User login
VIDEO: Mobile stroke units aren’t just expensive toys
SAN DIEGO – Mobile stroke units are specially equipped ambulance units designed to respond and deliver treatment to stroke patients as swiftly as possible. They are outfitted with a portable CT scanner, a mobile lab, and specialized personnel, including a telemedicine unit to assist with diagnosis. If a patient is experiencing an ischemic stroke, the unit can deliver thrombolytic therapy on the spot, circumventing travel to an emergency department.
But are they cost effective? There are 13 active units in the United States, and they’re not cheap. They cost about $3.5 million to build and operate over 5 years, according to James Grotta, MD, a neurologist with the Memorial Hermann Medical Group and director of stroke research at Memorial Hermann–Texas Medical Center, both in Houston.
In a video interview at the annual meeting of the American Neurological Association, Dr. Grotta described how his group is studying the impact of mobile stroke units on time to treatment and the long-term costs and cost savings associated with them in an ongoing clinical trial that is comparing outcomes in patients eligible for tissue plasminogen activator when treated by a mobile stroke unit versus standard prehospital triage and transport by emergency medical services. The study is comparing outcomes when the mobile stroke unit and emergency medical services are the primary responders on alternating weeks. Primary outcomes include cost-effectiveness, the change in Rankin scale score from baseline to 90 days, and the diagnostic agreement between a vascular neurologist in the mobile stroke unit and a telemedicine vascular neurologist consulted from the unit.
Mobile stroke units can even supplement existing health care in case of an emergency. Dr. Grotta also recounted how one unit assisted during the aftermath of Hurricane Harvey.
SAN DIEGO – Mobile stroke units are specially equipped ambulance units designed to respond and deliver treatment to stroke patients as swiftly as possible. They are outfitted with a portable CT scanner, a mobile lab, and specialized personnel, including a telemedicine unit to assist with diagnosis. If a patient is experiencing an ischemic stroke, the unit can deliver thrombolytic therapy on the spot, circumventing travel to an emergency department.
But are they cost effective? There are 13 active units in the United States, and they’re not cheap. They cost about $3.5 million to build and operate over 5 years, according to James Grotta, MD, a neurologist with the Memorial Hermann Medical Group and director of stroke research at Memorial Hermann–Texas Medical Center, both in Houston.
In a video interview at the annual meeting of the American Neurological Association, Dr. Grotta described how his group is studying the impact of mobile stroke units on time to treatment and the long-term costs and cost savings associated with them in an ongoing clinical trial that is comparing outcomes in patients eligible for tissue plasminogen activator when treated by a mobile stroke unit versus standard prehospital triage and transport by emergency medical services. The study is comparing outcomes when the mobile stroke unit and emergency medical services are the primary responders on alternating weeks. Primary outcomes include cost-effectiveness, the change in Rankin scale score from baseline to 90 days, and the diagnostic agreement between a vascular neurologist in the mobile stroke unit and a telemedicine vascular neurologist consulted from the unit.
Mobile stroke units can even supplement existing health care in case of an emergency. Dr. Grotta also recounted how one unit assisted during the aftermath of Hurricane Harvey.
SAN DIEGO – Mobile stroke units are specially equipped ambulance units designed to respond and deliver treatment to stroke patients as swiftly as possible. They are outfitted with a portable CT scanner, a mobile lab, and specialized personnel, including a telemedicine unit to assist with diagnosis. If a patient is experiencing an ischemic stroke, the unit can deliver thrombolytic therapy on the spot, circumventing travel to an emergency department.
But are they cost effective? There are 13 active units in the United States, and they’re not cheap. They cost about $3.5 million to build and operate over 5 years, according to James Grotta, MD, a neurologist with the Memorial Hermann Medical Group and director of stroke research at Memorial Hermann–Texas Medical Center, both in Houston.
In a video interview at the annual meeting of the American Neurological Association, Dr. Grotta described how his group is studying the impact of mobile stroke units on time to treatment and the long-term costs and cost savings associated with them in an ongoing clinical trial that is comparing outcomes in patients eligible for tissue plasminogen activator when treated by a mobile stroke unit versus standard prehospital triage and transport by emergency medical services. The study is comparing outcomes when the mobile stroke unit and emergency medical services are the primary responders on alternating weeks. Primary outcomes include cost-effectiveness, the change in Rankin scale score from baseline to 90 days, and the diagnostic agreement between a vascular neurologist in the mobile stroke unit and a telemedicine vascular neurologist consulted from the unit.
Mobile stroke units can even supplement existing health care in case of an emergency. Dr. Grotta also recounted how one unit assisted during the aftermath of Hurricane Harvey.
AT ANA 2017
SHM’s RADEO Program aids safer opioid prescribing
In January 2017, the U.S. Centers for Medicare & Medicaid Services honored SHM for its hospital patient safety and quality improvement efforts. A big reason for the plaudits was the society’s successful program and implementation toolkit called Reducing Adverse Drug Events related to Opioids (RADEO), now in its second phase.
Kevin Vuernick, senior project manager of SHM’s Center for Hospital Innovation and Improvement, says that the freely available RADEO guide explains how to develop and carry out quality improvement projects related to inpatient opioid prescribing. One of the first steps was devising interventions that hospitalists could implement to reduce opioid-related adverse events. An independent evaluator will help analyze the program’s data, best practices, and outcomes.
Keri Holmes-Maybank, MD, MSCR, FHM, an academic hospitalist at the Medical University of South Carolina, Charleston, said that the RADEO guide has a been a “phenomenal” resource. Dr. Holmes-Maybank, who led her medical center’s involvement in RADEO’s first round, says the guide helped her identify areas that her institution could work on. For one project, the medical university implemented the Pasero Opioid-Induced Sedation Scale to help prevent adverse opioid-related events, such as life-threatening respiratory depression. For a second project, the center combined existing discharge information into a more complete document that could be given to patients to educate them and their caregivers better.
St. Anthony Hospital in Oklahoma City first used RADEO to revisit how it was evaluating patients’ pain and then widened the scope to reassess how it was managing its opioid treatment and narcotic use. “We just kept swinging at the tree, trying to hit the low-hanging fruit and seeing what we could improve upon,” said Matthew Jared, MD, a hospitalist at St. Anthony and its program lead during its involvement in phase one of RADEO.
Dr. Jared is hoping to build on the momentum with a plan to develop better in-house protocols for monitoring pain, employing alternative treatments, and establishing clear lines of communication. “That’s our next step forward: really taking what we’ve learned and beginning to implement it into a holistic type of pain management within the hospital that each physician can tailor to the individual patient but still have the framework to support them,” he said. This ambitious plan is precisely the goal of RADEO, Mr. Vuernick said: providing the catalyst for change not just for hospital medicine but also for entire institutions.
In January 2017, the U.S. Centers for Medicare & Medicaid Services honored SHM for its hospital patient safety and quality improvement efforts. A big reason for the plaudits was the society’s successful program and implementation toolkit called Reducing Adverse Drug Events related to Opioids (RADEO), now in its second phase.
Kevin Vuernick, senior project manager of SHM’s Center for Hospital Innovation and Improvement, says that the freely available RADEO guide explains how to develop and carry out quality improvement projects related to inpatient opioid prescribing. One of the first steps was devising interventions that hospitalists could implement to reduce opioid-related adverse events. An independent evaluator will help analyze the program’s data, best practices, and outcomes.
Keri Holmes-Maybank, MD, MSCR, FHM, an academic hospitalist at the Medical University of South Carolina, Charleston, said that the RADEO guide has a been a “phenomenal” resource. Dr. Holmes-Maybank, who led her medical center’s involvement in RADEO’s first round, says the guide helped her identify areas that her institution could work on. For one project, the medical university implemented the Pasero Opioid-Induced Sedation Scale to help prevent adverse opioid-related events, such as life-threatening respiratory depression. For a second project, the center combined existing discharge information into a more complete document that could be given to patients to educate them and their caregivers better.
St. Anthony Hospital in Oklahoma City first used RADEO to revisit how it was evaluating patients’ pain and then widened the scope to reassess how it was managing its opioid treatment and narcotic use. “We just kept swinging at the tree, trying to hit the low-hanging fruit and seeing what we could improve upon,” said Matthew Jared, MD, a hospitalist at St. Anthony and its program lead during its involvement in phase one of RADEO.
Dr. Jared is hoping to build on the momentum with a plan to develop better in-house protocols for monitoring pain, employing alternative treatments, and establishing clear lines of communication. “That’s our next step forward: really taking what we’ve learned and beginning to implement it into a holistic type of pain management within the hospital that each physician can tailor to the individual patient but still have the framework to support them,” he said. This ambitious plan is precisely the goal of RADEO, Mr. Vuernick said: providing the catalyst for change not just for hospital medicine but also for entire institutions.
In January 2017, the U.S. Centers for Medicare & Medicaid Services honored SHM for its hospital patient safety and quality improvement efforts. A big reason for the plaudits was the society’s successful program and implementation toolkit called Reducing Adverse Drug Events related to Opioids (RADEO), now in its second phase.
Kevin Vuernick, senior project manager of SHM’s Center for Hospital Innovation and Improvement, says that the freely available RADEO guide explains how to develop and carry out quality improvement projects related to inpatient opioid prescribing. One of the first steps was devising interventions that hospitalists could implement to reduce opioid-related adverse events. An independent evaluator will help analyze the program’s data, best practices, and outcomes.
Keri Holmes-Maybank, MD, MSCR, FHM, an academic hospitalist at the Medical University of South Carolina, Charleston, said that the RADEO guide has a been a “phenomenal” resource. Dr. Holmes-Maybank, who led her medical center’s involvement in RADEO’s first round, says the guide helped her identify areas that her institution could work on. For one project, the medical university implemented the Pasero Opioid-Induced Sedation Scale to help prevent adverse opioid-related events, such as life-threatening respiratory depression. For a second project, the center combined existing discharge information into a more complete document that could be given to patients to educate them and their caregivers better.
St. Anthony Hospital in Oklahoma City first used RADEO to revisit how it was evaluating patients’ pain and then widened the scope to reassess how it was managing its opioid treatment and narcotic use. “We just kept swinging at the tree, trying to hit the low-hanging fruit and seeing what we could improve upon,” said Matthew Jared, MD, a hospitalist at St. Anthony and its program lead during its involvement in phase one of RADEO.
Dr. Jared is hoping to build on the momentum with a plan to develop better in-house protocols for monitoring pain, employing alternative treatments, and establishing clear lines of communication. “That’s our next step forward: really taking what we’ve learned and beginning to implement it into a holistic type of pain management within the hospital that each physician can tailor to the individual patient but still have the framework to support them,” he said. This ambitious plan is precisely the goal of RADEO, Mr. Vuernick said: providing the catalyst for change not just for hospital medicine but also for entire institutions.
Sorting out syncope signs and symptoms in kids remains essential
CHICAGO – Syncope often is misdiagnosed in pediatric patients complaining of chest pain, and a new guideline released in 2017 could guide clinicians toward a more accurate differential diagnosis and help them know when immediate referral to cardiology or the emergency department is warranted.
“There are recent guidelines published this year which are helpful,” said Dr. Barbara Deal, the Getz Professor of Cardiology at Northwestern University in Chicago. “,” which is defined as transient loss of consciousness.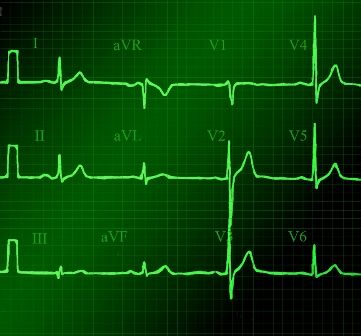
“Once you establish it is a simple vasovagal [cause], you need to educate patients on conditions that would promote this and the need to be anticipatory,” Dr. Deal said at the annual meeting of the American Academy of Pediatrics. “Further testing with an echo[cardiogram] should be done if you suspect heart disease or a rhythm disorder.”
Chest pain and syncope are common complaints that can lead to significant anxiety for patients, parents, and pediatric providers. The greatest cause of this anxiety is the prospect of a fatal or near-fatal event. An abnormal cardiac examination, any associated palpitations, and a history of urinary incontinence or traumatic injury are reasons to worry, she said. “Any of these should prompt an urgent consult to cardiology or the emergency department.”
Cardiac causes of chest pain include reflex or vasovagal syncope or even a more serious cardiac cause, such as arrhythmic or structural issues, Dr. Deal said. Symptoms that appear with exertion or stress also are very worrisome. “You would know if they have a heart murmur or stenosis – it’s these other things that don’t present with a cardiac abnormality: hypertrophic cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy,” she said. “If they have symptoms on exertion, pay attention. This is not good.”
Syncope often is misdiagnosed, Dr. Deal said. Approximately 35%-48% of patients classified as having syncope do not have actual syncope;rather they experience dizziness rather than a loss of consciousness. In a study of 194 children, for example, the leading etiologies diagnosed after evaluation for syncope included simple fainting in 49%, a vasopressor/vasovagal response in 14%, and possible seizure in 14% (J Am Coll Cardiol. 1997;29[6]:1039-45). Seven percent were diagnosed with syncope not otherwise specified in this series. Some other causes included psychogenic or orthostatic ones, hyperventilation, dysmenorrhea, vertigo, dehydration, trauma, stress, exhaustion, or an infectious condition.*
“When should you be thinking life-threatening syncope?” Dr. Deal asked. Arrhythmic disorders that are heritable, such as an ion channelopathy, are an example. “They don’t always feel the racing heartbeat, but they feel something is not right; they feel a sense of impending doom. Some families report signs during exercise like swimming, seizures, gargling noises, or unusual symptoms on awakening.”
