User login
‘Self-anesthetizing’ to cope with grief
CASE Grieving, delusional
Mr. M, age 51, is brought to the emergency department (ED) because of new-onset delusions and decreased self-care over the last 2 weeks following the sudden death of his wife. He has become expansive and grandiose, with pressured speech, increased energy, and markedly reduced sleep. Mr. M is preoccupied with the idea that he is “the first to survive a human reboot process” and says that his and his wife’s bodies and brains had been “split apart.” Mr. M has limited his food and fluid intake and lost 15 lb within the past 2 to 3 weeks.
Mr. M has no history of any affective, psychotic, or other major mental disorders or treatment. He reports that he has regularly used Cannabis over the last 10 years, and a few years ago, he started occasionally using nitrous oxide (N2O). He says that in the week following his wife’s death, he used N2O almost daily and in copious amounts. In an attempt to “self-anesthetize” himself after his wife’s funeral, he isolated himself in his bedroom and used escalating amounts of Cannabis and N2O, while continually working on a book about their life together.
At first, Mr. M shows little emotion and describes his situation as “interesting and fascinating.” He mentions that he thinks he might have been “psychotic” the week after his wife’s death, but he shows no sustained insight and immediately relapses into psychotic thinking. Over several hours in the ED, he is tearful and sad about his wife’s death. Mr. M recalls a similar experience of grief after his mother died when he was a teenager, but at that time he did not abuse substances or have psychotic symptoms. He is fully alert, fully oriented, and has no significant deficits of attention or memory.
[polldaddy:9859135]
The authors’ observations
Grief was a precipitating event, but by itself grief cannot explain psychosis. Psychotic depression is a possibility, but Mr. M’s psychotic features are incongruent with his mood. Mania would be a diagnosis of exclusion. Mr. M had no prior history of major affective illness. Mr. M was abusing Cannabis, which might independently contribute to psychosis1; however, he had been using it recreationally for 10 years without psychiatric problems. N2O, however, can cause symptoms consistent with Mr. M’s presentation.
[polldaddy:9859140]
EVALUATION Laboratory tests
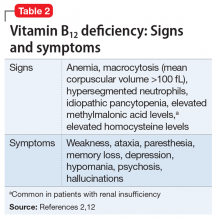
Mr. M’s physical examination is notable only for an elevated blood pressure of 196/120 mm Hg. Neurologic examination is normal. Toxicology is positive for cannabinoids and negative for amphetamines, cocaine, opiates, and phencyclidine. Chemistries are normal except for a potassium of 3.4 mEq/L (reference range, 3.7 to 5.2 mEq/L) and a blood urine nitrogen of 25 mg/dL (reference range, 6 to 20 mg/dL), which are consistent with reduced food and fluid intake. Mr. M shows no signs of anemia. Hematocrit is 42% and mean corpuscular volume is 90 fL. Syphilis screen is negative; a head CT scan is unremarkable.
The authors’ observations
N2O, also known as “laughing gas,” is routinely used by dentists and pediatric anesthesiologists, and has other medical uses. Some studies have examined an adjunctive use of N2O for pain control in the ED and during colonoscopies.3,4
In the 2013 U.S. National Survey on Drug Use and Health, 16% of respondents reported lifetime illicit use of N2O.5,6 It is readily available in tanks used in medicine and industry and in small dispensers called “whippits” that can be legally purchased. Acute effects of N2O include euphoric mood, numbness, feeling of warmth, dizziness, and auditory hallucinations.7 The anesthetic effects of N2O are linked to endogenous release of opiates, and recent research links its anxiolytic activity to the facilitation of GABAergic inhibitory and N-methyl-
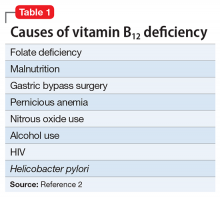
Beginning with a 1960 report of a series of patients with “megaloblastic madness,”17 there have been calls for increased awareness of the potential for vitamin B12 deficiency–induced psychiatric disorders, even in the absence of other hematologic or neurologic sequelae that would alert clinicians of the deficiency. In a case series of 141 patients with a broad array of neurologic and psychiatric symptoms associated with vitamin B12 deficiency, 40 (28%) patients had no anemia or macrocytosis.2
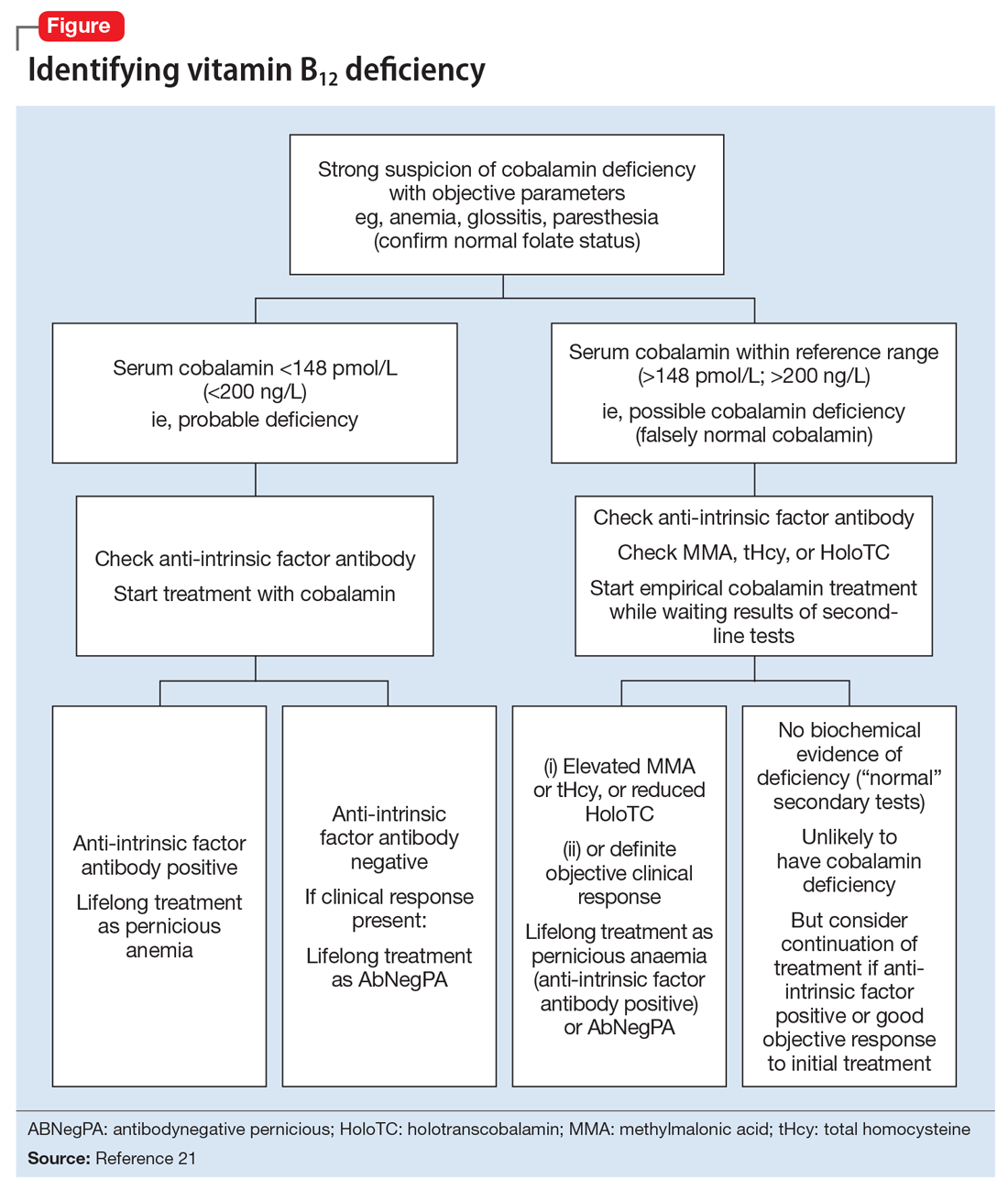
Vitamin B12-responsive psychosis has been reported as the sole manifestation of illness, without associated neurologic or hematologic symptoms, in only a few case reports. Vitamin B12 levels in these cases ranged from 75 to 236 pg/mL (reference range, 160 to 950 pg/mL).18-20 In all of these cases, the vitamin B12 deficiency was traced to dietary causes. The clinical evaluation of suspected vitamin B12 deficiency is outlined in the Figure.21 Mr. M had used Cannabis recreationally for a long time, and his Cannabis use acutely escalated with use of N2O. Long-term use of Cannabis alone is a risk factor for psychotic illness.22 Combined abuse of Cannabis and N2O has been reported to provoke psychotic illness. In a case report of a 22-year-old male who was treated for paranoid delusions, using Cannabis and 100 cartridges of N2O daily was associated with low vitamin B12 and elevated homocysteine and methylmalonic acid levels.23
Cannabis use may have played a role in Mr. M’s escalating N2O use. In a study comparing 9 active Cannabis users with 9 non-using controls, users rated the subjective effects of N2O as more intense than non-users.24 In our patient’s case, Cannabis may have played a role in both sustaining his escalating N2O abuse and potentiating its psychotomimetic effects.
It also is possible that Mr. M may have been “self-medicating” his grief with N2O. In a recent placebo-controlled crossover trial of 20 patients with treatment-resistant depression, Nagele et al25 found a significant rapid and week-long antidepressant effect of subanesthetic N2O use. A model involving NMDA receptor activation has been proposed.25,26 Zorumski et al26 further reviewed possible antidepressant mechanisms of N2O. They compared N2O with ketamine as an NMDA receptor antagonist, but also noted its distinct effects on glutaminergic and GABAergic neurotransmitter systems as well as other receptors and channels.26 However, illicit use of N2O poses toxicity dangers and has no current indication for psychiatric treatment.
TREATMENT Supplementation
Mr. M is diagnosed with substance-induced psychotic disorder. His symptoms were precipitated by an acute increase in N2O use, which has been shown to cause vitamin B12 deficiency, which we consider was likely a primary contributor to his presentation. Other potential contributing factors are premorbid hyperthymic temperament, a possible propensity to psychotic thinking under stress, the sudden death of his wife, acute grief, the potentiating role of Cannabis, dehydration, and general malnutrition. The death of a loved one is associated with an increased risk of developing substance use disorders.27
During a 15-day psychiatric hospitalization, Mr. M is given olanzapine, increased to 15 mg/d and oral vitamin B12, 1,000 mcg/d for 4 days, then IM cyanocobalamin for 7 days. Mr. M’s symptoms steadily improve, with normalization of sleep and near-total resolution of delusions. On hospital Day 14, his vitamin B12 levels are within normal limits (844 pg/mL). At discharge, Mr. M shows residual mild grandiosity, with limited insight into his illness and what caused it, but frank delusional ideation has clearly receded. He still shows some signs of grief. Mr. M is advised to stop using Cannabis and N2O and about the potential consequences of continued use.
The authors’ observations
For patients with vitamin B12 deficiency, guidelines from the National Health Service in the United Kingdom and the British Society for Haematology recommend treatment with IM hydroxocobalamin, 1,000 IU, 3 times weekly, for 2 weeks.21,28 For patients with neurologic symptoms, the British National Foundation recommends treatment with IM hydroxocobalamin, 1,000 IU, on alternative days until there is no further improvement.21
This case is a reminder for clinicians to screen for inhalant use, specifically N2O, which can precipitate vitamin B12 deficiency with psychiatric symptoms as the only presenting concern. Clinicians should consider measuring vitamin B12 levels in psychiatric patients at risk of deficiency of this nutrient, including older adults, vegetarians, and those with alimentary disorders.29,30 Dietary sources of vitamin B12 include meat, milk, egg, fish, and shellfish.31 The body can store a total of 2 to 5 mg of vitamin B12; thus, it takes 2 to 5 years to develop vitamin B12 deficiency from malabsorption and can take as long as 20 years to develop vitamin B12 deficiency from vegetarianism.32 However, by chemically inactivating vitamin B12, N2O causes a rapid functional deficiency, as was seen in our patient.
OUTCOME Improved insight
At a 1-week follow-up appointment with a psychiatrist, Mr. M has no evident psychotic symptoms. He reports that he has not used Cannabis or N2O, and he discontinues olanzapine following this visit. Two weeks later, Mr. M shows no psychotic or affective symptoms other than grief, which is appropriately expressed. His insight has improved. He commits to not using Cannabis, N2O, or any other illicit substances. Mr. M is referred back to his long-standing primary care provider with the understanding that if any psychiatric symptoms recur he will see a psychiatrist again.
1. Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol. 2005;19(2):187-194.
2. Lindenbaum J, Healton EB, Savage DG, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318(26)1720-1728.
3. Herres J, Chudnofsky CR, Manur R, et al. The use of inhaled nitrous oxide for analgesia in adult ED patients: a pilot study. Am J Emerg Med. 2016;34(2):269-273.
4. Aboumarzouk OM, Agarwal T, Syed Nong Chek SA, et al. Nitrous oxide for colonoscopy. Cochrane Database Syst Rev. 2011;(8):CD008506.
5. National Institute on Drug Abuse. Drug facts: inhalants. http://www.drugabuse.gov/publications/drugfacts/inhalants. Updated February 2017. Accessed September 30, 2017.
6. SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health 2012 and 2013: Table 1.88C. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs2013.pdf. Published September 4, 2017. Accessed September 30, 2017.
7. Brouette T, Anton R. Clinical review of inhalants. Am J Addict. 2001;10(1):79-94.
8. Emmanouil DE, Quock RM. Advances in understanding the actions of nitrous oxide. Anesth Prog. 2007;54(1):9-18.
9. Garakani A, Jaffe RJ, Savla D, et al. Neurologic, psychiatric, and other medical manifestations of nitrous oxide abuse: a systematic review of the case literature. Am J Addict. 2016;25(5):358-369.
10. Hathout L, El-Saden S. Nitrous oxide-induced B12 deficiency myelopathy: perspectives on the clinical biochemistry of vitamin B12. J Neurol Sci. 2011;301(1-2):1-8.
11. van Tonder SV, Ruck A, van der Westhuyzen J, et al. Dissociation of methionine synthetase (EC 2.1.1.13) activity and impairment of DNA synthesis in fruit bats (Rousettus aegyptiacus) with nitrous oxide-induced vitamin B12 deficiency. Br J Nutr. 1986;55(1):187-192.
12. Schrier SL, Mentzer WC, Tirnauer JS. Diagnosis and treatment of vitamin B12 and folate deficiency. UpToDate. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-vitamin-b12-and-folate-deficiency. Updated September 30, 2011. Accessed September 8, 2015.
13. Sethi NK, Mullin P, Torgovnick J, et al. Nitrous oxide “whippit” abuse presenting with cobalamin responsive psychosis. J Med Toxicol. 2006;2(2):71-74.
14. Cousaert C, Heylens G, Audenaert K. Laughing gas abuse is no joke. An overview of the implications for psychiatric practice. Clin Neurol Neurosurg. 2013;115(7):859-862.
15. Brodsky L, Zuniga J. Nitrous oxide: a psychotogenic agent. Compr Psychiatry. 1975;16(2):185-188.
16. Wong SL, Harrison R, Mattman A, et al. Nitrous oxide (N2O)-induced acute psychosis. Can J Neurol Sci. 2014;41(5):672-674.
17. Smith AD. Megaloblastic madness. Br Med J. 1960;2(5216):1840-1845.
18. Masalha R, Chudakov B, Muhamad M, et al. Cobalamin-responsive psychosis as the sole manifestation of vitamin B12 deficiency. Isr Med Associ J. 2001;3(9):701-703.
19. Kuo SC, Yeh SB, Yeh YW, et al. Schizophrenia-like psychotic episode precipitated by cobalamin deficiency. Gen Hosp Psychiatry. 2009;31(6):586-588.
20. Raveendranathan D, Shiva L, Venkatasubramanian G, et al. Vitamin B12 deficiency masquerading as clozapine-resistant psychotic symptoms in schizophrenia. J Neuropsychiatry Clin Neurosci. 2013;25(2):E34-E35.
21. Devalia V, Hamilton MS, Molloy AM; British Committee for Standards in Haematology. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol. 2014;166(4):496-513.
22. Moore THM, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319-328.
23. Garakani A, Welch AK, Jaffe RJ, et al. Psychosis and low cyanocobalamin in a patient abusing nitrous oxide and cannabis. Psychosomatics. 2014;55(6):715-719.
24. Yajnik S, Thapar P, Lichtor JL, et al. Effects of marijuana history on the subjective, psychomotor, and reinforcing effects of nitrous oxide in human. Drug Alcohol Depend. 1994;36(3):227-236.
25. Nagele P, Duma A, Kopec M, et al. Nitrous oxide for treatment-resistant major depression: a proof-of-concept trial. Biol Psychiatry. 2015;78(1):10-18.
26. Zorumski CF, Nagele P, Mennerick S, et al. Treatment-resistant major depression: rationale for NMDA receptors as targets and nitrous oxide as therapy. Front Psychiatry. 2015;6:172.
27. Shear MK. Clinical practice. Complicated grief. N Engl J Med. 2015;372(2):153-160.
28. Knechtli CJC, Crowe JN. Guidelines for the investigation & management of vitamin B12 deficiency. Royal United Hospital Bath, National Health Service. http://www.ruh.nhs.uk/For_Clinicians/departments_ruh/Pathology/documents/haematology/B12_-_advice_on_investigation_management.pdf. Accessed June 14, 2016.
29. Jayaram N, Rao MG, Narashima A, et al. Vitamin B12 levels and psychiatric symptomatology: a case series. J Neuropsychiatry Clin Neurosci. 2013;25(2):150-152.
30. Marks PW, Zukerberg LR. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 30-2004. A 37-year-old woman with paresthesias of the arms and legs. N Engl J Med. 2004;351(13):1333-1341.
31. Watanabe F. Vitamin B12 sources and bioavailablility. Exp Biol Med (Maywood). 2007;232(10):1266-1274.
32. Green R, Kinsella LJ. Current concepts in the diagnosis of cobalamin deficiency. Neurology. 1995;45(8):1435-1440.
CASE Grieving, delusional
Mr. M, age 51, is brought to the emergency department (ED) because of new-onset delusions and decreased self-care over the last 2 weeks following the sudden death of his wife. He has become expansive and grandiose, with pressured speech, increased energy, and markedly reduced sleep. Mr. M is preoccupied with the idea that he is “the first to survive a human reboot process” and says that his and his wife’s bodies and brains had been “split apart.” Mr. M has limited his food and fluid intake and lost 15 lb within the past 2 to 3 weeks.
Mr. M has no history of any affective, psychotic, or other major mental disorders or treatment. He reports that he has regularly used Cannabis over the last 10 years, and a few years ago, he started occasionally using nitrous oxide (N2O). He says that in the week following his wife’s death, he used N2O almost daily and in copious amounts. In an attempt to “self-anesthetize” himself after his wife’s funeral, he isolated himself in his bedroom and used escalating amounts of Cannabis and N2O, while continually working on a book about their life together.
At first, Mr. M shows little emotion and describes his situation as “interesting and fascinating.” He mentions that he thinks he might have been “psychotic” the week after his wife’s death, but he shows no sustained insight and immediately relapses into psychotic thinking. Over several hours in the ED, he is tearful and sad about his wife’s death. Mr. M recalls a similar experience of grief after his mother died when he was a teenager, but at that time he did not abuse substances or have psychotic symptoms. He is fully alert, fully oriented, and has no significant deficits of attention or memory.
[polldaddy:9859135]
The authors’ observations
Grief was a precipitating event, but by itself grief cannot explain psychosis. Psychotic depression is a possibility, but Mr. M’s psychotic features are incongruent with his mood. Mania would be a diagnosis of exclusion. Mr. M had no prior history of major affective illness. Mr. M was abusing Cannabis, which might independently contribute to psychosis1; however, he had been using it recreationally for 10 years without psychiatric problems. N2O, however, can cause symptoms consistent with Mr. M’s presentation.
[polldaddy:9859140]
EVALUATION Laboratory tests
Mr. M’s physical examination is notable only for an elevated blood pressure of 196/120 mm Hg. Neurologic examination is normal. Toxicology is positive for cannabinoids and negative for amphetamines, cocaine, opiates, and phencyclidine. Chemistries are normal except for a potassium of 3.4 mEq/L (reference range, 3.7 to 5.2 mEq/L) and a blood urine nitrogen of 25 mg/dL (reference range, 6 to 20 mg/dL), which are consistent with reduced food and fluid intake. Mr. M shows no signs of anemia. Hematocrit is 42% and mean corpuscular volume is 90 fL. Syphilis screen is negative; a head CT scan is unremarkable.
The authors’ observations
N2O, also known as “laughing gas,” is routinely used by dentists and pediatric anesthesiologists, and has other medical uses. Some studies have examined an adjunctive use of N2O for pain control in the ED and during colonoscopies.3,4
In the 2013 U.S. National Survey on Drug Use and Health, 16% of respondents reported lifetime illicit use of N2O.5,6 It is readily available in tanks used in medicine and industry and in small dispensers called “whippits” that can be legally purchased. Acute effects of N2O include euphoric mood, numbness, feeling of warmth, dizziness, and auditory hallucinations.7 The anesthetic effects of N2O are linked to endogenous release of opiates, and recent research links its anxiolytic activity to the facilitation of GABAergic inhibitory and N-methyl-
Beginning with a 1960 report of a series of patients with “megaloblastic madness,”17 there have been calls for increased awareness of the potential for vitamin B12 deficiency–induced psychiatric disorders, even in the absence of other hematologic or neurologic sequelae that would alert clinicians of the deficiency. In a case series of 141 patients with a broad array of neurologic and psychiatric symptoms associated with vitamin B12 deficiency, 40 (28%) patients had no anemia or macrocytosis.2
Vitamin B12-responsive psychosis has been reported as the sole manifestation of illness, without associated neurologic or hematologic symptoms, in only a few case reports. Vitamin B12 levels in these cases ranged from 75 to 236 pg/mL (reference range, 160 to 950 pg/mL).18-20 In all of these cases, the vitamin B12 deficiency was traced to dietary causes. The clinical evaluation of suspected vitamin B12 deficiency is outlined in the Figure.21 Mr. M had used Cannabis recreationally for a long time, and his Cannabis use acutely escalated with use of N2O. Long-term use of Cannabis alone is a risk factor for psychotic illness.22 Combined abuse of Cannabis and N2O has been reported to provoke psychotic illness. In a case report of a 22-year-old male who was treated for paranoid delusions, using Cannabis and 100 cartridges of N2O daily was associated with low vitamin B12 and elevated homocysteine and methylmalonic acid levels.23

Cannabis use may have played a role in Mr. M’s escalating N2O use. In a study comparing 9 active Cannabis users with 9 non-using controls, users rated the subjective effects of N2O as more intense than non-users.24 In our patient’s case, Cannabis may have played a role in both sustaining his escalating N2O abuse and potentiating its psychotomimetic effects.
It also is possible that Mr. M may have been “self-medicating” his grief with N2O. In a recent placebo-controlled crossover trial of 20 patients with treatment-resistant depression, Nagele et al25 found a significant rapid and week-long antidepressant effect of subanesthetic N2O use. A model involving NMDA receptor activation has been proposed.25,26 Zorumski et al26 further reviewed possible antidepressant mechanisms of N2O. They compared N2O with ketamine as an NMDA receptor antagonist, but also noted its distinct effects on glutaminergic and GABAergic neurotransmitter systems as well as other receptors and channels.26 However, illicit use of N2O poses toxicity dangers and has no current indication for psychiatric treatment.
TREATMENT Supplementation
Mr. M is diagnosed with substance-induced psychotic disorder. His symptoms were precipitated by an acute increase in N2O use, which has been shown to cause vitamin B12 deficiency, which we consider was likely a primary contributor to his presentation. Other potential contributing factors are premorbid hyperthymic temperament, a possible propensity to psychotic thinking under stress, the sudden death of his wife, acute grief, the potentiating role of Cannabis, dehydration, and general malnutrition. The death of a loved one is associated with an increased risk of developing substance use disorders.27
During a 15-day psychiatric hospitalization, Mr. M is given olanzapine, increased to 15 mg/d and oral vitamin B12, 1,000 mcg/d for 4 days, then IM cyanocobalamin for 7 days. Mr. M’s symptoms steadily improve, with normalization of sleep and near-total resolution of delusions. On hospital Day 14, his vitamin B12 levels are within normal limits (844 pg/mL). At discharge, Mr. M shows residual mild grandiosity, with limited insight into his illness and what caused it, but frank delusional ideation has clearly receded. He still shows some signs of grief. Mr. M is advised to stop using Cannabis and N2O and about the potential consequences of continued use.
