User login
New narcolepsy drug passes phase 3 test
SAN DIEGO – The selective dopamine and norepinephrine reuptake inhibitor solriamfetol is effective in reducing sleepiness in patients with narcolepsy, according to results of a phase 3 study.
At 150-mg and 300-mg doses, the drug had statistically significant effects on objective and subjective measures.
There are wake-promoting drugs available, such as amphetamine-related drugs that are often used off label, but addiction liability is a concern. The nonamphetamine modafinil has been approved by the Food and Drug Administration since 1998.
Jazz Pharmaceuticals is in the process of submitting solriamfetol for FDA evaluation. If approved, the drug will add to the options available for narcolepsy patients. “All of the available drugs have some limitations. Some have more abuse liability than others. Some have more robust wake-promoting properties than others. We haven’t done any head-to-head comparisons, so I can’t tell you how we will stack up,” Philip Jochelson, MD, said in an interview. Dr. Jochelson is vice president of clinical development at Jazz Pharmaceuticals and presented the results of the study at a poster session at the annual meeting of the American Neurological Association.
An earlier study showed the drug had less abuse potential than the schedule IV stimulant phentermine. That’s not surprising given the drug’s mechanism of action, Dr. Jochelson said. Amphetamine-based drugs stimulate dopamine release, which can prompt a dopamine surge that people equate with a high, he said. Solriamfetol also affects dopamine, but it is a reuptake inhibitor, so it doesn’t produce a surge.
If the drug gains approval, it remains to be seen how it will be classified on the Drug Enforcement Agency Controlled Substance scale. “Where it will fall in that spectrum is speculative at this point,” said Dr. Jochelson.
In the current study, 236 adults (aged 18-75 years) with type 1 narcolepsy were randomized to once-daily placebo, 75 mg solriamfetol, 150 mg solriamfetol, or 300 mg solriamfetol; 27.1% of patients in the 300-mg group discontinued, compared with 7.3% in the 150-mg group, 16.9% in the 75-mg group, and 10.3% in the placebo group. The mean change from baseline on the Maintenance of Wakefulness Test was statistically significant in the 300-mg group (12.3 minutes vs. 2.1 minutes for placebo, P less than .0001) and the 150-mg group (9.8 minutes vs. 2.1 minutes, P less than .0001) but not the 75-mg group (4.7 minutes vs. 2.1 minutes).
The drug also outperformed placebo at week 12 on the Epworth Sleepiness Scale. The mean change in the 300-mg group was –6.4 vs. –1.6 for placebo (P less than .001), –5.4 in the 150-mg group (P less than .0001), and –3.8 in the 75-mg group (P less than .05).
By both Maintenance of Wakefulness Test and Epworth Sleepiness Scale measures, the 150-mg and 300-mg solriamfetol groups had statistically significant differences as early as week 1.
The drug had some adverse effects, which were expected based on its pharmacologic profile. These included increases in headache (5.1% with placebo, 10.2% with 75 mg, 23.7% with 150 mg, 30.5% with 300 mg), nausea (1.7% for placebo, 5.1% for 75 mg, 10.2% for 150 mg, 16.9% for 300 mg), anxiety (1.7% with placebo, 1.7% with 75 mg, 5.1% with 150 mg, 8.5% with 300 mg), and insomnia (0% for placebo, 3.4% for 75 mg, 0% for 150 mg, 5.1% for 300 mg). Other adverse events occurring in at least 5% of patients were decreased appetite, nasopharyngitis, and dry mouth.
The study was funded by Jazz Pharmaceuticals. Dr. Jochelson is an employee of Jazz.
SAN DIEGO – The selective dopamine and norepinephrine reuptake inhibitor solriamfetol is effective in reducing sleepiness in patients with narcolepsy, according to results of a phase 3 study.
At 150-mg and 300-mg doses, the drug had statistically significant effects on objective and subjective measures.
There are wake-promoting drugs available, such as amphetamine-related drugs that are often used off label, but addiction liability is a concern. The nonamphetamine modafinil has been approved by the Food and Drug Administration since 1998.
Jazz Pharmaceuticals is in the process of submitting solriamfetol for FDA evaluation. If approved, the drug will add to the options available for narcolepsy patients. “All of the available drugs have some limitations. Some have more abuse liability than others. Some have more robust wake-promoting properties than others. We haven’t done any head-to-head comparisons, so I can’t tell you how we will stack up,” Philip Jochelson, MD, said in an interview. Dr. Jochelson is vice president of clinical development at Jazz Pharmaceuticals and presented the results of the study at a poster session at the annual meeting of the American Neurological Association.
An earlier study showed the drug had less abuse potential than the schedule IV stimulant phentermine. That’s not surprising given the drug’s mechanism of action, Dr. Jochelson said. Amphetamine-based drugs stimulate dopamine release, which can prompt a dopamine surge that people equate with a high, he said. Solriamfetol also affects dopamine, but it is a reuptake inhibitor, so it doesn’t produce a surge.
If the drug gains approval, it remains to be seen how it will be classified on the Drug Enforcement Agency Controlled Substance scale. “Where it will fall in that spectrum is speculative at this point,” said Dr. Jochelson.
In the current study, 236 adults (aged 18-75 years) with type 1 narcolepsy were randomized to once-daily placebo, 75 mg solriamfetol, 150 mg solriamfetol, or 300 mg solriamfetol; 27.1% of patients in the 300-mg group discontinued, compared with 7.3% in the 150-mg group, 16.9% in the 75-mg group, and 10.3% in the placebo group. The mean change from baseline on the Maintenance of Wakefulness Test was statistically significant in the 300-mg group (12.3 minutes vs. 2.1 minutes for placebo, P less than .0001) and the 150-mg group (9.8 minutes vs. 2.1 minutes, P less than .0001) but not the 75-mg group (4.7 minutes vs. 2.1 minutes).
The drug also outperformed placebo at week 12 on the Epworth Sleepiness Scale. The mean change in the 300-mg group was –6.4 vs. –1.6 for placebo (P less than .001), –5.4 in the 150-mg group (P less than .0001), and –3.8 in the 75-mg group (P less than .05).
By both Maintenance of Wakefulness Test and Epworth Sleepiness Scale measures, the 150-mg and 300-mg solriamfetol groups had statistically significant differences as early as week 1.
The drug had some adverse effects, which were expected based on its pharmacologic profile. These included increases in headache (5.1% with placebo, 10.2% with 75 mg, 23.7% with 150 mg, 30.5% with 300 mg), nausea (1.7% for placebo, 5.1% for 75 mg, 10.2% for 150 mg, 16.9% for 300 mg), anxiety (1.7% with placebo, 1.7% with 75 mg, 5.1% with 150 mg, 8.5% with 300 mg), and insomnia (0% for placebo, 3.4% for 75 mg, 0% for 150 mg, 5.1% for 300 mg). Other adverse events occurring in at least 5% of patients were decreased appetite, nasopharyngitis, and dry mouth.
The study was funded by Jazz Pharmaceuticals. Dr. Jochelson is an employee of Jazz.
SAN DIEGO – The selective dopamine and norepinephrine reuptake inhibitor solriamfetol is effective in reducing sleepiness in patients with narcolepsy, according to results of a phase 3 study.
At 150-mg and 300-mg doses, the drug had statistically significant effects on objective and subjective measures.
There are wake-promoting drugs available, such as amphetamine-related drugs that are often used off label, but addiction liability is a concern. The nonamphetamine modafinil has been approved by the Food and Drug Administration since 1998.
Jazz Pharmaceuticals is in the process of submitting solriamfetol for FDA evaluation. If approved, the drug will add to the options available for narcolepsy patients. “All of the available drugs have some limitations. Some have more abuse liability than others. Some have more robust wake-promoting properties than others. We haven’t done any head-to-head comparisons, so I can’t tell you how we will stack up,” Philip Jochelson, MD, said in an interview. Dr. Jochelson is vice president of clinical development at Jazz Pharmaceuticals and presented the results of the study at a poster session at the annual meeting of the American Neurological Association.
An earlier study showed the drug had less abuse potential than the schedule IV stimulant phentermine. That’s not surprising given the drug’s mechanism of action, Dr. Jochelson said. Amphetamine-based drugs stimulate dopamine release, which can prompt a dopamine surge that people equate with a high, he said. Solriamfetol also affects dopamine, but it is a reuptake inhibitor, so it doesn’t produce a surge.
If the drug gains approval, it remains to be seen how it will be classified on the Drug Enforcement Agency Controlled Substance scale. “Where it will fall in that spectrum is speculative at this point,” said Dr. Jochelson.
In the current study, 236 adults (aged 18-75 years) with type 1 narcolepsy were randomized to once-daily placebo, 75 mg solriamfetol, 150 mg solriamfetol, or 300 mg solriamfetol; 27.1% of patients in the 300-mg group discontinued, compared with 7.3% in the 150-mg group, 16.9% in the 75-mg group, and 10.3% in the placebo group. The mean change from baseline on the Maintenance of Wakefulness Test was statistically significant in the 300-mg group (12.3 minutes vs. 2.1 minutes for placebo, P less than .0001) and the 150-mg group (9.8 minutes vs. 2.1 minutes, P less than .0001) but not the 75-mg group (4.7 minutes vs. 2.1 minutes).
The drug also outperformed placebo at week 12 on the Epworth Sleepiness Scale. The mean change in the 300-mg group was –6.4 vs. –1.6 for placebo (P less than .001), –5.4 in the 150-mg group (P less than .0001), and –3.8 in the 75-mg group (P less than .05).
By both Maintenance of Wakefulness Test and Epworth Sleepiness Scale measures, the 150-mg and 300-mg solriamfetol groups had statistically significant differences as early as week 1.
The drug had some adverse effects, which were expected based on its pharmacologic profile. These included increases in headache (5.1% with placebo, 10.2% with 75 mg, 23.7% with 150 mg, 30.5% with 300 mg), nausea (1.7% for placebo, 5.1% for 75 mg, 10.2% for 150 mg, 16.9% for 300 mg), anxiety (1.7% with placebo, 1.7% with 75 mg, 5.1% with 150 mg, 8.5% with 300 mg), and insomnia (0% for placebo, 3.4% for 75 mg, 0% for 150 mg, 5.1% for 300 mg). Other adverse events occurring in at least 5% of patients were decreased appetite, nasopharyngitis, and dry mouth.
The study was funded by Jazz Pharmaceuticals. Dr. Jochelson is an employee of Jazz.
AT ANA 2017
Key clinical point: Solriamfetol outperformed placebo on both objective and subjective sleep measures.
Major finding: At 12 weeks, 150 mg increased Maintenance of Wakefulness Test score to 9.8 minutes, compared with 2.1 minutes in the placebo group.
Data source: A randomized, controlled trial (n = 236).
Disclosures: The study was funded by Jazz Pharmaceuticals. Dr. Jochelson is an employee of Jazz.
ADMIRE CD trial: Stem cells promote long-term fistula remission
ORLANDO – A single treatment with a suspension of allogeneic expanded adipose-derived mesenchymal stem cells, or Cx601, promotes long-term combined remission of complex perianal fistulas in patients with Crohn’s disease, according to 52-week results from the phase 3 ADMIRE CD trial.
Combined remission – a stringent endpoint consisting of closure of all treated external openings that were draining at baseline and of an absence of collections more than 2 cm of treated perianal fistulas as confirmed by blinded central MRI – was achieved in 56.3% of 103 treated patients, compared with 38.6% of 101 patients who received placebo (P = .010), Daniel C. Baumgart, MD, reported at the World Congress of Gastroenterology at ACG 2017.
This parallel-group, double-blind, multicenter study included patients who had draining, treatment-refractory, complex perianal fistulas and inactive or mildly active luminal Crohn’s disease (Crohn’s Disease Activity Index scores of 220 or less) at baseline. Those randomized to the Cx601 group received a single intralesional injection of 120 million expanded adipose-derived stem cells and standard of care. Those in the control arm received a placebo injection plus standard of care, said Dr. Baumgart of Charité Medical School, which is affiliated with both Humboldt University in Berlin and the Free University of Berlin.
Prior to receiving treatment or placebo, the patients underwent fistula curettage and, if indicated, seton placement and subsequent removal, he noted, adding that baseline concomitant medications, including immunosuppressants and anti–tumor necrosis factors, were continued without dose or regimen modification and that antibiotics were allowed for up to 4 weeks.
The 52-week findings were evaluated in the modified intention-to-treat population of patients who were randomized, were treated, and had at least one postbaseline efficacy assessment (61.8% of the study population). These findings showed that Cx601 is associated with even better outcomes at 1 year than those reported at 24 weeks; those prior results, published in The Lancet in July 2016, showed combined remission rates in the modified intention-to-treat population of 51% vs. 36% for placebo.
Furthermore, 75% of treated patients who achieved combined remission at 24 weeks maintained that remission at 52 weeks, compared with 55.9% of those in the placebo group (P = .052), Dr. Baumgart said.
Sensitivity analyses in this long-term assessment supported the long-term effectiveness of Cx601 over that of the control treatment, which provided evidence on the robustness of its advantage, he said, noting that safety results were also encouraging.
“The safety profile was similar to week 24; there were no new safety signals there at all,” he said.
The findings are of note because existing therapies for complex perianal fistulas in Crohn’s disease are often ineffective, he said, adding that fistulas occur in up to 50% of Crohn’s disease patients and that 70%-80% are complex and difficult to treat. Most are refractory to conventional anti–tumor necrosis factor therapies, and 60%-70% of patients relapse, he explained.
“If we’re honest, very few medications have been properly studied,” he said, adding that fistula patients often are excluded from industry trials.
“So [the ADMIRE CD trial] is new, design-wise, and has addressed a true medical need,” he said.
He attributed the good placebo response in this trial to the ongoing standard of care treatment in both groups, as well as to the team approach to care used in the trial. He also noted that a number of questions regarding the use of Cx601 remain to be answered, including the when the ideal retreatment time point should be, whether the treatment can be used for rectovaginal fistulas, and which patients should not receive treatment.
“So there is a lot to learn still, but I think it’s a revolutionary step forward, compared to what we have today, due to the trial design, which I think is robust, and also the encouraging outcomes,” he said.
The ADMIRE CD trial was sponsored by TiGenix SAU. Dr. Baumgart has received consulting fees and nonfinancial support from AbbVie, Biogen, and BMS.
ORLANDO – A single treatment with a suspension of allogeneic expanded adipose-derived mesenchymal stem cells, or Cx601, promotes long-term combined remission of complex perianal fistulas in patients with Crohn’s disease, according to 52-week results from the phase 3 ADMIRE CD trial.
Combined remission – a stringent endpoint consisting of closure of all treated external openings that were draining at baseline and of an absence of collections more than 2 cm of treated perianal fistulas as confirmed by blinded central MRI – was achieved in 56.3% of 103 treated patients, compared with 38.6% of 101 patients who received placebo (P = .010), Daniel C. Baumgart, MD, reported at the World Congress of Gastroenterology at ACG 2017.
This parallel-group, double-blind, multicenter study included patients who had draining, treatment-refractory, complex perianal fistulas and inactive or mildly active luminal Crohn’s disease (Crohn’s Disease Activity Index scores of 220 or less) at baseline. Those randomized to the Cx601 group received a single intralesional injection of 120 million expanded adipose-derived stem cells and standard of care. Those in the control arm received a placebo injection plus standard of care, said Dr. Baumgart of Charité Medical School, which is affiliated with both Humboldt University in Berlin and the Free University of Berlin.
Prior to receiving treatment or placebo, the patients underwent fistula curettage and, if indicated, seton placement and subsequent removal, he noted, adding that baseline concomitant medications, including immunosuppressants and anti–tumor necrosis factors, were continued without dose or regimen modification and that antibiotics were allowed for up to 4 weeks.
The 52-week findings were evaluated in the modified intention-to-treat population of patients who were randomized, were treated, and had at least one postbaseline efficacy assessment (61.8% of the study population). These findings showed that Cx601 is associated with even better outcomes at 1 year than those reported at 24 weeks; those prior results, published in The Lancet in July 2016, showed combined remission rates in the modified intention-to-treat population of 51% vs. 36% for placebo.
Furthermore, 75% of treated patients who achieved combined remission at 24 weeks maintained that remission at 52 weeks, compared with 55.9% of those in the placebo group (P = .052), Dr. Baumgart said.
Sensitivity analyses in this long-term assessment supported the long-term effectiveness of Cx601 over that of the control treatment, which provided evidence on the robustness of its advantage, he said, noting that safety results were also encouraging.
“The safety profile was similar to week 24; there were no new safety signals there at all,” he said.
The findings are of note because existing therapies for complex perianal fistulas in Crohn’s disease are often ineffective, he said, adding that fistulas occur in up to 50% of Crohn’s disease patients and that 70%-80% are complex and difficult to treat. Most are refractory to conventional anti–tumor necrosis factor therapies, and 60%-70% of patients relapse, he explained.
“If we’re honest, very few medications have been properly studied,” he said, adding that fistula patients often are excluded from industry trials.
“So [the ADMIRE CD trial] is new, design-wise, and has addressed a true medical need,” he said.
He attributed the good placebo response in this trial to the ongoing standard of care treatment in both groups, as well as to the team approach to care used in the trial. He also noted that a number of questions regarding the use of Cx601 remain to be answered, including the when the ideal retreatment time point should be, whether the treatment can be used for rectovaginal fistulas, and which patients should not receive treatment.
“So there is a lot to learn still, but I think it’s a revolutionary step forward, compared to what we have today, due to the trial design, which I think is robust, and also the encouraging outcomes,” he said.
The ADMIRE CD trial was sponsored by TiGenix SAU. Dr. Baumgart has received consulting fees and nonfinancial support from AbbVie, Biogen, and BMS.
ORLANDO – A single treatment with a suspension of allogeneic expanded adipose-derived mesenchymal stem cells, or Cx601, promotes long-term combined remission of complex perianal fistulas in patients with Crohn’s disease, according to 52-week results from the phase 3 ADMIRE CD trial.
Combined remission – a stringent endpoint consisting of closure of all treated external openings that were draining at baseline and of an absence of collections more than 2 cm of treated perianal fistulas as confirmed by blinded central MRI – was achieved in 56.3% of 103 treated patients, compared with 38.6% of 101 patients who received placebo (P = .010), Daniel C. Baumgart, MD, reported at the World Congress of Gastroenterology at ACG 2017.
This parallel-group, double-blind, multicenter study included patients who had draining, treatment-refractory, complex perianal fistulas and inactive or mildly active luminal Crohn’s disease (Crohn’s Disease Activity Index scores of 220 or less) at baseline. Those randomized to the Cx601 group received a single intralesional injection of 120 million expanded adipose-derived stem cells and standard of care. Those in the control arm received a placebo injection plus standard of care, said Dr. Baumgart of Charité Medical School, which is affiliated with both Humboldt University in Berlin and the Free University of Berlin.
Prior to receiving treatment or placebo, the patients underwent fistula curettage and, if indicated, seton placement and subsequent removal, he noted, adding that baseline concomitant medications, including immunosuppressants and anti–tumor necrosis factors, were continued without dose or regimen modification and that antibiotics were allowed for up to 4 weeks.
The 52-week findings were evaluated in the modified intention-to-treat population of patients who were randomized, were treated, and had at least one postbaseline efficacy assessment (61.8% of the study population). These findings showed that Cx601 is associated with even better outcomes at 1 year than those reported at 24 weeks; those prior results, published in The Lancet in July 2016, showed combined remission rates in the modified intention-to-treat population of 51% vs. 36% for placebo.
Furthermore, 75% of treated patients who achieved combined remission at 24 weeks maintained that remission at 52 weeks, compared with 55.9% of those in the placebo group (P = .052), Dr. Baumgart said.
Sensitivity analyses in this long-term assessment supported the long-term effectiveness of Cx601 over that of the control treatment, which provided evidence on the robustness of its advantage, he said, noting that safety results were also encouraging.
“The safety profile was similar to week 24; there were no new safety signals there at all,” he said.
The findings are of note because existing therapies for complex perianal fistulas in Crohn’s disease are often ineffective, he said, adding that fistulas occur in up to 50% of Crohn’s disease patients and that 70%-80% are complex and difficult to treat. Most are refractory to conventional anti–tumor necrosis factor therapies, and 60%-70% of patients relapse, he explained.
“If we’re honest, very few medications have been properly studied,” he said, adding that fistula patients often are excluded from industry trials.
“So [the ADMIRE CD trial] is new, design-wise, and has addressed a true medical need,” he said.
He attributed the good placebo response in this trial to the ongoing standard of care treatment in both groups, as well as to the team approach to care used in the trial. He also noted that a number of questions regarding the use of Cx601 remain to be answered, including the when the ideal retreatment time point should be, whether the treatment can be used for rectovaginal fistulas, and which patients should not receive treatment.
“So there is a lot to learn still, but I think it’s a revolutionary step forward, compared to what we have today, due to the trial design, which I think is robust, and also the encouraging outcomes,” he said.
The ADMIRE CD trial was sponsored by TiGenix SAU. Dr. Baumgart has received consulting fees and nonfinancial support from AbbVie, Biogen, and BMS.
AT THE WORLD CONGRESS OF GASTROENTEROLOGY
Key clinical point:
Major finding: Combined remission was achieved in 56.3% of treated patients, compared with 38.6% of controls.
Data source: The phase 3 ADMIRE CD trial of 204 patients.
Disclosures: The ADMIRE CD trial was sponsored by TiGenix SAU. Dr. Baumgart has received consulting fees and nonfinancial support from AbbVie, Biogen, and Bristol-Myers Squibb.
Sneak Peek: The Hospital Leader blog – Oct. 2017
You Have Lowered Length of Stay. Congratulations: You’re Fired.
For several decades, providers working within hospitals have had incentives to reduce stay durations and keep patient flow tip-top. Diagnosis Related Group (DRG)–based and capitated payments expedited that shift.
Accompanying the change, physicians became more aware of the potential repercussions of sicker and quicker discharges. They began to monitor their care and, as best as possible, use what measures they could as a proxy for quality (readmissions and hospital-acquired conditions). Providers balanced the harms of a continued stay with the benefits of added days, not to mention the need for cost savings.
I recognize this because of the cognitive dissonance providers now experience because of the mixed messages delivered by hospital leaders.
