User login
Unsuspected Lymphomatoid Granulomatosis in a Patient With Antisynthetase Syndrome
Lymphomatoid granulomatosis (LYG) is a rare Epstein-Barr virus (EBV)–related extranodal angiocentric lymphoproliferative disorder. Most patients are adults in the fifth decade of life, and men are twice as likely as women to be affected.1 The most common site of involvement is the lungs, which has been observed in more than 90% of patients.2 The skin is the most common extrapulmonary site of involvement with variable manifestations including “rash,” subcutaneous nodules, and ulceration. Although a small subset of patients experience remission without treatment, most patients report a progressive course with median survival of less than 2 years.1,2 Clinical diagnosis often is challenging due to underrecognition of this rare condition by multidisciplinary physicians.
Case Report
A 60-year-old woman presented with fatigue, night sweats, poor appetite, unintentional weight loss, and dyspnea with minor exertion of 2 weeks’ duration. Her medical history was remarkable for antisynthetase syndrome manifested as polymyositis and interstitial lung disease, as well as recurrent breast cancer treated with wide excision, chemotherapy, and radiation therapy completed 2 months prior. Antisynthetase syndrome was controlled with azathioprine for 2 years, which was stopped during chemotherapy but restarted to treat worsened myalgia 4 months prior to presentation. Two weeks prior to hospital admission, she was treated with antibiotics at an outside hospital for presumed pneumonia without improvement. Upon admission to our hospital she was pancytopenic. Chest computed tomography showed interval development of extensive patchy ground-glass opacities in all lung lobes with areas of confluent consolidation. Broad infectious workup was negative. Given the time course of presentation and anterior accentuation of the lung infiltrates, the greatest clinical concern was radiation pneumonitis followed by drug toxicity. A bone marrow biopsy was hypocellular but without evidence of malignancy. Her pancytopenia was thought to be induced by azathioprine and/or antibiotics. Antibiotics were discontinued and prednisone was started for treatment of presumed radiation pneumonitis.
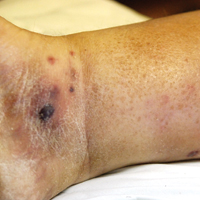
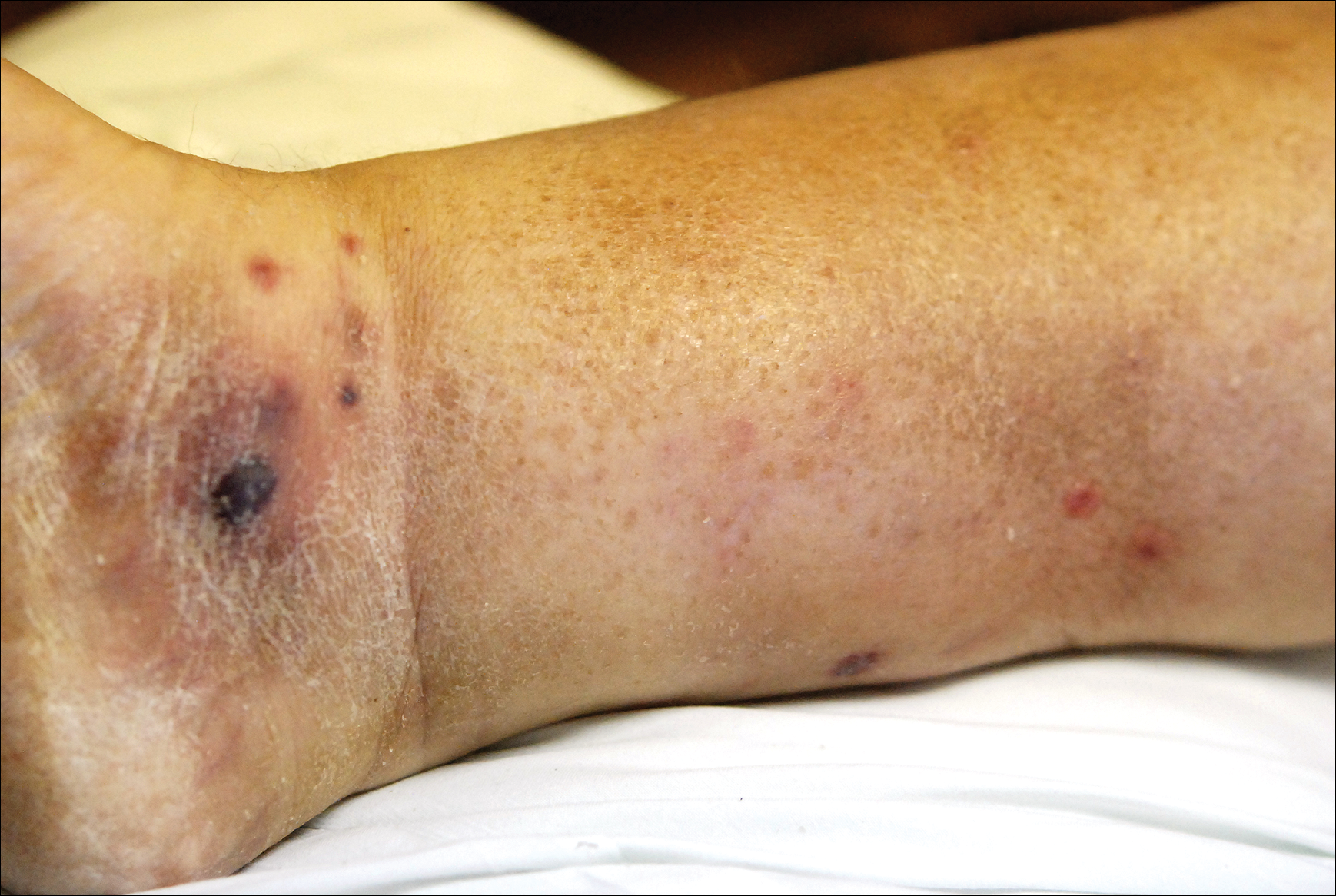
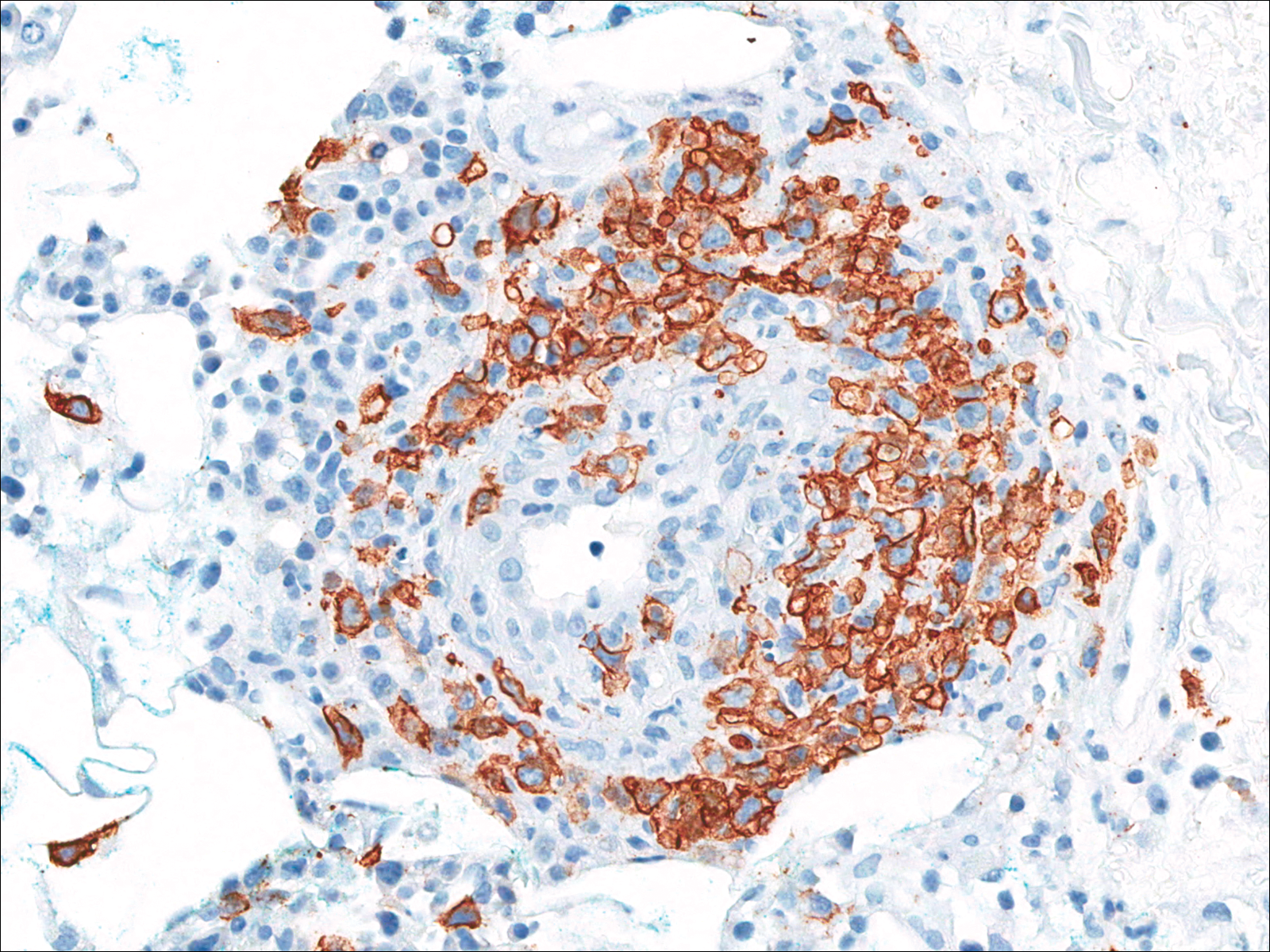
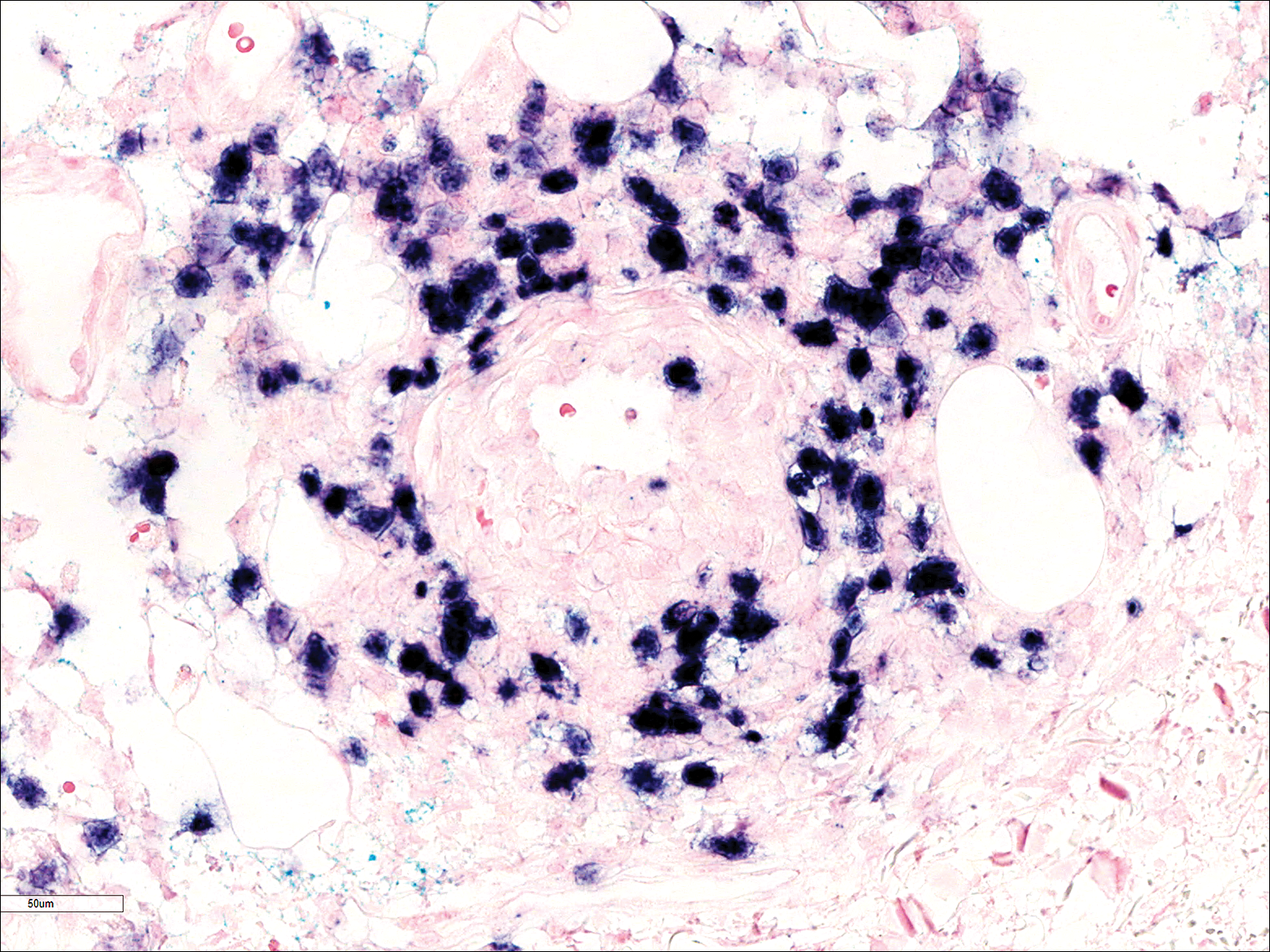
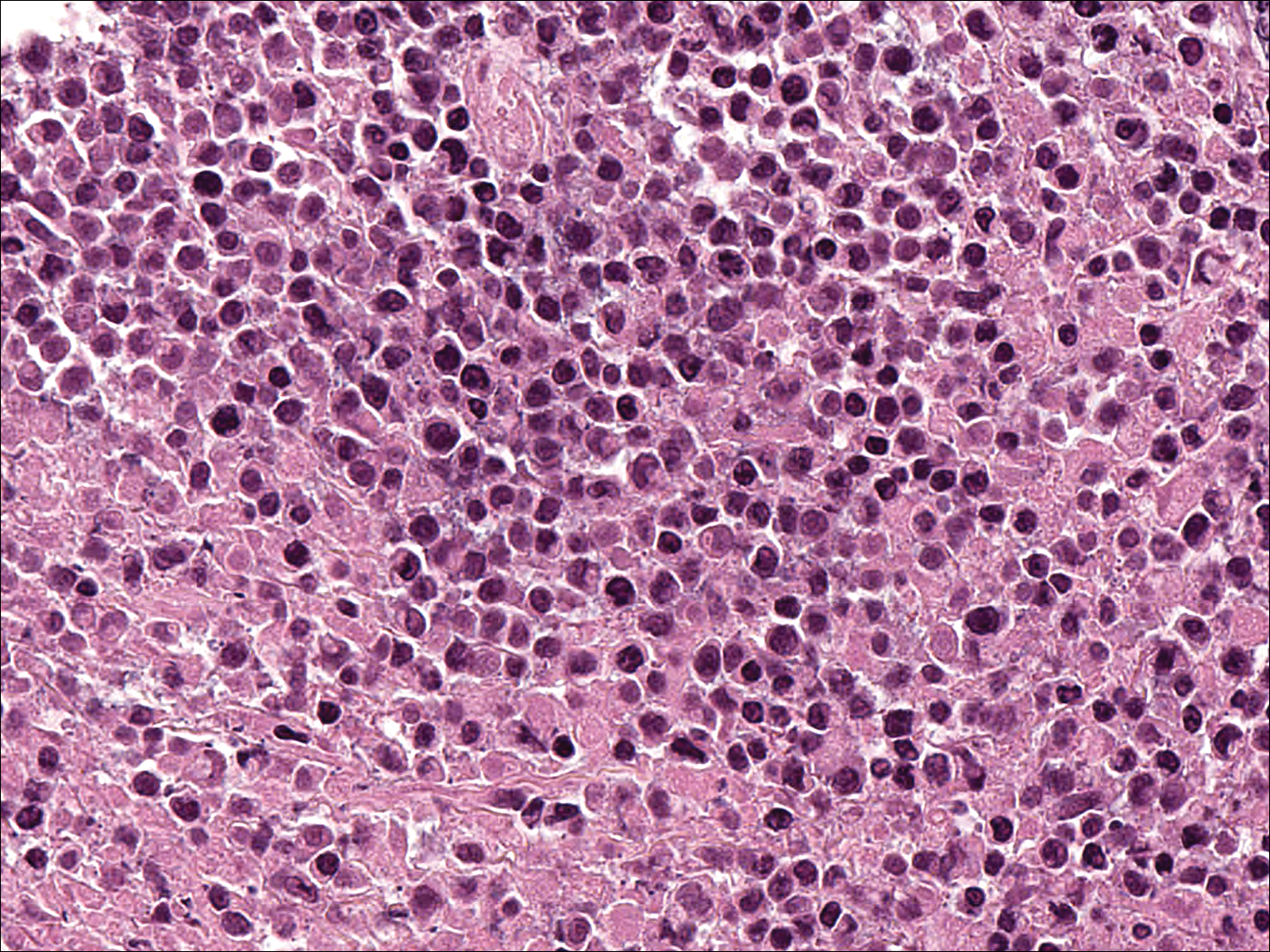
A few days later, the patient developed new skin lesions and worsening bilateral leg edema. There were multiple small erythematous and hemorrhagic papules, macules, and blisters on the medial aspect of the right lower leg and ankle, each measuring less than 1 cm in diameter (Figure 1). The clinical differential diagnosis included vasculitis related to an underlying collagen vascular disease, atypical edema blisters, and drug hypersensitivity reaction. A punch biopsy of one of the lesions showed a moderately dense superficial and deep perivascular lymphoid infiltrate with marked papillary dermal edema and early subepidermal split (Figure 2). The infiltrate was comprised of small- to medium-sized lymphocytes admixed with large cells, histiocytes, and plasma cells (Figure 3). Immunohistochemistry revealed a predominance of CD3+ and CD4+ small- to medium-sized T cells. CD20 highlighted the large angiocentric B cells (Figure 4), which also were positive on EBV-encoded small RNA (EBER) in situ hybridization (Figure 5). A diagnosis of LYG was rendered. Approximately 40 to 50 EBV-positive large B cells were present per high-power field (HPF), consistent with grade 2 disease.
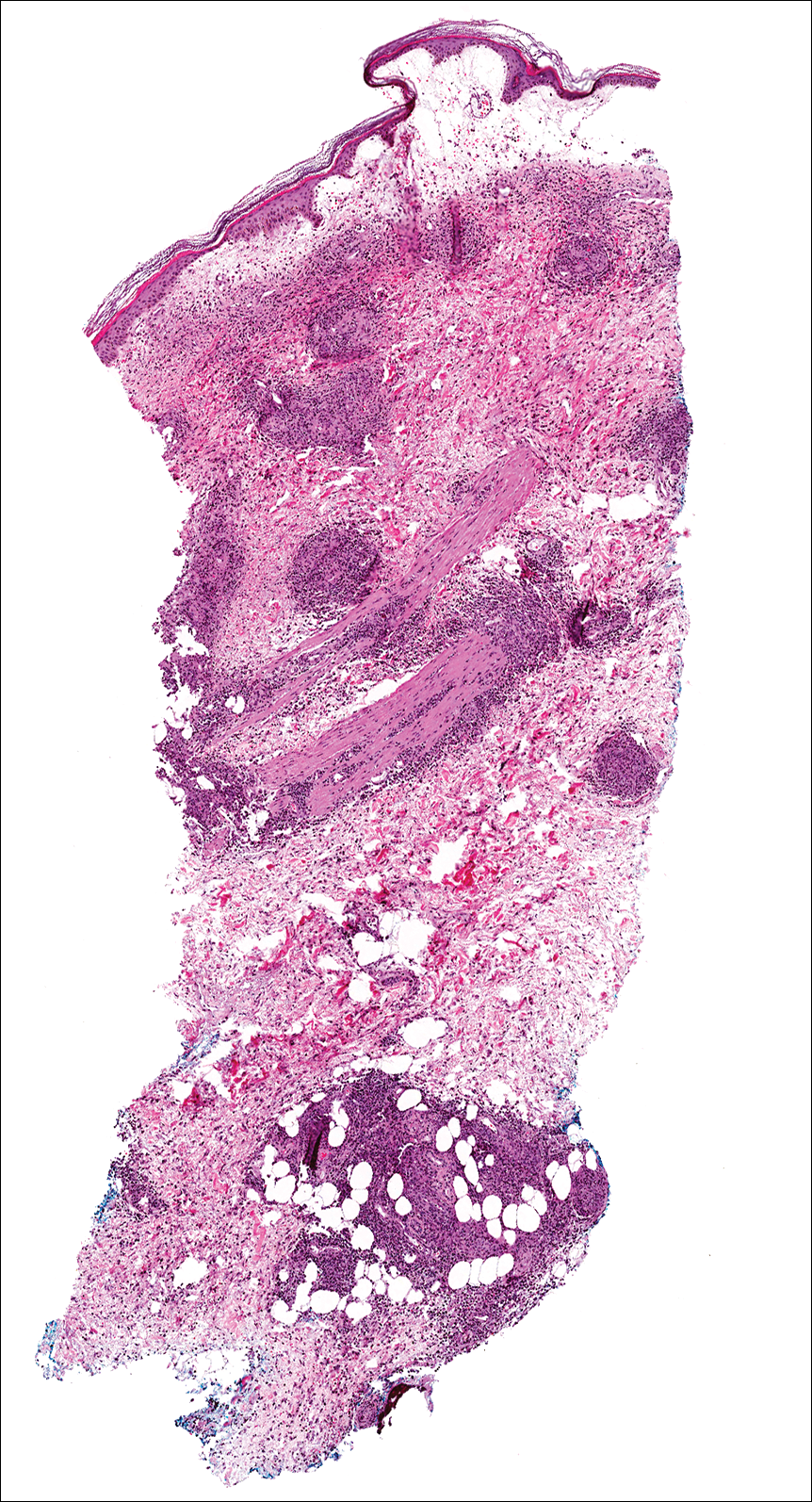
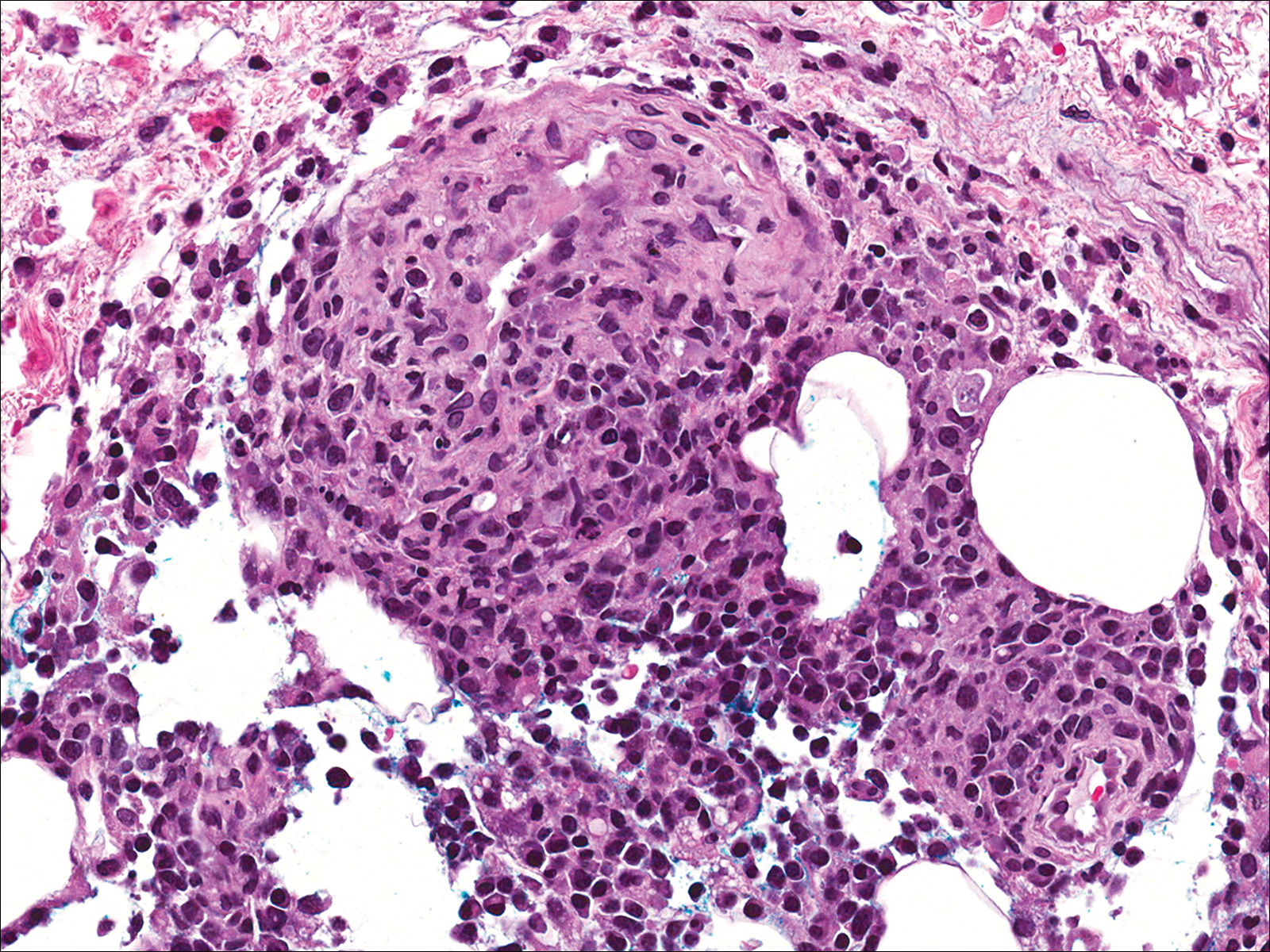
Soon after diagnosis, follow-up computed tomography of the chest, abdomen, and pelvis revealed suspicious lesions in the kidneys, liver, spleen, and inguinal and iliac lymph nodes. The ground-glass opacities in the lungs continued to progress, with 2 additional nodules noted in the right upper and lower lobes. Four days later, core needle biopsies of the right inguinal lymph node showed a large B-cell lymphoma with extensive necrosis (Figure 6). EBER in situ hybridization was suboptimal, probably due to extensive necrosis.
