User login
Government uncertainty drives jump in ACA silver plan insurance premiums
Silver plans on the Affordable Care Act insurance exchanges in 2018 will see an average premium increase of 34% nationwide, according to new research from Avalere Health.
“Plans are raising premiums in 2018 to account for market uncertainty and the federal government’s failure to pay for cost-sharing reductions,” Caroline Pearson, senior vice president at Avalere, said in a statement. “These premium increases may allow insurers to remain in the market and enrollees in all regions to have access to coverage.”
The expected premium changes are highly variable by state. Iowa has the highest change in its silver plans, with an average premium increase of 69% for its silver plans, while at the other end of the spectrum, Alaska is actually seeing a 22% decrease.
“These rates may change prior to open enrollment depending on how states respond to the elimination of CSR [cost-sharing reduction] funding for the 2018 plan year,” Avalere notes in its new analysis, adding that states may allow plans to refile for rate hikes now that CSR funding is likely dead. “In states where this occurs, it is expected that the newly updated rates will be substantially higher for the 2018 plan year.”
There was a glimmer of hope that the CSR payments would resume after a compromise was reached in the Senate Health, Education, Labor & Pensions Committee by Chairman Lamar Alexander (R-Tenn.) and Ranking Member Patty Murray (D-Wash.) that would offer 2 years of funding along with flexibility in the waiver program to allow states to tweak Affordable Care Act requirements, which is supported by AGA (http://www.gastro.org/news_items/aga-supports-alexander-murray-agreement-to-stabilize-individual-market). However, Speaker Paul Ryan (R-Wisc.) said the House would not be taking on any more health care action for the remainder of the year.
A spokeswoman from America’s Health Insurance Plans said in an interview that, although the CSR payments are no more, premium tax credits still exist to help lower-income individuals obtain insurance coverage.
Silver plans on the Affordable Care Act insurance exchanges in 2018 will see an average premium increase of 34% nationwide, according to new research from Avalere Health.
“Plans are raising premiums in 2018 to account for market uncertainty and the federal government’s failure to pay for cost-sharing reductions,” Caroline Pearson, senior vice president at Avalere, said in a statement. “These premium increases may allow insurers to remain in the market and enrollees in all regions to have access to coverage.”
The expected premium changes are highly variable by state. Iowa has the highest change in its silver plans, with an average premium increase of 69% for its silver plans, while at the other end of the spectrum, Alaska is actually seeing a 22% decrease.
“These rates may change prior to open enrollment depending on how states respond to the elimination of CSR [cost-sharing reduction] funding for the 2018 plan year,” Avalere notes in its new analysis, adding that states may allow plans to refile for rate hikes now that CSR funding is likely dead. “In states where this occurs, it is expected that the newly updated rates will be substantially higher for the 2018 plan year.”
There was a glimmer of hope that the CSR payments would resume after a compromise was reached in the Senate Health, Education, Labor & Pensions Committee by Chairman Lamar Alexander (R-Tenn.) and Ranking Member Patty Murray (D-Wash.) that would offer 2 years of funding along with flexibility in the waiver program to allow states to tweak Affordable Care Act requirements, which is supported by AGA (http://www.gastro.org/news_items/aga-supports-alexander-murray-agreement-to-stabilize-individual-market). However, Speaker Paul Ryan (R-Wisc.) said the House would not be taking on any more health care action for the remainder of the year.
A spokeswoman from America’s Health Insurance Plans said in an interview that, although the CSR payments are no more, premium tax credits still exist to help lower-income individuals obtain insurance coverage.
Silver plans on the Affordable Care Act insurance exchanges in 2018 will see an average premium increase of 34% nationwide, according to new research from Avalere Health.
“Plans are raising premiums in 2018 to account for market uncertainty and the federal government’s failure to pay for cost-sharing reductions,” Caroline Pearson, senior vice president at Avalere, said in a statement. “These premium increases may allow insurers to remain in the market and enrollees in all regions to have access to coverage.”
The expected premium changes are highly variable by state. Iowa has the highest change in its silver plans, with an average premium increase of 69% for its silver plans, while at the other end of the spectrum, Alaska is actually seeing a 22% decrease.
“These rates may change prior to open enrollment depending on how states respond to the elimination of CSR [cost-sharing reduction] funding for the 2018 plan year,” Avalere notes in its new analysis, adding that states may allow plans to refile for rate hikes now that CSR funding is likely dead. “In states where this occurs, it is expected that the newly updated rates will be substantially higher for the 2018 plan year.”
There was a glimmer of hope that the CSR payments would resume after a compromise was reached in the Senate Health, Education, Labor & Pensions Committee by Chairman Lamar Alexander (R-Tenn.) and Ranking Member Patty Murray (D-Wash.) that would offer 2 years of funding along with flexibility in the waiver program to allow states to tweak Affordable Care Act requirements, which is supported by AGA (http://www.gastro.org/news_items/aga-supports-alexander-murray-agreement-to-stabilize-individual-market). However, Speaker Paul Ryan (R-Wisc.) said the House would not be taking on any more health care action for the remainder of the year.
A spokeswoman from America’s Health Insurance Plans said in an interview that, although the CSR payments are no more, premium tax credits still exist to help lower-income individuals obtain insurance coverage.
DDSEP® 8 Quick quiz - November 2017 Question 1
Answer: C
Rationale: The patient presents with acute gallstone pancreatitis. In patients with gallstone pancreatitis and evidence of cholangitis, ERCP with sphincterotomy and stone extraction should be performed. The patient’s fever, jaundice, and right upper-quadrant pain are sufficient to make the diagnosis of cholangitis. It is too early in the course of the disease to evaluate for pancreatic necrosis. Typically, triglyceride levels above 1,000 mg/dL are required to induce pancreatitis. Finally, while the patient has cholelithiasis, there is no evidence of cholecystitis. Therefore, a HIDA scan is not warranted.
Reference
1. Behrns K.E., Ashley S.W., Hunter J.G., Carr-Locke D. Early ERCP for gallstone pancreatitis: for whom and when? J Gastrointest Surg. 2008;12(4):629-33.
Answer: C
Rationale: The patient presents with acute gallstone pancreatitis. In patients with gallstone pancreatitis and evidence of cholangitis, ERCP with sphincterotomy and stone extraction should be performed. The patient’s fever, jaundice, and right upper-quadrant pain are sufficient to make the diagnosis of cholangitis. It is too early in the course of the disease to evaluate for pancreatic necrosis. Typically, triglyceride levels above 1,000 mg/dL are required to induce pancreatitis. Finally, while the patient has cholelithiasis, there is no evidence of cholecystitis. Therefore, a HIDA scan is not warranted.
Reference
1. Behrns K.E., Ashley S.W., Hunter J.G., Carr-Locke D. Early ERCP for gallstone pancreatitis: for whom and when? J Gastrointest Surg. 2008;12(4):629-33.
Answer: C
Rationale: The patient presents with acute gallstone pancreatitis. In patients with gallstone pancreatitis and evidence of cholangitis, ERCP with sphincterotomy and stone extraction should be performed. The patient’s fever, jaundice, and right upper-quadrant pain are sufficient to make the diagnosis of cholangitis. It is too early in the course of the disease to evaluate for pancreatic necrosis. Typically, triglyceride levels above 1,000 mg/dL are required to induce pancreatitis. Finally, while the patient has cholelithiasis, there is no evidence of cholecystitis. Therefore, a HIDA scan is not warranted.
Reference
1. Behrns K.E., Ashley S.W., Hunter J.G., Carr-Locke D. Early ERCP for gallstone pancreatitis: for whom and when? J Gastrointest Surg. 2008;12(4):629-33.
A 50-year-old woman with no past medical history presents to the emergency department with the acute onset of severe epigastric pain and vomiting. She is afebrile with a blood pressure of 100/50 mm Hg, and pulse of 110 bpm. Physical exam shows right upper-quadrant and epigastric tenderness to palpation without rebound. Labs demonstrate a WBC count of 17,000/mm3, hemoglobin of 16 g/dL, creatinine of 1.4 mg/dL, ALT of 215 U/L, AST of 190 U/L, a total bilirubin of 2.1 mg/dL, and triglycerides of 492 mg/dL. Right upper-quadrant ultrasound reveals gallstones and a 1.2-cm common bile duct. The following day, despite being hydrated aggressively, the patient develops a fever and becomes jaundiced with worsening abdominal pain.
What’s Eating You? Scabies in the Developing World
Scabies is caused by the mite Sarcoptes scabiei var hominis.1 It is in the arthropod class Arachnida, subclass Acari, and family Sarcoptidae.2 Historically, scabies was first described in the Old Testament and by Aristotle,2 but the causative organism was not identified until 1687 using a light microscope.3 Scabies affects all age groups, races, and social classes and is globally widespread. It is most prevalent in developing tropical countries.1 It is estimated that 300 million individuals worldwide are infested with scabies mites annually, with the highest burden in young children.4-7 In industrialized societies, infections often are seen in young adults and in institutional settings such as nursing homes.8 Scabies disproportionately impacts impoverished communities with crowded living conditions, poor hygiene and nutrition, and substandard housing.5,9 Controlling the spread of the disease in these communities presents challenges but is important because of the connection between scabies and chronic kidney disease.10 As such, scabies represents a major health problem in the developing world and has been the focus of major health initiatives.1,11
Identifying Characteristics
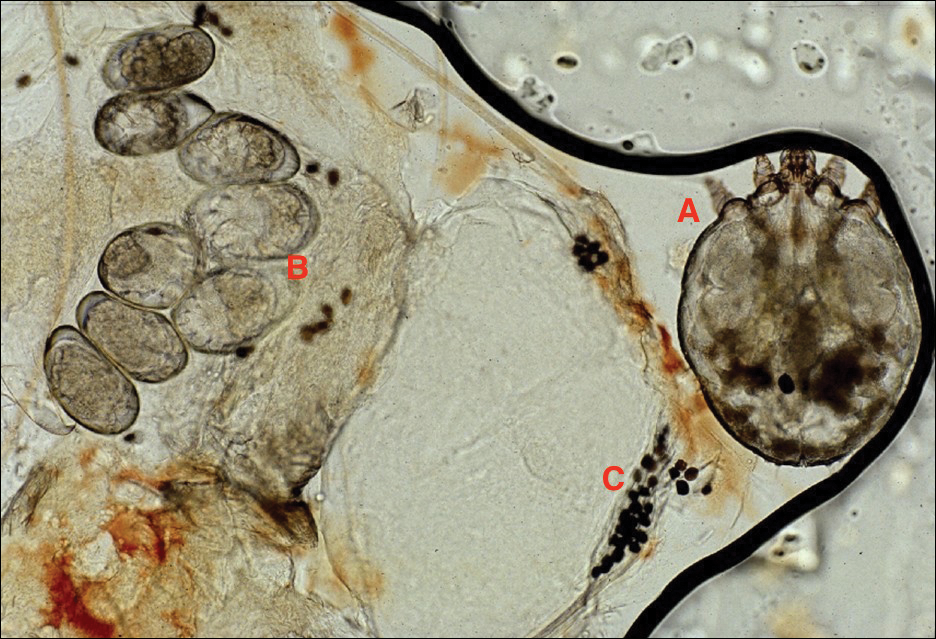
Adult females are 0.4-mm long and 0.3-mm wide, with males being smaller. Adult nymphs have 8 legs and larvae have 6 legs. Scabies mites are distinguishable from other arachnids by the position of a distinct gnathosoma and the lack of a division between the abdomen and cephalothorax.12 They are ovoid with a small anterior cephalic and caudal thoracoabdominal portion with hairlike projections coming off from the rudimentary legs. They can crawl as fast as 2.5 cm per minute on warm skin.2 The life cycle of the mite begins after mating: the male mite dies, and the female lays up to 3 eggs per day, which hatch in 3 to 4 days,2 in skin burrows within the stratum granulosum.12 Maturation from larva to adult takes 10 to 14 days.12 A female mite can live for 4 to 6 weeks and can produce up to 40 ova (Figure 1).
Disease Transmission
Without a host, mites are able to survive and remain capable of infestation for 24 to 36 hours at 21°C and 40% to 80% relative humidity. Lower temperatures and higher humidity prolong survival, but infectivity decreases the longer they are without a host.13
An adult human with ordinary scabies will have an average of 12 adult female mites on the body surface at a given time.14 However, hundreds of mites can be found in neglected children in underprivileged communities and millions in patients with crusted scabies.13 Transmission of typical scabies requires close direct skin-to-skin contact for 15 to 20 minutes.2,8 Transmission from clothing or fomites are an unlikely source of infestation with the exception of patients who are heavily infested such as in crusted scabies.12 In adults, sexual contact is an important method of transmission,12 and patients with scabies should be screened for other sexually transmitted diseases.8
Clinical Manifestations
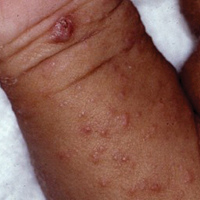
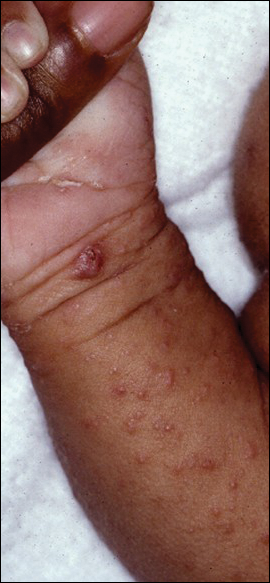
Signs of scabies on the skin include burrows, erythematous papules, and generalized pruritus (Figure 2).12 The scalp, face, and neck frequently are involved in infants and children,2 and the hands, wrists, elbows, genitalia, axillae, umbilicus, belt line, nipples, and buttocks commonly are involved in adults.12 Itching is characteristically worse at night.8 In tropical climates, patients with scabies are predisposed to secondary bacterial skin infections, particularly Streptococcus pyogenes (group A streptococci). The association between scabies and pyoderma caused by group A streptococci has been well established.15,16 Mika et al10 suggested that local complement inhibition plays an important role in the development of pyoderma in scabies-infested skin.
Prevention and Control in the Developing World
Low-cost diagnostic equipment can play a key role in the definitive diagnosis and management of scabies outbreaks in the developing world. Micali et al28 found that a $30 videomicroscope was as effective in scabies diagnosis as a $20,000 videodermatoscope. Because of the low cost of benzyl benzoate, it is commonly used as a first-line drug in many parts of the world,13 whereas permethrin cream 5% is the standard treatment in the developed world.29 Recognition of the role of scabies in patients with pyoderma is key, and one study indicated clinically apparent scabies went unnoticed by physicians in 52% of patients presenting with skin lesions.30 Drug shortages also can contribute to a high prevalence of scabies infestation in the community.31 Mass treatment with ivermectin has proven to be an effective means of reducing the prevalence of many parasitic diseases,1,32,33 and it shows great promise for crusted scabies, institutional outbreaks, and mass administration in highly endemic communites.8 However, there is evidence of ivermectin tolerance among mites, which could undermine the success of mass drug administration.34 Another important consideration is population mobility and the risk for rapid reintroduction of scabies infection across regions.35
Complicating disease control are the socioeconomic factors associated with scabies in the developing world. Families with scabies infestation typically do not own their homes, are less likely to have constant electricity, have a lower monthly income, and live in substandard housing.20 Families can spend a substantial part of their household income on treatment, impacting what they can spend on food.8,11 In addition to medication, control of scabies requires community education and involvement, along with access to primary care and attention to living conditions and environmental factors.34,36
- Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373:2305-2313.
- Hicks MI, Elston DM. Scabies. Dermatol Ther. 2009;22:279-292.
- Ramos-e-Silva M. Giovan Cosimo Bonomo (1663-1696): discoverer of the etiology of scabies. Int J Dermatol. 1998;37:625-630.
- Chung SD, Wang KH, Huang CC, et al. Scabies increased the risk of chronic kidney disease: a 5-year follow-up study. J Eur Acad Dermatol Venereol. 2014;28:286-292.
- Wong SS, Poon RW, Chau S, et al. Development of conventional and real-time quantitative PCR assays for diagnosis and monitoring of scabies. J Clin Microbiol. 2015;53:2095-2102.
- Kearns TM, Speare R, Cheng AC, et al. Impact of an ivermectin mass drug administration on scabies prevalence in a remote Australian aboriginal community. PLoS Negl Trop Dis. 2015;9:e0004151.
- Gilmore SJ. Control strategies for endemic childhood scabies. PLoS One. 2011;6:e15990.
- Hay RJ, Steer AC, Engelman D, Walton S. Scabies in the developing world—its prevalence, complications, and management. Clin Microbiol Infect. 2012;18:313-323.
- Hoy WE, White AV, Dowling A, et al. Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int. 2012;81:1026-1032.
- Mika A, Reynolds SL, Pickering D, et al. Complement inhibitors from scabies mites promote streptococcal growth—a novel mechanism in infected epidermis? PLoS Negl Trop Dis. 2012;6:e1563.
- McLean FE. The elimination of scabies: a task for our generation. Int J Dermatol. 2013;52:1215-1223.
- Hengge UR, Currie BJ, Jäger G, et al. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006;6:769-779.
- Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767-1774.
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622.
- Yeoh DK, Bowen AC, Carapetis JR. Impetigo and scabies—disease burden and modern treatment strategies [published online May 11, 2016]. J Infect. 2016;(72 suppl):S61-S67.
- Bowen AC, Mahé A, Hay RJ, et al. The global epidemiology of impetigo: a systematic review of the population prevalence of impetigo and pyoderma. PLoS One. 2015;10:e0136789.
- Bowen AC, Tong SY, Chatfield MD, et al. The microbiology of impetigo in indigenous children: associations between Streptococcus pyogenes, Staphylococcus aureus, scabies, and nasal carriage. BMC Infect Dis. 2014;14:727.
- Sesso R, Pinto SW. Five-year follow-up of patients with epidemic glomerulonephritis due to Streptococcus zooepidemicus. Nephrol Dial Transplant. 2005;20:1808-1812.
- Singh GR. Glomerulonephritis and managing the risks of chronic renal disease. Pediatr Clin North Am. 2009;56:1363-1382.
- La Vincente S, Kearns T, Connors C, et al. Community management of endemic scabies in remote aboriginal communities of northern Australia: low treatment uptake and high ongoing acquisition. PLoS Negl Trop Dis. 2009;3:e444.
- Clucas DB, Carville KS, Connors C, et al. Disease burden and health-care clinic attendances for young children in remote aboriginal communities of northern Australia. Bull World Health Organ. 2008;86:275-281.
- Stanton B, Khanam S, Nazrul H, et al. Scabies in urban Bangladesh. J Trop Med Hyg. 1987;90:219-226.
- Heukelbach J, de Oliveira FA, Feldmeier H. Ecoparasitoses and public health in Brazil: challenges for control [in Portuguese]. Cad Saude Publica. 2003;19:1535-1540.
