User login
U.S. judge orders Philips to cease AED manufacturing
A U.S. District Judge has ordered Philips North America, as well as two Philips officers, to cease manufacturing and distribution of automatic external defibrillators (AEDs) until they can comply with federal regulations in a consent decree, according to a statement from the Food and Drug Administration.
In a complaint filed with the decree, Philips North America in Andover, Mass., which operates as Philips Medical Systems and Philips Healthcare, sold compromised automatic external defibrillators and Q-CPR Meters in violation of current Federal Food, Drug and Cosmetic (FD&C) Act good manufacturing practice requirements. The injunction also applies to Carla Kriwet and Ojas Buch of the Patient Care and Monitoring Solutions business group, according to the statement.
“AEDs are life-saving tools and are designed to be used by the general public or professionals in an emergency. People rely on these devices to work when needed. By not adequately addressing corrective and preventative actions with their AEDs in a timely manner, Philips distributed adulterated products that put people at risk,” Melinda Plaisier, associate commissioner for regulatory affairs at the FDA said in the press release.
In an Oct. 11 statement, Carla Kriwet, head of Connected Care & Health Informatics at Royal Philips, said “We are committed to delivering high-quality, innovative products and solutions, and we take this matter very seriously. We are fully prepared to fulfill the terms of the decree, and we hope to resume the suspended defibrillator production in the course of 2018.”
Ms. Kriwet added that in the past several years Philips has made significant investments in its quality procedures and leadership.
The company recommends that Philips defibrillators currently in use by customers should remain in use, and should not be taken out of service as Philips has no reason to believe they pose a risk to patients.
A U.S. District Judge has ordered Philips North America, as well as two Philips officers, to cease manufacturing and distribution of automatic external defibrillators (AEDs) until they can comply with federal regulations in a consent decree, according to a statement from the Food and Drug Administration.
In a complaint filed with the decree, Philips North America in Andover, Mass., which operates as Philips Medical Systems and Philips Healthcare, sold compromised automatic external defibrillators and Q-CPR Meters in violation of current Federal Food, Drug and Cosmetic (FD&C) Act good manufacturing practice requirements. The injunction also applies to Carla Kriwet and Ojas Buch of the Patient Care and Monitoring Solutions business group, according to the statement.
“AEDs are life-saving tools and are designed to be used by the general public or professionals in an emergency. People rely on these devices to work when needed. By not adequately addressing corrective and preventative actions with their AEDs in a timely manner, Philips distributed adulterated products that put people at risk,” Melinda Plaisier, associate commissioner for regulatory affairs at the FDA said in the press release.
In an Oct. 11 statement, Carla Kriwet, head of Connected Care & Health Informatics at Royal Philips, said “We are committed to delivering high-quality, innovative products and solutions, and we take this matter very seriously. We are fully prepared to fulfill the terms of the decree, and we hope to resume the suspended defibrillator production in the course of 2018.”
Ms. Kriwet added that in the past several years Philips has made significant investments in its quality procedures and leadership.
The company recommends that Philips defibrillators currently in use by customers should remain in use, and should not be taken out of service as Philips has no reason to believe they pose a risk to patients.
A U.S. District Judge has ordered Philips North America, as well as two Philips officers, to cease manufacturing and distribution of automatic external defibrillators (AEDs) until they can comply with federal regulations in a consent decree, according to a statement from the Food and Drug Administration.
In a complaint filed with the decree, Philips North America in Andover, Mass., which operates as Philips Medical Systems and Philips Healthcare, sold compromised automatic external defibrillators and Q-CPR Meters in violation of current Federal Food, Drug and Cosmetic (FD&C) Act good manufacturing practice requirements. The injunction also applies to Carla Kriwet and Ojas Buch of the Patient Care and Monitoring Solutions business group, according to the statement.
“AEDs are life-saving tools and are designed to be used by the general public or professionals in an emergency. People rely on these devices to work when needed. By not adequately addressing corrective and preventative actions with their AEDs in a timely manner, Philips distributed adulterated products that put people at risk,” Melinda Plaisier, associate commissioner for regulatory affairs at the FDA said in the press release.
In an Oct. 11 statement, Carla Kriwet, head of Connected Care & Health Informatics at Royal Philips, said “We are committed to delivering high-quality, innovative products and solutions, and we take this matter very seriously. We are fully prepared to fulfill the terms of the decree, and we hope to resume the suspended defibrillator production in the course of 2018.”
Ms. Kriwet added that in the past several years Philips has made significant investments in its quality procedures and leadership.
The company recommends that Philips defibrillators currently in use by customers should remain in use, and should not be taken out of service as Philips has no reason to believe they pose a risk to patients.
The race is on for a Zika vaccine
WASHINGTON – A DNA vaccine developed at the National Institute of Allergy and Infectious Diseases Vaccine Research Center – one of five National Institutes of Health Zika vaccine candidates – has entered phase 2 testing in a trial underway in Brazil, Peru, Ecuador, Mexico, and Texas.
“The DNA vaccine is a simple 21st century way of developing vaccines that I think will become one of the major [methods of the future] for emerging infections, as opposed to growing a virus and inactivating or attenuating it,” Anthony S. Fauci, MD, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America. With Zika “this is the vaccine that is ahead of all the others.”
Will it be possible to test efficacy, given the declining prevalence of Zika across the Americas, and will it be too late to prevent more disease? Dr. Fauci, director of NIAID, said that’s a concern, and that an accelerated approval based on a bridging of animal efficacy data with human safety and immunogenicity data might be possible.
The Southern hemisphere is “entering their summer, so it’s conceivable there will be an uptick in Zika. … We’ll just need to wait and see,” he said.
Sexual transmission
The Zika virus is part of a “long line of arboviruses that have threatened us in the Americas,” but infection with the organism is “the first – and may be the only – arthropod-borne or mosquito-borne infection that is also sexually transmitted,” Dr. Fauci said.
Sexual contact as an important mode of viral transmission “has been documented very clearly through a number of studies in which individuals clearly had no exposure to mosquitoes but were in fact a sexual partner of someone who got infected,” he said. And recent research suggests that the “female reproductive tract is a preferentially permissive site for Zika replication, which adds to the concern about sexual transmission.”
He cited a study published in July 2017 in PLOS Pathogens in which the Zika virus was found to preferentially replicate in the reproductive tract of female rhesus macaques who received vaginal inoculations of the virus.
Zika virus was “detected in the reproductive tract before it was detected in plasma, and replication levels in the reproductive tract did not reflect viral levels in other parts of the body,” according to the author summary. The kinetics of virus replication and dissemination after intravaginal inoculation were markedly different from what was previously seen in macaques infected with the Zika virus by subcutaneous infection, the report noted (PLOS Pathogens 13[7]:e1006537).
Dr. Fauci briefly described this and several other studies and findings that he said exemplify growing knowledge of the infection. He pointed to a prospective observational study that documents episodes of oligospermia in 15 men who presented with infection in 2016 in the French Caribbean (Lancet Infect Dis. 2017;17:1200-08).
Sperm counts fell in some of the study participants by about 50% between days 7 and 60 post infection, and the counts “recovered somewhat” by day 120. “We’re still following patients in prospective studies to determine if there’s a long-term effect in men,” he said.
In the meantime, he said, research in mice has shown that “without a doubt, Zika infection damages the testes,” Dr. Fauci said, noting that the mouse model is proving to be a good model for studying Zika’s effects. “They become oligospermic and have testicular atrophy.”
Maternal-fetal transmission
Regarding maternal-fetal transmission, there’s evidence that placental trophoblasts “are exquisitely permissive for Zika virus replication,” he said.
In another recent study, primary human placental trophoblasts from nonexposed donors were found to be infected by the Zika virus ex-vivo and permissive for viral RNA replication, compared with dengue virus, a fellow flavivirus (Sci Rep. 2017;7:41389. doi: 10.1038/srep41389).
However, Yoel Sadovsky, MD, who also presented at the meeting, explained that his lab’s ex-vivo studies show that primary human trophoblasts have inherent resistance to a number of viruses – and that trophoblasts are refractory to direct infection with the Zika virus. “We don’t think the trophoblasts are very permissive at all,” he said.
Moreover, trophoblasts appear to confer their antiviral effects to other nontrophoblast cells by releasing a particular type of interferon – type III interferon IFN1 – and by delivering certain micro-RNAs (C19MC miRNAs) that are packaged within trophoblast-derived nanovesicles called exosomes, said Dr. Sadovsky, scientific director of the Magee-Womens Research Institute and professor of ob.gyn., reproductive sciences, microbiology, and molecular genetics at the University of Pittsburgh.
WASHINGTON – A DNA vaccine developed at the National Institute of Allergy and Infectious Diseases Vaccine Research Center – one of five National Institutes of Health Zika vaccine candidates – has entered phase 2 testing in a trial underway in Brazil, Peru, Ecuador, Mexico, and Texas.
“The DNA vaccine is a simple 21st century way of developing vaccines that I think will become one of the major [methods of the future] for emerging infections, as opposed to growing a virus and inactivating or attenuating it,” Anthony S. Fauci, MD, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America. With Zika “this is the vaccine that is ahead of all the others.”
Will it be possible to test efficacy, given the declining prevalence of Zika across the Americas, and will it be too late to prevent more disease? Dr. Fauci, director of NIAID, said that’s a concern, and that an accelerated approval based on a bridging of animal efficacy data with human safety and immunogenicity data might be possible.
The Southern hemisphere is “entering their summer, so it’s conceivable there will be an uptick in Zika. … We’ll just need to wait and see,” he said.
Sexual transmission
The Zika virus is part of a “long line of arboviruses that have threatened us in the Americas,” but infection with the organism is “the first – and may be the only – arthropod-borne or mosquito-borne infection that is also sexually transmitted,” Dr. Fauci said.
Sexual contact as an important mode of viral transmission “has been documented very clearly through a number of studies in which individuals clearly had no exposure to mosquitoes but were in fact a sexual partner of someone who got infected,” he said. And recent research suggests that the “female reproductive tract is a preferentially permissive site for Zika replication, which adds to the concern about sexual transmission.”
He cited a study published in July 2017 in PLOS Pathogens in which the Zika virus was found to preferentially replicate in the reproductive tract of female rhesus macaques who received vaginal inoculations of the virus.
Zika virus was “detected in the reproductive tract before it was detected in plasma, and replication levels in the reproductive tract did not reflect viral levels in other parts of the body,” according to the author summary. The kinetics of virus replication and dissemination after intravaginal inoculation were markedly different from what was previously seen in macaques infected with the Zika virus by subcutaneous infection, the report noted (PLOS Pathogens 13[7]:e1006537).
Dr. Fauci briefly described this and several other studies and findings that he said exemplify growing knowledge of the infection. He pointed to a prospective observational study that documents episodes of oligospermia in 15 men who presented with infection in 2016 in the French Caribbean (Lancet Infect Dis. 2017;17:1200-08).
Sperm counts fell in some of the study participants by about 50% between days 7 and 60 post infection, and the counts “recovered somewhat” by day 120. “We’re still following patients in prospective studies to determine if there’s a long-term effect in men,” he said.
In the meantime, he said, research in mice has shown that “without a doubt, Zika infection damages the testes,” Dr. Fauci said, noting that the mouse model is proving to be a good model for studying Zika’s effects. “They become oligospermic and have testicular atrophy.”
Maternal-fetal transmission
Regarding maternal-fetal transmission, there’s evidence that placental trophoblasts “are exquisitely permissive for Zika virus replication,” he said.
In another recent study, primary human placental trophoblasts from nonexposed donors were found to be infected by the Zika virus ex-vivo and permissive for viral RNA replication, compared with dengue virus, a fellow flavivirus (Sci Rep. 2017;7:41389. doi: 10.1038/srep41389).
However, Yoel Sadovsky, MD, who also presented at the meeting, explained that his lab’s ex-vivo studies show that primary human trophoblasts have inherent resistance to a number of viruses – and that trophoblasts are refractory to direct infection with the Zika virus. “We don’t think the trophoblasts are very permissive at all,” he said.
Moreover, trophoblasts appear to confer their antiviral effects to other nontrophoblast cells by releasing a particular type of interferon – type III interferon IFN1 – and by delivering certain micro-RNAs (C19MC miRNAs) that are packaged within trophoblast-derived nanovesicles called exosomes, said Dr. Sadovsky, scientific director of the Magee-Womens Research Institute and professor of ob.gyn., reproductive sciences, microbiology, and molecular genetics at the University of Pittsburgh.
WASHINGTON – A DNA vaccine developed at the National Institute of Allergy and Infectious Diseases Vaccine Research Center – one of five National Institutes of Health Zika vaccine candidates – has entered phase 2 testing in a trial underway in Brazil, Peru, Ecuador, Mexico, and Texas.
“The DNA vaccine is a simple 21st century way of developing vaccines that I think will become one of the major [methods of the future] for emerging infections, as opposed to growing a virus and inactivating or attenuating it,” Anthony S. Fauci, MD, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America. With Zika “this is the vaccine that is ahead of all the others.”
Will it be possible to test efficacy, given the declining prevalence of Zika across the Americas, and will it be too late to prevent more disease? Dr. Fauci, director of NIAID, said that’s a concern, and that an accelerated approval based on a bridging of animal efficacy data with human safety and immunogenicity data might be possible.
The Southern hemisphere is “entering their summer, so it’s conceivable there will be an uptick in Zika. … We’ll just need to wait and see,” he said.
Sexual transmission
The Zika virus is part of a “long line of arboviruses that have threatened us in the Americas,” but infection with the organism is “the first – and may be the only – arthropod-borne or mosquito-borne infection that is also sexually transmitted,” Dr. Fauci said.
Sexual contact as an important mode of viral transmission “has been documented very clearly through a number of studies in which individuals clearly had no exposure to mosquitoes but were in fact a sexual partner of someone who got infected,” he said. And recent research suggests that the “female reproductive tract is a preferentially permissive site for Zika replication, which adds to the concern about sexual transmission.”
He cited a study published in July 2017 in PLOS Pathogens in which the Zika virus was found to preferentially replicate in the reproductive tract of female rhesus macaques who received vaginal inoculations of the virus.
Zika virus was “detected in the reproductive tract before it was detected in plasma, and replication levels in the reproductive tract did not reflect viral levels in other parts of the body,” according to the author summary. The kinetics of virus replication and dissemination after intravaginal inoculation were markedly different from what was previously seen in macaques infected with the Zika virus by subcutaneous infection, the report noted (PLOS Pathogens 13[7]:e1006537).
Dr. Fauci briefly described this and several other studies and findings that he said exemplify growing knowledge of the infection. He pointed to a prospective observational study that documents episodes of oligospermia in 15 men who presented with infection in 2016 in the French Caribbean (Lancet Infect Dis. 2017;17:1200-08).
Sperm counts fell in some of the study participants by about 50% between days 7 and 60 post infection, and the counts “recovered somewhat” by day 120. “We’re still following patients in prospective studies to determine if there’s a long-term effect in men,” he said.
In the meantime, he said, research in mice has shown that “without a doubt, Zika infection damages the testes,” Dr. Fauci said, noting that the mouse model is proving to be a good model for studying Zika’s effects. “They become oligospermic and have testicular atrophy.”
Maternal-fetal transmission
Regarding maternal-fetal transmission, there’s evidence that placental trophoblasts “are exquisitely permissive for Zika virus replication,” he said.
In another recent study, primary human placental trophoblasts from nonexposed donors were found to be infected by the Zika virus ex-vivo and permissive for viral RNA replication, compared with dengue virus, a fellow flavivirus (Sci Rep. 2017;7:41389. doi: 10.1038/srep41389).
However, Yoel Sadovsky, MD, who also presented at the meeting, explained that his lab’s ex-vivo studies show that primary human trophoblasts have inherent resistance to a number of viruses – and that trophoblasts are refractory to direct infection with the Zika virus. “We don’t think the trophoblasts are very permissive at all,” he said.
Moreover, trophoblasts appear to confer their antiviral effects to other nontrophoblast cells by releasing a particular type of interferon – type III interferon IFN1 – and by delivering certain micro-RNAs (C19MC miRNAs) that are packaged within trophoblast-derived nanovesicles called exosomes, said Dr. Sadovsky, scientific director of the Magee-Womens Research Institute and professor of ob.gyn., reproductive sciences, microbiology, and molecular genetics at the University of Pittsburgh.
AT DPSG-NA 2017
FDA: Ultrasound surgical devices are contraindicated for uterine fibroid removal
, according to the Food and Drug Administration.
These devices have the potential to disseminate undetected tumor tissue, and there are no proven preoperative screening methods for detecting uterine sarcoma in uterine fibroids that otherwise appear to be benign, FDA officials wrote in a guidance document issued Oct. 30. The FDA is calling for new product labeling for these devices within 120 days.
The devices deliver ultrasonic energy through an oscillating tip, which leads to tissue fragmentation. This can lead to tissue dissemination that cannot be eliminated by suction/aspiration. In advanced cancers, the risk of dissemination may be outweighed by the benefits of the devices, including the debulking effect with no thermal collateral damage, as well as avoidance of the need for organ removal or resection.
The devices are currently labeled in a way that suggests they could be used in removing uterine fibroids, though the agency said that it is not aware that they are used for this purpose.
The agency recommended against their use in uterine fibroids, in part because there are alternative treatment options available. But the American College of Obstetricians and Gynecologists has challenged that assertion. When the FDA first issued a draft notice of the labeling guidance in November 2016, ACOG commented that abdominal hysterectomy is the alternative treatment option and is associated with significant morbidity and mortality beyond that seen with minimally invasive techniques. ACOG urged the FDA to prioritize informed consent and the weighing of risks and benefits.
Ultrasonic surgical aspirator devices are used for a wide range of surgical applications, but the recommendations apply specifically to laparoscopic surgery, open surgery, and gynecologic surgery.
, according to the Food and Drug Administration.
These devices have the potential to disseminate undetected tumor tissue, and there are no proven preoperative screening methods for detecting uterine sarcoma in uterine fibroids that otherwise appear to be benign, FDA officials wrote in a guidance document issued Oct. 30. The FDA is calling for new product labeling for these devices within 120 days.
The devices deliver ultrasonic energy through an oscillating tip, which leads to tissue fragmentation. This can lead to tissue dissemination that cannot be eliminated by suction/aspiration. In advanced cancers, the risk of dissemination may be outweighed by the benefits of the devices, including the debulking effect with no thermal collateral damage, as well as avoidance of the need for organ removal or resection.
The devices are currently labeled in a way that suggests they could be used in removing uterine fibroids, though the agency said that it is not aware that they are used for this purpose.
The agency recommended against their use in uterine fibroids, in part because there are alternative treatment options available. But the American College of Obstetricians and Gynecologists has challenged that assertion. When the FDA first issued a draft notice of the labeling guidance in November 2016, ACOG commented that abdominal hysterectomy is the alternative treatment option and is associated with significant morbidity and mortality beyond that seen with minimally invasive techniques. ACOG urged the FDA to prioritize informed consent and the weighing of risks and benefits.
Ultrasonic surgical aspirator devices are used for a wide range of surgical applications, but the recommendations apply specifically to laparoscopic surgery, open surgery, and gynecologic surgery.
, according to the Food and Drug Administration.
These devices have the potential to disseminate undetected tumor tissue, and there are no proven preoperative screening methods for detecting uterine sarcoma in uterine fibroids that otherwise appear to be benign, FDA officials wrote in a guidance document issued Oct. 30. The FDA is calling for new product labeling for these devices within 120 days.
The devices deliver ultrasonic energy through an oscillating tip, which leads to tissue fragmentation. This can lead to tissue dissemination that cannot be eliminated by suction/aspiration. In advanced cancers, the risk of dissemination may be outweighed by the benefits of the devices, including the debulking effect with no thermal collateral damage, as well as avoidance of the need for organ removal or resection.
The devices are currently labeled in a way that suggests they could be used in removing uterine fibroids, though the agency said that it is not aware that they are used for this purpose.
The agency recommended against their use in uterine fibroids, in part because there are alternative treatment options available. But the American College of Obstetricians and Gynecologists has challenged that assertion. When the FDA first issued a draft notice of the labeling guidance in November 2016, ACOG commented that abdominal hysterectomy is the alternative treatment option and is associated with significant morbidity and mortality beyond that seen with minimally invasive techniques. ACOG urged the FDA to prioritize informed consent and the weighing of risks and benefits.
Ultrasonic surgical aspirator devices are used for a wide range of surgical applications, but the recommendations apply specifically to laparoscopic surgery, open surgery, and gynecologic surgery.
Ulcerative Sarcoidosis: A Prototypical Presentation and Review
Sarcoidosis is a multisystem granulomatous disorder of unknown etiology that primarily affects the lungs and lymphatic system but also may involve the skin, eyes, liver, spleen, muscles, bones, and nervous system.1 Cutaneous symptoms of sarcoidosis occur in approximately 25% of patients and are classified as specific and nonspecific, with specific lesions demonstrating noncaseating granuloma formation, which is typical of sarcoidosis.2 Nonspecific lesions primarily include erythema nodosum and calcinosis cutis. Specific lesions commonly present as reddish brown infiltrated plaques that may be annular, polycyclic, or serpiginous.1,3 They also may appear as yellowish brown or violaceous maculopapular lesions. However, specific lesions may present in a wide variety of morphologies, most often papules, nodules, subcutaneous infiltrates, and lupus pernio.4 Additionally, atypical cutaneous manifestations of sarcoidosis include erythroderma; scarring alopecia; nail dystrophy; and verrucous, ichthyosiform, psoriasiform, hypopigmented, or ulcerative skin lesions.3-5 Among these many potential clinical presentations, ulcerative sarcoidosis is quite uncommon.
We report a case of a patient who presented with classic clinical and histopathological findings of ulcerative sarcoidosis to highlight the prototypical presentation of a rare condition. We also review 34 additional cases of ulcerative sarcoidosis published in the English-language literature based on a PubMed search of articles indexed for MEDLINE using the term ulcerative sarcoid.4-32 Analyzing this historical information, the scope of this unusual form of cutaneous sarcoidosis can be better understood, recognized, and treated. Although current standard-of-care treatments are most often successful, there is a paucity of definitive clinical trials to justify and verify comparative therapeutic efficacy.
Case Report
A 49-year-old black man with known pulmonary sarcoidosis, idiopathic (human immunodeficiency virus–negative) CD4 depletion syndrome, and chronic kidney disease presented with persistent bilateral ulcers of the legs of 1 month’s duration. The lesions first appeared as multiple “dark spots” on the legs. After the patient applied homemade aloe vera extract under occlusion for 1 to 2 days, the lesions became painful and began to ulcerate approximately 3 months prior to presentation. The patient applied a combination of a topical first aid antibiotic ointment, Epsom salts, and hydrogen peroxide without any improvement. A current review of systems was negative.
The patient’s medical history was notable for sarcoidosis diagnosed more than 10 years prior. During this time, he had intermittently been treated elsewhere with low-dose oral prednisone (5 mg once daily), hydroxychloroquine (200 mg twice daily), and an inhaled steroid as needed. He had a history of human immunodeficiency virus–negative, idiopathic CD4 depletion syndrome, which had been complicated by cryptococcal meningitis 7 years prior to presentation. He also had renal insufficiency, with baseline creatinine levels ranging from 1.4 to 1.7 mg/dL (reference range, 0.6–1.2 mg/dL). There was no personal or family history of known or suspected inflammatory bowel disease.
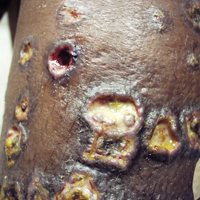
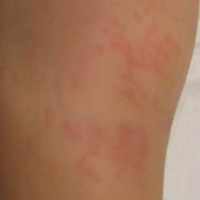
On physical examination, numerous discrete, coalescing, punched out–appearing ulcerations with foul-smelling, greenish yellow, purulent drainage were present bilaterally on the legs (Figure 1). The ulcers had a rolled border with a moderate amount of seemingly nonviable necrotic tissue. A number of hyperpigmented round papules, patches, and plaques also were present on the proximal legs. Laboratory evaluation revealed a CD4 count of 151 cc/mm3 (reference range, 500–1600 cc/mm3) and mildly elevated calcium of 10.7 mg/dL (reference range, 8.2–10.2 mg/dL).
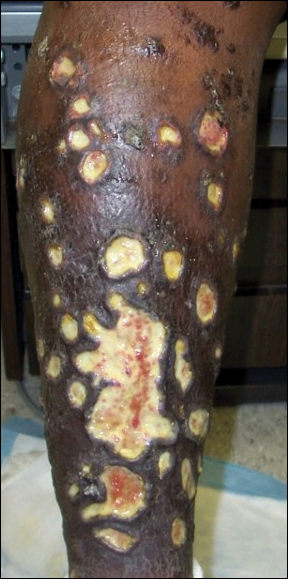
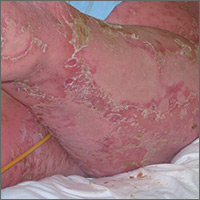
Aerobic, anaerobic, mycobacterial, and fungal cultures of the purulent exudate were obtained. Given a high suspicion for secondary infection of the exogenous wound sites, doxycycline (100 mg twice daily) and topical mupir-ocin were initiated. Gram stain revealed few to moderate polymorphonuclear cells and many gram-positive cocci in pairs, chains, and clusters, along with many gram-negative rods. Bacterial culture grew Pseudomonas aeruginosa, Enterococcus species group G streptococci, and methicillin-resistant Staphylococcus aureus–positive staphylococci. Ciprofloxacin (500 mg twice daily) was then initiated, but the ulcers showed absolutely no clinical improvement and in fact worsened both in number and depth (Figure 2) over subsequent clinic visits during the next 3 months, even after amoxicillin (500 mg 3 times daily) was added. The patient was admitted for treatment with intravenous antibiotics after additional wound cultures revealed fluoroquinolone-resistant Pseudomonas.
