User login
FDA to rely on foreign authorities’ inspections
The US Food and Drug Administration (FDA) will now recognize 8 European drug regulatory authorities as capable of conducting inspections of manufacturing facilities that meet FDA requirements.
This move is a step toward successful implementation of the amended Pharmaceutical Annex to the 1998 US-European Union (EU) Mutual Recognition Agreement.
This agreement enables US and EU regulators to utilize each other’s good manufacturing practice (GMP) inspections of pharmaceutical manufacturing facilities.
In June, the European Commission determined that the FDA “has the capability, capacity, and procedures in place to carry out GMP inspections at a level equivalent to the EU.”
Now, the FDA has followed suit. The agency said it will rely on inspections of manufacturing facilities conducted by regulatory authorities located in Austria, Croatia, France, Italy, Malta, Spain, Sweden, and the United Kingdom.
“Beginning November 1, we will take the unprecedented and significant step forward in realizing the key benefits of the Mutual Recognition Agreement with our European counterparts in that we will now rely on the inspectional data obtained by these 8 regulatory agencies,” said Dara Corrigan, the FDA’s acting deputy commissioner for global regulatory operations and policy.
“The progress made so far puts us on track to meet our goal of completing all 28 capability assessments in the EU by July 2019.”
The completion of these capability assessments is intended to reduce duplication of drug inspections and allow regulators to devote more resources to other manufacturing facilities in countries where there may be greater risk.
The FDA believes that, ultimately, the prioritization of such inspections will help identify potential drug quality problems more quickly and prevent poor quality drugs from entering the US market.
“At a time in which medical product manufacturing is truly a global enterprise, there is much to be gained by partnering with regulatory counterparts to reduce duplicative efforts and maximize global resources while realizing the greatest bang for our collective inspectional buck,” said FDA Commissioner Scott Gottlieb, MD.
“By partnering with these countries, we can create greater efficiencies and better fulfill our public health goals, relying on the expertise of our colleagues and refocusing our resources on inspections in higher-risk countries.” ![]()
The US Food and Drug Administration (FDA) will now recognize 8 European drug regulatory authorities as capable of conducting inspections of manufacturing facilities that meet FDA requirements.
This move is a step toward successful implementation of the amended Pharmaceutical Annex to the 1998 US-European Union (EU) Mutual Recognition Agreement.
This agreement enables US and EU regulators to utilize each other’s good manufacturing practice (GMP) inspections of pharmaceutical manufacturing facilities.
In June, the European Commission determined that the FDA “has the capability, capacity, and procedures in place to carry out GMP inspections at a level equivalent to the EU.”
Now, the FDA has followed suit. The agency said it will rely on inspections of manufacturing facilities conducted by regulatory authorities located in Austria, Croatia, France, Italy, Malta, Spain, Sweden, and the United Kingdom.
“Beginning November 1, we will take the unprecedented and significant step forward in realizing the key benefits of the Mutual Recognition Agreement with our European counterparts in that we will now rely on the inspectional data obtained by these 8 regulatory agencies,” said Dara Corrigan, the FDA’s acting deputy commissioner for global regulatory operations and policy.
“The progress made so far puts us on track to meet our goal of completing all 28 capability assessments in the EU by July 2019.”
The completion of these capability assessments is intended to reduce duplication of drug inspections and allow regulators to devote more resources to other manufacturing facilities in countries where there may be greater risk.
The FDA believes that, ultimately, the prioritization of such inspections will help identify potential drug quality problems more quickly and prevent poor quality drugs from entering the US market.
“At a time in which medical product manufacturing is truly a global enterprise, there is much to be gained by partnering with regulatory counterparts to reduce duplicative efforts and maximize global resources while realizing the greatest bang for our collective inspectional buck,” said FDA Commissioner Scott Gottlieb, MD.
“By partnering with these countries, we can create greater efficiencies and better fulfill our public health goals, relying on the expertise of our colleagues and refocusing our resources on inspections in higher-risk countries.” ![]()
The US Food and Drug Administration (FDA) will now recognize 8 European drug regulatory authorities as capable of conducting inspections of manufacturing facilities that meet FDA requirements.
This move is a step toward successful implementation of the amended Pharmaceutical Annex to the 1998 US-European Union (EU) Mutual Recognition Agreement.
This agreement enables US and EU regulators to utilize each other’s good manufacturing practice (GMP) inspections of pharmaceutical manufacturing facilities.
In June, the European Commission determined that the FDA “has the capability, capacity, and procedures in place to carry out GMP inspections at a level equivalent to the EU.”
Now, the FDA has followed suit. The agency said it will rely on inspections of manufacturing facilities conducted by regulatory authorities located in Austria, Croatia, France, Italy, Malta, Spain, Sweden, and the United Kingdom.
“Beginning November 1, we will take the unprecedented and significant step forward in realizing the key benefits of the Mutual Recognition Agreement with our European counterparts in that we will now rely on the inspectional data obtained by these 8 regulatory agencies,” said Dara Corrigan, the FDA’s acting deputy commissioner for global regulatory operations and policy.
“The progress made so far puts us on track to meet our goal of completing all 28 capability assessments in the EU by July 2019.”
The completion of these capability assessments is intended to reduce duplication of drug inspections and allow regulators to devote more resources to other manufacturing facilities in countries where there may be greater risk.
The FDA believes that, ultimately, the prioritization of such inspections will help identify potential drug quality problems more quickly and prevent poor quality drugs from entering the US market.
“At a time in which medical product manufacturing is truly a global enterprise, there is much to be gained by partnering with regulatory counterparts to reduce duplicative efforts and maximize global resources while realizing the greatest bang for our collective inspectional buck,” said FDA Commissioner Scott Gottlieb, MD.
“By partnering with these countries, we can create greater efficiencies and better fulfill our public health goals, relying on the expertise of our colleagues and refocusing our resources on inspections in higher-risk countries.” ![]()
Beyond medication for the Tx of chronic pain
A tribute to David Warfield Stires, JFP’s founding publisher
The recent passing of the founding publisher of The Journal of Family Practice, David Warfield Stires, is an occasion to honor and celebrate his support of, and dedication to, the specialty of family medicine.
David and I began working together in 1970. That was one year after family medicine was recognized as the 20th medical specialty in the United States. It was also a year after I left my solo rural family practice in Mount Shasta, Calif. to convert the general practice residency at Sonoma County Hospital, Santa Rosa, to a 3-year family practice residency affiliated with the University of California San Francisco School of Medicine.
In 1970, I’d just completed my first book manuscript, “The Modern Family Doctor and Changing Medical Practice,” and I went searching for a publisher for it. After 2 rejections, I approached David, who was the president of Appleton-Century-Crofts, the second largest medical publisher in the country. He grew up in a small town near Canton, Ohio, and his father had been a general practitioner and a real country doctor. David immediately saw the value of my book, and our lifelong friendship began.
There was no academic journal in the field of family medicine at that time. The only thing that came close was the American Academy of Family Physicians’ journal for summary CME articles, American Family Physician. As we got to talking, David saw the need to expand the field’s literature base to articulate its academic discipline and report original research. We soon held an organizational meeting of a new editorial board in San Francisco. And in 1974, The Journal of Family Practice was “born” with Appleton-Century-Crofts as its publisher.
Because we had very little startup funding, we depended on advertising to enable us to send the journal to all general and family physicians in the United States. In those early years, advertising income was sufficient to maintain the journal. But with increasing pressure to bring in more and more ad dollars, JFP was bought and sold over the next 16 years. And in 1990, I left as editor and began my stint as editor of the Journal of the American Board of Family Practice (now Family Medicine).
After more than 30 years in publishing, David and his wife, Wendy, moved to Albuquerque, New Mexico, where he pursued his lifelong interest in photography, and where his work was regularly shown in galleries. He and I saw each other frequently over the years, often visiting in the Pacific Northwest. Beyond the many books that he published, he was most proud of creating JFP.
Today, 43 years later, David’s legacy lives on in a vibrant journal and medical specialty. Thank you, David, for your lifelong support of family medicine and for your friendship.
John Geyman, MD
Friday Harbor, Wash.
Editor’s response
Dr. John Geyman’s tribute to The Journal of Family Practice’s founding publisher, David Warfield Stires, provides me with the opportunity to do 2 things.
First, to thank John for his visionary leadership in founding and guiding the successful development of the first research journal for family medicine in the United States. (In 1970, family medicine was called “family practice,” hence our name The Journal of Family Practice—a name we have maintained over the years because of its “recognition factor.”) Much of the original US family medicine research of the 1970s, ‘80s, and ‘90s was published in JFP. I still remember the thrill of having my first research study published in JFP in 1983.1
Second, I want to remind our readers that although our focus has changed to mostly evidence-based clinical reviews, we remain firmly rooted in practical research that informs the everyday practice of family medicine and primary care. We still publish (albeit a limited number) of original research studies that have high practical value to primary care, such as a recent article on the use of medical scribes.2 This is largely due to the foresight and vision of pioneers in this field like David Warfield Stires and Dr. John Geyman.
John Hickner, MD, MSc
1. Messimer S, Hickner J. Oral fluoride supplementation: improving practitioner compliance by using a protocol. J Fam Pract. 1983;17:821-825.
2. Earls ST, Savageau JA, Begley S, et al. Can scribes boost FPs’ efficiency and job satisfaction? J Fam Pract. 2017;66:206-214.
The recent passing of the founding publisher of The Journal of Family Practice, David Warfield Stires, is an occasion to honor and celebrate his support of, and dedication to, the specialty of family medicine.
David and I began working together in 1970. That was one year after family medicine was recognized as the 20th medical specialty in the United States. It was also a year after I left my solo rural family practice in Mount Shasta, Calif. to convert the general practice residency at Sonoma County Hospital, Santa Rosa, to a 3-year family practice residency affiliated with the University of California San Francisco School of Medicine.
In 1970, I’d just completed my first book manuscript, “The Modern Family Doctor and Changing Medical Practice,” and I went searching for a publisher for it. After 2 rejections, I approached David, who was the president of Appleton-Century-Crofts, the second largest medical publisher in the country. He grew up in a small town near Canton, Ohio, and his father had been a general practitioner and a real country doctor. David immediately saw the value of my book, and our lifelong friendship began.
There was no academic journal in the field of family medicine at that time. The only thing that came close was the American Academy of Family Physicians’ journal for summary CME articles, American Family Physician. As we got to talking, David saw the need to expand the field’s literature base to articulate its academic discipline and report original research. We soon held an organizational meeting of a new editorial board in San Francisco. And in 1974, The Journal of Family Practice was “born” with Appleton-Century-Crofts as its publisher.
Because we had very little startup funding, we depended on advertising to enable us to send the journal to all general and family physicians in the United States. In those early years, advertising income was sufficient to maintain the journal. But with increasing pressure to bring in more and more ad dollars, JFP was bought and sold over the next 16 years. And in 1990, I left as editor and began my stint as editor of the Journal of the American Board of Family Practice (now Family Medicine).
After more than 30 years in publishing, David and his wife, Wendy, moved to Albuquerque, New Mexico, where he pursued his lifelong interest in photography, and where his work was regularly shown in galleries. He and I saw each other frequently over the years, often visiting in the Pacific Northwest. Beyond the many books that he published, he was most proud of creating JFP.
Today, 43 years later, David’s legacy lives on in a vibrant journal and medical specialty. Thank you, David, for your lifelong support of family medicine and for your friendship.
John Geyman, MD
Friday Harbor, Wash.
Editor’s response
Dr. John Geyman’s tribute to The Journal of Family Practice’s founding publisher, David Warfield Stires, provides me with the opportunity to do 2 things.
First, to thank John for his visionary leadership in founding and guiding the successful development of the first research journal for family medicine in the United States. (In 1970, family medicine was called “family practice,” hence our name The Journal of Family Practice—a name we have maintained over the years because of its “recognition factor.”) Much of the original US family medicine research of the 1970s, ‘80s, and ‘90s was published in JFP. I still remember the thrill of having my first research study published in JFP in 1983.1
Second, I want to remind our readers that although our focus has changed to mostly evidence-based clinical reviews, we remain firmly rooted in practical research that informs the everyday practice of family medicine and primary care. We still publish (albeit a limited number) of original research studies that have high practical value to primary care, such as a recent article on the use of medical scribes.2 This is largely due to the foresight and vision of pioneers in this field like David Warfield Stires and Dr. John Geyman.
John Hickner, MD, MSc
The recent passing of the founding publisher of The Journal of Family Practice, David Warfield Stires, is an occasion to honor and celebrate his support of, and dedication to, the specialty of family medicine.
David and I began working together in 1970. That was one year after family medicine was recognized as the 20th medical specialty in the United States. It was also a year after I left my solo rural family practice in Mount Shasta, Calif. to convert the general practice residency at Sonoma County Hospital, Santa Rosa, to a 3-year family practice residency affiliated with the University of California San Francisco School of Medicine.
In 1970, I’d just completed my first book manuscript, “The Modern Family Doctor and Changing Medical Practice,” and I went searching for a publisher for it. After 2 rejections, I approached David, who was the president of Appleton-Century-Crofts, the second largest medical publisher in the country. He grew up in a small town near Canton, Ohio, and his father had been a general practitioner and a real country doctor. David immediately saw the value of my book, and our lifelong friendship began.
There was no academic journal in the field of family medicine at that time. The only thing that came close was the American Academy of Family Physicians’ journal for summary CME articles, American Family Physician. As we got to talking, David saw the need to expand the field’s literature base to articulate its academic discipline and report original research. We soon held an organizational meeting of a new editorial board in San Francisco. And in 1974, The Journal of Family Practice was “born” with Appleton-Century-Crofts as its publisher.
Because we had very little startup funding, we depended on advertising to enable us to send the journal to all general and family physicians in the United States. In those early years, advertising income was sufficient to maintain the journal. But with increasing pressure to bring in more and more ad dollars, JFP was bought and sold over the next 16 years. And in 1990, I left as editor and began my stint as editor of the Journal of the American Board of Family Practice (now Family Medicine).
After more than 30 years in publishing, David and his wife, Wendy, moved to Albuquerque, New Mexico, where he pursued his lifelong interest in photography, and where his work was regularly shown in galleries. He and I saw each other frequently over the years, often visiting in the Pacific Northwest. Beyond the many books that he published, he was most proud of creating JFP.
Today, 43 years later, David’s legacy lives on in a vibrant journal and medical specialty. Thank you, David, for your lifelong support of family medicine and for your friendship.
John Geyman, MD
Friday Harbor, Wash.
Editor’s response
Dr. John Geyman’s tribute to The Journal of Family Practice’s founding publisher, David Warfield Stires, provides me with the opportunity to do 2 things.
First, to thank John for his visionary leadership in founding and guiding the successful development of the first research journal for family medicine in the United States. (In 1970, family medicine was called “family practice,” hence our name The Journal of Family Practice—a name we have maintained over the years because of its “recognition factor.”) Much of the original US family medicine research of the 1970s, ‘80s, and ‘90s was published in JFP. I still remember the thrill of having my first research study published in JFP in 1983.1
Second, I want to remind our readers that although our focus has changed to mostly evidence-based clinical reviews, we remain firmly rooted in practical research that informs the everyday practice of family medicine and primary care. We still publish (albeit a limited number) of original research studies that have high practical value to primary care, such as a recent article on the use of medical scribes.2 This is largely due to the foresight and vision of pioneers in this field like David Warfield Stires and Dr. John Geyman.
John Hickner, MD, MSc
1. Messimer S, Hickner J. Oral fluoride supplementation: improving practitioner compliance by using a protocol. J Fam Pract. 1983;17:821-825.
2. Earls ST, Savageau JA, Begley S, et al. Can scribes boost FPs’ efficiency and job satisfaction? J Fam Pract. 2017;66:206-214.
1. Messimer S, Hickner J. Oral fluoride supplementation: improving practitioner compliance by using a protocol. J Fam Pract. 1983;17:821-825.
2. Earls ST, Savageau JA, Begley S, et al. Can scribes boost FPs’ efficiency and job satisfaction? J Fam Pract. 2017;66:206-214.
Vaping marijuana?
Cannavaping—the inhalation of a cannabis-containing aerosol, created by a battery-driven, heated atomizer in e-cigarettes or similar devices1—is touted as a less expensive and safer alternative to smoking marijuana. It’s also gaining in popularity.2 One study of Connecticut high school students found that 5.4% had used e-cigarettes to vaporize cannabis.3 But what do we know about this new way to get high?
We know that those who wish to cannavape can easily obtain e-cigarettes from gas stations and tobacco shops. They then have to obtain a cartridge, filled with either hash oil or tetrahydrocannabinol-infused wax, to attach to the e-cigarette. These cartridges are available for purchase in states that have legalized the sale of marijuana. They also find their way into states where the sale of marijuana is not legal, and are purchased illegally for the purpose of cannavaping.
And while cannavaping does appear to reduce the cost of smoking marijuana,4 it has not been widely researched, nor determined to be safe.5
In fact, although marijuana has several important therapeutic and medicinal purposes, cannavaping the substance can result in medical concerns.6 The vaping aerosols of some compounds can induce lung pathology and may be carcinogenic, since they often contain a number of dangerous toxins.4
Chronic marijuana use can increase the likelihood of motor vehicles accidents, cognitive impairment, psychoses, and demotivation.4 It may predispose certain individuals to use other drugs and tobacco products and could increase the consumption of marijuana.4,5 Increased consumption could have a detrimental effect on intellect and behavior when used chronically—especially in youngsters, whose nervous systems are not yet fully matured.7-9
Because cannavaping has potentially deleterious effects, more regulations on the manufacture, distribution, access, and use are indicated—at least until research sheds more light on issues surrounding this practice.