- Edison L, Beaudoin A, Goh L, et al. Scabies and bacterial superinfection among American Samoan children, 2011-2012. PLoS One. 2015;10:e0139336.
- Steer AC, Jenney AW, Kado J, et al. High burden of impetigo and scabies in a tropical country. PLoS Negl Trop Dis. 2009;3:e467.
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967.
- Romani L, Koroivueta J, Steer AC, et al. Scabies and impetigo prevalence and risk factors in Fiji: a national survey. PLoS Negl Trop Dis. 2015;9:e0003452.
- Micali G, Lacarrubba F, Verzì AE, et al. Low-cost equipment for diagnosis and management of endemic scabies outbreaks in underserved populations. Clin Infect Dis. 2015;60:327-329.
- Pasay C, Walton S, Fischer K, et al. PCR-based assay to survey for knockdown resistance to pyrethroid acaricides in human scabies mites (Sarcoptes scabiei var hominis). Am J Trop Med Hyg. 2006;74:649-657.
- Heukelbach J, van Haeff E, Rump B, et al. Parasitic skin diseases: health care-seeking in a slum in north-east Brazil. Trop Med Int Health. 2003;8:368-373.
- Potter EV, Mayon-White R, Poon-King T, et al. Acute glomerulonephritis as a complication of scabies. In: Orkin M, Maibach HI, eds. Cutaneous Infestations and Insect Bites. New York, NY: Marcel Dekker; 1985.
- Mahé A. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689.
- Steer AC, Romani L, Kaldor JM. Mass drug administration for scabies control. N Engl J Med. 2016;374:1690.
- Mounsey KE, Holt DC, McCarthy JS, et al. Longitudinal evidence of increasing in vitro tolerance of scabies mites to ivermectin in scabies-endemic communities. Arch Dermatol. 2009;145:840-841.
- Currie BJ. Scabies and global control of neglected tropical diseases. N Engl J Med. 2015;373:2371-2372.
- O’Donnell V, Morris S, Ward J. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689-1690.
Scabies is caused by the mite Sarcoptes scabiei var hominis.1 It is in the arthropod class Arachnida, subclass Acari, and family Sarcoptidae.2 Historically, scabies was first described in the Old Testament and by Aristotle,2 but the causative organism was not identified until 1687 using a light microscope.3 Scabies affects all age groups, races, and social classes and is globally widespread. It is most prevalent in developing tropical countries.1 It is estimated that 300 million individuals worldwide are infested with scabies mites annually, with the highest burden in young children.4-7 In industrialized societies, infections often are seen in young adults and in institutional settings such as nursing homes.8 Scabies disproportionately impacts impoverished communities with crowded living conditions, poor hygiene and nutrition, and substandard housing.5,9 Controlling the spread of the disease in these communities presents challenges but is important because of the connection between scabies and chronic kidney disease.10 As such, scabies represents a major health problem in the developing world and has been the focus of major health initiatives.1,11
Identifying Characteristics
Adult females are 0.4-mm long and 0.3-mm wide, with males being smaller. Adult nymphs have 8 legs and larvae have 6 legs. Scabies mites are distinguishable from other arachnids by the position of a distinct gnathosoma and the lack of a division between the abdomen and cephalothorax.12 They are ovoid with a small anterior cephalic and caudal thoracoabdominal portion with hairlike projections coming off from the rudimentary legs. They can crawl as fast as 2.5 cm per minute on warm skin.2 The life cycle of the mite begins after mating: the male mite dies, and the female lays up to 3 eggs per day, which hatch in 3 to 4 days,2 in skin burrows within the stratum granulosum.12 Maturation from larva to adult takes 10 to 14 days.12 A female mite can live for 4 to 6 weeks and can produce up to 40 ova (Figure 1).

Disease Transmission
Without a host, mites are able to survive and remain capable of infestation for 24 to 36 hours at 21°C and 40% to 80% relative humidity. Lower temperatures and higher humidity prolong survival, but infectivity decreases the longer they are without a host.13
An adult human with ordinary scabies will have an average of 12 adult female mites on the body surface at a given time.14 However, hundreds of mites can be found in neglected children in underprivileged communities and millions in patients with crusted scabies.13 Transmission of typical scabies requires close direct skin-to-skin contact for 15 to 20 minutes.2,8 Transmission from clothing or fomites are an unlikely source of infestation with the exception of patients who are heavily infested such as in crusted scabies.12 In adults, sexual contact is an important method of transmission,12 and patients with scabies should be screened for other sexually transmitted diseases.8
Clinical Manifestations
Signs of scabies on the skin include burrows, erythematous papules, and generalized pruritus (Figure 2).12 The scalp, face, and neck frequently are involved in infants and children,2 and the hands, wrists, elbows, genitalia, axillae, umbilicus, belt line, nipples, and buttocks commonly are involved in adults.12 Itching is characteristically worse at night.8 In tropical climates, patients with scabies are predisposed to secondary bacterial skin infections, particularly Streptococcus pyogenes (group A streptococci). The association between scabies and pyoderma caused by group A streptococci has been well established.15,16 Mika et al10 suggested that local complement inhibition plays an important role in the development of pyoderma in scabies-infested skin.

Prevention and Control in the Developing World
Low-cost diagnostic equipment can play a key role in the definitive diagnosis and management of scabies outbreaks in the developing world. Micali et al28 found that a $30 videomicroscope was as effective in scabies diagnosis as a $20,000 videodermatoscope. Because of the low cost of benzyl benzoate, it is commonly used as a first-line drug in many parts of the world,13 whereas permethrin cream 5% is the standard treatment in the developed world.29 Recognition of the role of scabies in patients with pyoderma is key, and one study indicated clinically apparent scabies went unnoticed by physicians in 52% of patients presenting with skin lesions.30 Drug shortages also can contribute to a high prevalence of scabies infestation in the community.31 Mass treatment with ivermectin has proven to be an effective means of reducing the prevalence of many parasitic diseases,1,32,33 and it shows great promise for crusted scabies, institutional outbreaks, and mass administration in highly endemic communites.8 However, there is evidence of ivermectin tolerance among mites, which could undermine the success of mass drug administration.34 Another important consideration is population mobility and the risk for rapid reintroduction of scabies infection across regions.35
Complicating disease control are the socioeconomic factors associated with scabies in the developing world. Families with scabies infestation typically do not own their homes, are less likely to have constant electricity, have a lower monthly income, and live in substandard housing.20 Families can spend a substantial part of their household income on treatment, impacting what they can spend on food.8,11 In addition to medication, control of scabies requires community education and involvement, along with access to primary care and attention to living conditions and environmental factors.34,36
Scabies is caused by the mite Sarcoptes scabiei var hominis.1 It is in the arthropod class Arachnida, subclass Acari, and family Sarcoptidae.2 Historically, scabies was first described in the Old Testament and by Aristotle,2 but the causative organism was not identified until 1687 using a light microscope.3 Scabies affects all age groups, races, and social classes and is globally widespread. It is most prevalent in developing tropical countries.1 It is estimated that 300 million individuals worldwide are infested with scabies mites annually, with the highest burden in young children.4-7 In industrialized societies, infections often are seen in young adults and in institutional settings such as nursing homes.8 Scabies disproportionately impacts impoverished communities with crowded living conditions, poor hygiene and nutrition, and substandard housing.5,9 Controlling the spread of the disease in these communities presents challenges but is important because of the connection between scabies and chronic kidney disease.10 As such, scabies represents a major health problem in the developing world and has been the focus of major health initiatives.1,11
Identifying Characteristics
Adult females are 0.4-mm long and 0.3-mm wide, with males being smaller. Adult nymphs have 8 legs and larvae have 6 legs. Scabies mites are distinguishable from other arachnids by the position of a distinct gnathosoma and the lack of a division between the abdomen and cephalothorax.12 They are ovoid with a small anterior cephalic and caudal thoracoabdominal portion with hairlike projections coming off from the rudimentary legs. They can crawl as fast as 2.5 cm per minute on warm skin.2 The life cycle of the mite begins after mating: the male mite dies, and the female lays up to 3 eggs per day, which hatch in 3 to 4 days,2 in skin burrows within the stratum granulosum.12 Maturation from larva to adult takes 10 to 14 days.12 A female mite can live for 4 to 6 weeks and can produce up to 40 ova (Figure 1).

Disease Transmission
Without a host, mites are able to survive and remain capable of infestation for 24 to 36 hours at 21°C and 40% to 80% relative humidity. Lower temperatures and higher humidity prolong survival, but infectivity decreases the longer they are without a host.13
An adult human with ordinary scabies will have an average of 12 adult female mites on the body surface at a given time.14 However, hundreds of mites can be found in neglected children in underprivileged communities and millions in patients with crusted scabies.13 Transmission of typical scabies requires close direct skin-to-skin contact for 15 to 20 minutes.2,8 Transmission from clothing or fomites are an unlikely source of infestation with the exception of patients who are heavily infested such as in crusted scabies.12 In adults, sexual contact is an important method of transmission,12 and patients with scabies should be screened for other sexually transmitted diseases.8
Clinical Manifestations
Signs of scabies on the skin include burrows, erythematous papules, and generalized pruritus (Figure 2).12 The scalp, face, and neck frequently are involved in infants and children,2 and the hands, wrists, elbows, genitalia, axillae, umbilicus, belt line, nipples, and buttocks commonly are involved in adults.12 Itching is characteristically worse at night.8 In tropical climates, patients with scabies are predisposed to secondary bacterial skin infections, particularly Streptococcus pyogenes (group A streptococci). The association between scabies and pyoderma caused by group A streptococci has been well established.15,16 Mika et al10 suggested that local complement inhibition plays an important role in the development of pyoderma in scabies-infested skin.

Prevention and Control in the Developing World
Low-cost diagnostic equipment can play a key role in the definitive diagnosis and management of scabies outbreaks in the developing world. Micali et al28 found that a $30 videomicroscope was as effective in scabies diagnosis as a $20,000 videodermatoscope. Because of the low cost of benzyl benzoate, it is commonly used as a first-line drug in many parts of the world,13 whereas permethrin cream 5% is the standard treatment in the developed world.29 Recognition of the role of scabies in patients with pyoderma is key, and one study indicated clinically apparent scabies went unnoticed by physicians in 52% of patients presenting with skin lesions.30 Drug shortages also can contribute to a high prevalence of scabies infestation in the community.31 Mass treatment with ivermectin has proven to be an effective means of reducing the prevalence of many parasitic diseases,1,32,33 and it shows great promise for crusted scabies, institutional outbreaks, and mass administration in highly endemic communites.8 However, there is evidence of ivermectin tolerance among mites, which could undermine the success of mass drug administration.34 Another important consideration is population mobility and the risk for rapid reintroduction of scabies infection across regions.35
Complicating disease control are the socioeconomic factors associated with scabies in the developing world. Families with scabies infestation typically do not own their homes, are less likely to have constant electricity, have a lower monthly income, and live in substandard housing.20 Families can spend a substantial part of their household income on treatment, impacting what they can spend on food.8,11 In addition to medication, control of scabies requires community education and involvement, along with access to primary care and attention to living conditions and environmental factors.34,36
- Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373:2305-2313.
- Hicks MI, Elston DM. Scabies. Dermatol Ther. 2009;22:279-292.
- Ramos-e-Silva M. Giovan Cosimo Bonomo (1663-1696): discoverer of the etiology of scabies. Int J Dermatol. 1998;37:625-630.
- Chung SD, Wang KH, Huang CC, et al. Scabies increased the risk of chronic kidney disease: a 5-year follow-up study. J Eur Acad Dermatol Venereol. 2014;28:286-292.
- Wong SS, Poon RW, Chau S, et al. Development of conventional and real-time quantitative PCR assays for diagnosis and monitoring of scabies. J Clin Microbiol. 2015;53:2095-2102.
- Kearns TM, Speare R, Cheng AC, et al. Impact of an ivermectin mass drug administration on scabies prevalence in a remote Australian aboriginal community. PLoS Negl Trop Dis. 2015;9:e0004151.
- Gilmore SJ. Control strategies for endemic childhood scabies. PLoS One. 2011;6:e15990.
- Hay RJ, Steer AC, Engelman D, Walton S. Scabies in the developing world—its prevalence, complications, and management. Clin Microbiol Infect. 2012;18:313-323.
- Hoy WE, White AV, Dowling A, et al. Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int. 2012;81:1026-1032.
- Mika A, Reynolds SL, Pickering D, et al. Complement inhibitors from scabies mites promote streptococcal growth—a novel mechanism in infected epidermis? PLoS Negl Trop Dis. 2012;6:e1563.
- McLean FE. The elimination of scabies: a task for our generation. Int J Dermatol. 2013;52:1215-1223.
- Hengge UR, Currie BJ, Jäger G, et al. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006;6:769-779.
- Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767-1774.
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622.
- Yeoh DK, Bowen AC, Carapetis JR. Impetigo and scabies—disease burden and modern treatment strategies [published online May 11, 2016]. J Infect. 2016;(72 suppl):S61-S67.
- Bowen AC, Mahé A, Hay RJ, et al. The global epidemiology of impetigo: a systematic review of the population prevalence of impetigo and pyoderma. PLoS One. 2015;10:e0136789.
- Bowen AC, Tong SY, Chatfield MD, et al. The microbiology of impetigo in indigenous children: associations between Streptococcus pyogenes, Staphylococcus aureus, scabies, and nasal carriage. BMC Infect Dis. 2014;14:727.
- Sesso R, Pinto SW. Five-year follow-up of patients with epidemic glomerulonephritis due to Streptococcus zooepidemicus. Nephrol Dial Transplant. 2005;20:1808-1812.
- Singh GR. Glomerulonephritis and managing the risks of chronic renal disease. Pediatr Clin North Am. 2009;56:1363-1382.
- La Vincente S, Kearns T, Connors C, et al. Community management of endemic scabies in remote aboriginal communities of northern Australia: low treatment uptake and high ongoing acquisition. PLoS Negl Trop Dis. 2009;3:e444.
- Clucas DB, Carville KS, Connors C, et al. Disease burden and health-care clinic attendances for young children in remote aboriginal communities of northern Australia. Bull World Health Organ. 2008;86:275-281.
- Stanton B, Khanam S, Nazrul H, et al. Scabies in urban Bangladesh. J Trop Med Hyg. 1987;90:219-226.
- Heukelbach J, de Oliveira FA, Feldmeier H. Ecoparasitoses and public health in Brazil: challenges for control [in Portuguese]. Cad Saude Publica. 2003;19:1535-1540.
- Edison L, Beaudoin A, Goh L, et al. Scabies and bacterial superinfection among American Samoan children, 2011-2012. PLoS One. 2015;10:e0139336.
- Steer AC, Jenney AW, Kado J, et al. High burden of impetigo and scabies in a tropical country. PLoS Negl Trop Dis. 2009;3:e467.
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967.
- Romani L, Koroivueta J, Steer AC, et al. Scabies and impetigo prevalence and risk factors in Fiji: a national survey. PLoS Negl Trop Dis. 2015;9:e0003452.
- Micali G, Lacarrubba F, Verzì AE, et al. Low-cost equipment for diagnosis and management of endemic scabies outbreaks in underserved populations. Clin Infect Dis. 2015;60:327-329.
- Pasay C, Walton S, Fischer K, et al. PCR-based assay to survey for knockdown resistance to pyrethroid acaricides in human scabies mites (Sarcoptes scabiei var hominis). Am J Trop Med Hyg. 2006;74:649-657.
- Heukelbach J, van Haeff E, Rump B, et al. Parasitic skin diseases: health care-seeking in a slum in north-east Brazil. Trop Med Int Health. 2003;8:368-373.
- Potter EV, Mayon-White R, Poon-King T, et al. Acute glomerulonephritis as a complication of scabies. In: Orkin M, Maibach HI, eds. Cutaneous Infestations and Insect Bites. New York, NY: Marcel Dekker; 1985.
- Mahé A. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689.
- Steer AC, Romani L, Kaldor JM. Mass drug administration for scabies control. N Engl J Med. 2016;374:1690.
- Mounsey KE, Holt DC, McCarthy JS, et al. Longitudinal evidence of increasing in vitro tolerance of scabies mites to ivermectin in scabies-endemic communities. Arch Dermatol. 2009;145:840-841.
- Currie BJ. Scabies and global control of neglected tropical diseases. N Engl J Med. 2015;373:2371-2372.
- O’Donnell V, Morris S, Ward J. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689-1690.
- Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373:2305-2313.
- Hicks MI, Elston DM. Scabies. Dermatol Ther. 2009;22:279-292.
- Ramos-e-Silva M. Giovan Cosimo Bonomo (1663-1696): discoverer of the etiology of scabies. Int J Dermatol. 1998;37:625-630.
- Chung SD, Wang KH, Huang CC, et al. Scabies increased the risk of chronic kidney disease: a 5-year follow-up study. J Eur Acad Dermatol Venereol. 2014;28:286-292.
- Wong SS, Poon RW, Chau S, et al. Development of conventional and real-time quantitative PCR assays for diagnosis and monitoring of scabies. J Clin Microbiol. 2015;53:2095-2102.
- Kearns TM, Speare R, Cheng AC, et al. Impact of an ivermectin mass drug administration on scabies prevalence in a remote Australian aboriginal community. PLoS Negl Trop Dis. 2015;9:e0004151.
- Gilmore SJ. Control strategies for endemic childhood scabies. PLoS One. 2011;6:e15990.
- Hay RJ, Steer AC, Engelman D, Walton S. Scabies in the developing world—its prevalence, complications, and management. Clin Microbiol Infect. 2012;18:313-323.
- Hoy WE, White AV, Dowling A, et al. Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int. 2012;81:1026-1032.
- Mika A, Reynolds SL, Pickering D, et al. Complement inhibitors from scabies mites promote streptococcal growth—a novel mechanism in infected epidermis? PLoS Negl Trop Dis. 2012;6:e1563.
- McLean FE. The elimination of scabies: a task for our generation. Int J Dermatol. 2013;52:1215-1223.
- Hengge UR, Currie BJ, Jäger G, et al. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006;6:769-779.
- Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767-1774.
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622.
- Yeoh DK, Bowen AC, Carapetis JR. Impetigo and scabies—disease burden and modern treatment strategies [published online May 11, 2016]. J Infect. 2016;(72 suppl):S61-S67.
- Bowen AC, Mahé A, Hay RJ, et al. The global epidemiology of impetigo: a systematic review of the population prevalence of impetigo and pyoderma. PLoS One. 2015;10:e0136789.
- Bowen AC, Tong SY, Chatfield MD, et al. The microbiology of impetigo in indigenous children: associations between Streptococcus pyogenes, Staphylococcus aureus, scabies, and nasal carriage. BMC Infect Dis. 2014;14:727.