She was started on etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin (EPOCH) for 5 days before developing Klebsiella pneumoniae sepsis and acute kidney injury. She was transferred to the critical care unit due to increasing oxygen requirement. Despite medical interventions, she continued to decompensate and elected to transition to palliative care. She died 6 weeks after the initial presentation. Her family did not request an autopsy.
Comment
Lymphomatoid granulomatosis is a rare lymphoproliferative disorder associated with various immunocompromised states including primary immunodeficiency disorders, human immunodeficiency virus infection, and immunosuppression for organ transplantation and autoimmune diseases. Our patient was receiving azathioprine for antisynthetase syndrome, which put her at risk for EBV infection and LYG. Azathioprine rarely has been reported as a possible culprit of LYG,3,4 but there are no known reported cases that were related to antisynthetase syndrome. There are multiple reports of development of LYG in patients receiving methotrexate for rheumatoid arthritis.5-10 Other iatrogenic causes reported in the literature include thiopurines11,12 and imatinib.13,14
The clinical diagnosis of our patient was particularly challenging given her complicated medical history including interstitial lung disease, predisposition to infection secondary to immunosuppression, and recent radiation therapy to the chest. This case illustrates the importance of maintaining a high index of suspicion for LYG in immunosuppressed patients presenting with lung infiltrates.
Presentation
Radiologically, LYG typically manifests as nodular densities accentuated in the lower lung lobes, which may become confluent.15 Because the nodular pattern in LYG is nonspecific and may mimic sarcoidosis, hypersensitivity pneumonitis, vasculitis, and infectious and neoplastic diseases,16 open lung biopsy often is required to establish the diagnosis in the absence of more accessible lesions.
Cutaneous lesions are seen in 40% to 50% of patients2 and may be the presenting sign of LYG. In a retrospective study, 16% (3/19) of LYG patients presented with cutaneous lesions months before diagnostic pulmonary lesions were identified.17 The skin is the most accessible site for biopsy, allowing definitive tissue diagnosis even when the condition is not clinically suspected. Therefore, dermatologists and dermatopathologists should be aware of this rare entity.
The clinical morphologies of the skin lesions are nonspecific, ranging from erythematous papules and subcutaneous nodules to indurated plaques. Ulceration may be present. The lesions may be widely disseminated or limited to the arms and legs. Our patient presented with erythematous and hemorrhagic papules, macules, and blisters on the lower leg. The hemorrhagic and blistering nature of some of these lesions in our patient may be attributable to thrombocytopenia and lymphedema in addition to LYG.
Histopathology and Differential
The skin biopsy from our patient demonstrated typical features of LYG, namely EBV-positive neoplastic large B cells in a background of predominating reactive T cells.18 The neoplastic large cells frequently invade blood vessels, leading to luminal narrowing without necrosis of the vessel walls. Grading is based on the density of EBV-positive large B cells: grade 1 is defined as fewer than 5 cells per HPF; grade 2, 5 to 50 cells per HPF; and grade 3, more than 50 cells per HPF.18 Grade 2 or 3 disease predicts worse outcome,2 as observed in our case. It is important for pathologists and clinicians to be aware that the proportion of EBV-positive large B cells is variable even within a single lesion; therefore, more than 1 biopsy may be necessary for appropriate grading and management.1,17 Additionally, skin biopsy may have a lower sensitivity for detecting EBV-positive B cells compared to lung biopsy, possibly due to sampling error in small biopsies.17
The histopathologic features of LYG frequently overlap with other lymphomas. Due to the abundance of T cells, LYG may be misclassified as T-cell/histiocyte-rich large B-cell lymphoma.19 Because the latter is not associated with EBV, EBER in situ hybridization is helpful in distinguishing the 2 conditions. On the other hand, EBER in situ hybridization has no value in discriminating LYG and extranodal natural killer (NK)/T-cell lymphoma, as both are EBV driven. Unlike LYG, the neoplastic EBV-positive cells in extranodal NK/T-cell lymphoma make up the majority of the infiltrate and exhibit an NK-cell immunophenotype (positive CD56 and cytoplasmic CD3 epsilon).20 Pulmonary involvement also is uncommon in NK/T-cell lymphoma.
Aside from lymphomas, LYG also resembles granulomatosis with polyangiitis (GPA)(formerly known as Wegener granulomatosis). Clinically, both LYG and GPA can present with constitutional symptoms, as well as lung, kidney, and skin lesions. The 2 conditions differ microscopically, with leukocytoclastic vasculitis and necrotizing granulomatous inflammation being characteristic of GPA but absent in LYG.1,21 Neutrophils and eosinophils are much more likely to be present in GPA.22,23
Disease Progression
Although LYG is an extranodal disease, there is a 7% to 45% risk of progression to nodal lymphoma in patients with high-grade disease.2,22,24 Our patient progressed to nodal large B-cell lymphoma shortly after the diagnosis of high-grade LYG. She developed additional lesions in the liver, spleen, and kidneys, and ultimately succumbed to the disease. Prior studies have shown higher mortality in patients with bilateral lung involvement and neurologic abnormalities, whereas cutaneous involvement does not affect outcome.2
Treatment
A prospective study used an initial treatment regimen of cyclophosphamide and prednisone but mortality was high.24 More recently, chemotherapy regimens including CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), CVP (cyclophosphamide, vincristine, and prednisone), CVP or CHOP combined with rituximab, C-MOPP (cyclophosphamide, vincristine, prednisone, and procarbazine), EPOCH, and rituximab with high-dose cytarabine have been used with variable success for grades 2 and 3 LYG.17,23,25,26 Antiviral and immunomodulatory (interferon alfa) therapy has been used to induce remission in a majority of patients with grades 1 or 2 LYG.3,17,27,28 There is a report of successful treatment of relapsed LYG with the retinoid agent bexarotene.29 Autologous or allogeneic stem cell transplantation was effective for some patients with refractory or relapsed LYG.30 Further studies are needed to clarify optimal treatment of LYG, especially high-grade disease.
Conclusion
We report a rare case of LYG in a patient with antisynthetase syndrome, which highlights the critical role of skin biopsy in establishing the diagnosis of LYG when the clinical and radiologic presentations are obscured by other comorbidities. Dermatologists should be familiar with this rare disease and maintain a low threshold for biopsy in immunocompromised patients presenting with nodular lung infiltrates and/or nonspecific skin lesions.
- Katzenstein AL, Doxtader E, Narendra S. Lymphomatoid granulomatosis: insights gained over 4 decades. Am J Surg Pathol. 2010;34:E35-E48.
- Katzenstein AL, Carrington CB, Liebow AA. Lymphomatoid granulomatosis: a clinicopathologic study of 152 cases. Cancer. 1979;43:360-373.
- Connors W, Griffiths C, Patel J, et al. Lymphomatoid granulomatosis associated with azathioprine therapy in Crohn disease. BMC Gastroenterol. 2014;14:127.
- Katherine Martin L, Porcu P, Baiocchi RA, et al. Primary central nervous system lymphomatoid granulomatosis in a patient receiving azathioprine therapy. Clin Adv Hematol Oncol. 2009;7:65-68.
- Barakat A, Grover K, Peshin R. Rituximab for pulmonary lymphomatoid granulomatosis which developed as a complication of methotrexate and azathioprine therapy for rheumatoid arthritis. Springerplus. 2014;3:751.
- Kobayashi S, Kikuchi Y, Sato K, et al. Reversible iatrogenic, MTX-associated EBV-driven lymphoproliferation with histopathological features of a lymphomatoid granulomatosis in a patient with rheumatoid arthritis. Ann Hematol. 2013;92:1561-1564.
- Kameda H, Okuyama A, Tamaru J, et al. Lymphomatoid granulomatosis and diffuse alveolar damage associated with methotrexate therapy in a patient with rheumatoid arthritis. Clin Rheumatol. 2007;26:1585-1589.
- Oiwa H, Mihara K, Kan T, et al. Grade 3 lymphomatoid granulomatosis in a patient receiving methotrexate therapy for rheumatoid arthritis. Intern Med. 2014;53:1873-1875.
- Blanchart K, Paciencia M, Seguin A, et al. Fatal pulmonary lymphomatoid granulomatosis in a patient taking methotrexate for rheumatoid arthritis. Minerva Anestesiol. 2014;80:119-120.
- Schalk E, Krogel C, Scheinpflug K, et al. Lymphomatoid granulomatosis in a patient with rheumatoid arthritis receiving methotrexate: successful treatment with the anti-CD20 antibody mabthera. Onkologie. 2009;32:440-441.
- Subramaniam K, Cherian M, Jain S, et al. Two rare cases of Epstein-Barr virus-associated lymphoproliferative disorders in inflammatory bowel disease patients on thiopurines and other immunosuppressive medications. Intern Med J. 2013;43:1339-1342.
- Destombe S, Bouron-DalSoglio D, Rougemont AL, et al. Lymphomatoid granulomatosis: a unique complication of Crohn disease and its treatment in pediatrics. J Pediatr Gastroenterol Nutr. 2010;50:559-561.
- Yazdi AS, Metzler G, Weyrauch S, et al. Lymphomatoid granulomatosis induced by imatinib treatment. Arch Dermatol. 2007;143:1222-1223.
- Salmons N, Gregg RJ, Pallalau A, et al. Lymphomatoid granulomatosis in a patient previously diagnosed with a gastrointestinal stromal tumour and treated with imatinib. J Clin Pathol. 2007;60:199-201.
- Dee PM, Arora NS, Innes DJ Jr. The pulmonary manifestations of lymphomatoid granulomatosis. Radiology. 1982;143:613-618.
- Rezai P, Hart EM, Patel SK. Case 169: lymphomatoid granulomatosis. Radiology. 2011;259:604-609.
- Beaty MW, Toro J, Sorbara L, et al. Cutaneous lymphomatoid granulomatosis: correlation of clinical and biologic features. Am J Surg Pathol. 2001;25:1111-1120.
- Pittaluga S, Wilson WH, Jaffe E. Lymphomatoid granulomatosis. In: Swerdlow S, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: International Agency for Research on Cancer; 2008:247-249.
- Abramson JS. T-cell/histiocyte-rich B-cell lymphoma: biology, diagnosis, and management. Oncologist. 2006;11:384-392.
- Jaffe E. Nasal and nasal-type T/NK cell lymphoma: a unique form of lymphoma associated with the Epstein-Barr virus. Histopathology. 1995;27:581-583.
- Barksdale SK, Hallahan CW, Kerr GS, et al. Cutaneous pathology in Wegener’s granulomatosis: a clinicopathologic study of 74 biopsies in 46 patients. Am J Surg Pathol. 1995;19:161-172.
- Koss MN, Hochholzer L, Langloss JM, et al. Lymphomatoid granulomatosis: a clinicopathologic study of 42 patients. Pathology. 1986;18:283-288.
- Aoki T, Harada Y, Matsubara E, et al. Long-term remission after multiple relapses in an elderly patient with lymphomatoid granulomatosis after rituximab and high-dose cytarabine chemotherapy without stem-cell transplantation. J Clin Oncol. 2013;31:E390-E393.
- Fauci AS, Haynes BF, Costa J, et al. Lymphomatoid granulomatosis: prospective clinical and therapeutic experience over 10 years. N Engl J Med. 1982;306:68-74.
- Jung KH, Sung HJ, Lee JH, et al. A case of pulmonary lymphomatoid granulomatosis successfully treated by combination chemotherapy with rituximab. Chemotherapy. 2009;55:386-390.
- Hernandez-Marques C, Lassaletta A, Torrelo A, et al. Rituximab in lymphomatoid granulomatosis. J Pediatr Hematol Oncol. 2014;36:E69-E74.
- Wilson WH, Gutierrez M, Raffeld M, et al. Lymphomatoid granulomatosis: phase 2 study of dose-adjusted interferon-alfa or EPOCH chemotherapy. Blood. 1999;94:599A.
- Wilson WH, Kingma DW, Raffeld M, et al. Association of lymphomatoid granulomatosis with Epstein-Barr viral infection of B lymphocytes and response to interferon-alpha 2b. Blood. 1996;87:4531-4537.
- Berg SE, Downs LH, Torigian DA, et al. Successful treatment of relapsed lymphomatoid granulomatosis with bexarotene. Cancer Biol Ther. 2008;7:1544-1546.
- Siegloch K, Schmitz N, Wu HS, et al. Hematopoietic stem cell transplantation in patients with lymphomatoid granulomatosis: a European group for blood and marrow transplantation report. Biol Blood Marrow Transplant. 2013;19:1522-1525.
Lymphomatoid granulomatosis (LYG) is a rare Epstein-Barr virus (EBV)–related extranodal angiocentric lymphoproliferative disorder. Most patients are adults in the fifth decade of life, and men are twice as likely as women to be affected.1 The most common site of involvement is the lungs, which has been observed in more than 90% of patients.2 The skin is the most common extrapulmonary site of involvement with variable manifestations including “rash,” subcutaneous nodules, and ulceration. Although a small subset of patients experience remission without treatment, most patients report a progressive course with median survival of less than 2 years.1,2 Clinical diagnosis often is challenging due to underrecognition of this rare condition by multidisciplinary physicians.
Case Report
A 60-year-old woman presented with fatigue, night sweats, poor appetite, unintentional weight loss, and dyspnea with minor exertion of 2 weeks’ duration. Her medical history was remarkable for antisynthetase syndrome manifested as polymyositis and interstitial lung disease, as well as recurrent breast cancer treated with wide excision, chemotherapy, and radiation therapy completed 2 months prior. Antisynthetase syndrome was controlled with azathioprine for 2 years, which was stopped during chemotherapy but restarted to treat worsened myalgia 4 months prior to presentation. Two weeks prior to hospital admission, she was treated with antibiotics at an outside hospital for presumed pneumonia without improvement. Upon admission to our hospital she was pancytopenic. Chest computed tomography showed interval development of extensive patchy ground-glass opacities in all lung lobes with areas of confluent consolidation. Broad infectious workup was negative. Given the time course of presentation and anterior accentuation of the lung infiltrates, the greatest clinical concern was radiation pneumonitis followed by drug toxicity. A bone marrow biopsy was hypocellular but without evidence of malignancy. Her pancytopenia was thought to be induced by azathioprine and/or antibiotics. Antibiotics were discontinued and prednisone was started for treatment of presumed radiation pneumonitis.
A few days later, the patient developed new skin lesions and worsening bilateral leg edema. There were multiple small erythematous and hemorrhagic papules, macules, and blisters on the medial aspect of the right lower leg and ankle, each measuring less than 1 cm in diameter (Figure 1). The clinical differential diagnosis included vasculitis related to an underlying collagen vascular disease, atypical edema blisters, and drug hypersensitivity reaction. A punch biopsy of one of the lesions showed a moderately dense superficial and deep perivascular lymphoid infiltrate with marked papillary dermal edema and early subepidermal split (Figure 2). The infiltrate was comprised of small- to medium-sized lymphocytes admixed with large cells, histiocytes, and plasma cells (Figure 3). Immunohistochemistry revealed a predominance of CD3+ and CD4+ small- to medium-sized T cells. CD20 highlighted the large angiocentric B cells (Figure 4), which also were positive on EBV-encoded small RNA (EBER) in situ hybridization (Figure 5). A diagnosis of LYG was rendered. Approximately 40 to 50 EBV-positive large B cells were present per high-power field (HPF), consistent with grade 2 disease.





Soon after diagnosis, follow-up computed tomography of the chest, abdomen, and pelvis revealed suspicious lesions in the kidneys, liver, spleen, and inguinal and iliac lymph nodes. The ground-glass opacities in the lungs continued to progress, with 2 additional nodules noted in the right upper and lower lobes. Four days later, core needle biopsies of the right inguinal lymph node showed a large B-cell lymphoma with extensive necrosis (Figure 6). EBER in situ hybridization was suboptimal, probably due to extensive necrosis.

She was started on etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin (EPOCH) for 5 days before developing Klebsiella pneumoniae sepsis and acute kidney injury. She was transferred to the critical care unit due to increasing oxygen requirement. Despite medical interventions, she continued to decompensate and elected to transition to palliative care. She died 6 weeks after the initial presentation. Her family did not request an autopsy.
Comment
Lymphomatoid granulomatosis is a rare lymphoproliferative disorder associated with various immunocompromised states including primary immunodeficiency disorders, human immunodeficiency virus infection, and immunosuppression for organ transplantation and autoimmune diseases. Our patient was receiving azathioprine for antisynthetase syndrome, which put her at risk for EBV infection and LYG. Azathioprine rarely has been reported as a possible culprit of LYG,3,4 but there are no known reported cases that were related to antisynthetase syndrome. There are multiple reports of development of LYG in patients receiving methotrexate for rheumatoid arthritis.5-10 Other iatrogenic causes reported in the literature include thiopurines11,12 and imatinib.13,14
The clinical diagnosis of our patient was particularly challenging given her complicated medical history including interstitial lung disease, predisposition to infection secondary to immunosuppression, and recent radiation therapy to the chest. This case illustrates the importance of maintaining a high index of suspicion for LYG in immunosuppressed patients presenting with lung infiltrates.
Presentation
Radiologically, LYG typically manifests as nodular densities accentuated in the lower lung lobes, which may become confluent.15 Because the nodular pattern in LYG is nonspecific and may mimic sarcoidosis, hypersensitivity pneumonitis, vasculitis, and infectious and neoplastic diseases,16 open lung biopsy often is required to establish the diagnosis in the absence of more accessible lesions.
Cutaneous lesions are seen in 40% to 50% of patients2 and may be the presenting sign of LYG. In a retrospective study, 16% (3/19) of LYG patients presented with cutaneous lesions months before diagnostic pulmonary lesions were identified.17 The skin is the most accessible site for biopsy, allowing definitive tissue diagnosis even when the condition is not clinically suspected. Therefore, dermatologists and dermatopathologists should be aware of this rare entity.
The clinical morphologies of the skin lesions are nonspecific, ranging from erythematous papules and subcutaneous nodules to indurated plaques. Ulceration may be present. The lesions may be widely disseminated or limited to the arms and legs. Our patient presented with erythematous and hemorrhagic papules, macules, and blisters on the lower leg. The hemorrhagic and blistering nature of some of these lesions in our patient may be attributable to thrombocytopenia and lymphedema in addition to LYG.
Histopathology and Differential
The skin biopsy from our patient demonstrated typical features of LYG, namely EBV-positive neoplastic large B cells in a background of predominating reactive T cells.18 The neoplastic large cells frequently invade blood vessels, leading to luminal narrowing without necrosis of the vessel walls. Grading is based on the density of EBV-positive large B cells: grade 1 is defined as fewer than 5 cells per HPF; grade 2, 5 to 50 cells per HPF; and grade 3, more than 50 cells per HPF.18 Grade 2 or 3 disease predicts worse outcome,2 as observed in our case. It is important for pathologists and clinicians to be aware that the proportion of EBV-positive large B cells is variable even within a single lesion; therefore, more than 1 biopsy may be necessary for appropriate grading and management.1,17 Additionally, skin biopsy may have a lower sensitivity for detecting EBV-positive B cells compared to lung biopsy, possibly due to sampling error in small biopsies.17
The histopathologic features of LYG frequently overlap with other lymphomas. Due to the abundance of T cells, LYG may be misclassified as T-cell/histiocyte-rich large B-cell lymphoma.19 Because the latter is not associated with EBV, EBER in situ hybridization is helpful in distinguishing the 2 conditions. On the other hand, EBER in situ hybridization has no value in discriminating LYG and extranodal natural killer (NK)/T-cell lymphoma, as both are EBV driven. Unlike LYG, the neoplastic EBV-positive cells in extranodal NK/T-cell lymphoma make up the majority of the infiltrate and exhibit an NK-cell immunophenotype (positive CD56 and cytoplasmic CD3 epsilon).20 Pulmonary involvement also is uncommon in NK/T-cell lymphoma.
Aside from lymphomas, LYG also resembles granulomatosis with polyangiitis (GPA)(formerly known as Wegener granulomatosis). Clinically, both LYG and GPA can present with constitutional symptoms, as well as lung, kidney, and skin lesions. The 2 conditions differ microscopically, with leukocytoclastic vasculitis and necrotizing granulomatous inflammation being characteristic of GPA but absent in LYG.1,21 Neutrophils and eosinophils are much more likely to be present in GPA.22,23
Disease Progression
Although LYG is an extranodal disease, there is a 7% to 45% risk of progression to nodal lymphoma in patients with high-grade disease.2,22,24 Our patient progressed to nodal large B-cell lymphoma shortly after the diagnosis of high-grade LYG. She developed additional lesions in the liver, spleen, and kidneys, and ultimately succumbed to the disease. Prior studies have shown higher mortality in patients with bilateral lung involvement and neurologic abnormalities, whereas cutaneous involvement does not affect outcome.2
Treatment
A prospective study used an initial treatment regimen of cyclophosphamide and prednisone but mortality was high.24 More recently, chemotherapy regimens including CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), CVP (cyclophosphamide, vincristine, and prednisone), CVP or CHOP combined with rituximab, C-MOPP (cyclophosphamide, vincristine, prednisone, and procarbazine), EPOCH, and rituximab with high-dose cytarabine have been used with variable success for grades 2 and 3 LYG.17,23,25,26 Antiviral and immunomodulatory (interferon alfa) therapy has been used to induce remission in a majority of patients with grades 1 or 2 LYG.3,17,27,28 There is a report of successful treatment of relapsed LYG with the retinoid agent bexarotene.29 Autologous or allogeneic stem cell transplantation was effective for some patients with refractory or relapsed LYG.30 Further studies are needed to clarify optimal treatment of LYG, especially high-grade disease.