Dr. Deal noted that ages 3-24 years are “the problem territory for cardiac arrest.” In this age group, 43%-55% of cardiac arrests are associated with hypertrophic cardiomyopathy or arrhythmias; about one in three will have prior syncope. “These causes are not detectable on physical examination and often are not detectable with ECG only,” adding to the differential diagnosis challenges.*
Refer to a pediatric cardiologist
For this reason and others, referral to a pediatric cardiologist is indicated instead of a consult with an adult cardiologist, Dr. Deal said. “You know how your kids will start with ‘no offense.’ Like, ‘no offense, Mom, but your hair looks awful.’ With moderate offense intended, having an adult cardiologist read a pediatric ECG and clear them is not adequate. They will be looking for ischemia or A-fib [atrial fibrillation]; they’re not looking for long QT syndrome, arrhythmogenic cardiomyopathy, or abnormal T waves.”
Early detection of long QT syndrome is optimal, Dr. Deal said, because symptom onset often is between infancy and age 7 years. In addition, mortality is highest in the first 2 decades of life. “I think this could be why adult cardiologists are not as concerned as we are,” Dr. Deal said. “The bad ones die before they reach adulthood.”
Ruling out a cardiac cause
The 2017 joint guideline defined syncope as a transient loss of consciousness. “By definition, you pass out, you’re not aware of where you are, and you cannot hear,” Dr. Deal said. If a patient reports they could hear people talking, they may have lost postural tone, but they did not have syncope, she added.
“Sometimes, we see teenagers who are said to pass out and are unresponsive for 5 minutes, 10 minutes, or 20 minutes. I’m usually relieved to hear that because that gets cardiac off the hook,” Dr. Deal said. “There is nothing cardiac that makes you pass out for 20 minutes, unless people are resuscitating you.” She added, “I’m not suggesting it’s not a significant problem that you need to get to the bottom of.”
Noncardiac etiologies can be neurologic, metabolic, drug-induced, or psychogenic. “This is where the detective work comes in.”
Keep clinical suspicion high for psychogenic syncope, Dr. Deal said. Psychogenic episodes stem from significant psychological stress, often something so profoundly bothersome that they cannot cope, such as sexual abuse.
A helpful tip for diagnosing the less worrisome vasovagal syncope is asking whether a patient was sitting to standing or standing a long time before an episode, Dr. Deal said. “A common complaint is that the family went to airport, got up early, didn’t eat, and ended up standing for a long time. Kids will say they don’t feel well, they fall down, and all hell breaks loose.” Other causes of vasovagal syncope include stress, pain, or a situational trigger, for example, when a person faints during a blood draw or immunization.
The 2017 guideline also set forth the evidence behind various medications used to lower the risk of syncope. However, Dr. Deal said, “If syncope only happens every 3 years when you go to an airport, it’s probably not worth daily therapy to prevent that.”
‘The world’s most boring test’
For the most part, lifestyle measures should work to address vasovagal syncope. A tilt table test can be useful to aid the differential diagnosis, but it’s recommended only when the etiology is unclear, Dr. Deal said. On the plus side, the tilt table test allows clinicians to reproduce symptoms in a controlled environment. On the downside, she added, “It’s the world’s most boring test. It’s a challenge for the cardiologist to stay awake. It’s very boring, until all hell breaks loose.”
“I find this helpful in the setting of kids with seizures and for kids with this atypical syncope where you just cannot convince the family that these 20-minute episodes of loss of consciousness are not near-death episodes.”
Dr. Deal had no relevant financial disclosures.
*This article was updated on 10/25/2017.
CHICAGO – Syncope often is misdiagnosed in pediatric patients complaining of chest pain, and a new guideline released in 2017 could guide clinicians toward a more accurate differential diagnosis and help them know when immediate referral to cardiology or the emergency department is warranted.
“There are recent guidelines published this year which are helpful,” said Dr. Barbara Deal, the Getz Professor of Cardiology at Northwestern University in Chicago. “,” which is defined as transient loss of consciousness.
“Once you establish it is a simple vasovagal [cause], you need to educate patients on conditions that would promote this and the need to be anticipatory,” Dr. Deal said at the annual meeting of the American Academy of Pediatrics. “Further testing with an echo[cardiogram] should be done if you suspect heart disease or a rhythm disorder.”
Chest pain and syncope are common complaints that can lead to significant anxiety for patients, parents, and pediatric providers. The greatest cause of this anxiety is the prospect of a fatal or near-fatal event. An abnormal cardiac examination, any associated palpitations, and a history of urinary incontinence or traumatic injury are reasons to worry, she said. “Any of these should prompt an urgent consult to cardiology or the emergency department.”
Cardiac causes of chest pain include reflex or vasovagal syncope or even a more serious cardiac cause, such as arrhythmic or structural issues, Dr. Deal said. Symptoms that appear with exertion or stress also are very worrisome. “You would know if they have a heart murmur or stenosis – it’s these other things that don’t present with a cardiac abnormality: hypertrophic cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy,” she said. “If they have symptoms on exertion, pay attention. This is not good.”
Syncope often is misdiagnosed, Dr. Deal said. Approximately 35%-48% of patients classified as having syncope do not have actual syncope;rather they experience dizziness rather than a loss of consciousness. In a study of 194 children, for example, the leading etiologies diagnosed after evaluation for syncope included simple fainting in 49%, a vasopressor/vasovagal response in 14%, and possible seizure in 14% (J Am Coll Cardiol. 1997;29[6]:1039-45). Seven percent were diagnosed with syncope not otherwise specified in this series. Some other causes included psychogenic or orthostatic ones, hyperventilation, dysmenorrhea, vertigo, dehydration, trauma, stress, exhaustion, or an infectious condition.*
“When should you be thinking life-threatening syncope?” Dr. Deal asked. Arrhythmic disorders that are heritable, such as an ion channelopathy, are an example. “They don’t always feel the racing heartbeat, but they feel something is not right; they feel a sense of impending doom. Some families report signs during exercise like swimming, seizures, gargling noises, or unusual symptoms on awakening.”
Dr. Deal noted that ages 3-24 years are “the problem territory for cardiac arrest.” In this age group, 43%-55% of cardiac arrests are associated with hypertrophic cardiomyopathy or arrhythmias; about one in three will have prior syncope. “These causes are not detectable on physical examination and often are not detectable with ECG only,” adding to the differential diagnosis challenges.*
Refer to a pediatric cardiologist
For this reason and others, referral to a pediatric cardiologist is indicated instead of a consult with an adult cardiologist, Dr. Deal said. “You know how your kids will start with ‘no offense.’ Like, ‘no offense, Mom, but your hair looks awful.’ With moderate offense intended, having an adult cardiologist read a pediatric ECG and clear them is not adequate. They will be looking for ischemia or A-fib [atrial fibrillation]; they’re not looking for long QT syndrome, arrhythmogenic cardiomyopathy, or abnormal T waves.”
Early detection of long QT syndrome is optimal, Dr. Deal said, because symptom onset often is between infancy and age 7 years. In addition, mortality is highest in the first 2 decades of life. “I think this could be why adult cardiologists are not as concerned as we are,” Dr. Deal said. “The bad ones die before they reach adulthood.”
Ruling out a cardiac cause
The 2017 joint guideline defined syncope as a transient loss of consciousness. “By definition, you pass out, you’re not aware of where you are, and you cannot hear,” Dr. Deal said. If a patient reports they could hear people talking, they may have lost postural tone, but they did not have syncope, she added.
“Sometimes, we see teenagers who are said to pass out and are unresponsive for 5 minutes, 10 minutes, or 20 minutes. I’m usually relieved to hear that because that gets cardiac off the hook,” Dr. Deal said. “There is nothing cardiac that makes you pass out for 20 minutes, unless people are resuscitating you.” She added, “I’m not suggesting it’s not a significant problem that you need to get to the bottom of.”
Noncardiac etiologies can be neurologic, metabolic, drug-induced, or psychogenic. “This is where the detective work comes in.”
Keep clinical suspicion high for psychogenic syncope, Dr. Deal said. Psychogenic episodes stem from significant psychological stress, often something so profoundly bothersome that they cannot cope, such as sexual abuse.
A helpful tip for diagnosing the less worrisome vasovagal syncope is asking whether a patient was sitting to standing or standing a long time before an episode, Dr. Deal said. “A common complaint is that the family went to airport, got up early, didn’t eat, and ended up standing for a long time. Kids will say they don’t feel well, they fall down, and all hell breaks loose.” Other causes of vasovagal syncope include stress, pain, or a situational trigger, for example, when a person faints during a blood draw or immunization.
The 2017 guideline also set forth the evidence behind various medications used to lower the risk of syncope. However, Dr. Deal said, “If syncope only happens every 3 years when you go to an airport, it’s probably not worth daily therapy to prevent that.”
‘The world’s most boring test’
For the most part, lifestyle measures should work to address vasovagal syncope. A tilt table test can be useful to aid the differential diagnosis, but it’s recommended only when the etiology is unclear, Dr. Deal said. On the plus side, the tilt table test allows clinicians to reproduce symptoms in a controlled environment. On the downside, she added, “It’s the world’s most boring test. It’s a challenge for the cardiologist to stay awake. It’s very boring, until all hell breaks loose.”
“I find this helpful in the setting of kids with seizures and for kids with this atypical syncope where you just cannot convince the family that these 20-minute episodes of loss of consciousness are not near-death episodes.”
Dr. Deal had no relevant financial disclosures.
*This article was updated on 10/25/2017.
CHICAGO – Syncope often is misdiagnosed in pediatric patients complaining of chest pain, and a new guideline released in 2017 could guide clinicians toward a more accurate differential diagnosis and help them know when immediate referral to cardiology or the emergency department is warranted.
“There are recent guidelines published this year which are helpful,” said Dr. Barbara Deal, the Getz Professor of Cardiology at Northwestern University in Chicago. “,” which is defined as transient loss of consciousness.
“Once you establish it is a simple vasovagal [cause], you need to educate patients on conditions that would promote this and the need to be anticipatory,” Dr. Deal said at the annual meeting of the American Academy of Pediatrics. “Further testing with an echo[cardiogram] should be done if you suspect heart disease or a rhythm disorder.”
Chest pain and syncope are common complaints that can lead to significant anxiety for patients, parents, and pediatric providers. The greatest cause of this anxiety is the prospect of a fatal or near-fatal event. An abnormal cardiac examination, any associated palpitations, and a history of urinary incontinence or traumatic injury are reasons to worry, she said. “Any of these should prompt an urgent consult to cardiology or the emergency department.”
Cardiac causes of chest pain include reflex or vasovagal syncope or even a more serious cardiac cause, such as arrhythmic or structural issues, Dr. Deal said. Symptoms that appear with exertion or stress also are very worrisome. “You would know if they have a heart murmur or stenosis – it’s these other things that don’t present with a cardiac abnormality: hypertrophic cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy,” she said. “If they have symptoms on exertion, pay attention. This is not good.”
Syncope often is misdiagnosed, Dr. Deal said. Approximately 35%-48% of patients classified as having syncope do not have actual syncope;rather they experience dizziness rather than a loss of consciousness. In a study of 194 children, for example, the leading etiologies diagnosed after evaluation for syncope included simple fainting in 49%, a vasopressor/vasovagal response in 14%, and possible seizure in 14% (J Am Coll Cardiol. 1997;29[6]:1039-45). Seven percent were diagnosed with syncope not otherwise specified in this series. Some other causes included psychogenic or orthostatic ones, hyperventilation, dysmenorrhea, vertigo, dehydration, trauma, stress, exhaustion, or an infectious condition.*
“When should you be thinking life-threatening syncope?” Dr. Deal asked. Arrhythmic disorders that are heritable, such as an ion channelopathy, are an example. “They don’t always feel the racing heartbeat, but they feel something is not right; they feel a sense of impending doom. Some families report signs during exercise like swimming, seizures, gargling noises, or unusual symptoms on awakening.”
Dr. Deal noted that ages 3-24 years are “the problem territory for cardiac arrest.” In this age group, 43%-55% of cardiac arrests are associated with hypertrophic cardiomyopathy or arrhythmias; about one in three will have prior syncope. “These causes are not detectable on physical examination and often are not detectable with ECG only,” adding to the differential diagnosis challenges.*
Refer to a pediatric cardiologist
For this reason and others, referral to a pediatric cardiologist is indicated instead of a consult with an adult cardiologist, Dr. Deal said. “You know how your kids will start with ‘no offense.’ Like, ‘no offense, Mom, but your hair looks awful.’ With moderate offense intended, having an adult cardiologist read a pediatric ECG and clear them is not adequate. They will be looking for ischemia or A-fib [atrial fibrillation]; they’re not looking for long QT syndrome, arrhythmogenic cardiomyopathy, or abnormal T waves.”
Early detection of long QT syndrome is optimal, Dr. Deal said, because symptom onset often is between infancy and age 7 years. In addition, mortality is highest in the first 2 decades of life. “I think this could be why adult cardiologists are not as concerned as we are,” Dr. Deal said. “The bad ones die before they reach adulthood.”
