User login
Visit ACS Central during Clinical Congress
Make the most of your American College of Surgeons (ACS) Clinical Congress 2017 experience by visiting the new ACS Central area designed specifically for our members. ACS Central visitors will have opportunities to meet College staff; learn about the latest ACS programs, products, and services; purchase ACS materials; and make ACS Central a convenient gathering space during the meeting. While there, you can also update your member profile and receive a flash drive with your own professional photo to keep.
ACS Central also will house the ACS Theatre, which will feature presentations on the following new ACS programs and products during lunch hours Monday through Wednesday:
• Monday: The Surgeon Specific Registry
• Tuesday: Improving Surgical Care and Recovery (ISCR)
• Wednesday: Optimal Resources for Surgical Quality and Safety (the “red book”)
The ACS Theatre also will be used for meet-and-greets with ACS leaders. Posters outlining the schedule for the day will be located throughout ACS Central, and alerts will be sent out through the app and social media.
ACS Central will be open 9:00 am–4:30 pm Monday through Wednesday in the San Diego Convention Center, Exhibit Hall.
The following select ACS Programs also will have a presence Sunday through Thursday in the main lobby of the San Diego Convention Center and in the Registration Area:
• ACS Foundation and Fellows Leadership Society
• American College of Surgeons Professional Association-SurgeonsPAC
• Mobile Connect
• Become a Member/Member Services booth, where you can join the ACS, pay your membership dues, or get answers to questions about your membership
In addition, MyCME and Webcast Sales Booths will be located throughout the Convention Center and open the same hours as registration Monday through Thursday. To view other conference resources, go to the Clinical Congress 2017 Resources web page at facs.org/clincon2017/resources .
Make the most of your American College of Surgeons (ACS) Clinical Congress 2017 experience by visiting the new ACS Central area designed specifically for our members. ACS Central visitors will have opportunities to meet College staff; learn about the latest ACS programs, products, and services; purchase ACS materials; and make ACS Central a convenient gathering space during the meeting. While there, you can also update your member profile and receive a flash drive with your own professional photo to keep.
ACS Central also will house the ACS Theatre, which will feature presentations on the following new ACS programs and products during lunch hours Monday through Wednesday:
• Monday: The Surgeon Specific Registry
• Tuesday: Improving Surgical Care and Recovery (ISCR)
• Wednesday: Optimal Resources for Surgical Quality and Safety (the “red book”)
The ACS Theatre also will be used for meet-and-greets with ACS leaders. Posters outlining the schedule for the day will be located throughout ACS Central, and alerts will be sent out through the app and social media.
ACS Central will be open 9:00 am–4:30 pm Monday through Wednesday in the San Diego Convention Center, Exhibit Hall.
The following select ACS Programs also will have a presence Sunday through Thursday in the main lobby of the San Diego Convention Center and in the Registration Area:
• ACS Foundation and Fellows Leadership Society
• American College of Surgeons Professional Association-SurgeonsPAC
• Mobile Connect
• Become a Member/Member Services booth, where you can join the ACS, pay your membership dues, or get answers to questions about your membership
In addition, MyCME and Webcast Sales Booths will be located throughout the Convention Center and open the same hours as registration Monday through Thursday. To view other conference resources, go to the Clinical Congress 2017 Resources web page at facs.org/clincon2017/resources .
Make the most of your American College of Surgeons (ACS) Clinical Congress 2017 experience by visiting the new ACS Central area designed specifically for our members. ACS Central visitors will have opportunities to meet College staff; learn about the latest ACS programs, products, and services; purchase ACS materials; and make ACS Central a convenient gathering space during the meeting. While there, you can also update your member profile and receive a flash drive with your own professional photo to keep.
ACS Central also will house the ACS Theatre, which will feature presentations on the following new ACS programs and products during lunch hours Monday through Wednesday:
• Monday: The Surgeon Specific Registry
• Tuesday: Improving Surgical Care and Recovery (ISCR)
• Wednesday: Optimal Resources for Surgical Quality and Safety (the “red book”)
The ACS Theatre also will be used for meet-and-greets with ACS leaders. Posters outlining the schedule for the day will be located throughout ACS Central, and alerts will be sent out through the app and social media.
ACS Central will be open 9:00 am–4:30 pm Monday through Wednesday in the San Diego Convention Center, Exhibit Hall.
The following select ACS Programs also will have a presence Sunday through Thursday in the main lobby of the San Diego Convention Center and in the Registration Area:
• ACS Foundation and Fellows Leadership Society
• American College of Surgeons Professional Association-SurgeonsPAC
• Mobile Connect
• Become a Member/Member Services booth, where you can join the ACS, pay your membership dues, or get answers to questions about your membership
In addition, MyCME and Webcast Sales Booths will be located throughout the Convention Center and open the same hours as registration Monday through Thursday. To view other conference resources, go to the Clinical Congress 2017 Resources web page at facs.org/clincon2017/resources .
ACS scores victory for trauma research
The American College of Surgeons (ACS) has been working with members of the U.S. Senate Committee on Appropriations to advocate for inclusion of trauma research language in the Labor, Health and Human Services, Education, and Related Agencies (Labor-HHS) Appropriations Bill for fiscal year 2018. More specifically, the ACS is requesting that the committee report language stress the importance of trauma research and encourage the National Institutes of Health to establish a trauma research agenda to minimize death, disability, and injury by ensuring that patient-specific trauma care is based on scientifically validated findings. Committee report language is included in appropriations legislation to guide the administration and departments in their support of the committee’s priorities. The report and bill await further action in the Senate. The bill contains base discretionary funding for the agencies.
For more information about the College’s policy positions on trauma, contact Justin Rosen, ACS Congressional Lobbyist, at [email protected] or 202-672-1528.
The American College of Surgeons (ACS) has been working with members of the U.S. Senate Committee on Appropriations to advocate for inclusion of trauma research language in the Labor, Health and Human Services, Education, and Related Agencies (Labor-HHS) Appropriations Bill for fiscal year 2018. More specifically, the ACS is requesting that the committee report language stress the importance of trauma research and encourage the National Institutes of Health to establish a trauma research agenda to minimize death, disability, and injury by ensuring that patient-specific trauma care is based on scientifically validated findings. Committee report language is included in appropriations legislation to guide the administration and departments in their support of the committee’s priorities. The report and bill await further action in the Senate. The bill contains base discretionary funding for the agencies.
For more information about the College’s policy positions on trauma, contact Justin Rosen, ACS Congressional Lobbyist, at [email protected] or 202-672-1528.
The American College of Surgeons (ACS) has been working with members of the U.S. Senate Committee on Appropriations to advocate for inclusion of trauma research language in the Labor, Health and Human Services, Education, and Related Agencies (Labor-HHS) Appropriations Bill for fiscal year 2018. More specifically, the ACS is requesting that the committee report language stress the importance of trauma research and encourage the National Institutes of Health to establish a trauma research agenda to minimize death, disability, and injury by ensuring that patient-specific trauma care is based on scientifically validated findings. Committee report language is included in appropriations legislation to guide the administration and departments in their support of the committee’s priorities. The report and bill await further action in the Senate. The bill contains base discretionary funding for the agencies.
For more information about the College’s policy positions on trauma, contact Justin Rosen, ACS Congressional Lobbyist, at [email protected] or 202-672-1528.
The right choice? Surgery “offered” or “recommended”?
The story was not unusual for a late-night surgical consultation request by the emergency department. The patient was a 32-year-old man who had presented to the emergency room with crampy abdominal pain. He initially had felt distended, but – during the 3 hours since his presentation to the hospital – the pain and distention had resolved. A CT of the abdomen and pelvis was obtained shortly after the patient arrived in the emergency department. The study showed some dilated small bowel along with a worrisome spiral pattern of the mesentery that suggested a midgut volvulus. The finding was surprising to the surgical resident examining the patient given that the patient now had a soft and nontender abdomen on exam. The white blood cell count was not elevated, and the electrolyte levels were all normal.
When presented with this recommendation, the patient declined the recommended surgery. He stated that he felt fine and that this would be a bad time to have an operation and miss work. The patient ultimately left the hospital only to present 5 days later with peritonitis. When he was emergently explored on the second admission, he was found to have a significant amount of gangrenous small bowel that required resection.
The case was presented at the M and M (morbidity and mortality) conference the following week. When asked about the prior hospital admission, the resident reported that the patient had been “offered surgery,” and in the context of “shared decision making,” the patient had chosen to go home. This characterization of the interactions with the patient raised concern among several of the attending surgeons present. Was the patient only “offered” surgery, or was he strongly recommended to have surgery to avoid potentially risking his life? Was the patient’s refusal to have surgery despite the risks actually a case of “shared decision making”?
