User login
Cancer screening in elderly: When to just say no
ESTES PARK, COLO. – A simple walking speed measurement over a 20-foot distance is an invaluable guide to physiologic age as part of individualized decision making about when to stop cancer screening in elderly patients, according to Jeff Wallace, MD, professor of geriatric medicine at the University of Colorado at Denver.
“If you have one measurement to assess ‘am I aging well?’ it’s your gait speed. A lot of us in geriatrics are advocating evaluation of gait speed in all patients as a fifth vital sign. It’s probably more useful than blood pressure in some of the older adults coming into our clinics,” he said at a conference on internal medicine sponsored by the University of Colorado.
Dr. Wallace also gave a shout-out to the ePrognosis cancer-screening decision tool, available free at www.eprognosis.org, as an aid in shared decision-making conversations regarding when to stop cancer screening. This tool, developed by researchers at the University of California, San Francisco, allows physicians to plug key individual patient characteristics into its model, including comorbid conditions, functional status, and body mass index, and then spits out data-driven estimated benefits and harms a patient can expect from advanced-age screening for colon or breast cancer.
Of course, guidelines as to when to stop screening for various cancers are available from the U.S. Preventive Services Task Force, the American Cancer Society, and specialty societies. However, it’s important that nongeriatricians understand the serious limitations of those guidelines.
“We’re not guidelines followers in the geriatrics world because the guidelines don’t apply to most of our patients,” he explained. “We hate guidelines in geriatrics because few studies – and no lung cancer or breast cancer trials – enroll patients over age 75 with comorbid conditions. Also, most of these guidelines do not incorporate patient preferences, which probably should be a primary goal. So we’re left extrapolating.“
Regrettably, though, “it turns out most Americans are drinking the Kool-Aid when it comes to patient preferences. It’s amazing how much cancer screening is going on in this country. We’re doing a lot more than we should,” said Dr. Wallace.
All of that is clearly overscreening. Experts unanimously agree that if someone is not going to live for 10 years, that person is not likely to benefit from cancer screening. The one exception is lung cancer screening of high-risk patients, where there are data to show that annual low-dose CT screening is beneficial in those with even a 5-year life expectancy.
As part of the Choosing Wisely program, the American Geriatric Society has advocated that physicians “don’t recommend screening for breast, colorectal, prostate, or lung cancer without considering life expectancy and the risks of testing, overdiagnosis, and overtreatment.”
That’s where gait speed and ePrognosis come in handy in discussions with patients regarding what they can realistically expect from cancer screening at an advanced age.
The importance of gait speed was highlighted in a pooled analysis of nine cohort studies totaling more than 34,000 community-dwelling adults aged 65 years and older with 6-21 years of follow-up. Investigators at the University of Pittsburgh identified a strong relationship between gait speed and survival. Every 0.1-m/sec made a significant difference (JAMA. 2011 Jan 5;305[1]:50-8).
A gait speed evaluation is simple: The patient is asked to walk 20 feet at a normal speed, not racing. For men age 75, the Pittsburgh investigators found, gait speed predicted 10-year survival across a range of 19%-87%. The median speed was 0.8 m/sec, or about 1.8 mph, so a middle-of-the-pack walker ought to stop all cancer screening by age 75. A fast-walking older man won’t reach a 10-year remaining life expectancy until he’s in his early to mid-80s; a slow walker reaches that life expectancy as early as his late 60s, depending upon just how slow he walks. A woman at age 80 with an average gait speed has roughly 10 years of remaining life, factoring in plus or minus 5 years from that landmark depending upon whether she is a faster- or slower-than-average walker, Dr. Wallace explained.
The U.S. Preventive Services Task Force currently recommends colon cancer screening routinely for 50- to 75-year-olds, declaring in accord with other groups that this strategy has a high certainty of substantial net benefit. But the USPSTF also recommends selective screening for those aged 76-85, with a weaker C recommendation (JAMA. 2016 Jun 21;315[23]:2564-75).
What are the practical implications of that recommendation for selective screening after age 75?
Investigators at Harvard Medical School and the University of Oslo recently took a closer look. Their population-based, prospective, observational study included 1,355,692 Medicare beneficiaries aged 70-79 years at average risk for colorectal cancer who had not had a colonoscopy within the previous 5 years.
The investigators demonstrated that the benefit of screening colonoscopy decreased with age. For patients aged 70-74, the 8-year risk of colorectal cancer was 2.19% in those who were screened, compared with 2.62% in those who weren’t, for an absolute 0.43% difference. The number needed to be screened to detect one additional case of colorectal cancer was 283. Among those aged 75-79, the number needed to be screened climbed to 714 (Ann Intern Med. 2017 Jan 3;166[1]18-26).
Moreover, the risk of colonoscopy-related adverse events also climbed with age. These included perforations, falls while racing to the bathroom during the preprocedural bowel prep, and the humiliation of fecal incontinence. The excess 30-day risk for any adverse event in the colonoscopy group was 5.6 events per 1,000 patients aged 70-74 and 10.3 per 1,000 in 75- to 79-year-olds.
In a similar vein, Mara A. Schonberg, MD, of Harvard Medical School, Boston, has shed light on the risks and benefits of biannual mammographic screening for breast cancer in 70- to 79-year-olds, a practice recommended in American Cancer Society guidelines for women who are in overall good health and have at least a 10-year life expectancy.
She estimated that 2 women per 1,000 screened would avoid death due to breast cancer, for a number needed to screen of 500. But roughly 200 of those 1,000 women would experience a false-positive mammogram, and 20-40 of those false-positive imaging studies would result in a breast biopsy. Also, roughly 30% of the screen-detected cancers would not otherwise become apparent in an older woman’s lifetime, yet nearly all of the malignancies would undergo breast cancer therapy (J Am Geriatr Soc. 2016 Dec;64[12]:2413-8).
Dr. Schonberg’s research speaks to Dr. Wallace.
“It’s breast cancer therapy: It’s procedures; it’s medicalizing the patient’s whole life and creating a high degree of angst when she’s 75 or 80,” he said.
As to when to ‘just say no’ to cancer screening, Dr. Wallace said his answer is after age 65 for cervical cancer screening in women with at least two normal screens in the past 10 years or a prior total hysterectomy for a benign indication. All of the guidelines agree on that, although the American Congress of Obstetricians and Gynecologists recommends in addition that women with cervical intraepithelial neoplasia 2 be screened for the next 20 years.
For prostate cancer, Dr. Wallace recommends his colleagues just say no to screening at age 70 and above because harm is more likely than benefit to ensue.
“I don’t know about you, but I have a ton of patients over age 70 asking me for PSAs. That’s one place I won’t do any screening. I tell them I know you’re in great shape for 76 and you think it’s a good idea, but I think it’s bad medicine and I won’t do it. Even the American Urological Association says don’t do it after age 70,” he said.
For prostate cancer screening at age 55-69, however, patient preference rules the day, he added.
He draws the line at any cancer screening in patients aged 90 or over. Mean survival at age 90 is another 4-5 years. Only 11% of 90-year-old women will reach 100.
“Everybody has to die eventually,” he mused.
Dr. Wallace reported having no financial conflicts regarding his presentation.
ESTES PARK, COLO. – A simple walking speed measurement over a 20-foot distance is an invaluable guide to physiologic age as part of individualized decision making about when to stop cancer screening in elderly patients, according to Jeff Wallace, MD, professor of geriatric medicine at the University of Colorado at Denver.
“If you have one measurement to assess ‘am I aging well?’ it’s your gait speed. A lot of us in geriatrics are advocating evaluation of gait speed in all patients as a fifth vital sign. It’s probably more useful than blood pressure in some of the older adults coming into our clinics,” he said at a conference on internal medicine sponsored by the University of Colorado.
Dr. Wallace also gave a shout-out to the ePrognosis cancer-screening decision tool, available free at www.eprognosis.org, as an aid in shared decision-making conversations regarding when to stop cancer screening. This tool, developed by researchers at the University of California, San Francisco, allows physicians to plug key individual patient characteristics into its model, including comorbid conditions, functional status, and body mass index, and then spits out data-driven estimated benefits and harms a patient can expect from advanced-age screening for colon or breast cancer.
Of course, guidelines as to when to stop screening for various cancers are available from the U.S. Preventive Services Task Force, the American Cancer Society, and specialty societies. However, it’s important that nongeriatricians understand the serious limitations of those guidelines.
“We’re not guidelines followers in the geriatrics world because the guidelines don’t apply to most of our patients,” he explained. “We hate guidelines in geriatrics because few studies – and no lung cancer or breast cancer trials – enroll patients over age 75 with comorbid conditions. Also, most of these guidelines do not incorporate patient preferences, which probably should be a primary goal. So we’re left extrapolating.“
Regrettably, though, “it turns out most Americans are drinking the Kool-Aid when it comes to patient preferences. It’s amazing how much cancer screening is going on in this country. We’re doing a lot more than we should,” said Dr. Wallace.
All of that is clearly overscreening. Experts unanimously agree that if someone is not going to live for 10 years, that person is not likely to benefit from cancer screening. The one exception is lung cancer screening of high-risk patients, where there are data to show that annual low-dose CT screening is beneficial in those with even a 5-year life expectancy.
As part of the Choosing Wisely program, the American Geriatric Society has advocated that physicians “don’t recommend screening for breast, colorectal, prostate, or lung cancer without considering life expectancy and the risks of testing, overdiagnosis, and overtreatment.”
That’s where gait speed and ePrognosis come in handy in discussions with patients regarding what they can realistically expect from cancer screening at an advanced age.
The importance of gait speed was highlighted in a pooled analysis of nine cohort studies totaling more than 34,000 community-dwelling adults aged 65 years and older with 6-21 years of follow-up. Investigators at the University of Pittsburgh identified a strong relationship between gait speed and survival. Every 0.1-m/sec made a significant difference (JAMA. 2011 Jan 5;305[1]:50-8).
A gait speed evaluation is simple: The patient is asked to walk 20 feet at a normal speed, not racing. For men age 75, the Pittsburgh investigators found, gait speed predicted 10-year survival across a range of 19%-87%. The median speed was 0.8 m/sec, or about 1.8 mph, so a middle-of-the-pack walker ought to stop all cancer screening by age 75. A fast-walking older man won’t reach a 10-year remaining life expectancy until he’s in his early to mid-80s; a slow walker reaches that life expectancy as early as his late 60s, depending upon just how slow he walks. A woman at age 80 with an average gait speed has roughly 10 years of remaining life, factoring in plus or minus 5 years from that landmark depending upon whether she is a faster- or slower-than-average walker, Dr. Wallace explained.
The U.S. Preventive Services Task Force currently recommends colon cancer screening routinely for 50- to 75-year-olds, declaring in accord with other groups that this strategy has a high certainty of substantial net benefit. But the USPSTF also recommends selective screening for those aged 76-85, with a weaker C recommendation (JAMA. 2016 Jun 21;315[23]:2564-75).
What are the practical implications of that recommendation for selective screening after age 75?
Investigators at Harvard Medical School and the University of Oslo recently took a closer look. Their population-based, prospective, observational study included 1,355,692 Medicare beneficiaries aged 70-79 years at average risk for colorectal cancer who had not had a colonoscopy within the previous 5 years.
The investigators demonstrated that the benefit of screening colonoscopy decreased with age. For patients aged 70-74, the 8-year risk of colorectal cancer was 2.19% in those who were screened, compared with 2.62% in those who weren’t, for an absolute 0.43% difference. The number needed to be screened to detect one additional case of colorectal cancer was 283. Among those aged 75-79, the number needed to be screened climbed to 714 (Ann Intern Med. 2017 Jan 3;166[1]18-26).
Moreover, the risk of colonoscopy-related adverse events also climbed with age. These included perforations, falls while racing to the bathroom during the preprocedural bowel prep, and the humiliation of fecal incontinence. The excess 30-day risk for any adverse event in the colonoscopy group was 5.6 events per 1,000 patients aged 70-74 and 10.3 per 1,000 in 75- to 79-year-olds.
In a similar vein, Mara A. Schonberg, MD, of Harvard Medical School, Boston, has shed light on the risks and benefits of biannual mammographic screening for breast cancer in 70- to 79-year-olds, a practice recommended in American Cancer Society guidelines for women who are in overall good health and have at least a 10-year life expectancy.
She estimated that 2 women per 1,000 screened would avoid death due to breast cancer, for a number needed to screen of 500. But roughly 200 of those 1,000 women would experience a false-positive mammogram, and 20-40 of those false-positive imaging studies would result in a breast biopsy. Also, roughly 30% of the screen-detected cancers would not otherwise become apparent in an older woman’s lifetime, yet nearly all of the malignancies would undergo breast cancer therapy (J Am Geriatr Soc. 2016 Dec;64[12]:2413-8).
Dr. Schonberg’s research speaks to Dr. Wallace.
“It’s breast cancer therapy: It’s procedures; it’s medicalizing the patient’s whole life and creating a high degree of angst when she’s 75 or 80,” he said.
As to when to ‘just say no’ to cancer screening, Dr. Wallace said his answer is after age 65 for cervical cancer screening in women with at least two normal screens in the past 10 years or a prior total hysterectomy for a benign indication. All of the guidelines agree on that, although the American Congress of Obstetricians and Gynecologists recommends in addition that women with cervical intraepithelial neoplasia 2 be screened for the next 20 years.
For prostate cancer, Dr. Wallace recommends his colleagues just say no to screening at age 70 and above because harm is more likely than benefit to ensue.
“I don’t know about you, but I have a ton of patients over age 70 asking me for PSAs. That’s one place I won’t do any screening. I tell them I know you’re in great shape for 76 and you think it’s a good idea, but I think it’s bad medicine and I won’t do it. Even the American Urological Association says don’t do it after age 70,” he said.
For prostate cancer screening at age 55-69, however, patient preference rules the day, he added.
He draws the line at any cancer screening in patients aged 90 or over. Mean survival at age 90 is another 4-5 years. Only 11% of 90-year-old women will reach 100.
“Everybody has to die eventually,” he mused.
Dr. Wallace reported having no financial conflicts regarding his presentation.
ESTES PARK, COLO. – A simple walking speed measurement over a 20-foot distance is an invaluable guide to physiologic age as part of individualized decision making about when to stop cancer screening in elderly patients, according to Jeff Wallace, MD, professor of geriatric medicine at the University of Colorado at Denver.
“If you have one measurement to assess ‘am I aging well?’ it’s your gait speed. A lot of us in geriatrics are advocating evaluation of gait speed in all patients as a fifth vital sign. It’s probably more useful than blood pressure in some of the older adults coming into our clinics,” he said at a conference on internal medicine sponsored by the University of Colorado.
Dr. Wallace also gave a shout-out to the ePrognosis cancer-screening decision tool, available free at www.eprognosis.org, as an aid in shared decision-making conversations regarding when to stop cancer screening. This tool, developed by researchers at the University of California, San Francisco, allows physicians to plug key individual patient characteristics into its model, including comorbid conditions, functional status, and body mass index, and then spits out data-driven estimated benefits and harms a patient can expect from advanced-age screening for colon or breast cancer.
Of course, guidelines as to when to stop screening for various cancers are available from the U.S. Preventive Services Task Force, the American Cancer Society, and specialty societies. However, it’s important that nongeriatricians understand the serious limitations of those guidelines.
“We’re not guidelines followers in the geriatrics world because the guidelines don’t apply to most of our patients,” he explained. “We hate guidelines in geriatrics because few studies – and no lung cancer or breast cancer trials – enroll patients over age 75 with comorbid conditions. Also, most of these guidelines do not incorporate patient preferences, which probably should be a primary goal. So we’re left extrapolating.“
Regrettably, though, “it turns out most Americans are drinking the Kool-Aid when it comes to patient preferences. It’s amazing how much cancer screening is going on in this country. We’re doing a lot more than we should,” said Dr. Wallace.
All of that is clearly overscreening. Experts unanimously agree that if someone is not going to live for 10 years, that person is not likely to benefit from cancer screening. The one exception is lung cancer screening of high-risk patients, where there are data to show that annual low-dose CT screening is beneficial in those with even a 5-year life expectancy.
As part of the Choosing Wisely program, the American Geriatric Society has advocated that physicians “don’t recommend screening for breast, colorectal, prostate, or lung cancer without considering life expectancy and the risks of testing, overdiagnosis, and overtreatment.”
That’s where gait speed and ePrognosis come in handy in discussions with patients regarding what they can realistically expect from cancer screening at an advanced age.
The importance of gait speed was highlighted in a pooled analysis of nine cohort studies totaling more than 34,000 community-dwelling adults aged 65 years and older with 6-21 years of follow-up. Investigators at the University of Pittsburgh identified a strong relationship between gait speed and survival. Every 0.1-m/sec made a significant difference (JAMA. 2011 Jan 5;305[1]:50-8).
A gait speed evaluation is simple: The patient is asked to walk 20 feet at a normal speed, not racing. For men age 75, the Pittsburgh investigators found, gait speed predicted 10-year survival across a range of 19%-87%. The median speed was 0.8 m/sec, or about 1.8 mph, so a middle-of-the-pack walker ought to stop all cancer screening by age 75. A fast-walking older man won’t reach a 10-year remaining life expectancy until he’s in his early to mid-80s; a slow walker reaches that life expectancy as early as his late 60s, depending upon just how slow he walks. A woman at age 80 with an average gait speed has roughly 10 years of remaining life, factoring in plus or minus 5 years from that landmark depending upon whether she is a faster- or slower-than-average walker, Dr. Wallace explained.
