User login
Using Contingency Management for the Treatment of Substance Use Disorders in Real-World Settings
From UConn Health, Farmington, CT.
Abstract
- Objective: To discuss the efficacy and generalizability of contingency management (CM) for the treatment of substance use disorders and design considerations for those considering implementing in clinical settings.
- Methods: Review of the literature.
- Results: CM is an efficacious treatment for substance abuse disorders that is widely generalizable across substance use disorders and patient characteristics. CM can be implemented in a number of treatment programs, including residential and outpatient settings, and it can be administered in both individual and group formats. Abstinence and attendance are the most commonly targeted behaviors in substance abuse treatment settings. Design features, including the selection of target behaviors, delivery methods, and reinforcers, are discussed. Schedule parameters, such as frequency, magnitude, immediacy, and escalation of reinforcement, are associated with overall impact of the CM program and are important considerations for those interested in tailoring CM protocols to their needs.
- Conclusion: CM is an efficacious option that is applicable to most substance abuse treatment patients. A number of demonstrations of real-world implementation have been published and suggest CM can be adapted with success to clinic settings. In adopting CM protocols, clinics should aim for those protocols with established efficacy; however, if adaptations are necessary, careful consideration should be given to modifications to minimize risks of undermining CM’s effects.
Key words: incentives; reinforcement; substance abuse treatment; dissemination; implementation.
Contingency management (CM) is a behavioral intervention that is efficacious in the treatment of substance use disorders (SUDs). CM uses a behavior analytic framework and applies principles of learning theory, particularly operant conditioning theory, to change client behavior(s) [1–5]. In basic terms, operant conditioning principles suggests that whether a behavior continues or not is a function of consequences [6]. Reinforced behaviors are more likely to occur in the future. Substance abuse can be viewed as a behavior maintained by the reinforcing effects of the drug itself [5], including the feel-good aspects of intoxication or relaxation and the amelioration of withdrawal symptoms. CM extends these same principles of to a treatment context, such that reinforcers for abstinent behavior are introduced to compete with the reinforcing effects of continued drug use [5].
In CM’s application to substance abuse treatment, drug-negative samples or treatment attendance are reinforced using tangible incentives with the goal of motivating continued abstinence and/or treatment engagement. When clients demonstrate these target behaviors, they earn incentives in the form of goods or services of value to the client, such as small electronics, gift cards, and toiletries. Despite the promising effects observed in research trials, real-world implementation efforts have not kept pace [7–9]. This review briefly discusses CM’s efficacy and highlights key features for professionals considering adopting this intervention. Demonstration efforts that illustrate how CM can be effectively implemented within the constraints and limitations of non-research, clinical settings are also presented.
Efficacy of CM
CM’s efficacy spans a number of SUDs, including cocaine, opioids, alcohol, nicotine, and marijuana [10–13], making it amenable for treatment of most SUD clinic populations. It generates larger effect sizes than other SUD treatments, including cognitive behavioral therapy [14], and it has been evaluated in a wide range of settings. Large-scale evaluations have been conducted in both intensive outpatient [15] and methadone maintenance [16] settings as part of the National Institute on Drug Abuse Clinical Trials Network, demonstrating consistent benefits of CM when added to treatment as usual. In the first of these 2 studies, Petry et al [15] randomized 415 stimulant users from 1 of 8 intensive outpatient clinics to treatment as usual or treatment as usual plus CM for alcohol and stimulant abstinence. CM participants submitted more substance-negative urine and breath samples, achieved continuous abstinence at significantly higher rates, and had longer treatment retention compared to those receiving treatment as usual. The parallel study [16] focused on stimulant use in clients from methadone maintenance clinics and found similar benefits of CM on stimulant abstinence. Beyond these settings, CM has been applied in a number of other contexts, including drop-in centers [17], vocational rehabilitation [18,19], job-skills training [20], and residential programs [21–23]. In addition, several group-based adaptations have been explored [17,24–27].
CM benefits most clients and generalizes across several demographic variables, including gender [28,29], race [30], housing status [31], and income levels [32–34]. Among clinical characteristics, CM is efficacious for those with co-occurring SUDs [35], other substance use [36], psychiatric disorders [37–39], medical problems [40–42], and history of transactional sex [43].
Design Considerations
Design features, including what behavior will be reinforced and how to do so, are among the first decision points for clinicians interested in implementing CM. One of the advantages of CM is that it has a high degree of flexibility in design, which means that it can be readily tailored to client populations and clinic needs. However, this flexibility can lead clinicians astray from the foundational principles of CM and unknowingly weaken the impact of the program. Below, some key considerations for CM protocol design are reviewed. For additional coverage of these topics, readers are referred to additional articles [1,2] or Petry’s comprehensive book on implementing CM [44]. In this review, published examples of CM’s application in real-world settings are presented, highlighting how CM has been adapted in these clinical efforts.
Target Behaviors
The selection of the target behavior will drive many of the subsequent program design decisions. As such, it is important to identify this feature early. Target behaviors must be achievable, objectively verifiable, and well defined. The most common CM targets are drug abstinence or therapy session attendance. CM has also been used to target other behaviors, such as medication adherence [45,46], treatment-related activities [47,48], and exercise [49–51]. Client self-report of behaviors or vaguely defined behaviors (eg, “good participation”) should be avoided. While some of the decisions related to CM protocols are flexible, the use of objectively verifiable target behaviors is a core feature that should not be neglected. If the behavior of interest cannot be objectively verified, an alternate behavior should be chosen.
Selection of the target behavior is often considered in hand with defining which population is eligible to participate in the CM program. Client characteristics are often forefront in this decision, but clinic-driven logistical issues or unmet needs may also play a role. A real-world example of this decision process is evident in the nationwide rollout of CM among the intensive outpatient programs within the Veterans Administration (VA). The VA identified a treatment need for those with stimulant use disorders, as this group did not have efficacious pharmacotherapy options available that targeted stimulant use. As such, the VA applied CM to patients with a focus on stimulant abstinence as the behavioral target [52]. For others, the decision may revolve around addressing underutilization of specific treatment resources (eg, outpatient groups, vocational rehabilitation) [53–56] or treatment needs among certain subgroups of clients, such as adolescents [57–59].
For abstinence targets, clinics would need to select one or more specific substances as the focus of the CM program. In general, targeting a single substance rather than multiple substances is more effective [10,13], is more straightforward for clients to understand, and allows more clients to access the reinforcers. Exposure to the reinforcers is necessary for CM to work; thus, setting a goal that is achievable for most clients should be a priority. Requiring abstinence from multiple substances means that some clients may never experience the reinforcer and thus cannot benefit from its effects at all. Some clinicians or administrators may initially have reservations about reinforcing single drug abstinence in the event that other drug use continues. However, targeting a single substance for reinforcement often results in reduced use of other substances [60]. Clinicians may find that this makes intuitive sense; a client with cocaine use disorder who is trying to maintain cocaine abstinence over a long period is likely to avoid using alcohol or other substances that might lead to relapse. For abstinence, objective verification through urine or breath specimens using tests that include validity checks is relatively straightforward.
Attendance is a popular target for clinics in part because it does not require additional staff time to collect specimen samples and it was the most commonly reported target behavior in samples of SUD providers who use incentives [61,62]. Objective verification of attendance is usually via a staff member, but expectations must be clear to both parties. Clinics should consider potential problems that may arise (eg, arriving late, leaving group early, excused absences) and carefully define and communicate expectations for the CM program. Piloting [19] the CM program with a small group of clients may be valuable in trouble-shooting challenges before wider implementation.
In a recent study [55], clients earned reinforcers for attending clinician-led group counseling sessions and/or the in-clinic patient-led Methadone Anonymous (MA) groups. This non-research, clinical effort addressed historically poor therapy attendance at the clinic, and attendance rates were compared before, during, and after the CM program. CM increased attendance to both groups in the short-term after implementation, but effects were more robust for the MA groups in which increased attendance persisted 3 months following the withdrawal of the contingencies. Overall effects of this program were modest, but they are notable given the use of an ultra-low cost approach.
Delivery Methods
The majority of CM studies used voucher or prize-based methods. Head-to-head comparisons suggest that they are comparable in efficacy [63–65], and each has advantages and disadvantages that may make one option more appealing for a given clinic. Voucher programs are generally straightforward to administer. Clients earn vouchers for each instance of the target behavior. The value of the vouchers typically increases with consecutive performance. The schedule used in the influential Higgins et al studies [66,67] started at $2.50 for the first cocaine-negative sample and increased by $0.50 for each subsequent consecutive cocaine-negative sample. Earned vouchers are exchanged for goods or services selected by the client, increasing the likelihood that the selected items will be highly desirable and allowing for a wide range of client preferences. Clients appear to prefer this approach when given a choice between set schedules or those that introduce an element of chance (ie, prize-based CM, discussed below) [68]. However, voucher programs can be costly (~$1000 per client over 12 weeks) and may require more staff time to fulfill individual requests for specific items. However, staff burden related to shopping can be reduced by limiting these individual requests and using an on-site stocked cabinet of goods similar to prize-CM programs.
Prize-based CM is similar but introduces probabilistic earnings and variability in prize magnitude. Rather than earning vouchers, clients earn draws from a fishbowl for each instance of the target behavior, again typically in an escalating manner. For example, a client may earn one draw from the fishbowl for the first cocaine-negative sample, 2 draws for the second consecutive negative sample, 3 draws for the third, and so on. A typical fishbowl is composed of 500 slips, some noting prizes and some having no prize value. Typically, half the slips in the bowl are non-monetary “good jobs.” The remaining half are small prizes worth about $1 in value (eg, food coupons, bus tokens, small toiletries), large prizes worth about $20 in value (eg, small electronics, gift certificates), and one slip is the jumbo prize worth about $100. When a client draws a winning slip, they select a prize from that category (ie, small, large, jumbo) from an onsite, stocked cabinet. Due to the probabilistic feature of prize-based CM, overall costs of the program can be substantially lower than typical voucher programs, with average maximum expected earnings ranging $250 t $450 per client over a 12-week treatment period [15,16,65,69]. Advantages of this method include potentially lower costs and minimal shopping demands (a once-monthly shopping trip to restock the cabinet will usually suffice) while maintaining comparable efficacy. Relative to voucher programs, prize-based CM involves additional administration time to allow for drawing slips from the fishbowl, which can be compounded when multiple clients want to draw at the same time such as in a group setting. Many of the group-based CM adaptations address this issue by limiting the number of clients who can draw for prizes in a given group or by limiting the number of draws per client [25,27,54].
Reinforcers
Regardless of whether selecting voucher or prize CM, reinforcers are critically important to the success of the program. Reinforcers must be desirable. One of the quickest ways to undermine a CM program is lack of variety or undesirable reinforcers. If stocking a cabinet with prizes onsite, care should be taken to have numerous options within each of the small and large prize categories that are appealing to a wide range of clients. Since a client who is consistently earning draws will choose prizes often, it is imperative to include enough variety so that even these clients find desirable items each time they select a prize. Clients should be asked regularly if they have suggestions for prizes; one program [54] found suggestion boxes useful for encouraging clients to voice their preferences. Donations can be solicited from local businesses to reduce costs [53], and low-cost but high-value options, such as clinic privileges, can also be explored. Petry [1,44] provides some suggestions of the latter, and Amass and Kamien [70] describe their successful strategies to fund and sustain a clinic-based CM program through community donations. Some clinics may already have tangible goods, such as gas or metro cards, that are offered to clients based on need rather than behavior [53]. These existing resources might be redirected to a CM program, in which these goods are contingent on abstinence or attendance, if appropriate.
Schedule Parameters
Once the target behavior, client population, and CM delivery methods are selected, the next step is to design the reinforcement schedule. The following schedule parameters apply to both voucher and prize-based CM systems. The more closely a clinical program adheres to the parameters of effective protocols, the more likely the program is to generate comparable outcomes. If there is a parameter or design feature that is incompatible with clinic needs, modifications can be introduced. However, each deviation away from the ideal has a chance of undermining the success of the CM program. Any changes and their potential impacts should considered carefully, and consultation with a CM expert may aid in the development of successful and efficacious clinic-based protocols. Of note, a meta-analysis [13] of CM studies found that researcher involvement in the planning and design of CM programs is associated with larger treatment effects. CM researchers are especially attuned to the potential impacts and pitfalls associated with modifying CM protocols, and they can be valuable resources for clinics interested in tailoring a CM program to their specific needs. Several examples of clinical demonstration projects that used researcher input are available [19,53,71].
Magnitude
Incentive magnitude was directly related to the size of treatment effects in a meta-analysis [11] of CM studies. Although not all studies find significant differences in outcomes related to magnitude [65,72], the bulk of evidence suggests magnitude is an important parameter and is related to effect size for both voucher [73–75] and prize-based CM [69,76] systems. Thus, although clinics may have restrictive budgets, severely undercutting the magnitude of rewards is not usually the solution as it can undermine treatment effects [76]. Donations can reduce overall costs [53,57,70], and other protocol features discussed below, such as capping the amount of reinforcement available, can reduce the overall magnitude available per patient.
Another approach, used in group-based CM, limits the number of patients who earn prizes per week [25,27]. For example, in a 2011 study by Petry et al, clients added slips with their name to a bowl for attendance and negative samples. Once all names were collected in the bowl, the group leader would pull a specified number of slips (eg, 3 slips per group). These individuals were eligible to draw from the prize bowl for prizes. This approach was associated with longer durations of consecutive abstinence and better treatment attendance relative to treatment as usual. However, clinics can control the overall program costs by limiting the number of patients eligible for prizes.
Frequency
Frequent reinforcement opportunities are ideal, and more frequent assessment is associated with larger treatment effects [10]. However, a number of factors, including which target behavior is selected and logistical issues specific to the clinic such as when groups meet, will play a role in determining the frequency of CM sessions. For abstinence targets, the substance targeted and type of test will largely determine the frequency of CM sessions. The goal would be to test at a frequency that would detect most or nearly all instances of use. For cocaine or opioids, this equates to testing 2 to 3 times weekly. Breath samples for alcohol or cigarette smoking would necessitate testing daily or multiple times per day to detect most instances of use because these tests have short windows of detection. CM protocols based on these breath tests have often had daily or twice daily CM sessions [77,78]; technological adaptations [77,79,80] or residential settings [21,23] may reduce burden to the client for assessment of these substances. Tapering the number of breath tests over time or transitioning from daily breath tests to once or twice weekly urine testing after abstinence is established is another approach [81,82].
Marijuana, on the other hand, poses difficulties because it is detectable in urine samples for up to 2 weeks following use. If relying solely on urine results for reinforcement, clients may not test negative for several days or weeks after last use, resulting in a delay of reinforcement. To address this issue, some CM programs targeting marijuana abstinence initially reinforce attendance in the first 2 weeks and then transition to reinforcing marijuana-negative drug samples for the remainder of the treatment period [48].
In general, more frequent CM sessions can translate to higher costs; however, infrequent reinforcement (ie, less than weekly) is not as effective [45]. In real-world applications, clinics often need to balance feasibility and costs with the ideal CM schedule. In abstinence-based CM, this compromise may result in a testing schedule that may not capture all instances of use. For example, while thrice-weekly testing may be ideal for cocaine or opioids, a twice-weekly schedule may be selected because it lowers costs and is more consistent with clinic schedules.
Immediacy
In general, clinics should aim to deliver reinforcement as immediately as possible, as delays between the target behavior and reinforcement are associated with decreased treatment effects [10,11,83]. For drug abstinence, onsite urine testing systems that provide immediate results are preferred over sending samples for laboratory testing. Clinics that do not have access to or who cannot afford specimen testing that allows onsite collection and immediate results might consider other options for target behaviors, such as attendance.
Immediacy of reinforcement is also important when targeting attendance. One clinic [53] implemented a program that offered a $50 incentive if clients attended 1 month of group therapy sessions. This approach was not effective and no clients earned the incentive for several months. After consultation, the clinic revised the incentive program to a daily drawing for attendance using the fishbowl method, thereby decreasing the delay between the behavior and its consequence. This example illustrates not only problems with delayed reinforcement but also the common mistake of setting expectations for the target behavior too high. Attending a month of group therapy sessions is a high bar that few patients will achieve, resulting in a system that mostly rewards those already doing well [19]. In contrast, attending a single group session in order to earn reinforcers is a reachable goal and increases the likelihood that more clients are exposed to the reinforcers. These small steps (ie, attending a single group or submitting a single drug negative urine) encourage initiation of the behavior(s) targeted. Other features, such as escalation (discussed next), aim to sustain the behavior over time.
Escalation
Escalation involves increasing the amount of reinforcement for each consecutive target behavior. In the voucher programs, the amount earned per negative sample may increase for each consecutive negative sample (eg, $2.50 for the first negative sample, $3.00 for the second, $3.50 for the third, and so on). For prize-based programs, the number of draws escalates with consecutive performance (eg, 1 draw for the first group attended, 2 draws for the second, 3 for the third, and so on). Protocols that include escalation generate larger effects than those that have a set, flat incentive amount even when total costs are the same across comparison conditions [73].
Escalating schedules usually include a reset feature. Following a positive or refused sample or unexcused absence, the amount earned for the next negative sample is reduced to the initial amount and begins escalating anew with consecutive negative samples. Some schedules allow for a rapid reset in which after a specified period of time or consecutive performance, the value jumps to the value achieved when the relapse occurred [66].
Despite its consistent inclusion in CM protocols from randomized clinical trials, our data [61] suggest that more than half of providers using incentives in treatment as part of a clinical effort do not use escalating reinforcers. Escalating schedules require more careful tracking of client progress, possibly contributing to lower uptake of this design feature in clinical practice. Development of simple tracking forms can minimize this challenge.
Another drawback of escalation pertaining to prize-based CM is that escalating schedules can affect the duration of CM sessions when clients are drawing a large number of slips each session and escalation can increase costs of the overall program. Capping the number of draws will help mitigate both issues. For example, once a client reaches 10 draws for group attendance, they continue earning 10 draws for each consecutive session attended with no further escalation.
Putting It All Together
CM sessions can be conducted as stand-alone sessions or incorporated into group or individual therapy sessions. Many clinicians will find the latter approach sets a positive tone for the therapy session given CM's focus on what the client is doing well. Starting the treatment session with the CM component often naturally leads into a discussion of relevant therapeutic issues, such as effective coping, slips, or triggers. The CM session length can be variable, but it is typically under 10 minutes. Thus, the CM component need not dominate the clinical session or content. CM sessions for abstinence are scheduled according to a set schedule (eg, Mondays and Thursdays) and often coincide with other treatment aspects (eg, before or after group therapy on Mondays and Thursdays). CM sessions for attendance also generally follow a set schedule (eg, client expected to attend Monday and Wednesday group therapy sessions). The duration of the CM protocol can also vary, with most clinical trials ranging from 12 to 24 weeks. Very short durations are unlikely to produce lasting behavior change, particularly with complex behaviors such as abstinence. Petry [44] recommends no less than 8 weeks duration and a maximum duration of 24 weeks.
As discussed, CM offers many opportunities for tailoring to optimize its fit within the existing structure of clinics. However, this flexibility must be viewed together with an understanding of the principles that impact CM's efficacy. Specific recommendations for CM protocol development will depend on the behavior targeted, the delivery methods, and format (eg, individual versus group settings). For these reasons, consultation with a CM expert is ideal. However, some general guidelines for developing a CM program that incorporate the principles discussed above include an 8- to 12-week program that (1) provides sufficient magnitude to compete with the behavior you are attempting to change, (2) offers frequent opportunities for reinforcement (eg, 2-3 times/wk for opioids or stimulant abstinence, 1-2 times/week for attendance targets; not less than weekly for most behaviors), (3) delivers the reinforcement immediately or very close in time with the behavior (eg, reinforce attendance at the beginning of the group, use onsite urine testing and reinforce immediately after testing), and (4) incorporates escalating and reset features into the schedule.
Clinician Training and Supervision
Training in CM is an important part of the implementation process. Studies [62,84–87] have identified a number of perceived barriers to and negative beliefs about CM, including philosophical and logistical concerns. Tangible incentives, the core of most CM protocols, are generally viewed less favorably than social or nonspecified incentives [84,86,87]. Philosophical concerns relate to CM’s inability to address the underlying causes of addiction, that it does not address multiple behaviors, and that it may undermine internal motivation for sobriety [62,84]. An additional objection relates to paying someone to do what they should do on their own [86]. Logistical and practical concerns often represent implementation barriers such as costs and access to training and supervision, but they also reflect concern for what happens when contingencies are withdrawn, that clients may sell or trade prizes for drugs, and worries that CM’s evidence does not generalize to clinic populations [62].
Many of these beliefs reflect a limited understanding of CM, and addressing these misperceptions is a first step toward reducing resistance to implementation efforts. For example, a substantial body of literature points to CM’s wide generalizability across a range of characteristics, clients that sell or trade prizes for drugs are likely to disrupt their chain of negative samples or attendance, and most studies do not find negative impacts of CM on intrinsic motivation [88–90]. Fortunately, CM training appears to be an effective way to address negative beliefs. In the VA implementation effort [52], training workshops decreased perceived barriers and increased positive impressions of CM [91]. In other training efforts, brief educational materials were effective in changing perceptions of CM’s efficacy [92].
Beyond initial training, supervision of CM delivery is likely to be necessary [93,94]. Clinician skill in delivering CM is related to client outcomes [93,95] and relatively simple adherence measures are available for monitoring [96,97]. However, the best methods for training and supervision of CM have yet to be established. The VA initiative was developed in consultation with CM experts and employed ongoing phone consultation following initial training workshops [52,91]. This approach represented significant investment by the VA toward staff training and CM protocol development that may not be achievable for individual clinics. As attention to CM’s dissemination and implementation has grown, some free resources have been developed. Promoting Awareness of Motivational Incentives (PAMI; www.bettertxoutcomes.org/bettertxoutcomes/PAMI.html) is a collaborative initiative sponsored by the National Institute of Drug Abuse and the Substance Abuse and Mental Health Services Administration. It offers free resources and training materials.
Conclusion
Overall, CM is a highly efficacious treatment for SUDs that generalizes to most clients. Despite a robust evidence base, CM’s implementation in clinical settings lags behind other empirically supported treatments [92]. At least in part, CM’s costs, which include not only staff training and adherence monitoring (as with other treatments), but also costs of the incentives themselves, may contribute to slow uptake in clinical settings. Clinics often do not have the resources available for CM within their operating budgets. However, a growing number of projects [19,52,53,55–57,70,71] illustrate CM implementation within routine clinical care, and increased revenue from improved attendance to treatment groups may be one mechanism through which to fund a CM program [54,56,57]. These projects are valuable not only for demonstrating that CM can be efficacious outside the research setting, but also for highlighting how implementation barriers can be overcome. Continued efforts of this nature are likely to be particularly valuable for clinicians and administrators considering adopting CM within clinical settings.
1. Petry NM. A comprehensive guide to the application of contingency management procedures in clinical settings. Drug Alcohol Depend 2000;58:9–25.
2. Stitzer M, Petry N. Contingency management for treatment of substance abuse. Annu Rev Clin Psychol 2006;2:411–34.
3. Meredith SE, Jarvis BP, Raiff BR, et al. The ABCs of incentive-based treatment in health care: A behavior analytic framework to inform research and practice. Psychol Res Behav Manag 2014;7:103–14.
4. Bigelow GE, Silverman K. Theoretical and empirical foundations of contingency management treatments for drug abuse. In: Higgins ST, Silverman K, editors. Motivating behavior change among illicit drug users. Washington DC: American Psychological Association; 1999: 15–31.
5. Higgins ST, Budney AJ, Bickel WK. Applying behavioral concepts and principles to the treatment of cocaine dependence. Drug Alcohol Depend 1994;34:87–97.
6. Skinner BF. Science and human behavior. New York: Macmillan; 1953.
7. Herbeck DM, Hser YI, Teruya C. Empirically supported substance abuse treatment approaches: A survey of treatment providers’ perspectives and practices. Addict Behav 2008;33:699–712.
8. McGovern MP, Fox TS, Xie H, Drake RE. A survey of clinical practices and readiness to adopt evidence-based practices: Dissemination research in an addiction treatment system. J Subst Abuse Treat 2004;26:305–12.
9. Willenbring ML, Kivlahan D, Kenny M, et al. Beliefs about evidence-based practices in addiction treatment: A survey of Veterans Administration program leaders. J Subst Abuse Treat 2004;26:79–85.
10. Griffith JD, Rowan-Szal GA, Roark RR, Simpson DD. Contingency management in outpatient methadone treatment: A meta-analysis. Drug Alcohol Depend 2000;58:55–66.
11. Lussier JP, Heil SH, Mongeon JA, et al. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction 2006;101:192–203.
12. Benishek LA, Dugosh KL, Kirby KC, et al. Prize-based contingency management for the treatment of substance abusers: A meta-analysis. Addiction 2014;109:1426–36.
13. Prendergast M, Podus D, Finney J, et al. Contingency management for treatment of substance use disorders: A meta-analysis. Addiction 2006;101:1546–60.
14. Dutra L, Stathopoulou G, Basden SL, et al. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry 2008;165:179–87.
15. Petry NM, Peirce JM, Stitzer ML, et al. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: A National Drug Abuse Treatment Clinical Trials Network study. Arch Gen Psychiatry 2005;62:1148–56.
16. Peirce JM, Petry NM, Stitzer ML, et al. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment - A National Drug Abuse Treatment Clinical Trials Network study. Arch Gen Psychiatry 2006;63:201–8.
17. Petry NM, Martin B, Finocche C. Contingency management in group treatment: a demonstration project in an HIV drop-in center. J Subst Abuse Treat 2001;21:89–96.
18. Drebing CE, Van Ormer EA, Mueller L, et al. Adding contingency management intervention to vocational rehabilitation: outcomes for dually diagnosed veterans. J Rehabil Res Dev 2007;44:851–65.
19. Kellogg SH, Burns M, Coleman P, et al. Something of value: The introduction of contingency management interventions into the New York City Health and Hospital Addiction Treatment Service. J Subst Abuse Treat 2005;28:57–65.
20. Koffarnus MN, Wong CJ, Fingerhood M, et al. Monetary incentives to reinforce engagement and achievement in a job-skills training program for homeless, unemployed adults. J Appl Behav Anal 2013;46:582–91.
21. Rohsenow D, Martin R, Tidey JW, et al. Treating smokers in substance treatment with contingent vouchers, nicotine replacement, and brief advice adapted for sobriety settings. J Subst Abuse Treat 2017.
22. Hunt YM, Rash CJ, Burke RS, Parker JD. Smoking cessation in recovery: Comparing two different cognitive behavioral treatments. Addict Disord Their Treat 2010;9:64–74.
23. Alessi SM, Petry NM, Urso J. Contingency management promotes smoking reductions in residential substance abuse patients. J Appl Behav Anal 2008;41:617–22.
24. Petry NM, Weinstock J, Alessi SM, et al. Group-based randomized trial of contingencies for health and abstinence in HIV patients. J Consult Clin Psychol 2010;78:89.
25. Petry NM, Weinstock J, Alessi SM. A randomized trial of contingency management delivered in the context of group counseling. J Consult Clin Psychol 2011;79:686–96.
26. Ledgerwood DM, Alessi SM, Hanson T, et al. Contingency management for attendance to group substance abuse treatment administered by clinicians in community clinics. J Appl Behav Anal 2008;41:517–26.
27. Alessi SM, Hanson T, Wieners M, Petry NM. Low-cost contingency management in community clinics: Delivering incentives partially in group therapy. Exp Clin Psychopharmacol 2007;15:293–300.
28. Burch AE, Rash CJ, Petry NM. Sex effects in cocaine-using methadone patients randomized to contingency management interventions. Exp Clin Psychopharmacol 2015;23:284–90.
29. Rash CJ, Petry NM. Contingency management treatments are equally efficacious for both sexes in intensive outpatient settings. Exp Clin Psychopharmacol 2015;23:369–76.
30. Barry D, Sullivan B, Petry NM. Comparable efficacy of contingency management for cocaine dependence among African American, Hispanic, and White methadone maintenance clients. Psychol Addict Behav 2009;23:168–74.
31. Rash CJ, Alessi SM, Petry NM. Substance abuse treatment patients in housing programs respond to contingency management interventions. J Subst Abuse Treat 2017;72:97–102.
32. Rash CJ, Olmstead TA, Petry NM. Income does not affect response to contingency management treatments among community substance abuse treatment-seekers. Drug Alcohol Depend 2009;104:249–53.
33. Rash CJ, Andrade LF, Petry NM. Income received during treatment does not affect response to contingency management treatments in cocaine-dependent outpatients. Drug Alcohol Depend 2013;132:528–34.
34. Secades-Villa R, García-Fernández G, Peña-Suárez E, et al. Contingency management is effective across cocaine-dependent outpatients with different socioeconomic status. J Subst Abuse Treat 2013;44:349–54.
35. Rash CJ, Alessi SM, Petry NM. Cocaine abusers with and without alcohol dependence respond equally well to contingency management treatments. Exp Clin Psychopharmacol 2008;16:275–81.
36. Alessi SM, Rash C, Petry NM. Contingency management is efficacious and improves outcomes in cocaine patients with pretreatment marijuana use. Drug Alcohol Depend 2011;118:62–7.
37. Ford JD, Hawke J, Alessi S, et al. Psychological trauma and PTSD symptoms as predictors of substance dependence treatment outcomes. Behav Res Ther 2007;45:2417–31.
38. Weinstock J, Alessi SM, Petry NM. Regardless of psychiatric severity the addition of contingency management to standard treatment improves retention and drug use outcomes. Drug Alcohol Depend 2007;87:288–96.
39. García-Fernández G, Secades-Villa R, García-Rodríguez O, et al. Contingency management improves outcomes in cocaine-dependent outpatients with depressive symptoms. Exp Clin Psychopharmacol 2013;21:482–9.
40. Walter KN, Petry NM. Patients with diabetes respond well to contingency management treatment targeting alcohol and substance use. Psychol Health Med 2015;20:916–26.
41. Burch AE, Morasco BJ, Petry NM. Patients undergoing substance abuse treatment and receiving financial sssistance for a physical disability respond well to contingency management treatment. J Subst Abuse Treat 2015;58:67–71.
42. Burch AE, Rash CJ, Petry NM. Cocaine-using substance abuse treatment patients with and without HIV respond well to contingency management treatment. J Subst Abuse Treat 2017;77:21–5.
43. Rash CJ, Burki M, Montezuma-Rusca JM, Petry NM. A retrospective and prospective analysis of trading sex for drugs or money in women substance abuse treatment patients. Drug Alcohol Depend 2016;162:182–9.
44. Petry NM. Contingency management for substance abuse treatment: A guide to implementing this evidence-based practice. New York: Routledge; 2012.
45. Petry NM, Rash CJ, Byrne S, et al. Financial reinforcers for improving medication adherence: Findings from a meta-analysis. Am J Med 2012;125:888–96.
46. Herrmann ES, Matusiewicz AK, Stitzer ML, et al. Contingency management interventions for HIV, tuberculosis, and hepatitis control among individuals with substance use disorders: a systematized review. J Subst Abuse Treat 2017;72:117–25.
47. Petry NM, Alessi SM, Carroll KM, et al. Contingency management treatments: Reinforcing abstinence versus adherence with goal-related activities. J Consult Clin Psychol 2006;74:592–601.
48. Litt MD, Kadden RM, Petry NM. Behavioral treatment for marijuana dependence: Randomized trial of contingency management and self-efficacy enhancement. Addict Behav 2013;38:1764–75.
49. Kurti AN, Dallery J. A laboratory-based evaluation of exercise plus contingency management for reducing cigarette smoking. Drug Alcohol Depend 2014;144:201–9.
50. Weinstock J, Capizzi J, Weber SM, et al. Exercise as an intervention for sedentary hazardous drinking college students: A pilot study. Ment Health Phys Act 2014;7:55–62.
51. Mitchell MS, Goodman JM, Alter DA, et al. Financial incentives for exercise adherence in adults: Systematic review and meta-analysis. Am J Prev Med 2013;45:658–67.
52. Petry NM, Dephilippis D, Rash CJ, et al. Nationwide dissemination of contingency management: The Veterans Administration initiative. Am J Addict 2014;23:205–10.
53. Walker R, Rosvall T, Field CA, et al. Disseminating contingency management to increase attendance in two community substance abuse treatment centers: Lessons learned. J Subst Abuse Treat 2010;39:202–9.
54. Sigmon SC, Stitzer ML. Use of a low-cost incentive intervention to improve counseling attendance among methadone-maintained patients. J Subst Abuse Treat 2005;29:253–8.
55. Kropp F, Lewis D, Winhusen T. The effectiveness of ultra-low magnitude reinforcers: Findings from a “real-world” application of contingency management. J Subst Abuse Treat 2017;72:111–6.
56. Fitzsimons H, Tuten M, Borsuk C, et al. Clinician-delivered contingency management increases engagement and attendance in drug and alcohol treatment. Drug Alcohol Depend 2015;152:62–7.
57. Lott DC, Jencius S. Effectiveness of very low-cost contingency management in a community adolescent treatment program. Drug Alcohol Depend 2009;102:162–5.
58. Henggeler SW, Chapman JE, Rowland MD, et al. Statewide adoption and initial implementation of contingency management for substance-abusing adolescents. 2008;76:556–67.
59. Henggeler SW, Chapman JE, Rowland MD, et al. If you build it , they will come: Statewide practitioner interest in contingency management for youths. 2007;32:121–31.
60. Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes, and they will come: contingency management for treatment of alcohol dependence. J Consult Clin Psychol 2000;68:250–7.
61. Rash CJ, Petry NM, Alessi SM. Examining implementation of contingency management in real-world settings [Abstract]. Alcohol Clin Exp Res 2016;20:103A.
62. Rash CJ, Petry NM, Kirby KC, et al. Identifying provider beliefs related to contingency management adoption using the Contingency Management Beliefs Questionnaire. Drug Alcohol Depend 2012;121:205–12.
63. Petry NM, Alessi SM, Marx J, et al. Vouchers versus prizes: Contingency management treatment of substance abusers in community settings. J Consult Clin Psychol 2005;73:1005–14.
64. Petry NM, Alessi SM, Hanson T, Sierra S. Randomized trial of contingent prizes versus vouchers in cocaine-using methadone patients. J Consult Clin Psychol 2007;75:983–91.
65. Petry NM, Alessi SM, Barry D, Carroll KM. Standard magnitude prize reinforcers can be as efficacious as larger magnitude reinforcers in cocaine-dependent methadone patients. J Consult Clin Psychol 2015;83:464–72.
66. Higgins ST, Budney AJ, Bickel WK, et al. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Arch Gen Psychiatry 1994;51:568.
67. Higgins ST, Wong CJ, Badger GJ, et al. Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1 year of follow-up. J Consult Clin Psychol 2000;68:64–72.
68. Hartzler B, Garrett S. Interest and preferences for contingency management design among addiction treatment clientele. Am J Drug Alcohol Abuse 2016;42:287–95.
69. Petry NM, Barry D, Alessi SM, et al. A randomized trial adapting contingency management targets based on initial abstinence status of cocaine-dependent patients. J Consult Clin Psychol 2012;80:276–85.
70. Amass L, Kamien J. A tale of two cities: Financing two voucher programs for substance abusers through community donations. Exp Clin Psychopharmacol 2004;12:147–55.
71. Hartzler B. Building a bonfire that remains stoked: Sustainment of a contingency management intervention developed through collaborative design. Subst Abuse Treat Prev Policy 2015;10:30.
72. Carroll KM, Sinha R, Nich C, et al. Contingency management to enhance naltrexone treatment of opioid dependence: a randomized clinical trial of reinforcement magnitude. Exp Clin Psychopharmacol 2002;10:54–63.
73. Roll JM, Shoptaw S. Contingency management: Schedule effects. Psychiatry Res 2006;144:91–3.
74. Silverman K, Chutuape MA, Bigelow GE, Stitzer ML. Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: Effects of reinforcement magnitude. Psychopharmacology (Berl) 1999;146:128–38.
75. Businelle MS, Rash CJ, Burke RS, Parker JD. Using vouchers to increase continuing care participation in veterans: does magnitude matter? Am J Addict 2009;18:122–9.
76. Petry NM, Tedford J, Austin M, et al. Prize reinforcement contingency management for treating cocaine users: How low can we go, and with whom? Addiction 2004;99:349–60.
77. Alessi SM, Petry NM. A randomized study of cellphone technology to reinforce alcohol abstinence in the natural environment. Addiction 2013;108:900–9.
78. Alessi SM, Petry NM. Smoking reductions and increased self-efficacy in a randomized controlled trial of smoking abstinence--contingent incentives in residential substance abuse treatment patients. Nicotine Tob Res 2014;16:1436–45.
79. Dougherty DM, Hill-Kapturczak N, Liang Y, et al. Use of continuous transdermal alcohol monitoring during a contingency management procedure to reduce excessive alcohol use. Drug Alcohol Depend 2014;142:301–6.
80. Dallery J, Meredith S, Jarvis B, Nuzzo PA. Internet-based group contingency management to promote smoking abstinence. Exp Clin Psychopharmacol 2015;23:176–83.
81. Higgins ST, Washio Y, Lopez AA, et al. Examining two different schedules of financial incentives for smoking cessation among pregnant women. Prev Med (Baltim) 2014;68:51–7.
82. Higgins ST, Heil SH, Solomon LJ, et al. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine Tob Res 2004;6:1015–20.
83. Packer RR, Howell DN, McPherson S, Roll JM. Investigating reinforcer magnitude and reinforcer delay: A contingency management analog study. Exp Clin Psychopharmacol 2012;20:287–92.
84. Kirby KC, Benishek LA, Dugosh KL, Kerwin ME. Substance abuse treatment providers’ beliefs and objections regarding contingency management: Implications for dissemination. Drug Alcohol Depend 2006;85:19–27.
85. Cameron J, Ritter A. Contingency management: Perspectives of Australian service providers. 2007;26:183–9.
86. Hartzler B, Rabun C. Community opioid treatment perspectives on contingency management: Perceived feasibility, effectiveness, and transportability of social and financial incentives. J Subst Abuse Treat 2013;45:242–8.
87. Aletraris L, Shelton JS, Roman PM. Counselor attitudes toward contingency management for substance use disorder: Effectiveness, acceptability, and endorsement of incentives for treatment attendance and abstinence. J Subst Abuse Treat 2015;57:41–8.
88. Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. J Consult Clin Psychol 2000;68:1051–61.
89. Ledgerwood DM, Petry NM. Does contingency management affect motivation to change substance use? Drug Alcohol Depend 2006;83:65–72.
90. Litt MD, Kadden RM, Kabela-Cormier E, Petry NM. Coping skills training and contingency management treatments for marijuana dependence: Exploring mechanisms of behavior change. Addiction 2008;103:638–48.
91. Rash CJ, DePhilippis D, McKay JR, et al. Training workshops positively impact beliefs about contingency management in a nationwide dissemination effort. J Subst Abuse Treat 2013;45:306–12.
92. Benishek LA, Kirby KC, Dugosh KL, Padovano A. Beliefs about the empirical support of drug abuse treatment interventions: A survey of outpatient treatment providers. Drug Alcohol Depend 2010;107:202–8.
93. Petry NM, Alessi SM, Ledgerwood DM. Contingency management delivered by community therapists in outpatient settings. Drug Alcohol Depend 2012;122:86–92.
94. Petry NM, Alessi SM, Ledgerwood DM. A randomized trial of contingency management delivered by community therapists. J Consult Clin Psychol 2012;80:286–98.
95. Hartzler B, Beadnell B, Donovan D. Predictive validity of addiction treatment clinicians’ post-training contingency management skills for subsequent clinical outcomes. J Subst Abuse Treat 2017.
96. Hartzler B. Adapting the Helpful Responses Questionnaire to assess communication skills involved in delivering contingency management: Preliminary psychometrics. J Subst Abuse Treat 2014;55:52–7.
97. Petry NM, Alessi SM, Ledgerwood DM, Sierra S. Psychometric properties of the Contingency Management Competence Scale. Drug Alcohol Depend 2010;109:167–74.
From UConn Health, Farmington, CT.
Abstract
- Objective: To discuss the efficacy and generalizability of contingency management (CM) for the treatment of substance use disorders and design considerations for those considering implementing in clinical settings.
- Methods: Review of the literature.
- Results: CM is an efficacious treatment for substance abuse disorders that is widely generalizable across substance use disorders and patient characteristics. CM can be implemented in a number of treatment programs, including residential and outpatient settings, and it can be administered in both individual and group formats. Abstinence and attendance are the most commonly targeted behaviors in substance abuse treatment settings. Design features, including the selection of target behaviors, delivery methods, and reinforcers, are discussed. Schedule parameters, such as frequency, magnitude, immediacy, and escalation of reinforcement, are associated with overall impact of the CM program and are important considerations for those interested in tailoring CM protocols to their needs.
- Conclusion: CM is an efficacious option that is applicable to most substance abuse treatment patients. A number of demonstrations of real-world implementation have been published and suggest CM can be adapted with success to clinic settings. In adopting CM protocols, clinics should aim for those protocols with established efficacy; however, if adaptations are necessary, careful consideration should be given to modifications to minimize risks of undermining CM’s effects.
Key words: incentives; reinforcement; substance abuse treatment; dissemination; implementation.
Contingency management (CM) is a behavioral intervention that is efficacious in the treatment of substance use disorders (SUDs). CM uses a behavior analytic framework and applies principles of learning theory, particularly operant conditioning theory, to change client behavior(s) [1–5]. In basic terms, operant conditioning principles suggests that whether a behavior continues or not is a function of consequences [6]. Reinforced behaviors are more likely to occur in the future. Substance abuse can be viewed as a behavior maintained by the reinforcing effects of the drug itself [5], including the feel-good aspects of intoxication or relaxation and the amelioration of withdrawal symptoms. CM extends these same principles of to a treatment context, such that reinforcers for abstinent behavior are introduced to compete with the reinforcing effects of continued drug use [5].
In CM’s application to substance abuse treatment, drug-negative samples or treatment attendance are reinforced using tangible incentives with the goal of motivating continued abstinence and/or treatment engagement. When clients demonstrate these target behaviors, they earn incentives in the form of goods or services of value to the client, such as small electronics, gift cards, and toiletries. Despite the promising effects observed in research trials, real-world implementation efforts have not kept pace [7–9]. This review briefly discusses CM’s efficacy and highlights key features for professionals considering adopting this intervention. Demonstration efforts that illustrate how CM can be effectively implemented within the constraints and limitations of non-research, clinical settings are also presented.
Efficacy of CM
CM’s efficacy spans a number of SUDs, including cocaine, opioids, alcohol, nicotine, and marijuana [10–13], making it amenable for treatment of most SUD clinic populations. It generates larger effect sizes than other SUD treatments, including cognitive behavioral therapy [14], and it has been evaluated in a wide range of settings. Large-scale evaluations have been conducted in both intensive outpatient [15] and methadone maintenance [16] settings as part of the National Institute on Drug Abuse Clinical Trials Network, demonstrating consistent benefits of CM when added to treatment as usual. In the first of these 2 studies, Petry et al [15] randomized 415 stimulant users from 1 of 8 intensive outpatient clinics to treatment as usual or treatment as usual plus CM for alcohol and stimulant abstinence. CM participants submitted more substance-negative urine and breath samples, achieved continuous abstinence at significantly higher rates, and had longer treatment retention compared to those receiving treatment as usual. The parallel study [16] focused on stimulant use in clients from methadone maintenance clinics and found similar benefits of CM on stimulant abstinence. Beyond these settings, CM has been applied in a number of other contexts, including drop-in centers [17], vocational rehabilitation [18,19], job-skills training [20], and residential programs [21–23]. In addition, several group-based adaptations have been explored [17,24–27].