These questions are but a few of the many that can arise when language is used indiscriminately. Although, in the contemporary era of “patient-centered decision making,” it is common to think about every recommendation as an offer of alternative therapies, I worry that describing the interaction in this fashion is potentially misleading. Patients should not be offered potentially life-saving treatments – those treatments should be strongly recommended. Certainly, we must accept that patients can refuse even the most strongly recommended treatments, but a patient’s refusal to follow a strong recommendation for surgery should not be characterized as shared decision making. “Shared decision making” suggests that there are medically acceptable choices that the physician has offered the patient, from which the patient can make a choice based on his or her preferences and values. The case above is not shared decision making but one of respecting the patient’s autonomous choices even if we do not agree with the choices made.
There are undoubtedly situations in which there is a choice among reasonable medical options. When there is a such a choice to be made, as surgeons, we should help our patients understand the options so that they can make a decision that best fits with their values and goals. However, when the only choice is whether to have the recommended surgery or decline it, we have now moved beyond shared decision making. In that circumstance, we should strongly recommend what we believe is the better option while still respecting the autonomy of patients to decline our recommendation.
Shared decision making often is viewed as the pinnacle of ethical practice – that is, involving patients in the decisions to be made about their own health. Although I agree that we do want to educate our patients and encourage them to make what we consider safe medical decisions, when we recommend a safe choice and the patient declines it, we are no longer talking about shared decision making. In such circumstances, we are in the realm of respecting patients’ choices even when we disagree with those choices. Our responsibility as surgeons is to recommend what we think is safe but respect their choices whether we agree or not. We should do more than simply offer surgery when it is potentially life threatening not to have it.
Dr. Angelos is the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, chief of endocrine surgery, and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
The story was not unusual for a late-night surgical consultation request by the emergency department. The patient was a 32-year-old man who had presented to the emergency room with crampy abdominal pain. He initially had felt distended, but – during the 3 hours since his presentation to the hospital – the pain and distention had resolved. A CT of the abdomen and pelvis was obtained shortly after the patient arrived in the emergency department. The study showed some dilated small bowel along with a worrisome spiral pattern of the mesentery that suggested a midgut volvulus. The finding was surprising to the surgical resident examining the patient given that the patient now had a soft and nontender abdomen on exam. The white blood cell count was not elevated, and the electrolyte levels were all normal.
When presented with this recommendation, the patient declined the recommended surgery. He stated that he felt fine and that this would be a bad time to have an operation and miss work. The patient ultimately left the hospital only to present 5 days later with peritonitis. When he was emergently explored on the second admission, he was found to have a significant amount of gangrenous small bowel that required resection.
The case was presented at the M and M (morbidity and mortality) conference the following week. When asked about the prior hospital admission, the resident reported that the patient had been “offered surgery,” and in the context of “shared decision making,” the patient had chosen to go home. This characterization of the interactions with the patient raised concern among several of the attending surgeons present. Was the patient only “offered” surgery, or was he strongly recommended to have surgery to avoid potentially risking his life? Was the patient’s refusal to have surgery despite the risks actually a case of “shared decision making”?
These questions are but a few of the many that can arise when language is used indiscriminately. Although, in the contemporary era of “patient-centered decision making,” it is common to think about every recommendation as an offer of alternative therapies, I worry that describing the interaction in this fashion is potentially misleading. Patients should not be offered potentially life-saving treatments – those treatments should be strongly recommended. Certainly, we must accept that patients can refuse even the most strongly recommended treatments, but a patient’s refusal to follow a strong recommendation for surgery should not be characterized as shared decision making. “Shared decision making” suggests that there are medically acceptable choices that the physician has offered the patient, from which the patient can make a choice based on his or her preferences and values. The case above is not shared decision making but one of respecting the patient’s autonomous choices even if we do not agree with the choices made.
There are undoubtedly situations in which there is a choice among reasonable medical options. When there is a such a choice to be made, as surgeons, we should help our patients understand the options so that they can make a decision that best fits with their values and goals. However, when the only choice is whether to have the recommended surgery or decline it, we have now moved beyond shared decision making. In that circumstance, we should strongly recommend what we believe is the better option while still respecting the autonomy of patients to decline our recommendation.
Shared decision making often is viewed as the pinnacle of ethical practice – that is, involving patients in the decisions to be made about their own health. Although I agree that we do want to educate our patients and encourage them to make what we consider safe medical decisions, when we recommend a safe choice and the patient declines it, we are no longer talking about shared decision making. In such circumstances, we are in the realm of respecting patients’ choices even when we disagree with those choices. Our responsibility as surgeons is to recommend what we think is safe but respect their choices whether we agree or not. We should do more than simply offer surgery when it is potentially life threatening not to have it.
Dr. Angelos is the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, chief of endocrine surgery, and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
The story was not unusual for a late-night surgical consultation request by the emergency department. The patient was a 32-year-old man who had presented to the emergency room with crampy abdominal pain. He initially had felt distended, but – during the 3 hours since his presentation to the hospital – the pain and distention had resolved. A CT of the abdomen and pelvis was obtained shortly after the patient arrived in the emergency department. The study showed some dilated small bowel along with a worrisome spiral pattern of the mesentery that suggested a midgut volvulus. The finding was surprising to the surgical resident examining the patient given that the patient now had a soft and nontender abdomen on exam. The white blood cell count was not elevated, and the electrolyte levels were all normal.
When presented with this recommendation, the patient declined the recommended surgery. He stated that he felt fine and that this would be a bad time to have an operation and miss work. The patient ultimately left the hospital only to present 5 days later with peritonitis. When he was emergently explored on the second admission, he was found to have a significant amount of gangrenous small bowel that required resection.
The case was presented at the M and M (morbidity and mortality) conference the following week. When asked about the prior hospital admission, the resident reported that the patient had been “offered surgery,” and in the context of “shared decision making,” the patient had chosen to go home. This characterization of the interactions with the patient raised concern among several of the attending surgeons present. Was the patient only “offered” surgery, or was he strongly recommended to have surgery to avoid potentially risking his life? Was the patient’s refusal to have surgery despite the risks actually a case of “shared decision making”?
These questions are but a few of the many that can arise when language is used indiscriminately. Although, in the contemporary era of “patient-centered decision making,” it is common to think about every recommendation as an offer of alternative therapies, I worry that describing the interaction in this fashion is potentially misleading. Patients should not be offered potentially life-saving treatments – those treatments should be strongly recommended. Certainly, we must accept that patients can refuse even the most strongly recommended treatments, but a patient’s refusal to follow a strong recommendation for surgery should not be characterized as shared decision making. “Shared decision making” suggests that there are medically acceptable choices that the physician has offered the patient, from which the patient can make a choice based on his or her preferences and values. The case above is not shared decision making but one of respecting the patient’s autonomous choices even if we do not agree with the choices made.
There are undoubtedly situations in which there is a choice among reasonable medical options. When there is a such a choice to be made, as surgeons, we should help our patients understand the options so that they can make a decision that best fits with their values and goals. However, when the only choice is whether to have the recommended surgery or decline it, we have now moved beyond shared decision making. In that circumstance, we should strongly recommend what we believe is the better option while still respecting the autonomy of patients to decline our recommendation.
Shared decision making often is viewed as the pinnacle of ethical practice – that is, involving patients in the decisions to be made about their own health. Although I agree that we do want to educate our patients and encourage them to make what we consider safe medical decisions, when we recommend a safe choice and the patient declines it, we are no longer talking about shared decision making. In such circumstances, we are in the realm of respecting patients’ choices even when we disagree with those choices. Our responsibility as surgeons is to recommend what we think is safe but respect their choices whether we agree or not. We should do more than simply offer surgery when it is potentially life threatening not to have it.
Dr. Angelos is the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, chief of endocrine surgery, and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
From the Editors: Halsted, Holmes, and penguins
Is it not ironic that in a profession that is always seeking answers – What does this patient have? Is that mass malignant? What’s the best way to make a diagnosis? – too much information has become a major problem?
Unlike William Stewart Halsted or Theodor Billroth, who blazed surgical trails in an age when much was unknown, today’s surgeons face a jungle of information obscuring the trail ahead. Every morning we wake up to another 30 or 40 unread emails. Journals multiply on our desks. The books we need to read pile up and spill over onto our desks, bookshelves, and side tables. Sometimes, it makes one long for the old days when definitive answers might not be found in the literature. These days, we know it is likely that someone has published exactly what we need at any particular moment, and yet finding it in the jungle of information can be a great challenge.
Another outcome of too much information is the accumulation in our brains of unsorted bits of medical/surgical knowledge. Some of those bits are pearls, and others are just gum wrappers that take up space. It becomes an overwhelming task of ranking, sorting, prioritizing, and discarding.