On the one hand, the DRG-driven system that we have binds the hospital’s bottom line – and that is not going away. On the other, we are paying more attention to excessive costs in post-acute settings, that is, subacute facilities when home health will do or more intense acute rehabilitation rather than the subacute route.
Making determinations as to whether a certain course is proper, whether a patient will be safe, whether families can provide adequate agency and backing, and whether we can avail community services takes time. Sicker and quicker; mindful of short-term outcomes; worked when we had postdischarge blinders on. As we remove such obstacles, and payment incentives change to cover broader intervals of time, we have to adapt. And that means leadership must realize that the practices that held hospitals in sound financial stead in years past are heading toward extinction – or, at best, falling out of favor.
Compare the costs of routine hospital care with the added expense of post-acute care, then multiply that extra expense times an aging, dependent population, and you add billions of dollars to the recovery tab. Some of these expenses are necessary, and some are not; a stay at a skilled nursing facility, for example, doubles the cost of an episode.
Read the full post at hospitalleader.org.
Also on The Hospital Leader …
- Why 7 On/7 Off Doesn’t Meet the Needs of Long-Stay Hospital Patients by Lauren Doctoroff, MD, FHM
- Is It Time for Health Policy M&Ms? by Chris Moriates, MD
- George Carlin Predicts Hospital Planning Strategy by Jordan Messler, MD, SFHM
- Many Paths to a Richer Job by Leslie Flores, MHA, MPH, SFHM
- A New Face for Online Modules by Chris Moriates, MD
You Have Lowered Length of Stay. Congratulations: You’re Fired.
For several decades, providers working within hospitals have had incentives to reduce stay durations and keep patient flow tip-top. Diagnosis Related Group (DRG)–based and capitated payments expedited that shift.
Accompanying the change, physicians became more aware of the potential repercussions of sicker and quicker discharges. They began to monitor their care and, as best as possible, use what measures they could as a proxy for quality (readmissions and hospital-acquired conditions). Providers balanced the harms of a continued stay with the benefits of added days, not to mention the need for cost savings.
I recognize this because of the cognitive dissonance providers now experience because of the mixed messages delivered by hospital leaders.
On the one hand, the DRG-driven system that we have binds the hospital’s bottom line – and that is not going away. On the other, we are paying more attention to excessive costs in post-acute settings, that is, subacute facilities when home health will do or more intense acute rehabilitation rather than the subacute route.
Making determinations as to whether a certain course is proper, whether a patient will be safe, whether families can provide adequate agency and backing, and whether we can avail community services takes time. Sicker and quicker; mindful of short-term outcomes; worked when we had postdischarge blinders on. As we remove such obstacles, and payment incentives change to cover broader intervals of time, we have to adapt. And that means leadership must realize that the practices that held hospitals in sound financial stead in years past are heading toward extinction – or, at best, falling out of favor.
Compare the costs of routine hospital care with the added expense of post-acute care, then multiply that extra expense times an aging, dependent population, and you add billions of dollars to the recovery tab. Some of these expenses are necessary, and some are not; a stay at a skilled nursing facility, for example, doubles the cost of an episode.
Read the full post at hospitalleader.org.
Also on The Hospital Leader …
- Why 7 On/7 Off Doesn’t Meet the Needs of Long-Stay Hospital Patients by Lauren Doctoroff, MD, FHM
- Is It Time for Health Policy M&Ms? by Chris Moriates, MD
- George Carlin Predicts Hospital Planning Strategy by Jordan Messler, MD, SFHM
- Many Paths to a Richer Job by Leslie Flores, MHA, MPH, SFHM
- A New Face for Online Modules by Chris Moriates, MD
You Have Lowered Length of Stay. Congratulations: You’re Fired.
For several decades, providers working within hospitals have had incentives to reduce stay durations and keep patient flow tip-top. Diagnosis Related Group (DRG)–based and capitated payments expedited that shift.
Accompanying the change, physicians became more aware of the potential repercussions of sicker and quicker discharges. They began to monitor their care and, as best as possible, use what measures they could as a proxy for quality (readmissions and hospital-acquired conditions). Providers balanced the harms of a continued stay with the benefits of added days, not to mention the need for cost savings.
I recognize this because of the cognitive dissonance providers now experience because of the mixed messages delivered by hospital leaders.
On the one hand, the DRG-driven system that we have binds the hospital’s bottom line – and that is not going away. On the other, we are paying more attention to excessive costs in post-acute settings, that is, subacute facilities when home health will do or more intense acute rehabilitation rather than the subacute route.
Making determinations as to whether a certain course is proper, whether a patient will be safe, whether families can provide adequate agency and backing, and whether we can avail community services takes time. Sicker and quicker; mindful of short-term outcomes; worked when we had postdischarge blinders on. As we remove such obstacles, and payment incentives change to cover broader intervals of time, we have to adapt. And that means leadership must realize that the practices that held hospitals in sound financial stead in years past are heading toward extinction – or, at best, falling out of favor.
Compare the costs of routine hospital care with the added expense of post-acute care, then multiply that extra expense times an aging, dependent population, and you add billions of dollars to the recovery tab. Some of these expenses are necessary, and some are not; a stay at a skilled nursing facility, for example, doubles the cost of an episode.
Read the full post at hospitalleader.org.
Also on The Hospital Leader …
- Why 7 On/7 Off Doesn’t Meet the Needs of Long-Stay Hospital Patients by Lauren Doctoroff, MD, FHM
- Is It Time for Health Policy M&Ms? by Chris Moriates, MD
- George Carlin Predicts Hospital Planning Strategy by Jordan Messler, MD, SFHM
- Many Paths to a Richer Job by Leslie Flores, MHA, MPH, SFHM
- A New Face for Online Modules by Chris Moriates, MD
How Does Cladribine Compare With Other MS Therapies?
New data confirm cladribine’s efficacy as a treatment for relapsing-remitting multiple sclerosis (MS), according to results published online ahead of print August 1 in Multiple Sclerosis Journal. The drug’s effect on relapses is comparable to that of fingolimod, and its effect on disability accumulation is comparable to those of interferon β and fingolimod. Compared with interferon, fingolimod, and natalizumab, cladribine may be associated with superior recovery from disability.
A phase III trial demonstrated that, compared with placebo, cladribine reduced relapse rate and increased the likelihood of remaining free from three-month confirmed disability progression in patients with relapsing-remitting MS. The European Medicines Agency approved the therapy in August 2017. Cladribine’s potential position in the treatment landscape is unclear, however, because no direct comparisons of cladribine with other MS therapies are available.
An Analysis of Matched Cohorts
Tomas Kalincik, MD, PhD, Professor of Medicine at Royal Melbourne Hospital in Australia, and colleagues conducted a propensity score–matched analysis of observational data from MSBase, including patients from the Australian Cladribine Product Familiarization Program, to compare the effectiveness of cladribine to that of interferon β, fingolimod, and natalizumab. Eligible participants had relapsing-remitting MS, received one of the study medications as monotherapy for one or more years, and had no prior exposure to alemtuzumab, mitoxantrone, rituximab, or hematopoietic stem cell transplantation.
Patients received 3.5 mg/kg of oral cladribine, 44 μg of subcutaneous interferon β-1a three times weekly, 0.5 mg/day of oral fingolimod, or 300 μg of IV natalizumab every four weeks. Data were recorded during routine clinical practice. The primary end points were the proportion of patients free from relapses, disability accumulation, and disability improvement while on study therapy.
Cladribine Was Associated With Superior Disability Improvement
The researchers included 37 patients treated with cladribine, 1,940 patients treated with interferon β, 1,892 patients treated with fingolimod, and 1,410 patients treated with natalizumab in their analysis. The investigators noted only small differences in baseline characteristics between the matched cohorts.
Compared with participants receiving interferon β, patients receiving cladribine were less likely to have a relapse during the first year of treatment (hazard ratio [HR], 0.6). The proportion of relapse-free patients was 86% in the cladribine group and 70% in the interferon β group. The probability of disability accumulation was similar for these drugs (HR, 0.41), but the cladribine group was more likely to have disability improvement (HR, 15).
The proportion of relapse-free patients at one year was 79% in the cladribine and fingolimod groups, and cumulative hazards of a relapse did not differ between the two groups (HR, 1.2). The probability of disability accumulation was similar for cladribine and fingolimod (HR, 1.8), but the probability of disability improvement was greater for cladribine (HR, 3.9).
The probability of relapse was higher with cladribine than with natalizumab (HR, 1.8), but the proportions of relapse-free patients at the end of year one were 80% and 81%, respectively. The probability of disability accumulation was greater in the cladribine group than in the natalizumab group (HR, 2.5). The probability of disability improvement was greater among patients receiving cladribine than among those receiving natalizumab (HR, 4). Sensitivity analyses largely confirmed the results of the primary analyses.
“Six-month confirmed improvement of disability was observed in 10%–20% of the cladribine cohort during the first year, which was superior to all three comparator therapies. This [finding] is of interest in the context of the comparison to natalizumab, which is known to be associated with a marked improvement in disability early after its commencement,” said Dr. Kalincik and colleagues. “Improvement in disability in a cohort with this profile is unexpected.”
The study’s main limitation is the small size of the cladribine cohort, said the authors. Another limitation is the brief duration of follow-up for the cladribine group, which precludes conclusions about long-term outcomes. Nevertheless, the comparative effectiveness results “represent timely information about the role of cladribine in the management of MS,” Dr. Kalincik concluded.
—Erik Greb
Suggested Reading
Kalincik T, Jokubaitis V, Spelman T, et al. Cladribine versus fingolimod, natalizumab and interferon β for multiple sclerosis. Mult Scler. 2017 Aug 1 [Epub ahead of print].
New data confirm cladribine’s efficacy as a treatment for relapsing-remitting multiple sclerosis (MS), according to results published online ahead of print August 1 in Multiple Sclerosis Journal. The drug’s effect on relapses is comparable to that of fingolimod, and its effect on disability accumulation is comparable to those of interferon β and fingolimod. Compared with interferon, fingolimod, and natalizumab, cladribine may be associated with superior recovery from disability.
A phase III trial demonstrated that, compared with placebo, cladribine reduced relapse rate and increased the likelihood of remaining free from three-month confirmed disability progression in patients with relapsing-remitting MS. The European Medicines Agency approved the therapy in August 2017. Cladribine’s potential position in the treatment landscape is unclear, however, because no direct comparisons of cladribine with other MS therapies are available.
An Analysis of Matched Cohorts
Tomas Kalincik, MD, PhD, Professor of Medicine at Royal Melbourne Hospital in Australia, and colleagues conducted a propensity score–matched analysis of observational data from MSBase, including patients from the Australian Cladribine Product Familiarization Program, to compare the effectiveness of cladribine to that of interferon β, fingolimod, and natalizumab. Eligible participants had relapsing-remitting MS, received one of the study medications as monotherapy for one or more years, and had no prior exposure to alemtuzumab, mitoxantrone, rituximab, or hematopoietic stem cell transplantation.
Patients received 3.5 mg/kg of oral cladribine, 44 μg of subcutaneous interferon β-1a three times weekly, 0.5 mg/day of oral fingolimod, or 300 μg of IV natalizumab every four weeks. Data were recorded during routine clinical practice. The primary end points were the proportion of patients free from relapses, disability accumulation, and disability improvement while on study therapy.
Cladribine Was Associated With Superior Disability Improvement
The researchers included 37 patients treated with cladribine, 1,940 patients treated with interferon β, 1,892 patients treated with fingolimod, and 1,410 patients treated with natalizumab in their analysis. The investigators noted only small differences in baseline characteristics between the matched cohorts.
Compared with participants receiving interferon β, patients receiving cladribine were less likely to have a relapse during the first year of treatment (hazard ratio [HR], 0.6). The proportion of relapse-free patients was 86% in the cladribine group and 70% in the interferon β group. The probability of disability accumulation was similar for these drugs (HR, 0.41), but the cladribine group was more likely to have disability improvement (HR, 15).
The proportion of relapse-free patients at one year was 79% in the cladribine and fingolimod groups, and cumulative hazards of a relapse did not differ between the two groups (HR, 1.2). The probability of disability accumulation was similar for cladribine and fingolimod (HR, 1.8), but the probability of disability improvement was greater for cladribine (HR, 3.9).
The probability of relapse was higher with cladribine than with natalizumab (HR, 1.8), but the proportions of relapse-free patients at the end of year one were 80% and 81%, respectively. The probability of disability accumulation was greater in the cladribine group than in the natalizumab group (HR, 2.5). The probability of disability improvement was greater among patients receiving cladribine than among those receiving natalizumab (HR, 4). Sensitivity analyses largely confirmed the results of the primary analyses.
“Six-month confirmed improvement of disability was observed in 10%–20% of the cladribine cohort during the first year, which was superior to all three comparator therapies. This [finding] is of interest in the context of the comparison to natalizumab, which is known to be associated with a marked improvement in disability early after its commencement,” said Dr. Kalincik and colleagues. “Improvement in disability in a cohort with this profile is unexpected.”
The study’s main limitation is the small size of the cladribine cohort, said the authors. Another limitation is the brief duration of follow-up for the cladribine group, which precludes conclusions about long-term outcomes. Nevertheless, the comparative effectiveness results “represent timely information about the role of cladribine in the management of MS,” Dr. Kalincik concluded.
—Erik Greb
Suggested Reading
Kalincik T, Jokubaitis V, Spelman T, et al. Cladribine versus fingolimod, natalizumab and interferon β for multiple sclerosis. Mult Scler. 2017 Aug 1 [Epub ahead of print].
New data confirm cladribine’s efficacy as a treatment for relapsing-remitting multiple sclerosis (MS), according to results published online ahead of print August 1 in Multiple Sclerosis Journal. The drug’s effect on relapses is comparable to that of fingolimod, and its effect on disability accumulation is comparable to those of interferon β and fingolimod. Compared with interferon, fingolimod, and natalizumab, cladribine may be associated with superior recovery from disability.
A phase III trial demonstrated that, compared with placebo, cladribine reduced relapse rate and increased the likelihood of remaining free from three-month confirmed disability progression in patients with relapsing-remitting MS. The European Medicines Agency approved the therapy in August 2017. Cladribine’s potential position in the treatment landscape is unclear, however, because no direct comparisons of cladribine with other MS therapies are available.
An Analysis of Matched Cohorts
Tomas Kalincik, MD, PhD, Professor of Medicine at Royal Melbourne Hospital in Australia, and colleagues conducted a propensity score–matched analysis of observational data from MSBase, including patients from the Australian Cladribine Product Familiarization Program, to compare the effectiveness of cladribine to that of interferon β, fingolimod, and natalizumab. Eligible participants had relapsing-remitting MS, received one of the study medications as monotherapy for one or more years, and had no prior exposure to alemtuzumab, mitoxantrone, rituximab, or hematopoietic stem cell transplantation.
Patients received 3.5 mg/kg of oral cladribine, 44 μg of subcutaneous interferon β-1a three times weekly, 0.5 mg/day of oral fingolimod, or 300 μg of IV natalizumab every four weeks. Data were recorded during routine clinical practice. The primary end points were the proportion of patients free from relapses, disability accumulation, and disability improvement while on study therapy.
Cladribine Was Associated With Superior Disability Improvement
The researchers included 37 patients treated with cladribine, 1,940 patients treated with interferon β, 1,892 patients treated with fingolimod, and 1,410 patients treated with natalizumab in their analysis. The investigators noted only small differences in baseline characteristics between the matched cohorts.
Compared with participants receiving interferon β, patients receiving cladribine were less likely to have a relapse during the first year of treatment (hazard ratio [HR], 0.6). The proportion of relapse-free patients was 86% in the cladribine group and 70% in the interferon β group. The probability of disability accumulation was similar for these drugs (HR, 0.41), but the cladribine group was more likely to have disability improvement (HR, 15).
The proportion of relapse-free patients at one year was 79% in the cladribine and fingolimod groups, and cumulative hazards of a relapse did not differ between the two groups (HR, 1.2). The probability of disability accumulation was similar for cladribine and fingolimod (HR, 1.8), but the probability of disability improvement was greater for cladribine (HR, 3.9).
The probability of relapse was higher with cladribine than with natalizumab (HR, 1.8), but the proportions of relapse-free patients at the end of year one were 80% and 81%, respectively. The probability of disability accumulation was greater in the cladribine group than in the natalizumab group (HR, 2.5). The probability of disability improvement was greater among patients receiving cladribine than among those receiving natalizumab (HR, 4). Sensitivity analyses largely confirmed the results of the primary analyses.
“Six-month confirmed improvement of disability was observed in 10%–20% of the cladribine cohort during the first year, which was superior to all three comparator therapies. This [finding] is of interest in the context of the comparison to natalizumab, which is known to be associated with a marked improvement in disability early after its commencement,” said Dr. Kalincik and colleagues. “Improvement in disability in a cohort with this profile is unexpected.”
The study’s main limitation is the small size of the cladribine cohort, said the authors. Another limitation is the brief duration of follow-up for the cladribine group, which precludes conclusions about long-term outcomes. Nevertheless, the comparative effectiveness results “represent timely information about the role of cladribine in the management of MS,” Dr. Kalincik concluded.
—Erik Greb
Suggested Reading
Kalincik T, Jokubaitis V, Spelman T, et al. Cladribine versus fingolimod, natalizumab and interferon β for multiple sclerosis. Mult Scler. 2017 Aug 1 [Epub ahead of print].
Junior surgical trainees hew closer to surgery risk calculators than do faculty members
SAN DIEGO – Researchers say that they’ve developed an easy and inexpensive way to instantly track divergences in thinking by faculty and students as they ponder cases presented in Mortality and Morbidity (M&M) conferences. They’ve already produced an intriguing early finding: Interns and junior residents hew more closely than do their elders to estimates provided by a surgical risk calculator.
The research has the potential to shed light on problems in the much-maligned M&M, says study leader Ira Leeds, MD, of Johns Hopkins University, Baltimore. He presented the study findings at the annual Clinical Congress of the American College of Surgeons.
“This project demonstrates that educational technologies can reveal important gaps in surgical education,” said Dr. Leeds, who made comments during his presentation and in an interview.
At issue: The M&M conference, a mainstay of medical education. “This has been defined as the ‘golden hour’ of surgical education,” Dr. Leeds said. “By discussing someone else’s complications, you can learn how to handle your own in the future.”
However, he added, “there’s very little evidence that we’re currently learning from M&M.”
Dr. Leeds and his colleagues are studying the M&M’s role in medical education to see if it can be improved. The new study, a prospective time-series analysis of weekly M&M conferences, aims to understand the potential value of a real-time feedback system. The idea is to develop a way to alert participants to discrepancies in their perceptions about cases.
The researchers turned to a company called Poll Everywhere, whose technology allowed them to collect instant opinions about M&M cases from those in attendance. During 2016-2017, 110 faculty, residents, and interns used Poll Everywhere’s smartphone app to do two things – make guesses about the root causes of adverse events and estimate the risk of complications from surgical procedures over the next 30 days.
“We can see all the results streaming in real time,” said Dr. Leeds, noting that the service cost $600 per year.
The participants, about two-thirds of whom were male, included faculty (35%), fellows and senior residents (28%), and interns and junior residents (37%). They’d been trained an average of 9 years.
The 34 M&M cases represented a mixture of surgical specialties, including oncology, trauma, transplant, and others.
In terms of the root cause analysis, the technology allowed researchers to instantly detect if the guesses of faculty and students were far apart.
The researchers also compared the risk estimates from the participants to those provided by the NSQIP Risk Calculator. They found that the participants tended to boost their estimate of risk, compared with the calculator, by an absolute mean difference of 7.7 percentage points.
“They were overestimating risk by nearly 8 percentage points,” Dr. Leeds said. This isn’t surprising, since other research has revealed a trend toward overestimation of risk by physicians, compared with calculators, he added.
There wasn’t a major difference between the general level of higher estimation of risk among faculty and senior residents (mean of 8.6 and 7.2 percentage points higher than the calculator, respectively). But interns and junior residents estimated risk higher than the calculator by a mean of 4.9 percentage points.
What’s going on? Are the less experienced staff members outperforming their teachers? Another possibility, Dr. Leeds said, is that “the senior surgeons are better picking up on nuances that aren’t being captured by predictive models or the underdeveloped intuition of a junior trainee.”
Rachel Dawn Aufforth, MD, of Johns Hopkins Medicine, who served as discussant for the presentation by Dr. Leeds, said she looks forward to seeing if this technology can improve resident education. She also wondered why estimates via the risk calculator were chosen as a baseline, especially considering that surgeons tend to estimate higher levels of risk.
“One of the things we’ve been trying to do is look at time-series differences,” Dr. Leeds said. “Are they getting better over an academic year? And does that vary by faculty, especially for interns? The calculator isn’t changing or learning on its own.”
In the big picture, the study shows that “collecting real-time risk estimates and root cause assignment is feasible and can be performed as part of routine M&M conferences,” he said.
The study was funded in part by Johns Hopkins University School of Medicine Institute for Excellence in Education. Dr. Leeds reports no relevant disclosures.
SAN DIEGO – Researchers say that they’ve developed an easy and inexpensive way to instantly track divergences in thinking by faculty and students as they ponder cases presented in Mortality and Morbidity (M&M) conferences. They’ve already produced an intriguing early finding: Interns and junior residents hew more closely than do their elders to estimates provided by a surgical risk calculator.
The research has the potential to shed light on problems in the much-maligned M&M, says study leader Ira Leeds, MD, of Johns Hopkins University, Baltimore. He presented the study findings at the annual Clinical Congress of the American College of Surgeons.
“This project demonstrates that educational technologies can reveal important gaps in surgical education,” said Dr. Leeds, who made comments during his presentation and in an interview.
At issue: The M&M conference, a mainstay of medical education. “This has been defined as the ‘golden hour’ of surgical education,” Dr. Leeds said. “By discussing someone else’s complications, you can learn how to handle your own in the future.”
However, he added, “there’s very little evidence that we’re currently learning from M&M.”
Dr. Leeds and his colleagues are studying the M&M’s role in medical education to see if it can be improved. The new study, a prospective time-series analysis of weekly M&M conferences, aims to understand the potential value of a real-time feedback system. The idea is to develop a way to alert participants to discrepancies in their perceptions about cases.