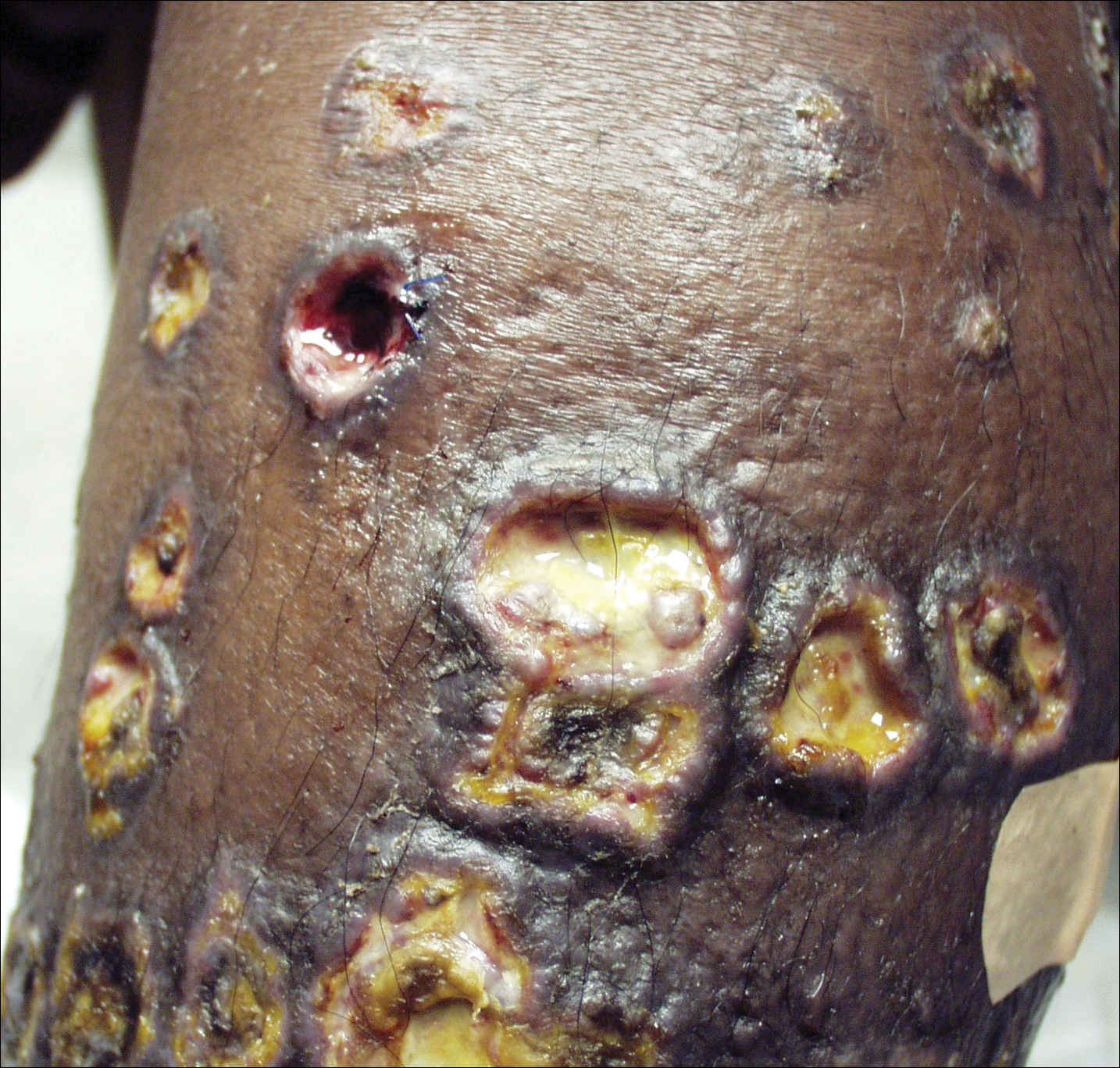
Punch biopsies of the ulcers showed nonspecific acute inflammation and tissue necrosis in the active ulcers with nonnecrotizing granulomatous inflammation extending into the deep dermis, with many Langerhans-type giant cells present in the palpable ulcer borders (Figure 3). Neither birefringent particles nor asteroid bodies were observed. Tissue Gram stains did not reveal evidence of bacterial infection. Special stains for acid-fast and fungal organisms (ie, periodic acid–Schiff, Gomori methenamine-silver, Fite, acid-fast bacilli) were similarly negative. Tissue cultures obtained on deep biopsy revealed only rare colonies of P aeruginosa and no isolates on anaerobic, mycobacterial, or fungal cultures. Polymerase chain reaction for mycobacteria and common endemic fungi also was negative. In the absence of infection and considering his history of known sarcoidosis, these histologic features were consistent with ulcerative sarcoidosis. The patient was started on prednisone (60 mg once daily) and hydroxychloroquine (200 mg twice daily). The prednisone was tapered to 20 mg once daily over a 2-year period, at which point 90% of the ulcers had healed. He was continued on hydroxychloroquine at the initial dose, and at a 3-year follow-up his ulcers had healed completely without relapse.
Comment
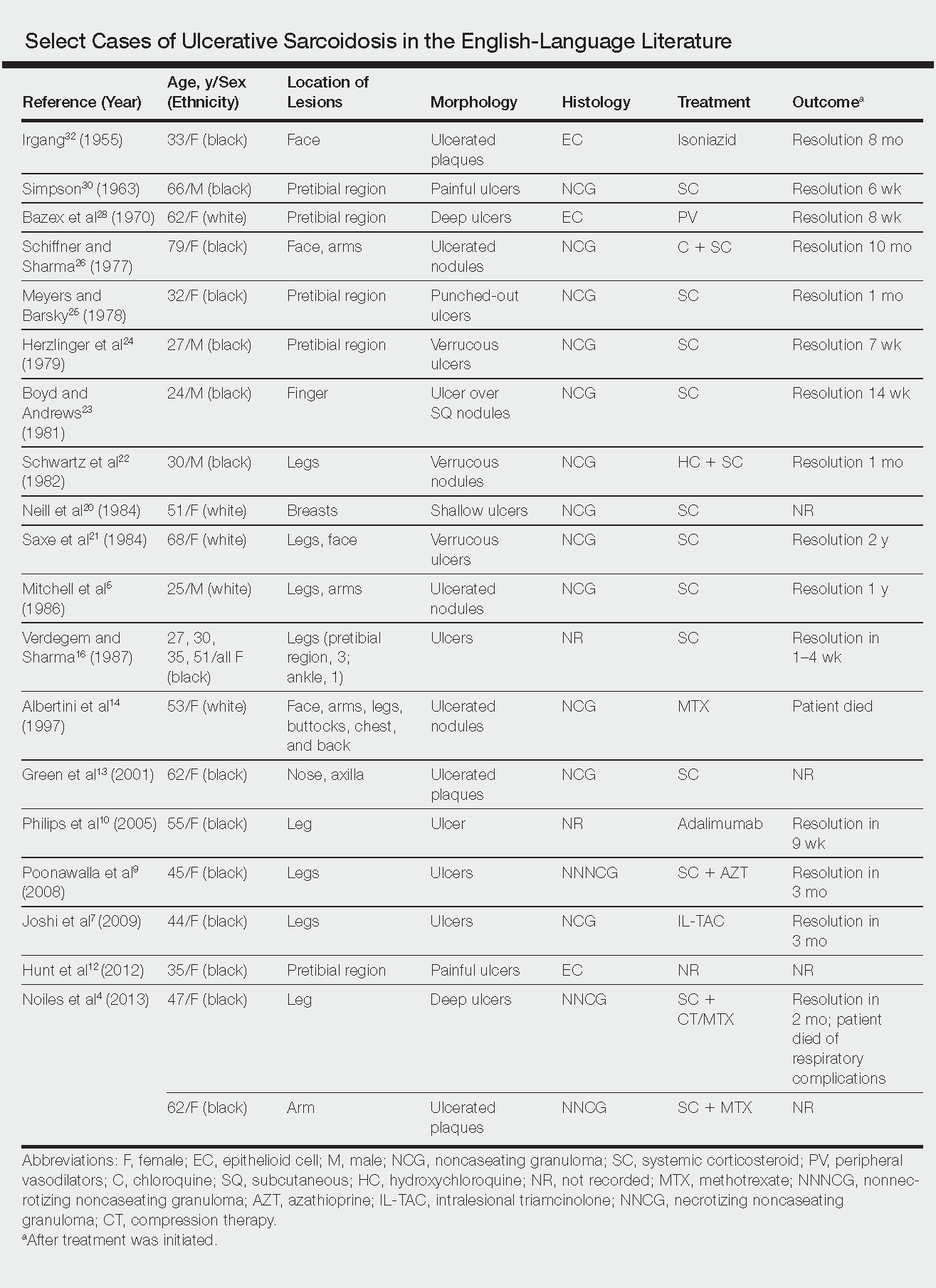
Ulcerative sarcoidosis is rare, seen worldwide in only 5% of patients with cutaneous sarcoidosis.33 However, cases have been encountered worldwide, with reports emanating from Japan, China, Germany, France, and Russia, among others.6,34-55 We reviewed 34 cases from the English-language literature based on a PubMed search of articles indexed for MEDLINE using the term ulcerative sarcoid and examined patient demographics, clinical presentation, histological findings, treatment type, and outcome. Key references are presented in the Table. Disease prevalence previously has been estimated as being 3-times more common in women than men1; in our literature review, we found a female to male ratio of 3.25 to 1. Additionally, ulcerative sarcoidosis is reported to be twice as common in black versus white individuals.33 In our literature review, when race was reported, 66% (21/32) of patients were black. Disease prevalence has been reported to peak at 20 to 40 years of age.3 In this review, the average age of presentation was 45 years (age range, 24–79 years).
Ulceration may arise de novo but more commonly arises in preexisting scars or cutaneous lesions. There are 2 distinct patterns seen in ulcerative sarcoidosis.4 The first is characterized by ulceration within necrotic yellow plaques.2 The second pattern is characterized by violaceous nodules arising in an annular confluent pattern that eventually ulcerate.4 This presentation commonly mimics or may be mimicked by multiple disease states, including sporotrichosis, tuberculosis, stasis dermatitis with venous ulceration, and even metastatic breast cancer.7,46,55,56 Regardless of presentation, the legs are the most common location of ulcer formation.1,33 In our review, 85% (29/34) of cases presented with involvement of the legs, including our own case. Other locations of ulcer formation have included the face, arms, trunk, and genital area.
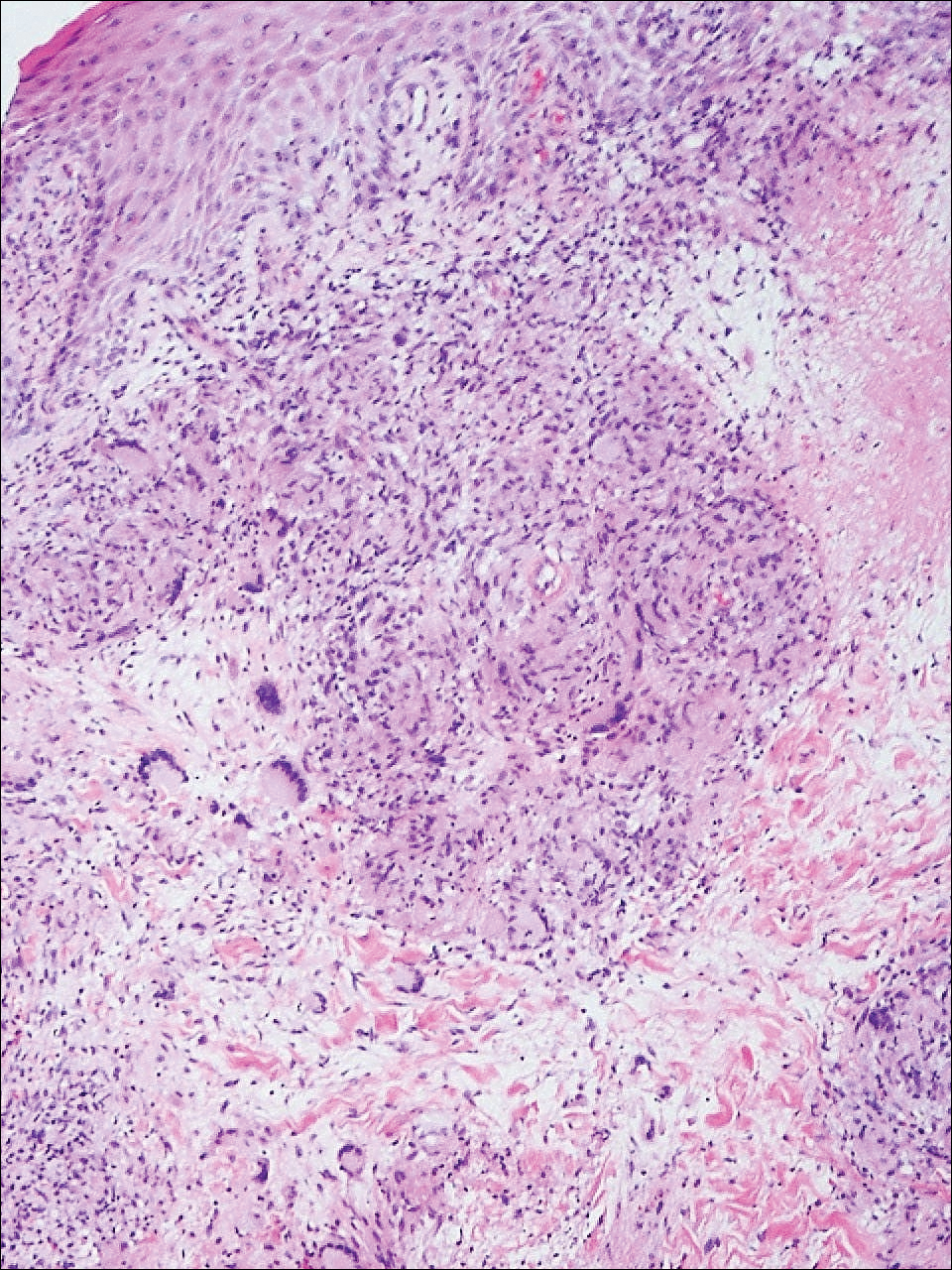
On histologic examination of ulcerative sarcoidosis, epithelioid granulomas composed of multinucleated giant cells, histiocytes, and scant numbers of lymphocytes are present.1,3 These formations are the noncaseating granulomas typical of sarcoidosis (Table). All of the cases in our review of the literature were described as either a collection of epithelioid granulomas with giant cell formation or noncaseating granulomas. There also have been reports of atypical features including necrotizing granulomas and granulomatous vasculitis.4,8,9,50 The histologic differential diagnosis in this case also would primarily include an infectious granulomatous process and less so an id reaction, rosacea, a paraneoplastic phenomenon, foreign body granulomas, and metastatic Crohn disease. The presence of ulceration, the large number of lesions, and the anatomic distribution help rule out most of these alternate diagnostic considerations. Diligent extensive workup was done in our patient to insure it was not an infection.
The goals of treatment include symptomatic relief, improvement in objective parameters of disease activity, and prevention of disease progression and subsequent disability.33,57 Fortunately, the majority of sarcoidosis patients with cutaneous symptoms achieve full recovery within months to years.33 Our literature review indicated that 81% (22/27) of patients with ulcerative lesions experienced full resolution within 1 year of treatment. Of those that did not (19% [5/27]), the patients were either lost to follow-up or died from other complications of sarcoidosis.
The widely accepted standard therapy for cutaneous sarcoidosis includes topical, intralesional, and systemic corticosteroids; antimalarials; and methotrexate.33,57 Steroids and methotrexate act by suppressing granuloma formation, while antimalarials prevent antigen presentation (presumably part of the pathogenesis).33 For mild to moderate disease, topical and intralesional steroids may be all that is necessary.33,57 Systemic steroids are used for disfiguring, destructive, and widespread lesions that have been refractory to local and other systemic therapies.33,57 Steroids are tapered gradually depending on the patient’s response, as it is common for patients to relapse below a certain dose.33,57 Antimalarials (chloroquine or hydroxychloroquine) and methotrexate are considered adjunct treatments for patients who are either steroid unresponsive or who are unable to tolerate corticosteroid treatment due to adverse events.33,57
Standard therapy is complicated by the side effects of treatment. Use of corticosteroids may lead to gastrointestinal tract upset, increased appetite, mood disturbances, impaired wound healing, hyperglycemia, hypertension, cushingoid features, and acne.57 Antimalarials can cause nausea, anorexia, and agranulocytosis, and chloroquine therapy in particular can lead to blurred vision, corneal deposits, and central retinopathy.33,57 Methotrexate is associated with hematologic, gastrointestinal tract, pulmonary, and hepatic toxicities well known to most practitioners.
Because of the variable clinical response of patients to standard therapy and their associated toxicities, other treatment options have been used including pentoxifylline, tetracyclines, isotretinoin, leflunomide, thalidomide, infliximab, adalimumab, allopurinol, and the pulsed dye or CO2 laser.10,33,57 In nonhealing ulcers, split-thickness grafting and a bilayered bioengineered skin substitute have been used with good results in conjunction with ongoing systemic therapy.11,47 Additionally, nanoparticle silver burn paste has been used successfully, with resolution of ulcers within 2 weeks in the Chinese literature.53
All of these treatment recommendations are based on historically accepted modalities. Controlled trials with longitudinal follow-up are needed to provide justification for the current standard of care.34
- Howard A, White CR Jr. Non-infectious granulomas. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Spain: Elsevier; 2008:1421-1435.
- Doherty CB, Rosen T. Evidence-based therapy for cutaneous sarcoidosis. Drugs. 2008;68:1361-1383.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Noiles K, Beleznay K, Crawford RI, et al. Sarcoidosis can present with necrotizing granulomas histologically: two cases of ulcerated sarcoidosis and review of the literature. J Cutan Med Surg. 2013;17:377-378.
- Mitchell IC, Sweatman MC, Rustin MH, et al. Ulcerative and hypopigmented sarcoidosis. J Am Acad Dermatol. 1986;15:1062-1065.
- Yoo SS, Mimouni D, Nikolskaia OV, et al. Clinicopathologic features of ulcerative-atrophic sarcoidosis. Int J Dermatol. 2004;43:108-112.
- Joshi SS, Romanelli R, Kirsner RS. Sarcoidosis mimicking a venous ulcer: a case report. Ostomy Wound Manage. 2009;55:46-48.
- Petri M, Barr E, Cho K, et al. Overlap of granulomatous vasculitis and sarcoidosis: presentation with uveitis, eosinophilia, leg ulcers, sinusitis and past foot drop. J Rheumatol. 1988;15:1171-1173.
- Poonawalla T, Colome-Grimmer MI, Kelly B. Ulcerative sarcoidosis in the legs with granulomatous vasculitis. Clin Exp Dermatol. 2008;33:282-286.
- Philips MA, Lynch J, Azmi FH. Ulcerative sarcoidosis responding to adalimumab. J Am Acad Dermatol. 2005;53:917.
- Collison DW, Novice F, Banse L, et al. Split-thickness skin grafting in extensive ulcerative sarcoidosis. J Dermatol Surg Oncol. 1989;15:679-683.
- Hunt RD, Gonzalez ME, Robinson M, et al. Ulcerative sarcoidosis. Dermatol Online J. 2012;18:29.
- Green JJ, Lawrence N, Heymann WR. Generalized ulcerative sarcoidosis induced by therapy with the flashlamp-pumped pulsed dye. Arch Dermatol. 2001;137:507-508.
- Albertini JG, Tyler W, Miller OF. Ulcerative sarcoidosis. case report and review of the literature. Arch Dermatol. 1997;133:215-219.
- Thomas J, Williams DW. Peritoneal involvement and ulcerative skin plaques in sarcoidosis: a case report. Sarcoidosis. 1989;6:161-162.
- Verdegem TD, Sharma OP. Cutaneous ulcers in sarcoidosis. Arch Dermatol. 1987;123:1531-1534.
- Gupta AK, Haberman HF, From GL, et al. Sarcoidosis with extensive cutaneous ulceration. unusual clinical presentation. Dermatologica. 1987;174:135-139.
- Hruza GJ, Kerdel FA. Generalized atrophic sarcoidosis with ulcerations. Arch Dermatol. 1986;122:320-322.
- Muhlemann MF, Walker NP, Tan LB, et al. Elephantine sarcoidosis presenting as ulcerating lymphoedema. J R Soc Med. 1985;78:260-261.
- Neill SM, Smith NP, Eady RA. Ulcerative sarcoidosis: a rare manifestation of a common disease. Clin Exp Dermatol. 1984;9:277-279.
- Saxe N, Benatar SR, Bok L, et al. Sarcoidosis with leg ulcers and annular facial lesions. Arch Dermatol. 1984;120:93-96.
- Schwartz RA, Robertson DB, Tierney LM, et al. Generalized ulcerative sarcoidosis. Arch Dermatol. 1982;118:931-933.
- Boyd RE, Andrews BS. Sarcoidosis presenting as cutaneous ulceration, subcutaneous nodules and chronic arthritis. J Rheumatol. 1981;8:311-316.
- Herzlinger DC, Marland AM, Barr RJ. Verrucous ulcerative skin lesions in sarcoidosis. an unusual clinical presentation. Cutis. 1979;23:569-572.
- Meyers M, Barsky S. Ulcerative sarcoidosis. Arch Dermatol. 1978;114:447.
- Schiffner J, Sharma OP. Ulcerative sarcoidosis. report of an unusual case. Arch Dermatol. 1977;113:676-677.
- Williamson DM. Sarcoidosis with atrophic lesions and ulcers of the legs. Br J Dermatol. 1971;84:92-93.
- Bazex A, Dupre A, Christol B, et al. Sarcoidosis with atrophic lesions and ulcers and the presence in some sarcoid granulomata of orceinophil fibres. Br J Dermatol. 1970;83:255-262.
- Brodkin RH. Leg ulcers. a report of two cases caused by sarcoidosis. Acta Derm Venereol. 1969;49:584-587.
- Simpson JR. Sarcoidosis with erythrodermia and ulceration. Br J Dermatol. 1963;75:193-198.
- Irgang S. Ulcerative cutaneous lesion in sarcoidosis; report of a case with clinical resemblance to lupus vulgaris. Harlem Hosp Bull. 1956;8:134-139.
- Irgang S. Ulcerative cutaneous lesions in sarcoidosis; report of a case with clinical resemblance to papulonecrotic tuberculide. Br J Dermatol. 1955;67:255-260.
- Hoffman MD. Atypical ulcers. Dermatol Ther. 2013;26:222-235.
- Hopf B, Krebs A. Ulcera cruris as a rare manifestation of sarcoidosis. Dermatologica. 1974;113:55-62.
- Metz J, Hartmann A, Hautkr Z. Ulcerative form of skin sarcoidosis. Z Hautkr. 1977;52:890-896.
- Berenbeĭn BA, Malygina LA, Tiutiunnikova IA. Ulcerative form of skin sarcoidosis [in Russian]. Vestn Dermatol Venerol. 1984;4:50-53.
- Takahashi N, Hoshino M, Takase T, et al. A case of ulcerative sarcoidosis [in Japanese]. Nihon Hifuka Gakkai Zasshi. 1985;95:1049-1054.
- Schamroth JM. Sarcoidosis with severe extensive skin ulceration. Int J Dermatol. 1985;24:451-452.
- Porteau L, Dromer C, Le Guennec P, et al. Ulcer lesions in sarcoidosis: apropos of a case [in French]. Ann Med Interne (Paris). 1997;148:105-106.
- de La Blanchardière A, Bachmeyer C, Toutous L, et al. Cutaneous ulcerations in sarcoidosis [in French]. Rev Med Interne. 1995;16:927-929.
- Mitsuishi T, Nogita T, Kawashima M. Psoriasiform sarcoidosis with ulceration. Int J Dermatol. 1992;31:339-340.
- Rodionov AN, Samtsov AV. The ulcerative form of skin sarcoidosis [in Russian]. Vestn Dermatol Venerol. 1990;7:68-71.
- Jacyk WK. Cutaneous sarcoidosis in black South Africans. Int J Dermatol. 1999;38:841-845.
- Gungor E, Artuz F, Alli N, et al. Ulcerative sarcoidosis. J Eur Acad Dermatol Venereol. 1999;12:78-79.
- Schleinitz N, Luc M, Genot S, et al. Ulcerative cutaneous lesions: a rare manifestation of sarcoidosis [in French]. Rev Med Interne. 2005;26:758-759.
- Klocker J, Duckers J, Morse R, et al. Ulcerative cutaneous sarcoidosis masquerading as metastatic carcinoma of the breast. Age Ageing. 2002;31:77-79.
- Streit M, Bohlen LM, Braathen LR. Ulcerative sarcoidosis successfully treated with apligraf. Dermatology. 2001;202:367-370.
- Ichiki Y, Kitajima Y. Ulcerative sarcoidosis: case report and review of the Japanese literature. Acta Derm Venereol. 2008;88:526-528
- Meyersburg D, Schön MP, Bertsch HP, et al. Uncommon cutaneous ulcerative and systemic sarcoidosis. successful treatment with hydroxychloroquine and compression therapy [in German]. Hautarzt. 2011;62:691-695.
- Wei CH, Huang YH, Shih YC, et al. Sarcoidosis with cutaneous granulomatous vasculitis. Australas J Dermatol. 2010;51:198-201.
- Kluger N, Girard C, Durand L, et al. Leg ulcers revealing systemic sarcoidosis with splenomegaly and thrombocytopenia. Int J Dermatol. 2013;52:1425-1427.
- Jun L, Jia-Wei L, Hong-Zhong J. Ulcerative sarcoidosis. Int J Dermatol. 2014;53:E315-E316.
- Chen JH, Wang TT, Lin ZQ. Successful application of a novel dressing for the treatment of ulcerative cutaneous sarcoidosis. Chin Med J. 2013;126:3400.
- Ri G, Yoshikawa E, Shigekiyo T, et al. Takayasu artertitis and ulcerative sarcoidosis. Intern Med. 2015;54:1075-1080.
- Spiliopoulou I, Foka A, Bounas A, et al. Mycobacterium kansasii cutaneous infection in a patient with sarcoidosis treated with anti-TNF agents. Acta Clin Belg. 2014;69:229-231.
- Yang DJ, Krishnan RS, Guillen DR, et al. Disseminated sporotrichosis mimicking sarcoidosis. Int J Dermatol. 2006;45:450-453.