Conclusion
We report a rare case of LYG in a patient with antisynthetase syndrome, which highlights the critical role of skin biopsy in establishing the diagnosis of LYG when the clinical and radiologic presentations are obscured by other comorbidities. Dermatologists should be familiar with this rare disease and maintain a low threshold for biopsy in immunocompromised patients presenting with nodular lung infiltrates and/or nonspecific skin lesions.
Lymphomatoid granulomatosis (LYG) is a rare Epstein-Barr virus (EBV)–related extranodal angiocentric lymphoproliferative disorder. Most patients are adults in the fifth decade of life, and men are twice as likely as women to be affected.1 The most common site of involvement is the lungs, which has been observed in more than 90% of patients.2 The skin is the most common extrapulmonary site of involvement with variable manifestations including “rash,” subcutaneous nodules, and ulceration. Although a small subset of patients experience remission without treatment, most patients report a progressive course with median survival of less than 2 years.1,2 Clinical diagnosis often is challenging due to underrecognition of this rare condition by multidisciplinary physicians.
Case Report
A 60-year-old woman presented with fatigue, night sweats, poor appetite, unintentional weight loss, and dyspnea with minor exertion of 2 weeks’ duration. Her medical history was remarkable for antisynthetase syndrome manifested as polymyositis and interstitial lung disease, as well as recurrent breast cancer treated with wide excision, chemotherapy, and radiation therapy completed 2 months prior. Antisynthetase syndrome was controlled with azathioprine for 2 years, which was stopped during chemotherapy but restarted to treat worsened myalgia 4 months prior to presentation. Two weeks prior to hospital admission, she was treated with antibiotics at an outside hospital for presumed pneumonia without improvement. Upon admission to our hospital she was pancytopenic. Chest computed tomography showed interval development of extensive patchy ground-glass opacities in all lung lobes with areas of confluent consolidation. Broad infectious workup was negative. Given the time course of presentation and anterior accentuation of the lung infiltrates, the greatest clinical concern was radiation pneumonitis followed by drug toxicity. A bone marrow biopsy was hypocellular but without evidence of malignancy. Her pancytopenia was thought to be induced by azathioprine and/or antibiotics. Antibiotics were discontinued and prednisone was started for treatment of presumed radiation pneumonitis.
A few days later, the patient developed new skin lesions and worsening bilateral leg edema. There were multiple small erythematous and hemorrhagic papules, macules, and blisters on the medial aspect of the right lower leg and ankle, each measuring less than 1 cm in diameter (Figure 1). The clinical differential diagnosis included vasculitis related to an underlying collagen vascular disease, atypical edema blisters, and drug hypersensitivity reaction. A punch biopsy of one of the lesions showed a moderately dense superficial and deep perivascular lymphoid infiltrate with marked papillary dermal edema and early subepidermal split (Figure 2). The infiltrate was comprised of small- to medium-sized lymphocytes admixed with large cells, histiocytes, and plasma cells (Figure 3). Immunohistochemistry revealed a predominance of CD3+ and CD4+ small- to medium-sized T cells. CD20 highlighted the large angiocentric B cells (Figure 4), which also were positive on EBV-encoded small RNA (EBER) in situ hybridization (Figure 5). A diagnosis of LYG was rendered. Approximately 40 to 50 EBV-positive large B cells were present per high-power field (HPF), consistent with grade 2 disease.





Soon after diagnosis, follow-up computed tomography of the chest, abdomen, and pelvis revealed suspicious lesions in the kidneys, liver, spleen, and inguinal and iliac lymph nodes. The ground-glass opacities in the lungs continued to progress, with 2 additional nodules noted in the right upper and lower lobes. Four days later, core needle biopsies of the right inguinal lymph node showed a large B-cell lymphoma with extensive necrosis (Figure 6). EBER in situ hybridization was suboptimal, probably due to extensive necrosis.

She was started on etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin (EPOCH) for 5 days before developing Klebsiella pneumoniae sepsis and acute kidney injury. She was transferred to the critical care unit due to increasing oxygen requirement. Despite medical interventions, she continued to decompensate and elected to transition to palliative care. She died 6 weeks after the initial presentation. Her family did not request an autopsy.
Comment
Lymphomatoid granulomatosis is a rare lymphoproliferative disorder associated with various immunocompromised states including primary immunodeficiency disorders, human immunodeficiency virus infection, and immunosuppression for organ transplantation and autoimmune diseases. Our patient was receiving azathioprine for antisynthetase syndrome, which put her at risk for EBV infection and LYG. Azathioprine rarely has been reported as a possible culprit of LYG,3,4 but there are no known reported cases that were related to antisynthetase syndrome. There are multiple reports of development of LYG in patients receiving methotrexate for rheumatoid arthritis.5-10 Other iatrogenic causes reported in the literature include thiopurines11,12 and imatinib.13,14
The clinical diagnosis of our patient was particularly challenging given her complicated medical history including interstitial lung disease, predisposition to infection secondary to immunosuppression, and recent radiation therapy to the chest. This case illustrates the importance of maintaining a high index of suspicion for LYG in immunosuppressed patients presenting with lung infiltrates.
Presentation
Radiologically, LYG typically manifests as nodular densities accentuated in the lower lung lobes, which may become confluent.15 Because the nodular pattern in LYG is nonspecific and may mimic sarcoidosis, hypersensitivity pneumonitis, vasculitis, and infectious and neoplastic diseases,16 open lung biopsy often is required to establish the diagnosis in the absence of more accessible lesions.
Cutaneous lesions are seen in 40% to 50% of patients2 and may be the presenting sign of LYG. In a retrospective study, 16% (3/19) of LYG patients presented with cutaneous lesions months before diagnostic pulmonary lesions were identified.17 The skin is the most accessible site for biopsy, allowing definitive tissue diagnosis even when the condition is not clinically suspected. Therefore, dermatologists and dermatopathologists should be aware of this rare entity.
The clinical morphologies of the skin lesions are nonspecific, ranging from erythematous papules and subcutaneous nodules to indurated plaques. Ulceration may be present. The lesions may be widely disseminated or limited to the arms and legs. Our patient presented with erythematous and hemorrhagic papules, macules, and blisters on the lower leg. The hemorrhagic and blistering nature of some of these lesions in our patient may be attributable to thrombocytopenia and lymphedema in addition to LYG.
Histopathology and Differential
The skin biopsy from our patient demonstrated typical features of LYG, namely EBV-positive neoplastic large B cells in a background of predominating reactive T cells.18 The neoplastic large cells frequently invade blood vessels, leading to luminal narrowing without necrosis of the vessel walls. Grading is based on the density of EBV-positive large B cells: grade 1 is defined as fewer than 5 cells per HPF; grade 2, 5 to 50 cells per HPF; and grade 3, more than 50 cells per HPF.18 Grade 2 or 3 disease predicts worse outcome,2 as observed in our case. It is important for pathologists and clinicians to be aware that the proportion of EBV-positive large B cells is variable even within a single lesion; therefore, more than 1 biopsy may be necessary for appropriate grading and management.1,17 Additionally, skin biopsy may have a lower sensitivity for detecting EBV-positive B cells compared to lung biopsy, possibly due to sampling error in small biopsies.17
The histopathologic features of LYG frequently overlap with other lymphomas. Due to the abundance of T cells, LYG may be misclassified as T-cell/histiocyte-rich large B-cell lymphoma.19 Because the latter is not associated with EBV, EBER in situ hybridization is helpful in distinguishing the 2 conditions. On the other hand, EBER in situ hybridization has no value in discriminating LYG and extranodal natural killer (NK)/T-cell lymphoma, as both are EBV driven. Unlike LYG, the neoplastic EBV-positive cells in extranodal NK/T-cell lymphoma make up the majority of the infiltrate and exhibit an NK-cell immunophenotype (positive CD56 and cytoplasmic CD3 epsilon).20 Pulmonary involvement also is uncommon in NK/T-cell lymphoma.
Aside from lymphomas, LYG also resembles granulomatosis with polyangiitis (GPA)(formerly known as Wegener granulomatosis). Clinically, both LYG and GPA can present with constitutional symptoms, as well as lung, kidney, and skin lesions. The 2 conditions differ microscopically, with leukocytoclastic vasculitis and necrotizing granulomatous inflammation being characteristic of GPA but absent in LYG.1,21 Neutrophils and eosinophils are much more likely to be present in GPA.22,23
Disease Progression
Although LYG is an extranodal disease, there is a 7% to 45% risk of progression to nodal lymphoma in patients with high-grade disease.2,22,24 Our patient progressed to nodal large B-cell lymphoma shortly after the diagnosis of high-grade LYG. She developed additional lesions in the liver, spleen, and kidneys, and ultimately succumbed to the disease. Prior studies have shown higher mortality in patients with bilateral lung involvement and neurologic abnormalities, whereas cutaneous involvement does not affect outcome.2
Treatment
A prospective study used an initial treatment regimen of cyclophosphamide and prednisone but mortality was high.24 More recently, chemotherapy regimens including CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), CVP (cyclophosphamide, vincristine, and prednisone), CVP or CHOP combined with rituximab, C-MOPP (cyclophosphamide, vincristine, prednisone, and procarbazine), EPOCH, and rituximab with high-dose cytarabine have been used with variable success for grades 2 and 3 LYG.17,23,25,26 Antiviral and immunomodulatory (interferon alfa) therapy has been used to induce remission in a majority of patients with grades 1 or 2 LYG.3,17,27,28 There is a report of successful treatment of relapsed LYG with the retinoid agent bexarotene.29 Autologous or allogeneic stem cell transplantation was effective for some patients with refractory or relapsed LYG.30 Further studies are needed to clarify optimal treatment of LYG, especially high-grade disease.
Conclusion
We report a rare case of LYG in a patient with antisynthetase syndrome, which highlights the critical role of skin biopsy in establishing the diagnosis of LYG when the clinical and radiologic presentations are obscured by other comorbidities. Dermatologists should be familiar with this rare disease and maintain a low threshold for biopsy in immunocompromised patients presenting with nodular lung infiltrates and/or nonspecific skin lesions.
- Katzenstein AL, Doxtader E, Narendra S. Lymphomatoid granulomatosis: insights gained over 4 decades. Am J Surg Pathol. 2010;34:E35-E48.
- Katzenstein AL, Carrington CB, Liebow AA. Lymphomatoid granulomatosis: a clinicopathologic study of 152 cases. Cancer. 1979;43:360-373.
- Connors W, Griffiths C, Patel J, et al. Lymphomatoid granulomatosis associated with azathioprine therapy in Crohn disease. BMC Gastroenterol. 2014;14:127.
- Katherine Martin L, Porcu P, Baiocchi RA, et al. Primary central nervous system lymphomatoid granulomatosis in a patient receiving azathioprine therapy. Clin Adv Hematol Oncol. 2009;7:65-68.
- Barakat A, Grover K, Peshin R. Rituximab for pulmonary lymphomatoid granulomatosis which developed as a complication of methotrexate and azathioprine therapy for rheumatoid arthritis. Springerplus. 2014;3:751.
- Kobayashi S, Kikuchi Y, Sato K, et al. Reversible iatrogenic, MTX-associated EBV-driven lymphoproliferation with histopathological features of a lymphomatoid granulomatosis in a patient with rheumatoid arthritis. Ann Hematol. 2013;92:1561-1564.
- Kameda H, Okuyama A, Tamaru J, et al. Lymphomatoid granulomatosis and diffuse alveolar damage associated with methotrexate therapy in a patient with rheumatoid arthritis. Clin Rheumatol. 2007;26:1585-1589.
- Oiwa H, Mihara K, Kan T, et al. Grade 3 lymphomatoid granulomatosis in a patient receiving methotrexate therapy for rheumatoid arthritis. Intern Med. 2014;53:1873-1875.
- Blanchart K, Paciencia M, Seguin A, et al. Fatal pulmonary lymphomatoid granulomatosis in a patient taking methotrexate for rheumatoid arthritis. Minerva Anestesiol. 2014;80:119-120.
- Schalk E, Krogel C, Scheinpflug K, et al. Lymphomatoid granulomatosis in a patient with rheumatoid arthritis receiving methotrexate: successful treatment with the anti-CD20 antibody mabthera. Onkologie. 2009;32:440-441.
- Subramaniam K, Cherian M, Jain S, et al. Two rare cases of Epstein-Barr virus-associated lymphoproliferative disorders in inflammatory bowel disease patients on thiopurines and other immunosuppressive medications. Intern Med J. 2013;43:1339-1342.
- Destombe S, Bouron-DalSoglio D, Rougemont AL, et al. Lymphomatoid granulomatosis: a unique complication of Crohn disease and its treatment in pediatrics. J Pediatr Gastroenterol Nutr. 2010;50:559-561.
- Yazdi AS, Metzler G, Weyrauch S, et al. Lymphomatoid granulomatosis induced by imatinib treatment. Arch Dermatol. 2007;143:1222-1223.
- Salmons N, Gregg RJ, Pallalau A, et al. Lymphomatoid granulomatosis in a patient previously diagnosed with a gastrointestinal stromal tumour and treated with imatinib. J Clin Pathol. 2007;60:199-201.
- Dee PM, Arora NS, Innes DJ Jr. The pulmonary manifestations of lymphomatoid granulomatosis. Radiology. 1982;143:613-618.
- Rezai P, Hart EM, Patel SK. Case 169: lymphomatoid granulomatosis. Radiology. 2011;259:604-609.
- Beaty MW, Toro J, Sorbara L, et al. Cutaneous lymphomatoid granulomatosis: correlation of clinical and biologic features. Am J Surg Pathol. 2001;25:1111-1120.
- Pittaluga S, Wilson WH, Jaffe E. Lymphomatoid granulomatosis. In: Swerdlow S, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: International Agency for Research on Cancer; 2008:247-249.
- Abramson JS. T-cell/histiocyte-rich B-cell lymphoma: biology, diagnosis, and management. Oncologist. 2006;11:384-392.
- Jaffe E. Nasal and nasal-type T/NK cell lymphoma: a unique form of lymphoma associated with the Epstein-Barr virus. Histopathology. 1995;27:581-583.
- Barksdale SK, Hallahan CW, Kerr GS, et al. Cutaneous pathology in Wegener’s granulomatosis: a clinicopathologic study of 74 biopsies in 46 patients. Am J Surg Pathol. 1995;19:161-172.
- Koss MN, Hochholzer L, Langloss JM, et al. Lymphomatoid granulomatosis: a clinicopathologic study of 42 patients. Pathology. 1986;18:283-288.
- Aoki T, Harada Y, Matsubara E, et al. Long-term remission after multiple relapses in an elderly patient with lymphomatoid granulomatosis after rituximab and high-dose cytarabine chemotherapy without stem-cell transplantation. J Clin Oncol. 2013;31:E390-E393.
- Fauci AS, Haynes BF, Costa J, et al. Lymphomatoid granulomatosis: prospective clinical and therapeutic experience over 10 years. N Engl J Med. 1982;306:68-74.
- Jung KH, Sung HJ, Lee JH, et al. A case of pulmonary lymphomatoid granulomatosis successfully treated by combination chemotherapy with rituximab. Chemotherapy. 2009;55:386-390.
- Hernandez-Marques C, Lassaletta A, Torrelo A, et al. Rituximab in lymphomatoid granulomatosis. J Pediatr Hematol Oncol. 2014;36:E69-E74.
- Wilson WH, Gutierrez M, Raffeld M, et al. Lymphomatoid granulomatosis: phase 2 study of dose-adjusted interferon-alfa or EPOCH chemotherapy. Blood. 1999;94:599A.
- Wilson WH, Kingma DW, Raffeld M, et al. Association of lymphomatoid granulomatosis with Epstein-Barr viral infection of B lymphocytes and response to interferon-alpha 2b. Blood. 1996;87:4531-4537.
- Berg SE, Downs LH, Torigian DA, et al. Successful treatment of relapsed lymphomatoid granulomatosis with bexarotene. Cancer Biol Ther. 2008;7:1544-1546.
- Siegloch K, Schmitz N, Wu HS, et al. Hematopoietic stem cell transplantation in patients with lymphomatoid granulomatosis: a European group for blood and marrow transplantation report. Biol Blood Marrow Transplant. 2013;19:1522-1525.
- Katzenstein AL, Doxtader E, Narendra S. Lymphomatoid granulomatosis: insights gained over 4 decades. Am J Surg Pathol. 2010;34:E35-E48.
- Katzenstein AL, Carrington CB, Liebow AA. Lymphomatoid granulomatosis: a clinicopathologic study of 152 cases. Cancer. 1979;43:360-373.
- Connors W, Griffiths C, Patel J, et al. Lymphomatoid granulomatosis associated with azathioprine therapy in Crohn disease. BMC Gastroenterol. 2014;14:127.
- Katherine Martin L, Porcu P, Baiocchi RA, et al. Primary central nervous system lymphomatoid granulomatosis in a patient receiving azathioprine therapy. Clin Adv Hematol Oncol. 2009;7:65-68.
- Barakat A, Grover K, Peshin R. Rituximab for pulmonary lymphomatoid granulomatosis which developed as a complication of methotrexate and azathioprine therapy for rheumatoid arthritis. Springerplus. 2014;3:751.
- Kobayashi S, Kikuchi Y, Sato K, et al. Reversible iatrogenic, MTX-associated EBV-driven lymphoproliferation with histopathological features of a lymphomatoid granulomatosis in a patient with rheumatoid arthritis. Ann Hematol. 2013;92:1561-1564.
- Kameda H, Okuyama A, Tamaru J, et al. Lymphomatoid granulomatosis and diffuse alveolar damage associated with methotrexate therapy in a patient with rheumatoid arthritis. Clin Rheumatol. 2007;26:1585-1589.
- Oiwa H, Mihara K, Kan T, et al. Grade 3 lymphomatoid granulomatosis in a patient receiving methotrexate therapy for rheumatoid arthritis. Intern Med. 2014;53:1873-1875.
- Blanchart K, Paciencia M, Seguin A, et al. Fatal pulmonary lymphomatoid granulomatosis in a patient taking methotrexate for rheumatoid arthritis. Minerva Anestesiol. 2014;80:119-120.
- Schalk E, Krogel C, Scheinpflug K, et al. Lymphomatoid granulomatosis in a patient with rheumatoid arthritis receiving methotrexate: successful treatment with the anti-CD20 antibody mabthera. Onkologie. 2009;32:440-441.
- Subramaniam K, Cherian M, Jain S, et al. Two rare cases of Epstein-Barr virus-associated lymphoproliferative disorders in inflammatory bowel disease patients on thiopurines and other immunosuppressive medications. Intern Med J. 2013;43:1339-1342.
- Destombe S, Bouron-DalSoglio D, Rougemont AL, et al. Lymphomatoid granulomatosis: a unique complication of Crohn disease and its treatment in pediatrics. J Pediatr Gastroenterol Nutr. 2010;50:559-561.
- Yazdi AS, Metzler G, Weyrauch S, et al. Lymphomatoid granulomatosis induced by imatinib treatment. Arch Dermatol. 2007;143:1222-1223.
- Salmons N, Gregg RJ, Pallalau A, et al. Lymphomatoid granulomatosis in a patient previously diagnosed with a gastrointestinal stromal tumour and treated with imatinib. J Clin Pathol. 2007;60:199-201.
- Dee PM, Arora NS, Innes DJ Jr. The pulmonary manifestations of lymphomatoid granulomatosis. Radiology. 1982;143:613-618.
- Rezai P, Hart EM, Patel SK. Case 169: lymphomatoid granulomatosis. Radiology. 2011;259:604-609.
- Beaty MW, Toro J, Sorbara L, et al. Cutaneous lymphomatoid granulomatosis: correlation of clinical and biologic features. Am J Surg Pathol. 2001;25:1111-1120.
- Pittaluga S, Wilson WH, Jaffe E. Lymphomatoid granulomatosis. In: Swerdlow S, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: International Agency for Research on Cancer; 2008:247-249.
- Abramson JS. T-cell/histiocyte-rich B-cell lymphoma: biology, diagnosis, and management. Oncologist. 2006;11:384-392.
- Jaffe E. Nasal and nasal-type T/NK cell lymphoma: a unique form of lymphoma associated with the Epstein-Barr virus. Histopathology. 1995;27:581-583.
- Barksdale SK, Hallahan CW, Kerr GS, et al. Cutaneous pathology in Wegener’s granulomatosis: a clinicopathologic study of 74 biopsies in 46 patients. Am J Surg Pathol. 1995;19:161-172.
- Koss MN, Hochholzer L, Langloss JM, et al. Lymphomatoid granulomatosis: a clinicopathologic study of 42 patients. Pathology. 1986;18:283-288.
- Aoki T, Harada Y, Matsubara E, et al. Long-term remission after multiple relapses in an elderly patient with lymphomatoid granulomatosis after rituximab and high-dose cytarabine chemotherapy without stem-cell transplantation. J Clin Oncol. 2013;31:E390-E393.
- Fauci AS, Haynes BF, Costa J, et al. Lymphomatoid granulomatosis: prospective clinical and therapeutic experience over 10 years. N Engl J Med. 1982;306:68-74.
- Jung KH, Sung HJ, Lee JH, et al. A case of pulmonary lymphomatoid granulomatosis successfully treated by combination chemotherapy with rituximab. Chemotherapy. 2009;55:386-390.
- Hernandez-Marques C, Lassaletta A, Torrelo A, et al. Rituximab in lymphomatoid granulomatosis. J Pediatr Hematol Oncol. 2014;36:E69-E74.
- Wilson WH, Gutierrez M, Raffeld M, et al. Lymphomatoid granulomatosis: phase 2 study of dose-adjusted interferon-alfa or EPOCH chemotherapy. Blood. 1999;94:599A.
- Wilson WH, Kingma DW, Raffeld M, et al. Association of lymphomatoid granulomatosis with Epstein-Barr viral infection of B lymphocytes and response to interferon-alpha 2b. Blood. 1996;87:4531-4537.
- Berg SE, Downs LH, Torigian DA, et al. Successful treatment of relapsed lymphomatoid granulomatosis with bexarotene. Cancer Biol Ther. 2008;7:1544-1546.
- Siegloch K, Schmitz N, Wu HS, et al. Hematopoietic stem cell transplantation in patients with lymphomatoid granulomatosis: a European group for blood and marrow transplantation report. Biol Blood Marrow Transplant. 2013;19:1522-1525.
Practice Points
- Lymphomatoid granulomatosis (LYG) is a rare extranodal angiocentric large B-cell lymphoma driven by the Epstein-Barr virus.
- Lymphomatoid granulomatosis should be suspected when immunocompromised patients present with nodular lung infiltrates and/or nonspecific skin lesions.
- Skin biopsy serves a critical role in establishing the diagnosis of LYG, especially when clinical and radiologic findings are obscured by other comorbidities.
Expert Panel: Little support for delaying cosmetic procedures after isotretinoin
In most cases, there is little evidence to support delaying cosmetic procedures, such as laser therapy or chemical peels, in patients who have recently been treated with isotretinoin for acne, according to a consensus statement from the American Society of Dermatologic Surgery (ASDS).
An expert panel convened by the ASDS issued specific recommendations that supported safe, early initiation of cosmetic procedures in most cases. It noted that the likelihood of any potential harms from initiating cosmetic procedures after recent isotretinoin treatment is “low to very low” and that such harms have been reported only in case reports and case series.
Notable exceptions included dermabrasion and full-face ablative resurfacing; the experts recommended against having such procedures within 6 months of isotretinoin use because of potentially increased risks of adverse events in some patients.
“Potential benefits of this guideline include early access to scar treatments for many patients who are at the highest risk for scarring and, thereby, potentially improved patient quality of life,” Abigail Waldman, MD, of the department of dermatology at Brigham and Women’s Hospital, Boston, and her coauthors wrote in the consensus statement (Dermatol Surg. 2017 Oct;43[10]:1249-62). This is the first consensus statement document published by the ASDS to address this topic.