The authors’ observations
For patients with vitamin B12 deficiency, guidelines from the National Health Service in the United Kingdom and the British Society for Haematology recommend treatment with IM hydroxocobalamin, 1,000 IU, 3 times weekly, for 2 weeks.21,28 For patients with neurologic symptoms, the British National Foundation recommends treatment with IM hydroxocobalamin, 1,000 IU, on alternative days until there is no further improvement.21
This case is a reminder for clinicians to screen for inhalant use, specifically N2O, which can precipitate vitamin B12 deficiency with psychiatric symptoms as the only presenting concern. Clinicians should consider measuring vitamin B12 levels in psychiatric patients at risk of deficiency of this nutrient, including older adults, vegetarians, and those with alimentary disorders.29,30 Dietary sources of vitamin B12 include meat, milk, egg, fish, and shellfish.31 The body can store a total of 2 to 5 mg of vitamin B12; thus, it takes 2 to 5 years to develop vitamin B12 deficiency from malabsorption and can take as long as 20 years to develop vitamin B12 deficiency from vegetarianism.32 However, by chemically inactivating vitamin B12, N2O causes a rapid functional deficiency, as was seen in our patient.
OUTCOME Improved insight
At a 1-week follow-up appointment with a psychiatrist, Mr. M has no evident psychotic symptoms. He reports that he has not used Cannabis or N2O, and he discontinues olanzapine following this visit. Two weeks later, Mr. M shows no psychotic or affective symptoms other than grief, which is appropriately expressed. His insight has improved. He commits to not using Cannabis, N2O, or any other illicit substances. Mr. M is referred back to his long-standing primary care provider with the understanding that if any psychiatric symptoms recur he will see a psychiatrist again.
CASE Grieving, delusional
Mr. M, age 51, is brought to the emergency department (ED) because of new-onset delusions and decreased self-care over the last 2 weeks following the sudden death of his wife. He has become expansive and grandiose, with pressured speech, increased energy, and markedly reduced sleep. Mr. M is preoccupied with the idea that he is “the first to survive a human reboot process” and says that his and his wife’s bodies and brains had been “split apart.” Mr. M has limited his food and fluid intake and lost 15 lb within the past 2 to 3 weeks.
Mr. M has no history of any affective, psychotic, or other major mental disorders or treatment. He reports that he has regularly used Cannabis over the last 10 years, and a few years ago, he started occasionally using nitrous oxide (N2O). He says that in the week following his wife’s death, he used N2O almost daily and in copious amounts. In an attempt to “self-anesthetize” himself after his wife’s funeral, he isolated himself in his bedroom and used escalating amounts of Cannabis and N2O, while continually working on a book about their life together.
At first, Mr. M shows little emotion and describes his situation as “interesting and fascinating.” He mentions that he thinks he might have been “psychotic” the week after his wife’s death, but he shows no sustained insight and immediately relapses into psychotic thinking. Over several hours in the ED, he is tearful and sad about his wife’s death. Mr. M recalls a similar experience of grief after his mother died when he was a teenager, but at that time he did not abuse substances or have psychotic symptoms. He is fully alert, fully oriented, and has no significant deficits of attention or memory.
[polldaddy:9859135]
The authors’ observations
Grief was a precipitating event, but by itself grief cannot explain psychosis. Psychotic depression is a possibility, but Mr. M’s psychotic features are incongruent with his mood. Mania would be a diagnosis of exclusion. Mr. M had no prior history of major affective illness. Mr. M was abusing Cannabis, which might independently contribute to psychosis1; however, he had been using it recreationally for 10 years without psychiatric problems. N2O, however, can cause symptoms consistent with Mr. M’s presentation.
[polldaddy:9859140]
EVALUATION Laboratory tests
Mr. M’s physical examination is notable only for an elevated blood pressure of 196/120 mm Hg. Neurologic examination is normal. Toxicology is positive for cannabinoids and negative for amphetamines, cocaine, opiates, and phencyclidine. Chemistries are normal except for a potassium of 3.4 mEq/L (reference range, 3.7 to 5.2 mEq/L) and a blood urine nitrogen of 25 mg/dL (reference range, 6 to 20 mg/dL), which are consistent with reduced food and fluid intake. Mr. M shows no signs of anemia. Hematocrit is 42% and mean corpuscular volume is 90 fL. Syphilis screen is negative; a head CT scan is unremarkable.
The authors’ observations
N2O, also known as “laughing gas,” is routinely used by dentists and pediatric anesthesiologists, and has other medical uses. Some studies have examined an adjunctive use of N2O for pain control in the ED and during colonoscopies.3,4
In the 2013 U.S. National Survey on Drug Use and Health, 16% of respondents reported lifetime illicit use of N2O.5,6 It is readily available in tanks used in medicine and industry and in small dispensers called “whippits” that can be legally purchased. Acute effects of N2O include euphoric mood, numbness, feeling of warmth, dizziness, and auditory hallucinations.7 The anesthetic effects of N2O are linked to endogenous release of opiates, and recent research links its anxiolytic activity to the facilitation of GABAergic inhibitory and N-methyl-
Beginning with a 1960 report of a series of patients with “megaloblastic madness,”17 there have been calls for increased awareness of the potential for vitamin B12 deficiency–induced psychiatric disorders, even in the absence of other hematologic or neurologic sequelae that would alert clinicians of the deficiency. In a case series of 141 patients with a broad array of neurologic and psychiatric symptoms associated with vitamin B12 deficiency, 40 (28%) patients had no anemia or macrocytosis.2
Vitamin B12-responsive psychosis has been reported as the sole manifestation of illness, without associated neurologic or hematologic symptoms, in only a few case reports. Vitamin B12 levels in these cases ranged from 75 to 236 pg/mL (reference range, 160 to 950 pg/mL).18-20 In all of these cases, the vitamin B12 deficiency was traced to dietary causes. The clinical evaluation of suspected vitamin B12 deficiency is outlined in the Figure.21 Mr. M had used Cannabis recreationally for a long time, and his Cannabis use acutely escalated with use of N2O. Long-term use of Cannabis alone is a risk factor for psychotic illness.22 Combined abuse of Cannabis and N2O has been reported to provoke psychotic illness. In a case report of a 22-year-old male who was treated for paranoid delusions, using Cannabis and 100 cartridges of N2O daily was associated with low vitamin B12 and elevated homocysteine and methylmalonic acid levels.23

Cannabis use may have played a role in Mr. M’s escalating N2O use. In a study comparing 9 active Cannabis users with 9 non-using controls, users rated the subjective effects of N2O as more intense than non-users.24 In our patient’s case, Cannabis may have played a role in both sustaining his escalating N2O abuse and potentiating its psychotomimetic effects.
It also is possible that Mr. M may have been “self-medicating” his grief with N2O. In a recent placebo-controlled crossover trial of 20 patients with treatment-resistant depression, Nagele et al25 found a significant rapid and week-long antidepressant effect of subanesthetic N2O use. A model involving NMDA receptor activation has been proposed.25,26 Zorumski et al26 further reviewed possible antidepressant mechanisms of N2O. They compared N2O with ketamine as an NMDA receptor antagonist, but also noted its distinct effects on glutaminergic and GABAergic neurotransmitter systems as well as other receptors and channels.26 However, illicit use of N2O poses toxicity dangers and has no current indication for psychiatric treatment.
TREATMENT Supplementation
Mr. M is diagnosed with substance-induced psychotic disorder. His symptoms were precipitated by an acute increase in N2O use, which has been shown to cause vitamin B12 deficiency, which we consider was likely a primary contributor to his presentation. Other potential contributing factors are premorbid hyperthymic temperament, a possible propensity to psychotic thinking under stress, the sudden death of his wife, acute grief, the potentiating role of Cannabis, dehydration, and general malnutrition. The death of a loved one is associated with an increased risk of developing substance use disorders.27
During a 15-day psychiatric hospitalization, Mr. M is given olanzapine, increased to 15 mg/d and oral vitamin B12, 1,000 mcg/d for 4 days, then IM cyanocobalamin for 7 days. Mr. M’s symptoms steadily improve, with normalization of sleep and near-total resolution of delusions. On hospital Day 14, his vitamin B12 levels are within normal limits (844 pg/mL). At discharge, Mr. M shows residual mild grandiosity, with limited insight into his illness and what caused it, but frank delusional ideation has clearly receded. He still shows some signs of grief. Mr. M is advised to stop using Cannabis and N2O and about the potential consequences of continued use.
The authors’ observations
For patients with vitamin B12 deficiency, guidelines from the National Health Service in the United Kingdom and the British Society for Haematology recommend treatment with IM hydroxocobalamin, 1,000 IU, 3 times weekly, for 2 weeks.21,28 For patients with neurologic symptoms, the British National Foundation recommends treatment with IM hydroxocobalamin, 1,000 IU, on alternative days until there is no further improvement.21
This case is a reminder for clinicians to screen for inhalant use, specifically N2O, which can precipitate vitamin B12 deficiency with psychiatric symptoms as the only presenting concern. Clinicians should consider measuring vitamin B12 levels in psychiatric patients at risk of deficiency of this nutrient, including older adults, vegetarians, and those with alimentary disorders.29,30 Dietary sources of vitamin B12 include meat, milk, egg, fish, and shellfish.31 The body can store a total of 2 to 5 mg of vitamin B12; thus, it takes 2 to 5 years to develop vitamin B12 deficiency from malabsorption and can take as long as 20 years to develop vitamin B12 deficiency from vegetarianism.32 However, by chemically inactivating vitamin B12, N2O causes a rapid functional deficiency, as was seen in our patient.
OUTCOME Improved insight
At a 1-week follow-up appointment with a psychiatrist, Mr. M has no evident psychotic symptoms. He reports that he has not used Cannabis or N2O, and he discontinues olanzapine following this visit. Two weeks later, Mr. M shows no psychotic or affective symptoms other than grief, which is appropriately expressed. His insight has improved. He commits to not using Cannabis, N2O, or any other illicit substances. Mr. M is referred back to his long-standing primary care provider with the understanding that if any psychiatric symptoms recur he will see a psychiatrist again.
1. Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol. 2005;19(2):187-194.
2. Lindenbaum J, Healton EB, Savage DG, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318(26)1720-1728.
3. Herres J, Chudnofsky CR, Manur R, et al. The use of inhaled nitrous oxide for analgesia in adult ED patients: a pilot study. Am J Emerg Med. 2016;34(2):269-273.
4. Aboumarzouk OM, Agarwal T, Syed Nong Chek SA, et al. Nitrous oxide for colonoscopy. Cochrane Database Syst Rev. 2011;(8):CD008506.
5. National Institute on Drug Abuse. Drug facts: inhalants. http://www.drugabuse.gov/publications/drugfacts/inhalants. Updated February 2017. Accessed September 30, 2017.
6. SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health 2012 and 2013: Table 1.88C. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs2013.pdf. Published September 4, 2017. Accessed September 30, 2017.
7. Brouette T, Anton R. Clinical review of inhalants. Am J Addict. 2001;10(1):79-94.
8. Emmanouil DE, Quock RM. Advances in understanding the actions of nitrous oxide. Anesth Prog. 2007;54(1):9-18.
9. Garakani A, Jaffe RJ, Savla D, et al. Neurologic, psychiatric, and other medical manifestations of nitrous oxide abuse: a systematic review of the case literature. Am J Addict. 2016;25(5):358-369.
10. Hathout L, El-Saden S. Nitrous oxide-induced B12 deficiency myelopathy: perspectives on the clinical biochemistry of vitamin B12. J Neurol Sci. 2011;301(1-2):1-8.
11. van Tonder SV, Ruck A, van der Westhuyzen J, et al. Dissociation of methionine synthetase (EC 2.1.1.13) activity and impairment of DNA synthesis in fruit bats (Rousettus aegyptiacus) with nitrous oxide-induced vitamin B12 deficiency. Br J Nutr. 1986;55(1):187-192.
12. Schrier SL, Mentzer WC, Tirnauer JS. Diagnosis and treatment of vitamin B12 and folate deficiency. UpToDate. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-vitamin-b12-and-folate-deficiency. Updated September 30, 2011. Accessed September 8, 2015.
13. Sethi NK, Mullin P, Torgovnick J, et al. Nitrous oxide “whippit” abuse presenting with cobalamin responsive psychosis. J Med Toxicol. 2006;2(2):71-74.
14. Cousaert C, Heylens G, Audenaert K. Laughing gas abuse is no joke. An overview of the implications for psychiatric practice. Clin Neurol Neurosurg. 2013;115(7):859-862.
15. Brodsky L, Zuniga J. Nitrous oxide: a psychotogenic agent. Compr Psychiatry. 1975;16(2):185-188.
16. Wong SL, Harrison R, Mattman A, et al. Nitrous oxide (N2O)-induced acute psychosis. Can J Neurol Sci. 2014;41(5):672-674.
17. Smith AD. Megaloblastic madness. Br Med J. 1960;2(5216):1840-1845.
18. Masalha R, Chudakov B, Muhamad M, et al. Cobalamin-responsive psychosis as the sole manifestation of vitamin B12 deficiency. Isr Med Associ J. 2001;3(9):701-703.
19. Kuo SC, Yeh SB, Yeh YW, et al. Schizophrenia-like psychotic episode precipitated by cobalamin deficiency. Gen Hosp Psychiatry. 2009;31(6):586-588.
20. Raveendranathan D, Shiva L, Venkatasubramanian G, et al. Vitamin B12 deficiency masquerading as clozapine-resistant psychotic symptoms in schizophrenia. J Neuropsychiatry Clin Neurosci. 2013;25(2):E34-E35.
21. Devalia V, Hamilton MS, Molloy AM; British Committee for Standards in Haematology. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol. 2014;166(4):496-513.
22. Moore THM, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319-328.
23. Garakani A, Welch AK, Jaffe RJ, et al. Psychosis and low cyanocobalamin in a patient abusing nitrous oxide and cannabis. Psychosomatics. 2014;55(6):715-719.
24. Yajnik S, Thapar P, Lichtor JL, et al. Effects of marijuana history on the subjective, psychomotor, and reinforcing effects of nitrous oxide in human. Drug Alcohol Depend. 1994;36(3):227-236.
25. Nagele P, Duma A, Kopec M, et al. Nitrous oxide for treatment-resistant major depression: a proof-of-concept trial. Biol Psychiatry. 2015;78(1):10-18.
26. Zorumski CF, Nagele P, Mennerick S, et al. Treatment-resistant major depression: rationale for NMDA receptors as targets and nitrous oxide as therapy. Front Psychiatry. 2015;6:172.
27. Shear MK. Clinical practice. Complicated grief. N Engl J Med. 2015;372(2):153-160.
28. Knechtli CJC, Crowe JN. Guidelines for the investigation & management of vitamin B12 deficiency. Royal United Hospital Bath, National Health Service. http://www.ruh.nhs.uk/For_Clinicians/departments_ruh/Pathology/documents/haematology/B12_-_advice_on_investigation_management.pdf. Accessed June 14, 2016.
29. Jayaram N, Rao MG, Narashima A, et al. Vitamin B12 levels and psychiatric symptomatology: a case series. J Neuropsychiatry Clin Neurosci. 2013;25(2):150-152.
30. Marks PW, Zukerberg LR. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 30-2004. A 37-year-old woman with paresthesias of the arms and legs. N Engl J Med. 2004;351(13):1333-1341.
31. Watanabe F. Vitamin B12 sources and bioavailablility. Exp Biol Med (Maywood). 2007;232(10):1266-1274.
32. Green R, Kinsella LJ. Current concepts in the diagnosis of cobalamin deficiency. Neurology. 1995;45(8):1435-1440.
1. Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol. 2005;19(2):187-194.
2. Lindenbaum J, Healton EB, Savage DG, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318(26)1720-1728.
3. Herres J, Chudnofsky CR, Manur R, et al. The use of inhaled nitrous oxide for analgesia in adult ED patients: a pilot study. Am J Emerg Med. 2016;34(2):269-273.
4. Aboumarzouk OM, Agarwal T, Syed Nong Chek SA, et al. Nitrous oxide for colonoscopy. Cochrane Database Syst Rev. 2011;(8):CD008506.
5. National Institute on Drug Abuse. Drug facts: inhalants. http://www.drugabuse.gov/publications/drugfacts/inhalants. Updated February 2017. Accessed September 30, 2017.
6. SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health 2012 and 2013: Table 1.88C. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs2013.pdf. Published September 4, 2017. Accessed September 30, 2017.
7. Brouette T, Anton R. Clinical review of inhalants. Am J Addict. 2001;10(1):79-94.
8. Emmanouil DE, Quock RM. Advances in understanding the actions of nitrous oxide. Anesth Prog. 2007;54(1):9-18.
9. Garakani A, Jaffe RJ, Savla D, et al. Neurologic, psychiatric, and other medical manifestations of nitrous oxide abuse: a systematic review of the case literature. Am J Addict. 2016;25(5):358-369.
10. Hathout L, El-Saden S. Nitrous oxide-induced B12 deficiency myelopathy: perspectives on the clinical biochemistry of vitamin B12. J Neurol Sci. 2011;301(1-2):1-8.
11. van Tonder SV, Ruck A, van der Westhuyzen J, et al. Dissociation of methionine synthetase (EC 2.1.1.13) activity and impairment of DNA synthesis in fruit bats (Rousettus aegyptiacus) with nitrous oxide-induced vitamin B12 deficiency. Br J Nutr. 1986;55(1):187-192.
12. Schrier SL, Mentzer WC, Tirnauer JS. Diagnosis and treatment of vitamin B12 and folate deficiency. UpToDate. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-vitamin-b12-and-folate-deficiency. Updated September 30, 2011. Accessed September 8, 2015.
13. Sethi NK, Mullin P, Torgovnick J, et al. Nitrous oxide “whippit” abuse presenting with cobalamin responsive psychosis. J Med Toxicol. 2006;2(2):71-74.
14. Cousaert C, Heylens G, Audenaert K. Laughing gas abuse is no joke. An overview of the implications for psychiatric practice. Clin Neurol Neurosurg. 2013;115(7):859-862.
15. Brodsky L, Zuniga J. Nitrous oxide: a psychotogenic agent. Compr Psychiatry. 1975;16(2):185-188.
16. Wong SL, Harrison R, Mattman A, et al. Nitrous oxide (N2O)-induced acute psychosis. Can J Neurol Sci. 2014;41(5):672-674.
17. Smith AD. Megaloblastic madness. Br Med J. 1960;2(5216):1840-1845.
18. Masalha R, Chudakov B, Muhamad M, et al. Cobalamin-responsive psychosis as the sole manifestation of vitamin B12 deficiency. Isr Med Associ J. 2001;3(9):701-703.
19. Kuo SC, Yeh SB, Yeh YW, et al. Schizophrenia-like psychotic episode precipitated by cobalamin deficiency. Gen Hosp Psychiatry. 2009;31(6):586-588.
20. Raveendranathan D, Shiva L, Venkatasubramanian G, et al. Vitamin B12 deficiency masquerading as clozapine-resistant psychotic symptoms in schizophrenia. J Neuropsychiatry Clin Neurosci. 2013;25(2):E34-E35.
21. Devalia V, Hamilton MS, Molloy AM; British Committee for Standards in Haematology. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol. 2014;166(4):496-513.
22. Moore THM, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319-328.
23. Garakani A, Welch AK, Jaffe RJ, et al. Psychosis and low cyanocobalamin in a patient abusing nitrous oxide and cannabis. Psychosomatics. 2014;55(6):715-719.
24. Yajnik S, Thapar P, Lichtor JL, et al. Effects of marijuana history on the subjective, psychomotor, and reinforcing effects of nitrous oxide in human. Drug Alcohol Depend. 1994;36(3):227-236.
25. Nagele P, Duma A, Kopec M, et al. Nitrous oxide for treatment-resistant major depression: a proof-of-concept trial. Biol Psychiatry. 2015;78(1):10-18.
26. Zorumski CF, Nagele P, Mennerick S, et al. Treatment-resistant major depression: rationale for NMDA receptors as targets and nitrous oxide as therapy. Front Psychiatry. 2015;6:172.
27. Shear MK. Clinical practice. Complicated grief. N Engl J Med. 2015;372(2):153-160.
28. Knechtli CJC, Crowe JN. Guidelines for the investigation & management of vitamin B12 deficiency. Royal United Hospital Bath, National Health Service. http://www.ruh.nhs.uk/For_Clinicians/departments_ruh/Pathology/documents/haematology/B12_-_advice_on_investigation_management.pdf. Accessed June 14, 2016.
29. Jayaram N, Rao MG, Narashima A, et al. Vitamin B12 levels and psychiatric symptomatology: a case series. J Neuropsychiatry Clin Neurosci. 2013;25(2):150-152.
30. Marks PW, Zukerberg LR. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 30-2004. A 37-year-old woman with paresthesias of the arms and legs. N Engl J Med. 2004;351(13):1333-1341.
31. Watanabe F. Vitamin B12 sources and bioavailablility. Exp Biol Med (Maywood). 2007;232(10):1266-1274.
32. Green R, Kinsella LJ. Current concepts in the diagnosis of cobalamin deficiency. Neurology. 1995;45(8):1435-1440.
The art of psychopharmacology: Avoiding medication changes and slowing down
As physicians, we are cognizant of the importance of patient-centered care, active listening, empathy, and patience—the so-called “hidden curriculum of medicine.”1 However, our attempts to centralize these concepts may be overshadowed by the deeply rooted drive to treat and fix. At times, we are simply treating uncertainty, whether it be diagnostic uncertainty or the uncertainty arising from clinical responses and outcomes that are far from binary. Definitive actions, such as adding medications or altering dosages, may appear to both patients and physicians to be a step closer to a “cure.” However, watchful waiting, re-evaluation, and accepting uncertainty are the true skills of effective care.
Be savvy about psychopharmacology
Psychotropics can take weeks to months to reach their full potential, and have varying responses and adverse effects. Beware of changing regimens prematurely, and keep in mind basic, yet crucial, pharmacokinetic concepts (eg, 4 to 5 half-lives to reach steady state, variations in metabolism). Receptor binding and dosing heuristics are notably common in psychiatry. Although such concepts are important to grasp, there is no one-size-fits-all rule. The brain simply does not possess the heart’s machine-like, linear functioning. Therefore, targeting individual parts (ie, receptors) will not equate to fixing the whole organ systematically or predictably.
Is the patient truly treatment-resistant?
Even the best treatment regimen has no clinical benefit if the patient cannot afford the prescription or does not take the medication. If cost is an impediment, switch from brand name drugs to generic formulations or to older medications in the same class. Before declaring the patient “treatment-resistant” and making medication changes, assess for compliance. This may require assistance from collateral informants. Ask family members to count the number of pills remaining in the bottle, and call the pharmacy to find out the last refill dates. If the patient exhibits a partial response to what should be a therapeutic dose, consider obtaining drug plasma levels to rule out rapid metabolism before deeming the medication trial a failure.2
Medications as liabilities
Overreliance on medications can result in the medications becoming liabilities. The polypharmacy problem is not unique to psychiatry.3 However, psychiatric patients may be more likely to inadvertently use medications in a maladaptive manner and disrupt the fundamental goals of long-term care. Avoid making medication adjustments in response to a patient’s life stressors and normative situational reactions. Doing so is a disservice to patients, because we are robbing them of chances to develop necessary coping skills and defenses. This can be overtly damaging in certain patient populations, such as those with borderline personality disorder, who may use medication adjustments as a crutch during crises.4
Treat the patient, not yourself
We physicians mean well in prescribing evidence-based treatments; however, if the symptoms or adverse effects are not bothersome or cause functional impairment, we risk losing sight of the patient’s goals in treatment and imposing our own instead. Displacing the treatment focus can alienate the patient, harm the therapeutic alliance, and result in “pill fatigue.” For example, we may be tempted to treat antipsychotic-induced tardive dyskinesia, even if the patient is not concerned about abnormal movements. Although we see this adverse effect
Change does not happen overnight
Picking a treatment option out of a lineup of choices, à la UWorld questions, does not always translate into patients agreeing with the suggested treatment, let alone the idea of receiving treatment at all. Motivational interviewing is our chance to shine in such situations and the reason why we are physicians, rather than answer-picking bots. Patients cannot change if they are not ready. However, we should be ready to roll with resistance while looking for signs of readiness to change. We must accept that it may take a week, a month, a year, or even longer for patients to align with our plan of action. The only futile decision is deeming our efforts as futile while discounting the benefits of incremental care.
