User login
Idelalisib efficacy against CLL tarnished by toxicity
NEW YORK – PI3K inhibitors are highly active against B-cell malignancies, but this class of drugs, led by
Idelalisib is a potent inhibitor of the delta isoform of phosphatidylinositol 3-kinase (PI3K) that in a phase 1 trial was associated at higher dose levels with a median progression-free survival (PFS) of 32 months in patients with CLL who had received a median of five prior lines of therapy, noted Jennifer R. Brown, MD, PhD, director of the CLL center at the Dana-Farber Cancer Institute in Boston.
“This is really a very effective drug. So what’s happened? Why aren’t we using it more?” she asked rhetorically at an international congress on hematologic malignancies.
“This relates to a pattern of toxicities that has becoming increasingly familiar to us,” she added.
There is increasing evidence to suggest that the toxicities associated with idelalisib are immune mediated, indicating both the need for caution among clinicians who think about prescribing the drug, and a potential future use for this and other PI3K inhibitors as immunomodulatory agents, Dr. Brown said.
Registration trial toxicities
Among 760 patients enrolled in trials for the idelalisib registration programs, grade 3 or greater diarrhea and/or colitis and transaminitis each occurred in 14% of patients, rash occurred in 6%, and pneumonitis of any grade was seen in 3%.
Among patients with relapsed disease, transaminitis was often self-limiting and usually resolved when the drug was withheld, and about 75% of patients were successfully restarted on idelalisib at the same or lower dose, Dr. Brown noted.
Rashes, which can occur any time with therapy, were also successfully managed by withholding drug and then rechallenging, with the addition of corticosteroids as necessary.
Patients who developed drug-related pneumonitis were less likely than those with other toxicities to be rechallenged, and most required steroids until the infections resolved.
“The steroid responsiveness of many of these side effects suggested that they were autoimmune,” Dr. Brown said.
Drugs only work when you take them
The toxicities seen with idelalisib have had a marked effect on the use of the drug. In registration trials for idelalisib in combination with rituximab or ofatumumab (Arzerra), each of which had at least 2 years of follow-up, only 22.5% of 369 patients remained on idelalisib, primarily because of toxicities rather than disease progression. The combined 2-year progression in these trials was 13.3% In contrast, 40.7% of patients discontinued idelalisib because of adverse events.
Out to about 7 months, survival rates for patients who discontinued idelalisib because of disease progression or adverse events were roughly similar, but survival for the patients who stopped because of side effects began to plateau out to 2 years, Dr. Brown noted.
As of March 2016, 23.2% of patients who received idelalisib in clinical trials in combination with other agents as second- or third-line therapy had died, compared with 31% of controls, indicating a clear survival benefit with the drug.
“This is probably because the benefit of disease control in that setting overwhelmed the adverse event or infections problem,” she said.
Many of the deaths in registration trials were related to opportunistic infections, including Pneumocystis jiroveci pneumonia, fungal infection, and cytomegalovirus.
“Idelalisib, I think, is a prototypical delta inhibitor with a pattern of immune-mediated toxicity that remains unpredictable and can be severe. We now have pretty good data, based on the Gilead [sponsor] trials, that younger age and less prior therapy predispose to this toxicity,” Dr. Brown said.
Evidence is less robust, but growing, that mutated IGHV and a decrease in regulatory T cells may be also be risk factors for immune-mediated toxicities with idelalisib. Immune modulation with the drug may also account for associated neutropenia, sepsis, and opportunistic infections seen with idelalisib therapy, she added.
So how to use it?
Currently, the best uses for idelalisib and other PI3K inhibitors in CLL appear to be in single-agent therapy in patients with relapsed disease who cannot tolerate a Bruton’s tyrosine kinase (BTK) inhibitor such as ibrutinib (Imbruvica) or in patients whose disease has progressed on a BTK inhibitor.
“Where I think about this drug is in older, more heavily pretreated patients, who are generally at less risk for toxicities, and if they have significant comorbidities that may impact BTK-inhibitor tolerability, usually cardiac,” Dr. Brown said.
Future expansion of PI3K inhibitors in B-cell malignancies may require identifying a biomarker for tolerance, alternative dosing schedules, or identification of an idelalisib/drug X combination that might mitigate the toxicity, she said.
The immune-activation properties of PI3K-delta inhibitors suggests that they might also play a role as antitumor immunomodulatory agents in treatment of both hematologic malignancies and solid tumors, Dr. Brown concluded.
Idelalisib trials were sponsored by Gilead Sciences. Dr. Brown disclosed serving as a consultant for Gilead and other companies.
NEW YORK – PI3K inhibitors are highly active against B-cell malignancies, but this class of drugs, led by
Idelalisib is a potent inhibitor of the delta isoform of phosphatidylinositol 3-kinase (PI3K) that in a phase 1 trial was associated at higher dose levels with a median progression-free survival (PFS) of 32 months in patients with CLL who had received a median of five prior lines of therapy, noted Jennifer R. Brown, MD, PhD, director of the CLL center at the Dana-Farber Cancer Institute in Boston.
“This is really a very effective drug. So what’s happened? Why aren’t we using it more?” she asked rhetorically at an international congress on hematologic malignancies.
“This relates to a pattern of toxicities that has becoming increasingly familiar to us,” she added.
There is increasing evidence to suggest that the toxicities associated with idelalisib are immune mediated, indicating both the need for caution among clinicians who think about prescribing the drug, and a potential future use for this and other PI3K inhibitors as immunomodulatory agents, Dr. Brown said.
Registration trial toxicities
Among 760 patients enrolled in trials for the idelalisib registration programs, grade 3 or greater diarrhea and/or colitis and transaminitis each occurred in 14% of patients, rash occurred in 6%, and pneumonitis of any grade was seen in 3%.
Among patients with relapsed disease, transaminitis was often self-limiting and usually resolved when the drug was withheld, and about 75% of patients were successfully restarted on idelalisib at the same or lower dose, Dr. Brown noted.
Rashes, which can occur any time with therapy, were also successfully managed by withholding drug and then rechallenging, with the addition of corticosteroids as necessary.
Patients who developed drug-related pneumonitis were less likely than those with other toxicities to be rechallenged, and most required steroids until the infections resolved.
“The steroid responsiveness of many of these side effects suggested that they were autoimmune,” Dr. Brown said.
Drugs only work when you take them
The toxicities seen with idelalisib have had a marked effect on the use of the drug. In registration trials for idelalisib in combination with rituximab or ofatumumab (Arzerra), each of which had at least 2 years of follow-up, only 22.5% of 369 patients remained on idelalisib, primarily because of toxicities rather than disease progression. The combined 2-year progression in these trials was 13.3% In contrast, 40.7% of patients discontinued idelalisib because of adverse events.
Out to about 7 months, survival rates for patients who discontinued idelalisib because of disease progression or adverse events were roughly similar, but survival for the patients who stopped because of side effects began to plateau out to 2 years, Dr. Brown noted.
As of March 2016, 23.2% of patients who received idelalisib in clinical trials in combination with other agents as second- or third-line therapy had died, compared with 31% of controls, indicating a clear survival benefit with the drug.
“This is probably because the benefit of disease control in that setting overwhelmed the adverse event or infections problem,” she said.
Many of the deaths in registration trials were related to opportunistic infections, including Pneumocystis jiroveci pneumonia, fungal infection, and cytomegalovirus.
“Idelalisib, I think, is a prototypical delta inhibitor with a pattern of immune-mediated toxicity that remains unpredictable and can be severe. We now have pretty good data, based on the Gilead [sponsor] trials, that younger age and less prior therapy predispose to this toxicity,” Dr. Brown said.
Evidence is less robust, but growing, that mutated IGHV and a decrease in regulatory T cells may be also be risk factors for immune-mediated toxicities with idelalisib. Immune modulation with the drug may also account for associated neutropenia, sepsis, and opportunistic infections seen with idelalisib therapy, she added.
So how to use it?
Currently, the best uses for idelalisib and other PI3K inhibitors in CLL appear to be in single-agent therapy in patients with relapsed disease who cannot tolerate a Bruton’s tyrosine kinase (BTK) inhibitor such as ibrutinib (Imbruvica) or in patients whose disease has progressed on a BTK inhibitor.
“Where I think about this drug is in older, more heavily pretreated patients, who are generally at less risk for toxicities, and if they have significant comorbidities that may impact BTK-inhibitor tolerability, usually cardiac,” Dr. Brown said.
Future expansion of PI3K inhibitors in B-cell malignancies may require identifying a biomarker for tolerance, alternative dosing schedules, or identification of an idelalisib/drug X combination that might mitigate the toxicity, she said.
The immune-activation properties of PI3K-delta inhibitors suggests that they might also play a role as antitumor immunomodulatory agents in treatment of both hematologic malignancies and solid tumors, Dr. Brown concluded.
Idelalisib trials were sponsored by Gilead Sciences. Dr. Brown disclosed serving as a consultant for Gilead and other companies.
NEW YORK – PI3K inhibitors are highly active against B-cell malignancies, but this class of drugs, led by
Idelalisib is a potent inhibitor of the delta isoform of phosphatidylinositol 3-kinase (PI3K) that in a phase 1 trial was associated at higher dose levels with a median progression-free survival (PFS) of 32 months in patients with CLL who had received a median of five prior lines of therapy, noted Jennifer R. Brown, MD, PhD, director of the CLL center at the Dana-Farber Cancer Institute in Boston.
“This is really a very effective drug. So what’s happened? Why aren’t we using it more?” she asked rhetorically at an international congress on hematologic malignancies.
“This relates to a pattern of toxicities that has becoming increasingly familiar to us,” she added.
There is increasing evidence to suggest that the toxicities associated with idelalisib are immune mediated, indicating both the need for caution among clinicians who think about prescribing the drug, and a potential future use for this and other PI3K inhibitors as immunomodulatory agents, Dr. Brown said.
Registration trial toxicities
Among 760 patients enrolled in trials for the idelalisib registration programs, grade 3 or greater diarrhea and/or colitis and transaminitis each occurred in 14% of patients, rash occurred in 6%, and pneumonitis of any grade was seen in 3%.
Among patients with relapsed disease, transaminitis was often self-limiting and usually resolved when the drug was withheld, and about 75% of patients were successfully restarted on idelalisib at the same or lower dose, Dr. Brown noted.
Rashes, which can occur any time with therapy, were also successfully managed by withholding drug and then rechallenging, with the addition of corticosteroids as necessary.
Patients who developed drug-related pneumonitis were less likely than those with other toxicities to be rechallenged, and most required steroids until the infections resolved.
“The steroid responsiveness of many of these side effects suggested that they were autoimmune,” Dr. Brown said.
Drugs only work when you take them
The toxicities seen with idelalisib have had a marked effect on the use of the drug. In registration trials for idelalisib in combination with rituximab or ofatumumab (Arzerra), each of which had at least 2 years of follow-up, only 22.5% of 369 patients remained on idelalisib, primarily because of toxicities rather than disease progression. The combined 2-year progression in these trials was 13.3% In contrast, 40.7% of patients discontinued idelalisib because of adverse events.
Out to about 7 months, survival rates for patients who discontinued idelalisib because of disease progression or adverse events were roughly similar, but survival for the patients who stopped because of side effects began to plateau out to 2 years, Dr. Brown noted.
As of March 2016, 23.2% of patients who received idelalisib in clinical trials in combination with other agents as second- or third-line therapy had died, compared with 31% of controls, indicating a clear survival benefit with the drug.
“This is probably because the benefit of disease control in that setting overwhelmed the adverse event or infections problem,” she said.
Many of the deaths in registration trials were related to opportunistic infections, including Pneumocystis jiroveci pneumonia, fungal infection, and cytomegalovirus.
“Idelalisib, I think, is a prototypical delta inhibitor with a pattern of immune-mediated toxicity that remains unpredictable and can be severe. We now have pretty good data, based on the Gilead [sponsor] trials, that younger age and less prior therapy predispose to this toxicity,” Dr. Brown said.
Evidence is less robust, but growing, that mutated IGHV and a decrease in regulatory T cells may be also be risk factors for immune-mediated toxicities with idelalisib. Immune modulation with the drug may also account for associated neutropenia, sepsis, and opportunistic infections seen with idelalisib therapy, she added.
So how to use it?
Currently, the best uses for idelalisib and other PI3K inhibitors in CLL appear to be in single-agent therapy in patients with relapsed disease who cannot tolerate a Bruton’s tyrosine kinase (BTK) inhibitor such as ibrutinib (Imbruvica) or in patients whose disease has progressed on a BTK inhibitor.
“Where I think about this drug is in older, more heavily pretreated patients, who are generally at less risk for toxicities, and if they have significant comorbidities that may impact BTK-inhibitor tolerability, usually cardiac,” Dr. Brown said.
Future expansion of PI3K inhibitors in B-cell malignancies may require identifying a biomarker for tolerance, alternative dosing schedules, or identification of an idelalisib/drug X combination that might mitigate the toxicity, she said.
The immune-activation properties of PI3K-delta inhibitors suggests that they might also play a role as antitumor immunomodulatory agents in treatment of both hematologic malignancies and solid tumors, Dr. Brown concluded.
Idelalisib trials were sponsored by Gilead Sciences. Dr. Brown disclosed serving as a consultant for Gilead and other companies.
EXPERT ANALYSIS FROM LYMPHOMA & MYELOMA
Precision medicine’s future in rheumatic diseases outlined at ACR 2017
The treatment and prevention of rheumatic diseases through a precision medicine approach that takes into account individual variability in genes, environment, and lifestyle has not yet become a reality in clinical practice, but researchers in the field hope to demonstrate its potential at a course before and a session during the annual meeting of the American College of Rheumatology in San Diego.
In a 2-day premeeting course that begins on Friday, Nov. 3, a multidisciplinary faculty will discuss the technologies involved in precision medicine research; the complexities and challenges of identifying optimal drug treatments for rheumatic diseases; past, ongoing, and future research initiatives in precision medicine affecting rheumatic diseases; and how large data-set analysis and collaborative networks of researchers can shape its future.
Later, during the meeting on the afternoon of Monday, Nov. 6, an ACR session will look at how precision medicine research in patients with systemic lupus erythematosus (SLE) has laid groundwork to improve clinical trial design and the implementation of more tailored treatment for SLE and other complex autoimmune diseases.
The first day of the 2-day premeeting course will cover current approaches to studying familial and pediatric rheumatic diseases as well as an outline of how precision medicine could affect treatment approaches to SLE. Later in the day, presentations will focus on examples of personalized medicine approaches that have been derived from studies of cancer genomics and familial syndromes and the genetic underpinning of different responses to medications. The impact of direct-to-consumer genetic testing will also be discussed. Abstract presentations at the end of the day will focus on new genetic biomarkers that differentiate active disease from remission in granulomatosis with polyangiitis and mutations related to cancer-associated myositis.