Isotretinoin was approved by the Food and Drug Administration in 1982 for treating severe and nodulocystic acne. Because of a perceived higher risk of scarring or irritation associated with isotretinoin use, standard clinical practice has been to avoid performing laser procedures, chemical peels, waxing, dermabrasion, and incisional or excisional cutaneous surgeries on patients within 6 months of their using isotretinoin, according to the authors. A warning regarding the potential for scarring with cosmetic procedures meant to smooth the skin is even included in the patient information leaflet for isotretinoin.
“This is in contradistinction to the observation that nodulocystic or severe inflammatory acne patients who have recently completed treatment with isotretinoin are among those most likely to benefit from treatment of their acne scars with modalities such as laser, dermabrasion, or chemical peels,” the experts wrote in the consensus recommendations.
Following a review of the 36 source documents, the task force concluded that, for patients currently or recently receiving isotretinoin, evidence was “insufficient” to justify delaying treatment with superficial chemical peels, vascular lasers, and nonablative modalities, such as hair removal lasers and lights. They also stated that superficial and focal dermabrasion “may also be safe when performed by a well-trained clinician” in a clinical setting.
The panel recommendations covered the following four key areas:
- Dermabrasion. Treating specific facial areas while the patient is on isotretinoin or within 6 months of discontinuation “is not associated with increased risk of scar or delay in wound healing, and there is no evidence in the literature that supports a need to delay treatment,” they wrote. In contrast, they did not recommend full-face or mechanical dermabrasion with rotary devices within the 6-month window because it may be “associated with increased risk of adverse events in selected patients.”
- Lasers and energy devices. Similarly, the panel found no evidence that would justify delaying use of vascular lasers, hair removal lasers and lights, and nonablative or ablative fractional devices among patients recently treated with isotretinoin. However, they said fully ablative treatment of the entire face or regions other than the face should “generally be avoided until 6 months after completion of isotretinoin treatment because of the likely elevated risk of avoidable adverse events.”
- Chemical peels. Patients currently on isotretinoin or who have recently discontinued it can safely undergo superficial chemical peels, according to the panel. For medium or deep chemical peels, there was “insufficient data … to preclude a recommendation in this case,” the panel wrote.
- Other surgeries. Because of the risk of dry eyes, isotretinoin should be discontinued prior to laser eye surgery. For incisional and excisional cutaneous surgery, the data on isotretinoin were insufficient to make any recommendations, the experts concluded, though they acknowledged that in some cases, the surgeries may be “medically necessary.”
Most of these recommendations were based on case series and cohort studies, the panel said, rather than higher-quality, randomized clinical trials, which are “generally impractical and not likely forthcoming in this setting.” Moreover, they cautioned that insufficient evidence to make a recommendation should not be misconstrued as a confirmation of safety or a warning about risk.
Overall, the results of the analysis suggested that “procedural interventions during or soon after isotretinoin treatment can safely and effectively address acne scarring and similar disorders, thus providing relief to patients without the need for protracted waiting,” the authors wrote.
In August, another expert panel’s recommendations were published, which concluded that skin procedures, including superficial chemical peels, laser hair removal, minor cutaneous surgery, manual dermabrasion, and fractional ablative and fractional nonablative laser procedures, can be performed safely on patients who have recently been or are currently being treated with isotretinoin (JAMA Dermatol. 2017 Aug 1;153[8]:802-9).
The authors of the ASDS statement reported no relevant financial conflicts.
In most cases, there is little evidence to support delaying cosmetic procedures, such as laser therapy or chemical peels, in patients who have recently been treated with isotretinoin for acne, according to a consensus statement from the American Society of Dermatologic Surgery (ASDS).
An expert panel convened by the ASDS issued specific recommendations that supported safe, early initiation of cosmetic procedures in most cases. It noted that the likelihood of any potential harms from initiating cosmetic procedures after recent isotretinoin treatment is “low to very low” and that such harms have been reported only in case reports and case series.
Notable exceptions included dermabrasion and full-face ablative resurfacing; the experts recommended against having such procedures within 6 months of isotretinoin use because of potentially increased risks of adverse events in some patients.
“Potential benefits of this guideline include early access to scar treatments for many patients who are at the highest risk for scarring and, thereby, potentially improved patient quality of life,” Abigail Waldman, MD, of the department of dermatology at Brigham and Women’s Hospital, Boston, and her coauthors wrote in the consensus statement (Dermatol Surg. 2017 Oct;43[10]:1249-62). This is the first consensus statement document published by the ASDS to address this topic.
Isotretinoin was approved by the Food and Drug Administration in 1982 for treating severe and nodulocystic acne. Because of a perceived higher risk of scarring or irritation associated with isotretinoin use, standard clinical practice has been to avoid performing laser procedures, chemical peels, waxing, dermabrasion, and incisional or excisional cutaneous surgeries on patients within 6 months of their using isotretinoin, according to the authors. A warning regarding the potential for scarring with cosmetic procedures meant to smooth the skin is even included in the patient information leaflet for isotretinoin.
“This is in contradistinction to the observation that nodulocystic or severe inflammatory acne patients who have recently completed treatment with isotretinoin are among those most likely to benefit from treatment of their acne scars with modalities such as laser, dermabrasion, or chemical peels,” the experts wrote in the consensus recommendations.
Following a review of the 36 source documents, the task force concluded that, for patients currently or recently receiving isotretinoin, evidence was “insufficient” to justify delaying treatment with superficial chemical peels, vascular lasers, and nonablative modalities, such as hair removal lasers and lights. They also stated that superficial and focal dermabrasion “may also be safe when performed by a well-trained clinician” in a clinical setting.
The panel recommendations covered the following four key areas:
- Dermabrasion. Treating specific facial areas while the patient is on isotretinoin or within 6 months of discontinuation “is not associated with increased risk of scar or delay in wound healing, and there is no evidence in the literature that supports a need to delay treatment,” they wrote. In contrast, they did not recommend full-face or mechanical dermabrasion with rotary devices within the 6-month window because it may be “associated with increased risk of adverse events in selected patients.”
- Lasers and energy devices. Similarly, the panel found no evidence that would justify delaying use of vascular lasers, hair removal lasers and lights, and nonablative or ablative fractional devices among patients recently treated with isotretinoin. However, they said fully ablative treatment of the entire face or regions other than the face should “generally be avoided until 6 months after completion of isotretinoin treatment because of the likely elevated risk of avoidable adverse events.”
- Chemical peels. Patients currently on isotretinoin or who have recently discontinued it can safely undergo superficial chemical peels, according to the panel. For medium or deep chemical peels, there was “insufficient data … to preclude a recommendation in this case,” the panel wrote.
- Other surgeries. Because of the risk of dry eyes, isotretinoin should be discontinued prior to laser eye surgery. For incisional and excisional cutaneous surgery, the data on isotretinoin were insufficient to make any recommendations, the experts concluded, though they acknowledged that in some cases, the surgeries may be “medically necessary.”
Most of these recommendations were based on case series and cohort studies, the panel said, rather than higher-quality, randomized clinical trials, which are “generally impractical and not likely forthcoming in this setting.” Moreover, they cautioned that insufficient evidence to make a recommendation should not be misconstrued as a confirmation of safety or a warning about risk.
Overall, the results of the analysis suggested that “procedural interventions during or soon after isotretinoin treatment can safely and effectively address acne scarring and similar disorders, thus providing relief to patients without the need for protracted waiting,” the authors wrote.
In August, another expert panel’s recommendations were published, which concluded that skin procedures, including superficial chemical peels, laser hair removal, minor cutaneous surgery, manual dermabrasion, and fractional ablative and fractional nonablative laser procedures, can be performed safely on patients who have recently been or are currently being treated with isotretinoin (JAMA Dermatol. 2017 Aug 1;153[8]:802-9).
The authors of the ASDS statement reported no relevant financial conflicts.
In most cases, there is little evidence to support delaying cosmetic procedures, such as laser therapy or chemical peels, in patients who have recently been treated with isotretinoin for acne, according to a consensus statement from the American Society of Dermatologic Surgery (ASDS).
An expert panel convened by the ASDS issued specific recommendations that supported safe, early initiation of cosmetic procedures in most cases. It noted that the likelihood of any potential harms from initiating cosmetic procedures after recent isotretinoin treatment is “low to very low” and that such harms have been reported only in case reports and case series.
Notable exceptions included dermabrasion and full-face ablative resurfacing; the experts recommended against having such procedures within 6 months of isotretinoin use because of potentially increased risks of adverse events in some patients.
“Potential benefits of this guideline include early access to scar treatments for many patients who are at the highest risk for scarring and, thereby, potentially improved patient quality of life,” Abigail Waldman, MD, of the department of dermatology at Brigham and Women’s Hospital, Boston, and her coauthors wrote in the consensus statement (Dermatol Surg. 2017 Oct;43[10]:1249-62). This is the first consensus statement document published by the ASDS to address this topic.
Isotretinoin was approved by the Food and Drug Administration in 1982 for treating severe and nodulocystic acne. Because of a perceived higher risk of scarring or irritation associated with isotretinoin use, standard clinical practice has been to avoid performing laser procedures, chemical peels, waxing, dermabrasion, and incisional or excisional cutaneous surgeries on patients within 6 months of their using isotretinoin, according to the authors. A warning regarding the potential for scarring with cosmetic procedures meant to smooth the skin is even included in the patient information leaflet for isotretinoin.
“This is in contradistinction to the observation that nodulocystic or severe inflammatory acne patients who have recently completed treatment with isotretinoin are among those most likely to benefit from treatment of their acne scars with modalities such as laser, dermabrasion, or chemical peels,” the experts wrote in the consensus recommendations.
Following a review of the 36 source documents, the task force concluded that, for patients currently or recently receiving isotretinoin, evidence was “insufficient” to justify delaying treatment with superficial chemical peels, vascular lasers, and nonablative modalities, such as hair removal lasers and lights. They also stated that superficial and focal dermabrasion “may also be safe when performed by a well-trained clinician” in a clinical setting.
The panel recommendations covered the following four key areas:
- Dermabrasion. Treating specific facial areas while the patient is on isotretinoin or within 6 months of discontinuation “is not associated with increased risk of scar or delay in wound healing, and there is no evidence in the literature that supports a need to delay treatment,” they wrote. In contrast, they did not recommend full-face or mechanical dermabrasion with rotary devices within the 6-month window because it may be “associated with increased risk of adverse events in selected patients.”
- Lasers and energy devices. Similarly, the panel found no evidence that would justify delaying use of vascular lasers, hair removal lasers and lights, and nonablative or ablative fractional devices among patients recently treated with isotretinoin. However, they said fully ablative treatment of the entire face or regions other than the face should “generally be avoided until 6 months after completion of isotretinoin treatment because of the likely elevated risk of avoidable adverse events.”
- Chemical peels. Patients currently on isotretinoin or who have recently discontinued it can safely undergo superficial chemical peels, according to the panel. For medium or deep chemical peels, there was “insufficient data … to preclude a recommendation in this case,” the panel wrote.
- Other surgeries. Because of the risk of dry eyes, isotretinoin should be discontinued prior to laser eye surgery. For incisional and excisional cutaneous surgery, the data on isotretinoin were insufficient to make any recommendations, the experts concluded, though they acknowledged that in some cases, the surgeries may be “medically necessary.”
Most of these recommendations were based on case series and cohort studies, the panel said, rather than higher-quality, randomized clinical trials, which are “generally impractical and not likely forthcoming in this setting.” Moreover, they cautioned that insufficient evidence to make a recommendation should not be misconstrued as a confirmation of safety or a warning about risk.
Overall, the results of the analysis suggested that “procedural interventions during or soon after isotretinoin treatment can safely and effectively address acne scarring and similar disorders, thus providing relief to patients without the need for protracted waiting,” the authors wrote.
In August, another expert panel’s recommendations were published, which concluded that skin procedures, including superficial chemical peels, laser hair removal, minor cutaneous surgery, manual dermabrasion, and fractional ablative and fractional nonablative laser procedures, can be performed safely on patients who have recently been or are currently being treated with isotretinoin (JAMA Dermatol. 2017 Aug 1;153[8]:802-9).
The authors of the ASDS statement reported no relevant financial conflicts.
FROM DERMATOLOGIC SURGERY
Key clinical point: Contrary to current recommendations,
Major finding: Experts convened by the American Society of Dermatologic Surgery found that, in most cases, the likelihood of potential harms of initiating cosmetic procedures after recent isotretinoin use is “low to very low,” and those that did occur were reported only in case reports and case series rather than in higher-quality clinical trials.
Data source: A consensus review of 36 source documents obtained by a literature review, the results of which were then validated by peer review.
Disclosures: The authors reported no relevant financial conflicts.
Vesiculobullous and Pustular Diseases in Newborns
Vesiculobullous eruptions in neonates can readily generate anxiety from parents/guardians and pediatricians over both infectious and noninfectious causes. The role of the dermatology resident is critical to help diminish fear over common vesicular presentations or to escalate care in rarer situations if a more obscure or ominous diagnosis is clouding the patient’s clinical presentation and well-being. This article summarizes both common and uncommon vesiculobullous neonatal diseases to augment precise and efficient diagnoses in this vulnerable patient population.
Steps for Evaluating a Vesiculopustular Eruption
Receiving a consultation for a newborn with widespread vesicles can be a daunting scenario for a dermatology resident. Fear of missing an ominous diagnosis or aggressively treating a newborn for an erroneous infection when the diagnosis is actually a benign presentation can lead to an anxiety-provoking situation. Additionally, performing a procedure on a newborn can cause personal uneasiness. Dr. Lawrence A. Schachner, an eminent pediatric dermatologist at the University of Miami Miller School of Medicine (Miami, Florida), recently lectured on 5 key steps (Table 1) for the evaluation of a vesiculobullous eruption in the newborn to maximize the accuracy of diagnosis and patient care.1
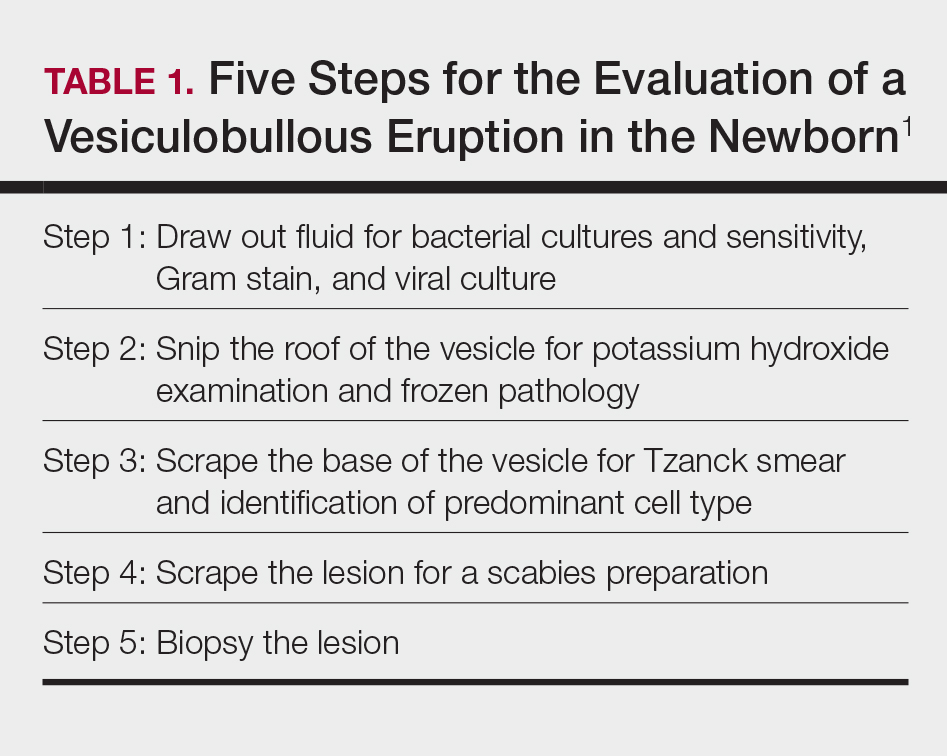
First, draw out the fluid from the vesicle to send for bacterial and viral culture as well as Gram stain. Second, snip the roof of the vesicle to perform potassium hydroxide examination for yeast or fungi and frozen pathology when indicated. Third, use the base of the vesicle to obtain cells for a Tzanck smear to identify the predominant cell infiltrate, such as multinucleated giant cells in herpes simplex virus or eosinophils in erythema toxicum neonatorum (ETN). Fourth, a mineral oil preparation can be performed on several lesions, especially if a burrow is observed, to rule out bullous scabies in the appropriate clinical presentation. Lastly, a perilesional or lesional punch biopsy can be performed if the above steps have not yet clinched the diagnosis.2 By utilizing these steps, the resident efficiently utilizes 1 lesion to narrow down a formidable differential list of bullous disorders in the newborn.
Specific Diagnoses
A number of common diagnoses can present during the newborn period and can usually be readily diagnosed by clinical manifestations alone; a summary of these eruptions is provided in Table 2. Erythema toxicum neonatorum is the most common pustular eruption in neonates and presents in up to 50% of full-term infants at days 1 to 2 of life. Inflammatory pustules surrounded by characteristic blotchy erythema are displayed on the face, trunk, arms, and legs, usually sparing the palms and soles.3 Erythema toxicum neonatorum typically is a clinical diagnosis; however, it can be confirmed by demonstrating the predominance of eosinophils on Tzanck smear.
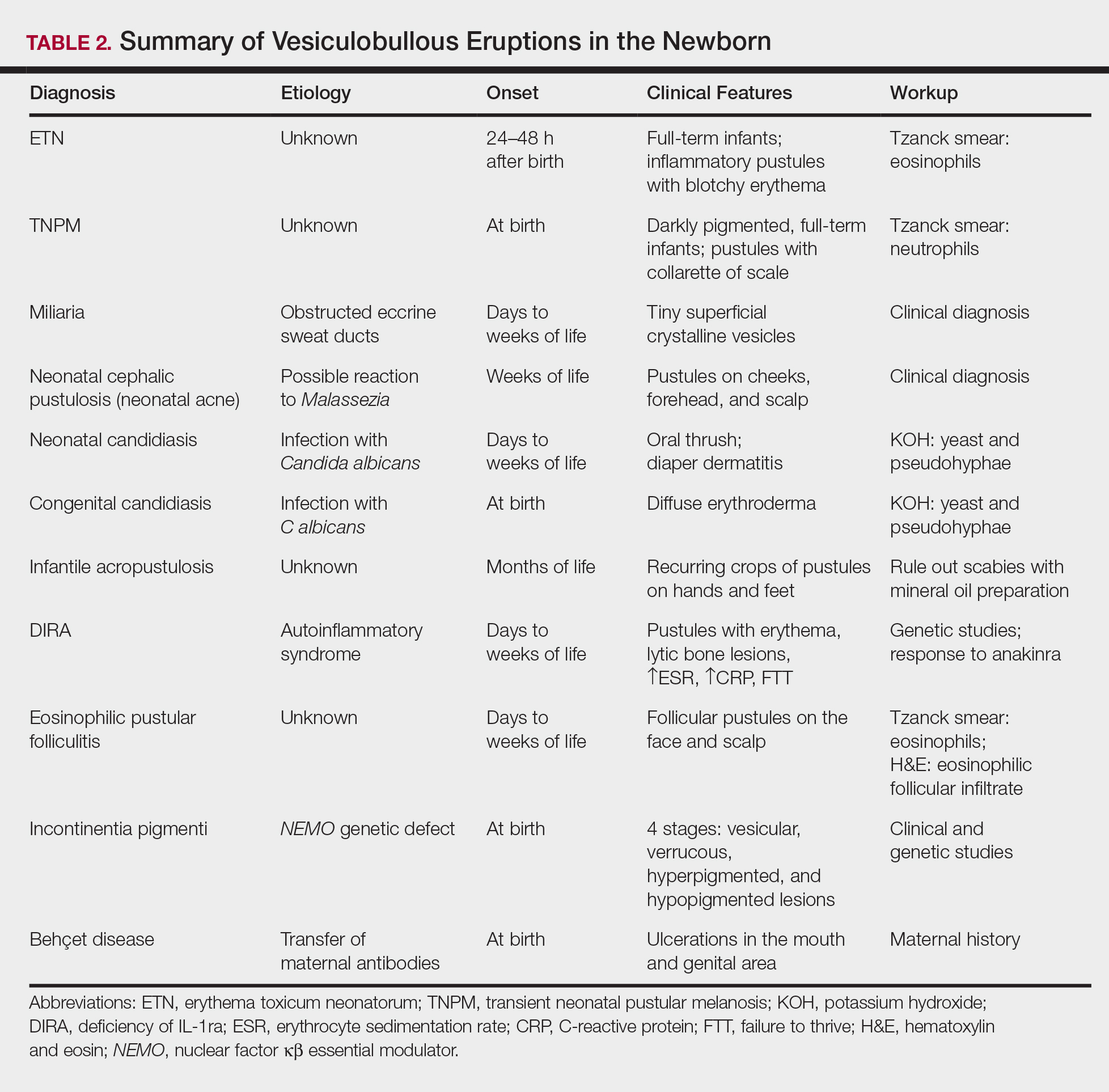
Transient neonatal pustular melanosis (TNPM) also presents in full-term infants; usually favors darkly pigmented neonates; and exhibits either pustules with a collarette of scale that lack surrounding erythema or with residual brown macules on the face, genitals, and acral surfaces. Postinflammatory pigmentary alteration on lesion clearance is another clue to diagnosis. Similarly, it is a clinical diagnosis but can be confirmed with a Tzanck smear demonstrating neutrophils as the major cell infiltrate.
In a prospective 1-year multicenter study performed by Reginatto et al,4 2831 neonates born in southern Brazil underwent a skin examination by a dermatologist within 72 hours of birth to characterize the prevalence and demographics of ETN and TNPM. They found a 21.3% (602 cases) prevalence of ETN compared to a 3.4% (97 cases) prevalence of TNPM, but they noted that most patients were white, and thus the diagnosis of TNPM likely is less prevalent in this group, as it favors darkly pigmented individuals. Additional predisposing factors associated with ETN were male gender, an Apgar score of 8 to 10 at 1 minute, non–neonatal intensive care unit (NICU) patients, and lack of gestational risk factors. The TNPM population was much smaller, though the authors were able to conclude that the disease also was correlated with healthy, non-NICU patients. The authors hypothesized that there may be a role of immune system maturity in the pathogenesis of ETN and thus dermatology residents should be aware of the setting of their consultation.4 A NICU consultation for ETN should raise suspicion, as ETN and TNPM favor healthy infants who likely are not residing in the NICU; we are reminded of the target populations for these disease processes.
Additional common causes of vesicular eruptions in neonates can likewise be diagnosed chiefly with clinical inspection. Miliaria presents with tiny superficial crystalline vesicles on the neck and back of newborns due to elevated temperature and resultant obstruction of the eccrine sweat ducts. Reassurance can be provided, as spontaneous resolution occurs with cooling and limitation of occlusive clothing and swaddling.2
Infants at a few weeks of life may present with a noncomedonal pustular eruption on the cheeks, forehead, and scalp commonly known as neonatal acne or neonatal cephalic pustulosis. The driving factor is thought to be an abnormal response to Malassezia and can be treated with ketoconazole cream or expectant management.2
Cutaneous candidiasis is the most common infectious cause of vesicles in the neonate and can present in 2 fashions. Neonatal candidiasis is common, presenting a week after birth and manifesting as oral thrush and red plaques with satellite pustules in the diaper area. Congenital candidiasis is due to infection in utero, presents prior to 1 week of life, exhibits diffuse erythroderma, and requires timely parenteral antifungals.5 Newborns and preterm infants are at higher risk for systemic disease, while full-term infants may experience a mild course of skin-limited lesions.
