User login
FDA approves lower dose of rivaroxaban
The US Food and Drug Administration (FDA) has approved use of a 10 mg once-daily dose of the factor Xa inhibitor rivaroxaban (XARELTO®).
This dose is now approved to reduce the risk of recurrent venous thromboembolism (VTE) in patients who have received at least 6 months of standard anticoagulant therapy.
With this approval, the rivaroxaban prescribing information provides instructions for physicians to begin treatment with rivaroxaban at 15 mg, dosed twice daily, for the first 21 days after a VTE occurrence.
On day 22 through at least day 180, the dose decreases to 20 mg once daily. After at least 180 days (6 months), physicians can prescribe 10 mg once daily in patients at continued risk for deep vein thrombosis and/or pulmonary embolism.
The FDA’s approval of a 10 mg once-daily dose of rivaroxaban follows a priority review designation from the FDA and is based on data from the EINSTEIN CHOICE study.
Results from EINSTEIN CHOICE were presented at the American College of Cardiology’s 66th Annual Scientific Session and published in NEJM in March.
Patients enrolled in this phase 3 study had confirmed VTE and were treated initially with standard anticoagulant therapy for 6 to 12 months.
During the study, 3365 patients received rivaroxaban at 10 mg, rivaroxaban at 20 mg, or aspirin at 100 mg once daily for up to 12 months of extended treatment.
Both rivaroxaban doses were superior to aspirin in preventing fatal or non-fatal recurrent VTE, the study’s primary efficacy endpoint.
The rate of recurrent VTE was 1.2% in the 10 mg rivaroxaban arm (hazard ratio [HR]=0.26; 95% CI, 0.14 to 0.47; P<0.001), 1.5% in the 20 mg rivaroxaban arm (HR=0.34; 95% CI, 0.20 to 0.59; P<0.001), and 4.4% in the aspirin arm. Fatal VTE occurred in 0%, 0.2%, and 0.2% of patients, respectively.
The primary safety endpoint was major bleeding as defined by the International Society on Thrombosis and Haemostasis.
The rate of major bleeding was 0.4% for the 10 mg rivaroxaban arm (HR=1.64; 95% CI, 0.39 to 6.84; P=0.50), 0.5% for the 20 mg rivaroxaban arm (HR=2.01; 95% CI, 0.50 to 8.04; P=0.32), and 0.3% for the aspirin arm. ![]()
The US Food and Drug Administration (FDA) has approved use of a 10 mg once-daily dose of the factor Xa inhibitor rivaroxaban (XARELTO®).
This dose is now approved to reduce the risk of recurrent venous thromboembolism (VTE) in patients who have received at least 6 months of standard anticoagulant therapy.
With this approval, the rivaroxaban prescribing information provides instructions for physicians to begin treatment with rivaroxaban at 15 mg, dosed twice daily, for the first 21 days after a VTE occurrence.
On day 22 through at least day 180, the dose decreases to 20 mg once daily. After at least 180 days (6 months), physicians can prescribe 10 mg once daily in patients at continued risk for deep vein thrombosis and/or pulmonary embolism.
The FDA’s approval of a 10 mg once-daily dose of rivaroxaban follows a priority review designation from the FDA and is based on data from the EINSTEIN CHOICE study.
Results from EINSTEIN CHOICE were presented at the American College of Cardiology’s 66th Annual Scientific Session and published in NEJM in March.
Patients enrolled in this phase 3 study had confirmed VTE and were treated initially with standard anticoagulant therapy for 6 to 12 months.
During the study, 3365 patients received rivaroxaban at 10 mg, rivaroxaban at 20 mg, or aspirin at 100 mg once daily for up to 12 months of extended treatment.
Both rivaroxaban doses were superior to aspirin in preventing fatal or non-fatal recurrent VTE, the study’s primary efficacy endpoint.
The rate of recurrent VTE was 1.2% in the 10 mg rivaroxaban arm (hazard ratio [HR]=0.26; 95% CI, 0.14 to 0.47; P<0.001), 1.5% in the 20 mg rivaroxaban arm (HR=0.34; 95% CI, 0.20 to 0.59; P<0.001), and 4.4% in the aspirin arm. Fatal VTE occurred in 0%, 0.2%, and 0.2% of patients, respectively.
The primary safety endpoint was major bleeding as defined by the International Society on Thrombosis and Haemostasis.
The rate of major bleeding was 0.4% for the 10 mg rivaroxaban arm (HR=1.64; 95% CI, 0.39 to 6.84; P=0.50), 0.5% for the 20 mg rivaroxaban arm (HR=2.01; 95% CI, 0.50 to 8.04; P=0.32), and 0.3% for the aspirin arm. ![]()
The US Food and Drug Administration (FDA) has approved use of a 10 mg once-daily dose of the factor Xa inhibitor rivaroxaban (XARELTO®).
This dose is now approved to reduce the risk of recurrent venous thromboembolism (VTE) in patients who have received at least 6 months of standard anticoagulant therapy.
With this approval, the rivaroxaban prescribing information provides instructions for physicians to begin treatment with rivaroxaban at 15 mg, dosed twice daily, for the first 21 days after a VTE occurrence.
On day 22 through at least day 180, the dose decreases to 20 mg once daily. After at least 180 days (6 months), physicians can prescribe 10 mg once daily in patients at continued risk for deep vein thrombosis and/or pulmonary embolism.
The FDA’s approval of a 10 mg once-daily dose of rivaroxaban follows a priority review designation from the FDA and is based on data from the EINSTEIN CHOICE study.
Results from EINSTEIN CHOICE were presented at the American College of Cardiology’s 66th Annual Scientific Session and published in NEJM in March.
Patients enrolled in this phase 3 study had confirmed VTE and were treated initially with standard anticoagulant therapy for 6 to 12 months.
During the study, 3365 patients received rivaroxaban at 10 mg, rivaroxaban at 20 mg, or aspirin at 100 mg once daily for up to 12 months of extended treatment.
Both rivaroxaban doses were superior to aspirin in preventing fatal or non-fatal recurrent VTE, the study’s primary efficacy endpoint.
The rate of recurrent VTE was 1.2% in the 10 mg rivaroxaban arm (hazard ratio [HR]=0.26; 95% CI, 0.14 to 0.47; P<0.001), 1.5% in the 20 mg rivaroxaban arm (HR=0.34; 95% CI, 0.20 to 0.59; P<0.001), and 4.4% in the aspirin arm. Fatal VTE occurred in 0%, 0.2%, and 0.2% of patients, respectively.
The primary safety endpoint was major bleeding as defined by the International Society on Thrombosis and Haemostasis.
The rate of major bleeding was 0.4% for the 10 mg rivaroxaban arm (HR=1.64; 95% CI, 0.39 to 6.84; P=0.50), 0.5% for the 20 mg rivaroxaban arm (HR=2.01; 95% CI, 0.50 to 8.04; P=0.32), and 0.3% for the aspirin arm. ![]()
Reduce maternal morbidity by the expeditious and decisive treatment of severe hypertension in pregnancy
Obstetrician-gynecologists are deeply committed to reducing maternal mortality and severe morbidity. Hypertensive diseases of pregnancy, including preeclampsia and eclampsia, are important contributors to both maternal mortality and severe morbidity. Among US live births from 2011–2013 there were 1,078 pregnancy-related maternal deaths, and 10% were attributed to preeclampsia or eclampsia.1 Hypertensive disease of pregnancy is also a major cause of severe maternal morbidity, with an increased risk of acute renal failure, respiratory failure, and cerebrovascular events.2 Preeclampsia is associated with a 4-fold increased risk of thrombocytopenia and coagulopathy and a 2-fold increased risk of postpartum hemorrhage.3
Severe hypertension is defined as a systolic blood pressure (BP) ≥160 mm Hg or a diastolic BP ≥110 mm Hg on 2 measurements within 15 minutes.4,5 Severe hypertensive disease of pregnancy is a common clinical problem in obstetrics, requiring clinicians to respond expeditiously and decisively to minimize adverse maternal outcomes. Following the identification of severe hypertension, a diagnosis and management plan should be initiated within 30 to 60 minutes.4 Some experts recommend that treatment be initiated within 15 minutes of identifying severe hypertension in a pregnant woman.6
The American College of Obstetricians and Gynecologists recommends that obstetric programs adopt standardized guidelines for the management of women with preeclampsia or eclampsia.4 The National Partnership for Maternal Safety recommends that all obstetric programs develop care bundles to respond to severe hypertension.5 Key points in managing severe hypertension are summarized below.
Related article:
2017 Update on obstetrics: Preeclampsia prevention
1. Expeditiously initiate treatment of severe hypertension…
…with intravenous (IV) labetalol (administered as 20 mg/40 mg/80 mg sequential doses as needed) or hydralazine (administered as 10 mg/10 mg/20 mg/40 mg sequential doses as needed). Our preferred agent is labetalol, administered as a 20-mg IV infusion over 2 minutes. If the patient’s BP remains elevated 10 min after the initial dose, administer labetalol 40 mg as an IV infusion over 2 min. If her BP remains elevated 10 min after this dose, administer 80 mg of labetalol. If the BP continues to be elevated, hydralazine treatment can be initiated as described below.
Occasionally there are national shortages of labetalol or a patient has a low heart rate or contraindication such as heart disease or asthma prohibiting its use. If labetalol is not available, we use hydralazine administered as a 10-mg IV bolus over 2 min. If the BP remains elevated, every 20 min, an escalating dose of hydralazine is administered, first by repeating the 10-mg dose, then administering 20 mg, and finally 40 mg.
For women without IV access, we use oral nifedipine 10 mg to control hypertension only while awaiting the placement of an IV. If BP remains elevated after 30 min, a second dose of oral nifedipine 20 mg can be given with a plan to transition to IV agents as soon as possible. The risks of maternal tachycardia or overshoot hypotension with immediate release oral nifedipine limit its use in our clinical practice to this circumstance.
Once the BP is controlled, start maintenance oral hypertension therapy. Our first-line agent is labetalol 200 mg twice per day with a maximum dose of 800 mg 3 times daily (2,400 mg maximal daily dose).
2. Initiate treatment with magnesium sulfate
If the patient’s BP is ≥160/110 mm Hg or if her BP is ≥140/90 mm Hg with coexisting symptoms of severe preeclampsia (for example a severe headache), initiate magnesium sulfate treatment. A standard regimen is magnesium sulfate 4 to 6 g administered as an IV bolus over 20 min followed by the IV infusion of 2 g per hour. In our clinical opinion, if you plan on initiating IV antihypertensive treatment for severe hypertension you also should strongly consider starting magnesium sulfate to reduce the risk of an eclamptic seizure.
We also start magnesium sulfate therapy for women with severe hypertension and clinical symptoms or laboratory signs of preeclampsia even in the absence of proteinuria. Approximately 2% of women with preeclampsia will develop an eclamptic seizure and magnesium sulfate treatment significantly reduces the risk of seizure and may also reduce maternal mortality.7,8
Magnesium sulfate is contra-indicated in women with myasthenia gravis. In women with renal dysfunction, the loading dose can be given, but the continuous magnesium sulfate infusion should not be initiated until serum magnesium levels are assessed.
3. Consider administering maternal betamethasone
Treatment with betamethasone advances fetal maturation if the pregnancy is preterm (for example, <34 weeks of gestation). A major cause of neonatal morbidity and mortality for pregnancy complicated by severe hypertensive disease is premature delivery. Maternal glucocorticoid treatment reduces the risk of neonatal morbidity and mortality if preterm delivery is anticipated. However, do not delay delivery for antenatal corticosteroids for women with severe and persistent hypertension or symptoms of preeclampsia that do not resolve following treatment.
We also consider women with eclampsia, placental abruption, pulmonary edema, or severe laboratory derangements too unstable to delay delivery for 48 hours to achieve the maximum benefit of steroid treatment. If antenatal corticosteroids are administered in the late preterm period between 34 0/7 weeks and 36 6/7 weeks of gestation, obstetric management should not be altered and delivery should not be delayed.9
Related article:
Start offering antenatal corticosteroids to women delivering between 34 0/7 and 36 6/7 weeks of gestation to improve newborn outcomes
4. Preeclampsia plus a severe headache is a toxic combination
For patients with this constellation either have a plan for delivery or keep them under close surveillance. Occasionally a woman >20 weeks pregnant with new onset hypertension and a headache is seen in an emergency department and is not assessed for proteinuria or other preeclampsia laboratory abnormalities. If the woman is diagnosed as having a migraine or tension headache and discharged home with a headache medicine they are at high risk for serious morbidity, including stroke.
Read about preeclampsia and thrombocytopenia, HELLP syndrome, more.
5. Preeclampsia plus thrombocytopenia complicates anesthesia options
If the platelet count falls too low (for instance, <70,000 platelets per µL), many anesthesiologists will not provide a regional anesthetic for delivery because of the risk of peridural bleeding. In addition, a low platelet count (<50,000 platelets per µL) significantly increases the risk of obstetric hemorrhage. Transfer of the patient to an obstetrics unit with a full-service blood bank capable of supporting multiple platelet transfusions may be warranted.
6. Preeclampsia plus dyspnea or chest pain increases the risk of severe maternal morbidity
Authors of a prospective study of 2,023 women with preeclampsia reported an increase in adverse maternal outcomes when the following factors were present: early gestational age, dyspnea, chest pain, oxygen saturation of SpO2 <93%, thrombocytopenia, elevated creatinine, or elevated aspartate transaminase concentration.10 If dyspnea is present, the patient may have pulmonary edema, pulmonary embolism, heart failure, acute asthma, or pneumonia. If the patient has chest pain the differential diagnosis includes pulmonary embolism, cardiac ischemia, cardiomyopathy, or another cardiac disease.
Consider obtaining a chest radiograph for pregnant women with dyspnea and a computed tomography pulmonary angiogram or lung scintigraphy (ventilation perfusion scan) if the chest radiograph is normal for women with chest pain.6,11 We obtain a transthoracic echocardiogram in cases of pulmonary edema to evaluate for the possibility of peripartum cardiomyopathy.
7. HELLP syndrome
The triad of hemolysis, elevated liver enzymes, and low platelet count (HELLP) is associated with an increased risk of maternal mortality and severe morbidity.12 In a study of 171 women with HELLP, factors that increased the risk for adverse maternal outcomes included12:
- aspartate aminotransferase (AST) levels >316 U/L
- alanine aminotransferase (ALT) levels >217 U/L
- total bilirubin levels >2.0 mg/dL
- lactate dehydrogenase (LDH) levels >1,290 U/L
- blood urea nitrogen test results >44 mg/dL
- platelet count <50,000 platelets per µL.
The clinical course of HELLP syndrome is characterized by progression and the potential for sudden and catastrophic deterioration. For example, some women with HELLP will suddenly develop a ruptured liver, pulmonary edema, or a stroke. The Society for Maternal-Fetal Medicine recommends against expectant management of women with HELLP syndrome.13
Related article:
Optimal obstetric care for women aged 40 and older
8. Delivery or expectant management?
Currently the only cure for preeclampsia is delivery. The Society for Maternal-Fetal Medicine recommends against expectant management of severe preeclampsia if certain problems occur (BOX).13 For women with preeclampsia who are less than 34 weeks’ gestation and do not have a contraindication to expectant management, consider transferring the patient to a tertiary maternal care center. In our practice, pregnant women with a hypertensive disorder are scheduled for an induction of labor and delivery at 37 weeks’ gestation.
The Society for Maternal-Fetal Medicine recommends delivery (not expectant management) in the presence of severe preeclampsia if any of the following are present13:
- eclampsia
- pulmonary edema
- disseminated intravascular coagulation
- renal insufficiency
- abruptio placentae
- abnormal fetal testing
- HELLP syndrome or persistent symptoms of severe preeclampsia.
In the United States, major obstetric causes of pregnancy-related death include sepsis, venous thromboembolism-pulmonary embolism, hemorrhage, and hypertensive disease of pregnancy. Other important causes of pregnancy-related death include cardiac disease, stroke, and pre-existing major medical disease including advanced cancer. In the United States there are approximately 17 pregnancy-related maternal deaths per 100,000 live births.1 Obstetricians are dedicated to reducing this excessively high rate of maternal death.
Given the US maternal death rate of 1 maternity death per 5,880 live births, over the course of a 40-year career, most obstetrician-gynecologists will have 1 or 2 of their pregnant patients die. From the perspective of an individual clinician, maternal death is an extremely rare event, with 1 death during every 20 years of practice. However, from a population perspective, maternal death in the United States is all too common compared to other developed countries. We can only reduce the rate of maternal death by working in interdisciplinary teams to ensure our obstetrics units are prepared to expeditiously diagnose and treat the most common obstetric causes of death and severe morbidity.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130(2):366–373.
- Kuklina EV, Ayala C, Callaghan WM. Hyper-tensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113(6):1299–1306.
- Stevens S, Shih T, Incerti D, et al. Short-term costs of preeclampsia to the United States health care system. Am J Obstet Gynecol. 2017;217(3):237–248.e16.
- Committee on Obstetric Practice. Committee Opinion No. 692: Emergent therapy for acute-onset, severe hypertension during pregnancy and the postpartum period. Obstet Gynecol. 2017;129(4):e90–e95.
- Bernstein PS, Martin JN Jr, Barton JR, et al. National Partnership for Maternal Safety: Consensus bundle on severe hypertension during pregnancy and the postpartum period. Obstet Gynecol. 2017;130(2):347–357.
- Clark SL, Hankins GD. Preventing maternal death: 10 clinical diamonds. Obstet Gynecol. 2012;119(2 pt 1):360–364.
- Thornton C, Dahlen H, Korda A, Hennessy A. The incidence of preeclampsia and eclampsia and associated maternal mortality in Australia from population-linked datasets: 2000-2008. Am J Obstet Gynecol. 2013;208(6):476.e1–e5.
- Altman D, Carroli G, Duley L, et al; Magpie Trial Collaboration Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359(9321):1877–1890.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374(14):1311–1320.
- von Dadelszen P, Payne B, Li J, et al; PIERS Study Group. Prediction of adverse maternal outcomes in pre-eclampsia: development and validation of the full PIERS model. Lancet. 2011;377(9761):219–227.
- Shahir K, Goodman LR, Tali A, Thorsen KM, Hellman RS. Pulmonary embolism in pregnancy: CT pulmonary angiography versus perfusion scanning. AJR Am J Roentgenol. 2010;195(3):W214–W220.
- Erkilinç S, Eyi EG. Factors contributing to adverse maternal outcomes in patients with HELLP syndrome. J Matern Fetal Neonatal Med. 2017:1–7. doi:10.1080/14767058.2017.1359528.
- Publications Committee, Society for Maternal-Fetal Medicine, Sibai BM. Evaluation and management of severe preeclampsia before 34 weeks’ gestation. Am J Obstet Gynecol. 2011;205(3):191–198
Obstetrician-gynecologists are deeply committed to reducing maternal mortality and severe morbidity. Hypertensive diseases of pregnancy, including preeclampsia and eclampsia, are important contributors to both maternal mortality and severe morbidity. Among US live births from 2011–2013 there were 1,078 pregnancy-related maternal deaths, and 10% were attributed to preeclampsia or eclampsia.1 Hypertensive disease of pregnancy is also a major cause of severe maternal morbidity, with an increased risk of acute renal failure, respiratory failure, and cerebrovascular events.2 Preeclampsia is associated with a 4-fold increased risk of thrombocytopenia and coagulopathy and a 2-fold increased risk of postpartum hemorrhage.3
Severe hypertension is defined as a systolic blood pressure (BP) ≥160 mm Hg or a diastolic BP ≥110 mm Hg on 2 measurements within 15 minutes.4,5 Severe hypertensive disease of pregnancy is a common clinical problem in obstetrics, requiring clinicians to respond expeditiously and decisively to minimize adverse maternal outcomes. Following the identification of severe hypertension, a diagnosis and management plan should be initiated within 30 to 60 minutes.4 Some experts recommend that treatment be initiated within 15 minutes of identifying severe hypertension in a pregnant woman.6
The American College of Obstetricians and Gynecologists recommends that obstetric programs adopt standardized guidelines for the management of women with preeclampsia or eclampsia.4 The National Partnership for Maternal Safety recommends that all obstetric programs develop care bundles to respond to severe hypertension.5 Key points in managing severe hypertension are summarized below.
Related article:
2017 Update on obstetrics: Preeclampsia prevention
1. Expeditiously initiate treatment of severe hypertension…
…with intravenous (IV) labetalol (administered as 20 mg/40 mg/80 mg sequential doses as needed) or hydralazine (administered as 10 mg/10 mg/20 mg/40 mg sequential doses as needed). Our preferred agent is labetalol, administered as a 20-mg IV infusion over 2 minutes. If the patient’s BP remains elevated 10 min after the initial dose, administer labetalol 40 mg as an IV infusion over 2 min. If her BP remains elevated 10 min after this dose, administer 80 mg of labetalol. If the BP continues to be elevated, hydralazine treatment can be initiated as described below.
Occasionally there are national shortages of labetalol or a patient has a low heart rate or contraindication such as heart disease or asthma prohibiting its use. If labetalol is not available, we use hydralazine administered as a 10-mg IV bolus over 2 min. If the BP remains elevated, every 20 min, an escalating dose of hydralazine is administered, first by repeating the 10-mg dose, then administering 20 mg, and finally 40 mg.
For women without IV access, we use oral nifedipine 10 mg to control hypertension only while awaiting the placement of an IV. If BP remains elevated after 30 min, a second dose of oral nifedipine 20 mg can be given with a plan to transition to IV agents as soon as possible. The risks of maternal tachycardia or overshoot hypotension with immediate release oral nifedipine limit its use in our clinical practice to this circumstance.
Once the BP is controlled, start maintenance oral hypertension therapy. Our first-line agent is labetalol 200 mg twice per day with a maximum dose of 800 mg 3 times daily (2,400 mg maximal daily dose).
2. Initiate treatment with magnesium sulfate
If the patient’s BP is ≥160/110 mm Hg or if her BP is ≥140/90 mm Hg with coexisting symptoms of severe preeclampsia (for example a severe headache), initiate magnesium sulfate treatment. A standard regimen is magnesium sulfate 4 to 6 g administered as an IV bolus over 20 min followed by the IV infusion of 2 g per hour. In our clinical opinion, if you plan on initiating IV antihypertensive treatment for severe hypertension you also should strongly consider starting magnesium sulfate to reduce the risk of an eclamptic seizure.
We also start magnesium sulfate therapy for women with severe hypertension and clinical symptoms or laboratory signs of preeclampsia even in the absence of proteinuria. Approximately 2% of women with preeclampsia will develop an eclamptic seizure and magnesium sulfate treatment significantly reduces the risk of seizure and may also reduce maternal mortality.7,8
Magnesium sulfate is contra-indicated in women with myasthenia gravis. In women with renal dysfunction, the loading dose can be given, but the continuous magnesium sulfate infusion should not be initiated until serum magnesium levels are assessed.
3. Consider administering maternal betamethasone
Treatment with betamethasone advances fetal maturation if the pregnancy is preterm (for example, <34 weeks of gestation). A major cause of neonatal morbidity and mortality for pregnancy complicated by severe hypertensive disease is premature delivery. Maternal glucocorticoid treatment reduces the risk of neonatal morbidity and mortality if preterm delivery is anticipated. However, do not delay delivery for antenatal corticosteroids for women with severe and persistent hypertension or symptoms of preeclampsia that do not resolve following treatment.
We also consider women with eclampsia, placental abruption, pulmonary edema, or severe laboratory derangements too unstable to delay delivery for 48 hours to achieve the maximum benefit of steroid treatment. If antenatal corticosteroids are administered in the late preterm period between 34 0/7 weeks and 36 6/7 weeks of gestation, obstetric management should not be altered and delivery should not be delayed.9
Related article:
Start offering antenatal corticosteroids to women delivering between 34 0/7 and 36 6/7 weeks of gestation to improve newborn outcomes
4. Preeclampsia plus a severe headache is a toxic combination
For patients with this constellation either have a plan for delivery or keep them under close surveillance. Occasionally a woman >20 weeks pregnant with new onset hypertension and a headache is seen in an emergency department and is not assessed for proteinuria or other preeclampsia laboratory abnormalities. If the woman is diagnosed as having a migraine or tension headache and discharged home with a headache medicine they are at high risk for serious morbidity, including stroke.
Read about preeclampsia and thrombocytopenia, HELLP syndrome, more.
5. Preeclampsia plus thrombocytopenia complicates anesthesia options
If the platelet count falls too low (for instance, <70,000 platelets per µL), many anesthesiologists will not provide a regional anesthetic for delivery because of the risk of peridural bleeding. In addition, a low platelet count (<50,000 platelets per µL) significantly increases the risk of obstetric hemorrhage. Transfer of the patient to an obstetrics unit with a full-service blood bank capable of supporting multiple platelet transfusions may be warranted.
6. Preeclampsia plus dyspnea or chest pain increases the risk of severe maternal morbidity
Authors of a prospective study of 2,023 women with preeclampsia reported an increase in adverse maternal outcomes when the following factors were present: early gestational age, dyspnea, chest pain, oxygen saturation of SpO2 <93%, thrombocytopenia, elevated creatinine, or elevated aspartate transaminase concentration.10 If dyspnea is present, the patient may have pulmonary edema, pulmonary embolism, heart failure, acute asthma, or pneumonia. If the patient has chest pain the differential diagnosis includes pulmonary embolism, cardiac ischemia, cardiomyopathy, or another cardiac disease.
Consider obtaining a chest radiograph for pregnant women with dyspnea and a computed tomography pulmonary angiogram or lung scintigraphy (ventilation perfusion scan) if the chest radiograph is normal for women with chest pain.6,11 We obtain a transthoracic echocardiogram in cases of pulmonary edema to evaluate for the possibility of peripartum cardiomyopathy.
7. HELLP syndrome
The triad of hemolysis, elevated liver enzymes, and low platelet count (HELLP) is associated with an increased risk of maternal mortality and severe morbidity.12 In a study of 171 women with HELLP, factors that increased the risk for adverse maternal outcomes included12:
- aspartate aminotransferase (AST) levels >316 U/L
- alanine aminotransferase (ALT) levels >217 U/L
- total bilirubin levels >2.0 mg/dL
- lactate dehydrogenase (LDH) levels >1,290 U/L
- blood urea nitrogen test results >44 mg/dL
- platelet count <50,000 platelets per µL.
The clinical course of HELLP syndrome is characterized by progression and the potential for sudden and catastrophic deterioration. For example, some women with HELLP will suddenly develop a ruptured liver, pulmonary edema, or a stroke. The Society for Maternal-Fetal Medicine recommends against expectant management of women with HELLP syndrome.13
Related article:
Optimal obstetric care for women aged 40 and older
8. Delivery or expectant management?
Currently the only cure for preeclampsia is delivery. The Society for Maternal-Fetal Medicine recommends against expectant management of severe preeclampsia if certain problems occur (BOX).13 For women with preeclampsia who are less than 34 weeks’ gestation and do not have a contraindication to expectant management, consider transferring the patient to a tertiary maternal care center. In our practice, pregnant women with a hypertensive disorder are scheduled for an induction of labor and delivery at 37 weeks’ gestation.
The Society for Maternal-Fetal Medicine recommends delivery (not expectant management) in the presence of severe preeclampsia if any of the following are present13:
- eclampsia
- pulmonary edema
- disseminated intravascular coagulation
- renal insufficiency
- abruptio placentae
- abnormal fetal testing
- HELLP syndrome or persistent symptoms of severe preeclampsia.
In the United States, major obstetric causes of pregnancy-related death include sepsis, venous thromboembolism-pulmonary embolism, hemorrhage, and hypertensive disease of pregnancy. Other important causes of pregnancy-related death include cardiac disease, stroke, and pre-existing major medical disease including advanced cancer. In the United States there are approximately 17 pregnancy-related maternal deaths per 100,000 live births.1 Obstetricians are dedicated to reducing this excessively high rate of maternal death.
Given the US maternal death rate of 1 maternity death per 5,880 live births, over the course of a 40-year career, most obstetrician-gynecologists will have 1 or 2 of their pregnant patients die. From the perspective of an individual clinician, maternal death is an extremely rare event, with 1 death during every 20 years of practice. However, from a population perspective, maternal death in the United States is all too common compared to other developed countries. We can only reduce the rate of maternal death by working in interdisciplinary teams to ensure our obstetrics units are prepared to expeditiously diagnose and treat the most common obstetric causes of death and severe morbidity.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Obstetrician-gynecologists are deeply committed to reducing maternal mortality and severe morbidity. Hypertensive diseases of pregnancy, including preeclampsia and eclampsia, are important contributors to both maternal mortality and severe morbidity. Among US live births from 2011–2013 there were 1,078 pregnancy-related maternal deaths, and 10% were attributed to preeclampsia or eclampsia.1 Hypertensive disease of pregnancy is also a major cause of severe maternal morbidity, with an increased risk of acute renal failure, respiratory failure, and cerebrovascular events.2 Preeclampsia is associated with a 4-fold increased risk of thrombocytopenia and coagulopathy and a 2-fold increased risk of postpartum hemorrhage.3
Severe hypertension is defined as a systolic blood pressure (BP) ≥160 mm Hg or a diastolic BP ≥110 mm Hg on 2 measurements within 15 minutes.4,5 Severe hypertensive disease of pregnancy is a common clinical problem in obstetrics, requiring clinicians to respond expeditiously and decisively to minimize adverse maternal outcomes. Following the identification of severe hypertension, a diagnosis and management plan should be initiated within 30 to 60 minutes.4 Some experts recommend that treatment be initiated within 15 minutes of identifying severe hypertension in a pregnant woman.6
The American College of Obstetricians and Gynecologists recommends that obstetric programs adopt standardized guidelines for the management of women with preeclampsia or eclampsia.4 The National Partnership for Maternal Safety recommends that all obstetric programs develop care bundles to respond to severe hypertension.5 Key points in managing severe hypertension are summarized below.
Related article:
2017 Update on obstetrics: Preeclampsia prevention
1. Expeditiously initiate treatment of severe hypertension…
…with intravenous (IV) labetalol (administered as 20 mg/40 mg/80 mg sequential doses as needed) or hydralazine (administered as 10 mg/10 mg/20 mg/40 mg sequential doses as needed). Our preferred agent is labetalol, administered as a 20-mg IV infusion over 2 minutes. If the patient’s BP remains elevated 10 min after the initial dose, administer labetalol 40 mg as an IV infusion over 2 min. If her BP remains elevated 10 min after this dose, administer 80 mg of labetalol. If the BP continues to be elevated, hydralazine treatment can be initiated as described below.
Occasionally there are national shortages of labetalol or a patient has a low heart rate or contraindication such as heart disease or asthma prohibiting its use. If labetalol is not available, we use hydralazine administered as a 10-mg IV bolus over 2 min. If the BP remains elevated, every 20 min, an escalating dose of hydralazine is administered, first by repeating the 10-mg dose, then administering 20 mg, and finally 40 mg.
For women without IV access, we use oral nifedipine 10 mg to control hypertension only while awaiting the placement of an IV. If BP remains elevated after 30 min, a second dose of oral nifedipine 20 mg can be given with a plan to transition to IV agents as soon as possible. The risks of maternal tachycardia or overshoot hypotension with immediate release oral nifedipine limit its use in our clinical practice to this circumstance.
Once the BP is controlled, start maintenance oral hypertension therapy. Our first-line agent is labetalol 200 mg twice per day with a maximum dose of 800 mg 3 times daily (2,400 mg maximal daily dose).
2. Initiate treatment with magnesium sulfate
If the patient’s BP is ≥160/110 mm Hg or if her BP is ≥140/90 mm Hg with coexisting symptoms of severe preeclampsia (for example a severe headache), initiate magnesium sulfate treatment. A standard regimen is magnesium sulfate 4 to 6 g administered as an IV bolus over 20 min followed by the IV infusion of 2 g per hour. In our clinical opinion, if you plan on initiating IV antihypertensive treatment for severe hypertension you also should strongly consider starting magnesium sulfate to reduce the risk of an eclamptic seizure.
We also start magnesium sulfate therapy for women with severe hypertension and clinical symptoms or laboratory signs of preeclampsia even in the absence of proteinuria. Approximately 2% of women with preeclampsia will develop an eclamptic seizure and magnesium sulfate treatment significantly reduces the risk of seizure and may also reduce maternal mortality.7,8
Magnesium sulfate is contra-indicated in women with myasthenia gravis. In women with renal dysfunction, the loading dose can be given, but the continuous magnesium sulfate infusion should not be initiated until serum magnesium levels are assessed.
3. Consider administering maternal betamethasone
Treatment with betamethasone advances fetal maturation if the pregnancy is preterm (for example, <34 weeks of gestation). A major cause of neonatal morbidity and mortality for pregnancy complicated by severe hypertensive disease is premature delivery. Maternal glucocorticoid treatment reduces the risk of neonatal morbidity and mortality if preterm delivery is anticipated. However, do not delay delivery for antenatal corticosteroids for women with severe and persistent hypertension or symptoms of preeclampsia that do not resolve following treatment.
We also consider women with eclampsia, placental abruption, pulmonary edema, or severe laboratory derangements too unstable to delay delivery for 48 hours to achieve the maximum benefit of steroid treatment. If antenatal corticosteroids are administered in the late preterm period between 34 0/7 weeks and 36 6/7 weeks of gestation, obstetric management should not be altered and delivery should not be delayed.9
Related article:
Start offering antenatal corticosteroids to women delivering between 34 0/7 and 36 6/7 weeks of gestation to improve newborn outcomes
4. Preeclampsia plus a severe headache is a toxic combination
For patients with this constellation either have a plan for delivery or keep them under close surveillance. Occasionally a woman >20 weeks pregnant with new onset hypertension and a headache is seen in an emergency department and is not assessed for proteinuria or other preeclampsia laboratory abnormalities. If the woman is diagnosed as having a migraine or tension headache and discharged home with a headache medicine they are at high risk for serious morbidity, including stroke.
Read about preeclampsia and thrombocytopenia, HELLP syndrome, more.
5. Preeclampsia plus thrombocytopenia complicates anesthesia options
If the platelet count falls too low (for instance, <70,000 platelets per µL), many anesthesiologists will not provide a regional anesthetic for delivery because of the risk of peridural bleeding. In addition, a low platelet count (<50,000 platelets per µL) significantly increases the risk of obstetric hemorrhage. Transfer of the patient to an obstetrics unit with a full-service blood bank capable of supporting multiple platelet transfusions may be warranted.
6. Preeclampsia plus dyspnea or chest pain increases the risk of severe maternal morbidity
Authors of a prospective study of 2,023 women with preeclampsia reported an increase in adverse maternal outcomes when the following factors were present: early gestational age, dyspnea, chest pain, oxygen saturation of SpO2 <93%, thrombocytopenia, elevated creatinine, or elevated aspartate transaminase concentration.10 If dyspnea is present, the patient may have pulmonary edema, pulmonary embolism, heart failure, acute asthma, or pneumonia. If the patient has chest pain the differential diagnosis includes pulmonary embolism, cardiac ischemia, cardiomyopathy, or another cardiac disease.
Consider obtaining a chest radiograph for pregnant women with dyspnea and a computed tomography pulmonary angiogram or lung scintigraphy (ventilation perfusion scan) if the chest radiograph is normal for women with chest pain.6,11 We obtain a transthoracic echocardiogram in cases of pulmonary edema to evaluate for the possibility of peripartum cardiomyopathy.
7. HELLP syndrome
The triad of hemolysis, elevated liver enzymes, and low platelet count (HELLP) is associated with an increased risk of maternal mortality and severe morbidity.12 In a study of 171 women with HELLP, factors that increased the risk for adverse maternal outcomes included12:
- aspartate aminotransferase (AST) levels >316 U/L
- alanine aminotransferase (ALT) levels >217 U/L
- total bilirubin levels >2.0 mg/dL
- lactate dehydrogenase (LDH) levels >1,290 U/L
- blood urea nitrogen test results >44 mg/dL
- platelet count <50,000 platelets per µL.
The clinical course of HELLP syndrome is characterized by progression and the potential for sudden and catastrophic deterioration. For example, some women with HELLP will suddenly develop a ruptured liver, pulmonary edema, or a stroke. The Society for Maternal-Fetal Medicine recommends against expectant management of women with HELLP syndrome.13
Related article:
Optimal obstetric care for women aged 40 and older
8. Delivery or expectant management?
Currently the only cure for preeclampsia is delivery. The Society for Maternal-Fetal Medicine recommends against expectant management of severe preeclampsia if certain problems occur (BOX).13 For women with preeclampsia who are less than 34 weeks’ gestation and do not have a contraindication to expectant management, consider transferring the patient to a tertiary maternal care center. In our practice, pregnant women with a hypertensive disorder are scheduled for an induction of labor and delivery at 37 weeks’ gestation.
The Society for Maternal-Fetal Medicine recommends delivery (not expectant management) in the presence of severe preeclampsia if any of the following are present13:
- eclampsia
- pulmonary edema
- disseminated intravascular coagulation
- renal insufficiency
- abruptio placentae
- abnormal fetal testing
- HELLP syndrome or persistent symptoms of severe preeclampsia.
In the United States, major obstetric causes of pregnancy-related death include sepsis, venous thromboembolism-pulmonary embolism, hemorrhage, and hypertensive disease of pregnancy. Other important causes of pregnancy-related death include cardiac disease, stroke, and pre-existing major medical disease including advanced cancer. In the United States there are approximately 17 pregnancy-related maternal deaths per 100,000 live births.1 Obstetricians are dedicated to reducing this excessively high rate of maternal death.
Given the US maternal death rate of 1 maternity death per 5,880 live births, over the course of a 40-year career, most obstetrician-gynecologists will have 1 or 2 of their pregnant patients die. From the perspective of an individual clinician, maternal death is an extremely rare event, with 1 death during every 20 years of practice. However, from a population perspective, maternal death in the United States is all too common compared to other developed countries. We can only reduce the rate of maternal death by working in interdisciplinary teams to ensure our obstetrics units are prepared to expeditiously diagnose and treat the most common obstetric causes of death and severe morbidity.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130(2):366–373.
- Kuklina EV, Ayala C, Callaghan WM. Hyper-tensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113(6):1299–1306.
- Stevens S, Shih T, Incerti D, et al. Short-term costs of preeclampsia to the United States health care system. Am J Obstet Gynecol. 2017;217(3):237–248.e16.
- Committee on Obstetric Practice. Committee Opinion No. 692: Emergent therapy for acute-onset, severe hypertension during pregnancy and the postpartum period. Obstet Gynecol. 2017;129(4):e90–e95.
- Bernstein PS, Martin JN Jr, Barton JR, et al. National Partnership for Maternal Safety: Consensus bundle on severe hypertension during pregnancy and the postpartum period. Obstet Gynecol. 2017;130(2):347–357.
- Clark SL, Hankins GD. Preventing maternal death: 10 clinical diamonds. Obstet Gynecol. 2012;119(2 pt 1):360–364.
- Thornton C, Dahlen H, Korda A, Hennessy A. The incidence of preeclampsia and eclampsia and associated maternal mortality in Australia from population-linked datasets: 2000-2008. Am J Obstet Gynecol. 2013;208(6):476.e1–e5.
- Altman D, Carroli G, Duley L, et al; Magpie Trial Collaboration Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359(9321):1877–1890.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374(14):1311–1320.
- von Dadelszen P, Payne B, Li J, et al; PIERS Study Group. Prediction of adverse maternal outcomes in pre-eclampsia: development and validation of the full PIERS model. Lancet. 2011;377(9761):219–227.
- Shahir K, Goodman LR, Tali A, Thorsen KM, Hellman RS. Pulmonary embolism in pregnancy: CT pulmonary angiography versus perfusion scanning. AJR Am J Roentgenol. 2010;195(3):W214–W220.
- Erkilinç S, Eyi EG. Factors contributing to adverse maternal outcomes in patients with HELLP syndrome. J Matern Fetal Neonatal Med. 2017:1–7. doi:10.1080/14767058.2017.1359528.
- Publications Committee, Society for Maternal-Fetal Medicine, Sibai BM. Evaluation and management of severe preeclampsia before 34 weeks’ gestation. Am J Obstet Gynecol. 2011;205(3):191–198
- Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130(2):366–373.
- Kuklina EV, Ayala C, Callaghan WM. Hyper-tensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113(6):1299–1306.
- Stevens S, Shih T, Incerti D, et al. Short-term costs of preeclampsia to the United States health care system. Am J Obstet Gynecol. 2017;217(3):237–248.e16.
- Committee on Obstetric Practice. Committee Opinion No. 692: Emergent therapy for acute-onset, severe hypertension during pregnancy and the postpartum period. Obstet Gynecol. 2017;129(4):e90–e95.
- Bernstein PS, Martin JN Jr, Barton JR, et al. National Partnership for Maternal Safety: Consensus bundle on severe hypertension during pregnancy and the postpartum period. Obstet Gynecol. 2017;130(2):347–357.
- Clark SL, Hankins GD. Preventing maternal death: 10 clinical diamonds. Obstet Gynecol. 2012;119(2 pt 1):360–364.
- Thornton C, Dahlen H, Korda A, Hennessy A. The incidence of preeclampsia and eclampsia and associated maternal mortality in Australia from population-linked datasets: 2000-2008. Am J Obstet Gynecol. 2013;208(6):476.e1–e5.
- Altman D, Carroli G, Duley L, et al; Magpie Trial Collaboration Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359(9321):1877–1890.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374(14):1311–1320.
- von Dadelszen P, Payne B, Li J, et al; PIERS Study Group. Prediction of adverse maternal outcomes in pre-eclampsia: development and validation of the full PIERS model. Lancet. 2011;377(9761):219–227.
- Shahir K, Goodman LR, Tali A, Thorsen KM, Hellman RS. Pulmonary embolism in pregnancy: CT pulmonary angiography versus perfusion scanning. AJR Am J Roentgenol. 2010;195(3):W214–W220.
- Erkilinç S, Eyi EG. Factors contributing to adverse maternal outcomes in patients with HELLP syndrome. J Matern Fetal Neonatal Med. 2017:1–7. doi:10.1080/14767058.2017.1359528.
- Publications Committee, Society for Maternal-Fetal Medicine, Sibai BM. Evaluation and management of severe preeclampsia before 34 weeks’ gestation. Am J Obstet Gynecol. 2011;205(3):191–198
Breast cancer screening: Is the controversy of benefits versus harms resolved?
Breast cancer is the most common cancer and the second leading cause of cancer death in women in the United States, with an estimated 252,710 new cases and 40,610 deaths in 2017.1 Breast cancer mortality is prevented by the use of regular screening mammography, as demonstrated by randomized controlled trials (20% reduction), incidence-based mortality studies (38% to 40% reduction), and service screening studies (48% to 49% reduction).2
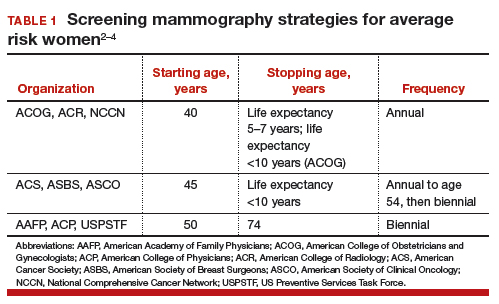
Controversy continues, however, on when to start mammography screening, when to stop screening, and the frequency with which screening should be performed for women at average risk for breast cancer. Indeed, 3 national recommendations—written by the American College of Obstetricians and Gynecologists (ACOG), the American Cancer Society (ACS), and the US Preventive Services Task Force (USPSTF)—offer different guidelines for mammography screening (TABLE 1).2–4
There are 2 principal reasons for the controversy over screening:
- mammography has both benefits and harms, and individuals place differential weight on the importance of these relative to each other
- randomized controlled trials on screening mammography did not include all of the starting age, stopping age, and screening intervals that are included in screening recommendations.
New comparison of recommendations
An ongoing project funded by the National Cancer Institute, known as the Cancer Intervention and Surveillance Modeling Network (CISNET), models different starting and stopping ages and screening intervals for mammography to assess their impact on both benefits (mortality improvement, life-years gained) and harms (callbacks, benign breast biopsies). Recently, Arleo and colleagues used CISNET model data to compare the breast cancer screening recommendations from ACOG, the ACS, and the USPSTF, focusing on the differential effect on benefits and harms.5
Benefits vs harms of screening in perspective
Without question, the principal goal of cancer screening strategies is to effectively and efficiently reduce cancer mortality. Because mammography screening has both benefits and harms, a clear understanding of the relative frequency of these events among the different screening recommendations should be an important element in patient counseling.
Based on CISNET-modeled estimates, TABLE 2, illustrates the differences in both benefits and harms of the 3 screening strategies. With all strategies, there is a clear benefit in both fewer breast cancer–related deaths and life-years gained per 1,000 women screened.
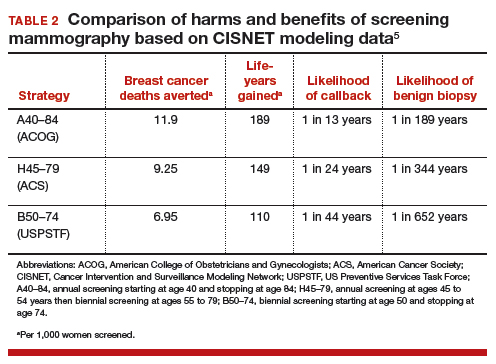
The greatest benefit is seen in the A40–84 group, that is, women who undergo the most intensive screening strategy with annual screening starting at age 40 and ending at age 84 (ACOG) compared with the USPSTF’s least intensive screening strategy, B50–74, which includes biennial screening starting at age 50 and stopping at age 74; benefits of the ACS’s H45–79 strategy (annual screening at ages 45 to 54 years then biennial screening at ages 55 to 79) were in-between. Not surprisingly, the A40–84 screening strategy was also associated with the most harms, with more recalls and benign breast biopsies; the least harms occurred with the USPSTF strategy, with the ACS strategy again in-between in terms of harms.
Related articles:
Breast density and optimal screening for breast cancer
To further demonstrate differences between the 3 strategies, CISNET also modeled results by looking at all women born in a single birth year cohort (1960) who were still alive at age 40 (2.468 million women). The modeling estimates the number of women who would die from breast cancer without screening mammography and compares that with the number of women who would die from breast cancer using any of the 3 screening strategies. Using this 1960 birth year cohort analysis, there would be approximately 12,000 fewer breast cancer deaths using the ACOG-recommended screening strategy compared with the USPSTF-recommended approach.4
These data show that while there are more harms associated with the most intense screening recommendation, the less frequent screening recommendations will result in higher mortality and more life-years lost. It is reasonable to assume that most patients would value mortality reduction and life-years gained over a likelihood of more benign biopsies or callbacks. As a result, each of the guidelines recommends that by age 40, women at average risk for breast cancer should be counseled and offered mammography screening based on their personal values.
Read about how Dr. Pearlman counsels his patients on screening.
My counseling approach on screening
Notably, the Women’s Preventive Services Initiative recommends that average risk women initiate mammography screening no earlier than age 40 and no later than age 50.6 This creates more flexibility around starting time for screening. In the population of women that I personally counsel, we discuss that fewer women (1 in 68) will experience breast cancer in their 40s compared with in their 50s (1 in 43); therefore as a population, more women will benefit from screening mammography in their 50s. However, there is clear evidence of mortality benefit for a woman in either decade should she develop breast cancer.
We also discuss that the frequency of harms is fairly comparable in either decade, but women who choose to start screening at age 50 will obviously not experience any callbacks or screening-associated benign breast biopsies in their 40s. With this understanding of benefits and harms, most (but not all) average risk women in my practice choose to start screening at age 40.
Related articles:
Breast cancer screening: My practices and response to the USPSTF guidelines
Be mindful of study limitations
The study by Arleo and colleagues has several weaknesses.5
Simulation studies/computer models have limitations. They are only as accurate as the assumptions that are used in the model. However, CISNET modeling has the benefit of having 6 different models with different assumptions on mortality, efficacy of mammography, and efficacy of treatment, and Arleo and colleagues’ analysis takes the mean of these 6 different models.5 It is reassuring to know that the modeling results are consistent with virtually all studies that show that annual screening mammography has a mortality benefit for women in their 40s.
Cost differences are not included. The actual cost of differences between the strategies is difficult to calculate and was not analyzed in this study. While it is easy to calculate the “front end” costs in a study like this (for example, how many more mammograms or biopsies in the different strategies), it is very difficult to calculate the “back end” costs (such as avoided chemotherapy or end-of-life care).
Overtreatment and overdiagnosis have been discussed extensively with regard to the different screening strategies. For example, approximately 80% of women with ductal carcinoma in situ (DCIS) have these tumors detected on screening mammography, and DCIS is not an obligate precursor to invasive breast cancer. Because the natural history of DCIS cannot be predicted, treatment is recommended for all women with DCIS, even though many of these tumors will remain indolent and never cause harm. As a result, concerns have been raised that more intensive screening strategies may result in more overdiagnosis and overtreatment compared with less intensive strategies.
Increasingly, this argument has been questioned, since the prevailing thought is that DCIS does not regress or disappear on mammography. In other words, if DCIS is present at age 40, it will be detected whenever screening starts (age 40, 45, or 50), and age of starting screening or the screening interval will not impact overdiagnosis or overtreatment.7
Related articles:
More than one-third of tumors found on breast cancer screening represent overdiagnosis
Counsel patients, offer screening at age 40
While 3 different breast cancer mammography screening strategies are recommended in the United States, the study by Arleo and colleagues suggests that based on CISNET data, the A40–84 strategy appears to be the most effective at reducing breast cancer mortality and resulting in the most life-years gained. This strategy also requires the most lifetime mammograms and results in the most callbacks and benign biopsies. Women should be offered annual screening mammography starting at age 40 and should start no later than age 50 after receiving counseling about benefits and harms.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Cancer Facts & Figures 2017. American Cancer Society website. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Accessed October 4, 2017.
- Oeffinger KC, Frontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 179: Breast cancer risk assessment and screening in average risk women. Obstet Gynecol. 2017;130(1):e1–e16.
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(4):279–296.
- Arleo EK, Hendrick RE, Helvie MA, Sickles EA. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123(19):3673–3680.
- Women’s Preventive Services Initiative. Breast cancer screening for average-risk women. https://www.womenspreventivehealth.org/recommendations/breast-cancer-screening-for-average-risk-women/. Published 2016. Accessed October 4, 2017.
- Arleo EK, Monticciolo DL, Monsees B, McGinty G, Sickles EA. Persistent untreated screening-detected breast cancer: an argument against delaying screening or increasing the interval between screenings. J Am Coll Radiol. 2017;14(7):863–867.
Breast cancer is the most common cancer and the second leading cause of cancer death in women in the United States, with an estimated 252,710 new cases and 40,610 deaths in 2017.1 Breast cancer mortality is prevented by the use of regular screening mammography, as demonstrated by randomized controlled trials (20% reduction), incidence-based mortality studies (38% to 40% reduction), and service screening studies (48% to 49% reduction).2
Controversy continues, however, on when to start mammography screening, when to stop screening, and the frequency with which screening should be performed for women at average risk for breast cancer. Indeed, 3 national recommendations—written by the American College of Obstetricians and Gynecologists (ACOG), the American Cancer Society (ACS), and the US Preventive Services Task Force (USPSTF)—offer different guidelines for mammography screening (TABLE 1).2–4