A friend of mine years of ago called his brain an iceberg on which thousands of penguins stand. The penguins just kept coming and, finally, in order to learn anything new, he had to push some penguins off the iceberg. We have a lot of penguins on our icebergs these days.
This brings to mind many doctors’ favorite fictional character, Sherlock Holmes. That denizen of 221B Baker Street was a master at data management. He always had the right information available in his head relevant for the mystery at hand. How did he do it? Recall that Dr. Watson (a surgeon, I might add) was intermittently shocked by what Holmes didn’t know, to which the tobacco- and opiate-addicted hero would reply that he purposely forgot things that did not help him solve his cases.
And so, what is the modern surgeon – who must keep in the forefront of his or her mind every best practice, algorithm, and guideline – to do in this age of too much information? Like Holmes, we need to sort what is critical from what is not and let go of those items that no longer are germane. We then need to triage the vast amount of information delivered to us yearly, weekly, monthly, daily, hourly. The stream of little notes flashing at you from your black mirror (the screen of your mobile device) needs to be controlled lest it control you.
So, might I suggest a few strategies I have used to triage the flow of information? For hourly and daily information, I tend to ignore everything except ACS NewsScope and the ACS Communities items I find most interesting. For monthly information, I tend to use ACS Surgery News (plug intended) because it is “news” – the stuff that just happened in the meeting sphere or has not yet hit print (sorry, e-publication). The Journal of the American College of Surgeons is another monthly source that is reliable.
You may see a theme here. I’ve used the American College of Surgeons as my main filter. What gets through the editors of these outlets generally is viable and useful information. That’s what I need to know for right now. Such knowledge allows me to push those penguins no longer needed off my iceberg and greet the new ones with joy. We often wonder what the benefit of membership in the College may be. For me, these filters are worth the price of admission.
Dr. Hughes is clinical professor in the department of surgery and director of medical education at the Kansas University School of Medicine, Salina Campus, and Co-Editor of ACS Surgery News.
Is it not ironic that in a profession that is always seeking answers – What does this patient have? Is that mass malignant? What’s the best way to make a diagnosis? – too much information has become a major problem?
Unlike William Stewart Halsted or Theodor Billroth, who blazed surgical trails in an age when much was unknown, today’s surgeons face a jungle of information obscuring the trail ahead. Every morning we wake up to another 30 or 40 unread emails. Journals multiply on our desks. The books we need to read pile up and spill over onto our desks, bookshelves, and side tables. Sometimes, it makes one long for the old days when definitive answers might not be found in the literature. These days, we know it is likely that someone has published exactly what we need at any particular moment, and yet finding it in the jungle of information can be a great challenge.
Another outcome of too much information is the accumulation in our brains of unsorted bits of medical/surgical knowledge. Some of those bits are pearls, and others are just gum wrappers that take up space. It becomes an overwhelming task of ranking, sorting, prioritizing, and discarding.
A friend of mine years of ago called his brain an iceberg on which thousands of penguins stand. The penguins just kept coming and, finally, in order to learn anything new, he had to push some penguins off the iceberg. We have a lot of penguins on our icebergs these days.
This brings to mind many doctors’ favorite fictional character, Sherlock Holmes. That denizen of 221B Baker Street was a master at data management. He always had the right information available in his head relevant for the mystery at hand. How did he do it? Recall that Dr. Watson (a surgeon, I might add) was intermittently shocked by what Holmes didn’t know, to which the tobacco- and opiate-addicted hero would reply that he purposely forgot things that did not help him solve his cases.
And so, what is the modern surgeon – who must keep in the forefront of his or her mind every best practice, algorithm, and guideline – to do in this age of too much information? Like Holmes, we need to sort what is critical from what is not and let go of those items that no longer are germane. We then need to triage the vast amount of information delivered to us yearly, weekly, monthly, daily, hourly. The stream of little notes flashing at you from your black mirror (the screen of your mobile device) needs to be controlled lest it control you.
So, might I suggest a few strategies I have used to triage the flow of information? For hourly and daily information, I tend to ignore everything except ACS NewsScope and the ACS Communities items I find most interesting. For monthly information, I tend to use ACS Surgery News (plug intended) because it is “news” – the stuff that just happened in the meeting sphere or has not yet hit print (sorry, e-publication). The Journal of the American College of Surgeons is another monthly source that is reliable.
You may see a theme here. I’ve used the American College of Surgeons as my main filter. What gets through the editors of these outlets generally is viable and useful information. That’s what I need to know for right now. Such knowledge allows me to push those penguins no longer needed off my iceberg and greet the new ones with joy. We often wonder what the benefit of membership in the College may be. For me, these filters are worth the price of admission.
Dr. Hughes is clinical professor in the department of surgery and director of medical education at the Kansas University School of Medicine, Salina Campus, and Co-Editor of ACS Surgery News.
Is it not ironic that in a profession that is always seeking answers – What does this patient have? Is that mass malignant? What’s the best way to make a diagnosis? – too much information has become a major problem?
Unlike William Stewart Halsted or Theodor Billroth, who blazed surgical trails in an age when much was unknown, today’s surgeons face a jungle of information obscuring the trail ahead. Every morning we wake up to another 30 or 40 unread emails. Journals multiply on our desks. The books we need to read pile up and spill over onto our desks, bookshelves, and side tables. Sometimes, it makes one long for the old days when definitive answers might not be found in the literature. These days, we know it is likely that someone has published exactly what we need at any particular moment, and yet finding it in the jungle of information can be a great challenge.
Another outcome of too much information is the accumulation in our brains of unsorted bits of medical/surgical knowledge. Some of those bits are pearls, and others are just gum wrappers that take up space. It becomes an overwhelming task of ranking, sorting, prioritizing, and discarding.
A friend of mine years of ago called his brain an iceberg on which thousands of penguins stand. The penguins just kept coming and, finally, in order to learn anything new, he had to push some penguins off the iceberg. We have a lot of penguins on our icebergs these days.
This brings to mind many doctors’ favorite fictional character, Sherlock Holmes. That denizen of 221B Baker Street was a master at data management. He always had the right information available in his head relevant for the mystery at hand. How did he do it? Recall that Dr. Watson (a surgeon, I might add) was intermittently shocked by what Holmes didn’t know, to which the tobacco- and opiate-addicted hero would reply that he purposely forgot things that did not help him solve his cases.
And so, what is the modern surgeon – who must keep in the forefront of his or her mind every best practice, algorithm, and guideline – to do in this age of too much information? Like Holmes, we need to sort what is critical from what is not and let go of those items that no longer are germane. We then need to triage the vast amount of information delivered to us yearly, weekly, monthly, daily, hourly. The stream of little notes flashing at you from your black mirror (the screen of your mobile device) needs to be controlled lest it control you.
So, might I suggest a few strategies I have used to triage the flow of information? For hourly and daily information, I tend to ignore everything except ACS NewsScope and the ACS Communities items I find most interesting. For monthly information, I tend to use ACS Surgery News (plug intended) because it is “news” – the stuff that just happened in the meeting sphere or has not yet hit print (sorry, e-publication). The Journal of the American College of Surgeons is another monthly source that is reliable.
You may see a theme here. I’ve used the American College of Surgeons as my main filter. What gets through the editors of these outlets generally is viable and useful information. That’s what I need to know for right now. Such knowledge allows me to push those penguins no longer needed off my iceberg and greet the new ones with joy. We often wonder what the benefit of membership in the College may be. For me, these filters are worth the price of admission.
Dr. Hughes is clinical professor in the department of surgery and director of medical education at the Kansas University School of Medicine, Salina Campus, and Co-Editor of ACS Surgery News.
VIDEO: Sildenafil improves cerebrovascular reactivity in chronic TBI
SAN DIEGO – The healthy brain is a master of autoregulation, continuously adjusting blood flow to meet metabolic demand.
But in traumatic brain injury, cerebrovascular reactivity (CVR) breaks down; blood vessels don’t dilate as they should to deliver nutrients and oxygen, leading to progressive neurologic decline.
Sildenafil (Viagra) – a vasodilator in injured blood vessels – might help, according to ongoing research at the University of Pennsylvania, Philadelphia.
Researchers there gave sildenafil to inpatients with persistent symptoms at least 6 months after traumatic brain injury and measured CVR by a novel MRI technique an hour later. “Sildenafil was able to correct the deficit in CVR in many cases. We are hopeful this could be a useful therapy,” said principal investigator Ramon Diaz-Arrastia, MD, a professor of neurology at the university.
He explained the work in an interview at annual meeting of the American Neurological Association. The next step is to see if sildenafil helps CVR in acute traumatic brain injury, and in people who have had multiple, mild brain traumas, including professional athletes.
SAN DIEGO – The healthy brain is a master of autoregulation, continuously adjusting blood flow to meet metabolic demand.
