the month, 2 had 3 weeks of influenza activity, and 3 had 2 weeks of influenza activity (Table 1).
Hospitalizations
A total of 452,209 hospital discharges occurred in pediatric and internal medicine departments during the influenza seasons that were evaluated. The mean monthly rate of hospitalizations (as defined in M
The mean monthly rate of hospitalizations for all ages due to the diagnosis of respiratory/cardiovascular diseases and influenza/pneumonia was 18.6% and 60.8% higher, respectively, during influenza seasons compared with the preceding summers (panels B and C in Figure). These differences were statistically significant (panels B and C in Figure; Supplementary Table 1).
The increase in mean monthly hospitalization rates for patients with a diagnosis of respiratory/cardiovascular diseases and pneumonia/influenza was highest among infants <1 year and children aged 1-4 years (panels B and C in Figure; Supplementary Table 1). Increases were also observed among other age groups. However, they were more modest and reached statistical significance for respiratory/cardiovascular diseases in the age groups of ≤34 years and ≥75 years (panel B in Figure; Supplementary Table 1). The increases in mean monthly hospitalization rates for pneumonia/influenza were statistically significant in all age groups and were greater than 40% among adults ≥55 years (panel C in Figure; Supplementary Table 1).
Statistically significant decreases in mean monthly hospitalization rates during influenza seasons were observed for all diagnoses in the 5-54 age groups (panel A in Figure; Supplementary Table 1). Decreases were not seen for the diagnoses of respiratory/cardiovascular diseases or pneumonia/influenza (panels B and C in Figure; Supplementary Table 1).
Hospitalization Days
The mean monthly rate of hospitalization days per 100,000 residents showed a similar trend to that of the hospitalization rates (panels A, B, and C in Figure; Supplementary Table 2), with the most prominent increases observed among infants and children <5 years and adults ≥65 years.
The mean monthly rate of hospitalization days per 100,000 during influenza seasons for all ages due to all diagnoses was 8% higher (P < 0.001) as compared with the summer seasons (panel A in Figure; Supplementary Table 2). Statistically significant increases were also found among patients diagnosed with respiratory/cardiovascular diseases and for influenza/pneumonia (panels B and C in Figure; Supplementary Table 2).
Children <5 years of age showed the largest increases during the influenza season as compared with the summer, with an up to 155.9% increase in the mean monthly rate of hospitalization days due to influenza/pneumonia (panel C in Figure; Supplementary Table 2), and an up to 206.6% increase for respiratory/cardiovascular diseases in infants <1 year of age (panel B in Figure; Supplementary Table 2). In adults, the largest increases were observed among those ≥75 years; the rates for influenza/pneumonia increased by about 40% (panel C in Figure; Supplementary Table 2), and the rates for respiratory/cardiovascular diseases increased by 14.8%-20.7% as compared with the summer months (panel B in Figure; Supplementary Table 2).
Statistically significant decreases in monthly mean rate of hospitalization days during influenza seasons were observed for all diagnoses in the 5-54 age groups (panel A in Figure; Supplementary Table 2). Decreases were not seen for the diagnoses of respiratory/cardiovascular diseases or influenza/pneumonia (panels B and C in Figure; Supplementary Table 2).
Hospital Length of Stay
The longest mean monthly LOS due to all diagnoses (for both influenza and summer seasons) was observed in adults ≥65 years of age (Table 2). The longest mean monthly LOS due to influenza/pneumonia (for both influenza and summer seasons) was observed in adults ≥55 years or older, and for the diagnosis of respiratory/cardiovascular diseases, infants <1 year and adults ≥55 years had the longest LOS.
The differences between influenza and summer seasons in mean monthly LOS were mostly small or not observed in any of the diagnostic categories examined. The mean monthly LOS due to a diagnosis of influenza/pneumonia was shorter during the influenza seasons than summer seasons in most age groups. These differences were statistically significant in children <5 years and adults ≥45 years (Table 2).
The mean LOS due to respiratory/cardiovascular diseases was significantly shorter during influenza seasons than summer seasons in children under 5.
Bed Occupancy
Mean bed occupancy was significantly higher during influenza seasons compared with the preceding summer seasons, both in internal medicine and pediatric departments (Table 3). The differences were higher in pediatric departments as compared with internal medicine departments for most years evaluated.
DISCUSSION
Our study demonstrates trends of excess hospitalizations during influenza as compared with summer seasons and identifies patient groups that contribute mostly to changes in hospital burden between these seasons.