Steven Lippman, MD; Devina Singh, MD
Louisville, KY
1. Varlet V, Concha-Lozano N, Berthlet A, et al. Drug vaping applied to cannabis: is “cannavaping” a therapeutic alternative to marijuana? Sci Rep. 2016;6:25599.
2. Giroud C, de Cesare M, Berthet A, et al. E-cigarettes: a review of new trends in cannabis use. Int J Environ Res Public Health. 2015;12:9988-10008.
3. Morean ME, Kong G, Camenga DR, et al. High school students’ use of electronic cigarettes to vaporize cannabis. Pediatrics. 2015;136:611-616.
4. Budney AJ, Sargent JD, Lee DC. Vaping cannabis (marijuana): parallel concerns to e-cigs? Addiction. 2015;110:1699-1704.
5. Cox B. Can the research community respond adequately to the health risks of vaping? Addiction. 2015;110:1709-1709.
6. Rong C, Lee Y, Carmona NE, et al. Cannabidiol in medical marijuana: research vistas and potential opportunities. Pharmacol Res. 2017;121:213-218.
7. Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Curr Drug Abuse Rev. 2008;1:99-111.
8. Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci USA. 2012;109:E2657-2664.
9. Castellanos-Ryan N, Pingault J, Parent S, et al. Adolescent cannabis use, change in neurocognitive function, and high-school graduation: a longitudinal study from early adolescence to young adulthood. Dev Psychopathol . 2017;29:1253-1266.
Cannavaping—the inhalation of a cannabis-containing aerosol, created by a battery-driven, heated atomizer in e-cigarettes or similar devices1—is touted as a less expensive and safer alternative to smoking marijuana. It’s also gaining in popularity.2 One study of Connecticut high school students found that 5.4% had used e-cigarettes to vaporize cannabis.3 But what do we know about this new way to get high?
We know that those who wish to cannavape can easily obtain e-cigarettes from gas stations and tobacco shops. They then have to obtain a cartridge, filled with either hash oil or tetrahydrocannabinol-infused wax, to attach to the e-cigarette. These cartridges are available for purchase in states that have legalized the sale of marijuana. They also find their way into states where the sale of marijuana is not legal, and are purchased illegally for the purpose of cannavaping.
And while cannavaping does appear to reduce the cost of smoking marijuana,4 it has not been widely researched, nor determined to be safe.5
In fact, although marijuana has several important therapeutic and medicinal purposes, cannavaping the substance can result in medical concerns.6 The vaping aerosols of some compounds can induce lung pathology and may be carcinogenic, since they often contain a number of dangerous toxins.4
Chronic marijuana use can increase the likelihood of motor vehicles accidents, cognitive impairment, psychoses, and demotivation.4 It may predispose certain individuals to use other drugs and tobacco products and could increase the consumption of marijuana.4,5 Increased consumption could have a detrimental effect on intellect and behavior when used chronically—especially in youngsters, whose nervous systems are not yet fully matured.7-9
Because cannavaping has potentially deleterious effects, more regulations on the manufacture, distribution, access, and use are indicated—at least until research sheds more light on issues surrounding this practice.
Steven Lippman, MD; Devina Singh, MD
Louisville, KY
Cannavaping—the inhalation of a cannabis-containing aerosol, created by a battery-driven, heated atomizer in e-cigarettes or similar devices1—is touted as a less expensive and safer alternative to smoking marijuana. It’s also gaining in popularity.2 One study of Connecticut high school students found that 5.4% had used e-cigarettes to vaporize cannabis.3 But what do we know about this new way to get high?
We know that those who wish to cannavape can easily obtain e-cigarettes from gas stations and tobacco shops. They then have to obtain a cartridge, filled with either hash oil or tetrahydrocannabinol-infused wax, to attach to the e-cigarette. These cartridges are available for purchase in states that have legalized the sale of marijuana. They also find their way into states where the sale of marijuana is not legal, and are purchased illegally for the purpose of cannavaping.
And while cannavaping does appear to reduce the cost of smoking marijuana,4 it has not been widely researched, nor determined to be safe.5
In fact, although marijuana has several important therapeutic and medicinal purposes, cannavaping the substance can result in medical concerns.6 The vaping aerosols of some compounds can induce lung pathology and may be carcinogenic, since they often contain a number of dangerous toxins.4
Chronic marijuana use can increase the likelihood of motor vehicles accidents, cognitive impairment, psychoses, and demotivation.4 It may predispose certain individuals to use other drugs and tobacco products and could increase the consumption of marijuana.4,5 Increased consumption could have a detrimental effect on intellect and behavior when used chronically—especially in youngsters, whose nervous systems are not yet fully matured.7-9
Because cannavaping has potentially deleterious effects, more regulations on the manufacture, distribution, access, and use are indicated—at least until research sheds more light on issues surrounding this practice.
Steven Lippman, MD; Devina Singh, MD
Louisville, KY
1. Varlet V, Concha-Lozano N, Berthlet A, et al. Drug vaping applied to cannabis: is “cannavaping” a therapeutic alternative to marijuana? Sci Rep. 2016;6:25599.
2. Giroud C, de Cesare M, Berthet A, et al. E-cigarettes: a review of new trends in cannabis use. Int J Environ Res Public Health. 2015;12:9988-10008.
3. Morean ME, Kong G, Camenga DR, et al. High school students’ use of electronic cigarettes to vaporize cannabis. Pediatrics. 2015;136:611-616.
4. Budney AJ, Sargent JD, Lee DC. Vaping cannabis (marijuana): parallel concerns to e-cigs? Addiction. 2015;110:1699-1704.
5. Cox B. Can the research community respond adequately to the health risks of vaping? Addiction. 2015;110:1709-1709.
6. Rong C, Lee Y, Carmona NE, et al. Cannabidiol in medical marijuana: research vistas and potential opportunities. Pharmacol Res. 2017;121:213-218.
7. Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Curr Drug Abuse Rev. 2008;1:99-111.
8. Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci USA. 2012;109:E2657-2664.
9. Castellanos-Ryan N, Pingault J, Parent S, et al. Adolescent cannabis use, change in neurocognitive function, and high-school graduation: a longitudinal study from early adolescence to young adulthood. Dev Psychopathol . 2017;29:1253-1266.
1. Varlet V, Concha-Lozano N, Berthlet A, et al. Drug vaping applied to cannabis: is “cannavaping” a therapeutic alternative to marijuana? Sci Rep. 2016;6:25599.
2. Giroud C, de Cesare M, Berthet A, et al. E-cigarettes: a review of new trends in cannabis use. Int J Environ Res Public Health. 2015;12:9988-10008.
3. Morean ME, Kong G, Camenga DR, et al. High school students’ use of electronic cigarettes to vaporize cannabis. Pediatrics. 2015;136:611-616.
4. Budney AJ, Sargent JD, Lee DC. Vaping cannabis (marijuana): parallel concerns to e-cigs? Addiction. 2015;110:1699-1704.
5. Cox B. Can the research community respond adequately to the health risks of vaping? Addiction. 2015;110:1709-1709.
6. Rong C, Lee Y, Carmona NE, et al. Cannabidiol in medical marijuana: research vistas and potential opportunities. Pharmacol Res. 2017;121:213-218.
7. Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Curr Drug Abuse Rev. 2008;1:99-111.
8. Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci USA. 2012;109:E2657-2664.
9. Castellanos-Ryan N, Pingault J, Parent S, et al. Adolescent cannabis use, change in neurocognitive function, and high-school graduation: a longitudinal study from early adolescence to young adulthood. Dev Psychopathol . 2017;29:1253-1266.
The benefits—and limits—of PPIs with warfarin regimens
ILLUSTRATIVE CASE
A 60-year-old man establishes care with you. He has well-controlled osteoarthritis (as long as he takes his low-dose daily aspirin) and chronic atrial fibrillation, for which he takes warfarin. His international normalized ratio (INR) is consistently within the recommended target range of 2 to 3. He feels well and has never had gastroesophageal reflux disease (GERD) or a gastrointestinal (GI) bleed. Should you recommend a proton pump inhibitor (PPI) to decrease the likelihood of a future upper GI bleed?
Anticoagulation therapy creates a dilemma—the need to balance the benefits of preventing embolization with the risks of serious bleeding. Concurrent use of nonsteroidal anti-inflammatory drugs (NSAIDs), aspirin, and other antiplatelet agents further increases the risk of the latter.2
Physicians have long used PPIs to treat upper GI bleeds. They prevent acid secretion and are the most efficacious drugs for healing peptic ulcers.3,4 However, while previous case-control studies show that PPIs reduce the risk of upper GI bleeds in patients taking antiplatelet agents or NSAIDs, they do not show a statistically significant benefit for patients taking warfarin.5,6 Further reflecting the confusion and uncertainty surrounding this issue is that while one expert consensus report recommends that patients taking dual warfarin and antiplatelet agent/NSAID therapy take a PPI to decrease the risk of upper GI bleeding,2 other guidelines regarding anticoagulant therapy do not address this clinical question.2,7,8
[polldaddy:9860876]
STUDY SUMMARY
Study lends support to PPI use in a high-risk group
This retrospective cohort study sought to answer the question: “Does PPI co-therapy decrease the rate of serious upper GI bleeds in patients taking warfarin?” Researchers examined rates of hospitalization for upper GI bleeding for Medicare and Medicaid patients taking warfarin with and without PPI co-therapy (tracked via prescription fill dates). They also evaluated concomitant use of NSAIDs and antiplatelet agents.
The authors excluded patients with a recent history of a severe bleed or certain illnesses that would predispose a patient to GI bleeding (such as esophageal varices). Patients with risk factors for an upper GI bleed (such as abdominal pain, peptic ulcer disease, anemia, etc.) were more likely to be taking PPI co-therapy. Researchers analyzed the effect of PPI co-therapy in patients with and without these additional risk factors.
Results. The study followed over 75,000 person-years of active warfarin therapy (more than 52,000 person-years in the Medicaid cohort and more than 23,000 person-years in the Medicare cohort). Hospitalizations due to upper GI bleeding occurred at a rate of 127/10,000 person-years (incidence was similar in both the Medicaid and Medicare groups).
Looking at all patients taking warfarin (regardless of whether or not they were also taking an NSAID or antiplatelet agent), PPI co-therapy reduced the risk of hospitalization for upper GI bleeding by 24% (adjusted hazard ratio [HR]=0.76; 95% confidence interval [CI], 0.63 to 0.91), which translates into 29 fewer hospitalizations per 10,000 person-years. The number needed to treat (NNT) was 345 person-years, meaning 345 patients taking warfarin would have to take a PPI for one year to prevent one hospitalization for an upper GI bleed. As one might expect, PPI co-therapy did not significantly reduce the risk of lower GI, other GI, or non-GI bleeding.
In patients taking both warfarin and concurrent antiplatelet agents or NSAIDs, PPI co-therapy reduced the risk of hospitalization for upper GI bleeding by about half (HR=0.55; 95% CI, 0.39-0.77). Hospitalizations decreased by 128/10,000 person-years (95% CI, -66 to -173), yielding an NNT of 78 person-years. For patients taking warfarin but not antiplatelet agents or NSAIDs, PPI co-therapy did not significantly decrease the risk of hospitalization for upper GI bleeding (HR=0.86; 95% CI, 0.70-1.06).
Additional risk factors for GI bleeds. Researchers also looked at patients who had additional risk factors for GI bleeds (other than the exclusion criteria). For patients taking both warfarin and an antiplatelet agent/NSAID, PPI co-therapy decreased the risk of upper GI bleeding whether or not the patients had other bleeding risk factors. Again, for patients who had additional bleeding risk factors, but were not taking an antiplatelet agent or NSAID, PPI therapy showed no statistically significant effect.
WHAT’S NEW
PPIs offer benefits, but not to patients taking warfarin alone
The statistically significant results in this large observational study suggest that PPI co-therapy is beneficial in reducing the risk of upper GI bleeding in patients taking warfarin plus an antiplatelet agent/NSAID, but that PPI co-therapy provides no benefit to patients taking warfarin exclusively.
CAVEATS
Study was good, but it wasn’t a randomized controlled trial
This study is observational, and not a randomized control trial (RCT). Therefore, unknown confounding variables may have skewed results. For example, patients could have taken over-the-counter medications that influenced or obscured results, but were not included in the data analysis (misclassification bias).
At best, we can infer a correlation between PPIs and decreased risk of upper GI bleeds. We need RCTs to determine whether PPIs cause a lower risk.
Don’t overlook the risk of PPIs. This study assessed the ability of PPIs to prevent bleeds, but did not address the risks of long-term PPI therapy. Adverse effects of PPIs include an increased risk of pneumonia, infection with Clostridium difficile, hip and spine fractures, anemia, and possibly chronic kidney disease and dementia.9-11 In addition, cost-analysis studies of PPI therapy are limited and inconsistent.12 Therefore, it’s best to make decisions regarding PPIs after discussing other risks and benefits.
What about DOACs? Another consideration is the option to prescribe a direct oral anticoagulant (DOAC), such as dabigatran, rivaroxaban, or apixaban, instead of warfarin. DOACs are at least as effective as warfarin at preventing stroke in patients with atrial fibrillation and may even be safer.13 Dabigatran 110 mg causes fewer “major bleeding” events than warfarin.13 Rivaroxaban has been shown to result in fewer fatal bleeding events than warfarin due to fatal intracranial bleeds, although it is associated with more GI bleedding.13 Compared with warfarin, apixaban is associated with fewer GI bleeds and lower bleeding rates overall.13 Further research is warranted to determine if PPI therapy is beneficial to patients taking DOACs.
CHALLENGES TO IMPLEMENTATION
It’s still a balancing act
When chronic anticoagulation is necessary, physicians and patients must attempt to prevent thrombotic events while minimizing the risk of GI bleeds. PPIs may be beneficial in preventing upper GI bleeds in patients taking dual warfarin and antiplatelet therapy, but the long-term consequences of PPI therapy should not be ignored.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Ray WA, Chung CP, Murray KT, et al. Association of proton pump inhibitors with reduced risk of warfarin-related serious upper gastrointestinal bleeding. Gastroenterology. 2016;151:1105-1112.
2. Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52:1502-1517.
3. Salas M, Ward A, Caro J. Are proton pump inhibitors the first choice for acute treatment of gastric ulcers? A meta analysis of randomized clinical rials. BMC Gastroenterol. 2002;2:17.
4. Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10:528-534.
5. Lanas A, García-Rodríguez LA, Arroyo MT, et al. Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol. 2007;102:507-515.
6. Lin KJ, Hernández-Díaz S, García Rodríguez LA. Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti-inflammatory therapy. Gastroenterology. 2011;141:71-79.
7. Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):160S-198S.
8. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):257S-298S.
9. Ament PW, Dicola DB, James ME. Reducing adverse effects of proton pump inhibitors. Am Fam Physician. 2012;86:66-70.
10. Gomm W, von HK, Thome F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73:410-416.
11. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176:238-246.
12. Smeets HM, Hoes AW, de Wit NJ. Effectiveness and costs of implementation strategies to reduce acid suppressive drug prescriptions: a systematic review. BMC Health Serv Res. 2007;7:177.
13. Hanley CM, Kowey PR. Are the novel anticoagulants better than warfarin for patients with atrial fibrillation? J Thorac Dis. 2015;7:165-171.
ILLUSTRATIVE CASE
A 60-year-old man establishes care with you. He has well-controlled osteoarthritis (as long as he takes his low-dose daily aspirin) and chronic atrial fibrillation, for which he takes warfarin. His international normalized ratio (INR) is consistently within the recommended target range of 2 to 3. He feels well and has never had gastroesophageal reflux disease (GERD) or a gastrointestinal (GI) bleed. Should you recommend a proton pump inhibitor (PPI) to decrease the likelihood of a future upper GI bleed?
Anticoagulation therapy creates a dilemma—the need to balance the benefits of preventing embolization with the risks of serious bleeding. Concurrent use of nonsteroidal anti-inflammatory drugs (NSAIDs), aspirin, and other antiplatelet agents further increases the risk of the latter.2
Physicians have long used PPIs to treat upper GI bleeds. They prevent acid secretion and are the most efficacious drugs for healing peptic ulcers.3,4 However, while previous case-control studies show that PPIs reduce the risk of upper GI bleeds in patients taking antiplatelet agents or NSAIDs, they do not show a statistically significant benefit for patients taking warfarin.5,6 Further reflecting the confusion and uncertainty surrounding this issue is that while one expert consensus report recommends that patients taking dual warfarin and antiplatelet agent/NSAID therapy take a PPI to decrease the risk of upper GI bleeding,2 other guidelines regarding anticoagulant therapy do not address this clinical question.2,7,8
[polldaddy:9860876]
STUDY SUMMARY
Study lends support to PPI use in a high-risk group
This retrospective cohort study sought to answer the question: “Does PPI co-therapy decrease the rate of serious upper GI bleeds in patients taking warfarin?” Researchers examined rates of hospitalization for upper GI bleeding for Medicare and Medicaid patients taking warfarin with and without PPI co-therapy (tracked via prescription fill dates). They also evaluated concomitant use of NSAIDs and antiplatelet agents.
