User login
Inotuzumab ozogamicin tied to sinusoidal obstruction syndrome in ALL
Inotuzumab ozogamicin therapy significantly increased the risk of sinusoidal obstruction syndrome (veno-occlusive disease) among adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL), especially when they also received follow-up hematopoietic stem cell transplantation, according to a safety analysis from the INO-VATE trial.
After a median of 9 weeks of treatment, 13% of 164 patients who received inotuzumab ozogamicin (Besponsa, Wyeth/Pfizer) developed sinusoidal obstruction syndrome, compared with less than 1% of 143 patients who received standard care, reported Hagop M. Kantarjian, MD, of the University of Texas MD Anderson Cancer Center in Houston, and his associates (Lancet Haematol. 2017 Jul 4. doi: 10.1016/ S2352-3026[17]30103-5).
Follow-up treatment with HSCT increased the risk of sinusoidal obstruction syndrome in both the intervention (22%) and standard-care (3%) groups. Among patients who did not undergo HSCT, rates of this adverse event were 3% and 0%, respectively. Five patients died from sinusoidal obstruction syndrome, all of whom received both inotuzumab ozogamicin and HSCT. The findings earned the newly approved regimen a boxed warning for severe hepatotoxicity.
The open-label, phase 3, multicenter INO-VATE study included 326 adults with CD22-positive, Philadelphia chromosome–negative or Philadelphia chromosome–positive relapsed or refractory B-cell precursor ALL. The safety analysis included 305 patients. Rates of treatment-emergent hepatotoxicities, of all grades, were 51% with inotuzumab ozogamicin and 34% with standard care. Most adverse hepatic events were grade 1-2 liver-related laboratory abnormalities, but 8% of inotuzumab ozogamicin recipients developed grade 3 or higher sinusoidal obstruction syndrome, versus less than 1% of the control group.
“After follow-up HSCT, the frequency of sinusoidal obstruction syndrome was 50% or higher in the following subgroups: patients aged 65 years or older, patients with last available pre-HSCT serum bilirubin concentration more than or equal to the upper limit of normal, and patients who received conditioning regimens with two alkylating agents,” Dr. Kantarjian and his fellow investigators wrote. Conditioning regimens that included thiotepa markedly increased the risk of sinusoidal obstruction syndrome. Additional risk factors included HSCT before study enrollment, history of liver disease, and a final pre-HSCT platelet count of less than 100 × 109 platelets per L.
Rates of sinusoidal obstruction syndrome were 42% with four to six cycles of inotuzumab ozogamicin, 23% with three cycles, 19% with two cycles, and 8% with one cycle, said the investigators. In multivariate analysis, conditioning with two alkylating agents (P = .02 compared with one alkylating agent) and pre-HSCT bilirubin of at least the upper limit of normal (P = .01) significantly increased the risk of sinusoidal obstruction syndrome during or after treatment with inotuzumab ozogamicin.
Notably, inotuzumab ozogamicin did not significantly increase the chances of survival compared with standard care among patients who also received follow-up HSCT (hazard ratio, 1.3; 97.5% confidence interval, 0.66 to 2.3; P = 0.77). Among HSCT recipients, the chances of surviving to 24 months were 39% (95% CI, 28%-50%) with inotuzumab ozogamicin and 29% (11%-49%) with standard care. Nonetheless, HSCT “offers possibility of cure in the relapsed or recurrent [ALL] setting,” the researchers wrote. Clinicians should be especially wary of sinusoidal obstruction syndrome if patients are 65 years or older, received HSCT before inotuzumab ozogamicin treatment, or have a baseline history of liver disease, they said. Strategies to minimize risk include shortening the duration of inotuzumab ozogamicin treatment and avoiding conditioning regimens that contain two alkylating agents.
Pfizer funded and collaborated in the trial. Dr. Kantarjian disclosed ties to Pfizer and numerous other pharmaceutical companies.
Inotuzumab ozogamicin therapy significantly increased the risk of sinusoidal obstruction syndrome (veno-occlusive disease) among adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL), especially when they also received follow-up hematopoietic stem cell transplantation, according to a safety analysis from the INO-VATE trial.
After a median of 9 weeks of treatment, 13% of 164 patients who received inotuzumab ozogamicin (Besponsa, Wyeth/Pfizer) developed sinusoidal obstruction syndrome, compared with less than 1% of 143 patients who received standard care, reported Hagop M. Kantarjian, MD, of the University of Texas MD Anderson Cancer Center in Houston, and his associates (Lancet Haematol. 2017 Jul 4. doi: 10.1016/ S2352-3026[17]30103-5).
Follow-up treatment with HSCT increased the risk of sinusoidal obstruction syndrome in both the intervention (22%) and standard-care (3%) groups. Among patients who did not undergo HSCT, rates of this adverse event were 3% and 0%, respectively. Five patients died from sinusoidal obstruction syndrome, all of whom received both inotuzumab ozogamicin and HSCT. The findings earned the newly approved regimen a boxed warning for severe hepatotoxicity.
The open-label, phase 3, multicenter INO-VATE study included 326 adults with CD22-positive, Philadelphia chromosome–negative or Philadelphia chromosome–positive relapsed or refractory B-cell precursor ALL. The safety analysis included 305 patients. Rates of treatment-emergent hepatotoxicities, of all grades, were 51% with inotuzumab ozogamicin and 34% with standard care. Most adverse hepatic events were grade 1-2 liver-related laboratory abnormalities, but 8% of inotuzumab ozogamicin recipients developed grade 3 or higher sinusoidal obstruction syndrome, versus less than 1% of the control group.
“After follow-up HSCT, the frequency of sinusoidal obstruction syndrome was 50% or higher in the following subgroups: patients aged 65 years or older, patients with last available pre-HSCT serum bilirubin concentration more than or equal to the upper limit of normal, and patients who received conditioning regimens with two alkylating agents,” Dr. Kantarjian and his fellow investigators wrote. Conditioning regimens that included thiotepa markedly increased the risk of sinusoidal obstruction syndrome. Additional risk factors included HSCT before study enrollment, history of liver disease, and a final pre-HSCT platelet count of less than 100 × 109 platelets per L.
Rates of sinusoidal obstruction syndrome were 42% with four to six cycles of inotuzumab ozogamicin, 23% with three cycles, 19% with two cycles, and 8% with one cycle, said the investigators. In multivariate analysis, conditioning with two alkylating agents (P = .02 compared with one alkylating agent) and pre-HSCT bilirubin of at least the upper limit of normal (P = .01) significantly increased the risk of sinusoidal obstruction syndrome during or after treatment with inotuzumab ozogamicin.
Notably, inotuzumab ozogamicin did not significantly increase the chances of survival compared with standard care among patients who also received follow-up HSCT (hazard ratio, 1.3; 97.5% confidence interval, 0.66 to 2.3; P = 0.77). Among HSCT recipients, the chances of surviving to 24 months were 39% (95% CI, 28%-50%) with inotuzumab ozogamicin and 29% (11%-49%) with standard care. Nonetheless, HSCT “offers possibility of cure in the relapsed or recurrent [ALL] setting,” the researchers wrote. Clinicians should be especially wary of sinusoidal obstruction syndrome if patients are 65 years or older, received HSCT before inotuzumab ozogamicin treatment, or have a baseline history of liver disease, they said. Strategies to minimize risk include shortening the duration of inotuzumab ozogamicin treatment and avoiding conditioning regimens that contain two alkylating agents.
Pfizer funded and collaborated in the trial. Dr. Kantarjian disclosed ties to Pfizer and numerous other pharmaceutical companies.
Inotuzumab ozogamicin therapy significantly increased the risk of sinusoidal obstruction syndrome (veno-occlusive disease) among adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL), especially when they also received follow-up hematopoietic stem cell transplantation, according to a safety analysis from the INO-VATE trial.
After a median of 9 weeks of treatment, 13% of 164 patients who received inotuzumab ozogamicin (Besponsa, Wyeth/Pfizer) developed sinusoidal obstruction syndrome, compared with less than 1% of 143 patients who received standard care, reported Hagop M. Kantarjian, MD, of the University of Texas MD Anderson Cancer Center in Houston, and his associates (Lancet Haematol. 2017 Jul 4. doi: 10.1016/ S2352-3026[17]30103-5).
Follow-up treatment with HSCT increased the risk of sinusoidal obstruction syndrome in both the intervention (22%) and standard-care (3%) groups. Among patients who did not undergo HSCT, rates of this adverse event were 3% and 0%, respectively. Five patients died from sinusoidal obstruction syndrome, all of whom received both inotuzumab ozogamicin and HSCT. The findings earned the newly approved regimen a boxed warning for severe hepatotoxicity.
The open-label, phase 3, multicenter INO-VATE study included 326 adults with CD22-positive, Philadelphia chromosome–negative or Philadelphia chromosome–positive relapsed or refractory B-cell precursor ALL. The safety analysis included 305 patients. Rates of treatment-emergent hepatotoxicities, of all grades, were 51% with inotuzumab ozogamicin and 34% with standard care. Most adverse hepatic events were grade 1-2 liver-related laboratory abnormalities, but 8% of inotuzumab ozogamicin recipients developed grade 3 or higher sinusoidal obstruction syndrome, versus less than 1% of the control group.
“After follow-up HSCT, the frequency of sinusoidal obstruction syndrome was 50% or higher in the following subgroups: patients aged 65 years or older, patients with last available pre-HSCT serum bilirubin concentration more than or equal to the upper limit of normal, and patients who received conditioning regimens with two alkylating agents,” Dr. Kantarjian and his fellow investigators wrote. Conditioning regimens that included thiotepa markedly increased the risk of sinusoidal obstruction syndrome. Additional risk factors included HSCT before study enrollment, history of liver disease, and a final pre-HSCT platelet count of less than 100 × 109 platelets per L.
Rates of sinusoidal obstruction syndrome were 42% with four to six cycles of inotuzumab ozogamicin, 23% with three cycles, 19% with two cycles, and 8% with one cycle, said the investigators. In multivariate analysis, conditioning with two alkylating agents (P = .02 compared with one alkylating agent) and pre-HSCT bilirubin of at least the upper limit of normal (P = .01) significantly increased the risk of sinusoidal obstruction syndrome during or after treatment with inotuzumab ozogamicin.
Notably, inotuzumab ozogamicin did not significantly increase the chances of survival compared with standard care among patients who also received follow-up HSCT (hazard ratio, 1.3; 97.5% confidence interval, 0.66 to 2.3; P = 0.77). Among HSCT recipients, the chances of surviving to 24 months were 39% (95% CI, 28%-50%) with inotuzumab ozogamicin and 29% (11%-49%) with standard care. Nonetheless, HSCT “offers possibility of cure in the relapsed or recurrent [ALL] setting,” the researchers wrote. Clinicians should be especially wary of sinusoidal obstruction syndrome if patients are 65 years or older, received HSCT before inotuzumab ozogamicin treatment, or have a baseline history of liver disease, they said. Strategies to minimize risk include shortening the duration of inotuzumab ozogamicin treatment and avoiding conditioning regimens that contain two alkylating agents.
Pfizer funded and collaborated in the trial. Dr. Kantarjian disclosed ties to Pfizer and numerous other pharmaceutical companies.
FROM LANCET HAEMATOLOGY
Key clinical point: Treatment with inotuzumab ozogamicin (Besponsa, Wyeth/Pfizer) led to sinusoidal obstructive syndrome (veno-occlusive disease), especially after follow-up hematopoietic stem cell transplantation, compared with standard care for relapsed or refractory acute lymphoblastic leukemia.
Major finding: After a median of 9 weeks of treatment, rates of sinusoidal obstructive syndrome were 13% among inotuzumab ozogamicin recipients overall, 22% among those who also received HSCT, and less than 1% in the standard-care group.
Data source: A prespecified safety analysis of INO-VATE, an open-label, phase 3, multicenter trial of 326 adults with Philadelphia chromosome–negative or Philadelphia chromosome–positive relapsed or refractory B-cell precursor ALL.