1. Hafferty FW, Gaufberg EH, O’Donnell JF. The role of the hidden curriculum in “on doctoring” courses. AMA J Ethics. 2015;17(2):130-139.
2. Horvitz-Lennon M, Mattke S, Predmore Z, et al. The role of antipsychotic plasma levels in the treatment of schizophrenia. Am J Psychiatry. 2017;174(5):421-426.
3. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831.
4. Gunderson JG. The emergence of a generalist model to meet public health needs for patients with borderline personality disorder. Am J Psychiatry. 2016;173(5):452-458.
5. Kikkert MJ, Schene AH, Koeter MW, et al. Medication adherence in schizophrenia: exploring patients’, carers’ and professionals’ views. Schizophr Bull. 2005;32(4):786-794.
As physicians, we are cognizant of the importance of patient-centered care, active listening, empathy, and patience—the so-called “hidden curriculum of medicine.”1 However, our attempts to centralize these concepts may be overshadowed by the deeply rooted drive to treat and fix. At times, we are simply treating uncertainty, whether it be diagnostic uncertainty or the uncertainty arising from clinical responses and outcomes that are far from binary. Definitive actions, such as adding medications or altering dosages, may appear to both patients and physicians to be a step closer to a “cure.” However, watchful waiting, re-evaluation, and accepting uncertainty are the true skills of effective care.
Be savvy about psychopharmacology
Psychotropics can take weeks to months to reach their full potential, and have varying responses and adverse effects. Beware of changing regimens prematurely, and keep in mind basic, yet crucial, pharmacokinetic concepts (eg, 4 to 5 half-lives to reach steady state, variations in metabolism). Receptor binding and dosing heuristics are notably common in psychiatry. Although such concepts are important to grasp, there is no one-size-fits-all rule. The brain simply does not possess the heart’s machine-like, linear functioning. Therefore, targeting individual parts (ie, receptors) will not equate to fixing the whole organ systematically or predictably.
Is the patient truly treatment-resistant?
Even the best treatment regimen has no clinical benefit if the patient cannot afford the prescription or does not take the medication. If cost is an impediment, switch from brand name drugs to generic formulations or to older medications in the same class. Before declaring the patient “treatment-resistant” and making medication changes, assess for compliance. This may require assistance from collateral informants. Ask family members to count the number of pills remaining in the bottle, and call the pharmacy to find out the last refill dates. If the patient exhibits a partial response to what should be a therapeutic dose, consider obtaining drug plasma levels to rule out rapid metabolism before deeming the medication trial a failure.2
Medications as liabilities
Overreliance on medications can result in the medications becoming liabilities. The polypharmacy problem is not unique to psychiatry.3 However, psychiatric patients may be more likely to inadvertently use medications in a maladaptive manner and disrupt the fundamental goals of long-term care. Avoid making medication adjustments in response to a patient’s life stressors and normative situational reactions. Doing so is a disservice to patients, because we are robbing them of chances to develop necessary coping skills and defenses. This can be overtly damaging in certain patient populations, such as those with borderline personality disorder, who may use medication adjustments as a crutch during crises.4
Treat the patient, not yourself
We physicians mean well in prescribing evidence-based treatments; however, if the symptoms or adverse effects are not bothersome or cause functional impairment, we risk losing sight of the patient’s goals in treatment and imposing our own instead. Displacing the treatment focus can alienate the patient, harm the therapeutic alliance, and result in “pill fatigue.” For example, we may be tempted to treat antipsychotic-induced tardive dyskinesia, even if the patient is not concerned about abnormal movements. Although we see this adverse effect
Change does not happen overnight
Picking a treatment option out of a lineup of choices, à la UWorld questions, does not always translate into patients agreeing with the suggested treatment, let alone the idea of receiving treatment at all. Motivational interviewing is our chance to shine in such situations and the reason why we are physicians, rather than answer-picking bots. Patients cannot change if they are not ready. However, we should be ready to roll with resistance while looking for signs of readiness to change. We must accept that it may take a week, a month, a year, or even longer for patients to align with our plan of action. The only futile decision is deeming our efforts as futile while discounting the benefits of incremental care.
As physicians, we are cognizant of the importance of patient-centered care, active listening, empathy, and patience—the so-called “hidden curriculum of medicine.”1 However, our attempts to centralize these concepts may be overshadowed by the deeply rooted drive to treat and fix. At times, we are simply treating uncertainty, whether it be diagnostic uncertainty or the uncertainty arising from clinical responses and outcomes that are far from binary. Definitive actions, such as adding medications or altering dosages, may appear to both patients and physicians to be a step closer to a “cure.” However, watchful waiting, re-evaluation, and accepting uncertainty are the true skills of effective care.
Be savvy about psychopharmacology
Psychotropics can take weeks to months to reach their full potential, and have varying responses and adverse effects. Beware of changing regimens prematurely, and keep in mind basic, yet crucial, pharmacokinetic concepts (eg, 4 to 5 half-lives to reach steady state, variations in metabolism). Receptor binding and dosing heuristics are notably common in psychiatry. Although such concepts are important to grasp, there is no one-size-fits-all rule. The brain simply does not possess the heart’s machine-like, linear functioning. Therefore, targeting individual parts (ie, receptors) will not equate to fixing the whole organ systematically or predictably.
Is the patient truly treatment-resistant?
Even the best treatment regimen has no clinical benefit if the patient cannot afford the prescription or does not take the medication. If cost is an impediment, switch from brand name drugs to generic formulations or to older medications in the same class. Before declaring the patient “treatment-resistant” and making medication changes, assess for compliance. This may require assistance from collateral informants. Ask family members to count the number of pills remaining in the bottle, and call the pharmacy to find out the last refill dates. If the patient exhibits a partial response to what should be a therapeutic dose, consider obtaining drug plasma levels to rule out rapid metabolism before deeming the medication trial a failure.2
Medications as liabilities
Overreliance on medications can result in the medications becoming liabilities. The polypharmacy problem is not unique to psychiatry.3 However, psychiatric patients may be more likely to inadvertently use medications in a maladaptive manner and disrupt the fundamental goals of long-term care. Avoid making medication adjustments in response to a patient’s life stressors and normative situational reactions. Doing so is a disservice to patients, because we are robbing them of chances to develop necessary coping skills and defenses. This can be overtly damaging in certain patient populations, such as those with borderline personality disorder, who may use medication adjustments as a crutch during crises.4
Treat the patient, not yourself
We physicians mean well in prescribing evidence-based treatments; however, if the symptoms or adverse effects are not bothersome or cause functional impairment, we risk losing sight of the patient’s goals in treatment and imposing our own instead. Displacing the treatment focus can alienate the patient, harm the therapeutic alliance, and result in “pill fatigue.” For example, we may be tempted to treat antipsychotic-induced tardive dyskinesia, even if the patient is not concerned about abnormal movements. Although we see this adverse effect
Change does not happen overnight
Picking a treatment option out of a lineup of choices, à la UWorld questions, does not always translate into patients agreeing with the suggested treatment, let alone the idea of receiving treatment at all. Motivational interviewing is our chance to shine in such situations and the reason why we are physicians, rather than answer-picking bots. Patients cannot change if they are not ready. However, we should be ready to roll with resistance while looking for signs of readiness to change. We must accept that it may take a week, a month, a year, or even longer for patients to align with our plan of action. The only futile decision is deeming our efforts as futile while discounting the benefits of incremental care.
1. Hafferty FW, Gaufberg EH, O’Donnell JF. The role of the hidden curriculum in “on doctoring” courses. AMA J Ethics. 2015;17(2):130-139.
2. Horvitz-Lennon M, Mattke S, Predmore Z, et al. The role of antipsychotic plasma levels in the treatment of schizophrenia. Am J Psychiatry. 2017;174(5):421-426.
3. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831.
4. Gunderson JG. The emergence of a generalist model to meet public health needs for patients with borderline personality disorder. Am J Psychiatry. 2016;173(5):452-458.
5. Kikkert MJ, Schene AH, Koeter MW, et al. Medication adherence in schizophrenia: exploring patients’, carers’ and professionals’ views. Schizophr Bull. 2005;32(4):786-794.
1. Hafferty FW, Gaufberg EH, O’Donnell JF. The role of the hidden curriculum in “on doctoring” courses. AMA J Ethics. 2015;17(2):130-139.
2. Horvitz-Lennon M, Mattke S, Predmore Z, et al. The role of antipsychotic plasma levels in the treatment of schizophrenia. Am J Psychiatry. 2017;174(5):421-426.
3. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831.
4. Gunderson JG. The emergence of a generalist model to meet public health needs for patients with borderline personality disorder. Am J Psychiatry. 2016;173(5):452-458.
5. Kikkert MJ, Schene AH, Koeter MW, et al. Medication adherence in schizophrenia: exploring patients’, carers’ and professionals’ views. Schizophr Bull. 2005;32(4):786-794.
Providing psychotherapy? Keep these principles in mind
Although the biological aspects of psychiatry are crucial, psychotherapy is an integral part of psychiatry. Unfortunately, the emphasis on psychotherapy training in psychiatry residency programs has declined compared with a decade or more ago. In an era of dwindling psychotherapy training and resources, the quality and type of psychotherapy training has become more variable. In addition to helping maintain the therapeutic alliance, nuanced psychotherapy by a trained professional can be transformational by helping patients to:
- process complex life events and emotions
- feel understood
- overcome psychological barriers to recovery
- enhance self-esteem.
When providing psychotherapy for adult patients, consider these basic, but salient points that are often overlooked.
Refrain from making life decisions for patients, except in exceptional circumstances, such as in situations of abuse and other crises.1 Telling an adult patient what to do about life decisions that he finds challenging fits more under life coaching than psychotherapy. Through therapy, patients should be helped in processing the pros and cons of certain decisions and in navigating the decision-making process to arrive at a decision that makes the most sense to them. Also, it’s not uncommon for therapeutic relationships to rupture when therapists give advice such as suggesting that a patient divorce his spouse, date a certain individual, or have children.
There are many reasons why giving advice in psychotherapy is not recommended. Giving advice can be an impediment to the therapeutic process.2 What is good advice for one patient may not be good for another. Therapists who give advice often do so from their own lens and perspective. This perspective may not only be different from the patient’s priorities and life circumstances, but the therapist also may have inadequate information about the patient’s situation,1,2 which could lead to providing advice that could even harm the patient. In addition, providing advice might prevent a patient from gaining adequate agency or self-directedness while promoting an unhealthy dependence on the therapist and reinforcing the patient’s self-doubt or lack of confidence. In these cases, the patient may later resent the therapist for the advice.
Address the ‘here and now.’1 Pay attention to immediate issues or themes that emerge, and address them with the patient gently and thoughtfully, as appropriate. Ignoring these may create risks of missing vital, underlying material that could reveal more of the patient’s inner world, as these themes can sometimes reflect other themes of the patient’s life outside of treatment.
Acknowledging and empathizing, when appropriate, are key initial steps that help decrease resistance and facilitate the therapeutic process.
Explore the affect. Paying attention to the patient’s emotional state is critical.3 This holds true for all types of psychotherapy. For example, if a patient suddenly becomes tearful when telling his story or describing recent events, this is usually a sign that the subject matter affects or holds value to the patient in a significant or meaningful way and should be further explored.
‘Meet the patient where they are.’ This doesn’t mean you should yield to the patient or give in to his demands. It implies that you should assess the patient’s readiness for a particular intervention and devise interventions from that standpoint, exploring the patient’s ambivalence, noticing resistance, and continuing to acknowledge and empathize with where the patient is in life or treatment. When utilized judiciously, this technique can help the therapist align with the patient, and help the patient move forward through resistance and ambivalence.
Be nonjudgmental and empathetic. Patients place trust in their therapists when they disclose thoughts or emotions that are sensitive, meaningful, or close to the heart. A nonjudgmental response helps the patient accept his experiences and emotions. Being empathetic requires putting oneself in another’s shoes; it does not mean agreeing with the patient. Of course, if you learn that your patient abused a child or an older adult, you are required to report it to the appropriate state agency. In addition, follow the duty to warn and protect in case of any other safety issues, as appropriate.
Do not assume. Open-ended questions and exploration are key. For example, a patient told her resident therapist that her father recently passed away. The therapist expressed to the patient how hard this must be for her. However, the patient said she was relieved by her father’s death, because he had been abusive to her for years. Because of the therapist’s comment, the patient doubted her own reaction and felt guilty for not being more upset about her father’s death.
Avoid over-identifying with your patient. If you find yourself over-identifying with a patient because you have a common background or life events, seek supervision. Over-identification not only can pose barriers to objectively identifying patterns and trends in the patient’s behavior or presentation but also can increase the risk of crossing boundaries or even minimizing the patient’s experience. Exercise caution if you find yourself wanting to be liked by your patient; this is a common mistake among beginning therapists.4
Seek supervision. If you are feeling angry, frustrated, indifferent, or overly attached toward a patient, recognize this countertransference and seek consultation or supervision from an experienced colleague or supervisor. These emotions can be valuable tools that shed light not only on the patient’s life and the session itself, but also help you identify any other factors, such as your own feelings or experiences, that might be contributing to these reactions.
1. Yalom ID. The gift of therapy: an open letter to a new generation of therapists and their patients. New York, NY: HarperCollins Publishers; 2002:46-73,142-145.
2. Bender S, Messner E. Management of impasses. In: Bender S, Messner E. Becoming a therapist: what do I say, and why? New York, NY: The Guilford Press; 2003:235-258.
3.
4. Buckley P, Karasu TB, Charles E. Common mistakes in psychotherapy. Am J Psychiatry. 1979;136(12):1578-1580.
Although the biological aspects of psychiatry are crucial, psychotherapy is an integral part of psychiatry. Unfortunately, the emphasis on psychotherapy training in psychiatry residency programs has declined compared with a decade or more ago. In an era of dwindling psychotherapy training and resources, the quality and type of psychotherapy training has become more variable. In addition to helping maintain the therapeutic alliance, nuanced psychotherapy by a trained professional can be transformational by helping patients to:
- process complex life events and emotions
- feel understood
- overcome psychological barriers to recovery
- enhance self-esteem.
When providing psychotherapy for adult patients, consider these basic, but salient points that are often overlooked.
Refrain from making life decisions for patients, except in exceptional circumstances, such as in situations of abuse and other crises.1 Telling an adult patient what to do about life decisions that he finds challenging fits more under life coaching than psychotherapy. Through therapy, patients should be helped in processing the pros and cons of certain decisions and in navigating the decision-making process to arrive at a decision that makes the most sense to them. Also, it’s not uncommon for therapeutic relationships to rupture when therapists give advice such as suggesting that a patient divorce his spouse, date a certain individual, or have children.
There are many reasons why giving advice in psychotherapy is not recommended. Giving advice can be an impediment to the therapeutic process.2 What is good advice for one patient may not be good for another. Therapists who give advice often do so from their own lens and perspective. This perspective may not only be different from the patient’s priorities and life circumstances, but the therapist also may have inadequate information about the patient’s situation,1,2 which could lead to providing advice that could even harm the patient. In addition, providing advice might prevent a patient from gaining adequate agency or self-directedness while promoting an unhealthy dependence on the therapist and reinforcing the patient’s self-doubt or lack of confidence. In these cases, the patient may later resent the therapist for the advice.
Address the ‘here and now.’1 Pay attention to immediate issues or themes that emerge, and address them with the patient gently and thoughtfully, as appropriate. Ignoring these may create risks of missing vital, underlying material that could reveal more of the patient’s inner world, as these themes can sometimes reflect other themes of the patient’s life outside of treatment.
Acknowledging and empathizing, when appropriate, are key initial steps that help decrease resistance and facilitate the therapeutic process.
Explore the affect. Paying attention to the patient’s emotional state is critical.3 This holds true for all types of psychotherapy. For example, if a patient suddenly becomes tearful when telling his story or describing recent events, this is usually a sign that the subject matter affects or holds value to the patient in a significant or meaningful way and should be further explored.
‘Meet the patient where they are.’ This doesn’t mean you should yield to the patient or give in to his demands. It implies that you should assess the patient’s readiness for a particular intervention and devise interventions from that standpoint, exploring the patient’s ambivalence, noticing resistance, and continuing to acknowledge and empathize with where the patient is in life or treatment. When utilized judiciously, this technique can help the therapist align with the patient, and help the patient move forward through resistance and ambivalence.
Be nonjudgmental and empathetic. Patients place trust in their therapists when they disclose thoughts or emotions that are sensitive, meaningful, or close to the heart. A nonjudgmental response helps the patient accept his experiences and emotions. Being empathetic requires putting oneself in another’s shoes; it does not mean agreeing with the patient. Of course, if you learn that your patient abused a child or an older adult, you are required to report it to the appropriate state agency. In addition, follow the duty to warn and protect in case of any other safety issues, as appropriate.
Do not assume. Open-ended questions and exploration are key. For example, a patient told her resident therapist that her father recently passed away. The therapist expressed to the patient how hard this must be for her. However, the patient said she was relieved by her father’s death, because he had been abusive to her for years. Because of the therapist’s comment, the patient doubted her own reaction and felt guilty for not being more upset about her father’s death.
Avoid over-identifying with your patient. If you find yourself over-identifying with a patient because you have a common background or life events, seek supervision. Over-identification not only can pose barriers to objectively identifying patterns and trends in the patient’s behavior or presentation but also can increase the risk of crossing boundaries or even minimizing the patient’s experience. Exercise caution if you find yourself wanting to be liked by your patient; this is a common mistake among beginning therapists.4
Seek supervision. If you are feeling angry, frustrated, indifferent, or overly attached toward a patient, recognize this countertransference and seek consultation or supervision from an experienced colleague or supervisor. These emotions can be valuable tools that shed light not only on the patient’s life and the session itself, but also help you identify any other factors, such as your own feelings or experiences, that might be contributing to these reactions.
Although the biological aspects of psychiatry are crucial, psychotherapy is an integral part of psychiatry. Unfortunately, the emphasis on psychotherapy training in psychiatry residency programs has declined compared with a decade or more ago. In an era of dwindling psychotherapy training and resources, the quality and type of psychotherapy training has become more variable. In addition to helping maintain the therapeutic alliance, nuanced psychotherapy by a trained professional can be transformational by helping patients to:
- process complex life events and emotions
- feel understood
- overcome psychological barriers to recovery
- enhance self-esteem.
When providing psychotherapy for adult patients, consider these basic, but salient points that are often overlooked.
Refrain from making life decisions for patients, except in exceptional circumstances, such as in situations of abuse and other crises.1 Telling an adult patient what to do about life decisions that he finds challenging fits more under life coaching than psychotherapy. Through therapy, patients should be helped in processing the pros and cons of certain decisions and in navigating the decision-making process to arrive at a decision that makes the most sense to them. Also, it’s not uncommon for therapeutic relationships to rupture when therapists give advice such as suggesting that a patient divorce his spouse, date a certain individual, or have children.
There are many reasons why giving advice in psychotherapy is not recommended. Giving advice can be an impediment to the therapeutic process.2 What is good advice for one patient may not be good for another. Therapists who give advice often do so from their own lens and perspective. This perspective may not only be different from the patient’s priorities and life circumstances, but the therapist also may have inadequate information about the patient’s situation,1,2 which could lead to providing advice that could even harm the patient. In addition, providing advice might prevent a patient from gaining adequate agency or self-directedness while promoting an unhealthy dependence on the therapist and reinforcing the patient’s self-doubt or lack of confidence. In these cases, the patient may later resent the therapist for the advice.
Address the ‘here and now.’1 Pay attention to immediate issues or themes that emerge, and address them with the patient gently and thoughtfully, as appropriate. Ignoring these may create risks of missing vital, underlying material that could reveal more of the patient’s inner world, as these themes can sometimes reflect other themes of the patient’s life outside of treatment.
Acknowledging and empathizing, when appropriate, are key initial steps that help decrease resistance and facilitate the therapeutic process.
Explore the affect. Paying attention to the patient’s emotional state is critical.3 This holds true for all types of psychotherapy. For example, if a patient suddenly becomes tearful when telling his story or describing recent events, this is usually a sign that the subject matter affects or holds value to the patient in a significant or meaningful way and should be further explored.
‘Meet the patient where they are.’ This doesn’t mean you should yield to the patient or give in to his demands. It implies that you should assess the patient’s readiness for a particular intervention and devise interventions from that standpoint, exploring the patient’s ambivalence, noticing resistance, and continuing to acknowledge and empathize with where the patient is in life or treatment. When utilized judiciously, this technique can help the therapist align with the patient, and help the patient move forward through resistance and ambivalence.
Be nonjudgmental and empathetic. Patients place trust in their therapists when they disclose thoughts or emotions that are sensitive, meaningful, or close to the heart. A nonjudgmental response helps the patient accept his experiences and emotions. Being empathetic requires putting oneself in another’s shoes; it does not mean agreeing with the patient. Of course, if you learn that your patient abused a child or an older adult, you are required to report it to the appropriate state agency. In addition, follow the duty to warn and protect in case of any other safety issues, as appropriate.
Do not assume. Open-ended questions and exploration are key. For example, a patient told her resident therapist that her father recently passed away. The therapist expressed to the patient how hard this must be for her. However, the patient said she was relieved by her father’s death, because he had been abusive to her for years. Because of the therapist’s comment, the patient doubted her own reaction and felt guilty for not being more upset about her father’s death.
Avoid over-identifying with your patient. If you find yourself over-identifying with a patient because you have a common background or life events, seek supervision. Over-identification not only can pose barriers to objectively identifying patterns and trends in the patient’s behavior or presentation but also can increase the risk of crossing boundaries or even minimizing the patient’s experience. Exercise caution if you find yourself wanting to be liked by your patient; this is a common mistake among beginning therapists.4
Seek supervision. If you are feeling angry, frustrated, indifferent, or overly attached toward a patient, recognize this countertransference and seek consultation or supervision from an experienced colleague or supervisor. These emotions can be valuable tools that shed light not only on the patient’s life and the session itself, but also help you identify any other factors, such as your own feelings or experiences, that might be contributing to these reactions.
1. Yalom ID. The gift of therapy: an open letter to a new generation of therapists and their patients. New York, NY: HarperCollins Publishers; 2002:46-73,142-145.
2. Bender S, Messner E. Management of impasses. In: Bender S, Messner E. Becoming a therapist: what do I say, and why? New York, NY: The Guilford Press; 2003:235-258.
3.
4. Buckley P, Karasu TB, Charles E. Common mistakes in psychotherapy. Am J Psychiatry. 1979;136(12):1578-1580.
1. Yalom ID. The gift of therapy: an open letter to a new generation of therapists and their patients. New York, NY: HarperCollins Publishers; 2002:46-73,142-145.
2. Bender S, Messner E. Management of impasses. In: Bender S, Messner E. Becoming a therapist: what do I say, and why? New York, NY: The Guilford Press; 2003:235-258.
3.
4. Buckley P, Karasu TB, Charles E. Common mistakes in psychotherapy. Am J Psychiatry. 1979;136(12):1578-1580.
Use the ABCs when managing problem behaviors in autism
Despite a lack of evidence, polypharmacy often is used to treat autism spectrum disorder (ASD),1 while educational techniques are underutilized. Compared with the general population, children with ASD may be more prone to the adverse effects of the medications used to treat symptoms, such as antipsychotics and antidepressants.2 Therefore, when addressing problem behaviors, such as tantrums, aggressiveness, or self-injury, in a patient with ASD, before prescribing a medication, consider the ABCs of these behaviors.3
Antecedents. What happened before the behavior occurred? Where and when did the behavior occur? Was the individual unable to get a desired tangible item, such as a preferred food, toy, or another object? Was the individual told complete a task that he (she) did not want to do? Did the individual see someone else getting attention?
Behaviors. What behavior(s) occurred after each antecedent?
Consequences. What happened after the behavior occurred? Did the caregiver give the individual the item he wanted? Was the individual able to get out of doing work that he did not want to do or become the center of attention?
Having parents document the ABCs is useful not only for finding out why a behavior occurred, but also for objectively determining if and how a medication is affecting the frequency of a behavior. Charts that parents can use to document ABC data are available online (eg, http://www.positivelyautism.com/downloads/datasheet_abc.pdf). Once this data is collected, it can be used to implement appropriate interventions, which I describe as DEFG.
Differential reinforcement of other behaviors is a procedure that provides positive reinforcement for not engaging in a problem behavior or for staying on task. For example, use a token board to reward positive behaviors, with physical tokens or written marks. However, some patients require immediate reinforcement. I suggest that parents or caregivers carry small pieces of preferred food to give to the patient to reinforce positive behavior.