Overall, the present study demonstrates differences between influenza and summer seasons for all measures tested: hospitalizations, hospitalization days, LOS, and bed occupancy. These differences were due primarily to excess number of hospitalizations and hospitalization days, rather than to longer LOS.
Our results concerning hospitalizations for all diagnoses are consistent with a United States report showing about 5% more hospitalizations following emergency department visits during winter compared with summer.12
The increase in hospitalizations and total hospitalization days in older age groups reflects the probability of severe diseases in a population with multiple comorbidities, and is consistent with a 90% influenza-related mortality due to respiratory and cardiovascular diseases reported in patients 65 and older.13 The increase in hospitalization and total hospitalization days in the age groups <5 years during influenza seasons are consistent with studies showing that the risk of children to contract influenza is higher than that of adults surrounding them. In this regard, outbreak investigations during the 2009 influenza pandemic showed that influenza attack rates in children were higher than those of adults.14
Nationwide studies from Singapore and Taiwan also showed more hospitalizations related to influenza in young children and older adults.15,16
The increase in hospitalization days for all patients should be interpreted while taking into account the mean monthly LOS per patient (Table 2). In most age groups, a small decrease in the mean LOS for individual patients with the diagnosis of influenza/pneumonia was observed (Table 2). This decrease may suggest a need to shorten hospitalization slightly in order to accommodate new patients. Similarly, the decrease in hospitalization rates from all diagnoses during influenza seasons in the 5-54 years age groups (Figure) may stem, at least in part, from the shortage of available hospital beds due to patient overload. Additional study is required to further explore these decreases and their possible effects on morbidity and mortality.
Influenza vaccine guidelines in Israel following the 2009 influenza pandemic recommend influenza vaccination for all individuals age 6 months and older. However, influenza vaccination in Israel has remained low. Specifically, vaccination rates among children below the age of 5 years have been approximately 21%, as compared with 60%-65% in adults 65 years and over.17 Given the low rate of vaccination in children, we believe that there would be minimal or no difference in hospitalization of children under the age of 5 years, between the pre- and postpandemic years. Israel has started a school-based influenza vaccination program for the 2016-2017 influenza season in an effort to increase childhood influenza vaccination. It would be important to see if the expansion and continuation of the program would have an effect on influenza season hospitalizations.
Our study has several advantages. To the best of our knowledge, it is the first study examining differences in hospital burden between influenza and summer seasons on a national level. As such, it constitutes one of the largest studies on the subject. In addition, our study relies on original data, rather than estimates. Analysis of specific months of each year in which influenza virus circulates provides a targeted analysis of influenza seasons, rather than the entire winter season. The comparison with summer months is of great importance for preparatory plans by health systems, as it takes into account the degree of variation between the seasons. The analysis of 6 influenza seasons in our study intended to take into account season-to-season disease variability. Such variability among influenza seasons has been described previously due to changes in the virus itself, the population immune status, and the weather.18
We used several diagnosis categories to evaluate different aspects of hospital burden. Although the category of “all diagnoses” provided a broad assessment of hospital burden, influenza/pneumonia or pulmonary/cardiovascular disease constituted a more specific measure of influenza-associated burden.
Evaluating LOS added to the accuracy of hospital burden estimates, and our age-group analysis highlighted the specific age groups responsible for changes in hospital burden. Thus, the use of several measures to assess influenza season morbidity provides a comprehensive picture of the hospitalization dynamics between influenza and summer seasons. In this regard, the trends observed in our study for hospitalizations and total hospitalization days correspond to those observed in bed occupancy, especially for hospitalization rates due to all causes.
Our study has several limitations. We did not rely on laboratory diagnosis of influenza to determine burden. Because obtaining specimens for viral detection is usually based on individual clinical judgement, and patients hospitalized with influenza-related complications can often test negative for the virus due to time elapsed from disease onset, relying on a laboratory-based analysis may lead to underestimation of hospital burden. On the other hand, it is possible that patients with morbidity not specifically related to influenza were included in our analysis. Respiratory syncytial virus (RSV), for example, can also cause respiratory illness during the fall and winter.19 However, in Israel, RSV epidemic usually occurs before the influenza epidemic.17,20 Thus, it is expected that only a small percentage of hospital admissions due to RSV would occur during the influenza season. Another limitation of our study relates to the small number of months in the beginning and end of influenza seasons in which influenza activity was recorded only during part of the month. Thus, hospital burden may have been underestimated during these “incomplete” months. Future studies using time series analysis methods will contribute to a more accurate estimation of such differences, as well as account for variability in influenza activity.