- Badgwell C, Rosen T. Cutaneous sarcoidosis therapy updated. J Am Acad Dermatol. 2007;56:69-83.
Sarcoidosis is a multisystem granulomatous disorder of unknown etiology that primarily affects the lungs and lymphatic system but also may involve the skin, eyes, liver, spleen, muscles, bones, and nervous system.1 Cutaneous symptoms of sarcoidosis occur in approximately 25% of patients and are classified as specific and nonspecific, with specific lesions demonstrating noncaseating granuloma formation, which is typical of sarcoidosis.2 Nonspecific lesions primarily include erythema nodosum and calcinosis cutis. Specific lesions commonly present as reddish brown infiltrated plaques that may be annular, polycyclic, or serpiginous.1,3 They also may appear as yellowish brown or violaceous maculopapular lesions. However, specific lesions may present in a wide variety of morphologies, most often papules, nodules, subcutaneous infiltrates, and lupus pernio.4 Additionally, atypical cutaneous manifestations of sarcoidosis include erythroderma; scarring alopecia; nail dystrophy; and verrucous, ichthyosiform, psoriasiform, hypopigmented, or ulcerative skin lesions.3-5 Among these many potential clinical presentations, ulcerative sarcoidosis is quite uncommon.
We report a case of a patient who presented with classic clinical and histopathological findings of ulcerative sarcoidosis to highlight the prototypical presentation of a rare condition. We also review 34 additional cases of ulcerative sarcoidosis published in the English-language literature based on a PubMed search of articles indexed for MEDLINE using the term ulcerative sarcoid.4-32 Analyzing this historical information, the scope of this unusual form of cutaneous sarcoidosis can be better understood, recognized, and treated. Although current standard-of-care treatments are most often successful, there is a paucity of definitive clinical trials to justify and verify comparative therapeutic efficacy.
Case Report
A 49-year-old black man with known pulmonary sarcoidosis, idiopathic (human immunodeficiency virus–negative) CD4 depletion syndrome, and chronic kidney disease presented with persistent bilateral ulcers of the legs of 1 month’s duration. The lesions first appeared as multiple “dark spots” on the legs. After the patient applied homemade aloe vera extract under occlusion for 1 to 2 days, the lesions became painful and began to ulcerate approximately 3 months prior to presentation. The patient applied a combination of a topical first aid antibiotic ointment, Epsom salts, and hydrogen peroxide without any improvement. A current review of systems was negative.
The patient’s medical history was notable for sarcoidosis diagnosed more than 10 years prior. During this time, he had intermittently been treated elsewhere with low-dose oral prednisone (5 mg once daily), hydroxychloroquine (200 mg twice daily), and an inhaled steroid as needed. He had a history of human immunodeficiency virus–negative, idiopathic CD4 depletion syndrome, which had been complicated by cryptococcal meningitis 7 years prior to presentation. He also had renal insufficiency, with baseline creatinine levels ranging from 1.4 to 1.7 mg/dL (reference range, 0.6–1.2 mg/dL). There was no personal or family history of known or suspected inflammatory bowel disease.
On physical examination, numerous discrete, coalescing, punched out–appearing ulcerations with foul-smelling, greenish yellow, purulent drainage were present bilaterally on the legs (Figure 1). The ulcers had a rolled border with a moderate amount of seemingly nonviable necrotic tissue. A number of hyperpigmented round papules, patches, and plaques also were present on the proximal legs. Laboratory evaluation revealed a CD4 count of 151 cc/mm3 (reference range, 500–1600 cc/mm3) and mildly elevated calcium of 10.7 mg/dL (reference range, 8.2–10.2 mg/dL).

Aerobic, anaerobic, mycobacterial, and fungal cultures of the purulent exudate were obtained. Given a high suspicion for secondary infection of the exogenous wound sites, doxycycline (100 mg twice daily) and topical mupir-ocin were initiated. Gram stain revealed few to moderate polymorphonuclear cells and many gram-positive cocci in pairs, chains, and clusters, along with many gram-negative rods. Bacterial culture grew Pseudomonas aeruginosa, Enterococcus species group G streptococci, and methicillin-resistant Staphylococcus aureus–positive staphylococci. Ciprofloxacin (500 mg twice daily) was then initiated, but the ulcers showed absolutely no clinical improvement and in fact worsened both in number and depth (Figure 2) over subsequent clinic visits during the next 3 months, even after amoxicillin (500 mg 3 times daily) was added. The patient was admitted for treatment with intravenous antibiotics after additional wound cultures revealed fluoroquinolone-resistant Pseudomonas.

Punch biopsies of the ulcers showed nonspecific acute inflammation and tissue necrosis in the active ulcers with nonnecrotizing granulomatous inflammation extending into the deep dermis, with many Langerhans-type giant cells present in the palpable ulcer borders (Figure 3). Neither birefringent particles nor asteroid bodies were observed. Tissue Gram stains did not reveal evidence of bacterial infection. Special stains for acid-fast and fungal organisms (ie, periodic acid–Schiff, Gomori methenamine-silver, Fite, acid-fast bacilli) were similarly negative. Tissue cultures obtained on deep biopsy revealed only rare colonies of P aeruginosa and no isolates on anaerobic, mycobacterial, or fungal cultures. Polymerase chain reaction for mycobacteria and common endemic fungi also was negative. In the absence of infection and considering his history of known sarcoidosis, these histologic features were consistent with ulcerative sarcoidosis. The patient was started on prednisone (60 mg once daily) and hydroxychloroquine (200 mg twice daily). The prednisone was tapered to 20 mg once daily over a 2-year period, at which point 90% of the ulcers had healed. He was continued on hydroxychloroquine at the initial dose, and at a 3-year follow-up his ulcers had healed completely without relapse.

Comment
Ulcerative sarcoidosis is rare, seen worldwide in only 5% of patients with cutaneous sarcoidosis.33 However, cases have been encountered worldwide, with reports emanating from Japan, China, Germany, France, and Russia, among others.6,34-55 We reviewed 34 cases from the English-language literature based on a PubMed search of articles indexed for MEDLINE using the term ulcerative sarcoid and examined patient demographics, clinical presentation, histological findings, treatment type, and outcome. Key references are presented in the Table. Disease prevalence previously has been estimated as being 3-times more common in women than men1; in our literature review, we found a female to male ratio of 3.25 to 1. Additionally, ulcerative sarcoidosis is reported to be twice as common in black versus white individuals.33 In our literature review, when race was reported, 66% (21/32) of patients were black. Disease prevalence has been reported to peak at 20 to 40 years of age.3 In this review, the average age of presentation was 45 years (age range, 24–79 years).
Ulceration may arise de novo but more commonly arises in preexisting scars or cutaneous lesions. There are 2 distinct patterns seen in ulcerative sarcoidosis.4 The first is characterized by ulceration within necrotic yellow plaques.2 The second pattern is characterized by violaceous nodules arising in an annular confluent pattern that eventually ulcerate.4 This presentation commonly mimics or may be mimicked by multiple disease states, including sporotrichosis, tuberculosis, stasis dermatitis with venous ulceration, and even metastatic breast cancer.7,46,55,56 Regardless of presentation, the legs are the most common location of ulcer formation.1,33 In our review, 85% (29/34) of cases presented with involvement of the legs, including our own case. Other locations of ulcer formation have included the face, arms, trunk, and genital area.
On histologic examination of ulcerative sarcoidosis, epithelioid granulomas composed of multinucleated giant cells, histiocytes, and scant numbers of lymphocytes are present.1,3 These formations are the noncaseating granulomas typical of sarcoidosis (Table). All of the cases in our review of the literature were described as either a collection of epithelioid granulomas with giant cell formation or noncaseating granulomas. There also have been reports of atypical features including necrotizing granulomas and granulomatous vasculitis.4,8,9,50 The histologic differential diagnosis in this case also would primarily include an infectious granulomatous process and less so an id reaction, rosacea, a paraneoplastic phenomenon, foreign body granulomas, and metastatic Crohn disease. The presence of ulceration, the large number of lesions, and the anatomic distribution help rule out most of these alternate diagnostic considerations. Diligent extensive workup was done in our patient to insure it was not an infection.

The goals of treatment include symptomatic relief, improvement in objective parameters of disease activity, and prevention of disease progression and subsequent disability.33,57 Fortunately, the majority of sarcoidosis patients with cutaneous symptoms achieve full recovery within months to years.33 Our literature review indicated that 81% (22/27) of patients with ulcerative lesions experienced full resolution within 1 year of treatment. Of those that did not (19% [5/27]), the patients were either lost to follow-up or died from other complications of sarcoidosis.
The widely accepted standard therapy for cutaneous sarcoidosis includes topical, intralesional, and systemic corticosteroids; antimalarials; and methotrexate.33,57 Steroids and methotrexate act by suppressing granuloma formation, while antimalarials prevent antigen presentation (presumably part of the pathogenesis).33 For mild to moderate disease, topical and intralesional steroids may be all that is necessary.33,57 Systemic steroids are used for disfiguring, destructive, and widespread lesions that have been refractory to local and other systemic therapies.33,57 Steroids are tapered gradually depending on the patient’s response, as it is common for patients to relapse below a certain dose.33,57 Antimalarials (chloroquine or hydroxychloroquine) and methotrexate are considered adjunct treatments for patients who are either steroid unresponsive or who are unable to tolerate corticosteroid treatment due to adverse events.33,57
Standard therapy is complicated by the side effects of treatment. Use of corticosteroids may lead to gastrointestinal tract upset, increased appetite, mood disturbances, impaired wound healing, hyperglycemia, hypertension, cushingoid features, and acne.57 Antimalarials can cause nausea, anorexia, and agranulocytosis, and chloroquine therapy in particular can lead to blurred vision, corneal deposits, and central retinopathy.33,57 Methotrexate is associated with hematologic, gastrointestinal tract, pulmonary, and hepatic toxicities well known to most practitioners.
Because of the variable clinical response of patients to standard therapy and their associated toxicities, other treatment options have been used including pentoxifylline, tetracyclines, isotretinoin, leflunomide, thalidomide, infliximab, adalimumab, allopurinol, and the pulsed dye or CO2 laser.10,33,57 In nonhealing ulcers, split-thickness grafting and a bilayered bioengineered skin substitute have been used with good results in conjunction with ongoing systemic therapy.11,47 Additionally, nanoparticle silver burn paste has been used successfully, with resolution of ulcers within 2 weeks in the Chinese literature.53
All of these treatment recommendations are based on historically accepted modalities. Controlled trials with longitudinal follow-up are needed to provide justification for the current standard of care.34
Sarcoidosis is a multisystem granulomatous disorder of unknown etiology that primarily affects the lungs and lymphatic system but also may involve the skin, eyes, liver, spleen, muscles, bones, and nervous system.1 Cutaneous symptoms of sarcoidosis occur in approximately 25% of patients and are classified as specific and nonspecific, with specific lesions demonstrating noncaseating granuloma formation, which is typical of sarcoidosis.2 Nonspecific lesions primarily include erythema nodosum and calcinosis cutis. Specific lesions commonly present as reddish brown infiltrated plaques that may be annular, polycyclic, or serpiginous.1,3 They also may appear as yellowish brown or violaceous maculopapular lesions. However, specific lesions may present in a wide variety of morphologies, most often papules, nodules, subcutaneous infiltrates, and lupus pernio.4 Additionally, atypical cutaneous manifestations of sarcoidosis include erythroderma; scarring alopecia; nail dystrophy; and verrucous, ichthyosiform, psoriasiform, hypopigmented, or ulcerative skin lesions.3-5 Among these many potential clinical presentations, ulcerative sarcoidosis is quite uncommon.
We report a case of a patient who presented with classic clinical and histopathological findings of ulcerative sarcoidosis to highlight the prototypical presentation of a rare condition. We also review 34 additional cases of ulcerative sarcoidosis published in the English-language literature based on a PubMed search of articles indexed for MEDLINE using the term ulcerative sarcoid.4-32 Analyzing this historical information, the scope of this unusual form of cutaneous sarcoidosis can be better understood, recognized, and treated. Although current standard-of-care treatments are most often successful, there is a paucity of definitive clinical trials to justify and verify comparative therapeutic efficacy.
Case Report
A 49-year-old black man with known pulmonary sarcoidosis, idiopathic (human immunodeficiency virus–negative) CD4 depletion syndrome, and chronic kidney disease presented with persistent bilateral ulcers of the legs of 1 month’s duration. The lesions first appeared as multiple “dark spots” on the legs. After the patient applied homemade aloe vera extract under occlusion for 1 to 2 days, the lesions became painful and began to ulcerate approximately 3 months prior to presentation. The patient applied a combination of a topical first aid antibiotic ointment, Epsom salts, and hydrogen peroxide without any improvement. A current review of systems was negative.
The patient’s medical history was notable for sarcoidosis diagnosed more than 10 years prior. During this time, he had intermittently been treated elsewhere with low-dose oral prednisone (5 mg once daily), hydroxychloroquine (200 mg twice daily), and an inhaled steroid as needed. He had a history of human immunodeficiency virus–negative, idiopathic CD4 depletion syndrome, which had been complicated by cryptococcal meningitis 7 years prior to presentation. He also had renal insufficiency, with baseline creatinine levels ranging from 1.4 to 1.7 mg/dL (reference range, 0.6–1.2 mg/dL). There was no personal or family history of known or suspected inflammatory bowel disease.
On physical examination, numerous discrete, coalescing, punched out–appearing ulcerations with foul-smelling, greenish yellow, purulent drainage were present bilaterally on the legs (Figure 1). The ulcers had a rolled border with a moderate amount of seemingly nonviable necrotic tissue. A number of hyperpigmented round papules, patches, and plaques also were present on the proximal legs. Laboratory evaluation revealed a CD4 count of 151 cc/mm3 (reference range, 500–1600 cc/mm3) and mildly elevated calcium of 10.7 mg/dL (reference range, 8.2–10.2 mg/dL).

Aerobic, anaerobic, mycobacterial, and fungal cultures of the purulent exudate were obtained. Given a high suspicion for secondary infection of the exogenous wound sites, doxycycline (100 mg twice daily) and topical mupir-ocin were initiated. Gram stain revealed few to moderate polymorphonuclear cells and many gram-positive cocci in pairs, chains, and clusters, along with many gram-negative rods. Bacterial culture grew Pseudomonas aeruginosa, Enterococcus species group G streptococci, and methicillin-resistant Staphylococcus aureus–positive staphylococci. Ciprofloxacin (500 mg twice daily) was then initiated, but the ulcers showed absolutely no clinical improvement and in fact worsened both in number and depth (Figure 2) over subsequent clinic visits during the next 3 months, even after amoxicillin (500 mg 3 times daily) was added. The patient was admitted for treatment with intravenous antibiotics after additional wound cultures revealed fluoroquinolone-resistant Pseudomonas.

Punch biopsies of the ulcers showed nonspecific acute inflammation and tissue necrosis in the active ulcers with nonnecrotizing granulomatous inflammation extending into the deep dermis, with many Langerhans-type giant cells present in the palpable ulcer borders (Figure 3). Neither birefringent particles nor asteroid bodies were observed. Tissue Gram stains did not reveal evidence of bacterial infection. Special stains for acid-fast and fungal organisms (ie, periodic acid–Schiff, Gomori methenamine-silver, Fite, acid-fast bacilli) were similarly negative. Tissue cultures obtained on deep biopsy revealed only rare colonies of P aeruginosa and no isolates on anaerobic, mycobacterial, or fungal cultures. Polymerase chain reaction for mycobacteria and common endemic fungi also was negative. In the absence of infection and considering his history of known sarcoidosis, these histologic features were consistent with ulcerative sarcoidosis. The patient was started on prednisone (60 mg once daily) and hydroxychloroquine (200 mg twice daily). The prednisone was tapered to 20 mg once daily over a 2-year period, at which point 90% of the ulcers had healed. He was continued on hydroxychloroquine at the initial dose, and at a 3-year follow-up his ulcers had healed completely without relapse.

Comment
Ulcerative sarcoidosis is rare, seen worldwide in only 5% of patients with cutaneous sarcoidosis.33 However, cases have been encountered worldwide, with reports emanating from Japan, China, Germany, France, and Russia, among others.6,34-55 We reviewed 34 cases from the English-language literature based on a PubMed search of articles indexed for MEDLINE using the term ulcerative sarcoid and examined patient demographics, clinical presentation, histological findings, treatment type, and outcome. Key references are presented in the Table. Disease prevalence previously has been estimated as being 3-times more common in women than men1; in our literature review, we found a female to male ratio of 3.25 to 1. Additionally, ulcerative sarcoidosis is reported to be twice as common in black versus white individuals.33 In our literature review, when race was reported, 66% (21/32) of patients were black. Disease prevalence has been reported to peak at 20 to 40 years of age.3 In this review, the average age of presentation was 45 years (age range, 24–79 years).
Ulceration may arise de novo but more commonly arises in preexisting scars or cutaneous lesions. There are 2 distinct patterns seen in ulcerative sarcoidosis.4 The first is characterized by ulceration within necrotic yellow plaques.2 The second pattern is characterized by violaceous nodules arising in an annular confluent pattern that eventually ulcerate.4 This presentation commonly mimics or may be mimicked by multiple disease states, including sporotrichosis, tuberculosis, stasis dermatitis with venous ulceration, and even metastatic breast cancer.7,46,55,56 Regardless of presentation, the legs are the most common location of ulcer formation.1,33 In our review, 85% (29/34) of cases presented with involvement of the legs, including our own case. Other locations of ulcer formation have included the face, arms, trunk, and genital area.
On histologic examination of ulcerative sarcoidosis, epithelioid granulomas composed of multinucleated giant cells, histiocytes, and scant numbers of lymphocytes are present.1,3 These formations are the noncaseating granulomas typical of sarcoidosis (Table). All of the cases in our review of the literature were described as either a collection of epithelioid granulomas with giant cell formation or noncaseating granulomas. There also have been reports of atypical features including necrotizing granulomas and granulomatous vasculitis.4,8,9,50 The histologic differential diagnosis in this case also would primarily include an infectious granulomatous process and less so an id reaction, rosacea, a paraneoplastic phenomenon, foreign body granulomas, and metastatic Crohn disease. The presence of ulceration, the large number of lesions, and the anatomic distribution help rule out most of these alternate diagnostic considerations. Diligent extensive workup was done in our patient to insure it was not an infection.

The goals of treatment include symptomatic relief, improvement in objective parameters of disease activity, and prevention of disease progression and subsequent disability.33,57 Fortunately, the majority of sarcoidosis patients with cutaneous symptoms achieve full recovery within months to years.33 Our literature review indicated that 81% (22/27) of patients with ulcerative lesions experienced full resolution within 1 year of treatment. Of those that did not (19% [5/27]), the patients were either lost to follow-up or died from other complications of sarcoidosis.
The widely accepted standard therapy for cutaneous sarcoidosis includes topical, intralesional, and systemic corticosteroids; antimalarials; and methotrexate.33,57 Steroids and methotrexate act by suppressing granuloma formation, while antimalarials prevent antigen presentation (presumably part of the pathogenesis).33 For mild to moderate disease, topical and intralesional steroids may be all that is necessary.33,57 Systemic steroids are used for disfiguring, destructive, and widespread lesions that have been refractory to local and other systemic therapies.33,57 Steroids are tapered gradually depending on the patient’s response, as it is common for patients to relapse below a certain dose.33,57 Antimalarials (chloroquine or hydroxychloroquine) and methotrexate are considered adjunct treatments for patients who are either steroid unresponsive or who are unable to tolerate corticosteroid treatment due to adverse events.33,57
Standard therapy is complicated by the side effects of treatment. Use of corticosteroids may lead to gastrointestinal tract upset, increased appetite, mood disturbances, impaired wound healing, hyperglycemia, hypertension, cushingoid features, and acne.57 Antimalarials can cause nausea, anorexia, and agranulocytosis, and chloroquine therapy in particular can lead to blurred vision, corneal deposits, and central retinopathy.33,57 Methotrexate is associated with hematologic, gastrointestinal tract, pulmonary, and hepatic toxicities well known to most practitioners.
Because of the variable clinical response of patients to standard therapy and their associated toxicities, other treatment options have been used including pentoxifylline, tetracyclines, isotretinoin, leflunomide, thalidomide, infliximab, adalimumab, allopurinol, and the pulsed dye or CO2 laser.10,33,57 In nonhealing ulcers, split-thickness grafting and a bilayered bioengineered skin substitute have been used with good results in conjunction with ongoing systemic therapy.11,47 Additionally, nanoparticle silver burn paste has been used successfully, with resolution of ulcers within 2 weeks in the Chinese literature.53
All of these treatment recommendations are based on historically accepted modalities. Controlled trials with longitudinal follow-up are needed to provide justification for the current standard of care.34
- Howard A, White CR Jr. Non-infectious granulomas. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Spain: Elsevier; 2008:1421-1435.
- Doherty CB, Rosen T. Evidence-based therapy for cutaneous sarcoidosis. Drugs. 2008;68:1361-1383.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Noiles K, Beleznay K, Crawford RI, et al. Sarcoidosis can present with necrotizing granulomas histologically: two cases of ulcerated sarcoidosis and review of the literature. J Cutan Med Surg. 2013;17:377-378.
- Mitchell IC, Sweatman MC, Rustin MH, et al. Ulcerative and hypopigmented sarcoidosis. J Am Acad Dermatol. 1986;15:1062-1065.
- Yoo SS, Mimouni D, Nikolskaia OV, et al. Clinicopathologic features of ulcerative-atrophic sarcoidosis. Int J Dermatol. 2004;43:108-112.
- Joshi SS, Romanelli R, Kirsner RS. Sarcoidosis mimicking a venous ulcer: a case report. Ostomy Wound Manage. 2009;55:46-48.
- Petri M, Barr E, Cho K, et al. Overlap of granulomatous vasculitis and sarcoidosis: presentation with uveitis, eosinophilia, leg ulcers, sinusitis and past foot drop. J Rheumatol. 1988;15:1171-1173.
- Poonawalla T, Colome-Grimmer MI, Kelly B. Ulcerative sarcoidosis in the legs with granulomatous vasculitis. Clin Exp Dermatol. 2008;33:282-286.
- Philips MA, Lynch J, Azmi FH. Ulcerative sarcoidosis responding to adalimumab. J Am Acad Dermatol. 2005;53:917.
- Collison DW, Novice F, Banse L, et al. Split-thickness skin grafting in extensive ulcerative sarcoidosis. J Dermatol Surg Oncol. 1989;15:679-683.
- Hunt RD, Gonzalez ME, Robinson M, et al. Ulcerative sarcoidosis. Dermatol Online J. 2012;18:29.
- Green JJ, Lawrence N, Heymann WR. Generalized ulcerative sarcoidosis induced by therapy with the flashlamp-pumped pulsed dye. Arch Dermatol. 2001;137:507-508.
- Albertini JG, Tyler W, Miller OF. Ulcerative sarcoidosis. case report and review of the literature. Arch Dermatol. 1997;133:215-219.
- Thomas J, Williams DW. Peritoneal involvement and ulcerative skin plaques in sarcoidosis: a case report. Sarcoidosis. 1989;6:161-162.
- Verdegem TD, Sharma OP. Cutaneous ulcers in sarcoidosis. Arch Dermatol. 1987;123:1531-1534.
- Gupta AK, Haberman HF, From GL, et al. Sarcoidosis with extensive cutaneous ulceration. unusual clinical presentation. Dermatologica. 1987;174:135-139.
- Hruza GJ, Kerdel FA. Generalized atrophic sarcoidosis with ulcerations. Arch Dermatol. 1986;122:320-322.
- Muhlemann MF, Walker NP, Tan LB, et al. Elephantine sarcoidosis presenting as ulcerating lymphoedema. J R Soc Med. 1985;78:260-261.
- Neill SM, Smith NP, Eady RA. Ulcerative sarcoidosis: a rare manifestation of a common disease. Clin Exp Dermatol. 1984;9:277-279.
- Saxe N, Benatar SR, Bok L, et al. Sarcoidosis with leg ulcers and annular facial lesions. Arch Dermatol. 1984;120:93-96.
- Schwartz RA, Robertson DB, Tierney LM, et al. Generalized ulcerative sarcoidosis. Arch Dermatol. 1982;118:931-933.
- Boyd RE, Andrews BS. Sarcoidosis presenting as cutaneous ulceration, subcutaneous nodules and chronic arthritis. J Rheumatol. 1981;8:311-316.
- Herzlinger DC, Marland AM, Barr RJ. Verrucous ulcerative skin lesions in sarcoidosis. an unusual clinical presentation. Cutis. 1979;23:569-572.
- Meyers M, Barsky S. Ulcerative sarcoidosis. Arch Dermatol. 1978;114:447.
- Schiffner J, Sharma OP. Ulcerative sarcoidosis. report of an unusual case. Arch Dermatol. 1977;113:676-677.
- Williamson DM. Sarcoidosis with atrophic lesions and ulcers of the legs. Br J Dermatol. 1971;84:92-93.
- Bazex A, Dupre A, Christol B, et al. Sarcoidosis with atrophic lesions and ulcers and the presence in some sarcoid granulomata of orceinophil fibres. Br J Dermatol. 1970;83:255-262.
- Brodkin RH. Leg ulcers. a report of two cases caused by sarcoidosis. Acta Derm Venereol. 1969;49:584-587.
- Simpson JR. Sarcoidosis with erythrodermia and ulceration. Br J Dermatol. 1963;75:193-198.
- Irgang S. Ulcerative cutaneous lesion in sarcoidosis; report of a case with clinical resemblance to lupus vulgaris. Harlem Hosp Bull. 1956;8:134-139.
- Irgang S. Ulcerative cutaneous lesions in sarcoidosis; report of a case with clinical resemblance to papulonecrotic tuberculide. Br J Dermatol. 1955;67:255-260.
- Hoffman MD. Atypical ulcers. Dermatol Ther. 2013;26:222-235.
- Hopf B, Krebs A. Ulcera cruris as a rare manifestation of sarcoidosis. Dermatologica. 1974;113:55-62.
- Metz J, Hartmann A, Hautkr Z. Ulcerative form of skin sarcoidosis. Z Hautkr. 1977;52:890-896.