The authors excluded patients with a recent history of a severe bleed or certain illnesses that would predispose a patient to GI bleeding (such as esophageal varices). Patients with risk factors for an upper GI bleed (such as abdominal pain, peptic ulcer disease, anemia, etc.) were more likely to be taking PPI co-therapy. Researchers analyzed the effect of PPI co-therapy in patients with and without these additional risk factors.
Results. The study followed over 75,000 person-years of active warfarin therapy (more than 52,000 person-years in the Medicaid cohort and more than 23,000 person-years in the Medicare cohort). Hospitalizations due to upper GI bleeding occurred at a rate of 127/10,000 person-years (incidence was similar in both the Medicaid and Medicare groups).
Looking at all patients taking warfarin (regardless of whether or not they were also taking an NSAID or antiplatelet agent), PPI co-therapy reduced the risk of hospitalization for upper GI bleeding by 24% (adjusted hazard ratio [HR]=0.76; 95% confidence interval [CI], 0.63 to 0.91), which translates into 29 fewer hospitalizations per 10,000 person-years. The number needed to treat (NNT) was 345 person-years, meaning 345 patients taking warfarin would have to take a PPI for one year to prevent one hospitalization for an upper GI bleed. As one might expect, PPI co-therapy did not significantly reduce the risk of lower GI, other GI, or non-GI bleeding.
In patients taking both warfarin and concurrent antiplatelet agents or NSAIDs, PPI co-therapy reduced the risk of hospitalization for upper GI bleeding by about half (HR=0.55; 95% CI, 0.39-0.77). Hospitalizations decreased by 128/10,000 person-years (95% CI, -66 to -173), yielding an NNT of 78 person-years. For patients taking warfarin but not antiplatelet agents or NSAIDs, PPI co-therapy did not significantly decrease the risk of hospitalization for upper GI bleeding (HR=0.86; 95% CI, 0.70-1.06).
Additional risk factors for GI bleeds. Researchers also looked at patients who had additional risk factors for GI bleeds (other than the exclusion criteria). For patients taking both warfarin and an antiplatelet agent/NSAID, PPI co-therapy decreased the risk of upper GI bleeding whether or not the patients had other bleeding risk factors. Again, for patients who had additional bleeding risk factors, but were not taking an antiplatelet agent or NSAID, PPI therapy showed no statistically significant effect.
WHAT’S NEW
PPIs offer benefits, but not to patients taking warfarin alone
The statistically significant results in this large observational study suggest that PPI co-therapy is beneficial in reducing the risk of upper GI bleeding in patients taking warfarin plus an antiplatelet agent/NSAID, but that PPI co-therapy provides no benefit to patients taking warfarin exclusively.
CAVEATS
Study was good, but it wasn’t a randomized controlled trial
This study is observational, and not a randomized control trial (RCT). Therefore, unknown confounding variables may have skewed results. For example, patients could have taken over-the-counter medications that influenced or obscured results, but were not included in the data analysis (misclassification bias).
At best, we can infer a correlation between PPIs and decreased risk of upper GI bleeds. We need RCTs to determine whether PPIs cause a lower risk.
Don’t overlook the risk of PPIs. This study assessed the ability of PPIs to prevent bleeds, but did not address the risks of long-term PPI therapy. Adverse effects of PPIs include an increased risk of pneumonia, infection with Clostridium difficile, hip and spine fractures, anemia, and possibly chronic kidney disease and dementia.9-11 In addition, cost-analysis studies of PPI therapy are limited and inconsistent.12 Therefore, it’s best to make decisions regarding PPIs after discussing other risks and benefits.
What about DOACs? Another consideration is the option to prescribe a direct oral anticoagulant (DOAC), such as dabigatran, rivaroxaban, or apixaban, instead of warfarin. DOACs are at least as effective as warfarin at preventing stroke in patients with atrial fibrillation and may even be safer.13 Dabigatran 110 mg causes fewer “major bleeding” events than warfarin.13 Rivaroxaban has been shown to result in fewer fatal bleeding events than warfarin due to fatal intracranial bleeds, although it is associated with more GI bleedding.13 Compared with warfarin, apixaban is associated with fewer GI bleeds and lower bleeding rates overall.13 Further research is warranted to determine if PPI therapy is beneficial to patients taking DOACs.
CHALLENGES TO IMPLEMENTATION
It’s still a balancing act
When chronic anticoagulation is necessary, physicians and patients must attempt to prevent thrombotic events while minimizing the risk of GI bleeds. PPIs may be beneficial in preventing upper GI bleeds in patients taking dual warfarin and antiplatelet therapy, but the long-term consequences of PPI therapy should not be ignored.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 60-year-old man establishes care with you. He has well-controlled osteoarthritis (as long as he takes his low-dose daily aspirin) and chronic atrial fibrillation, for which he takes warfarin. His international normalized ratio (INR) is consistently within the recommended target range of 2 to 3. He feels well and has never had gastroesophageal reflux disease (GERD) or a gastrointestinal (GI) bleed. Should you recommend a proton pump inhibitor (PPI) to decrease the likelihood of a future upper GI bleed?
Anticoagulation therapy creates a dilemma—the need to balance the benefits of preventing embolization with the risks of serious bleeding. Concurrent use of nonsteroidal anti-inflammatory drugs (NSAIDs), aspirin, and other antiplatelet agents further increases the risk of the latter.2
Physicians have long used PPIs to treat upper GI bleeds. They prevent acid secretion and are the most efficacious drugs for healing peptic ulcers.3,4 However, while previous case-control studies show that PPIs reduce the risk of upper GI bleeds in patients taking antiplatelet agents or NSAIDs, they do not show a statistically significant benefit for patients taking warfarin.5,6 Further reflecting the confusion and uncertainty surrounding this issue is that while one expert consensus report recommends that patients taking dual warfarin and antiplatelet agent/NSAID therapy take a PPI to decrease the risk of upper GI bleeding,2 other guidelines regarding anticoagulant therapy do not address this clinical question.2,7,8
[polldaddy:9860876]
STUDY SUMMARY
Study lends support to PPI use in a high-risk group
This retrospective cohort study sought to answer the question: “Does PPI co-therapy decrease the rate of serious upper GI bleeds in patients taking warfarin?” Researchers examined rates of hospitalization for upper GI bleeding for Medicare and Medicaid patients taking warfarin with and without PPI co-therapy (tracked via prescription fill dates). They also evaluated concomitant use of NSAIDs and antiplatelet agents.
The authors excluded patients with a recent history of a severe bleed or certain illnesses that would predispose a patient to GI bleeding (such as esophageal varices). Patients with risk factors for an upper GI bleed (such as abdominal pain, peptic ulcer disease, anemia, etc.) were more likely to be taking PPI co-therapy. Researchers analyzed the effect of PPI co-therapy in patients with and without these additional risk factors.
Results. The study followed over 75,000 person-years of active warfarin therapy (more than 52,000 person-years in the Medicaid cohort and more than 23,000 person-years in the Medicare cohort). Hospitalizations due to upper GI bleeding occurred at a rate of 127/10,000 person-years (incidence was similar in both the Medicaid and Medicare groups).
Looking at all patients taking warfarin (regardless of whether or not they were also taking an NSAID or antiplatelet agent), PPI co-therapy reduced the risk of hospitalization for upper GI bleeding by 24% (adjusted hazard ratio [HR]=0.76; 95% confidence interval [CI], 0.63 to 0.91), which translates into 29 fewer hospitalizations per 10,000 person-years. The number needed to treat (NNT) was 345 person-years, meaning 345 patients taking warfarin would have to take a PPI for one year to prevent one hospitalization for an upper GI bleed. As one might expect, PPI co-therapy did not significantly reduce the risk of lower GI, other GI, or non-GI bleeding.
In patients taking both warfarin and concurrent antiplatelet agents or NSAIDs, PPI co-therapy reduced the risk of hospitalization for upper GI bleeding by about half (HR=0.55; 95% CI, 0.39-0.77). Hospitalizations decreased by 128/10,000 person-years (95% CI, -66 to -173), yielding an NNT of 78 person-years. For patients taking warfarin but not antiplatelet agents or NSAIDs, PPI co-therapy did not significantly decrease the risk of hospitalization for upper GI bleeding (HR=0.86; 95% CI, 0.70-1.06).
Additional risk factors for GI bleeds. Researchers also looked at patients who had additional risk factors for GI bleeds (other than the exclusion criteria). For patients taking both warfarin and an antiplatelet agent/NSAID, PPI co-therapy decreased the risk of upper GI bleeding whether or not the patients had other bleeding risk factors. Again, for patients who had additional bleeding risk factors, but were not taking an antiplatelet agent or NSAID, PPI therapy showed no statistically significant effect.
WHAT’S NEW
PPIs offer benefits, but not to patients taking warfarin alone
The statistically significant results in this large observational study suggest that PPI co-therapy is beneficial in reducing the risk of upper GI bleeding in patients taking warfarin plus an antiplatelet agent/NSAID, but that PPI co-therapy provides no benefit to patients taking warfarin exclusively.
CAVEATS
Study was good, but it wasn’t a randomized controlled trial
This study is observational, and not a randomized control trial (RCT). Therefore, unknown confounding variables may have skewed results. For example, patients could have taken over-the-counter medications that influenced or obscured results, but were not included in the data analysis (misclassification bias).
At best, we can infer a correlation between PPIs and decreased risk of upper GI bleeds. We need RCTs to determine whether PPIs cause a lower risk.
Don’t overlook the risk of PPIs. This study assessed the ability of PPIs to prevent bleeds, but did not address the risks of long-term PPI therapy. Adverse effects of PPIs include an increased risk of pneumonia, infection with Clostridium difficile, hip and spine fractures, anemia, and possibly chronic kidney disease and dementia.9-11 In addition, cost-analysis studies of PPI therapy are limited and inconsistent.12 Therefore, it’s best to make decisions regarding PPIs after discussing other risks and benefits.
What about DOACs? Another consideration is the option to prescribe a direct oral anticoagulant (DOAC), such as dabigatran, rivaroxaban, or apixaban, instead of warfarin. DOACs are at least as effective as warfarin at preventing stroke in patients with atrial fibrillation and may even be safer.13 Dabigatran 110 mg causes fewer “major bleeding” events than warfarin.13 Rivaroxaban has been shown to result in fewer fatal bleeding events than warfarin due to fatal intracranial bleeds, although it is associated with more GI bleedding.13 Compared with warfarin, apixaban is associated with fewer GI bleeds and lower bleeding rates overall.13 Further research is warranted to determine if PPI therapy is beneficial to patients taking DOACs.
CHALLENGES TO IMPLEMENTATION
It’s still a balancing act
When chronic anticoagulation is necessary, physicians and patients must attempt to prevent thrombotic events while minimizing the risk of GI bleeds. PPIs may be beneficial in preventing upper GI bleeds in patients taking dual warfarin and antiplatelet therapy, but the long-term consequences of PPI therapy should not be ignored.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Ray WA, Chung CP, Murray KT, et al. Association of proton pump inhibitors with reduced risk of warfarin-related serious upper gastrointestinal bleeding. Gastroenterology. 2016;151:1105-1112.
2. Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52:1502-1517.
3. Salas M, Ward A, Caro J. Are proton pump inhibitors the first choice for acute treatment of gastric ulcers? A meta analysis of randomized clinical rials. BMC Gastroenterol. 2002;2:17.
4. Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10:528-534.
5. Lanas A, García-Rodríguez LA, Arroyo MT, et al. Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol. 2007;102:507-515.
6. Lin KJ, Hernández-Díaz S, García Rodríguez LA. Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti-inflammatory therapy. Gastroenterology. 2011;141:71-79.
7. Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):160S-198S.
8. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):257S-298S.
9. Ament PW, Dicola DB, James ME. Reducing adverse effects of proton pump inhibitors. Am Fam Physician. 2012;86:66-70.
10. Gomm W, von HK, Thome F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73:410-416.
11. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176:238-246.
12. Smeets HM, Hoes AW, de Wit NJ. Effectiveness and costs of implementation strategies to reduce acid suppressive drug prescriptions: a systematic review. BMC Health Serv Res. 2007;7:177.
13. Hanley CM, Kowey PR. Are the novel anticoagulants better than warfarin for patients with atrial fibrillation? J Thorac Dis. 2015;7:165-171.
1. Ray WA, Chung CP, Murray KT, et al. Association of proton pump inhibitors with reduced risk of warfarin-related serious upper gastrointestinal bleeding. Gastroenterology. 2016;151:1105-1112.
2. Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52:1502-1517.
3. Salas M, Ward A, Caro J. Are proton pump inhibitors the first choice for acute treatment of gastric ulcers? A meta analysis of randomized clinical rials. BMC Gastroenterol. 2002;2:17.
4. Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10:528-534.
5. Lanas A, García-Rodríguez LA, Arroyo MT, et al. Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol. 2007;102:507-515.
6. Lin KJ, Hernández-Díaz S, García Rodríguez LA. Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti-inflammatory therapy. Gastroenterology. 2011;141:71-79.
7. Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):160S-198S.
8. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):257S-298S.
9. Ament PW, Dicola DB, James ME. Reducing adverse effects of proton pump inhibitors. Am Fam Physician. 2012;86:66-70.
10. Gomm W, von HK, Thome F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73:410-416.
11. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176:238-246.
12. Smeets HM, Hoes AW, de Wit NJ. Effectiveness and costs of implementation strategies to reduce acid suppressive drug prescriptions: a systematic review. BMC Health Serv Res. 2007;7:177.
13. Hanley CM, Kowey PR. Are the novel anticoagulants better than warfarin for patients with atrial fibrillation? J Thorac Dis. 2015;7:165-171.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Prescribe a proton pump inhibitor for patients taking dual antiplatelet/antithrombotic therapy to reduce the risk of upper gastrointestinal bleeding.
STRENGTH OF RECOMMENDATION
B: Based on a cohort study
Ray WA, Chung CP, Murray KT, et al. Association of proton pump inhibitors with reduced risk of warfarin-related serious upper gastrointestinal bleeding. Gastroenterology. 2016;151:1105-1112.1
Treat gun violence like the public health crisis it is
Last month’s mass shooting in Las Vegas, which killed 59 people and wounded 500, was committed by a single individual who legally purchased an arsenal that allowed him to fire hundreds of high-caliber bullets within minutes into a large crowd. This is just the latest in a series of high-profile mass killings that appear to be increasing in frequency.1
As terrifying as mass murders are, they account for only a small fraction of gun-related mortality. Everyday about 80 people in the United States are killed by a gun, usually by someone they know or by themselves (almost two-thirds of gun-related mortality involves suicide).2 No other developed country even comes close to our rate of gun-related violence.2
What to do? Recall anti-smoking efforts. Gun violence is a public health issue that should be addressed with tried and proven public health methods. A couple of examples from history hold valuable lessons. While tobacco-related mortality and morbidity remain public health concerns, we have made marked improvements and saved many lives through a series of public health interventions including increasing the price of tobacco products, restricting advertising and sales to minors, and prohibiting smoking in public areas, to name a few.3
These interventions occurred because the public recognized the threat of tobacco and was willing to adopt them. This was not always the case. During the first half of my life, smoking in public, including indoors at public events and even on airplanes, was accepted, and the “rights of smokers” were respected. This now seems inconceivable. Public health interventions work, and public perceptions and attitudes can change.
Consider inroads made in driver safety, too. We have also made marked improvements in motor vehicle crash-related deaths and injuries.4 For decades, we have recorded hundreds of data points on every car crash resulting in a death in a comprehensive database—the Fatality Analysis Reporting System (FARS). These data have been used by researchers to identify causes of crashes and crash-related deaths and have led to improvements in car design and road safety. Additional factors leading to improved road safety include restrictions on the age at which one can drive and on drinking alcohol and driving.
We can achieve similar improvements in gun-related mortality if we establish and maintain a comprehensive database, encourage and fund research, and are willing to adopt some commonsense product improvements and ownership restrictions that, nevertheless, preserve the right for most to responsibly own a firearm.
Don’t you think it’s time?
1. Blair JP, Schweit KW. A study of active shooter incidents in the United States between 2000 and 2013. Texas State University and the Federal Bureau of Investigation, US Department of Justice, Washington, DC. 2014. Available at: https://www.fbi.gov/file-repository/active-shooter-study-2000-2013-1.pdf. Accessed October 16, 2017.
2. Wintemute GJ. The epidemiology of firearm violence in the twenty-first century United States. Annu Rev Public Health. 2015;36:5-19.
3. Centers for Disease Control and Prevention. Tobacco use—United States, 1900-1999. MMWR Morb Mortal Wkly Rep. 1999;48:986-993.
4. Centers for Disease Control and Prevention. Achievements in public health, 1900-1999 motor-vehicle safety: a 20th century public health achievement. MMWR Morb Mortal Wkly Rep. 1999;48:369-374.