Ruling out a cardiac cause
The 2017 joint guideline defined syncope as a transient loss of consciousness. “By definition, you pass out, you’re not aware of where you are, and you cannot hear,” Dr. Deal said. If a patient reports they could hear people talking, they may have lost postural tone, but they did not have syncope, she added.
“Sometimes, we see teenagers who are said to pass out and are unresponsive for 5 minutes, 10 minutes, or 20 minutes. I’m usually relieved to hear that because that gets cardiac off the hook,” Dr. Deal said. “There is nothing cardiac that makes you pass out for 20 minutes, unless people are resuscitating you.” She added, “I’m not suggesting it’s not a significant problem that you need to get to the bottom of.”
Noncardiac etiologies can be neurologic, metabolic, drug-induced, or psychogenic. “This is where the detective work comes in.”
Keep clinical suspicion high for psychogenic syncope, Dr. Deal said. Psychogenic episodes stem from significant psychological stress, often something so profoundly bothersome that they cannot cope, such as sexual abuse.
A helpful tip for diagnosing the less worrisome vasovagal syncope is asking whether a patient was sitting to standing or standing a long time before an episode, Dr. Deal said. “A common complaint is that the family went to airport, got up early, didn’t eat, and ended up standing for a long time. Kids will say they don’t feel well, they fall down, and all hell breaks loose.” Other causes of vasovagal syncope include stress, pain, or a situational trigger, for example, when a person faints during a blood draw or immunization.
The 2017 guideline also set forth the evidence behind various medications used to lower the risk of syncope. However, Dr. Deal said, “If syncope only happens every 3 years when you go to an airport, it’s probably not worth daily therapy to prevent that.”
‘The world’s most boring test’
For the most part, lifestyle measures should work to address vasovagal syncope. A tilt table test can be useful to aid the differential diagnosis, but it’s recommended only when the etiology is unclear, Dr. Deal said. On the plus side, the tilt table test allows clinicians to reproduce symptoms in a controlled environment. On the downside, she added, “It’s the world’s most boring test. It’s a challenge for the cardiologist to stay awake. It’s very boring, until all hell breaks loose.”
“I find this helpful in the setting of kids with seizures and for kids with this atypical syncope where you just cannot convince the family that these 20-minute episodes of loss of consciousness are not near-death episodes.”
Dr. Deal had no relevant financial disclosures.
*This article was updated on 10/25/2017.
EXPERT ANALYSIS FROM AAP 2017
VIDEO: Endoscopy surpasses surgery for acute necrotizing pancreatitis
ORLANDO – An endoscopic approach to treatment of acute necrotizing pancreatitis was substantially safer than was minimally invasive surgical treatment in a randomized study of 66 patients.
Performing drainage and necrosectomy endoscopically in 34 patients with necrotizing pancreatitis that was symptomatic, infected, or both resulted in a 12% rate of major adverse events over the 3 months following intervention compared with a 38% rate among 32 similar patients who underwent laparoscopic drainage followed by either internal debridement or video-assisted retroperitoneal debridement, Ji Young Bang, MD, said at the World Congress of Gastroenterology at ACG 2017.
This statistically significant reduction in the study’s primary endpoint was driven primarily by a major reduction in the incidence of pancreaticocutaneous fistula, which occurred in none of the endoscopy patients and in eight (25%) of the surgery patients, and a smaller reduction in enterocutaneous fistula, which occurred in none of the endoscopy patients and in four (13%) of the patients treated surgically, said Dr. Bang, a gastroenterologist at the Center for Interventional Endoscopy at Florida Hospital, Orlando.
Based on these results, the endoscopic approach “is the treatment of the future,” Dr. Bang said in a video interview. Although the randomized study had a modest number of patients, it was adequately powered to address the hypothesis that endoscopy caused fewer major adverse events than did minimally invasive surgery, and hence the findings should have “an important clinical impact” on the choice of endoscopy or a minimally invasive surgical approach. But Dr. Bang also stressed that a successful endoscopic approach as obtained in this study requires treatment at a center that can offer multidisciplinary expertise from gastrointestinal endoscopists, surgeons, and radiologists, as well as infectious disease physicians, to minimize infections.
Prior to this study, results from the Pancreatitis, Endoscopic Transgastric vs Primary Necrosectomy in Patients With Infected Necrosis (PENGUIN) study run in the Netherlands had also shown significantly fewer adverse events with endoscopic treatment compared with laparoscopic surgery in 20 randomized patients (JAMA. 2012 Mar 14;307[10]:1053-61).
The study reported by Dr. Bang, the Minimally Invasive Surgery vs. Endoscopy Randomized (MISER) trial, enrolled patients with an average necrotic collection size of about 11 cm. The average age of the patients was 59 years. Nearly half of the patients had confirmed infected necrosis. More than 90% had American Society of Anesthesiologists class III or IV disease, and about half had systemic inflammatory response syndrome. All patients had disease that was amenable to both the endoscopic and minimally invasive surgical approaches.
The study’s primary endpoint included several other adverse events in addition to fistulas during 3-month follow-up: death, new-onset organ failure or multiple systemic dysfunction, visceral perforation, and intra-abdominal bleeding. The incidence of each of these outcomes was about the same in the two study arms.
The results also showed that endoscopy was significantly better than surgery for several other secondary outcomes, including new-onset systemic inflammatory response syndrome as well as the prevalence of this complication 3 days after intervention (21% compared with 66%), days in the ICU, average total procedure and hospitalization cost ($76,000 compared with $117,000), and physical quality of life 3 months after treatment. For all other measured outcomes the endoscopic approach and surgical approach produced similar outcomes, and no outcome measured showed that endoscopy was significantly inferior to surgery, Dr. Bang reported.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
ORLANDO – An endoscopic approach to treatment of acute necrotizing pancreatitis was substantially safer than was minimally invasive surgical treatment in a randomized study of 66 patients.
Performing drainage and necrosectomy endoscopically in 34 patients with necrotizing pancreatitis that was symptomatic, infected, or both resulted in a 12% rate of major adverse events over the 3 months following intervention compared with a 38% rate among 32 similar patients who underwent laparoscopic drainage followed by either internal debridement or video-assisted retroperitoneal debridement, Ji Young Bang, MD, said at the World Congress of Gastroenterology at ACG 2017.
This statistically significant reduction in the study’s primary endpoint was driven primarily by a major reduction in the incidence of pancreaticocutaneous fistula, which occurred in none of the endoscopy patients and in eight (25%) of the surgery patients, and a smaller reduction in enterocutaneous fistula, which occurred in none of the endoscopy patients and in four (13%) of the patients treated surgically, said Dr. Bang, a gastroenterologist at the Center for Interventional Endoscopy at Florida Hospital, Orlando.
Based on these results, the endoscopic approach “is the treatment of the future,” Dr. Bang said in a video interview. Although the randomized study had a modest number of patients, it was adequately powered to address the hypothesis that endoscopy caused fewer major adverse events than did minimally invasive surgery, and hence the findings should have “an important clinical impact” on the choice of endoscopy or a minimally invasive surgical approach. But Dr. Bang also stressed that a successful endoscopic approach as obtained in this study requires treatment at a center that can offer multidisciplinary expertise from gastrointestinal endoscopists, surgeons, and radiologists, as well as infectious disease physicians, to minimize infections.
Prior to this study, results from the Pancreatitis, Endoscopic Transgastric vs Primary Necrosectomy in Patients With Infected Necrosis (PENGUIN) study run in the Netherlands had also shown significantly fewer adverse events with endoscopic treatment compared with laparoscopic surgery in 20 randomized patients (JAMA. 2012 Mar 14;307[10]:1053-61).
The study reported by Dr. Bang, the Minimally Invasive Surgery vs. Endoscopy Randomized (MISER) trial, enrolled patients with an average necrotic collection size of about 11 cm. The average age of the patients was 59 years. Nearly half of the patients had confirmed infected necrosis. More than 90% had American Society of Anesthesiologists class III or IV disease, and about half had systemic inflammatory response syndrome. All patients had disease that was amenable to both the endoscopic and minimally invasive surgical approaches.
The study’s primary endpoint included several other adverse events in addition to fistulas during 3-month follow-up: death, new-onset organ failure or multiple systemic dysfunction, visceral perforation, and intra-abdominal bleeding. The incidence of each of these outcomes was about the same in the two study arms.
The results also showed that endoscopy was significantly better than surgery for several other secondary outcomes, including new-onset systemic inflammatory response syndrome as well as the prevalence of this complication 3 days after intervention (21% compared with 66%), days in the ICU, average total procedure and hospitalization cost ($76,000 compared with $117,000), and physical quality of life 3 months after treatment. For all other measured outcomes the endoscopic approach and surgical approach produced similar outcomes, and no outcome measured showed that endoscopy was significantly inferior to surgery, Dr. Bang reported.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
ORLANDO – An endoscopic approach to treatment of acute necrotizing pancreatitis was substantially safer than was minimally invasive surgical treatment in a randomized study of 66 patients.
Performing drainage and necrosectomy endoscopically in 34 patients with necrotizing pancreatitis that was symptomatic, infected, or both resulted in a 12% rate of major adverse events over the 3 months following intervention compared with a 38% rate among 32 similar patients who underwent laparoscopic drainage followed by either internal debridement or video-assisted retroperitoneal debridement, Ji Young Bang, MD, said at the World Congress of Gastroenterology at ACG 2017.
This statistically significant reduction in the study’s primary endpoint was driven primarily by a major reduction in the incidence of pancreaticocutaneous fistula, which occurred in none of the endoscopy patients and in eight (25%) of the surgery patients, and a smaller reduction in enterocutaneous fistula, which occurred in none of the endoscopy patients and in four (13%) of the patients treated surgically, said Dr. Bang, a gastroenterologist at the Center for Interventional Endoscopy at Florida Hospital, Orlando.
Based on these results, the endoscopic approach “is the treatment of the future,” Dr. Bang said in a video interview. Although the randomized study had a modest number of patients, it was adequately powered to address the hypothesis that endoscopy caused fewer major adverse events than did minimally invasive surgery, and hence the findings should have “an important clinical impact” on the choice of endoscopy or a minimally invasive surgical approach. But Dr. Bang also stressed that a successful endoscopic approach as obtained in this study requires treatment at a center that can offer multidisciplinary expertise from gastrointestinal endoscopists, surgeons, and radiologists, as well as infectious disease physicians, to minimize infections.
Prior to this study, results from the Pancreatitis, Endoscopic Transgastric vs Primary Necrosectomy in Patients With Infected Necrosis (PENGUIN) study run in the Netherlands had also shown significantly fewer adverse events with endoscopic treatment compared with laparoscopic surgery in 20 randomized patients (JAMA. 2012 Mar 14;307[10]:1053-61).
The study reported by Dr. Bang, the Minimally Invasive Surgery vs. Endoscopy Randomized (MISER) trial, enrolled patients with an average necrotic collection size of about 11 cm. The average age of the patients was 59 years. Nearly half of the patients had confirmed infected necrosis. More than 90% had American Society of Anesthesiologists class III or IV disease, and about half had systemic inflammatory response syndrome. All patients had disease that was amenable to both the endoscopic and minimally invasive surgical approaches.
The study’s primary endpoint included several other adverse events in addition to fistulas during 3-month follow-up: death, new-onset organ failure or multiple systemic dysfunction, visceral perforation, and intra-abdominal bleeding. The incidence of each of these outcomes was about the same in the two study arms.
The results also showed that endoscopy was significantly better than surgery for several other secondary outcomes, including new-onset systemic inflammatory response syndrome as well as the prevalence of this complication 3 days after intervention (21% compared with 66%), days in the ICU, average total procedure and hospitalization cost ($76,000 compared with $117,000), and physical quality of life 3 months after treatment. For all other measured outcomes the endoscopic approach and surgical approach produced similar outcomes, and no outcome measured showed that endoscopy was significantly inferior to surgery, Dr. Bang reported.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
AT THE WORLD CONGRESS OF GASTROENTEROLOGY
Key clinical point:
Major finding: Major adverse events occurred in 12% of patients treated endoscopically and in 38% of patients treated surgically.
Data source: MISER, a multicenter randomized study of 66 evaluable patients.
Disclosures: MISER received no commercial funding. Dr. Bang had no disclosures.
Exercise reduces T1DM mortality risk
LISBON – Exercise was associated with a lower risk of all-cause mortality in patients with type 1 diabetes mellitus, regardless of whether or not they also had chronic kidney disease, according to an analysis of data from a large ongoing population study.
Fully adjusted hazard ratios comparing low versus moderate-to-high amounts of physical activity, intensity, frequency, and duration were a respective 1.63, 2.17, 2.07, and 1.86 in patients without CKD.
The corresponding HRs in patients with comorbid CKD were 1.47, 1.39, 1.90, and 1.49, although only the total exercise amount and frequency were statistically significant in this study group.
“So far, we know little about exercise and mortality in type 1 diabetes in a prospective setting,” Dr. Tikkanen-Dolenc added. There have been two large studies – the Pittsburgh Study (Diabetes. 1984;33:271-6) and the EURODIAB study (Diabetologia. 2013;56:82-91) – that have been conducted previously. The first showed a benefit of greater participation in team sports and leisure time physical activity (LTPA) in men only, and the second showed a borderline inverse association between a higher amount of LTPA and mortality in both sexes, she said. There are even fewer data specifically in patients with comorbid CKD, although exercise is recommended and appears to be safe, she said.