There are 2 principal reasons for the controversy over screening:
- mammography has both benefits and harms, and individuals place differential weight on the importance of these relative to each other
- randomized controlled trials on screening mammography did not include all of the starting age, stopping age, and screening intervals that are included in screening recommendations.
New comparison of recommendations
An ongoing project funded by the National Cancer Institute, known as the Cancer Intervention and Surveillance Modeling Network (CISNET), models different starting and stopping ages and screening intervals for mammography to assess their impact on both benefits (mortality improvement, life-years gained) and harms (callbacks, benign breast biopsies). Recently, Arleo and colleagues used CISNET model data to compare the breast cancer screening recommendations from ACOG, the ACS, and the USPSTF, focusing on the differential effect on benefits and harms.5
Benefits vs harms of screening in perspective
Without question, the principal goal of cancer screening strategies is to effectively and efficiently reduce cancer mortality. Because mammography screening has both benefits and harms, a clear understanding of the relative frequency of these events among the different screening recommendations should be an important element in patient counseling.
Based on CISNET-modeled estimates, TABLE 2, illustrates the differences in both benefits and harms of the 3 screening strategies. With all strategies, there is a clear benefit in both fewer breast cancer–related deaths and life-years gained per 1,000 women screened.

The greatest benefit is seen in the A40–84 group, that is, women who undergo the most intensive screening strategy with annual screening starting at age 40 and ending at age 84 (ACOG) compared with the USPSTF’s least intensive screening strategy, B50–74, which includes biennial screening starting at age 50 and stopping at age 74; benefits of the ACS’s H45–79 strategy (annual screening at ages 45 to 54 years then biennial screening at ages 55 to 79) were in-between. Not surprisingly, the A40–84 screening strategy was also associated with the most harms, with more recalls and benign breast biopsies; the least harms occurred with the USPSTF strategy, with the ACS strategy again in-between in terms of harms.
Related articles:
Breast density and optimal screening for breast cancer
To further demonstrate differences between the 3 strategies, CISNET also modeled results by looking at all women born in a single birth year cohort (1960) who were still alive at age 40 (2.468 million women). The modeling estimates the number of women who would die from breast cancer without screening mammography and compares that with the number of women who would die from breast cancer using any of the 3 screening strategies. Using this 1960 birth year cohort analysis, there would be approximately 12,000 fewer breast cancer deaths using the ACOG-recommended screening strategy compared with the USPSTF-recommended approach.4
These data show that while there are more harms associated with the most intense screening recommendation, the less frequent screening recommendations will result in higher mortality and more life-years lost. It is reasonable to assume that most patients would value mortality reduction and life-years gained over a likelihood of more benign biopsies or callbacks. As a result, each of the guidelines recommends that by age 40, women at average risk for breast cancer should be counseled and offered mammography screening based on their personal values.
Read about how Dr. Pearlman counsels his patients on screening.
My counseling approach on screening
Notably, the Women’s Preventive Services Initiative recommends that average risk women initiate mammography screening no earlier than age 40 and no later than age 50.6 This creates more flexibility around starting time for screening. In the population of women that I personally counsel, we discuss that fewer women (1 in 68) will experience breast cancer in their 40s compared with in their 50s (1 in 43); therefore as a population, more women will benefit from screening mammography in their 50s. However, there is clear evidence of mortality benefit for a woman in either decade should she develop breast cancer.
We also discuss that the frequency of harms is fairly comparable in either decade, but women who choose to start screening at age 50 will obviously not experience any callbacks or screening-associated benign breast biopsies in their 40s. With this understanding of benefits and harms, most (but not all) average risk women in my practice choose to start screening at age 40.
Related articles:
Breast cancer screening: My practices and response to the USPSTF guidelines
Be mindful of study limitations
The study by Arleo and colleagues has several weaknesses.5
Simulation studies/computer models have limitations. They are only as accurate as the assumptions that are used in the model. However, CISNET modeling has the benefit of having 6 different models with different assumptions on mortality, efficacy of mammography, and efficacy of treatment, and Arleo and colleagues’ analysis takes the mean of these 6 different models.5 It is reassuring to know that the modeling results are consistent with virtually all studies that show that annual screening mammography has a mortality benefit for women in their 40s.
Cost differences are not included. The actual cost of differences between the strategies is difficult to calculate and was not analyzed in this study. While it is easy to calculate the “front end” costs in a study like this (for example, how many more mammograms or biopsies in the different strategies), it is very difficult to calculate the “back end” costs (such as avoided chemotherapy or end-of-life care).
Overtreatment and overdiagnosis have been discussed extensively with regard to the different screening strategies. For example, approximately 80% of women with ductal carcinoma in situ (DCIS) have these tumors detected on screening mammography, and DCIS is not an obligate precursor to invasive breast cancer. Because the natural history of DCIS cannot be predicted, treatment is recommended for all women with DCIS, even though many of these tumors will remain indolent and never cause harm. As a result, concerns have been raised that more intensive screening strategies may result in more overdiagnosis and overtreatment compared with less intensive strategies.
Increasingly, this argument has been questioned, since the prevailing thought is that DCIS does not regress or disappear on mammography. In other words, if DCIS is present at age 40, it will be detected whenever screening starts (age 40, 45, or 50), and age of starting screening or the screening interval will not impact overdiagnosis or overtreatment.7
Related articles:
More than one-third of tumors found on breast cancer screening represent overdiagnosis
Counsel patients, offer screening at age 40
While 3 different breast cancer mammography screening strategies are recommended in the United States, the study by Arleo and colleagues suggests that based on CISNET data, the A40–84 strategy appears to be the most effective at reducing breast cancer mortality and resulting in the most life-years gained. This strategy also requires the most lifetime mammograms and results in the most callbacks and benign biopsies. Women should be offered annual screening mammography starting at age 40 and should start no later than age 50 after receiving counseling about benefits and harms.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Breast cancer is the most common cancer and the second leading cause of cancer death in women in the United States, with an estimated 252,710 new cases and 40,610 deaths in 2017.1 Breast cancer mortality is prevented by the use of regular screening mammography, as demonstrated by randomized controlled trials (20% reduction), incidence-based mortality studies (38% to 40% reduction), and service screening studies (48% to 49% reduction).2
Controversy continues, however, on when to start mammography screening, when to stop screening, and the frequency with which screening should be performed for women at average risk for breast cancer. Indeed, 3 national recommendations—written by the American College of Obstetricians and Gynecologists (ACOG), the American Cancer Society (ACS), and the US Preventive Services Task Force (USPSTF)—offer different guidelines for mammography screening (TABLE 1).2–4

There are 2 principal reasons for the controversy over screening:
- mammography has both benefits and harms, and individuals place differential weight on the importance of these relative to each other
- randomized controlled trials on screening mammography did not include all of the starting age, stopping age, and screening intervals that are included in screening recommendations.
New comparison of recommendations
An ongoing project funded by the National Cancer Institute, known as the Cancer Intervention and Surveillance Modeling Network (CISNET), models different starting and stopping ages and screening intervals for mammography to assess their impact on both benefits (mortality improvement, life-years gained) and harms (callbacks, benign breast biopsies). Recently, Arleo and colleagues used CISNET model data to compare the breast cancer screening recommendations from ACOG, the ACS, and the USPSTF, focusing on the differential effect on benefits and harms.5
Benefits vs harms of screening in perspective
Without question, the principal goal of cancer screening strategies is to effectively and efficiently reduce cancer mortality. Because mammography screening has both benefits and harms, a clear understanding of the relative frequency of these events among the different screening recommendations should be an important element in patient counseling.
Based on CISNET-modeled estimates, TABLE 2, illustrates the differences in both benefits and harms of the 3 screening strategies. With all strategies, there is a clear benefit in both fewer breast cancer–related deaths and life-years gained per 1,000 women screened.

