User login
Over 40% of Americans have experience with medical errors
More than 40% of adults have either experienced a medical error or been involved in the care of someone who did, according to a recent national survey.
Specifically, 21% of American adults said that they have personally experienced a medical error and 31% said that they have been involved in the care of another person who experienced an error. The combined total, which includes some overlap, was 41% in the survey conducted by the Institute for Healthcare Improvement (IHI)/National Patient Safety Foundation (NPSF) and National Opinion Research Center (NORC), a nonpartisan research institution at the University of Chicago, .
Medical errors were defined for respondents as “mistakes [that] sometimes result in no harm, while other times they may result in additional or prolonged treatment, emotional distress, disability, or death,” investigators at IHI/NPSF and NORC said in their report.
A misdiagnosed medical problem was the most common type of medical error, reported by 59% of those with error experience. The next most common type of error was a mistake during a test, surgery, or treatment, which was mentioned by 46% of those with error experience, followed by a diagnosis that didn’t make sense (42%), lack of respect (39%), and incorrect instructions about follow-up care (29%), the IHI/HPSF and NORC report indicated.
Feelings of disrespect were more common among younger respondents: 46% of those aged 18-44 years said they were not treated with respect by a health care provider, compared with 34% of those aged 45 years and older. No differences in disrespect were seen with regard to socioeconomic status, health literacy, or English language proficiency. Those who spoke a language other than English at home, however, were more than twice as likely to get the wrong medication from a physician (34%) than were those who did not (15%), the report showed.
The survey, which had a sampling error of plus or minus 3.2 percentage points, was conducted between May 12, 2017, and June 26, 2017, and involved 2,536 respondents. It was conducted with support from Medtronic.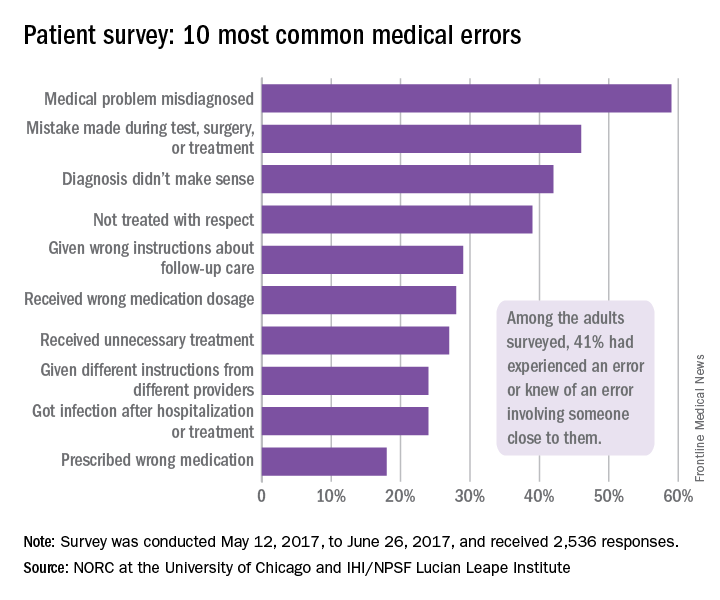
More than 40% of adults have either experienced a medical error or been involved in the care of someone who did, according to a recent national survey.
Specifically, 21% of American adults said that they have personally experienced a medical error and 31% said that they have been involved in the care of another person who experienced an error. The combined total, which includes some overlap, was 41% in the survey conducted by the Institute for Healthcare Improvement (IHI)/National Patient Safety Foundation (NPSF) and National Opinion Research Center (NORC), a nonpartisan research institution at the University of Chicago, .
Medical errors were defined for respondents as “mistakes [that] sometimes result in no harm, while other times they may result in additional or prolonged treatment, emotional distress, disability, or death,” investigators at IHI/NPSF and NORC said in their report.
A misdiagnosed medical problem was the most common type of medical error, reported by 59% of those with error experience. The next most common type of error was a mistake during a test, surgery, or treatment, which was mentioned by 46% of those with error experience, followed by a diagnosis that didn’t make sense (42%), lack of respect (39%), and incorrect instructions about follow-up care (29%), the IHI/HPSF and NORC report indicated.
Feelings of disrespect were more common among younger respondents: 46% of those aged 18-44 years said they were not treated with respect by a health care provider, compared with 34% of those aged 45 years and older. No differences in disrespect were seen with regard to socioeconomic status, health literacy, or English language proficiency. Those who spoke a language other than English at home, however, were more than twice as likely to get the wrong medication from a physician (34%) than were those who did not (15%), the report showed.
The survey, which had a sampling error of plus or minus 3.2 percentage points, was conducted between May 12, 2017, and June 26, 2017, and involved 2,536 respondents. It was conducted with support from Medtronic.
More than 40% of adults have either experienced a medical error or been involved in the care of someone who did, according to a recent national survey.
Specifically, 21% of American adults said that they have personally experienced a medical error and 31% said that they have been involved in the care of another person who experienced an error. The combined total, which includes some overlap, was 41% in the survey conducted by the Institute for Healthcare Improvement (IHI)/National Patient Safety Foundation (NPSF) and National Opinion Research Center (NORC), a nonpartisan research institution at the University of Chicago, .
Medical errors were defined for respondents as “mistakes [that] sometimes result in no harm, while other times they may result in additional or prolonged treatment, emotional distress, disability, or death,” investigators at IHI/NPSF and NORC said in their report.
A misdiagnosed medical problem was the most common type of medical error, reported by 59% of those with error experience. The next most common type of error was a mistake during a test, surgery, or treatment, which was mentioned by 46% of those with error experience, followed by a diagnosis that didn’t make sense (42%), lack of respect (39%), and incorrect instructions about follow-up care (29%), the IHI/HPSF and NORC report indicated.
Feelings of disrespect were more common among younger respondents: 46% of those aged 18-44 years said they were not treated with respect by a health care provider, compared with 34% of those aged 45 years and older. No differences in disrespect were seen with regard to socioeconomic status, health literacy, or English language proficiency. Those who spoke a language other than English at home, however, were more than twice as likely to get the wrong medication from a physician (34%) than were those who did not (15%), the report showed.
The survey, which had a sampling error of plus or minus 3.2 percentage points, was conducted between May 12, 2017, and June 26, 2017, and involved 2,536 respondents. It was conducted with support from Medtronic.
Clinical pearls from NAMS 2017
Visit the NAMS annual meetings website
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Visit the NAMS annual meetings website
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Visit the NAMS annual meetings website
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Two Senators reach deal on a health law fix
After nearly 2 months of negotiations, key senators said on Oct. 17 that they have reached a bipartisan deal on a proposal intended to stabilize the Affordable Care Act’s insurance market, which has been rocked by recent actions by President Donald Trump.
Sens. Lamar Alexander (R-Tenn.) and Patty Murray (D-Wash.), respectively the chairman and the top Democrat of the Senate Health, Education, Labor, and Pensions Committee, negotiated the emerging deal. The milestone agreement, they said, would guarantee payment of cost-sharing reduction subsidies that help some policyholders with low incomes afford their deductibles and other out-of-pocket costs for 2 years, 2018 and 2019.
President Trump announced on Oct. 12 that he would stop funding the subsidies, which also have been the subject of a long-running lawsuit.
Even if it fails to become law, the deal marks a singular achievement that has been almost completely missing in Congress for the past 8 years – a bipartisan compromise on how to make the nation’s health insurance system work.
“This is an agreement I am proud to support,” Sen. Murray said on the Senate floor, “because of the message it sends about how to get things done.”
The proposal – which will require 60 votes to pass the Senate and agreement from a still-dubious House of Representatives – also would restore $110 million in ACA outreach funding cut by the Trump administration. That funding would help guide eligible individuals to sign up for coverage on the health insurance exchanges during the open enrollment period that runs from Nov. 1 to Dec. 15.
In exchange for those provisions, urged by Democrats and state officials, Republicans would win some changes to make it easier for states to apply for waivers that would let them experiment with alternative ways to provide and subsidize health insurance. The deal also would allow the sale of less comprehensive catastrophic plans in the health exchanges. Currently, such plans can be sold only to those under age 30 years.
On the Senate floor, Sen. Alexander said, “This agreement avoids chaos. I don’t know a Democrat or a Republican who benefits from chaos.”