Dr. Tikkanen-Dolenc and her associates have previously shown that diabetic nephropathy largely accounts for the increased mortality risk in T1DM (Diabetes. 2009;58:1651-8), and that the intensity of exercise rather than the total amount could be important (Diabetologia. 2015;58:929-36). They have also found that high intensity and frequency of LTPA was associated with a decreased risk of cardiovascular events in patients with T1DM (Diabetologia. 2017;60:574-80). Now, they wanted to look more specifically at how LTPA might be associated with mortality in T1DM and also do a separate investigation of what happens when there is concomitant loss of kidney function.
Patients included in the analysis were part of the Finnish Diabetic Nephropathy (FinnDiane) Study, which is a nationwide study being conducted in 90 centers in Finland to look for risk factors and mechanisms behind diabetic complications. To date, the study involves around 5,000 participants, and 2,369 were included in the present analysis. Of these, 310 also had CKD, which was defined by an estimated glomerular filtration rate of 60 mL/min per 1.73 m2 or lower.
A previously validated questionnaire was used to measure LTPA. The total LTPA was calculated by measuring the time spent doing an activity by the intensity index expressed in metabolic equivalents (MET). The latter is a widely used unit in exercise research, Dr. Tikkanen-Dolenc said, and gives a measure of the ratio of the metabolic rate during activity to the rate at rest.
Over a follow-up of 11 years, 270 patients died and 2,099 were alive. Patients who died were significantly (P less than .001) older (50 vs. 38 years), had a longer duration of diabetes (33 vs. 22 years), higher systolic blood pressure (146 vs. 134 mm Hg), lower high-density lipoprotein cholesterol (1.38 vs. 1.44 mmol/L), and higher triglycerides (1.25 vs. 0.96 mmol/L). They were also more sedentary, with baseline LTPAs of 8.6 versus 17.2 MET/h (P less than .001). These factors were taken into account while analyzing the data in multiple ways for static and dynamic risk factors.
“These are great data, and this is one of the best clinical studies at the meeting,” observed Viktor Jörgens, MD, former executive director of the EASD and of the European Foundation for the Study of Diabetes, during the post-presentation discussion.
Dr. Jörgens suggested, however, that there was perhaps one important caveat before doctors around the globe started encouraging their patients to exercise more: the level of patient education around the risk for severe hypoglycemia with increasing exercise and routine availability of blood glucose monitoring.
“The problem with severe exercise in type 1 is severe hypoglycemia, and I know Finland is one of the leading countries for patient education and intensified insulin therapy,” Dr. Jörgens cautioned. “Therefore I assume that most of your patients were well educated on blood glucose monitoring, and knew everything about exercise and reducing the dosage [of insulin therapy].” Not all countries may have such high levels of patient education of monitoring, he suggested.
Dr. Tikkanen-Dolenc responded that “of course patient education is needed, such as on continuous glucose monitoring, and there is a risk, but when we look to current recommendations, we still do recommend exercise, even in type 1 diabetes, and it appears to be safe, but that’s a good point and that’s something we need to note.”
Neither Dr. Tikkanen-Dolenc or Dr. Jörgens had anything to disclose.
LISBON – Exercise was associated with a lower risk of all-cause mortality in patients with type 1 diabetes mellitus, regardless of whether or not they also had chronic kidney disease, according to an analysis of data from a large ongoing population study.
Fully adjusted hazard ratios comparing low versus moderate-to-high amounts of physical activity, intensity, frequency, and duration were a respective 1.63, 2.17, 2.07, and 1.86 in patients without CKD.
The corresponding HRs in patients with comorbid CKD were 1.47, 1.39, 1.90, and 1.49, although only the total exercise amount and frequency were statistically significant in this study group.
“So far, we know little about exercise and mortality in type 1 diabetes in a prospective setting,” Dr. Tikkanen-Dolenc added. There have been two large studies – the Pittsburgh Study (Diabetes. 1984;33:271-6) and the EURODIAB study (Diabetologia. 2013;56:82-91) – that have been conducted previously. The first showed a benefit of greater participation in team sports and leisure time physical activity (LTPA) in men only, and the second showed a borderline inverse association between a higher amount of LTPA and mortality in both sexes, she said. There are even fewer data specifically in patients with comorbid CKD, although exercise is recommended and appears to be safe, she said.
Dr. Tikkanen-Dolenc and her associates have previously shown that diabetic nephropathy largely accounts for the increased mortality risk in T1DM (Diabetes. 2009;58:1651-8), and that the intensity of exercise rather than the total amount could be important (Diabetologia. 2015;58:929-36). They have also found that high intensity and frequency of LTPA was associated with a decreased risk of cardiovascular events in patients with T1DM (Diabetologia. 2017;60:574-80). Now, they wanted to look more specifically at how LTPA might be associated with mortality in T1DM and also do a separate investigation of what happens when there is concomitant loss of kidney function.
Patients included in the analysis were part of the Finnish Diabetic Nephropathy (FinnDiane) Study, which is a nationwide study being conducted in 90 centers in Finland to look for risk factors and mechanisms behind diabetic complications. To date, the study involves around 5,000 participants, and 2,369 were included in the present analysis. Of these, 310 also had CKD, which was defined by an estimated glomerular filtration rate of 60 mL/min per 1.73 m2 or lower.
A previously validated questionnaire was used to measure LTPA. The total LTPA was calculated by measuring the time spent doing an activity by the intensity index expressed in metabolic equivalents (MET). The latter is a widely used unit in exercise research, Dr. Tikkanen-Dolenc said, and gives a measure of the ratio of the metabolic rate during activity to the rate at rest.
Over a follow-up of 11 years, 270 patients died and 2,099 were alive. Patients who died were significantly (P less than .001) older (50 vs. 38 years), had a longer duration of diabetes (33 vs. 22 years), higher systolic blood pressure (146 vs. 134 mm Hg), lower high-density lipoprotein cholesterol (1.38 vs. 1.44 mmol/L), and higher triglycerides (1.25 vs. 0.96 mmol/L). They were also more sedentary, with baseline LTPAs of 8.6 versus 17.2 MET/h (P less than .001). These factors were taken into account while analyzing the data in multiple ways for static and dynamic risk factors.
“These are great data, and this is one of the best clinical studies at the meeting,” observed Viktor Jörgens, MD, former executive director of the EASD and of the European Foundation for the Study of Diabetes, during the post-presentation discussion.
Dr. Jörgens suggested, however, that there was perhaps one important caveat before doctors around the globe started encouraging their patients to exercise more: the level of patient education around the risk for severe hypoglycemia with increasing exercise and routine availability of blood glucose monitoring.
“The problem with severe exercise in type 1 is severe hypoglycemia, and I know Finland is one of the leading countries for patient education and intensified insulin therapy,” Dr. Jörgens cautioned. “Therefore I assume that most of your patients were well educated on blood glucose monitoring, and knew everything about exercise and reducing the dosage [of insulin therapy].” Not all countries may have such high levels of patient education of monitoring, he suggested.
Dr. Tikkanen-Dolenc responded that “of course patient education is needed, such as on continuous glucose monitoring, and there is a risk, but when we look to current recommendations, we still do recommend exercise, even in type 1 diabetes, and it appears to be safe, but that’s a good point and that’s something we need to note.”
Neither Dr. Tikkanen-Dolenc or Dr. Jörgens had anything to disclose.
LISBON – Exercise was associated with a lower risk of all-cause mortality in patients with type 1 diabetes mellitus, regardless of whether or not they also had chronic kidney disease, according to an analysis of data from a large ongoing population study.
Fully adjusted hazard ratios comparing low versus moderate-to-high amounts of physical activity, intensity, frequency, and duration were a respective 1.63, 2.17, 2.07, and 1.86 in patients without CKD.
The corresponding HRs in patients with comorbid CKD were 1.47, 1.39, 1.90, and 1.49, although only the total exercise amount and frequency were statistically significant in this study group.
“So far, we know little about exercise and mortality in type 1 diabetes in a prospective setting,” Dr. Tikkanen-Dolenc added. There have been two large studies – the Pittsburgh Study (Diabetes. 1984;33:271-6) and the EURODIAB study (Diabetologia. 2013;56:82-91) – that have been conducted previously. The first showed a benefit of greater participation in team sports and leisure time physical activity (LTPA) in men only, and the second showed a borderline inverse association between a higher amount of LTPA and mortality in both sexes, she said. There are even fewer data specifically in patients with comorbid CKD, although exercise is recommended and appears to be safe, she said.
Dr. Tikkanen-Dolenc and her associates have previously shown that diabetic nephropathy largely accounts for the increased mortality risk in T1DM (Diabetes. 2009;58:1651-8), and that the intensity of exercise rather than the total amount could be important (Diabetologia. 2015;58:929-36). They have also found that high intensity and frequency of LTPA was associated with a decreased risk of cardiovascular events in patients with T1DM (Diabetologia. 2017;60:574-80). Now, they wanted to look more specifically at how LTPA might be associated with mortality in T1DM and also do a separate investigation of what happens when there is concomitant loss of kidney function.
Patients included in the analysis were part of the Finnish Diabetic Nephropathy (FinnDiane) Study, which is a nationwide study being conducted in 90 centers in Finland to look for risk factors and mechanisms behind diabetic complications. To date, the study involves around 5,000 participants, and 2,369 were included in the present analysis. Of these, 310 also had CKD, which was defined by an estimated glomerular filtration rate of 60 mL/min per 1.73 m2 or lower.
A previously validated questionnaire was used to measure LTPA. The total LTPA was calculated by measuring the time spent doing an activity by the intensity index expressed in metabolic equivalents (MET). The latter is a widely used unit in exercise research, Dr. Tikkanen-Dolenc said, and gives a measure of the ratio of the metabolic rate during activity to the rate at rest.
Over a follow-up of 11 years, 270 patients died and 2,099 were alive. Patients who died were significantly (P less than .001) older (50 vs. 38 years), had a longer duration of diabetes (33 vs. 22 years), higher systolic blood pressure (146 vs. 134 mm Hg), lower high-density lipoprotein cholesterol (1.38 vs. 1.44 mmol/L), and higher triglycerides (1.25 vs. 0.96 mmol/L). They were also more sedentary, with baseline LTPAs of 8.6 versus 17.2 MET/h (P less than .001). These factors were taken into account while analyzing the data in multiple ways for static and dynamic risk factors.
“These are great data, and this is one of the best clinical studies at the meeting,” observed Viktor Jörgens, MD, former executive director of the EASD and of the European Foundation for the Study of Diabetes, during the post-presentation discussion.
Dr. Jörgens suggested, however, that there was perhaps one important caveat before doctors around the globe started encouraging their patients to exercise more: the level of patient education around the risk for severe hypoglycemia with increasing exercise and routine availability of blood glucose monitoring.
“The problem with severe exercise in type 1 is severe hypoglycemia, and I know Finland is one of the leading countries for patient education and intensified insulin therapy,” Dr. Jörgens cautioned. “Therefore I assume that most of your patients were well educated on blood glucose monitoring, and knew everything about exercise and reducing the dosage [of insulin therapy].” Not all countries may have such high levels of patient education of monitoring, he suggested.
Dr. Tikkanen-Dolenc responded that “of course patient education is needed, such as on continuous glucose monitoring, and there is a risk, but when we look to current recommendations, we still do recommend exercise, even in type 1 diabetes, and it appears to be safe, but that’s a good point and that’s something we need to note.”
Neither Dr. Tikkanen-Dolenc or Dr. Jörgens had anything to disclose.
AT EASD 2017
Key clinical point: Exercise was associated with a lower risk of all-cause mortality in patients with type 1 diabetes mellitus, even in those with chronic kidney disease.
Major finding: Increasing exercise intensity and frequency was inversely associated with increased mortality in patients with T1DM and CKD (hazard ratios, 1.47 and 1.90, respectively).
Data source: The Finnish Diabetic Nephropathy Study; 2,369 patients with T1DM were included in the analyses.
Disclosures: The presenting author and commentator had no disclosures.
MI, stroke risk from HFrEF surpasses HFpEF
DALLAS – Patients newly diagnosed with heart failure with reduced ejection fraction had about an 8% incidence of MIs during the subsequent 9 months, and a 5% incidence of ischemic strokes in a retrospective review of more than 1,600 community-dwelling U.S. patients.
The MI and ischemic stroke incidence rates in heart failure patients with reduced ejection fraction (HFrEF) were both significantly higher than in more than 4,000 patients with heart failure with preserved ejection fraction (HFpEF), Gregg C. Fonarow, MD, said while presenting a poster at the annual scientific meeting of the Heart Failure Society of America.
The findings suggest that greater attention is needed to reduce the risks for MI and stroke in HFrEF patients, suggested Dr. Fonarow, professor and cochief of cardiology at the University of California, Los Angeles, and his associates in their poster.
The study used claims data collected during July 2009-September 2016 from more than 10 million people enrolled in the United Health Group, who received care at more than 650 hospitals and about 6,600 clinics. The study included all patients diagnosed with heart failure during a hospital or emergency room visit and who had no history of a heart failure diagnosis or episode during the preceding 18 months, a left ventricular ejection fraction measurement made close to the time of the index encounter, and no stroke or MI apparent at the time of the index event. The study included 1,622 patients with HFrEF, defined as a left ventricular ejection fraction of less than 40%, 4,288 with HFpEF, defined as an ejection fraction of 50% or more, and 1,095 with heart failure with a borderline ejection fraction of 40%-49%.