The greatest benefit is seen in the A40–84 group, that is, women who undergo the most intensive screening strategy with annual screening starting at age 40 and ending at age 84 (ACOG) compared with the USPSTF’s least intensive screening strategy, B50–74, which includes biennial screening starting at age 50 and stopping at age 74; benefits of the ACS’s H45–79 strategy (annual screening at ages 45 to 54 years then biennial screening at ages 55 to 79) were in-between. Not surprisingly, the A40–84 screening strategy was also associated with the most harms, with more recalls and benign breast biopsies; the least harms occurred with the USPSTF strategy, with the ACS strategy again in-between in terms of harms.
Related articles:
Breast density and optimal screening for breast cancer
To further demonstrate differences between the 3 strategies, CISNET also modeled results by looking at all women born in a single birth year cohort (1960) who were still alive at age 40 (2.468 million women). The modeling estimates the number of women who would die from breast cancer without screening mammography and compares that with the number of women who would die from breast cancer using any of the 3 screening strategies. Using this 1960 birth year cohort analysis, there would be approximately 12,000 fewer breast cancer deaths using the ACOG-recommended screening strategy compared with the USPSTF-recommended approach.4
These data show that while there are more harms associated with the most intense screening recommendation, the less frequent screening recommendations will result in higher mortality and more life-years lost. It is reasonable to assume that most patients would value mortality reduction and life-years gained over a likelihood of more benign biopsies or callbacks. As a result, each of the guidelines recommends that by age 40, women at average risk for breast cancer should be counseled and offered mammography screening based on their personal values.
Read about how Dr. Pearlman counsels his patients on screening.
My counseling approach on screening
Notably, the Women’s Preventive Services Initiative recommends that average risk women initiate mammography screening no earlier than age 40 and no later than age 50.6 This creates more flexibility around starting time for screening. In the population of women that I personally counsel, we discuss that fewer women (1 in 68) will experience breast cancer in their 40s compared with in their 50s (1 in 43); therefore as a population, more women will benefit from screening mammography in their 50s. However, there is clear evidence of mortality benefit for a woman in either decade should she develop breast cancer.
We also discuss that the frequency of harms is fairly comparable in either decade, but women who choose to start screening at age 50 will obviously not experience any callbacks or screening-associated benign breast biopsies in their 40s. With this understanding of benefits and harms, most (but not all) average risk women in my practice choose to start screening at age 40.
Related articles:
Breast cancer screening: My practices and response to the USPSTF guidelines
Be mindful of study limitations
The study by Arleo and colleagues has several weaknesses.5
Simulation studies/computer models have limitations. They are only as accurate as the assumptions that are used in the model. However, CISNET modeling has the benefit of having 6 different models with different assumptions on mortality, efficacy of mammography, and efficacy of treatment, and Arleo and colleagues’ analysis takes the mean of these 6 different models.5 It is reassuring to know that the modeling results are consistent with virtually all studies that show that annual screening mammography has a mortality benefit for women in their 40s.
Cost differences are not included. The actual cost of differences between the strategies is difficult to calculate and was not analyzed in this study. While it is easy to calculate the “front end” costs in a study like this (for example, how many more mammograms or biopsies in the different strategies), it is very difficult to calculate the “back end” costs (such as avoided chemotherapy or end-of-life care).
Overtreatment and overdiagnosis have been discussed extensively with regard to the different screening strategies. For example, approximately 80% of women with ductal carcinoma in situ (DCIS) have these tumors detected on screening mammography, and DCIS is not an obligate precursor to invasive breast cancer. Because the natural history of DCIS cannot be predicted, treatment is recommended for all women with DCIS, even though many of these tumors will remain indolent and never cause harm. As a result, concerns have been raised that more intensive screening strategies may result in more overdiagnosis and overtreatment compared with less intensive strategies.
Increasingly, this argument has been questioned, since the prevailing thought is that DCIS does not regress or disappear on mammography. In other words, if DCIS is present at age 40, it will be detected whenever screening starts (age 40, 45, or 50), and age of starting screening or the screening interval will not impact overdiagnosis or overtreatment.7
Related articles:
More than one-third of tumors found on breast cancer screening represent overdiagnosis
Counsel patients, offer screening at age 40
While 3 different breast cancer mammography screening strategies are recommended in the United States, the study by Arleo and colleagues suggests that based on CISNET data, the A40–84 strategy appears to be the most effective at reducing breast cancer mortality and resulting in the most life-years gained. This strategy also requires the most lifetime mammograms and results in the most callbacks and benign biopsies. Women should be offered annual screening mammography starting at age 40 and should start no later than age 50 after receiving counseling about benefits and harms.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Cancer Facts & Figures 2017. American Cancer Society website. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Accessed October 4, 2017.
- Oeffinger KC, Frontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 179: Breast cancer risk assessment and screening in average risk women. Obstet Gynecol. 2017;130(1):e1–e16.
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(4):279–296.
- Arleo EK, Hendrick RE, Helvie MA, Sickles EA. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123(19):3673–3680.
- Women’s Preventive Services Initiative. Breast cancer screening for average-risk women. https://www.womenspreventivehealth.org/recommendations/breast-cancer-screening-for-average-risk-women/. Published 2016. Accessed October 4, 2017.
- Arleo EK, Monticciolo DL, Monsees B, McGinty G, Sickles EA. Persistent untreated screening-detected breast cancer: an argument against delaying screening or increasing the interval between screenings. J Am Coll Radiol. 2017;14(7):863–867.
- Cancer Facts & Figures 2017. American Cancer Society website. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Accessed October 4, 2017.
- Oeffinger KC, Frontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 179: Breast cancer risk assessment and screening in average risk women. Obstet Gynecol. 2017;130(1):e1–e16.
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(4):279–296.
- Arleo EK, Hendrick RE, Helvie MA, Sickles EA. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123(19):3673–3680.
- Women’s Preventive Services Initiative. Breast cancer screening for average-risk women. https://www.womenspreventivehealth.org/recommendations/breast-cancer-screening-for-average-risk-women/. Published 2016. Accessed October 4, 2017.
- Arleo EK, Monticciolo DL, Monsees B, McGinty G, Sickles EA. Persistent untreated screening-detected breast cancer: an argument against delaying screening or increasing the interval between screenings. J Am Coll Radiol. 2017;14(7):863–867.
Genital herpes: Diagnostic and management considerations in pregnant women
Genital herpes is a common infection caused by herpes simplex virus type 1 (HSV-1) or herpes simplex virus type 2 (HSV-2). Although life-threatening health consequences of HSV infection after infancy are uncommon, women with genital herpes remain at risk for recurrent symptoms, which can be associated with significant physical and psychosocial distress. These patients also can transmit the disease to their partners and neonates, and have a 2- to 3-fold increased risk of HIV acquisition. In this article, we review the diagnosis and management of genital herpes in pregnant women.
CASE Asymptomatic pregnant patient tests positive for herpes
Sarah is a healthy 32-year-old (G1P0) presenting at 8 weeks’ gestation for her first prenatal visit. She requests HSV testing as she learned that genital herpes is common and it can be transmitted to the baby. You order the HSV-2 IgG assay from your laboratory, which performs the HerpeSelect HSV-2 enzyme immunoassay as the standard test. The test result is positive, with an index value of 2.2 (the manufacturer defines an index value >1.1 as positive). Repeat testing in 4 weeks returns positive results again, with an index value of 2.8.
The patient is distressed at this news. She has no history of genital lesions or symptoms consistent with genital herpes and is worried that her husband has been unfaithful. How would you manage this case?
How prevalent is HSV?
Genital herpes is a chronic viral infection transmitted through close contact with a person who is shedding the virus from genital or oral mucosa. In the United States, the National Health and Nutrition Examination Survey indicated an HSV-2 seroprevalence of 16% among persons aged 14 to 49 in 2005–2010, a decline from 21% in 1988–1991.1 The prevalence among women is twice as high as among men, at 20% versus 11%, respectively. Among those with HSV-2, 87% are not aware that they are infected; they are at risk of infecting their partners, however.1
In the same age group, the prevalence of HSV-1 is 54%.2 The seroprevalence of HSV-1 in adolescents declined from 39% in 1999–2004 to 30% in 2005–2010, resulting in a high number of young people who are seronegative at the time of sexual debut. Concurrently, genital HSV-1 has emerged as a frequent cause of first-episode genital herpes, often associated with oral-genital contact during sexual debut.2,3
When evaluating patients for possible genital herpes provide general educational messages regarding HSV infection and obtain a detailed medical and sexual history to determine the best diagnostic approach.
What are the clinical features of genital HSV infection?
The clinical manifestations of genital herpes vary according to whether the infection is primary, nonprimary first episode, or recurrent.
Primary infection. During primary infection,which occurs 4 to 12 days after sexual exposure and in the absence of pre-existing antibodies to HSV-1 or HSV-2, patients may experience genital and systemic symptoms (FIGURE and TABLE 1). Since this infection usually occurs in otherwise healthy people, for many, this is the most severe disease that they have experienced. However, most patients with primary infection develop mild, atypical, or completely asymptomatic presentation and are not diagnosed at the time of HSV acquisition. Whether primary infection is caused by HSV-1 or HSV-2 cannot be differentiated based on the clinical presentation alone.
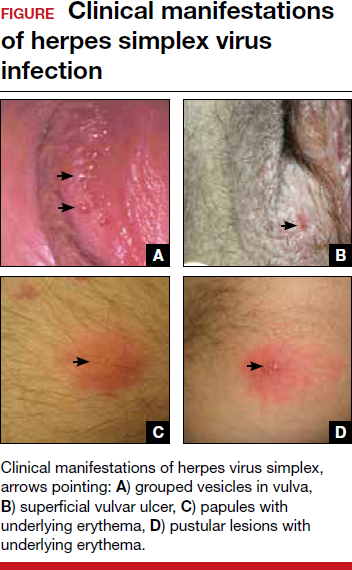
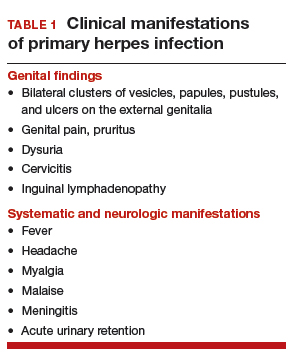
Nonprimary first episode infection. In a nonprimary infection, newly acquired infection with HSV-1 or HSV-2 occurs in a person with pre-existing antibodies to the other virus. Almost always, this means new HSV-2 infection in a HSV-1 seropositive person, as prior HSV-2 infection appears to protect against HSV-1 acquisition. In general, the clinical presentation of nonprimary infection is somewhat milder and the rate of complications is lower, but clinically the overlap is great, and antibody tests are needed to define whether the patient has primary or nonprimary infection.4
Recurrent genital herpes infection occurs in most patients with genital herpes. The rate of recurrence is low in patients with genital HSV-1 and often high in patients with genital HSV-2 infection. The median number of recurrences is 1 in the first year of genital HSV-1 infection, and many patients will not have any recurrences following the first year. By contrast, in patients with genital HSV-2 infection, the median number of recurrences is 4, and a high rate of recurrences can continue for many years. Prodromal symptoms (localized irritation, paresthesias, and pruritus) can precede recurrences, which usually present with fewer lesions and last a shorter time than primary infection. Recurrent genital lesions tend to heal in approximately 5 to 10 days in the absence of antiviral treatment, and systemic symptoms are uncommon.5
Asymptomatic viral shedding. After resolution of a primary HSV infection, people shed the virus in the genital tract despite symptom absence. Asymptomatic shedding tends to be more frequent and prolonged with primary genital HSV-2 infection compared with HSV-1 infection.6,7 The frequency of HSV shedding is highest in the first year of infection, and decreases subsequently.8 However, it is likely to persist intermittently for many years. Because the natural history is so strikingly different in genital HSV-1 versus HSV-2, identification of the viral type is important for prognostic information.
The first HSV episode does not necessarily indicate a new or recent infection—in about 25% of persons it represents the first recognized genital herpes episode. Additional serologic and virologic evaluation can be pursued to determine if the first episode represents a new infection.
Read about the diagnostic tests for genital HSV.
What diagnostic tests are available for genital herpes?
Most HSV infections are clinically silent. Therefore, laboratory tests are required to diagnose the infection. Even if symptoms are present, diagnoses based only on clinical presentation have a 20% false-positive rate. Always confirm diagnosis by laboratory assay.9 Furthermore, couples that are discordant for HSV-2 by history are often concordant by serologic assays, as the transmission already has occurred but was not recognized. In these cases, the direction of transmission cannot be determined, and stable couples often experience relief learning that they are not discordant.
Related article:
Effective treatment of recurrent bacterial vaginosis
Several laboratory tools for HSV diagnosis based on direct viral detection and antibody detection can be used in clinical settings (TABLE 2). Among patients with symptomatic genital herpes, a sample from the lesion can be used to confirm and identify viral type. Because polymerase chain reaction (PCR) is substantially more sensitive than viral culture and increasingly available it has emerged as the preferred test.9 Viral culture is highly specific (>99%), but sensitivity varies according to collection technique and stage of the lesions. (The test is less sensitive when lesions are healing.)9,10 Antigen detection by immunofluorescence (direct fluorescent antibody) detects HSV from active lesions with high specificity, but sensitivity is low. Cytologic identification of infected cells (using Tzanck or Pap test) has limited utility for diagnosis due to low sensitivity and specificity.9
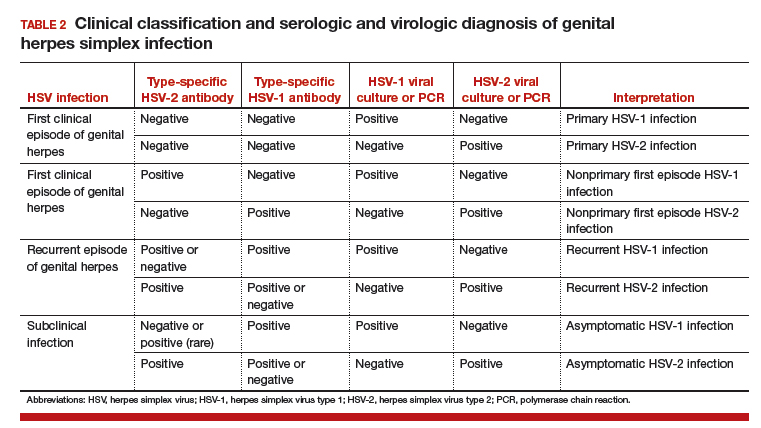
Type-specific antibodies to HSV develop during the first several weeks after acquisition and persist indefinitely.11 Most accurate type-specific serologic tests are based on detection of glycoprotein G1 and glycoprotein G2 for HSV-1 and HSV-2, respectively.
HerpeSelect HSV-2 enzyme immunoassay (EIA) is one of the most commonly used tests in the United States. The manufacturer considers results with index values 1.1 or greater as showing HSV-2 infection. Unfortunately, low positive results, often with a defined index value of 1.1 to 3.5, are frequently false positive. These low positive values should be confirmed with another test, such as Western blot.9
Western blot has been considered the gold standard assay for HSV-1 and HSV-2 antibody detection; this test is available at the University of Washington in Seattle. When comparing the HSV-1 EIA and HSV-2 EIA with the Western blot assay in clinical practice, the estimated sensitivity and specificity are 70.2% and 91.6%, respectively, for HSV-1 and 91.9% and 57.4%, respectively, for HSV-2.12
HerpeSelect HSV-2 Immunoblot testing should not be considered as confirmatory because this assay detects the same antigen as the HSV-2 EIA. Serologic tests based on detection of HSV-IgM should not be used for diagnosis of genital herpes as IgM response can present during a new infection or HSV reactivation and because IgM responses are not type-specific. Clearly, more accurate commercial type-specific antibody tests are needed.
Specific HSV antibodies can take up to 12 weeks to develop. Therefore, repeat serologic testing for patients in whom initial HSV antibody results are negative yet recent genital herpes acquisition is suspected.11 A confirmed positive HSV-2 antibody test indicates anogenital infection, even in a person who lacks genital symptoms. This finding became evident through a study of 53 HSV-2 seropositive patients who lacked a history of genital herpes. Patients were followed for 3 months, and all but 1 developed either virologic or clinical (or both) evidence of genital herpes.13
In the absence of genital or orolabial symptoms among individuals with positive HSV-1, serologic testing cannot distinguish anogenital from orolabial infection. Most of these infections may represent oral HSV-1 infection; however, given increasing occurrence of genital HSV-1 infection, this could also represent a genital infection.
What are the clinical uses of type-specific HSV serology?
Type-specific serologic tests are helpful in diagnosing patients with atypical or asymptomatic infection and managing the care of persons whose sex partners have genital herpes. Serologic testing can be useful to confirm a clinical diagnosis of HSV, to determine whether atypical lesions or symptoms are attributable to HSV, and as part of evaluation for sexually transmitted diseases in select patients. Screening for HSV-1 and HSV-2 in the general population is not supported by the Centers for Disease Control and Prevention (CDC) or the US Preventive Services Task Force (USPSTF) for several reasons9,10:
- suboptimal performance of commercial HSV antibody tests
- low positive predictive value of these tests in low prevalence HSV settings
- lack of widely available confirmatory testing
- lack of cost-effectiveness
- potential for psychological harm.
Read about treating HSV infection during pregnancy.
Case Continued…
Because Sarah did not have a history of genital herpes, a serum sample was tested by the University of Washington Western blot. The results indicated that Sarah is seronegative for HSV-1 and HSV-2.
Sarah, who is now at 16 weeks’ gestation, returns for evaluation of new genital pain. On examination, she has several shallow ulcerations on the labia and bilateral tender inguinal adenopathy. Her husband recently had cold sores. She is anxious and would like to know if she has genital herpes and if her baby is at risk for HSV infection. You swab the base of a lesion for HSV PCR testing and start antiviral treatment.
Treating HSV infection during pregnancy
Women presenting with a new genital ulcer consistent with HSV should receive empiric antiviral treatment while awaiting confirmatory diagnostic laboratory testing, even during pregnancy. Antiviral therapy with acyclovir, valacyclovir, and famciclovir is the backbone of management of most symptomatic patients with herpes. Antiviral drugs can reduce signs and symptoms of first or recurrent genital herpes and can be used for daily suppressive therapy to prevent recurrences. These drugs do not eradicate the infection or alter the risk of frequency or severity after the drug is discontinued.
Antiviral advantages/disadvantages. Acyclovir is the least expensive drug, but valacyclovir is the most convenient therapy given its less frequent dosing. Acyclovir and valacyclovir are equally efficacious in treating first-episode genital herpes infection with respect to duration of viral shedding, time of healing, duration of pain, and time to symptom clearance. Two randomized clinical trials showed similar benefits of acyclovir and valacyclovir for suppressive therapy management of genital herpes.14,15 Only 1 study compared the efficacy of famciclovir to valacyclovir for suppression and showed that valacyclovir was more effective.16 The cost of famciclovir is usually higher, and it has the least data on use in pregnant women. Acyclovir therapy can be safely used throughout pregnancy and during breastfeeding.9 Antiviral regimens for the treatment of genital HSV in pregnant and nonpregnant women recommended by the CDC are summarized in TABLE 3.17
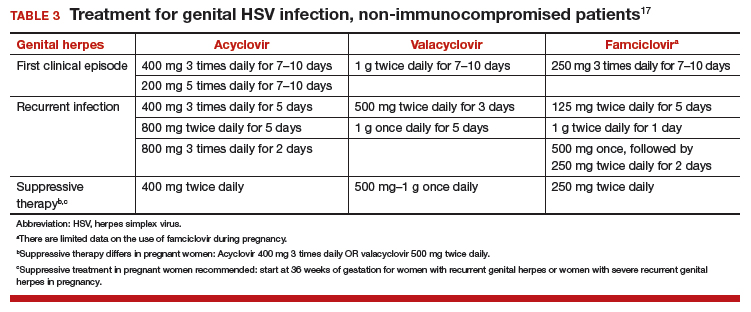
Related article:
5 ways to reduce infection risk during pregnancy
Will your patient’s infant develop neonatal herpes infection?
Neonatal herpes is a potentially devastating infection that results from exposure to HSV from the maternal genital tract at vaginal delivery. Most cases occur in infants born to women who lack a history of genital herpes.18 In a large cohort study conducted in Washington State, isolation of HSV at the time of labor was strongly associated with vertical transmission (odds ratio [OR], 346).19 The risk of neonatal herpes increased among women shedding HSV-1 compared with HSV-2 (OR, 16.5). The highest risk of transmission to the neonate is in women who acquire genital herpes in a period close to the delivery (30% to 50% risk of transmission), compared with women with a prenatal history of herpes or who acquired herpes early in pregnancy (about 1% to 3% risk of transmission), most likely due to protective HSV-specific maternal antibodies and lower viral load during reactivation versus primary infection.18
Neonatal HSV-1 infection also has been reported in neonates born to women with primary HSV-1 gingivostomatitis during pregnancy; 70% of these women had oral clinical symptoms during the peripartum period.20 Potential mechanisms are exposure to infected genital secretions, direct maternal hematogenous spread, or oral shedding from close contacts.
Although prenatal HSV screening is not recommended by the CDC or USPSTF, serologic testing could be helpful when identifying appropriate pregnancy management for women with a prior history of HSV infection. It also could be beneficial in identifying women without HSV to guide counseling prevention for HSV acquisition. In patients presenting with active genital lesions, viral-specific diagnostic evaluation should be obtained. In those with a history of laboratory confirmed genital herpes, no additional testing is warranted.
Preventing neonatal herpes
There are no prevention strategies for neonatal herpes in the United States, and the incidence of neonatal herpes has not changed in several decades.10 The current treatment guidelines focus on managing women who may be at risk for HSV acquisition during pregnancy and the management of genital lesions in women during pregnancy.9,10,21
When the partner has HSV. Women who have no history of genital herpes or who are seronegative for HSV-2 should avoid intercourse during the third trimester with a partner known to have genital herpes.9 Those who have no history of orolabial herpes or who are seronegative for HSV-1 and have a seropositive partner should avoid receptive oral-genital contact and genital intercourse.9 Condoms can reduce but not eliminate the risk of HSV transmission; to effectively avoid genital herpes infection, abstinence is recommended.
When the patient has HSV. When managing the care of a pregnant woman with genital herpes evaluate for clinical symptoms and timing of infection or recurrence relative to time of delivery:
- Monitor women with a mild recurrence of HSV during the first 35 weeks of pregnancy without antiviral treatment, as most of the recurrent episodes of genital herpes are short.
- Consider antivirals for women with severe symptoms or multiple recurrences.
- Offer women with a history of genital lesions suppressive antiviral therapy at 36 weeks of gestation until delivery.21
In a meta-analysis of 7 randomized trials, 1,249 women with a history of genital herpes prior to or during pregnancy received prophylaxis with either acyclovir or valacyclovir versus placebo or no treatment at 36 weeks of gestation. Antiviral therapy reduced the risk of HSV recurrence at delivery (relative risk [RR], 0.28), cesarean delivery in those with recurrent genital herpes (RR, 0.3), and asymptomatic shedding at delivery (RR, 0.14).22 No data are available regarding the effectiveness of this approach to prevention of neonatal HSV, and case reports confirm neonatal HSV in infants born to women who received suppressive antiviral therapy at the end of pregnancy.23
When cesarean delivery is warranted. At the time of delivery, ask all women about symptoms of genital herpes, including prodromal symptoms, and examine them for genital lesions. For women with active lesions or prodromal symptoms, offer cesarean delivery at the onset of labor or rupture of membranes—this recommendation is supported by the CDC and the American College of Obstetricians and Gynecologists.9,21 The protective effect of cesarean delivery was evaluated in a large cohort study that found: among women who were shedding HSV at the time of delivery, neonates born by cesarean delivery were less likely to develop HSV infection compared with those born through vaginal delivery (1.2% vs 7.7%, respectively).19 Cesarean delivery is not indicated in patients with a history of HSV without clinical recurrence or prodrome at delivery, as such women have a very low risk of transmitting the infection to the neonate.24
Avoid transcervical antepartum obstetric procedures to reduce the risk of placenta or membrane HSV infection; however, transabdominal invasive procedures can be performed safely, even in the presence of active genital lesions.21 Intrapartum procedures that can cause fetal skin disruption, such as use of fetal scalp electrode or forceps, are risk factors for HSV transmission and should be avoided in women with a history of genital herpes.
Related articles:
8 common questions about newborn circumcision
Case Resolved
Sarah’s genital lesion PCR results returned positive for HSV-1. She probably acquired the infection from oral-genital sex with her husband who likely has oral HSV-1, given the history of cold sores. You treat Sarah with acyclovir 400 mg 3 times per day for 7 days. At 36 weeks’ gestation, Sarah begins suppressive antiviral therapy until delivery. She spontaneously labors at 39 weeks’ gestation; at that time, she has no genital lesions and she delivers vaginally a healthy baby.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Fanfair RN, Zaidi A, Taylor LD, Xu F, Gottlieb S, Markowitz L. Trends in seroprevalence of herpes simplex virus type 2 among non-Hispanic blacks and non-Hispanic whites aged 14 to 49 years–United States, 1988 to 2010. Sex Transm Dis. 2013;40(11):860–864.
- Bradley H, Markowitz LE, Gibson T, McQuillan GM. Seroprevalence of herpes simplex virus types 1 and 2–United States, 1999-2010. J Infect Dis. 2014;209(3):325–333.
- Bernstein DI, Bellamy AR, Hook EW, 3rd, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infect Dis. 2013;56(3):344–351.
- Kimberlin DW, Rouse DJ. Clinical practice. Genital herpes. N Engl J Med. 2004;350(19):1970–1977.
- Corey L, Adams HG, Brown ZA, Holmes KK. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med. 1983;98(6):958–972.
- Wald A, Zeh J, Selke S, Ashley RL, Corey L. Virologic characteristics of subclinical and symptomatic genital herpes infections. N Engl J Med. 1995;333(12):770–775.
- Reeves WC, Corey L, Adams HG, Vontver LA, Holmes KK. Risk of recurrence after first episodes of genital herpes. Relation to HSV type and antibody response. N Engl J Med. 1981;305(6):315–319.
- Phipps W, Saracino M, Magaret A, et al. Persistent genital herpes simplex virus-2 shedding years following the first clinical episode. J Infect Dis. 2011;203(2):180–187.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1–137.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Task Force. Serologic screening for genital herpes infection: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316(23):2525–2530.
- Gupta R, Warren T, Wald A. Genital herpes. Lancet. 2007;370(9605):2127–2137.
- Agyemang E, Le QA, Warren T, et al. Performance of commercial enzyme-linked immunoassays 1 (EIA) for diagnosis of herpes simplex virus-1 and herpes simplex virus-2 infection in a clinical setting. Sex Transm Dis. 2017; doi:10.1097/olq.0000000000000689.
- Wald A, Zeh J, Selke S, et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med. 2000;342(12):844–850.
- Gupta R, Wald A, Krantz E, et al. Valacyclovir and acyclovir for suppression of shedding of herpes simplex virus in the genital tract. J Infect Dis. 2004;190(8):1374–1381.
- Reitano M, Tyring S, Lang W, et al. Valaciclovir for the suppression of recurrent genital herpes simplex virus infection: a large-scale dose range-finding study. International Valaciclovir HSV Study Group. J Infect Dis. 1998;178(3): 603–610.
- Wald A, Selke S, Warren T, et al. Comparative efficacy of famciclovir and valacyclovir for suppression of recurrent genital herpes and viral shedding. Sex Transm Dis. 2006;33(9):529–533.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015 [published correction appears in MMWR Recomm Rep. 2015;64(33):924]. MMWR Recomm Rep. 2015;64(RR-03):1–137.
- Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med. 2009;361(14):1376–1385.
- Brown ZA, Wald A, Morrow RA, Selke S, Zeh J, Corey L. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289(2):203–209.
- Healy SA, Mohan KM, Melvin AJ, Wald A. Primary maternal herpes simplex virus-1 gingivostomatitis during pregnancy and neonatal herpes: case series and literature review. J Pediatric Infect Dis Soc. 2012;1(4):299–305.
- American College of Obstetricians and Gynecoloigsts Committee on Practice Bulletins. ACOG Practice Bulletin No. 82: Management of herpes in pregnancy. Obstet Gynecol. 2007;109(6):1489–1498.
- Hollier LM, Wendel GD. Third trimester antiviral prophylaxis for preventing maternal genital herpes simplex virus (HSV) recurrences and neonatal infection. Cochrane Database Syst Rev. 2008(1):CD004946.
- Pinninti SG, Angara R, Feja KN, et al. Neonatal herpes disease following maternal antenatal antiviral suppressive therapy: a multicenter case series. J Pediatr. 2012;161(1):134–138.e1–e3.
- Vontver LA, Hickok DE, Brown Z, Reid L, Corey L. Recurrent genital herpes simplex virus infection in pregnancy: infant outcome and frequency of asymptomatic recurrences. American journal of obstetrics and gynecology. 1982;143(1):75–84.
Genital herpes is a common infection caused by herpes simplex virus type 1 (HSV-1) or herpes simplex virus type 2 (HSV-2). Although life-threatening health consequences of HSV infection after infancy are uncommon, women with genital herpes remain at risk for recurrent symptoms, which can be associated with significant physical and psychosocial distress. These patients also can transmit the disease to their partners and neonates, and have a 2- to 3-fold increased risk of HIV acquisition. In this article, we review the diagnosis and management of genital herpes in pregnant women.
CASE Asymptomatic pregnant patient tests positive for herpes
Sarah is a healthy 32-year-old (G1P0) presenting at 8 weeks’ gestation for her first prenatal visit. She requests HSV testing as she learned that genital herpes is common and it can be transmitted to the baby. You order the HSV-2 IgG assay from your laboratory, which performs the HerpeSelect HSV-2 enzyme immunoassay as the standard test. The test result is positive, with an index value of 2.2 (the manufacturer defines an index value >1.1 as positive). Repeat testing in 4 weeks returns positive results again, with an index value of 2.8.
The patient is distressed at this news. She has no history of genital lesions or symptoms consistent with genital herpes and is worried that her husband has been unfaithful. How would you manage this case?
How prevalent is HSV?
Genital herpes is a chronic viral infection transmitted through close contact with a person who is shedding the virus from genital or oral mucosa. In the United States, the National Health and Nutrition Examination Survey indicated an HSV-2 seroprevalence of 16% among persons aged 14 to 49 in 2005–2010, a decline from 21% in 1988–1991.1 The prevalence among women is twice as high as among men, at 20% versus 11%, respectively. Among those with HSV-2, 87% are not aware that they are infected; they are at risk of infecting their partners, however.1
In the same age group, the prevalence of HSV-1 is 54%.2 The seroprevalence of HSV-1 in adolescents declined from 39% in 1999–2004 to 30% in 2005–2010, resulting in a high number of young people who are seronegative at the time of sexual debut. Concurrently, genital HSV-1 has emerged as a frequent cause of first-episode genital herpes, often associated with oral-genital contact during sexual debut.2,3
When evaluating patients for possible genital herpes provide general educational messages regarding HSV infection and obtain a detailed medical and sexual history to determine the best diagnostic approach.
What are the clinical features of genital HSV infection?
The clinical manifestations of genital herpes vary according to whether the infection is primary, nonprimary first episode, or recurrent.
Primary infection. During primary infection,which occurs 4 to 12 days after sexual exposure and in the absence of pre-existing antibodies to HSV-1 or HSV-2, patients may experience genital and systemic symptoms (FIGURE and TABLE 1). Since this infection usually occurs in otherwise healthy people, for many, this is the most severe disease that they have experienced. However, most patients with primary infection develop mild, atypical, or completely asymptomatic presentation and are not diagnosed at the time of HSV acquisition. Whether primary infection is caused by HSV-1 or HSV-2 cannot be differentiated based on the clinical presentation alone.


Nonprimary first episode infection. In a nonprimary infection, newly acquired infection with HSV-1 or HSV-2 occurs in a person with pre-existing antibodies to the other virus. Almost always, this means new HSV-2 infection in a HSV-1 seropositive person, as prior HSV-2 infection appears to protect against HSV-1 acquisition. In general, the clinical presentation of nonprimary infection is somewhat milder and the rate of complications is lower, but clinically the overlap is great, and antibody tests are needed to define whether the patient has primary or nonprimary infection.4
Recurrent genital herpes infection occurs in most patients with genital herpes. The rate of recurrence is low in patients with genital HSV-1 and often high in patients with genital HSV-2 infection. The median number of recurrences is 1 in the first year of genital HSV-1 infection, and many patients will not have any recurrences following the first year. By contrast, in patients with genital HSV-2 infection, the median number of recurrences is 4, and a high rate of recurrences can continue for many years. Prodromal symptoms (localized irritation, paresthesias, and pruritus) can precede recurrences, which usually present with fewer lesions and last a shorter time than primary infection. Recurrent genital lesions tend to heal in approximately 5 to 10 days in the absence of antiviral treatment, and systemic symptoms are uncommon.5
Asymptomatic viral shedding. After resolution of a primary HSV infection, people shed the virus in the genital tract despite symptom absence. Asymptomatic shedding tends to be more frequent and prolonged with primary genital HSV-2 infection compared with HSV-1 infection.6,7 The frequency of HSV shedding is highest in the first year of infection, and decreases subsequently.8 However, it is likely to persist intermittently for many years. Because the natural history is so strikingly different in genital HSV-1 versus HSV-2, identification of the viral type is important for prognostic information.
The first HSV episode does not necessarily indicate a new or recent infection—in about 25% of persons it represents the first recognized genital herpes episode. Additional serologic and virologic evaluation can be pursued to determine if the first episode represents a new infection.
Read about the diagnostic tests for genital HSV.
What diagnostic tests are available for genital herpes?
Most HSV infections are clinically silent. Therefore, laboratory tests are required to diagnose the infection. Even if symptoms are present, diagnoses based only on clinical presentation have a 20% false-positive rate. Always confirm diagnosis by laboratory assay.9 Furthermore, couples that are discordant for HSV-2 by history are often concordant by serologic assays, as the transmission already has occurred but was not recognized. In these cases, the direction of transmission cannot be determined, and stable couples often experience relief learning that they are not discordant.
Related article:
Effective treatment of recurrent bacterial vaginosis
Several laboratory tools for HSV diagnosis based on direct viral detection and antibody detection can be used in clinical settings (TABLE 2). Among patients with symptomatic genital herpes, a sample from the lesion can be used to confirm and identify viral type. Because polymerase chain reaction (PCR) is substantially more sensitive than viral culture and increasingly available it has emerged as the preferred test.9 Viral culture is highly specific (>99%), but sensitivity varies according to collection technique and stage of the lesions. (The test is less sensitive when lesions are healing.)9,10 Antigen detection by immunofluorescence (direct fluorescent antibody) detects HSV from active lesions with high specificity, but sensitivity is low. Cytologic identification of infected cells (using Tzanck or Pap test) has limited utility for diagnosis due to low sensitivity and specificity.9