But in traumatic brain injury, cerebrovascular reactivity (CVR) breaks down; blood vessels don’t dilate as they should to deliver nutrients and oxygen, leading to progressive neurologic decline.
Sildenafil (Viagra) – a vasodilator in injured blood vessels – might help, according to ongoing research at the University of Pennsylvania, Philadelphia.
Researchers there gave sildenafil to inpatients with persistent symptoms at least 6 months after traumatic brain injury and measured CVR by a novel MRI technique an hour later. “Sildenafil was able to correct the deficit in CVR in many cases. We are hopeful this could be a useful therapy,” said principal investigator Ramon Diaz-Arrastia, MD, a professor of neurology at the university.
He explained the work in an interview at annual meeting of the American Neurological Association. The next step is to see if sildenafil helps CVR in acute traumatic brain injury, and in people who have had multiple, mild brain traumas, including professional athletes.
SAN DIEGO – The healthy brain is a master of autoregulation, continuously adjusting blood flow to meet metabolic demand.
But in traumatic brain injury, cerebrovascular reactivity (CVR) breaks down; blood vessels don’t dilate as they should to deliver nutrients and oxygen, leading to progressive neurologic decline.
Sildenafil (Viagra) – a vasodilator in injured blood vessels – might help, according to ongoing research at the University of Pennsylvania, Philadelphia.
Researchers there gave sildenafil to inpatients with persistent symptoms at least 6 months after traumatic brain injury and measured CVR by a novel MRI technique an hour later. “Sildenafil was able to correct the deficit in CVR in many cases. We are hopeful this could be a useful therapy,” said principal investigator Ramon Diaz-Arrastia, MD, a professor of neurology at the university.
He explained the work in an interview at annual meeting of the American Neurological Association. The next step is to see if sildenafil helps CVR in acute traumatic brain injury, and in people who have had multiple, mild brain traumas, including professional athletes.
AT ANA 2017
SGLT inhibition back on track in phase 3 type T1DM trial
LISBON – Despite some earlier concerns over safety, sodium-glucose cotransporter (SGLT) inhibitors appear to be back on track for adjunctive use in people with type 1 diabetes (T1DM), according to phase 3 study findings presented at the annual meeting of the European Association for the Study of Diabetes.
Results of the DEPICT-1 study, showed improved glycemic control, greater weight loss, and no increased risk for either hypoglycemia or ketoacidosis in people treated with the SGLT2 inhibitor dapagliflozin (Farxiga) versus those treated with placebo.

When researchers compared the two dapagliflozin doses with placebo, the mean difference in body weight from baseline to week 24 was –2.96% and –3.72% (both P less than .0001).
Rates of any hypoglycemia in the placebo, 5-mg dapagliflozin, and 10-mg dapagliflozin groups were a respective 79.6%, 79.4%, and 79.4%, and rates of severe hypoglycemia were 7.3%, 7.6%, and 6.4%.
Diabetic ketoacidosis occurred in a respective 1.2%, 1.4%, and 1.7% of patients treated with placebo, dapagliflozin 5 mg, and dapagliflozin 10 mg, respectively.
“Based on this 24-week study, dapagliflozin may be considered as a good candidate as an adjunct to insulin to improve glycemic control in type 1 diabetes,” said the lead investigator for the DEPICT-1 trial, Paresh Dandona, MD, PhD, at the EASD 2017 meeting.
“There may also be other potential long-term cardiovascular and renal benefits, which have recently been demonstrated in type 2 diabetes,” suggested Dr. Dandona, who is a distinguished professor of medicine and chief of the division of endocrinology at the State University of New York at Buffalo. “Of course, that’s an open question, which needs to be investigated and determined in future.”
DEPICT-1 is the first phase 3, randomized, blinded, clinical trial of a selective SGLT2 inhibitor in T1DM, the study’s investigators said in an early online publication (Lancet Diabetes Endocrinol. 2017 Sep 13. doi: 10.1016/S2213-8587[17]30308-X). The study was conducted at 143 sites in 17 centers and involved 833 people with T1DM who were not achieving optimal blood glucose control.
Adults with an HbA1c of 7.7% or higher who had been taking insulin for at least 12 months were recruited and underwent an 8-week lead-in period to optimize their diabetes management before being randomized to take placebo or dapagliflozin 5 mg or 10 mg for 52 weeks. The primary endpoint was the change in HbA1c at Week 24.
DEPICT-1 “provides encouraging short-term data for the efficacy of adjunct SGLT2 inhibition in type 1 diabetes but might also provide insights into how the risk of ketoacidosis can be minimized,” John Petrie, MD, commented in an editorial that accompanied the published findings.
Dr. Petrie, professor of diabetic medicine at the Institute of Cardiovascular and Medical Sciences at the University of Glasgow, observed, however, that the results needed to be considered in the context of similar, and also recently presented, findings from the inTandem3 trial (N Engl J Med. 2017 Sep 13. doi: 10.1056/NEJMoa1708337) with the investigational dual SGLT1/SGLT2 inhibitor sotagliflozin. Other trials are expected to report soon with another SGLT2 inhibitor, empagliflozin.
In inTandem3, sotagliflozin helped more people who had T1DM and were on stable insulin to achieve an HbA1c level of 7% or lower at week 24 with no episodes of severe hypoglycemia or diabetic ketoacidosis, compared with those who took insulin alone (28.6% vs. 15.2%; P less than .001). However, the overall rates of ketoacidosis were higher in the patients treated with sotagliflozin than in those treated with placebo, at 3% versus 0.6%, respectively.
In both inTandem3 and DEPICT-1, strategies were in place to help carefully monitor and manage ketoacidosis. Dr. Petrie observed that patients in the latter trial were given a meter that measured both their blood glucose and ketones and were seen in a clinic regularly to assess the risk of ketoacidosis. These frequent visits, which occurred every 2 weeks, were shown to have an independent effect on HbA1c, he pointed out.
“Nevertheless, the investigators kept real-world clinical practice in mind by providing a very simple rule that insulin doses should be reduced by no more than 20% when study medication was started and that they should subsequently be titrated back towards the initial dose,” Dr. Petrie said. “This rule seemed to be quite effective in mitigating ketoacidosis and is feasible to implement in modern clinical practice since meters that measure ketones are increasingly available.
So does this mean a license for SGLT-targeting agents in T1DM could be coming soon? Not yet, Dr. Petrie suggests. “Regulators are likely to await at least the results of the 12-month follow-up, and data from other ongoing trials, before considering an indication for SGLT2 inhibitors in type 1 diabetes,” he said.
Adjunct treatment with these drugs also may require that individuals have “a good understanding of the early-warning symptoms of ketoacidosis” and be prepared to undertake regular home monitoring of both their blood glucose and ketones, as well as having a high level of communication with their diabetes health care providers.
AstraZeneca and Bristol-Myers Squibb funded the study.
Dr. Dandona received research support from AstraZeneca, Novo Nordisk, Boehringer Ingelheim, and AbbVie and was a consultant to AstraZeneca, Novo Nordisk, Sanofi-Aventis, and others.
Dr. Petrie disclosed receiving personal fees and travel expenses from Novo Nordisk; grants and personal fees from Sanofi-Aventis, Quintiles, and Janssen; nonfinancial support from Merck and Itamar Medical; and personal fees from Lilly, ACI Clinical, and Pfizer.
LISBON – Despite some earlier concerns over safety, sodium-glucose cotransporter (SGLT) inhibitors appear to be back on track for adjunctive use in people with type 1 diabetes (T1DM), according to phase 3 study findings presented at the annual meeting of the European Association for the Study of Diabetes.
Results of the DEPICT-1 study, showed improved glycemic control, greater weight loss, and no increased risk for either hypoglycemia or ketoacidosis in people treated with the SGLT2 inhibitor dapagliflozin (Farxiga) versus those treated with placebo.

When researchers compared the two dapagliflozin doses with placebo, the mean difference in body weight from baseline to week 24 was –2.96% and –3.72% (both P less than .0001).
Rates of any hypoglycemia in the placebo, 5-mg dapagliflozin, and 10-mg dapagliflozin groups were a respective 79.6%, 79.4%, and 79.4%, and rates of severe hypoglycemia were 7.3%, 7.6%, and 6.4%.
Diabetic ketoacidosis occurred in a respective 1.2%, 1.4%, and 1.7% of patients treated with placebo, dapagliflozin 5 mg, and dapagliflozin 10 mg, respectively.
“Based on this 24-week study, dapagliflozin may be considered as a good candidate as an adjunct to insulin to improve glycemic control in type 1 diabetes,” said the lead investigator for the DEPICT-1 trial, Paresh Dandona, MD, PhD, at the EASD 2017 meeting.