The U.S. Preventive Services Task Force currently recommends colon cancer screening routinely for 50- to 75-year-olds, declaring in accord with other groups that this strategy has a high certainty of substantial net benefit. But the USPSTF also recommends selective screening for those aged 76-85, with a weaker C recommendation (JAMA. 2016 Jun 21;315[23]:2564-75).
What are the practical implications of that recommendation for selective screening after age 75?
Investigators at Harvard Medical School and the University of Oslo recently took a closer look. Their population-based, prospective, observational study included 1,355,692 Medicare beneficiaries aged 70-79 years at average risk for colorectal cancer who had not had a colonoscopy within the previous 5 years.
The investigators demonstrated that the benefit of screening colonoscopy decreased with age. For patients aged 70-74, the 8-year risk of colorectal cancer was 2.19% in those who were screened, compared with 2.62% in those who weren’t, for an absolute 0.43% difference. The number needed to be screened to detect one additional case of colorectal cancer was 283. Among those aged 75-79, the number needed to be screened climbed to 714 (Ann Intern Med. 2017 Jan 3;166[1]18-26).
Moreover, the risk of colonoscopy-related adverse events also climbed with age. These included perforations, falls while racing to the bathroom during the preprocedural bowel prep, and the humiliation of fecal incontinence. The excess 30-day risk for any adverse event in the colonoscopy group was 5.6 events per 1,000 patients aged 70-74 and 10.3 per 1,000 in 75- to 79-year-olds.
In a similar vein, Mara A. Schonberg, MD, of Harvard Medical School, Boston, has shed light on the risks and benefits of biannual mammographic screening for breast cancer in 70- to 79-year-olds, a practice recommended in American Cancer Society guidelines for women who are in overall good health and have at least a 10-year life expectancy.
She estimated that 2 women per 1,000 screened would avoid death due to breast cancer, for a number needed to screen of 500. But roughly 200 of those 1,000 women would experience a false-positive mammogram, and 20-40 of those false-positive imaging studies would result in a breast biopsy. Also, roughly 30% of the screen-detected cancers would not otherwise become apparent in an older woman’s lifetime, yet nearly all of the malignancies would undergo breast cancer therapy (J Am Geriatr Soc. 2016 Dec;64[12]:2413-8).
Dr. Schonberg’s research speaks to Dr. Wallace.
“It’s breast cancer therapy: It’s procedures; it’s medicalizing the patient’s whole life and creating a high degree of angst when she’s 75 or 80,” he said.
As to when to ‘just say no’ to cancer screening, Dr. Wallace said his answer is after age 65 for cervical cancer screening in women with at least two normal screens in the past 10 years or a prior total hysterectomy for a benign indication. All of the guidelines agree on that, although the American Congress of Obstetricians and Gynecologists recommends in addition that women with cervical intraepithelial neoplasia 2 be screened for the next 20 years.
For prostate cancer, Dr. Wallace recommends his colleagues just say no to screening at age 70 and above because harm is more likely than benefit to ensue.
“I don’t know about you, but I have a ton of patients over age 70 asking me for PSAs. That’s one place I won’t do any screening. I tell them I know you’re in great shape for 76 and you think it’s a good idea, but I think it’s bad medicine and I won’t do it. Even the American Urological Association says don’t do it after age 70,” he said.
For prostate cancer screening at age 55-69, however, patient preference rules the day, he added.
He draws the line at any cancer screening in patients aged 90 or over. Mean survival at age 90 is another 4-5 years. Only 11% of 90-year-old women will reach 100.
“Everybody has to die eventually,” he mused.
Dr. Wallace reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM THE ANNUAL INTERNAL MEDICINE PROGRAM
New acellular pertussis vaccine may solve waning immunogenicity problem
MADRID – A novel, monovalent, acellular pertussis vaccine containing a recombinant, genetically inactivated pertussis toxin displayed markedly greater sustained immunogenicity than the widely used Sanofi Pasteur Tdap, known as Adacel, which is used as a booster vaccination of adolescents and young adults, in a pivotal phase 3, randomized trial, Simonetta Viviani, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Our interpretation of these results is that they open up a new way to approach pertussis vaccination,” declared Dr. Viviani, director of clinical development at BioNet-Asia, a Bangkok-based biotech vaccine company.
The impetus for developing new acellular pertussis vaccines is the documented resurgence of pertussis.
“One suggested approach has been to replace chemically inactivated PT with a genetically inactivated PT. The rationale for that is the epitopes of the PT are conserved in the genetically modified PT toxin, as opposed to being destroyed in the chemical inactivation process,” Dr. Viviani explained.
The significant phase 3 trial included 450 Thai 12- to 17-year-olds who were randomized to a single 0.5-mL dose of Pertagen, Boostagen, or Adacel. Both Pertagen and Boostagen contain 5 mcg of the genetically inactivated PT and 5 mcg of filamentous hemagglutinin.
The seroconversion rate, defined as the proportion of subjects who reached at least a fourfold increase in titers of PT and filamentous-hemagglutinin antibodies over baseline, was far superior at both 28 days and 1 year in subjects who got Pertagen or Boostagen, compared with those who received Adacel.
Session chair Ulrich Heininger, MD, declared, “This is really, really exciting.”
It now will be very important that the monovalent Pertagen vaccine be formally studied in pregnant women, he observed.
“Since we’d like to immunize women in every pregnancy and they don’t necessarily need the Td component of Tdap every time, a monovalent vaccine might open a new path for acceptance,” commented Dr. Heininger, professor of pediatric infectious diseases at University Children’s Hospital in Basel, Switz.
Dr. Viviani said that a study in pregnant women is now in the early planning stages.
The study was sponsored by BioNet-Asia and Mahidol University. Dr. Viviani is a BioNet employee.
MADRID – A novel, monovalent, acellular pertussis vaccine containing a recombinant, genetically inactivated pertussis toxin displayed markedly greater sustained immunogenicity than the widely used Sanofi Pasteur Tdap, known as Adacel, which is used as a booster vaccination of adolescents and young adults, in a pivotal phase 3, randomized trial, Simonetta Viviani, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Our interpretation of these results is that they open up a new way to approach pertussis vaccination,” declared Dr. Viviani, director of clinical development at BioNet-Asia, a Bangkok-based biotech vaccine company.
The impetus for developing new acellular pertussis vaccines is the documented resurgence of pertussis.
“One suggested approach has been to replace chemically inactivated PT with a genetically inactivated PT. The rationale for that is the epitopes of the PT are conserved in the genetically modified PT toxin, as opposed to being destroyed in the chemical inactivation process,” Dr. Viviani explained.
The significant phase 3 trial included 450 Thai 12- to 17-year-olds who were randomized to a single 0.5-mL dose of Pertagen, Boostagen, or Adacel. Both Pertagen and Boostagen contain 5 mcg of the genetically inactivated PT and 5 mcg of filamentous hemagglutinin.
The seroconversion rate, defined as the proportion of subjects who reached at least a fourfold increase in titers of PT and filamentous-hemagglutinin antibodies over baseline, was far superior at both 28 days and 1 year in subjects who got Pertagen or Boostagen, compared with those who received Adacel.
Session chair Ulrich Heininger, MD, declared, “This is really, really exciting.”
It now will be very important that the monovalent Pertagen vaccine be formally studied in pregnant women, he observed.
“Since we’d like to immunize women in every pregnancy and they don’t necessarily need the Td component of Tdap every time, a monovalent vaccine might open a new path for acceptance,” commented Dr. Heininger, professor of pediatric infectious diseases at University Children’s Hospital in Basel, Switz.
Dr. Viviani said that a study in pregnant women is now in the early planning stages.
The study was sponsored by BioNet-Asia and Mahidol University. Dr. Viviani is a BioNet employee.
MADRID – A novel, monovalent, acellular pertussis vaccine containing a recombinant, genetically inactivated pertussis toxin displayed markedly greater sustained immunogenicity than the widely used Sanofi Pasteur Tdap, known as Adacel, which is used as a booster vaccination of adolescents and young adults, in a pivotal phase 3, randomized trial, Simonetta Viviani, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Our interpretation of these results is that they open up a new way to approach pertussis vaccination,” declared Dr. Viviani, director of clinical development at BioNet-Asia, a Bangkok-based biotech vaccine company.
The impetus for developing new acellular pertussis vaccines is the documented resurgence of pertussis.
“One suggested approach has been to replace chemically inactivated PT with a genetically inactivated PT. The rationale for that is the epitopes of the PT are conserved in the genetically modified PT toxin, as opposed to being destroyed in the chemical inactivation process,” Dr. Viviani explained.
The significant phase 3 trial included 450 Thai 12- to 17-year-olds who were randomized to a single 0.5-mL dose of Pertagen, Boostagen, or Adacel. Both Pertagen and Boostagen contain 5 mcg of the genetically inactivated PT and 5 mcg of filamentous hemagglutinin.
The seroconversion rate, defined as the proportion of subjects who reached at least a fourfold increase in titers of PT and filamentous-hemagglutinin antibodies over baseline, was far superior at both 28 days and 1 year in subjects who got Pertagen or Boostagen, compared with those who received Adacel.
Session chair Ulrich Heininger, MD, declared, “This is really, really exciting.”
It now will be very important that the monovalent Pertagen vaccine be formally studied in pregnant women, he observed.
“Since we’d like to immunize women in every pregnancy and they don’t necessarily need the Td component of Tdap every time, a monovalent vaccine might open a new path for acceptance,” commented Dr. Heininger, professor of pediatric infectious diseases at University Children’s Hospital in Basel, Switz.
Dr. Viviani said that a study in pregnant women is now in the early planning stages.
The study was sponsored by BioNet-Asia and Mahidol University. Dr. Viviani is a BioNet employee.
AT ESPID 2017
Key clinical point:
Major finding: One year after teens received a single dose of a novel acellular pertussis vaccine, they had a geometric mean titer of PT neutralizing antibody of 77 IU/mL, compared with just 12 IU/mL in adolescents who received a conventional Tdap vaccine.
Data source: This randomized, triple-arm, pivotal phase 3 clinical trial included 450 Thai 12- to 17-year-olds followed for 1 year after receiving a single dose of a novel monovalent pertussis vaccine or a novel Tdap vaccine, both of which contain genetically inactivated pertussis toxin, or, instead of those, a widely utilized conventional Tdap vaccine.
Disclosures: The study was sponsored by BioNet-Asia and Mahidol University. Dr. Viviani is a BioNet employee.
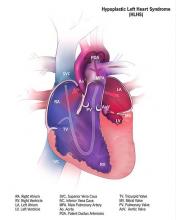
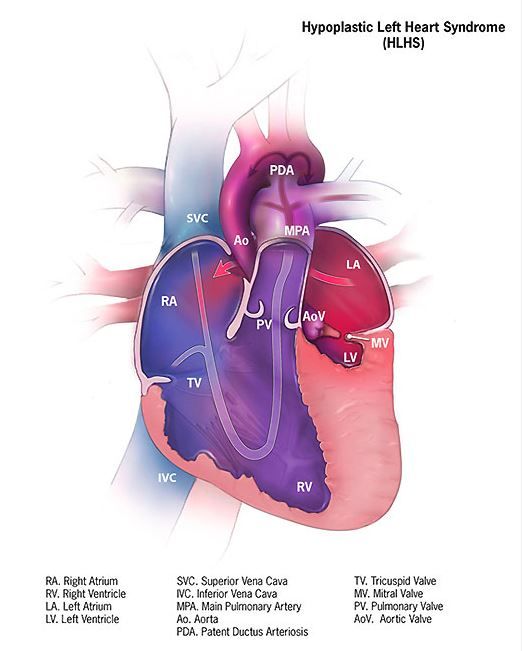
Cerebral NIRS may be flawed for assessing infant brains after stage 1 palliation of HLHS
The regional oxygenation index (rSO2) based on near infrared spectroscopy (NIRS) measurement is frequently used to assess the adequacy of oxygen delivery after stage 1 palliation of hypoplastic left heart syndrome (HLHS). However, a recent study showed that cerebral rSO2 has low sensitivity and should not be considered reassuring even at rSO2 of 50 or greater. In addition, values below 30 were not found to be sensitive for detecting compromised oxygen delivery, according to a report published online in the Journal of Thoracic and Cardiovascular Surgery.
Erin Rescoe, MD, of Boston Children’s Hospital, and her colleagues at Harvard Medical School, Boston, performed a retrospective study of 73 neonates assessed with cerebral venous oxyhemoglobin saturation (ScvO2) measured by co-oximetry from the internal jugular vein, which is considered the preferred method for assessing the adequacy of tissue oxygen delivery, compared with cerebral rSO2 after stage 1 palliation of HLHS (doi: 10.1016/j.jtcvs.2017.03.154).
To determine the suggested benefit of NIRS as an effective trend monitor, the researchers used their interpolated data to examine changes in rSO2 and changes in ScvO2 at hourly intervals and compared these values.Of particular concern is the result showing that, in all instances where ScvO2 was less than 30%, rSO2 was greater than 30%. In terms of the sensitivity (the true positive rate) and specificity (the true negative rate) of using NIRS, time-matched pairs of rSO2 and ScvO2 showed that the receiver operating characteristic curves for rSO2 as a diagnostic test to detect ScvO2 less than 30%, less than 40%, and less than 50% were 0.82, 0.84, and 0.87, respectively, showing good specificity, with a value of rSO2 less than 30% indicating that ScvO2 will be less than 30% 99% of the time.
“However, the sensitivity of rSO2 in the range of clinical interest in detecting ScvO2 less than 30% is extremely low,” according to the researchers. Thus, NIRS is likely to produce false negatives, missing patients with clinically low postoperative oxygen saturation.
In fact, rSO2 was less than 30% less than 1% of the time that ScvO2 was less than 30%. Similar results were seen in comparing values at the less than 40% mark (equivalent less than 1% of the time). Better results showed at the less than 50% mark, with equivalence seen 46% of the time.
NIRS measures a composite of arterial and venous blood, according to Dr. Rescoe and her colleagues. Therefore, to do a more direct comparison, they adjusted their NIRS results by calculating an rSO2-based ScvO2 designed to remove arterial contamination from the rSO2 signal: rSO2-based ScvO2 = (rSO2 arterial oxygen saturation x 0.3)/0.7.
This significantly improved the sensitivity of rSO2 to detect ScvO2 at less than 30% to 6.5%, to 29% for rSO2 at less than 40%, and 77.4% for rSO2 less than 50%.
The researchers “were surprised by the extremely low sensitivity of cerebral NIRS to detect even the most severe aberrations in DO2” (i.e., ScvO2 less than 30%, which has been found to be associated with poor outcomes).
“Cerebral rSO2 in isolation should not be used to detect low ScvO2, because its sensitivity is low, although correction of rSO2 for arterial contamination significantly improves sensitivity. Cerebral rSO2 of 50 or greater should not be considered reassuring with regard to ScvO2, although values less than 30 are specific for low ScvO2,” the researchers concluded.
The study was sponsored by the Gerber Foundation, the Hess Family Philanthropic Fund, and Boston Children’s Hospital Heart Center Strategic Investment Fund. The authors disclosed that they had no financial conflicts.
The use of postoperative cerebral venous oxygen saturation monitoring (ScvO2) through an internal jugular vein catheter allows better monitoring of circulation, which may lead to better outcomes, but it is invasive and challenging. NIRS, being noninvasive, has proved attractive, but clinical interpretation in terms of both absolute values and trends is difficult, Edward Buratto, MBBS, and his colleagues noted in their invited commentary (doi: 10.1016/j.jtcvs.2017.04.061).
Dr. Rescoe and her colleagues have analyzed the correlation of NIRS-derived data with ScvO2 measured by co-oximetry from the internal jugular vein in 73 neonates after stage 1 palliation for hypoplastic left heart syndrome. They demonstrated that cerebral rSO2 correlated poorly with low ScvO2, and they suggest that cerebral rSO2 not be used in isolation. This problem was somewhat ameliorated by correction of the signal for arterial contamination. NIRS appears to be too valuable a tool to be simply discarded, they said, suggesting that a perioperative risk assessment that would include multisite NIRS and hemodynamic monitoring might still allow early determination of low-cardiac output.
“Two numbers are better than one,” wrote Dr. Buratto and his colleagues. “Whether the NIRS technology will add any useful information to a simple bedside assessment by an astute clinician is yet to be seen.”
Edward Buratto, MBBS, Steve Horton, PhD, and Igor E. Konstantinov, MD, are from the Department of Cardiothoracic Surgery, The Royal Children’s Hospital; the Department of Pediatrics, University of Melbourne; and Murdoch Children’s Research Institute, Melbourne. They reported having no financial conflicts of interest.
The use of postoperative cerebral venous oxygen saturation monitoring (ScvO2) through an internal jugular vein catheter allows better monitoring of circulation, which may lead to better outcomes, but it is invasive and challenging. NIRS, being noninvasive, has proved attractive, but clinical interpretation in terms of both absolute values and trends is difficult, Edward Buratto, MBBS, and his colleagues noted in their invited commentary (doi: 10.1016/j.jtcvs.2017.04.061).