Type-specific antibodies to HSV develop during the first several weeks after acquisition and persist indefinitely.11 Most accurate type-specific serologic tests are based on detection of glycoprotein G1 and glycoprotein G2 for HSV-1 and HSV-2, respectively.
HerpeSelect HSV-2 enzyme immunoassay (EIA) is one of the most commonly used tests in the United States. The manufacturer considers results with index values 1.1 or greater as showing HSV-2 infection. Unfortunately, low positive results, often with a defined index value of 1.1 to 3.5, are frequently false positive. These low positive values should be confirmed with another test, such as Western blot.9
Western blot has been considered the gold standard assay for HSV-1 and HSV-2 antibody detection; this test is available at the University of Washington in Seattle. When comparing the HSV-1 EIA and HSV-2 EIA with the Western blot assay in clinical practice, the estimated sensitivity and specificity are 70.2% and 91.6%, respectively, for HSV-1 and 91.9% and 57.4%, respectively, for HSV-2.12
HerpeSelect HSV-2 Immunoblot testing should not be considered as confirmatory because this assay detects the same antigen as the HSV-2 EIA. Serologic tests based on detection of HSV-IgM should not be used for diagnosis of genital herpes as IgM response can present during a new infection or HSV reactivation and because IgM responses are not type-specific. Clearly, more accurate commercial type-specific antibody tests are needed.
Specific HSV antibodies can take up to 12 weeks to develop. Therefore, repeat serologic testing for patients in whom initial HSV antibody results are negative yet recent genital herpes acquisition is suspected.11 A confirmed positive HSV-2 antibody test indicates anogenital infection, even in a person who lacks genital symptoms. This finding became evident through a study of 53 HSV-2 seropositive patients who lacked a history of genital herpes. Patients were followed for 3 months, and all but 1 developed either virologic or clinical (or both) evidence of genital herpes.13
In the absence of genital or orolabial symptoms among individuals with positive HSV-1, serologic testing cannot distinguish anogenital from orolabial infection. Most of these infections may represent oral HSV-1 infection; however, given increasing occurrence of genital HSV-1 infection, this could also represent a genital infection.
What are the clinical uses of type-specific HSV serology?
Type-specific serologic tests are helpful in diagnosing patients with atypical or asymptomatic infection and managing the care of persons whose sex partners have genital herpes. Serologic testing can be useful to confirm a clinical diagnosis of HSV, to determine whether atypical lesions or symptoms are attributable to HSV, and as part of evaluation for sexually transmitted diseases in select patients. Screening for HSV-1 and HSV-2 in the general population is not supported by the Centers for Disease Control and Prevention (CDC) or the US Preventive Services Task Force (USPSTF) for several reasons9,10:
- suboptimal performance of commercial HSV antibody tests
- low positive predictive value of these tests in low prevalence HSV settings
- lack of widely available confirmatory testing
- lack of cost-effectiveness
- potential for psychological harm.
Read about treating HSV infection during pregnancy.
Case Continued…
Because Sarah did not have a history of genital herpes, a serum sample was tested by the University of Washington Western blot. The results indicated that Sarah is seronegative for HSV-1 and HSV-2.
Sarah, who is now at 16 weeks’ gestation, returns for evaluation of new genital pain. On examination, she has several shallow ulcerations on the labia and bilateral tender inguinal adenopathy. Her husband recently had cold sores. She is anxious and would like to know if she has genital herpes and if her baby is at risk for HSV infection. You swab the base of a lesion for HSV PCR testing and start antiviral treatment.
Treating HSV infection during pregnancy
Women presenting with a new genital ulcer consistent with HSV should receive empiric antiviral treatment while awaiting confirmatory diagnostic laboratory testing, even during pregnancy. Antiviral therapy with acyclovir, valacyclovir, and famciclovir is the backbone of management of most symptomatic patients with herpes. Antiviral drugs can reduce signs and symptoms of first or recurrent genital herpes and can be used for daily suppressive therapy to prevent recurrences. These drugs do not eradicate the infection or alter the risk of frequency or severity after the drug is discontinued.
Antiviral advantages/disadvantages. Acyclovir is the least expensive drug, but valacyclovir is the most convenient therapy given its less frequent dosing. Acyclovir and valacyclovir are equally efficacious in treating first-episode genital herpes infection with respect to duration of viral shedding, time of healing, duration of pain, and time to symptom clearance. Two randomized clinical trials showed similar benefits of acyclovir and valacyclovir for suppressive therapy management of genital herpes.14,15 Only 1 study compared the efficacy of famciclovir to valacyclovir for suppression and showed that valacyclovir was more effective.16 The cost of famciclovir is usually higher, and it has the least data on use in pregnant women. Acyclovir therapy can be safely used throughout pregnancy and during breastfeeding.9 Antiviral regimens for the treatment of genital HSV in pregnant and nonpregnant women recommended by the CDC are summarized in TABLE 3.17

Related article:
5 ways to reduce infection risk during pregnancy
Will your patient’s infant develop neonatal herpes infection?
Neonatal herpes is a potentially devastating infection that results from exposure to HSV from the maternal genital tract at vaginal delivery. Most cases occur in infants born to women who lack a history of genital herpes.18 In a large cohort study conducted in Washington State, isolation of HSV at the time of labor was strongly associated with vertical transmission (odds ratio [OR], 346).19 The risk of neonatal herpes increased among women shedding HSV-1 compared with HSV-2 (OR, 16.5). The highest risk of transmission to the neonate is in women who acquire genital herpes in a period close to the delivery (30% to 50% risk of transmission), compared with women with a prenatal history of herpes or who acquired herpes early in pregnancy (about 1% to 3% risk of transmission), most likely due to protective HSV-specific maternal antibodies and lower viral load during reactivation versus primary infection.18
Neonatal HSV-1 infection also has been reported in neonates born to women with primary HSV-1 gingivostomatitis during pregnancy; 70% of these women had oral clinical symptoms during the peripartum period.20 Potential mechanisms are exposure to infected genital secretions, direct maternal hematogenous spread, or oral shedding from close contacts.
Although prenatal HSV screening is not recommended by the CDC or USPSTF, serologic testing could be helpful when identifying appropriate pregnancy management for women with a prior history of HSV infection. It also could be beneficial in identifying women without HSV to guide counseling prevention for HSV acquisition. In patients presenting with active genital lesions, viral-specific diagnostic evaluation should be obtained. In those with a history of laboratory confirmed genital herpes, no additional testing is warranted.
Preventing neonatal herpes
There are no prevention strategies for neonatal herpes in the United States, and the incidence of neonatal herpes has not changed in several decades.10 The current treatment guidelines focus on managing women who may be at risk for HSV acquisition during pregnancy and the management of genital lesions in women during pregnancy.9,10,21
When the partner has HSV. Women who have no history of genital herpes or who are seronegative for HSV-2 should avoid intercourse during the third trimester with a partner known to have genital herpes.9 Those who have no history of orolabial herpes or who are seronegative for HSV-1 and have a seropositive partner should avoid receptive oral-genital contact and genital intercourse.9 Condoms can reduce but not eliminate the risk of HSV transmission; to effectively avoid genital herpes infection, abstinence is recommended.
When the patient has HSV. When managing the care of a pregnant woman with genital herpes evaluate for clinical symptoms and timing of infection or recurrence relative to time of delivery:
- Monitor women with a mild recurrence of HSV during the first 35 weeks of pregnancy without antiviral treatment, as most of the recurrent episodes of genital herpes are short.
- Consider antivirals for women with severe symptoms or multiple recurrences.
- Offer women with a history of genital lesions suppressive antiviral therapy at 36 weeks of gestation until delivery.21
In a meta-analysis of 7 randomized trials, 1,249 women with a history of genital herpes prior to or during pregnancy received prophylaxis with either acyclovir or valacyclovir versus placebo or no treatment at 36 weeks of gestation. Antiviral therapy reduced the risk of HSV recurrence at delivery (relative risk [RR], 0.28), cesarean delivery in those with recurrent genital herpes (RR, 0.3), and asymptomatic shedding at delivery (RR, 0.14).22 No data are available regarding the effectiveness of this approach to prevention of neonatal HSV, and case reports confirm neonatal HSV in infants born to women who received suppressive antiviral therapy at the end of pregnancy.23
When cesarean delivery is warranted. At the time of delivery, ask all women about symptoms of genital herpes, including prodromal symptoms, and examine them for genital lesions. For women with active lesions or prodromal symptoms, offer cesarean delivery at the onset of labor or rupture of membranes—this recommendation is supported by the CDC and the American College of Obstetricians and Gynecologists.9,21 The protective effect of cesarean delivery was evaluated in a large cohort study that found: among women who were shedding HSV at the time of delivery, neonates born by cesarean delivery were less likely to develop HSV infection compared with those born through vaginal delivery (1.2% vs 7.7%, respectively).19 Cesarean delivery is not indicated in patients with a history of HSV without clinical recurrence or prodrome at delivery, as such women have a very low risk of transmitting the infection to the neonate.24
Avoid transcervical antepartum obstetric procedures to reduce the risk of placenta or membrane HSV infection; however, transabdominal invasive procedures can be performed safely, even in the presence of active genital lesions.21 Intrapartum procedures that can cause fetal skin disruption, such as use of fetal scalp electrode or forceps, are risk factors for HSV transmission and should be avoided in women with a history of genital herpes.
Related articles:
8 common questions about newborn circumcision
Case Resolved
Sarah’s genital lesion PCR results returned positive for HSV-1. She probably acquired the infection from oral-genital sex with her husband who likely has oral HSV-1, given the history of cold sores. You treat Sarah with acyclovir 400 mg 3 times per day for 7 days. At 36 weeks’ gestation, Sarah begins suppressive antiviral therapy until delivery. She spontaneously labors at 39 weeks’ gestation; at that time, she has no genital lesions and she delivers vaginally a healthy baby.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Genital herpes is a common infection caused by herpes simplex virus type 1 (HSV-1) or herpes simplex virus type 2 (HSV-2). Although life-threatening health consequences of HSV infection after infancy are uncommon, women with genital herpes remain at risk for recurrent symptoms, which can be associated with significant physical and psychosocial distress. These patients also can transmit the disease to their partners and neonates, and have a 2- to 3-fold increased risk of HIV acquisition. In this article, we review the diagnosis and management of genital herpes in pregnant women.
CASE Asymptomatic pregnant patient tests positive for herpes
Sarah is a healthy 32-year-old (G1P0) presenting at 8 weeks’ gestation for her first prenatal visit. She requests HSV testing as she learned that genital herpes is common and it can be transmitted to the baby. You order the HSV-2 IgG assay from your laboratory, which performs the HerpeSelect HSV-2 enzyme immunoassay as the standard test. The test result is positive, with an index value of 2.2 (the manufacturer defines an index value >1.1 as positive). Repeat testing in 4 weeks returns positive results again, with an index value of 2.8.
The patient is distressed at this news. She has no history of genital lesions or symptoms consistent with genital herpes and is worried that her husband has been unfaithful. How would you manage this case?
How prevalent is HSV?
Genital herpes is a chronic viral infection transmitted through close contact with a person who is shedding the virus from genital or oral mucosa. In the United States, the National Health and Nutrition Examination Survey indicated an HSV-2 seroprevalence of 16% among persons aged 14 to 49 in 2005–2010, a decline from 21% in 1988–1991.1 The prevalence among women is twice as high as among men, at 20% versus 11%, respectively. Among those with HSV-2, 87% are not aware that they are infected; they are at risk of infecting their partners, however.1
In the same age group, the prevalence of HSV-1 is 54%.2 The seroprevalence of HSV-1 in adolescents declined from 39% in 1999–2004 to 30% in 2005–2010, resulting in a high number of young people who are seronegative at the time of sexual debut. Concurrently, genital HSV-1 has emerged as a frequent cause of first-episode genital herpes, often associated with oral-genital contact during sexual debut.2,3
When evaluating patients for possible genital herpes provide general educational messages regarding HSV infection and obtain a detailed medical and sexual history to determine the best diagnostic approach.
What are the clinical features of genital HSV infection?
The clinical manifestations of genital herpes vary according to whether the infection is primary, nonprimary first episode, or recurrent.
Primary infection. During primary infection,which occurs 4 to 12 days after sexual exposure and in the absence of pre-existing antibodies to HSV-1 or HSV-2, patients may experience genital and systemic symptoms (FIGURE and TABLE 1). Since this infection usually occurs in otherwise healthy people, for many, this is the most severe disease that they have experienced. However, most patients with primary infection develop mild, atypical, or completely asymptomatic presentation and are not diagnosed at the time of HSV acquisition. Whether primary infection is caused by HSV-1 or HSV-2 cannot be differentiated based on the clinical presentation alone.


Nonprimary first episode infection. In a nonprimary infection, newly acquired infection with HSV-1 or HSV-2 occurs in a person with pre-existing antibodies to the other virus. Almost always, this means new HSV-2 infection in a HSV-1 seropositive person, as prior HSV-2 infection appears to protect against HSV-1 acquisition. In general, the clinical presentation of nonprimary infection is somewhat milder and the rate of complications is lower, but clinically the overlap is great, and antibody tests are needed to define whether the patient has primary or nonprimary infection.4
Recurrent genital herpes infection occurs in most patients with genital herpes. The rate of recurrence is low in patients with genital HSV-1 and often high in patients with genital HSV-2 infection. The median number of recurrences is 1 in the first year of genital HSV-1 infection, and many patients will not have any recurrences following the first year. By contrast, in patients with genital HSV-2 infection, the median number of recurrences is 4, and a high rate of recurrences can continue for many years. Prodromal symptoms (localized irritation, paresthesias, and pruritus) can precede recurrences, which usually present with fewer lesions and last a shorter time than primary infection. Recurrent genital lesions tend to heal in approximately 5 to 10 days in the absence of antiviral treatment, and systemic symptoms are uncommon.5
Asymptomatic viral shedding. After resolution of a primary HSV infection, people shed the virus in the genital tract despite symptom absence. Asymptomatic shedding tends to be more frequent and prolonged with primary genital HSV-2 infection compared with HSV-1 infection.6,7 The frequency of HSV shedding is highest in the first year of infection, and decreases subsequently.8 However, it is likely to persist intermittently for many years. Because the natural history is so strikingly different in genital HSV-1 versus HSV-2, identification of the viral type is important for prognostic information.
The first HSV episode does not necessarily indicate a new or recent infection—in about 25% of persons it represents the first recognized genital herpes episode. Additional serologic and virologic evaluation can be pursued to determine if the first episode represents a new infection.
Read about the diagnostic tests for genital HSV.
What diagnostic tests are available for genital herpes?
Most HSV infections are clinically silent. Therefore, laboratory tests are required to diagnose the infection. Even if symptoms are present, diagnoses based only on clinical presentation have a 20% false-positive rate. Always confirm diagnosis by laboratory assay.9 Furthermore, couples that are discordant for HSV-2 by history are often concordant by serologic assays, as the transmission already has occurred but was not recognized. In these cases, the direction of transmission cannot be determined, and stable couples often experience relief learning that they are not discordant.
Related article:
Effective treatment of recurrent bacterial vaginosis
Several laboratory tools for HSV diagnosis based on direct viral detection and antibody detection can be used in clinical settings (TABLE 2). Among patients with symptomatic genital herpes, a sample from the lesion can be used to confirm and identify viral type. Because polymerase chain reaction (PCR) is substantially more sensitive than viral culture and increasingly available it has emerged as the preferred test.9 Viral culture is highly specific (>99%), but sensitivity varies according to collection technique and stage of the lesions. (The test is less sensitive when lesions are healing.)9,10 Antigen detection by immunofluorescence (direct fluorescent antibody) detects HSV from active lesions with high specificity, but sensitivity is low. Cytologic identification of infected cells (using Tzanck or Pap test) has limited utility for diagnosis due to low sensitivity and specificity.9

Type-specific antibodies to HSV develop during the first several weeks after acquisition and persist indefinitely.11 Most accurate type-specific serologic tests are based on detection of glycoprotein G1 and glycoprotein G2 for HSV-1 and HSV-2, respectively.
HerpeSelect HSV-2 enzyme immunoassay (EIA) is one of the most commonly used tests in the United States. The manufacturer considers results with index values 1.1 or greater as showing HSV-2 infection. Unfortunately, low positive results, often with a defined index value of 1.1 to 3.5, are frequently false positive. These low positive values should be confirmed with another test, such as Western blot.9
Western blot has been considered the gold standard assay for HSV-1 and HSV-2 antibody detection; this test is available at the University of Washington in Seattle. When comparing the HSV-1 EIA and HSV-2 EIA with the Western blot assay in clinical practice, the estimated sensitivity and specificity are 70.2% and 91.6%, respectively, for HSV-1 and 91.9% and 57.4%, respectively, for HSV-2.12
HerpeSelect HSV-2 Immunoblot testing should not be considered as confirmatory because this assay detects the same antigen as the HSV-2 EIA. Serologic tests based on detection of HSV-IgM should not be used for diagnosis of genital herpes as IgM response can present during a new infection or HSV reactivation and because IgM responses are not type-specific. Clearly, more accurate commercial type-specific antibody tests are needed.
Specific HSV antibodies can take up to 12 weeks to develop. Therefore, repeat serologic testing for patients in whom initial HSV antibody results are negative yet recent genital herpes acquisition is suspected.11 A confirmed positive HSV-2 antibody test indicates anogenital infection, even in a person who lacks genital symptoms. This finding became evident through a study of 53 HSV-2 seropositive patients who lacked a history of genital herpes. Patients were followed for 3 months, and all but 1 developed either virologic or clinical (or both) evidence of genital herpes.13
In the absence of genital or orolabial symptoms among individuals with positive HSV-1, serologic testing cannot distinguish anogenital from orolabial infection. Most of these infections may represent oral HSV-1 infection; however, given increasing occurrence of genital HSV-1 infection, this could also represent a genital infection.
What are the clinical uses of type-specific HSV serology?
Type-specific serologic tests are helpful in diagnosing patients with atypical or asymptomatic infection and managing the care of persons whose sex partners have genital herpes. Serologic testing can be useful to confirm a clinical diagnosis of HSV, to determine whether atypical lesions or symptoms are attributable to HSV, and as part of evaluation for sexually transmitted diseases in select patients. Screening for HSV-1 and HSV-2 in the general population is not supported by the Centers for Disease Control and Prevention (CDC) or the US Preventive Services Task Force (USPSTF) for several reasons9,10:
- suboptimal performance of commercial HSV antibody tests
- low positive predictive value of these tests in low prevalence HSV settings
- lack of widely available confirmatory testing
- lack of cost-effectiveness
- potential for psychological harm.
Read about treating HSV infection during pregnancy.
Case Continued…
Because Sarah did not have a history of genital herpes, a serum sample was tested by the University of Washington Western blot. The results indicated that Sarah is seronegative for HSV-1 and HSV-2.
Sarah, who is now at 16 weeks’ gestation, returns for evaluation of new genital pain. On examination, she has several shallow ulcerations on the labia and bilateral tender inguinal adenopathy. Her husband recently had cold sores. She is anxious and would like to know if she has genital herpes and if her baby is at risk for HSV infection. You swab the base of a lesion for HSV PCR testing and start antiviral treatment.
Treating HSV infection during pregnancy
Women presenting with a new genital ulcer consistent with HSV should receive empiric antiviral treatment while awaiting confirmatory diagnostic laboratory testing, even during pregnancy. Antiviral therapy with acyclovir, valacyclovir, and famciclovir is the backbone of management of most symptomatic patients with herpes. Antiviral drugs can reduce signs and symptoms of first or recurrent genital herpes and can be used for daily suppressive therapy to prevent recurrences. These drugs do not eradicate the infection or alter the risk of frequency or severity after the drug is discontinued.
Antiviral advantages/disadvantages. Acyclovir is the least expensive drug, but valacyclovir is the most convenient therapy given its less frequent dosing. Acyclovir and valacyclovir are equally efficacious in treating first-episode genital herpes infection with respect to duration of viral shedding, time of healing, duration of pain, and time to symptom clearance. Two randomized clinical trials showed similar benefits of acyclovir and valacyclovir for suppressive therapy management of genital herpes.14,15 Only 1 study compared the efficacy of famciclovir to valacyclovir for suppression and showed that valacyclovir was more effective.16 The cost of famciclovir is usually higher, and it has the least data on use in pregnant women. Acyclovir therapy can be safely used throughout pregnancy and during breastfeeding.9 Antiviral regimens for the treatment of genital HSV in pregnant and nonpregnant women recommended by the CDC are summarized in TABLE 3.17

Related article:
5 ways to reduce infection risk during pregnancy
Will your patient’s infant develop neonatal herpes infection?
Neonatal herpes is a potentially devastating infection that results from exposure to HSV from the maternal genital tract at vaginal delivery. Most cases occur in infants born to women who lack a history of genital herpes.18 In a large cohort study conducted in Washington State, isolation of HSV at the time of labor was strongly associated with vertical transmission (odds ratio [OR], 346).19 The risk of neonatal herpes increased among women shedding HSV-1 compared with HSV-2 (OR, 16.5). The highest risk of transmission to the neonate is in women who acquire genital herpes in a period close to the delivery (30% to 50% risk of transmission), compared with women with a prenatal history of herpes or who acquired herpes early in pregnancy (about 1% to 3% risk of transmission), most likely due to protective HSV-specific maternal antibodies and lower viral load during reactivation versus primary infection.18
Neonatal HSV-1 infection also has been reported in neonates born to women with primary HSV-1 gingivostomatitis during pregnancy; 70% of these women had oral clinical symptoms during the peripartum period.20 Potential mechanisms are exposure to infected genital secretions, direct maternal hematogenous spread, or oral shedding from close contacts.
Although prenatal HSV screening is not recommended by the CDC or USPSTF, serologic testing could be helpful when identifying appropriate pregnancy management for women with a prior history of HSV infection. It also could be beneficial in identifying women without HSV to guide counseling prevention for HSV acquisition. In patients presenting with active genital lesions, viral-specific diagnostic evaluation should be obtained. In those with a history of laboratory confirmed genital herpes, no additional testing is warranted.
Preventing neonatal herpes
There are no prevention strategies for neonatal herpes in the United States, and the incidence of neonatal herpes has not changed in several decades.10 The current treatment guidelines focus on managing women who may be at risk for HSV acquisition during pregnancy and the management of genital lesions in women during pregnancy.9,10,21
When the partner has HSV. Women who have no history of genital herpes or who are seronegative for HSV-2 should avoid intercourse during the third trimester with a partner known to have genital herpes.9 Those who have no history of orolabial herpes or who are seronegative for HSV-1 and have a seropositive partner should avoid receptive oral-genital contact and genital intercourse.9 Condoms can reduce but not eliminate the risk of HSV transmission; to effectively avoid genital herpes infection, abstinence is recommended.
When the patient has HSV. When managing the care of a pregnant woman with genital herpes evaluate for clinical symptoms and timing of infection or recurrence relative to time of delivery:
- Monitor women with a mild recurrence of HSV during the first 35 weeks of pregnancy without antiviral treatment, as most of the recurrent episodes of genital herpes are short.
- Consider antivirals for women with severe symptoms or multiple recurrences.
- Offer women with a history of genital lesions suppressive antiviral therapy at 36 weeks of gestation until delivery.21
In a meta-analysis of 7 randomized trials, 1,249 women with a history of genital herpes prior to or during pregnancy received prophylaxis with either acyclovir or valacyclovir versus placebo or no treatment at 36 weeks of gestation. Antiviral therapy reduced the risk of HSV recurrence at delivery (relative risk [RR], 0.28), cesarean delivery in those with recurrent genital herpes (RR, 0.3), and asymptomatic shedding at delivery (RR, 0.14).22 No data are available regarding the effectiveness of this approach to prevention of neonatal HSV, and case reports confirm neonatal HSV in infants born to women who received suppressive antiviral therapy at the end of pregnancy.23
When cesarean delivery is warranted. At the time of delivery, ask all women about symptoms of genital herpes, including prodromal symptoms, and examine them for genital lesions. For women with active lesions or prodromal symptoms, offer cesarean delivery at the onset of labor or rupture of membranes—this recommendation is supported by the CDC and the American College of Obstetricians and Gynecologists.9,21 The protective effect of cesarean delivery was evaluated in a large cohort study that found: among women who were shedding HSV at the time of delivery, neonates born by cesarean delivery were less likely to develop HSV infection compared with those born through vaginal delivery (1.2% vs 7.7%, respectively).19 Cesarean delivery is not indicated in patients with a history of HSV without clinical recurrence or prodrome at delivery, as such women have a very low risk of transmitting the infection to the neonate.24
Avoid transcervical antepartum obstetric procedures to reduce the risk of placenta or membrane HSV infection; however, transabdominal invasive procedures can be performed safely, even in the presence of active genital lesions.21 Intrapartum procedures that can cause fetal skin disruption, such as use of fetal scalp electrode or forceps, are risk factors for HSV transmission and should be avoided in women with a history of genital herpes.
Related articles:
8 common questions about newborn circumcision
Case Resolved
Sarah’s genital lesion PCR results returned positive for HSV-1. She probably acquired the infection from oral-genital sex with her husband who likely has oral HSV-1, given the history of cold sores. You treat Sarah with acyclovir 400 mg 3 times per day for 7 days. At 36 weeks’ gestation, Sarah begins suppressive antiviral therapy until delivery. She spontaneously labors at 39 weeks’ gestation; at that time, she has no genital lesions and she delivers vaginally a healthy baby.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Fanfair RN, Zaidi A, Taylor LD, Xu F, Gottlieb S, Markowitz L. Trends in seroprevalence of herpes simplex virus type 2 among non-Hispanic blacks and non-Hispanic whites aged 14 to 49 years–United States, 1988 to 2010. Sex Transm Dis. 2013;40(11):860–864.
- Bradley H, Markowitz LE, Gibson T, McQuillan GM. Seroprevalence of herpes simplex virus types 1 and 2–United States, 1999-2010. J Infect Dis. 2014;209(3):325–333.
- Bernstein DI, Bellamy AR, Hook EW, 3rd, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infect Dis. 2013;56(3):344–351.
- Kimberlin DW, Rouse DJ. Clinical practice. Genital herpes. N Engl J Med. 2004;350(19):1970–1977.
- Corey L, Adams HG, Brown ZA, Holmes KK. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med. 1983;98(6):958–972.
- Wald A, Zeh J, Selke S, Ashley RL, Corey L. Virologic characteristics of subclinical and symptomatic genital herpes infections. N Engl J Med. 1995;333(12):770–775.
- Reeves WC, Corey L, Adams HG, Vontver LA, Holmes KK. Risk of recurrence after first episodes of genital herpes. Relation to HSV type and antibody response. N Engl J Med. 1981;305(6):315–319.
- Phipps W, Saracino M, Magaret A, et al. Persistent genital herpes simplex virus-2 shedding years following the first clinical episode. J Infect Dis. 2011;203(2):180–187.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1–137.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Task Force. Serologic screening for genital herpes infection: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316(23):2525–2530.
- Gupta R, Warren T, Wald A. Genital herpes. Lancet. 2007;370(9605):2127–2137.
- Agyemang E, Le QA, Warren T, et al. Performance of commercial enzyme-linked immunoassays 1 (EIA) for diagnosis of herpes simplex virus-1 and herpes simplex virus-2 infection in a clinical setting. Sex Transm Dis. 2017; doi:10.1097/olq.0000000000000689.
- Wald A, Zeh J, Selke S, et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med. 2000;342(12):844–850.
- Gupta R, Wald A, Krantz E, et al. Valacyclovir and acyclovir for suppression of shedding of herpes simplex virus in the genital tract. J Infect Dis. 2004;190(8):1374–1381.
- Reitano M, Tyring S, Lang W, et al. Valaciclovir for the suppression of recurrent genital herpes simplex virus infection: a large-scale dose range-finding study. International Valaciclovir HSV Study Group. J Infect Dis. 1998;178(3): 603–610.
- Wald A, Selke S, Warren T, et al. Comparative efficacy of famciclovir and valacyclovir for suppression of recurrent genital herpes and viral shedding. Sex Transm Dis. 2006;33(9):529–533.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015 [published correction appears in MMWR Recomm Rep. 2015;64(33):924]. MMWR Recomm Rep. 2015;64(RR-03):1–137.
- Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med. 2009;361(14):1376–1385.
- Brown ZA, Wald A, Morrow RA, Selke S, Zeh J, Corey L. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289(2):203–209.
- Healy SA, Mohan KM, Melvin AJ, Wald A. Primary maternal herpes simplex virus-1 gingivostomatitis during pregnancy and neonatal herpes: case series and literature review. J Pediatric Infect Dis Soc. 2012;1(4):299–305.
- American College of Obstetricians and Gynecoloigsts Committee on Practice Bulletins. ACOG Practice Bulletin No. 82: Management of herpes in pregnancy. Obstet Gynecol. 2007;109(6):1489–1498.
- Hollier LM, Wendel GD. Third trimester antiviral prophylaxis for preventing maternal genital herpes simplex virus (HSV) recurrences and neonatal infection. Cochrane Database Syst Rev. 2008(1):CD004946.
- Pinninti SG, Angara R, Feja KN, et al. Neonatal herpes disease following maternal antenatal antiviral suppressive therapy: a multicenter case series. J Pediatr. 2012;161(1):134–138.e1–e3.
- Vontver LA, Hickok DE, Brown Z, Reid L, Corey L. Recurrent genital herpes simplex virus infection in pregnancy: infant outcome and frequency of asymptomatic recurrences. American journal of obstetrics and gynecology. 1982;143(1):75–84.
- Fanfair RN, Zaidi A, Taylor LD, Xu F, Gottlieb S, Markowitz L. Trends in seroprevalence of herpes simplex virus type 2 among non-Hispanic blacks and non-Hispanic whites aged 14 to 49 years–United States, 1988 to 2010. Sex Transm Dis. 2013;40(11):860–864.
- Bradley H, Markowitz LE, Gibson T, McQuillan GM. Seroprevalence of herpes simplex virus types 1 and 2–United States, 1999-2010. J Infect Dis. 2014;209(3):325–333.
- Bernstein DI, Bellamy AR, Hook EW, 3rd, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infect Dis. 2013;56(3):344–351.
- Kimberlin DW, Rouse DJ. Clinical practice. Genital herpes. N Engl J Med. 2004;350(19):1970–1977.
- Corey L, Adams HG, Brown ZA, Holmes KK. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med. 1983;98(6):958–972.
- Wald A, Zeh J, Selke S, Ashley RL, Corey L. Virologic characteristics of subclinical and symptomatic genital herpes infections. N Engl J Med. 1995;333(12):770–775.
- Reeves WC, Corey L, Adams HG, Vontver LA, Holmes KK. Risk of recurrence after first episodes of genital herpes. Relation to HSV type and antibody response. N Engl J Med. 1981;305(6):315–319.
- Phipps W, Saracino M, Magaret A, et al. Persistent genital herpes simplex virus-2 shedding years following the first clinical episode. J Infect Dis. 2011;203(2):180–187.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1–137.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Task Force. Serologic screening for genital herpes infection: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316(23):2525–2530.
- Gupta R, Warren T, Wald A. Genital herpes. Lancet. 2007;370(9605):2127–2137.
- Agyemang E, Le QA, Warren T, et al. Performance of commercial enzyme-linked immunoassays 1 (EIA) for diagnosis of herpes simplex virus-1 and herpes simplex virus-2 infection in a clinical setting. Sex Transm Dis. 2017; doi:10.1097/olq.0000000000000689.
- Wald A, Zeh J, Selke S, et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med. 2000;342(12):844–850.
- Gupta R, Wald A, Krantz E, et al. Valacyclovir and acyclovir for suppression of shedding of herpes simplex virus in the genital tract. J Infect Dis. 2004;190(8):1374–1381.
- Reitano M, Tyring S, Lang W, et al. Valaciclovir for the suppression of recurrent genital herpes simplex virus infection: a large-scale dose range-finding study. International Valaciclovir HSV Study Group. J Infect Dis. 1998;178(3): 603–610.
- Wald A, Selke S, Warren T, et al. Comparative efficacy of famciclovir and valacyclovir for suppression of recurrent genital herpes and viral shedding. Sex Transm Dis. 2006;33(9):529–533.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015 [published correction appears in MMWR Recomm Rep. 2015;64(33):924]. MMWR Recomm Rep. 2015;64(RR-03):1–137.
- Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med. 2009;361(14):1376–1385.
- Brown ZA, Wald A, Morrow RA, Selke S, Zeh J, Corey L. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289(2):203–209.
- Healy SA, Mohan KM, Melvin AJ, Wald A. Primary maternal herpes simplex virus-1 gingivostomatitis during pregnancy and neonatal herpes: case series and literature review. J Pediatric Infect Dis Soc. 2012;1(4):299–305.
- American College of Obstetricians and Gynecoloigsts Committee on Practice Bulletins. ACOG Practice Bulletin No. 82: Management of herpes in pregnancy. Obstet Gynecol. 2007;109(6):1489–1498.
- Hollier LM, Wendel GD. Third trimester antiviral prophylaxis for preventing maternal genital herpes simplex virus (HSV) recurrences and neonatal infection. Cochrane Database Syst Rev. 2008(1):CD004946.
- Pinninti SG, Angara R, Feja KN, et al. Neonatal herpes disease following maternal antenatal antiviral suppressive therapy: a multicenter case series. J Pediatr. 2012;161(1):134–138.e1–e3.
- Vontver LA, Hickok DE, Brown Z, Reid L, Corey L. Recurrent genital herpes simplex virus infection in pregnancy: infant outcome and frequency of asymptomatic recurrences. American journal of obstetrics and gynecology. 1982;143(1):75–84.
Bag-mask ventilation for CPR deflates in large RCT
BARCELONA – Bag-mask ventilation for airway management during resuscitation of patients with out-of-hospital cardiac arrest was considerably less safe and yet no more effective than endotracheal intubation in a large randomized trial, Frederic Adnet, MD, reported at the annual congress of the European Society of Cardiology.
This was an unexpected result.