Exercise. A review of 18 studies reported that physical exercise, such as jogging, weight training, and bike riding, can help reduce problem behaviors in individuals with ASD.4 Among 64 participants with ASD, there was a decrease in aggression, stereotypy, off-task behavior, and elopement, and improvements in on-task and motor behavior such as playing catch.
Function. Refer to the ABCs to determine why a specific problem behavior is occurring. Each behavior can have 1 or multiple functions; therefore, develop a plan specific to the reason the patient engages in the behavior. For example, if the individual engages in a behavior to avoid a task, the parent or caregiver can give individual tokens that the individual can later exchange for a break, instead of engaging in the problem behavior to avoid the task. If a behavior appears to be done for attention, instruct the caregivers to provide frequent periods of attention when the individual engages in positive behaviors.
Go to the appropriate placement. By law, persons age ≤21 have the right to an education and to make meaningful progress. If a patient with ASD exhibits behaviors that interfere with learning, he is entitled to a placement that can provide intensive applied behavior analysis. If you feel that the child needs a different school, write an evaluation for the parent or guardian to submit to the school district and clearly outline the patient’s needs and requirements.
1. Spencer D, Marshall J, Post B, et al. Psychotropic medication use and polypharmacy in children with autism spectrum disorders. Pediatrics. 2013;132(5):833-840.
2. Azeem MW, Imran N, Khawaja IS. Autism spectrum disorder: an update. Psychiatr Ann. 2016;46(1):58-62.
3. Pratt C, Dubie M. Observing behavior using A-B-C data. Indiana Resource Center for Autism. https://www.iidc.indiana.edu/pages/observing-behavior-using-a-b-c-data. Accessed October 4, 2017.
4. Lang R, Kern Koegel LK, Ashbaugh K, et al. Physical exercise and individuals with autism spectrum disorders: a systematic review. Res Autism Spectr Dis. 2010;4(4):565-576.
Despite a lack of evidence, polypharmacy often is used to treat autism spectrum disorder (ASD),1 while educational techniques are underutilized. Compared with the general population, children with ASD may be more prone to the adverse effects of the medications used to treat symptoms, such as antipsychotics and antidepressants.2 Therefore, when addressing problem behaviors, such as tantrums, aggressiveness, or self-injury, in a patient with ASD, before prescribing a medication, consider the ABCs of these behaviors.3
Antecedents. What happened before the behavior occurred? Where and when did the behavior occur? Was the individual unable to get a desired tangible item, such as a preferred food, toy, or another object? Was the individual told complete a task that he (she) did not want to do? Did the individual see someone else getting attention?
Behaviors. What behavior(s) occurred after each antecedent?
Consequences. What happened after the behavior occurred? Did the caregiver give the individual the item he wanted? Was the individual able to get out of doing work that he did not want to do or become the center of attention?
Having parents document the ABCs is useful not only for finding out why a behavior occurred, but also for objectively determining if and how a medication is affecting the frequency of a behavior. Charts that parents can use to document ABC data are available online (eg, http://www.positivelyautism.com/downloads/datasheet_abc.pdf). Once this data is collected, it can be used to implement appropriate interventions, which I describe as DEFG.
Differential reinforcement of other behaviors is a procedure that provides positive reinforcement for not engaging in a problem behavior or for staying on task. For example, use a token board to reward positive behaviors, with physical tokens or written marks. However, some patients require immediate reinforcement. I suggest that parents or caregivers carry small pieces of preferred food to give to the patient to reinforce positive behavior.
Exercise. A review of 18 studies reported that physical exercise, such as jogging, weight training, and bike riding, can help reduce problem behaviors in individuals with ASD.4 Among 64 participants with ASD, there was a decrease in aggression, stereotypy, off-task behavior, and elopement, and improvements in on-task and motor behavior such as playing catch.
Function. Refer to the ABCs to determine why a specific problem behavior is occurring. Each behavior can have 1 or multiple functions; therefore, develop a plan specific to the reason the patient engages in the behavior. For example, if the individual engages in a behavior to avoid a task, the parent or caregiver can give individual tokens that the individual can later exchange for a break, instead of engaging in the problem behavior to avoid the task. If a behavior appears to be done for attention, instruct the caregivers to provide frequent periods of attention when the individual engages in positive behaviors.
Go to the appropriate placement. By law, persons age ≤21 have the right to an education and to make meaningful progress. If a patient with ASD exhibits behaviors that interfere with learning, he is entitled to a placement that can provide intensive applied behavior analysis. If you feel that the child needs a different school, write an evaluation for the parent or guardian to submit to the school district and clearly outline the patient’s needs and requirements.
Despite a lack of evidence, polypharmacy often is used to treat autism spectrum disorder (ASD),1 while educational techniques are underutilized. Compared with the general population, children with ASD may be more prone to the adverse effects of the medications used to treat symptoms, such as antipsychotics and antidepressants.2 Therefore, when addressing problem behaviors, such as tantrums, aggressiveness, or self-injury, in a patient with ASD, before prescribing a medication, consider the ABCs of these behaviors.3
Antecedents. What happened before the behavior occurred? Where and when did the behavior occur? Was the individual unable to get a desired tangible item, such as a preferred food, toy, or another object? Was the individual told complete a task that he (she) did not want to do? Did the individual see someone else getting attention?
Behaviors. What behavior(s) occurred after each antecedent?
Consequences. What happened after the behavior occurred? Did the caregiver give the individual the item he wanted? Was the individual able to get out of doing work that he did not want to do or become the center of attention?
Having parents document the ABCs is useful not only for finding out why a behavior occurred, but also for objectively determining if and how a medication is affecting the frequency of a behavior. Charts that parents can use to document ABC data are available online (eg, http://www.positivelyautism.com/downloads/datasheet_abc.pdf). Once this data is collected, it can be used to implement appropriate interventions, which I describe as DEFG.
Differential reinforcement of other behaviors is a procedure that provides positive reinforcement for not engaging in a problem behavior or for staying on task. For example, use a token board to reward positive behaviors, with physical tokens or written marks. However, some patients require immediate reinforcement. I suggest that parents or caregivers carry small pieces of preferred food to give to the patient to reinforce positive behavior.
Exercise. A review of 18 studies reported that physical exercise, such as jogging, weight training, and bike riding, can help reduce problem behaviors in individuals with ASD.4 Among 64 participants with ASD, there was a decrease in aggression, stereotypy, off-task behavior, and elopement, and improvements in on-task and motor behavior such as playing catch.
Function. Refer to the ABCs to determine why a specific problem behavior is occurring. Each behavior can have 1 or multiple functions; therefore, develop a plan specific to the reason the patient engages in the behavior. For example, if the individual engages in a behavior to avoid a task, the parent or caregiver can give individual tokens that the individual can later exchange for a break, instead of engaging in the problem behavior to avoid the task. If a behavior appears to be done for attention, instruct the caregivers to provide frequent periods of attention when the individual engages in positive behaviors.
Go to the appropriate placement. By law, persons age ≤21 have the right to an education and to make meaningful progress. If a patient with ASD exhibits behaviors that interfere with learning, he is entitled to a placement that can provide intensive applied behavior analysis. If you feel that the child needs a different school, write an evaluation for the parent or guardian to submit to the school district and clearly outline the patient’s needs and requirements.
1. Spencer D, Marshall J, Post B, et al. Psychotropic medication use and polypharmacy in children with autism spectrum disorders. Pediatrics. 2013;132(5):833-840.
2. Azeem MW, Imran N, Khawaja IS. Autism spectrum disorder: an update. Psychiatr Ann. 2016;46(1):58-62.
3. Pratt C, Dubie M. Observing behavior using A-B-C data. Indiana Resource Center for Autism. https://www.iidc.indiana.edu/pages/observing-behavior-using-a-b-c-data. Accessed October 4, 2017.
4. Lang R, Kern Koegel LK, Ashbaugh K, et al. Physical exercise and individuals with autism spectrum disorders: a systematic review. Res Autism Spectr Dis. 2010;4(4):565-576.
1. Spencer D, Marshall J, Post B, et al. Psychotropic medication use and polypharmacy in children with autism spectrum disorders. Pediatrics. 2013;132(5):833-840.
2. Azeem MW, Imran N, Khawaja IS. Autism spectrum disorder: an update. Psychiatr Ann. 2016;46(1):58-62.
3. Pratt C, Dubie M. Observing behavior using A-B-C data. Indiana Resource Center for Autism. https://www.iidc.indiana.edu/pages/observing-behavior-using-a-b-c-data. Accessed October 4, 2017.
4. Lang R, Kern Koegel LK, Ashbaugh K, et al. Physical exercise and individuals with autism spectrum disorders: a systematic review. Res Autism Spectr Dis. 2010;4(4):565-576.
Employment contracts: What to check before you sign
Most psychiatrists are required to sign an employment contract before taking a job, but few of us have received any training on reviewing such contracts. We often rely on coworkers and attorneys to navigate this process for us. However, the contract is crucial, because it outlines your employer’s clinical and administrative expectations for the position, and it gives you the opportunity to lay out what you want.1 Because an employment contract is legally binding, you should thoroughly read it and look for clauses that may not work in your best interest. Although not a complete list, the following items should be reviewed before signing a contract.1,2
Benefits. Make sure you are offered a reasonable salary, but balance the dollar amount with benefits such as:
- continuing medical education allowances
- educational loan forgiveness
- health/malpractice/disability insurance
- retirement benefits
- compensation for call schedule.
In some cases, there may be a delay before you are eligible to obtain certain benefits.
Work expectations. Many contracts state that the position is “full-time” or have other nonspecific parameters for work expectations. You should inquire about objective work parameters, such as duty hours, the average frequency of the current call schedule, timeframe for completing medical documentation, and penalties for not meeting clinical or administrative requirements, so you are not surprised by:
- working longer-than-planned shifts
- performing on-call duties
- working on days that you were not expecting
- having your credentialing status placed in jeopardy.
Some group practices allow for a half-day of no scheduled appointments with patients, so you can complete paperwork and return phone calls.
Noncompete clause. This restricts you from working within a certain geographic area or for a competing employer for a finite time period after the contract terminates or expires. A noncompete clause could restrict you from practicing within a large geographical area, especially if the job is located in a densely populated area. Some noncompete clauses do not include a temporal or geographic restriction, but can limit your ability to bring patients with you to a new practice or facility when the contract expires.
Malpractice insurance. Two types of malpractice insurance are occurrence and claims-made:
- Occurrence insurance protects you whenever an action is brought against you, even if the action is brought after the contract terminates or expires.
- Claims-made insurance provides coverage if the policy with the same insurer was in effect when the malpractice was committed and when the actual action was commenced.
Although claims-made insurance is less expensive, it can leave you without coverage should you leave your employer and no longer maintain the same insurance policy. Claims-made can be converted into occurrence through the purchase of a tail endorsement. If the employer does not offer you tail coverage, then it is your responsibility to pay for this insurance, which can be expensive.
Termination language. Every contract features a termination section that lists potential causes for terminating your employment. This list is usually not exhaustive, but it sets the framework for a realistic view of reasonable causes. Contracts also commonly contain provisions that permit termination “without cause” after notice of termination is provided. Although you could negotiate for more notice time, “without cause” clauses are unlikely to be removed from the contract.
1. Claussen K. Eight physician employment contract items you need to know about. The Doctor Weighs In. https://thedoctorweighsin.com/8-physician-employment-contract-items-you-need-to-know-about. Published March 8, 2017. Accessed October 11, 2017.
2. Blustein AE, Keller LB. Physician employment contracts: what you need to know before you sign. J Am Acad Dermatol. https://www.aad.org/members/publications/directions-in-residency/archiveyment-contracts-what-you-need-to-know-before-you-sign. Accessed October 11, 2017.
Most psychiatrists are required to sign an employment contract before taking a job, but few of us have received any training on reviewing such contracts. We often rely on coworkers and attorneys to navigate this process for us. However, the contract is crucial, because it outlines your employer’s clinical and administrative expectations for the position, and it gives you the opportunity to lay out what you want.1 Because an employment contract is legally binding, you should thoroughly read it and look for clauses that may not work in your best interest. Although not a complete list, the following items should be reviewed before signing a contract.1,2
Benefits. Make sure you are offered a reasonable salary, but balance the dollar amount with benefits such as:
- continuing medical education allowances
- educational loan forgiveness
- health/malpractice/disability insurance
- retirement benefits
- compensation for call schedule.
In some cases, there may be a delay before you are eligible to obtain certain benefits.
Work expectations. Many contracts state that the position is “full-time” or have other nonspecific parameters for work expectations. You should inquire about objective work parameters, such as duty hours, the average frequency of the current call schedule, timeframe for completing medical documentation, and penalties for not meeting clinical or administrative requirements, so you are not surprised by:
- working longer-than-planned shifts
- performing on-call duties
- working on days that you were not expecting
- having your credentialing status placed in jeopardy.
Some group practices allow for a half-day of no scheduled appointments with patients, so you can complete paperwork and return phone calls.
Noncompete clause. This restricts you from working within a certain geographic area or for a competing employer for a finite time period after the contract terminates or expires. A noncompete clause could restrict you from practicing within a large geographical area, especially if the job is located in a densely populated area. Some noncompete clauses do not include a temporal or geographic restriction, but can limit your ability to bring patients with you to a new practice or facility when the contract expires.
Malpractice insurance. Two types of malpractice insurance are occurrence and claims-made:
- Occurrence insurance protects you whenever an action is brought against you, even if the action is brought after the contract terminates or expires.
- Claims-made insurance provides coverage if the policy with the same insurer was in effect when the malpractice was committed and when the actual action was commenced.
Although claims-made insurance is less expensive, it can leave you without coverage should you leave your employer and no longer maintain the same insurance policy. Claims-made can be converted into occurrence through the purchase of a tail endorsement. If the employer does not offer you tail coverage, then it is your responsibility to pay for this insurance, which can be expensive.
Termination language. Every contract features a termination section that lists potential causes for terminating your employment. This list is usually not exhaustive, but it sets the framework for a realistic view of reasonable causes. Contracts also commonly contain provisions that permit termination “without cause” after notice of termination is provided. Although you could negotiate for more notice time, “without cause” clauses are unlikely to be removed from the contract.
Most psychiatrists are required to sign an employment contract before taking a job, but few of us have received any training on reviewing such contracts. We often rely on coworkers and attorneys to navigate this process for us. However, the contract is crucial, because it outlines your employer’s clinical and administrative expectations for the position, and it gives you the opportunity to lay out what you want.1 Because an employment contract is legally binding, you should thoroughly read it and look for clauses that may not work in your best interest. Although not a complete list, the following items should be reviewed before signing a contract.1,2
Benefits. Make sure you are offered a reasonable salary, but balance the dollar amount with benefits such as:
- continuing medical education allowances
- educational loan forgiveness
- health/malpractice/disability insurance
- retirement benefits
- compensation for call schedule.
In some cases, there may be a delay before you are eligible to obtain certain benefits.
Work expectations. Many contracts state that the position is “full-time” or have other nonspecific parameters for work expectations. You should inquire about objective work parameters, such as duty hours, the average frequency of the current call schedule, timeframe for completing medical documentation, and penalties for not meeting clinical or administrative requirements, so you are not surprised by:
- working longer-than-planned shifts
- performing on-call duties
- working on days that you were not expecting
- having your credentialing status placed in jeopardy.
Some group practices allow for a half-day of no scheduled appointments with patients, so you can complete paperwork and return phone calls.
Noncompete clause. This restricts you from working within a certain geographic area or for a competing employer for a finite time period after the contract terminates or expires. A noncompete clause could restrict you from practicing within a large geographical area, especially if the job is located in a densely populated area. Some noncompete clauses do not include a temporal or geographic restriction, but can limit your ability to bring patients with you to a new practice or facility when the contract expires.
Malpractice insurance. Two types of malpractice insurance are occurrence and claims-made:
- Occurrence insurance protects you whenever an action is brought against you, even if the action is brought after the contract terminates or expires.
- Claims-made insurance provides coverage if the policy with the same insurer was in effect when the malpractice was committed and when the actual action was commenced.
Although claims-made insurance is less expensive, it can leave you without coverage should you leave your employer and no longer maintain the same insurance policy. Claims-made can be converted into occurrence through the purchase of a tail endorsement. If the employer does not offer you tail coverage, then it is your responsibility to pay for this insurance, which can be expensive.
Termination language. Every contract features a termination section that lists potential causes for terminating your employment. This list is usually not exhaustive, but it sets the framework for a realistic view of reasonable causes. Contracts also commonly contain provisions that permit termination “without cause” after notice of termination is provided. Although you could negotiate for more notice time, “without cause” clauses are unlikely to be removed from the contract.
1. Claussen K. Eight physician employment contract items you need to know about. The Doctor Weighs In. https://thedoctorweighsin.com/8-physician-employment-contract-items-you-need-to-know-about. Published March 8, 2017. Accessed October 11, 2017.
2. Blustein AE, Keller LB. Physician employment contracts: what you need to know before you sign. J Am Acad Dermatol. https://www.aad.org/members/publications/directions-in-residency/archiveyment-contracts-what-you-need-to-know-before-you-sign. Accessed October 11, 2017.
1. Claussen K. Eight physician employment contract items you need to know about. The Doctor Weighs In. https://thedoctorweighsin.com/8-physician-employment-contract-items-you-need-to-know-about. Published March 8, 2017. Accessed October 11, 2017.
2. Blustein AE, Keller LB. Physician employment contracts: what you need to know before you sign. J Am Acad Dermatol. https://www.aad.org/members/publications/directions-in-residency/archiveyment-contracts-what-you-need-to-know-before-you-sign. Accessed October 11, 2017.
3 Approaches to PMS
Throughout my 40 years in private psychiatric practice, I have found some treatments for premenstrual syndrome (PMS) that were not mentioned in “Etiology of premenstrual dysphoric disorder: 5 interwoven pieces” (Evidence-Based Reviews, Current Psychiatry. September 2017, p. 20-28).
This started in 1972 when I was serving in the Army in Oklahoma. A 28-year-old woman with severe PMS had been treated by internal medicine, an OB/GYN, and endocrinology, all to no avail. Three days before her menses began, she would start driving north. When menses commenced, she would find herself in Nebraska and have to call her husband so he could wire her money to come back.
Through my evaluation, I found that she would gain 10 lb before her menses. I prescribed a diuretic and instructed her to start taking it when she began swelling and to stop taking it after her menses began. This alleviated all of her symptoms. If a woman gains more than 3 to 5 lb, her brain also will swell, along with everything else. Because the brain is encapsulated in the skull, the swelling puts pressure on the brain, which might have been the cause of these brief psychotic episodes.
If a woman who develops PMS does not experience significant weight gain, the first thing I try is vitamin B6, 100 mg/d, prior to menses. Vitamin B6 is a cofactor in the production of numerous neurotransmitters. I found that prescribing vitamin B6 would alleviate about 20% of PMS symptoms. If the patient has a personal or family history of affective disorder, I often try antidepressants prior to menses, which alleviate approximately another 20% of her symptoms. If none of the previous 3 factors are present, I often add a low dose of progesterone, which appears to help. If all else fails, I will try a low dose of lithium, 300 mg/d, before menses. This also seems to have some positive effect.
I have not written an article about these approaches to PMS, although I have discussed them with OB/GYNs, who never seem to follow these recommendations. Because I am not university-based, I have not been able to put thes
Throughout my 40 years in private psychiatric practice, I have found some treatments for premenstrual syndrome (PMS) that were not mentioned in “Etiology of premenstrual dysphoric disorder: 5 interwoven pieces” (Evidence-Based Reviews, Current Psychiatry. September 2017, p. 20-28).
This started in 1972 when I was serving in the Army in Oklahoma. A 28-year-old woman with severe PMS had been treated by internal medicine, an OB/GYN, and endocrinology, all to no avail. Three days before her menses began, she would start driving north. When menses commenced, she would find herself in Nebraska and have to call her husband so he could wire her money to come back.
Through my evaluation, I found that she would gain 10 lb before her menses. I prescribed a diuretic and instructed her to start taking it when she began swelling and to stop taking it after her menses began. This alleviated all of her symptoms. If a woman gains more than 3 to 5 lb, her brain also will swell, along with everything else. Because the brain is encapsulated in the skull, the swelling puts pressure on the brain, which might have been the cause of these brief psychotic episodes.
If a woman who develops PMS does not experience significant weight gain, the first thing I try is vitamin B6, 100 mg/d, prior to menses. Vitamin B6 is a cofactor in the production of numerous neurotransmitters. I found that prescribing vitamin B6 would alleviate about 20% of PMS symptoms. If the patient has a personal or family history of affective disorder, I often try antidepressants prior to menses, which alleviate approximately another 20% of her symptoms. If none of the previous 3 factors are present, I often add a low dose of progesterone, which appears to help. If all else fails, I will try a low dose of lithium, 300 mg/d, before menses. This also seems to have some positive effect.
I have not written an article about these approaches to PMS, although I have discussed them with OB/GYNs, who never seem to follow these recommendations. Because I am not university-based, I have not been able to put thes
Throughout my 40 years in private psychiatric practice, I have found some treatments for premenstrual syndrome (PMS) that were not mentioned in “Etiology of premenstrual dysphoric disorder: 5 interwoven pieces” (Evidence-Based Reviews, Current Psychiatry. September 2017, p. 20-28).
This started in 1972 when I was serving in the Army in Oklahoma. A 28-year-old woman with severe PMS had been treated by internal medicine, an OB/GYN, and endocrinology, all to no avail. Three days before her menses began, she would start driving north. When menses commenced, she would find herself in Nebraska and have to call her husband so he could wire her money to come back.
Through my evaluation, I found that she would gain 10 lb before her menses. I prescribed a diuretic and instructed her to start taking it when she began swelling and to stop taking it after her menses began. This alleviated all of her symptoms. If a woman gains more than 3 to 5 lb, her brain also will swell, along with everything else. Because the brain is encapsulated in the skull, the swelling puts pressure on the brain, which might have been the cause of these brief psychotic episodes.
If a woman who develops PMS does not experience significant weight gain, the first thing I try is vitamin B6, 100 mg/d, prior to menses. Vitamin B6 is a cofactor in the production of numerous neurotransmitters. I found that prescribing vitamin B6 would alleviate about 20% of PMS symptoms. If the patient has a personal or family history of affective disorder, I often try antidepressants prior to menses, which alleviate approximately another 20% of her symptoms. If none of the previous 3 factors are present, I often add a low dose of progesterone, which appears to help. If all else fails, I will try a low dose of lithium, 300 mg/d, before menses. This also seems to have some positive effect.
I have not written an article about these approaches to PMS, although I have discussed them with OB/GYNs, who never seem to follow these recommendations. Because I am not university-based, I have not been able to put thes
What’s causing my older patient’s cognitive decline?
A 68-year-old woman with a history of well-controlled hypertension and diabetes presents to the office for routine follow-up. She says she has adhered to her current medications, and her blood pressure and hemoglobin A1c remain at goal. At the close of the visit, she mentions that she is worried she may be developing dementia. She says she has been having difficulty finding the right word in conversation and needs to write things down more than she used to.
What might be causing this patient’s changes in cognition?
In primary care settings, when patients complain of memory loss, there is a 20% to 30% chance they will be found to have mild cognitive impairment (MCI) or some level of dementia.1 Given the significant consequences of dementia, it’s important to maximize opportunities to distinguish those with age-related changes in cognition or reversible causes of memory loss from those who have irreversible pathologic changes.
Age-related changes in cognition
Changes in cognition associated with aging vary considerably among individuals and across domains of cognition. By their 7th decade, most people experience a decline in processing speed and working memory.2 However, some individuals retain excellent function into their 80s and perform as well as younger adults.3
Changes long thought to be due to brain senescence may, in fact, be related to the effects of age-related medical conditions on the brain’s function.4 Consistent with this theory is the observation that cognitive changes tend to occur earlier in individuals with cardiovascular disease, diabetes, and cancer.2 What constitutes a normal change depends on an individual’s baseline cognitive function, educational background, medical comorbidities, and the potential impact of sensory impairment on performance.

General cognitive trends with aging. Awareness of normal changes in an aging population is useful when assessing patients concerned about their memory. In general, an individual’s ability to maintain attention to a single task is preserved into late life. Ability to perform tasks requiring divided attention or attention-switching tends to decline.3 This has implications for driving, given the need to constantly switch one’s attention in response to the environment and the ability to sort relevant from irrelevant information.