Disclosures: Pfizer funded and collaborated in the trial. Dr. Kantarjian disclosed ties to Pfizer and numerous other pharmaceutical companies.
Opioids overprescribed in elective hernia repair patients
A patient-centered study of postoperative analgesic needs found that surgeons may be overprescribing opioids for pain management after hernia repair operations.
The growing opioid public health crisis – and potential contribution of pain control prescriptions to the crisis – has prompted empirical work on surgeons’ prescribing and actual patient use of opioids (Ann Surg. 2017 Jul 10. doi: 10.1097/SLA.0000000000002365. [Epub ahead of print];J Arthroplasty. 2017;32[8]2395-2398). Excess pain medications are thought to raise the risk of patient dependence and to be potentially diverted to nonpatients’ use. A recent study (Ann Surg. 2017;265:709-16) found that a median of 30 opioid tablets were routinely prescribed by surgeons for pain management after hernia repair.
Konstantinos Mylonas, MD, a research fellow at Massachusetts General Hospital, Boston, and his coinvestigators conducted a prospective, observational study of 185 patients who had an outpatient inguinal hernia repair between October 2015 and September 2016. Participants completed a survey on their pain levels and opioid use during the 2- to 3-week period between their procedure and follow-up appointment (Surgery. 2017 Aug 1. doi: 10.1016/j.surg.2017.06.017).
For postop pain control, the patients were given 10 Vicodin (hydrocodone 5 mg/acetaminophen 325 mg) tablets, although all were advised that they may not require the medication but could instead use acetaminophen or ibuprofen as needed.
Of the 185 patients who were surveyed, 159 (86%) reported taking 4 or less of the 10 opioid tablets prescribed to them, with 110 (60%) reporting taking no tablets at all
While 13 (7%) of the patients did report using nine or more of the prescribed Vicodin tablets, none of the patients surveyed were still taking the pain medication within 7 days of their follow-up appointment.
When asked about how pain affected their returning to daily activities, 123 patients (66.5%) reported not having any pain interference, 42 (22.7%) mentioned slight problems, and 5 (2.7%) were noticeably affected. No patients were unable to return to their daily activities, and those 111 (75%) of the 147 patients who were employed were able to return to work within 3 days of surgery, according to the investigators.
As might be expected, those patients who experienced higher pain levels and persistent pain took more Vicodin tablets, but only one patient required a refill of the original prescription.
Patients were also not blinded to the study, which may have caused them to either take less opioid medication or not report their intake accurately. But the findings suggest that opioid prescribing could be tailored much more narrowly to patients’ needs and to individual procedures than is currently common practice. “Although our study was limited to a single procedure, performed by a single surgeon in a high-volume center, implementing patient-centered, procedure-specific opioid administration strategies may be conceivable across a variety of surgical disciplines,” the investigators concluded.
For the 750,000 inguinal hernia repairs done in the United States each year, prescribing 4 instead of 30 opioid tablets would decrease the number of opioid analgesics dispensed annually for hernia operation from 22.5 million to 3 million,” the investigators wrote. “As a result, 20 million fewer tablets per year would be available for potential diversion and abuse or as a stimulus for the start of opioid dependency.”
The researchers reported no relevant financial disclosures.
[email protected]
On Twitter @eaztweets
A patient-centered study of postoperative analgesic needs found that surgeons may be overprescribing opioids for pain management after hernia repair operations.
The growing opioid public health crisis – and potential contribution of pain control prescriptions to the crisis – has prompted empirical work on surgeons’ prescribing and actual patient use of opioids (Ann Surg. 2017 Jul 10. doi: 10.1097/SLA.0000000000002365. [Epub ahead of print];J Arthroplasty. 2017;32[8]2395-2398). Excess pain medications are thought to raise the risk of patient dependence and to be potentially diverted to nonpatients’ use. A recent study (Ann Surg. 2017;265:709-16) found that a median of 30 opioid tablets were routinely prescribed by surgeons for pain management after hernia repair.
Konstantinos Mylonas, MD, a research fellow at Massachusetts General Hospital, Boston, and his coinvestigators conducted a prospective, observational study of 185 patients who had an outpatient inguinal hernia repair between October 2015 and September 2016. Participants completed a survey on their pain levels and opioid use during the 2- to 3-week period between their procedure and follow-up appointment (Surgery. 2017 Aug 1. doi: 10.1016/j.surg.2017.06.017).
For postop pain control, the patients were given 10 Vicodin (hydrocodone 5 mg/acetaminophen 325 mg) tablets, although all were advised that they may not require the medication but could instead use acetaminophen or ibuprofen as needed.
Of the 185 patients who were surveyed, 159 (86%) reported taking 4 or less of the 10 opioid tablets prescribed to them, with 110 (60%) reporting taking no tablets at all
While 13 (7%) of the patients did report using nine or more of the prescribed Vicodin tablets, none of the patients surveyed were still taking the pain medication within 7 days of their follow-up appointment.
When asked about how pain affected their returning to daily activities, 123 patients (66.5%) reported not having any pain interference, 42 (22.7%) mentioned slight problems, and 5 (2.7%) were noticeably affected. No patients were unable to return to their daily activities, and those 111 (75%) of the 147 patients who were employed were able to return to work within 3 days of surgery, according to the investigators.
As might be expected, those patients who experienced higher pain levels and persistent pain took more Vicodin tablets, but only one patient required a refill of the original prescription.
Patients were also not blinded to the study, which may have caused them to either take less opioid medication or not report their intake accurately. But the findings suggest that opioid prescribing could be tailored much more narrowly to patients’ needs and to individual procedures than is currently common practice. “Although our study was limited to a single procedure, performed by a single surgeon in a high-volume center, implementing patient-centered, procedure-specific opioid administration strategies may be conceivable across a variety of surgical disciplines,” the investigators concluded.
For the 750,000 inguinal hernia repairs done in the United States each year, prescribing 4 instead of 30 opioid tablets would decrease the number of opioid analgesics dispensed annually for hernia operation from 22.5 million to 3 million,” the investigators wrote. “As a result, 20 million fewer tablets per year would be available for potential diversion and abuse or as a stimulus for the start of opioid dependency.”
The researchers reported no relevant financial disclosures.
[email protected]
On Twitter @eaztweets
A patient-centered study of postoperative analgesic needs found that surgeons may be overprescribing opioids for pain management after hernia repair operations.
The growing opioid public health crisis – and potential contribution of pain control prescriptions to the crisis – has prompted empirical work on surgeons’ prescribing and actual patient use of opioids (Ann Surg. 2017 Jul 10. doi: 10.1097/SLA.0000000000002365. [Epub ahead of print];J Arthroplasty. 2017;32[8]2395-2398). Excess pain medications are thought to raise the risk of patient dependence and to be potentially diverted to nonpatients’ use. A recent study (Ann Surg. 2017;265:709-16) found that a median of 30 opioid tablets were routinely prescribed by surgeons for pain management after hernia repair.
Konstantinos Mylonas, MD, a research fellow at Massachusetts General Hospital, Boston, and his coinvestigators conducted a prospective, observational study of 185 patients who had an outpatient inguinal hernia repair between October 2015 and September 2016. Participants completed a survey on their pain levels and opioid use during the 2- to 3-week period between their procedure and follow-up appointment (Surgery. 2017 Aug 1. doi: 10.1016/j.surg.2017.06.017).
For postop pain control, the patients were given 10 Vicodin (hydrocodone 5 mg/acetaminophen 325 mg) tablets, although all were advised that they may not require the medication but could instead use acetaminophen or ibuprofen as needed.
Of the 185 patients who were surveyed, 159 (86%) reported taking 4 or less of the 10 opioid tablets prescribed to them, with 110 (60%) reporting taking no tablets at all
While 13 (7%) of the patients did report using nine or more of the prescribed Vicodin tablets, none of the patients surveyed were still taking the pain medication within 7 days of their follow-up appointment.
When asked about how pain affected their returning to daily activities, 123 patients (66.5%) reported not having any pain interference, 42 (22.7%) mentioned slight problems, and 5 (2.7%) were noticeably affected. No patients were unable to return to their daily activities, and those 111 (75%) of the 147 patients who were employed were able to return to work within 3 days of surgery, according to the investigators.
As might be expected, those patients who experienced higher pain levels and persistent pain took more Vicodin tablets, but only one patient required a refill of the original prescription.
Patients were also not blinded to the study, which may have caused them to either take less opioid medication or not report their intake accurately. But the findings suggest that opioid prescribing could be tailored much more narrowly to patients’ needs and to individual procedures than is currently common practice. “Although our study was limited to a single procedure, performed by a single surgeon in a high-volume center, implementing patient-centered, procedure-specific opioid administration strategies may be conceivable across a variety of surgical disciplines,” the investigators concluded.
For the 750,000 inguinal hernia repairs done in the United States each year, prescribing 4 instead of 30 opioid tablets would decrease the number of opioid analgesics dispensed annually for hernia operation from 22.5 million to 3 million,” the investigators wrote. “As a result, 20 million fewer tablets per year would be available for potential diversion and abuse or as a stimulus for the start of opioid dependency.”
The researchers reported no relevant financial disclosures.
[email protected]
On Twitter @eaztweets
FROM SURGERY
Key clinical point:
Major finding: Of 185 patients surveyed, 159 (86%) reported taking 4 or less opioid tablets of a 10-tablet prescription.
Data source: Observational study of 185 elective inguinal hernia repair patients between October 2015 and September 2016.
Disclosures: Investigators reported no relevant financial disclosures.
Dispensing hormonal contraceptives in 1-year supplies saves state $43 million and avoids 15,000 unintended pregnancies
Recent insurance coverage legislation enacted in California is expected to result in 15,000 fewer unintended pregnancies, 2,000 fewer miscarriages, and 7,000 fewer abortions, according to an analysis of the mandate’s potential impact by investigators at the University of California.1
Enacted in September 2016, the contraceptive supply legislation known as SB 999 requires health plans and insurers to cover a year-long supply of hormonal contraceptive pills, patches, and rings (formulations approved by the US Food and Drug Administration). Clinicians can now prescribe and pharmacists can dispense up to a 12-month supply at one time. California joins 5 other states and Washington, DC, that have such mandates.
Having a year’s worth of contraceptives on hand is anticipated to reduce the interruption in contraception use that may occur with a 30- or 90-day supply that needs frequent refilling and thereby lower the unplanned pregnancy rate as well as associated health care costs.
Understanding the legislation’s impact on the population’s health outcomes will be useful for other states considering similar proposed legislation.
Details of the study
In a short communication published in Contraception, McMenamin and colleagues described how University of California faculty and researchers, engaged by the California Health Benefits Review Program, assessed the utilization and cost implications of SB 999 and arrived at their estimated projections.1
The assessment was based on a literature review (including current use of hormonal contraceptives, unintended pregnancy rates among contraceptive users, and assumptions about shifts in dispensing patterns for contraceptive supplies), a survey of the state’s 5 largest health insurance providers, and a claims database review of the utilization and cost implications of SB 999.
Two scenarios. Projections for the use and costs of hormonal contraceptives were made for 2 situations: whether SB 999 was enacted into law or not. Approximately 25 million Californians would be affected by the legislation, including 744,000 who used hormonal contraceptives in 2016.
To calculate their projections, the researchers used a baseline estimate of a 9% unintended pregnancy rate among current users of hormonal contraceptives (or 67,000 unintended pregnancies leading to 28,000 live births, 9,000 miscarriages, and 30,000 abortions).2 They also used a previously reported 30% reduction in the odds of unintended pregnancy with 12-month dispensing.3
Impact of shift in dispensing patterns
With SB 999 versus without SB 999, a 30% reduction in the odds of unintended pregnancies would lead to 6,000 fewer live births, 2,000 fewer miscarriages, and 7,000 fewer abortions.