The second day of the course on Saturday, Nov. 4, will focus on the technology behind precision medicine, followed by data analysis and integration into clinical practice. Technology presentations aim to demonstrate how transcriptional analysis of single immune cells and epigenetics in small numbers of cells can provide information about disease activity and prognosis. Examples of how multiple types of data can be integrated to form a picture of the pathogenesis of diseases such as rheumatoid arthritis will be discussed. Two abstract presentations will serve to show how analysis of tissue-specific serum biomarkers can identify rheumatoid arthritis patients with structural progression and how the presence of a specific antibody can predict better response to anti–tumor necrosis factor treatment and better disease control over time. In the afternoon, sessions will focus on analyzing single-cell data in key cell subpopulations in rheumatic disease and specifically address immune cell interactions across systems in SLE.
On Monday at 1:00 p.m., Maria Virginia Pascual, PhD, director of the Drukier Institute for Children’s Health and the Ronay A. Menschel Professor of Pediatrics at Cornell University in New York will speak in the ACR session, “Precision Medicine for Rheumatic Disease: Closer Than You Think?” Dr. Pascual will provide an overview of her lab’s recent work in transcriptional profiling of pediatric SLE patients, which demonstrates the potential value of immunomonitoring in order to stratify patients into discrete molecular groups to make clinical trial design better and offer more targeted therapies.
The treatment and prevention of rheumatic diseases through a precision medicine approach that takes into account individual variability in genes, environment, and lifestyle has not yet become a reality in clinical practice, but researchers in the field hope to demonstrate its potential at a course before and a session during the annual meeting of the American College of Rheumatology in San Diego.
In a 2-day premeeting course that begins on Friday, Nov. 3, a multidisciplinary faculty will discuss the technologies involved in precision medicine research; the complexities and challenges of identifying optimal drug treatments for rheumatic diseases; past, ongoing, and future research initiatives in precision medicine affecting rheumatic diseases; and how large data-set analysis and collaborative networks of researchers can shape its future.
Later, during the meeting on the afternoon of Monday, Nov. 6, an ACR session will look at how precision medicine research in patients with systemic lupus erythematosus (SLE) has laid groundwork to improve clinical trial design and the implementation of more tailored treatment for SLE and other complex autoimmune diseases.
The first day of the 2-day premeeting course will cover current approaches to studying familial and pediatric rheumatic diseases as well as an outline of how precision medicine could affect treatment approaches to SLE. Later in the day, presentations will focus on examples of personalized medicine approaches that have been derived from studies of cancer genomics and familial syndromes and the genetic underpinning of different responses to medications. The impact of direct-to-consumer genetic testing will also be discussed. Abstract presentations at the end of the day will focus on new genetic biomarkers that differentiate active disease from remission in granulomatosis with polyangiitis and mutations related to cancer-associated myositis.
The second day of the course on Saturday, Nov. 4, will focus on the technology behind precision medicine, followed by data analysis and integration into clinical practice. Technology presentations aim to demonstrate how transcriptional analysis of single immune cells and epigenetics in small numbers of cells can provide information about disease activity and prognosis. Examples of how multiple types of data can be integrated to form a picture of the pathogenesis of diseases such as rheumatoid arthritis will be discussed. Two abstract presentations will serve to show how analysis of tissue-specific serum biomarkers can identify rheumatoid arthritis patients with structural progression and how the presence of a specific antibody can predict better response to anti–tumor necrosis factor treatment and better disease control over time. In the afternoon, sessions will focus on analyzing single-cell data in key cell subpopulations in rheumatic disease and specifically address immune cell interactions across systems in SLE.
On Monday at 1:00 p.m., Maria Virginia Pascual, PhD, director of the Drukier Institute for Children’s Health and the Ronay A. Menschel Professor of Pediatrics at Cornell University in New York will speak in the ACR session, “Precision Medicine for Rheumatic Disease: Closer Than You Think?” Dr. Pascual will provide an overview of her lab’s recent work in transcriptional profiling of pediatric SLE patients, which demonstrates the potential value of immunomonitoring in order to stratify patients into discrete molecular groups to make clinical trial design better and offer more targeted therapies.
The treatment and prevention of rheumatic diseases through a precision medicine approach that takes into account individual variability in genes, environment, and lifestyle has not yet become a reality in clinical practice, but researchers in the field hope to demonstrate its potential at a course before and a session during the annual meeting of the American College of Rheumatology in San Diego.
In a 2-day premeeting course that begins on Friday, Nov. 3, a multidisciplinary faculty will discuss the technologies involved in precision medicine research; the complexities and challenges of identifying optimal drug treatments for rheumatic diseases; past, ongoing, and future research initiatives in precision medicine affecting rheumatic diseases; and how large data-set analysis and collaborative networks of researchers can shape its future.
Later, during the meeting on the afternoon of Monday, Nov. 6, an ACR session will look at how precision medicine research in patients with systemic lupus erythematosus (SLE) has laid groundwork to improve clinical trial design and the implementation of more tailored treatment for SLE and other complex autoimmune diseases.
The first day of the 2-day premeeting course will cover current approaches to studying familial and pediatric rheumatic diseases as well as an outline of how precision medicine could affect treatment approaches to SLE. Later in the day, presentations will focus on examples of personalized medicine approaches that have been derived from studies of cancer genomics and familial syndromes and the genetic underpinning of different responses to medications. The impact of direct-to-consumer genetic testing will also be discussed. Abstract presentations at the end of the day will focus on new genetic biomarkers that differentiate active disease from remission in granulomatosis with polyangiitis and mutations related to cancer-associated myositis.
The second day of the course on Saturday, Nov. 4, will focus on the technology behind precision medicine, followed by data analysis and integration into clinical practice. Technology presentations aim to demonstrate how transcriptional analysis of single immune cells and epigenetics in small numbers of cells can provide information about disease activity and prognosis. Examples of how multiple types of data can be integrated to form a picture of the pathogenesis of diseases such as rheumatoid arthritis will be discussed. Two abstract presentations will serve to show how analysis of tissue-specific serum biomarkers can identify rheumatoid arthritis patients with structural progression and how the presence of a specific antibody can predict better response to anti–tumor necrosis factor treatment and better disease control over time. In the afternoon, sessions will focus on analyzing single-cell data in key cell subpopulations in rheumatic disease and specifically address immune cell interactions across systems in SLE.
On Monday at 1:00 p.m., Maria Virginia Pascual, PhD, director of the Drukier Institute for Children’s Health and the Ronay A. Menschel Professor of Pediatrics at Cornell University in New York will speak in the ACR session, “Precision Medicine for Rheumatic Disease: Closer Than You Think?” Dr. Pascual will provide an overview of her lab’s recent work in transcriptional profiling of pediatric SLE patients, which demonstrates the potential value of immunomonitoring in order to stratify patients into discrete molecular groups to make clinical trial design better and offer more targeted therapies.
FROM ACR 2017
Gene therapy granted breakthrough designation
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation to valoctocogene roxaparvovec (formerly BMN 270) for the treatment of patients with hemophilia A.
Valoctocogene roxaparvovec is a recombinant adeno-associated virus vector coding for human coagulation factor VIII (FVIII).
The breakthrough designation is intended to expedite the development and review of valoctocogene roxaparvovec.
The designation entitles BioMarin Pharmaceutical Inc., the company developing valoctocogene roxaparvovec, to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as rolling submission and priority review.
To earn breakthrough designation from the FDA, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
The FDA granted breakthrough designation to valoctocogene roxaparvovec based on data from an ongoing phase 1/2 trial. Results from this study were presented at the ISTH 2017 Congress.
The data suggest that valoctocogene roxaparvovec can allow patients with severe hemophilia A to maintain normal FVIII levels for over a year.
The highest dose of valoctocogene roxaparvovec, 6e13 vg/kg, reduced patients’ mean annual FVIII infusions by 94% and their mean annualized bleed rate by 97%, when compared to FVIII prophylaxis.
None of the patients in this study developed FVIII inhibitors. The most common adverse event was alanine aminotransferase (ALT) elevations.
In fact, the increase in ALT levels exceeded a pre-specified threshold, which resulted in the study being placed on hold last year. However, the hold was ultimately lifted. And all patients with ALT increases have either stopped receiving corticosteroids or are being tapered off of them.
BioMarin said it expects to initiate enrollment of a global phase 3 program for valoctocogene roxaparvovec before the end of this year.
The program includes 2 studies of valoctocogene roxaparvovec, one with a 4e13 vg/kg dose and one with a 6e13 vg/kg dose. The studies will each likely include approximately 40 patients.
Valoctocogene roxaparvovec also has orphan designation from the FDA. ![]()
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation to valoctocogene roxaparvovec (formerly BMN 270) for the treatment of patients with hemophilia A.
Valoctocogene roxaparvovec is a recombinant adeno-associated virus vector coding for human coagulation factor VIII (FVIII).
The breakthrough designation is intended to expedite the development and review of valoctocogene roxaparvovec.
The designation entitles BioMarin Pharmaceutical Inc., the company developing valoctocogene roxaparvovec, to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as rolling submission and priority review.
To earn breakthrough designation from the FDA, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
The FDA granted breakthrough designation to valoctocogene roxaparvovec based on data from an ongoing phase 1/2 trial. Results from this study were presented at the ISTH 2017 Congress.
The data suggest that valoctocogene roxaparvovec can allow patients with severe hemophilia A to maintain normal FVIII levels for over a year.
The highest dose of valoctocogene roxaparvovec, 6e13 vg/kg, reduced patients’ mean annual FVIII infusions by 94% and their mean annualized bleed rate by 97%, when compared to FVIII prophylaxis.
None of the patients in this study developed FVIII inhibitors. The most common adverse event was alanine aminotransferase (ALT) elevations.
In fact, the increase in ALT levels exceeded a pre-specified threshold, which resulted in the study being placed on hold last year. However, the hold was ultimately lifted. And all patients with ALT increases have either stopped receiving corticosteroids or are being tapered off of them.
BioMarin said it expects to initiate enrollment of a global phase 3 program for valoctocogene roxaparvovec before the end of this year.
The program includes 2 studies of valoctocogene roxaparvovec, one with a 4e13 vg/kg dose and one with a 6e13 vg/kg dose. The studies will each likely include approximately 40 patients.
Valoctocogene roxaparvovec also has orphan designation from the FDA. ![]()
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation to valoctocogene roxaparvovec (formerly BMN 270) for the treatment of patients with hemophilia A.
Valoctocogene roxaparvovec is a recombinant adeno-associated virus vector coding for human coagulation factor VIII (FVIII).
The breakthrough designation is intended to expedite the development and review of valoctocogene roxaparvovec.
The designation entitles BioMarin Pharmaceutical Inc., the company developing valoctocogene roxaparvovec, to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as rolling submission and priority review.
To earn breakthrough designation from the FDA, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
The FDA granted breakthrough designation to valoctocogene roxaparvovec based on data from an ongoing phase 1/2 trial. Results from this study were presented at the ISTH 2017 Congress.
The data suggest that valoctocogene roxaparvovec can allow patients with severe hemophilia A to maintain normal FVIII levels for over a year.
The highest dose of valoctocogene roxaparvovec, 6e13 vg/kg, reduced patients’ mean annual FVIII infusions by 94% and their mean annualized bleed rate by 97%, when compared to FVIII prophylaxis.
None of the patients in this study developed FVIII inhibitors. The most common adverse event was alanine aminotransferase (ALT) elevations.
In fact, the increase in ALT levels exceeded a pre-specified threshold, which resulted in the study being placed on hold last year. However, the hold was ultimately lifted. And all patients with ALT increases have either stopped receiving corticosteroids or are being tapered off of them.
BioMarin said it expects to initiate enrollment of a global phase 3 program for valoctocogene roxaparvovec before the end of this year.
The program includes 2 studies of valoctocogene roxaparvovec, one with a 4e13 vg/kg dose and one with a 6e13 vg/kg dose. The studies will each likely include approximately 40 patients.
Valoctocogene roxaparvovec also has orphan designation from the FDA. ![]()
Bryostatin Is Safe in Patients With Moderate to Severe Alzheimer’s Disease
LONDON—Bryostatin is safe and tolerable in patients with moderate to severe Alzheimer’s disease, according to the results of a phase II study presented at the 2017 Alzheimer’s Association International Conference. The drug also may provide sustained improvements in cognition and activities of daily living. The study results provide grounds for future trials with longer treatment durations to assess bryostatin’s efficacy, said Martin R. Farlow, MD, Vice-Chairman for Research in the Department of Neurology at Indiana University School of Medicine in Indianapolis.
Bryostatin activates protein kinase C ε (PKCε), an enzyme that promotes synaptogenesis and learning. Preclinical studies indicate that bryostatin enhances synaptogenesis, inhibits apoptosis, reduces amyloid plaque formation, and inhibits tau phosphorylation. In transgenic mouse models of Alzheimer’s disease, bryostatin improved learning and memory retention. The drug has a novel, potentially regenerative mechanism of action, compared with other therapies for Alzheimer’s disease.
An Improvement Over Standard of Care?
Dr. Farlow and colleagues conducted a phase II trial to assess the safety and tolerability of bryostatin. Eligible patients had advanced Alzheimer’s disease with a Mini-Mental State Exam (MMSE) score of between 4 and 15. Participants were receiving stable standard-of-care therapy with cholinesterase inhibitors, memantine, or both. In the double-blind study, the researchers randomized participants to 20 mg of bryostatin, 40 mg of bryostatin, or placebo. Participants received IV treatment every other week for 12 weeks. Investigators evaluated patients at weeks 5, 9, and 13.
The trial’s secondary objectives were to assess drug dosing and to evaluate bryostatin’s efficacy at week 13. The primary efficacy measure was the Severe Impairment Battery (SIB), and the secondary efficacy measure was the Alzheimer’s Disease Cooperative Study-Activities of Daily Living-Severe Impairment Version (ADCS-ADL-SIV).
In all, 49 participants received 20 mg of bryostatin, 48 received 40 mg of bryostatin, and 50 received placebo. Participants’ mean age was 71.6, and mean MMSE score was 10.2. Approximately 52% of participants were women, and the study arms were well balanced. Eight patients in the 20-mg group, 18 patients in the 40-mg group, and nine controls withdrew from the study. Withdrawal of consent in the 40-mg group accounted for the majority of study withdrawals.
Lower Dose Had Greater Tolerability
The rate of treatment-emergent adverse events was 65% in the 20-mg group, 83% in the 40-mg group, and 58% among controls. Myalgia was more common in the 20-mg group (2%) and the 40-mg group (9%) than among controls (0%). Infusion-site reactions occurred in 6% of controls, 17% of the 20-mg group, and 15% of the 40-mg group. The rates of decreased appetite, decreased weight, and falls were similar in the 20-mg group and controls, but higher in the 40-mg group.
In the modified intention-to-treat (mITT) analysis, the difference from controls in SIB at week 13 was 1.9 in the 20-mg group and 0.8 in the 40-mg group. Among participants who completed the study, the difference from controls in SIB at week 13 was 2.6 in the 20-mg group and 1.5 in the 40-mg group. Improvement in SIB for the 20-mg group, compared with controls, was apparent by week 5. This improvement persisted through week 13.
In the mITT analysis, the difference from controls in ADCS-ADL-SIV at week 13 was 1.4 in the 20-mg group and 0.8 in the 40-mg group. Among participants who completed the study, the difference from controls in ADCS-ADL-SIV at week 13 was 1.6 in the 20-mg group and 1.1 in the 40-mg group.