CM benefits most clients and generalizes across several demographic variables, including gender [28,29], race [30], housing status [31], and income levels [32–34]. Among clinical characteristics, CM is efficacious for those with co-occurring SUDs [35], other substance use [36], psychiatric disorders [37–39], medical problems [40–42], and history of transactional sex [43].
Design Considerations
Design features, including what behavior will be reinforced and how to do so, are among the first decision points for clinicians interested in implementing CM. One of the advantages of CM is that it has a high degree of flexibility in design, which means that it can be readily tailored to client populations and clinic needs. However, this flexibility can lead clinicians astray from the foundational principles of CM and unknowingly weaken the impact of the program. Below, some key considerations for CM protocol design are reviewed. For additional coverage of these topics, readers are referred to additional articles [1,2] or Petry’s comprehensive book on implementing CM [44]. In this review, published examples of CM’s application in real-world settings are presented, highlighting how CM has been adapted in these clinical efforts.
Target Behaviors
The selection of the target behavior will drive many of the subsequent program design decisions. As such, it is important to identify this feature early. Target behaviors must be achievable, objectively verifiable, and well defined. The most common CM targets are drug abstinence or therapy session attendance. CM has also been used to target other behaviors, such as medication adherence [45,46], treatment-related activities [47,48], and exercise [49–51]. Client self-report of behaviors or vaguely defined behaviors (eg, “good participation”) should be avoided. While some of the decisions related to CM protocols are flexible, the use of objectively verifiable target behaviors is a core feature that should not be neglected. If the behavior of interest cannot be objectively verified, an alternate behavior should be chosen.
Selection of the target behavior is often considered in hand with defining which population is eligible to participate in the CM program. Client characteristics are often forefront in this decision, but clinic-driven logistical issues or unmet needs may also play a role. A real-world example of this decision process is evident in the nationwide rollout of CM among the intensive outpatient programs within the Veterans Administration (VA). The VA identified a treatment need for those with stimulant use disorders, as this group did not have efficacious pharmacotherapy options available that targeted stimulant use. As such, the VA applied CM to patients with a focus on stimulant abstinence as the behavioral target [52]. For others, the decision may revolve around addressing underutilization of specific treatment resources (eg, outpatient groups, vocational rehabilitation) [53–56] or treatment needs among certain subgroups of clients, such as adolescents [57–59].
For abstinence targets, clinics would need to select one or more specific substances as the focus of the CM program. In general, targeting a single substance rather than multiple substances is more effective [10,13], is more straightforward for clients to understand, and allows more clients to access the reinforcers. Exposure to the reinforcers is necessary for CM to work; thus, setting a goal that is achievable for most clients should be a priority. Requiring abstinence from multiple substances means that some clients may never experience the reinforcer and thus cannot benefit from its effects at all. Some clinicians or administrators may initially have reservations about reinforcing single drug abstinence in the event that other drug use continues. However, targeting a single substance for reinforcement often results in reduced use of other substances [60]. Clinicians may find that this makes intuitive sense; a client with cocaine use disorder who is trying to maintain cocaine abstinence over a long period is likely to avoid using alcohol or other substances that might lead to relapse. For abstinence, objective verification through urine or breath specimens using tests that include validity checks is relatively straightforward.
Attendance is a popular target for clinics in part because it does not require additional staff time to collect specimen samples and it was the most commonly reported target behavior in samples of SUD providers who use incentives [61,62]. Objective verification of attendance is usually via a staff member, but expectations must be clear to both parties. Clinics should consider potential problems that may arise (eg, arriving late, leaving group early, excused absences) and carefully define and communicate expectations for the CM program. Piloting [19] the CM program with a small group of clients may be valuable in trouble-shooting challenges before wider implementation.
In a recent study [55], clients earned reinforcers for attending clinician-led group counseling sessions and/or the in-clinic patient-led Methadone Anonymous (MA) groups. This non-research, clinical effort addressed historically poor therapy attendance at the clinic, and attendance rates were compared before, during, and after the CM program. CM increased attendance to both groups in the short-term after implementation, but effects were more robust for the MA groups in which increased attendance persisted 3 months following the withdrawal of the contingencies. Overall effects of this program were modest, but they are notable given the use of an ultra-low cost approach.
Delivery Methods
The majority of CM studies used voucher or prize-based methods. Head-to-head comparisons suggest that they are comparable in efficacy [63–65], and each has advantages and disadvantages that may make one option more appealing for a given clinic. Voucher programs are generally straightforward to administer. Clients earn vouchers for each instance of the target behavior. The value of the vouchers typically increases with consecutive performance. The schedule used in the influential Higgins et al studies [66,67] started at $2.50 for the first cocaine-negative sample and increased by $0.50 for each subsequent consecutive cocaine-negative sample. Earned vouchers are exchanged for goods or services selected by the client, increasing the likelihood that the selected items will be highly desirable and allowing for a wide range of client preferences. Clients appear to prefer this approach when given a choice between set schedules or those that introduce an element of chance (ie, prize-based CM, discussed below) [68]. However, voucher programs can be costly (~$1000 per client over 12 weeks) and may require more staff time to fulfill individual requests for specific items. However, staff burden related to shopping can be reduced by limiting these individual requests and using an on-site stocked cabinet of goods similar to prize-CM programs.
Prize-based CM is similar but introduces probabilistic earnings and variability in prize magnitude. Rather than earning vouchers, clients earn draws from a fishbowl for each instance of the target behavior, again typically in an escalating manner. For example, a client may earn one draw from the fishbowl for the first cocaine-negative sample, 2 draws for the second consecutive negative sample, 3 draws for the third, and so on. A typical fishbowl is composed of 500 slips, some noting prizes and some having no prize value. Typically, half the slips in the bowl are non-monetary “good jobs.” The remaining half are small prizes worth about $1 in value (eg, food coupons, bus tokens, small toiletries), large prizes worth about $20 in value (eg, small electronics, gift certificates), and one slip is the jumbo prize worth about $100. When a client draws a winning slip, they select a prize from that category (ie, small, large, jumbo) from an onsite, stocked cabinet. Due to the probabilistic feature of prize-based CM, overall costs of the program can be substantially lower than typical voucher programs, with average maximum expected earnings ranging $250 t $450 per client over a 12-week treatment period [15,16,65,69]. Advantages of this method include potentially lower costs and minimal shopping demands (a once-monthly shopping trip to restock the cabinet will usually suffice) while maintaining comparable efficacy. Relative to voucher programs, prize-based CM involves additional administration time to allow for drawing slips from the fishbowl, which can be compounded when multiple clients want to draw at the same time such as in a group setting. Many of the group-based CM adaptations address this issue by limiting the number of clients who can draw for prizes in a given group or by limiting the number of draws per client [25,27,54].
Reinforcers
Regardless of whether selecting voucher or prize CM, reinforcers are critically important to the success of the program. Reinforcers must be desirable. One of the quickest ways to undermine a CM program is lack of variety or undesirable reinforcers. If stocking a cabinet with prizes onsite, care should be taken to have numerous options within each of the small and large prize categories that are appealing to a wide range of clients. Since a client who is consistently earning draws will choose prizes often, it is imperative to include enough variety so that even these clients find desirable items each time they select a prize. Clients should be asked regularly if they have suggestions for prizes; one program [54] found suggestion boxes useful for encouraging clients to voice their preferences. Donations can be solicited from local businesses to reduce costs [53], and low-cost but high-value options, such as clinic privileges, can also be explored. Petry [1,44] provides some suggestions of the latter, and Amass and Kamien [70] describe their successful strategies to fund and sustain a clinic-based CM program through community donations. Some clinics may already have tangible goods, such as gas or metro cards, that are offered to clients based on need rather than behavior [53]. These existing resources might be redirected to a CM program, in which these goods are contingent on abstinence or attendance, if appropriate.
Schedule Parameters
Once the target behavior, client population, and CM delivery methods are selected, the next step is to design the reinforcement schedule. The following schedule parameters apply to both voucher and prize-based CM systems. The more closely a clinical program adheres to the parameters of effective protocols, the more likely the program is to generate comparable outcomes. If there is a parameter or design feature that is incompatible with clinic needs, modifications can be introduced. However, each deviation away from the ideal has a chance of undermining the success of the CM program. Any changes and their potential impacts should considered carefully, and consultation with a CM expert may aid in the development of successful and efficacious clinic-based protocols. Of note, a meta-analysis [13] of CM studies found that researcher involvement in the planning and design of CM programs is associated with larger treatment effects. CM researchers are especially attuned to the potential impacts and pitfalls associated with modifying CM protocols, and they can be valuable resources for clinics interested in tailoring a CM program to their specific needs. Several examples of clinical demonstration projects that used researcher input are available [19,53,71].
Magnitude
Incentive magnitude was directly related to the size of treatment effects in a meta-analysis [11] of CM studies. Although not all studies find significant differences in outcomes related to magnitude [65,72], the bulk of evidence suggests magnitude is an important parameter and is related to effect size for both voucher [73–75] and prize-based CM [69,76] systems. Thus, although clinics may have restrictive budgets, severely undercutting the magnitude of rewards is not usually the solution as it can undermine treatment effects [76]. Donations can reduce overall costs [53,57,70], and other protocol features discussed below, such as capping the amount of reinforcement available, can reduce the overall magnitude available per patient.
Another approach, used in group-based CM, limits the number of patients who earn prizes per week [25,27]. For example, in a 2011 study by Petry et al, clients added slips with their name to a bowl for attendance and negative samples. Once all names were collected in the bowl, the group leader would pull a specified number of slips (eg, 3 slips per group). These individuals were eligible to draw from the prize bowl for prizes. This approach was associated with longer durations of consecutive abstinence and better treatment attendance relative to treatment as usual. However, clinics can control the overall program costs by limiting the number of patients eligible for prizes.
Frequency
Frequent reinforcement opportunities are ideal, and more frequent assessment is associated with larger treatment effects [10]. However, a number of factors, including which target behavior is selected and logistical issues specific to the clinic such as when groups meet, will play a role in determining the frequency of CM sessions. For abstinence targets, the substance targeted and type of test will largely determine the frequency of CM sessions. The goal would be to test at a frequency that would detect most or nearly all instances of use. For cocaine or opioids, this equates to testing 2 to 3 times weekly. Breath samples for alcohol or cigarette smoking would necessitate testing daily or multiple times per day to detect most instances of use because these tests have short windows of detection. CM protocols based on these breath tests have often had daily or twice daily CM sessions [77,78]; technological adaptations [77,79,80] or residential settings [21,23] may reduce burden to the client for assessment of these substances. Tapering the number of breath tests over time or transitioning from daily breath tests to once or twice weekly urine testing after abstinence is established is another approach [81,82].
Marijuana, on the other hand, poses difficulties because it is detectable in urine samples for up to 2 weeks following use. If relying solely on urine results for reinforcement, clients may not test negative for several days or weeks after last use, resulting in a delay of reinforcement. To address this issue, some CM programs targeting marijuana abstinence initially reinforce attendance in the first 2 weeks and then transition to reinforcing marijuana-negative drug samples for the remainder of the treatment period [48].
In general, more frequent CM sessions can translate to higher costs; however, infrequent reinforcement (ie, less than weekly) is not as effective [45]. In real-world applications, clinics often need to balance feasibility and costs with the ideal CM schedule. In abstinence-based CM, this compromise may result in a testing schedule that may not capture all instances of use. For example, while thrice-weekly testing may be ideal for cocaine or opioids, a twice-weekly schedule may be selected because it lowers costs and is more consistent with clinic schedules.
Immediacy
In general, clinics should aim to deliver reinforcement as immediately as possible, as delays between the target behavior and reinforcement are associated with decreased treatment effects [10,11,83]. For drug abstinence, onsite urine testing systems that provide immediate results are preferred over sending samples for laboratory testing. Clinics that do not have access to or who cannot afford specimen testing that allows onsite collection and immediate results might consider other options for target behaviors, such as attendance.
Immediacy of reinforcement is also important when targeting attendance. One clinic [53] implemented a program that offered a $50 incentive if clients attended 1 month of group therapy sessions. This approach was not effective and no clients earned the incentive for several months. After consultation, the clinic revised the incentive program to a daily drawing for attendance using the fishbowl method, thereby decreasing the delay between the behavior and its consequence. This example illustrates not only problems with delayed reinforcement but also the common mistake of setting expectations for the target behavior too high. Attending a month of group therapy sessions is a high bar that few patients will achieve, resulting in a system that mostly rewards those already doing well [19]. In contrast, attending a single group session in order to earn reinforcers is a reachable goal and increases the likelihood that more clients are exposed to the reinforcers. These small steps (ie, attending a single group or submitting a single drug negative urine) encourage initiation of the behavior(s) targeted. Other features, such as escalation (discussed next), aim to sustain the behavior over time.
Escalation
Escalation involves increasing the amount of reinforcement for each consecutive target behavior. In the voucher programs, the amount earned per negative sample may increase for each consecutive negative sample (eg, $2.50 for the first negative sample, $3.00 for the second, $3.50 for the third, and so on). For prize-based programs, the number of draws escalates with consecutive performance (eg, 1 draw for the first group attended, 2 draws for the second, 3 for the third, and so on). Protocols that include escalation generate larger effects than those that have a set, flat incentive amount even when total costs are the same across comparison conditions [73].
Escalating schedules usually include a reset feature. Following a positive or refused sample or unexcused absence, the amount earned for the next negative sample is reduced to the initial amount and begins escalating anew with consecutive negative samples. Some schedules allow for a rapid reset in which after a specified period of time or consecutive performance, the value jumps to the value achieved when the relapse occurred [66].
Despite its consistent inclusion in CM protocols from randomized clinical trials, our data [61] suggest that more than half of providers using incentives in treatment as part of a clinical effort do not use escalating reinforcers. Escalating schedules require more careful tracking of client progress, possibly contributing to lower uptake of this design feature in clinical practice. Development of simple tracking forms can minimize this challenge.
Another drawback of escalation pertaining to prize-based CM is that escalating schedules can affect the duration of CM sessions when clients are drawing a large number of slips each session and escalation can increase costs of the overall program. Capping the number of draws will help mitigate both issues. For example, once a client reaches 10 draws for group attendance, they continue earning 10 draws for each consecutive session attended with no further escalation.
Putting It All Together
CM sessions can be conducted as stand-alone sessions or incorporated into group or individual therapy sessions. Many clinicians will find the latter approach sets a positive tone for the therapy session given CM's focus on what the client is doing well. Starting the treatment session with the CM component often naturally leads into a discussion of relevant therapeutic issues, such as effective coping, slips, or triggers. The CM session length can be variable, but it is typically under 10 minutes. Thus, the CM component need not dominate the clinical session or content. CM sessions for abstinence are scheduled according to a set schedule (eg, Mondays and Thursdays) and often coincide with other treatment aspects (eg, before or after group therapy on Mondays and Thursdays). CM sessions for attendance also generally follow a set schedule (eg, client expected to attend Monday and Wednesday group therapy sessions). The duration of the CM protocol can also vary, with most clinical trials ranging from 12 to 24 weeks. Very short durations are unlikely to produce lasting behavior change, particularly with complex behaviors such as abstinence. Petry [44] recommends no less than 8 weeks duration and a maximum duration of 24 weeks.
As discussed, CM offers many opportunities for tailoring to optimize its fit within the existing structure of clinics. However, this flexibility must be viewed together with an understanding of the principles that impact CM's efficacy. Specific recommendations for CM protocol development will depend on the behavior targeted, the delivery methods, and format (eg, individual versus group settings). For these reasons, consultation with a CM expert is ideal. However, some general guidelines for developing a CM program that incorporate the principles discussed above include an 8- to 12-week program that (1) provides sufficient magnitude to compete with the behavior you are attempting to change, (2) offers frequent opportunities for reinforcement (eg, 2-3 times/wk for opioids or stimulant abstinence, 1-2 times/week for attendance targets; not less than weekly for most behaviors), (3) delivers the reinforcement immediately or very close in time with the behavior (eg, reinforce attendance at the beginning of the group, use onsite urine testing and reinforce immediately after testing), and (4) incorporates escalating and reset features into the schedule.
Clinician Training and Supervision
Training in CM is an important part of the implementation process. Studies [62,84–87] have identified a number of perceived barriers to and negative beliefs about CM, including philosophical and logistical concerns. Tangible incentives, the core of most CM protocols, are generally viewed less favorably than social or nonspecified incentives [84,86,87]. Philosophical concerns relate to CM’s inability to address the underlying causes of addiction, that it does not address multiple behaviors, and that it may undermine internal motivation for sobriety [62,84]. An additional objection relates to paying someone to do what they should do on their own [86]. Logistical and practical concerns often represent implementation barriers such as costs and access to training and supervision, but they also reflect concern for what happens when contingencies are withdrawn, that clients may sell or trade prizes for drugs, and worries that CM’s evidence does not generalize to clinic populations [62].
Many of these beliefs reflect a limited understanding of CM, and addressing these misperceptions is a first step toward reducing resistance to implementation efforts. For example, a substantial body of literature points to CM’s wide generalizability across a range of characteristics, clients that sell or trade prizes for drugs are likely to disrupt their chain of negative samples or attendance, and most studies do not find negative impacts of CM on intrinsic motivation [88–90]. Fortunately, CM training appears to be an effective way to address negative beliefs. In the VA implementation effort [52], training workshops decreased perceived barriers and increased positive impressions of CM [91]. In other training efforts, brief educational materials were effective in changing perceptions of CM’s efficacy [92].
Beyond initial training, supervision of CM delivery is likely to be necessary [93,94]. Clinician skill in delivering CM is related to client outcomes [93,95] and relatively simple adherence measures are available for monitoring [96,97]. However, the best methods for training and supervision of CM have yet to be established. The VA initiative was developed in consultation with CM experts and employed ongoing phone consultation following initial training workshops [52,91]. This approach represented significant investment by the VA toward staff training and CM protocol development that may not be achievable for individual clinics. As attention to CM’s dissemination and implementation has grown, some free resources have been developed. Promoting Awareness of Motivational Incentives (PAMI; www.bettertxoutcomes.org/bettertxoutcomes/PAMI.html) is a collaborative initiative sponsored by the National Institute of Drug Abuse and the Substance Abuse and Mental Health Services Administration. It offers free resources and training materials.
Conclusion
Overall, CM is a highly efficacious treatment for SUDs that generalizes to most clients. Despite a robust evidence base, CM’s implementation in clinical settings lags behind other empirically supported treatments [92]. At least in part, CM’s costs, which include not only staff training and adherence monitoring (as with other treatments), but also costs of the incentives themselves, may contribute to slow uptake in clinical settings. Clinics often do not have the resources available for CM within their operating budgets. However, a growing number of projects [19,52,53,55–57,70,71] illustrate CM implementation within routine clinical care, and increased revenue from improved attendance to treatment groups may be one mechanism through which to fund a CM program [54,56,57]. These projects are valuable not only for demonstrating that CM can be efficacious outside the research setting, but also for highlighting how implementation barriers can be overcome. Continued efforts of this nature are likely to be particularly valuable for clinicians and administrators considering adopting CM within clinical settings.
From UConn Health, Farmington, CT.
Abstract
- Objective: To discuss the efficacy and generalizability of contingency management (CM) for the treatment of substance use disorders and design considerations for those considering implementing in clinical settings.
- Methods: Review of the literature.
- Results: CM is an efficacious treatment for substance abuse disorders that is widely generalizable across substance use disorders and patient characteristics. CM can be implemented in a number of treatment programs, including residential and outpatient settings, and it can be administered in both individual and group formats. Abstinence and attendance are the most commonly targeted behaviors in substance abuse treatment settings. Design features, including the selection of target behaviors, delivery methods, and reinforcers, are discussed. Schedule parameters, such as frequency, magnitude, immediacy, and escalation of reinforcement, are associated with overall impact of the CM program and are important considerations for those interested in tailoring CM protocols to their needs.
- Conclusion: CM is an efficacious option that is applicable to most substance abuse treatment patients. A number of demonstrations of real-world implementation have been published and suggest CM can be adapted with success to clinic settings. In adopting CM protocols, clinics should aim for those protocols with established efficacy; however, if adaptations are necessary, careful consideration should be given to modifications to minimize risks of undermining CM’s effects.
Key words: incentives; reinforcement; substance abuse treatment; dissemination; implementation.
Contingency management (CM) is a behavioral intervention that is efficacious in the treatment of substance use disorders (SUDs). CM uses a behavior analytic framework and applies principles of learning theory, particularly operant conditioning theory, to change client behavior(s) [1–5]. In basic terms, operant conditioning principles suggests that whether a behavior continues or not is a function of consequences [6]. Reinforced behaviors are more likely to occur in the future. Substance abuse can be viewed as a behavior maintained by the reinforcing effects of the drug itself [5], including the feel-good aspects of intoxication or relaxation and the amelioration of withdrawal symptoms. CM extends these same principles of to a treatment context, such that reinforcers for abstinent behavior are introduced to compete with the reinforcing effects of continued drug use [5].
In CM’s application to substance abuse treatment, drug-negative samples or treatment attendance are reinforced using tangible incentives with the goal of motivating continued abstinence and/or treatment engagement. When clients demonstrate these target behaviors, they earn incentives in the form of goods or services of value to the client, such as small electronics, gift cards, and toiletries. Despite the promising effects observed in research trials, real-world implementation efforts have not kept pace [7–9]. This review briefly discusses CM’s efficacy and highlights key features for professionals considering adopting this intervention. Demonstration efforts that illustrate how CM can be effectively implemented within the constraints and limitations of non-research, clinical settings are also presented.
Efficacy of CM
CM’s efficacy spans a number of SUDs, including cocaine, opioids, alcohol, nicotine, and marijuana [10–13], making it amenable for treatment of most SUD clinic populations. It generates larger effect sizes than other SUD treatments, including cognitive behavioral therapy [14], and it has been evaluated in a wide range of settings. Large-scale evaluations have been conducted in both intensive outpatient [15] and methadone maintenance [16] settings as part of the National Institute on Drug Abuse Clinical Trials Network, demonstrating consistent benefits of CM when added to treatment as usual. In the first of these 2 studies, Petry et al [15] randomized 415 stimulant users from 1 of 8 intensive outpatient clinics to treatment as usual or treatment as usual plus CM for alcohol and stimulant abstinence. CM participants submitted more substance-negative urine and breath samples, achieved continuous abstinence at significantly higher rates, and had longer treatment retention compared to those receiving treatment as usual. The parallel study [16] focused on stimulant use in clients from methadone maintenance clinics and found similar benefits of CM on stimulant abstinence. Beyond these settings, CM has been applied in a number of other contexts, including drop-in centers [17], vocational rehabilitation [18,19], job-skills training [20], and residential programs [21–23]. In addition, several group-based adaptations have been explored [17,24–27].
CM benefits most clients and generalizes across several demographic variables, including gender [28,29], race [30], housing status [31], and income levels [32–34]. Among clinical characteristics, CM is efficacious for those with co-occurring SUDs [35], other substance use [36], psychiatric disorders [37–39], medical problems [40–42], and history of transactional sex [43].
Design Considerations
Design features, including what behavior will be reinforced and how to do so, are among the first decision points for clinicians interested in implementing CM. One of the advantages of CM is that it has a high degree of flexibility in design, which means that it can be readily tailored to client populations and clinic needs. However, this flexibility can lead clinicians astray from the foundational principles of CM and unknowingly weaken the impact of the program. Below, some key considerations for CM protocol design are reviewed. For additional coverage of these topics, readers are referred to additional articles [1,2] or Petry’s comprehensive book on implementing CM [44]. In this review, published examples of CM’s application in real-world settings are presented, highlighting how CM has been adapted in these clinical efforts.
Target Behaviors
The selection of the target behavior will drive many of the subsequent program design decisions. As such, it is important to identify this feature early. Target behaviors must be achievable, objectively verifiable, and well defined. The most common CM targets are drug abstinence or therapy session attendance. CM has also been used to target other behaviors, such as medication adherence [45,46], treatment-related activities [47,48], and exercise [49–51]. Client self-report of behaviors or vaguely defined behaviors (eg, “good participation”) should be avoided. While some of the decisions related to CM protocols are flexible, the use of objectively verifiable target behaviors is a core feature that should not be neglected. If the behavior of interest cannot be objectively verified, an alternate behavior should be chosen.
Selection of the target behavior is often considered in hand with defining which population is eligible to participate in the CM program. Client characteristics are often forefront in this decision, but clinic-driven logistical issues or unmet needs may also play a role. A real-world example of this decision process is evident in the nationwide rollout of CM among the intensive outpatient programs within the Veterans Administration (VA). The VA identified a treatment need for those with stimulant use disorders, as this group did not have efficacious pharmacotherapy options available that targeted stimulant use. As such, the VA applied CM to patients with a focus on stimulant abstinence as the behavioral target [52]. For others, the decision may revolve around addressing underutilization of specific treatment resources (eg, outpatient groups, vocational rehabilitation) [53–56] or treatment needs among certain subgroups of clients, such as adolescents [57–59].
For abstinence targets, clinics would need to select one or more specific substances as the focus of the CM program. In general, targeting a single substance rather than multiple substances is more effective [10,13], is more straightforward for clients to understand, and allows more clients to access the reinforcers. Exposure to the reinforcers is necessary for CM to work; thus, setting a goal that is achievable for most clients should be a priority. Requiring abstinence from multiple substances means that some clients may never experience the reinforcer and thus cannot benefit from its effects at all. Some clinicians or administrators may initially have reservations about reinforcing single drug abstinence in the event that other drug use continues. However, targeting a single substance for reinforcement often results in reduced use of other substances [60]. Clinicians may find that this makes intuitive sense; a client with cocaine use disorder who is trying to maintain cocaine abstinence over a long period is likely to avoid using alcohol or other substances that might lead to relapse. For abstinence, objective verification through urine or breath specimens using tests that include validity checks is relatively straightforward.
Attendance is a popular target for clinics in part because it does not require additional staff time to collect specimen samples and it was the most commonly reported target behavior in samples of SUD providers who use incentives [61,62]. Objective verification of attendance is usually via a staff member, but expectations must be clear to both parties. Clinics should consider potential problems that may arise (eg, arriving late, leaving group early, excused absences) and carefully define and communicate expectations for the CM program. Piloting [19] the CM program with a small group of clients may be valuable in trouble-shooting challenges before wider implementation.
In a recent study [55], clients earned reinforcers for attending clinician-led group counseling sessions and/or the in-clinic patient-led Methadone Anonymous (MA) groups. This non-research, clinical effort addressed historically poor therapy attendance at the clinic, and attendance rates were compared before, during, and after the CM program. CM increased attendance to both groups in the short-term after implementation, but effects were more robust for the MA groups in which increased attendance persisted 3 months following the withdrawal of the contingencies. Overall effects of this program were modest, but they are notable given the use of an ultra-low cost approach.
Delivery Methods
The majority of CM studies used voucher or prize-based methods. Head-to-head comparisons suggest that they are comparable in efficacy [63–65], and each has advantages and disadvantages that may make one option more appealing for a given clinic. Voucher programs are generally straightforward to administer. Clients earn vouchers for each instance of the target behavior. The value of the vouchers typically increases with consecutive performance. The schedule used in the influential Higgins et al studies [66,67] started at $2.50 for the first cocaine-negative sample and increased by $0.50 for each subsequent consecutive cocaine-negative sample. Earned vouchers are exchanged for goods or services selected by the client, increasing the likelihood that the selected items will be highly desirable and allowing for a wide range of client preferences. Clients appear to prefer this approach when given a choice between set schedules or those that introduce an element of chance (ie, prize-based CM, discussed below) [68]. However, voucher programs can be costly (~$1000 per client over 12 weeks) and may require more staff time to fulfill individual requests for specific items. However, staff burden related to shopping can be reduced by limiting these individual requests and using an on-site stocked cabinet of goods similar to prize-CM programs.
Prize-based CM is similar but introduces probabilistic earnings and variability in prize magnitude. Rather than earning vouchers, clients earn draws from a fishbowl for each instance of the target behavior, again typically in an escalating manner. For example, a client may earn one draw from the fishbowl for the first cocaine-negative sample, 2 draws for the second consecutive negative sample, 3 draws for the third, and so on. A typical fishbowl is composed of 500 slips, some noting prizes and some having no prize value. Typically, half the slips in the bowl are non-monetary “good jobs.” The remaining half are small prizes worth about $1 in value (eg, food coupons, bus tokens, small toiletries), large prizes worth about $20 in value (eg, small electronics, gift certificates), and one slip is the jumbo prize worth about $100. When a client draws a winning slip, they select a prize from that category (ie, small, large, jumbo) from an onsite, stocked cabinet. Due to the probabilistic feature of prize-based CM, overall costs of the program can be substantially lower than typical voucher programs, with average maximum expected earnings ranging $250 t $450 per client over a 12-week treatment period [15,16,65,69]. Advantages of this method include potentially lower costs and minimal shopping demands (a once-monthly shopping trip to restock the cabinet will usually suffice) while maintaining comparable efficacy. Relative to voucher programs, prize-based CM involves additional administration time to allow for drawing slips from the fishbowl, which can be compounded when multiple clients want to draw at the same time such as in a group setting. Many of the group-based CM adaptations address this issue by limiting the number of clients who can draw for prizes in a given group or by limiting the number of draws per client [25,27,54].
Reinforcers
Regardless of whether selecting voucher or prize CM, reinforcers are critically important to the success of the program. Reinforcers must be desirable. One of the quickest ways to undermine a CM program is lack of variety or undesirable reinforcers. If stocking a cabinet with prizes onsite, care should be taken to have numerous options within each of the small and large prize categories that are appealing to a wide range of clients. Since a client who is consistently earning draws will choose prizes often, it is imperative to include enough variety so that even these clients find desirable items each time they select a prize. Clients should be asked regularly if they have suggestions for prizes; one program [54] found suggestion boxes useful for encouraging clients to voice their preferences. Donations can be solicited from local businesses to reduce costs [53], and low-cost but high-value options, such as clinic privileges, can also be explored. Petry [1,44] provides some suggestions of the latter, and Amass and Kamien [70] describe their successful strategies to fund and sustain a clinic-based CM program through community donations. Some clinics may already have tangible goods, such as gas or metro cards, that are offered to clients based on need rather than behavior [53]. These existing resources might be redirected to a CM program, in which these goods are contingent on abstinence or attendance, if appropriate.
Schedule Parameters
Once the target behavior, client population, and CM delivery methods are selected, the next step is to design the reinforcement schedule. The following schedule parameters apply to both voucher and prize-based CM systems. The more closely a clinical program adheres to the parameters of effective protocols, the more likely the program is to generate comparable outcomes. If there is a parameter or design feature that is incompatible with clinic needs, modifications can be introduced. However, each deviation away from the ideal has a chance of undermining the success of the CM program. Any changes and their potential impacts should considered carefully, and consultation with a CM expert may aid in the development of successful and efficacious clinic-based protocols. Of note, a meta-analysis [13] of CM studies found that researcher involvement in the planning and design of CM programs is associated with larger treatment effects. CM researchers are especially attuned to the potential impacts and pitfalls associated with modifying CM protocols, and they can be valuable resources for clinics interested in tailoring a CM program to their specific needs. Several examples of clinical demonstration projects that used researcher input are available [19,53,71].
Magnitude
Incentive magnitude was directly related to the size of treatment effects in a meta-analysis [11] of CM studies. Although not all studies find significant differences in outcomes related to magnitude [65,72], the bulk of evidence suggests magnitude is an important parameter and is related to effect size for both voucher [73–75] and prize-based CM [69,76] systems. Thus, although clinics may have restrictive budgets, severely undercutting the magnitude of rewards is not usually the solution as it can undermine treatment effects [76]. Donations can reduce overall costs [53,57,70], and other protocol features discussed below, such as capping the amount of reinforcement available, can reduce the overall magnitude available per patient.
Another approach, used in group-based CM, limits the number of patients who earn prizes per week [25,27]. For example, in a 2011 study by Petry et al, clients added slips with their name to a bowl for attendance and negative samples. Once all names were collected in the bowl, the group leader would pull a specified number of slips (eg, 3 slips per group). These individuals were eligible to draw from the prize bowl for prizes. This approach was associated with longer durations of consecutive abstinence and better treatment attendance relative to treatment as usual. However, clinics can control the overall program costs by limiting the number of patients eligible for prizes.
Frequency
Frequent reinforcement opportunities are ideal, and more frequent assessment is associated with larger treatment effects [10]. However, a number of factors, including which target behavior is selected and logistical issues specific to the clinic such as when groups meet, will play a role in determining the frequency of CM sessions. For abstinence targets, the substance targeted and type of test will largely determine the frequency of CM sessions. The goal would be to test at a frequency that would detect most or nearly all instances of use. For cocaine or opioids, this equates to testing 2 to 3 times weekly. Breath samples for alcohol or cigarette smoking would necessitate testing daily or multiple times per day to detect most instances of use because these tests have short windows of detection. CM protocols based on these breath tests have often had daily or twice daily CM sessions [77,78]; technological adaptations [77,79,80] or residential settings [21,23] may reduce burden to the client for assessment of these substances. Tapering the number of breath tests over time or transitioning from daily breath tests to once or twice weekly urine testing after abstinence is established is another approach [81,82].
Marijuana, on the other hand, poses difficulties because it is detectable in urine samples for up to 2 weeks following use. If relying solely on urine results for reinforcement, clients may not test negative for several days or weeks after last use, resulting in a delay of reinforcement. To address this issue, some CM programs targeting marijuana abstinence initially reinforce attendance in the first 2 weeks and then transition to reinforcing marijuana-negative drug samples for the remainder of the treatment period [48].
In general, more frequent CM sessions can translate to higher costs; however, infrequent reinforcement (ie, less than weekly) is not as effective [45]. In real-world applications, clinics often need to balance feasibility and costs with the ideal CM schedule. In abstinence-based CM, this compromise may result in a testing schedule that may not capture all instances of use. For example, while thrice-weekly testing may be ideal for cocaine or opioids, a twice-weekly schedule may be selected because it lowers costs and is more consistent with clinic schedules.
Immediacy
In general, clinics should aim to deliver reinforcement as immediately as possible, as delays between the target behavior and reinforcement are associated with decreased treatment effects [10,11,83]. For drug abstinence, onsite urine testing systems that provide immediate results are preferred over sending samples for laboratory testing. Clinics that do not have access to or who cannot afford specimen testing that allows onsite collection and immediate results might consider other options for target behaviors, such as attendance.
Immediacy of reinforcement is also important when targeting attendance. One clinic [53] implemented a program that offered a $50 incentive if clients attended 1 month of group therapy sessions. This approach was not effective and no clients earned the incentive for several months. After consultation, the clinic revised the incentive program to a daily drawing for attendance using the fishbowl method, thereby decreasing the delay between the behavior and its consequence. This example illustrates not only problems with delayed reinforcement but also the common mistake of setting expectations for the target behavior too high. Attending a month of group therapy sessions is a high bar that few patients will achieve, resulting in a system that mostly rewards those already doing well [19]. In contrast, attending a single group session in order to earn reinforcers is a reachable goal and increases the likelihood that more clients are exposed to the reinforcers. These small steps (ie, attending a single group or submitting a single drug negative urine) encourage initiation of the behavior(s) targeted. Other features, such as escalation (discussed next), aim to sustain the behavior over time.
Escalation
Escalation involves increasing the amount of reinforcement for each consecutive target behavior. In the voucher programs, the amount earned per negative sample may increase for each consecutive negative sample (eg, $2.50 for the first negative sample, $3.00 for the second, $3.50 for the third, and so on). For prize-based programs, the number of draws escalates with consecutive performance (eg, 1 draw for the first group attended, 2 draws for the second, 3 for the third, and so on). Protocols that include escalation generate larger effects than those that have a set, flat incentive amount even when total costs are the same across comparison conditions [73].
Escalating schedules usually include a reset feature. Following a positive or refused sample or unexcused absence, the amount earned for the next negative sample is reduced to the initial amount and begins escalating anew with consecutive negative samples. Some schedules allow for a rapid reset in which after a specified period of time or consecutive performance, the value jumps to the value achieved when the relapse occurred [66].
Despite its consistent inclusion in CM protocols from randomized clinical trials, our data [61] suggest that more than half of providers using incentives in treatment as part of a clinical effort do not use escalating reinforcers. Escalating schedules require more careful tracking of client progress, possibly contributing to lower uptake of this design feature in clinical practice. Development of simple tracking forms can minimize this challenge.
Another drawback of escalation pertaining to prize-based CM is that escalating schedules can affect the duration of CM sessions when clients are drawing a large number of slips each session and escalation can increase costs of the overall program. Capping the number of draws will help mitigate both issues. For example, once a client reaches 10 draws for group attendance, they continue earning 10 draws for each consecutive session attended with no further escalation.
Putting It All Together
CM sessions can be conducted as stand-alone sessions or incorporated into group or individual therapy sessions. Many clinicians will find the latter approach sets a positive tone for the therapy session given CM's focus on what the client is doing well. Starting the treatment session with the CM component often naturally leads into a discussion of relevant therapeutic issues, such as effective coping, slips, or triggers. The CM session length can be variable, but it is typically under 10 minutes. Thus, the CM component need not dominate the clinical session or content. CM sessions for abstinence are scheduled according to a set schedule (eg, Mondays and Thursdays) and often coincide with other treatment aspects (eg, before or after group therapy on Mondays and Thursdays). CM sessions for attendance also generally follow a set schedule (eg, client expected to attend Monday and Wednesday group therapy sessions). The duration of the CM protocol can also vary, with most clinical trials ranging from 12 to 24 weeks. Very short durations are unlikely to produce lasting behavior change, particularly with complex behaviors such as abstinence. Petry [44] recommends no less than 8 weeks duration and a maximum duration of 24 weeks.
As discussed, CM offers many opportunities for tailoring to optimize its fit within the existing structure of clinics. However, this flexibility must be viewed together with an understanding of the principles that impact CM's efficacy. Specific recommendations for CM protocol development will depend on the behavior targeted, the delivery methods, and format (eg, individual versus group settings). For these reasons, consultation with a CM expert is ideal. However, some general guidelines for developing a CM program that incorporate the principles discussed above include an 8- to 12-week program that (1) provides sufficient magnitude to compete with the behavior you are attempting to change, (2) offers frequent opportunities for reinforcement (eg, 2-3 times/wk for opioids or stimulant abstinence, 1-2 times/week for attendance targets; not less than weekly for most behaviors), (3) delivers the reinforcement immediately or very close in time with the behavior (eg, reinforce attendance at the beginning of the group, use onsite urine testing and reinforce immediately after testing), and (4) incorporates escalating and reset features into the schedule.
Clinician Training and Supervision
Training in CM is an important part of the implementation process. Studies [62,84–87] have identified a number of perceived barriers to and negative beliefs about CM, including philosophical and logistical concerns. Tangible incentives, the core of most CM protocols, are generally viewed less favorably than social or nonspecified incentives [84,86,87]. Philosophical concerns relate to CM’s inability to address the underlying causes of addiction, that it does not address multiple behaviors, and that it may undermine internal motivation for sobriety [62,84]. An additional objection relates to paying someone to do what they should do on their own [86]. Logistical and practical concerns often represent implementation barriers such as costs and access to training and supervision, but they also reflect concern for what happens when contingencies are withdrawn, that clients may sell or trade prizes for drugs, and worries that CM’s evidence does not generalize to clinic populations [62].
Many of these beliefs reflect a limited understanding of CM, and addressing these misperceptions is a first step toward reducing resistance to implementation efforts. For example, a substantial body of literature points to CM’s wide generalizability across a range of characteristics, clients that sell or trade prizes for drugs are likely to disrupt their chain of negative samples or attendance, and most studies do not find negative impacts of CM on intrinsic motivation [88–90]. Fortunately, CM training appears to be an effective way to address negative beliefs. In the VA implementation effort [52], training workshops decreased perceived barriers and increased positive impressions of CM [91]. In other training efforts, brief educational materials were effective in changing perceptions of CM’s efficacy [92].
Beyond initial training, supervision of CM delivery is likely to be necessary [93,94]. Clinician skill in delivering CM is related to client outcomes [93,95] and relatively simple adherence measures are available for monitoring [96,97]. However, the best methods for training and supervision of CM have yet to be established. The VA initiative was developed in consultation with CM experts and employed ongoing phone consultation following initial training workshops [52,91]. This approach represented significant investment by the VA toward staff training and CM protocol development that may not be achievable for individual clinics. As attention to CM’s dissemination and implementation has grown, some free resources have been developed. Promoting Awareness of Motivational Incentives (PAMI; www.bettertxoutcomes.org/bettertxoutcomes/PAMI.html) is a collaborative initiative sponsored by the National Institute of Drug Abuse and the Substance Abuse and Mental Health Services Administration. It offers free resources and training materials.
Conclusion
Overall, CM is a highly efficacious treatment for SUDs that generalizes to most clients. Despite a robust evidence base, CM’s implementation in clinical settings lags behind other empirically supported treatments [92]. At least in part, CM’s costs, which include not only staff training and adherence monitoring (as with other treatments), but also costs of the incentives themselves, may contribute to slow uptake in clinical settings. Clinics often do not have the resources available for CM within their operating budgets. However, a growing number of projects [19,52,53,55–57,70,71] illustrate CM implementation within routine clinical care, and increased revenue from improved attendance to treatment groups may be one mechanism through which to fund a CM program [54,56,57]. These projects are valuable not only for demonstrating that CM can be efficacious outside the research setting, but also for highlighting how implementation barriers can be overcome. Continued efforts of this nature are likely to be particularly valuable for clinicians and administrators considering adopting CM within clinical settings.
1. Petry NM. A comprehensive guide to the application of contingency management procedures in clinical settings. Drug Alcohol Depend 2000;58:9–25.
2. Stitzer M, Petry N. Contingency management for treatment of substance abuse. Annu Rev Clin Psychol 2006;2:411–34.
3. Meredith SE, Jarvis BP, Raiff BR, et al. The ABCs of incentive-based treatment in health care: A behavior analytic framework to inform research and practice. Psychol Res Behav Manag 2014;7:103–14.
4. Bigelow GE, Silverman K. Theoretical and empirical foundations of contingency management treatments for drug abuse. In: Higgins ST, Silverman K, editors. Motivating behavior change among illicit drug users. Washington DC: American Psychological Association; 1999: 15–31.
5. Higgins ST, Budney AJ, Bickel WK. Applying behavioral concepts and principles to the treatment of cocaine dependence. Drug Alcohol Depend 1994;34:87–97.
6. Skinner BF. Science and human behavior. New York: Macmillan; 1953.
7. Herbeck DM, Hser YI, Teruya C. Empirically supported substance abuse treatment approaches: A survey of treatment providers’ perspectives and practices. Addict Behav 2008;33:699–712.
8. McGovern MP, Fox TS, Xie H, Drake RE. A survey of clinical practices and readiness to adopt evidence-based practices: Dissemination research in an addiction treatment system. J Subst Abuse Treat 2004;26:305–12.
9. Willenbring ML, Kivlahan D, Kenny M, et al. Beliefs about evidence-based practices in addiction treatment: A survey of Veterans Administration program leaders. J Subst Abuse Treat 2004;26:79–85.