- Sesso R, Pinto SW. Five-year follow-up of patients with epidemic glomerulonephritis due to Streptococcus zooepidemicus. Nephrol Dial Transplant. 2005;20:1808-1812.
- Singh GR. Glomerulonephritis and managing the risks of chronic renal disease. Pediatr Clin North Am. 2009;56:1363-1382.
- La Vincente S, Kearns T, Connors C, et al. Community management of endemic scabies in remote aboriginal communities of northern Australia: low treatment uptake and high ongoing acquisition. PLoS Negl Trop Dis. 2009;3:e444.
- Clucas DB, Carville KS, Connors C, et al. Disease burden and health-care clinic attendances for young children in remote aboriginal communities of northern Australia. Bull World Health Organ. 2008;86:275-281.
- Stanton B, Khanam S, Nazrul H, et al. Scabies in urban Bangladesh. J Trop Med Hyg. 1987;90:219-226.
- Heukelbach J, de Oliveira FA, Feldmeier H. Ecoparasitoses and public health in Brazil: challenges for control [in Portuguese]. Cad Saude Publica. 2003;19:1535-1540.
- Edison L, Beaudoin A, Goh L, et al. Scabies and bacterial superinfection among American Samoan children, 2011-2012. PLoS One. 2015;10:e0139336.
- Steer AC, Jenney AW, Kado J, et al. High burden of impetigo and scabies in a tropical country. PLoS Negl Trop Dis. 2009;3:e467.
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967.
- Romani L, Koroivueta J, Steer AC, et al. Scabies and impetigo prevalence and risk factors in Fiji: a national survey. PLoS Negl Trop Dis. 2015;9:e0003452.
- Micali G, Lacarrubba F, Verzì AE, et al. Low-cost equipment for diagnosis and management of endemic scabies outbreaks in underserved populations. Clin Infect Dis. 2015;60:327-329.
- Pasay C, Walton S, Fischer K, et al. PCR-based assay to survey for knockdown resistance to pyrethroid acaricides in human scabies mites (Sarcoptes scabiei var hominis). Am J Trop Med Hyg. 2006;74:649-657.
- Heukelbach J, van Haeff E, Rump B, et al. Parasitic skin diseases: health care-seeking in a slum in north-east Brazil. Trop Med Int Health. 2003;8:368-373.
- Potter EV, Mayon-White R, Poon-King T, et al. Acute glomerulonephritis as a complication of scabies. In: Orkin M, Maibach HI, eds. Cutaneous Infestations and Insect Bites. New York, NY: Marcel Dekker; 1985.
- Mahé A. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689.
- Steer AC, Romani L, Kaldor JM. Mass drug administration for scabies control. N Engl J Med. 2016;374:1690.
- Mounsey KE, Holt DC, McCarthy JS, et al. Longitudinal evidence of increasing in vitro tolerance of scabies mites to ivermectin in scabies-endemic communities. Arch Dermatol. 2009;145:840-841.
- Currie BJ. Scabies and global control of neglected tropical diseases. N Engl J Med. 2015;373:2371-2372.
- O’Donnell V, Morris S, Ward J. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689-1690.
Practice Points
- Scabies infestation is one of the world’s leading causes of chronic kidney disease.
- Ivermectin can be used to treat mass infestations, and older topical therapies also are commonly used.
FDA approves acalabrutinib for second-line treatment of MCL
The Food and Drug Administration has granted accelerated approval to acalabrutinib, a Bruton tyrosine kinase inhibitor, for the treatment of adults with mantle cell lymphoma (MCL) who have received at least one prior therapy.
Approval was based on an 81% overall response rate in a phase 2, single-arm trial (ACE-LY-004) of 124 patients with MCL who had received at least one prior treatment (40% complete response, 41% partial response), the FDA said in a statement.
Serious adverse effects include hemorrhage and atrial fibrillation. Second primary malignancies have occurred in some patients, according to the FDA.
Acalabrutinib will be marketed as Calquence by AstraZeneca. The company is currently enrolling patients in a phase 3 trial evaluating acalabrutinib as a first-line treatment for patients with MCL in combination with bendamustine and rituximab.
[email protected]
On Twitter @NikolaidesLaura
The Food and Drug Administration has granted accelerated approval to acalabrutinib, a Bruton tyrosine kinase inhibitor, for the treatment of adults with mantle cell lymphoma (MCL) who have received at least one prior therapy.
Approval was based on an 81% overall response rate in a phase 2, single-arm trial (ACE-LY-004) of 124 patients with MCL who had received at least one prior treatment (40% complete response, 41% partial response), the FDA said in a statement.
Serious adverse effects include hemorrhage and atrial fibrillation. Second primary malignancies have occurred in some patients, according to the FDA.
Acalabrutinib will be marketed as Calquence by AstraZeneca. The company is currently enrolling patients in a phase 3 trial evaluating acalabrutinib as a first-line treatment for patients with MCL in combination with bendamustine and rituximab.
[email protected]
On Twitter @NikolaidesLaura
The Food and Drug Administration has granted accelerated approval to acalabrutinib, a Bruton tyrosine kinase inhibitor, for the treatment of adults with mantle cell lymphoma (MCL) who have received at least one prior therapy.
Approval was based on an 81% overall response rate in a phase 2, single-arm trial (ACE-LY-004) of 124 patients with MCL who had received at least one prior treatment (40% complete response, 41% partial response), the FDA said in a statement.
Serious adverse effects include hemorrhage and atrial fibrillation. Second primary malignancies have occurred in some patients, according to the FDA.
Acalabrutinib will be marketed as Calquence by AstraZeneca. The company is currently enrolling patients in a phase 3 trial evaluating acalabrutinib as a first-line treatment for patients with MCL in combination with bendamustine and rituximab.
[email protected]
On Twitter @NikolaidesLaura
Syphilis and the Dermatologist
Once upon a time, and long ago, dermatology journals included “syphilology” in their names. The first dermatologic journal published in the United States was the American Journal of Syphilology and Dermatology.1 In October 1882 the Journal of Cutaneous and Venereal Diseases appeared and subsequently renamed several times from 1882 to 1919: Journal of Cutaneous Diseases and Genitourinary Diseases and the Journal of Cutaneous Diseases, Including Syphilis. When the American Medical Association (AMA) assumed control, this publication obtained a new name: Archives of Dermatology and Syphilology; in January 1955 syphilology was deleted from the title. According to an editorial in that issue, the rationale for dropping the word syphilology was as follows: “The diagnosis and treatment of patients with syphilis is no longer an important part of dermatologic practice. . . . Few dermatologists now have patients with syphilis; in fact, there are decidedly fewer patients with syphilis, and so continuance of the old label, ‘Syphilology,’ on this publication seems no longer warranted.”1 Needless to say, this decision ignored the obvious fact that the majority of dermatologists traditionally were well trained in and clinically practiced venereology, particularly the management of syphilis,2,3 which makes sense, considering that many of the clinical manifestations of syphilis involve the skin, hair, and oral mucosa. My own mentor and former Baylor College of Medicine dermatology department chair, Dr. John Knox, authored 3 dozen major publications regarding the diagnosis, treatment, and immunology of syphilis. During his chairmanship, all residents were required to rotate in the Harris County sexually transmitted disease (STD) clinic on a weekly basis.
I am confident that the decision to drop “syphilology” from the journal title also was based on the unduly optimistic assumption that syphilis would soon become a rare disease due to the availability of penicillin. Indeed, the Centers for Disease Control and Prevention in the United States has periodically announced strategic programs designed to eradicate syphilis!4 This rosy outlook reached a fever pitch in 2000 when the number of cases (5979) and the incidence (2.1 cases per 100,000 population) of primary and secondary syphilis reached an all-time low in the United States.5
Unfortunately, no one could accurately predict the future. Although the number of cases and incidence of early infectious syphilis have fluctuated widely since the 1940s, we currently are in a dire period of syphilis resurgence; the largest number of cases (27,814) and the highest incidence rate of primary and secondary syphilis (8.7 cases per 100,000 population) since 1994 were reported in 2016,6 which illustrates the inability of public health initiatives to eliminate syphilis, largely due to the inability of health authorities, health care providers, teachers, parents, clergy, and peer groups to alter sexual behaviors or modify other socioeconomic factors.7 Thus, syphilis lives on! Nobody could have predicted the easy availability of oral contraceptives and the ensuing sexual revolution of the 1960s or the advent of erectile dysfunction drugs decades later that led to increasing STDs among older patients.8 Nobody could have predicted the wholesale acceptance of casual sexual intercourse as popularized on television and in the movies or the pervasive use of sexual images in advertising. Nobody could have predicted the modern phenomena of “booty-call relationships,” “friends with benefits,” and “sexting,” or the nearly ubiquitous and increasingly legal use of noninjectable mind-altering drugs, all of which facilitate the perpetuation of STDs.9-11 Finally, those who removed “syphilology” from that journal title certainly did not foresee the worldwide epidemic now known as human immunodeficiency virus/AIDS, which has most assuredly helped keep syphilis a modern day menace.12-14
How have dermatologists been impacted? Our journals and our teachers have deemphasized STDs, including syphilis, in modern times, yet we are faced with a disease carrying serious, if not often fatal, consequences that is simply refusing to disappear (contrary to wishful thinking). Dermatologists are, however, in a perfect epidemiological position to help in the war against Treponema pallidum, the bacterium that causes syphilis. We frequently see adolescent patients for warts and acne, and we often diagnose and help care for patients with human immunodeficiency virus. We obliterate actinic keratoses and perform cosmetic procedures on those who rely on erectile dysfunction drugs (or their partners do). Who better than a dermatologist to recognize in these high-risk constituencies, and others, that patchy hair loss may represent syphilitic alopecia and that extragenital chancres can mimic nonmelanoma skin cancer? Who better than the dermatologist to distinguish between oral mucous patches and orolabial herpes? Who better than the dermatologist to diagnose the annular syphilid of the face, or ostraceous, florid nodular, or ulceronecrotic lesions of lues maligna? Who better than the dermatologist to differentiate condylomata lata from external genital warts?
I would suggest that the responsible dermatologist become reacquainted with syphilis, in all its various manifestations. I would further suggest that our dermatology training centers spend more time diligently teaching residents about syphilis and other STDs. In conclusion, I fervently hope that organized dermatology will once again dutifully consider venereal disease to be a critical part of our specialty’s skill set.
- Editorial. AMA Arch Dermatol. 1955;71:1.
- Shelley WB. Major contributors to American dermatology—1876 to 1926. Arch Dermatol. 1976;112:1642-1646.
- Lobitz WC Jr. Major contributions of American dermatologists—1926 to 1976. Arch Dermatol. 1976;112:1646-1650.
- Hook EW 3rd. Elimination of syphilis transmission in the United States: historic perspectives and practical considerations. Trans Am ClinClimatol Assoc. 1999;110:195-203.
- Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2000. Atlanta, GA: Department of Health and Human Services; 2001.
- 2016 Sexually transmitted diseases surveillance: syphilis. Centers for Disease Control and Prevention website. https://www.cdc.gov/std/stats16/syphilis.htm. Updated September 26, 2017. Accessed October 20, 2017.
- Shockman S, Buescher LS, Stone SP. Syphilis in the United States. Clin Dermatol. 2014;32:213-218.
- Jena AB, Goldman DP, Kamdar A, et al. Sexually transmitted diseases among users of erectile dysfunction drugs: analysis of claims data. Ann Intern Med. 2010;153:1-7.
- Jonason PK, Li NP, Richardson J. Positioning the booty-call relationship on the spectrum of relationships: sexual but more emotional than one-night stands. J Sex Res. 2011;48:486-495.
- Temple JR, Choi H. Longitudinal association between teen sexting and sexual behavior. Pediatrics. 2014;134:E1287-E1292.
- Regan R, Dyer TP, Gooding T, et al. Associations between drug use and sexual risks among heterosexual men in the Philippines [published online July 22, 2013]. Int J STD AIDS. 2013;24:969-976.
- Flagg EW, Weinstock HS, Frazier EL, et al. Bacterial sexually transmitted infections among HIV-infected patients in the United States: estimates from the Medical Monitoring Project. Sex Transm Dis. 2015;42:171-179.
- Shilaih M, Marzel A, Braun DL, et al; Swiss HIV Cohort Study. Factors associated with syphilis incidence in the HIV-infected in the era of highly active antiretrovirals. Medicine (Baltimore). 2017;96:E5849.
- Salado-Rasmussen K. Syphilis and HIV co-infection. epidemiology, treatment and molecular typing of Treponema pallidum. Dan Med J. 2015;62:B5176.
Once upon a time, and long ago, dermatology journals included “syphilology” in their names. The first dermatologic journal published in the United States was the American Journal of Syphilology and Dermatology.1 In October 1882 the Journal of Cutaneous and Venereal Diseases appeared and subsequently renamed several times from 1882 to 1919: Journal of Cutaneous Diseases and Genitourinary Diseases and the Journal of Cutaneous Diseases, Including Syphilis. When the American Medical Association (AMA) assumed control, this publication obtained a new name: Archives of Dermatology and Syphilology; in January 1955 syphilology was deleted from the title. According to an editorial in that issue, the rationale for dropping the word syphilology was as follows: “The diagnosis and treatment of patients with syphilis is no longer an important part of dermatologic practice. . . . Few dermatologists now have patients with syphilis; in fact, there are decidedly fewer patients with syphilis, and so continuance of the old label, ‘Syphilology,’ on this publication seems no longer warranted.”1 Needless to say, this decision ignored the obvious fact that the majority of dermatologists traditionally were well trained in and clinically practiced venereology, particularly the management of syphilis,2,3 which makes sense, considering that many of the clinical manifestations of syphilis involve the skin, hair, and oral mucosa. My own mentor and former Baylor College of Medicine dermatology department chair, Dr. John Knox, authored 3 dozen major publications regarding the diagnosis, treatment, and immunology of syphilis. During his chairmanship, all residents were required to rotate in the Harris County sexually transmitted disease (STD) clinic on a weekly basis.
I am confident that the decision to drop “syphilology” from the journal title also was based on the unduly optimistic assumption that syphilis would soon become a rare disease due to the availability of penicillin. Indeed, the Centers for Disease Control and Prevention in the United States has periodically announced strategic programs designed to eradicate syphilis!4 This rosy outlook reached a fever pitch in 2000 when the number of cases (5979) and the incidence (2.1 cases per 100,000 population) of primary and secondary syphilis reached an all-time low in the United States.5
Unfortunately, no one could accurately predict the future. Although the number of cases and incidence of early infectious syphilis have fluctuated widely since the 1940s, we currently are in a dire period of syphilis resurgence; the largest number of cases (27,814) and the highest incidence rate of primary and secondary syphilis (8.7 cases per 100,000 population) since 1994 were reported in 2016,6 which illustrates the inability of public health initiatives to eliminate syphilis, largely due to the inability of health authorities, health care providers, teachers, parents, clergy, and peer groups to alter sexual behaviors or modify other socioeconomic factors.7 Thus, syphilis lives on! Nobody could have predicted the easy availability of oral contraceptives and the ensuing sexual revolution of the 1960s or the advent of erectile dysfunction drugs decades later that led to increasing STDs among older patients.8 Nobody could have predicted the wholesale acceptance of casual sexual intercourse as popularized on television and in the movies or the pervasive use of sexual images in advertising. Nobody could have predicted the modern phenomena of “booty-call relationships,” “friends with benefits,” and “sexting,” or the nearly ubiquitous and increasingly legal use of noninjectable mind-altering drugs, all of which facilitate the perpetuation of STDs.9-11 Finally, those who removed “syphilology” from that journal title certainly did not foresee the worldwide epidemic now known as human immunodeficiency virus/AIDS, which has most assuredly helped keep syphilis a modern day menace.12-14
How have dermatologists been impacted? Our journals and our teachers have deemphasized STDs, including syphilis, in modern times, yet we are faced with a disease carrying serious, if not often fatal, consequences that is simply refusing to disappear (contrary to wishful thinking). Dermatologists are, however, in a perfect epidemiological position to help in the war against Treponema pallidum, the bacterium that causes syphilis. We frequently see adolescent patients for warts and acne, and we often diagnose and help care for patients with human immunodeficiency virus. We obliterate actinic keratoses and perform cosmetic procedures on those who rely on erectile dysfunction drugs (or their partners do). Who better than a dermatologist to recognize in these high-risk constituencies, and others, that patchy hair loss may represent syphilitic alopecia and that extragenital chancres can mimic nonmelanoma skin cancer? Who better than the dermatologist to distinguish between oral mucous patches and orolabial herpes? Who better than the dermatologist to diagnose the annular syphilid of the face, or ostraceous, florid nodular, or ulceronecrotic lesions of lues maligna? Who better than the dermatologist to differentiate condylomata lata from external genital warts?
I would suggest that the responsible dermatologist become reacquainted with syphilis, in all its various manifestations. I would further suggest that our dermatology training centers spend more time diligently teaching residents about syphilis and other STDs. In conclusion, I fervently hope that organized dermatology will once again dutifully consider venereal disease to be a critical part of our specialty’s skill set.
Once upon a time, and long ago, dermatology journals included “syphilology” in their names. The first dermatologic journal published in the United States was the American Journal of Syphilology and Dermatology.1 In October 1882 the Journal of Cutaneous and Venereal Diseases appeared and subsequently renamed several times from 1882 to 1919: Journal of Cutaneous Diseases and Genitourinary Diseases and the Journal of Cutaneous Diseases, Including Syphilis. When the American Medical Association (AMA) assumed control, this publication obtained a new name: Archives of Dermatology and Syphilology; in January 1955 syphilology was deleted from the title. According to an editorial in that issue, the rationale for dropping the word syphilology was as follows: “The diagnosis and treatment of patients with syphilis is no longer an important part of dermatologic practice. . . . Few dermatologists now have patients with syphilis; in fact, there are decidedly fewer patients with syphilis, and so continuance of the old label, ‘Syphilology,’ on this publication seems no longer warranted.”1 Needless to say, this decision ignored the obvious fact that the majority of dermatologists traditionally were well trained in and clinically practiced venereology, particularly the management of syphilis,2,3 which makes sense, considering that many of the clinical manifestations of syphilis involve the skin, hair, and oral mucosa. My own mentor and former Baylor College of Medicine dermatology department chair, Dr. John Knox, authored 3 dozen major publications regarding the diagnosis, treatment, and immunology of syphilis. During his chairmanship, all residents were required to rotate in the Harris County sexually transmitted disease (STD) clinic on a weekly basis.