Last month’s mass shooting in Las Vegas, which killed 59 people and wounded 500, was committed by a single individual who legally purchased an arsenal that allowed him to fire hundreds of high-caliber bullets within minutes into a large crowd. This is just the latest in a series of high-profile mass killings that appear to be increasing in frequency.1
As terrifying as mass murders are, they account for only a small fraction of gun-related mortality. Everyday about 80 people in the United States are killed by a gun, usually by someone they know or by themselves (almost two-thirds of gun-related mortality involves suicide).2 No other developed country even comes close to our rate of gun-related violence.2
What to do? Recall anti-smoking efforts. Gun violence is a public health issue that should be addressed with tried and proven public health methods. A couple of examples from history hold valuable lessons. While tobacco-related mortality and morbidity remain public health concerns, we have made marked improvements and saved many lives through a series of public health interventions including increasing the price of tobacco products, restricting advertising and sales to minors, and prohibiting smoking in public areas, to name a few.3
These interventions occurred because the public recognized the threat of tobacco and was willing to adopt them. This was not always the case. During the first half of my life, smoking in public, including indoors at public events and even on airplanes, was accepted, and the “rights of smokers” were respected. This now seems inconceivable. Public health interventions work, and public perceptions and attitudes can change.
Consider inroads made in driver safety, too. We have also made marked improvements in motor vehicle crash-related deaths and injuries.4 For decades, we have recorded hundreds of data points on every car crash resulting in a death in a comprehensive database—the Fatality Analysis Reporting System (FARS). These data have been used by researchers to identify causes of crashes and crash-related deaths and have led to improvements in car design and road safety. Additional factors leading to improved road safety include restrictions on the age at which one can drive and on drinking alcohol and driving.
We can achieve similar improvements in gun-related mortality if we establish and maintain a comprehensive database, encourage and fund research, and are willing to adopt some commonsense product improvements and ownership restrictions that, nevertheless, preserve the right for most to responsibly own a firearm.
Don’t you think it’s time?
Last month’s mass shooting in Las Vegas, which killed 59 people and wounded 500, was committed by a single individual who legally purchased an arsenal that allowed him to fire hundreds of high-caliber bullets within minutes into a large crowd. This is just the latest in a series of high-profile mass killings that appear to be increasing in frequency.1
As terrifying as mass murders are, they account for only a small fraction of gun-related mortality. Everyday about 80 people in the United States are killed by a gun, usually by someone they know or by themselves (almost two-thirds of gun-related mortality involves suicide).2 No other developed country even comes close to our rate of gun-related violence.2
What to do? Recall anti-smoking efforts. Gun violence is a public health issue that should be addressed with tried and proven public health methods. A couple of examples from history hold valuable lessons. While tobacco-related mortality and morbidity remain public health concerns, we have made marked improvements and saved many lives through a series of public health interventions including increasing the price of tobacco products, restricting advertising and sales to minors, and prohibiting smoking in public areas, to name a few.3
These interventions occurred because the public recognized the threat of tobacco and was willing to adopt them. This was not always the case. During the first half of my life, smoking in public, including indoors at public events and even on airplanes, was accepted, and the “rights of smokers” were respected. This now seems inconceivable. Public health interventions work, and public perceptions and attitudes can change.
Consider inroads made in driver safety, too. We have also made marked improvements in motor vehicle crash-related deaths and injuries.4 For decades, we have recorded hundreds of data points on every car crash resulting in a death in a comprehensive database—the Fatality Analysis Reporting System (FARS). These data have been used by researchers to identify causes of crashes and crash-related deaths and have led to improvements in car design and road safety. Additional factors leading to improved road safety include restrictions on the age at which one can drive and on drinking alcohol and driving.
We can achieve similar improvements in gun-related mortality if we establish and maintain a comprehensive database, encourage and fund research, and are willing to adopt some commonsense product improvements and ownership restrictions that, nevertheless, preserve the right for most to responsibly own a firearm.
Don’t you think it’s time?
1. Blair JP, Schweit KW. A study of active shooter incidents in the United States between 2000 and 2013. Texas State University and the Federal Bureau of Investigation, US Department of Justice, Washington, DC. 2014. Available at: https://www.fbi.gov/file-repository/active-shooter-study-2000-2013-1.pdf. Accessed October 16, 2017.
2. Wintemute GJ. The epidemiology of firearm violence in the twenty-first century United States. Annu Rev Public Health. 2015;36:5-19.
3. Centers for Disease Control and Prevention. Tobacco use—United States, 1900-1999. MMWR Morb Mortal Wkly Rep. 1999;48:986-993.
4. Centers for Disease Control and Prevention. Achievements in public health, 1900-1999 motor-vehicle safety: a 20th century public health achievement. MMWR Morb Mortal Wkly Rep. 1999;48:369-374.
1. Blair JP, Schweit KW. A study of active shooter incidents in the United States between 2000 and 2013. Texas State University and the Federal Bureau of Investigation, US Department of Justice, Washington, DC. 2014. Available at: https://www.fbi.gov/file-repository/active-shooter-study-2000-2013-1.pdf. Accessed October 16, 2017.
2. Wintemute GJ. The epidemiology of firearm violence in the twenty-first century United States. Annu Rev Public Health. 2015;36:5-19.
3. Centers for Disease Control and Prevention. Tobacco use—United States, 1900-1999. MMWR Morb Mortal Wkly Rep. 1999;48:986-993.
4. Centers for Disease Control and Prevention. Achievements in public health, 1900-1999 motor-vehicle safety: a 20th century public health achievement. MMWR Morb Mortal Wkly Rep. 1999;48:369-374.
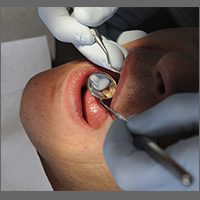
What is the optimal frequency for dental checkups for children and adults?
EVIDENCE SUMMARY
A systematic review featured a single RCT (n=185) comparing the effect of a 12-month vs 24-month interval between dental visits on dental caries in low-risk 3- to 5-year-old children with primary teeth and young adults, ages 16 to 20 years, with permanent teeth.1 The outcomes of caries (ie, decayed, missing, filled surfaces increment) between the 12- and 24-month visits both in younger children (mean difference [MD]= -0.90; 95% confidence interval [CI], -1.96 to 0.16) and young adults (MD= -0.86; 95% CI, -1.75 to 0.03) did not differ.
Gingivitis: Not an issue when visits were delayed in healthy adults
Another systematic review (3 RCTs; N=836) evaluated the benefits associated with scaling and polishing in the prevention of gingivitis (primary outcome measure).2 One RCT (n=207) compared scaling and polishing at 6- and 12-month intervals to no treatment for 24 months in adults with healthy dental histories. There was no difference in the percentage of index teeth with bleeding in the 6-month or 12-month treatment groups compared to the group that received no treatment for 24 months (MD= -2%; 95% CI, -10% to 6% and MD= -1%; 95% CI, -9% to 7%, respectively).
2 visits/year prevents tooth loss in high-risk patients
A retrospective cohort study (N=5117) using 16 years of data evaluated the association between one or 2 preventive dental visits per year and tooth extraction events in adults at low risk and those at high risk for progressive periodontitis.3 Those at high risk had at least one of the following risk factors: smoking, diabetes, or interleukin-1 genotype. Low-risk patients had no difference in tooth loss with one visit compared to 2 visits annually (absolute risk reduction [ARR]=2.6%; 95% CI, 0.5%-5.8%; P=.092); however, high-risk patients had fewer events with 2 annual visits (number needed to treat [NNT]=19; ARR 5.2%; 95% CI, 1.8%-8.4%; P=.002).
Visits before age 3 likely benefit only those at high risk
A systematic review of 4 retrospective cohort studies (N=77,291) analyzed the impact of early preventive dental visits (EPDV) on the frequency of future preventive and non-preventive dental visits and related expenditures using data from insurance claims and a kindergarten state dental registry.4 One study (n=11,394) used dental disease status at kindergarten (defined as the count of decayed, missing [molar teeth only], and filled primary teeth) as an outcome measure. Children who received EPDV before age 24 months had a comparable number of caries to those who had EPDV at 24 to 36 months. The authors concluded that EPDV before age 3 years is likely to benefit only children at high risk, and that evidence for a first dental visit by age one year is weak.
RECOMMENDATIONS
The National Institute for Health and Care Excellence recommends preventive dental visit intervals based on individual risk (12 months as the longest interval under age 18 years and 24 months as the longest interval for those 18 years and older at low risk).5 The American Dental Association recommends preventive dental visits at intervals determined by individual risk.6 The American Academy of Pediatric Dentistry recommends a first exam by age one year and preventive dental visits every 6 months through adolescence or as indicated by individual risk.7 The US Preventive Services Task Force states there is insufficient evidence to recommend routine dental screening by primary care physicians in children up to age 5 years.8
1. Riley P, Worthington HV, Clarkson JE, et al. Recall intervals for oral health in primary care patients. Cochrane Database Syst Rev. 2013;12:CD004346.
2. Worthington HV, Clarkson JE, Bryan G, et al. Routine scale and polish for periodontal health in adults. Cochrane Database Syst Rev. 2013;11:CD004625.
3. Giannobile WV, Braun TM, Caplis AK, et al. Patient stratification for preventive care in dentistry. J Dent Res. 2013;92:694-701.
4. Bhaskar V, McGraw KA, Divaris K. The importance of preventive dental visits from a young age: systematic review and current perspectives. Clin Cosmetic Investig Dent. 2014;6:21-27.
5. National Institute for Health and Care Excellence. Dental checks: intervals between oral health reviews. Available at: https://www.nice.org.uk/guidance/cg19. Accessed March 22, 2016.
6. American Dental Association. American Dental Association Statement on Regular Dental Visits. 2013. Available at: http://www.ada.org/en/press-room/news-releases/2013-archive/june/american-dental-association-statement-on-regular-dental-visits. Accessed March 22, 2016.
7. American Academy of Pediatric Dentistry. Guideline on periodicity of examination, preventive dental services, anticipatory guidance/counseling, and oral treatment for infants, children and adolescents. Pediatr Dent. 2013;35:E148-E156.
8. Moyer VA; US Preventive Services Task Force. Prevention of dental caries in children from birth through age 5 years: US Preventive Services Task Force recommendation statement. Pediatrics. 2014;133:1102-1111.
EVIDENCE SUMMARY
A systematic review featured a single RCT (n=185) comparing the effect of a 12-month vs 24-month interval between dental visits on dental caries in low-risk 3- to 5-year-old children with primary teeth and young adults, ages 16 to 20 years, with permanent teeth.1 The outcomes of caries (ie, decayed, missing, filled surfaces increment) between the 12- and 24-month visits both in younger children (mean difference [MD]= -0.90; 95% confidence interval [CI], -1.96 to 0.16) and young adults (MD= -0.86; 95% CI, -1.75 to 0.03) did not differ.
Gingivitis: Not an issue when visits were delayed in healthy adults
Another systematic review (3 RCTs; N=836) evaluated the benefits associated with scaling and polishing in the prevention of gingivitis (primary outcome measure).2 One RCT (n=207) compared scaling and polishing at 6- and 12-month intervals to no treatment for 24 months in adults with healthy dental histories. There was no difference in the percentage of index teeth with bleeding in the 6-month or 12-month treatment groups compared to the group that received no treatment for 24 months (MD= -2%; 95% CI, -10% to 6% and MD= -1%; 95% CI, -9% to 7%, respectively).
2 visits/year prevents tooth loss in high-risk patients
A retrospective cohort study (N=5117) using 16 years of data evaluated the association between one or 2 preventive dental visits per year and tooth extraction events in adults at low risk and those at high risk for progressive periodontitis.3 Those at high risk had at least one of the following risk factors: smoking, diabetes, or interleukin-1 genotype. Low-risk patients had no difference in tooth loss with one visit compared to 2 visits annually (absolute risk reduction [ARR]=2.6%; 95% CI, 0.5%-5.8%; P=.092); however, high-risk patients had fewer events with 2 annual visits (number needed to treat [NNT]=19; ARR 5.2%; 95% CI, 1.8%-8.4%; P=.002).
Visits before age 3 likely benefit only those at high risk
A systematic review of 4 retrospective cohort studies (N=77,291) analyzed the impact of early preventive dental visits (EPDV) on the frequency of future preventive and non-preventive dental visits and related expenditures using data from insurance claims and a kindergarten state dental registry.4 One study (n=11,394) used dental disease status at kindergarten (defined as the count of decayed, missing [molar teeth only], and filled primary teeth) as an outcome measure. Children who received EPDV before age 24 months had a comparable number of caries to those who had EPDV at 24 to 36 months. The authors concluded that EPDV before age 3 years is likely to benefit only children at high risk, and that evidence for a first dental visit by age one year is weak.
RECOMMENDATIONS
The National Institute for Health and Care Excellence recommends preventive dental visit intervals based on individual risk (12 months as the longest interval under age 18 years and 24 months as the longest interval for those 18 years and older at low risk).5 The American Dental Association recommends preventive dental visits at intervals determined by individual risk.6 The American Academy of Pediatric Dentistry recommends a first exam by age one year and preventive dental visits every 6 months through adolescence or as indicated by individual risk.7 The US Preventive Services Task Force states there is insufficient evidence to recommend routine dental screening by primary care physicians in children up to age 5 years.8
EVIDENCE SUMMARY
A systematic review featured a single RCT (n=185) comparing the effect of a 12-month vs 24-month interval between dental visits on dental caries in low-risk 3- to 5-year-old children with primary teeth and young adults, ages 16 to 20 years, with permanent teeth.1 The outcomes of caries (ie, decayed, missing, filled surfaces increment) between the 12- and 24-month visits both in younger children (mean difference [MD]= -0.90; 95% confidence interval [CI], -1.96 to 0.16) and young adults (MD= -0.86; 95% CI, -1.75 to 0.03) did not differ.
Gingivitis: Not an issue when visits were delayed in healthy adults
Another systematic review (3 RCTs; N=836) evaluated the benefits associated with scaling and polishing in the prevention of gingivitis (primary outcome measure).2 One RCT (n=207) compared scaling and polishing at 6- and 12-month intervals to no treatment for 24 months in adults with healthy dental histories. There was no difference in the percentage of index teeth with bleeding in the 6-month or 12-month treatment groups compared to the group that received no treatment for 24 months (MD= -2%; 95% CI, -10% to 6% and MD= -1%; 95% CI, -9% to 7%, respectively).
2 visits/year prevents tooth loss in high-risk patients
A retrospective cohort study (N=5117) using 16 years of data evaluated the association between one or 2 preventive dental visits per year and tooth extraction events in adults at low risk and those at high risk for progressive periodontitis.3 Those at high risk had at least one of the following risk factors: smoking, diabetes, or interleukin-1 genotype. Low-risk patients had no difference in tooth loss with one visit compared to 2 visits annually (absolute risk reduction [ARR]=2.6%; 95% CI, 0.5%-5.8%; P=.092); however, high-risk patients had fewer events with 2 annual visits (number needed to treat [NNT]=19; ARR 5.2%; 95% CI, 1.8%-8.4%; P=.002).
Visits before age 3 likely benefit only those at high risk
A systematic review of 4 retrospective cohort studies (N=77,291) analyzed the impact of early preventive dental visits (EPDV) on the frequency of future preventive and non-preventive dental visits and related expenditures using data from insurance claims and a kindergarten state dental registry.4 One study (n=11,394) used dental disease status at kindergarten (defined as the count of decayed, missing [molar teeth only], and filled primary teeth) as an outcome measure. Children who received EPDV before age 24 months had a comparable number of caries to those who had EPDV at 24 to 36 months. The authors concluded that EPDV before age 3 years is likely to benefit only children at high risk, and that evidence for a first dental visit by age one year is weak.
RECOMMENDATIONS
The National Institute for Health and Care Excellence recommends preventive dental visit intervals based on individual risk (12 months as the longest interval under age 18 years and 24 months as the longest interval for those 18 years and older at low risk).5 The American Dental Association recommends preventive dental visits at intervals determined by individual risk.6 The American Academy of Pediatric Dentistry recommends a first exam by age one year and preventive dental visits every 6 months through adolescence or as indicated by individual risk.7 The US Preventive Services Task Force states there is insufficient evidence to recommend routine dental screening by primary care physicians in children up to age 5 years.8
1. Riley P, Worthington HV, Clarkson JE, et al. Recall intervals for oral health in primary care patients. Cochrane Database Syst Rev. 2013;12:CD004346.
2. Worthington HV, Clarkson JE, Bryan G, et al. Routine scale and polish for periodontal health in adults. Cochrane Database Syst Rev. 2013;11:CD004625.
3. Giannobile WV, Braun TM, Caplis AK, et al. Patient stratification for preventive care in dentistry. J Dent Res. 2013;92:694-701.
4. Bhaskar V, McGraw KA, Divaris K. The importance of preventive dental visits from a young age: systematic review and current perspectives. Clin Cosmetic Investig Dent. 2014;6:21-27.
5. National Institute for Health and Care Excellence. Dental checks: intervals between oral health reviews. Available at: https://www.nice.org.uk/guidance/cg19. Accessed March 22, 2016.
6. American Dental Association. American Dental Association Statement on Regular Dental Visits. 2013. Available at: http://www.ada.org/en/press-room/news-releases/2013-archive/june/american-dental-association-statement-on-regular-dental-visits. Accessed March 22, 2016.
7. American Academy of Pediatric Dentistry. Guideline on periodicity of examination, preventive dental services, anticipatory guidance/counseling, and oral treatment for infants, children and adolescents. Pediatr Dent. 2013;35:E148-E156.