10. Griffith JD, Rowan-Szal GA, Roark RR, Simpson DD. Contingency management in outpatient methadone treatment: A meta-analysis. Drug Alcohol Depend 2000;58:55–66.
11. Lussier JP, Heil SH, Mongeon JA, et al. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction 2006;101:192–203.
12. Benishek LA, Dugosh KL, Kirby KC, et al. Prize-based contingency management for the treatment of substance abusers: A meta-analysis. Addiction 2014;109:1426–36.
13. Prendergast M, Podus D, Finney J, et al. Contingency management for treatment of substance use disorders: A meta-analysis. Addiction 2006;101:1546–60.
14. Dutra L, Stathopoulou G, Basden SL, et al. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry 2008;165:179–87.
15. Petry NM, Peirce JM, Stitzer ML, et al. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: A National Drug Abuse Treatment Clinical Trials Network study. Arch Gen Psychiatry 2005;62:1148–56.
16. Peirce JM, Petry NM, Stitzer ML, et al. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment - A National Drug Abuse Treatment Clinical Trials Network study. Arch Gen Psychiatry 2006;63:201–8.
17. Petry NM, Martin B, Finocche C. Contingency management in group treatment: a demonstration project in an HIV drop-in center. J Subst Abuse Treat 2001;21:89–96.
18. Drebing CE, Van Ormer EA, Mueller L, et al. Adding contingency management intervention to vocational rehabilitation: outcomes for dually diagnosed veterans. J Rehabil Res Dev 2007;44:851–65.
19. Kellogg SH, Burns M, Coleman P, et al. Something of value: The introduction of contingency management interventions into the New York City Health and Hospital Addiction Treatment Service. J Subst Abuse Treat 2005;28:57–65.
20. Koffarnus MN, Wong CJ, Fingerhood M, et al. Monetary incentives to reinforce engagement and achievement in a job-skills training program for homeless, unemployed adults. J Appl Behav Anal 2013;46:582–91.
21. Rohsenow D, Martin R, Tidey JW, et al. Treating smokers in substance treatment with contingent vouchers, nicotine replacement, and brief advice adapted for sobriety settings. J Subst Abuse Treat 2017.
22. Hunt YM, Rash CJ, Burke RS, Parker JD. Smoking cessation in recovery: Comparing two different cognitive behavioral treatments. Addict Disord Their Treat 2010;9:64–74.
23. Alessi SM, Petry NM, Urso J. Contingency management promotes smoking reductions in residential substance abuse patients. J Appl Behav Anal 2008;41:617–22.
24. Petry NM, Weinstock J, Alessi SM, et al. Group-based randomized trial of contingencies for health and abstinence in HIV patients. J Consult Clin Psychol 2010;78:89.
25. Petry NM, Weinstock J, Alessi SM. A randomized trial of contingency management delivered in the context of group counseling. J Consult Clin Psychol 2011;79:686–96.
26. Ledgerwood DM, Alessi SM, Hanson T, et al. Contingency management for attendance to group substance abuse treatment administered by clinicians in community clinics. J Appl Behav Anal 2008;41:517–26.
27. Alessi SM, Hanson T, Wieners M, Petry NM. Low-cost contingency management in community clinics: Delivering incentives partially in group therapy. Exp Clin Psychopharmacol 2007;15:293–300.
28. Burch AE, Rash CJ, Petry NM. Sex effects in cocaine-using methadone patients randomized to contingency management interventions. Exp Clin Psychopharmacol 2015;23:284–90.
29. Rash CJ, Petry NM. Contingency management treatments are equally efficacious for both sexes in intensive outpatient settings. Exp Clin Psychopharmacol 2015;23:369–76.
30. Barry D, Sullivan B, Petry NM. Comparable efficacy of contingency management for cocaine dependence among African American, Hispanic, and White methadone maintenance clients. Psychol Addict Behav 2009;23:168–74.
31. Rash CJ, Alessi SM, Petry NM. Substance abuse treatment patients in housing programs respond to contingency management interventions. J Subst Abuse Treat 2017;72:97–102.
32. Rash CJ, Olmstead TA, Petry NM. Income does not affect response to contingency management treatments among community substance abuse treatment-seekers. Drug Alcohol Depend 2009;104:249–53.
33. Rash CJ, Andrade LF, Petry NM. Income received during treatment does not affect response to contingency management treatments in cocaine-dependent outpatients. Drug Alcohol Depend 2013;132:528–34.
34. Secades-Villa R, García-Fernández G, Peña-Suárez E, et al. Contingency management is effective across cocaine-dependent outpatients with different socioeconomic status. J Subst Abuse Treat 2013;44:349–54.
35. Rash CJ, Alessi SM, Petry NM. Cocaine abusers with and without alcohol dependence respond equally well to contingency management treatments. Exp Clin Psychopharmacol 2008;16:275–81.
36. Alessi SM, Rash C, Petry NM. Contingency management is efficacious and improves outcomes in cocaine patients with pretreatment marijuana use. Drug Alcohol Depend 2011;118:62–7.
37. Ford JD, Hawke J, Alessi S, et al. Psychological trauma and PTSD symptoms as predictors of substance dependence treatment outcomes. Behav Res Ther 2007;45:2417–31.
38. Weinstock J, Alessi SM, Petry NM. Regardless of psychiatric severity the addition of contingency management to standard treatment improves retention and drug use outcomes. Drug Alcohol Depend 2007;87:288–96.
39. García-Fernández G, Secades-Villa R, García-Rodríguez O, et al. Contingency management improves outcomes in cocaine-dependent outpatients with depressive symptoms. Exp Clin Psychopharmacol 2013;21:482–9.
40. Walter KN, Petry NM. Patients with diabetes respond well to contingency management treatment targeting alcohol and substance use. Psychol Health Med 2015;20:916–26.
41. Burch AE, Morasco BJ, Petry NM. Patients undergoing substance abuse treatment and receiving financial sssistance for a physical disability respond well to contingency management treatment. J Subst Abuse Treat 2015;58:67–71.
42. Burch AE, Rash CJ, Petry NM. Cocaine-using substance abuse treatment patients with and without HIV respond well to contingency management treatment. J Subst Abuse Treat 2017;77:21–5.
43. Rash CJ, Burki M, Montezuma-Rusca JM, Petry NM. A retrospective and prospective analysis of trading sex for drugs or money in women substance abuse treatment patients. Drug Alcohol Depend 2016;162:182–9.
44. Petry NM. Contingency management for substance abuse treatment: A guide to implementing this evidence-based practice. New York: Routledge; 2012.
45. Petry NM, Rash CJ, Byrne S, et al. Financial reinforcers for improving medication adherence: Findings from a meta-analysis. Am J Med 2012;125:888–96.
46. Herrmann ES, Matusiewicz AK, Stitzer ML, et al. Contingency management interventions for HIV, tuberculosis, and hepatitis control among individuals with substance use disorders: a systematized review. J Subst Abuse Treat 2017;72:117–25.
47. Petry NM, Alessi SM, Carroll KM, et al. Contingency management treatments: Reinforcing abstinence versus adherence with goal-related activities. J Consult Clin Psychol 2006;74:592–601.
48. Litt MD, Kadden RM, Petry NM. Behavioral treatment for marijuana dependence: Randomized trial of contingency management and self-efficacy enhancement. Addict Behav 2013;38:1764–75.
49. Kurti AN, Dallery J. A laboratory-based evaluation of exercise plus contingency management for reducing cigarette smoking. Drug Alcohol Depend 2014;144:201–9.
50. Weinstock J, Capizzi J, Weber SM, et al. Exercise as an intervention for sedentary hazardous drinking college students: A pilot study. Ment Health Phys Act 2014;7:55–62.
51. Mitchell MS, Goodman JM, Alter DA, et al. Financial incentives for exercise adherence in adults: Systematic review and meta-analysis. Am J Prev Med 2013;45:658–67.
52. Petry NM, Dephilippis D, Rash CJ, et al. Nationwide dissemination of contingency management: The Veterans Administration initiative. Am J Addict 2014;23:205–10.
53. Walker R, Rosvall T, Field CA, et al. Disseminating contingency management to increase attendance in two community substance abuse treatment centers: Lessons learned. J Subst Abuse Treat 2010;39:202–9.
54. Sigmon SC, Stitzer ML. Use of a low-cost incentive intervention to improve counseling attendance among methadone-maintained patients. J Subst Abuse Treat 2005;29:253–8.
55. Kropp F, Lewis D, Winhusen T. The effectiveness of ultra-low magnitude reinforcers: Findings from a “real-world” application of contingency management. J Subst Abuse Treat 2017;72:111–6.
56. Fitzsimons H, Tuten M, Borsuk C, et al. Clinician-delivered contingency management increases engagement and attendance in drug and alcohol treatment. Drug Alcohol Depend 2015;152:62–7.
57. Lott DC, Jencius S. Effectiveness of very low-cost contingency management in a community adolescent treatment program. Drug Alcohol Depend 2009;102:162–5.
58. Henggeler SW, Chapman JE, Rowland MD, et al. Statewide adoption and initial implementation of contingency management for substance-abusing adolescents. 2008;76:556–67.
59. Henggeler SW, Chapman JE, Rowland MD, et al. If you build it , they will come: Statewide practitioner interest in contingency management for youths. 2007;32:121–31.
60. Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes, and they will come: contingency management for treatment of alcohol dependence. J Consult Clin Psychol 2000;68:250–7.
61. Rash CJ, Petry NM, Alessi SM. Examining implementation of contingency management in real-world settings [Abstract]. Alcohol Clin Exp Res 2016;20:103A.
62. Rash CJ, Petry NM, Kirby KC, et al. Identifying provider beliefs related to contingency management adoption using the Contingency Management Beliefs Questionnaire. Drug Alcohol Depend 2012;121:205–12.
63. Petry NM, Alessi SM, Marx J, et al. Vouchers versus prizes: Contingency management treatment of substance abusers in community settings. J Consult Clin Psychol 2005;73:1005–14.
64. Petry NM, Alessi SM, Hanson T, Sierra S. Randomized trial of contingent prizes versus vouchers in cocaine-using methadone patients. J Consult Clin Psychol 2007;75:983–91.
65. Petry NM, Alessi SM, Barry D, Carroll KM. Standard magnitude prize reinforcers can be as efficacious as larger magnitude reinforcers in cocaine-dependent methadone patients. J Consult Clin Psychol 2015;83:464–72.
66. Higgins ST, Budney AJ, Bickel WK, et al. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Arch Gen Psychiatry 1994;51:568.
67. Higgins ST, Wong CJ, Badger GJ, et al. Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1 year of follow-up. J Consult Clin Psychol 2000;68:64–72.
68. Hartzler B, Garrett S. Interest and preferences for contingency management design among addiction treatment clientele. Am J Drug Alcohol Abuse 2016;42:287–95.
69. Petry NM, Barry D, Alessi SM, et al. A randomized trial adapting contingency management targets based on initial abstinence status of cocaine-dependent patients. J Consult Clin Psychol 2012;80:276–85.
70. Amass L, Kamien J. A tale of two cities: Financing two voucher programs for substance abusers through community donations. Exp Clin Psychopharmacol 2004;12:147–55.
71. Hartzler B. Building a bonfire that remains stoked: Sustainment of a contingency management intervention developed through collaborative design. Subst Abuse Treat Prev Policy 2015;10:30.
72. Carroll KM, Sinha R, Nich C, et al. Contingency management to enhance naltrexone treatment of opioid dependence: a randomized clinical trial of reinforcement magnitude. Exp Clin Psychopharmacol 2002;10:54–63.
73. Roll JM, Shoptaw S. Contingency management: Schedule effects. Psychiatry Res 2006;144:91–3.
74. Silverman K, Chutuape MA, Bigelow GE, Stitzer ML. Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: Effects of reinforcement magnitude. Psychopharmacology (Berl) 1999;146:128–38.
75. Businelle MS, Rash CJ, Burke RS, Parker JD. Using vouchers to increase continuing care participation in veterans: does magnitude matter? Am J Addict 2009;18:122–9.
76. Petry NM, Tedford J, Austin M, et al. Prize reinforcement contingency management for treating cocaine users: How low can we go, and with whom? Addiction 2004;99:349–60.
77. Alessi SM, Petry NM. A randomized study of cellphone technology to reinforce alcohol abstinence in the natural environment. Addiction 2013;108:900–9.
78. Alessi SM, Petry NM. Smoking reductions and increased self-efficacy in a randomized controlled trial of smoking abstinence--contingent incentives in residential substance abuse treatment patients. Nicotine Tob Res 2014;16:1436–45.
79. Dougherty DM, Hill-Kapturczak N, Liang Y, et al. Use of continuous transdermal alcohol monitoring during a contingency management procedure to reduce excessive alcohol use. Drug Alcohol Depend 2014;142:301–6.
80. Dallery J, Meredith S, Jarvis B, Nuzzo PA. Internet-based group contingency management to promote smoking abstinence. Exp Clin Psychopharmacol 2015;23:176–83.
81. Higgins ST, Washio Y, Lopez AA, et al. Examining two different schedules of financial incentives for smoking cessation among pregnant women. Prev Med (Baltim) 2014;68:51–7.
82. Higgins ST, Heil SH, Solomon LJ, et al. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine Tob Res 2004;6:1015–20.
83. Packer RR, Howell DN, McPherson S, Roll JM. Investigating reinforcer magnitude and reinforcer delay: A contingency management analog study. Exp Clin Psychopharmacol 2012;20:287–92.
84. Kirby KC, Benishek LA, Dugosh KL, Kerwin ME. Substance abuse treatment providers’ beliefs and objections regarding contingency management: Implications for dissemination. Drug Alcohol Depend 2006;85:19–27.
85. Cameron J, Ritter A. Contingency management: Perspectives of Australian service providers. 2007;26:183–9.
86. Hartzler B, Rabun C. Community opioid treatment perspectives on contingency management: Perceived feasibility, effectiveness, and transportability of social and financial incentives. J Subst Abuse Treat 2013;45:242–8.
87. Aletraris L, Shelton JS, Roman PM. Counselor attitudes toward contingency management for substance use disorder: Effectiveness, acceptability, and endorsement of incentives for treatment attendance and abstinence. J Subst Abuse Treat 2015;57:41–8.
88. Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. J Consult Clin Psychol 2000;68:1051–61.
89. Ledgerwood DM, Petry NM. Does contingency management affect motivation to change substance use? Drug Alcohol Depend 2006;83:65–72.
90. Litt MD, Kadden RM, Kabela-Cormier E, Petry NM. Coping skills training and contingency management treatments for marijuana dependence: Exploring mechanisms of behavior change. Addiction 2008;103:638–48.
91. Rash CJ, DePhilippis D, McKay JR, et al. Training workshops positively impact beliefs about contingency management in a nationwide dissemination effort. J Subst Abuse Treat 2013;45:306–12.
92. Benishek LA, Kirby KC, Dugosh KL, Padovano A. Beliefs about the empirical support of drug abuse treatment interventions: A survey of outpatient treatment providers. Drug Alcohol Depend 2010;107:202–8.
93. Petry NM, Alessi SM, Ledgerwood DM. Contingency management delivered by community therapists in outpatient settings. Drug Alcohol Depend 2012;122:86–92.
94. Petry NM, Alessi SM, Ledgerwood DM. A randomized trial of contingency management delivered by community therapists. J Consult Clin Psychol 2012;80:286–98.
95. Hartzler B, Beadnell B, Donovan D. Predictive validity of addiction treatment clinicians’ post-training contingency management skills for subsequent clinical outcomes. J Subst Abuse Treat 2017.
96. Hartzler B. Adapting the Helpful Responses Questionnaire to assess communication skills involved in delivering contingency management: Preliminary psychometrics. J Subst Abuse Treat 2014;55:52–7.
97. Petry NM, Alessi SM, Ledgerwood DM, Sierra S. Psychometric properties of the Contingency Management Competence Scale. Drug Alcohol Depend 2010;109:167–74.
1. Petry NM. A comprehensive guide to the application of contingency management procedures in clinical settings. Drug Alcohol Depend 2000;58:9–25.
2. Stitzer M, Petry N. Contingency management for treatment of substance abuse. Annu Rev Clin Psychol 2006;2:411–34.
3. Meredith SE, Jarvis BP, Raiff BR, et al. The ABCs of incentive-based treatment in health care: A behavior analytic framework to inform research and practice. Psychol Res Behav Manag 2014;7:103–14.
4. Bigelow GE, Silverman K. Theoretical and empirical foundations of contingency management treatments for drug abuse. In: Higgins ST, Silverman K, editors. Motivating behavior change among illicit drug users. Washington DC: American Psychological Association; 1999: 15–31.
5. Higgins ST, Budney AJ, Bickel WK. Applying behavioral concepts and principles to the treatment of cocaine dependence. Drug Alcohol Depend 1994;34:87–97.
6. Skinner BF. Science and human behavior. New York: Macmillan; 1953.
7. Herbeck DM, Hser YI, Teruya C. Empirically supported substance abuse treatment approaches: A survey of treatment providers’ perspectives and practices. Addict Behav 2008;33:699–712.
8. McGovern MP, Fox TS, Xie H, Drake RE. A survey of clinical practices and readiness to adopt evidence-based practices: Dissemination research in an addiction treatment system. J Subst Abuse Treat 2004;26:305–12.
9. Willenbring ML, Kivlahan D, Kenny M, et al. Beliefs about evidence-based practices in addiction treatment: A survey of Veterans Administration program leaders. J Subst Abuse Treat 2004;26:79–85.
10. Griffith JD, Rowan-Szal GA, Roark RR, Simpson DD. Contingency management in outpatient methadone treatment: A meta-analysis. Drug Alcohol Depend 2000;58:55–66.
11. Lussier JP, Heil SH, Mongeon JA, et al. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction 2006;101:192–203.
12. Benishek LA, Dugosh KL, Kirby KC, et al. Prize-based contingency management for the treatment of substance abusers: A meta-analysis. Addiction 2014;109:1426–36.
13. Prendergast M, Podus D, Finney J, et al. Contingency management for treatment of substance use disorders: A meta-analysis. Addiction 2006;101:1546–60.
14. Dutra L, Stathopoulou G, Basden SL, et al. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry 2008;165:179–87.
15. Petry NM, Peirce JM, Stitzer ML, et al. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: A National Drug Abuse Treatment Clinical Trials Network study. Arch Gen Psychiatry 2005;62:1148–56.
16. Peirce JM, Petry NM, Stitzer ML, et al. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment - A National Drug Abuse Treatment Clinical Trials Network study. Arch Gen Psychiatry 2006;63:201–8.
17. Petry NM, Martin B, Finocche C. Contingency management in group treatment: a demonstration project in an HIV drop-in center. J Subst Abuse Treat 2001;21:89–96.
18. Drebing CE, Van Ormer EA, Mueller L, et al. Adding contingency management intervention to vocational rehabilitation: outcomes for dually diagnosed veterans. J Rehabil Res Dev 2007;44:851–65.
19. Kellogg SH, Burns M, Coleman P, et al. Something of value: The introduction of contingency management interventions into the New York City Health and Hospital Addiction Treatment Service. J Subst Abuse Treat 2005;28:57–65.
20. Koffarnus MN, Wong CJ, Fingerhood M, et al. Monetary incentives to reinforce engagement and achievement in a job-skills training program for homeless, unemployed adults. J Appl Behav Anal 2013;46:582–91.
21. Rohsenow D, Martin R, Tidey JW, et al. Treating smokers in substance treatment with contingent vouchers, nicotine replacement, and brief advice adapted for sobriety settings. J Subst Abuse Treat 2017.
22. Hunt YM, Rash CJ, Burke RS, Parker JD. Smoking cessation in recovery: Comparing two different cognitive behavioral treatments. Addict Disord Their Treat 2010;9:64–74.
23. Alessi SM, Petry NM, Urso J. Contingency management promotes smoking reductions in residential substance abuse patients. J Appl Behav Anal 2008;41:617–22.
24. Petry NM, Weinstock J, Alessi SM, et al. Group-based randomized trial of contingencies for health and abstinence in HIV patients. J Consult Clin Psychol 2010;78:89.
25. Petry NM, Weinstock J, Alessi SM. A randomized trial of contingency management delivered in the context of group counseling. J Consult Clin Psychol 2011;79:686–96.
26. Ledgerwood DM, Alessi SM, Hanson T, et al. Contingency management for attendance to group substance abuse treatment administered by clinicians in community clinics. J Appl Behav Anal 2008;41:517–26.
27. Alessi SM, Hanson T, Wieners M, Petry NM. Low-cost contingency management in community clinics: Delivering incentives partially in group therapy. Exp Clin Psychopharmacol 2007;15:293–300.
28. Burch AE, Rash CJ, Petry NM. Sex effects in cocaine-using methadone patients randomized to contingency management interventions. Exp Clin Psychopharmacol 2015;23:284–90.
29. Rash CJ, Petry NM. Contingency management treatments are equally efficacious for both sexes in intensive outpatient settings. Exp Clin Psychopharmacol 2015;23:369–76.
30. Barry D, Sullivan B, Petry NM. Comparable efficacy of contingency management for cocaine dependence among African American, Hispanic, and White methadone maintenance clients. Psychol Addict Behav 2009;23:168–74.
31. Rash CJ, Alessi SM, Petry NM. Substance abuse treatment patients in housing programs respond to contingency management interventions. J Subst Abuse Treat 2017;72:97–102.
32. Rash CJ, Olmstead TA, Petry NM. Income does not affect response to contingency management treatments among community substance abuse treatment-seekers. Drug Alcohol Depend 2009;104:249–53.
33. Rash CJ, Andrade LF, Petry NM. Income received during treatment does not affect response to contingency management treatments in cocaine-dependent outpatients. Drug Alcohol Depend 2013;132:528–34.
34. Secades-Villa R, García-Fernández G, Peña-Suárez E, et al. Contingency management is effective across cocaine-dependent outpatients with different socioeconomic status. J Subst Abuse Treat 2013;44:349–54.
35. Rash CJ, Alessi SM, Petry NM. Cocaine abusers with and without alcohol dependence respond equally well to contingency management treatments. Exp Clin Psychopharmacol 2008;16:275–81.
36. Alessi SM, Rash C, Petry NM. Contingency management is efficacious and improves outcomes in cocaine patients with pretreatment marijuana use. Drug Alcohol Depend 2011;118:62–7.
37. Ford JD, Hawke J, Alessi S, et al. Psychological trauma and PTSD symptoms as predictors of substance dependence treatment outcomes. Behav Res Ther 2007;45:2417–31.
38. Weinstock J, Alessi SM, Petry NM. Regardless of psychiatric severity the addition of contingency management to standard treatment improves retention and drug use outcomes. Drug Alcohol Depend 2007;87:288–96.
39. García-Fernández G, Secades-Villa R, García-Rodríguez O, et al. Contingency management improves outcomes in cocaine-dependent outpatients with depressive symptoms. Exp Clin Psychopharmacol 2013;21:482–9.
40. Walter KN, Petry NM. Patients with diabetes respond well to contingency management treatment targeting alcohol and substance use. Psychol Health Med 2015;20:916–26.
41. Burch AE, Morasco BJ, Petry NM. Patients undergoing substance abuse treatment and receiving financial sssistance for a physical disability respond well to contingency management treatment. J Subst Abuse Treat 2015;58:67–71.
42. Burch AE, Rash CJ, Petry NM. Cocaine-using substance abuse treatment patients with and without HIV respond well to contingency management treatment. J Subst Abuse Treat 2017;77:21–5.
43. Rash CJ, Burki M, Montezuma-Rusca JM, Petry NM. A retrospective and prospective analysis of trading sex for drugs or money in women substance abuse treatment patients. Drug Alcohol Depend 2016;162:182–9.
44. Petry NM. Contingency management for substance abuse treatment: A guide to implementing this evidence-based practice. New York: Routledge; 2012.
45. Petry NM, Rash CJ, Byrne S, et al. Financial reinforcers for improving medication adherence: Findings from a meta-analysis. Am J Med 2012;125:888–96.
46. Herrmann ES, Matusiewicz AK, Stitzer ML, et al. Contingency management interventions for HIV, tuberculosis, and hepatitis control among individuals with substance use disorders: a systematized review. J Subst Abuse Treat 2017;72:117–25.
47. Petry NM, Alessi SM, Carroll KM, et al. Contingency management treatments: Reinforcing abstinence versus adherence with goal-related activities. J Consult Clin Psychol 2006;74:592–601.
48. Litt MD, Kadden RM, Petry NM. Behavioral treatment for marijuana dependence: Randomized trial of contingency management and self-efficacy enhancement. Addict Behav 2013;38:1764–75.
49. Kurti AN, Dallery J. A laboratory-based evaluation of exercise plus contingency management for reducing cigarette smoking. Drug Alcohol Depend 2014;144:201–9.
50. Weinstock J, Capizzi J, Weber SM, et al. Exercise as an intervention for sedentary hazardous drinking college students: A pilot study. Ment Health Phys Act 2014;7:55–62.
51. Mitchell MS, Goodman JM, Alter DA, et al. Financial incentives for exercise adherence in adults: Systematic review and meta-analysis. Am J Prev Med 2013;45:658–67.
52. Petry NM, Dephilippis D, Rash CJ, et al. Nationwide dissemination of contingency management: The Veterans Administration initiative. Am J Addict 2014;23:205–10.
53. Walker R, Rosvall T, Field CA, et al. Disseminating contingency management to increase attendance in two community substance abuse treatment centers: Lessons learned. J Subst Abuse Treat 2010;39:202–9.
54. Sigmon SC, Stitzer ML. Use of a low-cost incentive intervention to improve counseling attendance among methadone-maintained patients. J Subst Abuse Treat 2005;29:253–8.
55. Kropp F, Lewis D, Winhusen T. The effectiveness of ultra-low magnitude reinforcers: Findings from a “real-world” application of contingency management. J Subst Abuse Treat 2017;72:111–6.
56. Fitzsimons H, Tuten M, Borsuk C, et al. Clinician-delivered contingency management increases engagement and attendance in drug and alcohol treatment. Drug Alcohol Depend 2015;152:62–7.
57. Lott DC, Jencius S. Effectiveness of very low-cost contingency management in a community adolescent treatment program. Drug Alcohol Depend 2009;102:162–5.
58. Henggeler SW, Chapman JE, Rowland MD, et al. Statewide adoption and initial implementation of contingency management for substance-abusing adolescents. 2008;76:556–67.
59. Henggeler SW, Chapman JE, Rowland MD, et al. If you build it , they will come: Statewide practitioner interest in contingency management for youths. 2007;32:121–31.
60. Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes, and they will come: contingency management for treatment of alcohol dependence. J Consult Clin Psychol 2000;68:250–7.
61. Rash CJ, Petry NM, Alessi SM. Examining implementation of contingency management in real-world settings [Abstract]. Alcohol Clin Exp Res 2016;20:103A.
62. Rash CJ, Petry NM, Kirby KC, et al. Identifying provider beliefs related to contingency management adoption using the Contingency Management Beliefs Questionnaire. Drug Alcohol Depend 2012;121:205–12.
63. Petry NM, Alessi SM, Marx J, et al. Vouchers versus prizes: Contingency management treatment of substance abusers in community settings. J Consult Clin Psychol 2005;73:1005–14.
64. Petry NM, Alessi SM, Hanson T, Sierra S. Randomized trial of contingent prizes versus vouchers in cocaine-using methadone patients. J Consult Clin Psychol 2007;75:983–91.
65. Petry NM, Alessi SM, Barry D, Carroll KM. Standard magnitude prize reinforcers can be as efficacious as larger magnitude reinforcers in cocaine-dependent methadone patients. J Consult Clin Psychol 2015;83:464–72.
66. Higgins ST, Budney AJ, Bickel WK, et al. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Arch Gen Psychiatry 1994;51:568.
67. Higgins ST, Wong CJ, Badger GJ, et al. Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1 year of follow-up. J Consult Clin Psychol 2000;68:64–72.
68. Hartzler B, Garrett S. Interest and preferences for contingency management design among addiction treatment clientele. Am J Drug Alcohol Abuse 2016;42:287–95.
69. Petry NM, Barry D, Alessi SM, et al. A randomized trial adapting contingency management targets based on initial abstinence status of cocaine-dependent patients. J Consult Clin Psychol 2012;80:276–85.
70. Amass L, Kamien J. A tale of two cities: Financing two voucher programs for substance abusers through community donations. Exp Clin Psychopharmacol 2004;12:147–55.
71. Hartzler B. Building a bonfire that remains stoked: Sustainment of a contingency management intervention developed through collaborative design. Subst Abuse Treat Prev Policy 2015;10:30.
72. Carroll KM, Sinha R, Nich C, et al. Contingency management to enhance naltrexone treatment of opioid dependence: a randomized clinical trial of reinforcement magnitude. Exp Clin Psychopharmacol 2002;10:54–63.
73. Roll JM, Shoptaw S. Contingency management: Schedule effects. Psychiatry Res 2006;144:91–3.
74. Silverman K, Chutuape MA, Bigelow GE, Stitzer ML. Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: Effects of reinforcement magnitude. Psychopharmacology (Berl) 1999;146:128–38.
75. Businelle MS, Rash CJ, Burke RS, Parker JD. Using vouchers to increase continuing care participation in veterans: does magnitude matter? Am J Addict 2009;18:122–9.
76. Petry NM, Tedford J, Austin M, et al. Prize reinforcement contingency management for treating cocaine users: How low can we go, and with whom? Addiction 2004;99:349–60.
77. Alessi SM, Petry NM. A randomized study of cellphone technology to reinforce alcohol abstinence in the natural environment. Addiction 2013;108:900–9.
78. Alessi SM, Petry NM. Smoking reductions and increased self-efficacy in a randomized controlled trial of smoking abstinence--contingent incentives in residential substance abuse treatment patients. Nicotine Tob Res 2014;16:1436–45.
79. Dougherty DM, Hill-Kapturczak N, Liang Y, et al. Use of continuous transdermal alcohol monitoring during a contingency management procedure to reduce excessive alcohol use. Drug Alcohol Depend 2014;142:301–6.
80. Dallery J, Meredith S, Jarvis B, Nuzzo PA. Internet-based group contingency management to promote smoking abstinence. Exp Clin Psychopharmacol 2015;23:176–83.
81. Higgins ST, Washio Y, Lopez AA, et al. Examining two different schedules of financial incentives for smoking cessation among pregnant women. Prev Med (Baltim) 2014;68:51–7.
82. Higgins ST, Heil SH, Solomon LJ, et al. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine Tob Res 2004;6:1015–20.
83. Packer RR, Howell DN, McPherson S, Roll JM. Investigating reinforcer magnitude and reinforcer delay: A contingency management analog study. Exp Clin Psychopharmacol 2012;20:287–92.
84. Kirby KC, Benishek LA, Dugosh KL, Kerwin ME. Substance abuse treatment providers’ beliefs and objections regarding contingency management: Implications for dissemination. Drug Alcohol Depend 2006;85:19–27.
85. Cameron J, Ritter A. Contingency management: Perspectives of Australian service providers. 2007;26:183–9.
86. Hartzler B, Rabun C. Community opioid treatment perspectives on contingency management: Perceived feasibility, effectiveness, and transportability of social and financial incentives. J Subst Abuse Treat 2013;45:242–8.
87. Aletraris L, Shelton JS, Roman PM. Counselor attitudes toward contingency management for substance use disorder: Effectiveness, acceptability, and endorsement of incentives for treatment attendance and abstinence. J Subst Abuse Treat 2015;57:41–8.
88. Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. J Consult Clin Psychol 2000;68:1051–61.
89. Ledgerwood DM, Petry NM. Does contingency management affect motivation to change substance use? Drug Alcohol Depend 2006;83:65–72.
90. Litt MD, Kadden RM, Kabela-Cormier E, Petry NM. Coping skills training and contingency management treatments for marijuana dependence: Exploring mechanisms of behavior change. Addiction 2008;103:638–48.
91. Rash CJ, DePhilippis D, McKay JR, et al. Training workshops positively impact beliefs about contingency management in a nationwide dissemination effort. J Subst Abuse Treat 2013;45:306–12.
92. Benishek LA, Kirby KC, Dugosh KL, Padovano A. Beliefs about the empirical support of drug abuse treatment interventions: A survey of outpatient treatment providers. Drug Alcohol Depend 2010;107:202–8.
93. Petry NM, Alessi SM, Ledgerwood DM. Contingency management delivered by community therapists in outpatient settings. Drug Alcohol Depend 2012;122:86–92.
94. Petry NM, Alessi SM, Ledgerwood DM. A randomized trial of contingency management delivered by community therapists. J Consult Clin Psychol 2012;80:286–98.
95. Hartzler B, Beadnell B, Donovan D. Predictive validity of addiction treatment clinicians’ post-training contingency management skills for subsequent clinical outcomes. J Subst Abuse Treat 2017.
96. Hartzler B. Adapting the Helpful Responses Questionnaire to assess communication skills involved in delivering contingency management: Preliminary psychometrics. J Subst Abuse Treat 2014;55:52–7.
97. Petry NM, Alessi SM, Ledgerwood DM, Sierra S. Psychometric properties of the Contingency Management Competence Scale. Drug Alcohol Depend 2010;109:167–74.
Flashback to 2016
For the final installment of this series, we “flashback” to our April 2016 issue, which featured a study examining 30-day complications among commercially insured adults undergoing colonoscopy with and without anesthesia-assisted sedation using Marketscan data (2008-2011).
Several letters to the editor challenged the methods used in this systematic review/meta-analysis, such that this question remains largely unresolved. What is clear is that we continue to lack an adequate understanding of which patients are most likely to benefit from anesthesia-assisted sedation, whether due to increased risk of failing standard sedation or increased risk of complications with standard sedation. This lack of clarity, as manifested in poorly specified guidelines, has fueled likely inappropriate allocation of monitored anesthesia care to low risk-patients (driven by a complex interplay of patient, provider, organizational, and economic factors), which has contributed to ballooning health care costs
Megan A. Adams, MS, JD, MSc, is a clinical lecturer in the division of gastroenterology at the University of Michigan, a gastroenterologist at the Ann Arbor Mich, VA, and an investigator in the VA Ann Arbor Center for Clinical Management Research. She is an associate editor of GI & Hepatology News.
For the final installment of this series, we “flashback” to our April 2016 issue, which featured a study examining 30-day complications among commercially insured adults undergoing colonoscopy with and without anesthesia-assisted sedation using Marketscan data (2008-2011).
Several letters to the editor challenged the methods used in this systematic review/meta-analysis, such that this question remains largely unresolved. What is clear is that we continue to lack an adequate understanding of which patients are most likely to benefit from anesthesia-assisted sedation, whether due to increased risk of failing standard sedation or increased risk of complications with standard sedation. This lack of clarity, as manifested in poorly specified guidelines, has fueled likely inappropriate allocation of monitored anesthesia care to low risk-patients (driven by a complex interplay of patient, provider, organizational, and economic factors), which has contributed to ballooning health care costs
Megan A. Adams, MS, JD, MSc, is a clinical lecturer in the division of gastroenterology at the University of Michigan, a gastroenterologist at the Ann Arbor Mich, VA, and an investigator in the VA Ann Arbor Center for Clinical Management Research. She is an associate editor of GI & Hepatology News.
For the final installment of this series, we “flashback” to our April 2016 issue, which featured a study examining 30-day complications among commercially insured adults undergoing colonoscopy with and without anesthesia-assisted sedation using Marketscan data (2008-2011).
Several letters to the editor challenged the methods used in this systematic review/meta-analysis, such that this question remains largely unresolved. What is clear is that we continue to lack an adequate understanding of which patients are most likely to benefit from anesthesia-assisted sedation, whether due to increased risk of failing standard sedation or increased risk of complications with standard sedation. This lack of clarity, as manifested in poorly specified guidelines, has fueled likely inappropriate allocation of monitored anesthesia care to low risk-patients (driven by a complex interplay of patient, provider, organizational, and economic factors), which has contributed to ballooning health care costs
Megan A. Adams, MS, JD, MSc, is a clinical lecturer in the division of gastroenterology at the University of Michigan, a gastroenterologist at the Ann Arbor Mich, VA, and an investigator in the VA Ann Arbor Center for Clinical Management Research. She is an associate editor of GI & Hepatology News.
Oral semaglutide achieves outcomes similar to those of subcutaneous semaglutide
, according to a phase 2, placebo-controlled trial published in the Oct. 17 edition of JAMA.
In a parallel-group, dosage-finding 26-week trial, 632 patients with type 2 diabetes and poor glycemic control were randomized to either 2.5 mg, 5 mg, 10 mg, 20 mg, or 40 mg of once-daily oral semaglutide escalated over 4 weeks (standard escalation); 40 mg escalated over 8 weeks; 40 mg escalated over 2 weeks; once-weekly subcutaneous semaglutide (1 mg) for 26 weeks, or oral placebo.
The study found that all patients who had received oral or subcutaneous semaglutide showed significantly reduced mean hemoglobin A1c levels compared with placebo. Almost all patients treated with semaglutide – both oral and subcutaneous – showed a reduction in HbA1c levels while only 74% of patients in the placebo group did (JAMA 2017 Oct 17;318[15]:1460-70. doi: 10.1001/jama.2017.14752).
The estimated treatment difference compared with placebo at week 26 ranged from –0.4% for the 2.5-mg group to –1.6% for the 40-mg-over-4-weeks group, and –1.6% for the subcutaneous group.
Researchers also saw a dose-dependent decrease in mean body weight from baseline to 26 weeks, ranging from –0.9 kg in the 2.5-mg group to –5.7 kg in the 40-mg standard escalation group, compared with –1.2 kg in the placebo group. The difference between treatment and placebo was significant only for doses at or above 10 mg.
The most common adverse events reported were mild to moderate gastrointestinal problems, mostly associated with oral and subcutaneous semaglutide, which are a known side effect of GLP-1 receptor agonists. There was also a higher rate of premature discontinuation of treatment due to gastrointestinal effects in the semaglutide-treated patients.
However, the overall rate of hypoglycemic episodes was low, and was similar in both semaglutide groups and the placebo group.
There were three cases of acute pancreatitis in patients treated with semaglutide, and the treatment was also associated with a significant increase in heart rate, compared with placebo.
“A longer study duration may have demonstrated the maximum HbA1c level and weight reductions in the groups administered the higher doses of the medication,” wrote Melanie Davies, MD, professor of diabetes medicine at the University of Leicester, England, and her coauthors. “Future trials should assess the efficacy of oral semaglutide in patients with a high baseline HbA1c level to explore its potential in patients who are less well controlled, and in combination with other glucose-lowering agents.”
Semaglutide manufacturer Novo Nordisk provided editorial support. Three authors declared board membership and consultancy fees from the company, as well as institutional grants, lecture fees, and other funding from other pharmaceutical companies. Two authors declared shares in Novo Nordisk. One author declared a patent relating to semaglutide, and two authors declared funding from pharmaceutical companies including Novo Nordisk.
, according to a phase 2, placebo-controlled trial published in the Oct. 17 edition of JAMA.
In a parallel-group, dosage-finding 26-week trial, 632 patients with type 2 diabetes and poor glycemic control were randomized to either 2.5 mg, 5 mg, 10 mg, 20 mg, or 40 mg of once-daily oral semaglutide escalated over 4 weeks (standard escalation); 40 mg escalated over 8 weeks; 40 mg escalated over 2 weeks; once-weekly subcutaneous semaglutide (1 mg) for 26 weeks, or oral placebo.
The study found that all patients who had received oral or subcutaneous semaglutide showed significantly reduced mean hemoglobin A1c levels compared with placebo. Almost all patients treated with semaglutide – both oral and subcutaneous – showed a reduction in HbA1c levels while only 74% of patients in the placebo group did (JAMA 2017 Oct 17;318[15]:1460-70. doi: 10.1001/jama.2017.14752).
The estimated treatment difference compared with placebo at week 26 ranged from –0.4% for the 2.5-mg group to –1.6% for the 40-mg-over-4-weeks group, and –1.6% for the subcutaneous group.
Researchers also saw a dose-dependent decrease in mean body weight from baseline to 26 weeks, ranging from –0.9 kg in the 2.5-mg group to –5.7 kg in the 40-mg standard escalation group, compared with –1.2 kg in the placebo group. The difference between treatment and placebo was significant only for doses at or above 10 mg.
The most common adverse events reported were mild to moderate gastrointestinal problems, mostly associated with oral and subcutaneous semaglutide, which are a known side effect of GLP-1 receptor agonists. There was also a higher rate of premature discontinuation of treatment due to gastrointestinal effects in the semaglutide-treated patients.
However, the overall rate of hypoglycemic episodes was low, and was similar in both semaglutide groups and the placebo group.
There were three cases of acute pancreatitis in patients treated with semaglutide, and the treatment was also associated with a significant increase in heart rate, compared with placebo.
“A longer study duration may have demonstrated the maximum HbA1c level and weight reductions in the groups administered the higher doses of the medication,” wrote Melanie Davies, MD, professor of diabetes medicine at the University of Leicester, England, and her coauthors. “Future trials should assess the efficacy of oral semaglutide in patients with a high baseline HbA1c level to explore its potential in patients who are less well controlled, and in combination with other glucose-lowering agents.”
Semaglutide manufacturer Novo Nordisk provided editorial support. Three authors declared board membership and consultancy fees from the company, as well as institutional grants, lecture fees, and other funding from other pharmaceutical companies. Two authors declared shares in Novo Nordisk. One author declared a patent relating to semaglutide, and two authors declared funding from pharmaceutical companies including Novo Nordisk.
, according to a phase 2, placebo-controlled trial published in the Oct. 17 edition of JAMA.
In a parallel-group, dosage-finding 26-week trial, 632 patients with type 2 diabetes and poor glycemic control were randomized to either 2.5 mg, 5 mg, 10 mg, 20 mg, or 40 mg of once-daily oral semaglutide escalated over 4 weeks (standard escalation); 40 mg escalated over 8 weeks; 40 mg escalated over 2 weeks; once-weekly subcutaneous semaglutide (1 mg) for 26 weeks, or oral placebo.
The study found that all patients who had received oral or subcutaneous semaglutide showed significantly reduced mean hemoglobin A1c levels compared with placebo. Almost all patients treated with semaglutide – both oral and subcutaneous – showed a reduction in HbA1c levels while only 74% of patients in the placebo group did (JAMA 2017 Oct 17;318[15]:1460-70. doi: 10.1001/jama.2017.14752).
The estimated treatment difference compared with placebo at week 26 ranged from –0.4% for the 2.5-mg group to –1.6% for the 40-mg-over-4-weeks group, and –1.6% for the subcutaneous group.
Researchers also saw a dose-dependent decrease in mean body weight from baseline to 26 weeks, ranging from –0.9 kg in the 2.5-mg group to –5.7 kg in the 40-mg standard escalation group, compared with –1.2 kg in the placebo group. The difference between treatment and placebo was significant only for doses at or above 10 mg.
The most common adverse events reported were mild to moderate gastrointestinal problems, mostly associated with oral and subcutaneous semaglutide, which are a known side effect of GLP-1 receptor agonists. There was also a higher rate of premature discontinuation of treatment due to gastrointestinal effects in the semaglutide-treated patients.
However, the overall rate of hypoglycemic episodes was low, and was similar in both semaglutide groups and the placebo group.
There were three cases of acute pancreatitis in patients treated with semaglutide, and the treatment was also associated with a significant increase in heart rate, compared with placebo.