It is imperative to rule out other infectious etiologies in ill-appearing neonates with vesicles such as herpes simplex virus, bacterial infections, syphilis, and vertically transmitted TORCH (toxoplasmosis, other infections rubella, cytomegalovirus infection, and herpes simplex) diagnoses.6 Herpes simplex virus classically presents with grouped vesicles on an erythematous base; however, such characteristic lesions may be subtle in the newborn. The site of skin involvement usually is the area that first comes into contact with maternal lesions, such as the face for a newborn delivered in a cephalic presentation.2 It is critical to be cognizant of this diagnosis, as a delay in antiviral therapy can result in neurologic consequences due to disseminated disease.
If the clinical picture of vesiculobullous disease in the newborn is not as clear, less common causes must be considered. Infantile acropustulosis presents with recurring crops of pustules on the hands and feet at several months of age. The most common differential diagnosis is scabies; therefore, a mineral oil preparation should be performed to rule out this common mimicker. Potent topical corticosteroids are first-line therapy, and episodes generally resolve with time.
Another mimicker of pustules in neonates includes deficiency of IL-1ra, a rare entity described in 2009.7 Deficiency of IL-1ra is an autoinflammatory syndrome of skin and bone due to unopposed action of IL-1 with life-threatening inflammation; infants present with pustules, lytic bone lesions, elevated erythrocyte sedimentation rate and C-reactive protein, and failure to thrive.8 The characteristic mutation was discovered when the infants dramatically responded to therapy with anakinra, an IL-1ra.
Eosinophilic pustular folliculitis is an additional pustular dermatosis that manifests with lesions predominately in the head and neck area, and unlike the adult population, it usually is self-resolving and not associated with other comorbidities in newborns.2
Incontinentia pigmenti is an X-linked dominant syndrome due to a genetic mutation in NEMO, nuclear factor κβ essential modulator, which protects against apoptosis.3 Incontinentia pigmenti presents in newborn girls shortly after birth with vesicles in a blaschkoid distribution before evolving through 4 unique stages of vesicular lesions, verrucous lesions, hyperpigmentation, and ultimately resolves with residual hypopigmentation in the affected area.
Lastly, neonatal Behçet disease can present with vesicles in the mouth and genital region due to transfer of maternal antibodies. It is self-limiting in nature and would be readily diagnosed with a known maternal history, though judicious screening for infections may be needed in specific settings.2
Conclusion
In summary, a vast array of benign and worrisome dermatoses present in the neonatal period. A thorough history and physical examination, including the temporality of the lesions, the health status of the newborn, and the maternal history, can help delineate the diagnosis. The 5-step method presented can further elucidate the underlying mechanism and reduce an overwhelming differential diagnosis list by reviewing each finding yielded from each step. Dermatology residents should feel comfortable addressing this unique patient population to ameliorate unclear cutaneous diagnoses for pediatricians.
Acknowledgment
A special thank you to Lawrence A. Schachner, MD (Miami, Florida), for his help providing resources and guidance for this topic.
- Schachner L. Vesiculopustular dermatosis in neonates and infants. Lecture presented at: University of Miami Department of Dermatology & Cutaneous Surgery Grand Rounds; August 23, 2017; Miami, Florida.
- Eichenfield LF, Lee PW, Larraide M, et al. Neonatal skin and skin disorders. In: Schachner LA, Hansen RC, eds. Pediatric Dermatology. 4th ed. Philadelphia, PA: Elsevier Mosby; 2011:299-373.
- Goddard DS, Gilliam AE, Frieden IJ. Vesiculobullous and erosive diseases in the newborn. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:523-537.
- Reginatto FP, Muller FM, Peruzzo J, et al. Epidemiology and predisposing factors for erythema toxicum neonatorum and transient neonatal pustular melanosis: a multicenter study [published online May 25, 2017]. Pediatr Dermatol. 2017;34:422-426.
- Aruna C, Seetharam K. Congenital candidiasis. Indian Dermatol Online J. 2014;5(suppl 1):S44-S47.
- O’Connor NR, McLaughlin MR, Ham P. Newborn skin: part I. common rashes. Am Fam Physician. 2008;77:47-52.
- Reddy S, Jia S, Geoffrey R, et al. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N Engl J Med. 2009;360:2438-2444.
- Minkis K, Aksentijevich I, Goldbach-Mansky R, et al. Interleukin 1 receptor antagonist deficiency presenting as infantile pustulosis mimicking infantile pustular psoriasis. Arch Dermatol. 2012;148:747-752.
Vesiculobullous eruptions in neonates can readily generate anxiety from parents/guardians and pediatricians over both infectious and noninfectious causes. The role of the dermatology resident is critical to help diminish fear over common vesicular presentations or to escalate care in rarer situations if a more obscure or ominous diagnosis is clouding the patient’s clinical presentation and well-being. This article summarizes both common and uncommon vesiculobullous neonatal diseases to augment precise and efficient diagnoses in this vulnerable patient population.
Steps for Evaluating a Vesiculopustular Eruption
Receiving a consultation for a newborn with widespread vesicles can be a daunting scenario for a dermatology resident. Fear of missing an ominous diagnosis or aggressively treating a newborn for an erroneous infection when the diagnosis is actually a benign presentation can lead to an anxiety-provoking situation. Additionally, performing a procedure on a newborn can cause personal uneasiness. Dr. Lawrence A. Schachner, an eminent pediatric dermatologist at the University of Miami Miller School of Medicine (Miami, Florida), recently lectured on 5 key steps (Table 1) for the evaluation of a vesiculobullous eruption in the newborn to maximize the accuracy of diagnosis and patient care.1

First, draw out the fluid from the vesicle to send for bacterial and viral culture as well as Gram stain. Second, snip the roof of the vesicle to perform potassium hydroxide examination for yeast or fungi and frozen pathology when indicated. Third, use the base of the vesicle to obtain cells for a Tzanck smear to identify the predominant cell infiltrate, such as multinucleated giant cells in herpes simplex virus or eosinophils in erythema toxicum neonatorum (ETN). Fourth, a mineral oil preparation can be performed on several lesions, especially if a burrow is observed, to rule out bullous scabies in the appropriate clinical presentation. Lastly, a perilesional or lesional punch biopsy can be performed if the above steps have not yet clinched the diagnosis.2 By utilizing these steps, the resident efficiently utilizes 1 lesion to narrow down a formidable differential list of bullous disorders in the newborn.
Specific Diagnoses
A number of common diagnoses can present during the newborn period and can usually be readily diagnosed by clinical manifestations alone; a summary of these eruptions is provided in Table 2. Erythema toxicum neonatorum is the most common pustular eruption in neonates and presents in up to 50% of full-term infants at days 1 to 2 of life. Inflammatory pustules surrounded by characteristic blotchy erythema are displayed on the face, trunk, arms, and legs, usually sparing the palms and soles.3 Erythema toxicum neonatorum typically is a clinical diagnosis; however, it can be confirmed by demonstrating the predominance of eosinophils on Tzanck smear.

Transient neonatal pustular melanosis (TNPM) also presents in full-term infants; usually favors darkly pigmented neonates; and exhibits either pustules with a collarette of scale that lack surrounding erythema or with residual brown macules on the face, genitals, and acral surfaces. Postinflammatory pigmentary alteration on lesion clearance is another clue to diagnosis. Similarly, it is a clinical diagnosis but can be confirmed with a Tzanck smear demonstrating neutrophils as the major cell infiltrate.
In a prospective 1-year multicenter study performed by Reginatto et al,4 2831 neonates born in southern Brazil underwent a skin examination by a dermatologist within 72 hours of birth to characterize the prevalence and demographics of ETN and TNPM. They found a 21.3% (602 cases) prevalence of ETN compared to a 3.4% (97 cases) prevalence of TNPM, but they noted that most patients were white, and thus the diagnosis of TNPM likely is less prevalent in this group, as it favors darkly pigmented individuals. Additional predisposing factors associated with ETN were male gender, an Apgar score of 8 to 10 at 1 minute, non–neonatal intensive care unit (NICU) patients, and lack of gestational risk factors. The TNPM population was much smaller, though the authors were able to conclude that the disease also was correlated with healthy, non-NICU patients. The authors hypothesized that there may be a role of immune system maturity in the pathogenesis of ETN and thus dermatology residents should be aware of the setting of their consultation.4 A NICU consultation for ETN should raise suspicion, as ETN and TNPM favor healthy infants who likely are not residing in the NICU; we are reminded of the target populations for these disease processes.
Additional common causes of vesicular eruptions in neonates can likewise be diagnosed chiefly with clinical inspection. Miliaria presents with tiny superficial crystalline vesicles on the neck and back of newborns due to elevated temperature and resultant obstruction of the eccrine sweat ducts. Reassurance can be provided, as spontaneous resolution occurs with cooling and limitation of occlusive clothing and swaddling.2
Infants at a few weeks of life may present with a noncomedonal pustular eruption on the cheeks, forehead, and scalp commonly known as neonatal acne or neonatal cephalic pustulosis. The driving factor is thought to be an abnormal response to Malassezia and can be treated with ketoconazole cream or expectant management.2
Cutaneous candidiasis is the most common infectious cause of vesicles in the neonate and can present in 2 fashions. Neonatal candidiasis is common, presenting a week after birth and manifesting as oral thrush and red plaques with satellite pustules in the diaper area. Congenital candidiasis is due to infection in utero, presents prior to 1 week of life, exhibits diffuse erythroderma, and requires timely parenteral antifungals.5 Newborns and preterm infants are at higher risk for systemic disease, while full-term infants may experience a mild course of skin-limited lesions.
It is imperative to rule out other infectious etiologies in ill-appearing neonates with vesicles such as herpes simplex virus, bacterial infections, syphilis, and vertically transmitted TORCH (toxoplasmosis, other infections rubella, cytomegalovirus infection, and herpes simplex) diagnoses.6 Herpes simplex virus classically presents with grouped vesicles on an erythematous base; however, such characteristic lesions may be subtle in the newborn. The site of skin involvement usually is the area that first comes into contact with maternal lesions, such as the face for a newborn delivered in a cephalic presentation.2 It is critical to be cognizant of this diagnosis, as a delay in antiviral therapy can result in neurologic consequences due to disseminated disease.
If the clinical picture of vesiculobullous disease in the newborn is not as clear, less common causes must be considered. Infantile acropustulosis presents with recurring crops of pustules on the hands and feet at several months of age. The most common differential diagnosis is scabies; therefore, a mineral oil preparation should be performed to rule out this common mimicker. Potent topical corticosteroids are first-line therapy, and episodes generally resolve with time.
Another mimicker of pustules in neonates includes deficiency of IL-1ra, a rare entity described in 2009.7 Deficiency of IL-1ra is an autoinflammatory syndrome of skin and bone due to unopposed action of IL-1 with life-threatening inflammation; infants present with pustules, lytic bone lesions, elevated erythrocyte sedimentation rate and C-reactive protein, and failure to thrive.8 The characteristic mutation was discovered when the infants dramatically responded to therapy with anakinra, an IL-1ra.
Eosinophilic pustular folliculitis is an additional pustular dermatosis that manifests with lesions predominately in the head and neck area, and unlike the adult population, it usually is self-resolving and not associated with other comorbidities in newborns.2
Incontinentia pigmenti is an X-linked dominant syndrome due to a genetic mutation in NEMO, nuclear factor κβ essential modulator, which protects against apoptosis.3 Incontinentia pigmenti presents in newborn girls shortly after birth with vesicles in a blaschkoid distribution before evolving through 4 unique stages of vesicular lesions, verrucous lesions, hyperpigmentation, and ultimately resolves with residual hypopigmentation in the affected area.
Lastly, neonatal Behçet disease can present with vesicles in the mouth and genital region due to transfer of maternal antibodies. It is self-limiting in nature and would be readily diagnosed with a known maternal history, though judicious screening for infections may be needed in specific settings.2
Conclusion
In summary, a vast array of benign and worrisome dermatoses present in the neonatal period. A thorough history and physical examination, including the temporality of the lesions, the health status of the newborn, and the maternal history, can help delineate the diagnosis. The 5-step method presented can further elucidate the underlying mechanism and reduce an overwhelming differential diagnosis list by reviewing each finding yielded from each step. Dermatology residents should feel comfortable addressing this unique patient population to ameliorate unclear cutaneous diagnoses for pediatricians.
Acknowledgment
A special thank you to Lawrence A. Schachner, MD (Miami, Florida), for his help providing resources and guidance for this topic.
Vesiculobullous eruptions in neonates can readily generate anxiety from parents/guardians and pediatricians over both infectious and noninfectious causes. The role of the dermatology resident is critical to help diminish fear over common vesicular presentations or to escalate care in rarer situations if a more obscure or ominous diagnosis is clouding the patient’s clinical presentation and well-being. This article summarizes both common and uncommon vesiculobullous neonatal diseases to augment precise and efficient diagnoses in this vulnerable patient population.
Steps for Evaluating a Vesiculopustular Eruption
Receiving a consultation for a newborn with widespread vesicles can be a daunting scenario for a dermatology resident. Fear of missing an ominous diagnosis or aggressively treating a newborn for an erroneous infection when the diagnosis is actually a benign presentation can lead to an anxiety-provoking situation. Additionally, performing a procedure on a newborn can cause personal uneasiness. Dr. Lawrence A. Schachner, an eminent pediatric dermatologist at the University of Miami Miller School of Medicine (Miami, Florida), recently lectured on 5 key steps (Table 1) for the evaluation of a vesiculobullous eruption in the newborn to maximize the accuracy of diagnosis and patient care.1

First, draw out the fluid from the vesicle to send for bacterial and viral culture as well as Gram stain. Second, snip the roof of the vesicle to perform potassium hydroxide examination for yeast or fungi and frozen pathology when indicated. Third, use the base of the vesicle to obtain cells for a Tzanck smear to identify the predominant cell infiltrate, such as multinucleated giant cells in herpes simplex virus or eosinophils in erythema toxicum neonatorum (ETN). Fourth, a mineral oil preparation can be performed on several lesions, especially if a burrow is observed, to rule out bullous scabies in the appropriate clinical presentation. Lastly, a perilesional or lesional punch biopsy can be performed if the above steps have not yet clinched the diagnosis.2 By utilizing these steps, the resident efficiently utilizes 1 lesion to narrow down a formidable differential list of bullous disorders in the newborn.
Specific Diagnoses
A number of common diagnoses can present during the newborn period and can usually be readily diagnosed by clinical manifestations alone; a summary of these eruptions is provided in Table 2. Erythema toxicum neonatorum is the most common pustular eruption in neonates and presents in up to 50% of full-term infants at days 1 to 2 of life. Inflammatory pustules surrounded by characteristic blotchy erythema are displayed on the face, trunk, arms, and legs, usually sparing the palms and soles.3 Erythema toxicum neonatorum typically is a clinical diagnosis; however, it can be confirmed by demonstrating the predominance of eosinophils on Tzanck smear.

Transient neonatal pustular melanosis (TNPM) also presents in full-term infants; usually favors darkly pigmented neonates; and exhibits either pustules with a collarette of scale that lack surrounding erythema or with residual brown macules on the face, genitals, and acral surfaces. Postinflammatory pigmentary alteration on lesion clearance is another clue to diagnosis. Similarly, it is a clinical diagnosis but can be confirmed with a Tzanck smear demonstrating neutrophils as the major cell infiltrate.
In a prospective 1-year multicenter study performed by Reginatto et al,4 2831 neonates born in southern Brazil underwent a skin examination by a dermatologist within 72 hours of birth to characterize the prevalence and demographics of ETN and TNPM. They found a 21.3% (602 cases) prevalence of ETN compared to a 3.4% (97 cases) prevalence of TNPM, but they noted that most patients were white, and thus the diagnosis of TNPM likely is less prevalent in this group, as it favors darkly pigmented individuals. Additional predisposing factors associated with ETN were male gender, an Apgar score of 8 to 10 at 1 minute, non–neonatal intensive care unit (NICU) patients, and lack of gestational risk factors. The TNPM population was much smaller, though the authors were able to conclude that the disease also was correlated with healthy, non-NICU patients. The authors hypothesized that there may be a role of immune system maturity in the pathogenesis of ETN and thus dermatology residents should be aware of the setting of their consultation.4 A NICU consultation for ETN should raise suspicion, as ETN and TNPM favor healthy infants who likely are not residing in the NICU; we are reminded of the target populations for these disease processes.
Additional common causes of vesicular eruptions in neonates can likewise be diagnosed chiefly with clinical inspection. Miliaria presents with tiny superficial crystalline vesicles on the neck and back of newborns due to elevated temperature and resultant obstruction of the eccrine sweat ducts. Reassurance can be provided, as spontaneous resolution occurs with cooling and limitation of occlusive clothing and swaddling.2
Infants at a few weeks of life may present with a noncomedonal pustular eruption on the cheeks, forehead, and scalp commonly known as neonatal acne or neonatal cephalic pustulosis. The driving factor is thought to be an abnormal response to Malassezia and can be treated with ketoconazole cream or expectant management.2
Cutaneous candidiasis is the most common infectious cause of vesicles in the neonate and can present in 2 fashions. Neonatal candidiasis is common, presenting a week after birth and manifesting as oral thrush and red plaques with satellite pustules in the diaper area. Congenital candidiasis is due to infection in utero, presents prior to 1 week of life, exhibits diffuse erythroderma, and requires timely parenteral antifungals.5 Newborns and preterm infants are at higher risk for systemic disease, while full-term infants may experience a mild course of skin-limited lesions.
It is imperative to rule out other infectious etiologies in ill-appearing neonates with vesicles such as herpes simplex virus, bacterial infections, syphilis, and vertically transmitted TORCH (toxoplasmosis, other infections rubella, cytomegalovirus infection, and herpes simplex) diagnoses.6 Herpes simplex virus classically presents with grouped vesicles on an erythematous base; however, such characteristic lesions may be subtle in the newborn. The site of skin involvement usually is the area that first comes into contact with maternal lesions, such as the face for a newborn delivered in a cephalic presentation.2 It is critical to be cognizant of this diagnosis, as a delay in antiviral therapy can result in neurologic consequences due to disseminated disease.
If the clinical picture of vesiculobullous disease in the newborn is not as clear, less common causes must be considered. Infantile acropustulosis presents with recurring crops of pustules on the hands and feet at several months of age. The most common differential diagnosis is scabies; therefore, a mineral oil preparation should be performed to rule out this common mimicker. Potent topical corticosteroids are first-line therapy, and episodes generally resolve with time.
Another mimicker of pustules in neonates includes deficiency of IL-1ra, a rare entity described in 2009.7 Deficiency of IL-1ra is an autoinflammatory syndrome of skin and bone due to unopposed action of IL-1 with life-threatening inflammation; infants present with pustules, lytic bone lesions, elevated erythrocyte sedimentation rate and C-reactive protein, and failure to thrive.8 The characteristic mutation was discovered when the infants dramatically responded to therapy with anakinra, an IL-1ra.
Eosinophilic pustular folliculitis is an additional pustular dermatosis that manifests with lesions predominately in the head and neck area, and unlike the adult population, it usually is self-resolving and not associated with other comorbidities in newborns.2
Incontinentia pigmenti is an X-linked dominant syndrome due to a genetic mutation in NEMO, nuclear factor κβ essential modulator, which protects against apoptosis.3 Incontinentia pigmenti presents in newborn girls shortly after birth with vesicles in a blaschkoid distribution before evolving through 4 unique stages of vesicular lesions, verrucous lesions, hyperpigmentation, and ultimately resolves with residual hypopigmentation in the affected area.
Lastly, neonatal Behçet disease can present with vesicles in the mouth and genital region due to transfer of maternal antibodies. It is self-limiting in nature and would be readily diagnosed with a known maternal history, though judicious screening for infections may be needed in specific settings.2
Conclusion
In summary, a vast array of benign and worrisome dermatoses present in the neonatal period. A thorough history and physical examination, including the temporality of the lesions, the health status of the newborn, and the maternal history, can help delineate the diagnosis. The 5-step method presented can further elucidate the underlying mechanism and reduce an overwhelming differential diagnosis list by reviewing each finding yielded from each step. Dermatology residents should feel comfortable addressing this unique patient population to ameliorate unclear cutaneous diagnoses for pediatricians.
Acknowledgment
A special thank you to Lawrence A. Schachner, MD (Miami, Florida), for his help providing resources and guidance for this topic.
- Schachner L. Vesiculopustular dermatosis in neonates and infants. Lecture presented at: University of Miami Department of Dermatology & Cutaneous Surgery Grand Rounds; August 23, 2017; Miami, Florida.
- Eichenfield LF, Lee PW, Larraide M, et al. Neonatal skin and skin disorders. In: Schachner LA, Hansen RC, eds. Pediatric Dermatology. 4th ed. Philadelphia, PA: Elsevier Mosby; 2011:299-373.
- Goddard DS, Gilliam AE, Frieden IJ. Vesiculobullous and erosive diseases in the newborn. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:523-537.
- Reginatto FP, Muller FM, Peruzzo J, et al. Epidemiology and predisposing factors for erythema toxicum neonatorum and transient neonatal pustular melanosis: a multicenter study [published online May 25, 2017]. Pediatr Dermatol. 2017;34:422-426.
- Aruna C, Seetharam K. Congenital candidiasis. Indian Dermatol Online J. 2014;5(suppl 1):S44-S47.
- O’Connor NR, McLaughlin MR, Ham P. Newborn skin: part I. common rashes. Am Fam Physician. 2008;77:47-52.
- Reddy S, Jia S, Geoffrey R, et al. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N Engl J Med. 2009;360:2438-2444.
- Minkis K, Aksentijevich I, Goldbach-Mansky R, et al. Interleukin 1 receptor antagonist deficiency presenting as infantile pustulosis mimicking infantile pustular psoriasis. Arch Dermatol. 2012;148:747-752.
- Schachner L. Vesiculopustular dermatosis in neonates and infants. Lecture presented at: University of Miami Department of Dermatology & Cutaneous Surgery Grand Rounds; August 23, 2017; Miami, Florida.
- Eichenfield LF, Lee PW, Larraide M, et al. Neonatal skin and skin disorders. In: Schachner LA, Hansen RC, eds. Pediatric Dermatology. 4th ed. Philadelphia, PA: Elsevier Mosby; 2011:299-373.
- Goddard DS, Gilliam AE, Frieden IJ. Vesiculobullous and erosive diseases in the newborn. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:523-537.
- Reginatto FP, Muller FM, Peruzzo J, et al. Epidemiology and predisposing factors for erythema toxicum neonatorum and transient neonatal pustular melanosis: a multicenter study [published online May 25, 2017]. Pediatr Dermatol. 2017;34:422-426.
- Aruna C, Seetharam K. Congenital candidiasis. Indian Dermatol Online J. 2014;5(suppl 1):S44-S47.
- O’Connor NR, McLaughlin MR, Ham P. Newborn skin: part I. common rashes. Am Fam Physician. 2008;77:47-52.
- Reddy S, Jia S, Geoffrey R, et al. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N Engl J Med. 2009;360:2438-2444.
- Minkis K, Aksentijevich I, Goldbach-Mansky R, et al. Interleukin 1 receptor antagonist deficiency presenting as infantile pustulosis mimicking infantile pustular psoriasis. Arch Dermatol. 2012;148:747-752.
Early evidence shows that surgery can alter gut microbiome
SAN DIEGO – Surgery appears to stimulate abrupt changes in both the skin and gut microbiome, which in some patients may increase the risk of surgical site infections and anastomotic leaks. With that knowledge, researchers are exploring the very first steps toward a presurgical microbiome optimization protocol, Heidi Nelson, MD, FACS, said at the annual clinical congress of the American College of Surgeons.
It’s very early in the journey, said Dr. Nelson, the Fred C. Andersen Professor of Surgery at Mayo Clinic, Rochester. Minn. And it won’t be a straightforward path: The human microbiome appears to be nearly as individually unique as the human fingerprint, so presurgical protocols might have to be individually tailored to each patient.
Dr. Nelson comoderated a session exploring this topic with John Alverdy, MD, FACS, of the University of Chicago. The panel discussed human and animal studies suggesting that the stress of surgery, when combined with subclinical ischemia and any baseline physiologic stress (chronic illness or radiation, for example) can cause some commensals to begin producing collagenase – a change that endangers even surgically sound anastomoses.