Senate Majority Leader Mitch McConnell (R-Ky.) reserved judgment about the deal.
Both parties still have some major disagreements when it comes to health care, Senate Minority Leader Chuck Schumer (D-N.Y.) told reporters on Oct. 17, but “I think there’s a growing consensus that in the short term we need stability in the markets. So we’ve achieved stability if this agreement becomes law.”
More than 60 senators have already participated in the meetings that led to the deal, Sen. Alexander said on the Senate floor. But the path to passage in the House is uncertain – with many conservatives vehemently opposed to anything that could be construed as helping the ACA succeed.
Rep. Mark Walker (R-N.C.), chairman of the conservative Republican Study Committee, tweeted on Oct. 17: “The GOP should focus on repealing & replacing Obamacare, not trying to save it. This bailout is unacceptable.”
Both Sen. Murray and Sen. Alexander said that they were still struggling over language to make sure that if the cost-sharing payments are resumed, insurers would not receive a windfall by keeping both those payments and the higher premiums that many states are allowing in anticipation of the payments being ended.
“We want to make sure that the cost-sharing payments go to the benefit of consumers, not the insurance companies,” Sen. Alexander said.
President Trump, who as recently as Oct. 16 called the cost-sharing subsidies “a payoff” to insurance companies, took credit for the negotiations. “If I didn’t cut the CSRs, they wouldn’t be meeting,” he said. That was not, in fact, the case. The negotiations had picked up some weeks ago after being called off earlier in September while the Senate tried for one last-ditch repeal vote.
On Oct. 13, White House Budget Director Mick Mulvaney told Politico that the president would not allow a short-term fix, calling a restoration of the cost-sharing reduction funds “corporate welfare and bailouts for the insurance companies.”
But on Oct. 17 the president hailed the deal. “We think it’s going to not only save money, but give people much better health care with a very, very much smaller premium spike,” he told reporters.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
After nearly 2 months of negotiations, key senators said on Oct. 17 that they have reached a bipartisan deal on a proposal intended to stabilize the Affordable Care Act’s insurance market, which has been rocked by recent actions by President Donald Trump.
Sens. Lamar Alexander (R-Tenn.) and Patty Murray (D-Wash.), respectively the chairman and the top Democrat of the Senate Health, Education, Labor, and Pensions Committee, negotiated the emerging deal. The milestone agreement, they said, would guarantee payment of cost-sharing reduction subsidies that help some policyholders with low incomes afford their deductibles and other out-of-pocket costs for 2 years, 2018 and 2019.
President Trump announced on Oct. 12 that he would stop funding the subsidies, which also have been the subject of a long-running lawsuit.
Even if it fails to become law, the deal marks a singular achievement that has been almost completely missing in Congress for the past 8 years – a bipartisan compromise on how to make the nation’s health insurance system work.
“This is an agreement I am proud to support,” Sen. Murray said on the Senate floor, “because of the message it sends about how to get things done.”
The proposal – which will require 60 votes to pass the Senate and agreement from a still-dubious House of Representatives – also would restore $110 million in ACA outreach funding cut by the Trump administration. That funding would help guide eligible individuals to sign up for coverage on the health insurance exchanges during the open enrollment period that runs from Nov. 1 to Dec. 15.
In exchange for those provisions, urged by Democrats and state officials, Republicans would win some changes to make it easier for states to apply for waivers that would let them experiment with alternative ways to provide and subsidize health insurance. The deal also would allow the sale of less comprehensive catastrophic plans in the health exchanges. Currently, such plans can be sold only to those under age 30 years.
On the Senate floor, Sen. Alexander said, “This agreement avoids chaos. I don’t know a Democrat or a Republican who benefits from chaos.”
Senate Majority Leader Mitch McConnell (R-Ky.) reserved judgment about the deal.
Both parties still have some major disagreements when it comes to health care, Senate Minority Leader Chuck Schumer (D-N.Y.) told reporters on Oct. 17, but “I think there’s a growing consensus that in the short term we need stability in the markets. So we’ve achieved stability if this agreement becomes law.”
More than 60 senators have already participated in the meetings that led to the deal, Sen. Alexander said on the Senate floor. But the path to passage in the House is uncertain – with many conservatives vehemently opposed to anything that could be construed as helping the ACA succeed.
Rep. Mark Walker (R-N.C.), chairman of the conservative Republican Study Committee, tweeted on Oct. 17: “The GOP should focus on repealing & replacing Obamacare, not trying to save it. This bailout is unacceptable.”
Both Sen. Murray and Sen. Alexander said that they were still struggling over language to make sure that if the cost-sharing payments are resumed, insurers would not receive a windfall by keeping both those payments and the higher premiums that many states are allowing in anticipation of the payments being ended.
“We want to make sure that the cost-sharing payments go to the benefit of consumers, not the insurance companies,” Sen. Alexander said.
President Trump, who as recently as Oct. 16 called the cost-sharing subsidies “a payoff” to insurance companies, took credit for the negotiations. “If I didn’t cut the CSRs, they wouldn’t be meeting,” he said. That was not, in fact, the case. The negotiations had picked up some weeks ago after being called off earlier in September while the Senate tried for one last-ditch repeal vote.
On Oct. 13, White House Budget Director Mick Mulvaney told Politico that the president would not allow a short-term fix, calling a restoration of the cost-sharing reduction funds “corporate welfare and bailouts for the insurance companies.”
But on Oct. 17 the president hailed the deal. “We think it’s going to not only save money, but give people much better health care with a very, very much smaller premium spike,” he told reporters.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
After nearly 2 months of negotiations, key senators said on Oct. 17 that they have reached a bipartisan deal on a proposal intended to stabilize the Affordable Care Act’s insurance market, which has been rocked by recent actions by President Donald Trump.
Sens. Lamar Alexander (R-Tenn.) and Patty Murray (D-Wash.), respectively the chairman and the top Democrat of the Senate Health, Education, Labor, and Pensions Committee, negotiated the emerging deal. The milestone agreement, they said, would guarantee payment of cost-sharing reduction subsidies that help some policyholders with low incomes afford their deductibles and other out-of-pocket costs for 2 years, 2018 and 2019.
President Trump announced on Oct. 12 that he would stop funding the subsidies, which also have been the subject of a long-running lawsuit.
Even if it fails to become law, the deal marks a singular achievement that has been almost completely missing in Congress for the past 8 years – a bipartisan compromise on how to make the nation’s health insurance system work.
“This is an agreement I am proud to support,” Sen. Murray said on the Senate floor, “because of the message it sends about how to get things done.”
The proposal – which will require 60 votes to pass the Senate and agreement from a still-dubious House of Representatives – also would restore $110 million in ACA outreach funding cut by the Trump administration. That funding would help guide eligible individuals to sign up for coverage on the health insurance exchanges during the open enrollment period that runs from Nov. 1 to Dec. 15.
In exchange for those provisions, urged by Democrats and state officials, Republicans would win some changes to make it easier for states to apply for waivers that would let them experiment with alternative ways to provide and subsidize health insurance. The deal also would allow the sale of less comprehensive catastrophic plans in the health exchanges. Currently, such plans can be sold only to those under age 30 years.
On the Senate floor, Sen. Alexander said, “This agreement avoids chaos. I don’t know a Democrat or a Republican who benefits from chaos.”
Senate Majority Leader Mitch McConnell (R-Ky.) reserved judgment about the deal.
Both parties still have some major disagreements when it comes to health care, Senate Minority Leader Chuck Schumer (D-N.Y.) told reporters on Oct. 17, but “I think there’s a growing consensus that in the short term we need stability in the markets. So we’ve achieved stability if this agreement becomes law.”
More than 60 senators have already participated in the meetings that led to the deal, Sen. Alexander said on the Senate floor. But the path to passage in the House is uncertain – with many conservatives vehemently opposed to anything that could be construed as helping the ACA succeed.
Rep. Mark Walker (R-N.C.), chairman of the conservative Republican Study Committee, tweeted on Oct. 17: “The GOP should focus on repealing & replacing Obamacare, not trying to save it. This bailout is unacceptable.”
Both Sen. Murray and Sen. Alexander said that they were still struggling over language to make sure that if the cost-sharing payments are resumed, insurers would not receive a windfall by keeping both those payments and the higher premiums that many states are allowing in anticipation of the payments being ended.