“A longer study duration may have demonstrated the maximum HbA1c level and weight reductions in the groups administered the higher doses of the medication,” wrote Melanie Davies, MD, professor of diabetes medicine at the University of Leicester, England, and her coauthors. “Future trials should assess the efficacy of oral semaglutide in patients with a high baseline HbA1c level to explore its potential in patients who are less well controlled, and in combination with other glucose-lowering agents.”
Semaglutide manufacturer Novo Nordisk provided editorial support. Three authors declared board membership and consultancy fees from the company, as well as institutional grants, lecture fees, and other funding from other pharmaceutical companies. Two authors declared shares in Novo Nordisk. One author declared a patent relating to semaglutide, and two authors declared funding from pharmaceutical companies including Novo Nordisk.
FROM JAMA
Key clinical point: Oral semaglutide achieves similar glucose control in patients with type 2 diabetes when compared with subcutaneous semaglutide.
Major finding: The estimated treatment difference in HbA1c compared with placebo ranged from –0.4% to –1.6% in the oral semaglutide group, and –1.6% for the subcutaneous group
Data source: A phase 2, randomized, parallel-group, dosage-finding trial of 632 patients with type 2 diabetes.
Disclosures: Semaglutide manufacturer Novo Nordisk provided editorial support. Three authors declared board membership and consultancy fees from the company, as well as institutional grants, lecture fees and other funding from other pharmaceutical companies. Two authors declared shares in Novo Nordisk. One author declared a patent relating to semaglutide, and two authors declared funding from pharmaceutical companies including Novo Nordisk.
Management of Patients with HIV and Hepatitis B Coinfection
From UT Southwestern Medical Center, Dallas, TX.
Abstract
- Objective: To review the literature on and provide evidence-based recommendations for management of HIV/ hepatitis B (HBV) coinfection.
- Methods: Review of the literature for clinical trials, guidelines, and cohort studies on HIV/HBV disease management.
- Results: HIV patients should be evaluated for viral hepatitis. Those who do not have evidence of immunity should be vaccinated and monitored for response. Those who have HIV/HBV should have additional serologies checked to evaluate for hepatitis B e antigen status and level of viremia. All HIV/HBV coinfected patients should be started on antiretroviral therapy with tenofovir-based regimens. Those with HIV/HBV and cirrhosis should be screened for hepatocellular cancer every 6 months.
- Conclusion: HIV patients should be vaccinated against hepatitis B; those with coinfection should be treated for both viruses. It is important to monitor for treatment response to both HIV and HBV and liver disease complications.
Key words: incentives; reinforcement; substance abuse treatment; dissemination; implementation.
Morbidity and mortality for HIV-infected patients remain high compared to uninfected patients despite effective virologic suppression. Major contributors to illness and death among these patients include cardiovascular disease, non–AIDS-defining malignancies, and chronic liver disease, specifically viral hepatitis [1]. Hepatitis B virus (HBV) infection is one of the main causes of chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma (HCC) globally. Because HIV and HBV can both be acquired through injection drug use and sexual transmission, coinfection occurs frequently. The Joint United Nations Program on HIV/AIDS estimates that 10% of the 33 million HIV-positive patients worldwide have simultaneous chronic HBV infection [2].
HIV/HBV coinfection significantly impacts the natural history, progression, and mortality related to both viruses. HIV infection accelerates HBV-related liver impairment, leading to earlier cirrhosis, end-stage liver disease, and HCC. Conversely, chronic HBV does not have a considerable influence in the progression of HIV; however, antiretroviral treatment (ART) toxicities and/or HBV flares due to immune reconstitution inflammatory syndrome (IRIS) or HBV itself can lead to increased liver-related complications [3,4].
Case Patient 1
Initial Presentation and History
An asymptomatic 38-year-old man diagnosed with HIV infection 1 month ago presents for his initial visit to establish HIV care. The patient is a man who has sex with men (MSM) and is currently sexually active with multiple partners. He reports inconsistent use of condoms. One month ago he underwent routine screening and was found to be HIV-positive. At the time of diagnosis, the patient’s baseline CD4 cell count was 328 cells/µL and his viral load was 182,600 copies/mL. The patient wants to discuss the implications of his new diagnosis of HIV and recommendations for further testing and treatment. He is especially interested in HBV screening, since one of his recent partners was known to be positive. The patient has no relevant past medical history. He does not recall the details of his childhood vaccinations. He denies smoking and injection drug use but reports moderate alcohol consumption.
Physical Examination and Laboratory Testing
Physical examination is normal. Laboratory studies show normal complete blood count (CBC) and renal and liver function test results, and a baseline HIV genotype does not show resistance. Hepatitis A total antibody (anti-HAV) testing is positive, while tests for hepatitis B surface antigen (HBsAg), antibody to hepatitis B surface antigen (anti-HBs), antibody to hepatitis B core antigen (anti-HBc), and hepatitis C antibody (anti-HCV) are negative.
• Which screening tests for HBV should be performed in HIV-infected patients?
Routine screening of all HIV-infected patients for hepatitis A virus, HBV, and hepatitis C virus (HCV) is recommended [5]. Routine HBV screening involves obtaining serologies for HBsAg, anti-HBs, and anti-HBc. With these results, patients can be classified into categories of either active infection, immunity, or no evidence of prior exposure (Table 1).
• How effective is HBV vaccination in the HIV population?
Vaccination
Available HBV vaccines in the United States include 2 single-agent vaccines (Recombivax HB [Merck, Whitehouse Station, NJ] and Engerix-B [GlaxoSmithKline, Research Triangle Park, NC]) as well as a combination HAV/HBV vaccine (Twinrix [GlaxoSmithKline]). For adults (age ≥ 20 years) with an immunocompromising condition such as HIV infection, current Centers for Disease Control and Prevention guidelines recommend three 40 µg/mL doses of single-agent vaccine administered at 0, 1, and 6 months (Recombivax HB), or four 40 µg/mL doses of single-agent vaccine (2 doses of 20 µg/mL administered simultaneously) at 0, 1, 2, and 6 months (Engerix-B) [7].
The immunogenicity to HBV vaccination in HIV-positive patients is decreased, reflected by lower antibody titers, waning immunity, and seroconversion rates of 18% to 65% [8–11]. Factors associated with poor response include low CD4 cell counts, detectable HIV RNA, coinfection with HCV, and the general health status of the patient [8]. Ideally, HBV vaccination should occur prior to decline in CD4 cell count below 350 cells/µL. However, guidelines do not recommend deferring vaccination since some patients with advanced HIV disease will seroconvert [6].
Anti-HBs titers should be checked 1 month after completion of the vaccine series to confirm protective antibody titers. For patients with quantitative anti-HBs levels < 10 IU/mL, a second vaccine cycle is recommended. Some specialists may defer revaccination until a sustained increase in CD4 count is achieved on ART.
Two randomized controlled trials have demonstrated that 4 doses of double-dose (40 µg/mL) vaccine generate higher anti-HBs levels than 3 doses of standard-dose (20 µg/mL) vaccine in HIV-infected adults [12,13]. Another study showed that HIV patients with CD4 counts > 350 cells/µL had better responses when immunized with double- dose vaccines on the usual 3-dose series [14]. Currently, the CDC recommends giving a double dose for either a 3-dose schedule or a 4-dose schedule. However, it remains unclear what dosing schedule to use if a patient fails to respond. Likely waiting until the CD4 cell count has increased and HIV viral load is suppressed will be important to seeing a response.
• What approach for HBV prevention should be taken in this patient?
The patient’s serologies confirm no prior exposure to HBV, and he should be offered HBV vaccination. His CD4 cell count is below 350/µL and he has ongoing HIV viremia, which increases his risk for an inadequate response. However, vaccination should not be delayed, particularly given his high risk of sexual transmission. The patient should be counseled regarding all high-risk behaviors. As discussed above, 3 or 4 doses of the higher dose vaccine (40 µg/mL) should be administered depending on what type of recombinant vaccine is available. An anti-HBs level should be checked 1 month after completion. A full repeat vaccine series using the 40 µg/mL dose should be considered for nonresponders who initially received a standard vaccine series. Experts also recommend checking annual anti-HBs levels to monitor for waning immunity, with a booster dose given if the anti-HBs level drops below the protective range.
• What vaccination strategy should be used in patients with isolated positive anti-HBc?
The clinical implications of an isolated positive anti-HBc for vaccination are still uncertain. This serologic pattern may represent a false-positive test, remote HBV infection with loss of anti-HBs, or occult HBV infection with undetectable HBsAg. The latter scenario appears more commonly in HIV-infected patients, particularly with concomitant HCV infection [15].
A recent study suggested that patients with an isolated positive anti-HBc with a negative anamnestic antibody response (anti-HBs titer of < 10 IU/mL 4 weeks after a single 20 µg dose of recombinant HBV vaccine) should be further vaccinated with the double-dose for a 3-dose schedule [6]. Additionally, another study followed HIV/HCV coinfected patients for 9.5 years and found that an isolated positive anti-HBc was not associated with accelerated liver disease progression [16].
Treatment of HIV-1 Infection
Current HIV guidelines recommend initiation of ART for all HIV-infected patients regardless of their CD4 count [17]. ART for a treatment-naïve patient usually consists of 2 nucleotide/nucleoside reverse-transcriptase inhibitors (NRTIs; the “backbone”) combined with a third agent (the “anchor”), which can be a nonnucleoside reverse-transcriptase inhibitor (NNRTI), a protease inhibitor (PI) boosted with a boosting agent, or an integrase strand-transfer inhibitor (INSTI) [18,19].
There are numerous studies indicating that incident HBV risk can be reduced by placing those at risk for HBV acquisition on ART containing a combination of tenofovir disoproxil fumarate (TDF), lamivudine, or emtricitabine [20,21]. Another study in MSM found those on ART with HIV viral load < 400 copies/mL were protected from developing HBV compared to those not on ART [22]. Given this patient’s higher risk for HBV acquisition, placing him on emtricitabine/TDF backbone as part of ART could be protective against incident HBV [23].
Case Patient 2
Initial Presentation and History
A 32-year-old man diagnosed with HIV infection 8 years ago now on ART presents for follow-up. The patient is an MSM with a history of inconsistent condom use. At the time of HIV diagnosis 8 years ago, the patient had a CD4 cell count of 250 cells/µL and an HIV viral load of 648,000 copies/mL. The patient was initiated on lamivudine/zidovudine and lopinavir/ritonavir, and he achieved complete virologic suppression at 20 weeks, with a CD4 cell count of 455 cells/µL at 1 year. The patient has remained on this regimen without major side effects; however, he reports frequent missed doses over the last 2 years due to “pill fatigue.” Previous testing for HBV and HCV at the time of his initial HIV diagnosis was negative, but he failed to complete the HBV vaccine series. He denies alcohol or injection drug use.
Physical Examination and Laboratory Testing
Physical examination is normal. Laboratory studies reveal normal electrolytes and renal function, hemoglobin of 11 g/dL, and platelet count of 235,000/µL. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels are 34 U/L and 44 U/L, respectively, with an INR of 1.1 and albumin level of 3.4 g/dL. CD4 cell count is 320 cells/µL with an HIV viral load of 24,500 copies/mL. Viral hepatitis serologies show anti-HAV positive, anti-HCV negative, HBsAg positive, anti-HBc IgG positive, hepatitis Be antigen (HBeAg) negative, and hepatitis B e antibody (anti-HBe) positive. HBV DNA viral load is 685,000 IU/mL.
• What is the natural history of HIV/HBV coinfection?
Chronic HBV infection affects about 10% of HIV-infected patients globally. Epidemiologic studies indicate that HIV-infected patients have higher rates of reactivation and progression to chronic HBV infection and chronic liver disease than HIV-negative patients [24–26]. Coinfected patients demonstrate higher serum HBV DNA levels, which lead to more rapid progression of hepatic fibrosis and may increase the risk of cirrhosis and HCC [24,27,28]. HIV infection, however, can mediate the necroinflammatory response through blunting of the immune response that drives pathogenesis in HBV infection. Aminotransferase levels may be only slightly elevated or even normal, particularly in the severely immune suppressed. However, elevation in liver enzymes (hepatitis flare) can occur if a patient stops ART, or if HBV resistance develops. Patients being treated for HIV/HBV coinfection should be counseled that stopping HIV treatment puts them at risk of developing a hepatitis flare.
Large cohort studies of HIV/HBV coinfected patients indicate increased risk of liver-related mortality, most pronounced with lower CD4 cell counts [1,28,29]. Introduction of ART appears to increase rather than attenuate this liver-related mortality, possibly by decreasing AIDS-related mortality and allowing more time for liver disease progression. Finally, a recent meta-analysis including 12,382 patients demonstrated a significant effect of HIV/HBV coinfection on all-cause mortality, with a pooled effect estimate of 1.36 [30].
• What diagnostic testing should be done in coinfected patients?
Diagnostic Testing and Evaluation
Persistence of HBsAg for more than 6 months is diagnostic of chronic HBV infection and warrants further serologic evaluation. Patients with chronic HBV infection should be tested for HBeAg, anti-HBe, and HBV DNA levels and have AST and ALT levels measured. Elevation of AST and ALT can be seen with untreated HBV. For those on treatment, a hepatitis flare could be caused by abrupt discontinuation of HBV treatment, development of HBV resistance, or superinfection with another viral pathogen.
HBeAg is a marker of active viral replication and is associated with higher levels of HBV DNA and active liver disease. Seroconversion, or loss of HBeAg and development of anti-HBe, heralds a favorable treatment response for those who were initially HBeAg-positive. However, some HBV variants have precore or core promoter mutations that lead to higher levels of HBV DNA in the absence of HBeAg. This highlights the importance of monitoring HBV DNA levels in all patients with chronic HBV infection. Favorable response to therapy in HBeAg-negative patients is marked by normalization of aminotransferases and HBV DNA suppression.Hepatitis D virus (HDV) is a defective virus particle that can only replicate in the presence of HBV. Coinfected patients should be tested for anti-HDV if they are injection drug users or are from a high-prevalence region such as the Mediterranean and Amazon basins. Newly acquired HDV infection should also be considered in the context of hepatitis flares.
Although these routine diagnostic tests are essential for management, studies show low adherence rates to HBV testing guidelines by HIV providers [31,32].
• What is the role of HBV genotype and resistance testing?
HBV can be classified into 10 different genotypes, A through J, based on genomic sequence variations. Each genotype has a distinct ethnic and geographic distribution, with genotypes A and D predominating in North America. HBV genotyping appears to have important prognostic as well as treatment implications [19]. However, data are still preliminary and the guidelines do not recommend routine genotype testing.
Resistance testing in HBV allows for detection of mutations that decrease effectiveness of antivirals. Exposure to lamivudine can lead to mutations in the YMDD region of the HBV DNA polymerase, resulting in drug resistance. Resistance to lamivudine develops at a rate of approximately 25% after 1 year of drug exposure in HIV/HBV coinfection [33]. On the other hand, studies have shown that entecavir is active against HIV and, most importantly, selects for the M184V mutation in HIV. M184V is associated with lamivudine resistance for HIV treatment, thus limiting treatment options [34,35]. After these findings, the FDA advised against monotherapy with entecavir in patients with HIV/HBV coinfection. The case patient was on lamivudine during the time of HBV acquisition, and therefore YMDD mutation must be taken into consideration for therapy purposes. He should be switched to a regimen that suppresses both viruses. The development of drug resistance should be assessed in all patients with persistent or breakthrough HBV viremia on ART, particularly the nucleoside analogues. HIV providers treating HIV/HBV coinfection should regularly monitor both HIV and HBV viral load to assess for therapeutic efficacy.
• What is the role of liver biopsy in this patient?
Liver biopsy should be considered in all coinfected patients as it remains the gold standard for determining the activity and severity of chronic hepatitis B. However, because it carries inherent risks and is not required prior to treatment in all patients, the decision should be individualized. Liver biopsy can be useful to assess baseline liver histology and may be warranted to rule out significant coexisting genetic or metabolic liver disease. Currently, noninvasive methods to assess liver fibrosis either using elastography or various combinations of serum biomarkers are being evaluated [36] and may be considered in lieu of a liver biopsy [37]. One study compared the accuracy of elastography with liver biopsy in HIV/HBV coinfected patients and demonstrated that the former was proficient in discriminating between absence or mild fibrosis and moderate to severe fibrosis [38]. In general, this test has high accuracy in detecting minimal fibrosis from advanced fibrosis or cirrhosis. For the group in the middle, further investigation with additional methods must be considered [37]. Finally, a recent retrospective study involving 70 HIV/HBV coinfected individuals showed fibrosis regression suggesting beneficial effects of long-term ART on liver stiffness, [39] but further studies are needed to confirm these findings.
Case 2 Continued
The patient has now been diagnosed with chronic HBV infection. His diagnostic testing is negative for HBeAg and reveals modest HBV viremia with abnormal aminotransferases less than 2 times the upper limit of normal. Drug resistance testing reveals a mutation in the YMDD region indicative of HBV lamivudine resistance. HIV genotype demonstrates a wild-type virus without resistance. The patient asks what treatment options exist.
• What medications are currently available to treat hepatitis B?
Treatment
All patients with HIV/HBV coinfection should receive treatment to suppress both viruses, and ART needs to include 2 drugs active against HBV, ideally emtricitabine and TDF. This approach prevents drug resistance, slows progression of HBV infection, and reduces the incidence of IRIS [5]. TDF has been associated with decreased renal function and bone mineral density. Recently, tenofovir alafenamide fumarate (TAF) was approved for the treatment of HIV and HBV. A dose of 10 mg daily is given when coadministered with ritonavir, cobicistat, or protease inhibitors, but a dose of 25 mg should be given when administered with NNRTIs or integrase inhibitors. Compared to TDF, TAF shows less accumulation of tenofovir in kidneys and bones and consequently has reduced renal and bone mineral density effects [40]. All patients should be on a TDF- or TAF-based HIV regimen if they have chronic HBV.
Currently, the following antiviral drugs are FDA-approved for the treatment of HBV infection: interferon alfa-2b, pegylated interferon (peginterferon) alfa-2a, lamivudine and emtricitabine, entecavir, adefovir, TDF, TAF, and telbivudine. Telbivudine and adefovir are no longer recommended due to their association with higher incidence of toxicity and higher rates of HBV treatment failure. A summary of available HBV treatment options is outlined in Table 2.
Interferon
Standard interferon alfa-2b blocks HBV replication through interaction with viral proteins and stimulation of host cellular immunity. Peginterferon alfa-2a has proven efficacy in HBV-monoinfected patients, but efficacy data in HIV/HBV coinfected patients is lacking [41,42]. Studies in hepatitis C treatment demonstrate the safety of peginterferon alfa-2a use in HIV-positive patients and indicate that HIV viral suppression occurs with peginterferon without evidence of selection of resistance mutations that affect future ART options [43]. For HIV/HBV coinfected patients not on ART who will receive only therapy for HBV (which is infrequent since all HIV patients should be on ART), pegylated interferon-alfa-2a alone for 48 weeks is the only option that will not cause ART-associated HIV drug resistance [5]. Interferon therapy is contraindicated in patients with decompensated cirrhosis and should be used with caution in patients with active depression, uncontrolled diabetes, and cardiac and pulmonary disease.
Lamivudine and Emtricitabine
Lamivudine is a nucleoside analogue with efficacy against both HIV and HBV. Clinical trials in HIV/HBV-infected patients have shown up to 87% of patients achieve undetectable HBV DNA levels and about 25% achieve HBeAg seroconversion after 1 to 2 years of therapy [44,45]. However, the major issue limiting use of lamivudine is its low genetic barrier to resistance. Mutation of the YMDD motif of the HBV DNA polymerase confers HBV resistance. HIV/HBV coinfected patients develop resistance at rates of up to 94% after 4 years of therapy [33], heralded by rebounds in HBV DNA levels and often hepatitis flares or precipitation of hepatic failure [16]. Because of resistance, lamivudine monotherapy should be avoided; even in patients on ART, abrupt withdrawal of lamivudine or the development of HBV resistance should be closely monitored.
Emtricitabine is another nucleoside analogue with properties and efficacy similar to lamivudine. It is frequently used as a combination pill with TDF (Truvada) in coinfected patients. The same concerns regarding monotherapy and the emergence of resistance that exist for lamivudine apply to emtricitabine.
Tenofovir
TDF, a nucleotide analogue, is one of the preferred first-line agents for HIV treatment and has proven efficacy against both wild-type and lamivudine-resistant HBV. Since it was first used for HIV, TDF has been more extensively studied in coinfected patients compared to most other agents. In a meta-analysis of patients with HIV/HBV coinfection, TDF suppressed HBV viral load to undetectable titers in approximately 90% of patients [46]. Tenofovir is available in 2 preparations: TDF and TAF. TDF has been reported to cause renal impairment as well as bone loss. TAF has shown less renal toxicity and less bone damage [40,47]. In 2016, TAF became available as part as 4 regimens: stand-alone TAF, elvitegravir-cobicistat-emtricitabine-TAF, rilpivirine-emtricitabine-TAF, and TAF-emtricitabine.
TDF and TAF both suppress HIV. Two large randomized trials of HBV monoinfection demonstrate that TAF is noninferior to TDF for the treatment of naïve and treatment-experienced patients [48,49].
Entecavir
Entecavir, a guanosine analogue, is a potent HBV DNA polymerase inhibitor that results in greater virologic suppression compared to lamivudine and retains activity against lamivudine-resistant HBV [50]. Importantly, entecavir shares some cross-resistance with lamivudine, so an entecavir dose of 1 mg daily is recommended in lamivudine-experienced patients compared to 0.5 mg daily in lamivudine-naïve patients. Entecavir requires dose reduction for patients with creatinine clearance less than 50 mL/min, although it is not associated with renal toxicity. A 1-log10 reduction in HIV RNA levels as well as emergence of M184V mutations on entecavir monotherapy has been reported [51,52]. M184V confers HIV resistance to lamivudine and emtricitabine. Therefore, entecavir should not be used as monotherapy in HIV-coinfected patients and/or patients with evidence of lamivudine-resistant HBV.
Combination Therapy
Recent updates in the guidelines recommend that since emtricitabine, lamivudine, TDF, and TAF are active against both viruses, patients with coinfection should start ART with a fixed-dose combination of TDF/emtricitabine or TAF/emtricitabine or the individual drug combination TDF plus lamivudine [53]. Most experts recommend the use of combination HBV therapy in patients on ART, particularly with lamivudine given the high rates of resistance.
• When should HBV treatment be started in patients with coinfection?
An ART regimen containing TDF (creatinine clearance > 50 mL/min) or TAF (creatinine clearance > 30 mL/min) with lamivudine or emtricitabine should be used in all HIV/HBV patients as soon as the infection is diagnosed. If TDF or TAF cannot be used, the alternate recommended regimen for HBV is entecavir plus a fully suppressive ART. In those with decreased renal function, entecavir should be adjusted to renal function [19].
Although control of viremia is feasible, clearance of infection as marked by loss of HBsAg and development of anti-HBs is unlikely to occur in the majority of patients. Therefore, the goals of treatment focus on prevention of chronic liver disease complications by suppressing viral replication, which can halt disease progression. A suggested algorithm for the management of coinfected patients is provided (Figure).
• What is the duration of therapy for hepatitis B?
Most patients with HIV/HBV coinfection will require lifelong treatment. All patients on HBV therapy as a part of ART should continue HBV therapy, regardless of seroconversion status. Also, patients should be educated and advised against self-discontinuation as it may trigger hepatitis exacerbations and/or hepatic failure.
Case Patient 3
Initial Presentation and History
Two months after starting treatment for HIV and chronic HBV infection, a 46-year-old Hispanic woman presents to clinic with jaundice and right upper quadrant (RUQ) pain. The patient was recently diagnosed with HIV infection and was naïve to treatment with ART. Her CD4 cell count was 50 cells/µL, and her HIV viral load was 743,000 copies/mL, with no baseline mutations on HIV genotype. The patient was also diagnosed with chronic HBV infection with positive HBsAg and HBeAg and negative HBc IgM serologies, as well as an HBV DNA level of 87 million IU/mL. Routine blood work revealed normal renal function and serum transaminases. The decision was made to start the patient on darunavir/ritonavir and TDF/emtricitabine. The patient was also started on sulfamethoxazole/trimethoprim and azithromycin for opportunistic infection prophylaxis.
Physical Examination and Laboratory Testing
Examination is remarkable for mild tenderness in the RUQ and icteric sclera. Laboratory testing demonstrates the following: AST, 1523 U/L; ALT, 795 U/L; albumin, 2.8 mg/dL; and total bilirubin, 3.5 mg/dL. Her CD4 count has increased to 565 cells/µL, and her HIV viral load is 4320 copies/mL. Results of repeat hepatitis serologies are as follows: HBsAg positive, anti-HBc IgM positive, and an HBV DNA level of 4.2 million IU/mL. Testing for hepatitis A, C, and D is negative, and RUQ sonogram reveals no gallstones.
• What monitoring should be done for coinfected patients on HBV therapy?
Monitoring
Providers should routinely monitor patients’ response to HIV/HBV therapy. Initially, all coinfected patients should have liver function tests and HBV DNA levels checked every 12 weeks on therapy. Frequent monitoring allows early detection of HBV drug resistance as well as drug-related hepatotoxicity. In HBeAg-positive coinfected patients who achieve HBV DNA suppression, HBeAg and anti-HBe testing should be performed every 6 to 12 months to assess for seroconversion. In HBeAg-negative patients, only HBV DNA and liver function tests are needed. HBV virologic failure is defined as a greater than 1-log10 rise in HBV DNA levels or development of viremia in a patient with a previously suppressed DNA level on therapy.
Typically, virologic failure results from either the development of drug resistance or abrupt withdrawal of active HBV therapy due to patient nonadherence or changes to the ART regimen. Virologic failure can result in a rise in serum aminotransferases as well as decompensation in patients with significant underlying liver disease. Due to this risk, providers must counsel patients about the importance of adherence to therapy and should continue medications active against HBV when making a change in ART regimens, unless HBV drug resistance dictates a change in HBV therapy.
• What is the likely cause of this patient’s hepatitis “flare”?
Several studies indicate that patients with HIV/HBV coinfection are at increased risk of drug-related hepatotoxicity and grade 4 liver enzyme elevations [54,55].The first 3 months after initiation of ART is a particularly vulnerable time for liver injury. The differential diagnosis for an acute hepatitis “flare” following the initiation of ART is broad and includes the following: development of HBV drug resistance [16]; withdrawal of HBV-active medications due to nonadherence [54]; ART-related hepatotoxicity; superimposed infection with HAV, HCV, or HDV; other opportunistic infections including cytomegalovirus and mycobacterium avium complex; or IRIS, resulting in an exaggerated cytotoxic response by the recovering immune system [56,57]. Complete evaluation is critical to distinguish between the possible causes.
In this case, several clues point toward HBV-related IRIS as the most likely cause for the hepatitis “flare.” A low pretreatment CD4 cell count with a rapid rise after initiation of ART is associated with a higher rate of IRIS [57]. Serologic testing and imaging excluded superinfection with another hepatotropic virus or biliary tract disease. Appropriate declines in HIV viral load and HBV DNA levels imply patient adherence to therapy and argue against the development of HBV drug resistance. Finally, the emergence of anti-HBc IgM positivity signals HBV reactivation, which is commonly seen in patients with HBV-related IRIS [57]. The preferred treatment for HBV-related IRIS involves continuation of therapy, frequently leading to normalization of aminotransferases and subsequent HBeAg seroconversion. Because IRIS usually manifests within the first 6 to 12 weeks after starting ART, liver enzymes should be monitored closely during this period.
• What health maintenance should be done for coinfected patients?
All patients with HIV/HBV coinfection should be monitored for evidence of portal hypertension or cirrhosis and, if these conditions exist, should undergo endoscopic screening for esophageal varices as well as evaluation of ascites and encephalopathy. Patients with HBV are at increased risk for the development of HCC even in the absence of cirrhosis. A recent study showed low rates of HCC screening in HIV/HBV patients by HIV providers [58]. Whether HIV coinfection potentiates HCC risk is uncertain, though coinfected patients present at younger ages and with more symptoms than HIV-negative comparators [59]. Other risk factors for HCC include HCV infection, alcohol abuse, diabetes, obesity, exposure to environmental toxins, and cirrhosis of any etiology (most commonly non-alcoholic fatty liver disease, primary sclerosing cholangitis, primary biliary cirrhosis and hemochromatosis) [60].
The American Association of Liver Diseases (AASLD) guidelines recommend hepatic ultrasound screening every 6 months in all patients with cirrhosis or chronic HBV who are at increased risk (Asian men over the age of 40 years, Asian women over the age of 50 years, African or North American blacks, and patients with family history of HCC) [61]. They should also be referred for an esophagogastroduodenoscopy to evaluate for esophageal varices. In addition, all HIV/HBV coinfected patients with decompensated liver disease should be evaluated for transplantation. HIV infection is not a contraindication for liver transplant with the use of ART. However, since transplantation does not cure HBV infection, post-transplant HBV immune globulin and HBV treatment are required. Contemporary data suggest comparable survival rates after transplant in coinfected patients compared to HBV-monoinfected patients [51].
Summary
Routine screening with HBsAg, anti-HBs, and anti-HBc serologies is recommended for all HIV-positive individuals. Patients without evidence of prior exposure or vaccination and those with isolated anti-HBc should be offered vaccination. HIV-positive adults should receive three or four 40 µg/mL doses of single agent vaccine depending on the recombinant vaccine type available. Anti-HBsAg titers should be checked 1 month after completion of the immunization series. If quantitative anti-HBsAg levels are < 10 IU/mL, patients should receive a second vaccine cycle.
Patients who test positive for HBsAg should be tested for HBeAg, anti-HBe, and HBV DNA levels and have AST and ALT levels checked as well. All patients with HIV/HBV coinfection should start treatment as soon as HIV infection is diagnosed. ART needs to include 2 drugs against HBV, and therefore a fixed-dose combination of TDF/emtricitabine or TAF/emtricitabine or the individual combination of TDF plus lamivudine should be used.
Coinfected patients on treatment should have liver function tests as well as HBV DNA every 12 weeks. In HBeAg-positive coinfected individuals who achieve HBV DNA suppression, HBeAg and anti-HBe testing should be performed every 6 to 12 months to assess for seroconversion. HBV virologic failure is defined as a greater than 1-log10 rise in HBV DNA levels or development of viremia in a patient with a previously suppressed DNA level on therapy. Those with virologic failure should be tested for HBV resistance thorough HBV genotype. Coinfected patients with cirrhosis should receive ultrasound screening every 6 months for evidence of HCC and esophagogastroduodenoscopy to evaluate for esophageal varices.
1. Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med 2006;166:1632–41.
2. Kourtis AP, Bulterys M, Hu DJ, Jamieson DJ. HIV-HBV coinfection—a global change. N Engl J Med 2012;366:1749–52.
3. Bellini C, Keise O, Chave JP, et al. Liver enzyme elevation after lamivudine withdrawal in HIV-hepatitis B virus co-infected patients: the Swiss HIV Cohort Study. HIV Med 2009;10:12–8.
4. Law WP, Dore GJ, Duncombe CJ, et al. Risk of severe hepatotoxicity associated with antiretroviral therapy in the HIV-NAT Cohort, Thailand, 1996-2001. AIDS 2003;17:2191–18.
5. Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Accessed 6 Dec 2016 at http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Q1–Q17.
6. Piroth L, Launay O, Michel ML, et al. Vaccination against hepatitis B virus (HBV) in HIV-1-infected patients with isolated anti-HBV core antibody: the ANRS HB EP03 CISOVAC prospective study. J Infect Dis 2016;213:1735–42.
7. Centers for Disease Control and Prevention. Recommended adult immunization schedule for adults aged 19 years or older, by vaccine and age group. United States, 2016. Accessed 22 Dec 2016 at www.cdc.gov/vaccines/schedules/hcp/imz/adult.html.
8. Rey D, Krantz V, Partisani M, et al. Increasing the number of hepatitis B vaccine injections augments anti-HBs response rate in HIV-infected patients. Effects on HIV-1 viral load. Vaccine 2000;18:1161–5.
9. Overton ET, Sungkanuparph S, Powderly WG, et al. Undetectable plasma HIV RNA load predicts success after hepatitis B vaccination in HIV-infected persons. Clin Infect Dis 2005;41:1045–8.
10. Fonseca MO, Pang LW, de Paula Cavalheiro N, et al. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine 2005;23:2902–8.
11. Launay O, van der Vliet D, Rosenberg AR, et al. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA 2011;305:1432–40.
12. Chaiklang K, Wipasa J, Chaiwarith R, et al. Comparison of immunogenicity and safety of four doses and four double doses vs. standard doses of hepatitis B vaccination in HIV-infected adults: a randomized, controlled trial. PLoS One 2013;8:e80409.
13. Launay O, van der Vliet D, Rosenberg AR, et al. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA 2011;305:1432–40.
14. Fonseca MO, Pang LW, de Paula Cavalheiro N, et al. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine 2005;23:2902–8.
15. Gandhi RT, Wurcel A, Lee H, et al. Response to hepatitis B vaccine in HIV-1-positive subjects who test positive for isolated antibody to hepatitis B core antigen: implications for hepatitis B vaccine strategies. J Infect Dis 2005;191:1435–41.
16. French AL, Hotton A, Young M, et al. Isolated hepatitis B core antibody status is not associated with accelerated liver disease progression in HIV/hepatitis C coinfection. J Acquir Immune Defic Syndr 2016;72:274–80.
17. INSIGHT START Study Group, Lundgren JD, Babiker GA, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373:795–807.
18. Sebaaly JC, Kelley D. Single-tablet regimens for the treatment of HIV-1 infection. Ann Pharmacother 2017;51:332–44.
19. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Accessed 6 Dec 2016 at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf.
20. Heuft MM, Houba SM, van den Berk GE, et al. Protective effect of hepatitis B virus-active antiretroviral therapy against primary hepatitis B virus infection. AIDS 2014;28:999–1005.
21. Gatanaga H, Hayashida T, Tanuma J, Oka S. Prophylactic effect of antiretroviral therapy on Hepatitis B virus infection. Clin Infect Dis 2013;56:1812–9.
22. Falade-Nwulia O, Seaberg EC, Snider AE, et al. Incident hepatitis B virus infection in HIV-infected and HIV-uninfected men who have sex with men from pre-HAART to HAART periods: a cohort study. Ann Intern Med 2015;163:673–80.
23. Shilaih M, Marzel A, Scherrer AU, et al. Dually active HIV/HBV antiretrovirals as protection against incident hepatitis B infections: potential for prophylaxis. J Infect Dis 2016;214:599–606.
24. Colin JF, Cazals-Hatem D, Loriot MA, et al. Influence of human immunodeficiency virus infection on chronic hepatitis B in homosexual men. Hepatology 1999;29:1306–10.
25. Gilson RJ, Hawkins AE, Beecham MR, et al. Interactions between HIV and hepatitis B virus in homosexual men: effects on the natural history of infection. AIDS 1997;11:597–606.
26. Bodsworth N, Donovan B, Nightingale BN. The effect of concurrent human immunodeficiency virus infection on chronic hepatitis B: a
study of 150 homosexual men. J Infect Dis 1989;160:577–82.
27. Di Martino V, Thevenot T, Colin JF, et al. Influence of HIV infection on the response to interferon therapy and the long-term outcome of chronic hepatitis B. Gastroenterology 2002;123:1812–22.
28. Thio CL, Seaberg EC, Skolasky R Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002;360(9349):1921–6.
29. Konopnicki D, Mocroft A, de Wit S, et al. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS 2005;19:593–601.
30. Nikolopoulos GK, Paraskevis D, Hatzitheodorou E, et al. Impact of hepatitis B virus infection on the progression of AIDS and mortality in HIV-infected individuals: a cohort study and meta-analysis. Clin Infect Dis 2009;48:1763–71.
31. Jain MK, Opio CK, Osuagwu CC, et al. Do HIV care providers appropriately manage hepatitis B in coinfected patients treated with antiretroviral therapy? Clin Infect Dis 2007;44:996–1000.
32. Hearn B, Chasan R, Bichoupan K, et al. Low adherence of HIV providers to practice guidelines for hepatocellular carcinoma screening in HIV/hepatitis B coinfection. Clin Infect Dis 2015;61:1742–8.
33. Matthews GV, Bartholomeusz A, Locarnini S, et al. Characteristics of drug resistant HBV in an international collaborative study of HIV-HBV-infected individuals on extended lamivudine therapy. AIDS 2006;20:863–70.
34. McMahon M, Jilek B, Brennan T, Thio C. The HBV drug entecavir: effects on HIV-1 replication and resistance. N Engl J Med 2007;356:2614–21.
35. Jain M, Zoellner C. Entecavir can select for M184V of HIV-1: a case of an HIV/hepatitis B (HBV) naive patient treated for chronic HBV. AIDS 2007; 21:2365–6.
36. Bottero J, Lacombe K, Guechot J, et al. Performance of 11 biomarkers for liver fibrosis assessment in HIV/HBV coinfected patients. J Hepatol 2009;50:1074–83.
37. Moreno S, Garcia-Samaniego J, Moreno A, et al. Noninvasive diagnosis of liver fibrosis in patients with HIV infection and HCV/HBV co-infection. J Viral Hepat 2009;16:249–58.
38. Miailhes P, Pradat P, Chevallier M, et al. Proficiency of transient elastography compared to liver biopsy for the assessment of fibrosis in HIV/HBV-coinfected patients. J Viral Hepat 2011;18:61–9.
39. Audsley J, Robson C, Aitchison S, et al. Liver fibrosis regression measured by transient elastography in human immunodeficiency virus (HIV)-hepatitis B virus (HBV) coinfected individuals on long-term HBV-active combination antiretroviral therapy. Open Forum Infect Dis 2016;3:ofw035.
40. Achhra AC, Nugent M, Mocroft A, et al. Chronic kidney disease and antiretroviral therapy in HIV-positive individuals: recent developments. Curr HIV/AIDS Rep 2016;13:149–57.
41. Marcellin P, Lau GK, Bonino F, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2004;351:1206–17.
42. Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 2005;352:2682–95.
43. Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med 2004;351:438–50.44. Dore GJ, Cooper DA, Barrett C, et al. Dual efficacy of lamivudine treatment in human immunodeficiency virus/hepatitis B virus-coinfected persons in a randomized, controlled study (CAESAR). The CAESAR Coordinating Committee. J Infect Dis 1999;180:607–13.
45. Hoff J, Bani-Sadr F, Gassin M, Raffi F. Evaluation of chronic hepatitis B virus (HBV) infection in coinfected patients receiving lamivudine as a component of antihuman immunodeficiency virus regimens. Clin Infect Dis 2001;32:963–9.
46. Price H, Dunn D, PIllary D, et al. Suppresion of HBV by tenofovir in HIV/HBV coinfected patients: a systemic review and meta-analysis. PLoS One 2013;8:e68152.
47. Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with eltegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomized, double-blind, phase 3, non-inferiority trial. Lancet 2015;385:2606–15.
48. Chan HLY, Fung S, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAG-positive chronic hepatitis B infection. A randomized, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 2016;1:185–95.
49. Buti M, Gane E, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAG-negative chronic hepatitis B infection. A randomized, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 2016;1:196–206.
50. Chang TT, Lai CL. Hepatitis B virus with primary resistance to adefovir. N Engl J Med 2006;355:322–3.
51. McMahon MA, Jilek BL, Brennan TP, et al. The HBV drug entecavir -- effects on HIV-1 replication and resistance. N Engl J Med 2007;356:2614–21.
52. Jain MK, Zoellner CL. Entecavir can select for M184V of HIV-1: a case of an HIV/hepatitis B (HBV) naive patient treated for chronic HBV. AIDS 2007;21:2365–6.
53. Nuesch R, Ananworanich J, Srasuebkul P, et al. Interruptions of tenofovir/emtricitabine-based antiretroviral therapy in patients with HIV/hepatitis B virus co-infection. AIDS 2008;22:152–4.
54. Gaglio PJ, Sterling R, Daniels E, Tedaldi E. Hepatitis B virus and HIV coinfection: results of a survey on treatment practices and recommendations for therapy. Clin Infect Dis 2007;45:618–23.
55. Reisler RB, Han C, Burman WJ, et al. Grade 4 events are as important as AIDS events in the era of HAART. J Acquir Immune Defic Syndr 2003;34:379–86.
56. Drake A, Mijch A, Sasadeusz J. Immune reconstitution hepatitis in HIV and hepatitis B coinfection, despite lamivudine therapy as part of HAART. Clin Infect Dis 2004;39:129–32.
57. Jain MK, Parekh NK, Hester J, Lee WM. Aminotransferase elevation in HIV/hepatitis B virus co-infected patients treated with two active hepatitis B virus drugs. AIDS Patient Care STDS 2006;20:817–22.
58. Hearn B, Chasan R, Bichoupan K, et al. Low adherence of HIV providers to practice guidelines for hepatocellular carcinoma screening in HIV/hepatitis B coinfection. Clin Infect Dis 2015;611742–8.
59. Brau N, Fox RK, Xiao P, et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a U.S.–Canadian multicenter study. J Hepatol 2007;47:527–37.
60. Yang JD, Harmsen WS, Slettedahl SW, et al. Factors that affect risk for hepatocellular carcinoma and effects of surveillance. Clin Gastroenterol Hepatol 2011;9:617–23.
61. Bruix J, Sherman M. AASLD Practice Guideline. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–2.
From UT Southwestern Medical Center, Dallas, TX.
Abstract
- Objective: To review the literature on and provide evidence-based recommendations for management of HIV/ hepatitis B (HBV) coinfection.
- Methods: Review of the literature for clinical trials, guidelines, and cohort studies on HIV/HBV disease management.
- Results: HIV patients should be evaluated for viral hepatitis. Those who do not have evidence of immunity should be vaccinated and monitored for response. Those who have HIV/HBV should have additional serologies checked to evaluate for hepatitis B e antigen status and level of viremia. All HIV/HBV coinfected patients should be started on antiretroviral therapy with tenofovir-based regimens. Those with HIV/HBV and cirrhosis should be screened for hepatocellular cancer every 6 months.
- Conclusion: HIV patients should be vaccinated against hepatitis B; those with coinfection should be treated for both viruses. It is important to monitor for treatment response to both HIV and HBV and liver disease complications.
Key words: incentives; reinforcement; substance abuse treatment; dissemination; implementation.
Morbidity and mortality for HIV-infected patients remain high compared to uninfected patients despite effective virologic suppression. Major contributors to illness and death among these patients include cardiovascular disease, non–AIDS-defining malignancies, and chronic liver disease, specifically viral hepatitis [1]. Hepatitis B virus (HBV) infection is one of the main causes of chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma (HCC) globally. Because HIV and HBV can both be acquired through injection drug use and sexual transmission, coinfection occurs frequently. The Joint United Nations Program on HIV/AIDS estimates that 10% of the 33 million HIV-positive patients worldwide have simultaneous chronic HBV infection [2].
HIV/HBV coinfection significantly impacts the natural history, progression, and mortality related to both viruses. HIV infection accelerates HBV-related liver impairment, leading to earlier cirrhosis, end-stage liver disease, and HCC. Conversely, chronic HBV does not have a considerable influence in the progression of HIV; however, antiretroviral treatment (ART) toxicities and/or HBV flares due to immune reconstitution inflammatory syndrome (IRIS) or HBV itself can lead to increased liver-related complications [3,4].