Remote memory, semantic memory (factual information), and procedural memory (knowledge of skills and procedures) tend to remain intact with aging.4 Short-term memory (simple maintenance of information over a short period of time) shows little change with aging. However, working memory, which requires the manipulation of information in short-term memory, declines.
A simple demonstration of this is that performance on digit span testing tends to remain preserved (7±2), but digit span backwards declines. Holding digits in mind in the order they are received can be achieved through rehearsal. But to reverse the order requires reorganization of the information, and this ability declines with aging.3
Prospective memory (remembering to do things in the future) often requires increased dependence on external aids, such as a to-do list.3 The capacity to learn and recall new information declines. Even when given repeated opportunity to practice, older adults demonstrate a slower learning curve and lower total amount learned.4 Therefore, it becomes easier relying on well-learned cognitive processes such as cooking a familiar meal or relying on previously used principles for decision making.2
Language comprehension and vocabulary remain stable over time. However, difficulty with spontaneous word finding—the “tip-of-the-tongue” phenomenon—tends to increase. In contrast to the dysnomia related to dementia, the word-finding difficulties with normal aging typically improve with cues, indicating that the problem is in retrieval of information rather than storage. Verbal fluency, the rate at which words from a single category can be produced, shows decline. This is particularly true in tests of semantic verbal fluency (name all the animals you can think of); phonemic fluency (words that start with a certain letter) tends to be preserved.4
Some studies using neurocognitive testing have suggested a decline in executive functioning. But, in general, aging has little impact on “real world” executive functions that are required for planning and executing tasks.4 On the whole, cognitive changes related to aging typically do not interfere with an individual’s ability to function independently.
Mild cognitive impairment/mild neurocognitive disorder
Originally conceived as a precursor to Alzheimer’s dementia,5 mild cognitive impairment (MCI) is a diagnosis that has evolved to describe a heterogeneous syndrome of abnormal cognition characterized by:6-8
- a suspected change in cognition expressed by the patient, an acquaintance who knows the patient well, or a clinician;
- objectively measured impairment in one or more cognitive domains beyond what would be expected based on an individual’s age and educational background;
- preservation of functional abilities; and
- a lack of findings that would fulfill criteria for dementia.
In the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM V), this concept is identified as mild neurocognitive disorder, with the additional caveats that an individual’s cognitive deficits do not occur exclusively in the context of delirium and are not better explained by another mental disorder such as depression or schizophrenia.9
An accurate assessment of cognitive change is best measured against the individual’s baseline, which may necessitate the report of a reliable acquaintance. An assessment of functional abilities is also critical. Mild problems in performing complex functions (bill paying, shopping, etc) could be present and still allow a patient to meet the criteria for MCI. An individual may take more time, be less efficient, or make more errors than before; however, independence with minimal aid or assistance is preserved. It
MCI can be divided into 4 subtypes depending upon the cognitive domains affected (complex attention, executive function, learning and memory, language, visuospatial, social cognition):
- Amnestic MCI single domain, if only memory is affected.
- Amnestic MCI multiple domain, if memory and any other cognitive domains are affected.
- Non-amnestic MCI single domain, if any other cognitive domain aside from memory is the only one affected.
- Non-amnestic MCI multiple domain, if multiple domains other than memory are affected.
These distinctions may provide clues to the underlying cause of dysfunction and provide prognostic information regarding the risk of progression to dementia.6,7
Prevalence estimates for MCI vary widely due to differences in definitions used and populations studied. The best estimate is 5% to 10% prevalence among those ages 65 to 69 years old, and 12% to 25% among those ages 80 to 84.10 Similarly, estimates of the rate of progression to dementia vary. Among MCI populations identified through referral sources such as memory centers, the rate of progression to dementia has been 10% to 15% per year.11 In epidemiologic studies of general populations, the rate has been 6% to 10% per year.11 The rate of development of dementia among normal subjects is 1% to 2% per year.5
Dementia/major neurocognitive disorder
The primary feature distinguishing MCI/mild neurocognitive disorder from dementia or major neurocognitive disorder is a patient’s functional status. The core clinical criteria for all-cause dementia are cognitive or neurobehavioral symptoms that: 12
- interfere with work or usual daily function,
- represent a change from the prior baseline function,
- are not explained by delirium or a psychiatric illness, and
- include detectable impairment in 2 cognitive domains.
Criteria outlined in the DSM-V for major neurocognitive disorder are essentially the same but describe the functional change criteria as cognitive changes that “interfere with independence in everyday activities.”9 The DSM-V elaborates: “at a minimum, requiring assistance with complex instrumental activities of daily living such as paying bills or managing medications.”
Assessing functional status accurately in clinical practice typically requires the assistance of a collateral informant who knows the patient well. The Informant Questionnaire on Cognitive Decline in the Elderly (https://www.alz.org/documents_custom/shortiqcode_english.pdf) is one validated assessment tool that can be used for this purpose.13 With this self-administered form, the informant answers 16 questions regarding changes in the patient’s performance of different activities over the 10 years prior. Alternatively, a structured interview based on indices of activities of daily living (ADLs) and instrumental activities of daily living (IADLs) as listed in TABLE 1 can be employed.14,15
Review of the various causes of dementia is beyond the scope of this article, but a list of common diagnoses is presented in TABLE 2.
Dementia syndrome of depression (pseudodementia)
Elderly patients with depression commonly complain of memory impairment, and this interaction between depression and dementia has been investigated for decades. The term “pseudodementia” has been used since 1961 to describe signs of dementia in a patient with any psychiatric illness,16 but it has since been refined to apply solely to depression. The prevalence of depression among older adults varies depending on the population studied and how depression is defined. Approximately 2% to 3% of community-dwelling elders meet criteria for major depression, with 10% to 30% showing some symptoms of depression.17,18
Twenty percent to 40% of elderly patients diagnosed with depression will have evidence of cognitive impairment.19-21 Most improve with antidepressive treatment, though evidence of cognitive impairment may continue for some.19
A broad range of cognitive deficits have been associated with depression. Most consistently described are deficits in processing speed,22-25 attention,26-28 and executive function.22,25-29 Memory deficits can be apparent with tests of delayed recall, but recognition (the ability to identify items from a list) generally is preserved.26,28-30
Distinguishing between pseudodementia and true dementia can be challenging. An increased severity of deficits, particularly with delayed recall, is more indicative of dementia.31 Additionally, on clock drawing tasks, individuals with depression perform more comparably to controls than do those with true dementia.32
A 2013 meta-analysis reported a significant association of late-life depression with subsequent development of dementia, with an odds ratio (OR) of 1.85. The risk of subsequently developing vascular dementia (OR=2.52) was significantly higher than that for Alzheimer’s disease (OR=1.65). Individuals with evidence of reversible cognitive impairment at the time of diagnosis of depression seem to be particularly vulnerable, with dementia developing in 43% to 71%, compared with rates of 12% to 18% among elders diagnosed with depression but lacking signs of cognitive impairment.20,21
Other causes of reversible dementia
A meta-analysis performed in 1988 found that 11% of cases of dementia were reversible.33 However, an update using the same methodology in 2003 revealed the number had dropped to less than 1%.34 In the latest meta-analysis, one of the authors’ leading hypotheses for the dramatic decline in apparent prevalence was a significant shift in the study population from the inpatient to outpatient setting. In studies of community-based populations used in the re-analysis, the reported prevalence of reversibility was near zero.34
Metabolic abnormalities—most often B12 deficiency and hypothyroidism—are commonly cited as potential causes of dementia. Four systematic reviews, including one conducted by the Cochrane Collaborative, concluded there is a lack of evidence that treating low vitamin B12 in individuals with dementia improves cognition.35,36 There is some evidence, though, of a time-limited window for successful treatment within 12 months of the onset of symptoms.37,38 A study reviewing causes of dementia in nearly 3000 individuals found one case of reversible dementia attributable to hypothyroidism.39 A subsequent review reached similar conclusions about the lack of data to support the notion that treatment of hypothyroidism reverses dementia.40
Similarly, imaging for cerebral tumors, subdural hematomas, or normal-pressure hydrocephalus rarely identifies these as a cause of dementia.41 This is particularly true of unselected community-based populations, as there are typically signs or symptoms suggesting an intracranial pathology.
Numerous medications have been implicated in causing acute confusional states, and there is some evidence for their role in chronic confusion (TABLE 3).42,43 In my experience, many who experience adverse effects on cognition with medications will also have an underlying neurodegenerative process, and symptoms do not completely resolve with withdrawal of the offending agent.
Further assessment of the patient yielded a score of 29/30 on the Montreal Cognitive Assessment* and a zero on the Patient Health Questionnaire-2. Careful review of her daily function revealed no significant deficits in ADL or IADL performance, and her daughter confirmed that she had not observed any significant decline in her mother’s function. There was no significant family history of dementia. The patient was reassured that her cognitive changes were normal and age related.
Unfortunately, few data support specific interventions to reduce this patient’s risk of developing dementia. She was commended for keeping her blood pressure and blood sugar levels under control, thereby reducing her risk of vascular disease.
She and her daughter were directed to the Alzheimer’s Association Web site (alz.org) as a resource for information about signs and symptoms to watch for and for caregiving resources, should they be needed. She was briefly counseled to eliminate distractions to improve her ability to complete tasks and improve recall along with rehearsing or writing down information that she wished to retain.
Finally, she was counseled to remain physically, cognitively, and socially active as these are factors generally associated with healthy aging, have some evidence to support efficacy in reducing the risk of cognitive decline,44,45 and are unlikely to be of harm.
*The Montreal Cognitive Assessment is a validated office-based tool for the evaluation of cognitive impairment that is highly sensitive for the detection of mild cognitive impairment.
CORRESPONDENCE
Ian M. Deutchki, MD, Professor of Family Medicine and Geriatrics, University of Rochester Medical Center, 777 S. Clinton Avenue, Rochester, NY 14620; [email protected].
1. Mitchell AJ. The clinical significance of subjective memory complaints in the diagnosis of mild cognitive impairment and dementia: a meta-analysis. Int J Geriatr Psychiatry. 2008;23:1191-1202.
2. Burnette V, Howell T. Cognitive changes in aging. In: Capezuti EA, Malone ML, Katz PR, et al, eds. The Encyclopedia of Elder Care. New York, NY, USA: Springer Publishing Company; 2013.
3. Glisky EL. Changes in cognitive function in human aging. In: Riddle DR, ed. Brain Aging: Models, Methods, and Mechanisms. Boca Raton, FL: Taylor & Francis Group, LLC; 2007:4-20.
4. Craft S, Cholerton B, Reger M. Cognitive changes associated with normal and pathological aging. In: Halter JB, Ouslander JG, Tinetti ME, Studenski S, et al, eds. Hazzard’s Geriatric Medicine and Gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:751-766.
5. Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303-308.
6. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183-194.
7. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240-246.
8. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270-279.
9. Neurocognitive disorders. In: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
10. Ward A. Arrighi HM, Michels S, et al. Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement. 2012;8:14-21.
11. Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66:1447-1455.
12. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263-269.
13. Jorm AF. A short form of the informant questionnaire on cognitive decline in the elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145-153.
14. Katz S, Downs TD, Cash HR, et al. Progress in development of the index of ADL. Gerontologist. 1970;10:20-30.
15. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179-186.
16. Kiloh LG. Pseudo-dementia. Acta Psychiatr Scand. 1961;37:336-351.
17. Beekman AT, Copeland JR, Prince MJ. Review of community prevalence of depression in later life. Br J Psychiatry. 1999;174:307-311.
18. Birrer RB, Vemuri SP. Depression in later life: a diagnostic and therapeutic challenge. Am Fam Physician. 2004;69:2375-2382.
19. Butters MA, Becker JT, Nebes RD, et al. Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry. 2000;157:1949-1954.
20. Alexopoulos GS, Meyers BS, Young RC, et al. The course of geriatric depression with “reversible dementia”: a controlled study. Am J Psychiatry. 1993;150:1693-1699.
21. Saez-Fonseca JA, Lee L, Walker Z. Long-term outcome of depressive pseudodementia in the elderly. J Affect Disord. 2007;101:123-129.
22. Dillon C, Allegri RF, Serrano CM, et al. Late- versus early-onset geriatric depression in a memory research center. Neuropsychiatr Dis Treat. 2009;5:517-526.
23. Lockwood KA, Alexopoulos GS, van Gorp WG. Executive dysfunction in geriatric depression. Am J Psychiatry. 2002;159:1119-1126.
24. Shimada H, Park H, Makizako H, et al. Depressive symptoms and cognitive performance in older adults. J Psychiatr Res. 2014;57:149-156.
25. Butters MA, Whyte EM, Nebes RD, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61:587-595.
26. Dillon C, Machnicki G, Serrano CM, et al. Clinical manifestations of geriatric depression in a memory clinic: toward a proposed subtyping of geriatric depression. J Affect Disord. 2011;134:177-187.
27. Rapp MA, Dahlman K, Sano M, et al. Neuropsychological differences between late-onset and recurrent geriatric major depression. Am J Psychiatry. 2005;162:691-698.
28. Zihl J, Reppermund S, Thum S, et al. Neuropsychological profiles in MCI and in depression: differential cognitive dysfunction patterns or similar final common pathway disorder? J Psychiatr Res. 2010;44:647-654.
29. Dillon C, Tartaglini MF, Stefani D, et al. Geriatric depression and its relation with cognitive impairment and dementia. Arch Gerontol Geriatr. 2014;59:450-456.
30. Wright SL, Persad C. Distinguishing between depression and dementia in older persons: neuropsychological and neuropathological correlates. J Geriatr Psychiatry Neurol. 2007;20:189-198.
31. Visser PJ, Verhey FR, Ponds RW, et al. Distinction between preclinical Alzheimer’s disease and depression. J Am Geriatr Soc. 2000;48:479-484.
32. Bodner T, Delazer M, Kemmler G, et al. Clock drawing, clock reading, clock setting, and judgment of clock faces in elderly people with dementia and depression. J Am Geriatr Soc. 2004;52:1146-1150.
33. Clarfield AM. The reversible dementias: do they reverse? Ann Intern Med. 1988;109:476-486.
34. Clarfield AM. The decreasing prevalence of reversible dementias: an updated meta-analysis. Arch Intern Med. 2003;163:2219-2229.
35. Malouf R, Areosa Sastre A. Vitamin B12 for cognition. Cochrane Database Syst Rev. 2003;(3):CD004326.
36. Health Quality Ontario. Vitamin B12 and cognitive function: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13:1-45.
37. Abyad A. Prevalence of vitamin B12 deficiency among demented patients and cognitive recovery with cobalamin replacement. J Nutr Health Aging. 2002;6:254-260.
38. Martin DC, Francis J, Protetch J, et al. Time dependency of cognitive recovery with cobalamin replacement: Report of a pilot study. J Am Geriatr Soc. 1992;40:168-172.
39. Clarnette RM, Patterson CJ. Hypothyroidism: does treatment cure dementia? J Geriatr Psychiatry Neurol. 1994;7:23-27.
40. Dugbartey AT. Neurocognitive aspects of hypothyroidism. Arch Intern Med. 1998;158:1413-1418.
41. Alexander EM, Wagner EH, Buchner DM, et al. Do surgical brain lesions present as isolated dementia? A population-based study. J Am Geriatr Soc. 1995;43:138-143.
42. Moore AR, O’Keeffe ST. Drug-induced cognitive impairment in the elderly. Drugs Aging. 1999;15:15-28.
43. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American geriatrics society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
44. Middleton LE, Yaffe K. Promising strategies for the prevention of dementia. Arch Neurol. 2009;66:1210-1215.
45. Shatenstein B, Barberger-Gateau P, Mecocci P. Prevention of age-related cognitive decline: which strategies, when, and for whom? J Alzheimers Dis. 2015;48:35-53.
A 68-year-old woman with a history of well-controlled hypertension and diabetes presents to the office for routine follow-up. She says she has adhered to her current medications, and her blood pressure and hemoglobin A1c remain at goal. At the close of the visit, she mentions that she is worried she may be developing dementia. She says she has been having difficulty finding the right word in conversation and needs to write things down more than she used to.
What might be causing this patient’s changes in cognition?
In primary care settings, when patients complain of memory loss, there is a 20% to 30% chance they will be found to have mild cognitive impairment (MCI) or some level of dementia.1 Given the significant consequences of dementia, it’s important to maximize opportunities to distinguish those with age-related changes in cognition or reversible causes of memory loss from those who have irreversible pathologic changes.
Age-related changes in cognition
Changes in cognition associated with aging vary considerably among individuals and across domains of cognition. By their 7th decade, most people experience a decline in processing speed and working memory.2 However, some individuals retain excellent function into their 80s and perform as well as younger adults.3
Changes long thought to be due to brain senescence may, in fact, be related to the effects of age-related medical conditions on the brain’s function.4 Consistent with this theory is the observation that cognitive changes tend to occur earlier in individuals with cardiovascular disease, diabetes, and cancer.2 What constitutes a normal change depends on an individual’s baseline cognitive function, educational background, medical comorbidities, and the potential impact of sensory impairment on performance.

General cognitive trends with aging. Awareness of normal changes in an aging population is useful when assessing patients concerned about their memory. In general, an individual’s ability to maintain attention to a single task is preserved into late life. Ability to perform tasks requiring divided attention or attention-switching tends to decline.3 This has implications for driving, given the need to constantly switch one’s attention in response to the environment and the ability to sort relevant from irrelevant information.
Remote memory, semantic memory (factual information), and procedural memory (knowledge of skills and procedures) tend to remain intact with aging.4 Short-term memory (simple maintenance of information over a short period of time) shows little change with aging. However, working memory, which requires the manipulation of information in short-term memory, declines.
A simple demonstration of this is that performance on digit span testing tends to remain preserved (7±2), but digit span backwards declines. Holding digits in mind in the order they are received can be achieved through rehearsal. But to reverse the order requires reorganization of the information, and this ability declines with aging.3
Prospective memory (remembering to do things in the future) often requires increased dependence on external aids, such as a to-do list.3 The capacity to learn and recall new information declines. Even when given repeated opportunity to practice, older adults demonstrate a slower learning curve and lower total amount learned.4 Therefore, it becomes easier relying on well-learned cognitive processes such as cooking a familiar meal or relying on previously used principles for decision making.2
Language comprehension and vocabulary remain stable over time. However, difficulty with spontaneous word finding—the “tip-of-the-tongue” phenomenon—tends to increase. In contrast to the dysnomia related to dementia, the word-finding difficulties with normal aging typically improve with cues, indicating that the problem is in retrieval of information rather than storage. Verbal fluency, the rate at which words from a single category can be produced, shows decline. This is particularly true in tests of semantic verbal fluency (name all the animals you can think of); phonemic fluency (words that start with a certain letter) tends to be preserved.4
Some studies using neurocognitive testing have suggested a decline in executive functioning. But, in general, aging has little impact on “real world” executive functions that are required for planning and executing tasks.4 On the whole, cognitive changes related to aging typically do not interfere with an individual’s ability to function independently.
Mild cognitive impairment/mild neurocognitive disorder
Originally conceived as a precursor to Alzheimer’s dementia,5 mild cognitive impairment (MCI) is a diagnosis that has evolved to describe a heterogeneous syndrome of abnormal cognition characterized by:6-8
- a suspected change in cognition expressed by the patient, an acquaintance who knows the patient well, or a clinician;
- objectively measured impairment in one or more cognitive domains beyond what would be expected based on an individual’s age and educational background;
- preservation of functional abilities; and
- a lack of findings that would fulfill criteria for dementia.
In the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM V), this concept is identified as mild neurocognitive disorder, with the additional caveats that an individual’s cognitive deficits do not occur exclusively in the context of delirium and are not better explained by another mental disorder such as depression or schizophrenia.9
An accurate assessment of cognitive change is best measured against the individual’s baseline, which may necessitate the report of a reliable acquaintance. An assessment of functional abilities is also critical. Mild problems in performing complex functions (bill paying, shopping, etc) could be present and still allow a patient to meet the criteria for MCI. An individual may take more time, be less efficient, or make more errors than before; however, independence with minimal aid or assistance is preserved. It
MCI can be divided into 4 subtypes depending upon the cognitive domains affected (complex attention, executive function, learning and memory, language, visuospatial, social cognition):
- Amnestic MCI single domain, if only memory is affected.
- Amnestic MCI multiple domain, if memory and any other cognitive domains are affected.
- Non-amnestic MCI single domain, if any other cognitive domain aside from memory is the only one affected.
- Non-amnestic MCI multiple domain, if multiple domains other than memory are affected.
These distinctions may provide clues to the underlying cause of dysfunction and provide prognostic information regarding the risk of progression to dementia.6,7
Prevalence estimates for MCI vary widely due to differences in definitions used and populations studied. The best estimate is 5% to 10% prevalence among those ages 65 to 69 years old, and 12% to 25% among those ages 80 to 84.10 Similarly, estimates of the rate of progression to dementia vary. Among MCI populations identified through referral sources such as memory centers, the rate of progression to dementia has been 10% to 15% per year.11 In epidemiologic studies of general populations, the rate has been 6% to 10% per year.11 The rate of development of dementia among normal subjects is 1% to 2% per year.5
Dementia/major neurocognitive disorder
The primary feature distinguishing MCI/mild neurocognitive disorder from dementia or major neurocognitive disorder is a patient’s functional status. The core clinical criteria for all-cause dementia are cognitive or neurobehavioral symptoms that: 12
- interfere with work or usual daily function,
- represent a change from the prior baseline function,
- are not explained by delirium or a psychiatric illness, and
- include detectable impairment in 2 cognitive domains.
Criteria outlined in the DSM-V for major neurocognitive disorder are essentially the same but describe the functional change criteria as cognitive changes that “interfere with independence in everyday activities.”9 The DSM-V elaborates: “at a minimum, requiring assistance with complex instrumental activities of daily living such as paying bills or managing medications.”
Assessing functional status accurately in clinical practice typically requires the assistance of a collateral informant who knows the patient well. The Informant Questionnaire on Cognitive Decline in the Elderly (https://www.alz.org/documents_custom/shortiqcode_english.pdf) is one validated assessment tool that can be used for this purpose.13 With this self-administered form, the informant answers 16 questions regarding changes in the patient’s performance of different activities over the 10 years prior. Alternatively, a structured interview based on indices of activities of daily living (ADLs) and instrumental activities of daily living (IADLs) as listed in TABLE 1 can be employed.14,15

Review of the various causes of dementia is beyond the scope of this article, but a list of common diagnoses is presented in TABLE 2.

Dementia syndrome of depression (pseudodementia)
Elderly patients with depression commonly complain of memory impairment, and this interaction between depression and dementia has been investigated for decades. The term “pseudodementia” has been used since 1961 to describe signs of dementia in a patient with any psychiatric illness,16 but it has since been refined to apply solely to depression. The prevalence of depression among older adults varies depending on the population studied and how depression is defined. Approximately 2% to 3% of community-dwelling elders meet criteria for major depression, with 10% to 30% showing some symptoms of depression.17,18
Twenty percent to 40% of elderly patients diagnosed with depression will have evidence of cognitive impairment.19-21 Most improve with antidepressive treatment, though evidence of cognitive impairment may continue for some.19
A broad range of cognitive deficits have been associated with depression. Most consistently described are deficits in processing speed,22-25 attention,26-28 and executive function.22,25-29 Memory deficits can be apparent with tests of delayed recall, but recognition (the ability to identify items from a list) generally is preserved.26,28-30
Distinguishing between pseudodementia and true dementia can be challenging. An increased severity of deficits, particularly with delayed recall, is more indicative of dementia.31 Additionally, on clock drawing tasks, individuals with depression perform more comparably to controls than do those with true dementia.32
A 2013 meta-analysis reported a significant association of late-life depression with subsequent development of dementia, with an odds ratio (OR) of 1.85. The risk of subsequently developing vascular dementia (OR=2.52) was significantly higher than that for Alzheimer’s disease (OR=1.65). Individuals with evidence of reversible cognitive impairment at the time of diagnosis of depression seem to be particularly vulnerable, with dementia developing in 43% to 71%, compared with rates of 12% to 18% among elders diagnosed with depression but lacking signs of cognitive impairment.20,21
Other causes of reversible dementia
A meta-analysis performed in 1988 found that 11% of cases of dementia were reversible.33 However, an update using the same methodology in 2003 revealed the number had dropped to less than 1%.34 In the latest meta-analysis, one of the authors’ leading hypotheses for the dramatic decline in apparent prevalence was a significant shift in the study population from the inpatient to outpatient setting. In studies of community-based populations used in the re-analysis, the reported prevalence of reversibility was near zero.34
Metabolic abnormalities—most often B12 deficiency and hypothyroidism—are commonly cited as potential causes of dementia. Four systematic reviews, including one conducted by the Cochrane Collaborative, concluded there is a lack of evidence that treating low vitamin B12 in individuals with dementia improves cognition.35,36 There is some evidence, though, of a time-limited window for successful treatment within 12 months of the onset of symptoms.37,38 A study reviewing causes of dementia in nearly 3000 individuals found one case of reversible dementia attributable to hypothyroidism.39 A subsequent review reached similar conclusions about the lack of data to support the notion that treatment of hypothyroidism reverses dementia.40
Similarly, imaging for cerebral tumors, subdural hematomas, or normal-pressure hydrocephalus rarely identifies these as a cause of dementia.41 This is particularly true of unselected community-based populations, as there are typically signs or symptoms suggesting an intracranial pathology.