Dr. Rescoe and her colleagues have analyzed the correlation of NIRS-derived data with ScvO2 measured by co-oximetry from the internal jugular vein in 73 neonates after stage 1 palliation for hypoplastic left heart syndrome. They demonstrated that cerebral rSO2 correlated poorly with low ScvO2, and they suggest that cerebral rSO2 not be used in isolation. This problem was somewhat ameliorated by correction of the signal for arterial contamination. NIRS appears to be too valuable a tool to be simply discarded, they said, suggesting that a perioperative risk assessment that would include multisite NIRS and hemodynamic monitoring might still allow early determination of low-cardiac output.
“Two numbers are better than one,” wrote Dr. Buratto and his colleagues. “Whether the NIRS technology will add any useful information to a simple bedside assessment by an astute clinician is yet to be seen.”
Edward Buratto, MBBS, Steve Horton, PhD, and Igor E. Konstantinov, MD, are from the Department of Cardiothoracic Surgery, The Royal Children’s Hospital; the Department of Pediatrics, University of Melbourne; and Murdoch Children’s Research Institute, Melbourne. They reported having no financial conflicts of interest.
The use of postoperative cerebral venous oxygen saturation monitoring (ScvO2) through an internal jugular vein catheter allows better monitoring of circulation, which may lead to better outcomes, but it is invasive and challenging. NIRS, being noninvasive, has proved attractive, but clinical interpretation in terms of both absolute values and trends is difficult, Edward Buratto, MBBS, and his colleagues noted in their invited commentary (doi: 10.1016/j.jtcvs.2017.04.061).
Dr. Rescoe and her colleagues have analyzed the correlation of NIRS-derived data with ScvO2 measured by co-oximetry from the internal jugular vein in 73 neonates after stage 1 palliation for hypoplastic left heart syndrome. They demonstrated that cerebral rSO2 correlated poorly with low ScvO2, and they suggest that cerebral rSO2 not be used in isolation. This problem was somewhat ameliorated by correction of the signal for arterial contamination. NIRS appears to be too valuable a tool to be simply discarded, they said, suggesting that a perioperative risk assessment that would include multisite NIRS and hemodynamic monitoring might still allow early determination of low-cardiac output.
“Two numbers are better than one,” wrote Dr. Buratto and his colleagues. “Whether the NIRS technology will add any useful information to a simple bedside assessment by an astute clinician is yet to be seen.”
Edward Buratto, MBBS, Steve Horton, PhD, and Igor E. Konstantinov, MD, are from the Department of Cardiothoracic Surgery, The Royal Children’s Hospital; the Department of Pediatrics, University of Melbourne; and Murdoch Children’s Research Institute, Melbourne. They reported having no financial conflicts of interest.
The regional oxygenation index (rSO2) based on near infrared spectroscopy (NIRS) measurement is frequently used to assess the adequacy of oxygen delivery after stage 1 palliation of hypoplastic left heart syndrome (HLHS). However, a recent study showed that cerebral rSO2 has low sensitivity and should not be considered reassuring even at rSO2 of 50 or greater. In addition, values below 30 were not found to be sensitive for detecting compromised oxygen delivery, according to a report published online in the Journal of Thoracic and Cardiovascular Surgery.
Erin Rescoe, MD, of Boston Children’s Hospital, and her colleagues at Harvard Medical School, Boston, performed a retrospective study of 73 neonates assessed with cerebral venous oxyhemoglobin saturation (ScvO2) measured by co-oximetry from the internal jugular vein, which is considered the preferred method for assessing the adequacy of tissue oxygen delivery, compared with cerebral rSO2 after stage 1 palliation of HLHS (doi: 10.1016/j.jtcvs.2017.03.154).
To determine the suggested benefit of NIRS as an effective trend monitor, the researchers used their interpolated data to examine changes in rSO2 and changes in ScvO2 at hourly intervals and compared these values.Of particular concern is the result showing that, in all instances where ScvO2 was less than 30%, rSO2 was greater than 30%. In terms of the sensitivity (the true positive rate) and specificity (the true negative rate) of using NIRS, time-matched pairs of rSO2 and ScvO2 showed that the receiver operating characteristic curves for rSO2 as a diagnostic test to detect ScvO2 less than 30%, less than 40%, and less than 50% were 0.82, 0.84, and 0.87, respectively, showing good specificity, with a value of rSO2 less than 30% indicating that ScvO2 will be less than 30% 99% of the time.
“However, the sensitivity of rSO2 in the range of clinical interest in detecting ScvO2 less than 30% is extremely low,” according to the researchers. Thus, NIRS is likely to produce false negatives, missing patients with clinically low postoperative oxygen saturation.
In fact, rSO2 was less than 30% less than 1% of the time that ScvO2 was less than 30%. Similar results were seen in comparing values at the less than 40% mark (equivalent less than 1% of the time). Better results showed at the less than 50% mark, with equivalence seen 46% of the time.
NIRS measures a composite of arterial and venous blood, according to Dr. Rescoe and her colleagues. Therefore, to do a more direct comparison, they adjusted their NIRS results by calculating an rSO2-based ScvO2 designed to remove arterial contamination from the rSO2 signal: rSO2-based ScvO2 = (rSO2 arterial oxygen saturation x 0.3)/0.7.
This significantly improved the sensitivity of rSO2 to detect ScvO2 at less than 30% to 6.5%, to 29% for rSO2 at less than 40%, and 77.4% for rSO2 less than 50%.
The researchers “were surprised by the extremely low sensitivity of cerebral NIRS to detect even the most severe aberrations in DO2” (i.e., ScvO2 less than 30%, which has been found to be associated with poor outcomes).
“Cerebral rSO2 in isolation should not be used to detect low ScvO2, because its sensitivity is low, although correction of rSO2 for arterial contamination significantly improves sensitivity. Cerebral rSO2 of 50 or greater should not be considered reassuring with regard to ScvO2, although values less than 30 are specific for low ScvO2,” the researchers concluded.
The study was sponsored by the Gerber Foundation, the Hess Family Philanthropic Fund, and Boston Children’s Hospital Heart Center Strategic Investment Fund. The authors disclosed that they had no financial conflicts.
The regional oxygenation index (rSO2) based on near infrared spectroscopy (NIRS) measurement is frequently used to assess the adequacy of oxygen delivery after stage 1 palliation of hypoplastic left heart syndrome (HLHS). However, a recent study showed that cerebral rSO2 has low sensitivity and should not be considered reassuring even at rSO2 of 50 or greater. In addition, values below 30 were not found to be sensitive for detecting compromised oxygen delivery, according to a report published online in the Journal of Thoracic and Cardiovascular Surgery.
Erin Rescoe, MD, of Boston Children’s Hospital, and her colleagues at Harvard Medical School, Boston, performed a retrospective study of 73 neonates assessed with cerebral venous oxyhemoglobin saturation (ScvO2) measured by co-oximetry from the internal jugular vein, which is considered the preferred method for assessing the adequacy of tissue oxygen delivery, compared with cerebral rSO2 after stage 1 palliation of HLHS (doi: 10.1016/j.jtcvs.2017.03.154).
To determine the suggested benefit of NIRS as an effective trend monitor, the researchers used their interpolated data to examine changes in rSO2 and changes in ScvO2 at hourly intervals and compared these values.Of particular concern is the result showing that, in all instances where ScvO2 was less than 30%, rSO2 was greater than 30%. In terms of the sensitivity (the true positive rate) and specificity (the true negative rate) of using NIRS, time-matched pairs of rSO2 and ScvO2 showed that the receiver operating characteristic curves for rSO2 as a diagnostic test to detect ScvO2 less than 30%, less than 40%, and less than 50% were 0.82, 0.84, and 0.87, respectively, showing good specificity, with a value of rSO2 less than 30% indicating that ScvO2 will be less than 30% 99% of the time.
“However, the sensitivity of rSO2 in the range of clinical interest in detecting ScvO2 less than 30% is extremely low,” according to the researchers. Thus, NIRS is likely to produce false negatives, missing patients with clinically low postoperative oxygen saturation.
In fact, rSO2 was less than 30% less than 1% of the time that ScvO2 was less than 30%. Similar results were seen in comparing values at the less than 40% mark (equivalent less than 1% of the time). Better results showed at the less than 50% mark, with equivalence seen 46% of the time.
NIRS measures a composite of arterial and venous blood, according to Dr. Rescoe and her colleagues. Therefore, to do a more direct comparison, they adjusted their NIRS results by calculating an rSO2-based ScvO2 designed to remove arterial contamination from the rSO2 signal: rSO2-based ScvO2 = (rSO2 arterial oxygen saturation x 0.3)/0.7.
This significantly improved the sensitivity of rSO2 to detect ScvO2 at less than 30% to 6.5%, to 29% for rSO2 at less than 40%, and 77.4% for rSO2 less than 50%.
The researchers “were surprised by the extremely low sensitivity of cerebral NIRS to detect even the most severe aberrations in DO2” (i.e., ScvO2 less than 30%, which has been found to be associated with poor outcomes).
“Cerebral rSO2 in isolation should not be used to detect low ScvO2, because its sensitivity is low, although correction of rSO2 for arterial contamination significantly improves sensitivity. Cerebral rSO2 of 50 or greater should not be considered reassuring with regard to ScvO2, although values less than 30 are specific for low ScvO2,” the researchers concluded.
The study was sponsored by the Gerber Foundation, the Hess Family Philanthropic Fund, and Boston Children’s Hospital Heart Center Strategic Investment Fund. The authors disclosed that they had no financial conflicts.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point:
Major finding: In terms of sensitivity, rSO2 was less than 30% less than 1% of the time that ScvO2 was less than 30%.
Data source: A retrospective single institution study of 73 neonates assessed after stage 1 palliation
Disclosures: The study was sponsored by the Gerber Foundation, the Hess Family Philanthropic Fund, and Boston Children’s Hospital Heart Center Strategic Investment Fund. The authors disclosed that they had no financial conflicts.
Cognitive impairment in MS may affect fitness to drive motor vehicles
Assessing fitness to drive a motor vehicle is an important part of clinical practice for multiple sclerosis, but MS and cognitive impairment alone don’t indicate that a patient will fail a formal road test. However, the results from a recent study indicate that one cognitive test may be able to predict the patients who would fail road tests though only with a high false-positive rate.
During 2015-2016, Sarah A. Morrow, MD, of Western University, London, Ont., and her coauthors recruited licensed drivers aged 18-59 who were diagnosed with MS. They had low physical disability (Expanded Disability Status Scale score of less than 4) but cognitive impairment in both processing speed and either memory or executive function. This set of requirements, along with the need for signed informed consent, yielded a smaller sample size (36) than the researchers’ goal, a possible weakness of the study, the investigators said (Mult Scler. 2017 Aug 7. doi: 10.1177/1352458517723991).
The researchers noted that, if a patient does not show impairment on the BVMTR-IR, then they will definitely pass the road test, but they also noted that showing impairment is much less likely to predict a failed test. Instead, impairment both on this test and on the Symbol Digit Modalities Test, which measures processing speed, should indicate the need for a formal driving assessment.
“This study further contributes to the clinician’s ability to identify [persons with MS] in whom fitness-to-drive should be addressed,” the investigators wrote.
Assessing fitness to drive a motor vehicle is an important part of clinical practice for multiple sclerosis, but MS and cognitive impairment alone don’t indicate that a patient will fail a formal road test. However, the results from a recent study indicate that one cognitive test may be able to predict the patients who would fail road tests though only with a high false-positive rate.
During 2015-2016, Sarah A. Morrow, MD, of Western University, London, Ont., and her coauthors recruited licensed drivers aged 18-59 who were diagnosed with MS. They had low physical disability (Expanded Disability Status Scale score of less than 4) but cognitive impairment in both processing speed and either memory or executive function. This set of requirements, along with the need for signed informed consent, yielded a smaller sample size (36) than the researchers’ goal, a possible weakness of the study, the investigators said (Mult Scler. 2017 Aug 7. doi: 10.1177/1352458517723991).
The researchers noted that, if a patient does not show impairment on the BVMTR-IR, then they will definitely pass the road test, but they also noted that showing impairment is much less likely to predict a failed test. Instead, impairment both on this test and on the Symbol Digit Modalities Test, which measures processing speed, should indicate the need for a formal driving assessment.
“This study further contributes to the clinician’s ability to identify [persons with MS] in whom fitness-to-drive should be addressed,” the investigators wrote.
Assessing fitness to drive a motor vehicle is an important part of clinical practice for multiple sclerosis, but MS and cognitive impairment alone don’t indicate that a patient will fail a formal road test. However, the results from a recent study indicate that one cognitive test may be able to predict the patients who would fail road tests though only with a high false-positive rate.
During 2015-2016, Sarah A. Morrow, MD, of Western University, London, Ont., and her coauthors recruited licensed drivers aged 18-59 who were diagnosed with MS. They had low physical disability (Expanded Disability Status Scale score of less than 4) but cognitive impairment in both processing speed and either memory or executive function. This set of requirements, along with the need for signed informed consent, yielded a smaller sample size (36) than the researchers’ goal, a possible weakness of the study, the investigators said (Mult Scler. 2017 Aug 7. doi: 10.1177/1352458517723991).
The researchers noted that, if a patient does not show impairment on the BVMTR-IR, then they will definitely pass the road test, but they also noted that showing impairment is much less likely to predict a failed test. Instead, impairment both on this test and on the Symbol Digit Modalities Test, which measures processing speed, should indicate the need for a formal driving assessment.
“This study further contributes to the clinician’s ability to identify [persons with MS] in whom fitness-to-drive should be addressed,” the investigators wrote.
FROM MULTIPLE SCLEROSIS JOURNAL
ERAS program cuts complications after radical cystectomy
NEW YORK – Prior to October 2014, urology patients undergoing radical cystectomy at a 950-bed, tertiary care hospital experienced postoperative morbidity at a relative high rate, according to NSQIP data.
“Despite improvements in surgical techniques and perioperative care protocols, the rate of the overall morbidity for radical cystectomy was higher than we would like to see,” said Tracey Hong, RN, BScN, of the Clinical Quality and Patient Safety Department at Vancouver (B.C.) General Hospital. “We took this as an opportunity to improve our patient outcomes and experience.”
Vancouver General joined the American College of Surgeons National Surgical Improvement Quality Program (ACS NSQIP) in 2011. The enhanced recovery after surgery (ERAS) perioperative protocol the institution adopted in late 2014 was associated with a 32% decrease in overall morbidity. The rate dropped from 31.3% in the pre-ERAS study period from May 2011 to September 2014, to 21.1% after implementation, from October 2014 to September 2016, according to a study Ms. Hong presented at the American College of Surgeons Quality and Safety Conference.
The investigators compared outcomes between all 92 people undergoing elective radical cystectomy during the first time period to 152 consecutive patients treated under the ERAS protocol. Median length of stay decreased from 8 days before ERAS to 7 days after, a significant difference (P less than .05).
The researchers also assessed outcomes based on how adherent clinicians were to 12 key elements of the 26-item ERAS initiative. These elements included preoperative counseling, preoperative anesthesia consultation, and carbohydrate loading on the morning of surgery. Intraoperatively, they tracked normothermia, use of multimodal anesthesia, use of goal-directed fluid therapy using a monitor, timely antibiotics, and adequate postoperative nausea and vomiting prophylaxis. The four postoperative key measures were mobilization at least once by postoperative day 0, full fluids and mobilization twice on postoperative day 1, and starting solid food by postoperative day 4.
A total 52% of the ERAS cases were associated with 75% or greater adherence to these 12 key items. Adherence with the intraoperative fluid therapy and all the postoperative elements proved to be the most challenging, Ms. Hong said.
The more adherent cases experienced a lower overall postoperative morbidity rate, 15.2%, compared with 27.4% among the less adherent group. The 15.2% morbidity among the more adherent cases also compared favorably with the 31.1% rate for cases prior to ERAS adoption.
“We will continue working on improving compliance,” Ms. Hong said. “We need to increase adherence to goal-directed fluid therapy and the postoperative components,” Ms. Hong said.
Three main strategies remain essential to the ongoing success of the ERAS program, Ms. Hong said. Empowering patients to be active participants and to engage in their own health outcomes is one. “Second, we involve a multidisciplinary team at an early stage so they take ownership and get engaged in the program,” she said. “Last but not least, we continue to measure the outcomes in 100% of cases.”
Continuous auditing and sharing results with the team on a regular basis will be necessary to maintain engagement in the ERAS protocol going forward, Ms. Hong added. “Tenacity is vital.”
Ms. Hong had no relevant financial disclosures.
NEW YORK – Prior to October 2014, urology patients undergoing radical cystectomy at a 950-bed, tertiary care hospital experienced postoperative morbidity at a relative high rate, according to NSQIP data.
“Despite improvements in surgical techniques and perioperative care protocols, the rate of the overall morbidity for radical cystectomy was higher than we would like to see,” said Tracey Hong, RN, BScN, of the Clinical Quality and Patient Safety Department at Vancouver (B.C.) General Hospital. “We took this as an opportunity to improve our patient outcomes and experience.”
Vancouver General joined the American College of Surgeons National Surgical Improvement Quality Program (ACS NSQIP) in 2011. The enhanced recovery after surgery (ERAS) perioperative protocol the institution adopted in late 2014 was associated with a 32% decrease in overall morbidity. The rate dropped from 31.3% in the pre-ERAS study period from May 2011 to September 2014, to 21.1% after implementation, from October 2014 to September 2016, according to a study Ms. Hong presented at the American College of Surgeons Quality and Safety Conference.