Case Patient 1
Initial Presentation and History
An asymptomatic 38-year-old man diagnosed with HIV infection 1 month ago presents for his initial visit to establish HIV care. The patient is a man who has sex with men (MSM) and is currently sexually active with multiple partners. He reports inconsistent use of condoms. One month ago he underwent routine screening and was found to be HIV-positive. At the time of diagnosis, the patient’s baseline CD4 cell count was 328 cells/µL and his viral load was 182,600 copies/mL. The patient wants to discuss the implications of his new diagnosis of HIV and recommendations for further testing and treatment. He is especially interested in HBV screening, since one of his recent partners was known to be positive. The patient has no relevant past medical history. He does not recall the details of his childhood vaccinations. He denies smoking and injection drug use but reports moderate alcohol consumption.
Physical Examination and Laboratory Testing
Physical examination is normal. Laboratory studies show normal complete blood count (CBC) and renal and liver function test results, and a baseline HIV genotype does not show resistance. Hepatitis A total antibody (anti-HAV) testing is positive, while tests for hepatitis B surface antigen (HBsAg), antibody to hepatitis B surface antigen (anti-HBs), antibody to hepatitis B core antigen (anti-HBc), and hepatitis C antibody (anti-HCV) are negative.
• Which screening tests for HBV should be performed in HIV-infected patients?
Routine screening of all HIV-infected patients for hepatitis A virus, HBV, and hepatitis C virus (HCV) is recommended [5]. Routine HBV screening involves obtaining serologies for HBsAg, anti-HBs, and anti-HBc. With these results, patients can be classified into categories of either active infection, immunity, or no evidence of prior exposure (Table 1).
• How effective is HBV vaccination in the HIV population?
Vaccination
Available HBV vaccines in the United States include 2 single-agent vaccines (Recombivax HB [Merck, Whitehouse Station, NJ] and Engerix-B [GlaxoSmithKline, Research Triangle Park, NC]) as well as a combination HAV/HBV vaccine (Twinrix [GlaxoSmithKline]). For adults (age ≥ 20 years) with an immunocompromising condition such as HIV infection, current Centers for Disease Control and Prevention guidelines recommend three 40 µg/mL doses of single-agent vaccine administered at 0, 1, and 6 months (Recombivax HB), or four 40 µg/mL doses of single-agent vaccine (2 doses of 20 µg/mL administered simultaneously) at 0, 1, 2, and 6 months (Engerix-B) [7].
The immunogenicity to HBV vaccination in HIV-positive patients is decreased, reflected by lower antibody titers, waning immunity, and seroconversion rates of 18% to 65% [8–11]. Factors associated with poor response include low CD4 cell counts, detectable HIV RNA, coinfection with HCV, and the general health status of the patient [8]. Ideally, HBV vaccination should occur prior to decline in CD4 cell count below 350 cells/µL. However, guidelines do not recommend deferring vaccination since some patients with advanced HIV disease will seroconvert [6].
Anti-HBs titers should be checked 1 month after completion of the vaccine series to confirm protective antibody titers. For patients with quantitative anti-HBs levels < 10 IU/mL, a second vaccine cycle is recommended. Some specialists may defer revaccination until a sustained increase in CD4 count is achieved on ART.
Two randomized controlled trials have demonstrated that 4 doses of double-dose (40 µg/mL) vaccine generate higher anti-HBs levels than 3 doses of standard-dose (20 µg/mL) vaccine in HIV-infected adults [12,13]. Another study showed that HIV patients with CD4 counts > 350 cells/µL had better responses when immunized with double- dose vaccines on the usual 3-dose series [14]. Currently, the CDC recommends giving a double dose for either a 3-dose schedule or a 4-dose schedule. However, it remains unclear what dosing schedule to use if a patient fails to respond. Likely waiting until the CD4 cell count has increased and HIV viral load is suppressed will be important to seeing a response.
• What approach for HBV prevention should be taken in this patient?
The patient’s serologies confirm no prior exposure to HBV, and he should be offered HBV vaccination. His CD4 cell count is below 350/µL and he has ongoing HIV viremia, which increases his risk for an inadequate response. However, vaccination should not be delayed, particularly given his high risk of sexual transmission. The patient should be counseled regarding all high-risk behaviors. As discussed above, 3 or 4 doses of the higher dose vaccine (40 µg/mL) should be administered depending on what type of recombinant vaccine is available. An anti-HBs level should be checked 1 month after completion. A full repeat vaccine series using the 40 µg/mL dose should be considered for nonresponders who initially received a standard vaccine series. Experts also recommend checking annual anti-HBs levels to monitor for waning immunity, with a booster dose given if the anti-HBs level drops below the protective range.
• What vaccination strategy should be used in patients with isolated positive anti-HBc?
The clinical implications of an isolated positive anti-HBc for vaccination are still uncertain. This serologic pattern may represent a false-positive test, remote HBV infection with loss of anti-HBs, or occult HBV infection with undetectable HBsAg. The latter scenario appears more commonly in HIV-infected patients, particularly with concomitant HCV infection [15].
A recent study suggested that patients with an isolated positive anti-HBc with a negative anamnestic antibody response (anti-HBs titer of < 10 IU/mL 4 weeks after a single 20 µg dose of recombinant HBV vaccine) should be further vaccinated with the double-dose for a 3-dose schedule [6]. Additionally, another study followed HIV/HCV coinfected patients for 9.5 years and found that an isolated positive anti-HBc was not associated with accelerated liver disease progression [16].
Treatment of HIV-1 Infection
Current HIV guidelines recommend initiation of ART for all HIV-infected patients regardless of their CD4 count [17]. ART for a treatment-naïve patient usually consists of 2 nucleotide/nucleoside reverse-transcriptase inhibitors (NRTIs; the “backbone”) combined with a third agent (the “anchor”), which can be a nonnucleoside reverse-transcriptase inhibitor (NNRTI), a protease inhibitor (PI) boosted with a boosting agent, or an integrase strand-transfer inhibitor (INSTI) [18,19].
There are numerous studies indicating that incident HBV risk can be reduced by placing those at risk for HBV acquisition on ART containing a combination of tenofovir disoproxil fumarate (TDF), lamivudine, or emtricitabine [20,21]. Another study in MSM found those on ART with HIV viral load < 400 copies/mL were protected from developing HBV compared to those not on ART [22]. Given this patient’s higher risk for HBV acquisition, placing him on emtricitabine/TDF backbone as part of ART could be protective against incident HBV [23].
Case Patient 2
Initial Presentation and History
A 32-year-old man diagnosed with HIV infection 8 years ago now on ART presents for follow-up. The patient is an MSM with a history of inconsistent condom use. At the time of HIV diagnosis 8 years ago, the patient had a CD4 cell count of 250 cells/µL and an HIV viral load of 648,000 copies/mL. The patient was initiated on lamivudine/zidovudine and lopinavir/ritonavir, and he achieved complete virologic suppression at 20 weeks, with a CD4 cell count of 455 cells/µL at 1 year. The patient has remained on this regimen without major side effects; however, he reports frequent missed doses over the last 2 years due to “pill fatigue.” Previous testing for HBV and HCV at the time of his initial HIV diagnosis was negative, but he failed to complete the HBV vaccine series. He denies alcohol or injection drug use.
Physical Examination and Laboratory Testing
Physical examination is normal. Laboratory studies reveal normal electrolytes and renal function, hemoglobin of 11 g/dL, and platelet count of 235,000/µL. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels are 34 U/L and 44 U/L, respectively, with an INR of 1.1 and albumin level of 3.4 g/dL. CD4 cell count is 320 cells/µL with an HIV viral load of 24,500 copies/mL. Viral hepatitis serologies show anti-HAV positive, anti-HCV negative, HBsAg positive, anti-HBc IgG positive, hepatitis Be antigen (HBeAg) negative, and hepatitis B e antibody (anti-HBe) positive. HBV DNA viral load is 685,000 IU/mL.
• What is the natural history of HIV/HBV coinfection?
Chronic HBV infection affects about 10% of HIV-infected patients globally. Epidemiologic studies indicate that HIV-infected patients have higher rates of reactivation and progression to chronic HBV infection and chronic liver disease than HIV-negative patients [24–26]. Coinfected patients demonstrate higher serum HBV DNA levels, which lead to more rapid progression of hepatic fibrosis and may increase the risk of cirrhosis and HCC [24,27,28]. HIV infection, however, can mediate the necroinflammatory response through blunting of the immune response that drives pathogenesis in HBV infection. Aminotransferase levels may be only slightly elevated or even normal, particularly in the severely immune suppressed. However, elevation in liver enzymes (hepatitis flare) can occur if a patient stops ART, or if HBV resistance develops. Patients being treated for HIV/HBV coinfection should be counseled that stopping HIV treatment puts them at risk of developing a hepatitis flare.
Large cohort studies of HIV/HBV coinfected patients indicate increased risk of liver-related mortality, most pronounced with lower CD4 cell counts [1,28,29]. Introduction of ART appears to increase rather than attenuate this liver-related mortality, possibly by decreasing AIDS-related mortality and allowing more time for liver disease progression. Finally, a recent meta-analysis including 12,382 patients demonstrated a significant effect of HIV/HBV coinfection on all-cause mortality, with a pooled effect estimate of 1.36 [30].
• What diagnostic testing should be done in coinfected patients?
Diagnostic Testing and Evaluation
Persistence of HBsAg for more than 6 months is diagnostic of chronic HBV infection and warrants further serologic evaluation. Patients with chronic HBV infection should be tested for HBeAg, anti-HBe, and HBV DNA levels and have AST and ALT levels measured. Elevation of AST and ALT can be seen with untreated HBV. For those on treatment, a hepatitis flare could be caused by abrupt discontinuation of HBV treatment, development of HBV resistance, or superinfection with another viral pathogen.
HBeAg is a marker of active viral replication and is associated with higher levels of HBV DNA and active liver disease. Seroconversion, or loss of HBeAg and development of anti-HBe, heralds a favorable treatment response for those who were initially HBeAg-positive. However, some HBV variants have precore or core promoter mutations that lead to higher levels of HBV DNA in the absence of HBeAg. This highlights the importance of monitoring HBV DNA levels in all patients with chronic HBV infection. Favorable response to therapy in HBeAg-negative patients is marked by normalization of aminotransferases and HBV DNA suppression.Hepatitis D virus (HDV) is a defective virus particle that can only replicate in the presence of HBV. Coinfected patients should be tested for anti-HDV if they are injection drug users or are from a high-prevalence region such as the Mediterranean and Amazon basins. Newly acquired HDV infection should also be considered in the context of hepatitis flares.
Although these routine diagnostic tests are essential for management, studies show low adherence rates to HBV testing guidelines by HIV providers [31,32].
• What is the role of HBV genotype and resistance testing?
HBV can be classified into 10 different genotypes, A through J, based on genomic sequence variations. Each genotype has a distinct ethnic and geographic distribution, with genotypes A and D predominating in North America. HBV genotyping appears to have important prognostic as well as treatment implications [19]. However, data are still preliminary and the guidelines do not recommend routine genotype testing.
Resistance testing in HBV allows for detection of mutations that decrease effectiveness of antivirals. Exposure to lamivudine can lead to mutations in the YMDD region of the HBV DNA polymerase, resulting in drug resistance. Resistance to lamivudine develops at a rate of approximately 25% after 1 year of drug exposure in HIV/HBV coinfection [33]. On the other hand, studies have shown that entecavir is active against HIV and, most importantly, selects for the M184V mutation in HIV. M184V is associated with lamivudine resistance for HIV treatment, thus limiting treatment options [34,35]. After these findings, the FDA advised against monotherapy with entecavir in patients with HIV/HBV coinfection. The case patient was on lamivudine during the time of HBV acquisition, and therefore YMDD mutation must be taken into consideration for therapy purposes. He should be switched to a regimen that suppresses both viruses. The development of drug resistance should be assessed in all patients with persistent or breakthrough HBV viremia on ART, particularly the nucleoside analogues. HIV providers treating HIV/HBV coinfection should regularly monitor both HIV and HBV viral load to assess for therapeutic efficacy.
• What is the role of liver biopsy in this patient?
Liver biopsy should be considered in all coinfected patients as it remains the gold standard for determining the activity and severity of chronic hepatitis B. However, because it carries inherent risks and is not required prior to treatment in all patients, the decision should be individualized. Liver biopsy can be useful to assess baseline liver histology and may be warranted to rule out significant coexisting genetic or metabolic liver disease. Currently, noninvasive methods to assess liver fibrosis either using elastography or various combinations of serum biomarkers are being evaluated [36] and may be considered in lieu of a liver biopsy [37]. One study compared the accuracy of elastography with liver biopsy in HIV/HBV coinfected patients and demonstrated that the former was proficient in discriminating between absence or mild fibrosis and moderate to severe fibrosis [38]. In general, this test has high accuracy in detecting minimal fibrosis from advanced fibrosis or cirrhosis. For the group in the middle, further investigation with additional methods must be considered [37]. Finally, a recent retrospective study involving 70 HIV/HBV coinfected individuals showed fibrosis regression suggesting beneficial effects of long-term ART on liver stiffness, [39] but further studies are needed to confirm these findings.
Case 2 Continued
The patient has now been diagnosed with chronic HBV infection. His diagnostic testing is negative for HBeAg and reveals modest HBV viremia with abnormal aminotransferases less than 2 times the upper limit of normal. Drug resistance testing reveals a mutation in the YMDD region indicative of HBV lamivudine resistance. HIV genotype demonstrates a wild-type virus without resistance. The patient asks what treatment options exist.
• What medications are currently available to treat hepatitis B?
Treatment
All patients with HIV/HBV coinfection should receive treatment to suppress both viruses, and ART needs to include 2 drugs active against HBV, ideally emtricitabine and TDF. This approach prevents drug resistance, slows progression of HBV infection, and reduces the incidence of IRIS [5]. TDF has been associated with decreased renal function and bone mineral density. Recently, tenofovir alafenamide fumarate (TAF) was approved for the treatment of HIV and HBV. A dose of 10 mg daily is given when coadministered with ritonavir, cobicistat, or protease inhibitors, but a dose of 25 mg should be given when administered with NNRTIs or integrase inhibitors. Compared to TDF, TAF shows less accumulation of tenofovir in kidneys and bones and consequently has reduced renal and bone mineral density effects [40]. All patients should be on a TDF- or TAF-based HIV regimen if they have chronic HBV.
Currently, the following antiviral drugs are FDA-approved for the treatment of HBV infection: interferon alfa-2b, pegylated interferon (peginterferon) alfa-2a, lamivudine and emtricitabine, entecavir, adefovir, TDF, TAF, and telbivudine. Telbivudine and adefovir are no longer recommended due to their association with higher incidence of toxicity and higher rates of HBV treatment failure. A summary of available HBV treatment options is outlined in Table 2.
Interferon
Standard interferon alfa-2b blocks HBV replication through interaction with viral proteins and stimulation of host cellular immunity. Peginterferon alfa-2a has proven efficacy in HBV-monoinfected patients, but efficacy data in HIV/HBV coinfected patients is lacking [41,42]. Studies in hepatitis C treatment demonstrate the safety of peginterferon alfa-2a use in HIV-positive patients and indicate that HIV viral suppression occurs with peginterferon without evidence of selection of resistance mutations that affect future ART options [43]. For HIV/HBV coinfected patients not on ART who will receive only therapy for HBV (which is infrequent since all HIV patients should be on ART), pegylated interferon-alfa-2a alone for 48 weeks is the only option that will not cause ART-associated HIV drug resistance [5]. Interferon therapy is contraindicated in patients with decompensated cirrhosis and should be used with caution in patients with active depression, uncontrolled diabetes, and cardiac and pulmonary disease.
Lamivudine and Emtricitabine
Lamivudine is a nucleoside analogue with efficacy against both HIV and HBV. Clinical trials in HIV/HBV-infected patients have shown up to 87% of patients achieve undetectable HBV DNA levels and about 25% achieve HBeAg seroconversion after 1 to 2 years of therapy [44,45]. However, the major issue limiting use of lamivudine is its low genetic barrier to resistance. Mutation of the YMDD motif of the HBV DNA polymerase confers HBV resistance. HIV/HBV coinfected patients develop resistance at rates of up to 94% after 4 years of therapy [33], heralded by rebounds in HBV DNA levels and often hepatitis flares or precipitation of hepatic failure [16]. Because of resistance, lamivudine monotherapy should be avoided; even in patients on ART, abrupt withdrawal of lamivudine or the development of HBV resistance should be closely monitored.
Emtricitabine is another nucleoside analogue with properties and efficacy similar to lamivudine. It is frequently used as a combination pill with TDF (Truvada) in coinfected patients. The same concerns regarding monotherapy and the emergence of resistance that exist for lamivudine apply to emtricitabine.
Tenofovir
TDF, a nucleotide analogue, is one of the preferred first-line agents for HIV treatment and has proven efficacy against both wild-type and lamivudine-resistant HBV. Since it was first used for HIV, TDF has been more extensively studied in coinfected patients compared to most other agents. In a meta-analysis of patients with HIV/HBV coinfection, TDF suppressed HBV viral load to undetectable titers in approximately 90% of patients [46]. Tenofovir is available in 2 preparations: TDF and TAF. TDF has been reported to cause renal impairment as well as bone loss. TAF has shown less renal toxicity and less bone damage [40,47]. In 2016, TAF became available as part as 4 regimens: stand-alone TAF, elvitegravir-cobicistat-emtricitabine-TAF, rilpivirine-emtricitabine-TAF, and TAF-emtricitabine.
TDF and TAF both suppress HIV. Two large randomized trials of HBV monoinfection demonstrate that TAF is noninferior to TDF for the treatment of naïve and treatment-experienced patients [48,49].
Entecavir
Entecavir, a guanosine analogue, is a potent HBV DNA polymerase inhibitor that results in greater virologic suppression compared to lamivudine and retains activity against lamivudine-resistant HBV [50]. Importantly, entecavir shares some cross-resistance with lamivudine, so an entecavir dose of 1 mg daily is recommended in lamivudine-experienced patients compared to 0.5 mg daily in lamivudine-naïve patients. Entecavir requires dose reduction for patients with creatinine clearance less than 50 mL/min, although it is not associated with renal toxicity. A 1-log10 reduction in HIV RNA levels as well as emergence of M184V mutations on entecavir monotherapy has been reported [51,52]. M184V confers HIV resistance to lamivudine and emtricitabine. Therefore, entecavir should not be used as monotherapy in HIV-coinfected patients and/or patients with evidence of lamivudine-resistant HBV.
Combination Therapy
Recent updates in the guidelines recommend that since emtricitabine, lamivudine, TDF, and TAF are active against both viruses, patients with coinfection should start ART with a fixed-dose combination of TDF/emtricitabine or TAF/emtricitabine or the individual drug combination TDF plus lamivudine [53]. Most experts recommend the use of combination HBV therapy in patients on ART, particularly with lamivudine given the high rates of resistance.
• When should HBV treatment be started in patients with coinfection?
An ART regimen containing TDF (creatinine clearance > 50 mL/min) or TAF (creatinine clearance > 30 mL/min) with lamivudine or emtricitabine should be used in all HIV/HBV patients as soon as the infection is diagnosed. If TDF or TAF cannot be used, the alternate recommended regimen for HBV is entecavir plus a fully suppressive ART. In those with decreased renal function, entecavir should be adjusted to renal function [19].
Although control of viremia is feasible, clearance of infection as marked by loss of HBsAg and development of anti-HBs is unlikely to occur in the majority of patients. Therefore, the goals of treatment focus on prevention of chronic liver disease complications by suppressing viral replication, which can halt disease progression. A suggested algorithm for the management of coinfected patients is provided (Figure).
• What is the duration of therapy for hepatitis B?
Most patients with HIV/HBV coinfection will require lifelong treatment. All patients on HBV therapy as a part of ART should continue HBV therapy, regardless of seroconversion status. Also, patients should be educated and advised against self-discontinuation as it may trigger hepatitis exacerbations and/or hepatic failure.
Case Patient 3
Initial Presentation and History
Two months after starting treatment for HIV and chronic HBV infection, a 46-year-old Hispanic woman presents to clinic with jaundice and right upper quadrant (RUQ) pain. The patient was recently diagnosed with HIV infection and was naïve to treatment with ART. Her CD4 cell count was 50 cells/µL, and her HIV viral load was 743,000 copies/mL, with no baseline mutations on HIV genotype. The patient was also diagnosed with chronic HBV infection with positive HBsAg and HBeAg and negative HBc IgM serologies, as well as an HBV DNA level of 87 million IU/mL. Routine blood work revealed normal renal function and serum transaminases. The decision was made to start the patient on darunavir/ritonavir and TDF/emtricitabine. The patient was also started on sulfamethoxazole/trimethoprim and azithromycin for opportunistic infection prophylaxis.
Physical Examination and Laboratory Testing
Examination is remarkable for mild tenderness in the RUQ and icteric sclera. Laboratory testing demonstrates the following: AST, 1523 U/L; ALT, 795 U/L; albumin, 2.8 mg/dL; and total bilirubin, 3.5 mg/dL. Her CD4 count has increased to 565 cells/µL, and her HIV viral load is 4320 copies/mL. Results of repeat hepatitis serologies are as follows: HBsAg positive, anti-HBc IgM positive, and an HBV DNA level of 4.2 million IU/mL. Testing for hepatitis A, C, and D is negative, and RUQ sonogram reveals no gallstones.
• What monitoring should be done for coinfected patients on HBV therapy?
Monitoring
Providers should routinely monitor patients’ response to HIV/HBV therapy. Initially, all coinfected patients should have liver function tests and HBV DNA levels checked every 12 weeks on therapy. Frequent monitoring allows early detection of HBV drug resistance as well as drug-related hepatotoxicity. In HBeAg-positive coinfected patients who achieve HBV DNA suppression, HBeAg and anti-HBe testing should be performed every 6 to 12 months to assess for seroconversion. In HBeAg-negative patients, only HBV DNA and liver function tests are needed. HBV virologic failure is defined as a greater than 1-log10 rise in HBV DNA levels or development of viremia in a patient with a previously suppressed DNA level on therapy.
Typically, virologic failure results from either the development of drug resistance or abrupt withdrawal of active HBV therapy due to patient nonadherence or changes to the ART regimen. Virologic failure can result in a rise in serum aminotransferases as well as decompensation in patients with significant underlying liver disease. Due to this risk, providers must counsel patients about the importance of adherence to therapy and should continue medications active against HBV when making a change in ART regimens, unless HBV drug resistance dictates a change in HBV therapy.
• What is the likely cause of this patient’s hepatitis “flare”?
Several studies indicate that patients with HIV/HBV coinfection are at increased risk of drug-related hepatotoxicity and grade 4 liver enzyme elevations [54,55].The first 3 months after initiation of ART is a particularly vulnerable time for liver injury. The differential diagnosis for an acute hepatitis “flare” following the initiation of ART is broad and includes the following: development of HBV drug resistance [16]; withdrawal of HBV-active medications due to nonadherence [54]; ART-related hepatotoxicity; superimposed infection with HAV, HCV, or HDV; other opportunistic infections including cytomegalovirus and mycobacterium avium complex; or IRIS, resulting in an exaggerated cytotoxic response by the recovering immune system [56,57]. Complete evaluation is critical to distinguish between the possible causes.
In this case, several clues point toward HBV-related IRIS as the most likely cause for the hepatitis “flare.” A low pretreatment CD4 cell count with a rapid rise after initiation of ART is associated with a higher rate of IRIS [57]. Serologic testing and imaging excluded superinfection with another hepatotropic virus or biliary tract disease. Appropriate declines in HIV viral load and HBV DNA levels imply patient adherence to therapy and argue against the development of HBV drug resistance. Finally, the emergence of anti-HBc IgM positivity signals HBV reactivation, which is commonly seen in patients with HBV-related IRIS [57]. The preferred treatment for HBV-related IRIS involves continuation of therapy, frequently leading to normalization of aminotransferases and subsequent HBeAg seroconversion. Because IRIS usually manifests within the first 6 to 12 weeks after starting ART, liver enzymes should be monitored closely during this period.
• What health maintenance should be done for coinfected patients?
All patients with HIV/HBV coinfection should be monitored for evidence of portal hypertension or cirrhosis and, if these conditions exist, should undergo endoscopic screening for esophageal varices as well as evaluation of ascites and encephalopathy. Patients with HBV are at increased risk for the development of HCC even in the absence of cirrhosis. A recent study showed low rates of HCC screening in HIV/HBV patients by HIV providers [58]. Whether HIV coinfection potentiates HCC risk is uncertain, though coinfected patients present at younger ages and with more symptoms than HIV-negative comparators [59]. Other risk factors for HCC include HCV infection, alcohol abuse, diabetes, obesity, exposure to environmental toxins, and cirrhosis of any etiology (most commonly non-alcoholic fatty liver disease, primary sclerosing cholangitis, primary biliary cirrhosis and hemochromatosis) [60].
The American Association of Liver Diseases (AASLD) guidelines recommend hepatic ultrasound screening every 6 months in all patients with cirrhosis or chronic HBV who are at increased risk (Asian men over the age of 40 years, Asian women over the age of 50 years, African or North American blacks, and patients with family history of HCC) [61]. They should also be referred for an esophagogastroduodenoscopy to evaluate for esophageal varices. In addition, all HIV/HBV coinfected patients with decompensated liver disease should be evaluated for transplantation. HIV infection is not a contraindication for liver transplant with the use of ART. However, since transplantation does not cure HBV infection, post-transplant HBV immune globulin and HBV treatment are required. Contemporary data suggest comparable survival rates after transplant in coinfected patients compared to HBV-monoinfected patients [51].
Summary
Routine screening with HBsAg, anti-HBs, and anti-HBc serologies is recommended for all HIV-positive individuals. Patients without evidence of prior exposure or vaccination and those with isolated anti-HBc should be offered vaccination. HIV-positive adults should receive three or four 40 µg/mL doses of single agent vaccine depending on the recombinant vaccine type available. Anti-HBsAg titers should be checked 1 month after completion of the immunization series. If quantitative anti-HBsAg levels are < 10 IU/mL, patients should receive a second vaccine cycle.
Patients who test positive for HBsAg should be tested for HBeAg, anti-HBe, and HBV DNA levels and have AST and ALT levels checked as well. All patients with HIV/HBV coinfection should start treatment as soon as HIV infection is diagnosed. ART needs to include 2 drugs against HBV, and therefore a fixed-dose combination of TDF/emtricitabine or TAF/emtricitabine or the individual combination of TDF plus lamivudine should be used.
Coinfected patients on treatment should have liver function tests as well as HBV DNA every 12 weeks. In HBeAg-positive coinfected individuals who achieve HBV DNA suppression, HBeAg and anti-HBe testing should be performed every 6 to 12 months to assess for seroconversion. HBV virologic failure is defined as a greater than 1-log10 rise in HBV DNA levels or development of viremia in a patient with a previously suppressed DNA level on therapy. Those with virologic failure should be tested for HBV resistance thorough HBV genotype. Coinfected patients with cirrhosis should receive ultrasound screening every 6 months for evidence of HCC and esophagogastroduodenoscopy to evaluate for esophageal varices.
From UT Southwestern Medical Center, Dallas, TX.
Abstract
- Objective: To review the literature on and provide evidence-based recommendations for management of HIV/ hepatitis B (HBV) coinfection.
- Methods: Review of the literature for clinical trials, guidelines, and cohort studies on HIV/HBV disease management.
- Results: HIV patients should be evaluated for viral hepatitis. Those who do not have evidence of immunity should be vaccinated and monitored for response. Those who have HIV/HBV should have additional serologies checked to evaluate for hepatitis B e antigen status and level of viremia. All HIV/HBV coinfected patients should be started on antiretroviral therapy with tenofovir-based regimens. Those with HIV/HBV and cirrhosis should be screened for hepatocellular cancer every 6 months.
- Conclusion: HIV patients should be vaccinated against hepatitis B; those with coinfection should be treated for both viruses. It is important to monitor for treatment response to both HIV and HBV and liver disease complications.
Key words: incentives; reinforcement; substance abuse treatment; dissemination; implementation.
Morbidity and mortality for HIV-infected patients remain high compared to uninfected patients despite effective virologic suppression. Major contributors to illness and death among these patients include cardiovascular disease, non–AIDS-defining malignancies, and chronic liver disease, specifically viral hepatitis [1]. Hepatitis B virus (HBV) infection is one of the main causes of chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma (HCC) globally. Because HIV and HBV can both be acquired through injection drug use and sexual transmission, coinfection occurs frequently. The Joint United Nations Program on HIV/AIDS estimates that 10% of the 33 million HIV-positive patients worldwide have simultaneous chronic HBV infection [2].
HIV/HBV coinfection significantly impacts the natural history, progression, and mortality related to both viruses. HIV infection accelerates HBV-related liver impairment, leading to earlier cirrhosis, end-stage liver disease, and HCC. Conversely, chronic HBV does not have a considerable influence in the progression of HIV; however, antiretroviral treatment (ART) toxicities and/or HBV flares due to immune reconstitution inflammatory syndrome (IRIS) or HBV itself can lead to increased liver-related complications [3,4].
Case Patient 1
Initial Presentation and History
An asymptomatic 38-year-old man diagnosed with HIV infection 1 month ago presents for his initial visit to establish HIV care. The patient is a man who has sex with men (MSM) and is currently sexually active with multiple partners. He reports inconsistent use of condoms. One month ago he underwent routine screening and was found to be HIV-positive. At the time of diagnosis, the patient’s baseline CD4 cell count was 328 cells/µL and his viral load was 182,600 copies/mL. The patient wants to discuss the implications of his new diagnosis of HIV and recommendations for further testing and treatment. He is especially interested in HBV screening, since one of his recent partners was known to be positive. The patient has no relevant past medical history. He does not recall the details of his childhood vaccinations. He denies smoking and injection drug use but reports moderate alcohol consumption.
Physical Examination and Laboratory Testing
Physical examination is normal. Laboratory studies show normal complete blood count (CBC) and renal and liver function test results, and a baseline HIV genotype does not show resistance. Hepatitis A total antibody (anti-HAV) testing is positive, while tests for hepatitis B surface antigen (HBsAg), antibody to hepatitis B surface antigen (anti-HBs), antibody to hepatitis B core antigen (anti-HBc), and hepatitis C antibody (anti-HCV) are negative.
• Which screening tests for HBV should be performed in HIV-infected patients?
Routine screening of all HIV-infected patients for hepatitis A virus, HBV, and hepatitis C virus (HCV) is recommended [5]. Routine HBV screening involves obtaining serologies for HBsAg, anti-HBs, and anti-HBc. With these results, patients can be classified into categories of either active infection, immunity, or no evidence of prior exposure (Table 1).
• How effective is HBV vaccination in the HIV population?
Vaccination
Available HBV vaccines in the United States include 2 single-agent vaccines (Recombivax HB [Merck, Whitehouse Station, NJ] and Engerix-B [GlaxoSmithKline, Research Triangle Park, NC]) as well as a combination HAV/HBV vaccine (Twinrix [GlaxoSmithKline]). For adults (age ≥ 20 years) with an immunocompromising condition such as HIV infection, current Centers for Disease Control and Prevention guidelines recommend three 40 µg/mL doses of single-agent vaccine administered at 0, 1, and 6 months (Recombivax HB), or four 40 µg/mL doses of single-agent vaccine (2 doses of 20 µg/mL administered simultaneously) at 0, 1, 2, and 6 months (Engerix-B) [7].
The immunogenicity to HBV vaccination in HIV-positive patients is decreased, reflected by lower antibody titers, waning immunity, and seroconversion rates of 18% to 65% [8–11]. Factors associated with poor response include low CD4 cell counts, detectable HIV RNA, coinfection with HCV, and the general health status of the patient [8]. Ideally, HBV vaccination should occur prior to decline in CD4 cell count below 350 cells/µL. However, guidelines do not recommend deferring vaccination since some patients with advanced HIV disease will seroconvert [6].
Anti-HBs titers should be checked 1 month after completion of the vaccine series to confirm protective antibody titers. For patients with quantitative anti-HBs levels < 10 IU/mL, a second vaccine cycle is recommended. Some specialists may defer revaccination until a sustained increase in CD4 count is achieved on ART.
Two randomized controlled trials have demonstrated that 4 doses of double-dose (40 µg/mL) vaccine generate higher anti-HBs levels than 3 doses of standard-dose (20 µg/mL) vaccine in HIV-infected adults [12,13]. Another study showed that HIV patients with CD4 counts > 350 cells/µL had better responses when immunized with double- dose vaccines on the usual 3-dose series [14]. Currently, the CDC recommends giving a double dose for either a 3-dose schedule or a 4-dose schedule. However, it remains unclear what dosing schedule to use if a patient fails to respond. Likely waiting until the CD4 cell count has increased and HIV viral load is suppressed will be important to seeing a response.
• What approach for HBV prevention should be taken in this patient?
The patient’s serologies confirm no prior exposure to HBV, and he should be offered HBV vaccination. His CD4 cell count is below 350/µL and he has ongoing HIV viremia, which increases his risk for an inadequate response. However, vaccination should not be delayed, particularly given his high risk of sexual transmission. The patient should be counseled regarding all high-risk behaviors. As discussed above, 3 or 4 doses of the higher dose vaccine (40 µg/mL) should be administered depending on what type of recombinant vaccine is available. An anti-HBs level should be checked 1 month after completion. A full repeat vaccine series using the 40 µg/mL dose should be considered for nonresponders who initially received a standard vaccine series. Experts also recommend checking annual anti-HBs levels to monitor for waning immunity, with a booster dose given if the anti-HBs level drops below the protective range.
• What vaccination strategy should be used in patients with isolated positive anti-HBc?
The clinical implications of an isolated positive anti-HBc for vaccination are still uncertain. This serologic pattern may represent a false-positive test, remote HBV infection with loss of anti-HBs, or occult HBV infection with undetectable HBsAg. The latter scenario appears more commonly in HIV-infected patients, particularly with concomitant HCV infection [15].
A recent study suggested that patients with an isolated positive anti-HBc with a negative anamnestic antibody response (anti-HBs titer of < 10 IU/mL 4 weeks after a single 20 µg dose of recombinant HBV vaccine) should be further vaccinated with the double-dose for a 3-dose schedule [6]. Additionally, another study followed HIV/HCV coinfected patients for 9.5 years and found that an isolated positive anti-HBc was not associated with accelerated liver disease progression [16].
Treatment of HIV-1 Infection
Current HIV guidelines recommend initiation of ART for all HIV-infected patients regardless of their CD4 count [17]. ART for a treatment-naïve patient usually consists of 2 nucleotide/nucleoside reverse-transcriptase inhibitors (NRTIs; the “backbone”) combined with a third agent (the “anchor”), which can be a nonnucleoside reverse-transcriptase inhibitor (NNRTI), a protease inhibitor (PI) boosted with a boosting agent, or an integrase strand-transfer inhibitor (INSTI) [18,19].
There are numerous studies indicating that incident HBV risk can be reduced by placing those at risk for HBV acquisition on ART containing a combination of tenofovir disoproxil fumarate (TDF), lamivudine, or emtricitabine [20,21]. Another study in MSM found those on ART with HIV viral load < 400 copies/mL were protected from developing HBV compared to those not on ART [22]. Given this patient’s higher risk for HBV acquisition, placing him on emtricitabine/TDF backbone as part of ART could be protective against incident HBV [23].
Case Patient 2
Initial Presentation and History
A 32-year-old man diagnosed with HIV infection 8 years ago now on ART presents for follow-up. The patient is an MSM with a history of inconsistent condom use. At the time of HIV diagnosis 8 years ago, the patient had a CD4 cell count of 250 cells/µL and an HIV viral load of 648,000 copies/mL. The patient was initiated on lamivudine/zidovudine and lopinavir/ritonavir, and he achieved complete virologic suppression at 20 weeks, with a CD4 cell count of 455 cells/µL at 1 year. The patient has remained on this regimen without major side effects; however, he reports frequent missed doses over the last 2 years due to “pill fatigue.” Previous testing for HBV and HCV at the time of his initial HIV diagnosis was negative, but he failed to complete the HBV vaccine series. He denies alcohol or injection drug use.
Physical Examination and Laboratory Testing
Physical examination is normal. Laboratory studies reveal normal electrolytes and renal function, hemoglobin of 11 g/dL, and platelet count of 235,000/µL. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels are 34 U/L and 44 U/L, respectively, with an INR of 1.1 and albumin level of 3.4 g/dL. CD4 cell count is 320 cells/µL with an HIV viral load of 24,500 copies/mL. Viral hepatitis serologies show anti-HAV positive, anti-HCV negative, HBsAg positive, anti-HBc IgG positive, hepatitis Be antigen (HBeAg) negative, and hepatitis B e antibody (anti-HBe) positive. HBV DNA viral load is 685,000 IU/mL.
• What is the natural history of HIV/HBV coinfection?
Chronic HBV infection affects about 10% of HIV-infected patients globally. Epidemiologic studies indicate that HIV-infected patients have higher rates of reactivation and progression to chronic HBV infection and chronic liver disease than HIV-negative patients [24–26]. Coinfected patients demonstrate higher serum HBV DNA levels, which lead to more rapid progression of hepatic fibrosis and may increase the risk of cirrhosis and HCC [24,27,28]. HIV infection, however, can mediate the necroinflammatory response through blunting of the immune response that drives pathogenesis in HBV infection. Aminotransferase levels may be only slightly elevated or even normal, particularly in the severely immune suppressed. However, elevation in liver enzymes (hepatitis flare) can occur if a patient stops ART, or if HBV resistance develops. Patients being treated for HIV/HBV coinfection should be counseled that stopping HIV treatment puts them at risk of developing a hepatitis flare.
Large cohort studies of HIV/HBV coinfected patients indicate increased risk of liver-related mortality, most pronounced with lower CD4 cell counts [1,28,29]. Introduction of ART appears to increase rather than attenuate this liver-related mortality, possibly by decreasing AIDS-related mortality and allowing more time for liver disease progression. Finally, a recent meta-analysis including 12,382 patients demonstrated a significant effect of HIV/HBV coinfection on all-cause mortality, with a pooled effect estimate of 1.36 [30].
• What diagnostic testing should be done in coinfected patients?
Diagnostic Testing and Evaluation
Persistence of HBsAg for more than 6 months is diagnostic of chronic HBV infection and warrants further serologic evaluation. Patients with chronic HBV infection should be tested for HBeAg, anti-HBe, and HBV DNA levels and have AST and ALT levels measured. Elevation of AST and ALT can be seen with untreated HBV. For those on treatment, a hepatitis flare could be caused by abrupt discontinuation of HBV treatment, development of HBV resistance, or superinfection with another viral pathogen.
HBeAg is a marker of active viral replication and is associated with higher levels of HBV DNA and active liver disease. Seroconversion, or loss of HBeAg and development of anti-HBe, heralds a favorable treatment response for those who were initially HBeAg-positive. However, some HBV variants have precore or core promoter mutations that lead to higher levels of HBV DNA in the absence of HBeAg. This highlights the importance of monitoring HBV DNA levels in all patients with chronic HBV infection. Favorable response to therapy in HBeAg-negative patients is marked by normalization of aminotransferases and HBV DNA suppression.Hepatitis D virus (HDV) is a defective virus particle that can only replicate in the presence of HBV. Coinfected patients should be tested for anti-HDV if they are injection drug users or are from a high-prevalence region such as the Mediterranean and Amazon basins. Newly acquired HDV infection should also be considered in the context of hepatitis flares.
Although these routine diagnostic tests are essential for management, studies show low adherence rates to HBV testing guidelines by HIV providers [31,32].
• What is the role of HBV genotype and resistance testing?
HBV can be classified into 10 different genotypes, A through J, based on genomic sequence variations. Each genotype has a distinct ethnic and geographic distribution, with genotypes A and D predominating in North America. HBV genotyping appears to have important prognostic as well as treatment implications [19]. However, data are still preliminary and the guidelines do not recommend routine genotype testing.
Resistance testing in HBV allows for detection of mutations that decrease effectiveness of antivirals. Exposure to lamivudine can lead to mutations in the YMDD region of the HBV DNA polymerase, resulting in drug resistance. Resistance to lamivudine develops at a rate of approximately 25% after 1 year of drug exposure in HIV/HBV coinfection [33]. On the other hand, studies have shown that entecavir is active against HIV and, most importantly, selects for the M184V mutation in HIV. M184V is associated with lamivudine resistance for HIV treatment, thus limiting treatment options [34,35]. After these findings, the FDA advised against monotherapy with entecavir in patients with HIV/HBV coinfection. The case patient was on lamivudine during the time of HBV acquisition, and therefore YMDD mutation must be taken into consideration for therapy purposes. He should be switched to a regimen that suppresses both viruses. The development of drug resistance should be assessed in all patients with persistent or breakthrough HBV viremia on ART, particularly the nucleoside analogues. HIV providers treating HIV/HBV coinfection should regularly monitor both HIV and HBV viral load to assess for therapeutic efficacy.
• What is the role of liver biopsy in this patient?
Liver biopsy should be considered in all coinfected patients as it remains the gold standard for determining the activity and severity of chronic hepatitis B. However, because it carries inherent risks and is not required prior to treatment in all patients, the decision should be individualized. Liver biopsy can be useful to assess baseline liver histology and may be warranted to rule out significant coexisting genetic or metabolic liver disease. Currently, noninvasive methods to assess liver fibrosis either using elastography or various combinations of serum biomarkers are being evaluated [36] and may be considered in lieu of a liver biopsy [37]. One study compared the accuracy of elastography with liver biopsy in HIV/HBV coinfected patients and demonstrated that the former was proficient in discriminating between absence or mild fibrosis and moderate to severe fibrosis [38]. In general, this test has high accuracy in detecting minimal fibrosis from advanced fibrosis or cirrhosis. For the group in the middle, further investigation with additional methods must be considered [37]. Finally, a recent retrospective study involving 70 HIV/HBV coinfected individuals showed fibrosis regression suggesting beneficial effects of long-term ART on liver stiffness, [39] but further studies are needed to confirm these findings.
Case 2 Continued
The patient has now been diagnosed with chronic HBV infection. His diagnostic testing is negative for HBeAg and reveals modest HBV viremia with abnormal aminotransferases less than 2 times the upper limit of normal. Drug resistance testing reveals a mutation in the YMDD region indicative of HBV lamivudine resistance. HIV genotype demonstrates a wild-type virus without resistance. The patient asks what treatment options exist.
• What medications are currently available to treat hepatitis B?
Treatment
All patients with HIV/HBV coinfection should receive treatment to suppress both viruses, and ART needs to include 2 drugs active against HBV, ideally emtricitabine and TDF. This approach prevents drug resistance, slows progression of HBV infection, and reduces the incidence of IRIS [5]. TDF has been associated with decreased renal function and bone mineral density. Recently, tenofovir alafenamide fumarate (TAF) was approved for the treatment of HIV and HBV. A dose of 10 mg daily is given when coadministered with ritonavir, cobicistat, or protease inhibitors, but a dose of 25 mg should be given when administered with NNRTIs or integrase inhibitors. Compared to TDF, TAF shows less accumulation of tenofovir in kidneys and bones and consequently has reduced renal and bone mineral density effects [40]. All patients should be on a TDF- or TAF-based HIV regimen if they have chronic HBV.