“We want to make sure that the cost-sharing payments go to the benefit of consumers, not the insurance companies,” Sen. Alexander said.
President Trump, who as recently as Oct. 16 called the cost-sharing subsidies “a payoff” to insurance companies, took credit for the negotiations. “If I didn’t cut the CSRs, they wouldn’t be meeting,” he said. That was not, in fact, the case. The negotiations had picked up some weeks ago after being called off earlier in September while the Senate tried for one last-ditch repeal vote.
On Oct. 13, White House Budget Director Mick Mulvaney told Politico that the president would not allow a short-term fix, calling a restoration of the cost-sharing reduction funds “corporate welfare and bailouts for the insurance companies.”
But on Oct. 17 the president hailed the deal. “We think it’s going to not only save money, but give people much better health care with a very, very much smaller premium spike,” he told reporters.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
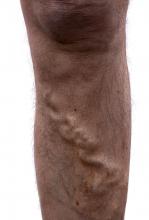
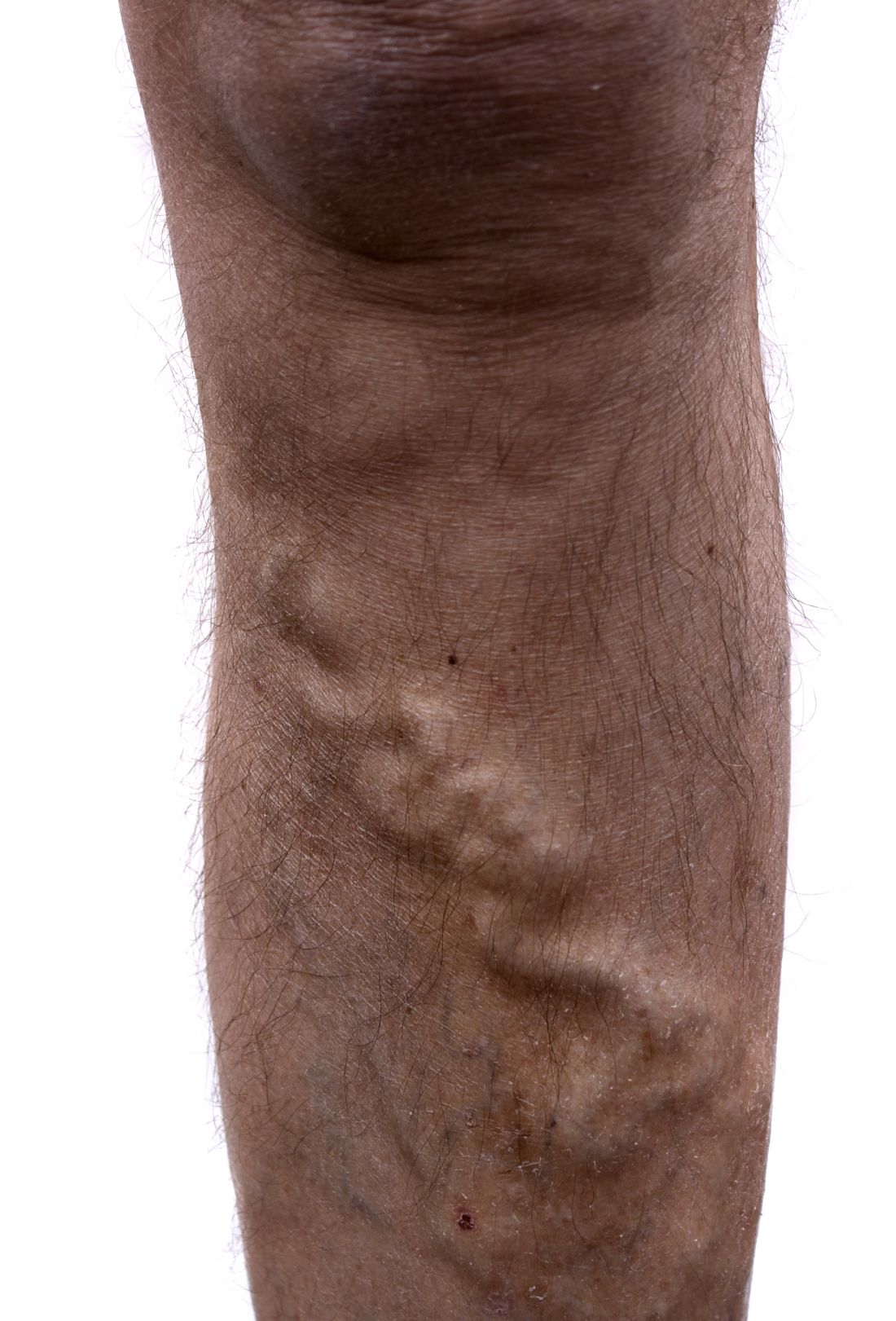
Study: Varicose vein procedures should be offered to patients 65 years and older
Older and younger patients benefited from varicose vein procedures, a finding that calls into question the use of compression therapy as the primary treatment for patients aged 65 years and older, according to the results of a prospectively maintained database study of all patients in the Vascular Quality Initiative Varicose Vein Registry–participating centers.
Procedures for varicose veins in 1,068 patients aged 65 years or older showed similar improvement in Clinical, Etiology, Anatomy, and Pathophysiology class and Venous Clinical Severity Score, compared with a group of 2,691 younger patients, according to a report published in Journal of Vascular Surgery: Venous and Lymphatic Disorders. Among patients younger than 65 years, 57.4% had an improvement, compared with 52% of patients aged 65 years or older.
However, the younger patients had more improvement in patient-reported outcomes, according to Danielle C. Sutzko, MD, of the University of Michigan, Ann Arbor, and her colleagues (J Vasc Surg Venous Lymphat Disord. 2017 Sep;5[5]:647-57).
One of the main limitations of the study was the fact that only 62% of procedures within the Vascular Quality Initiative Varicose Vein Registry had follow-up.
“Patients older than 65 years appear to benefit from appropriate varicose vein procedures and should not be denied interventions on their varicose veins and venous insufficiency on the basis of their age only,” the researchers concluded.
Dr. Sutzko and her colleagues reported having no conflicts of interest.
Older and younger patients benefited from varicose vein procedures, a finding that calls into question the use of compression therapy as the primary treatment for patients aged 65 years and older, according to the results of a prospectively maintained database study of all patients in the Vascular Quality Initiative Varicose Vein Registry–participating centers.
Procedures for varicose veins in 1,068 patients aged 65 years or older showed similar improvement in Clinical, Etiology, Anatomy, and Pathophysiology class and Venous Clinical Severity Score, compared with a group of 2,691 younger patients, according to a report published in Journal of Vascular Surgery: Venous and Lymphatic Disorders. Among patients younger than 65 years, 57.4% had an improvement, compared with 52% of patients aged 65 years or older.
However, the younger patients had more improvement in patient-reported outcomes, according to Danielle C. Sutzko, MD, of the University of Michigan, Ann Arbor, and her colleagues (J Vasc Surg Venous Lymphat Disord. 2017 Sep;5[5]:647-57).
One of the main limitations of the study was the fact that only 62% of procedures within the Vascular Quality Initiative Varicose Vein Registry had follow-up.
“Patients older than 65 years appear to benefit from appropriate varicose vein procedures and should not be denied interventions on their varicose veins and venous insufficiency on the basis of their age only,” the researchers concluded.
Dr. Sutzko and her colleagues reported having no conflicts of interest.
Older and younger patients benefited from varicose vein procedures, a finding that calls into question the use of compression therapy as the primary treatment for patients aged 65 years and older, according to the results of a prospectively maintained database study of all patients in the Vascular Quality Initiative Varicose Vein Registry–participating centers.
Procedures for varicose veins in 1,068 patients aged 65 years or older showed similar improvement in Clinical, Etiology, Anatomy, and Pathophysiology class and Venous Clinical Severity Score, compared with a group of 2,691 younger patients, according to a report published in Journal of Vascular Surgery: Venous and Lymphatic Disorders. Among patients younger than 65 years, 57.4% had an improvement, compared with 52% of patients aged 65 years or older.
However, the younger patients had more improvement in patient-reported outcomes, according to Danielle C. Sutzko, MD, of the University of Michigan, Ann Arbor, and her colleagues (J Vasc Surg Venous Lymphat Disord. 2017 Sep;5[5]:647-57).