Several large, well-respected observational registry studies had strongly suggested that bag-mask ventilation is associated with a superior survival rate with good neurologic outcome. As a result, many in the resuscitation science field have been moving closer to replacing endotracheal intubation as the standard of care in favor of bag-mask ventilation. But this first-of-its-kind, large, randomized trial to formally address the issue showed virtually identical rates of day-28 survival with good neurologic outcome in the two study arms. Plus, bag-mask ventilation had a significantly higher complication rate.
“So, at this time, we will not yet change our technique,” according to coinvestigator Eric Vicaut, MD, of Fernand Widal Hospital in Paris.
This major prospective randomized trial included 2,043 patients with out-of-hospital cardiac arrest at 20 centers in France and Belgium. The primary endpoint – day-28 survival with good neurologic status as defined by a Glasgow-Pittsburgh Cerebral Performance Scale score of 2 or less – occurred in 4.2% of the bag-mask ventilation group and 4.1% of the endotracheal intubation group.
However, the rate of aspiration or regurgitation of gastric contents was significantly higher in the bag-mask ventilation group by a margin of 14.9% to 7.7%. Moreover, the bag-mask ventilation technique failed in 6.3% of patients, compared with a 2.5% endotracheal intubation failure rate.
Discussant Susanna Price, MD, praised the study as a high-quality, well-conducted randomized trial, adding that it’s just the sort of study that the field of resuscitation science had needed for a long time. Indeed, most guidelines in the field are based on faint supporting evidence. That may be one reason why good outcomes of out-of-hospital cardiac arrest are so disappointingly low: The worldwide average is roughly 7%, with huge differences between countries.
“It is really very depressing sometimes when one looks at the percentage of patients who actually return to normal life and normal functional neurologic status and, indeed, whose relatives get back to work,” commented Dr. Price, a cardiologist and intensivist at Royal Brompton Hospital in London.
“This is a huge study for resuscitation science,” she continued. “Prehospital airway management is currently a very hot topic. Bear in mind that in the United States, roughly 88% of cardiac arrests happen in the home.”
“This trial does challenge the current feeling that bag-mask ventilation is definitely superior to advanced airway interventions,” Dr. Price added.
For her, the study contained three surprises, she continued. One was the high bag-mask failure rate in the hands of very experienced operators. Another was the high complication rate associated with the device, again even in expert hands. Also, contrary to numerous published reports, the chest compression rate in this RCT was not better with bag mask ventilation.
“The study did not demonstrate that endotracheal intubation interrupts chest compressions. In fact, chest compression pauses were actually significantly more frequent in the bag-mask ventilation group than with endotracheal intubation,” she observed.
It’s worth noting that in the French EMS system, a physician experienced in cardiopulmonary resuscitation is typically on board for ambulance runs. This creates an element of uncertainty as to the generalizability of the study findings to EMS systems where paramedics who may be less proficient in endotracheal intubation are the first responders. Indeed, whether endotracheal intubation will stack up as favorably as it did against bag-mask ventilation in this randomized trial when tested in other settings where airway management is left in the hands of paramedics is an open question that’s the topic of ongoing studies, Dr. Price noted.
Dr. Adnet and Dr. Vicaut reported having no financial conflicts regarding their study, which was funded by the French Ministry of Health.
[email protected]
BARCELONA – Bag-mask ventilation for airway management during resuscitation of patients with out-of-hospital cardiac arrest was considerably less safe and yet no more effective than endotracheal intubation in a large randomized trial, Frederic Adnet, MD, reported at the annual congress of the European Society of Cardiology.
This was an unexpected result.

Several large, well-respected observational registry studies had strongly suggested that bag-mask ventilation is associated with a superior survival rate with good neurologic outcome. As a result, many in the resuscitation science field have been moving closer to replacing endotracheal intubation as the standard of care in favor of bag-mask ventilation. But this first-of-its-kind, large, randomized trial to formally address the issue showed virtually identical rates of day-28 survival with good neurologic outcome in the two study arms. Plus, bag-mask ventilation had a significantly higher complication rate.
“So, at this time, we will not yet change our technique,” according to coinvestigator Eric Vicaut, MD, of Fernand Widal Hospital in Paris.
This major prospective randomized trial included 2,043 patients with out-of-hospital cardiac arrest at 20 centers in France and Belgium. The primary endpoint – day-28 survival with good neurologic status as defined by a Glasgow-Pittsburgh Cerebral Performance Scale score of 2 or less – occurred in 4.2% of the bag-mask ventilation group and 4.1% of the endotracheal intubation group.
However, the rate of aspiration or regurgitation of gastric contents was significantly higher in the bag-mask ventilation group by a margin of 14.9% to 7.7%. Moreover, the bag-mask ventilation technique failed in 6.3% of patients, compared with a 2.5% endotracheal intubation failure rate.
Discussant Susanna Price, MD, praised the study as a high-quality, well-conducted randomized trial, adding that it’s just the sort of study that the field of resuscitation science had needed for a long time. Indeed, most guidelines in the field are based on faint supporting evidence. That may be one reason why good outcomes of out-of-hospital cardiac arrest are so disappointingly low: The worldwide average is roughly 7%, with huge differences between countries.
“It is really very depressing sometimes when one looks at the percentage of patients who actually return to normal life and normal functional neurologic status and, indeed, whose relatives get back to work,” commented Dr. Price, a cardiologist and intensivist at Royal Brompton Hospital in London.
“This is a huge study for resuscitation science,” she continued. “Prehospital airway management is currently a very hot topic. Bear in mind that in the United States, roughly 88% of cardiac arrests happen in the home.”
“This trial does challenge the current feeling that bag-mask ventilation is definitely superior to advanced airway interventions,” Dr. Price added.
For her, the study contained three surprises, she continued. One was the high bag-mask failure rate in the hands of very experienced operators. Another was the high complication rate associated with the device, again even in expert hands. Also, contrary to numerous published reports, the chest compression rate in this RCT was not better with bag mask ventilation.
“The study did not demonstrate that endotracheal intubation interrupts chest compressions. In fact, chest compression pauses were actually significantly more frequent in the bag-mask ventilation group than with endotracheal intubation,” she observed.
It’s worth noting that in the French EMS system, a physician experienced in cardiopulmonary resuscitation is typically on board for ambulance runs. This creates an element of uncertainty as to the generalizability of the study findings to EMS systems where paramedics who may be less proficient in endotracheal intubation are the first responders. Indeed, whether endotracheal intubation will stack up as favorably as it did against bag-mask ventilation in this randomized trial when tested in other settings where airway management is left in the hands of paramedics is an open question that’s the topic of ongoing studies, Dr. Price noted.
Dr. Adnet and Dr. Vicaut reported having no financial conflicts regarding their study, which was funded by the French Ministry of Health.
[email protected]
BARCELONA – Bag-mask ventilation for airway management during resuscitation of patients with out-of-hospital cardiac arrest was considerably less safe and yet no more effective than endotracheal intubation in a large randomized trial, Frederic Adnet, MD, reported at the annual congress of the European Society of Cardiology.
This was an unexpected result.

Several large, well-respected observational registry studies had strongly suggested that bag-mask ventilation is associated with a superior survival rate with good neurologic outcome. As a result, many in the resuscitation science field have been moving closer to replacing endotracheal intubation as the standard of care in favor of bag-mask ventilation. But this first-of-its-kind, large, randomized trial to formally address the issue showed virtually identical rates of day-28 survival with good neurologic outcome in the two study arms. Plus, bag-mask ventilation had a significantly higher complication rate.
“So, at this time, we will not yet change our technique,” according to coinvestigator Eric Vicaut, MD, of Fernand Widal Hospital in Paris.
This major prospective randomized trial included 2,043 patients with out-of-hospital cardiac arrest at 20 centers in France and Belgium. The primary endpoint – day-28 survival with good neurologic status as defined by a Glasgow-Pittsburgh Cerebral Performance Scale score of 2 or less – occurred in 4.2% of the bag-mask ventilation group and 4.1% of the endotracheal intubation group.
However, the rate of aspiration or regurgitation of gastric contents was significantly higher in the bag-mask ventilation group by a margin of 14.9% to 7.7%. Moreover, the bag-mask ventilation technique failed in 6.3% of patients, compared with a 2.5% endotracheal intubation failure rate.
Discussant Susanna Price, MD, praised the study as a high-quality, well-conducted randomized trial, adding that it’s just the sort of study that the field of resuscitation science had needed for a long time. Indeed, most guidelines in the field are based on faint supporting evidence. That may be one reason why good outcomes of out-of-hospital cardiac arrest are so disappointingly low: The worldwide average is roughly 7%, with huge differences between countries.
“It is really very depressing sometimes when one looks at the percentage of patients who actually return to normal life and normal functional neurologic status and, indeed, whose relatives get back to work,” commented Dr. Price, a cardiologist and intensivist at Royal Brompton Hospital in London.
“This is a huge study for resuscitation science,” she continued. “Prehospital airway management is currently a very hot topic. Bear in mind that in the United States, roughly 88% of cardiac arrests happen in the home.”
“This trial does challenge the current feeling that bag-mask ventilation is definitely superior to advanced airway interventions,” Dr. Price added.
For her, the study contained three surprises, she continued. One was the high bag-mask failure rate in the hands of very experienced operators. Another was the high complication rate associated with the device, again even in expert hands. Also, contrary to numerous published reports, the chest compression rate in this RCT was not better with bag mask ventilation.
“The study did not demonstrate that endotracheal intubation interrupts chest compressions. In fact, chest compression pauses were actually significantly more frequent in the bag-mask ventilation group than with endotracheal intubation,” she observed.
It’s worth noting that in the French EMS system, a physician experienced in cardiopulmonary resuscitation is typically on board for ambulance runs. This creates an element of uncertainty as to the generalizability of the study findings to EMS systems where paramedics who may be less proficient in endotracheal intubation are the first responders. Indeed, whether endotracheal intubation will stack up as favorably as it did against bag-mask ventilation in this randomized trial when tested in other settings where airway management is left in the hands of paramedics is an open question that’s the topic of ongoing studies, Dr. Price noted.
Dr. Adnet and Dr. Vicaut reported having no financial conflicts regarding their study, which was funded by the French Ministry of Health.
[email protected]
AT THE ESC CONGRESS 2017
Key clinical point:
Major finding: Rates of survival with good neurologic outcome 28 days after out-of-hospital cardiac arrest were similarly low whether airway management during CPR relied upon endotracheal intubation or bag-mask ventilation, but the latter strategy had a significantly higher complication rate.
Data source: This prospective randomized trial included 2,043 patients with out-of-hospital cardiac arrest at 20 centers in France and Belgium.
Disclosures: The study was funded by the French Ministry of Health. The presenter reported having no financial conflicts.
2017 Update on minimally invasive gynecologic surgery
Gynecologic surgeons who trained in the early 1990s may feel that the practice of gynecologic surgery seemed simpler back then. There were really only 2 ways to perform a hysterectomy: vaginally (TVH—total vaginal hysterectomy) and abdominally (TAH—total abdominal hysterectomy). Global endometrial ablation devices were not an established treatment for abnormal uterine bleeding, and therapeutic advancements such as hormonally laden intrauterine devices, vaginal mesh kits, and surgical robots did not exist. The options in the surgical toolbox were limited, and the general expectation in residency was long hours. During that period, consistent exposure to the operating room and case volume allowed one to graduate confidant in one’s surgical skills.
The changing landscape of gynecologic surgery
Fast-forward to 2017. Now, so many variables affect the ability to produce a well-trained gynecologic surgeon. In fact, in 2015 Guntupalli and colleagues studied the preparedness of ObGyn residents for fellowship training in the 4 subspecialties of female pelvic medicine and reconstructive surgery, gynecologic oncology, maternal-fetal medicine, and reproductive endocrinology-infertility.1 Through a validated survey of fellowship program directors, the authors found that only 20% of first-year fellows were able to perform a vaginal hysterectomy independently, and 46%, an abdominal hysterectomy. Barely 50% of first-year fellows in all subspecialties studied could independently set up a retractor for laparotomy and appropriately pack and mobilize the bowel for pelvic surgery.1
Today the hysterectomy procedure has become the proverbial alphabet soup. Trainees are confronted with having to learn not only the TVH and the TAH but also the LAVH (laparoscopic-assisted vaginal hysterectomy), LSH (laparoscopic supracervical hysterectomy), TLH (total laparoscopic hysterectomy), and RALH (robot-assisted laparoscopic hysterectomy).2 With a mandated 80-hour residency workweek restriction and an increasing number of minimally invasive hysterectomies performed nationally, a perfect storm exists for critically evaluating the current paradigm of resident and fellow surgical training.3
One may wonder if current controversies surrounding many of the technologic advancements in gynecologic surgery result from inadequate training and too many treatment options or from flaws in the actual devices. A “see one, do one, teach one” approach to assimilating surgical skills is no longer an accepted approach, and although the “10,000-hour rule” of focused practice to attain expertise makes sense, how can a trainee gain enough exposure to achieve competency?
Related article:
The Extracorporeal C-Incision Tissue Extraction (ExCITE) technique
Simulation: A creditable training tactic
This is where simulation—whether low or high fidelity—potentially can fill in some of those training gaps. Simulation in medicine is a proven instructional design strategy in which learning is an active and experiential process. Studies clearly have shown that simulation-based medical education (SBME) with deliberate practice is superior to traditional clinical medical education in achieving specific clinical skill acquisition goals.4
This special Update on minimally invasive gynecologic surgery offers a 30,000-foot overview of the current state of simulation in gynecologic surgical training. Equally important to this conversation is the process by which a trained individual can obtain the appropriate credentials and subsequent privileging to perform various surgical procedures. Simulation has begun to play a significant role not only in an individual’s initial credentialing and privileging in surgery but also in maintaining those privileges.
Read about the evolving role of simulation in gyn surgery training.
Simulation's evolving role in gyn surgery training
Recently, the traditional model of gynecologic surgical training has been impacted by the exponential growth of technology (surgical devices), increased surgical options, and the limited work hours of trainees. As a result, simulation-based medical education has been identified as a potential solution to address deficits in surgical training. Fortunately, all modalities of surgery are now amenable to improvements in surgical education via simulation.5
Although basic skill training in the standard areas of hand-eye coordination, tissue handling, and instrument use still is prerequisite, the integration of both low- and high-fidelity simulation technologies--with enhanced functionality--now allows for a more comprehensive approach to understanding surgical anatomy. In addition, simulation training provides the opportunity for independent practice of full surgical procedures and, in many instances, offers objective and instantaneous assessment feedback for the learner. This discussion highlights some of the relevant literature on simulation training and the impact of surgical simulation on hysteroscopy and laparoscopy.
Box trainers and virtual reality simulators in hysteroscopy training
Hysteroscopic surgery allows direct endoscopic visualization of the uterine cavity for both diagnostic and therapeutic purposes. While the majority of these procedures are generally low risk, operative hysteroscopic experience minimizes the possibility of significant procedure-related complications, such as uterine perforation.5 The literature repeatedly shows that significant differences exist in skill and sense of preparedness between the novice or inexperienced surgeon (resident trainee) and the expert in hysteroscopic surgery.6-8
Both low- and high-fidelity hysteroscopic simulators can be used to fine-tune operator skills. Low-fidelity simulators such as box trainers, which focus on skills like endometrial ablation and hysteroscopic resection with energy, have been shown to measurably improve performance, and they are well-received by participants. Low-fidelity simulations that incorporate vegetable/fruit or animal models (for example, porcine bladders and cattle uteri) have also been employed with success.9
On the high-fidelity end, surgical trainees can now experience hysteroscopic surgery simulation through virtual reality simulators, which have evolved with improvements in technology (FIGURE 1). Many high-fidelity simulators have been developed, and technical skill and theoretical knowledge improve with their use. Overall, trainees have provided positive feedback regarding the realism and training capacity afforded by virtual reality simultors.10,11
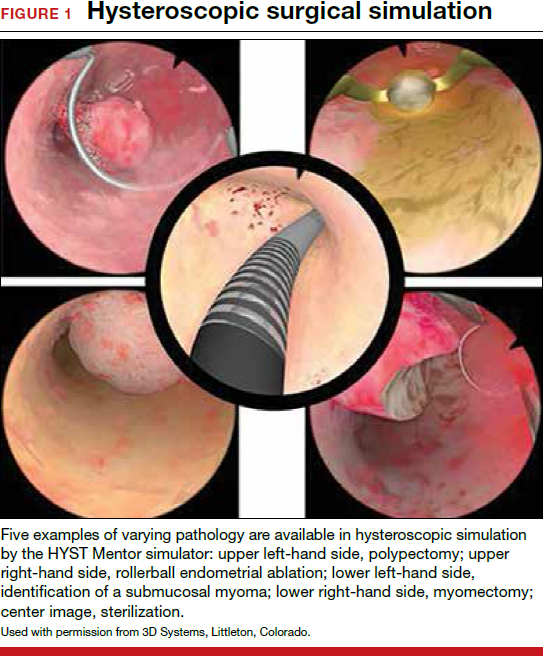
Various simulators are equipped with complete training curriculums that focus on essential surgical skills. Common troubleshooting techniques taught via simulator include establishing and maintaining clear views, detecting and coagulating bleeding sources, fluid management and handling, and instrument failure. Learners can perform these sessions repeatedly, independent of their respective starting skill level. On completion of simulation training, the trainee is given objective performance assessments on economy of motion, visualization, safety, fluid handling, and other skills.
Related article:
ExCITE: Minimally invasive tissue extraction made simple with simulation
Learning the complexities of laparoscopy through simulation
Laparoscopic surgery (both conventional and robot assisted) allows for a minimally invasive, cost-effective, and rapid-recovery approach to the management of many common gynecologic conditions. In both approaches, the learning curve to reach competency is steep. Conventional laparoscopy requires unique surgical skills, including adapting to a 2-dimensional field with altered depth perception; this creates challenges in spatial reasoning and achieving proficiency in video-eye-hand coordination as a result of the fulcrum effect inherent in laparoscopic instrumentation. This is further complicated by the essential dexterity required to complete dissections and suturing.12,13
Robot-assisted laparoscopic surgery requires significant modifications to adapt to a 3-dimensional view. In addition, this approach incorporates another level of complexity (and challenge to attaining mastery), namely, using remotely controlled multiple instrument arms with no tactile feedback.
Importantly, some residency training programs are structured unevenly, emphasizing one or the other surgical modality.14 As a result, this propagates certain skills--or lack thereof--on graduation, and thus highlights potential areas of laparoscopic training that need to be improved and enhanced.
Increasing the learning potential
The growing integration of low- and high-fidelity simulation training in laparoscopic surgery has led to improved skill acquisition.12,13,15,16 A well-established low-fidelity simulation model is the Fundamentals of Laparoscopic Surgery module, through which trainees are taught vital psychomotor skills via a validated box trainer that is also supported by a cognitive component (FIGURE 2).17,18
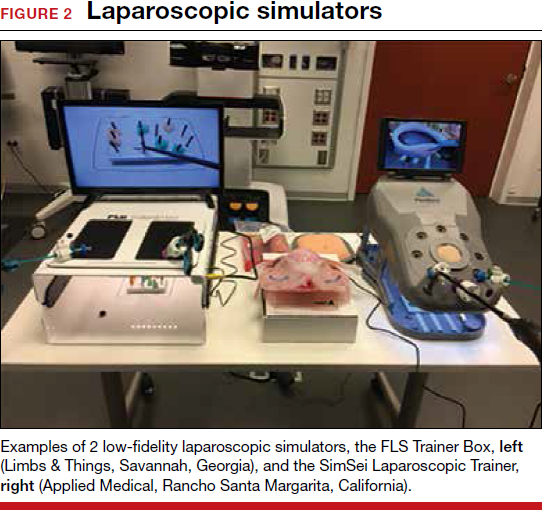
The advent of laparoscopic virtual reality training systems has raised the learning potential further, even for experienced surgeons. Some benefits of virtual reality simulation in conventional laparoscopy include education on an interactive 3D pelvis, step-by-step procedural guidance, a comprehensive return of performance metrics on vital laparoscopic skills, and the incorporation of advanced skills such as laparoscopic suturing, complex dissections, and lysis of adhesions.
In the arena of robot-assisted procedures, simulation modules are available for learning fundamental skill development in hand-eye coordination, depth perception, bimanual manipulation, camera navigation, and wrist articulation.
In both conventional and robot-assisted laparoscopy simulation pathways, complete procedural curriculums (for example, hysterectomy with adnexectomy) are available. Thus, learners can start a procedure or technique at a point applicable to them, practice repeatedly until competency, and eventually become proficient (FIGURE 3).
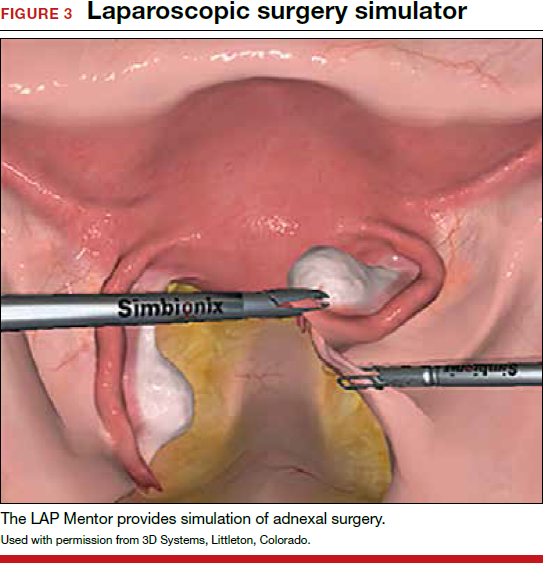
Generally, high-fidelity computerized simulators provide a comprehensive performance report on completion of training, along with a complete recording of the trainee's encounter during accruement of skill. Most importantly, laparoscopic training via simulation has been validated to translate into improved operating room performance by impacting operating times, safety profiles, and surgical skill growth.15,19
Related article:
Complete colpectomy & colpocleisis: Model for simulation
Simulation is a mainstream training tool
The skills gap between expert surgeons and new trainees continues to widen. A comprehensive educational pathway that provides an optimistic safety profile, abides by time constraints, and elevates skill sets will fall to simulation-based surgical training.20,21 Surgical competence is defined not simply by observation and Halstedian technique but by a combination of cognitive and behavioral abilities as well as perceptual and psychomotor skills. It is impractical to expect current learners to become proficient in visuospatial and tactile perception and to demonstrate technical competency without supplementing their training.22-24 Ultimately, as experience with both low- and high-fidelity surgical simulation grows, the predictive validity of this type of training pathway will become more readily apparent. In other words, improved performance in the simulated environment will translate into improved performance in the operating room.
Read about how gyn surgery simulation is being incorporated into credentialing and privileging
Incorporating gyn surgery simulation into credentialing and privileging
Over the last 25 years surgeons have seen unprecedented changes in technology that have revolutionized our surgical approaches to common gynecologic conditions. In the past, granting surgical privileges was pretty straightforward. Surgeons were granted privileges based on successfully completing their training, and subsequent renewal of those privileges was based on not having any significant misadventures or complications. With the advent of laparoscopy, hysteroscopy, and then robot-assisted surgery, training surgeons and verifying their competency has become much more complicated. The variety of surgical approaches now being taught coupled with reduced resident training time and decreasing case volumes have significantly impacted the traditional methodologies of surgical training.25,26
Related article:
How the AAGL is trying to improve outcomes for patients undergoing robot-assisted gynecologic surgery
High-tech surgery demands high-tech training
The development of high-tech surgical approaches has been accompanied by the natural development of simulation models to help with training. Initially, inanimate models, animal labs, and cadavers were used. Over the last 15 years, several innovative companies have developed virtual reality simulation platforms for laparoscopy, hysteroscopy, and even robotics.27 These virtual reality simulators allow students to develop the psychomotor skills necessary to perform minimally invasive procedures and to practice those skills until they can demonstrate proficiency before operating on a live patient.
Most would agree that the key to learning a surgical skill is to "practice, practice, practice."28 Many studies have shown that improvement in surgical outcomes is clearly related to a surgeon's case volume.29,30 But with case volumes decreased, simulation has evolved as the best training alternative. Current surgical simulators enable a student to engage in "deliberate practice"; that is, to have tasks with well-defined goals, to be motivated to improve, and to receive immediate feedback along with opportunities for repetition and refinements of performance.
Simulation allows students to try different surgical techniques and to use "deliberate practice" avoidance of errors in a controlled, safe situation that provides immediate performance feedback.31 Currently, virtual reality simulators are available for hysteroscopy, laparoscopy, and robot-assisted gynecologic applications. Early models focused solely on developing a learner's psychomotor skills necessary to safely perform minimally invasive surgeries. Newer simulators add a cognitive component to help students learn specific procedures, such as adnexectomy and hysterectomy.32
Based on the aviation simulator training model, the AAGL endorsed a Gynecologic Robotic Surgery Credentialing and Privileging Guideline in 2014; this guidance relies heavily on simulation for initial training as well as for subsequent annual recertification.33 Many institutions, including the MultiCare Health System in Tacoma, Washington, require all surgeons--even high-volume surgeons--to demonstrate proficiency annually by passing required robotic simulation exercises at least 2 times consecutively in order to maintain robotic surgery privileges.34
A work-around for a simulation drawback
Using simulation for recertification has been criticized because, although it can confirm that a surgeon is skilled enough to operate the tool, it does not evaluate surgical judgment or technique. In response, crowdsourced review of an individual surgeon's surgical videos has proven to be a useful, dependable way to give a surgeon direct feedback regarding his or her performance on a live patient.35 Many institutions now use this technology not only for initial training but also for helping surgeons improve with direct feedback from master surgeon reviewers. Other institutions have considered replacing annual re-credentialing case volume requirements with this technology, which actually assesses competence in a more accurate way.36
Related article:
Flight plan for robotic surgery credentialing: New AAGL guidelines
A new flight plan
The bottom line is that the training and annual recertification of future surgeons now mimics closely the pathway that all airplane pilots are required to follow.
Initial training will require mastery of surgical techniques using a simulator before taking a "solo flight" on a live patient.
Maintenance of privileges now requires either large case volumes or skills testing on a simulator. Many institutions now also require an annual "check ride," such as a crowdsourced video review of a surgeon's cases, as described above.
Re-credentialing. Just as the "see one, do one, teach one" model is now part of our historical legacy, re-credentialing simply by avoiding misadventures and staying out of trouble will go the way of paper medical records. Our future will certainly require an annual objective evaluation of good surgical judgment and surgical technique proficiency. Surgical simulation will be the norm for all of us.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Guntupalli SR, Doo DW, Guy M, et al. Preparedness of obstetrics and gynecology residents for fellowship training. Obstet Gynecol. 2015;126(3):559–568.
- Pulliam SJ, Berkowitz LR. Smaller pieces of the hysterectomy pie: current challenges in resident surgical education. Obstet Gynecol. 2009;113(2 pt 1):395–398.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
- McGaghie WC, Issenberg SB, Cohen ER, Barsuk JH, Wayne DB. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86(6):706–711.
- Smith ML. Simulation and education in gynecologic surgery. Obstet Gynecol Clin North Am. 2011;38(4):733–740.
- Raymond E, Ternamian A, Leyland N, Tolomiczenko G. Endoscopy teaching in Canada: a survey of obstetrics and gynecology program directors and graduating residents. J Minim Invasive Gynecol. 2006;13(1):10–16.
- Goff BA, VanBlaricom A, Mandel L, Chinn M, Nielsen P. Comparison of objective, structured assessment of technical skills with a virtual reality hysteroscopy trainer and standard latex hysteroscopy model. J Reprod Med. 2007;52(5):407–412.
- Singhi A. Comparison of complications rates in endoscopic surgery performed by a clinical assistant vs an experienced endoscopic surgeon. J Gynecol Endosc Surg. 2009;1(1):40–46.
- Savran MM, Sorensen SM, Konge L, Tolsgaard MG, Bjerrum F. Training and assessment of hysteroscopic skills: a systematic review. J Surg Ed. 2016;73(5):906–918.
- Panel P, Bajka M, Le Tohic A, Ghoneimi AE, Chis C, Cotin S. Hysteroscopic placement of tubal sterilization implants: virtual reality simulator training. Surg Endosc. 2012;26(7):1986–1996.
- Bajka M, Tuchschmid S, Streich M, Fink D, Szekely G, Harders M. Evaluation of a new virtual-reality training simulator for hysteroscopy. Surg Endosc. 2009;23(9):2026–2033.
- Scott DJ, Bergen PC, Rege RV, et al. Laparoscopic training on bench models: better and more cost effective than operating room experience? J Am Coll Surg. 2000;191(3):272–283.
- Scott-Conner CE, Hall TJ, Anglin BL, et al. The integration of laparoscopy into a surgical residency and implications for the training environment. Surg Endosc. 1994;8(9):1054–1057.
- Berkowitz RL, Minkoff H. A call for change in a changing world. Obstet Gynecol. 2016;127(1):153–156.
- Larsen CR, Oestergaard J, Ottesen BS, Soerensen JL. The efficacy of virtual reality simulation training in laparoscopy: a systematic review of randomized trials. Acta Obstet Gynecol Scand. 2012;91(9):1015–1028.
- Aggarwal R, Ward J, Balasundaram I, Sains P, Athanasiou T, Darzi A. Proving the effectiveness of virtual reality simulation for training in laparoscopic surgery. AnnSurg. 2007;246(5):771–779.
- Oropesa I, Sanchez-Gonzalez P, Lamata P, et al. Methods and tools for objective assessment of psychomotor skills in laparoscopic surgery. J Surg Res. 2011;171(1):e81–e95.
- Rooney DM, Brissman IC, Finks JF, Gauger PG. Fundamentals of Laparoscopic Surgery manual test: is videotaped performance assessment an option? J Surg Educ. 2015;72(1):90–95.
- Seymour NE, Gallagher AG, Roman SA, et al. Virtual reality training improves operating room performance: results of a randomized, double-blinded study. Ann Surg. 2002;236(4):458–463, 63–64.
- Aggarwal R, Tully A, Grantcharov T, et al. Virtual reality simulation training can improve technical skills during laparoscopic salpingectomy for ectopic pregnancy. BJOG. 2006;113(12):1382–1387.
- Darzi A, Smith S, Taffinder N. Assessing operative skill. Needs to become more objective. BMJ. 1999;318(7188):887–888.
- Moorthy K, Munz Y, Sarker SK, Darzi A. Objective assessment of technical skills in surgery. BMJ. 2003;327(7422):1032–1037.
- Grantcharov TP, Bardram L, Funch-Jensen P, Rosenberg J. Assessment of technical surgical skills. Eur J Surg. 2002;168(3):139–144.
- Wanzel KR, Hamstra SJ, Caminiti MF, Anastakis DJ, Grober ED, Reznick RK. Visual-spatial ability correlates with efficiency of hand motion and successful surgical performance. Surgery. 2003;134(5):750–757.
- Einarsson JI, Young A, Tsien L, Sangi-Haghpeykar H. Perceived proficiency in endoscopic techniques among senior obstetrics and gynecology residents. J Am Assoc Gynecol Laparosc. 2002;9(2):158–164.
- Cohen SL, Hinchcliffe E. Is surgical training in ob-gyn residency adequate? Contemp ObGyn. . Published July 22, 2016. Accessed October 18, 2017.
- Bric JD, Lumbard DC, Frelich MJ, Gould JC. Current state of virtual reality simulation in robotic surgery training: a review. Surg Endosc. 2016;30(6):2169–2178.
- Gladwell M. Outliers: The Story of Success. New York, New York: Little Brown and Co; 2008.
- Boyd LR, Novetsky AP, Curtain JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116(4):909–915.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effects of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119(4):709–716.
- Kotsis SV, Chung KC. Application of the “see one, do one, teach one” concept in surgical training. Plast Reconstr Surg. 2013;131(5):1194–1201.
- Maestro AR Hysterectomy Module. Mimic simulation website. http://www.mimicsimulation.com/hysterectomy/. Accessed October 18, 2017.
- AAGL. Guidelines for privileging for robotic-assisted gynecologic laparoscopy. J Minim Invasiv Gynecol, 2014;21(2):157–167.
- Lenihan JP Jr. Navigating credentialing and privileging and learning curves in robotics with an evidence and experienced-based approach. Clin Obstet Gynecol. 2011;54(3):382–390.
- Polin MR, Siddiqui NY, Comstock BA, et al. . Am J Obstet Gynecol. 2016;215(5):644.e1–644.e7.
- Continuous People Improvement. C-SATS website. https://www.csats.com/customers-main/. Accessed October 18, 2017.
Gynecologic surgeons who trained in the early 1990s may feel that the practice of gynecologic surgery seemed simpler back then. There were really only 2 ways to perform a hysterectomy: vaginally (TVH—total vaginal hysterectomy) and abdominally (TAH—total abdominal hysterectomy). Global endometrial ablation devices were not an established treatment for abnormal uterine bleeding, and therapeutic advancements such as hormonally laden intrauterine devices, vaginal mesh kits, and surgical robots did not exist. The options in the surgical toolbox were limited, and the general expectation in residency was long hours. During that period, consistent exposure to the operating room and case volume allowed one to graduate confidant in one’s surgical skills.