Currently, the following antiviral drugs are FDA-approved for the treatment of HBV infection: interferon alfa-2b, pegylated interferon (peginterferon) alfa-2a, lamivudine and emtricitabine, entecavir, adefovir, TDF, TAF, and telbivudine. Telbivudine and adefovir are no longer recommended due to their association with higher incidence of toxicity and higher rates of HBV treatment failure. A summary of available HBV treatment options is outlined in Table 2.
Interferon
Standard interferon alfa-2b blocks HBV replication through interaction with viral proteins and stimulation of host cellular immunity. Peginterferon alfa-2a has proven efficacy in HBV-monoinfected patients, but efficacy data in HIV/HBV coinfected patients is lacking [41,42]. Studies in hepatitis C treatment demonstrate the safety of peginterferon alfa-2a use in HIV-positive patients and indicate that HIV viral suppression occurs with peginterferon without evidence of selection of resistance mutations that affect future ART options [43]. For HIV/HBV coinfected patients not on ART who will receive only therapy for HBV (which is infrequent since all HIV patients should be on ART), pegylated interferon-alfa-2a alone for 48 weeks is the only option that will not cause ART-associated HIV drug resistance [5]. Interferon therapy is contraindicated in patients with decompensated cirrhosis and should be used with caution in patients with active depression, uncontrolled diabetes, and cardiac and pulmonary disease.
Lamivudine and Emtricitabine
Lamivudine is a nucleoside analogue with efficacy against both HIV and HBV. Clinical trials in HIV/HBV-infected patients have shown up to 87% of patients achieve undetectable HBV DNA levels and about 25% achieve HBeAg seroconversion after 1 to 2 years of therapy [44,45]. However, the major issue limiting use of lamivudine is its low genetic barrier to resistance. Mutation of the YMDD motif of the HBV DNA polymerase confers HBV resistance. HIV/HBV coinfected patients develop resistance at rates of up to 94% after 4 years of therapy [33], heralded by rebounds in HBV DNA levels and often hepatitis flares or precipitation of hepatic failure [16]. Because of resistance, lamivudine monotherapy should be avoided; even in patients on ART, abrupt withdrawal of lamivudine or the development of HBV resistance should be closely monitored.
Emtricitabine is another nucleoside analogue with properties and efficacy similar to lamivudine. It is frequently used as a combination pill with TDF (Truvada) in coinfected patients. The same concerns regarding monotherapy and the emergence of resistance that exist for lamivudine apply to emtricitabine.
Tenofovir
TDF, a nucleotide analogue, is one of the preferred first-line agents for HIV treatment and has proven efficacy against both wild-type and lamivudine-resistant HBV. Since it was first used for HIV, TDF has been more extensively studied in coinfected patients compared to most other agents. In a meta-analysis of patients with HIV/HBV coinfection, TDF suppressed HBV viral load to undetectable titers in approximately 90% of patients [46]. Tenofovir is available in 2 preparations: TDF and TAF. TDF has been reported to cause renal impairment as well as bone loss. TAF has shown less renal toxicity and less bone damage [40,47]. In 2016, TAF became available as part as 4 regimens: stand-alone TAF, elvitegravir-cobicistat-emtricitabine-TAF, rilpivirine-emtricitabine-TAF, and TAF-emtricitabine.
TDF and TAF both suppress HIV. Two large randomized trials of HBV monoinfection demonstrate that TAF is noninferior to TDF for the treatment of naïve and treatment-experienced patients [48,49].
Entecavir
Entecavir, a guanosine analogue, is a potent HBV DNA polymerase inhibitor that results in greater virologic suppression compared to lamivudine and retains activity against lamivudine-resistant HBV [50]. Importantly, entecavir shares some cross-resistance with lamivudine, so an entecavir dose of 1 mg daily is recommended in lamivudine-experienced patients compared to 0.5 mg daily in lamivudine-naïve patients. Entecavir requires dose reduction for patients with creatinine clearance less than 50 mL/min, although it is not associated with renal toxicity. A 1-log10 reduction in HIV RNA levels as well as emergence of M184V mutations on entecavir monotherapy has been reported [51,52]. M184V confers HIV resistance to lamivudine and emtricitabine. Therefore, entecavir should not be used as monotherapy in HIV-coinfected patients and/or patients with evidence of lamivudine-resistant HBV.
Combination Therapy
Recent updates in the guidelines recommend that since emtricitabine, lamivudine, TDF, and TAF are active against both viruses, patients with coinfection should start ART with a fixed-dose combination of TDF/emtricitabine or TAF/emtricitabine or the individual drug combination TDF plus lamivudine [53]. Most experts recommend the use of combination HBV therapy in patients on ART, particularly with lamivudine given the high rates of resistance.
• When should HBV treatment be started in patients with coinfection?
An ART regimen containing TDF (creatinine clearance > 50 mL/min) or TAF (creatinine clearance > 30 mL/min) with lamivudine or emtricitabine should be used in all HIV/HBV patients as soon as the infection is diagnosed. If TDF or TAF cannot be used, the alternate recommended regimen for HBV is entecavir plus a fully suppressive ART. In those with decreased renal function, entecavir should be adjusted to renal function [19].
Although control of viremia is feasible, clearance of infection as marked by loss of HBsAg and development of anti-HBs is unlikely to occur in the majority of patients. Therefore, the goals of treatment focus on prevention of chronic liver disease complications by suppressing viral replication, which can halt disease progression. A suggested algorithm for the management of coinfected patients is provided (Figure).
• What is the duration of therapy for hepatitis B?
Most patients with HIV/HBV coinfection will require lifelong treatment. All patients on HBV therapy as a part of ART should continue HBV therapy, regardless of seroconversion status. Also, patients should be educated and advised against self-discontinuation as it may trigger hepatitis exacerbations and/or hepatic failure.
Case Patient 3
Initial Presentation and History
Two months after starting treatment for HIV and chronic HBV infection, a 46-year-old Hispanic woman presents to clinic with jaundice and right upper quadrant (RUQ) pain. The patient was recently diagnosed with HIV infection and was naïve to treatment with ART. Her CD4 cell count was 50 cells/µL, and her HIV viral load was 743,000 copies/mL, with no baseline mutations on HIV genotype. The patient was also diagnosed with chronic HBV infection with positive HBsAg and HBeAg and negative HBc IgM serologies, as well as an HBV DNA level of 87 million IU/mL. Routine blood work revealed normal renal function and serum transaminases. The decision was made to start the patient on darunavir/ritonavir and TDF/emtricitabine. The patient was also started on sulfamethoxazole/trimethoprim and azithromycin for opportunistic infection prophylaxis.
Physical Examination and Laboratory Testing
Examination is remarkable for mild tenderness in the RUQ and icteric sclera. Laboratory testing demonstrates the following: AST, 1523 U/L; ALT, 795 U/L; albumin, 2.8 mg/dL; and total bilirubin, 3.5 mg/dL. Her CD4 count has increased to 565 cells/µL, and her HIV viral load is 4320 copies/mL. Results of repeat hepatitis serologies are as follows: HBsAg positive, anti-HBc IgM positive, and an HBV DNA level of 4.2 million IU/mL. Testing for hepatitis A, C, and D is negative, and RUQ sonogram reveals no gallstones.
• What monitoring should be done for coinfected patients on HBV therapy?
Monitoring
Providers should routinely monitor patients’ response to HIV/HBV therapy. Initially, all coinfected patients should have liver function tests and HBV DNA levels checked every 12 weeks on therapy. Frequent monitoring allows early detection of HBV drug resistance as well as drug-related hepatotoxicity. In HBeAg-positive coinfected patients who achieve HBV DNA suppression, HBeAg and anti-HBe testing should be performed every 6 to 12 months to assess for seroconversion. In HBeAg-negative patients, only HBV DNA and liver function tests are needed. HBV virologic failure is defined as a greater than 1-log10 rise in HBV DNA levels or development of viremia in a patient with a previously suppressed DNA level on therapy.
Typically, virologic failure results from either the development of drug resistance or abrupt withdrawal of active HBV therapy due to patient nonadherence or changes to the ART regimen. Virologic failure can result in a rise in serum aminotransferases as well as decompensation in patients with significant underlying liver disease. Due to this risk, providers must counsel patients about the importance of adherence to therapy and should continue medications active against HBV when making a change in ART regimens, unless HBV drug resistance dictates a change in HBV therapy.
• What is the likely cause of this patient’s hepatitis “flare”?
Several studies indicate that patients with HIV/HBV coinfection are at increased risk of drug-related hepatotoxicity and grade 4 liver enzyme elevations [54,55].The first 3 months after initiation of ART is a particularly vulnerable time for liver injury. The differential diagnosis for an acute hepatitis “flare” following the initiation of ART is broad and includes the following: development of HBV drug resistance [16]; withdrawal of HBV-active medications due to nonadherence [54]; ART-related hepatotoxicity; superimposed infection with HAV, HCV, or HDV; other opportunistic infections including cytomegalovirus and mycobacterium avium complex; or IRIS, resulting in an exaggerated cytotoxic response by the recovering immune system [56,57]. Complete evaluation is critical to distinguish between the possible causes.
In this case, several clues point toward HBV-related IRIS as the most likely cause for the hepatitis “flare.” A low pretreatment CD4 cell count with a rapid rise after initiation of ART is associated with a higher rate of IRIS [57]. Serologic testing and imaging excluded superinfection with another hepatotropic virus or biliary tract disease. Appropriate declines in HIV viral load and HBV DNA levels imply patient adherence to therapy and argue against the development of HBV drug resistance. Finally, the emergence of anti-HBc IgM positivity signals HBV reactivation, which is commonly seen in patients with HBV-related IRIS [57]. The preferred treatment for HBV-related IRIS involves continuation of therapy, frequently leading to normalization of aminotransferases and subsequent HBeAg seroconversion. Because IRIS usually manifests within the first 6 to 12 weeks after starting ART, liver enzymes should be monitored closely during this period.
• What health maintenance should be done for coinfected patients?
All patients with HIV/HBV coinfection should be monitored for evidence of portal hypertension or cirrhosis and, if these conditions exist, should undergo endoscopic screening for esophageal varices as well as evaluation of ascites and encephalopathy. Patients with HBV are at increased risk for the development of HCC even in the absence of cirrhosis. A recent study showed low rates of HCC screening in HIV/HBV patients by HIV providers [58]. Whether HIV coinfection potentiates HCC risk is uncertain, though coinfected patients present at younger ages and with more symptoms than HIV-negative comparators [59]. Other risk factors for HCC include HCV infection, alcohol abuse, diabetes, obesity, exposure to environmental toxins, and cirrhosis of any etiology (most commonly non-alcoholic fatty liver disease, primary sclerosing cholangitis, primary biliary cirrhosis and hemochromatosis) [60].
The American Association of Liver Diseases (AASLD) guidelines recommend hepatic ultrasound screening every 6 months in all patients with cirrhosis or chronic HBV who are at increased risk (Asian men over the age of 40 years, Asian women over the age of 50 years, African or North American blacks, and patients with family history of HCC) [61]. They should also be referred for an esophagogastroduodenoscopy to evaluate for esophageal varices. In addition, all HIV/HBV coinfected patients with decompensated liver disease should be evaluated for transplantation. HIV infection is not a contraindication for liver transplant with the use of ART. However, since transplantation does not cure HBV infection, post-transplant HBV immune globulin and HBV treatment are required. Contemporary data suggest comparable survival rates after transplant in coinfected patients compared to HBV-monoinfected patients [51].
Summary
Routine screening with HBsAg, anti-HBs, and anti-HBc serologies is recommended for all HIV-positive individuals. Patients without evidence of prior exposure or vaccination and those with isolated anti-HBc should be offered vaccination. HIV-positive adults should receive three or four 40 µg/mL doses of single agent vaccine depending on the recombinant vaccine type available. Anti-HBsAg titers should be checked 1 month after completion of the immunization series. If quantitative anti-HBsAg levels are < 10 IU/mL, patients should receive a second vaccine cycle.
Patients who test positive for HBsAg should be tested for HBeAg, anti-HBe, and HBV DNA levels and have AST and ALT levels checked as well. All patients with HIV/HBV coinfection should start treatment as soon as HIV infection is diagnosed. ART needs to include 2 drugs against HBV, and therefore a fixed-dose combination of TDF/emtricitabine or TAF/emtricitabine or the individual combination of TDF plus lamivudine should be used.
Coinfected patients on treatment should have liver function tests as well as HBV DNA every 12 weeks. In HBeAg-positive coinfected individuals who achieve HBV DNA suppression, HBeAg and anti-HBe testing should be performed every 6 to 12 months to assess for seroconversion. HBV virologic failure is defined as a greater than 1-log10 rise in HBV DNA levels or development of viremia in a patient with a previously suppressed DNA level on therapy. Those with virologic failure should be tested for HBV resistance thorough HBV genotype. Coinfected patients with cirrhosis should receive ultrasound screening every 6 months for evidence of HCC and esophagogastroduodenoscopy to evaluate for esophageal varices.
1. Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med 2006;166:1632–41.
2. Kourtis AP, Bulterys M, Hu DJ, Jamieson DJ. HIV-HBV coinfection—a global change. N Engl J Med 2012;366:1749–52.
3. Bellini C, Keise O, Chave JP, et al. Liver enzyme elevation after lamivudine withdrawal in HIV-hepatitis B virus co-infected patients: the Swiss HIV Cohort Study. HIV Med 2009;10:12–8.
4. Law WP, Dore GJ, Duncombe CJ, et al. Risk of severe hepatotoxicity associated with antiretroviral therapy in the HIV-NAT Cohort, Thailand, 1996-2001. AIDS 2003;17:2191–18.
5. Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Accessed 6 Dec 2016 at http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Q1–Q17.
6. Piroth L, Launay O, Michel ML, et al. Vaccination against hepatitis B virus (HBV) in HIV-1-infected patients with isolated anti-HBV core antibody: the ANRS HB EP03 CISOVAC prospective study. J Infect Dis 2016;213:1735–42.
7. Centers for Disease Control and Prevention. Recommended adult immunization schedule for adults aged 19 years or older, by vaccine and age group. United States, 2016. Accessed 22 Dec 2016 at www.cdc.gov/vaccines/schedules/hcp/imz/adult.html.
8. Rey D, Krantz V, Partisani M, et al. Increasing the number of hepatitis B vaccine injections augments anti-HBs response rate in HIV-infected patients. Effects on HIV-1 viral load. Vaccine 2000;18:1161–5.
9. Overton ET, Sungkanuparph S, Powderly WG, et al. Undetectable plasma HIV RNA load predicts success after hepatitis B vaccination in HIV-infected persons. Clin Infect Dis 2005;41:1045–8.
10. Fonseca MO, Pang LW, de Paula Cavalheiro N, et al. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine 2005;23:2902–8.
11. Launay O, van der Vliet D, Rosenberg AR, et al. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA 2011;305:1432–40.
12. Chaiklang K, Wipasa J, Chaiwarith R, et al. Comparison of immunogenicity and safety of four doses and four double doses vs. standard doses of hepatitis B vaccination in HIV-infected adults: a randomized, controlled trial. PLoS One 2013;8:e80409.
13. Launay O, van der Vliet D, Rosenberg AR, et al. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA 2011;305:1432–40.
14. Fonseca MO, Pang LW, de Paula Cavalheiro N, et al. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine 2005;23:2902–8.
15. Gandhi RT, Wurcel A, Lee H, et al. Response to hepatitis B vaccine in HIV-1-positive subjects who test positive for isolated antibody to hepatitis B core antigen: implications for hepatitis B vaccine strategies. J Infect Dis 2005;191:1435–41.
16. French AL, Hotton A, Young M, et al. Isolated hepatitis B core antibody status is not associated with accelerated liver disease progression in HIV/hepatitis C coinfection. J Acquir Immune Defic Syndr 2016;72:274–80.
17. INSIGHT START Study Group, Lundgren JD, Babiker GA, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373:795–807.
18. Sebaaly JC, Kelley D. Single-tablet regimens for the treatment of HIV-1 infection. Ann Pharmacother 2017;51:332–44.
19. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Accessed 6 Dec 2016 at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf.
20. Heuft MM, Houba SM, van den Berk GE, et al. Protective effect of hepatitis B virus-active antiretroviral therapy against primary hepatitis B virus infection. AIDS 2014;28:999–1005.
21. Gatanaga H, Hayashida T, Tanuma J, Oka S. Prophylactic effect of antiretroviral therapy on Hepatitis B virus infection. Clin Infect Dis 2013;56:1812–9.
22. Falade-Nwulia O, Seaberg EC, Snider AE, et al. Incident hepatitis B virus infection in HIV-infected and HIV-uninfected men who have sex with men from pre-HAART to HAART periods: a cohort study. Ann Intern Med 2015;163:673–80.
23. Shilaih M, Marzel A, Scherrer AU, et al. Dually active HIV/HBV antiretrovirals as protection against incident hepatitis B infections: potential for prophylaxis. J Infect Dis 2016;214:599–606.
24. Colin JF, Cazals-Hatem D, Loriot MA, et al. Influence of human immunodeficiency virus infection on chronic hepatitis B in homosexual men. Hepatology 1999;29:1306–10.
25. Gilson RJ, Hawkins AE, Beecham MR, et al. Interactions between HIV and hepatitis B virus in homosexual men: effects on the natural history of infection. AIDS 1997;11:597–606.
26. Bodsworth N, Donovan B, Nightingale BN. The effect of concurrent human immunodeficiency virus infection on chronic hepatitis B: a
study of 150 homosexual men. J Infect Dis 1989;160:577–82.
27. Di Martino V, Thevenot T, Colin JF, et al. Influence of HIV infection on the response to interferon therapy and the long-term outcome of chronic hepatitis B. Gastroenterology 2002;123:1812–22.
28. Thio CL, Seaberg EC, Skolasky R Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002;360(9349):1921–6.
29. Konopnicki D, Mocroft A, de Wit S, et al. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS 2005;19:593–601.
30. Nikolopoulos GK, Paraskevis D, Hatzitheodorou E, et al. Impact of hepatitis B virus infection on the progression of AIDS and mortality in HIV-infected individuals: a cohort study and meta-analysis. Clin Infect Dis 2009;48:1763–71.
31. Jain MK, Opio CK, Osuagwu CC, et al. Do HIV care providers appropriately manage hepatitis B in coinfected patients treated with antiretroviral therapy? Clin Infect Dis 2007;44:996–1000.
32. Hearn B, Chasan R, Bichoupan K, et al. Low adherence of HIV providers to practice guidelines for hepatocellular carcinoma screening in HIV/hepatitis B coinfection. Clin Infect Dis 2015;61:1742–8.
33. Matthews GV, Bartholomeusz A, Locarnini S, et al. Characteristics of drug resistant HBV in an international collaborative study of HIV-HBV-infected individuals on extended lamivudine therapy. AIDS 2006;20:863–70.
34. McMahon M, Jilek B, Brennan T, Thio C. The HBV drug entecavir: effects on HIV-1 replication and resistance. N Engl J Med 2007;356:2614–21.
35. Jain M, Zoellner C. Entecavir can select for M184V of HIV-1: a case of an HIV/hepatitis B (HBV) naive patient treated for chronic HBV. AIDS 2007; 21:2365–6.
36. Bottero J, Lacombe K, Guechot J, et al. Performance of 11 biomarkers for liver fibrosis assessment in HIV/HBV coinfected patients. J Hepatol 2009;50:1074–83.
37. Moreno S, Garcia-Samaniego J, Moreno A, et al. Noninvasive diagnosis of liver fibrosis in patients with HIV infection and HCV/HBV co-infection. J Viral Hepat 2009;16:249–58.
38. Miailhes P, Pradat P, Chevallier M, et al. Proficiency of transient elastography compared to liver biopsy for the assessment of fibrosis in HIV/HBV-coinfected patients. J Viral Hepat 2011;18:61–9.
39. Audsley J, Robson C, Aitchison S, et al. Liver fibrosis regression measured by transient elastography in human immunodeficiency virus (HIV)-hepatitis B virus (HBV) coinfected individuals on long-term HBV-active combination antiretroviral therapy. Open Forum Infect Dis 2016;3:ofw035.
40. Achhra AC, Nugent M, Mocroft A, et al. Chronic kidney disease and antiretroviral therapy in HIV-positive individuals: recent developments. Curr HIV/AIDS Rep 2016;13:149–57.
41. Marcellin P, Lau GK, Bonino F, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2004;351:1206–17.
42. Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 2005;352:2682–95.
43. Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med 2004;351:438–50.44. Dore GJ, Cooper DA, Barrett C, et al. Dual efficacy of lamivudine treatment in human immunodeficiency virus/hepatitis B virus-coinfected persons in a randomized, controlled study (CAESAR). The CAESAR Coordinating Committee. J Infect Dis 1999;180:607–13.
45. Hoff J, Bani-Sadr F, Gassin M, Raffi F. Evaluation of chronic hepatitis B virus (HBV) infection in coinfected patients receiving lamivudine as a component of antihuman immunodeficiency virus regimens. Clin Infect Dis 2001;32:963–9.
46. Price H, Dunn D, PIllary D, et al. Suppresion of HBV by tenofovir in HIV/HBV coinfected patients: a systemic review and meta-analysis. PLoS One 2013;8:e68152.
47. Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with eltegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomized, double-blind, phase 3, non-inferiority trial. Lancet 2015;385:2606–15.
48. Chan HLY, Fung S, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAG-positive chronic hepatitis B infection. A randomized, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 2016;1:185–95.
49. Buti M, Gane E, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAG-negative chronic hepatitis B infection. A randomized, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 2016;1:196–206.
50. Chang TT, Lai CL. Hepatitis B virus with primary resistance to adefovir. N Engl J Med 2006;355:322–3.
51. McMahon MA, Jilek BL, Brennan TP, et al. The HBV drug entecavir -- effects on HIV-1 replication and resistance. N Engl J Med 2007;356:2614–21.
52. Jain MK, Zoellner CL. Entecavir can select for M184V of HIV-1: a case of an HIV/hepatitis B (HBV) naive patient treated for chronic HBV. AIDS 2007;21:2365–6.
53. Nuesch R, Ananworanich J, Srasuebkul P, et al. Interruptions of tenofovir/emtricitabine-based antiretroviral therapy in patients with HIV/hepatitis B virus co-infection. AIDS 2008;22:152–4.
54. Gaglio PJ, Sterling R, Daniels E, Tedaldi E. Hepatitis B virus and HIV coinfection: results of a survey on treatment practices and recommendations for therapy. Clin Infect Dis 2007;45:618–23.
55. Reisler RB, Han C, Burman WJ, et al. Grade 4 events are as important as AIDS events in the era of HAART. J Acquir Immune Defic Syndr 2003;34:379–86.
56. Drake A, Mijch A, Sasadeusz J. Immune reconstitution hepatitis in HIV and hepatitis B coinfection, despite lamivudine therapy as part of HAART. Clin Infect Dis 2004;39:129–32.
57. Jain MK, Parekh NK, Hester J, Lee WM. Aminotransferase elevation in HIV/hepatitis B virus co-infected patients treated with two active hepatitis B virus drugs. AIDS Patient Care STDS 2006;20:817–22.
58. Hearn B, Chasan R, Bichoupan K, et al. Low adherence of HIV providers to practice guidelines for hepatocellular carcinoma screening in HIV/hepatitis B coinfection. Clin Infect Dis 2015;611742–8.
59. Brau N, Fox RK, Xiao P, et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a U.S.–Canadian multicenter study. J Hepatol 2007;47:527–37.
60. Yang JD, Harmsen WS, Slettedahl SW, et al. Factors that affect risk for hepatocellular carcinoma and effects of surveillance. Clin Gastroenterol Hepatol 2011;9:617–23.
61. Bruix J, Sherman M. AASLD Practice Guideline. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–2.
1. Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med 2006;166:1632–41.
2. Kourtis AP, Bulterys M, Hu DJ, Jamieson DJ. HIV-HBV coinfection—a global change. N Engl J Med 2012;366:1749–52.
3. Bellini C, Keise O, Chave JP, et al. Liver enzyme elevation after lamivudine withdrawal in HIV-hepatitis B virus co-infected patients: the Swiss HIV Cohort Study. HIV Med 2009;10:12–8.
4. Law WP, Dore GJ, Duncombe CJ, et al. Risk of severe hepatotoxicity associated with antiretroviral therapy in the HIV-NAT Cohort, Thailand, 1996-2001. AIDS 2003;17:2191–18.
5. Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Accessed 6 Dec 2016 at http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Q1–Q17.
6. Piroth L, Launay O, Michel ML, et al. Vaccination against hepatitis B virus (HBV) in HIV-1-infected patients with isolated anti-HBV core antibody: the ANRS HB EP03 CISOVAC prospective study. J Infect Dis 2016;213:1735–42.
7. Centers for Disease Control and Prevention. Recommended adult immunization schedule for adults aged 19 years or older, by vaccine and age group. United States, 2016. Accessed 22 Dec 2016 at www.cdc.gov/vaccines/schedules/hcp/imz/adult.html.
8. Rey D, Krantz V, Partisani M, et al. Increasing the number of hepatitis B vaccine injections augments anti-HBs response rate in HIV-infected patients. Effects on HIV-1 viral load. Vaccine 2000;18:1161–5.
9. Overton ET, Sungkanuparph S, Powderly WG, et al. Undetectable plasma HIV RNA load predicts success after hepatitis B vaccination in HIV-infected persons. Clin Infect Dis 2005;41:1045–8.
10. Fonseca MO, Pang LW, de Paula Cavalheiro N, et al. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine 2005;23:2902–8.
11. Launay O, van der Vliet D, Rosenberg AR, et al. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA 2011;305:1432–40.
12. Chaiklang K, Wipasa J, Chaiwarith R, et al. Comparison of immunogenicity and safety of four doses and four double doses vs. standard doses of hepatitis B vaccination in HIV-infected adults: a randomized, controlled trial. PLoS One 2013;8:e80409.
13. Launay O, van der Vliet D, Rosenberg AR, et al. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA 2011;305:1432–40.
14. Fonseca MO, Pang LW, de Paula Cavalheiro N, et al. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine 2005;23:2902–8.
15. Gandhi RT, Wurcel A, Lee H, et al. Response to hepatitis B vaccine in HIV-1-positive subjects who test positive for isolated antibody to hepatitis B core antigen: implications for hepatitis B vaccine strategies. J Infect Dis 2005;191:1435–41.
16. French AL, Hotton A, Young M, et al. Isolated hepatitis B core antibody status is not associated with accelerated liver disease progression in HIV/hepatitis C coinfection. J Acquir Immune Defic Syndr 2016;72:274–80.
17. INSIGHT START Study Group, Lundgren JD, Babiker GA, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373:795–807.
18. Sebaaly JC, Kelley D. Single-tablet regimens for the treatment of HIV-1 infection. Ann Pharmacother 2017;51:332–44.
19. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Accessed 6 Dec 2016 at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf.
20. Heuft MM, Houba SM, van den Berk GE, et al. Protective effect of hepatitis B virus-active antiretroviral therapy against primary hepatitis B virus infection. AIDS 2014;28:999–1005.
21. Gatanaga H, Hayashida T, Tanuma J, Oka S. Prophylactic effect of antiretroviral therapy on Hepatitis B virus infection. Clin Infect Dis 2013;56:1812–9.
22. Falade-Nwulia O, Seaberg EC, Snider AE, et al. Incident hepatitis B virus infection in HIV-infected and HIV-uninfected men who have sex with men from pre-HAART to HAART periods: a cohort study. Ann Intern Med 2015;163:673–80.
23. Shilaih M, Marzel A, Scherrer AU, et al. Dually active HIV/HBV antiretrovirals as protection against incident hepatitis B infections: potential for prophylaxis. J Infect Dis 2016;214:599–606.
24. Colin JF, Cazals-Hatem D, Loriot MA, et al. Influence of human immunodeficiency virus infection on chronic hepatitis B in homosexual men. Hepatology 1999;29:1306–10.
25. Gilson RJ, Hawkins AE, Beecham MR, et al. Interactions between HIV and hepatitis B virus in homosexual men: effects on the natural history of infection. AIDS 1997;11:597–606.
26. Bodsworth N, Donovan B, Nightingale BN. The effect of concurrent human immunodeficiency virus infection on chronic hepatitis B: a
study of 150 homosexual men. J Infect Dis 1989;160:577–82.
27. Di Martino V, Thevenot T, Colin JF, et al. Influence of HIV infection on the response to interferon therapy and the long-term outcome of chronic hepatitis B. Gastroenterology 2002;123:1812–22.
28. Thio CL, Seaberg EC, Skolasky R Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002;360(9349):1921–6.
29. Konopnicki D, Mocroft A, de Wit S, et al. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS 2005;19:593–601.
30. Nikolopoulos GK, Paraskevis D, Hatzitheodorou E, et al. Impact of hepatitis B virus infection on the progression of AIDS and mortality in HIV-infected individuals: a cohort study and meta-analysis. Clin Infect Dis 2009;48:1763–71.
31. Jain MK, Opio CK, Osuagwu CC, et al. Do HIV care providers appropriately manage hepatitis B in coinfected patients treated with antiretroviral therapy? Clin Infect Dis 2007;44:996–1000.
32. Hearn B, Chasan R, Bichoupan K, et al. Low adherence of HIV providers to practice guidelines for hepatocellular carcinoma screening in HIV/hepatitis B coinfection. Clin Infect Dis 2015;61:1742–8.
33. Matthews GV, Bartholomeusz A, Locarnini S, et al. Characteristics of drug resistant HBV in an international collaborative study of HIV-HBV-infected individuals on extended lamivudine therapy. AIDS 2006;20:863–70.
34. McMahon M, Jilek B, Brennan T, Thio C. The HBV drug entecavir: effects on HIV-1 replication and resistance. N Engl J Med 2007;356:2614–21.
35. Jain M, Zoellner C. Entecavir can select for M184V of HIV-1: a case of an HIV/hepatitis B (HBV) naive patient treated for chronic HBV. AIDS 2007; 21:2365–6.
36. Bottero J, Lacombe K, Guechot J, et al. Performance of 11 biomarkers for liver fibrosis assessment in HIV/HBV coinfected patients. J Hepatol 2009;50:1074–83.
37. Moreno S, Garcia-Samaniego J, Moreno A, et al. Noninvasive diagnosis of liver fibrosis in patients with HIV infection and HCV/HBV co-infection. J Viral Hepat 2009;16:249–58.
38. Miailhes P, Pradat P, Chevallier M, et al. Proficiency of transient elastography compared to liver biopsy for the assessment of fibrosis in HIV/HBV-coinfected patients. J Viral Hepat 2011;18:61–9.
39. Audsley J, Robson C, Aitchison S, et al. Liver fibrosis regression measured by transient elastography in human immunodeficiency virus (HIV)-hepatitis B virus (HBV) coinfected individuals on long-term HBV-active combination antiretroviral therapy. Open Forum Infect Dis 2016;3:ofw035.
40. Achhra AC, Nugent M, Mocroft A, et al. Chronic kidney disease and antiretroviral therapy in HIV-positive individuals: recent developments. Curr HIV/AIDS Rep 2016;13:149–57.
41. Marcellin P, Lau GK, Bonino F, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2004;351:1206–17.
42. Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 2005;352:2682–95.
43. Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med 2004;351:438–50.44. Dore GJ, Cooper DA, Barrett C, et al. Dual efficacy of lamivudine treatment in human immunodeficiency virus/hepatitis B virus-coinfected persons in a randomized, controlled study (CAESAR). The CAESAR Coordinating Committee. J Infect Dis 1999;180:607–13.
45. Hoff J, Bani-Sadr F, Gassin M, Raffi F. Evaluation of chronic hepatitis B virus (HBV) infection in coinfected patients receiving lamivudine as a component of antihuman immunodeficiency virus regimens. Clin Infect Dis 2001;32:963–9.
46. Price H, Dunn D, PIllary D, et al. Suppresion of HBV by tenofovir in HIV/HBV coinfected patients: a systemic review and meta-analysis. PLoS One 2013;8:e68152.
47. Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with eltegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomized, double-blind, phase 3, non-inferiority trial. Lancet 2015;385:2606–15.
48. Chan HLY, Fung S, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAG-positive chronic hepatitis B infection. A randomized, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 2016;1:185–95.
49. Buti M, Gane E, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAG-negative chronic hepatitis B infection. A randomized, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 2016;1:196–206.
50. Chang TT, Lai CL. Hepatitis B virus with primary resistance to adefovir. N Engl J Med 2006;355:322–3.
51. McMahon MA, Jilek BL, Brennan TP, et al. The HBV drug entecavir -- effects on HIV-1 replication and resistance. N Engl J Med 2007;356:2614–21.
52. Jain MK, Zoellner CL. Entecavir can select for M184V of HIV-1: a case of an HIV/hepatitis B (HBV) naive patient treated for chronic HBV. AIDS 2007;21:2365–6.
53. Nuesch R, Ananworanich J, Srasuebkul P, et al. Interruptions of tenofovir/emtricitabine-based antiretroviral therapy in patients with HIV/hepatitis B virus co-infection. AIDS 2008;22:152–4.
54. Gaglio PJ, Sterling R, Daniels E, Tedaldi E. Hepatitis B virus and HIV coinfection: results of a survey on treatment practices and recommendations for therapy. Clin Infect Dis 2007;45:618–23.
55. Reisler RB, Han C, Burman WJ, et al. Grade 4 events are as important as AIDS events in the era of HAART. J Acquir Immune Defic Syndr 2003;34:379–86.
56. Drake A, Mijch A, Sasadeusz J. Immune reconstitution hepatitis in HIV and hepatitis B coinfection, despite lamivudine therapy as part of HAART. Clin Infect Dis 2004;39:129–32.
57. Jain MK, Parekh NK, Hester J, Lee WM. Aminotransferase elevation in HIV/hepatitis B virus co-infected patients treated with two active hepatitis B virus drugs. AIDS Patient Care STDS 2006;20:817–22.
58. Hearn B, Chasan R, Bichoupan K, et al. Low adherence of HIV providers to practice guidelines for hepatocellular carcinoma screening in HIV/hepatitis B coinfection. Clin Infect Dis 2015;611742–8.
59. Brau N, Fox RK, Xiao P, et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a U.S.–Canadian multicenter study. J Hepatol 2007;47:527–37.
60. Yang JD, Harmsen WS, Slettedahl SW, et al. Factors that affect risk for hepatocellular carcinoma and effects of surveillance. Clin Gastroenterol Hepatol 2011;9:617–23.
61. Bruix J, Sherman M. AASLD Practice Guideline. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–2.
The Impact of Two-Person Indwelling Urinary Catheter Insertion in the Emergency Department Using Technical and Socioadaptive Interventions
From Tampa General Hospital, Tampa, FL.
Abstract
- Objective: To decrease insertion-related catheter-associated urinary tract infections (CAUTIs) attributed to the emergency department (ED) as well as facility-wide within a large teaching hospital.
- Methods: Recommendations from the Agency for Healthcare Research and Quality (AHRQ) toolkit for reducing CAUTIs in hospital units were used to implement both technical and socioadaptive changes focused on prevention of insertion-related CAUTIs in the ED through a trial that required 2 licensed personnel for insertion of all urinary catheters. The process would include a safety time-out to confirm catheter appropriateness and review of the proper steps for insertion as a means to encompass and hardwire both the technical and socioadaptive aspects of the Comprehensive Unit-based Safety Project methodology into ED practice.
- Results: There was a 75% decrease in CAUTI rates following the intervention (P = 0.05). This reduction was sustained for at least 1 year following implementation.
- Conclusion: Using AHRQ recommendations to implement socioadaptive and technical changes through 2-person insertion of urinary catheters yielded a significant and sustainable decrease in insertion-related CAUTI rates and utilization of indwelling urinary catheters in the ED at Tampa General Hospital.
Key words: catheter-associated urinary tract infections; infection prevention; quality improvement; change model.
Each year an estimated 721,800 health care–associated infections occur in U.S. acute care hospitals, resulting in approximately 75,000 deaths [1]. Catheter-associated urinary tract infections (CAUTIs) account for an estimated 449,334 of health care–associated infections s annually [2]. The direct medical cost per CAUTI ranges from $749 to $1007, resulting in direct costs to U.S. facilities of over $340 million annually [2]. Although CAUTIs are one of the most common health care–associated infections, the literature has shown that following well established prevention guidelines can greatly reduce their incidence.
Since most health care–associated infections are preventable and cause unnecessary patient harm, there is pressure from regulatory bodies to prevent such events during a patient’s hospitalization. Prevention of CAUTIs is a Joint Commission National Patient Safety Goal, and as of 2008 the Centers for Medicare and Medicaid Services (CMS) does not reimburse hospitals for the cost of additional care as a result of a CAUTI. Additionally, facility CAUTI data is included in the CMS value-based purchasing program, which can withhold payments to hospitals based on performance, as well as the inpatient quality reporting program, which requires public reporting of CAUTI to receive a higher annual payment.
Even before the external pressures of regulatory bodies, Tampa General Hospital has strived to protect patients by preventing infections through implementing best practices via multidisciplinary committees to maximize impact. Tampa General Hospital, a private not-for-profit level 1 trauma center located in downtown Tampa, Florida, is a teaching facility affiliated with the University of South Florida Morsani College of Medicine. It is licensed for more than 1000 beds and serves 12 surrounding counties with a population in excess of 4 million.
Background
CAUTI data had been collected in all of the intensive care units at the hospital for several years, benchmarked against national unit-specific rates, with feedback provided to committees and the hospital board. However, in 2006, a multidisciplinary committee chaired by the chief operating officer known as Committee Targeting Zero (CTZ) was formed to review best practices and analyze all device-associated infection rates in an effort to reduce hospital-acquired infections. To target reduction of the CAUTI rate, a Foley stabilization device and renewed focus on hand hygiene were implemented, and CAUTI rates were reduced by over 50% by the end of 2007.
When CAUTI rates began to climb in 2008, additional interventions were implemented under the direction of CTZ, including a literature review for CAUTI prevention for any new or novel prevention strategies, reporting of each CAUTI to leadership of the attributed unit at the time of identification, ongoing surveillance of the appropriateness of indwelling urinary catheters at the unit level with feedback to CTZ, and mandatory education focused on infection of CAUTI and proper insertion for all staff inserting indwelling urinary catheters. Additionally, in 2009 an evaluation of an antibiotic-coated Foley catheter was implemented to further decrease rates, resulting in a statistically significant 42% reduction in the CAUTI rate as compared to 2008. Other prevention strategies instituted between 2010 and 2012 included increased availability of condom catheters, a closed system urine culture collection kit, and computer-based learning module for all staff inserting indwelling urinary catheters.
In 2013, the hospital included CAUTI prevention as part of a facility-wide initiative to decrease patient harm. A CAUTI committee led by senior leadership was convened to address CAUTI rates that exceeded national benchmarks. The multidisciplinary team began as a subcommittee of CTZ and was chaired by the chief nursing officer with the support of the chief operations officer and included representation from the infection prevention department and nursing unit leadership. After reviewing the Healthcare Infection Control Practices Advisory Committee’s (HICPAC) guideline for prevention of CAUTIs [3], the committee focused its efforts on appropriate indications for insertion and timely removal, aseptic insertion, and proper maintenance of indwelling urinary catheters.
The key accomplishments of the CAUTI committee during 2014 included development of a comprehensive genitourinary management policy, incorporation of CAUTI prevention into new employee orientation for all patient care staff, aseptic indwelling urinary catheter insertion competency check-off with return demonstration (teachback methodology) for all nursing staff, and reinforcement of insertion criteria and daily assessment for necessity with documentation of indications, and removal via nurse-driven protocol when necessary. Additionally, a requirement to document indications for ordering urine cultures and a pop-up reminder in the electronic medical record for patients with an indwelling urinary catheter requiring indications to continue, both targeted towards physicians and advanced practice providers, were implemented.
In conjunction with the technical changes, additional strategies were executed with the intent of facilitating a culture of patient safety and reinforcing the aforementioned technical changes. In 2014, the hospital implemented Franklin Covey’s “The Speed of Trust” methodology [4] and its associated 13 behaviors hospital-wide. Additionally, several of the inpatient units participated in a quality improvement project with either the Florida Hospital Engagement Network (HEN) [5] or the Agency for Healthcare Research and Quality (AHRQ) Comprehensive Unit-based Safety Program (CUSP) [6] national project. Physician engagement and education was accomplished through a white paper written by the infection prevention department, summarizing the current state of CAUTI within the facility and highlighting strategies to reduce infection, including evidence-based guidelines on ordering urine cultures.
In an attempt to target ongoing improvement strategies, CAUTIs were categorized as either insertion-related, occurring within 7 days of insertion, or maintenance-related, occurring greater than 7 days of insertion; the date of insertion was considered day 1. A review of the facility CAUTI data demonstrated that an opportunity to reduce insertion-related CAUTIs existed and a high volume of urinary catheters were inserted in the emergency department (ED). Therefore, ED leadership agreed to participate in the CUSP initiative for EDs beginning April 2014. The goals of the CUSP initiative include using best practices for CAUTI prevention through the implementation of both technical and socioadaptive changes.
Methods
CUSP Initiative
The CUSP initiative focuses specifically on improving processes for determining catheter appropriateness and promoting proper insertion techniques in addition to changes in culture to facilitate teamwork and communication amongst frontline staff and improve collaboration between the ED and inpatient units. To participate in the project, a multidisciplinary team that included ED leadership, infection prevention department, and nursing clinical quality and research specialists was established.
The team designed an intervention that required 2 licensed personnel for insertion of all urinary catheters. The process would include a safety time-out consisting of a pause before inserting the indwelling urinary catheter to confirm catheter appropriateness and review of the proper steps for insertion as a means to encompass and hardwire both the technical and socioadaptive aspects of the CUSP methodology into ED practice.
Rollout Using 4Es
January through March 2015 was the implementation period during which education and validation of practices were conducted. The 4Es model created by the Johns Hopkins University Quality and Safety Research Group was used to roll out the changes in practice to the ED staff; the 4Es are Engagement, Education, Execution, and Evaluation [7]. To engage staff, the scope of CAUTIs, including the implications to both patients and to the health care system as a whole, were presented from a local (hospital) and a national perspective. Education was achieved by outlining the new process in ED staff education sessions, as well as through handouts, emails, and during shift change huddles. The content included a checklist (Figure 1) staff would use to follow proper aseptic technique as well as reminders of the intent of the project.
The process was executed through the use of a safety time-out completed by the 2 personnel (nurses) involved in the procedure prior to insertion of an indwelling urinary catheter. The time-out consisted of reviewing the insertion criteria to determine appropriateness for placement and the proper steps for insertion per hospital policy. The catheter was then inserted by one person while the second was solely responsible to assure compliance with proper aseptic technique. The procedure was stopped if aseptic technique was compromised. The indications for insertion and/or maintaining the urinary catheter are based on the HICPAC guidelines [3] and include the following:
- Acute urinary retention/obstruction
- Urologic, urethral or extensive abdominal surgical procedure
- Critically ill patient with unstable vital signs and requires close urine output monitoring (ICU patient receiving aggressive diuretic therapy, vasopressor/inotropic therapy, paralytic therapy, aggressive fluid management or titrated vasoactive medications)
- Stage 3 or 4 sacral or perineal pressure ulcer in a patient with incontinence
- End of life comfort
- Prevention of further trauma due to a difficult insertion
- Prolonged immobility due to unstable spinal fracture or pelvic fracture and inability to use bedpan.