“It’s well known that bacteria can change their function in response to host stress,” said Dr. Shogan, a colorectal surgeon at the University of Chicago. “They recognize these factors and change their entire function. In our work, we found that Enterococcus began to express a tissue-destroying phenotype in response to subclinical ischemia related to surgery.”
The pathogenic flip doesn’t occur unless there are a couple of predisposing factors, he theorized. “There have to be multiple stresses involved. These could include smoking, steroids, obesity, and prior exposure to radiation – all things that we commonly see in our colorectal surgery patients. But when the right situation developed, we can see a proliferation of collagen-destroying bacteria that predispose to leaks.”
The skin microbiome is altered as well, with areas around abdominal incisions beginning to express gut flora, which increase the risk of a surgical site infection, said Andrew Yeh, MD, a general surgery resident at the University of Pittsburgh.
He presented data on 28 colorectal surgery patients, detailing perioperative changes in the chest and abdominal skin microbiome. All of the subjects were adults undergoing colon resection who had not been on any antibiotics at least 1 month before surgery. Skin sampling was performed before and after opening, with additional postoperative skin samples taken daily while the patient was in the hospital recovering. Dr. Yeh had DNA/RNA data on 431 samples taken from this group.
“We saw increases in Staphylococcus and Bacteroides on the skin – normally part of the gut microflora – in relative abundance, while Corynebacterium, a normal constituent of the skin microbiome, had decreased.”
These are all very early observations, though, and the surgical community is nowhere near being able to make any specific presurgical recommendations to optimize the microbiome, or postsurgical recommendations to manage it, said Neil Hyman, MD, FACS, professor of surgery at the University of Chicago.
While it does appear that good bacteria “gone bad” are associated with anastomotic leaks, he agreed that the right constellation of factors has to be in place for this to happen, including “the right bacteria [Enterococcus], the right virulence genes [collagenase], the right activating cures [long operation, blood loss], and the wrong microbiome [altered by smoking, chemotherapy, radiation, or other chronic stressors].”
“I think it’s safe to say that developing collagenase-producing bacteria at an anastomosis site is a bad thing, but the individual genetic makeup of every patient makes any one-size-fits-all protocol approach to treatment really problematic,” Dr. Hyman said.
None of the presenters had any financial disclosures.
[email protected]
On Twitter @Alz_Gal
SAN DIEGO – Surgery appears to stimulate abrupt changes in both the skin and gut microbiome, which in some patients may increase the risk of surgical site infections and anastomotic leaks. With that knowledge, researchers are exploring the very first steps toward a presurgical microbiome optimization protocol, Heidi Nelson, MD, FACS, said at the annual clinical congress of the American College of Surgeons.
It’s very early in the journey, said Dr. Nelson, the Fred C. Andersen Professor of Surgery at Mayo Clinic, Rochester. Minn. And it won’t be a straightforward path: The human microbiome appears to be nearly as individually unique as the human fingerprint, so presurgical protocols might have to be individually tailored to each patient.
Dr. Nelson comoderated a session exploring this topic with John Alverdy, MD, FACS, of the University of Chicago. The panel discussed human and animal studies suggesting that the stress of surgery, when combined with subclinical ischemia and any baseline physiologic stress (chronic illness or radiation, for example) can cause some commensals to begin producing collagenase – a change that endangers even surgically sound anastomoses.
“It’s well known that bacteria can change their function in response to host stress,” said Dr. Shogan, a colorectal surgeon at the University of Chicago. “They recognize these factors and change their entire function. In our work, we found that Enterococcus began to express a tissue-destroying phenotype in response to subclinical ischemia related to surgery.”
The pathogenic flip doesn’t occur unless there are a couple of predisposing factors, he theorized. “There have to be multiple stresses involved. These could include smoking, steroids, obesity, and prior exposure to radiation – all things that we commonly see in our colorectal surgery patients. But when the right situation developed, we can see a proliferation of collagen-destroying bacteria that predispose to leaks.”
The skin microbiome is altered as well, with areas around abdominal incisions beginning to express gut flora, which increase the risk of a surgical site infection, said Andrew Yeh, MD, a general surgery resident at the University of Pittsburgh.
He presented data on 28 colorectal surgery patients, detailing perioperative changes in the chest and abdominal skin microbiome. All of the subjects were adults undergoing colon resection who had not been on any antibiotics at least 1 month before surgery. Skin sampling was performed before and after opening, with additional postoperative skin samples taken daily while the patient was in the hospital recovering. Dr. Yeh had DNA/RNA data on 431 samples taken from this group.
“We saw increases in Staphylococcus and Bacteroides on the skin – normally part of the gut microflora – in relative abundance, while Corynebacterium, a normal constituent of the skin microbiome, had decreased.”
These are all very early observations, though, and the surgical community is nowhere near being able to make any specific presurgical recommendations to optimize the microbiome, or postsurgical recommendations to manage it, said Neil Hyman, MD, FACS, professor of surgery at the University of Chicago.
While it does appear that good bacteria “gone bad” are associated with anastomotic leaks, he agreed that the right constellation of factors has to be in place for this to happen, including “the right bacteria [Enterococcus], the right virulence genes [collagenase], the right activating cures [long operation, blood loss], and the wrong microbiome [altered by smoking, chemotherapy, radiation, or other chronic stressors].”
“I think it’s safe to say that developing collagenase-producing bacteria at an anastomosis site is a bad thing, but the individual genetic makeup of every patient makes any one-size-fits-all protocol approach to treatment really problematic,” Dr. Hyman said.
None of the presenters had any financial disclosures.
[email protected]
On Twitter @Alz_Gal
SAN DIEGO – Surgery appears to stimulate abrupt changes in both the skin and gut microbiome, which in some patients may increase the risk of surgical site infections and anastomotic leaks. With that knowledge, researchers are exploring the very first steps toward a presurgical microbiome optimization protocol, Heidi Nelson, MD, FACS, said at the annual clinical congress of the American College of Surgeons.
It’s very early in the journey, said Dr. Nelson, the Fred C. Andersen Professor of Surgery at Mayo Clinic, Rochester. Minn. And it won’t be a straightforward path: The human microbiome appears to be nearly as individually unique as the human fingerprint, so presurgical protocols might have to be individually tailored to each patient.
Dr. Nelson comoderated a session exploring this topic with John Alverdy, MD, FACS, of the University of Chicago. The panel discussed human and animal studies suggesting that the stress of surgery, when combined with subclinical ischemia and any baseline physiologic stress (chronic illness or radiation, for example) can cause some commensals to begin producing collagenase – a change that endangers even surgically sound anastomoses.
“It’s well known that bacteria can change their function in response to host stress,” said Dr. Shogan, a colorectal surgeon at the University of Chicago. “They recognize these factors and change their entire function. In our work, we found that Enterococcus began to express a tissue-destroying phenotype in response to subclinical ischemia related to surgery.”
The pathogenic flip doesn’t occur unless there are a couple of predisposing factors, he theorized. “There have to be multiple stresses involved. These could include smoking, steroids, obesity, and prior exposure to radiation – all things that we commonly see in our colorectal surgery patients. But when the right situation developed, we can see a proliferation of collagen-destroying bacteria that predispose to leaks.”
The skin microbiome is altered as well, with areas around abdominal incisions beginning to express gut flora, which increase the risk of a surgical site infection, said Andrew Yeh, MD, a general surgery resident at the University of Pittsburgh.
He presented data on 28 colorectal surgery patients, detailing perioperative changes in the chest and abdominal skin microbiome. All of the subjects were adults undergoing colon resection who had not been on any antibiotics at least 1 month before surgery. Skin sampling was performed before and after opening, with additional postoperative skin samples taken daily while the patient was in the hospital recovering. Dr. Yeh had DNA/RNA data on 431 samples taken from this group.
“We saw increases in Staphylococcus and Bacteroides on the skin – normally part of the gut microflora – in relative abundance, while Corynebacterium, a normal constituent of the skin microbiome, had decreased.”
These are all very early observations, though, and the surgical community is nowhere near being able to make any specific presurgical recommendations to optimize the microbiome, or postsurgical recommendations to manage it, said Neil Hyman, MD, FACS, professor of surgery at the University of Chicago.
While it does appear that good bacteria “gone bad” are associated with anastomotic leaks, he agreed that the right constellation of factors has to be in place for this to happen, including “the right bacteria [Enterococcus], the right virulence genes [collagenase], the right activating cures [long operation, blood loss], and the wrong microbiome [altered by smoking, chemotherapy, radiation, or other chronic stressors].”
“I think it’s safe to say that developing collagenase-producing bacteria at an anastomosis site is a bad thing, but the individual genetic makeup of every patient makes any one-size-fits-all protocol approach to treatment really problematic,” Dr. Hyman said.
None of the presenters had any financial disclosures.
[email protected]
On Twitter @Alz_Gal
EXPERT ANALYSIS FROM THE ACS CLINICAL CONGRESS
Guselkumab tops adalimumab for psychiatric comorbidities in psoriasis
GENEVA – Guselkumab for treatment of moderate to severe plaque psoriasis generated significantly greater improvement in comorbid anxiety and depression than adalimumab in the head-to-head VOYAGE 2 randomized trial, Kenneth B. Gordon, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The population enrolled in VOYAGE 2, which did not preselect to enrich the study population for anxiety and depression, reflected how common these mental health problems are among psoriasis patients. Fully 38.6% of the 992 randomized patients had a baseline Hospital Anxiety and Depression Score-Anxiety (HADS-A) of 8 or more (considered clinically important anxiety) on the 0-21 scale, and 27.7% had a HADS-D score of at least 8 (indicative of clinically meaningful depression), noted Dr. Gordon, professor and chair of dermatology at the Medical College of Wisconsin, Milwaukee.
The on-treatment reductions in psychiatric symptoms in VOYAGE 2 correlated closely with greater improvement in psoriasis, he added. For example, regardless of which drug they were on, patients with a baseline HADS-D of 8 or more averaged a 3.6-point reduction in HADS-D at week 24 if, at that point, they had a Psoriasis Area Severity Index (PASI) response of 75-89, a 4.0-point improvement if they had a PASI 90-99 response, and a 5.2-point reduction in HADS-D if they had a PASI 100 response.
VOYAGE 2 was a phase 3, double-blind, multicenter, placebo-controlled comparison of guselkumab (Tremfya), a human monoclonal antibody targeting the interleukin-23 p 19 subunit, and the tumor necrosis factor-alpha inhibitor adalimumab (Humira) in their standard dosing. Participants were randomized 2:1:1 to guselkumab, adalimumab, or placebo. The study’s primary outcomes have previously been reported (J Am Acad Dermatol. 2017 Mar;76[3]:418-31). For example, at week 24 the guselkumab group demonstrated PASI 75, 90, and 100 responses of 89.1%, 75.2%, and 44.2%, respectively, compared with adalimumab, with PASI 75, 90, and 100 responses of 71%, 54.8%, and 26.6%.
Serial measurement of anxiety and depression symptoms using the HADS-A and HADS-D were part of the study protocol. The mean baseline HADS-A and HADS-D scores were 6.8 and 5.3. At week 24, the mean reduction in the HADS-A score was 2.0 points in the guselkumab group, compared with 1.0 point in the adalimumab arm. HADS-D scores improved by 1.7 and 1.1 points, respectively, in the overall guselkumab and adalimumab groups.
More importantly, among patients with a baseline HADS-A of 8 or higher, by week 16 of the trial, the HADS-A score had dropped below 8 in 51.4% of those on guselkumab, compared with 44.9% of patients on adalimumab and 25.9% on placebo, Dr. Gordon reported. Similarly, 59.2% of guselkumab-treated patients with a high baseline HADS-D improved to a score below 8 at week 16, compared with 54.3% on adalimumab and 27% on placebo.
At week 24, 58.4% of guselkumab-treated patients with a high baseline HADS-A and 59.4% of those with a high HADS-D had scores below 8. Again, these responses were significantly better than the 42.9% and 46.4% rates, respectively, in the adalimumab arm.
VOYAGE 2 was sponsored by Janssen, which markets guselkumab, approved by the Food and Drug Administration in July 2017 for treating adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Dr. Gordon reported receiving research support from and serving as a consultant to Janssen and numerous other pharmaceutical companies.
GENEVA – Guselkumab for treatment of moderate to severe plaque psoriasis generated significantly greater improvement in comorbid anxiety and depression than adalimumab in the head-to-head VOYAGE 2 randomized trial, Kenneth B. Gordon, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The population enrolled in VOYAGE 2, which did not preselect to enrich the study population for anxiety and depression, reflected how common these mental health problems are among psoriasis patients. Fully 38.6% of the 992 randomized patients had a baseline Hospital Anxiety and Depression Score-Anxiety (HADS-A) of 8 or more (considered clinically important anxiety) on the 0-21 scale, and 27.7% had a HADS-D score of at least 8 (indicative of clinically meaningful depression), noted Dr. Gordon, professor and chair of dermatology at the Medical College of Wisconsin, Milwaukee.
The on-treatment reductions in psychiatric symptoms in VOYAGE 2 correlated closely with greater improvement in psoriasis, he added. For example, regardless of which drug they were on, patients with a baseline HADS-D of 8 or more averaged a 3.6-point reduction in HADS-D at week 24 if, at that point, they had a Psoriasis Area Severity Index (PASI) response of 75-89, a 4.0-point improvement if they had a PASI 90-99 response, and a 5.2-point reduction in HADS-D if they had a PASI 100 response.
VOYAGE 2 was a phase 3, double-blind, multicenter, placebo-controlled comparison of guselkumab (Tremfya), a human monoclonal antibody targeting the interleukin-23 p 19 subunit, and the tumor necrosis factor-alpha inhibitor adalimumab (Humira) in their standard dosing. Participants were randomized 2:1:1 to guselkumab, adalimumab, or placebo. The study’s primary outcomes have previously been reported (J Am Acad Dermatol. 2017 Mar;76[3]:418-31). For example, at week 24 the guselkumab group demonstrated PASI 75, 90, and 100 responses of 89.1%, 75.2%, and 44.2%, respectively, compared with adalimumab, with PASI 75, 90, and 100 responses of 71%, 54.8%, and 26.6%.
Serial measurement of anxiety and depression symptoms using the HADS-A and HADS-D were part of the study protocol. The mean baseline HADS-A and HADS-D scores were 6.8 and 5.3. At week 24, the mean reduction in the HADS-A score was 2.0 points in the guselkumab group, compared with 1.0 point in the adalimumab arm. HADS-D scores improved by 1.7 and 1.1 points, respectively, in the overall guselkumab and adalimumab groups.
More importantly, among patients with a baseline HADS-A of 8 or higher, by week 16 of the trial, the HADS-A score had dropped below 8 in 51.4% of those on guselkumab, compared with 44.9% of patients on adalimumab and 25.9% on placebo, Dr. Gordon reported. Similarly, 59.2% of guselkumab-treated patients with a high baseline HADS-D improved to a score below 8 at week 16, compared with 54.3% on adalimumab and 27% on placebo.
At week 24, 58.4% of guselkumab-treated patients with a high baseline HADS-A and 59.4% of those with a high HADS-D had scores below 8. Again, these responses were significantly better than the 42.9% and 46.4% rates, respectively, in the adalimumab arm.
VOYAGE 2 was sponsored by Janssen, which markets guselkumab, approved by the Food and Drug Administration in July 2017 for treating adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Dr. Gordon reported receiving research support from and serving as a consultant to Janssen and numerous other pharmaceutical companies.
GENEVA – Guselkumab for treatment of moderate to severe plaque psoriasis generated significantly greater improvement in comorbid anxiety and depression than adalimumab in the head-to-head VOYAGE 2 randomized trial, Kenneth B. Gordon, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The population enrolled in VOYAGE 2, which did not preselect to enrich the study population for anxiety and depression, reflected how common these mental health problems are among psoriasis patients. Fully 38.6% of the 992 randomized patients had a baseline Hospital Anxiety and Depression Score-Anxiety (HADS-A) of 8 or more (considered clinically important anxiety) on the 0-21 scale, and 27.7% had a HADS-D score of at least 8 (indicative of clinically meaningful depression), noted Dr. Gordon, professor and chair of dermatology at the Medical College of Wisconsin, Milwaukee.
The on-treatment reductions in psychiatric symptoms in VOYAGE 2 correlated closely with greater improvement in psoriasis, he added. For example, regardless of which drug they were on, patients with a baseline HADS-D of 8 or more averaged a 3.6-point reduction in HADS-D at week 24 if, at that point, they had a Psoriasis Area Severity Index (PASI) response of 75-89, a 4.0-point improvement if they had a PASI 90-99 response, and a 5.2-point reduction in HADS-D if they had a PASI 100 response.
VOYAGE 2 was a phase 3, double-blind, multicenter, placebo-controlled comparison of guselkumab (Tremfya), a human monoclonal antibody targeting the interleukin-23 p 19 subunit, and the tumor necrosis factor-alpha inhibitor adalimumab (Humira) in their standard dosing. Participants were randomized 2:1:1 to guselkumab, adalimumab, or placebo. The study’s primary outcomes have previously been reported (J Am Acad Dermatol. 2017 Mar;76[3]:418-31). For example, at week 24 the guselkumab group demonstrated PASI 75, 90, and 100 responses of 89.1%, 75.2%, and 44.2%, respectively, compared with adalimumab, with PASI 75, 90, and 100 responses of 71%, 54.8%, and 26.6%.
Serial measurement of anxiety and depression symptoms using the HADS-A and HADS-D were part of the study protocol. The mean baseline HADS-A and HADS-D scores were 6.8 and 5.3. At week 24, the mean reduction in the HADS-A score was 2.0 points in the guselkumab group, compared with 1.0 point in the adalimumab arm. HADS-D scores improved by 1.7 and 1.1 points, respectively, in the overall guselkumab and adalimumab groups.
More importantly, among patients with a baseline HADS-A of 8 or higher, by week 16 of the trial, the HADS-A score had dropped below 8 in 51.4% of those on guselkumab, compared with 44.9% of patients on adalimumab and 25.9% on placebo, Dr. Gordon reported. Similarly, 59.2% of guselkumab-treated patients with a high baseline HADS-D improved to a score below 8 at week 16, compared with 54.3% on adalimumab and 27% on placebo.
At week 24, 58.4% of guselkumab-treated patients with a high baseline HADS-A and 59.4% of those with a high HADS-D had scores below 8. Again, these responses were significantly better than the 42.9% and 46.4% rates, respectively, in the adalimumab arm.
VOYAGE 2 was sponsored by Janssen, which markets guselkumab, approved by the Food and Drug Administration in July 2017 for treating adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Dr. Gordon reported receiving research support from and serving as a consultant to Janssen and numerous other pharmaceutical companies.
AT THE EADV CONGRESS
Key clinical point:
Major finding: Fifty-nine percent of gesulkumab-treated patients with moderate to severe psoriasis and clinically significant depression at baseline were below the depression threshold at week 24, compared with 46% of adalimumab-treated patients.
Data source: A phase 3, double-blind, multicenter, placebo-controlled comparison of guselkumab and adalimumab in 992 randomized patients with moderate to severe psoriasis.
Disclosures: VOYAGE 2 was sponsored by Janssen, which markets guselkumab. The presenter reported receiving research support from and serving as a consultant to Janssen and numerous other pharmaceutical companies.
Statement Offers Guidance for Management of Brain AVMs
Recent studies have helped clarify the natural history risks of intracranial hemorrhage (ICH) and seizure in patients with unruptured brain arteriovenous malformations (AVMs). In addition, researchers have completed the first randomized trial of interventional therapy versus conservative management for unruptured brain AVMs. Nevertheless, data to guide treatment decisions remain limited, and “considerable uncertainties … face physicians managing patients with ruptured and unruptured brain AVMs,” according to a scientific statement for healthcare professionals from the American Heart Association (AHA) and American Stroke Association (ASA). Optimal management of unruptured brain AVMs is a subject of debate, and discussion of treatment options should include careful consideration of natural history risks, the relative risks of intervention strategies, and patients’ life expectancy, the authors said.
The scientific statement on the management of brain AVMs was published in the August issue of Stroke. It updates the American Heart Association’s 2001 statement on this topic. The report reviews epidemiology, diagnosis, natural history, treatment, and management of ruptured and unruptured brain AVMs, as well as areas requiring more evidence. The American Academy of Neurology affirmed the value of the statement as an educational tool for neurologists. The Society of NeuroInterventional Surgery endorsed it, and the American Association of Neurological Surgeons/Congress of Neurological Surgeons Joint Cerebrovascular Section affirmed its educational benefit.
An Uncommon Lesion
Brain AVMs are uncommon, may present with spontaneous ICH, seizures, or headache, and typically present in young adults. Neurologists may identify incidental asymptomatic AVMs when patients undergo brain imaging for other reasons. The primary goal of treatment is prevention of hemorrhagic stroke.
Colin P. Derdeyn, MD, Professor of Neurology and Radiology at the University of Iowa in Iowa City and chair of the group that wrote the scientific statement, and colleagues searched all English-language articles on brain AVMs in humans that had been published following the 2001 statement. “Advances … include the accumulation of new data related to epidemiology, biology, imaging, outcomes with treatment, and introduction of new embolic agents,” the authors said. “Most notable of these data are the results of the first randomized trial of intervention for unruptured brain AVMs, the ARUBA trial (A Randomized Trial of Unruptured Brain Arteriovenous Malformations).”
ARUBA enrolled 226 adult patients with unruptured brain AVMs between 2007 and 2013. Patients were randomized to receive medical management alone or medical management with interventional therapy (eg, resection, embolization, or stereotactic radiosurgery alone or in combination).
A preplanned interim analysis found that after a mean follow-up of 33 months, the risk of stroke or death was more than three times higher in the intervention group (30.7%) than in the medical management group (10.1%). The analysis included data from 224 participants at 39 sites worldwide. On the recommendation of the ARUBA Data and Safety Monitoring Board, the National Institute of Neurological Disorders and Stroke stopped enrollment and stated that “under the experimental conditions in this trial, the interim analysis … shows that medical management is superior to intervention in patients with unruptured brain AVMs.”
Although ARUBA and an observational study by Al-Shahi Salman et al support a conservative approach to management, the studies had relatively short follow-up, considering the long duration of hemorrhage risk for patients with untreated brain AVMs. Furthermore, complication rates in the treatment arms in ARUBA were much higher than expected, the authors said. “The primary end points of ARUBA [ie, stroke or death] in the intervention group for Spetzler-Martin (SM) grade I (14.3%), II (43.3%), and III (57.1%) AVMs are higher than would have been expected in contemporary series, particularly for surgery or radiosurgery performed alone,” the authors said.
Additional studies are needed to better define long-term risks of stroke and seizure and to identify specific predictors of hemorrhagic stroke. “Long-term follow-up of participants in the ARUBA trial will be valuable for determining whether the superiority of conservative management over intervention observed in that study persists in the long term,” they said. “Further randomized controlled trials are justified to investigate the reproducibility of the findings of ARUBA and to investigate whether the balance of risk between conservative management and intervention is different in specific groups (eg, patients with SM grade I brain AVM).”
Recommendations for Management
Neurologists can inform patients about natural history risks, which are reliably quantified over approximately 10 years for ICH and approximately five years for epileptic seizure, the authors said. The annual risk of a first-ever ICH from an unruptured brain AVM is approximately 1%. The five-year risk of developing a first seizure is approximately 8%, and the five-year risk of developing epilepsy after a first seizure is approximately 58%.
Discussion of treatment options should consider natural history risks “weighed carefully against the relative risks of different intervention strategies and life expectancy,” the authors said. The SM grading scale is useful for predicting the risk of surgical resection.
For the management of patients with ruptured brain AVMs, the authors recommend that the evaluation of underlying brain AVMs and management of an initial hemorrhage follow the AHA and ASA’s 2015 spontaneous ICH management guidelines. The annual risk of recurrent ICH from a ruptured brain AVM is approximately 5%, and increasing age, deep venous drainage, arterial aneurysms, and female sex may increase the risk.