The drug’s biochemistry explains these results, according to Daniel Alkon, MD, President and Chief Scientific Officer of Neurotrope Bioscience, the company that is developing bryostatin. Upon administration, bryostatin first triggers a brief period of activation of PKCε, followed by downregulation of PKCε, and finally de novo synthesis of PKCε. “If you give a high enough dose, you can actually trigger so much downregulation that it overwhelms the activation,” said Dr. Alkon. The 40-mg dose appeared to trigger more downregulation in humans than it had in animals, thus establishing a maximum dosing limit. Previous biochemical studies have shown that bryostatin’s synaptogenic benefits can outlast brief periods of PKCε activation by hours.
The number of patients studied was suitable for assessing safety, but the length of the trial was insufficient for assessing efficacy, said Dr. Farlow. “IV administration obviously is not ideal,” and subcutaneous injection or oral administration could improve the compound’s clinical utility, he said.
“With a safe dose that did show evidence of a sustained benefit for these patients, we are encouraged to optimize dosing further in our next trials,” said Dr. Alkon. “We are hoping that we get not only a larger improvement with further optimization and with fewer drugs already on board (that could interfere with bryostatin efficacy), but a much more prolonged improvement.”
—Erik Greb
Suggested Reading
Hongpaisan J, Sun MK, Alkon DL. PKC ε activation prevents synaptic loss, Aβ elevation, and cognitive deficits in Alzheimer’s disease transgenic mice. J Neurosci. 2011;31(2):630-643.
LONDON—Bryostatin is safe and tolerable in patients with moderate to severe Alzheimer’s disease, according to the results of a phase II study presented at the 2017 Alzheimer’s Association International Conference. The drug also may provide sustained improvements in cognition and activities of daily living. The study results provide grounds for future trials with longer treatment durations to assess bryostatin’s efficacy, said Martin R. Farlow, MD, Vice-Chairman for Research in the Department of Neurology at Indiana University School of Medicine in Indianapolis.
Bryostatin activates protein kinase C ε (PKCε), an enzyme that promotes synaptogenesis and learning. Preclinical studies indicate that bryostatin enhances synaptogenesis, inhibits apoptosis, reduces amyloid plaque formation, and inhibits tau phosphorylation. In transgenic mouse models of Alzheimer’s disease, bryostatin improved learning and memory retention. The drug has a novel, potentially regenerative mechanism of action, compared with other therapies for Alzheimer’s disease.
An Improvement Over Standard of Care?
Dr. Farlow and colleagues conducted a phase II trial to assess the safety and tolerability of bryostatin. Eligible patients had advanced Alzheimer’s disease with a Mini-Mental State Exam (MMSE) score of between 4 and 15. Participants were receiving stable standard-of-care therapy with cholinesterase inhibitors, memantine, or both. In the double-blind study, the researchers randomized participants to 20 mg of bryostatin, 40 mg of bryostatin, or placebo. Participants received IV treatment every other week for 12 weeks. Investigators evaluated patients at weeks 5, 9, and 13.
The trial’s secondary objectives were to assess drug dosing and to evaluate bryostatin’s efficacy at week 13. The primary efficacy measure was the Severe Impairment Battery (SIB), and the secondary efficacy measure was the Alzheimer’s Disease Cooperative Study-Activities of Daily Living-Severe Impairment Version (ADCS-ADL-SIV).
In all, 49 participants received 20 mg of bryostatin, 48 received 40 mg of bryostatin, and 50 received placebo. Participants’ mean age was 71.6, and mean MMSE score was 10.2. Approximately 52% of participants were women, and the study arms were well balanced. Eight patients in the 20-mg group, 18 patients in the 40-mg group, and nine controls withdrew from the study. Withdrawal of consent in the 40-mg group accounted for the majority of study withdrawals.
Lower Dose Had Greater Tolerability
The rate of treatment-emergent adverse events was 65% in the 20-mg group, 83% in the 40-mg group, and 58% among controls. Myalgia was more common in the 20-mg group (2%) and the 40-mg group (9%) than among controls (0%). Infusion-site reactions occurred in 6% of controls, 17% of the 20-mg group, and 15% of the 40-mg group. The rates of decreased appetite, decreased weight, and falls were similar in the 20-mg group and controls, but higher in the 40-mg group.
In the modified intention-to-treat (mITT) analysis, the difference from controls in SIB at week 13 was 1.9 in the 20-mg group and 0.8 in the 40-mg group. Among participants who completed the study, the difference from controls in SIB at week 13 was 2.6 in the 20-mg group and 1.5 in the 40-mg group. Improvement in SIB for the 20-mg group, compared with controls, was apparent by week 5. This improvement persisted through week 13.
In the mITT analysis, the difference from controls in ADCS-ADL-SIV at week 13 was 1.4 in the 20-mg group and 0.8 in the 40-mg group. Among participants who completed the study, the difference from controls in ADCS-ADL-SIV at week 13 was 1.6 in the 20-mg group and 1.1 in the 40-mg group.
The drug’s biochemistry explains these results, according to Daniel Alkon, MD, President and Chief Scientific Officer of Neurotrope Bioscience, the company that is developing bryostatin. Upon administration, bryostatin first triggers a brief period of activation of PKCε, followed by downregulation of PKCε, and finally de novo synthesis of PKCε. “If you give a high enough dose, you can actually trigger so much downregulation that it overwhelms the activation,” said Dr. Alkon. The 40-mg dose appeared to trigger more downregulation in humans than it had in animals, thus establishing a maximum dosing limit. Previous biochemical studies have shown that bryostatin’s synaptogenic benefits can outlast brief periods of PKCε activation by hours.
The number of patients studied was suitable for assessing safety, but the length of the trial was insufficient for assessing efficacy, said Dr. Farlow. “IV administration obviously is not ideal,” and subcutaneous injection or oral administration could improve the compound’s clinical utility, he said.
“With a safe dose that did show evidence of a sustained benefit for these patients, we are encouraged to optimize dosing further in our next trials,” said Dr. Alkon. “We are hoping that we get not only a larger improvement with further optimization and with fewer drugs already on board (that could interfere with bryostatin efficacy), but a much more prolonged improvement.”
—Erik Greb
Suggested Reading
Hongpaisan J, Sun MK, Alkon DL. PKC ε activation prevents synaptic loss, Aβ elevation, and cognitive deficits in Alzheimer’s disease transgenic mice. J Neurosci. 2011;31(2):630-643.
LONDON—Bryostatin is safe and tolerable in patients with moderate to severe Alzheimer’s disease, according to the results of a phase II study presented at the 2017 Alzheimer’s Association International Conference. The drug also may provide sustained improvements in cognition and activities of daily living. The study results provide grounds for future trials with longer treatment durations to assess bryostatin’s efficacy, said Martin R. Farlow, MD, Vice-Chairman for Research in the Department of Neurology at Indiana University School of Medicine in Indianapolis.
Bryostatin activates protein kinase C ε (PKCε), an enzyme that promotes synaptogenesis and learning. Preclinical studies indicate that bryostatin enhances synaptogenesis, inhibits apoptosis, reduces amyloid plaque formation, and inhibits tau phosphorylation. In transgenic mouse models of Alzheimer’s disease, bryostatin improved learning and memory retention. The drug has a novel, potentially regenerative mechanism of action, compared with other therapies for Alzheimer’s disease.
An Improvement Over Standard of Care?
Dr. Farlow and colleagues conducted a phase II trial to assess the safety and tolerability of bryostatin. Eligible patients had advanced Alzheimer’s disease with a Mini-Mental State Exam (MMSE) score of between 4 and 15. Participants were receiving stable standard-of-care therapy with cholinesterase inhibitors, memantine, or both. In the double-blind study, the researchers randomized participants to 20 mg of bryostatin, 40 mg of bryostatin, or placebo. Participants received IV treatment every other week for 12 weeks. Investigators evaluated patients at weeks 5, 9, and 13.
The trial’s secondary objectives were to assess drug dosing and to evaluate bryostatin’s efficacy at week 13. The primary efficacy measure was the Severe Impairment Battery (SIB), and the secondary efficacy measure was the Alzheimer’s Disease Cooperative Study-Activities of Daily Living-Severe Impairment Version (ADCS-ADL-SIV).
In all, 49 participants received 20 mg of bryostatin, 48 received 40 mg of bryostatin, and 50 received placebo. Participants’ mean age was 71.6, and mean MMSE score was 10.2. Approximately 52% of participants were women, and the study arms were well balanced. Eight patients in the 20-mg group, 18 patients in the 40-mg group, and nine controls withdrew from the study. Withdrawal of consent in the 40-mg group accounted for the majority of study withdrawals.
Lower Dose Had Greater Tolerability
The rate of treatment-emergent adverse events was 65% in the 20-mg group, 83% in the 40-mg group, and 58% among controls. Myalgia was more common in the 20-mg group (2%) and the 40-mg group (9%) than among controls (0%). Infusion-site reactions occurred in 6% of controls, 17% of the 20-mg group, and 15% of the 40-mg group. The rates of decreased appetite, decreased weight, and falls were similar in the 20-mg group and controls, but higher in the 40-mg group.
In the modified intention-to-treat (mITT) analysis, the difference from controls in SIB at week 13 was 1.9 in the 20-mg group and 0.8 in the 40-mg group. Among participants who completed the study, the difference from controls in SIB at week 13 was 2.6 in the 20-mg group and 1.5 in the 40-mg group. Improvement in SIB for the 20-mg group, compared with controls, was apparent by week 5. This improvement persisted through week 13.
In the mITT analysis, the difference from controls in ADCS-ADL-SIV at week 13 was 1.4 in the 20-mg group and 0.8 in the 40-mg group. Among participants who completed the study, the difference from controls in ADCS-ADL-SIV at week 13 was 1.6 in the 20-mg group and 1.1 in the 40-mg group.
The drug’s biochemistry explains these results, according to Daniel Alkon, MD, President and Chief Scientific Officer of Neurotrope Bioscience, the company that is developing bryostatin. Upon administration, bryostatin first triggers a brief period of activation of PKCε, followed by downregulation of PKCε, and finally de novo synthesis of PKCε. “If you give a high enough dose, you can actually trigger so much downregulation that it overwhelms the activation,” said Dr. Alkon. The 40-mg dose appeared to trigger more downregulation in humans than it had in animals, thus establishing a maximum dosing limit. Previous biochemical studies have shown that bryostatin’s synaptogenic benefits can outlast brief periods of PKCε activation by hours.
The number of patients studied was suitable for assessing safety, but the length of the trial was insufficient for assessing efficacy, said Dr. Farlow. “IV administration obviously is not ideal,” and subcutaneous injection or oral administration could improve the compound’s clinical utility, he said.
“With a safe dose that did show evidence of a sustained benefit for these patients, we are encouraged to optimize dosing further in our next trials,” said Dr. Alkon. “We are hoping that we get not only a larger improvement with further optimization and with fewer drugs already on board (that could interfere with bryostatin efficacy), but a much more prolonged improvement.”
—Erik Greb
Suggested Reading
Hongpaisan J, Sun MK, Alkon DL. PKC ε activation prevents synaptic loss, Aβ elevation, and cognitive deficits in Alzheimer’s disease transgenic mice. J Neurosci. 2011;31(2):630-643.
Blood Test May Detect Amyloid Plaques in the Brain
LONDON—Plasma β-amyloid concentrations may have sufficient specificity to screen for amyloid plaques in the brain, according to research presented at the 2017 Alzheimer’s Association International Conference. A blood test for CNS amyloidosis could accelerate clinical trial enrollment. It also could aid in Alzheimer’s disease diagnosis and be incorporated into regular patient screening if effective antiamyloid therapies are developed, said Randall Bateman, MD.
Studies indicate that Alzheimer’s disease pathology occurs over 20 to 30 years. A presymptomatic stage is characterized by 15 to 20 years of amyloid deposition. Symptoms manifest in the last seven to 10 years, and this symptomatic stage is characterized by tau tangle pathology, said Dr. Bateman, Charles F. and Joanne Knight Distinguished Professor of Neurology at Washington University School of Medicine in St. Louis.
Although CSF testing and PET scans can detect brain amyloid deposition, researchers have sought more practical and less expensive tests to detect amyloid plaques in patients who have or are at increased risk of Alzheimer’s disease.
Seeking a Blood Biomarker
To investigate the potential of a plasma-based β-amyloid biomarker, Dr. Bateman and colleagues adapted their Stable Isotope Label Kinetics (SILK) protocol to analyze the turnover kinetics and absolute amounts of β-amyloid 38, β-amyloid 40, and β-amyloid 42 in blood plasma. Dr. Bateman has equity in a company, C2N Diagnostics, that has licensed the assay technology, and he receives royalty income from its use.
The researchers included 41 older adults in the study. Participants had clinically diagnosed late-onset Alzheimer’s disease or were cognitively normal age-matched controls. All participants underwent brain PET amyloid imaging or CSF amyloid measurement to detect brain amyloidosis.
Eighteen patients were amyloid positive, and 23 patients were amyloid negative. The majority of the participants were cognitively normal. Participants’ Clinical Dementia Rating scores ranged from 0 to 2, and the average Mini-Mental State Examination score was about 27.
Participants received a bolus of 13C6-leucine label and underwent blood sampling over 24 hours. Researchers blinded to participants’ amyloid status immediately processed blood samples to plasma. They immunoprecipitated β-amyloid isoforms with an anti-β-amyloid antibody and analyzed them using high-resolution liquid chromatography–mass spectrometry.
The turnover rate of β-amyloid 42, compared with that of β-amyloid 40, in blood was faster in participants with β-amyloid plaques than in participants without β-amyloid plaques. This difference in turnover rate is the same characteristic signature of amyloidosis that appears in CSF, Dr. Bateman said. In blood, however, the turnover occurs more rapidly, and the magnitude of the difference is less than that in CSF, he said.
In addition, the investigators compared the concentrations of β-amyloid isoforms. The ratio of β-amyloid 42 to β-amyloid 40 was lower in patients with amyloidosis than in patients without amyloidosis. “This [result] is consistent with what we see in CSF, again, at a lower magnitude of difference,” he said. An analysis indicated that there is an important physiologic relationship between amyloid measures in the CSF and those in blood.
High Sensitivity
The researchers then assessed the potential of using blood β-amyloid concentrations to distinguish between patients who are amyloid positive and patients who are amyloid negative. Using a blood β-amyloid 42:40 ratio of less than 0.1243 as a cutoff, the test provided an area under the receiver operating characteristic curve (AUC) of approximately 0.89, “which puts this potentially in the range of being a good screening test for amyloidosis,” Dr. Bateman said.
The test identified the vast majority of people who were amyloid positive and the majority of people who were amyloid negative. “However, there are some amyloid negative individuals who would be incorrectly classified as being amyloid positive, and so confirmatory tests would be needed if this [measure] were to be used as a screening test,” Dr. Bateman said.
Validation Cohort
The researchers conducted a double-blind validation study using 164 samples. The validation study identified a highly statistically significant difference between participants who were amyloid positive and participants who were amyloid negative. In the validation cohort, the test had an AUC of approximately 0.76.