The changing landscape of gynecologic surgery
Fast-forward to 2017. Now, so many variables affect the ability to produce a well-trained gynecologic surgeon. In fact, in 2015 Guntupalli and colleagues studied the preparedness of ObGyn residents for fellowship training in the 4 subspecialties of female pelvic medicine and reconstructive surgery, gynecologic oncology, maternal-fetal medicine, and reproductive endocrinology-infertility.1 Through a validated survey of fellowship program directors, the authors found that only 20% of first-year fellows were able to perform a vaginal hysterectomy independently, and 46%, an abdominal hysterectomy. Barely 50% of first-year fellows in all subspecialties studied could independently set up a retractor for laparotomy and appropriately pack and mobilize the bowel for pelvic surgery.1
Today the hysterectomy procedure has become the proverbial alphabet soup. Trainees are confronted with having to learn not only the TVH and the TAH but also the LAVH (laparoscopic-assisted vaginal hysterectomy), LSH (laparoscopic supracervical hysterectomy), TLH (total laparoscopic hysterectomy), and RALH (robot-assisted laparoscopic hysterectomy).2 With a mandated 80-hour residency workweek restriction and an increasing number of minimally invasive hysterectomies performed nationally, a perfect storm exists for critically evaluating the current paradigm of resident and fellow surgical training.3
One may wonder if current controversies surrounding many of the technologic advancements in gynecologic surgery result from inadequate training and too many treatment options or from flaws in the actual devices. A “see one, do one, teach one” approach to assimilating surgical skills is no longer an accepted approach, and although the “10,000-hour rule” of focused practice to attain expertise makes sense, how can a trainee gain enough exposure to achieve competency?
Related article:
The Extracorporeal C-Incision Tissue Extraction (ExCITE) technique
Simulation: A creditable training tactic
This is where simulation—whether low or high fidelity—potentially can fill in some of those training gaps. Simulation in medicine is a proven instructional design strategy in which learning is an active and experiential process. Studies clearly have shown that simulation-based medical education (SBME) with deliberate practice is superior to traditional clinical medical education in achieving specific clinical skill acquisition goals.4
This special Update on minimally invasive gynecologic surgery offers a 30,000-foot overview of the current state of simulation in gynecologic surgical training. Equally important to this conversation is the process by which a trained individual can obtain the appropriate credentials and subsequent privileging to perform various surgical procedures. Simulation has begun to play a significant role not only in an individual’s initial credentialing and privileging in surgery but also in maintaining those privileges.
Read about the evolving role of simulation in gyn surgery training.
Simulation's evolving role in gyn surgery training
Recently, the traditional model of gynecologic surgical training has been impacted by the exponential growth of technology (surgical devices), increased surgical options, and the limited work hours of trainees. As a result, simulation-based medical education has been identified as a potential solution to address deficits in surgical training. Fortunately, all modalities of surgery are now amenable to improvements in surgical education via simulation.5
Although basic skill training in the standard areas of hand-eye coordination, tissue handling, and instrument use still is prerequisite, the integration of both low- and high-fidelity simulation technologies--with enhanced functionality--now allows for a more comprehensive approach to understanding surgical anatomy. In addition, simulation training provides the opportunity for independent practice of full surgical procedures and, in many instances, offers objective and instantaneous assessment feedback for the learner. This discussion highlights some of the relevant literature on simulation training and the impact of surgical simulation on hysteroscopy and laparoscopy.
Box trainers and virtual reality simulators in hysteroscopy training
Hysteroscopic surgery allows direct endoscopic visualization of the uterine cavity for both diagnostic and therapeutic purposes. While the majority of these procedures are generally low risk, operative hysteroscopic experience minimizes the possibility of significant procedure-related complications, such as uterine perforation.5 The literature repeatedly shows that significant differences exist in skill and sense of preparedness between the novice or inexperienced surgeon (resident trainee) and the expert in hysteroscopic surgery.6-8
Both low- and high-fidelity hysteroscopic simulators can be used to fine-tune operator skills. Low-fidelity simulators such as box trainers, which focus on skills like endometrial ablation and hysteroscopic resection with energy, have been shown to measurably improve performance, and they are well-received by participants. Low-fidelity simulations that incorporate vegetable/fruit or animal models (for example, porcine bladders and cattle uteri) have also been employed with success.9
On the high-fidelity end, surgical trainees can now experience hysteroscopic surgery simulation through virtual reality simulators, which have evolved with improvements in technology (FIGURE 1). Many high-fidelity simulators have been developed, and technical skill and theoretical knowledge improve with their use. Overall, trainees have provided positive feedback regarding the realism and training capacity afforded by virtual reality simultors.10,11

Various simulators are equipped with complete training curriculums that focus on essential surgical skills. Common troubleshooting techniques taught via simulator include establishing and maintaining clear views, detecting and coagulating bleeding sources, fluid management and handling, and instrument failure. Learners can perform these sessions repeatedly, independent of their respective starting skill level. On completion of simulation training, the trainee is given objective performance assessments on economy of motion, visualization, safety, fluid handling, and other skills.
Related article:
ExCITE: Minimally invasive tissue extraction made simple with simulation
Learning the complexities of laparoscopy through simulation
Laparoscopic surgery (both conventional and robot assisted) allows for a minimally invasive, cost-effective, and rapid-recovery approach to the management of many common gynecologic conditions. In both approaches, the learning curve to reach competency is steep. Conventional laparoscopy requires unique surgical skills, including adapting to a 2-dimensional field with altered depth perception; this creates challenges in spatial reasoning and achieving proficiency in video-eye-hand coordination as a result of the fulcrum effect inherent in laparoscopic instrumentation. This is further complicated by the essential dexterity required to complete dissections and suturing.12,13
Robot-assisted laparoscopic surgery requires significant modifications to adapt to a 3-dimensional view. In addition, this approach incorporates another level of complexity (and challenge to attaining mastery), namely, using remotely controlled multiple instrument arms with no tactile feedback.
Importantly, some residency training programs are structured unevenly, emphasizing one or the other surgical modality.14 As a result, this propagates certain skills--or lack thereof--on graduation, and thus highlights potential areas of laparoscopic training that need to be improved and enhanced.
Increasing the learning potential
The growing integration of low- and high-fidelity simulation training in laparoscopic surgery has led to improved skill acquisition.12,13,15,16 A well-established low-fidelity simulation model is the Fundamentals of Laparoscopic Surgery module, through which trainees are taught vital psychomotor skills via a validated box trainer that is also supported by a cognitive component (FIGURE 2).17,18

The advent of laparoscopic virtual reality training systems has raised the learning potential further, even for experienced surgeons. Some benefits of virtual reality simulation in conventional laparoscopy include education on an interactive 3D pelvis, step-by-step procedural guidance, a comprehensive return of performance metrics on vital laparoscopic skills, and the incorporation of advanced skills such as laparoscopic suturing, complex dissections, and lysis of adhesions.
In the arena of robot-assisted procedures, simulation modules are available for learning fundamental skill development in hand-eye coordination, depth perception, bimanual manipulation, camera navigation, and wrist articulation.
In both conventional and robot-assisted laparoscopy simulation pathways, complete procedural curriculums (for example, hysterectomy with adnexectomy) are available. Thus, learners can start a procedure or technique at a point applicable to them, practice repeatedly until competency, and eventually become proficient (FIGURE 3).

Generally, high-fidelity computerized simulators provide a comprehensive performance report on completion of training, along with a complete recording of the trainee's encounter during accruement of skill. Most importantly, laparoscopic training via simulation has been validated to translate into improved operating room performance by impacting operating times, safety profiles, and surgical skill growth.15,19
Related article:
Complete colpectomy & colpocleisis: Model for simulation
Simulation is a mainstream training tool
The skills gap between expert surgeons and new trainees continues to widen. A comprehensive educational pathway that provides an optimistic safety profile, abides by time constraints, and elevates skill sets will fall to simulation-based surgical training.20,21 Surgical competence is defined not simply by observation and Halstedian technique but by a combination of cognitive and behavioral abilities as well as perceptual and psychomotor skills. It is impractical to expect current learners to become proficient in visuospatial and tactile perception and to demonstrate technical competency without supplementing their training.22-24 Ultimately, as experience with both low- and high-fidelity surgical simulation grows, the predictive validity of this type of training pathway will become more readily apparent. In other words, improved performance in the simulated environment will translate into improved performance in the operating room.
Read about how gyn surgery simulation is being incorporated into credentialing and privileging
Incorporating gyn surgery simulation into credentialing and privileging
Over the last 25 years surgeons have seen unprecedented changes in technology that have revolutionized our surgical approaches to common gynecologic conditions. In the past, granting surgical privileges was pretty straightforward. Surgeons were granted privileges based on successfully completing their training, and subsequent renewal of those privileges was based on not having any significant misadventures or complications. With the advent of laparoscopy, hysteroscopy, and then robot-assisted surgery, training surgeons and verifying their competency has become much more complicated. The variety of surgical approaches now being taught coupled with reduced resident training time and decreasing case volumes have significantly impacted the traditional methodologies of surgical training.25,26
Related article:
How the AAGL is trying to improve outcomes for patients undergoing robot-assisted gynecologic surgery
High-tech surgery demands high-tech training
The development of high-tech surgical approaches has been accompanied by the natural development of simulation models to help with training. Initially, inanimate models, animal labs, and cadavers were used. Over the last 15 years, several innovative companies have developed virtual reality simulation platforms for laparoscopy, hysteroscopy, and even robotics.27 These virtual reality simulators allow students to develop the psychomotor skills necessary to perform minimally invasive procedures and to practice those skills until they can demonstrate proficiency before operating on a live patient.
Most would agree that the key to learning a surgical skill is to "practice, practice, practice."28 Many studies have shown that improvement in surgical outcomes is clearly related to a surgeon's case volume.29,30 But with case volumes decreased, simulation has evolved as the best training alternative. Current surgical simulators enable a student to engage in "deliberate practice"; that is, to have tasks with well-defined goals, to be motivated to improve, and to receive immediate feedback along with opportunities for repetition and refinements of performance.
Simulation allows students to try different surgical techniques and to use "deliberate practice" avoidance of errors in a controlled, safe situation that provides immediate performance feedback.31 Currently, virtual reality simulators are available for hysteroscopy, laparoscopy, and robot-assisted gynecologic applications. Early models focused solely on developing a learner's psychomotor skills necessary to safely perform minimally invasive surgeries. Newer simulators add a cognitive component to help students learn specific procedures, such as adnexectomy and hysterectomy.32
Based on the aviation simulator training model, the AAGL endorsed a Gynecologic Robotic Surgery Credentialing and Privileging Guideline in 2014; this guidance relies heavily on simulation for initial training as well as for subsequent annual recertification.33 Many institutions, including the MultiCare Health System in Tacoma, Washington, require all surgeons--even high-volume surgeons--to demonstrate proficiency annually by passing required robotic simulation exercises at least 2 times consecutively in order to maintain robotic surgery privileges.34
A work-around for a simulation drawback
Using simulation for recertification has been criticized because, although it can confirm that a surgeon is skilled enough to operate the tool, it does not evaluate surgical judgment or technique. In response, crowdsourced review of an individual surgeon's surgical videos has proven to be a useful, dependable way to give a surgeon direct feedback regarding his or her performance on a live patient.35 Many institutions now use this technology not only for initial training but also for helping surgeons improve with direct feedback from master surgeon reviewers. Other institutions have considered replacing annual re-credentialing case volume requirements with this technology, which actually assesses competence in a more accurate way.36
Related article:
Flight plan for robotic surgery credentialing: New AAGL guidelines
A new flight plan
The bottom line is that the training and annual recertification of future surgeons now mimics closely the pathway that all airplane pilots are required to follow.
Initial training will require mastery of surgical techniques using a simulator before taking a "solo flight" on a live patient.
Maintenance of privileges now requires either large case volumes or skills testing on a simulator. Many institutions now also require an annual "check ride," such as a crowdsourced video review of a surgeon's cases, as described above.
Re-credentialing. Just as the "see one, do one, teach one" model is now part of our historical legacy, re-credentialing simply by avoiding misadventures and staying out of trouble will go the way of paper medical records. Our future will certainly require an annual objective evaluation of good surgical judgment and surgical technique proficiency. Surgical simulation will be the norm for all of us.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Gynecologic surgeons who trained in the early 1990s may feel that the practice of gynecologic surgery seemed simpler back then. There were really only 2 ways to perform a hysterectomy: vaginally (TVH—total vaginal hysterectomy) and abdominally (TAH—total abdominal hysterectomy). Global endometrial ablation devices were not an established treatment for abnormal uterine bleeding, and therapeutic advancements such as hormonally laden intrauterine devices, vaginal mesh kits, and surgical robots did not exist. The options in the surgical toolbox were limited, and the general expectation in residency was long hours. During that period, consistent exposure to the operating room and case volume allowed one to graduate confidant in one’s surgical skills.

The changing landscape of gynecologic surgery
Fast-forward to 2017. Now, so many variables affect the ability to produce a well-trained gynecologic surgeon. In fact, in 2015 Guntupalli and colleagues studied the preparedness of ObGyn residents for fellowship training in the 4 subspecialties of female pelvic medicine and reconstructive surgery, gynecologic oncology, maternal-fetal medicine, and reproductive endocrinology-infertility.1 Through a validated survey of fellowship program directors, the authors found that only 20% of first-year fellows were able to perform a vaginal hysterectomy independently, and 46%, an abdominal hysterectomy. Barely 50% of first-year fellows in all subspecialties studied could independently set up a retractor for laparotomy and appropriately pack and mobilize the bowel for pelvic surgery.1
Today the hysterectomy procedure has become the proverbial alphabet soup. Trainees are confronted with having to learn not only the TVH and the TAH but also the LAVH (laparoscopic-assisted vaginal hysterectomy), LSH (laparoscopic supracervical hysterectomy), TLH (total laparoscopic hysterectomy), and RALH (robot-assisted laparoscopic hysterectomy).2 With a mandated 80-hour residency workweek restriction and an increasing number of minimally invasive hysterectomies performed nationally, a perfect storm exists for critically evaluating the current paradigm of resident and fellow surgical training.3
One may wonder if current controversies surrounding many of the technologic advancements in gynecologic surgery result from inadequate training and too many treatment options or from flaws in the actual devices. A “see one, do one, teach one” approach to assimilating surgical skills is no longer an accepted approach, and although the “10,000-hour rule” of focused practice to attain expertise makes sense, how can a trainee gain enough exposure to achieve competency?
Related article:
The Extracorporeal C-Incision Tissue Extraction (ExCITE) technique
Simulation: A creditable training tactic
This is where simulation—whether low or high fidelity—potentially can fill in some of those training gaps. Simulation in medicine is a proven instructional design strategy in which learning is an active and experiential process. Studies clearly have shown that simulation-based medical education (SBME) with deliberate practice is superior to traditional clinical medical education in achieving specific clinical skill acquisition goals.4
This special Update on minimally invasive gynecologic surgery offers a 30,000-foot overview of the current state of simulation in gynecologic surgical training. Equally important to this conversation is the process by which a trained individual can obtain the appropriate credentials and subsequent privileging to perform various surgical procedures. Simulation has begun to play a significant role not only in an individual’s initial credentialing and privileging in surgery but also in maintaining those privileges.
Read about the evolving role of simulation in gyn surgery training.
Simulation's evolving role in gyn surgery training
Recently, the traditional model of gynecologic surgical training has been impacted by the exponential growth of technology (surgical devices), increased surgical options, and the limited work hours of trainees. As a result, simulation-based medical education has been identified as a potential solution to address deficits in surgical training. Fortunately, all modalities of surgery are now amenable to improvements in surgical education via simulation.5
Although basic skill training in the standard areas of hand-eye coordination, tissue handling, and instrument use still is prerequisite, the integration of both low- and high-fidelity simulation technologies--with enhanced functionality--now allows for a more comprehensive approach to understanding surgical anatomy. In addition, simulation training provides the opportunity for independent practice of full surgical procedures and, in many instances, offers objective and instantaneous assessment feedback for the learner. This discussion highlights some of the relevant literature on simulation training and the impact of surgical simulation on hysteroscopy and laparoscopy.
Box trainers and virtual reality simulators in hysteroscopy training
Hysteroscopic surgery allows direct endoscopic visualization of the uterine cavity for both diagnostic and therapeutic purposes. While the majority of these procedures are generally low risk, operative hysteroscopic experience minimizes the possibility of significant procedure-related complications, such as uterine perforation.5 The literature repeatedly shows that significant differences exist in skill and sense of preparedness between the novice or inexperienced surgeon (resident trainee) and the expert in hysteroscopic surgery.6-8
Both low- and high-fidelity hysteroscopic simulators can be used to fine-tune operator skills. Low-fidelity simulators such as box trainers, which focus on skills like endometrial ablation and hysteroscopic resection with energy, have been shown to measurably improve performance, and they are well-received by participants. Low-fidelity simulations that incorporate vegetable/fruit or animal models (for example, porcine bladders and cattle uteri) have also been employed with success.9
On the high-fidelity end, surgical trainees can now experience hysteroscopic surgery simulation through virtual reality simulators, which have evolved with improvements in technology (FIGURE 1). Many high-fidelity simulators have been developed, and technical skill and theoretical knowledge improve with their use. Overall, trainees have provided positive feedback regarding the realism and training capacity afforded by virtual reality simultors.10,11

Various simulators are equipped with complete training curriculums that focus on essential surgical skills. Common troubleshooting techniques taught via simulator include establishing and maintaining clear views, detecting and coagulating bleeding sources, fluid management and handling, and instrument failure. Learners can perform these sessions repeatedly, independent of their respective starting skill level. On completion of simulation training, the trainee is given objective performance assessments on economy of motion, visualization, safety, fluid handling, and other skills.
Related article:
ExCITE: Minimally invasive tissue extraction made simple with simulation
Learning the complexities of laparoscopy through simulation
Laparoscopic surgery (both conventional and robot assisted) allows for a minimally invasive, cost-effective, and rapid-recovery approach to the management of many common gynecologic conditions. In both approaches, the learning curve to reach competency is steep. Conventional laparoscopy requires unique surgical skills, including adapting to a 2-dimensional field with altered depth perception; this creates challenges in spatial reasoning and achieving proficiency in video-eye-hand coordination as a result of the fulcrum effect inherent in laparoscopic instrumentation. This is further complicated by the essential dexterity required to complete dissections and suturing.12,13
Robot-assisted laparoscopic surgery requires significant modifications to adapt to a 3-dimensional view. In addition, this approach incorporates another level of complexity (and challenge to attaining mastery), namely, using remotely controlled multiple instrument arms with no tactile feedback.
Importantly, some residency training programs are structured unevenly, emphasizing one or the other surgical modality.14 As a result, this propagates certain skills--or lack thereof--on graduation, and thus highlights potential areas of laparoscopic training that need to be improved and enhanced.
Increasing the learning potential
The growing integration of low- and high-fidelity simulation training in laparoscopic surgery has led to improved skill acquisition.12,13,15,16 A well-established low-fidelity simulation model is the Fundamentals of Laparoscopic Surgery module, through which trainees are taught vital psychomotor skills via a validated box trainer that is also supported by a cognitive component (FIGURE 2).17,18

The advent of laparoscopic virtual reality training systems has raised the learning potential further, even for experienced surgeons. Some benefits of virtual reality simulation in conventional laparoscopy include education on an interactive 3D pelvis, step-by-step procedural guidance, a comprehensive return of performance metrics on vital laparoscopic skills, and the incorporation of advanced skills such as laparoscopic suturing, complex dissections, and lysis of adhesions.
In the arena of robot-assisted procedures, simulation modules are available for learning fundamental skill development in hand-eye coordination, depth perception, bimanual manipulation, camera navigation, and wrist articulation.
In both conventional and robot-assisted laparoscopy simulation pathways, complete procedural curriculums (for example, hysterectomy with adnexectomy) are available. Thus, learners can start a procedure or technique at a point applicable to them, practice repeatedly until competency, and eventually become proficient (FIGURE 3).