The researchers turned to a company called Poll Everywhere, whose technology allowed them to collect instant opinions about M&M cases from those in attendance. During 2016-2017, 110 faculty, residents, and interns used Poll Everywhere’s smartphone app to do two things – make guesses about the root causes of adverse events and estimate the risk of complications from surgical procedures over the next 30 days.
“We can see all the results streaming in real time,” said Dr. Leeds, noting that the service cost $600 per year.
The participants, about two-thirds of whom were male, included faculty (35%), fellows and senior residents (28%), and interns and junior residents (37%). They’d been trained an average of 9 years.
The 34 M&M cases represented a mixture of surgical specialties, including oncology, trauma, transplant, and others.
In terms of the root cause analysis, the technology allowed researchers to instantly detect if the guesses of faculty and students were far apart.
The researchers also compared the risk estimates from the participants to those provided by the NSQIP Risk Calculator. They found that the participants tended to boost their estimate of risk, compared with the calculator, by an absolute mean difference of 7.7 percentage points.
“They were overestimating risk by nearly 8 percentage points,” Dr. Leeds said. This isn’t surprising, since other research has revealed a trend toward overestimation of risk by physicians, compared with calculators, he added.
There wasn’t a major difference between the general level of higher estimation of risk among faculty and senior residents (mean of 8.6 and 7.2 percentage points higher than the calculator, respectively). But interns and junior residents estimated risk higher than the calculator by a mean of 4.9 percentage points.
What’s going on? Are the less experienced staff members outperforming their teachers? Another possibility, Dr. Leeds said, is that “the senior surgeons are better picking up on nuances that aren’t being captured by predictive models or the underdeveloped intuition of a junior trainee.”
Rachel Dawn Aufforth, MD, of Johns Hopkins Medicine, who served as discussant for the presentation by Dr. Leeds, said she looks forward to seeing if this technology can improve resident education. She also wondered why estimates via the risk calculator were chosen as a baseline, especially considering that surgeons tend to estimate higher levels of risk.
“One of the things we’ve been trying to do is look at time-series differences,” Dr. Leeds said. “Are they getting better over an academic year? And does that vary by faculty, especially for interns? The calculator isn’t changing or learning on its own.”
In the big picture, the study shows that “collecting real-time risk estimates and root cause assignment is feasible and can be performed as part of routine M&M conferences,” he said.
The study was funded in part by Johns Hopkins University School of Medicine Institute for Excellence in Education. Dr. Leeds reports no relevant disclosures.
SAN DIEGO – Researchers say that they’ve developed an easy and inexpensive way to instantly track divergences in thinking by faculty and students as they ponder cases presented in Mortality and Morbidity (M&M) conferences. They’ve already produced an intriguing early finding: Interns and junior residents hew more closely than do their elders to estimates provided by a surgical risk calculator.
The research has the potential to shed light on problems in the much-maligned M&M, says study leader Ira Leeds, MD, of Johns Hopkins University, Baltimore. He presented the study findings at the annual Clinical Congress of the American College of Surgeons.
“This project demonstrates that educational technologies can reveal important gaps in surgical education,” said Dr. Leeds, who made comments during his presentation and in an interview.
At issue: The M&M conference, a mainstay of medical education. “This has been defined as the ‘golden hour’ of surgical education,” Dr. Leeds said. “By discussing someone else’s complications, you can learn how to handle your own in the future.”
However, he added, “there’s very little evidence that we’re currently learning from M&M.”
Dr. Leeds and his colleagues are studying the M&M’s role in medical education to see if it can be improved. The new study, a prospective time-series analysis of weekly M&M conferences, aims to understand the potential value of a real-time feedback system. The idea is to develop a way to alert participants to discrepancies in their perceptions about cases.
The researchers turned to a company called Poll Everywhere, whose technology allowed them to collect instant opinions about M&M cases from those in attendance. During 2016-2017, 110 faculty, residents, and interns used Poll Everywhere’s smartphone app to do two things – make guesses about the root causes of adverse events and estimate the risk of complications from surgical procedures over the next 30 days.
“We can see all the results streaming in real time,” said Dr. Leeds, noting that the service cost $600 per year.
The participants, about two-thirds of whom were male, included faculty (35%), fellows and senior residents (28%), and interns and junior residents (37%). They’d been trained an average of 9 years.
The 34 M&M cases represented a mixture of surgical specialties, including oncology, trauma, transplant, and others.
In terms of the root cause analysis, the technology allowed researchers to instantly detect if the guesses of faculty and students were far apart.
The researchers also compared the risk estimates from the participants to those provided by the NSQIP Risk Calculator. They found that the participants tended to boost their estimate of risk, compared with the calculator, by an absolute mean difference of 7.7 percentage points.
“They were overestimating risk by nearly 8 percentage points,” Dr. Leeds said. This isn’t surprising, since other research has revealed a trend toward overestimation of risk by physicians, compared with calculators, he added.
There wasn’t a major difference between the general level of higher estimation of risk among faculty and senior residents (mean of 8.6 and 7.2 percentage points higher than the calculator, respectively). But interns and junior residents estimated risk higher than the calculator by a mean of 4.9 percentage points.
What’s going on? Are the less experienced staff members outperforming their teachers? Another possibility, Dr. Leeds said, is that “the senior surgeons are better picking up on nuances that aren’t being captured by predictive models or the underdeveloped intuition of a junior trainee.”
Rachel Dawn Aufforth, MD, of Johns Hopkins Medicine, who served as discussant for the presentation by Dr. Leeds, said she looks forward to seeing if this technology can improve resident education. She also wondered why estimates via the risk calculator were chosen as a baseline, especially considering that surgeons tend to estimate higher levels of risk.
“One of the things we’ve been trying to do is look at time-series differences,” Dr. Leeds said. “Are they getting better over an academic year? And does that vary by faculty, especially for interns? The calculator isn’t changing or learning on its own.”
In the big picture, the study shows that “collecting real-time risk estimates and root cause assignment is feasible and can be performed as part of routine M&M conferences,” he said.
The study was funded in part by Johns Hopkins University School of Medicine Institute for Excellence in Education. Dr. Leeds reports no relevant disclosures.
AT THE ACS CLINICAL CONGRESS
DiaRem score predicts remission of type 2 diabetes after sleeve gastrectomy
SAN DIEGO – The DiaRem score was effective in predicting remission of type 2 diabetes following laparoscopic sleeve gastrectomy, results from a single-center study showed.
Developed by clinicians at Geisinger Clinic, the DiaRem is a simple score that helps predict remission of type 2 diabetes in severely obese subjects with metabolic syndrome who undergo Roux-en-Y gastric bypass surgery (Lancet Diabetes Endocrinol 2014;2[1]:38-45). The DiaRem score spans from 0 to 22 and is divided into five groups corresponding to five probability ranges for type 2 diabetes remission: 0-2 (88%-99%), 3-7 (64%-88%), 8-12 (23%-49%), 13-17 (11%-33%), 18-22 (2%-16%). In an effort to assess the feasibility of using the DiaRem score to predict remission of type 2 diabetes after laparoscopic sleeve gastrectomy, Raul J. Rosenthal, MD, FACS, and his associates conducted a 4-year retrospective review of 162 patients at the Cleveland Clinic Florida, Weston. “This is the first report that uses the DiaRem score for similar subjects that underwent sleeve gastrectomy instead,” Dr. Rosenthal said in an interview in advance of the annual clinical congress of the American College of Surgeons.
The mean age of the 162 patients was 55 years, 61% were women, 74% were non-Hispanic, their mean body mass index was 43.2 kg/m2, 33% had a preoperative hemoglobin A1c level between 7% and 8.9%, and 22% had an HbA1c of 9%. All had a minimum follow-up of 1 year after their laparoscopic sleeve gastrectomy and 67% had follow-up of 3 years or more, said Dr. Rosenthal, professor and chairman of the department of general surgery at Cleveland Clinic Florida.
Based on results of the DiaRem scores, 58% of patients achieved complete remission of type 2 diabetes, 6% achieved partial remission, and 36% had no remission. Specifically, 96% had DiaRem scores between 0 and 2; 92% had scores between 3 and 7; 50% had scores between 8 and 12, 20% had scores between 13 and 17, and 24% had scores between 18 and 22. “We were pleased to find out that 58% of patients that underwent sleeve gastrectomy achieved complete remission of type 2 diabetes mellitus,” said Dr. Rosenthal, who also directs the clinic’s bariatric and metabolic institute. “This compares favorably to previous reports in which patients achieved 33% of complete remission after gastric bypass.” The researchers also found that 84% of patients achieved remission in 12 months and the rest in 3 years. They observed medication reduction in 93% of the patients.
“Sleeve gastrectomy is a valid bariatric-metabolic procedure in patients with type 2 diabetes,” Dr. Rosenthal concluded. “The main limitation of this study is that is it a retrospective one, and we do not have a control group of patients that underwent gastric bypass or medical treatment to compare.”
The findings were presented at the meeting by Emanuele Lo Menzo, MD. Dr. Rosenthal disclosed that he is a consultant for Medtronic. Dr. Lo Menzo reported having no financial disclosures.
SAN DIEGO – The DiaRem score was effective in predicting remission of type 2 diabetes following laparoscopic sleeve gastrectomy, results from a single-center study showed.
Developed by clinicians at Geisinger Clinic, the DiaRem is a simple score that helps predict remission of type 2 diabetes in severely obese subjects with metabolic syndrome who undergo Roux-en-Y gastric bypass surgery (Lancet Diabetes Endocrinol 2014;2[1]:38-45). The DiaRem score spans from 0 to 22 and is divided into five groups corresponding to five probability ranges for type 2 diabetes remission: 0-2 (88%-99%), 3-7 (64%-88%), 8-12 (23%-49%), 13-17 (11%-33%), 18-22 (2%-16%). In an effort to assess the feasibility of using the DiaRem score to predict remission of type 2 diabetes after laparoscopic sleeve gastrectomy, Raul J. Rosenthal, MD, FACS, and his associates conducted a 4-year retrospective review of 162 patients at the Cleveland Clinic Florida, Weston. “This is the first report that uses the DiaRem score for similar subjects that underwent sleeve gastrectomy instead,” Dr. Rosenthal said in an interview in advance of the annual clinical congress of the American College of Surgeons.
The mean age of the 162 patients was 55 years, 61% were women, 74% were non-Hispanic, their mean body mass index was 43.2 kg/m2, 33% had a preoperative hemoglobin A1c level between 7% and 8.9%, and 22% had an HbA1c of 9%. All had a minimum follow-up of 1 year after their laparoscopic sleeve gastrectomy and 67% had follow-up of 3 years or more, said Dr. Rosenthal, professor and chairman of the department of general surgery at Cleveland Clinic Florida.
Based on results of the DiaRem scores, 58% of patients achieved complete remission of type 2 diabetes, 6% achieved partial remission, and 36% had no remission. Specifically, 96% had DiaRem scores between 0 and 2; 92% had scores between 3 and 7; 50% had scores between 8 and 12, 20% had scores between 13 and 17, and 24% had scores between 18 and 22. “We were pleased to find out that 58% of patients that underwent sleeve gastrectomy achieved complete remission of type 2 diabetes mellitus,” said Dr. Rosenthal, who also directs the clinic’s bariatric and metabolic institute. “This compares favorably to previous reports in which patients achieved 33% of complete remission after gastric bypass.” The researchers also found that 84% of patients achieved remission in 12 months and the rest in 3 years. They observed medication reduction in 93% of the patients.
“Sleeve gastrectomy is a valid bariatric-metabolic procedure in patients with type 2 diabetes,” Dr. Rosenthal concluded. “The main limitation of this study is that is it a retrospective one, and we do not have a control group of patients that underwent gastric bypass or medical treatment to compare.”
The findings were presented at the meeting by Emanuele Lo Menzo, MD. Dr. Rosenthal disclosed that he is a consultant for Medtronic. Dr. Lo Menzo reported having no financial disclosures.
SAN DIEGO – The DiaRem score was effective in predicting remission of type 2 diabetes following laparoscopic sleeve gastrectomy, results from a single-center study showed.
Developed by clinicians at Geisinger Clinic, the DiaRem is a simple score that helps predict remission of type 2 diabetes in severely obese subjects with metabolic syndrome who undergo Roux-en-Y gastric bypass surgery (Lancet Diabetes Endocrinol 2014;2[1]:38-45). The DiaRem score spans from 0 to 22 and is divided into five groups corresponding to five probability ranges for type 2 diabetes remission: 0-2 (88%-99%), 3-7 (64%-88%), 8-12 (23%-49%), 13-17 (11%-33%), 18-22 (2%-16%). In an effort to assess the feasibility of using the DiaRem score to predict remission of type 2 diabetes after laparoscopic sleeve gastrectomy, Raul J. Rosenthal, MD, FACS, and his associates conducted a 4-year retrospective review of 162 patients at the Cleveland Clinic Florida, Weston. “This is the first report that uses the DiaRem score for similar subjects that underwent sleeve gastrectomy instead,” Dr. Rosenthal said in an interview in advance of the annual clinical congress of the American College of Surgeons.
The mean age of the 162 patients was 55 years, 61% were women, 74% were non-Hispanic, their mean body mass index was 43.2 kg/m2, 33% had a preoperative hemoglobin A1c level between 7% and 8.9%, and 22% had an HbA1c of 9%. All had a minimum follow-up of 1 year after their laparoscopic sleeve gastrectomy and 67% had follow-up of 3 years or more, said Dr. Rosenthal, professor and chairman of the department of general surgery at Cleveland Clinic Florida.
Based on results of the DiaRem scores, 58% of patients achieved complete remission of type 2 diabetes, 6% achieved partial remission, and 36% had no remission. Specifically, 96% had DiaRem scores between 0 and 2; 92% had scores between 3 and 7; 50% had scores between 8 and 12, 20% had scores between 13 and 17, and 24% had scores between 18 and 22. “We were pleased to find out that 58% of patients that underwent sleeve gastrectomy achieved complete remission of type 2 diabetes mellitus,” said Dr. Rosenthal, who also directs the clinic’s bariatric and metabolic institute. “This compares favorably to previous reports in which patients achieved 33% of complete remission after gastric bypass.” The researchers also found that 84% of patients achieved remission in 12 months and the rest in 3 years. They observed medication reduction in 93% of the patients.
“Sleeve gastrectomy is a valid bariatric-metabolic procedure in patients with type 2 diabetes,” Dr. Rosenthal concluded. “The main limitation of this study is that is it a retrospective one, and we do not have a control group of patients that underwent gastric bypass or medical treatment to compare.”
The findings were presented at the meeting by Emanuele Lo Menzo, MD. Dr. Rosenthal disclosed that he is a consultant for Medtronic. Dr. Lo Menzo reported having no financial disclosures.
AT THE ACS CLINICAL CONGRESS
Key clinical point: The DiaRem score is useful in predicting remission of type 2 diabetes following laparoscopic sleeve gastrectomy.
Major finding: Results of the DiaRem scores indicated that 58% of patients achieved complete remission of type 2 diabetes.
Study details: A retrospective analysis of 162 patients who underwent laparoscopic sleeve gastrectomy.
Disclosures: Dr. Rosenthal disclosed that he is a consultant for Medtronic. Dr. Lo Menzo reported having no financial disclosures.
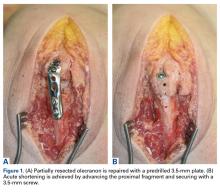
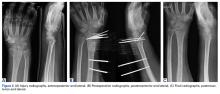
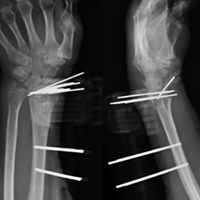
Acute Shortening Versus Bridging Plate for Highly Comminuted Olecranon Fractures
Take-Home Points
- The ulnohumeral joint can tolerate substantial articular surface loss without compromising stability.
- Consider BP as an alternative to AS in unreconstructable olecranon fractures.
- Both BP and AS of olecranon fractures maintain elbow stability.
- BP has the advantage of maintaining elbow range of motion.
Olecranon fractures constitute about 10% of all forearm fractures.1 Many are low-energy fractures in osteoporotic bone in the elderly.1,2 Unstable fractures require operative fixation in which the goal is restoration of articular congruity and stability.3 Various fixation methods are used to treat unstable olecranon fractures, and outcomes are good overall.3-21 However, severely comminuted olecranon fractures, especially in osteoporotic bone, pose a unique challenge, where reconstruction may not be feasible.9 Although the articular surface can be reconstructed in most cases, reconstruction is not feasible with severe comminution or low bone mineral density. When articular congruity is no longer possible, the primary goal of fixation becomes elbow stability. Postoperative stability is linked to favorable outcomes, as it allows patients to engage in early range-of-motion (ROM) exercises, which improves joint function.5,21,22
When treating these severely comminuted olecranon fractures, surgeons have 2 options: bridge plating (BP) and acute shortening (AS). In BP, a plate is used to restore the length of the olecranon. The plate is spanned over the comminuted segment with fixation at proximal and distal pieces but without open reduction of the comminuted pieces.8 This process may be performed with or without bone grafting.21 Although any bony defect between the proximal and distal pieces may be filled, there is now a gap in articular congruity within the sigmoid notch. One concern with this fixation method is that joint stability is lost when this gap becomes too large. Surgeons therefore may decide to forgo BP and perform AS instead, as long as the coronoid is intact.21 In AS, often referred to as olecranon excision, comminuted fragments are removed and the triceps muscle advanced distally. AS constructs, often reserved for older, less active patients, yield acceptable results in this population.5 However, the long-term effects of AS in young, active patients are unclear, and biomechanical studies suggest reduced triceps muscle strength.23
Surgeons have had no studies guiding them in deciding which construct to use, BP or AS, in severely comminuted olecranon fractures in which the articular surface cannot be reconstructed.
We conducted a biomechanical study to determine the percentage loss of articular surface at which a BP construct becomes significantly clinically unstable. We also compared BP stability and AS stability for each percentage loss of articular surface and compared initial elbow ROM with the 2 methods. We hypothesized that, at a certain percentage loss of articular congruity, the BP construct would become too unstable and would require conversion to the AS construct.
Materials and Methods
Specimen Preparation
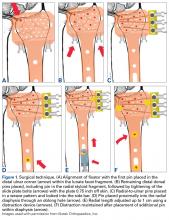
Eight fresh-frozen paired cadaveric upper limbs (2 male, 2 female; mean age, 61.8 years; age range, 56-74 years) were obtained from donors with no history of elbow trauma or prior surgery. Specimens were stored at –20°C, thawed to room temperature before testing, and, using clinical and radiographic evaluation, screened for abnormalities.
Each specimen was positioned with the arm draped in the lateral decubitus position, as in typical olecranon fracture surgery. A standard posterior approach to the olecranon was made with a midline posterior longitudinal skin incision. Subcutaneous flaps were developed, and the subcutaneous border of the proximal olecranon was exposed, preserving the medial and lateral collateral ligaments as well as the extensor mechanism. Baseline maximum flexion and extension of the elbow as well as olecranon length were measured with fluoroscopy (BV Pulsera, Philips) and ImageJ software (National Institutes of Health).
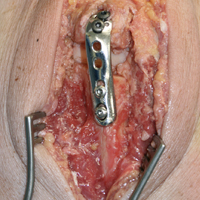
To ensure reproducible anatomical reduction during plating, a 3.5-mm 4-hole nonlocking periarticular anatomically contoured plate (Zimmer Biomet) was applied posteriorly to the intact olecranon through a longitudinal slit in the distal triceps tendon. The plate was predrilled to house 4 nonlocking screws, 2 proximal and 2 distal.
Fracture Generation and Testing of Fixation Constructs
Analysis
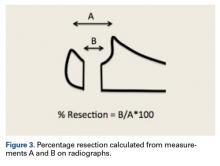
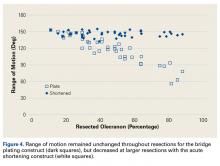
ImageJ software was used to analyze the C-arm radiographs. Measurements were divided into 4 groups of joint surface loss caused by the resections: 0% to 20%, 20% to 40%, 40% to 60%, and >60%. Differences in ROM between the BP and AS constructs were analyzed with a Wilcoxon signed rank test with statistical significance set at P < .05 (Prism 6; GraphPad Software).
Results
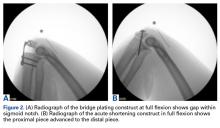
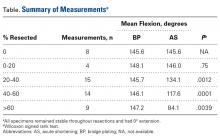
As many as 6 serial resections were made before the proximal fragment of the olecranon was judged too small to be secured to a plate with at least 2 screws. Only 7 specimens were large enough for the fifth cut, and only 4 were large enough for the sixth cut. After the final resection, mean loss of olecranon length was 77.3% (range, 63.7%-88%; median, 80.6%). All elbow specimens remained stable to manual valgus and varus testing in full extension, 30° of flexion, and full flexion in both supination and pronation. There was no medial or lateral opening of the ulnohumeral joint on fluoroscopy throughout testing, for either the BP or the AS constructs. There was no anterior or posterior subluxation throughout the entire ROM.
Discussion
Our goal in this study was to determine the maximum articular surface loss that can be tolerated before a BP construct becomes unstable. This finding applies to situations in which the degree of comminution makes reconstruction of the articular surface impossible. Contrary to our hypothesis, the ulnohumeral joint remained stable despite extensive loss of congruity within the sigmoid notch. In 1 specimen, the joint remained stable at 88% loss of olecranon. However, the 2 constructs had different ROM results: ROM was significantly lower at more resections with AS but remained unchanged from baseline with BP.
Dorsal plating has become standard treatment for comminuted olecranon fractures, and many studies, both clinical and biomechanical, have reported favorable results, good functional outcomes, and acceptable ROM.3,7,10,13,18-20,25 However, the multiple studies on the use of various plates in comminuted olecranon fractures did not address whether articular congruity was maintained during reductions or how much articular surface was reconstructed. Although we may reasonably assume larger studies included cases with some unmeasured loss of articular congruity, it is difficult to directly compare our findings with those of other studies. In addition, it is possible those studies did not include fractures that were deemed unfit for BP (because of very severe comminution) and underwent AS instead. Only 1 case series has focused on BP without complete articular reconstruction.8 The cases in that series had good outcomes with good stability—consistent with our finding of extreme comminution in a worst-case scenario.