The blood test requires validation in larger, multicenter studies, Dr. Bateman said. Amyloid buildup does not necessarily mean that a patient has or will develop Alzheimer’s disease, “but a test like this can help identify those who are at risk,” he said.
A plasma β-amyloid test could lead to shorter and less costly clinical trials and allow investigators to test more therapeutics, he said. In addition, a blood test someday could “facilitate widespread treatment when effective antiamyloid therapeutics are developed,” Dr. Bateman said. Similar to screening for and treating high cholesterol to reduce the risk of heart attack and stroke, “a person may … get a blood test to find out if the amyloid protein is building up in the brain, a
—Jake Remaly
Suggested Reading
Huang Y, Potter R, Sigurdson W, et al. β-amyloid dynamics in human plasma. Arch Neurol. 2012;69(12):1591-1597.
Ovod V, Ramsey KN, Mawuenyega KG, et al. Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement. 2017;13(8):841-849.
Patterson BW, Elbert DL, Mawuenyega KG, et al. Age and amyloid effects on human central nervous system amyloid-beta kinetics. Ann Neurol. 2015;78(3):439-453.
LONDON—Plasma β-amyloid concentrations may have sufficient specificity to screen for amyloid plaques in the brain, according to research presented at the 2017 Alzheimer’s Association International Conference. A blood test for CNS amyloidosis could accelerate clinical trial enrollment. It also could aid in Alzheimer’s disease diagnosis and be incorporated into regular patient screening if effective antiamyloid therapies are developed, said Randall Bateman, MD.
Studies indicate that Alzheimer’s disease pathology occurs over 20 to 30 years. A presymptomatic stage is characterized by 15 to 20 years of amyloid deposition. Symptoms manifest in the last seven to 10 years, and this symptomatic stage is characterized by tau tangle pathology, said Dr. Bateman, Charles F. and Joanne Knight Distinguished Professor of Neurology at Washington University School of Medicine in St. Louis.
Although CSF testing and PET scans can detect brain amyloid deposition, researchers have sought more practical and less expensive tests to detect amyloid plaques in patients who have or are at increased risk of Alzheimer’s disease.
Seeking a Blood Biomarker
To investigate the potential of a plasma-based β-amyloid biomarker, Dr. Bateman and colleagues adapted their Stable Isotope Label Kinetics (SILK) protocol to analyze the turnover kinetics and absolute amounts of β-amyloid 38, β-amyloid 40, and β-amyloid 42 in blood plasma. Dr. Bateman has equity in a company, C2N Diagnostics, that has licensed the assay technology, and he receives royalty income from its use.
The researchers included 41 older adults in the study. Participants had clinically diagnosed late-onset Alzheimer’s disease or were cognitively normal age-matched controls. All participants underwent brain PET amyloid imaging or CSF amyloid measurement to detect brain amyloidosis.
Eighteen patients were amyloid positive, and 23 patients were amyloid negative. The majority of the participants were cognitively normal. Participants’ Clinical Dementia Rating scores ranged from 0 to 2, and the average Mini-Mental State Examination score was about 27.
Participants received a bolus of 13C6-leucine label and underwent blood sampling over 24 hours. Researchers blinded to participants’ amyloid status immediately processed blood samples to plasma. They immunoprecipitated β-amyloid isoforms with an anti-β-amyloid antibody and analyzed them using high-resolution liquid chromatography–mass spectrometry.
The turnover rate of β-amyloid 42, compared with that of β-amyloid 40, in blood was faster in participants with β-amyloid plaques than in participants without β-amyloid plaques. This difference in turnover rate is the same characteristic signature of amyloidosis that appears in CSF, Dr. Bateman said. In blood, however, the turnover occurs more rapidly, and the magnitude of the difference is less than that in CSF, he said.
In addition, the investigators compared the concentrations of β-amyloid isoforms. The ratio of β-amyloid 42 to β-amyloid 40 was lower in patients with amyloidosis than in patients without amyloidosis. “This [result] is consistent with what we see in CSF, again, at a lower magnitude of difference,” he said. An analysis indicated that there is an important physiologic relationship between amyloid measures in the CSF and those in blood.
High Sensitivity
The researchers then assessed the potential of using blood β-amyloid concentrations to distinguish between patients who are amyloid positive and patients who are amyloid negative. Using a blood β-amyloid 42:40 ratio of less than 0.1243 as a cutoff, the test provided an area under the receiver operating characteristic curve (AUC) of approximately 0.89, “which puts this potentially in the range of being a good screening test for amyloidosis,” Dr. Bateman said.
The test identified the vast majority of people who were amyloid positive and the majority of people who were amyloid negative. “However, there are some amyloid negative individuals who would be incorrectly classified as being amyloid positive, and so confirmatory tests would be needed if this [measure] were to be used as a screening test,” Dr. Bateman said.
Validation Cohort
The researchers conducted a double-blind validation study using 164 samples. The validation study identified a highly statistically significant difference between participants who were amyloid positive and participants who were amyloid negative. In the validation cohort, the test had an AUC of approximately 0.76.
The blood test requires validation in larger, multicenter studies, Dr. Bateman said. Amyloid buildup does not necessarily mean that a patient has or will develop Alzheimer’s disease, “but a test like this can help identify those who are at risk,” he said.
A plasma β-amyloid test could lead to shorter and less costly clinical trials and allow investigators to test more therapeutics, he said. In addition, a blood test someday could “facilitate widespread treatment when effective antiamyloid therapeutics are developed,” Dr. Bateman said. Similar to screening for and treating high cholesterol to reduce the risk of heart attack and stroke, “a person may … get a blood test to find out if the amyloid protein is building up in the brain, a
—Jake Remaly
Suggested Reading
Huang Y, Potter R, Sigurdson W, et al. β-amyloid dynamics in human plasma. Arch Neurol. 2012;69(12):1591-1597.
Ovod V, Ramsey KN, Mawuenyega KG, et al. Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement. 2017;13(8):841-849.
Patterson BW, Elbert DL, Mawuenyega KG, et al. Age and amyloid effects on human central nervous system amyloid-beta kinetics. Ann Neurol. 2015;78(3):439-453.
LONDON—Plasma β-amyloid concentrations may have sufficient specificity to screen for amyloid plaques in the brain, according to research presented at the 2017 Alzheimer’s Association International Conference. A blood test for CNS amyloidosis could accelerate clinical trial enrollment. It also could aid in Alzheimer’s disease diagnosis and be incorporated into regular patient screening if effective antiamyloid therapies are developed, said Randall Bateman, MD.
Studies indicate that Alzheimer’s disease pathology occurs over 20 to 30 years. A presymptomatic stage is characterized by 15 to 20 years of amyloid deposition. Symptoms manifest in the last seven to 10 years, and this symptomatic stage is characterized by tau tangle pathology, said Dr. Bateman, Charles F. and Joanne Knight Distinguished Professor of Neurology at Washington University School of Medicine in St. Louis.
Although CSF testing and PET scans can detect brain amyloid deposition, researchers have sought more practical and less expensive tests to detect amyloid plaques in patients who have or are at increased risk of Alzheimer’s disease.
Seeking a Blood Biomarker
To investigate the potential of a plasma-based β-amyloid biomarker, Dr. Bateman and colleagues adapted their Stable Isotope Label Kinetics (SILK) protocol to analyze the turnover kinetics and absolute amounts of β-amyloid 38, β-amyloid 40, and β-amyloid 42 in blood plasma. Dr. Bateman has equity in a company, C2N Diagnostics, that has licensed the assay technology, and he receives royalty income from its use.
The researchers included 41 older adults in the study. Participants had clinically diagnosed late-onset Alzheimer’s disease or were cognitively normal age-matched controls. All participants underwent brain PET amyloid imaging or CSF amyloid measurement to detect brain amyloidosis.
Eighteen patients were amyloid positive, and 23 patients were amyloid negative. The majority of the participants were cognitively normal. Participants’ Clinical Dementia Rating scores ranged from 0 to 2, and the average Mini-Mental State Examination score was about 27.
Participants received a bolus of 13C6-leucine label and underwent blood sampling over 24 hours. Researchers blinded to participants’ amyloid status immediately processed blood samples to plasma. They immunoprecipitated β-amyloid isoforms with an anti-β-amyloid antibody and analyzed them using high-resolution liquid chromatography–mass spectrometry.
The turnover rate of β-amyloid 42, compared with that of β-amyloid 40, in blood was faster in participants with β-amyloid plaques than in participants without β-amyloid plaques. This difference in turnover rate is the same characteristic signature of amyloidosis that appears in CSF, Dr. Bateman said. In blood, however, the turnover occurs more rapidly, and the magnitude of the difference is less than that in CSF, he said.
In addition, the investigators compared the concentrations of β-amyloid isoforms. The ratio of β-amyloid 42 to β-amyloid 40 was lower in patients with amyloidosis than in patients without amyloidosis. “This [result] is consistent with what we see in CSF, again, at a lower magnitude of difference,” he said. An analysis indicated that there is an important physiologic relationship between amyloid measures in the CSF and those in blood.
High Sensitivity
The researchers then assessed the potential of using blood β-amyloid concentrations to distinguish between patients who are amyloid positive and patients who are amyloid negative. Using a blood β-amyloid 42:40 ratio of less than 0.1243 as a cutoff, the test provided an area under the receiver operating characteristic curve (AUC) of approximately 0.89, “which puts this potentially in the range of being a good screening test for amyloidosis,” Dr. Bateman said.
The test identified the vast majority of people who were amyloid positive and the majority of people who were amyloid negative. “However, there are some amyloid negative individuals who would be incorrectly classified as being amyloid positive, and so confirmatory tests would be needed if this [measure] were to be used as a screening test,” Dr. Bateman said.
Validation Cohort
The researchers conducted a double-blind validation study using 164 samples. The validation study identified a highly statistically significant difference between participants who were amyloid positive and participants who were amyloid negative. In the validation cohort, the test had an AUC of approximately 0.76.
The blood test requires validation in larger, multicenter studies, Dr. Bateman said. Amyloid buildup does not necessarily mean that a patient has or will develop Alzheimer’s disease, “but a test like this can help identify those who are at risk,” he said.
A plasma β-amyloid test could lead to shorter and less costly clinical trials and allow investigators to test more therapeutics, he said. In addition, a blood test someday could “facilitate widespread treatment when effective antiamyloid therapeutics are developed,” Dr. Bateman said. Similar to screening for and treating high cholesterol to reduce the risk of heart attack and stroke, “a person may … get a blood test to find out if the amyloid protein is building up in the brain, a
—Jake Remaly
Suggested Reading
Huang Y, Potter R, Sigurdson W, et al. β-amyloid dynamics in human plasma. Arch Neurol. 2012;69(12):1591-1597.
Ovod V, Ramsey KN, Mawuenyega KG, et al. Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement. 2017;13(8):841-849.
Patterson BW, Elbert DL, Mawuenyega KG, et al. Age and amyloid effects on human central nervous system amyloid-beta kinetics. Ann Neurol. 2015;78(3):439-453.
IDSA updates infectious diarrhea guidelines
Molecular-based diagnostic tests for enteric pathogens are highly sensitive but may require expert input to assess their clinical and public health implications, according to new guidelines from the Infectious Diseases Society of America.
Among the specific areas on which the guidelines focus are assessment of the diagnostic needs of patients who have been traveling, those in health care settings, including long-term care facilities, and dealing with immunocompromised patients, especially those with acquired immune deficiency syndrome (AIDS).
“Differentiating colonization from active infection, obtaining antimicrobial susceptibility results, providing optimal management, and preventing transmission are areas in need of additional research as nonculture diagnostics replace traditional culture-based methods,” writes Andi L. Shane, MD, of Emory University and Children’s Healthcare in Atlanta, with her associates in Clinical Infectious Diseases. Performing a complete physical exam and taking a thorough exposure history remain crucial in this era of rapid molecular tests in order to identify and treat infectious diarrhea and interrupt the chain of transmission, the experts emphasize (Clin Infect Dis. 2017 Oct 19. doi: 10.1093/cid/cix669).
The rise of culture-independent diagnostic tests also has important public health implications, the experts state. Pulsed-field gel electrophoresis and whole-genome sequencing are essential for rapidly detecting outbreaks, but they must be performed on clinical isolates. “Continuing to detect and respond to such outbreaks is a vital part of making our food and water systems safer,” the authors add. “As culture-independent diagnostic panels become used more frequently, public health departments may request that specimens be cultured in public health laboratories if unable to be cultured in the clinical diagnostic laboratory.” Clinicians should continue submitting isolates for subtyping of notifiable pathogens, such as Salmonella, Shiga toxin–producing Escherichia coli, Shigella, and Listeria, according to the guidelines.
The guidelines also recommend a broad differential diagnosis in immunocompromised people with diarrhea, and people with AIDS who have persistent diarrhea should undergo additional testing for other organisms including, Cryptosporidium, Cyclospora, Cystoisospora, Microsporidia, Mycobacterium avium complex, and Cytomegalovirus.
The IDSA last updated its guidelines on infectious diarrhea in 2001. The current iteration includes 60 specific recommendations and five tables that stratify pathogens by exposures, clinical signs and symptoms, postinfectious sequelae, laboratory diagnostics, and antimicrobial therapy. Two additional tables list other sources of guidelines and provide detailed recommendations on rehydration therapy. Most patients with diarrhea do not need to be tested for infectious pathogens, with exceptions such as children younger than 5 years, the elderly, immunocompromised patients, and patients with bloody diarrhea, severe abdominal pain or tenderness, or signs of sepsis. Even when patients do not need to be tested, they should receive oral rehydration solution to correct mild to moderate dehydration or intravenous rehydration if they cannot tolerate oral therapy.
The World Health Organization defines diarrhea as loose or liquid stools occurring three or more times in 24 hours or more often than normal. Rapid molecular tests most often identify norovirus in these patients. Infectious diarrhea remains most common in children under age 5 years, but the advent of rotavirus vaccines over the past decade has decreased its incidence in this age group.
The work was funded by IDSA. Dr. Shane disclosed research grants from the Division of Microbiology and Infectious Diseases of the National Institute of Allergy and Infectious Diseases, salary support from the Gerber Foundation, honoraria from SLACK, and travel support from International Scientific Association for Probiotics and Prebiotics.
This article was updated 11/3/17.
Molecular-based diagnostic tests for enteric pathogens are highly sensitive but may require expert input to assess their clinical and public health implications, according to new guidelines from the Infectious Diseases Society of America.
Among the specific areas on which the guidelines focus are assessment of the diagnostic needs of patients who have been traveling, those in health care settings, including long-term care facilities, and dealing with immunocompromised patients, especially those with acquired immune deficiency syndrome (AIDS).
“Differentiating colonization from active infection, obtaining antimicrobial susceptibility results, providing optimal management, and preventing transmission are areas in need of additional research as nonculture diagnostics replace traditional culture-based methods,” writes Andi L. Shane, MD, of Emory University and Children’s Healthcare in Atlanta, with her associates in Clinical Infectious Diseases. Performing a complete physical exam and taking a thorough exposure history remain crucial in this era of rapid molecular tests in order to identify and treat infectious diarrhea and interrupt the chain of transmission, the experts emphasize (Clin Infect Dis. 2017 Oct 19. doi: 10.1093/cid/cix669).