The investigators compared outcomes between all 92 people undergoing elective radical cystectomy during the first time period to 152 consecutive patients treated under the ERAS protocol. Median length of stay decreased from 8 days before ERAS to 7 days after, a significant difference (P less than .05).
The researchers also assessed outcomes based on how adherent clinicians were to 12 key elements of the 26-item ERAS initiative. These elements included preoperative counseling, preoperative anesthesia consultation, and carbohydrate loading on the morning of surgery. Intraoperatively, they tracked normothermia, use of multimodal anesthesia, use of goal-directed fluid therapy using a monitor, timely antibiotics, and adequate postoperative nausea and vomiting prophylaxis. The four postoperative key measures were mobilization at least once by postoperative day 0, full fluids and mobilization twice on postoperative day 1, and starting solid food by postoperative day 4.
A total 52% of the ERAS cases were associated with 75% or greater adherence to these 12 key items. Adherence with the intraoperative fluid therapy and all the postoperative elements proved to be the most challenging, Ms. Hong said.
The more adherent cases experienced a lower overall postoperative morbidity rate, 15.2%, compared with 27.4% among the less adherent group. The 15.2% morbidity among the more adherent cases also compared favorably with the 31.1% rate for cases prior to ERAS adoption.
“We will continue working on improving compliance,” Ms. Hong said. “We need to increase adherence to goal-directed fluid therapy and the postoperative components,” Ms. Hong said.
Three main strategies remain essential to the ongoing success of the ERAS program, Ms. Hong said. Empowering patients to be active participants and to engage in their own health outcomes is one. “Second, we involve a multidisciplinary team at an early stage so they take ownership and get engaged in the program,” she said. “Last but not least, we continue to measure the outcomes in 100% of cases.”
Continuous auditing and sharing results with the team on a regular basis will be necessary to maintain engagement in the ERAS protocol going forward, Ms. Hong added. “Tenacity is vital.”
Ms. Hong had no relevant financial disclosures.
NEW YORK – Prior to October 2014, urology patients undergoing radical cystectomy at a 950-bed, tertiary care hospital experienced postoperative morbidity at a relative high rate, according to NSQIP data.
“Despite improvements in surgical techniques and perioperative care protocols, the rate of the overall morbidity for radical cystectomy was higher than we would like to see,” said Tracey Hong, RN, BScN, of the Clinical Quality and Patient Safety Department at Vancouver (B.C.) General Hospital. “We took this as an opportunity to improve our patient outcomes and experience.”
Vancouver General joined the American College of Surgeons National Surgical Improvement Quality Program (ACS NSQIP) in 2011. The enhanced recovery after surgery (ERAS) perioperative protocol the institution adopted in late 2014 was associated with a 32% decrease in overall morbidity. The rate dropped from 31.3% in the pre-ERAS study period from May 2011 to September 2014, to 21.1% after implementation, from October 2014 to September 2016, according to a study Ms. Hong presented at the American College of Surgeons Quality and Safety Conference.
The investigators compared outcomes between all 92 people undergoing elective radical cystectomy during the first time period to 152 consecutive patients treated under the ERAS protocol. Median length of stay decreased from 8 days before ERAS to 7 days after, a significant difference (P less than .05).
The researchers also assessed outcomes based on how adherent clinicians were to 12 key elements of the 26-item ERAS initiative. These elements included preoperative counseling, preoperative anesthesia consultation, and carbohydrate loading on the morning of surgery. Intraoperatively, they tracked normothermia, use of multimodal anesthesia, use of goal-directed fluid therapy using a monitor, timely antibiotics, and adequate postoperative nausea and vomiting prophylaxis. The four postoperative key measures were mobilization at least once by postoperative day 0, full fluids and mobilization twice on postoperative day 1, and starting solid food by postoperative day 4.
A total 52% of the ERAS cases were associated with 75% or greater adherence to these 12 key items. Adherence with the intraoperative fluid therapy and all the postoperative elements proved to be the most challenging, Ms. Hong said.
The more adherent cases experienced a lower overall postoperative morbidity rate, 15.2%, compared with 27.4% among the less adherent group. The 15.2% morbidity among the more adherent cases also compared favorably with the 31.1% rate for cases prior to ERAS adoption.
“We will continue working on improving compliance,” Ms. Hong said. “We need to increase adherence to goal-directed fluid therapy and the postoperative components,” Ms. Hong said.
Three main strategies remain essential to the ongoing success of the ERAS program, Ms. Hong said. Empowering patients to be active participants and to engage in their own health outcomes is one. “Second, we involve a multidisciplinary team at an early stage so they take ownership and get engaged in the program,” she said. “Last but not least, we continue to measure the outcomes in 100% of cases.”
Continuous auditing and sharing results with the team on a regular basis will be necessary to maintain engagement in the ERAS protocol going forward, Ms. Hong added. “Tenacity is vital.”
Ms. Hong had no relevant financial disclosures.
AT THE ACS QUALITY & SAFETY CONFERENCE
Key clinical point: An enhanced recovery after surgery protocol can reduce postoperative morbidity after radical cystectomy.
Major finding: Investigators report a 32% decrease in overall morbidity after adoption of ERAS pathways.
Data source: Comparison between 92 patients before and 152 patients after implementation of ERAS protocol.
Disclosures: Tracey Hong, BScN, had no relevant financial disclosures.
Everything We Say and Do: Digging deep brings empathy and sincere communication
Editor’s note: “Everything We Say and Do” is an informational series developed by SHM’s Patient Experience Committee to provide readers with thoughtful and actionable communication tactics that have great potential to positively impact patients’ experience of care. Each article will focus on how the contributor applies one or more of the “key communication” tactics in practice to maintain provider accountability for “everything we say and do that affects our patients’ thoughts, feelings, and well-being.”
What I say and do
I find a way to connect with my patients to express sincere appreciation.
A recent “Everything We Say and Do” column focused on an important element of high-impact physician-patient communication: closing the encounter by thanking the patient. Evidence suggests that patients feel more valued by their providers when expressions of gratitude are offered. However, it is not always easy to find a genuine and sincere way to incorporate a “thank you” at the end of a visit.
Why I do it
The physician-patient relationship is an inherently hierarchical one. Recognizing that the encounter represents a meeting of two people who equally stand to gain from the interaction goes a long way to strengthen trust, improve communication, and enhance the therapeutic effect.
How I do it
Many who don’t regularly experience serious illness firsthand take good health for granted. I appreciate my patients for reminding me to cherish my own good health. My patients offer me glimpses of hope as I watch them and their families rally through the trials that serious illness brings; in addition, they provide me inspiration and ideas for how I will handle these issues myself someday.
Some in other fields feel unfulfilled with their work as they contemplate their professional legacy. On the contrary, our patients validate our sense of purpose and strengthen our self-worth, as they allow us to participate in one of the noblest endeavors – caring for the sick. The unique insights physicians garner from patients via our intimate access to the private struggles and fears that all humans suffer, but rarely share, should strengthen our empathy for the greater human condition and enhance our own personal relationships.
Recalibrating my perspective makes it easier to harness and express sincere gratitude to patients, and enhances my ability to connect on a deeper level with those I serve.
Greg Seymann is clinical professor and vice chief for academic affairs, UCSD Division of Hospital Medicine.
Editor’s note: “Everything We Say and Do” is an informational series developed by SHM’s Patient Experience Committee to provide readers with thoughtful and actionable communication tactics that have great potential to positively impact patients’ experience of care. Each article will focus on how the contributor applies one or more of the “key communication” tactics in practice to maintain provider accountability for “everything we say and do that affects our patients’ thoughts, feelings, and well-being.”
What I say and do
I find a way to connect with my patients to express sincere appreciation.
A recent “Everything We Say and Do” column focused on an important element of high-impact physician-patient communication: closing the encounter by thanking the patient. Evidence suggests that patients feel more valued by their providers when expressions of gratitude are offered. However, it is not always easy to find a genuine and sincere way to incorporate a “thank you” at the end of a visit.
Why I do it
The physician-patient relationship is an inherently hierarchical one. Recognizing that the encounter represents a meeting of two people who equally stand to gain from the interaction goes a long way to strengthen trust, improve communication, and enhance the therapeutic effect.
How I do it
Many who don’t regularly experience serious illness firsthand take good health for granted. I appreciate my patients for reminding me to cherish my own good health. My patients offer me glimpses of hope as I watch them and their families rally through the trials that serious illness brings; in addition, they provide me inspiration and ideas for how I will handle these issues myself someday.
Some in other fields feel unfulfilled with their work as they contemplate their professional legacy. On the contrary, our patients validate our sense of purpose and strengthen our self-worth, as they allow us to participate in one of the noblest endeavors – caring for the sick. The unique insights physicians garner from patients via our intimate access to the private struggles and fears that all humans suffer, but rarely share, should strengthen our empathy for the greater human condition and enhance our own personal relationships.
Recalibrating my perspective makes it easier to harness and express sincere gratitude to patients, and enhances my ability to connect on a deeper level with those I serve.
Greg Seymann is clinical professor and vice chief for academic affairs, UCSD Division of Hospital Medicine.
Editor’s note: “Everything We Say and Do” is an informational series developed by SHM’s Patient Experience Committee to provide readers with thoughtful and actionable communication tactics that have great potential to positively impact patients’ experience of care. Each article will focus on how the contributor applies one or more of the “key communication” tactics in practice to maintain provider accountability for “everything we say and do that affects our patients’ thoughts, feelings, and well-being.”
What I say and do
I find a way to connect with my patients to express sincere appreciation.
A recent “Everything We Say and Do” column focused on an important element of high-impact physician-patient communication: closing the encounter by thanking the patient. Evidence suggests that patients feel more valued by their providers when expressions of gratitude are offered. However, it is not always easy to find a genuine and sincere way to incorporate a “thank you” at the end of a visit.
Why I do it
The physician-patient relationship is an inherently hierarchical one. Recognizing that the encounter represents a meeting of two people who equally stand to gain from the interaction goes a long way to strengthen trust, improve communication, and enhance the therapeutic effect.
How I do it
Many who don’t regularly experience serious illness firsthand take good health for granted. I appreciate my patients for reminding me to cherish my own good health. My patients offer me glimpses of hope as I watch them and their families rally through the trials that serious illness brings; in addition, they provide me inspiration and ideas for how I will handle these issues myself someday.
Some in other fields feel unfulfilled with their work as they contemplate their professional legacy. On the contrary, our patients validate our sense of purpose and strengthen our self-worth, as they allow us to participate in one of the noblest endeavors – caring for the sick. The unique insights physicians garner from patients via our intimate access to the private struggles and fears that all humans suffer, but rarely share, should strengthen our empathy for the greater human condition and enhance our own personal relationships.
Recalibrating my perspective makes it easier to harness and express sincere gratitude to patients, and enhances my ability to connect on a deeper level with those I serve.
Greg Seymann is clinical professor and vice chief for academic affairs, UCSD Division of Hospital Medicine.
Key steps to preventing patient injury
Physicians often encounter a “black box” in terms of where to focus their patient safety efforts. Obstetricians care for two patients – mothers and babies – simultaneously, which presents significant challenges. Safe obstetric care requires a multidisciplinary team with good communication skills. At the same time, gynecologic procedures are becoming more complex, and caring for a patient with complications can be quite challenging.
Breakdowns in communication often lead to adverse outcomes. Using closed obstetric malpractice claims allows practicing clinicians to understand where to focus their improvement efforts. The Doctors Company recently performed an analysis of 944 closed obstetric-gynecologic claims using a lexicon that is common to many other insurers. The study found that across patient claims, communication-related issues were common to nearly all allegations of ob.gyn.-related patient injury. The claims data revealed that key communication failures were in two major areas: communications with our patients and communication among team members, including the physician.
Physician-patient communication
Consider this example: Dr. S. is caring for a patient at term. At delivery, the infant has no heart rate and no respiratory effort and undergoes a full code and has APGAR scores of 0,0, and 3. While the indication for cesarean delivery was a nonreassuring fetal status, this was a surprising outcome. The team sent the placenta to the pathologist and there was an unusual finding of an amniotic web and the umbilical cord showed venous necrosis. A family meeting was held with the pathologist to explain the findings and the relationship to the baby’s need for support. While the presentation of the information did not change the outcome for the infant, it helped the patients and family to understand how the neonatal outcome occurred and that it was remote from delivery.
Common factors contributing to communication issues between physicians and patients/families include inadequate consent for treatment options; poor patient rapport, including unsympathetic responses to the patient; and language barriers, according to The Doctors Company’s clinical guide to improving patient safety and managing risks.
A fundamental step to address these factors is early education for patients and families about the risks and benefits of treatment, which is essential to reinforce understanding of potential interventions. Informed consent is a critical element of early education, but is often done in a rush, in a busy office setting – making it a critical risk factor. Having the informed consent conversation when the decision is made to go to surgery is important, but encouraging patients to bring forward questions and allowing their loved ones to have a clear understanding of their recovery process is also very helpful. When an unexpected complication occurs, having had a complete discussion about risks can help to mitigate an otherwise difficult situation.
We are often busy and stressed when complications arise, and the reflex is to withdraw from the patient who has suffered the complication. Demonstrating genuine empathy toward these patients allows the human side of physician-patient interaction to become apparent, which not only can reduce the patient’s and the family’s anger and frustration at a complicated situation but also can reduce the legal risk for the physician.
Provider communication
Consider another example: Ms. G is a 36-year old G1P0 who presented to the labor and delivery unit with severe preeclampsia. Failure to communicate a clear plan of action for management of her severe hypertension to all team members led to undertreated hypertension, a major stroke, and maternal death.
Both obstetrics and gynecology are specialties that require multidisciplinary teams, including nursing, anesthesia colleagues, consultants, and if at a teaching hospital, residents and medical and nursing students. Teamwork training initiatives can encourage providers to move out of their individual silos, practice more collaboratively, and share information across disciplines in a succinct and timely fashion. Understanding patients’ plans of care and competing resource demands allows a team to manage risk together.
Having a shared mental model and situation awareness across the team also creates a safety net for patients. This type of shared information is something highly reliable teams train on and communicate across disciplines, as this is not often taught in medical or nursing schools. Having clear roles and responsibilities allows professionals to create and understand expectations, especially around sharing safety concerns.
In situ simulations of high-risk situations both in labor and delivery and in the operating rooms are wonderful and instructive ways to practice teamwork behaviors. This type of exercise allows staff to practice skills together while discovering system failures in a nonthreatening environment. Later debriefing these activities allows staff to reflect on their own behavior and learn from one another, as well as to identify systems that need additional work.
Addressing patient safety risks effectively means focusing our energy and efforts toward underlying vulnerabilities that place patients at risk and increase liability for doctors. Sharing the results of lessons learned, through the evaluation of malpractice claims, helps to identify areas of vulnerabilities. Working to improve our communication with patients, families, and other providers, we can systematically lower risk to the patient and lower the risk of litigation to physicians.
Dr. Mann, an ob.gyn., is an assistant professor, part-time, at Harvard Medical School in Boston, and is a national consultant in patient safety and quality improvement in the field of obstetrics. She is a member of the Obstetrics Advisory Board of The Doctors Company. She is also a consultant at Harvard’s Risk Management Foundation, Dana-Farber Cancer Institute, and many institutions across the United States.
Physicians often encounter a “black box” in terms of where to focus their patient safety efforts. Obstetricians care for two patients – mothers and babies – simultaneously, which presents significant challenges. Safe obstetric care requires a multidisciplinary team with good communication skills. At the same time, gynecologic procedures are becoming more complex, and caring for a patient with complications can be quite challenging.
Breakdowns in communication often lead to adverse outcomes. Using closed obstetric malpractice claims allows practicing clinicians to understand where to focus their improvement efforts. The Doctors Company recently performed an analysis of 944 closed obstetric-gynecologic claims using a lexicon that is common to many other insurers. The study found that across patient claims, communication-related issues were common to nearly all allegations of ob.gyn.-related patient injury. The claims data revealed that key communication failures were in two major areas: communications with our patients and communication among team members, including the physician.
Physician-patient communication
Consider this example: Dr. S. is caring for a patient at term. At delivery, the infant has no heart rate and no respiratory effort and undergoes a full code and has APGAR scores of 0,0, and 3. While the indication for cesarean delivery was a nonreassuring fetal status, this was a surprising outcome. The team sent the placenta to the pathologist and there was an unusual finding of an amniotic web and the umbilical cord showed venous necrosis. A family meeting was held with the pathologist to explain the findings and the relationship to the baby’s need for support. While the presentation of the information did not change the outcome for the infant, it helped the patients and family to understand how the neonatal outcome occurred and that it was remote from delivery.
Common factors contributing to communication issues between physicians and patients/families include inadequate consent for treatment options; poor patient rapport, including unsympathetic responses to the patient; and language barriers, according to The Doctors Company’s clinical guide to improving patient safety and managing risks.
A fundamental step to address these factors is early education for patients and families about the risks and benefits of treatment, which is essential to reinforce understanding of potential interventions. Informed consent is a critical element of early education, but is often done in a rush, in a busy office setting – making it a critical risk factor. Having the informed consent conversation when the decision is made to go to surgery is important, but encouraging patients to bring forward questions and allowing their loved ones to have a clear understanding of their recovery process is also very helpful. When an unexpected complication occurs, having had a complete discussion about risks can help to mitigate an otherwise difficult situation.