Our results clearly highlight the issues that challenge hospitals in Israel, and possibly other countries, during influenza seasons, such as the most affected age groups and the shortening of hospital stay. Thus, our findings are most relevant for hospital preparedness towards influenza seasons, particularly in terms of the need for additional hospital beds and personnel.
Acknowledgment
We would like to thank Anneke ifrah for English language editing
Disclosure
All authors report no conflict of interest relevant to this article. No financial support was provided relevant to this article.
Influenza-associated morbidity poses a significant hospital burden.1 A study from the United States estimated that seasonal influenza is responsible for 3.1 million hospitalization days per year.2
Assessment of hospital burden during influenza seasons presents a challenge due to several possible factors, such as inaccurate recording of diagnosis3 and incomplete age group data. Although great emphasis has historically been placed on older age groups, a study from England and Wales showed that the number of hospitalizations and deaths resulting from influenza was significantly higher in children as compared with adults.4 Moreover, excess visits to emergency departments in New York City because of fever and respiratory morbidity during influenza seasons were found mostly among school-age children, whereas in adults, the surplus was small to nonexistent.5
Studies examining influenza-related hospitalizations evaluated numbers and rates of hospitalization.6-11 However, information regarding length of hospitalizations, hospitalizations during the influenza season that were not influenza related, or comparisons between influenza seasons and summer seasons is scarce. These determinants are of great importance for hospital preparedness towards influenza seasons. The aim of the current study was to estimate excess hospitalizations and length of hospitalization during influenza seasons, as compared with the summer, in different age groups and selected diagnoses in Israel.
METHODS
Data Sources
Hospitalization data of internal medicine and pediatric departments in 28 acute care hospitals in Israel between 2005 and 2013 were obtained from the National Hospital Discharges Database managed by the Health Information Division (HID) in the Israel Ministry of Health (MOH). The information included number of discharges (including in-hospital deaths), number of hospitalization days, and the mean length of stay (LOS) per discharge for all diagnoses and for primary or secondary diagnoses of respiratory/cardiovascular disease (ICD9 390-519) and influenza/pneumonia (ICD9 480-487).
Bed occupancy rates for internal medicine and pediatric departments were based on the National Patient Flow Database managed by the HID.
The 2009-2010 pandemic influenza season was excluded from analysis due to different morbidity patterns and timing (April 2009 until August 2010) as compared with seasonal influenza.
Data Classification
Hospitalizations data were analyzed for all ages, for specific age groups (the first year of life [0], ages 1-4, 5-14, 15-24, 25-34, 35-44, 45-54, 55-64, 65-74, 75-84, and 85 years and older), for all diagnoses, and for primary or secondary discharge diagnosis of respiratory/cardiovascular disease (ICD9 390-519) and influenza/pneumonia (ICD9 480-487).
Duration of Influenza Season
The beginning and the end of the influenza season were determined by the National Influenza surveillance program, which includes on average 22 community sentinel clinics, throughout Israel, each influenza season. These clinics send nose-throat samples from a convenience sample of patients with influenza-like illness (ILI), from week 40 of each year until the end of the influenza season in the subsequent year. These samples are analyzed for the presence of influenza virus by real-time reverse transcription polymerase chain reaction (RT-PCR) at the Central Virology Laboratory of Israel. Based on influenza virus detection in nose-throat samples from patients with ILI attending the community sentinel clinics, we determined the first and last month of each influenza season. The first month in which positive influenza samples were identified in sequence was defined as the first month of the season. The month in which the sequence of positive influenza samples stopped was defined as the last month of the season.
The 2009-2010 pandemic influenza season was excluded from analysis due to different morbidity patterns and timing (April 2009 until August 2010) as compared with seasonal influenza.
Data Analysis
Rates. Rates of monthly hospitalizations and monthly hospitalization days were calculated per 100,000 residents for all ages and for the specific age groups. Estimated average population sizes in different years for all ages and for specific age groups were obtained from the Central Bureau of Statistics (http://www.cbs.gov.il/reader/shnaton/templ_shnaton.html?num_tab=st02_01&CYear=2014). Monthly LOS was not converted to rates.
Hospitalizations. Mean monthly rate of hospitalizations during influenza and summer seasons was calculated by dividing the sum of hospital discharge rates during influenza/summer seasons of the entire evaluation period (2005/2006 to 2012/2013) by the number of influenza/summer activity months of that period.
Hospitalization Days. The measure “hospitalization days” refers to the hospitalization days of all patients who were discharged during influenza seasons. Mean monthly rate of hospitalization days during the influenza season and summer season was calculated using the procedure described for monthly mean rate of hospitalizations.