8. Moyer VA; US Preventive Services Task Force. Prevention of dental caries in children from birth through age 5 years: US Preventive Services Task Force recommendation statement. Pediatrics. 2014;133:1102-1111.
1. Riley P, Worthington HV, Clarkson JE, et al. Recall intervals for oral health in primary care patients. Cochrane Database Syst Rev. 2013;12:CD004346.
2. Worthington HV, Clarkson JE, Bryan G, et al. Routine scale and polish for periodontal health in adults. Cochrane Database Syst Rev. 2013;11:CD004625.
3. Giannobile WV, Braun TM, Caplis AK, et al. Patient stratification for preventive care in dentistry. J Dent Res. 2013;92:694-701.
4. Bhaskar V, McGraw KA, Divaris K. The importance of preventive dental visits from a young age: systematic review and current perspectives. Clin Cosmetic Investig Dent. 2014;6:21-27.
5. National Institute for Health and Care Excellence. Dental checks: intervals between oral health reviews. Available at: https://www.nice.org.uk/guidance/cg19. Accessed March 22, 2016.
6. American Dental Association. American Dental Association Statement on Regular Dental Visits. 2013. Available at: http://www.ada.org/en/press-room/news-releases/2013-archive/june/american-dental-association-statement-on-regular-dental-visits. Accessed March 22, 2016.
7. American Academy of Pediatric Dentistry. Guideline on periodicity of examination, preventive dental services, anticipatory guidance/counseling, and oral treatment for infants, children and adolescents. Pediatr Dent. 2013;35:E148-E156.
8. Moyer VA; US Preventive Services Task Force. Prevention of dental caries in children from birth through age 5 years: US Preventive Services Task Force recommendation statement. Pediatrics. 2014;133:1102-1111.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
It is unclear, but studies suggest that it should be based largely on individual risk. The American Academy of Pediatric Dentistry recommends a 6-month interval for preventive dental visits (strength of recommendation [SOR]: C, expert opinion), but a 24-month interval does not result in an increased incidence of dental caries in healthy children and young adults or increased incidence of gingivitis in healthy adults (SOR: B, a single randomized controlled trial [RCT]). In adults with risk factors (eg, smoking or diabetes), visits at 6-month intervals are associated with a lower incidence of tooth loss (SOR: C, a retrospective cohort study). Children with risk factors (eg, caries) may benefit from a first dental visit by age 3 years (SOR: C, a retrospective cohort study).
Diffuse skin rash, altered mental status
A 74-year-old Caucasian man presented to the hospital with intractable back and chest pain, a diffuse skin rash, and altered mental status. He said that 2 days ago, he’d gone to a different local hospital for treatment of back pain and a headache that had begun 3 days earlier. He was treated with intravenous hydromorphone and sent home with a prescription for meperidine. He said that several hours after being treated with the hydromorphone, the rash developed on his head and then spread to his trunk and upper extremities.
On physical examination, the patient was afebrile. He had numerous erythematous papules and vesicles in various stages of development on his scalp, face, neck, chest (FIGURE), abdomen, back, upper extremities, and groin. The lesions continued to spread and eventually involved his posterior oropharynx. The patient also developed conjunctivitis.
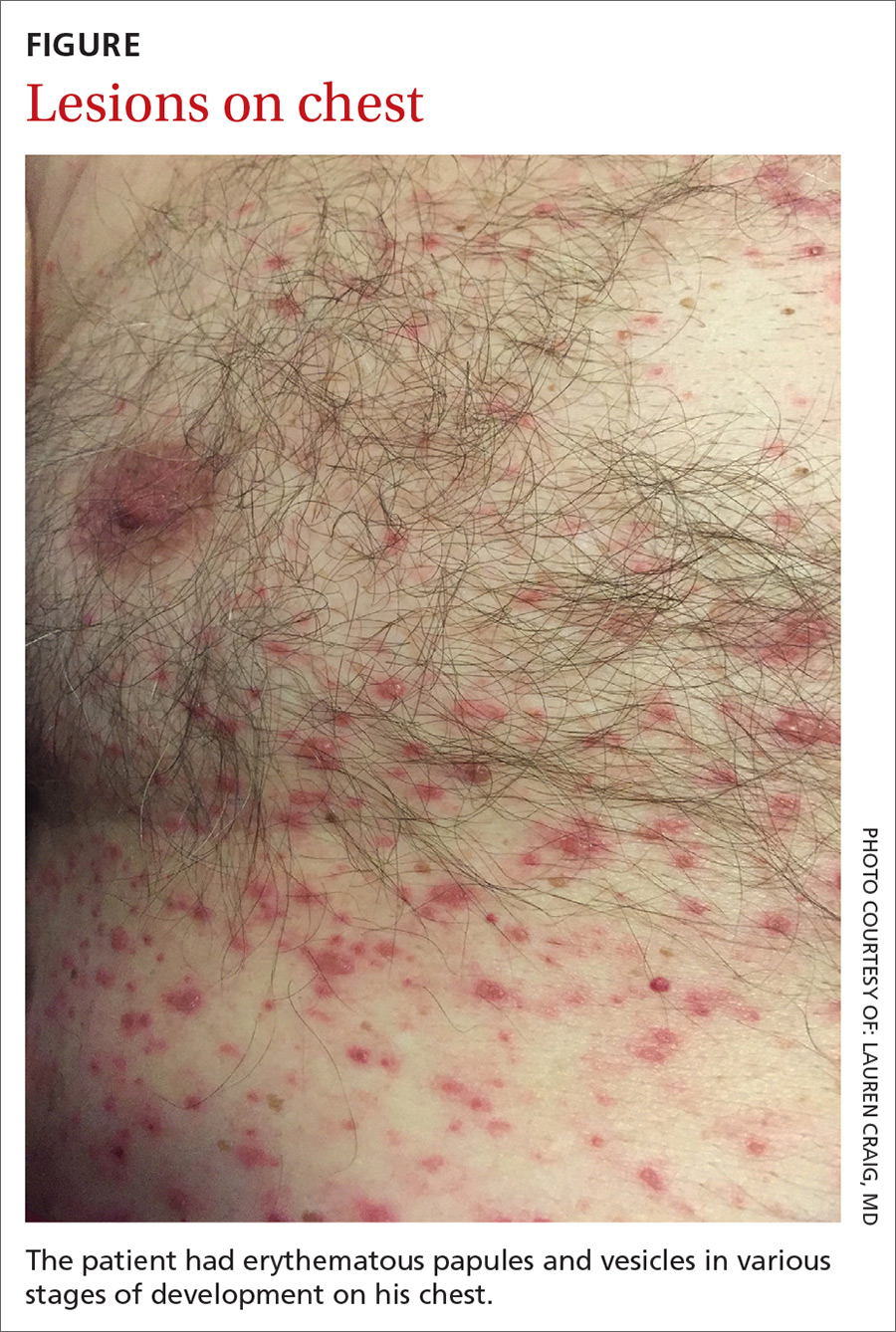
Laboratory findings included a white blood cell count of 4000/mcL (normal: 4500-11,000/mcL) with 65.9% segmented neutrophils (normal: 40%-60%), and 16.7% lymphocytes (normal: 20%-40%). Lab tests also revealed an aspartate aminotransferase level of 263 U/L (normal: 10-40 U/L), alanine aminotransferase of 236 U/L (normal: 7-56 U/L), and lactate dehydrogenase of 628 U/L (normal: 140-280 U/L).
The patient’s medical history was significant for hypertension, osteoarthritis, and IgG-kappa multiple myeloma, which had been treated with multiple chemotherapy regimens that included lenalidomide. Five years earlier, he’d undergone an autologous bone marrow transplant (BMT). At the time of presentation, the patient was being treated with daratumumab; he received his most recent treatment approximately one month earlier. Other medications included amlodipine, esomeprazole, and escitalopram.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Disseminated varicella-zoster virus infection
Because of the patient’s immunocompromised state, his presentation with altered mental status and diffuse rash was concerning. On hospital Day 2, a sample was taken from one of his skin lesions. Polymerase chain reaction (PCR) detected varicella-zoster virus (VZV), and we diagnosed disseminated VZV infection. On hospital Day 3, we performed a lumbar puncture because of worsening confusion and discovered that the cerebrospinal fluid was also positive for VZV.
Disseminated VZV is the most common cause of late infection in patients who have received an allogenic BMT; it is usually due to reactivation of the virus.1 In one study of 1186 patients who underwent BMT, 52% developed VZV infection within 5 years.2 Disseminated VZV may also involve visceral organs, causing pneumonitis, pancreatitis, hepatitis, or encephalitis. Mortality rates for disseminated VZV are as high as 50%.3 Because of this, physicians should be vigilant when patients who have received a BMT present with a rash and signs of systemic involvement.
Two reliable tests. Even when lesions are classic for VZV, the diagnosis must be confirmed by laboratory testing. Real-time PCR assay is a rapid and highly sensitive test for diagnosing VZV.4 Another rapid test that can be used to confirm the clinical diagnosis of VZV is a direct fluorescent antibody assay, which is becoming more widely available.
In contrast, the sensitivity of viral culture for VZV has been reported to be as low as 20%.5 Viral culture also takes much longer and has a significantly lower yield compared with newer methods.6 A biopsy of skin lesions will reveal multinucleated giant cells, but cannot differentiate between herpes simplex virus (HSV) and VZV.7
These lesions can be mimicked
When a rash develops following the use of intravenous hydromorphone, as occurred with our patient, a drug reaction must be ruled out. A drug reaction can cause almost any skin manifestation and may present as vesicles, a macular rash, a papular rash, or diffuse erythema. In this case, drug rash was ruled out by the positive VZV PCR.
Viral exanthems can also present in a variety of ways. They may cause a macular, papular, or vesicular rash.
Prompt management is crucial
Prompt treatment of VZV with acyclovir improves outcomes, but death may still occur, even with early diagnosis.3 Immunocompromised patients with VZV should be closely monitored for secondary infections, which may rapidly progress and become fatal.8 The Centers for Disease Control and Prevention recommends both airborne and contact precautions for patients with disseminated VZV until all lesions are dry and crusted.9
While the live zoster vaccine is approved for prevention of shingles in patients <60 years of age, it is contraindicated in patients with a history of primary or acquired immunodeficiency states including leukemia, lymphoma, or other malignant neoplasms affecting bone marrow.
Our patient. On admission, he was treated with intravenous (IV) acyclovir 10 mg/kg TID; IV vancomycin 15 mg/kg every 12 hours; and IV ceftriaxone 2 g/d. Slowly, his mental status returned to baseline, and his rash and conjunctivitis resolved. We discharged him on hospital Day 12. He was transitioned to oral valacyclovir 1000 mg TID. Including both inpatient and outpatient treatment, the patient received 3 weeks (total) of acyclovir/valacyclovir therapy.
CORRESPONDENCE
Caitlyn T. Reed, MD, School of Medicine, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216; [email protected].
1. Locksley RM, Flournoy N, Sullivan KM, et al. Infection with varicella-zoster virus after marrow transplantation. J Infect Dis. 1985;152:1172-1181.
2. Han CS, Miller W, Haake R, et al. Varicella zoster infection after bone marrow transplantation: incidence, risk factors and complications. Bone Marrow Transplant. 1994;13:277-283.
3. David DS, Tegtmeier BR, O’Donnell MR, at el. Visceral varicella-zoster after bone marrow transplantation: report of a case series and review of the literature. Am J Gastroenterol. 1998;93:810-813.
4. Harbecke R, Oxman MN, Arnold BA, et al. A real-time PCR assay to identify and discriminate among wild-type and vaccine strains of varicella-zoster virus and herpes simplex virus in clinical specimens, and comparison with the clinical diagnoses. J Med Virol. 2009;81:1310-1322.
5. Sauerbrei A, Eichhorn U, Schacke M, et al. Laboratory diagnosis of herpes zoster. J Clin Virol. 1999;14:31-36.
6. Gnann JW Jr, Whitley RJ. Clinical practice. Herpes zoster. N Engl J Med. 2002;347;340-346.
7. Mendoza N, Madkan V, Sra K, et al. Human herpesviruses. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd edition. China: Elsevier Limited; 2012:1321-1343.
8. Woznowski M, Quack I, Bölke E, et al. Fulminant staphylococcus lugdunensis septicaemia following a pelvic varicella-zoster virus infection in an immune-deficient patient: a case report. Eur J Med Res. 2010;15:410-414.
9. Centers for Disease Control and Prevention. Preventing varicella in healthcare settings. Available at: http://www.cdc.gov/chickenpox/hcp/healthcare-setting.html. Accessed October 6,2017.
A 74-year-old Caucasian man presented to the hospital with intractable back and chest pain, a diffuse skin rash, and altered mental status. He said that 2 days ago, he’d gone to a different local hospital for treatment of back pain and a headache that had begun 3 days earlier. He was treated with intravenous hydromorphone and sent home with a prescription for meperidine. He said that several hours after being treated with the hydromorphone, the rash developed on his head and then spread to his trunk and upper extremities.
On physical examination, the patient was afebrile. He had numerous erythematous papules and vesicles in various stages of development on his scalp, face, neck, chest (FIGURE), abdomen, back, upper extremities, and groin. The lesions continued to spread and eventually involved his posterior oropharynx. The patient also developed conjunctivitis.

Laboratory findings included a white blood cell count of 4000/mcL (normal: 4500-11,000/mcL) with 65.9% segmented neutrophils (normal: 40%-60%), and 16.7% lymphocytes (normal: 20%-40%). Lab tests also revealed an aspartate aminotransferase level of 263 U/L (normal: 10-40 U/L), alanine aminotransferase of 236 U/L (normal: 7-56 U/L), and lactate dehydrogenase of 628 U/L (normal: 140-280 U/L).
The patient’s medical history was significant for hypertension, osteoarthritis, and IgG-kappa multiple myeloma, which had been treated with multiple chemotherapy regimens that included lenalidomide. Five years earlier, he’d undergone an autologous bone marrow transplant (BMT). At the time of presentation, the patient was being treated with daratumumab; he received his most recent treatment approximately one month earlier. Other medications included amlodipine, esomeprazole, and escitalopram.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Disseminated varicella-zoster virus infection
Because of the patient’s immunocompromised state, his presentation with altered mental status and diffuse rash was concerning. On hospital Day 2, a sample was taken from one of his skin lesions. Polymerase chain reaction (PCR) detected varicella-zoster virus (VZV), and we diagnosed disseminated VZV infection. On hospital Day 3, we performed a lumbar puncture because of worsening confusion and discovered that the cerebrospinal fluid was also positive for VZV.
Disseminated VZV is the most common cause of late infection in patients who have received an allogenic BMT; it is usually due to reactivation of the virus.1 In one study of 1186 patients who underwent BMT, 52% developed VZV infection within 5 years.2 Disseminated VZV may also involve visceral organs, causing pneumonitis, pancreatitis, hepatitis, or encephalitis. Mortality rates for disseminated VZV are as high as 50%.3 Because of this, physicians should be vigilant when patients who have received a BMT present with a rash and signs of systemic involvement.
Two reliable tests. Even when lesions are classic for VZV, the diagnosis must be confirmed by laboratory testing. Real-time PCR assay is a rapid and highly sensitive test for diagnosing VZV.4 Another rapid test that can be used to confirm the clinical diagnosis of VZV is a direct fluorescent antibody assay, which is becoming more widely available.
In contrast, the sensitivity of viral culture for VZV has been reported to be as low as 20%.5 Viral culture also takes much longer and has a significantly lower yield compared with newer methods.6 A biopsy of skin lesions will reveal multinucleated giant cells, but cannot differentiate between herpes simplex virus (HSV) and VZV.7
These lesions can be mimicked
When a rash develops following the use of intravenous hydromorphone, as occurred with our patient, a drug reaction must be ruled out. A drug reaction can cause almost any skin manifestation and may present as vesicles, a macular rash, a papular rash, or diffuse erythema. In this case, drug rash was ruled out by the positive VZV PCR.
Viral exanthems can also present in a variety of ways. They may cause a macular, papular, or vesicular rash.
Prompt management is crucial
Prompt treatment of VZV with acyclovir improves outcomes, but death may still occur, even with early diagnosis.3 Immunocompromised patients with VZV should be closely monitored for secondary infections, which may rapidly progress and become fatal.8 The Centers for Disease Control and Prevention recommends both airborne and contact precautions for patients with disseminated VZV until all lesions are dry and crusted.9
While the live zoster vaccine is approved for prevention of shingles in patients <60 years of age, it is contraindicated in patients with a history of primary or acquired immunodeficiency states including leukemia, lymphoma, or other malignant neoplasms affecting bone marrow.
Our patient. On admission, he was treated with intravenous (IV) acyclovir 10 mg/kg TID; IV vancomycin 15 mg/kg every 12 hours; and IV ceftriaxone 2 g/d. Slowly, his mental status returned to baseline, and his rash and conjunctivitis resolved. We discharged him on hospital Day 12. He was transitioned to oral valacyclovir 1000 mg TID. Including both inpatient and outpatient treatment, the patient received 3 weeks (total) of acyclovir/valacyclovir therapy.
CORRESPONDENCE
Caitlyn T. Reed, MD, School of Medicine, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216; [email protected].