I am confident that the decision to drop “syphilology” from the journal title also was based on the unduly optimistic assumption that syphilis would soon become a rare disease due to the availability of penicillin. Indeed, the Centers for Disease Control and Prevention in the United States has periodically announced strategic programs designed to eradicate syphilis!4 This rosy outlook reached a fever pitch in 2000 when the number of cases (5979) and the incidence (2.1 cases per 100,000 population) of primary and secondary syphilis reached an all-time low in the United States.5
Unfortunately, no one could accurately predict the future. Although the number of cases and incidence of early infectious syphilis have fluctuated widely since the 1940s, we currently are in a dire period of syphilis resurgence; the largest number of cases (27,814) and the highest incidence rate of primary and secondary syphilis (8.7 cases per 100,000 population) since 1994 were reported in 2016,6 which illustrates the inability of public health initiatives to eliminate syphilis, largely due to the inability of health authorities, health care providers, teachers, parents, clergy, and peer groups to alter sexual behaviors or modify other socioeconomic factors.7 Thus, syphilis lives on! Nobody could have predicted the easy availability of oral contraceptives and the ensuing sexual revolution of the 1960s or the advent of erectile dysfunction drugs decades later that led to increasing STDs among older patients.8 Nobody could have predicted the wholesale acceptance of casual sexual intercourse as popularized on television and in the movies or the pervasive use of sexual images in advertising. Nobody could have predicted the modern phenomena of “booty-call relationships,” “friends with benefits,” and “sexting,” or the nearly ubiquitous and increasingly legal use of noninjectable mind-altering drugs, all of which facilitate the perpetuation of STDs.9-11 Finally, those who removed “syphilology” from that journal title certainly did not foresee the worldwide epidemic now known as human immunodeficiency virus/AIDS, which has most assuredly helped keep syphilis a modern day menace.12-14
How have dermatologists been impacted? Our journals and our teachers have deemphasized STDs, including syphilis, in modern times, yet we are faced with a disease carrying serious, if not often fatal, consequences that is simply refusing to disappear (contrary to wishful thinking). Dermatologists are, however, in a perfect epidemiological position to help in the war against Treponema pallidum, the bacterium that causes syphilis. We frequently see adolescent patients for warts and acne, and we often diagnose and help care for patients with human immunodeficiency virus. We obliterate actinic keratoses and perform cosmetic procedures on those who rely on erectile dysfunction drugs (or their partners do). Who better than a dermatologist to recognize in these high-risk constituencies, and others, that patchy hair loss may represent syphilitic alopecia and that extragenital chancres can mimic nonmelanoma skin cancer? Who better than the dermatologist to distinguish between oral mucous patches and orolabial herpes? Who better than the dermatologist to diagnose the annular syphilid of the face, or ostraceous, florid nodular, or ulceronecrotic lesions of lues maligna? Who better than the dermatologist to differentiate condylomata lata from external genital warts?
I would suggest that the responsible dermatologist become reacquainted with syphilis, in all its various manifestations. I would further suggest that our dermatology training centers spend more time diligently teaching residents about syphilis and other STDs. In conclusion, I fervently hope that organized dermatology will once again dutifully consider venereal disease to be a critical part of our specialty’s skill set.
- Editorial. AMA Arch Dermatol. 1955;71:1.
- Shelley WB. Major contributors to American dermatology—1876 to 1926. Arch Dermatol. 1976;112:1642-1646.
- Lobitz WC Jr. Major contributions of American dermatologists—1926 to 1976. Arch Dermatol. 1976;112:1646-1650.
- Hook EW 3rd. Elimination of syphilis transmission in the United States: historic perspectives and practical considerations. Trans Am ClinClimatol Assoc. 1999;110:195-203.
- Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2000. Atlanta, GA: Department of Health and Human Services; 2001.
- 2016 Sexually transmitted diseases surveillance: syphilis. Centers for Disease Control and Prevention website. https://www.cdc.gov/std/stats16/syphilis.htm. Updated September 26, 2017. Accessed October 20, 2017.
- Shockman S, Buescher LS, Stone SP. Syphilis in the United States. Clin Dermatol. 2014;32:213-218.
- Jena AB, Goldman DP, Kamdar A, et al. Sexually transmitted diseases among users of erectile dysfunction drugs: analysis of claims data. Ann Intern Med. 2010;153:1-7.
- Jonason PK, Li NP, Richardson J. Positioning the booty-call relationship on the spectrum of relationships: sexual but more emotional than one-night stands. J Sex Res. 2011;48:486-495.
- Temple JR, Choi H. Longitudinal association between teen sexting and sexual behavior. Pediatrics. 2014;134:E1287-E1292.
- Regan R, Dyer TP, Gooding T, et al. Associations between drug use and sexual risks among heterosexual men in the Philippines [published online July 22, 2013]. Int J STD AIDS. 2013;24:969-976.
- Flagg EW, Weinstock HS, Frazier EL, et al. Bacterial sexually transmitted infections among HIV-infected patients in the United States: estimates from the Medical Monitoring Project. Sex Transm Dis. 2015;42:171-179.
- Shilaih M, Marzel A, Braun DL, et al; Swiss HIV Cohort Study. Factors associated with syphilis incidence in the HIV-infected in the era of highly active antiretrovirals. Medicine (Baltimore). 2017;96:E5849.
- Salado-Rasmussen K. Syphilis and HIV co-infection. epidemiology, treatment and molecular typing of Treponema pallidum. Dan Med J. 2015;62:B5176.
- Editorial. AMA Arch Dermatol. 1955;71:1.
- Shelley WB. Major contributors to American dermatology—1876 to 1926. Arch Dermatol. 1976;112:1642-1646.
- Lobitz WC Jr. Major contributions of American dermatologists—1926 to 1976. Arch Dermatol. 1976;112:1646-1650.
- Hook EW 3rd. Elimination of syphilis transmission in the United States: historic perspectives and practical considerations. Trans Am ClinClimatol Assoc. 1999;110:195-203.
- Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2000. Atlanta, GA: Department of Health and Human Services; 2001.
- 2016 Sexually transmitted diseases surveillance: syphilis. Centers for Disease Control and Prevention website. https://www.cdc.gov/std/stats16/syphilis.htm. Updated September 26, 2017. Accessed October 20, 2017.
- Shockman S, Buescher LS, Stone SP. Syphilis in the United States. Clin Dermatol. 2014;32:213-218.
- Jena AB, Goldman DP, Kamdar A, et al. Sexually transmitted diseases among users of erectile dysfunction drugs: analysis of claims data. Ann Intern Med. 2010;153:1-7.
- Jonason PK, Li NP, Richardson J. Positioning the booty-call relationship on the spectrum of relationships: sexual but more emotional than one-night stands. J Sex Res. 2011;48:486-495.
- Temple JR, Choi H. Longitudinal association between teen sexting and sexual behavior. Pediatrics. 2014;134:E1287-E1292.
- Regan R, Dyer TP, Gooding T, et al. Associations between drug use and sexual risks among heterosexual men in the Philippines [published online July 22, 2013]. Int J STD AIDS. 2013;24:969-976.
- Flagg EW, Weinstock HS, Frazier EL, et al. Bacterial sexually transmitted infections among HIV-infected patients in the United States: estimates from the Medical Monitoring Project. Sex Transm Dis. 2015;42:171-179.
- Shilaih M, Marzel A, Braun DL, et al; Swiss HIV Cohort Study. Factors associated with syphilis incidence in the HIV-infected in the era of highly active antiretrovirals. Medicine (Baltimore). 2017;96:E5849.
- Salado-Rasmussen K. Syphilis and HIV co-infection. epidemiology, treatment and molecular typing of Treponema pallidum. Dan Med J. 2015;62:B5176.
Left main distal bifurcation? Double kiss and crush it
DENVER – A planned two-stent, double-kissing crush PCI technique proved superior to the widely utilized provisional stenting strategy for treatment of unprotected distal left main bifurcation lesions in the randomized DKCRUSH-V trial, Shao-Liang Chen, MD, reported at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
The study randomized 482 patients with unprotected true distal left main bifurcation lesions to one of the two PCI strategies at 26 centers in five countries, including the United States. Roughly 80% of the left main lesions were categorized as Medina 1,1,1.
The primary outcome was the 1-year composite rate of target lesion failure (TLF), defined as cardiac death, target vessel MI, or clinically driven target lesion revascularization. The rate was 10.7% in patients assigned to provisional stenting and 5.0% with double kissing (DK) crush. This clinically and statistically significant difference was driven by a sharp reduction in target vessel MI in the DK crush group: 0.4%, compared with 2.9% in the provisional stenting group.
Moreover, the DK crush group’s 3.8% rate of clinically driven target lesion revascularization and 7.1% rate of angiographic restenosis within the left main complex were both less than half the rates in the provisional stenting group, he added at the meeting, which was sponsored by the Cardiovascular Research Foundation.
The absolute benefit for the DK crush strategy was greatest in the roughly 30% of patients with complex bifurcations, defined as those with an ostial side branch lesion length of at least 10 mm and 70% diameter stenosis while meeting at least two of six minor criteria. The 1-year TLF rate in such patients was 18.2% with provisional stenting versus 7% with DK crush. For simple bifurcations, the TLF rates were 8% versus 1.9%.
The results favored DK crush in all examined subgroups, including those based upon age, gender, SYNTAX score, distal angle, and diabetes status.
Forty-seven percent of patients in the provisional stenting arm received a second stent, typically needed as a bailout for the side branch. Most of the excess target vessel MIs and other TLF events in the provisional stenting group occurred in patients who got a second stent.
The DK crush is an advanced technique with numerous steps involving multiple vessel wirings, rewirings, and stent crushing. It can be challenging to perform. It took an average of 82 minutes, 16 minutes longer than provisional stenting – a difference in procedural time that interventional cardiologists don’t take lightly. It also entailed an average of 36 mL more contrast media than the 191 mL for provisional stenting.
In an earlier multicenter, randomized, prospective trial – DKCRUSH-III – Dr. Chen and his coinvestigators showed that the DK crush technique provides superior outcomes compared with culotte stenting, another widely used treatment strategy for distal left main bifurcation lesions (J Am Coll Cardiol. 2013 Apr 9;61[14]:1482-8).
“The take home message on the surface of the data would be that we should consider this particular two-stent bifurcation technique as perhaps the treatment of choice for true distal bifurcation lesions,” said Gregg W. Stone, MD, who was a coinvestigator in DKCRUSH-V and chaired the late-breaking clinical trial session where Dr. Chen presented the results.
“This technique theoretically gives you the largest amount of laminar flow both in the main vessel and the side branch,” added Dr. Stone, professor of medicine and director of cardiovascular research and education at Columbia University Medical Center in New York.
Simultaneously with Dr. Chen’s presentation at TCT 2017, the DKCRUSH-V results were published online (J Am Coll Cardiol. 2017 Oct 30. doi: 10.1016/j.jacc.2017.09.1066). In an accompanying editorial, Emmanouil S. Brilakis, MD, declared that DK crush should become the preferred strategy for treating unprotected left main bifurcation lesions.
It’s not a technique for the average interventionalist, though. It should be performed in high-volume centers of excellence accustomed to performing complex PCIs. Indeed, the DKCRUSH-V trial required the primary operators to have performed at least 300 PCIs per year for 5 years, including 20 left main PCIs per year or more. To put that in perspective, the median annual PCI volume in the United States is 59 cases, noted Dr. Brilakis of the Minneapolis Heart Institute (J Am Coll Cardiol. 2017 Oct. 30. doi: 10.1016/j.jacc.2017.09.1083).
At TCT 2017 in Denver, not everyone found the DKCRUSH-V findings persuasive.
“I’m quite surprised by the results,” said panel discussant David Hildick-Smith, MD.
“There’s something we have yet to understand about the divergence between the results that are coming out of China and the results coming out of Europe. Almost everything coming out of Europe tends to suggest that provisional stenting is better, while in Chinese hands the DK crush technique has proved to be an extremely successful strategy,” said Dr. Hildick-Smith, professor of interventional cardiology and director of the cardiac research unit at the Brighton and Sussex (England) Medical School.
He added that he intends to reserve judgment as to the preferred strategy until the results of the ongoing European Bifurcation Club Left Main Study (EBC MAIN) become available late in 2018. EBC MAIN is comparing the DK crush, culotte, and other strategies, with the choice of technique left to the operator’s discretion.
The DKCRUSH-V trial was supported by the National Science Foundation of China, the Nanjing Municipal Medical Development Project, Microport, Abbott Vascular, and Medtronic. Dr. Chen and Dr. Stone reported having no financial conflicts of interest regarding the study.
DENVER – A planned two-stent, double-kissing crush PCI technique proved superior to the widely utilized provisional stenting strategy for treatment of unprotected distal left main bifurcation lesions in the randomized DKCRUSH-V trial, Shao-Liang Chen, MD, reported at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
The study randomized 482 patients with unprotected true distal left main bifurcation lesions to one of the two PCI strategies at 26 centers in five countries, including the United States. Roughly 80% of the left main lesions were categorized as Medina 1,1,1.
The primary outcome was the 1-year composite rate of target lesion failure (TLF), defined as cardiac death, target vessel MI, or clinically driven target lesion revascularization. The rate was 10.7% in patients assigned to provisional stenting and 5.0% with double kissing (DK) crush. This clinically and statistically significant difference was driven by a sharp reduction in target vessel MI in the DK crush group: 0.4%, compared with 2.9% in the provisional stenting group.
Moreover, the DK crush group’s 3.8% rate of clinically driven target lesion revascularization and 7.1% rate of angiographic restenosis within the left main complex were both less than half the rates in the provisional stenting group, he added at the meeting, which was sponsored by the Cardiovascular Research Foundation.
The absolute benefit for the DK crush strategy was greatest in the roughly 30% of patients with complex bifurcations, defined as those with an ostial side branch lesion length of at least 10 mm and 70% diameter stenosis while meeting at least two of six minor criteria. The 1-year TLF rate in such patients was 18.2% with provisional stenting versus 7% with DK crush. For simple bifurcations, the TLF rates were 8% versus 1.9%.
The results favored DK crush in all examined subgroups, including those based upon age, gender, SYNTAX score, distal angle, and diabetes status.
Forty-seven percent of patients in the provisional stenting arm received a second stent, typically needed as a bailout for the side branch. Most of the excess target vessel MIs and other TLF events in the provisional stenting group occurred in patients who got a second stent.
The DK crush is an advanced technique with numerous steps involving multiple vessel wirings, rewirings, and stent crushing. It can be challenging to perform. It took an average of 82 minutes, 16 minutes longer than provisional stenting – a difference in procedural time that interventional cardiologists don’t take lightly. It also entailed an average of 36 mL more contrast media than the 191 mL for provisional stenting.
In an earlier multicenter, randomized, prospective trial – DKCRUSH-III – Dr. Chen and his coinvestigators showed that the DK crush technique provides superior outcomes compared with culotte stenting, another widely used treatment strategy for distal left main bifurcation lesions (J Am Coll Cardiol. 2013 Apr 9;61[14]:1482-8).
“The take home message on the surface of the data would be that we should consider this particular two-stent bifurcation technique as perhaps the treatment of choice for true distal bifurcation lesions,” said Gregg W. Stone, MD, who was a coinvestigator in DKCRUSH-V and chaired the late-breaking clinical trial session where Dr. Chen presented the results.
“This technique theoretically gives you the largest amount of laminar flow both in the main vessel and the side branch,” added Dr. Stone, professor of medicine and director of cardiovascular research and education at Columbia University Medical Center in New York.
Simultaneously with Dr. Chen’s presentation at TCT 2017, the DKCRUSH-V results were published online (J Am Coll Cardiol. 2017 Oct 30. doi: 10.1016/j.jacc.2017.09.1066). In an accompanying editorial, Emmanouil S. Brilakis, MD, declared that DK crush should become the preferred strategy for treating unprotected left main bifurcation lesions.
It’s not a technique for the average interventionalist, though. It should be performed in high-volume centers of excellence accustomed to performing complex PCIs. Indeed, the DKCRUSH-V trial required the primary operators to have performed at least 300 PCIs per year for 5 years, including 20 left main PCIs per year or more. To put that in perspective, the median annual PCI volume in the United States is 59 cases, noted Dr. Brilakis of the Minneapolis Heart Institute (J Am Coll Cardiol. 2017 Oct. 30. doi: 10.1016/j.jacc.2017.09.1083).
At TCT 2017 in Denver, not everyone found the DKCRUSH-V findings persuasive.
“I’m quite surprised by the results,” said panel discussant David Hildick-Smith, MD.
“There’s something we have yet to understand about the divergence between the results that are coming out of China and the results coming out of Europe. Almost everything coming out of Europe tends to suggest that provisional stenting is better, while in Chinese hands the DK crush technique has proved to be an extremely successful strategy,” said Dr. Hildick-Smith, professor of interventional cardiology and director of the cardiac research unit at the Brighton and Sussex (England) Medical School.
He added that he intends to reserve judgment as to the preferred strategy until the results of the ongoing European Bifurcation Club Left Main Study (EBC MAIN) become available late in 2018. EBC MAIN is comparing the DK crush, culotte, and other strategies, with the choice of technique left to the operator’s discretion.
The DKCRUSH-V trial was supported by the National Science Foundation of China, the Nanjing Municipal Medical Development Project, Microport, Abbott Vascular, and Medtronic. Dr. Chen and Dr. Stone reported having no financial conflicts of interest regarding the study.
DENVER – A planned two-stent, double-kissing crush PCI technique proved superior to the widely utilized provisional stenting strategy for treatment of unprotected distal left main bifurcation lesions in the randomized DKCRUSH-V trial, Shao-Liang Chen, MD, reported at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
The study randomized 482 patients with unprotected true distal left main bifurcation lesions to one of the two PCI strategies at 26 centers in five countries, including the United States. Roughly 80% of the left main lesions were categorized as Medina 1,1,1.
The primary outcome was the 1-year composite rate of target lesion failure (TLF), defined as cardiac death, target vessel MI, or clinically driven target lesion revascularization. The rate was 10.7% in patients assigned to provisional stenting and 5.0% with double kissing (DK) crush. This clinically and statistically significant difference was driven by a sharp reduction in target vessel MI in the DK crush group: 0.4%, compared with 2.9% in the provisional stenting group.
Moreover, the DK crush group’s 3.8% rate of clinically driven target lesion revascularization and 7.1% rate of angiographic restenosis within the left main complex were both less than half the rates in the provisional stenting group, he added at the meeting, which was sponsored by the Cardiovascular Research Foundation.
The absolute benefit for the DK crush strategy was greatest in the roughly 30% of patients with complex bifurcations, defined as those with an ostial side branch lesion length of at least 10 mm and 70% diameter stenosis while meeting at least two of six minor criteria. The 1-year TLF rate in such patients was 18.2% with provisional stenting versus 7% with DK crush. For simple bifurcations, the TLF rates were 8% versus 1.9%.
The results favored DK crush in all examined subgroups, including those based upon age, gender, SYNTAX score, distal angle, and diabetes status.
Forty-seven percent of patients in the provisional stenting arm received a second stent, typically needed as a bailout for the side branch. Most of the excess target vessel MIs and other TLF events in the provisional stenting group occurred in patients who got a second stent.