Complete elbow stability after AS is consistent with findings in the literature.4,6,12,14,16 As AS is reserved for severely comminuted fractures and bone resections,21,23,26 our findings can be compared with the earlier findings. In AS, either the proximal pieces or the intermediate pieces are removed to create a smaller but congruent articular surface, with less concern for nonunion.21 When the proximal piece is removed, the triceps muscle is advanced to the ulnar shaft, creating a slinglike structure for the trochlea.4,11,16,23 When the intermediate piece is removed, the proximal piece is advanced to the shaft along with the triceps.12,14,27 In either technique, the triceps muscle is advanced distally, potentially affecting its extensibility and moment arm.23
Although small in numbers, case series and retrospective reviews have found that AS has good outcomes,4,14,16 whereas our study found significantly decreased ROM. A few patients in these studies lost ROM or triceps strength,12,14,16 but the cause, AS or fracture severity, is unclear. It is possible only 0% to 20% of the olecranon was resected in those cases, whereas our study found no significant change in ROM. It is also possible that cadaveric muscles do not stretch as well as muscles in vivo. Biomechanical studies have demonstrated changes in triceps stretch and strength,23,26 but perhaps these changes are subclinical or overcome with therapy and time.12,14 There are no data regarding whether patients who undergo AS (vs another fixation method) need more physical therapy. In extreme resection, some reduction in ROM is expected.13
The ulnohumeral joint is a primary static stabilizer of the elbow joint.28-30 Recent studies on the role of the ulnohumeral joint in elbow stability have focused mainly on the coronoid process in the setting of dislocation.28,29,31,32 According to these studies, 50% of the coronoid must remain intact for the elbow to be stable when all other stabilizers are intact.32 In our study, resections preserved the coronoid and the ligamentous stabilizers of the elbow. It is therefore possible that the elbow joint remained stable despite the considerable articular surface loss. Although the term ulnohumeral joint refers to both the coronoid and the remaining articular surface, our findings support the coronoid as a primary stabilizer and the remaining articular surface as a secondary static stabilizer.
This study had several limitations. First, its fractures were simulated by serial resection of only the middle portion of the olecranon. In reality, comminution could extend farther proximally or distally and involve the surrounding tissues, which help stabilize the elbow. However, our focus was on loss of articular surface and stability, so keeping surrounding structures intact avoided confounding factors that could contribute to stability. A second possible limitation is that the implant used here may be different from the implant used in a clinical setting. However, our focus was not on fixation quality, and stability alone should not be affected by plate type. Third, stability was measured not quantitatively but instead subjectively under manual stress and fluoroscopy. We chose this method because it mimics what happens during surgery and is the clinical standard for stability assessment.24 Fourth, soft-tissue properties of the cadaver models used in this biomechanical study may differ from soft-tissue properties in vivo. This study could not evaluate possible long-term complications, such as posttraumatic arthritis and heterotopic ossification.5,10 There are no long-term studies comparing BP and other olecranon fixation methods in terms of postoperative elbow arthritis.
Conclusion
The ulnohumeral joint can tolerate substantial articular surface loss without compromising stability. As a result, in the management of highly comminuted olecranon fractures, BP may be considered before AS is performed. Quality and amount of intact proximal bone, rather than degree of comminution, may be more important factors in deciding which fixation method to use.
This biomechanical study is the first to focus on olecranon fracture BP without complete reconstruction of the articular surface. When treating a highly comminuted olecranon fracture that has an unreconstructible articular surface, surgeons may consider BP with or without bone graft, as well as AS. Our study findings suggest that, though both constructs maintain elbow stability, BP may have the advantage of maintaining ROM too. BP can avoid effects on triceps and elbow ROM, which may be more important in younger, more active patients. Clinical correlates are needed to validate these findings, as overall outcomes may be affected by concurrent fractures and injuries to surrounding structures.
1. Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691-697.
2. Duckworth AD, Clement ND, Aitken SA, Court-Brown CM, McQueen MM. The epidemiology of fractures of the proximal ulna. Injury. 2012;43(3):343-346.
3. Bailey CS, MacDermid J, Patterson SD, King GJ. Outcome of plate fixation of olecranon fractures. J Orthop Trauma. 2001;15(8):542-548.
4. Adler S, Fay GF, Macausland WR Jr. Treatment of olecranon fractures. Indications for excision of the olecranon fragment and repair of the triceps tendon. J Trauma. 1962;2:597-602.
5. Baecher N, Edwards S. Olecranon fractures. J Hand Surg Am. 2013;38(3):593-604.
6. Bell TH, Ferreira LM, McDonald CP, Johnson JA, King GJW. Contribution of the olecranon to elbow stability: an in vitro biomechanical study. J Bone Joint Surg Am. 2010;92(4):949-957.
7. Buijze G, Kloen P. Clinical evaluation of locking compression plate fixation for comminuted olecranon fractures. J Bone Joint Surg Am. 2009;91(10):2416-2420.
8. Cervera-Irimia J, Tomé-Bermejo F, Gómez-Bermejo MA, Holgado-Moreno E, Stratenwerth EG. Treatment of comminuted olecranon fractures with olecranon plate and structural iliac crest graft. Acta Orthop Belg. 2012;78(6):703-707.
9. Edwards SG, Martin BD, Fu RH, et al. Comparison of olecranon plate fixation in osteoporotic bone: do current technologies and designs make a difference? J Orthop Trauma. 2011;25(5):306-311.
10. Erturer RE, Sever C, Sonmez MM, Ozcelik IB, Akman S, Ozturk I. Results of open reduction and plate osteosynthesis in comminuted fracture of the olecranon. J Shoulder Elbow Surg. 2011;20(3):449-454.
11. Estourgie RJ, Tinnemans JG. Treatment of grossly comminuted fractures of the olecranon by excision. Neth J Surg. 1982;34(3):127-129.
12. Fern ED, Brown JN. Olecranon advancement osteotomy in the management of severely comminuted olecranon fractures. Injury. 1993;24(4):267-269.
13. Gordon MJ, Budoff JE, Yeh ML, Luo ZP, Noble PC. Comminuted olecranon fractures: a comparison of plating methods. J Shoulder Elbow Surg. 2006;15(1):94-99.
14. Iannuzzi N, Dahners L. Excision and advancement in the treatment of comminuted olecranon fractures. J Orthop Trauma. 2009;23(3):226-228.
15. Ikeda M, Fukushima Y, Kobayashi Y, Oka Y. Comminuted fractures of the olecranon. Management by bone graft from the iliac crest and multiple tension-band wiring. J Bone Joint Surg Br. 2001;83(6):805-808.
16. McKeever FM, Buck RM. Fracture of the olecranon process of the ulna; treatment by excision of fragment and repair of triceps tendon. JAMA. 1947;135(1):1-5.
17. Rommens PM, Küchle R, Schneider RU, Reuter M. Olecranon fractures in adults: factors influencing outcome. Injury. 2004;35(11):1149-1157.
18. Siebenlist S, Torsiglieri T, Kraus T, Burghardt RD, Stöckle U, Lucke M. Comminuted fractures of the proximal ulna—preliminary results with an anatomically preshaped locking compression plate (LCP) system. Injury. 2010;41(12):1306-1311.
19. Tarallo L, Mugnai R, Adani R, Capra F, Zambianchi F, Catani F. Simple and comminuted displaced olecranon fractures: a clinical comparison between tension band wiring and plate fixation techniques. Arch Orthop Trauma Surg. 2014;134(8):1107-1114.
20. Wang Y, Tao R, Xu H, Cao Y, Zhou Z, Xu S. Mid-term outcomes of contoured plating for comminuted fractures of the olecranon. Orthop Surg. 2011;3(3):176-180.
21. Newman SD, Mauffrey C, Krikler S. Olecranon fractures. Injury. 2009;40(6):575-581.
22. Boyer MI, Galatz LM, Borrelli J, Axelrod TS, Ricci WM. Intra-articular fractures of the upper extremity: new concepts in surgical treatment. Instr Course Lect. 2003;52:591-605.
23. Didonna ML, Fernandez JJ, Lim TH, Hastings H, Cohen MS. Partial olecranon excision: the relationship between triceps insertion site and extension strength of the elbow. J Hand Surg Am. 2003;28(1):117-122.
24. Trumble T, Cornwall R, Budoff J. Core Knowledge in Orthopaedics: Hand, Elbow, and Shoulder. Philadelphia, PA: Mosby; 2006.
25. Simpson NS, Goodman LA, Jupiter JB. Contoured LCDC plating of the proximal ulna. Injury. 1996;27(6):411-417.
26. Ferreira LM, Bell TH, Johnson JA, King GJ. The effect of triceps repair techniques following olecranon excision on elbow stability and extension strength: an in vitro biomechanical study. J Orthop Trauma. 2011;25(7):420-424.
27. Colton CL. Fractures of the olecranon in adults: classification and management. Injury. 1973;5(2):121-129.
28. Hull JR, Owen JR, Fern SE, Wayne JS, Boardman ND 3rd. Role of the coronoid process in varus osteoarticular stability of the elbow. J Shoulder Elbow Surg. 2005;14(4):441-446.
29. Morrey BF, An KN. Stability of the elbow: osseous constraints. J Shoulder Elbow Surg. 2005;14(1 suppl S):174S-178S.
30. Williams G, Ramsey M, Wiesel S. Operative Techniques in Shoulder and Elbow Surgery. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
31. Schneeberger AG, Sadowski MM, Jacob HA. Coronoid process and radial head as posterolateral rotatory stabilizers of the elbow. J Bone Joint Surg Am. 2004;86(5):975-982.
32. Closkey RF, Goode JR, Kirschenbaum D, Cody RP. The role of the coronoid process in elbow stability. A biomechanical analysis of axial loading. J Bone Joint Surg Am. 2000;82(12):1749-1753.
Take-Home Points
- The ulnohumeral joint can tolerate substantial articular surface loss without compromising stability.
- Consider BP as an alternative to AS in unreconstructable olecranon fractures.
- Both BP and AS of olecranon fractures maintain elbow stability.
- BP has the advantage of maintaining elbow range of motion.
Olecranon fractures constitute about 10% of all forearm fractures.1 Many are low-energy fractures in osteoporotic bone in the elderly.1,2 Unstable fractures require operative fixation in which the goal is restoration of articular congruity and stability.3 Various fixation methods are used to treat unstable olecranon fractures, and outcomes are good overall.3-21 However, severely comminuted olecranon fractures, especially in osteoporotic bone, pose a unique challenge, where reconstruction may not be feasible.9 Although the articular surface can be reconstructed in most cases, reconstruction is not feasible with severe comminution or low bone mineral density. When articular congruity is no longer possible, the primary goal of fixation becomes elbow stability. Postoperative stability is linked to favorable outcomes, as it allows patients to engage in early range-of-motion (ROM) exercises, which improves joint function.5,21,22
When treating these severely comminuted olecranon fractures, surgeons have 2 options: bridge plating (BP) and acute shortening (AS). In BP, a plate is used to restore the length of the olecranon. The plate is spanned over the comminuted segment with fixation at proximal and distal pieces but without open reduction of the comminuted pieces.8 This process may be performed with or without bone grafting.21 Although any bony defect between the proximal and distal pieces may be filled, there is now a gap in articular congruity within the sigmoid notch. One concern with this fixation method is that joint stability is lost when this gap becomes too large. Surgeons therefore may decide to forgo BP and perform AS instead, as long as the coronoid is intact.21 In AS, often referred to as olecranon excision, comminuted fragments are removed and the triceps muscle advanced distally. AS constructs, often reserved for older, less active patients, yield acceptable results in this population.5 However, the long-term effects of AS in young, active patients are unclear, and biomechanical studies suggest reduced triceps muscle strength.23
Surgeons have had no studies guiding them in deciding which construct to use, BP or AS, in severely comminuted olecranon fractures in which the articular surface cannot be reconstructed.
We conducted a biomechanical study to determine the percentage loss of articular surface at which a BP construct becomes significantly clinically unstable. We also compared BP stability and AS stability for each percentage loss of articular surface and compared initial elbow ROM with the 2 methods. We hypothesized that, at a certain percentage loss of articular congruity, the BP construct would become too unstable and would require conversion to the AS construct.
Materials and Methods
Specimen Preparation
Eight fresh-frozen paired cadaveric upper limbs (2 male, 2 female; mean age, 61.8 years; age range, 56-74 years) were obtained from donors with no history of elbow trauma or prior surgery. Specimens were stored at –20°C, thawed to room temperature before testing, and, using clinical and radiographic evaluation, screened for abnormalities.
Each specimen was positioned with the arm draped in the lateral decubitus position, as in typical olecranon fracture surgery. A standard posterior approach to the olecranon was made with a midline posterior longitudinal skin incision. Subcutaneous flaps were developed, and the subcutaneous border of the proximal olecranon was exposed, preserving the medial and lateral collateral ligaments as well as the extensor mechanism. Baseline maximum flexion and extension of the elbow as well as olecranon length were measured with fluoroscopy (BV Pulsera, Philips) and ImageJ software (National Institutes of Health).
To ensure reproducible anatomical reduction during plating, a 3.5-mm 4-hole nonlocking periarticular anatomically contoured plate (Zimmer Biomet) was applied posteriorly to the intact olecranon through a longitudinal slit in the distal triceps tendon. The plate was predrilled to house 4 nonlocking screws, 2 proximal and 2 distal.
Fracture Generation and Testing of Fixation Constructs
Analysis
ImageJ software was used to analyze the C-arm radiographs. Measurements were divided into 4 groups of joint surface loss caused by the resections: 0% to 20%, 20% to 40%, 40% to 60%, and >60%. Differences in ROM between the BP and AS constructs were analyzed with a Wilcoxon signed rank test with statistical significance set at P < .05 (Prism 6; GraphPad Software).
Results
As many as 6 serial resections were made before the proximal fragment of the olecranon was judged too small to be secured to a plate with at least 2 screws. Only 7 specimens were large enough for the fifth cut, and only 4 were large enough for the sixth cut. After the final resection, mean loss of olecranon length was 77.3% (range, 63.7%-88%; median, 80.6%). All elbow specimens remained stable to manual valgus and varus testing in full extension, 30° of flexion, and full flexion in both supination and pronation. There was no medial or lateral opening of the ulnohumeral joint on fluoroscopy throughout testing, for either the BP or the AS constructs. There was no anterior or posterior subluxation throughout the entire ROM.
Discussion
Our goal in this study was to determine the maximum articular surface loss that can be tolerated before a BP construct becomes unstable. This finding applies to situations in which the degree of comminution makes reconstruction of the articular surface impossible. Contrary to our hypothesis, the ulnohumeral joint remained stable despite extensive loss of congruity within the sigmoid notch. In 1 specimen, the joint remained stable at 88% loss of olecranon. However, the 2 constructs had different ROM results: ROM was significantly lower at more resections with AS but remained unchanged from baseline with BP.
Dorsal plating has become standard treatment for comminuted olecranon fractures, and many studies, both clinical and biomechanical, have reported favorable results, good functional outcomes, and acceptable ROM.3,7,10,13,18-20,25 However, the multiple studies on the use of various plates in comminuted olecranon fractures did not address whether articular congruity was maintained during reductions or how much articular surface was reconstructed. Although we may reasonably assume larger studies included cases with some unmeasured loss of articular congruity, it is difficult to directly compare our findings with those of other studies. In addition, it is possible those studies did not include fractures that were deemed unfit for BP (because of very severe comminution) and underwent AS instead. Only 1 case series has focused on BP without complete articular reconstruction.8 The cases in that series had good outcomes with good stability—consistent with our finding of extreme comminution in a worst-case scenario.
Complete elbow stability after AS is consistent with findings in the literature.4,6,12,14,16 As AS is reserved for severely comminuted fractures and bone resections,21,23,26 our findings can be compared with the earlier findings. In AS, either the proximal pieces or the intermediate pieces are removed to create a smaller but congruent articular surface, with less concern for nonunion.21 When the proximal piece is removed, the triceps muscle is advanced to the ulnar shaft, creating a slinglike structure for the trochlea.4,11,16,23 When the intermediate piece is removed, the proximal piece is advanced to the shaft along with the triceps.12,14,27 In either technique, the triceps muscle is advanced distally, potentially affecting its extensibility and moment arm.23
Although small in numbers, case series and retrospective reviews have found that AS has good outcomes,4,14,16 whereas our study found significantly decreased ROM. A few patients in these studies lost ROM or triceps strength,12,14,16 but the cause, AS or fracture severity, is unclear. It is possible only 0% to 20% of the olecranon was resected in those cases, whereas our study found no significant change in ROM. It is also possible that cadaveric muscles do not stretch as well as muscles in vivo. Biomechanical studies have demonstrated changes in triceps stretch and strength,23,26 but perhaps these changes are subclinical or overcome with therapy and time.12,14 There are no data regarding whether patients who undergo AS (vs another fixation method) need more physical therapy. In extreme resection, some reduction in ROM is expected.13
The ulnohumeral joint is a primary static stabilizer of the elbow joint.28-30 Recent studies on the role of the ulnohumeral joint in elbow stability have focused mainly on the coronoid process in the setting of dislocation.28,29,31,32 According to these studies, 50% of the coronoid must remain intact for the elbow to be stable when all other stabilizers are intact.32 In our study, resections preserved the coronoid and the ligamentous stabilizers of the elbow. It is therefore possible that the elbow joint remained stable despite the considerable articular surface loss. Although the term ulnohumeral joint refers to both the coronoid and the remaining articular surface, our findings support the coronoid as a primary stabilizer and the remaining articular surface as a secondary static stabilizer.
This study had several limitations. First, its fractures were simulated by serial resection of only the middle portion of the olecranon. In reality, comminution could extend farther proximally or distally and involve the surrounding tissues, which help stabilize the elbow. However, our focus was on loss of articular surface and stability, so keeping surrounding structures intact avoided confounding factors that could contribute to stability. A second possible limitation is that the implant used here may be different from the implant used in a clinical setting. However, our focus was not on fixation quality, and stability alone should not be affected by plate type. Third, stability was measured not quantitatively but instead subjectively under manual stress and fluoroscopy. We chose this method because it mimics what happens during surgery and is the clinical standard for stability assessment.24 Fourth, soft-tissue properties of the cadaver models used in this biomechanical study may differ from soft-tissue properties in vivo. This study could not evaluate possible long-term complications, such as posttraumatic arthritis and heterotopic ossification.5,10 There are no long-term studies comparing BP and other olecranon fixation methods in terms of postoperative elbow arthritis.
Conclusion
The ulnohumeral joint can tolerate substantial articular surface loss without compromising stability. As a result, in the management of highly comminuted olecranon fractures, BP may be considered before AS is performed. Quality and amount of intact proximal bone, rather than degree of comminution, may be more important factors in deciding which fixation method to use.
This biomechanical study is the first to focus on olecranon fracture BP without complete reconstruction of the articular surface. When treating a highly comminuted olecranon fracture that has an unreconstructible articular surface, surgeons may consider BP with or without bone graft, as well as AS. Our study findings suggest that, though both constructs maintain elbow stability, BP may have the advantage of maintaining ROM too. BP can avoid effects on triceps and elbow ROM, which may be more important in younger, more active patients. Clinical correlates are needed to validate these findings, as overall outcomes may be affected by concurrent fractures and injuries to surrounding structures.
Take-Home Points
- The ulnohumeral joint can tolerate substantial articular surface loss without compromising stability.
- Consider BP as an alternative to AS in unreconstructable olecranon fractures.
- Both BP and AS of olecranon fractures maintain elbow stability.
- BP has the advantage of maintaining elbow range of motion.
Olecranon fractures constitute about 10% of all forearm fractures.1 Many are low-energy fractures in osteoporotic bone in the elderly.1,2 Unstable fractures require operative fixation in which the goal is restoration of articular congruity and stability.3 Various fixation methods are used to treat unstable olecranon fractures, and outcomes are good overall.3-21 However, severely comminuted olecranon fractures, especially in osteoporotic bone, pose a unique challenge, where reconstruction may not be feasible.9 Although the articular surface can be reconstructed in most cases, reconstruction is not feasible with severe comminution or low bone mineral density. When articular congruity is no longer possible, the primary goal of fixation becomes elbow stability. Postoperative stability is linked to favorable outcomes, as it allows patients to engage in early range-of-motion (ROM) exercises, which improves joint function.5,21,22
When treating these severely comminuted olecranon fractures, surgeons have 2 options: bridge plating (BP) and acute shortening (AS). In BP, a plate is used to restore the length of the olecranon. The plate is spanned over the comminuted segment with fixation at proximal and distal pieces but without open reduction of the comminuted pieces.8 This process may be performed with or without bone grafting.21 Although any bony defect between the proximal and distal pieces may be filled, there is now a gap in articular congruity within the sigmoid notch. One concern with this fixation method is that joint stability is lost when this gap becomes too large. Surgeons therefore may decide to forgo BP and perform AS instead, as long as the coronoid is intact.21 In AS, often referred to as olecranon excision, comminuted fragments are removed and the triceps muscle advanced distally. AS constructs, often reserved for older, less active patients, yield acceptable results in this population.5 However, the long-term effects of AS in young, active patients are unclear, and biomechanical studies suggest reduced triceps muscle strength.23
Surgeons have had no studies guiding them in deciding which construct to use, BP or AS, in severely comminuted olecranon fractures in which the articular surface cannot be reconstructed.
We conducted a biomechanical study to determine the percentage loss of articular surface at which a BP construct becomes significantly clinically unstable. We also compared BP stability and AS stability for each percentage loss of articular surface and compared initial elbow ROM with the 2 methods. We hypothesized that, at a certain percentage loss of articular congruity, the BP construct would become too unstable and would require conversion to the AS construct.
Materials and Methods
Specimen Preparation
Eight fresh-frozen paired cadaveric upper limbs (2 male, 2 female; mean age, 61.8 years; age range, 56-74 years) were obtained from donors with no history of elbow trauma or prior surgery. Specimens were stored at –20°C, thawed to room temperature before testing, and, using clinical and radiographic evaluation, screened for abnormalities.
Each specimen was positioned with the arm draped in the lateral decubitus position, as in typical olecranon fracture surgery. A standard posterior approach to the olecranon was made with a midline posterior longitudinal skin incision. Subcutaneous flaps were developed, and the subcutaneous border of the proximal olecranon was exposed, preserving the medial and lateral collateral ligaments as well as the extensor mechanism. Baseline maximum flexion and extension of the elbow as well as olecranon length were measured with fluoroscopy (BV Pulsera, Philips) and ImageJ software (National Institutes of Health).