The legislation would also reduce projected health care expenditures. Total net health care costs would decrease by 0.03%, for a savings of about $43 million, due to avoidance of unintended pregnancies and related medical costs.
Benefits will be even greater over time
The authors noted that the reductions in unintended pregnancies and associated health care costs with the implementation of SB 999 may be even greater in later years as beneficial health outcomes and cost savings accrue over time.
This study’s findings provide support for the implementation of similar legislation in other states.
- McMenamin SB, Charles SA, Tabatabaeepour N, Shigekawa E, Corbett G. Implications of dispensing self-administered hormonal contraceptives in a 1-year supply: a California case study. Contraception. 2017;95(5):449–451.
- Trussel J. Contraceptive failure in the United States. Contraception. 2011;83(5):397–404.
- Foster DG, Hulett D, Bradsberry M, Darney P, Policar M. Number of oral contraceptive pill packages dispensed and subsequent unintended pregnancies. Obstet Gynecol. 2011;117(3):566–572.
Recent insurance coverage legislation enacted in California is expected to result in 15,000 fewer unintended pregnancies, 2,000 fewer miscarriages, and 7,000 fewer abortions, according to an analysis of the mandate’s potential impact by investigators at the University of California.1
Enacted in September 2016, the contraceptive supply legislation known as SB 999 requires health plans and insurers to cover a year-long supply of hormonal contraceptive pills, patches, and rings (formulations approved by the US Food and Drug Administration). Clinicians can now prescribe and pharmacists can dispense up to a 12-month supply at one time. California joins 5 other states and Washington, DC, that have such mandates.
Having a year’s worth of contraceptives on hand is anticipated to reduce the interruption in contraception use that may occur with a 30- or 90-day supply that needs frequent refilling and thereby lower the unplanned pregnancy rate as well as associated health care costs.
Understanding the legislation’s impact on the population’s health outcomes will be useful for other states considering similar proposed legislation.
Details of the study
In a short communication published in Contraception, McMenamin and colleagues described how University of California faculty and researchers, engaged by the California Health Benefits Review Program, assessed the utilization and cost implications of SB 999 and arrived at their estimated projections.1
The assessment was based on a literature review (including current use of hormonal contraceptives, unintended pregnancy rates among contraceptive users, and assumptions about shifts in dispensing patterns for contraceptive supplies), a survey of the state’s 5 largest health insurance providers, and a claims database review of the utilization and cost implications of SB 999.
Two scenarios. Projections for the use and costs of hormonal contraceptives were made for 2 situations: whether SB 999 was enacted into law or not. Approximately 25 million Californians would be affected by the legislation, including 744,000 who used hormonal contraceptives in 2016.
To calculate their projections, the researchers used a baseline estimate of a 9% unintended pregnancy rate among current users of hormonal contraceptives (or 67,000 unintended pregnancies leading to 28,000 live births, 9,000 miscarriages, and 30,000 abortions).2 They also used a previously reported 30% reduction in the odds of unintended pregnancy with 12-month dispensing.3
Impact of shift in dispensing patterns
With SB 999 versus without SB 999, a 30% reduction in the odds of unintended pregnancies would lead to 6,000 fewer live births, 2,000 fewer miscarriages, and 7,000 fewer abortions.
The legislation would also reduce projected health care expenditures. Total net health care costs would decrease by 0.03%, for a savings of about $43 million, due to avoidance of unintended pregnancies and related medical costs.
Benefits will be even greater over time
The authors noted that the reductions in unintended pregnancies and associated health care costs with the implementation of SB 999 may be even greater in later years as beneficial health outcomes and cost savings accrue over time.
This study’s findings provide support for the implementation of similar legislation in other states.
Recent insurance coverage legislation enacted in California is expected to result in 15,000 fewer unintended pregnancies, 2,000 fewer miscarriages, and 7,000 fewer abortions, according to an analysis of the mandate’s potential impact by investigators at the University of California.1
Enacted in September 2016, the contraceptive supply legislation known as SB 999 requires health plans and insurers to cover a year-long supply of hormonal contraceptive pills, patches, and rings (formulations approved by the US Food and Drug Administration). Clinicians can now prescribe and pharmacists can dispense up to a 12-month supply at one time. California joins 5 other states and Washington, DC, that have such mandates.
Having a year’s worth of contraceptives on hand is anticipated to reduce the interruption in contraception use that may occur with a 30- or 90-day supply that needs frequent refilling and thereby lower the unplanned pregnancy rate as well as associated health care costs.
Understanding the legislation’s impact on the population’s health outcomes will be useful for other states considering similar proposed legislation.
Details of the study
In a short communication published in Contraception, McMenamin and colleagues described how University of California faculty and researchers, engaged by the California Health Benefits Review Program, assessed the utilization and cost implications of SB 999 and arrived at their estimated projections.1
The assessment was based on a literature review (including current use of hormonal contraceptives, unintended pregnancy rates among contraceptive users, and assumptions about shifts in dispensing patterns for contraceptive supplies), a survey of the state’s 5 largest health insurance providers, and a claims database review of the utilization and cost implications of SB 999.
Two scenarios. Projections for the use and costs of hormonal contraceptives were made for 2 situations: whether SB 999 was enacted into law or not. Approximately 25 million Californians would be affected by the legislation, including 744,000 who used hormonal contraceptives in 2016.
To calculate their projections, the researchers used a baseline estimate of a 9% unintended pregnancy rate among current users of hormonal contraceptives (or 67,000 unintended pregnancies leading to 28,000 live births, 9,000 miscarriages, and 30,000 abortions).2 They also used a previously reported 30% reduction in the odds of unintended pregnancy with 12-month dispensing.3
Impact of shift in dispensing patterns
With SB 999 versus without SB 999, a 30% reduction in the odds of unintended pregnancies would lead to 6,000 fewer live births, 2,000 fewer miscarriages, and 7,000 fewer abortions.
The legislation would also reduce projected health care expenditures. Total net health care costs would decrease by 0.03%, for a savings of about $43 million, due to avoidance of unintended pregnancies and related medical costs.
Benefits will be even greater over time
The authors noted that the reductions in unintended pregnancies and associated health care costs with the implementation of SB 999 may be even greater in later years as beneficial health outcomes and cost savings accrue over time.
This study’s findings provide support for the implementation of similar legislation in other states.
- McMenamin SB, Charles SA, Tabatabaeepour N, Shigekawa E, Corbett G. Implications of dispensing self-administered hormonal contraceptives in a 1-year supply: a California case study. Contraception. 2017;95(5):449–451.
- Trussel J. Contraceptive failure in the United States. Contraception. 2011;83(5):397–404.
- Foster DG, Hulett D, Bradsberry M, Darney P, Policar M. Number of oral contraceptive pill packages dispensed and subsequent unintended pregnancies. Obstet Gynecol. 2011;117(3):566–572.
- McMenamin SB, Charles SA, Tabatabaeepour N, Shigekawa E, Corbett G. Implications of dispensing self-administered hormonal contraceptives in a 1-year supply: a California case study. Contraception. 2017;95(5):449–451.
- Trussel J. Contraceptive failure in the United States. Contraception. 2011;83(5):397–404.
- Foster DG, Hulett D, Bradsberry M, Darney P, Policar M. Number of oral contraceptive pill packages dispensed and subsequent unintended pregnancies. Obstet Gynecol. 2011;117(3):566–572.
‘Flakka’: A low-cost, dangerous high
Use of α-pyrrolidinovalerophenone (α-PVP), a psychostimulant related to cathinone derivatives (“bath salts”), has been reported in the United States, especially in Florida.1 Known by the street names “flakka” or “gravel,” α-PVP is inexpensive, with a single dose (typically 100 mg) costing as little as $5.2 Alpha-PVP can be consumed via ingestion, injection, insufflation, or inhalation in vaporized forms, such as E-cigarettes, which deliver the drug quickly into the bloodstream and can make it easy to overdose.1 The low cost of this drug makes it likely to be abused. Here we review the mechanism of action and effects of α-PVP and summarize treatment options.
Mechanism of action
Alpha-PVP is a structural parent of 3,4-methylenedioxypyrovalerone (MDPV)—the first widely abused synthetic cathinone.3 Much like cocaine, α-PVP stimulates the CNS by acting as a potent dopamine and norepinephrine reuptake inhibitor. However, unlike cocaine, it lacks any action on serotonin transporters. The pyrrolidine ring in MDPV and α-PVP is responsible for the highly potent dopamine reuptake inhibitor action of these agents.3
A wide range of adverse effects
Use of α-PVP results in a state of “excited delirium,” with symptoms such as hyperthermia, hallucinations, paranoia, violent aggression, and self-harm.1 Alpha-PVP is known to cause rhabdomyolysis.4 Some studies have reported cardiovascular effects, such as arterial hypertension, palpitations, dyspnea, vasoconstriction, arrhythmia, myocardial infarction (MI), and myocarditis.5 Alpha-PVP also may result in neurologic symptoms, including headache, mydriasis, lightheadedness, paresthesia, seizures, dystonic movements, tremor, amnesia, dysgeusia, cerebral edema, motor automatisms, muscle spasm, nystagmus, parkinsonism, and stroke.5 Death may occur by cardiac arrest, renal damage, or suicide.
Case reports. The effects of α-PVP have been documented in the literature:
- A 17-year-old girl was brought to an emergency department in Florida with acute onset of bizarre behavior, agitation, and altered mental status. It took 6 days and repeated administrations of olanzapine and lorazepam for the patient to become calm, alert, and oriented.2
- ST-elevated MI with several intracardiac thrombi was reported in a 41-year-old woman who used α-PVP.4
- In 2015, 18 deaths related to α-PVP use were reported in South Florida.5
- Deaths related to α-PVP use also have been reported in Japan and Australia.5
Treatment options
There are no treatment guidelines for α-PVP-related psychiatric symptoms. Case reports describe remission of symptoms following aggressive treatment with antipsychotics and benzodiazepines.2 Guidelines for treatment of stimulant-induced behavioral and psychotic symptoms6 may be considered for patients who have used α-PVP.
Reassurance and supportive care are the basic principles of such interventions. A quiet environment and benzodiazepines may provide relief of agitation. Antipsychotics may be helpful if a patient exhibits psychotic symptoms.
Similar drugs may emerge
In 2014, the DEA classified α-PVP as a Schedule I substance. Laws against the import of such substances via the Internet or other means also may help control the spread of this drug. However, chemically similar drugs that may elude drug screens are continually emerging. The lack of evidence-based guidelines on recognizing and managing intoxication, withdrawal, and long-term effects of α-PVP and other “designer drugs” calls for greater research in this emerging area of substance use disorders.
1. National Institute on Drug Abuse. “Flakka” (alpha-PVP). https://www.drugabuse.gov/emerging-trends/flakka-alpha-pvp. Accessed July 26, 2017.
2. Crespi C. Flakka-induced prolonged psychosis. Case Rep Psychiatry. 2016;2016:3460849. doi: 10.1155/2016/3460849.
3. Glennon RA, Young R. Neurobiology of 3,4-methylenedioxypyrovalerone (MDPV) and α-pyrrolidinovalerophenone (α-PVP). Brain Res Bull. 2016;126(pt 1):111-126.
4. Cherry SV, Rodriguez YF. Synthetic stimulant reaching epidemic proportions: flakka-induced ST-elevation myocardial infarction with intracardiac thrombi. J Cardiothorac Vasc Anesth. 2017;31(1):e13-e14.
5. Katselou M, Papoutsis I, Nikolaou P, et al. α-PVP (“flakka”): a new synthetic cathinone invades the drug arena. Forensic Toxicol. 2016;34(1):41-50.
6. Sadock BJ, Sadock VA, Ruiz P. Hallucinogen-related disorders. In: Sadock BJ, Sadock VA, Ruiz P. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry. 11th ed. Philadelphia, PA: Wolters Kluwer; 2015:648-655.