The DK crush is an advanced technique with numerous steps involving multiple vessel wirings, rewirings, and stent crushing. It can be challenging to perform. It took an average of 82 minutes, 16 minutes longer than provisional stenting – a difference in procedural time that interventional cardiologists don’t take lightly. It also entailed an average of 36 mL more contrast media than the 191 mL for provisional stenting.
In an earlier multicenter, randomized, prospective trial – DKCRUSH-III – Dr. Chen and his coinvestigators showed that the DK crush technique provides superior outcomes compared with culotte stenting, another widely used treatment strategy for distal left main bifurcation lesions (J Am Coll Cardiol. 2013 Apr 9;61[14]:1482-8).
“The take home message on the surface of the data would be that we should consider this particular two-stent bifurcation technique as perhaps the treatment of choice for true distal bifurcation lesions,” said Gregg W. Stone, MD, who was a coinvestigator in DKCRUSH-V and chaired the late-breaking clinical trial session where Dr. Chen presented the results.
“This technique theoretically gives you the largest amount of laminar flow both in the main vessel and the side branch,” added Dr. Stone, professor of medicine and director of cardiovascular research and education at Columbia University Medical Center in New York.
Simultaneously with Dr. Chen’s presentation at TCT 2017, the DKCRUSH-V results were published online (J Am Coll Cardiol. 2017 Oct 30. doi: 10.1016/j.jacc.2017.09.1066). In an accompanying editorial, Emmanouil S. Brilakis, MD, declared that DK crush should become the preferred strategy for treating unprotected left main bifurcation lesions.
It’s not a technique for the average interventionalist, though. It should be performed in high-volume centers of excellence accustomed to performing complex PCIs. Indeed, the DKCRUSH-V trial required the primary operators to have performed at least 300 PCIs per year for 5 years, including 20 left main PCIs per year or more. To put that in perspective, the median annual PCI volume in the United States is 59 cases, noted Dr. Brilakis of the Minneapolis Heart Institute (J Am Coll Cardiol. 2017 Oct. 30. doi: 10.1016/j.jacc.2017.09.1083).
At TCT 2017 in Denver, not everyone found the DKCRUSH-V findings persuasive.
“I’m quite surprised by the results,” said panel discussant David Hildick-Smith, MD.
“There’s something we have yet to understand about the divergence between the results that are coming out of China and the results coming out of Europe. Almost everything coming out of Europe tends to suggest that provisional stenting is better, while in Chinese hands the DK crush technique has proved to be an extremely successful strategy,” said Dr. Hildick-Smith, professor of interventional cardiology and director of the cardiac research unit at the Brighton and Sussex (England) Medical School.
He added that he intends to reserve judgment as to the preferred strategy until the results of the ongoing European Bifurcation Club Left Main Study (EBC MAIN) become available late in 2018. EBC MAIN is comparing the DK crush, culotte, and other strategies, with the choice of technique left to the operator’s discretion.
The DKCRUSH-V trial was supported by the National Science Foundation of China, the Nanjing Municipal Medical Development Project, Microport, Abbott Vascular, and Medtronic. Dr. Chen and Dr. Stone reported having no financial conflicts of interest regarding the study.
AT TCT 2017
Key clinical point:
Major finding: The 1-year composite rate of target lesion failure in patients treated with the planned two-stent double-kissing crush strategy was 5%, significantly less than the 10.7% rate with provisional stenting.
Data source: The DKCRUSH-V study randomized 482 patients with unprotected true distal left main bifurcation lesions to one of the two PCI strategies at 26 centers in five countries.
Disclosures: The study was supported by the National Science Foundation of China, the Nanjing Municipal Medical Development Project, Microport, Abbott Vascular, and Medtronic. The presenter reported having no financial conflicts of interest.
Children and trauma: How Sesame Street can help
Nearly half of American children have faced one adverse childhood experience (ACE), according to new analysis of the 2016 National Survey of Children’s Health, and more than 20% have had two ACEs or more. This may include abuse or neglect, witnessing violence, parental substance abuse, mental illness, or incarceration. And from news headlines, we are all too aware of other traumas children face, such as natural disasters and mass violence.
But we know that children are remarkably resilient, and trauma does not have to define their trajectory. With the right tools and support, the effects of trauma can be mitigated, and children can build coping skills and resiliency for a healthy, promising future.
When we began hearing from community service partners and child development experts that there was a critical need for resources to help children cope with trauma, we felt we could help.
Traumatic experiences can disrupt brain development, but when children have hope, when they feel seen and heard by caring adults who can guide them through those crucial resilience-building techniques, the impact of ACEs can be mitigated, and children can be set on the road to healing and stability.
With support from the Robert Wood Johnson Foundation and other funders, To do this, we enlisted the pediatric community and professionals in the field, grounding our approach in the latest research. Then we used our proven model to produce resources that could engage and comfort children while building coping skills and foster crucial nurturing connections between children and the adults in their lives.
Our free materials – some are targeted for children and others are for providers – include videos, storybooks, and digital activities in English and Spanish. They are all available at sesamestreetincommunities.org/topics/traumatic-experiences.
In one video called “Comfy Cozy Nest,” when Big Bird faces an unspecified difficult situation, he learns to think of his nest as a “safe space” with comforting items like his teddy bear and Granny Bird’s birdseed cookies. This is a place he can go in his imagination to make himself feel safe. In others, Elmo builds a blanket fort to feel secure and the Count teaches Cookie Monster a breathing strategy to help him relax.
In addition to engaging materials for children, providers can find professional development workshops, webinars, and other adult-facing content that includes, as part of our trauma content, a powerful animation to help parents and caregivers understand the impact of domestic violence from a child’s perspective.
No one plays a more vital role in children’s health and well-being than pediatricians, nurse practitioners, and family physicians. Our hope is that Sesame Street in Communities will allow us to work together, to help children everywhere grow smarter, stronger, and kinder.
Dr. Betancourt is the senior vice president for U.S. social impact at Sesame Workshop, the nonprofit media and educational organization behind Sesame Street, in New York.
Nearly half of American children have faced one adverse childhood experience (ACE), according to new analysis of the 2016 National Survey of Children’s Health, and more than 20% have had two ACEs or more. This may include abuse or neglect, witnessing violence, parental substance abuse, mental illness, or incarceration. And from news headlines, we are all too aware of other traumas children face, such as natural disasters and mass violence.
But we know that children are remarkably resilient, and trauma does not have to define their trajectory. With the right tools and support, the effects of trauma can be mitigated, and children can build coping skills and resiliency for a healthy, promising future.
When we began hearing from community service partners and child development experts that there was a critical need for resources to help children cope with trauma, we felt we could help.
Traumatic experiences can disrupt brain development, but when children have hope, when they feel seen and heard by caring adults who can guide them through those crucial resilience-building techniques, the impact of ACEs can be mitigated, and children can be set on the road to healing and stability.
With support from the Robert Wood Johnson Foundation and other funders, To do this, we enlisted the pediatric community and professionals in the field, grounding our approach in the latest research. Then we used our proven model to produce resources that could engage and comfort children while building coping skills and foster crucial nurturing connections between children and the adults in their lives.
Our free materials – some are targeted for children and others are for providers – include videos, storybooks, and digital activities in English and Spanish. They are all available at sesamestreetincommunities.org/topics/traumatic-experiences.
In one video called “Comfy Cozy Nest,” when Big Bird faces an unspecified difficult situation, he learns to think of his nest as a “safe space” with comforting items like his teddy bear and Granny Bird’s birdseed cookies. This is a place he can go in his imagination to make himself feel safe. In others, Elmo builds a blanket fort to feel secure and the Count teaches Cookie Monster a breathing strategy to help him relax.
In addition to engaging materials for children, providers can find professional development workshops, webinars, and other adult-facing content that includes, as part of our trauma content, a powerful animation to help parents and caregivers understand the impact of domestic violence from a child’s perspective.
No one plays a more vital role in children’s health and well-being than pediatricians, nurse practitioners, and family physicians. Our hope is that Sesame Street in Communities will allow us to work together, to help children everywhere grow smarter, stronger, and kinder.
Dr. Betancourt is the senior vice president for U.S. social impact at Sesame Workshop, the nonprofit media and educational organization behind Sesame Street, in New York.
Nearly half of American children have faced one adverse childhood experience (ACE), according to new analysis of the 2016 National Survey of Children’s Health, and more than 20% have had two ACEs or more. This may include abuse or neglect, witnessing violence, parental substance abuse, mental illness, or incarceration. And from news headlines, we are all too aware of other traumas children face, such as natural disasters and mass violence.
But we know that children are remarkably resilient, and trauma does not have to define their trajectory. With the right tools and support, the effects of trauma can be mitigated, and children can build coping skills and resiliency for a healthy, promising future.
When we began hearing from community service partners and child development experts that there was a critical need for resources to help children cope with trauma, we felt we could help.
Traumatic experiences can disrupt brain development, but when children have hope, when they feel seen and heard by caring adults who can guide them through those crucial resilience-building techniques, the impact of ACEs can be mitigated, and children can be set on the road to healing and stability.
With support from the Robert Wood Johnson Foundation and other funders, To do this, we enlisted the pediatric community and professionals in the field, grounding our approach in the latest research. Then we used our proven model to produce resources that could engage and comfort children while building coping skills and foster crucial nurturing connections between children and the adults in their lives.
Our free materials – some are targeted for children and others are for providers – include videos, storybooks, and digital activities in English and Spanish. They are all available at sesamestreetincommunities.org/topics/traumatic-experiences.
In one video called “Comfy Cozy Nest,” when Big Bird faces an unspecified difficult situation, he learns to think of his nest as a “safe space” with comforting items like his teddy bear and Granny Bird’s birdseed cookies. This is a place he can go in his imagination to make himself feel safe. In others, Elmo builds a blanket fort to feel secure and the Count teaches Cookie Monster a breathing strategy to help him relax.
In addition to engaging materials for children, providers can find professional development workshops, webinars, and other adult-facing content that includes, as part of our trauma content, a powerful animation to help parents and caregivers understand the impact of domestic violence from a child’s perspective.
No one plays a more vital role in children’s health and well-being than pediatricians, nurse practitioners, and family physicians. Our hope is that Sesame Street in Communities will allow us to work together, to help children everywhere grow smarter, stronger, and kinder.
Dr. Betancourt is the senior vice president for U.S. social impact at Sesame Workshop, the nonprofit media and educational organization behind Sesame Street, in New York.
Low tryptophan levels linked to IBD
Patients with inflammatory bowel disease (IBD) had significantly lower serum levels of the essential amino acid tryptophan than healthy controls in a large study reported in the December issue of Gastroenterology (doi: 10.1053/j.gastro.2017.08.028).
Serum tryptophan levels also correlated inversely with both disease activity and C-reactive protein levels in patients with IBD, reported Susanna Nikolaus, MD, of University Hospital Schleswig-Holstein, Kiel, Germany, with her associates. “Tryptophan deficiency could contribute to development of IBD. Studies are needed to determine whether modification of intestinal tryptophan pathways affects [its] severity,” they wrote.
Several small case series have reported low levels of tryptophan in IBD and other autoimmune disorders, the investigators noted. Removing tryptophan from the diet has been found to increase susceptibility to colitis in mice, and supplementing with tryptophan or some of its metabolites has the opposite effect. For this study, the researchers used high-performance liquid chromatography to quantify tryptophan levels in serum samples from 535 consecutive patients with IBD and 100 matched controls. They used mass spectrometry to measure metabolites of tryptophan, enzyme-linked immunosorbent assay to measure interleukin-22 (IL-22) levels, and 16S rDNA amplicon sequencing to correlate tryptophan levels with fecal microbiota species. Finally, they used real-time polymerase chain reaction to measure levels of mRNA encoding tryptophan metabolites in colonic biopsy specimens.
Serum tryptophan levels were significantly lower in patients with IBD than controls (P = 5.3 x 10–6). The difference was starker in patients with Crohn’s disease (P = 1.1 x 10–10 vs. controls) compared with those with ulcerative colitis (P = 2.8 x 10–3 vs. controls), the investigators noted. Serum tryptophan levels also correlated inversely with disease activity in patients with Crohn’s disease (P = .01), while patients with ulcerative colitis showed a similar but nonsignificant trend (P = .07). Low tryptophan levels were associated with marked, statistically significant increases in C-reactive protein levels in both Crohn’s disease and ulcerative colitis. Tryptophan level also correlated inversely with leukocyte count, although the trend was less pronounced (P = .04).IBD was associated with several aberrations in the tryptophan kynurenine pathway, which is the primary means of catabolizing the amino acid. For example, compared with controls, patients with active IBD had significantly lower levels of mRNA encoding tryptophan 2,3-dioxygenase-2 (TDO2, a key enzyme in the kynurenine pathway) and solute carrier family 6 member 19 (SLC6A19, also called B0AT1, a neutral amino acid transporter). Patients with IBD also had significantly higher levels of indoleamine 2,3-dioxygenase 1 (IDO1), which catalyzes the initial, rate-limiting oxidation of tryptophan to kynurenine. Accordingly, patients with IBD had a significantly higher ratio of kynurenine to tryptophan than did controls, and this abnormality was associated with disease activity, especially in Crohn’s disease (P = .03).
Patients with IBD who had relatively higher tryptophan levels also tended to have more diverse gut microbiota than did patients with lower serum tryptophan levels, although differences among groups were not statistically significant, the investigators said. Serum concentration of IL-22 also correlated with disease activity in patients with IBD, and infliximab responders had a “significant and sustained increase” of tryptophan levels over time, compared with nonresponders.
Potsdam dietary questionnaires found no link between disease activity and dietary consumption of tryptophan, the researchers said. Additionally, they found no links between serum tryptophan levels and age, smoking status, or disease complications, such as fistulae or abscess formation.The investigators acknowledged grant support from the DFG Excellence Cluster “Inflammation at Interfaces” and BMBF e-med SYSINFLAME and H2020 SysCID. One coinvestigator reported employment by CONARIS Research Institute AG, which helps develop drugs with inflammatory indications. The other investigators had no conflicts of interest.
In this interesting study, Nikolaus et al. found an association of decreased serum tryptophan in patients with inflammatory bowel disease (IBD), compared with control subjects. The authors also found an inverse correlation of serum tryptophan levels in patients with C-reactive protein in both ulcerative colitis and Crohn's disease and with active disease as defined by clinical disease activity scores in Crohn's disease. A validated food-frequency questionnaire found no difference in tryptophan consumption based on disease activity in a subset of patients, decreasing the likelihood that this association is secondary to altered dietary intake only and may be related to other mechanisms.
Sara Horst, MD, MPH, is an assistant professor, division of gastroenterology, hepatology & nutrition, Inflammatory Bowel Disease Center, Vanderbilt University Medical Center, Nashville, Tenn. She had no relevant conflicts of interest.
In this interesting study, Nikolaus et al. found an association of decreased serum tryptophan in patients with inflammatory bowel disease (IBD), compared with control subjects. The authors also found an inverse correlation of serum tryptophan levels in patients with C-reactive protein in both ulcerative colitis and Crohn's disease and with active disease as defined by clinical disease activity scores in Crohn's disease. A validated food-frequency questionnaire found no difference in tryptophan consumption based on disease activity in a subset of patients, decreasing the likelihood that this association is secondary to altered dietary intake only and may be related to other mechanisms.
Sara Horst, MD, MPH, is an assistant professor, division of gastroenterology, hepatology & nutrition, Inflammatory Bowel Disease Center, Vanderbilt University Medical Center, Nashville, Tenn. She had no relevant conflicts of interest.
In this interesting study, Nikolaus et al. found an association of decreased serum tryptophan in patients with inflammatory bowel disease (IBD), compared with control subjects. The authors also found an inverse correlation of serum tryptophan levels in patients with C-reactive protein in both ulcerative colitis and Crohn's disease and with active disease as defined by clinical disease activity scores in Crohn's disease. A validated food-frequency questionnaire found no difference in tryptophan consumption based on disease activity in a subset of patients, decreasing the likelihood that this association is secondary to altered dietary intake only and may be related to other mechanisms.
Sara Horst, MD, MPH, is an assistant professor, division of gastroenterology, hepatology & nutrition, Inflammatory Bowel Disease Center, Vanderbilt University Medical Center, Nashville, Tenn. She had no relevant conflicts of interest.
Patients with inflammatory bowel disease (IBD) had significantly lower serum levels of the essential amino acid tryptophan than healthy controls in a large study reported in the December issue of Gastroenterology (doi: 10.1053/j.gastro.2017.08.028).
Serum tryptophan levels also correlated inversely with both disease activity and C-reactive protein levels in patients with IBD, reported Susanna Nikolaus, MD, of University Hospital Schleswig-Holstein, Kiel, Germany, with her associates. “Tryptophan deficiency could contribute to development of IBD. Studies are needed to determine whether modification of intestinal tryptophan pathways affects [its] severity,” they wrote.
Several small case series have reported low levels of tryptophan in IBD and other autoimmune disorders, the investigators noted. Removing tryptophan from the diet has been found to increase susceptibility to colitis in mice, and supplementing with tryptophan or some of its metabolites has the opposite effect. For this study, the researchers used high-performance liquid chromatography to quantify tryptophan levels in serum samples from 535 consecutive patients with IBD and 100 matched controls. They used mass spectrometry to measure metabolites of tryptophan, enzyme-linked immunosorbent assay to measure interleukin-22 (IL-22) levels, and 16S rDNA amplicon sequencing to correlate tryptophan levels with fecal microbiota species. Finally, they used real-time polymerase chain reaction to measure levels of mRNA encoding tryptophan metabolites in colonic biopsy specimens.
Serum tryptophan levels were significantly lower in patients with IBD than controls (P = 5.3 x 10–6). The difference was starker in patients with Crohn’s disease (P = 1.1 x 10–10 vs. controls) compared with those with ulcerative colitis (P = 2.8 x 10–3 vs. controls), the investigators noted. Serum tryptophan levels also correlated inversely with disease activity in patients with Crohn’s disease (P = .01), while patients with ulcerative colitis showed a similar but nonsignificant trend (P = .07). Low tryptophan levels were associated with marked, statistically significant increases in C-reactive protein levels in both Crohn’s disease and ulcerative colitis. Tryptophan level also correlated inversely with leukocyte count, although the trend was less pronounced (P = .04).IBD was associated with several aberrations in the tryptophan kynurenine pathway, which is the primary means of catabolizing the amino acid. For example, compared with controls, patients with active IBD had significantly lower levels of mRNA encoding tryptophan 2,3-dioxygenase-2 (TDO2, a key enzyme in the kynurenine pathway) and solute carrier family 6 member 19 (SLC6A19, also called B0AT1, a neutral amino acid transporter). Patients with IBD also had significantly higher levels of indoleamine 2,3-dioxygenase 1 (IDO1), which catalyzes the initial, rate-limiting oxidation of tryptophan to kynurenine. Accordingly, patients with IBD had a significantly higher ratio of kynurenine to tryptophan than did controls, and this abnormality was associated with disease activity, especially in Crohn’s disease (P = .03).