- Berenbeĭn BA, Malygina LA, Tiutiunnikova IA. Ulcerative form of skin sarcoidosis [in Russian]. Vestn Dermatol Venerol. 1984;4:50-53.
- Takahashi N, Hoshino M, Takase T, et al. A case of ulcerative sarcoidosis [in Japanese]. Nihon Hifuka Gakkai Zasshi. 1985;95:1049-1054.
- Schamroth JM. Sarcoidosis with severe extensive skin ulceration. Int J Dermatol. 1985;24:451-452.
- Porteau L, Dromer C, Le Guennec P, et al. Ulcer lesions in sarcoidosis: apropos of a case [in French]. Ann Med Interne (Paris). 1997;148:105-106.
- de La Blanchardière A, Bachmeyer C, Toutous L, et al. Cutaneous ulcerations in sarcoidosis [in French]. Rev Med Interne. 1995;16:927-929.
- Mitsuishi T, Nogita T, Kawashima M. Psoriasiform sarcoidosis with ulceration. Int J Dermatol. 1992;31:339-340.
- Rodionov AN, Samtsov AV. The ulcerative form of skin sarcoidosis [in Russian]. Vestn Dermatol Venerol. 1990;7:68-71.
- Jacyk WK. Cutaneous sarcoidosis in black South Africans. Int J Dermatol. 1999;38:841-845.
- Gungor E, Artuz F, Alli N, et al. Ulcerative sarcoidosis. J Eur Acad Dermatol Venereol. 1999;12:78-79.
- Schleinitz N, Luc M, Genot S, et al. Ulcerative cutaneous lesions: a rare manifestation of sarcoidosis [in French]. Rev Med Interne. 2005;26:758-759.
- Klocker J, Duckers J, Morse R, et al. Ulcerative cutaneous sarcoidosis masquerading as metastatic carcinoma of the breast. Age Ageing. 2002;31:77-79.
- Streit M, Bohlen LM, Braathen LR. Ulcerative sarcoidosis successfully treated with apligraf. Dermatology. 2001;202:367-370.
- Ichiki Y, Kitajima Y. Ulcerative sarcoidosis: case report and review of the Japanese literature. Acta Derm Venereol. 2008;88:526-528
- Meyersburg D, Schön MP, Bertsch HP, et al. Uncommon cutaneous ulcerative and systemic sarcoidosis. successful treatment with hydroxychloroquine and compression therapy [in German]. Hautarzt. 2011;62:691-695.
- Wei CH, Huang YH, Shih YC, et al. Sarcoidosis with cutaneous granulomatous vasculitis. Australas J Dermatol. 2010;51:198-201.
- Kluger N, Girard C, Durand L, et al. Leg ulcers revealing systemic sarcoidosis with splenomegaly and thrombocytopenia. Int J Dermatol. 2013;52:1425-1427.
- Jun L, Jia-Wei L, Hong-Zhong J. Ulcerative sarcoidosis. Int J Dermatol. 2014;53:E315-E316.
- Chen JH, Wang TT, Lin ZQ. Successful application of a novel dressing for the treatment of ulcerative cutaneous sarcoidosis. Chin Med J. 2013;126:3400.
- Ri G, Yoshikawa E, Shigekiyo T, et al. Takayasu artertitis and ulcerative sarcoidosis. Intern Med. 2015;54:1075-1080.
- Spiliopoulou I, Foka A, Bounas A, et al. Mycobacterium kansasii cutaneous infection in a patient with sarcoidosis treated with anti-TNF agents. Acta Clin Belg. 2014;69:229-231.
- Yang DJ, Krishnan RS, Guillen DR, et al. Disseminated sporotrichosis mimicking sarcoidosis. Int J Dermatol. 2006;45:450-453.
- Badgwell C, Rosen T. Cutaneous sarcoidosis therapy updated. J Am Acad Dermatol. 2007;56:69-83.
- Howard A, White CR Jr. Non-infectious granulomas. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Spain: Elsevier; 2008:1421-1435.
- Doherty CB, Rosen T. Evidence-based therapy for cutaneous sarcoidosis. Drugs. 2008;68:1361-1383.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Noiles K, Beleznay K, Crawford RI, et al. Sarcoidosis can present with necrotizing granulomas histologically: two cases of ulcerated sarcoidosis and review of the literature. J Cutan Med Surg. 2013;17:377-378.
- Mitchell IC, Sweatman MC, Rustin MH, et al. Ulcerative and hypopigmented sarcoidosis. J Am Acad Dermatol. 1986;15:1062-1065.
- Yoo SS, Mimouni D, Nikolskaia OV, et al. Clinicopathologic features of ulcerative-atrophic sarcoidosis. Int J Dermatol. 2004;43:108-112.
- Joshi SS, Romanelli R, Kirsner RS. Sarcoidosis mimicking a venous ulcer: a case report. Ostomy Wound Manage. 2009;55:46-48.
- Petri M, Barr E, Cho K, et al. Overlap of granulomatous vasculitis and sarcoidosis: presentation with uveitis, eosinophilia, leg ulcers, sinusitis and past foot drop. J Rheumatol. 1988;15:1171-1173.
- Poonawalla T, Colome-Grimmer MI, Kelly B. Ulcerative sarcoidosis in the legs with granulomatous vasculitis. Clin Exp Dermatol. 2008;33:282-286.
- Philips MA, Lynch J, Azmi FH. Ulcerative sarcoidosis responding to adalimumab. J Am Acad Dermatol. 2005;53:917.
- Collison DW, Novice F, Banse L, et al. Split-thickness skin grafting in extensive ulcerative sarcoidosis. J Dermatol Surg Oncol. 1989;15:679-683.
- Hunt RD, Gonzalez ME, Robinson M, et al. Ulcerative sarcoidosis. Dermatol Online J. 2012;18:29.
- Green JJ, Lawrence N, Heymann WR. Generalized ulcerative sarcoidosis induced by therapy with the flashlamp-pumped pulsed dye. Arch Dermatol. 2001;137:507-508.
- Albertini JG, Tyler W, Miller OF. Ulcerative sarcoidosis. case report and review of the literature. Arch Dermatol. 1997;133:215-219.
- Thomas J, Williams DW. Peritoneal involvement and ulcerative skin plaques in sarcoidosis: a case report. Sarcoidosis. 1989;6:161-162.
- Verdegem TD, Sharma OP. Cutaneous ulcers in sarcoidosis. Arch Dermatol. 1987;123:1531-1534.
- Gupta AK, Haberman HF, From GL, et al. Sarcoidosis with extensive cutaneous ulceration. unusual clinical presentation. Dermatologica. 1987;174:135-139.
- Hruza GJ, Kerdel FA. Generalized atrophic sarcoidosis with ulcerations. Arch Dermatol. 1986;122:320-322.
- Muhlemann MF, Walker NP, Tan LB, et al. Elephantine sarcoidosis presenting as ulcerating lymphoedema. J R Soc Med. 1985;78:260-261.
- Neill SM, Smith NP, Eady RA. Ulcerative sarcoidosis: a rare manifestation of a common disease. Clin Exp Dermatol. 1984;9:277-279.
- Saxe N, Benatar SR, Bok L, et al. Sarcoidosis with leg ulcers and annular facial lesions. Arch Dermatol. 1984;120:93-96.
- Schwartz RA, Robertson DB, Tierney LM, et al. Generalized ulcerative sarcoidosis. Arch Dermatol. 1982;118:931-933.
- Boyd RE, Andrews BS. Sarcoidosis presenting as cutaneous ulceration, subcutaneous nodules and chronic arthritis. J Rheumatol. 1981;8:311-316.
- Herzlinger DC, Marland AM, Barr RJ. Verrucous ulcerative skin lesions in sarcoidosis. an unusual clinical presentation. Cutis. 1979;23:569-572.
- Meyers M, Barsky S. Ulcerative sarcoidosis. Arch Dermatol. 1978;114:447.
- Schiffner J, Sharma OP. Ulcerative sarcoidosis. report of an unusual case. Arch Dermatol. 1977;113:676-677.
- Williamson DM. Sarcoidosis with atrophic lesions and ulcers of the legs. Br J Dermatol. 1971;84:92-93.
- Bazex A, Dupre A, Christol B, et al. Sarcoidosis with atrophic lesions and ulcers and the presence in some sarcoid granulomata of orceinophil fibres. Br J Dermatol. 1970;83:255-262.
- Brodkin RH. Leg ulcers. a report of two cases caused by sarcoidosis. Acta Derm Venereol. 1969;49:584-587.
- Simpson JR. Sarcoidosis with erythrodermia and ulceration. Br J Dermatol. 1963;75:193-198.
- Irgang S. Ulcerative cutaneous lesion in sarcoidosis; report of a case with clinical resemblance to lupus vulgaris. Harlem Hosp Bull. 1956;8:134-139.
- Irgang S. Ulcerative cutaneous lesions in sarcoidosis; report of a case with clinical resemblance to papulonecrotic tuberculide. Br J Dermatol. 1955;67:255-260.
- Hoffman MD. Atypical ulcers. Dermatol Ther. 2013;26:222-235.
- Hopf B, Krebs A. Ulcera cruris as a rare manifestation of sarcoidosis. Dermatologica. 1974;113:55-62.
- Metz J, Hartmann A, Hautkr Z. Ulcerative form of skin sarcoidosis. Z Hautkr. 1977;52:890-896.
- Berenbeĭn BA, Malygina LA, Tiutiunnikova IA. Ulcerative form of skin sarcoidosis [in Russian]. Vestn Dermatol Venerol. 1984;4:50-53.
- Takahashi N, Hoshino M, Takase T, et al. A case of ulcerative sarcoidosis [in Japanese]. Nihon Hifuka Gakkai Zasshi. 1985;95:1049-1054.
- Schamroth JM. Sarcoidosis with severe extensive skin ulceration. Int J Dermatol. 1985;24:451-452.
- Porteau L, Dromer C, Le Guennec P, et al. Ulcer lesions in sarcoidosis: apropos of a case [in French]. Ann Med Interne (Paris). 1997;148:105-106.
- de La Blanchardière A, Bachmeyer C, Toutous L, et al. Cutaneous ulcerations in sarcoidosis [in French]. Rev Med Interne. 1995;16:927-929.
- Mitsuishi T, Nogita T, Kawashima M. Psoriasiform sarcoidosis with ulceration. Int J Dermatol. 1992;31:339-340.
- Rodionov AN, Samtsov AV. The ulcerative form of skin sarcoidosis [in Russian]. Vestn Dermatol Venerol. 1990;7:68-71.
- Jacyk WK. Cutaneous sarcoidosis in black South Africans. Int J Dermatol. 1999;38:841-845.
- Gungor E, Artuz F, Alli N, et al. Ulcerative sarcoidosis. J Eur Acad Dermatol Venereol. 1999;12:78-79.
- Schleinitz N, Luc M, Genot S, et al. Ulcerative cutaneous lesions: a rare manifestation of sarcoidosis [in French]. Rev Med Interne. 2005;26:758-759.
- Klocker J, Duckers J, Morse R, et al. Ulcerative cutaneous sarcoidosis masquerading as metastatic carcinoma of the breast. Age Ageing. 2002;31:77-79.
- Streit M, Bohlen LM, Braathen LR. Ulcerative sarcoidosis successfully treated with apligraf. Dermatology. 2001;202:367-370.
- Ichiki Y, Kitajima Y. Ulcerative sarcoidosis: case report and review of the Japanese literature. Acta Derm Venereol. 2008;88:526-528
- Meyersburg D, Schön MP, Bertsch HP, et al. Uncommon cutaneous ulcerative and systemic sarcoidosis. successful treatment with hydroxychloroquine and compression therapy [in German]. Hautarzt. 2011;62:691-695.
- Wei CH, Huang YH, Shih YC, et al. Sarcoidosis with cutaneous granulomatous vasculitis. Australas J Dermatol. 2010;51:198-201.
- Kluger N, Girard C, Durand L, et al. Leg ulcers revealing systemic sarcoidosis with splenomegaly and thrombocytopenia. Int J Dermatol. 2013;52:1425-1427.
- Jun L, Jia-Wei L, Hong-Zhong J. Ulcerative sarcoidosis. Int J Dermatol. 2014;53:E315-E316.
- Chen JH, Wang TT, Lin ZQ. Successful application of a novel dressing for the treatment of ulcerative cutaneous sarcoidosis. Chin Med J. 2013;126:3400.
- Ri G, Yoshikawa E, Shigekiyo T, et al. Takayasu artertitis and ulcerative sarcoidosis. Intern Med. 2015;54:1075-1080.
- Spiliopoulou I, Foka A, Bounas A, et al. Mycobacterium kansasii cutaneous infection in a patient with sarcoidosis treated with anti-TNF agents. Acta Clin Belg. 2014;69:229-231.
- Yang DJ, Krishnan RS, Guillen DR, et al. Disseminated sporotrichosis mimicking sarcoidosis. Int J Dermatol. 2006;45:450-453.
- Badgwell C, Rosen T. Cutaneous sarcoidosis therapy updated. J Am Acad Dermatol. 2007;56:69-83.
Practice Points
- Sarcoidosis can present as a primary ulcerative disease.
- Suspect ulcerative sarcoidosis when ulcerations are seen on the leg.
- Systemic corticosteroids may be the most effective treatment of ulcerative sarcoidosis.
Drug-eluting balloon is as good as drug-eluting stent for in-stent restenosis
DENVER – Treatment of coronary in-stent restenosis using a paclitaxel-eluting balloon proved noninferior to an everolimus-eluting stent in terms of minimal lumen diameter at 6 months in the DARE trial, Jose P.S. Henriques, MD, reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
The two forms of device therapy also yielded similar rates of adverse clinical events, including target vessel revascularization, at 12 months, he said.
The DARE (Drug-Eluting Balloon for In-Stent Restenosis) trial included 278 patients with in-stent restenosis (ISR) randomized to the SeQuent Please paclitaxel-eluting balloon or Xience everolimus-eluting stent at high-volume Dutch percutaneous coronary intervention centers. The trial was unique in that it included a mix of patients with in-stent restenosis involving DES and bare-metal stents. Indeed, 44% of participants had ISR in a bare-metal stent. These older-model stents are still used in patients who require a shorter duration of dual-antiplatelet therapy, so the DARE population reflects real-world clinical practice better than do prior studies restricted to ISR in only one stent type or the other, according to the cardiologist.
The primary outcome in this noninferiority trial was the in-segment minimal lumen diameter at 6-month angiographic follow-up. The mean diameter was 1.71 mm in the drug-eluting balloon (DEB) group and closely similar at 1.74 mm in the DES group. There was greater acute gain with the drug-eluting stent, but it was canceled out by greater late loss by 6 months.
Moreover, the 12-month composite clinical event rate composed of death, target vessel MI, and target vessel revascularization was 10.9% in the DEB recipients and 9.2% with the DES, a nonsignificant difference. Of note, target vessel revascularization occurred in 8.8% of the DEB group and was similar at 7.1% in the DES recipients, although the DARE trial wasn’t powered to detect differences in clinical events.
These results confirm the European Society of Cardiology’s class 1A recommendation for DEB as well as DES for ISR, Dr. Henriques said at the meeting sponsored by the Cardiovascular Research Foundation.
U.S. guidelines don’t address DEB for the treatment of coronary ISR. That’s because the devices, which have long been available in Europe, aren’t approved for use in the coronary tree in the United States. They are available in the United States only for treatment of peripheral vascular disease. And no U.S. clinical trials of DEBs in the coronary tree are planned.
“I wish the U.S. Food and Drug Administration was listening to the DARE results because we really would like to see this technology in the U.S.,” said Roxana Mehran, MD, who moderated a press conference where the DARE findings were highlighted.
David J. Cohen, MD, director of cardiovascular research at Saint Luke’s Mid America Heart Institute in Kansas City, Mo., commented, “This type of device, obviously with it being similar in performance to drug-eluting stents, would be a very welcome addition to our armamentarium, because one of the things I don’t like to do as a coronary interventionalist is to line up multiple stents inside each other.”
“Making club sandwiches out of patients’ arteries with stent after stent is not a good idea. We know that,” added Dr. Mehran, professor of medicine and director of interventional cardiovascular research and clinical trials at Mount Sinai School of Medicine in New York.
Cindy L. Grines, MD, chair of cardiology at the Hofstra Northwell School of Medicine in Hempstead, N.Y., said DEBs “would absolutely be welcome” if they were available to cardiologists in the United States.
“When you have repeated episodes of in-stent restenosis, you can start with a vessel that’s 3 mm in diameter; then when it restenoses and you place a second stent inside there, all of a sudden – even if you have a great stent result – you can be down to 2.25 mm. And then the next time you need to treat it for restenosis, you’re down to a very tiny lumen. That’s the big problem with trying to treat in-stent restenosis with more stents,” she explained.
The DEBs are expensive, and ISR has become so uncommon with the use of the current generation of drug-eluting stents that the device companies have little incentive to do the studies required to be able to market DEBs in the United States.
“I think the FDA should consider in-stent restenosis as an orphan disease. We really should be able to get a drug-eluting balloon approved in this country based on the data over in Europe,” Dr. Grines said.
Dr. Henriques reported receiving research grants from B. Braun, which markets the paclitaxel-eluting stent in Europe, as well as from Abbott Vascular.
DENVER – Treatment of coronary in-stent restenosis using a paclitaxel-eluting balloon proved noninferior to an everolimus-eluting stent in terms of minimal lumen diameter at 6 months in the DARE trial, Jose P.S. Henriques, MD, reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
The two forms of device therapy also yielded similar rates of adverse clinical events, including target vessel revascularization, at 12 months, he said.
The DARE (Drug-Eluting Balloon for In-Stent Restenosis) trial included 278 patients with in-stent restenosis (ISR) randomized to the SeQuent Please paclitaxel-eluting balloon or Xience everolimus-eluting stent at high-volume Dutch percutaneous coronary intervention centers. The trial was unique in that it included a mix of patients with in-stent restenosis involving DES and bare-metal stents. Indeed, 44% of participants had ISR in a bare-metal stent. These older-model stents are still used in patients who require a shorter duration of dual-antiplatelet therapy, so the DARE population reflects real-world clinical practice better than do prior studies restricted to ISR in only one stent type or the other, according to the cardiologist.
The primary outcome in this noninferiority trial was the in-segment minimal lumen diameter at 6-month angiographic follow-up. The mean diameter was 1.71 mm in the drug-eluting balloon (DEB) group and closely similar at 1.74 mm in the DES group. There was greater acute gain with the drug-eluting stent, but it was canceled out by greater late loss by 6 months.
Moreover, the 12-month composite clinical event rate composed of death, target vessel MI, and target vessel revascularization was 10.9% in the DEB recipients and 9.2% with the DES, a nonsignificant difference. Of note, target vessel revascularization occurred in 8.8% of the DEB group and was similar at 7.1% in the DES recipients, although the DARE trial wasn’t powered to detect differences in clinical events.
These results confirm the European Society of Cardiology’s class 1A recommendation for DEB as well as DES for ISR, Dr. Henriques said at the meeting sponsored by the Cardiovascular Research Foundation.
U.S. guidelines don’t address DEB for the treatment of coronary ISR. That’s because the devices, which have long been available in Europe, aren’t approved for use in the coronary tree in the United States. They are available in the United States only for treatment of peripheral vascular disease. And no U.S. clinical trials of DEBs in the coronary tree are planned.
“I wish the U.S. Food and Drug Administration was listening to the DARE results because we really would like to see this technology in the U.S.,” said Roxana Mehran, MD, who moderated a press conference where the DARE findings were highlighted.
David J. Cohen, MD, director of cardiovascular research at Saint Luke’s Mid America Heart Institute in Kansas City, Mo., commented, “This type of device, obviously with it being similar in performance to drug-eluting stents, would be a very welcome addition to our armamentarium, because one of the things I don’t like to do as a coronary interventionalist is to line up multiple stents inside each other.”
“Making club sandwiches out of patients’ arteries with stent after stent is not a good idea. We know that,” added Dr. Mehran, professor of medicine and director of interventional cardiovascular research and clinical trials at Mount Sinai School of Medicine in New York.
Cindy L. Grines, MD, chair of cardiology at the Hofstra Northwell School of Medicine in Hempstead, N.Y., said DEBs “would absolutely be welcome” if they were available to cardiologists in the United States.
“When you have repeated episodes of in-stent restenosis, you can start with a vessel that’s 3 mm in diameter; then when it restenoses and you place a second stent inside there, all of a sudden – even if you have a great stent result – you can be down to 2.25 mm. And then the next time you need to treat it for restenosis, you’re down to a very tiny lumen. That’s the big problem with trying to treat in-stent restenosis with more stents,” she explained.
The DEBs are expensive, and ISR has become so uncommon with the use of the current generation of drug-eluting stents that the device companies have little incentive to do the studies required to be able to market DEBs in the United States.
“I think the FDA should consider in-stent restenosis as an orphan disease. We really should be able to get a drug-eluting balloon approved in this country based on the data over in Europe,” Dr. Grines said.
Dr. Henriques reported receiving research grants from B. Braun, which markets the paclitaxel-eluting stent in Europe, as well as from Abbott Vascular.
DENVER – Treatment of coronary in-stent restenosis using a paclitaxel-eluting balloon proved noninferior to an everolimus-eluting stent in terms of minimal lumen diameter at 6 months in the DARE trial, Jose P.S. Henriques, MD, reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
The two forms of device therapy also yielded similar rates of adverse clinical events, including target vessel revascularization, at 12 months, he said.
The DARE (Drug-Eluting Balloon for In-Stent Restenosis) trial included 278 patients with in-stent restenosis (ISR) randomized to the SeQuent Please paclitaxel-eluting balloon or Xience everolimus-eluting stent at high-volume Dutch percutaneous coronary intervention centers. The trial was unique in that it included a mix of patients with in-stent restenosis involving DES and bare-metal stents. Indeed, 44% of participants had ISR in a bare-metal stent. These older-model stents are still used in patients who require a shorter duration of dual-antiplatelet therapy, so the DARE population reflects real-world clinical practice better than do prior studies restricted to ISR in only one stent type or the other, according to the cardiologist.
The primary outcome in this noninferiority trial was the in-segment minimal lumen diameter at 6-month angiographic follow-up. The mean diameter was 1.71 mm in the drug-eluting balloon (DEB) group and closely similar at 1.74 mm in the DES group. There was greater acute gain with the drug-eluting stent, but it was canceled out by greater late loss by 6 months.
Moreover, the 12-month composite clinical event rate composed of death, target vessel MI, and target vessel revascularization was 10.9% in the DEB recipients and 9.2% with the DES, a nonsignificant difference. Of note, target vessel revascularization occurred in 8.8% of the DEB group and was similar at 7.1% in the DES recipients, although the DARE trial wasn’t powered to detect differences in clinical events.
These results confirm the European Society of Cardiology’s class 1A recommendation for DEB as well as DES for ISR, Dr. Henriques said at the meeting sponsored by the Cardiovascular Research Foundation.
U.S. guidelines don’t address DEB for the treatment of coronary ISR. That’s because the devices, which have long been available in Europe, aren’t approved for use in the coronary tree in the United States. They are available in the United States only for treatment of peripheral vascular disease. And no U.S. clinical trials of DEBs in the coronary tree are planned.
“I wish the U.S. Food and Drug Administration was listening to the DARE results because we really would like to see this technology in the U.S.,” said Roxana Mehran, MD, who moderated a press conference where the DARE findings were highlighted.
David J. Cohen, MD, director of cardiovascular research at Saint Luke’s Mid America Heart Institute in Kansas City, Mo., commented, “This type of device, obviously with it being similar in performance to drug-eluting stents, would be a very welcome addition to our armamentarium, because one of the things I don’t like to do as a coronary interventionalist is to line up multiple stents inside each other.”
“Making club sandwiches out of patients’ arteries with stent after stent is not a good idea. We know that,” added Dr. Mehran, professor of medicine and director of interventional cardiovascular research and clinical trials at Mount Sinai School of Medicine in New York.
Cindy L. Grines, MD, chair of cardiology at the Hofstra Northwell School of Medicine in Hempstead, N.Y., said DEBs “would absolutely be welcome” if they were available to cardiologists in the United States.
“When you have repeated episodes of in-stent restenosis, you can start with a vessel that’s 3 mm in diameter; then when it restenoses and you place a second stent inside there, all of a sudden – even if you have a great stent result – you can be down to 2.25 mm. And then the next time you need to treat it for restenosis, you’re down to a very tiny lumen. That’s the big problem with trying to treat in-stent restenosis with more stents,” she explained.
The DEBs are expensive, and ISR has become so uncommon with the use of the current generation of drug-eluting stents that the device companies have little incentive to do the studies required to be able to market DEBs in the United States.
“I think the FDA should consider in-stent restenosis as an orphan disease. We really should be able to get a drug-eluting balloon approved in this country based on the data over in Europe,” Dr. Grines said.
Dr. Henriques reported receiving research grants from B. Braun, which markets the paclitaxel-eluting stent in Europe, as well as from Abbott Vascular.
AT TCT 2017
Key clinical point:
Major finding: The mean 6-month in-segment minimal lumen diameter following treatment of in-stent restenosis with a paclitaxel-eluting balloon was 1.71 mm and was similar at 1.74 mm in patients treated using an everolimus-eluting stent.
Data source: A prospective, multicenter Dutch randomized trial including 278 patients with in-stent restenosis.
Disclosures: The study was sponsored by the University of Amsterdam and financially supported by a research grant from B. Braun. The presenter reported receiving research grants from that company and Abbott Vascular.
In MI with cardiogenic shock, PCI of only culprit lesions is safer
In patients with acute myocardial infarction and multivessel coronary artery disease with cardiogenic shock, 30-day rates of death and renal-replacement therapy were lower when patients underwent percutaneous coronary intervention (PCI) of the culprit lesion as opposed to multivessel PCI.
The difference appeared to be driven by mortality, as the renal endpoint alone was not significant.