Numerous medications have been implicated in causing acute confusional states, and there is some evidence for their role in chronic confusion (TABLE 3).42,43 In my experience, many who experience adverse effects on cognition with medications will also have an underlying neurodegenerative process, and symptoms do not completely resolve with withdrawal of the offending agent.

Further assessment of the patient yielded a score of 29/30 on the Montreal Cognitive Assessment* and a zero on the Patient Health Questionnaire-2. Careful review of her daily function revealed no significant deficits in ADL or IADL performance, and her daughter confirmed that she had not observed any significant decline in her mother’s function. There was no significant family history of dementia. The patient was reassured that her cognitive changes were normal and age related.
Unfortunately, few data support specific interventions to reduce this patient’s risk of developing dementia. She was commended for keeping her blood pressure and blood sugar levels under control, thereby reducing her risk of vascular disease.
She and her daughter were directed to the Alzheimer’s Association Web site (alz.org) as a resource for information about signs and symptoms to watch for and for caregiving resources, should they be needed. She was briefly counseled to eliminate distractions to improve her ability to complete tasks and improve recall along with rehearsing or writing down information that she wished to retain.
Finally, she was counseled to remain physically, cognitively, and socially active as these are factors generally associated with healthy aging, have some evidence to support efficacy in reducing the risk of cognitive decline,44,45 and are unlikely to be of harm.
*The Montreal Cognitive Assessment is a validated office-based tool for the evaluation of cognitive impairment that is highly sensitive for the detection of mild cognitive impairment.
CORRESPONDENCE
Ian M. Deutchki, MD, Professor of Family Medicine and Geriatrics, University of Rochester Medical Center, 777 S. Clinton Avenue, Rochester, NY 14620; [email protected].
A 68-year-old woman with a history of well-controlled hypertension and diabetes presents to the office for routine follow-up. She says she has adhered to her current medications, and her blood pressure and hemoglobin A1c remain at goal. At the close of the visit, she mentions that she is worried she may be developing dementia. She says she has been having difficulty finding the right word in conversation and needs to write things down more than she used to.
What might be causing this patient’s changes in cognition?
In primary care settings, when patients complain of memory loss, there is a 20% to 30% chance they will be found to have mild cognitive impairment (MCI) or some level of dementia.1 Given the significant consequences of dementia, it’s important to maximize opportunities to distinguish those with age-related changes in cognition or reversible causes of memory loss from those who have irreversible pathologic changes.
Age-related changes in cognition
Changes in cognition associated with aging vary considerably among individuals and across domains of cognition. By their 7th decade, most people experience a decline in processing speed and working memory.2 However, some individuals retain excellent function into their 80s and perform as well as younger adults.3
Changes long thought to be due to brain senescence may, in fact, be related to the effects of age-related medical conditions on the brain’s function.4 Consistent with this theory is the observation that cognitive changes tend to occur earlier in individuals with cardiovascular disease, diabetes, and cancer.2 What constitutes a normal change depends on an individual’s baseline cognitive function, educational background, medical comorbidities, and the potential impact of sensory impairment on performance.

General cognitive trends with aging. Awareness of normal changes in an aging population is useful when assessing patients concerned about their memory. In general, an individual’s ability to maintain attention to a single task is preserved into late life. Ability to perform tasks requiring divided attention or attention-switching tends to decline.3 This has implications for driving, given the need to constantly switch one’s attention in response to the environment and the ability to sort relevant from irrelevant information.
Remote memory, semantic memory (factual information), and procedural memory (knowledge of skills and procedures) tend to remain intact with aging.4 Short-term memory (simple maintenance of information over a short period of time) shows little change with aging. However, working memory, which requires the manipulation of information in short-term memory, declines.
A simple demonstration of this is that performance on digit span testing tends to remain preserved (7±2), but digit span backwards declines. Holding digits in mind in the order they are received can be achieved through rehearsal. But to reverse the order requires reorganization of the information, and this ability declines with aging.3
Prospective memory (remembering to do things in the future) often requires increased dependence on external aids, such as a to-do list.3 The capacity to learn and recall new information declines. Even when given repeated opportunity to practice, older adults demonstrate a slower learning curve and lower total amount learned.4 Therefore, it becomes easier relying on well-learned cognitive processes such as cooking a familiar meal or relying on previously used principles for decision making.2
Language comprehension and vocabulary remain stable over time. However, difficulty with spontaneous word finding—the “tip-of-the-tongue” phenomenon—tends to increase. In contrast to the dysnomia related to dementia, the word-finding difficulties with normal aging typically improve with cues, indicating that the problem is in retrieval of information rather than storage. Verbal fluency, the rate at which words from a single category can be produced, shows decline. This is particularly true in tests of semantic verbal fluency (name all the animals you can think of); phonemic fluency (words that start with a certain letter) tends to be preserved.4
Some studies using neurocognitive testing have suggested a decline in executive functioning. But, in general, aging has little impact on “real world” executive functions that are required for planning and executing tasks.4 On the whole, cognitive changes related to aging typically do not interfere with an individual’s ability to function independently.
Mild cognitive impairment/mild neurocognitive disorder
Originally conceived as a precursor to Alzheimer’s dementia,5 mild cognitive impairment (MCI) is a diagnosis that has evolved to describe a heterogeneous syndrome of abnormal cognition characterized by:6-8
- a suspected change in cognition expressed by the patient, an acquaintance who knows the patient well, or a clinician;
- objectively measured impairment in one or more cognitive domains beyond what would be expected based on an individual’s age and educational background;
- preservation of functional abilities; and
- a lack of findings that would fulfill criteria for dementia.
In the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM V), this concept is identified as mild neurocognitive disorder, with the additional caveats that an individual’s cognitive deficits do not occur exclusively in the context of delirium and are not better explained by another mental disorder such as depression or schizophrenia.9
An accurate assessment of cognitive change is best measured against the individual’s baseline, which may necessitate the report of a reliable acquaintance. An assessment of functional abilities is also critical. Mild problems in performing complex functions (bill paying, shopping, etc) could be present and still allow a patient to meet the criteria for MCI. An individual may take more time, be less efficient, or make more errors than before; however, independence with minimal aid or assistance is preserved. It
MCI can be divided into 4 subtypes depending upon the cognitive domains affected (complex attention, executive function, learning and memory, language, visuospatial, social cognition):
- Amnestic MCI single domain, if only memory is affected.
- Amnestic MCI multiple domain, if memory and any other cognitive domains are affected.
- Non-amnestic MCI single domain, if any other cognitive domain aside from memory is the only one affected.
- Non-amnestic MCI multiple domain, if multiple domains other than memory are affected.
These distinctions may provide clues to the underlying cause of dysfunction and provide prognostic information regarding the risk of progression to dementia.6,7
Prevalence estimates for MCI vary widely due to differences in definitions used and populations studied. The best estimate is 5% to 10% prevalence among those ages 65 to 69 years old, and 12% to 25% among those ages 80 to 84.10 Similarly, estimates of the rate of progression to dementia vary. Among MCI populations identified through referral sources such as memory centers, the rate of progression to dementia has been 10% to 15% per year.11 In epidemiologic studies of general populations, the rate has been 6% to 10% per year.11 The rate of development of dementia among normal subjects is 1% to 2% per year.5
Dementia/major neurocognitive disorder
The primary feature distinguishing MCI/mild neurocognitive disorder from dementia or major neurocognitive disorder is a patient’s functional status. The core clinical criteria for all-cause dementia are cognitive or neurobehavioral symptoms that: 12
- interfere with work or usual daily function,
- represent a change from the prior baseline function,
- are not explained by delirium or a psychiatric illness, and
- include detectable impairment in 2 cognitive domains.
Criteria outlined in the DSM-V for major neurocognitive disorder are essentially the same but describe the functional change criteria as cognitive changes that “interfere with independence in everyday activities.”9 The DSM-V elaborates: “at a minimum, requiring assistance with complex instrumental activities of daily living such as paying bills or managing medications.”
Assessing functional status accurately in clinical practice typically requires the assistance of a collateral informant who knows the patient well. The Informant Questionnaire on Cognitive Decline in the Elderly (https://www.alz.org/documents_custom/shortiqcode_english.pdf) is one validated assessment tool that can be used for this purpose.13 With this self-administered form, the informant answers 16 questions regarding changes in the patient’s performance of different activities over the 10 years prior. Alternatively, a structured interview based on indices of activities of daily living (ADLs) and instrumental activities of daily living (IADLs) as listed in TABLE 1 can be employed.14,15

Review of the various causes of dementia is beyond the scope of this article, but a list of common diagnoses is presented in TABLE 2.

Dementia syndrome of depression (pseudodementia)
Elderly patients with depression commonly complain of memory impairment, and this interaction between depression and dementia has been investigated for decades. The term “pseudodementia” has been used since 1961 to describe signs of dementia in a patient with any psychiatric illness,16 but it has since been refined to apply solely to depression. The prevalence of depression among older adults varies depending on the population studied and how depression is defined. Approximately 2% to 3% of community-dwelling elders meet criteria for major depression, with 10% to 30% showing some symptoms of depression.17,18
Twenty percent to 40% of elderly patients diagnosed with depression will have evidence of cognitive impairment.19-21 Most improve with antidepressive treatment, though evidence of cognitive impairment may continue for some.19
A broad range of cognitive deficits have been associated with depression. Most consistently described are deficits in processing speed,22-25 attention,26-28 and executive function.22,25-29 Memory deficits can be apparent with tests of delayed recall, but recognition (the ability to identify items from a list) generally is preserved.26,28-30
Distinguishing between pseudodementia and true dementia can be challenging. An increased severity of deficits, particularly with delayed recall, is more indicative of dementia.31 Additionally, on clock drawing tasks, individuals with depression perform more comparably to controls than do those with true dementia.32
A 2013 meta-analysis reported a significant association of late-life depression with subsequent development of dementia, with an odds ratio (OR) of 1.85. The risk of subsequently developing vascular dementia (OR=2.52) was significantly higher than that for Alzheimer’s disease (OR=1.65). Individuals with evidence of reversible cognitive impairment at the time of diagnosis of depression seem to be particularly vulnerable, with dementia developing in 43% to 71%, compared with rates of 12% to 18% among elders diagnosed with depression but lacking signs of cognitive impairment.20,21
Other causes of reversible dementia
A meta-analysis performed in 1988 found that 11% of cases of dementia were reversible.33 However, an update using the same methodology in 2003 revealed the number had dropped to less than 1%.34 In the latest meta-analysis, one of the authors’ leading hypotheses for the dramatic decline in apparent prevalence was a significant shift in the study population from the inpatient to outpatient setting. In studies of community-based populations used in the re-analysis, the reported prevalence of reversibility was near zero.34
Metabolic abnormalities—most often B12 deficiency and hypothyroidism—are commonly cited as potential causes of dementia. Four systematic reviews, including one conducted by the Cochrane Collaborative, concluded there is a lack of evidence that treating low vitamin B12 in individuals with dementia improves cognition.35,36 There is some evidence, though, of a time-limited window for successful treatment within 12 months of the onset of symptoms.37,38 A study reviewing causes of dementia in nearly 3000 individuals found one case of reversible dementia attributable to hypothyroidism.39 A subsequent review reached similar conclusions about the lack of data to support the notion that treatment of hypothyroidism reverses dementia.40
Similarly, imaging for cerebral tumors, subdural hematomas, or normal-pressure hydrocephalus rarely identifies these as a cause of dementia.41 This is particularly true of unselected community-based populations, as there are typically signs or symptoms suggesting an intracranial pathology.
Numerous medications have been implicated in causing acute confusional states, and there is some evidence for their role in chronic confusion (TABLE 3).42,43 In my experience, many who experience adverse effects on cognition with medications will also have an underlying neurodegenerative process, and symptoms do not completely resolve with withdrawal of the offending agent.

Further assessment of the patient yielded a score of 29/30 on the Montreal Cognitive Assessment* and a zero on the Patient Health Questionnaire-2. Careful review of her daily function revealed no significant deficits in ADL or IADL performance, and her daughter confirmed that she had not observed any significant decline in her mother’s function. There was no significant family history of dementia. The patient was reassured that her cognitive changes were normal and age related.
Unfortunately, few data support specific interventions to reduce this patient’s risk of developing dementia. She was commended for keeping her blood pressure and blood sugar levels under control, thereby reducing her risk of vascular disease.
She and her daughter were directed to the Alzheimer’s Association Web site (alz.org) as a resource for information about signs and symptoms to watch for and for caregiving resources, should they be needed. She was briefly counseled to eliminate distractions to improve her ability to complete tasks and improve recall along with rehearsing or writing down information that she wished to retain.
Finally, she was counseled to remain physically, cognitively, and socially active as these are factors generally associated with healthy aging, have some evidence to support efficacy in reducing the risk of cognitive decline,44,45 and are unlikely to be of harm.
*The Montreal Cognitive Assessment is a validated office-based tool for the evaluation of cognitive impairment that is highly sensitive for the detection of mild cognitive impairment.
CORRESPONDENCE
Ian M. Deutchki, MD, Professor of Family Medicine and Geriatrics, University of Rochester Medical Center, 777 S. Clinton Avenue, Rochester, NY 14620; [email protected].
1. Mitchell AJ. The clinical significance of subjective memory complaints in the diagnosis of mild cognitive impairment and dementia: a meta-analysis. Int J Geriatr Psychiatry. 2008;23:1191-1202.
2. Burnette V, Howell T. Cognitive changes in aging. In: Capezuti EA, Malone ML, Katz PR, et al, eds. The Encyclopedia of Elder Care. New York, NY, USA: Springer Publishing Company; 2013.
3. Glisky EL. Changes in cognitive function in human aging. In: Riddle DR, ed. Brain Aging: Models, Methods, and Mechanisms. Boca Raton, FL: Taylor & Francis Group, LLC; 2007:4-20.
4. Craft S, Cholerton B, Reger M. Cognitive changes associated with normal and pathological aging. In: Halter JB, Ouslander JG, Tinetti ME, Studenski S, et al, eds. Hazzard’s Geriatric Medicine and Gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:751-766.
5. Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303-308.
6. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183-194.
7. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240-246.
8. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270-279.
9. Neurocognitive disorders. In: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
10. Ward A. Arrighi HM, Michels S, et al. Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement. 2012;8:14-21.
11. Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66:1447-1455.
12. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263-269.
13. Jorm AF. A short form of the informant questionnaire on cognitive decline in the elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145-153.
14. Katz S, Downs TD, Cash HR, et al. Progress in development of the index of ADL. Gerontologist. 1970;10:20-30.
15. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179-186.
16. Kiloh LG. Pseudo-dementia. Acta Psychiatr Scand. 1961;37:336-351.
17. Beekman AT, Copeland JR, Prince MJ. Review of community prevalence of depression in later life. Br J Psychiatry. 1999;174:307-311.
18. Birrer RB, Vemuri SP. Depression in later life: a diagnostic and therapeutic challenge. Am Fam Physician. 2004;69:2375-2382.
19. Butters MA, Becker JT, Nebes RD, et al. Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry. 2000;157:1949-1954.
20. Alexopoulos GS, Meyers BS, Young RC, et al. The course of geriatric depression with “reversible dementia”: a controlled study. Am J Psychiatry. 1993;150:1693-1699.
21. Saez-Fonseca JA, Lee L, Walker Z. Long-term outcome of depressive pseudodementia in the elderly. J Affect Disord. 2007;101:123-129.
22. Dillon C, Allegri RF, Serrano CM, et al. Late- versus early-onset geriatric depression in a memory research center. Neuropsychiatr Dis Treat. 2009;5:517-526.
23. Lockwood KA, Alexopoulos GS, van Gorp WG. Executive dysfunction in geriatric depression. Am J Psychiatry. 2002;159:1119-1126.
24. Shimada H, Park H, Makizako H, et al. Depressive symptoms and cognitive performance in older adults. J Psychiatr Res. 2014;57:149-156.
25. Butters MA, Whyte EM, Nebes RD, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61:587-595.
26. Dillon C, Machnicki G, Serrano CM, et al. Clinical manifestations of geriatric depression in a memory clinic: toward a proposed subtyping of geriatric depression. J Affect Disord. 2011;134:177-187.
27. Rapp MA, Dahlman K, Sano M, et al. Neuropsychological differences between late-onset and recurrent geriatric major depression. Am J Psychiatry. 2005;162:691-698.
28. Zihl J, Reppermund S, Thum S, et al. Neuropsychological profiles in MCI and in depression: differential cognitive dysfunction patterns or similar final common pathway disorder? J Psychiatr Res. 2010;44:647-654.
29. Dillon C, Tartaglini MF, Stefani D, et al. Geriatric depression and its relation with cognitive impairment and dementia. Arch Gerontol Geriatr. 2014;59:450-456.
30. Wright SL, Persad C. Distinguishing between depression and dementia in older persons: neuropsychological and neuropathological correlates. J Geriatr Psychiatry Neurol. 2007;20:189-198.
31. Visser PJ, Verhey FR, Ponds RW, et al. Distinction between preclinical Alzheimer’s disease and depression. J Am Geriatr Soc. 2000;48:479-484.
32. Bodner T, Delazer M, Kemmler G, et al. Clock drawing, clock reading, clock setting, and judgment of clock faces in elderly people with dementia and depression. J Am Geriatr Soc. 2004;52:1146-1150.
33. Clarfield AM. The reversible dementias: do they reverse? Ann Intern Med. 1988;109:476-486.
34. Clarfield AM. The decreasing prevalence of reversible dementias: an updated meta-analysis. Arch Intern Med. 2003;163:2219-2229.
35. Malouf R, Areosa Sastre A. Vitamin B12 for cognition. Cochrane Database Syst Rev. 2003;(3):CD004326.
36. Health Quality Ontario. Vitamin B12 and cognitive function: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13:1-45.
37. Abyad A. Prevalence of vitamin B12 deficiency among demented patients and cognitive recovery with cobalamin replacement. J Nutr Health Aging. 2002;6:254-260.
38. Martin DC, Francis J, Protetch J, et al. Time dependency of cognitive recovery with cobalamin replacement: Report of a pilot study. J Am Geriatr Soc. 1992;40:168-172.
39. Clarnette RM, Patterson CJ. Hypothyroidism: does treatment cure dementia? J Geriatr Psychiatry Neurol. 1994;7:23-27.
40. Dugbartey AT. Neurocognitive aspects of hypothyroidism. Arch Intern Med. 1998;158:1413-1418.
41. Alexander EM, Wagner EH, Buchner DM, et al. Do surgical brain lesions present as isolated dementia? A population-based study. J Am Geriatr Soc. 1995;43:138-143.
42. Moore AR, O’Keeffe ST. Drug-induced cognitive impairment in the elderly. Drugs Aging. 1999;15:15-28.
43. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American geriatrics society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
44. Middleton LE, Yaffe K. Promising strategies for the prevention of dementia. Arch Neurol. 2009;66:1210-1215.
45. Shatenstein B, Barberger-Gateau P, Mecocci P. Prevention of age-related cognitive decline: which strategies, when, and for whom? J Alzheimers Dis. 2015;48:35-53.
1. Mitchell AJ. The clinical significance of subjective memory complaints in the diagnosis of mild cognitive impairment and dementia: a meta-analysis. Int J Geriatr Psychiatry. 2008;23:1191-1202.
2. Burnette V, Howell T. Cognitive changes in aging. In: Capezuti EA, Malone ML, Katz PR, et al, eds. The Encyclopedia of Elder Care. New York, NY, USA: Springer Publishing Company; 2013.
3. Glisky EL. Changes in cognitive function in human aging. In: Riddle DR, ed. Brain Aging: Models, Methods, and Mechanisms. Boca Raton, FL: Taylor & Francis Group, LLC; 2007:4-20.
4. Craft S, Cholerton B, Reger M. Cognitive changes associated with normal and pathological aging. In: Halter JB, Ouslander JG, Tinetti ME, Studenski S, et al, eds. Hazzard’s Geriatric Medicine and Gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:751-766.
5. Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303-308.
6. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183-194.
7. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240-246.
8. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270-279.
9. Neurocognitive disorders. In: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
10. Ward A. Arrighi HM, Michels S, et al. Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement. 2012;8:14-21.
11. Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66:1447-1455.
12. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263-269.
13. Jorm AF. A short form of the informant questionnaire on cognitive decline in the elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145-153.
14. Katz S, Downs TD, Cash HR, et al. Progress in development of the index of ADL. Gerontologist. 1970;10:20-30.
15. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179-186.
16. Kiloh LG. Pseudo-dementia. Acta Psychiatr Scand. 1961;37:336-351.
17. Beekman AT, Copeland JR, Prince MJ. Review of community prevalence of depression in later life. Br J Psychiatry. 1999;174:307-311.
18. Birrer RB, Vemuri SP. Depression in later life: a diagnostic and therapeutic challenge. Am Fam Physician. 2004;69:2375-2382.
19. Butters MA, Becker JT, Nebes RD, et al. Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry. 2000;157:1949-1954.
20. Alexopoulos GS, Meyers BS, Young RC, et al. The course of geriatric depression with “reversible dementia”: a controlled study. Am J Psychiatry. 1993;150:1693-1699.
21. Saez-Fonseca JA, Lee L, Walker Z. Long-term outcome of depressive pseudodementia in the elderly. J Affect Disord. 2007;101:123-129.
22. Dillon C, Allegri RF, Serrano CM, et al. Late- versus early-onset geriatric depression in a memory research center. Neuropsychiatr Dis Treat. 2009;5:517-526.
23. Lockwood KA, Alexopoulos GS, van Gorp WG. Executive dysfunction in geriatric depression. Am J Psychiatry. 2002;159:1119-1126.
24. Shimada H, Park H, Makizako H, et al. Depressive symptoms and cognitive performance in older adults. J Psychiatr Res. 2014;57:149-156.
25. Butters MA, Whyte EM, Nebes RD, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61:587-595.
26. Dillon C, Machnicki G, Serrano CM, et al. Clinical manifestations of geriatric depression in a memory clinic: toward a proposed subtyping of geriatric depression. J Affect Disord. 2011;134:177-187.
27. Rapp MA, Dahlman K, Sano M, et al. Neuropsychological differences between late-onset and recurrent geriatric major depression. Am J Psychiatry. 2005;162:691-698.
28. Zihl J, Reppermund S, Thum S, et al. Neuropsychological profiles in MCI and in depression: differential cognitive dysfunction patterns or similar final common pathway disorder? J Psychiatr Res. 2010;44:647-654.
29. Dillon C, Tartaglini MF, Stefani D, et al. Geriatric depression and its relation with cognitive impairment and dementia. Arch Gerontol Geriatr. 2014;59:450-456.
30. Wright SL, Persad C. Distinguishing between depression and dementia in older persons: neuropsychological and neuropathological correlates. J Geriatr Psychiatry Neurol. 2007;20:189-198.
31. Visser PJ, Verhey FR, Ponds RW, et al. Distinction between preclinical Alzheimer’s disease and depression. J Am Geriatr Soc. 2000;48:479-484.
32. Bodner T, Delazer M, Kemmler G, et al. Clock drawing, clock reading, clock setting, and judgment of clock faces in elderly people with dementia and depression. J Am Geriatr Soc. 2004;52:1146-1150.
33. Clarfield AM. The reversible dementias: do they reverse? Ann Intern Med. 1988;109:476-486.
34. Clarfield AM. The decreasing prevalence of reversible dementias: an updated meta-analysis. Arch Intern Med. 2003;163:2219-2229.
35. Malouf R, Areosa Sastre A. Vitamin B12 for cognition. Cochrane Database Syst Rev. 2003;(3):CD004326.
36. Health Quality Ontario. Vitamin B12 and cognitive function: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13:1-45.