The HFrEF patients had an average ejection fraction of 28%, they averaged 72 years old, 36% were women, and 8% had a prior stroke. The HFpEF patients averaged 74 years old, their average ejection fraction was 61%, 55% were women, and 11% had a prior stroke. Follow-up data on all patients were available for an average of nearly 9 months following their index heart failure event, with some patients followed as long as 1 year.
During follow-up, the incidence of ischemic stroke was 5.4% in the HFrEF patients and 3.9% in those with HFpEF, a difference that worked out to a statistically significant 40% higher ischemic stroke rate in HFrEF patients after adjustment for baseline differences between the two patient groups, Dr. Fonarow reported. The patients with a borderline ejection fraction had a 3.7% stroke incidence that fell short of a significant difference, compared with the HFrEF patient.The rate of new MIs during follow-up was 7.5% in the HFrEF patients and 3.2% in the HFpEF patients, a statistically significant 2.5-fold relatively higher MI rate with HFrEF, a statistically significant difference after adjustments. The MI incidence in patients with a borderline ejection fraction was 5.9%
[email protected]
On Twitter @mitchelzoler
DALLAS – Patients newly diagnosed with heart failure with reduced ejection fraction had about an 8% incidence of MIs during the subsequent 9 months, and a 5% incidence of ischemic strokes in a retrospective review of more than 1,600 community-dwelling U.S. patients.
The MI and ischemic stroke incidence rates in heart failure patients with reduced ejection fraction (HFrEF) were both significantly higher than in more than 4,000 patients with heart failure with preserved ejection fraction (HFpEF), Gregg C. Fonarow, MD, said while presenting a poster at the annual scientific meeting of the Heart Failure Society of America.
The findings suggest that greater attention is needed to reduce the risks for MI and stroke in HFrEF patients, suggested Dr. Fonarow, professor and cochief of cardiology at the University of California, Los Angeles, and his associates in their poster.
The study used claims data collected during July 2009-September 2016 from more than 10 million people enrolled in the United Health Group, who received care at more than 650 hospitals and about 6,600 clinics. The study included all patients diagnosed with heart failure during a hospital or emergency room visit and who had no history of a heart failure diagnosis or episode during the preceding 18 months, a left ventricular ejection fraction measurement made close to the time of the index encounter, and no stroke or MI apparent at the time of the index event. The study included 1,622 patients with HFrEF, defined as a left ventricular ejection fraction of less than 40%, 4,288 with HFpEF, defined as an ejection fraction of 50% or more, and 1,095 with heart failure with a borderline ejection fraction of 40%-49%.
The HFrEF patients had an average ejection fraction of 28%, they averaged 72 years old, 36% were women, and 8% had a prior stroke. The HFpEF patients averaged 74 years old, their average ejection fraction was 61%, 55% were women, and 11% had a prior stroke. Follow-up data on all patients were available for an average of nearly 9 months following their index heart failure event, with some patients followed as long as 1 year.
During follow-up, the incidence of ischemic stroke was 5.4% in the HFrEF patients and 3.9% in those with HFpEF, a difference that worked out to a statistically significant 40% higher ischemic stroke rate in HFrEF patients after adjustment for baseline differences between the two patient groups, Dr. Fonarow reported. The patients with a borderline ejection fraction had a 3.7% stroke incidence that fell short of a significant difference, compared with the HFrEF patient.The rate of new MIs during follow-up was 7.5% in the HFrEF patients and 3.2% in the HFpEF patients, a statistically significant 2.5-fold relatively higher MI rate with HFrEF, a statistically significant difference after adjustments. The MI incidence in patients with a borderline ejection fraction was 5.9%
[email protected]
On Twitter @mitchelzoler
DALLAS – Patients newly diagnosed with heart failure with reduced ejection fraction had about an 8% incidence of MIs during the subsequent 9 months, and a 5% incidence of ischemic strokes in a retrospective review of more than 1,600 community-dwelling U.S. patients.
The MI and ischemic stroke incidence rates in heart failure patients with reduced ejection fraction (HFrEF) were both significantly higher than in more than 4,000 patients with heart failure with preserved ejection fraction (HFpEF), Gregg C. Fonarow, MD, said while presenting a poster at the annual scientific meeting of the Heart Failure Society of America.
The findings suggest that greater attention is needed to reduce the risks for MI and stroke in HFrEF patients, suggested Dr. Fonarow, professor and cochief of cardiology at the University of California, Los Angeles, and his associates in their poster.
The study used claims data collected during July 2009-September 2016 from more than 10 million people enrolled in the United Health Group, who received care at more than 650 hospitals and about 6,600 clinics. The study included all patients diagnosed with heart failure during a hospital or emergency room visit and who had no history of a heart failure diagnosis or episode during the preceding 18 months, a left ventricular ejection fraction measurement made close to the time of the index encounter, and no stroke or MI apparent at the time of the index event. The study included 1,622 patients with HFrEF, defined as a left ventricular ejection fraction of less than 40%, 4,288 with HFpEF, defined as an ejection fraction of 50% or more, and 1,095 with heart failure with a borderline ejection fraction of 40%-49%.
The HFrEF patients had an average ejection fraction of 28%, they averaged 72 years old, 36% were women, and 8% had a prior stroke. The HFpEF patients averaged 74 years old, their average ejection fraction was 61%, 55% were women, and 11% had a prior stroke. Follow-up data on all patients were available for an average of nearly 9 months following their index heart failure event, with some patients followed as long as 1 year.
During follow-up, the incidence of ischemic stroke was 5.4% in the HFrEF patients and 3.9% in those with HFpEF, a difference that worked out to a statistically significant 40% higher ischemic stroke rate in HFrEF patients after adjustment for baseline differences between the two patient groups, Dr. Fonarow reported. The patients with a borderline ejection fraction had a 3.7% stroke incidence that fell short of a significant difference, compared with the HFrEF patient.The rate of new MIs during follow-up was 7.5% in the HFrEF patients and 3.2% in the HFpEF patients, a statistically significant 2.5-fold relatively higher MI rate with HFrEF, a statistically significant difference after adjustments. The MI incidence in patients with a borderline ejection fraction was 5.9%
[email protected]
On Twitter @mitchelzoler
AT THE HFSA ANNUAL SCIENTIFIC MEETING
Key clinical point:
Major finding: HFrEF patients had a 40% higher incidence of stroke and a 2.5-fold higher incidence of MI, compared with HFpEF patients.
Data source: Retrospective review of 7,005 U.S. patients newly diagnosed with heart failure.
Disclosures: The study was funded by Janssen. Dr. Fonarow had no relevant disclosures.
Keynote 040: Pembrolizumab misses efficacy endpoint in advanced HNSCC
MADRID – Although pembrolizumab (Keytruda) was associated with a 19% reduction in the risk of death compared with the standard of care in patients with relapsed or metastatic head and neck squamous cell carcinoma (HNSCC) in the KEYNOTE 040 trial, the immune checkpoint inhibitor just missed meeting its primary efficacy endpoint of an improvement in overall survival.
The fault may lie in the confounding of overall survival results when patients who were initially assigned to standard of care were crossed over to subsequent therapy with a checkpoint inhibitor after the study ended, said lead investigator Ezra Cohen, MD, of Moores Cancer Center at UC San Diego Health Sciences in La Jolla, Calif.
“This was a trial that clearly did not meet its primary endpoint, but was felt to confer some benefit – at least in the opinion of this investigator – to pembrolizumab vs. standard of care,” he said at the European Society for Medical Oncology Congress.
The byzantine statistical design of the trial, while it may warm a mathematician’s heart, imposed stringent restrictions on the data that may also have led to the ultimate failure of the programmed death-1 (PD-1) inhibitor to meet the efficacy endpoint, he said.
In July 2017, Merck, which markets pembrolizumab, announced the failure in a press release, promising to present the data in a future medical meeting. Dr. Cohen’s ESMO presentation was the fulfillment of that promise.
In the trial, patients with squamous cell carcinomas of the oral cavity, oropharynx, hypopharynx, or larynx with disease progression after a platinum-containing chemotherapy regimen, or recurrence/disease progress within 3-6 months of multimodal therapy using platinum, were randomly assigned to therapy with either pembrolizumab 200 mg intravenously every 3 weeks for 2 years, or to standard of care at the investigator’s choice: either methotrexate 40 mg/m2 weekly, docetaxel 75 mg/m2 every 3 weeks, or cetuximab (Erbitux) 250 mg/m2 weekly after a loading dose of 400 mg per m2.
Some math required
Dr. Cohen explained that the statistical design of the trial involved a multiplicity strategy using a family-wise alpha strictly controlled at 0.025. The alpha was allocated in a stepwise fashion. The study hypothesis was that pembrolizumab would have an overall survival (OS) advantage with a one-side alpha, and if that was met, OS would then be looked at in specific cohorts according to expression of the PD ligand 1 (PD-L1) on cells, followed by evaluation of objective response rates and progression-free survival in each subgroup.
The final analysis was to be performed after 380 OS events had occurred among 495 randomized patients. The prespecified efficacy boundary for OS in the intention-to-treat (ITT) population was .0175, translating into a hazard ratio (HR) of 0.80.
Median OS in the ITT population was 8.4 months for the pembrolizumab arm, compared with 7.1 months for the standard-of-care arm. This translated into an HR of 0.81 (P = .0204), which do not reach the aforementioned efficacy boundary.
Rates of 1-year overall survival were 37% in the experimental arm, vs. 27% in the standard-of-care arm.
“When one looks at the biomarker-specific cohorts, we can see that these differences are further exaggerated in favor of pembrolizumab, Dr. Cohen said.
An analysis stratified by patients with PD-L1 expression on 1% or more of cells vs. less than 1% showed median overall OS of 8.7 months for those with higher levels of expression, vs. 7.1 months for expression levels below 1% (HR 0.75, P = .0078).
Similarly, an analysis comparing patients with a tumor proportion score (TPS; expression of PD-L1 in the membranes of 50% or more of tumor cells) with patients whose tumors had lower TPS levels showed a median OS of 11.6 months with pembrolizumab, vs. 7.9 months with standard of care (HR 0.54, P = .0017).
Objective response rates were also significantly higher with pembrolizumab in the patients with higher levels of both overall PD-L1 expression (17.3% vs. 9.9% with standard care), and in patients with 50% or more of tumor cells expressing membrane PD-L1 (26.6% vs. 65%, P = .0009).
In an exploratory analysis, the investigators also found that among patients in the standard of care arm who went on to receive a checkpoint inhibitor as subsequent therapy, the median OS was 20.1 months, compared with 9.8 months for patients who received other subsequent therapies, and 4.8 months for those who did not receive subsequent therapy, suggesting that crossover to a checkpoint inhibitor may have diluted overall survival differences between the trial arms, Dr. Cohen said.
Treatment-related adverse events of any grade were more frequent in the standard of care arm, except for hypothyroidism, which was substantially more frequent with pembrolizumab. The incidence of immune-mediated adverse events other than hypothyroidism was generally similar between the treatment arms.
“I think pembrolizumab, despite not meeting the primary endpoint of overall survival, showed evidence of activity and a [good] safety profile. So I think this study is borderline possible, since there is a 19% reduction in the risk of death, with a hazard ratio borderline to the statistical hypothesis that was initially planned,” said invited discussant Sandrine Faivre, MD, PhD, of Bichat-Beaujon University Hospitals in Paris.
Although pembrolizumab did not reach the primary efficacy endpoint in the ITT population, among patients with TPS of 50% or greater, the benefit with the PD-1 inhibitor was “exquisite” she said, adding that this subgroup of patients made up only 26% of the ITT population.
The study was funded by Merck. Dr. Cohen disclosed stock ownership in Human Longevity, Inc, and being an advisory board member for AstraZeneca, Bristol-Myers Squibb, Human Longevity, Merck, and Pfizer. Dr. Faivre disclosed unspecified relationships with Bayer Pharma, Bristol-Myers Squibb, Eli Lilly, Ipsen, Merck, Serono, and Novartis.
MADRID – Although pembrolizumab (Keytruda) was associated with a 19% reduction in the risk of death compared with the standard of care in patients with relapsed or metastatic head and neck squamous cell carcinoma (HNSCC) in the KEYNOTE 040 trial, the immune checkpoint inhibitor just missed meeting its primary efficacy endpoint of an improvement in overall survival.
The fault may lie in the confounding of overall survival results when patients who were initially assigned to standard of care were crossed over to subsequent therapy with a checkpoint inhibitor after the study ended, said lead investigator Ezra Cohen, MD, of Moores Cancer Center at UC San Diego Health Sciences in La Jolla, Calif.
“This was a trial that clearly did not meet its primary endpoint, but was felt to confer some benefit – at least in the opinion of this investigator – to pembrolizumab vs. standard of care,” he said at the European Society for Medical Oncology Congress.
The byzantine statistical design of the trial, while it may warm a mathematician’s heart, imposed stringent restrictions on the data that may also have led to the ultimate failure of the programmed death-1 (PD-1) inhibitor to meet the efficacy endpoint, he said.
In July 2017, Merck, which markets pembrolizumab, announced the failure in a press release, promising to present the data in a future medical meeting. Dr. Cohen’s ESMO presentation was the fulfillment of that promise.
In the trial, patients with squamous cell carcinomas of the oral cavity, oropharynx, hypopharynx, or larynx with disease progression after a platinum-containing chemotherapy regimen, or recurrence/disease progress within 3-6 months of multimodal therapy using platinum, were randomly assigned to therapy with either pembrolizumab 200 mg intravenously every 3 weeks for 2 years, or to standard of care at the investigator’s choice: either methotrexate 40 mg/m2 weekly, docetaxel 75 mg/m2 every 3 weeks, or cetuximab (Erbitux) 250 mg/m2 weekly after a loading dose of 400 mg per m2.