One of the main limitations of the study was the fact that only 62% of procedures within the Vascular Quality Initiative Varicose Vein Registry had follow-up.
“Patients older than 65 years appear to benefit from appropriate varicose vein procedures and should not be denied interventions on their varicose veins and venous insufficiency on the basis of their age only,” the researchers concluded.
Dr. Sutzko and her colleagues reported having no conflicts of interest.
FROM JOURNAL OF VASCULAR SURGERY: VENOUS AND LYMPHATIC DISORDERS
Key clinical point:
Major finding: Among patients younger than 65, 57.4% had an improvement, compared to 52% of patients aged 65 years or older.
Data source: Prospectively captured data for all patients in the Vascular Quality Initiative Varicose Vein Registry–participating centers.
Disclosures: Dr. Sutzko and her colleagues reported having no conflicts of interest.
Vedolizumab improves social satisfaction among IBD patients
ORLANDO – Vedolizumab therapy was associated with significant improvements in social satisfaction scores and steroid-free remission rates in biologic-naive patients with inflammatory bowel diseases (IBD) in a large prospective cohort.
The Internet-based cohort – Crohn’s & Colitis Foundation of America (CCFA) Partners – includes more than 15,000 IBD patients. For the current study, researchers evaluated 348 participants with Crohn’s disease or ulcerative colitis who initiated vedolizumab therapy between 2014 and 2017 and who had at least 6 months’ follow-up.
The difference in social satisfaction T scores was also improved among biologic-exposed patients (45.8 vs. 47.2, respectively), but the difference did not reach statistical significance, said Dr. Long of the University of North Carolina, Chapel Hill.
Improvements were also seen for numerous other measures, including anxiety, depression, fatigue, pain interference, and sleep disturbance – for both biologic-naive and -exposed patients – but the differences were not significant.
“But these [patient-reported outcomes] are clearly improving,” she said, explaining that trends toward minimally clinically important differences were seen for multiple measures.
As for steroid-free remission, the rate improved from 20% to 45% from baseline to 6-12 months among biologic-naive patients, and from 24% to 30% among biologic-exposed patients, Dr. Long said.
Vedolizumab in this real-world cohort was predominantly used in patients with refractory disease and prior biologic exposure.
The CCFA cohort provides an important glimpse into the effects of vedolizumab on patient-reported outcomes in real-world settings, Dr. Long said, noting that while vedolizumab has demonstrated important quality of life improvements in IBD clinical trials, little has been known about the effects of vedolizumab on quality of life in real-world settings.
The finding with respect to social satisfaction is particularly important, she said.
“These are sick patients. [These scores show that] they’re able to leave the house, they’re able to do the things they want to do,” she said. “It has made a big impact to be able to address this.”
This study was funded by Takeda Pharmaceuticals USA. CCFA Partners is supported by the Crohn’s & Colitis Foundation and the Patient Centered Outcomes Research Institute.
ORLANDO – Vedolizumab therapy was associated with significant improvements in social satisfaction scores and steroid-free remission rates in biologic-naive patients with inflammatory bowel diseases (IBD) in a large prospective cohort.
The Internet-based cohort – Crohn’s & Colitis Foundation of America (CCFA) Partners – includes more than 15,000 IBD patients. For the current study, researchers evaluated 348 participants with Crohn’s disease or ulcerative colitis who initiated vedolizumab therapy between 2014 and 2017 and who had at least 6 months’ follow-up.
The difference in social satisfaction T scores was also improved among biologic-exposed patients (45.8 vs. 47.2, respectively), but the difference did not reach statistical significance, said Dr. Long of the University of North Carolina, Chapel Hill.
Improvements were also seen for numerous other measures, including anxiety, depression, fatigue, pain interference, and sleep disturbance – for both biologic-naive and -exposed patients – but the differences were not significant.
“But these [patient-reported outcomes] are clearly improving,” she said, explaining that trends toward minimally clinically important differences were seen for multiple measures.
As for steroid-free remission, the rate improved from 20% to 45% from baseline to 6-12 months among biologic-naive patients, and from 24% to 30% among biologic-exposed patients, Dr. Long said.
Vedolizumab in this real-world cohort was predominantly used in patients with refractory disease and prior biologic exposure.
The CCFA cohort provides an important glimpse into the effects of vedolizumab on patient-reported outcomes in real-world settings, Dr. Long said, noting that while vedolizumab has demonstrated important quality of life improvements in IBD clinical trials, little has been known about the effects of vedolizumab on quality of life in real-world settings.
The finding with respect to social satisfaction is particularly important, she said.
“These are sick patients. [These scores show that] they’re able to leave the house, they’re able to do the things they want to do,” she said. “It has made a big impact to be able to address this.”
This study was funded by Takeda Pharmaceuticals USA. CCFA Partners is supported by the Crohn’s & Colitis Foundation and the Patient Centered Outcomes Research Institute.
ORLANDO – Vedolizumab therapy was associated with significant improvements in social satisfaction scores and steroid-free remission rates in biologic-naive patients with inflammatory bowel diseases (IBD) in a large prospective cohort.
The Internet-based cohort – Crohn’s & Colitis Foundation of America (CCFA) Partners – includes more than 15,000 IBD patients. For the current study, researchers evaluated 348 participants with Crohn’s disease or ulcerative colitis who initiated vedolizumab therapy between 2014 and 2017 and who had at least 6 months’ follow-up.
The difference in social satisfaction T scores was also improved among biologic-exposed patients (45.8 vs. 47.2, respectively), but the difference did not reach statistical significance, said Dr. Long of the University of North Carolina, Chapel Hill.
Improvements were also seen for numerous other measures, including anxiety, depression, fatigue, pain interference, and sleep disturbance – for both biologic-naive and -exposed patients – but the differences were not significant.
“But these [patient-reported outcomes] are clearly improving,” she said, explaining that trends toward minimally clinically important differences were seen for multiple measures.
As for steroid-free remission, the rate improved from 20% to 45% from baseline to 6-12 months among biologic-naive patients, and from 24% to 30% among biologic-exposed patients, Dr. Long said.
Vedolizumab in this real-world cohort was predominantly used in patients with refractory disease and prior biologic exposure.
The CCFA cohort provides an important glimpse into the effects of vedolizumab on patient-reported outcomes in real-world settings, Dr. Long said, noting that while vedolizumab has demonstrated important quality of life improvements in IBD clinical trials, little has been known about the effects of vedolizumab on quality of life in real-world settings.
The finding with respect to social satisfaction is particularly important, she said.
“These are sick patients. [These scores show that] they’re able to leave the house, they’re able to do the things they want to do,” she said. “It has made a big impact to be able to address this.”
This study was funded by Takeda Pharmaceuticals USA. CCFA Partners is supported by the Crohn’s & Colitis Foundation and the Patient Centered Outcomes Research Institute.
AT THE WORLD CONGRESS OF GASTROENTEROLOGY
Key clinical point:
Major finding: T scores in biologic-naive patients improved significantly (46.1 before treatment vs. 51.0 after 6 months).
Data source: A prospective cohort study of 348 patients.
Disclosures: This study was funded by Takeda Pharmaceuticals USA. CCFA Partners is supported by the Crohn’s and Colitis Foundation and the Patient Centered Outcomes Research Institute.
The Future of Choice & VA Health Care
In late August, the President signed legislation that provided $2.1 billion to extend a program that gives veterans enrolled in the VHA a “Choice” in where they receive care. In the next few months, Congress will consider various plans to redesign the Veterans Choice Program. As policy makers consider these options, they should assess not only the plan’s ability to remedy any problems in veterans’ access to care, but also its broader impact. Congress must ensure that the next Choice Program does not compromise VHA’s overall quality of health care services delivered to veterans—care that has been demonstrated, with geographic variations, to be equal to, and often superior to, non-VA care.
Launched in 2014 as part of the Veterans Access, Choice and Accountability Act, the temporary Choice Program was meant to remedy a crisis of limited capacity, access, and excessive delays reported at many VHA facilities. The program offered non-VA options to veterans who had to wait long or travel far for their care. To date, the program has provided health care services to more than 1.6 million veterans.