A 74-year-old Caucasian man presented to the hospital with intractable back and chest pain, a diffuse skin rash, and altered mental status. He said that 2 days ago, he’d gone to a different local hospital for treatment of back pain and a headache that had begun 3 days earlier. He was treated with intravenous hydromorphone and sent home with a prescription for meperidine. He said that several hours after being treated with the hydromorphone, the rash developed on his head and then spread to his trunk and upper extremities.
On physical examination, the patient was afebrile. He had numerous erythematous papules and vesicles in various stages of development on his scalp, face, neck, chest (FIGURE), abdomen, back, upper extremities, and groin. The lesions continued to spread and eventually involved his posterior oropharynx. The patient also developed conjunctivitis.

Laboratory findings included a white blood cell count of 4000/mcL (normal: 4500-11,000/mcL) with 65.9% segmented neutrophils (normal: 40%-60%), and 16.7% lymphocytes (normal: 20%-40%). Lab tests also revealed an aspartate aminotransferase level of 263 U/L (normal: 10-40 U/L), alanine aminotransferase of 236 U/L (normal: 7-56 U/L), and lactate dehydrogenase of 628 U/L (normal: 140-280 U/L).
The patient’s medical history was significant for hypertension, osteoarthritis, and IgG-kappa multiple myeloma, which had been treated with multiple chemotherapy regimens that included lenalidomide. Five years earlier, he’d undergone an autologous bone marrow transplant (BMT). At the time of presentation, the patient was being treated with daratumumab; he received his most recent treatment approximately one month earlier. Other medications included amlodipine, esomeprazole, and escitalopram.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Disseminated varicella-zoster virus infection
Because of the patient’s immunocompromised state, his presentation with altered mental status and diffuse rash was concerning. On hospital Day 2, a sample was taken from one of his skin lesions. Polymerase chain reaction (PCR) detected varicella-zoster virus (VZV), and we diagnosed disseminated VZV infection. On hospital Day 3, we performed a lumbar puncture because of worsening confusion and discovered that the cerebrospinal fluid was also positive for VZV.
Disseminated VZV is the most common cause of late infection in patients who have received an allogenic BMT; it is usually due to reactivation of the virus.1 In one study of 1186 patients who underwent BMT, 52% developed VZV infection within 5 years.2 Disseminated VZV may also involve visceral organs, causing pneumonitis, pancreatitis, hepatitis, or encephalitis. Mortality rates for disseminated VZV are as high as 50%.3 Because of this, physicians should be vigilant when patients who have received a BMT present with a rash and signs of systemic involvement.
Two reliable tests. Even when lesions are classic for VZV, the diagnosis must be confirmed by laboratory testing. Real-time PCR assay is a rapid and highly sensitive test for diagnosing VZV.4 Another rapid test that can be used to confirm the clinical diagnosis of VZV is a direct fluorescent antibody assay, which is becoming more widely available.
In contrast, the sensitivity of viral culture for VZV has been reported to be as low as 20%.5 Viral culture also takes much longer and has a significantly lower yield compared with newer methods.6 A biopsy of skin lesions will reveal multinucleated giant cells, but cannot differentiate between herpes simplex virus (HSV) and VZV.7
These lesions can be mimicked
When a rash develops following the use of intravenous hydromorphone, as occurred with our patient, a drug reaction must be ruled out. A drug reaction can cause almost any skin manifestation and may present as vesicles, a macular rash, a papular rash, or diffuse erythema. In this case, drug rash was ruled out by the positive VZV PCR.
Viral exanthems can also present in a variety of ways. They may cause a macular, papular, or vesicular rash.
Prompt management is crucial
Prompt treatment of VZV with acyclovir improves outcomes, but death may still occur, even with early diagnosis.3 Immunocompromised patients with VZV should be closely monitored for secondary infections, which may rapidly progress and become fatal.8 The Centers for Disease Control and Prevention recommends both airborne and contact precautions for patients with disseminated VZV until all lesions are dry and crusted.9
While the live zoster vaccine is approved for prevention of shingles in patients <60 years of age, it is contraindicated in patients with a history of primary or acquired immunodeficiency states including leukemia, lymphoma, or other malignant neoplasms affecting bone marrow.
Our patient. On admission, he was treated with intravenous (IV) acyclovir 10 mg/kg TID; IV vancomycin 15 mg/kg every 12 hours; and IV ceftriaxone 2 g/d. Slowly, his mental status returned to baseline, and his rash and conjunctivitis resolved. We discharged him on hospital Day 12. He was transitioned to oral valacyclovir 1000 mg TID. Including both inpatient and outpatient treatment, the patient received 3 weeks (total) of acyclovir/valacyclovir therapy.
CORRESPONDENCE
Caitlyn T. Reed, MD, School of Medicine, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216; [email protected].
1. Locksley RM, Flournoy N, Sullivan KM, et al. Infection with varicella-zoster virus after marrow transplantation. J Infect Dis. 1985;152:1172-1181.
2. Han CS, Miller W, Haake R, et al. Varicella zoster infection after bone marrow transplantation: incidence, risk factors and complications. Bone Marrow Transplant. 1994;13:277-283.
3. David DS, Tegtmeier BR, O’Donnell MR, at el. Visceral varicella-zoster after bone marrow transplantation: report of a case series and review of the literature. Am J Gastroenterol. 1998;93:810-813.
4. Harbecke R, Oxman MN, Arnold BA, et al. A real-time PCR assay to identify and discriminate among wild-type and vaccine strains of varicella-zoster virus and herpes simplex virus in clinical specimens, and comparison with the clinical diagnoses. J Med Virol. 2009;81:1310-1322.
5. Sauerbrei A, Eichhorn U, Schacke M, et al. Laboratory diagnosis of herpes zoster. J Clin Virol. 1999;14:31-36.
6. Gnann JW Jr, Whitley RJ. Clinical practice. Herpes zoster. N Engl J Med. 2002;347;340-346.
7. Mendoza N, Madkan V, Sra K, et al. Human herpesviruses. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd edition. China: Elsevier Limited; 2012:1321-1343.
8. Woznowski M, Quack I, Bölke E, et al. Fulminant staphylococcus lugdunensis septicaemia following a pelvic varicella-zoster virus infection in an immune-deficient patient: a case report. Eur J Med Res. 2010;15:410-414.
9. Centers for Disease Control and Prevention. Preventing varicella in healthcare settings. Available at: http://www.cdc.gov/chickenpox/hcp/healthcare-setting.html. Accessed October 6,2017.
1. Locksley RM, Flournoy N, Sullivan KM, et al. Infection with varicella-zoster virus after marrow transplantation. J Infect Dis. 1985;152:1172-1181.
2. Han CS, Miller W, Haake R, et al. Varicella zoster infection after bone marrow transplantation: incidence, risk factors and complications. Bone Marrow Transplant. 1994;13:277-283.
3. David DS, Tegtmeier BR, O’Donnell MR, at el. Visceral varicella-zoster after bone marrow transplantation: report of a case series and review of the literature. Am J Gastroenterol. 1998;93:810-813.
4. Harbecke R, Oxman MN, Arnold BA, et al. A real-time PCR assay to identify and discriminate among wild-type and vaccine strains of varicella-zoster virus and herpes simplex virus in clinical specimens, and comparison with the clinical diagnoses. J Med Virol. 2009;81:1310-1322.
5. Sauerbrei A, Eichhorn U, Schacke M, et al. Laboratory diagnosis of herpes zoster. J Clin Virol. 1999;14:31-36.
6. Gnann JW Jr, Whitley RJ. Clinical practice. Herpes zoster. N Engl J Med. 2002;347;340-346.
7. Mendoza N, Madkan V, Sra K, et al. Human herpesviruses. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd edition. China: Elsevier Limited; 2012:1321-1343.
8. Woznowski M, Quack I, Bölke E, et al. Fulminant staphylococcus lugdunensis septicaemia following a pelvic varicella-zoster virus infection in an immune-deficient patient: a case report. Eur J Med Res. 2010;15:410-414.
9. Centers for Disease Control and Prevention. Preventing varicella in healthcare settings. Available at: http://www.cdc.gov/chickenpox/hcp/healthcare-setting.html. Accessed October 6,2017.
Swollen toes
A 15-month-old black male was brought to the pediatric emergency department by his grandmother because she was concerned about his 2 swollen big toes. The patient’s grandmother said that the swelling began 36 hours prior and that her grandson’s big toes had continued to increase in size. She denied trauma, bites, or unusual exposures and said that although her grandson had been fussier than usual that day, he was eating and drinking normally and had normal urine output.
The patient had a history of developmental delay, but was otherwise healthy. He had no rashes, and there was no recent history of vomiting, diarrhea, difficulty breathing, or fever.
Examination of the patient’s skin revealed diffuse edema and erythema of the bilateral great toes (FIGURE 1A), with large overlying bullae extending from the dorsal surface of the base of the great toes around to the plantar (volar) surface of the foot (FIGURE 1B). The bullae on the plantar surface were approximately 4 cm long, extending from the tip of the toes proximally to the region of the head of the first metatarsal.
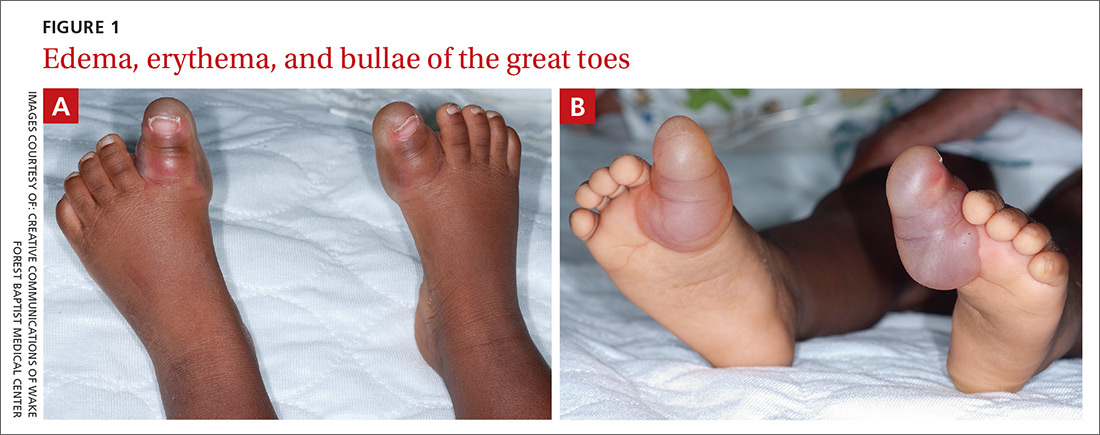
The patient’s vital signs were notable for a rectal temperature of 100.2° F and a heart rate of 180 beats per minute.
Initial lab tests included a complete blood count (CBC), blood cultures, and urinalysis with urine culture. The CBC revealed a white blood count of 27,000/mcL (normal: 6000-17,500/mcL). Both wound culture and herpes simplex viral culture were negative. An intranasal surveillance culture for methicillin-resistant Staphylococcus aureus (MRSA) was also negative.
Given the patient’s fever and leukocytosis, a 100-mg dose of intravenous clindamycin was administered.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Blistering distal dactylitis
We made a clinical diagnosis of blistering distal dactylitis (BDD), a condition typically caused by infection with Gram-positive bacteria. BDD is generally described as a localized infection of the volar fat pads of one or more fingers. The infection may also occur more proximally on the hand or involve the thumbs or toes.1
Who’s at risk? BDD occurs among children ages 2 to 16 years, although it has been reported in infants as young as 6 months and in adults. No cases have occurred among the elderly.2-7
The most common etiologic agents are group A beta-hemolytic Streptococci. Less commonly reported agents include Staphylococcus aureus, S. epidermidis, group B Streptococci, and MRSA.1,6,8 The presence of multiple bullae may be predictive of infection with S. aureus.9
A clinical diagnosis
Diagnosis is usually made on clinical grounds based on the presence of large, tense, superficial, and typically painful bullae, the base of which may be erythematous. Culture of the blister fluid and the base of an unroofed blister may confirm the presence of a Streptococcus or Staphylococcus species.
Lab tests are typically not required to confirm a diagnosis of BDD. However, wound cultures of blister fluid, rapid antigen testing for group A beta-hemolytic Streptococci, and viral culture or polymerase chain reaction testing for herpes simplex virus may be considered.
Rule these conditions out
Lesions similar to those seen with BDD can be caused by the following infections and irritants:4,5,8
Herpetic whitlow is caused by a herpes simplex virus infection. It presents as a cluster of painful vesicles or ulcers with an erythematous base on the distal part of a finger or toe.
Bullous impetigo is the result of a staphylococcal infection, which produces an epidermolytic toxin leading to bulla formation. Lesions may occur anywhere on the body but are most common on the face.
Irritant or allergic contact dermatitis results from an external topical exposure and is typically localized to the area of contact. The reaction is an eczematous eruption that may include bullae.
Treatment is typically empiric
Treatment of BDD includes wound care with wet-to-dry saline dressings, incision and drainage of the bulla(e), and a systemic beta-lactamase-resistant antibiotic. Topical antibiotics alone are not recommended.7
Our patient was transitioned from intravenous to oral clindamycin, 100 mg every 8 hours, and the bullae were incised and drained. His leukocytosis resolved within 24 hours, and he continued to do well. At follow-up one week later, the patient’s blisters were healing well, and he was playful and eating and drinking normally.
CORRESPONDENCE
C. Randall Clinch, DO, MS, Wake Forest University School of Medicine, 1 Medical Center Blvd, Winston-Salem, NC 27157; [email protected].
1. Hays GC, Mullard JE. Blistering distal dactylitis: a clinically recognizable streptococcal infection. Pediatrics. 1975;56:129-131.
2. Schneider JA, Parlette HL 3rd. Blistering distal dactylitis: a manifestation of group A beta-hemolytic streptococcal infection. Arch Dermatol. 1982;118:879-880.
3. Scheinfeld NS. Is blistering distal dactylitis a variant of bullous impetigo? Clin Exp Dermatol. 2007;32:314-316.
4. Kollipara R, Downing C, Lee M, et al. Blistering distal dactylitis in an adult. J Cutan Med Surg. 2015;19:397-399.
5. Fretzayas A, Moustaki M, Tsagris V, et al. MRSA blistering distal dactylitis and review of reported cases. Pediatr Dermatol. 2011;28:433-435.
6. Lyon M, Doehring MC. Blistering distal dactylitis: a case series in children under nine months of age. J Emerg Med. 2004;26:421-423.
7. Frieden IJ. Blistering dactylitis caused by group B streptococci. Pediatr Dermatol. 1989;6:300-302.
8. Woroszylski A, Durán C, Tamayo L, et al. Staphylococcal blistering dactylitis: report of two patients. Pediatr Dermatol. 1996;13:292-293.
9. Norcross MC Jr, Mitchell DF. Blistering distal dactylitis caused by Staphylococcus aureus. Cutis. 1993;51:353-354 .
A 15-month-old black male was brought to the pediatric emergency department by his grandmother because she was concerned about his 2 swollen big toes. The patient’s grandmother said that the swelling began 36 hours prior and that her grandson’s big toes had continued to increase in size. She denied trauma, bites, or unusual exposures and said that although her grandson had been fussier than usual that day, he was eating and drinking normally and had normal urine output.
The patient had a history of developmental delay, but was otherwise healthy. He had no rashes, and there was no recent history of vomiting, diarrhea, difficulty breathing, or fever.
Examination of the patient’s skin revealed diffuse edema and erythema of the bilateral great toes (FIGURE 1A), with large overlying bullae extending from the dorsal surface of the base of the great toes around to the plantar (volar) surface of the foot (FIGURE 1B). The bullae on the plantar surface were approximately 4 cm long, extending from the tip of the toes proximally to the region of the head of the first metatarsal.

The patient’s vital signs were notable for a rectal temperature of 100.2° F and a heart rate of 180 beats per minute.
Initial lab tests included a complete blood count (CBC), blood cultures, and urinalysis with urine culture. The CBC revealed a white blood count of 27,000/mcL (normal: 6000-17,500/mcL). Both wound culture and herpes simplex viral culture were negative. An intranasal surveillance culture for methicillin-resistant Staphylococcus aureus (MRSA) was also negative.
Given the patient’s fever and leukocytosis, a 100-mg dose of intravenous clindamycin was administered.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Blistering distal dactylitis
We made a clinical diagnosis of blistering distal dactylitis (BDD), a condition typically caused by infection with Gram-positive bacteria. BDD is generally described as a localized infection of the volar fat pads of one or more fingers. The infection may also occur more proximally on the hand or involve the thumbs or toes.1
Who’s at risk? BDD occurs among children ages 2 to 16 years, although it has been reported in infants as young as 6 months and in adults. No cases have occurred among the elderly.2-7
The most common etiologic agents are group A beta-hemolytic Streptococci. Less commonly reported agents include Staphylococcus aureus, S. epidermidis, group B Streptococci, and MRSA.1,6,8 The presence of multiple bullae may be predictive of infection with S. aureus.9
A clinical diagnosis
Diagnosis is usually made on clinical grounds based on the presence of large, tense, superficial, and typically painful bullae, the base of which may be erythematous. Culture of the blister fluid and the base of an unroofed blister may confirm the presence of a Streptococcus or Staphylococcus species.
Lab tests are typically not required to confirm a diagnosis of BDD. However, wound cultures of blister fluid, rapid antigen testing for group A beta-hemolytic Streptococci, and viral culture or polymerase chain reaction testing for herpes simplex virus may be considered.