As many as 80% of patients with cardiogenic shock also present with multivessel coronary artery disease, and this is associated with worse mortality. It is unclear whether immediate PCI of clinically important stenoses of major nonculprit coronary arteries is of benefit, and previous randomized trials comparing the procedures did not look at patients with cardiogenic shock, Holger Thiele, MD, reported at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
European guidelines suggest that PCI of nonculprit lesions should be considered in patients with cardiogenic shock, while U.S. guidelines offer no opinion, but recent appropriate use criteria recommend revascularization of a nonculprit artery if cardiogenic shock continues after the culprit artery has been repaired. It is thought that immediate revascularization of all coronary arteries with clinically important stenoses might improve overall myocardial perfusion and function in patients with cardiogenic shock, but the procedure could also have drawbacks, including additional ischemia, volume overload, and renal impairment from higher doses of contrast material.
To better understand outcomes in these patients, the Culprit Lesion Only PCI versus Multivessel PCI in Cardiogenic Shock (CULPRIT-SHOCK) trial randomized 706 patients to culprit-only PCI or multivessel PCI, in which PCI was performed on all major coronary arteries with more than 70% stenosis. Patients receiving culprit-only PCI could also undergo optional staged revascularization due to residual ischemic lesions, symptoms, or clinical or neurologic status.
At 30 days, death and/or renal-replacement therapy occurred in 45.9% of the culprit-only group, compared to 55.4% in the multivessel group (relative risk, 0.83; 95% confidence interval, 0.71-0.96; P = .01). A per-protocol analysis showed similar results (RR, 0.81; 95% CI, 0.69-0.96; P =.01), as did an analysis of the as-treated population (RR, 0.83; 95% CI, 0.72-0.97; P = .02).
All-cause mortality was lower in the culprit-only group (43.3% versus 51.6%; RR, 0.84; 95% CI, 0.72-0.98; P=.03). The rate of renal-replacement therapy was higher in the multivessel group (16.4% versus 11.6%), but this did not reach statistical significance (P = .07).
There were no statistically significant differences between the two groups with respect to recurrent myocardial infarction, rehospitalization for heart failure, bleeding, or stroke, Dr. Thiele reported at the meeting, which was sponsored by the Cardiovascular Research Foundation.
Some limitations of the study included its unblinded nature, and the fact that 75 patients originally assigned to one treatment category crossed over to the other, including 14 in the culprit-lesion only category who underwent immediate multivessel PCI. This suggests that treatment strategy may need to be adopted to a patient’s clinical circumstances.
The CULPRIT-SHOCK results were published online at the time of Dr. Thiele’s presentation (N Engl J Med. 2017 Oct 30. doi:10/056/NEJMoa1710261).
Several of the study’s authors reported financial ties to the pharmaceutical industry.
This study’s findings reinforce those of previous trials that had suggested that multivessel percutaneous coronary intervention has higher early mortality than culprit-lesion-only PCI.
The study provides compelling evidence that culprit-lesion-only PCI should be the preferred treatment choice over multivessel PCI in patients with cardiogenic shock.
A previous meta-analysis of patients with uncomplicated ST-segment elevation myocardial infarction showed lower rates of mortality or MI with initial multivessel PCI. The disagreement between the two studies suggests that patients with cardiogenic shock may experience greater risk of these adverse outcomes during multivessel PCI procedures.
Future clinical trials should test individual multivessel revascularization strategies to reduce mortality in MI patients with cardiogenic shock, such as coronary artery bypass grafting (CABG) and venoarterial extracorporeal membrane oxygenation (ECMO).
Judith Hochman, MD, and Stuart Katz, MD, of New York University Langone Health, made these comments in an accompanying editorial (N Engl J Med. 2017 Oct 30. doi: 10.1056/nejme1713341). Dr. Hochman had no relevant disclosures. Dr. Katz has consulted for Novartis, Amgen, and Regeneron, and has received funding from Amgen, American Regent, and Janssen.
This study’s findings reinforce those of previous trials that had suggested that multivessel percutaneous coronary intervention has higher early mortality than culprit-lesion-only PCI.
The study provides compelling evidence that culprit-lesion-only PCI should be the preferred treatment choice over multivessel PCI in patients with cardiogenic shock.
A previous meta-analysis of patients with uncomplicated ST-segment elevation myocardial infarction showed lower rates of mortality or MI with initial multivessel PCI. The disagreement between the two studies suggests that patients with cardiogenic shock may experience greater risk of these adverse outcomes during multivessel PCI procedures.
Future clinical trials should test individual multivessel revascularization strategies to reduce mortality in MI patients with cardiogenic shock, such as coronary artery bypass grafting (CABG) and venoarterial extracorporeal membrane oxygenation (ECMO).
Judith Hochman, MD, and Stuart Katz, MD, of New York University Langone Health, made these comments in an accompanying editorial (N Engl J Med. 2017 Oct 30. doi: 10.1056/nejme1713341). Dr. Hochman had no relevant disclosures. Dr. Katz has consulted for Novartis, Amgen, and Regeneron, and has received funding from Amgen, American Regent, and Janssen.
This study’s findings reinforce those of previous trials that had suggested that multivessel percutaneous coronary intervention has higher early mortality than culprit-lesion-only PCI.
The study provides compelling evidence that culprit-lesion-only PCI should be the preferred treatment choice over multivessel PCI in patients with cardiogenic shock.
A previous meta-analysis of patients with uncomplicated ST-segment elevation myocardial infarction showed lower rates of mortality or MI with initial multivessel PCI. The disagreement between the two studies suggests that patients with cardiogenic shock may experience greater risk of these adverse outcomes during multivessel PCI procedures.
Future clinical trials should test individual multivessel revascularization strategies to reduce mortality in MI patients with cardiogenic shock, such as coronary artery bypass grafting (CABG) and venoarterial extracorporeal membrane oxygenation (ECMO).
Judith Hochman, MD, and Stuart Katz, MD, of New York University Langone Health, made these comments in an accompanying editorial (N Engl J Med. 2017 Oct 30. doi: 10.1056/nejme1713341). Dr. Hochman had no relevant disclosures. Dr. Katz has consulted for Novartis, Amgen, and Regeneron, and has received funding from Amgen, American Regent, and Janssen.
In patients with acute myocardial infarction and multivessel coronary artery disease with cardiogenic shock, 30-day rates of death and renal-replacement therapy were lower when patients underwent percutaneous coronary intervention (PCI) of the culprit lesion as opposed to multivessel PCI.
The difference appeared to be driven by mortality, as the renal endpoint alone was not significant.
As many as 80% of patients with cardiogenic shock also present with multivessel coronary artery disease, and this is associated with worse mortality. It is unclear whether immediate PCI of clinically important stenoses of major nonculprit coronary arteries is of benefit, and previous randomized trials comparing the procedures did not look at patients with cardiogenic shock, Holger Thiele, MD, reported at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
European guidelines suggest that PCI of nonculprit lesions should be considered in patients with cardiogenic shock, while U.S. guidelines offer no opinion, but recent appropriate use criteria recommend revascularization of a nonculprit artery if cardiogenic shock continues after the culprit artery has been repaired. It is thought that immediate revascularization of all coronary arteries with clinically important stenoses might improve overall myocardial perfusion and function in patients with cardiogenic shock, but the procedure could also have drawbacks, including additional ischemia, volume overload, and renal impairment from higher doses of contrast material.
To better understand outcomes in these patients, the Culprit Lesion Only PCI versus Multivessel PCI in Cardiogenic Shock (CULPRIT-SHOCK) trial randomized 706 patients to culprit-only PCI or multivessel PCI, in which PCI was performed on all major coronary arteries with more than 70% stenosis. Patients receiving culprit-only PCI could also undergo optional staged revascularization due to residual ischemic lesions, symptoms, or clinical or neurologic status.
At 30 days, death and/or renal-replacement therapy occurred in 45.9% of the culprit-only group, compared to 55.4% in the multivessel group (relative risk, 0.83; 95% confidence interval, 0.71-0.96; P = .01). A per-protocol analysis showed similar results (RR, 0.81; 95% CI, 0.69-0.96; P =.01), as did an analysis of the as-treated population (RR, 0.83; 95% CI, 0.72-0.97; P = .02).
All-cause mortality was lower in the culprit-only group (43.3% versus 51.6%; RR, 0.84; 95% CI, 0.72-0.98; P=.03). The rate of renal-replacement therapy was higher in the multivessel group (16.4% versus 11.6%), but this did not reach statistical significance (P = .07).
There were no statistically significant differences between the two groups with respect to recurrent myocardial infarction, rehospitalization for heart failure, bleeding, or stroke, Dr. Thiele reported at the meeting, which was sponsored by the Cardiovascular Research Foundation.
Some limitations of the study included its unblinded nature, and the fact that 75 patients originally assigned to one treatment category crossed over to the other, including 14 in the culprit-lesion only category who underwent immediate multivessel PCI. This suggests that treatment strategy may need to be adopted to a patient’s clinical circumstances.
The CULPRIT-SHOCK results were published online at the time of Dr. Thiele’s presentation (N Engl J Med. 2017 Oct 30. doi:10/056/NEJMoa1710261).
Several of the study’s authors reported financial ties to the pharmaceutical industry.
In patients with acute myocardial infarction and multivessel coronary artery disease with cardiogenic shock, 30-day rates of death and renal-replacement therapy were lower when patients underwent percutaneous coronary intervention (PCI) of the culprit lesion as opposed to multivessel PCI.
The difference appeared to be driven by mortality, as the renal endpoint alone was not significant.
As many as 80% of patients with cardiogenic shock also present with multivessel coronary artery disease, and this is associated with worse mortality. It is unclear whether immediate PCI of clinically important stenoses of major nonculprit coronary arteries is of benefit, and previous randomized trials comparing the procedures did not look at patients with cardiogenic shock, Holger Thiele, MD, reported at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
European guidelines suggest that PCI of nonculprit lesions should be considered in patients with cardiogenic shock, while U.S. guidelines offer no opinion, but recent appropriate use criteria recommend revascularization of a nonculprit artery if cardiogenic shock continues after the culprit artery has been repaired. It is thought that immediate revascularization of all coronary arteries with clinically important stenoses might improve overall myocardial perfusion and function in patients with cardiogenic shock, but the procedure could also have drawbacks, including additional ischemia, volume overload, and renal impairment from higher doses of contrast material.
To better understand outcomes in these patients, the Culprit Lesion Only PCI versus Multivessel PCI in Cardiogenic Shock (CULPRIT-SHOCK) trial randomized 706 patients to culprit-only PCI or multivessel PCI, in which PCI was performed on all major coronary arteries with more than 70% stenosis. Patients receiving culprit-only PCI could also undergo optional staged revascularization due to residual ischemic lesions, symptoms, or clinical or neurologic status.
At 30 days, death and/or renal-replacement therapy occurred in 45.9% of the culprit-only group, compared to 55.4% in the multivessel group (relative risk, 0.83; 95% confidence interval, 0.71-0.96; P = .01). A per-protocol analysis showed similar results (RR, 0.81; 95% CI, 0.69-0.96; P =.01), as did an analysis of the as-treated population (RR, 0.83; 95% CI, 0.72-0.97; P = .02).
All-cause mortality was lower in the culprit-only group (43.3% versus 51.6%; RR, 0.84; 95% CI, 0.72-0.98; P=.03). The rate of renal-replacement therapy was higher in the multivessel group (16.4% versus 11.6%), but this did not reach statistical significance (P = .07).
There were no statistically significant differences between the two groups with respect to recurrent myocardial infarction, rehospitalization for heart failure, bleeding, or stroke, Dr. Thiele reported at the meeting, which was sponsored by the Cardiovascular Research Foundation.
Some limitations of the study included its unblinded nature, and the fact that 75 patients originally assigned to one treatment category crossed over to the other, including 14 in the culprit-lesion only category who underwent immediate multivessel PCI. This suggests that treatment strategy may need to be adopted to a patient’s clinical circumstances.
The CULPRIT-SHOCK results were published online at the time of Dr. Thiele’s presentation (N Engl J Med. 2017 Oct 30. doi:10/056/NEJMoa1710261).
Several of the study’s authors reported financial ties to the pharmaceutical industry.
FROM TCT 2017
Key clinical point: The combined rate of 30-day mortality and renal-replacement therapy was lower when the culprit lesion alone was treated.
Major finding: The culprit-only PCI group had a relative risk of death or renal-replacement therapy of 0.83.
Data source: CULPRIT-SHOCK, a randomized, controlled trial of 706 patients.
Disclosures: Some of the study’s authors reported financial ties to the pharmaceutical industry. Dr. Katz has consulted for Novartis, Amgen, and Regeneron, and has received funding from Amgen, American Regent, and Janssen.
Red, peeling skin
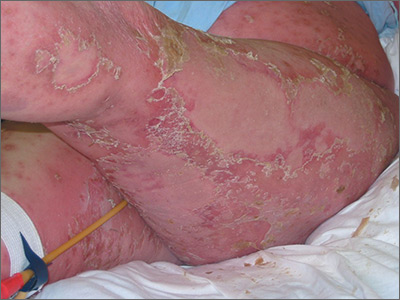
The FP diagnosed erythroderma, which can be life-threatening because of dehydration. The severe exfoliation (sheets of skin peeling off) impairs the skin’s barrier function, which leads to dehydration and makes the patient susceptible to infection. (This is similar to a patient with second-degree burns.)
At the hospital, the patient received supportive therapy with intravenous fluids and urine and blood cultures to check for infections. A Foley catheter was inserted to measure urine output. A dermatology colleague was available for consultation and performed a 4-mm punch biopsy to determine the etiology of the erythroderma, and a dermatopathologist was called.
The following labs were ordered in anticipation of cyclosporine therapy: complete blood count, chemistry panel, uric acid, magnesium level, QuantiFERON TB gold, hepatitis C antibody, and hepatitis B surface antigen, surface antibody, and core antibody.
The FP prescribed 0.1% triamcinolone ointment using the wet pajama technique: the ointment is applied over the red areas and then covered with wet hospital gowns overnight. The cloth is made wet with lukewarm water and wrung out so that it is not dripping with water. A dry blanket is applied over the wet layer of clothing. The patient then attempts to sleep in this and only removes it if she becomes chilled or is unable to sleep at all.
Results of the punch biopsy were consistent with erythrodermic psoriasis. The patient did not have a history of renal disease or hypertension, and lab tests returned sufficiently normal, so cyclosporine was not contraindicated. Oral cyclosporine was started while the patient was in the hospital. She recovered rapidly and was discharged with ongoing cyclosporine treatment and topical steroids. The patient followed up with the dermatologist for treatment.
Photo courtesy of Jack Resneck Sr, MD. Text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Henderson D. Erythroderma. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 915-921.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP diagnosed erythroderma, which can be life-threatening because of dehydration. The severe exfoliation (sheets of skin peeling off) impairs the skin’s barrier function, which leads to dehydration and makes the patient susceptible to infection. (This is similar to a patient with second-degree burns.)
At the hospital, the patient received supportive therapy with intravenous fluids and urine and blood cultures to check for infections. A Foley catheter was inserted to measure urine output. A dermatology colleague was available for consultation and performed a 4-mm punch biopsy to determine the etiology of the erythroderma, and a dermatopathologist was called.
The following labs were ordered in anticipation of cyclosporine therapy: complete blood count, chemistry panel, uric acid, magnesium level, QuantiFERON TB gold, hepatitis C antibody, and hepatitis B surface antigen, surface antibody, and core antibody.
The FP prescribed 0.1% triamcinolone ointment using the wet pajama technique: the ointment is applied over the red areas and then covered with wet hospital gowns overnight. The cloth is made wet with lukewarm water and wrung out so that it is not dripping with water. A dry blanket is applied over the wet layer of clothing. The patient then attempts to sleep in this and only removes it if she becomes chilled or is unable to sleep at all.
Results of the punch biopsy were consistent with erythrodermic psoriasis. The patient did not have a history of renal disease or hypertension, and lab tests returned sufficiently normal, so cyclosporine was not contraindicated. Oral cyclosporine was started while the patient was in the hospital. She recovered rapidly and was discharged with ongoing cyclosporine treatment and topical steroids. The patient followed up with the dermatologist for treatment.
Photo courtesy of Jack Resneck Sr, MD. Text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Henderson D. Erythroderma. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 915-921.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP diagnosed erythroderma, which can be life-threatening because of dehydration. The severe exfoliation (sheets of skin peeling off) impairs the skin’s barrier function, which leads to dehydration and makes the patient susceptible to infection. (This is similar to a patient with second-degree burns.)
At the hospital, the patient received supportive therapy with intravenous fluids and urine and blood cultures to check for infections. A Foley catheter was inserted to measure urine output. A dermatology colleague was available for consultation and performed a 4-mm punch biopsy to determine the etiology of the erythroderma, and a dermatopathologist was called.
The following labs were ordered in anticipation of cyclosporine therapy: complete blood count, chemistry panel, uric acid, magnesium level, QuantiFERON TB gold, hepatitis C antibody, and hepatitis B surface antigen, surface antibody, and core antibody.
The FP prescribed 0.1% triamcinolone ointment using the wet pajama technique: the ointment is applied over the red areas and then covered with wet hospital gowns overnight. The cloth is made wet with lukewarm water and wrung out so that it is not dripping with water. A dry blanket is applied over the wet layer of clothing. The patient then attempts to sleep in this and only removes it if she becomes chilled or is unable to sleep at all.
Results of the punch biopsy were consistent with erythrodermic psoriasis. The patient did not have a history of renal disease or hypertension, and lab tests returned sufficiently normal, so cyclosporine was not contraindicated. Oral cyclosporine was started while the patient was in the hospital. She recovered rapidly and was discharged with ongoing cyclosporine treatment and topical steroids. The patient followed up with the dermatologist for treatment.
Photo courtesy of Jack Resneck Sr, MD. Text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Henderson D. Erythroderma. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 915-921.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Effects of Platelet-Rich Plasma and Indomethacin on Biomechanics of Rotator Cuff Repair
Take-Home Points
- The optimal centrifugation protocol for production of rat PRP is 1300 rpm for 5 minutes.
- PRP administration in RCR improves tendon biomechanics in a rat model.
- Administration of NSAIDs following RCR has no significant effect on tendon biomechanical properties.
- NSAIDs may be co-administered with PRP without reducing efficacy of PRP.
- The role of PRP and NSAIDs in human RCR remains unclear.
Rotator cuff tears are a common source of shoulder pain and disability among older adults and athletes. Full-thickness tears alone occur in up to 30% of adults older than 60 years.1 Surgical repair is plagued by an unpredictable rate of recurrence (range, 11%-94%).1-10 As a result of improved suture materials, knotting patterns, and anchor designs, hardware issues are no longer the primary cause of rotator cuff repair (RCR) failures; now the principal mode of failure is biologic.2 Animal model studies have found that, after injury and subsequent healing, the tendon–bone interface remains abnormal.11 Rotator cuff research therefore has focused largely on biological enhancement of tendon-to-bone healing.
One means of biological augmentation is autologous platelet-rich plasma (PRP), which has supraphysiologic concentrations of platelets and their secreted growth factors. Although there is no consensus on the long-term efficacy of PRP, some studies suggest PRP accelerates healing over short and intermediate terms, which may contribute to a more rapid decrease in pain and more rapid return to normal activities.12-18 Similarly, systemic nonsteroidal anti-inflammatory drugs (NSAIDs) have long been used to treat musculoskeletal injuries, including rotator cuff pathology. However, NSAIDs inhibit cyclooxygenase activity, and clinical and experimental data have shown that cyclooxygenase 2 function is crucial in normal tendon-to-bone healing.19-21
Comprehensive studies have been conducted on the efficacy of both PRP and NSAIDs, but the interaction of concurrently used PRP and NSAIDs has not been determined. As many physicians use both modalities in the treatment of soft-tissue injuries, it is important to study the potential interactions when coadministered. Prior studies in small animal models suggest NSAIDs may impair tendon-to-bone healing in RCR, but there is no evidence regarding the effect of NSAIDs on the efficacy of PRP treatment.21
We conducted a study to determine the interaction of PRP and NSAIDs when used as adjuncts to RCR in a rat model. We hypothesized that PRP would increase the strength of RCR and that NSAIDs would interfere with the effects of PRP. A preliminary study objective was to determine an appropriate centrifugation protocol for producing PRP from rat blood, for use in this study and in future rat-based studies of PRP.
Materials and Methods
Part A: Pretesting Determination of PRP Centrifugation Protocol
Fourteen adult male Fischer rats were used in part A of this study, which was conducted to determine an appropriate PRP centrifugation protocol. Traditional PRP centrifugation protocols are established for human blood, but rat red blood cells (RBCs) and human RBCs differ in size.22 In our preliminary study, we wanted to determine the adjusted centrifuge speed and duration for producing clinically optimal PRP from rats. Clinically optimal PRP has reduced levels of RBCs, which decrease platelet affinity. Although the role of leukocytes in PRP preparations is debated, reducing the number of white blood cells (WBCs) decreases the number of matrix metalloproteinases and reactive oxygen species that may lead to inflammation. We used the platelet index (ratio of platelets to WBCs) and the RBC count to quantify the quality of our PRP sample.
Each rat in part A was anesthetized while supine. We used the Autologous Conditioned Plasma (ACP) system (Arthrex), which requires only 1 centrifugation cycle to create PRP. About 9 mL or 10 mL of blood was obtained by cardiac aspiration using an ACP Double Syringe (Arthrex). After blood retrieval, a thoracotomy was performed to confirm each rat’s death.
Part B: Determining the Effects of PRP and NSAIDs on RCR in a Rat Model
Operative Cohort. Of the 34 Fischer rats used in part B of this study, 6 were used as blood donors for PRP production, and the other 28 underwent bilateral rotator cuff surgeries. We used donor rats to maximize the amount of PRP retrieval, allocating about 1 donor rat per 5 operative rats. Fischer rats are an inbred strain, so the PRP from a donor Fischer rat simulates autologous blood in other Fischer rats. Use of allogenic blood is consistent with prior rat PRP studies.23,24
Operative Technique. Each bilateral surgery was performed by a single board-certified shoulder surgeon, and the anesthetic and surgical protocols were followed as approved by the home institution’s Institutional Animal Care and Use Committee. Before surgery, blood was harvested for PRP production from donor rats, as described earlier, and centrifuged for 5 minutes × 1300 rpm. After anesthetic induction and skin incision, the deltoid muscle was cut to expose the acromion and underlying rotator cuff. The distal supraspinatus tendon was sharply detached from the greater tuberosity. A bone-tunnel RCR was performed by drilling a transverse tunnel across the greater tuberosity and affixing the tendon to its footprint with a 5-0 polypropylene suture (Prolene; Ethicon). Each rat was then randomly assigned to receive 50 µL of donor PRP injected in 1 operative shoulder and saline in the contralateral shoulder. Injections were made in the supraspinatus tendon at its attachment to the humerus. Deltoid and skin were closed with 4-0 polyglactin (Vicryl) suture (Ethicon) and staples, respectively.
Tendon Preparation. Immediately post mortem, each shoulder was grossly dissected to isolate the supraspinatus muscle attached to the humerus. Shoulders were then frozen in 0.15-M saline solution until specified biomechanical testing dates.
On day of dimensional/biomechanical testing, each specimen was thawed at room temperature and finely dissected under a microscope (Stemi 200-C; Car Zeiss). After dissection, the humeral shaft was embedded in polymethylmethacrylate within a test tube. The free end of the supraspinatus tendon was glued within a “tab” of waterproofed emery cloth, leaving about 2 mm of tendon between the tab and the greater tuberosity.
Biomechanical Analysis. A 5848 MicroTester (Instron) was used for biomechanical testing. Each tabbed tendon, held by a pneumatic clamp attached to the MicroTester, was tested in a preconditioning phase and then a ramp-to-failure phase. A constant drip of 0.15-M saline was run through the apparatus to simulate physiologic hydration of tissue. After the embedded specimen was secure within the loading apparatus, an initial tensile preload of 0.2 N was applied. After preloading, the tendon was run through a preconditioning phase to account for viscoelastic relaxation. Immediately after preconditioning, each tendon was subjected to failure testing at a ramp rate of 0.1 mm/s. Force data were collected as a function of displacement, allowing for the calculation of 4 biomechanical parameters: failure force, tendon stiffness and normalized stiffness, energy to failure, and total energy. Tendon stiffness is the slope of a curve-fit line of the initial peak; failure force is the force of the highest peak; energy to failure is the area under the curve (AUC) to the highest peak; and total energy is the AUC from the start of failure ramping to the point at which the tendon is torn off completely. Two-way ANOVA was used to assess the differences between treatment groups and diet groups for all parameters. Statistical significance was set at P < .05.
A power analysis was performed to determine ability to detect differences between cohorts. For power of 80% and P = .05, a difference of 16% of the mean could be detected for failure force, 30% for energy to failure, 14% for total energy to failure, and 24% for stiffness. In addition, a difference of 4% of the mean could be detected for tendon length, 6% for width, and 10% for thickness.
Results
Across all collective treatment-diet groups and biomechanical parameters, there was only 1 statistically significant difference. Mean (SD) energy to failure was significantly higher (P = .03) in shoulders treated with PRP, 11.7 (7.3) N-mm, than in those treated without PRP, 8.7 (4.6) N-mm (Figure 4). There were no statistically significant differences between shoulders treated with indomethacin and those treated without indomethacin (Table 3), and no statistically significant relationships between treatment and drug for any other biomechanical parameter (Figures 5-7).
Discussion
Our preliminary objective in this study was to determine the optimal centrifugation protocol for producing rat-based PRP. Optimal PRP requires a dense concentration of platelets as well as reduced levels of RBCs and WBCs.25 We used the platelet index to quantify the quality of our PRP samples, and we obtained the highest platelet index for the protocol of 5 minutes × 1300 rpm. This finding may be useful in later rat studies involving PRP.
The primary objective of this study was to assess the effect of the interaction of PRP and NSAIDs on RCR. PRP has been found to augment RCR,12,26,27 but indomethacin may impair healing.21,25 We hypothesized that shoulders treated with PRP would have more biomechanical strength than control shoulders and that indomethacin would decrease biomechanical strength.