37. Abyad A. Prevalence of vitamin B12 deficiency among demented patients and cognitive recovery with cobalamin replacement. J Nutr Health Aging. 2002;6:254-260.
38. Martin DC, Francis J, Protetch J, et al. Time dependency of cognitive recovery with cobalamin replacement: Report of a pilot study. J Am Geriatr Soc. 1992;40:168-172.
39. Clarnette RM, Patterson CJ. Hypothyroidism: does treatment cure dementia? J Geriatr Psychiatry Neurol. 1994;7:23-27.
40. Dugbartey AT. Neurocognitive aspects of hypothyroidism. Arch Intern Med. 1998;158:1413-1418.
41. Alexander EM, Wagner EH, Buchner DM, et al. Do surgical brain lesions present as isolated dementia? A population-based study. J Am Geriatr Soc. 1995;43:138-143.
42. Moore AR, O’Keeffe ST. Drug-induced cognitive impairment in the elderly. Drugs Aging. 1999;15:15-28.
43. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American geriatrics society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
44. Middleton LE, Yaffe K. Promising strategies for the prevention of dementia. Arch Neurol. 2009;66:1210-1215.
45. Shatenstein B, Barberger-Gateau P, Mecocci P. Prevention of age-related cognitive decline: which strategies, when, and for whom? J Alzheimers Dis. 2015;48:35-53.
From The Journal of Family Practice | 2017;66(11):670-676.
PRACTICE RECOMMENDATIONS
› Evaluate cognitive domain involvement in cases of suspected mild cognitive impairment; findings could suggest an underlying cause and indicate risk of progression to dementia. C
› Consider the severity of a cognitive deficit (eg, delayed recall) when depression is diagnosed; a marked deficit is usually more indicative of true dementia than pseudodementia. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
FDA approves drug to treat rel/ref MCL
The US Food and Drug Administration (FDA) has granted accelerated approval to the BTK inhibitor acalabrutinib (Calquence, formerly ACP-196).
The drug is now approved to treat adults with mantle cell lymphoma (MCL) who have received at least 1 prior therapy.
The FDA’s accelerated approval pathway is used for drugs intended to treat serious conditions where there is unmet medical need and when said drugs have demonstrated effects that suggest they will provide a clinical benefit to patients.
This means further study is required to verify and describe the anticipated clinical benefits of acalabrutinib, which was approved based on the overall response rate observed in a phase 2 trial.
The company developing acalabrutinib, AstraZeneca Pharmaceuticals LP, is currently conducting the necessary additional research.
The FDA previously granted AstraZeneca priority review, breakthrough therapy, and orphan drug designations for acalabrutinib as a treatment for MCL.
Phase 2 trial
The FDA approved acalabrutinib based on results of the phase 2 ACE-LY-004 trial. This single-arm trial enrolled 124 adults with relapsed or refractory MCL.
According to AstraZeneca, acalabrutinib produced an overall response rate of 80%, with 40% of patients achieving a complete response and 40% experiencing a partial response.
The most common adverse events (AEs) of any grade (occurring in at least 20% of patients) were anemia (46%), thrombocytopenia (44%), headache (39%), neutropenia (36%), diarrhea (31%), fatigue (28%), myalgia (21%), and bruising (21%).
Dosage reductions due to AEs occurred in 1.6% of patients. Discontinuations due to AEs occurred in 6.5% of patients. Increases in creatinine 1.5 to 3 times the upper limit of normal occurred in 4.8% of patients.
According to AstraZeneca, full results from ACE-LY-004 have been submitted for presentation at an upcoming medical meeting.
This will be the first MCL trial data to be presented from the acalabrutinib development program, which includes both monotherapy and combination therapies in hematologic and solid tumor malignancies. ![]()
The US Food and Drug Administration (FDA) has granted accelerated approval to the BTK inhibitor acalabrutinib (Calquence, formerly ACP-196).
The drug is now approved to treat adults with mantle cell lymphoma (MCL) who have received at least 1 prior therapy.
The FDA’s accelerated approval pathway is used for drugs intended to treat serious conditions where there is unmet medical need and when said drugs have demonstrated effects that suggest they will provide a clinical benefit to patients.
This means further study is required to verify and describe the anticipated clinical benefits of acalabrutinib, which was approved based on the overall response rate observed in a phase 2 trial.
The company developing acalabrutinib, AstraZeneca Pharmaceuticals LP, is currently conducting the necessary additional research.
The FDA previously granted AstraZeneca priority review, breakthrough therapy, and orphan drug designations for acalabrutinib as a treatment for MCL.
Phase 2 trial
The FDA approved acalabrutinib based on results of the phase 2 ACE-LY-004 trial. This single-arm trial enrolled 124 adults with relapsed or refractory MCL.
According to AstraZeneca, acalabrutinib produced an overall response rate of 80%, with 40% of patients achieving a complete response and 40% experiencing a partial response.
The most common adverse events (AEs) of any grade (occurring in at least 20% of patients) were anemia (46%), thrombocytopenia (44%), headache (39%), neutropenia (36%), diarrhea (31%), fatigue (28%), myalgia (21%), and bruising (21%).
Dosage reductions due to AEs occurred in 1.6% of patients. Discontinuations due to AEs occurred in 6.5% of patients. Increases in creatinine 1.5 to 3 times the upper limit of normal occurred in 4.8% of patients.
According to AstraZeneca, full results from ACE-LY-004 have been submitted for presentation at an upcoming medical meeting.
This will be the first MCL trial data to be presented from the acalabrutinib development program, which includes both monotherapy and combination therapies in hematologic and solid tumor malignancies. ![]()
The US Food and Drug Administration (FDA) has granted accelerated approval to the BTK inhibitor acalabrutinib (Calquence, formerly ACP-196).
The drug is now approved to treat adults with mantle cell lymphoma (MCL) who have received at least 1 prior therapy.
The FDA’s accelerated approval pathway is used for drugs intended to treat serious conditions where there is unmet medical need and when said drugs have demonstrated effects that suggest they will provide a clinical benefit to patients.
This means further study is required to verify and describe the anticipated clinical benefits of acalabrutinib, which was approved based on the overall response rate observed in a phase 2 trial.
The company developing acalabrutinib, AstraZeneca Pharmaceuticals LP, is currently conducting the necessary additional research.
The FDA previously granted AstraZeneca priority review, breakthrough therapy, and orphan drug designations for acalabrutinib as a treatment for MCL.
Phase 2 trial
The FDA approved acalabrutinib based on results of the phase 2 ACE-LY-004 trial. This single-arm trial enrolled 124 adults with relapsed or refractory MCL.
According to AstraZeneca, acalabrutinib produced an overall response rate of 80%, with 40% of patients achieving a complete response and 40% experiencing a partial response.
The most common adverse events (AEs) of any grade (occurring in at least 20% of patients) were anemia (46%), thrombocytopenia (44%), headache (39%), neutropenia (36%), diarrhea (31%), fatigue (28%), myalgia (21%), and bruising (21%).
Dosage reductions due to AEs occurred in 1.6% of patients. Discontinuations due to AEs occurred in 6.5% of patients. Increases in creatinine 1.5 to 3 times the upper limit of normal occurred in 4.8% of patients.
According to AstraZeneca, full results from ACE-LY-004 have been submitted for presentation at an upcoming medical meeting.
This will be the first MCL trial data to be presented from the acalabrutinib development program, which includes both monotherapy and combination therapies in hematologic and solid tumor malignancies. ![]()
Treating an Alzheimer’s patient? 6 tips from a patient’s spouse
What’s it like to be the caregiver for an Alzheimer’s patient? In my case, it was like being both married and widowed at the same time. Or as a person in my support group once put it: It’s a life filled with grief on the installment plan.
My wife, Clare, struggled for nearly 10 years with Alzheimer’s disease before passing away in April 2016—just one month shy of her 70th birthday and 2 months shy of our 49th wedding anniversary.
Our experience was gut-wrenching, but not unique for families coping with Alzheimer’s disease. Life as a caregiver is one of non-stop daily stress, with much sadness and anxiety, often accompanied by periods of mild or serious depression. Doubt, guilt, frustration, and many other emotions lead many caregivers to take anti-anxiety or antidepressant medication, meet regularly with therapists, take sleeping pills, or experience significant weight gain or loss. Stress drove me to my comfort foods, and I gained nearly 100 pounds while caring for Clare. Only in the last few months have I been able to start taking off that weight.
Helping a loved one who has Alzheimer’s with even the basic activities of daily living—hygiene, dressing, eating—becomes progressively difficult. Caring for a loved one who is confused, no longer remembers your name or who you are, or can occasionally become aggressive, is emotionally painful.
After being Clare’s 24/7 caregiver for 6 years, I agreed that placement in an assisted living facility was in her best interest. My role morphed from primary caregiver to primary care advocate, but the stress did not lessen. I met regularly with facility staff to ensure proper care because many staff members were not sufficiently motivated, educated, or trained to consistently provide proper care for individuals with Alzheimer’s disease.
Financial stress weighs heavily on caregivers. Unless one qualifies for Medicaid, is very wealthy, or is lucky enough to have outstanding long-term health care insurance and prescription drug coverage, caregiving costs can be astronomical. For someone with Alzheimer’s in a community such as Long Island, NY, assisted living facilities charge between $7000 and $10,000 per month, and nursing homes between $15,000 and $18,000 per month. Home health aides working 24/7 also cost around $15,000 per month. Caregiving costs can drain not just the patient’s bank account, but can wipe out the retirement life savings of the surviving caregiver.
Once Clare went into assisted living, I dealt with the daily loneliness and the enormous lifestyle changes. Being alone in my bed those first few nights after placement was painful beyond words, and learning to live alone for the first time after many years of marriage brought incredible sadness. It is no surprise to me that research points to caregiver stress as an independent risk factor for elderly caregiver mortality.1
My experience navigating the health care system with my wife included numerous challenges and instances of unnecessary frustration. My hope in providing the following suggestions is that they will help you help other families like mine.
1. Listen carefully to caregivers
When Clare first exhibited symptoms suggestive of Alzheimer’s, I started logging them and presented written summaries to doctors at each visit. But unless Clare exhibited those same symptoms in the presence of her doctors, my observations were routinely ignored. I’d try to discuss concerns—eg, Clare getting lost while driving to familiar locations, experiencing increased aphasia—but the doctors didn’t read my logs or listen carefully to what I was trying to tell them. The January/February 2017 AARP Bulletin2 noted studies showing that doctors listen for about 23 seconds before interrupting patients, but it also cited a 2001 South Carolina study3 that found patients spoke, uninterrupted, for an average of 12 seconds before being interrupted by a resident.
I eventually did learn that early Alzheimer’s symptoms can be easily misinterpreted as signs of stress, anxiety, or depression. But that underscores the need for doctors to listen carefully to caregivers, especially spouse caregivers who observe behaviors 24/7 that may not be present in a quick office visit or revealed on a brief cognitive screening test.
2. Stay up to date on screening tools that detect Alzheimer’s
The Mini-Mental State Examination, or MMSE, is the most frequently used cognitive screening tool, in part because it can be administered in less than 10 minutes. Although unquestionably valuable, a Cochrane review “did not find evidence supporting a substantial role of MMSE as a stand-alone single-administration test in the identification of MCI [mild-cognitive impairment] patients who could develop dementia.”4
Time-pressured doctors might consider using the AD8 screening interview, an informant questionnaire that takes only 2 to 3 minutes to administer, but has demonstrated superior sensitivity in detecting early dementia compared with the MMSE.5 In addition, a study in the December 2016 issue of the Journal of Alzheimer’s Disease 6 confirmed the usefulness of the Sniffin’ Sticks Odor Identification Test whereby patients try to identify 16 different odors. I can attest to Clare’s rapidly deteriorating senses of taste and smell as her disease progressed.
“Results suggest that a simple odor identification test can be a useful supplementary tool for clinically categorizing MCI and Alzheimer’s, and even for identifying people who are at the highest risk of worsening,” according to principal investigator, David R. Roalf, PhD.7
Prompted by prior studies that have linked a weakening sense of smell to Alzheimer’s, doctors in a few larger dementia clinics have already begun using smell tests in their assessments. One possible reason the practice has not yet become common, however, is that the tests take about 5 to 8 minutes to administer. Roalf and his colleagues are hoping to develop a shorter test that will work as well as the longer ones. “We’re hoping to shorten the Sniffin’ Sticks test … down to 3 minutes or so … We think that will encourage more neurology clinics to do this type of screening.”7
Is 5 minutes too much time to take to administer a valuable screening test?
3. Be candid when speaking with patients and their caregivers
A survey reported in Time magazine on March 24, 2015, found that as many as 64% of doctors do not share a diagnosis of Alzheimer’s with their patients because of “fear of causing emotional distress in their patients” due to a lack of effective treatment or cure, and because of a “lack of time and resources to fully explain what the diagnosis means.”8
But Alzheimer’s patients and their caregivers need as much time as possible to plan accordingly, especially if they have not already discussed and finalized end-of-life planning (will, living will, health care proxy, durable power of attorney), preferences for staying at home with aides or being placed in a facility, or wishes to take final trips or enjoy final activities together before cognitive impairment worsens. Withholding a diagnosis can rob patients and caregivers of that valuable planning time.
4. Connect caregivers to resources and support groups
Information on the stages of the disease, available local support groups, and online resources are extremely helpful. Of the 15 people in my spouse support group, only one or 2 were referred there by a doctor. Become familiar with local support groups because that is where caregivers discuss common needs, learn and share helpful caregiving strategies and techniques, and find emotional support from others walking in similar shoes.
5. Help caregivers take away the car keys
When to take away the car keys is an extremely difficult emotional decision that often leads to heated arguments. People with Alzheimer’s rightfully fear losing their independence and only reluctantly accept they can no longer drive safely. But their caregivers worry about them getting lost or causing an accident or, worse, a death. Even though some people with Alzheimer’s can continue to drive safely for a while, the ever-worsening cognitive decline with the disease sooner or later leads to impaired judgment and the inability to drive safely.
If caregivers have already observed issues with their loved one’s driving ability and ask you to intervene, please help remove a major cause of caregiver stress while also making our roads safer. And please do not routinely refer people with Alzheimer’s to driving test facilities. A person with Alzheimer’s may do very well at the particular moment of the test, yet might fail that same test if it was given an hour earlier or later.
6. Manage expectations of what medications can do
None of the current FDA-approved medications have proven to have any long-term positive effects on Alzheimer’s. Clinical trial data show that these meds may be able to slow the rate of disease progression for some people who take them, but even then the benefit is short-lived. Yet many doctors, year after year, renew these “expensive bottles of hope,” as I call them, when the thousands of dollars needed to buy them could be much better spent on day-care programs or personal aides. A candid disclosure to patients and caregivers would enable better decision-making.
1. Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;282:2215-2219.
2. Patural A. How to talk so your doctor will listen. AARP Bulletin. January/February 2017. Available at: http://www.aarp.org/health/healthy-living/info-2016/talk-to-doctor-patient-relationship.html. Accessed September 25, 2017.
3. Rhoades DR, McFarland KF, Finch WH, et al. Speaking and interruptions during primary care office visits. Fam Med. 2001;33:528-532.
4. Arevalo-Rodriguez I, Smailagic N, Roque I Figuls M, et al. Mini-Mental State Examination (MMSE) for the detection of Alzheimer’s disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev. 2015;(3):CD010783.
5. Galvin JE, Fagan AM, Holtzman DM, et al. Relationship of dementia screening tests with biomarkers of Alzheimer's disease. Brain. 2010;133:3290-3300.
6. Quarmley M, Moberg PJ, Mechanic-Hamilton D, et al. Odor identification screening improves diagnostic classification in incipient Alzheimer’s disease. J Alzheimers Dis. 2017;55:1497-1507.
7. Penn study confirms that “sniff test” may be useful in diagnosing early Alzheimer’s disease. December 21, 2016. Available at: http://www.j-alz.com/content/penn-study-confirms-%E2%80%9Csniff-test%E2%80%9D-may-be-useful-diagnosing-early-alzheimer%E2%80%99s-disease. Accessed October 12, 2017.
8. Park A. Many doctors don’t tell patients they have Alzheimer’s. Time. March 24, 2015. Available at: http://time.com/3755176/doctors-diagnose-alzheimers-dont-tell/. Accessed September 25, 2017.
What’s it like to be the caregiver for an Alzheimer’s patient? In my case, it was like being both married and widowed at the same time. Or as a person in my support group once put it: It’s a life filled with grief on the installment plan.
My wife, Clare, struggled for nearly 10 years with Alzheimer’s disease before passing away in April 2016—just one month shy of her 70th birthday and 2 months shy of our 49th wedding anniversary.
Our experience was gut-wrenching, but not unique for families coping with Alzheimer’s disease. Life as a caregiver is one of non-stop daily stress, with much sadness and anxiety, often accompanied by periods of mild or serious depression. Doubt, guilt, frustration, and many other emotions lead many caregivers to take anti-anxiety or antidepressant medication, meet regularly with therapists, take sleeping pills, or experience significant weight gain or loss. Stress drove me to my comfort foods, and I gained nearly 100 pounds while caring for Clare. Only in the last few months have I been able to start taking off that weight.
Helping a loved one who has Alzheimer’s with even the basic activities of daily living—hygiene, dressing, eating—becomes progressively difficult. Caring for a loved one who is confused, no longer remembers your name or who you are, or can occasionally become aggressive, is emotionally painful.
After being Clare’s 24/7 caregiver for 6 years, I agreed that placement in an assisted living facility was in her best interest. My role morphed from primary caregiver to primary care advocate, but the stress did not lessen. I met regularly with facility staff to ensure proper care because many staff members were not sufficiently motivated, educated, or trained to consistently provide proper care for individuals with Alzheimer’s disease.
Financial stress weighs heavily on caregivers. Unless one qualifies for Medicaid, is very wealthy, or is lucky enough to have outstanding long-term health care insurance and prescription drug coverage, caregiving costs can be astronomical. For someone with Alzheimer’s in a community such as Long Island, NY, assisted living facilities charge between $7000 and $10,000 per month, and nursing homes between $15,000 and $18,000 per month. Home health aides working 24/7 also cost around $15,000 per month. Caregiving costs can drain not just the patient’s bank account, but can wipe out the retirement life savings of the surviving caregiver.
Once Clare went into assisted living, I dealt with the daily loneliness and the enormous lifestyle changes. Being alone in my bed those first few nights after placement was painful beyond words, and learning to live alone for the first time after many years of marriage brought incredible sadness. It is no surprise to me that research points to caregiver stress as an independent risk factor for elderly caregiver mortality.1
My experience navigating the health care system with my wife included numerous challenges and instances of unnecessary frustration. My hope in providing the following suggestions is that they will help you help other families like mine.
1. Listen carefully to caregivers
When Clare first exhibited symptoms suggestive of Alzheimer’s, I started logging them and presented written summaries to doctors at each visit. But unless Clare exhibited those same symptoms in the presence of her doctors, my observations were routinely ignored. I’d try to discuss concerns—eg, Clare getting lost while driving to familiar locations, experiencing increased aphasia—but the doctors didn’t read my logs or listen carefully to what I was trying to tell them. The January/February 2017 AARP Bulletin2 noted studies showing that doctors listen for about 23 seconds before interrupting patients, but it also cited a 2001 South Carolina study3 that found patients spoke, uninterrupted, for an average of 12 seconds before being interrupted by a resident.
I eventually did learn that early Alzheimer’s symptoms can be easily misinterpreted as signs of stress, anxiety, or depression. But that underscores the need for doctors to listen carefully to caregivers, especially spouse caregivers who observe behaviors 24/7 that may not be present in a quick office visit or revealed on a brief cognitive screening test.
2. Stay up to date on screening tools that detect Alzheimer’s
The Mini-Mental State Examination, or MMSE, is the most frequently used cognitive screening tool, in part because it can be administered in less than 10 minutes. Although unquestionably valuable, a Cochrane review “did not find evidence supporting a substantial role of MMSE as a stand-alone single-administration test in the identification of MCI [mild-cognitive impairment] patients who could develop dementia.”4
Time-pressured doctors might consider using the AD8 screening interview, an informant questionnaire that takes only 2 to 3 minutes to administer, but has demonstrated superior sensitivity in detecting early dementia compared with the MMSE.5 In addition, a study in the December 2016 issue of the Journal of Alzheimer’s Disease 6 confirmed the usefulness of the Sniffin’ Sticks Odor Identification Test whereby patients try to identify 16 different odors. I can attest to Clare’s rapidly deteriorating senses of taste and smell as her disease progressed.
“Results suggest that a simple odor identification test can be a useful supplementary tool for clinically categorizing MCI and Alzheimer’s, and even for identifying people who are at the highest risk of worsening,” according to principal investigator, David R. Roalf, PhD.7
Prompted by prior studies that have linked a weakening sense of smell to Alzheimer’s, doctors in a few larger dementia clinics have already begun using smell tests in their assessments. One possible reason the practice has not yet become common, however, is that the tests take about 5 to 8 minutes to administer. Roalf and his colleagues are hoping to develop a shorter test that will work as well as the longer ones. “We’re hoping to shorten the Sniffin’ Sticks test … down to 3 minutes or so … We think that will encourage more neurology clinics to do this type of screening.”7
Is 5 minutes too much time to take to administer a valuable screening test?
3. Be candid when speaking with patients and their caregivers
A survey reported in Time magazine on March 24, 2015, found that as many as 64% of doctors do not share a diagnosis of Alzheimer’s with their patients because of “fear of causing emotional distress in their patients” due to a lack of effective treatment or cure, and because of a “lack of time and resources to fully explain what the diagnosis means.”8
But Alzheimer’s patients and their caregivers need as much time as possible to plan accordingly, especially if they have not already discussed and finalized end-of-life planning (will, living will, health care proxy, durable power of attorney), preferences for staying at home with aides or being placed in a facility, or wishes to take final trips or enjoy final activities together before cognitive impairment worsens. Withholding a diagnosis can rob patients and caregivers of that valuable planning time.
4. Connect caregivers to resources and support groups
Information on the stages of the disease, available local support groups, and online resources are extremely helpful. Of the 15 people in my spouse support group, only one or 2 were referred there by a doctor. Become familiar with local support groups because that is where caregivers discuss common needs, learn and share helpful caregiving strategies and techniques, and find emotional support from others walking in similar shoes.
5. Help caregivers take away the car keys
When to take away the car keys is an extremely difficult emotional decision that often leads to heated arguments. People with Alzheimer’s rightfully fear losing their independence and only reluctantly accept they can no longer drive safely. But their caregivers worry about them getting lost or causing an accident or, worse, a death. Even though some people with Alzheimer’s can continue to drive safely for a while, the ever-worsening cognitive decline with the disease sooner or later leads to impaired judgment and the inability to drive safely.
If caregivers have already observed issues with their loved one’s driving ability and ask you to intervene, please help remove a major cause of caregiver stress while also making our roads safer. And please do not routinely refer people with Alzheimer’s to driving test facilities. A person with Alzheimer’s may do very well at the particular moment of the test, yet might fail that same test if it was given an hour earlier or later.
6. Manage expectations of what medications can do
None of the current FDA-approved medications have proven to have any long-term positive effects on Alzheimer’s. Clinical trial data show that these meds may be able to slow the rate of disease progression for some people who take them, but even then the benefit is short-lived. Yet many doctors, year after year, renew these “expensive bottles of hope,” as I call them, when the thousands of dollars needed to buy them could be much better spent on day-care programs or personal aides. A candid disclosure to patients and caregivers would enable better decision-making.
What’s it like to be the caregiver for an Alzheimer’s patient? In my case, it was like being both married and widowed at the same time. Or as a person in my support group once put it: It’s a life filled with grief on the installment plan.
My wife, Clare, struggled for nearly 10 years with Alzheimer’s disease before passing away in April 2016—just one month shy of her 70th birthday and 2 months shy of our 49th wedding anniversary.
Our experience was gut-wrenching, but not unique for families coping with Alzheimer’s disease. Life as a caregiver is one of non-stop daily stress, with much sadness and anxiety, often accompanied by periods of mild or serious depression. Doubt, guilt, frustration, and many other emotions lead many caregivers to take anti-anxiety or antidepressant medication, meet regularly with therapists, take sleeping pills, or experience significant weight gain or loss. Stress drove me to my comfort foods, and I gained nearly 100 pounds while caring for Clare. Only in the last few months have I been able to start taking off that weight.
Helping a loved one who has Alzheimer’s with even the basic activities of daily living—hygiene, dressing, eating—becomes progressively difficult. Caring for a loved one who is confused, no longer remembers your name or who you are, or can occasionally become aggressive, is emotionally painful.