As Senate and House VA committees began to draft new authorizing language for the program, many have spoken out about these issues and highlighted the unique importance of the VHA’s comprehensive, integrated model of care—one that is focused on the specific problems of veterans. NOVA, alongside its partners—Association of VA Psychologist Leaders, Association of VA Social Workers, and the organization Fighting for Veterans Healthcare—has provided their thoughts on the best solution to continue providing veterans timely access to this type of high-quality health care.
Congress must ensure far more than simply preserving the VHA’s innovative, integrated-care model. It must guarantee that the VHA’s system for clinically training the majority of U.S. health care professionals is maintained. The program funding must include a robust research department whose mission not only benefits veterans, but also the health care provided to every American. It must ensure that the community has the capacity to absorb an influx of veterans in a timely manner.
Community providers must be required to meet VHA’s elevated standards, use evidence-based treatments driven by measurement-based care, have knowledge of military culture and competence in veteran-specific problems, perform needed screenings, and be subject to the same training and continuing education requirements as VHA providers.
Given that non-VA care is more expensive than VHA care, Congress must ensure that any Choice care that veterans are offered is done so judiciously. Otherwise, the cost of Choice could wind up eroding VHA’s level of services. Finally, Congress also must ensure that the VHA is improved, not dismantled. As surveys and studies have shown, this is what the majority of veterans prefer and what they have been promised by administration and congressional leaders.
As VA nurses providing and coordinating care for veterans, we have a stake in how Choice and all community care is provided. As an organization, NOVA understands that community providers are a crucial part of an integrated network set up to provide care where there are shortages, but VHA must remain the first point of access and coordinator of that care.
Any new legislation addressing community-integrated care must include measures that hold providers accountable for performance and timeliness of care and services. It also must take into account the VHA’s unparalleled integration of primary and mental health care and the many wraparound services that are offered veterans.
Finally, the congressional budgeting process must include adequate funding for both VHA services and its integrated-community care accounts. The practice of reallocating funds from VHA health care accounts to pay for non-VA care cannot continue.
Making significant, lasting improvements in how VHA provides health care within its facilities and with partners in the community is unquestionably the right thing to do. It honors the sacred obligation we owe to veterans. Congress must be willing to invest in the VHA and provide veterans with the type of high-quality, veteran-centered care that serves their complex needs.
In late August, the President signed legislation that provided $2.1 billion to extend a program that gives veterans enrolled in the VHA a “Choice” in where they receive care. In the next few months, Congress will consider various plans to redesign the Veterans Choice Program. As policy makers consider these options, they should assess not only the plan’s ability to remedy any problems in veterans’ access to care, but also its broader impact. Congress must ensure that the next Choice Program does not compromise VHA’s overall quality of health care services delivered to veterans—care that has been demonstrated, with geographic variations, to be equal to, and often superior to, non-VA care.
Launched in 2014 as part of the Veterans Access, Choice and Accountability Act, the temporary Choice Program was meant to remedy a crisis of limited capacity, access, and excessive delays reported at many VHA facilities. The program offered non-VA options to veterans who had to wait long or travel far for their care. To date, the program has provided health care services to more than 1.6 million veterans.
As Senate and House VA committees began to draft new authorizing language for the program, many have spoken out about these issues and highlighted the unique importance of the VHA’s comprehensive, integrated model of care—one that is focused on the specific problems of veterans. NOVA, alongside its partners—Association of VA Psychologist Leaders, Association of VA Social Workers, and the organization Fighting for Veterans Healthcare—has provided their thoughts on the best solution to continue providing veterans timely access to this type of high-quality health care.
Congress must ensure far more than simply preserving the VHA’s innovative, integrated-care model. It must guarantee that the VHA’s system for clinically training the majority of U.S. health care professionals is maintained. The program funding must include a robust research department whose mission not only benefits veterans, but also the health care provided to every American. It must ensure that the community has the capacity to absorb an influx of veterans in a timely manner.
Community providers must be required to meet VHA’s elevated standards, use evidence-based treatments driven by measurement-based care, have knowledge of military culture and competence in veteran-specific problems, perform needed screenings, and be subject to the same training and continuing education requirements as VHA providers.
Given that non-VA care is more expensive than VHA care, Congress must ensure that any Choice care that veterans are offered is done so judiciously. Otherwise, the cost of Choice could wind up eroding VHA’s level of services. Finally, Congress also must ensure that the VHA is improved, not dismantled. As surveys and studies have shown, this is what the majority of veterans prefer and what they have been promised by administration and congressional leaders.
As VA nurses providing and coordinating care for veterans, we have a stake in how Choice and all community care is provided. As an organization, NOVA understands that community providers are a crucial part of an integrated network set up to provide care where there are shortages, but VHA must remain the first point of access and coordinator of that care.
Any new legislation addressing community-integrated care must include measures that hold providers accountable for performance and timeliness of care and services. It also must take into account the VHA’s unparalleled integration of primary and mental health care and the many wraparound services that are offered veterans.
Finally, the congressional budgeting process must include adequate funding for both VHA services and its integrated-community care accounts. The practice of reallocating funds from VHA health care accounts to pay for non-VA care cannot continue.
Making significant, lasting improvements in how VHA provides health care within its facilities and with partners in the community is unquestionably the right thing to do. It honors the sacred obligation we owe to veterans. Congress must be willing to invest in the VHA and provide veterans with the type of high-quality, veteran-centered care that serves their complex needs.
In late August, the President signed legislation that provided $2.1 billion to extend a program that gives veterans enrolled in the VHA a “Choice” in where they receive care. In the next few months, Congress will consider various plans to redesign the Veterans Choice Program. As policy makers consider these options, they should assess not only the plan’s ability to remedy any problems in veterans’ access to care, but also its broader impact. Congress must ensure that the next Choice Program does not compromise VHA’s overall quality of health care services delivered to veterans—care that has been demonstrated, with geographic variations, to be equal to, and often superior to, non-VA care.
Launched in 2014 as part of the Veterans Access, Choice and Accountability Act, the temporary Choice Program was meant to remedy a crisis of limited capacity, access, and excessive delays reported at many VHA facilities. The program offered non-VA options to veterans who had to wait long or travel far for their care. To date, the program has provided health care services to more than 1.6 million veterans.
As Senate and House VA committees began to draft new authorizing language for the program, many have spoken out about these issues and highlighted the unique importance of the VHA’s comprehensive, integrated model of care—one that is focused on the specific problems of veterans. NOVA, alongside its partners—Association of VA Psychologist Leaders, Association of VA Social Workers, and the organization Fighting for Veterans Healthcare—has provided their thoughts on the best solution to continue providing veterans timely access to this type of high-quality health care.
Congress must ensure far more than simply preserving the VHA’s innovative, integrated-care model. It must guarantee that the VHA’s system for clinically training the majority of U.S. health care professionals is maintained. The program funding must include a robust research department whose mission not only benefits veterans, but also the health care provided to every American. It must ensure that the community has the capacity to absorb an influx of veterans in a timely manner.
Community providers must be required to meet VHA’s elevated standards, use evidence-based treatments driven by measurement-based care, have knowledge of military culture and competence in veteran-specific problems, perform needed screenings, and be subject to the same training and continuing education requirements as VHA providers.
Given that non-VA care is more expensive than VHA care, Congress must ensure that any Choice care that veterans are offered is done so judiciously. Otherwise, the cost of Choice could wind up eroding VHA’s level of services. Finally, Congress also must ensure that the VHA is improved, not dismantled. As surveys and studies have shown, this is what the majority of veterans prefer and what they have been promised by administration and congressional leaders.
As VA nurses providing and coordinating care for veterans, we have a stake in how Choice and all community care is provided. As an organization, NOVA understands that community providers are a crucial part of an integrated network set up to provide care where there are shortages, but VHA must remain the first point of access and coordinator of that care.
Any new legislation addressing community-integrated care must include measures that hold providers accountable for performance and timeliness of care and services. It also must take into account the VHA’s unparalleled integration of primary and mental health care and the many wraparound services that are offered veterans.
Finally, the congressional budgeting process must include adequate funding for both VHA services and its integrated-community care accounts. The practice of reallocating funds from VHA health care accounts to pay for non-VA care cannot continue.
Making significant, lasting improvements in how VHA provides health care within its facilities and with partners in the community is unquestionably the right thing to do. It honors the sacred obligation we owe to veterans. Congress must be willing to invest in the VHA and provide veterans with the type of high-quality, veteran-centered care that serves their complex needs.