Patients with IBD who had relatively higher tryptophan levels also tended to have more diverse gut microbiota than did patients with lower serum tryptophan levels, although differences among groups were not statistically significant, the investigators said. Serum concentration of IL-22 also correlated with disease activity in patients with IBD, and infliximab responders had a “significant and sustained increase” of tryptophan levels over time, compared with nonresponders.
Potsdam dietary questionnaires found no link between disease activity and dietary consumption of tryptophan, the researchers said. Additionally, they found no links between serum tryptophan levels and age, smoking status, or disease complications, such as fistulae or abscess formation.The investigators acknowledged grant support from the DFG Excellence Cluster “Inflammation at Interfaces” and BMBF e-med SYSINFLAME and H2020 SysCID. One coinvestigator reported employment by CONARIS Research Institute AG, which helps develop drugs with inflammatory indications. The other investigators had no conflicts of interest.
Patients with inflammatory bowel disease (IBD) had significantly lower serum levels of the essential amino acid tryptophan than healthy controls in a large study reported in the December issue of Gastroenterology (doi: 10.1053/j.gastro.2017.08.028).
Serum tryptophan levels also correlated inversely with both disease activity and C-reactive protein levels in patients with IBD, reported Susanna Nikolaus, MD, of University Hospital Schleswig-Holstein, Kiel, Germany, with her associates. “Tryptophan deficiency could contribute to development of IBD. Studies are needed to determine whether modification of intestinal tryptophan pathways affects [its] severity,” they wrote.
Several small case series have reported low levels of tryptophan in IBD and other autoimmune disorders, the investigators noted. Removing tryptophan from the diet has been found to increase susceptibility to colitis in mice, and supplementing with tryptophan or some of its metabolites has the opposite effect. For this study, the researchers used high-performance liquid chromatography to quantify tryptophan levels in serum samples from 535 consecutive patients with IBD and 100 matched controls. They used mass spectrometry to measure metabolites of tryptophan, enzyme-linked immunosorbent assay to measure interleukin-22 (IL-22) levels, and 16S rDNA amplicon sequencing to correlate tryptophan levels with fecal microbiota species. Finally, they used real-time polymerase chain reaction to measure levels of mRNA encoding tryptophan metabolites in colonic biopsy specimens.
Serum tryptophan levels were significantly lower in patients with IBD than controls (P = 5.3 x 10–6). The difference was starker in patients with Crohn’s disease (P = 1.1 x 10–10 vs. controls) compared with those with ulcerative colitis (P = 2.8 x 10–3 vs. controls), the investigators noted. Serum tryptophan levels also correlated inversely with disease activity in patients with Crohn’s disease (P = .01), while patients with ulcerative colitis showed a similar but nonsignificant trend (P = .07). Low tryptophan levels were associated with marked, statistically significant increases in C-reactive protein levels in both Crohn’s disease and ulcerative colitis. Tryptophan level also correlated inversely with leukocyte count, although the trend was less pronounced (P = .04).IBD was associated with several aberrations in the tryptophan kynurenine pathway, which is the primary means of catabolizing the amino acid. For example, compared with controls, patients with active IBD had significantly lower levels of mRNA encoding tryptophan 2,3-dioxygenase-2 (TDO2, a key enzyme in the kynurenine pathway) and solute carrier family 6 member 19 (SLC6A19, also called B0AT1, a neutral amino acid transporter). Patients with IBD also had significantly higher levels of indoleamine 2,3-dioxygenase 1 (IDO1), which catalyzes the initial, rate-limiting oxidation of tryptophan to kynurenine. Accordingly, patients with IBD had a significantly higher ratio of kynurenine to tryptophan than did controls, and this abnormality was associated with disease activity, especially in Crohn’s disease (P = .03).
Patients with IBD who had relatively higher tryptophan levels also tended to have more diverse gut microbiota than did patients with lower serum tryptophan levels, although differences among groups were not statistically significant, the investigators said. Serum concentration of IL-22 also correlated with disease activity in patients with IBD, and infliximab responders had a “significant and sustained increase” of tryptophan levels over time, compared with nonresponders.
Potsdam dietary questionnaires found no link between disease activity and dietary consumption of tryptophan, the researchers said. Additionally, they found no links between serum tryptophan levels and age, smoking status, or disease complications, such as fistulae or abscess formation.The investigators acknowledged grant support from the DFG Excellence Cluster “Inflammation at Interfaces” and BMBF e-med SYSINFLAME and H2020 SysCID. One coinvestigator reported employment by CONARIS Research Institute AG, which helps develop drugs with inflammatory indications. The other investigators had no conflicts of interest.
FROM GASTROENTEROLOGY
Key clinical point: Patients with inflammatory bowel disease had significantly lower serum tryptophan levels than healthy controls.
Major finding: Serum tryptophan levels also correlated inversely with disease activity and C-reactive protein levels in patients with IBD.
Data source: An analysis of serum samples from 535 consecutive patients with IBD and 100 matched controls.
Disclosures: The investigators acknowledged grant support from the DFG Excellence Cluster “Inflammation at Interfaces” and BMBF e-med SYSINFLAME and H2020 SysCID. One coinvestigator reported employment by CONARIS Research Institute AG, which helps develop therapies with inflammatory indications. The other investigators had no conflicts of interest.
Constructing an inflammatory bowel disease patient–centered medical home
Inflammatory bowel diseases (IBDs) including Crohn’s disease and ulcerative colitis are life-long chronic diseases with high morbidity. There has been remarkable progress in the understanding of disease pathophysiology, leading to new medical therapies and surgical approaches for the management of IBD. These trends have resulted in a marked increase in the cost of IBD care, with current estimates ranging from $14 to $31 billion in both direct and indirect costs in the United States.1
IBD patients have unique behavioral, preventive, and therapeutic care requirements.2,3 Because of the complexity of care, there is a large degree of segmentation and fragmentation of IBD management across health care systems and among multiple providers. This siloed approach often falls short of seamless, efficient, high-quality, patient-centered care.
To address the increasing costs and fragmentation of chronic disease management, population-based health care has emerged as a new concept with an emphasis on reward for value, not volume. Two such examples of population-based health care include accountable care organizations and patient-centered medical homes. This concept relies on the development of new payment models and shifts the risk to the providers.4,5 Primary care providers play a central coordinating role in these new models.6,7 However, the role of specialists is less well defined, with limited sharing of risk for the care and costs of populations.
The IBD specialty medical home (SMH) implemented at the University of Pittsburgh Medical Center (UPMC) is an example of a new model of care. The IBD SMH is constructed to align incentives and provide up-front resources to manage a population of patients with IBD optimally – including treatment of their inflammatory disease, coexisting pain, and psychological issues.8-10 In the case of the IBD SMH, the gastroenterologist is the principal provider for a cohort of IBD patients. The gastroenterologist is responsible for the coordination and management of health care of this population and places the IBD patient at the center of the medical universe.
In this article, we draw from our rich partnership between the UPMC Health Plan (HP) and Health System to describe the construction and deployment of the IBD SMH. Although this model is new and we still are learning, we already have seen an improvement in the overall quality of life, decreased utilization, and reduction in total cost of care for this IBD SMH population.
Constructing an IBD medical home: where to begin?
In conjunction with the UPMC HP, we designed and established an IBD patient-centered SMH, designated in July 2015 as UPMC Total Care–Inflammatory Bowel Disease.11 The development of the medical home was facilitated by our unique integrated delivery and finance system. The UPMC HP provided important utilization data on their IBD population, which allowed for focused enrollment of the highest-utilizer patients. In addition, the UPMC HP funded positions that we hired directly as employees of our IBD SMH. These positions included the following: two nurse coordinators, two certified nurse practitioners, a dietitian, a social worker, and a psychiatrist. The UPMC HP also provided their own HP employees to work with our IBD SMH: The rare and chronic disease team included two nurses and a social worker who made house calls for a select group of patients (identified based on the frequency of their health care use). The HP also provided health coaches who worked directly with our patients on lifestyle modifications, such as smoking cessation and exercise programs. Finally, the UPMC HP worked with the IBD SMH to provide support for a variety of operational functions. Examples of these important efforts included data analytics through their department of health economics, regular collaboration to assist the provider team in modifying the program, publicizing the IBD SMH to their members, and facilitating approval of IBD medications through their pharmacy department.
We acknowledge that the development and implementation of an IBD SMH will vary from region to region and depend on the relationship of payers and providers. Thus, the blueprint of our UPMC IBD Medical Home may not be replicated readily in other centers or regions. However, there are several core elements that we believe are necessary in constructing any SMH: 1) a payer willing to partner with the provider, 2) a patient population with specific characteristics, 3) a physician champion, and 4) prespecified goals and measures of success.
Payer or health plan
A SMH is based on the premise that providers and payers working together can achieve more efficient, high-quality care for patients than either party working alone. Payers have essential resources for infrastructure support, preventive services delivery, marketing and engagement expertise, large databases for risk stratification and gap closure, and care management capacity to be a valuable partner. In the short term, philanthropy, grants, and crowd-sourcing options can be used to provide initial support for components of the SMH; however, these rarely are sustainable long-term options. Thus, the most critical collaboration necessary to considering a SMH is between payer(s) (insurance company or health plan) and the specialty provider.
Ideally, the local environment should consist of a single or a few large payers to ease SMH implementation. UPMC is a large integrated delivery (25 hospitals and more than 600 clinics) and financing system (more than 3 million members and is the dominant payer in the region), with a history of leveraging payer–provider partnerships to achieve better patient care, education, and research, and thus served as an ideal collaborator in the design and launch of the IBD SMH. Most physicians in the United States do not work in an integrated payer–provider health delivery system, and partnering with a large regional payer with an interest in specialty population-based chronic care is reasonable for constructing an SMH in your medical neighborhood.
Patient population
In addition to having a collaborative health plan with large population coverage, there must exist a substantial IBD population managed by gastroenterologists. There must be a sufficient number of high-utilizer, high-cost members to justify up-front capital expenditure and return on investment. To determine the feasibility and utility of creating an IBD SMH at UPMC, we collected baseline data on the following: 1) the number of IBD patients within our IBD center and health plan, 2) a hotspotting analysis for our Pennsylvania counties, and 3) health care utilization of the IBD population of interest. At the time of the SMH inception, there were 6,319 Crohn’s disease and ulcerative colitis patients (including all insurance plans) in our center, with more than 3,500 members insured by our HP. There was a 30% increase in new IBD patients to our center in the 3 years before starting the IBD SMH, and the HP had a 27% increase in overall IBD members. Based on a regional hotspotting analysis, $24.3 million of the annual total of $36.9 million was related to hospitalization costs from our IBD patients. The high-utilizer patients accounted for most of the total cost of care for our HP; 16% accounted for 48% of the per-member per-month cost and 29% accounted for 79% of the total annual cost. These baseline data supported justification for an IBD SMH.
Although there is no absolute minimum number of members (patients) required, and the SMH model can be scaled to various IBD populations, we believe that at least 1,000 patients covered by a single insurer must exist. The justification for the 1,000 patients is an estimate of the number of high-utilizer patients who would be required to justify a cost savings, and ultimately a return on investment. We calculated that at least 300 high-utilizer patients would need to be included in our IBD SMH to show a reduction in health care utilization and total cost of care. Therefore, if we assume that approximately 30% of any chronic disease population drives the majority of cost and represents the highest utilizers, we estimated that at least 1,000 patients should be covered by a single insurer.
For development of an SMH, there are two approaches that may be taken: Design the medical home for the entire Health Plan’s population of patients with the disease of interest, or focus only on the high-utilizing, most expensive patients. The latter will include a more complex and challenging cohort of patients, but likely will provide the opportunity to show a reduction in utilization and total cost of care than a broader all-comers population approach.
Physician champions
A successful SMH requires a physician (or health care provider) champion. IBD care within the SMH is unique and distinct from gastroenterologists’ classic training and specialty care. In addition to addressing the biologic disease, the emphasis is on whole-patient care: preventive care, behavioral medicine, socioeconomic considerations of the patient, and provision of care for nongastrointestinal symptoms and diseases. In an SMH, the specialist must be willing to incorporate and address all facets of health care to improve patient outcomes.
Goals and measures of success
To ensure successful deployment of an SMH, it is important to establish shared payer–provider goals and metrics during the construction phase of the medical home. These goals should include an enrollment target number for each year, quality improvement metrics, patient experience outcomes, and metrics for a reduction in health care utilization and total cost of care. Examples of our IBD SMH year 1 and year 2 goals are outlined in Supplementary Table 1 (at http://dx.doi.org/10.1016/j.cgh.2017.05.026). In the first year of our IBD SMH, we were able to achieve our goals, and publication of our results is forthcoming. We have enrolled more than 325 patients, retained 90%, reduced emergency room visits and hospitalizations by 50%, and significantly improved quality of life. Most of our patients have been assigned an HP coach and use the electronic medical portal to communicate with the medical home. Our patient satisfaction for physician communication was 99%.
Key components of the IBD medical home
Based on our experience, we believe the following are key components of a successful IBD SMH: 1) team-based care with physician extenders, nurse coordinators, schedulers, social workers, and dietitians as essential members of the IBD SMH; 2) effective care coordination to reduce barriers to comprehensive biopsychosocial care; 3) tracking of process and outcome metrics of interest; 4) appropriate use of technology to enhance clinical care; and 5) care access (e.g., open-access appointments), after-hours care, and follow-up care after emergency room visits and hospitalizations (Table 1).
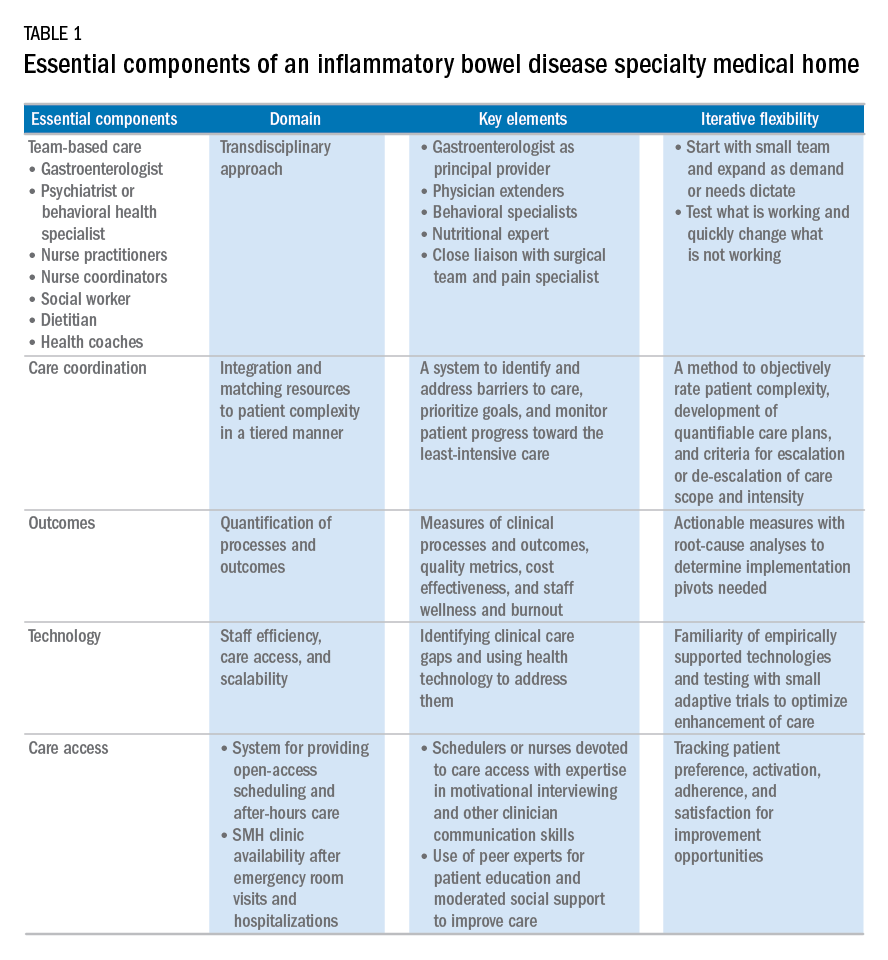
Although the eventual goal of an IBD SMH is to consolidate health care for all IBD patients, the initial launch stages are more likely to succeed if the SMH focuses on the subgroup of IBD patients who use health care excessively, often in an unplanned fashion (e.g., emergency department visits or hospitalizations). In conjunction with a payer, it is easy to identify the most costly IBD patients in a cohort. For example, for initial enrollment, the UPMC IBD SMH selected patients between the ages of 18 and 55 years, with confirmed Crohn’s disease or ulcerative colitis, and evidence that IBD was a primary driver of patients’ health care utilization; the latter was defined if the majority of health care expenditures in the prior year was related to IBD (as judged by International Classification of Diseases, 9th and 10th revisions, primary and secondary diagnoses).
Team-based care
A central component to our IBD SMH was the creation of an integrated team. Supplementary Table 2 (at http://dx.doi.org/10.1016/j.cgh.2017.05.026) describes various positions that are vital for a successful SMH. For a team approach to be most effective, there needs to be clear definitions or roles and role overlaps so that team members can work as a cohesive, organized, and efficient unit. Physician extenders are critical to the model’s success and are trained to make routine IBD care decisions, provide basic primary care, and coordinate care with the gastroenterologist to meet patient needs. The staff-to-patient ratio requirements may vary from region to region and from SMH to SMH. The nurse coordinators and physician extenders assume the burden of day-to-day patient care, and are supervised by the gastroenterologist and psychiatrist. In our UPMC IBD SMH, the ratio of one nurse coordinator and one certified nurse practitioner per 500 patients is sufficient. In addition, one social worker, one dietitian, one scheduler, one gastroenterologist, and one psychiatrist per 1,000 patients is our current model. To date, we have enrolled more than 500 patients, and through funding from our UPMC HP, we have just hired our second nurse coordinator and second nurse practitioner in anticipation of 1,000 patients by year 4.