The rise of culture-independent diagnostic tests also has important public health implications, the experts state. Pulsed-field gel electrophoresis and whole-genome sequencing are essential for rapidly detecting outbreaks, but they must be performed on clinical isolates. “Continuing to detect and respond to such outbreaks is a vital part of making our food and water systems safer,” the authors add. “As culture-independent diagnostic panels become used more frequently, public health departments may request that specimens be cultured in public health laboratories if unable to be cultured in the clinical diagnostic laboratory.” Clinicians should continue submitting isolates for subtyping of notifiable pathogens, such as Salmonella, Shiga toxin–producing Escherichia coli, Shigella, and Listeria, according to the guidelines.
The guidelines also recommend a broad differential diagnosis in immunocompromised people with diarrhea, and people with AIDS who have persistent diarrhea should undergo additional testing for other organisms including, Cryptosporidium, Cyclospora, Cystoisospora, Microsporidia, Mycobacterium avium complex, and Cytomegalovirus.
The IDSA last updated its guidelines on infectious diarrhea in 2001. The current iteration includes 60 specific recommendations and five tables that stratify pathogens by exposures, clinical signs and symptoms, postinfectious sequelae, laboratory diagnostics, and antimicrobial therapy. Two additional tables list other sources of guidelines and provide detailed recommendations on rehydration therapy. Most patients with diarrhea do not need to be tested for infectious pathogens, with exceptions such as children younger than 5 years, the elderly, immunocompromised patients, and patients with bloody diarrhea, severe abdominal pain or tenderness, or signs of sepsis. Even when patients do not need to be tested, they should receive oral rehydration solution to correct mild to moderate dehydration or intravenous rehydration if they cannot tolerate oral therapy.
The World Health Organization defines diarrhea as loose or liquid stools occurring three or more times in 24 hours or more often than normal. Rapid molecular tests most often identify norovirus in these patients. Infectious diarrhea remains most common in children under age 5 years, but the advent of rotavirus vaccines over the past decade has decreased its incidence in this age group.
The work was funded by IDSA. Dr. Shane disclosed research grants from the Division of Microbiology and Infectious Diseases of the National Institute of Allergy and Infectious Diseases, salary support from the Gerber Foundation, honoraria from SLACK, and travel support from International Scientific Association for Probiotics and Prebiotics.
This article was updated 11/3/17.
Molecular-based diagnostic tests for enteric pathogens are highly sensitive but may require expert input to assess their clinical and public health implications, according to new guidelines from the Infectious Diseases Society of America.
Among the specific areas on which the guidelines focus are assessment of the diagnostic needs of patients who have been traveling, those in health care settings, including long-term care facilities, and dealing with immunocompromised patients, especially those with acquired immune deficiency syndrome (AIDS).
“Differentiating colonization from active infection, obtaining antimicrobial susceptibility results, providing optimal management, and preventing transmission are areas in need of additional research as nonculture diagnostics replace traditional culture-based methods,” writes Andi L. Shane, MD, of Emory University and Children’s Healthcare in Atlanta, with her associates in Clinical Infectious Diseases. Performing a complete physical exam and taking a thorough exposure history remain crucial in this era of rapid molecular tests in order to identify and treat infectious diarrhea and interrupt the chain of transmission, the experts emphasize (Clin Infect Dis. 2017 Oct 19. doi: 10.1093/cid/cix669).
The rise of culture-independent diagnostic tests also has important public health implications, the experts state. Pulsed-field gel electrophoresis and whole-genome sequencing are essential for rapidly detecting outbreaks, but they must be performed on clinical isolates. “Continuing to detect and respond to such outbreaks is a vital part of making our food and water systems safer,” the authors add. “As culture-independent diagnostic panels become used more frequently, public health departments may request that specimens be cultured in public health laboratories if unable to be cultured in the clinical diagnostic laboratory.” Clinicians should continue submitting isolates for subtyping of notifiable pathogens, such as Salmonella, Shiga toxin–producing Escherichia coli, Shigella, and Listeria, according to the guidelines.
The guidelines also recommend a broad differential diagnosis in immunocompromised people with diarrhea, and people with AIDS who have persistent diarrhea should undergo additional testing for other organisms including, Cryptosporidium, Cyclospora, Cystoisospora, Microsporidia, Mycobacterium avium complex, and Cytomegalovirus.
The IDSA last updated its guidelines on infectious diarrhea in 2001. The current iteration includes 60 specific recommendations and five tables that stratify pathogens by exposures, clinical signs and symptoms, postinfectious sequelae, laboratory diagnostics, and antimicrobial therapy. Two additional tables list other sources of guidelines and provide detailed recommendations on rehydration therapy. Most patients with diarrhea do not need to be tested for infectious pathogens, with exceptions such as children younger than 5 years, the elderly, immunocompromised patients, and patients with bloody diarrhea, severe abdominal pain or tenderness, or signs of sepsis. Even when patients do not need to be tested, they should receive oral rehydration solution to correct mild to moderate dehydration or intravenous rehydration if they cannot tolerate oral therapy.
The World Health Organization defines diarrhea as loose or liquid stools occurring three or more times in 24 hours or more often than normal. Rapid molecular tests most often identify norovirus in these patients. Infectious diarrhea remains most common in children under age 5 years, but the advent of rotavirus vaccines over the past decade has decreased its incidence in this age group.
The work was funded by IDSA. Dr. Shane disclosed research grants from the Division of Microbiology and Infectious Diseases of the National Institute of Allergy and Infectious Diseases, salary support from the Gerber Foundation, honoraria from SLACK, and travel support from International Scientific Association for Probiotics and Prebiotics.
This article was updated 11/3/17.
FROM CLINICAL INFECTIOUS DISEASES
Trials Clarify Benefit of PFO Closure After Stroke
Among patients with a patent foramen ovale (PFO) who have had a cryptogenic stroke, closure of the PFO reduces the risk of recurrent stroke, compared with medical therapy alone, according to three studies published in the September 14 issue of the New England Journal of Medicine. PFO closure, however, entailed increased risk of atrial fibrillation and, in two of the studies, venous thromboembolism.
In prior trials, PFO closure did not have a statistically significant effect on stroke risk, although the results suggested potential benefit. In an editorial accompanying the new trial results, Allan H. Ropper, MD, said that various limitations of the prior studies, including inclusion of patients whose prior stroke would not benefit from PFO closure, could help explain the positive results of the current studies—the CLOSE and Gore REDUCE trials and extended follow-up from the RESPECT trial. Dr. Ropper is Professor of Neurology at Harvard Medical School and Raymond D. Adams Master Clinician and Executive Vice Chairman of the Department of Neurology at Brigham and Women’s Hospital in Boston.
Persuasive Effect
Together, the trials indicate the importance of considering the characteristics of a PFO when assessing whether a patient may be an appropriate candidate for the procedure, Dr. Ropper said. In the CLOSE trial, patients had to have a large interatrial shunt at rest or an atrial septal aneurysm. While rates of stroke in the PFO closure groups in all of the studies were low, “in the CLOSE trial, no patient in the PFO closure group had a stroke, whereas stroke occurred in 6% of the patients in the antiplatelet-only group.”
The Gore REDUCE trial represented a “middle ground” in that most patients (81%) had a moderate or large interatrial shunt. In that trial, PFO closure was associated with significantly reduced risk of recurrent stroke, compared with antiplatelet therapy alone (1.4% vs 5.4%).
The primary analysis of the RESPECT trial, published in 2013, suggested a potential benefit, but the primary result was not statistically significant. The trial nevertheless provided a reasonable assurance of safety and effectiveness, and the FDA approved the device studied in the trial, the Amplatzer PFO Occluder (St. Jude Medical, St. Paul), in October 2016. The primary analysis included a median of 2.1 years of follow-up, whereas the current analysis included a median of 5.9 years of follow-up. Through the extended follow-up, 3.6% of patients in the PFO closure group had recurrent ischemic stroke, compared with 5.8% of patients in the medical therapy group.
“Therefore, in patients who have had a stroke, are younger than 60 years of age, and have a PFO with characteristics that are highly likely to allow paradoxical embolism to occur, the effect of closure becomes persuasive,” Dr. Ropper said. “Restricting PFO closure entirely to patients with high-risk characteristics of the PFO may perhaps be too conservative, but the boundaries of the features that support the procedure are becoming clearer.”
CLOSE
In the CLOSE trial, Jean-Louis Mas, MD, Professor of Neurology at Paris Descartes University and Head of the Neurology Department at Sainte-Anne Hospital in Paris, and colleagues enrolled patients who had had a recent cryptogenic stroke attributed to a PFO with an associated atrial septal aneurysm or large interatrial shunt.
The investigators assigned in a 1:1:1 ratio patients age 16 to 60 to transcatheter PFO closure plus long-term antiplatelet therapy, antiplatelet therapy alone, or oral anticoagulation. The primary outcome was occurrence of stroke. In all, 663 patients were randomized and followed for a mean of 5.3 years.
“No stroke occurred among the 238 patients in the PFO closure group, whereas stroke occurred in 14 of the 235 patients in the antiplatelet-only group (hazard ratio, 0.03),” the researchers said. “Procedural complication from PFO closure occurred in 14 patients (5.9%). The rate of atrial fibrillation was higher in the PFO closure group than in the antiplatelet-only group (4.6% vs 0.9%). The number of serious adverse events did not differ significantly between the treatment groups.” The trial was underpowered to determine the effects of oral anticoagulant therapy versus antiplatelet therapy.
Unlike in prior studies, CLOSE “included only patients who had PFO features that have been associated with cryptogenic stroke” and researchers “used a standardized evaluation to define a previous cryptogenic stroke,” the authors noted.
REDUCE
In the Gore REDUCE trial, investigators enrolled patients with a PFO who had had a cryptogenic stroke. Lars Søndergaard, MD, of the Department of Cardiology at Rigshospitalet, University of Copenhagen, and colleagues randomly assigned patients 2:1 to undergo PFO closure (with the Helex Septal Occluder or Cardioform Septal Occluder; W.L. Gore and Associates, Newark, Delaware) plus antiplatelet therapy or to receive antiplatelet therapy alone. Patients underwent brain imaging at baseline and 24 months. Coprimary end points were freedom from clinical evidence of ischemic stroke through at least 24 months after randomization and the 24-month incidence of new brain infarction (ie, clinical ischemic stroke or silent brain infarction on imaging).
The investigators enrolled 664 patients (mean age, 45.2). During a median follow-up of 3.2 years, clinical ischemic stroke occurred in six of 441 patients (1.4%) in the PFO closure group and in 12 of 223 patients (5.4%) in the antiplatelet-only group (hazard ratio, 0.23). The incidence of new brain infarctions was lower in the PFO closure group than in the antiplatelet-only group (5.7% vs 11.3%), but the incidence of silent brain infarction did not differ significantly between the study groups.
Serious adverse events occurred in 23.1% of the patients in the PFO closure group and 27.8% of the patients in the antiplatelet-only group. Serious device-related adverse events occurred in six patients (1.4%) in the PFO closure group. Atrial fibrillation or flutter occurred in significantly more patients in the PFO closure group than in the antiplatelet-only group (6.6% vs 0.4%). Most cases of atrial fibrillation or flutter were detected within 45 days after the procedure and resolved within two weeks after onset.
RESPECT
In RESPECT, among patients age 18 to 60 who had had a cryptogenic ischemic stroke, closure of a PFO was associated with a lower rate of recurrent ischemic strokes than medical therapy alone during extended follow-up.
Jeffrey L. Saver, MD, Director of the University of California, Los Angeles Comprehensive Stroke Center and Professor of Neurology at the David Geffen School of Medicine at UCLA, and colleagues randomly assigned patients who had a PFO and had had a cryptogenic ischemic stroke to undergo closure of the PFO with the Amplatzer PFO Occluder or to receive medical therapy alone (ie, aspirin, warfarin, clopidogrel, or aspirin combined with extended-release dipyridamole). After the device was implanted, patients in the PFO closure group received aspirin plus clopidogrel daily for one month, followed by aspirin monotherapy for five months. Subsequent antithrombotic therapy was at the site investigator’s discretion.
The investigators enrolled 980 patients (mean age, 45.9) at 69 sites and followed patients for a median of 5.9 years. In the intention-to-treat population, recurrent ischemic stroke occurred in 18 patients in the PFO closure group (3.6%) and in 28 patients in the medical-therapy group (5.8%), resulting in rates of 0.58 events per 100 patient-years and 1.07 events per 100 patient-years, respectively (hazard ratio with PFO closure vs medical therapy, 0.55).
“The relative difference in the rate of recurrent ischemic stroke between PFO closure and medical therapy alone was large (45% lower with PFO closure), but the absolute difference was small (0.49 fewer events per 100 patient-years with PFO closure),” the authors said. “Nonetheless, the cumulative absolute benefit had clinical relevance, since patients in this trial were younger … than the general population of patients who have stroke and thus faced a longer period of risk for recurrent stroke.”
Venous thromboembolism (ie, pulmonary embolism and deep-vein thrombosis) was more common in the PFO closure group than in the medical-therapy group. Among patients in the PFO closure group, those with a history of overt deep-vein thrombosis had a higher incidence of venous thromboembolic events. A lower intensity of antithrombotic therapy, including less common use of anticoagulant agents, in the PFO closure group, compared with the medical therapy group, “may have contributed to the higher rate of venous thromboembolism in the PFO closure group,” the researchers said. “These findings provide indirect support for the recent revision in the national management guidelines that endorsed lifelong anticoagulation therapy in patients with overt deep-vein thrombosis.”
In addition, seven periprocedural events of atrial fibrillation occurred in the PFO closure group, all of which resolved before the patients’ discharge from the hospital.
Assessment by Neurologist and Cardiologist Is Key
“The key to appropriate device use is comprehensive clinical assessment by both a neurologist and a cardiologist to confirm the diagnosis of ischemic stroke and exclude other possible causes,” said Andrew Farb, MD, of the FDA’s Center for Devices and Radiological Health in Silver Spring, Maryland, and colleagues, in a perspective accompanying the RESPECT trial results. The Amplatzer PFO Occluder’s
—Jake Remaly
Suggested Reading
Farb A, Ibrahim NG, Zuckerman BD. Patent foramen ovale after cryptogenic stroke - assessing the evidence for closure. N Engl J Med. 2017;377(11):1006-1009.
Mas JL, Derumeaux G, Guillon B, et al; CLOSE Investigators. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med. 2017;377(11):1011-1021.
Ropper AH. Tipping point for patent foramen ovale closure. N Engl J Med. 2017;377(11):1093-1095.
Saver JL, Carroll JD, Thaler DE, et al; RESPECT Investigators. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. 2017;377(11):1022-1032.
Søndergaard L, Kasner SE, Rhodes JF, et al; Gore REDUCE Clinical Study Investigators. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. 2017;377(11):1033-1042.
Among patients with a patent foramen ovale (PFO) who have had a cryptogenic stroke, closure of the PFO reduces the risk of recurrent stroke, compared with medical therapy alone, according to three studies published in the September 14 issue of the New England Journal of Medicine. PFO closure, however, entailed increased risk of atrial fibrillation and, in two of the studies, venous thromboembolism.