Length of Stay. The measure “length of stay” refers to the number of days that individual patients stayed in the hospital during an admission in the evaluated seasons.
Mean monthly LOS during the influenza and summer seasons for all patients (in both internal medicine and pediatric departments) and by age group was calculated by dividing the sum of monthly LOS during influenza seasons/summer season of the entire evaluation period (2005/2006 to 2012/2013 except for the 2009/2010 season) by the number of influenza/summer activity months of that period.
LOS for each specific month of the evaluation period for a single patient was calculated by dividing the number of hospitalization days of all patients that were discharged that month (stratified by age group) by the number of discharges in the same month.
Bed Occupancy. Bed occupancy rates for internal medicine and pediatric departments of the seasons evaluated were computed as a weighted rate based on the hospitalization days and licensed inpatient beds for the period of each influenza and summer season. The calculation took into account the number of days of each month and was based on the monthly reporting of hospital inpatient days in these departments and on the number of inpatient beds according to standard license documents issued by the MOH for each hospital.
Difference Between Influenza and Summer Seasons. Differences in mean monthly rates of hospitalizations, mean monthly rate of hospitalization days, and LOS during influenza seasons and the preceding summer were calculated as absolute numbers per month and as a percentage. The difference between bed occupancy during the influenza seasons and the preceding summers was expressed in percentage. Differences were computed for all diagnoses and for ICD9 480-487 and 390-519.
Statistical Analysis
Mean and standard deviation for monthly hospitalization rates, rates of monthly hospitalization days, and for LOS were calculated for all the influenza and summer seasons that were evaluated. Differences and statistical significance for these parameters were evaluated using a two-tailed Wilcoxon-Mann-Whitney test adjusted for ties, with 95% confidence interval for mean locations. The null hypothesis of the Wilcoxon test used was that the mean ranks of the influenza and summer season observations were equal.
Mean of bed occupancy percentage was calculated for influenza and summer seasons, with the difference and statistical significance being evaluated using a χ2 test. P value of < 0.05 was considered statistically significant. SAS Version 9.1 and R program version 3.3.1 software were used for analysis.
RESULTS
Influenza Seasons
The length of influenza seasons varied, with the shortest season lasting 3 months (2006-2007) and the longest season lasting six months (2010-2011 and 2011-2012; Table 1). Of the 14 first and last months of the 7 influenza seasons, 9 had influenza activity throughout the month, 2 had 3 weeks of influenza activity, and 3 had 2 weeks of influenza activity (Table 1).
Hospitalizations
A total of 452,209 hospital discharges occurred in pediatric and internal medicine departments during the influenza seasons that were evaluated. The mean monthly rate of hospitalizations (as defined in M
The mean monthly rate of hospitalizations for all ages due to the diagnosis of respiratory/cardiovascular diseases and influenza/pneumonia was 18.6% and 60.8% higher, respectively, during influenza seasons compared with the preceding summers (panels B and C in Figure). These differences were statistically significant (panels B and C in Figure; Supplementary Table 1).
The increase in mean monthly hospitalization rates for patients with a diagnosis of respiratory/cardiovascular diseases and pneumonia/influenza was highest among infants <1 year and children aged 1-4 years (panels B and C in Figure; Supplementary Table 1). Increases were also observed among other age groups. However, they were more modest and reached statistical significance for respiratory/cardiovascular diseases in the age groups of ≤34 years and ≥75 years (panel B in Figure; Supplementary Table 1). The increases in mean monthly hospitalization rates for pneumonia/influenza were statistically significant in all age groups and were greater than 40% among adults ≥55 years (panel C in Figure; Supplementary Table 1).
Statistically significant decreases in mean monthly hospitalization rates during influenza seasons were observed for all diagnoses in the 5-54 age groups (panel A in Figure; Supplementary Table 1). Decreases were not seen for the diagnoses of respiratory/cardiovascular diseases or pneumonia/influenza (panels B and C in Figure; Supplementary Table 1).
Hospitalization Days
The mean monthly rate of hospitalization days per 100,000 residents showed a similar trend to that of the hospitalization rates (panels A, B, and C in Figure; Supplementary Table 2), with the most prominent increases observed among infants and children <5 years and adults ≥65 years.
The mean monthly rate of hospitalization days per 100,000 during influenza seasons for all ages due to all diagnoses was 8% higher (P < 0.001) as compared with the summer seasons (panel A in Figure; Supplementary Table 2). Statistically significant increases were also found among patients diagnosed with respiratory/cardiovascular diseases and for influenza/pneumonia (panels B and C in Figure; Supplementary Table 2).