“There may also be other potential long-term cardiovascular and renal benefits, which have recently been demonstrated in type 2 diabetes,” suggested Dr. Dandona, who is a distinguished professor of medicine and chief of the division of endocrinology at the State University of New York at Buffalo. “Of course, that’s an open question, which needs to be investigated and determined in future.”
DEPICT-1 is the first phase 3, randomized, blinded, clinical trial of a selective SGLT2 inhibitor in T1DM, the study’s investigators said in an early online publication (Lancet Diabetes Endocrinol. 2017 Sep 13. doi: 10.1016/S2213-8587[17]30308-X). The study was conducted at 143 sites in 17 centers and involved 833 people with T1DM who were not achieving optimal blood glucose control.
Adults with an HbA1c of 7.7% or higher who had been taking insulin for at least 12 months were recruited and underwent an 8-week lead-in period to optimize their diabetes management before being randomized to take placebo or dapagliflozin 5 mg or 10 mg for 52 weeks. The primary endpoint was the change in HbA1c at Week 24.
DEPICT-1 “provides encouraging short-term data for the efficacy of adjunct SGLT2 inhibition in type 1 diabetes but might also provide insights into how the risk of ketoacidosis can be minimized,” John Petrie, MD, commented in an editorial that accompanied the published findings.
Dr. Petrie, professor of diabetic medicine at the Institute of Cardiovascular and Medical Sciences at the University of Glasgow, observed, however, that the results needed to be considered in the context of similar, and also recently presented, findings from the inTandem3 trial (N Engl J Med. 2017 Sep 13. doi: 10.1056/NEJMoa1708337) with the investigational dual SGLT1/SGLT2 inhibitor sotagliflozin. Other trials are expected to report soon with another SGLT2 inhibitor, empagliflozin.
In inTandem3, sotagliflozin helped more people who had T1DM and were on stable insulin to achieve an HbA1c level of 7% or lower at week 24 with no episodes of severe hypoglycemia or diabetic ketoacidosis, compared with those who took insulin alone (28.6% vs. 15.2%; P less than .001). However, the overall rates of ketoacidosis were higher in the patients treated with sotagliflozin than in those treated with placebo, at 3% versus 0.6%, respectively.
In both inTandem3 and DEPICT-1, strategies were in place to help carefully monitor and manage ketoacidosis. Dr. Petrie observed that patients in the latter trial were given a meter that measured both their blood glucose and ketones and were seen in a clinic regularly to assess the risk of ketoacidosis. These frequent visits, which occurred every 2 weeks, were shown to have an independent effect on HbA1c, he pointed out.
“Nevertheless, the investigators kept real-world clinical practice in mind by providing a very simple rule that insulin doses should be reduced by no more than 20% when study medication was started and that they should subsequently be titrated back towards the initial dose,” Dr. Petrie said. “This rule seemed to be quite effective in mitigating ketoacidosis and is feasible to implement in modern clinical practice since meters that measure ketones are increasingly available.
So does this mean a license for SGLT-targeting agents in T1DM could be coming soon? Not yet, Dr. Petrie suggests. “Regulators are likely to await at least the results of the 12-month follow-up, and data from other ongoing trials, before considering an indication for SGLT2 inhibitors in type 1 diabetes,” he said.
Adjunct treatment with these drugs also may require that individuals have “a good understanding of the early-warning symptoms of ketoacidosis” and be prepared to undertake regular home monitoring of both their blood glucose and ketones, as well as having a high level of communication with their diabetes health care providers.
AstraZeneca and Bristol-Myers Squibb funded the study.
Dr. Dandona received research support from AstraZeneca, Novo Nordisk, Boehringer Ingelheim, and AbbVie and was a consultant to AstraZeneca, Novo Nordisk, Sanofi-Aventis, and others.
Dr. Petrie disclosed receiving personal fees and travel expenses from Novo Nordisk; grants and personal fees from Sanofi-Aventis, Quintiles, and Janssen; nonfinancial support from Merck and Itamar Medical; and personal fees from Lilly, ACI Clinical, and Pfizer.
LISBON – Despite some earlier concerns over safety, sodium-glucose cotransporter (SGLT) inhibitors appear to be back on track for adjunctive use in people with type 1 diabetes (T1DM), according to phase 3 study findings presented at the annual meeting of the European Association for the Study of Diabetes.
Results of the DEPICT-1 study, showed improved glycemic control, greater weight loss, and no increased risk for either hypoglycemia or ketoacidosis in people treated with the SGLT2 inhibitor dapagliflozin (Farxiga) versus those treated with placebo.

When researchers compared the two dapagliflozin doses with placebo, the mean difference in body weight from baseline to week 24 was –2.96% and –3.72% (both P less than .0001).
Rates of any hypoglycemia in the placebo, 5-mg dapagliflozin, and 10-mg dapagliflozin groups were a respective 79.6%, 79.4%, and 79.4%, and rates of severe hypoglycemia were 7.3%, 7.6%, and 6.4%.
Diabetic ketoacidosis occurred in a respective 1.2%, 1.4%, and 1.7% of patients treated with placebo, dapagliflozin 5 mg, and dapagliflozin 10 mg, respectively.
“Based on this 24-week study, dapagliflozin may be considered as a good candidate as an adjunct to insulin to improve glycemic control in type 1 diabetes,” said the lead investigator for the DEPICT-1 trial, Paresh Dandona, MD, PhD, at the EASD 2017 meeting.
“There may also be other potential long-term cardiovascular and renal benefits, which have recently been demonstrated in type 2 diabetes,” suggested Dr. Dandona, who is a distinguished professor of medicine and chief of the division of endocrinology at the State University of New York at Buffalo. “Of course, that’s an open question, which needs to be investigated and determined in future.”
DEPICT-1 is the first phase 3, randomized, blinded, clinical trial of a selective SGLT2 inhibitor in T1DM, the study’s investigators said in an early online publication (Lancet Diabetes Endocrinol. 2017 Sep 13. doi: 10.1016/S2213-8587[17]30308-X). The study was conducted at 143 sites in 17 centers and involved 833 people with T1DM who were not achieving optimal blood glucose control.
Adults with an HbA1c of 7.7% or higher who had been taking insulin for at least 12 months were recruited and underwent an 8-week lead-in period to optimize their diabetes management before being randomized to take placebo or dapagliflozin 5 mg or 10 mg for 52 weeks. The primary endpoint was the change in HbA1c at Week 24.
DEPICT-1 “provides encouraging short-term data for the efficacy of adjunct SGLT2 inhibition in type 1 diabetes but might also provide insights into how the risk of ketoacidosis can be minimized,” John Petrie, MD, commented in an editorial that accompanied the published findings.
Dr. Petrie, professor of diabetic medicine at the Institute of Cardiovascular and Medical Sciences at the University of Glasgow, observed, however, that the results needed to be considered in the context of similar, and also recently presented, findings from the inTandem3 trial (N Engl J Med. 2017 Sep 13. doi: 10.1056/NEJMoa1708337) with the investigational dual SGLT1/SGLT2 inhibitor sotagliflozin. Other trials are expected to report soon with another SGLT2 inhibitor, empagliflozin.
In inTandem3, sotagliflozin helped more people who had T1DM and were on stable insulin to achieve an HbA1c level of 7% or lower at week 24 with no episodes of severe hypoglycemia or diabetic ketoacidosis, compared with those who took insulin alone (28.6% vs. 15.2%; P less than .001). However, the overall rates of ketoacidosis were higher in the patients treated with sotagliflozin than in those treated with placebo, at 3% versus 0.6%, respectively.
In both inTandem3 and DEPICT-1, strategies were in place to help carefully monitor and manage ketoacidosis. Dr. Petrie observed that patients in the latter trial were given a meter that measured both their blood glucose and ketones and were seen in a clinic regularly to assess the risk of ketoacidosis. These frequent visits, which occurred every 2 weeks, were shown to have an independent effect on HbA1c, he pointed out.
“Nevertheless, the investigators kept real-world clinical practice in mind by providing a very simple rule that insulin doses should be reduced by no more than 20% when study medication was started and that they should subsequently be titrated back towards the initial dose,” Dr. Petrie said. “This rule seemed to be quite effective in mitigating ketoacidosis and is feasible to implement in modern clinical practice since meters that measure ketones are increasingly available.
So does this mean a license for SGLT-targeting agents in T1DM could be coming soon? Not yet, Dr. Petrie suggests. “Regulators are likely to await at least the results of the 12-month follow-up, and data from other ongoing trials, before considering an indication for SGLT2 inhibitors in type 1 diabetes,” he said.
Adjunct treatment with these drugs also may require that individuals have “a good understanding of the early-warning symptoms of ketoacidosis” and be prepared to undertake regular home monitoring of both their blood glucose and ketones, as well as having a high level of communication with their diabetes health care providers.