VIDEO: Gastroenterologist survey shows opportunity to expand Lynch syndrome testing
ORLANDO – A large percentage of U.S. gastroenterologists said that they don’t routinely order genetic testing for Lynch syndrome for patients with early-onset colorectal cancer, often because the physicians believe that the test is too expensive, or because they are unfamiliar with interpreting or applying the results, according to survey replies from 442 gastroenterologists.
Another factor hindering broader screening for Lynch syndrome (also known as hereditary nonpolyposis colorectal cancer) is that many of the surveyed gastroenterologists did not see themselves as having primary responsibility for ordering Lynch syndrome testing in patients who develop colorectal cancer before reaching age 50 years, Jordan J. Karlitz, MD, and his associates reported in a poster at the World Congress of Gastroenterology at ACG 2017.
The survey results showed that only a third of the survey respondents believed it primarily was the attending gastroenterologist’s responsibility to order testing for Lynch syndrome using either a microsatellite DNA instability test or by immunohistochemistry. A larger percentage, 38%, said that ordering one of these tests was something that a pathologist should arrange, 15% said it was primarily the responsibility of the attending medical oncologist, and the remaining respondents cited a surgeon or genetic counselor as having primary responsibility for ordering the test.
This absence of a clear consensus on who orders the test shows a “diffusion of responsibility” that often means testing is never ordered, Dr. Karlitz said in a video interview. What’s needed instead is “reflex testing” that’s done automatically for appropriate patients, an approach that has become standard at several U.S. medical centers, he noted.
The survey Dr. Karlitz and his associates ran stemmed from a report they published in 2015 that focused on management of the 274 patients diagnosed with early-onset colorectal cancer in Louisiana during 2011, defined as cancers diagnosed in patients aged 50 years or younger. Data collected in the Louisiana Tumor Registry showed that Lynch syndrome testing occurred for only 23% of these patients, the researchers reported (Am J Gastroenterol. 2015 Jul;110[7]:948-55).
To better understand the underpinnings of this low testing rate they sent a survey about Lynch syndrome testing by email in March 2017 to nearly 12,000 physicians on the membership roster of the American College of Gastroenterology. They received 455 replies, with 442 (97%) of the responses from gastroenterologists. When asked why they might not order Lynch syndrome testing for patients with early-onset colorectal cancer, 22% said the cost of testing was prohibitive, 18% blamed their lack of familiarity with the Lynch syndrome tests and how to properly interpret their results, and 15% attributed their decision to a lack of easy access to genetic counseling for their patients, with additional reasons cited by fewer respondents.
Dr. Karlitz noted that current recommendations from the National Comprehensive Cancer Network call for Lynch syndrome testing for all patients who develop colorectal cancer regardless of their age at diagnosis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
ORLANDO – A large percentage of U.S. gastroenterologists said that they don’t routinely order genetic testing for Lynch syndrome for patients with early-onset colorectal cancer, often because the physicians believe that the test is too expensive, or because they are unfamiliar with interpreting or applying the results, according to survey replies from 442 gastroenterologists.
Another factor hindering broader screening for Lynch syndrome (also known as hereditary nonpolyposis colorectal cancer) is that many of the surveyed gastroenterologists did not see themselves as having primary responsibility for ordering Lynch syndrome testing in patients who develop colorectal cancer before reaching age 50 years, Jordan J. Karlitz, MD, and his associates reported in a poster at the World Congress of Gastroenterology at ACG 2017.
The survey results showed that only a third of the survey respondents believed it primarily was the attending gastroenterologist’s responsibility to order testing for Lynch syndrome using either a microsatellite DNA instability test or by immunohistochemistry. A larger percentage, 38%, said that ordering one of these tests was something that a pathologist should arrange, 15% said it was primarily the responsibility of the attending medical oncologist, and the remaining respondents cited a surgeon or genetic counselor as having primary responsibility for ordering the test.
This absence of a clear consensus on who orders the test shows a “diffusion of responsibility” that often means testing is never ordered, Dr. Karlitz said in a video interview. What’s needed instead is “reflex testing” that’s done automatically for appropriate patients, an approach that has become standard at several U.S. medical centers, he noted.
The survey Dr. Karlitz and his associates ran stemmed from a report they published in 2015 that focused on management of the 274 patients diagnosed with early-onset colorectal cancer in Louisiana during 2011, defined as cancers diagnosed in patients aged 50 years or younger. Data collected in the Louisiana Tumor Registry showed that Lynch syndrome testing occurred for only 23% of these patients, the researchers reported (Am J Gastroenterol. 2015 Jul;110[7]:948-55).
To better understand the underpinnings of this low testing rate they sent a survey about Lynch syndrome testing by email in March 2017 to nearly 12,000 physicians on the membership roster of the American College of Gastroenterology. They received 455 replies, with 442 (97%) of the responses from gastroenterologists. When asked why they might not order Lynch syndrome testing for patients with early-onset colorectal cancer, 22% said the cost of testing was prohibitive, 18% blamed their lack of familiarity with the Lynch syndrome tests and how to properly interpret their results, and 15% attributed their decision to a lack of easy access to genetic counseling for their patients, with additional reasons cited by fewer respondents.
Dr. Karlitz noted that current recommendations from the National Comprehensive Cancer Network call for Lynch syndrome testing for all patients who develop colorectal cancer regardless of their age at diagnosis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
ORLANDO – A large percentage of U.S. gastroenterologists said that they don’t routinely order genetic testing for Lynch syndrome for patients with early-onset colorectal cancer, often because the physicians believe that the test is too expensive, or because they are unfamiliar with interpreting or applying the results, according to survey replies from 442 gastroenterologists.
Another factor hindering broader screening for Lynch syndrome (also known as hereditary nonpolyposis colorectal cancer) is that many of the surveyed gastroenterologists did not see themselves as having primary responsibility for ordering Lynch syndrome testing in patients who develop colorectal cancer before reaching age 50 years, Jordan J. Karlitz, MD, and his associates reported in a poster at the World Congress of Gastroenterology at ACG 2017.
The survey results showed that only a third of the survey respondents believed it primarily was the attending gastroenterologist’s responsibility to order testing for Lynch syndrome using either a microsatellite DNA instability test or by immunohistochemistry. A larger percentage, 38%, said that ordering one of these tests was something that a pathologist should arrange, 15% said it was primarily the responsibility of the attending medical oncologist, and the remaining respondents cited a surgeon or genetic counselor as having primary responsibility for ordering the test.
This absence of a clear consensus on who orders the test shows a “diffusion of responsibility” that often means testing is never ordered, Dr. Karlitz said in a video interview. What’s needed instead is “reflex testing” that’s done automatically for appropriate patients, an approach that has become standard at several U.S. medical centers, he noted.
The survey Dr. Karlitz and his associates ran stemmed from a report they published in 2015 that focused on management of the 274 patients diagnosed with early-onset colorectal cancer in Louisiana during 2011, defined as cancers diagnosed in patients aged 50 years or younger. Data collected in the Louisiana Tumor Registry showed that Lynch syndrome testing occurred for only 23% of these patients, the researchers reported (Am J Gastroenterol. 2015 Jul;110[7]:948-55).
To better understand the underpinnings of this low testing rate they sent a survey about Lynch syndrome testing by email in March 2017 to nearly 12,000 physicians on the membership roster of the American College of Gastroenterology. They received 455 replies, with 442 (97%) of the responses from gastroenterologists. When asked why they might not order Lynch syndrome testing for patients with early-onset colorectal cancer, 22% said the cost of testing was prohibitive, 18% blamed their lack of familiarity with the Lynch syndrome tests and how to properly interpret their results, and 15% attributed their decision to a lack of easy access to genetic counseling for their patients, with additional reasons cited by fewer respondents.
Dr. Karlitz noted that current recommendations from the National Comprehensive Cancer Network call for Lynch syndrome testing for all patients who develop colorectal cancer regardless of their age at diagnosis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
AT THE WORLD CONGRESS OF GASTROENTEROLOGY
Key clinical point:
Major finding: Among gastroenterologist survey respondents, one-third said they had primary responsibility for ordering Lynch syndrome testing.
Data source: Survey emailed to members of the American College of Gastroenterology and completed by 455 physicians and surgeons.