Use of α-pyrrolidinovalerophenone (α-PVP), a psychostimulant related to cathinone derivatives (“bath salts”), has been reported in the United States, especially in Florida.1 Known by the street names “flakka” or “gravel,” α-PVP is inexpensive, with a single dose (typically 100 mg) costing as little as $5.2 Alpha-PVP can be consumed via ingestion, injection, insufflation, or inhalation in vaporized forms, such as E-cigarettes, which deliver the drug quickly into the bloodstream and can make it easy to overdose.1 The low cost of this drug makes it likely to be abused. Here we review the mechanism of action and effects of α-PVP and summarize treatment options.
Mechanism of action
Alpha-PVP is a structural parent of 3,4-methylenedioxypyrovalerone (MDPV)—the first widely abused synthetic cathinone.3 Much like cocaine, α-PVP stimulates the CNS by acting as a potent dopamine and norepinephrine reuptake inhibitor. However, unlike cocaine, it lacks any action on serotonin transporters. The pyrrolidine ring in MDPV and α-PVP is responsible for the highly potent dopamine reuptake inhibitor action of these agents.3
A wide range of adverse effects
Use of α-PVP results in a state of “excited delirium,” with symptoms such as hyperthermia, hallucinations, paranoia, violent aggression, and self-harm.1 Alpha-PVP is known to cause rhabdomyolysis.4 Some studies have reported cardiovascular effects, such as arterial hypertension, palpitations, dyspnea, vasoconstriction, arrhythmia, myocardial infarction (MI), and myocarditis.5 Alpha-PVP also may result in neurologic symptoms, including headache, mydriasis, lightheadedness, paresthesia, seizures, dystonic movements, tremor, amnesia, dysgeusia, cerebral edema, motor automatisms, muscle spasm, nystagmus, parkinsonism, and stroke.5 Death may occur by cardiac arrest, renal damage, or suicide.
Case reports. The effects of α-PVP have been documented in the literature:
- A 17-year-old girl was brought to an emergency department in Florida with acute onset of bizarre behavior, agitation, and altered mental status. It took 6 days and repeated administrations of olanzapine and lorazepam for the patient to become calm, alert, and oriented.2
- ST-elevated MI with several intracardiac thrombi was reported in a 41-year-old woman who used α-PVP.4
- In 2015, 18 deaths related to α-PVP use were reported in South Florida.5
- Deaths related to α-PVP use also have been reported in Japan and Australia.5
Treatment options
There are no treatment guidelines for α-PVP-related psychiatric symptoms. Case reports describe remission of symptoms following aggressive treatment with antipsychotics and benzodiazepines.2 Guidelines for treatment of stimulant-induced behavioral and psychotic symptoms6 may be considered for patients who have used α-PVP.
Reassurance and supportive care are the basic principles of such interventions. A quiet environment and benzodiazepines may provide relief of agitation. Antipsychotics may be helpful if a patient exhibits psychotic symptoms.
Similar drugs may emerge
In 2014, the DEA classified α-PVP as a Schedule I substance. Laws against the import of such substances via the Internet or other means also may help control the spread of this drug. However, chemically similar drugs that may elude drug screens are continually emerging. The lack of evidence-based guidelines on recognizing and managing intoxication, withdrawal, and long-term effects of α-PVP and other “designer drugs” calls for greater research in this emerging area of substance use disorders.
Use of α-pyrrolidinovalerophenone (α-PVP), a psychostimulant related to cathinone derivatives (“bath salts”), has been reported in the United States, especially in Florida.1 Known by the street names “flakka” or “gravel,” α-PVP is inexpensive, with a single dose (typically 100 mg) costing as little as $5.2 Alpha-PVP can be consumed via ingestion, injection, insufflation, or inhalation in vaporized forms, such as E-cigarettes, which deliver the drug quickly into the bloodstream and can make it easy to overdose.1 The low cost of this drug makes it likely to be abused. Here we review the mechanism of action and effects of α-PVP and summarize treatment options.
Mechanism of action
Alpha-PVP is a structural parent of 3,4-methylenedioxypyrovalerone (MDPV)—the first widely abused synthetic cathinone.3 Much like cocaine, α-PVP stimulates the CNS by acting as a potent dopamine and norepinephrine reuptake inhibitor. However, unlike cocaine, it lacks any action on serotonin transporters. The pyrrolidine ring in MDPV and α-PVP is responsible for the highly potent dopamine reuptake inhibitor action of these agents.3
A wide range of adverse effects
Use of α-PVP results in a state of “excited delirium,” with symptoms such as hyperthermia, hallucinations, paranoia, violent aggression, and self-harm.1 Alpha-PVP is known to cause rhabdomyolysis.4 Some studies have reported cardiovascular effects, such as arterial hypertension, palpitations, dyspnea, vasoconstriction, arrhythmia, myocardial infarction (MI), and myocarditis.5 Alpha-PVP also may result in neurologic symptoms, including headache, mydriasis, lightheadedness, paresthesia, seizures, dystonic movements, tremor, amnesia, dysgeusia, cerebral edema, motor automatisms, muscle spasm, nystagmus, parkinsonism, and stroke.5 Death may occur by cardiac arrest, renal damage, or suicide.
Case reports. The effects of α-PVP have been documented in the literature:
- A 17-year-old girl was brought to an emergency department in Florida with acute onset of bizarre behavior, agitation, and altered mental status. It took 6 days and repeated administrations of olanzapine and lorazepam for the patient to become calm, alert, and oriented.2
- ST-elevated MI with several intracardiac thrombi was reported in a 41-year-old woman who used α-PVP.4
- In 2015, 18 deaths related to α-PVP use were reported in South Florida.5
- Deaths related to α-PVP use also have been reported in Japan and Australia.5
Treatment options
There are no treatment guidelines for α-PVP-related psychiatric symptoms. Case reports describe remission of symptoms following aggressive treatment with antipsychotics and benzodiazepines.2 Guidelines for treatment of stimulant-induced behavioral and psychotic symptoms6 may be considered for patients who have used α-PVP.
Reassurance and supportive care are the basic principles of such interventions. A quiet environment and benzodiazepines may provide relief of agitation. Antipsychotics may be helpful if a patient exhibits psychotic symptoms.
Similar drugs may emerge
In 2014, the DEA classified α-PVP as a Schedule I substance. Laws against the import of such substances via the Internet or other means also may help control the spread of this drug. However, chemically similar drugs that may elude drug screens are continually emerging. The lack of evidence-based guidelines on recognizing and managing intoxication, withdrawal, and long-term effects of α-PVP and other “designer drugs” calls for greater research in this emerging area of substance use disorders.
1. National Institute on Drug Abuse. “Flakka” (alpha-PVP). https://www.drugabuse.gov/emerging-trends/flakka-alpha-pvp. Accessed July 26, 2017.
2. Crespi C. Flakka-induced prolonged psychosis. Case Rep Psychiatry. 2016;2016:3460849. doi: 10.1155/2016/3460849.
3. Glennon RA, Young R. Neurobiology of 3,4-methylenedioxypyrovalerone (MDPV) and α-pyrrolidinovalerophenone (α-PVP). Brain Res Bull. 2016;126(pt 1):111-126.
4. Cherry SV, Rodriguez YF. Synthetic stimulant reaching epidemic proportions: flakka-induced ST-elevation myocardial infarction with intracardiac thrombi. J Cardiothorac Vasc Anesth. 2017;31(1):e13-e14.
5. Katselou M, Papoutsis I, Nikolaou P, et al. α-PVP (“flakka”): a new synthetic cathinone invades the drug arena. Forensic Toxicol. 2016;34(1):41-50.
6. Sadock BJ, Sadock VA, Ruiz P. Hallucinogen-related disorders. In: Sadock BJ, Sadock VA, Ruiz P. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry. 11th ed. Philadelphia, PA: Wolters Kluwer; 2015:648-655.
1. National Institute on Drug Abuse. “Flakka” (alpha-PVP). https://www.drugabuse.gov/emerging-trends/flakka-alpha-pvp. Accessed July 26, 2017.
2. Crespi C. Flakka-induced prolonged psychosis. Case Rep Psychiatry. 2016;2016:3460849. doi: 10.1155/2016/3460849.
3. Glennon RA, Young R. Neurobiology of 3,4-methylenedioxypyrovalerone (MDPV) and α-pyrrolidinovalerophenone (α-PVP). Brain Res Bull. 2016;126(pt 1):111-126.
4. Cherry SV, Rodriguez YF. Synthetic stimulant reaching epidemic proportions: flakka-induced ST-elevation myocardial infarction with intracardiac thrombi. J Cardiothorac Vasc Anesth. 2017;31(1):e13-e14.
5. Katselou M, Papoutsis I, Nikolaou P, et al. α-PVP (“flakka”): a new synthetic cathinone invades the drug arena. Forensic Toxicol. 2016;34(1):41-50.
6. Sadock BJ, Sadock VA, Ruiz P. Hallucinogen-related disorders. In: Sadock BJ, Sadock VA, Ruiz P. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry. 11th ed. Philadelphia, PA: Wolters Kluwer; 2015:648-655.
Confronting hate and violence against the LGBT community
It may be unusual for an LGBT health columnist to mention the horrendous events that occurred in Charlottesville, Va., in August 2017. It clearly was a demonstration of hate and violence against racial and ethnic minorities. Unfortunately, the LGBT community – especially LGBT communities of color – are often a target of that kind of hate and violence. This has a detrimental effect on the health of the LGBT community, and I believe that health care providers have a responsibility to address this hate and violence to promote the well-being of this marginalized community.
It cannot be overstated that LGBT individuals frequently experience anti-gay and anti-trans violence. According to the 2015 Federal Bureau of Investigation Hate Crime Statistics, about a fifth of hate crimes reported were based on sexual orientation or gender identity.1 In addition, LGBT youth are eight times as likely to experience bullying at school because of their sexual orientation or gender identity.2 Furthermore, on many surveys on anti-LGBT violence, people of color comprise more than half of the victims.3 There is a strong association between exposure to this violence and the health outcomes of LGBT youth. A study by Russell et al. showed that LGBT youth who were victims of physical violence at school are more likely to be depressed and suicidal and more likely to be diagnosed with an STD,4 and another study showed that LGBT youth who experienced anti-LGBT violence are more likely to engage in substance use.5 The health outcomes from anti-LGBT violence are not limited to the adolescent period – adolescents who experienced this kind of violence are more likely to report higher levels of depression as adults.6 Although researchers still are trying to determine the exact mechanism for these relationships, the most cited (and sensible) explanation is that exposure to anti-LGBT stigma, discrimination, and violence leads to a toxic environment, which in turn increases the risk for mental health problems and maladaptive coping mechanisms (such as substance use) as a response to such an environment.7
What can we do to stand up to the hate and violence against marginalized groups, such as the LGBT community? First, make your office a safe space. With the recent brazen display of hate and violence going around, members the LGBT community are desperate to feel protected. A good place to start is a guide by Advocates for Youth. Second, educate yourself and others. The title physician means “teacher,” and I feel it is your responsibility to teach your peers, colleagues, and the public about how anti-LGBT violence affects the health of LGBT individuals. To be an effective teacher, you need to be up to date on the research on how hatred and intolerance affects the health of the LGBT community. A good place to start is the Human Rights Campaign, which has accurate statistics on anti-LGBT violence and resources to address this problem. Finally, be an advocate. You don’t need to be in the streets with picket signs, nor do you necessarily need to lead the charge against anti-LGBT hate and violence – others will be at the front lines. What you can do is to call for your local, state, and federal government to institute policies that address anti-LGBT violence. Many medical organizations have resources that help health care providers engage with policy makers (check out the American Academy of Pediatrics advocacy page for these resources). Many of our elected officials take our professional opinions seriously.
Anti-gay and anti-trans violence is all too common in the LGBT community, especially violence against LGBT people of color, and this violence can adversely affect their health. Health care providers have a responsibility and the influence to confront these nexuses of hate and intolerance. You don’t need to do something heroic to accomplish this. You are members of a privileged and respected group of professionals, so small actions can coalesce into something that has a large impact on the health and well-being of the communities you serve.