To ensure reproducible anatomical reduction during plating, a 3.5-mm 4-hole nonlocking periarticular anatomically contoured plate (Zimmer Biomet) was applied posteriorly to the intact olecranon through a longitudinal slit in the distal triceps tendon. The plate was predrilled to house 4 nonlocking screws, 2 proximal and 2 distal.
Fracture Generation and Testing of Fixation Constructs
Analysis
ImageJ software was used to analyze the C-arm radiographs. Measurements were divided into 4 groups of joint surface loss caused by the resections: 0% to 20%, 20% to 40%, 40% to 60%, and >60%. Differences in ROM between the BP and AS constructs were analyzed with a Wilcoxon signed rank test with statistical significance set at P < .05 (Prism 6; GraphPad Software).
Results
As many as 6 serial resections were made before the proximal fragment of the olecranon was judged too small to be secured to a plate with at least 2 screws. Only 7 specimens were large enough for the fifth cut, and only 4 were large enough for the sixth cut. After the final resection, mean loss of olecranon length was 77.3% (range, 63.7%-88%; median, 80.6%). All elbow specimens remained stable to manual valgus and varus testing in full extension, 30° of flexion, and full flexion in both supination and pronation. There was no medial or lateral opening of the ulnohumeral joint on fluoroscopy throughout testing, for either the BP or the AS constructs. There was no anterior or posterior subluxation throughout the entire ROM.
Discussion
Our goal in this study was to determine the maximum articular surface loss that can be tolerated before a BP construct becomes unstable. This finding applies to situations in which the degree of comminution makes reconstruction of the articular surface impossible. Contrary to our hypothesis, the ulnohumeral joint remained stable despite extensive loss of congruity within the sigmoid notch. In 1 specimen, the joint remained stable at 88% loss of olecranon. However, the 2 constructs had different ROM results: ROM was significantly lower at more resections with AS but remained unchanged from baseline with BP.
Dorsal plating has become standard treatment for comminuted olecranon fractures, and many studies, both clinical and biomechanical, have reported favorable results, good functional outcomes, and acceptable ROM.3,7,10,13,18-20,25 However, the multiple studies on the use of various plates in comminuted olecranon fractures did not address whether articular congruity was maintained during reductions or how much articular surface was reconstructed. Although we may reasonably assume larger studies included cases with some unmeasured loss of articular congruity, it is difficult to directly compare our findings with those of other studies. In addition, it is possible those studies did not include fractures that were deemed unfit for BP (because of very severe comminution) and underwent AS instead. Only 1 case series has focused on BP without complete articular reconstruction.8 The cases in that series had good outcomes with good stability—consistent with our finding of extreme comminution in a worst-case scenario.
Complete elbow stability after AS is consistent with findings in the literature.4,6,12,14,16 As AS is reserved for severely comminuted fractures and bone resections,21,23,26 our findings can be compared with the earlier findings. In AS, either the proximal pieces or the intermediate pieces are removed to create a smaller but congruent articular surface, with less concern for nonunion.21 When the proximal piece is removed, the triceps muscle is advanced to the ulnar shaft, creating a slinglike structure for the trochlea.4,11,16,23 When the intermediate piece is removed, the proximal piece is advanced to the shaft along with the triceps.12,14,27 In either technique, the triceps muscle is advanced distally, potentially affecting its extensibility and moment arm.23
Although small in numbers, case series and retrospective reviews have found that AS has good outcomes,4,14,16 whereas our study found significantly decreased ROM. A few patients in these studies lost ROM or triceps strength,12,14,16 but the cause, AS or fracture severity, is unclear. It is possible only 0% to 20% of the olecranon was resected in those cases, whereas our study found no significant change in ROM. It is also possible that cadaveric muscles do not stretch as well as muscles in vivo. Biomechanical studies have demonstrated changes in triceps stretch and strength,23,26 but perhaps these changes are subclinical or overcome with therapy and time.12,14 There are no data regarding whether patients who undergo AS (vs another fixation method) need more physical therapy. In extreme resection, some reduction in ROM is expected.13
The ulnohumeral joint is a primary static stabilizer of the elbow joint.28-30 Recent studies on the role of the ulnohumeral joint in elbow stability have focused mainly on the coronoid process in the setting of dislocation.28,29,31,32 According to these studies, 50% of the coronoid must remain intact for the elbow to be stable when all other stabilizers are intact.32 In our study, resections preserved the coronoid and the ligamentous stabilizers of the elbow. It is therefore possible that the elbow joint remained stable despite the considerable articular surface loss. Although the term ulnohumeral joint refers to both the coronoid and the remaining articular surface, our findings support the coronoid as a primary stabilizer and the remaining articular surface as a secondary static stabilizer.
This study had several limitations. First, its fractures were simulated by serial resection of only the middle portion of the olecranon. In reality, comminution could extend farther proximally or distally and involve the surrounding tissues, which help stabilize the elbow. However, our focus was on loss of articular surface and stability, so keeping surrounding structures intact avoided confounding factors that could contribute to stability. A second possible limitation is that the implant used here may be different from the implant used in a clinical setting. However, our focus was not on fixation quality, and stability alone should not be affected by plate type. Third, stability was measured not quantitatively but instead subjectively under manual stress and fluoroscopy. We chose this method because it mimics what happens during surgery and is the clinical standard for stability assessment.24 Fourth, soft-tissue properties of the cadaver models used in this biomechanical study may differ from soft-tissue properties in vivo. This study could not evaluate possible long-term complications, such as posttraumatic arthritis and heterotopic ossification.5,10 There are no long-term studies comparing BP and other olecranon fixation methods in terms of postoperative elbow arthritis.
Conclusion
The ulnohumeral joint can tolerate substantial articular surface loss without compromising stability. As a result, in the management of highly comminuted olecranon fractures, BP may be considered before AS is performed. Quality and amount of intact proximal bone, rather than degree of comminution, may be more important factors in deciding which fixation method to use.
This biomechanical study is the first to focus on olecranon fracture BP without complete reconstruction of the articular surface. When treating a highly comminuted olecranon fracture that has an unreconstructible articular surface, surgeons may consider BP with or without bone graft, as well as AS. Our study findings suggest that, though both constructs maintain elbow stability, BP may have the advantage of maintaining ROM too. BP can avoid effects on triceps and elbow ROM, which may be more important in younger, more active patients. Clinical correlates are needed to validate these findings, as overall outcomes may be affected by concurrent fractures and injuries to surrounding structures.
1. Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691-697.
2. Duckworth AD, Clement ND, Aitken SA, Court-Brown CM, McQueen MM. The epidemiology of fractures of the proximal ulna. Injury. 2012;43(3):343-346.
3. Bailey CS, MacDermid J, Patterson SD, King GJ. Outcome of plate fixation of olecranon fractures. J Orthop Trauma. 2001;15(8):542-548.
4. Adler S, Fay GF, Macausland WR Jr. Treatment of olecranon fractures. Indications for excision of the olecranon fragment and repair of the triceps tendon. J Trauma. 1962;2:597-602.
5. Baecher N, Edwards S. Olecranon fractures. J Hand Surg Am. 2013;38(3):593-604.
6. Bell TH, Ferreira LM, McDonald CP, Johnson JA, King GJW. Contribution of the olecranon to elbow stability: an in vitro biomechanical study. J Bone Joint Surg Am. 2010;92(4):949-957.
7. Buijze G, Kloen P. Clinical evaluation of locking compression plate fixation for comminuted olecranon fractures. J Bone Joint Surg Am. 2009;91(10):2416-2420.
8. Cervera-Irimia J, Tomé-Bermejo F, Gómez-Bermejo MA, Holgado-Moreno E, Stratenwerth EG. Treatment of comminuted olecranon fractures with olecranon plate and structural iliac crest graft. Acta Orthop Belg. 2012;78(6):703-707.
9. Edwards SG, Martin BD, Fu RH, et al. Comparison of olecranon plate fixation in osteoporotic bone: do current technologies and designs make a difference? J Orthop Trauma. 2011;25(5):306-311.
10. Erturer RE, Sever C, Sonmez MM, Ozcelik IB, Akman S, Ozturk I. Results of open reduction and plate osteosynthesis in comminuted fracture of the olecranon. J Shoulder Elbow Surg. 2011;20(3):449-454.
11. Estourgie RJ, Tinnemans JG. Treatment of grossly comminuted fractures of the olecranon by excision. Neth J Surg. 1982;34(3):127-129.
12. Fern ED, Brown JN. Olecranon advancement osteotomy in the management of severely comminuted olecranon fractures. Injury. 1993;24(4):267-269.
13. Gordon MJ, Budoff JE, Yeh ML, Luo ZP, Noble PC. Comminuted olecranon fractures: a comparison of plating methods. J Shoulder Elbow Surg. 2006;15(1):94-99.
14. Iannuzzi N, Dahners L. Excision and advancement in the treatment of comminuted olecranon fractures. J Orthop Trauma. 2009;23(3):226-228.
15. Ikeda M, Fukushima Y, Kobayashi Y, Oka Y. Comminuted fractures of the olecranon. Management by bone graft from the iliac crest and multiple tension-band wiring. J Bone Joint Surg Br. 2001;83(6):805-808.
16. McKeever FM, Buck RM. Fracture of the olecranon process of the ulna; treatment by excision of fragment and repair of triceps tendon. JAMA. 1947;135(1):1-5.
17. Rommens PM, Küchle R, Schneider RU, Reuter M. Olecranon fractures in adults: factors influencing outcome. Injury. 2004;35(11):1149-1157.
18. Siebenlist S, Torsiglieri T, Kraus T, Burghardt RD, Stöckle U, Lucke M. Comminuted fractures of the proximal ulna—preliminary results with an anatomically preshaped locking compression plate (LCP) system. Injury. 2010;41(12):1306-1311.
19. Tarallo L, Mugnai R, Adani R, Capra F, Zambianchi F, Catani F. Simple and comminuted displaced olecranon fractures: a clinical comparison between tension band wiring and plate fixation techniques. Arch Orthop Trauma Surg. 2014;134(8):1107-1114.
20. Wang Y, Tao R, Xu H, Cao Y, Zhou Z, Xu S. Mid-term outcomes of contoured plating for comminuted fractures of the olecranon. Orthop Surg. 2011;3(3):176-180.
21. Newman SD, Mauffrey C, Krikler S. Olecranon fractures. Injury. 2009;40(6):575-581.
22. Boyer MI, Galatz LM, Borrelli J, Axelrod TS, Ricci WM. Intra-articular fractures of the upper extremity: new concepts in surgical treatment. Instr Course Lect. 2003;52:591-605.
23. Didonna ML, Fernandez JJ, Lim TH, Hastings H, Cohen MS. Partial olecranon excision: the relationship between triceps insertion site and extension strength of the elbow. J Hand Surg Am. 2003;28(1):117-122.
24. Trumble T, Cornwall R, Budoff J. Core Knowledge in Orthopaedics: Hand, Elbow, and Shoulder. Philadelphia, PA: Mosby; 2006.
25. Simpson NS, Goodman LA, Jupiter JB. Contoured LCDC plating of the proximal ulna. Injury. 1996;27(6):411-417.
26. Ferreira LM, Bell TH, Johnson JA, King GJ. The effect of triceps repair techniques following olecranon excision on elbow stability and extension strength: an in vitro biomechanical study. J Orthop Trauma. 2011;25(7):420-424.
27. Colton CL. Fractures of the olecranon in adults: classification and management. Injury. 1973;5(2):121-129.
28. Hull JR, Owen JR, Fern SE, Wayne JS, Boardman ND 3rd. Role of the coronoid process in varus osteoarticular stability of the elbow. J Shoulder Elbow Surg. 2005;14(4):441-446.
29. Morrey BF, An KN. Stability of the elbow: osseous constraints. J Shoulder Elbow Surg. 2005;14(1 suppl S):174S-178S.
30. Williams G, Ramsey M, Wiesel S. Operative Techniques in Shoulder and Elbow Surgery. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
31. Schneeberger AG, Sadowski MM, Jacob HA. Coronoid process and radial head as posterolateral rotatory stabilizers of the elbow. J Bone Joint Surg Am. 2004;86(5):975-982.
32. Closkey RF, Goode JR, Kirschenbaum D, Cody RP. The role of the coronoid process in elbow stability. A biomechanical analysis of axial loading. J Bone Joint Surg Am. 2000;82(12):1749-1753.
1. Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691-697.
2. Duckworth AD, Clement ND, Aitken SA, Court-Brown CM, McQueen MM. The epidemiology of fractures of the proximal ulna. Injury. 2012;43(3):343-346.
3. Bailey CS, MacDermid J, Patterson SD, King GJ. Outcome of plate fixation of olecranon fractures. J Orthop Trauma. 2001;15(8):542-548.
4. Adler S, Fay GF, Macausland WR Jr. Treatment of olecranon fractures. Indications for excision of the olecranon fragment and repair of the triceps tendon. J Trauma. 1962;2:597-602.
5. Baecher N, Edwards S. Olecranon fractures. J Hand Surg Am. 2013;38(3):593-604.
6. Bell TH, Ferreira LM, McDonald CP, Johnson JA, King GJW. Contribution of the olecranon to elbow stability: an in vitro biomechanical study. J Bone Joint Surg Am. 2010;92(4):949-957.
7. Buijze G, Kloen P. Clinical evaluation of locking compression plate fixation for comminuted olecranon fractures. J Bone Joint Surg Am. 2009;91(10):2416-2420.
8. Cervera-Irimia J, Tomé-Bermejo F, Gómez-Bermejo MA, Holgado-Moreno E, Stratenwerth EG. Treatment of comminuted olecranon fractures with olecranon plate and structural iliac crest graft. Acta Orthop Belg. 2012;78(6):703-707.
9. Edwards SG, Martin BD, Fu RH, et al. Comparison of olecranon plate fixation in osteoporotic bone: do current technologies and designs make a difference? J Orthop Trauma. 2011;25(5):306-311.
10. Erturer RE, Sever C, Sonmez MM, Ozcelik IB, Akman S, Ozturk I. Results of open reduction and plate osteosynthesis in comminuted fracture of the olecranon. J Shoulder Elbow Surg. 2011;20(3):449-454.
11. Estourgie RJ, Tinnemans JG. Treatment of grossly comminuted fractures of the olecranon by excision. Neth J Surg. 1982;34(3):127-129.
12. Fern ED, Brown JN. Olecranon advancement osteotomy in the management of severely comminuted olecranon fractures. Injury. 1993;24(4):267-269.
13. Gordon MJ, Budoff JE, Yeh ML, Luo ZP, Noble PC. Comminuted olecranon fractures: a comparison of plating methods. J Shoulder Elbow Surg. 2006;15(1):94-99.
14. Iannuzzi N, Dahners L. Excision and advancement in the treatment of comminuted olecranon fractures. J Orthop Trauma. 2009;23(3):226-228.
15. Ikeda M, Fukushima Y, Kobayashi Y, Oka Y. Comminuted fractures of the olecranon. Management by bone graft from the iliac crest and multiple tension-band wiring. J Bone Joint Surg Br. 2001;83(6):805-808.
16. McKeever FM, Buck RM. Fracture of the olecranon process of the ulna; treatment by excision of fragment and repair of triceps tendon. JAMA. 1947;135(1):1-5.
17. Rommens PM, Küchle R, Schneider RU, Reuter M. Olecranon fractures in adults: factors influencing outcome. Injury. 2004;35(11):1149-1157.
18. Siebenlist S, Torsiglieri T, Kraus T, Burghardt RD, Stöckle U, Lucke M. Comminuted fractures of the proximal ulna—preliminary results with an anatomically preshaped locking compression plate (LCP) system. Injury. 2010;41(12):1306-1311.
19. Tarallo L, Mugnai R, Adani R, Capra F, Zambianchi F, Catani F. Simple and comminuted displaced olecranon fractures: a clinical comparison between tension band wiring and plate fixation techniques. Arch Orthop Trauma Surg. 2014;134(8):1107-1114.
20. Wang Y, Tao R, Xu H, Cao Y, Zhou Z, Xu S. Mid-term outcomes of contoured plating for comminuted fractures of the olecranon. Orthop Surg. 2011;3(3):176-180.
21. Newman SD, Mauffrey C, Krikler S. Olecranon fractures. Injury. 2009;40(6):575-581.
22. Boyer MI, Galatz LM, Borrelli J, Axelrod TS, Ricci WM. Intra-articular fractures of the upper extremity: new concepts in surgical treatment. Instr Course Lect. 2003;52:591-605.
23. Didonna ML, Fernandez JJ, Lim TH, Hastings H, Cohen MS. Partial olecranon excision: the relationship between triceps insertion site and extension strength of the elbow. J Hand Surg Am. 2003;28(1):117-122.
24. Trumble T, Cornwall R, Budoff J. Core Knowledge in Orthopaedics: Hand, Elbow, and Shoulder. Philadelphia, PA: Mosby; 2006.
25. Simpson NS, Goodman LA, Jupiter JB. Contoured LCDC plating of the proximal ulna. Injury. 1996;27(6):411-417.
26. Ferreira LM, Bell TH, Johnson JA, King GJ. The effect of triceps repair techniques following olecranon excision on elbow stability and extension strength: an in vitro biomechanical study. J Orthop Trauma. 2011;25(7):420-424.
27. Colton CL. Fractures of the olecranon in adults: classification and management. Injury. 1973;5(2):121-129.
28. Hull JR, Owen JR, Fern SE, Wayne JS, Boardman ND 3rd. Role of the coronoid process in varus osteoarticular stability of the elbow. J Shoulder Elbow Surg. 2005;14(4):441-446.
29. Morrey BF, An KN. Stability of the elbow: osseous constraints. J Shoulder Elbow Surg. 2005;14(1 suppl S):174S-178S.
30. Williams G, Ramsey M, Wiesel S. Operative Techniques in Shoulder and Elbow Surgery. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
31. Schneeberger AG, Sadowski MM, Jacob HA. Coronoid process and radial head as posterolateral rotatory stabilizers of the elbow. J Bone Joint Surg Am. 2004;86(5):975-982.
32. Closkey RF, Goode JR, Kirschenbaum D, Cody RP. The role of the coronoid process in elbow stability. A biomechanical analysis of axial loading. J Bone Joint Surg Am. 2000;82(12):1749-1753.
Deep remission or long-term control? Choice is key in early CLL
SAN FRANCISCO – Pursue a deep remission that allows a patient to stay treatment free for some period of time, or go for long-term disease control that might not allow for a drug holiday?
It’s a key decision facing physicians in the frontline setting of chronic lymphocytic leukemia, William Wierda, MD, PhD, said at the annual congress on Hematologic Malignancies held by the National Comprehensive Cancer Network.
For those with del(17p) or TP53 mutations, it’s probably best to aim for durable disease control with ibrutinib or high-dose methylprednisolone, plus an anti-CD20 monoclonal antibody, explained Dr. Wierda, medical director of the department of leukemia at MD Anderson Cancer Center, Houston.
The decision is more uncertain for those who are older or frail, he added.
“This is really where we need to select the option based on what our preference is, what our patient’s preference is, and have an understanding of the durability and toxicities with remission with the oral agent versus the toxicities and responses with regard to the chemoimmunotherapy regimens,” Dr. Wierda explained.
For those who are younger and fit, a chemoimmunotherapy regimen likely makes the most sense for those with a mutated IgHV gene, he said, because those patients have been shown to have a better prognosis on the fludarabine-cyclophosphamide-rituximab (FCR) combination.
Those with an unmutated IgHV gene probably should be approached differently, he added. “I know if they get FCR treatment, they will eventually relapse and progress. So, saving chemoimmunotherapy for later is an important endpoint.”
For relapsed patients who’ve had prior chemoimmunotherapy or who have del(17p) or TP53 mutations, options include ibrutinib, venetoclax with or without rituximab, idelalisib with or without rituximab, high-dose methylprednisolone plus an anti-CD20 monoclonal antibody, or lenalidomide plus an anti-CD20 monoclonal antibody.
For patients who’ve already had prior experience with a BTK inhibitor such as ibrutinib, Dr. Wierda suggested venetoclax, idelalisib with or without rituximab, chemoimmunotherapy if they’ve had no prior treatment, or high-dose methylprednisolone with an anti-CD20 monoclonal antibody.
It’s important to keep in mind ibrutinib’s effectiveness in that setting, Dr. Wierda noted. “You can effectively salvage patients with ibrutinib nearly as effectively as you can in the frontline setting.”
A recent study found that, for patients refractory to a kinase inhibitor, switching to a different kinase inhibitor was better than chemoimmunotherapy combinations. Researchers also found that using venetoclax after ibrutinib failure could be better than idelalisib (Ann Oncol. 2017 May 1;28[5]:1050-6)
Trials underway are testing first-line chemoimmunotherapy regimens to reach minimal residual disease-negativity, Dr. Wierda said, and examining combinations in the sequencing of small-molecule inhibitors for patients who have the unmutated IgHV gene.
“We’re also looking at consolidation strategies and have a definite interest in making progress for Richter’s transformation,” he added, an uncommon phenomenon that, in most cases, involves slow-growing CLL becoming aggressive diffuse large B-cell lymphoma. “We don’t know as much as we should know about it, and we have very few effective therapies for it.”
Dr. Wierda reported financial relationships with AbbVie, Celgene, Genentech, Merck, Novartis, Roche, and other companies.
SAN FRANCISCO – Pursue a deep remission that allows a patient to stay treatment free for some period of time, or go for long-term disease control that might not allow for a drug holiday?
It’s a key decision facing physicians in the frontline setting of chronic lymphocytic leukemia, William Wierda, MD, PhD, said at the annual congress on Hematologic Malignancies held by the National Comprehensive Cancer Network.
For those with del(17p) or TP53 mutations, it’s probably best to aim for durable disease control with ibrutinib or high-dose methylprednisolone, plus an anti-CD20 monoclonal antibody, explained Dr. Wierda, medical director of the department of leukemia at MD Anderson Cancer Center, Houston.
The decision is more uncertain for those who are older or frail, he added.