Disclosures: Dr. Karlitz has been a speaker on behalf of Myriad Genetics, a company that markets genetic tests for Lynch syndrome.
FDA Approves Patient-Assisted Mammography
Women of all ages and sizes will be glad to know that they now have some say in the amount of pressure applied to the breast during a mammography. The FDA has cleared Senographe Pristina with Self-Compression, the first patient-assisted 2D digital mammography system.
Digital mammograms use a computer along with x-rays. During an exam with the new system, the technologist positions the patient and initiates compression, then guides the patient in using the handheld wireless remote control to adjust the compression to a comfortable level. The technologist makes the final decision on whether the compression is adequate.
A clinical validation demonstrated that the addition of a remote to allow self-compression did not negatively affect image quality. Nor did allowing the patient to help with adjustments make the exam take significantly longer.
Women of all ages and sizes will be glad to know that they now have some say in the amount of pressure applied to the breast during a mammography. The FDA has cleared Senographe Pristina with Self-Compression, the first patient-assisted 2D digital mammography system.
Digital mammograms use a computer along with x-rays. During an exam with the new system, the technologist positions the patient and initiates compression, then guides the patient in using the handheld wireless remote control to adjust the compression to a comfortable level. The technologist makes the final decision on whether the compression is adequate.
A clinical validation demonstrated that the addition of a remote to allow self-compression did not negatively affect image quality. Nor did allowing the patient to help with adjustments make the exam take significantly longer.
Women of all ages and sizes will be glad to know that they now have some say in the amount of pressure applied to the breast during a mammography. The FDA has cleared Senographe Pristina with Self-Compression, the first patient-assisted 2D digital mammography system.
Digital mammograms use a computer along with x-rays. During an exam with the new system, the technologist positions the patient and initiates compression, then guides the patient in using the handheld wireless remote control to adjust the compression to a comfortable level. The technologist makes the final decision on whether the compression is adequate.
A clinical validation demonstrated that the addition of a remote to allow self-compression did not negatively affect image quality. Nor did allowing the patient to help with adjustments make the exam take significantly longer.
Blood donors’ pregnancy history may impact recipients’ risk of death
In a large study, men who received blood from women with a history of pregnancy had an increased risk of death after transfusion.
However, receiving blood from a woman who was never pregnant did not carry the same risk.
And female recipients of blood transfusions had a similar risk of death whether they received blood from women with or without a history of pregnancy.
Rutger A. Middelburg, PhD, of Sanquin Research in Leiden, Netherlands, and his colleagues reported these results in JAMA.
The researchers noted that the most common cause of transfusion-related mortality is transfusion-related acute lung injury, which has been associated with transfusions from female donors, specifically those with a history of pregnancy.
Therefore, Dr Middleburg and his colleagues set out to determine whether an increased risk of mortality after red blood cell transfusions could depend on the donor’s history of pregnancy.
The team studied first-time transfusion recipients at 6 major Dutch hospitals.
When the researchers looked only at patients who received blood from a single type of donor (male/female with pregnancy history/female without), there was a significantly higher risk of death among men who received blood from females with a history of pregnancy.
Male recipients
There were 1722 deaths among the 12,212 males who only received blood from male donors (hazard ratio [HR]=1.00).
There were 1873 deaths among the 13,669 males who only received blood from females with a history of pregnancy (HR=1.13, P=0.03).
And there were 1831 deaths among the 13,538 men who only received blood from females without a history of pregnancy (HR=0.93, P=0.29).
Female recipients
There were 1752 deaths among the 13,332 females who only received blood from male donors (HR=1.00).
There were 1871 deaths among the 14,770 females who only received blood from females with a history of pregnancy (HR=0.99, P=0.92).
And there were 1868 deaths among the 14,685 females who only received blood from females with no history of pregnancy (HR=1.01, P=0.92).
Role of age
The researchers also found the association between donor pregnancy history and recipient death was only observed for men younger than 51.
There were 107 deaths among the 2251 males ages 0 to 17 who received blood from male donors (HR=1.00). And there were 124 deaths among the 2556 males ages 0 to 17 who received blood from females with a history of pregnancy (HR=1.63, P=0.04).
There were 84 deaths among the 1170 males ages 18 to 50 who received blood from male donors (HR=1.00). And there were 94 deaths among the 1296 males ages 18 to 50 who received blood from females with a history of pregnancy (HR=1.50, P=0.06).
There were 598 deaths among the 4292 males ages 51 to 70 who received blood from male donors (HR=1.00). And there were 645 deaths among the 4775 males ages 51 to 70 who received blood from females with a history of pregnancy (HR=1.10, P=0.31).
There were 933 deaths among the 4499 males ages 71 and older who received blood from male donors (HR=1.00). And there were 1010 deaths among the 5042 males ages 71 and older who received blood from females with a history of pregnancy (HR=1.06, P=0.47).
The researchers said more work is required to replicate these findings, determine their clinical significance, and identify the underlying mechanism. ![]()
In a large study, men who received blood from women with a history of pregnancy had an increased risk of death after transfusion.
However, receiving blood from a woman who was never pregnant did not carry the same risk.
And female recipients of blood transfusions had a similar risk of death whether they received blood from women with or without a history of pregnancy.
Rutger A. Middelburg, PhD, of Sanquin Research in Leiden, Netherlands, and his colleagues reported these results in JAMA.
The researchers noted that the most common cause of transfusion-related mortality is transfusion-related acute lung injury, which has been associated with transfusions from female donors, specifically those with a history of pregnancy.
Therefore, Dr Middleburg and his colleagues set out to determine whether an increased risk of mortality after red blood cell transfusions could depend on the donor’s history of pregnancy.
The team studied first-time transfusion recipients at 6 major Dutch hospitals.
When the researchers looked only at patients who received blood from a single type of donor (male/female with pregnancy history/female without), there was a significantly higher risk of death among men who received blood from females with a history of pregnancy.
Male recipients
There were 1722 deaths among the 12,212 males who only received blood from male donors (hazard ratio [HR]=1.00).
There were 1873 deaths among the 13,669 males who only received blood from females with a history of pregnancy (HR=1.13, P=0.03).
And there were 1831 deaths among the 13,538 men who only received blood from females without a history of pregnancy (HR=0.93, P=0.29).
Female recipients
There were 1752 deaths among the 13,332 females who only received blood from male donors (HR=1.00).
There were 1871 deaths among the 14,770 females who only received blood from females with a history of pregnancy (HR=0.99, P=0.92).
And there were 1868 deaths among the 14,685 females who only received blood from females with no history of pregnancy (HR=1.01, P=0.92).
Role of age
The researchers also found the association between donor pregnancy history and recipient death was only observed for men younger than 51.
There were 107 deaths among the 2251 males ages 0 to 17 who received blood from male donors (HR=1.00). And there were 124 deaths among the 2556 males ages 0 to 17 who received blood from females with a history of pregnancy (HR=1.63, P=0.04).
There were 84 deaths among the 1170 males ages 18 to 50 who received blood from male donors (HR=1.00). And there were 94 deaths among the 1296 males ages 18 to 50 who received blood from females with a history of pregnancy (HR=1.50, P=0.06).
There were 598 deaths among the 4292 males ages 51 to 70 who received blood from male donors (HR=1.00). And there were 645 deaths among the 4775 males ages 51 to 70 who received blood from females with a history of pregnancy (HR=1.10, P=0.31).
There were 933 deaths among the 4499 males ages 71 and older who received blood from male donors (HR=1.00). And there were 1010 deaths among the 5042 males ages 71 and older who received blood from females with a history of pregnancy (HR=1.06, P=0.47).
The researchers said more work is required to replicate these findings, determine their clinical significance, and identify the underlying mechanism. ![]()
In a large study, men who received blood from women with a history of pregnancy had an increased risk of death after transfusion.
However, receiving blood from a woman who was never pregnant did not carry the same risk.
And female recipients of blood transfusions had a similar risk of death whether they received blood from women with or without a history of pregnancy.
Rutger A. Middelburg, PhD, of Sanquin Research in Leiden, Netherlands, and his colleagues reported these results in JAMA.
The researchers noted that the most common cause of transfusion-related mortality is transfusion-related acute lung injury, which has been associated with transfusions from female donors, specifically those with a history of pregnancy.