In prior trials, PFO closure did not have a statistically significant effect on stroke risk, although the results suggested potential benefit. In an editorial accompanying the new trial results, Allan H. Ropper, MD, said that various limitations of the prior studies, including inclusion of patients whose prior stroke would not benefit from PFO closure, could help explain the positive results of the current studies—the CLOSE and Gore REDUCE trials and extended follow-up from the RESPECT trial. Dr. Ropper is Professor of Neurology at Harvard Medical School and Raymond D. Adams Master Clinician and Executive Vice Chairman of the Department of Neurology at Brigham and Women’s Hospital in Boston.
Persuasive Effect
Together, the trials indicate the importance of considering the characteristics of a PFO when assessing whether a patient may be an appropriate candidate for the procedure, Dr. Ropper said. In the CLOSE trial, patients had to have a large interatrial shunt at rest or an atrial septal aneurysm. While rates of stroke in the PFO closure groups in all of the studies were low, “in the CLOSE trial, no patient in the PFO closure group had a stroke, whereas stroke occurred in 6% of the patients in the antiplatelet-only group.”
The Gore REDUCE trial represented a “middle ground” in that most patients (81%) had a moderate or large interatrial shunt. In that trial, PFO closure was associated with significantly reduced risk of recurrent stroke, compared with antiplatelet therapy alone (1.4% vs 5.4%).
The primary analysis of the RESPECT trial, published in 2013, suggested a potential benefit, but the primary result was not statistically significant. The trial nevertheless provided a reasonable assurance of safety and effectiveness, and the FDA approved the device studied in the trial, the Amplatzer PFO Occluder (St. Jude Medical, St. Paul), in October 2016. The primary analysis included a median of 2.1 years of follow-up, whereas the current analysis included a median of 5.9 years of follow-up. Through the extended follow-up, 3.6% of patients in the PFO closure group had recurrent ischemic stroke, compared with 5.8% of patients in the medical therapy group.
“Therefore, in patients who have had a stroke, are younger than 60 years of age, and have a PFO with characteristics that are highly likely to allow paradoxical embolism to occur, the effect of closure becomes persuasive,” Dr. Ropper said. “Restricting PFO closure entirely to patients with high-risk characteristics of the PFO may perhaps be too conservative, but the boundaries of the features that support the procedure are becoming clearer.”
CLOSE
In the CLOSE trial, Jean-Louis Mas, MD, Professor of Neurology at Paris Descartes University and Head of the Neurology Department at Sainte-Anne Hospital in Paris, and colleagues enrolled patients who had had a recent cryptogenic stroke attributed to a PFO with an associated atrial septal aneurysm or large interatrial shunt.
The investigators assigned in a 1:1:1 ratio patients age 16 to 60 to transcatheter PFO closure plus long-term antiplatelet therapy, antiplatelet therapy alone, or oral anticoagulation. The primary outcome was occurrence of stroke. In all, 663 patients were randomized and followed for a mean of 5.3 years.
“No stroke occurred among the 238 patients in the PFO closure group, whereas stroke occurred in 14 of the 235 patients in the antiplatelet-only group (hazard ratio, 0.03),” the researchers said. “Procedural complication from PFO closure occurred in 14 patients (5.9%). The rate of atrial fibrillation was higher in the PFO closure group than in the antiplatelet-only group (4.6% vs 0.9%). The number of serious adverse events did not differ significantly between the treatment groups.” The trial was underpowered to determine the effects of oral anticoagulant therapy versus antiplatelet therapy.
Unlike in prior studies, CLOSE “included only patients who had PFO features that have been associated with cryptogenic stroke” and researchers “used a standardized evaluation to define a previous cryptogenic stroke,” the authors noted.
REDUCE
In the Gore REDUCE trial, investigators enrolled patients with a PFO who had had a cryptogenic stroke. Lars Søndergaard, MD, of the Department of Cardiology at Rigshospitalet, University of Copenhagen, and colleagues randomly assigned patients 2:1 to undergo PFO closure (with the Helex Septal Occluder or Cardioform Septal Occluder; W.L. Gore and Associates, Newark, Delaware) plus antiplatelet therapy or to receive antiplatelet therapy alone. Patients underwent brain imaging at baseline and 24 months. Coprimary end points were freedom from clinical evidence of ischemic stroke through at least 24 months after randomization and the 24-month incidence of new brain infarction (ie, clinical ischemic stroke or silent brain infarction on imaging).
The investigators enrolled 664 patients (mean age, 45.2). During a median follow-up of 3.2 years, clinical ischemic stroke occurred in six of 441 patients (1.4%) in the PFO closure group and in 12 of 223 patients (5.4%) in the antiplatelet-only group (hazard ratio, 0.23). The incidence of new brain infarctions was lower in the PFO closure group than in the antiplatelet-only group (5.7% vs 11.3%), but the incidence of silent brain infarction did not differ significantly between the study groups.
Serious adverse events occurred in 23.1% of the patients in the PFO closure group and 27.8% of the patients in the antiplatelet-only group. Serious device-related adverse events occurred in six patients (1.4%) in the PFO closure group. Atrial fibrillation or flutter occurred in significantly more patients in the PFO closure group than in the antiplatelet-only group (6.6% vs 0.4%). Most cases of atrial fibrillation or flutter were detected within 45 days after the procedure and resolved within two weeks after onset.
RESPECT
In RESPECT, among patients age 18 to 60 who had had a cryptogenic ischemic stroke, closure of a PFO was associated with a lower rate of recurrent ischemic strokes than medical therapy alone during extended follow-up.
Jeffrey L. Saver, MD, Director of the University of California, Los Angeles Comprehensive Stroke Center and Professor of Neurology at the David Geffen School of Medicine at UCLA, and colleagues randomly assigned patients who had a PFO and had had a cryptogenic ischemic stroke to undergo closure of the PFO with the Amplatzer PFO Occluder or to receive medical therapy alone (ie, aspirin, warfarin, clopidogrel, or aspirin combined with extended-release dipyridamole). After the device was implanted, patients in the PFO closure group received aspirin plus clopidogrel daily for one month, followed by aspirin monotherapy for five months. Subsequent antithrombotic therapy was at the site investigator’s discretion.
The investigators enrolled 980 patients (mean age, 45.9) at 69 sites and followed patients for a median of 5.9 years. In the intention-to-treat population, recurrent ischemic stroke occurred in 18 patients in the PFO closure group (3.6%) and in 28 patients in the medical-therapy group (5.8%), resulting in rates of 0.58 events per 100 patient-years and 1.07 events per 100 patient-years, respectively (hazard ratio with PFO closure vs medical therapy, 0.55).
“The relative difference in the rate of recurrent ischemic stroke between PFO closure and medical therapy alone was large (45% lower with PFO closure), but the absolute difference was small (0.49 fewer events per 100 patient-years with PFO closure),” the authors said. “Nonetheless, the cumulative absolute benefit had clinical relevance, since patients in this trial were younger … than the general population of patients who have stroke and thus faced a longer period of risk for recurrent stroke.”
Venous thromboembolism (ie, pulmonary embolism and deep-vein thrombosis) was more common in the PFO closure group than in the medical-therapy group. Among patients in the PFO closure group, those with a history of overt deep-vein thrombosis had a higher incidence of venous thromboembolic events. A lower intensity of antithrombotic therapy, including less common use of anticoagulant agents, in the PFO closure group, compared with the medical therapy group, “may have contributed to the higher rate of venous thromboembolism in the PFO closure group,” the researchers said. “These findings provide indirect support for the recent revision in the national management guidelines that endorsed lifelong anticoagulation therapy in patients with overt deep-vein thrombosis.”
In addition, seven periprocedural events of atrial fibrillation occurred in the PFO closure group, all of which resolved before the patients’ discharge from the hospital.
Assessment by Neurologist and Cardiologist Is Key
“The key to appropriate device use is comprehensive clinical assessment by both a neurologist and a cardiologist to confirm the diagnosis of ischemic stroke and exclude other possible causes,” said Andrew Farb, MD, of the FDA’s Center for Devices and Radiological Health in Silver Spring, Maryland, and colleagues, in a perspective accompanying the RESPECT trial results. The Amplatzer PFO Occluder’s
—Jake Remaly
Suggested Reading
Farb A, Ibrahim NG, Zuckerman BD. Patent foramen ovale after cryptogenic stroke - assessing the evidence for closure. N Engl J Med. 2017;377(11):1006-1009.
Mas JL, Derumeaux G, Guillon B, et al; CLOSE Investigators. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med. 2017;377(11):1011-1021.
Ropper AH. Tipping point for patent foramen ovale closure. N Engl J Med. 2017;377(11):1093-1095.
Saver JL, Carroll JD, Thaler DE, et al; RESPECT Investigators. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. 2017;377(11):1022-1032.
Søndergaard L, Kasner SE, Rhodes JF, et al; Gore REDUCE Clinical Study Investigators. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. 2017;377(11):1033-1042.
Among patients with a patent foramen ovale (PFO) who have had a cryptogenic stroke, closure of the PFO reduces the risk of recurrent stroke, compared with medical therapy alone, according to three studies published in the September 14 issue of the New England Journal of Medicine. PFO closure, however, entailed increased risk of atrial fibrillation and, in two of the studies, venous thromboembolism.
In prior trials, PFO closure did not have a statistically significant effect on stroke risk, although the results suggested potential benefit. In an editorial accompanying the new trial results, Allan H. Ropper, MD, said that various limitations of the prior studies, including inclusion of patients whose prior stroke would not benefit from PFO closure, could help explain the positive results of the current studies—the CLOSE and Gore REDUCE trials and extended follow-up from the RESPECT trial. Dr. Ropper is Professor of Neurology at Harvard Medical School and Raymond D. Adams Master Clinician and Executive Vice Chairman of the Department of Neurology at Brigham and Women’s Hospital in Boston.
Persuasive Effect
Together, the trials indicate the importance of considering the characteristics of a PFO when assessing whether a patient may be an appropriate candidate for the procedure, Dr. Ropper said. In the CLOSE trial, patients had to have a large interatrial shunt at rest or an atrial septal aneurysm. While rates of stroke in the PFO closure groups in all of the studies were low, “in the CLOSE trial, no patient in the PFO closure group had a stroke, whereas stroke occurred in 6% of the patients in the antiplatelet-only group.”
The Gore REDUCE trial represented a “middle ground” in that most patients (81%) had a moderate or large interatrial shunt. In that trial, PFO closure was associated with significantly reduced risk of recurrent stroke, compared with antiplatelet therapy alone (1.4% vs 5.4%).
The primary analysis of the RESPECT trial, published in 2013, suggested a potential benefit, but the primary result was not statistically significant. The trial nevertheless provided a reasonable assurance of safety and effectiveness, and the FDA approved the device studied in the trial, the Amplatzer PFO Occluder (St. Jude Medical, St. Paul), in October 2016. The primary analysis included a median of 2.1 years of follow-up, whereas the current analysis included a median of 5.9 years of follow-up. Through the extended follow-up, 3.6% of patients in the PFO closure group had recurrent ischemic stroke, compared with 5.8% of patients in the medical therapy group.
“Therefore, in patients who have had a stroke, are younger than 60 years of age, and have a PFO with characteristics that are highly likely to allow paradoxical embolism to occur, the effect of closure becomes persuasive,” Dr. Ropper said. “Restricting PFO closure entirely to patients with high-risk characteristics of the PFO may perhaps be too conservative, but the boundaries of the features that support the procedure are becoming clearer.”
CLOSE
In the CLOSE trial, Jean-Louis Mas, MD, Professor of Neurology at Paris Descartes University and Head of the Neurology Department at Sainte-Anne Hospital in Paris, and colleagues enrolled patients who had had a recent cryptogenic stroke attributed to a PFO with an associated atrial septal aneurysm or large interatrial shunt.
The investigators assigned in a 1:1:1 ratio patients age 16 to 60 to transcatheter PFO closure plus long-term antiplatelet therapy, antiplatelet therapy alone, or oral anticoagulation. The primary outcome was occurrence of stroke. In all, 663 patients were randomized and followed for a mean of 5.3 years.
“No stroke occurred among the 238 patients in the PFO closure group, whereas stroke occurred in 14 of the 235 patients in the antiplatelet-only group (hazard ratio, 0.03),” the researchers said. “Procedural complication from PFO closure occurred in 14 patients (5.9%). The rate of atrial fibrillation was higher in the PFO closure group than in the antiplatelet-only group (4.6% vs 0.9%). The number of serious adverse events did not differ significantly between the treatment groups.” The trial was underpowered to determine the effects of oral anticoagulant therapy versus antiplatelet therapy.
Unlike in prior studies, CLOSE “included only patients who had PFO features that have been associated with cryptogenic stroke” and researchers “used a standardized evaluation to define a previous cryptogenic stroke,” the authors noted.
REDUCE
In the Gore REDUCE trial, investigators enrolled patients with a PFO who had had a cryptogenic stroke. Lars Søndergaard, MD, of the Department of Cardiology at Rigshospitalet, University of Copenhagen, and colleagues randomly assigned patients 2:1 to undergo PFO closure (with the Helex Septal Occluder or Cardioform Septal Occluder; W.L. Gore and Associates, Newark, Delaware) plus antiplatelet therapy or to receive antiplatelet therapy alone. Patients underwent brain imaging at baseline and 24 months. Coprimary end points were freedom from clinical evidence of ischemic stroke through at least 24 months after randomization and the 24-month incidence of new brain infarction (ie, clinical ischemic stroke or silent brain infarction on imaging).
The investigators enrolled 664 patients (mean age, 45.2). During a median follow-up of 3.2 years, clinical ischemic stroke occurred in six of 441 patients (1.4%) in the PFO closure group and in 12 of 223 patients (5.4%) in the antiplatelet-only group (hazard ratio, 0.23). The incidence of new brain infarctions was lower in the PFO closure group than in the antiplatelet-only group (5.7% vs 11.3%), but the incidence of silent brain infarction did not differ significantly between the study groups.
Serious adverse events occurred in 23.1% of the patients in the PFO closure group and 27.8% of the patients in the antiplatelet-only group. Serious device-related adverse events occurred in six patients (1.4%) in the PFO closure group. Atrial fibrillation or flutter occurred in significantly more patients in the PFO closure group than in the antiplatelet-only group (6.6% vs 0.4%). Most cases of atrial fibrillation or flutter were detected within 45 days after the procedure and resolved within two weeks after onset.
RESPECT
In RESPECT, among patients age 18 to 60 who had had a cryptogenic ischemic stroke, closure of a PFO was associated with a lower rate of recurrent ischemic strokes than medical therapy alone during extended follow-up.
Jeffrey L. Saver, MD, Director of the University of California, Los Angeles Comprehensive Stroke Center and Professor of Neurology at the David Geffen School of Medicine at UCLA, and colleagues randomly assigned patients who had a PFO and had had a cryptogenic ischemic stroke to undergo closure of the PFO with the Amplatzer PFO Occluder or to receive medical therapy alone (ie, aspirin, warfarin, clopidogrel, or aspirin combined with extended-release dipyridamole). After the device was implanted, patients in the PFO closure group received aspirin plus clopidogrel daily for one month, followed by aspirin monotherapy for five months. Subsequent antithrombotic therapy was at the site investigator’s discretion.