Our data showed increased energy to failure of the rotator cuff with PRP injections (P = .03). All other biomechanical parameters showed no significant differences with PRP treatment, though there were statistically insignificant trends of increased total energy, failure force, and stiffness in the PRP cohorts. There were no statistically significant differences between the indomethacin and no-indomethacin groups, and indomethacin had no effect on the efficacy of PRP treatment. It should be noted that the measurements of total energy, energy to failure, and failure force best reflect the strength of the tendon–bone interface. Other biomechanical measures, such as stiffness and normalized stiffness, are physical properties of the tendon itself and apply less to enthesis strength, which was the primary focus of this study.
Beck and colleagues23 studied the effect of allogeneic PRP on RCR in a rat model. They tested biomechanical and histologic outcomes 7, 14, and 21 days after surgery. There was no significant difference in failure load between the 2 groups at any time point. Compared with failure strain in the control group, failure strain in the PRP group was decreased at 7 days, normalized at 14 days, and increased at 21 days. The authors hypothesized that increased tendon failure strain at 21 days may have reduced forces being transmitted to the suture fixation site, which may be clinically significant and warrants further investigation. In a similar study, by Dolkart and colleagues,28 intraoperative PRP administration enhanced the maximal load-to-failure and stiffness of rats’ repaired rotator cuffs. On histologic examination, tendons treated with PRP (vs control tendons) had more organized collagen. Although these studies have limitations similar to our study, these results further support improved tendon-to-bone healing with PRP.
In clinical application, Barber and colleagues26 found that, compared with controls, suturing PRP fibrin matrix into the rotator cuff during repair decreased the incidence of magnetic resonance imaging–detected retears. However, in 2 prospective, randomized trials, Castricini and colleagues29 and Weber and colleagues30 found that use of PRP in RCR did not improve outcomes. All 3 studies differ from ours in that they used fibrin matrix. However, Ersen and colleagues31 found no difference in the effects of PRP on rotator cuff healing between injection and fibrin matrix; PRP improved biomechanical properties of repaired rotator cuff independent of administration method. In a meta-analysis of PRP supplementation in RCR, Warth and colleagues32 found a statistically significant improvement in retear rates for tears >3 cm repaired with a double-row technique, but otherwise no overall improvement in retear rates or outcome scores with PRP. The authors acknowledged that the significant heterogeneity of the studies in their meta-analysis may have affected the quality of their data.
Although our study provides some insight into the effectiveness of PRP in tendon repair, the lack of standardization in PRP preparation and time points tested makes comparisons with similar studies difficult.33 Recent reports have emphasized that not all PRP separation systems yield similar products.33 Platelet concentrations, and therefore platelet-derived growth factor concentrations, differ between systems and may yield different clinical outcomes. Our decision to use leukocyte-reduced PRP is supported by a meta-analysis by Riboh and colleagues,34 who reviewed the literature on the effect of leukocyte concentration on the efficacy of PRP products. They found that, in the treatment of knee osteoarthritis, use of leukocyte-poor PRP resulted in improved functional outcomes scores in comparison with placebo, but this improvement did not occur with leukocyte-rich PRP. However, there is still no consensus on optimal preparation, dosing, and route of administration of PRP, and preparations described in the literature vary.
This study also assessed the interaction of PRP and NSAIDs. Although there were no statistically significant differences between treatment and diet, shoulders treated with indomethacin alone showed a trend toward weaker biomechanical parameters in comparison with shoulders treated with saline alone, with PRP alone, or with both PRP and indomethacin. A larger sample would be needed to establish statistical significance. These trends are not surprising, as Cohen and colleagues21 found that NSAIDs, specifically indomethacin and celecoxib, significantly inhibited rotator cuff tendon-to-bone healing. The authors also found that a 2-week course of indomethacin was sufficient to significantly inhibit tendon-to-bone healing. In fact, although the drugs were discontinued after 14 days, biomechanical properties were negatively affected up to 8 weeks after repair. Our results differ from theirs even though the 2 studies used similar doses and administration protocols.
One strength of this study was that all surgeries were performed by a single board-certified surgeon using a standardized technique. In addition, a control group was established, and personnel and techniques for all fine dissections and biomechanical tests were consistent throughout. Blinded randomization and diet normalization, as well as adequate power for detecting significant effects, strengthened the study as well.
The study had several limitations. First, whereas most human rotator cuff tears are chronic, we used a model of acute injury and repair. As acute tears that are immediately repaired are more likely to heal, detection of differences between cohorts is less likely. However, using an acute model is still the most reliable strategy for inducing a controlled injury with reproducible severity. Second, we analyzed data at only 1 time point, which may not provide an accurate representation of long-term effects. Third, systemic administration of indomethacin did not allow for intra-rat shoulder comparisons of the different drug groups. Fourth, although it is possible that the dosage of NSAID was insufficient to produce significant differences in biomechanics, our dosage was consistent with that used in a study that found a significant effect on tendon healing.21
Conclusion
Our study found that the strength of the supraspinatus tendon enthesis as defined by energy to failure was increased with intratendinous PRP injection. Indomethacin showed no statistical effect, but there was a trend toward reduced strength after repair. However, the extent to which coadministration of indomethacin affects PRP remains unclear, and these data cannot necessarily be extrapolated to the typical human rotator cuff tear caused by chronic repetitive stress.
1. Kinsella KG, Velkoff VA. An Aging World: 2001. Washington, DC: US Government Printing Office; 2001. https://www.census.gov/prod/2001pubs/p95-01-1.pdf. Published November 2001. Accessed September 24, 2017.
2. Gamradt SC, Rodeo SA, Warren RF. Platelet rich plasma in rotator cuff repair. Tech Orthop. 2007;22(1):26-33.
3. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
4. Harryman DT, Mack LA, Wang KY. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
5. Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15(3):290-299.
6. Boileau P, Brassart N, Watkinson DJ, Carles M. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
7. Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82(4):505-515.
8. Lafosse L, Brozska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2007;89(7):1533-1541.
9. Levy O, Venkateswaran B, Even T, Ravenscroft M, Copeland S. Mid-term clinical and sonographic outcome of arthroscopic repair of the rotator cuff. J Bone Joint Surg Br. 2008;90(10):1341-1347.
10. Zumstein MA, Jost B, Hempel J, Hodler J, Gerber C. The clinical and structural long-term results of open repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2008;90(11):2423-2431.
11. Gerber C, Schneeberger AG, Perren SM, Nyffeler RW. Experimental rotator cuff repair. A preliminary study. J Bone Joint Surg Am. 1999;81(9):1281-1290.
12. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
13. Akeda K, An HS, Okuma M, et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage. 2006;14(12):1272-1280.
14. de Mos M, van der Windt AE, Jahr H, et al. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008;36(6):1171-1178.
15. Harmon KG. Muscle injuries and PRP: what does the science say? Br J Sports Med. 2010;44(9):616-617.
16. Kasten P, Vogel J, Geiger F, Niemeyer P, Luginbühl R, Szalay K. The effect of platelet-rich plasma on healing in critical-size long-bone defects. Biomaterials. 2008;29(29):3983-3992.
17. Mei-Dan O, Mann G, Maffulli N. Platelet-rich plasma: any substance into it? Br J Sports Med. 2010;44(9):618-619.
18. Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25(8):1007-1017.
19. Virchenko O, Skoglund B, Aspenberg P. Parecoxib impairs early tendon repair but improves later remodeling. Am J Sports Med. 2004;32(7):1743-1747.
20. Aspenberg P. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2004;22(3):684.
21. Cohen DB, Kawamura S, Ehteshami JR, Rodeo SA. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;34(3):362-369.
22. Balazs T, Grice HC, Airth JM. On counting the blood cells of the rat with an electronic counter. Can J Comp Med Vet Sci. 1960;24(9):273-275.
23. Beck J, Evans D, Tonino PM, Yong S, Callaci JJ. The biomechanical and histologic effects of platelet-rich plasma on rat rotator cuff repairs. Am J Sports Med. 2012;40(9):2037-2044.
24. Aspenberg P, Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand. 2004;75(1):93-99.
25. Chechik O, Dolkart O, Mozes G, Rak O, Alhajajra F, Maman E. Timing matters: NSAIDs interfere with the late proliferation stage of a repaired rotator cuff tendon healing in rats. Arch Orthop Trauma Surg. 2014;134(4):515-520.
26. Barber FA, Hrnack SA, Snyder SJ, Hapa O. Rotator cuff repair healing influenced by platelet-rich plasma construct augmentation. Arthroscopy. 2011;27(8):1029-1035.
27. Randelli PS, Arrigoni P, Cabitza P, Volpi P, Maffulli N. Autologous platelet rich plasma for arthroscopic rotator cuff repair. A pilot study. Disabil Rehabil. 2008;30(20-22):1584-1589.
28. Dolkart O, Chechik O, Zarfati Y, Brosh T, Alhajajra F, Maman E. A single dose of platelet-rich plasma improves the organization and strength of a surgically repaired rotator cuff tendon in rats. Arch Orthop Trauma Surg. 2014;134(9):1271-1277.
29. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
30. Weber SC, Kauffman JI, Parise C, Weber SJ, Katz SD. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. Am J Sports Med. 2013;41(2):263-270.
31. Ersen A, Demirhan M, Atalar AC, Kapicioğlu M, Baysal G. Platelet-rich plasma for enhancing surgical rotator cuff repair: evaluation and comparison of two application methods in a rat model. Arch Orthop Trauma Surg. 2014;134(3):405-411.
32. Warth RJ, Dornan GJ, James EW, Horan MP, Millett PJ. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. 2015;31(2):306-320.
33. Bergeson AG, Tashjian RZ, Greis PE, Crim J, Stoddard GJ, Burks RT. Effects of platelet-rich fibrin matrix on repair integrity of at-risk rotator cuff tears. Am J Sports Med. 2012;40(2):286-293.
34. Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med. 2016;44(3):792-800.
Take-Home Points
- The optimal centrifugation protocol for production of rat PRP is 1300 rpm for 5 minutes.
- PRP administration in RCR improves tendon biomechanics in a rat model.
- Administration of NSAIDs following RCR has no significant effect on tendon biomechanical properties.
- NSAIDs may be co-administered with PRP without reducing efficacy of PRP.
- The role of PRP and NSAIDs in human RCR remains unclear.
Rotator cuff tears are a common source of shoulder pain and disability among older adults and athletes. Full-thickness tears alone occur in up to 30% of adults older than 60 years.1 Surgical repair is plagued by an unpredictable rate of recurrence (range, 11%-94%).1-10 As a result of improved suture materials, knotting patterns, and anchor designs, hardware issues are no longer the primary cause of rotator cuff repair (RCR) failures; now the principal mode of failure is biologic.2 Animal model studies have found that, after injury and subsequent healing, the tendon–bone interface remains abnormal.11 Rotator cuff research therefore has focused largely on biological enhancement of tendon-to-bone healing.
One means of biological augmentation is autologous platelet-rich plasma (PRP), which has supraphysiologic concentrations of platelets and their secreted growth factors. Although there is no consensus on the long-term efficacy of PRP, some studies suggest PRP accelerates healing over short and intermediate terms, which may contribute to a more rapid decrease in pain and more rapid return to normal activities.12-18 Similarly, systemic nonsteroidal anti-inflammatory drugs (NSAIDs) have long been used to treat musculoskeletal injuries, including rotator cuff pathology. However, NSAIDs inhibit cyclooxygenase activity, and clinical and experimental data have shown that cyclooxygenase 2 function is crucial in normal tendon-to-bone healing.19-21
Comprehensive studies have been conducted on the efficacy of both PRP and NSAIDs, but the interaction of concurrently used PRP and NSAIDs has not been determined. As many physicians use both modalities in the treatment of soft-tissue injuries, it is important to study the potential interactions when coadministered. Prior studies in small animal models suggest NSAIDs may impair tendon-to-bone healing in RCR, but there is no evidence regarding the effect of NSAIDs on the efficacy of PRP treatment.21
We conducted a study to determine the interaction of PRP and NSAIDs when used as adjuncts to RCR in a rat model. We hypothesized that PRP would increase the strength of RCR and that NSAIDs would interfere with the effects of PRP. A preliminary study objective was to determine an appropriate centrifugation protocol for producing PRP from rat blood, for use in this study and in future rat-based studies of PRP.
Materials and Methods
Part A: Pretesting Determination of PRP Centrifugation Protocol
Fourteen adult male Fischer rats were used in part A of this study, which was conducted to determine an appropriate PRP centrifugation protocol. Traditional PRP centrifugation protocols are established for human blood, but rat red blood cells (RBCs) and human RBCs differ in size.22 In our preliminary study, we wanted to determine the adjusted centrifuge speed and duration for producing clinically optimal PRP from rats. Clinically optimal PRP has reduced levels of RBCs, which decrease platelet affinity. Although the role of leukocytes in PRP preparations is debated, reducing the number of white blood cells (WBCs) decreases the number of matrix metalloproteinases and reactive oxygen species that may lead to inflammation. We used the platelet index (ratio of platelets to WBCs) and the RBC count to quantify the quality of our PRP sample.
Each rat in part A was anesthetized while supine. We used the Autologous Conditioned Plasma (ACP) system (Arthrex), which requires only 1 centrifugation cycle to create PRP. About 9 mL or 10 mL of blood was obtained by cardiac aspiration using an ACP Double Syringe (Arthrex). After blood retrieval, a thoracotomy was performed to confirm each rat’s death.
Part B: Determining the Effects of PRP and NSAIDs on RCR in a Rat Model
Operative Cohort. Of the 34 Fischer rats used in part B of this study, 6 were used as blood donors for PRP production, and the other 28 underwent bilateral rotator cuff surgeries. We used donor rats to maximize the amount of PRP retrieval, allocating about 1 donor rat per 5 operative rats. Fischer rats are an inbred strain, so the PRP from a donor Fischer rat simulates autologous blood in other Fischer rats. Use of allogenic blood is consistent with prior rat PRP studies.23,24
Operative Technique. Each bilateral surgery was performed by a single board-certified shoulder surgeon, and the anesthetic and surgical protocols were followed as approved by the home institution’s Institutional Animal Care and Use Committee. Before surgery, blood was harvested for PRP production from donor rats, as described earlier, and centrifuged for 5 minutes × 1300 rpm. After anesthetic induction and skin incision, the deltoid muscle was cut to expose the acromion and underlying rotator cuff. The distal supraspinatus tendon was sharply detached from the greater tuberosity. A bone-tunnel RCR was performed by drilling a transverse tunnel across the greater tuberosity and affixing the tendon to its footprint with a 5-0 polypropylene suture (Prolene; Ethicon). Each rat was then randomly assigned to receive 50 µL of donor PRP injected in 1 operative shoulder and saline in the contralateral shoulder. Injections were made in the supraspinatus tendon at its attachment to the humerus. Deltoid and skin were closed with 4-0 polyglactin (Vicryl) suture (Ethicon) and staples, respectively.
Tendon Preparation. Immediately post mortem, each shoulder was grossly dissected to isolate the supraspinatus muscle attached to the humerus. Shoulders were then frozen in 0.15-M saline solution until specified biomechanical testing dates.
On day of dimensional/biomechanical testing, each specimen was thawed at room temperature and finely dissected under a microscope (Stemi 200-C; Car Zeiss). After dissection, the humeral shaft was embedded in polymethylmethacrylate within a test tube. The free end of the supraspinatus tendon was glued within a “tab” of waterproofed emery cloth, leaving about 2 mm of tendon between the tab and the greater tuberosity.
Biomechanical Analysis. A 5848 MicroTester (Instron) was used for biomechanical testing. Each tabbed tendon, held by a pneumatic clamp attached to the MicroTester, was tested in a preconditioning phase and then a ramp-to-failure phase. A constant drip of 0.15-M saline was run through the apparatus to simulate physiologic hydration of tissue. After the embedded specimen was secure within the loading apparatus, an initial tensile preload of 0.2 N was applied. After preloading, the tendon was run through a preconditioning phase to account for viscoelastic relaxation. Immediately after preconditioning, each tendon was subjected to failure testing at a ramp rate of 0.1 mm/s. Force data were collected as a function of displacement, allowing for the calculation of 4 biomechanical parameters: failure force, tendon stiffness and normalized stiffness, energy to failure, and total energy. Tendon stiffness is the slope of a curve-fit line of the initial peak; failure force is the force of the highest peak; energy to failure is the area under the curve (AUC) to the highest peak; and total energy is the AUC from the start of failure ramping to the point at which the tendon is torn off completely. Two-way ANOVA was used to assess the differences between treatment groups and diet groups for all parameters. Statistical significance was set at P < .05.
A power analysis was performed to determine ability to detect differences between cohorts. For power of 80% and P = .05, a difference of 16% of the mean could be detected for failure force, 30% for energy to failure, 14% for total energy to failure, and 24% for stiffness. In addition, a difference of 4% of the mean could be detected for tendon length, 6% for width, and 10% for thickness.
Results
Across all collective treatment-diet groups and biomechanical parameters, there was only 1 statistically significant difference. Mean (SD) energy to failure was significantly higher (P = .03) in shoulders treated with PRP, 11.7 (7.3) N-mm, than in those treated without PRP, 8.7 (4.6) N-mm (Figure 4). There were no statistically significant differences between shoulders treated with indomethacin and those treated without indomethacin (Table 3), and no statistically significant relationships between treatment and drug for any other biomechanical parameter (Figures 5-7).
Discussion
Our preliminary objective in this study was to determine the optimal centrifugation protocol for producing rat-based PRP. Optimal PRP requires a dense concentration of platelets as well as reduced levels of RBCs and WBCs.25 We used the platelet index to quantify the quality of our PRP samples, and we obtained the highest platelet index for the protocol of 5 minutes × 1300 rpm. This finding may be useful in later rat studies involving PRP.
The primary objective of this study was to assess the effect of the interaction of PRP and NSAIDs on RCR. PRP has been found to augment RCR,12,26,27 but indomethacin may impair healing.21,25 We hypothesized that shoulders treated with PRP would have more biomechanical strength than control shoulders and that indomethacin would decrease biomechanical strength.
Our data showed increased energy to failure of the rotator cuff with PRP injections (P = .03). All other biomechanical parameters showed no significant differences with PRP treatment, though there were statistically insignificant trends of increased total energy, failure force, and stiffness in the PRP cohorts. There were no statistically significant differences between the indomethacin and no-indomethacin groups, and indomethacin had no effect on the efficacy of PRP treatment. It should be noted that the measurements of total energy, energy to failure, and failure force best reflect the strength of the tendon–bone interface. Other biomechanical measures, such as stiffness and normalized stiffness, are physical properties of the tendon itself and apply less to enthesis strength, which was the primary focus of this study.
Beck and colleagues23 studied the effect of allogeneic PRP on RCR in a rat model. They tested biomechanical and histologic outcomes 7, 14, and 21 days after surgery. There was no significant difference in failure load between the 2 groups at any time point. Compared with failure strain in the control group, failure strain in the PRP group was decreased at 7 days, normalized at 14 days, and increased at 21 days. The authors hypothesized that increased tendon failure strain at 21 days may have reduced forces being transmitted to the suture fixation site, which may be clinically significant and warrants further investigation. In a similar study, by Dolkart and colleagues,28 intraoperative PRP administration enhanced the maximal load-to-failure and stiffness of rats’ repaired rotator cuffs. On histologic examination, tendons treated with PRP (vs control tendons) had more organized collagen. Although these studies have limitations similar to our study, these results further support improved tendon-to-bone healing with PRP.
In clinical application, Barber and colleagues26 found that, compared with controls, suturing PRP fibrin matrix into the rotator cuff during repair decreased the incidence of magnetic resonance imaging–detected retears. However, in 2 prospective, randomized trials, Castricini and colleagues29 and Weber and colleagues30 found that use of PRP in RCR did not improve outcomes. All 3 studies differ from ours in that they used fibrin matrix. However, Ersen and colleagues31 found no difference in the effects of PRP on rotator cuff healing between injection and fibrin matrix; PRP improved biomechanical properties of repaired rotator cuff independent of administration method. In a meta-analysis of PRP supplementation in RCR, Warth and colleagues32 found a statistically significant improvement in retear rates for tears >3 cm repaired with a double-row technique, but otherwise no overall improvement in retear rates or outcome scores with PRP. The authors acknowledged that the significant heterogeneity of the studies in their meta-analysis may have affected the quality of their data.
Although our study provides some insight into the effectiveness of PRP in tendon repair, the lack of standardization in PRP preparation and time points tested makes comparisons with similar studies difficult.33 Recent reports have emphasized that not all PRP separation systems yield similar products.33 Platelet concentrations, and therefore platelet-derived growth factor concentrations, differ between systems and may yield different clinical outcomes. Our decision to use leukocyte-reduced PRP is supported by a meta-analysis by Riboh and colleagues,34 who reviewed the literature on the effect of leukocyte concentration on the efficacy of PRP products. They found that, in the treatment of knee osteoarthritis, use of leukocyte-poor PRP resulted in improved functional outcomes scores in comparison with placebo, but this improvement did not occur with leukocyte-rich PRP. However, there is still no consensus on optimal preparation, dosing, and route of administration of PRP, and preparations described in the literature vary.
This study also assessed the interaction of PRP and NSAIDs. Although there were no statistically significant differences between treatment and diet, shoulders treated with indomethacin alone showed a trend toward weaker biomechanical parameters in comparison with shoulders treated with saline alone, with PRP alone, or with both PRP and indomethacin. A larger sample would be needed to establish statistical significance. These trends are not surprising, as Cohen and colleagues21 found that NSAIDs, specifically indomethacin and celecoxib, significantly inhibited rotator cuff tendon-to-bone healing. The authors also found that a 2-week course of indomethacin was sufficient to significantly inhibit tendon-to-bone healing. In fact, although the drugs were discontinued after 14 days, biomechanical properties were negatively affected up to 8 weeks after repair. Our results differ from theirs even though the 2 studies used similar doses and administration protocols.
One strength of this study was that all surgeries were performed by a single board-certified surgeon using a standardized technique. In addition, a control group was established, and personnel and techniques for all fine dissections and biomechanical tests were consistent throughout. Blinded randomization and diet normalization, as well as adequate power for detecting significant effects, strengthened the study as well.
The study had several limitations. First, whereas most human rotator cuff tears are chronic, we used a model of acute injury and repair. As acute tears that are immediately repaired are more likely to heal, detection of differences between cohorts is less likely. However, using an acute model is still the most reliable strategy for inducing a controlled injury with reproducible severity. Second, we analyzed data at only 1 time point, which may not provide an accurate representation of long-term effects. Third, systemic administration of indomethacin did not allow for intra-rat shoulder comparisons of the different drug groups. Fourth, although it is possible that the dosage of NSAID was insufficient to produce significant differences in biomechanics, our dosage was consistent with that used in a study that found a significant effect on tendon healing.21
Conclusion
Our study found that the strength of the supraspinatus tendon enthesis as defined by energy to failure was increased with intratendinous PRP injection. Indomethacin showed no statistical effect, but there was a trend toward reduced strength after repair. However, the extent to which coadministration of indomethacin affects PRP remains unclear, and these data cannot necessarily be extrapolated to the typical human rotator cuff tear caused by chronic repetitive stress.
Take-Home Points
- The optimal centrifugation protocol for production of rat PRP is 1300 rpm for 5 minutes.
- PRP administration in RCR improves tendon biomechanics in a rat model.
- Administration of NSAIDs following RCR has no significant effect on tendon biomechanical properties.
- NSAIDs may be co-administered with PRP without reducing efficacy of PRP.
- The role of PRP and NSAIDs in human RCR remains unclear.
Rotator cuff tears are a common source of shoulder pain and disability among older adults and athletes. Full-thickness tears alone occur in up to 30% of adults older than 60 years.1 Surgical repair is plagued by an unpredictable rate of recurrence (range, 11%-94%).1-10 As a result of improved suture materials, knotting patterns, and anchor designs, hardware issues are no longer the primary cause of rotator cuff repair (RCR) failures; now the principal mode of failure is biologic.2 Animal model studies have found that, after injury and subsequent healing, the tendon–bone interface remains abnormal.11 Rotator cuff research therefore has focused largely on biological enhancement of tendon-to-bone healing.
One means of biological augmentation is autologous platelet-rich plasma (PRP), which has supraphysiologic concentrations of platelets and their secreted growth factors. Although there is no consensus on the long-term efficacy of PRP, some studies suggest PRP accelerates healing over short and intermediate terms, which may contribute to a more rapid decrease in pain and more rapid return to normal activities.12-18 Similarly, systemic nonsteroidal anti-inflammatory drugs (NSAIDs) have long been used to treat musculoskeletal injuries, including rotator cuff pathology. However, NSAIDs inhibit cyclooxygenase activity, and clinical and experimental data have shown that cyclooxygenase 2 function is crucial in normal tendon-to-bone healing.19-21
Comprehensive studies have been conducted on the efficacy of both PRP and NSAIDs, but the interaction of concurrently used PRP and NSAIDs has not been determined. As many physicians use both modalities in the treatment of soft-tissue injuries, it is important to study the potential interactions when coadministered. Prior studies in small animal models suggest NSAIDs may impair tendon-to-bone healing in RCR, but there is no evidence regarding the effect of NSAIDs on the efficacy of PRP treatment.21
We conducted a study to determine the interaction of PRP and NSAIDs when used as adjuncts to RCR in a rat model. We hypothesized that PRP would increase the strength of RCR and that NSAIDs would interfere with the effects of PRP. A preliminary study objective was to determine an appropriate centrifugation protocol for producing PRP from rat blood, for use in this study and in future rat-based studies of PRP.
Materials and Methods
Part A: Pretesting Determination of PRP Centrifugation Protocol
Fourteen adult male Fischer rats were used in part A of this study, which was conducted to determine an appropriate PRP centrifugation protocol. Traditional PRP centrifugation protocols are established for human blood, but rat red blood cells (RBCs) and human RBCs differ in size.22 In our preliminary study, we wanted to determine the adjusted centrifuge speed and duration for producing clinically optimal PRP from rats. Clinically optimal PRP has reduced levels of RBCs, which decrease platelet affinity. Although the role of leukocytes in PRP preparations is debated, reducing the number of white blood cells (WBCs) decreases the number of matrix metalloproteinases and reactive oxygen species that may lead to inflammation. We used the platelet index (ratio of platelets to WBCs) and the RBC count to quantify the quality of our PRP sample.