Generally, high-fidelity computerized simulators provide a comprehensive performance report on completion of training, along with a complete recording of the trainee's encounter during accruement of skill. Most importantly, laparoscopic training via simulation has been validated to translate into improved operating room performance by impacting operating times, safety profiles, and surgical skill growth.15,19
Related article:
Complete colpectomy & colpocleisis: Model for simulation
Simulation is a mainstream training tool
The skills gap between expert surgeons and new trainees continues to widen. A comprehensive educational pathway that provides an optimistic safety profile, abides by time constraints, and elevates skill sets will fall to simulation-based surgical training.20,21 Surgical competence is defined not simply by observation and Halstedian technique but by a combination of cognitive and behavioral abilities as well as perceptual and psychomotor skills. It is impractical to expect current learners to become proficient in visuospatial and tactile perception and to demonstrate technical competency without supplementing their training.22-24 Ultimately, as experience with both low- and high-fidelity surgical simulation grows, the predictive validity of this type of training pathway will become more readily apparent. In other words, improved performance in the simulated environment will translate into improved performance in the operating room.
Read about how gyn surgery simulation is being incorporated into credentialing and privileging
Incorporating gyn surgery simulation into credentialing and privileging
Over the last 25 years surgeons have seen unprecedented changes in technology that have revolutionized our surgical approaches to common gynecologic conditions. In the past, granting surgical privileges was pretty straightforward. Surgeons were granted privileges based on successfully completing their training, and subsequent renewal of those privileges was based on not having any significant misadventures or complications. With the advent of laparoscopy, hysteroscopy, and then robot-assisted surgery, training surgeons and verifying their competency has become much more complicated. The variety of surgical approaches now being taught coupled with reduced resident training time and decreasing case volumes have significantly impacted the traditional methodologies of surgical training.25,26
Related article:
How the AAGL is trying to improve outcomes for patients undergoing robot-assisted gynecologic surgery
High-tech surgery demands high-tech training
The development of high-tech surgical approaches has been accompanied by the natural development of simulation models to help with training. Initially, inanimate models, animal labs, and cadavers were used. Over the last 15 years, several innovative companies have developed virtual reality simulation platforms for laparoscopy, hysteroscopy, and even robotics.27 These virtual reality simulators allow students to develop the psychomotor skills necessary to perform minimally invasive procedures and to practice those skills until they can demonstrate proficiency before operating on a live patient.
Most would agree that the key to learning a surgical skill is to "practice, practice, practice."28 Many studies have shown that improvement in surgical outcomes is clearly related to a surgeon's case volume.29,30 But with case volumes decreased, simulation has evolved as the best training alternative. Current surgical simulators enable a student to engage in "deliberate practice"; that is, to have tasks with well-defined goals, to be motivated to improve, and to receive immediate feedback along with opportunities for repetition and refinements of performance.
Simulation allows students to try different surgical techniques and to use "deliberate practice" avoidance of errors in a controlled, safe situation that provides immediate performance feedback.31 Currently, virtual reality simulators are available for hysteroscopy, laparoscopy, and robot-assisted gynecologic applications. Early models focused solely on developing a learner's psychomotor skills necessary to safely perform minimally invasive surgeries. Newer simulators add a cognitive component to help students learn specific procedures, such as adnexectomy and hysterectomy.32
Based on the aviation simulator training model, the AAGL endorsed a Gynecologic Robotic Surgery Credentialing and Privileging Guideline in 2014; this guidance relies heavily on simulation for initial training as well as for subsequent annual recertification.33 Many institutions, including the MultiCare Health System in Tacoma, Washington, require all surgeons--even high-volume surgeons--to demonstrate proficiency annually by passing required robotic simulation exercises at least 2 times consecutively in order to maintain robotic surgery privileges.34
A work-around for a simulation drawback
Using simulation for recertification has been criticized because, although it can confirm that a surgeon is skilled enough to operate the tool, it does not evaluate surgical judgment or technique. In response, crowdsourced review of an individual surgeon's surgical videos has proven to be a useful, dependable way to give a surgeon direct feedback regarding his or her performance on a live patient.35 Many institutions now use this technology not only for initial training but also for helping surgeons improve with direct feedback from master surgeon reviewers. Other institutions have considered replacing annual re-credentialing case volume requirements with this technology, which actually assesses competence in a more accurate way.36
Related article:
Flight plan for robotic surgery credentialing: New AAGL guidelines
A new flight plan
The bottom line is that the training and annual recertification of future surgeons now mimics closely the pathway that all airplane pilots are required to follow.
Initial training will require mastery of surgical techniques using a simulator before taking a "solo flight" on a live patient.
Maintenance of privileges now requires either large case volumes or skills testing on a simulator. Many institutions now also require an annual "check ride," such as a crowdsourced video review of a surgeon's cases, as described above.
Re-credentialing. Just as the "see one, do one, teach one" model is now part of our historical legacy, re-credentialing simply by avoiding misadventures and staying out of trouble will go the way of paper medical records. Our future will certainly require an annual objective evaluation of good surgical judgment and surgical technique proficiency. Surgical simulation will be the norm for all of us.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Guntupalli SR, Doo DW, Guy M, et al. Preparedness of obstetrics and gynecology residents for fellowship training. Obstet Gynecol. 2015;126(3):559–568.
- Pulliam SJ, Berkowitz LR. Smaller pieces of the hysterectomy pie: current challenges in resident surgical education. Obstet Gynecol. 2009;113(2 pt 1):395–398.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
- McGaghie WC, Issenberg SB, Cohen ER, Barsuk JH, Wayne DB. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86(6):706–711.
- Smith ML. Simulation and education in gynecologic surgery. Obstet Gynecol Clin North Am. 2011;38(4):733–740.
- Raymond E, Ternamian A, Leyland N, Tolomiczenko G. Endoscopy teaching in Canada: a survey of obstetrics and gynecology program directors and graduating residents. J Minim Invasive Gynecol. 2006;13(1):10–16.
- Goff BA, VanBlaricom A, Mandel L, Chinn M, Nielsen P. Comparison of objective, structured assessment of technical skills with a virtual reality hysteroscopy trainer and standard latex hysteroscopy model. J Reprod Med. 2007;52(5):407–412.
- Singhi A. Comparison of complications rates in endoscopic surgery performed by a clinical assistant vs an experienced endoscopic surgeon. J Gynecol Endosc Surg. 2009;1(1):40–46.
- Savran MM, Sorensen SM, Konge L, Tolsgaard MG, Bjerrum F. Training and assessment of hysteroscopic skills: a systematic review. J Surg Ed. 2016;73(5):906–918.
- Panel P, Bajka M, Le Tohic A, Ghoneimi AE, Chis C, Cotin S. Hysteroscopic placement of tubal sterilization implants: virtual reality simulator training. Surg Endosc. 2012;26(7):1986–1996.
- Bajka M, Tuchschmid S, Streich M, Fink D, Szekely G, Harders M. Evaluation of a new virtual-reality training simulator for hysteroscopy. Surg Endosc. 2009;23(9):2026–2033.
- Scott DJ, Bergen PC, Rege RV, et al. Laparoscopic training on bench models: better and more cost effective than operating room experience? J Am Coll Surg. 2000;191(3):272–283.
- Scott-Conner CE, Hall TJ, Anglin BL, et al. The integration of laparoscopy into a surgical residency and implications for the training environment. Surg Endosc. 1994;8(9):1054–1057.
- Berkowitz RL, Minkoff H. A call for change in a changing world. Obstet Gynecol. 2016;127(1):153–156.
- Larsen CR, Oestergaard J, Ottesen BS, Soerensen JL. The efficacy of virtual reality simulation training in laparoscopy: a systematic review of randomized trials. Acta Obstet Gynecol Scand. 2012;91(9):1015–1028.
- Aggarwal R, Ward J, Balasundaram I, Sains P, Athanasiou T, Darzi A. Proving the effectiveness of virtual reality simulation for training in laparoscopic surgery. AnnSurg. 2007;246(5):771–779.
- Oropesa I, Sanchez-Gonzalez P, Lamata P, et al. Methods and tools for objective assessment of psychomotor skills in laparoscopic surgery. J Surg Res. 2011;171(1):e81–e95.
- Rooney DM, Brissman IC, Finks JF, Gauger PG. Fundamentals of Laparoscopic Surgery manual test: is videotaped performance assessment an option? J Surg Educ. 2015;72(1):90–95.
- Seymour NE, Gallagher AG, Roman SA, et al. Virtual reality training improves operating room performance: results of a randomized, double-blinded study. Ann Surg. 2002;236(4):458–463, 63–64.
- Aggarwal R, Tully A, Grantcharov T, et al. Virtual reality simulation training can improve technical skills during laparoscopic salpingectomy for ectopic pregnancy. BJOG. 2006;113(12):1382–1387.
- Darzi A, Smith S, Taffinder N. Assessing operative skill. Needs to become more objective. BMJ. 1999;318(7188):887–888.
- Moorthy K, Munz Y, Sarker SK, Darzi A. Objective assessment of technical skills in surgery. BMJ. 2003;327(7422):1032–1037.
- Grantcharov TP, Bardram L, Funch-Jensen P, Rosenberg J. Assessment of technical surgical skills. Eur J Surg. 2002;168(3):139–144.
- Wanzel KR, Hamstra SJ, Caminiti MF, Anastakis DJ, Grober ED, Reznick RK. Visual-spatial ability correlates with efficiency of hand motion and successful surgical performance. Surgery. 2003;134(5):750–757.
- Einarsson JI, Young A, Tsien L, Sangi-Haghpeykar H. Perceived proficiency in endoscopic techniques among senior obstetrics and gynecology residents. J Am Assoc Gynecol Laparosc. 2002;9(2):158–164.
- Cohen SL, Hinchcliffe E. Is surgical training in ob-gyn residency adequate? Contemp ObGyn. . Published July 22, 2016. Accessed October 18, 2017.
- Bric JD, Lumbard DC, Frelich MJ, Gould JC. Current state of virtual reality simulation in robotic surgery training: a review. Surg Endosc. 2016;30(6):2169–2178.
- Gladwell M. Outliers: The Story of Success. New York, New York: Little Brown and Co; 2008.
- Boyd LR, Novetsky AP, Curtain JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116(4):909–915.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effects of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119(4):709–716.
- Kotsis SV, Chung KC. Application of the “see one, do one, teach one” concept in surgical training. Plast Reconstr Surg. 2013;131(5):1194–1201.
- Maestro AR Hysterectomy Module. Mimic simulation website. http://www.mimicsimulation.com/hysterectomy/. Accessed October 18, 2017.
- AAGL. Guidelines for privileging for robotic-assisted gynecologic laparoscopy. J Minim Invasiv Gynecol, 2014;21(2):157–167.
- Lenihan JP Jr. Navigating credentialing and privileging and learning curves in robotics with an evidence and experienced-based approach. Clin Obstet Gynecol. 2011;54(3):382–390.
- Polin MR, Siddiqui NY, Comstock BA, et al. . Am J Obstet Gynecol. 2016;215(5):644.e1–644.e7.
- Continuous People Improvement. C-SATS website. https://www.csats.com/customers-main/. Accessed October 18, 2017.
- Guntupalli SR, Doo DW, Guy M, et al. Preparedness of obstetrics and gynecology residents for fellowship training. Obstet Gynecol. 2015;126(3):559–568.
- Pulliam SJ, Berkowitz LR. Smaller pieces of the hysterectomy pie: current challenges in resident surgical education. Obstet Gynecol. 2009;113(2 pt 1):395–398.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
- McGaghie WC, Issenberg SB, Cohen ER, Barsuk JH, Wayne DB. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86(6):706–711.
- Smith ML. Simulation and education in gynecologic surgery. Obstet Gynecol Clin North Am. 2011;38(4):733–740.
- Raymond E, Ternamian A, Leyland N, Tolomiczenko G. Endoscopy teaching in Canada: a survey of obstetrics and gynecology program directors and graduating residents. J Minim Invasive Gynecol. 2006;13(1):10–16.
- Goff BA, VanBlaricom A, Mandel L, Chinn M, Nielsen P. Comparison of objective, structured assessment of technical skills with a virtual reality hysteroscopy trainer and standard latex hysteroscopy model. J Reprod Med. 2007;52(5):407–412.
- Singhi A. Comparison of complications rates in endoscopic surgery performed by a clinical assistant vs an experienced endoscopic surgeon. J Gynecol Endosc Surg. 2009;1(1):40–46.
- Savran MM, Sorensen SM, Konge L, Tolsgaard MG, Bjerrum F. Training and assessment of hysteroscopic skills: a systematic review. J Surg Ed. 2016;73(5):906–918.
- Panel P, Bajka M, Le Tohic A, Ghoneimi AE, Chis C, Cotin S. Hysteroscopic placement of tubal sterilization implants: virtual reality simulator training. Surg Endosc. 2012;26(7):1986–1996.
- Bajka M, Tuchschmid S, Streich M, Fink D, Szekely G, Harders M. Evaluation of a new virtual-reality training simulator for hysteroscopy. Surg Endosc. 2009;23(9):2026–2033.
- Scott DJ, Bergen PC, Rege RV, et al. Laparoscopic training on bench models: better and more cost effective than operating room experience? J Am Coll Surg. 2000;191(3):272–283.
- Scott-Conner CE, Hall TJ, Anglin BL, et al. The integration of laparoscopy into a surgical residency and implications for the training environment. Surg Endosc. 1994;8(9):1054–1057.
- Berkowitz RL, Minkoff H. A call for change in a changing world. Obstet Gynecol. 2016;127(1):153–156.
- Larsen CR, Oestergaard J, Ottesen BS, Soerensen JL. The efficacy of virtual reality simulation training in laparoscopy: a systematic review of randomized trials. Acta Obstet Gynecol Scand. 2012;91(9):1015–1028.
- Aggarwal R, Ward J, Balasundaram I, Sains P, Athanasiou T, Darzi A. Proving the effectiveness of virtual reality simulation for training in laparoscopic surgery. AnnSurg. 2007;246(5):771–779.
- Oropesa I, Sanchez-Gonzalez P, Lamata P, et al. Methods and tools for objective assessment of psychomotor skills in laparoscopic surgery. J Surg Res. 2011;171(1):e81–e95.
- Rooney DM, Brissman IC, Finks JF, Gauger PG. Fundamentals of Laparoscopic Surgery manual test: is videotaped performance assessment an option? J Surg Educ. 2015;72(1):90–95.
- Seymour NE, Gallagher AG, Roman SA, et al. Virtual reality training improves operating room performance: results of a randomized, double-blinded study. Ann Surg. 2002;236(4):458–463, 63–64.
- Aggarwal R, Tully A, Grantcharov T, et al. Virtual reality simulation training can improve technical skills during laparoscopic salpingectomy for ectopic pregnancy. BJOG. 2006;113(12):1382–1387.
- Darzi A, Smith S, Taffinder N. Assessing operative skill. Needs to become more objective. BMJ. 1999;318(7188):887–888.
- Moorthy K, Munz Y, Sarker SK, Darzi A. Objective assessment of technical skills in surgery. BMJ. 2003;327(7422):1032–1037.
- Grantcharov TP, Bardram L, Funch-Jensen P, Rosenberg J. Assessment of technical surgical skills. Eur J Surg. 2002;168(3):139–144.
- Wanzel KR, Hamstra SJ, Caminiti MF, Anastakis DJ, Grober ED, Reznick RK. Visual-spatial ability correlates with efficiency of hand motion and successful surgical performance. Surgery. 2003;134(5):750–757.
- Einarsson JI, Young A, Tsien L, Sangi-Haghpeykar H. Perceived proficiency in endoscopic techniques among senior obstetrics and gynecology residents. J Am Assoc Gynecol Laparosc. 2002;9(2):158–164.
- Cohen SL, Hinchcliffe E. Is surgical training in ob-gyn residency adequate? Contemp ObGyn. . Published July 22, 2016. Accessed October 18, 2017.
- Bric JD, Lumbard DC, Frelich MJ, Gould JC. Current state of virtual reality simulation in robotic surgery training: a review. Surg Endosc. 2016;30(6):2169–2178.
- Gladwell M. Outliers: The Story of Success. New York, New York: Little Brown and Co; 2008.
- Boyd LR, Novetsky AP, Curtain JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116(4):909–915.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effects of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119(4):709–716.
- Kotsis SV, Chung KC. Application of the “see one, do one, teach one” concept in surgical training. Plast Reconstr Surg. 2013;131(5):1194–1201.
- Maestro AR Hysterectomy Module. Mimic simulation website. http://www.mimicsimulation.com/hysterectomy/. Accessed October 18, 2017.
- AAGL. Guidelines for privileging for robotic-assisted gynecologic laparoscopy. J Minim Invasiv Gynecol, 2014;21(2):157–167.
- Lenihan JP Jr. Navigating credentialing and privileging and learning curves in robotics with an evidence and experienced-based approach. Clin Obstet Gynecol. 2011;54(3):382–390.
- Polin MR, Siddiqui NY, Comstock BA, et al. . Am J Obstet Gynecol. 2016;215(5):644.e1–644.e7.
- Continuous People Improvement. C-SATS website. https://www.csats.com/customers-main/. Accessed October 18, 2017.
AMA: Patient mix has become less uninsured since 2012
Uninsured patients made up a smaller share of the average physician’s practice in 2016 than in 2012, according to a survey by the American Medical Association.
For the average practice in 2016, 6.1% of patients were uninsured, compared with 6.9% in 2012. That significant drop of 0.8 percentage points was accompanied by significant increases in the number of patients covered by Medicaid and by private insurance, the AMA said in a report released Oct. 30.
“The overall picture from new physician-reported data is of more patients covered and fewer uninsured, but the findings also indicate that the improvement along those lines was concentrated in states that expanded their Medicaid programs under the ACA,” said David O. Barbe, MD, AMA president.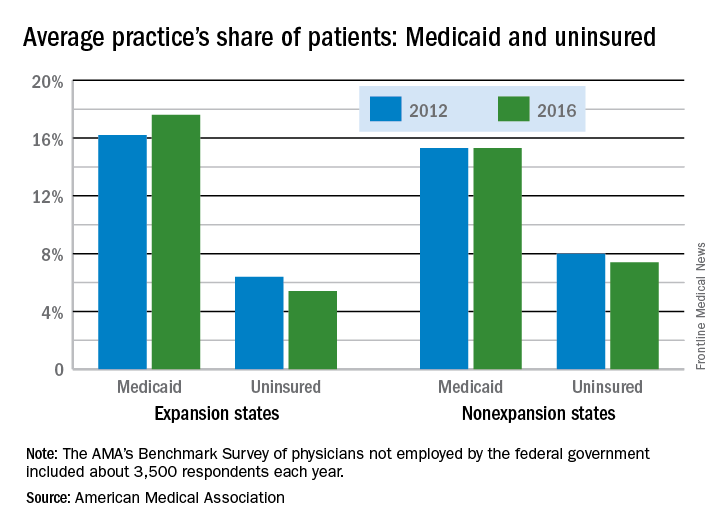
In the 31 states and the District of Columbia that expanded Medicaid, the average patient share dropped from 6.4% uninsured in 2012 to 5.4% in 2016, which was significant. In the states that did not expand Medicaid, the average share of uninsured patients went from 8.0% to 7.4% over that period, a drop that did not reach significance, the AMA said.
The changes involving Medicaid itself were of somewhat greater magnitude, in both directions: nonexpansion states saw a smaller increase and expansion states had a larger increase. In nonexpansion states the average share of a practice’s patients covered by Medicaid basically held steady at 15.3% (there was actually a very slight increase, but the AMA reported the figures for both 2012 and 2016 as 15.3%). In expansion states, the average Medicaid share went from 16.2% to 17.6% – a statistically significant increase of 1.4 percentage points, the AMA analysis shows.
The AMA report covers data from its 2012 and 2016 Benchmark Surveys, which each year involved approximately 3,500 physicians in patient care who were not employed by the federal government.
Uninsured patients made up a smaller share of the average physician’s practice in 2016 than in 2012, according to a survey by the American Medical Association.
For the average practice in 2016, 6.1% of patients were uninsured, compared with 6.9% in 2012. That significant drop of 0.8 percentage points was accompanied by significant increases in the number of patients covered by Medicaid and by private insurance, the AMA said in a report released Oct. 30.
“The overall picture from new physician-reported data is of more patients covered and fewer uninsured, but the findings also indicate that the improvement along those lines was concentrated in states that expanded their Medicaid programs under the ACA,” said David O. Barbe, MD, AMA president.
In the 31 states and the District of Columbia that expanded Medicaid, the average patient share dropped from 6.4% uninsured in 2012 to 5.4% in 2016, which was significant. In the states that did not expand Medicaid, the average share of uninsured patients went from 8.0% to 7.4% over that period, a drop that did not reach significance, the AMA said.
The changes involving Medicaid itself were of somewhat greater magnitude, in both directions: nonexpansion states saw a smaller increase and expansion states had a larger increase. In nonexpansion states the average share of a practice’s patients covered by Medicaid basically held steady at 15.3% (there was actually a very slight increase, but the AMA reported the figures for both 2012 and 2016 as 15.3%). In expansion states, the average Medicaid share went from 16.2% to 17.6% – a statistically significant increase of 1.4 percentage points, the AMA analysis shows.
The AMA report covers data from its 2012 and 2016 Benchmark Surveys, which each year involved approximately 3,500 physicians in patient care who were not employed by the federal government.
Uninsured patients made up a smaller share of the average physician’s practice in 2016 than in 2012, according to a survey by the American Medical Association.
For the average practice in 2016, 6.1% of patients were uninsured, compared with 6.9% in 2012. That significant drop of 0.8 percentage points was accompanied by significant increases in the number of patients covered by Medicaid and by private insurance, the AMA said in a report released Oct. 30.
“The overall picture from new physician-reported data is of more patients covered and fewer uninsured, but the findings also indicate that the improvement along those lines was concentrated in states that expanded their Medicaid programs under the ACA,” said David O. Barbe, MD, AMA president.
In the 31 states and the District of Columbia that expanded Medicaid, the average patient share dropped from 6.4% uninsured in 2012 to 5.4% in 2016, which was significant. In the states that did not expand Medicaid, the average share of uninsured patients went from 8.0% to 7.4% over that period, a drop that did not reach significance, the AMA said.
The changes involving Medicaid itself were of somewhat greater magnitude, in both directions: nonexpansion states saw a smaller increase and expansion states had a larger increase. In nonexpansion states the average share of a practice’s patients covered by Medicaid basically held steady at 15.3% (there was actually a very slight increase, but the AMA reported the figures for both 2012 and 2016 as 15.3%). In expansion states, the average Medicaid share went from 16.2% to 17.6% – a statistically significant increase of 1.4 percentage points, the AMA analysis shows.
The AMA report covers data from its 2012 and 2016 Benchmark Surveys, which each year involved approximately 3,500 physicians in patient care who were not employed by the federal government.
Study highlights disparities in U.S. lupus mortality
Mortality from systemic lupus erythematosus has declined since 1968 in the United States, but not as markedly as rates of death from other causes, according to a study in Annals of Internal Medicine.
Furthermore, systemic lupus erythematosus (SLE) mortality varied significantly by sex, race, and geographic region, noted Eric Y. Yen, MD, and his associates at the University of California, Los Angeles. Mortality from SLE was highest among females, black individuals, and those who lived in the South. Multivariable analyses confirmed that sex, race, and geographic region were independent risk factors for SLE mortality and that race modified relationships among SLE mortality, sex, and geographic region.
Between 1968 and 2013, there were 50,249 deaths from SLE and more than 100.8 million deaths from other causes in the United States, the researchers said. Mortality from other causes continuously dropped over the study period, but SLE mortality dropped only between 1968 and 1975 before rising continuously for 24 years. Only in 1999 did SLE mortality begin to fall again. Consequently, the ratio of SLE mortality to mortality from other causes rose by 34.6% overall between 1968 and 2013, and rose by 62.5% among blacks and by 58.6% among southerners.
After the researchers accounted for age, sex, race or ethnicity, and geographic region, the risk of death from SLE dropped significantly during 2004 through 2008, compared with 1999 through 2003, and declined even more between 2009 and 2013. Female sex, racial or ethnic minority status, residing in the South or West, and being older than 65 years all independently increased the risk of dying from SLE.
Although the South had the highest SLE mortality among whites, the West had the highest SLE mortality among all other races and ethnicities, the investigators determined. Previous research has identified pockets of increased SLE mortality in Alabama, Arkansas, Louisiana, and New Mexico, and has shown that poverty is a stronger predictor of SLE mortality than race, they noted. “Geographic differences in the quality of care of patients with lupus nephritis have also been reported, with more patients in the Northeast receiving standard-of-care medications,” they wrote. “Interactions between genetic and non-genetic factors associated with race/ethnicity and geographic differences in environment, such as increased sunlight exposure, socioeconomic factors, and access to medical care, might also influence SLE mortality.”
The National Institutes of Health, the Lupus Foundation of America, and the Rheumatology Research Foundation funded the study. The investigators reported having no conflicts of interest.
Mortality from systemic lupus erythematosus has declined since 1968 in the United States, but not as markedly as rates of death from other causes, according to a study in Annals of Internal Medicine.
Furthermore, systemic lupus erythematosus (SLE) mortality varied significantly by sex, race, and geographic region, noted Eric Y. Yen, MD, and his associates at the University of California, Los Angeles. Mortality from SLE was highest among females, black individuals, and those who lived in the South. Multivariable analyses confirmed that sex, race, and geographic region were independent risk factors for SLE mortality and that race modified relationships among SLE mortality, sex, and geographic region.
Between 1968 and 2013, there were 50,249 deaths from SLE and more than 100.8 million deaths from other causes in the United States, the researchers said. Mortality from other causes continuously dropped over the study period, but SLE mortality dropped only between 1968 and 1975 before rising continuously for 24 years. Only in 1999 did SLE mortality begin to fall again. Consequently, the ratio of SLE mortality to mortality from other causes rose by 34.6% overall between 1968 and 2013, and rose by 62.5% among blacks and by 58.6% among southerners.
After the researchers accounted for age, sex, race or ethnicity, and geographic region, the risk of death from SLE dropped significantly during 2004 through 2008, compared with 1999 through 2003, and declined even more between 2009 and 2013. Female sex, racial or ethnic minority status, residing in the South or West, and being older than 65 years all independently increased the risk of dying from SLE.
Although the South had the highest SLE mortality among whites, the West had the highest SLE mortality among all other races and ethnicities, the investigators determined. Previous research has identified pockets of increased SLE mortality in Alabama, Arkansas, Louisiana, and New Mexico, and has shown that poverty is a stronger predictor of SLE mortality than race, they noted. “Geographic differences in the quality of care of patients with lupus nephritis have also been reported, with more patients in the Northeast receiving standard-of-care medications,” they wrote. “Interactions between genetic and non-genetic factors associated with race/ethnicity and geographic differences in environment, such as increased sunlight exposure, socioeconomic factors, and access to medical care, might also influence SLE mortality.”
The National Institutes of Health, the Lupus Foundation of America, and the Rheumatology Research Foundation funded the study. The investigators reported having no conflicts of interest.
Mortality from systemic lupus erythematosus has declined since 1968 in the United States, but not as markedly as rates of death from other causes, according to a study in Annals of Internal Medicine.
Furthermore, systemic lupus erythematosus (SLE) mortality varied significantly by sex, race, and geographic region, noted Eric Y. Yen, MD, and his associates at the University of California, Los Angeles. Mortality from SLE was highest among females, black individuals, and those who lived in the South. Multivariable analyses confirmed that sex, race, and geographic region were independent risk factors for SLE mortality and that race modified relationships among SLE mortality, sex, and geographic region.
Between 1968 and 2013, there were 50,249 deaths from SLE and more than 100.8 million deaths from other causes in the United States, the researchers said. Mortality from other causes continuously dropped over the study period, but SLE mortality dropped only between 1968 and 1975 before rising continuously for 24 years. Only in 1999 did SLE mortality begin to fall again. Consequently, the ratio of SLE mortality to mortality from other causes rose by 34.6% overall between 1968 and 2013, and rose by 62.5% among blacks and by 58.6% among southerners.
After the researchers accounted for age, sex, race or ethnicity, and geographic region, the risk of death from SLE dropped significantly during 2004 through 2008, compared with 1999 through 2003, and declined even more between 2009 and 2013. Female sex, racial or ethnic minority status, residing in the South or West, and being older than 65 years all independently increased the risk of dying from SLE.
Although the South had the highest SLE mortality among whites, the West had the highest SLE mortality among all other races and ethnicities, the investigators determined. Previous research has identified pockets of increased SLE mortality in Alabama, Arkansas, Louisiana, and New Mexico, and has shown that poverty is a stronger predictor of SLE mortality than race, they noted. “Geographic differences in the quality of care of patients with lupus nephritis have also been reported, with more patients in the Northeast receiving standard-of-care medications,” they wrote. “Interactions between genetic and non-genetic factors associated with race/ethnicity and geographic differences in environment, such as increased sunlight exposure, socioeconomic factors, and access to medical care, might also influence SLE mortality.”
The National Institutes of Health, the Lupus Foundation of America, and the Rheumatology Research Foundation funded the study. The investigators reported having no conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Mortality from systemic lupus erythematosus has declined since 1968, but not as markedly as rates of death from other causes.
Major finding: The ratio of SLE mortality to mortality from other causes rose by nearly 35% between 1968 and 2013.
Data source: Analyses of the Centers for Disease Control and Prevention’s National Vital Statistics System and CDC WONDER.
Disclosures: The National Institutes of Health, the Lupus Foundation of America, and the Rheumatology Research Foundation funded the study. The investigators reported having no conflicts of interest.
Business law critical to your practice
It is no surprise that the law is playing an ever more important role in the practice of medicine. Concerns about legal issues are a source of stress for ObGyns, including increasing worries about the economics of professional liability, the anxiety of defending a legal claim, and ambiguity about what is required for compliance.1 In this article my goal is to demystify some of the most important legal principles affecting your practice and provide suggestions for avoiding legal problems.
Medical malpractice: A form of negligence
Most ObGyns instinctively think first of medical malpractice when “legal problems” are mentioned—not an unreasonable response because obstetrics has a high incidence of malpractice claims. In one study, 77% of the American College of Obstetricians and Gynecologists (ACOG) Fellows reported that they have been sued.2
At its core, malpractice is a form of negligence, or, medical practice that falls below the quality of care that a reasonably careful practitioner would provide under the circumstances. When practice falls below that “standard of care,” and it causes injury, there may be malpractice liability. Insurance usually covers the cost of defending malpractice lawsuits and paying liability (although liability is the result of a minority of malpractice suits). There are, however, collateral consequences, including the time, stress, and disruption associated with defending the suit. In addition, malpractice may trigger review by the institutions with which the physician is associated, or in extreme cases, by licensing authorities. Large malpractice settlements or verdicts must be reported to the National Practitioner Database (sometimes colloquially referred to the “problem physician” database) or a similar state database.
This article is the third installment of the new series, "The Business of Medicine," edited by Joseph Sanfilippo, MD, MBA. In September, David Kim, MD, MBA, MPH, offered marketing strategies using social media. Last month, Dr. Sanfilippo presented ways to ensure patient satisfaction and service excellence in your practice. Watch next time for "Accounting 101." Other featured topics will include investing in your practice, billing and coding, gaining the competitive advantage, understanding "best practices," and striving for cost-effective care.
Related article:
Who is liable when a surgical error occurs?
Regulation and reimbursement (“compliance”) policies
The practice of medicine is closely regulated by federal and state bodies. Many regulations apply through reimbursement policies related to Medicare and Medicaid. While malpractice liability may, at worst, result in a financial award (with the cost of defense and any award paid by insurance), regulatory problems may result in a number of unpleasant consequences, most of which are not covered by insurance. In addition to loss of reimbursement, civil penalties (even criminalpenalties in extreme cases), loss of hospital privileges, licensure discipline, and loss of Medicare-Medicaid eligibility may result from regulatory noncompliance.3
There are multivolume sets discussing these legal requirements, so here we will look only at a tiny tip of the regulatory iceberg by mentioning some common regulatory areas.
Fraud and abuse laws refer to a bundle of federal (and some state) statutes and regulations that are intended to ensure that public-funded programs such as Medicare and Medicaid are not cheated or overpaying for services. It is a violation to provide low-quality services to government-funded programs. Proper payment and coding and ensuring that services were actually performed by the professional listed (not someone else) are examples of traps for the unwary. Submitting inaccurate records may result in action to recover incorrect payments and in civil penalties. In extreme cases where there is intentional misrepresentation, there have been criminal charges and loss of future Medicare-Medicaid eligibility.
Anti-kickback, self-referral, and Stark limitations are intended to avoid unnecessary or overpriced services. When someone is receiving a benefit for ordering or recommending a product or service, it is reasonable to expect that an incentive might affect the decision to order it, likely resulting in unnecessary or suboptimal services. It is illegal to receive a kickback for using, ordering, or recommending a product or service (a pharmaceutical company could not pay a physician $10 for each prescription written for its product). It is also illegal for physicians to refer patients to other entities in which they have a financial interest (a physician could not refer a patient to a lab in which the physician has partial ownership). The Stark laws and state prohibitions on self-referral have complex series of “safe harbor” exceptions in an ocean of prohibitions.4
HIPAA and confidentiality regulations are intended to protect patient privacy. The Health Insurance Portability and Accountability Act of 1996 (HIPAA) has extensive regulations concerning both privacy and security. The medical community is well-versed in HIPAA regulations and sensitive (perhaps hypersensitive) to its requirements. Most states have patient privacy regulations that apply in addition to HIPAA and are commonly less well known.
Protecting patient confidentiality is an ethical, legal, and licensure obligation. Protecting patient confidentiality is, therefore, general duty and not tied to a specific federal program.5
Related article:
Patient with a breast mass: Why did she pursue litigation?
Insurance Fraud is the private side of fraud and abuse. Submitting private insurance claims that are false or a misrepresentation of service is generally a violation of the contract between the provider and the insurance company. It may also be a crime—it is, after all, a form of theft. Serious fraud may result in the loss of the license to practice.
The False Claims Act and Whistleblower laws make it a civil offense (and, in extreme cases, a criminal offense), to present to the government a false claim for payment of services. It may be false in the sense that the service was not provided or in the sense that service was of inadequate quality. These statutes (both federal and state) also allow for a private whistleblower to receive some of the proceeds if he or she helps the government recoup wrongful payments. Disgruntled former employees are a common source of whistleblowing.6
Abuse-reporting statutes are part of every state’s law but vary considerably. They require certain professions, including physicians, to report known or suspected abuse of children, dependent adults, and often, other groups. The failure to make required reports can result in civil liability or even (rarely) criminal charges.
Read about how organizational law affects ObGyns.
How organizational and commercial law affects ObGyns
Physicians are generally members of organizations that are engaged in the business of health care (even nonprofit organizations have business interests). There are 2 major legal building blocks of these business relationships: contracts and agency.7
Contracts are agreements between 2 or more persons or entities that carry with them legally enforceable obligations. The 3 common elements are an offer by one party, acceptance by another, and consideration (exchanging one thing of value for another). Contracts are binding in the sense that, if there is a breach of the promise by one party, the other party may seek monetary damages for the loss of the benefit of the bargain (and in limited circumstances, require that the contract be performed).
Agency is essentially the mechanism that allows a person to legally work for or on behalf of another. A “principal” authorizes an agent to take actions for, and bind, the principal. All employment, partnership, and “agent” relationships create an agency. The principal is generally responsible for the actions of the agent—at least within the scope of the agent’s authority. For example, the principal is responsible for the torts (civil liability resulting from the breach of a socially imposed duty, but generally not arising from a contract) of an agent doing the principal’s business. The agent has the obligation to act in good faith for the benefit of the principal and to abide by the instructions of the principal.
Corporate structures
There are a variety of corporate organizational structures; the basic types are corporations, partnerships, and unincorporated associations. These generally are available to nonprofit and for-profit organizations. As a general matter, corporations limit the owners’ personal liability; partnerships have tax advantages. A number of laws now allow the creation of entities that have both liability and tax advantages (subchapter S corporations, limited liability companies, and limited liability partnerships).
Other areas of business law
Employment law, which now affects almost every aspect of hiring, dismissal, payment, and fringe benefits, is not a single law but a series of state and federal statutes, regulations, and court decisions.8
Competition is regulated through a number of antitrust laws as well as fair business practices. These affect the ability of health care entities to merge, fix prices, and split markets.9
There are literally hundreds of other laws that affect the way health care entities can operate. Conducting a careful compliance review is of considerable importance.10
Read about the dos and don’ts of preventive law.
Dos and don’ts of preventive law
The business of medicine is subject to many laws and keeping track of all of these is generally beyond the expertise of the ObGyn. Here are a few practical suggestions for thriving in this legal milieu.
Understanding the law
DO establish an ongoing relationship with an attorney you can trust who is knowledgeable in health law. Consult with this attorney not only on an as-needed basis but also for an “annual checkup” of legal issues affecting your practice.
DON’T guess what the law is. Laws vary from state to state and change frequently. Taking curbstone advice or suggestions from a podcast is a good way to develop problems.
Error reduction
DO take risk management seriously. Implement plans to improve patient safety and reduce errors.11
DON’T ignore angry or hostile patients. Their hostility may be directed at you—an undesirable state. The same goes for disgruntled (or former) employees, who may become whistleblowers.
Insurance
DO review your insurance coverage annually, preferably with an expert or your attorney. Insurance policies and your insurance needs change frequently.
DON’T assume you have all the insurance you need or that insurance will cover all legal claims arising from your practice. Intentional torts, some antitrust claims, licensure discipline, and civil fines, for example, may not be covered.
Informed consent and ethics
DO use the informed consent process as a means of improving communication between you and your patients to address their concerns and discuss expectations. Autonomy is a basic ethical value of medicine and informed consent helps to achieve that goal.
DON’T ignore ethics. Ethical obligations are not just essential to maintaining a license, hospital privileges, and professional standing.12 They also help guide you toward good practice that avoids liability.
Related articles:
Informed consent: The more you know, the more you and your patient are protected
Compliance, disputes, and arbitration
DO engage in continuing compliance review. That includes understanding the contracts and professional arrangements in which you practice and all of the requirements of third-party payers (especially government entities). There are a wide range of other compliance obligations that require ongoing attention.
DON’T sign arbitration agreements without understanding exactly what you are agreeing to. There are advantages to arbitration,13 but there are disadvantages, too.14 The courts generally enforce arbitration agreements, even ones that are unfair or one-sided.15
The law need not be a mystery or the enemy. Preventive law, like preventive medicine, can make all the difference.16
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Carpentieri AM, Lumalcuri JJ, Shaw J, Joseph GF Jr. Overview of the 2015 American Congress of Obstetricians and Gynecologists’ Survey on Professional Liability. https://www.acog.org/-/media/Departments/Professional-Liability/2015PLSurveyNationalSummary11315.pdf?dmc=1&ts=20171003T150028497. Published November 3, 2015. Accessed October 3, 2017.
- American College of Obstetrics and Gynecology Committee on Professional Liability. ACOG Opinion No. 551: Coping with the stress of professional liability litigation. Obstet Gynecol. 2013;121(1):220–222.
- Teitlebaum JB, Wilensky SE. Essential of Health Policy and Law. 2nd ed. Burlington, MA: Jones & Bartlett Learning; 2012:31–43, 127–134.
- Fabrikant R, Kalb PE, Bucy PH, Hopson MD. Health Care Fraud: Enforcement and Compliance. Newark, NJ: Law Journal Press; 2017;4:44–140.
- Health Information Privacy. Department of Health and Human Services. https://www.hhs.gov/hipaa. Updated 2017. Accessed October 3, 2017.
- Kropf S. Healthcare Fraud 101: The False Claims Act. ObGyn.Net. http://www.obgyn.net/blog/healthcare-fraud-101-false-claims-act. Published March 10, 2017. Accessed October 3, 2017.
- Smith SR, Sanfilippo JS. Applied Business Law. In: Sanfilippo JS, Bieber EJ, Javitch DG, Siegrist RB, eds. MBA for Healthcare. New York, NY: Oxford University Press; 2016:91–126.
- Todd MK. The Physician Employment Contract Handbook: A Guide to Structuring Equitable Arrangements. 2nd ed. New York, NY: Productivity Press; 2011:67–77, 93–118.
- Federal Trade Commission. Competition in the Health Care Marketplace. https://www.ftc.gov/tips-advice/competition-guidance/industry-guidance/health-care. Updated 2017. Accessed October 3, 2017.
- Shwayder JM. What is new in medical-legal issues in obstetrics and gynecology?: Best articles from the past 2 years. Obstet Gynecol. 2016;128(6):1441–1442.
- Sanfilippo JS, Smith SR. Risk Management. In: Sanfilippo JS, Bieber EJ, Javitch DG, Siegrist RB, eds. MBA for Healthcare. New York, NY: Oxford University Press; 2016:277–298.
- Smith SR, Sanfilippo JS. Ethics and the Business of the Healthcare Professional. In: Sanfilippo JS, Bieber EJ, Javitch DG, Siegrist RB, eds. MBA for Healthcare. New York, NY: Oxford University Press; 2016:71–89.
- Knag PE, Kagan DJ. Why arbitration is the preferred dispute resolution vehicle for most integrated delivery system disputes. Dispute Resolution J. 2016;71(3):127–137.
- Larson DA, Dahl D. Medical malpractice arbitration: Not business as usual. Yearbook Arbitration Mediation. 2016;8:69–92.
- Trantina TL. What law applies to an agreement to arbitrate? American Bar Association. Dispute Resolution Magazine. Fall 2015:29–31.
- Curran M. Preventative law: Interdisciplinary from medical-legal partnership. NYU Rev Law Social Change. 2014;38(4):595–606.
It is no surprise that the law is playing an ever more important role in the practice of medicine. Concerns about legal issues are a source of stress for ObGyns, including increasing worries about the economics of professional liability, the anxiety of defending a legal claim, and ambiguity about what is required for compliance.1 In this article my goal is to demystify some of the most important legal principles affecting your practice and provide suggestions for avoiding legal problems.
Medical malpractice: A form of negligence
Most ObGyns instinctively think first of medical malpractice when “legal problems” are mentioned—not an unreasonable response because obstetrics has a high incidence of malpractice claims. In one study, 77% of the American College of Obstetricians and Gynecologists (ACOG) Fellows reported that they have been sued.2
At its core, malpractice is a form of negligence, or, medical practice that falls below the quality of care that a reasonably careful practitioner would provide under the circumstances. When practice falls below that “standard of care,” and it causes injury, there may be malpractice liability. Insurance usually covers the cost of defending malpractice lawsuits and paying liability (although liability is the result of a minority of malpractice suits). There are, however, collateral consequences, including the time, stress, and disruption associated with defending the suit. In addition, malpractice may trigger review by the institutions with which the physician is associated, or in extreme cases, by licensing authorities. Large malpractice settlements or verdicts must be reported to the National Practitioner Database (sometimes colloquially referred to the “problem physician” database) or a similar state database.
This article is the third installment of the new series, "The Business of Medicine," edited by Joseph Sanfilippo, MD, MBA. In September, David Kim, MD, MBA, MPH, offered marketing strategies using social media. Last month, Dr. Sanfilippo presented ways to ensure patient satisfaction and service excellence in your practice. Watch next time for "Accounting 101." Other featured topics will include investing in your practice, billing and coding, gaining the competitive advantage, understanding "best practices," and striving for cost-effective care.
Related article:
Who is liable when a surgical error occurs?
Regulation and reimbursement (“compliance”) policies
The practice of medicine is closely regulated by federal and state bodies. Many regulations apply through reimbursement policies related to Medicare and Medicaid. While malpractice liability may, at worst, result in a financial award (with the cost of defense and any award paid by insurance), regulatory problems may result in a number of unpleasant consequences, most of which are not covered by insurance. In addition to loss of reimbursement, civil penalties (even criminalpenalties in extreme cases), loss of hospital privileges, licensure discipline, and loss of Medicare-Medicaid eligibility may result from regulatory noncompliance.3
There are multivolume sets discussing these legal requirements, so here we will look only at a tiny tip of the regulatory iceberg by mentioning some common regulatory areas.
Fraud and abuse laws refer to a bundle of federal (and some state) statutes and regulations that are intended to ensure that public-funded programs such as Medicare and Medicaid are not cheated or overpaying for services. It is a violation to provide low-quality services to government-funded programs. Proper payment and coding and ensuring that services were actually performed by the professional listed (not someone else) are examples of traps for the unwary. Submitting inaccurate records may result in action to recover incorrect payments and in civil penalties. In extreme cases where there is intentional misrepresentation, there have been criminal charges and loss of future Medicare-Medicaid eligibility.
Anti-kickback, self-referral, and Stark limitations are intended to avoid unnecessary or overpriced services. When someone is receiving a benefit for ordering or recommending a product or service, it is reasonable to expect that an incentive might affect the decision to order it, likely resulting in unnecessary or suboptimal services. It is illegal to receive a kickback for using, ordering, or recommending a product or service (a pharmaceutical company could not pay a physician $10 for each prescription written for its product). It is also illegal for physicians to refer patients to other entities in which they have a financial interest (a physician could not refer a patient to a lab in which the physician has partial ownership). The Stark laws and state prohibitions on self-referral have complex series of “safe harbor” exceptions in an ocean of prohibitions.4
HIPAA and confidentiality regulations are intended to protect patient privacy. The Health Insurance Portability and Accountability Act of 1996 (HIPAA) has extensive regulations concerning both privacy and security. The medical community is well-versed in HIPAA regulations and sensitive (perhaps hypersensitive) to its requirements. Most states have patient privacy regulations that apply in addition to HIPAA and are commonly less well known.
Protecting patient confidentiality is an ethical, legal, and licensure obligation. Protecting patient confidentiality is, therefore, general duty and not tied to a specific federal program.5
Related article:
Patient with a breast mass: Why did she pursue litigation?
Insurance Fraud is the private side of fraud and abuse. Submitting private insurance claims that are false or a misrepresentation of service is generally a violation of the contract between the provider and the insurance company. It may also be a crime—it is, after all, a form of theft. Serious fraud may result in the loss of the license to practice.
The False Claims Act and Whistleblower laws make it a civil offense (and, in extreme cases, a criminal offense), to present to the government a false claim for payment of services. It may be false in the sense that the service was not provided or in the sense that service was of inadequate quality. These statutes (both federal and state) also allow for a private whistleblower to receive some of the proceeds if he or she helps the government recoup wrongful payments. Disgruntled former employees are a common source of whistleblowing.6
Abuse-reporting statutes are part of every state’s law but vary considerably. They require certain professions, including physicians, to report known or suspected abuse of children, dependent adults, and often, other groups. The failure to make required reports can result in civil liability or even (rarely) criminal charges.
Read about how organizational law affects ObGyns.
How organizational and commercial law affects ObGyns
Physicians are generally members of organizations that are engaged in the business of health care (even nonprofit organizations have business interests). There are 2 major legal building blocks of these business relationships: contracts and agency.7
Contracts are agreements between 2 or more persons or entities that carry with them legally enforceable obligations. The 3 common elements are an offer by one party, acceptance by another, and consideration (exchanging one thing of value for another). Contracts are binding in the sense that, if there is a breach of the promise by one party, the other party may seek monetary damages for the loss of the benefit of the bargain (and in limited circumstances, require that the contract be performed).