The investigators enrolled 980 patients (mean age, 45.9) at 69 sites and followed patients for a median of 5.9 years. In the intention-to-treat population, recurrent ischemic stroke occurred in 18 patients in the PFO closure group (3.6%) and in 28 patients in the medical-therapy group (5.8%), resulting in rates of 0.58 events per 100 patient-years and 1.07 events per 100 patient-years, respectively (hazard ratio with PFO closure vs medical therapy, 0.55).
“The relative difference in the rate of recurrent ischemic stroke between PFO closure and medical therapy alone was large (45% lower with PFO closure), but the absolute difference was small (0.49 fewer events per 100 patient-years with PFO closure),” the authors said. “Nonetheless, the cumulative absolute benefit had clinical relevance, since patients in this trial were younger … than the general population of patients who have stroke and thus faced a longer period of risk for recurrent stroke.”
Venous thromboembolism (ie, pulmonary embolism and deep-vein thrombosis) was more common in the PFO closure group than in the medical-therapy group. Among patients in the PFO closure group, those with a history of overt deep-vein thrombosis had a higher incidence of venous thromboembolic events. A lower intensity of antithrombotic therapy, including less common use of anticoagulant agents, in the PFO closure group, compared with the medical therapy group, “may have contributed to the higher rate of venous thromboembolism in the PFO closure group,” the researchers said. “These findings provide indirect support for the recent revision in the national management guidelines that endorsed lifelong anticoagulation therapy in patients with overt deep-vein thrombosis.”
In addition, seven periprocedural events of atrial fibrillation occurred in the PFO closure group, all of which resolved before the patients’ discharge from the hospital.
Assessment by Neurologist and Cardiologist Is Key
“The key to appropriate device use is comprehensive clinical assessment by both a neurologist and a cardiologist to confirm the diagnosis of ischemic stroke and exclude other possible causes,” said Andrew Farb, MD, of the FDA’s Center for Devices and Radiological Health in Silver Spring, Maryland, and colleagues, in a perspective accompanying the RESPECT trial results. The Amplatzer PFO Occluder’s
—Jake Remaly
Suggested Reading
Farb A, Ibrahim NG, Zuckerman BD. Patent foramen ovale after cryptogenic stroke - assessing the evidence for closure. N Engl J Med. 2017;377(11):1006-1009.
Mas JL, Derumeaux G, Guillon B, et al; CLOSE Investigators. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med. 2017;377(11):1011-1021.
Ropper AH. Tipping point for patent foramen ovale closure. N Engl J Med. 2017;377(11):1093-1095.
Saver JL, Carroll JD, Thaler DE, et al; RESPECT Investigators. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. 2017;377(11):1022-1032.
Søndergaard L, Kasner SE, Rhodes JF, et al; Gore REDUCE Clinical Study Investigators. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. 2017;377(11):1033-1042.
Fentanyl analogues an increasing factor in opioid deaths
Fentanyl analogues were involved in 14% of opioid overdose deaths in the second half of 2016, according to an analysis of 10 states reporting to the Enhanced State Opioid Overdose Surveillance program.
“Illicitly manufactured fentanyl is a key factor driving opioid overdose deaths and … fentanyl analogues [such as carfentanil, furanylfentanyl, and acetylfentanyl] are increasingly contributing to a complex illicit opioid market with significant public health implications,” investigators said in a report from the Centers for Disease Control and Prevention (MMWR 2017 Oct 27;66[early release]:1-6).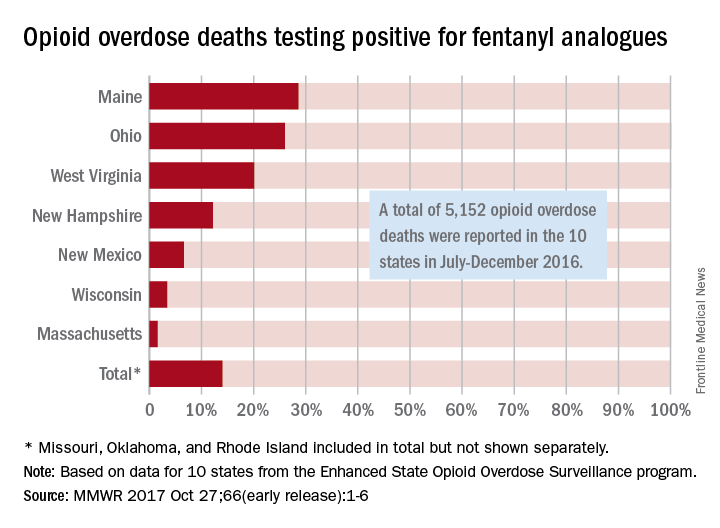
The overall rate of 14% represents 720 of the 5,152 total opioid deaths occurring in 10 states over the 6-month study period, they noted.
Of the 10 states in the analysis, Maine had the largest proportion (28.6%) of opioid overdose deaths involving fentanyl analogues, with Ohio second at 26% and West Virginia third at 20.1%. Ohio had the largest overall number of analogue-involved overdose deaths, however, at 531 during July-December 2016. At 1.6%, Massachusetts had the lowest rate of fentanyl analogue–involved deaths among the seven states for which separate figures were given, the published data show. (Three states – Missouri [22 counties], Oklahoma, and Rhode Island – were grouped together and had a combined rate of 1%.)
More than half of the overdose deaths involving fentanyl or a fentanyl analogue also involved heroin, cocaine, or methamphetamine, which means that almost half of the deaths “did not test positive for other illicit opioids, suggesting that fentanyl and fentanyl analogues might be emerging as unique illicit products,” the investigators wrote, adding that the fentanyl analogue situation “might mirror the rapidly rising trajectory of fentanyl overdose deaths that began in 2013 and become a major factor in opioid overdose deaths.”
Fentanyl analogues were involved in 14% of opioid overdose deaths in the second half of 2016, according to an analysis of 10 states reporting to the Enhanced State Opioid Overdose Surveillance program.
“Illicitly manufactured fentanyl is a key factor driving opioid overdose deaths and … fentanyl analogues [such as carfentanil, furanylfentanyl, and acetylfentanyl] are increasingly contributing to a complex illicit opioid market with significant public health implications,” investigators said in a report from the Centers for Disease Control and Prevention (MMWR 2017 Oct 27;66[early release]:1-6).
The overall rate of 14% represents 720 of the 5,152 total opioid deaths occurring in 10 states over the 6-month study period, they noted.
Of the 10 states in the analysis, Maine had the largest proportion (28.6%) of opioid overdose deaths involving fentanyl analogues, with Ohio second at 26% and West Virginia third at 20.1%. Ohio had the largest overall number of analogue-involved overdose deaths, however, at 531 during July-December 2016. At 1.6%, Massachusetts had the lowest rate of fentanyl analogue–involved deaths among the seven states for which separate figures were given, the published data show. (Three states – Missouri [22 counties], Oklahoma, and Rhode Island – were grouped together and had a combined rate of 1%.)
More than half of the overdose deaths involving fentanyl or a fentanyl analogue also involved heroin, cocaine, or methamphetamine, which means that almost half of the deaths “did not test positive for other illicit opioids, suggesting that fentanyl and fentanyl analogues might be emerging as unique illicit products,” the investigators wrote, adding that the fentanyl analogue situation “might mirror the rapidly rising trajectory of fentanyl overdose deaths that began in 2013 and become a major factor in opioid overdose deaths.”
Fentanyl analogues were involved in 14% of opioid overdose deaths in the second half of 2016, according to an analysis of 10 states reporting to the Enhanced State Opioid Overdose Surveillance program.
“Illicitly manufactured fentanyl is a key factor driving opioid overdose deaths and … fentanyl analogues [such as carfentanil, furanylfentanyl, and acetylfentanyl] are increasingly contributing to a complex illicit opioid market with significant public health implications,” investigators said in a report from the Centers for Disease Control and Prevention (MMWR 2017 Oct 27;66[early release]:1-6).
The overall rate of 14% represents 720 of the 5,152 total opioid deaths occurring in 10 states over the 6-month study period, they noted.
Of the 10 states in the analysis, Maine had the largest proportion (28.6%) of opioid overdose deaths involving fentanyl analogues, with Ohio second at 26% and West Virginia third at 20.1%. Ohio had the largest overall number of analogue-involved overdose deaths, however, at 531 during July-December 2016. At 1.6%, Massachusetts had the lowest rate of fentanyl analogue–involved deaths among the seven states for which separate figures were given, the published data show. (Three states – Missouri [22 counties], Oklahoma, and Rhode Island – were grouped together and had a combined rate of 1%.)
More than half of the overdose deaths involving fentanyl or a fentanyl analogue also involved heroin, cocaine, or methamphetamine, which means that almost half of the deaths “did not test positive for other illicit opioids, suggesting that fentanyl and fentanyl analogues might be emerging as unique illicit products,” the investigators wrote, adding that the fentanyl analogue situation “might mirror the rapidly rising trajectory of fentanyl overdose deaths that began in 2013 and become a major factor in opioid overdose deaths.”
FROM MMWR
Are Two Antithrombotic Agents Better Than Three?
Dual antithrombotic therapy with dabigatran and a P2Y12 inhibitor (eg, clopidogrel or ticagrelor) is associated with a lower risk of bleeding, compared with standard triple antithrombotic therapy, after percutaneous coronary intervention (PCI) for patients with atrial fibrillation, according to research published online ahead of print August 27 in the New England Journal of Medicine. Dual therapy also is noninferior to triple therapy regarding the risk of thromboembolic events.
“Patients who received two anticlotting medications—including one of a newer class of drug—had fewer bleeding events without being more at risk for a stroke or other cardiac events,” said Christopher P. Cannon, MD, Professor of Medicine at Harvard Medical School and a cardiovascular medicine specialist at Brigham and Women’s Hospital in Boston, and colleagues.
Standard triple antithrombotic therapy (ie, warfarin plus two antiplatelet agents) has been associated with a high risk of bleeding, thus prompting researchers to seek a better approach to treatment. One emerging therapy omits aspirin from the standard regimen and uses a single P2Y12 inhibitor in combination with an oral anticoagulant. A moderate-sized trial found that this form of dual-therapy lowered the risk of bleeding, compared with standard triple therapy. Another trial supported standard triple therapy for a shorter duration. Finally, a recent trial found that the risk of bleeding was lower with a regimen of reduced-dose rivaroxaban plus a P2Y12 inhibitor than with standard triple therapy.
Dual Therapy Versus Triple Therapy
To compare two regimens of dual antithrombotic therapy that include dabigatran with a regimen of triple antithrombotic therapy that includes warfarin, Dr. Cannon and colleagues conducted the RE-DUAL PCI trial. Eligible participants were 18 or older, had nonvalvular atrial fibrillation, and had successfully undergone PCI with a bare-metal or drug-eluting stent within the previous 120 hours. Patients with bioprosthetic or mechanical heart valves, severe renal insufficiency, or other major coexisting conditions were excluded.
All patients in the United States and nonelderly patients in other countries were randomized 1:1:1 to receive triple therapy with warfarin plus a PSY12 inhibitor and aspirin for one to three months, or to receive dual therapy with dabigatran (110 mg or 150 mg twice daily) plus a P2Y12 inhibitor and no aspirin. Outside the US, elderly patients (ie, 70 or older in Japan and 80 or older elsewhere) were randomized 1:1 to receive 110 mg of dual therapy or triple therapy.
The primary end point was the first major or clinically relevant nonmajor bleeding event. A major secondary end point was the composite of thromboembolic events (ie, myocardial infarction, stroke, or systemic embolism), death, or unplanned revascularization. Other secondary end points included a composite of thromboembolic events or death, as well as the individual thromboembolic events and definite stent thrombosis.
No Significant Difference in Serious Adverse Events Between Groups
Between July 21, 2014, and October 31, 2016, 2,725 participants underwent randomization at 414 sites in 41 countries. The mean age of participants was 70.8. The mean duration of treatment with the trial anticoagulant was 12.3 months, and the mean duration of follow-up was 14 months. Six patients were lost to follow-up. Most patients received clopidogrel as their P2Y12 inhibitor, and 12% received ticagrelor.
The incidence of the primary end point was 15.4% in the 110-mg dual-therapy group, compared with 26.9% in the triple-therapy group, and 20.2% in the 150-mg dual-therapy group, compared with 25.7% in the corresponding triple-therapy group. The incidence of the composite efficacy end point was 13.7% in both dual-therapy groups combined, compared with 13.4% in the triple-therapy group.
Researchers found no significant difference between groups in the rate of serious adverse events. Fatal serious adverse events occurred during treatment in 38 patients in the 100-mg dual-therapy group, 24 patients in the 150-mg dual-therapy group, and 41 patients in the triple-therapy group.
“We now have new information to help select the right treatment for individual patients,” said Dr. Cannon. “With respect to the results for both the bleeding and thromboembolic-event end points, we may only speculate on the relative contributions of the omission of aspirin and the type of oral anticoagulant in the dual-therapy groups and the triple-therapy group. A trial conducted with a formal two-by-two factorial design would be able to discern these contributions, and one such trial is ongoing.”
One limitation of this trial was that researchers enrolled a smaller number of patients than initially planned. The power of the trial to examine efficacy according to dabigatran dose consequently was limited, said the authors.
This study was supported by Boehringer Ingelheim.
—Erica Tricarico
Suggested Reading
Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017 Aug 27 [Epub ahead of print].
Dual antithrombotic therapy with dabigatran and a P2Y12 inhibitor (eg, clopidogrel or ticagrelor) is associated with a lower risk of bleeding, compared with standard triple antithrombotic therapy, after percutaneous coronary intervention (PCI) for patients with atrial fibrillation, according to research published online ahead of print August 27 in the New England Journal of Medicine. Dual therapy also is noninferior to triple therapy regarding the risk of thromboembolic events.
“Patients who received two anticlotting medications—including one of a newer class of drug—had fewer bleeding events without being more at risk for a stroke or other cardiac events,” said Christopher P. Cannon, MD, Professor of Medicine at Harvard Medical School and a cardiovascular medicine specialist at Brigham and Women’s Hospital in Boston, and colleagues.
Standard triple antithrombotic therapy (ie, warfarin plus two antiplatelet agents) has been associated with a high risk of bleeding, thus prompting researchers to seek a better approach to treatment. One emerging therapy omits aspirin from the standard regimen and uses a single P2Y12 inhibitor in combination with an oral anticoagulant. A moderate-sized trial found that this form of dual-therapy lowered the risk of bleeding, compared with standard triple therapy. Another trial supported standard triple therapy for a shorter duration. Finally, a recent trial found that the risk of bleeding was lower with a regimen of reduced-dose rivaroxaban plus a P2Y12 inhibitor than with standard triple therapy.
Dual Therapy Versus Triple Therapy
To compare two regimens of dual antithrombotic therapy that include dabigatran with a regimen of triple antithrombotic therapy that includes warfarin, Dr. Cannon and colleagues conducted the RE-DUAL PCI trial. Eligible participants were 18 or older, had nonvalvular atrial fibrillation, and had successfully undergone PCI with a bare-metal or drug-eluting stent within the previous 120 hours. Patients with bioprosthetic or mechanical heart valves, severe renal insufficiency, or other major coexisting conditions were excluded.