Resources
• Advocates for Youth. Creating Safe Space for GLBTQ Youth: A Toolkit
• Human Rights Campaign. www.hrc.org/resources/
• American Academy of Pediatrics advocacy page: www.aap.org/en-us/advocacy-and-policy/
References
1. U.S. Department of Justice Federal Bureau of Investigation. Uniform Crime Report Hate Crime Statistics, 2015.
2. J Interpers Violence. 2017. doi: 10.1177/0886260517718830.
3. National Coalition of Anti-Violence Programs (NCAVP). Lesbian, Gay, Bisexual, Transgender, Queer and HIV-Affected Hate Violence in 2016.
4. J Sch Health. 2011 May;81(5):223-30.
5. Prev Sci. 2015 Jul;16(5):734-43.
6. Dev Psychol. 2010 Nov;46(6):1580-9.
7. Psychol Bull. 2003 Sep;129(5):674-97.
8. Gallup. Americans Rate Healthcare Providers High on Honesty, Ethics. 2016.
9. The Hippocratic Oath Today. 2001 or Do. No. Harm.
It may be unusual for an LGBT health columnist to mention the horrendous events that occurred in Charlottesville, Va., in August 2017. It clearly was a demonstration of hate and violence against racial and ethnic minorities. Unfortunately, the LGBT community – especially LGBT communities of color – are often a target of that kind of hate and violence. This has a detrimental effect on the health of the LGBT community, and I believe that health care providers have a responsibility to address this hate and violence to promote the well-being of this marginalized community.
It cannot be overstated that LGBT individuals frequently experience anti-gay and anti-trans violence. According to the 2015 Federal Bureau of Investigation Hate Crime Statistics, about a fifth of hate crimes reported were based on sexual orientation or gender identity.1 In addition, LGBT youth are eight times as likely to experience bullying at school because of their sexual orientation or gender identity.2 Furthermore, on many surveys on anti-LGBT violence, people of color comprise more than half of the victims.3 There is a strong association between exposure to this violence and the health outcomes of LGBT youth. A study by Russell et al. showed that LGBT youth who were victims of physical violence at school are more likely to be depressed and suicidal and more likely to be diagnosed with an STD,4 and another study showed that LGBT youth who experienced anti-LGBT violence are more likely to engage in substance use.5 The health outcomes from anti-LGBT violence are not limited to the adolescent period – adolescents who experienced this kind of violence are more likely to report higher levels of depression as adults.6 Although researchers still are trying to determine the exact mechanism for these relationships, the most cited (and sensible) explanation is that exposure to anti-LGBT stigma, discrimination, and violence leads to a toxic environment, which in turn increases the risk for mental health problems and maladaptive coping mechanisms (such as substance use) as a response to such an environment.7
What can we do to stand up to the hate and violence against marginalized groups, such as the LGBT community? First, make your office a safe space. With the recent brazen display of hate and violence going around, members the LGBT community are desperate to feel protected. A good place to start is a guide by Advocates for Youth. Second, educate yourself and others. The title physician means “teacher,” and I feel it is your responsibility to teach your peers, colleagues, and the public about how anti-LGBT violence affects the health of LGBT individuals. To be an effective teacher, you need to be up to date on the research on how hatred and intolerance affects the health of the LGBT community. A good place to start is the Human Rights Campaign, which has accurate statistics on anti-LGBT violence and resources to address this problem. Finally, be an advocate. You don’t need to be in the streets with picket signs, nor do you necessarily need to lead the charge against anti-LGBT hate and violence – others will be at the front lines. What you can do is to call for your local, state, and federal government to institute policies that address anti-LGBT violence. Many medical organizations have resources that help health care providers engage with policy makers (check out the American Academy of Pediatrics advocacy page for these resources). Many of our elected officials take our professional opinions seriously.
Anti-gay and anti-trans violence is all too common in the LGBT community, especially violence against LGBT people of color, and this violence can adversely affect their health. Health care providers have a responsibility and the influence to confront these nexuses of hate and intolerance. You don’t need to do something heroic to accomplish this. You are members of a privileged and respected group of professionals, so small actions can coalesce into something that has a large impact on the health and well-being of the communities you serve.
Resources
• Advocates for Youth. Creating Safe Space for GLBTQ Youth: A Toolkit
• Human Rights Campaign. www.hrc.org/resources/
• American Academy of Pediatrics advocacy page: www.aap.org/en-us/advocacy-and-policy/
References
1. U.S. Department of Justice Federal Bureau of Investigation. Uniform Crime Report Hate Crime Statistics, 2015.
2. J Interpers Violence. 2017. doi: 10.1177/0886260517718830.
3. National Coalition of Anti-Violence Programs (NCAVP). Lesbian, Gay, Bisexual, Transgender, Queer and HIV-Affected Hate Violence in 2016.
4. J Sch Health. 2011 May;81(5):223-30.
5. Prev Sci. 2015 Jul;16(5):734-43.
6. Dev Psychol. 2010 Nov;46(6):1580-9.
7. Psychol Bull. 2003 Sep;129(5):674-97.
8. Gallup. Americans Rate Healthcare Providers High on Honesty, Ethics. 2016.
9. The Hippocratic Oath Today. 2001 or Do. No. Harm.
It may be unusual for an LGBT health columnist to mention the horrendous events that occurred in Charlottesville, Va., in August 2017. It clearly was a demonstration of hate and violence against racial and ethnic minorities. Unfortunately, the LGBT community – especially LGBT communities of color – are often a target of that kind of hate and violence. This has a detrimental effect on the health of the LGBT community, and I believe that health care providers have a responsibility to address this hate and violence to promote the well-being of this marginalized community.
It cannot be overstated that LGBT individuals frequently experience anti-gay and anti-trans violence. According to the 2015 Federal Bureau of Investigation Hate Crime Statistics, about a fifth of hate crimes reported were based on sexual orientation or gender identity.1 In addition, LGBT youth are eight times as likely to experience bullying at school because of their sexual orientation or gender identity.2 Furthermore, on many surveys on anti-LGBT violence, people of color comprise more than half of the victims.3 There is a strong association between exposure to this violence and the health outcomes of LGBT youth. A study by Russell et al. showed that LGBT youth who were victims of physical violence at school are more likely to be depressed and suicidal and more likely to be diagnosed with an STD,4 and another study showed that LGBT youth who experienced anti-LGBT violence are more likely to engage in substance use.5 The health outcomes from anti-LGBT violence are not limited to the adolescent period – adolescents who experienced this kind of violence are more likely to report higher levels of depression as adults.6 Although researchers still are trying to determine the exact mechanism for these relationships, the most cited (and sensible) explanation is that exposure to anti-LGBT stigma, discrimination, and violence leads to a toxic environment, which in turn increases the risk for mental health problems and maladaptive coping mechanisms (such as substance use) as a response to such an environment.7
What can we do to stand up to the hate and violence against marginalized groups, such as the LGBT community? First, make your office a safe space. With the recent brazen display of hate and violence going around, members the LGBT community are desperate to feel protected. A good place to start is a guide by Advocates for Youth. Second, educate yourself and others. The title physician means “teacher,” and I feel it is your responsibility to teach your peers, colleagues, and the public about how anti-LGBT violence affects the health of LGBT individuals. To be an effective teacher, you need to be up to date on the research on how hatred and intolerance affects the health of the LGBT community. A good place to start is the Human Rights Campaign, which has accurate statistics on anti-LGBT violence and resources to address this problem. Finally, be an advocate. You don’t need to be in the streets with picket signs, nor do you necessarily need to lead the charge against anti-LGBT hate and violence – others will be at the front lines. What you can do is to call for your local, state, and federal government to institute policies that address anti-LGBT violence. Many medical organizations have resources that help health care providers engage with policy makers (check out the American Academy of Pediatrics advocacy page for these resources). Many of our elected officials take our professional opinions seriously.
Anti-gay and anti-trans violence is all too common in the LGBT community, especially violence against LGBT people of color, and this violence can adversely affect their health. Health care providers have a responsibility and the influence to confront these nexuses of hate and intolerance. You don’t need to do something heroic to accomplish this. You are members of a privileged and respected group of professionals, so small actions can coalesce into something that has a large impact on the health and well-being of the communities you serve.
Resources
• Advocates for Youth. Creating Safe Space for GLBTQ Youth: A Toolkit
• Human Rights Campaign. www.hrc.org/resources/
• American Academy of Pediatrics advocacy page: www.aap.org/en-us/advocacy-and-policy/
References
1. U.S. Department of Justice Federal Bureau of Investigation. Uniform Crime Report Hate Crime Statistics, 2015.
2. J Interpers Violence. 2017. doi: 10.1177/0886260517718830.
3. National Coalition of Anti-Violence Programs (NCAVP). Lesbian, Gay, Bisexual, Transgender, Queer and HIV-Affected Hate Violence in 2016.
4. J Sch Health. 2011 May;81(5):223-30.
5. Prev Sci. 2015 Jul;16(5):734-43.
6. Dev Psychol. 2010 Nov;46(6):1580-9.
7. Psychol Bull. 2003 Sep;129(5):674-97.
8. Gallup. Americans Rate Healthcare Providers High on Honesty, Ethics. 2016.
9. The Hippocratic Oath Today. 2001 or Do. No. Harm.
Cerebral palsy rate down in children born very or moderately preterm
, even in children who were born moderately preterm.
The researchers gathered data for cerebral palsy from the medical questionnaire, including information on head control, sitting, standing, walking, and quality of gait; trunk and limb tone; and any abnormal neurologic signs. Development was assessed using the second version of the 24-month Ages and Stages Questionnaire (ASQ), which is completed by parents.
Of 4,199 neonates born between 22 and 34 weeks’ gestation in 2011 enrolled in the EPIPAGE-2 study who lived until a median of 24.2 months corrected age, the rate of cerebral palsy dropped from 7% to 4% in those born between 24-26 and 27-31 weeks’ gestation, reported Véronique Pierrat, MD, PhD, of the Epidemiology and Biostatistics Sorbonne Paris Cité Research Center, INSERM, in Paris, and her associates. At 32-34 weeks’ gestation, the cerebral palsy rate was 1%. Only one child born at 22-23 weeks’ gestation lived beyond the neonatal period. Fewer than 1% of the children overall had severe auditory or visual impairment.
ASQ analysis was considered for 2,506 children, after excluding children with cerebral palsy, deafness or blindness, or severe congenital brain malformations. ASQ scores were below threshold in 50%, 41%, and 36% of children born at 24-26, 27-31, and 32-34 weeks’ gestation, respectively. “The domains most frequently scoring below threshold were communication and personal-social in all gestational age groups. Proportions of children scoring below the threshold in either of these domains decreased with increasing gestational age but still were 18% and 13%, respectively, at 32-34 weeks’ gestation,” the investigators said.
Only 1% or fewer of the children in this cohort had severe gastrointestinal or respiratory disabilities.
In a comparison of 1997 data and this 2011 data after adjustment for baseline characteristics in children born at 22-31 weeks’ gestation, survival increased by a mean 6% , and survival without neuromotor or sensory impairment by 7%; in children born at 24-31 weeks’ gestation, cerebral palsy decreased by a mean 3%. No statistically significant changes were found between the two periods for survival, survival without neuromotor or sensory disabilities, and rates of cerebral palsy in children born at 24 weeks’ gestation, but “noticeable improvements were seen at 25-26 weeks and, to a lesser extent, at 27-31 weeks,” Dr. Pierrat and her associates said. At 32-34 weeks’ gestation, the cerebral palsy rate dropped by 3%, but survival and survival without severe neuromotor or sensory impairment were similar between the two time periods.