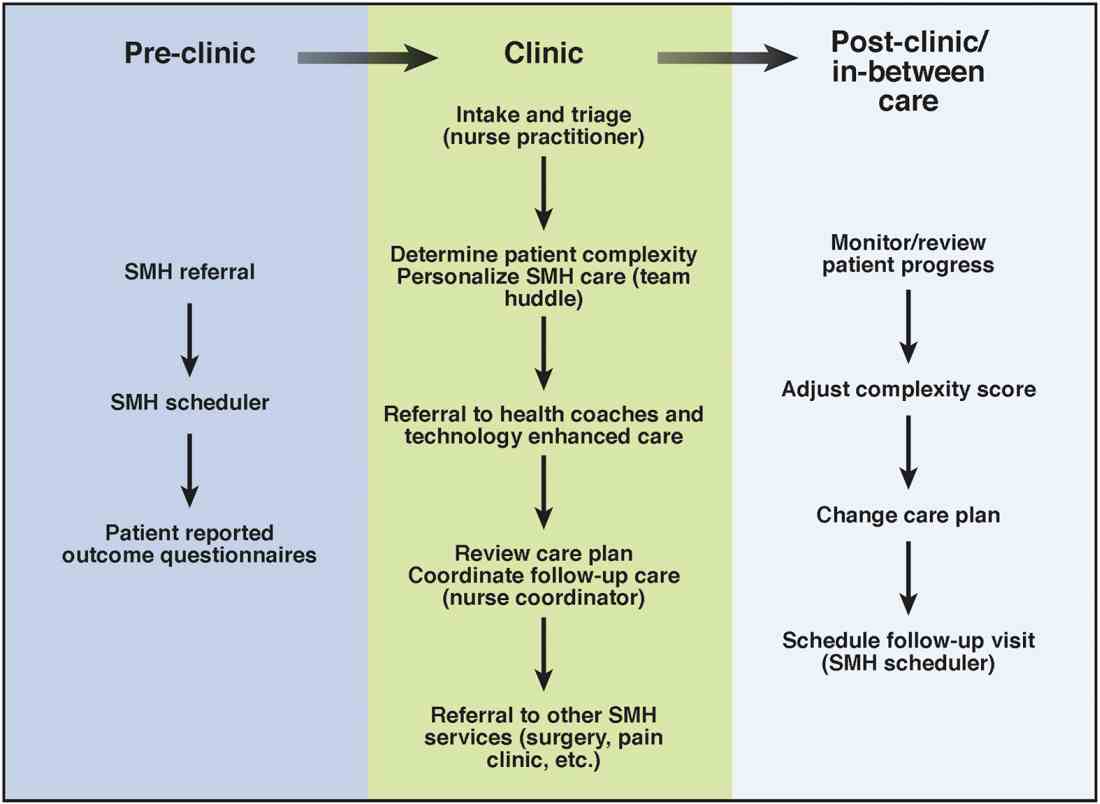
Care coordination and incorporation of technology
The team composition is organized to provide tiered care for optimal efficiency. For such a stepped care model to be effective and scalable, two components are essential. The first component is a care coordination system that allows for the reliable classification of the biological, psychological, social, and health systems barriers faced by patients. To this end, our SMH developed an IBD-specific complexity grid (Supplementary Table 3; at http://dx.doi.org/10.1016/j.cgh.2017.05.026) that was derived from a primary care model.12 The second component is the use of technology-enhanced care to scale delivery of services in a population health model. Examples of technology in our SMH include the use of telemedicine/telepsychiatry by secure video, health coach virtual visits, remote monitoring, and provider-assisted behavioral interventions that patients can access on their smart phones.
New payment models for specialty medical homes
The SMH transitions away from relative value unit–based reimbursement and toward a value-based paradigm. In the SMH, the gastroenterologist serves as the principal medical provider for the IBD patient. Both providers and payers will be able to refer patients to the SMH. Data on quality metrics will be tracked and physician extenders and nurse coordinators will help ensure that goals are met. Quality improvement, preventive medicine, telemedicine, and point-of-contact mental health care will replace the volume-based relative value unit system.
Alternative payment models will be required to support the SMH. Because of the novel nature of the SMH, the optimal payment model has yet to be determined, but probably will include either a shared savings or global cap approach, with an emphasis on the total cost of care reduction. This means that the specialist in the SMH must be aware of all care, and the cost of care, that the patient receives. Biologics and other IBD therapy costs are high and will continue to increase. The sustainable model must be sufficiently supple to not disincentivize the provider to use proven and effective, albeit expensive, therapy for patients who need it most. A close working relationship between the SMH providers and the health plan chief pharmacy officer will be essential. We expect that appropriate use of medications will lead to a medical cost offset with improved IBD outcomes, a reduction in health care utilization, and optimized work and life productivity.
Conclusions
In new models of care, specialty providers partner with payers in a patient-centered system to provide principal care for patients with chronic diseases, including IBD, in an effort to reduce costs and provide efficient, high-quality care. These models will require close collaborations with payers, a sufficiently large patient population, a physician champion, and a multidisciplinary staff targeting various aspects of health care. Successful implementation of such models will help reduce costs of care while improving the patient-centered experience and outcomes.
Supplementary material
To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://dx.doi.org/10.1016/j.cgh.2017.05.026.
References
1. Mehta F. Report: economic implications of inflammatory bowel disease and its management. Am J Manag Care. 2016;22(Suppl):s51-60.
2. Mikocka-Walus A., Knowles S.R., Keefer L., et al. Controversies revisited: a systematic review of the comorbidity of depression and anxiety with inflammatory bowel diseases. Inflamm Bowel Dis. 2016;22:752-62.
3. Regueiro M., Greer J.B., Szigethy E. Etiology and treatment of pain and psychosocial issues in patients with inflammatory bowel diseases. Gastroenterology. 2017;152:430-9.e4.
4. Silow-Carroll S., Edwards J.N., Rodin D. How Colorado, Minnesota, and Vermont are reforming care delivery and payment to improve health and lower costs. Issue Brief. (Commonw Fund) 2013;10:1-9.
5. Fogelman C., Gates T. A primary care perspective on U.S. health care: part 2: thinking globally, acting locally. J Lancaster Gen Hospital. 2013;8:101-5.
6. Rosenthal M.B., Sinaiko A.D., Eastman D., et al. Impact of the Rochester medical home initiative on primary care practices, quality, utilization, and costs. Med Care. 2015;53:967-73.
7. Friedberg M.W., Rosenthal M.B., Werner R.M., et al. Effects of a medical home and shared savings intervention on quality and utilization of care. JAMA Intern Med. 2015;175:1362-8.
8. Fernandes S.M., Sanders L.M. Patient-centered medical home for patients with complex congenital heart disease. Curr Opin Pediatr. 2015;27:581-6.
9. Mikocka-Walus A.A., Andrews J.M., Bernstein C.N., et al. Integrated models of care in managing inflammatory bowel disease: a discussion. Inflamm Bowel Dis. 2012;18:1582-7.
10. Kessler R., Miller B.F., Kelly M., et al. Mental health, substance abuse, and health behavior services in patient-centered medical homes. J Am Board Fam Med. 2014;27:637-44.
11. Regueiro M.D., McAnallen S.E., Greer J.B., et al. The inflammatory bowel disease specialty medical home: a new model of patient centered care. Inflamm Bowel Dis. 2016;22:1971-80.
12. Lobo E., Ventura T., Navio M., et al. Identification of components of health complexity on internal medicine units by means of the INTERMED method. Int J Clin Pract. 2015;69:1377-86.
Dr. Regueiro and Dr. Click are in the division of gastroenterology, hepatology and nutrition, University of Pittsburgh Medical Center; Ms. Holder, Dr. Shrank, and Ms. McAnAllen are in the Insurance Services Division, University of Pittsburgh Medical Center, and Dr. Szigethy is in the Department of Psychiatry, University of Pittsburgh School of Medicine. Dr. Regueiro serves as a consultant for, on advisory boards for, and receives research support from Abbvie, Janssen, and Takeda; and Dr. Szigethy serves as a consultant for Abbvie. The remaining authors disclose no conflicts.
Inflammatory bowel diseases (IBDs) including Crohn’s disease and ulcerative colitis are life-long chronic diseases with high morbidity. There has been remarkable progress in the understanding of disease pathophysiology, leading to new medical therapies and surgical approaches for the management of IBD. These trends have resulted in a marked increase in the cost of IBD care, with current estimates ranging from $14 to $31 billion in both direct and indirect costs in the United States.1
IBD patients have unique behavioral, preventive, and therapeutic care requirements.2,3 Because of the complexity of care, there is a large degree of segmentation and fragmentation of IBD management across health care systems and among multiple providers. This siloed approach often falls short of seamless, efficient, high-quality, patient-centered care.
To address the increasing costs and fragmentation of chronic disease management, population-based health care has emerged as a new concept with an emphasis on reward for value, not volume. Two such examples of population-based health care include accountable care organizations and patient-centered medical homes. This concept relies on the development of new payment models and shifts the risk to the providers.4,5 Primary care providers play a central coordinating role in these new models.6,7 However, the role of specialists is less well defined, with limited sharing of risk for the care and costs of populations.
The IBD specialty medical home (SMH) implemented at the University of Pittsburgh Medical Center (UPMC) is an example of a new model of care. The IBD SMH is constructed to align incentives and provide up-front resources to manage a population of patients with IBD optimally – including treatment of their inflammatory disease, coexisting pain, and psychological issues.8-10 In the case of the IBD SMH, the gastroenterologist is the principal provider for a cohort of IBD patients. The gastroenterologist is responsible for the coordination and management of health care of this population and places the IBD patient at the center of the medical universe.
In this article, we draw from our rich partnership between the UPMC Health Plan (HP) and Health System to describe the construction and deployment of the IBD SMH. Although this model is new and we still are learning, we already have seen an improvement in the overall quality of life, decreased utilization, and reduction in total cost of care for this IBD SMH population.
Constructing an IBD medical home: where to begin?
In conjunction with the UPMC HP, we designed and established an IBD patient-centered SMH, designated in July 2015 as UPMC Total Care–Inflammatory Bowel Disease.11 The development of the medical home was facilitated by our unique integrated delivery and finance system. The UPMC HP provided important utilization data on their IBD population, which allowed for focused enrollment of the highest-utilizer patients. In addition, the UPMC HP funded positions that we hired directly as employees of our IBD SMH. These positions included the following: two nurse coordinators, two certified nurse practitioners, a dietitian, a social worker, and a psychiatrist. The UPMC HP also provided their own HP employees to work with our IBD SMH: The rare and chronic disease team included two nurses and a social worker who made house calls for a select group of patients (identified based on the frequency of their health care use). The HP also provided health coaches who worked directly with our patients on lifestyle modifications, such as smoking cessation and exercise programs. Finally, the UPMC HP worked with the IBD SMH to provide support for a variety of operational functions. Examples of these important efforts included data analytics through their department of health economics, regular collaboration to assist the provider team in modifying the program, publicizing the IBD SMH to their members, and facilitating approval of IBD medications through their pharmacy department.
We acknowledge that the development and implementation of an IBD SMH will vary from region to region and depend on the relationship of payers and providers. Thus, the blueprint of our UPMC IBD Medical Home may not be replicated readily in other centers or regions. However, there are several core elements that we believe are necessary in constructing any SMH: 1) a payer willing to partner with the provider, 2) a patient population with specific characteristics, 3) a physician champion, and 4) prespecified goals and measures of success.
Payer or health plan
A SMH is based on the premise that providers and payers working together can achieve more efficient, high-quality care for patients than either party working alone. Payers have essential resources for infrastructure support, preventive services delivery, marketing and engagement expertise, large databases for risk stratification and gap closure, and care management capacity to be a valuable partner. In the short term, philanthropy, grants, and crowd-sourcing options can be used to provide initial support for components of the SMH; however, these rarely are sustainable long-term options. Thus, the most critical collaboration necessary to considering a SMH is between payer(s) (insurance company or health plan) and the specialty provider.
Ideally, the local environment should consist of a single or a few large payers to ease SMH implementation. UPMC is a large integrated delivery (25 hospitals and more than 600 clinics) and financing system (more than 3 million members and is the dominant payer in the region), with a history of leveraging payer–provider partnerships to achieve better patient care, education, and research, and thus served as an ideal collaborator in the design and launch of the IBD SMH. Most physicians in the United States do not work in an integrated payer–provider health delivery system, and partnering with a large regional payer with an interest in specialty population-based chronic care is reasonable for constructing an SMH in your medical neighborhood.
Patient population
In addition to having a collaborative health plan with large population coverage, there must exist a substantial IBD population managed by gastroenterologists. There must be a sufficient number of high-utilizer, high-cost members to justify up-front capital expenditure and return on investment. To determine the feasibility and utility of creating an IBD SMH at UPMC, we collected baseline data on the following: 1) the number of IBD patients within our IBD center and health plan, 2) a hotspotting analysis for our Pennsylvania counties, and 3) health care utilization of the IBD population of interest. At the time of the SMH inception, there were 6,319 Crohn’s disease and ulcerative colitis patients (including all insurance plans) in our center, with more than 3,500 members insured by our HP. There was a 30% increase in new IBD patients to our center in the 3 years before starting the IBD SMH, and the HP had a 27% increase in overall IBD members. Based on a regional hotspotting analysis, $24.3 million of the annual total of $36.9 million was related to hospitalization costs from our IBD patients. The high-utilizer patients accounted for most of the total cost of care for our HP; 16% accounted for 48% of the per-member per-month cost and 29% accounted for 79% of the total annual cost. These baseline data supported justification for an IBD SMH.
Although there is no absolute minimum number of members (patients) required, and the SMH model can be scaled to various IBD populations, we believe that at least 1,000 patients covered by a single insurer must exist. The justification for the 1,000 patients is an estimate of the number of high-utilizer patients who would be required to justify a cost savings, and ultimately a return on investment. We calculated that at least 300 high-utilizer patients would need to be included in our IBD SMH to show a reduction in health care utilization and total cost of care. Therefore, if we assume that approximately 30% of any chronic disease population drives the majority of cost and represents the highest utilizers, we estimated that at least 1,000 patients should be covered by a single insurer.
For development of an SMH, there are two approaches that may be taken: Design the medical home for the entire Health Plan’s population of patients with the disease of interest, or focus only on the high-utilizing, most expensive patients. The latter will include a more complex and challenging cohort of patients, but likely will provide the opportunity to show a reduction in utilization and total cost of care than a broader all-comers population approach.
Physician champions
A successful SMH requires a physician (or health care provider) champion. IBD care within the SMH is unique and distinct from gastroenterologists’ classic training and specialty care. In addition to addressing the biologic disease, the emphasis is on whole-patient care: preventive care, behavioral medicine, socioeconomic considerations of the patient, and provision of care for nongastrointestinal symptoms and diseases. In an SMH, the specialist must be willing to incorporate and address all facets of health care to improve patient outcomes.
Goals and measures of success
To ensure successful deployment of an SMH, it is important to establish shared payer–provider goals and metrics during the construction phase of the medical home. These goals should include an enrollment target number for each year, quality improvement metrics, patient experience outcomes, and metrics for a reduction in health care utilization and total cost of care. Examples of our IBD SMH year 1 and year 2 goals are outlined in Supplementary Table 1 (at http://dx.doi.org/10.1016/j.cgh.2017.05.026). In the first year of our IBD SMH, we were able to achieve our goals, and publication of our results is forthcoming. We have enrolled more than 325 patients, retained 90%, reduced emergency room visits and hospitalizations by 50%, and significantly improved quality of life. Most of our patients have been assigned an HP coach and use the electronic medical portal to communicate with the medical home. Our patient satisfaction for physician communication was 99%.
Key components of the IBD medical home
Based on our experience, we believe the following are key components of a successful IBD SMH: 1) team-based care with physician extenders, nurse coordinators, schedulers, social workers, and dietitians as essential members of the IBD SMH; 2) effective care coordination to reduce barriers to comprehensive biopsychosocial care; 3) tracking of process and outcome metrics of interest; 4) appropriate use of technology to enhance clinical care; and 5) care access (e.g., open-access appointments), after-hours care, and follow-up care after emergency room visits and hospitalizations (Table 1).

Although the eventual goal of an IBD SMH is to consolidate health care for all IBD patients, the initial launch stages are more likely to succeed if the SMH focuses on the subgroup of IBD patients who use health care excessively, often in an unplanned fashion (e.g., emergency department visits or hospitalizations). In conjunction with a payer, it is easy to identify the most costly IBD patients in a cohort. For example, for initial enrollment, the UPMC IBD SMH selected patients between the ages of 18 and 55 years, with confirmed Crohn’s disease or ulcerative colitis, and evidence that IBD was a primary driver of patients’ health care utilization; the latter was defined if the majority of health care expenditures in the prior year was related to IBD (as judged by International Classification of Diseases, 9th and 10th revisions, primary and secondary diagnoses).
Team-based care
A central component to our IBD SMH was the creation of an integrated team. Supplementary Table 2 (at http://dx.doi.org/10.1016/j.cgh.2017.05.026) describes various positions that are vital for a successful SMH. For a team approach to be most effective, there needs to be clear definitions or roles and role overlaps so that team members can work as a cohesive, organized, and efficient unit. Physician extenders are critical to the model’s success and are trained to make routine IBD care decisions, provide basic primary care, and coordinate care with the gastroenterologist to meet patient needs. The staff-to-patient ratio requirements may vary from region to region and from SMH to SMH. The nurse coordinators and physician extenders assume the burden of day-to-day patient care, and are supervised by the gastroenterologist and psychiatrist. In our UPMC IBD SMH, the ratio of one nurse coordinator and one certified nurse practitioner per 500 patients is sufficient. In addition, one social worker, one dietitian, one scheduler, one gastroenterologist, and one psychiatrist per 1,000 patients is our current model. To date, we have enrolled more than 500 patients, and through funding from our UPMC HP, we have just hired our second nurse coordinator and second nurse practitioner in anticipation of 1,000 patients by year 4.

Care coordination and incorporation of technology
The team composition is organized to provide tiered care for optimal efficiency. For such a stepped care model to be effective and scalable, two components are essential. The first component is a care coordination system that allows for the reliable classification of the biological, psychological, social, and health systems barriers faced by patients. To this end, our SMH developed an IBD-specific complexity grid (Supplementary Table 3; at http://dx.doi.org/10.1016/j.cgh.2017.05.026) that was derived from a primary care model.12 The second component is the use of technology-enhanced care to scale delivery of services in a population health model. Examples of technology in our SMH include the use of telemedicine/telepsychiatry by secure video, health coach virtual visits, remote monitoring, and provider-assisted behavioral interventions that patients can access on their smart phones.
New payment models for specialty medical homes
The SMH transitions away from relative value unit–based reimbursement and toward a value-based paradigm. In the SMH, the gastroenterologist serves as the principal medical provider for the IBD patient. Both providers and payers will be able to refer patients to the SMH. Data on quality metrics will be tracked and physician extenders and nurse coordinators will help ensure that goals are met. Quality improvement, preventive medicine, telemedicine, and point-of-contact mental health care will replace the volume-based relative value unit system.
Alternative payment models will be required to support the SMH. Because of the novel nature of the SMH, the optimal payment model has yet to be determined, but probably will include either a shared savings or global cap approach, with an emphasis on the total cost of care reduction. This means that the specialist in the SMH must be aware of all care, and the cost of care, that the patient receives. Biologics and other IBD therapy costs are high and will continue to increase. The sustainable model must be sufficiently supple to not disincentivize the provider to use proven and effective, albeit expensive, therapy for patients who need it most. A close working relationship between the SMH providers and the health plan chief pharmacy officer will be essential. We expect that appropriate use of medications will lead to a medical cost offset with improved IBD outcomes, a reduction in health care utilization, and optimized work and life productivity.