All patients in the United States and nonelderly patients in other countries were randomized 1:1:1 to receive triple therapy with warfarin plus a PSY12 inhibitor and aspirin for one to three months, or to receive dual therapy with dabigatran (110 mg or 150 mg twice daily) plus a P2Y12 inhibitor and no aspirin. Outside the US, elderly patients (ie, 70 or older in Japan and 80 or older elsewhere) were randomized 1:1 to receive 110 mg of dual therapy or triple therapy.
The primary end point was the first major or clinically relevant nonmajor bleeding event. A major secondary end point was the composite of thromboembolic events (ie, myocardial infarction, stroke, or systemic embolism), death, or unplanned revascularization. Other secondary end points included a composite of thromboembolic events or death, as well as the individual thromboembolic events and definite stent thrombosis.
No Significant Difference in Serious Adverse Events Between Groups
Between July 21, 2014, and October 31, 2016, 2,725 participants underwent randomization at 414 sites in 41 countries. The mean age of participants was 70.8. The mean duration of treatment with the trial anticoagulant was 12.3 months, and the mean duration of follow-up was 14 months. Six patients were lost to follow-up. Most patients received clopidogrel as their P2Y12 inhibitor, and 12% received ticagrelor.
The incidence of the primary end point was 15.4% in the 110-mg dual-therapy group, compared with 26.9% in the triple-therapy group, and 20.2% in the 150-mg dual-therapy group, compared with 25.7% in the corresponding triple-therapy group. The incidence of the composite efficacy end point was 13.7% in both dual-therapy groups combined, compared with 13.4% in the triple-therapy group.
Researchers found no significant difference between groups in the rate of serious adverse events. Fatal serious adverse events occurred during treatment in 38 patients in the 100-mg dual-therapy group, 24 patients in the 150-mg dual-therapy group, and 41 patients in the triple-therapy group.
“We now have new information to help select the right treatment for individual patients,” said Dr. Cannon. “With respect to the results for both the bleeding and thromboembolic-event end points, we may only speculate on the relative contributions of the omission of aspirin and the type of oral anticoagulant in the dual-therapy groups and the triple-therapy group. A trial conducted with a formal two-by-two factorial design would be able to discern these contributions, and one such trial is ongoing.”
One limitation of this trial was that researchers enrolled a smaller number of patients than initially planned. The power of the trial to examine efficacy according to dabigatran dose consequently was limited, said the authors.
This study was supported by Boehringer Ingelheim.
—Erica Tricarico
Suggested Reading
Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017 Aug 27 [Epub ahead of print].
Dual antithrombotic therapy with dabigatran and a P2Y12 inhibitor (eg, clopidogrel or ticagrelor) is associated with a lower risk of bleeding, compared with standard triple antithrombotic therapy, after percutaneous coronary intervention (PCI) for patients with atrial fibrillation, according to research published online ahead of print August 27 in the New England Journal of Medicine. Dual therapy also is noninferior to triple therapy regarding the risk of thromboembolic events.
“Patients who received two anticlotting medications—including one of a newer class of drug—had fewer bleeding events without being more at risk for a stroke or other cardiac events,” said Christopher P. Cannon, MD, Professor of Medicine at Harvard Medical School and a cardiovascular medicine specialist at Brigham and Women’s Hospital in Boston, and colleagues.
Standard triple antithrombotic therapy (ie, warfarin plus two antiplatelet agents) has been associated with a high risk of bleeding, thus prompting researchers to seek a better approach to treatment. One emerging therapy omits aspirin from the standard regimen and uses a single P2Y12 inhibitor in combination with an oral anticoagulant. A moderate-sized trial found that this form of dual-therapy lowered the risk of bleeding, compared with standard triple therapy. Another trial supported standard triple therapy for a shorter duration. Finally, a recent trial found that the risk of bleeding was lower with a regimen of reduced-dose rivaroxaban plus a P2Y12 inhibitor than with standard triple therapy.
Dual Therapy Versus Triple Therapy
To compare two regimens of dual antithrombotic therapy that include dabigatran with a regimen of triple antithrombotic therapy that includes warfarin, Dr. Cannon and colleagues conducted the RE-DUAL PCI trial. Eligible participants were 18 or older, had nonvalvular atrial fibrillation, and had successfully undergone PCI with a bare-metal or drug-eluting stent within the previous 120 hours. Patients with bioprosthetic or mechanical heart valves, severe renal insufficiency, or other major coexisting conditions were excluded.
All patients in the United States and nonelderly patients in other countries were randomized 1:1:1 to receive triple therapy with warfarin plus a PSY12 inhibitor and aspirin for one to three months, or to receive dual therapy with dabigatran (110 mg or 150 mg twice daily) plus a P2Y12 inhibitor and no aspirin. Outside the US, elderly patients (ie, 70 or older in Japan and 80 or older elsewhere) were randomized 1:1 to receive 110 mg of dual therapy or triple therapy.
The primary end point was the first major or clinically relevant nonmajor bleeding event. A major secondary end point was the composite of thromboembolic events (ie, myocardial infarction, stroke, or systemic embolism), death, or unplanned revascularization. Other secondary end points included a composite of thromboembolic events or death, as well as the individual thromboembolic events and definite stent thrombosis.
No Significant Difference in Serious Adverse Events Between Groups
Between July 21, 2014, and October 31, 2016, 2,725 participants underwent randomization at 414 sites in 41 countries. The mean age of participants was 70.8. The mean duration of treatment with the trial anticoagulant was 12.3 months, and the mean duration of follow-up was 14 months. Six patients were lost to follow-up. Most patients received clopidogrel as their P2Y12 inhibitor, and 12% received ticagrelor.
The incidence of the primary end point was 15.4% in the 110-mg dual-therapy group, compared with 26.9% in the triple-therapy group, and 20.2% in the 150-mg dual-therapy group, compared with 25.7% in the corresponding triple-therapy group. The incidence of the composite efficacy end point was 13.7% in both dual-therapy groups combined, compared with 13.4% in the triple-therapy group.
Researchers found no significant difference between groups in the rate of serious adverse events. Fatal serious adverse events occurred during treatment in 38 patients in the 100-mg dual-therapy group, 24 patients in the 150-mg dual-therapy group, and 41 patients in the triple-therapy group.
“We now have new information to help select the right treatment for individual patients,” said Dr. Cannon. “With respect to the results for both the bleeding and thromboembolic-event end points, we may only speculate on the relative contributions of the omission of aspirin and the type of oral anticoagulant in the dual-therapy groups and the triple-therapy group. A trial conducted with a formal two-by-two factorial design would be able to discern these contributions, and one such trial is ongoing.”
One limitation of this trial was that researchers enrolled a smaller number of patients than initially planned. The power of the trial to examine efficacy according to dabigatran dose consequently was limited, said the authors.
This study was supported by Boehringer Ingelheim.
—Erica Tricarico
Suggested Reading
Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017 Aug 27 [Epub ahead of print].
Loss of Functional Connectivity May Cause Hallucinations in Parkinson’s Disease
Visual hallucinations in Parkinson’s disease may arise from a global loss of network efficiency in the brain that disturbs visual attention and visual processing, according to research published online ahead of print September 27 in Radiology. A specific pattern of brain disconnection on fMRI may therefore help predict the development of visual hallucinations in patients with Parkinson’s disease.
An imperfect understanding of the pathophysiology behind visual hallucinations in Parkinson’s disease has hindered the development of effective treatments. Previous fMRI studies of these symptoms focused on task-based imaging, but visual hallucinations are associated with the development of cognitive decline, which could have influenced participants’ ability to perform specific tasks in the imager.
Dagmar H. Hepp, MD, of the Department of Neurology at VU University Medical Center in Amsterdam, and colleagues retrospectively examined resting-state fMRI data for 55 patients with Parkinson’s disease and 15 healthy controls who participated in a prospective cohort study. Of the participants with Parkinson’s disease, 15 had visual hallucinations. Dr. Hepp’s team calculated functional connectivity between 47 brain regions of interest. They compared whole-brain and region-specific means of connectivity using a general linear model with false discovery rate control for multiple comparisons.
In eight regions in the occipital lobe and paracentral area, functional connectivity was lower in all patients with Parkinson’s disease, compared with controls. Compared with controls, patients with Parkinson’s disease and visual hallucinations—but not patients with Parkinson’s disease without visual hallucinations—had nine brain regions with reduced functional connectivity. These regions were in the frontal cortex (ie, the superior frontal gyrus), temporal cortex (eg, the superior temporal gyrus), rolandic operculum, occipital cortex, and striatum. Connectivity of the superior temporal gyrus was correlated with orientation, attention, praxis, perception, and intraextra dimensional set shifting. Loss of functional connectivity of the rolandic operculum correlated with lower cognitive test scores in the subdomains of praxis and perception.
The superior frontal gyrus contributes to the allocation and maintenance of visuospatial attention and inhibitory control. This region also may allow an individual to reflect on sensory experience and judge its possible significance in relation to the self. Investigators believe that the superior temporal gyrus influences visual attention and controls the dorsal and ventral visual streams. Disconnection of frontal and temporal areas may impair the discrimination of external perceptions from internally generated information.
“Our findings argue against the notion that a single specific functional brain region or network is the neural substrate of visual hallucinations in Parkinson’s disease, but rather supply further evidence for a more global loss of network efficiency, which could drive disturbed attentional and visual processing and thereby lead to visual hallucinations in Parkinson’s disease,” the authors concluded.
—Erik Greb
Suggested Reading
Hepp DH, Foncke EMJ, Olde Dubbelink KTE, et al. Loss of functional connectivity in patients with Parkinson disease and visual hallucinations. Radiology. 2017 Sep 27 [Epub ahead of print].
Visual hallucinations in Parkinson’s disease may arise from a global loss of network efficiency in the brain that disturbs visual attention and visual processing, according to research published online ahead of print September 27 in Radiology. A specific pattern of brain disconnection on fMRI may therefore help predict the development of visual hallucinations in patients with Parkinson’s disease.
An imperfect understanding of the pathophysiology behind visual hallucinations in Parkinson’s disease has hindered the development of effective treatments. Previous fMRI studies of these symptoms focused on task-based imaging, but visual hallucinations are associated with the development of cognitive decline, which could have influenced participants’ ability to perform specific tasks in the imager.
Dagmar H. Hepp, MD, of the Department of Neurology at VU University Medical Center in Amsterdam, and colleagues retrospectively examined resting-state fMRI data for 55 patients with Parkinson’s disease and 15 healthy controls who participated in a prospective cohort study. Of the participants with Parkinson’s disease, 15 had visual hallucinations. Dr. Hepp’s team calculated functional connectivity between 47 brain regions of interest. They compared whole-brain and region-specific means of connectivity using a general linear model with false discovery rate control for multiple comparisons.
In eight regions in the occipital lobe and paracentral area, functional connectivity was lower in all patients with Parkinson’s disease, compared with controls. Compared with controls, patients with Parkinson’s disease and visual hallucinations—but not patients with Parkinson’s disease without visual hallucinations—had nine brain regions with reduced functional connectivity. These regions were in the frontal cortex (ie, the superior frontal gyrus), temporal cortex (eg, the superior temporal gyrus), rolandic operculum, occipital cortex, and striatum. Connectivity of the superior temporal gyrus was correlated with orientation, attention, praxis, perception, and intraextra dimensional set shifting. Loss of functional connectivity of the rolandic operculum correlated with lower cognitive test scores in the subdomains of praxis and perception.
The superior frontal gyrus contributes to the allocation and maintenance of visuospatial attention and inhibitory control. This region also may allow an individual to reflect on sensory experience and judge its possible significance in relation to the self. Investigators believe that the superior temporal gyrus influences visual attention and controls the dorsal and ventral visual streams. Disconnection of frontal and temporal areas may impair the discrimination of external perceptions from internally generated information.
“Our findings argue against the notion that a single specific functional brain region or network is the neural substrate of visual hallucinations in Parkinson’s disease, but rather supply further evidence for a more global loss of network efficiency, which could drive disturbed attentional and visual processing and thereby lead to visual hallucinations in Parkinson’s disease,” the authors concluded.
—Erik Greb
Suggested Reading
Hepp DH, Foncke EMJ, Olde Dubbelink KTE, et al. Loss of functional connectivity in patients with Parkinson disease and visual hallucinations. Radiology. 2017 Sep 27 [Epub ahead of print].
Visual hallucinations in Parkinson’s disease may arise from a global loss of network efficiency in the brain that disturbs visual attention and visual processing, according to research published online ahead of print September 27 in Radiology. A specific pattern of brain disconnection on fMRI may therefore help predict the development of visual hallucinations in patients with Parkinson’s disease.
An imperfect understanding of the pathophysiology behind visual hallucinations in Parkinson’s disease has hindered the development of effective treatments. Previous fMRI studies of these symptoms focused on task-based imaging, but visual hallucinations are associated with the development of cognitive decline, which could have influenced participants’ ability to perform specific tasks in the imager.
Dagmar H. Hepp, MD, of the Department of Neurology at VU University Medical Center in Amsterdam, and colleagues retrospectively examined resting-state fMRI data for 55 patients with Parkinson’s disease and 15 healthy controls who participated in a prospective cohort study. Of the participants with Parkinson’s disease, 15 had visual hallucinations. Dr. Hepp’s team calculated functional connectivity between 47 brain regions of interest. They compared whole-brain and region-specific means of connectivity using a general linear model with false discovery rate control for multiple comparisons.
In eight regions in the occipital lobe and paracentral area, functional connectivity was lower in all patients with Parkinson’s disease, compared with controls. Compared with controls, patients with Parkinson’s disease and visual hallucinations—but not patients with Parkinson’s disease without visual hallucinations—had nine brain regions with reduced functional connectivity. These regions were in the frontal cortex (ie, the superior frontal gyrus), temporal cortex (eg, the superior temporal gyrus), rolandic operculum, occipital cortex, and striatum. Connectivity of the superior temporal gyrus was correlated with orientation, attention, praxis, perception, and intraextra dimensional set shifting. Loss of functional connectivity of the rolandic operculum correlated with lower cognitive test scores in the subdomains of praxis and perception.
The superior frontal gyrus contributes to the allocation and maintenance of visuospatial attention and inhibitory control. This region also may allow an individual to reflect on sensory experience and judge its possible significance in relation to the self. Investigators believe that the superior temporal gyrus influences visual attention and controls the dorsal and ventral visual streams. Disconnection of frontal and temporal areas may impair the discrimination of external perceptions from internally generated information.
“Our findings argue against the notion that a single specific functional brain region or network is the neural substrate of visual hallucinations in Parkinson’s disease, but rather supply further evidence for a more global loss of network efficiency, which could drive disturbed attentional and visual processing and thereby lead to visual hallucinations in Parkinson’s disease,” the authors concluded.
—Erik Greb
Suggested Reading
Hepp DH, Foncke EMJ, Olde Dubbelink KTE, et al. Loss of functional connectivity in patients with Parkinson disease and visual hallucinations. Radiology. 2017 Sep 27 [Epub ahead of print].
