In regard to the 2011 data, “the proportion of infants at risk of developmental delay was high, even for those born at 32-34 weeks’ gestation. Our results invite questioning perinatal strategies in France, and in countries with similar recommendations. However, improving outcomes at extremely low gestational age requires a complex change in philosophy of care and close cooperation not only between obstetricians and neonatologists, but also developmental specialists, parent associations, and policy makers,” Dr. Pierrat and her associates concluded.
The investigators said they had no relevant financial disclosures. The study was funded by the French Institute of Public Health Research/Institute of Public Health and several of its partners, the PREMUP Foundation, Fondation de France, and Fondation pour la Recherche Médicale.
, even in children who were born moderately preterm.
The researchers gathered data for cerebral palsy from the medical questionnaire, including information on head control, sitting, standing, walking, and quality of gait; trunk and limb tone; and any abnormal neurologic signs. Development was assessed using the second version of the 24-month Ages and Stages Questionnaire (ASQ), which is completed by parents.
Of 4,199 neonates born between 22 and 34 weeks’ gestation in 2011 enrolled in the EPIPAGE-2 study who lived until a median of 24.2 months corrected age, the rate of cerebral palsy dropped from 7% to 4% in those born between 24-26 and 27-31 weeks’ gestation, reported Véronique Pierrat, MD, PhD, of the Epidemiology and Biostatistics Sorbonne Paris Cité Research Center, INSERM, in Paris, and her associates. At 32-34 weeks’ gestation, the cerebral palsy rate was 1%. Only one child born at 22-23 weeks’ gestation lived beyond the neonatal period. Fewer than 1% of the children overall had severe auditory or visual impairment.
ASQ analysis was considered for 2,506 children, after excluding children with cerebral palsy, deafness or blindness, or severe congenital brain malformations. ASQ scores were below threshold in 50%, 41%, and 36% of children born at 24-26, 27-31, and 32-34 weeks’ gestation, respectively. “The domains most frequently scoring below threshold were communication and personal-social in all gestational age groups. Proportions of children scoring below the threshold in either of these domains decreased with increasing gestational age but still were 18% and 13%, respectively, at 32-34 weeks’ gestation,” the investigators said.
Only 1% or fewer of the children in this cohort had severe gastrointestinal or respiratory disabilities.
In a comparison of 1997 data and this 2011 data after adjustment for baseline characteristics in children born at 22-31 weeks’ gestation, survival increased by a mean 6% , and survival without neuromotor or sensory impairment by 7%; in children born at 24-31 weeks’ gestation, cerebral palsy decreased by a mean 3%. No statistically significant changes were found between the two periods for survival, survival without neuromotor or sensory disabilities, and rates of cerebral palsy in children born at 24 weeks’ gestation, but “noticeable improvements were seen at 25-26 weeks and, to a lesser extent, at 27-31 weeks,” Dr. Pierrat and her associates said. At 32-34 weeks’ gestation, the cerebral palsy rate dropped by 3%, but survival and survival without severe neuromotor or sensory impairment were similar between the two time periods.
In regard to the 2011 data, “the proportion of infants at risk of developmental delay was high, even for those born at 32-34 weeks’ gestation. Our results invite questioning perinatal strategies in France, and in countries with similar recommendations. However, improving outcomes at extremely low gestational age requires a complex change in philosophy of care and close cooperation not only between obstetricians and neonatologists, but also developmental specialists, parent associations, and policy makers,” Dr. Pierrat and her associates concluded.
The investigators said they had no relevant financial disclosures. The study was funded by the French Institute of Public Health Research/Institute of Public Health and several of its partners, the PREMUP Foundation, Fondation de France, and Fondation pour la Recherche Médicale.
, even in children who were born moderately preterm.
The researchers gathered data for cerebral palsy from the medical questionnaire, including information on head control, sitting, standing, walking, and quality of gait; trunk and limb tone; and any abnormal neurologic signs. Development was assessed using the second version of the 24-month Ages and Stages Questionnaire (ASQ), which is completed by parents.
Of 4,199 neonates born between 22 and 34 weeks’ gestation in 2011 enrolled in the EPIPAGE-2 study who lived until a median of 24.2 months corrected age, the rate of cerebral palsy dropped from 7% to 4% in those born between 24-26 and 27-31 weeks’ gestation, reported Véronique Pierrat, MD, PhD, of the Epidemiology and Biostatistics Sorbonne Paris Cité Research Center, INSERM, in Paris, and her associates. At 32-34 weeks’ gestation, the cerebral palsy rate was 1%. Only one child born at 22-23 weeks’ gestation lived beyond the neonatal period. Fewer than 1% of the children overall had severe auditory or visual impairment.
ASQ analysis was considered for 2,506 children, after excluding children with cerebral palsy, deafness or blindness, or severe congenital brain malformations. ASQ scores were below threshold in 50%, 41%, and 36% of children born at 24-26, 27-31, and 32-34 weeks’ gestation, respectively. “The domains most frequently scoring below threshold were communication and personal-social in all gestational age groups. Proportions of children scoring below the threshold in either of these domains decreased with increasing gestational age but still were 18% and 13%, respectively, at 32-34 weeks’ gestation,” the investigators said.
Only 1% or fewer of the children in this cohort had severe gastrointestinal or respiratory disabilities.
In a comparison of 1997 data and this 2011 data after adjustment for baseline characteristics in children born at 22-31 weeks’ gestation, survival increased by a mean 6% , and survival without neuromotor or sensory impairment by 7%; in children born at 24-31 weeks’ gestation, cerebral palsy decreased by a mean 3%. No statistically significant changes were found between the two periods for survival, survival without neuromotor or sensory disabilities, and rates of cerebral palsy in children born at 24 weeks’ gestation, but “noticeable improvements were seen at 25-26 weeks and, to a lesser extent, at 27-31 weeks,” Dr. Pierrat and her associates said. At 32-34 weeks’ gestation, the cerebral palsy rate dropped by 3%, but survival and survival without severe neuromotor or sensory impairment were similar between the two time periods.
In regard to the 2011 data, “the proportion of infants at risk of developmental delay was high, even for those born at 32-34 weeks’ gestation. Our results invite questioning perinatal strategies in France, and in countries with similar recommendations. However, improving outcomes at extremely low gestational age requires a complex change in philosophy of care and close cooperation not only between obstetricians and neonatologists, but also developmental specialists, parent associations, and policy makers,” Dr. Pierrat and her associates concluded.
The investigators said they had no relevant financial disclosures. The study was funded by the French Institute of Public Health Research/Institute of Public Health and several of its partners, the PREMUP Foundation, Fondation de France, and Fondation pour la Recherche Médicale.
FROM BMJ
Key clinical point: Cerebral palsy rates are down in children born very and moderately preterm, but the risk of developmental delay remains high.
Major finding: ASQ scores were below threshold in 50%, 41%, and 36% of children born at 24-26, 27-31, and 32-34 weeks’ gestation, respectively.
Data source: A national population study of 4,199 French neonates born between 22 and 34 weeks’ gestation in 2011.
Disclosures: The investigators said they had no relevant financial disclosures. The study was funded by the French Institute of Public Health Research/Institute of Public Health and several of its partners, the PREMUP Foundation, Fondation de France, and Fondation pour la Recherche Médicale.
Standardized infection ratio for CLABSI almost halved since 2009
The standardized infection ratio (SIR) for central line–associated bloodstream infections dropped 42% from 2009 to 2014, according to the Agency for Healthcare Research and Quality.
For acute care hospitalizations, the SIR for central line–associated bloodstream infections (CLABSIs) fell from 0.854 in 2009 to 0.495 in 2014. Over that same time period, the SIR for surgical site infections involving Surgical Care Improvement Project procedures decreased from 0.981 to 0.827 – almost 16%, the AHRQ said in its annual National Healthcare Quality and Disparities Report.
From 2010 to 2014, the SIR for catheter-associated urinary tract infections increased 6.7% from 0.937 to 1.000, but that change was not significant. For laboratory-identified hospital-onset Clostridium difficile infection, the SIR dropped from 0.963 to 0.924 – about 4% – from 2012 to 2014, the AHRQ reported using data from the National Center for Emerging and Zoonotic Infectious Diseases and the National Healthcare Safety Network.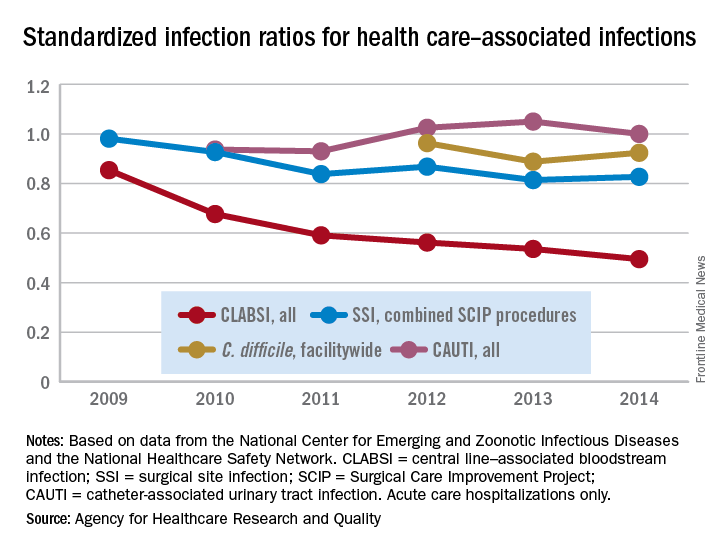
The standardized infection ratio (SIR) for central line–associated bloodstream infections dropped 42% from 2009 to 2014, according to the Agency for Healthcare Research and Quality.
For acute care hospitalizations, the SIR for central line–associated bloodstream infections (CLABSIs) fell from 0.854 in 2009 to 0.495 in 2014. Over that same time period, the SIR for surgical site infections involving Surgical Care Improvement Project procedures decreased from 0.981 to 0.827 – almost 16%, the AHRQ said in its annual National Healthcare Quality and Disparities Report.
From 2010 to 2014, the SIR for catheter-associated urinary tract infections increased 6.7% from 0.937 to 1.000, but that change was not significant. For laboratory-identified hospital-onset Clostridium difficile infection, the SIR dropped from 0.963 to 0.924 – about 4% – from 2012 to 2014, the AHRQ reported using data from the National Center for Emerging and Zoonotic Infectious Diseases and the National Healthcare Safety Network.
The standardized infection ratio (SIR) for central line–associated bloodstream infections dropped 42% from 2009 to 2014, according to the Agency for Healthcare Research and Quality.
For acute care hospitalizations, the SIR for central line–associated bloodstream infections (CLABSIs) fell from 0.854 in 2009 to 0.495 in 2014. Over that same time period, the SIR for surgical site infections involving Surgical Care Improvement Project procedures decreased from 0.981 to 0.827 – almost 16%, the AHRQ said in its annual National Healthcare Quality and Disparities Report.
From 2010 to 2014, the SIR for catheter-associated urinary tract infections increased 6.7% from 0.937 to 1.000, but that change was not significant. For laboratory-identified hospital-onset Clostridium difficile infection, the SIR dropped from 0.963 to 0.924 – about 4% – from 2012 to 2014, the AHRQ reported using data from the National Center for Emerging and Zoonotic Infectious Diseases and the National Healthcare Safety Network.
Rare Case of Orbital Involvement from Multiple Myeloma
An orbital mass is often the “tip of the iceberg”—it may be secondary to systemic malignancy, warn clinicians from University Sains Malaysia-Health Campus and Hospital Sultanah Bahiyah, both in Malaysia. Orbital metastases usually originate from lung and breast cancers, but these authors report on an unusual case of a patient whose orbital involvement stemmed from multiple myeloma (MM).
Related: A Mysterious Massive Hemorrhage
The 85-year-old woman presented with right-eye proptosis, reduced visual acuity and diplopia. She had been bedridden with chronic back pain but had no symptoms of thyroid disorder or malignancy. Cardiovascular, breast, abdominal, and neurologic examinations were normal. She had no palpable lymph nodes. Blood investigations for infective and inflammatory causes were unremarkable.