Each rat in part A was anesthetized while supine. We used the Autologous Conditioned Plasma (ACP) system (Arthrex), which requires only 1 centrifugation cycle to create PRP. About 9 mL or 10 mL of blood was obtained by cardiac aspiration using an ACP Double Syringe (Arthrex). After blood retrieval, a thoracotomy was performed to confirm each rat’s death.
Part B: Determining the Effects of PRP and NSAIDs on RCR in a Rat Model
Operative Cohort. Of the 34 Fischer rats used in part B of this study, 6 were used as blood donors for PRP production, and the other 28 underwent bilateral rotator cuff surgeries. We used donor rats to maximize the amount of PRP retrieval, allocating about 1 donor rat per 5 operative rats. Fischer rats are an inbred strain, so the PRP from a donor Fischer rat simulates autologous blood in other Fischer rats. Use of allogenic blood is consistent with prior rat PRP studies.23,24
Operative Technique. Each bilateral surgery was performed by a single board-certified shoulder surgeon, and the anesthetic and surgical protocols were followed as approved by the home institution’s Institutional Animal Care and Use Committee. Before surgery, blood was harvested for PRP production from donor rats, as described earlier, and centrifuged for 5 minutes × 1300 rpm. After anesthetic induction and skin incision, the deltoid muscle was cut to expose the acromion and underlying rotator cuff. The distal supraspinatus tendon was sharply detached from the greater tuberosity. A bone-tunnel RCR was performed by drilling a transverse tunnel across the greater tuberosity and affixing the tendon to its footprint with a 5-0 polypropylene suture (Prolene; Ethicon). Each rat was then randomly assigned to receive 50 µL of donor PRP injected in 1 operative shoulder and saline in the contralateral shoulder. Injections were made in the supraspinatus tendon at its attachment to the humerus. Deltoid and skin were closed with 4-0 polyglactin (Vicryl) suture (Ethicon) and staples, respectively.
Tendon Preparation. Immediately post mortem, each shoulder was grossly dissected to isolate the supraspinatus muscle attached to the humerus. Shoulders were then frozen in 0.15-M saline solution until specified biomechanical testing dates.
On day of dimensional/biomechanical testing, each specimen was thawed at room temperature and finely dissected under a microscope (Stemi 200-C; Car Zeiss). After dissection, the humeral shaft was embedded in polymethylmethacrylate within a test tube. The free end of the supraspinatus tendon was glued within a “tab” of waterproofed emery cloth, leaving about 2 mm of tendon between the tab and the greater tuberosity.
Biomechanical Analysis. A 5848 MicroTester (Instron) was used for biomechanical testing. Each tabbed tendon, held by a pneumatic clamp attached to the MicroTester, was tested in a preconditioning phase and then a ramp-to-failure phase. A constant drip of 0.15-M saline was run through the apparatus to simulate physiologic hydration of tissue. After the embedded specimen was secure within the loading apparatus, an initial tensile preload of 0.2 N was applied. After preloading, the tendon was run through a preconditioning phase to account for viscoelastic relaxation. Immediately after preconditioning, each tendon was subjected to failure testing at a ramp rate of 0.1 mm/s. Force data were collected as a function of displacement, allowing for the calculation of 4 biomechanical parameters: failure force, tendon stiffness and normalized stiffness, energy to failure, and total energy. Tendon stiffness is the slope of a curve-fit line of the initial peak; failure force is the force of the highest peak; energy to failure is the area under the curve (AUC) to the highest peak; and total energy is the AUC from the start of failure ramping to the point at which the tendon is torn off completely. Two-way ANOVA was used to assess the differences between treatment groups and diet groups for all parameters. Statistical significance was set at P < .05.
A power analysis was performed to determine ability to detect differences between cohorts. For power of 80% and P = .05, a difference of 16% of the mean could be detected for failure force, 30% for energy to failure, 14% for total energy to failure, and 24% for stiffness. In addition, a difference of 4% of the mean could be detected for tendon length, 6% for width, and 10% for thickness.
Results
Across all collective treatment-diet groups and biomechanical parameters, there was only 1 statistically significant difference. Mean (SD) energy to failure was significantly higher (P = .03) in shoulders treated with PRP, 11.7 (7.3) N-mm, than in those treated without PRP, 8.7 (4.6) N-mm (Figure 4). There were no statistically significant differences between shoulders treated with indomethacin and those treated without indomethacin (Table 3), and no statistically significant relationships between treatment and drug for any other biomechanical parameter (Figures 5-7).
Discussion
Our preliminary objective in this study was to determine the optimal centrifugation protocol for producing rat-based PRP. Optimal PRP requires a dense concentration of platelets as well as reduced levels of RBCs and WBCs.25 We used the platelet index to quantify the quality of our PRP samples, and we obtained the highest platelet index for the protocol of 5 minutes × 1300 rpm. This finding may be useful in later rat studies involving PRP.
The primary objective of this study was to assess the effect of the interaction of PRP and NSAIDs on RCR. PRP has been found to augment RCR,12,26,27 but indomethacin may impair healing.21,25 We hypothesized that shoulders treated with PRP would have more biomechanical strength than control shoulders and that indomethacin would decrease biomechanical strength.
Our data showed increased energy to failure of the rotator cuff with PRP injections (P = .03). All other biomechanical parameters showed no significant differences with PRP treatment, though there were statistically insignificant trends of increased total energy, failure force, and stiffness in the PRP cohorts. There were no statistically significant differences between the indomethacin and no-indomethacin groups, and indomethacin had no effect on the efficacy of PRP treatment. It should be noted that the measurements of total energy, energy to failure, and failure force best reflect the strength of the tendon–bone interface. Other biomechanical measures, such as stiffness and normalized stiffness, are physical properties of the tendon itself and apply less to enthesis strength, which was the primary focus of this study.
Beck and colleagues23 studied the effect of allogeneic PRP on RCR in a rat model. They tested biomechanical and histologic outcomes 7, 14, and 21 days after surgery. There was no significant difference in failure load between the 2 groups at any time point. Compared with failure strain in the control group, failure strain in the PRP group was decreased at 7 days, normalized at 14 days, and increased at 21 days. The authors hypothesized that increased tendon failure strain at 21 days may have reduced forces being transmitted to the suture fixation site, which may be clinically significant and warrants further investigation. In a similar study, by Dolkart and colleagues,28 intraoperative PRP administration enhanced the maximal load-to-failure and stiffness of rats’ repaired rotator cuffs. On histologic examination, tendons treated with PRP (vs control tendons) had more organized collagen. Although these studies have limitations similar to our study, these results further support improved tendon-to-bone healing with PRP.
In clinical application, Barber and colleagues26 found that, compared with controls, suturing PRP fibrin matrix into the rotator cuff during repair decreased the incidence of magnetic resonance imaging–detected retears. However, in 2 prospective, randomized trials, Castricini and colleagues29 and Weber and colleagues30 found that use of PRP in RCR did not improve outcomes. All 3 studies differ from ours in that they used fibrin matrix. However, Ersen and colleagues31 found no difference in the effects of PRP on rotator cuff healing between injection and fibrin matrix; PRP improved biomechanical properties of repaired rotator cuff independent of administration method. In a meta-analysis of PRP supplementation in RCR, Warth and colleagues32 found a statistically significant improvement in retear rates for tears >3 cm repaired with a double-row technique, but otherwise no overall improvement in retear rates or outcome scores with PRP. The authors acknowledged that the significant heterogeneity of the studies in their meta-analysis may have affected the quality of their data.
Although our study provides some insight into the effectiveness of PRP in tendon repair, the lack of standardization in PRP preparation and time points tested makes comparisons with similar studies difficult.33 Recent reports have emphasized that not all PRP separation systems yield similar products.33 Platelet concentrations, and therefore platelet-derived growth factor concentrations, differ between systems and may yield different clinical outcomes. Our decision to use leukocyte-reduced PRP is supported by a meta-analysis by Riboh and colleagues,34 who reviewed the literature on the effect of leukocyte concentration on the efficacy of PRP products. They found that, in the treatment of knee osteoarthritis, use of leukocyte-poor PRP resulted in improved functional outcomes scores in comparison with placebo, but this improvement did not occur with leukocyte-rich PRP. However, there is still no consensus on optimal preparation, dosing, and route of administration of PRP, and preparations described in the literature vary.
This study also assessed the interaction of PRP and NSAIDs. Although there were no statistically significant differences between treatment and diet, shoulders treated with indomethacin alone showed a trend toward weaker biomechanical parameters in comparison with shoulders treated with saline alone, with PRP alone, or with both PRP and indomethacin. A larger sample would be needed to establish statistical significance. These trends are not surprising, as Cohen and colleagues21 found that NSAIDs, specifically indomethacin and celecoxib, significantly inhibited rotator cuff tendon-to-bone healing. The authors also found that a 2-week course of indomethacin was sufficient to significantly inhibit tendon-to-bone healing. In fact, although the drugs were discontinued after 14 days, biomechanical properties were negatively affected up to 8 weeks after repair. Our results differ from theirs even though the 2 studies used similar doses and administration protocols.
One strength of this study was that all surgeries were performed by a single board-certified surgeon using a standardized technique. In addition, a control group was established, and personnel and techniques for all fine dissections and biomechanical tests were consistent throughout. Blinded randomization and diet normalization, as well as adequate power for detecting significant effects, strengthened the study as well.
The study had several limitations. First, whereas most human rotator cuff tears are chronic, we used a model of acute injury and repair. As acute tears that are immediately repaired are more likely to heal, detection of differences between cohorts is less likely. However, using an acute model is still the most reliable strategy for inducing a controlled injury with reproducible severity. Second, we analyzed data at only 1 time point, which may not provide an accurate representation of long-term effects. Third, systemic administration of indomethacin did not allow for intra-rat shoulder comparisons of the different drug groups. Fourth, although it is possible that the dosage of NSAID was insufficient to produce significant differences in biomechanics, our dosage was consistent with that used in a study that found a significant effect on tendon healing.21
Conclusion
Our study found that the strength of the supraspinatus tendon enthesis as defined by energy to failure was increased with intratendinous PRP injection. Indomethacin showed no statistical effect, but there was a trend toward reduced strength after repair. However, the extent to which coadministration of indomethacin affects PRP remains unclear, and these data cannot necessarily be extrapolated to the typical human rotator cuff tear caused by chronic repetitive stress.
1. Kinsella KG, Velkoff VA. An Aging World: 2001. Washington, DC: US Government Printing Office; 2001. https://www.census.gov/prod/2001pubs/p95-01-1.pdf. Published November 2001. Accessed September 24, 2017.
2. Gamradt SC, Rodeo SA, Warren RF. Platelet rich plasma in rotator cuff repair. Tech Orthop. 2007;22(1):26-33.
3. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
4. Harryman DT, Mack LA, Wang KY. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
5. Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15(3):290-299.
6. Boileau P, Brassart N, Watkinson DJ, Carles M. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
7. Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82(4):505-515.
8. Lafosse L, Brozska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2007;89(7):1533-1541.
9. Levy O, Venkateswaran B, Even T, Ravenscroft M, Copeland S. Mid-term clinical and sonographic outcome of arthroscopic repair of the rotator cuff. J Bone Joint Surg Br. 2008;90(10):1341-1347.
10. Zumstein MA, Jost B, Hempel J, Hodler J, Gerber C. The clinical and structural long-term results of open repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2008;90(11):2423-2431.
11. Gerber C, Schneeberger AG, Perren SM, Nyffeler RW. Experimental rotator cuff repair. A preliminary study. J Bone Joint Surg Am. 1999;81(9):1281-1290.
12. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
13. Akeda K, An HS, Okuma M, et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage. 2006;14(12):1272-1280.
14. de Mos M, van der Windt AE, Jahr H, et al. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008;36(6):1171-1178.
15. Harmon KG. Muscle injuries and PRP: what does the science say? Br J Sports Med. 2010;44(9):616-617.
16. Kasten P, Vogel J, Geiger F, Niemeyer P, Luginbühl R, Szalay K. The effect of platelet-rich plasma on healing in critical-size long-bone defects. Biomaterials. 2008;29(29):3983-3992.
17. Mei-Dan O, Mann G, Maffulli N. Platelet-rich plasma: any substance into it? Br J Sports Med. 2010;44(9):618-619.
18. Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25(8):1007-1017.
19. Virchenko O, Skoglund B, Aspenberg P. Parecoxib impairs early tendon repair but improves later remodeling. Am J Sports Med. 2004;32(7):1743-1747.
20. Aspenberg P. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2004;22(3):684.
21. Cohen DB, Kawamura S, Ehteshami JR, Rodeo SA. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;34(3):362-369.
22. Balazs T, Grice HC, Airth JM. On counting the blood cells of the rat with an electronic counter. Can J Comp Med Vet Sci. 1960;24(9):273-275.
23. Beck J, Evans D, Tonino PM, Yong S, Callaci JJ. The biomechanical and histologic effects of platelet-rich plasma on rat rotator cuff repairs. Am J Sports Med. 2012;40(9):2037-2044.
24. Aspenberg P, Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand. 2004;75(1):93-99.
25. Chechik O, Dolkart O, Mozes G, Rak O, Alhajajra F, Maman E. Timing matters: NSAIDs interfere with the late proliferation stage of a repaired rotator cuff tendon healing in rats. Arch Orthop Trauma Surg. 2014;134(4):515-520.
26. Barber FA, Hrnack SA, Snyder SJ, Hapa O. Rotator cuff repair healing influenced by platelet-rich plasma construct augmentation. Arthroscopy. 2011;27(8):1029-1035.
27. Randelli PS, Arrigoni P, Cabitza P, Volpi P, Maffulli N. Autologous platelet rich plasma for arthroscopic rotator cuff repair. A pilot study. Disabil Rehabil. 2008;30(20-22):1584-1589.
28. Dolkart O, Chechik O, Zarfati Y, Brosh T, Alhajajra F, Maman E. A single dose of platelet-rich plasma improves the organization and strength of a surgically repaired rotator cuff tendon in rats. Arch Orthop Trauma Surg. 2014;134(9):1271-1277.
29. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
30. Weber SC, Kauffman JI, Parise C, Weber SJ, Katz SD. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. Am J Sports Med. 2013;41(2):263-270.
31. Ersen A, Demirhan M, Atalar AC, Kapicioğlu M, Baysal G. Platelet-rich plasma for enhancing surgical rotator cuff repair: evaluation and comparison of two application methods in a rat model. Arch Orthop Trauma Surg. 2014;134(3):405-411.
32. Warth RJ, Dornan GJ, James EW, Horan MP, Millett PJ. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. 2015;31(2):306-320.
33. Bergeson AG, Tashjian RZ, Greis PE, Crim J, Stoddard GJ, Burks RT. Effects of platelet-rich fibrin matrix on repair integrity of at-risk rotator cuff tears. Am J Sports Med. 2012;40(2):286-293.
34. Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med. 2016;44(3):792-800.
1. Kinsella KG, Velkoff VA. An Aging World: 2001. Washington, DC: US Government Printing Office; 2001. https://www.census.gov/prod/2001pubs/p95-01-1.pdf. Published November 2001. Accessed September 24, 2017.
2. Gamradt SC, Rodeo SA, Warren RF. Platelet rich plasma in rotator cuff repair. Tech Orthop. 2007;22(1):26-33.
3. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
4. Harryman DT, Mack LA, Wang KY. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
5. Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15(3):290-299.
6. Boileau P, Brassart N, Watkinson DJ, Carles M. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
7. Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82(4):505-515.
8. Lafosse L, Brozska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2007;89(7):1533-1541.
9. Levy O, Venkateswaran B, Even T, Ravenscroft M, Copeland S. Mid-term clinical and sonographic outcome of arthroscopic repair of the rotator cuff. J Bone Joint Surg Br. 2008;90(10):1341-1347.
10. Zumstein MA, Jost B, Hempel J, Hodler J, Gerber C. The clinical and structural long-term results of open repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2008;90(11):2423-2431.
11. Gerber C, Schneeberger AG, Perren SM, Nyffeler RW. Experimental rotator cuff repair. A preliminary study. J Bone Joint Surg Am. 1999;81(9):1281-1290.
12. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
13. Akeda K, An HS, Okuma M, et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage. 2006;14(12):1272-1280.
14. de Mos M, van der Windt AE, Jahr H, et al. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008;36(6):1171-1178.
15. Harmon KG. Muscle injuries and PRP: what does the science say? Br J Sports Med. 2010;44(9):616-617.
16. Kasten P, Vogel J, Geiger F, Niemeyer P, Luginbühl R, Szalay K. The effect of platelet-rich plasma on healing in critical-size long-bone defects. Biomaterials. 2008;29(29):3983-3992.
17. Mei-Dan O, Mann G, Maffulli N. Platelet-rich plasma: any substance into it? Br J Sports Med. 2010;44(9):618-619.
18. Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25(8):1007-1017.
19. Virchenko O, Skoglund B, Aspenberg P. Parecoxib impairs early tendon repair but improves later remodeling. Am J Sports Med. 2004;32(7):1743-1747.
20. Aspenberg P. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2004;22(3):684.
21. Cohen DB, Kawamura S, Ehteshami JR, Rodeo SA. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;34(3):362-369.
22. Balazs T, Grice HC, Airth JM. On counting the blood cells of the rat with an electronic counter. Can J Comp Med Vet Sci. 1960;24(9):273-275.
23. Beck J, Evans D, Tonino PM, Yong S, Callaci JJ. The biomechanical and histologic effects of platelet-rich plasma on rat rotator cuff repairs. Am J Sports Med. 2012;40(9):2037-2044.
24. Aspenberg P, Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand. 2004;75(1):93-99.
25. Chechik O, Dolkart O, Mozes G, Rak O, Alhajajra F, Maman E. Timing matters: NSAIDs interfere with the late proliferation stage of a repaired rotator cuff tendon healing in rats. Arch Orthop Trauma Surg. 2014;134(4):515-520.
26. Barber FA, Hrnack SA, Snyder SJ, Hapa O. Rotator cuff repair healing influenced by platelet-rich plasma construct augmentation. Arthroscopy. 2011;27(8):1029-1035.
27. Randelli PS, Arrigoni P, Cabitza P, Volpi P, Maffulli N. Autologous platelet rich plasma for arthroscopic rotator cuff repair. A pilot study. Disabil Rehabil. 2008;30(20-22):1584-1589.
28. Dolkart O, Chechik O, Zarfati Y, Brosh T, Alhajajra F, Maman E. A single dose of platelet-rich plasma improves the organization and strength of a surgically repaired rotator cuff tendon in rats. Arch Orthop Trauma Surg. 2014;134(9):1271-1277.
29. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
30. Weber SC, Kauffman JI, Parise C, Weber SJ, Katz SD. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. Am J Sports Med. 2013;41(2):263-270.
31. Ersen A, Demirhan M, Atalar AC, Kapicioğlu M, Baysal G. Platelet-rich plasma for enhancing surgical rotator cuff repair: evaluation and comparison of two application methods in a rat model. Arch Orthop Trauma Surg. 2014;134(3):405-411.
32. Warth RJ, Dornan GJ, James EW, Horan MP, Millett PJ. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. 2015;31(2):306-320.
33. Bergeson AG, Tashjian RZ, Greis PE, Crim J, Stoddard GJ, Burks RT. Effects of platelet-rich fibrin matrix on repair integrity of at-risk rotator cuff tears. Am J Sports Med. 2012;40(2):286-293.
34. Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med. 2016;44(3):792-800.
VIDEO: Balanced crystalloids protect kidney better than saline
TORONTO – Treatment with balanced crystalloid IV fluids cut adverse renal events modestly but with statistical significance, compared with 0.9% saline in hospitalized patients in a pair of single-center randomized trials with more than 29,000 total patients.
Despite showing a number needed to treat with balanced crystalloids of roughly 100 to prevent one major renal event, compared with saline, the scope of IV fluid use makes even this relatively small improvement potentially important to tens of thousands of patients annually.
“It’s a small but clinically important difference,” Wesley H. Self, MD, said at the CHEST annual meeting.
“These fluids are used every day and in millions of patients annually in the United States and worldwide. There is no functional cost difference between them, and now we have the data to show that [balanced crystalloid fluids] produce a better patient outcome. It’s reasonable to consider changing practice,” based on the results, said Matthew W. Semler, MD, a pulmonologist at Vanderbilt University Medical Center in Nashville, Tenn., who led one of the two trials.
At Vanderbilt, where the two studies ran, “we’ve changed our practice and are transitioning from primarily using saline to primarily balanced crystalloid,” Dr. Semler said in a video interview. The main limitation to changing practice now because of the results is that the two trials both ran at a single center.
The findings Dr. Semler reported came from the Isotonic Solutions and Major Adverse Renal Events Trial (SMART), which randomized 7,860 ICU patients to treatment with 0.9% saline fluid and 7,942 ICU patients to treatment with balanced crystalloid fluid, either lactated Ringer’s or Plasma-Lyte A. The study’s primary endpoint was the combined 30-day rate of in-hospital death, incident need for renal replacement therapy, or at least a doubling of the patient’s baseline creatinine level, a marker of persistent renal dysfunction.
This outcome occurred in 14.3% of patients on balanced crystalloid fluid and 15.4% on saline, a 1.1% statistically significant absolute difference. The endpoint components showed that patients treated with balanced crystalloid had 0.8% less in-hospital death and 0.4% less incident renal replacement therapy; both of these between-group differences were close to having statistical significance. The two treatment groups showed less difference in the rate of persistent renal dysfunction.
The second trial had an identical design but ran instead in the emergency department. The Saline Against Lactated Ringers or Plasmalyte in the Emergency Department (SALT-ED) trial randomized 6,708 to receive balanced crystalloid and 6,639 to receive saline. The combined primary renal endpoint was 0.9% less frequent with balanced crystalloid fluid, a statistically significant difference, Dr. Self, an emergency medicine physician at Vanderbilt, reported at the meeting. In this study the between-group differences for both incident renal replacement therapy and persistent renal dysfunction were statistically significant in favor of balanced crystalloid, but the between-group mortality difference was not significantly different.
The reason why balanced crystalloid fluid produced better renal outcomes than saline remains unclear. Both Dr. Semler and Dr. Self noted that the two balanced crystalloid fluids used in the study have chloride levels that closely match normal plasma levels, but the chloride concentration in 0.9% saline is about 50% higher than plasma. Some researchers have hypothesized, based on animal findings, that this difference may influence inflammation, blood pressure, acute kidney injury, and renal vasoconstriction.
The SMART and SALT-ED trials received no commercial funding. Dr. Semler had no disclosures. Dr. Self has been a consultant to Abbott Point of Care, BioTest, Cempra, Ferring, Gilead, and Pfizer.
[email protected]
On Twitter @mitchelzoler
Bennett P. deBoisblanc, MD , is professor of medicine at Louisiana State University Health and director of Critical Care Services at the Medical Center of Louisiana in New Orleans. He had no disclosures. He made these comments from the floor during discussion of the two reports.
Bennett P. deBoisblanc, MD , is professor of medicine at Louisiana State University Health and director of Critical Care Services at the Medical Center of Louisiana in New Orleans. He had no disclosures. He made these comments from the floor during discussion of the two reports.
Bennett P. deBoisblanc, MD , is professor of medicine at Louisiana State University Health and director of Critical Care Services at the Medical Center of Louisiana in New Orleans. He had no disclosures. He made these comments from the floor during discussion of the two reports.
TORONTO – Treatment with balanced crystalloid IV fluids cut adverse renal events modestly but with statistical significance, compared with 0.9% saline in hospitalized patients in a pair of single-center randomized trials with more than 29,000 total patients.
Despite showing a number needed to treat with balanced crystalloids of roughly 100 to prevent one major renal event, compared with saline, the scope of IV fluid use makes even this relatively small improvement potentially important to tens of thousands of patients annually.
“It’s a small but clinically important difference,” Wesley H. Self, MD, said at the CHEST annual meeting.
“These fluids are used every day and in millions of patients annually in the United States and worldwide. There is no functional cost difference between them, and now we have the data to show that [balanced crystalloid fluids] produce a better patient outcome. It’s reasonable to consider changing practice,” based on the results, said Matthew W. Semler, MD, a pulmonologist at Vanderbilt University Medical Center in Nashville, Tenn., who led one of the two trials.
At Vanderbilt, where the two studies ran, “we’ve changed our practice and are transitioning from primarily using saline to primarily balanced crystalloid,” Dr. Semler said in a video interview. The main limitation to changing practice now because of the results is that the two trials both ran at a single center.
The findings Dr. Semler reported came from the Isotonic Solutions and Major Adverse Renal Events Trial (SMART), which randomized 7,860 ICU patients to treatment with 0.9% saline fluid and 7,942 ICU patients to treatment with balanced crystalloid fluid, either lactated Ringer’s or Plasma-Lyte A. The study’s primary endpoint was the combined 30-day rate of in-hospital death, incident need for renal replacement therapy, or at least a doubling of the patient’s baseline creatinine level, a marker of persistent renal dysfunction.
This outcome occurred in 14.3% of patients on balanced crystalloid fluid and 15.4% on saline, a 1.1% statistically significant absolute difference. The endpoint components showed that patients treated with balanced crystalloid had 0.8% less in-hospital death and 0.4% less incident renal replacement therapy; both of these between-group differences were close to having statistical significance. The two treatment groups showed less difference in the rate of persistent renal dysfunction.
The second trial had an identical design but ran instead in the emergency department. The Saline Against Lactated Ringers or Plasmalyte in the Emergency Department (SALT-ED) trial randomized 6,708 to receive balanced crystalloid and 6,639 to receive saline. The combined primary renal endpoint was 0.9% less frequent with balanced crystalloid fluid, a statistically significant difference, Dr. Self, an emergency medicine physician at Vanderbilt, reported at the meeting. In this study the between-group differences for both incident renal replacement therapy and persistent renal dysfunction were statistically significant in favor of balanced crystalloid, but the between-group mortality difference was not significantly different.
The reason why balanced crystalloid fluid produced better renal outcomes than saline remains unclear. Both Dr. Semler and Dr. Self noted that the two balanced crystalloid fluids used in the study have chloride levels that closely match normal plasma levels, but the chloride concentration in 0.9% saline is about 50% higher than plasma. Some researchers have hypothesized, based on animal findings, that this difference may influence inflammation, blood pressure, acute kidney injury, and renal vasoconstriction.