Children <5 years of age showed the largest increases during the influenza season as compared with the summer, with an up to 155.9% increase in the mean monthly rate of hospitalization days due to influenza/pneumonia (panel C in Figure; Supplementary Table 2), and an up to 206.6% increase for respiratory/cardiovascular diseases in infants <1 year of age (panel B in Figure; Supplementary Table 2). In adults, the largest increases were observed among those ≥75 years; the rates for influenza/pneumonia increased by about 40% (panel C in Figure; Supplementary Table 2), and the rates for respiratory/cardiovascular diseases increased by 14.8%-20.7% as compared with the summer months (panel B in Figure; Supplementary Table 2).
Statistically significant decreases in monthly mean rate of hospitalization days during influenza seasons were observed for all diagnoses in the 5-54 age groups (panel A in Figure; Supplementary Table 2). Decreases were not seen for the diagnoses of respiratory/cardiovascular diseases or influenza/pneumonia (panels B and C in Figure; Supplementary Table 2).
Hospital Length of Stay
The longest mean monthly LOS due to all diagnoses (for both influenza and summer seasons) was observed in adults ≥65 years of age (Table 2). The longest mean monthly LOS due to influenza/pneumonia (for both influenza and summer seasons) was observed in adults ≥55 years or older, and for the diagnosis of respiratory/cardiovascular diseases, infants <1 year and adults ≥55 years had the longest LOS.
The differences between influenza and summer seasons in mean monthly LOS were mostly small or not observed in any of the diagnostic categories examined. The mean monthly LOS due to a diagnosis of influenza/pneumonia was shorter during the influenza seasons than summer seasons in most age groups. These differences were statistically significant in children <5 years and adults ≥45 years (Table 2).
The mean LOS due to respiratory/cardiovascular diseases was significantly shorter during influenza seasons than summer seasons in children under 5.
Bed Occupancy
Mean bed occupancy was significantly higher during influenza seasons compared with the preceding summer seasons, both in internal medicine and pediatric departments (Table 3). The differences were higher in pediatric departments as compared with internal medicine departments for most years evaluated.
DISCUSSION
Our study demonstrates trends of excess hospitalizations during influenza as compared with summer seasons and identifies patient groups that contribute mostly to changes in hospital burden between these seasons.
Overall, the present study demonstrates differences between influenza and summer seasons for all measures tested: hospitalizations, hospitalization days, LOS, and bed occupancy. These differences were due primarily to excess number of hospitalizations and hospitalization days, rather than to longer LOS.
Our results concerning hospitalizations for all diagnoses are consistent with a United States report showing about 5% more hospitalizations following emergency department visits during winter compared with summer.12
The increase in hospitalizations and total hospitalization days in older age groups reflects the probability of severe diseases in a population with multiple comorbidities, and is consistent with a 90% influenza-related mortality due to respiratory and cardiovascular diseases reported in patients 65 and older.13 The increase in hospitalization and total hospitalization days in the age groups <5 years during influenza seasons are consistent with studies showing that the risk of children to contract influenza is higher than that of adults surrounding them. In this regard, outbreak investigations during the 2009 influenza pandemic showed that influenza attack rates in children were higher than those of adults.14
Nationwide studies from Singapore and Taiwan also showed more hospitalizations related to influenza in young children and older adults.15,16
The increase in hospitalization days for all patients should be interpreted while taking into account the mean monthly LOS per patient (Table 2). In most age groups, a small decrease in the mean LOS for individual patients with the diagnosis of influenza/pneumonia was observed (Table 2). This decrease may suggest a need to shorten hospitalization slightly in order to accommodate new patients. Similarly, the decrease in hospitalization rates from all diagnoses during influenza seasons in the 5-54 years age groups (Figure) may stem, at least in part, from the shortage of available hospital beds due to patient overload. Additional study is required to further explore these decreases and their possible effects on morbidity and mortality.
Influenza vaccine guidelines in Israel following the 2009 influenza pandemic recommend influenza vaccination for all individuals age 6 months and older. However, influenza vaccination in Israel has remained low. Specifically, vaccination rates among children below the age of 5 years have been approximately 21%, as compared with 60%-65% in adults 65 years and over.17 Given the low rate of vaccination in children, we believe that there would be minimal or no difference in hospitalization of children under the age of 5 years, between the pre- and postpandemic years. Israel has started a school-based influenza vaccination program for the 2016-2017 influenza season in an effort to increase childhood influenza vaccination. It would be important to see if the expansion and continuation of the program would have an effect on influenza season hospitalizations.