Agency is essentially the mechanism that allows a person to legally work for or on behalf of another. A “principal” authorizes an agent to take actions for, and bind, the principal. All employment, partnership, and “agent” relationships create an agency. The principal is generally responsible for the actions of the agent—at least within the scope of the agent’s authority. For example, the principal is responsible for the torts (civil liability resulting from the breach of a socially imposed duty, but generally not arising from a contract) of an agent doing the principal’s business. The agent has the obligation to act in good faith for the benefit of the principal and to abide by the instructions of the principal.
Corporate structures
There are a variety of corporate organizational structures; the basic types are corporations, partnerships, and unincorporated associations. These generally are available to nonprofit and for-profit organizations. As a general matter, corporations limit the owners’ personal liability; partnerships have tax advantages. A number of laws now allow the creation of entities that have both liability and tax advantages (subchapter S corporations, limited liability companies, and limited liability partnerships).
Other areas of business law
Employment law, which now affects almost every aspect of hiring, dismissal, payment, and fringe benefits, is not a single law but a series of state and federal statutes, regulations, and court decisions.8
Competition is regulated through a number of antitrust laws as well as fair business practices. These affect the ability of health care entities to merge, fix prices, and split markets.9
There are literally hundreds of other laws that affect the way health care entities can operate. Conducting a careful compliance review is of considerable importance.10
Read about the dos and don’ts of preventive law.
Dos and don’ts of preventive law
The business of medicine is subject to many laws and keeping track of all of these is generally beyond the expertise of the ObGyn. Here are a few practical suggestions for thriving in this legal milieu.
Understanding the law
DO establish an ongoing relationship with an attorney you can trust who is knowledgeable in health law. Consult with this attorney not only on an as-needed basis but also for an “annual checkup” of legal issues affecting your practice.
DON’T guess what the law is. Laws vary from state to state and change frequently. Taking curbstone advice or suggestions from a podcast is a good way to develop problems.
Error reduction
DO take risk management seriously. Implement plans to improve patient safety and reduce errors.11
DON’T ignore angry or hostile patients. Their hostility may be directed at you—an undesirable state. The same goes for disgruntled (or former) employees, who may become whistleblowers.
Insurance
DO review your insurance coverage annually, preferably with an expert or your attorney. Insurance policies and your insurance needs change frequently.
DON’T assume you have all the insurance you need or that insurance will cover all legal claims arising from your practice. Intentional torts, some antitrust claims, licensure discipline, and civil fines, for example, may not be covered.
Informed consent and ethics
DO use the informed consent process as a means of improving communication between you and your patients to address their concerns and discuss expectations. Autonomy is a basic ethical value of medicine and informed consent helps to achieve that goal.
DON’T ignore ethics. Ethical obligations are not just essential to maintaining a license, hospital privileges, and professional standing.12 They also help guide you toward good practice that avoids liability.
Related articles:
Informed consent: The more you know, the more you and your patient are protected
Compliance, disputes, and arbitration
DO engage in continuing compliance review. That includes understanding the contracts and professional arrangements in which you practice and all of the requirements of third-party payers (especially government entities). There are a wide range of other compliance obligations that require ongoing attention.
DON’T sign arbitration agreements without understanding exactly what you are agreeing to. There are advantages to arbitration,13 but there are disadvantages, too.14 The courts generally enforce arbitration agreements, even ones that are unfair or one-sided.15
The law need not be a mystery or the enemy. Preventive law, like preventive medicine, can make all the difference.16
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
It is no surprise that the law is playing an ever more important role in the practice of medicine. Concerns about legal issues are a source of stress for ObGyns, including increasing worries about the economics of professional liability, the anxiety of defending a legal claim, and ambiguity about what is required for compliance.1 In this article my goal is to demystify some of the most important legal principles affecting your practice and provide suggestions for avoiding legal problems.
Medical malpractice: A form of negligence
Most ObGyns instinctively think first of medical malpractice when “legal problems” are mentioned—not an unreasonable response because obstetrics has a high incidence of malpractice claims. In one study, 77% of the American College of Obstetricians and Gynecologists (ACOG) Fellows reported that they have been sued.2
At its core, malpractice is a form of negligence, or, medical practice that falls below the quality of care that a reasonably careful practitioner would provide under the circumstances. When practice falls below that “standard of care,” and it causes injury, there may be malpractice liability. Insurance usually covers the cost of defending malpractice lawsuits and paying liability (although liability is the result of a minority of malpractice suits). There are, however, collateral consequences, including the time, stress, and disruption associated with defending the suit. In addition, malpractice may trigger review by the institutions with which the physician is associated, or in extreme cases, by licensing authorities. Large malpractice settlements or verdicts must be reported to the National Practitioner Database (sometimes colloquially referred to the “problem physician” database) or a similar state database.
This article is the third installment of the new series, "The Business of Medicine," edited by Joseph Sanfilippo, MD, MBA. In September, David Kim, MD, MBA, MPH, offered marketing strategies using social media. Last month, Dr. Sanfilippo presented ways to ensure patient satisfaction and service excellence in your practice. Watch next time for "Accounting 101." Other featured topics will include investing in your practice, billing and coding, gaining the competitive advantage, understanding "best practices," and striving for cost-effective care.
Related article:
Who is liable when a surgical error occurs?
Regulation and reimbursement (“compliance”) policies
The practice of medicine is closely regulated by federal and state bodies. Many regulations apply through reimbursement policies related to Medicare and Medicaid. While malpractice liability may, at worst, result in a financial award (with the cost of defense and any award paid by insurance), regulatory problems may result in a number of unpleasant consequences, most of which are not covered by insurance. In addition to loss of reimbursement, civil penalties (even criminalpenalties in extreme cases), loss of hospital privileges, licensure discipline, and loss of Medicare-Medicaid eligibility may result from regulatory noncompliance.3
There are multivolume sets discussing these legal requirements, so here we will look only at a tiny tip of the regulatory iceberg by mentioning some common regulatory areas.
Fraud and abuse laws refer to a bundle of federal (and some state) statutes and regulations that are intended to ensure that public-funded programs such as Medicare and Medicaid are not cheated or overpaying for services. It is a violation to provide low-quality services to government-funded programs. Proper payment and coding and ensuring that services were actually performed by the professional listed (not someone else) are examples of traps for the unwary. Submitting inaccurate records may result in action to recover incorrect payments and in civil penalties. In extreme cases where there is intentional misrepresentation, there have been criminal charges and loss of future Medicare-Medicaid eligibility.
Anti-kickback, self-referral, and Stark limitations are intended to avoid unnecessary or overpriced services. When someone is receiving a benefit for ordering or recommending a product or service, it is reasonable to expect that an incentive might affect the decision to order it, likely resulting in unnecessary or suboptimal services. It is illegal to receive a kickback for using, ordering, or recommending a product or service (a pharmaceutical company could not pay a physician $10 for each prescription written for its product). It is also illegal for physicians to refer patients to other entities in which they have a financial interest (a physician could not refer a patient to a lab in which the physician has partial ownership). The Stark laws and state prohibitions on self-referral have complex series of “safe harbor” exceptions in an ocean of prohibitions.4
HIPAA and confidentiality regulations are intended to protect patient privacy. The Health Insurance Portability and Accountability Act of 1996 (HIPAA) has extensive regulations concerning both privacy and security. The medical community is well-versed in HIPAA regulations and sensitive (perhaps hypersensitive) to its requirements. Most states have patient privacy regulations that apply in addition to HIPAA and are commonly less well known.
Protecting patient confidentiality is an ethical, legal, and licensure obligation. Protecting patient confidentiality is, therefore, general duty and not tied to a specific federal program.5
Related article:
Patient with a breast mass: Why did she pursue litigation?
Insurance Fraud is the private side of fraud and abuse. Submitting private insurance claims that are false or a misrepresentation of service is generally a violation of the contract between the provider and the insurance company. It may also be a crime—it is, after all, a form of theft. Serious fraud may result in the loss of the license to practice.
The False Claims Act and Whistleblower laws make it a civil offense (and, in extreme cases, a criminal offense), to present to the government a false claim for payment of services. It may be false in the sense that the service was not provided or in the sense that service was of inadequate quality. These statutes (both federal and state) also allow for a private whistleblower to receive some of the proceeds if he or she helps the government recoup wrongful payments. Disgruntled former employees are a common source of whistleblowing.6
Abuse-reporting statutes are part of every state’s law but vary considerably. They require certain professions, including physicians, to report known or suspected abuse of children, dependent adults, and often, other groups. The failure to make required reports can result in civil liability or even (rarely) criminal charges.
Read about how organizational law affects ObGyns.
How organizational and commercial law affects ObGyns
Physicians are generally members of organizations that are engaged in the business of health care (even nonprofit organizations have business interests). There are 2 major legal building blocks of these business relationships: contracts and agency.7
Contracts are agreements between 2 or more persons or entities that carry with them legally enforceable obligations. The 3 common elements are an offer by one party, acceptance by another, and consideration (exchanging one thing of value for another). Contracts are binding in the sense that, if there is a breach of the promise by one party, the other party may seek monetary damages for the loss of the benefit of the bargain (and in limited circumstances, require that the contract be performed).

Agency is essentially the mechanism that allows a person to legally work for or on behalf of another. A “principal” authorizes an agent to take actions for, and bind, the principal. All employment, partnership, and “agent” relationships create an agency. The principal is generally responsible for the actions of the agent—at least within the scope of the agent’s authority. For example, the principal is responsible for the torts (civil liability resulting from the breach of a socially imposed duty, but generally not arising from a contract) of an agent doing the principal’s business. The agent has the obligation to act in good faith for the benefit of the principal and to abide by the instructions of the principal.
Corporate structures
There are a variety of corporate organizational structures; the basic types are corporations, partnerships, and unincorporated associations. These generally are available to nonprofit and for-profit organizations. As a general matter, corporations limit the owners’ personal liability; partnerships have tax advantages. A number of laws now allow the creation of entities that have both liability and tax advantages (subchapter S corporations, limited liability companies, and limited liability partnerships).
Other areas of business law
Employment law, which now affects almost every aspect of hiring, dismissal, payment, and fringe benefits, is not a single law but a series of state and federal statutes, regulations, and court decisions.8
Competition is regulated through a number of antitrust laws as well as fair business practices. These affect the ability of health care entities to merge, fix prices, and split markets.9
There are literally hundreds of other laws that affect the way health care entities can operate. Conducting a careful compliance review is of considerable importance.10
Read about the dos and don’ts of preventive law.
Dos and don’ts of preventive law
The business of medicine is subject to many laws and keeping track of all of these is generally beyond the expertise of the ObGyn. Here are a few practical suggestions for thriving in this legal milieu.
Understanding the law
DO establish an ongoing relationship with an attorney you can trust who is knowledgeable in health law. Consult with this attorney not only on an as-needed basis but also for an “annual checkup” of legal issues affecting your practice.
DON’T guess what the law is. Laws vary from state to state and change frequently. Taking curbstone advice or suggestions from a podcast is a good way to develop problems.
Error reduction
DO take risk management seriously. Implement plans to improve patient safety and reduce errors.11
DON’T ignore angry or hostile patients. Their hostility may be directed at you—an undesirable state. The same goes for disgruntled (or former) employees, who may become whistleblowers.
Insurance
DO review your insurance coverage annually, preferably with an expert or your attorney. Insurance policies and your insurance needs change frequently.
DON’T assume you have all the insurance you need or that insurance will cover all legal claims arising from your practice. Intentional torts, some antitrust claims, licensure discipline, and civil fines, for example, may not be covered.
Informed consent and ethics
DO use the informed consent process as a means of improving communication between you and your patients to address their concerns and discuss expectations. Autonomy is a basic ethical value of medicine and informed consent helps to achieve that goal.
DON’T ignore ethics. Ethical obligations are not just essential to maintaining a license, hospital privileges, and professional standing.12 They also help guide you toward good practice that avoids liability.
Related articles:
Informed consent: The more you know, the more you and your patient are protected
Compliance, disputes, and arbitration
DO engage in continuing compliance review. That includes understanding the contracts and professional arrangements in which you practice and all of the requirements of third-party payers (especially government entities). There are a wide range of other compliance obligations that require ongoing attention.
DON’T sign arbitration agreements without understanding exactly what you are agreeing to. There are advantages to arbitration,13 but there are disadvantages, too.14 The courts generally enforce arbitration agreements, even ones that are unfair or one-sided.15
The law need not be a mystery or the enemy. Preventive law, like preventive medicine, can make all the difference.16
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Carpentieri AM, Lumalcuri JJ, Shaw J, Joseph GF Jr. Overview of the 2015 American Congress of Obstetricians and Gynecologists’ Survey on Professional Liability. https://www.acog.org/-/media/Departments/Professional-Liability/2015PLSurveyNationalSummary11315.pdf?dmc=1&ts=20171003T150028497. Published November 3, 2015. Accessed October 3, 2017.
- American College of Obstetrics and Gynecology Committee on Professional Liability. ACOG Opinion No. 551: Coping with the stress of professional liability litigation. Obstet Gynecol. 2013;121(1):220–222.
- Teitlebaum JB, Wilensky SE. Essential of Health Policy and Law. 2nd ed. Burlington, MA: Jones & Bartlett Learning; 2012:31–43, 127–134.
- Fabrikant R, Kalb PE, Bucy PH, Hopson MD. Health Care Fraud: Enforcement and Compliance. Newark, NJ: Law Journal Press; 2017;4:44–140.
- Health Information Privacy. Department of Health and Human Services. https://www.hhs.gov/hipaa. Updated 2017. Accessed October 3, 2017.
- Kropf S. Healthcare Fraud 101: The False Claims Act. ObGyn.Net. http://www.obgyn.net/blog/healthcare-fraud-101-false-claims-act. Published March 10, 2017. Accessed October 3, 2017.
- Smith SR, Sanfilippo JS. Applied Business Law. In: Sanfilippo JS, Bieber EJ, Javitch DG, Siegrist RB, eds. MBA for Healthcare. New York, NY: Oxford University Press; 2016:91–126.
- Todd MK. The Physician Employment Contract Handbook: A Guide to Structuring Equitable Arrangements. 2nd ed. New York, NY: Productivity Press; 2011:67–77, 93–118.
- Federal Trade Commission. Competition in the Health Care Marketplace. https://www.ftc.gov/tips-advice/competition-guidance/industry-guidance/health-care. Updated 2017. Accessed October 3, 2017.
- Shwayder JM. What is new in medical-legal issues in obstetrics and gynecology?: Best articles from the past 2 years. Obstet Gynecol. 2016;128(6):1441–1442.
- Sanfilippo JS, Smith SR. Risk Management. In: Sanfilippo JS, Bieber EJ, Javitch DG, Siegrist RB, eds. MBA for Healthcare. New York, NY: Oxford University Press; 2016:277–298.
- Smith SR, Sanfilippo JS. Ethics and the Business of the Healthcare Professional. In: Sanfilippo JS, Bieber EJ, Javitch DG, Siegrist RB, eds. MBA for Healthcare. New York, NY: Oxford University Press; 2016:71–89.
- Knag PE, Kagan DJ. Why arbitration is the preferred dispute resolution vehicle for most integrated delivery system disputes. Dispute Resolution J. 2016;71(3):127–137.
- Larson DA, Dahl D. Medical malpractice arbitration: Not business as usual. Yearbook Arbitration Mediation. 2016;8:69–92.
- Trantina TL. What law applies to an agreement to arbitrate? American Bar Association. Dispute Resolution Magazine. Fall 2015:29–31.
- Curran M. Preventative law: Interdisciplinary from medical-legal partnership. NYU Rev Law Social Change. 2014;38(4):595–606.
- Carpentieri AM, Lumalcuri JJ, Shaw J, Joseph GF Jr. Overview of the 2015 American Congress of Obstetricians and Gynecologists’ Survey on Professional Liability. https://www.acog.org/-/media/Departments/Professional-Liability/2015PLSurveyNationalSummary11315.pdf?dmc=1&ts=20171003T150028497. Published November 3, 2015. Accessed October 3, 2017.
- American College of Obstetrics and Gynecology Committee on Professional Liability. ACOG Opinion No. 551: Coping with the stress of professional liability litigation. Obstet Gynecol. 2013;121(1):220–222.
- Teitlebaum JB, Wilensky SE. Essential of Health Policy and Law. 2nd ed. Burlington, MA: Jones & Bartlett Learning; 2012:31–43, 127–134.
- Fabrikant R, Kalb PE, Bucy PH, Hopson MD. Health Care Fraud: Enforcement and Compliance. Newark, NJ: Law Journal Press; 2017;4:44–140.
- Health Information Privacy. Department of Health and Human Services. https://www.hhs.gov/hipaa. Updated 2017. Accessed October 3, 2017.
- Kropf S. Healthcare Fraud 101: The False Claims Act. ObGyn.Net. http://www.obgyn.net/blog/healthcare-fraud-101-false-claims-act. Published March 10, 2017. Accessed October 3, 2017.
- Smith SR, Sanfilippo JS. Applied Business Law. In: Sanfilippo JS, Bieber EJ, Javitch DG, Siegrist RB, eds. MBA for Healthcare. New York, NY: Oxford University Press; 2016:91–126.
- Todd MK. The Physician Employment Contract Handbook: A Guide to Structuring Equitable Arrangements. 2nd ed. New York, NY: Productivity Press; 2011:67–77, 93–118.
- Federal Trade Commission. Competition in the Health Care Marketplace. https://www.ftc.gov/tips-advice/competition-guidance/industry-guidance/health-care. Updated 2017. Accessed October 3, 2017.
- Shwayder JM. What is new in medical-legal issues in obstetrics and gynecology?: Best articles from the past 2 years. Obstet Gynecol. 2016;128(6):1441–1442.
- Sanfilippo JS, Smith SR. Risk Management. In: Sanfilippo JS, Bieber EJ, Javitch DG, Siegrist RB, eds. MBA for Healthcare. New York, NY: Oxford University Press; 2016:277–298.
- Smith SR, Sanfilippo JS. Ethics and the Business of the Healthcare Professional. In: Sanfilippo JS, Bieber EJ, Javitch DG, Siegrist RB, eds. MBA for Healthcare. New York, NY: Oxford University Press; 2016:71–89.
- Knag PE, Kagan DJ. Why arbitration is the preferred dispute resolution vehicle for most integrated delivery system disputes. Dispute Resolution J. 2016;71(3):127–137.
- Larson DA, Dahl D. Medical malpractice arbitration: Not business as usual. Yearbook Arbitration Mediation. 2016;8:69–92.
- Trantina TL. What law applies to an agreement to arbitrate? American Bar Association. Dispute Resolution Magazine. Fall 2015:29–31.
- Curran M. Preventative law: Interdisciplinary from medical-legal partnership. NYU Rev Law Social Change. 2014;38(4):595–606.
In-hospital outcomes are better for vaccinated H1N1 patients
TORONTO – Patients who received an influenza vaccination but still required hospitalization for H1N1 influenza had better outcomes, compared with unvaccinated patients, according to findings from a retrospective study.
In the hospital, vaccinated patients had significantly lower rates of acute kidney injury (6% vs. 35%; P = .038) and were more likely to be satisfactorily managed with noninvasive mechanical ventilation (41% vs. 6%; P = .004).
Dr. Chandak and her colleagues studied 72 cases of seasonal influenza requiring hospitalization from September 2015 to April 2016 at Berkshire Medical Center, a 300-bed teaching hospital in western Massachusetts. Based on rapid polymerase chain reaction testing, 51 of these patients were positive for H1N1, of which 38 had received a seasonal flu vaccine.
H1N1 patients who had received vaccination were significantly older (70.4 years vs. 59.6 years; P = .016) and were more often smokers (76% vs. 38%; P = .017), compared with patients who were unvaccinated.
The finding that the unvaccinated patients were younger and still had poorer outcomes, “emphasizes the need for widespread vaccination,” Dr. Chandak said.
There were several parameters that trended in favor of vaccination, but did not reach statistical significance due to the relatively small sample size, Dr. Chandak said. These included a trend towards more ICU admission in the unvaccinated, compared with vaccinated patients (21% and 12%, respectively; P = .699), a longer ICU stay (1.7 days and 0.2 days; P = .144), more multiorgan dysfunction syndrome (12% and 6%; P = .654), and more acute respiratory distress syndrome (6% and 0%; P = .547). Vasopressors were needed in a similar proportion of patients (12% of both groups).
During the 2009-2010 flu season, H1N1 was the cause of about 61 million cases of influenza in the United States, 274,000 hospitalizations, and 12,470 deaths, Dr. Chandak reported.
Since the 2010-2011 influenza season, the trivalent influenza vaccine has included antigen from the 2009 pandemic H1N1 influenza A virus. This has prevented between 700,000 and 1.5 million cases of H1N1, up to 10,000 hospitalizations, and as many as 500 deaths, according to surveillance data (Emerg Infect Dis. 2013;19[3]:439-48).
The viral subtype made a strong reappearance in the 2015-2016 flu season when it was again the predominant viral subtype of the season, according to the CDC. Most studies have looked at the effectiveness of the vaccine, but have not studied critical care outcomes in vaccinated versus unvaccinated patients, Dr. Chandak noted.
Dr. Chandak reported having no financial disclosures.
Daniel Ouellette, MD, FCCP, comments: “I never take the flu vaccine,” my patient stated, following my suggestion that she be inoculated. “It makes me sick.”
I reflected on the cases of influenza patients that I took care of the previous year in the ICU: the 50-year-old man with no comorbidities who died in respiratory failure; the 32-year-old pregnant woman who survived a 3-month hospitalization during which she was treated with ECMO and suffered irreversible kidney failure. “I take it every year,” I told her.
While the influenza vaccine may not prevent all cases of influenza, those who develop influenza may have an attenuated illness. Data from Chandak and colleagues affirm improved outcomes in patients who receive the vaccine and still develop influenza.
Daniel Ouellette, MD, FCCP, comments: “I never take the flu vaccine,” my patient stated, following my suggestion that she be inoculated. “It makes me sick.”
I reflected on the cases of influenza patients that I took care of the previous year in the ICU: the 50-year-old man with no comorbidities who died in respiratory failure; the 32-year-old pregnant woman who survived a 3-month hospitalization during which she was treated with ECMO and suffered irreversible kidney failure. “I take it every year,” I told her.
While the influenza vaccine may not prevent all cases of influenza, those who develop influenza may have an attenuated illness. Data from Chandak and colleagues affirm improved outcomes in patients who receive the vaccine and still develop influenza.
Daniel Ouellette, MD, FCCP, comments: “I never take the flu vaccine,” my patient stated, following my suggestion that she be inoculated. “It makes me sick.”
I reflected on the cases of influenza patients that I took care of the previous year in the ICU: the 50-year-old man with no comorbidities who died in respiratory failure; the 32-year-old pregnant woman who survived a 3-month hospitalization during which she was treated with ECMO and suffered irreversible kidney failure. “I take it every year,” I told her.
While the influenza vaccine may not prevent all cases of influenza, those who develop influenza may have an attenuated illness. Data from Chandak and colleagues affirm improved outcomes in patients who receive the vaccine and still develop influenza.
TORONTO – Patients who received an influenza vaccination but still required hospitalization for H1N1 influenza had better outcomes, compared with unvaccinated patients, according to findings from a retrospective study.
In the hospital, vaccinated patients had significantly lower rates of acute kidney injury (6% vs. 35%; P = .038) and were more likely to be satisfactorily managed with noninvasive mechanical ventilation (41% vs. 6%; P = .004).
Dr. Chandak and her colleagues studied 72 cases of seasonal influenza requiring hospitalization from September 2015 to April 2016 at Berkshire Medical Center, a 300-bed teaching hospital in western Massachusetts. Based on rapid polymerase chain reaction testing, 51 of these patients were positive for H1N1, of which 38 had received a seasonal flu vaccine.
H1N1 patients who had received vaccination were significantly older (70.4 years vs. 59.6 years; P = .016) and were more often smokers (76% vs. 38%; P = .017), compared with patients who were unvaccinated.
The finding that the unvaccinated patients were younger and still had poorer outcomes, “emphasizes the need for widespread vaccination,” Dr. Chandak said.
There were several parameters that trended in favor of vaccination, but did not reach statistical significance due to the relatively small sample size, Dr. Chandak said. These included a trend towards more ICU admission in the unvaccinated, compared with vaccinated patients (21% and 12%, respectively; P = .699), a longer ICU stay (1.7 days and 0.2 days; P = .144), more multiorgan dysfunction syndrome (12% and 6%; P = .654), and more acute respiratory distress syndrome (6% and 0%; P = .547). Vasopressors were needed in a similar proportion of patients (12% of both groups).
During the 2009-2010 flu season, H1N1 was the cause of about 61 million cases of influenza in the United States, 274,000 hospitalizations, and 12,470 deaths, Dr. Chandak reported.
Since the 2010-2011 influenza season, the trivalent influenza vaccine has included antigen from the 2009 pandemic H1N1 influenza A virus. This has prevented between 700,000 and 1.5 million cases of H1N1, up to 10,000 hospitalizations, and as many as 500 deaths, according to surveillance data (Emerg Infect Dis. 2013;19[3]:439-48).
The viral subtype made a strong reappearance in the 2015-2016 flu season when it was again the predominant viral subtype of the season, according to the CDC. Most studies have looked at the effectiveness of the vaccine, but have not studied critical care outcomes in vaccinated versus unvaccinated patients, Dr. Chandak noted.
Dr. Chandak reported having no financial disclosures.
TORONTO – Patients who received an influenza vaccination but still required hospitalization for H1N1 influenza had better outcomes, compared with unvaccinated patients, according to findings from a retrospective study.
In the hospital, vaccinated patients had significantly lower rates of acute kidney injury (6% vs. 35%; P = .038) and were more likely to be satisfactorily managed with noninvasive mechanical ventilation (41% vs. 6%; P = .004).
Dr. Chandak and her colleagues studied 72 cases of seasonal influenza requiring hospitalization from September 2015 to April 2016 at Berkshire Medical Center, a 300-bed teaching hospital in western Massachusetts. Based on rapid polymerase chain reaction testing, 51 of these patients were positive for H1N1, of which 38 had received a seasonal flu vaccine.
H1N1 patients who had received vaccination were significantly older (70.4 years vs. 59.6 years; P = .016) and were more often smokers (76% vs. 38%; P = .017), compared with patients who were unvaccinated.
The finding that the unvaccinated patients were younger and still had poorer outcomes, “emphasizes the need for widespread vaccination,” Dr. Chandak said.
There were several parameters that trended in favor of vaccination, but did not reach statistical significance due to the relatively small sample size, Dr. Chandak said. These included a trend towards more ICU admission in the unvaccinated, compared with vaccinated patients (21% and 12%, respectively; P = .699), a longer ICU stay (1.7 days and 0.2 days; P = .144), more multiorgan dysfunction syndrome (12% and 6%; P = .654), and more acute respiratory distress syndrome (6% and 0%; P = .547). Vasopressors were needed in a similar proportion of patients (12% of both groups).
During the 2009-2010 flu season, H1N1 was the cause of about 61 million cases of influenza in the United States, 274,000 hospitalizations, and 12,470 deaths, Dr. Chandak reported.
Since the 2010-2011 influenza season, the trivalent influenza vaccine has included antigen from the 2009 pandemic H1N1 influenza A virus. This has prevented between 700,000 and 1.5 million cases of H1N1, up to 10,000 hospitalizations, and as many as 500 deaths, according to surveillance data (Emerg Infect Dis. 2013;19[3]:439-48).
The viral subtype made a strong reappearance in the 2015-2016 flu season when it was again the predominant viral subtype of the season, according to the CDC. Most studies have looked at the effectiveness of the vaccine, but have not studied critical care outcomes in vaccinated versus unvaccinated patients, Dr. Chandak noted.
Dr. Chandak reported having no financial disclosures.
AT CHEST 2017
Key clinical point:
Major finding: Unvaccinated patients had a significantly higher risk of acute kidney injury (35% vs. 6%; P = .038) and were less likely to be managed with noninvasive mechanical ventilation (6% vs. 41%; P = .004).
Data source: Retrospective analysis including 72 reported influenza cases, 51 (71%) testing positive for H1N1.
Disclosures: Dr. Chandak reported having no financial disclosures.