We are often busy and stressed when complications arise, and the reflex is to withdraw from the patient who has suffered the complication. Demonstrating genuine empathy toward these patients allows the human side of physician-patient interaction to become apparent, which not only can reduce the patient’s and the family’s anger and frustration at a complicated situation but also can reduce the legal risk for the physician.
Provider communication
Consider another example: Ms. G is a 36-year old G1P0 who presented to the labor and delivery unit with severe preeclampsia. Failure to communicate a clear plan of action for management of her severe hypertension to all team members led to undertreated hypertension, a major stroke, and maternal death.
Both obstetrics and gynecology are specialties that require multidisciplinary teams, including nursing, anesthesia colleagues, consultants, and if at a teaching hospital, residents and medical and nursing students. Teamwork training initiatives can encourage providers to move out of their individual silos, practice more collaboratively, and share information across disciplines in a succinct and timely fashion. Understanding patients’ plans of care and competing resource demands allows a team to manage risk together.
Having a shared mental model and situation awareness across the team also creates a safety net for patients. This type of shared information is something highly reliable teams train on and communicate across disciplines, as this is not often taught in medical or nursing schools. Having clear roles and responsibilities allows professionals to create and understand expectations, especially around sharing safety concerns.
In situ simulations of high-risk situations both in labor and delivery and in the operating rooms are wonderful and instructive ways to practice teamwork behaviors. This type of exercise allows staff to practice skills together while discovering system failures in a nonthreatening environment. Later debriefing these activities allows staff to reflect on their own behavior and learn from one another, as well as to identify systems that need additional work.
Addressing patient safety risks effectively means focusing our energy and efforts toward underlying vulnerabilities that place patients at risk and increase liability for doctors. Sharing the results of lessons learned, through the evaluation of malpractice claims, helps to identify areas of vulnerabilities. Working to improve our communication with patients, families, and other providers, we can systematically lower risk to the patient and lower the risk of litigation to physicians.
Dr. Mann, an ob.gyn., is an assistant professor, part-time, at Harvard Medical School in Boston, and is a national consultant in patient safety and quality improvement in the field of obstetrics. She is a member of the Obstetrics Advisory Board of The Doctors Company. She is also a consultant at Harvard’s Risk Management Foundation, Dana-Farber Cancer Institute, and many institutions across the United States.
Physicians often encounter a “black box” in terms of where to focus their patient safety efforts. Obstetricians care for two patients – mothers and babies – simultaneously, which presents significant challenges. Safe obstetric care requires a multidisciplinary team with good communication skills. At the same time, gynecologic procedures are becoming more complex, and caring for a patient with complications can be quite challenging.
Breakdowns in communication often lead to adverse outcomes. Using closed obstetric malpractice claims allows practicing clinicians to understand where to focus their improvement efforts. The Doctors Company recently performed an analysis of 944 closed obstetric-gynecologic claims using a lexicon that is common to many other insurers. The study found that across patient claims, communication-related issues were common to nearly all allegations of ob.gyn.-related patient injury. The claims data revealed that key communication failures were in two major areas: communications with our patients and communication among team members, including the physician.
Physician-patient communication
Consider this example: Dr. S. is caring for a patient at term. At delivery, the infant has no heart rate and no respiratory effort and undergoes a full code and has APGAR scores of 0,0, and 3. While the indication for cesarean delivery was a nonreassuring fetal status, this was a surprising outcome. The team sent the placenta to the pathologist and there was an unusual finding of an amniotic web and the umbilical cord showed venous necrosis. A family meeting was held with the pathologist to explain the findings and the relationship to the baby’s need for support. While the presentation of the information did not change the outcome for the infant, it helped the patients and family to understand how the neonatal outcome occurred and that it was remote from delivery.
Common factors contributing to communication issues between physicians and patients/families include inadequate consent for treatment options; poor patient rapport, including unsympathetic responses to the patient; and language barriers, according to The Doctors Company’s clinical guide to improving patient safety and managing risks.
A fundamental step to address these factors is early education for patients and families about the risks and benefits of treatment, which is essential to reinforce understanding of potential interventions. Informed consent is a critical element of early education, but is often done in a rush, in a busy office setting – making it a critical risk factor. Having the informed consent conversation when the decision is made to go to surgery is important, but encouraging patients to bring forward questions and allowing their loved ones to have a clear understanding of their recovery process is also very helpful. When an unexpected complication occurs, having had a complete discussion about risks can help to mitigate an otherwise difficult situation.
We are often busy and stressed when complications arise, and the reflex is to withdraw from the patient who has suffered the complication. Demonstrating genuine empathy toward these patients allows the human side of physician-patient interaction to become apparent, which not only can reduce the patient’s and the family’s anger and frustration at a complicated situation but also can reduce the legal risk for the physician.
Provider communication
Consider another example: Ms. G is a 36-year old G1P0 who presented to the labor and delivery unit with severe preeclampsia. Failure to communicate a clear plan of action for management of her severe hypertension to all team members led to undertreated hypertension, a major stroke, and maternal death.
Both obstetrics and gynecology are specialties that require multidisciplinary teams, including nursing, anesthesia colleagues, consultants, and if at a teaching hospital, residents and medical and nursing students. Teamwork training initiatives can encourage providers to move out of their individual silos, practice more collaboratively, and share information across disciplines in a succinct and timely fashion. Understanding patients’ plans of care and competing resource demands allows a team to manage risk together.
Having a shared mental model and situation awareness across the team also creates a safety net for patients. This type of shared information is something highly reliable teams train on and communicate across disciplines, as this is not often taught in medical or nursing schools. Having clear roles and responsibilities allows professionals to create and understand expectations, especially around sharing safety concerns.
In situ simulations of high-risk situations both in labor and delivery and in the operating rooms are wonderful and instructive ways to practice teamwork behaviors. This type of exercise allows staff to practice skills together while discovering system failures in a nonthreatening environment. Later debriefing these activities allows staff to reflect on their own behavior and learn from one another, as well as to identify systems that need additional work.
Addressing patient safety risks effectively means focusing our energy and efforts toward underlying vulnerabilities that place patients at risk and increase liability for doctors. Sharing the results of lessons learned, through the evaluation of malpractice claims, helps to identify areas of vulnerabilities. Working to improve our communication with patients, families, and other providers, we can systematically lower risk to the patient and lower the risk of litigation to physicians.
Dr. Mann, an ob.gyn., is an assistant professor, part-time, at Harvard Medical School in Boston, and is a national consultant in patient safety and quality improvement in the field of obstetrics. She is a member of the Obstetrics Advisory Board of The Doctors Company. She is also a consultant at Harvard’s Risk Management Foundation, Dana-Farber Cancer Institute, and many institutions across the United States.
The Role of Methicillin-Resistant Staphylococcus aureus Polymerase Chain Reaction Nasal Swabs in Clinical Decision Making
Methicillin-resistant Staphylococcus aureus (MRSA) is a Gram positive, round bacterium. The bacteria has evolved to withstand attacks from antibiotics and has made MRSA resistant to traditional antibiotics, such as β-lactams, resulting in difficult-to-treat infections. The presence of a genetic mutation within the mecA gene, which codes for the penicillin-binding protein 2a (PBP2a), differentiates MRSA from methicillin-susceptible Staphylococcus aureus (MSSA). Presence of the PBP2a protein allows Staphylococcus aureus (S aureus)to overcome β-lactam antibiotics’ method of killing by allowing the bacteria to continue to divide and grow.
β-lactam antibiotics cause cell death in susceptible isolates by binding to penicillin-binding proteins, which inhibits transpeptidation within the cell wall via inactivation of the penicillin-binding protein. By inhibiting cell wall synthesis, the cell loses its integrity and leaks its contents, causing cell death. Penicillin-binding protein 2a is a modified protein that has a low affinity for β-lactam antibiotics, allowing MRSA to survive and making it dangerous and difficult to eradicate.
First described in 1961, MRSA’s prevalence steadily increased in the following decades. It is the most common cause of skin and soft tissue infections presenting to emergency departments in the U.S.1 About 20% of bloodstream infections are caused by S aureus, and in 2003, nearly two-thirds of hospital-onset S aureus infections were methicillin-resistant in U.S. intensive-care units (ICUs).2 It has been shown that patients with MRSA bacteremia have worse overall outcomes, including increased mortality, greater lengths of stay, and increased costs, compared with those with MSSA infections.2,3 In 2011, MRSA infections caused an estimated 11,000 deaths, making fast and accurate detection of MRSA a crucial step in appropriate antimicrobial therapy selection.4
Currently, the Clinical and Laboratory Standards Institute (CLSI) recommends testing for MRSA by using phenotypic or genotypic methods. Phenotypic methods test for the observable characteristics of an organism, whereas a genotypic method identifies the specific gene that the organism carries. Recommended phenotypic methods include the latex agglutination test for PBP2a, the cefoxitin disk screen test, and a plate containing 6 μg/mL of oxacillin in Mueller-Hinton agar supplemented with sodium chloride.5 These methods have varying sensitivity and specificity and take between 48 to 72 hours to provide a result.
Within the past 15 years, a newer, genotypic, method of MRSA detection was approved by the FDA with high sensitivity and specificity. This method uses polymerase chain reaction (PCR) to identify the mecA gene. Polymerase chain reaction is a technique used to copy and amplify a specific segment of DNA, making thousands to millions of copies. If present, the MRSA PCR amplifies the mecA gene that makes S aureus resistant to methicillin and other β-lactams, which confirms that the specimen contains MRSA. The FDA has approved the use of MRSA PCR nasal swabs to detect MRSA in patients at risk of nasal colonization. While previously discussed methods may take between 2 and 3 days to confirm presence of MRSA, PCR can identify MRSA in about 1 hour.6
If a S aureus infection is suspected, empiric therapy often includes coverage of both MSSA and MRSA, due to the high morbidity and mortality associated with these infections. However, continuing an unneeded or unduly broad antibiotic, such as those that cover MRSA, can cause unintended consequences, such as toxicities, emerging resistance, or selection for pathogenic organisms.7 Therefore, empiric broad antibiotic therapy should be de-escalated as soon as possible, which further emphasizes the need for quick and accurate detection of the infecting organism. De-escalation of therapy can lead to a shorter length of stay and decreased mortality.8,9 Conversely, quick identification of infections caused by MRSA would allow therapy to be broadened to cover MRSA in infected patients, which could potentially decrease patient morbidity and mortality.
Nasal MRSA PCR Colonization
Rapid identification of a causative organism is crucial to determine appropriate antibiotic therapy. Fortunately, PCR is a very rapid method of detecting MRSA, and the use of MRSA PCR nasal swabs may be an effective way to predict whether MRSA is the organism causing an infection at various anatomical sites. If a patient has a suspected infection on admission, a MRSA PCR nasal swab often is completed to determine whether a patient’s nares are colonized with MRSA. However, there is no clear consensus in the literature regarding the correlation between MRSA nasal colonization and an infection caused by MRSA, making it difficult for clinicians to confidently de-escalate therapy on a negative MRSA PCR or broaden therapy on a positive result. The purpose of this literature review was to determine whether a MRSA PCR nasal swab can be used as a surrogate marker for MRSA infections at various sites.
Pneumonia has many potential causative organisms, many of which are covered empirically with guideline-directed therapy. The predictive power of MRSA PCR nasal swabs may allow clinicians to prescribe earlier directed therapy. A retrospective cohort study performed at a tertiary care center looked at the clinical usefulness of a MRSA PCR nasal swab in the treatment of pneumonia.10 Patients were included in the trial if they had a MRSA PCR nasal swab within 1 month of their blood or sputum culture as well as confirmed pneumonia. After analysis of 435 patients, the MRSA PCR nasal swab showed the following performance characteristics for detecting culture-proven MRSA: 88.0% sensitivity, 90.1% specificity, 35.4% positive predictive value (PPV), and 99.2% negative predictive value (NPV). Due to the high negative predictive value, the results indicated that discontinuation of MRSA antibiotic coverage would be appropriate for noncritically ill patients with pneumonia who had a negative MRSA PCR nasal swab.
Another retrospective study was performed by Johnson and colleagues to determine the association between MRSA PCR nasal swabs and the causative organism in pneumonia.11 Patients were included in the trial if they had a MRSA PCR nasal swab and a lower respiratory culture yielding S aureus within 48 hours of hospital admission. After analysis of 72 patients, MRSA PCR nasal swabs demonstrated the following diagnostic characteristics for detecting culture-proven MRSA: 93.3% sensitivity, 95.2% specificity, 93.3%PPV, and 95.2% NPV. These results suggest that early nasal swab MRSA PCR tests can predict the absence of MRSA reliably and may help guide the discontinuation of MRSA-directed empiric antibiotic therapy.
In addition, Giancola retrospectively studied the relationship between MRSA PCR nasal swabs and the likelihood of pneumonia caused by MRSA in intensive and intermediate care units.12 An analysis of 200 patients revealed high concordance between respiratory cultures and MRSA PCR nasal swab results with the following characteristics: 90.5% sensitivity, 79.9% specificity, 34.5% PPV, and 98.6% NPV. These test characteristics suggested that MRSA PCR nasal swabs might be a useful stewardship tool to allow for discontinuation of anti-MRSA therapy in critically ill patients with confirmed pneumonia.
Another retrospective analysis conducted by Baby and colleagues took a different approach to determine the clinical usefulness of MRSA PCR nasal swabs in the treatment of pneumonia.13 The primary outcome, mean duration of MRSA-targeted therapy, was reduced by 46.6 hours in the group who received a pharmacist-ordered MRSA PCR nasal swab compared with the group that did not receive a MRSA PCR nasal swab (P < .01) Per protocol, pharmacists were authorized to order a MRSA PCR nasal swab for patients who were prescribed vancomycin or linezolid for pneumonia. On receipt of the MRSA PCR nasal swab results, pharmacists were instructed to recommend discontinuation of anti-MRSA therapy if the PCR was negative for MRSA.
Results of this study indicated there were no significant differences in time to clinical improvement between preprotocol and postprotocol implementation (1.8 days vs 2.3 days, respectively; P = .54), length of stay (11.0 days vs 8.2 days, respectively; P = .22), or mortality (14.8% vs 6.7%, respectively; P = .41). The MRSA PCR nasal swabs allowed for a reduction in duration of anti-MRSA therapy without adverse effects on outcomes and provided a statistically significant reduction in the incidence of acute kidney injury during therapy in the postprotocol implementation group (26% vs 3.3%; P = .02), likely due to decreased exposure to vancomycin. Collectively, these studies indicate that MRSA PCR nasal swabs can be clinically useful in making decisions regarding discontinuation of MRSA-targeted therapy in pneumonia when MRSA PCR nasal swabs are negative.
A wider variety of infection sites were studied in a 2008 retrospective review of nearly 5,800 MRSA PCR nasal swabs taken within 24 hours (before or after) of a clinical culture that resulted growth of any organism.14 The goal of this study was to determine whether MRSA nasal colonization could predict MRSA involvement at various suspected infection sites. Overall, 217 patients (67.2%) with positive MRSA clinical cultures had a positive MRSA PCR nasal swab. The concordance between MRSA PCR nasal swabs and infection sites was highest with positive urine cultures (77%) and lowest in “other” infection sites (60%, primarily abdomen, buttock, and breast). Respiratory infections showed a 75% concordance between MRSA PCR nasal swabs and infection sites, as well as the following characteristics: 75% sensitivity, 90% specificity, 30% PPV, and 98% NPV. Additionally, infection site concordance was higher when clinical cultures grew clindamycin-resistant MRSA (71.3%) vs clindamycin-susceptible MRSA (59.3%; P = .04).
Overall, a positive MRSA PCR nasal swab increased the likelihood of MRSA at the primary infection site but was not clinically significant or consistent across infection sites. As seen in other studies, a negative MRSA PCR nasal swab could be useful for lowering concern for MRSA involvement in the primary infection, as evidenced by the following characteristics for all infection sites: 67% sensitivity, 90% specificity, 27% PPV, and 98% NPV.
Sarkionda and colleagues evaluated the clinical usefulness of MRSA PCR nasal swabs in the ICU setting in patients with a lower respiratory tract infection (RTI) or bloodstream infection.15 A total of 749 patients received a MRSA PCR nasal swab before admission to the ICU and were included in this study. The concordance between MRSA PCR nasal swabs and the causative organism was analyzed in patients who developed a MRSA lower respiratory infection (N = 120) and a MRSA bloodstream infection (N = 78) and demonstrated the following characteristics: 24.2% sensitivity, 78.5% specificity, 17.7% PPV, and 84.4% NPV; and 23.1% sensitivity, 78.2% specificity, 11.0% PPV, and 89.7% NPV, respectively. The authors concluded that the MRSA nasal swab results are not useful for making decisions regarding the need of empiric antimicrobial therapy targeting MRSA infections in lower respiratory infections and bloodstream infections. However, due to the high NPV in this study, one might conclude that negative MRSA PCR nasal swabs could still be used to de-escalate therapy, which is in agreement with the results from Dangerfield and Johnson.10,11
Similarly, results from a retrospective chart review demonstrated a lack of predictive value by the MRSA PCR nasal swab.16 Of 1,203 adult patients admitted to an ICU at a single center, 57 positive MRSA colonized and 122 negative MRSA colonized patients’ charts were randomly selected. The presence of MRSA lower RTI or bloodstream infections was found to be 3.51% vs 2.46% in the colonized and noncolonized groups, respectively (P = .46). These results led to the conclusion that a positive MRSA PCR nasal swab alone should not be used to make decisions regarding empiric MRSA antibiotic coverage.