Our study has several advantages. To the best of our knowledge, it is the first study examining differences in hospital burden between influenza and summer seasons on a national level. As such, it constitutes one of the largest studies on the subject. In addition, our study relies on original data, rather than estimates. Analysis of specific months of each year in which influenza virus circulates provides a targeted analysis of influenza seasons, rather than the entire winter season. The comparison with summer months is of great importance for preparatory plans by health systems, as it takes into account the degree of variation between the seasons. The analysis of 6 influenza seasons in our study intended to take into account season-to-season disease variability. Such variability among influenza seasons has been described previously due to changes in the virus itself, the population immune status, and the weather.18
We used several diagnosis categories to evaluate different aspects of hospital burden. Although the category of “all diagnoses” provided a broad assessment of hospital burden, influenza/pneumonia or pulmonary/cardiovascular disease constituted a more specific measure of influenza-associated burden.
Evaluating LOS added to the accuracy of hospital burden estimates, and our age-group analysis highlighted the specific age groups responsible for changes in hospital burden. Thus, the use of several measures to assess influenza season morbidity provides a comprehensive picture of the hospitalization dynamics between influenza and summer seasons. In this regard, the trends observed in our study for hospitalizations and total hospitalization days correspond to those observed in bed occupancy, especially for hospitalization rates due to all causes.
Our study has several limitations. We did not rely on laboratory diagnosis of influenza to determine burden. Because obtaining specimens for viral detection is usually based on individual clinical judgement, and patients hospitalized with influenza-related complications can often test negative for the virus due to time elapsed from disease onset, relying on a laboratory-based analysis may lead to underestimation of hospital burden. On the other hand, it is possible that patients with morbidity not specifically related to influenza were included in our analysis. Respiratory syncytial virus (RSV), for example, can also cause respiratory illness during the fall and winter.19 However, in Israel, RSV epidemic usually occurs before the influenza epidemic.17,20 Thus, it is expected that only a small percentage of hospital admissions due to RSV would occur during the influenza season. Another limitation of our study relates to the small number of months in the beginning and end of influenza seasons in which influenza activity was recorded only during part of the month. Thus, hospital burden may have been underestimated during these “incomplete” months. Future studies using time series analysis methods will contribute to a more accurate estimation of such differences, as well as account for variability in influenza activity.
Our results clearly highlight the issues that challenge hospitals in Israel, and possibly other countries, during influenza seasons, such as the most affected age groups and the shortening of hospital stay. Thus, our findings are most relevant for hospital preparedness towards influenza seasons, particularly in terms of the need for additional hospital beds and personnel.
Acknowledgment
We would like to thank Anneke ifrah for English language editing
Disclosure
All authors report no conflict of interest relevant to this article. No financial support was provided relevant to this article.
1. Bromberg M, Kaufman Z, Mandelboim M, et al. [Clinical and virological surveillance of influenza in Israel--implementation during pandemic influenza]. Harefuah. 2009;148(9):577-582, 659. PubMed
2. Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25(27):5086-5096. Epub 2007/06/05. doi: 10.1016/j.vaccine.2007.03.046. PubMed
3. De Pascale G, Bittner EA. Influenza-associated critical illness: estimating the burden and the burden of estimation. Crit Care Med. 2014;42(11):2441-2442. Epub 2014/10/17. doi: 10.1097/ccm.0000000000000589. PubMed