“This is really where we need to select the option based on what our preference is, what our patient’s preference is, and have an understanding of the durability and toxicities with remission with the oral agent versus the toxicities and responses with regard to the chemoimmunotherapy regimens,” Dr. Wierda explained.
For those who are younger and fit, a chemoimmunotherapy regimen likely makes the most sense for those with a mutated IgHV gene, he said, because those patients have been shown to have a better prognosis on the fludarabine-cyclophosphamide-rituximab (FCR) combination.
Those with an unmutated IgHV gene probably should be approached differently, he added. “I know if they get FCR treatment, they will eventually relapse and progress. So, saving chemoimmunotherapy for later is an important endpoint.”
For relapsed patients who’ve had prior chemoimmunotherapy or who have del(17p) or TP53 mutations, options include ibrutinib, venetoclax with or without rituximab, idelalisib with or without rituximab, high-dose methylprednisolone plus an anti-CD20 monoclonal antibody, or lenalidomide plus an anti-CD20 monoclonal antibody.
For patients who’ve already had prior experience with a BTK inhibitor such as ibrutinib, Dr. Wierda suggested venetoclax, idelalisib with or without rituximab, chemoimmunotherapy if they’ve had no prior treatment, or high-dose methylprednisolone with an anti-CD20 monoclonal antibody.
It’s important to keep in mind ibrutinib’s effectiveness in that setting, Dr. Wierda noted. “You can effectively salvage patients with ibrutinib nearly as effectively as you can in the frontline setting.”
A recent study found that, for patients refractory to a kinase inhibitor, switching to a different kinase inhibitor was better than chemoimmunotherapy combinations. Researchers also found that using venetoclax after ibrutinib failure could be better than idelalisib (Ann Oncol. 2017 May 1;28[5]:1050-6)
Trials underway are testing first-line chemoimmunotherapy regimens to reach minimal residual disease-negativity, Dr. Wierda said, and examining combinations in the sequencing of small-molecule inhibitors for patients who have the unmutated IgHV gene.
“We’re also looking at consolidation strategies and have a definite interest in making progress for Richter’s transformation,” he added, an uncommon phenomenon that, in most cases, involves slow-growing CLL becoming aggressive diffuse large B-cell lymphoma. “We don’t know as much as we should know about it, and we have very few effective therapies for it.”
Dr. Wierda reported financial relationships with AbbVie, Celgene, Genentech, Merck, Novartis, Roche, and other companies.
SAN FRANCISCO – Pursue a deep remission that allows a patient to stay treatment free for some period of time, or go for long-term disease control that might not allow for a drug holiday?
It’s a key decision facing physicians in the frontline setting of chronic lymphocytic leukemia, William Wierda, MD, PhD, said at the annual congress on Hematologic Malignancies held by the National Comprehensive Cancer Network.
For those with del(17p) or TP53 mutations, it’s probably best to aim for durable disease control with ibrutinib or high-dose methylprednisolone, plus an anti-CD20 monoclonal antibody, explained Dr. Wierda, medical director of the department of leukemia at MD Anderson Cancer Center, Houston.
The decision is more uncertain for those who are older or frail, he added.
“This is really where we need to select the option based on what our preference is, what our patient’s preference is, and have an understanding of the durability and toxicities with remission with the oral agent versus the toxicities and responses with regard to the chemoimmunotherapy regimens,” Dr. Wierda explained.
For those who are younger and fit, a chemoimmunotherapy regimen likely makes the most sense for those with a mutated IgHV gene, he said, because those patients have been shown to have a better prognosis on the fludarabine-cyclophosphamide-rituximab (FCR) combination.
Those with an unmutated IgHV gene probably should be approached differently, he added. “I know if they get FCR treatment, they will eventually relapse and progress. So, saving chemoimmunotherapy for later is an important endpoint.”
For relapsed patients who’ve had prior chemoimmunotherapy or who have del(17p) or TP53 mutations, options include ibrutinib, venetoclax with or without rituximab, idelalisib with or without rituximab, high-dose methylprednisolone plus an anti-CD20 monoclonal antibody, or lenalidomide plus an anti-CD20 monoclonal antibody.
For patients who’ve already had prior experience with a BTK inhibitor such as ibrutinib, Dr. Wierda suggested venetoclax, idelalisib with or without rituximab, chemoimmunotherapy if they’ve had no prior treatment, or high-dose methylprednisolone with an anti-CD20 monoclonal antibody.
It’s important to keep in mind ibrutinib’s effectiveness in that setting, Dr. Wierda noted. “You can effectively salvage patients with ibrutinib nearly as effectively as you can in the frontline setting.”
A recent study found that, for patients refractory to a kinase inhibitor, switching to a different kinase inhibitor was better than chemoimmunotherapy combinations. Researchers also found that using venetoclax after ibrutinib failure could be better than idelalisib (Ann Oncol. 2017 May 1;28[5]:1050-6)
Trials underway are testing first-line chemoimmunotherapy regimens to reach minimal residual disease-negativity, Dr. Wierda said, and examining combinations in the sequencing of small-molecule inhibitors for patients who have the unmutated IgHV gene.
“We’re also looking at consolidation strategies and have a definite interest in making progress for Richter’s transformation,” he added, an uncommon phenomenon that, in most cases, involves slow-growing CLL becoming aggressive diffuse large B-cell lymphoma. “We don’t know as much as we should know about it, and we have very few effective therapies for it.”
Dr. Wierda reported financial relationships with AbbVie, Celgene, Genentech, Merck, Novartis, Roche, and other companies.
AT NCCN HEMATOLOGIC MALIGNANCIES CONGRESS
Treating Unstable Distal Radius Fractures With a Nonspanning External Fixation Device: Comparison With Volar Locking Plates in Historical Control Group
Take-Home Points
- Clinical and radiographic outcomes of patients treated with non-spanning external fixation are comparable to those treated with open reduction and internal volar locked plate fixation.
- Non-spanning external fixation can lead to satisfactory outcomes based on the following features: fragment specific fixation, subchondral support, fixed angle strength, limited dissection, distraction/length adjustment, joint distraction avoidance, and ability to perform early rehabilitation.
- Non-spanning external fixation should be considered as a treatment option for complicated unstable comminuted intra-articular distal radius fractures, specifically in the elderly.
In the United States, distal radius fractures (DRFs) are among the most common fractures, comprising about 15% of all extremity fractures.1 With a DRF, the primary treatment goal is anatomical reduction with restoration of radiographic parameters and stable fixation of the fracture to restore wrist function.
This fracture type has a variety of treatment alternatives, including nonoperative closed reduction and casting of stable fractures, open reduction and internal fixation (ORIF) with dorsal or volar locking plates, and external fixation. Optimal surgical management of unstable DRFs remains controversial.2 Closed reduction with percutaneous pinning or external fixation has become less common with a trend toward using volar locking plates for internal fixation.3
External fixation of DRFs traditionally has involved either spanning or simple nonspanning devices. Spanning fixation is particularly useful in open or highly comminuted fractures with an unstable soft-tissue envelope. In the past, nonspanning external fixation typically was reserved for fractures with a noncomminuted extra-articular distal fragment to which several large pins or Kirschner wires (K-wires) could be secured. The Non-Bridging External Fixator (NBX; Nutek Orthopaedics) may be used in cases that traditionally might be treated with locked plating or fragment-specific fixation. Specifically, this device is indicated for comminuted intra-articular DRFs in which bone quality may be less than ideal. The NBX, also suitable in open fractures with a stable soft-tissue envelope, can restore and maintain articular alignment by providing subchondral support and stability with fragment-specific fixation. A key advantage of this type of external fixation is that it involves percutaneous fixation and allows for early postoperative range of motion (ROM).
Numerous studies have found excellent outcomes of treating unstable DRFs with ORIF with volar locking plates.4-6 However, few studies have compared the clinical and radiographic outcomes of ORIF with those of nonspanning external fixation in the treatment of unstable comminuted intra-articular DRFs. Windolf and colleagues7 found that, in cadaveric unstable intra-articular DRFs, nonspanning external fixation with multiplanar K-wires had biomechanical characteristics comparable to those of volar locking plates. Other suitable DRF treatment options have been found: an alternative nonbridging external fixator with multiplanar K-wires (Gradl and colleagues8) and the Cross-Pin Fixation system (A.M. Surgical) (Mirza and colleagues9).
We conducted a study to compare functional and radiographic outcomes of unstable comminuted intra-articular DRFs treated with a nonspanning external fixation device (NBX) with outcomes achieved with volar locking plates in a historical control group.
Materials and Methods
This retrospective case-control study was approved by our Institutional Review Board and conducted at 2 institutions. Included in the study were 25 consecutive patients (2 institutions) who underwent closed reduction and external fixation (CREF) with NBX as treatment for unstable DRFs (diagnosis based on radiographic parameters or inability to maintain acceptable alignment after closed reduction and casting). Of these 25 patients, 11 were available for clinical follow-up and medical records review; the other 14 were not available for followup but had their charts reviewed for radiographic data and treatment details. Six of the 14 patients declined to participate in the study, and the other 8 were lost to follow-up because of nonstandardized follow-up protocols. Patients were excluded from the study if their final follow-up had not occurred, or if it occurred before 6 months. For their participation in clinical follow-up, patients received nominal time compensation and mileage reimbursement through a grant from the NBX manufacturer.
The 25 patients underwent CREF with NBX between November 2008 and March 2013. Indications for external fixation consideration were intra-articular extension or significant comminution in patients with poor soft tissue or in patients who wanted to avoid invasive surgery or a permanent implant. Of the 11 patients who agreed to participate in the study, 7 were women and 4 were men; mean age was 64 years (range, 15-81 years). Of the 14 patients unable to follow up, 11 were women and 3 were men; mean age was 63 years (range, 26-89 years). At the last available follow-up, each of the 25 patients was doing well, was satisfied with treatment received and function regained, and had a healed DRF. In almost every case, the mechanism of injury was a fall onto an outstretched hand; most fractures were type C per AO (Arbeitsgemeinschaft für Osteosynthesefragen) classification (Table 1).
The surgical technique for this nonspanning external fixator involves closed reduction with longitudinal traction using ligamentotaxis to grossly align the fracture fragments, with small adjustments made throughout the procedure. A dorsally placed radiolucent fixator is used with fluoroscopic guidance to percutaneously affix a subchondral raft of smooth bicortical .062-inch K-wires. The fixator’s abundant pin holes allow for each specific distal fragment to be captured by pins that are a part of the external fixation construct. Furthermore, radially based pins that use a side bar allow for a “weave” of fixation. Radial length is then obtained and maintained by attaching the distal complex to proximal pins in the radial diaphysis. After pins are cut and wrist and digits are taken through full ROM to ensure smooth tracking, fluoroscopy is used to confirm final fracture fixation and alignment (Figure 1).
In ideal scenarios with good fixation, patients can begin gentle ROM exercises within 1 week after surgery. This regimen can progress to more aggressive motion exercises and even light strengthening (Figure 2).
The 11 clinical follow-up patients underwent directed clinical examination, including ROM and strength evaluation, by Dr. Dwyer and Dr. Crosby. Follow-up also included completion of questionnaires and review of radiographs.
During the clinical follow-up, a standard goniometer was used to evaluate active ROM (wrist flexion and extension and wrist radial and ulnar deviation, measured down the long axis of the forearm and the index ray), and forearm pronation and supination were measured from the 90° elbow flexion position using the humerus as the reference point with the shoulders in 0° of flexion, abduction, and external rotation. In addition, a calibrated dynamometer (Sammons Preston) was used to measure grip strength (position 3) and key pinch strength, and the average of 3 trials of each strength test was calculated. ROM and strength values were calculated as percentages of the contralateral (uninjured) side, as these ratios are more sensitive in detecting clinical changes.10 A 10% adjustment for dominant hand grip strength in right-handed patients was used for this comparison.11
Union (osseous bridging across fracture site on 2 of 3 views), radial height, radial inclination, and volar tilt were measured on standard posteroanterior and lateral radiographs taken at several points: time of injury, postreduction and/or preoperative, initial postoperative, and final follow-up. All radiographic measurements were independently taken by Dr. Dwyer and Dr. Crosby, who used a digital goniometer and ruler (Siemens Medical Solutions) or, when necessary, manual instruments. Means of the original and independent measurements were used for calculations.
The Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire, the Mayo wrist score, and the patient-rated wrist evaluation were used to assess activities of daily living, pain, and quality of life after surgery. Mayo wrist scores were adjusted for unemployed patients; work status was replaced with return to normal activities.
Complications of surgical treatment were evaluated. Major complications evaluated were loss of reduction, malunion, nonunion, deep infection, neuropathy, and tendon rupture. Minor complication possibilities were transient extensor tendon irritation, superficial infection, and finger stiffness. Also noted were 1 patient who subsequently required another procedure and 7 patients who were immobilized after external fixation removal.
We compared our study group’s outcomes with those of historical control patients who underwent fixation with internal volar locking plates. The 2 groups had similar demographic characteristics. To obtain the historical controls, we used the key words distal, radi*, volar, and plat* in a PubMed search. From the 169 citations found, we removed biomechanical cadaver studies, studies that focused on patients with demographics and fracture types dissimilar from our patient population’s, and studies that focused on special circumstances, such as complications or patient characteristics. Eight studies remained for historical comparison.
Results
Radiographic Outcomes
On the injury radiographs, mean volar tilt was –16.7° (range, 2° to –42°), mean radial inclination was 14.1° (range, –1° to 44°), and mean radial height was 5.3 mm (range, –2 mm to 11 mm). Minor improvement after reduction was noted. All patients had intraoperative or postoperative radiographs with external fixation in place (Figure 3).
On the final (post-fixation removal) radiographs, mean volar tilt was 3.3° (range, –16° to 21°), mean radial inclination was 20.7° (range, 0° to 31°), and mean radial height was 7.5 mm (range, 0 mm to 13 mm). Comparison of the injury and final means revealed correction of ~20° for volar tilt, 6° for radial inclination, and 2 mm for radial height. All but 5 patients had type C fractures (AO classification).
Clinical Outcomes
Eleven patients underwent clinical evaluation (functional assessment, physical examination). Mean DASH score was 11.4 (SD, 10.5; range, 0-27.3), mean Mayo wrist score was 79.0 (SD, 12.2; range, 65-100), and mean patient-rated wrist evaluation was 12.2 (SD, 11.9; range, 0-25.5). There was no statistical difference in DASH scores between this group and the historical control group (Table 3). ROM was measured under active effort. In our group, mean wrist flexion was 69.3° (86% of contralateral side), and mean extension was 64.0° (94%). Mean radial deviation of the wrist was 47.4° (135% of relative normal for patient), and mean ulnar deviation was 29.2° (101%). Mean (SD) pronation was 84.6° (4.7°), and mean (SD) supination was 82.3° (8.5°), or about 100% of contralateral pronosupination.
For each hand, 3 grip strength values and 3 key pinch strength values were obtained. These values were averaged, and the injury and contralateral sides were compared. Mean grip strength was 49.6 pounds (85% of contralateral), and mean key pinch strength was 14.0 pounds (97%).
Complications
Of the 25 patients, 6 (24%) had a pin-tract infection treated with oral antibiotics. One of these infections resulted in the removal of the entire fixator. One (4%) of the 25 patients reported transient hypoesthesia of the dorsal first webspace, and 3 (12%) reported pain at the pin sites.
Although all fractures achieved complete bony union, 1 patient (4%) had a refracture on the same fracture line after a fall within 6 weeks after fixator removal; this refracture was successfully treated with a cast worn for 6 weeks. Of the 3 patients with complete follow-up (27%) who lost reduction with external fixation in place, 2 had radiographic parameters maintained within acceptable limits, and 1 (9%) had a malunion with –16° volar tilt.
Our study patients had no tendon rupture, tendon irritation, or stiffness. By contrast, fixation with volar locking plates has been associated with extensor tendon and flexor tendon injury, flexor pollicis rupture, carpal tunnel syndrome, complex regional pain syndrome, loss of reduction, and hardware failure.19 Flexor pollicis longus ruptures that occur after volar plate fixation of DRFs are often attributed to plate positioning.20-22
Discussion
With volar locking plate internal fixation on the rise, CREF has become less widely used.3 This is especially true for comminuted and intra-articular fractures—most earlier external fixators required either spanning of the wrist or limited fixation in the distal articular fragment. Although many studies have found excellent outcomes of ORIF with volar locking plates in the treatment of unstable DRFs,4,6 few studies have compared volar locking plate ORIF with nonspanning external fixation for unstable comminuted intra-articular DRFs. Both Gradl and colleagues,8 using a nonbridging external fixator with multiplanar K-wires, and Mirza and colleagues,9 using the Cross-Pin Fixation system, found wrist function, quality-of-life, and radiographic outcomes similar to those of volar plate fixation in the treatment of DRFs. A comparative meta-analysis by Margaliot and colleagues17 revealed no superiority of internal fixation over external fixation for unstable DRFs, given the similarity in wrist function, radiographic, and subjective outcomes.
At a mean follow-up of 12.8 months (range, 6-23 months), our retrospective study found that the functional and radiographic outcomes of treating unstable comminuted DRFs with a nonspanning external fixator were similar to those reported in similarly matched control studies. Although followup of >2 years has been shown to be unnecessary,23-25 small differences may have been detected with interval results over these 2 years. The effect of selection bias on our study results should be considered in light of patients’ involvement in selecting fixation type. Our results parallel those of the temporal studies of Rozental and colleagues5 and Wei and colleagues12 (Table 2) while allowing for patients to return to function with limited morbidity and complications, similar to Orbay and Fernandez15 though with a less invasive procedure.
Although we found patient-rated outcome measure values analogous to those of the volar plate fixation group and bridging external fixator group in the study by Wright and colleagues,6 we did not measure intra-articular step-off. Another variable not addressed here was operative time. The nonspanning external fixator treatment that we investigated should undergo further study. A randomized prospective study that includes the additional outcome measures of intra-articular step-off and operative time is warranted.
We found that our study patients, who had their comminuted intra-articular DRFs treated with a nonspanning external fixator, and similar historical control patients, treated with volar locking plate internal fixation, had similar clinical and radiographic outcomes at final follow-up. There was no statistically significant difference in measured outcomes—wrist flexion and extension, radial deviation, pronation and supination, volar tilt, radial height, radial inclination, DASH scores—between the 2 groups. Compared with the historical control group, the external fixator group had significantly more postoperative ulnar deviation.
Given the functional and radiographic outcomes found at final follow-up in this study, we recommend considering a nonspanning external fixator in the treatment of unstable complex comminuted intra-articular DRFs, particularly those that occur in the elderly.
1. Sanders WE. Distal radius fractures. In: Manske PR, ed. Hand Surgery Update. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1996:117-123.
2. Shin EK, Jupiter JB. Current concepts in the management of distal radius fractures. Acta Chir Orthop Traumatol Cech. 2007;74(4):233-246.
3. Koval KJ, Harrast JJ, Anglen JO, Weinstein JN. Fractures of the distal part of the radius. The evolution of practice over time. Where’s the evidence? J Bone Joint Surg Am. 2008;90(9):1855-1861.
4. Sammer DM, Kawamura K, Chung KC. Outcomes using an internal osteotomy and distraction device for corrective osteotomy of distal radius malunions requiring correction in multiple planes. J Hand Surg Am. 2006;31(10):1567-1577.
5. Rozental TD, Blazar PE, Franko OI, Chacko AT, Earp BE, Day CS. Functional outcomes for unstable distal radial fractures treated with open reduction and internal fixation or closed reduction and percutaneous fixation. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(8):1837-1846.
6. Wright TW, Horodyski M, Smith DW. Functional outcome of unstable distal radius fractures: ORIF with a volar fixed-angle tine plate versus external fixation. J Hand Surg Am. 2005;30(2):289-299.
7. Windolf M, Schwieger K, Ockert B, Jupiter JB, Gradl G. A novel non-bridging external fixator construct versus volar angular stable plating for the fixation of intra-articular fractures of the distal radius—a biomechanical study. Injury. 2010;41(2):204-209.
8. Gradl G, Gradl G, Wendt M, Mittlmeier T, Kundt G, Jupiter JB. Non-bridging external fixation employing multiplanar K-wires versus volar locked plating for dorsally displaced fractures of the distal radius. Arch Orthop Trauma Surg. 2013;133(5):595-602.
9. Mirza A, Jupiter JB, Reinhart MK, Meyer P. Fractures of the distal radius treated with cross-pin fixation and a nonbridging external fixator, the CPX system: a preliminary report. J Hand Surg Am. 2009;34(4):603-616.
10. MacDermid JC, Richards RS, Donner A, Bellamy N, Roth JH. Responsiveness of the Short Form-36, Disability of the Arm, Shoulder, and Hand questionnaire, patient-rated wrist evaluation, and physical impairment measurements in evaluating recovery after a distal radius fracture. J Hand Surg Am. 2000;25(2):330-340.
11. Petersen P, Petrick M, Connor H, Conklin D. Grip strength and hand dominance: challenging the 10% rule. Am J Occup Ther. 1989;43(7):444-447.
12. Wei DH, Raizman NM, Bottino CJ, Jobin CM, Strauch RJ, Rosenwasser MP. Unstable distal radial fractures treated with external fixation, a radial column plate, or a volar plate. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1568-1577.
13. Rozental TD, Blazar PE. Functional outcome and complications after volar plating for dorsally displaced, unstable fractures of the distal radius. J Hand Surg Am. 2006;31(3):359-365.
14. Osada D, Kamei S, Masuzaki K, Takai M, Kameda M, Tamai K. Prospective study of distal radius fractures treated with a volar locking plate system. J Hand Surg Am. 2008;33(5):691-700.
15. Orbay JL, Fernandez DL. Volar fixed-angle plate fixation for unstable distal radius fractures in the elderly patient. J Hand Surg Am. 2004;29(1):96-102.
16. Rein S, Schikore H, Schneiders W, Amlang M, Zwipp H. Results of dorsal or volar plate fixation of AO type C3 distal radius fractures: a retrospective study. J Hand Surg Am. 2007;32(7):954-961.
17. Margaliot Z, Haase SC, Kotsis SV, Kim HM, Chung KC. A meta-analysis of outcomes of external fixation versus plate osteosynthesis for unstable distal radius fractures. J Hand Surg Am. 2005;30(6):1185-1199.