AstraZeneca and Bristol-Myers Squibb funded the study.
Dr. Dandona received research support from AstraZeneca, Novo Nordisk, Boehringer Ingelheim, and AbbVie and was a consultant to AstraZeneca, Novo Nordisk, Sanofi-Aventis, and others.
Dr. Petrie disclosed receiving personal fees and travel expenses from Novo Nordisk; grants and personal fees from Sanofi-Aventis, Quintiles, and Janssen; nonfinancial support from Merck and Itamar Medical; and personal fees from Lilly, ACI Clinical, and Pfizer.
AT EASD 2017
Key clinical point:
Major finding: Dapagliflozin 5 mg and 10 mg reduced HbA1c from Week 0 to Week 24 more (0.42% and 0.45%, respectively) than did placebo (both P less than .0001).
Data source: A phase 3 randomized, double-blind, parallel-controlled, multicenter trial conducted in 833 people with type 1 diabetes.
Disclosures: AstraZeneca and Bristol-Myers Squibb funded the study. Dr. Dandona disclosed acting as a consultant to AstraZeneca, Novo Nordisk, Sanofi-Aventis, Merck, Intarcia, and AbbVie, as well as receiving research support from AstraZeneca, Novo Nordisk, Boehringer Ingelheim, and AbbVie. Dr. Petrie disclosed receiving personal fees and travel expenses from Novo Nordisk; grants and personal fees from Sanofi-Aventis, Quintiles, and Janssen; nonfinancial support from Merck and Itamar Medical; and personal fees from Lilly, ACI Clinical, and Pfizer.
Fentanyl in the cath lab questioned
BARCELONA – The current routine use of intravenous fentanyl in the cardiac catheterization lab for patient comfort during coronary angiography has been called into question by the results of a double-blind randomized trial presented at the annual congress of the European Society of Cardiology.
The trial, known as PACIFY, showed that IV fentanyl delayed absorption of the oral P2Y12 inhibitor ticagrelor (Brilinta) by up to 4 hours. That’s a disturbing finding that could account for the relatively high risk of stent thrombosis in the first hours after percutaneous coronary intervention, according to lead investigator John W. McEvoy, MD, a cardiologist at Johns Hopkins University in Baltimore.
“These data challenge the routine and nonselective use of fentanyl for cardiac catheterization and PCI, particularly when rapid platelet inhibition is desirable,” he said, adding, “This would represent a significant change in U.S. cath lab practice.”
PACIFY (Platelet Aggregation After Ticagrelor Inhibition and Fentanyl) was a single-center trial in which 212 patients undergoing PCI were randomized in double-blind fashion to fentanyl or no fentanyl on top of a local anesthetic and IV midazolam (Versed). In addition, the 70 subjects undergoing PCI with stent placement received a 180-mg loading dose of ticagrelor intraprocedurally.
The primary endpoint was ticagrelor plasma concentration during the first 24 hours after the drug’s administration. Secondary endpoints were patients’ self-reported maximum pain during the procedure and platelet inhibition at 2 hours.
The plasma concentration time area under the curve over the course of 24 hours was superior in the no-fentanyl group by a margin of 3,441 ng/mL–1 per hour to 2,016 ng/mL–1 per hour. Moreover, 37% of fentanyl recipients displayed high platelet reactivity at 2 hours as measured by light transmission platelet aggregometry, compared with none of the no-fentanyl controls.
Pain was similarly well controlled in both treatment arms, casting doubt on the widespread belief among U.S. interventionalists that routine administration of fentanyl in the cath lab is necessary for patient comfort. Patients in the control arm could receive bailout fentanyl upon request; only two did so.
Dr. McEvoy reported having no financial conflicts regarding this study, which was conducted free of commercial support.
BARCELONA – The current routine use of intravenous fentanyl in the cardiac catheterization lab for patient comfort during coronary angiography has been called into question by the results of a double-blind randomized trial presented at the annual congress of the European Society of Cardiology.
The trial, known as PACIFY, showed that IV fentanyl delayed absorption of the oral P2Y12 inhibitor ticagrelor (Brilinta) by up to 4 hours. That’s a disturbing finding that could account for the relatively high risk of stent thrombosis in the first hours after percutaneous coronary intervention, according to lead investigator John W. McEvoy, MD, a cardiologist at Johns Hopkins University in Baltimore.
“These data challenge the routine and nonselective use of fentanyl for cardiac catheterization and PCI, particularly when rapid platelet inhibition is desirable,” he said, adding, “This would represent a significant change in U.S. cath lab practice.”
PACIFY (Platelet Aggregation After Ticagrelor Inhibition and Fentanyl) was a single-center trial in which 212 patients undergoing PCI were randomized in double-blind fashion to fentanyl or no fentanyl on top of a local anesthetic and IV midazolam (Versed). In addition, the 70 subjects undergoing PCI with stent placement received a 180-mg loading dose of ticagrelor intraprocedurally.
The primary endpoint was ticagrelor plasma concentration during the first 24 hours after the drug’s administration. Secondary endpoints were patients’ self-reported maximum pain during the procedure and platelet inhibition at 2 hours.
The plasma concentration time area under the curve over the course of 24 hours was superior in the no-fentanyl group by a margin of 3,441 ng/mL–1 per hour to 2,016 ng/mL–1 per hour. Moreover, 37% of fentanyl recipients displayed high platelet reactivity at 2 hours as measured by light transmission platelet aggregometry, compared with none of the no-fentanyl controls.
Pain was similarly well controlled in both treatment arms, casting doubt on the widespread belief among U.S. interventionalists that routine administration of fentanyl in the cath lab is necessary for patient comfort. Patients in the control arm could receive bailout fentanyl upon request; only two did so.
Dr. McEvoy reported having no financial conflicts regarding this study, which was conducted free of commercial support.
BARCELONA – The current routine use of intravenous fentanyl in the cardiac catheterization lab for patient comfort during coronary angiography has been called into question by the results of a double-blind randomized trial presented at the annual congress of the European Society of Cardiology.
The trial, known as PACIFY, showed that IV fentanyl delayed absorption of the oral P2Y12 inhibitor ticagrelor (Brilinta) by up to 4 hours. That’s a disturbing finding that could account for the relatively high risk of stent thrombosis in the first hours after percutaneous coronary intervention, according to lead investigator John W. McEvoy, MD, a cardiologist at Johns Hopkins University in Baltimore.
“These data challenge the routine and nonselective use of fentanyl for cardiac catheterization and PCI, particularly when rapid platelet inhibition is desirable,” he said, adding, “This would represent a significant change in U.S. cath lab practice.”
PACIFY (Platelet Aggregation After Ticagrelor Inhibition and Fentanyl) was a single-center trial in which 212 patients undergoing PCI were randomized in double-blind fashion to fentanyl or no fentanyl on top of a local anesthetic and IV midazolam (Versed). In addition, the 70 subjects undergoing PCI with stent placement received a 180-mg loading dose of ticagrelor intraprocedurally.
The primary endpoint was ticagrelor plasma concentration during the first 24 hours after the drug’s administration. Secondary endpoints were patients’ self-reported maximum pain during the procedure and platelet inhibition at 2 hours.
The plasma concentration time area under the curve over the course of 24 hours was superior in the no-fentanyl group by a margin of 3,441 ng/mL–1 per hour to 2,016 ng/mL–1 per hour. Moreover, 37% of fentanyl recipients displayed high platelet reactivity at 2 hours as measured by light transmission platelet aggregometry, compared with none of the no-fentanyl controls.
Pain was similarly well controlled in both treatment arms, casting doubt on the widespread belief among U.S. interventionalists that routine administration of fentanyl in the cath lab is necessary for patient comfort. Patients in the control arm could receive bailout fentanyl upon request; only two did so.
Dr. McEvoy reported having no financial conflicts regarding this study, which was conducted free of commercial support.
AT THE ESC CONGRESS 2017
Key clinical point:
Major finding: High platelet reactivity at 2 hours was present in 37% of patients who underwent coronary angiography with IV fentanyl and in none randomized to going without the opiate.
Data source: PACIFY, a single-center, double-blind, randomized trial included 212 patients undergoing coronary angiography.
Disclosures: The presenter reported having no financial conflicts regarding this study, which was conducted free of commercial support.
Flu study shows overall efficacy of LAIV, but weakness for one strain
Trivalent and quadrivalent inactivated influenza vaccine (IIV) and quadrivalent live attenuated influenza vaccine (LAIV) all gave statistically significant protection against any flu in U.S. children aged 2-17 years in 2015-2016, Katherine A. Poehling, MD, of Wake Forest University, Winston-Salem, N.C., and her associates reported in a study of more than 1,000 children.
“This study also adds to the clinical evidence suggesting that ,” the researchers concluded.