4. Pitman RJ, Melegaro A, Gelb D, Siddiqui MR, Gay NJ, Edmunds WJ. Assessing the burden of influenza and other respiratory infections in England and Wales. J Infect. 2007;54(6):530-538. Epub 2006/11/14. doi: 10.1016/j.jinf.2006.09.017. PubMed
5. Olson DR, Heffernan RT, Paladini M, Konty K, Weiss D, Mostashari F. Monitoring the impact of influenza by age: emergency department fever and respiratory complaint surveillance in New York City. PLoS Med. 2007;4(8):e247. doi: 10.1371/journal.pmed.0040247. PubMed
6. Sheu SM, Tsai CF, Yang HY, Pai HW, Chen SC. Comparison of age-specific hospitalization during pandemic and seasonal influenza periods from 2009 to 2012 in Taiwan: a nationwide population-based study. BMC Infect Dis. 2016;16:88. doi: 10.1186/s12879-016-1438-x. PubMed
7. Goldstein E, Greene SK, Olson DR, Hanage WP, Lipsitch M. Estimating the hospitalization burden associated with influenza and respiratory syncytial virus in New York City, 2003-2011. Influenza Other Respir Viruses. 2015;9(5):225-233. doi: 10.1111/irv.12325. PubMed
8. Matias G, Taylor RJ, Haguinet F, Schuck-Paim C, Lustig RL, Fleming DM. Modelling estimates of age-specific influenza-related hospitalisation and mortality in the United Kingdom. BMC Public Health. 2016;16:481. doi: 10.1186/s12889-016-3128-4. PubMed
9. Reed C, Chaves SS, Daily Kirley P, et al. Estimating influenza disease burden from population-based surveillance data in the United States. PLoS One. 2015;10(3):e0118369. doi: 10.1371/journal.pone.0118369. PubMed
10. Hirve S, Krishnan A, Dawood FS, et al. Incidence of influenza-associated hospitalization in rural communities in western and northern India, 2010-2012: a multi-site population-based study. J Infect. 2015;70(2):160-170. doi: 10.1016/j.jinf.2014.08.015. PubMed
11. Chaves SS, Perez A, Farley MM, et al. The burden of influenza hospitalizations in infants from 2003 to 2012, United States. Pediatr Infect Dis J. 2014;33(9):912-919. doi: 10.1097/inf.0000000000000321. PubMed
12. Pitts SR, Niska RW, Xu J, Burt CW. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. Natl Health Stat Report. 2008;(7):1-38. PubMed
13. Linhart Y, Shohat T, Bromberg M, Mendelson E, Dictiar R, Green MS. Excess mortality from seasonal influenza is negligible below the age of 50 in Israel: implications for vaccine policy. Infection. 2011;39(5):399-404. doi: 10.1007/s15010-011-0153-1. PubMed
14. Glatman-Freedman A, Portelli I, Jacobs SK, et al. Attack rates assessment of the 2009 pandemic H1N1 influenza A in children and their contacts: a systematic review and meta-analysis. PLoS One. 2012;7(11):e50228. doi: 10.1371/journal.pone.0050228. PubMed
15. Ang LW, Lim C, Lee VJ, et al. Influenza-associated hospitalizations, Singapore, 2004-2008 and 2010-2012. Emerg Infect Dis. 2014;20(10). doi: 10.3201/eid2010.131768. PubMed
16. Sheu SM, Tsai CF, Yang HY, Pai HW, Chen SC. Comparison of age-specific hospitalization during pandemic and seasonal influenza periods from 2009 to 2012 in Taiwan: a nationwide population-based study. BMC Infect Dis. 2016;16(1):88. doi: 10.1186/s12879-016-1438-x. PubMed
17. Israel Center for Disease Control. Summary Report - The 2015/2016 Influenza Season. https://www.health.gov.il/PublicationsFiles/flu2015-2016e.pdf. Accessed July 18, 2017.
18. Yaari R, Katriel G, Huppert A, Axelsen JB, Stone L. Modelling seasonal influenza: the role of weather and punctuated antigenic drift. J R Soc Interface. 2013;10(84):20130298. doi: 10.1098/rsif.2013.0298. PubMed
19. Hirsh S, Hindiyeh M, Kolet L, et al. Epidemiological changes of respiratory syncytial virus (RSV) infections in Israel. PLoS One. 2014;9(3):e90515. doi: 10.1371/journal.pone.0090515. PubMed
20. Miguez A, Iftimi A, Montes F. Temporal association between the influenza virus and respiratory syncytial virus (RSV): RSV as a predictor of seasonal influenza. Epidemiol Infect. 2016;144(12):2621-32. doi: 10.1017/s095026881600090x. PubMed
1. Bromberg M, Kaufman Z, Mandelboim M, et al. [Clinical and virological surveillance of influenza in Israel--implementation during pandemic influenza]. Harefuah. 2009;148(9):577-582, 659. PubMed
2. Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25(27):5086-5096. Epub 2007/06/05. doi: 10.1016/j.vaccine.2007.03.046. PubMed