—Jake Remaly
Suggested Reading
Al-Shahi Salman R, White PM, Counsell CE, et al. Outcome after conservative management or intervention for unruptured brain arteriovenous malformations. JAMA. 2014;311(16):1661-1669.
Derdeyn CP, Zipfel GJ, Albuquerque FC, et al. Management of brain arteriovenous malformations: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48(8):e200-e224.
Hemphill JC 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032-2060.
Mohr JP, Parides MK, Stapf C, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014;383(9917):614-621.
Recent studies have helped clarify the natural history risks of intracranial hemorrhage (ICH) and seizure in patients with unruptured brain arteriovenous malformations (AVMs). In addition, researchers have completed the first randomized trial of interventional therapy versus conservative management for unruptured brain AVMs. Nevertheless, data to guide treatment decisions remain limited, and “considerable uncertainties … face physicians managing patients with ruptured and unruptured brain AVMs,” according to a scientific statement for healthcare professionals from the American Heart Association (AHA) and American Stroke Association (ASA). Optimal management of unruptured brain AVMs is a subject of debate, and discussion of treatment options should include careful consideration of natural history risks, the relative risks of intervention strategies, and patients’ life expectancy, the authors said.
The scientific statement on the management of brain AVMs was published in the August issue of Stroke. It updates the American Heart Association’s 2001 statement on this topic. The report reviews epidemiology, diagnosis, natural history, treatment, and management of ruptured and unruptured brain AVMs, as well as areas requiring more evidence. The American Academy of Neurology affirmed the value of the statement as an educational tool for neurologists. The Society of NeuroInterventional Surgery endorsed it, and the American Association of Neurological Surgeons/Congress of Neurological Surgeons Joint Cerebrovascular Section affirmed its educational benefit.
An Uncommon Lesion
Brain AVMs are uncommon, may present with spontaneous ICH, seizures, or headache, and typically present in young adults. Neurologists may identify incidental asymptomatic AVMs when patients undergo brain imaging for other reasons. The primary goal of treatment is prevention of hemorrhagic stroke.
Colin P. Derdeyn, MD, Professor of Neurology and Radiology at the University of Iowa in Iowa City and chair of the group that wrote the scientific statement, and colleagues searched all English-language articles on brain AVMs in humans that had been published following the 2001 statement. “Advances … include the accumulation of new data related to epidemiology, biology, imaging, outcomes with treatment, and introduction of new embolic agents,” the authors said. “Most notable of these data are the results of the first randomized trial of intervention for unruptured brain AVMs, the ARUBA trial (A Randomized Trial of Unruptured Brain Arteriovenous Malformations).”
ARUBA enrolled 226 adult patients with unruptured brain AVMs between 2007 and 2013. Patients were randomized to receive medical management alone or medical management with interventional therapy (eg, resection, embolization, or stereotactic radiosurgery alone or in combination).
A preplanned interim analysis found that after a mean follow-up of 33 months, the risk of stroke or death was more than three times higher in the intervention group (30.7%) than in the medical management group (10.1%). The analysis included data from 224 participants at 39 sites worldwide. On the recommendation of the ARUBA Data and Safety Monitoring Board, the National Institute of Neurological Disorders and Stroke stopped enrollment and stated that “under the experimental conditions in this trial, the interim analysis … shows that medical management is superior to intervention in patients with unruptured brain AVMs.”
Although ARUBA and an observational study by Al-Shahi Salman et al support a conservative approach to management, the studies had relatively short follow-up, considering the long duration of hemorrhage risk for patients with untreated brain AVMs. Furthermore, complication rates in the treatment arms in ARUBA were much higher than expected, the authors said. “The primary end points of ARUBA [ie, stroke or death] in the intervention group for Spetzler-Martin (SM) grade I (14.3%), II (43.3%), and III (57.1%) AVMs are higher than would have been expected in contemporary series, particularly for surgery or radiosurgery performed alone,” the authors said.
Additional studies are needed to better define long-term risks of stroke and seizure and to identify specific predictors of hemorrhagic stroke. “Long-term follow-up of participants in the ARUBA trial will be valuable for determining whether the superiority of conservative management over intervention observed in that study persists in the long term,” they said. “Further randomized controlled trials are justified to investigate the reproducibility of the findings of ARUBA and to investigate whether the balance of risk between conservative management and intervention is different in specific groups (eg, patients with SM grade I brain AVM).”
Recommendations for Management
Neurologists can inform patients about natural history risks, which are reliably quantified over approximately 10 years for ICH and approximately five years for epileptic seizure, the authors said. The annual risk of a first-ever ICH from an unruptured brain AVM is approximately 1%. The five-year risk of developing a first seizure is approximately 8%, and the five-year risk of developing epilepsy after a first seizure is approximately 58%.
Discussion of treatment options should consider natural history risks “weighed carefully against the relative risks of different intervention strategies and life expectancy,” the authors said. The SM grading scale is useful for predicting the risk of surgical resection.
For the management of patients with ruptured brain AVMs, the authors recommend that the evaluation of underlying brain AVMs and management of an initial hemorrhage follow the AHA and ASA’s 2015 spontaneous ICH management guidelines. The annual risk of recurrent ICH from a ruptured brain AVM is approximately 5%, and increasing age, deep venous drainage, arterial aneurysms, and female sex may increase the risk.
—Jake Remaly
Suggested Reading
Al-Shahi Salman R, White PM, Counsell CE, et al. Outcome after conservative management or intervention for unruptured brain arteriovenous malformations. JAMA. 2014;311(16):1661-1669.
Derdeyn CP, Zipfel GJ, Albuquerque FC, et al. Management of brain arteriovenous malformations: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48(8):e200-e224.
Hemphill JC 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032-2060.
Mohr JP, Parides MK, Stapf C, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014;383(9917):614-621.
Recent studies have helped clarify the natural history risks of intracranial hemorrhage (ICH) and seizure in patients with unruptured brain arteriovenous malformations (AVMs). In addition, researchers have completed the first randomized trial of interventional therapy versus conservative management for unruptured brain AVMs. Nevertheless, data to guide treatment decisions remain limited, and “considerable uncertainties … face physicians managing patients with ruptured and unruptured brain AVMs,” according to a scientific statement for healthcare professionals from the American Heart Association (AHA) and American Stroke Association (ASA). Optimal management of unruptured brain AVMs is a subject of debate, and discussion of treatment options should include careful consideration of natural history risks, the relative risks of intervention strategies, and patients’ life expectancy, the authors said.
The scientific statement on the management of brain AVMs was published in the August issue of Stroke. It updates the American Heart Association’s 2001 statement on this topic. The report reviews epidemiology, diagnosis, natural history, treatment, and management of ruptured and unruptured brain AVMs, as well as areas requiring more evidence. The American Academy of Neurology affirmed the value of the statement as an educational tool for neurologists. The Society of NeuroInterventional Surgery endorsed it, and the American Association of Neurological Surgeons/Congress of Neurological Surgeons Joint Cerebrovascular Section affirmed its educational benefit.
An Uncommon Lesion
Brain AVMs are uncommon, may present with spontaneous ICH, seizures, or headache, and typically present in young adults. Neurologists may identify incidental asymptomatic AVMs when patients undergo brain imaging for other reasons. The primary goal of treatment is prevention of hemorrhagic stroke.
Colin P. Derdeyn, MD, Professor of Neurology and Radiology at the University of Iowa in Iowa City and chair of the group that wrote the scientific statement, and colleagues searched all English-language articles on brain AVMs in humans that had been published following the 2001 statement. “Advances … include the accumulation of new data related to epidemiology, biology, imaging, outcomes with treatment, and introduction of new embolic agents,” the authors said. “Most notable of these data are the results of the first randomized trial of intervention for unruptured brain AVMs, the ARUBA trial (A Randomized Trial of Unruptured Brain Arteriovenous Malformations).”
ARUBA enrolled 226 adult patients with unruptured brain AVMs between 2007 and 2013. Patients were randomized to receive medical management alone or medical management with interventional therapy (eg, resection, embolization, or stereotactic radiosurgery alone or in combination).
A preplanned interim analysis found that after a mean follow-up of 33 months, the risk of stroke or death was more than three times higher in the intervention group (30.7%) than in the medical management group (10.1%). The analysis included data from 224 participants at 39 sites worldwide. On the recommendation of the ARUBA Data and Safety Monitoring Board, the National Institute of Neurological Disorders and Stroke stopped enrollment and stated that “under the experimental conditions in this trial, the interim analysis … shows that medical management is superior to intervention in patients with unruptured brain AVMs.”
Although ARUBA and an observational study by Al-Shahi Salman et al support a conservative approach to management, the studies had relatively short follow-up, considering the long duration of hemorrhage risk for patients with untreated brain AVMs. Furthermore, complication rates in the treatment arms in ARUBA were much higher than expected, the authors said. “The primary end points of ARUBA [ie, stroke or death] in the intervention group for Spetzler-Martin (SM) grade I (14.3%), II (43.3%), and III (57.1%) AVMs are higher than would have been expected in contemporary series, particularly for surgery or radiosurgery performed alone,” the authors said.
Additional studies are needed to better define long-term risks of stroke and seizure and to identify specific predictors of hemorrhagic stroke. “Long-term follow-up of participants in the ARUBA trial will be valuable for determining whether the superiority of conservative management over intervention observed in that study persists in the long term,” they said. “Further randomized controlled trials are justified to investigate the reproducibility of the findings of ARUBA and to investigate whether the balance of risk between conservative management and intervention is different in specific groups (eg, patients with SM grade I brain AVM).”
Recommendations for Management
Neurologists can inform patients about natural history risks, which are reliably quantified over approximately 10 years for ICH and approximately five years for epileptic seizure, the authors said. The annual risk of a first-ever ICH from an unruptured brain AVM is approximately 1%. The five-year risk of developing a first seizure is approximately 8%, and the five-year risk of developing epilepsy after a first seizure is approximately 58%.
Discussion of treatment options should consider natural history risks “weighed carefully against the relative risks of different intervention strategies and life expectancy,” the authors said. The SM grading scale is useful for predicting the risk of surgical resection.
For the management of patients with ruptured brain AVMs, the authors recommend that the evaluation of underlying brain AVMs and management of an initial hemorrhage follow the AHA and ASA’s 2015 spontaneous ICH management guidelines. The annual risk of recurrent ICH from a ruptured brain AVM is approximately 5%, and increasing age, deep venous drainage, arterial aneurysms, and female sex may increase the risk.
—Jake Remaly
Suggested Reading
Al-Shahi Salman R, White PM, Counsell CE, et al. Outcome after conservative management or intervention for unruptured brain arteriovenous malformations. JAMA. 2014;311(16):1661-1669.
Derdeyn CP, Zipfel GJ, Albuquerque FC, et al. Management of brain arteriovenous malformations: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48(8):e200-e224.
Hemphill JC 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032-2060.
Mohr JP, Parides MK, Stapf C, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014;383(9917):614-621.
Vascular risks heightened in fibrotic NAFLD
WASHINGTON – The true impact of advanced fibrosis and cirrhosis advancing to serious hepatic and nonhepatic complications in nonalcoholic fatty liver disease remains somewhat a mystery, but a multinational prospective cohort study has found that patients with bridging fibrosis are at risk of nonhepatic malignancies and vascular problems while patients with cirrhosis are prone to predominantly liver-related complications that result in higher rates of death and liver transplant, according to a report presented at the annual meeting of the American Association for the Study of Liver Diseases.
“NAFLD patients with biopsy-proven cirrhosis have a higher mortality and liver-related complications than those with bridging fibrosis, whereas vascular events and nonhepatic malignancies are the commonest complications in those with bridging fibrosis,” said Eduardo Vilar-Gomez, MD, PhD, of Indiana University, Indianapolis. “Among compensated cirrhotic patients, those with Child-Pugh (CP) A6 are at highest risk of liver-related outcomes and mortality.”
Dr. Vilar-Gomez reported on a multicenter, international prospective cohort study of 458 NAFLD patients, 159 with biopsy-proven bridging fibrosis and 299 with compensated cirrhosis (222 with CP A5 and 77 with CP A6) who were followed at six academic centers in four different countries between April 1995 and December 2016.
During a mean follow-up of 5.7 years, 41 patients died, 43 had liver transplants, 90 had initial hepatic decompensation events, 42 were diagnosed with hepatocellular carcinoma (HCC), 14 had major vascular events, and 30 were diagnosed with nonhepatic cancers. The overall death or transplant rates were 3% (four) in the bridging fibrosis group, 14% (32) in the cirrhosis and CP A5 group, and 62% (48) in the cirrhosis and CP A6 group.
Liver-related death rates were also highest among patients with cirrhosis, Dr. Vilar-Gomez said. While the four deaths in the fibrosis patients were evenly split between liver- and non–liver-related causes, 79% of deaths (15) in the cirrhosis and CP A5 patients were liver related, as were all 18 deaths in the cirrhosis and CP A6 group.
“Cirrhotic patients displayed the highest rates of hepatic outcomes overall as compared to those with bridging fibrosis,” Dr. Vilar-Gomez said. Rates of hepatic decompensation were 3% (5) for bridging fibrosis, 18% (40) for cirrhosis and CP A5, and 58% (48) for cirrhosis and CP A6. Likewise, HCC rates were also highest in cirrhotic patients: 9% (21) and 25% (19) for those with CP A5 and CP A6 disease, respectively, vs. 1% (2) for those with bridging fibrosis.
But nonhepatic outcomes were highest among the bridging fibrosis patients, Dr. Vilar-Gomez noted. “These include higher rates of major vascular events – 5% (eight) for those with bridging fibrosis vs. 2% (five) and 1% (one) for those with cirrhosis and CP A5 or CP A6 disease, respectively,” he said. “Rates of nonhepatic malignancies were comparable: 8% (13) for those with bridging fibrosis, 5% (10) for those with cirrhosis and CP5 disease and 9% (7) for those with cirrhosis and CP6 disease.
“Cirrhosis increased the risk of death/transplant, decompensation, and hepatocellular carcinoma by 4.3-, 4.5-, and 3.9-fold, respectively,” Dr. Vilar-Gomez said, “but was associated with a lower risk of vascular events.”
He noted that the study identified a number of risk factors associated with overall mortality and severity of liver disease in cirrhosis compared with bridging fibrosis. Cirrhosis patients tended to be 2-3 years older, had lower body mass index and blood pressure, had higher rates of type 2 diabetes, had lower lipid levels, and had worse liver and renal function.
“Type 2 diabetes and steatosis severity on liver histology, among other factors, were independent predictors for liver-related outcomes,” Dr. Vilar-Gomez said. The findings have implications in assessing patients with advanced NAFLD and for the design and interpretation of clinical trials, he added.
Dr. Vilar-Gomez had no financial relationships to disclose.
WASHINGTON – The true impact of advanced fibrosis and cirrhosis advancing to serious hepatic and nonhepatic complications in nonalcoholic fatty liver disease remains somewhat a mystery, but a multinational prospective cohort study has found that patients with bridging fibrosis are at risk of nonhepatic malignancies and vascular problems while patients with cirrhosis are prone to predominantly liver-related complications that result in higher rates of death and liver transplant, according to a report presented at the annual meeting of the American Association for the Study of Liver Diseases.
“NAFLD patients with biopsy-proven cirrhosis have a higher mortality and liver-related complications than those with bridging fibrosis, whereas vascular events and nonhepatic malignancies are the commonest complications in those with bridging fibrosis,” said Eduardo Vilar-Gomez, MD, PhD, of Indiana University, Indianapolis. “Among compensated cirrhotic patients, those with Child-Pugh (CP) A6 are at highest risk of liver-related outcomes and mortality.”
Dr. Vilar-Gomez reported on a multicenter, international prospective cohort study of 458 NAFLD patients, 159 with biopsy-proven bridging fibrosis and 299 with compensated cirrhosis (222 with CP A5 and 77 with CP A6) who were followed at six academic centers in four different countries between April 1995 and December 2016.
During a mean follow-up of 5.7 years, 41 patients died, 43 had liver transplants, 90 had initial hepatic decompensation events, 42 were diagnosed with hepatocellular carcinoma (HCC), 14 had major vascular events, and 30 were diagnosed with nonhepatic cancers. The overall death or transplant rates were 3% (four) in the bridging fibrosis group, 14% (32) in the cirrhosis and CP A5 group, and 62% (48) in the cirrhosis and CP A6 group.
Liver-related death rates were also highest among patients with cirrhosis, Dr. Vilar-Gomez said. While the four deaths in the fibrosis patients were evenly split between liver- and non–liver-related causes, 79% of deaths (15) in the cirrhosis and CP A5 patients were liver related, as were all 18 deaths in the cirrhosis and CP A6 group.
“Cirrhotic patients displayed the highest rates of hepatic outcomes overall as compared to those with bridging fibrosis,” Dr. Vilar-Gomez said. Rates of hepatic decompensation were 3% (5) for bridging fibrosis, 18% (40) for cirrhosis and CP A5, and 58% (48) for cirrhosis and CP A6. Likewise, HCC rates were also highest in cirrhotic patients: 9% (21) and 25% (19) for those with CP A5 and CP A6 disease, respectively, vs. 1% (2) for those with bridging fibrosis.
But nonhepatic outcomes were highest among the bridging fibrosis patients, Dr. Vilar-Gomez noted. “These include higher rates of major vascular events – 5% (eight) for those with bridging fibrosis vs. 2% (five) and 1% (one) for those with cirrhosis and CP A5 or CP A6 disease, respectively,” he said. “Rates of nonhepatic malignancies were comparable: 8% (13) for those with bridging fibrosis, 5% (10) for those with cirrhosis and CP5 disease and 9% (7) for those with cirrhosis and CP6 disease.
“Cirrhosis increased the risk of death/transplant, decompensation, and hepatocellular carcinoma by 4.3-, 4.5-, and 3.9-fold, respectively,” Dr. Vilar-Gomez said, “but was associated with a lower risk of vascular events.”
He noted that the study identified a number of risk factors associated with overall mortality and severity of liver disease in cirrhosis compared with bridging fibrosis. Cirrhosis patients tended to be 2-3 years older, had lower body mass index and blood pressure, had higher rates of type 2 diabetes, had lower lipid levels, and had worse liver and renal function.
“Type 2 diabetes and steatosis severity on liver histology, among other factors, were independent predictors for liver-related outcomes,” Dr. Vilar-Gomez said. The findings have implications in assessing patients with advanced NAFLD and for the design and interpretation of clinical trials, he added.
Dr. Vilar-Gomez had no financial relationships to disclose.
WASHINGTON – The true impact of advanced fibrosis and cirrhosis advancing to serious hepatic and nonhepatic complications in nonalcoholic fatty liver disease remains somewhat a mystery, but a multinational prospective cohort study has found that patients with bridging fibrosis are at risk of nonhepatic malignancies and vascular problems while patients with cirrhosis are prone to predominantly liver-related complications that result in higher rates of death and liver transplant, according to a report presented at the annual meeting of the American Association for the Study of Liver Diseases.
“NAFLD patients with biopsy-proven cirrhosis have a higher mortality and liver-related complications than those with bridging fibrosis, whereas vascular events and nonhepatic malignancies are the commonest complications in those with bridging fibrosis,” said Eduardo Vilar-Gomez, MD, PhD, of Indiana University, Indianapolis. “Among compensated cirrhotic patients, those with Child-Pugh (CP) A6 are at highest risk of liver-related outcomes and mortality.”
Dr. Vilar-Gomez reported on a multicenter, international prospective cohort study of 458 NAFLD patients, 159 with biopsy-proven bridging fibrosis and 299 with compensated cirrhosis (222 with CP A5 and 77 with CP A6) who were followed at six academic centers in four different countries between April 1995 and December 2016.
During a mean follow-up of 5.7 years, 41 patients died, 43 had liver transplants, 90 had initial hepatic decompensation events, 42 were diagnosed with hepatocellular carcinoma (HCC), 14 had major vascular events, and 30 were diagnosed with nonhepatic cancers. The overall death or transplant rates were 3% (four) in the bridging fibrosis group, 14% (32) in the cirrhosis and CP A5 group, and 62% (48) in the cirrhosis and CP A6 group.
Liver-related death rates were also highest among patients with cirrhosis, Dr. Vilar-Gomez said. While the four deaths in the fibrosis patients were evenly split between liver- and non–liver-related causes, 79% of deaths (15) in the cirrhosis and CP A5 patients were liver related, as were all 18 deaths in the cirrhosis and CP A6 group.
“Cirrhotic patients displayed the highest rates of hepatic outcomes overall as compared to those with bridging fibrosis,” Dr. Vilar-Gomez said. Rates of hepatic decompensation were 3% (5) for bridging fibrosis, 18% (40) for cirrhosis and CP A5, and 58% (48) for cirrhosis and CP A6. Likewise, HCC rates were also highest in cirrhotic patients: 9% (21) and 25% (19) for those with CP A5 and CP A6 disease, respectively, vs. 1% (2) for those with bridging fibrosis.
But nonhepatic outcomes were highest among the bridging fibrosis patients, Dr. Vilar-Gomez noted. “These include higher rates of major vascular events – 5% (eight) for those with bridging fibrosis vs. 2% (five) and 1% (one) for those with cirrhosis and CP A5 or CP A6 disease, respectively,” he said. “Rates of nonhepatic malignancies were comparable: 8% (13) for those with bridging fibrosis, 5% (10) for those with cirrhosis and CP5 disease and 9% (7) for those with cirrhosis and CP6 disease.
“Cirrhosis increased the risk of death/transplant, decompensation, and hepatocellular carcinoma by 4.3-, 4.5-, and 3.9-fold, respectively,” Dr. Vilar-Gomez said, “but was associated with a lower risk of vascular events.”
He noted that the study identified a number of risk factors associated with overall mortality and severity of liver disease in cirrhosis compared with bridging fibrosis. Cirrhosis patients tended to be 2-3 years older, had lower body mass index and blood pressure, had higher rates of type 2 diabetes, had lower lipid levels, and had worse liver and renal function.
“Type 2 diabetes and steatosis severity on liver histology, among other factors, were independent predictors for liver-related outcomes,” Dr. Vilar-Gomez said. The findings have implications in assessing patients with advanced NAFLD and for the design and interpretation of clinical trials, he added.
Dr. Vilar-Gomez had no financial relationships to disclose.
AT THE LIVER MEETING 2017
Key clinical point: Cirrhotic patients with advanced nonalcoholic fatty liver disease are at higher risk for liver-related adverse outcomes whereas patients with bridging fibrosis are more prone to nonhepatic malignancies and vascular events.
Major finding: Overall death or transplant rates were 3% in the bridging fibrosis group, 14% in the cirrhosis and Child-Pugh (CP) A5 group, and 62% in the cirrhosis and CP A6 group
Data source: Multicenter, international prospective cohort study of 458 NAFLD patients with biopsy-proven bridging fibrosis or compensated cirrhosis.
Disclosures: Dr. Vilar-Gomez had no financial relationships to disclose.
Opioids Are Overprescribed for Migraine
Opiates are overused, and acute analgesics with high-quality evidence may be underused, for the treatment of migraine, according to research published online ahead of print June 26 in Cephalalgia. In addition, migraine may be undertreated with preventive medications. These prescribing patterns do not significantly differ by patients’ race, however.
Analyzing Nationally Representative Data
In the US, racial disparities in migraine burden have been reported. Migraine in African Americans is more frequent, more severe, more likely to become chronic, and associated with more depression and lower quality of life, compared with migraine in non-Hispanic whites. Lower-quality medication treatment for migraine may explain this difference, but little is known about the quality of migraine prescribing patterns in the US or whether racial differences exist.
Dr. Charleston and James F. Burke, MD, Associate Professor of Neurology at the University of Michigan Medical School, hypothesized that racial differences in the use of high-quality abortive, prophylactic medications would exist in ambulatory care settings, and that the quality of medical treatment for migraine disorders would be suboptimal in minority populations. To test these hypotheses, they analyzed all headache visits for patients over age 18 in the National Ambulatory Medical Care Survey (NAMCS) from 2006 to 2013.