“The 2015-2016 season northern hemisphere trivalent IIV included A/California/7/2009 (H1N1)-like virus, a new A/Switzerland/9715293/2013 (H3N2)-like virus, and a new B/Phuket/3073/2013-like virus (Yamagata lineage),” the investigators noted. “Quadrivalent IIV was similar to trivalent IIV and also included B/Brisbane/60/2008-like virus (Victoria lineage). LAIV was similar to quadrivalent IIV, except that it contained A/Bolivia/559/2013.”
Of the 1,012 children enrolled, 59% were unvaccinated, 10% were given LAIV, 10% received trivalent IIV, 20% were given quadrivalent IIV, and 1% received IIV of “unknown valence.”
Vaccine efficacy against any influenza was 46% for LAIV and 65% for IIV, compared with no vaccination. But only IIV gave “significant protection against influenza A(H1N1)pdm09 strains in the total study population,” Dr. Poehling and her associates said. Vaccine efficacy against influenza A(H1N1)pdm09 strains was 50% for LAIV and 71% for IIV.
Read more in Clinical Infectious Diseases (2017 Oct 4. doi: 10.1093/cid/cix869).
Trivalent and quadrivalent inactivated influenza vaccine (IIV) and quadrivalent live attenuated influenza vaccine (LAIV) all gave statistically significant protection against any flu in U.S. children aged 2-17 years in 2015-2016, Katherine A. Poehling, MD, of Wake Forest University, Winston-Salem, N.C., and her associates reported in a study of more than 1,000 children.
“This study also adds to the clinical evidence suggesting that ,” the researchers concluded.
“The 2015-2016 season northern hemisphere trivalent IIV included A/California/7/2009 (H1N1)-like virus, a new A/Switzerland/9715293/2013 (H3N2)-like virus, and a new B/Phuket/3073/2013-like virus (Yamagata lineage),” the investigators noted. “Quadrivalent IIV was similar to trivalent IIV and also included B/Brisbane/60/2008-like virus (Victoria lineage). LAIV was similar to quadrivalent IIV, except that it contained A/Bolivia/559/2013.”
Of the 1,012 children enrolled, 59% were unvaccinated, 10% were given LAIV, 10% received trivalent IIV, 20% were given quadrivalent IIV, and 1% received IIV of “unknown valence.”
Vaccine efficacy against any influenza was 46% for LAIV and 65% for IIV, compared with no vaccination. But only IIV gave “significant protection against influenza A(H1N1)pdm09 strains in the total study population,” Dr. Poehling and her associates said. Vaccine efficacy against influenza A(H1N1)pdm09 strains was 50% for LAIV and 71% for IIV.
Read more in Clinical Infectious Diseases (2017 Oct 4. doi: 10.1093/cid/cix869).
Trivalent and quadrivalent inactivated influenza vaccine (IIV) and quadrivalent live attenuated influenza vaccine (LAIV) all gave statistically significant protection against any flu in U.S. children aged 2-17 years in 2015-2016, Katherine A. Poehling, MD, of Wake Forest University, Winston-Salem, N.C., and her associates reported in a study of more than 1,000 children.
“This study also adds to the clinical evidence suggesting that ,” the researchers concluded.
“The 2015-2016 season northern hemisphere trivalent IIV included A/California/7/2009 (H1N1)-like virus, a new A/Switzerland/9715293/2013 (H3N2)-like virus, and a new B/Phuket/3073/2013-like virus (Yamagata lineage),” the investigators noted. “Quadrivalent IIV was similar to trivalent IIV and also included B/Brisbane/60/2008-like virus (Victoria lineage). LAIV was similar to quadrivalent IIV, except that it contained A/Bolivia/559/2013.”
Of the 1,012 children enrolled, 59% were unvaccinated, 10% were given LAIV, 10% received trivalent IIV, 20% were given quadrivalent IIV, and 1% received IIV of “unknown valence.”
Vaccine efficacy against any influenza was 46% for LAIV and 65% for IIV, compared with no vaccination. But only IIV gave “significant protection against influenza A(H1N1)pdm09 strains in the total study population,” Dr. Poehling and her associates said. Vaccine efficacy against influenza A(H1N1)pdm09 strains was 50% for LAIV and 71% for IIV.
Read more in Clinical Infectious Diseases (2017 Oct 4. doi: 10.1093/cid/cix869).
FROM CLINICAL INFECTIOUS DISEASES
Pilot study: Novel spray powder stops GI bleeding
ORLANDO – TC-325 (Hemospray), a proprietary mineral powder blend developed for endoscopic hemostasis, promoted immediate hemostasis and prevented rebleeding in patients with malignant gastrointestinal bleeding in a randomized pilot trial.
Nine of 10 patients randomized to receive treatment with TC-325 experienced immediate hemostasis, compared with 4 of 10 patients randomized to receive standard of care (usually argon plasma coagulation, sometimes with radiation therapy), Alan Barkun, MD, of McGill University, Montreal reported at the World Congress of Gastroenterology at ACG 2017.
Five of six patients in the standard of care group who did not achieve immediate hemostasis crossed over to TC-325. Hemostasis was then achieved at index endoscopy in 80% of these crossovers, said Dr. Barkun, whose work received the 2017 GI Bleeding Category Award at the congress.
“So a total of 15 patients were treated with Hemospray among both groups, and 100% of them achieved immediate hemostasis,” he said. “We also assessed feasibility of recruitment and randomization, and it was indeed demonstrated in the context of this feasibility trial.”
Secondary measures, including the use of additional hemostatic approaches, blood transfusions, length of stay, and mortality, among others, did not differ between the two groups.
“This pilot trial is the first to assess TC-325 in patients with malignant bleeding, allowing us to plan for adequate powering and demonstrating feasibility for a larger multicenter, randomized, controlled trial,” he said. “Although this trial was not powered to seek statistically significant differences, the observed results suggest that TC-325 may indeed be a promising hemostatic modality in managing patients with malignant bleeding in achieving both immediate hemostasis and in our minds, surprisingly, perhaps delayed rebleeding.”
Hemospray, which is approved in Canada for upper/lower gastrointestinal bleeding of any etiology, as well as in Mexico and in some countries in Europe, Asia, and South America, works by forming a mechanical barrier over the bleeding site. The powder absorbs water, then acts both cohesively and adhesively to form that barrier, according to information from Cook Medical, which developed the product. It is not currently approved for this indication in the United States.
“An adequately powered randomized, controlled trial is now needed to better determine any beneficial downstream effect on subsequent rebleeding and health care resource use when compared to existing standard of care,” he concluded.
Dr. Barkun is an advisory committee/board member and consultant for Cook Medical and has received grant/research support from the company.
ORLANDO – TC-325 (Hemospray), a proprietary mineral powder blend developed for endoscopic hemostasis, promoted immediate hemostasis and prevented rebleeding in patients with malignant gastrointestinal bleeding in a randomized pilot trial.
Nine of 10 patients randomized to receive treatment with TC-325 experienced immediate hemostasis, compared with 4 of 10 patients randomized to receive standard of care (usually argon plasma coagulation, sometimes with radiation therapy), Alan Barkun, MD, of McGill University, Montreal reported at the World Congress of Gastroenterology at ACG 2017.
Five of six patients in the standard of care group who did not achieve immediate hemostasis crossed over to TC-325. Hemostasis was then achieved at index endoscopy in 80% of these crossovers, said Dr. Barkun, whose work received the 2017 GI Bleeding Category Award at the congress.
“So a total of 15 patients were treated with Hemospray among both groups, and 100% of them achieved immediate hemostasis,” he said. “We also assessed feasibility of recruitment and randomization, and it was indeed demonstrated in the context of this feasibility trial.”
Secondary measures, including the use of additional hemostatic approaches, blood transfusions, length of stay, and mortality, among others, did not differ between the two groups.
“This pilot trial is the first to assess TC-325 in patients with malignant bleeding, allowing us to plan for adequate powering and demonstrating feasibility for a larger multicenter, randomized, controlled trial,” he said. “Although this trial was not powered to seek statistically significant differences, the observed results suggest that TC-325 may indeed be a promising hemostatic modality in managing patients with malignant bleeding in achieving both immediate hemostasis and in our minds, surprisingly, perhaps delayed rebleeding.”
Hemospray, which is approved in Canada for upper/lower gastrointestinal bleeding of any etiology, as well as in Mexico and in some countries in Europe, Asia, and South America, works by forming a mechanical barrier over the bleeding site. The powder absorbs water, then acts both cohesively and adhesively to form that barrier, according to information from Cook Medical, which developed the product. It is not currently approved for this indication in the United States.
“An adequately powered randomized, controlled trial is now needed to better determine any beneficial downstream effect on subsequent rebleeding and health care resource use when compared to existing standard of care,” he concluded.