During the implementation period, a process measure was used to evaluate the rollout. The compliance rate of returned insertion checklists versus the total number of insertions was calculated weekly and tracked over time. Although compliance was low at first, through several Plan-Do-Study-Act (PDSA) cycles conducted on a weekly basis, compliance steadily increased during the implementation period. Staff were also kept abreast of the compliance rates and progress of the project with weekly email updates and periodically in daily huddles during shift change.
Rollout Using 4Es
In parallel to the CUSP framework, the ED leadership team discretely used 6 of “The Speed of Trust” behaviors most relevant to the project to help drive the new process including get better, practice accountability, keep commitments, clarify expectations, deliver results, and create transparency. Get better was used to motivate staff to action in order to deliver the highest quality of care to our patients. Practice accountability was exercised by having the staff sign the checklist used in the new process. Deliver results was supported by the timely feedback of data to frontline staff to show whether the goal was being met. Clarifying expectations was demonstrated through feedback from weekly PDSA rapid cycles and constant reinforcement that all insertions must involve 2 personnel. Keeping commitments was established with an agreement amongst the staff and leadership to keep patients safe and deliver high quality care. Creating transparency was exemplified by explaining the initiative clearly to each patient and their family and allowing for any questions.
Outcomes Measurement
During the post-intervention period, progress was evaluated using 2 outcome measures: the insertion-related CAUTI rate and the catheter utilization ratio. National Healthcare Safety Network (NHSN) 2014 and 2015 criteria was used to identify any CAUTI [8] and for the purposes of this project, the insertion-related CAUTI rate was defined as the number of CAUTIs occurring ≤ 7 days after insertion, with the date of insertion being day 1, per 1000 catheters inserted in the ED. The utilization ratio was calculated from the number of catheters inserted per patient ED visits. The insertion-related CAUTI rates for the pre- and post-intervention periods were compared after excluding 2014 yeast CAUTIs to adjust for changes in the 2015 National Healthcare Safety Network CAUTI criteria, which removed yeast as an organism for CAUTI. The utilization ratio was also calculated and compared between pre- and post-intervention periods. All statistical analysis was done using the NHSN statistics calculator.
Results
During the pre-intervention period (April–December 2014) there were 10 infections and 1450 catheters inserted, which equates to an insertion-related CAUTI rate of 6.9/1,000 catheters. In the post-intervention period (April–December 2015), there were 2 infections and 1180 catheters placed, or an insertion-related CAUTI rate of 1.7/1000 catheters (Table 1)—a 75% decrease from the pre-intervention rate (P = 0.05).
Additionally, the utilization ratio was calculated for 2014 and 2015 based on the number of catheter insertions per total patient ED visits in each year (Table 2). In 2014 the utilization ratio was 2.2 and in 2015 the utilization ratio was 1.7, representing a 23% reduction (P < 0.01).
Following the post-intervention period, insertion-related rates and device utilization were also monitored in 2016. There were a total of 97,004 patient visits to the ED in 2016 with 1530 catheters inserted and 3 insertion-related CAUTIs attributed to the ED. The insertion-related CAUTI rate was 2.0/1000 catheters, which is statistically no different from the post-intervention period rate. The utilization ratio was 1.6, which is less than the post-intervention period (P < 0.01).
Discussion
As highlighted in the AHRQ toolkit [5], the project confirmed that using both technical and socioadaptive methodologies yielded a significant and sustainable impact on CAUTIs and utilization of indwelling urinary catheters. Prior to initiating the project, a review of the literature did not show any previous studies involving the insertion of urinary catheters by 2 licensed personnel. Since then, an acute care facility published data demonstrating a sustainable 39% reduction of CAUTI rates in an inpatient post-surgical unit within 6 months after the implementation of 2-person urinary catheter insertion [9]. The facility had also done extensive education and training on the CAUTI prevention best-practices prior to implementing the new insertion practices.
A key measure of success in regards to implementing cultural and technical changes is the sustainability of the results yielded after implementation. According to the AHRQ CAUTI toolkit, several specific strategies are necessary to successfully sustain prevention efforts. Implementing changes in the ED at our hospital in alignment with the goal of creating a culture of safety, incorporating the changes into daily work flow, employing both technical and socioadaptive interventions, empowering staff to stop the procedure if there are any concerns, and monitoring and communicating outcomes all ensure that the changes in practice will be sustained. Additionally, there is an engaged interdisciplinary CAUTI committee that continues to meet regularly as well as required yearly computer-based education for all frontline staff, and a “Safety Day” education session for all newly hired nurses where competency is assessed and validated for proper insertion and maintenance of a urinary catheter.
Initially, barriers for implementation included limited staff to ensure the presence of 2 licensed personnel for every urinary catheter insertion, lack of ability to collect checklist data in the electronic medical record and run compliance reports, and availability of the checklists at the onset of implementation. The staffing limitation seemed to work in favor of meeting the goals of the project, as staff were less likely to insert indwelling urinary catheters for inappropriate indications. In regards to the checklists, the barriers identified via the PDSA rapid cycles included inadequate locations to obtain checklists for use during insertion and drop-off locations for checklists after use. To increase availability and convenience, brightly colored folders labeled “FOLEY!” containing the checklists were placed both on the outside of the supply management stations and on the doors exiting the supply rooms where indwelling urinary catheter kits were located. Rounds were made on these folders approximately 1 to 2 times per week to be sure they remained full. In addition, more locations for dropping off completed forms were placed at all nursing stations as opposed to a single drop off location.
A limitation of the project is that there are not established metrics for infection rates in any outpatient setting nor are there established criteria to differentiate between insertion- and maintenance-related infections. While the metrics were created for the purposes of the project, they are easily reproducible within other health care facilities to track infection rates associated with outpatient areas. Additionally, by ensuring indications are met and proper insertion occurs in ED patients, the overall hospital’s CAUTI infection rate and standardized infection ratio are impacted, which are comparable across facilities. The criteria for differentiating between insertion and maintenance related infections was established in an attempt to define where the biggest vulnerabilities were with insertion versus maintenance. Days from insertion to infection were tracked for all infections, and arbitrarily a 7-day cutoff was used to consider the infection potentially insertion-related, as no evidence has been published to define this previously.
The lessons learned both during implementation of the changes in practice and the impact it can have on infection rates are valuable. Moving forward, Tampa General Hospital plans to spread dual personnel indwelling urinary catheter insertion as a best practice, first targeting inpatient units identified with the highest number of insertion-related infections as well as high device utilization ratios.
1. Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care–associated infections. N Engl J Med 2014;370:1198–208.
2. Scott, RD. Center for Disease Control and Prevention. The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention. 2009. Accessed at https://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf.
3. Gould CV, Umscheid CA, Agarwal RK, et al. The Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for prevention of catheter-associated urinary tract infections. 2009. Accessed at http://www.cdc.gov/hicpac/pdf/CAUTI/CAUTIguideline2009final.pdf.
4. Covey SMR, Merrill RR. The speed of trust: the one thing that changes everything. New York: Free Press; 2008.
5. Florida Hospital Association Hospital Engagement Network. Update, March 2015. Florida Hospital Association, Orlando, FL. Accessed at www.fha.org/showDocument.aspx?f=2015HEN-Brief-Web.pdf.
6. Agency for Healthcare Research and Quality (AHRQ). Toolkit for reducing catheter-associated urinary tract infections in hospital units: implementation guide. 2014. Accessed at https://www.ahrq.gov/professionals/quality-patient-safety/hais/tools/cauti-hospitals/index.html.
7. Pronovost PJ, Berenholtz SM, Goeschel CA, et al. Creating high reliability in health care organizations. Health Serv Res 2006;41(4 Pt 2):1599–617.
8. Centers for Disease Control National Healthcare Safety Network. Urinary tract infection (catheter-associated urinary tract infection [CAUTI] and non-catheter-associated urinary tract infection [UTI]) and other urinary system infection [USI]) events. 2014. Accessed at https://www.cdc.gov/nhsn/pdfs/pscmanual/7psccauticurrent.pdf.
9. Belizario SM, Preventing urinary tract infections with a two-person catheter insertion procedure. Nursing 2015;45:67–9.
From Tampa General Hospital, Tampa, FL.
Abstract
- Objective: To decrease insertion-related catheter-associated urinary tract infections (CAUTIs) attributed to the emergency department (ED) as well as facility-wide within a large teaching hospital.
- Methods: Recommendations from the Agency for Healthcare Research and Quality (AHRQ) toolkit for reducing CAUTIs in hospital units were used to implement both technical and socioadaptive changes focused on prevention of insertion-related CAUTIs in the ED through a trial that required 2 licensed personnel for insertion of all urinary catheters. The process would include a safety time-out to confirm catheter appropriateness and review of the proper steps for insertion as a means to encompass and hardwire both the technical and socioadaptive aspects of the Comprehensive Unit-based Safety Project methodology into ED practice.
- Results: There was a 75% decrease in CAUTI rates following the intervention (P = 0.05). This reduction was sustained for at least 1 year following implementation.
- Conclusion: Using AHRQ recommendations to implement socioadaptive and technical changes through 2-person insertion of urinary catheters yielded a significant and sustainable decrease in insertion-related CAUTI rates and utilization of indwelling urinary catheters in the ED at Tampa General Hospital.
Key words: catheter-associated urinary tract infections; infection prevention; quality improvement; change model.
Each year an estimated 721,800 health care–associated infections occur in U.S. acute care hospitals, resulting in approximately 75,000 deaths [1]. Catheter-associated urinary tract infections (CAUTIs) account for an estimated 449,334 of health care–associated infections s annually [2]. The direct medical cost per CAUTI ranges from $749 to $1007, resulting in direct costs to U.S. facilities of over $340 million annually [2]. Although CAUTIs are one of the most common health care–associated infections, the literature has shown that following well established prevention guidelines can greatly reduce their incidence.
Since most health care–associated infections are preventable and cause unnecessary patient harm, there is pressure from regulatory bodies to prevent such events during a patient’s hospitalization. Prevention of CAUTIs is a Joint Commission National Patient Safety Goal, and as of 2008 the Centers for Medicare and Medicaid Services (CMS) does not reimburse hospitals for the cost of additional care as a result of a CAUTI. Additionally, facility CAUTI data is included in the CMS value-based purchasing program, which can withhold payments to hospitals based on performance, as well as the inpatient quality reporting program, which requires public reporting of CAUTI to receive a higher annual payment.
Even before the external pressures of regulatory bodies, Tampa General Hospital has strived to protect patients by preventing infections through implementing best practices via multidisciplinary committees to maximize impact. Tampa General Hospital, a private not-for-profit level 1 trauma center located in downtown Tampa, Florida, is a teaching facility affiliated with the University of South Florida Morsani College of Medicine. It is licensed for more than 1000 beds and serves 12 surrounding counties with a population in excess of 4 million.
Background
CAUTI data had been collected in all of the intensive care units at the hospital for several years, benchmarked against national unit-specific rates, with feedback provided to committees and the hospital board. However, in 2006, a multidisciplinary committee chaired by the chief operating officer known as Committee Targeting Zero (CTZ) was formed to review best practices and analyze all device-associated infection rates in an effort to reduce hospital-acquired infections. To target reduction of the CAUTI rate, a Foley stabilization device and renewed focus on hand hygiene were implemented, and CAUTI rates were reduced by over 50% by the end of 2007.
When CAUTI rates began to climb in 2008, additional interventions were implemented under the direction of CTZ, including a literature review for CAUTI prevention for any new or novel prevention strategies, reporting of each CAUTI to leadership of the attributed unit at the time of identification, ongoing surveillance of the appropriateness of indwelling urinary catheters at the unit level with feedback to CTZ, and mandatory education focused on infection of CAUTI and proper insertion for all staff inserting indwelling urinary catheters. Additionally, in 2009 an evaluation of an antibiotic-coated Foley catheter was implemented to further decrease rates, resulting in a statistically significant 42% reduction in the CAUTI rate as compared to 2008. Other prevention strategies instituted between 2010 and 2012 included increased availability of condom catheters, a closed system urine culture collection kit, and computer-based learning module for all staff inserting indwelling urinary catheters.
In 2013, the hospital included CAUTI prevention as part of a facility-wide initiative to decrease patient harm. A CAUTI committee led by senior leadership was convened to address CAUTI rates that exceeded national benchmarks. The multidisciplinary team began as a subcommittee of CTZ and was chaired by the chief nursing officer with the support of the chief operations officer and included representation from the infection prevention department and nursing unit leadership. After reviewing the Healthcare Infection Control Practices Advisory Committee’s (HICPAC) guideline for prevention of CAUTIs [3], the committee focused its efforts on appropriate indications for insertion and timely removal, aseptic insertion, and proper maintenance of indwelling urinary catheters.
The key accomplishments of the CAUTI committee during 2014 included development of a comprehensive genitourinary management policy, incorporation of CAUTI prevention into new employee orientation for all patient care staff, aseptic indwelling urinary catheter insertion competency check-off with return demonstration (teachback methodology) for all nursing staff, and reinforcement of insertion criteria and daily assessment for necessity with documentation of indications, and removal via nurse-driven protocol when necessary. Additionally, a requirement to document indications for ordering urine cultures and a pop-up reminder in the electronic medical record for patients with an indwelling urinary catheter requiring indications to continue, both targeted towards physicians and advanced practice providers, were implemented.
In conjunction with the technical changes, additional strategies were executed with the intent of facilitating a culture of patient safety and reinforcing the aforementioned technical changes. In 2014, the hospital implemented Franklin Covey’s “The Speed of Trust” methodology [4] and its associated 13 behaviors hospital-wide. Additionally, several of the inpatient units participated in a quality improvement project with either the Florida Hospital Engagement Network (HEN) [5] or the Agency for Healthcare Research and Quality (AHRQ) Comprehensive Unit-based Safety Program (CUSP) [6] national project. Physician engagement and education was accomplished through a white paper written by the infection prevention department, summarizing the current state of CAUTI within the facility and highlighting strategies to reduce infection, including evidence-based guidelines on ordering urine cultures.
In an attempt to target ongoing improvement strategies, CAUTIs were categorized as either insertion-related, occurring within 7 days of insertion, or maintenance-related, occurring greater than 7 days of insertion; the date of insertion was considered day 1. A review of the facility CAUTI data demonstrated that an opportunity to reduce insertion-related CAUTIs existed and a high volume of urinary catheters were inserted in the emergency department (ED). Therefore, ED leadership agreed to participate in the CUSP initiative for EDs beginning April 2014. The goals of the CUSP initiative include using best practices for CAUTI prevention through the implementation of both technical and socioadaptive changes.
Methods
CUSP Initiative
The CUSP initiative focuses specifically on improving processes for determining catheter appropriateness and promoting proper insertion techniques in addition to changes in culture to facilitate teamwork and communication amongst frontline staff and improve collaboration between the ED and inpatient units. To participate in the project, a multidisciplinary team that included ED leadership, infection prevention department, and nursing clinical quality and research specialists was established.
The team designed an intervention that required 2 licensed personnel for insertion of all urinary catheters. The process would include a safety time-out consisting of a pause before inserting the indwelling urinary catheter to confirm catheter appropriateness and review of the proper steps for insertion as a means to encompass and hardwire both the technical and socioadaptive aspects of the CUSP methodology into ED practice.
Rollout Using 4Es
January through March 2015 was the implementation period during which education and validation of practices were conducted. The 4Es model created by the Johns Hopkins University Quality and Safety Research Group was used to roll out the changes in practice to the ED staff; the 4Es are Engagement, Education, Execution, and Evaluation [7]. To engage staff, the scope of CAUTIs, including the implications to both patients and to the health care system as a whole, were presented from a local (hospital) and a national perspective. Education was achieved by outlining the new process in ED staff education sessions, as well as through handouts, emails, and during shift change huddles. The content included a checklist (Figure 1) staff would use to follow proper aseptic technique as well as reminders of the intent of the project.
The process was executed through the use of a safety time-out completed by the 2 personnel (nurses) involved in the procedure prior to insertion of an indwelling urinary catheter. The time-out consisted of reviewing the insertion criteria to determine appropriateness for placement and the proper steps for insertion per hospital policy. The catheter was then inserted by one person while the second was solely responsible to assure compliance with proper aseptic technique. The procedure was stopped if aseptic technique was compromised. The indications for insertion and/or maintaining the urinary catheter are based on the HICPAC guidelines [3] and include the following:
- Acute urinary retention/obstruction
- Urologic, urethral or extensive abdominal surgical procedure
- Critically ill patient with unstable vital signs and requires close urine output monitoring (ICU patient receiving aggressive diuretic therapy, vasopressor/inotropic therapy, paralytic therapy, aggressive fluid management or titrated vasoactive medications)
- Stage 3 or 4 sacral or perineal pressure ulcer in a patient with incontinence
- End of life comfort
- Prevention of further trauma due to a difficult insertion
- Prolonged immobility due to unstable spinal fracture or pelvic fracture and inability to use bedpan.
During the implementation period, a process measure was used to evaluate the rollout. The compliance rate of returned insertion checklists versus the total number of insertions was calculated weekly and tracked over time. Although compliance was low at first, through several Plan-Do-Study-Act (PDSA) cycles conducted on a weekly basis, compliance steadily increased during the implementation period. Staff were also kept abreast of the compliance rates and progress of the project with weekly email updates and periodically in daily huddles during shift change.
Rollout Using 4Es
In parallel to the CUSP framework, the ED leadership team discretely used 6 of “The Speed of Trust” behaviors most relevant to the project to help drive the new process including get better, practice accountability, keep commitments, clarify expectations, deliver results, and create transparency. Get better was used to motivate staff to action in order to deliver the highest quality of care to our patients. Practice accountability was exercised by having the staff sign the checklist used in the new process. Deliver results was supported by the timely feedback of data to frontline staff to show whether the goal was being met. Clarifying expectations was demonstrated through feedback from weekly PDSA rapid cycles and constant reinforcement that all insertions must involve 2 personnel. Keeping commitments was established with an agreement amongst the staff and leadership to keep patients safe and deliver high quality care. Creating transparency was exemplified by explaining the initiative clearly to each patient and their family and allowing for any questions.
Outcomes Measurement
During the post-intervention period, progress was evaluated using 2 outcome measures: the insertion-related CAUTI rate and the catheter utilization ratio. National Healthcare Safety Network (NHSN) 2014 and 2015 criteria was used to identify any CAUTI [8] and for the purposes of this project, the insertion-related CAUTI rate was defined as the number of CAUTIs occurring ≤ 7 days after insertion, with the date of insertion being day 1, per 1000 catheters inserted in the ED. The utilization ratio was calculated from the number of catheters inserted per patient ED visits. The insertion-related CAUTI rates for the pre- and post-intervention periods were compared after excluding 2014 yeast CAUTIs to adjust for changes in the 2015 National Healthcare Safety Network CAUTI criteria, which removed yeast as an organism for CAUTI. The utilization ratio was also calculated and compared between pre- and post-intervention periods. All statistical analysis was done using the NHSN statistics calculator.
Results
During the pre-intervention period (April–December 2014) there were 10 infections and 1450 catheters inserted, which equates to an insertion-related CAUTI rate of 6.9/1,000 catheters. In the post-intervention period (April–December 2015), there were 2 infections and 1180 catheters placed, or an insertion-related CAUTI rate of 1.7/1000 catheters (Table 1)—a 75% decrease from the pre-intervention rate (P = 0.05).
Additionally, the utilization ratio was calculated for 2014 and 2015 based on the number of catheter insertions per total patient ED visits in each year (Table 2). In 2014 the utilization ratio was 2.2 and in 2015 the utilization ratio was 1.7, representing a 23% reduction (P < 0.01).
Following the post-intervention period, insertion-related rates and device utilization were also monitored in 2016. There were a total of 97,004 patient visits to the ED in 2016 with 1530 catheters inserted and 3 insertion-related CAUTIs attributed to the ED. The insertion-related CAUTI rate was 2.0/1000 catheters, which is statistically no different from the post-intervention period rate. The utilization ratio was 1.6, which is less than the post-intervention period (P < 0.01).
Discussion
As highlighted in the AHRQ toolkit [5], the project confirmed that using both technical and socioadaptive methodologies yielded a significant and sustainable impact on CAUTIs and utilization of indwelling urinary catheters. Prior to initiating the project, a review of the literature did not show any previous studies involving the insertion of urinary catheters by 2 licensed personnel. Since then, an acute care facility published data demonstrating a sustainable 39% reduction of CAUTI rates in an inpatient post-surgical unit within 6 months after the implementation of 2-person urinary catheter insertion [9]. The facility had also done extensive education and training on the CAUTI prevention best-practices prior to implementing the new insertion practices.
A key measure of success in regards to implementing cultural and technical changes is the sustainability of the results yielded after implementation. According to the AHRQ CAUTI toolkit, several specific strategies are necessary to successfully sustain prevention efforts. Implementing changes in the ED at our hospital in alignment with the goal of creating a culture of safety, incorporating the changes into daily work flow, employing both technical and socioadaptive interventions, empowering staff to stop the procedure if there are any concerns, and monitoring and communicating outcomes all ensure that the changes in practice will be sustained. Additionally, there is an engaged interdisciplinary CAUTI committee that continues to meet regularly as well as required yearly computer-based education for all frontline staff, and a “Safety Day” education session for all newly hired nurses where competency is assessed and validated for proper insertion and maintenance of a urinary catheter.
Initially, barriers for implementation included limited staff to ensure the presence of 2 licensed personnel for every urinary catheter insertion, lack of ability to collect checklist data in the electronic medical record and run compliance reports, and availability of the checklists at the onset of implementation. The staffing limitation seemed to work in favor of meeting the goals of the project, as staff were less likely to insert indwelling urinary catheters for inappropriate indications. In regards to the checklists, the barriers identified via the PDSA rapid cycles included inadequate locations to obtain checklists for use during insertion and drop-off locations for checklists after use. To increase availability and convenience, brightly colored folders labeled “FOLEY!” containing the checklists were placed both on the outside of the supply management stations and on the doors exiting the supply rooms where indwelling urinary catheter kits were located. Rounds were made on these folders approximately 1 to 2 times per week to be sure they remained full. In addition, more locations for dropping off completed forms were placed at all nursing stations as opposed to a single drop off location.
A limitation of the project is that there are not established metrics for infection rates in any outpatient setting nor are there established criteria to differentiate between insertion- and maintenance-related infections. While the metrics were created for the purposes of the project, they are easily reproducible within other health care facilities to track infection rates associated with outpatient areas. Additionally, by ensuring indications are met and proper insertion occurs in ED patients, the overall hospital’s CAUTI infection rate and standardized infection ratio are impacted, which are comparable across facilities. The criteria for differentiating between insertion and maintenance related infections was established in an attempt to define where the biggest vulnerabilities were with insertion versus maintenance. Days from insertion to infection were tracked for all infections, and arbitrarily a 7-day cutoff was used to consider the infection potentially insertion-related, as no evidence has been published to define this previously.
The lessons learned both during implementation of the changes in practice and the impact it can have on infection rates are valuable. Moving forward, Tampa General Hospital plans to spread dual personnel indwelling urinary catheter insertion as a best practice, first targeting inpatient units identified with the highest number of insertion-related infections as well as high device utilization ratios.
From Tampa General Hospital, Tampa, FL.
Abstract
- Objective: To decrease insertion-related catheter-associated urinary tract infections (CAUTIs) attributed to the emergency department (ED) as well as facility-wide within a large teaching hospital.
- Methods: Recommendations from the Agency for Healthcare Research and Quality (AHRQ) toolkit for reducing CAUTIs in hospital units were used to implement both technical and socioadaptive changes focused on prevention of insertion-related CAUTIs in the ED through a trial that required 2 licensed personnel for insertion of all urinary catheters. The process would include a safety time-out to confirm catheter appropriateness and review of the proper steps for insertion as a means to encompass and hardwire both the technical and socioadaptive aspects of the Comprehensive Unit-based Safety Project methodology into ED practice.
- Results: There was a 75% decrease in CAUTI rates following the intervention (P = 0.05). This reduction was sustained for at least 1 year following implementation.
- Conclusion: Using AHRQ recommendations to implement socioadaptive and technical changes through 2-person insertion of urinary catheters yielded a significant and sustainable decrease in insertion-related CAUTI rates and utilization of indwelling urinary catheters in the ED at Tampa General Hospital.
Key words: catheter-associated urinary tract infections; infection prevention; quality improvement; change model.
Each year an estimated 721,800 health care–associated infections occur in U.S. acute care hospitals, resulting in approximately 75,000 deaths [1]. Catheter-associated urinary tract infections (CAUTIs) account for an estimated 449,334 of health care–associated infections s annually [2]. The direct medical cost per CAUTI ranges from $749 to $1007, resulting in direct costs to U.S. facilities of over $340 million annually [2]. Although CAUTIs are one of the most common health care–associated infections, the literature has shown that following well established prevention guidelines can greatly reduce their incidence.
Since most health care–associated infections are preventable and cause unnecessary patient harm, there is pressure from regulatory bodies to prevent such events during a patient’s hospitalization. Prevention of CAUTIs is a Joint Commission National Patient Safety Goal, and as of 2008 the Centers for Medicare and Medicaid Services (CMS) does not reimburse hospitals for the cost of additional care as a result of a CAUTI. Additionally, facility CAUTI data is included in the CMS value-based purchasing program, which can withhold payments to hospitals based on performance, as well as the inpatient quality reporting program, which requires public reporting of CAUTI to receive a higher annual payment.
Even before the external pressures of regulatory bodies, Tampa General Hospital has strived to protect patients by preventing infections through implementing best practices via multidisciplinary committees to maximize impact. Tampa General Hospital, a private not-for-profit level 1 trauma center located in downtown Tampa, Florida, is a teaching facility affiliated with the University of South Florida Morsani College of Medicine. It is licensed for more than 1000 beds and serves 12 surrounding counties with a population in excess of 4 million.
Background
CAUTI data had been collected in all of the intensive care units at the hospital for several years, benchmarked against national unit-specific rates, with feedback provided to committees and the hospital board. However, in 2006, a multidisciplinary committee chaired by the chief operating officer known as Committee Targeting Zero (CTZ) was formed to review best practices and analyze all device-associated infection rates in an effort to reduce hospital-acquired infections. To target reduction of the CAUTI rate, a Foley stabilization device and renewed focus on hand hygiene were implemented, and CAUTI rates were reduced by over 50% by the end of 2007.
When CAUTI rates began to climb in 2008, additional interventions were implemented under the direction of CTZ, including a literature review for CAUTI prevention for any new or novel prevention strategies, reporting of each CAUTI to leadership of the attributed unit at the time of identification, ongoing surveillance of the appropriateness of indwelling urinary catheters at the unit level with feedback to CTZ, and mandatory education focused on infection of CAUTI and proper insertion for all staff inserting indwelling urinary catheters. Additionally, in 2009 an evaluation of an antibiotic-coated Foley catheter was implemented to further decrease rates, resulting in a statistically significant 42% reduction in the CAUTI rate as compared to 2008. Other prevention strategies instituted between 2010 and 2012 included increased availability of condom catheters, a closed system urine culture collection kit, and computer-based learning module for all staff inserting indwelling urinary catheters.
In 2013, the hospital included CAUTI prevention as part of a facility-wide initiative to decrease patient harm. A CAUTI committee led by senior leadership was convened to address CAUTI rates that exceeded national benchmarks. The multidisciplinary team began as a subcommittee of CTZ and was chaired by the chief nursing officer with the support of the chief operations officer and included representation from the infection prevention department and nursing unit leadership. After reviewing the Healthcare Infection Control Practices Advisory Committee’s (HICPAC) guideline for prevention of CAUTIs [3], the committee focused its efforts on appropriate indications for insertion and timely removal, aseptic insertion, and proper maintenance of indwelling urinary catheters.
The key accomplishments of the CAUTI committee during 2014 included development of a comprehensive genitourinary management policy, incorporation of CAUTI prevention into new employee orientation for all patient care staff, aseptic indwelling urinary catheter insertion competency check-off with return demonstration (teachback methodology) for all nursing staff, and reinforcement of insertion criteria and daily assessment for necessity with documentation of indications, and removal via nurse-driven protocol when necessary. Additionally, a requirement to document indications for ordering urine cultures and a pop-up reminder in the electronic medical record for patients with an indwelling urinary catheter requiring indications to continue, both targeted towards physicians and advanced practice providers, were implemented.
In conjunction with the technical changes, additional strategies were executed with the intent of facilitating a culture of patient safety and reinforcing the aforementioned technical changes. In 2014, the hospital implemented Franklin Covey’s “The Speed of Trust” methodology [4] and its associated 13 behaviors hospital-wide. Additionally, several of the inpatient units participated in a quality improvement project with either the Florida Hospital Engagement Network (HEN) [5] or the Agency for Healthcare Research and Quality (AHRQ) Comprehensive Unit-based Safety Program (CUSP) [6] national project. Physician engagement and education was accomplished through a white paper written by the infection prevention department, summarizing the current state of CAUTI within the facility and highlighting strategies to reduce infection, including evidence-based guidelines on ordering urine cultures.
In an attempt to target ongoing improvement strategies, CAUTIs were categorized as either insertion-related, occurring within 7 days of insertion, or maintenance-related, occurring greater than 7 days of insertion; the date of insertion was considered day 1. A review of the facility CAUTI data demonstrated that an opportunity to reduce insertion-related CAUTIs existed and a high volume of urinary catheters were inserted in the emergency department (ED). Therefore, ED leadership agreed to participate in the CUSP initiative for EDs beginning April 2014. The goals of the CUSP initiative include using best practices for CAUTI prevention through the implementation of both technical and socioadaptive changes.
Methods
CUSP Initiative
The CUSP initiative focuses specifically on improving processes for determining catheter appropriateness and promoting proper insertion techniques in addition to changes in culture to facilitate teamwork and communication amongst frontline staff and improve collaboration between the ED and inpatient units. To participate in the project, a multidisciplinary team that included ED leadership, infection prevention department, and nursing clinical quality and research specialists was established.
The team designed an intervention that required 2 licensed personnel for insertion of all urinary catheters. The process would include a safety time-out consisting of a pause before inserting the indwelling urinary catheter to confirm catheter appropriateness and review of the proper steps for insertion as a means to encompass and hardwire both the technical and socioadaptive aspects of the CUSP methodology into ED practice.
Rollout Using 4Es
January through March 2015 was the implementation period during which education and validation of practices were conducted. The 4Es model created by the Johns Hopkins University Quality and Safety Research Group was used to roll out the changes in practice to the ED staff; the 4Es are Engagement, Education, Execution, and Evaluation [7]. To engage staff, the scope of CAUTIs, including the implications to both patients and to the health care system as a whole, were presented from a local (hospital) and a national perspective. Education was achieved by outlining the new process in ED staff education sessions, as well as through handouts, emails, and during shift change huddles. The content included a checklist (Figure 1) staff would use to follow proper aseptic technique as well as reminders of the intent of the project.
The process was executed through the use of a safety time-out completed by the 2 personnel (nurses) involved in the procedure prior to insertion of an indwelling urinary catheter. The time-out consisted of reviewing the insertion criteria to determine appropriateness for placement and the proper steps for insertion per hospital policy. The catheter was then inserted by one person while the second was solely responsible to assure compliance with proper aseptic technique. The procedure was stopped if aseptic technique was compromised. The indications for insertion and/or maintaining the urinary catheter are based on the HICPAC guidelines [3] and include the following:
- Acute urinary retention/obstruction
- Urologic, urethral or extensive abdominal surgical procedure
- Critically ill patient with unstable vital signs and requires close urine output monitoring (ICU patient receiving aggressive diuretic therapy, vasopressor/inotropic therapy, paralytic therapy, aggressive fluid management or titrated vasoactive medications)
- Stage 3 or 4 sacral or perineal pressure ulcer in a patient with incontinence
- End of life comfort
- Prevention of further trauma due to a difficult insertion
- Prolonged immobility due to unstable spinal fracture or pelvic fracture and inability to use bedpan.
During the implementation period, a process measure was used to evaluate the rollout. The compliance rate of returned insertion checklists versus the total number of insertions was calculated weekly and tracked over time. Although compliance was low at first, through several Plan-Do-Study-Act (PDSA) cycles conducted on a weekly basis, compliance steadily increased during the implementation period. Staff were also kept abreast of the compliance rates and progress of the project with weekly email updates and periodically in daily huddles during shift change.
Rollout Using 4Es
In parallel to the CUSP framework, the ED leadership team discretely used 6 of “The Speed of Trust” behaviors most relevant to the project to help drive the new process including get better, practice accountability, keep commitments, clarify expectations, deliver results, and create transparency. Get better was used to motivate staff to action in order to deliver the highest quality of care to our patients. Practice accountability was exercised by having the staff sign the checklist used in the new process. Deliver results was supported by the timely feedback of data to frontline staff to show whether the goal was being met. Clarifying expectations was demonstrated through feedback from weekly PDSA rapid cycles and constant reinforcement that all insertions must involve 2 personnel. Keeping commitments was established with an agreement amongst the staff and leadership to keep patients safe and deliver high quality care. Creating transparency was exemplified by explaining the initiative clearly to each patient and their family and allowing for any questions.
Outcomes Measurement
During the post-intervention period, progress was evaluated using 2 outcome measures: the insertion-related CAUTI rate and the catheter utilization ratio. National Healthcare Safety Network (NHSN) 2014 and 2015 criteria was used to identify any CAUTI [8] and for the purposes of this project, the insertion-related CAUTI rate was defined as the number of CAUTIs occurring ≤ 7 days after insertion, with the date of insertion being day 1, per 1000 catheters inserted in the ED. The utilization ratio was calculated from the number of catheters inserted per patient ED visits. The insertion-related CAUTI rates for the pre- and post-intervention periods were compared after excluding 2014 yeast CAUTIs to adjust for changes in the 2015 National Healthcare Safety Network CAUTI criteria, which removed yeast as an organism for CAUTI. The utilization ratio was also calculated and compared between pre- and post-intervention periods. All statistical analysis was done using the NHSN statistics calculator.
Results
During the pre-intervention period (April–December 2014) there were 10 infections and 1450 catheters inserted, which equates to an insertion-related CAUTI rate of 6.9/1,000 catheters. In the post-intervention period (April–December 2015), there were 2 infections and 1180 catheters placed, or an insertion-related CAUTI rate of 1.7/1000 catheters (Table 1)—a 75% decrease from the pre-intervention rate (P = 0.05).
Additionally, the utilization ratio was calculated for 2014 and 2015 based on the number of catheter insertions per total patient ED visits in each year (Table 2). In 2014 the utilization ratio was 2.2 and in 2015 the utilization ratio was 1.7, representing a 23% reduction (P < 0.01).
Following the post-intervention period, insertion-related rates and device utilization were also monitored in 2016. There were a total of 97,004 patient visits to the ED in 2016 with 1530 catheters inserted and 3 insertion-related CAUTIs attributed to the ED. The insertion-related CAUTI rate was 2.0/1000 catheters, which is statistically no different from the post-intervention period rate. The utilization ratio was 1.6, which is less than the post-intervention period (P < 0.01).
Discussion
As highlighted in the AHRQ toolkit [5], the project confirmed that using both technical and socioadaptive methodologies yielded a significant and sustainable impact on CAUTIs and utilization of indwelling urinary catheters. Prior to initiating the project, a review of the literature did not show any previous studies involving the insertion of urinary catheters by 2 licensed personnel. Since then, an acute care facility published data demonstrating a sustainable 39% reduction of CAUTI rates in an inpatient post-surgical unit within 6 months after the implementation of 2-person urinary catheter insertion [9]. The facility had also done extensive education and training on the CAUTI prevention best-practices prior to implementing the new insertion practices.
A key measure of success in regards to implementing cultural and technical changes is the sustainability of the results yielded after implementation. According to the AHRQ CAUTI toolkit, several specific strategies are necessary to successfully sustain prevention efforts. Implementing changes in the ED at our hospital in alignment with the goal of creating a culture of safety, incorporating the changes into daily work flow, employing both technical and socioadaptive interventions, empowering staff to stop the procedure if there are any concerns, and monitoring and communicating outcomes all ensure that the changes in practice will be sustained. Additionally, there is an engaged interdisciplinary CAUTI committee that continues to meet regularly as well as required yearly computer-based education for all frontline staff, and a “Safety Day” education session for all newly hired nurses where competency is assessed and validated for proper insertion and maintenance of a urinary catheter.
Initially, barriers for implementation included limited staff to ensure the presence of 2 licensed personnel for every urinary catheter insertion, lack of ability to collect checklist data in the electronic medical record and run compliance reports, and availability of the checklists at the onset of implementation. The staffing limitation seemed to work in favor of meeting the goals of the project, as staff were less likely to insert indwelling urinary catheters for inappropriate indications. In regards to the checklists, the barriers identified via the PDSA rapid cycles included inadequate locations to obtain checklists for use during insertion and drop-off locations for checklists after use. To increase availability and convenience, brightly colored folders labeled “FOLEY!” containing the checklists were placed both on the outside of the supply management stations and on the doors exiting the supply rooms where indwelling urinary catheter kits were located. Rounds were made on these folders approximately 1 to 2 times per week to be sure they remained full. In addition, more locations for dropping off completed forms were placed at all nursing stations as opposed to a single drop off location.
A limitation of the project is that there are not established metrics for infection rates in any outpatient setting nor are there established criteria to differentiate between insertion- and maintenance-related infections. While the metrics were created for the purposes of the project, they are easily reproducible within other health care facilities to track infection rates associated with outpatient areas. Additionally, by ensuring indications are met and proper insertion occurs in ED patients, the overall hospital’s CAUTI infection rate and standardized infection ratio are impacted, which are comparable across facilities. The criteria for differentiating between insertion and maintenance related infections was established in an attempt to define where the biggest vulnerabilities were with insertion versus maintenance. Days from insertion to infection were tracked for all infections, and arbitrarily a 7-day cutoff was used to consider the infection potentially insertion-related, as no evidence has been published to define this previously.
The lessons learned both during implementation of the changes in practice and the impact it can have on infection rates are valuable. Moving forward, Tampa General Hospital plans to spread dual personnel indwelling urinary catheter insertion as a best practice, first targeting inpatient units identified with the highest number of insertion-related infections as well as high device utilization ratios.
1. Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care–associated infections. N Engl J Med 2014;370:1198–208.
2. Scott, RD. Center for Disease Control and Prevention. The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention. 2009. Accessed at https://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf.
3. Gould CV, Umscheid CA, Agarwal RK, et al. The Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for prevention of catheter-associated urinary tract infections. 2009. Accessed at http://www.cdc.gov/hicpac/pdf/CAUTI/CAUTIguideline2009final.pdf.
4. Covey SMR, Merrill RR. The speed of trust: the one thing that changes everything. New York: Free Press; 2008.
5. Florida Hospital Association Hospital Engagement Network. Update, March 2015. Florida Hospital Association, Orlando, FL. Accessed at www.fha.org/showDocument.aspx?f=2015HEN-Brief-Web.pdf.
6. Agency for Healthcare Research and Quality (AHRQ). Toolkit for reducing catheter-associated urinary tract infections in hospital units: implementation guide. 2014. Accessed at https://www.ahrq.gov/professionals/quality-patient-safety/hais/tools/cauti-hospitals/index.html.
7. Pronovost PJ, Berenholtz SM, Goeschel CA, et al. Creating high reliability in health care organizations. Health Serv Res 2006;41(4 Pt 2):1599–617.
8. Centers for Disease Control National Healthcare Safety Network. Urinary tract infection (catheter-associated urinary tract infection [CAUTI] and non-catheter-associated urinary tract infection [UTI]) and other urinary system infection [USI]) events. 2014. Accessed at https://www.cdc.gov/nhsn/pdfs/pscmanual/7psccauticurrent.pdf.
9. Belizario SM, Preventing urinary tract infections with a two-person catheter insertion procedure. Nursing 2015;45:67–9.
1. Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care–associated infections. N Engl J Med 2014;370:1198–208.
2. Scott, RD. Center for Disease Control and Prevention. The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention. 2009. Accessed at https://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf.
3. Gould CV, Umscheid CA, Agarwal RK, et al. The Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for prevention of catheter-associated urinary tract infections. 2009. Accessed at http://www.cdc.gov/hicpac/pdf/CAUTI/CAUTIguideline2009final.pdf.
4. Covey SMR, Merrill RR. The speed of trust: the one thing that changes everything. New York: Free Press; 2008.
5. Florida Hospital Association Hospital Engagement Network. Update, March 2015. Florida Hospital Association, Orlando, FL. Accessed at www.fha.org/showDocument.aspx?f=2015HEN-Brief-Web.pdf.
6. Agency for Healthcare Research and Quality (AHRQ). Toolkit for reducing catheter-associated urinary tract infections in hospital units: implementation guide. 2014. Accessed at https://www.ahrq.gov/professionals/quality-patient-safety/hais/tools/cauti-hospitals/index.html.
7. Pronovost PJ, Berenholtz SM, Goeschel CA, et al. Creating high reliability in health care organizations. Health Serv Res 2006;41(4 Pt 2):1599–617.
8. Centers for Disease Control National Healthcare Safety Network. Urinary tract infection (catheter-associated urinary tract infection [CAUTI] and non-catheter-associated urinary tract infection [UTI]) and other urinary system infection [USI]) events. 2014. Accessed at https://www.cdc.gov/nhsn/pdfs/pscmanual/7psccauticurrent.pdf.
9. Belizario SM, Preventing urinary tract infections with a two-person catheter insertion procedure. Nursing 2015;45:67–9.
Staph bloodstream infection algorithm shortens treatment course
SAN DIEGO – By standardizing antibiotic treatment for simple and uncomplicated staphylococcal bloodstream infections (BSI), an algorithm effectively shortens therapy and simplifies decision making, according to results of a multinational randomized trial.
“The data from the study confirm that the algorithm achieves more with less and verify that shorter antibiotic courses are sufficient,” reported Thomas L. Holland, MD, an infectious disease specialist at Duke University Hospital in Durham, N.C.
In this study, the patients randomized to algorithm treatment received vancomycin (or daptomycin in allergic or intolerant patients), which was administered for periods of duration based on clinical features. In those randomized to SOC, the choice and duration of antibiotics were left to the discretion of the treating physician without restrictions.
In those with simple staphylococcal BSI, the algorithm called for no further antibiotics beyond what had already been administered empirically prior to randomization. Key features of simple coagulase-negative staphylococcal (CONS) BSIs include absence of fever or evidence of metastatic infection, as well as no more than one positive follow-up blood culture. The key features of simple Staphylococcus aureus BSI are similar. However, no positive blood cultures are required for S. aureus BSI to be classified as simple.
“In the algorithm arm, no antibiotics were given to those with simple staphylococcal BSI unless antibiotics had been given prior to randomization,” Dr. Holland explained, but he acknowledged that empiric antibiotics prior to randomization reflect “clinical reality.”
In those with uncomplicated rather than simple staphylococcal BSI, vancomycin was given for 5 days to those with CONS BSI and for 14 days to those with S. aureus BSI. In those who were randomized and then subsequently found to have a complicated infection, defined as multiple positive blood cultures or evidence of metastatic infection, patients received as few as 7 days or as many as 28 days of antibiotics, “reflecting the heterogeneity of these infections,” Dr. Holland reported at an annual scientific meeting on infectious diseases.
The coprimary endpoints were treatment success at test of cure and treatment safety, both of which were adjudicated by an external committee consisting of infectious disease specialists blinded to the therapy.