3. De Pascale G, Bittner EA. Influenza-associated critical illness: estimating the burden and the burden of estimation. Crit Care Med. 2014;42(11):2441-2442. Epub 2014/10/17. doi: 10.1097/ccm.0000000000000589. PubMed
4. Pitman RJ, Melegaro A, Gelb D, Siddiqui MR, Gay NJ, Edmunds WJ. Assessing the burden of influenza and other respiratory infections in England and Wales. J Infect. 2007;54(6):530-538. Epub 2006/11/14. doi: 10.1016/j.jinf.2006.09.017. PubMed
5. Olson DR, Heffernan RT, Paladini M, Konty K, Weiss D, Mostashari F. Monitoring the impact of influenza by age: emergency department fever and respiratory complaint surveillance in New York City. PLoS Med. 2007;4(8):e247. doi: 10.1371/journal.pmed.0040247. PubMed
6. Sheu SM, Tsai CF, Yang HY, Pai HW, Chen SC. Comparison of age-specific hospitalization during pandemic and seasonal influenza periods from 2009 to 2012 in Taiwan: a nationwide population-based study. BMC Infect Dis. 2016;16:88. doi: 10.1186/s12879-016-1438-x. PubMed
7. Goldstein E, Greene SK, Olson DR, Hanage WP, Lipsitch M. Estimating the hospitalization burden associated with influenza and respiratory syncytial virus in New York City, 2003-2011. Influenza Other Respir Viruses. 2015;9(5):225-233. doi: 10.1111/irv.12325. PubMed
8. Matias G, Taylor RJ, Haguinet F, Schuck-Paim C, Lustig RL, Fleming DM. Modelling estimates of age-specific influenza-related hospitalisation and mortality in the United Kingdom. BMC Public Health. 2016;16:481. doi: 10.1186/s12889-016-3128-4. PubMed
9. Reed C, Chaves SS, Daily Kirley P, et al. Estimating influenza disease burden from population-based surveillance data in the United States. PLoS One. 2015;10(3):e0118369. doi: 10.1371/journal.pone.0118369. PubMed
10. Hirve S, Krishnan A, Dawood FS, et al. Incidence of influenza-associated hospitalization in rural communities in western and northern India, 2010-2012: a multi-site population-based study. J Infect. 2015;70(2):160-170. doi: 10.1016/j.jinf.2014.08.015. PubMed
11. Chaves SS, Perez A, Farley MM, et al. The burden of influenza hospitalizations in infants from 2003 to 2012, United States. Pediatr Infect Dis J. 2014;33(9):912-919. doi: 10.1097/inf.0000000000000321. PubMed
12. Pitts SR, Niska RW, Xu J, Burt CW. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. Natl Health Stat Report. 2008;(7):1-38. PubMed
13. Linhart Y, Shohat T, Bromberg M, Mendelson E, Dictiar R, Green MS. Excess mortality from seasonal influenza is negligible below the age of 50 in Israel: implications for vaccine policy. Infection. 2011;39(5):399-404. doi: 10.1007/s15010-011-0153-1. PubMed
14. Glatman-Freedman A, Portelli I, Jacobs SK, et al. Attack rates assessment of the 2009 pandemic H1N1 influenza A in children and their contacts: a systematic review and meta-analysis. PLoS One. 2012;7(11):e50228. doi: 10.1371/journal.pone.0050228. PubMed
15. Ang LW, Lim C, Lee VJ, et al. Influenza-associated hospitalizations, Singapore, 2004-2008 and 2010-2012. Emerg Infect Dis. 2014;20(10). doi: 10.3201/eid2010.131768. PubMed
16. Sheu SM, Tsai CF, Yang HY, Pai HW, Chen SC. Comparison of age-specific hospitalization during pandemic and seasonal influenza periods from 2009 to 2012 in Taiwan: a nationwide population-based study. BMC Infect Dis. 2016;16(1):88. doi: 10.1186/s12879-016-1438-x. PubMed
17. Israel Center for Disease Control. Summary Report - The 2015/2016 Influenza Season. https://www.health.gov.il/PublicationsFiles/flu2015-2016e.pdf. Accessed July 18, 2017.
18. Yaari R, Katriel G, Huppert A, Axelsen JB, Stone L. Modelling seasonal influenza: the role of weather and punctuated antigenic drift. J R Soc Interface. 2013;10(84):20130298. doi: 10.1098/rsif.2013.0298. PubMed
19. Hirsh S, Hindiyeh M, Kolet L, et al. Epidemiological changes of respiratory syncytial virus (RSV) infections in Israel. PLoS One. 2014;9(3):e90515. doi: 10.1371/journal.pone.0090515. PubMed
20. Miguez A, Iftimi A, Montes F. Temporal association between the influenza virus and respiratory syncytial virus (RSV): RSV as a predictor of seasonal influenza. Epidemiol Infect. 2016;144(12):2621-32. doi: 10.1017/s095026881600090x. PubMed
© 2017 Society of Hospital Medicine