However, a chest radiograph showed osteopenic bones, a pathologic fracture of the right clavicle, and an opacity obscuring the left retrocardiac region, suggesting a mass in the lower lobe of the left lung. The patient declined further imaging but underwent biopsy for the right orbital mass. Histopathologic examination revealed cells suggestive of MM. She was diagnosed with osseous plasmacytoma.
Orbital involvement in MM may be the first manifestation of systemic disease, the clinicians say. The diagnosis is usually based on clinical suspicion. Patients tend to present with nonspecific symptoms like back pain and fatigue. Computed tomography scanning is the imaging modality of choice, the authors say, but in older patients the findings may be hard to interpret. Thinning of the bone, for instance, may mimic metastases. Biopsy provides a definitive diagnosis and guides further management.
Related: Less Lenalidomide May Be More in Frail Elderly Multiple Myeloma Patients
Orbital involvement in MM is rare but treatable. Discovery of a plasmacytoma should always prompt investigation for systemic involvement, the authors advise, because the treatment and prognosis differ between the two. In their patient, proptosis secondary to the orbital plasmacytoma led them to discover end-organ damage in the form of multiple bone lesions. Solitary plasmacytoma would be treated with radiotherapy and resection; active MM with end-organ damage requires systemic chemotherapy.
Getting to the root of the problem can be difficult when the presentation is “insidious” and clinical features are nonspecific, the authors say. Patient and thorough investigation can make the difference in resolving the diagnostic imaging challenges.
Source:
Tai E, Sim SK, Haron J, Wan Hitam WH. BMJ Case Rep. 2017;2017: pii: bcr-2017-220895.
doi: 10.1136/bcr-2017-220895.
An orbital mass is often the “tip of the iceberg”—it may be secondary to systemic malignancy, warn clinicians from University Sains Malaysia-Health Campus and Hospital Sultanah Bahiyah, both in Malaysia. Orbital metastases usually originate from lung and breast cancers, but these authors report on an unusual case of a patient whose orbital involvement stemmed from multiple myeloma (MM).
Related: A Mysterious Massive Hemorrhage
The 85-year-old woman presented with right-eye proptosis, reduced visual acuity and diplopia. She had been bedridden with chronic back pain but had no symptoms of thyroid disorder or malignancy. Cardiovascular, breast, abdominal, and neurologic examinations were normal. She had no palpable lymph nodes. Blood investigations for infective and inflammatory causes were unremarkable.
However, a chest radiograph showed osteopenic bones, a pathologic fracture of the right clavicle, and an opacity obscuring the left retrocardiac region, suggesting a mass in the lower lobe of the left lung. The patient declined further imaging but underwent biopsy for the right orbital mass. Histopathologic examination revealed cells suggestive of MM. She was diagnosed with osseous plasmacytoma.
Orbital involvement in MM may be the first manifestation of systemic disease, the clinicians say. The diagnosis is usually based on clinical suspicion. Patients tend to present with nonspecific symptoms like back pain and fatigue. Computed tomography scanning is the imaging modality of choice, the authors say, but in older patients the findings may be hard to interpret. Thinning of the bone, for instance, may mimic metastases. Biopsy provides a definitive diagnosis and guides further management.
Related: Less Lenalidomide May Be More in Frail Elderly Multiple Myeloma Patients
Orbital involvement in MM is rare but treatable. Discovery of a plasmacytoma should always prompt investigation for systemic involvement, the authors advise, because the treatment and prognosis differ between the two. In their patient, proptosis secondary to the orbital plasmacytoma led them to discover end-organ damage in the form of multiple bone lesions. Solitary plasmacytoma would be treated with radiotherapy and resection; active MM with end-organ damage requires systemic chemotherapy.
Getting to the root of the problem can be difficult when the presentation is “insidious” and clinical features are nonspecific, the authors say. Patient and thorough investigation can make the difference in resolving the diagnostic imaging challenges.
Source:
Tai E, Sim SK, Haron J, Wan Hitam WH. BMJ Case Rep. 2017;2017: pii: bcr-2017-220895.
doi: 10.1136/bcr-2017-220895.
An orbital mass is often the “tip of the iceberg”—it may be secondary to systemic malignancy, warn clinicians from University Sains Malaysia-Health Campus and Hospital Sultanah Bahiyah, both in Malaysia. Orbital metastases usually originate from lung and breast cancers, but these authors report on an unusual case of a patient whose orbital involvement stemmed from multiple myeloma (MM).
Related: A Mysterious Massive Hemorrhage
The 85-year-old woman presented with right-eye proptosis, reduced visual acuity and diplopia. She had been bedridden with chronic back pain but had no symptoms of thyroid disorder or malignancy. Cardiovascular, breast, abdominal, and neurologic examinations were normal. She had no palpable lymph nodes. Blood investigations for infective and inflammatory causes were unremarkable.
However, a chest radiograph showed osteopenic bones, a pathologic fracture of the right clavicle, and an opacity obscuring the left retrocardiac region, suggesting a mass in the lower lobe of the left lung. The patient declined further imaging but underwent biopsy for the right orbital mass. Histopathologic examination revealed cells suggestive of MM. She was diagnosed with osseous plasmacytoma.
Orbital involvement in MM may be the first manifestation of systemic disease, the clinicians say. The diagnosis is usually based on clinical suspicion. Patients tend to present with nonspecific symptoms like back pain and fatigue. Computed tomography scanning is the imaging modality of choice, the authors say, but in older patients the findings may be hard to interpret. Thinning of the bone, for instance, may mimic metastases. Biopsy provides a definitive diagnosis and guides further management.
Related: Less Lenalidomide May Be More in Frail Elderly Multiple Myeloma Patients
Orbital involvement in MM is rare but treatable. Discovery of a plasmacytoma should always prompt investigation for systemic involvement, the authors advise, because the treatment and prognosis differ between the two. In their patient, proptosis secondary to the orbital plasmacytoma led them to discover end-organ damage in the form of multiple bone lesions. Solitary plasmacytoma would be treated with radiotherapy and resection; active MM with end-organ damage requires systemic chemotherapy.
Getting to the root of the problem can be difficult when the presentation is “insidious” and clinical features are nonspecific, the authors say. Patient and thorough investigation can make the difference in resolving the diagnostic imaging challenges.
Source:
Tai E, Sim SK, Haron J, Wan Hitam WH. BMJ Case Rep. 2017;2017: pii: bcr-2017-220895.
doi: 10.1136/bcr-2017-220895.
Vitamin C could help treat TET2-mutant leukemias
Preclinical research suggests high-dose vitamin C may be effective against TET2-mutant leukemias.
Investigators found that vitamin C mimics TET2 restoration, thereby suppressing leukemic colony formation, inhibiting leukemia progression in mice, and enhancing leukemia cells’ sensitivity to treatment with a PARP inhibitor.
“We’re excited by the prospect that high-dose vitamin C might become a safe treatment for blood diseases caused by TET2-deficient leukemia stem cells, most likely in combination with other targeted therapies,” said study author Benjamin G. Neel, MD, PhD, of NYU School of Medicine in New York, New York.
Dr Neel and his colleagues reported their findings in Cell.
Previous research had shown that vitamin C could stimulate the activity of TET2 as well as TET1 and TET3.
Because only 1 copy of the TET2 gene in each stem cell is usually affected in TET2-mutant blood diseases, the investigators hypothesized that high doses of vitamin C might reverse the effects of TET2 deficiency by turning up the action of the remaining functional gene.
Indeed, the team found that vitamin C had the same effect as restoring TET2 function genetically. By promoting DNA demethylation, high-dose vitamin C induced stem cells to mature and suppressed the growth of leukemic stem cells (LSCs) implanted in mice.
“Interestingly, we also found that vitamin C treatment had an effect on leukemic stem cells that resembled damage to their DNA,” said study author Luisa Cimmino, PhD, of NYU School of Medicine.
“For this reason, we decided to combine vitamin C with a PARP inhibitor, a drug type known to cause cancer cell death by blocking the repair of DNA damage, and already approved for treating certain patients with ovarian cancer.”
The combination had an enhanced effect on LSCs, further shifting them from self-renewal back toward maturity and cell death.
Dr Cimmino said these results suggest vitamin C might also be effective against leukemias without TET2 mutations. As vitamin C turns up any TET2 activity normally in place, it might drive LSCs without TET2 mutations toward death as well. ![]()
Preclinical research suggests high-dose vitamin C may be effective against TET2-mutant leukemias.
Investigators found that vitamin C mimics TET2 restoration, thereby suppressing leukemic colony formation, inhibiting leukemia progression in mice, and enhancing leukemia cells’ sensitivity to treatment with a PARP inhibitor.
“We’re excited by the prospect that high-dose vitamin C might become a safe treatment for blood diseases caused by TET2-deficient leukemia stem cells, most likely in combination with other targeted therapies,” said study author Benjamin G. Neel, MD, PhD, of NYU School of Medicine in New York, New York.
Dr Neel and his colleagues reported their findings in Cell.
Previous research had shown that vitamin C could stimulate the activity of TET2 as well as TET1 and TET3.
Because only 1 copy of the TET2 gene in each stem cell is usually affected in TET2-mutant blood diseases, the investigators hypothesized that high doses of vitamin C might reverse the effects of TET2 deficiency by turning up the action of the remaining functional gene.
Indeed, the team found that vitamin C had the same effect as restoring TET2 function genetically. By promoting DNA demethylation, high-dose vitamin C induced stem cells to mature and suppressed the growth of leukemic stem cells (LSCs) implanted in mice.
“Interestingly, we also found that vitamin C treatment had an effect on leukemic stem cells that resembled damage to their DNA,” said study author Luisa Cimmino, PhD, of NYU School of Medicine.
“For this reason, we decided to combine vitamin C with a PARP inhibitor, a drug type known to cause cancer cell death by blocking the repair of DNA damage, and already approved for treating certain patients with ovarian cancer.”
The combination had an enhanced effect on LSCs, further shifting them from self-renewal back toward maturity and cell death.
Dr Cimmino said these results suggest vitamin C might also be effective against leukemias without TET2 mutations. As vitamin C turns up any TET2 activity normally in place, it might drive LSCs without TET2 mutations toward death as well. ![]()
Preclinical research suggests high-dose vitamin C may be effective against TET2-mutant leukemias.
Investigators found that vitamin C mimics TET2 restoration, thereby suppressing leukemic colony formation, inhibiting leukemia progression in mice, and enhancing leukemia cells’ sensitivity to treatment with a PARP inhibitor.
“We’re excited by the prospect that high-dose vitamin C might become a safe treatment for blood diseases caused by TET2-deficient leukemia stem cells, most likely in combination with other targeted therapies,” said study author Benjamin G. Neel, MD, PhD, of NYU School of Medicine in New York, New York.
Dr Neel and his colleagues reported their findings in Cell.
Previous research had shown that vitamin C could stimulate the activity of TET2 as well as TET1 and TET3.
Because only 1 copy of the TET2 gene in each stem cell is usually affected in TET2-mutant blood diseases, the investigators hypothesized that high doses of vitamin C might reverse the effects of TET2 deficiency by turning up the action of the remaining functional gene.
Indeed, the team found that vitamin C had the same effect as restoring TET2 function genetically. By promoting DNA demethylation, high-dose vitamin C induced stem cells to mature and suppressed the growth of leukemic stem cells (LSCs) implanted in mice.
“Interestingly, we also found that vitamin C treatment had an effect on leukemic stem cells that resembled damage to their DNA,” said study author Luisa Cimmino, PhD, of NYU School of Medicine.
“For this reason, we decided to combine vitamin C with a PARP inhibitor, a drug type known to cause cancer cell death by blocking the repair of DNA damage, and already approved for treating certain patients with ovarian cancer.”
The combination had an enhanced effect on LSCs, further shifting them from self-renewal back toward maturity and cell death.