There were 509 patients from 16 sites in both the United States and Spain. CONS BSIs represented approximately 75% of patients in both arms. The complicated staphylococcal infections, which also were evenly distributed in the two arms, were included in the intention-to-treat analysis. Of complicated staphylococcal infections in this study, the pathogen was CONS in 34 instances and S. aureus in 37.
Treatment success was achieved in 82.0% and 81.5% of patients in the algorithm and SOC arms, respectively. Significant adverse events occurred in 32.9% and 28.3% of patients, respectively. Neither difference approached statistical significance.
“In other words, the algorithm was as effective and safe as standard of care,” Dr. Holland confirmed.
However, the median duration of treatment was reduced substantially for those randomized to the algorithm arm, compared with that seen in the standard of care arm. Among evaluable patients without complicated BSI, the mean duration of therapy was 4.4 days in the algorithm group vs. 6.2 days in the SOC group (P = .003). Most of this nearly 2-day reduction in treatment was achieved in uncomplicated CONS BSI patients. In this group, the mean days of treatment were 5.3 days and 8.4 days for the algorithm and SOC groups, respectively. In the uncomplicated S. aureus group, the reduction (from 15.9 to 15.3 days) was not significant.
“The study has several messages. For one, it suggests that patients who meet the criteria of simple staphylococcal BSI can be managed safely with monitoring alone. In addition, this study “provides the best evidence to date that 14 days of vancomycin from the first negative blood culture is sufficient in uncomplicated S. aureus bloodstream infections,” Dr. Holland stated.
“For many, these data will validate what they are already doing,” said Dr. Holland, who reported that the algorithm is now being applied routinely at his institution. “The value is that we now have a randomized trial to demonstrate that shorter therapy can be provided in uncomplicated staphylococcal blood stream infections without increasing risk of serious adverse events.”
SAN DIEGO – By standardizing antibiotic treatment for simple and uncomplicated staphylococcal bloodstream infections (BSI), an algorithm effectively shortens therapy and simplifies decision making, according to results of a multinational randomized trial.
“The data from the study confirm that the algorithm achieves more with less and verify that shorter antibiotic courses are sufficient,” reported Thomas L. Holland, MD, an infectious disease specialist at Duke University Hospital in Durham, N.C.
In this study, the patients randomized to algorithm treatment received vancomycin (or daptomycin in allergic or intolerant patients), which was administered for periods of duration based on clinical features. In those randomized to SOC, the choice and duration of antibiotics were left to the discretion of the treating physician without restrictions.
In those with simple staphylococcal BSI, the algorithm called for no further antibiotics beyond what had already been administered empirically prior to randomization. Key features of simple coagulase-negative staphylococcal (CONS) BSIs include absence of fever or evidence of metastatic infection, as well as no more than one positive follow-up blood culture. The key features of simple Staphylococcus aureus BSI are similar. However, no positive blood cultures are required for S. aureus BSI to be classified as simple.
“In the algorithm arm, no antibiotics were given to those with simple staphylococcal BSI unless antibiotics had been given prior to randomization,” Dr. Holland explained, but he acknowledged that empiric antibiotics prior to randomization reflect “clinical reality.”
In those with uncomplicated rather than simple staphylococcal BSI, vancomycin was given for 5 days to those with CONS BSI and for 14 days to those with S. aureus BSI. In those who were randomized and then subsequently found to have a complicated infection, defined as multiple positive blood cultures or evidence of metastatic infection, patients received as few as 7 days or as many as 28 days of antibiotics, “reflecting the heterogeneity of these infections,” Dr. Holland reported at an annual scientific meeting on infectious diseases.
The coprimary endpoints were treatment success at test of cure and treatment safety, both of which were adjudicated by an external committee consisting of infectious disease specialists blinded to the therapy.
There were 509 patients from 16 sites in both the United States and Spain. CONS BSIs represented approximately 75% of patients in both arms. The complicated staphylococcal infections, which also were evenly distributed in the two arms, were included in the intention-to-treat analysis. Of complicated staphylococcal infections in this study, the pathogen was CONS in 34 instances and S. aureus in 37.
Treatment success was achieved in 82.0% and 81.5% of patients in the algorithm and SOC arms, respectively. Significant adverse events occurred in 32.9% and 28.3% of patients, respectively. Neither difference approached statistical significance.
“In other words, the algorithm was as effective and safe as standard of care,” Dr. Holland confirmed.
However, the median duration of treatment was reduced substantially for those randomized to the algorithm arm, compared with that seen in the standard of care arm. Among evaluable patients without complicated BSI, the mean duration of therapy was 4.4 days in the algorithm group vs. 6.2 days in the SOC group (P = .003). Most of this nearly 2-day reduction in treatment was achieved in uncomplicated CONS BSI patients. In this group, the mean days of treatment were 5.3 days and 8.4 days for the algorithm and SOC groups, respectively. In the uncomplicated S. aureus group, the reduction (from 15.9 to 15.3 days) was not significant.
“The study has several messages. For one, it suggests that patients who meet the criteria of simple staphylococcal BSI can be managed safely with monitoring alone. In addition, this study “provides the best evidence to date that 14 days of vancomycin from the first negative blood culture is sufficient in uncomplicated S. aureus bloodstream infections,” Dr. Holland stated.
“For many, these data will validate what they are already doing,” said Dr. Holland, who reported that the algorithm is now being applied routinely at his institution. “The value is that we now have a randomized trial to demonstrate that shorter therapy can be provided in uncomplicated staphylococcal blood stream infections without increasing risk of serious adverse events.”
SAN DIEGO – By standardizing antibiotic treatment for simple and uncomplicated staphylococcal bloodstream infections (BSI), an algorithm effectively shortens therapy and simplifies decision making, according to results of a multinational randomized trial.
“The data from the study confirm that the algorithm achieves more with less and verify that shorter antibiotic courses are sufficient,” reported Thomas L. Holland, MD, an infectious disease specialist at Duke University Hospital in Durham, N.C.
In this study, the patients randomized to algorithm treatment received vancomycin (or daptomycin in allergic or intolerant patients), which was administered for periods of duration based on clinical features. In those randomized to SOC, the choice and duration of antibiotics were left to the discretion of the treating physician without restrictions.
In those with simple staphylococcal BSI, the algorithm called for no further antibiotics beyond what had already been administered empirically prior to randomization. Key features of simple coagulase-negative staphylococcal (CONS) BSIs include absence of fever or evidence of metastatic infection, as well as no more than one positive follow-up blood culture. The key features of simple Staphylococcus aureus BSI are similar. However, no positive blood cultures are required for S. aureus BSI to be classified as simple.
“In the algorithm arm, no antibiotics were given to those with simple staphylococcal BSI unless antibiotics had been given prior to randomization,” Dr. Holland explained, but he acknowledged that empiric antibiotics prior to randomization reflect “clinical reality.”
In those with uncomplicated rather than simple staphylococcal BSI, vancomycin was given for 5 days to those with CONS BSI and for 14 days to those with S. aureus BSI. In those who were randomized and then subsequently found to have a complicated infection, defined as multiple positive blood cultures or evidence of metastatic infection, patients received as few as 7 days or as many as 28 days of antibiotics, “reflecting the heterogeneity of these infections,” Dr. Holland reported at an annual scientific meeting on infectious diseases.
The coprimary endpoints were treatment success at test of cure and treatment safety, both of which were adjudicated by an external committee consisting of infectious disease specialists blinded to the therapy.
There were 509 patients from 16 sites in both the United States and Spain. CONS BSIs represented approximately 75% of patients in both arms. The complicated staphylococcal infections, which also were evenly distributed in the two arms, were included in the intention-to-treat analysis. Of complicated staphylococcal infections in this study, the pathogen was CONS in 34 instances and S. aureus in 37.
Treatment success was achieved in 82.0% and 81.5% of patients in the algorithm and SOC arms, respectively. Significant adverse events occurred in 32.9% and 28.3% of patients, respectively. Neither difference approached statistical significance.
“In other words, the algorithm was as effective and safe as standard of care,” Dr. Holland confirmed.
However, the median duration of treatment was reduced substantially for those randomized to the algorithm arm, compared with that seen in the standard of care arm. Among evaluable patients without complicated BSI, the mean duration of therapy was 4.4 days in the algorithm group vs. 6.2 days in the SOC group (P = .003). Most of this nearly 2-day reduction in treatment was achieved in uncomplicated CONS BSI patients. In this group, the mean days of treatment were 5.3 days and 8.4 days for the algorithm and SOC groups, respectively. In the uncomplicated S. aureus group, the reduction (from 15.9 to 15.3 days) was not significant.
“The study has several messages. For one, it suggests that patients who meet the criteria of simple staphylococcal BSI can be managed safely with monitoring alone. In addition, this study “provides the best evidence to date that 14 days of vancomycin from the first negative blood culture is sufficient in uncomplicated S. aureus bloodstream infections,” Dr. Holland stated.
“For many, these data will validate what they are already doing,” said Dr. Holland, who reported that the algorithm is now being applied routinely at his institution. “The value is that we now have a randomized trial to demonstrate that shorter therapy can be provided in uncomplicated staphylococcal blood stream infections without increasing risk of serious adverse events.”
AT ID WEEK 2017
Key clinical point:
Major finding: With no change in outcome or differences in adverse events, the algorithm reduced average antibiotic duration by 1.9 days (P = .003).
Data source: A randomized, prospective, multicenter trial of 509 patients randomized at 16 sites in the United States and Spain.
Disclosures: Dr. Holland reports no financial relationships relevant to this study.
Readmission rates linked to hospital quality measures
Poorer-performing hospitals have higher readmission rates than better-performing hospitals for patients with similar diagnoses, a study shows.
Lead author Harlan M. Krumholz, MD, of Yale University, New Haven, Conn., and his colleagues analyzed Centers for Medicare and Medicaid Services hospital-wide readmission data and divided data from July 2014 through June 2015 into two random samples. Researchers used the first sample to calculate the risk-standardized readmission rate within 30 days for each hospital and classified hospitals into performance quartiles, with a lower readmission rate indicating better performance. The second study sample included patients who had two admissions for similar diagnoses at different hospitals that occurred more than 1 month and less than 1 year apart. Researchers compared the observed readmission rates among patients who had been admitted to hospitals in different performance quartiles. The analysis included all discharges occurring from July 1, 2014, through June 30, 2015, from short-term acute care or critical access hospitals in the United States involving Medicare patients who were aged 65 years or older.
Results found that among the patients hospitalized more than once for similar diagnoses at different hospitals, the readmission rate was significantly higher among patients admitted to the worst-performing quartile of hospitals than among those admitted to the best-performing quartile (absolute difference in readmission rate, 2.0 percentage points; 95% confidence interval, 0.4-3.5; P = .001) (N Engl J Med. 2017. doi: 10.1056/NEJMsa1702321). The differences in the comparisons of the other quartiles were smaller and not significant, according to the study. 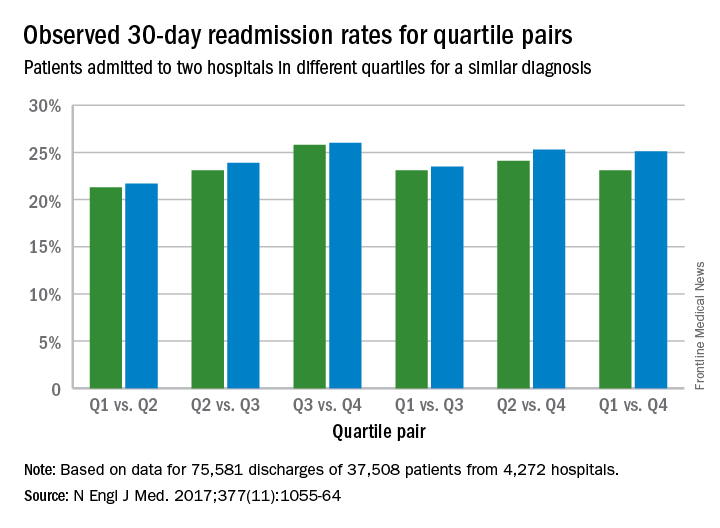
The findings suggest that hospital quality contributes at least in part to readmission rates, independent of patient factors, study authors concluded.
“This study addresses a persistent concern that national readmission measures may reflect differences in unmeasured factors rather than in hospital performance,” they said. “The findings suggest that hospital quality contributes at least in part to readmission rates, independent of patient factors. By studying patients who were admitted twice within 1 year with similar diagnoses to different hospitals, this study design was able to isolate hospital signals of performance while minimizing differences among the patients. In these cases, because the same patients had similar admissions at two hospitals, the characteristics of the patients, including their level of social disadvantage, level of education, or degree of underlying illness, were broadly the same. The alignment of the differences that we observed with the results of the CMS hospital-wide readmission measure also adds to evidence that the readmission measure classifies true differences in performance.”
Dr. Krumholz and seven coauthors reported receiving support from contracts with the Center for Medicare and Medicaid Services to develop and reevaluate performance measures that are used for public reporting.
[email protected]
On Twitter @legal_med
The Hospital Readmission Reduction Program (HRRP) was established in 2011 by a provision in the Affordable Care Act (ACA) requiring Medicare to reduce payments to hospitals with relatively high readmission rates for patients in traditional Medicare. Since the inception of the HRRP, readmission rates have declined across all measured diagnostic categories resulting in estimates of 565,000 fewer Medicare readmissions through 2015.1 These reductions seem to be driven by penalties demonstrated by the fact that readmissions fell more quickly at hospitals that had readmission penalties than at other hospitals. Although the severity and fairness of the penalties can be debated, the HRRP has been successful in achieving the goal of reducing readmissions.
Despite these declines seen in most hospitals, readmission rates have not declined among all hospitals. Hospitals that have higher proportions of low-income Medicare patients have not had as significant reduction in readmissions as their counterparts.2 One of the biggest complaints leveled at the HRRP program is that it is indifferent to the socioeconomic circumstances of a hospital’s patient population. In many of these hospitals, efforts to reduce readmissions have been seen as futile exercises in a patient population with complex social needs.
A study published in the journal Health Affairs found that socioeconomic factors do appear to drive many of the difference in readmission rates between safety net hospitals and their more prosperous peers. However, it also suggested that hospital performance may play a factor as well.3
The NEJM article, Hospital-Readmission Risk – Isolating Hospital Effects from Patient Effects confirms this. This well-designed review determined that hospitals, independent of a patient’s socioeconomic status, had an impact on the likelihood of patient being readmitted. The more complicated question of what higher functioning hospitals did to reduce readmissions was not addressed. It is certain that some hospitals will face greater challenges in reducing readmissions. It is difficult to determine which socioeconomic factors play the biggest role in driving readmission rates and even more difficult to change them. This study also demonstrates that despite challenging conditions, reductions in readmissions can occur.
As the primary focus and leader of health care in most communities, hospitals are best equipped to reach into the community and to develop successful transition programs that limit readmissions and begin to addressee complex social needs. Of course this must be a coordinated effort among many groups, but the hospital and its organization is in the right position to take a leading role. It is essential that hospitalists, who are on the front lines of this process, play a significant role.Many hospitals with patients who have complex needs are rising to the occasion. Motivated by the HRRP, unique innovations to improve care transitions out of hospitals are being developed. Hospitals that are serving low socioeconomic populations are finding innovative ways to reduce readmissions. These include identifying high-risk social conditions driving readmissions, intensive discharge planning, and deploying community health care workers. A key component of this has been addressing the opioid epidemic.Despite some opposition, the HHRP has worked by aligning financial incentives with good health care. The program was successful not by developing complicated metrics, but rather by simply providing financial incentives for good care and then allowing innovation to develop independently. Hopefully this study further promotes these efforts
Kevin Conrad, MD, is medical director of community affairs and healthy policy at Ochsner Health System, New Orleans.
References
1. Zuckerman RB et al. Readmissions, observation, and the hospital readmissions reduction program. N Engl J Med. 2016 April 21;374:1543-1551.
2. Jencks SF et al. Hospitalizations among patients in the Medicare Fee-for-Service Program. N Engl J Med. 2009.360(14):1418-1428; Epstein AM et al. The relationship between hospital admission rates and rehospitalizations. N Engl J Med. 2011. 365(24):2287-2295.
3. Kahn C et al. Assessing Medicare’s hospital Pay-for-Performance programs and whether they are achieving their goals. Health Affairs. 2015 Aug;34(8).
The Hospital Readmission Reduction Program (HRRP) was established in 2011 by a provision in the Affordable Care Act (ACA) requiring Medicare to reduce payments to hospitals with relatively high readmission rates for patients in traditional Medicare. Since the inception of the HRRP, readmission rates have declined across all measured diagnostic categories resulting in estimates of 565,000 fewer Medicare readmissions through 2015.1 These reductions seem to be driven by penalties demonstrated by the fact that readmissions fell more quickly at hospitals that had readmission penalties than at other hospitals. Although the severity and fairness of the penalties can be debated, the HRRP has been successful in achieving the goal of reducing readmissions.
Despite these declines seen in most hospitals, readmission rates have not declined among all hospitals. Hospitals that have higher proportions of low-income Medicare patients have not had as significant reduction in readmissions as their counterparts.2 One of the biggest complaints leveled at the HRRP program is that it is indifferent to the socioeconomic circumstances of a hospital’s patient population. In many of these hospitals, efforts to reduce readmissions have been seen as futile exercises in a patient population with complex social needs.
A study published in the journal Health Affairs found that socioeconomic factors do appear to drive many of the difference in readmission rates between safety net hospitals and their more prosperous peers. However, it also suggested that hospital performance may play a factor as well.3
The NEJM article, Hospital-Readmission Risk – Isolating Hospital Effects from Patient Effects confirms this. This well-designed review determined that hospitals, independent of a patient’s socioeconomic status, had an impact on the likelihood of patient being readmitted. The more complicated question of what higher functioning hospitals did to reduce readmissions was not addressed. It is certain that some hospitals will face greater challenges in reducing readmissions. It is difficult to determine which socioeconomic factors play the biggest role in driving readmission rates and even more difficult to change them. This study also demonstrates that despite challenging conditions, reductions in readmissions can occur.
As the primary focus and leader of health care in most communities, hospitals are best equipped to reach into the community and to develop successful transition programs that limit readmissions and begin to addressee complex social needs. Of course this must be a coordinated effort among many groups, but the hospital and its organization is in the right position to take a leading role. It is essential that hospitalists, who are on the front lines of this process, play a significant role.Many hospitals with patients who have complex needs are rising to the occasion. Motivated by the HRRP, unique innovations to improve care transitions out of hospitals are being developed. Hospitals that are serving low socioeconomic populations are finding innovative ways to reduce readmissions. These include identifying high-risk social conditions driving readmissions, intensive discharge planning, and deploying community health care workers. A key component of this has been addressing the opioid epidemic.Despite some opposition, the HHRP has worked by aligning financial incentives with good health care. The program was successful not by developing complicated metrics, but rather by simply providing financial incentives for good care and then allowing innovation to develop independently. Hopefully this study further promotes these efforts
Kevin Conrad, MD, is medical director of community affairs and healthy policy at Ochsner Health System, New Orleans.
References
1. Zuckerman RB et al. Readmissions, observation, and the hospital readmissions reduction program. N Engl J Med. 2016 April 21;374:1543-1551.
2. Jencks SF et al. Hospitalizations among patients in the Medicare Fee-for-Service Program. N Engl J Med. 2009.360(14):1418-1428; Epstein AM et al. The relationship between hospital admission rates and rehospitalizations. N Engl J Med. 2011. 365(24):2287-2295.
3. Kahn C et al. Assessing Medicare’s hospital Pay-for-Performance programs and whether they are achieving their goals. Health Affairs. 2015 Aug;34(8).
The Hospital Readmission Reduction Program (HRRP) was established in 2011 by a provision in the Affordable Care Act (ACA) requiring Medicare to reduce payments to hospitals with relatively high readmission rates for patients in traditional Medicare. Since the inception of the HRRP, readmission rates have declined across all measured diagnostic categories resulting in estimates of 565,000 fewer Medicare readmissions through 2015.1 These reductions seem to be driven by penalties demonstrated by the fact that readmissions fell more quickly at hospitals that had readmission penalties than at other hospitals. Although the severity and fairness of the penalties can be debated, the HRRP has been successful in achieving the goal of reducing readmissions.
Despite these declines seen in most hospitals, readmission rates have not declined among all hospitals. Hospitals that have higher proportions of low-income Medicare patients have not had as significant reduction in readmissions as their counterparts.2 One of the biggest complaints leveled at the HRRP program is that it is indifferent to the socioeconomic circumstances of a hospital’s patient population. In many of these hospitals, efforts to reduce readmissions have been seen as futile exercises in a patient population with complex social needs.
A study published in the journal Health Affairs found that socioeconomic factors do appear to drive many of the difference in readmission rates between safety net hospitals and their more prosperous peers. However, it also suggested that hospital performance may play a factor as well.3
The NEJM article, Hospital-Readmission Risk – Isolating Hospital Effects from Patient Effects confirms this. This well-designed review determined that hospitals, independent of a patient’s socioeconomic status, had an impact on the likelihood of patient being readmitted. The more complicated question of what higher functioning hospitals did to reduce readmissions was not addressed. It is certain that some hospitals will face greater challenges in reducing readmissions. It is difficult to determine which socioeconomic factors play the biggest role in driving readmission rates and even more difficult to change them. This study also demonstrates that despite challenging conditions, reductions in readmissions can occur.
As the primary focus and leader of health care in most communities, hospitals are best equipped to reach into the community and to develop successful transition programs that limit readmissions and begin to addressee complex social needs. Of course this must be a coordinated effort among many groups, but the hospital and its organization is in the right position to take a leading role. It is essential that hospitalists, who are on the front lines of this process, play a significant role.Many hospitals with patients who have complex needs are rising to the occasion. Motivated by the HRRP, unique innovations to improve care transitions out of hospitals are being developed. Hospitals that are serving low socioeconomic populations are finding innovative ways to reduce readmissions. These include identifying high-risk social conditions driving readmissions, intensive discharge planning, and deploying community health care workers. A key component of this has been addressing the opioid epidemic.Despite some opposition, the HHRP has worked by aligning financial incentives with good health care. The program was successful not by developing complicated metrics, but rather by simply providing financial incentives for good care and then allowing innovation to develop independently. Hopefully this study further promotes these efforts
Kevin Conrad, MD, is medical director of community affairs and healthy policy at Ochsner Health System, New Orleans.
References
1. Zuckerman RB et al. Readmissions, observation, and the hospital readmissions reduction program. N Engl J Med. 2016 April 21;374:1543-1551.
2. Jencks SF et al. Hospitalizations among patients in the Medicare Fee-for-Service Program. N Engl J Med. 2009.360(14):1418-1428; Epstein AM et al. The relationship between hospital admission rates and rehospitalizations. N Engl J Med. 2011. 365(24):2287-2295.
3. Kahn C et al. Assessing Medicare’s hospital Pay-for-Performance programs and whether they are achieving their goals. Health Affairs. 2015 Aug;34(8).
Poorer-performing hospitals have higher readmission rates than better-performing hospitals for patients with similar diagnoses, a study shows.
Lead author Harlan M. Krumholz, MD, of Yale University, New Haven, Conn., and his colleagues analyzed Centers for Medicare and Medicaid Services hospital-wide readmission data and divided data from July 2014 through June 2015 into two random samples. Researchers used the first sample to calculate the risk-standardized readmission rate within 30 days for each hospital and classified hospitals into performance quartiles, with a lower readmission rate indicating better performance. The second study sample included patients who had two admissions for similar diagnoses at different hospitals that occurred more than 1 month and less than 1 year apart. Researchers compared the observed readmission rates among patients who had been admitted to hospitals in different performance quartiles. The analysis included all discharges occurring from July 1, 2014, through June 30, 2015, from short-term acute care or critical access hospitals in the United States involving Medicare patients who were aged 65 years or older.
Results found that among the patients hospitalized more than once for similar diagnoses at different hospitals, the readmission rate was significantly higher among patients admitted to the worst-performing quartile of hospitals than among those admitted to the best-performing quartile (absolute difference in readmission rate, 2.0 percentage points; 95% confidence interval, 0.4-3.5; P = .001) (N Engl J Med. 2017. doi: 10.1056/NEJMsa1702321). The differences in the comparisons of the other quartiles were smaller and not significant, according to the study. 
The findings suggest that hospital quality contributes at least in part to readmission rates, independent of patient factors, study authors concluded.
“This study addresses a persistent concern that national readmission measures may reflect differences in unmeasured factors rather than in hospital performance,” they said. “The findings suggest that hospital quality contributes at least in part to readmission rates, independent of patient factors. By studying patients who were admitted twice within 1 year with similar diagnoses to different hospitals, this study design was able to isolate hospital signals of performance while minimizing differences among the patients. In these cases, because the same patients had similar admissions at two hospitals, the characteristics of the patients, including their level of social disadvantage, level of education, or degree of underlying illness, were broadly the same. The alignment of the differences that we observed with the results of the CMS hospital-wide readmission measure also adds to evidence that the readmission measure classifies true differences in performance.”
Dr. Krumholz and seven coauthors reported receiving support from contracts with the Center for Medicare and Medicaid Services to develop and reevaluate performance measures that are used for public reporting.
[email protected]
On Twitter @legal_med
Poorer-performing hospitals have higher readmission rates than better-performing hospitals for patients with similar diagnoses, a study shows.
Lead author Harlan M. Krumholz, MD, of Yale University, New Haven, Conn., and his colleagues analyzed Centers for Medicare and Medicaid Services hospital-wide readmission data and divided data from July 2014 through June 2015 into two random samples. Researchers used the first sample to calculate the risk-standardized readmission rate within 30 days for each hospital and classified hospitals into performance quartiles, with a lower readmission rate indicating better performance. The second study sample included patients who had two admissions for similar diagnoses at different hospitals that occurred more than 1 month and less than 1 year apart. Researchers compared the observed readmission rates among patients who had been admitted to hospitals in different performance quartiles. The analysis included all discharges occurring from July 1, 2014, through June 30, 2015, from short-term acute care or critical access hospitals in the United States involving Medicare patients who were aged 65 years or older.
Results found that among the patients hospitalized more than once for similar diagnoses at different hospitals, the readmission rate was significantly higher among patients admitted to the worst-performing quartile of hospitals than among those admitted to the best-performing quartile (absolute difference in readmission rate, 2.0 percentage points; 95% confidence interval, 0.4-3.5; P = .001) (N Engl J Med. 2017. doi: 10.1056/NEJMsa1702321). The differences in the comparisons of the other quartiles were smaller and not significant, according to the study. 
The findings suggest that hospital quality contributes at least in part to readmission rates, independent of patient factors, study authors concluded.
“This study addresses a persistent concern that national readmission measures may reflect differences in unmeasured factors rather than in hospital performance,” they said. “The findings suggest that hospital quality contributes at least in part to readmission rates, independent of patient factors. By studying patients who were admitted twice within 1 year with similar diagnoses to different hospitals, this study design was able to isolate hospital signals of performance while minimizing differences among the patients. In these cases, because the same patients had similar admissions at two hospitals, the characteristics of the patients, including their level of social disadvantage, level of education, or degree of underlying illness, were broadly the same. The alignment of the differences that we observed with the results of the CMS hospital-wide readmission measure also adds to evidence that the readmission measure classifies true differences in performance.”
Dr. Krumholz and seven coauthors reported receiving support from contracts with the Center for Medicare and Medicaid Services to develop and reevaluate performance measures that are used for public reporting.
[email protected]
On Twitter @legal_med
Key clinical point:
Major finding: The readmission rate was significantly higher among patients admitted to the worst-performing quartile of hospitals than among those admitted to the best-performing quartile (absolute difference in readmission rate, 2.0 percentage points).
Data source: Analysis of Centers for Medicare and Medicaid Services hospital-wide readmission data from July 2014 through June 2015.
Disclosures: Dr. Krumholz and seven coauthors reported receiving support from contracts with the Center for Medicare and Medicaid Services to develop and reevaluate performance measures that are used for public reporting.
Over 40% of Americans have experience with medical errors
More than 40% of adults have either experienced a medical error or been involved in the care of someone who did, according to a recent national survey.
Specifically, 21% of American adults said that they have personally experienced a medical error and 31% said that they have been involved in the care of another person who experienced an error. The combined total, which includes some overlap, was 41% in the survey conducted by the Institute for Healthcare Improvement (IHI)/National Patient Safety Foundation (NPSF) and National Opinion Research Center (NORC), a nonpartisan research institution at the University of Chicago, .
Medical errors were defined for respondents as “mistakes [that] sometimes result in no harm, while other times they may result in additional or prolonged treatment, emotional distress, disability, or death,” investigators at IHI/NPSF and NORC said in their report.
A misdiagnosed medical problem was the most common type of medical error, reported by 59% of those with error experience. The next most common type of error was a mistake during a test, surgery, or treatment, which was mentioned by 46% of those with error experience, followed by a diagnosis that didn’t make sense (42%), lack of respect (39%), and incorrect instructions about follow-up care (29%), the IHI/HPSF and NORC report indicated.
Feelings of disrespect were more common among younger respondents: 46% of those aged 18-44 years said they were not treated with respect by a health care provider, compared with 34% of those aged 45 years and older. No differences in disrespect were seen with regard to socioeconomic status, health literacy, or English language proficiency. Those who spoke a language other than English at home, however, were more than twice as likely to get the wrong medication from a physician (34%) than were those who did not (15%), the report showed.
The survey, which had a sampling error of plus or minus 3.2 percentage points, was conducted between May 12, 2017, and June 26, 2017, and involved 2,536 respondents. It was conducted with support from Medtronic.
More than 40% of adults have either experienced a medical error or been involved in the care of someone who did, according to a recent national survey.
Specifically, 21% of American adults said that they have personally experienced a medical error and 31% said that they have been involved in the care of another person who experienced an error. The combined total, which includes some overlap, was 41% in the survey conducted by the Institute for Healthcare Improvement (IHI)/National Patient Safety Foundation (NPSF) and National Opinion Research Center (NORC), a nonpartisan research institution at the University of Chicago, .
Medical errors were defined for respondents as “mistakes [that] sometimes result in no harm, while other times they may result in additional or prolonged treatment, emotional distress, disability, or death,” investigators at IHI/NPSF and NORC said in their report.
A misdiagnosed medical problem was the most common type of medical error, reported by 59% of those with error experience. The next most common type of error was a mistake during a test, surgery, or treatment, which was mentioned by 46% of those with error experience, followed by a diagnosis that didn’t make sense (42%), lack of respect (39%), and incorrect instructions about follow-up care (29%), the IHI/HPSF and NORC report indicated.
Feelings of disrespect were more common among younger respondents: 46% of those aged 18-44 years said they were not treated with respect by a health care provider, compared with 34% of those aged 45 years and older. No differences in disrespect were seen with regard to socioeconomic status, health literacy, or English language proficiency. Those who spoke a language other than English at home, however, were more than twice as likely to get the wrong medication from a physician (34%) than were those who did not (15%), the report showed.
The survey, which had a sampling error of plus or minus 3.2 percentage points, was conducted between May 12, 2017, and June 26, 2017, and involved 2,536 respondents. It was conducted with support from Medtronic.
More than 40% of adults have either experienced a medical error or been involved in the care of someone who did, according to a recent national survey.
Specifically, 21% of American adults said that they have personally experienced a medical error and 31% said that they have been involved in the care of another person who experienced an error. The combined total, which includes some overlap, was 41% in the survey conducted by the Institute for Healthcare Improvement (IHI)/National Patient Safety Foundation (NPSF) and National Opinion Research Center (NORC), a nonpartisan research institution at the University of Chicago, .
Medical errors were defined for respondents as “mistakes [that] sometimes result in no harm, while other times they may result in additional or prolonged treatment, emotional distress, disability, or death,” investigators at IHI/NPSF and NORC said in their report.
A misdiagnosed medical problem was the most common type of medical error, reported by 59% of those with error experience. The next most common type of error was a mistake during a test, surgery, or treatment, which was mentioned by 46% of those with error experience, followed by a diagnosis that didn’t make sense (42%), lack of respect (39%), and incorrect instructions about follow-up care (29%), the IHI/HPSF and NORC report indicated.
Feelings of disrespect were more common among younger respondents: 46% of those aged 18-44 years said they were not treated with respect by a health care provider, compared with 34% of those aged 45 years and older. No differences in disrespect were seen with regard to socioeconomic status, health literacy, or English language proficiency. Those who spoke a language other than English at home, however, were more than twice as likely to get the wrong medication from a physician (34%) than were those who did not (15%), the report showed.
The survey, which had a sampling error of plus or minus 3.2 percentage points, was conducted between May 12, 2017, and June 26, 2017, and involved 2,536 respondents. It was conducted with support from Medtronic.
Clinical pearls from NAMS 2017
Visit the NAMS annual meetings website
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Visit the NAMS annual meetings website
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Visit the NAMS annual meetings website
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Two Senators reach deal on a health law fix
After nearly 2 months of negotiations, key senators said on Oct. 17 that they have reached a bipartisan deal on a proposal intended to stabilize the Affordable Care Act’s insurance market, which has been rocked by recent actions by President Donald Trump.
Sens. Lamar Alexander (R-Tenn.) and Patty Murray (D-Wash.), respectively the chairman and the top Democrat of the Senate Health, Education, Labor, and Pensions Committee, negotiated the emerging deal. The milestone agreement, they said, would guarantee payment of cost-sharing reduction subsidies that help some policyholders with low incomes afford their deductibles and other out-of-pocket costs for 2 years, 2018 and 2019.
President Trump announced on Oct. 12 that he would stop funding the subsidies, which also have been the subject of a long-running lawsuit.
Even if it fails to become law, the deal marks a singular achievement that has been almost completely missing in Congress for the past 8 years – a bipartisan compromise on how to make the nation’s health insurance system work.
“This is an agreement I am proud to support,” Sen. Murray said on the Senate floor, “because of the message it sends about how to get things done.”
The proposal – which will require 60 votes to pass the Senate and agreement from a still-dubious House of Representatives – also would restore $110 million in ACA outreach funding cut by the Trump administration. That funding would help guide eligible individuals to sign up for coverage on the health insurance exchanges during the open enrollment period that runs from Nov. 1 to Dec. 15.
In exchange for those provisions, urged by Democrats and state officials, Republicans would win some changes to make it easier for states to apply for waivers that would let them experiment with alternative ways to provide and subsidize health insurance. The deal also would allow the sale of less comprehensive catastrophic plans in the health exchanges. Currently, such plans can be sold only to those under age 30 years.
On the Senate floor, Sen. Alexander said, “This agreement avoids chaos. I don’t know a Democrat or a Republican who benefits from chaos.”
Senate Majority Leader Mitch McConnell (R-Ky.) reserved judgment about the deal.
Both parties still have some major disagreements when it comes to health care, Senate Minority Leader Chuck Schumer (D-N.Y.) told reporters on Oct. 17, but “I think there’s a growing consensus that in the short term we need stability in the markets. So we’ve achieved stability if this agreement becomes law.”
More than 60 senators have already participated in the meetings that led to the deal, Sen. Alexander said on the Senate floor. But the path to passage in the House is uncertain – with many conservatives vehemently opposed to anything that could be construed as helping the ACA succeed.
Rep. Mark Walker (R-N.C.), chairman of the conservative Republican Study Committee, tweeted on Oct. 17: “The GOP should focus on repealing & replacing Obamacare, not trying to save it. This bailout is unacceptable.”
Both Sen. Murray and Sen. Alexander said that they were still struggling over language to make sure that if the cost-sharing payments are resumed, insurers would not receive a windfall by keeping both those payments and the higher premiums that many states are allowing in anticipation of the payments being ended.
“We want to make sure that the cost-sharing payments go to the benefit of consumers, not the insurance companies,” Sen. Alexander said.
President Trump, who as recently as Oct. 16 called the cost-sharing subsidies “a payoff” to insurance companies, took credit for the negotiations. “If I didn’t cut the CSRs, they wouldn’t be meeting,” he said. That was not, in fact, the case. The negotiations had picked up some weeks ago after being called off earlier in September while the Senate tried for one last-ditch repeal vote.
On Oct. 13, White House Budget Director Mick Mulvaney told Politico that the president would not allow a short-term fix, calling a restoration of the cost-sharing reduction funds “corporate welfare and bailouts for the insurance companies.”
But on Oct. 17 the president hailed the deal. “We think it’s going to not only save money, but give people much better health care with a very, very much smaller premium spike,” he told reporters.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
After nearly 2 months of negotiations, key senators said on Oct. 17 that they have reached a bipartisan deal on a proposal intended to stabilize the Affordable Care Act’s insurance market, which has been rocked by recent actions by President Donald Trump.
Sens. Lamar Alexander (R-Tenn.) and Patty Murray (D-Wash.), respectively the chairman and the top Democrat of the Senate Health, Education, Labor, and Pensions Committee, negotiated the emerging deal. The milestone agreement, they said, would guarantee payment of cost-sharing reduction subsidies that help some policyholders with low incomes afford their deductibles and other out-of-pocket costs for 2 years, 2018 and 2019.
President Trump announced on Oct. 12 that he would stop funding the subsidies, which also have been the subject of a long-running lawsuit.
Even if it fails to become law, the deal marks a singular achievement that has been almost completely missing in Congress for the past 8 years – a bipartisan compromise on how to make the nation’s health insurance system work.
“This is an agreement I am proud to support,” Sen. Murray said on the Senate floor, “because of the message it sends about how to get things done.”
The proposal – which will require 60 votes to pass the Senate and agreement from a still-dubious House of Representatives – also would restore $110 million in ACA outreach funding cut by the Trump administration. That funding would help guide eligible individuals to sign up for coverage on the health insurance exchanges during the open enrollment period that runs from Nov. 1 to Dec. 15.
In exchange for those provisions, urged by Democrats and state officials, Republicans would win some changes to make it easier for states to apply for waivers that would let them experiment with alternative ways to provide and subsidize health insurance. The deal also would allow the sale of less comprehensive catastrophic plans in the health exchanges. Currently, such plans can be sold only to those under age 30 years.
On the Senate floor, Sen. Alexander said, “This agreement avoids chaos. I don’t know a Democrat or a Republican who benefits from chaos.”
Senate Majority Leader Mitch McConnell (R-Ky.) reserved judgment about the deal.
Both parties still have some major disagreements when it comes to health care, Senate Minority Leader Chuck Schumer (D-N.Y.) told reporters on Oct. 17, but “I think there’s a growing consensus that in the short term we need stability in the markets. So we’ve achieved stability if this agreement becomes law.”
More than 60 senators have already participated in the meetings that led to the deal, Sen. Alexander said on the Senate floor. But the path to passage in the House is uncertain – with many conservatives vehemently opposed to anything that could be construed as helping the ACA succeed.
Rep. Mark Walker (R-N.C.), chairman of the conservative Republican Study Committee, tweeted on Oct. 17: “The GOP should focus on repealing & replacing Obamacare, not trying to save it. This bailout is unacceptable.”
Both Sen. Murray and Sen. Alexander said that they were still struggling over language to make sure that if the cost-sharing payments are resumed, insurers would not receive a windfall by keeping both those payments and the higher premiums that many states are allowing in anticipation of the payments being ended.
“We want to make sure that the cost-sharing payments go to the benefit of consumers, not the insurance companies,” Sen. Alexander said.
President Trump, who as recently as Oct. 16 called the cost-sharing subsidies “a payoff” to insurance companies, took credit for the negotiations. “If I didn’t cut the CSRs, they wouldn’t be meeting,” he said. That was not, in fact, the case. The negotiations had picked up some weeks ago after being called off earlier in September while the Senate tried for one last-ditch repeal vote.
On Oct. 13, White House Budget Director Mick Mulvaney told Politico that the president would not allow a short-term fix, calling a restoration of the cost-sharing reduction funds “corporate welfare and bailouts for the insurance companies.”
But on Oct. 17 the president hailed the deal. “We think it’s going to not only save money, but give people much better health care with a very, very much smaller premium spike,” he told reporters.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
After nearly 2 months of negotiations, key senators said on Oct. 17 that they have reached a bipartisan deal on a proposal intended to stabilize the Affordable Care Act’s insurance market, which has been rocked by recent actions by President Donald Trump.
Sens. Lamar Alexander (R-Tenn.) and Patty Murray (D-Wash.), respectively the chairman and the top Democrat of the Senate Health, Education, Labor, and Pensions Committee, negotiated the emerging deal. The milestone agreement, they said, would guarantee payment of cost-sharing reduction subsidies that help some policyholders with low incomes afford their deductibles and other out-of-pocket costs for 2 years, 2018 and 2019.
President Trump announced on Oct. 12 that he would stop funding the subsidies, which also have been the subject of a long-running lawsuit.
Even if it fails to become law, the deal marks a singular achievement that has been almost completely missing in Congress for the past 8 years – a bipartisan compromise on how to make the nation’s health insurance system work.
“This is an agreement I am proud to support,” Sen. Murray said on the Senate floor, “because of the message it sends about how to get things done.”
The proposal – which will require 60 votes to pass the Senate and agreement from a still-dubious House of Representatives – also would restore $110 million in ACA outreach funding cut by the Trump administration. That funding would help guide eligible individuals to sign up for coverage on the health insurance exchanges during the open enrollment period that runs from Nov. 1 to Dec. 15.
In exchange for those provisions, urged by Democrats and state officials, Republicans would win some changes to make it easier for states to apply for waivers that would let them experiment with alternative ways to provide and subsidize health insurance. The deal also would allow the sale of less comprehensive catastrophic plans in the health exchanges. Currently, such plans can be sold only to those under age 30 years.
On the Senate floor, Sen. Alexander said, “This agreement avoids chaos. I don’t know a Democrat or a Republican who benefits from chaos.”
Senate Majority Leader Mitch McConnell (R-Ky.) reserved judgment about the deal.
Both parties still have some major disagreements when it comes to health care, Senate Minority Leader Chuck Schumer (D-N.Y.) told reporters on Oct. 17, but “I think there’s a growing consensus that in the short term we need stability in the markets. So we’ve achieved stability if this agreement becomes law.”
More than 60 senators have already participated in the meetings that led to the deal, Sen. Alexander said on the Senate floor. But the path to passage in the House is uncertain – with many conservatives vehemently opposed to anything that could be construed as helping the ACA succeed.
Rep. Mark Walker (R-N.C.), chairman of the conservative Republican Study Committee, tweeted on Oct. 17: “The GOP should focus on repealing & replacing Obamacare, not trying to save it. This bailout is unacceptable.”
Both Sen. Murray and Sen. Alexander said that they were still struggling over language to make sure that if the cost-sharing payments are resumed, insurers would not receive a windfall by keeping both those payments and the higher premiums that many states are allowing in anticipation of the payments being ended.
“We want to make sure that the cost-sharing payments go to the benefit of consumers, not the insurance companies,” Sen. Alexander said.
President Trump, who as recently as Oct. 16 called the cost-sharing subsidies “a payoff” to insurance companies, took credit for the negotiations. “If I didn’t cut the CSRs, they wouldn’t be meeting,” he said. That was not, in fact, the case. The negotiations had picked up some weeks ago after being called off earlier in September while the Senate tried for one last-ditch repeal vote.
On Oct. 13, White House Budget Director Mick Mulvaney told Politico that the president would not allow a short-term fix, calling a restoration of the cost-sharing reduction funds “corporate welfare and bailouts for the insurance companies.”
But on Oct. 17 the president hailed the deal. “We think it’s going to not only save money, but give people much better health care with a very, very much smaller premium spike,” he told reporters.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.