An alternative approach to MRSA surveillance was taken by Harris in a prospective cohort of 12,080 adults with a suspected infection on admission to a non-ICU.17 Patients were screened with a 2-question tool to determine whether they were high risk for a MRSA infection. The 2 questions were “Have you been admitted to any health care facility in the last 12 months?” and “Do you have a skin infection (eg, boil, abscess, spider bite, or cellulitis) at this time?” If patients answered yes to either question, they were considered high risk, and a MRSA PCR nasal swab was ordered.
Patients who answered no to both questions were considered low risk and did not receive a MRSA PCR nasal swab. In total, 623 of 5,609 patients (11.1%) identified as high risk had a positive MRSA PCR nasal swab, and 148 of these 623 patients (23.8%) developed a MRSA-positive clinical culture. Only 121 of 4,986 patients (2.4%) who were high risk and had a negative MRSA PCR nasal swab went on to develop a MRSA-positive clinical culture (98% NPV). Additionally, 104 of 6,741 patients (1.6%) who answered no to both screening questions developed a MRSA-positive clinical culture (98% NPV). Results indicated that a high percentage of patients who were at high risk for MRSA (yes response to either question) and had a positive MRSA PCR nasal swab also had a positive clinical culture for MRSA. Conversely, a very small percentage of high-risk patients with a negative MRSA PCR nasal swab developed a positive clinical culture for MRSA.
The screening tool proved very effective as the low-risk group had the lowest number of patients (1.6%) develop a positive clinical culture for MRSA. It may be deduced that combination use of MRSA colonization testing via PCR nasal swabs in conjunction with a screening tool may be an effective method to identify patients in whom anti-MRSA therapy can be safely discontinued.
Conclusion
Based on the results of previously described studies, sufficient data may exist to support the discontinuation of MRSA-targeted therapy in noncritically ill patients with confirmed or suspected pneumonia and a negative MRSA PCR nasal swab. Insufficient evidence exists, however, to support a broadening of antimicrobial therapy to include anti-MRSA coverage in individuals with a positive MRSA PCR nasal swab, regardless of the infection site.
Clinical judgment should be used when determining empiric antimicrobial therapy and for appropriateness of de-escalation of therapy in critically ill patients. Once a patient stabilizes, a negative MRSA PCR nasal swab could be considered as supporting evidence to discontinue anti-MRSA therapy, especially in patients with lower respiratory infections, such as pneumonia.
1. Moran GJ, Krishnadasan A, Gorwitz RJ, et al; EMERGEncy ID Net Study Group. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666-674.
2. Klevens RM, Morrison MA, Nadle J, et al; Active Bacterial Core surveillance (ABCs) MRSA Investigators. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298(15):1763-1771.
3. Cosgrove SE, Fowler VG Jr. Management of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46(suppl 5):S386-S393.
4. Dantes R, Mu Y, Belflower R, et al; Emerging Infections Program-Active Bacterial Core Surveillance MRSA Surveillance Investigators. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173(21):1970-1978.
5. Pillai MM, Latha R, Sarkar G. Detection of methicillin resistance in Staphylococcus aureus by polymerase chain reaction and conventional methods: a comparative study. J Lab Physicians. 2012;4(2):83-88.
6. Peterson LR, Liesenfeld O, Woods CW, et al. Multicenter evaluation of the LightCycler methicillin-resistant Staphylococcus aureus (MRSA) advanced test as a rapid method for detection of MRSA in nasal surveillance swabs. J Clin Microbiol. 2010;48(5):1661-1666.
7. File TM Jr, Srinivasan A, Bartlett JG. Antimicrobial stewardship: important for patient and public health. Clin Infect Dis. 2014;59(suppl 3):S93-S96.
8. Viasus D, Simonetti AF, Garcia-Vidal C, Niubó J, Dorca J, Carratalà J. Impact of antibiotic de-escalation on clinical outcomes in community-acquired pneumococcal pneumonia. J Antimicrob Chemother. 2017;72(2):547-5553.
9. Paul M, Dickstein Y, Raz-Pasteur A. Antibiotic de-escalation for bloodstream infections and pneumonia: a systematic review and meta-analysis. Clin Microbiol Infect. 2016;22(12):960-967.
10. Dangerfield B, Chung A, Webb B, Seville MT. Predictive value of methicillin-resistant Staphylococcus aureus (MRSA) nasal swab PCR assay for MRSA pneumonia. Antimicrob Agents Chemother. 2014;58(2):859-864.
11. Johnson JA, Wright ME, Sheperd LA, Musher DM, Dang BN. Nasal methicillin-resistant Staphylococcus aureus polymerase chain reaction a potential use in guiding antibiotic therapy for pneumonia. Perm J. 2015;19(1):34-36.
12. Giancola SE, Nguyen AT, Le B, et al. Clinical utility of a nasal swab methicillin-resistant Staphylococcus aureus polymerase chain reaction test in intensive and intermediate care unit patients with pneumonia. Diagn Microbiol Infect Dis. 2016;86(3):307-310.
13. Baby N, Faust AC, Smith T, Sheperd LA, Knoll L, Goodman EL. Nasal methicillin-resistant Staphylococcus aureus (MRSA) PCR testing reduces the duration of MRSA-targeted therapy in patients with suspected MRSA pneumonia. Antimicrob Agents Chemother. 2017;61(4):e02432-16.
14. Robicsek A, Suseno M, Beaumont JL, Thomson RB Jr, Peterson LR. Prediction of methicillin-resistant Staphylococcus aureus involvement in disease sites by concomitant nasal sampling. J Clin Microbiol. 2008;46(2):588-592.
15. Sarkionda KV, Micek ST, Dohery JA, Reichley RM, Warren D, Kollef MH. Methicillin-resistant Staphylococcus aureus nasal colonization is a poor predictor of intensive care unit-acquired methicillin-resistant Staphylococcus aureus infections requiring antibiotic treatment. Crit Care Med. 2010;38(10):1991-1995.
16. Ghidey F, Igbinosa O, Igbinosa E. Nasal colonization of methicillin resistant Staphylococcus aureus (MRSA) does not predict subsequent infection in the intensive care unit. Beni-Seuf University J Basic Appl Sci
17. Harris, AD, Furuno JP, Roghmann MC, et al. Targeted surveillance of methicillin-resistant Staphylococcus aureus and its potential use to guide empiric antibiotic therapy. Antimicrob Agents Chemother. 2010;54(8):3143-3148.
Methicillin-resistant Staphylococcus aureus (MRSA) is a Gram positive, round bacterium. The bacteria has evolved to withstand attacks from antibiotics and has made MRSA resistant to traditional antibiotics, such as β-lactams, resulting in difficult-to-treat infections. The presence of a genetic mutation within the mecA gene, which codes for the penicillin-binding protein 2a (PBP2a), differentiates MRSA from methicillin-susceptible Staphylococcus aureus (MSSA). Presence of the PBP2a protein allows Staphylococcus aureus (S aureus)to overcome β-lactam antibiotics’ method of killing by allowing the bacteria to continue to divide and grow.
β-lactam antibiotics cause cell death in susceptible isolates by binding to penicillin-binding proteins, which inhibits transpeptidation within the cell wall via inactivation of the penicillin-binding protein. By inhibiting cell wall synthesis, the cell loses its integrity and leaks its contents, causing cell death. Penicillin-binding protein 2a is a modified protein that has a low affinity for β-lactam antibiotics, allowing MRSA to survive and making it dangerous and difficult to eradicate.
First described in 1961, MRSA’s prevalence steadily increased in the following decades. It is the most common cause of skin and soft tissue infections presenting to emergency departments in the U.S.1 About 20% of bloodstream infections are caused by S aureus, and in 2003, nearly two-thirds of hospital-onset S aureus infections were methicillin-resistant in U.S. intensive-care units (ICUs).2 It has been shown that patients with MRSA bacteremia have worse overall outcomes, including increased mortality, greater lengths of stay, and increased costs, compared with those with MSSA infections.2,3 In 2011, MRSA infections caused an estimated 11,000 deaths, making fast and accurate detection of MRSA a crucial step in appropriate antimicrobial therapy selection.4
Currently, the Clinical and Laboratory Standards Institute (CLSI) recommends testing for MRSA by using phenotypic or genotypic methods. Phenotypic methods test for the observable characteristics of an organism, whereas a genotypic method identifies the specific gene that the organism carries. Recommended phenotypic methods include the latex agglutination test for PBP2a, the cefoxitin disk screen test, and a plate containing 6 μg/mL of oxacillin in Mueller-Hinton agar supplemented with sodium chloride.5 These methods have varying sensitivity and specificity and take between 48 to 72 hours to provide a result.
Within the past 15 years, a newer, genotypic, method of MRSA detection was approved by the FDA with high sensitivity and specificity. This method uses polymerase chain reaction (PCR) to identify the mecA gene. Polymerase chain reaction is a technique used to copy and amplify a specific segment of DNA, making thousands to millions of copies. If present, the MRSA PCR amplifies the mecA gene that makes S aureus resistant to methicillin and other β-lactams, which confirms that the specimen contains MRSA. The FDA has approved the use of MRSA PCR nasal swabs to detect MRSA in patients at risk of nasal colonization. While previously discussed methods may take between 2 and 3 days to confirm presence of MRSA, PCR can identify MRSA in about 1 hour.6
If a S aureus infection is suspected, empiric therapy often includes coverage of both MSSA and MRSA, due to the high morbidity and mortality associated with these infections. However, continuing an unneeded or unduly broad antibiotic, such as those that cover MRSA, can cause unintended consequences, such as toxicities, emerging resistance, or selection for pathogenic organisms.7 Therefore, empiric broad antibiotic therapy should be de-escalated as soon as possible, which further emphasizes the need for quick and accurate detection of the infecting organism. De-escalation of therapy can lead to a shorter length of stay and decreased mortality.8,9 Conversely, quick identification of infections caused by MRSA would allow therapy to be broadened to cover MRSA in infected patients, which could potentially decrease patient morbidity and mortality.
Nasal MRSA PCR Colonization
Rapid identification of a causative organism is crucial to determine appropriate antibiotic therapy. Fortunately, PCR is a very rapid method of detecting MRSA, and the use of MRSA PCR nasal swabs may be an effective way to predict whether MRSA is the organism causing an infection at various anatomical sites. If a patient has a suspected infection on admission, a MRSA PCR nasal swab often is completed to determine whether a patient’s nares are colonized with MRSA. However, there is no clear consensus in the literature regarding the correlation between MRSA nasal colonization and an infection caused by MRSA, making it difficult for clinicians to confidently de-escalate therapy on a negative MRSA PCR or broaden therapy on a positive result. The purpose of this literature review was to determine whether a MRSA PCR nasal swab can be used as a surrogate marker for MRSA infections at various sites.
Pneumonia has many potential causative organisms, many of which are covered empirically with guideline-directed therapy. The predictive power of MRSA PCR nasal swabs may allow clinicians to prescribe earlier directed therapy. A retrospective cohort study performed at a tertiary care center looked at the clinical usefulness of a MRSA PCR nasal swab in the treatment of pneumonia.10 Patients were included in the trial if they had a MRSA PCR nasal swab within 1 month of their blood or sputum culture as well as confirmed pneumonia. After analysis of 435 patients, the MRSA PCR nasal swab showed the following performance characteristics for detecting culture-proven MRSA: 88.0% sensitivity, 90.1% specificity, 35.4% positive predictive value (PPV), and 99.2% negative predictive value (NPV). Due to the high negative predictive value, the results indicated that discontinuation of MRSA antibiotic coverage would be appropriate for noncritically ill patients with pneumonia who had a negative MRSA PCR nasal swab.
Another retrospective study was performed by Johnson and colleagues to determine the association between MRSA PCR nasal swabs and the causative organism in pneumonia.11 Patients were included in the trial if they had a MRSA PCR nasal swab and a lower respiratory culture yielding S aureus within 48 hours of hospital admission. After analysis of 72 patients, MRSA PCR nasal swabs demonstrated the following diagnostic characteristics for detecting culture-proven MRSA: 93.3% sensitivity, 95.2% specificity, 93.3%PPV, and 95.2% NPV. These results suggest that early nasal swab MRSA PCR tests can predict the absence of MRSA reliably and may help guide the discontinuation of MRSA-directed empiric antibiotic therapy.
In addition, Giancola retrospectively studied the relationship between MRSA PCR nasal swabs and the likelihood of pneumonia caused by MRSA in intensive and intermediate care units.12 An analysis of 200 patients revealed high concordance between respiratory cultures and MRSA PCR nasal swab results with the following characteristics: 90.5% sensitivity, 79.9% specificity, 34.5% PPV, and 98.6% NPV. These test characteristics suggested that MRSA PCR nasal swabs might be a useful stewardship tool to allow for discontinuation of anti-MRSA therapy in critically ill patients with confirmed pneumonia.
Another retrospective analysis conducted by Baby and colleagues took a different approach to determine the clinical usefulness of MRSA PCR nasal swabs in the treatment of pneumonia.13 The primary outcome, mean duration of MRSA-targeted therapy, was reduced by 46.6 hours in the group who received a pharmacist-ordered MRSA PCR nasal swab compared with the group that did not receive a MRSA PCR nasal swab (P < .01) Per protocol, pharmacists were authorized to order a MRSA PCR nasal swab for patients who were prescribed vancomycin or linezolid for pneumonia. On receipt of the MRSA PCR nasal swab results, pharmacists were instructed to recommend discontinuation of anti-MRSA therapy if the PCR was negative for MRSA.
Results of this study indicated there were no significant differences in time to clinical improvement between preprotocol and postprotocol implementation (1.8 days vs 2.3 days, respectively; P = .54), length of stay (11.0 days vs 8.2 days, respectively; P = .22), or mortality (14.8% vs 6.7%, respectively; P = .41). The MRSA PCR nasal swabs allowed for a reduction in duration of anti-MRSA therapy without adverse effects on outcomes and provided a statistically significant reduction in the incidence of acute kidney injury during therapy in the postprotocol implementation group (26% vs 3.3%; P = .02), likely due to decreased exposure to vancomycin. Collectively, these studies indicate that MRSA PCR nasal swabs can be clinically useful in making decisions regarding discontinuation of MRSA-targeted therapy in pneumonia when MRSA PCR nasal swabs are negative.
A wider variety of infection sites were studied in a 2008 retrospective review of nearly 5,800 MRSA PCR nasal swabs taken within 24 hours (before or after) of a clinical culture that resulted growth of any organism.14 The goal of this study was to determine whether MRSA nasal colonization could predict MRSA involvement at various suspected infection sites. Overall, 217 patients (67.2%) with positive MRSA clinical cultures had a positive MRSA PCR nasal swab. The concordance between MRSA PCR nasal swabs and infection sites was highest with positive urine cultures (77%) and lowest in “other” infection sites (60%, primarily abdomen, buttock, and breast). Respiratory infections showed a 75% concordance between MRSA PCR nasal swabs and infection sites, as well as the following characteristics: 75% sensitivity, 90% specificity, 30% PPV, and 98% NPV. Additionally, infection site concordance was higher when clinical cultures grew clindamycin-resistant MRSA (71.3%) vs clindamycin-susceptible MRSA (59.3%; P = .04).
Overall, a positive MRSA PCR nasal swab increased the likelihood of MRSA at the primary infection site but was not clinically significant or consistent across infection sites. As seen in other studies, a negative MRSA PCR nasal swab could be useful for lowering concern for MRSA involvement in the primary infection, as evidenced by the following characteristics for all infection sites: 67% sensitivity, 90% specificity, 27% PPV, and 98% NPV.
Sarkionda and colleagues evaluated the clinical usefulness of MRSA PCR nasal swabs in the ICU setting in patients with a lower respiratory tract infection (RTI) or bloodstream infection.15 A total of 749 patients received a MRSA PCR nasal swab before admission to the ICU and were included in this study. The concordance between MRSA PCR nasal swabs and the causative organism was analyzed in patients who developed a MRSA lower respiratory infection (N = 120) and a MRSA bloodstream infection (N = 78) and demonstrated the following characteristics: 24.2% sensitivity, 78.5% specificity, 17.7% PPV, and 84.4% NPV; and 23.1% sensitivity, 78.2% specificity, 11.0% PPV, and 89.7% NPV, respectively. The authors concluded that the MRSA nasal swab results are not useful for making decisions regarding the need of empiric antimicrobial therapy targeting MRSA infections in lower respiratory infections and bloodstream infections. However, due to the high NPV in this study, one might conclude that negative MRSA PCR nasal swabs could still be used to de-escalate therapy, which is in agreement with the results from Dangerfield and Johnson.10,11
Similarly, results from a retrospective chart review demonstrated a lack of predictive value by the MRSA PCR nasal swab.16 Of 1,203 adult patients admitted to an ICU at a single center, 57 positive MRSA colonized and 122 negative MRSA colonized patients’ charts were randomly selected. The presence of MRSA lower RTI or bloodstream infections was found to be 3.51% vs 2.46% in the colonized and noncolonized groups, respectively (P = .46). These results led to the conclusion that a positive MRSA PCR nasal swab alone should not be used to make decisions regarding empiric MRSA antibiotic coverage.