Dr Cimmino said these results suggest vitamin C might also be effective against leukemias without TET2 mutations. As vitamin C turns up any TET2 activity normally in place, it might drive LSCs without TET2 mutations toward death as well. ![]()
AAP releases revised guidelines on screening, treatment of hypertension
which includes revised BP tables based on normal-weight children only.
The document, published Aug. 21 in Pediatrics, is the first update since 2004, and recommends significant changes in both screening and treatment of hypertension (HTN).
The guidelines also include a simplified screening table for initial screening, which lists the 90th percentile BP for age and sex, for children at the fifth percentile of height. These values give the table a negative predictive value of greater than 99%, although the committee stressed that the table should only be used for screening, and not for diagnosis.
“To diagnose elevated BP or HTN, it is important to locate the actual cutoffs in the complete BP tables because the [systolic] BP and [diastolic] BP cutoffs may be as much as 9 mm Hg higher depending on a child’s age and length or height,” wrote Joseph T. Flynn, MD, and his colleagues on the AAP subcommittee on screening and management of high blood pressure in children.
To ensure consistency between these guidelines and the 2017 adult guidelines from the American Heart Association and American College of Cardiology, the committee also decided to replace the term “prehypertension” with “elevated blood pressure.”
Similarly, the committee recommended adopting revised stage 1 and stage 2 HTN criteria, to enable the staging scheme for children aged 13 years and over to “seamlessly interface” with the 2017 AHA and ACC adult guidelines.
“There are still no data to identify a specific level of BP in childhood that leads to adverse [cardiovascular] outcomes in adulthood,” the committee wrote. “Therefore, the subcommittee decided to maintain a statistical definition for childhood HTN.”
In terms of screening children for hypertension, the guidelines review committee made the recommendation that BP be measured annually in children and adolescents aged 3 years or above. However, if the child is at greater risk of hypertension because of obesity, medications known to increase BP – such as stimulants for ADHD – renal disease, a history of aortic arch obstruction or coarctation, or have diabetes, they should have their BP measured at every health care encounter.
They also stressed it was important to take more than one measurement over time before diagnosing HTN, and to use appropriately-sized cuffs to ensure an accurate measurement.
If a child or adolescent has confirmed auscultatory BP readings at or above the 95th percentile on three different visits, this justifies a diagnosis of HTN, they wrote.
The committee strongly recommended the routine performance of ambulatory BP monitoring in patients with high-risk conditions, such as diabetes, secondary hypertension, or renal disease, to look for abnormal circadian BP patterns that might point to an increased risk of target organ damage.
They also issued revised recommendations on when to perform echocardiography in children newly diagnosed with HTN.
“It is recommended that echocardiography be performed to assess for cardiac target organ damage (left ventricular mass, geometry, and function) at the time of consideration of pharmacologic treatment of HTN,” they wrote, suggesting repeat echocardiography could be used to monitor target organ damage at 6-12 month intervals.
They offered a revised definition of left ventricular HTN as a left ventricular mass greater than 51 g/m2.7 for children and adolescents older than 8 years (greater than 115 g/body surface area for boys and greater than 95 g/body surface area for girls).
While previous treatment recommendations used a treatment target of a systolic and diastolic BP below the 95th percentile in children without chronic kidney disease, new evidence prompted a revised recommendation of a target either below the 90th percentile or less than 130/80 mm Hg.
“Longitudinal studies on BP from childhood to adulthood that include indirect measures of [cardiovascular] injury indicate that the risk for subsequent [cardiovascular disease] in early adulthood increases as the BP level in adolescence exceeds 120/80 mm Hg,” they wrote. “In addition, there is some evidence that targeting a BP less than 90th percentile results in reductions in [left ventricular mass index] and prevalence of [left ventricular hypertrophy].”
In hypertensive children and adolescents who have failed lifestyle modifications, such as physical activity, weight loss, and stress reduction (particularly those who have left ventricular hypertrophy, symptomatic HTN, or stage 2 HTN without a clearly modifiable factor such as obesity), pharmacologic treatment with an angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, long-acting calcium channel blocker, or thiazide diuretic is indicated, according to the revised guidelines.
The guidelines were supported by the American Academy of Pediatrics and endorsed by the American Heart Association. No conflicts of interest were declared.
which includes revised BP tables based on normal-weight children only.
The document, published Aug. 21 in Pediatrics, is the first update since 2004, and recommends significant changes in both screening and treatment of hypertension (HTN).
The guidelines also include a simplified screening table for initial screening, which lists the 90th percentile BP for age and sex, for children at the fifth percentile of height. These values give the table a negative predictive value of greater than 99%, although the committee stressed that the table should only be used for screening, and not for diagnosis.
“To diagnose elevated BP or HTN, it is important to locate the actual cutoffs in the complete BP tables because the [systolic] BP and [diastolic] BP cutoffs may be as much as 9 mm Hg higher depending on a child’s age and length or height,” wrote Joseph T. Flynn, MD, and his colleagues on the AAP subcommittee on screening and management of high blood pressure in children.
To ensure consistency between these guidelines and the 2017 adult guidelines from the American Heart Association and American College of Cardiology, the committee also decided to replace the term “prehypertension” with “elevated blood pressure.”
Similarly, the committee recommended adopting revised stage 1 and stage 2 HTN criteria, to enable the staging scheme for children aged 13 years and over to “seamlessly interface” with the 2017 AHA and ACC adult guidelines.
“There are still no data to identify a specific level of BP in childhood that leads to adverse [cardiovascular] outcomes in adulthood,” the committee wrote. “Therefore, the subcommittee decided to maintain a statistical definition for childhood HTN.”
In terms of screening children for hypertension, the guidelines review committee made the recommendation that BP be measured annually in children and adolescents aged 3 years or above. However, if the child is at greater risk of hypertension because of obesity, medications known to increase BP – such as stimulants for ADHD – renal disease, a history of aortic arch obstruction or coarctation, or have diabetes, they should have their BP measured at every health care encounter.
They also stressed it was important to take more than one measurement over time before diagnosing HTN, and to use appropriately-sized cuffs to ensure an accurate measurement.
If a child or adolescent has confirmed auscultatory BP readings at or above the 95th percentile on three different visits, this justifies a diagnosis of HTN, they wrote.
The committee strongly recommended the routine performance of ambulatory BP monitoring in patients with high-risk conditions, such as diabetes, secondary hypertension, or renal disease, to look for abnormal circadian BP patterns that might point to an increased risk of target organ damage.
They also issued revised recommendations on when to perform echocardiography in children newly diagnosed with HTN.
“It is recommended that echocardiography be performed to assess for cardiac target organ damage (left ventricular mass, geometry, and function) at the time of consideration of pharmacologic treatment of HTN,” they wrote, suggesting repeat echocardiography could be used to monitor target organ damage at 6-12 month intervals.
They offered a revised definition of left ventricular HTN as a left ventricular mass greater than 51 g/m2.7 for children and adolescents older than 8 years (greater than 115 g/body surface area for boys and greater than 95 g/body surface area for girls).
While previous treatment recommendations used a treatment target of a systolic and diastolic BP below the 95th percentile in children without chronic kidney disease, new evidence prompted a revised recommendation of a target either below the 90th percentile or less than 130/80 mm Hg.
“Longitudinal studies on BP from childhood to adulthood that include indirect measures of [cardiovascular] injury indicate that the risk for subsequent [cardiovascular disease] in early adulthood increases as the BP level in adolescence exceeds 120/80 mm Hg,” they wrote. “In addition, there is some evidence that targeting a BP less than 90th percentile results in reductions in [left ventricular mass index] and prevalence of [left ventricular hypertrophy].”
In hypertensive children and adolescents who have failed lifestyle modifications, such as physical activity, weight loss, and stress reduction (particularly those who have left ventricular hypertrophy, symptomatic HTN, or stage 2 HTN without a clearly modifiable factor such as obesity), pharmacologic treatment with an angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, long-acting calcium channel blocker, or thiazide diuretic is indicated, according to the revised guidelines.
The guidelines were supported by the American Academy of Pediatrics and endorsed by the American Heart Association. No conflicts of interest were declared.
which includes revised BP tables based on normal-weight children only.
The document, published Aug. 21 in Pediatrics, is the first update since 2004, and recommends significant changes in both screening and treatment of hypertension (HTN).
The guidelines also include a simplified screening table for initial screening, which lists the 90th percentile BP for age and sex, for children at the fifth percentile of height. These values give the table a negative predictive value of greater than 99%, although the committee stressed that the table should only be used for screening, and not for diagnosis.
“To diagnose elevated BP or HTN, it is important to locate the actual cutoffs in the complete BP tables because the [systolic] BP and [diastolic] BP cutoffs may be as much as 9 mm Hg higher depending on a child’s age and length or height,” wrote Joseph T. Flynn, MD, and his colleagues on the AAP subcommittee on screening and management of high blood pressure in children.
To ensure consistency between these guidelines and the 2017 adult guidelines from the American Heart Association and American College of Cardiology, the committee also decided to replace the term “prehypertension” with “elevated blood pressure.”
Similarly, the committee recommended adopting revised stage 1 and stage 2 HTN criteria, to enable the staging scheme for children aged 13 years and over to “seamlessly interface” with the 2017 AHA and ACC adult guidelines.
“There are still no data to identify a specific level of BP in childhood that leads to adverse [cardiovascular] outcomes in adulthood,” the committee wrote. “Therefore, the subcommittee decided to maintain a statistical definition for childhood HTN.”
In terms of screening children for hypertension, the guidelines review committee made the recommendation that BP be measured annually in children and adolescents aged 3 years or above. However, if the child is at greater risk of hypertension because of obesity, medications known to increase BP – such as stimulants for ADHD – renal disease, a history of aortic arch obstruction or coarctation, or have diabetes, they should have their BP measured at every health care encounter.
They also stressed it was important to take more than one measurement over time before diagnosing HTN, and to use appropriately-sized cuffs to ensure an accurate measurement.
If a child or adolescent has confirmed auscultatory BP readings at or above the 95th percentile on three different visits, this justifies a diagnosis of HTN, they wrote.
The committee strongly recommended the routine performance of ambulatory BP monitoring in patients with high-risk conditions, such as diabetes, secondary hypertension, or renal disease, to look for abnormal circadian BP patterns that might point to an increased risk of target organ damage.
They also issued revised recommendations on when to perform echocardiography in children newly diagnosed with HTN.
“It is recommended that echocardiography be performed to assess for cardiac target organ damage (left ventricular mass, geometry, and function) at the time of consideration of pharmacologic treatment of HTN,” they wrote, suggesting repeat echocardiography could be used to monitor target organ damage at 6-12 month intervals.
They offered a revised definition of left ventricular HTN as a left ventricular mass greater than 51 g/m2.7 for children and adolescents older than 8 years (greater than 115 g/body surface area for boys and greater than 95 g/body surface area for girls).
While previous treatment recommendations used a treatment target of a systolic and diastolic BP below the 95th percentile in children without chronic kidney disease, new evidence prompted a revised recommendation of a target either below the 90th percentile or less than 130/80 mm Hg.
“Longitudinal studies on BP from childhood to adulthood that include indirect measures of [cardiovascular] injury indicate that the risk for subsequent [cardiovascular disease] in early adulthood increases as the BP level in adolescence exceeds 120/80 mm Hg,” they wrote. “In addition, there is some evidence that targeting a BP less than 90th percentile results in reductions in [left ventricular mass index] and prevalence of [left ventricular hypertrophy].”
In hypertensive children and adolescents who have failed lifestyle modifications, such as physical activity, weight loss, and stress reduction (particularly those who have left ventricular hypertrophy, symptomatic HTN, or stage 2 HTN without a clearly modifiable factor such as obesity), pharmacologic treatment with an angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, long-acting calcium channel blocker, or thiazide diuretic is indicated, according to the revised guidelines.
The guidelines were supported by the American Academy of Pediatrics and endorsed by the American Heart Association. No conflicts of interest were declared.
FROM PEDIATRICS