Some math required
Dr. Cohen explained that the statistical design of the trial involved a multiplicity strategy using a family-wise alpha strictly controlled at 0.025. The alpha was allocated in a stepwise fashion. The study hypothesis was that pembrolizumab would have an overall survival (OS) advantage with a one-side alpha, and if that was met, OS would then be looked at in specific cohorts according to expression of the PD ligand 1 (PD-L1) on cells, followed by evaluation of objective response rates and progression-free survival in each subgroup.
The final analysis was to be performed after 380 OS events had occurred among 495 randomized patients. The prespecified efficacy boundary for OS in the intention-to-treat (ITT) population was .0175, translating into a hazard ratio (HR) of 0.80.
Median OS in the ITT population was 8.4 months for the pembrolizumab arm, compared with 7.1 months for the standard-of-care arm. This translated into an HR of 0.81 (P = .0204), which do not reach the aforementioned efficacy boundary.
Rates of 1-year overall survival were 37% in the experimental arm, vs. 27% in the standard-of-care arm.
“When one looks at the biomarker-specific cohorts, we can see that these differences are further exaggerated in favor of pembrolizumab, Dr. Cohen said.
An analysis stratified by patients with PD-L1 expression on 1% or more of cells vs. less than 1% showed median overall OS of 8.7 months for those with higher levels of expression, vs. 7.1 months for expression levels below 1% (HR 0.75, P = .0078).
Similarly, an analysis comparing patients with a tumor proportion score (TPS; expression of PD-L1 in the membranes of 50% or more of tumor cells) with patients whose tumors had lower TPS levels showed a median OS of 11.6 months with pembrolizumab, vs. 7.9 months with standard of care (HR 0.54, P = .0017).
Objective response rates were also significantly higher with pembrolizumab in the patients with higher levels of both overall PD-L1 expression (17.3% vs. 9.9% with standard care), and in patients with 50% or more of tumor cells expressing membrane PD-L1 (26.6% vs. 65%, P = .0009).
In an exploratory analysis, the investigators also found that among patients in the standard of care arm who went on to receive a checkpoint inhibitor as subsequent therapy, the median OS was 20.1 months, compared with 9.8 months for patients who received other subsequent therapies, and 4.8 months for those who did not receive subsequent therapy, suggesting that crossover to a checkpoint inhibitor may have diluted overall survival differences between the trial arms, Dr. Cohen said.
Treatment-related adverse events of any grade were more frequent in the standard of care arm, except for hypothyroidism, which was substantially more frequent with pembrolizumab. The incidence of immune-mediated adverse events other than hypothyroidism was generally similar between the treatment arms.
“I think pembrolizumab, despite not meeting the primary endpoint of overall survival, showed evidence of activity and a [good] safety profile. So I think this study is borderline possible, since there is a 19% reduction in the risk of death, with a hazard ratio borderline to the statistical hypothesis that was initially planned,” said invited discussant Sandrine Faivre, MD, PhD, of Bichat-Beaujon University Hospitals in Paris.
Although pembrolizumab did not reach the primary efficacy endpoint in the ITT population, among patients with TPS of 50% or greater, the benefit with the PD-1 inhibitor was “exquisite” she said, adding that this subgroup of patients made up only 26% of the ITT population.
The study was funded by Merck. Dr. Cohen disclosed stock ownership in Human Longevity, Inc, and being an advisory board member for AstraZeneca, Bristol-Myers Squibb, Human Longevity, Merck, and Pfizer. Dr. Faivre disclosed unspecified relationships with Bayer Pharma, Bristol-Myers Squibb, Eli Lilly, Ipsen, Merck, Serono, and Novartis.
MADRID – Although pembrolizumab (Keytruda) was associated with a 19% reduction in the risk of death compared with the standard of care in patients with relapsed or metastatic head and neck squamous cell carcinoma (HNSCC) in the KEYNOTE 040 trial, the immune checkpoint inhibitor just missed meeting its primary efficacy endpoint of an improvement in overall survival.
The fault may lie in the confounding of overall survival results when patients who were initially assigned to standard of care were crossed over to subsequent therapy with a checkpoint inhibitor after the study ended, said lead investigator Ezra Cohen, MD, of Moores Cancer Center at UC San Diego Health Sciences in La Jolla, Calif.
“This was a trial that clearly did not meet its primary endpoint, but was felt to confer some benefit – at least in the opinion of this investigator – to pembrolizumab vs. standard of care,” he said at the European Society for Medical Oncology Congress.
The byzantine statistical design of the trial, while it may warm a mathematician’s heart, imposed stringent restrictions on the data that may also have led to the ultimate failure of the programmed death-1 (PD-1) inhibitor to meet the efficacy endpoint, he said.
In July 2017, Merck, which markets pembrolizumab, announced the failure in a press release, promising to present the data in a future medical meeting. Dr. Cohen’s ESMO presentation was the fulfillment of that promise.
In the trial, patients with squamous cell carcinomas of the oral cavity, oropharynx, hypopharynx, or larynx with disease progression after a platinum-containing chemotherapy regimen, or recurrence/disease progress within 3-6 months of multimodal therapy using platinum, were randomly assigned to therapy with either pembrolizumab 200 mg intravenously every 3 weeks for 2 years, or to standard of care at the investigator’s choice: either methotrexate 40 mg/m2 weekly, docetaxel 75 mg/m2 every 3 weeks, or cetuximab (Erbitux) 250 mg/m2 weekly after a loading dose of 400 mg per m2.
Some math required
Dr. Cohen explained that the statistical design of the trial involved a multiplicity strategy using a family-wise alpha strictly controlled at 0.025. The alpha was allocated in a stepwise fashion. The study hypothesis was that pembrolizumab would have an overall survival (OS) advantage with a one-side alpha, and if that was met, OS would then be looked at in specific cohorts according to expression of the PD ligand 1 (PD-L1) on cells, followed by evaluation of objective response rates and progression-free survival in each subgroup.
The final analysis was to be performed after 380 OS events had occurred among 495 randomized patients. The prespecified efficacy boundary for OS in the intention-to-treat (ITT) population was .0175, translating into a hazard ratio (HR) of 0.80.
Median OS in the ITT population was 8.4 months for the pembrolizumab arm, compared with 7.1 months for the standard-of-care arm. This translated into an HR of 0.81 (P = .0204), which do not reach the aforementioned efficacy boundary.
Rates of 1-year overall survival were 37% in the experimental arm, vs. 27% in the standard-of-care arm.
“When one looks at the biomarker-specific cohorts, we can see that these differences are further exaggerated in favor of pembrolizumab, Dr. Cohen said.
An analysis stratified by patients with PD-L1 expression on 1% or more of cells vs. less than 1% showed median overall OS of 8.7 months for those with higher levels of expression, vs. 7.1 months for expression levels below 1% (HR 0.75, P = .0078).
Similarly, an analysis comparing patients with a tumor proportion score (TPS; expression of PD-L1 in the membranes of 50% or more of tumor cells) with patients whose tumors had lower TPS levels showed a median OS of 11.6 months with pembrolizumab, vs. 7.9 months with standard of care (HR 0.54, P = .0017).
Objective response rates were also significantly higher with pembrolizumab in the patients with higher levels of both overall PD-L1 expression (17.3% vs. 9.9% with standard care), and in patients with 50% or more of tumor cells expressing membrane PD-L1 (26.6% vs. 65%, P = .0009).
In an exploratory analysis, the investigators also found that among patients in the standard of care arm who went on to receive a checkpoint inhibitor as subsequent therapy, the median OS was 20.1 months, compared with 9.8 months for patients who received other subsequent therapies, and 4.8 months for those who did not receive subsequent therapy, suggesting that crossover to a checkpoint inhibitor may have diluted overall survival differences between the trial arms, Dr. Cohen said.
Treatment-related adverse events of any grade were more frequent in the standard of care arm, except for hypothyroidism, which was substantially more frequent with pembrolizumab. The incidence of immune-mediated adverse events other than hypothyroidism was generally similar between the treatment arms.
“I think pembrolizumab, despite not meeting the primary endpoint of overall survival, showed evidence of activity and a [good] safety profile. So I think this study is borderline possible, since there is a 19% reduction in the risk of death, with a hazard ratio borderline to the statistical hypothesis that was initially planned,” said invited discussant Sandrine Faivre, MD, PhD, of Bichat-Beaujon University Hospitals in Paris.
Although pembrolizumab did not reach the primary efficacy endpoint in the ITT population, among patients with TPS of 50% or greater, the benefit with the PD-1 inhibitor was “exquisite” she said, adding that this subgroup of patients made up only 26% of the ITT population.
The study was funded by Merck. Dr. Cohen disclosed stock ownership in Human Longevity, Inc, and being an advisory board member for AstraZeneca, Bristol-Myers Squibb, Human Longevity, Merck, and Pfizer. Dr. Faivre disclosed unspecified relationships with Bayer Pharma, Bristol-Myers Squibb, Eli Lilly, Ipsen, Merck, Serono, and Novartis.
AT ESMO 2017
Key clinical point: Pembrolizumab was associated with a 19% reduction in the risk of death in patients with advanced HNSCC, but was not significantly better than standard of care.
Major finding: Median overall survival in the ITT population was 8.4 months with pembrolizumab arm, vs 7.1 months for the standard-of-care arm. The difference did not reach the prespecified efficacy boundary.
Data source: Randomized phase 3 trial of 495 patients with relapsed or metastatic head and neck squamous cell carcinoma.
Disclosures: Merck funded the study. Dr. Cohen disclosed stock ownership in Human Longevity, and being an advisory board member for AstraZeneca, Bristol-Myers Squibb, Human Longevity, Merck, and Pfizer. Dr. Faivre disclosed unspecified relationships with Bayer Pharma, Bristol-Myers Squibb, Eli Lilly, Ipsen, Merck, Serono, and Novartis.
Flu shots and persuasion
Compliant patients are all alike; every noncompliant patient is obstinate in his or her own way. Because of this, persuading patients to make good choices is rarely easy and never universal.
At Kaiser Permanente, we have begun in earnest providing flu shots. Every department participates (even dermatology) with a goal of vaccinating every eligible patient. Most patients want their shot. When patients decline, it’s game on. A rare few decline for justifiable reasons such as an allergy. Most say “no” for flawed reasons: “I never get the flu,” “The shot always gives me the flu,” and “I don’t believe in vaccines,” are common ones.
Fortunately, we can help them. Here are techniques I learned while working on my MBA that I’ve found useful in persuading patients to make better choices:
- The “everyone is doing it” technique. At KP, we’ve put up boards with the iconic goal thermometer showing how many flu shots we need to reach our objective. When patients see we’ve given over 1,000 shots in dermatology in just 2 weeks, this technique helps convince them. Patients prefer to be like others rather than to stand out, particularly when there is uncertainty.
- The “this is who you are technique.” Patients hate to be seen as inconsistent. In fact, we are all more likely to make a choice seen as consistent with who we are rather than change our mind, even if doing so is a better choice. Highlight how they have previously shown good decision making and healthy behaviors and point out how getting vaccinated is consonant with who they are. For example: “Being a vegan, you are clearly someone who takes care of her health. Getting the vaccine is similar to choosing to eat plants. It’s what healthy people like you do.”
- The “well, that’s not like you” technique. Here, you point out how their choice is inconsistent with their previous choices. You might say, “Why would you get the hepatitis A vaccine last week and not the flu shot today?” Like the previous technique, this creates cognitive dissonance. You might soften the approach by saying, “You might have thought this,” or “I’m sure you didn’t realize.”
- The emotional decision approach. Making the risk seem real and imminent can combat future discounting. One example might be: “We have had several people hospitalized and one death from the flu in San Diego already.” Use stories and descriptive language to make the risk salient.
- The use your authority approach. The long coat does matter. A more modern version of the paternalistic physician is referred to as “asymmetric” or “light paternalism,” and we should recognize that it might be used to save a life. One example is: “I advise you to get the flu shot because I care about you, and I’m worried you might end up in the hospital or worse if you don’t get it.” There’s a reason why tobacco companies once used doctors in white coats to sell cigarettes – we can be quite persuasive.
“A great deal of literature has been distributed, casting discredit upon the value of vaccination ... I do not see how any one ... who is familiar with the history of the subject, and who has any capacity left for clear judgment, can doubt its value.” – William Osler
Dr. Benabio is director of health care transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Compliant patients are all alike; every noncompliant patient is obstinate in his or her own way. Because of this, persuading patients to make good choices is rarely easy and never universal.
At Kaiser Permanente, we have begun in earnest providing flu shots. Every department participates (even dermatology) with a goal of vaccinating every eligible patient. Most patients want their shot. When patients decline, it’s game on. A rare few decline for justifiable reasons such as an allergy. Most say “no” for flawed reasons: “I never get the flu,” “The shot always gives me the flu,” and “I don’t believe in vaccines,” are common ones.
Fortunately, we can help them. Here are techniques I learned while working on my MBA that I’ve found useful in persuading patients to make better choices:
- The “everyone is doing it” technique. At KP, we’ve put up boards with the iconic goal thermometer showing how many flu shots we need to reach our objective. When patients see we’ve given over 1,000 shots in dermatology in just 2 weeks, this technique helps convince them. Patients prefer to be like others rather than to stand out, particularly when there is uncertainty.