Therefore, Dr Middleburg and his colleagues set out to determine whether an increased risk of mortality after red blood cell transfusions could depend on the donor’s history of pregnancy.
The team studied first-time transfusion recipients at 6 major Dutch hospitals.
When the researchers looked only at patients who received blood from a single type of donor (male/female with pregnancy history/female without), there was a significantly higher risk of death among men who received blood from females with a history of pregnancy.
Male recipients
There were 1722 deaths among the 12,212 males who only received blood from male donors (hazard ratio [HR]=1.00).
There were 1873 deaths among the 13,669 males who only received blood from females with a history of pregnancy (HR=1.13, P=0.03).
And there were 1831 deaths among the 13,538 men who only received blood from females without a history of pregnancy (HR=0.93, P=0.29).
Female recipients
There were 1752 deaths among the 13,332 females who only received blood from male donors (HR=1.00).
There were 1871 deaths among the 14,770 females who only received blood from females with a history of pregnancy (HR=0.99, P=0.92).
And there were 1868 deaths among the 14,685 females who only received blood from females with no history of pregnancy (HR=1.01, P=0.92).
Role of age
The researchers also found the association between donor pregnancy history and recipient death was only observed for men younger than 51.
There were 107 deaths among the 2251 males ages 0 to 17 who received blood from male donors (HR=1.00). And there were 124 deaths among the 2556 males ages 0 to 17 who received blood from females with a history of pregnancy (HR=1.63, P=0.04).
There were 84 deaths among the 1170 males ages 18 to 50 who received blood from male donors (HR=1.00). And there were 94 deaths among the 1296 males ages 18 to 50 who received blood from females with a history of pregnancy (HR=1.50, P=0.06).
There were 598 deaths among the 4292 males ages 51 to 70 who received blood from male donors (HR=1.00). And there were 645 deaths among the 4775 males ages 51 to 70 who received blood from females with a history of pregnancy (HR=1.10, P=0.31).
There were 933 deaths among the 4499 males ages 71 and older who received blood from male donors (HR=1.00). And there were 1010 deaths among the 5042 males ages 71 and older who received blood from females with a history of pregnancy (HR=1.06, P=0.47).
The researchers said more work is required to replicate these findings, determine their clinical significance, and identify the underlying mechanism. ![]()
Study shows similar safety with DOACs and warfarin
New research suggests patients with venous thromboembolism (VTE) have similar safety outcomes whether they receive direct oral anticoagulants (DOACs) or warfarin.
The population-based study showed there was no significant difference in the risk of major bleeding and all-cause mortality at 90 days whether patients received warfarin or DOACs (apixaban, dabigatran, and rivaroxaban).
Brenda R. Hemmelgarn, MD, PhD, of the University of Calgary in Alberta, Canada, and her colleagues reported results from this study in The BMJ.
The researchers noted that recent clinical trials have shown similar effectiveness and a reduced or similar risk of major bleeding complications for DOACs compared with warfarin. However, clinical trials involve a highly selected group of patients, so the rate of safety events reported in trials may not reflect those seen in everyday clinical practice.
With this in mind, Dr Hemmelgarn and her colleagues conducted a population-based study to determine the safety of DOAC and warfarin use in adults diagnosed with VTE between January 1, 2009, and March 31, 2016.
Using healthcare data from 6 jurisdictions in Canada and the US, the researchers identified 59,525 adults with a new diagnosis of VTE and a prescription for a DOAC (n=12,489) or warfarin (n=47,036) within 30 days of diagnosis.
Participants were followed for an average of 85.2 days. Of the 59,525 participants, 1967 (3.3%) had a major bleed, and 1029 (1.7%) died during the follow-up period.
Bleeding rates at 30 days ranged between 0.2% and 2.9% for DOACs and 0.2% and 2.9% for warfarin.
Bleeding rates at 60 days ranged between 0.4% and 4.3% for DOACs and 0.4% and 4.3% for warfarin.
The hazard ratio for major bleeding at 90 days was 0.92 (favoring DOACs). The hazard ratio for all-cause mortality at 90 days was 0.99.
The researchers said there was no evidence of heterogeneity across treatment centers, between patients with and without chronic kidney disease, across age groups, or between male and female patients.
The team noted that this is an observational study, so no firm conclusions can be drawn about cause and effect. And they could not rule out the possibility that their results may be due to confounding factors. ![]()
New research suggests patients with venous thromboembolism (VTE) have similar safety outcomes whether they receive direct oral anticoagulants (DOACs) or warfarin.
The population-based study showed there was no significant difference in the risk of major bleeding and all-cause mortality at 90 days whether patients received warfarin or DOACs (apixaban, dabigatran, and rivaroxaban).
Brenda R. Hemmelgarn, MD, PhD, of the University of Calgary in Alberta, Canada, and her colleagues reported results from this study in The BMJ.
The researchers noted that recent clinical trials have shown similar effectiveness and a reduced or similar risk of major bleeding complications for DOACs compared with warfarin. However, clinical trials involve a highly selected group of patients, so the rate of safety events reported in trials may not reflect those seen in everyday clinical practice.
With this in mind, Dr Hemmelgarn and her colleagues conducted a population-based study to determine the safety of DOAC and warfarin use in adults diagnosed with VTE between January 1, 2009, and March 31, 2016.
Using healthcare data from 6 jurisdictions in Canada and the US, the researchers identified 59,525 adults with a new diagnosis of VTE and a prescription for a DOAC (n=12,489) or warfarin (n=47,036) within 30 days of diagnosis.
Participants were followed for an average of 85.2 days. Of the 59,525 participants, 1967 (3.3%) had a major bleed, and 1029 (1.7%) died during the follow-up period.
Bleeding rates at 30 days ranged between 0.2% and 2.9% for DOACs and 0.2% and 2.9% for warfarin.
Bleeding rates at 60 days ranged between 0.4% and 4.3% for DOACs and 0.4% and 4.3% for warfarin.
The hazard ratio for major bleeding at 90 days was 0.92 (favoring DOACs). The hazard ratio for all-cause mortality at 90 days was 0.99.
The researchers said there was no evidence of heterogeneity across treatment centers, between patients with and without chronic kidney disease, across age groups, or between male and female patients.
The team noted that this is an observational study, so no firm conclusions can be drawn about cause and effect. And they could not rule out the possibility that their results may be due to confounding factors. ![]()
New research suggests patients with venous thromboembolism (VTE) have similar safety outcomes whether they receive direct oral anticoagulants (DOACs) or warfarin.
The population-based study showed there was no significant difference in the risk of major bleeding and all-cause mortality at 90 days whether patients received warfarin or DOACs (apixaban, dabigatran, and rivaroxaban).
Brenda R. Hemmelgarn, MD, PhD, of the University of Calgary in Alberta, Canada, and her colleagues reported results from this study in The BMJ.
The researchers noted that recent clinical trials have shown similar effectiveness and a reduced or similar risk of major bleeding complications for DOACs compared with warfarin. However, clinical trials involve a highly selected group of patients, so the rate of safety events reported in trials may not reflect those seen in everyday clinical practice.
With this in mind, Dr Hemmelgarn and her colleagues conducted a population-based study to determine the safety of DOAC and warfarin use in adults diagnosed with VTE between January 1, 2009, and March 31, 2016.
Using healthcare data from 6 jurisdictions in Canada and the US, the researchers identified 59,525 adults with a new diagnosis of VTE and a prescription for a DOAC (n=12,489) or warfarin (n=47,036) within 30 days of diagnosis.
Participants were followed for an average of 85.2 days. Of the 59,525 participants, 1967 (3.3%) had a major bleed, and 1029 (1.7%) died during the follow-up period.
Bleeding rates at 30 days ranged between 0.2% and 2.9% for DOACs and 0.2% and 2.9% for warfarin.
Bleeding rates at 60 days ranged between 0.4% and 4.3% for DOACs and 0.4% and 4.3% for warfarin.
The hazard ratio for major bleeding at 90 days was 0.92 (favoring DOACs). The hazard ratio for all-cause mortality at 90 days was 0.99.
The researchers said there was no evidence of heterogeneity across treatment centers, between patients with and without chronic kidney disease, across age groups, or between male and female patients.
The team noted that this is an observational study, so no firm conclusions can be drawn about cause and effect. And they could not rule out the possibility that their results may be due to confounding factors. ![]()