The investigators defined quality of migraine care by using the American Academy of Neurology Headache Quality Measure Set. Researchers then assigned patients to one of three race or ethnicity categories—non-Hispanic white, African American, and Hispanic. Patients who reported other races or ethnicities were excluded.
The researchers assigned patients to one of four abortive agent categories—no abortive agent prescribed, all high-quality abortive agents (ie, triptans or dihydroergotamine), some low-quality abortive agents, or any opiate agent. In addition, the investigators assigned patients to one of four prophylactic agent categories—no prophylactic agent, all high-quality prophylaxis, some low-quality prophylaxis, or all low-quality prophylaxis. Finally, researchers made racial comparisons using descriptive statistics after applying NAMCS survey weights.
Prophylactic Agents Are Likely Underused
A total of 2,860 visits were included in the study, representing approximately 50 million migraine visits in the US from 2006 to 2013; 76% of patients were non-Hispanic white, 10% were African American, and 10% were Hispanic. Hispanic patients were more likely to be seen in a given practice for the first time, compared with non-Hispanic whites or African Americans. Researchers also observed a trend toward less private insurance and more Medicaid among African American and Hispanic patients, compared with non-Hispanic white patients.
Although overall migraine prescribing patterns were suboptimal, researchers did not find any major differences by race. “It is possible, though, that more modest racial differences in abortive prescribing exist, but that we lacked statistical power to identify those differences,” said Drs. Charleston and Burke.
In all, 41.3% of African Americans, 40.8% of non-Hispanic whites, and 41.2% of Hispanic patients received no prophylactic treatment. A total of 18.8% of African Americans, 11.9% of non-Hispanic whites, and 6.9% of Hispanic patients received exclusively Level A prophylaxis.
Forty-seven percent of African Americans, 38.2% of non-Hispanic whites, and 36.3% of Hispanic patients received no abortive treatments. In all, 15.3% of African Americans, 19.4% of non-Hispanic whites, and 17.7% of Hispanic patients received exclusively Level A abortive agents. About 15.2% of patients had a prescription for opiates, but no racial differences were observed.
In addition, African Americans were 4% less likely to receive all high-quality abortive agents, and Hispanic patients were 5% less likely to receive all high-quality prophylactic agents, compared with non-Hispanic whites. Neither of these differences was statistically significant, said the researchers.
“Understanding what drives these prescribing patterns and improving overall migraine prescribing should be a central concern for headache care practitioners and quality-improvement initiatives, and a target of future interventions,” said Drs. Charleston and Burke.
One limitation of this study was that researchers had no details on headache severity or duration. Another limitation was that the researchers did not control for socioeconomic status.
—Erica Tricarico
Suggested Reading
Charleston IV L, Burke JF. Do racial/ethnic disparities exist in recommended migraine treatments in US ambulatory care? Cephalalgia. 2017 Jun 26 [Epub ahead of print].
Opiates are overused, and acute analgesics with high-quality evidence may be underused, for the treatment of migraine, according to research published online ahead of print June 26 in Cephalalgia. In addition, migraine may be undertreated with preventive medications. These prescribing patterns do not significantly differ by patients’ race, however.
Analyzing Nationally Representative Data
In the US, racial disparities in migraine burden have been reported. Migraine in African Americans is more frequent, more severe, more likely to become chronic, and associated with more depression and lower quality of life, compared with migraine in non-Hispanic whites. Lower-quality medication treatment for migraine may explain this difference, but little is known about the quality of migraine prescribing patterns in the US or whether racial differences exist.
Dr. Charleston and James F. Burke, MD, Associate Professor of Neurology at the University of Michigan Medical School, hypothesized that racial differences in the use of high-quality abortive, prophylactic medications would exist in ambulatory care settings, and that the quality of medical treatment for migraine disorders would be suboptimal in minority populations. To test these hypotheses, they analyzed all headache visits for patients over age 18 in the National Ambulatory Medical Care Survey (NAMCS) from 2006 to 2013.
The investigators defined quality of migraine care by using the American Academy of Neurology Headache Quality Measure Set. Researchers then assigned patients to one of three race or ethnicity categories—non-Hispanic white, African American, and Hispanic. Patients who reported other races or ethnicities were excluded.
The researchers assigned patients to one of four abortive agent categories—no abortive agent prescribed, all high-quality abortive agents (ie, triptans or dihydroergotamine), some low-quality abortive agents, or any opiate agent. In addition, the investigators assigned patients to one of four prophylactic agent categories—no prophylactic agent, all high-quality prophylaxis, some low-quality prophylaxis, or all low-quality prophylaxis. Finally, researchers made racial comparisons using descriptive statistics after applying NAMCS survey weights.
Prophylactic Agents Are Likely Underused
A total of 2,860 visits were included in the study, representing approximately 50 million migraine visits in the US from 2006 to 2013; 76% of patients were non-Hispanic white, 10% were African American, and 10% were Hispanic. Hispanic patients were more likely to be seen in a given practice for the first time, compared with non-Hispanic whites or African Americans. Researchers also observed a trend toward less private insurance and more Medicaid among African American and Hispanic patients, compared with non-Hispanic white patients.
Although overall migraine prescribing patterns were suboptimal, researchers did not find any major differences by race. “It is possible, though, that more modest racial differences in abortive prescribing exist, but that we lacked statistical power to identify those differences,” said Drs. Charleston and Burke.
In all, 41.3% of African Americans, 40.8% of non-Hispanic whites, and 41.2% of Hispanic patients received no prophylactic treatment. A total of 18.8% of African Americans, 11.9% of non-Hispanic whites, and 6.9% of Hispanic patients received exclusively Level A prophylaxis.
Forty-seven percent of African Americans, 38.2% of non-Hispanic whites, and 36.3% of Hispanic patients received no abortive treatments. In all, 15.3% of African Americans, 19.4% of non-Hispanic whites, and 17.7% of Hispanic patients received exclusively Level A abortive agents. About 15.2% of patients had a prescription for opiates, but no racial differences were observed.
In addition, African Americans were 4% less likely to receive all high-quality abortive agents, and Hispanic patients were 5% less likely to receive all high-quality prophylactic agents, compared with non-Hispanic whites. Neither of these differences was statistically significant, said the researchers.
“Understanding what drives these prescribing patterns and improving overall migraine prescribing should be a central concern for headache care practitioners and quality-improvement initiatives, and a target of future interventions,” said Drs. Charleston and Burke.
One limitation of this study was that researchers had no details on headache severity or duration. Another limitation was that the researchers did not control for socioeconomic status.
—Erica Tricarico
Suggested Reading
Charleston IV L, Burke JF. Do racial/ethnic disparities exist in recommended migraine treatments in US ambulatory care? Cephalalgia. 2017 Jun 26 [Epub ahead of print].
Opiates are overused, and acute analgesics with high-quality evidence may be underused, for the treatment of migraine, according to research published online ahead of print June 26 in Cephalalgia. In addition, migraine may be undertreated with preventive medications. These prescribing patterns do not significantly differ by patients’ race, however.
Analyzing Nationally Representative Data
In the US, racial disparities in migraine burden have been reported. Migraine in African Americans is more frequent, more severe, more likely to become chronic, and associated with more depression and lower quality of life, compared with migraine in non-Hispanic whites. Lower-quality medication treatment for migraine may explain this difference, but little is known about the quality of migraine prescribing patterns in the US or whether racial differences exist.
Dr. Charleston and James F. Burke, MD, Associate Professor of Neurology at the University of Michigan Medical School, hypothesized that racial differences in the use of high-quality abortive, prophylactic medications would exist in ambulatory care settings, and that the quality of medical treatment for migraine disorders would be suboptimal in minority populations. To test these hypotheses, they analyzed all headache visits for patients over age 18 in the National Ambulatory Medical Care Survey (NAMCS) from 2006 to 2013.
The investigators defined quality of migraine care by using the American Academy of Neurology Headache Quality Measure Set. Researchers then assigned patients to one of three race or ethnicity categories—non-Hispanic white, African American, and Hispanic. Patients who reported other races or ethnicities were excluded.
The researchers assigned patients to one of four abortive agent categories—no abortive agent prescribed, all high-quality abortive agents (ie, triptans or dihydroergotamine), some low-quality abortive agents, or any opiate agent. In addition, the investigators assigned patients to one of four prophylactic agent categories—no prophylactic agent, all high-quality prophylaxis, some low-quality prophylaxis, or all low-quality prophylaxis. Finally, researchers made racial comparisons using descriptive statistics after applying NAMCS survey weights.
Prophylactic Agents Are Likely Underused
A total of 2,860 visits were included in the study, representing approximately 50 million migraine visits in the US from 2006 to 2013; 76% of patients were non-Hispanic white, 10% were African American, and 10% were Hispanic. Hispanic patients were more likely to be seen in a given practice for the first time, compared with non-Hispanic whites or African Americans. Researchers also observed a trend toward less private insurance and more Medicaid among African American and Hispanic patients, compared with non-Hispanic white patients.
Although overall migraine prescribing patterns were suboptimal, researchers did not find any major differences by race. “It is possible, though, that more modest racial differences in abortive prescribing exist, but that we lacked statistical power to identify those differences,” said Drs. Charleston and Burke.
In all, 41.3% of African Americans, 40.8% of non-Hispanic whites, and 41.2% of Hispanic patients received no prophylactic treatment. A total of 18.8% of African Americans, 11.9% of non-Hispanic whites, and 6.9% of Hispanic patients received exclusively Level A prophylaxis.
Forty-seven percent of African Americans, 38.2% of non-Hispanic whites, and 36.3% of Hispanic patients received no abortive treatments. In all, 15.3% of African Americans, 19.4% of non-Hispanic whites, and 17.7% of Hispanic patients received exclusively Level A abortive agents. About 15.2% of patients had a prescription for opiates, but no racial differences were observed.
In addition, African Americans were 4% less likely to receive all high-quality abortive agents, and Hispanic patients were 5% less likely to receive all high-quality prophylactic agents, compared with non-Hispanic whites. Neither of these differences was statistically significant, said the researchers.
“Understanding what drives these prescribing patterns and improving overall migraine prescribing should be a central concern for headache care practitioners and quality-improvement initiatives, and a target of future interventions,” said Drs. Charleston and Burke.
One limitation of this study was that researchers had no details on headache severity or duration. Another limitation was that the researchers did not control for socioeconomic status.
—Erica Tricarico
Suggested Reading
Charleston IV L, Burke JF. Do racial/ethnic disparities exist in recommended migraine treatments in US ambulatory care? Cephalalgia. 2017 Jun 26 [Epub ahead of print].
The Exam of the Future: What Should Residents Expect?
Understanding people is complex, yet essential for effective leadership
Editor’s note: Each month, Society of Hospital Medicine puts the spotlight on some of our most active members who are making substantial contributions to hospital medicine. Log on to www.hospitalmedicine.org/getinvolved for more information on how you can lend your expertise to help SHM improve the care of hospitalized patients.
What are the requirements to become a Master in Hospital Medicine, and how has this designation been beneficial to your career?
I have been an SHM member since the early years (early 2000s, I think), and I became a Master in Hospital Medicine (MHM) in 2013. I see the MHM designation as recognizing accomplishments that have been critical in advancing the field of hospital medicine and SHM as a society.
I would guess that my contributions to the SHM Board, being SHM president, cofounding (with others) the Academic Hospitalist Academy, founding (with others) the Quality Safety Educators Academy, and being the founding chair of the American Board of Internal Medicine’s Focused Practice in Hospital Medicine pathway were probably what led to my induction.
The salient question probably isn’t “How has this designation been beneficial to my career?” but, rather, “How, after receiving the MHM designation, has my career benefited hospital medicine and SHM?” To my mind, there are some awards in life that recognize excellence in the completion of a task. They herald the end of a finite game: a “best research project” award, for example. But then there are a special few recognitions that, while they recognize past contributions, focus more upon the future than the past. They are infinite recognitions, because implicitly, they are recognitions of “promise” as much as achievement. They convey the organization’s trust in, and high expectations for, the recipient. In sum, they are simultaneously an honor and an obligation … an obligation and an expectation that the recipient will continue to do even more. In academic parlance, being “tenured” is a good example; for the Society of Hospital Medicine, the equivalent is the MHM recognition. I have done a lot for SHM, but the MHM designation obligates me to do even more. Honoring that obligation is what I plan to do with my career.
How did you become involved with SHM’s Leadership Academy, and how has the program developed over the years?
I started doing a 1-hour talk when the Mastering Teamwork course started. I did that for a couple of years but, as my career was evolving into higher-level institutional and hospital leadership, there was much more to talk about than I could fit into 1 hour.
The core of my leadership message is based in the “character ethic” (being better than who you are) and not the popular “personality ethic” (looking better than you are). So it’s that … plus all of the leadership mistakes I have made along the way. And that’s a lot of mistakes … enough to fill 9 hours of Mastering Teamwork.
In your opinion, what are some of the main takeaways for those who participate in SHM’s Leadership Academy?
Two of the three core components of great leadership are having a mission and purpose and being sincere. Leadership Academy can’t deliver the first two, so participants do have to come prepared to be trained.
Understanding people is the third core component, and mastering that skill is really complex. It is not something you can do with a clever slogan and a new lapel pin. It comes in many forms: teamwork, communication, networking, dealing with crisis, orchestrating change, etc. But at its core, Leadership Academy is all about training future leaders in how to understand people … and to develop the skills to inspire, motivate, and move their team to greater heights. Because at its core, leadership is about getting people to go places they otherwise didn’t want to go and to do things that they didn’t already want to do. And, to do that, you have to understand people.
As an active SHM member of many years, what advice do you have for members who wish to get more involved?
You have to start somewhere, and you have to see the entry level years as investing in yourself. There will be sacrifice involved, so don’t expect immediate returns on the investment, and the first few years might not be that fun.
Every year, there is a call for committee membership, and you need to get involved in one or more of those committees. Find the most senior hospitalist, who is the most involved in SHM, and tell her that you want to be on an SHM committee, and could she nominate you? If you do not have that luxury, then pay attention at the SHM annual conference. The SHM president-elect is responsible for building out the SHM committee nominees; as president, you are always looking to find enthusiastic people to be on the committees. Receiving emails from enthusiastic members is more welcome than you might think. As soon as that person is announced, find her email and start making the request to be on a committee. Be open to the assignment: Even if it is not your favorite committee, being there is more important than not.
But remember, networking and reputation are “two tailed.” You can improve your reputation by meaningful and consistent participation on a committee (leading to higher and better leadership opportunities), but you can also tarnish it by being assigned to a committee and not doing anything. You do that once, and there is a high probability that you will not be asked back again.
Great strategy, at the end of the day, is always putting yourself in a position with the maximum number of options. The key to personal development strategy is networking. The more people you know, the higher the probability that your email box will light up with the “Hey, do you want to collaborate on this project together?” sort of emails. Attend the annual conferences, attend the SHM Academies (Leadership, Quality and Safety Educators Academy, Academic Hospitalist Academy, etc.). Build genuine relationships with the people you meet there, and the rest will work out just fine.
Ms. Steele is the marketing communications specialist at the Society of Hospital Medicine.
Editor’s note: Each month, Society of Hospital Medicine puts the spotlight on some of our most active members who are making substantial contributions to hospital medicine. Log on to www.hospitalmedicine.org/getinvolved for more information on how you can lend your expertise to help SHM improve the care of hospitalized patients.
What are the requirements to become a Master in Hospital Medicine, and how has this designation been beneficial to your career?
I have been an SHM member since the early years (early 2000s, I think), and I became a Master in Hospital Medicine (MHM) in 2013. I see the MHM designation as recognizing accomplishments that have been critical in advancing the field of hospital medicine and SHM as a society.
I would guess that my contributions to the SHM Board, being SHM president, cofounding (with others) the Academic Hospitalist Academy, founding (with others) the Quality Safety Educators Academy, and being the founding chair of the American Board of Internal Medicine’s Focused Practice in Hospital Medicine pathway were probably what led to my induction.
The salient question probably isn’t “How has this designation been beneficial to my career?” but, rather, “How, after receiving the MHM designation, has my career benefited hospital medicine and SHM?” To my mind, there are some awards in life that recognize excellence in the completion of a task. They herald the end of a finite game: a “best research project” award, for example. But then there are a special few recognitions that, while they recognize past contributions, focus more upon the future than the past. They are infinite recognitions, because implicitly, they are recognitions of “promise” as much as achievement. They convey the organization’s trust in, and high expectations for, the recipient. In sum, they are simultaneously an honor and an obligation … an obligation and an expectation that the recipient will continue to do even more. In academic parlance, being “tenured” is a good example; for the Society of Hospital Medicine, the equivalent is the MHM recognition. I have done a lot for SHM, but the MHM designation obligates me to do even more. Honoring that obligation is what I plan to do with my career.
How did you become involved with SHM’s Leadership Academy, and how has the program developed over the years?
I started doing a 1-hour talk when the Mastering Teamwork course started. I did that for a couple of years but, as my career was evolving into higher-level institutional and hospital leadership, there was much more to talk about than I could fit into 1 hour.
The core of my leadership message is based in the “character ethic” (being better than who you are) and not the popular “personality ethic” (looking better than you are). So it’s that … plus all of the leadership mistakes I have made along the way. And that’s a lot of mistakes … enough to fill 9 hours of Mastering Teamwork.
In your opinion, what are some of the main takeaways for those who participate in SHM’s Leadership Academy?
Two of the three core components of great leadership are having a mission and purpose and being sincere. Leadership Academy can’t deliver the first two, so participants do have to come prepared to be trained.
Understanding people is the third core component, and mastering that skill is really complex. It is not something you can do with a clever slogan and a new lapel pin. It comes in many forms: teamwork, communication, networking, dealing with crisis, orchestrating change, etc. But at its core, Leadership Academy is all about training future leaders in how to understand people … and to develop the skills to inspire, motivate, and move their team to greater heights. Because at its core, leadership is about getting people to go places they otherwise didn’t want to go and to do things that they didn’t already want to do. And, to do that, you have to understand people.
As an active SHM member of many years, what advice do you have for members who wish to get more involved?
You have to start somewhere, and you have to see the entry level years as investing in yourself. There will be sacrifice involved, so don’t expect immediate returns on the investment, and the first few years might not be that fun.
Every year, there is a call for committee membership, and you need to get involved in one or more of those committees. Find the most senior hospitalist, who is the most involved in SHM, and tell her that you want to be on an SHM committee, and could she nominate you? If you do not have that luxury, then pay attention at the SHM annual conference. The SHM president-elect is responsible for building out the SHM committee nominees; as president, you are always looking to find enthusiastic people to be on the committees. Receiving emails from enthusiastic members is more welcome than you might think. As soon as that person is announced, find her email and start making the request to be on a committee. Be open to the assignment: Even if it is not your favorite committee, being there is more important than not.
But remember, networking and reputation are “two tailed.” You can improve your reputation by meaningful and consistent participation on a committee (leading to higher and better leadership opportunities), but you can also tarnish it by being assigned to a committee and not doing anything. You do that once, and there is a high probability that you will not be asked back again.
Great strategy, at the end of the day, is always putting yourself in a position with the maximum number of options. The key to personal development strategy is networking. The more people you know, the higher the probability that your email box will light up with the “Hey, do you want to collaborate on this project together?” sort of emails. Attend the annual conferences, attend the SHM Academies (Leadership, Quality and Safety Educators Academy, Academic Hospitalist Academy, etc.). Build genuine relationships with the people you meet there, and the rest will work out just fine.
Ms. Steele is the marketing communications specialist at the Society of Hospital Medicine.
Editor’s note: Each month, Society of Hospital Medicine puts the spotlight on some of our most active members who are making substantial contributions to hospital medicine. Log on to www.hospitalmedicine.org/getinvolved for more information on how you can lend your expertise to help SHM improve the care of hospitalized patients.
What are the requirements to become a Master in Hospital Medicine, and how has this designation been beneficial to your career?
I have been an SHM member since the early years (early 2000s, I think), and I became a Master in Hospital Medicine (MHM) in 2013. I see the MHM designation as recognizing accomplishments that have been critical in advancing the field of hospital medicine and SHM as a society.
I would guess that my contributions to the SHM Board, being SHM president, cofounding (with others) the Academic Hospitalist Academy, founding (with others) the Quality Safety Educators Academy, and being the founding chair of the American Board of Internal Medicine’s Focused Practice in Hospital Medicine pathway were probably what led to my induction.
The salient question probably isn’t “How has this designation been beneficial to my career?” but, rather, “How, after receiving the MHM designation, has my career benefited hospital medicine and SHM?” To my mind, there are some awards in life that recognize excellence in the completion of a task. They herald the end of a finite game: a “best research project” award, for example. But then there are a special few recognitions that, while they recognize past contributions, focus more upon the future than the past. They are infinite recognitions, because implicitly, they are recognitions of “promise” as much as achievement. They convey the organization’s trust in, and high expectations for, the recipient. In sum, they are simultaneously an honor and an obligation … an obligation and an expectation that the recipient will continue to do even more. In academic parlance, being “tenured” is a good example; for the Society of Hospital Medicine, the equivalent is the MHM recognition. I have done a lot for SHM, but the MHM designation obligates me to do even more. Honoring that obligation is what I plan to do with my career.
How did you become involved with SHM’s Leadership Academy, and how has the program developed over the years?
I started doing a 1-hour talk when the Mastering Teamwork course started. I did that for a couple of years but, as my career was evolving into higher-level institutional and hospital leadership, there was much more to talk about than I could fit into 1 hour.
The core of my leadership message is based in the “character ethic” (being better than who you are) and not the popular “personality ethic” (looking better than you are). So it’s that … plus all of the leadership mistakes I have made along the way. And that’s a lot of mistakes … enough to fill 9 hours of Mastering Teamwork.
In your opinion, what are some of the main takeaways for those who participate in SHM’s Leadership Academy?
Two of the three core components of great leadership are having a mission and purpose and being sincere. Leadership Academy can’t deliver the first two, so participants do have to come prepared to be trained.
Understanding people is the third core component, and mastering that skill is really complex. It is not something you can do with a clever slogan and a new lapel pin. It comes in many forms: teamwork, communication, networking, dealing with crisis, orchestrating change, etc. But at its core, Leadership Academy is all about training future leaders in how to understand people … and to develop the skills to inspire, motivate, and move their team to greater heights. Because at its core, leadership is about getting people to go places they otherwise didn’t want to go and to do things that they didn’t already want to do. And, to do that, you have to understand people.
As an active SHM member of many years, what advice do you have for members who wish to get more involved?
You have to start somewhere, and you have to see the entry level years as investing in yourself. There will be sacrifice involved, so don’t expect immediate returns on the investment, and the first few years might not be that fun.
Every year, there is a call for committee membership, and you need to get involved in one or more of those committees. Find the most senior hospitalist, who is the most involved in SHM, and tell her that you want to be on an SHM committee, and could she nominate you? If you do not have that luxury, then pay attention at the SHM annual conference. The SHM president-elect is responsible for building out the SHM committee nominees; as president, you are always looking to find enthusiastic people to be on the committees. Receiving emails from enthusiastic members is more welcome than you might think. As soon as that person is announced, find her email and start making the request to be on a committee. Be open to the assignment: Even if it is not your favorite committee, being there is more important than not.
But remember, networking and reputation are “two tailed.” You can improve your reputation by meaningful and consistent participation on a committee (leading to higher and better leadership opportunities), but you can also tarnish it by being assigned to a committee and not doing anything. You do that once, and there is a high probability that you will not be asked back again.
Great strategy, at the end of the day, is always putting yourself in a position with the maximum number of options. The key to personal development strategy is networking. The more people you know, the higher the probability that your email box will light up with the “Hey, do you want to collaborate on this project together?” sort of emails. Attend the annual conferences, attend the SHM Academies (Leadership, Quality and Safety Educators Academy, Academic Hospitalist Academy, etc.). Build genuine relationships with the people you meet there, and the rest will work out just fine.
Ms. Steele is the marketing communications specialist at the Society of Hospital Medicine.