- The “this is who you are technique.” Patients hate to be seen as inconsistent. In fact, we are all more likely to make a choice seen as consistent with who we are rather than change our mind, even if doing so is a better choice. Highlight how they have previously shown good decision making and healthy behaviors and point out how getting vaccinated is consonant with who they are. For example: “Being a vegan, you are clearly someone who takes care of her health. Getting the vaccine is similar to choosing to eat plants. It’s what healthy people like you do.”
- The “well, that’s not like you” technique. Here, you point out how their choice is inconsistent with their previous choices. You might say, “Why would you get the hepatitis A vaccine last week and not the flu shot today?” Like the previous technique, this creates cognitive dissonance. You might soften the approach by saying, “You might have thought this,” or “I’m sure you didn’t realize.”
- The emotional decision approach. Making the risk seem real and imminent can combat future discounting. One example might be: “We have had several people hospitalized and one death from the flu in San Diego already.” Use stories and descriptive language to make the risk salient.
- The use your authority approach. The long coat does matter. A more modern version of the paternalistic physician is referred to as “asymmetric” or “light paternalism,” and we should recognize that it might be used to save a life. One example is: “I advise you to get the flu shot because I care about you, and I’m worried you might end up in the hospital or worse if you don’t get it.” There’s a reason why tobacco companies once used doctors in white coats to sell cigarettes – we can be quite persuasive.
“A great deal of literature has been distributed, casting discredit upon the value of vaccination ... I do not see how any one ... who is familiar with the history of the subject, and who has any capacity left for clear judgment, can doubt its value.” – William Osler
Dr. Benabio is director of health care transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Compliant patients are all alike; every noncompliant patient is obstinate in his or her own way. Because of this, persuading patients to make good choices is rarely easy and never universal.
At Kaiser Permanente, we have begun in earnest providing flu shots. Every department participates (even dermatology) with a goal of vaccinating every eligible patient. Most patients want their shot. When patients decline, it’s game on. A rare few decline for justifiable reasons such as an allergy. Most say “no” for flawed reasons: “I never get the flu,” “The shot always gives me the flu,” and “I don’t believe in vaccines,” are common ones.
Fortunately, we can help them. Here are techniques I learned while working on my MBA that I’ve found useful in persuading patients to make better choices:
- The “everyone is doing it” technique. At KP, we’ve put up boards with the iconic goal thermometer showing how many flu shots we need to reach our objective. When patients see we’ve given over 1,000 shots in dermatology in just 2 weeks, this technique helps convince them. Patients prefer to be like others rather than to stand out, particularly when there is uncertainty.
- The “this is who you are technique.” Patients hate to be seen as inconsistent. In fact, we are all more likely to make a choice seen as consistent with who we are rather than change our mind, even if doing so is a better choice. Highlight how they have previously shown good decision making and healthy behaviors and point out how getting vaccinated is consonant with who they are. For example: “Being a vegan, you are clearly someone who takes care of her health. Getting the vaccine is similar to choosing to eat plants. It’s what healthy people like you do.”
- The “well, that’s not like you” technique. Here, you point out how their choice is inconsistent with their previous choices. You might say, “Why would you get the hepatitis A vaccine last week and not the flu shot today?” Like the previous technique, this creates cognitive dissonance. You might soften the approach by saying, “You might have thought this,” or “I’m sure you didn’t realize.”
- The emotional decision approach. Making the risk seem real and imminent can combat future discounting. One example might be: “We have had several people hospitalized and one death from the flu in San Diego already.” Use stories and descriptive language to make the risk salient.
- The use your authority approach. The long coat does matter. A more modern version of the paternalistic physician is referred to as “asymmetric” or “light paternalism,” and we should recognize that it might be used to save a life. One example is: “I advise you to get the flu shot because I care about you, and I’m worried you might end up in the hospital or worse if you don’t get it.” There’s a reason why tobacco companies once used doctors in white coats to sell cigarettes – we can be quite persuasive.
“A great deal of literature has been distributed, casting discredit upon the value of vaccination ... I do not see how any one ... who is familiar with the history of the subject, and who has any capacity left for clear judgment, can doubt its value.” – William Osler
Dr. Benabio is director of health care transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Cascade of costs could push new gene therapy above $1 million per patient
Outrage over the high cost of cancer care has focused on skyrocketing drug prices, including the $475,000 price tag for the country’s first gene therapy, Novartis’ Kymriah (tisagenlecleucel), a leukemia treatment approved in August.
But the total costs of tisagenlecleucel and the 21 similar drugs in development – known as CAR T-cell therapies – will be far higher than many have imagined, reaching $1 million or more per patient, according to leading cancer experts. The next CAR T-cell drug could be approved as soon as November.
Although Kymriah’s price tag has “shattered oncology drug pricing norms,” said Leonard Saltz, MD, chief of gastrointestinal oncology at Memorial Sloan Kettering Cancer Center in New York, “the sticker price is just the starting point.”
These therapies lead to a cascade of costs, propelled by serious side effects that require sophisticated management, Dr. Saltz said. For this class of drugs, Dr, Saltz advised consumers to “think of the $475,000 as parts, not labor.”
Hagop Kantarjian, MD, leukemia specialist and professor at the University of Texas MD Anderson Cancer Center, estimates tisagenlecleucel’s total cost could reach $1.5 million.
CAR T-cell therapy is expensive because of the unique way that it works. Doctors harvest patients’ immune cells, genetically alter them to rev up their ability to fight cancer, then reinfuse them into patients.
Taking the brakes off the immune system, Dr. Kantarjian said, can lead to life-threatening complications that require lengthy hospitalizations and expensive medications, which are prescribed in addition to conventional cancer therapy, rather than in place of it.
Keith D. Eaton, MD, a Seattle oncologist, said he ran up medical bills of $500,000 when he participated in a clinical trial of CAR T cells in 2013, even though all patients in the study received the medication for free. Dr. Eaton, who was diagnosed with acute lymphoblastic leukemia (ALL), spent nearly 2 months in the hospital.
Like Dr. Eaton, nearly half of patients who receive CAR T cells develop cytokine storm. Other serious side effects include strokelike symptoms and coma.
The cytokine storm felt like “the worst flu of your life,” said Dr. Eaton, now aged 51 years. His fever spiked so high that a hospital nurse assumed the thermometer was broken. Dr. Eaton replied, “It’s not broken. My temperature is too high to register on the thermometer.”
Although Dr. Eaton recovered, he wasn’t done with treatment. His doctors recommended a bone marrow transplant, another harrowing procedure, at a cost of hundreds of thousands of dollars.
Dr. Eaton said he feels fortunate to be healthy today, with tests showing no evidence of leukemia. His insurer paid for almost everything.
Kymriah’s sticker price is especially “outrageous” given its relatively low manufacturing costs, said Walid F. Gellad, MD, codirector of the Center for Pharmaceutical Policy and Prescribing at the University of Pittsburgh.
The gene therapy process used to create tisagenlecleucel costs about $15,000, according to a 2012 presentation by Carl H. June, MD, who pioneered CAR T-cell research at the University of Pennsylvania in Philadelphia. Dr. June could not be reached for comment.
To quell unrest about price, Novartis has offered patients and insurers a new twist on the money-back guarantee.
Novartis will charge for the drug only if patients go into remission within 1 month of treatment. In a key clinical trial, 83% of the children and young adults treated with tisagenlecleucel went into remission within 3 months. Novartis calls the plan “outcomes-based pricing.”
Novartis is “working through the specific details” of how the pricing plan will affect the Centers for Medicare & Medicaid Services, which pays for care for many cancer patients, company spokesperson Julie Masow said. “There are many hurdles” to this type of pricing plan but, Ms. Masow said, “Novartis is committed to making this happen.”
She also said that Kymriah’s manufacturing costs are much higher than $15,000, although she didn’t cite a specific dollar amount. She noted that Novartis has invested heavily in the technology, designing “an innovative manufacturing facility and process specifically for cellular therapies.”
As for Kymriah-related hospital and medication charges, “costs will vary from patient to patient and treatment center to treatment center, based on the level of care each patient requires,” Ms. Masow said. “Kymriah is a one-time treatment that has shown remarkable early, deep, and durable responses in these children who are very sick and often out of options.”
Some doctors said tisagenlecleucel, which could be used by about 600 patients a year, offers an incalculable benefit for desperately ill young people. The drug is approved for children and young adults with B-cell ALL who already have been treated with at least two other cancer therapies.
“A kid’s life is priceless,” said Michelle Hermiston, MD, director of pediatric immunotherapy at Benioff Children’s Hospital, at the University of California, San Francisco. “Any given kid has the potential to make financial impacts over a lifetime that far outweigh the cost of their cure. From this perspective, every child in my mind deserves the best curative therapy we can offer.”
Other cancer doctors say the Novartis plan is no bargain.
About 36% of patients who go into remission with tisagenlecleucel relapse within 1 year, said Vinay Prasad, MD, of Oregon Health & Science University, Portland. Many of these patients will need additional treatment, said Dr. Prasad, who wrote an editorial about tisagenlecleucel’s price Oct. 4 in Nature.
“If you’ve paid half a million dollars for drugs and half a million dollars for care, and a year later your cancer is back, is that a good deal?” asked Dr. Saltz, who cowrote a recent editorial on tisagenlecleucel’s price in JAMA.
Steve Miller, MD, chief medical officer for Express Scripts, said it would be more fair to judge Kymriah’s success after 6 months of treatment, rather than 1 month. Dr. Prasad goes even further. He said Novartis should issue refunds for any patient who relapses within 3 years.
A consumer-advocate group called Patients for Affordable Drugs also has said that tisagenlecleucel costs too much, given that the federal government spent more than $200 million over 2 decades to support the basic research into CAR T-cell therapy, long before Novartis bought the rights.
Rep. Lloyd Doggett (D-Texas) wrote a letter to the Medicare program’s director last month asking for details on how the Novartis payment deal will work.
“As Big Pharma continues to put price gouging before patient access, companies will point more and more proudly at their pricing agreements,” Rep. Doggett wrote. “But taxpayers deserve to know more about how these agreements will work – whether they will actually save the government money, defray these massive costs, and ensure that they can access lifesaving medications.”
KHN’s coverage related to aging & improving care of older adults is supported by The John A. Hartford Foundation. Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
Outrage over the high cost of cancer care has focused on skyrocketing drug prices, including the $475,000 price tag for the country’s first gene therapy, Novartis’ Kymriah (tisagenlecleucel), a leukemia treatment approved in August.
But the total costs of tisagenlecleucel and the 21 similar drugs in development – known as CAR T-cell therapies – will be far higher than many have imagined, reaching $1 million or more per patient, according to leading cancer experts. The next CAR T-cell drug could be approved as soon as November.
Although Kymriah’s price tag has “shattered oncology drug pricing norms,” said Leonard Saltz, MD, chief of gastrointestinal oncology at Memorial Sloan Kettering Cancer Center in New York, “the sticker price is just the starting point.”
These therapies lead to a cascade of costs, propelled by serious side effects that require sophisticated management, Dr. Saltz said. For this class of drugs, Dr, Saltz advised consumers to “think of the $475,000 as parts, not labor.”
Hagop Kantarjian, MD, leukemia specialist and professor at the University of Texas MD Anderson Cancer Center, estimates tisagenlecleucel’s total cost could reach $1.5 million.
CAR T-cell therapy is expensive because of the unique way that it works. Doctors harvest patients’ immune cells, genetically alter them to rev up their ability to fight cancer, then reinfuse them into patients.
Taking the brakes off the immune system, Dr. Kantarjian said, can lead to life-threatening complications that require lengthy hospitalizations and expensive medications, which are prescribed in addition to conventional cancer therapy, rather than in place of it.
Keith D. Eaton, MD, a Seattle oncologist, said he ran up medical bills of $500,000 when he participated in a clinical trial of CAR T cells in 2013, even though all patients in the study received the medication for free. Dr. Eaton, who was diagnosed with acute lymphoblastic leukemia (ALL), spent nearly 2 months in the hospital.
Like Dr. Eaton, nearly half of patients who receive CAR T cells develop cytokine storm. Other serious side effects include strokelike symptoms and coma.
The cytokine storm felt like “the worst flu of your life,” said Dr. Eaton, now aged 51 years. His fever spiked so high that a hospital nurse assumed the thermometer was broken. Dr. Eaton replied, “It’s not broken. My temperature is too high to register on the thermometer.”
Although Dr. Eaton recovered, he wasn’t done with treatment. His doctors recommended a bone marrow transplant, another harrowing procedure, at a cost of hundreds of thousands of dollars.
Dr. Eaton said he feels fortunate to be healthy today, with tests showing no evidence of leukemia. His insurer paid for almost everything.
Kymriah’s sticker price is especially “outrageous” given its relatively low manufacturing costs, said Walid F. Gellad, MD, codirector of the Center for Pharmaceutical Policy and Prescribing at the University of Pittsburgh.
The gene therapy process used to create tisagenlecleucel costs about $15,000, according to a 2012 presentation by Carl H. June, MD, who pioneered CAR T-cell research at the University of Pennsylvania in Philadelphia. Dr. June could not be reached for comment.
To quell unrest about price, Novartis has offered patients and insurers a new twist on the money-back guarantee.