An alternative approach to MRSA surveillance was taken by Harris in a prospective cohort of 12,080 adults with a suspected infection on admission to a non-ICU.17 Patients were screened with a 2-question tool to determine whether they were high risk for a MRSA infection. The 2 questions were “Have you been admitted to any health care facility in the last 12 months?” and “Do you have a skin infection (eg, boil, abscess, spider bite, or cellulitis) at this time?” If patients answered yes to either question, they were considered high risk, and a MRSA PCR nasal swab was ordered.
Patients who answered no to both questions were considered low risk and did not receive a MRSA PCR nasal swab. In total, 623 of 5,609 patients (11.1%) identified as high risk had a positive MRSA PCR nasal swab, and 148 of these 623 patients (23.8%) developed a MRSA-positive clinical culture. Only 121 of 4,986 patients (2.4%) who were high risk and had a negative MRSA PCR nasal swab went on to develop a MRSA-positive clinical culture (98% NPV). Additionally, 104 of 6,741 patients (1.6%) who answered no to both screening questions developed a MRSA-positive clinical culture (98% NPV). Results indicated that a high percentage of patients who were at high risk for MRSA (yes response to either question) and had a positive MRSA PCR nasal swab also had a positive clinical culture for MRSA. Conversely, a very small percentage of high-risk patients with a negative MRSA PCR nasal swab developed a positive clinical culture for MRSA.
The screening tool proved very effective as the low-risk group had the lowest number of patients (1.6%) develop a positive clinical culture for MRSA. It may be deduced that combination use of MRSA colonization testing via PCR nasal swabs in conjunction with a screening tool may be an effective method to identify patients in whom anti-MRSA therapy can be safely discontinued.
Conclusion
Based on the results of previously described studies, sufficient data may exist to support the discontinuation of MRSA-targeted therapy in noncritically ill patients with confirmed or suspected pneumonia and a negative MRSA PCR nasal swab. Insufficient evidence exists, however, to support a broadening of antimicrobial therapy to include anti-MRSA coverage in individuals with a positive MRSA PCR nasal swab, regardless of the infection site.
Clinical judgment should be used when determining empiric antimicrobial therapy and for appropriateness of de-escalation of therapy in critically ill patients. Once a patient stabilizes, a negative MRSA PCR nasal swab could be considered as supporting evidence to discontinue anti-MRSA therapy, especially in patients with lower respiratory infections, such as pneumonia.
Methicillin-resistant Staphylococcus aureus (MRSA) is a Gram positive, round bacterium. The bacteria has evolved to withstand attacks from antibiotics and has made MRSA resistant to traditional antibiotics, such as β-lactams, resulting in difficult-to-treat infections. The presence of a genetic mutation within the mecA gene, which codes for the penicillin-binding protein 2a (PBP2a), differentiates MRSA from methicillin-susceptible Staphylococcus aureus (MSSA). Presence of the PBP2a protein allows Staphylococcus aureus (S aureus)to overcome β-lactam antibiotics’ method of killing by allowing the bacteria to continue to divide and grow.
β-lactam antibiotics cause cell death in susceptible isolates by binding to penicillin-binding proteins, which inhibits transpeptidation within the cell wall via inactivation of the penicillin-binding protein. By inhibiting cell wall synthesis, the cell loses its integrity and leaks its contents, causing cell death. Penicillin-binding protein 2a is a modified protein that has a low affinity for β-lactam antibiotics, allowing MRSA to survive and making it dangerous and difficult to eradicate.
First described in 1961, MRSA’s prevalence steadily increased in the following decades. It is the most common cause of skin and soft tissue infections presenting to emergency departments in the U.S.1 About 20% of bloodstream infections are caused by S aureus, and in 2003, nearly two-thirds of hospital-onset S aureus infections were methicillin-resistant in U.S. intensive-care units (ICUs).2 It has been shown that patients with MRSA bacteremia have worse overall outcomes, including increased mortality, greater lengths of stay, and increased costs, compared with those with MSSA infections.2,3 In 2011, MRSA infections caused an estimated 11,000 deaths, making fast and accurate detection of MRSA a crucial step in appropriate antimicrobial therapy selection.4
Currently, the Clinical and Laboratory Standards Institute (CLSI) recommends testing for MRSA by using phenotypic or genotypic methods. Phenotypic methods test for the observable characteristics of an organism, whereas a genotypic method identifies the specific gene that the organism carries. Recommended phenotypic methods include the latex agglutination test for PBP2a, the cefoxitin disk screen test, and a plate containing 6 μg/mL of oxacillin in Mueller-Hinton agar supplemented with sodium chloride.5 These methods have varying sensitivity and specificity and take between 48 to 72 hours to provide a result.
Within the past 15 years, a newer, genotypic, method of MRSA detection was approved by the FDA with high sensitivity and specificity. This method uses polymerase chain reaction (PCR) to identify the mecA gene. Polymerase chain reaction is a technique used to copy and amplify a specific segment of DNA, making thousands to millions of copies. If present, the MRSA PCR amplifies the mecA gene that makes S aureus resistant to methicillin and other β-lactams, which confirms that the specimen contains MRSA. The FDA has approved the use of MRSA PCR nasal swabs to detect MRSA in patients at risk of nasal colonization. While previously discussed methods may take between 2 and 3 days to confirm presence of MRSA, PCR can identify MRSA in about 1 hour.6
If a S aureus infection is suspected, empiric therapy often includes coverage of both MSSA and MRSA, due to the high morbidity and mortality associated with these infections. However, continuing an unneeded or unduly broad antibiotic, such as those that cover MRSA, can cause unintended consequences, such as toxicities, emerging resistance, or selection for pathogenic organisms.7 Therefore, empiric broad antibiotic therapy should be de-escalated as soon as possible, which further emphasizes the need for quick and accurate detection of the infecting organism. De-escalation of therapy can lead to a shorter length of stay and decreased mortality.8,9 Conversely, quick identification of infections caused by MRSA would allow therapy to be broadened to cover MRSA in infected patients, which could potentially decrease patient morbidity and mortality.
Nasal MRSA PCR Colonization
Rapid identification of a causative organism is crucial to determine appropriate antibiotic therapy. Fortunately, PCR is a very rapid method of detecting MRSA, and the use of MRSA PCR nasal swabs may be an effective way to predict whether MRSA is the organism causing an infection at various anatomical sites. If a patient has a suspected infection on admission, a MRSA PCR nasal swab often is completed to determine whether a patient’s nares are colonized with MRSA. However, there is no clear consensus in the literature regarding the correlation between MRSA nasal colonization and an infection caused by MRSA, making it difficult for clinicians to confidently de-escalate therapy on a negative MRSA PCR or broaden therapy on a positive result. The purpose of this literature review was to determine whether a MRSA PCR nasal swab can be used as a surrogate marker for MRSA infections at various sites.
Pneumonia has many potential causative organisms, many of which are covered empirically with guideline-directed therapy. The predictive power of MRSA PCR nasal swabs may allow clinicians to prescribe earlier directed therapy. A retrospective cohort study performed at a tertiary care center looked at the clinical usefulness of a MRSA PCR nasal swab in the treatment of pneumonia.10 Patients were included in the trial if they had a MRSA PCR nasal swab within 1 month of their blood or sputum culture as well as confirmed pneumonia. After analysis of 435 patients, the MRSA PCR nasal swab showed the following performance characteristics for detecting culture-proven MRSA: 88.0% sensitivity, 90.1% specificity, 35.4% positive predictive value (PPV), and 99.2% negative predictive value (NPV). Due to the high negative predictive value, the results indicated that discontinuation of MRSA antibiotic coverage would be appropriate for noncritically ill patients with pneumonia who had a negative MRSA PCR nasal swab.
Another retrospective study was performed by Johnson and colleagues to determine the association between MRSA PCR nasal swabs and the causative organism in pneumonia.11 Patients were included in the trial if they had a MRSA PCR nasal swab and a lower respiratory culture yielding S aureus within 48 hours of hospital admission. After analysis of 72 patients, MRSA PCR nasal swabs demonstrated the following diagnostic characteristics for detecting culture-proven MRSA: 93.3% sensitivity, 95.2% specificity, 93.3%PPV, and 95.2% NPV. These results suggest that early nasal swab MRSA PCR tests can predict the absence of MRSA reliably and may help guide the discontinuation of MRSA-directed empiric antibiotic therapy.
In addition, Giancola retrospectively studied the relationship between MRSA PCR nasal swabs and the likelihood of pneumonia caused by MRSA in intensive and intermediate care units.12 An analysis of 200 patients revealed high concordance between respiratory cultures and MRSA PCR nasal swab results with the following characteristics: 90.5% sensitivity, 79.9% specificity, 34.5% PPV, and 98.6% NPV. These test characteristics suggested that MRSA PCR nasal swabs might be a useful stewardship tool to allow for discontinuation of anti-MRSA therapy in critically ill patients with confirmed pneumonia.
Another retrospective analysis conducted by Baby and colleagues took a different approach to determine the clinical usefulness of MRSA PCR nasal swabs in the treatment of pneumonia.13 The primary outcome, mean duration of MRSA-targeted therapy, was reduced by 46.6 hours in the group who received a pharmacist-ordered MRSA PCR nasal swab compared with the group that did not receive a MRSA PCR nasal swab (P < .01) Per protocol, pharmacists were authorized to order a MRSA PCR nasal swab for patients who were prescribed vancomycin or linezolid for pneumonia. On receipt of the MRSA PCR nasal swab results, pharmacists were instructed to recommend discontinuation of anti-MRSA therapy if the PCR was negative for MRSA.
Results of this study indicated there were no significant differences in time to clinical improvement between preprotocol and postprotocol implementation (1.8 days vs 2.3 days, respectively; P = .54), length of stay (11.0 days vs 8.2 days, respectively; P = .22), or mortality (14.8% vs 6.7%, respectively; P = .41). The MRSA PCR nasal swabs allowed for a reduction in duration of anti-MRSA therapy without adverse effects on outcomes and provided a statistically significant reduction in the incidence of acute kidney injury during therapy in the postprotocol implementation group (26% vs 3.3%; P = .02), likely due to decreased exposure to vancomycin. Collectively, these studies indicate that MRSA PCR nasal swabs can be clinically useful in making decisions regarding discontinuation of MRSA-targeted therapy in pneumonia when MRSA PCR nasal swabs are negative.
A wider variety of infection sites were studied in a 2008 retrospective review of nearly 5,800 MRSA PCR nasal swabs taken within 24 hours (before or after) of a clinical culture that resulted growth of any organism.14 The goal of this study was to determine whether MRSA nasal colonization could predict MRSA involvement at various suspected infection sites. Overall, 217 patients (67.2%) with positive MRSA clinical cultures had a positive MRSA PCR nasal swab. The concordance between MRSA PCR nasal swabs and infection sites was highest with positive urine cultures (77%) and lowest in “other” infection sites (60%, primarily abdomen, buttock, and breast). Respiratory infections showed a 75% concordance between MRSA PCR nasal swabs and infection sites, as well as the following characteristics: 75% sensitivity, 90% specificity, 30% PPV, and 98% NPV. Additionally, infection site concordance was higher when clinical cultures grew clindamycin-resistant MRSA (71.3%) vs clindamycin-susceptible MRSA (59.3%; P = .04).
Overall, a positive MRSA PCR nasal swab increased the likelihood of MRSA at the primary infection site but was not clinically significant or consistent across infection sites. As seen in other studies, a negative MRSA PCR nasal swab could be useful for lowering concern for MRSA involvement in the primary infection, as evidenced by the following characteristics for all infection sites: 67% sensitivity, 90% specificity, 27% PPV, and 98% NPV.
Sarkionda and colleagues evaluated the clinical usefulness of MRSA PCR nasal swabs in the ICU setting in patients with a lower respiratory tract infection (RTI) or bloodstream infection.15 A total of 749 patients received a MRSA PCR nasal swab before admission to the ICU and were included in this study. The concordance between MRSA PCR nasal swabs and the causative organism was analyzed in patients who developed a MRSA lower respiratory infection (N = 120) and a MRSA bloodstream infection (N = 78) and demonstrated the following characteristics: 24.2% sensitivity, 78.5% specificity, 17.7% PPV, and 84.4% NPV; and 23.1% sensitivity, 78.2% specificity, 11.0% PPV, and 89.7% NPV, respectively. The authors concluded that the MRSA nasal swab results are not useful for making decisions regarding the need of empiric antimicrobial therapy targeting MRSA infections in lower respiratory infections and bloodstream infections. However, due to the high NPV in this study, one might conclude that negative MRSA PCR nasal swabs could still be used to de-escalate therapy, which is in agreement with the results from Dangerfield and Johnson.10,11
Similarly, results from a retrospective chart review demonstrated a lack of predictive value by the MRSA PCR nasal swab.16 Of 1,203 adult patients admitted to an ICU at a single center, 57 positive MRSA colonized and 122 negative MRSA colonized patients’ charts were randomly selected. The presence of MRSA lower RTI or bloodstream infections was found to be 3.51% vs 2.46% in the colonized and noncolonized groups, respectively (P = .46). These results led to the conclusion that a positive MRSA PCR nasal swab alone should not be used to make decisions regarding empiric MRSA antibiotic coverage.
An alternative approach to MRSA surveillance was taken by Harris in a prospective cohort of 12,080 adults with a suspected infection on admission to a non-ICU.17 Patients were screened with a 2-question tool to determine whether they were high risk for a MRSA infection. The 2 questions were “Have you been admitted to any health care facility in the last 12 months?” and “Do you have a skin infection (eg, boil, abscess, spider bite, or cellulitis) at this time?” If patients answered yes to either question, they were considered high risk, and a MRSA PCR nasal swab was ordered.
Patients who answered no to both questions were considered low risk and did not receive a MRSA PCR nasal swab. In total, 623 of 5,609 patients (11.1%) identified as high risk had a positive MRSA PCR nasal swab, and 148 of these 623 patients (23.8%) developed a MRSA-positive clinical culture. Only 121 of 4,986 patients (2.4%) who were high risk and had a negative MRSA PCR nasal swab went on to develop a MRSA-positive clinical culture (98% NPV). Additionally, 104 of 6,741 patients (1.6%) who answered no to both screening questions developed a MRSA-positive clinical culture (98% NPV). Results indicated that a high percentage of patients who were at high risk for MRSA (yes response to either question) and had a positive MRSA PCR nasal swab also had a positive clinical culture for MRSA. Conversely, a very small percentage of high-risk patients with a negative MRSA PCR nasal swab developed a positive clinical culture for MRSA.
The screening tool proved very effective as the low-risk group had the lowest number of patients (1.6%) develop a positive clinical culture for MRSA. It may be deduced that combination use of MRSA colonization testing via PCR nasal swabs in conjunction with a screening tool may be an effective method to identify patients in whom anti-MRSA therapy can be safely discontinued.
Conclusion
Based on the results of previously described studies, sufficient data may exist to support the discontinuation of MRSA-targeted therapy in noncritically ill patients with confirmed or suspected pneumonia and a negative MRSA PCR nasal swab. Insufficient evidence exists, however, to support a broadening of antimicrobial therapy to include anti-MRSA coverage in individuals with a positive MRSA PCR nasal swab, regardless of the infection site.
Clinical judgment should be used when determining empiric antimicrobial therapy and for appropriateness of de-escalation of therapy in critically ill patients. Once a patient stabilizes, a negative MRSA PCR nasal swab could be considered as supporting evidence to discontinue anti-MRSA therapy, especially in patients with lower respiratory infections, such as pneumonia.
1. Moran GJ, Krishnadasan A, Gorwitz RJ, et al; EMERGEncy ID Net Study Group. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666-674.
2. Klevens RM, Morrison MA, Nadle J, et al; Active Bacterial Core surveillance (ABCs) MRSA Investigators. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298(15):1763-1771.
3. Cosgrove SE, Fowler VG Jr. Management of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46(suppl 5):S386-S393.
4. Dantes R, Mu Y, Belflower R, et al; Emerging Infections Program-Active Bacterial Core Surveillance MRSA Surveillance Investigators. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173(21):1970-1978.
5. Pillai MM, Latha R, Sarkar G. Detection of methicillin resistance in Staphylococcus aureus by polymerase chain reaction and conventional methods: a comparative study. J Lab Physicians. 2012;4(2):83-88.
6. Peterson LR, Liesenfeld O, Woods CW, et al. Multicenter evaluation of the LightCycler methicillin-resistant Staphylococcus aureus (MRSA) advanced test as a rapid method for detection of MRSA in nasal surveillance swabs. J Clin Microbiol. 2010;48(5):1661-1666.
7. File TM Jr, Srinivasan A, Bartlett JG. Antimicrobial stewardship: important for patient and public health. Clin Infect Dis. 2014;59(suppl 3):S93-S96.
8. Viasus D, Simonetti AF, Garcia-Vidal C, Niubó J, Dorca J, Carratalà J. Impact of antibiotic de-escalation on clinical outcomes in community-acquired pneumococcal pneumonia. J Antimicrob Chemother. 2017;72(2):547-5553.
9. Paul M, Dickstein Y, Raz-Pasteur A. Antibiotic de-escalation for bloodstream infections and pneumonia: a systematic review and meta-analysis. Clin Microbiol Infect. 2016;22(12):960-967.
10. Dangerfield B, Chung A, Webb B, Seville MT. Predictive value of methicillin-resistant Staphylococcus aureus (MRSA) nasal swab PCR assay for MRSA pneumonia. Antimicrob Agents Chemother. 2014;58(2):859-864.