After being Clare’s 24/7 caregiver for 6 years, I agreed that placement in an assisted living facility was in her best interest. My role morphed from primary caregiver to primary care advocate, but the stress did not lessen. I met regularly with facility staff to ensure proper care because many staff members were not sufficiently motivated, educated, or trained to consistently provide proper care for individuals with Alzheimer’s disease.
Financial stress weighs heavily on caregivers. Unless one qualifies for Medicaid, is very wealthy, or is lucky enough to have outstanding long-term health care insurance and prescription drug coverage, caregiving costs can be astronomical. For someone with Alzheimer’s in a community such as Long Island, NY, assisted living facilities charge between $7000 and $10,000 per month, and nursing homes between $15,000 and $18,000 per month. Home health aides working 24/7 also cost around $15,000 per month. Caregiving costs can drain not just the patient’s bank account, but can wipe out the retirement life savings of the surviving caregiver.
Once Clare went into assisted living, I dealt with the daily loneliness and the enormous lifestyle changes. Being alone in my bed those first few nights after placement was painful beyond words, and learning to live alone for the first time after many years of marriage brought incredible sadness. It is no surprise to me that research points to caregiver stress as an independent risk factor for elderly caregiver mortality.1
My experience navigating the health care system with my wife included numerous challenges and instances of unnecessary frustration. My hope in providing the following suggestions is that they will help you help other families like mine.
1. Listen carefully to caregivers
When Clare first exhibited symptoms suggestive of Alzheimer’s, I started logging them and presented written summaries to doctors at each visit. But unless Clare exhibited those same symptoms in the presence of her doctors, my observations were routinely ignored. I’d try to discuss concerns—eg, Clare getting lost while driving to familiar locations, experiencing increased aphasia—but the doctors didn’t read my logs or listen carefully to what I was trying to tell them. The January/February 2017 AARP Bulletin2 noted studies showing that doctors listen for about 23 seconds before interrupting patients, but it also cited a 2001 South Carolina study3 that found patients spoke, uninterrupted, for an average of 12 seconds before being interrupted by a resident.
I eventually did learn that early Alzheimer’s symptoms can be easily misinterpreted as signs of stress, anxiety, or depression. But that underscores the need for doctors to listen carefully to caregivers, especially spouse caregivers who observe behaviors 24/7 that may not be present in a quick office visit or revealed on a brief cognitive screening test.
2. Stay up to date on screening tools that detect Alzheimer’s
The Mini-Mental State Examination, or MMSE, is the most frequently used cognitive screening tool, in part because it can be administered in less than 10 minutes. Although unquestionably valuable, a Cochrane review “did not find evidence supporting a substantial role of MMSE as a stand-alone single-administration test in the identification of MCI [mild-cognitive impairment] patients who could develop dementia.”4
Time-pressured doctors might consider using the AD8 screening interview, an informant questionnaire that takes only 2 to 3 minutes to administer, but has demonstrated superior sensitivity in detecting early dementia compared with the MMSE.5 In addition, a study in the December 2016 issue of the Journal of Alzheimer’s Disease 6 confirmed the usefulness of the Sniffin’ Sticks Odor Identification Test whereby patients try to identify 16 different odors. I can attest to Clare’s rapidly deteriorating senses of taste and smell as her disease progressed.
“Results suggest that a simple odor identification test can be a useful supplementary tool for clinically categorizing MCI and Alzheimer’s, and even for identifying people who are at the highest risk of worsening,” according to principal investigator, David R. Roalf, PhD.7
Prompted by prior studies that have linked a weakening sense of smell to Alzheimer’s, doctors in a few larger dementia clinics have already begun using smell tests in their assessments. One possible reason the practice has not yet become common, however, is that the tests take about 5 to 8 minutes to administer. Roalf and his colleagues are hoping to develop a shorter test that will work as well as the longer ones. “We’re hoping to shorten the Sniffin’ Sticks test … down to 3 minutes or so … We think that will encourage more neurology clinics to do this type of screening.”7
Is 5 minutes too much time to take to administer a valuable screening test?
3. Be candid when speaking with patients and their caregivers
A survey reported in Time magazine on March 24, 2015, found that as many as 64% of doctors do not share a diagnosis of Alzheimer’s with their patients because of “fear of causing emotional distress in their patients” due to a lack of effective treatment or cure, and because of a “lack of time and resources to fully explain what the diagnosis means.”8
But Alzheimer’s patients and their caregivers need as much time as possible to plan accordingly, especially if they have not already discussed and finalized end-of-life planning (will, living will, health care proxy, durable power of attorney), preferences for staying at home with aides or being placed in a facility, or wishes to take final trips or enjoy final activities together before cognitive impairment worsens. Withholding a diagnosis can rob patients and caregivers of that valuable planning time.
4. Connect caregivers to resources and support groups
Information on the stages of the disease, available local support groups, and online resources are extremely helpful. Of the 15 people in my spouse support group, only one or 2 were referred there by a doctor. Become familiar with local support groups because that is where caregivers discuss common needs, learn and share helpful caregiving strategies and techniques, and find emotional support from others walking in similar shoes.
5. Help caregivers take away the car keys
When to take away the car keys is an extremely difficult emotional decision that often leads to heated arguments. People with Alzheimer’s rightfully fear losing their independence and only reluctantly accept they can no longer drive safely. But their caregivers worry about them getting lost or causing an accident or, worse, a death. Even though some people with Alzheimer’s can continue to drive safely for a while, the ever-worsening cognitive decline with the disease sooner or later leads to impaired judgment and the inability to drive safely.
If caregivers have already observed issues with their loved one’s driving ability and ask you to intervene, please help remove a major cause of caregiver stress while also making our roads safer. And please do not routinely refer people with Alzheimer’s to driving test facilities. A person with Alzheimer’s may do very well at the particular moment of the test, yet might fail that same test if it was given an hour earlier or later.
6. Manage expectations of what medications can do
None of the current FDA-approved medications have proven to have any long-term positive effects on Alzheimer’s. Clinical trial data show that these meds may be able to slow the rate of disease progression for some people who take them, but even then the benefit is short-lived. Yet many doctors, year after year, renew these “expensive bottles of hope,” as I call them, when the thousands of dollars needed to buy them could be much better spent on day-care programs or personal aides. A candid disclosure to patients and caregivers would enable better decision-making.
1. Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;282:2215-2219.
2. Patural A. How to talk so your doctor will listen. AARP Bulletin. January/February 2017. Available at: http://www.aarp.org/health/healthy-living/info-2016/talk-to-doctor-patient-relationship.html. Accessed September 25, 2017.
3. Rhoades DR, McFarland KF, Finch WH, et al. Speaking and interruptions during primary care office visits. Fam Med. 2001;33:528-532.
4. Arevalo-Rodriguez I, Smailagic N, Roque I Figuls M, et al. Mini-Mental State Examination (MMSE) for the detection of Alzheimer’s disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev. 2015;(3):CD010783.
5. Galvin JE, Fagan AM, Holtzman DM, et al. Relationship of dementia screening tests with biomarkers of Alzheimer's disease. Brain. 2010;133:3290-3300.
6. Quarmley M, Moberg PJ, Mechanic-Hamilton D, et al. Odor identification screening improves diagnostic classification in incipient Alzheimer’s disease. J Alzheimers Dis. 2017;55:1497-1507.
7. Penn study confirms that “sniff test” may be useful in diagnosing early Alzheimer’s disease. December 21, 2016. Available at: http://www.j-alz.com/content/penn-study-confirms-%E2%80%9Csniff-test%E2%80%9D-may-be-useful-diagnosing-early-alzheimer%E2%80%99s-disease. Accessed October 12, 2017.
8. Park A. Many doctors don’t tell patients they have Alzheimer’s. Time. March 24, 2015. Available at: http://time.com/3755176/doctors-diagnose-alzheimers-dont-tell/. Accessed September 25, 2017.
1. Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;282:2215-2219.
2. Patural A. How to talk so your doctor will listen. AARP Bulletin. January/February 2017. Available at: http://www.aarp.org/health/healthy-living/info-2016/talk-to-doctor-patient-relationship.html. Accessed September 25, 2017.
3. Rhoades DR, McFarland KF, Finch WH, et al. Speaking and interruptions during primary care office visits. Fam Med. 2001;33:528-532.
4. Arevalo-Rodriguez I, Smailagic N, Roque I Figuls M, et al. Mini-Mental State Examination (MMSE) for the detection of Alzheimer’s disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev. 2015;(3):CD010783.
5. Galvin JE, Fagan AM, Holtzman DM, et al. Relationship of dementia screening tests with biomarkers of Alzheimer's disease. Brain. 2010;133:3290-3300.
6. Quarmley M, Moberg PJ, Mechanic-Hamilton D, et al. Odor identification screening improves diagnostic classification in incipient Alzheimer’s disease. J Alzheimers Dis. 2017;55:1497-1507.
7. Penn study confirms that “sniff test” may be useful in diagnosing early Alzheimer’s disease. December 21, 2016. Available at: http://www.j-alz.com/content/penn-study-confirms-%E2%80%9Csniff-test%E2%80%9D-may-be-useful-diagnosing-early-alzheimer%E2%80%99s-disease. Accessed October 12, 2017.
8. Park A. Many doctors don’t tell patients they have Alzheimer’s. Time. March 24, 2015. Available at: http://time.com/3755176/doctors-diagnose-alzheimers-dont-tell/. Accessed September 25, 2017.
Speaker advises caution in adding mAbs upfront in MM
NEW YORK, NY—Despite the attraction of incorporating monoclonal antibodies (mAbs) into upfront therapy for multiple myeloma (MM), a speaker at Lymphoma & Myeloma 2017 suggested mAbs are “not quite ready” for this use.
MAbs, particularly daratumumab, have shown single-agent activity in refractory MM and have been feasibly added to proteasome inhibitors and immunomodulatory drugs.
MAbs may even have the potential to enhance induction and shorten the time to minimal residual disease negativity.
“So the tendency is to simply add them to frontline therapy,” said the speaker, Joseph Mikhael, MD, from Mayo Clinic Arizona in Scottsdale.
However, he noted that there is little long-term experience with these agents.
“I’m going to suggest to you that they’re not quite ready [for upfront use] but will likely be ready in the future,” Dr Mikhael said. “We’ve had such a revolution in myeloma the last decade that it’s just easy for us to say, ‘Oh, throw it in there, just like we did, frankly, with rituximab in the lymphoma days. We added it to CVP, we added it to CHOP, we added it to bendamustine. It didn’t matter what we added it to, it just upgraded the response.”
“And, sometimes, I think we have the same approach with daratumumab or elotuzumab or some of the other mAbs that we have. I think we just have to do so cautiously.”
At present, the combination of a proteasome inhibitor and an immunomodulatory drug are the standard of care upfront in transplant-eligible and -ineligible MM patients.
Daratumumab plus KRd
Dr Mikhael described the experience of daratumumab added to carfilzomib, lenalidomide, and dexamethasone (KRd) in patients with newly diagnosed MM in the MMY1001 study.
Twenty-two patients were enrolled on the study, 91% achieved a very good partial response (VGPR) or better, and 43% achieved a complete response (CR). The depth of response improved with the duration of treatment.
Eight patients (36%) discontinued treatment.
Dr Mikhael emphasized that the preliminary data included very small numbers.
“There is a little bit of a yellow flag that pops up here,” he added, “when I see that 36% discontinued treatment, even in small numbers.”
The safety profile was consistent with previous reports for daratumumab or KRd.
The most common hematologic grade 3-4 treatment-emergent adverse events (AEs) occurring in 30% or more of patients were lymphopenia (64%), thrombocytopenia (9%), anemia (9%), leukopenia (9%), and neutropenia (14%).
Diarrhea (14%), cough (5%), fatigue (5%), insomnia (5%), and increased ALT (9%) were the most common grade 3-4 nonhematologic treatment-emergent AEs occurring in 30% or more of patients.
The treatment had no adverse impact on stem cell collection.
Elotuzumab plus VRd
Turning to elotuzumab in combination with bortezomib, lenalidomide, and dexamethasone (VRd), Dr Mikhael reviewed the phase 2a study (NCT02375555) presented at ASCO 2017 (abstract 8002).
Forty-one patients were enrolled on the study.
The overall response rate after 4 cycles was 100%, with 24% achieving a CR, 47% achieving a VGPR, and 29% a partial response.
Fatigue (60%), neuropathy (55%), musculoskeletal/joint pain (55%), infection (50%), back/neck pain (48%), diarrhea (45%), edema (38%), constipation (38%), cough (35%), mood alteration (35%), rash (35%), and insomnia (30%) occurred in 30% or more of patients.
“So again, not shocking,” Dr Mikhael said, “there was fatigue, there was neuropathy, and there were infections in 50% of patients.”
Grade 4 or greater AEs included thrombocytopenia, hyperglycemia, sepsis, cardiac arrest, and respiratory failure.
“However, here, [we have] maybe not even a yellow flag but a red flag of caution that there were 2 patients who died,” Dr Mikhael noted.
One patient died on study due to respiratory failure and sepsis that arose during cycle 2.
The other patient died more than 30 days after discontinuing study therapy due to febrile neutropenia and hypotension related to sepsis, followed by renal failure.
“Again, in a study that has such small numbers, I don’t want to overstate the case . . ., we don’t want to overreact, but whenever there is death involved, obviously, we have to be particularly cautious,” Dr Mikhael said.
Put into the context of 3 other VRd studies, he noted, the response rate with elotuzumab and VRd is relatively similar but not as good as the phase 3 study of VRd, which was a much larger study of 350 patients.
The situation with daratumumab and KRd is similar to elotuzumab, Dr Mikhael pointed out.
The initial response rates are impressive, but, when compared to other studies, “71% VGPR is good, only after 4 cycles, but we know that, in other studies, after a few more cycles, it was significantly higher.”
Cost
Dr Mikhael also considered cost in his assessment of daratumumab and elotuzumab integrated into frontline regimens.
Adding elotuzumab to VRd would almost double the cost of 12 weeks of therapy. And adding daratumumab to KRd would increase the cost even more.
“These costs are real,” Dr Mikhael said, “and, ultimately, if it’s the best thing for our patients, that’s what we are going to do. But until we have that convincing evidence, I think it’s critical to keep that in perspective. I would suggest that VRd, in many respects, is the standard of care for most patients.”
In terms of adding a mAb upfront, he said, “I don’t think we’re there yet. Do I think, in time, we will be? Quite likely, but I don’t think we are there yet.” ![]()
NEW YORK, NY—Despite the attraction of incorporating monoclonal antibodies (mAbs) into upfront therapy for multiple myeloma (MM), a speaker at Lymphoma & Myeloma 2017 suggested mAbs are “not quite ready” for this use.
MAbs, particularly daratumumab, have shown single-agent activity in refractory MM and have been feasibly added to proteasome inhibitors and immunomodulatory drugs.
MAbs may even have the potential to enhance induction and shorten the time to minimal residual disease negativity.
“So the tendency is to simply add them to frontline therapy,” said the speaker, Joseph Mikhael, MD, from Mayo Clinic Arizona in Scottsdale.
However, he noted that there is little long-term experience with these agents.
“I’m going to suggest to you that they’re not quite ready [for upfront use] but will likely be ready in the future,” Dr Mikhael said. “We’ve had such a revolution in myeloma the last decade that it’s just easy for us to say, ‘Oh, throw it in there, just like we did, frankly, with rituximab in the lymphoma days. We added it to CVP, we added it to CHOP, we added it to bendamustine. It didn’t matter what we added it to, it just upgraded the response.”
“And, sometimes, I think we have the same approach with daratumumab or elotuzumab or some of the other mAbs that we have. I think we just have to do so cautiously.”
At present, the combination of a proteasome inhibitor and an immunomodulatory drug are the standard of care upfront in transplant-eligible and -ineligible MM patients.
Daratumumab plus KRd
Dr Mikhael described the experience of daratumumab added to carfilzomib, lenalidomide, and dexamethasone (KRd) in patients with newly diagnosed MM in the MMY1001 study.
Twenty-two patients were enrolled on the study, 91% achieved a very good partial response (VGPR) or better, and 43% achieved a complete response (CR). The depth of response improved with the duration of treatment.
Eight patients (36%) discontinued treatment.
Dr Mikhael emphasized that the preliminary data included very small numbers.
“There is a little bit of a yellow flag that pops up here,” he added, “when I see that 36% discontinued treatment, even in small numbers.”
The safety profile was consistent with previous reports for daratumumab or KRd.
The most common hematologic grade 3-4 treatment-emergent adverse events (AEs) occurring in 30% or more of patients were lymphopenia (64%), thrombocytopenia (9%), anemia (9%), leukopenia (9%), and neutropenia (14%).
Diarrhea (14%), cough (5%), fatigue (5%), insomnia (5%), and increased ALT (9%) were the most common grade 3-4 nonhematologic treatment-emergent AEs occurring in 30% or more of patients.
The treatment had no adverse impact on stem cell collection.
Elotuzumab plus VRd
Turning to elotuzumab in combination with bortezomib, lenalidomide, and dexamethasone (VRd), Dr Mikhael reviewed the phase 2a study (NCT02375555) presented at ASCO 2017 (abstract 8002).
Forty-one patients were enrolled on the study.
The overall response rate after 4 cycles was 100%, with 24% achieving a CR, 47% achieving a VGPR, and 29% a partial response.
Fatigue (60%), neuropathy (55%), musculoskeletal/joint pain (55%), infection (50%), back/neck pain (48%), diarrhea (45%), edema (38%), constipation (38%), cough (35%), mood alteration (35%), rash (35%), and insomnia (30%) occurred in 30% or more of patients.
“So again, not shocking,” Dr Mikhael said, “there was fatigue, there was neuropathy, and there were infections in 50% of patients.”
Grade 4 or greater AEs included thrombocytopenia, hyperglycemia, sepsis, cardiac arrest, and respiratory failure.
“However, here, [we have] maybe not even a yellow flag but a red flag of caution that there were 2 patients who died,” Dr Mikhael noted.
One patient died on study due to respiratory failure and sepsis that arose during cycle 2.
The other patient died more than 30 days after discontinuing study therapy due to febrile neutropenia and hypotension related to sepsis, followed by renal failure.
“Again, in a study that has such small numbers, I don’t want to overstate the case . . ., we don’t want to overreact, but whenever there is death involved, obviously, we have to be particularly cautious,” Dr Mikhael said.
Put into the context of 3 other VRd studies, he noted, the response rate with elotuzumab and VRd is relatively similar but not as good as the phase 3 study of VRd, which was a much larger study of 350 patients.
The situation with daratumumab and KRd is similar to elotuzumab, Dr Mikhael pointed out.
The initial response rates are impressive, but, when compared to other studies, “71% VGPR is good, only after 4 cycles, but we know that, in other studies, after a few more cycles, it was significantly higher.”
Cost
Dr Mikhael also considered cost in his assessment of daratumumab and elotuzumab integrated into frontline regimens.
Adding elotuzumab to VRd would almost double the cost of 12 weeks of therapy. And adding daratumumab to KRd would increase the cost even more.
“These costs are real,” Dr Mikhael said, “and, ultimately, if it’s the best thing for our patients, that’s what we are going to do. But until we have that convincing evidence, I think it’s critical to keep that in perspective. I would suggest that VRd, in many respects, is the standard of care for most patients.”
In terms of adding a mAb upfront, he said, “I don’t think we’re there yet. Do I think, in time, we will be? Quite likely, but I don’t think we are there yet.” ![]()
NEW YORK, NY—Despite the attraction of incorporating monoclonal antibodies (mAbs) into upfront therapy for multiple myeloma (MM), a speaker at Lymphoma & Myeloma 2017 suggested mAbs are “not quite ready” for this use.
MAbs, particularly daratumumab, have shown single-agent activity in refractory MM and have been feasibly added to proteasome inhibitors and immunomodulatory drugs.
MAbs may even have the potential to enhance induction and shorten the time to minimal residual disease negativity.
“So the tendency is to simply add them to frontline therapy,” said the speaker, Joseph Mikhael, MD, from Mayo Clinic Arizona in Scottsdale.
However, he noted that there is little long-term experience with these agents.
“I’m going to suggest to you that they’re not quite ready [for upfront use] but will likely be ready in the future,” Dr Mikhael said. “We’ve had such a revolution in myeloma the last decade that it’s just easy for us to say, ‘Oh, throw it in there, just like we did, frankly, with rituximab in the lymphoma days. We added it to CVP, we added it to CHOP, we added it to bendamustine. It didn’t matter what we added it to, it just upgraded the response.”
“And, sometimes, I think we have the same approach with daratumumab or elotuzumab or some of the other mAbs that we have. I think we just have to do so cautiously.”
At present, the combination of a proteasome inhibitor and an immunomodulatory drug are the standard of care upfront in transplant-eligible and -ineligible MM patients.
Daratumumab plus KRd
Dr Mikhael described the experience of daratumumab added to carfilzomib, lenalidomide, and dexamethasone (KRd) in patients with newly diagnosed MM in the MMY1001 study.
Twenty-two patients were enrolled on the study, 91% achieved a very good partial response (VGPR) or better, and 43% achieved a complete response (CR). The depth of response improved with the duration of treatment.
Eight patients (36%) discontinued treatment.
Dr Mikhael emphasized that the preliminary data included very small numbers.
“There is a little bit of a yellow flag that pops up here,” he added, “when I see that 36% discontinued treatment, even in small numbers.”
The safety profile was consistent with previous reports for daratumumab or KRd.
The most common hematologic grade 3-4 treatment-emergent adverse events (AEs) occurring in 30% or more of patients were lymphopenia (64%), thrombocytopenia (9%), anemia (9%), leukopenia (9%), and neutropenia (14%).
Diarrhea (14%), cough (5%), fatigue (5%), insomnia (5%), and increased ALT (9%) were the most common grade 3-4 nonhematologic treatment-emergent AEs occurring in 30% or more of patients.
The treatment had no adverse impact on stem cell collection.
Elotuzumab plus VRd
Turning to elotuzumab in combination with bortezomib, lenalidomide, and dexamethasone (VRd), Dr Mikhael reviewed the phase 2a study (NCT02375555) presented at ASCO 2017 (abstract 8002).
Forty-one patients were enrolled on the study.
The overall response rate after 4 cycles was 100%, with 24% achieving a CR, 47% achieving a VGPR, and 29% a partial response.
Fatigue (60%), neuropathy (55%), musculoskeletal/joint pain (55%), infection (50%), back/neck pain (48%), diarrhea (45%), edema (38%), constipation (38%), cough (35%), mood alteration (35%), rash (35%), and insomnia (30%) occurred in 30% or more of patients.
“So again, not shocking,” Dr Mikhael said, “there was fatigue, there was neuropathy, and there were infections in 50% of patients.”
Grade 4 or greater AEs included thrombocytopenia, hyperglycemia, sepsis, cardiac arrest, and respiratory failure.
“However, here, [we have] maybe not even a yellow flag but a red flag of caution that there were 2 patients who died,” Dr Mikhael noted.
One patient died on study due to respiratory failure and sepsis that arose during cycle 2.
The other patient died more than 30 days after discontinuing study therapy due to febrile neutropenia and hypotension related to sepsis, followed by renal failure.
“Again, in a study that has such small numbers, I don’t want to overstate the case . . ., we don’t want to overreact, but whenever there is death involved, obviously, we have to be particularly cautious,” Dr Mikhael said.
Put into the context of 3 other VRd studies, he noted, the response rate with elotuzumab and VRd is relatively similar but not as good as the phase 3 study of VRd, which was a much larger study of 350 patients.
The situation with daratumumab and KRd is similar to elotuzumab, Dr Mikhael pointed out.
The initial response rates are impressive, but, when compared to other studies, “71% VGPR is good, only after 4 cycles, but we know that, in other studies, after a few more cycles, it was significantly higher.”
Cost
Dr Mikhael also considered cost in his assessment of daratumumab and elotuzumab integrated into frontline regimens.
Adding elotuzumab to VRd would almost double the cost of 12 weeks of therapy. And adding daratumumab to KRd would increase the cost even more.
“These costs are real,” Dr Mikhael said, “and, ultimately, if it’s the best thing for our patients, that’s what we are going to do. But until we have that convincing evidence, I think it’s critical to keep that in perspective. I would suggest that VRd, in many respects, is the standard of care for most patients.”
In terms of adding a mAb upfront, he said, “I don’t think we’re there yet. Do I think, in time, we will be? Quite likely, but I don’t think we are there yet.” ![]()