The SMART and SALT-ED trials received no commercial funding. Dr. Semler had no disclosures. Dr. Self has been a consultant to Abbott Point of Care, BioTest, Cempra, Ferring, Gilead, and Pfizer.
[email protected]
On Twitter @mitchelzoler
TORONTO – Treatment with balanced crystalloid IV fluids cut adverse renal events modestly but with statistical significance, compared with 0.9% saline in hospitalized patients in a pair of single-center randomized trials with more than 29,000 total patients.
Despite showing a number needed to treat with balanced crystalloids of roughly 100 to prevent one major renal event, compared with saline, the scope of IV fluid use makes even this relatively small improvement potentially important to tens of thousands of patients annually.
“It’s a small but clinically important difference,” Wesley H. Self, MD, said at the CHEST annual meeting.
“These fluids are used every day and in millions of patients annually in the United States and worldwide. There is no functional cost difference between them, and now we have the data to show that [balanced crystalloid fluids] produce a better patient outcome. It’s reasonable to consider changing practice,” based on the results, said Matthew W. Semler, MD, a pulmonologist at Vanderbilt University Medical Center in Nashville, Tenn., who led one of the two trials.
At Vanderbilt, where the two studies ran, “we’ve changed our practice and are transitioning from primarily using saline to primarily balanced crystalloid,” Dr. Semler said in a video interview. The main limitation to changing practice now because of the results is that the two trials both ran at a single center.
The findings Dr. Semler reported came from the Isotonic Solutions and Major Adverse Renal Events Trial (SMART), which randomized 7,860 ICU patients to treatment with 0.9% saline fluid and 7,942 ICU patients to treatment with balanced crystalloid fluid, either lactated Ringer’s or Plasma-Lyte A. The study’s primary endpoint was the combined 30-day rate of in-hospital death, incident need for renal replacement therapy, or at least a doubling of the patient’s baseline creatinine level, a marker of persistent renal dysfunction.
This outcome occurred in 14.3% of patients on balanced crystalloid fluid and 15.4% on saline, a 1.1% statistically significant absolute difference. The endpoint components showed that patients treated with balanced crystalloid had 0.8% less in-hospital death and 0.4% less incident renal replacement therapy; both of these between-group differences were close to having statistical significance. The two treatment groups showed less difference in the rate of persistent renal dysfunction.
The second trial had an identical design but ran instead in the emergency department. The Saline Against Lactated Ringers or Plasmalyte in the Emergency Department (SALT-ED) trial randomized 6,708 to receive balanced crystalloid and 6,639 to receive saline. The combined primary renal endpoint was 0.9% less frequent with balanced crystalloid fluid, a statistically significant difference, Dr. Self, an emergency medicine physician at Vanderbilt, reported at the meeting. In this study the between-group differences for both incident renal replacement therapy and persistent renal dysfunction were statistically significant in favor of balanced crystalloid, but the between-group mortality difference was not significantly different.
The reason why balanced crystalloid fluid produced better renal outcomes than saline remains unclear. Both Dr. Semler and Dr. Self noted that the two balanced crystalloid fluids used in the study have chloride levels that closely match normal plasma levels, but the chloride concentration in 0.9% saline is about 50% higher than plasma. Some researchers have hypothesized, based on animal findings, that this difference may influence inflammation, blood pressure, acute kidney injury, and renal vasoconstriction.
The SMART and SALT-ED trials received no commercial funding. Dr. Semler had no disclosures. Dr. Self has been a consultant to Abbott Point of Care, BioTest, Cempra, Ferring, Gilead, and Pfizer.
[email protected]
On Twitter @mitchelzoler
AT CHEST 2017
Key clinical point:
Major finding: Balanced crystalloids reduced combined adverse renal events by 1.1% in ICU patients and 0.9% in ED patients.
Data source: The SMART and SALT-ED trials, both single-center studies with a total of 29,149 patients.
Disclosures: The SMART and SALT-ED trials received no commercial funding. Dr. Semler had no disclosures. Dr. Self has been a consultant to Abbott Point of Care, BioTest, Cempra, Ferring, Gilead, and Pfizer.
Atypical Herpes Zoster Presentation in a Healthy Vaccinated Pediatric Patient
Varicella-zoster virus (VZV) is a neurotropic human herpesvirus that causes varicella (chicken pox) and herpes zoster (shingles). During infection, the virus invades the dorsal root ganglia and establishes permanent latency. It can later reactivate and travel through sensory nerves to the skin where localized viral replication causes herpes zoster (HZ), which manifests with pain in a unilateral dermatomal distribution followed closely by an eruption of grouped macules and papules that evolve into vesicles on an erythematous base.1 These lesions form pustules and crusts over 7 to 10 days and heal completely within 4 weeks. Although postherpetic neuralgia is rare in children, the pain associated with HZ can last months or years.1,2
Universal childhood vaccination against VZV has existed in the United States since 1995, with a 2-dose vaccine regimen recommended by the CDC since 2007. Consequently, primary varicella infection in children is uncommon, and the majority of cases now occur in the vaccinated population.3 However, breakthrough varicella infection and postvaccination HZ are rare due to the long-lasting immunity and low virulence of the attenuated vaccine strain. We recount the case of a 6-year-old vaccinated girl with a unique presentation of HZ with no known primary varicella infection.
Case Report
A healthy 6-year-old girl presented with a stabbing burning pain in the left thigh extending down the calf of 4 days’ duration. The intense pain made walking difficult and responded minimally to ibuprofen and naproxen. Poor appetite, nausea, colicky abdominal pain, and fever (temperature, 38°C) accompanied the pain. Three days after the pain began she developed a pruritic rash on the same leg. Notably, she reported falling on a rosebush and sustaining a thorn prick in the left thigh 3 days prior to the onset of pain. Before presenting to our dermatology clinic, she was seen by a pediatrician, an emergency department physician, and an infectious disease specialist. The initial workup included a complete blood cell count, C-reactive protein test, erythrocyte sedimentation rate test, and hip and femur radiograph, which were all unremarkable. She was referred to dermatology with a differential diagnosis of sporotrichosis, contact dermatitis, reactive arthritis, viral myalgia, and Legg-Calvé-Perthes disease.
Physical examination revealed a well-appearing child with pink eczematous patches and plaques extending from the left side of the lower back to the mid shin in an L5 distribution (Figure). The left thigh was tender to palpation, and nontender left inguinal lymphadenopathy was present. A single isolated 2-mm vesicle was found on the anterior aspect of the left lower leg. Direct fluorescent antibody testing of vesicle fluid was positive for VZV antigen, confirming the diagnosis of HZ.
The patient’s mother confirmed that she had no obvious history of VZV. She had received VZV vaccinations in the left leg and arm at 1 and 4 years of age, respectively. She was treated with acyclovir (80 mg/kg daily at 6-hour intervals for 5 days) with immediate improvement in symptoms and resolution of the rash by day 5 of treatment. She experienced intermittent burning pain in the leg throughout the course of treatment, which resolved shortly thereafter.
Comment
Herpes zoster is rare in young healthy children, and its incidence has decreased since the introduction of universal varicella vaccination.4 Reported incidence rates in vaccinated children vary from approximately 15 to 93 per 100,000 person-years,5,6 and the reported relative risk is 0.08 to 0.36 in vaccinated compared to unvaccinated children.6,7 No correlations with gender, race, or ethnicity and postvaccination HZ have been observed.5,8 Reported intervals between vaccination and HZ presentation are as short as 3 months and as long as 11 years.9 Although HZ is uncommon in immunocompetent children, the diagnosis of HZ itself is not an indication for formal workup for an underlying immunodeficiency or malignancy.10
Both wild-type and vaccine-strain VZV establish latent infection and can cause HZ in vaccinated children. Direct fluorescent antibody testing or polymerase chain reaction of HZ lesions can be used to identify VZV. Genotyping can distinguish the wild-type versus the vaccine strain but is not required for clinical management.3 In previously vaccinated children with HZ, approximately half present with wild-type and half with vaccine-strain VZV. In approximately half of wild-type cases, prior clinical varicella infection also occurred.8
Regardless of virus strain, vaccinated children typically present with the characteristic painful, vesicular, dermatomal HZ rash.8,9 This presentation can be milder with less pain and fewer vesicles than with unvaccinated cases.6 When vaccine-strain HZ occurs, the rash often presents at or near the site of initial vaccination, which typically is the arm or thigh.3,4,6,9 The vaccine strain has lower virulence than the wild-type virus. Eight cases of vaccine-strain zoster severe enough to cause neurological complications such as meningitis or encephalitis have been reported in children, with 6 cases reported in healthy children.9,11-17 Antiviral drugs hasten the healing of the HZ rash and shorten the duration of associated pain.1
Although pediatric HZ is uncommon, all physicians should be aware of possible atypical presentations in healthy vaccinated children to appropriately and quickly manage treatment.
- Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84:274-280.
- Hillebrand K, Bricout H, Schulze-Rath R, et al. Incidence of herpes zoster and its complications in Germany, 2005-2009. J Infect. 2015;70:178-186.
- Lopez A, Schmid S, Bialek S. Varicella. In: Centers for Disease Control and Prevention. Manual for the Surveillance of Vaccine-Preventable Diseases. 5th ed. 2011:1-16.
- Tanuseputroa P, Zagorskia B, Chanc KJ, et al. Population-based incidence of herpes zoster after introduction of a publicly funded varicella vaccination program. Vaccine. 2011;29:8580- 8584.
- Wen SY, Liu WL. Epidemiology of pediatric herpes zoster after varicella infection: a population-based study. Pediatrics. 2015;135:565-571.
- Civen R, Chaves SS, Jumaan A, et al. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr Infect Dis J. 2009;28:954-959.
- Stein M, Cohen R, Bromberg M, et al. Herpes zoster in a partially vaccinated pediatric population in Central Israel. Pediatr Infect Dis J. 2012;31:906-909.
- Weinmann S, Chun C, Schmid DS, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005-2009. J Infect Dis. 2013;208:1859-1868.
- Horien C, Grose C. Neurovirulence of varicella and the live attenuated varicella vaccine virus. Semin Pediatr Neurol. 2012;19:124-129.
- Petursson G, Helgason S, Gudmundsson S, et al. Herpes zoster in children and adolescents. Pediatr Infect Dis J. 1998;17:905-908.
- Levin MJ, Dahl KM, Weinberg A, et al. Development of resistance to acyclovir during chronic infection with the Oka vaccine strain of varicella-zoster virus in an immunosuppressed child. J Infect Dis. 2003;188:954-959.
- Chaves SS, Haber P, Walton K, et al. Safety of varicella vaccine after licensure in the United States: experience from reports to the vaccine adverse event reporting system, 1995-2005. J Infect Dis. 2008;197(suppl 2):S170-S177.
- Levin MJ, DeBiasi RL, Bostik V, et al. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella zoster virus vaccine. J Infect Dis. 2008;198:1444-1447.
- Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792-795.
- Chouliaras G, Spoulou V, Quinlivan M, et al. Vaccine-associated herpes zoster ophthalmicus and encephalitis in an immunocompetent child. Pediatrics. 2010;125:e969-e972.
- Pahud BA, Glaser CA, Dekker CL, et al. Varicella zoster disease of the central nervous system: epidemiological, clinical, and laboratory features 10 years after the introduction of the varicella vaccine. J Infect Dis. 2011;203:316-323.
- Han JY, Hanson DC, Way SS. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30:266-268.
Varicella-zoster virus (VZV) is a neurotropic human herpesvirus that causes varicella (chicken pox) and herpes zoster (shingles). During infection, the virus invades the dorsal root ganglia and establishes permanent latency. It can later reactivate and travel through sensory nerves to the skin where localized viral replication causes herpes zoster (HZ), which manifests with pain in a unilateral dermatomal distribution followed closely by an eruption of grouped macules and papules that evolve into vesicles on an erythematous base.1 These lesions form pustules and crusts over 7 to 10 days and heal completely within 4 weeks. Although postherpetic neuralgia is rare in children, the pain associated with HZ can last months or years.1,2
Universal childhood vaccination against VZV has existed in the United States since 1995, with a 2-dose vaccine regimen recommended by the CDC since 2007. Consequently, primary varicella infection in children is uncommon, and the majority of cases now occur in the vaccinated population.3 However, breakthrough varicella infection and postvaccination HZ are rare due to the long-lasting immunity and low virulence of the attenuated vaccine strain. We recount the case of a 6-year-old vaccinated girl with a unique presentation of HZ with no known primary varicella infection.
Case Report
A healthy 6-year-old girl presented with a stabbing burning pain in the left thigh extending down the calf of 4 days’ duration. The intense pain made walking difficult and responded minimally to ibuprofen and naproxen. Poor appetite, nausea, colicky abdominal pain, and fever (temperature, 38°C) accompanied the pain. Three days after the pain began she developed a pruritic rash on the same leg. Notably, she reported falling on a rosebush and sustaining a thorn prick in the left thigh 3 days prior to the onset of pain. Before presenting to our dermatology clinic, she was seen by a pediatrician, an emergency department physician, and an infectious disease specialist. The initial workup included a complete blood cell count, C-reactive protein test, erythrocyte sedimentation rate test, and hip and femur radiograph, which were all unremarkable. She was referred to dermatology with a differential diagnosis of sporotrichosis, contact dermatitis, reactive arthritis, viral myalgia, and Legg-Calvé-Perthes disease.
Physical examination revealed a well-appearing child with pink eczematous patches and plaques extending from the left side of the lower back to the mid shin in an L5 distribution (Figure). The left thigh was tender to palpation, and nontender left inguinal lymphadenopathy was present. A single isolated 2-mm vesicle was found on the anterior aspect of the left lower leg. Direct fluorescent antibody testing of vesicle fluid was positive for VZV antigen, confirming the diagnosis of HZ.

The patient’s mother confirmed that she had no obvious history of VZV. She had received VZV vaccinations in the left leg and arm at 1 and 4 years of age, respectively. She was treated with acyclovir (80 mg/kg daily at 6-hour intervals for 5 days) with immediate improvement in symptoms and resolution of the rash by day 5 of treatment. She experienced intermittent burning pain in the leg throughout the course of treatment, which resolved shortly thereafter.
Comment
Herpes zoster is rare in young healthy children, and its incidence has decreased since the introduction of universal varicella vaccination.4 Reported incidence rates in vaccinated children vary from approximately 15 to 93 per 100,000 person-years,5,6 and the reported relative risk is 0.08 to 0.36 in vaccinated compared to unvaccinated children.6,7 No correlations with gender, race, or ethnicity and postvaccination HZ have been observed.5,8 Reported intervals between vaccination and HZ presentation are as short as 3 months and as long as 11 years.9 Although HZ is uncommon in immunocompetent children, the diagnosis of HZ itself is not an indication for formal workup for an underlying immunodeficiency or malignancy.10
Both wild-type and vaccine-strain VZV establish latent infection and can cause HZ in vaccinated children. Direct fluorescent antibody testing or polymerase chain reaction of HZ lesions can be used to identify VZV. Genotyping can distinguish the wild-type versus the vaccine strain but is not required for clinical management.3 In previously vaccinated children with HZ, approximately half present with wild-type and half with vaccine-strain VZV. In approximately half of wild-type cases, prior clinical varicella infection also occurred.8
Regardless of virus strain, vaccinated children typically present with the characteristic painful, vesicular, dermatomal HZ rash.8,9 This presentation can be milder with less pain and fewer vesicles than with unvaccinated cases.6 When vaccine-strain HZ occurs, the rash often presents at or near the site of initial vaccination, which typically is the arm or thigh.3,4,6,9 The vaccine strain has lower virulence than the wild-type virus. Eight cases of vaccine-strain zoster severe enough to cause neurological complications such as meningitis or encephalitis have been reported in children, with 6 cases reported in healthy children.9,11-17 Antiviral drugs hasten the healing of the HZ rash and shorten the duration of associated pain.1
Although pediatric HZ is uncommon, all physicians should be aware of possible atypical presentations in healthy vaccinated children to appropriately and quickly manage treatment.
Varicella-zoster virus (VZV) is a neurotropic human herpesvirus that causes varicella (chicken pox) and herpes zoster (shingles). During infection, the virus invades the dorsal root ganglia and establishes permanent latency. It can later reactivate and travel through sensory nerves to the skin where localized viral replication causes herpes zoster (HZ), which manifests with pain in a unilateral dermatomal distribution followed closely by an eruption of grouped macules and papules that evolve into vesicles on an erythematous base.1 These lesions form pustules and crusts over 7 to 10 days and heal completely within 4 weeks. Although postherpetic neuralgia is rare in children, the pain associated with HZ can last months or years.1,2
Universal childhood vaccination against VZV has existed in the United States since 1995, with a 2-dose vaccine regimen recommended by the CDC since 2007. Consequently, primary varicella infection in children is uncommon, and the majority of cases now occur in the vaccinated population.3 However, breakthrough varicella infection and postvaccination HZ are rare due to the long-lasting immunity and low virulence of the attenuated vaccine strain. We recount the case of a 6-year-old vaccinated girl with a unique presentation of HZ with no known primary varicella infection.
Case Report
A healthy 6-year-old girl presented with a stabbing burning pain in the left thigh extending down the calf of 4 days’ duration. The intense pain made walking difficult and responded minimally to ibuprofen and naproxen. Poor appetite, nausea, colicky abdominal pain, and fever (temperature, 38°C) accompanied the pain. Three days after the pain began she developed a pruritic rash on the same leg. Notably, she reported falling on a rosebush and sustaining a thorn prick in the left thigh 3 days prior to the onset of pain. Before presenting to our dermatology clinic, she was seen by a pediatrician, an emergency department physician, and an infectious disease specialist. The initial workup included a complete blood cell count, C-reactive protein test, erythrocyte sedimentation rate test, and hip and femur radiograph, which were all unremarkable. She was referred to dermatology with a differential diagnosis of sporotrichosis, contact dermatitis, reactive arthritis, viral myalgia, and Legg-Calvé-Perthes disease.
Physical examination revealed a well-appearing child with pink eczematous patches and plaques extending from the left side of the lower back to the mid shin in an L5 distribution (Figure). The left thigh was tender to palpation, and nontender left inguinal lymphadenopathy was present. A single isolated 2-mm vesicle was found on the anterior aspect of the left lower leg. Direct fluorescent antibody testing of vesicle fluid was positive for VZV antigen, confirming the diagnosis of HZ.

The patient’s mother confirmed that she had no obvious history of VZV. She had received VZV vaccinations in the left leg and arm at 1 and 4 years of age, respectively. She was treated with acyclovir (80 mg/kg daily at 6-hour intervals for 5 days) with immediate improvement in symptoms and resolution of the rash by day 5 of treatment. She experienced intermittent burning pain in the leg throughout the course of treatment, which resolved shortly thereafter.
Comment
Herpes zoster is rare in young healthy children, and its incidence has decreased since the introduction of universal varicella vaccination.4 Reported incidence rates in vaccinated children vary from approximately 15 to 93 per 100,000 person-years,5,6 and the reported relative risk is 0.08 to 0.36 in vaccinated compared to unvaccinated children.6,7 No correlations with gender, race, or ethnicity and postvaccination HZ have been observed.5,8 Reported intervals between vaccination and HZ presentation are as short as 3 months and as long as 11 years.9 Although HZ is uncommon in immunocompetent children, the diagnosis of HZ itself is not an indication for formal workup for an underlying immunodeficiency or malignancy.10
Both wild-type and vaccine-strain VZV establish latent infection and can cause HZ in vaccinated children. Direct fluorescent antibody testing or polymerase chain reaction of HZ lesions can be used to identify VZV. Genotyping can distinguish the wild-type versus the vaccine strain but is not required for clinical management.3 In previously vaccinated children with HZ, approximately half present with wild-type and half with vaccine-strain VZV. In approximately half of wild-type cases, prior clinical varicella infection also occurred.8
Regardless of virus strain, vaccinated children typically present with the characteristic painful, vesicular, dermatomal HZ rash.8,9 This presentation can be milder with less pain and fewer vesicles than with unvaccinated cases.6 When vaccine-strain HZ occurs, the rash often presents at or near the site of initial vaccination, which typically is the arm or thigh.3,4,6,9 The vaccine strain has lower virulence than the wild-type virus. Eight cases of vaccine-strain zoster severe enough to cause neurological complications such as meningitis or encephalitis have been reported in children, with 6 cases reported in healthy children.9,11-17 Antiviral drugs hasten the healing of the HZ rash and shorten the duration of associated pain.1
Although pediatric HZ is uncommon, all physicians should be aware of possible atypical presentations in healthy vaccinated children to appropriately and quickly manage treatment.
- Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84:274-280.
- Hillebrand K, Bricout H, Schulze-Rath R, et al. Incidence of herpes zoster and its complications in Germany, 2005-2009. J Infect. 2015;70:178-186.
- Lopez A, Schmid S, Bialek S. Varicella. In: Centers for Disease Control and Prevention. Manual for the Surveillance of Vaccine-Preventable Diseases. 5th ed. 2011:1-16.
- Tanuseputroa P, Zagorskia B, Chanc KJ, et al. Population-based incidence of herpes zoster after introduction of a publicly funded varicella vaccination program. Vaccine. 2011;29:8580- 8584.
- Wen SY, Liu WL. Epidemiology of pediatric herpes zoster after varicella infection: a population-based study. Pediatrics. 2015;135:565-571.
- Civen R, Chaves SS, Jumaan A, et al. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr Infect Dis J. 2009;28:954-959.
- Stein M, Cohen R, Bromberg M, et al. Herpes zoster in a partially vaccinated pediatric population in Central Israel. Pediatr Infect Dis J. 2012;31:906-909.
- Weinmann S, Chun C, Schmid DS, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005-2009. J Infect Dis. 2013;208:1859-1868.
- Horien C, Grose C. Neurovirulence of varicella and the live attenuated varicella vaccine virus. Semin Pediatr Neurol. 2012;19:124-129.
- Petursson G, Helgason S, Gudmundsson S, et al. Herpes zoster in children and adolescents. Pediatr Infect Dis J. 1998;17:905-908.
- Levin MJ, Dahl KM, Weinberg A, et al. Development of resistance to acyclovir during chronic infection with the Oka vaccine strain of varicella-zoster virus in an immunosuppressed child. J Infect Dis. 2003;188:954-959.
- Chaves SS, Haber P, Walton K, et al. Safety of varicella vaccine after licensure in the United States: experience from reports to the vaccine adverse event reporting system, 1995-2005. J Infect Dis. 2008;197(suppl 2):S170-S177.
- Levin MJ, DeBiasi RL, Bostik V, et al. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella zoster virus vaccine. J Infect Dis. 2008;198:1444-1447.
- Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792-795.
- Chouliaras G, Spoulou V, Quinlivan M, et al. Vaccine-associated herpes zoster ophthalmicus and encephalitis in an immunocompetent child. Pediatrics. 2010;125:e969-e972.
- Pahud BA, Glaser CA, Dekker CL, et al. Varicella zoster disease of the central nervous system: epidemiological, clinical, and laboratory features 10 years after the introduction of the varicella vaccine. J Infect Dis. 2011;203:316-323.
- Han JY, Hanson DC, Way SS. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30:266-268.
- Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84:274-280.
- Hillebrand K, Bricout H, Schulze-Rath R, et al. Incidence of herpes zoster and its complications in Germany, 2005-2009. J Infect. 2015;70:178-186.
- Lopez A, Schmid S, Bialek S. Varicella. In: Centers for Disease Control and Prevention. Manual for the Surveillance of Vaccine-Preventable Diseases. 5th ed. 2011:1-16.
- Tanuseputroa P, Zagorskia B, Chanc KJ, et al. Population-based incidence of herpes zoster after introduction of a publicly funded varicella vaccination program. Vaccine. 2011;29:8580- 8584.
- Wen SY, Liu WL. Epidemiology of pediatric herpes zoster after varicella infection: a population-based study. Pediatrics. 2015;135:565-571.
- Civen R, Chaves SS, Jumaan A, et al. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr Infect Dis J. 2009;28:954-959.
- Stein M, Cohen R, Bromberg M, et al. Herpes zoster in a partially vaccinated pediatric population in Central Israel. Pediatr Infect Dis J. 2012;31:906-909.
- Weinmann S, Chun C, Schmid DS, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005-2009. J Infect Dis. 2013;208:1859-1868.
- Horien C, Grose C. Neurovirulence of varicella and the live attenuated varicella vaccine virus. Semin Pediatr Neurol. 2012;19:124-129.
- Petursson G, Helgason S, Gudmundsson S, et al. Herpes zoster in children and adolescents. Pediatr Infect Dis J. 1998;17:905-908.
- Levin MJ, Dahl KM, Weinberg A, et al. Development of resistance to acyclovir during chronic infection with the Oka vaccine strain of varicella-zoster virus in an immunosuppressed child. J Infect Dis. 2003;188:954-959.
- Chaves SS, Haber P, Walton K, et al. Safety of varicella vaccine after licensure in the United States: experience from reports to the vaccine adverse event reporting system, 1995-2005. J Infect Dis. 2008;197(suppl 2):S170-S177.
- Levin MJ, DeBiasi RL, Bostik V, et al. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella zoster virus vaccine. J Infect Dis. 2008;198:1444-1447.
- Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792-795.
- Chouliaras G, Spoulou V, Quinlivan M, et al. Vaccine-associated herpes zoster ophthalmicus and encephalitis in an immunocompetent child. Pediatrics. 2010;125:e969-e972.
- Pahud BA, Glaser CA, Dekker CL, et al. Varicella zoster disease of the central nervous system: epidemiological, clinical, and laboratory features 10 years after the introduction of the varicella vaccine. J Infect Dis. 2011;203:316-323.
- Han JY, Hanson DC, Way SS. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30:266-268.
Practice Points
- Both wild-type and vaccine-strain varicella-zoster virus (VZV) can establish latency in dorsal root ganglia and can cause herpes zoster (HZ) in vaccinated children.
- When HZ due to a vaccine strain of VZV occurs, the rash often presents near the site of initial vaccination.
- Although most cases of HZ in vaccinated children present with a characteristic HZ rash, physicians should be aware of the possibility for atypical presentations.