Rule these conditions out
Lesions similar to those seen with BDD can be caused by the following infections and irritants:4,5,8
Herpetic whitlow is caused by a herpes simplex virus infection. It presents as a cluster of painful vesicles or ulcers with an erythematous base on the distal part of a finger or toe.
Bullous impetigo is the result of a staphylococcal infection, which produces an epidermolytic toxin leading to bulla formation. Lesions may occur anywhere on the body but are most common on the face.
Irritant or allergic contact dermatitis results from an external topical exposure and is typically localized to the area of contact. The reaction is an eczematous eruption that may include bullae.
Treatment is typically empiric
Treatment of BDD includes wound care with wet-to-dry saline dressings, incision and drainage of the bulla(e), and a systemic beta-lactamase-resistant antibiotic. Topical antibiotics alone are not recommended.7
Our patient was transitioned from intravenous to oral clindamycin, 100 mg every 8 hours, and the bullae were incised and drained. His leukocytosis resolved within 24 hours, and he continued to do well. At follow-up one week later, the patient’s blisters were healing well, and he was playful and eating and drinking normally.
CORRESPONDENCE
C. Randall Clinch, DO, MS, Wake Forest University School of Medicine, 1 Medical Center Blvd, Winston-Salem, NC 27157; [email protected].
A 15-month-old black male was brought to the pediatric emergency department by his grandmother because she was concerned about his 2 swollen big toes. The patient’s grandmother said that the swelling began 36 hours prior and that her grandson’s big toes had continued to increase in size. She denied trauma, bites, or unusual exposures and said that although her grandson had been fussier than usual that day, he was eating and drinking normally and had normal urine output.
The patient had a history of developmental delay, but was otherwise healthy. He had no rashes, and there was no recent history of vomiting, diarrhea, difficulty breathing, or fever.
Examination of the patient’s skin revealed diffuse edema and erythema of the bilateral great toes (FIGURE 1A), with large overlying bullae extending from the dorsal surface of the base of the great toes around to the plantar (volar) surface of the foot (FIGURE 1B). The bullae on the plantar surface were approximately 4 cm long, extending from the tip of the toes proximally to the region of the head of the first metatarsal.

The patient’s vital signs were notable for a rectal temperature of 100.2° F and a heart rate of 180 beats per minute.
Initial lab tests included a complete blood count (CBC), blood cultures, and urinalysis with urine culture. The CBC revealed a white blood count of 27,000/mcL (normal: 6000-17,500/mcL). Both wound culture and herpes simplex viral culture were negative. An intranasal surveillance culture for methicillin-resistant Staphylococcus aureus (MRSA) was also negative.
Given the patient’s fever and leukocytosis, a 100-mg dose of intravenous clindamycin was administered.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Blistering distal dactylitis
We made a clinical diagnosis of blistering distal dactylitis (BDD), a condition typically caused by infection with Gram-positive bacteria. BDD is generally described as a localized infection of the volar fat pads of one or more fingers. The infection may also occur more proximally on the hand or involve the thumbs or toes.1
Who’s at risk? BDD occurs among children ages 2 to 16 years, although it has been reported in infants as young as 6 months and in adults. No cases have occurred among the elderly.2-7
The most common etiologic agents are group A beta-hemolytic Streptococci. Less commonly reported agents include Staphylococcus aureus, S. epidermidis, group B Streptococci, and MRSA.1,6,8 The presence of multiple bullae may be predictive of infection with S. aureus.9
A clinical diagnosis
Diagnosis is usually made on clinical grounds based on the presence of large, tense, superficial, and typically painful bullae, the base of which may be erythematous. Culture of the blister fluid and the base of an unroofed blister may confirm the presence of a Streptococcus or Staphylococcus species.
Lab tests are typically not required to confirm a diagnosis of BDD. However, wound cultures of blister fluid, rapid antigen testing for group A beta-hemolytic Streptococci, and viral culture or polymerase chain reaction testing for herpes simplex virus may be considered.
Rule these conditions out
Lesions similar to those seen with BDD can be caused by the following infections and irritants:4,5,8
Herpetic whitlow is caused by a herpes simplex virus infection. It presents as a cluster of painful vesicles or ulcers with an erythematous base on the distal part of a finger or toe.
Bullous impetigo is the result of a staphylococcal infection, which produces an epidermolytic toxin leading to bulla formation. Lesions may occur anywhere on the body but are most common on the face.
Irritant or allergic contact dermatitis results from an external topical exposure and is typically localized to the area of contact. The reaction is an eczematous eruption that may include bullae.
Treatment is typically empiric
Treatment of BDD includes wound care with wet-to-dry saline dressings, incision and drainage of the bulla(e), and a systemic beta-lactamase-resistant antibiotic. Topical antibiotics alone are not recommended.7
Our patient was transitioned from intravenous to oral clindamycin, 100 mg every 8 hours, and the bullae were incised and drained. His leukocytosis resolved within 24 hours, and he continued to do well. At follow-up one week later, the patient’s blisters were healing well, and he was playful and eating and drinking normally.
CORRESPONDENCE
C. Randall Clinch, DO, MS, Wake Forest University School of Medicine, 1 Medical Center Blvd, Winston-Salem, NC 27157; [email protected].
1. Hays GC, Mullard JE. Blistering distal dactylitis: a clinically recognizable streptococcal infection. Pediatrics. 1975;56:129-131.
2. Schneider JA, Parlette HL 3rd. Blistering distal dactylitis: a manifestation of group A beta-hemolytic streptococcal infection. Arch Dermatol. 1982;118:879-880.
3. Scheinfeld NS. Is blistering distal dactylitis a variant of bullous impetigo? Clin Exp Dermatol. 2007;32:314-316.
4. Kollipara R, Downing C, Lee M, et al. Blistering distal dactylitis in an adult. J Cutan Med Surg. 2015;19:397-399.
5. Fretzayas A, Moustaki M, Tsagris V, et al. MRSA blistering distal dactylitis and review of reported cases. Pediatr Dermatol. 2011;28:433-435.
6. Lyon M, Doehring MC. Blistering distal dactylitis: a case series in children under nine months of age. J Emerg Med. 2004;26:421-423.
7. Frieden IJ. Blistering dactylitis caused by group B streptococci. Pediatr Dermatol. 1989;6:300-302.
8. Woroszylski A, Durán C, Tamayo L, et al. Staphylococcal blistering dactylitis: report of two patients. Pediatr Dermatol. 1996;13:292-293.
9. Norcross MC Jr, Mitchell DF. Blistering distal dactylitis caused by Staphylococcus aureus. Cutis. 1993;51:353-354 .
1. Hays GC, Mullard JE. Blistering distal dactylitis: a clinically recognizable streptococcal infection. Pediatrics. 1975;56:129-131.
2. Schneider JA, Parlette HL 3rd. Blistering distal dactylitis: a manifestation of group A beta-hemolytic streptococcal infection. Arch Dermatol. 1982;118:879-880.
3. Scheinfeld NS. Is blistering distal dactylitis a variant of bullous impetigo? Clin Exp Dermatol. 2007;32:314-316.
4. Kollipara R, Downing C, Lee M, et al. Blistering distal dactylitis in an adult. J Cutan Med Surg. 2015;19:397-399.
5. Fretzayas A, Moustaki M, Tsagris V, et al. MRSA blistering distal dactylitis and review of reported cases. Pediatr Dermatol. 2011;28:433-435.
6. Lyon M, Doehring MC. Blistering distal dactylitis: a case series in children under nine months of age. J Emerg Med. 2004;26:421-423.
7. Frieden IJ. Blistering dactylitis caused by group B streptococci. Pediatr Dermatol. 1989;6:300-302.
8. Woroszylski A, Durán C, Tamayo L, et al. Staphylococcal blistering dactylitis: report of two patients. Pediatr Dermatol. 1996;13:292-293.
9. Norcross MC Jr, Mitchell DF. Blistering distal dactylitis caused by Staphylococcus aureus. Cutis. 1993;51:353-354 .
Hip pain • difficulty walking • tenderness along the anteromedial thigh and groin • Dx?
THE CASE
A 14-year-old Caucasian boy presented to our clinic with a complaint of left anterior hip pain. The patient had been running during a flag football match when he suddenly developed a sharp, stabbing pain in his left hip. He said he felt a “pop” in his left groin while his left foot was planted and he was cutting to the right. The patient said this was followed by worsening pain with ambulation and hip flexion.
The patient had considerable difficulty walking into the exam room. On physical examination, he had significant tenderness to palpation along the anteromedial thigh and groin. The patient’s strength was 1/5 with left hip flexion. There was apparent muscle firing, but no significant leg movement. He had full passive range of motion and there was no soft-tissue swelling, erythema, or other integumentary changes.
THE DIAGNOSIS
Plain radiographs revealed a lesser trochanter avulsion fracture with a 2-cm displacement (FIGURE 1).
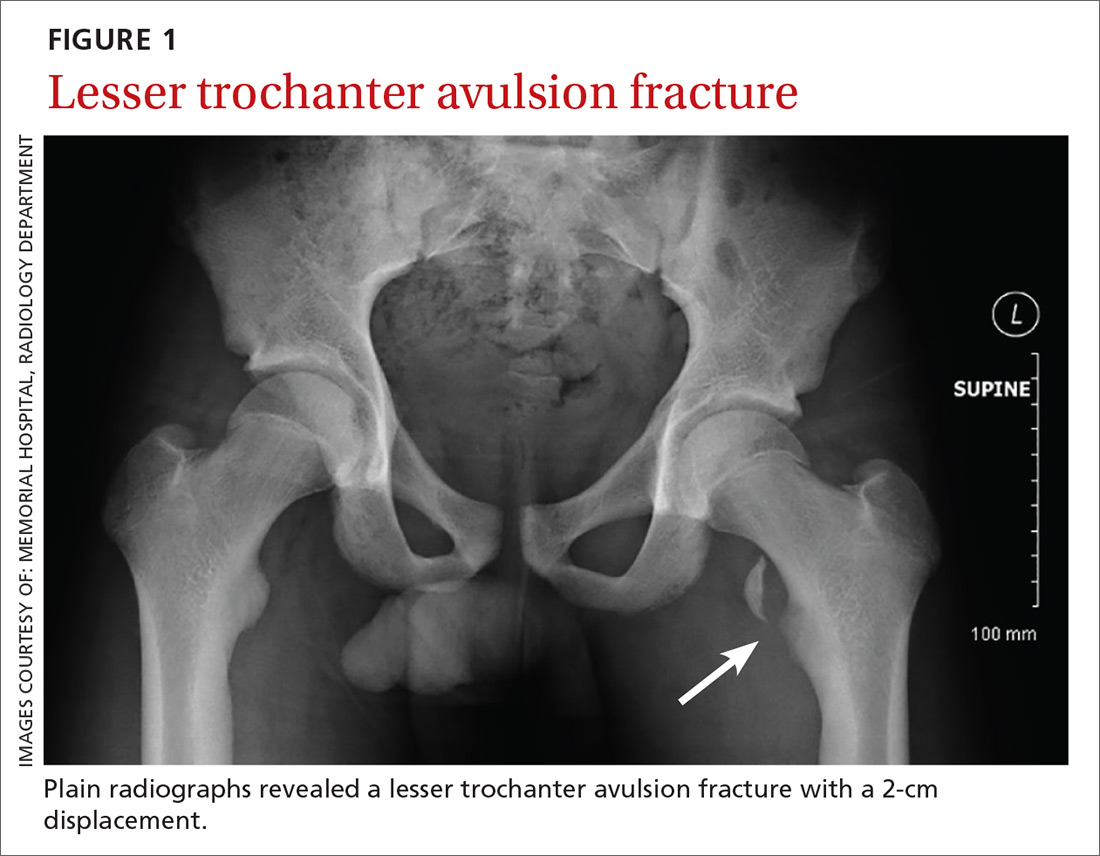
DISCUSSION
Pelvic and proximal femur avulsion fractures tend to occur during the second decade of life.1,2 They’re more frequently seen in boys and adolescent athletes, especially those involved in soccer and gymnastics.3,4
Anterior superior iliac spine (ASIS), ischial tuberosity (IT), and anterior inferior iliac spine (AIIS) avulsion fractures are more prevalent,4 while lesser trochanter avulsion fractures are more rare. In one review of 1126 children with femoral neck and proximal 1/3 femoral shaft fractures, only 3 of them had lesser trochanter avulsion fractures.5
Clinical presentation. Presenting symptoms of lesser trochanter avulsion fractures can be vague, but are usually localized to the groin and medial hip region. Patients will demonstrate pain and weakness with hip flexion.3,6 There may be signs of inflammation, tenderness, and ecchymosis near the site of injury.
On physical exam, a positive Ludloff sign helps localize the injury to the iliopsoas muscle, which inserts at the lesser trochanter and is involved in hip flexion.3,6,7 The Ludloff test is performed by flexing the patient’s hip while he/she is in a seated position.
BIOMECHANICS OF AVULSION FRACTURES
Perhaps surprisingly, the majority of avulsion injuries in children and adolescents are the result of non-contact athletic movement and indirect trauma.4 In children, muscles and tendons are often stronger than their bones,7 and physes—structurally weak regions—are particularly predisposed to fractures.2,4,6
The mechanism of injury in children and adolescents is commonly a sudden, forceful contraction of the iliopsoas muscle.6,7 While similar movement in adults will produce tendon sprains and muscle strains, children often experience a complete avulsion fracture.7 So uncommon are these fractures among adults that an adult patient presenting with one should receive further work-up for underlying pathology such as malignancy.8,9
While other hip and femur avulsion fractures in children and adolescents involve different muscle groups, the etiologic mechanism—forceful muscle contraction—is usually the same.2,4,7 IT injuries are often seen with sudden, aggressive lengthening of the hamstring muscles, whereas injuries to the ASIS and AIIS are the result of abrupt eccentric contraction of hip extensor muscles while the knee is flexed.4
DIFFERENTIAL DIAGNOSIS
There are several entities that can mimic a lesser trochanter avulsion fracture including Legg-Calve-Perthes disease (LCPD), slipped capital femoral epiphysis (SCFE), snapping hip with the iliofemoral ligament, iliopsoas tendonitis, referred pain from the gastrointestinal region, and a genito-urologic etiology.1,7,10
Diagnostic studies. Physical exam findings of severe pain and reduced strength are clear indications for obtaining baseline imaging. Baseline radiographs are key to the diagnosis of avulsion fractures. They help differentiate between more benign fractures, such as a nondisplaced avulsion fracture, and more substantial conditions, such as LCPD and SCFE, which require significantly different approaches to treatment and follow-up.1,7
Anteroposterior, oblique, and axial views of the pelvis all assist in assessing avulsion fractures radiographically.3,4,7 In the event that an avulsion fracture is not radiographically visible, but is still suspected, additional imaging should be obtained.10 A computerized tomography (CT) scan is an appropriate follow-up, given its meticulous detail of bony anatomy.3,10 Alternatively, if physes have yet to ossify or there are concerns about soft tissue injury, magnetic resonance imaging can be useful.3,7,10
MANAGEMENT
The majority of lesser trochanter avulsion fractures are managed conservatively with rest, nonsteroidal anti-inflammatory drugs (NSAIDs), and physical therapy. Patients are often placed on non-weight bearing activity for up to 6 weeks while the fracture repairs and forms a new union.7 Current management strategies have moved away from immobilization with splints and braces.
In rare instances when the fragment is displaced >2 cm, or there is inadequate healing or pain relief after 3 months of supportive care, surgery may be required.1 With appropriate diagnosis and medical care, the injured athlete should fully recover with no impairment or chronic pain.2
Our patient was placed on non-weight-bearing activity and treated with NSAIDs and acetaminophen. We advanced him to weight-bearing activities 4 weeks after injury. After 8 weeks of conservative management, he returned to competitive play with no further complications (FIGURE 2).