Conclusions
In new models of care, specialty providers partner with payers in a patient-centered system to provide principal care for patients with chronic diseases, including IBD, in an effort to reduce costs and provide efficient, high-quality care. These models will require close collaborations with payers, a sufficiently large patient population, a physician champion, and a multidisciplinary staff targeting various aspects of health care. Successful implementation of such models will help reduce costs of care while improving the patient-centered experience and outcomes.
Supplementary material
To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://dx.doi.org/10.1016/j.cgh.2017.05.026.
References
1. Mehta F. Report: economic implications of inflammatory bowel disease and its management. Am J Manag Care. 2016;22(Suppl):s51-60.
2. Mikocka-Walus A., Knowles S.R., Keefer L., et al. Controversies revisited: a systematic review of the comorbidity of depression and anxiety with inflammatory bowel diseases. Inflamm Bowel Dis. 2016;22:752-62.
3. Regueiro M., Greer J.B., Szigethy E. Etiology and treatment of pain and psychosocial issues in patients with inflammatory bowel diseases. Gastroenterology. 2017;152:430-9.e4.
4. Silow-Carroll S., Edwards J.N., Rodin D. How Colorado, Minnesota, and Vermont are reforming care delivery and payment to improve health and lower costs. Issue Brief. (Commonw Fund) 2013;10:1-9.
5. Fogelman C., Gates T. A primary care perspective on U.S. health care: part 2: thinking globally, acting locally. J Lancaster Gen Hospital. 2013;8:101-5.
6. Rosenthal M.B., Sinaiko A.D., Eastman D., et al. Impact of the Rochester medical home initiative on primary care practices, quality, utilization, and costs. Med Care. 2015;53:967-73.
7. Friedberg M.W., Rosenthal M.B., Werner R.M., et al. Effects of a medical home and shared savings intervention on quality and utilization of care. JAMA Intern Med. 2015;175:1362-8.
8. Fernandes S.M., Sanders L.M. Patient-centered medical home for patients with complex congenital heart disease. Curr Opin Pediatr. 2015;27:581-6.
9. Mikocka-Walus A.A., Andrews J.M., Bernstein C.N., et al. Integrated models of care in managing inflammatory bowel disease: a discussion. Inflamm Bowel Dis. 2012;18:1582-7.
10. Kessler R., Miller B.F., Kelly M., et al. Mental health, substance abuse, and health behavior services in patient-centered medical homes. J Am Board Fam Med. 2014;27:637-44.
11. Regueiro M.D., McAnallen S.E., Greer J.B., et al. The inflammatory bowel disease specialty medical home: a new model of patient centered care. Inflamm Bowel Dis. 2016;22:1971-80.
12. Lobo E., Ventura T., Navio M., et al. Identification of components of health complexity on internal medicine units by means of the INTERMED method. Int J Clin Pract. 2015;69:1377-86.
Dr. Regueiro and Dr. Click are in the division of gastroenterology, hepatology and nutrition, University of Pittsburgh Medical Center; Ms. Holder, Dr. Shrank, and Ms. McAnAllen are in the Insurance Services Division, University of Pittsburgh Medical Center, and Dr. Szigethy is in the Department of Psychiatry, University of Pittsburgh School of Medicine. Dr. Regueiro serves as a consultant for, on advisory boards for, and receives research support from Abbvie, Janssen, and Takeda; and Dr. Szigethy serves as a consultant for Abbvie. The remaining authors disclose no conflicts.
Inflammatory bowel diseases (IBDs) including Crohn’s disease and ulcerative colitis are life-long chronic diseases with high morbidity. There has been remarkable progress in the understanding of disease pathophysiology, leading to new medical therapies and surgical approaches for the management of IBD. These trends have resulted in a marked increase in the cost of IBD care, with current estimates ranging from $14 to $31 billion in both direct and indirect costs in the United States.1
IBD patients have unique behavioral, preventive, and therapeutic care requirements.2,3 Because of the complexity of care, there is a large degree of segmentation and fragmentation of IBD management across health care systems and among multiple providers. This siloed approach often falls short of seamless, efficient, high-quality, patient-centered care.
To address the increasing costs and fragmentation of chronic disease management, population-based health care has emerged as a new concept with an emphasis on reward for value, not volume. Two such examples of population-based health care include accountable care organizations and patient-centered medical homes. This concept relies on the development of new payment models and shifts the risk to the providers.4,5 Primary care providers play a central coordinating role in these new models.6,7 However, the role of specialists is less well defined, with limited sharing of risk for the care and costs of populations.
The IBD specialty medical home (SMH) implemented at the University of Pittsburgh Medical Center (UPMC) is an example of a new model of care. The IBD SMH is constructed to align incentives and provide up-front resources to manage a population of patients with IBD optimally – including treatment of their inflammatory disease, coexisting pain, and psychological issues.8-10 In the case of the IBD SMH, the gastroenterologist is the principal provider for a cohort of IBD patients. The gastroenterologist is responsible for the coordination and management of health care of this population and places the IBD patient at the center of the medical universe.
In this article, we draw from our rich partnership between the UPMC Health Plan (HP) and Health System to describe the construction and deployment of the IBD SMH. Although this model is new and we still are learning, we already have seen an improvement in the overall quality of life, decreased utilization, and reduction in total cost of care for this IBD SMH population.
Constructing an IBD medical home: where to begin?
In conjunction with the UPMC HP, we designed and established an IBD patient-centered SMH, designated in July 2015 as UPMC Total Care–Inflammatory Bowel Disease.11 The development of the medical home was facilitated by our unique integrated delivery and finance system. The UPMC HP provided important utilization data on their IBD population, which allowed for focused enrollment of the highest-utilizer patients. In addition, the UPMC HP funded positions that we hired directly as employees of our IBD SMH. These positions included the following: two nurse coordinators, two certified nurse practitioners, a dietitian, a social worker, and a psychiatrist. The UPMC HP also provided their own HP employees to work with our IBD SMH: The rare and chronic disease team included two nurses and a social worker who made house calls for a select group of patients (identified based on the frequency of their health care use). The HP also provided health coaches who worked directly with our patients on lifestyle modifications, such as smoking cessation and exercise programs. Finally, the UPMC HP worked with the IBD SMH to provide support for a variety of operational functions. Examples of these important efforts included data analytics through their department of health economics, regular collaboration to assist the provider team in modifying the program, publicizing the IBD SMH to their members, and facilitating approval of IBD medications through their pharmacy department.
We acknowledge that the development and implementation of an IBD SMH will vary from region to region and depend on the relationship of payers and providers. Thus, the blueprint of our UPMC IBD Medical Home may not be replicated readily in other centers or regions. However, there are several core elements that we believe are necessary in constructing any SMH: 1) a payer willing to partner with the provider, 2) a patient population with specific characteristics, 3) a physician champion, and 4) prespecified goals and measures of success.
Payer or health plan
A SMH is based on the premise that providers and payers working together can achieve more efficient, high-quality care for patients than either party working alone. Payers have essential resources for infrastructure support, preventive services delivery, marketing and engagement expertise, large databases for risk stratification and gap closure, and care management capacity to be a valuable partner. In the short term, philanthropy, grants, and crowd-sourcing options can be used to provide initial support for components of the SMH; however, these rarely are sustainable long-term options. Thus, the most critical collaboration necessary to considering a SMH is between payer(s) (insurance company or health plan) and the specialty provider.
Ideally, the local environment should consist of a single or a few large payers to ease SMH implementation. UPMC is a large integrated delivery (25 hospitals and more than 600 clinics) and financing system (more than 3 million members and is the dominant payer in the region), with a history of leveraging payer–provider partnerships to achieve better patient care, education, and research, and thus served as an ideal collaborator in the design and launch of the IBD SMH. Most physicians in the United States do not work in an integrated payer–provider health delivery system, and partnering with a large regional payer with an interest in specialty population-based chronic care is reasonable for constructing an SMH in your medical neighborhood.
Patient population
In addition to having a collaborative health plan with large population coverage, there must exist a substantial IBD population managed by gastroenterologists. There must be a sufficient number of high-utilizer, high-cost members to justify up-front capital expenditure and return on investment. To determine the feasibility and utility of creating an IBD SMH at UPMC, we collected baseline data on the following: 1) the number of IBD patients within our IBD center and health plan, 2) a hotspotting analysis for our Pennsylvania counties, and 3) health care utilization of the IBD population of interest. At the time of the SMH inception, there were 6,319 Crohn’s disease and ulcerative colitis patients (including all insurance plans) in our center, with more than 3,500 members insured by our HP. There was a 30% increase in new IBD patients to our center in the 3 years before starting the IBD SMH, and the HP had a 27% increase in overall IBD members. Based on a regional hotspotting analysis, $24.3 million of the annual total of $36.9 million was related to hospitalization costs from our IBD patients. The high-utilizer patients accounted for most of the total cost of care for our HP; 16% accounted for 48% of the per-member per-month cost and 29% accounted for 79% of the total annual cost. These baseline data supported justification for an IBD SMH.
Although there is no absolute minimum number of members (patients) required, and the SMH model can be scaled to various IBD populations, we believe that at least 1,000 patients covered by a single insurer must exist. The justification for the 1,000 patients is an estimate of the number of high-utilizer patients who would be required to justify a cost savings, and ultimately a return on investment. We calculated that at least 300 high-utilizer patients would need to be included in our IBD SMH to show a reduction in health care utilization and total cost of care. Therefore, if we assume that approximately 30% of any chronic disease population drives the majority of cost and represents the highest utilizers, we estimated that at least 1,000 patients should be covered by a single insurer.
For development of an SMH, there are two approaches that may be taken: Design the medical home for the entire Health Plan’s population of patients with the disease of interest, or focus only on the high-utilizing, most expensive patients. The latter will include a more complex and challenging cohort of patients, but likely will provide the opportunity to show a reduction in utilization and total cost of care than a broader all-comers population approach.
Physician champions
A successful SMH requires a physician (or health care provider) champion. IBD care within the SMH is unique and distinct from gastroenterologists’ classic training and specialty care. In addition to addressing the biologic disease, the emphasis is on whole-patient care: preventive care, behavioral medicine, socioeconomic considerations of the patient, and provision of care for nongastrointestinal symptoms and diseases. In an SMH, the specialist must be willing to incorporate and address all facets of health care to improve patient outcomes.
Goals and measures of success
To ensure successful deployment of an SMH, it is important to establish shared payer–provider goals and metrics during the construction phase of the medical home. These goals should include an enrollment target number for each year, quality improvement metrics, patient experience outcomes, and metrics for a reduction in health care utilization and total cost of care. Examples of our IBD SMH year 1 and year 2 goals are outlined in Supplementary Table 1 (at http://dx.doi.org/10.1016/j.cgh.2017.05.026). In the first year of our IBD SMH, we were able to achieve our goals, and publication of our results is forthcoming. We have enrolled more than 325 patients, retained 90%, reduced emergency room visits and hospitalizations by 50%, and significantly improved quality of life. Most of our patients have been assigned an HP coach and use the electronic medical portal to communicate with the medical home. Our patient satisfaction for physician communication was 99%.
Key components of the IBD medical home
Based on our experience, we believe the following are key components of a successful IBD SMH: 1) team-based care with physician extenders, nurse coordinators, schedulers, social workers, and dietitians as essential members of the IBD SMH; 2) effective care coordination to reduce barriers to comprehensive biopsychosocial care; 3) tracking of process and outcome metrics of interest; 4) appropriate use of technology to enhance clinical care; and 5) care access (e.g., open-access appointments), after-hours care, and follow-up care after emergency room visits and hospitalizations (Table 1).

Although the eventual goal of an IBD SMH is to consolidate health care for all IBD patients, the initial launch stages are more likely to succeed if the SMH focuses on the subgroup of IBD patients who use health care excessively, often in an unplanned fashion (e.g., emergency department visits or hospitalizations). In conjunction with a payer, it is easy to identify the most costly IBD patients in a cohort. For example, for initial enrollment, the UPMC IBD SMH selected patients between the ages of 18 and 55 years, with confirmed Crohn’s disease or ulcerative colitis, and evidence that IBD was a primary driver of patients’ health care utilization; the latter was defined if the majority of health care expenditures in the prior year was related to IBD (as judged by International Classification of Diseases, 9th and 10th revisions, primary and secondary diagnoses).
Team-based care
A central component to our IBD SMH was the creation of an integrated team. Supplementary Table 2 (at http://dx.doi.org/10.1016/j.cgh.2017.05.026) describes various positions that are vital for a successful SMH. For a team approach to be most effective, there needs to be clear definitions or roles and role overlaps so that team members can work as a cohesive, organized, and efficient unit. Physician extenders are critical to the model’s success and are trained to make routine IBD care decisions, provide basic primary care, and coordinate care with the gastroenterologist to meet patient needs. The staff-to-patient ratio requirements may vary from region to region and from SMH to SMH. The nurse coordinators and physician extenders assume the burden of day-to-day patient care, and are supervised by the gastroenterologist and psychiatrist. In our UPMC IBD SMH, the ratio of one nurse coordinator and one certified nurse practitioner per 500 patients is sufficient. In addition, one social worker, one dietitian, one scheduler, one gastroenterologist, and one psychiatrist per 1,000 patients is our current model. To date, we have enrolled more than 500 patients, and through funding from our UPMC HP, we have just hired our second nurse coordinator and second nurse practitioner in anticipation of 1,000 patients by year 4.

Care coordination and incorporation of technology
The team composition is organized to provide tiered care for optimal efficiency. For such a stepped care model to be effective and scalable, two components are essential. The first component is a care coordination system that allows for the reliable classification of the biological, psychological, social, and health systems barriers faced by patients. To this end, our SMH developed an IBD-specific complexity grid (Supplementary Table 3; at http://dx.doi.org/10.1016/j.cgh.2017.05.026) that was derived from a primary care model.12 The second component is the use of technology-enhanced care to scale delivery of services in a population health model. Examples of technology in our SMH include the use of telemedicine/telepsychiatry by secure video, health coach virtual visits, remote monitoring, and provider-assisted behavioral interventions that patients can access on their smart phones.
New payment models for specialty medical homes
The SMH transitions away from relative value unit–based reimbursement and toward a value-based paradigm. In the SMH, the gastroenterologist serves as the principal medical provider for the IBD patient. Both providers and payers will be able to refer patients to the SMH. Data on quality metrics will be tracked and physician extenders and nurse coordinators will help ensure that goals are met. Quality improvement, preventive medicine, telemedicine, and point-of-contact mental health care will replace the volume-based relative value unit system.
Alternative payment models will be required to support the SMH. Because of the novel nature of the SMH, the optimal payment model has yet to be determined, but probably will include either a shared savings or global cap approach, with an emphasis on the total cost of care reduction. This means that the specialist in the SMH must be aware of all care, and the cost of care, that the patient receives. Biologics and other IBD therapy costs are high and will continue to increase. The sustainable model must be sufficiently supple to not disincentivize the provider to use proven and effective, albeit expensive, therapy for patients who need it most. A close working relationship between the SMH providers and the health plan chief pharmacy officer will be essential. We expect that appropriate use of medications will lead to a medical cost offset with improved IBD outcomes, a reduction in health care utilization, and optimized work and life productivity.
Conclusions
In new models of care, specialty providers partner with payers in a patient-centered system to provide principal care for patients with chronic diseases, including IBD, in an effort to reduce costs and provide efficient, high-quality care. These models will require close collaborations with payers, a sufficiently large patient population, a physician champion, and a multidisciplinary staff targeting various aspects of health care. Successful implementation of such models will help reduce costs of care while improving the patient-centered experience and outcomes.
Supplementary material
To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://dx.doi.org/10.1016/j.cgh.2017.05.026.
References
1. Mehta F. Report: economic implications of inflammatory bowel disease and its management. Am J Manag Care. 2016;22(Suppl):s51-60.
2. Mikocka-Walus A., Knowles S.R., Keefer L., et al. Controversies revisited: a systematic review of the comorbidity of depression and anxiety with inflammatory bowel diseases. Inflamm Bowel Dis. 2016;22:752-62.
3. Regueiro M., Greer J.B., Szigethy E. Etiology and treatment of pain and psychosocial issues in patients with inflammatory bowel diseases. Gastroenterology. 2017;152:430-9.e4.
4. Silow-Carroll S., Edwards J.N., Rodin D. How Colorado, Minnesota, and Vermont are reforming care delivery and payment to improve health and lower costs. Issue Brief. (Commonw Fund) 2013;10:1-9.
5. Fogelman C., Gates T. A primary care perspective on U.S. health care: part 2: thinking globally, acting locally. J Lancaster Gen Hospital. 2013;8:101-5.
6. Rosenthal M.B., Sinaiko A.D., Eastman D., et al. Impact of the Rochester medical home initiative on primary care practices, quality, utilization, and costs. Med Care. 2015;53:967-73.
7. Friedberg M.W., Rosenthal M.B., Werner R.M., et al. Effects of a medical home and shared savings intervention on quality and utilization of care. JAMA Intern Med. 2015;175:1362-8.
8. Fernandes S.M., Sanders L.M. Patient-centered medical home for patients with complex congenital heart disease. Curr Opin Pediatr. 2015;27:581-6.
9. Mikocka-Walus A.A., Andrews J.M., Bernstein C.N., et al. Integrated models of care in managing inflammatory bowel disease: a discussion. Inflamm Bowel Dis. 2012;18:1582-7.
10. Kessler R., Miller B.F., Kelly M., et al. Mental health, substance abuse, and health behavior services in patient-centered medical homes. J Am Board Fam Med. 2014;27:637-44.
11. Regueiro M.D., McAnallen S.E., Greer J.B., et al. The inflammatory bowel disease specialty medical home: a new model of patient centered care. Inflamm Bowel Dis. 2016;22:1971-80.
12. Lobo E., Ventura T., Navio M., et al. Identification of components of health complexity on internal medicine units by means of the INTERMED method. Int J Clin Pract. 2015;69:1377-86.
Dr. Regueiro and Dr. Click are in the division of gastroenterology, hepatology and nutrition, University of Pittsburgh Medical Center; Ms. Holder, Dr. Shrank, and Ms. McAnAllen are in the Insurance Services Division, University of Pittsburgh Medical Center, and Dr. Szigethy is in the Department of Psychiatry, University of Pittsburgh School of Medicine. Dr. Regueiro serves as a consultant for, on advisory boards for, and receives research support from Abbvie, Janssen, and Takeda; and Dr. Szigethy serves as a consultant for Abbvie. The remaining authors disclose no conflicts.