11. Johnson JA, Wright ME, Sheperd LA, Musher DM, Dang BN. Nasal methicillin-resistant Staphylococcus aureus polymerase chain reaction a potential use in guiding antibiotic therapy for pneumonia. Perm J. 2015;19(1):34-36.
12. Giancola SE, Nguyen AT, Le B, et al. Clinical utility of a nasal swab methicillin-resistant Staphylococcus aureus polymerase chain reaction test in intensive and intermediate care unit patients with pneumonia. Diagn Microbiol Infect Dis. 2016;86(3):307-310.
13. Baby N, Faust AC, Smith T, Sheperd LA, Knoll L, Goodman EL. Nasal methicillin-resistant Staphylococcus aureus (MRSA) PCR testing reduces the duration of MRSA-targeted therapy in patients with suspected MRSA pneumonia. Antimicrob Agents Chemother. 2017;61(4):e02432-16.
14. Robicsek A, Suseno M, Beaumont JL, Thomson RB Jr, Peterson LR. Prediction of methicillin-resistant Staphylococcus aureus involvement in disease sites by concomitant nasal sampling. J Clin Microbiol. 2008;46(2):588-592.
15. Sarkionda KV, Micek ST, Dohery JA, Reichley RM, Warren D, Kollef MH. Methicillin-resistant Staphylococcus aureus nasal colonization is a poor predictor of intensive care unit-acquired methicillin-resistant Staphylococcus aureus infections requiring antibiotic treatment. Crit Care Med. 2010;38(10):1991-1995.
16. Ghidey F, Igbinosa O, Igbinosa E. Nasal colonization of methicillin resistant Staphylococcus aureus (MRSA) does not predict subsequent infection in the intensive care unit. Beni-Seuf University J Basic Appl Sci
17. Harris, AD, Furuno JP, Roghmann MC, et al. Targeted surveillance of methicillin-resistant Staphylococcus aureus and its potential use to guide empiric antibiotic therapy. Antimicrob Agents Chemother. 2010;54(8):3143-3148.
1. Moran GJ, Krishnadasan A, Gorwitz RJ, et al; EMERGEncy ID Net Study Group. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666-674.
2. Klevens RM, Morrison MA, Nadle J, et al; Active Bacterial Core surveillance (ABCs) MRSA Investigators. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298(15):1763-1771.
3. Cosgrove SE, Fowler VG Jr. Management of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46(suppl 5):S386-S393.
4. Dantes R, Mu Y, Belflower R, et al; Emerging Infections Program-Active Bacterial Core Surveillance MRSA Surveillance Investigators. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173(21):1970-1978.
5. Pillai MM, Latha R, Sarkar G. Detection of methicillin resistance in Staphylococcus aureus by polymerase chain reaction and conventional methods: a comparative study. J Lab Physicians. 2012;4(2):83-88.
6. Peterson LR, Liesenfeld O, Woods CW, et al. Multicenter evaluation of the LightCycler methicillin-resistant Staphylococcus aureus (MRSA) advanced test as a rapid method for detection of MRSA in nasal surveillance swabs. J Clin Microbiol. 2010;48(5):1661-1666.
7. File TM Jr, Srinivasan A, Bartlett JG. Antimicrobial stewardship: important for patient and public health. Clin Infect Dis. 2014;59(suppl 3):S93-S96.
8. Viasus D, Simonetti AF, Garcia-Vidal C, Niubó J, Dorca J, Carratalà J. Impact of antibiotic de-escalation on clinical outcomes in community-acquired pneumococcal pneumonia. J Antimicrob Chemother. 2017;72(2):547-5553.
9. Paul M, Dickstein Y, Raz-Pasteur A. Antibiotic de-escalation for bloodstream infections and pneumonia: a systematic review and meta-analysis. Clin Microbiol Infect. 2016;22(12):960-967.
10. Dangerfield B, Chung A, Webb B, Seville MT. Predictive value of methicillin-resistant Staphylococcus aureus (MRSA) nasal swab PCR assay for MRSA pneumonia. Antimicrob Agents Chemother. 2014;58(2):859-864.
11. Johnson JA, Wright ME, Sheperd LA, Musher DM, Dang BN. Nasal methicillin-resistant Staphylococcus aureus polymerase chain reaction a potential use in guiding antibiotic therapy for pneumonia. Perm J. 2015;19(1):34-36.
12. Giancola SE, Nguyen AT, Le B, et al. Clinical utility of a nasal swab methicillin-resistant Staphylococcus aureus polymerase chain reaction test in intensive and intermediate care unit patients with pneumonia. Diagn Microbiol Infect Dis. 2016;86(3):307-310.
13. Baby N, Faust AC, Smith T, Sheperd LA, Knoll L, Goodman EL. Nasal methicillin-resistant Staphylococcus aureus (MRSA) PCR testing reduces the duration of MRSA-targeted therapy in patients with suspected MRSA pneumonia. Antimicrob Agents Chemother. 2017;61(4):e02432-16.
14. Robicsek A, Suseno M, Beaumont JL, Thomson RB Jr, Peterson LR. Prediction of methicillin-resistant Staphylococcus aureus involvement in disease sites by concomitant nasal sampling. J Clin Microbiol. 2008;46(2):588-592.
15. Sarkionda KV, Micek ST, Dohery JA, Reichley RM, Warren D, Kollef MH. Methicillin-resistant Staphylococcus aureus nasal colonization is a poor predictor of intensive care unit-acquired methicillin-resistant Staphylococcus aureus infections requiring antibiotic treatment. Crit Care Med. 2010;38(10):1991-1995.
16. Ghidey F, Igbinosa O, Igbinosa E. Nasal colonization of methicillin resistant Staphylococcus aureus (MRSA) does not predict subsequent infection in the intensive care unit. Beni-Seuf University J Basic Appl Sci
17. Harris, AD, Furuno JP, Roghmann MC, et al. Targeted surveillance of methicillin-resistant Staphylococcus aureus and its potential use to guide empiric antibiotic therapy. Antimicrob Agents Chemother. 2010;54(8):3143-3148.
Elder Patients With Diabetes at Higher Risk for Cancer Surgery
Patients with diabetes—especially the elderly—are at high risk for morbidity and mortality due to cancer, studies have shown. Researchers from Specialist District Hospital and University of Rzeszow in Poland say their study “sheds some new light” in demonstrating another kind of association between diabetes and cancer in older patients: a higher risk of hospitalization with surgery due to cancer.
They analyzed data on 7,694 patients aged > 45 years hospitalized in a surgery ward. Of those, 652 were diagnosed with cancer and 370 with diabetes; 93 patients had both. The most common kind of cancer was urinary bladder cancer. The researchers note that their surgical unit has a large urology subdivision. Patients with other site-specific cancers are usually referred to more specialized clinical units.
Diabetes was the strongest predictor of risk among the variables analyzed, although urban residence also was a significant predictor. Risk of hospitalization due to cancer doubled among diabetic patients aged 45 to 65 years and was > 5 times higher among patients aged > 65 years, compared with the nondiabetic patients. The highest risk of hospitalization for site-specific cancers was among patients with kidney and breast cancers.
The researchers say their findings suggest that it is “advisable to make major efforts” for early detection and early radical treatment in older patients with diabetes.
Source:
Dᾳbrowski M, Grindecka A. Arch Med Sci. 2017;13(5):1025-1030.
doi: 10.5114/aoms.2016.58666.
Patients with diabetes—especially the elderly—are at high risk for morbidity and mortality due to cancer, studies have shown. Researchers from Specialist District Hospital and University of Rzeszow in Poland say their study “sheds some new light” in demonstrating another kind of association between diabetes and cancer in older patients: a higher risk of hospitalization with surgery due to cancer.
They analyzed data on 7,694 patients aged > 45 years hospitalized in a surgery ward. Of those, 652 were diagnosed with cancer and 370 with diabetes; 93 patients had both. The most common kind of cancer was urinary bladder cancer. The researchers note that their surgical unit has a large urology subdivision. Patients with other site-specific cancers are usually referred to more specialized clinical units.
Diabetes was the strongest predictor of risk among the variables analyzed, although urban residence also was a significant predictor. Risk of hospitalization due to cancer doubled among diabetic patients aged 45 to 65 years and was > 5 times higher among patients aged > 65 years, compared with the nondiabetic patients. The highest risk of hospitalization for site-specific cancers was among patients with kidney and breast cancers.
The researchers say their findings suggest that it is “advisable to make major efforts” for early detection and early radical treatment in older patients with diabetes.
Source:
Dᾳbrowski M, Grindecka A. Arch Med Sci. 2017;13(5):1025-1030.
doi: 10.5114/aoms.2016.58666.
Patients with diabetes—especially the elderly—are at high risk for morbidity and mortality due to cancer, studies have shown. Researchers from Specialist District Hospital and University of Rzeszow in Poland say their study “sheds some new light” in demonstrating another kind of association between diabetes and cancer in older patients: a higher risk of hospitalization with surgery due to cancer.
They analyzed data on 7,694 patients aged > 45 years hospitalized in a surgery ward. Of those, 652 were diagnosed with cancer and 370 with diabetes; 93 patients had both. The most common kind of cancer was urinary bladder cancer. The researchers note that their surgical unit has a large urology subdivision. Patients with other site-specific cancers are usually referred to more specialized clinical units.
Diabetes was the strongest predictor of risk among the variables analyzed, although urban residence also was a significant predictor. Risk of hospitalization due to cancer doubled among diabetic patients aged 45 to 65 years and was > 5 times higher among patients aged > 65 years, compared with the nondiabetic patients. The highest risk of hospitalization for site-specific cancers was among patients with kidney and breast cancers.
The researchers say their findings suggest that it is “advisable to make major efforts” for early detection and early radical treatment in older patients with diabetes.
Source:
Dᾳbrowski M, Grindecka A. Arch Med Sci. 2017;13(5):1025-1030.
doi: 10.5114/aoms.2016.58666.
FDA approves inotuzumab ozogamicin for rel/ref ALL
The US Food and Drug Administration (FDA) has approved inotuzumab ozogamicin (Besponsa®) for the treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).
The labeling for inotuzumab ozogamicin includes a boxed warning stating that the drug poses a risk of hepatotoxicity, including hepatic veno-occlusive disease (or sinusoidal obstruction syndrome), as well as an increased risk of post-transplant non-relapse mortality.
The full prescribing information for inotuzumab ozogamicin is available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761040s000lbl.pdf.
Inotuzumab ozogamicin is an antibody-drug conjugate that consists of a monoclonal antibody targeting CD22 and a cytotoxic agent known as calicheamicin.
The product originates from a collaboration between Pfizer and Celltech (now UCB), but Pfizer has sole responsibility for all manufacturing and clinical development activities.
Inotuzumab ozogamicin was reviewed and approved under the FDA’s breakthrough therapy designation and priority review programs.
The application for inotuzumab ozogamicin was supported by results from the phase 3 INO-VATE trial, which were published in NEJM in June 2016.
The trial enrolled 326 adults with relapsed or refractory B-cell ALL. Patients received inotuzumab ozogamicin or 1 of 3 chemotherapy regimens (high-dose cytarabine, cytarabine plus mitoxantrone, or fludarabine, cytarabine, and granulocyte colony-stimulating factor).
The rate of complete remission, including incomplete hematologic recovery, was 80.7% in the inotuzumab arm and 29.4% in the chemotherapy arm (P<0.001). The median duration of remission was 4.6 months and 3.1 months, respectively (P=0.03).
Forty-one percent of patients treated with inotuzumab and 11% of those who received chemotherapy proceeded to stem cell transplant directly after treatment (P<0.001).
The median progression-free survival was 5.0 months in the inotuzumab arm and 1.8 months in the chemotherapy arm (P<0.001).
The median overall survival was 7.7 months and 6.7 months, respectively (P=0.04). This did not meet the prespecified boundary of significance (P=0.0208).
Liver-related adverse events were more common in the inotuzumab arm than the chemotherapy arm. The most frequent of these were increased aspartate aminotransferase level (20% vs 10%), hyperbilirubinemia (15% vs 10%), and increased alanine aminotransferase level (14% vs 11%).
Veno-occlusive liver disease occurred in 11% of patients in the inotuzumab arm and 1% in the chemotherapy arm.
There were 17 deaths during treatment in the inotuzumab arm and 11 in the chemotherapy arm. Four deaths were considered related to inotuzumab, and 2 were thought to be related to chemotherapy. ![]()
The US Food and Drug Administration (FDA) has approved inotuzumab ozogamicin (Besponsa®) for the treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).
The labeling for inotuzumab ozogamicin includes a boxed warning stating that the drug poses a risk of hepatotoxicity, including hepatic veno-occlusive disease (or sinusoidal obstruction syndrome), as well as an increased risk of post-transplant non-relapse mortality.
The full prescribing information for inotuzumab ozogamicin is available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761040s000lbl.pdf.
Inotuzumab ozogamicin is an antibody-drug conjugate that consists of a monoclonal antibody targeting CD22 and a cytotoxic agent known as calicheamicin.
The product originates from a collaboration between Pfizer and Celltech (now UCB), but Pfizer has sole responsibility for all manufacturing and clinical development activities.
Inotuzumab ozogamicin was reviewed and approved under the FDA’s breakthrough therapy designation and priority review programs.
The application for inotuzumab ozogamicin was supported by results from the phase 3 INO-VATE trial, which were published in NEJM in June 2016.
The trial enrolled 326 adults with relapsed or refractory B-cell ALL. Patients received inotuzumab ozogamicin or 1 of 3 chemotherapy regimens (high-dose cytarabine, cytarabine plus mitoxantrone, or fludarabine, cytarabine, and granulocyte colony-stimulating factor).
The rate of complete remission, including incomplete hematologic recovery, was 80.7% in the inotuzumab arm and 29.4% in the chemotherapy arm (P<0.001). The median duration of remission was 4.6 months and 3.1 months, respectively (P=0.03).
Forty-one percent of patients treated with inotuzumab and 11% of those who received chemotherapy proceeded to stem cell transplant directly after treatment (P<0.001).
The median progression-free survival was 5.0 months in the inotuzumab arm and 1.8 months in the chemotherapy arm (P<0.001).
The median overall survival was 7.7 months and 6.7 months, respectively (P=0.04). This did not meet the prespecified boundary of significance (P=0.0208).
Liver-related adverse events were more common in the inotuzumab arm than the chemotherapy arm. The most frequent of these were increased aspartate aminotransferase level (20% vs 10%), hyperbilirubinemia (15% vs 10%), and increased alanine aminotransferase level (14% vs 11%).
Veno-occlusive liver disease occurred in 11% of patients in the inotuzumab arm and 1% in the chemotherapy arm.
There were 17 deaths during treatment in the inotuzumab arm and 11 in the chemotherapy arm. Four deaths were considered related to inotuzumab, and 2 were thought to be related to chemotherapy. ![]()
The US Food and Drug Administration (FDA) has approved inotuzumab ozogamicin (Besponsa®) for the treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).
The labeling for inotuzumab ozogamicin includes a boxed warning stating that the drug poses a risk of hepatotoxicity, including hepatic veno-occlusive disease (or sinusoidal obstruction syndrome), as well as an increased risk of post-transplant non-relapse mortality.
The full prescribing information for inotuzumab ozogamicin is available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761040s000lbl.pdf.
Inotuzumab ozogamicin is an antibody-drug conjugate that consists of a monoclonal antibody targeting CD22 and a cytotoxic agent known as calicheamicin.
The product originates from a collaboration between Pfizer and Celltech (now UCB), but Pfizer has sole responsibility for all manufacturing and clinical development activities.
Inotuzumab ozogamicin was reviewed and approved under the FDA’s breakthrough therapy designation and priority review programs.
The application for inotuzumab ozogamicin was supported by results from the phase 3 INO-VATE trial, which were published in NEJM in June 2016.
The trial enrolled 326 adults with relapsed or refractory B-cell ALL. Patients received inotuzumab ozogamicin or 1 of 3 chemotherapy regimens (high-dose cytarabine, cytarabine plus mitoxantrone, or fludarabine, cytarabine, and granulocyte colony-stimulating factor).
The rate of complete remission, including incomplete hematologic recovery, was 80.7% in the inotuzumab arm and 29.4% in the chemotherapy arm (P<0.001). The median duration of remission was 4.6 months and 3.1 months, respectively (P=0.03).
Forty-one percent of patients treated with inotuzumab and 11% of those who received chemotherapy proceeded to stem cell transplant directly after treatment (P<0.001).
The median progression-free survival was 5.0 months in the inotuzumab arm and 1.8 months in the chemotherapy arm (P<0.001).
The median overall survival was 7.7 months and 6.7 months, respectively (P=0.04). This did not meet the prespecified boundary of significance (P=0.0208).
Liver-related adverse events were more common in the inotuzumab arm than the chemotherapy arm. The most frequent of these were increased aspartate aminotransferase level (20% vs 10%), hyperbilirubinemia (15% vs 10%), and increased alanine aminotransferase level (14% vs 11%).
Veno-occlusive liver disease occurred in 11% of patients in the inotuzumab arm and 1% in the chemotherapy arm.
There were 17 deaths during treatment in the inotuzumab arm and 11 in the chemotherapy arm. Four deaths were considered related to inotuzumab, and 2 were thought to be related to chemotherapy. ![]()