Novartis will charge for the drug only if patients go into remission within 1 month of treatment. In a key clinical trial, 83% of the children and young adults treated with tisagenlecleucel went into remission within 3 months. Novartis calls the plan “outcomes-based pricing.”
Novartis is “working through the specific details” of how the pricing plan will affect the Centers for Medicare & Medicaid Services, which pays for care for many cancer patients, company spokesperson Julie Masow said. “There are many hurdles” to this type of pricing plan but, Ms. Masow said, “Novartis is committed to making this happen.”
She also said that Kymriah’s manufacturing costs are much higher than $15,000, although she didn’t cite a specific dollar amount. She noted that Novartis has invested heavily in the technology, designing “an innovative manufacturing facility and process specifically for cellular therapies.”
As for Kymriah-related hospital and medication charges, “costs will vary from patient to patient and treatment center to treatment center, based on the level of care each patient requires,” Ms. Masow said. “Kymriah is a one-time treatment that has shown remarkable early, deep, and durable responses in these children who are very sick and often out of options.”
Some doctors said tisagenlecleucel, which could be used by about 600 patients a year, offers an incalculable benefit for desperately ill young people. The drug is approved for children and young adults with B-cell ALL who already have been treated with at least two other cancer therapies.
“A kid’s life is priceless,” said Michelle Hermiston, MD, director of pediatric immunotherapy at Benioff Children’s Hospital, at the University of California, San Francisco. “Any given kid has the potential to make financial impacts over a lifetime that far outweigh the cost of their cure. From this perspective, every child in my mind deserves the best curative therapy we can offer.”
Other cancer doctors say the Novartis plan is no bargain.
About 36% of patients who go into remission with tisagenlecleucel relapse within 1 year, said Vinay Prasad, MD, of Oregon Health & Science University, Portland. Many of these patients will need additional treatment, said Dr. Prasad, who wrote an editorial about tisagenlecleucel’s price Oct. 4 in Nature.
“If you’ve paid half a million dollars for drugs and half a million dollars for care, and a year later your cancer is back, is that a good deal?” asked Dr. Saltz, who cowrote a recent editorial on tisagenlecleucel’s price in JAMA.
Steve Miller, MD, chief medical officer for Express Scripts, said it would be more fair to judge Kymriah’s success after 6 months of treatment, rather than 1 month. Dr. Prasad goes even further. He said Novartis should issue refunds for any patient who relapses within 3 years.
A consumer-advocate group called Patients for Affordable Drugs also has said that tisagenlecleucel costs too much, given that the federal government spent more than $200 million over 2 decades to support the basic research into CAR T-cell therapy, long before Novartis bought the rights.
Rep. Lloyd Doggett (D-Texas) wrote a letter to the Medicare program’s director last month asking for details on how the Novartis payment deal will work.
“As Big Pharma continues to put price gouging before patient access, companies will point more and more proudly at their pricing agreements,” Rep. Doggett wrote. “But taxpayers deserve to know more about how these agreements will work – whether they will actually save the government money, defray these massive costs, and ensure that they can access lifesaving medications.”
KHN’s coverage related to aging & improving care of older adults is supported by The John A. Hartford Foundation. Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
Outrage over the high cost of cancer care has focused on skyrocketing drug prices, including the $475,000 price tag for the country’s first gene therapy, Novartis’ Kymriah (tisagenlecleucel), a leukemia treatment approved in August.
But the total costs of tisagenlecleucel and the 21 similar drugs in development – known as CAR T-cell therapies – will be far higher than many have imagined, reaching $1 million or more per patient, according to leading cancer experts. The next CAR T-cell drug could be approved as soon as November.
Although Kymriah’s price tag has “shattered oncology drug pricing norms,” said Leonard Saltz, MD, chief of gastrointestinal oncology at Memorial Sloan Kettering Cancer Center in New York, “the sticker price is just the starting point.”
These therapies lead to a cascade of costs, propelled by serious side effects that require sophisticated management, Dr. Saltz said. For this class of drugs, Dr, Saltz advised consumers to “think of the $475,000 as parts, not labor.”
Hagop Kantarjian, MD, leukemia specialist and professor at the University of Texas MD Anderson Cancer Center, estimates tisagenlecleucel’s total cost could reach $1.5 million.
CAR T-cell therapy is expensive because of the unique way that it works. Doctors harvest patients’ immune cells, genetically alter them to rev up their ability to fight cancer, then reinfuse them into patients.
Taking the brakes off the immune system, Dr. Kantarjian said, can lead to life-threatening complications that require lengthy hospitalizations and expensive medications, which are prescribed in addition to conventional cancer therapy, rather than in place of it.
Keith D. Eaton, MD, a Seattle oncologist, said he ran up medical bills of $500,000 when he participated in a clinical trial of CAR T cells in 2013, even though all patients in the study received the medication for free. Dr. Eaton, who was diagnosed with acute lymphoblastic leukemia (ALL), spent nearly 2 months in the hospital.
Like Dr. Eaton, nearly half of patients who receive CAR T cells develop cytokine storm. Other serious side effects include strokelike symptoms and coma.
The cytokine storm felt like “the worst flu of your life,” said Dr. Eaton, now aged 51 years. His fever spiked so high that a hospital nurse assumed the thermometer was broken. Dr. Eaton replied, “It’s not broken. My temperature is too high to register on the thermometer.”
Although Dr. Eaton recovered, he wasn’t done with treatment. His doctors recommended a bone marrow transplant, another harrowing procedure, at a cost of hundreds of thousands of dollars.
Dr. Eaton said he feels fortunate to be healthy today, with tests showing no evidence of leukemia. His insurer paid for almost everything.
Kymriah’s sticker price is especially “outrageous” given its relatively low manufacturing costs, said Walid F. Gellad, MD, codirector of the Center for Pharmaceutical Policy and Prescribing at the University of Pittsburgh.
The gene therapy process used to create tisagenlecleucel costs about $15,000, according to a 2012 presentation by Carl H. June, MD, who pioneered CAR T-cell research at the University of Pennsylvania in Philadelphia. Dr. June could not be reached for comment.
To quell unrest about price, Novartis has offered patients and insurers a new twist on the money-back guarantee.
Novartis will charge for the drug only if patients go into remission within 1 month of treatment. In a key clinical trial, 83% of the children and young adults treated with tisagenlecleucel went into remission within 3 months. Novartis calls the plan “outcomes-based pricing.”
Novartis is “working through the specific details” of how the pricing plan will affect the Centers for Medicare & Medicaid Services, which pays for care for many cancer patients, company spokesperson Julie Masow said. “There are many hurdles” to this type of pricing plan but, Ms. Masow said, “Novartis is committed to making this happen.”
She also said that Kymriah’s manufacturing costs are much higher than $15,000, although she didn’t cite a specific dollar amount. She noted that Novartis has invested heavily in the technology, designing “an innovative manufacturing facility and process specifically for cellular therapies.”
As for Kymriah-related hospital and medication charges, “costs will vary from patient to patient and treatment center to treatment center, based on the level of care each patient requires,” Ms. Masow said. “Kymriah is a one-time treatment that has shown remarkable early, deep, and durable responses in these children who are very sick and often out of options.”
Some doctors said tisagenlecleucel, which could be used by about 600 patients a year, offers an incalculable benefit for desperately ill young people. The drug is approved for children and young adults with B-cell ALL who already have been treated with at least two other cancer therapies.
“A kid’s life is priceless,” said Michelle Hermiston, MD, director of pediatric immunotherapy at Benioff Children’s Hospital, at the University of California, San Francisco. “Any given kid has the potential to make financial impacts over a lifetime that far outweigh the cost of their cure. From this perspective, every child in my mind deserves the best curative therapy we can offer.”
Other cancer doctors say the Novartis plan is no bargain.
About 36% of patients who go into remission with tisagenlecleucel relapse within 1 year, said Vinay Prasad, MD, of Oregon Health & Science University, Portland. Many of these patients will need additional treatment, said Dr. Prasad, who wrote an editorial about tisagenlecleucel’s price Oct. 4 in Nature.
“If you’ve paid half a million dollars for drugs and half a million dollars for care, and a year later your cancer is back, is that a good deal?” asked Dr. Saltz, who cowrote a recent editorial on tisagenlecleucel’s price in JAMA.
Steve Miller, MD, chief medical officer for Express Scripts, said it would be more fair to judge Kymriah’s success after 6 months of treatment, rather than 1 month. Dr. Prasad goes even further. He said Novartis should issue refunds for any patient who relapses within 3 years.
A consumer-advocate group called Patients for Affordable Drugs also has said that tisagenlecleucel costs too much, given that the federal government spent more than $200 million over 2 decades to support the basic research into CAR T-cell therapy, long before Novartis bought the rights.
Rep. Lloyd Doggett (D-Texas) wrote a letter to the Medicare program’s director last month asking for details on how the Novartis payment deal will work.
“As Big Pharma continues to put price gouging before patient access, companies will point more and more proudly at their pricing agreements,” Rep. Doggett wrote. “But taxpayers deserve to know more about how these agreements will work – whether they will actually save the government money, defray these massive costs, and ensure that they can access lifesaving medications.”
KHN’s coverage related to aging & improving care of older adults is supported by The John A. Hartford Foundation. Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
VIDEO: Expert roundtable on hormone therapy
PHILADELPHIA – The updated hormone therapy position statement from the North American Menopause Society tells clinicians to move away from “lowest dose for the shortest time” and toward prescribing the appropriate dose, formulation, and route of administration to meet treatment goals.
At the group’s annual meeting, some of the authors of the position statement outlined the latest evidence for the safety of hormone therapy and special clinical considerations based on age or time of menopause and unique health risks like breast cancer. Additionally, the authors said there should not be an arbitrary “stop date” for hormone therapy.
The authors also discussed the risks of using compounded bioidentical hormones to treat menopausal symptoms.
This roundtable discussion includes JoAnn V. Pinkerton, MD, NAMS executive director and professor of obstetrics and gynecology at the University of Virginia, Charlottesville; Andrew Kaunitz, MD, associate chairman of the department of obstetrics and gynecology at the University of Florida, Jacksonville; and Cynthia A. Stuenkel, MD, clinical professor of medicine at the University of California, San Diego.
Dr. Pinkerton reported institutional research support from TherapeuticsMD. Dr. Kaunitz reported consultant/advisory board work for Allergan, Amag Pharmaceuticals, Bayer, Mithra Pharmaceuticals, Pfizer, and Shionogi. He has also received grant/research support from Bayer, Radius Health, TherapeuticsMD, and Millendo Therapeutics. Dr. Stuenkel reported having no financial disclosures.
[email protected]
On Twitter @maryellenny
PHILADELPHIA – The updated hormone therapy position statement from the North American Menopause Society tells clinicians to move away from “lowest dose for the shortest time” and toward prescribing the appropriate dose, formulation, and route of administration to meet treatment goals.
At the group’s annual meeting, some of the authors of the position statement outlined the latest evidence for the safety of hormone therapy and special clinical considerations based on age or time of menopause and unique health risks like breast cancer. Additionally, the authors said there should not be an arbitrary “stop date” for hormone therapy.
The authors also discussed the risks of using compounded bioidentical hormones to treat menopausal symptoms.
This roundtable discussion includes JoAnn V. Pinkerton, MD, NAMS executive director and professor of obstetrics and gynecology at the University of Virginia, Charlottesville; Andrew Kaunitz, MD, associate chairman of the department of obstetrics and gynecology at the University of Florida, Jacksonville; and Cynthia A. Stuenkel, MD, clinical professor of medicine at the University of California, San Diego.
Dr. Pinkerton reported institutional research support from TherapeuticsMD. Dr. Kaunitz reported consultant/advisory board work for Allergan, Amag Pharmaceuticals, Bayer, Mithra Pharmaceuticals, Pfizer, and Shionogi. He has also received grant/research support from Bayer, Radius Health, TherapeuticsMD, and Millendo Therapeutics. Dr. Stuenkel reported having no financial disclosures.
[email protected]
On Twitter @maryellenny
PHILADELPHIA – The updated hormone therapy position statement from the North American Menopause Society tells clinicians to move away from “lowest dose for the shortest time” and toward prescribing the appropriate dose, formulation, and route of administration to meet treatment goals.
At the group’s annual meeting, some of the authors of the position statement outlined the latest evidence for the safety of hormone therapy and special clinical considerations based on age or time of menopause and unique health risks like breast cancer. Additionally, the authors said there should not be an arbitrary “stop date” for hormone therapy.
The authors also discussed the risks of using compounded bioidentical hormones to treat menopausal symptoms.
This roundtable discussion includes JoAnn V. Pinkerton, MD, NAMS executive director and professor of obstetrics and gynecology at the University of Virginia, Charlottesville; Andrew Kaunitz, MD, associate chairman of the department of obstetrics and gynecology at the University of Florida, Jacksonville; and Cynthia A. Stuenkel, MD, clinical professor of medicine at the University of California, San Diego.
Dr. Pinkerton reported institutional research support from TherapeuticsMD. Dr. Kaunitz reported consultant/advisory board work for Allergan, Amag Pharmaceuticals, Bayer, Mithra Pharmaceuticals, Pfizer, and Shionogi. He has also received grant/research support from Bayer, Radius Health, TherapeuticsMD, and Millendo Therapeutics. Dr. Stuenkel reported having no financial disclosures.
[email protected]
On Twitter @maryellenny
EXPERT ANALYSIS FROM NAMS 2017