18. Anderson RL. Practical Statistics for Analytical Chemists. New York, NY: Van Nostrand Reinhold; 1987.
19. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
20. Cross AW, Schmidt CC. Flexor tendon injuries following locked volar plating of distal radius fractures. J Hand Surg Am. 2008;33(2):164-167.
21. Bell JS, Wollstein R, Citron ND. Rupture of flexor pollicis longus tendon: a complication of volar plating of the distal radius. J Bone Joint Surg Br. 1998;80(2):225-226.
22. Klug RA, Press CM, Gonzalez MH. Rupture of the flexor pollicis longus tendon after volar fixed-angle plating of a distal radius fracture: a case report. J Hand Surg Am. 2007;32(7):984-988.
23. Kreder HJ, Hanel DP, Agel J, et al. Indirect reduction and percutaneous fixation versus open reduction and internal fixation for displaced intra-articular fractures of the distal radius: a randomised, controlled trial. J Bone Joint Surg Br. 2005;87(6):829-836.
24. Catalano LW 3rd, Cole RJ, Gelberman RH, Evanoff BA, Gilula LA, Borrelli J Jr. Displaced intra-articular fractures of the distal aspect of the radius. Long-term results in young adults after open reduction and internal fixation. J Bone Joint Surg Am. 1997;79(9):1290-1302.
25. Goldfarb CA, Rudzki JR, Catalano LW, Hughes M, Borrelli J Jr. Fifteen-year outcome of displaced intra-articular fractures of the distal radius. J Hand Surg Am. 2006;31(4):633-639.
Take-Home Points
- Clinical and radiographic outcomes of patients treated with non-spanning external fixation are comparable to those treated with open reduction and internal volar locked plate fixation.
- Non-spanning external fixation can lead to satisfactory outcomes based on the following features: fragment specific fixation, subchondral support, fixed angle strength, limited dissection, distraction/length adjustment, joint distraction avoidance, and ability to perform early rehabilitation.
- Non-spanning external fixation should be considered as a treatment option for complicated unstable comminuted intra-articular distal radius fractures, specifically in the elderly.
In the United States, distal radius fractures (DRFs) are among the most common fractures, comprising about 15% of all extremity fractures.1 With a DRF, the primary treatment goal is anatomical reduction with restoration of radiographic parameters and stable fixation of the fracture to restore wrist function.
This fracture type has a variety of treatment alternatives, including nonoperative closed reduction and casting of stable fractures, open reduction and internal fixation (ORIF) with dorsal or volar locking plates, and external fixation. Optimal surgical management of unstable DRFs remains controversial.2 Closed reduction with percutaneous pinning or external fixation has become less common with a trend toward using volar locking plates for internal fixation.3
External fixation of DRFs traditionally has involved either spanning or simple nonspanning devices. Spanning fixation is particularly useful in open or highly comminuted fractures with an unstable soft-tissue envelope. In the past, nonspanning external fixation typically was reserved for fractures with a noncomminuted extra-articular distal fragment to which several large pins or Kirschner wires (K-wires) could be secured. The Non-Bridging External Fixator (NBX; Nutek Orthopaedics) may be used in cases that traditionally might be treated with locked plating or fragment-specific fixation. Specifically, this device is indicated for comminuted intra-articular DRFs in which bone quality may be less than ideal. The NBX, also suitable in open fractures with a stable soft-tissue envelope, can restore and maintain articular alignment by providing subchondral support and stability with fragment-specific fixation. A key advantage of this type of external fixation is that it involves percutaneous fixation and allows for early postoperative range of motion (ROM).
Numerous studies have found excellent outcomes of treating unstable DRFs with ORIF with volar locking plates.4-6 However, few studies have compared the clinical and radiographic outcomes of ORIF with those of nonspanning external fixation in the treatment of unstable comminuted intra-articular DRFs. Windolf and colleagues7 found that, in cadaveric unstable intra-articular DRFs, nonspanning external fixation with multiplanar K-wires had biomechanical characteristics comparable to those of volar locking plates. Other suitable DRF treatment options have been found: an alternative nonbridging external fixator with multiplanar K-wires (Gradl and colleagues8) and the Cross-Pin Fixation system (A.M. Surgical) (Mirza and colleagues9).
We conducted a study to compare functional and radiographic outcomes of unstable comminuted intra-articular DRFs treated with a nonspanning external fixation device (NBX) with outcomes achieved with volar locking plates in a historical control group.
Materials and Methods
This retrospective case-control study was approved by our Institutional Review Board and conducted at 2 institutions. Included in the study were 25 consecutive patients (2 institutions) who underwent closed reduction and external fixation (CREF) with NBX as treatment for unstable DRFs (diagnosis based on radiographic parameters or inability to maintain acceptable alignment after closed reduction and casting). Of these 25 patients, 11 were available for clinical follow-up and medical records review; the other 14 were not available for followup but had their charts reviewed for radiographic data and treatment details. Six of the 14 patients declined to participate in the study, and the other 8 were lost to follow-up because of nonstandardized follow-up protocols. Patients were excluded from the study if their final follow-up had not occurred, or if it occurred before 6 months. For their participation in clinical follow-up, patients received nominal time compensation and mileage reimbursement through a grant from the NBX manufacturer.
The 25 patients underwent CREF with NBX between November 2008 and March 2013. Indications for external fixation consideration were intra-articular extension or significant comminution in patients with poor soft tissue or in patients who wanted to avoid invasive surgery or a permanent implant. Of the 11 patients who agreed to participate in the study, 7 were women and 4 were men; mean age was 64 years (range, 15-81 years). Of the 14 patients unable to follow up, 11 were women and 3 were men; mean age was 63 years (range, 26-89 years). At the last available follow-up, each of the 25 patients was doing well, was satisfied with treatment received and function regained, and had a healed DRF. In almost every case, the mechanism of injury was a fall onto an outstretched hand; most fractures were type C per AO (Arbeitsgemeinschaft für Osteosynthesefragen) classification (Table 1).
The surgical technique for this nonspanning external fixator involves closed reduction with longitudinal traction using ligamentotaxis to grossly align the fracture fragments, with small adjustments made throughout the procedure. A dorsally placed radiolucent fixator is used with fluoroscopic guidance to percutaneously affix a subchondral raft of smooth bicortical .062-inch K-wires. The fixator’s abundant pin holes allow for each specific distal fragment to be captured by pins that are a part of the external fixation construct. Furthermore, radially based pins that use a side bar allow for a “weave” of fixation. Radial length is then obtained and maintained by attaching the distal complex to proximal pins in the radial diaphysis. After pins are cut and wrist and digits are taken through full ROM to ensure smooth tracking, fluoroscopy is used to confirm final fracture fixation and alignment (Figure 1).
In ideal scenarios with good fixation, patients can begin gentle ROM exercises within 1 week after surgery. This regimen can progress to more aggressive motion exercises and even light strengthening (Figure 2).
The 11 clinical follow-up patients underwent directed clinical examination, including ROM and strength evaluation, by Dr. Dwyer and Dr. Crosby. Follow-up also included completion of questionnaires and review of radiographs.
During the clinical follow-up, a standard goniometer was used to evaluate active ROM (wrist flexion and extension and wrist radial and ulnar deviation, measured down the long axis of the forearm and the index ray), and forearm pronation and supination were measured from the 90° elbow flexion position using the humerus as the reference point with the shoulders in 0° of flexion, abduction, and external rotation. In addition, a calibrated dynamometer (Sammons Preston) was used to measure grip strength (position 3) and key pinch strength, and the average of 3 trials of each strength test was calculated. ROM and strength values were calculated as percentages of the contralateral (uninjured) side, as these ratios are more sensitive in detecting clinical changes.10 A 10% adjustment for dominant hand grip strength in right-handed patients was used for this comparison.11
Union (osseous bridging across fracture site on 2 of 3 views), radial height, radial inclination, and volar tilt were measured on standard posteroanterior and lateral radiographs taken at several points: time of injury, postreduction and/or preoperative, initial postoperative, and final follow-up. All radiographic measurements were independently taken by Dr. Dwyer and Dr. Crosby, who used a digital goniometer and ruler (Siemens Medical Solutions) or, when necessary, manual instruments. Means of the original and independent measurements were used for calculations.
The Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire, the Mayo wrist score, and the patient-rated wrist evaluation were used to assess activities of daily living, pain, and quality of life after surgery. Mayo wrist scores were adjusted for unemployed patients; work status was replaced with return to normal activities.
Complications of surgical treatment were evaluated. Major complications evaluated were loss of reduction, malunion, nonunion, deep infection, neuropathy, and tendon rupture. Minor complication possibilities were transient extensor tendon irritation, superficial infection, and finger stiffness. Also noted were 1 patient who subsequently required another procedure and 7 patients who were immobilized after external fixation removal.
We compared our study group’s outcomes with those of historical control patients who underwent fixation with internal volar locking plates. The 2 groups had similar demographic characteristics. To obtain the historical controls, we used the key words distal, radi*, volar, and plat* in a PubMed search. From the 169 citations found, we removed biomechanical cadaver studies, studies that focused on patients with demographics and fracture types dissimilar from our patient population’s, and studies that focused on special circumstances, such as complications or patient characteristics. Eight studies remained for historical comparison.
Results
Radiographic Outcomes
On the injury radiographs, mean volar tilt was –16.7° (range, 2° to –42°), mean radial inclination was 14.1° (range, –1° to 44°), and mean radial height was 5.3 mm (range, –2 mm to 11 mm). Minor improvement after reduction was noted. All patients had intraoperative or postoperative radiographs with external fixation in place (Figure 3).
On the final (post-fixation removal) radiographs, mean volar tilt was 3.3° (range, –16° to 21°), mean radial inclination was 20.7° (range, 0° to 31°), and mean radial height was 7.5 mm (range, 0 mm to 13 mm). Comparison of the injury and final means revealed correction of ~20° for volar tilt, 6° for radial inclination, and 2 mm for radial height. All but 5 patients had type C fractures (AO classification).
Clinical Outcomes
Eleven patients underwent clinical evaluation (functional assessment, physical examination). Mean DASH score was 11.4 (SD, 10.5; range, 0-27.3), mean Mayo wrist score was 79.0 (SD, 12.2; range, 65-100), and mean patient-rated wrist evaluation was 12.2 (SD, 11.9; range, 0-25.5). There was no statistical difference in DASH scores between this group and the historical control group (Table 3). ROM was measured under active effort. In our group, mean wrist flexion was 69.3° (86% of contralateral side), and mean extension was 64.0° (94%). Mean radial deviation of the wrist was 47.4° (135% of relative normal for patient), and mean ulnar deviation was 29.2° (101%). Mean (SD) pronation was 84.6° (4.7°), and mean (SD) supination was 82.3° (8.5°), or about 100% of contralateral pronosupination.
For each hand, 3 grip strength values and 3 key pinch strength values were obtained. These values were averaged, and the injury and contralateral sides were compared. Mean grip strength was 49.6 pounds (85% of contralateral), and mean key pinch strength was 14.0 pounds (97%).
Complications
Of the 25 patients, 6 (24%) had a pin-tract infection treated with oral antibiotics. One of these infections resulted in the removal of the entire fixator. One (4%) of the 25 patients reported transient hypoesthesia of the dorsal first webspace, and 3 (12%) reported pain at the pin sites.
Although all fractures achieved complete bony union, 1 patient (4%) had a refracture on the same fracture line after a fall within 6 weeks after fixator removal; this refracture was successfully treated with a cast worn for 6 weeks. Of the 3 patients with complete follow-up (27%) who lost reduction with external fixation in place, 2 had radiographic parameters maintained within acceptable limits, and 1 (9%) had a malunion with –16° volar tilt.
Our study patients had no tendon rupture, tendon irritation, or stiffness. By contrast, fixation with volar locking plates has been associated with extensor tendon and flexor tendon injury, flexor pollicis rupture, carpal tunnel syndrome, complex regional pain syndrome, loss of reduction, and hardware failure.19 Flexor pollicis longus ruptures that occur after volar plate fixation of DRFs are often attributed to plate positioning.20-22
Discussion
With volar locking plate internal fixation on the rise, CREF has become less widely used.3 This is especially true for comminuted and intra-articular fractures—most earlier external fixators required either spanning of the wrist or limited fixation in the distal articular fragment. Although many studies have found excellent outcomes of ORIF with volar locking plates in the treatment of unstable DRFs,4,6 few studies have compared volar locking plate ORIF with nonspanning external fixation for unstable comminuted intra-articular DRFs. Both Gradl and colleagues,8 using a nonbridging external fixator with multiplanar K-wires, and Mirza and colleagues,9 using the Cross-Pin Fixation system, found wrist function, quality-of-life, and radiographic outcomes similar to those of volar plate fixation in the treatment of DRFs. A comparative meta-analysis by Margaliot and colleagues17 revealed no superiority of internal fixation over external fixation for unstable DRFs, given the similarity in wrist function, radiographic, and subjective outcomes.
At a mean follow-up of 12.8 months (range, 6-23 months), our retrospective study found that the functional and radiographic outcomes of treating unstable comminuted DRFs with a nonspanning external fixator were similar to those reported in similarly matched control studies. Although followup of >2 years has been shown to be unnecessary,23-25 small differences may have been detected with interval results over these 2 years. The effect of selection bias on our study results should be considered in light of patients’ involvement in selecting fixation type. Our results parallel those of the temporal studies of Rozental and colleagues5 and Wei and colleagues12 (Table 2) while allowing for patients to return to function with limited morbidity and complications, similar to Orbay and Fernandez15 though with a less invasive procedure.
Although we found patient-rated outcome measure values analogous to those of the volar plate fixation group and bridging external fixator group in the study by Wright and colleagues,6 we did not measure intra-articular step-off. Another variable not addressed here was operative time. The nonspanning external fixator treatment that we investigated should undergo further study. A randomized prospective study that includes the additional outcome measures of intra-articular step-off and operative time is warranted.
We found that our study patients, who had their comminuted intra-articular DRFs treated with a nonspanning external fixator, and similar historical control patients, treated with volar locking plate internal fixation, had similar clinical and radiographic outcomes at final follow-up. There was no statistically significant difference in measured outcomes—wrist flexion and extension, radial deviation, pronation and supination, volar tilt, radial height, radial inclination, DASH scores—between the 2 groups. Compared with the historical control group, the external fixator group had significantly more postoperative ulnar deviation.
Given the functional and radiographic outcomes found at final follow-up in this study, we recommend considering a nonspanning external fixator in the treatment of unstable complex comminuted intra-articular DRFs, particularly those that occur in the elderly.
Take-Home Points
- Clinical and radiographic outcomes of patients treated with non-spanning external fixation are comparable to those treated with open reduction and internal volar locked plate fixation.
- Non-spanning external fixation can lead to satisfactory outcomes based on the following features: fragment specific fixation, subchondral support, fixed angle strength, limited dissection, distraction/length adjustment, joint distraction avoidance, and ability to perform early rehabilitation.
- Non-spanning external fixation should be considered as a treatment option for complicated unstable comminuted intra-articular distal radius fractures, specifically in the elderly.
In the United States, distal radius fractures (DRFs) are among the most common fractures, comprising about 15% of all extremity fractures.1 With a DRF, the primary treatment goal is anatomical reduction with restoration of radiographic parameters and stable fixation of the fracture to restore wrist function.
This fracture type has a variety of treatment alternatives, including nonoperative closed reduction and casting of stable fractures, open reduction and internal fixation (ORIF) with dorsal or volar locking plates, and external fixation. Optimal surgical management of unstable DRFs remains controversial.2 Closed reduction with percutaneous pinning or external fixation has become less common with a trend toward using volar locking plates for internal fixation.3
External fixation of DRFs traditionally has involved either spanning or simple nonspanning devices. Spanning fixation is particularly useful in open or highly comminuted fractures with an unstable soft-tissue envelope. In the past, nonspanning external fixation typically was reserved for fractures with a noncomminuted extra-articular distal fragment to which several large pins or Kirschner wires (K-wires) could be secured. The Non-Bridging External Fixator (NBX; Nutek Orthopaedics) may be used in cases that traditionally might be treated with locked plating or fragment-specific fixation. Specifically, this device is indicated for comminuted intra-articular DRFs in which bone quality may be less than ideal. The NBX, also suitable in open fractures with a stable soft-tissue envelope, can restore and maintain articular alignment by providing subchondral support and stability with fragment-specific fixation. A key advantage of this type of external fixation is that it involves percutaneous fixation and allows for early postoperative range of motion (ROM).
Numerous studies have found excellent outcomes of treating unstable DRFs with ORIF with volar locking plates.4-6 However, few studies have compared the clinical and radiographic outcomes of ORIF with those of nonspanning external fixation in the treatment of unstable comminuted intra-articular DRFs. Windolf and colleagues7 found that, in cadaveric unstable intra-articular DRFs, nonspanning external fixation with multiplanar K-wires had biomechanical characteristics comparable to those of volar locking plates. Other suitable DRF treatment options have been found: an alternative nonbridging external fixator with multiplanar K-wires (Gradl and colleagues8) and the Cross-Pin Fixation system (A.M. Surgical) (Mirza and colleagues9).
We conducted a study to compare functional and radiographic outcomes of unstable comminuted intra-articular DRFs treated with a nonspanning external fixation device (NBX) with outcomes achieved with volar locking plates in a historical control group.
Materials and Methods
This retrospective case-control study was approved by our Institutional Review Board and conducted at 2 institutions. Included in the study were 25 consecutive patients (2 institutions) who underwent closed reduction and external fixation (CREF) with NBX as treatment for unstable DRFs (diagnosis based on radiographic parameters or inability to maintain acceptable alignment after closed reduction and casting). Of these 25 patients, 11 were available for clinical follow-up and medical records review; the other 14 were not available for followup but had their charts reviewed for radiographic data and treatment details. Six of the 14 patients declined to participate in the study, and the other 8 were lost to follow-up because of nonstandardized follow-up protocols. Patients were excluded from the study if their final follow-up had not occurred, or if it occurred before 6 months. For their participation in clinical follow-up, patients received nominal time compensation and mileage reimbursement through a grant from the NBX manufacturer.
The 25 patients underwent CREF with NBX between November 2008 and March 2013. Indications for external fixation consideration were intra-articular extension or significant comminution in patients with poor soft tissue or in patients who wanted to avoid invasive surgery or a permanent implant. Of the 11 patients who agreed to participate in the study, 7 were women and 4 were men; mean age was 64 years (range, 15-81 years). Of the 14 patients unable to follow up, 11 were women and 3 were men; mean age was 63 years (range, 26-89 years). At the last available follow-up, each of the 25 patients was doing well, was satisfied with treatment received and function regained, and had a healed DRF. In almost every case, the mechanism of injury was a fall onto an outstretched hand; most fractures were type C per AO (Arbeitsgemeinschaft für Osteosynthesefragen) classification (Table 1).
The surgical technique for this nonspanning external fixator involves closed reduction with longitudinal traction using ligamentotaxis to grossly align the fracture fragments, with small adjustments made throughout the procedure. A dorsally placed radiolucent fixator is used with fluoroscopic guidance to percutaneously affix a subchondral raft of smooth bicortical .062-inch K-wires. The fixator’s abundant pin holes allow for each specific distal fragment to be captured by pins that are a part of the external fixation construct. Furthermore, radially based pins that use a side bar allow for a “weave” of fixation. Radial length is then obtained and maintained by attaching the distal complex to proximal pins in the radial diaphysis. After pins are cut and wrist and digits are taken through full ROM to ensure smooth tracking, fluoroscopy is used to confirm final fracture fixation and alignment (Figure 1).
In ideal scenarios with good fixation, patients can begin gentle ROM exercises within 1 week after surgery. This regimen can progress to more aggressive motion exercises and even light strengthening (Figure 2).
The 11 clinical follow-up patients underwent directed clinical examination, including ROM and strength evaluation, by Dr. Dwyer and Dr. Crosby. Follow-up also included completion of questionnaires and review of radiographs.
During the clinical follow-up, a standard goniometer was used to evaluate active ROM (wrist flexion and extension and wrist radial and ulnar deviation, measured down the long axis of the forearm and the index ray), and forearm pronation and supination were measured from the 90° elbow flexion position using the humerus as the reference point with the shoulders in 0° of flexion, abduction, and external rotation. In addition, a calibrated dynamometer (Sammons Preston) was used to measure grip strength (position 3) and key pinch strength, and the average of 3 trials of each strength test was calculated. ROM and strength values were calculated as percentages of the contralateral (uninjured) side, as these ratios are more sensitive in detecting clinical changes.10 A 10% adjustment for dominant hand grip strength in right-handed patients was used for this comparison.11
Union (osseous bridging across fracture site on 2 of 3 views), radial height, radial inclination, and volar tilt were measured on standard posteroanterior and lateral radiographs taken at several points: time of injury, postreduction and/or preoperative, initial postoperative, and final follow-up. All radiographic measurements were independently taken by Dr. Dwyer and Dr. Crosby, who used a digital goniometer and ruler (Siemens Medical Solutions) or, when necessary, manual instruments. Means of the original and independent measurements were used for calculations.
The Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire, the Mayo wrist score, and the patient-rated wrist evaluation were used to assess activities of daily living, pain, and quality of life after surgery. Mayo wrist scores were adjusted for unemployed patients; work status was replaced with return to normal activities.
Complications of surgical treatment were evaluated. Major complications evaluated were loss of reduction, malunion, nonunion, deep infection, neuropathy, and tendon rupture. Minor complication possibilities were transient extensor tendon irritation, superficial infection, and finger stiffness. Also noted were 1 patient who subsequently required another procedure and 7 patients who were immobilized after external fixation removal.
We compared our study group’s outcomes with those of historical control patients who underwent fixation with internal volar locking plates. The 2 groups had similar demographic characteristics. To obtain the historical controls, we used the key words distal, radi*, volar, and plat* in a PubMed search. From the 169 citations found, we removed biomechanical cadaver studies, studies that focused on patients with demographics and fracture types dissimilar from our patient population’s, and studies that focused on special circumstances, such as complications or patient characteristics. Eight studies remained for historical comparison.
Results
Radiographic Outcomes
On the injury radiographs, mean volar tilt was –16.7° (range, 2° to –42°), mean radial inclination was 14.1° (range, –1° to 44°), and mean radial height was 5.3 mm (range, –2 mm to 11 mm). Minor improvement after reduction was noted. All patients had intraoperative or postoperative radiographs with external fixation in place (Figure 3).