Dr. Barkun is an advisory committee/board member and consultant for Cook Medical and has received grant/research support from the company.
ORLANDO – TC-325 (Hemospray), a proprietary mineral powder blend developed for endoscopic hemostasis, promoted immediate hemostasis and prevented rebleeding in patients with malignant gastrointestinal bleeding in a randomized pilot trial.
Nine of 10 patients randomized to receive treatment with TC-325 experienced immediate hemostasis, compared with 4 of 10 patients randomized to receive standard of care (usually argon plasma coagulation, sometimes with radiation therapy), Alan Barkun, MD, of McGill University, Montreal reported at the World Congress of Gastroenterology at ACG 2017.
Five of six patients in the standard of care group who did not achieve immediate hemostasis crossed over to TC-325. Hemostasis was then achieved at index endoscopy in 80% of these crossovers, said Dr. Barkun, whose work received the 2017 GI Bleeding Category Award at the congress.
“So a total of 15 patients were treated with Hemospray among both groups, and 100% of them achieved immediate hemostasis,” he said. “We also assessed feasibility of recruitment and randomization, and it was indeed demonstrated in the context of this feasibility trial.”
Secondary measures, including the use of additional hemostatic approaches, blood transfusions, length of stay, and mortality, among others, did not differ between the two groups.
“This pilot trial is the first to assess TC-325 in patients with malignant bleeding, allowing us to plan for adequate powering and demonstrating feasibility for a larger multicenter, randomized, controlled trial,” he said. “Although this trial was not powered to seek statistically significant differences, the observed results suggest that TC-325 may indeed be a promising hemostatic modality in managing patients with malignant bleeding in achieving both immediate hemostasis and in our minds, surprisingly, perhaps delayed rebleeding.”
Hemospray, which is approved in Canada for upper/lower gastrointestinal bleeding of any etiology, as well as in Mexico and in some countries in Europe, Asia, and South America, works by forming a mechanical barrier over the bleeding site. The powder absorbs water, then acts both cohesively and adhesively to form that barrier, according to information from Cook Medical, which developed the product. It is not currently approved for this indication in the United States.
“An adequately powered randomized, controlled trial is now needed to better determine any beneficial downstream effect on subsequent rebleeding and health care resource use when compared to existing standard of care,” he concluded.
Dr. Barkun is an advisory committee/board member and consultant for Cook Medical and has received grant/research support from the company.
AT THE WORLD CONGRESS OF GASTROENTEROLOGY
Key clinical point:
Major finding: All 15 patients treated with Hemospray achieved immediate hemostasis.
Data source: A randomized pilot study of 20 patients.
Disclosures: Dr. Barkun is an advisory committee/board member and consultant for Cook Medical and has received grant/research support from the company.
Make The Diagnosis - November 2017
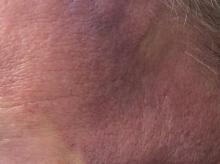
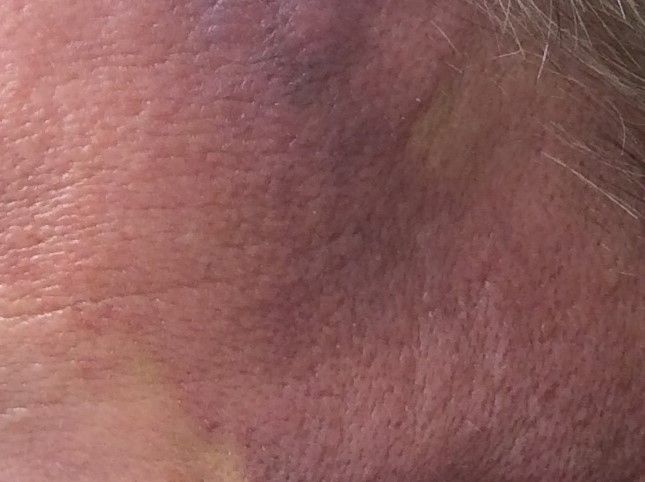
Angiosarcoma is also known as malignant hemangioendothelioma, hemangiosarcoma, and lymphangiosarcoma. It is an uncommon, high-grade malignant vascular neoplasm of the inner lining of blood vessels. Unlike most sarcomas, it occurs more superficially, most often on the head and neck (particularly on the scalp). This neoplasm occurs twice as often in males as it does in females. Angiosarcomas can occur in the breast after radiation therapy, as well as in the liver and spleen, but 60% are cutaneous.
Clinical exam findings may show a violaceous lesion similar to a bruise on the head and neck that does not heal or bleeds when scratched; this is of particular concern when the lesion has appeared in an area of prior radiation therapy. Deeper tumors may be felt as a soft nodule. Ulceration may be present. Biopsy of the lesion will show hyperchromatic, pleomorphic tumor cells that dissect between collagen bundles with endothelial cells that are multilayered along with hemorrhage. Malignant cells stain positive for CD31, CD34, ERG, and FLI1.
For localized disease, surgery with wide local excision plus adjuvant radiation therapy can be used. For metastatic disease, chemotherapy is the treatment modality of choice. Unfortunately, prognosis is poor, with a 5-year survival rate of about 35% in nonmetastatic angiosarcoma cases. The majority of recurrences – approximately 75% – occur within 24 months of local treatment.
This case and photo were submitted by Parteek Singla, MD, of the division of dermatology at Washington University and at Barnes-Jewish Hospital, both in St. Louis, and by Susannah McClain, MD, of Three Rivers Dermatology, Coraopolis, Pa.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
Angiosarcoma is also known as malignant hemangioendothelioma, hemangiosarcoma, and lymphangiosarcoma. It is an uncommon, high-grade malignant vascular neoplasm of the inner lining of blood vessels. Unlike most sarcomas, it occurs more superficially, most often on the head and neck (particularly on the scalp). This neoplasm occurs twice as often in males as it does in females. Angiosarcomas can occur in the breast after radiation therapy, as well as in the liver and spleen, but 60% are cutaneous.
Clinical exam findings may show a violaceous lesion similar to a bruise on the head and neck that does not heal or bleeds when scratched; this is of particular concern when the lesion has appeared in an area of prior radiation therapy. Deeper tumors may be felt as a soft nodule. Ulceration may be present. Biopsy of the lesion will show hyperchromatic, pleomorphic tumor cells that dissect between collagen bundles with endothelial cells that are multilayered along with hemorrhage. Malignant cells stain positive for CD31, CD34, ERG, and FLI1.
For localized disease, surgery with wide local excision plus adjuvant radiation therapy can be used. For metastatic disease, chemotherapy is the treatment modality of choice. Unfortunately, prognosis is poor, with a 5-year survival rate of about 35% in nonmetastatic angiosarcoma cases. The majority of recurrences – approximately 75% – occur within 24 months of local treatment.
This case and photo were submitted by Parteek Singla, MD, of the division of dermatology at Washington University and at Barnes-Jewish Hospital, both in St. Louis, and by Susannah McClain, MD, of Three Rivers Dermatology, Coraopolis, Pa.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
Angiosarcoma is also known as malignant hemangioendothelioma, hemangiosarcoma, and lymphangiosarcoma. It is an uncommon, high-grade malignant vascular neoplasm of the inner lining of blood vessels. Unlike most sarcomas, it occurs more superficially, most often on the head and neck (particularly on the scalp). This neoplasm occurs twice as often in males as it does in females. Angiosarcomas can occur in the breast after radiation therapy, as well as in the liver and spleen, but 60% are cutaneous.
Clinical exam findings may show a violaceous lesion similar to a bruise on the head and neck that does not heal or bleeds when scratched; this is of particular concern when the lesion has appeared in an area of prior radiation therapy. Deeper tumors may be felt as a soft nodule. Ulceration may be present. Biopsy of the lesion will show hyperchromatic, pleomorphic tumor cells that dissect between collagen bundles with endothelial cells that are multilayered along with hemorrhage. Malignant cells stain positive for CD31, CD34, ERG, and FLI1.
For localized disease, surgery with wide local excision plus adjuvant radiation therapy can be used. For metastatic disease, chemotherapy is the treatment modality of choice. Unfortunately, prognosis is poor, with a 5-year survival rate of about 35% in nonmetastatic angiosarcoma cases. The majority of recurrences – approximately 75% – occur within 24 months of local treatment.
This case and photo were submitted by Parteek Singla, MD, of the division of dermatology at Washington University and at Barnes-Jewish Hospital, both in St. Louis, and by Susannah McClain, MD, of Three Rivers Dermatology, Coraopolis, Pa.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
A 62-year-old healthy man presented with a skin lesion located on the left scalp. The lesion was swollen and painful and had been present for 4 months. This lesion had not been treated in the past.