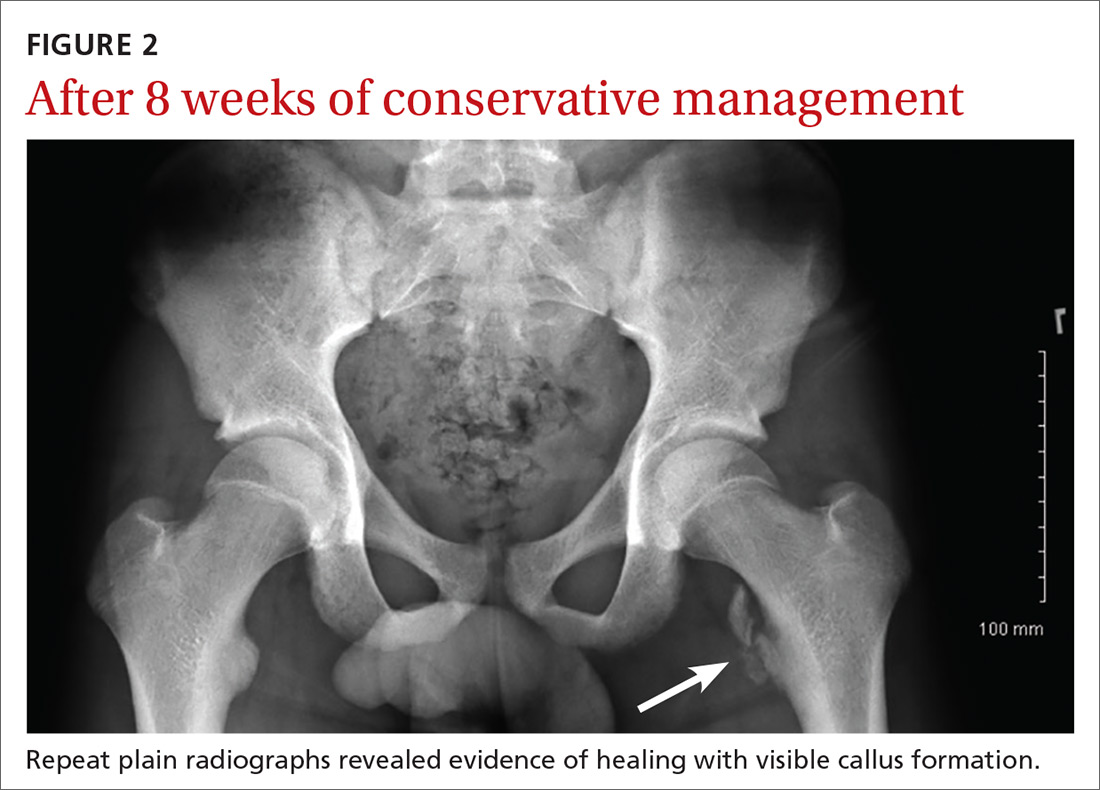
THE TAKEAWAY
Pelvic and proximal femur avulsion fractures occur more often in child and adolescent athletes. As this population becomes increasingly competitive in athletics, the risk of injury increases. Infrequent fractures such as lesser trochanter avulsion fractures may become more common, as well. The majority of avulsion fractures don’t require surgical intervention, but it’s important to obtain baseline radiographs to rule out other injuries or pathologies that may lead to poor prognoses if they are left untreated.
1. Byrne A, Reidy D. Acute groin pain in an adolescent sprinter: a case report. Int J Clin Pediatr. 2012;1:46-48.
2. Fernbach SK, Wilkinson RH. Avulsion injuries of the pelvis and proximal femur. AJR Am J Roentgenol. 1981;137:581-584.
3. McKinney BI, Nelson C, Carrion W. Apophyseal avulsion fractures of the hip and pelvis. Orthopedics. 2009;32:42.
4. Rossi F, Dragoni S. Acute avulsion fractures of the pelvis in adolescent competitive athletes: prevalence, location and sports distribution of 203 cases collected. Skeletal Radiol. 2001;30:127-131.
5. Theologis TN, Epps H, Latz K, et al. Isolated fractures of the lesser trochanter in children. Injury. 1997;28:363-364.
6. Paluska SA. An overview of hip injuries in running. Sports Med. 2005;35:991-1014.
7. Vazquez E, Kim TY, Young TP. Avulsion fracture of the lesser trochanter: an unusual cause of hip pain in an adolescent. CJEM. 2013;15:123-125.
8. Afra R, Boardman DL, Kabo JM, et al. Avulsion fracture of the lesser trochanter as a result of a preliminary malignant tumor of bone. A report of four cases. J Bone Joint Surg Am. 1999;81:1299-1304.
9. DePasse JM, Varner K, Cosculluela P, et al. Atraumatic avulsion of the distal iliopsoas tendon: an unusual cause of hip pain. Orthopedics. 2010;33.
10. Suarez JC, Ely EE, Mutnal AB, et al. Comprehensive approach to the evaluation of groin pain. J Am Acad Orthop Surg. 2013;21:558-570.
THE CASE
A 14-year-old Caucasian boy presented to our clinic with a complaint of left anterior hip pain. The patient had been running during a flag football match when he suddenly developed a sharp, stabbing pain in his left hip. He said he felt a “pop” in his left groin while his left foot was planted and he was cutting to the right. The patient said this was followed by worsening pain with ambulation and hip flexion.
The patient had considerable difficulty walking into the exam room. On physical examination, he had significant tenderness to palpation along the anteromedial thigh and groin. The patient’s strength was 1/5 with left hip flexion. There was apparent muscle firing, but no significant leg movement. He had full passive range of motion and there was no soft-tissue swelling, erythema, or other integumentary changes.
THE DIAGNOSIS
Plain radiographs revealed a lesser trochanter avulsion fracture with a 2-cm displacement (FIGURE 1).

DISCUSSION
Pelvic and proximal femur avulsion fractures tend to occur during the second decade of life.1,2 They’re more frequently seen in boys and adolescent athletes, especially those involved in soccer and gymnastics.3,4
Anterior superior iliac spine (ASIS), ischial tuberosity (IT), and anterior inferior iliac spine (AIIS) avulsion fractures are more prevalent,4 while lesser trochanter avulsion fractures are more rare. In one review of 1126 children with femoral neck and proximal 1/3 femoral shaft fractures, only 3 of them had lesser trochanter avulsion fractures.5
Clinical presentation. Presenting symptoms of lesser trochanter avulsion fractures can be vague, but are usually localized to the groin and medial hip region. Patients will demonstrate pain and weakness with hip flexion.3,6 There may be signs of inflammation, tenderness, and ecchymosis near the site of injury.
On physical exam, a positive Ludloff sign helps localize the injury to the iliopsoas muscle, which inserts at the lesser trochanter and is involved in hip flexion.3,6,7 The Ludloff test is performed by flexing the patient’s hip while he/she is in a seated position.
BIOMECHANICS OF AVULSION FRACTURES
Perhaps surprisingly, the majority of avulsion injuries in children and adolescents are the result of non-contact athletic movement and indirect trauma.4 In children, muscles and tendons are often stronger than their bones,7 and physes—structurally weak regions—are particularly predisposed to fractures.2,4,6
The mechanism of injury in children and adolescents is commonly a sudden, forceful contraction of the iliopsoas muscle.6,7 While similar movement in adults will produce tendon sprains and muscle strains, children often experience a complete avulsion fracture.7 So uncommon are these fractures among adults that an adult patient presenting with one should receive further work-up for underlying pathology such as malignancy.8,9
While other hip and femur avulsion fractures in children and adolescents involve different muscle groups, the etiologic mechanism—forceful muscle contraction—is usually the same.2,4,7 IT injuries are often seen with sudden, aggressive lengthening of the hamstring muscles, whereas injuries to the ASIS and AIIS are the result of abrupt eccentric contraction of hip extensor muscles while the knee is flexed.4
DIFFERENTIAL DIAGNOSIS
There are several entities that can mimic a lesser trochanter avulsion fracture including Legg-Calve-Perthes disease (LCPD), slipped capital femoral epiphysis (SCFE), snapping hip with the iliofemoral ligament, iliopsoas tendonitis, referred pain from the gastrointestinal region, and a genito-urologic etiology.1,7,10
Diagnostic studies. Physical exam findings of severe pain and reduced strength are clear indications for obtaining baseline imaging. Baseline radiographs are key to the diagnosis of avulsion fractures. They help differentiate between more benign fractures, such as a nondisplaced avulsion fracture, and more substantial conditions, such as LCPD and SCFE, which require significantly different approaches to treatment and follow-up.1,7
Anteroposterior, oblique, and axial views of the pelvis all assist in assessing avulsion fractures radiographically.3,4,7 In the event that an avulsion fracture is not radiographically visible, but is still suspected, additional imaging should be obtained.10 A computerized tomography (CT) scan is an appropriate follow-up, given its meticulous detail of bony anatomy.3,10 Alternatively, if physes have yet to ossify or there are concerns about soft tissue injury, magnetic resonance imaging can be useful.3,7,10
MANAGEMENT
The majority of lesser trochanter avulsion fractures are managed conservatively with rest, nonsteroidal anti-inflammatory drugs (NSAIDs), and physical therapy. Patients are often placed on non-weight bearing activity for up to 6 weeks while the fracture repairs and forms a new union.7 Current management strategies have moved away from immobilization with splints and braces.
In rare instances when the fragment is displaced >2 cm, or there is inadequate healing or pain relief after 3 months of supportive care, surgery may be required.1 With appropriate diagnosis and medical care, the injured athlete should fully recover with no impairment or chronic pain.2
Our patient was placed on non-weight-bearing activity and treated with NSAIDs and acetaminophen. We advanced him to weight-bearing activities 4 weeks after injury. After 8 weeks of conservative management, he returned to competitive play with no further complications (FIGURE 2).

THE TAKEAWAY
Pelvic and proximal femur avulsion fractures occur more often in child and adolescent athletes. As this population becomes increasingly competitive in athletics, the risk of injury increases. Infrequent fractures such as lesser trochanter avulsion fractures may become more common, as well. The majority of avulsion fractures don’t require surgical intervention, but it’s important to obtain baseline radiographs to rule out other injuries or pathologies that may lead to poor prognoses if they are left untreated.
THE CASE
A 14-year-old Caucasian boy presented to our clinic with a complaint of left anterior hip pain. The patient had been running during a flag football match when he suddenly developed a sharp, stabbing pain in his left hip. He said he felt a “pop” in his left groin while his left foot was planted and he was cutting to the right. The patient said this was followed by worsening pain with ambulation and hip flexion.
The patient had considerable difficulty walking into the exam room. On physical examination, he had significant tenderness to palpation along the anteromedial thigh and groin. The patient’s strength was 1/5 with left hip flexion. There was apparent muscle firing, but no significant leg movement. He had full passive range of motion and there was no soft-tissue swelling, erythema, or other integumentary changes.
THE DIAGNOSIS
Plain radiographs revealed a lesser trochanter avulsion fracture with a 2-cm displacement (FIGURE 1).

DISCUSSION
Pelvic and proximal femur avulsion fractures tend to occur during the second decade of life.1,2 They’re more frequently seen in boys and adolescent athletes, especially those involved in soccer and gymnastics.3,4
Anterior superior iliac spine (ASIS), ischial tuberosity (IT), and anterior inferior iliac spine (AIIS) avulsion fractures are more prevalent,4 while lesser trochanter avulsion fractures are more rare. In one review of 1126 children with femoral neck and proximal 1/3 femoral shaft fractures, only 3 of them had lesser trochanter avulsion fractures.5
Clinical presentation. Presenting symptoms of lesser trochanter avulsion fractures can be vague, but are usually localized to the groin and medial hip region. Patients will demonstrate pain and weakness with hip flexion.3,6 There may be signs of inflammation, tenderness, and ecchymosis near the site of injury.
On physical exam, a positive Ludloff sign helps localize the injury to the iliopsoas muscle, which inserts at the lesser trochanter and is involved in hip flexion.3,6,7 The Ludloff test is performed by flexing the patient’s hip while he/she is in a seated position.
BIOMECHANICS OF AVULSION FRACTURES
Perhaps surprisingly, the majority of avulsion injuries in children and adolescents are the result of non-contact athletic movement and indirect trauma.4 In children, muscles and tendons are often stronger than their bones,7 and physes—structurally weak regions—are particularly predisposed to fractures.2,4,6
The mechanism of injury in children and adolescents is commonly a sudden, forceful contraction of the iliopsoas muscle.6,7 While similar movement in adults will produce tendon sprains and muscle strains, children often experience a complete avulsion fracture.7 So uncommon are these fractures among adults that an adult patient presenting with one should receive further work-up for underlying pathology such as malignancy.8,9
While other hip and femur avulsion fractures in children and adolescents involve different muscle groups, the etiologic mechanism—forceful muscle contraction—is usually the same.2,4,7 IT injuries are often seen with sudden, aggressive lengthening of the hamstring muscles, whereas injuries to the ASIS and AIIS are the result of abrupt eccentric contraction of hip extensor muscles while the knee is flexed.4
DIFFERENTIAL DIAGNOSIS
There are several entities that can mimic a lesser trochanter avulsion fracture including Legg-Calve-Perthes disease (LCPD), slipped capital femoral epiphysis (SCFE), snapping hip with the iliofemoral ligament, iliopsoas tendonitis, referred pain from the gastrointestinal region, and a genito-urologic etiology.1,7,10
Diagnostic studies. Physical exam findings of severe pain and reduced strength are clear indications for obtaining baseline imaging. Baseline radiographs are key to the diagnosis of avulsion fractures. They help differentiate between more benign fractures, such as a nondisplaced avulsion fracture, and more substantial conditions, such as LCPD and SCFE, which require significantly different approaches to treatment and follow-up.1,7
Anteroposterior, oblique, and axial views of the pelvis all assist in assessing avulsion fractures radiographically.3,4,7 In the event that an avulsion fracture is not radiographically visible, but is still suspected, additional imaging should be obtained.10 A computerized tomography (CT) scan is an appropriate follow-up, given its meticulous detail of bony anatomy.3,10 Alternatively, if physes have yet to ossify or there are concerns about soft tissue injury, magnetic resonance imaging can be useful.3,7,10
MANAGEMENT
The majority of lesser trochanter avulsion fractures are managed conservatively with rest, nonsteroidal anti-inflammatory drugs (NSAIDs), and physical therapy. Patients are often placed on non-weight bearing activity for up to 6 weeks while the fracture repairs and forms a new union.7 Current management strategies have moved away from immobilization with splints and braces.
In rare instances when the fragment is displaced >2 cm, or there is inadequate healing or pain relief after 3 months of supportive care, surgery may be required.1 With appropriate diagnosis and medical care, the injured athlete should fully recover with no impairment or chronic pain.2
Our patient was placed on non-weight-bearing activity and treated with NSAIDs and acetaminophen. We advanced him to weight-bearing activities 4 weeks after injury. After 8 weeks of conservative management, he returned to competitive play with no further complications (FIGURE 2).

THE TAKEAWAY
Pelvic and proximal femur avulsion fractures occur more often in child and adolescent athletes. As this population becomes increasingly competitive in athletics, the risk of injury increases. Infrequent fractures such as lesser trochanter avulsion fractures may become more common, as well. The majority of avulsion fractures don’t require surgical intervention, but it’s important to obtain baseline radiographs to rule out other injuries or pathologies that may lead to poor prognoses if they are left untreated.
1. Byrne A, Reidy D. Acute groin pain in an adolescent sprinter: a case report. Int J Clin Pediatr. 2012;1:46-48.
2. Fernbach SK, Wilkinson RH. Avulsion injuries of the pelvis and proximal femur. AJR Am J Roentgenol. 1981;137:581-584.
3. McKinney BI, Nelson C, Carrion W. Apophyseal avulsion fractures of the hip and pelvis. Orthopedics. 2009;32:42.
4. Rossi F, Dragoni S. Acute avulsion fractures of the pelvis in adolescent competitive athletes: prevalence, location and sports distribution of 203 cases collected. Skeletal Radiol. 2001;30:127-131.
5. Theologis TN, Epps H, Latz K, et al. Isolated fractures of the lesser trochanter in children. Injury. 1997;28:363-364.
6. Paluska SA. An overview of hip injuries in running. Sports Med. 2005;35:991-1014.
7. Vazquez E, Kim TY, Young TP. Avulsion fracture of the lesser trochanter: an unusual cause of hip pain in an adolescent. CJEM. 2013;15:123-125.
8. Afra R, Boardman DL, Kabo JM, et al. Avulsion fracture of the lesser trochanter as a result of a preliminary malignant tumor of bone. A report of four cases. J Bone Joint Surg Am. 1999;81:1299-1304.
9. DePasse JM, Varner K, Cosculluela P, et al. Atraumatic avulsion of the distal iliopsoas tendon: an unusual cause of hip pain. Orthopedics. 2010;33.
10. Suarez JC, Ely EE, Mutnal AB, et al. Comprehensive approach to the evaluation of groin pain. J Am Acad Orthop Surg. 2013;21:558-570.
1. Byrne A, Reidy D. Acute groin pain in an adolescent sprinter: a case report. Int J Clin Pediatr. 2012;1:46-48.
2. Fernbach SK, Wilkinson RH. Avulsion injuries of the pelvis and proximal femur. AJR Am J Roentgenol. 1981;137:581-584.
3. McKinney BI, Nelson C, Carrion W. Apophyseal avulsion fractures of the hip and pelvis. Orthopedics. 2009;32:42.
4. Rossi F, Dragoni S. Acute avulsion fractures of the pelvis in adolescent competitive athletes: prevalence, location and sports distribution of 203 cases collected. Skeletal Radiol. 2001;30:127-131.
5. Theologis TN, Epps H, Latz K, et al. Isolated fractures of the lesser trochanter in children. Injury. 1997;28:363-364.
6. Paluska SA. An overview of hip injuries in running. Sports Med. 2005;35:991-1014.
7. Vazquez E, Kim TY, Young TP. Avulsion fracture of the lesser trochanter: an unusual cause of hip pain in an adolescent. CJEM. 2013;15:123-125.
8. Afra R, Boardman DL, Kabo JM, et al. Avulsion fracture of the lesser trochanter as a result of a preliminary malignant tumor of bone. A report of four cases. J Bone Joint Surg Am. 1999;81:1299-1304.
9. DePasse JM, Varner K, Cosculluela P, et al. Atraumatic avulsion of the distal iliopsoas tendon: an unusual cause of hip pain. Orthopedics. 2010;33.
10. Suarez JC, Ely EE, Mutnal AB, et al. Comprehensive approach to the evaluation of groin pain. J Am Acad Orthop Surg. 2013;21:558-570.