On the final (post-fixation removal) radiographs, mean volar tilt was 3.3° (range, –16° to 21°), mean radial inclination was 20.7° (range, 0° to 31°), and mean radial height was 7.5 mm (range, 0 mm to 13 mm). Comparison of the injury and final means revealed correction of ~20° for volar tilt, 6° for radial inclination, and 2 mm for radial height. All but 5 patients had type C fractures (AO classification).
Clinical Outcomes
Eleven patients underwent clinical evaluation (functional assessment, physical examination). Mean DASH score was 11.4 (SD, 10.5; range, 0-27.3), mean Mayo wrist score was 79.0 (SD, 12.2; range, 65-100), and mean patient-rated wrist evaluation was 12.2 (SD, 11.9; range, 0-25.5). There was no statistical difference in DASH scores between this group and the historical control group (Table 3). ROM was measured under active effort. In our group, mean wrist flexion was 69.3° (86% of contralateral side), and mean extension was 64.0° (94%). Mean radial deviation of the wrist was 47.4° (135% of relative normal for patient), and mean ulnar deviation was 29.2° (101%). Mean (SD) pronation was 84.6° (4.7°), and mean (SD) supination was 82.3° (8.5°), or about 100% of contralateral pronosupination.
For each hand, 3 grip strength values and 3 key pinch strength values were obtained. These values were averaged, and the injury and contralateral sides were compared. Mean grip strength was 49.6 pounds (85% of contralateral), and mean key pinch strength was 14.0 pounds (97%).
Complications
Of the 25 patients, 6 (24%) had a pin-tract infection treated with oral antibiotics. One of these infections resulted in the removal of the entire fixator. One (4%) of the 25 patients reported transient hypoesthesia of the dorsal first webspace, and 3 (12%) reported pain at the pin sites.
Although all fractures achieved complete bony union, 1 patient (4%) had a refracture on the same fracture line after a fall within 6 weeks after fixator removal; this refracture was successfully treated with a cast worn for 6 weeks. Of the 3 patients with complete follow-up (27%) who lost reduction with external fixation in place, 2 had radiographic parameters maintained within acceptable limits, and 1 (9%) had a malunion with –16° volar tilt.
Our study patients had no tendon rupture, tendon irritation, or stiffness. By contrast, fixation with volar locking plates has been associated with extensor tendon and flexor tendon injury, flexor pollicis rupture, carpal tunnel syndrome, complex regional pain syndrome, loss of reduction, and hardware failure.19 Flexor pollicis longus ruptures that occur after volar plate fixation of DRFs are often attributed to plate positioning.20-22
Discussion
With volar locking plate internal fixation on the rise, CREF has become less widely used.3 This is especially true for comminuted and intra-articular fractures—most earlier external fixators required either spanning of the wrist or limited fixation in the distal articular fragment. Although many studies have found excellent outcomes of ORIF with volar locking plates in the treatment of unstable DRFs,4,6 few studies have compared volar locking plate ORIF with nonspanning external fixation for unstable comminuted intra-articular DRFs. Both Gradl and colleagues,8 using a nonbridging external fixator with multiplanar K-wires, and Mirza and colleagues,9 using the Cross-Pin Fixation system, found wrist function, quality-of-life, and radiographic outcomes similar to those of volar plate fixation in the treatment of DRFs. A comparative meta-analysis by Margaliot and colleagues17 revealed no superiority of internal fixation over external fixation for unstable DRFs, given the similarity in wrist function, radiographic, and subjective outcomes.
At a mean follow-up of 12.8 months (range, 6-23 months), our retrospective study found that the functional and radiographic outcomes of treating unstable comminuted DRFs with a nonspanning external fixator were similar to those reported in similarly matched control studies. Although followup of >2 years has been shown to be unnecessary,23-25 small differences may have been detected with interval results over these 2 years. The effect of selection bias on our study results should be considered in light of patients’ involvement in selecting fixation type. Our results parallel those of the temporal studies of Rozental and colleagues5 and Wei and colleagues12 (Table 2) while allowing for patients to return to function with limited morbidity and complications, similar to Orbay and Fernandez15 though with a less invasive procedure.
Although we found patient-rated outcome measure values analogous to those of the volar plate fixation group and bridging external fixator group in the study by Wright and colleagues,6 we did not measure intra-articular step-off. Another variable not addressed here was operative time. The nonspanning external fixator treatment that we investigated should undergo further study. A randomized prospective study that includes the additional outcome measures of intra-articular step-off and operative time is warranted.
We found that our study patients, who had their comminuted intra-articular DRFs treated with a nonspanning external fixator, and similar historical control patients, treated with volar locking plate internal fixation, had similar clinical and radiographic outcomes at final follow-up. There was no statistically significant difference in measured outcomes—wrist flexion and extension, radial deviation, pronation and supination, volar tilt, radial height, radial inclination, DASH scores—between the 2 groups. Compared with the historical control group, the external fixator group had significantly more postoperative ulnar deviation.
Given the functional and radiographic outcomes found at final follow-up in this study, we recommend considering a nonspanning external fixator in the treatment of unstable complex comminuted intra-articular DRFs, particularly those that occur in the elderly.
1. Sanders WE. Distal radius fractures. In: Manske PR, ed. Hand Surgery Update. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1996:117-123.
2. Shin EK, Jupiter JB. Current concepts in the management of distal radius fractures. Acta Chir Orthop Traumatol Cech. 2007;74(4):233-246.
3. Koval KJ, Harrast JJ, Anglen JO, Weinstein JN. Fractures of the distal part of the radius. The evolution of practice over time. Where’s the evidence? J Bone Joint Surg Am. 2008;90(9):1855-1861.
4. Sammer DM, Kawamura K, Chung KC. Outcomes using an internal osteotomy and distraction device for corrective osteotomy of distal radius malunions requiring correction in multiple planes. J Hand Surg Am. 2006;31(10):1567-1577.
5. Rozental TD, Blazar PE, Franko OI, Chacko AT, Earp BE, Day CS. Functional outcomes for unstable distal radial fractures treated with open reduction and internal fixation or closed reduction and percutaneous fixation. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(8):1837-1846.
6. Wright TW, Horodyski M, Smith DW. Functional outcome of unstable distal radius fractures: ORIF with a volar fixed-angle tine plate versus external fixation. J Hand Surg Am. 2005;30(2):289-299.
7. Windolf M, Schwieger K, Ockert B, Jupiter JB, Gradl G. A novel non-bridging external fixator construct versus volar angular stable plating for the fixation of intra-articular fractures of the distal radius—a biomechanical study. Injury. 2010;41(2):204-209.
8. Gradl G, Gradl G, Wendt M, Mittlmeier T, Kundt G, Jupiter JB. Non-bridging external fixation employing multiplanar K-wires versus volar locked plating for dorsally displaced fractures of the distal radius. Arch Orthop Trauma Surg. 2013;133(5):595-602.
9. Mirza A, Jupiter JB, Reinhart MK, Meyer P. Fractures of the distal radius treated with cross-pin fixation and a nonbridging external fixator, the CPX system: a preliminary report. J Hand Surg Am. 2009;34(4):603-616.
10. MacDermid JC, Richards RS, Donner A, Bellamy N, Roth JH. Responsiveness of the Short Form-36, Disability of the Arm, Shoulder, and Hand questionnaire, patient-rated wrist evaluation, and physical impairment measurements in evaluating recovery after a distal radius fracture. J Hand Surg Am. 2000;25(2):330-340.
11. Petersen P, Petrick M, Connor H, Conklin D. Grip strength and hand dominance: challenging the 10% rule. Am J Occup Ther. 1989;43(7):444-447.
12. Wei DH, Raizman NM, Bottino CJ, Jobin CM, Strauch RJ, Rosenwasser MP. Unstable distal radial fractures treated with external fixation, a radial column plate, or a volar plate. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1568-1577.
13. Rozental TD, Blazar PE. Functional outcome and complications after volar plating for dorsally displaced, unstable fractures of the distal radius. J Hand Surg Am. 2006;31(3):359-365.
14. Osada D, Kamei S, Masuzaki K, Takai M, Kameda M, Tamai K. Prospective study of distal radius fractures treated with a volar locking plate system. J Hand Surg Am. 2008;33(5):691-700.
15. Orbay JL, Fernandez DL. Volar fixed-angle plate fixation for unstable distal radius fractures in the elderly patient. J Hand Surg Am. 2004;29(1):96-102.
16. Rein S, Schikore H, Schneiders W, Amlang M, Zwipp H. Results of dorsal or volar plate fixation of AO type C3 distal radius fractures: a retrospective study. J Hand Surg Am. 2007;32(7):954-961.
17. Margaliot Z, Haase SC, Kotsis SV, Kim HM, Chung KC. A meta-analysis of outcomes of external fixation versus plate osteosynthesis for unstable distal radius fractures. J Hand Surg Am. 2005;30(6):1185-1199.
18. Anderson RL. Practical Statistics for Analytical Chemists. New York, NY: Van Nostrand Reinhold; 1987.
19. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
20. Cross AW, Schmidt CC. Flexor tendon injuries following locked volar plating of distal radius fractures. J Hand Surg Am. 2008;33(2):164-167.
21. Bell JS, Wollstein R, Citron ND. Rupture of flexor pollicis longus tendon: a complication of volar plating of the distal radius. J Bone Joint Surg Br. 1998;80(2):225-226.
22. Klug RA, Press CM, Gonzalez MH. Rupture of the flexor pollicis longus tendon after volar fixed-angle plating of a distal radius fracture: a case report. J Hand Surg Am. 2007;32(7):984-988.
23. Kreder HJ, Hanel DP, Agel J, et al. Indirect reduction and percutaneous fixation versus open reduction and internal fixation for displaced intra-articular fractures of the distal radius: a randomised, controlled trial. J Bone Joint Surg Br. 2005;87(6):829-836.
24. Catalano LW 3rd, Cole RJ, Gelberman RH, Evanoff BA, Gilula LA, Borrelli J Jr. Displaced intra-articular fractures of the distal aspect of the radius. Long-term results in young adults after open reduction and internal fixation. J Bone Joint Surg Am. 1997;79(9):1290-1302.
25. Goldfarb CA, Rudzki JR, Catalano LW, Hughes M, Borrelli J Jr. Fifteen-year outcome of displaced intra-articular fractures of the distal radius. J Hand Surg Am. 2006;31(4):633-639.
1. Sanders WE. Distal radius fractures. In: Manske PR, ed. Hand Surgery Update. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1996:117-123.
2. Shin EK, Jupiter JB. Current concepts in the management of distal radius fractures. Acta Chir Orthop Traumatol Cech. 2007;74(4):233-246.
3. Koval KJ, Harrast JJ, Anglen JO, Weinstein JN. Fractures of the distal part of the radius. The evolution of practice over time. Where’s the evidence? J Bone Joint Surg Am. 2008;90(9):1855-1861.
4. Sammer DM, Kawamura K, Chung KC. Outcomes using an internal osteotomy and distraction device for corrective osteotomy of distal radius malunions requiring correction in multiple planes. J Hand Surg Am. 2006;31(10):1567-1577.
5. Rozental TD, Blazar PE, Franko OI, Chacko AT, Earp BE, Day CS. Functional outcomes for unstable distal radial fractures treated with open reduction and internal fixation or closed reduction and percutaneous fixation. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(8):1837-1846.
6. Wright TW, Horodyski M, Smith DW. Functional outcome of unstable distal radius fractures: ORIF with a volar fixed-angle tine plate versus external fixation. J Hand Surg Am. 2005;30(2):289-299.
7. Windolf M, Schwieger K, Ockert B, Jupiter JB, Gradl G. A novel non-bridging external fixator construct versus volar angular stable plating for the fixation of intra-articular fractures of the distal radius—a biomechanical study. Injury. 2010;41(2):204-209.
8. Gradl G, Gradl G, Wendt M, Mittlmeier T, Kundt G, Jupiter JB. Non-bridging external fixation employing multiplanar K-wires versus volar locked plating for dorsally displaced fractures of the distal radius. Arch Orthop Trauma Surg. 2013;133(5):595-602.
9. Mirza A, Jupiter JB, Reinhart MK, Meyer P. Fractures of the distal radius treated with cross-pin fixation and a nonbridging external fixator, the CPX system: a preliminary report. J Hand Surg Am. 2009;34(4):603-616.
10. MacDermid JC, Richards RS, Donner A, Bellamy N, Roth JH. Responsiveness of the Short Form-36, Disability of the Arm, Shoulder, and Hand questionnaire, patient-rated wrist evaluation, and physical impairment measurements in evaluating recovery after a distal radius fracture. J Hand Surg Am. 2000;25(2):330-340.
11. Petersen P, Petrick M, Connor H, Conklin D. Grip strength and hand dominance: challenging the 10% rule. Am J Occup Ther. 1989;43(7):444-447.
12. Wei DH, Raizman NM, Bottino CJ, Jobin CM, Strauch RJ, Rosenwasser MP. Unstable distal radial fractures treated with external fixation, a radial column plate, or a volar plate. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1568-1577.
13. Rozental TD, Blazar PE. Functional outcome and complications after volar plating for dorsally displaced, unstable fractures of the distal radius. J Hand Surg Am. 2006;31(3):359-365.
14. Osada D, Kamei S, Masuzaki K, Takai M, Kameda M, Tamai K. Prospective study of distal radius fractures treated with a volar locking plate system. J Hand Surg Am. 2008;33(5):691-700.
15. Orbay JL, Fernandez DL. Volar fixed-angle plate fixation for unstable distal radius fractures in the elderly patient. J Hand Surg Am. 2004;29(1):96-102.
16. Rein S, Schikore H, Schneiders W, Amlang M, Zwipp H. Results of dorsal or volar plate fixation of AO type C3 distal radius fractures: a retrospective study. J Hand Surg Am. 2007;32(7):954-961.
17. Margaliot Z, Haase SC, Kotsis SV, Kim HM, Chung KC. A meta-analysis of outcomes of external fixation versus plate osteosynthesis for unstable distal radius fractures. J Hand Surg Am. 2005;30(6):1185-1199.
18. Anderson RL. Practical Statistics for Analytical Chemists. New York, NY: Van Nostrand Reinhold; 1987.
19. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
20. Cross AW, Schmidt CC. Flexor tendon injuries following locked volar plating of distal radius fractures. J Hand Surg Am. 2008;33(2):164-167.
21. Bell JS, Wollstein R, Citron ND. Rupture of flexor pollicis longus tendon: a complication of volar plating of the distal radius. J Bone Joint Surg Br. 1998;80(2):225-226.
22. Klug RA, Press CM, Gonzalez MH. Rupture of the flexor pollicis longus tendon after volar fixed-angle plating of a distal radius fracture: a case report. J Hand Surg Am. 2007;32(7):984-988.
23. Kreder HJ, Hanel DP, Agel J, et al. Indirect reduction and percutaneous fixation versus open reduction and internal fixation for displaced intra-articular fractures of the distal radius: a randomised, controlled trial. J Bone Joint Surg Br. 2005;87(6):829-836.
24. Catalano LW 3rd, Cole RJ, Gelberman RH, Evanoff BA, Gilula LA, Borrelli J Jr. Displaced intra-articular fractures of the distal aspect of the radius. Long-term results in young adults after open reduction and internal fixation. J Bone Joint Surg Am. 1997;79(9):1290-1302.
25. Goldfarb CA, Rudzki JR, Catalano LW, Hughes M, Borrelli J Jr. Fifteen-year outcome of displaced intra-articular fractures of the distal radius. J Hand Surg Am. 2006;31(4):633-639.
VIDEO: SBO in bariatric patient can mean internal herniation
SAN DIEGO – You get a call from the emergency department at 3 a.m. A 48-year-old woman is presenting with fever, nausea, vomiting, and left upper quadrant pain. And the patient says she had a gastric bypass procedure 3 years ago.
Time to panic? Not necessarily, but things can, and occasionally do, go bad for these patients, even if they have had a long-stable bypass, Jennifer Choi, MD, FACS, said in a video interview at the annual clinical congress of the American College of Surgeons.
“We do have to remember that our bariatric surgery patients can develop all of the same kinds of problems that anyone else can,” said Dr. Choi, a general surgeon at Indiana University, Indianapolis. “Appendicitis, diverticulitis, abdominal wall hernias, and other common things do happen.”
In her book, though, a patient with a gastric bypass who presents with a combination of small-bowel obstruction and pain has an internal herniation until proven otherwise.
“The symptoms can be subtle, and they can either have been building for several weeks or have an acute onset,” Dr. Choi said. These can include nausea, dry heaves, bloating, or nonbilious vomiting. Pain is typically located in the left upper quadrant or mid-back, especially if the hernia is located at one of the two most common spots: Petersen’s defect. This is the point where the biliopancreatic loop tends to slip under the alimentary loop and become trapped. Imaging will show a typical swirling of blood vessels around the herniation, accompanied by dilated small bowel at the point of obstruction.
At the other common herniation point, the site of the jejunojejunostomy, the alimentary loop can slip under the biliopancreatic loop. On imaging, jejunum will be seen in the upper right quadrant.
Both of these can be surgical emergencies, Dr. Choi said. “This needs an operation sooner, rather than later. It needs to be reduced and repaired.”
She typically performs this laparoscopically, but said that some surgeons prefer an open approach, which is a perfectly sound option.
“The key to a successful repair is to start at the ileocecal valve, because it is consistent and fixed, and run the bowel from distal to proximal to reduce the internal hernia. Then close the defect with a permanent suture,” she said.
Chylous ascites is almost always present in these cases because the herniation traumatizes the lymphatic system, Dr. Choi added. “It doesn’t all always have to be removed at the time of surgery, but just be aware that this is definitely something we do see, almost all the time in bariatric patients with these internal hernias.”
Dr. Choi had no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @alz_gal
SAN DIEGO – You get a call from the emergency department at 3 a.m. A 48-year-old woman is presenting with fever, nausea, vomiting, and left upper quadrant pain. And the patient says she had a gastric bypass procedure 3 years ago.
Time to panic? Not necessarily, but things can, and occasionally do, go bad for these patients, even if they have had a long-stable bypass, Jennifer Choi, MD, FACS, said in a video interview at the annual clinical congress of the American College of Surgeons.
“We do have to remember that our bariatric surgery patients can develop all of the same kinds of problems that anyone else can,” said Dr. Choi, a general surgeon at Indiana University, Indianapolis. “Appendicitis, diverticulitis, abdominal wall hernias, and other common things do happen.”
In her book, though, a patient with a gastric bypass who presents with a combination of small-bowel obstruction and pain has an internal herniation until proven otherwise.
“The symptoms can be subtle, and they can either have been building for several weeks or have an acute onset,” Dr. Choi said. These can include nausea, dry heaves, bloating, or nonbilious vomiting. Pain is typically located in the left upper quadrant or mid-back, especially if the hernia is located at one of the two most common spots: Petersen’s defect. This is the point where the biliopancreatic loop tends to slip under the alimentary loop and become trapped. Imaging will show a typical swirling of blood vessels around the herniation, accompanied by dilated small bowel at the point of obstruction.
At the other common herniation point, the site of the jejunojejunostomy, the alimentary loop can slip under the biliopancreatic loop. On imaging, jejunum will be seen in the upper right quadrant.
Both of these can be surgical emergencies, Dr. Choi said. “This needs an operation sooner, rather than later. It needs to be reduced and repaired.”
She typically performs this laparoscopically, but said that some surgeons prefer an open approach, which is a perfectly sound option.
“The key to a successful repair is to start at the ileocecal valve, because it is consistent and fixed, and run the bowel from distal to proximal to reduce the internal hernia. Then close the defect with a permanent suture,” she said.
Chylous ascites is almost always present in these cases because the herniation traumatizes the lymphatic system, Dr. Choi added. “It doesn’t all always have to be removed at the time of surgery, but just be aware that this is definitely something we do see, almost all the time in bariatric patients with these internal hernias.”
Dr. Choi had no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @alz_gal
SAN DIEGO – You get a call from the emergency department at 3 a.m. A 48-year-old woman is presenting with fever, nausea, vomiting, and left upper quadrant pain. And the patient says she had a gastric bypass procedure 3 years ago.
Time to panic? Not necessarily, but things can, and occasionally do, go bad for these patients, even if they have had a long-stable bypass, Jennifer Choi, MD, FACS, said in a video interview at the annual clinical congress of the American College of Surgeons.
“We do have to remember that our bariatric surgery patients can develop all of the same kinds of problems that anyone else can,” said Dr. Choi, a general surgeon at Indiana University, Indianapolis. “Appendicitis, diverticulitis, abdominal wall hernias, and other common things do happen.”
In her book, though, a patient with a gastric bypass who presents with a combination of small-bowel obstruction and pain has an internal herniation until proven otherwise.
“The symptoms can be subtle, and they can either have been building for several weeks or have an acute onset,” Dr. Choi said. These can include nausea, dry heaves, bloating, or nonbilious vomiting. Pain is typically located in the left upper quadrant or mid-back, especially if the hernia is located at one of the two most common spots: Petersen’s defect. This is the point where the biliopancreatic loop tends to slip under the alimentary loop and become trapped. Imaging will show a typical swirling of blood vessels around the herniation, accompanied by dilated small bowel at the point of obstruction.
At the other common herniation point, the site of the jejunojejunostomy, the alimentary loop can slip under the biliopancreatic loop. On imaging, jejunum will be seen in the upper right quadrant.
Both of these can be surgical emergencies, Dr. Choi said. “This needs an operation sooner, rather than later. It needs to be reduced and repaired.”
She typically performs this laparoscopically, but said that some surgeons prefer an open approach, which is a perfectly sound option.
“The key to a successful repair is to start at the ileocecal valve, because it is consistent and fixed, and run the bowel from distal to proximal to reduce the internal hernia. Then close the defect with a permanent suture,” she said.
Chylous ascites is almost always present in these cases because the herniation traumatizes the lymphatic system, Dr. Choi added. “It doesn’t all always have to be removed at the time of surgery, but just be aware that this is definitely something we do see, almost all the time in bariatric patients with these internal hernias.”
Dr. Choi had no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @alz_gal
AT THE ACS Clinical Congress